User login
Studying in Dermatology Residency
Dermatology residency can feel like drinking from a firehose, in which one is bombarded with so much information that it is impossible to retain any content. This article provides an overview of available resources and a guide on how to tailor studying throughout one’s training.
Prior to Residency
There are several resources that provide an introduction to dermatology and are appropriate for all medical students, regardless of intended specialty. The American Academy of Dermatology offers a free basic dermatology curriculum (https://www.aad.org/member/education/residents/bdc), with a choice of a 2- or 4-week course consisting of modules such as skin examination, basic science of the skin, dermatologic therapies, and specific dermatologic conditions. VisualDx offers LearnDerm (https://www.visualdx.com/learnderm/), which includes a 5-part tutorial and quiz focused on the skin examination, morphology, and lesion distribution. Lookingbill and Marks’ Principles of Dermatology1 is a book at an appropriate level for a medical student to learn about the fundamentals of dermatology. These resources may be helpful for residents to review immediately before starting dermatology residency (toward the end of intern year for most).
First Year
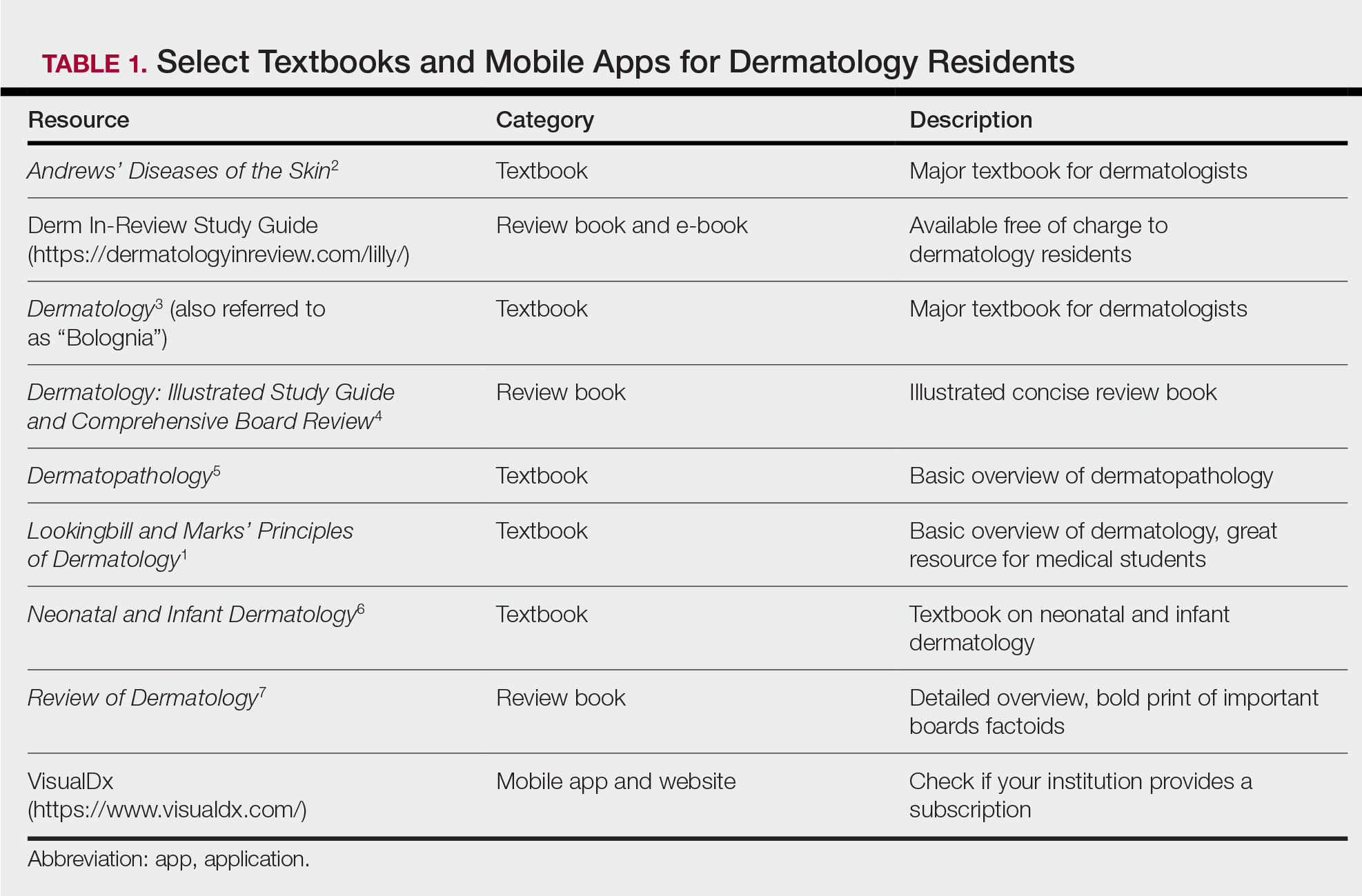
During the beginning of dermatology residency (postgraduate year [PGY] 2 for most), the fire hose of information feels most daunting. During this time, studying should focus on engendering a broad overview of dermatology. Most residencies maintain a textbook reading schedule, which provides a framework from which residents may structure their studying. Selection of a textbook tends to be program dependent. Even if the details of reading the textbook do not stick when reading it the first time, benefits include becoming familiar with what information one is expected to learn as a dermatologist and developing a strong foundation upon which one may continue to build. Based on my informal discussions with current residents, some reported that reading the textbook did not work well for them, citing too much minutiae in the textbooks and/or a preference for a more active learning approach. These residents instead focused on reading a review book for a broad overview, accompanied by a textbook or VisualDx when a more detailed reference is necessary. Table 1 provides a list of textbooks and mobile applications (apps) that residents may find helpful.
First-year residents may begin their efforts in synthesizing this new knowledge base toward the end of the year in preparation for the BASIC examination. The American Board of Dermatology provides a content outline as well as sample questions on their website (https://www.abderm.org/residents-and-fellows/exam-of-the-future-information-center.aspx#content), which may be used to guide more focused studying efforts during the weeks leading up to the examination.
Second Year
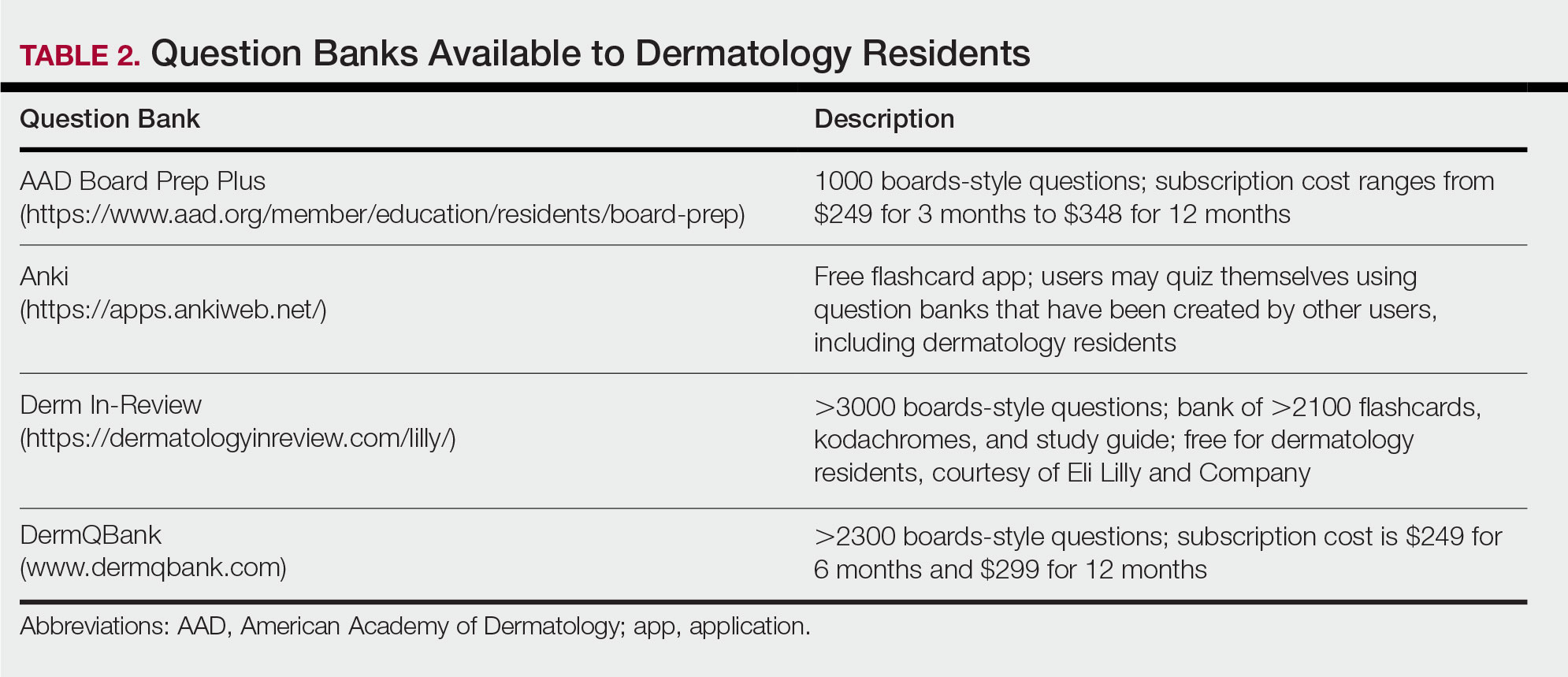
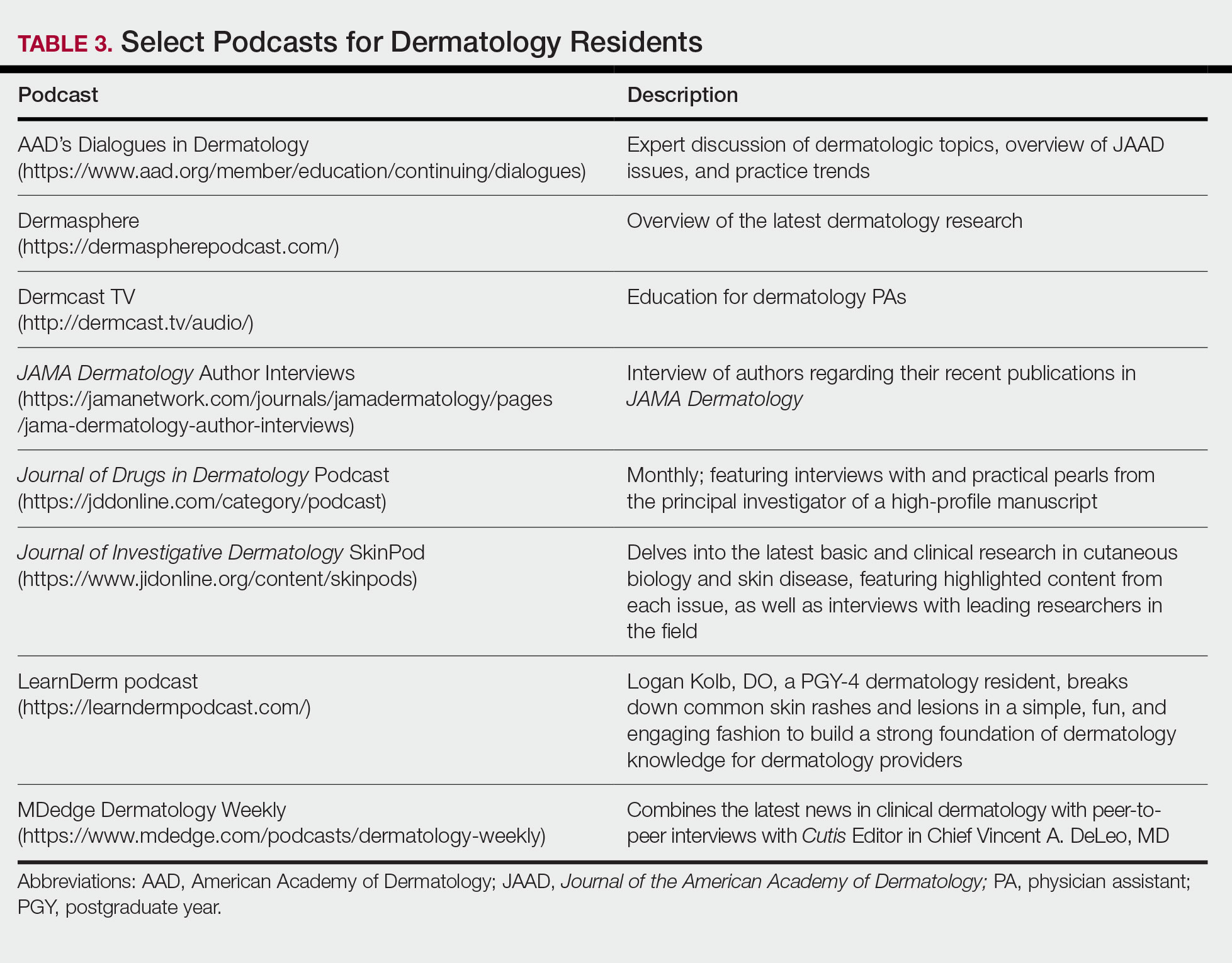
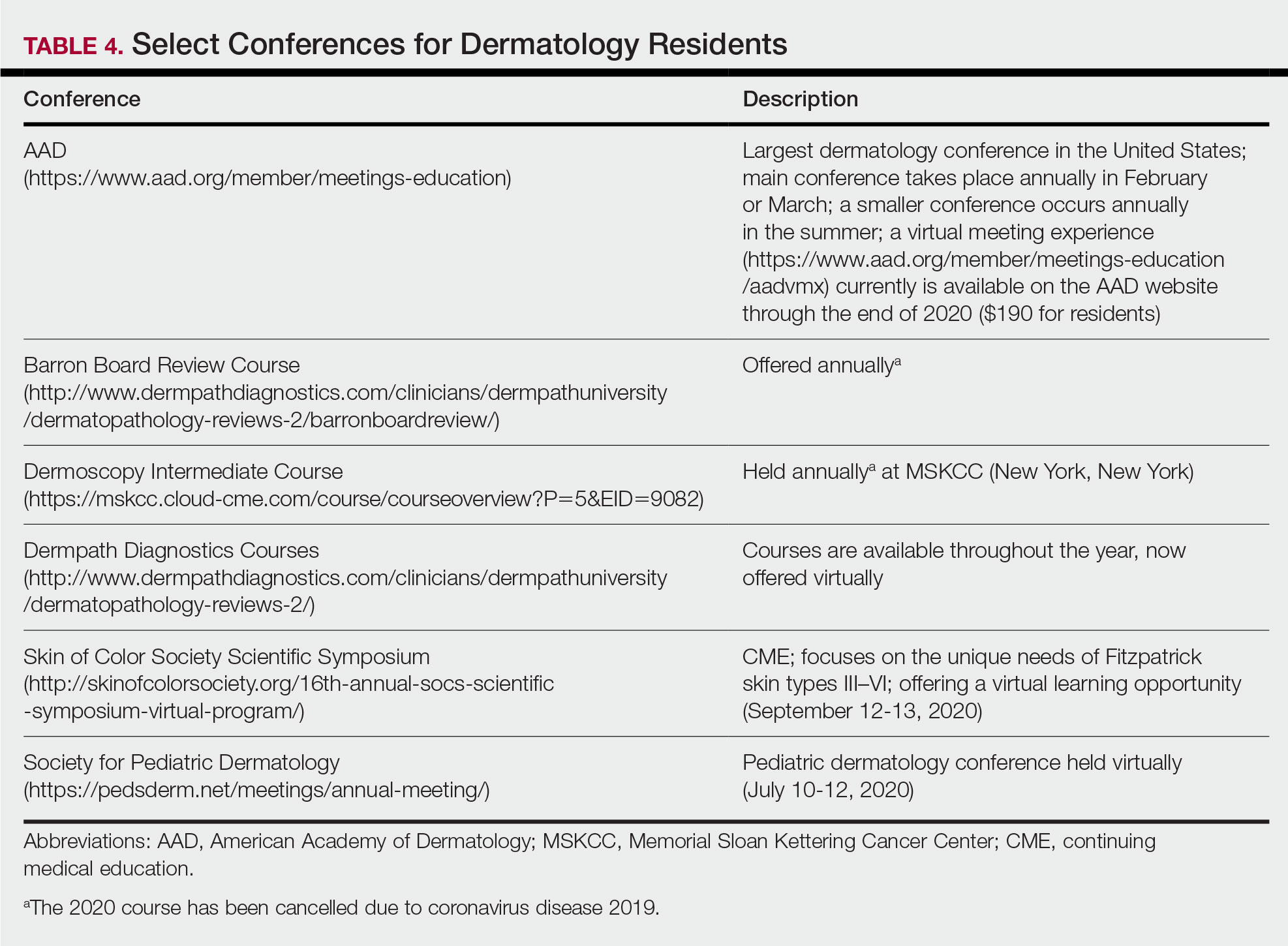
For second-year residents (PGY-3 for most) studying should focus on deepening and consolidating the broad foundation that was established during their first year. For many, this pursuit involves rereading the textbook chapters alongside more active learning measures, such as taking notes and quizzing oneself using flashcard apps and question banks (Table 2). Others may benefit from listening to podcasts (Table 3) or other sources utilizing audiovisual content, including attending conferences and other lectures virtually, which is becoming increasingly available in the setting of the coronavirus disease 2019 pandemic (Table 4). Because there are so many resources available to support these efforts, residents should be encouraged to try out a variety to determine what works best.
Toward the end of second year, studying may be tailored to preparing for the CORE examinations using the resources of one’s choice. Based on my discussions with current residents, a combination of reading review books, reviewing one’s personal notes, and quizzing through question banks and/or flashcard apps could be used.
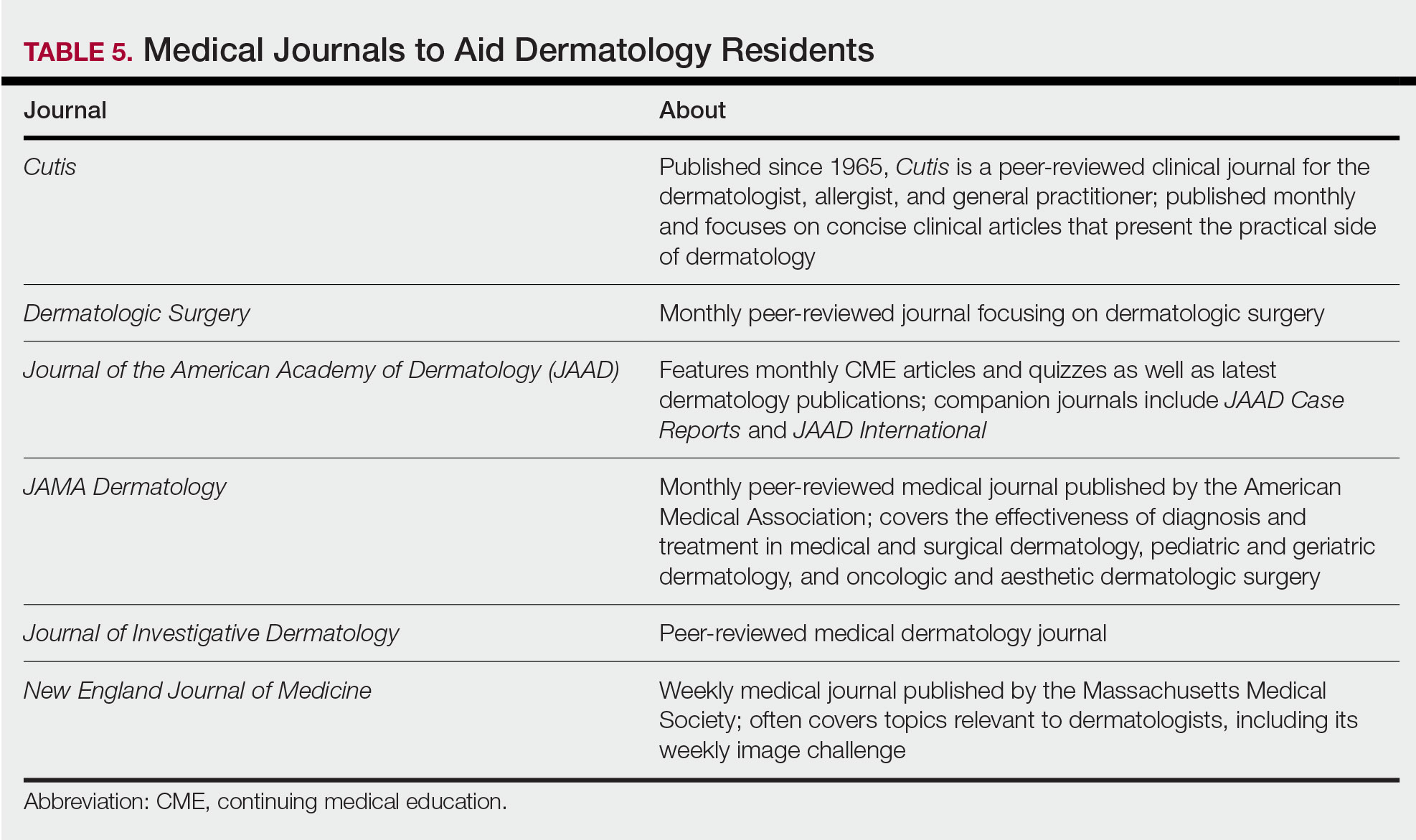
In addition to maintaining a consistent and organized study schedule, second-year residents should continue to read in depth on topics related to patients for whom they are caring and stay on top of the dermatology literature. Table 5 provides a list of medical journals that dermatology residents should aim to read. The Journal of the American Academy of Dermatology’s continuing medical education series (https://www.jaad.org/content/collection-cme) may be particularly helpful to residents. In this series, experts review a variety of dermatologic topics in depth paired with quiz questions.
Third Year
As a third-year resident (PGY-4 for most), studying should focus on deepening one’s knowledge base and beginning preparation for the boards examination. At this point, residents should stick to a limited selection of resources (ie, 1 textbook, 1 review book, 1 question bank) for in-depth study. More time should be spent on active learning, such as note-taking and question banks. Boards review courses historically have been available to dermatology residents, namely the Barron Board Review course and a plenary session at the American Academy of Dermatology Annual Conference (Table 4).
Consistent Habits
Studying strategies can and should differ throughout dermatology residency, though consistency is necessary throughout. It is helpful to plan study schedules in advance—yearly, monthly, weekly—and aim to stick to them as much as possible. Finding what works for each individual may take trial and error. For some, it may mean waking up early to study before work, whereas others may do better in the evenings. It also is helpful to utilize a combination of longer blocks of studying (ie, weekend days), with consistent shorter blocks of time during the week. Many residents also learn to take advantage of time spent commuting by listening to podcasts in the car or reading while on public transportation.
Final Thoughts
There are many resources available to support residents in their learning such as textbooks, journals, podcasts, flashcards, question banks, and more. The path to mastery will be individualized for each resident, likely using a unique combination of resources. The beginning of residency is a good time to explore a variety of resources to see what works best, whereas at the end studying becomes more targeted.
- Marks Jr JG, Miller JJ. Lookingbill and Marks’ Principles of Dermatology. 6th ed. China: Elsevier; 2019.
- James WD, Elston DM, Treat JR. Andrews’ Diseases of the Skin. 13th ed. China: Elsevier; 2019.
- Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. China: Elsevier; 2018.
- Jain S. Dermatology: Illustrated Study Guide and Comprehensive Board Review. New York, NY: Springer; 2012.
- Elston DM, Ferringer T, Ko C, et al. Dermatopathology. 2nd ed. China: Elsevier Saunders; 2014.
- Eichenfield LF, Frieden IJ, eds. Neonatal and Infant Dermatology. London, England: Saunders; 2015.
- Alikhan A, Hocker TLH, eds. Review of Dermatology. China: Elsevier; 2017.
Dermatology residency can feel like drinking from a firehose, in which one is bombarded with so much information that it is impossible to retain any content. This article provides an overview of available resources and a guide on how to tailor studying throughout one’s training.
Prior to Residency
There are several resources that provide an introduction to dermatology and are appropriate for all medical students, regardless of intended specialty. The American Academy of Dermatology offers a free basic dermatology curriculum (https://www.aad.org/member/education/residents/bdc), with a choice of a 2- or 4-week course consisting of modules such as skin examination, basic science of the skin, dermatologic therapies, and specific dermatologic conditions. VisualDx offers LearnDerm (https://www.visualdx.com/learnderm/), which includes a 5-part tutorial and quiz focused on the skin examination, morphology, and lesion distribution. Lookingbill and Marks’ Principles of Dermatology1 is a book at an appropriate level for a medical student to learn about the fundamentals of dermatology. These resources may be helpful for residents to review immediately before starting dermatology residency (toward the end of intern year for most).
First Year
During the beginning of dermatology residency (postgraduate year [PGY] 2 for most), the fire hose of information feels most daunting. During this time, studying should focus on engendering a broad overview of dermatology. Most residencies maintain a textbook reading schedule, which provides a framework from which residents may structure their studying. Selection of a textbook tends to be program dependent. Even if the details of reading the textbook do not stick when reading it the first time, benefits include becoming familiar with what information one is expected to learn as a dermatologist and developing a strong foundation upon which one may continue to build. Based on my informal discussions with current residents, some reported that reading the textbook did not work well for them, citing too much minutiae in the textbooks and/or a preference for a more active learning approach. These residents instead focused on reading a review book for a broad overview, accompanied by a textbook or VisualDx when a more detailed reference is necessary. Table 1 provides a list of textbooks and mobile applications (apps) that residents may find helpful.

First-year residents may begin their efforts in synthesizing this new knowledge base toward the end of the year in preparation for the BASIC examination. The American Board of Dermatology provides a content outline as well as sample questions on their website (https://www.abderm.org/residents-and-fellows/exam-of-the-future-information-center.aspx#content), which may be used to guide more focused studying efforts during the weeks leading up to the examination.
Second Year
For second-year residents (PGY-3 for most) studying should focus on deepening and consolidating the broad foundation that was established during their first year. For many, this pursuit involves rereading the textbook chapters alongside more active learning measures, such as taking notes and quizzing oneself using flashcard apps and question banks (Table 2). Others may benefit from listening to podcasts (Table 3) or other sources utilizing audiovisual content, including attending conferences and other lectures virtually, which is becoming increasingly available in the setting of the coronavirus disease 2019 pandemic (Table 4). Because there are so many resources available to support these efforts, residents should be encouraged to try out a variety to determine what works best.



Toward the end of second year, studying may be tailored to preparing for the CORE examinations using the resources of one’s choice. Based on my discussions with current residents, a combination of reading review books, reviewing one’s personal notes, and quizzing through question banks and/or flashcard apps could be used.
In addition to maintaining a consistent and organized study schedule, second-year residents should continue to read in depth on topics related to patients for whom they are caring and stay on top of the dermatology literature. Table 5 provides a list of medical journals that dermatology residents should aim to read. The Journal of the American Academy of Dermatology’s continuing medical education series (https://www.jaad.org/content/collection-cme) may be particularly helpful to residents. In this series, experts review a variety of dermatologic topics in depth paired with quiz questions.

Third Year
As a third-year resident (PGY-4 for most), studying should focus on deepening one’s knowledge base and beginning preparation for the boards examination. At this point, residents should stick to a limited selection of resources (ie, 1 textbook, 1 review book, 1 question bank) for in-depth study. More time should be spent on active learning, such as note-taking and question banks. Boards review courses historically have been available to dermatology residents, namely the Barron Board Review course and a plenary session at the American Academy of Dermatology Annual Conference (Table 4).
Consistent Habits
Studying strategies can and should differ throughout dermatology residency, though consistency is necessary throughout. It is helpful to plan study schedules in advance—yearly, monthly, weekly—and aim to stick to them as much as possible. Finding what works for each individual may take trial and error. For some, it may mean waking up early to study before work, whereas others may do better in the evenings. It also is helpful to utilize a combination of longer blocks of studying (ie, weekend days), with consistent shorter blocks of time during the week. Many residents also learn to take advantage of time spent commuting by listening to podcasts in the car or reading while on public transportation.
Final Thoughts
There are many resources available to support residents in their learning such as textbooks, journals, podcasts, flashcards, question banks, and more. The path to mastery will be individualized for each resident, likely using a unique combination of resources. The beginning of residency is a good time to explore a variety of resources to see what works best, whereas at the end studying becomes more targeted.
Dermatology residency can feel like drinking from a firehose, in which one is bombarded with so much information that it is impossible to retain any content. This article provides an overview of available resources and a guide on how to tailor studying throughout one’s training.
Prior to Residency
There are several resources that provide an introduction to dermatology and are appropriate for all medical students, regardless of intended specialty. The American Academy of Dermatology offers a free basic dermatology curriculum (https://www.aad.org/member/education/residents/bdc), with a choice of a 2- or 4-week course consisting of modules such as skin examination, basic science of the skin, dermatologic therapies, and specific dermatologic conditions. VisualDx offers LearnDerm (https://www.visualdx.com/learnderm/), which includes a 5-part tutorial and quiz focused on the skin examination, morphology, and lesion distribution. Lookingbill and Marks’ Principles of Dermatology1 is a book at an appropriate level for a medical student to learn about the fundamentals of dermatology. These resources may be helpful for residents to review immediately before starting dermatology residency (toward the end of intern year for most).
First Year
During the beginning of dermatology residency (postgraduate year [PGY] 2 for most), the fire hose of information feels most daunting. During this time, studying should focus on engendering a broad overview of dermatology. Most residencies maintain a textbook reading schedule, which provides a framework from which residents may structure their studying. Selection of a textbook tends to be program dependent. Even if the details of reading the textbook do not stick when reading it the first time, benefits include becoming familiar with what information one is expected to learn as a dermatologist and developing a strong foundation upon which one may continue to build. Based on my informal discussions with current residents, some reported that reading the textbook did not work well for them, citing too much minutiae in the textbooks and/or a preference for a more active learning approach. These residents instead focused on reading a review book for a broad overview, accompanied by a textbook or VisualDx when a more detailed reference is necessary. Table 1 provides a list of textbooks and mobile applications (apps) that residents may find helpful.

First-year residents may begin their efforts in synthesizing this new knowledge base toward the end of the year in preparation for the BASIC examination. The American Board of Dermatology provides a content outline as well as sample questions on their website (https://www.abderm.org/residents-and-fellows/exam-of-the-future-information-center.aspx#content), which may be used to guide more focused studying efforts during the weeks leading up to the examination.
Second Year
For second-year residents (PGY-3 for most) studying should focus on deepening and consolidating the broad foundation that was established during their first year. For many, this pursuit involves rereading the textbook chapters alongside more active learning measures, such as taking notes and quizzing oneself using flashcard apps and question banks (Table 2). Others may benefit from listening to podcasts (Table 3) or other sources utilizing audiovisual content, including attending conferences and other lectures virtually, which is becoming increasingly available in the setting of the coronavirus disease 2019 pandemic (Table 4). Because there are so many resources available to support these efforts, residents should be encouraged to try out a variety to determine what works best.



Toward the end of second year, studying may be tailored to preparing for the CORE examinations using the resources of one’s choice. Based on my discussions with current residents, a combination of reading review books, reviewing one’s personal notes, and quizzing through question banks and/or flashcard apps could be used.
In addition to maintaining a consistent and organized study schedule, second-year residents should continue to read in depth on topics related to patients for whom they are caring and stay on top of the dermatology literature. Table 5 provides a list of medical journals that dermatology residents should aim to read. The Journal of the American Academy of Dermatology’s continuing medical education series (https://www.jaad.org/content/collection-cme) may be particularly helpful to residents. In this series, experts review a variety of dermatologic topics in depth paired with quiz questions.

Third Year
As a third-year resident (PGY-4 for most), studying should focus on deepening one’s knowledge base and beginning preparation for the boards examination. At this point, residents should stick to a limited selection of resources (ie, 1 textbook, 1 review book, 1 question bank) for in-depth study. More time should be spent on active learning, such as note-taking and question banks. Boards review courses historically have been available to dermatology residents, namely the Barron Board Review course and a plenary session at the American Academy of Dermatology Annual Conference (Table 4).
Consistent Habits
Studying strategies can and should differ throughout dermatology residency, though consistency is necessary throughout. It is helpful to plan study schedules in advance—yearly, monthly, weekly—and aim to stick to them as much as possible. Finding what works for each individual may take trial and error. For some, it may mean waking up early to study before work, whereas others may do better in the evenings. It also is helpful to utilize a combination of longer blocks of studying (ie, weekend days), with consistent shorter blocks of time during the week. Many residents also learn to take advantage of time spent commuting by listening to podcasts in the car or reading while on public transportation.
Final Thoughts
There are many resources available to support residents in their learning such as textbooks, journals, podcasts, flashcards, question banks, and more. The path to mastery will be individualized for each resident, likely using a unique combination of resources. The beginning of residency is a good time to explore a variety of resources to see what works best, whereas at the end studying becomes more targeted.
- Marks Jr JG, Miller JJ. Lookingbill and Marks’ Principles of Dermatology. 6th ed. China: Elsevier; 2019.
- James WD, Elston DM, Treat JR. Andrews’ Diseases of the Skin. 13th ed. China: Elsevier; 2019.
- Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. China: Elsevier; 2018.
- Jain S. Dermatology: Illustrated Study Guide and Comprehensive Board Review. New York, NY: Springer; 2012.
- Elston DM, Ferringer T, Ko C, et al. Dermatopathology. 2nd ed. China: Elsevier Saunders; 2014.
- Eichenfield LF, Frieden IJ, eds. Neonatal and Infant Dermatology. London, England: Saunders; 2015.
- Alikhan A, Hocker TLH, eds. Review of Dermatology. China: Elsevier; 2017.
- Marks Jr JG, Miller JJ. Lookingbill and Marks’ Principles of Dermatology. 6th ed. China: Elsevier; 2019.
- James WD, Elston DM, Treat JR. Andrews’ Diseases of the Skin. 13th ed. China: Elsevier; 2019.
- Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. China: Elsevier; 2018.
- Jain S. Dermatology: Illustrated Study Guide and Comprehensive Board Review. New York, NY: Springer; 2012.
- Elston DM, Ferringer T, Ko C, et al. Dermatopathology. 2nd ed. China: Elsevier Saunders; 2014.
- Eichenfield LF, Frieden IJ, eds. Neonatal and Infant Dermatology. London, England: Saunders; 2015.
- Alikhan A, Hocker TLH, eds. Review of Dermatology. China: Elsevier; 2017.
Resident Pearls
- Independent study is a large component of dermatology residency.
- Consistent habits and a tailored approach will support optimal learning for each dermatology resident.
- The beginning of residency is a good time to explore a variety of resources to see what works best. Toward the end of residency, as studying becomes more targeted, residents may benefit from sticking to the resources with which they are most comfortable.
Lifting the restrictions on mifepristone during COVID-19: A step in the right direction
Mifepristone is a safe, effective, and well-tolerated medication for managing miscarriage and for medical abortion when combined with misoprostol.1,2 Since the US Food and Drug Administration (FDA) approved its use in 2000, more than 4 million women have used this medication.3 The combination of mifepristone with misoprostol was used for 39% of all US abortions in 2017.4 Approximately 10% of all clinically recognized pregnancies end in miscarriages, and many are safely managed with either misoprostol alone or with the combination of mifepristone and misoprostol.5
The issue
The prescription and distribution of mifepristone is highly regulated by the FDA via requirements outlined in the Risk Evaluation and Mitigation Strategies (REMS) drug safety program. The FDA may determine a REMS is necessary for a specific drug to ensure the benefits of a drug outweigh the potential risks. A REMS may include an informative package insert for patients, follow-up communication to prescribers—including letters, safety protocols or recommended laboratory tests, or Elements to Assure Safe Use (ETASU). ETASU are types of REMS that are placed on medications that have significant potential for serious adverse effects, and without such restrictions FDA approval would be rescinded.
Are mifepristone requirements fairly applied?
The 3 ETASU restrictions on the distribution of mifepristone are in-person dispensation, prescriber certification, and patient signatures on special forms.6 The in-person dispensing requirement is applied to only 16 other medications (one of which is Mifeprex, the brand version of mifepristone), and Mifeprex/mifepristone are the only ones deemed safe for self-administration—meaning that patients receive the drug from a clinic but then may take it at a site of their choosing. The prescriber certification requirement places expectations on providers to account for distribution of doses and keep records of serial numbers (in effect, having clinicians act as both physician and pharmacist, as most medications are distributed and recorded in pharmacies). The patient form was recommended for elimination in 2016 due to its duplicative information and burden on patients—a recommendation that was then overruled by the FDA commissioner.7
These 3 requirements placed on mifepristone specifically target dosages for use related to abortions and miscarriages. Mifepristone is used to treat other medical conditions, with much higher doses, without the same restrictions—in fact, the FDA has allowed much higher doses of mifepristone to be mailed directly to a patient when prescribed for different disorders. The American College of Obstetricians and Gynecologists (ACOG) has long opposed the burdensome REMS requirements on mifepristone for reproductive health indications.8
Arguments regarding the safety of mifepristone must be understood in the context of how the medication is taken, and the unique difference with other medications that must be administered by physicians or in health care facilities. Mifepristone is self-administered, and the desired effect—evacuation of uterine contents—typically occurs after a patient takes the accompanying medication misoprostol, which is some 24 to 72 hours later. This timeframe makes it highly unlikely that any patient would be in the presence of their provider at the time of medication effect, thus an in-person dispensing requirement has no medical bearing on the outcome of the health of the patient.
REMS changes during the COVID-19 pandemic
The coronavirus disease 2019 (COVID-19) pandemic has necessarily changed the structure of REMS and ETASU requirements for many medications, with changes made in order to mitigate viral transmission through the limitation of unnecessary visits to clinics or hospitals. The FDA announced in March of 2020 that it would not enforce pre-prescription requirements, such as laboratory or magnetic resonance imaging results, for many medications (including those more toxic than mifepristone), and that it would lift the requirement for in-person dispensation of several medications.9 Also in March 2020 the Department of Health and Human Services Secretary (HHS) and the Drug Enforcement Agency (DEA) activated a “telemedicine exception” to allow physicians to use telemedicine to satisfy mandatory requirements for prescribing controlled substances, including opioids.10
Despite repeated pleas from organizations, individuals, and physician groups, the FDA continued to enforce the REMS/ETASU for mifepristone as the pandemic decimated communities. Importantly, the pandemic has not had an equal effect on all communities, and the disparities highlighted in outcomes as related to COVID-19 are also reflected in disparities to access to reproductive choices.11 By enforcing REMS/ETASU for mifepristone during a global pandemic, the FDA has placed additional burden on women and people who menstruate. As offices and clinics have closed, and as many jobs have evaporated, additional barriers have emerged, such as lack of childcare, fewer transportation options, and decreased clinic appointments.
As the pandemic continues to affect communities in the United States, ACOG has issued guidance recommending assessment for eligibility for medical abortion remotely, and has encouraged the use of telemedicine and other remote interactions for its members and patients to limit transmission of the virus.
The lawsuit
On May 27, 2020, the American Civil Liberties Union (ACLU) (on behalf of ACOG, the Council of University Chairs of Obstetrics and Gynecology, New York State Academy of Family Physicians, SisterSong, and Honor MacNaughton, MD) filed a civil action against the FDA and HHS challenging the requirement for in-person dispensing of mifepristone and associated ETASU requirements during the COVID-19 pandemic. The plaintiffs sought this injunction based on the claim that these restrictions during the pandemic infringe on the constitutional rights to patients’ privacy and liberty and to equal protection of the law as protected by the Due Process Clause of the Fifth Amendment. Additionally, the ACLU and other organizations said these unnecessary restrictions place patients, providers, and staff at unnecessary risk of viral exposure amidst a global pandemic.
The verdict
On July 13, 2020, a federal court granted the preliminary injunction to suspend FDA’s enforcement of the in-person requirements of mifepristone for abortion during the COVID-19 pandemic. The court denied the motion for suspension of in-person restrictions as applied to miscarriage management. The preliminary injunction applies nationwide without geographic limitation. It will remain in effect until the end of the litigation or for 30 days following the expiration of the public health emergency.
What the outcome means
This injunction is a step in the right direction for patients and providers to allow for autonomy and clinical practice guided by clinician expertise. However, this ruling remains narrow. Patients must be counseled about mifepristone via telemedicine and sign a Patient Agreement Form, which must be returned electronically or by mail. Patients must receive a copy of the mifepristone medication guide, and dispensing of mifepristone must still be conducted by or under the supervision of a certified provider. The medication may not be dispensed by retail pharmacies, thus requiring providers to arrange for mailing of prescriptions to patients. Given state-based legal statutes regarding mailing of medications, this injunction may not lead to an immediate increase in access to care. In addition, patients seeking management for miscarriage must go to clinic to have mifepristone dispensed and thus risk exposure to viral transmission.
What now?
The regulation of mifepristone—in spite of excellent safety and specifically for the narrow purpose of administration in the setting of abortion and miscarriage care—is by definition a discriminatory practice against patients and providers. As clinicians, we are duty-bound to speak out against injustices to our practices and our patients. At a local level, we can work to implement safe practices in the setting of this injunction and continue to work on a national level to ensure this injunction becomes permanent and with more broad scope to eliminate all of the REMS requirements for mifepristone.
ACTION ITEMS
- Act locally! Are you an abortion provider? Contact your local ACLU (find them here) or lawyer in your area for assistance navigating the legal landscape to prescribe after this injunction.
- Act statewide! Press candidates in your state to stand up for science and data. Support legislative acts and bills that address combating discriminatory regulations.
- Act nationally! The President is responsible for appointing the Commissioner of the FDA and the Secretary of Health and Human Services (with Senate advice and consent). Who we elect matters. Seek out opportunities to become involved in increasing access to and awareness of voter registration and Election Day, and speak out against voter suppression. Make sure you are registered to vote here and check your area to review new recommendations amidst the pandemic.
- American College of Obstetricians and Gynecologists. Practice bulletin number 143: medical management of first trimester abortion. Obstet Gynecol. 2014;123:676-692.
- Schreiber CA, Crenin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Danco Laboratories. Mifeprex effectiveness and advantages. https://www.earlyoptionpill.com/is-mifeprex-right-for-me/effectiveness-advantages/ Accessed August 2, 2020.
- Jones RK, Witwer E, Jerman J. Abortion incidence and service availability in the United States, 2017. September 2019. https://www.guttmacher.org/report/abortion-incidence-service-availability-us-2017. Accessed September 10, 2020.
- American College of Obstetricians and Gynecologists. Practice bulletin no. 150: early pregnancy loss. Obstet Gynecol. 2015;125:1258-1267.
- US Food and Drug Administration. Risk evaluation and mitigation strategy (REMS) single shared system for mifepristone 200 mg. April 2019. https://www.accessdata.fda.gov/drugsatfda_docs/rems/Mifepristone_2019_04_11_REMS_Full.pdf. Accessed September 10, 2020.
- US Food and Drug Administration; Center for Drug Evaluation and Research. 2016 REMS Review, Summary Review 25. March 29, 2016. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/020687Orig1s020SumR.pdf. Accessed September 10, 2020.
- American College of Obstetricians and Gynecologists. Improving access to mifepristone for reproductive health indications. June 2018. https://www.acog.org/clinical-information/policy-and-position-statements/position-statements/2018/improving-access-to-mifepristone-for-reproductive-health-indications. Accessed August 2, 2020.
- US Food and Drug Administration. Policy for certain REMS requirements during the COVID-19 public health emergency: guidance for industry and health care professionals. March 2020. https://www.fda.gov/media/136317/download. Accessed September 10, 2020.
- US Department of Justice. US Drug Enforcement Administration. COVID-19 Information Page, Telemedicine. https://www.deadiversion.usdoj.gov/coronavirus.html#TELE. Accessed May 25, 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019: health equity considerations and racial and ethnic minority groups. https://www.cdc.gov/coronavirus/2019-ncov/community/health-equity/race-ethnicity.html. Updated July 24, 2020. Accessed September 10, 2020.
Mifepristone is a safe, effective, and well-tolerated medication for managing miscarriage and for medical abortion when combined with misoprostol.1,2 Since the US Food and Drug Administration (FDA) approved its use in 2000, more than 4 million women have used this medication.3 The combination of mifepristone with misoprostol was used for 39% of all US abortions in 2017.4 Approximately 10% of all clinically recognized pregnancies end in miscarriages, and many are safely managed with either misoprostol alone or with the combination of mifepristone and misoprostol.5
The issue
The prescription and distribution of mifepristone is highly regulated by the FDA via requirements outlined in the Risk Evaluation and Mitigation Strategies (REMS) drug safety program. The FDA may determine a REMS is necessary for a specific drug to ensure the benefits of a drug outweigh the potential risks. A REMS may include an informative package insert for patients, follow-up communication to prescribers—including letters, safety protocols or recommended laboratory tests, or Elements to Assure Safe Use (ETASU). ETASU are types of REMS that are placed on medications that have significant potential for serious adverse effects, and without such restrictions FDA approval would be rescinded.
Are mifepristone requirements fairly applied?
The 3 ETASU restrictions on the distribution of mifepristone are in-person dispensation, prescriber certification, and patient signatures on special forms.6 The in-person dispensing requirement is applied to only 16 other medications (one of which is Mifeprex, the brand version of mifepristone), and Mifeprex/mifepristone are the only ones deemed safe for self-administration—meaning that patients receive the drug from a clinic but then may take it at a site of their choosing. The prescriber certification requirement places expectations on providers to account for distribution of doses and keep records of serial numbers (in effect, having clinicians act as both physician and pharmacist, as most medications are distributed and recorded in pharmacies). The patient form was recommended for elimination in 2016 due to its duplicative information and burden on patients—a recommendation that was then overruled by the FDA commissioner.7
These 3 requirements placed on mifepristone specifically target dosages for use related to abortions and miscarriages. Mifepristone is used to treat other medical conditions, with much higher doses, without the same restrictions—in fact, the FDA has allowed much higher doses of mifepristone to be mailed directly to a patient when prescribed for different disorders. The American College of Obstetricians and Gynecologists (ACOG) has long opposed the burdensome REMS requirements on mifepristone for reproductive health indications.8
Arguments regarding the safety of mifepristone must be understood in the context of how the medication is taken, and the unique difference with other medications that must be administered by physicians or in health care facilities. Mifepristone is self-administered, and the desired effect—evacuation of uterine contents—typically occurs after a patient takes the accompanying medication misoprostol, which is some 24 to 72 hours later. This timeframe makes it highly unlikely that any patient would be in the presence of their provider at the time of medication effect, thus an in-person dispensing requirement has no medical bearing on the outcome of the health of the patient.
REMS changes during the COVID-19 pandemic
The coronavirus disease 2019 (COVID-19) pandemic has necessarily changed the structure of REMS and ETASU requirements for many medications, with changes made in order to mitigate viral transmission through the limitation of unnecessary visits to clinics or hospitals. The FDA announced in March of 2020 that it would not enforce pre-prescription requirements, such as laboratory or magnetic resonance imaging results, for many medications (including those more toxic than mifepristone), and that it would lift the requirement for in-person dispensation of several medications.9 Also in March 2020 the Department of Health and Human Services Secretary (HHS) and the Drug Enforcement Agency (DEA) activated a “telemedicine exception” to allow physicians to use telemedicine to satisfy mandatory requirements for prescribing controlled substances, including opioids.10
Despite repeated pleas from organizations, individuals, and physician groups, the FDA continued to enforce the REMS/ETASU for mifepristone as the pandemic decimated communities. Importantly, the pandemic has not had an equal effect on all communities, and the disparities highlighted in outcomes as related to COVID-19 are also reflected in disparities to access to reproductive choices.11 By enforcing REMS/ETASU for mifepristone during a global pandemic, the FDA has placed additional burden on women and people who menstruate. As offices and clinics have closed, and as many jobs have evaporated, additional barriers have emerged, such as lack of childcare, fewer transportation options, and decreased clinic appointments.
As the pandemic continues to affect communities in the United States, ACOG has issued guidance recommending assessment for eligibility for medical abortion remotely, and has encouraged the use of telemedicine and other remote interactions for its members and patients to limit transmission of the virus.
The lawsuit
On May 27, 2020, the American Civil Liberties Union (ACLU) (on behalf of ACOG, the Council of University Chairs of Obstetrics and Gynecology, New York State Academy of Family Physicians, SisterSong, and Honor MacNaughton, MD) filed a civil action against the FDA and HHS challenging the requirement for in-person dispensing of mifepristone and associated ETASU requirements during the COVID-19 pandemic. The plaintiffs sought this injunction based on the claim that these restrictions during the pandemic infringe on the constitutional rights to patients’ privacy and liberty and to equal protection of the law as protected by the Due Process Clause of the Fifth Amendment. Additionally, the ACLU and other organizations said these unnecessary restrictions place patients, providers, and staff at unnecessary risk of viral exposure amidst a global pandemic.
The verdict
On July 13, 2020, a federal court granted the preliminary injunction to suspend FDA’s enforcement of the in-person requirements of mifepristone for abortion during the COVID-19 pandemic. The court denied the motion for suspension of in-person restrictions as applied to miscarriage management. The preliminary injunction applies nationwide without geographic limitation. It will remain in effect until the end of the litigation or for 30 days following the expiration of the public health emergency.
What the outcome means
This injunction is a step in the right direction for patients and providers to allow for autonomy and clinical practice guided by clinician expertise. However, this ruling remains narrow. Patients must be counseled about mifepristone via telemedicine and sign a Patient Agreement Form, which must be returned electronically or by mail. Patients must receive a copy of the mifepristone medication guide, and dispensing of mifepristone must still be conducted by or under the supervision of a certified provider. The medication may not be dispensed by retail pharmacies, thus requiring providers to arrange for mailing of prescriptions to patients. Given state-based legal statutes regarding mailing of medications, this injunction may not lead to an immediate increase in access to care. In addition, patients seeking management for miscarriage must go to clinic to have mifepristone dispensed and thus risk exposure to viral transmission.
What now?
The regulation of mifepristone—in spite of excellent safety and specifically for the narrow purpose of administration in the setting of abortion and miscarriage care—is by definition a discriminatory practice against patients and providers. As clinicians, we are duty-bound to speak out against injustices to our practices and our patients. At a local level, we can work to implement safe practices in the setting of this injunction and continue to work on a national level to ensure this injunction becomes permanent and with more broad scope to eliminate all of the REMS requirements for mifepristone.
ACTION ITEMS
- Act locally! Are you an abortion provider? Contact your local ACLU (find them here) or lawyer in your area for assistance navigating the legal landscape to prescribe after this injunction.
- Act statewide! Press candidates in your state to stand up for science and data. Support legislative acts and bills that address combating discriminatory regulations.
- Act nationally! The President is responsible for appointing the Commissioner of the FDA and the Secretary of Health and Human Services (with Senate advice and consent). Who we elect matters. Seek out opportunities to become involved in increasing access to and awareness of voter registration and Election Day, and speak out against voter suppression. Make sure you are registered to vote here and check your area to review new recommendations amidst the pandemic.
Mifepristone is a safe, effective, and well-tolerated medication for managing miscarriage and for medical abortion when combined with misoprostol.1,2 Since the US Food and Drug Administration (FDA) approved its use in 2000, more than 4 million women have used this medication.3 The combination of mifepristone with misoprostol was used for 39% of all US abortions in 2017.4 Approximately 10% of all clinically recognized pregnancies end in miscarriages, and many are safely managed with either misoprostol alone or with the combination of mifepristone and misoprostol.5
The issue
The prescription and distribution of mifepristone is highly regulated by the FDA via requirements outlined in the Risk Evaluation and Mitigation Strategies (REMS) drug safety program. The FDA may determine a REMS is necessary for a specific drug to ensure the benefits of a drug outweigh the potential risks. A REMS may include an informative package insert for patients, follow-up communication to prescribers—including letters, safety protocols or recommended laboratory tests, or Elements to Assure Safe Use (ETASU). ETASU are types of REMS that are placed on medications that have significant potential for serious adverse effects, and without such restrictions FDA approval would be rescinded.
Are mifepristone requirements fairly applied?
The 3 ETASU restrictions on the distribution of mifepristone are in-person dispensation, prescriber certification, and patient signatures on special forms.6 The in-person dispensing requirement is applied to only 16 other medications (one of which is Mifeprex, the brand version of mifepristone), and Mifeprex/mifepristone are the only ones deemed safe for self-administration—meaning that patients receive the drug from a clinic but then may take it at a site of their choosing. The prescriber certification requirement places expectations on providers to account for distribution of doses and keep records of serial numbers (in effect, having clinicians act as both physician and pharmacist, as most medications are distributed and recorded in pharmacies). The patient form was recommended for elimination in 2016 due to its duplicative information and burden on patients—a recommendation that was then overruled by the FDA commissioner.7
These 3 requirements placed on mifepristone specifically target dosages for use related to abortions and miscarriages. Mifepristone is used to treat other medical conditions, with much higher doses, without the same restrictions—in fact, the FDA has allowed much higher doses of mifepristone to be mailed directly to a patient when prescribed for different disorders. The American College of Obstetricians and Gynecologists (ACOG) has long opposed the burdensome REMS requirements on mifepristone for reproductive health indications.8
Arguments regarding the safety of mifepristone must be understood in the context of how the medication is taken, and the unique difference with other medications that must be administered by physicians or in health care facilities. Mifepristone is self-administered, and the desired effect—evacuation of uterine contents—typically occurs after a patient takes the accompanying medication misoprostol, which is some 24 to 72 hours later. This timeframe makes it highly unlikely that any patient would be in the presence of their provider at the time of medication effect, thus an in-person dispensing requirement has no medical bearing on the outcome of the health of the patient.
REMS changes during the COVID-19 pandemic
The coronavirus disease 2019 (COVID-19) pandemic has necessarily changed the structure of REMS and ETASU requirements for many medications, with changes made in order to mitigate viral transmission through the limitation of unnecessary visits to clinics or hospitals. The FDA announced in March of 2020 that it would not enforce pre-prescription requirements, such as laboratory or magnetic resonance imaging results, for many medications (including those more toxic than mifepristone), and that it would lift the requirement for in-person dispensation of several medications.9 Also in March 2020 the Department of Health and Human Services Secretary (HHS) and the Drug Enforcement Agency (DEA) activated a “telemedicine exception” to allow physicians to use telemedicine to satisfy mandatory requirements for prescribing controlled substances, including opioids.10
Despite repeated pleas from organizations, individuals, and physician groups, the FDA continued to enforce the REMS/ETASU for mifepristone as the pandemic decimated communities. Importantly, the pandemic has not had an equal effect on all communities, and the disparities highlighted in outcomes as related to COVID-19 are also reflected in disparities to access to reproductive choices.11 By enforcing REMS/ETASU for mifepristone during a global pandemic, the FDA has placed additional burden on women and people who menstruate. As offices and clinics have closed, and as many jobs have evaporated, additional barriers have emerged, such as lack of childcare, fewer transportation options, and decreased clinic appointments.
As the pandemic continues to affect communities in the United States, ACOG has issued guidance recommending assessment for eligibility for medical abortion remotely, and has encouraged the use of telemedicine and other remote interactions for its members and patients to limit transmission of the virus.
The lawsuit
On May 27, 2020, the American Civil Liberties Union (ACLU) (on behalf of ACOG, the Council of University Chairs of Obstetrics and Gynecology, New York State Academy of Family Physicians, SisterSong, and Honor MacNaughton, MD) filed a civil action against the FDA and HHS challenging the requirement for in-person dispensing of mifepristone and associated ETASU requirements during the COVID-19 pandemic. The plaintiffs sought this injunction based on the claim that these restrictions during the pandemic infringe on the constitutional rights to patients’ privacy and liberty and to equal protection of the law as protected by the Due Process Clause of the Fifth Amendment. Additionally, the ACLU and other organizations said these unnecessary restrictions place patients, providers, and staff at unnecessary risk of viral exposure amidst a global pandemic.
The verdict
On July 13, 2020, a federal court granted the preliminary injunction to suspend FDA’s enforcement of the in-person requirements of mifepristone for abortion during the COVID-19 pandemic. The court denied the motion for suspension of in-person restrictions as applied to miscarriage management. The preliminary injunction applies nationwide without geographic limitation. It will remain in effect until the end of the litigation or for 30 days following the expiration of the public health emergency.
What the outcome means
This injunction is a step in the right direction for patients and providers to allow for autonomy and clinical practice guided by clinician expertise. However, this ruling remains narrow. Patients must be counseled about mifepristone via telemedicine and sign a Patient Agreement Form, which must be returned electronically or by mail. Patients must receive a copy of the mifepristone medication guide, and dispensing of mifepristone must still be conducted by or under the supervision of a certified provider. The medication may not be dispensed by retail pharmacies, thus requiring providers to arrange for mailing of prescriptions to patients. Given state-based legal statutes regarding mailing of medications, this injunction may not lead to an immediate increase in access to care. In addition, patients seeking management for miscarriage must go to clinic to have mifepristone dispensed and thus risk exposure to viral transmission.
What now?
The regulation of mifepristone—in spite of excellent safety and specifically for the narrow purpose of administration in the setting of abortion and miscarriage care—is by definition a discriminatory practice against patients and providers. As clinicians, we are duty-bound to speak out against injustices to our practices and our patients. At a local level, we can work to implement safe practices in the setting of this injunction and continue to work on a national level to ensure this injunction becomes permanent and with more broad scope to eliminate all of the REMS requirements for mifepristone.
ACTION ITEMS
- Act locally! Are you an abortion provider? Contact your local ACLU (find them here) or lawyer in your area for assistance navigating the legal landscape to prescribe after this injunction.
- Act statewide! Press candidates in your state to stand up for science and data. Support legislative acts and bills that address combating discriminatory regulations.
- Act nationally! The President is responsible for appointing the Commissioner of the FDA and the Secretary of Health and Human Services (with Senate advice and consent). Who we elect matters. Seek out opportunities to become involved in increasing access to and awareness of voter registration and Election Day, and speak out against voter suppression. Make sure you are registered to vote here and check your area to review new recommendations amidst the pandemic.
- American College of Obstetricians and Gynecologists. Practice bulletin number 143: medical management of first trimester abortion. Obstet Gynecol. 2014;123:676-692.
- Schreiber CA, Crenin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Danco Laboratories. Mifeprex effectiveness and advantages. https://www.earlyoptionpill.com/is-mifeprex-right-for-me/effectiveness-advantages/ Accessed August 2, 2020.
- Jones RK, Witwer E, Jerman J. Abortion incidence and service availability in the United States, 2017. September 2019. https://www.guttmacher.org/report/abortion-incidence-service-availability-us-2017. Accessed September 10, 2020.
- American College of Obstetricians and Gynecologists. Practice bulletin no. 150: early pregnancy loss. Obstet Gynecol. 2015;125:1258-1267.
- US Food and Drug Administration. Risk evaluation and mitigation strategy (REMS) single shared system for mifepristone 200 mg. April 2019. https://www.accessdata.fda.gov/drugsatfda_docs/rems/Mifepristone_2019_04_11_REMS_Full.pdf. Accessed September 10, 2020.
- US Food and Drug Administration; Center for Drug Evaluation and Research. 2016 REMS Review, Summary Review 25. March 29, 2016. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/020687Orig1s020SumR.pdf. Accessed September 10, 2020.
- American College of Obstetricians and Gynecologists. Improving access to mifepristone for reproductive health indications. June 2018. https://www.acog.org/clinical-information/policy-and-position-statements/position-statements/2018/improving-access-to-mifepristone-for-reproductive-health-indications. Accessed August 2, 2020.
- US Food and Drug Administration. Policy for certain REMS requirements during the COVID-19 public health emergency: guidance for industry and health care professionals. March 2020. https://www.fda.gov/media/136317/download. Accessed September 10, 2020.
- US Department of Justice. US Drug Enforcement Administration. COVID-19 Information Page, Telemedicine. https://www.deadiversion.usdoj.gov/coronavirus.html#TELE. Accessed May 25, 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019: health equity considerations and racial and ethnic minority groups. https://www.cdc.gov/coronavirus/2019-ncov/community/health-equity/race-ethnicity.html. Updated July 24, 2020. Accessed September 10, 2020.
- American College of Obstetricians and Gynecologists. Practice bulletin number 143: medical management of first trimester abortion. Obstet Gynecol. 2014;123:676-692.
- Schreiber CA, Crenin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Danco Laboratories. Mifeprex effectiveness and advantages. https://www.earlyoptionpill.com/is-mifeprex-right-for-me/effectiveness-advantages/ Accessed August 2, 2020.
- Jones RK, Witwer E, Jerman J. Abortion incidence and service availability in the United States, 2017. September 2019. https://www.guttmacher.org/report/abortion-incidence-service-availability-us-2017. Accessed September 10, 2020.
- American College of Obstetricians and Gynecologists. Practice bulletin no. 150: early pregnancy loss. Obstet Gynecol. 2015;125:1258-1267.
- US Food and Drug Administration. Risk evaluation and mitigation strategy (REMS) single shared system for mifepristone 200 mg. April 2019. https://www.accessdata.fda.gov/drugsatfda_docs/rems/Mifepristone_2019_04_11_REMS_Full.pdf. Accessed September 10, 2020.
- US Food and Drug Administration; Center for Drug Evaluation and Research. 2016 REMS Review, Summary Review 25. March 29, 2016. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/020687Orig1s020SumR.pdf. Accessed September 10, 2020.
- American College of Obstetricians and Gynecologists. Improving access to mifepristone for reproductive health indications. June 2018. https://www.acog.org/clinical-information/policy-and-position-statements/position-statements/2018/improving-access-to-mifepristone-for-reproductive-health-indications. Accessed August 2, 2020.
- US Food and Drug Administration. Policy for certain REMS requirements during the COVID-19 public health emergency: guidance for industry and health care professionals. March 2020. https://www.fda.gov/media/136317/download. Accessed September 10, 2020.
- US Department of Justice. US Drug Enforcement Administration. COVID-19 Information Page, Telemedicine. https://www.deadiversion.usdoj.gov/coronavirus.html#TELE. Accessed May 25, 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019: health equity considerations and racial and ethnic minority groups. https://www.cdc.gov/coronavirus/2019-ncov/community/health-equity/race-ethnicity.html. Updated July 24, 2020. Accessed September 10, 2020.
Strengthening faith during coronavirus: An Islamic perspective
"Ramadan will be depressing this year,” a patient told me as I entered the room for an evaluation. This is one of many similar reactions my patients expressed in March, when mosques began to close and social distancing parameters were put in place to limit the spread of coronavirus disease 2019 (COVID-19). Muslims began to adjust to new social norms, such as replacing warm hugs with waving hands from 6 feet away. They were suddenly advised to avoid century-long cultural practices, such as spending time with extended family, visiting the sick and the elderly, and meeting for Jummah (Friday) prayer at mosque. With increasing anxiety and uncertainty in the air, I began thinking about how the pandemic would psychologically affect Islamic spirituality, especially during Ramadan (the Islamic month of fasting) this year.
As a Muslim psychiatry resident working on an inpatient psychiatric unit and in a psychiatry consultation service during the COVID-19 pandemic, I often explore spirituality and faith with my patients as a way of providing supportive therapy for anxiety. Many of my Christian patients endorsed anxiety about how Easter would be “terrible” this year because they could not attend church. Upon hearing this, I realized that I could not picture a Ramadan during which I was not permitted to go to mosque. How was I supposed to provide supportive therapy for my patients when I also felt so uncertain? These concerns led me to take a step back and remind myself of what I frequently tell my patients when they feel hopeless: “With every difficulty, there comes an opportunity to gain a new perspective.”
A time for spirituality
When Ramadan began in April, many people who are Muslim and were working from home told me that it felt strange to have so much time during the day to pray, reflect, and read the Quran. Others mentioned that they enjoyed the peace of Iftar (breaking fast) at home, because they could avoid the hustle and bustle of this at mosque. Halfway through Ramadan, a Muslim patient I was treating reported that her “coronavirus anxiety” had improved as she began focusing her energy on Allah, rather than spending hours watching the news and obsessing over death tolls.
Due to the pandemic, many more opportunities for donating to those in need arose, which led my religious community to perform Zakat (providing charity) and send supplies to food banks in our area. Because of social distancing, Muslim families were able to spend more time preparing meals, learning together, and supporting each other. Although mosques were closed due to the pandemic, it seemed as though each home became its own gathering place for spirituality, gratitude, and self-reflection. By the end of Ramadan, the values of self-discipline, empathy, and patience became self-evident.
Increased attention to mental health among Muslims
Psychologically, I believe resilience has grown stronger among Muslims worldwide during this pandemic. Along with adopting a positive mindset, Muslims have committed to creating their own routines to combat anxiety during this stressful time. The Salat (praying 5 times a day) and Taharat (cleanliness) that Islam emphasizes have been helpful in creating structure to offset the uncertainty and fear that is associated with COVID-19.
The discussion of mental illness, which previously has been regarded as a culturally stigmatized topic, has been gaining significant recognition within Islamic communities. Depression, anxiety, and self-care are now emphasized during virtual sermons, and contact information for mental health hotlines and professionals are being rapidly disseminated. There is now a greater sense of encouragement for people of Islamic faith to seek psychiatric help when needed.
Although COVID-19 has limited some social and physical religious practices, this pandemic has helped to strengthen faith and spirituality not only among Muslims, but also people of other faiths. During periods of stress, change, and uncertainty, it is important to remember that “With every difficulty, there comes an opportunity to gain a new perspective.” Although mosques and churches continue to stay closed and anxiety persists, I can now confidently reassure my patients that through this experience we are becoming resilient and learning to value patience, gratitude, and empathy more than ever.
"Ramadan will be depressing this year,” a patient told me as I entered the room for an evaluation. This is one of many similar reactions my patients expressed in March, when mosques began to close and social distancing parameters were put in place to limit the spread of coronavirus disease 2019 (COVID-19). Muslims began to adjust to new social norms, such as replacing warm hugs with waving hands from 6 feet away. They were suddenly advised to avoid century-long cultural practices, such as spending time with extended family, visiting the sick and the elderly, and meeting for Jummah (Friday) prayer at mosque. With increasing anxiety and uncertainty in the air, I began thinking about how the pandemic would psychologically affect Islamic spirituality, especially during Ramadan (the Islamic month of fasting) this year.
As a Muslim psychiatry resident working on an inpatient psychiatric unit and in a psychiatry consultation service during the COVID-19 pandemic, I often explore spirituality and faith with my patients as a way of providing supportive therapy for anxiety. Many of my Christian patients endorsed anxiety about how Easter would be “terrible” this year because they could not attend church. Upon hearing this, I realized that I could not picture a Ramadan during which I was not permitted to go to mosque. How was I supposed to provide supportive therapy for my patients when I also felt so uncertain? These concerns led me to take a step back and remind myself of what I frequently tell my patients when they feel hopeless: “With every difficulty, there comes an opportunity to gain a new perspective.”
A time for spirituality
When Ramadan began in April, many people who are Muslim and were working from home told me that it felt strange to have so much time during the day to pray, reflect, and read the Quran. Others mentioned that they enjoyed the peace of Iftar (breaking fast) at home, because they could avoid the hustle and bustle of this at mosque. Halfway through Ramadan, a Muslim patient I was treating reported that her “coronavirus anxiety” had improved as she began focusing her energy on Allah, rather than spending hours watching the news and obsessing over death tolls.
Due to the pandemic, many more opportunities for donating to those in need arose, which led my religious community to perform Zakat (providing charity) and send supplies to food banks in our area. Because of social distancing, Muslim families were able to spend more time preparing meals, learning together, and supporting each other. Although mosques were closed due to the pandemic, it seemed as though each home became its own gathering place for spirituality, gratitude, and self-reflection. By the end of Ramadan, the values of self-discipline, empathy, and patience became self-evident.
Increased attention to mental health among Muslims
Psychologically, I believe resilience has grown stronger among Muslims worldwide during this pandemic. Along with adopting a positive mindset, Muslims have committed to creating their own routines to combat anxiety during this stressful time. The Salat (praying 5 times a day) and Taharat (cleanliness) that Islam emphasizes have been helpful in creating structure to offset the uncertainty and fear that is associated with COVID-19.
The discussion of mental illness, which previously has been regarded as a culturally stigmatized topic, has been gaining significant recognition within Islamic communities. Depression, anxiety, and self-care are now emphasized during virtual sermons, and contact information for mental health hotlines and professionals are being rapidly disseminated. There is now a greater sense of encouragement for people of Islamic faith to seek psychiatric help when needed.
Although COVID-19 has limited some social and physical religious practices, this pandemic has helped to strengthen faith and spirituality not only among Muslims, but also people of other faiths. During periods of stress, change, and uncertainty, it is important to remember that “With every difficulty, there comes an opportunity to gain a new perspective.” Although mosques and churches continue to stay closed and anxiety persists, I can now confidently reassure my patients that through this experience we are becoming resilient and learning to value patience, gratitude, and empathy more than ever.
"Ramadan will be depressing this year,” a patient told me as I entered the room for an evaluation. This is one of many similar reactions my patients expressed in March, when mosques began to close and social distancing parameters were put in place to limit the spread of coronavirus disease 2019 (COVID-19). Muslims began to adjust to new social norms, such as replacing warm hugs with waving hands from 6 feet away. They were suddenly advised to avoid century-long cultural practices, such as spending time with extended family, visiting the sick and the elderly, and meeting for Jummah (Friday) prayer at mosque. With increasing anxiety and uncertainty in the air, I began thinking about how the pandemic would psychologically affect Islamic spirituality, especially during Ramadan (the Islamic month of fasting) this year.
As a Muslim psychiatry resident working on an inpatient psychiatric unit and in a psychiatry consultation service during the COVID-19 pandemic, I often explore spirituality and faith with my patients as a way of providing supportive therapy for anxiety. Many of my Christian patients endorsed anxiety about how Easter would be “terrible” this year because they could not attend church. Upon hearing this, I realized that I could not picture a Ramadan during which I was not permitted to go to mosque. How was I supposed to provide supportive therapy for my patients when I also felt so uncertain? These concerns led me to take a step back and remind myself of what I frequently tell my patients when they feel hopeless: “With every difficulty, there comes an opportunity to gain a new perspective.”
A time for spirituality
When Ramadan began in April, many people who are Muslim and were working from home told me that it felt strange to have so much time during the day to pray, reflect, and read the Quran. Others mentioned that they enjoyed the peace of Iftar (breaking fast) at home, because they could avoid the hustle and bustle of this at mosque. Halfway through Ramadan, a Muslim patient I was treating reported that her “coronavirus anxiety” had improved as she began focusing her energy on Allah, rather than spending hours watching the news and obsessing over death tolls.
Due to the pandemic, many more opportunities for donating to those in need arose, which led my religious community to perform Zakat (providing charity) and send supplies to food banks in our area. Because of social distancing, Muslim families were able to spend more time preparing meals, learning together, and supporting each other. Although mosques were closed due to the pandemic, it seemed as though each home became its own gathering place for spirituality, gratitude, and self-reflection. By the end of Ramadan, the values of self-discipline, empathy, and patience became self-evident.
Increased attention to mental health among Muslims
Psychologically, I believe resilience has grown stronger among Muslims worldwide during this pandemic. Along with adopting a positive mindset, Muslims have committed to creating their own routines to combat anxiety during this stressful time. The Salat (praying 5 times a day) and Taharat (cleanliness) that Islam emphasizes have been helpful in creating structure to offset the uncertainty and fear that is associated with COVID-19.
The discussion of mental illness, which previously has been regarded as a culturally stigmatized topic, has been gaining significant recognition within Islamic communities. Depression, anxiety, and self-care are now emphasized during virtual sermons, and contact information for mental health hotlines and professionals are being rapidly disseminated. There is now a greater sense of encouragement for people of Islamic faith to seek psychiatric help when needed.
Although COVID-19 has limited some social and physical religious practices, this pandemic has helped to strengthen faith and spirituality not only among Muslims, but also people of other faiths. During periods of stress, change, and uncertainty, it is important to remember that “With every difficulty, there comes an opportunity to gain a new perspective.” Although mosques and churches continue to stay closed and anxiety persists, I can now confidently reassure my patients that through this experience we are becoming resilient and learning to value patience, gratitude, and empathy more than ever.
Journey from first name to last name: Pursuing my dream
After graduating from medical school in India, where I was born and raised, I came to the United States in 2009 to expand my medical knowledge. At that time, I completed my clinical skills exam and soon after began a volunteer rotation at New York-Presbyterian Queens Hospital. In those early days, as I made my rounds through the emergency department (ED) of the hospital, I would introduce myself as Dr. Siva, which is my first name; this is how the doctors back home in India would introduce themselves to patients. Little did I know that the same name convention was not necessarily used here in the United States. Nonetheless, in those formative days, I learned a great deal from listening to the unique stories of how my patients had ended up in the ED, and I quickly felt right at home getting to know them.
When I first came to the United States, I had limited knowledge of psychiatry because I had only had a few months of psychiatry rotations during medical school. But in 2012, while I served as a volunteer in a research and observership program at Beth Israel Medical Center, one of my colleagues who was a psychiatry resident piqued my interest in the specialty and motivated me to explore and learn more about the various treatment modalities, strategies, and nuances this new modern world of psychiatry had to offer.
So I began by attending training sessions and evening seminars at the New York Psychoanalytic Society & Institute, where I became interested in Sigmund Freud’s work on the development of psychoanalysis. From there, my appetite for knowledge only continued to grow, and I took every opportunity to participate in various learning exercises, present at poster sessions, and give lectures at national conferences. I read and absorbed significant theories and texts and interacted with and learned from colleagues and mentors as I strived to sculpt my mind, with the aim of becoming a well-rounded psychiatrist.
Overcoming challenges
As I worked to further my understanding of psychiatry and understand the different treatment modalities—my goals becoming more clear with each step of my journey—I faced a significant setback. I was unable to secure a residency position to officially enter the specialty. I was devastated in my pursuit to realize the American Dream. At that point, I had been in the United States for 4 years with the financial and emotional support of my parents back home in India. I continued to struggle; another 2 years passed, and I was still coming up empty in my search for a residency position.
In the meantime, I kept moving forward, with my sights firmly on learning more about psychiatry. This time, I sought out several projects, including one where I served as a research assistant (volunteer) for nonpharmacologic clinical trials in patients with bipolar disorder, and another where I served as a research assistant (volunteer) at a substance use disorder clinic at Columbia University. I was also accepted into the “Prelude to Training” program at the Psychoanalytic Association of New York, which is affiliated with the NYU Grossman School of Medicine. Through that program, I was introduced to psychodynamic thinking and practice, which gave me the valuable foundation of thinking beyond oneself.
Grit and determination
To further my education, I studied clinical and translational sciences at Creighton University in Omaha. I was given opportunities to discuss topics related to the historical aspects of and recent advances in psychoanalysis through my involvement with the Professional Reading Alliance on Psychoanalysis at The Circle for the Lacanian Orientation of Omaha. Then came the moment when all my dreams came to fruition—I was accepted into the psychiatry residency program at Creighton University.
Those 4 years of residency passed by in a flash! Recently, I began a neuromodulation fellowship at the University of Florida in Gainesville. Here, my journey continues, as I search for tools to help the disenfranchised and those in need of mental health support. After the neuromodulation fellowship, I plan to pursue a pain medicine fellowship.
Continue to: Through the years...
Through the years, I have grown both professionally and personally. I have also overcome the instinctual urge to introduce myself to patients by my first name and have adapted to the American style of using my last name, and now introduce myself as Dr. Koppolu.
My educational journey in a place far from home has impacted me in ways I never knew possible, and I believe my strength to continue the pursuit is rooted in my passion and ambition to become a psychiatrist. I never gave up working toward that dream—a dream that is slowly becoming a reality.
After graduating from medical school in India, where I was born and raised, I came to the United States in 2009 to expand my medical knowledge. At that time, I completed my clinical skills exam and soon after began a volunteer rotation at New York-Presbyterian Queens Hospital. In those early days, as I made my rounds through the emergency department (ED) of the hospital, I would introduce myself as Dr. Siva, which is my first name; this is how the doctors back home in India would introduce themselves to patients. Little did I know that the same name convention was not necessarily used here in the United States. Nonetheless, in those formative days, I learned a great deal from listening to the unique stories of how my patients had ended up in the ED, and I quickly felt right at home getting to know them.
When I first came to the United States, I had limited knowledge of psychiatry because I had only had a few months of psychiatry rotations during medical school. But in 2012, while I served as a volunteer in a research and observership program at Beth Israel Medical Center, one of my colleagues who was a psychiatry resident piqued my interest in the specialty and motivated me to explore and learn more about the various treatment modalities, strategies, and nuances this new modern world of psychiatry had to offer.
So I began by attending training sessions and evening seminars at the New York Psychoanalytic Society & Institute, where I became interested in Sigmund Freud’s work on the development of psychoanalysis. From there, my appetite for knowledge only continued to grow, and I took every opportunity to participate in various learning exercises, present at poster sessions, and give lectures at national conferences. I read and absorbed significant theories and texts and interacted with and learned from colleagues and mentors as I strived to sculpt my mind, with the aim of becoming a well-rounded psychiatrist.
Overcoming challenges
As I worked to further my understanding of psychiatry and understand the different treatment modalities—my goals becoming more clear with each step of my journey—I faced a significant setback. I was unable to secure a residency position to officially enter the specialty. I was devastated in my pursuit to realize the American Dream. At that point, I had been in the United States for 4 years with the financial and emotional support of my parents back home in India. I continued to struggle; another 2 years passed, and I was still coming up empty in my search for a residency position.
In the meantime, I kept moving forward, with my sights firmly on learning more about psychiatry. This time, I sought out several projects, including one where I served as a research assistant (volunteer) for nonpharmacologic clinical trials in patients with bipolar disorder, and another where I served as a research assistant (volunteer) at a substance use disorder clinic at Columbia University. I was also accepted into the “Prelude to Training” program at the Psychoanalytic Association of New York, which is affiliated with the NYU Grossman School of Medicine. Through that program, I was introduced to psychodynamic thinking and practice, which gave me the valuable foundation of thinking beyond oneself.
Grit and determination
To further my education, I studied clinical and translational sciences at Creighton University in Omaha. I was given opportunities to discuss topics related to the historical aspects of and recent advances in psychoanalysis through my involvement with the Professional Reading Alliance on Psychoanalysis at The Circle for the Lacanian Orientation of Omaha. Then came the moment when all my dreams came to fruition—I was accepted into the psychiatry residency program at Creighton University.
Those 4 years of residency passed by in a flash! Recently, I began a neuromodulation fellowship at the University of Florida in Gainesville. Here, my journey continues, as I search for tools to help the disenfranchised and those in need of mental health support. After the neuromodulation fellowship, I plan to pursue a pain medicine fellowship.
Continue to: Through the years...
Through the years, I have grown both professionally and personally. I have also overcome the instinctual urge to introduce myself to patients by my first name and have adapted to the American style of using my last name, and now introduce myself as Dr. Koppolu.
My educational journey in a place far from home has impacted me in ways I never knew possible, and I believe my strength to continue the pursuit is rooted in my passion and ambition to become a psychiatrist. I never gave up working toward that dream—a dream that is slowly becoming a reality.
After graduating from medical school in India, where I was born and raised, I came to the United States in 2009 to expand my medical knowledge. At that time, I completed my clinical skills exam and soon after began a volunteer rotation at New York-Presbyterian Queens Hospital. In those early days, as I made my rounds through the emergency department (ED) of the hospital, I would introduce myself as Dr. Siva, which is my first name; this is how the doctors back home in India would introduce themselves to patients. Little did I know that the same name convention was not necessarily used here in the United States. Nonetheless, in those formative days, I learned a great deal from listening to the unique stories of how my patients had ended up in the ED, and I quickly felt right at home getting to know them.
When I first came to the United States, I had limited knowledge of psychiatry because I had only had a few months of psychiatry rotations during medical school. But in 2012, while I served as a volunteer in a research and observership program at Beth Israel Medical Center, one of my colleagues who was a psychiatry resident piqued my interest in the specialty and motivated me to explore and learn more about the various treatment modalities, strategies, and nuances this new modern world of psychiatry had to offer.
So I began by attending training sessions and evening seminars at the New York Psychoanalytic Society & Institute, where I became interested in Sigmund Freud’s work on the development of psychoanalysis. From there, my appetite for knowledge only continued to grow, and I took every opportunity to participate in various learning exercises, present at poster sessions, and give lectures at national conferences. I read and absorbed significant theories and texts and interacted with and learned from colleagues and mentors as I strived to sculpt my mind, with the aim of becoming a well-rounded psychiatrist.
Overcoming challenges
As I worked to further my understanding of psychiatry and understand the different treatment modalities—my goals becoming more clear with each step of my journey—I faced a significant setback. I was unable to secure a residency position to officially enter the specialty. I was devastated in my pursuit to realize the American Dream. At that point, I had been in the United States for 4 years with the financial and emotional support of my parents back home in India. I continued to struggle; another 2 years passed, and I was still coming up empty in my search for a residency position.
In the meantime, I kept moving forward, with my sights firmly on learning more about psychiatry. This time, I sought out several projects, including one where I served as a research assistant (volunteer) for nonpharmacologic clinical trials in patients with bipolar disorder, and another where I served as a research assistant (volunteer) at a substance use disorder clinic at Columbia University. I was also accepted into the “Prelude to Training” program at the Psychoanalytic Association of New York, which is affiliated with the NYU Grossman School of Medicine. Through that program, I was introduced to psychodynamic thinking and practice, which gave me the valuable foundation of thinking beyond oneself.
Grit and determination
To further my education, I studied clinical and translational sciences at Creighton University in Omaha. I was given opportunities to discuss topics related to the historical aspects of and recent advances in psychoanalysis through my involvement with the Professional Reading Alliance on Psychoanalysis at The Circle for the Lacanian Orientation of Omaha. Then came the moment when all my dreams came to fruition—I was accepted into the psychiatry residency program at Creighton University.
Those 4 years of residency passed by in a flash! Recently, I began a neuromodulation fellowship at the University of Florida in Gainesville. Here, my journey continues, as I search for tools to help the disenfranchised and those in need of mental health support. After the neuromodulation fellowship, I plan to pursue a pain medicine fellowship.
Continue to: Through the years...
Through the years, I have grown both professionally and personally. I have also overcome the instinctual urge to introduce myself to patients by my first name and have adapted to the American style of using my last name, and now introduce myself as Dr. Koppolu.
My educational journey in a place far from home has impacted me in ways I never knew possible, and I believe my strength to continue the pursuit is rooted in my passion and ambition to become a psychiatrist. I never gave up working toward that dream—a dream that is slowly becoming a reality.
APPlying Knowledge: Evidence for and Regulation of Mobile Apps for Dermatologists
Since the first mobile application (app) was developed in the 1990s, apps have become increasingly integrated into medical practice and training. More than 5.5 million apps were downloadable in 2019,1 of which more than 300,000 were health related.2 In the United States, more than 80% of physicians reported using smartphones for professional purposes in 2016.3 As the complexity of apps and their purpose of use has evolved, regulatory bodies have not adapted adequately to monitor apps that have broad-reaching consequences in medicine.
We review the primary literature on PubMed behind health-related apps that impact dermatologists as well as the government regulation of these apps, with a focus on the 3 most prevalent dermatology-related apps used by dermatology residents in the United States: VisualDx, UpToDate, and Mohs Surgery Appropriate Use Criteria. This prevalence is according to a survey emailed to all dermatology residents in the United States by the American Academy of Dermatology (AAD) in 2019 (unpublished data).
VisualDx
VisualDx, which aims to improve diagnostic accuracy and patient safety, contains peer-reviewed data and more than 32,000 images of dermatologic conditions. The editorial board includes more than 50 physicians. It provides opportunities for continuing medical education credit, is used in more than 2300 medical settings, and costs $399.99 annually for a subscription with partial features. Prior to the launch of the app in 2010, some health science professionals noted that the website version lacked references to primary sources.4 The same issue carried over to the app, which has evolved to offer artificial intelligence (AI) analysis of photographed skin lesions. However, there are no peer-reviewed publications showing positive impact of the app on diagnostic skills among dermatology residents or on patient outcomes.
UpToDate
UpToDate is a web-based database created in the early 1990s. A corresponding app was created around 2010. Both internal and independent research has demonstrated improved outcomes, and the app is advertised as the only clinical decision support resource associated with improved outcomes, as shown in more than 80 publications.5 UpToDate covers more than 11,800 medical topics and contains more than 35,000 graphics. It cites primary sources and uses a published system for grading recommendation strength and evidence quality. The data are processed and produced by a team of more than 7100 physicians as authors, editors, and reviewers. The platform grants continuing medical education credit and is used by more than 1.9 million clinicians in more than 190 countries. A 1-year subscription for an individual US-based physician costs $559. An observational study assessed UpToDate articles for potential conflicts of interest between authors and their recommendations. Of the 6 articles that met inclusion criteria of discussing management of medical conditions that have controversial or mostly brand-name treatment options, all had conflicts of interest, such as naming drugs from companies with which the authors and/or editors had financial relationships.6
Mohs Surgery Appropriate Use Criteria
The Mohs Surgery Appropriate Use Criteria app is a free clinical decision-making tool based on a consensus statement published in 2012 by the AAD, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and American Society for Mohs Surgery.7 It helps guide management of more than 200 dermatologic scenarios. Critique has been made that the criteria are partly based on expert opinion and data largely from the United States and has not been revised to incorporate newer data.8 There are no publications regarding the app itself.
Regulation of Health-Related Apps
Health-related apps that are designed for utilization by health care providers can be a valuable tool. However, given their prevalence, cost, and potential impact on patient lives, these apps should be well regulated and researched. The general paucity of peer-reviewed literature demonstrating the utility, safety, quality, and accuracy of health-related apps commonly used by providers is a reflection of insufficient mobile health regulation in the United States.
There are 3 primary government agencies responsible for regulating mobile medical apps: the US Food and Drug Administration (FDA), Federal Trade Commission, and Office for Civil Rights.9 The FDA does not regulate all medical devices. Apps intended for use in the diagnosis, cure, mitigation, prevention, or treatment of a disease or condition are considered to be medical devices.10 The FDA regulates those apps only if they are judged to pose more than minimal risk. Apps that are designed only to provide easy access to information related to health conditions or treatment are considered to be minimal risk but can develop into a different risk level such as by offering AI.11 Although the FDA does update its approach to medical devices, including apps and AI- and machine learning–based software, the rate and direction of update has not kept pace with the rapid evolution of apps.12 In 2019, the FDA began piloting a precertification program that grants long-term approval to organizations that develop apps instead of reviewing each app product individually.13 This decrease in premarket oversight is intended to expedite innovation with the hopeful upside of improving patient outcomes but is inconsistent, with the FDA still reviewing other types of medical devices individually.
For apps that are already in use, the Federal Trade Commission only gets involved in response to deceptive or unfair acts or practices relating to privacy, data security, and false or misleading claims about safety or performance. It may be more beneficial for consumers if those apps had a more stringent initial approval process. The Office for Civil Rights enforces the Health Insurance Portability and Accountability Act when relevant to apps.
Nongovernment agencies also are involved in app regulation. The FDA believes sharing more regulatory responsibility with private industry would promote efficiency.14 Google does not allow apps that contain false or misleading health claims,15 and Apple may scrutinize medical apps that could provide inaccurate data or be used for diagnosing or treating patients.16 Xcertia, a nonprofit organization founded by the American Medical Association and others, develops standards for the security, privacy, content, and operability of health-related apps, but those standards have not been adopted by other parties. Ultimately, nongovernment agencies are not responsible for public health and do not boast the government’s ability to enforce rules or ensure public safety.
Final Thoughts
The AAD survey of US dermatology residents found that the top consideration when choosing apps was up-to-date and accurate information; however, the 3 most prevalent apps among those same respondents did not need government approval and are not required to contain up-to-date data or to improve clinical outcomes, similar to most other health-related apps. This discrepancy is concerning considering the increasing utilization of apps for physician education and health care delivery and the increasing complexity of those apps. In light of these results, the potential decrease in federal premarket regulation suggested by the FDA’s precertification program seems inappropriate. It is important for the government to take responsibility for regulating health-related apps and to find a balance between too much regulation delaying innovation and too little regulation hurting physician training and patient care. It also is important for providers to be aware of the evidence and oversight behind the technologies they use for professional purposes.
- Clement J. Number of apps available in leading app stores as of 1st quarter 2020. Statista website. https://www.statista.com/statistics/276623/number-of-apps-available-in-leading-app-stores/. Published May 4, 2020. Accessed July 23, 2020.
- mHealth App Economics 2017/2018. Current Status and Future Trends in Mobile Health. Berlin, Germany: Research 2 Guidance; 2018.
- Healthcare Client Services. Professional usage of smartphones by doctors. Kantar website. https://www.kantarmedia.com/us/thinking-and-resources/blog/professional-usage-of-smartphones-by-doctors-2016. Published November 16, 2016. Accessed July 23, 2020.
- Skhal KJ, Koffel J. VisualDx. J Med Libr Assoc. 2007;95:470-471.
- UpToDate is the only clinical decision support resource associated with improved outcomes. UpToDate website. https://www.uptodate.com/home/research. Accessed July 29, 2020.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Amber KT, Dhiman G, Goodman KW. Conflict of interest in online point-of-care clinical support websites. J Med Ethics. 2014;40:578-580.
- Croley JA, Joseph AK, Wagner RF Jr. Discrepancies in the Mohs micrographic surgery appropriate use criteria. J Am Acad Dermatol. 2020;82:E55.
- Mobile health apps interactive tool. Federal Trade Commission website. https://www.ftc.gov/tips-advice/business-center/guidance/mobile-health-apps-interactive-tool. Published April 2016. Accessed May 23, 2020.
- Federal Food, Drug, and Cosmetic Act, 21 USC §321 (2018).
- US Food and Drug Administration. Examples of software functions for which the FDA will exercise enforcement discretion. https://www.fda.gov/medical-devices/device-software-functions-including-mobile-medical-applications/examples-software-functions-which-fda-will-exercise-enforcement-discretion. Updated September 26, 2019. Accessed July 29, 2020.
- US Food and Drug Administration. Proposed regulatory framework for modifications to artificial intelligence/machine learning (AI/ML)‐based software as a medical device (SaMD). https://www.fda.gov/downloads/MedicalDevices/DigitalHealth/SoftwareasaMedicalDevice/UCM635052.pdf. Accessed July 23, 2020.
- US Food and Drug Administration. Digital health software precertification (pre-cert) program. https://www.fda.gov/medical-devices/digital-health/digital-health-software-precertification-pre-cert-program. Updated July 18, 2019. Accessed July 23, 2020.
- Gottlieb S. Fostering medical innovation: a plan for digital health devices. US Food and Drug Administration website. https://www.fda.gov/news-events/fda-voices/fostering-medical-innovation-plan-digital-health-devices. Published June 15, 2017. Accessed July 23, 2020.
- Restricted content: unapproved substances. Google Play website. https://play.google.com/about/restricted-content/unapproved-substances. Accessed July 23, 2020.
- App store review guidelines. Apple Developer website. https://developer.apple.com/app-store/review/guidelines. Updated March 4, 2020. Accessed July 23, 2020.
Since the first mobile application (app) was developed in the 1990s, apps have become increasingly integrated into medical practice and training. More than 5.5 million apps were downloadable in 2019,1 of which more than 300,000 were health related.2 In the United States, more than 80% of physicians reported using smartphones for professional purposes in 2016.3 As the complexity of apps and their purpose of use has evolved, regulatory bodies have not adapted adequately to monitor apps that have broad-reaching consequences in medicine.
We review the primary literature on PubMed behind health-related apps that impact dermatologists as well as the government regulation of these apps, with a focus on the 3 most prevalent dermatology-related apps used by dermatology residents in the United States: VisualDx, UpToDate, and Mohs Surgery Appropriate Use Criteria. This prevalence is according to a survey emailed to all dermatology residents in the United States by the American Academy of Dermatology (AAD) in 2019 (unpublished data).
VisualDx
VisualDx, which aims to improve diagnostic accuracy and patient safety, contains peer-reviewed data and more than 32,000 images of dermatologic conditions. The editorial board includes more than 50 physicians. It provides opportunities for continuing medical education credit, is used in more than 2300 medical settings, and costs $399.99 annually for a subscription with partial features. Prior to the launch of the app in 2010, some health science professionals noted that the website version lacked references to primary sources.4 The same issue carried over to the app, which has evolved to offer artificial intelligence (AI) analysis of photographed skin lesions. However, there are no peer-reviewed publications showing positive impact of the app on diagnostic skills among dermatology residents or on patient outcomes.
UpToDate
UpToDate is a web-based database created in the early 1990s. A corresponding app was created around 2010. Both internal and independent research has demonstrated improved outcomes, and the app is advertised as the only clinical decision support resource associated with improved outcomes, as shown in more than 80 publications.5 UpToDate covers more than 11,800 medical topics and contains more than 35,000 graphics. It cites primary sources and uses a published system for grading recommendation strength and evidence quality. The data are processed and produced by a team of more than 7100 physicians as authors, editors, and reviewers. The platform grants continuing medical education credit and is used by more than 1.9 million clinicians in more than 190 countries. A 1-year subscription for an individual US-based physician costs $559. An observational study assessed UpToDate articles for potential conflicts of interest between authors and their recommendations. Of the 6 articles that met inclusion criteria of discussing management of medical conditions that have controversial or mostly brand-name treatment options, all had conflicts of interest, such as naming drugs from companies with which the authors and/or editors had financial relationships.6
Mohs Surgery Appropriate Use Criteria
The Mohs Surgery Appropriate Use Criteria app is a free clinical decision-making tool based on a consensus statement published in 2012 by the AAD, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and American Society for Mohs Surgery.7 It helps guide management of more than 200 dermatologic scenarios. Critique has been made that the criteria are partly based on expert opinion and data largely from the United States and has not been revised to incorporate newer data.8 There are no publications regarding the app itself.
Regulation of Health-Related Apps
Health-related apps that are designed for utilization by health care providers can be a valuable tool. However, given their prevalence, cost, and potential impact on patient lives, these apps should be well regulated and researched. The general paucity of peer-reviewed literature demonstrating the utility, safety, quality, and accuracy of health-related apps commonly used by providers is a reflection of insufficient mobile health regulation in the United States.
There are 3 primary government agencies responsible for regulating mobile medical apps: the US Food and Drug Administration (FDA), Federal Trade Commission, and Office for Civil Rights.9 The FDA does not regulate all medical devices. Apps intended for use in the diagnosis, cure, mitigation, prevention, or treatment of a disease or condition are considered to be medical devices.10 The FDA regulates those apps only if they are judged to pose more than minimal risk. Apps that are designed only to provide easy access to information related to health conditions or treatment are considered to be minimal risk but can develop into a different risk level such as by offering AI.11 Although the FDA does update its approach to medical devices, including apps and AI- and machine learning–based software, the rate and direction of update has not kept pace with the rapid evolution of apps.12 In 2019, the FDA began piloting a precertification program that grants long-term approval to organizations that develop apps instead of reviewing each app product individually.13 This decrease in premarket oversight is intended to expedite innovation with the hopeful upside of improving patient outcomes but is inconsistent, with the FDA still reviewing other types of medical devices individually.
For apps that are already in use, the Federal Trade Commission only gets involved in response to deceptive or unfair acts or practices relating to privacy, data security, and false or misleading claims about safety or performance. It may be more beneficial for consumers if those apps had a more stringent initial approval process. The Office for Civil Rights enforces the Health Insurance Portability and Accountability Act when relevant to apps.
Nongovernment agencies also are involved in app regulation. The FDA believes sharing more regulatory responsibility with private industry would promote efficiency.14 Google does not allow apps that contain false or misleading health claims,15 and Apple may scrutinize medical apps that could provide inaccurate data or be used for diagnosing or treating patients.16 Xcertia, a nonprofit organization founded by the American Medical Association and others, develops standards for the security, privacy, content, and operability of health-related apps, but those standards have not been adopted by other parties. Ultimately, nongovernment agencies are not responsible for public health and do not boast the government’s ability to enforce rules or ensure public safety.
Final Thoughts
The AAD survey of US dermatology residents found that the top consideration when choosing apps was up-to-date and accurate information; however, the 3 most prevalent apps among those same respondents did not need government approval and are not required to contain up-to-date data or to improve clinical outcomes, similar to most other health-related apps. This discrepancy is concerning considering the increasing utilization of apps for physician education and health care delivery and the increasing complexity of those apps. In light of these results, the potential decrease in federal premarket regulation suggested by the FDA’s precertification program seems inappropriate. It is important for the government to take responsibility for regulating health-related apps and to find a balance between too much regulation delaying innovation and too little regulation hurting physician training and patient care. It also is important for providers to be aware of the evidence and oversight behind the technologies they use for professional purposes.
Since the first mobile application (app) was developed in the 1990s, apps have become increasingly integrated into medical practice and training. More than 5.5 million apps were downloadable in 2019,1 of which more than 300,000 were health related.2 In the United States, more than 80% of physicians reported using smartphones for professional purposes in 2016.3 As the complexity of apps and their purpose of use has evolved, regulatory bodies have not adapted adequately to monitor apps that have broad-reaching consequences in medicine.
We review the primary literature on PubMed behind health-related apps that impact dermatologists as well as the government regulation of these apps, with a focus on the 3 most prevalent dermatology-related apps used by dermatology residents in the United States: VisualDx, UpToDate, and Mohs Surgery Appropriate Use Criteria. This prevalence is according to a survey emailed to all dermatology residents in the United States by the American Academy of Dermatology (AAD) in 2019 (unpublished data).
VisualDx
VisualDx, which aims to improve diagnostic accuracy and patient safety, contains peer-reviewed data and more than 32,000 images of dermatologic conditions. The editorial board includes more than 50 physicians. It provides opportunities for continuing medical education credit, is used in more than 2300 medical settings, and costs $399.99 annually for a subscription with partial features. Prior to the launch of the app in 2010, some health science professionals noted that the website version lacked references to primary sources.4 The same issue carried over to the app, which has evolved to offer artificial intelligence (AI) analysis of photographed skin lesions. However, there are no peer-reviewed publications showing positive impact of the app on diagnostic skills among dermatology residents or on patient outcomes.
UpToDate
UpToDate is a web-based database created in the early 1990s. A corresponding app was created around 2010. Both internal and independent research has demonstrated improved outcomes, and the app is advertised as the only clinical decision support resource associated with improved outcomes, as shown in more than 80 publications.5 UpToDate covers more than 11,800 medical topics and contains more than 35,000 graphics. It cites primary sources and uses a published system for grading recommendation strength and evidence quality. The data are processed and produced by a team of more than 7100 physicians as authors, editors, and reviewers. The platform grants continuing medical education credit and is used by more than 1.9 million clinicians in more than 190 countries. A 1-year subscription for an individual US-based physician costs $559. An observational study assessed UpToDate articles for potential conflicts of interest between authors and their recommendations. Of the 6 articles that met inclusion criteria of discussing management of medical conditions that have controversial or mostly brand-name treatment options, all had conflicts of interest, such as naming drugs from companies with which the authors and/or editors had financial relationships.6
Mohs Surgery Appropriate Use Criteria
The Mohs Surgery Appropriate Use Criteria app is a free clinical decision-making tool based on a consensus statement published in 2012 by the AAD, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and American Society for Mohs Surgery.7 It helps guide management of more than 200 dermatologic scenarios. Critique has been made that the criteria are partly based on expert opinion and data largely from the United States and has not been revised to incorporate newer data.8 There are no publications regarding the app itself.
Regulation of Health-Related Apps
Health-related apps that are designed for utilization by health care providers can be a valuable tool. However, given their prevalence, cost, and potential impact on patient lives, these apps should be well regulated and researched. The general paucity of peer-reviewed literature demonstrating the utility, safety, quality, and accuracy of health-related apps commonly used by providers is a reflection of insufficient mobile health regulation in the United States.
There are 3 primary government agencies responsible for regulating mobile medical apps: the US Food and Drug Administration (FDA), Federal Trade Commission, and Office for Civil Rights.9 The FDA does not regulate all medical devices. Apps intended for use in the diagnosis, cure, mitigation, prevention, or treatment of a disease or condition are considered to be medical devices.10 The FDA regulates those apps only if they are judged to pose more than minimal risk. Apps that are designed only to provide easy access to information related to health conditions or treatment are considered to be minimal risk but can develop into a different risk level such as by offering AI.11 Although the FDA does update its approach to medical devices, including apps and AI- and machine learning–based software, the rate and direction of update has not kept pace with the rapid evolution of apps.12 In 2019, the FDA began piloting a precertification program that grants long-term approval to organizations that develop apps instead of reviewing each app product individually.13 This decrease in premarket oversight is intended to expedite innovation with the hopeful upside of improving patient outcomes but is inconsistent, with the FDA still reviewing other types of medical devices individually.
For apps that are already in use, the Federal Trade Commission only gets involved in response to deceptive or unfair acts or practices relating to privacy, data security, and false or misleading claims about safety or performance. It may be more beneficial for consumers if those apps had a more stringent initial approval process. The Office for Civil Rights enforces the Health Insurance Portability and Accountability Act when relevant to apps.
Nongovernment agencies also are involved in app regulation. The FDA believes sharing more regulatory responsibility with private industry would promote efficiency.14 Google does not allow apps that contain false or misleading health claims,15 and Apple may scrutinize medical apps that could provide inaccurate data or be used for diagnosing or treating patients.16 Xcertia, a nonprofit organization founded by the American Medical Association and others, develops standards for the security, privacy, content, and operability of health-related apps, but those standards have not been adopted by other parties. Ultimately, nongovernment agencies are not responsible for public health and do not boast the government’s ability to enforce rules or ensure public safety.
Final Thoughts
The AAD survey of US dermatology residents found that the top consideration when choosing apps was up-to-date and accurate information; however, the 3 most prevalent apps among those same respondents did not need government approval and are not required to contain up-to-date data or to improve clinical outcomes, similar to most other health-related apps. This discrepancy is concerning considering the increasing utilization of apps for physician education and health care delivery and the increasing complexity of those apps. In light of these results, the potential decrease in federal premarket regulation suggested by the FDA’s precertification program seems inappropriate. It is important for the government to take responsibility for regulating health-related apps and to find a balance between too much regulation delaying innovation and too little regulation hurting physician training and patient care. It also is important for providers to be aware of the evidence and oversight behind the technologies they use for professional purposes.
- Clement J. Number of apps available in leading app stores as of 1st quarter 2020. Statista website. https://www.statista.com/statistics/276623/number-of-apps-available-in-leading-app-stores/. Published May 4, 2020. Accessed July 23, 2020.
- mHealth App Economics 2017/2018. Current Status and Future Trends in Mobile Health. Berlin, Germany: Research 2 Guidance; 2018.
- Healthcare Client Services. Professional usage of smartphones by doctors. Kantar website. https://www.kantarmedia.com/us/thinking-and-resources/blog/professional-usage-of-smartphones-by-doctors-2016. Published November 16, 2016. Accessed July 23, 2020.
- Skhal KJ, Koffel J. VisualDx. J Med Libr Assoc. 2007;95:470-471.
- UpToDate is the only clinical decision support resource associated with improved outcomes. UpToDate website. https://www.uptodate.com/home/research. Accessed July 29, 2020.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Amber KT, Dhiman G, Goodman KW. Conflict of interest in online point-of-care clinical support websites. J Med Ethics. 2014;40:578-580.
- Croley JA, Joseph AK, Wagner RF Jr. Discrepancies in the Mohs micrographic surgery appropriate use criteria. J Am Acad Dermatol. 2020;82:E55.
- Mobile health apps interactive tool. Federal Trade Commission website. https://www.ftc.gov/tips-advice/business-center/guidance/mobile-health-apps-interactive-tool. Published April 2016. Accessed May 23, 2020.
- Federal Food, Drug, and Cosmetic Act, 21 USC §321 (2018).
- US Food and Drug Administration. Examples of software functions for which the FDA will exercise enforcement discretion. https://www.fda.gov/medical-devices/device-software-functions-including-mobile-medical-applications/examples-software-functions-which-fda-will-exercise-enforcement-discretion. Updated September 26, 2019. Accessed July 29, 2020.
- US Food and Drug Administration. Proposed regulatory framework for modifications to artificial intelligence/machine learning (AI/ML)‐based software as a medical device (SaMD). https://www.fda.gov/downloads/MedicalDevices/DigitalHealth/SoftwareasaMedicalDevice/UCM635052.pdf. Accessed July 23, 2020.
- US Food and Drug Administration. Digital health software precertification (pre-cert) program. https://www.fda.gov/medical-devices/digital-health/digital-health-software-precertification-pre-cert-program. Updated July 18, 2019. Accessed July 23, 2020.
- Gottlieb S. Fostering medical innovation: a plan for digital health devices. US Food and Drug Administration website. https://www.fda.gov/news-events/fda-voices/fostering-medical-innovation-plan-digital-health-devices. Published June 15, 2017. Accessed July 23, 2020.
- Restricted content: unapproved substances. Google Play website. https://play.google.com/about/restricted-content/unapproved-substances. Accessed July 23, 2020.
- App store review guidelines. Apple Developer website. https://developer.apple.com/app-store/review/guidelines. Updated March 4, 2020. Accessed July 23, 2020.
- Clement J. Number of apps available in leading app stores as of 1st quarter 2020. Statista website. https://www.statista.com/statistics/276623/number-of-apps-available-in-leading-app-stores/. Published May 4, 2020. Accessed July 23, 2020.
- mHealth App Economics 2017/2018. Current Status and Future Trends in Mobile Health. Berlin, Germany: Research 2 Guidance; 2018.
- Healthcare Client Services. Professional usage of smartphones by doctors. Kantar website. https://www.kantarmedia.com/us/thinking-and-resources/blog/professional-usage-of-smartphones-by-doctors-2016. Published November 16, 2016. Accessed July 23, 2020.
- Skhal KJ, Koffel J. VisualDx. J Med Libr Assoc. 2007;95:470-471.
- UpToDate is the only clinical decision support resource associated with improved outcomes. UpToDate website. https://www.uptodate.com/home/research. Accessed July 29, 2020.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Amber KT, Dhiman G, Goodman KW. Conflict of interest in online point-of-care clinical support websites. J Med Ethics. 2014;40:578-580.
- Croley JA, Joseph AK, Wagner RF Jr. Discrepancies in the Mohs micrographic surgery appropriate use criteria. J Am Acad Dermatol. 2020;82:E55.
- Mobile health apps interactive tool. Federal Trade Commission website. https://www.ftc.gov/tips-advice/business-center/guidance/mobile-health-apps-interactive-tool. Published April 2016. Accessed May 23, 2020.
- Federal Food, Drug, and Cosmetic Act, 21 USC §321 (2018).
- US Food and Drug Administration. Examples of software functions for which the FDA will exercise enforcement discretion. https://www.fda.gov/medical-devices/device-software-functions-including-mobile-medical-applications/examples-software-functions-which-fda-will-exercise-enforcement-discretion. Updated September 26, 2019. Accessed July 29, 2020.
- US Food and Drug Administration. Proposed regulatory framework for modifications to artificial intelligence/machine learning (AI/ML)‐based software as a medical device (SaMD). https://www.fda.gov/downloads/MedicalDevices/DigitalHealth/SoftwareasaMedicalDevice/UCM635052.pdf. Accessed July 23, 2020.
- US Food and Drug Administration. Digital health software precertification (pre-cert) program. https://www.fda.gov/medical-devices/digital-health/digital-health-software-precertification-pre-cert-program. Updated July 18, 2019. Accessed July 23, 2020.
- Gottlieb S. Fostering medical innovation: a plan for digital health devices. US Food and Drug Administration website. https://www.fda.gov/news-events/fda-voices/fostering-medical-innovation-plan-digital-health-devices. Published June 15, 2017. Accessed July 23, 2020.
- Restricted content: unapproved substances. Google Play website. https://play.google.com/about/restricted-content/unapproved-substances. Accessed July 23, 2020.
- App store review guidelines. Apple Developer website. https://developer.apple.com/app-store/review/guidelines. Updated March 4, 2020. Accessed July 23, 2020.
Practice Points
- Physicians who are selecting an app for self-education or patient care should take into consideration the strength of the evidence supporting the app as well as the rigor of any approval process the app had to undergo.
- Only a minority of health-related apps are regulated by the government. This regulation has not kept up with the evolution of app software and may become more indirect.
Wellness for the Dermatology Resident
Resident wellness is a topic that has become increasingly important in recent years due to physician burnout. A prior Cutis Resident Corner column discussed the prevalence of physician burnout and how it can affect dermatologists.1 When discussing resident burnout, dermatology may not be a specialty that immediately comes to mind, considering that dermatology is mostly outpatient based, with few emergencies and critically ill patients. In a JAMA study assessing levels of burnout by specialty, dermatology residents were the lowest at approximately 30%.2 However, this still means that 3 out of every 10 dermatology residents feel burnt out.
Burnout in Dermatology
In 2017, results from a survey of 112 dermatology residents in Canada about burnout was published in the British Journal of Dermatology.3 The numbers were staggering; the results showed that more than 50% of dermatology residents experienced high levels of emotional exhaustion and depersonalization, and 40% had low levels of personal accomplishment. Additionally, 52% experienced low or depressed mood, 20% reported feelings of hurting themselves within the last year, and more than 25% had high anxiety levels.3
Dermatology requires a high level of daily studying, which is a major source of stress for many dermatology residents. The survey of dermatology residents in Canada showed that the top stressor for 61% of survey respondents was studying, specifically for the board examination.3 Dermatology is an academically rigorous specialty. We are responsible for recognizing every disease process affecting the skin, including hundreds that are extremely uncommon. We must understand these disease processes at a molecular level from a basic science standpoint and at a microscopic level through our knowledge of dermatopathology. Much of what we see in clinic are bread-and-butter dermatologic conditions that do not necessarily correlate with the rare diseases that we study. This differs from other specialties in which residents learn much of their specialty knowledge through their clinical work.
Current Challenges
We are training in a uniquely challenging time, providing care for our patients amid the coronavirus disease 2019 pandemic. Many of us are dealing with constant levels of stress and worry about the health and safety of ourselves, along with our friends, families, and patients. Some residents have been redeployed to work in unfamiliar roles in the emergency department or hospital wards, while others adjust to new roles in teledermatology. I also cannot talk about resident wellness without recognizing the challenges faced by physicians who are racial and religious minorities. This is especially true for black physicians, as they face unconscious biases and microaggressions daily derived from implicit racism; this leads to discrimination in every area of life and ultimately harms their emotional and psychological well-being.4 Additionally, black physicians are underrepresented in dermatology, making up only 4.3% of dermatology residents in the 2013-2014 academic year.5,6 Underrepresentation can serve as a major stressor for racial and religious minorities and should be considered when addressing resident wellness to ensure their voices are heard and validated.
Focusing on Wellness
What can we do to improve wellness? A viewpoint published in JAMA Surgery in 2015 by Salles et al7 from the Stanford University Department of Surgery (Stanford, California) discussed their Balance in Life (BIL) program, which was established after one of their residency graduates tragically died by suicide shortly after graduating from residency. The BIL program addresses 4 different facets of well-being—professional, physical, psychological, and social—and lists the specific actions taken to improve these areas of well-being.7
I completed my transitional year residency at St. Vincent Hospital (Indianapolis, Indiana). The program emphasizes the importance of resident wellness. They established a department-sponsored well-being program to improve resident wellness,8 with its objectives aligning with the 4 areas of well-being that were outlined in the Stanford viewpoint.7 A short Q&A with me was published in the supplemental material as a way of highlighting their residents.9 I will outline the 4 areas of well-being, with suggestions based on the Stanford BIL program, the well-being innovation program at St. Vincent, and initiatives at my current dermatology residency program at the University of Wisconsin (UW) in Madison.
The 4 Areas of Well-being
Stanford BIL Program
One of the changes implemented was starting a resident mentorship program. Each junior resident selects a senior resident as a mentor with department-sponsored quarterly lunch meetings.7 Another initiative is a leadership training program, which includes an outdoor rope course each year focusing on leadership and team building.7
UW Dermatology
Monthly meetings are held with our program director and coordinator so that we can address any concerns or issues as they arise and brainstorm solutions together. During the coronavirus disease 2019 pandemic, we had weekly resident town halls with department leadership with transparency about our institution’s current status.
Physical Well-being
Stanford BIL Program
One method of improving physical well-being included stocking healthy snacks for residents and providing incoming residents with a guide of physicians, dentists, and fitness venues to promote regular health care. We have adopted the same at UW with healthy snacks available in our resident workroom.
St. Vincent Internal Medicine Wellness
There are monthly fitness challenges for a variety of physical wellness activities such as sleep, mindfulness minutes, nutrition, and step challenges.8
UW Dermatology
In addition to healthy snacks in our workroom, we also have various discounted fitness classes available for employees, along with discounts on gym memberships, kayak rentals, and city bike-share programs.
Psychological Well-Being
Stanford BIL Program
They enlisted a clinical psychologist available for residents to talk to regularly about any issues they face and to help manage stress in their lives.7
St. Vincent Internal Medicine Wellness
Faculty and coordinators provide S.A.F.E.—secure, affirming, friendly, and empathetic—zones to provide confidential and judgment-free support for residents.8 They also host photography competitions; residents submit photographs of nature, and the winning photographs are printed and displayed throughout the work area.
UW Dermatology
We have made changes to beautify our resident workroom with photograph collages of residents and other assorted décor to make it a more work-friendly space.
Social Well-being
Common themes highlighted by all 3 programs include the importance of socializing outside of the workplace, team-building activities, and resident retreats. Social media accounts on Instagram at St. Vincent (@stvimresidency) and at UW (@uwderm) highlight resident accomplishments and promote interconnectedness when residents are not together in clinics or hospitals.
Final Thoughts
Resident wellness will continue to be an important topic for discussion in the future, especially given the uncertain times right now during our training. Focusing on the 4 areas of well-being can help to prevent burnout and improve resident wellness.
- Croley JAA. #Dermlife and the burned-out resident. Cutis. 2019;104:E32-E33.
- Dyrbye LN, Burke SE, Hardeman RR, et al. Association of clinical specialty with symptoms of burnout and career choice regret among US resident physicians. JAMA. 2018;320:1114-1130.
- Shoimer I, Patten S, Mydlarski PR. Burnout in dermatology residents: a Canadian perspective [published online November 1, 2017]. Br J Dermatol. 2018;178:270-271.
- Grills CN, Aird EG, Rowe D. Breathe, baby, breathe: clearing the way for the emotional emancipation of black people. Cultural Studies & Critical Methodologies. 2016;16:333-343.
- Imadojemu S, James WD. Increasing African American representation in dermatology. JAMA Dermatol. 2016;152:15-16.
- Brotherton SE, Etzel SI. Graduate medical education, 2013-2014. JAMA. 2014;312:2427-2445.
- Salles A, Liebert CA, Greco RS. Promoting balance in the lives of resident physicians: a call to action. JAMA Surg. 2015;150:607-608.
- Fick L, Axon K, Potini Y, et al. Improving overall resident and faculty wellbeing through program-sponsored innovations. MedEdPublish. Published September 27, 2019. doi:10.15694/mep.2019.000184.1.
- St. Vincent Internal Medicine Residency Wellness Bulletin. https://www.mededpublish.org/manuscriptfiles/2586/Supplementary%20File%203_Wellness%20Bulletin.pdf. Published April 2018. Accessed August 5, 2020.
Resident wellness is a topic that has become increasingly important in recent years due to physician burnout. A prior Cutis Resident Corner column discussed the prevalence of physician burnout and how it can affect dermatologists.1 When discussing resident burnout, dermatology may not be a specialty that immediately comes to mind, considering that dermatology is mostly outpatient based, with few emergencies and critically ill patients. In a JAMA study assessing levels of burnout by specialty, dermatology residents were the lowest at approximately 30%.2 However, this still means that 3 out of every 10 dermatology residents feel burnt out.
Burnout in Dermatology
In 2017, results from a survey of 112 dermatology residents in Canada about burnout was published in the British Journal of Dermatology.3 The numbers were staggering; the results showed that more than 50% of dermatology residents experienced high levels of emotional exhaustion and depersonalization, and 40% had low levels of personal accomplishment. Additionally, 52% experienced low or depressed mood, 20% reported feelings of hurting themselves within the last year, and more than 25% had high anxiety levels.3
Dermatology requires a high level of daily studying, which is a major source of stress for many dermatology residents. The survey of dermatology residents in Canada showed that the top stressor for 61% of survey respondents was studying, specifically for the board examination.3 Dermatology is an academically rigorous specialty. We are responsible for recognizing every disease process affecting the skin, including hundreds that are extremely uncommon. We must understand these disease processes at a molecular level from a basic science standpoint and at a microscopic level through our knowledge of dermatopathology. Much of what we see in clinic are bread-and-butter dermatologic conditions that do not necessarily correlate with the rare diseases that we study. This differs from other specialties in which residents learn much of their specialty knowledge through their clinical work.
Current Challenges
We are training in a uniquely challenging time, providing care for our patients amid the coronavirus disease 2019 pandemic. Many of us are dealing with constant levels of stress and worry about the health and safety of ourselves, along with our friends, families, and patients. Some residents have been redeployed to work in unfamiliar roles in the emergency department or hospital wards, while others adjust to new roles in teledermatology. I also cannot talk about resident wellness without recognizing the challenges faced by physicians who are racial and religious minorities. This is especially true for black physicians, as they face unconscious biases and microaggressions daily derived from implicit racism; this leads to discrimination in every area of life and ultimately harms their emotional and psychological well-being.4 Additionally, black physicians are underrepresented in dermatology, making up only 4.3% of dermatology residents in the 2013-2014 academic year.5,6 Underrepresentation can serve as a major stressor for racial and religious minorities and should be considered when addressing resident wellness to ensure their voices are heard and validated.
Focusing on Wellness
What can we do to improve wellness? A viewpoint published in JAMA Surgery in 2015 by Salles et al7 from the Stanford University Department of Surgery (Stanford, California) discussed their Balance in Life (BIL) program, which was established after one of their residency graduates tragically died by suicide shortly after graduating from residency. The BIL program addresses 4 different facets of well-being—professional, physical, psychological, and social—and lists the specific actions taken to improve these areas of well-being.7
I completed my transitional year residency at St. Vincent Hospital (Indianapolis, Indiana). The program emphasizes the importance of resident wellness. They established a department-sponsored well-being program to improve resident wellness,8 with its objectives aligning with the 4 areas of well-being that were outlined in the Stanford viewpoint.7 A short Q&A with me was published in the supplemental material as a way of highlighting their residents.9 I will outline the 4 areas of well-being, with suggestions based on the Stanford BIL program, the well-being innovation program at St. Vincent, and initiatives at my current dermatology residency program at the University of Wisconsin (UW) in Madison.
The 4 Areas of Well-being
Stanford BIL Program
One of the changes implemented was starting a resident mentorship program. Each junior resident selects a senior resident as a mentor with department-sponsored quarterly lunch meetings.7 Another initiative is a leadership training program, which includes an outdoor rope course each year focusing on leadership and team building.7
UW Dermatology
Monthly meetings are held with our program director and coordinator so that we can address any concerns or issues as they arise and brainstorm solutions together. During the coronavirus disease 2019 pandemic, we had weekly resident town halls with department leadership with transparency about our institution’s current status.
Physical Well-being
Stanford BIL Program
One method of improving physical well-being included stocking healthy snacks for residents and providing incoming residents with a guide of physicians, dentists, and fitness venues to promote regular health care. We have adopted the same at UW with healthy snacks available in our resident workroom.
St. Vincent Internal Medicine Wellness
There are monthly fitness challenges for a variety of physical wellness activities such as sleep, mindfulness minutes, nutrition, and step challenges.8
UW Dermatology
In addition to healthy snacks in our workroom, we also have various discounted fitness classes available for employees, along with discounts on gym memberships, kayak rentals, and city bike-share programs.
Psychological Well-Being
Stanford BIL Program
They enlisted a clinical psychologist available for residents to talk to regularly about any issues they face and to help manage stress in their lives.7
St. Vincent Internal Medicine Wellness
Faculty and coordinators provide S.A.F.E.—secure, affirming, friendly, and empathetic—zones to provide confidential and judgment-free support for residents.8 They also host photography competitions; residents submit photographs of nature, and the winning photographs are printed and displayed throughout the work area.
UW Dermatology
We have made changes to beautify our resident workroom with photograph collages of residents and other assorted décor to make it a more work-friendly space.
Social Well-being
Common themes highlighted by all 3 programs include the importance of socializing outside of the workplace, team-building activities, and resident retreats. Social media accounts on Instagram at St. Vincent (@stvimresidency) and at UW (@uwderm) highlight resident accomplishments and promote interconnectedness when residents are not together in clinics or hospitals.
Final Thoughts
Resident wellness will continue to be an important topic for discussion in the future, especially given the uncertain times right now during our training. Focusing on the 4 areas of well-being can help to prevent burnout and improve resident wellness.
Resident wellness is a topic that has become increasingly important in recent years due to physician burnout. A prior Cutis Resident Corner column discussed the prevalence of physician burnout and how it can affect dermatologists.1 When discussing resident burnout, dermatology may not be a specialty that immediately comes to mind, considering that dermatology is mostly outpatient based, with few emergencies and critically ill patients. In a JAMA study assessing levels of burnout by specialty, dermatology residents were the lowest at approximately 30%.2 However, this still means that 3 out of every 10 dermatology residents feel burnt out.
Burnout in Dermatology
In 2017, results from a survey of 112 dermatology residents in Canada about burnout was published in the British Journal of Dermatology.3 The numbers were staggering; the results showed that more than 50% of dermatology residents experienced high levels of emotional exhaustion and depersonalization, and 40% had low levels of personal accomplishment. Additionally, 52% experienced low or depressed mood, 20% reported feelings of hurting themselves within the last year, and more than 25% had high anxiety levels.3
Dermatology requires a high level of daily studying, which is a major source of stress for many dermatology residents. The survey of dermatology residents in Canada showed that the top stressor for 61% of survey respondents was studying, specifically for the board examination.3 Dermatology is an academically rigorous specialty. We are responsible for recognizing every disease process affecting the skin, including hundreds that are extremely uncommon. We must understand these disease processes at a molecular level from a basic science standpoint and at a microscopic level through our knowledge of dermatopathology. Much of what we see in clinic are bread-and-butter dermatologic conditions that do not necessarily correlate with the rare diseases that we study. This differs from other specialties in which residents learn much of their specialty knowledge through their clinical work.
Current Challenges
We are training in a uniquely challenging time, providing care for our patients amid the coronavirus disease 2019 pandemic. Many of us are dealing with constant levels of stress and worry about the health and safety of ourselves, along with our friends, families, and patients. Some residents have been redeployed to work in unfamiliar roles in the emergency department or hospital wards, while others adjust to new roles in teledermatology. I also cannot talk about resident wellness without recognizing the challenges faced by physicians who are racial and religious minorities. This is especially true for black physicians, as they face unconscious biases and microaggressions daily derived from implicit racism; this leads to discrimination in every area of life and ultimately harms their emotional and psychological well-being.4 Additionally, black physicians are underrepresented in dermatology, making up only 4.3% of dermatology residents in the 2013-2014 academic year.5,6 Underrepresentation can serve as a major stressor for racial and religious minorities and should be considered when addressing resident wellness to ensure their voices are heard and validated.
Focusing on Wellness
What can we do to improve wellness? A viewpoint published in JAMA Surgery in 2015 by Salles et al7 from the Stanford University Department of Surgery (Stanford, California) discussed their Balance in Life (BIL) program, which was established after one of their residency graduates tragically died by suicide shortly after graduating from residency. The BIL program addresses 4 different facets of well-being—professional, physical, psychological, and social—and lists the specific actions taken to improve these areas of well-being.7
I completed my transitional year residency at St. Vincent Hospital (Indianapolis, Indiana). The program emphasizes the importance of resident wellness. They established a department-sponsored well-being program to improve resident wellness,8 with its objectives aligning with the 4 areas of well-being that were outlined in the Stanford viewpoint.7 A short Q&A with me was published in the supplemental material as a way of highlighting their residents.9 I will outline the 4 areas of well-being, with suggestions based on the Stanford BIL program, the well-being innovation program at St. Vincent, and initiatives at my current dermatology residency program at the University of Wisconsin (UW) in Madison.
The 4 Areas of Well-being
Stanford BIL Program
One of the changes implemented was starting a resident mentorship program. Each junior resident selects a senior resident as a mentor with department-sponsored quarterly lunch meetings.7 Another initiative is a leadership training program, which includes an outdoor rope course each year focusing on leadership and team building.7
UW Dermatology
Monthly meetings are held with our program director and coordinator so that we can address any concerns or issues as they arise and brainstorm solutions together. During the coronavirus disease 2019 pandemic, we had weekly resident town halls with department leadership with transparency about our institution’s current status.
Physical Well-being
Stanford BIL Program
One method of improving physical well-being included stocking healthy snacks for residents and providing incoming residents with a guide of physicians, dentists, and fitness venues to promote regular health care. We have adopted the same at UW with healthy snacks available in our resident workroom.
St. Vincent Internal Medicine Wellness
There are monthly fitness challenges for a variety of physical wellness activities such as sleep, mindfulness minutes, nutrition, and step challenges.8
UW Dermatology
In addition to healthy snacks in our workroom, we also have various discounted fitness classes available for employees, along with discounts on gym memberships, kayak rentals, and city bike-share programs.
Psychological Well-Being
Stanford BIL Program
They enlisted a clinical psychologist available for residents to talk to regularly about any issues they face and to help manage stress in their lives.7
St. Vincent Internal Medicine Wellness
Faculty and coordinators provide S.A.F.E.—secure, affirming, friendly, and empathetic—zones to provide confidential and judgment-free support for residents.8 They also host photography competitions; residents submit photographs of nature, and the winning photographs are printed and displayed throughout the work area.
UW Dermatology
We have made changes to beautify our resident workroom with photograph collages of residents and other assorted décor to make it a more work-friendly space.
Social Well-being
Common themes highlighted by all 3 programs include the importance of socializing outside of the workplace, team-building activities, and resident retreats. Social media accounts on Instagram at St. Vincent (@stvimresidency) and at UW (@uwderm) highlight resident accomplishments and promote interconnectedness when residents are not together in clinics or hospitals.
Final Thoughts
Resident wellness will continue to be an important topic for discussion in the future, especially given the uncertain times right now during our training. Focusing on the 4 areas of well-being can help to prevent burnout and improve resident wellness.
- Croley JAA. #Dermlife and the burned-out resident. Cutis. 2019;104:E32-E33.
- Dyrbye LN, Burke SE, Hardeman RR, et al. Association of clinical specialty with symptoms of burnout and career choice regret among US resident physicians. JAMA. 2018;320:1114-1130.
- Shoimer I, Patten S, Mydlarski PR. Burnout in dermatology residents: a Canadian perspective [published online November 1, 2017]. Br J Dermatol. 2018;178:270-271.
- Grills CN, Aird EG, Rowe D. Breathe, baby, breathe: clearing the way for the emotional emancipation of black people. Cultural Studies & Critical Methodologies. 2016;16:333-343.
- Imadojemu S, James WD. Increasing African American representation in dermatology. JAMA Dermatol. 2016;152:15-16.
- Brotherton SE, Etzel SI. Graduate medical education, 2013-2014. JAMA. 2014;312:2427-2445.
- Salles A, Liebert CA, Greco RS. Promoting balance in the lives of resident physicians: a call to action. JAMA Surg. 2015;150:607-608.
- Fick L, Axon K, Potini Y, et al. Improving overall resident and faculty wellbeing through program-sponsored innovations. MedEdPublish. Published September 27, 2019. doi:10.15694/mep.2019.000184.1.
- St. Vincent Internal Medicine Residency Wellness Bulletin. https://www.mededpublish.org/manuscriptfiles/2586/Supplementary%20File%203_Wellness%20Bulletin.pdf. Published April 2018. Accessed August 5, 2020.
- Croley JAA. #Dermlife and the burned-out resident. Cutis. 2019;104:E32-E33.
- Dyrbye LN, Burke SE, Hardeman RR, et al. Association of clinical specialty with symptoms of burnout and career choice regret among US resident physicians. JAMA. 2018;320:1114-1130.
- Shoimer I, Patten S, Mydlarski PR. Burnout in dermatology residents: a Canadian perspective [published online November 1, 2017]. Br J Dermatol. 2018;178:270-271.
- Grills CN, Aird EG, Rowe D. Breathe, baby, breathe: clearing the way for the emotional emancipation of black people. Cultural Studies & Critical Methodologies. 2016;16:333-343.
- Imadojemu S, James WD. Increasing African American representation in dermatology. JAMA Dermatol. 2016;152:15-16.
- Brotherton SE, Etzel SI. Graduate medical education, 2013-2014. JAMA. 2014;312:2427-2445.
- Salles A, Liebert CA, Greco RS. Promoting balance in the lives of resident physicians: a call to action. JAMA Surg. 2015;150:607-608.
- Fick L, Axon K, Potini Y, et al. Improving overall resident and faculty wellbeing through program-sponsored innovations. MedEdPublish. Published September 27, 2019. doi:10.15694/mep.2019.000184.1.
- St. Vincent Internal Medicine Residency Wellness Bulletin. https://www.mededpublish.org/manuscriptfiles/2586/Supplementary%20File%203_Wellness%20Bulletin.pdf. Published April 2018. Accessed August 5, 2020.
Resident Pearls
- Resident wellness is an important issue affecting resident physicians of all specialties, including dermatology.
- To improve wellness, changes can be made by targeting the following 4 areas of well-being: professional, physical, psychological, and social.
Assessing decision-making capacity
Medical and surgical inpatient teams often consult psychiatrists to help assess a patient’s decision-making capacity (DMC). Completing a DMC assessment can be a source of stress and frustration for psychiatry residents; however, the process can be significantly improved by following a consistent and focused psychiatric interview. In our institution, the most frequently used tool for assessing DMC is the Aid to Capacity Evaluation (ACE).1 The American Academy of Family Physicians offers a printer-friendly adaptation2 of the ACE assessment.
Reasons for a DMC consultation
When accepting a DMC consultation, make sure to specify for which medical decision the primary team would like the patient to be evaluated so that the consultation can be most helpful and specific to primary team’s concerns.3 The 4 most common reasons for a DMC consultation are:
Acute changes in mental status. These changes may be due to hypoxia, infection, medication, metabolic disturbances, or other medical conditions. Often, the diagnosis is delirium due to a medical condition, and assessing for DMC is deferred until the delirium resolves.
Refusal of a recommended treatment. One of the most frequent reasons for a DMC consultation is when a patient declines the primary team’s treatment recommendations. These recommendations may include medications, interventions (procedural or surgical), or discharge planning (such as transfer to a rehabilitation facility or skilled nursing care facility).
Consenting to a risky or invasive treatment too hastily. This occurs less often than the other 3 scenarios, most likely because capacity is rarely questioned when a patient’s decision aligns with the physicians’ recommendations.
Having risk factors for impaired decision-making. One of the most common reasons for involving psychiatry in a DMC consultation is when the patient has a risk factor that may impair his/her decision-making.
These risk factors include:
- a chronic psychiatric or neurologic condition
- a significant cultural or language barrier
- a low or unknown education level
- anxiety or discomfort with institutional health care settings
- age <18 or >85.
Continue to: When should you put off a DMC consultation?
When should you put off a DMC consultation?
There are situations in which a DMC consultation should be declined or postponed. A DMC consultation is not appropriate for patients who have a court-appointed legal guardian because these patients are considered legally incapacitated. Although such patients may still choose to participate in shared decision-making with the treatment team and communicate those decisions to their legal guardian, all treatment decisions are made by their legal guardian.
Consider delaying DMC consultations for patients with acute changes in mental status, including delirium, or those who are sedated or intubated. On the other hand, patients not excluded from a DMC assessment include those with mild or major neurocognitive disorders (such as Alzheimer’s disease or other types of dementia), intellectual disabilities, a durable power of attorney (DPOA), or legal charges. It is important to note that a diagnosis of a neurocognitive disorder does not preclude capacity, and capacity may therefore vary based on the complexity of medical decision.
Prepare for the interview
Prior to meeting the patient, familiarize yourself with his/her reason for admission, diagnosis, medical workup, treatment provided thus far, and proposed future treatment. If possible, attempt to gather the patient’s medical and psychiatric history from chart review, which may be obtained from outside medical records or inferred from the patient’s current medication list. Review the chart for any signs that the patient is in a delirious state, including the use of psychotropic medications for agitation, restraints, or a sitter at bedside. Finally, speak with the patient’s nurse before entering the patient’s room. This may provide valuable information regarding the patient’s willingness to participate (including any hostility or aggression toward clinicians), any barriers to the interview (such as hearing or language difficulties), and if any family or friends are present at the bedside.
During the interview
The structure of a decision-making capacity evaluation has been well documented elsewhere1,2 and, if completed, is sufficient for determining DMC. Asking the patient about additional psychiatric histories, including hospitalizations, suicide attempts, or a family history of psychiatric illness, may be counterproductive and frustrating for the patient, especially if the interview has already been lengthy and emotionally exhausting. Therefore, it is often appropriate to shorten the psychiatric interview to assess for symptoms of anxiety and depression, perform a brief suicide risk assessment, and assess for the presence of auditory or visual hallucinations, or delusions. This allows for a complete mental status exam while still focusing the interview on determining DMC. If time allows and the patient is willing to participate, it can be helpful to perform a Montreal Cognitive Assessment (MoCA) or Mini-Mental State Exam, although a score that indicates some level of cognitive impairment (<26 on MoCA4) does not necessarily preclude patient from having capacity for certain medical decisions. Additionally, if the patient does not understand a question, explain it and return to it later to check for comprehension.
Making a recommendation
Patients are presumed to be capable; therefore, if the determination is not clear, err on the side of capacity. Additional information may be helpful from the patient’s family, friends, DPOA, or guardian, especially as it pertains to the patient’s past wishes and medical decisions made in similar situations. If available and pertinent, review the patient’s advance health care directive.
Communicate your recommendations to the primary team clearly and concisely, including if the evaluation is incomplete, and if the patient will be seen again by the consult team, because this may determine disposition planning. Finally, if the patient is deemed to not have DMC, the primary team must then establish a surrogate decision maker on the patient’s behalf. Because the protocol and hierarchy of next-of-kin varies by state law and institution policy, it is essential to involve social work and case management
1. Joint Centre for Bioethics. Aid to capacity evaluation (ACE). http://www.jcb.utoronto.ca/tools/documents/ace.pdf. Published 1996. Accessed July 20, 2020.
2. American Academy of Family Physicians. Aid to capacity evaluation. https://www.aafp.org/afp/2001/0715/afp20010715p299-f2.pdf. Published 2000. Accessed July 20, 2020.
3. Tunzi M. Can the patient decide? Evaluating patient capacity in practice. Am Fam Physician. 2001;64(2):299-306.
4. Montreal Cognitive Assessment. FAQ. https://www.mocatest.org/faq/. Accessed July 20, 2020.
Medical and surgical inpatient teams often consult psychiatrists to help assess a patient’s decision-making capacity (DMC). Completing a DMC assessment can be a source of stress and frustration for psychiatry residents; however, the process can be significantly improved by following a consistent and focused psychiatric interview. In our institution, the most frequently used tool for assessing DMC is the Aid to Capacity Evaluation (ACE).1 The American Academy of Family Physicians offers a printer-friendly adaptation2 of the ACE assessment.
Reasons for a DMC consultation
When accepting a DMC consultation, make sure to specify for which medical decision the primary team would like the patient to be evaluated so that the consultation can be most helpful and specific to primary team’s concerns.3 The 4 most common reasons for a DMC consultation are:
Acute changes in mental status. These changes may be due to hypoxia, infection, medication, metabolic disturbances, or other medical conditions. Often, the diagnosis is delirium due to a medical condition, and assessing for DMC is deferred until the delirium resolves.
Refusal of a recommended treatment. One of the most frequent reasons for a DMC consultation is when a patient declines the primary team’s treatment recommendations. These recommendations may include medications, interventions (procedural or surgical), or discharge planning (such as transfer to a rehabilitation facility or skilled nursing care facility).
Consenting to a risky or invasive treatment too hastily. This occurs less often than the other 3 scenarios, most likely because capacity is rarely questioned when a patient’s decision aligns with the physicians’ recommendations.
Having risk factors for impaired decision-making. One of the most common reasons for involving psychiatry in a DMC consultation is when the patient has a risk factor that may impair his/her decision-making.
These risk factors include:
- a chronic psychiatric or neurologic condition
- a significant cultural or language barrier
- a low or unknown education level
- anxiety or discomfort with institutional health care settings
- age <18 or >85.
Continue to: When should you put off a DMC consultation?
When should you put off a DMC consultation?
There are situations in which a DMC consultation should be declined or postponed. A DMC consultation is not appropriate for patients who have a court-appointed legal guardian because these patients are considered legally incapacitated. Although such patients may still choose to participate in shared decision-making with the treatment team and communicate those decisions to their legal guardian, all treatment decisions are made by their legal guardian.
Consider delaying DMC consultations for patients with acute changes in mental status, including delirium, or those who are sedated or intubated. On the other hand, patients not excluded from a DMC assessment include those with mild or major neurocognitive disorders (such as Alzheimer’s disease or other types of dementia), intellectual disabilities, a durable power of attorney (DPOA), or legal charges. It is important to note that a diagnosis of a neurocognitive disorder does not preclude capacity, and capacity may therefore vary based on the complexity of medical decision.
Prepare for the interview
Prior to meeting the patient, familiarize yourself with his/her reason for admission, diagnosis, medical workup, treatment provided thus far, and proposed future treatment. If possible, attempt to gather the patient’s medical and psychiatric history from chart review, which may be obtained from outside medical records or inferred from the patient’s current medication list. Review the chart for any signs that the patient is in a delirious state, including the use of psychotropic medications for agitation, restraints, or a sitter at bedside. Finally, speak with the patient’s nurse before entering the patient’s room. This may provide valuable information regarding the patient’s willingness to participate (including any hostility or aggression toward clinicians), any barriers to the interview (such as hearing or language difficulties), and if any family or friends are present at the bedside.
During the interview
The structure of a decision-making capacity evaluation has been well documented elsewhere1,2 and, if completed, is sufficient for determining DMC. Asking the patient about additional psychiatric histories, including hospitalizations, suicide attempts, or a family history of psychiatric illness, may be counterproductive and frustrating for the patient, especially if the interview has already been lengthy and emotionally exhausting. Therefore, it is often appropriate to shorten the psychiatric interview to assess for symptoms of anxiety and depression, perform a brief suicide risk assessment, and assess for the presence of auditory or visual hallucinations, or delusions. This allows for a complete mental status exam while still focusing the interview on determining DMC. If time allows and the patient is willing to participate, it can be helpful to perform a Montreal Cognitive Assessment (MoCA) or Mini-Mental State Exam, although a score that indicates some level of cognitive impairment (<26 on MoCA4) does not necessarily preclude patient from having capacity for certain medical decisions. Additionally, if the patient does not understand a question, explain it and return to it later to check for comprehension.
Making a recommendation
Patients are presumed to be capable; therefore, if the determination is not clear, err on the side of capacity. Additional information may be helpful from the patient’s family, friends, DPOA, or guardian, especially as it pertains to the patient’s past wishes and medical decisions made in similar situations. If available and pertinent, review the patient’s advance health care directive.
Communicate your recommendations to the primary team clearly and concisely, including if the evaluation is incomplete, and if the patient will be seen again by the consult team, because this may determine disposition planning. Finally, if the patient is deemed to not have DMC, the primary team must then establish a surrogate decision maker on the patient’s behalf. Because the protocol and hierarchy of next-of-kin varies by state law and institution policy, it is essential to involve social work and case management
Medical and surgical inpatient teams often consult psychiatrists to help assess a patient’s decision-making capacity (DMC). Completing a DMC assessment can be a source of stress and frustration for psychiatry residents; however, the process can be significantly improved by following a consistent and focused psychiatric interview. In our institution, the most frequently used tool for assessing DMC is the Aid to Capacity Evaluation (ACE).1 The American Academy of Family Physicians offers a printer-friendly adaptation2 of the ACE assessment.
Reasons for a DMC consultation
When accepting a DMC consultation, make sure to specify for which medical decision the primary team would like the patient to be evaluated so that the consultation can be most helpful and specific to primary team’s concerns.3 The 4 most common reasons for a DMC consultation are:
Acute changes in mental status. These changes may be due to hypoxia, infection, medication, metabolic disturbances, or other medical conditions. Often, the diagnosis is delirium due to a medical condition, and assessing for DMC is deferred until the delirium resolves.
Refusal of a recommended treatment. One of the most frequent reasons for a DMC consultation is when a patient declines the primary team’s treatment recommendations. These recommendations may include medications, interventions (procedural or surgical), or discharge planning (such as transfer to a rehabilitation facility or skilled nursing care facility).
Consenting to a risky or invasive treatment too hastily. This occurs less often than the other 3 scenarios, most likely because capacity is rarely questioned when a patient’s decision aligns with the physicians’ recommendations.
Having risk factors for impaired decision-making. One of the most common reasons for involving psychiatry in a DMC consultation is when the patient has a risk factor that may impair his/her decision-making.
These risk factors include:
- a chronic psychiatric or neurologic condition
- a significant cultural or language barrier
- a low or unknown education level
- anxiety or discomfort with institutional health care settings
- age <18 or >85.
Continue to: When should you put off a DMC consultation?
When should you put off a DMC consultation?
There are situations in which a DMC consultation should be declined or postponed. A DMC consultation is not appropriate for patients who have a court-appointed legal guardian because these patients are considered legally incapacitated. Although such patients may still choose to participate in shared decision-making with the treatment team and communicate those decisions to their legal guardian, all treatment decisions are made by their legal guardian.
Consider delaying DMC consultations for patients with acute changes in mental status, including delirium, or those who are sedated or intubated. On the other hand, patients not excluded from a DMC assessment include those with mild or major neurocognitive disorders (such as Alzheimer’s disease or other types of dementia), intellectual disabilities, a durable power of attorney (DPOA), or legal charges. It is important to note that a diagnosis of a neurocognitive disorder does not preclude capacity, and capacity may therefore vary based on the complexity of medical decision.
Prepare for the interview
Prior to meeting the patient, familiarize yourself with his/her reason for admission, diagnosis, medical workup, treatment provided thus far, and proposed future treatment. If possible, attempt to gather the patient’s medical and psychiatric history from chart review, which may be obtained from outside medical records or inferred from the patient’s current medication list. Review the chart for any signs that the patient is in a delirious state, including the use of psychotropic medications for agitation, restraints, or a sitter at bedside. Finally, speak with the patient’s nurse before entering the patient’s room. This may provide valuable information regarding the patient’s willingness to participate (including any hostility or aggression toward clinicians), any barriers to the interview (such as hearing or language difficulties), and if any family or friends are present at the bedside.
During the interview
The structure of a decision-making capacity evaluation has been well documented elsewhere1,2 and, if completed, is sufficient for determining DMC. Asking the patient about additional psychiatric histories, including hospitalizations, suicide attempts, or a family history of psychiatric illness, may be counterproductive and frustrating for the patient, especially if the interview has already been lengthy and emotionally exhausting. Therefore, it is often appropriate to shorten the psychiatric interview to assess for symptoms of anxiety and depression, perform a brief suicide risk assessment, and assess for the presence of auditory or visual hallucinations, or delusions. This allows for a complete mental status exam while still focusing the interview on determining DMC. If time allows and the patient is willing to participate, it can be helpful to perform a Montreal Cognitive Assessment (MoCA) or Mini-Mental State Exam, although a score that indicates some level of cognitive impairment (<26 on MoCA4) does not necessarily preclude patient from having capacity for certain medical decisions. Additionally, if the patient does not understand a question, explain it and return to it later to check for comprehension.
Making a recommendation
Patients are presumed to be capable; therefore, if the determination is not clear, err on the side of capacity. Additional information may be helpful from the patient’s family, friends, DPOA, or guardian, especially as it pertains to the patient’s past wishes and medical decisions made in similar situations. If available and pertinent, review the patient’s advance health care directive.
Communicate your recommendations to the primary team clearly and concisely, including if the evaluation is incomplete, and if the patient will be seen again by the consult team, because this may determine disposition planning. Finally, if the patient is deemed to not have DMC, the primary team must then establish a surrogate decision maker on the patient’s behalf. Because the protocol and hierarchy of next-of-kin varies by state law and institution policy, it is essential to involve social work and case management
1. Joint Centre for Bioethics. Aid to capacity evaluation (ACE). http://www.jcb.utoronto.ca/tools/documents/ace.pdf. Published 1996. Accessed July 20, 2020.
2. American Academy of Family Physicians. Aid to capacity evaluation. https://www.aafp.org/afp/2001/0715/afp20010715p299-f2.pdf. Published 2000. Accessed July 20, 2020.
3. Tunzi M. Can the patient decide? Evaluating patient capacity in practice. Am Fam Physician. 2001;64(2):299-306.
4. Montreal Cognitive Assessment. FAQ. https://www.mocatest.org/faq/. Accessed July 20, 2020.
1. Joint Centre for Bioethics. Aid to capacity evaluation (ACE). http://www.jcb.utoronto.ca/tools/documents/ace.pdf. Published 1996. Accessed July 20, 2020.
2. American Academy of Family Physicians. Aid to capacity evaluation. https://www.aafp.org/afp/2001/0715/afp20010715p299-f2.pdf. Published 2000. Accessed July 20, 2020.
3. Tunzi M. Can the patient decide? Evaluating patient capacity in practice. Am Fam Physician. 2001;64(2):299-306.
4. Montreal Cognitive Assessment. FAQ. https://www.mocatest.org/faq/. Accessed July 20, 2020.
When the professional becomes personal
I arrived at the inpatient psychiatry unit to begin my last overnight call shift as an intern. When I received sign-out from the day-shift resident, Dr. A (all names have been changed to protect anonymity), I was surprised to learn that the medical student assigned for call that day, G, did not present for his shift. In my residency program, a third-year medical student was assigned to accompany an intern during call shifts. While this assignment was for the medical student’s “learning purposes,” the presence of a medical student was vital for the intern. The high volume of patients who needed to be seen while on call was difficult to manage, and the help of a medical student certainly lessened that burden.
During her shift, Dr. A had texted G regarding his missed shift, but he replied that he was busy with a research project and did not intend to attend his call shift. Dr. A and I agreed that G’s behavior was unusual, especially because we had both worked with him before and had found him to be highly motivated and competent. Dr. A said that she was going to follow up with the clerkship director about G’s missed shift. While I was initially angry about having to work the call shift alone, I was quickly overcome with patient care. I labored through the night, and then spent the next day sleeping and recovering.
The following morning, I was back on the inpatient unit to start the work week. I was performing my usual morning pre-rounding when I received shocking news: a medical student had completed suicide on the school’s campus. After processing this news, I asked for the name of the student. It was G.
I sat in disbelief. How could a medical student who was supposed to work with me just 2 days ago have completed suicide? How could such a bright and well-presenting student decide to end his life? I voiced these thoughts to my colleague, who responded, “He must have been preparing to do it instead of coming to your call shift.” This statement hit me like a ton of bricks. Should I have done more to reach out to him? If I had spoken with him, could I have intervened and arrested his planning?
Later, I found out that G had been diagnosed with bipolar disorder. He had shared his diagnosis with some of his classmates and his psychiatry attending. G had wanted to share his success story as someone with mental illness who could have a career in medicine. However, because mental illness carries stigma, the attending had warned G about possible negative consequences in professional settings if he chose to share his diagnosis openly. G expressed disappointment with this advice. Subsequently, he had appeared more withdrawn during his clerkship engagements.
As psychiatrists, we expect to have conversations with our patients about thoughts of suicide, but such discussions with our physician colleagues are far from the norm. We know that the rate of suicide in physicians is higher than in the general population1—particularly in women2—but stigma often prevents those who work in medicine from seeking treatment.3 Unfortunately, professional requirements and attitudes perpetuate barriers to accessing mental health care.4 As long as licensure concerns or other negative professional consequences persist, physicians will avoid seeking potentially life-saving treatment.
I felt guilty for having been angry at G for missing my call shift. I knew that the sequence of events could not be changed, but that did not stop me from wondering, “What if?” What if I had reached out? What if Dr. A had corresponded differently? If we want to prevent tragic outcomes like G’s, then we need to fix our flawed system. We need to allow physicians to seek treatment and share their experience without punitive professional consequences, because suicide is permanent, and there is no undoing.
1. Schernhammer ES, Colditz GA. Suicide rates among physicians: a quantitative and gender assessment (meta-analysis). Am J Psychiatry. 2004;161(12):2295-2302.
2. Duarte D, El-Hagrassy MM, Couto TCE, et al. Male and female physician suicidality: a systematic review and meta-analysis [published online March 4, 2020]. JAMA Psychiatry. 2020;77(6):1-11.
3. Worley LLM. Our fallen peers: a mandate for change. Acad Psychiatry. 2008;32(1):8-12.
4. Center C, Davis M, Detre T, et al. Confronting depression and suicide in physicians: a consensus statement. JAMA. 2003;289(23):3161-3166.
I arrived at the inpatient psychiatry unit to begin my last overnight call shift as an intern. When I received sign-out from the day-shift resident, Dr. A (all names have been changed to protect anonymity), I was surprised to learn that the medical student assigned for call that day, G, did not present for his shift. In my residency program, a third-year medical student was assigned to accompany an intern during call shifts. While this assignment was for the medical student’s “learning purposes,” the presence of a medical student was vital for the intern. The high volume of patients who needed to be seen while on call was difficult to manage, and the help of a medical student certainly lessened that burden.
During her shift, Dr. A had texted G regarding his missed shift, but he replied that he was busy with a research project and did not intend to attend his call shift. Dr. A and I agreed that G’s behavior was unusual, especially because we had both worked with him before and had found him to be highly motivated and competent. Dr. A said that she was going to follow up with the clerkship director about G’s missed shift. While I was initially angry about having to work the call shift alone, I was quickly overcome with patient care. I labored through the night, and then spent the next day sleeping and recovering.
The following morning, I was back on the inpatient unit to start the work week. I was performing my usual morning pre-rounding when I received shocking news: a medical student had completed suicide on the school’s campus. After processing this news, I asked for the name of the student. It was G.
I sat in disbelief. How could a medical student who was supposed to work with me just 2 days ago have completed suicide? How could such a bright and well-presenting student decide to end his life? I voiced these thoughts to my colleague, who responded, “He must have been preparing to do it instead of coming to your call shift.” This statement hit me like a ton of bricks. Should I have done more to reach out to him? If I had spoken with him, could I have intervened and arrested his planning?
Later, I found out that G had been diagnosed with bipolar disorder. He had shared his diagnosis with some of his classmates and his psychiatry attending. G had wanted to share his success story as someone with mental illness who could have a career in medicine. However, because mental illness carries stigma, the attending had warned G about possible negative consequences in professional settings if he chose to share his diagnosis openly. G expressed disappointment with this advice. Subsequently, he had appeared more withdrawn during his clerkship engagements.
As psychiatrists, we expect to have conversations with our patients about thoughts of suicide, but such discussions with our physician colleagues are far from the norm. We know that the rate of suicide in physicians is higher than in the general population1—particularly in women2—but stigma often prevents those who work in medicine from seeking treatment.3 Unfortunately, professional requirements and attitudes perpetuate barriers to accessing mental health care.4 As long as licensure concerns or other negative professional consequences persist, physicians will avoid seeking potentially life-saving treatment.
I felt guilty for having been angry at G for missing my call shift. I knew that the sequence of events could not be changed, but that did not stop me from wondering, “What if?” What if I had reached out? What if Dr. A had corresponded differently? If we want to prevent tragic outcomes like G’s, then we need to fix our flawed system. We need to allow physicians to seek treatment and share their experience without punitive professional consequences, because suicide is permanent, and there is no undoing.
I arrived at the inpatient psychiatry unit to begin my last overnight call shift as an intern. When I received sign-out from the day-shift resident, Dr. A (all names have been changed to protect anonymity), I was surprised to learn that the medical student assigned for call that day, G, did not present for his shift. In my residency program, a third-year medical student was assigned to accompany an intern during call shifts. While this assignment was for the medical student’s “learning purposes,” the presence of a medical student was vital for the intern. The high volume of patients who needed to be seen while on call was difficult to manage, and the help of a medical student certainly lessened that burden.
During her shift, Dr. A had texted G regarding his missed shift, but he replied that he was busy with a research project and did not intend to attend his call shift. Dr. A and I agreed that G’s behavior was unusual, especially because we had both worked with him before and had found him to be highly motivated and competent. Dr. A said that she was going to follow up with the clerkship director about G’s missed shift. While I was initially angry about having to work the call shift alone, I was quickly overcome with patient care. I labored through the night, and then spent the next day sleeping and recovering.
The following morning, I was back on the inpatient unit to start the work week. I was performing my usual morning pre-rounding when I received shocking news: a medical student had completed suicide on the school’s campus. After processing this news, I asked for the name of the student. It was G.
I sat in disbelief. How could a medical student who was supposed to work with me just 2 days ago have completed suicide? How could such a bright and well-presenting student decide to end his life? I voiced these thoughts to my colleague, who responded, “He must have been preparing to do it instead of coming to your call shift.” This statement hit me like a ton of bricks. Should I have done more to reach out to him? If I had spoken with him, could I have intervened and arrested his planning?
Later, I found out that G had been diagnosed with bipolar disorder. He had shared his diagnosis with some of his classmates and his psychiatry attending. G had wanted to share his success story as someone with mental illness who could have a career in medicine. However, because mental illness carries stigma, the attending had warned G about possible negative consequences in professional settings if he chose to share his diagnosis openly. G expressed disappointment with this advice. Subsequently, he had appeared more withdrawn during his clerkship engagements.
As psychiatrists, we expect to have conversations with our patients about thoughts of suicide, but such discussions with our physician colleagues are far from the norm. We know that the rate of suicide in physicians is higher than in the general population1—particularly in women2—but stigma often prevents those who work in medicine from seeking treatment.3 Unfortunately, professional requirements and attitudes perpetuate barriers to accessing mental health care.4 As long as licensure concerns or other negative professional consequences persist, physicians will avoid seeking potentially life-saving treatment.
I felt guilty for having been angry at G for missing my call shift. I knew that the sequence of events could not be changed, but that did not stop me from wondering, “What if?” What if I had reached out? What if Dr. A had corresponded differently? If we want to prevent tragic outcomes like G’s, then we need to fix our flawed system. We need to allow physicians to seek treatment and share their experience without punitive professional consequences, because suicide is permanent, and there is no undoing.
1. Schernhammer ES, Colditz GA. Suicide rates among physicians: a quantitative and gender assessment (meta-analysis). Am J Psychiatry. 2004;161(12):2295-2302.
2. Duarte D, El-Hagrassy MM, Couto TCE, et al. Male and female physician suicidality: a systematic review and meta-analysis [published online March 4, 2020]. JAMA Psychiatry. 2020;77(6):1-11.
3. Worley LLM. Our fallen peers: a mandate for change. Acad Psychiatry. 2008;32(1):8-12.
4. Center C, Davis M, Detre T, et al. Confronting depression and suicide in physicians: a consensus statement. JAMA. 2003;289(23):3161-3166.
1. Schernhammer ES, Colditz GA. Suicide rates among physicians: a quantitative and gender assessment (meta-analysis). Am J Psychiatry. 2004;161(12):2295-2302.
2. Duarte D, El-Hagrassy MM, Couto TCE, et al. Male and female physician suicidality: a systematic review and meta-analysis [published online March 4, 2020]. JAMA Psychiatry. 2020;77(6):1-11.
3. Worley LLM. Our fallen peers: a mandate for change. Acad Psychiatry. 2008;32(1):8-12.
4. Center C, Davis M, Detre T, et al. Confronting depression and suicide in physicians: a consensus statement. JAMA. 2003;289(23):3161-3166.
Applications for the CUTIS 2021 Resident Corner Column
The Cutis Editorial Board is now accepting applications for the 2021 Resident Corner column. The Editorial Board will select 2 to 3 residents to serve as the Resident Corner columnists for 1 year. Articles are posted online only at www.mdedge.com/dermatology but will be referenced in Index Medicus. All applicants must be current residents and will be in residency throughout 2021.
Columnists also will participate in a monthly resident takeover of our Dermatology Weekly podcast.
For consideration, send your curriculum vitae along with a brief (not to exceed 500 words) statement of why you enjoy Cutis and what you can offer your fellow residents in contributing a monthly column.
A signed letter of recommendation from the Director of the dermatology residency program also should be supplied.
All materials should be submitted via email to Melissa Sears ([email protected]) by October 15. The residents who are selected to write the column for the upcoming year will be notified by November 2.
We look forward to continuing to educate dermatology residents on topics that are most important to them!
The Cutis Editorial Board is now accepting applications for the 2021 Resident Corner column. The Editorial Board will select 2 to 3 residents to serve as the Resident Corner columnists for 1 year. Articles are posted online only at www.mdedge.com/dermatology but will be referenced in Index Medicus. All applicants must be current residents and will be in residency throughout 2021.
Columnists also will participate in a monthly resident takeover of our Dermatology Weekly podcast.
For consideration, send your curriculum vitae along with a brief (not to exceed 500 words) statement of why you enjoy Cutis and what you can offer your fellow residents in contributing a monthly column.
A signed letter of recommendation from the Director of the dermatology residency program also should be supplied.
All materials should be submitted via email to Melissa Sears ([email protected]) by October 15. The residents who are selected to write the column for the upcoming year will be notified by November 2.
We look forward to continuing to educate dermatology residents on topics that are most important to them!
The Cutis Editorial Board is now accepting applications for the 2021 Resident Corner column. The Editorial Board will select 2 to 3 residents to serve as the Resident Corner columnists for 1 year. Articles are posted online only at www.mdedge.com/dermatology but will be referenced in Index Medicus. All applicants must be current residents and will be in residency throughout 2021.
Columnists also will participate in a monthly resident takeover of our Dermatology Weekly podcast.
For consideration, send your curriculum vitae along with a brief (not to exceed 500 words) statement of why you enjoy Cutis and what you can offer your fellow residents in contributing a monthly column.
A signed letter of recommendation from the Director of the dermatology residency program also should be supplied.
All materials should be submitted via email to Melissa Sears ([email protected]) by October 15. The residents who are selected to write the column for the upcoming year will be notified by November 2.
We look forward to continuing to educate dermatology residents on topics that are most important to them!
Confronting the epidemic of racism in ObGyn practice
CASE Black woman in stable labor expresses fear
A 29-year-old Black woman (G1) at 39 0/7 weeks’ gestation presents to your labor and delivery unit reporting leaking fluid and contractions. She is found to have ruptured membranes and reassuring fetal testing. Her cervix is 4 cm dilated, and you recommend admission for expectant management of labor. She is otherwise healthy and has no significant medical history.
As you are finishing admitting this patient, you ask if she has any remaining questions. She asks quietly, “Am I going to die today?”
You provide reassurance of her stable clinical picture, then pause and ask the patient about her fears. She looks at you and says, “They didn’t believe Serena Williams, so why would they believe me?”
Your patient is referencing Serena Williams’ harrowing and public postpartum course, complicated by a pulmonary embolism and several reoperations.1 While many of us in the medical field may read this account as a story of challenges with an ultimate triumph, many expectant Black mothers hold Serena’s experience as a cautionary tale about deep-rooted inequities in our health care system that lead to potentially dangerous outcomes.
Disparities in care
They are right to be concerned. In the United States, Black mothers are 4 times more likely to die during or after pregnancy, mostly from preventable causes,2 and nearly 50% more likely to have a preterm delivery.3 These disparities extend beyond the delivery room to all aspects of ObGyn care. Black women are 2 to 3 times more likely to die from cervical cancer, and they are more likely to be diagnosed at a later stage, thus rendering treatment less effective.4 Black patients also have a higher burden of obesity, diabetes, and cardiac disease, and when they present to the hospital, receive evidence-based treatment at lower rates compared with White patients.5
Mourning the deaths of Ahmaud Arbery, Breonna Taylor, and George Floyd, amongst the many other Black lives taken unjustly in the United States, has highlighted egregious practices against people of color embedded within the systems meant to protect and serve our communities. We as ObGyn physicians must take professional onus to recognize a devastating but humbling truth—systemic racism has long pervaded our health care practices and systems, and now more than ever, we must do more to stand by and for our patients.
As ObGyns, we help support patients through some of the happiest, most vulnerable, and potentially most dire moments of their lives. We help patients through the birth of their children, reproductive struggles, gynecologic concerns, and cancer diagnoses. Many of us chose this field for the privilege of caring for patients at these critical moments in their lives, but we have often neglected the racism present in our practices, our hospital settings, and the medical system itself. We often fail to acknowledge our own implicit bias and the role that we play in contributing to acts and experiences of racism that our patients and our colleagues face on a daily basis.
Racism in our origins
The history of obstetrics and gynecology shows us a long record of physicians perpetrating injustices that target marginalized communities of color. Dr. James Sims, often given the title of “father of modern gynecology,” performed numerous experiments on unanesthetized Black female slaves to develop procedures for fistulae repair and other surgical techniques.6 Throughout the twentieth century, dating as recent as 1979, state laws written in the name of public safety forcibly sterilized women of color to control an “undesirable population.”7 When a patient of color declines a method of long-acting reversible contraception, birth control pills, or tubal ligation, do you take the time to reflect on the potential context of the patient’s decision?
It is critical to recognize the legacy that these acts have on our patients today, leading to a higher burden of disease and an understandable distrust of the medical system. The uncovering of the unethical practices of the National Institutions of Health‒funded Tuskegee syphilis study, in which hundreds of Black men with latent syphilis were passively monitored despite the knowledge of a proven treatment, has attributed to a measurable decrease in life expectancy among Black males.8 Even as we face the COVID-19 pandemic, the undercurrent of racism continues to do harm. Black patients are 5 times more likely to be hospitalized with COVID-19 than their White counterparts. This disparity, in part, is a product of a higher burden of comorbidities and the privilege associated with shelter-in-place policies, which disproportionately strain communities of color.9
We as a medical community need to do better for our patients. No matter how difficult to confront, each of us must acknowledge our own biases and our duty to combat persistent and perpetual racism in our medical system. We need to commit to amplifying the voices of our Black patients and colleagues. It is not enough to celebrate diversity for performance sake—it is time to recognize that diversity saves lives.
We have a responsibility to rectify these traditions of injustice and work toward a safer, more equitable, healthy future for our patients and their families. While this pledge may seem daunting, changes at individual and systems levels can make a difference for all patients that come through our doors. In addition, to honor our oath to “do no harm,” we must act; Black lives matter, and we are charged as medical providers to help our patients thrive, especially those from historically oppressed communities and who continue to suffer inexcusable injustices in health care and beyond.
Take action
Here is a collection of ways to institute an antiracist environment and more equitable care for your patients.
Self-reflect and educate
- Learn about the role racism plays in ObGyn and modern medicine. One place to start: read “Medical Bondage: Race, Gender and the Origins of American Gynecology” by Deidre Cooper Owens. Also check out articles and key readings curated by the Black Mamas Matter Alliance.
- Introduce and sustain antiracism training for all staff in your clinic or hospital system. To start, consider taking these free and quick implicit bias tests at a staff or department meeting.
- Familiarize yourself and your colleagues with facets of reproductive justice—the human right to have children, to not have children, and to nurture children in a safe and healthy environment—and incorporate these values in your practice. Request trainings in reproductive justice from community groups like Sister Song.
- Sign up for updates for state and national bills addressing health inequity and access to reproductive health services. Show your support by calling your congress-people, testifying, or donating to a cause that promotes these bills. You can stay up to date on national issues with government affairs newsletters from the American College of Obstetricians and Gynecologists. Sign up here.
- Continue the conversation and re-evaluate your personal and institution’s efforts to combat racism and social and reproductive injustices.
Provide access to high-quality reproductive health care
- Ask your patients what barriers they faced to come to your clinic and receive the care they needed. Consider incorporating the following screening tools regarding social determinants of health: PRAPARE screening tool, AAFP screening tool.
- Promote access to insurance and support programs, including nutrition, exercise and wellness, and safe home and school environments. Look up resources available to your patients by their zip codes using AAFP’s Neighborhood Navigator.
- Help patients access their medications at affordable prices in their neighborhoods by using free apps. Use the GoodRx app to identify discounts for prescriptions at various pharmacies, and search the Bedsider app to find out how your patients can get their birth control for free and delivered to their homes.
- Expand access to language services for patients who do not speak English as their first language. If working in a resource-limited setting, use the Google Translate app. Print out these free handouts for birth control fact sheets in different languages.
- Establish standardized protocols for common treatment paradigms to reduce the influence of bias in clinical scenarios. For example, institute a protocol for managing postoperative pain to ensure equal access to treatment.
- Institute the AIM (Alliance for Innovation on Maternal Health) patient safety bundle on the Reduction of Peripartum Racial/Ethnic Disparities. Learn more about AIM’s maternal safety and quality improvement initiative to reduce maternal morbidity and mortality here.
Support a diverse workforce
- Designate and/or hire a Diversity and Inclusion Officer at your institution to ensure that hiring practices actively achieve a diverse workforce and that employees feel supported in the work environment. Consider coalition-building between hospitals, like the UPHS-CHOP Alliance of Minority Physicians.
- Recruit diverse applicants by advertising positions to groups that focus on the advancement of underrepresented minorities in medicine. Engage with your local chapter of the National Medical Association and American Medical Women’s Association.
- Have a system in place for anonymous reporting of incidents involving bias or discrimination against staff, and develop a protocol to ensure action is taken in case of such incidents.
- Institute a recurring conference or Grand Rounds across disciplines to discuss the impacts of bias and discrimination on patients and providers at your institution. View examples of these conferences here.
- Ensure invited speakers and other educational opportunities are comprised of diverse representation.
- Create a work environment with safe spaces for the discussion of racism, discrimination, and bias.
- Haskell R. Serena Williams on motherhood, marriage, and making her comeback. January 10, 2018. https://www.vogue.com/article/serena-williams-vogue-cover-interview-february-2018. Accessed July 1, 2020.
- Louis JM, Menard MK, Gee RE. Racial and ethnic disparities in maternal morbidity and mortality. Obstet Gynecol. 2015;125:690-694.
- Sigurdson K, Mitchell B, Liu J, et al. Racial/ethnic disparities in neonatal intensive care: a systematic review. Pediatrics. 2019;144:e20183114.
- Garner EI. Cervical cancer: disparities in screening, treatment, and survival. Cancer Epidemiol Biomarkers Prev. 2003;12:242s-247s.
- Arora S, Stouffer GA, Kucharska‐Newton A, et al. Fifteen‐year trends in management and outcomes of non–ST‐segment–elevation myocardial infarction among black and white patients: the ARIC community surveillance study, 2000–2014. J Am Heart Assoc. 2018;7:e010203.
- Zellars R. Black subjectivity and the origins of American gynecology. May 31, 2018. https://www.aaihs.org/black-subjectivity-and-the-origins-of-american-gynecology/. Accessed June 28, 2020.
- Ko K. Unwanted sterilization and eugenics programs in the United States. January 29, 2016. https://www.pbs.org/independentlens/blog/unwanted-sterilization-and-eugenics-programs-in-the-united-states/. Accessed June 28, 2020.
- Alsan M, Wanamaker M. Tuskegee and the health of black men. Q J Econ. 2018;133:407-455.
- Hooper MW, Nápoles AM, Pérez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA. 2020 May 11. doi: 10.1001/jama.2020.8598.
CASE Black woman in stable labor expresses fear
A 29-year-old Black woman (G1) at 39 0/7 weeks’ gestation presents to your labor and delivery unit reporting leaking fluid and contractions. She is found to have ruptured membranes and reassuring fetal testing. Her cervix is 4 cm dilated, and you recommend admission for expectant management of labor. She is otherwise healthy and has no significant medical history.
As you are finishing admitting this patient, you ask if she has any remaining questions. She asks quietly, “Am I going to die today?”
You provide reassurance of her stable clinical picture, then pause and ask the patient about her fears. She looks at you and says, “They didn’t believe Serena Williams, so why would they believe me?”
Your patient is referencing Serena Williams’ harrowing and public postpartum course, complicated by a pulmonary embolism and several reoperations.1 While many of us in the medical field may read this account as a story of challenges with an ultimate triumph, many expectant Black mothers hold Serena’s experience as a cautionary tale about deep-rooted inequities in our health care system that lead to potentially dangerous outcomes.
Disparities in care
They are right to be concerned. In the United States, Black mothers are 4 times more likely to die during or after pregnancy, mostly from preventable causes,2 and nearly 50% more likely to have a preterm delivery.3 These disparities extend beyond the delivery room to all aspects of ObGyn care. Black women are 2 to 3 times more likely to die from cervical cancer, and they are more likely to be diagnosed at a later stage, thus rendering treatment less effective.4 Black patients also have a higher burden of obesity, diabetes, and cardiac disease, and when they present to the hospital, receive evidence-based treatment at lower rates compared with White patients.5
Mourning the deaths of Ahmaud Arbery, Breonna Taylor, and George Floyd, amongst the many other Black lives taken unjustly in the United States, has highlighted egregious practices against people of color embedded within the systems meant to protect and serve our communities. We as ObGyn physicians must take professional onus to recognize a devastating but humbling truth—systemic racism has long pervaded our health care practices and systems, and now more than ever, we must do more to stand by and for our patients.
As ObGyns, we help support patients through some of the happiest, most vulnerable, and potentially most dire moments of their lives. We help patients through the birth of their children, reproductive struggles, gynecologic concerns, and cancer diagnoses. Many of us chose this field for the privilege of caring for patients at these critical moments in their lives, but we have often neglected the racism present in our practices, our hospital settings, and the medical system itself. We often fail to acknowledge our own implicit bias and the role that we play in contributing to acts and experiences of racism that our patients and our colleagues face on a daily basis.
Racism in our origins
The history of obstetrics and gynecology shows us a long record of physicians perpetrating injustices that target marginalized communities of color. Dr. James Sims, often given the title of “father of modern gynecology,” performed numerous experiments on unanesthetized Black female slaves to develop procedures for fistulae repair and other surgical techniques.6 Throughout the twentieth century, dating as recent as 1979, state laws written in the name of public safety forcibly sterilized women of color to control an “undesirable population.”7 When a patient of color declines a method of long-acting reversible contraception, birth control pills, or tubal ligation, do you take the time to reflect on the potential context of the patient’s decision?
It is critical to recognize the legacy that these acts have on our patients today, leading to a higher burden of disease and an understandable distrust of the medical system. The uncovering of the unethical practices of the National Institutions of Health‒funded Tuskegee syphilis study, in which hundreds of Black men with latent syphilis were passively monitored despite the knowledge of a proven treatment, has attributed to a measurable decrease in life expectancy among Black males.8 Even as we face the COVID-19 pandemic, the undercurrent of racism continues to do harm. Black patients are 5 times more likely to be hospitalized with COVID-19 than their White counterparts. This disparity, in part, is a product of a higher burden of comorbidities and the privilege associated with shelter-in-place policies, which disproportionately strain communities of color.9
We as a medical community need to do better for our patients. No matter how difficult to confront, each of us must acknowledge our own biases and our duty to combat persistent and perpetual racism in our medical system. We need to commit to amplifying the voices of our Black patients and colleagues. It is not enough to celebrate diversity for performance sake—it is time to recognize that diversity saves lives.
We have a responsibility to rectify these traditions of injustice and work toward a safer, more equitable, healthy future for our patients and their families. While this pledge may seem daunting, changes at individual and systems levels can make a difference for all patients that come through our doors. In addition, to honor our oath to “do no harm,” we must act; Black lives matter, and we are charged as medical providers to help our patients thrive, especially those from historically oppressed communities and who continue to suffer inexcusable injustices in health care and beyond.
Take action
Here is a collection of ways to institute an antiracist environment and more equitable care for your patients.
Self-reflect and educate
- Learn about the role racism plays in ObGyn and modern medicine. One place to start: read “Medical Bondage: Race, Gender and the Origins of American Gynecology” by Deidre Cooper Owens. Also check out articles and key readings curated by the Black Mamas Matter Alliance.
- Introduce and sustain antiracism training for all staff in your clinic or hospital system. To start, consider taking these free and quick implicit bias tests at a staff or department meeting.
- Familiarize yourself and your colleagues with facets of reproductive justice—the human right to have children, to not have children, and to nurture children in a safe and healthy environment—and incorporate these values in your practice. Request trainings in reproductive justice from community groups like Sister Song.
- Sign up for updates for state and national bills addressing health inequity and access to reproductive health services. Show your support by calling your congress-people, testifying, or donating to a cause that promotes these bills. You can stay up to date on national issues with government affairs newsletters from the American College of Obstetricians and Gynecologists. Sign up here.
- Continue the conversation and re-evaluate your personal and institution’s efforts to combat racism and social and reproductive injustices.
Provide access to high-quality reproductive health care
- Ask your patients what barriers they faced to come to your clinic and receive the care they needed. Consider incorporating the following screening tools regarding social determinants of health: PRAPARE screening tool, AAFP screening tool.
- Promote access to insurance and support programs, including nutrition, exercise and wellness, and safe home and school environments. Look up resources available to your patients by their zip codes using AAFP’s Neighborhood Navigator.
- Help patients access their medications at affordable prices in their neighborhoods by using free apps. Use the GoodRx app to identify discounts for prescriptions at various pharmacies, and search the Bedsider app to find out how your patients can get their birth control for free and delivered to their homes.
- Expand access to language services for patients who do not speak English as their first language. If working in a resource-limited setting, use the Google Translate app. Print out these free handouts for birth control fact sheets in different languages.
- Establish standardized protocols for common treatment paradigms to reduce the influence of bias in clinical scenarios. For example, institute a protocol for managing postoperative pain to ensure equal access to treatment.
- Institute the AIM (Alliance for Innovation on Maternal Health) patient safety bundle on the Reduction of Peripartum Racial/Ethnic Disparities. Learn more about AIM’s maternal safety and quality improvement initiative to reduce maternal morbidity and mortality here.
Support a diverse workforce
- Designate and/or hire a Diversity and Inclusion Officer at your institution to ensure that hiring practices actively achieve a diverse workforce and that employees feel supported in the work environment. Consider coalition-building between hospitals, like the UPHS-CHOP Alliance of Minority Physicians.
- Recruit diverse applicants by advertising positions to groups that focus on the advancement of underrepresented minorities in medicine. Engage with your local chapter of the National Medical Association and American Medical Women’s Association.
- Have a system in place for anonymous reporting of incidents involving bias or discrimination against staff, and develop a protocol to ensure action is taken in case of such incidents.
- Institute a recurring conference or Grand Rounds across disciplines to discuss the impacts of bias and discrimination on patients and providers at your institution. View examples of these conferences here.
- Ensure invited speakers and other educational opportunities are comprised of diverse representation.
- Create a work environment with safe spaces for the discussion of racism, discrimination, and bias.
CASE Black woman in stable labor expresses fear
A 29-year-old Black woman (G1) at 39 0/7 weeks’ gestation presents to your labor and delivery unit reporting leaking fluid and contractions. She is found to have ruptured membranes and reassuring fetal testing. Her cervix is 4 cm dilated, and you recommend admission for expectant management of labor. She is otherwise healthy and has no significant medical history.
As you are finishing admitting this patient, you ask if she has any remaining questions. She asks quietly, “Am I going to die today?”
You provide reassurance of her stable clinical picture, then pause and ask the patient about her fears. She looks at you and says, “They didn’t believe Serena Williams, so why would they believe me?”
Your patient is referencing Serena Williams’ harrowing and public postpartum course, complicated by a pulmonary embolism and several reoperations.1 While many of us in the medical field may read this account as a story of challenges with an ultimate triumph, many expectant Black mothers hold Serena’s experience as a cautionary tale about deep-rooted inequities in our health care system that lead to potentially dangerous outcomes.
Disparities in care
They are right to be concerned. In the United States, Black mothers are 4 times more likely to die during or after pregnancy, mostly from preventable causes,2 and nearly 50% more likely to have a preterm delivery.3 These disparities extend beyond the delivery room to all aspects of ObGyn care. Black women are 2 to 3 times more likely to die from cervical cancer, and they are more likely to be diagnosed at a later stage, thus rendering treatment less effective.4 Black patients also have a higher burden of obesity, diabetes, and cardiac disease, and when they present to the hospital, receive evidence-based treatment at lower rates compared with White patients.5
Mourning the deaths of Ahmaud Arbery, Breonna Taylor, and George Floyd, amongst the many other Black lives taken unjustly in the United States, has highlighted egregious practices against people of color embedded within the systems meant to protect and serve our communities. We as ObGyn physicians must take professional onus to recognize a devastating but humbling truth—systemic racism has long pervaded our health care practices and systems, and now more than ever, we must do more to stand by and for our patients.
As ObGyns, we help support patients through some of the happiest, most vulnerable, and potentially most dire moments of their lives. We help patients through the birth of their children, reproductive struggles, gynecologic concerns, and cancer diagnoses. Many of us chose this field for the privilege of caring for patients at these critical moments in their lives, but we have often neglected the racism present in our practices, our hospital settings, and the medical system itself. We often fail to acknowledge our own implicit bias and the role that we play in contributing to acts and experiences of racism that our patients and our colleagues face on a daily basis.
Racism in our origins
The history of obstetrics and gynecology shows us a long record of physicians perpetrating injustices that target marginalized communities of color. Dr. James Sims, often given the title of “father of modern gynecology,” performed numerous experiments on unanesthetized Black female slaves to develop procedures for fistulae repair and other surgical techniques.6 Throughout the twentieth century, dating as recent as 1979, state laws written in the name of public safety forcibly sterilized women of color to control an “undesirable population.”7 When a patient of color declines a method of long-acting reversible contraception, birth control pills, or tubal ligation, do you take the time to reflect on the potential context of the patient’s decision?
It is critical to recognize the legacy that these acts have on our patients today, leading to a higher burden of disease and an understandable distrust of the medical system. The uncovering of the unethical practices of the National Institutions of Health‒funded Tuskegee syphilis study, in which hundreds of Black men with latent syphilis were passively monitored despite the knowledge of a proven treatment, has attributed to a measurable decrease in life expectancy among Black males.8 Even as we face the COVID-19 pandemic, the undercurrent of racism continues to do harm. Black patients are 5 times more likely to be hospitalized with COVID-19 than their White counterparts. This disparity, in part, is a product of a higher burden of comorbidities and the privilege associated with shelter-in-place policies, which disproportionately strain communities of color.9
We as a medical community need to do better for our patients. No matter how difficult to confront, each of us must acknowledge our own biases and our duty to combat persistent and perpetual racism in our medical system. We need to commit to amplifying the voices of our Black patients and colleagues. It is not enough to celebrate diversity for performance sake—it is time to recognize that diversity saves lives.
We have a responsibility to rectify these traditions of injustice and work toward a safer, more equitable, healthy future for our patients and their families. While this pledge may seem daunting, changes at individual and systems levels can make a difference for all patients that come through our doors. In addition, to honor our oath to “do no harm,” we must act; Black lives matter, and we are charged as medical providers to help our patients thrive, especially those from historically oppressed communities and who continue to suffer inexcusable injustices in health care and beyond.
Take action
Here is a collection of ways to institute an antiracist environment and more equitable care for your patients.
Self-reflect and educate
- Learn about the role racism plays in ObGyn and modern medicine. One place to start: read “Medical Bondage: Race, Gender and the Origins of American Gynecology” by Deidre Cooper Owens. Also check out articles and key readings curated by the Black Mamas Matter Alliance.
- Introduce and sustain antiracism training for all staff in your clinic or hospital system. To start, consider taking these free and quick implicit bias tests at a staff or department meeting.
- Familiarize yourself and your colleagues with facets of reproductive justice—the human right to have children, to not have children, and to nurture children in a safe and healthy environment—and incorporate these values in your practice. Request trainings in reproductive justice from community groups like Sister Song.
- Sign up for updates for state and national bills addressing health inequity and access to reproductive health services. Show your support by calling your congress-people, testifying, or donating to a cause that promotes these bills. You can stay up to date on national issues with government affairs newsletters from the American College of Obstetricians and Gynecologists. Sign up here.
- Continue the conversation and re-evaluate your personal and institution’s efforts to combat racism and social and reproductive injustices.
Provide access to high-quality reproductive health care
- Ask your patients what barriers they faced to come to your clinic and receive the care they needed. Consider incorporating the following screening tools regarding social determinants of health: PRAPARE screening tool, AAFP screening tool.
- Promote access to insurance and support programs, including nutrition, exercise and wellness, and safe home and school environments. Look up resources available to your patients by their zip codes using AAFP’s Neighborhood Navigator.
- Help patients access their medications at affordable prices in their neighborhoods by using free apps. Use the GoodRx app to identify discounts for prescriptions at various pharmacies, and search the Bedsider app to find out how your patients can get their birth control for free and delivered to their homes.
- Expand access to language services for patients who do not speak English as their first language. If working in a resource-limited setting, use the Google Translate app. Print out these free handouts for birth control fact sheets in different languages.
- Establish standardized protocols for common treatment paradigms to reduce the influence of bias in clinical scenarios. For example, institute a protocol for managing postoperative pain to ensure equal access to treatment.
- Institute the AIM (Alliance for Innovation on Maternal Health) patient safety bundle on the Reduction of Peripartum Racial/Ethnic Disparities. Learn more about AIM’s maternal safety and quality improvement initiative to reduce maternal morbidity and mortality here.
Support a diverse workforce
- Designate and/or hire a Diversity and Inclusion Officer at your institution to ensure that hiring practices actively achieve a diverse workforce and that employees feel supported in the work environment. Consider coalition-building between hospitals, like the UPHS-CHOP Alliance of Minority Physicians.
- Recruit diverse applicants by advertising positions to groups that focus on the advancement of underrepresented minorities in medicine. Engage with your local chapter of the National Medical Association and American Medical Women’s Association.
- Have a system in place for anonymous reporting of incidents involving bias or discrimination against staff, and develop a protocol to ensure action is taken in case of such incidents.
- Institute a recurring conference or Grand Rounds across disciplines to discuss the impacts of bias and discrimination on patients and providers at your institution. View examples of these conferences here.
- Ensure invited speakers and other educational opportunities are comprised of diverse representation.
- Create a work environment with safe spaces for the discussion of racism, discrimination, and bias.
- Haskell R. Serena Williams on motherhood, marriage, and making her comeback. January 10, 2018. https://www.vogue.com/article/serena-williams-vogue-cover-interview-february-2018. Accessed July 1, 2020.
- Louis JM, Menard MK, Gee RE. Racial and ethnic disparities in maternal morbidity and mortality. Obstet Gynecol. 2015;125:690-694.
- Sigurdson K, Mitchell B, Liu J, et al. Racial/ethnic disparities in neonatal intensive care: a systematic review. Pediatrics. 2019;144:e20183114.
- Garner EI. Cervical cancer: disparities in screening, treatment, and survival. Cancer Epidemiol Biomarkers Prev. 2003;12:242s-247s.
- Arora S, Stouffer GA, Kucharska‐Newton A, et al. Fifteen‐year trends in management and outcomes of non–ST‐segment–elevation myocardial infarction among black and white patients: the ARIC community surveillance study, 2000–2014. J Am Heart Assoc. 2018;7:e010203.
- Zellars R. Black subjectivity and the origins of American gynecology. May 31, 2018. https://www.aaihs.org/black-subjectivity-and-the-origins-of-american-gynecology/. Accessed June 28, 2020.
- Ko K. Unwanted sterilization and eugenics programs in the United States. January 29, 2016. https://www.pbs.org/independentlens/blog/unwanted-sterilization-and-eugenics-programs-in-the-united-states/. Accessed June 28, 2020.
- Alsan M, Wanamaker M. Tuskegee and the health of black men. Q J Econ. 2018;133:407-455.
- Hooper MW, Nápoles AM, Pérez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA. 2020 May 11. doi: 10.1001/jama.2020.8598.
- Haskell R. Serena Williams on motherhood, marriage, and making her comeback. January 10, 2018. https://www.vogue.com/article/serena-williams-vogue-cover-interview-february-2018. Accessed July 1, 2020.
- Louis JM, Menard MK, Gee RE. Racial and ethnic disparities in maternal morbidity and mortality. Obstet Gynecol. 2015;125:690-694.
- Sigurdson K, Mitchell B, Liu J, et al. Racial/ethnic disparities in neonatal intensive care: a systematic review. Pediatrics. 2019;144:e20183114.
- Garner EI. Cervical cancer: disparities in screening, treatment, and survival. Cancer Epidemiol Biomarkers Prev. 2003;12:242s-247s.
- Arora S, Stouffer GA, Kucharska‐Newton A, et al. Fifteen‐year trends in management and outcomes of non–ST‐segment–elevation myocardial infarction among black and white patients: the ARIC community surveillance study, 2000–2014. J Am Heart Assoc. 2018;7:e010203.
- Zellars R. Black subjectivity and the origins of American gynecology. May 31, 2018. https://www.aaihs.org/black-subjectivity-and-the-origins-of-american-gynecology/. Accessed June 28, 2020.
- Ko K. Unwanted sterilization and eugenics programs in the United States. January 29, 2016. https://www.pbs.org/independentlens/blog/unwanted-sterilization-and-eugenics-programs-in-the-united-states/. Accessed June 28, 2020.
- Alsan M, Wanamaker M. Tuskegee and the health of black men. Q J Econ. 2018;133:407-455.
- Hooper MW, Nápoles AM, Pérez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA. 2020 May 11. doi: 10.1001/jama.2020.8598.






