User login
Racing thoughts: What to consider
Have you ever had times in your life when you had a tremendous amount of energy, like too much energy, with racing thoughts? I initially ask patients this question when evaluating for bipolar disorder. Some patients insist that they have racing thoughts—thoughts occurring at a rate faster than they can be expressed through speech1—but not episodes of hyperactivity. This response suggests that some patients can have racing thoughts without a diagnosis of bipolar disorder.
Among the patients I treat, racing thoughts vary in severity, duration, and treatment. When untreated, a patient’s racing thoughts may range from a mild disturbance lasting a few days to a more severe disturbance occurring daily. In this article, I suggest treatments that may help ameliorate racing thoughts, and describe possible causes that include, but are not limited to, mood disorders.
Major depressive disorder
Many patients with major depressive disorder (MDD) have racing thoughts that often go unrecognized, and this symptom is associated with more severe depression.2 Those with a DSM-5 diagnosis of MDD with mixed features could experience prolonged racing thoughts during a major depressive episode.1 Untreated racing thoughts may explain why many patients with MDD do not improve with an antidepressant alone.3 These patients might benefit from augmentation with a mood stabilizer such as lithium4 or a second-generation antipsychotic.5
Other potential causes
Racing thoughts are a symptom, not a diagnosis. Apprehension and anxiety could cause racing thoughts that do not require treatment with a mood stabilizer or antipsychotic. Patients who often worry about having panic attacks or experience severe chronic stress may have racing thoughts. Also, some patients may be taking medications or illicit drugs or have a medical disorder that could cause symptoms of mania or hypomania that include racing thoughts (Table1).
In summary, when caring for a patient who reports having racing thoughts, consider:
- whether that patient actually does have racing thoughts
- the potential causes, severity, duration, and treatment of the racing thoughts
- the possibility that for a patient with MDD, augmenting an antidepressant with a mood stabilizer or antipsychotic could decrease racing thoughts, thereby helping to alleviate many cases of MDD.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Benazzi F. Unipolar depression with racing thoughts: a bipolar spectrum disorder? Psychiatry Clin Neurosci. 2005;59:570-575.
3. Undurraga J, Baldessarini RJ. Randomized, placebo-controlled trials of antidepressants for acute major depression: thirty-year meta-analytic review. Neuropsychopharmacology. 2012;37(4):851-864.
4. Bauer M, Adli M, Bschor T, et al. Lithium’s emerging role in the treatment of refractory major depressive episodes: augmentation of antidepressants. Neuropsychobiology. 2010;62(1):36-42.
5. Nelson JC, Papakostas GI. Atypical antipsychotic augmentation in major depressive disorder: a meta-analysis of placebo-controlled randomized trials. Am J Psychiatry. 2009;166(9):980-991.
Have you ever had times in your life when you had a tremendous amount of energy, like too much energy, with racing thoughts? I initially ask patients this question when evaluating for bipolar disorder. Some patients insist that they have racing thoughts—thoughts occurring at a rate faster than they can be expressed through speech1—but not episodes of hyperactivity. This response suggests that some patients can have racing thoughts without a diagnosis of bipolar disorder.
Among the patients I treat, racing thoughts vary in severity, duration, and treatment. When untreated, a patient’s racing thoughts may range from a mild disturbance lasting a few days to a more severe disturbance occurring daily. In this article, I suggest treatments that may help ameliorate racing thoughts, and describe possible causes that include, but are not limited to, mood disorders.
Major depressive disorder
Many patients with major depressive disorder (MDD) have racing thoughts that often go unrecognized, and this symptom is associated with more severe depression.2 Those with a DSM-5 diagnosis of MDD with mixed features could experience prolonged racing thoughts during a major depressive episode.1 Untreated racing thoughts may explain why many patients with MDD do not improve with an antidepressant alone.3 These patients might benefit from augmentation with a mood stabilizer such as lithium4 or a second-generation antipsychotic.5
Other potential causes
Racing thoughts are a symptom, not a diagnosis. Apprehension and anxiety could cause racing thoughts that do not require treatment with a mood stabilizer or antipsychotic. Patients who often worry about having panic attacks or experience severe chronic stress may have racing thoughts. Also, some patients may be taking medications or illicit drugs or have a medical disorder that could cause symptoms of mania or hypomania that include racing thoughts (Table1).
In summary, when caring for a patient who reports having racing thoughts, consider:
- whether that patient actually does have racing thoughts
- the potential causes, severity, duration, and treatment of the racing thoughts
- the possibility that for a patient with MDD, augmenting an antidepressant with a mood stabilizer or antipsychotic could decrease racing thoughts, thereby helping to alleviate many cases of MDD.
Have you ever had times in your life when you had a tremendous amount of energy, like too much energy, with racing thoughts? I initially ask patients this question when evaluating for bipolar disorder. Some patients insist that they have racing thoughts—thoughts occurring at a rate faster than they can be expressed through speech1—but not episodes of hyperactivity. This response suggests that some patients can have racing thoughts without a diagnosis of bipolar disorder.
Among the patients I treat, racing thoughts vary in severity, duration, and treatment. When untreated, a patient’s racing thoughts may range from a mild disturbance lasting a few days to a more severe disturbance occurring daily. In this article, I suggest treatments that may help ameliorate racing thoughts, and describe possible causes that include, but are not limited to, mood disorders.
Major depressive disorder
Many patients with major depressive disorder (MDD) have racing thoughts that often go unrecognized, and this symptom is associated with more severe depression.2 Those with a DSM-5 diagnosis of MDD with mixed features could experience prolonged racing thoughts during a major depressive episode.1 Untreated racing thoughts may explain why many patients with MDD do not improve with an antidepressant alone.3 These patients might benefit from augmentation with a mood stabilizer such as lithium4 or a second-generation antipsychotic.5
Other potential causes
Racing thoughts are a symptom, not a diagnosis. Apprehension and anxiety could cause racing thoughts that do not require treatment with a mood stabilizer or antipsychotic. Patients who often worry about having panic attacks or experience severe chronic stress may have racing thoughts. Also, some patients may be taking medications or illicit drugs or have a medical disorder that could cause symptoms of mania or hypomania that include racing thoughts (Table1).
In summary, when caring for a patient who reports having racing thoughts, consider:
- whether that patient actually does have racing thoughts
- the potential causes, severity, duration, and treatment of the racing thoughts
- the possibility that for a patient with MDD, augmenting an antidepressant with a mood stabilizer or antipsychotic could decrease racing thoughts, thereby helping to alleviate many cases of MDD.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Benazzi F. Unipolar depression with racing thoughts: a bipolar spectrum disorder? Psychiatry Clin Neurosci. 2005;59:570-575.
3. Undurraga J, Baldessarini RJ. Randomized, placebo-controlled trials of antidepressants for acute major depression: thirty-year meta-analytic review. Neuropsychopharmacology. 2012;37(4):851-864.
4. Bauer M, Adli M, Bschor T, et al. Lithium’s emerging role in the treatment of refractory major depressive episodes: augmentation of antidepressants. Neuropsychobiology. 2010;62(1):36-42.
5. Nelson JC, Papakostas GI. Atypical antipsychotic augmentation in major depressive disorder: a meta-analysis of placebo-controlled randomized trials. Am J Psychiatry. 2009;166(9):980-991.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Benazzi F. Unipolar depression with racing thoughts: a bipolar spectrum disorder? Psychiatry Clin Neurosci. 2005;59:570-575.
3. Undurraga J, Baldessarini RJ. Randomized, placebo-controlled trials of antidepressants for acute major depression: thirty-year meta-analytic review. Neuropsychopharmacology. 2012;37(4):851-864.
4. Bauer M, Adli M, Bschor T, et al. Lithium’s emerging role in the treatment of refractory major depressive episodes: augmentation of antidepressants. Neuropsychobiology. 2010;62(1):36-42.
5. Nelson JC, Papakostas GI. Atypical antipsychotic augmentation in major depressive disorder: a meta-analysis of placebo-controlled randomized trials. Am J Psychiatry. 2009;166(9):980-991.
Does your patient have postpartum OCD?
Childbirth is a trigger for first-onse
In one prospective study of 461 women who recently gave birth, researchers found the prevalence of OCD symptoms was 11% at 2 weeks postpartum.1 Mothers with OCD may have time-consuming or functionally impairing obsessions and/or compulsions that can include:
- anticipatory anxiety of contamination (eg, germs, illness)
- thoughts of accidental or intentional harm to their infant
- compulsions comprised of cleaning and checking behaviors
- avoidance of situations
- thought suppression.
Because both clinicians and patients may not be aware of the effects of childbirth on women with OCD, postpartum OCD may go underdiagnosed or be misdiagnosed as major depressive disorder (MDD) or an anxiety disorder. Additionally, women with OCD who lack insight or have delusional beliefs might be misdiagnosed with postpartum psychosis.
Here I offer methods to help effectively identify OCD in postpartum women, and suggest how to implement an individualized treatment approach.
Keys to identification and diagnosis
Mothers who present with postpartum anxiety or depression may have obsessions and compulsions. It is important to specifically screen for these symptoms because some mothers may be reluctant to discuss the content of their thoughts or behaviors.
Screen women who present with postpartum anxiety or depression for obsessions and compulsions by using questions based on DSM-5 criteria,2 such as:
- Do you have unpleasant thoughts, urges, or images that repeatedly enter your mind?
- Do you feel driven to perform certain behaviors or mental acts over and over again?
A validated scale, such as the Yale-Brown Obsessive Compulsive Scale (Y-BOCS),3 can also be used to screen for obsessive/compulsive symptoms in these patients.
Continue to: Evaluate women who endorse...
Evaluate women who endorse obsessions or compulsions for OCD. Women who meet diagnostic criteria for OCD should also be assessed for common psychiatric comorbidities, including MDD, anxiety disorders, or bipolar disorder. Obsessive-compulsive disorder with absent insight and delusional beliefs should be differentiated from postpartum psychosis, which is often a manifestation of bipolar disorder.
Treatment: What to consider
When selecting a treatment, consider factors such as symptom severity, psychiatric comorbidities, the patient’s insight into her OCD symptoms, patient preference, and breastfeeding status. Cognitive-behavioral therapy with exposure response prevention is indicated for patients with mild to moderate OCD. Pharmacotherapy should be reserved for individuals with severe OCD. Selective serotonin reuptake inhibitors (SSRIs) are the mainstay pharmacologic treatment of postpartum OCD; however, there are currently no randomized controlled trials of SSRIs for women with postpartum OCD. Augmentation with quetiapine should be considered for women who have an inadequate response to SSRIs.
Acknowledgment
The author thanks Christine Baczynski for her help with the preparation of this article.
1. Miller ES, Chu C, Gollan J, et al. Obsessive-compulsive symptoms during the postpartum period. A prospective cohort. J Reprod Med. 2013;58(3-4):115-122.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46(11):1006-1011.
Childbirth is a trigger for first-onse
In one prospective study of 461 women who recently gave birth, researchers found the prevalence of OCD symptoms was 11% at 2 weeks postpartum.1 Mothers with OCD may have time-consuming or functionally impairing obsessions and/or compulsions that can include:
- anticipatory anxiety of contamination (eg, germs, illness)
- thoughts of accidental or intentional harm to their infant
- compulsions comprised of cleaning and checking behaviors
- avoidance of situations
- thought suppression.
Because both clinicians and patients may not be aware of the effects of childbirth on women with OCD, postpartum OCD may go underdiagnosed or be misdiagnosed as major depressive disorder (MDD) or an anxiety disorder. Additionally, women with OCD who lack insight or have delusional beliefs might be misdiagnosed with postpartum psychosis.
Here I offer methods to help effectively identify OCD in postpartum women, and suggest how to implement an individualized treatment approach.
Keys to identification and diagnosis
Mothers who present with postpartum anxiety or depression may have obsessions and compulsions. It is important to specifically screen for these symptoms because some mothers may be reluctant to discuss the content of their thoughts or behaviors.
Screen women who present with postpartum anxiety or depression for obsessions and compulsions by using questions based on DSM-5 criteria,2 such as:
- Do you have unpleasant thoughts, urges, or images that repeatedly enter your mind?
- Do you feel driven to perform certain behaviors or mental acts over and over again?
A validated scale, such as the Yale-Brown Obsessive Compulsive Scale (Y-BOCS),3 can also be used to screen for obsessive/compulsive symptoms in these patients.
Continue to: Evaluate women who endorse...
Evaluate women who endorse obsessions or compulsions for OCD. Women who meet diagnostic criteria for OCD should also be assessed for common psychiatric comorbidities, including MDD, anxiety disorders, or bipolar disorder. Obsessive-compulsive disorder with absent insight and delusional beliefs should be differentiated from postpartum psychosis, which is often a manifestation of bipolar disorder.
Treatment: What to consider
When selecting a treatment, consider factors such as symptom severity, psychiatric comorbidities, the patient’s insight into her OCD symptoms, patient preference, and breastfeeding status. Cognitive-behavioral therapy with exposure response prevention is indicated for patients with mild to moderate OCD. Pharmacotherapy should be reserved for individuals with severe OCD. Selective serotonin reuptake inhibitors (SSRIs) are the mainstay pharmacologic treatment of postpartum OCD; however, there are currently no randomized controlled trials of SSRIs for women with postpartum OCD. Augmentation with quetiapine should be considered for women who have an inadequate response to SSRIs.
Acknowledgment
The author thanks Christine Baczynski for her help with the preparation of this article.
Childbirth is a trigger for first-onse
In one prospective study of 461 women who recently gave birth, researchers found the prevalence of OCD symptoms was 11% at 2 weeks postpartum.1 Mothers with OCD may have time-consuming or functionally impairing obsessions and/or compulsions that can include:
- anticipatory anxiety of contamination (eg, germs, illness)
- thoughts of accidental or intentional harm to their infant
- compulsions comprised of cleaning and checking behaviors
- avoidance of situations
- thought suppression.
Because both clinicians and patients may not be aware of the effects of childbirth on women with OCD, postpartum OCD may go underdiagnosed or be misdiagnosed as major depressive disorder (MDD) or an anxiety disorder. Additionally, women with OCD who lack insight or have delusional beliefs might be misdiagnosed with postpartum psychosis.
Here I offer methods to help effectively identify OCD in postpartum women, and suggest how to implement an individualized treatment approach.
Keys to identification and diagnosis
Mothers who present with postpartum anxiety or depression may have obsessions and compulsions. It is important to specifically screen for these symptoms because some mothers may be reluctant to discuss the content of their thoughts or behaviors.
Screen women who present with postpartum anxiety or depression for obsessions and compulsions by using questions based on DSM-5 criteria,2 such as:
- Do you have unpleasant thoughts, urges, or images that repeatedly enter your mind?
- Do you feel driven to perform certain behaviors or mental acts over and over again?
A validated scale, such as the Yale-Brown Obsessive Compulsive Scale (Y-BOCS),3 can also be used to screen for obsessive/compulsive symptoms in these patients.
Continue to: Evaluate women who endorse...
Evaluate women who endorse obsessions or compulsions for OCD. Women who meet diagnostic criteria for OCD should also be assessed for common psychiatric comorbidities, including MDD, anxiety disorders, or bipolar disorder. Obsessive-compulsive disorder with absent insight and delusional beliefs should be differentiated from postpartum psychosis, which is often a manifestation of bipolar disorder.
Treatment: What to consider
When selecting a treatment, consider factors such as symptom severity, psychiatric comorbidities, the patient’s insight into her OCD symptoms, patient preference, and breastfeeding status. Cognitive-behavioral therapy with exposure response prevention is indicated for patients with mild to moderate OCD. Pharmacotherapy should be reserved for individuals with severe OCD. Selective serotonin reuptake inhibitors (SSRIs) are the mainstay pharmacologic treatment of postpartum OCD; however, there are currently no randomized controlled trials of SSRIs for women with postpartum OCD. Augmentation with quetiapine should be considered for women who have an inadequate response to SSRIs.
Acknowledgment
The author thanks Christine Baczynski for her help with the preparation of this article.
1. Miller ES, Chu C, Gollan J, et al. Obsessive-compulsive symptoms during the postpartum period. A prospective cohort. J Reprod Med. 2013;58(3-4):115-122.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46(11):1006-1011.
1. Miller ES, Chu C, Gollan J, et al. Obsessive-compulsive symptoms during the postpartum period. A prospective cohort. J Reprod Med. 2013;58(3-4):115-122.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46(11):1006-1011.
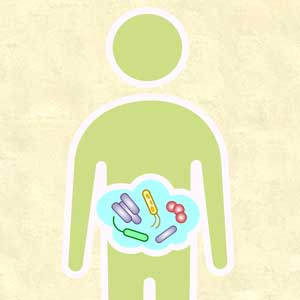
Gut microbiota and its implications for psychiatry: A review of 3 studies
The “human microbiota” describes all microorganisms within the human body, including bacteria, viruses, and eukaryotes. The related term “microbiome” refers to the complete catalog of these microbes and their genes.1 There is a growing awareness that the human microbiota plays an important role in maintaining mental health, and that a disruption in its composition can contribute to manifestations of psychiatric disorders. A growing body of evidence has also linked mental health outcomes to the gut microbiome, suggesting that the gut microbiota can modulate the gut-brain axis.2
Numerous neurotransmitters, including dopamine, serotonin, gamma-aminobutyric acid, and acetylcholine, are produced in the gastrointestinal (GI) tract, and our diet is vital in sustaining and replenishing them. At the same time, our brain regulates our GI tract by secretion of hormones such as oxytocin, leptin, ghrelin, neuropeptide Y, corticotrophin-releasing factor, and a plethora of others. Dysregulation of this microbiome can lead to both physical and mental illnesses. Symptoms of psychiatric disorders, such as depression, psychosis, anxiety, and autism, can be a consequence of this dysregulation.2
Our diet can also modify the gut microorganisms and therefore many of its metabolic pathways. More attention has been given to pre- and probiotics and their effects on DNA by epigenetic changes. One can quickly start to appreciate how this intricate crosstalk can lead to a variety of pathologic and psychiatric problems that have an adverse effect on autoimmune, inflammatory, metabolic, cognitive, and behavioral processes.2,3
Thus far, links have mostly been reported in animal models, and human studies are limited.4 Researchers are just beginning to elucidate how the microbiota affect gut-brain signaling in humans. Such mechanisms may include alterations in microbial composition, immune activation, vagus nerve signaling, alterations in tryptophan metabolism, production of specific microbial neuroactive metabolites, and bacterial cell wall sugars.5 The microbiota-gut-brain axis plays a part in regulating/programming the hypothalamic-pituitary-adrenal (HPA) axis throughout the life span.3 The interactions between the gut microbiome, the immune system, and the CNS are regulated through pathways that involve endocrine functions (HPA axis), the immune system, and metabolic factors.3,4 Recent research focusing on the gut microbiome has also given rise to international projects such as the Human Microbiome Project (Human Microbiome Project Consortium, 2012).3
Several studies have looked into psychiatry and inflammatory/immune pathways. Here we review 3 recent studies that have focused on the gut-brain axis (Table6-8).
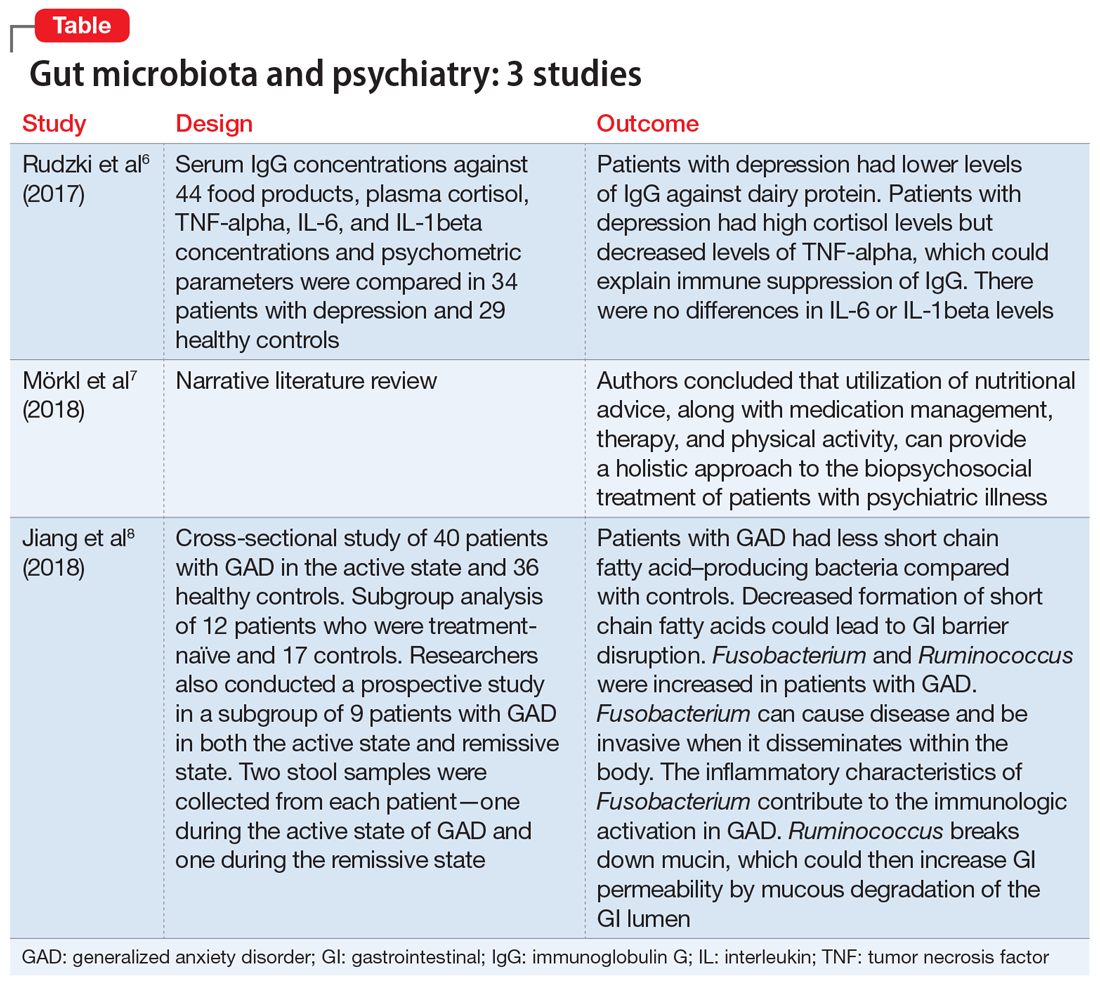
1. Rudzki L, Pawlak D, Pawlak K, et al. Immune suppression of IgG response against dairy proteins in major depression. BMC Psychiatry. 2017;17(1):268.
The aim of this study was to evaluate immunoglobulin G (IgG) response against 40 food products in patients with depression vs those in a control group, along with changes in inflammatory markers, psychological stress, and dietary variables.6
Study design
- N = 63, IgG levels against 44 food products, cortisol levels, tumor necrosis factor (TNF)-alpha, interleukin 6 (IL-6), and IL-1 beta levels were recorded. The psychological parameters of 34 participants with depression and 29 controls were compared using the Hamilton Depression Rating scale, (HAM-D-17), Perceived Stress scale, and Symptom Checklist scale. The study was conducted in Poland.
Continue to: Outcomes
Outcomes
- Patients who were depressed had lower IgG levels against dairy products compared to controls when there was high dairy consumption. However, there was no overall difference between patients and controls in mean IgG concentration against food products.
- Patients who were depressed had higher levels of cortisol. Levels of cortisol had a positive correlation with HAM-D-17 score. Patients with depression had lower levels of TNF-alpha.
Conclusion
- Patients with depression had lower levels of IgG against dairy protein. Patients with depression had high cortisol levels but decreased levels of TNF-alpha, which could explain an immune suppression of IgG in these patients. There were no differences in IL-6 or IL-1beta levels.
Hypercortisolemia is present in approximately 60% of patients with depression. Elevated cortisol levels have a negative effect on lymphocyte function. B-lymphocytes (CD 10+ and CD 19+) are sensitive to glucocorticoids. Studies in mice have demonstrated that elevated glucocorticoid levels are associated with a 50% decrease in serum B-lymphocytes, and this can be explained by downregulation of c-myc protein, which plays a role in cell proliferation and cell survival. Glucocorticoids also decrease levels of protein kinases that are vital for the cell cycle to continue, and they upregulate p27 and p21, which are cell cycle inhibitors. Therefore, if high cortisol suppresses B-lymphocyte production, this can explain how patients with depression have low IgG levels, since B-lymphocytes differentiate into plasma cells that will produce antibodies.6
Depression can trigger an inflammatory response by increasing levels of inflammatory cytokines, acute phase reactants, and oxidative molecules. The inflammatory response can lead to intestinal wall disruption, and therefore bacteria can migrate across the GI barrier, along with food antigens, which could then lead to food antigen hypersensitivity.6
The significance of diet
Many studies have looked into specific types of diets, such as the Mediterranean diet, the ketogenic diet, and the addition of supplements such as probiotics, omega-3 fatty acids, zinc, and multivitamins.7 The Mediterranean diet is high in fiber, nuts, legumes, and fish.7 The ketogenic diet includes a controlled amount of fat, but is low in protein and carbohydrates.7 The main point is that a balanced diet can have a positive effect on mental health.7 The Mediterranean diet has shown to decrease the incidence of cardiovascular disease and lower the risk of depression.7 In animal studies, the ketogenic diet has improved anxiety, depression, and autism.7 Diet clearly affects gut microbiota and, as a consequence, the body’s level of inflammation.7
Continue to: The following review...
The following review highlighted the significance of diet on gut microbiome and mental health.7
2. Mörkl S, Wagner-Skacel J, Lahousen T, et al. The role of nutrition and the gut- brain axis in psychiatry: a review of the literature. Neuropsychobiology. 2018;17: 1-9.
Study design
- These researchers provided a narrative review of the significance of a healthy diet and nutritional supplements on the gut microbiome and the treatment of patients with psychiatric illness.
Outcomes
- This review suggested dietary coaching as a nonpharmacologic treatment for patients with psychiatric illness.
Conclusion
- The utilization of nutritional advice, along with medication management, therapy, and physical activity, can provide a holistic approach to the biopsychosocial treatment of patients with psychiatric illness.
This review also emphasized the poor dietary trends of Westernized countries, which include calorie-dense, genetically altered, processed meals. As Mörkl et al7 noted, we are overfed but undernourished. Mörkl et al7 reviewed studies that involve dietary coaching as part of the treatment plan of patients with mental illness. In one of these studies, patients who received nutritional advice and coaching over 6 weeks had a 40% to 50% decrease in depressive symptoms. These effects persisted for 2 more years. Mörkl et al7 also reviewed an Italian study that found that providing nutritional advice in patients with affective disorders and psychosis helped improve symptom severity and sleep.7
Continue to: Mörkl et al...
Mörkl et al7 also reviewed dietary supplements. Some studies have linked use of omega-3 fatty acids with improvement in affective disorders, Alzheimer’s disease, and posttraumatic stress disorder, as well as cardiovascular conditions. Omega-3 fatty acids may exert beneficial effects by enhancing brain-derived neurotrophic factor and neurogenesis as well as by decreasing inflammation.7
Zinc supplementation can also improve depression, as it has been linked to cytokine variation and hippocampal neuronal growth. Vitamin B9 deficiency and vitamin D deficiency also have been associated with depression. Mörkl et al7 emphasized that a balanced diet that incorporates a variety of nutrients is more beneficial than supplementation of any individual vitamin alone.
Researchers have long emphasized the importance of a healthy balanced diet when treating patients with medical conditions such as cardiovascular or cerebrovascular diseases. Based on the studies Mörkl et al7 reviewed, the same emphasis should be communicated to our patients who suffer from psychiatric conditions.
The gut and anxiety
The gut microbiome has also been an area of research when studying generalized anxiety disorder (GAD).8
3. Jiang HY, Zhang X, Yu ZH, et al. Altered gut microbiota profile in patients with generalized anxiety disorder. J Psychiatr Res. 2018;104:130-136.
The aim of the study was to determine if there were changes in the composition of the gut microbiome in patients with GAD compared with healthy controls.8
Continue to: Study design
Study design
- A cross-sectional study of 76 patients in Zhejiang, China. Forty patients with GAD in the active state and 36 healthy controls were compared in terms of composition of GI microbacterial flora.
- Researchers also examined a subgroup of 12 patients who were treatment-naïve and 17 controls. Stool samples were collected from the 12 patients who were treatment-naïve before initiating medication.
- Researchers also conducted a prospective study in a subgroup of 9 patients with GAD in both the active state and remissive state. Two stool samples were collected from each patient—one during the active state of GAD and one during the remissive state—for a total of 18 samples. Stool samples analyzed with the use of polymerase chain reaction and microbial analysis.
- Patients completed the Hamilton Anxiety Rating (HAM-A) scale and were classified into groups. Those with HAM-A scores >14 were classified as being in the active state of GAD, and those with scores <7 were classified as being in the remissive state.
Outcomes
- Among the samples collected, 8 bacterial taxa were found in different amounts in patients with GAD and healthy controls. Bacteroidetes, Ruminococcus gnavus, and Fusobacterium were increased in patients with GAD compared with controls, while Faecalibacterium, Eubacterium rectale, Sutterella, Lachnospira, and Butyricicoccus were increased in healthy controls.
- Bacterial variety was notably lower in the 12 patients who were treatment-naïve compared with the control group.
- There was no notable difference in microbial composition between patients in the active vs remissive state.
Conclusion
- Patients with GAD had less short chain fatty acid–producing bacteria (Faecalibacterium, Eubacterium rectale, Sutterella, Lachnospira, and Butyricicoccus) compared with controls. Decreased formation of short chain fatty acids could lead to GI barrier disruption. Fusobacterium and Ruminococcus were increased in patients with GAD. Fusobacterium can cause disease and be invasive when it disseminates within the body. The inflammatory characteristics of Fusobacterium contribute to the immunologic activation in GAD. Ruminococcus breaks down mucin, which could then increase GI permeability by mucous degradation of the GI lumen.
Changes in food processing and manufacturing have led to changes in our diets. Changes in our normal GI microbacterial flora could lead to increased gut permeability, bacterial dissemination, and subsequent systemic inflammation. Research has shown that the composition of the microbiota changes across the life span.9 A balanced intake of nutrients is important for both our physical and mental health and safeguards the basis of gut microbiome regulation. A well-regulated gut microbiome ensures low levels of inflammation in the brain and body. Lifestyle modifications and dietary coaching could be practical interventions for patients with psychiatric conditions.5 Current advances in technology now offer precise analyses of thousands of metabolites, enabling metabolomics to offer the promise of discovering new drug targets and biomarkers that may help pave a way to precision medicine.
1. Dave M, Higgins PD, Middha S, et al. The human gut microbiome: current knowledge, challenges, and future directions. Transl Res. 2012;160:246-257.
2. Nasrallah HA. It takes guts to be mentally ill: microbiota and psychopathology. Current Psychiatry. 2018;17(9):4-6.
3. Malan-Muller S, Valles-Colomer M, Raes J, et al. The gut microbiome and mental health: implications for anxiety-and trauma-related disorders. OMICS. 2018;22(2):90-107.
4. Du Toit A. The gut microbiome and mental health. Nat Rev Microbiol. 2019;17(4):196.
5. Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13(10):701-712.
6. Rudzki L, Pawlak D, Pawlak K, et al. Immune suppression of IgG response against dairy proteins in major depression. BMC Psychiatry. 2017;17(1):268.
7. Mörkl S, Wagner-Skacel J, Lahousen T, et al. The role of nutrition and the gut-brain axis in psychiatry: a review of the literature. Neuropsychobiology. 2018;17:1-9.
8. Jiang HY, Zhang X, Yu ZH, et al. Altered gut microbiota profile in patients with generalized anxiety disorder. J Psychiatr Res. 2018;104:130-136.
9. Douglas-Escobar M, Elliott E, Neu J. Effect of intestinal microbial ecology on the developing brain. JAMA Pediatr. 2013;167(4):374-379.
The “human microbiota” describes all microorganisms within the human body, including bacteria, viruses, and eukaryotes. The related term “microbiome” refers to the complete catalog of these microbes and their genes.1 There is a growing awareness that the human microbiota plays an important role in maintaining mental health, and that a disruption in its composition can contribute to manifestations of psychiatric disorders. A growing body of evidence has also linked mental health outcomes to the gut microbiome, suggesting that the gut microbiota can modulate the gut-brain axis.2
Numerous neurotransmitters, including dopamine, serotonin, gamma-aminobutyric acid, and acetylcholine, are produced in the gastrointestinal (GI) tract, and our diet is vital in sustaining and replenishing them. At the same time, our brain regulates our GI tract by secretion of hormones such as oxytocin, leptin, ghrelin, neuropeptide Y, corticotrophin-releasing factor, and a plethora of others. Dysregulation of this microbiome can lead to both physical and mental illnesses. Symptoms of psychiatric disorders, such as depression, psychosis, anxiety, and autism, can be a consequence of this dysregulation.2
Our diet can also modify the gut microorganisms and therefore many of its metabolic pathways. More attention has been given to pre- and probiotics and their effects on DNA by epigenetic changes. One can quickly start to appreciate how this intricate crosstalk can lead to a variety of pathologic and psychiatric problems that have an adverse effect on autoimmune, inflammatory, metabolic, cognitive, and behavioral processes.2,3
Thus far, links have mostly been reported in animal models, and human studies are limited.4 Researchers are just beginning to elucidate how the microbiota affect gut-brain signaling in humans. Such mechanisms may include alterations in microbial composition, immune activation, vagus nerve signaling, alterations in tryptophan metabolism, production of specific microbial neuroactive metabolites, and bacterial cell wall sugars.5 The microbiota-gut-brain axis plays a part in regulating/programming the hypothalamic-pituitary-adrenal (HPA) axis throughout the life span.3 The interactions between the gut microbiome, the immune system, and the CNS are regulated through pathways that involve endocrine functions (HPA axis), the immune system, and metabolic factors.3,4 Recent research focusing on the gut microbiome has also given rise to international projects such as the Human Microbiome Project (Human Microbiome Project Consortium, 2012).3
Several studies have looked into psychiatry and inflammatory/immune pathways. Here we review 3 recent studies that have focused on the gut-brain axis (Table6-8).

1. Rudzki L, Pawlak D, Pawlak K, et al. Immune suppression of IgG response against dairy proteins in major depression. BMC Psychiatry. 2017;17(1):268.
The aim of this study was to evaluate immunoglobulin G (IgG) response against 40 food products in patients with depression vs those in a control group, along with changes in inflammatory markers, psychological stress, and dietary variables.6
Study design
- N = 63, IgG levels against 44 food products, cortisol levels, tumor necrosis factor (TNF)-alpha, interleukin 6 (IL-6), and IL-1 beta levels were recorded. The psychological parameters of 34 participants with depression and 29 controls were compared using the Hamilton Depression Rating scale, (HAM-D-17), Perceived Stress scale, and Symptom Checklist scale. The study was conducted in Poland.
Continue to: Outcomes
Outcomes
- Patients who were depressed had lower IgG levels against dairy products compared to controls when there was high dairy consumption. However, there was no overall difference between patients and controls in mean IgG concentration against food products.
- Patients who were depressed had higher levels of cortisol. Levels of cortisol had a positive correlation with HAM-D-17 score. Patients with depression had lower levels of TNF-alpha.
Conclusion
- Patients with depression had lower levels of IgG against dairy protein. Patients with depression had high cortisol levels but decreased levels of TNF-alpha, which could explain an immune suppression of IgG in these patients. There were no differences in IL-6 or IL-1beta levels.
Hypercortisolemia is present in approximately 60% of patients with depression. Elevated cortisol levels have a negative effect on lymphocyte function. B-lymphocytes (CD 10+ and CD 19+) are sensitive to glucocorticoids. Studies in mice have demonstrated that elevated glucocorticoid levels are associated with a 50% decrease in serum B-lymphocytes, and this can be explained by downregulation of c-myc protein, which plays a role in cell proliferation and cell survival. Glucocorticoids also decrease levels of protein kinases that are vital for the cell cycle to continue, and they upregulate p27 and p21, which are cell cycle inhibitors. Therefore, if high cortisol suppresses B-lymphocyte production, this can explain how patients with depression have low IgG levels, since B-lymphocytes differentiate into plasma cells that will produce antibodies.6
Depression can trigger an inflammatory response by increasing levels of inflammatory cytokines, acute phase reactants, and oxidative molecules. The inflammatory response can lead to intestinal wall disruption, and therefore bacteria can migrate across the GI barrier, along with food antigens, which could then lead to food antigen hypersensitivity.6
The significance of diet
Many studies have looked into specific types of diets, such as the Mediterranean diet, the ketogenic diet, and the addition of supplements such as probiotics, omega-3 fatty acids, zinc, and multivitamins.7 The Mediterranean diet is high in fiber, nuts, legumes, and fish.7 The ketogenic diet includes a controlled amount of fat, but is low in protein and carbohydrates.7 The main point is that a balanced diet can have a positive effect on mental health.7 The Mediterranean diet has shown to decrease the incidence of cardiovascular disease and lower the risk of depression.7 In animal studies, the ketogenic diet has improved anxiety, depression, and autism.7 Diet clearly affects gut microbiota and, as a consequence, the body’s level of inflammation.7
Continue to: The following review...
The following review highlighted the significance of diet on gut microbiome and mental health.7
2. Mörkl S, Wagner-Skacel J, Lahousen T, et al. The role of nutrition and the gut- brain axis in psychiatry: a review of the literature. Neuropsychobiology. 2018;17: 1-9.
Study design
- These researchers provided a narrative review of the significance of a healthy diet and nutritional supplements on the gut microbiome and the treatment of patients with psychiatric illness.
Outcomes
- This review suggested dietary coaching as a nonpharmacologic treatment for patients with psychiatric illness.
Conclusion
- The utilization of nutritional advice, along with medication management, therapy, and physical activity, can provide a holistic approach to the biopsychosocial treatment of patients with psychiatric illness.
This review also emphasized the poor dietary trends of Westernized countries, which include calorie-dense, genetically altered, processed meals. As Mörkl et al7 noted, we are overfed but undernourished. Mörkl et al7 reviewed studies that involve dietary coaching as part of the treatment plan of patients with mental illness. In one of these studies, patients who received nutritional advice and coaching over 6 weeks had a 40% to 50% decrease in depressive symptoms. These effects persisted for 2 more years. Mörkl et al7 also reviewed an Italian study that found that providing nutritional advice in patients with affective disorders and psychosis helped improve symptom severity and sleep.7
Continue to: Mörkl et al...
Mörkl et al7 also reviewed dietary supplements. Some studies have linked use of omega-3 fatty acids with improvement in affective disorders, Alzheimer’s disease, and posttraumatic stress disorder, as well as cardiovascular conditions. Omega-3 fatty acids may exert beneficial effects by enhancing brain-derived neurotrophic factor and neurogenesis as well as by decreasing inflammation.7
Zinc supplementation can also improve depression, as it has been linked to cytokine variation and hippocampal neuronal growth. Vitamin B9 deficiency and vitamin D deficiency also have been associated with depression. Mörkl et al7 emphasized that a balanced diet that incorporates a variety of nutrients is more beneficial than supplementation of any individual vitamin alone.
Researchers have long emphasized the importance of a healthy balanced diet when treating patients with medical conditions such as cardiovascular or cerebrovascular diseases. Based on the studies Mörkl et al7 reviewed, the same emphasis should be communicated to our patients who suffer from psychiatric conditions.
The gut and anxiety
The gut microbiome has also been an area of research when studying generalized anxiety disorder (GAD).8
3. Jiang HY, Zhang X, Yu ZH, et al. Altered gut microbiota profile in patients with generalized anxiety disorder. J Psychiatr Res. 2018;104:130-136.
The aim of the study was to determine if there were changes in the composition of the gut microbiome in patients with GAD compared with healthy controls.8
Continue to: Study design
Study design
- A cross-sectional study of 76 patients in Zhejiang, China. Forty patients with GAD in the active state and 36 healthy controls were compared in terms of composition of GI microbacterial flora.
- Researchers also examined a subgroup of 12 patients who were treatment-naïve and 17 controls. Stool samples were collected from the 12 patients who were treatment-naïve before initiating medication.
- Researchers also conducted a prospective study in a subgroup of 9 patients with GAD in both the active state and remissive state. Two stool samples were collected from each patient—one during the active state of GAD and one during the remissive state—for a total of 18 samples. Stool samples analyzed with the use of polymerase chain reaction and microbial analysis.
- Patients completed the Hamilton Anxiety Rating (HAM-A) scale and were classified into groups. Those with HAM-A scores >14 were classified as being in the active state of GAD, and those with scores <7 were classified as being in the remissive state.
Outcomes
- Among the samples collected, 8 bacterial taxa were found in different amounts in patients with GAD and healthy controls. Bacteroidetes, Ruminococcus gnavus, and Fusobacterium were increased in patients with GAD compared with controls, while Faecalibacterium, Eubacterium rectale, Sutterella, Lachnospira, and Butyricicoccus were increased in healthy controls.
- Bacterial variety was notably lower in the 12 patients who were treatment-naïve compared with the control group.
- There was no notable difference in microbial composition between patients in the active vs remissive state.
Conclusion
- Patients with GAD had less short chain fatty acid–producing bacteria (Faecalibacterium, Eubacterium rectale, Sutterella, Lachnospira, and Butyricicoccus) compared with controls. Decreased formation of short chain fatty acids could lead to GI barrier disruption. Fusobacterium and Ruminococcus were increased in patients with GAD. Fusobacterium can cause disease and be invasive when it disseminates within the body. The inflammatory characteristics of Fusobacterium contribute to the immunologic activation in GAD. Ruminococcus breaks down mucin, which could then increase GI permeability by mucous degradation of the GI lumen.
Changes in food processing and manufacturing have led to changes in our diets. Changes in our normal GI microbacterial flora could lead to increased gut permeability, bacterial dissemination, and subsequent systemic inflammation. Research has shown that the composition of the microbiota changes across the life span.9 A balanced intake of nutrients is important for both our physical and mental health and safeguards the basis of gut microbiome regulation. A well-regulated gut microbiome ensures low levels of inflammation in the brain and body. Lifestyle modifications and dietary coaching could be practical interventions for patients with psychiatric conditions.5 Current advances in technology now offer precise analyses of thousands of metabolites, enabling metabolomics to offer the promise of discovering new drug targets and biomarkers that may help pave a way to precision medicine.
The “human microbiota” describes all microorganisms within the human body, including bacteria, viruses, and eukaryotes. The related term “microbiome” refers to the complete catalog of these microbes and their genes.1 There is a growing awareness that the human microbiota plays an important role in maintaining mental health, and that a disruption in its composition can contribute to manifestations of psychiatric disorders. A growing body of evidence has also linked mental health outcomes to the gut microbiome, suggesting that the gut microbiota can modulate the gut-brain axis.2
Numerous neurotransmitters, including dopamine, serotonin, gamma-aminobutyric acid, and acetylcholine, are produced in the gastrointestinal (GI) tract, and our diet is vital in sustaining and replenishing them. At the same time, our brain regulates our GI tract by secretion of hormones such as oxytocin, leptin, ghrelin, neuropeptide Y, corticotrophin-releasing factor, and a plethora of others. Dysregulation of this microbiome can lead to both physical and mental illnesses. Symptoms of psychiatric disorders, such as depression, psychosis, anxiety, and autism, can be a consequence of this dysregulation.2
Our diet can also modify the gut microorganisms and therefore many of its metabolic pathways. More attention has been given to pre- and probiotics and their effects on DNA by epigenetic changes. One can quickly start to appreciate how this intricate crosstalk can lead to a variety of pathologic and psychiatric problems that have an adverse effect on autoimmune, inflammatory, metabolic, cognitive, and behavioral processes.2,3
Thus far, links have mostly been reported in animal models, and human studies are limited.4 Researchers are just beginning to elucidate how the microbiota affect gut-brain signaling in humans. Such mechanisms may include alterations in microbial composition, immune activation, vagus nerve signaling, alterations in tryptophan metabolism, production of specific microbial neuroactive metabolites, and bacterial cell wall sugars.5 The microbiota-gut-brain axis plays a part in regulating/programming the hypothalamic-pituitary-adrenal (HPA) axis throughout the life span.3 The interactions between the gut microbiome, the immune system, and the CNS are regulated through pathways that involve endocrine functions (HPA axis), the immune system, and metabolic factors.3,4 Recent research focusing on the gut microbiome has also given rise to international projects such as the Human Microbiome Project (Human Microbiome Project Consortium, 2012).3
Several studies have looked into psychiatry and inflammatory/immune pathways. Here we review 3 recent studies that have focused on the gut-brain axis (Table6-8).

1. Rudzki L, Pawlak D, Pawlak K, et al. Immune suppression of IgG response against dairy proteins in major depression. BMC Psychiatry. 2017;17(1):268.
The aim of this study was to evaluate immunoglobulin G (IgG) response against 40 food products in patients with depression vs those in a control group, along with changes in inflammatory markers, psychological stress, and dietary variables.6
Study design
- N = 63, IgG levels against 44 food products, cortisol levels, tumor necrosis factor (TNF)-alpha, interleukin 6 (IL-6), and IL-1 beta levels were recorded. The psychological parameters of 34 participants with depression and 29 controls were compared using the Hamilton Depression Rating scale, (HAM-D-17), Perceived Stress scale, and Symptom Checklist scale. The study was conducted in Poland.
Continue to: Outcomes
Outcomes
- Patients who were depressed had lower IgG levels against dairy products compared to controls when there was high dairy consumption. However, there was no overall difference between patients and controls in mean IgG concentration against food products.
- Patients who were depressed had higher levels of cortisol. Levels of cortisol had a positive correlation with HAM-D-17 score. Patients with depression had lower levels of TNF-alpha.
Conclusion
- Patients with depression had lower levels of IgG against dairy protein. Patients with depression had high cortisol levels but decreased levels of TNF-alpha, which could explain an immune suppression of IgG in these patients. There were no differences in IL-6 or IL-1beta levels.
Hypercortisolemia is present in approximately 60% of patients with depression. Elevated cortisol levels have a negative effect on lymphocyte function. B-lymphocytes (CD 10+ and CD 19+) are sensitive to glucocorticoids. Studies in mice have demonstrated that elevated glucocorticoid levels are associated with a 50% decrease in serum B-lymphocytes, and this can be explained by downregulation of c-myc protein, which plays a role in cell proliferation and cell survival. Glucocorticoids also decrease levels of protein kinases that are vital for the cell cycle to continue, and they upregulate p27 and p21, which are cell cycle inhibitors. Therefore, if high cortisol suppresses B-lymphocyte production, this can explain how patients with depression have low IgG levels, since B-lymphocytes differentiate into plasma cells that will produce antibodies.6
Depression can trigger an inflammatory response by increasing levels of inflammatory cytokines, acute phase reactants, and oxidative molecules. The inflammatory response can lead to intestinal wall disruption, and therefore bacteria can migrate across the GI barrier, along with food antigens, which could then lead to food antigen hypersensitivity.6
The significance of diet
Many studies have looked into specific types of diets, such as the Mediterranean diet, the ketogenic diet, and the addition of supplements such as probiotics, omega-3 fatty acids, zinc, and multivitamins.7 The Mediterranean diet is high in fiber, nuts, legumes, and fish.7 The ketogenic diet includes a controlled amount of fat, but is low in protein and carbohydrates.7 The main point is that a balanced diet can have a positive effect on mental health.7 The Mediterranean diet has shown to decrease the incidence of cardiovascular disease and lower the risk of depression.7 In animal studies, the ketogenic diet has improved anxiety, depression, and autism.7 Diet clearly affects gut microbiota and, as a consequence, the body’s level of inflammation.7
Continue to: The following review...
The following review highlighted the significance of diet on gut microbiome and mental health.7
2. Mörkl S, Wagner-Skacel J, Lahousen T, et al. The role of nutrition and the gut- brain axis in psychiatry: a review of the literature. Neuropsychobiology. 2018;17: 1-9.
Study design
- These researchers provided a narrative review of the significance of a healthy diet and nutritional supplements on the gut microbiome and the treatment of patients with psychiatric illness.
Outcomes
- This review suggested dietary coaching as a nonpharmacologic treatment for patients with psychiatric illness.
Conclusion
- The utilization of nutritional advice, along with medication management, therapy, and physical activity, can provide a holistic approach to the biopsychosocial treatment of patients with psychiatric illness.
This review also emphasized the poor dietary trends of Westernized countries, which include calorie-dense, genetically altered, processed meals. As Mörkl et al7 noted, we are overfed but undernourished. Mörkl et al7 reviewed studies that involve dietary coaching as part of the treatment plan of patients with mental illness. In one of these studies, patients who received nutritional advice and coaching over 6 weeks had a 40% to 50% decrease in depressive symptoms. These effects persisted for 2 more years. Mörkl et al7 also reviewed an Italian study that found that providing nutritional advice in patients with affective disorders and psychosis helped improve symptom severity and sleep.7
Continue to: Mörkl et al...
Mörkl et al7 also reviewed dietary supplements. Some studies have linked use of omega-3 fatty acids with improvement in affective disorders, Alzheimer’s disease, and posttraumatic stress disorder, as well as cardiovascular conditions. Omega-3 fatty acids may exert beneficial effects by enhancing brain-derived neurotrophic factor and neurogenesis as well as by decreasing inflammation.7
Zinc supplementation can also improve depression, as it has been linked to cytokine variation and hippocampal neuronal growth. Vitamin B9 deficiency and vitamin D deficiency also have been associated with depression. Mörkl et al7 emphasized that a balanced diet that incorporates a variety of nutrients is more beneficial than supplementation of any individual vitamin alone.
Researchers have long emphasized the importance of a healthy balanced diet when treating patients with medical conditions such as cardiovascular or cerebrovascular diseases. Based on the studies Mörkl et al7 reviewed, the same emphasis should be communicated to our patients who suffer from psychiatric conditions.
The gut and anxiety
The gut microbiome has also been an area of research when studying generalized anxiety disorder (GAD).8
3. Jiang HY, Zhang X, Yu ZH, et al. Altered gut microbiota profile in patients with generalized anxiety disorder. J Psychiatr Res. 2018;104:130-136.
The aim of the study was to determine if there were changes in the composition of the gut microbiome in patients with GAD compared with healthy controls.8
Continue to: Study design
Study design
- A cross-sectional study of 76 patients in Zhejiang, China. Forty patients with GAD in the active state and 36 healthy controls were compared in terms of composition of GI microbacterial flora.
- Researchers also examined a subgroup of 12 patients who were treatment-naïve and 17 controls. Stool samples were collected from the 12 patients who were treatment-naïve before initiating medication.
- Researchers also conducted a prospective study in a subgroup of 9 patients with GAD in both the active state and remissive state. Two stool samples were collected from each patient—one during the active state of GAD and one during the remissive state—for a total of 18 samples. Stool samples analyzed with the use of polymerase chain reaction and microbial analysis.
- Patients completed the Hamilton Anxiety Rating (HAM-A) scale and were classified into groups. Those with HAM-A scores >14 were classified as being in the active state of GAD, and those with scores <7 were classified as being in the remissive state.
Outcomes
- Among the samples collected, 8 bacterial taxa were found in different amounts in patients with GAD and healthy controls. Bacteroidetes, Ruminococcus gnavus, and Fusobacterium were increased in patients with GAD compared with controls, while Faecalibacterium, Eubacterium rectale, Sutterella, Lachnospira, and Butyricicoccus were increased in healthy controls.
- Bacterial variety was notably lower in the 12 patients who were treatment-naïve compared with the control group.
- There was no notable difference in microbial composition between patients in the active vs remissive state.
Conclusion
- Patients with GAD had less short chain fatty acid–producing bacteria (Faecalibacterium, Eubacterium rectale, Sutterella, Lachnospira, and Butyricicoccus) compared with controls. Decreased formation of short chain fatty acids could lead to GI barrier disruption. Fusobacterium and Ruminococcus were increased in patients with GAD. Fusobacterium can cause disease and be invasive when it disseminates within the body. The inflammatory characteristics of Fusobacterium contribute to the immunologic activation in GAD. Ruminococcus breaks down mucin, which could then increase GI permeability by mucous degradation of the GI lumen.
Changes in food processing and manufacturing have led to changes in our diets. Changes in our normal GI microbacterial flora could lead to increased gut permeability, bacterial dissemination, and subsequent systemic inflammation. Research has shown that the composition of the microbiota changes across the life span.9 A balanced intake of nutrients is important for both our physical and mental health and safeguards the basis of gut microbiome regulation. A well-regulated gut microbiome ensures low levels of inflammation in the brain and body. Lifestyle modifications and dietary coaching could be practical interventions for patients with psychiatric conditions.5 Current advances in technology now offer precise analyses of thousands of metabolites, enabling metabolomics to offer the promise of discovering new drug targets and biomarkers that may help pave a way to precision medicine.
1. Dave M, Higgins PD, Middha S, et al. The human gut microbiome: current knowledge, challenges, and future directions. Transl Res. 2012;160:246-257.
2. Nasrallah HA. It takes guts to be mentally ill: microbiota and psychopathology. Current Psychiatry. 2018;17(9):4-6.
3. Malan-Muller S, Valles-Colomer M, Raes J, et al. The gut microbiome and mental health: implications for anxiety-and trauma-related disorders. OMICS. 2018;22(2):90-107.
4. Du Toit A. The gut microbiome and mental health. Nat Rev Microbiol. 2019;17(4):196.
5. Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13(10):701-712.
6. Rudzki L, Pawlak D, Pawlak K, et al. Immune suppression of IgG response against dairy proteins in major depression. BMC Psychiatry. 2017;17(1):268.
7. Mörkl S, Wagner-Skacel J, Lahousen T, et al. The role of nutrition and the gut-brain axis in psychiatry: a review of the literature. Neuropsychobiology. 2018;17:1-9.
8. Jiang HY, Zhang X, Yu ZH, et al. Altered gut microbiota profile in patients with generalized anxiety disorder. J Psychiatr Res. 2018;104:130-136.
9. Douglas-Escobar M, Elliott E, Neu J. Effect of intestinal microbial ecology on the developing brain. JAMA Pediatr. 2013;167(4):374-379.
1. Dave M, Higgins PD, Middha S, et al. The human gut microbiome: current knowledge, challenges, and future directions. Transl Res. 2012;160:246-257.
2. Nasrallah HA. It takes guts to be mentally ill: microbiota and psychopathology. Current Psychiatry. 2018;17(9):4-6.
3. Malan-Muller S, Valles-Colomer M, Raes J, et al. The gut microbiome and mental health: implications for anxiety-and trauma-related disorders. OMICS. 2018;22(2):90-107.
4. Du Toit A. The gut microbiome and mental health. Nat Rev Microbiol. 2019;17(4):196.
5. Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13(10):701-712.
6. Rudzki L, Pawlak D, Pawlak K, et al. Immune suppression of IgG response against dairy proteins in major depression. BMC Psychiatry. 2017;17(1):268.
7. Mörkl S, Wagner-Skacel J, Lahousen T, et al. The role of nutrition and the gut-brain axis in psychiatry: a review of the literature. Neuropsychobiology. 2018;17:1-9.
8. Jiang HY, Zhang X, Yu ZH, et al. Altered gut microbiota profile in patients with generalized anxiety disorder. J Psychiatr Res. 2018;104:130-136.
9. Douglas-Escobar M, Elliott E, Neu J. Effect of intestinal microbial ecology on the developing brain. JAMA Pediatr. 2013;167(4):374-379.
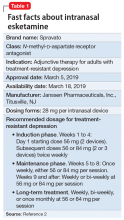
Intranasal esketamine
Treatment-resistant depression (TRD) is a common clinical struggle that practicing clinicians address on a daily basis. Major depressive disorder affects nearly 1 in 5 Americans at some point in their life and, by definition, impairs social and occupational functioning. Historic treatments have focused on the monoamine theories of depression—modulating the monoamines serotonin, norepinephrine, and/or dopamine. Limitations of currently available antidepressants include delayed onset of effect and low remission rates. To further complicate the matter, numerous studies have shown that with each subsequent antidepressant trial, patients have a decreasing likelihood of responding to subsequent antidepressant treatment options. For example, in the classic STAR*D trial, by the time a patient had not responded to the first 2 antidepressant options, the chance that they would respond to a third or fourth antidepressant had decreased to approximately 15% per antidepressant treatment course.1
To address the need for new treatments for patients with TRD, on March 5, 2019 the FDA-approved intranasal
How it works
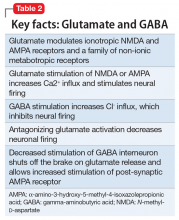
Modern research has looked beyond the monoamine system to explore the neuro-modulatory effects of glutamate and gamma-aminobutyric acid (GABA).3 The yin and yang of glutamate and GABA revolves around neural excitation vs neural inhibition at a local synaptic level. The primary effects of the glutamate and GABA systems (Table 2) can be broken down into several key areas of understanding.
Glutamate modulates ionotropic N-methyl-
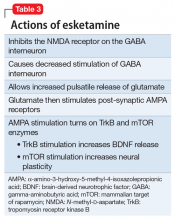
Esketamine, the S-enantiomer of ketamine, has a higher affinity for the NMDA receptor than the R-enantiomer and has been developed as an intranasal adjunctive treatment for TRD. Esketamine blocks NMDA receptors on GABA interneurons. This allows for increased pulsatile release of glutamate into the synapse. Intrasynaptic glutamate then stimulates postsynaptic AMPA receptors. Glutamate stimulation of postsynaptic AMPA receptors results in an intracellular cascade that activates the enzymes tropomyosin receptor kinase B (TrkB) and mammalian target of rapamycin (mTOR). TrkB stimulation results in increased production and release of BDNF. mTor stimulation increases neuronal membrane protein formation with subsequent increased neural plasticity. Taken together, preclinical models show that esketamine’s inhibition of the NMDA receptor on the GABA interneuron results in a cascade of increased BDNF release and synaptogenesis with increased neuroplasticity (Table 3).
Clinical implications
Treatment-resistant depression affects nearly one-third of patients currently receiving standard antidepressant treatment. Major depressive disorder is currently the second leading cause of disability for working adults within the United States and one of the largest causes of disability worldwide. The esketamine nasal spray could be beneficial for patients who have experienced TRD with standard monoamine antidepressants.
Supporting evidence
Clinical trials examining intranasal esketamine include both short- and long-term studies of patients with TRD.
Continue to: Esketamine was evaluated...
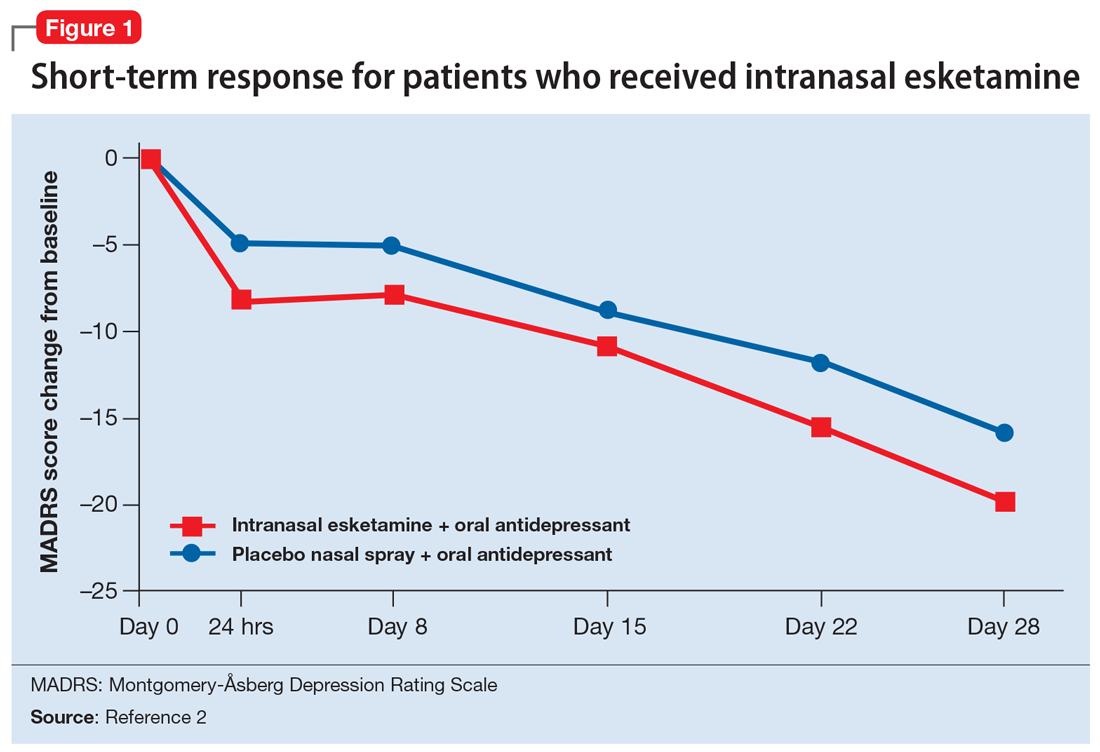
Esketamine was evaluated in a randomized, placebo-controlled, double-blind, multicenter, short-term (4-week) phase III study in adult patients age 18 to 65 with TRD (they had not responded to at least 2 different antidepressants of adequate dose and duration).4 After discontinuing prior antidepressant treatments, all patients were started on a newly initiated antidepressant and were also randomized to concomitant intranasal esketamine or intranasal placebo as follows:
- 114 patients were randomized to the intranasal esketamine plus newly initiated oral antidepressant arm
- 109 patients were randomized to the placebo nasal spray plus newly initiated oral antidepressant arm
- The mean baseline Montgomery-Åsberg Depression Rating Scale (MADRS) score for each group was 37 (ie, moderately to severely depressed).
Newly started antidepressants included esc
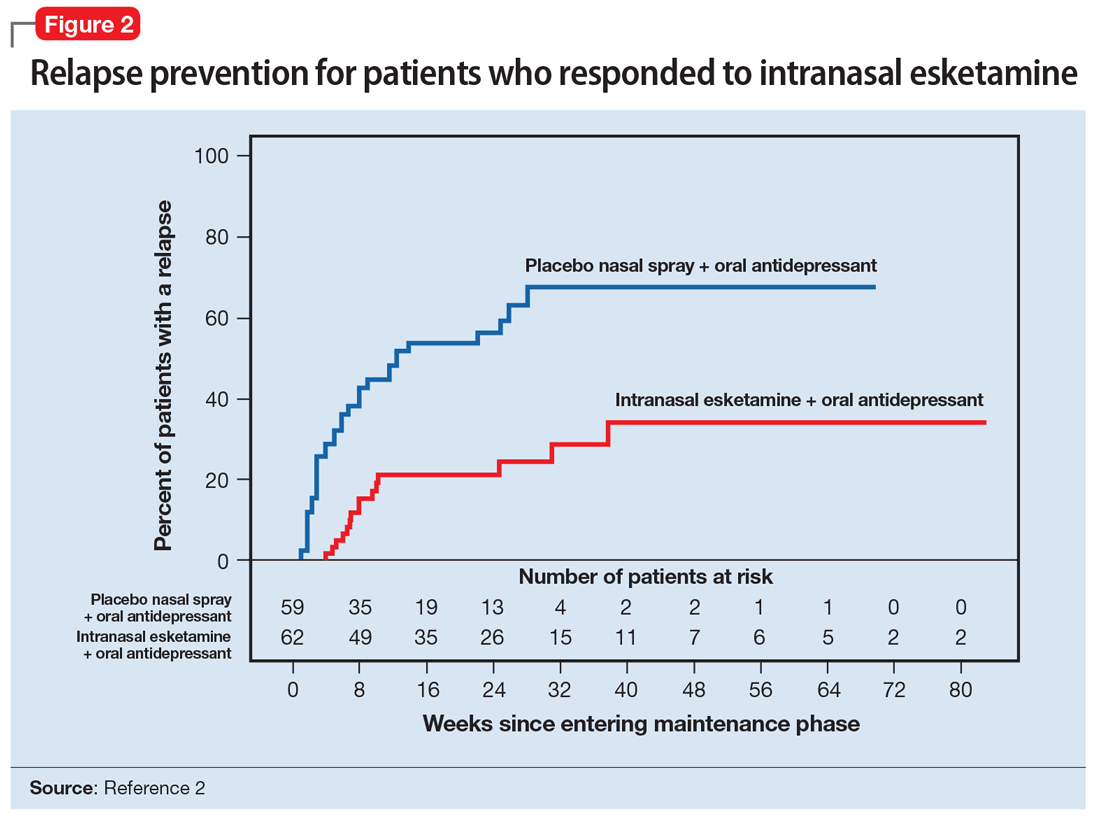
A long-term, double-blind multicenter maintenance-of-effect trial examined adults age 18 to 65 with TRD.5-6 Patients in this study were responders in 1 of 2 short-term studies or in an open-label direct enrollment study. Stable remission was defined as a MADRS total score <12 for at least 3 of the last 4 weeks of the study, and stable response was defined as a MADRS reduction of >50% but not in remission. After 16 weeks of intranasal esketamine plus an oral antidepressant, stable remitters and stable responders were then randomized separately to continue intranasal esketamine or switch to placebo nasal spray, with both groups continuing on their concomitant oral antidepressant. The primary study endpoint was time to relapse. Relapse was defined as a MADRS total score >22 for more than 2 consecutive weeks, hospitalization for worsening of depression, or any other clinically relevant event. The median age was 48, 66% were female, 90% were White and 4% were black. Patients in stable response or stable remission experienced a significantly longer time to relapse compared with patients who continued their oral antidepressant but were switched to placebo intranasal spray. In this remission response study, patients could receive intranasal treatment weekly or bi-weekly based on symptom severity (Figure 22).
Impact on driving. Two studies examined the impact of esketamine on driving performance. One examined adults with major depressive disorder and the other examined healthy participants. The effects of a single 84-mg dose of esketamine nasal spray on a patient’s ability to drive was assessed in 23 healthy adults. In this study, mirt
A second study evaluated the effects of repeated esketamine administration on driving performance in 25 adults with major depressive disorder. In this study, an ethanol-containing beverage was used as an active control. After administration of a single 84-mg dose of intranasal esketamine, driving performance was the same as a placebo at 18 hours. In the multiple dose phase, standard driving performance was similar for esketamine nasal spray and placebo at 6 hours postdose on Days 11, 18, and 25.
Continue to: Pharmacologic profile
Pharmacologic profile
Adverse events. The most common adverse events in patients treated with esketamine nasal spray were dissociation (41%), dizziness (29%), nausea (28%), sedation (23%), and vertigo (23%).2 The majority of these effects were short-term and resolved during the 2-hour observation period.
In addition to spontaneously reported events, sedation and dissociation were further monitored with specific scales. Sedation was measured with the Modified Observer’s Alertness and Sedation Scale. Using this scale, 50% of patients receiving 56 mg and 61% of patients receiving 84 mg of esketamine met criteria for sedation.
Similarly, dissociation/perceptional changes were measured with spontaneously reported events and also with the Clinician Administered Dissociative State Scale. On this scale, 61% of patients receiving the 56-mg dose, and 69% of patients receiving the 84-mg dose met criteria for dissociation/perceptional changes after dose administration.
Increases in blod pressure. Esketamine intranasal spray was associated with a 7 to 9 mm Hg increase in systolic blood pressure and a 4 to 6 mm Hg increase in diastolic blood pressure, both of which peaked 40 minutes post-dose.
Nausea and vomiting. Intranasal esketamine was associated with a 27% rate of nausea at 56 mg, and 32% at 84 mg, with a 6% rate of vomiting at 56 mg and 12% at 84 mg.
Continue to: Pharmacokinetics
Pharmacokinetics
Esketamine exposure increases from 28 to 84 mg in a fairly dose-proportional range. No accumulation of esketamine was observed in the plasma following twice-weekly administration. Bioavailability is approximately 48% following nasal administration. The Tmax for esketamine plasma concentration is 20 to 40 minutes after the last nasal spray. Protein binding of esketamine is approximately 43% to 45%. The brain-to-plasma ratio of noresketamine is 4 to 6 times lower than that of esketamine. The half-life of esketamine ranged from 7 to 12 hours. The mean half-life of nore
Potential drug interactions
Central nervous system depressants. Concomitant use of esketamine and other CNS depressants (ie, benzodiazepines, opioids, alcohol) may increase sedation. Patients receiving esketamine with concomitant use of other CNS depressants should be closely monitored for sedation.
Psychostimulants. Concomitant use of esketamine and psychostimulants (ie, amphetamines, methylphenidates, moda
Monoamine oxidase inhibitors. Concomitant use of esketamine with monoamine oxidase inhibitors may increase blood pressure. Closely monitor blood pressure with concomitant use of esketamine and monoamine oxidase inhibitors.
Use in special populations. Because of concerns of increased sedation, intranasal esketamine should be administered cautiously in patients receiving other CNS depressants, such as benzodiazepines. In patients with psychosis or a prior history of psychosis, esketamine should be used with increased caution and the risk/benefit ratio should be carefully considered.
Continue to: Because of potential teratogenicity...
Because of potential teratogenicity, esketamine is not recommended in women who are pregnant, may become pregnant, or who are currently nursing.
Intranasal esketamine was examined in a phase III trial of 194 patients age ≥65. At the end of 4 weeks, there was no statistically significant difference in groups on the MADRS, the primary efficacy endpoint. There were no overall differences in the safety profile in patients >65 years compared with younger patients; however, the mean esketamine Cmax and area under the curve were higher in older patients compared with younger adults. The mean esketamine half-life was longer in patients with moderate hepatic impairment.
Abuse liability
Esketamine is a CIII controlled substance and concerns about abuse, misuse, and diversion have been taken into account within the REMS drug safety program.2 Patients with a prior history of substance abuse or misuse should be considered with regard to the risk/benefit ratio.
The REMS drug safety program
Due to the nature of its usually transient adverse effects, including sedation, dissociation, hypertension, and nausea, intranasal esketamine will be administered through a REMS drug safety program at certified REMS treatment centers. Certified REMS treatment centers will receive training on how to safely and effectively counsel and monitor patients. Prior to treatment, patients will receive blood pressure monitoring and anticipated adverse effects will be discussed. Patients will be instructed to not eat solid food for 2 hours pre-dose and to not drink anything for 30 minutes prior.
A treatment session consists of nasal administration and a minimum 2-hour post-administration observation period. Blood pressure must be assessed prior to administration and if elevated, (ie, systolic blood pressure >140 mm Hg, diastolic >90 mm Hg), clinicians should consider the risk of short-term increases in blood pressure that may occur. Do not administer if increases in blood pressure or intracranial pressure pose a serious risk.
Continue to: After each intranasal...
After each intranasal administration the patient will be observed for 5 minutes before the second nasal inhaler is utilized and for another 5 minutes when the patient is receiving 84 mg (ie, each inhaler equals 28 mg). After administering, blood pressure should be reassessed at approximately 40 minutes, which corresponds to the Cmax of intranasal esketamine, and periodically thereafter as warranted.
The patient will then be monitored in a quiet environment for a minimum of 2 hours to make sure that dissociative phenomenon, sedation, and hypertensive reactions have normalized prior to discharge from a certified REMS treatment center.
Dosing and administration
Each intranasal device is primed for 2 infusions (1 in each nostril) for a total dose of 28 mg of esketamine. Combinations of devices can be used to adjust the dose as appropriate for individual patients. The recommended starting dose is 56 mg (ie, 2 devices, with a 5-minute gap between devices). The dose can be increased to 84 mg (ie, 3 intranasal devices spaced at 5-minute intervals) by the second dose based on clinical judgment.
The patient will be instructed to recline the head to a 45° angle, clear his or her nostrils prior to the first treatment, and then self-administer a dose to each nostril while holding the reciprocal nostril closed and inhaling. This process is then repeated every 5 minutes for each subsequent device, with a maximum total dose of 3 devices, or 84 mg (Figure 32). The patient will then be monitored for blood pressure, heart rate, and signs of psychologic or physiologic changes for the next 2 hours. Patients may not drive a car or operate any type of motor equipment until the following day after receiving a normal night’s sleep. Patients will be released from the REMS treatment center after 2 hours if both psychological and physical adverse effects have normalized.
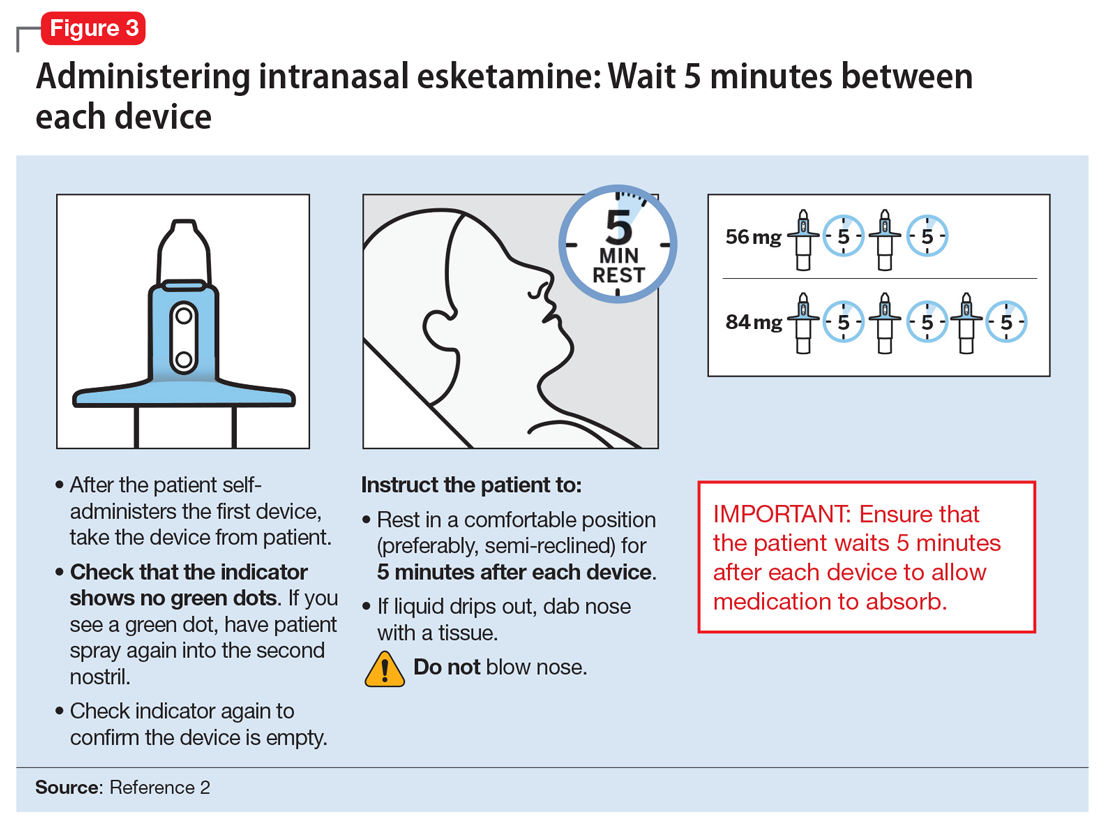
Missed treatment sessions. If a patient misses a treatment session and there is worsening of depressive symptoms, consider returning the patient to the previous dosing schedule (ie, every 2 weeks to once weekly, or weekly to twice weekly).
Continue to: Contraindications for...
Contraindications for intranasal esketamine include:
- aneurysmal vascular disease, including thoracic and abdominal aortic, intracranial, and peripheral arterial vessels, or arterial venous malformations
- history of intracerebral hemorrhage
- hypersensitivity to esketamine, ketamine, or any of the excipients.
Clinical considerations
Intranasal esketamine represents a unique delivery system for the first glutamatergic treatment approved for patients with TRD.
Why Rx? Treatment-resistant depression is found in nearly 1 out of 3 patients with currently available monoaminergic antidepressant treatment options. Patients with TRD are at increased risk of physical and psychological impairment, subsequent worsening of their condition, and social and occupational disability.
Bottom Line
Intranasal esketamine is the first glutamatergic treatment option FDA-approved for patients with treatment-resistant depression who have not responded to standard antidepressant treatment options. In short-term trials, intranasal esketamine significantly improved depressive symptoms as quickly as 24 hours after treatment, with significant improvement maintained through 4 weeks of ongoing administration. In addition, intranasal esketamine was shown to significantly decrease time to relapse for patients who had achieved stable remission or stable response.
Related Resource
- Sullivan MG. FDA approves intranasal esketamine for refractory major depressive disorder. Clinical Psychiatry News. https://www.mdedge.com/psychiatry/article/195712/depression/fda-approves-intranasal-esketamine-refractory-major-depressive. Published March 5, 2019.
Drug Brand Names
Armodafinil • Nuvigil
Duloxetine • Cymbalta
Escitalopram • Lexapro
Esketamine • Spravato
Mirtazapine • Remeron
Modafinil • Provigil
Sertraline • Zoloft
Venlafaxine • Effexor
1. Rush AG, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR D Report. Am J Psychiatry. 2006;163(11):1905-1917.
2. Spravato [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019.
3. Duman RS, Aghajanian GK, Sanacora G, et al. Synaptic plasticity and depression: new insights from stress and rapid-acting anti-depression. Nat Med. 2016;22(3):238-249.
4. Daly EJ, Singh JB, Fedgchin M, et al. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2018;75(2):139-148.
5. Daly EJ, Trivedi M, Janik A, et al. A randomized withdrawal, double-blind, multicenter study of esketamine nasal spray plus an oral antidepressant for relapse prevention in treatment-resistant depression. Poster presented at the 2018 American Society of Clinical Psychopharmacology Annual Meeting; May 2018; Miami, Florida.
6. Wajs E, Aluisio L, Morrison R, et al. Long-term safety of esketamine nasal spray plus oral antidepressants in patients with treatment-resistant depression: phase III open-label safety and efficacy study. Poster presented at the 2018 American Society of Clinical Psychopharmacology Annual Meeting; May 2018; Miami, Florida.
Treatment-resistant depression (TRD) is a common clinical struggle that practicing clinicians address on a daily basis. Major depressive disorder affects nearly 1 in 5 Americans at some point in their life and, by definition, impairs social and occupational functioning. Historic treatments have focused on the monoamine theories of depression—modulating the monoamines serotonin, norepinephrine, and/or dopamine. Limitations of currently available antidepressants include delayed onset of effect and low remission rates. To further complicate the matter, numerous studies have shown that with each subsequent antidepressant trial, patients have a decreasing likelihood of responding to subsequent antidepressant treatment options. For example, in the classic STAR*D trial, by the time a patient had not responded to the first 2 antidepressant options, the chance that they would respond to a third or fourth antidepressant had decreased to approximately 15% per antidepressant treatment course.1
To address the need for new treatments for patients with TRD, on March 5, 2019 the FDA-approved intranasal
How it works
Modern research has looked beyond the monoamine system to explore the neuro-modulatory effects of glutamate and gamma-aminobutyric acid (GABA).3 The yin and yang of glutamate and GABA revolves around neural excitation vs neural inhibition at a local synaptic level. The primary effects of the glutamate and GABA systems (Table 2) can be broken down into several key areas of understanding.
Glutamate modulates ionotropic N-methyl-
Esketamine, the S-enantiomer of ketamine, has a higher affinity for the NMDA receptor than the R-enantiomer and has been developed as an intranasal adjunctive treatment for TRD. Esketamine blocks NMDA receptors on GABA interneurons. This allows for increased pulsatile release of glutamate into the synapse. Intrasynaptic glutamate then stimulates postsynaptic AMPA receptors. Glutamate stimulation of postsynaptic AMPA receptors results in an intracellular cascade that activates the enzymes tropomyosin receptor kinase B (TrkB) and mammalian target of rapamycin (mTOR). TrkB stimulation results in increased production and release of BDNF. mTor stimulation increases neuronal membrane protein formation with subsequent increased neural plasticity. Taken together, preclinical models show that esketamine’s inhibition of the NMDA receptor on the GABA interneuron results in a cascade of increased BDNF release and synaptogenesis with increased neuroplasticity (Table 3).
Clinical implications
Treatment-resistant depression affects nearly one-third of patients currently receiving standard antidepressant treatment. Major depressive disorder is currently the second leading cause of disability for working adults within the United States and one of the largest causes of disability worldwide. The esketamine nasal spray could be beneficial for patients who have experienced TRD with standard monoamine antidepressants.
Supporting evidence
Clinical trials examining intranasal esketamine include both short- and long-term studies of patients with TRD.
Continue to: Esketamine was evaluated...
Esketamine was evaluated in a randomized, placebo-controlled, double-blind, multicenter, short-term (4-week) phase III study in adult patients age 18 to 65 with TRD (they had not responded to at least 2 different antidepressants of adequate dose and duration).4 After discontinuing prior antidepressant treatments, all patients were started on a newly initiated antidepressant and were also randomized to concomitant intranasal esketamine or intranasal placebo as follows:
- 114 patients were randomized to the intranasal esketamine plus newly initiated oral antidepressant arm
- 109 patients were randomized to the placebo nasal spray plus newly initiated oral antidepressant arm
- The mean baseline Montgomery-Åsberg Depression Rating Scale (MADRS) score for each group was 37 (ie, moderately to severely depressed).
Newly started antidepressants included esc

A long-term, double-blind multicenter maintenance-of-effect trial examined adults age 18 to 65 with TRD.5-6 Patients in this study were responders in 1 of 2 short-term studies or in an open-label direct enrollment study. Stable remission was defined as a MADRS total score <12 for at least 3 of the last 4 weeks of the study, and stable response was defined as a MADRS reduction of >50% but not in remission. After 16 weeks of intranasal esketamine plus an oral antidepressant, stable remitters and stable responders were then randomized separately to continue intranasal esketamine or switch to placebo nasal spray, with both groups continuing on their concomitant oral antidepressant. The primary study endpoint was time to relapse. Relapse was defined as a MADRS total score >22 for more than 2 consecutive weeks, hospitalization for worsening of depression, or any other clinically relevant event. The median age was 48, 66% were female, 90% were White and 4% were black. Patients in stable response or stable remission experienced a significantly longer time to relapse compared with patients who continued their oral antidepressant but were switched to placebo intranasal spray. In this remission response study, patients could receive intranasal treatment weekly or bi-weekly based on symptom severity (Figure 22).

Impact on driving. Two studies examined the impact of esketamine on driving performance. One examined adults with major depressive disorder and the other examined healthy participants. The effects of a single 84-mg dose of esketamine nasal spray on a patient’s ability to drive was assessed in 23 healthy adults. In this study, mirt
A second study evaluated the effects of repeated esketamine administration on driving performance in 25 adults with major depressive disorder. In this study, an ethanol-containing beverage was used as an active control. After administration of a single 84-mg dose of intranasal esketamine, driving performance was the same as a placebo at 18 hours. In the multiple dose phase, standard driving performance was similar for esketamine nasal spray and placebo at 6 hours postdose on Days 11, 18, and 25.
Continue to: Pharmacologic profile
Pharmacologic profile
Adverse events. The most common adverse events in patients treated with esketamine nasal spray were dissociation (41%), dizziness (29%), nausea (28%), sedation (23%), and vertigo (23%).2 The majority of these effects were short-term and resolved during the 2-hour observation period.
In addition to spontaneously reported events, sedation and dissociation were further monitored with specific scales. Sedation was measured with the Modified Observer’s Alertness and Sedation Scale. Using this scale, 50% of patients receiving 56 mg and 61% of patients receiving 84 mg of esketamine met criteria for sedation.
Similarly, dissociation/perceptional changes were measured with spontaneously reported events and also with the Clinician Administered Dissociative State Scale. On this scale, 61% of patients receiving the 56-mg dose, and 69% of patients receiving the 84-mg dose met criteria for dissociation/perceptional changes after dose administration.
Increases in blod pressure. Esketamine intranasal spray was associated with a 7 to 9 mm Hg increase in systolic blood pressure and a 4 to 6 mm Hg increase in diastolic blood pressure, both of which peaked 40 minutes post-dose.
Nausea and vomiting. Intranasal esketamine was associated with a 27% rate of nausea at 56 mg, and 32% at 84 mg, with a 6% rate of vomiting at 56 mg and 12% at 84 mg.
Continue to: Pharmacokinetics
Pharmacokinetics
Esketamine exposure increases from 28 to 84 mg in a fairly dose-proportional range. No accumulation of esketamine was observed in the plasma following twice-weekly administration. Bioavailability is approximately 48% following nasal administration. The Tmax for esketamine plasma concentration is 20 to 40 minutes after the last nasal spray. Protein binding of esketamine is approximately 43% to 45%. The brain-to-plasma ratio of noresketamine is 4 to 6 times lower than that of esketamine. The half-life of esketamine ranged from 7 to 12 hours. The mean half-life of nore
Potential drug interactions
Central nervous system depressants. Concomitant use of esketamine and other CNS depressants (ie, benzodiazepines, opioids, alcohol) may increase sedation. Patients receiving esketamine with concomitant use of other CNS depressants should be closely monitored for sedation.
Psychostimulants. Concomitant use of esketamine and psychostimulants (ie, amphetamines, methylphenidates, moda
Monoamine oxidase inhibitors. Concomitant use of esketamine with monoamine oxidase inhibitors may increase blood pressure. Closely monitor blood pressure with concomitant use of esketamine and monoamine oxidase inhibitors.
Use in special populations. Because of concerns of increased sedation, intranasal esketamine should be administered cautiously in patients receiving other CNS depressants, such as benzodiazepines. In patients with psychosis or a prior history of psychosis, esketamine should be used with increased caution and the risk/benefit ratio should be carefully considered.
Continue to: Because of potential teratogenicity...
Because of potential teratogenicity, esketamine is not recommended in women who are pregnant, may become pregnant, or who are currently nursing.
Intranasal esketamine was examined in a phase III trial of 194 patients age ≥65. At the end of 4 weeks, there was no statistically significant difference in groups on the MADRS, the primary efficacy endpoint. There were no overall differences in the safety profile in patients >65 years compared with younger patients; however, the mean esketamine Cmax and area under the curve were higher in older patients compared with younger adults. The mean esketamine half-life was longer in patients with moderate hepatic impairment.
Abuse liability
Esketamine is a CIII controlled substance and concerns about abuse, misuse, and diversion have been taken into account within the REMS drug safety program.2 Patients with a prior history of substance abuse or misuse should be considered with regard to the risk/benefit ratio.
The REMS drug safety program
Due to the nature of its usually transient adverse effects, including sedation, dissociation, hypertension, and nausea, intranasal esketamine will be administered through a REMS drug safety program at certified REMS treatment centers. Certified REMS treatment centers will receive training on how to safely and effectively counsel and monitor patients. Prior to treatment, patients will receive blood pressure monitoring and anticipated adverse effects will be discussed. Patients will be instructed to not eat solid food for 2 hours pre-dose and to not drink anything for 30 minutes prior.
A treatment session consists of nasal administration and a minimum 2-hour post-administration observation period. Blood pressure must be assessed prior to administration and if elevated, (ie, systolic blood pressure >140 mm Hg, diastolic >90 mm Hg), clinicians should consider the risk of short-term increases in blood pressure that may occur. Do not administer if increases in blood pressure or intracranial pressure pose a serious risk.
Continue to: After each intranasal...
After each intranasal administration the patient will be observed for 5 minutes before the second nasal inhaler is utilized and for another 5 minutes when the patient is receiving 84 mg (ie, each inhaler equals 28 mg). After administering, blood pressure should be reassessed at approximately 40 minutes, which corresponds to the Cmax of intranasal esketamine, and periodically thereafter as warranted.
The patient will then be monitored in a quiet environment for a minimum of 2 hours to make sure that dissociative phenomenon, sedation, and hypertensive reactions have normalized prior to discharge from a certified REMS treatment center.
Dosing and administration
Each intranasal device is primed for 2 infusions (1 in each nostril) for a total dose of 28 mg of esketamine. Combinations of devices can be used to adjust the dose as appropriate for individual patients. The recommended starting dose is 56 mg (ie, 2 devices, with a 5-minute gap between devices). The dose can be increased to 84 mg (ie, 3 intranasal devices spaced at 5-minute intervals) by the second dose based on clinical judgment.
The patient will be instructed to recline the head to a 45° angle, clear his or her nostrils prior to the first treatment, and then self-administer a dose to each nostril while holding the reciprocal nostril closed and inhaling. This process is then repeated every 5 minutes for each subsequent device, with a maximum total dose of 3 devices, or 84 mg (Figure 32). The patient will then be monitored for blood pressure, heart rate, and signs of psychologic or physiologic changes for the next 2 hours. Patients may not drive a car or operate any type of motor equipment until the following day after receiving a normal night’s sleep. Patients will be released from the REMS treatment center after 2 hours if both psychological and physical adverse effects have normalized.

Missed treatment sessions. If a patient misses a treatment session and there is worsening of depressive symptoms, consider returning the patient to the previous dosing schedule (ie, every 2 weeks to once weekly, or weekly to twice weekly).
Continue to: Contraindications for...
Contraindications for intranasal esketamine include:
- aneurysmal vascular disease, including thoracic and abdominal aortic, intracranial, and peripheral arterial vessels, or arterial venous malformations
- history of intracerebral hemorrhage
- hypersensitivity to esketamine, ketamine, or any of the excipients.
Clinical considerations
Intranasal esketamine represents a unique delivery system for the first glutamatergic treatment approved for patients with TRD.
Why Rx? Treatment-resistant depression is found in nearly 1 out of 3 patients with currently available monoaminergic antidepressant treatment options. Patients with TRD are at increased risk of physical and psychological impairment, subsequent worsening of their condition, and social and occupational disability.
Bottom Line
Intranasal esketamine is the first glutamatergic treatment option FDA-approved for patients with treatment-resistant depression who have not responded to standard antidepressant treatment options. In short-term trials, intranasal esketamine significantly improved depressive symptoms as quickly as 24 hours after treatment, with significant improvement maintained through 4 weeks of ongoing administration. In addition, intranasal esketamine was shown to significantly decrease time to relapse for patients who had achieved stable remission or stable response.
Related Resource
- Sullivan MG. FDA approves intranasal esketamine for refractory major depressive disorder. Clinical Psychiatry News. https://www.mdedge.com/psychiatry/article/195712/depression/fda-approves-intranasal-esketamine-refractory-major-depressive. Published March 5, 2019.
Drug Brand Names
Armodafinil • Nuvigil
Duloxetine • Cymbalta
Escitalopram • Lexapro
Esketamine • Spravato
Mirtazapine • Remeron
Modafinil • Provigil
Sertraline • Zoloft
Venlafaxine • Effexor
Treatment-resistant depression (TRD) is a common clinical struggle that practicing clinicians address on a daily basis. Major depressive disorder affects nearly 1 in 5 Americans at some point in their life and, by definition, impairs social and occupational functioning. Historic treatments have focused on the monoamine theories of depression—modulating the monoamines serotonin, norepinephrine, and/or dopamine. Limitations of currently available antidepressants include delayed onset of effect and low remission rates. To further complicate the matter, numerous studies have shown that with each subsequent antidepressant trial, patients have a decreasing likelihood of responding to subsequent antidepressant treatment options. For example, in the classic STAR*D trial, by the time a patient had not responded to the first 2 antidepressant options, the chance that they would respond to a third or fourth antidepressant had decreased to approximately 15% per antidepressant treatment course.1
To address the need for new treatments for patients with TRD, on March 5, 2019 the FDA-approved intranasal
How it works
Modern research has looked beyond the monoamine system to explore the neuro-modulatory effects of glutamate and gamma-aminobutyric acid (GABA).3 The yin and yang of glutamate and GABA revolves around neural excitation vs neural inhibition at a local synaptic level. The primary effects of the glutamate and GABA systems (Table 2) can be broken down into several key areas of understanding.
Glutamate modulates ionotropic N-methyl-
Esketamine, the S-enantiomer of ketamine, has a higher affinity for the NMDA receptor than the R-enantiomer and has been developed as an intranasal adjunctive treatment for TRD. Esketamine blocks NMDA receptors on GABA interneurons. This allows for increased pulsatile release of glutamate into the synapse. Intrasynaptic glutamate then stimulates postsynaptic AMPA receptors. Glutamate stimulation of postsynaptic AMPA receptors results in an intracellular cascade that activates the enzymes tropomyosin receptor kinase B (TrkB) and mammalian target of rapamycin (mTOR). TrkB stimulation results in increased production and release of BDNF. mTor stimulation increases neuronal membrane protein formation with subsequent increased neural plasticity. Taken together, preclinical models show that esketamine’s inhibition of the NMDA receptor on the GABA interneuron results in a cascade of increased BDNF release and synaptogenesis with increased neuroplasticity (Table 3).
Clinical implications
Treatment-resistant depression affects nearly one-third of patients currently receiving standard antidepressant treatment. Major depressive disorder is currently the second leading cause of disability for working adults within the United States and one of the largest causes of disability worldwide. The esketamine nasal spray could be beneficial for patients who have experienced TRD with standard monoamine antidepressants.
Supporting evidence
Clinical trials examining intranasal esketamine include both short- and long-term studies of patients with TRD.
Continue to: Esketamine was evaluated...
Esketamine was evaluated in a randomized, placebo-controlled, double-blind, multicenter, short-term (4-week) phase III study in adult patients age 18 to 65 with TRD (they had not responded to at least 2 different antidepressants of adequate dose and duration).4 After discontinuing prior antidepressant treatments, all patients were started on a newly initiated antidepressant and were also randomized to concomitant intranasal esketamine or intranasal placebo as follows:
- 114 patients were randomized to the intranasal esketamine plus newly initiated oral antidepressant arm
- 109 patients were randomized to the placebo nasal spray plus newly initiated oral antidepressant arm
- The mean baseline Montgomery-Åsberg Depression Rating Scale (MADRS) score for each group was 37 (ie, moderately to severely depressed).
Newly started antidepressants included esc

A long-term, double-blind multicenter maintenance-of-effect trial examined adults age 18 to 65 with TRD.5-6 Patients in this study were responders in 1 of 2 short-term studies or in an open-label direct enrollment study. Stable remission was defined as a MADRS total score <12 for at least 3 of the last 4 weeks of the study, and stable response was defined as a MADRS reduction of >50% but not in remission. After 16 weeks of intranasal esketamine plus an oral antidepressant, stable remitters and stable responders were then randomized separately to continue intranasal esketamine or switch to placebo nasal spray, with both groups continuing on their concomitant oral antidepressant. The primary study endpoint was time to relapse. Relapse was defined as a MADRS total score >22 for more than 2 consecutive weeks, hospitalization for worsening of depression, or any other clinically relevant event. The median age was 48, 66% were female, 90% were White and 4% were black. Patients in stable response or stable remission experienced a significantly longer time to relapse compared with patients who continued their oral antidepressant but were switched to placebo intranasal spray. In this remission response study, patients could receive intranasal treatment weekly or bi-weekly based on symptom severity (Figure 22).

Impact on driving. Two studies examined the impact of esketamine on driving performance. One examined adults with major depressive disorder and the other examined healthy participants. The effects of a single 84-mg dose of esketamine nasal spray on a patient’s ability to drive was assessed in 23 healthy adults. In this study, mirt
A second study evaluated the effects of repeated esketamine administration on driving performance in 25 adults with major depressive disorder. In this study, an ethanol-containing beverage was used as an active control. After administration of a single 84-mg dose of intranasal esketamine, driving performance was the same as a placebo at 18 hours. In the multiple dose phase, standard driving performance was similar for esketamine nasal spray and placebo at 6 hours postdose on Days 11, 18, and 25.
Continue to: Pharmacologic profile
Pharmacologic profile
Adverse events. The most common adverse events in patients treated with esketamine nasal spray were dissociation (41%), dizziness (29%), nausea (28%), sedation (23%), and vertigo (23%).2 The majority of these effects were short-term and resolved during the 2-hour observation period.
In addition to spontaneously reported events, sedation and dissociation were further monitored with specific scales. Sedation was measured with the Modified Observer’s Alertness and Sedation Scale. Using this scale, 50% of patients receiving 56 mg and 61% of patients receiving 84 mg of esketamine met criteria for sedation.
Similarly, dissociation/perceptional changes were measured with spontaneously reported events and also with the Clinician Administered Dissociative State Scale. On this scale, 61% of patients receiving the 56-mg dose, and 69% of patients receiving the 84-mg dose met criteria for dissociation/perceptional changes after dose administration.
Increases in blod pressure. Esketamine intranasal spray was associated with a 7 to 9 mm Hg increase in systolic blood pressure and a 4 to 6 mm Hg increase in diastolic blood pressure, both of which peaked 40 minutes post-dose.
Nausea and vomiting. Intranasal esketamine was associated with a 27% rate of nausea at 56 mg, and 32% at 84 mg, with a 6% rate of vomiting at 56 mg and 12% at 84 mg.
Continue to: Pharmacokinetics
Pharmacokinetics
Esketamine exposure increases from 28 to 84 mg in a fairly dose-proportional range. No accumulation of esketamine was observed in the plasma following twice-weekly administration. Bioavailability is approximately 48% following nasal administration. The Tmax for esketamine plasma concentration is 20 to 40 minutes after the last nasal spray. Protein binding of esketamine is approximately 43% to 45%. The brain-to-plasma ratio of noresketamine is 4 to 6 times lower than that of esketamine. The half-life of esketamine ranged from 7 to 12 hours. The mean half-life of nore
Potential drug interactions
Central nervous system depressants. Concomitant use of esketamine and other CNS depressants (ie, benzodiazepines, opioids, alcohol) may increase sedation. Patients receiving esketamine with concomitant use of other CNS depressants should be closely monitored for sedation.
Psychostimulants. Concomitant use of esketamine and psychostimulants (ie, amphetamines, methylphenidates, moda
Monoamine oxidase inhibitors. Concomitant use of esketamine with monoamine oxidase inhibitors may increase blood pressure. Closely monitor blood pressure with concomitant use of esketamine and monoamine oxidase inhibitors.
Use in special populations. Because of concerns of increased sedation, intranasal esketamine should be administered cautiously in patients receiving other CNS depressants, such as benzodiazepines. In patients with psychosis or a prior history of psychosis, esketamine should be used with increased caution and the risk/benefit ratio should be carefully considered.
Continue to: Because of potential teratogenicity...
Because of potential teratogenicity, esketamine is not recommended in women who are pregnant, may become pregnant, or who are currently nursing.
Intranasal esketamine was examined in a phase III trial of 194 patients age ≥65. At the end of 4 weeks, there was no statistically significant difference in groups on the MADRS, the primary efficacy endpoint. There were no overall differences in the safety profile in patients >65 years compared with younger patients; however, the mean esketamine Cmax and area under the curve were higher in older patients compared with younger adults. The mean esketamine half-life was longer in patients with moderate hepatic impairment.
Abuse liability
Esketamine is a CIII controlled substance and concerns about abuse, misuse, and diversion have been taken into account within the REMS drug safety program.2 Patients with a prior history of substance abuse or misuse should be considered with regard to the risk/benefit ratio.
The REMS drug safety program
Due to the nature of its usually transient adverse effects, including sedation, dissociation, hypertension, and nausea, intranasal esketamine will be administered through a REMS drug safety program at certified REMS treatment centers. Certified REMS treatment centers will receive training on how to safely and effectively counsel and monitor patients. Prior to treatment, patients will receive blood pressure monitoring and anticipated adverse effects will be discussed. Patients will be instructed to not eat solid food for 2 hours pre-dose and to not drink anything for 30 minutes prior.
A treatment session consists of nasal administration and a minimum 2-hour post-administration observation period. Blood pressure must be assessed prior to administration and if elevated, (ie, systolic blood pressure >140 mm Hg, diastolic >90 mm Hg), clinicians should consider the risk of short-term increases in blood pressure that may occur. Do not administer if increases in blood pressure or intracranial pressure pose a serious risk.
Continue to: After each intranasal...
After each intranasal administration the patient will be observed for 5 minutes before the second nasal inhaler is utilized and for another 5 minutes when the patient is receiving 84 mg (ie, each inhaler equals 28 mg). After administering, blood pressure should be reassessed at approximately 40 minutes, which corresponds to the Cmax of intranasal esketamine, and periodically thereafter as warranted.
The patient will then be monitored in a quiet environment for a minimum of 2 hours to make sure that dissociative phenomenon, sedation, and hypertensive reactions have normalized prior to discharge from a certified REMS treatment center.
Dosing and administration
Each intranasal device is primed for 2 infusions (1 in each nostril) for a total dose of 28 mg of esketamine. Combinations of devices can be used to adjust the dose as appropriate for individual patients. The recommended starting dose is 56 mg (ie, 2 devices, with a 5-minute gap between devices). The dose can be increased to 84 mg (ie, 3 intranasal devices spaced at 5-minute intervals) by the second dose based on clinical judgment.
The patient will be instructed to recline the head to a 45° angle, clear his or her nostrils prior to the first treatment, and then self-administer a dose to each nostril while holding the reciprocal nostril closed and inhaling. This process is then repeated every 5 minutes for each subsequent device, with a maximum total dose of 3 devices, or 84 mg (Figure 32). The patient will then be monitored for blood pressure, heart rate, and signs of psychologic or physiologic changes for the next 2 hours. Patients may not drive a car or operate any type of motor equipment until the following day after receiving a normal night’s sleep. Patients will be released from the REMS treatment center after 2 hours if both psychological and physical adverse effects have normalized.

Missed treatment sessions. If a patient misses a treatment session and there is worsening of depressive symptoms, consider returning the patient to the previous dosing schedule (ie, every 2 weeks to once weekly, or weekly to twice weekly).
Continue to: Contraindications for...
Contraindications for intranasal esketamine include:
- aneurysmal vascular disease, including thoracic and abdominal aortic, intracranial, and peripheral arterial vessels, or arterial venous malformations
- history of intracerebral hemorrhage
- hypersensitivity to esketamine, ketamine, or any of the excipients.
Clinical considerations
Intranasal esketamine represents a unique delivery system for the first glutamatergic treatment approved for patients with TRD.
Why Rx? Treatment-resistant depression is found in nearly 1 out of 3 patients with currently available monoaminergic antidepressant treatment options. Patients with TRD are at increased risk of physical and psychological impairment, subsequent worsening of their condition, and social and occupational disability.
Bottom Line
Intranasal esketamine is the first glutamatergic treatment option FDA-approved for patients with treatment-resistant depression who have not responded to standard antidepressant treatment options. In short-term trials, intranasal esketamine significantly improved depressive symptoms as quickly as 24 hours after treatment, with significant improvement maintained through 4 weeks of ongoing administration. In addition, intranasal esketamine was shown to significantly decrease time to relapse for patients who had achieved stable remission or stable response.
Related Resource
- Sullivan MG. FDA approves intranasal esketamine for refractory major depressive disorder. Clinical Psychiatry News. https://www.mdedge.com/psychiatry/article/195712/depression/fda-approves-intranasal-esketamine-refractory-major-depressive. Published March 5, 2019.
Drug Brand Names
Armodafinil • Nuvigil
Duloxetine • Cymbalta
Escitalopram • Lexapro
Esketamine • Spravato
Mirtazapine • Remeron
Modafinil • Provigil
Sertraline • Zoloft
Venlafaxine • Effexor
1. Rush AG, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR D Report. Am J Psychiatry. 2006;163(11):1905-1917.
2. Spravato [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019.
3. Duman RS, Aghajanian GK, Sanacora G, et al. Synaptic plasticity and depression: new insights from stress and rapid-acting anti-depression. Nat Med. 2016;22(3):238-249.
4. Daly EJ, Singh JB, Fedgchin M, et al. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2018;75(2):139-148.
5. Daly EJ, Trivedi M, Janik A, et al. A randomized withdrawal, double-blind, multicenter study of esketamine nasal spray plus an oral antidepressant for relapse prevention in treatment-resistant depression. Poster presented at the 2018 American Society of Clinical Psychopharmacology Annual Meeting; May 2018; Miami, Florida.
6. Wajs E, Aluisio L, Morrison R, et al. Long-term safety of esketamine nasal spray plus oral antidepressants in patients with treatment-resistant depression: phase III open-label safety and efficacy study. Poster presented at the 2018 American Society of Clinical Psychopharmacology Annual Meeting; May 2018; Miami, Florida.
1. Rush AG, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR D Report. Am J Psychiatry. 2006;163(11):1905-1917.
2. Spravato [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019.
3. Duman RS, Aghajanian GK, Sanacora G, et al. Synaptic plasticity and depression: new insights from stress and rapid-acting anti-depression. Nat Med. 2016;22(3):238-249.
4. Daly EJ, Singh JB, Fedgchin M, et al. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2018;75(2):139-148.
5. Daly EJ, Trivedi M, Janik A, et al. A randomized withdrawal, double-blind, multicenter study of esketamine nasal spray plus an oral antidepressant for relapse prevention in treatment-resistant depression. Poster presented at the 2018 American Society of Clinical Psychopharmacology Annual Meeting; May 2018; Miami, Florida.
6. Wajs E, Aluisio L, Morrison R, et al. Long-term safety of esketamine nasal spray plus oral antidepressants in patients with treatment-resistant depression: phase III open-label safety and efficacy study. Poster presented at the 2018 American Society of Clinical Psychopharmacology Annual Meeting; May 2018; Miami, Florida.
When a disaster disrupts access to psychiatric medications
In recent decades, disasters such as storms, earthquakes, and terrorism have occurred with increasing frequency. Disaster planners assess the needs and vulnerabilities of communities in order to save lives during these events. They focus on providing electricity and clean water and addressing other public health measures. What is not adequately planned for, in our opinion, is a disruption in the pharmaceutical supply chain, particularly supplies of psychiatric medications.
There is now a rich literature on disaster psychiatry.1-4 However, there’s been a lack of information about disrupted access to psychiatric medications. Disruptive behavior after Hurricanes Katrina, Maria, Rita, and others were a consequence of a lack of medications or difficulty obtaining medications following these disasters.5-7
This article discusses the pharmaceutical supply chain, the lack of stockpiles of psychiatric medications, and how clinicians can prepare themselves and their patients in the event a disaster strikes.
Supply chains
Each day, nearly 12 million prescriptions are filled in the United States, with gratifying swiftness, efficiency, and accuracy. Our confidence in the nation’s pharmaceutical dependability, however, rests squarely upon the strength and resilience of vast, interconnected supply chains that involve the myriad aspects of private industry—from manufacturing to shipping and transport to last-mile delivery from pharmacy to patient. The failure of any one of the links in any of these supply chains can result in the instant unavailability of critical medications.
Supply chains are fundamental to modern life and must fluctuate to address disruptions; however, common supplemental and gap-filling functions that address minor changes may be insufficient to mitigate supply chain disruptions during a disaster. While supply chains can be extremely complex and can vary significantly from product to product, all supply chains can generally be presented through the components found in Table 1.
All components within a supply chain, such as the transportation mechanisms between nodes, facilities, people, and communication networks, can affect a supply chain’s resilience. For a supply chain to be resilient, key players—in this case, psychiatrists and associated medical professionals—must be acutely aware of the supply chain elements within their vision and reasonable anticipation: known nodes and links, their potential vulnerabilities, and ways and means to mitigate expected disruption.
Recent natural disasters, especially Hurricanes Katrina, Sandy, Harvey, and Maria, have given both government emergency management (at all levels) and clinicians the opportunity to understand the full effects of broken pharmaceutical supply chains under varying and extreme circumstances.
Continue to: As stated in a...
As stated in a recent Department of Homeland Security health care supply chain report, “Pharmaceuticals are one of the top concerns for healthcare providers in terms of supply chain disruptions. They are prone to various supply chain problems, including limited sources, lack of alternatives, time sensitivity, frequent shortages, and minimal on-site inventories. Each stakeholder along the pharmaceutical supply chain faces challenges with understanding and planning for possible disruptions emerging further up the chain. The rapidly expanding use of just-in-time inventory practices by distributors and healthcare customers is creating an increasingly fragile supply-demand balance that could be highly disrupted by a major event either further up the supply chain or within the last mile of delivery.”8,9
No national stockpiles of psychiatric medications
The CDC maintains stockpiles of emergency medications, but these supplies focus on medications to combat infection. In these caches, there are no psychiatric medications other than diazepam, which is stocked for its ability to combat the effects of nerve agents.
In major storm-related events, such as Hurricane Katrina in New Orleans in 2005, the disruptions in all supply chains included psychiatric medications. In the aftermath, many people with addictions and/or severe mental illnesses did not receive either their drugs of choice and/or antimanic and antipsychotic medications. As a result, disruptive behavior became common, especially in the shelters.5-7
During a widespread public emergency, police and emergency services are often stretched very thin. In calmer times, police or emergency services may take a person with disruptive and aggressive behavior to a local emergency department. However, in times of chaos, such as during Hurricane Katrina, patients with aggressive or disruptive behaviors were forcefully incapacitated (ie, “tased”) or shot.
Withdrawal from antidepressants, opiates, alcohol, and benzodiazepines has its own risks. Withdrawal from alcohol or benzodiazepines can be life-threatening. Therefore, it is critically important that clinicians think about how to ensure their patients have a supply of their medications. This may imply stockpiling on a personal or community basis.
Continue to: What to consider before disruption
What to consider before disruption
Many psychiatrists, especially those who have not practiced through a local disaster, may have never contemplated how they would support their patients during a disruptive event. Psychiatrists should carefully consider the questions outlined in Table 2 before a disaster strikes.

Medication-specific issues
During major disasters, patients may not have access to their medications, or the medications may not be able to be fed into the health care system for dispersion. Other issues include closed pharmacies, expired medications as a result of limited refrigeration service, inability to deliver medications to an affected area, and the inability of manufacturing plants to produce medications. For example, after Hurricane Maria, sterile water was in short supply.
After a major disaster, clinicians often leave their communities because they cannot support themselves or their practices. Thus, clinicians may not be available to prescribe needed medications. Available clinicians—often primary care physicians—may not be aware of a patient’s medication history, or they may be uncomfortable prescribing psychiatric medications, especially antipsychotics.
Abrupt discontinuation of psychiatric medications can have severe consequences. Patients may experience withdrawal symptoms, worsening psychiatric symptoms, new-onset psychiatric symptoms, thoughts of harm to self or others, psychosis, or cravings. These issues may be particularly problematic for patients receiving antidepressants, antipsychotics, benzodiazepines, or medication-assisted treatment for opioid use disorder.
Antidepressants. Patients experiencing antidepressant withdrawal, particularly withdrawal from selective serotonin reuptake inhibitors or serotonin-norepinephrine reuptake inhibitors, may exhibit severe symptoms. In addition to the potential recurrence of depressive or anxiety symptoms and suicidal thoughts, patients may experience irritability, insomnia, headache, nausea, and electric shock–like sensations. Prescribing an antidepressant with a longer half-life could potentially prevent an abrupt withdrawal in the event a disaster occurs.
Continue to: Antipsychotics
Antipsychotics. Rapid or abrupt withdrawal of antipsychotics could lead to an increase in psychosis, paranoia, hallucinations, or delusions. Withdrawal of antipsychotics could also lead to agitation, restlessness, insomnia, paresthesia, and anxiety. If a known disaster is likely to occur, such as in the case of a hurricane forecast, clinicians may consider switching a patient a long-acting injectable antipsychotic to minimize the risk of withdrawal and symptom exacerbation.
Benzodiazepines. The abrupt withdrawal of benzodiazepines could result in symptoms that include rebound anxiety, insomnia, restlessness, muscle tension, irritability, nausea, malaise, blurred vision, diaphoresis, nightmares, and seizures. Additionally, many people use benzodiazepines recreationally, and their illicit supply may run out during disasters, which could lead to untreated withdrawal and violence in the community.
Clinicians need to develop action plans for any patients who are receiving scheduled benzodiazepine dosing in order to prevent abrupt withdrawal if a disaster occurs.
Opioids. Opioid cravings and withdrawal are also a major concern during times of disrupted supply. Patients receiving chronic opioid therapy may not be able to receive their maintenance medications, which could lead to withdrawal. Additionally, patients taking illicit opioids may also be at risk of withdrawal.
Early symptoms of opioid withdrawal include watery eyes, runny nose, sweating, anxiety and irritability, poor sleep, and muscle pain. Later symptoms could include cramping, diarrhea, vomiting, increased heart rate and blood pressure, restlessness, shakiness, chills, sweating, and dilated pupils.
Continue to: Contingency planning...
Contingency planning should be a part of the treatment plan for every patient receiving chronic opioid therapy who lives in an area where major disasters are likely to occur.
Medication-assisted treatment for opioid use disorder. Patients receiving treatment for opioid use disorder may be prescribed the partial opioid agonist buprenorphine, either by itself or in combination with the opioid antagonist naloxone. This could be particularly problematic to continue in a major disaster due to the lack of credentialed clinicians, limited supplies, and patients only receiving small amounts of the medication at a time due to the risk of diversion.
Symptoms of buprenorphine withdrawal are similar to those associated with opioid withdrawal. Developing a thoughtful plan in case of a disaster should be part of all buprenorphine prescribing. Patients should be aware of withdrawal symptoms and what to do if they run out of medication.
Additionally, emergency clinicians should have access to buprenorphine and buprenorphine/naloxone and the ability to prescribe them in disaster situations. As with all aspects of disaster response, it is wise to work out issues in advance.
Help your patients get ready
Advise your patients to prepare emergency kits that contain their psychiatric medications that they could quickly grab and go if needed. Because there may be times when it is not possible to gather all necessary medications, having even a small supply ready to go at a moment’s notice would be beneficial. If permitted, patients should also consider keeping medications in multiple locations, including at their place of work, home, or a family member’s home.
Continue to: Additionally, instruct patients...
Additionally, instruct patients to always carry a list of all medications they currently take. Ideally, this list should also include past medications and responses, allergies, and provider contact information. During a disaster, this information could prove vital to an emergency clinician. At a minimum, verify that your patient maintains a list of current medications.
Clinicians should develop emergency plans for all psychiatric medications they prescribe. Document and discuss with your patients any necessary considerations for patients who take medications that require more intensive monitoring, such as lithium or clozapine.
Clinicians, patients, emergency responders, and health care workers need to work together to prepare for major disasters to avoid withdrawal and other consequences of disrupted access to psychiatric medications.
Bottom Line
Consult with local public health officials to determine and develop contingency plans to provide psychiatric medications to your patients in the event of a disaster. Discuss treatment plans and contingency planning with patients, particularly those in regions most likely to be affected by a disaster. Instruct patients to refill medications prior to a foreseeable disaster and to maintain a personal stockpile of medications when appropriate.
Related Resources
- Ochi S, Hodgson S, Landeg O, et al. Disaster-driven evacuation and medication loss: A systematic literature review. PLoS Curr. 2014;6.b. doi: 10.1371/currents.dis.fa417630b566a0c7dfdbf945910edd96.
- Pate JE, Fisher JW. Disaster ethics: What are the ground rules? Current Psychiatry. 2007;6(6):69-78.
Drug Brand Names
Buprenorphine • Subutex
Buprenorphine/naloxone • Suboxone
Clozapine • Clozaril
Diazepam • Valium
Lithium • Eskalith, Lithobid
1. National Institute of Mental Health. Mental health and mass violence: evidence based early psychological intervention for victims/survivors of mass violence. A workshop to reach consensus on best practices. https://cpa.ca/docs/File/Emergencies/massviolence.pdf. Published 2002. Accessed March 11, 2019.
2. Ritchie EC, Friedman M, Watson P. Interventions following mass violence and disasters: strategies for mental health practice. New York, NY: Guilford Press; 2006.
3. Ritchie EC, O’Brien K, Grant M, et al. Disaster psychiatry. In: Stern TA, Rosenbaum JF, Fava M, et al. The Massachusetts General Hospital textbook of comprehensive clinical psychiatry, 2nd edition. Philadelphia, PA: Mosby/Elsevier; 2016:968-974.
4. Ritchie EC, Hamilton S. Early interventions and risk assessment following disaster. Psychiatric Annals. 2004;34(8):605-610.
5. Kessler RC, Galea S, Gruber MJ, et al. Trends in mental illness and suicidality after Hurricane Katrina. Mol Psychiatry. 2008;13(4):374-384.
6. Weisler RH, Barbee JG IV, Townsend MH. Mental health and recovery in the Gulf Coast after Hurricanes Katrina and Rita. JAMA. 2006;296(5):585-588.
7. Galea S, Brewin CR, Gruber M, et al. Exposure to hurricane-related stressors and mental illness after Hurricane Katrina. Arch Gen Psychiatry. 2007;64(12).1427-1434.
8. Federal Emergency Management Agency. Supply Chain Resilience Guide Department of Homeland Security. https://www.fema.gov/media-library-data/1544795397837-767851ba177c7097bf8672aadf8a93c9/NE_DRAFT_Supply_Chain_Resilience.pdf. Published December 17, 2018. Accessed January 2, 2019.
9. Durkin J, Telab M, Fitzmaurice P, et al. Only as strong as its weakest link: resilience of the healthcare supply chain in New York. https://www.hstoday.us/subject-matter-areas/emergency-preparedness/only-as-strong-as-its-weakest-link-the-resilience-of-the-healthcare-supply-chain-in-new-york/. Published October 26, 2018. Accessed February 14, 2019.
In recent decades, disasters such as storms, earthquakes, and terrorism have occurred with increasing frequency. Disaster planners assess the needs and vulnerabilities of communities in order to save lives during these events. They focus on providing electricity and clean water and addressing other public health measures. What is not adequately planned for, in our opinion, is a disruption in the pharmaceutical supply chain, particularly supplies of psychiatric medications.
There is now a rich literature on disaster psychiatry.1-4 However, there’s been a lack of information about disrupted access to psychiatric medications. Disruptive behavior after Hurricanes Katrina, Maria, Rita, and others were a consequence of a lack of medications or difficulty obtaining medications following these disasters.5-7
This article discusses the pharmaceutical supply chain, the lack of stockpiles of psychiatric medications, and how clinicians can prepare themselves and their patients in the event a disaster strikes.
Supply chains
Each day, nearly 12 million prescriptions are filled in the United States, with gratifying swiftness, efficiency, and accuracy. Our confidence in the nation’s pharmaceutical dependability, however, rests squarely upon the strength and resilience of vast, interconnected supply chains that involve the myriad aspects of private industry—from manufacturing to shipping and transport to last-mile delivery from pharmacy to patient. The failure of any one of the links in any of these supply chains can result in the instant unavailability of critical medications.
Supply chains are fundamental to modern life and must fluctuate to address disruptions; however, common supplemental and gap-filling functions that address minor changes may be insufficient to mitigate supply chain disruptions during a disaster. While supply chains can be extremely complex and can vary significantly from product to product, all supply chains can generally be presented through the components found in Table 1.

All components within a supply chain, such as the transportation mechanisms between nodes, facilities, people, and communication networks, can affect a supply chain’s resilience. For a supply chain to be resilient, key players—in this case, psychiatrists and associated medical professionals—must be acutely aware of the supply chain elements within their vision and reasonable anticipation: known nodes and links, their potential vulnerabilities, and ways and means to mitigate expected disruption.
Recent natural disasters, especially Hurricanes Katrina, Sandy, Harvey, and Maria, have given both government emergency management (at all levels) and clinicians the opportunity to understand the full effects of broken pharmaceutical supply chains under varying and extreme circumstances.
Continue to: As stated in a...
As stated in a recent Department of Homeland Security health care supply chain report, “Pharmaceuticals are one of the top concerns for healthcare providers in terms of supply chain disruptions. They are prone to various supply chain problems, including limited sources, lack of alternatives, time sensitivity, frequent shortages, and minimal on-site inventories. Each stakeholder along the pharmaceutical supply chain faces challenges with understanding and planning for possible disruptions emerging further up the chain. The rapidly expanding use of just-in-time inventory practices by distributors and healthcare customers is creating an increasingly fragile supply-demand balance that could be highly disrupted by a major event either further up the supply chain or within the last mile of delivery.”8,9
No national stockpiles of psychiatric medications
The CDC maintains stockpiles of emergency medications, but these supplies focus on medications to combat infection. In these caches, there are no psychiatric medications other than diazepam, which is stocked for its ability to combat the effects of nerve agents.
In major storm-related events, such as Hurricane Katrina in New Orleans in 2005, the disruptions in all supply chains included psychiatric medications. In the aftermath, many people with addictions and/or severe mental illnesses did not receive either their drugs of choice and/or antimanic and antipsychotic medications. As a result, disruptive behavior became common, especially in the shelters.5-7
During a widespread public emergency, police and emergency services are often stretched very thin. In calmer times, police or emergency services may take a person with disruptive and aggressive behavior to a local emergency department. However, in times of chaos, such as during Hurricane Katrina, patients with aggressive or disruptive behaviors were forcefully incapacitated (ie, “tased”) or shot.
Withdrawal from antidepressants, opiates, alcohol, and benzodiazepines has its own risks. Withdrawal from alcohol or benzodiazepines can be life-threatening. Therefore, it is critically important that clinicians think about how to ensure their patients have a supply of their medications. This may imply stockpiling on a personal or community basis.
Continue to: What to consider before disruption
What to consider before disruption
Many psychiatrists, especially those who have not practiced through a local disaster, may have never contemplated how they would support their patients during a disruptive event. Psychiatrists should carefully consider the questions outlined in Table 2 before a disaster strikes.

Medication-specific issues
During major disasters, patients may not have access to their medications, or the medications may not be able to be fed into the health care system for dispersion. Other issues include closed pharmacies, expired medications as a result of limited refrigeration service, inability to deliver medications to an affected area, and the inability of manufacturing plants to produce medications. For example, after Hurricane Maria, sterile water was in short supply.
After a major disaster, clinicians often leave their communities because they cannot support themselves or their practices. Thus, clinicians may not be available to prescribe needed medications. Available clinicians—often primary care physicians—may not be aware of a patient’s medication history, or they may be uncomfortable prescribing psychiatric medications, especially antipsychotics.
Abrupt discontinuation of psychiatric medications can have severe consequences. Patients may experience withdrawal symptoms, worsening psychiatric symptoms, new-onset psychiatric symptoms, thoughts of harm to self or others, psychosis, or cravings. These issues may be particularly problematic for patients receiving antidepressants, antipsychotics, benzodiazepines, or medication-assisted treatment for opioid use disorder.
Antidepressants. Patients experiencing antidepressant withdrawal, particularly withdrawal from selective serotonin reuptake inhibitors or serotonin-norepinephrine reuptake inhibitors, may exhibit severe symptoms. In addition to the potential recurrence of depressive or anxiety symptoms and suicidal thoughts, patients may experience irritability, insomnia, headache, nausea, and electric shock–like sensations. Prescribing an antidepressant with a longer half-life could potentially prevent an abrupt withdrawal in the event a disaster occurs.
Continue to: Antipsychotics
Antipsychotics. Rapid or abrupt withdrawal of antipsychotics could lead to an increase in psychosis, paranoia, hallucinations, or delusions. Withdrawal of antipsychotics could also lead to agitation, restlessness, insomnia, paresthesia, and anxiety. If a known disaster is likely to occur, such as in the case of a hurricane forecast, clinicians may consider switching a patient a long-acting injectable antipsychotic to minimize the risk of withdrawal and symptom exacerbation.
Benzodiazepines. The abrupt withdrawal of benzodiazepines could result in symptoms that include rebound anxiety, insomnia, restlessness, muscle tension, irritability, nausea, malaise, blurred vision, diaphoresis, nightmares, and seizures. Additionally, many people use benzodiazepines recreationally, and their illicit supply may run out during disasters, which could lead to untreated withdrawal and violence in the community.
Clinicians need to develop action plans for any patients who are receiving scheduled benzodiazepine dosing in order to prevent abrupt withdrawal if a disaster occurs.
Opioids. Opioid cravings and withdrawal are also a major concern during times of disrupted supply. Patients receiving chronic opioid therapy may not be able to receive their maintenance medications, which could lead to withdrawal. Additionally, patients taking illicit opioids may also be at risk of withdrawal.
Early symptoms of opioid withdrawal include watery eyes, runny nose, sweating, anxiety and irritability, poor sleep, and muscle pain. Later symptoms could include cramping, diarrhea, vomiting, increased heart rate and blood pressure, restlessness, shakiness, chills, sweating, and dilated pupils.
Continue to: Contingency planning...
Contingency planning should be a part of the treatment plan for every patient receiving chronic opioid therapy who lives in an area where major disasters are likely to occur.
Medication-assisted treatment for opioid use disorder. Patients receiving treatment for opioid use disorder may be prescribed the partial opioid agonist buprenorphine, either by itself or in combination with the opioid antagonist naloxone. This could be particularly problematic to continue in a major disaster due to the lack of credentialed clinicians, limited supplies, and patients only receiving small amounts of the medication at a time due to the risk of diversion.
Symptoms of buprenorphine withdrawal are similar to those associated with opioid withdrawal. Developing a thoughtful plan in case of a disaster should be part of all buprenorphine prescribing. Patients should be aware of withdrawal symptoms and what to do if they run out of medication.
Additionally, emergency clinicians should have access to buprenorphine and buprenorphine/naloxone and the ability to prescribe them in disaster situations. As with all aspects of disaster response, it is wise to work out issues in advance.
Help your patients get ready
Advise your patients to prepare emergency kits that contain their psychiatric medications that they could quickly grab and go if needed. Because there may be times when it is not possible to gather all necessary medications, having even a small supply ready to go at a moment’s notice would be beneficial. If permitted, patients should also consider keeping medications in multiple locations, including at their place of work, home, or a family member’s home.
Continue to: Additionally, instruct patients...
Additionally, instruct patients to always carry a list of all medications they currently take. Ideally, this list should also include past medications and responses, allergies, and provider contact information. During a disaster, this information could prove vital to an emergency clinician. At a minimum, verify that your patient maintains a list of current medications.
Clinicians should develop emergency plans for all psychiatric medications they prescribe. Document and discuss with your patients any necessary considerations for patients who take medications that require more intensive monitoring, such as lithium or clozapine.
Clinicians, patients, emergency responders, and health care workers need to work together to prepare for major disasters to avoid withdrawal and other consequences of disrupted access to psychiatric medications.
Bottom Line
Consult with local public health officials to determine and develop contingency plans to provide psychiatric medications to your patients in the event of a disaster. Discuss treatment plans and contingency planning with patients, particularly those in regions most likely to be affected by a disaster. Instruct patients to refill medications prior to a foreseeable disaster and to maintain a personal stockpile of medications when appropriate.
Related Resources
- Ochi S, Hodgson S, Landeg O, et al. Disaster-driven evacuation and medication loss: A systematic literature review. PLoS Curr. 2014;6.b. doi: 10.1371/currents.dis.fa417630b566a0c7dfdbf945910edd96.
- Pate JE, Fisher JW. Disaster ethics: What are the ground rules? Current Psychiatry. 2007;6(6):69-78.
Drug Brand Names
Buprenorphine • Subutex
Buprenorphine/naloxone • Suboxone
Clozapine • Clozaril
Diazepam • Valium
Lithium • Eskalith, Lithobid
In recent decades, disasters such as storms, earthquakes, and terrorism have occurred with increasing frequency. Disaster planners assess the needs and vulnerabilities of communities in order to save lives during these events. They focus on providing electricity and clean water and addressing other public health measures. What is not adequately planned for, in our opinion, is a disruption in the pharmaceutical supply chain, particularly supplies of psychiatric medications.
There is now a rich literature on disaster psychiatry.1-4 However, there’s been a lack of information about disrupted access to psychiatric medications. Disruptive behavior after Hurricanes Katrina, Maria, Rita, and others were a consequence of a lack of medications or difficulty obtaining medications following these disasters.5-7
This article discusses the pharmaceutical supply chain, the lack of stockpiles of psychiatric medications, and how clinicians can prepare themselves and their patients in the event a disaster strikes.
Supply chains
Each day, nearly 12 million prescriptions are filled in the United States, with gratifying swiftness, efficiency, and accuracy. Our confidence in the nation’s pharmaceutical dependability, however, rests squarely upon the strength and resilience of vast, interconnected supply chains that involve the myriad aspects of private industry—from manufacturing to shipping and transport to last-mile delivery from pharmacy to patient. The failure of any one of the links in any of these supply chains can result in the instant unavailability of critical medications.
Supply chains are fundamental to modern life and must fluctuate to address disruptions; however, common supplemental and gap-filling functions that address minor changes may be insufficient to mitigate supply chain disruptions during a disaster. While supply chains can be extremely complex and can vary significantly from product to product, all supply chains can generally be presented through the components found in Table 1.

All components within a supply chain, such as the transportation mechanisms between nodes, facilities, people, and communication networks, can affect a supply chain’s resilience. For a supply chain to be resilient, key players—in this case, psychiatrists and associated medical professionals—must be acutely aware of the supply chain elements within their vision and reasonable anticipation: known nodes and links, their potential vulnerabilities, and ways and means to mitigate expected disruption.
Recent natural disasters, especially Hurricanes Katrina, Sandy, Harvey, and Maria, have given both government emergency management (at all levels) and clinicians the opportunity to understand the full effects of broken pharmaceutical supply chains under varying and extreme circumstances.
Continue to: As stated in a...
As stated in a recent Department of Homeland Security health care supply chain report, “Pharmaceuticals are one of the top concerns for healthcare providers in terms of supply chain disruptions. They are prone to various supply chain problems, including limited sources, lack of alternatives, time sensitivity, frequent shortages, and minimal on-site inventories. Each stakeholder along the pharmaceutical supply chain faces challenges with understanding and planning for possible disruptions emerging further up the chain. The rapidly expanding use of just-in-time inventory practices by distributors and healthcare customers is creating an increasingly fragile supply-demand balance that could be highly disrupted by a major event either further up the supply chain or within the last mile of delivery.”8,9
No national stockpiles of psychiatric medications
The CDC maintains stockpiles of emergency medications, but these supplies focus on medications to combat infection. In these caches, there are no psychiatric medications other than diazepam, which is stocked for its ability to combat the effects of nerve agents.
In major storm-related events, such as Hurricane Katrina in New Orleans in 2005, the disruptions in all supply chains included psychiatric medications. In the aftermath, many people with addictions and/or severe mental illnesses did not receive either their drugs of choice and/or antimanic and antipsychotic medications. As a result, disruptive behavior became common, especially in the shelters.5-7
During a widespread public emergency, police and emergency services are often stretched very thin. In calmer times, police or emergency services may take a person with disruptive and aggressive behavior to a local emergency department. However, in times of chaos, such as during Hurricane Katrina, patients with aggressive or disruptive behaviors were forcefully incapacitated (ie, “tased”) or shot.
Withdrawal from antidepressants, opiates, alcohol, and benzodiazepines has its own risks. Withdrawal from alcohol or benzodiazepines can be life-threatening. Therefore, it is critically important that clinicians think about how to ensure their patients have a supply of their medications. This may imply stockpiling on a personal or community basis.
Continue to: What to consider before disruption
What to consider before disruption
Many psychiatrists, especially those who have not practiced through a local disaster, may have never contemplated how they would support their patients during a disruptive event. Psychiatrists should carefully consider the questions outlined in Table 2 before a disaster strikes.

Medication-specific issues
During major disasters, patients may not have access to their medications, or the medications may not be able to be fed into the health care system for dispersion. Other issues include closed pharmacies, expired medications as a result of limited refrigeration service, inability to deliver medications to an affected area, and the inability of manufacturing plants to produce medications. For example, after Hurricane Maria, sterile water was in short supply.
After a major disaster, clinicians often leave their communities because they cannot support themselves or their practices. Thus, clinicians may not be available to prescribe needed medications. Available clinicians—often primary care physicians—may not be aware of a patient’s medication history, or they may be uncomfortable prescribing psychiatric medications, especially antipsychotics.
Abrupt discontinuation of psychiatric medications can have severe consequences. Patients may experience withdrawal symptoms, worsening psychiatric symptoms, new-onset psychiatric symptoms, thoughts of harm to self or others, psychosis, or cravings. These issues may be particularly problematic for patients receiving antidepressants, antipsychotics, benzodiazepines, or medication-assisted treatment for opioid use disorder.
Antidepressants. Patients experiencing antidepressant withdrawal, particularly withdrawal from selective serotonin reuptake inhibitors or serotonin-norepinephrine reuptake inhibitors, may exhibit severe symptoms. In addition to the potential recurrence of depressive or anxiety symptoms and suicidal thoughts, patients may experience irritability, insomnia, headache, nausea, and electric shock–like sensations. Prescribing an antidepressant with a longer half-life could potentially prevent an abrupt withdrawal in the event a disaster occurs.
Continue to: Antipsychotics
Antipsychotics. Rapid or abrupt withdrawal of antipsychotics could lead to an increase in psychosis, paranoia, hallucinations, or delusions. Withdrawal of antipsychotics could also lead to agitation, restlessness, insomnia, paresthesia, and anxiety. If a known disaster is likely to occur, such as in the case of a hurricane forecast, clinicians may consider switching a patient a long-acting injectable antipsychotic to minimize the risk of withdrawal and symptom exacerbation.
Benzodiazepines. The abrupt withdrawal of benzodiazepines could result in symptoms that include rebound anxiety, insomnia, restlessness, muscle tension, irritability, nausea, malaise, blurred vision, diaphoresis, nightmares, and seizures. Additionally, many people use benzodiazepines recreationally, and their illicit supply may run out during disasters, which could lead to untreated withdrawal and violence in the community.
Clinicians need to develop action plans for any patients who are receiving scheduled benzodiazepine dosing in order to prevent abrupt withdrawal if a disaster occurs.
Opioids. Opioid cravings and withdrawal are also a major concern during times of disrupted supply. Patients receiving chronic opioid therapy may not be able to receive their maintenance medications, which could lead to withdrawal. Additionally, patients taking illicit opioids may also be at risk of withdrawal.
Early symptoms of opioid withdrawal include watery eyes, runny nose, sweating, anxiety and irritability, poor sleep, and muscle pain. Later symptoms could include cramping, diarrhea, vomiting, increased heart rate and blood pressure, restlessness, shakiness, chills, sweating, and dilated pupils.
Continue to: Contingency planning...
Contingency planning should be a part of the treatment plan for every patient receiving chronic opioid therapy who lives in an area where major disasters are likely to occur.
Medication-assisted treatment for opioid use disorder. Patients receiving treatment for opioid use disorder may be prescribed the partial opioid agonist buprenorphine, either by itself or in combination with the opioid antagonist naloxone. This could be particularly problematic to continue in a major disaster due to the lack of credentialed clinicians, limited supplies, and patients only receiving small amounts of the medication at a time due to the risk of diversion.
Symptoms of buprenorphine withdrawal are similar to those associated with opioid withdrawal. Developing a thoughtful plan in case of a disaster should be part of all buprenorphine prescribing. Patients should be aware of withdrawal symptoms and what to do if they run out of medication.
Additionally, emergency clinicians should have access to buprenorphine and buprenorphine/naloxone and the ability to prescribe them in disaster situations. As with all aspects of disaster response, it is wise to work out issues in advance.
Help your patients get ready
Advise your patients to prepare emergency kits that contain their psychiatric medications that they could quickly grab and go if needed. Because there may be times when it is not possible to gather all necessary medications, having even a small supply ready to go at a moment’s notice would be beneficial. If permitted, patients should also consider keeping medications in multiple locations, including at their place of work, home, or a family member’s home.
Continue to: Additionally, instruct patients...
Additionally, instruct patients to always carry a list of all medications they currently take. Ideally, this list should also include past medications and responses, allergies, and provider contact information. During a disaster, this information could prove vital to an emergency clinician. At a minimum, verify that your patient maintains a list of current medications.
Clinicians should develop emergency plans for all psychiatric medications they prescribe. Document and discuss with your patients any necessary considerations for patients who take medications that require more intensive monitoring, such as lithium or clozapine.
Clinicians, patients, emergency responders, and health care workers need to work together to prepare for major disasters to avoid withdrawal and other consequences of disrupted access to psychiatric medications.
Bottom Line
Consult with local public health officials to determine and develop contingency plans to provide psychiatric medications to your patients in the event of a disaster. Discuss treatment plans and contingency planning with patients, particularly those in regions most likely to be affected by a disaster. Instruct patients to refill medications prior to a foreseeable disaster and to maintain a personal stockpile of medications when appropriate.
Related Resources
- Ochi S, Hodgson S, Landeg O, et al. Disaster-driven evacuation and medication loss: A systematic literature review. PLoS Curr. 2014;6.b. doi: 10.1371/currents.dis.fa417630b566a0c7dfdbf945910edd96.
- Pate JE, Fisher JW. Disaster ethics: What are the ground rules? Current Psychiatry. 2007;6(6):69-78.
Drug Brand Names
Buprenorphine • Subutex
Buprenorphine/naloxone • Suboxone
Clozapine • Clozaril
Diazepam • Valium
Lithium • Eskalith, Lithobid
1. National Institute of Mental Health. Mental health and mass violence: evidence based early psychological intervention for victims/survivors of mass violence. A workshop to reach consensus on best practices. https://cpa.ca/docs/File/Emergencies/massviolence.pdf. Published 2002. Accessed March 11, 2019.
2. Ritchie EC, Friedman M, Watson P. Interventions following mass violence and disasters: strategies for mental health practice. New York, NY: Guilford Press; 2006.
3. Ritchie EC, O’Brien K, Grant M, et al. Disaster psychiatry. In: Stern TA, Rosenbaum JF, Fava M, et al. The Massachusetts General Hospital textbook of comprehensive clinical psychiatry, 2nd edition. Philadelphia, PA: Mosby/Elsevier; 2016:968-974.
4. Ritchie EC, Hamilton S. Early interventions and risk assessment following disaster. Psychiatric Annals. 2004;34(8):605-610.
5. Kessler RC, Galea S, Gruber MJ, et al. Trends in mental illness and suicidality after Hurricane Katrina. Mol Psychiatry. 2008;13(4):374-384.
6. Weisler RH, Barbee JG IV, Townsend MH. Mental health and recovery in the Gulf Coast after Hurricanes Katrina and Rita. JAMA. 2006;296(5):585-588.
7. Galea S, Brewin CR, Gruber M, et al. Exposure to hurricane-related stressors and mental illness after Hurricane Katrina. Arch Gen Psychiatry. 2007;64(12).1427-1434.
8. Federal Emergency Management Agency. Supply Chain Resilience Guide Department of Homeland Security. https://www.fema.gov/media-library-data/1544795397837-767851ba177c7097bf8672aadf8a93c9/NE_DRAFT_Supply_Chain_Resilience.pdf. Published December 17, 2018. Accessed January 2, 2019.
9. Durkin J, Telab M, Fitzmaurice P, et al. Only as strong as its weakest link: resilience of the healthcare supply chain in New York. https://www.hstoday.us/subject-matter-areas/emergency-preparedness/only-as-strong-as-its-weakest-link-the-resilience-of-the-healthcare-supply-chain-in-new-york/. Published October 26, 2018. Accessed February 14, 2019.
1. National Institute of Mental Health. Mental health and mass violence: evidence based early psychological intervention for victims/survivors of mass violence. A workshop to reach consensus on best practices. https://cpa.ca/docs/File/Emergencies/massviolence.pdf. Published 2002. Accessed March 11, 2019.
2. Ritchie EC, Friedman M, Watson P. Interventions following mass violence and disasters: strategies for mental health practice. New York, NY: Guilford Press; 2006.
3. Ritchie EC, O’Brien K, Grant M, et al. Disaster psychiatry. In: Stern TA, Rosenbaum JF, Fava M, et al. The Massachusetts General Hospital textbook of comprehensive clinical psychiatry, 2nd edition. Philadelphia, PA: Mosby/Elsevier; 2016:968-974.
4. Ritchie EC, Hamilton S. Early interventions and risk assessment following disaster. Psychiatric Annals. 2004;34(8):605-610.
5. Kessler RC, Galea S, Gruber MJ, et al. Trends in mental illness and suicidality after Hurricane Katrina. Mol Psychiatry. 2008;13(4):374-384.
6. Weisler RH, Barbee JG IV, Townsend MH. Mental health and recovery in the Gulf Coast after Hurricanes Katrina and Rita. JAMA. 2006;296(5):585-588.
7. Galea S, Brewin CR, Gruber M, et al. Exposure to hurricane-related stressors and mental illness after Hurricane Katrina. Arch Gen Psychiatry. 2007;64(12).1427-1434.
8. Federal Emergency Management Agency. Supply Chain Resilience Guide Department of Homeland Security. https://www.fema.gov/media-library-data/1544795397837-767851ba177c7097bf8672aadf8a93c9/NE_DRAFT_Supply_Chain_Resilience.pdf. Published December 17, 2018. Accessed January 2, 2019.
9. Durkin J, Telab M, Fitzmaurice P, et al. Only as strong as its weakest link: resilience of the healthcare supply chain in New York. https://www.hstoday.us/subject-matter-areas/emergency-preparedness/only-as-strong-as-its-weakest-link-the-resilience-of-the-healthcare-supply-chain-in-new-york/. Published October 26, 2018. Accessed February 14, 2019.
Cannabidiol (CBD) for schizophrenia: Promise or pipe dream?
Over the past few decades, it has become increasingly clear that cannabis use can increase the risk of developing a psychotic disorder and worsen the course of existing schizophrenia in a dose-dependent fashion.1-3 Beyond psychosis, although many patients with mental illness use cannabis for recreational purposes or as purported “self-medication,” currently available evidence suggests that marijuana is more likely to represent a harm than a benefit for psychiatric disorders4 (Box4-8). Our current state of knowledge therefore suggests that psychiatrists should caution their patients against using cannabis and prioritize interventions to reduce or discontinue use, especially among those with psychotic disorders.
Box
Data from California in 2006—a decade after the state’s legalization of “medical marijuana”—revealed that 23% of patients in a sample enrolled in medical marijuana clinics were receiving cannabis to treat a mental disorder.5 That was a striking statistic given the dearth of evidence to support a benefit of cannabis for psychiatric conditions at the time, leaving clinicians who provided the necessary recommendations to obtain medical marijuana largely unable to give informed consent about the risks and benefits, much less recommendations about specific products, routes of administration, or dosing. In 2019, we know considerably more about the interaction between cannabinoids and mental health, but research findings thus far warrant more caution than enthusiasm, with one recent review concluding that “whenever an association is observed between cannabis use and psychiatric disorders, the relationship is generally an adverse one.”4
Some critics have argued that the medical marijuana industry represents little more than a front for recreational use. In California and other states that have legalized recreational use, that claim has been rendered all but moot, although the public remains curious about the potential health benefits of cannabinoids and will likely continue to look to clinicians for advice. For those seeking guidance from evidence-based research, the existing state of knowledge can seem like a “Wild West” of anecdotal subjective reports, biased opinions, and uncontrolled clinical studies. Cannabis remains a Schedule I drug at the federal level, and quality clinical research has been limited to a relatively modest number of randomized controlled trials (RCTs), mostly involving FDA-approved cannabinoids rather than smoked cannabis. Randomized controlled trials that have involved smoked marijuana have generally involved low-potency delta-9-tetrahydrocannabinol (THC) cannabis that may not reflect the same therapeutic and adverse effects of the increasingly high potency cannabis now available on the street and in dispensaries.
In psychiatry, a few RCTs are underway exploring cannabis as a viable treatment for mental disorders (eg, posttraumatic stress disorder), but none have yet been completed or published. At best, retrospective studies to date have failed to support a consistent benefit of cannabis for any psychiatric disorder and at worst increasingly suggest a negative impact on psychotic, mood, and anxiety disorders.4,6 Meanwhile, synthetic cannabinoid receptor agonists (eg, “Spice” products) have come to represent a clear public health risk, with both medical and psychiatric toxicity.7
A more cautiously optimistic case for the therapeutic potential of cannabinoids in psychiatry could be made for cannabidiol (CBD), which may possess anxiolytic, antipsychotic, and neuroprotective properties.8 Based on its purported health benefits, it is possible that CBD may even gain widespread popularity as a food supplement. Because a pharmaceutically-manufactured form of CBD was recently FDA-approved for the treatment of seizures associated with Lennox-Gastaut syndrome and Dravet syndrome, off-label prescribing of CBD for psychiatric disorders can be anticipated. While there is not yet sufficient evidence about risks and benefits to justify CBD being recommended broadly in psychiatry, that same informational vacuum has not stopped eager patients from seeking approval for cannabis, and some physicians from providing it.
Despite that conclusion, because cannabis is classified as a Schedule I drug by the US Drug Enforcement Agency, clinical research investigating the risks and benefits of cannabis has been limited. It therefore remains possible that cannabis, or individual cannabinoids such as cannabidiol (CBD), may yet find a therapeutic niche in psychiatry. This article reviews evidence on CBD for the treatment of schizophrenia.
Cannabinergic drugs as potential antipsychotics
Although the bulk of evidence indicates a harmful effect of cannabis in individuals with or at risk for psychosis, there have been a few published cases of schizophrenia improving with dronabinol, an FDA-approved, synthetic form of delta-9-tetrahydrocannabinol (THC).9,10 THC is the constituent of cannabis that produces euphoric effects. These provocative findings have not been replicated in controlled clinical trials, but suggest at least the theoretical possibility of idiosyncratic benefits from THC for some individuals within the psychotic spectrum.
Still, given that most available evidence supports that THC has a harmful effect on psychosis and psychosis risk, researchers have instead performed randomized controlled trials (RCTs) to investigate a possible therapeutic role for medications that oppose the agonist effects of THC at cannabinoid type 1 (CB1) receptors. To date, 2 RCTs comparing rimonabant, a CB1 inverse agonist, with placebo (PLB) in patients with schizophrenia have failed to demonstrate any benefit for psychotic symptoms or cognitive deficits.11,12 A third trial examining rimonabant for people diagnosed with schizophrenia who were overweight found significant benefits for anxiety and depressive symptoms, but none for positive symptoms or the primary outcome of weight loss.13 While these results are discouraging, the role of THC in precipitating psychosis suggests that novel agents opposing the actions of THC on the cannabinoid system could have antipsychotic properties.14
Cannabidiol: An antipsychotic medication?
In contrast to THC, CBD has minimal euphorigenic properties and has recently been heralded in the popular press as a “miracle drug” with benefits for medical and psychiatric disorders alike.15 It has even been speculated that it could become a popular food supplement.16 In 2018, the FDA gave full approval to a pharmaceutically manufactured form of CBD (brand name: Epidiolex) as a novel treatment for 2 rare and severe forms of pediatric epilepsy, Lennox-Gastaut syndrome and Dravet syndrome,17 based on RCTs supporting its efficacy for these often refractory and life-threatening conditions.18-20
In psychiatry, there have not yet been enough robust clinical studies to support broad therapeutic claims for CBD as a treatment for any mental disorder.21 However, there is growing evidence that CBD has potential as an antipsychotic medication. In 1995, the first case report was published describing the efficacy of CBD, 1,500 mg/d, as standalone therapy in a single individual with schizophrenia.22 In 2006, the same research group followed up with a case series in which only 1 out of 3 patients with treatment-refractory schizophrenia improved with flexible dosing of CBD to a maximum dose of 1,280 mg/d.23
There have been 3 published RCTs exploring the efficacy of CBD in schizophrenia (Table24-26). The first study, published in 2012, included 39 adults with schizophrenia who were randomized to 800 mg/d of CBD or amisulpride (AMS), a second-generation antipsychotic that is popular in Europe but is not available in the United States.24 Over 4 weeks of randomized treatment, CBD resulted in as much improvement in overall symptoms and positive symptoms as AMS, and improvement of negative symptoms was significantly greater with CBD. Compared with patients treated with antipsychotic medication, patients who were treated with CBD had fewer extrapyramidal symptoms, less weight gain, and less prolactin elevation. This initial trial suggests that CBD might be as efficacious in schizophrenia as antipsychotic medication, without its burdensome adverse effects. However, this is the only RCT of CBD monotherapy published to date.
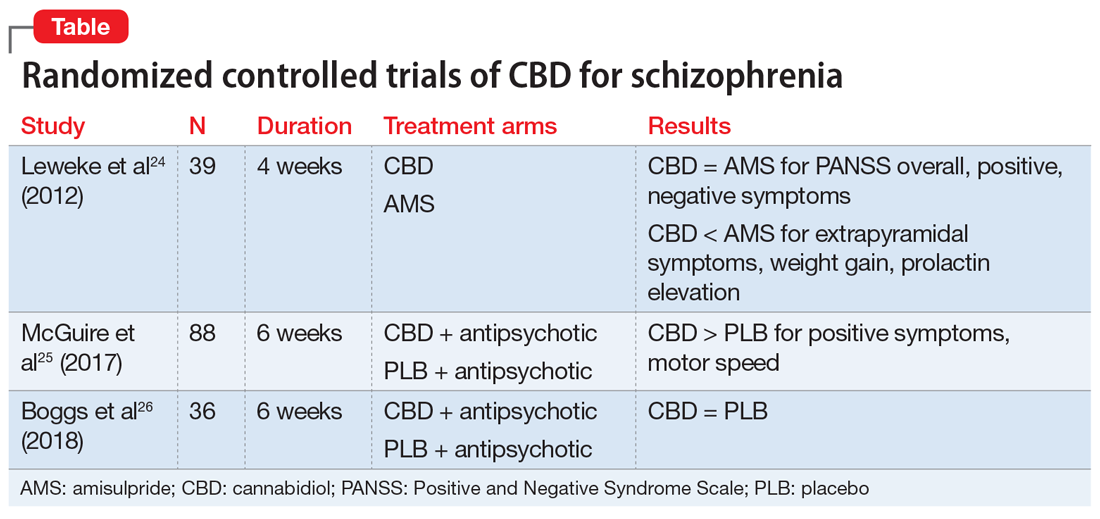
Continue to: Two other recently published RCTs...
Two other recently published RCTs compared CBD with PLB as add-on therapy to antipsychotics. McGuire et al25 compared CBD, 1,000 mg/d, to PLB over 6 weeks in 88 patients with schizophrenia. Positive symptom improvement was statistically greater with CBD than with PLB, although the magnitude of clinical change was modest (using the Positive and Negative Syndrome Scale [PANSS] positive symptom subscale: −3.2 points for CBD vs −1.7 points for PLB). Changes in PANSS total score and subscales for general and negative symptoms were not significantly different between treatment groups. There was also no significant difference in overall change in neurocognitive symptoms, although post-hoc analysis revealed significantly greater improvement in motor speed for patients treated with CBD. More than twice the number of patients treated with CBD were rated as “much improved” by the Clinical Global Impressions scale compared with patients treated with PLB, but this was not a statistically significant finding, and most patients experienced only “minimal” or “no improvement.” In terms of adverse events, there were no significant differences between patients in the CBD and PLB groups. Although this study is technically “positive” for CBD and suggests minimal adverse effects, it is not clear whether the statistically significant positive symptom improvements (+1.5 PANSS points for CBD over PLB) were clinically significant.
The most recently published placebo-controlled RCT of CBD as add-on therapy to antipsychotic medication included 36 patients with schizophrenia treated over 6 weeks.26 In this study, there was no benefit of CBD, 600 mg/d, on any PANSS score outcome (total, general, positive, or negative symptoms). For the primary outcome of the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery, there were no significant drug × time effects, and post-hoc analyses showed that only patients treated with PLB improved with time. Sedation was more common among patients treated with CBD compared with PLB.
Making sense of the data
There have been mixed results from the few case reports and 3 RCTs of patients with schizophrenia who were treated with CBD. How can we resolve these disparate findings? A few possible interpretations of the data that warrant clarification through additional research include:
Dosing. In the first case report with positive results, CBD was dosed at 1,500 mg/d,22 whereas in the subsequent case series with mixed results, the maximum allowable dose of CBD was 1,280 mg/d.23 Likewise, in the RCTs, positive results were found when CBD was dosed at 800 to 1,000 mg/d,24,25 but not at 600 mg/d.26 The efficacy of CBD for schizophrenia might depend on higher doses.
Treatment resistance. In the second case series in which only 1 out of 3 patients responded to treatment with CBD,23 the patients had demonstrated previous nonresponse to at least 2 first-generation antipsychotics (FGAs) and risperidone, 6 mg/d. In the RCTs, all patients were antipsychotic-responsive.24-26 Cannabidiol may not be as effective for patients with treatment-refractory schizophrenia as it is for patients with schizophrenia who respond to antipsychotics.
Continue to: Clinical stability
Clinical stability. Within the RCTs, the greatest response was observed in the study that enrolled patients who were hospitalized with acute symptoms of schizophrenia.23 In the 2 studies that found either modest or no benefit with CBD, the patients had been stabilized on antipsychotic medications prior to randomization. Cannabidiol may offer limited benefit as add-on therapy to patients who have already responded to antipsychotic treatment, where there is “less room” for additional improvement.
Monotherapy. Both the case reports22,23 and the RCT with the most robust positive findings24 involved treatment with CBD as monotherapy. For some patients with schizophrenia, CBD might be effective as standalone therapy as an alternative to antipsychotics that is better tolerated. Adding CBD to antipsychotic therapy might be redundant and therefore less effective.
Answering questions about CBD
Cannabidiol is becoming increasingly popular for its purported health benefits. The mixed results of the few studies published on CBD for schizophrenia place clinicians in a difficult position when attempting to answer questions about how cannabinoids might fit into treatment of patients with psychosis. Consider the following:
Is cannabis helpful for patients with schizophrenia? No. Aside from the few case reports suggesting that FDA-approved THC (dronabinol) can improve symptoms in some patients,9,10 most of the evidence from anecdotal reports and both experimental and observational studies indicate that cannabis, THC, and synthetic cannabinoids have a harmful effect in patients with or at risk for psychosis.1-3
If you are considering recommending some form of cannabis to patients with schizophrenia, what kind should you recommend? Recommending or encouraging cannabis use for patients with psychosis is ill-advised. Although certain types of cannabis might contain more THC (eg, Cannabis indica vs Cannabis sativa) or variable amounts of CBD, in general the amount of CBD in whole leaf cannabis is minimal, with the ratio of THC to CBD increasingly significantly over the past decade.3,27 Most forms of cannabis should therefore be avoided by individuals with or at risk for psychotic disorders.
Continue to: What about CBD oil and other CBD products sold in dispensaries?
What about CBD oil and other CBD products sold in dispensaries? Cannabidiol is increasingly available in various forms based on its ability to be designated as a legal hemp product (containing <0.3% THC) at the federal level or as a cannabinoid in states where cannabis is legal. However, several studies have now shown that cannabis products sold online or in dispensaries are often labeled inaccurately, with both under- and over-reporting of THC and CBD content.28-30 Some CBD products have been found to have almost no CBD at all.29,30 The unreliability of product labeling makes it difficult to predict the effects of CBD products that are not subject to FDA purity standards for medications or dietary supplements. It also raises questions about the sources of CBD and the reliability of dosing in the studies discussed above.
Why might CBD work as an antipsychotic? Although CBD has minimal affinity for cannabinoid receptors, it appears to act as a partial agonist of dopamine D2 receptors and an agonist at 5-HT1A receptors, with overall effects that decrease mesolimbic dopamine activity.31,32 In addition, CBD increases the availability of the endogenous cannabinoid anandamide, which may have antipsychotic properties.14,33
Now that the FDA has approved CBD manufactured by a pharmaceutical company, should it be prescribed “off-label” for patients with schizophrenia? This is the “million dollar question,” with insufficient evidence to provide a clear answer. It should now be possible to prescribe FDA-approved CBD for off-label purposes, including the treatment of schizophrenia and other psychiatric disorders. No doubt, some clinicians are already doing so. This will predictably yield more anecdotal evidence about efficacy and adverse effects in the future, but there is not yet adequate evidence to support an FDA indication for CBD in schizophrenia. Additional studies of CBD for schizophrenia are ongoing.
Bottom Line
Cannabidiol (CBD) is becoming increasingly popular based on its purported health benefits, but the evidence supporting a therapeutic role in psychiatry is preliminary at best. Although CBD is now available by prescription as an FDA-approved drug for the treatment of 2 rare forms of epilepsy, its benefits in patients with schizophrenia are uncertain based on mixed results in clinical trials.
Related Resources
- Clinicaltrials.gov. Studies of “cannabidiol” and “schizophrenia.” U.S. National Library of Medicine. https://clinicaltrials.gov/ct2/results?cond=Schizophrenia&term=cannabidiol.
- Grinspoon P. Cannabidiol (CBD) – what we know and what we don’t. Harvard Health Blog. https://www.health.harvard.edu/blog/cannabidiol-cbd-what-we-know-and-what-wedont-2018082414476. Published August 24, 2018.
Drug Brand Names
Cannabidiol • Epidiolex
Dronabinol • Marinol
Risperidone • Risperdal
1. Pierre JM. Cannabis, synthetic cannabinoids, and psychosis risk: what the evidence says. Current Psychiatry. 2011;10(9):49-58.
2. Radhakrishan R, Wilkinson ST, D’Souza DC. Gone to pot – a review of the association between cannabis and psychosis. Front Psychiatry. 2014;5:54.
3. Pierre JM. Risks of increasingly potent cannabis: joint effects of potency and frequency. Current Psychiatry. 2016;16(2):14-20.
4. Hanna RC, Perez JM, Ghose S. Cannabis and development of dual diagnoses: a literature review. Am J Drug Alcohol Abuse. 2017;43(4):442-255.
5. Nunberg H, Kilmer B, Pacula RL, et al. An analysis of applicants presenting to a medical marijuana specialty practice in California. J Drug Policy Anal. 2011;4(1):1.
6. Wilkinson ST, Radhakrishnan, D’Souza DC. A systematic review of the evidence for medical marijuana in psychiatric indications. J Clin Psychiatry. 2016;77(8):1050-1064.
7. Tournebize J, Gibaja V, Kahn JP. Acute effects of synthetic cannabinoids: Update 2015. Subst Abus. 2016;38(3):344-366.
8. Crippa JA, Guimarães FS, Campos A, et al. Translational investigation of the therapeutic potential of cannabidiol (CBD): toward a new age. Front Immunol. 2018;9:2009.
9. Schwarz G, Karajgi B. Improvement in refractory psychosis with dronabinol: four case reports. J Clin Psychiatry. 2010;71(11):1552-1553.
10. Schwarz G, Karajgi B, McCarthy R. Synthetic delta-9-tetrahydrocannabinol (dronabinol) can improve the symptoms of schizophrenia. J Clin Psychopharmacol. 2009;29(3):255-258.
11. Meltzer HY, Arvanitis L, Bauer D, et al. Placebo-controlled evaluation of four novel compounds for the treatment of schizophrenia and schizoaffective disorder. Am J Psychiatry. 2004;161(6):975-984.
12. Boggs DL, Kelly DL, McMahon RP, et al. Rimonabant for neurocognition in schizophrenia: a 16-week double blind placebo controlled trial. Schizophr Res. 2012;134(2-3):207-210.
13. Kelly DL, Gorelick DA, Conley RR, et al. Effects of cannabinoid-1 receptor antagonist rimonabant on psychiatric symptoms in overweight people with schizophrenia: a randomized, double-blind, pilot study. J Clin Psychopharmacol. 2011;31(1):86-91.
14. Leweke FM, Mueller JK, Lange B, et al. Therapeutic potential of cannabinoids in psychosis. Biol Psychiatry. 2016;79(7):604-612.
15. Halperin A. What is CBD? The ‘miracle’ cannabis compound that doesn’t get you high. The Guardian. https://www.theguardian.com/society/2018/may/28/what-is-cbd-cannabidiol-cannabis-medical-uses. Published May 28, 2018. Accessed April 3, 2019.
16. Pierre J. Coca, cola, and cannabis: psychoactive drugs as beverages. Psychology Today (blog) Psych Unseen. https://www.psychologytoday.com/us/blog/psych-unseen/201810/coca-cola-and-cannabis-psychoactive-drugs-beverages. Published October 1, 2018. Accessed April 3, 2019.
17. U.S. Food and Drug Administration. FDA approves first drug comprised of an active ingredient derived from marijuana to treat rare, severe forms of epilepsy. FDA News Release. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm611046.htm. Published June 25, 2018. Accessed April 3, 2019.
18. Devinsky O, Cross JH, Laux L, et al. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med. 2017;376:2011-2020.
19. Thiele EA, March ED, French JA, et al. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391(10125):1085-1096.
20. Devinsky O, Patel AD, Cross JH, et al. Effect of cannabidiol on drop seizures in the Lennox-Gastaut syndrome. N Engl J Med. 2018;378:1888-1897.
21. Khoury JM, Neves MCLD, Rogue MAV, et al. Is there a role of cannabidiol in psychiatry? World J Biol Psychiatry. 2017:1-16.
22. Zuardi AW, Morais SL, Guimares FS, et al. Antipsychotic effect of cannabidiol. J Clin Psychiatry. 1995;56(10):485-486.
23. Zuardi AW, Hallak JEC, Dursun SM. Cannabidiol monotherapy for treatment-resistant schizophrenia. J Psychopharmacol. 2006;20(5):683-686.
24. Leweke FM, Piomelli D, Pahlisch F, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry. 2012;2:e94. doi: 10.1038/tp.2012.15.
25. McGuire P, Robson P, Cubala WJ, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018;175(3):225-231.
26. Boggs DL, Surti I, Gupta A, et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacol. 2018;235(7):1923-1932.
27. ElSohly MA, Mehmedic Z, Foster S, et al. Changes in cannabis potency over the last 2 decades (1995-2014): analysis of current data in the United States. Biol Psychiatry. 2016; 79(7):613-619.
28. Vandrey R, Raber JC, Raber ME, et al. Cannabinoid dose and label accuracy in edible medical cannabis products. JAMA. 2015;313(24):2491-2492.
29. Ruth AC, Gryniewicz-Ruzicka CM, Trehy ML, et al. Consistency of label claims of internet-purchased hemp oil and cannabis products as determined using IMS and LC-MS: a marketplace study. J Reg Sci. 2016;3:1-6.
30. Bonn-Miller MO, Loflin MJE, Thomas BF, et al. Labeling accuracy of cannabidiol extracts sold online. JAMA. 2017;318(17):1708-1709.
31. Seeman P. Cannabidiol is a partial agonist at dopamine D2High receptors, predicting its antipsychotic clinical dose. Transl Psychiatry. 2016;6(10):e920. doi: 10.1038/tp.2016.195.
32. Renard J, Norris C, Rushlow W, et al. Neuronal and molecular effects of cannabidiol on the mesolimbic dopamine system: implications for novel schizophrenia treatments. Neurosci Biobehav Rev. 2017;157-165.
33. Gururajan A, Malone DT. Does cannabidiol have a role in the treatment of schizophrenia? Schizophr Res. 2016;176(2-3):281-290.
Over the past few decades, it has become increasingly clear that cannabis use can increase the risk of developing a psychotic disorder and worsen the course of existing schizophrenia in a dose-dependent fashion.1-3 Beyond psychosis, although many patients with mental illness use cannabis for recreational purposes or as purported “self-medication,” currently available evidence suggests that marijuana is more likely to represent a harm than a benefit for psychiatric disorders4 (Box4-8). Our current state of knowledge therefore suggests that psychiatrists should caution their patients against using cannabis and prioritize interventions to reduce or discontinue use, especially among those with psychotic disorders.
Box
Data from California in 2006—a decade after the state’s legalization of “medical marijuana”—revealed that 23% of patients in a sample enrolled in medical marijuana clinics were receiving cannabis to treat a mental disorder.5 That was a striking statistic given the dearth of evidence to support a benefit of cannabis for psychiatric conditions at the time, leaving clinicians who provided the necessary recommendations to obtain medical marijuana largely unable to give informed consent about the risks and benefits, much less recommendations about specific products, routes of administration, or dosing. In 2019, we know considerably more about the interaction between cannabinoids and mental health, but research findings thus far warrant more caution than enthusiasm, with one recent review concluding that “whenever an association is observed between cannabis use and psychiatric disorders, the relationship is generally an adverse one.”4
Some critics have argued that the medical marijuana industry represents little more than a front for recreational use. In California and other states that have legalized recreational use, that claim has been rendered all but moot, although the public remains curious about the potential health benefits of cannabinoids and will likely continue to look to clinicians for advice. For those seeking guidance from evidence-based research, the existing state of knowledge can seem like a “Wild West” of anecdotal subjective reports, biased opinions, and uncontrolled clinical studies. Cannabis remains a Schedule I drug at the federal level, and quality clinical research has been limited to a relatively modest number of randomized controlled trials (RCTs), mostly involving FDA-approved cannabinoids rather than smoked cannabis. Randomized controlled trials that have involved smoked marijuana have generally involved low-potency delta-9-tetrahydrocannabinol (THC) cannabis that may not reflect the same therapeutic and adverse effects of the increasingly high potency cannabis now available on the street and in dispensaries.
In psychiatry, a few RCTs are underway exploring cannabis as a viable treatment for mental disorders (eg, posttraumatic stress disorder), but none have yet been completed or published. At best, retrospective studies to date have failed to support a consistent benefit of cannabis for any psychiatric disorder and at worst increasingly suggest a negative impact on psychotic, mood, and anxiety disorders.4,6 Meanwhile, synthetic cannabinoid receptor agonists (eg, “Spice” products) have come to represent a clear public health risk, with both medical and psychiatric toxicity.7
A more cautiously optimistic case for the therapeutic potential of cannabinoids in psychiatry could be made for cannabidiol (CBD), which may possess anxiolytic, antipsychotic, and neuroprotective properties.8 Based on its purported health benefits, it is possible that CBD may even gain widespread popularity as a food supplement. Because a pharmaceutically-manufactured form of CBD was recently FDA-approved for the treatment of seizures associated with Lennox-Gastaut syndrome and Dravet syndrome, off-label prescribing of CBD for psychiatric disorders can be anticipated. While there is not yet sufficient evidence about risks and benefits to justify CBD being recommended broadly in psychiatry, that same informational vacuum has not stopped eager patients from seeking approval for cannabis, and some physicians from providing it.
Despite that conclusion, because cannabis is classified as a Schedule I drug by the US Drug Enforcement Agency, clinical research investigating the risks and benefits of cannabis has been limited. It therefore remains possible that cannabis, or individual cannabinoids such as cannabidiol (CBD), may yet find a therapeutic niche in psychiatry. This article reviews evidence on CBD for the treatment of schizophrenia.
Cannabinergic drugs as potential antipsychotics
Although the bulk of evidence indicates a harmful effect of cannabis in individuals with or at risk for psychosis, there have been a few published cases of schizophrenia improving with dronabinol, an FDA-approved, synthetic form of delta-9-tetrahydrocannabinol (THC).9,10 THC is the constituent of cannabis that produces euphoric effects. These provocative findings have not been replicated in controlled clinical trials, but suggest at least the theoretical possibility of idiosyncratic benefits from THC for some individuals within the psychotic spectrum.
Still, given that most available evidence supports that THC has a harmful effect on psychosis and psychosis risk, researchers have instead performed randomized controlled trials (RCTs) to investigate a possible therapeutic role for medications that oppose the agonist effects of THC at cannabinoid type 1 (CB1) receptors. To date, 2 RCTs comparing rimonabant, a CB1 inverse agonist, with placebo (PLB) in patients with schizophrenia have failed to demonstrate any benefit for psychotic symptoms or cognitive deficits.11,12 A third trial examining rimonabant for people diagnosed with schizophrenia who were overweight found significant benefits for anxiety and depressive symptoms, but none for positive symptoms or the primary outcome of weight loss.13 While these results are discouraging, the role of THC in precipitating psychosis suggests that novel agents opposing the actions of THC on the cannabinoid system could have antipsychotic properties.14
Cannabidiol: An antipsychotic medication?
In contrast to THC, CBD has minimal euphorigenic properties and has recently been heralded in the popular press as a “miracle drug” with benefits for medical and psychiatric disorders alike.15 It has even been speculated that it could become a popular food supplement.16 In 2018, the FDA gave full approval to a pharmaceutically manufactured form of CBD (brand name: Epidiolex) as a novel treatment for 2 rare and severe forms of pediatric epilepsy, Lennox-Gastaut syndrome and Dravet syndrome,17 based on RCTs supporting its efficacy for these often refractory and life-threatening conditions.18-20
In psychiatry, there have not yet been enough robust clinical studies to support broad therapeutic claims for CBD as a treatment for any mental disorder.21 However, there is growing evidence that CBD has potential as an antipsychotic medication. In 1995, the first case report was published describing the efficacy of CBD, 1,500 mg/d, as standalone therapy in a single individual with schizophrenia.22 In 2006, the same research group followed up with a case series in which only 1 out of 3 patients with treatment-refractory schizophrenia improved with flexible dosing of CBD to a maximum dose of 1,280 mg/d.23
There have been 3 published RCTs exploring the efficacy of CBD in schizophrenia (Table24-26). The first study, published in 2012, included 39 adults with schizophrenia who were randomized to 800 mg/d of CBD or amisulpride (AMS), a second-generation antipsychotic that is popular in Europe but is not available in the United States.24 Over 4 weeks of randomized treatment, CBD resulted in as much improvement in overall symptoms and positive symptoms as AMS, and improvement of negative symptoms was significantly greater with CBD. Compared with patients treated with antipsychotic medication, patients who were treated with CBD had fewer extrapyramidal symptoms, less weight gain, and less prolactin elevation. This initial trial suggests that CBD might be as efficacious in schizophrenia as antipsychotic medication, without its burdensome adverse effects. However, this is the only RCT of CBD monotherapy published to date.

Continue to: Two other recently published RCTs...
Two other recently published RCTs compared CBD with PLB as add-on therapy to antipsychotics. McGuire et al25 compared CBD, 1,000 mg/d, to PLB over 6 weeks in 88 patients with schizophrenia. Positive symptom improvement was statistically greater with CBD than with PLB, although the magnitude of clinical change was modest (using the Positive and Negative Syndrome Scale [PANSS] positive symptom subscale: −3.2 points for CBD vs −1.7 points for PLB). Changes in PANSS total score and subscales for general and negative symptoms were not significantly different between treatment groups. There was also no significant difference in overall change in neurocognitive symptoms, although post-hoc analysis revealed significantly greater improvement in motor speed for patients treated with CBD. More than twice the number of patients treated with CBD were rated as “much improved” by the Clinical Global Impressions scale compared with patients treated with PLB, but this was not a statistically significant finding, and most patients experienced only “minimal” or “no improvement.” In terms of adverse events, there were no significant differences between patients in the CBD and PLB groups. Although this study is technically “positive” for CBD and suggests minimal adverse effects, it is not clear whether the statistically significant positive symptom improvements (+1.5 PANSS points for CBD over PLB) were clinically significant.
The most recently published placebo-controlled RCT of CBD as add-on therapy to antipsychotic medication included 36 patients with schizophrenia treated over 6 weeks.26 In this study, there was no benefit of CBD, 600 mg/d, on any PANSS score outcome (total, general, positive, or negative symptoms). For the primary outcome of the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery, there were no significant drug × time effects, and post-hoc analyses showed that only patients treated with PLB improved with time. Sedation was more common among patients treated with CBD compared with PLB.
Making sense of the data
There have been mixed results from the few case reports and 3 RCTs of patients with schizophrenia who were treated with CBD. How can we resolve these disparate findings? A few possible interpretations of the data that warrant clarification through additional research include:
Dosing. In the first case report with positive results, CBD was dosed at 1,500 mg/d,22 whereas in the subsequent case series with mixed results, the maximum allowable dose of CBD was 1,280 mg/d.23 Likewise, in the RCTs, positive results were found when CBD was dosed at 800 to 1,000 mg/d,24,25 but not at 600 mg/d.26 The efficacy of CBD for schizophrenia might depend on higher doses.
Treatment resistance. In the second case series in which only 1 out of 3 patients responded to treatment with CBD,23 the patients had demonstrated previous nonresponse to at least 2 first-generation antipsychotics (FGAs) and risperidone, 6 mg/d. In the RCTs, all patients were antipsychotic-responsive.24-26 Cannabidiol may not be as effective for patients with treatment-refractory schizophrenia as it is for patients with schizophrenia who respond to antipsychotics.
Continue to: Clinical stability
Clinical stability. Within the RCTs, the greatest response was observed in the study that enrolled patients who were hospitalized with acute symptoms of schizophrenia.23 In the 2 studies that found either modest or no benefit with CBD, the patients had been stabilized on antipsychotic medications prior to randomization. Cannabidiol may offer limited benefit as add-on therapy to patients who have already responded to antipsychotic treatment, where there is “less room” for additional improvement.
Monotherapy. Both the case reports22,23 and the RCT with the most robust positive findings24 involved treatment with CBD as monotherapy. For some patients with schizophrenia, CBD might be effective as standalone therapy as an alternative to antipsychotics that is better tolerated. Adding CBD to antipsychotic therapy might be redundant and therefore less effective.
Answering questions about CBD
Cannabidiol is becoming increasingly popular for its purported health benefits. The mixed results of the few studies published on CBD for schizophrenia place clinicians in a difficult position when attempting to answer questions about how cannabinoids might fit into treatment of patients with psychosis. Consider the following:
Is cannabis helpful for patients with schizophrenia? No. Aside from the few case reports suggesting that FDA-approved THC (dronabinol) can improve symptoms in some patients,9,10 most of the evidence from anecdotal reports and both experimental and observational studies indicate that cannabis, THC, and synthetic cannabinoids have a harmful effect in patients with or at risk for psychosis.1-3
If you are considering recommending some form of cannabis to patients with schizophrenia, what kind should you recommend? Recommending or encouraging cannabis use for patients with psychosis is ill-advised. Although certain types of cannabis might contain more THC (eg, Cannabis indica vs Cannabis sativa) or variable amounts of CBD, in general the amount of CBD in whole leaf cannabis is minimal, with the ratio of THC to CBD increasingly significantly over the past decade.3,27 Most forms of cannabis should therefore be avoided by individuals with or at risk for psychotic disorders.
Continue to: What about CBD oil and other CBD products sold in dispensaries?
What about CBD oil and other CBD products sold in dispensaries? Cannabidiol is increasingly available in various forms based on its ability to be designated as a legal hemp product (containing <0.3% THC) at the federal level or as a cannabinoid in states where cannabis is legal. However, several studies have now shown that cannabis products sold online or in dispensaries are often labeled inaccurately, with both under- and over-reporting of THC and CBD content.28-30 Some CBD products have been found to have almost no CBD at all.29,30 The unreliability of product labeling makes it difficult to predict the effects of CBD products that are not subject to FDA purity standards for medications or dietary supplements. It also raises questions about the sources of CBD and the reliability of dosing in the studies discussed above.
Why might CBD work as an antipsychotic? Although CBD has minimal affinity for cannabinoid receptors, it appears to act as a partial agonist of dopamine D2 receptors and an agonist at 5-HT1A receptors, with overall effects that decrease mesolimbic dopamine activity.31,32 In addition, CBD increases the availability of the endogenous cannabinoid anandamide, which may have antipsychotic properties.14,33
Now that the FDA has approved CBD manufactured by a pharmaceutical company, should it be prescribed “off-label” for patients with schizophrenia? This is the “million dollar question,” with insufficient evidence to provide a clear answer. It should now be possible to prescribe FDA-approved CBD for off-label purposes, including the treatment of schizophrenia and other psychiatric disorders. No doubt, some clinicians are already doing so. This will predictably yield more anecdotal evidence about efficacy and adverse effects in the future, but there is not yet adequate evidence to support an FDA indication for CBD in schizophrenia. Additional studies of CBD for schizophrenia are ongoing.
Bottom Line
Cannabidiol (CBD) is becoming increasingly popular based on its purported health benefits, but the evidence supporting a therapeutic role in psychiatry is preliminary at best. Although CBD is now available by prescription as an FDA-approved drug for the treatment of 2 rare forms of epilepsy, its benefits in patients with schizophrenia are uncertain based on mixed results in clinical trials.
Related Resources
- Clinicaltrials.gov. Studies of “cannabidiol” and “schizophrenia.” U.S. National Library of Medicine. https://clinicaltrials.gov/ct2/results?cond=Schizophrenia&term=cannabidiol.
- Grinspoon P. Cannabidiol (CBD) – what we know and what we don’t. Harvard Health Blog. https://www.health.harvard.edu/blog/cannabidiol-cbd-what-we-know-and-what-wedont-2018082414476. Published August 24, 2018.
Drug Brand Names
Cannabidiol • Epidiolex
Dronabinol • Marinol
Risperidone • Risperdal
Over the past few decades, it has become increasingly clear that cannabis use can increase the risk of developing a psychotic disorder and worsen the course of existing schizophrenia in a dose-dependent fashion.1-3 Beyond psychosis, although many patients with mental illness use cannabis for recreational purposes or as purported “self-medication,” currently available evidence suggests that marijuana is more likely to represent a harm than a benefit for psychiatric disorders4 (Box4-8). Our current state of knowledge therefore suggests that psychiatrists should caution their patients against using cannabis and prioritize interventions to reduce or discontinue use, especially among those with psychotic disorders.
Box
Data from California in 2006—a decade after the state’s legalization of “medical marijuana”—revealed that 23% of patients in a sample enrolled in medical marijuana clinics were receiving cannabis to treat a mental disorder.5 That was a striking statistic given the dearth of evidence to support a benefit of cannabis for psychiatric conditions at the time, leaving clinicians who provided the necessary recommendations to obtain medical marijuana largely unable to give informed consent about the risks and benefits, much less recommendations about specific products, routes of administration, or dosing. In 2019, we know considerably more about the interaction between cannabinoids and mental health, but research findings thus far warrant more caution than enthusiasm, with one recent review concluding that “whenever an association is observed between cannabis use and psychiatric disorders, the relationship is generally an adverse one.”4
Some critics have argued that the medical marijuana industry represents little more than a front for recreational use. In California and other states that have legalized recreational use, that claim has been rendered all but moot, although the public remains curious about the potential health benefits of cannabinoids and will likely continue to look to clinicians for advice. For those seeking guidance from evidence-based research, the existing state of knowledge can seem like a “Wild West” of anecdotal subjective reports, biased opinions, and uncontrolled clinical studies. Cannabis remains a Schedule I drug at the federal level, and quality clinical research has been limited to a relatively modest number of randomized controlled trials (RCTs), mostly involving FDA-approved cannabinoids rather than smoked cannabis. Randomized controlled trials that have involved smoked marijuana have generally involved low-potency delta-9-tetrahydrocannabinol (THC) cannabis that may not reflect the same therapeutic and adverse effects of the increasingly high potency cannabis now available on the street and in dispensaries.
In psychiatry, a few RCTs are underway exploring cannabis as a viable treatment for mental disorders (eg, posttraumatic stress disorder), but none have yet been completed or published. At best, retrospective studies to date have failed to support a consistent benefit of cannabis for any psychiatric disorder and at worst increasingly suggest a negative impact on psychotic, mood, and anxiety disorders.4,6 Meanwhile, synthetic cannabinoid receptor agonists (eg, “Spice” products) have come to represent a clear public health risk, with both medical and psychiatric toxicity.7
A more cautiously optimistic case for the therapeutic potential of cannabinoids in psychiatry could be made for cannabidiol (CBD), which may possess anxiolytic, antipsychotic, and neuroprotective properties.8 Based on its purported health benefits, it is possible that CBD may even gain widespread popularity as a food supplement. Because a pharmaceutically-manufactured form of CBD was recently FDA-approved for the treatment of seizures associated with Lennox-Gastaut syndrome and Dravet syndrome, off-label prescribing of CBD for psychiatric disorders can be anticipated. While there is not yet sufficient evidence about risks and benefits to justify CBD being recommended broadly in psychiatry, that same informational vacuum has not stopped eager patients from seeking approval for cannabis, and some physicians from providing it.
Despite that conclusion, because cannabis is classified as a Schedule I drug by the US Drug Enforcement Agency, clinical research investigating the risks and benefits of cannabis has been limited. It therefore remains possible that cannabis, or individual cannabinoids such as cannabidiol (CBD), may yet find a therapeutic niche in psychiatry. This article reviews evidence on CBD for the treatment of schizophrenia.
Cannabinergic drugs as potential antipsychotics
Although the bulk of evidence indicates a harmful effect of cannabis in individuals with or at risk for psychosis, there have been a few published cases of schizophrenia improving with dronabinol, an FDA-approved, synthetic form of delta-9-tetrahydrocannabinol (THC).9,10 THC is the constituent of cannabis that produces euphoric effects. These provocative findings have not been replicated in controlled clinical trials, but suggest at least the theoretical possibility of idiosyncratic benefits from THC for some individuals within the psychotic spectrum.
Still, given that most available evidence supports that THC has a harmful effect on psychosis and psychosis risk, researchers have instead performed randomized controlled trials (RCTs) to investigate a possible therapeutic role for medications that oppose the agonist effects of THC at cannabinoid type 1 (CB1) receptors. To date, 2 RCTs comparing rimonabant, a CB1 inverse agonist, with placebo (PLB) in patients with schizophrenia have failed to demonstrate any benefit for psychotic symptoms or cognitive deficits.11,12 A third trial examining rimonabant for people diagnosed with schizophrenia who were overweight found significant benefits for anxiety and depressive symptoms, but none for positive symptoms or the primary outcome of weight loss.13 While these results are discouraging, the role of THC in precipitating psychosis suggests that novel agents opposing the actions of THC on the cannabinoid system could have antipsychotic properties.14
Cannabidiol: An antipsychotic medication?
In contrast to THC, CBD has minimal euphorigenic properties and has recently been heralded in the popular press as a “miracle drug” with benefits for medical and psychiatric disorders alike.15 It has even been speculated that it could become a popular food supplement.16 In 2018, the FDA gave full approval to a pharmaceutically manufactured form of CBD (brand name: Epidiolex) as a novel treatment for 2 rare and severe forms of pediatric epilepsy, Lennox-Gastaut syndrome and Dravet syndrome,17 based on RCTs supporting its efficacy for these often refractory and life-threatening conditions.18-20
In psychiatry, there have not yet been enough robust clinical studies to support broad therapeutic claims for CBD as a treatment for any mental disorder.21 However, there is growing evidence that CBD has potential as an antipsychotic medication. In 1995, the first case report was published describing the efficacy of CBD, 1,500 mg/d, as standalone therapy in a single individual with schizophrenia.22 In 2006, the same research group followed up with a case series in which only 1 out of 3 patients with treatment-refractory schizophrenia improved with flexible dosing of CBD to a maximum dose of 1,280 mg/d.23
There have been 3 published RCTs exploring the efficacy of CBD in schizophrenia (Table24-26). The first study, published in 2012, included 39 adults with schizophrenia who were randomized to 800 mg/d of CBD or amisulpride (AMS), a second-generation antipsychotic that is popular in Europe but is not available in the United States.24 Over 4 weeks of randomized treatment, CBD resulted in as much improvement in overall symptoms and positive symptoms as AMS, and improvement of negative symptoms was significantly greater with CBD. Compared with patients treated with antipsychotic medication, patients who were treated with CBD had fewer extrapyramidal symptoms, less weight gain, and less prolactin elevation. This initial trial suggests that CBD might be as efficacious in schizophrenia as antipsychotic medication, without its burdensome adverse effects. However, this is the only RCT of CBD monotherapy published to date.

Continue to: Two other recently published RCTs...
Two other recently published RCTs compared CBD with PLB as add-on therapy to antipsychotics. McGuire et al25 compared CBD, 1,000 mg/d, to PLB over 6 weeks in 88 patients with schizophrenia. Positive symptom improvement was statistically greater with CBD than with PLB, although the magnitude of clinical change was modest (using the Positive and Negative Syndrome Scale [PANSS] positive symptom subscale: −3.2 points for CBD vs −1.7 points for PLB). Changes in PANSS total score and subscales for general and negative symptoms were not significantly different between treatment groups. There was also no significant difference in overall change in neurocognitive symptoms, although post-hoc analysis revealed significantly greater improvement in motor speed for patients treated with CBD. More than twice the number of patients treated with CBD were rated as “much improved” by the Clinical Global Impressions scale compared with patients treated with PLB, but this was not a statistically significant finding, and most patients experienced only “minimal” or “no improvement.” In terms of adverse events, there were no significant differences between patients in the CBD and PLB groups. Although this study is technically “positive” for CBD and suggests minimal adverse effects, it is not clear whether the statistically significant positive symptom improvements (+1.5 PANSS points for CBD over PLB) were clinically significant.
The most recently published placebo-controlled RCT of CBD as add-on therapy to antipsychotic medication included 36 patients with schizophrenia treated over 6 weeks.26 In this study, there was no benefit of CBD, 600 mg/d, on any PANSS score outcome (total, general, positive, or negative symptoms). For the primary outcome of the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery, there were no significant drug × time effects, and post-hoc analyses showed that only patients treated with PLB improved with time. Sedation was more common among patients treated with CBD compared with PLB.
Making sense of the data
There have been mixed results from the few case reports and 3 RCTs of patients with schizophrenia who were treated with CBD. How can we resolve these disparate findings? A few possible interpretations of the data that warrant clarification through additional research include:
Dosing. In the first case report with positive results, CBD was dosed at 1,500 mg/d,22 whereas in the subsequent case series with mixed results, the maximum allowable dose of CBD was 1,280 mg/d.23 Likewise, in the RCTs, positive results were found when CBD was dosed at 800 to 1,000 mg/d,24,25 but not at 600 mg/d.26 The efficacy of CBD for schizophrenia might depend on higher doses.
Treatment resistance. In the second case series in which only 1 out of 3 patients responded to treatment with CBD,23 the patients had demonstrated previous nonresponse to at least 2 first-generation antipsychotics (FGAs) and risperidone, 6 mg/d. In the RCTs, all patients were antipsychotic-responsive.24-26 Cannabidiol may not be as effective for patients with treatment-refractory schizophrenia as it is for patients with schizophrenia who respond to antipsychotics.
Continue to: Clinical stability
Clinical stability. Within the RCTs, the greatest response was observed in the study that enrolled patients who were hospitalized with acute symptoms of schizophrenia.23 In the 2 studies that found either modest or no benefit with CBD, the patients had been stabilized on antipsychotic medications prior to randomization. Cannabidiol may offer limited benefit as add-on therapy to patients who have already responded to antipsychotic treatment, where there is “less room” for additional improvement.
Monotherapy. Both the case reports22,23 and the RCT with the most robust positive findings24 involved treatment with CBD as monotherapy. For some patients with schizophrenia, CBD might be effective as standalone therapy as an alternative to antipsychotics that is better tolerated. Adding CBD to antipsychotic therapy might be redundant and therefore less effective.
Answering questions about CBD
Cannabidiol is becoming increasingly popular for its purported health benefits. The mixed results of the few studies published on CBD for schizophrenia place clinicians in a difficult position when attempting to answer questions about how cannabinoids might fit into treatment of patients with psychosis. Consider the following:
Is cannabis helpful for patients with schizophrenia? No. Aside from the few case reports suggesting that FDA-approved THC (dronabinol) can improve symptoms in some patients,9,10 most of the evidence from anecdotal reports and both experimental and observational studies indicate that cannabis, THC, and synthetic cannabinoids have a harmful effect in patients with or at risk for psychosis.1-3
If you are considering recommending some form of cannabis to patients with schizophrenia, what kind should you recommend? Recommending or encouraging cannabis use for patients with psychosis is ill-advised. Although certain types of cannabis might contain more THC (eg, Cannabis indica vs Cannabis sativa) or variable amounts of CBD, in general the amount of CBD in whole leaf cannabis is minimal, with the ratio of THC to CBD increasingly significantly over the past decade.3,27 Most forms of cannabis should therefore be avoided by individuals with or at risk for psychotic disorders.
Continue to: What about CBD oil and other CBD products sold in dispensaries?
What about CBD oil and other CBD products sold in dispensaries? Cannabidiol is increasingly available in various forms based on its ability to be designated as a legal hemp product (containing <0.3% THC) at the federal level or as a cannabinoid in states where cannabis is legal. However, several studies have now shown that cannabis products sold online or in dispensaries are often labeled inaccurately, with both under- and over-reporting of THC and CBD content.28-30 Some CBD products have been found to have almost no CBD at all.29,30 The unreliability of product labeling makes it difficult to predict the effects of CBD products that are not subject to FDA purity standards for medications or dietary supplements. It also raises questions about the sources of CBD and the reliability of dosing in the studies discussed above.
Why might CBD work as an antipsychotic? Although CBD has minimal affinity for cannabinoid receptors, it appears to act as a partial agonist of dopamine D2 receptors and an agonist at 5-HT1A receptors, with overall effects that decrease mesolimbic dopamine activity.31,32 In addition, CBD increases the availability of the endogenous cannabinoid anandamide, which may have antipsychotic properties.14,33
Now that the FDA has approved CBD manufactured by a pharmaceutical company, should it be prescribed “off-label” for patients with schizophrenia? This is the “million dollar question,” with insufficient evidence to provide a clear answer. It should now be possible to prescribe FDA-approved CBD for off-label purposes, including the treatment of schizophrenia and other psychiatric disorders. No doubt, some clinicians are already doing so. This will predictably yield more anecdotal evidence about efficacy and adverse effects in the future, but there is not yet adequate evidence to support an FDA indication for CBD in schizophrenia. Additional studies of CBD for schizophrenia are ongoing.
Bottom Line
Cannabidiol (CBD) is becoming increasingly popular based on its purported health benefits, but the evidence supporting a therapeutic role in psychiatry is preliminary at best. Although CBD is now available by prescription as an FDA-approved drug for the treatment of 2 rare forms of epilepsy, its benefits in patients with schizophrenia are uncertain based on mixed results in clinical trials.
Related Resources
- Clinicaltrials.gov. Studies of “cannabidiol” and “schizophrenia.” U.S. National Library of Medicine. https://clinicaltrials.gov/ct2/results?cond=Schizophrenia&term=cannabidiol.
- Grinspoon P. Cannabidiol (CBD) – what we know and what we don’t. Harvard Health Blog. https://www.health.harvard.edu/blog/cannabidiol-cbd-what-we-know-and-what-wedont-2018082414476. Published August 24, 2018.
Drug Brand Names
Cannabidiol • Epidiolex
Dronabinol • Marinol
Risperidone • Risperdal
1. Pierre JM. Cannabis, synthetic cannabinoids, and psychosis risk: what the evidence says. Current Psychiatry. 2011;10(9):49-58.
2. Radhakrishan R, Wilkinson ST, D’Souza DC. Gone to pot – a review of the association between cannabis and psychosis. Front Psychiatry. 2014;5:54.
3. Pierre JM. Risks of increasingly potent cannabis: joint effects of potency and frequency. Current Psychiatry. 2016;16(2):14-20.
4. Hanna RC, Perez JM, Ghose S. Cannabis and development of dual diagnoses: a literature review. Am J Drug Alcohol Abuse. 2017;43(4):442-255.
5. Nunberg H, Kilmer B, Pacula RL, et al. An analysis of applicants presenting to a medical marijuana specialty practice in California. J Drug Policy Anal. 2011;4(1):1.
6. Wilkinson ST, Radhakrishnan, D’Souza DC. A systematic review of the evidence for medical marijuana in psychiatric indications. J Clin Psychiatry. 2016;77(8):1050-1064.
7. Tournebize J, Gibaja V, Kahn JP. Acute effects of synthetic cannabinoids: Update 2015. Subst Abus. 2016;38(3):344-366.
8. Crippa JA, Guimarães FS, Campos A, et al. Translational investigation of the therapeutic potential of cannabidiol (CBD): toward a new age. Front Immunol. 2018;9:2009.
9. Schwarz G, Karajgi B. Improvement in refractory psychosis with dronabinol: four case reports. J Clin Psychiatry. 2010;71(11):1552-1553.
10. Schwarz G, Karajgi B, McCarthy R. Synthetic delta-9-tetrahydrocannabinol (dronabinol) can improve the symptoms of schizophrenia. J Clin Psychopharmacol. 2009;29(3):255-258.
11. Meltzer HY, Arvanitis L, Bauer D, et al. Placebo-controlled evaluation of four novel compounds for the treatment of schizophrenia and schizoaffective disorder. Am J Psychiatry. 2004;161(6):975-984.
12. Boggs DL, Kelly DL, McMahon RP, et al. Rimonabant for neurocognition in schizophrenia: a 16-week double blind placebo controlled trial. Schizophr Res. 2012;134(2-3):207-210.
13. Kelly DL, Gorelick DA, Conley RR, et al. Effects of cannabinoid-1 receptor antagonist rimonabant on psychiatric symptoms in overweight people with schizophrenia: a randomized, double-blind, pilot study. J Clin Psychopharmacol. 2011;31(1):86-91.
14. Leweke FM, Mueller JK, Lange B, et al. Therapeutic potential of cannabinoids in psychosis. Biol Psychiatry. 2016;79(7):604-612.
15. Halperin A. What is CBD? The ‘miracle’ cannabis compound that doesn’t get you high. The Guardian. https://www.theguardian.com/society/2018/may/28/what-is-cbd-cannabidiol-cannabis-medical-uses. Published May 28, 2018. Accessed April 3, 2019.
16. Pierre J. Coca, cola, and cannabis: psychoactive drugs as beverages. Psychology Today (blog) Psych Unseen. https://www.psychologytoday.com/us/blog/psych-unseen/201810/coca-cola-and-cannabis-psychoactive-drugs-beverages. Published October 1, 2018. Accessed April 3, 2019.
17. U.S. Food and Drug Administration. FDA approves first drug comprised of an active ingredient derived from marijuana to treat rare, severe forms of epilepsy. FDA News Release. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm611046.htm. Published June 25, 2018. Accessed April 3, 2019.
18. Devinsky O, Cross JH, Laux L, et al. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med. 2017;376:2011-2020.
19. Thiele EA, March ED, French JA, et al. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391(10125):1085-1096.
20. Devinsky O, Patel AD, Cross JH, et al. Effect of cannabidiol on drop seizures in the Lennox-Gastaut syndrome. N Engl J Med. 2018;378:1888-1897.
21. Khoury JM, Neves MCLD, Rogue MAV, et al. Is there a role of cannabidiol in psychiatry? World J Biol Psychiatry. 2017:1-16.
22. Zuardi AW, Morais SL, Guimares FS, et al. Antipsychotic effect of cannabidiol. J Clin Psychiatry. 1995;56(10):485-486.
23. Zuardi AW, Hallak JEC, Dursun SM. Cannabidiol monotherapy for treatment-resistant schizophrenia. J Psychopharmacol. 2006;20(5):683-686.
24. Leweke FM, Piomelli D, Pahlisch F, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry. 2012;2:e94. doi: 10.1038/tp.2012.15.
25. McGuire P, Robson P, Cubala WJ, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018;175(3):225-231.
26. Boggs DL, Surti I, Gupta A, et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacol. 2018;235(7):1923-1932.
27. ElSohly MA, Mehmedic Z, Foster S, et al. Changes in cannabis potency over the last 2 decades (1995-2014): analysis of current data in the United States. Biol Psychiatry. 2016; 79(7):613-619.
28. Vandrey R, Raber JC, Raber ME, et al. Cannabinoid dose and label accuracy in edible medical cannabis products. JAMA. 2015;313(24):2491-2492.
29. Ruth AC, Gryniewicz-Ruzicka CM, Trehy ML, et al. Consistency of label claims of internet-purchased hemp oil and cannabis products as determined using IMS and LC-MS: a marketplace study. J Reg Sci. 2016;3:1-6.
30. Bonn-Miller MO, Loflin MJE, Thomas BF, et al. Labeling accuracy of cannabidiol extracts sold online. JAMA. 2017;318(17):1708-1709.
31. Seeman P. Cannabidiol is a partial agonist at dopamine D2High receptors, predicting its antipsychotic clinical dose. Transl Psychiatry. 2016;6(10):e920. doi: 10.1038/tp.2016.195.
32. Renard J, Norris C, Rushlow W, et al. Neuronal and molecular effects of cannabidiol on the mesolimbic dopamine system: implications for novel schizophrenia treatments. Neurosci Biobehav Rev. 2017;157-165.
33. Gururajan A, Malone DT. Does cannabidiol have a role in the treatment of schizophrenia? Schizophr Res. 2016;176(2-3):281-290.
1. Pierre JM. Cannabis, synthetic cannabinoids, and psychosis risk: what the evidence says. Current Psychiatry. 2011;10(9):49-58.
2. Radhakrishan R, Wilkinson ST, D’Souza DC. Gone to pot – a review of the association between cannabis and psychosis. Front Psychiatry. 2014;5:54.
3. Pierre JM. Risks of increasingly potent cannabis: joint effects of potency and frequency. Current Psychiatry. 2016;16(2):14-20.
4. Hanna RC, Perez JM, Ghose S. Cannabis and development of dual diagnoses: a literature review. Am J Drug Alcohol Abuse. 2017;43(4):442-255.
5. Nunberg H, Kilmer B, Pacula RL, et al. An analysis of applicants presenting to a medical marijuana specialty practice in California. J Drug Policy Anal. 2011;4(1):1.
6. Wilkinson ST, Radhakrishnan, D’Souza DC. A systematic review of the evidence for medical marijuana in psychiatric indications. J Clin Psychiatry. 2016;77(8):1050-1064.
7. Tournebize J, Gibaja V, Kahn JP. Acute effects of synthetic cannabinoids: Update 2015. Subst Abus. 2016;38(3):344-366.
8. Crippa JA, Guimarães FS, Campos A, et al. Translational investigation of the therapeutic potential of cannabidiol (CBD): toward a new age. Front Immunol. 2018;9:2009.
9. Schwarz G, Karajgi B. Improvement in refractory psychosis with dronabinol: four case reports. J Clin Psychiatry. 2010;71(11):1552-1553.
10. Schwarz G, Karajgi B, McCarthy R. Synthetic delta-9-tetrahydrocannabinol (dronabinol) can improve the symptoms of schizophrenia. J Clin Psychopharmacol. 2009;29(3):255-258.
11. Meltzer HY, Arvanitis L, Bauer D, et al. Placebo-controlled evaluation of four novel compounds for the treatment of schizophrenia and schizoaffective disorder. Am J Psychiatry. 2004;161(6):975-984.
12. Boggs DL, Kelly DL, McMahon RP, et al. Rimonabant for neurocognition in schizophrenia: a 16-week double blind placebo controlled trial. Schizophr Res. 2012;134(2-3):207-210.
13. Kelly DL, Gorelick DA, Conley RR, et al. Effects of cannabinoid-1 receptor antagonist rimonabant on psychiatric symptoms in overweight people with schizophrenia: a randomized, double-blind, pilot study. J Clin Psychopharmacol. 2011;31(1):86-91.
14. Leweke FM, Mueller JK, Lange B, et al. Therapeutic potential of cannabinoids in psychosis. Biol Psychiatry. 2016;79(7):604-612.
15. Halperin A. What is CBD? The ‘miracle’ cannabis compound that doesn’t get you high. The Guardian. https://www.theguardian.com/society/2018/may/28/what-is-cbd-cannabidiol-cannabis-medical-uses. Published May 28, 2018. Accessed April 3, 2019.
16. Pierre J. Coca, cola, and cannabis: psychoactive drugs as beverages. Psychology Today (blog) Psych Unseen. https://www.psychologytoday.com/us/blog/psych-unseen/201810/coca-cola-and-cannabis-psychoactive-drugs-beverages. Published October 1, 2018. Accessed April 3, 2019.
17. U.S. Food and Drug Administration. FDA approves first drug comprised of an active ingredient derived from marijuana to treat rare, severe forms of epilepsy. FDA News Release. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm611046.htm. Published June 25, 2018. Accessed April 3, 2019.
18. Devinsky O, Cross JH, Laux L, et al. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med. 2017;376:2011-2020.
19. Thiele EA, March ED, French JA, et al. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391(10125):1085-1096.
20. Devinsky O, Patel AD, Cross JH, et al. Effect of cannabidiol on drop seizures in the Lennox-Gastaut syndrome. N Engl J Med. 2018;378:1888-1897.
21. Khoury JM, Neves MCLD, Rogue MAV, et al. Is there a role of cannabidiol in psychiatry? World J Biol Psychiatry. 2017:1-16.
22. Zuardi AW, Morais SL, Guimares FS, et al. Antipsychotic effect of cannabidiol. J Clin Psychiatry. 1995;56(10):485-486.
23. Zuardi AW, Hallak JEC, Dursun SM. Cannabidiol monotherapy for treatment-resistant schizophrenia. J Psychopharmacol. 2006;20(5):683-686.
24. Leweke FM, Piomelli D, Pahlisch F, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry. 2012;2:e94. doi: 10.1038/tp.2012.15.
25. McGuire P, Robson P, Cubala WJ, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018;175(3):225-231.
26. Boggs DL, Surti I, Gupta A, et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacol. 2018;235(7):1923-1932.
27. ElSohly MA, Mehmedic Z, Foster S, et al. Changes in cannabis potency over the last 2 decades (1995-2014): analysis of current data in the United States. Biol Psychiatry. 2016; 79(7):613-619.
28. Vandrey R, Raber JC, Raber ME, et al. Cannabinoid dose and label accuracy in edible medical cannabis products. JAMA. 2015;313(24):2491-2492.
29. Ruth AC, Gryniewicz-Ruzicka CM, Trehy ML, et al. Consistency of label claims of internet-purchased hemp oil and cannabis products as determined using IMS and LC-MS: a marketplace study. J Reg Sci. 2016;3:1-6.
30. Bonn-Miller MO, Loflin MJE, Thomas BF, et al. Labeling accuracy of cannabidiol extracts sold online. JAMA. 2017;318(17):1708-1709.
31. Seeman P. Cannabidiol is a partial agonist at dopamine D2High receptors, predicting its antipsychotic clinical dose. Transl Psychiatry. 2016;6(10):e920. doi: 10.1038/tp.2016.195.
32. Renard J, Norris C, Rushlow W, et al. Neuronal and molecular effects of cannabidiol on the mesolimbic dopamine system: implications for novel schizophrenia treatments. Neurosci Biobehav Rev. 2017;157-165.
33. Gururajan A, Malone DT. Does cannabidiol have a role in the treatment of schizophrenia? Schizophr Res. 2016;176(2-3):281-290.
Pharmacologic Management of Malignant Bowel Obstruction: When Surgery Is Not an Option
Malignant bowel obstruction (MBO) is a catastrophic complication of cancer that often requires hospitalization and a multidisciplinary approach in its management. Hospitalists frequently collaborate with such specialties as Hematology/Oncology, Surgery, Palliative Medicine, and Interventional Radiology in arriving at a treatment plan.
Initial management is focused on hydration, bowel rest and decompression via nasogastric (NG) tube. Surgical resection or endoscopic stenting should be considered early.1 However, patients who present in the terminal stages may be poor candidates for these options due to diminished functional status, multiple areas of obstruction, complicated anatomy limiting intervention, or an associated large volume of ascites.
Presence of inoperable MBO portends a poor prognosis, often measured in weeks.2 Presentation often occurs in the context of a sentinel hospitalization, signifying a shift in disease course.3,4 It is essential for hospitalists to be familiar with noninvasive therapies for inoperable MBO given the increasing role of hospitalists in providing inpatient palliative care. Palliative pharmacologic management of MBO can reduce symptom burden during these terminal stages and will be the focus of this paper.
BACKGROUND AND PATHOPHYSIOLOGY
Malignant bowel obstruction occurs in about 3%-15% of patients with cancer.2 A consensus definition of MBO established the following specific criteria: (1) clinical evidence of bowel obstruction, (2) obstruction distal to the ligament of Treitz, and (3) the presence of primary intra-abdominal cancer with incurable disease or extra-abdominal cancer with peritoneal involvement.5 The most common malignancies are gastric, colorectal, and ovarian in origin.1,2 The most common extra-abdominal malignancies associated with MBO are breast, melanoma, and lung. MBO is most frequently diagnosed during the advanced stages of cancer.2 The obstruction can involve a partial or total blockage of the small or large intestine from either an intrinsic or extrinsic source. Peristalsis may also be impaired via direct tumor infiltration of the intestinal walls or within the enteric nervous system or celiac plexus. Other etiologies of MBO include peritoneal carcinomatosis and radiation-induced fibrosis.1,6 The obstruction can occur at a single level or involve multiple areas, which usually precludes surgical intervention.2
Symptoms of MBO can be insidious in onset and take several weeks to manifest. The most prevalent symptoms are nausea, vomiting, constipation, abdominal pain, and distension.2,6 The intermittent pattern of symptoms may evolve into continuous episodes with spontaneous remission in between. The etiology of symptoms can be attributed to distension proximal to the site of obstruction with concomitantly increased gastrointestinal and pancreaticobiliary secretions.
The distension creates a “hypertensive state” in the intestinal lumen causing enterochromaffin cells to release serotonin which activates the enteric nervous system and its effectors including substance P, nitric oxide, acetylcholine, somatostatin, and vasoactive intestinal peptide (VIP). These neurotransmitters stimulate the secretomotor actions that cause hypersecretion of mucus from cells of the intestinal crypts. Additional water and sodium secretions accumulate due to the expanded surface area of the bowel.1,2 Overloaded with luminal contents, the bowel attempts to overcome the obstruction by contracting, which leads to colicky abdominal pain. Tumor burden can also damage the intestinal epithelium and cause continuous pain.
INITIAL MANAGEMENT
Fluid resuscitation, electrolyte repletion, and a trial of NG tube decompression are part of the initial management of MBO (Figure ). While studies have shown that moderate intravenous hydration can minimize nausea and drowsiness, excessive fluids may worsen bowel edema and exacerbate vomiting.1,8 NG tube decompression is most effective in patients with proximal obstructions but some studies suggest it can decrease vomiting in patients with colonic obstructions as well.9 Computed tomography imaging can identify the extent of the tumor, the transition point of the obstruction, and any distant metastases. Surgery, Gastroenterology, and/or Interventional Radiology consultation should be obtained early to evaluate options for direct decompression. Hematology/Oncology and Radiation/Oncology referral may help delineate prognosis and achievable outcomes. Emergent exploratory surgery may be required in cases of bowel perforation or ischemia. Otherwise, a planned surgical resection should be considered in those with an isolated resectable lesion and acceptable perioperative risk. Colorectal or duodenal stents may be an option for those who are not surgical candidates or as a bridge to surgery.
As bowel obstruction is often a late manifestation of advanced malignancy, many patients may not be appropriate candidates for operative/interventional treatment due to malnutrition, comorbid conditions, or anatomic considerations. For these individuals, pharmacologic management is the mainstay of treatment. Additionally, the pharmacologic approaches detailed below may provide benefit as adjunctive therapy for patients undergoing procedural intervention.7 Consultation for early palliative care can improve symptom control as well as clarify goals of care.
PHARMACOLOGIC MANAGEMENT
Given the pathophysiology of MBO, pharmacologic therapies are focused on controlling nausea and pain while reducing bowel edema and secretions.
Antiemetic Agents
Nausea and vomiting in MBO are due to activation of vagal nerve fibers in the gastric wall and stimulation of the chemoreceptor trigger zone (CTZ).10 Dopamine antagonists have started to gain favor for MBO compared to more commonly used antiemetics such as the serotonin antagonists. Haloperidol should be considered as a first-line antiemetic in patients with MBO. Its potent D2-receptor antagonistic properties block receptors in the CTZ. The high affinity of the drug for only the D2-receptor makes it preferable to alternative agents in the same class such as chlorpromazine. However, haloperidol may cause or worsen QT prolongation and should be avoided in patients with Parkinson’s disease. The medication has less sedative and unwanted anticholinergic side effects due to its limited interaction with histaminergic and acetylcholine receptors.11 Haloperidol has been shown in the past to be efficacious for post-operative nausea but there are few randomized controlled trials in the terminally ill.12 Nonetheless, recent consensus guidelines from the Multinational Association of Supportive Care recommended haloperidol as the initial treatment of nausea for individuals with MBO based on available systematic reviews.10
Other dopamine antagonists remain good options, though they may cause additional side effects due to actions on other receptor types. Metoclopramide, another D2-receptor antagonist, has been shown to be effective in the treatment of nausea and vomiting due to advanced cancer.13 However as a prokinetic agent, this medication should be avoided in those with complete MBO and only considered in those with partial MBO.10,14
Olanzapine, an atypical antipsychotic, may also have a role in controlling nausea in patients with MBO. It functions as a 5-HT2A and D2-receptor antagonist, with a slightly greater affinity for the 5-HT2A receptor. Olanzapine thus can target two critical receptors playing a role in nausea and vomiting. A study of patients with incomplete bowel obstruction found the addition of olanzapine significantly decreased nausea and vomiting in patients who were refractory to other treatments including steroids and haloperidol.15 Olanzapine has the added advantage of single-day dosing as well as an oral disintegrating formulation.16
Intravenous and sublingual preparations of 5-HT3 receptor antagonists such as ondansetron are commonly used in the inpatient setting. These medications are potent antiemetics that exhibit their effects via pathways where serotonin acts as a neurotransmitter.17 An alternative agent, tropisetron, has shown promise when used alone or in conjunction with metoclopramide but is not currently available in the US.18 Granisetron is available in a transdermal formulation, which can be very convenient for patients with bowel obstruction. Its mechanism of action differs from ondansetron as it is an allosteric inhibitor rather than a competitive inhibitor.19 Granisetron needs more specific study with regards to its role in MBO.
Although haloperidol remains the initial choice, combination therapy can help to decrease the risk of extrapyramidal symptoms seen with higher doses of dopaminergic monotherapy.
Analgesics
Pain control is an essential part of the palliative treatment of MBO as bowel distention, secretions, and edema can cause rapid onset of pain. Parenteral step three opioids remain the optimal initial choice since patients are unable to take medications orally and may have compromised absorption. Opioids address both the colicky and continuous aspects of MBO pain.
Short-acting intravenous opioids such as morphine or hydromorphone may be scheduled every four hours with breakthrough dosing every hour in between. Alternatively, analgesics can be administered via a patient-controlled analgesia (PCA) pump.1 Although doses vary across patients, opioid-naïve patients can be initiated on a low dose therapy such as hydromorphone 0.2 mg IV/SC or morphine 1 mg IV/SC every four hours as needed for pain control.
Ongoing pain management for patients with MBO requires coordination of care. Many patients will elect to receive hospice care following discharge. Direct communication with palliative consultants and hospice providers can help facilitate a smooth transition. In patients for whom bowel obstruction resolves, transition to oral opioids based on morphine equivalent daily dose is indicated with further dose adjustment as patients may have reduced pain at this stage.
Options for patients with unresolved obstruction include transdermal and sublingual preparations as well as outpatient PCA with hospice support. Transdermal fentanyl patch can be useful but onset of peak levels occur within 8-12 hours.20 The patch is usually exchanged every 72 hours and is most effective when applied to areas containing adipose tissue which may limit its use in cachectic patients. The liquid preparation of methadone can be useful even in patients with unresolved MBO. Its lipophilic properties allow for ease of absorption.21 A baseline electrocardiogram (EKG) is recommended prior to methadone initiation due to the potential for QT prolongation. Methadone should not be a first-line option for opioid-naïve individuals due to its longer onset of action which limits rapid dose titration. Close collaboration with palliative medicine is highly recommended when using longer acting opioids.
Antisecretory Agents
Antisecretory agents are a mainstay of the pharmacologic management of inoperable MBO. Medications that reduce secretions and bowel edema include: somatostatin analogs, H2-blockers, proton pump inhibitors (PPIs), steroids, and anticholinergic agents. Table 2 summarizes the major studies comparing various antisecretory medications.
Octreotide, a somatostatin analog, has been increasingly used for the palliative treatment of MBO. The mechanism of action involves splanchnic vasoconstriction, reduction of intestinal and pancreatic secretions (via inhibition of VIP), decrease in gastric emptying, and slowing of smooth muscle contractions.22 Octreotide comes in an immediate-release formulation with an initial subcutaneous dose of 100 µg three or four times per day. Most patients will require 300-800 µg/day, maximum dose being up to 1 mg/day.22,23 A long-acting formulation, lanreotide, exists but can be difficult to obtain and may not provide the immediate relief needed in an acute care setting.
Initiation of octreotide should be considered in the presence of persistent symptoms. Studies have suggested that the benefit of octreotide is most apparent in the first three days of treatment (range 1-5 days).6,22,24 The medication should be discontinued if there is no clinical improvement such as reduction of NG tube output. Octreotide has been shown to be more efficacious than anticholinergic agents in reducing secretions as well as frequency of nausea and vomiting.8,25-28 Octreotide expedites NG tube removal, recovery of bowel function, and improvement in quality of life.29-32 The medication should also be considered in cases of recurrent MBO that previously responded to the medication.
Octreotide is considered the first-line agent in the palliative treatment of MBO, however the medication is costly. Recent studies suggest combination therapy with steroids and H2-blockers or PPIs may be an equally effective and less expensive alternative. The primary rationale for the use of steroids in MBO is their ability to decrease peritumoral edema and promote salt and water absorption from the intestine.1,2 PPIs and H2-blockers decrease distension, pain, and vomiting by reducing the volume of gastric secretions.33 A recent meta-analysis of phase 3 trials found both PPIs and H2-blockers to be effective in lowering volumes of gastric aspirates with ranitidine being slightly superior.34
Initial research into the utility of steroids in MBO garnered mixed results. One study showed marginal benefit for steroid plus octreotide combination therapy compared to octreotide, in a cohort of 27 patients.35 A subsequent review of practice patterns in the management of terminal MBO in Japan found that patients given steroids in combination with octreotide compared to octreotide alone were more likely to undergo early NG tube removal.36 A 1999 systematic review of corticosteroid treatment of MBO concluded low morbidity associated with the medications with a trend toward benefit that was not statistically significant.37 A 2015 study by Currow showed the addition of octreotide in patients already on a regime of dexamethasone and ranitidine did not improve the number of days free from vomiting but did reduce vomiting episodes in those with the most refractory symptoms.38
Collectively, the studies suggest that combination therapy with steroid and PPI or H2 blocker could be a less expensive option in the initial management of MBO. Alternatively, steroids may provide additional relief in patients with continued symptoms on octreotide and H2-blockers. Dexamethasone is preferable given its longer half-life and decreased propensity for sodium retention. Dosing of dexamethasone should be 8 mg IV once a day.38
Anticholinergic agents also reduce secretions. However, they are considered second-line therapy given their lower efficacy compared to other treatment options as well as their propensity to worsen cognitive function.1,2 Anticholinergics may benefit patients with continued symptoms who cannot tolerate the side effects of other treatments. Scopolamine, also known as hyoscine hydrobromide in the US, should be avoided as it crosses the blood-brain barrier. The quaternary formulation, scopolamine butylbromide (hyoscine butylbromide), does not pass this barrier but is currently not available in the US. Glycopyrrolate may be considered as it is also a quaternary ammonium compound that does not cross the blood-brain barrier. Several case reports have described its effectiveness in the resolution of refractory nausea and vomiting in combination with haloperidol and hydromorphone for symptom control.39 Effective oral care is imperative if anticholinergics are used in order to prevent the unpleasant feeling of dry mouth.
SUBSEQUENT SUPPORTIVE CARE
While initial management of MBO often requires placement of an NG tube, prolonged placement can increase the risk for erosions, aspiration, and sinus infections. Removal of the NG tube is most successful when secretions are minimal, but this may not happen unless the obstruction resolves. Some patients may elect to keep an NG tube if symptoms cannot be otherwise controlled by medications.
A venting gastrostomy tube can be considered as an alternative to prolonged NG tube placement. The tube may help alleviate distressing symptoms and can enhance the quality of life of patients by allowing the sensation of oral intake, though it will not allow for absorption of nutrients.40 Although a low risk procedure, patients may be too frail to undergo the procedure and may have postprocedure pain and complications. Anatomic abnormalities such as overlying bowel may also prevent the noninvasive percutaneous approach.
In patients with unresolved obstruction, oral intake should be reinitiated with caution with the patient’s wishes taken into account at all times. Some patients may prioritize the comfort derived from eating small amounts over any associated risks of increased nausea and vomiting.
Parenteral nutrition should be avoided in those with inoperable MBO in the advanced stages. The risks of infection, refeeding syndrome, and the discomfort of an intravenous line and intermittent testing may outweigh any benefits given the overall prognosis.41,42
CONCLUSION
Hospitalists are often involved in the initial care of patients with advanced malignancy who present with MBO. When interventions or surgeries to directly alleviate the obstruction are not possible, pharmacologic options are essential in managing burdensome symptoms and improving quality of life. Early Palliative Care referral can also assist with symptom management, emotional support, clarification of goals of care, and transition to the outpatient setting. While patients with inoperable MBO have a poor prognosis, hospitalists can play a vital role in alleviation of suffering in this devastating complication of advanced cancer.
Disclosures
The authors have nothing to disclose.
1. Ripamonti CI, Easson AM, Gerdes H. Management of malignant bowel obstruction. Eur J Cancer. 2008;44(8):1105-1115. doi: 10.1016/j.ejca.2008.02.028. PubMed
2. Tuca A, Guell E, Martinez-Losada E, Codorniu N. Malignant bowel obstruction in advanced cancer patients: epidemiology, management, and factors influencing spontaneous resolution. Cancer Manag Res. 2012;4:159-169. doi: 10.2147/CMAR.S29297. PubMed
3. Meier DE. Palliative care in hospitals. J Hosp Med. 2006;1(1):21-28. doi: 10.1002/jhm.3. PubMed
4. Lin RJ, Adelman RD, Diamond RR, Evans AT. The sentinel hospitalization and the role of palliative care. J Hosp Med. 2014;9(5):320-323. doi: 10.1002/jhm.2160. PubMed
5. Anthony T, Baron T, Mercadante S, et al. Report of the clinical protocol committee: development of randomized trials for malignant bowel obstruction. J Pain Symptom Manage. 2007;34(1 Suppl):S49-S59. doi: 10.1016/j.jpainsymman.2007.04.011. PubMed
6. Laval G, Marcelin-Benazech B, Guirimand F, et al. Recommendations for bowel obstruction with peritoneal carcinomatosis. J Pain Symptom Manage. 2014;48(1):75-91. doi: 10.1016/j.jpainsymman.2013.08.022. PubMed
7. Ferguson HJ, Ferguson CI, Speakman J, Ismail T. Management of intestinal obstruction in advanced malignancy. Ann Med Surg. 2015;4(3):264-270. doi: 10.1016/j.amsu.2015.07.018. PubMed
8. Ripamonti C, Mercadante S, Groff L, et al. Role of octreotide, scopolamine butylbromide, and hydration in symptom control of patients with inoperable bowel obstruction and nasogastric tubes: A prospective randomized trial. J Pain Symptom Manage. 2000;19(1):23-34. doi: 10.1016/S0885-3924(99)00147-5. PubMed
9. Rao W, Zhang X, Zhang J, et al. The role of nasogastric tube in decompression after elective colon and rectum surgery: a meta-analysis. Int J Colorectal Dis. 2011;26(4):423-429. doi: 10.1007/s00384-010-1093-4. PubMed
10. Walsh D, Davis M, Ripamonti C, et al. 2016 updated MASCC/ESMO consensus recommendations: management of nausea and vomiting in advanced cancer. Support Care Cancer. 2017;25(1):333-340. doi: 10.1007/s00520-016-3371-3. PubMed
11. Murray-Brown F, Dorman S. Haloperidol for the treatment of nausea and vomiting in palliative care patients. Cochrane Database Syst Rev. 2015;11(11):CD006271. doi: 10.1002/14651858.CD006271.pub3. PubMed
12. Digges M, Hussein A, Wilcock A, et al. Pharmacovigilance in hospice/palliative care: Net effect of haloperidol for nausea or vomiting. J Palliat Med. 2018;21(1):37-43. doi: 10.1089/jpm.2017.0159. PubMed
13. Bruera E, Belzile M, Neumann C, et al. A double-blind, crossover study of controlled-release metoclopramide and placebo for the chronic nausea and dyspepsia of advanced cancer. J Pain Symptom Manage. 2000;19(6):427-435. doi: 10.1016/S0885-3924(00)00138-X. PubMed
14. Gupta M, Davis M, LeGrand S, Walsh D, Lagman R. Nausea and vomiting in advanced cancer: the Cleveland clinic protocol. J Support Oncol. 2013;11(1):8-13. doi: 10.1016/j.suponc.2012.10.002. PubMed
15. Kaneishi K, Kawabata M, Morita T. Olanzapine for the relief of nausea in patients with advanced cancer and incomplete bowel obstruction. J Pain Symptom Manage. 2012;44(4):604-607. doi: 10.1016/j.jpainsymman.2011.10.023. PubMed
16. Prommer E. Olanzapine: palliative medicine update. Am J Hosp Palliat Care. 2013;30(1):75-82. doi: 10.1177/1049909112441241. PubMed
17. Currow DC, Coughlan M, Fardell B, Cooney NJ. Use of ondansetron in palliative medicine. J Pain Symptom Manage. 1997;13(5):302-307. doi: 10.1016/S0885-3924(97)00079-1. PubMed
18. Mystakidou K
19. Tuca A, Roca R, Sala C, et al. Efficacy of granisetron in the antiemetic control of nonsurgical intestinal obstruction in advanced cancer: A phase II clinical trial. J Pain Symptom Manage. 2009;37(2):259-270. doi: 10.1016/j.jpainsymman.2008.01.014. PubMed
20. Prommer E. The role of fentanyl in cancer-related pain. J Palliat Med. 2009;12(10):947-954. doi: 10.1089/jpm.2009.0051. PubMed
21. Shaiova LL, Berger A, Blinderman CD, et al. Consensus guideline on parenteral methadone use in pain and palliative care. Palliat Support Care. 2008;6(2):165-176. doi: 10.1017/S1478951508000254. PubMed
22. Murphy E, Prommer EE, Mihalyo M, Wilcock A. Octreotide. J Pain Symptom Manage. 2010;40(1):142-148. doi: 10.1016/j.jpainsymman.2010.05.002. PubMed
23. Prommer EE. Established and potential therapeutic applications of octreotide in palliative care. Support Care Cancer. 2008;16(10):1117-1123. doi: 10.1007/s00520-007-0399-4. PubMed
24. Mercadante S, Ferrera P, Villari P, Marrazzo A. Aggressive pharmacological treatment for reversing malignant bowel obstruction. J Pain Symptom Manage. 2004;28(4):412-416. doi: 10.1016/j.jpainsymman.2004.01.007. PubMed
25. Peng X, Wang P, Li S, Zhang G, Hu S. Randomized clinical trial comparing octreotide and scopolamine butylbromide in symptom control of patients with inoperable bowel obstruction due to advanced ovarian cancer. World J Surg Oncol. 2015;13:50. doi: 10.1186/s12957-015-0455-3. PubMed
26. Mercadante S, Ripamonti C, Casuccio A, Zecca E, Groff L. Comparison of octreotide and hyoscine butylbromide in controlling gastrointestinal symptoms due to malignant inoperable bowel obstruction. Support Care Cancer. 2000;8(3):188-191. doi: 10.1007/s005200050283. PubMed
27. Mystakidou K, Tsilika E, Kalaidopoulou O, et al. Comparison of octreotide administration vs conservative treatment in the management of inoperable bowel obstruction in patients with far advanced cancer: a randomized, double-blind, controlled clinical trial. Anticancer Res. 2002;22(2B):1187-1192. PubMed
28. Obita GP, Boland EG, Currow DC, Johnson MJ, Boland JW. Somatostatin analogues compared with placebo and other pharmacologic agents in the management of symptoms of inoperable malignant bowel obstruction: a systematic review. J Pain Symptom Manage. 2016;52(6):901-919. doi: 10.1016/j.jpainsymman.2016.05.032. PubMed
29. Watari H, Hosaka M, Wakui Y, et al. A prospective study on the efficacy of octreotide in the management of malignant bowel obstruction in gynecologic cancer. Int J Gynecol Cancer. 2012;22(4):692-696. doi: 10.1097/IGC.0b013e318244ce93. PubMed
30. Hisanaga T, Shinjo T, Morita T, et al. Multicenter prospective study on efficacy and safety of octreotide for inoperable malignant bowel obstruction. Jpn J Clin Oncol. 2010;40(8):739-745. doi: 10.1093/jjco/hyq048. PubMed
31. Laval G, Rousselot H, Toussaint-Martel S, et al. SALTO: a randomized, multicenter study assessing octreotide LAR in inoperable bowel obstruction. Bull Cancer. 2012;99(2):E1-E9. doi: 10.1684/bdc.2011.1535. PubMed
32. Mariani PP, Blumberg J, Landau A, et al. Symptomatic treatment with lanreotide microparticles in inoperable bowel obstruction resulting from peritoneal carcinomatosis: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2012;30(35):4337-4343. doi: 10.1200/JCO.2011.40.5712. PubMed
33. Clark K, Lam L, Currow D. Reducing gastric secretions--a role for histamine 2 antagonists or proton pump inhibitors in malignant bowel obstruction? Support Care Cancer. 2009;17(12):1463-1468. doi: 10.1007/s00520-009-0609-3. PubMed
34. Strand DS, Kim D, Peura DA. 25 years of proton pump inhibitors: a comprehensive review. Gut Liver. 2017;11(1):27-37. doi: 10.5009/gnl15502. PubMed
35. Murakami H, Matsumoto H, Nakamura M, Hirai T, Yamaguchi Y. Octreotide acetate-steroid combination therapy for malignant gastrointestinal obstruction. Anticancer Res. 2013;33(12):5557-5560. PubMed
36. Minoura T, Takeuchi M, Morita T, Kawakami K. Practice patterns of medications for patients with malignant bowel obstruction using a nationwide claims database and the association between treatment outcomes and concomitant use of H2-blockers/proton pump inhibitors and corticosteroids with octreotide. J Pain Symptom Manage. 2018;55(2):413-419. doi: 10.1016/j.jpainsymman.2017.10.019. PubMed
37. Feuer DJ, Broadley KE. Systematic review and meta-analysis of corticosteroids for the resolution of malignant bowel obstruction in advanced gynaecological and gastrointestinal cancers. Systematic Review Steering Committee. Ann Oncol. 1999;10(9):1035-1041. doi: 10.1023/A:1008361102808. PubMed
38. Currow DC, Quinn S, Agar M, et al. Double-blind, placebo-controlled, randomized trial of octreotide in malignant bowel obstruction. J Pain Symptom Manage. 2015;49(5):814-821. doi: 10.1016/j.jpainsymman.2014.09.013. PubMed
39. Davis MP, Furste A. Glycopyrrolate: a useful drug in the palliation of mechanical bowel obstruction. J Pain Symptom Manage. 1999;18(3):153-154. PubMed
40. Zucchi E, Fornasarig M, Martella L, et al. Decompressive percutaneous endoscopic gastrostomy in advanced cancer patients with small-bowel obstruction is feasible and effective: a large prospective study. Support Care Cancer. 2016;24(7):2877-2882. doi: 10.1007/s00520-016-3102-9. PubMed
41. Naghibi M, Smith TR, Elia M. A systematic review with meta-analysis of survival, quality of life and cost-effectiveness of home parenteral nutrition in patients with inoperable malignant bowel obstruction. Clin Nutr. 2015;34(5):825-837. doi: 10.1016/j.clnu.2014.09.010. PubMed
42. O’Connor B, Creedon B. Pharmacological treatment of bowel obstruction in cancer patients. Expert Opin Pharmacother. 2011;12(14):2205-2214. doi: 10.1517/14656566.2011.597382. PubMed
Malignant bowel obstruction (MBO) is a catastrophic complication of cancer that often requires hospitalization and a multidisciplinary approach in its management. Hospitalists frequently collaborate with such specialties as Hematology/Oncology, Surgery, Palliative Medicine, and Interventional Radiology in arriving at a treatment plan.
Initial management is focused on hydration, bowel rest and decompression via nasogastric (NG) tube. Surgical resection or endoscopic stenting should be considered early.1 However, patients who present in the terminal stages may be poor candidates for these options due to diminished functional status, multiple areas of obstruction, complicated anatomy limiting intervention, or an associated large volume of ascites.
Presence of inoperable MBO portends a poor prognosis, often measured in weeks.2 Presentation often occurs in the context of a sentinel hospitalization, signifying a shift in disease course.3,4 It is essential for hospitalists to be familiar with noninvasive therapies for inoperable MBO given the increasing role of hospitalists in providing inpatient palliative care. Palliative pharmacologic management of MBO can reduce symptom burden during these terminal stages and will be the focus of this paper.
BACKGROUND AND PATHOPHYSIOLOGY
Malignant bowel obstruction occurs in about 3%-15% of patients with cancer.2 A consensus definition of MBO established the following specific criteria: (1) clinical evidence of bowel obstruction, (2) obstruction distal to the ligament of Treitz, and (3) the presence of primary intra-abdominal cancer with incurable disease or extra-abdominal cancer with peritoneal involvement.5 The most common malignancies are gastric, colorectal, and ovarian in origin.1,2 The most common extra-abdominal malignancies associated with MBO are breast, melanoma, and lung. MBO is most frequently diagnosed during the advanced stages of cancer.2 The obstruction can involve a partial or total blockage of the small or large intestine from either an intrinsic or extrinsic source. Peristalsis may also be impaired via direct tumor infiltration of the intestinal walls or within the enteric nervous system or celiac plexus. Other etiologies of MBO include peritoneal carcinomatosis and radiation-induced fibrosis.1,6 The obstruction can occur at a single level or involve multiple areas, which usually precludes surgical intervention.2
Symptoms of MBO can be insidious in onset and take several weeks to manifest. The most prevalent symptoms are nausea, vomiting, constipation, abdominal pain, and distension.2,6 The intermittent pattern of symptoms may evolve into continuous episodes with spontaneous remission in between. The etiology of symptoms can be attributed to distension proximal to the site of obstruction with concomitantly increased gastrointestinal and pancreaticobiliary secretions.
The distension creates a “hypertensive state” in the intestinal lumen causing enterochromaffin cells to release serotonin which activates the enteric nervous system and its effectors including substance P, nitric oxide, acetylcholine, somatostatin, and vasoactive intestinal peptide (VIP). These neurotransmitters stimulate the secretomotor actions that cause hypersecretion of mucus from cells of the intestinal crypts. Additional water and sodium secretions accumulate due to the expanded surface area of the bowel.1,2 Overloaded with luminal contents, the bowel attempts to overcome the obstruction by contracting, which leads to colicky abdominal pain. Tumor burden can also damage the intestinal epithelium and cause continuous pain.
INITIAL MANAGEMENT
Fluid resuscitation, electrolyte repletion, and a trial of NG tube decompression are part of the initial management of MBO (Figure ). While studies have shown that moderate intravenous hydration can minimize nausea and drowsiness, excessive fluids may worsen bowel edema and exacerbate vomiting.1,8 NG tube decompression is most effective in patients with proximal obstructions but some studies suggest it can decrease vomiting in patients with colonic obstructions as well.9 Computed tomography imaging can identify the extent of the tumor, the transition point of the obstruction, and any distant metastases. Surgery, Gastroenterology, and/or Interventional Radiology consultation should be obtained early to evaluate options for direct decompression. Hematology/Oncology and Radiation/Oncology referral may help delineate prognosis and achievable outcomes. Emergent exploratory surgery may be required in cases of bowel perforation or ischemia. Otherwise, a planned surgical resection should be considered in those with an isolated resectable lesion and acceptable perioperative risk. Colorectal or duodenal stents may be an option for those who are not surgical candidates or as a bridge to surgery.
As bowel obstruction is often a late manifestation of advanced malignancy, many patients may not be appropriate candidates for operative/interventional treatment due to malnutrition, comorbid conditions, or anatomic considerations. For these individuals, pharmacologic management is the mainstay of treatment. Additionally, the pharmacologic approaches detailed below may provide benefit as adjunctive therapy for patients undergoing procedural intervention.7 Consultation for early palliative care can improve symptom control as well as clarify goals of care.
PHARMACOLOGIC MANAGEMENT
Given the pathophysiology of MBO, pharmacologic therapies are focused on controlling nausea and pain while reducing bowel edema and secretions.
Antiemetic Agents
Nausea and vomiting in MBO are due to activation of vagal nerve fibers in the gastric wall and stimulation of the chemoreceptor trigger zone (CTZ).10 Dopamine antagonists have started to gain favor for MBO compared to more commonly used antiemetics such as the serotonin antagonists. Haloperidol should be considered as a first-line antiemetic in patients with MBO. Its potent D2-receptor antagonistic properties block receptors in the CTZ. The high affinity of the drug for only the D2-receptor makes it preferable to alternative agents in the same class such as chlorpromazine. However, haloperidol may cause or worsen QT prolongation and should be avoided in patients with Parkinson’s disease. The medication has less sedative and unwanted anticholinergic side effects due to its limited interaction with histaminergic and acetylcholine receptors.11 Haloperidol has been shown in the past to be efficacious for post-operative nausea but there are few randomized controlled trials in the terminally ill.12 Nonetheless, recent consensus guidelines from the Multinational Association of Supportive Care recommended haloperidol as the initial treatment of nausea for individuals with MBO based on available systematic reviews.10
Other dopamine antagonists remain good options, though they may cause additional side effects due to actions on other receptor types. Metoclopramide, another D2-receptor antagonist, has been shown to be effective in the treatment of nausea and vomiting due to advanced cancer.13 However as a prokinetic agent, this medication should be avoided in those with complete MBO and only considered in those with partial MBO.10,14
Olanzapine, an atypical antipsychotic, may also have a role in controlling nausea in patients with MBO. It functions as a 5-HT2A and D2-receptor antagonist, with a slightly greater affinity for the 5-HT2A receptor. Olanzapine thus can target two critical receptors playing a role in nausea and vomiting. A study of patients with incomplete bowel obstruction found the addition of olanzapine significantly decreased nausea and vomiting in patients who were refractory to other treatments including steroids and haloperidol.15 Olanzapine has the added advantage of single-day dosing as well as an oral disintegrating formulation.16
Intravenous and sublingual preparations of 5-HT3 receptor antagonists such as ondansetron are commonly used in the inpatient setting. These medications are potent antiemetics that exhibit their effects via pathways where serotonin acts as a neurotransmitter.17 An alternative agent, tropisetron, has shown promise when used alone or in conjunction with metoclopramide but is not currently available in the US.18 Granisetron is available in a transdermal formulation, which can be very convenient for patients with bowel obstruction. Its mechanism of action differs from ondansetron as it is an allosteric inhibitor rather than a competitive inhibitor.19 Granisetron needs more specific study with regards to its role in MBO.
Although haloperidol remains the initial choice, combination therapy can help to decrease the risk of extrapyramidal symptoms seen with higher doses of dopaminergic monotherapy.
Analgesics
Pain control is an essential part of the palliative treatment of MBO as bowel distention, secretions, and edema can cause rapid onset of pain. Parenteral step three opioids remain the optimal initial choice since patients are unable to take medications orally and may have compromised absorption. Opioids address both the colicky and continuous aspects of MBO pain.
Short-acting intravenous opioids such as morphine or hydromorphone may be scheduled every four hours with breakthrough dosing every hour in between. Alternatively, analgesics can be administered via a patient-controlled analgesia (PCA) pump.1 Although doses vary across patients, opioid-naïve patients can be initiated on a low dose therapy such as hydromorphone 0.2 mg IV/SC or morphine 1 mg IV/SC every four hours as needed for pain control.
Ongoing pain management for patients with MBO requires coordination of care. Many patients will elect to receive hospice care following discharge. Direct communication with palliative consultants and hospice providers can help facilitate a smooth transition. In patients for whom bowel obstruction resolves, transition to oral opioids based on morphine equivalent daily dose is indicated with further dose adjustment as patients may have reduced pain at this stage.
Options for patients with unresolved obstruction include transdermal and sublingual preparations as well as outpatient PCA with hospice support. Transdermal fentanyl patch can be useful but onset of peak levels occur within 8-12 hours.20 The patch is usually exchanged every 72 hours and is most effective when applied to areas containing adipose tissue which may limit its use in cachectic patients. The liquid preparation of methadone can be useful even in patients with unresolved MBO. Its lipophilic properties allow for ease of absorption.21 A baseline electrocardiogram (EKG) is recommended prior to methadone initiation due to the potential for QT prolongation. Methadone should not be a first-line option for opioid-naïve individuals due to its longer onset of action which limits rapid dose titration. Close collaboration with palliative medicine is highly recommended when using longer acting opioids.
Antisecretory Agents
Antisecretory agents are a mainstay of the pharmacologic management of inoperable MBO. Medications that reduce secretions and bowel edema include: somatostatin analogs, H2-blockers, proton pump inhibitors (PPIs), steroids, and anticholinergic agents. Table 2 summarizes the major studies comparing various antisecretory medications.
Octreotide, a somatostatin analog, has been increasingly used for the palliative treatment of MBO. The mechanism of action involves splanchnic vasoconstriction, reduction of intestinal and pancreatic secretions (via inhibition of VIP), decrease in gastric emptying, and slowing of smooth muscle contractions.22 Octreotide comes in an immediate-release formulation with an initial subcutaneous dose of 100 µg three or four times per day. Most patients will require 300-800 µg/day, maximum dose being up to 1 mg/day.22,23 A long-acting formulation, lanreotide, exists but can be difficult to obtain and may not provide the immediate relief needed in an acute care setting.
Initiation of octreotide should be considered in the presence of persistent symptoms. Studies have suggested that the benefit of octreotide is most apparent in the first three days of treatment (range 1-5 days).6,22,24 The medication should be discontinued if there is no clinical improvement such as reduction of NG tube output. Octreotide has been shown to be more efficacious than anticholinergic agents in reducing secretions as well as frequency of nausea and vomiting.8,25-28 Octreotide expedites NG tube removal, recovery of bowel function, and improvement in quality of life.29-32 The medication should also be considered in cases of recurrent MBO that previously responded to the medication.
Octreotide is considered the first-line agent in the palliative treatment of MBO, however the medication is costly. Recent studies suggest combination therapy with steroids and H2-blockers or PPIs may be an equally effective and less expensive alternative. The primary rationale for the use of steroids in MBO is their ability to decrease peritumoral edema and promote salt and water absorption from the intestine.1,2 PPIs and H2-blockers decrease distension, pain, and vomiting by reducing the volume of gastric secretions.33 A recent meta-analysis of phase 3 trials found both PPIs and H2-blockers to be effective in lowering volumes of gastric aspirates with ranitidine being slightly superior.34
Initial research into the utility of steroids in MBO garnered mixed results. One study showed marginal benefit for steroid plus octreotide combination therapy compared to octreotide, in a cohort of 27 patients.35 A subsequent review of practice patterns in the management of terminal MBO in Japan found that patients given steroids in combination with octreotide compared to octreotide alone were more likely to undergo early NG tube removal.36 A 1999 systematic review of corticosteroid treatment of MBO concluded low morbidity associated with the medications with a trend toward benefit that was not statistically significant.37 A 2015 study by Currow showed the addition of octreotide in patients already on a regime of dexamethasone and ranitidine did not improve the number of days free from vomiting but did reduce vomiting episodes in those with the most refractory symptoms.38
Collectively, the studies suggest that combination therapy with steroid and PPI or H2 blocker could be a less expensive option in the initial management of MBO. Alternatively, steroids may provide additional relief in patients with continued symptoms on octreotide and H2-blockers. Dexamethasone is preferable given its longer half-life and decreased propensity for sodium retention. Dosing of dexamethasone should be 8 mg IV once a day.38
Anticholinergic agents also reduce secretions. However, they are considered second-line therapy given their lower efficacy compared to other treatment options as well as their propensity to worsen cognitive function.1,2 Anticholinergics may benefit patients with continued symptoms who cannot tolerate the side effects of other treatments. Scopolamine, also known as hyoscine hydrobromide in the US, should be avoided as it crosses the blood-brain barrier. The quaternary formulation, scopolamine butylbromide (hyoscine butylbromide), does not pass this barrier but is currently not available in the US. Glycopyrrolate may be considered as it is also a quaternary ammonium compound that does not cross the blood-brain barrier. Several case reports have described its effectiveness in the resolution of refractory nausea and vomiting in combination with haloperidol and hydromorphone for symptom control.39 Effective oral care is imperative if anticholinergics are used in order to prevent the unpleasant feeling of dry mouth.
SUBSEQUENT SUPPORTIVE CARE
While initial management of MBO often requires placement of an NG tube, prolonged placement can increase the risk for erosions, aspiration, and sinus infections. Removal of the NG tube is most successful when secretions are minimal, but this may not happen unless the obstruction resolves. Some patients may elect to keep an NG tube if symptoms cannot be otherwise controlled by medications.
A venting gastrostomy tube can be considered as an alternative to prolonged NG tube placement. The tube may help alleviate distressing symptoms and can enhance the quality of life of patients by allowing the sensation of oral intake, though it will not allow for absorption of nutrients.40 Although a low risk procedure, patients may be too frail to undergo the procedure and may have postprocedure pain and complications. Anatomic abnormalities such as overlying bowel may also prevent the noninvasive percutaneous approach.
In patients with unresolved obstruction, oral intake should be reinitiated with caution with the patient’s wishes taken into account at all times. Some patients may prioritize the comfort derived from eating small amounts over any associated risks of increased nausea and vomiting.
Parenteral nutrition should be avoided in those with inoperable MBO in the advanced stages. The risks of infection, refeeding syndrome, and the discomfort of an intravenous line and intermittent testing may outweigh any benefits given the overall prognosis.41,42
CONCLUSION
Hospitalists are often involved in the initial care of patients with advanced malignancy who present with MBO. When interventions or surgeries to directly alleviate the obstruction are not possible, pharmacologic options are essential in managing burdensome symptoms and improving quality of life. Early Palliative Care referral can also assist with symptom management, emotional support, clarification of goals of care, and transition to the outpatient setting. While patients with inoperable MBO have a poor prognosis, hospitalists can play a vital role in alleviation of suffering in this devastating complication of advanced cancer.
Disclosures
The authors have nothing to disclose.
Malignant bowel obstruction (MBO) is a catastrophic complication of cancer that often requires hospitalization and a multidisciplinary approach in its management. Hospitalists frequently collaborate with such specialties as Hematology/Oncology, Surgery, Palliative Medicine, and Interventional Radiology in arriving at a treatment plan.
Initial management is focused on hydration, bowel rest and decompression via nasogastric (NG) tube. Surgical resection or endoscopic stenting should be considered early.1 However, patients who present in the terminal stages may be poor candidates for these options due to diminished functional status, multiple areas of obstruction, complicated anatomy limiting intervention, or an associated large volume of ascites.
Presence of inoperable MBO portends a poor prognosis, often measured in weeks.2 Presentation often occurs in the context of a sentinel hospitalization, signifying a shift in disease course.3,4 It is essential for hospitalists to be familiar with noninvasive therapies for inoperable MBO given the increasing role of hospitalists in providing inpatient palliative care. Palliative pharmacologic management of MBO can reduce symptom burden during these terminal stages and will be the focus of this paper.
BACKGROUND AND PATHOPHYSIOLOGY
Malignant bowel obstruction occurs in about 3%-15% of patients with cancer.2 A consensus definition of MBO established the following specific criteria: (1) clinical evidence of bowel obstruction, (2) obstruction distal to the ligament of Treitz, and (3) the presence of primary intra-abdominal cancer with incurable disease or extra-abdominal cancer with peritoneal involvement.5 The most common malignancies are gastric, colorectal, and ovarian in origin.1,2 The most common extra-abdominal malignancies associated with MBO are breast, melanoma, and lung. MBO is most frequently diagnosed during the advanced stages of cancer.2 The obstruction can involve a partial or total blockage of the small or large intestine from either an intrinsic or extrinsic source. Peristalsis may also be impaired via direct tumor infiltration of the intestinal walls or within the enteric nervous system or celiac plexus. Other etiologies of MBO include peritoneal carcinomatosis and radiation-induced fibrosis.1,6 The obstruction can occur at a single level or involve multiple areas, which usually precludes surgical intervention.2
Symptoms of MBO can be insidious in onset and take several weeks to manifest. The most prevalent symptoms are nausea, vomiting, constipation, abdominal pain, and distension.2,6 The intermittent pattern of symptoms may evolve into continuous episodes with spontaneous remission in between. The etiology of symptoms can be attributed to distension proximal to the site of obstruction with concomitantly increased gastrointestinal and pancreaticobiliary secretions.
The distension creates a “hypertensive state” in the intestinal lumen causing enterochromaffin cells to release serotonin which activates the enteric nervous system and its effectors including substance P, nitric oxide, acetylcholine, somatostatin, and vasoactive intestinal peptide (VIP). These neurotransmitters stimulate the secretomotor actions that cause hypersecretion of mucus from cells of the intestinal crypts. Additional water and sodium secretions accumulate due to the expanded surface area of the bowel.1,2 Overloaded with luminal contents, the bowel attempts to overcome the obstruction by contracting, which leads to colicky abdominal pain. Tumor burden can also damage the intestinal epithelium and cause continuous pain.
INITIAL MANAGEMENT
Fluid resuscitation, electrolyte repletion, and a trial of NG tube decompression are part of the initial management of MBO (Figure ). While studies have shown that moderate intravenous hydration can minimize nausea and drowsiness, excessive fluids may worsen bowel edema and exacerbate vomiting.1,8 NG tube decompression is most effective in patients with proximal obstructions but some studies suggest it can decrease vomiting in patients with colonic obstructions as well.9 Computed tomography imaging can identify the extent of the tumor, the transition point of the obstruction, and any distant metastases. Surgery, Gastroenterology, and/or Interventional Radiology consultation should be obtained early to evaluate options for direct decompression. Hematology/Oncology and Radiation/Oncology referral may help delineate prognosis and achievable outcomes. Emergent exploratory surgery may be required in cases of bowel perforation or ischemia. Otherwise, a planned surgical resection should be considered in those with an isolated resectable lesion and acceptable perioperative risk. Colorectal or duodenal stents may be an option for those who are not surgical candidates or as a bridge to surgery.
As bowel obstruction is often a late manifestation of advanced malignancy, many patients may not be appropriate candidates for operative/interventional treatment due to malnutrition, comorbid conditions, or anatomic considerations. For these individuals, pharmacologic management is the mainstay of treatment. Additionally, the pharmacologic approaches detailed below may provide benefit as adjunctive therapy for patients undergoing procedural intervention.7 Consultation for early palliative care can improve symptom control as well as clarify goals of care.
PHARMACOLOGIC MANAGEMENT
Given the pathophysiology of MBO, pharmacologic therapies are focused on controlling nausea and pain while reducing bowel edema and secretions.
Antiemetic Agents
Nausea and vomiting in MBO are due to activation of vagal nerve fibers in the gastric wall and stimulation of the chemoreceptor trigger zone (CTZ).10 Dopamine antagonists have started to gain favor for MBO compared to more commonly used antiemetics such as the serotonin antagonists. Haloperidol should be considered as a first-line antiemetic in patients with MBO. Its potent D2-receptor antagonistic properties block receptors in the CTZ. The high affinity of the drug for only the D2-receptor makes it preferable to alternative agents in the same class such as chlorpromazine. However, haloperidol may cause or worsen QT prolongation and should be avoided in patients with Parkinson’s disease. The medication has less sedative and unwanted anticholinergic side effects due to its limited interaction with histaminergic and acetylcholine receptors.11 Haloperidol has been shown in the past to be efficacious for post-operative nausea but there are few randomized controlled trials in the terminally ill.12 Nonetheless, recent consensus guidelines from the Multinational Association of Supportive Care recommended haloperidol as the initial treatment of nausea for individuals with MBO based on available systematic reviews.10
Other dopamine antagonists remain good options, though they may cause additional side effects due to actions on other receptor types. Metoclopramide, another D2-receptor antagonist, has been shown to be effective in the treatment of nausea and vomiting due to advanced cancer.13 However as a prokinetic agent, this medication should be avoided in those with complete MBO and only considered in those with partial MBO.10,14
Olanzapine, an atypical antipsychotic, may also have a role in controlling nausea in patients with MBO. It functions as a 5-HT2A and D2-receptor antagonist, with a slightly greater affinity for the 5-HT2A receptor. Olanzapine thus can target two critical receptors playing a role in nausea and vomiting. A study of patients with incomplete bowel obstruction found the addition of olanzapine significantly decreased nausea and vomiting in patients who were refractory to other treatments including steroids and haloperidol.15 Olanzapine has the added advantage of single-day dosing as well as an oral disintegrating formulation.16
Intravenous and sublingual preparations of 5-HT3 receptor antagonists such as ondansetron are commonly used in the inpatient setting. These medications are potent antiemetics that exhibit their effects via pathways where serotonin acts as a neurotransmitter.17 An alternative agent, tropisetron, has shown promise when used alone or in conjunction with metoclopramide but is not currently available in the US.18 Granisetron is available in a transdermal formulation, which can be very convenient for patients with bowel obstruction. Its mechanism of action differs from ondansetron as it is an allosteric inhibitor rather than a competitive inhibitor.19 Granisetron needs more specific study with regards to its role in MBO.
Although haloperidol remains the initial choice, combination therapy can help to decrease the risk of extrapyramidal symptoms seen with higher doses of dopaminergic monotherapy.
Analgesics
Pain control is an essential part of the palliative treatment of MBO as bowel distention, secretions, and edema can cause rapid onset of pain. Parenteral step three opioids remain the optimal initial choice since patients are unable to take medications orally and may have compromised absorption. Opioids address both the colicky and continuous aspects of MBO pain.
Short-acting intravenous opioids such as morphine or hydromorphone may be scheduled every four hours with breakthrough dosing every hour in between. Alternatively, analgesics can be administered via a patient-controlled analgesia (PCA) pump.1 Although doses vary across patients, opioid-naïve patients can be initiated on a low dose therapy such as hydromorphone 0.2 mg IV/SC or morphine 1 mg IV/SC every four hours as needed for pain control.
Ongoing pain management for patients with MBO requires coordination of care. Many patients will elect to receive hospice care following discharge. Direct communication with palliative consultants and hospice providers can help facilitate a smooth transition. In patients for whom bowel obstruction resolves, transition to oral opioids based on morphine equivalent daily dose is indicated with further dose adjustment as patients may have reduced pain at this stage.
Options for patients with unresolved obstruction include transdermal and sublingual preparations as well as outpatient PCA with hospice support. Transdermal fentanyl patch can be useful but onset of peak levels occur within 8-12 hours.20 The patch is usually exchanged every 72 hours and is most effective when applied to areas containing adipose tissue which may limit its use in cachectic patients. The liquid preparation of methadone can be useful even in patients with unresolved MBO. Its lipophilic properties allow for ease of absorption.21 A baseline electrocardiogram (EKG) is recommended prior to methadone initiation due to the potential for QT prolongation. Methadone should not be a first-line option for opioid-naïve individuals due to its longer onset of action which limits rapid dose titration. Close collaboration with palliative medicine is highly recommended when using longer acting opioids.
Antisecretory Agents
Antisecretory agents are a mainstay of the pharmacologic management of inoperable MBO. Medications that reduce secretions and bowel edema include: somatostatin analogs, H2-blockers, proton pump inhibitors (PPIs), steroids, and anticholinergic agents. Table 2 summarizes the major studies comparing various antisecretory medications.
Octreotide, a somatostatin analog, has been increasingly used for the palliative treatment of MBO. The mechanism of action involves splanchnic vasoconstriction, reduction of intestinal and pancreatic secretions (via inhibition of VIP), decrease in gastric emptying, and slowing of smooth muscle contractions.22 Octreotide comes in an immediate-release formulation with an initial subcutaneous dose of 100 µg three or four times per day. Most patients will require 300-800 µg/day, maximum dose being up to 1 mg/day.22,23 A long-acting formulation, lanreotide, exists but can be difficult to obtain and may not provide the immediate relief needed in an acute care setting.
Initiation of octreotide should be considered in the presence of persistent symptoms. Studies have suggested that the benefit of octreotide is most apparent in the first three days of treatment (range 1-5 days).6,22,24 The medication should be discontinued if there is no clinical improvement such as reduction of NG tube output. Octreotide has been shown to be more efficacious than anticholinergic agents in reducing secretions as well as frequency of nausea and vomiting.8,25-28 Octreotide expedites NG tube removal, recovery of bowel function, and improvement in quality of life.29-32 The medication should also be considered in cases of recurrent MBO that previously responded to the medication.
Octreotide is considered the first-line agent in the palliative treatment of MBO, however the medication is costly. Recent studies suggest combination therapy with steroids and H2-blockers or PPIs may be an equally effective and less expensive alternative. The primary rationale for the use of steroids in MBO is their ability to decrease peritumoral edema and promote salt and water absorption from the intestine.1,2 PPIs and H2-blockers decrease distension, pain, and vomiting by reducing the volume of gastric secretions.33 A recent meta-analysis of phase 3 trials found both PPIs and H2-blockers to be effective in lowering volumes of gastric aspirates with ranitidine being slightly superior.34
Initial research into the utility of steroids in MBO garnered mixed results. One study showed marginal benefit for steroid plus octreotide combination therapy compared to octreotide, in a cohort of 27 patients.35 A subsequent review of practice patterns in the management of terminal MBO in Japan found that patients given steroids in combination with octreotide compared to octreotide alone were more likely to undergo early NG tube removal.36 A 1999 systematic review of corticosteroid treatment of MBO concluded low morbidity associated with the medications with a trend toward benefit that was not statistically significant.37 A 2015 study by Currow showed the addition of octreotide in patients already on a regime of dexamethasone and ranitidine did not improve the number of days free from vomiting but did reduce vomiting episodes in those with the most refractory symptoms.38
Collectively, the studies suggest that combination therapy with steroid and PPI or H2 blocker could be a less expensive option in the initial management of MBO. Alternatively, steroids may provide additional relief in patients with continued symptoms on octreotide and H2-blockers. Dexamethasone is preferable given its longer half-life and decreased propensity for sodium retention. Dosing of dexamethasone should be 8 mg IV once a day.38
Anticholinergic agents also reduce secretions. However, they are considered second-line therapy given their lower efficacy compared to other treatment options as well as their propensity to worsen cognitive function.1,2 Anticholinergics may benefit patients with continued symptoms who cannot tolerate the side effects of other treatments. Scopolamine, also known as hyoscine hydrobromide in the US, should be avoided as it crosses the blood-brain barrier. The quaternary formulation, scopolamine butylbromide (hyoscine butylbromide), does not pass this barrier but is currently not available in the US. Glycopyrrolate may be considered as it is also a quaternary ammonium compound that does not cross the blood-brain barrier. Several case reports have described its effectiveness in the resolution of refractory nausea and vomiting in combination with haloperidol and hydromorphone for symptom control.39 Effective oral care is imperative if anticholinergics are used in order to prevent the unpleasant feeling of dry mouth.
SUBSEQUENT SUPPORTIVE CARE
While initial management of MBO often requires placement of an NG tube, prolonged placement can increase the risk for erosions, aspiration, and sinus infections. Removal of the NG tube is most successful when secretions are minimal, but this may not happen unless the obstruction resolves. Some patients may elect to keep an NG tube if symptoms cannot be otherwise controlled by medications.
A venting gastrostomy tube can be considered as an alternative to prolonged NG tube placement. The tube may help alleviate distressing symptoms and can enhance the quality of life of patients by allowing the sensation of oral intake, though it will not allow for absorption of nutrients.40 Although a low risk procedure, patients may be too frail to undergo the procedure and may have postprocedure pain and complications. Anatomic abnormalities such as overlying bowel may also prevent the noninvasive percutaneous approach.
In patients with unresolved obstruction, oral intake should be reinitiated with caution with the patient’s wishes taken into account at all times. Some patients may prioritize the comfort derived from eating small amounts over any associated risks of increased nausea and vomiting.
Parenteral nutrition should be avoided in those with inoperable MBO in the advanced stages. The risks of infection, refeeding syndrome, and the discomfort of an intravenous line and intermittent testing may outweigh any benefits given the overall prognosis.41,42
CONCLUSION
Hospitalists are often involved in the initial care of patients with advanced malignancy who present with MBO. When interventions or surgeries to directly alleviate the obstruction are not possible, pharmacologic options are essential in managing burdensome symptoms and improving quality of life. Early Palliative Care referral can also assist with symptom management, emotional support, clarification of goals of care, and transition to the outpatient setting. While patients with inoperable MBO have a poor prognosis, hospitalists can play a vital role in alleviation of suffering in this devastating complication of advanced cancer.
Disclosures
The authors have nothing to disclose.
1. Ripamonti CI, Easson AM, Gerdes H. Management of malignant bowel obstruction. Eur J Cancer. 2008;44(8):1105-1115. doi: 10.1016/j.ejca.2008.02.028. PubMed
2. Tuca A, Guell E, Martinez-Losada E, Codorniu N. Malignant bowel obstruction in advanced cancer patients: epidemiology, management, and factors influencing spontaneous resolution. Cancer Manag Res. 2012;4:159-169. doi: 10.2147/CMAR.S29297. PubMed
3. Meier DE. Palliative care in hospitals. J Hosp Med. 2006;1(1):21-28. doi: 10.1002/jhm.3. PubMed
4. Lin RJ, Adelman RD, Diamond RR, Evans AT. The sentinel hospitalization and the role of palliative care. J Hosp Med. 2014;9(5):320-323. doi: 10.1002/jhm.2160. PubMed
5. Anthony T, Baron T, Mercadante S, et al. Report of the clinical protocol committee: development of randomized trials for malignant bowel obstruction. J Pain Symptom Manage. 2007;34(1 Suppl):S49-S59. doi: 10.1016/j.jpainsymman.2007.04.011. PubMed
6. Laval G, Marcelin-Benazech B, Guirimand F, et al. Recommendations for bowel obstruction with peritoneal carcinomatosis. J Pain Symptom Manage. 2014;48(1):75-91. doi: 10.1016/j.jpainsymman.2013.08.022. PubMed
7. Ferguson HJ, Ferguson CI, Speakman J, Ismail T. Management of intestinal obstruction in advanced malignancy. Ann Med Surg. 2015;4(3):264-270. doi: 10.1016/j.amsu.2015.07.018. PubMed
8. Ripamonti C, Mercadante S, Groff L, et al. Role of octreotide, scopolamine butylbromide, and hydration in symptom control of patients with inoperable bowel obstruction and nasogastric tubes: A prospective randomized trial. J Pain Symptom Manage. 2000;19(1):23-34. doi: 10.1016/S0885-3924(99)00147-5. PubMed
9. Rao W, Zhang X, Zhang J, et al. The role of nasogastric tube in decompression after elective colon and rectum surgery: a meta-analysis. Int J Colorectal Dis. 2011;26(4):423-429. doi: 10.1007/s00384-010-1093-4. PubMed
10. Walsh D, Davis M, Ripamonti C, et al. 2016 updated MASCC/ESMO consensus recommendations: management of nausea and vomiting in advanced cancer. Support Care Cancer. 2017;25(1):333-340. doi: 10.1007/s00520-016-3371-3. PubMed
11. Murray-Brown F, Dorman S. Haloperidol for the treatment of nausea and vomiting in palliative care patients. Cochrane Database Syst Rev. 2015;11(11):CD006271. doi: 10.1002/14651858.CD006271.pub3. PubMed
12. Digges M, Hussein A, Wilcock A, et al. Pharmacovigilance in hospice/palliative care: Net effect of haloperidol for nausea or vomiting. J Palliat Med. 2018;21(1):37-43. doi: 10.1089/jpm.2017.0159. PubMed
13. Bruera E, Belzile M, Neumann C, et al. A double-blind, crossover study of controlled-release metoclopramide and placebo for the chronic nausea and dyspepsia of advanced cancer. J Pain Symptom Manage. 2000;19(6):427-435. doi: 10.1016/S0885-3924(00)00138-X. PubMed
14. Gupta M, Davis M, LeGrand S, Walsh D, Lagman R. Nausea and vomiting in advanced cancer: the Cleveland clinic protocol. J Support Oncol. 2013;11(1):8-13. doi: 10.1016/j.suponc.2012.10.002. PubMed
15. Kaneishi K, Kawabata M, Morita T. Olanzapine for the relief of nausea in patients with advanced cancer and incomplete bowel obstruction. J Pain Symptom Manage. 2012;44(4):604-607. doi: 10.1016/j.jpainsymman.2011.10.023. PubMed
16. Prommer E. Olanzapine: palliative medicine update. Am J Hosp Palliat Care. 2013;30(1):75-82. doi: 10.1177/1049909112441241. PubMed
17. Currow DC, Coughlan M, Fardell B, Cooney NJ. Use of ondansetron in palliative medicine. J Pain Symptom Manage. 1997;13(5):302-307. doi: 10.1016/S0885-3924(97)00079-1. PubMed
18. Mystakidou K
19. Tuca A, Roca R, Sala C, et al. Efficacy of granisetron in the antiemetic control of nonsurgical intestinal obstruction in advanced cancer: A phase II clinical trial. J Pain Symptom Manage. 2009;37(2):259-270. doi: 10.1016/j.jpainsymman.2008.01.014. PubMed
20. Prommer E. The role of fentanyl in cancer-related pain. J Palliat Med. 2009;12(10):947-954. doi: 10.1089/jpm.2009.0051. PubMed
21. Shaiova LL, Berger A, Blinderman CD, et al. Consensus guideline on parenteral methadone use in pain and palliative care. Palliat Support Care. 2008;6(2):165-176. doi: 10.1017/S1478951508000254. PubMed
22. Murphy E, Prommer EE, Mihalyo M, Wilcock A. Octreotide. J Pain Symptom Manage. 2010;40(1):142-148. doi: 10.1016/j.jpainsymman.2010.05.002. PubMed
23. Prommer EE. Established and potential therapeutic applications of octreotide in palliative care. Support Care Cancer. 2008;16(10):1117-1123. doi: 10.1007/s00520-007-0399-4. PubMed
24. Mercadante S, Ferrera P, Villari P, Marrazzo A. Aggressive pharmacological treatment for reversing malignant bowel obstruction. J Pain Symptom Manage. 2004;28(4):412-416. doi: 10.1016/j.jpainsymman.2004.01.007. PubMed
25. Peng X, Wang P, Li S, Zhang G, Hu S. Randomized clinical trial comparing octreotide and scopolamine butylbromide in symptom control of patients with inoperable bowel obstruction due to advanced ovarian cancer. World J Surg Oncol. 2015;13:50. doi: 10.1186/s12957-015-0455-3. PubMed
26. Mercadante S, Ripamonti C, Casuccio A, Zecca E, Groff L. Comparison of octreotide and hyoscine butylbromide in controlling gastrointestinal symptoms due to malignant inoperable bowel obstruction. Support Care Cancer. 2000;8(3):188-191. doi: 10.1007/s005200050283. PubMed
27. Mystakidou K, Tsilika E, Kalaidopoulou O, et al. Comparison of octreotide administration vs conservative treatment in the management of inoperable bowel obstruction in patients with far advanced cancer: a randomized, double-blind, controlled clinical trial. Anticancer Res. 2002;22(2B):1187-1192. PubMed
28. Obita GP, Boland EG, Currow DC, Johnson MJ, Boland JW. Somatostatin analogues compared with placebo and other pharmacologic agents in the management of symptoms of inoperable malignant bowel obstruction: a systematic review. J Pain Symptom Manage. 2016;52(6):901-919. doi: 10.1016/j.jpainsymman.2016.05.032. PubMed
29. Watari H, Hosaka M, Wakui Y, et al. A prospective study on the efficacy of octreotide in the management of malignant bowel obstruction in gynecologic cancer. Int J Gynecol Cancer. 2012;22(4):692-696. doi: 10.1097/IGC.0b013e318244ce93. PubMed
30. Hisanaga T, Shinjo T, Morita T, et al. Multicenter prospective study on efficacy and safety of octreotide for inoperable malignant bowel obstruction. Jpn J Clin Oncol. 2010;40(8):739-745. doi: 10.1093/jjco/hyq048. PubMed
31. Laval G, Rousselot H, Toussaint-Martel S, et al. SALTO: a randomized, multicenter study assessing octreotide LAR in inoperable bowel obstruction. Bull Cancer. 2012;99(2):E1-E9. doi: 10.1684/bdc.2011.1535. PubMed
32. Mariani PP, Blumberg J, Landau A, et al. Symptomatic treatment with lanreotide microparticles in inoperable bowel obstruction resulting from peritoneal carcinomatosis: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2012;30(35):4337-4343. doi: 10.1200/JCO.2011.40.5712. PubMed
33. Clark K, Lam L, Currow D. Reducing gastric secretions--a role for histamine 2 antagonists or proton pump inhibitors in malignant bowel obstruction? Support Care Cancer. 2009;17(12):1463-1468. doi: 10.1007/s00520-009-0609-3. PubMed
34. Strand DS, Kim D, Peura DA. 25 years of proton pump inhibitors: a comprehensive review. Gut Liver. 2017;11(1):27-37. doi: 10.5009/gnl15502. PubMed
35. Murakami H, Matsumoto H, Nakamura M, Hirai T, Yamaguchi Y. Octreotide acetate-steroid combination therapy for malignant gastrointestinal obstruction. Anticancer Res. 2013;33(12):5557-5560. PubMed
36. Minoura T, Takeuchi M, Morita T, Kawakami K. Practice patterns of medications for patients with malignant bowel obstruction using a nationwide claims database and the association between treatment outcomes and concomitant use of H2-blockers/proton pump inhibitors and corticosteroids with octreotide. J Pain Symptom Manage. 2018;55(2):413-419. doi: 10.1016/j.jpainsymman.2017.10.019. PubMed
37. Feuer DJ, Broadley KE. Systematic review and meta-analysis of corticosteroids for the resolution of malignant bowel obstruction in advanced gynaecological and gastrointestinal cancers. Systematic Review Steering Committee. Ann Oncol. 1999;10(9):1035-1041. doi: 10.1023/A:1008361102808. PubMed
38. Currow DC, Quinn S, Agar M, et al. Double-blind, placebo-controlled, randomized trial of octreotide in malignant bowel obstruction. J Pain Symptom Manage. 2015;49(5):814-821. doi: 10.1016/j.jpainsymman.2014.09.013. PubMed
39. Davis MP, Furste A. Glycopyrrolate: a useful drug in the palliation of mechanical bowel obstruction. J Pain Symptom Manage. 1999;18(3):153-154. PubMed
40. Zucchi E, Fornasarig M, Martella L, et al. Decompressive percutaneous endoscopic gastrostomy in advanced cancer patients with small-bowel obstruction is feasible and effective: a large prospective study. Support Care Cancer. 2016;24(7):2877-2882. doi: 10.1007/s00520-016-3102-9. PubMed
41. Naghibi M, Smith TR, Elia M. A systematic review with meta-analysis of survival, quality of life and cost-effectiveness of home parenteral nutrition in patients with inoperable malignant bowel obstruction. Clin Nutr. 2015;34(5):825-837. doi: 10.1016/j.clnu.2014.09.010. PubMed
42. O’Connor B, Creedon B. Pharmacological treatment of bowel obstruction in cancer patients. Expert Opin Pharmacother. 2011;12(14):2205-2214. doi: 10.1517/14656566.2011.597382. PubMed
1. Ripamonti CI, Easson AM, Gerdes H. Management of malignant bowel obstruction. Eur J Cancer. 2008;44(8):1105-1115. doi: 10.1016/j.ejca.2008.02.028. PubMed
2. Tuca A, Guell E, Martinez-Losada E, Codorniu N. Malignant bowel obstruction in advanced cancer patients: epidemiology, management, and factors influencing spontaneous resolution. Cancer Manag Res. 2012;4:159-169. doi: 10.2147/CMAR.S29297. PubMed
3. Meier DE. Palliative care in hospitals. J Hosp Med. 2006;1(1):21-28. doi: 10.1002/jhm.3. PubMed
4. Lin RJ, Adelman RD, Diamond RR, Evans AT. The sentinel hospitalization and the role of palliative care. J Hosp Med. 2014;9(5):320-323. doi: 10.1002/jhm.2160. PubMed
5. Anthony T, Baron T, Mercadante S, et al. Report of the clinical protocol committee: development of randomized trials for malignant bowel obstruction. J Pain Symptom Manage. 2007;34(1 Suppl):S49-S59. doi: 10.1016/j.jpainsymman.2007.04.011. PubMed
6. Laval G, Marcelin-Benazech B, Guirimand F, et al. Recommendations for bowel obstruction with peritoneal carcinomatosis. J Pain Symptom Manage. 2014;48(1):75-91. doi: 10.1016/j.jpainsymman.2013.08.022. PubMed
7. Ferguson HJ, Ferguson CI, Speakman J, Ismail T. Management of intestinal obstruction in advanced malignancy. Ann Med Surg. 2015;4(3):264-270. doi: 10.1016/j.amsu.2015.07.018. PubMed
8. Ripamonti C, Mercadante S, Groff L, et al. Role of octreotide, scopolamine butylbromide, and hydration in symptom control of patients with inoperable bowel obstruction and nasogastric tubes: A prospective randomized trial. J Pain Symptom Manage. 2000;19(1):23-34. doi: 10.1016/S0885-3924(99)00147-5. PubMed
9. Rao W, Zhang X, Zhang J, et al. The role of nasogastric tube in decompression after elective colon and rectum surgery: a meta-analysis. Int J Colorectal Dis. 2011;26(4):423-429. doi: 10.1007/s00384-010-1093-4. PubMed
10. Walsh D, Davis M, Ripamonti C, et al. 2016 updated MASCC/ESMO consensus recommendations: management of nausea and vomiting in advanced cancer. Support Care Cancer. 2017;25(1):333-340. doi: 10.1007/s00520-016-3371-3. PubMed
11. Murray-Brown F, Dorman S. Haloperidol for the treatment of nausea and vomiting in palliative care patients. Cochrane Database Syst Rev. 2015;11(11):CD006271. doi: 10.1002/14651858.CD006271.pub3. PubMed
12. Digges M, Hussein A, Wilcock A, et al. Pharmacovigilance in hospice/palliative care: Net effect of haloperidol for nausea or vomiting. J Palliat Med. 2018;21(1):37-43. doi: 10.1089/jpm.2017.0159. PubMed
13. Bruera E, Belzile M, Neumann C, et al. A double-blind, crossover study of controlled-release metoclopramide and placebo for the chronic nausea and dyspepsia of advanced cancer. J Pain Symptom Manage. 2000;19(6):427-435. doi: 10.1016/S0885-3924(00)00138-X. PubMed
14. Gupta M, Davis M, LeGrand S, Walsh D, Lagman R. Nausea and vomiting in advanced cancer: the Cleveland clinic protocol. J Support Oncol. 2013;11(1):8-13. doi: 10.1016/j.suponc.2012.10.002. PubMed
15. Kaneishi K, Kawabata M, Morita T. Olanzapine for the relief of nausea in patients with advanced cancer and incomplete bowel obstruction. J Pain Symptom Manage. 2012;44(4):604-607. doi: 10.1016/j.jpainsymman.2011.10.023. PubMed
16. Prommer E. Olanzapine: palliative medicine update. Am J Hosp Palliat Care. 2013;30(1):75-82. doi: 10.1177/1049909112441241. PubMed
17. Currow DC, Coughlan M, Fardell B, Cooney NJ. Use of ondansetron in palliative medicine. J Pain Symptom Manage. 1997;13(5):302-307. doi: 10.1016/S0885-3924(97)00079-1. PubMed
18. Mystakidou K
19. Tuca A, Roca R, Sala C, et al. Efficacy of granisetron in the antiemetic control of nonsurgical intestinal obstruction in advanced cancer: A phase II clinical trial. J Pain Symptom Manage. 2009;37(2):259-270. doi: 10.1016/j.jpainsymman.2008.01.014. PubMed
20. Prommer E. The role of fentanyl in cancer-related pain. J Palliat Med. 2009;12(10):947-954. doi: 10.1089/jpm.2009.0051. PubMed
21. Shaiova LL, Berger A, Blinderman CD, et al. Consensus guideline on parenteral methadone use in pain and palliative care. Palliat Support Care. 2008;6(2):165-176. doi: 10.1017/S1478951508000254. PubMed
22. Murphy E, Prommer EE, Mihalyo M, Wilcock A. Octreotide. J Pain Symptom Manage. 2010;40(1):142-148. doi: 10.1016/j.jpainsymman.2010.05.002. PubMed
23. Prommer EE. Established and potential therapeutic applications of octreotide in palliative care. Support Care Cancer. 2008;16(10):1117-1123. doi: 10.1007/s00520-007-0399-4. PubMed
24. Mercadante S, Ferrera P, Villari P, Marrazzo A. Aggressive pharmacological treatment for reversing malignant bowel obstruction. J Pain Symptom Manage. 2004;28(4):412-416. doi: 10.1016/j.jpainsymman.2004.01.007. PubMed
25. Peng X, Wang P, Li S, Zhang G, Hu S. Randomized clinical trial comparing octreotide and scopolamine butylbromide in symptom control of patients with inoperable bowel obstruction due to advanced ovarian cancer. World J Surg Oncol. 2015;13:50. doi: 10.1186/s12957-015-0455-3. PubMed
26. Mercadante S, Ripamonti C, Casuccio A, Zecca E, Groff L. Comparison of octreotide and hyoscine butylbromide in controlling gastrointestinal symptoms due to malignant inoperable bowel obstruction. Support Care Cancer. 2000;8(3):188-191. doi: 10.1007/s005200050283. PubMed
27. Mystakidou K, Tsilika E, Kalaidopoulou O, et al. Comparison of octreotide administration vs conservative treatment in the management of inoperable bowel obstruction in patients with far advanced cancer: a randomized, double-blind, controlled clinical trial. Anticancer Res. 2002;22(2B):1187-1192. PubMed
28. Obita GP, Boland EG, Currow DC, Johnson MJ, Boland JW. Somatostatin analogues compared with placebo and other pharmacologic agents in the management of symptoms of inoperable malignant bowel obstruction: a systematic review. J Pain Symptom Manage. 2016;52(6):901-919. doi: 10.1016/j.jpainsymman.2016.05.032. PubMed
29. Watari H, Hosaka M, Wakui Y, et al. A prospective study on the efficacy of octreotide in the management of malignant bowel obstruction in gynecologic cancer. Int J Gynecol Cancer. 2012;22(4):692-696. doi: 10.1097/IGC.0b013e318244ce93. PubMed
30. Hisanaga T, Shinjo T, Morita T, et al. Multicenter prospective study on efficacy and safety of octreotide for inoperable malignant bowel obstruction. Jpn J Clin Oncol. 2010;40(8):739-745. doi: 10.1093/jjco/hyq048. PubMed
31. Laval G, Rousselot H, Toussaint-Martel S, et al. SALTO: a randomized, multicenter study assessing octreotide LAR in inoperable bowel obstruction. Bull Cancer. 2012;99(2):E1-E9. doi: 10.1684/bdc.2011.1535. PubMed
32. Mariani PP, Blumberg J, Landau A, et al. Symptomatic treatment with lanreotide microparticles in inoperable bowel obstruction resulting from peritoneal carcinomatosis: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2012;30(35):4337-4343. doi: 10.1200/JCO.2011.40.5712. PubMed
33. Clark K, Lam L, Currow D. Reducing gastric secretions--a role for histamine 2 antagonists or proton pump inhibitors in malignant bowel obstruction? Support Care Cancer. 2009;17(12):1463-1468. doi: 10.1007/s00520-009-0609-3. PubMed
34. Strand DS, Kim D, Peura DA. 25 years of proton pump inhibitors: a comprehensive review. Gut Liver. 2017;11(1):27-37. doi: 10.5009/gnl15502. PubMed
35. Murakami H, Matsumoto H, Nakamura M, Hirai T, Yamaguchi Y. Octreotide acetate-steroid combination therapy for malignant gastrointestinal obstruction. Anticancer Res. 2013;33(12):5557-5560. PubMed
36. Minoura T, Takeuchi M, Morita T, Kawakami K. Practice patterns of medications for patients with malignant bowel obstruction using a nationwide claims database and the association between treatment outcomes and concomitant use of H2-blockers/proton pump inhibitors and corticosteroids with octreotide. J Pain Symptom Manage. 2018;55(2):413-419. doi: 10.1016/j.jpainsymman.2017.10.019. PubMed
37. Feuer DJ, Broadley KE. Systematic review and meta-analysis of corticosteroids for the resolution of malignant bowel obstruction in advanced gynaecological and gastrointestinal cancers. Systematic Review Steering Committee. Ann Oncol. 1999;10(9):1035-1041. doi: 10.1023/A:1008361102808. PubMed
38. Currow DC, Quinn S, Agar M, et al. Double-blind, placebo-controlled, randomized trial of octreotide in malignant bowel obstruction. J Pain Symptom Manage. 2015;49(5):814-821. doi: 10.1016/j.jpainsymman.2014.09.013. PubMed
39. Davis MP, Furste A. Glycopyrrolate: a useful drug in the palliation of mechanical bowel obstruction. J Pain Symptom Manage. 1999;18(3):153-154. PubMed
40. Zucchi E, Fornasarig M, Martella L, et al. Decompressive percutaneous endoscopic gastrostomy in advanced cancer patients with small-bowel obstruction is feasible and effective: a large prospective study. Support Care Cancer. 2016;24(7):2877-2882. doi: 10.1007/s00520-016-3102-9. PubMed
41. Naghibi M, Smith TR, Elia M. A systematic review with meta-analysis of survival, quality of life and cost-effectiveness of home parenteral nutrition in patients with inoperable malignant bowel obstruction. Clin Nutr. 2015;34(5):825-837. doi: 10.1016/j.clnu.2014.09.010. PubMed
42. O’Connor B, Creedon B. Pharmacological treatment of bowel obstruction in cancer patients. Expert Opin Pharmacother. 2011;12(14):2205-2214. doi: 10.1517/14656566.2011.597382. PubMed
© 2019 Society of Hospital Medicine
Update in Hospital Medicine: Practical Lessons from Current Literature
Hospital medicine continues to expand with respect to the number of practitioners as well as the scope of the practice of those practitioners. In addition, the commitment to, and rigor of, scientific inquiry in the field continues to grow. The authors of this article conducted a review of the medical literature, including articles published between March 2017 and March 2018. The key articles reported studies with high methodological quality, clear findings, and a high potential for impact on clinical practice. The literature was independently reviewed by each author, and candidate works were chosen on the basis of relevance to hospital medicine and expected clinical impact. The articles were organized by subject matter, ranked by applicability to the audience, and selected to meet the time constraints of each talk. Twenty-nine articles were presented at the Update in Hospital Medicine at the 2018 Society of Hospital Medicine and Society of General Internal Medicine annual meetings (B Sharpe, A Burger at SGIM and B Slawski, C Cooper at SHM). Nine articles were included in this review through an iterative voting process. Each author ranked their top five articles from one to five. Points were tallied for each article, and the five articles with the highest points were included. A second round of voting identified the remaining four articles for inclusion. Ties were adjudicated by group discussion. Each article is summarized below, and their key points are highlighted in the table.
KEY PUBLICATIONS
Aspirin in Patients with Previous Percutaneous Coronary Intervention Undergoing Noncardiac Surgery. Graham MM et al. Ann Intern Med. 2018;168(4):237-244.1
Background
The Perioperative Ischemic Evaluation 2 (POISE-2) trial found that perioperative aspirin use had no significant effect on the risk of perioperative death and nonfatal myocardial infarction (MI) in patients who are at risk for vascular complications; however, the risk of major bleeding increased with aspirin use in these patients.2 Nevertheless, the POISE-2 trial did not specifically address the role of aspirin in patients who had undergone previous percutaneous coronary intervention (PCI).
Methods
A post hoc subgroup analysis of POISE-2 evaluated 470 PCI patients (234 aspirin-treated and 236 placebo-treated patients) aged >45 years, 90% of whom had stents. The administration of the study drug was initiated within four hours preoperatively and continued postoperatively. Patients who had bare metal stents placed within the six weeks prior to the study or drug-eluting stents placed within the preceding 12 months were excluded.
Findings
The composite endpoint of risk of death and nonfatal MI was 11.5% in the placebo group and 6% in aspirin-treated patients (HR 0.50; CI, 0.26-0.95). Most of the difference in primary outcome was attributed to an increase in nonfatal MI in the placebo group. Major and life-threatening bleeding were not substantially increased in PCI patients but increased in the overall POISE-2 trial (absolute risk increase 0.8% for major bleeding [95% CI, 0.1%-1.6%]; HR 1.22 [95% CI, 1.01-1.48]). Stent type had no effect on death and nonfatal MI.
Cautions
This was a non-prespecified subgroup analysis with a small sample size.
Implications
Perioperative aspirin use in patients with previous PCI appears to provide more benefit than harm, unless a substantial bleeding risk exists.
Association Between Wait Time and 30-Day Mortality in Adults Undergoing Hip Fracture Surgery. Pincus D et al. JAMA. 2017;318(20):1994-2003.3
Background
Wait times to hip fracture surgery have been associated with mortality in previous studies; however, the wait time associated with complications remains controversial.4,5
Methods
This retrospective cohort study of 42,230 adults modeled the probability of complications in accordance with wait time from hospital arrival to hip fracture surgery. It aimed to identify the optimal time window in which to conduct surgery before complications increased. This window to increased complications was used to define early and delayed surgery. The matched cohorts of early and delayed patients were then used to compare outcomes.
Findings
Overall 30-day mortality was 7%. Complication rates increased when wait times reached 24 hours. Comparing the propensity-matched early (<24 hours) and late (>24 hours) surgery patients revealed that late surgery patients had significantly higher 30-day mortality (6.5% vs 5.8%; % absolute RD 0.79; 95% CI, 0.23-1.35) than early surgery patients and the composite outcome of mortality or other medical complications (MI, DVT, PE, and pneumonia; 12.2% vs 10.1%; % absolute RD 2.16; 95% CI, 1.43-2.89).
Cautions
Only 34% of patients in this study had surgery within 24 hours. The observational cohort study design may result in unmeasured confounders, eg, less sick patients go to surgery more quickly than sicker patients.
Implications
A preoperative wait time of 24 hours appears to represent a threshold of increased risk for 30-day perioperative complications and mortality in hip fracture surgery.
When are Oral Antibiotics a Safe and Effective Choice for Bacterial Bloodstream Infections? An Evidence-Based Narrative Review. Hale AJ et al. J Hosp Med. 2018;13(5):328-335.6
Background
Bloodstream infections (BSIs) are significant causes of morbidity and mortality in the United States. Traditionally, clinicians have relied on intravenous antibiotics for treatment. A recent “Choosing Wisely®” initiative recommends that clinicians should use “oral formulations of highly bioavailable antimicrobials wherever possible.”7 Thus, the authors searched for evidence for scenarios wherein BSIs could be safely treated with oral antibiotics.
Methods
A narrative review was conducted given that robust clinical data for an extensive systematic review were insufficient.
Findings
Key decision points on the use of an oral antibiotic for a diagnosed BSI are as follows: (1) Source control must be attained prior to the consideration of oral antibiotics. (2) A highly bioavailable oral option to which the pathogen is sensitive must be available. (3) Patients must be able to comply with the therapy for the full course and not be on interfering medications. Good evidence for use of oral antibiotics against sensitive gram-negative bacilli other than Pseudomonas exists. Evidence for treating Streptococcus pneumoniae with early transition (within three days) to oral antibiotics is robust when treating bacteremia and pneumonia but not for other primary sites of infection. Evidence for the use of oral antibiotics for B-hemolytic streptococcus, including necrotizing fasciitis and Enterococcus, is insufficient. The evidence supports at least two weeks of IV antibiotics for the treatment of Staphylococcus aureus.
Cautions
This is a narrative review due to limited evidence.
Implications
The early use of oral antibiotics in the setting of bacteremia may be appropriate in select clinical situations.
Prevalence of Pulmonary Embolism in Patients with Syncope. Costantino et al. JAMA Intern Med. 2018;178(3):356-362.8
Background
Data on the prevalence of pulmonary embolism in patients presenting with syncope are conflicting.
Methods
This was a retrospective observational study involving five databases in four countries of >1.6 million adults identified through syncope ICD codes. The rates of pulmonary embolism at first evaluation and pulmonary embolism or venous thromboembolism within 90 days were calculated for emergency room patients and a hospitalized subgroup.
Findings
Pulmonary embolism was rare in patients with syncope, eg, less than 3% for hospitalized patients in this database study.
Cautions
The results of this study are based on the use of administrative databases to confirm the diagnosis of syncope. Additionally, the results include hospitalized and nonhospitalized patients. The design of this study differs significantly from those of the PESIT study, which showed a prevalence of 17% in hospitalized patients.9 The PESIT study specifically sought the diagnosis of pulmonary embolism even when other etiologies for syncope existed.
Implications
Ultimately, the clinical impetus to search for pulmonary embolism in hospitalized patients admitted with syncope will depend on individual presentations. The authors argued that pulmonary embolism is rare in syncope and much lower than 17% but should be considered in appropriate patients.
Balanced Crystalloids versus Saline in Noncritically Ill Patients. Self WH et al. N Engl J Med. 2018;378(9):819-828.10
Background
Data on the optimal composition of intravenous fluids (IVF) are limited. Limited experimental evidence suggests that IVF-induced hyperchloremia results in renal vasoconstriction and acute kidney injury.
Methods
This was a single-center, open-label, multiple crossover trial of >13,000 non-ICU hospitalized patients admitted from the Emergency Department. Patients were randomized to receive either only normal saline or a “balanced crystalloid,” eg, either Lactated Ringer’s or Plasmalyte. The primary outcome was hospital-free days. Secondary outcomes were major adverse kidney events (MAKE) at 30 days.
Findings
The study found no difference in the primary outcome of hospital-free days. However, balanced IVF resulted in a lower incidence of hyperchloremia and a slightly reduced incidence of MAKE 30 (4.7% vs 5.6%; adjusted OR 0.82).
Cautions
The incidence of acute kidney injury was low in this single-center ED population. This study, however, did not include hospitalized patients. The long-term effects on renal function could not be ascertained.
Implications
Hospital-free days after inpatient randomization to either normal saline or “balanced IVF” were not significantly different. “Balanced IVF” may be beneficial in select high renal-risk populations.
Speaker Introductions at Internal Medicine Grand Rounds: Forms of Address Reveal Speaker Bias. Files et al. J Womens Health. 2017;26(5):413-419.11
Background
Gender bias is known to contribute to leadership disparities between men and women in several academic medical centers.
Methods
This was a retrospective observational study reviewing video-archived introductions at Internal Medicine Grand Rounds at two connected institutions. All speakers had doctoral degrees. The outcome measured was the use of a speaker’s professional title during his/her introduction as a function of the introducer’s gender.
Findings
Women were more likely than men to introduce speakers of any gender by their professional title in the 321 forms of address analyzed (96% vs 66%, P < .001). When the introducer and speaker were of different genders, women were more likely to introduce male speakers with formal titles than men introducing female speakers (95% vs 49%, P < .001).
Cautions
This study was done at two associated academic institutions and may not reflect the practice or customs of physicians in other departments or institutions.
Implications
Despite the study’s limitations, it supports a theme of prevalent gender bias within academic medical institutions that may affect the outcomes of leadership, promotion, and scholarship.
Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism. Raskob GE et al. N Engl J Med. 2018;378(7):615-624.12
Background
Low-molecular-weight heparin (LMWH) is the standard of care for the treatment of venous thromboembolism (VTE) in patients with cancer. Direct oral anticoagulants have not been studied for this indication.
Methods
This open-label, noninferiority trial randomized patients with cancer and acute VTE to either LMWH for a minimum of five days followed by oral edoxaban vs subcutaneous dalteparin.
Findings
A total of 1,046 patients were included in the modified intention-to-treat analysis. Patients received treatment for six to twelve months total. A composite outcome of recurrent VTE or major bleed within 12 months occurred in 67 of 522 (12.8%) of patients in the edoxaban group vs 71 of 524 (13.5%) of patients in the dalteparin group (HR 0.91, 95% CI 0.70-1.36, P = .006 for noninferiority). Recurrent VTE occurred more commonly with dalteparin than with edoxaban (11.3% vs 7.9%), whereas major bleeding was less common with dalteparin than with edoxaban (4% vs 6.9%). The increased bleeding rate with edoxaban was predominantly in patients with an upper gastrointestinal (GI) malignancy.
Cautions
This was an open-label study. Patients in the edoxaban still received five days of LMWH prior to oral edoxaban. More patients in the edoxaban group continued treatment for the entire 12-month period, which contributes to the observed decreased bleeding and increased VTE rates in the dalteparin group.
Implications
Oral edoxaban is noninferior to subcutaneous dalteparin for the primary composite endpoint of VTE and bleeding. Notably, the patients in the edoxaban group experienced a lower rate of recurrent VTE and a higher rate of major bleeding than the patients in the dalteparin group. Additional caution about bleeding risk in those with a GI malignancy is recommended.
Can High-flow Nasal Cannula Reduce the Rate of Endotracheal Intubation in Adult Patients with Acute Respiratory Failure Compared with Conventional Oxygen Therapy and Noninvasive Positive Pressure Ventilation? Ni Y-N et al. Chest. 2017;151(4):764-775.13
Background
High-flow nasal cannula (HFNC) can deliver heated and humidified oxygen at rates of up to 60 L/min. Evidence on the benefits of HFNC over usual oxygen therapy or noninvasive positive pressure ventilation (NIPPV) is conflicting.
Methods
This systematic review and meta-analysis included 18 studies (12 RCTs, four retrospective, and two prospective cohort studies) with 3,881 patients with respiratory failure (medical and surgical causes). The included studies compared HFNC with usual oxygen therapy or NIPPV.
Findings
HFNC was associated with lower rates of endotracheal intubation (OR 0.47, 95% CI 0.27-0.84, P = .01) relative to oxygen therapy. Intubation rates did not differ between HFNC and NIPPV (OR 0.73, 95% CI 0.47-1.13, P = .16). No differences in ICU mortality or ICU length of stay (LOS) were found when HFNC was compared with either usual oxygen therapy or NIPPV.
Cautions
The significant heterogeneity in study design across studies is mainly attributable to varying causes of respiratory failure and differences in flow rate, oxygen concentration, and treatment duration across studies.
Implications
In patients with respiratory failure, HFNC may reduce intubation when compared with usual oxygen therapy and has similar ICU mortality when compared with usual oxygen and NIPPV.
Errors in the Diagnosis of Spinal Epidural Abscesses in the Era of Electronic Health Records. Bhise V et al. Am J Med. 2017;130(8):975-981.14
Background
Diagnostic errors are common in patients with spinal epidural abscess, but the main contributing factors are unclear.15
Methods
All patients who were newly diagnosed with spinal epidural abscess in 2013 were identified from the Veterans Affairs (VA) national database. Charts were reviewed for diagnostic delay and contributing factors, including the presence of “red flag” symptoms (eg, fever and neurological deficits).
Findings
Of the 119 patients with a new diagnosis of spinal epidural abscess, 66 (56%) had a diagnostic error. The median time to diagnosis in those with a diagnostic error was 12 days vs four days in those without error (P < .01). Common missed red flags in error cases included fever (n = 57, 86.4%), focal neurologic deficit (n = 54, 81.8%), and active infection (n = 54, 81.8%). Most errors occurred during the provider–patient encounter (eg, information not gathered during the history or physical). The magnitude of harm was serious for most patients (n = 40, 60.6%) and contributed to death in eight patients (12.1%).
Cautions
The study may not be generalizable because it was limited to the VA health system.
Implications
Diagnostic errors are common in patients with spinal epidural abscesses and can lead to serious harm. Health systems should build mechanisms to support providers in the evaluation of patients with back pain.
1. Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med. 2018;168(4):237-244. doi: 10.7326/M17-2341.
2. Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014;370(16):1494-1503. doi: 10.1056/NEJMoa1401105
3. Pincus D, Ravi B, Wasserstein D, et al. Association between wait time and 30-day mortality in adults undergoing hip fracture surgery. JAMA. 2017;318(20):1994-2003. doi: 10.1001/jama.2017.17606.
4. Simunovic N, Devereaux PJ, Sprague S, et al. Effect of early surgery after hip fracture on mortality and complications: systematic review and meta-analysis. CMAJ. 2010;182(15):1609-1616. doi: 10.1503/cmaj.092220.
5. Shiga T, Wajima Z, Ohe Y. Is operative delay associated with increased mortality of hip fracture patients? ystematic review, meta-analysis, and meta-regression. Can J Anaesth. 2008;55(3):146-154. doi: 10.1007/BF03016088.
6. Hale AJ, Snyder GM, Ahern JW, Eliopoulos G, Ricotta D, Alston WK. When are oral antibiotics a safe and effective choice for bacterial bloodstream infections? An evidence-based narrative review. J Hosp Med. 2018;13(5):328-335. doi: 10.12788/jhm.2949.
7. Lehmann C, Berner R, Bogner JR, et al. The “Choosing Wisely” initiative in infectious diseases. Infection. 2017;45(3):263-268. doi: 10.1007/s15010-017-0997-0.
8. Costantino G, Ruwald MH, Quinn J, et al. Prevalence of pulmonary embolism in patients with syncope. JAMA Intern Med. 2018;178(3):356-362. doi: 10.1001/jamainternmed.2017.8175.
9. Prandoni P, Lensing AW, Prins MH, et al. Prevalence of pulmonary embolism among patients hospitalized for syncope. N Engl J Med. 2016;375(16):1524-1531. doi: 10.1056/NEJMoa1602172
10. Self WH, Semler MW, Wanderer JP, et al. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. 2018;378(9):819-828. doi: 10.1056/NEJMoa1711586.
11. Files JA, Mayer AP, Ko MG, et al. Speaker introductions at internal medicine grand rounds: forms of address reveal gender bias. J Womens Health (Larchmt). 2017;26(5):413-419. doi: 10.1089/jwh.2016.6044.
12. Raskob GE, van Es N, Verhamme P, et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018;378(7):615-624. doi: 10.1056/NEJMoa1711948.
13. Ni YN, Luo J, Yu H, et al. Can high-flow nasal cannula reduce the rate of endotracheal intubation in adult patients with acute respiratory failure compared with conventional oxygen therapy and noninvasive positive pressure ventilation?: A systematic review and meta-analysis. Chest. 2017;151(4):764-775. doi: 10.1016/j.chest.2017.01.004.
14. Bhise V, Meyer AND, Singh H, et al. Errors in diagnosis of spinal epidural abscesses in the era of electronic health records. Am J Med. 2017;130(8):975-981. doi: 10.1016/j.amjmed.2017.03.009
15. Davis DP, Wold RM, Patel RJ, et al. The clinical presentation and impact of diagnostic delays on emergency department patients with spinal epidural abscess. J Emerg Med. 2004;26(3):285-291. doi: 10.1016/j.jemermed.2003.11.013.
Hospital medicine continues to expand with respect to the number of practitioners as well as the scope of the practice of those practitioners. In addition, the commitment to, and rigor of, scientific inquiry in the field continues to grow. The authors of this article conducted a review of the medical literature, including articles published between March 2017 and March 2018. The key articles reported studies with high methodological quality, clear findings, and a high potential for impact on clinical practice. The literature was independently reviewed by each author, and candidate works were chosen on the basis of relevance to hospital medicine and expected clinical impact. The articles were organized by subject matter, ranked by applicability to the audience, and selected to meet the time constraints of each talk. Twenty-nine articles were presented at the Update in Hospital Medicine at the 2018 Society of Hospital Medicine and Society of General Internal Medicine annual meetings (B Sharpe, A Burger at SGIM and B Slawski, C Cooper at SHM). Nine articles were included in this review through an iterative voting process. Each author ranked their top five articles from one to five. Points were tallied for each article, and the five articles with the highest points were included. A second round of voting identified the remaining four articles for inclusion. Ties were adjudicated by group discussion. Each article is summarized below, and their key points are highlighted in the table.
KEY PUBLICATIONS
Aspirin in Patients with Previous Percutaneous Coronary Intervention Undergoing Noncardiac Surgery. Graham MM et al. Ann Intern Med. 2018;168(4):237-244.1
Background
The Perioperative Ischemic Evaluation 2 (POISE-2) trial found that perioperative aspirin use had no significant effect on the risk of perioperative death and nonfatal myocardial infarction (MI) in patients who are at risk for vascular complications; however, the risk of major bleeding increased with aspirin use in these patients.2 Nevertheless, the POISE-2 trial did not specifically address the role of aspirin in patients who had undergone previous percutaneous coronary intervention (PCI).
Methods
A post hoc subgroup analysis of POISE-2 evaluated 470 PCI patients (234 aspirin-treated and 236 placebo-treated patients) aged >45 years, 90% of whom had stents. The administration of the study drug was initiated within four hours preoperatively and continued postoperatively. Patients who had bare metal stents placed within the six weeks prior to the study or drug-eluting stents placed within the preceding 12 months were excluded.
Findings
The composite endpoint of risk of death and nonfatal MI was 11.5% in the placebo group and 6% in aspirin-treated patients (HR 0.50; CI, 0.26-0.95). Most of the difference in primary outcome was attributed to an increase in nonfatal MI in the placebo group. Major and life-threatening bleeding were not substantially increased in PCI patients but increased in the overall POISE-2 trial (absolute risk increase 0.8% for major bleeding [95% CI, 0.1%-1.6%]; HR 1.22 [95% CI, 1.01-1.48]). Stent type had no effect on death and nonfatal MI.
Cautions
This was a non-prespecified subgroup analysis with a small sample size.
Implications
Perioperative aspirin use in patients with previous PCI appears to provide more benefit than harm, unless a substantial bleeding risk exists.
Association Between Wait Time and 30-Day Mortality in Adults Undergoing Hip Fracture Surgery. Pincus D et al. JAMA. 2017;318(20):1994-2003.3
Background
Wait times to hip fracture surgery have been associated with mortality in previous studies; however, the wait time associated with complications remains controversial.4,5
Methods
This retrospective cohort study of 42,230 adults modeled the probability of complications in accordance with wait time from hospital arrival to hip fracture surgery. It aimed to identify the optimal time window in which to conduct surgery before complications increased. This window to increased complications was used to define early and delayed surgery. The matched cohorts of early and delayed patients were then used to compare outcomes.
Findings
Overall 30-day mortality was 7%. Complication rates increased when wait times reached 24 hours. Comparing the propensity-matched early (<24 hours) and late (>24 hours) surgery patients revealed that late surgery patients had significantly higher 30-day mortality (6.5% vs 5.8%; % absolute RD 0.79; 95% CI, 0.23-1.35) than early surgery patients and the composite outcome of mortality or other medical complications (MI, DVT, PE, and pneumonia; 12.2% vs 10.1%; % absolute RD 2.16; 95% CI, 1.43-2.89).
Cautions
Only 34% of patients in this study had surgery within 24 hours. The observational cohort study design may result in unmeasured confounders, eg, less sick patients go to surgery more quickly than sicker patients.
Implications
A preoperative wait time of 24 hours appears to represent a threshold of increased risk for 30-day perioperative complications and mortality in hip fracture surgery.
When are Oral Antibiotics a Safe and Effective Choice for Bacterial Bloodstream Infections? An Evidence-Based Narrative Review. Hale AJ et al. J Hosp Med. 2018;13(5):328-335.6
Background
Bloodstream infections (BSIs) are significant causes of morbidity and mortality in the United States. Traditionally, clinicians have relied on intravenous antibiotics for treatment. A recent “Choosing Wisely®” initiative recommends that clinicians should use “oral formulations of highly bioavailable antimicrobials wherever possible.”7 Thus, the authors searched for evidence for scenarios wherein BSIs could be safely treated with oral antibiotics.
Methods
A narrative review was conducted given that robust clinical data for an extensive systematic review were insufficient.
Findings
Key decision points on the use of an oral antibiotic for a diagnosed BSI are as follows: (1) Source control must be attained prior to the consideration of oral antibiotics. (2) A highly bioavailable oral option to which the pathogen is sensitive must be available. (3) Patients must be able to comply with the therapy for the full course and not be on interfering medications. Good evidence for use of oral antibiotics against sensitive gram-negative bacilli other than Pseudomonas exists. Evidence for treating Streptococcus pneumoniae with early transition (within three days) to oral antibiotics is robust when treating bacteremia and pneumonia but not for other primary sites of infection. Evidence for the use of oral antibiotics for B-hemolytic streptococcus, including necrotizing fasciitis and Enterococcus, is insufficient. The evidence supports at least two weeks of IV antibiotics for the treatment of Staphylococcus aureus.
Cautions
This is a narrative review due to limited evidence.
Implications
The early use of oral antibiotics in the setting of bacteremia may be appropriate in select clinical situations.
Prevalence of Pulmonary Embolism in Patients with Syncope. Costantino et al. JAMA Intern Med. 2018;178(3):356-362.8
Background
Data on the prevalence of pulmonary embolism in patients presenting with syncope are conflicting.
Methods
This was a retrospective observational study involving five databases in four countries of >1.6 million adults identified through syncope ICD codes. The rates of pulmonary embolism at first evaluation and pulmonary embolism or venous thromboembolism within 90 days were calculated for emergency room patients and a hospitalized subgroup.
Findings
Pulmonary embolism was rare in patients with syncope, eg, less than 3% for hospitalized patients in this database study.
Cautions
The results of this study are based on the use of administrative databases to confirm the diagnosis of syncope. Additionally, the results include hospitalized and nonhospitalized patients. The design of this study differs significantly from those of the PESIT study, which showed a prevalence of 17% in hospitalized patients.9 The PESIT study specifically sought the diagnosis of pulmonary embolism even when other etiologies for syncope existed.
Implications
Ultimately, the clinical impetus to search for pulmonary embolism in hospitalized patients admitted with syncope will depend on individual presentations. The authors argued that pulmonary embolism is rare in syncope and much lower than 17% but should be considered in appropriate patients.
Balanced Crystalloids versus Saline in Noncritically Ill Patients. Self WH et al. N Engl J Med. 2018;378(9):819-828.10
Background
Data on the optimal composition of intravenous fluids (IVF) are limited. Limited experimental evidence suggests that IVF-induced hyperchloremia results in renal vasoconstriction and acute kidney injury.
Methods
This was a single-center, open-label, multiple crossover trial of >13,000 non-ICU hospitalized patients admitted from the Emergency Department. Patients were randomized to receive either only normal saline or a “balanced crystalloid,” eg, either Lactated Ringer’s or Plasmalyte. The primary outcome was hospital-free days. Secondary outcomes were major adverse kidney events (MAKE) at 30 days.
Findings
The study found no difference in the primary outcome of hospital-free days. However, balanced IVF resulted in a lower incidence of hyperchloremia and a slightly reduced incidence of MAKE 30 (4.7% vs 5.6%; adjusted OR 0.82).
Cautions
The incidence of acute kidney injury was low in this single-center ED population. This study, however, did not include hospitalized patients. The long-term effects on renal function could not be ascertained.
Implications
Hospital-free days after inpatient randomization to either normal saline or “balanced IVF” were not significantly different. “Balanced IVF” may be beneficial in select high renal-risk populations.
Speaker Introductions at Internal Medicine Grand Rounds: Forms of Address Reveal Speaker Bias. Files et al. J Womens Health. 2017;26(5):413-419.11
Background
Gender bias is known to contribute to leadership disparities between men and women in several academic medical centers.
Methods
This was a retrospective observational study reviewing video-archived introductions at Internal Medicine Grand Rounds at two connected institutions. All speakers had doctoral degrees. The outcome measured was the use of a speaker’s professional title during his/her introduction as a function of the introducer’s gender.
Findings
Women were more likely than men to introduce speakers of any gender by their professional title in the 321 forms of address analyzed (96% vs 66%, P < .001). When the introducer and speaker were of different genders, women were more likely to introduce male speakers with formal titles than men introducing female speakers (95% vs 49%, P < .001).
Cautions
This study was done at two associated academic institutions and may not reflect the practice or customs of physicians in other departments or institutions.
Implications
Despite the study’s limitations, it supports a theme of prevalent gender bias within academic medical institutions that may affect the outcomes of leadership, promotion, and scholarship.
Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism. Raskob GE et al. N Engl J Med. 2018;378(7):615-624.12
Background
Low-molecular-weight heparin (LMWH) is the standard of care for the treatment of venous thromboembolism (VTE) in patients with cancer. Direct oral anticoagulants have not been studied for this indication.
Methods
This open-label, noninferiority trial randomized patients with cancer and acute VTE to either LMWH for a minimum of five days followed by oral edoxaban vs subcutaneous dalteparin.
Findings
A total of 1,046 patients were included in the modified intention-to-treat analysis. Patients received treatment for six to twelve months total. A composite outcome of recurrent VTE or major bleed within 12 months occurred in 67 of 522 (12.8%) of patients in the edoxaban group vs 71 of 524 (13.5%) of patients in the dalteparin group (HR 0.91, 95% CI 0.70-1.36, P = .006 for noninferiority). Recurrent VTE occurred more commonly with dalteparin than with edoxaban (11.3% vs 7.9%), whereas major bleeding was less common with dalteparin than with edoxaban (4% vs 6.9%). The increased bleeding rate with edoxaban was predominantly in patients with an upper gastrointestinal (GI) malignancy.
Cautions
This was an open-label study. Patients in the edoxaban still received five days of LMWH prior to oral edoxaban. More patients in the edoxaban group continued treatment for the entire 12-month period, which contributes to the observed decreased bleeding and increased VTE rates in the dalteparin group.
Implications
Oral edoxaban is noninferior to subcutaneous dalteparin for the primary composite endpoint of VTE and bleeding. Notably, the patients in the edoxaban group experienced a lower rate of recurrent VTE and a higher rate of major bleeding than the patients in the dalteparin group. Additional caution about bleeding risk in those with a GI malignancy is recommended.
Can High-flow Nasal Cannula Reduce the Rate of Endotracheal Intubation in Adult Patients with Acute Respiratory Failure Compared with Conventional Oxygen Therapy and Noninvasive Positive Pressure Ventilation? Ni Y-N et al. Chest. 2017;151(4):764-775.13
Background
High-flow nasal cannula (HFNC) can deliver heated and humidified oxygen at rates of up to 60 L/min. Evidence on the benefits of HFNC over usual oxygen therapy or noninvasive positive pressure ventilation (NIPPV) is conflicting.
Methods
This systematic review and meta-analysis included 18 studies (12 RCTs, four retrospective, and two prospective cohort studies) with 3,881 patients with respiratory failure (medical and surgical causes). The included studies compared HFNC with usual oxygen therapy or NIPPV.
Findings
HFNC was associated with lower rates of endotracheal intubation (OR 0.47, 95% CI 0.27-0.84, P = .01) relative to oxygen therapy. Intubation rates did not differ between HFNC and NIPPV (OR 0.73, 95% CI 0.47-1.13, P = .16). No differences in ICU mortality or ICU length of stay (LOS) were found when HFNC was compared with either usual oxygen therapy or NIPPV.
Cautions
The significant heterogeneity in study design across studies is mainly attributable to varying causes of respiratory failure and differences in flow rate, oxygen concentration, and treatment duration across studies.
Implications
In patients with respiratory failure, HFNC may reduce intubation when compared with usual oxygen therapy and has similar ICU mortality when compared with usual oxygen and NIPPV.
Errors in the Diagnosis of Spinal Epidural Abscesses in the Era of Electronic Health Records. Bhise V et al. Am J Med. 2017;130(8):975-981.14
Background
Diagnostic errors are common in patients with spinal epidural abscess, but the main contributing factors are unclear.15
Methods
All patients who were newly diagnosed with spinal epidural abscess in 2013 were identified from the Veterans Affairs (VA) national database. Charts were reviewed for diagnostic delay and contributing factors, including the presence of “red flag” symptoms (eg, fever and neurological deficits).
Findings
Of the 119 patients with a new diagnosis of spinal epidural abscess, 66 (56%) had a diagnostic error. The median time to diagnosis in those with a diagnostic error was 12 days vs four days in those without error (P < .01). Common missed red flags in error cases included fever (n = 57, 86.4%), focal neurologic deficit (n = 54, 81.8%), and active infection (n = 54, 81.8%). Most errors occurred during the provider–patient encounter (eg, information not gathered during the history or physical). The magnitude of harm was serious for most patients (n = 40, 60.6%) and contributed to death in eight patients (12.1%).
Cautions
The study may not be generalizable because it was limited to the VA health system.
Implications
Diagnostic errors are common in patients with spinal epidural abscesses and can lead to serious harm. Health systems should build mechanisms to support providers in the evaluation of patients with back pain.
Hospital medicine continues to expand with respect to the number of practitioners as well as the scope of the practice of those practitioners. In addition, the commitment to, and rigor of, scientific inquiry in the field continues to grow. The authors of this article conducted a review of the medical literature, including articles published between March 2017 and March 2018. The key articles reported studies with high methodological quality, clear findings, and a high potential for impact on clinical practice. The literature was independently reviewed by each author, and candidate works were chosen on the basis of relevance to hospital medicine and expected clinical impact. The articles were organized by subject matter, ranked by applicability to the audience, and selected to meet the time constraints of each talk. Twenty-nine articles were presented at the Update in Hospital Medicine at the 2018 Society of Hospital Medicine and Society of General Internal Medicine annual meetings (B Sharpe, A Burger at SGIM and B Slawski, C Cooper at SHM). Nine articles were included in this review through an iterative voting process. Each author ranked their top five articles from one to five. Points were tallied for each article, and the five articles with the highest points were included. A second round of voting identified the remaining four articles for inclusion. Ties were adjudicated by group discussion. Each article is summarized below, and their key points are highlighted in the table.
KEY PUBLICATIONS
Aspirin in Patients with Previous Percutaneous Coronary Intervention Undergoing Noncardiac Surgery. Graham MM et al. Ann Intern Med. 2018;168(4):237-244.1
Background
The Perioperative Ischemic Evaluation 2 (POISE-2) trial found that perioperative aspirin use had no significant effect on the risk of perioperative death and nonfatal myocardial infarction (MI) in patients who are at risk for vascular complications; however, the risk of major bleeding increased with aspirin use in these patients.2 Nevertheless, the POISE-2 trial did not specifically address the role of aspirin in patients who had undergone previous percutaneous coronary intervention (PCI).
Methods
A post hoc subgroup analysis of POISE-2 evaluated 470 PCI patients (234 aspirin-treated and 236 placebo-treated patients) aged >45 years, 90% of whom had stents. The administration of the study drug was initiated within four hours preoperatively and continued postoperatively. Patients who had bare metal stents placed within the six weeks prior to the study or drug-eluting stents placed within the preceding 12 months were excluded.
Findings
The composite endpoint of risk of death and nonfatal MI was 11.5% in the placebo group and 6% in aspirin-treated patients (HR 0.50; CI, 0.26-0.95). Most of the difference in primary outcome was attributed to an increase in nonfatal MI in the placebo group. Major and life-threatening bleeding were not substantially increased in PCI patients but increased in the overall POISE-2 trial (absolute risk increase 0.8% for major bleeding [95% CI, 0.1%-1.6%]; HR 1.22 [95% CI, 1.01-1.48]). Stent type had no effect on death and nonfatal MI.
Cautions
This was a non-prespecified subgroup analysis with a small sample size.
Implications
Perioperative aspirin use in patients with previous PCI appears to provide more benefit than harm, unless a substantial bleeding risk exists.
Association Between Wait Time and 30-Day Mortality in Adults Undergoing Hip Fracture Surgery. Pincus D et al. JAMA. 2017;318(20):1994-2003.3
Background
Wait times to hip fracture surgery have been associated with mortality in previous studies; however, the wait time associated with complications remains controversial.4,5
Methods
This retrospective cohort study of 42,230 adults modeled the probability of complications in accordance with wait time from hospital arrival to hip fracture surgery. It aimed to identify the optimal time window in which to conduct surgery before complications increased. This window to increased complications was used to define early and delayed surgery. The matched cohorts of early and delayed patients were then used to compare outcomes.
Findings
Overall 30-day mortality was 7%. Complication rates increased when wait times reached 24 hours. Comparing the propensity-matched early (<24 hours) and late (>24 hours) surgery patients revealed that late surgery patients had significantly higher 30-day mortality (6.5% vs 5.8%; % absolute RD 0.79; 95% CI, 0.23-1.35) than early surgery patients and the composite outcome of mortality or other medical complications (MI, DVT, PE, and pneumonia; 12.2% vs 10.1%; % absolute RD 2.16; 95% CI, 1.43-2.89).
Cautions
Only 34% of patients in this study had surgery within 24 hours. The observational cohort study design may result in unmeasured confounders, eg, less sick patients go to surgery more quickly than sicker patients.
Implications
A preoperative wait time of 24 hours appears to represent a threshold of increased risk for 30-day perioperative complications and mortality in hip fracture surgery.
When are Oral Antibiotics a Safe and Effective Choice for Bacterial Bloodstream Infections? An Evidence-Based Narrative Review. Hale AJ et al. J Hosp Med. 2018;13(5):328-335.6
Background
Bloodstream infections (BSIs) are significant causes of morbidity and mortality in the United States. Traditionally, clinicians have relied on intravenous antibiotics for treatment. A recent “Choosing Wisely®” initiative recommends that clinicians should use “oral formulations of highly bioavailable antimicrobials wherever possible.”7 Thus, the authors searched for evidence for scenarios wherein BSIs could be safely treated with oral antibiotics.
Methods
A narrative review was conducted given that robust clinical data for an extensive systematic review were insufficient.
Findings
Key decision points on the use of an oral antibiotic for a diagnosed BSI are as follows: (1) Source control must be attained prior to the consideration of oral antibiotics. (2) A highly bioavailable oral option to which the pathogen is sensitive must be available. (3) Patients must be able to comply with the therapy for the full course and not be on interfering medications. Good evidence for use of oral antibiotics against sensitive gram-negative bacilli other than Pseudomonas exists. Evidence for treating Streptococcus pneumoniae with early transition (within three days) to oral antibiotics is robust when treating bacteremia and pneumonia but not for other primary sites of infection. Evidence for the use of oral antibiotics for B-hemolytic streptococcus, including necrotizing fasciitis and Enterococcus, is insufficient. The evidence supports at least two weeks of IV antibiotics for the treatment of Staphylococcus aureus.
Cautions
This is a narrative review due to limited evidence.
Implications
The early use of oral antibiotics in the setting of bacteremia may be appropriate in select clinical situations.
Prevalence of Pulmonary Embolism in Patients with Syncope. Costantino et al. JAMA Intern Med. 2018;178(3):356-362.8
Background
Data on the prevalence of pulmonary embolism in patients presenting with syncope are conflicting.
Methods
This was a retrospective observational study involving five databases in four countries of >1.6 million adults identified through syncope ICD codes. The rates of pulmonary embolism at first evaluation and pulmonary embolism or venous thromboembolism within 90 days were calculated for emergency room patients and a hospitalized subgroup.
Findings
Pulmonary embolism was rare in patients with syncope, eg, less than 3% for hospitalized patients in this database study.
Cautions
The results of this study are based on the use of administrative databases to confirm the diagnosis of syncope. Additionally, the results include hospitalized and nonhospitalized patients. The design of this study differs significantly from those of the PESIT study, which showed a prevalence of 17% in hospitalized patients.9 The PESIT study specifically sought the diagnosis of pulmonary embolism even when other etiologies for syncope existed.
Implications
Ultimately, the clinical impetus to search for pulmonary embolism in hospitalized patients admitted with syncope will depend on individual presentations. The authors argued that pulmonary embolism is rare in syncope and much lower than 17% but should be considered in appropriate patients.
Balanced Crystalloids versus Saline in Noncritically Ill Patients. Self WH et al. N Engl J Med. 2018;378(9):819-828.10
Background
Data on the optimal composition of intravenous fluids (IVF) are limited. Limited experimental evidence suggests that IVF-induced hyperchloremia results in renal vasoconstriction and acute kidney injury.
Methods
This was a single-center, open-label, multiple crossover trial of >13,000 non-ICU hospitalized patients admitted from the Emergency Department. Patients were randomized to receive either only normal saline or a “balanced crystalloid,” eg, either Lactated Ringer’s or Plasmalyte. The primary outcome was hospital-free days. Secondary outcomes were major adverse kidney events (MAKE) at 30 days.
Findings
The study found no difference in the primary outcome of hospital-free days. However, balanced IVF resulted in a lower incidence of hyperchloremia and a slightly reduced incidence of MAKE 30 (4.7% vs 5.6%; adjusted OR 0.82).
Cautions
The incidence of acute kidney injury was low in this single-center ED population. This study, however, did not include hospitalized patients. The long-term effects on renal function could not be ascertained.
Implications
Hospital-free days after inpatient randomization to either normal saline or “balanced IVF” were not significantly different. “Balanced IVF” may be beneficial in select high renal-risk populations.
Speaker Introductions at Internal Medicine Grand Rounds: Forms of Address Reveal Speaker Bias. Files et al. J Womens Health. 2017;26(5):413-419.11
Background
Gender bias is known to contribute to leadership disparities between men and women in several academic medical centers.
Methods
This was a retrospective observational study reviewing video-archived introductions at Internal Medicine Grand Rounds at two connected institutions. All speakers had doctoral degrees. The outcome measured was the use of a speaker’s professional title during his/her introduction as a function of the introducer’s gender.
Findings
Women were more likely than men to introduce speakers of any gender by their professional title in the 321 forms of address analyzed (96% vs 66%, P < .001). When the introducer and speaker were of different genders, women were more likely to introduce male speakers with formal titles than men introducing female speakers (95% vs 49%, P < .001).
Cautions
This study was done at two associated academic institutions and may not reflect the practice or customs of physicians in other departments or institutions.
Implications
Despite the study’s limitations, it supports a theme of prevalent gender bias within academic medical institutions that may affect the outcomes of leadership, promotion, and scholarship.
Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism. Raskob GE et al. N Engl J Med. 2018;378(7):615-624.12
Background
Low-molecular-weight heparin (LMWH) is the standard of care for the treatment of venous thromboembolism (VTE) in patients with cancer. Direct oral anticoagulants have not been studied for this indication.
Methods
This open-label, noninferiority trial randomized patients with cancer and acute VTE to either LMWH for a minimum of five days followed by oral edoxaban vs subcutaneous dalteparin.
Findings
A total of 1,046 patients were included in the modified intention-to-treat analysis. Patients received treatment for six to twelve months total. A composite outcome of recurrent VTE or major bleed within 12 months occurred in 67 of 522 (12.8%) of patients in the edoxaban group vs 71 of 524 (13.5%) of patients in the dalteparin group (HR 0.91, 95% CI 0.70-1.36, P = .006 for noninferiority). Recurrent VTE occurred more commonly with dalteparin than with edoxaban (11.3% vs 7.9%), whereas major bleeding was less common with dalteparin than with edoxaban (4% vs 6.9%). The increased bleeding rate with edoxaban was predominantly in patients with an upper gastrointestinal (GI) malignancy.
Cautions
This was an open-label study. Patients in the edoxaban still received five days of LMWH prior to oral edoxaban. More patients in the edoxaban group continued treatment for the entire 12-month period, which contributes to the observed decreased bleeding and increased VTE rates in the dalteparin group.
Implications
Oral edoxaban is noninferior to subcutaneous dalteparin for the primary composite endpoint of VTE and bleeding. Notably, the patients in the edoxaban group experienced a lower rate of recurrent VTE and a higher rate of major bleeding than the patients in the dalteparin group. Additional caution about bleeding risk in those with a GI malignancy is recommended.
Can High-flow Nasal Cannula Reduce the Rate of Endotracheal Intubation in Adult Patients with Acute Respiratory Failure Compared with Conventional Oxygen Therapy and Noninvasive Positive Pressure Ventilation? Ni Y-N et al. Chest. 2017;151(4):764-775.13
Background
High-flow nasal cannula (HFNC) can deliver heated and humidified oxygen at rates of up to 60 L/min. Evidence on the benefits of HFNC over usual oxygen therapy or noninvasive positive pressure ventilation (NIPPV) is conflicting.
Methods
This systematic review and meta-analysis included 18 studies (12 RCTs, four retrospective, and two prospective cohort studies) with 3,881 patients with respiratory failure (medical and surgical causes). The included studies compared HFNC with usual oxygen therapy or NIPPV.
Findings
HFNC was associated with lower rates of endotracheal intubation (OR 0.47, 95% CI 0.27-0.84, P = .01) relative to oxygen therapy. Intubation rates did not differ between HFNC and NIPPV (OR 0.73, 95% CI 0.47-1.13, P = .16). No differences in ICU mortality or ICU length of stay (LOS) were found when HFNC was compared with either usual oxygen therapy or NIPPV.
Cautions
The significant heterogeneity in study design across studies is mainly attributable to varying causes of respiratory failure and differences in flow rate, oxygen concentration, and treatment duration across studies.
Implications
In patients with respiratory failure, HFNC may reduce intubation when compared with usual oxygen therapy and has similar ICU mortality when compared with usual oxygen and NIPPV.
Errors in the Diagnosis of Spinal Epidural Abscesses in the Era of Electronic Health Records. Bhise V et al. Am J Med. 2017;130(8):975-981.14
Background
Diagnostic errors are common in patients with spinal epidural abscess, but the main contributing factors are unclear.15
Methods
All patients who were newly diagnosed with spinal epidural abscess in 2013 were identified from the Veterans Affairs (VA) national database. Charts were reviewed for diagnostic delay and contributing factors, including the presence of “red flag” symptoms (eg, fever and neurological deficits).
Findings
Of the 119 patients with a new diagnosis of spinal epidural abscess, 66 (56%) had a diagnostic error. The median time to diagnosis in those with a diagnostic error was 12 days vs four days in those without error (P < .01). Common missed red flags in error cases included fever (n = 57, 86.4%), focal neurologic deficit (n = 54, 81.8%), and active infection (n = 54, 81.8%). Most errors occurred during the provider–patient encounter (eg, information not gathered during the history or physical). The magnitude of harm was serious for most patients (n = 40, 60.6%) and contributed to death in eight patients (12.1%).
Cautions
The study may not be generalizable because it was limited to the VA health system.
Implications
Diagnostic errors are common in patients with spinal epidural abscesses and can lead to serious harm. Health systems should build mechanisms to support providers in the evaluation of patients with back pain.
1. Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med. 2018;168(4):237-244. doi: 10.7326/M17-2341.
2. Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014;370(16):1494-1503. doi: 10.1056/NEJMoa1401105
3. Pincus D, Ravi B, Wasserstein D, et al. Association between wait time and 30-day mortality in adults undergoing hip fracture surgery. JAMA. 2017;318(20):1994-2003. doi: 10.1001/jama.2017.17606.
4. Simunovic N, Devereaux PJ, Sprague S, et al. Effect of early surgery after hip fracture on mortality and complications: systematic review and meta-analysis. CMAJ. 2010;182(15):1609-1616. doi: 10.1503/cmaj.092220.
5. Shiga T, Wajima Z, Ohe Y. Is operative delay associated with increased mortality of hip fracture patients? ystematic review, meta-analysis, and meta-regression. Can J Anaesth. 2008;55(3):146-154. doi: 10.1007/BF03016088.
6. Hale AJ, Snyder GM, Ahern JW, Eliopoulos G, Ricotta D, Alston WK. When are oral antibiotics a safe and effective choice for bacterial bloodstream infections? An evidence-based narrative review. J Hosp Med. 2018;13(5):328-335. doi: 10.12788/jhm.2949.
7. Lehmann C, Berner R, Bogner JR, et al. The “Choosing Wisely” initiative in infectious diseases. Infection. 2017;45(3):263-268. doi: 10.1007/s15010-017-0997-0.
8. Costantino G, Ruwald MH, Quinn J, et al. Prevalence of pulmonary embolism in patients with syncope. JAMA Intern Med. 2018;178(3):356-362. doi: 10.1001/jamainternmed.2017.8175.
9. Prandoni P, Lensing AW, Prins MH, et al. Prevalence of pulmonary embolism among patients hospitalized for syncope. N Engl J Med. 2016;375(16):1524-1531. doi: 10.1056/NEJMoa1602172
10. Self WH, Semler MW, Wanderer JP, et al. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. 2018;378(9):819-828. doi: 10.1056/NEJMoa1711586.
11. Files JA, Mayer AP, Ko MG, et al. Speaker introductions at internal medicine grand rounds: forms of address reveal gender bias. J Womens Health (Larchmt). 2017;26(5):413-419. doi: 10.1089/jwh.2016.6044.
12. Raskob GE, van Es N, Verhamme P, et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018;378(7):615-624. doi: 10.1056/NEJMoa1711948.
13. Ni YN, Luo J, Yu H, et al. Can high-flow nasal cannula reduce the rate of endotracheal intubation in adult patients with acute respiratory failure compared with conventional oxygen therapy and noninvasive positive pressure ventilation?: A systematic review and meta-analysis. Chest. 2017;151(4):764-775. doi: 10.1016/j.chest.2017.01.004.
14. Bhise V, Meyer AND, Singh H, et al. Errors in diagnosis of spinal epidural abscesses in the era of electronic health records. Am J Med. 2017;130(8):975-981. doi: 10.1016/j.amjmed.2017.03.009
15. Davis DP, Wold RM, Patel RJ, et al. The clinical presentation and impact of diagnostic delays on emergency department patients with spinal epidural abscess. J Emerg Med. 2004;26(3):285-291. doi: 10.1016/j.jemermed.2003.11.013.
1. Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med. 2018;168(4):237-244. doi: 10.7326/M17-2341.
2. Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014;370(16):1494-1503. doi: 10.1056/NEJMoa1401105
3. Pincus D, Ravi B, Wasserstein D, et al. Association between wait time and 30-day mortality in adults undergoing hip fracture surgery. JAMA. 2017;318(20):1994-2003. doi: 10.1001/jama.2017.17606.
4. Simunovic N, Devereaux PJ, Sprague S, et al. Effect of early surgery after hip fracture on mortality and complications: systematic review and meta-analysis. CMAJ. 2010;182(15):1609-1616. doi: 10.1503/cmaj.092220.
5. Shiga T, Wajima Z, Ohe Y. Is operative delay associated with increased mortality of hip fracture patients? ystematic review, meta-analysis, and meta-regression. Can J Anaesth. 2008;55(3):146-154. doi: 10.1007/BF03016088.
6. Hale AJ, Snyder GM, Ahern JW, Eliopoulos G, Ricotta D, Alston WK. When are oral antibiotics a safe and effective choice for bacterial bloodstream infections? An evidence-based narrative review. J Hosp Med. 2018;13(5):328-335. doi: 10.12788/jhm.2949.
7. Lehmann C, Berner R, Bogner JR, et al. The “Choosing Wisely” initiative in infectious diseases. Infection. 2017;45(3):263-268. doi: 10.1007/s15010-017-0997-0.
8. Costantino G, Ruwald MH, Quinn J, et al. Prevalence of pulmonary embolism in patients with syncope. JAMA Intern Med. 2018;178(3):356-362. doi: 10.1001/jamainternmed.2017.8175.
9. Prandoni P, Lensing AW, Prins MH, et al. Prevalence of pulmonary embolism among patients hospitalized for syncope. N Engl J Med. 2016;375(16):1524-1531. doi: 10.1056/NEJMoa1602172
10. Self WH, Semler MW, Wanderer JP, et al. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. 2018;378(9):819-828. doi: 10.1056/NEJMoa1711586.
11. Files JA, Mayer AP, Ko MG, et al. Speaker introductions at internal medicine grand rounds: forms of address reveal gender bias. J Womens Health (Larchmt). 2017;26(5):413-419. doi: 10.1089/jwh.2016.6044.
12. Raskob GE, van Es N, Verhamme P, et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018;378(7):615-624. doi: 10.1056/NEJMoa1711948.
13. Ni YN, Luo J, Yu H, et al. Can high-flow nasal cannula reduce the rate of endotracheal intubation in adult patients with acute respiratory failure compared with conventional oxygen therapy and noninvasive positive pressure ventilation?: A systematic review and meta-analysis. Chest. 2017;151(4):764-775. doi: 10.1016/j.chest.2017.01.004.
14. Bhise V, Meyer AND, Singh H, et al. Errors in diagnosis of spinal epidural abscesses in the era of electronic health records. Am J Med. 2017;130(8):975-981. doi: 10.1016/j.amjmed.2017.03.009
15. Davis DP, Wold RM, Patel RJ, et al. The clinical presentation and impact of diagnostic delays on emergency department patients with spinal epidural abscess. J Emerg Med. 2004;26(3):285-291. doi: 10.1016/j.jemermed.2003.11.013.
© 2019 Society of Hospital Medicine
Spontaneous coronary artery dissection: An often unrecognized cause of acute coronary syndrome
A 12-lead electrocardiogram (Figure 1) showed ST-segment elevation of more than 2 mm in leads V2, V3, V4, and V5, with no reciprocal changes.
Based on the classic angiographic appearance and the absence of atherosclerotic disease in other coronary arteries, type 2 spontaneous coronary artery dissection (SCAD) was diagnosed.
CORONARY ARTERY WALL SEPARATION
SCAD is defined as a nontraumatic, noniatrogenic intramural hemorrhage leading to separation of the coronary arterial wall and the formation of a false lumen. The separation can occur between any of the coronary artery wall layers and may or may not involve an intimal tear. The bleeding may result in an intramural hematoma and possible narrowing of the arterial lumen. Depending on the severity of narrowing, blood supply to the myocardium could be compromised, resulting in symptoms of ischemia.1
SCAD usually involves a single coronary artery, although multiple coronary artery involvement has been reported.2
CASE CONTINUED: MANAGEMENT
The patient recovered completely and was discharged home with plans to return for outpatient imaging for fibromuscular dysplasia.
SCAD: RARE OR JUST RARELY RECOGNIZED?
SCAD appears to be a rare cause of acute coronary syndrome, but it is likely underdiagnosed and is becoming increasingly recognized worldwide. Typically, it affects women younger than 50, with women in general outnumbering men 9 to 1.3 Overall, SCAD causes up to 4% of acute myocardial infarctions, but in women age 50 or younger, it is responsible for 24% to 35% of acute myocardial infarctions, and the proportion is even higher in pregnant women.4
Not just pregnancy-associated
SCAD was previously thought to be mainly idiopathic and mostly affecting women peripartum. Current understanding paints a different picture: pregnancy-associated SCAD does not account for the majority of cases. That said, SCAD is the most common cause of myocardial infarction peripartum, with the third trimester and early postpartum period being the times of highest risk.5 SCAD development at those times is believed to be related to hormonal changes causing weakening of coronary artery walls.6
Weakening of the coronary artery wall also may occur in the setting of fibromuscular dysplasia, connective tissue disease, recurrent pregnancies, systemic inflammatory disease, hormonal therapy, and other disease states that cause arteriopathy. Exposure to a stressor in a patient with underlying risk factors can lead to either an intimal tear or rupture of the vasa vasorum, with subsequent formation of intramural hemorrhage and eventually SCAD.7 Stressors can be emotional or physical and can include labor and delivery, intense physical exercise, the Valsalva maneuver, and drug abuse.8
Presentation is variable
SCAD presentation depends on the degree of flow limitation and extent of the dissection. Presentation can range from asymptomatic to sudden cardiac death and can include signs and symptoms of acute coronary syndrome caused by ST-segment elevation or non-ST-segment elevation myocardial infarction.
DIAGNOSIS BY ANGIOGRAPHY
SCAD can be diagnosed by coronary angiography. There are 3 angiographic types:
Type 1 (about 25% of SCAD cases) has typical contrast dye staining of the arterial wall and multiple radiolucent luminal abnormalities, with or without dye hang-up.
Type 2 (about 70%) has diffuse, smooth narrowing of the coronary artery, with the left anterior descending artery the most frequently affected.8
Type 3 (about 5%) mimics atherosclerosis, with focal or tubular stenosis.9
Types 1 and 2 are usually easy to recognize. To diagnose type 2, intravenous nitroglycerin should first be administered to rule out coronary spasm.
Type 3 SCAD is more challenging to diagnose because its appearance on angiography is similar to that of atherosclerosis. For equivocal findings in any type, but especially in type 3, intravascular ultrasonography or optical coherence tomography can help.10 Optical coherence tomography is preferred because of superior image resolution, although ultrasonography offers better tissue penetration.11
MANAGE MOST CASES CONSERVATIVELY
Management algorithms for SCAD are available.8,12
The initial and most critical step is to make the correct diagnosis. Although the presentation of acute coronary syndrome caused by SCAD is often identical to that of atherosclerosis, the conditions have different pathophysiologies and thus require different management. Theoretically, systemic anticoagulation may worsen an intramural hemorrhage.
First-line therapy for most patients with SCAD is conservative management and close inpatient monitoring for 3 to 5 days.13 More aggressive management is indicated for any of the following:
- Left main or severe proximal 2-vessel dissection
- Hemodynamic instability
- Ongoing ischemic symptoms.
In a prospective cohort of 168 patients, 134 (80%) were initially treated conservatively; of those, in-hospital myocardial infarction recurred in 4.5%, a major cardiac event occurred within 2 years in 17%, and SCAD recurred in 13%.8
Observational data on patients with SCAD who had repeat angiography weeks to months after the initial event has shown that lesions heal in 70% to 97% of patients.12
WHEN TO CONSIDER AGGRESSIVE MANAGEMENT
Under the circumstances listed above, revascularization with PCI or coronary artery bypass grafting (CABG) should be considered, with choice of procedure determined by feasibility, technical considerations, and local expertise.
The American Heart Association recommendations are as follows12:
- For left main or severe proximal 2-vessel dissection in clinically stable patients, consider CABG
- For active ischemia or hemodynamic instability, consider PCI if feasible or perform urgent CABG.
A few series have shown that the prognosis with conservative management or CABG is better than with PCI.8,13,14 The success rate for revascularization with PCI is only about 60% because of challenges including risk of inducing iatrogenic dissection, passing the wire into the false lumen and worsening a dissection, and propagating an intramural hematoma with stenting and further compromising coronary blood flow. In addition, dissection tends to extend into distal arteries that are difficult to stent. There is also the risk of stent malapposition after resorption of the intramural hematoma, causing late stent thrombosis.7
SCREEN FOR OTHER VASCULAR PROBLEMS
Imaging of the renal, iliac, and cerebral vasculature is recommended for all patients with SCAD.12 Screening for fibromuscular dysplasia can be done with angiography, computed tomographic angiography (CTA), or magnetic resonance angiography (MRA).12
Multifocal fibromuscular dysplasia in extracoronary arteries occurs with SCAD in 25% to 86% of cases. In a single-center series of 115 patients with confirmed SCAD who underwent CTA from 2010 to 2014, extracoronary vascular abnormalities were found in 66%, with fibromuscular dysplasia being the most common type (45%).15 In another single-center study, 327 patients with SCAD were prospectively followed from 2012 to 2016 with screening for cerebrovascular, renal, and iliac fibromuscular dysplasia using CTA or catheter angiography. Fibromuscular dysplasia was found in 63%, and intracranial aneurysm was found in 14% of patients with fibromuscular dysplasia.9
SCAD can also be associated with connective tissue disorders such as Ehlers-Danlos syndrome type IV and Marfan syndrome.16,17
LONG-TERM MANAGEMENT
Patients with SCAD should start long-term aspirin and 1 year of clopidogrel. Statins are indicated for patients with hyperlipidemia8,18 but otherwise offer no clear benefit for SCAD alone. If there are no contraindications, a beta-adrenergic blocker should be considered, especially if left ventricular dysfunction or arrhythmias are present. Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers should also be considered with concomitant left ventricular dysfunction. Antianginal therapy can be used for post-SCAD chest pain syndromes.12
Repeat angiography is recommended only to evaluate recurrent symptoms, to confirm an unclear initial diagnosis, to assess for atherosclerosis-related stenosis, or to evaluate high-risk anatomy, eg, involvement of the left main coronary artery.12
Genetic testing is reserved for patients with a high clinical suspicion of connective tissue disease or systemic arteriopathy.19
- Garcia NA, Khan AN, Boppana RC, Smith HL. Spontaneous coronary artery dissection: a case series and literature review. J Community Hosp Intern Med Perspect 2014; 4(4). doi:10.3402/jchimp.v4.25261
- Lempereur M, Gin K, Saw J. Multivessel spontaneous coronary artery dissection mimicking atherosclerosis. JACC Cardiovasc Interv 2014; 7(7):e87–e88. doi:10.1016/j.jcin.2013.12.207
- Mahmoud AN, Taduru SS, Mentias A, et al. Trends of incidence, clinical presentation, and in-hospital mortality among women with acute myocardial infarction with or without spontaneous coronary artery dissection: a population-based analysis. JACC Cardiovasc Interv 2018; 11(1):80–90. doi:10.1016/j.jcin.2017.08.016
- Saw J. Pregnancy-associated spontaneous coronary artery dissection represents an exceptionally high-risk spontaneous coronary artery dissection cohort. Circ Cardiovasc Interv 2017; 10(3)pii:e005119. doi:10.1161/CIRCINTERVENTIONS.117.005119
- Elkayam U, Jalnapurkar S, Barakkat MN, et al. Pregnancy-associated acute myocardial infarction: a review of contemporary experience in 150 cases between 2006 and 2011. Circulation 2014; 129(16):1695–1702. doi:10.1161/CIRCULATIONAHA.113.002054
- Vijayaraghavan R, Verma S, Gupta N, Saw J. Pregnancy-related spontaneous coronary artery dissection. Circulation 2014; 130(21):1915–1920. doi:10.1161/CIRCULATIONAHA.114.011422
- Saw J, Mancini GBJ, Humphries KH. Contemporary review on spontaneous coronary artery dissection. J Am Coll Cardiol 2016; 68(3):297–312. doi:10.1016/j.jacc.2016.05.034
- Saw J, Aymong E, Sedlak T, et al. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv 2014; 7(5):645–655. doi:10.1161/CIRCINTERVENTIONS.114.001760
- Saw J, Humphries K ,Aymong E, et al. Spontaneous coronary artery dissection: clinical outcomes and risk of recurrence. J Am Coll Cardiol 2017; 70(9):1148–1158. doi:10.1016/j.jacc.2017.06.053
- Alfonso F, Bastante T, Cuesta J, Rodríguez D, Benedicto A, Rivero F. Spontaneous coronary artery dissection: novel insights on diagnosis and management. Cardiovasc Diagn Ther 2015; 5(2):133–140. doi:10.3978/j.issn.2223-3652.2015.03.05
- Kern MJ, Meier B. Evaluation of the culprit plaque and the physiological significance of coronary atherosclerotic narrowings. Circulation 2001; 103(25):3142–3149. pmid:11425782
- Hayes SN, Kim ESH, Saw J, et al; American Heart Association Council on Peripheral Vascular Disease; Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Genomic and Precision Medicine; and Stroke Council. Spontaneous coronary artery dissection: current state of the science: a scientific statement from the American Heart Association. Circulation 2018; 137(19):e523–e557. doi:10.1161/CIR.0000000000000564
- Tweet MS, Eleid MF, Best PJ, et al. Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circ Cardiovasc Interv 2014; 7(6):777–786. doi:10.1161/CIRCINTERVENTIONS.114.001659
- Tweet MS, Hayes SN, Pitta SR, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 2012; 126(5):579–588. doi:10.1161/CIRCULATIONAHA.112.105718
- Prasad M, Tweet MS, Hayes SN, et al. Prevalence of extracoronary vascular abnormalities and fibromuscular dysplasia in patients with spontaneous coronary artery dissection. Am J Cardiol 2015; 115(12):1672–1677. doi:10.1016/j.amjcard.2015.03.011
- Adès LC, Waltham RD, Chiodo AA, Bateman JF. Myocardial infarction resulting from coronary artery dissection in an adolescent with Ehlers-Danlos syndrome type IV due to a type III collagen mutation. Br Heart J 1995; 74(2):112–116. pmid:7546986
- Judge DP, Dietz HC. Marfan’s syndrome. Lancet 2005; 366(9501):1965–1976. doi:10.1016/S0140-6736(05)67789-6
- Saw J. Spontaneous coronary artery dissection. Can J Cardiol 2013; 29(9):1027–1033. doi:10.1016/j.cjca.2012.12.018
- Poloskey SL, Kim ES, Sanghani R, et al. Low yield of genetic testing for known vascular connective tissue disorders in patients with fibromuscular dysplasia. Vasc Med 2012; 17(6):371–378. doi:10.1177/1358863X12459650
A 12-lead electrocardiogram (Figure 1) showed ST-segment elevation of more than 2 mm in leads V2, V3, V4, and V5, with no reciprocal changes.
Based on the classic angiographic appearance and the absence of atherosclerotic disease in other coronary arteries, type 2 spontaneous coronary artery dissection (SCAD) was diagnosed.
CORONARY ARTERY WALL SEPARATION
SCAD is defined as a nontraumatic, noniatrogenic intramural hemorrhage leading to separation of the coronary arterial wall and the formation of a false lumen. The separation can occur between any of the coronary artery wall layers and may or may not involve an intimal tear. The bleeding may result in an intramural hematoma and possible narrowing of the arterial lumen. Depending on the severity of narrowing, blood supply to the myocardium could be compromised, resulting in symptoms of ischemia.1
SCAD usually involves a single coronary artery, although multiple coronary artery involvement has been reported.2
CASE CONTINUED: MANAGEMENT
The patient recovered completely and was discharged home with plans to return for outpatient imaging for fibromuscular dysplasia.
SCAD: RARE OR JUST RARELY RECOGNIZED?
SCAD appears to be a rare cause of acute coronary syndrome, but it is likely underdiagnosed and is becoming increasingly recognized worldwide. Typically, it affects women younger than 50, with women in general outnumbering men 9 to 1.3 Overall, SCAD causes up to 4% of acute myocardial infarctions, but in women age 50 or younger, it is responsible for 24% to 35% of acute myocardial infarctions, and the proportion is even higher in pregnant women.4
Not just pregnancy-associated
SCAD was previously thought to be mainly idiopathic and mostly affecting women peripartum. Current understanding paints a different picture: pregnancy-associated SCAD does not account for the majority of cases. That said, SCAD is the most common cause of myocardial infarction peripartum, with the third trimester and early postpartum period being the times of highest risk.5 SCAD development at those times is believed to be related to hormonal changes causing weakening of coronary artery walls.6
Weakening of the coronary artery wall also may occur in the setting of fibromuscular dysplasia, connective tissue disease, recurrent pregnancies, systemic inflammatory disease, hormonal therapy, and other disease states that cause arteriopathy. Exposure to a stressor in a patient with underlying risk factors can lead to either an intimal tear or rupture of the vasa vasorum, with subsequent formation of intramural hemorrhage and eventually SCAD.7 Stressors can be emotional or physical and can include labor and delivery, intense physical exercise, the Valsalva maneuver, and drug abuse.8
Presentation is variable
SCAD presentation depends on the degree of flow limitation and extent of the dissection. Presentation can range from asymptomatic to sudden cardiac death and can include signs and symptoms of acute coronary syndrome caused by ST-segment elevation or non-ST-segment elevation myocardial infarction.
DIAGNOSIS BY ANGIOGRAPHY
SCAD can be diagnosed by coronary angiography. There are 3 angiographic types:
Type 1 (about 25% of SCAD cases) has typical contrast dye staining of the arterial wall and multiple radiolucent luminal abnormalities, with or without dye hang-up.
Type 2 (about 70%) has diffuse, smooth narrowing of the coronary artery, with the left anterior descending artery the most frequently affected.8
Type 3 (about 5%) mimics atherosclerosis, with focal or tubular stenosis.9
Types 1 and 2 are usually easy to recognize. To diagnose type 2, intravenous nitroglycerin should first be administered to rule out coronary spasm.
Type 3 SCAD is more challenging to diagnose because its appearance on angiography is similar to that of atherosclerosis. For equivocal findings in any type, but especially in type 3, intravascular ultrasonography or optical coherence tomography can help.10 Optical coherence tomography is preferred because of superior image resolution, although ultrasonography offers better tissue penetration.11
MANAGE MOST CASES CONSERVATIVELY
Management algorithms for SCAD are available.8,12
The initial and most critical step is to make the correct diagnosis. Although the presentation of acute coronary syndrome caused by SCAD is often identical to that of atherosclerosis, the conditions have different pathophysiologies and thus require different management. Theoretically, systemic anticoagulation may worsen an intramural hemorrhage.
First-line therapy for most patients with SCAD is conservative management and close inpatient monitoring for 3 to 5 days.13 More aggressive management is indicated for any of the following:
- Left main or severe proximal 2-vessel dissection
- Hemodynamic instability
- Ongoing ischemic symptoms.
In a prospective cohort of 168 patients, 134 (80%) were initially treated conservatively; of those, in-hospital myocardial infarction recurred in 4.5%, a major cardiac event occurred within 2 years in 17%, and SCAD recurred in 13%.8
Observational data on patients with SCAD who had repeat angiography weeks to months after the initial event has shown that lesions heal in 70% to 97% of patients.12
WHEN TO CONSIDER AGGRESSIVE MANAGEMENT
Under the circumstances listed above, revascularization with PCI or coronary artery bypass grafting (CABG) should be considered, with choice of procedure determined by feasibility, technical considerations, and local expertise.
The American Heart Association recommendations are as follows12:
- For left main or severe proximal 2-vessel dissection in clinically stable patients, consider CABG
- For active ischemia or hemodynamic instability, consider PCI if feasible or perform urgent CABG.
A few series have shown that the prognosis with conservative management or CABG is better than with PCI.8,13,14 The success rate for revascularization with PCI is only about 60% because of challenges including risk of inducing iatrogenic dissection, passing the wire into the false lumen and worsening a dissection, and propagating an intramural hematoma with stenting and further compromising coronary blood flow. In addition, dissection tends to extend into distal arteries that are difficult to stent. There is also the risk of stent malapposition after resorption of the intramural hematoma, causing late stent thrombosis.7
SCREEN FOR OTHER VASCULAR PROBLEMS
Imaging of the renal, iliac, and cerebral vasculature is recommended for all patients with SCAD.12 Screening for fibromuscular dysplasia can be done with angiography, computed tomographic angiography (CTA), or magnetic resonance angiography (MRA).12
Multifocal fibromuscular dysplasia in extracoronary arteries occurs with SCAD in 25% to 86% of cases. In a single-center series of 115 patients with confirmed SCAD who underwent CTA from 2010 to 2014, extracoronary vascular abnormalities were found in 66%, with fibromuscular dysplasia being the most common type (45%).15 In another single-center study, 327 patients with SCAD were prospectively followed from 2012 to 2016 with screening for cerebrovascular, renal, and iliac fibromuscular dysplasia using CTA or catheter angiography. Fibromuscular dysplasia was found in 63%, and intracranial aneurysm was found in 14% of patients with fibromuscular dysplasia.9
SCAD can also be associated with connective tissue disorders such as Ehlers-Danlos syndrome type IV and Marfan syndrome.16,17
LONG-TERM MANAGEMENT
Patients with SCAD should start long-term aspirin and 1 year of clopidogrel. Statins are indicated for patients with hyperlipidemia8,18 but otherwise offer no clear benefit for SCAD alone. If there are no contraindications, a beta-adrenergic blocker should be considered, especially if left ventricular dysfunction or arrhythmias are present. Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers should also be considered with concomitant left ventricular dysfunction. Antianginal therapy can be used for post-SCAD chest pain syndromes.12
Repeat angiography is recommended only to evaluate recurrent symptoms, to confirm an unclear initial diagnosis, to assess for atherosclerosis-related stenosis, or to evaluate high-risk anatomy, eg, involvement of the left main coronary artery.12
Genetic testing is reserved for patients with a high clinical suspicion of connective tissue disease or systemic arteriopathy.19
A 12-lead electrocardiogram (Figure 1) showed ST-segment elevation of more than 2 mm in leads V2, V3, V4, and V5, with no reciprocal changes.
Based on the classic angiographic appearance and the absence of atherosclerotic disease in other coronary arteries, type 2 spontaneous coronary artery dissection (SCAD) was diagnosed.
CORONARY ARTERY WALL SEPARATION
SCAD is defined as a nontraumatic, noniatrogenic intramural hemorrhage leading to separation of the coronary arterial wall and the formation of a false lumen. The separation can occur between any of the coronary artery wall layers and may or may not involve an intimal tear. The bleeding may result in an intramural hematoma and possible narrowing of the arterial lumen. Depending on the severity of narrowing, blood supply to the myocardium could be compromised, resulting in symptoms of ischemia.1
SCAD usually involves a single coronary artery, although multiple coronary artery involvement has been reported.2
CASE CONTINUED: MANAGEMENT
The patient recovered completely and was discharged home with plans to return for outpatient imaging for fibromuscular dysplasia.
SCAD: RARE OR JUST RARELY RECOGNIZED?
SCAD appears to be a rare cause of acute coronary syndrome, but it is likely underdiagnosed and is becoming increasingly recognized worldwide. Typically, it affects women younger than 50, with women in general outnumbering men 9 to 1.3 Overall, SCAD causes up to 4% of acute myocardial infarctions, but in women age 50 or younger, it is responsible for 24% to 35% of acute myocardial infarctions, and the proportion is even higher in pregnant women.4
Not just pregnancy-associated
SCAD was previously thought to be mainly idiopathic and mostly affecting women peripartum. Current understanding paints a different picture: pregnancy-associated SCAD does not account for the majority of cases. That said, SCAD is the most common cause of myocardial infarction peripartum, with the third trimester and early postpartum period being the times of highest risk.5 SCAD development at those times is believed to be related to hormonal changes causing weakening of coronary artery walls.6
Weakening of the coronary artery wall also may occur in the setting of fibromuscular dysplasia, connective tissue disease, recurrent pregnancies, systemic inflammatory disease, hormonal therapy, and other disease states that cause arteriopathy. Exposure to a stressor in a patient with underlying risk factors can lead to either an intimal tear or rupture of the vasa vasorum, with subsequent formation of intramural hemorrhage and eventually SCAD.7 Stressors can be emotional or physical and can include labor and delivery, intense physical exercise, the Valsalva maneuver, and drug abuse.8
Presentation is variable
SCAD presentation depends on the degree of flow limitation and extent of the dissection. Presentation can range from asymptomatic to sudden cardiac death and can include signs and symptoms of acute coronary syndrome caused by ST-segment elevation or non-ST-segment elevation myocardial infarction.
DIAGNOSIS BY ANGIOGRAPHY
SCAD can be diagnosed by coronary angiography. There are 3 angiographic types:
Type 1 (about 25% of SCAD cases) has typical contrast dye staining of the arterial wall and multiple radiolucent luminal abnormalities, with or without dye hang-up.
Type 2 (about 70%) has diffuse, smooth narrowing of the coronary artery, with the left anterior descending artery the most frequently affected.8
Type 3 (about 5%) mimics atherosclerosis, with focal or tubular stenosis.9
Types 1 and 2 are usually easy to recognize. To diagnose type 2, intravenous nitroglycerin should first be administered to rule out coronary spasm.
Type 3 SCAD is more challenging to diagnose because its appearance on angiography is similar to that of atherosclerosis. For equivocal findings in any type, but especially in type 3, intravascular ultrasonography or optical coherence tomography can help.10 Optical coherence tomography is preferred because of superior image resolution, although ultrasonography offers better tissue penetration.11
MANAGE MOST CASES CONSERVATIVELY
Management algorithms for SCAD are available.8,12
The initial and most critical step is to make the correct diagnosis. Although the presentation of acute coronary syndrome caused by SCAD is often identical to that of atherosclerosis, the conditions have different pathophysiologies and thus require different management. Theoretically, systemic anticoagulation may worsen an intramural hemorrhage.
First-line therapy for most patients with SCAD is conservative management and close inpatient monitoring for 3 to 5 days.13 More aggressive management is indicated for any of the following:
- Left main or severe proximal 2-vessel dissection
- Hemodynamic instability
- Ongoing ischemic symptoms.
In a prospective cohort of 168 patients, 134 (80%) were initially treated conservatively; of those, in-hospital myocardial infarction recurred in 4.5%, a major cardiac event occurred within 2 years in 17%, and SCAD recurred in 13%.8
Observational data on patients with SCAD who had repeat angiography weeks to months after the initial event has shown that lesions heal in 70% to 97% of patients.12
WHEN TO CONSIDER AGGRESSIVE MANAGEMENT
Under the circumstances listed above, revascularization with PCI or coronary artery bypass grafting (CABG) should be considered, with choice of procedure determined by feasibility, technical considerations, and local expertise.
The American Heart Association recommendations are as follows12:
- For left main or severe proximal 2-vessel dissection in clinically stable patients, consider CABG
- For active ischemia or hemodynamic instability, consider PCI if feasible or perform urgent CABG.
A few series have shown that the prognosis with conservative management or CABG is better than with PCI.8,13,14 The success rate for revascularization with PCI is only about 60% because of challenges including risk of inducing iatrogenic dissection, passing the wire into the false lumen and worsening a dissection, and propagating an intramural hematoma with stenting and further compromising coronary blood flow. In addition, dissection tends to extend into distal arteries that are difficult to stent. There is also the risk of stent malapposition after resorption of the intramural hematoma, causing late stent thrombosis.7
SCREEN FOR OTHER VASCULAR PROBLEMS
Imaging of the renal, iliac, and cerebral vasculature is recommended for all patients with SCAD.12 Screening for fibromuscular dysplasia can be done with angiography, computed tomographic angiography (CTA), or magnetic resonance angiography (MRA).12
Multifocal fibromuscular dysplasia in extracoronary arteries occurs with SCAD in 25% to 86% of cases. In a single-center series of 115 patients with confirmed SCAD who underwent CTA from 2010 to 2014, extracoronary vascular abnormalities were found in 66%, with fibromuscular dysplasia being the most common type (45%).15 In another single-center study, 327 patients with SCAD were prospectively followed from 2012 to 2016 with screening for cerebrovascular, renal, and iliac fibromuscular dysplasia using CTA or catheter angiography. Fibromuscular dysplasia was found in 63%, and intracranial aneurysm was found in 14% of patients with fibromuscular dysplasia.9
SCAD can also be associated with connective tissue disorders such as Ehlers-Danlos syndrome type IV and Marfan syndrome.16,17
LONG-TERM MANAGEMENT
Patients with SCAD should start long-term aspirin and 1 year of clopidogrel. Statins are indicated for patients with hyperlipidemia8,18 but otherwise offer no clear benefit for SCAD alone. If there are no contraindications, a beta-adrenergic blocker should be considered, especially if left ventricular dysfunction or arrhythmias are present. Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers should also be considered with concomitant left ventricular dysfunction. Antianginal therapy can be used for post-SCAD chest pain syndromes.12
Repeat angiography is recommended only to evaluate recurrent symptoms, to confirm an unclear initial diagnosis, to assess for atherosclerosis-related stenosis, or to evaluate high-risk anatomy, eg, involvement of the left main coronary artery.12
Genetic testing is reserved for patients with a high clinical suspicion of connective tissue disease or systemic arteriopathy.19
- Garcia NA, Khan AN, Boppana RC, Smith HL. Spontaneous coronary artery dissection: a case series and literature review. J Community Hosp Intern Med Perspect 2014; 4(4). doi:10.3402/jchimp.v4.25261
- Lempereur M, Gin K, Saw J. Multivessel spontaneous coronary artery dissection mimicking atherosclerosis. JACC Cardiovasc Interv 2014; 7(7):e87–e88. doi:10.1016/j.jcin.2013.12.207
- Mahmoud AN, Taduru SS, Mentias A, et al. Trends of incidence, clinical presentation, and in-hospital mortality among women with acute myocardial infarction with or without spontaneous coronary artery dissection: a population-based analysis. JACC Cardiovasc Interv 2018; 11(1):80–90. doi:10.1016/j.jcin.2017.08.016
- Saw J. Pregnancy-associated spontaneous coronary artery dissection represents an exceptionally high-risk spontaneous coronary artery dissection cohort. Circ Cardiovasc Interv 2017; 10(3)pii:e005119. doi:10.1161/CIRCINTERVENTIONS.117.005119
- Elkayam U, Jalnapurkar S, Barakkat MN, et al. Pregnancy-associated acute myocardial infarction: a review of contemporary experience in 150 cases between 2006 and 2011. Circulation 2014; 129(16):1695–1702. doi:10.1161/CIRCULATIONAHA.113.002054
- Vijayaraghavan R, Verma S, Gupta N, Saw J. Pregnancy-related spontaneous coronary artery dissection. Circulation 2014; 130(21):1915–1920. doi:10.1161/CIRCULATIONAHA.114.011422
- Saw J, Mancini GBJ, Humphries KH. Contemporary review on spontaneous coronary artery dissection. J Am Coll Cardiol 2016; 68(3):297–312. doi:10.1016/j.jacc.2016.05.034
- Saw J, Aymong E, Sedlak T, et al. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv 2014; 7(5):645–655. doi:10.1161/CIRCINTERVENTIONS.114.001760
- Saw J, Humphries K ,Aymong E, et al. Spontaneous coronary artery dissection: clinical outcomes and risk of recurrence. J Am Coll Cardiol 2017; 70(9):1148–1158. doi:10.1016/j.jacc.2017.06.053
- Alfonso F, Bastante T, Cuesta J, Rodríguez D, Benedicto A, Rivero F. Spontaneous coronary artery dissection: novel insights on diagnosis and management. Cardiovasc Diagn Ther 2015; 5(2):133–140. doi:10.3978/j.issn.2223-3652.2015.03.05
- Kern MJ, Meier B. Evaluation of the culprit plaque and the physiological significance of coronary atherosclerotic narrowings. Circulation 2001; 103(25):3142–3149. pmid:11425782
- Hayes SN, Kim ESH, Saw J, et al; American Heart Association Council on Peripheral Vascular Disease; Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Genomic and Precision Medicine; and Stroke Council. Spontaneous coronary artery dissection: current state of the science: a scientific statement from the American Heart Association. Circulation 2018; 137(19):e523–e557. doi:10.1161/CIR.0000000000000564
- Tweet MS, Eleid MF, Best PJ, et al. Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circ Cardiovasc Interv 2014; 7(6):777–786. doi:10.1161/CIRCINTERVENTIONS.114.001659
- Tweet MS, Hayes SN, Pitta SR, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 2012; 126(5):579–588. doi:10.1161/CIRCULATIONAHA.112.105718
- Prasad M, Tweet MS, Hayes SN, et al. Prevalence of extracoronary vascular abnormalities and fibromuscular dysplasia in patients with spontaneous coronary artery dissection. Am J Cardiol 2015; 115(12):1672–1677. doi:10.1016/j.amjcard.2015.03.011
- Adès LC, Waltham RD, Chiodo AA, Bateman JF. Myocardial infarction resulting from coronary artery dissection in an adolescent with Ehlers-Danlos syndrome type IV due to a type III collagen mutation. Br Heart J 1995; 74(2):112–116. pmid:7546986
- Judge DP, Dietz HC. Marfan’s syndrome. Lancet 2005; 366(9501):1965–1976. doi:10.1016/S0140-6736(05)67789-6
- Saw J. Spontaneous coronary artery dissection. Can J Cardiol 2013; 29(9):1027–1033. doi:10.1016/j.cjca.2012.12.018
- Poloskey SL, Kim ES, Sanghani R, et al. Low yield of genetic testing for known vascular connective tissue disorders in patients with fibromuscular dysplasia. Vasc Med 2012; 17(6):371–378. doi:10.1177/1358863X12459650
- Garcia NA, Khan AN, Boppana RC, Smith HL. Spontaneous coronary artery dissection: a case series and literature review. J Community Hosp Intern Med Perspect 2014; 4(4). doi:10.3402/jchimp.v4.25261
- Lempereur M, Gin K, Saw J. Multivessel spontaneous coronary artery dissection mimicking atherosclerosis. JACC Cardiovasc Interv 2014; 7(7):e87–e88. doi:10.1016/j.jcin.2013.12.207
- Mahmoud AN, Taduru SS, Mentias A, et al. Trends of incidence, clinical presentation, and in-hospital mortality among women with acute myocardial infarction with or without spontaneous coronary artery dissection: a population-based analysis. JACC Cardiovasc Interv 2018; 11(1):80–90. doi:10.1016/j.jcin.2017.08.016
- Saw J. Pregnancy-associated spontaneous coronary artery dissection represents an exceptionally high-risk spontaneous coronary artery dissection cohort. Circ Cardiovasc Interv 2017; 10(3)pii:e005119. doi:10.1161/CIRCINTERVENTIONS.117.005119
- Elkayam U, Jalnapurkar S, Barakkat MN, et al. Pregnancy-associated acute myocardial infarction: a review of contemporary experience in 150 cases between 2006 and 2011. Circulation 2014; 129(16):1695–1702. doi:10.1161/CIRCULATIONAHA.113.002054
- Vijayaraghavan R, Verma S, Gupta N, Saw J. Pregnancy-related spontaneous coronary artery dissection. Circulation 2014; 130(21):1915–1920. doi:10.1161/CIRCULATIONAHA.114.011422
- Saw J, Mancini GBJ, Humphries KH. Contemporary review on spontaneous coronary artery dissection. J Am Coll Cardiol 2016; 68(3):297–312. doi:10.1016/j.jacc.2016.05.034
- Saw J, Aymong E, Sedlak T, et al. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv 2014; 7(5):645–655. doi:10.1161/CIRCINTERVENTIONS.114.001760
- Saw J, Humphries K ,Aymong E, et al. Spontaneous coronary artery dissection: clinical outcomes and risk of recurrence. J Am Coll Cardiol 2017; 70(9):1148–1158. doi:10.1016/j.jacc.2017.06.053
- Alfonso F, Bastante T, Cuesta J, Rodríguez D, Benedicto A, Rivero F. Spontaneous coronary artery dissection: novel insights on diagnosis and management. Cardiovasc Diagn Ther 2015; 5(2):133–140. doi:10.3978/j.issn.2223-3652.2015.03.05
- Kern MJ, Meier B. Evaluation of the culprit plaque and the physiological significance of coronary atherosclerotic narrowings. Circulation 2001; 103(25):3142–3149. pmid:11425782
- Hayes SN, Kim ESH, Saw J, et al; American Heart Association Council on Peripheral Vascular Disease; Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Genomic and Precision Medicine; and Stroke Council. Spontaneous coronary artery dissection: current state of the science: a scientific statement from the American Heart Association. Circulation 2018; 137(19):e523–e557. doi:10.1161/CIR.0000000000000564
- Tweet MS, Eleid MF, Best PJ, et al. Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circ Cardiovasc Interv 2014; 7(6):777–786. doi:10.1161/CIRCINTERVENTIONS.114.001659
- Tweet MS, Hayes SN, Pitta SR, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 2012; 126(5):579–588. doi:10.1161/CIRCULATIONAHA.112.105718
- Prasad M, Tweet MS, Hayes SN, et al. Prevalence of extracoronary vascular abnormalities and fibromuscular dysplasia in patients with spontaneous coronary artery dissection. Am J Cardiol 2015; 115(12):1672–1677. doi:10.1016/j.amjcard.2015.03.011
- Adès LC, Waltham RD, Chiodo AA, Bateman JF. Myocardial infarction resulting from coronary artery dissection in an adolescent with Ehlers-Danlos syndrome type IV due to a type III collagen mutation. Br Heart J 1995; 74(2):112–116. pmid:7546986
- Judge DP, Dietz HC. Marfan’s syndrome. Lancet 2005; 366(9501):1965–1976. doi:10.1016/S0140-6736(05)67789-6
- Saw J. Spontaneous coronary artery dissection. Can J Cardiol 2013; 29(9):1027–1033. doi:10.1016/j.cjca.2012.12.018
- Poloskey SL, Kim ES, Sanghani R, et al. Low yield of genetic testing for known vascular connective tissue disorders in patients with fibromuscular dysplasia. Vasc Med 2012; 17(6):371–378. doi:10.1177/1358863X12459650
KEY POINTS
- SCAD often presents with symptoms of acute coronary syndrome but can be asymptomatic or cause sudden death.
- Management is generally conservative, but a left main or severe proximal 2-vessel dissection, hemodynamic instability, or ongoing ischemic symptoms may warrant revascularization.
- All patients with SCAD should be screened for other vascular problems, especially fibromuscular dysplasia.
- Long-term aspirin therapy and 1 year of clopidogrel are recommended after an episode of SCAD.