User login
Surgical catastrophe: Offering a lifeline to the second victim
CASE A surgeon's story of patient loss
It was a Wednesday morning and Ms. M was my first case of the day. I knew her well, having delivered her 2 children. Now she had a 7-cm complex cyst on her right ovary, she was in pain, and she was possibly experiencing ovarian torsion. My resident took care of the paperwork, I met the patient in preop, answered her few questions, and reassured her husband that I would call him as soon as surgery was over. She was rolled to the operating room.
When I entered the OR, Ms. M was under general anesthesia, draped, and placed on the operating table in the usual position. I made a 5-mm incision at the umbilicus and inserted the trocar under direct visualization. There was blood and the camera became blurry. I removed the camera to clean it, and the anesthesiologist alerted me that there was sudden hypotension. I reinserted the camera and saw blood in the abdomen. I feared the worst—major vessel injury. I requested a scalpel and made a midline skin sub–umbilical incision, entered the peritoneal cavity, and observed blood everywhere. The massive transfusion protocol was activated and vascular surgery was called in. I could not find the source of the bleeding. Using a laparotomy towel I applied pressure on the aorta. The vascular surgeon arrived and pushed my resident away. He identified the source of the bleeding: The right common iliac artery was injured.
The patient coded, the anesthesiologist initiated CPR, bleeding continued, blood was being transfused, and after 20 long minutes of CPR the lifeless body of my patient could not hold any more. She was pronounced dead on the table.
At that moment, there were multiple victims: Ms. M lying on the surgical table; her family members, who did not know what was happening; and the surgical team members, who were looking at each other in denial and feeling that we had failed this patient, hoping that we would wake up from this nightmare.
Defining patient harm
Many patients experience harm each year because of an adverse medical event or preventable medical error.1 A 2013 report revealed that 210,000 to 440,000 deaths occur each year in the United States related to preventable patient harm.2 Although this fact is deeply disturbing, it is well known that modern health care is a high-risk industry.
Medical errors vary in terms of the degree of potential or actual damage. A “near miss” is any event that could have resulted in adverse consequences but did not (for example, an incorrect drug or dose ordered but not administered). On the other hand, an “adverse event” describes an error that resulted in some degree of patient harm or suffering.3
Related article:
Medical errors: Meeting ethical obligations and reducing liability with proper communication
For each patient who dies because of a medical error or a surgical complication, whether preventable or not, many clinicians are involved in the unfolding of the case. These events have a profound impact on well-intentioned, competent, and caring physicians, and they elicit intense emotional responses.4 When a patient experiences an unexpected adverse surgical outcome, the surgeons involved in their care may become “second victims.” They may feel that they have failed the patient and they second-guess their surgical skills and knowledge base; some express concern about their reputation and perhaps career choice.
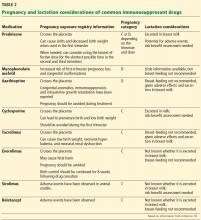
Psychological responses. It is importantto understand this process to ensure a healthy recovery. Psychological responses to an adverse medical event include guilt; distress, anxiety, and fear; frustration and anger; feelings of insufficiency; and long-standing suffering. Clinicians who experienced an adverse medical event have reported additional psychological as well as physical symptoms in the aftermath of the event (TABLE 1).5
Risk factors. Certain factors are associated with a greater emotional impact of an adverse medical event, including6:
- severity of the harm or leaving permanent sequelae
- death of a healthy patient or a child (for example, from a motor vehicle accident)
- self-blame for the error
- unexpected patient death (for example, a catastrophic complication after a relatively benign procedure)
- physicians-in-training responsible for the patient
- first death under a clinician’s watch.
While most research in the field of medical error focuses on systems or process improvement, it is important not to neglect the individual and personal aspects of the clinicians involved in the event. The health care system must include care for our injured colleagues, the so-called second victims.
Read about the steps to recovery for the second victim.
Steps in recovery for the second victim
Based on a semistructured interview of 31 physicians involved in adverse events, Scott and colleagues described the following 6 stages of healing5:
Chaos and accident response. Immediately after the event, the physician feels a sense of confusion, panic, and denial. How can this be happening to me? The physician is frequently distracted, immersed in self-reflection.
Intrusive reflections. This is a period of self-questioning. Thoughts of the event and different possible scenarios dominate the physician’s mind. What if I had done this or that?
Restoring personal integrity. During this phase, the physician seeks support from individuals with whom trusted relationships exist, such as colleagues, peers, close friends, and family members. Advice from a colleague who has your same level of expertise is precious. The second victim often fears that friends and family will not be understanding.
Enduring the inquisition. Root cause analysis and in-depth case review is an important part of the quality improvement process after an adverse event. A debriefing or departmental morbidity and mortality conference can trigger emotions and increase the sense of shame, guilt, and self-doubt. The second victim starts to wonder about repercussions that may affect job security, licensure, and future litigation.
Obtaining emotional first aid. At this stage, the second victim begins to heal, but it is important to obtain external help from a colleague, mentor, counselor, department chair, or loved ones. Many physicians express concerns about not knowing who is a “safe person” to trust in this situation. Often, second victims perceive that their loved ones just do not understand their professional life or should be protected from this situation.
Moving on. There is an urge to move forward with life and simply put the event behind. This is difficult, however. A second victim may follow one of these paths:
- drop out—stop practicing clinical medicine
- survive—maintain the same career but with significant residual emotional burden from the event
- thrive—make something good out of the unfortunate clinical experience.
Related article:
TRUST: How to build a support net for ObGyns affected by a medical error
All these programs offer immediate help to any clinician in psychological distress. They provide confidentiality, and the individual is reassured that he or she can safely use the service without further consequences (TABLE 2).10
The normal human response to an adverse medical event can lead to significant psychological consequences, long-term emotional incapacity, impaired performance of clinical care, and feelings of guilt, fear, isolation, or even suicide. At some point during his or her career, almost every physician will be involved in a serious adverse medical event and is at risk of experiencing strong emotional reactions. Health care facilities should have a support system in place to help clinicians cope with these stressful circumstances.
Use these 5 strategies to facilitate recovery
- Be determined. No matter how bad you feel about the event, you need to get up and moving.
- Avoid isolation. Get outside and interact with people. Avoid long periods in isolation. Bring your team together and talk about the event.
- Sleep well. Most symptoms of posttraumatic stress disorder occur at night. If you have trouble falling asleep or you wake up in the middle of the night with nightmares related to the event, attempt to regulate your body’s sleep schedule. Seek professional help if needed.
- Avoid negative coping habits. Sometimes people turn to alcohol, cigarettes, food, or drugs to cope. Although these strategies may help in the short term, they will do more harm than good over time.
- Enroll in activities that provide positive distraction. While the mind focuses on the traumatic event (this is normal), you need to get busy with such positive distractions as sports, going to the movies, and engaging in outdoor activities. Do things that you enjoy.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Kohn L. To err is human: an interview with the Institute of Medicine's Linda Kohn. Jt Comm J Qual Improv. 2000;26(4):227-234.
- James JT. A new, evidence-based estimate of patient harms associated with hospital care. J Patient Saf. 2013;9(3):122-128.
- Harrison R, Lawton R, Perlo J, Gardner P, Armitage G, Shapiro J. Emotion and coping in the aftermath of medical error: a cross-country exploration. J Patient Saf. 2015;11(1):28-35.
- Chan ST, Khong PC, Wang W. Psychological responses, coping and supporting needs of healthcare professionals as second victims. Int Nurs Rev. 2017;64(2):242-262.
- Scott SD, Hirschinger LE, Cox KR, McCoig M, Brandt J, Hall LW. The natural history of recovery for the healthcare provider "second victim" after adverse patient events. Qual Saf Health Care. 2009;18(5):325-330.
- Waterman AD, Garbutt J, Hazel E, et al. The emotional impact of medical errors on practicing physicians in the United States and Canada. Jt Comm J Qual Patient Saf. 2007;33(8):467-476.
- Shapiro J, Galowitz P. Peer support for clinicians: a programmatic approach. Acad Med. 2016;91(9):1200-1204.
- Edrees H, Connors C, Paine L, Norvell M, Taylor H, Wu AW. Implementing the RISE second victim support programme at the Johns Hopkins Hospital: a case study. BMJ Open. 2016;6(9):e011708.
- Johnson B. Code lavender: initiating holistic rapid response at the Cleveland Clinic. Beginnings. 2014;34(2):10-11.
- van Pelt F. Peer support: healthcare professionals supporting each other after adverse medical events. Qual Saf Health Care. 2008;17(4):249-252.
CASE A surgeon's story of patient loss
It was a Wednesday morning and Ms. M was my first case of the day. I knew her well, having delivered her 2 children. Now she had a 7-cm complex cyst on her right ovary, she was in pain, and she was possibly experiencing ovarian torsion. My resident took care of the paperwork, I met the patient in preop, answered her few questions, and reassured her husband that I would call him as soon as surgery was over. She was rolled to the operating room.
When I entered the OR, Ms. M was under general anesthesia, draped, and placed on the operating table in the usual position. I made a 5-mm incision at the umbilicus and inserted the trocar under direct visualization. There was blood and the camera became blurry. I removed the camera to clean it, and the anesthesiologist alerted me that there was sudden hypotension. I reinserted the camera and saw blood in the abdomen. I feared the worst—major vessel injury. I requested a scalpel and made a midline skin sub–umbilical incision, entered the peritoneal cavity, and observed blood everywhere. The massive transfusion protocol was activated and vascular surgery was called in. I could not find the source of the bleeding. Using a laparotomy towel I applied pressure on the aorta. The vascular surgeon arrived and pushed my resident away. He identified the source of the bleeding: The right common iliac artery was injured.
The patient coded, the anesthesiologist initiated CPR, bleeding continued, blood was being transfused, and after 20 long minutes of CPR the lifeless body of my patient could not hold any more. She was pronounced dead on the table.
At that moment, there were multiple victims: Ms. M lying on the surgical table; her family members, who did not know what was happening; and the surgical team members, who were looking at each other in denial and feeling that we had failed this patient, hoping that we would wake up from this nightmare.
Defining patient harm
Many patients experience harm each year because of an adverse medical event or preventable medical error.1 A 2013 report revealed that 210,000 to 440,000 deaths occur each year in the United States related to preventable patient harm.2 Although this fact is deeply disturbing, it is well known that modern health care is a high-risk industry.
Medical errors vary in terms of the degree of potential or actual damage. A “near miss” is any event that could have resulted in adverse consequences but did not (for example, an incorrect drug or dose ordered but not administered). On the other hand, an “adverse event” describes an error that resulted in some degree of patient harm or suffering.3
Related article:
Medical errors: Meeting ethical obligations and reducing liability with proper communication
For each patient who dies because of a medical error or a surgical complication, whether preventable or not, many clinicians are involved in the unfolding of the case. These events have a profound impact on well-intentioned, competent, and caring physicians, and they elicit intense emotional responses.4 When a patient experiences an unexpected adverse surgical outcome, the surgeons involved in their care may become “second victims.” They may feel that they have failed the patient and they second-guess their surgical skills and knowledge base; some express concern about their reputation and perhaps career choice.
Psychological responses. It is importantto understand this process to ensure a healthy recovery. Psychological responses to an adverse medical event include guilt; distress, anxiety, and fear; frustration and anger; feelings of insufficiency; and long-standing suffering. Clinicians who experienced an adverse medical event have reported additional psychological as well as physical symptoms in the aftermath of the event (TABLE 1).5
Risk factors. Certain factors are associated with a greater emotional impact of an adverse medical event, including6:
- severity of the harm or leaving permanent sequelae
- death of a healthy patient or a child (for example, from a motor vehicle accident)
- self-blame for the error
- unexpected patient death (for example, a catastrophic complication after a relatively benign procedure)
- physicians-in-training responsible for the patient
- first death under a clinician’s watch.
While most research in the field of medical error focuses on systems or process improvement, it is important not to neglect the individual and personal aspects of the clinicians involved in the event. The health care system must include care for our injured colleagues, the so-called second victims.
Read about the steps to recovery for the second victim.
Steps in recovery for the second victim
Based on a semistructured interview of 31 physicians involved in adverse events, Scott and colleagues described the following 6 stages of healing5:
Chaos and accident response. Immediately after the event, the physician feels a sense of confusion, panic, and denial. How can this be happening to me? The physician is frequently distracted, immersed in self-reflection.
Intrusive reflections. This is a period of self-questioning. Thoughts of the event and different possible scenarios dominate the physician’s mind. What if I had done this or that?
Restoring personal integrity. During this phase, the physician seeks support from individuals with whom trusted relationships exist, such as colleagues, peers, close friends, and family members. Advice from a colleague who has your same level of expertise is precious. The second victim often fears that friends and family will not be understanding.
Enduring the inquisition. Root cause analysis and in-depth case review is an important part of the quality improvement process after an adverse event. A debriefing or departmental morbidity and mortality conference can trigger emotions and increase the sense of shame, guilt, and self-doubt. The second victim starts to wonder about repercussions that may affect job security, licensure, and future litigation.
Obtaining emotional first aid. At this stage, the second victim begins to heal, but it is important to obtain external help from a colleague, mentor, counselor, department chair, or loved ones. Many physicians express concerns about not knowing who is a “safe person” to trust in this situation. Often, second victims perceive that their loved ones just do not understand their professional life or should be protected from this situation.
Moving on. There is an urge to move forward with life and simply put the event behind. This is difficult, however. A second victim may follow one of these paths:
- drop out—stop practicing clinical medicine
- survive—maintain the same career but with significant residual emotional burden from the event
- thrive—make something good out of the unfortunate clinical experience.
Related article:
TRUST: How to build a support net for ObGyns affected by a medical error
All these programs offer immediate help to any clinician in psychological distress. They provide confidentiality, and the individual is reassured that he or she can safely use the service without further consequences (TABLE 2).10
The normal human response to an adverse medical event can lead to significant psychological consequences, long-term emotional incapacity, impaired performance of clinical care, and feelings of guilt, fear, isolation, or even suicide. At some point during his or her career, almost every physician will be involved in a serious adverse medical event and is at risk of experiencing strong emotional reactions. Health care facilities should have a support system in place to help clinicians cope with these stressful circumstances.
Use these 5 strategies to facilitate recovery
- Be determined. No matter how bad you feel about the event, you need to get up and moving.
- Avoid isolation. Get outside and interact with people. Avoid long periods in isolation. Bring your team together and talk about the event.
- Sleep well. Most symptoms of posttraumatic stress disorder occur at night. If you have trouble falling asleep or you wake up in the middle of the night with nightmares related to the event, attempt to regulate your body’s sleep schedule. Seek professional help if needed.
- Avoid negative coping habits. Sometimes people turn to alcohol, cigarettes, food, or drugs to cope. Although these strategies may help in the short term, they will do more harm than good over time.
- Enroll in activities that provide positive distraction. While the mind focuses on the traumatic event (this is normal), you need to get busy with such positive distractions as sports, going to the movies, and engaging in outdoor activities. Do things that you enjoy.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
CASE A surgeon's story of patient loss
It was a Wednesday morning and Ms. M was my first case of the day. I knew her well, having delivered her 2 children. Now she had a 7-cm complex cyst on her right ovary, she was in pain, and she was possibly experiencing ovarian torsion. My resident took care of the paperwork, I met the patient in preop, answered her few questions, and reassured her husband that I would call him as soon as surgery was over. She was rolled to the operating room.
When I entered the OR, Ms. M was under general anesthesia, draped, and placed on the operating table in the usual position. I made a 5-mm incision at the umbilicus and inserted the trocar under direct visualization. There was blood and the camera became blurry. I removed the camera to clean it, and the anesthesiologist alerted me that there was sudden hypotension. I reinserted the camera and saw blood in the abdomen. I feared the worst—major vessel injury. I requested a scalpel and made a midline skin sub–umbilical incision, entered the peritoneal cavity, and observed blood everywhere. The massive transfusion protocol was activated and vascular surgery was called in. I could not find the source of the bleeding. Using a laparotomy towel I applied pressure on the aorta. The vascular surgeon arrived and pushed my resident away. He identified the source of the bleeding: The right common iliac artery was injured.
The patient coded, the anesthesiologist initiated CPR, bleeding continued, blood was being transfused, and after 20 long minutes of CPR the lifeless body of my patient could not hold any more. She was pronounced dead on the table.
At that moment, there were multiple victims: Ms. M lying on the surgical table; her family members, who did not know what was happening; and the surgical team members, who were looking at each other in denial and feeling that we had failed this patient, hoping that we would wake up from this nightmare.
Defining patient harm
Many patients experience harm each year because of an adverse medical event or preventable medical error.1 A 2013 report revealed that 210,000 to 440,000 deaths occur each year in the United States related to preventable patient harm.2 Although this fact is deeply disturbing, it is well known that modern health care is a high-risk industry.
Medical errors vary in terms of the degree of potential or actual damage. A “near miss” is any event that could have resulted in adverse consequences but did not (for example, an incorrect drug or dose ordered but not administered). On the other hand, an “adverse event” describes an error that resulted in some degree of patient harm or suffering.3
Related article:
Medical errors: Meeting ethical obligations and reducing liability with proper communication
For each patient who dies because of a medical error or a surgical complication, whether preventable or not, many clinicians are involved in the unfolding of the case. These events have a profound impact on well-intentioned, competent, and caring physicians, and they elicit intense emotional responses.4 When a patient experiences an unexpected adverse surgical outcome, the surgeons involved in their care may become “second victims.” They may feel that they have failed the patient and they second-guess their surgical skills and knowledge base; some express concern about their reputation and perhaps career choice.
Psychological responses. It is importantto understand this process to ensure a healthy recovery. Psychological responses to an adverse medical event include guilt; distress, anxiety, and fear; frustration and anger; feelings of insufficiency; and long-standing suffering. Clinicians who experienced an adverse medical event have reported additional psychological as well as physical symptoms in the aftermath of the event (TABLE 1).5
Risk factors. Certain factors are associated with a greater emotional impact of an adverse medical event, including6:
- severity of the harm or leaving permanent sequelae
- death of a healthy patient or a child (for example, from a motor vehicle accident)
- self-blame for the error
- unexpected patient death (for example, a catastrophic complication after a relatively benign procedure)
- physicians-in-training responsible for the patient
- first death under a clinician’s watch.
While most research in the field of medical error focuses on systems or process improvement, it is important not to neglect the individual and personal aspects of the clinicians involved in the event. The health care system must include care for our injured colleagues, the so-called second victims.
Read about the steps to recovery for the second victim.
Steps in recovery for the second victim
Based on a semistructured interview of 31 physicians involved in adverse events, Scott and colleagues described the following 6 stages of healing5:
Chaos and accident response. Immediately after the event, the physician feels a sense of confusion, panic, and denial. How can this be happening to me? The physician is frequently distracted, immersed in self-reflection.
Intrusive reflections. This is a period of self-questioning. Thoughts of the event and different possible scenarios dominate the physician’s mind. What if I had done this or that?
Restoring personal integrity. During this phase, the physician seeks support from individuals with whom trusted relationships exist, such as colleagues, peers, close friends, and family members. Advice from a colleague who has your same level of expertise is precious. The second victim often fears that friends and family will not be understanding.
Enduring the inquisition. Root cause analysis and in-depth case review is an important part of the quality improvement process after an adverse event. A debriefing or departmental morbidity and mortality conference can trigger emotions and increase the sense of shame, guilt, and self-doubt. The second victim starts to wonder about repercussions that may affect job security, licensure, and future litigation.
Obtaining emotional first aid. At this stage, the second victim begins to heal, but it is important to obtain external help from a colleague, mentor, counselor, department chair, or loved ones. Many physicians express concerns about not knowing who is a “safe person” to trust in this situation. Often, second victims perceive that their loved ones just do not understand their professional life or should be protected from this situation.
Moving on. There is an urge to move forward with life and simply put the event behind. This is difficult, however. A second victim may follow one of these paths:
- drop out—stop practicing clinical medicine
- survive—maintain the same career but with significant residual emotional burden from the event
- thrive—make something good out of the unfortunate clinical experience.
Related article:
TRUST: How to build a support net for ObGyns affected by a medical error
All these programs offer immediate help to any clinician in psychological distress. They provide confidentiality, and the individual is reassured that he or she can safely use the service without further consequences (TABLE 2).10
The normal human response to an adverse medical event can lead to significant psychological consequences, long-term emotional incapacity, impaired performance of clinical care, and feelings of guilt, fear, isolation, or even suicide. At some point during his or her career, almost every physician will be involved in a serious adverse medical event and is at risk of experiencing strong emotional reactions. Health care facilities should have a support system in place to help clinicians cope with these stressful circumstances.
Use these 5 strategies to facilitate recovery
- Be determined. No matter how bad you feel about the event, you need to get up and moving.
- Avoid isolation. Get outside and interact with people. Avoid long periods in isolation. Bring your team together and talk about the event.
- Sleep well. Most symptoms of posttraumatic stress disorder occur at night. If you have trouble falling asleep or you wake up in the middle of the night with nightmares related to the event, attempt to regulate your body’s sleep schedule. Seek professional help if needed.
- Avoid negative coping habits. Sometimes people turn to alcohol, cigarettes, food, or drugs to cope. Although these strategies may help in the short term, they will do more harm than good over time.
- Enroll in activities that provide positive distraction. While the mind focuses on the traumatic event (this is normal), you need to get busy with such positive distractions as sports, going to the movies, and engaging in outdoor activities. Do things that you enjoy.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Kohn L. To err is human: an interview with the Institute of Medicine's Linda Kohn. Jt Comm J Qual Improv. 2000;26(4):227-234.
- James JT. A new, evidence-based estimate of patient harms associated with hospital care. J Patient Saf. 2013;9(3):122-128.
- Harrison R, Lawton R, Perlo J, Gardner P, Armitage G, Shapiro J. Emotion and coping in the aftermath of medical error: a cross-country exploration. J Patient Saf. 2015;11(1):28-35.
- Chan ST, Khong PC, Wang W. Psychological responses, coping and supporting needs of healthcare professionals as second victims. Int Nurs Rev. 2017;64(2):242-262.
- Scott SD, Hirschinger LE, Cox KR, McCoig M, Brandt J, Hall LW. The natural history of recovery for the healthcare provider "second victim" after adverse patient events. Qual Saf Health Care. 2009;18(5):325-330.
- Waterman AD, Garbutt J, Hazel E, et al. The emotional impact of medical errors on practicing physicians in the United States and Canada. Jt Comm J Qual Patient Saf. 2007;33(8):467-476.
- Shapiro J, Galowitz P. Peer support for clinicians: a programmatic approach. Acad Med. 2016;91(9):1200-1204.
- Edrees H, Connors C, Paine L, Norvell M, Taylor H, Wu AW. Implementing the RISE second victim support programme at the Johns Hopkins Hospital: a case study. BMJ Open. 2016;6(9):e011708.
- Johnson B. Code lavender: initiating holistic rapid response at the Cleveland Clinic. Beginnings. 2014;34(2):10-11.
- van Pelt F. Peer support: healthcare professionals supporting each other after adverse medical events. Qual Saf Health Care. 2008;17(4):249-252.
- Kohn L. To err is human: an interview with the Institute of Medicine's Linda Kohn. Jt Comm J Qual Improv. 2000;26(4):227-234.
- James JT. A new, evidence-based estimate of patient harms associated with hospital care. J Patient Saf. 2013;9(3):122-128.
- Harrison R, Lawton R, Perlo J, Gardner P, Armitage G, Shapiro J. Emotion and coping in the aftermath of medical error: a cross-country exploration. J Patient Saf. 2015;11(1):28-35.
- Chan ST, Khong PC, Wang W. Psychological responses, coping and supporting needs of healthcare professionals as second victims. Int Nurs Rev. 2017;64(2):242-262.
- Scott SD, Hirschinger LE, Cox KR, McCoig M, Brandt J, Hall LW. The natural history of recovery for the healthcare provider "second victim" after adverse patient events. Qual Saf Health Care. 2009;18(5):325-330.
- Waterman AD, Garbutt J, Hazel E, et al. The emotional impact of medical errors on practicing physicians in the United States and Canada. Jt Comm J Qual Patient Saf. 2007;33(8):467-476.
- Shapiro J, Galowitz P. Peer support for clinicians: a programmatic approach. Acad Med. 2016;91(9):1200-1204.
- Edrees H, Connors C, Paine L, Norvell M, Taylor H, Wu AW. Implementing the RISE second victim support programme at the Johns Hopkins Hospital: a case study. BMJ Open. 2016;6(9):e011708.
- Johnson B. Code lavender: initiating holistic rapid response at the Cleveland Clinic. Beginnings. 2014;34(2):10-11.
- van Pelt F. Peer support: healthcare professionals supporting each other after adverse medical events. Qual Saf Health Care. 2008;17(4):249-252.
Fast Tracks
- A "near miss" is any event that could have resulted in adverse consequences but did not. An "adverse event" describes an error that resulted in some degree of patient harm or suffering.
- At some point in his or her career, almost every physician will be involved in a serious adverse medical event and is at risk of experiencing strong emotional reactions
A minimally invasive treatment for early GI cancers
The treatment of early esophageal, gastric, and colorectal cancer is changing.1 For many years, surgery was the mainstay of treatment for early-stage gastrointestinal cancer. Unfortunately, surgery leads to significant loss of function of the organ, resulting in increased morbidity and decreased quality of life.2
Endoscopic techniques, particularly endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD), have been developed and are widely used in Japan, where gastrointestinal cancer is more common than in the West. This article reviews the indications, complications, and outcomes of ESD for early gastrointestinal neoplasms, so that readers will recognize the subset of patients who would benefit from ESD in a Western setting.
ENDOSCOPIC MUCOSAL RESECTION AND SUBMUCOSAL DISSECTION
Since the first therapeutic polypectomy was performed in Japan in 1974, several endoscopic techniques for tumor resection have been developed.3
EMR, one of the most successful and widely used techniques, involves elevating the lesion either with submucosal injection of a solution or with cap suction, and then removing it with a snare.4 Most lesions smaller than 20 mm can be removed in one piece (en bloc).5 Larger lesions are removed in multiple pieces (ie, piecemeal). Unfortunately, some fibrotic lesions, which are usually difficult to lift, cannot be completely removed by EMR.
ESD was first performed in the late 1990s with the aim of overcoming the limitations of EMR in resecting large or fibrotic tumors en bloc.6,7 Since then, ESD technique has been standardized and training centers have been created, especially in Asia, where it is widely used for treatment of early gastric cancer.3,8–10 Since 2012 it has been covered by the Japanese National Health Insurance for treatment of early gastric cancer, and since 2014 for treatment of colorectal malignant tumors measuring 2 to 5 cm.11
Adoption of ESD has been slow in Western countries, where many patients are still referred for surgery or undergo EMR for removal of superficial neoplasms. Reasons for this slow adoption are that gastric cancer is much less common in Western countries, and also that ESD demands a high level of technical skill, is difficult to learn, and is expensive.3,12,13 However, small groups of Western endoscopists have become interested and are advocating it, first studying it on their own and then training in a Japanese center and learning from experts performing the procedure.
Therefore, in a Western setting, ESD should be performed in specialized endoscopy centers and offered to selected patients.1
CANDIDATES SHOULD HAVE EARLY-STAGE, SUPERFICIAL TUMORS
Ideal candidates for endoscopic resection are patients who have early cancer with a negligible risk of lymph node metastasis, such as cancer limited to the mucosa (stage T1a).7 Therefore, to determine the best treatment for a patient with a newly diagnosed gastrointestinal neoplasm, it is mandatory to estimate the depth of invasion.
The depth of invasion is directly correlated with lymph node involvement, which is ultimately the main predictive factor for long-term adverse outcomes of gastrointestinal tumors.4,14–17 Accurate multidisciplinary preprocedure estimations are mandatory, as incorrect evaluations may result in inappropriate therapy and residual cancer.18
Other factors that have been used to predict lymph node involvement include tumor size, macroscopic appearance, histologic differentiation, and lymphatic and vascular involvement.19 Some of these factors can be assessed by special endoscopic techniques (chromoendoscopy and narrow-band imaging with magnifying endoscopy) that allow accurate real-time estimation of the depth of invasion of the lesion.5,17,20–27 Evaluation of microsurface and microvascular arrangements is especially useful for determining the feasibility of ESD in gastric tumors, evaluation of intracapillary loops is useful in esophageal lesions, and assessment of mucosal pit patterns is useful for colorectal lesions.21–29
Endoscopic ultrasonography is another tool that has been used to estimate the depth of the tumor. Although it can differentiate between definite intramucosal and definite submucosal invasive cancers, its ability to confirm minute submucosal invasion is limited. Its use as the sole tumor staging modality is not encouraged, and it should always be used in conjunction with endoscopic evaluation.18
Though the aforementioned factors help stratify patients, pathologic staging is the best predictor of lymph node metastasis. ESD provides adequate specimens for accurate pathologic evaluation, as it removes lesions en bloc.30
All patients found to have risk factors for lymph node metastasis on endoscopic, ultrasonographic, or pathologic analysis should be referred for surgical evaluation.9,19,31,32
ENDOSCOPIC SUBMUCOSAL DISSECTION
Before the procedure, the patient’s physicians need to do the following:
Determine the best type of intervention (EMR, ESD, ablation, surgery) for the specific lesion.3 A multidisciplinary approach is encouraged, with involvement of the internist, gastroenterologist, and surgeon.
Plan for anesthesia, additional consultations, pre- and postprocedural hospital admission, and need for special equipment.33
During the procedure
Define the lateral extent of the lesion using magnification chromoendoscopy or narrow-band imaging. In the stomach, a biopsy sample should be taken from the worst-looking segment and from normal-looking mucosa. Multiple biopsies should be avoided to prevent subsequent fibrosis.33 In the colon, biopsy should be avoided.34
Identify and circumferentially mark the target lesion. Cautery or argon plasma coagulation can be used for making markings at a distance of 5 to 10 mm from the edges.33 This is done to recognize the borders of the lesion, because they can become distorted after submucosal injection.14 This step is unnecessary in colorectal cases, as tumor margins can be adequately visualized after chromoendoscopy.16,35
Lift the lesion by injecting saline, 0.5% hyaluronate, or glycerin to create a submucosal fluid cushion.19,33
Perform a circumferential incision lateral to the mucosal margins to allow for a normal tissue margin.33 Partial incision is performed for esophageal and colorectal ESD to avoid fluid leakage from the submucosal layer, achieving a sustained submucosal lift and safer dissection.16
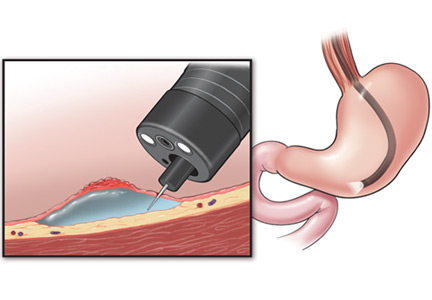
Submucosal dissection. The submucosal layer is dissected with an electrocautery knife until the lesion is completely removed. Dissection should be done carefully to keep the submucosal plane.33 Hemoclips or hemostat forceps can be used to control visible bleeding. The resected specimen is then stretched and fixed to a board using small pins for further histopathologic evaluation.35
Postprocedural monitoring. All patients should be admitted for overnight observation. Those who undergo gastric ESD should receive high-dose acid suppression, and the next day they can be started on a liquid diet.19
STOMACH CANCER
Indications for ESD for stomach cancer in the East
The incidence of gastric cancer is higher in Japan and Korea, where widespread screening programs have led to early identification and early treatment of this disease.36
Pathology studies37 of samples from patients with gastric cancer identified the following as risk factors for lymph node metastasis, which would make ESD unsuitable:
- Undifferentiated type
- Tumors larger than 2 cm
- Lymphatic or venous involvement
- Submucosal invasion
- Ulcerative change.
Based on these findings, the situations in which there was no risk of lymph node involvement (ie, when none of the above factors are present) were accepted as absolute indications for endoscopic resection of early gastric cancer.38 Further histologic studies identified a subset of patients with lesions with very low risk of lymph node metastasis, which outweighed the risk of surgery. Based on these findings, expanded criteria for gastric ESD were proposed,39,40 and the Japanese gastric cancer treatment guidelines now include these expanded preoperative indications9,17 (Table 1).
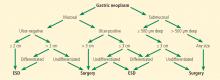
The Japanese Gastric Cancer Association has proposed a treatment algorithm based on the histopathologic evaluation after resection (Figure 2).9
Outcomes
In the largest series of patients who underwent curative ESD for early gastric cancer, the 5-year survival rate was 92.6%, the 5-year disease-specific survival rate was 99.9%, and the 5-year relative survival rate was 105%.41
Similarly, in a Japanese population-based survival analysis, the relative 5-year survival rate for localized gastric cancer was 94.4%.42 Rates of en bloc resection and complete resection with ESD are higher than those with EMR, resulting in a lower risk of local recurrence in selected patients who undergo ESD.8,43,44
Although rare, local recurrence after curative gastric ESD has been reported.45 The annual incidence of local recurrence has been estimated to be 0.84%.46
ESD entails a shorter hospital stay and requires fewer resources than surgery, resulting in lower medical costs (Table 2).44 Additionally, as endoscopic resection is associated with less morbidity, fewer procedure-related adverse events, and fewer complications, ESD could be used as the standard treatment for early gastric cancer.47,48
The Western perspective on endoscopic submucosal dissection for gastric cancer
Since the prevalence of gastric cancer in Western countries is significantly lower than in Japan and Korea, local data and experience are scarce. However, experts performing ESD in the West have adopted the indications of the Japan Gastroenterological Endoscopy Society. The European Society of Gastrointestinal Endoscopy recommends ESD for excision of most superficial gastric neoplasms, with EMR being preferred only in lesions smaller than 15 mm, Paris classification 0 or IIA.5,32
Patients with gastric lesions measuring 15 mm or larger should undergo high-quality endoscopy, preferably chromoendoscopy, to evaluate the mucosal patterns and determine the depth of invasion. If superficial involvement is confirmed, other imaging techniques are not routinely recommended.5 A surgery consult is also recommended.
ESOPHAGEAL CANCER
Indications for ESD for esophageal cancer in the East
Due to the success of ESD for early gastric cancer, this technique is now also used for superficial esophageal neoplasms.19,49 It should be done in a specialized center, as it is more technically difficult than gastric ESD: the esophageal lumen is narrow, the wall is thin, and the esophagus moves with respiration and heartbeat.50 A multidisciplinary approach including an endoscopist, a surgeon, and a pathologist is highly recommended for evaluation and treatment.
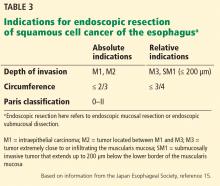
EMR is preferred for removal of mucosal cancer, in view of its safety profile and success rates. ESD can be considered in cases of lesions larger than 15 mm, poorly lifting tumors, and those with the possibility of submucosal invasion (Table 3).5,45,49,51
Circumference involvement is critical when determining eligible candidates, as a defect involving more than three-fourths of the esophageal circumference can lead to esophageal strictures.52 Controlled prospective studies have shown promising results from giving intralesional and oral steroids to prevent stricture after ESD, which could potentially overcome this size limitation.53,54
Outcomes for esophageal cancer
ESD has been shown to be safe and effective, achieving en bloc resection in 85% to 100% of patients.19,51 Its advantages over EMR include en bloc resection, complete resection, and high curative rates, resulting in higher recurrence-free survival.2,55,56 Although the incidence of complications such as bleeding, perforation, and stricture formation are higher with ESD, patients usually recover uneventfully.2,19,20
ESD in the esophagus: The Western perspective
As data on the efficacy of EMR vs ESD for the treatment of Barrett esophagus with adenocarcinoma are limited, EMR is the gold standard endoscopic technique for removal of visible esophageal dysplastic lesions.5,51,57 ESD can be considered for tumors larger than 15 mm, for poorly lifting lesions, and if there is suspicion of submucosal invasion.5
Patients should be evaluated by an experienced endoscopist, using an advanced imaging technique such as narrow-band imaging or chromoendoscopy. If suspicious features are found, endoscopic ultrasonography should be considered to confirm submucosal invasion or lymph node involvement.5
COLORECTAL CANCER
Indications for ESD for colorectal cancer in the East
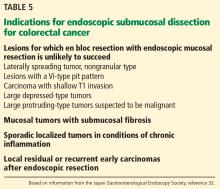
Colon cancer is one of the leading causes of cancer-related deaths worldwide.58 Since ESD has been found to be effective and safe in treating gastric cancer, it has also been used to remove large colorectal tumors.59 However, ESD is not universally accepted in the treatment of colorectal neoplasms due to its greater technical difficulty, longer procedural time, and higher risk of perforating the thinner colonic wall compared with EMR.21,60
Outcomes for colorectal cancer
Tumor size of 50 mm or larger is a risk factor for complications, while a high procedure volume at the center is a protective factor.60
Endoscopic treatment of colorectal cancer: The Western perspective
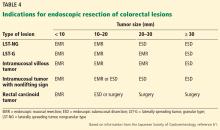
EMR is the gold standard for removal of superficial colorectal lesions. However, ESD can be considered if there is suspicion of superficial submucosal invasion, especially for lesions larger than 20 mm that cannot be resected en bloc by EMR.32 ESD can also be used for fibrotic lesions not amenable to complete EMR removal, or as a salvage procedure after recurrence after EMR.67 Proper selection of cases is critical.1
Patients who have a superficial colonic lesion should be evaluated by means of high-definition endoscopy and chromoendoscopy to assess the mucosal pattern and establish feasibility of endoscopic resection. If submucosal invasion is suspected, staging with endoscopic ultrasonography or magnetic resonance imaging should be considered.5
FOLLOW-UP AFTER ESD
Endoscopic surveillance after the procedure is recommended, given the persistent risk of metachronous cancer after curative ESD due to its organ-sparing quality.68 Surveillance endoscopy aims to achieve early detection and subsequent endoscopic resection of metachronous lesions.
Histopathologic evaluation assessing the presence of malignant cells in the margins of a resected sample is mandatory for determining the next step in treatment. If margins are negative, follow-up endoscopy can be done every 6 to 12 months. If margins are positive, the approach includes surgery, reattempting ESD or endoscopic surveillance in 3 or 6 months.3,32 Although the surveillance strategy varies according to individual risk of metachronous cancer, it should be continued indefinitely.68
COMPLICATIONS OF ESD
The most common procedure-related complications of ESD are bleeding, perforation, and stricture. Most intraprocedural adverse events can be managed endoscopically.69
Bleeding
Most bleeding occurs during the procedure or early after it and can be controlled with electrocautery.49,69 No episodes of massive bleeding, defined as causing clinical symptoms and requiring transfusion or surgery, have been reported.20,43,55
In gastric ESD, delayed bleeding rates have ranged from 0 to 15.6%.69 Bleeding may be prevented with endoscopic coagulation of visible vessels after dissection has been completed and by proton pump inhibitor therapy.70,71 Excessive coagulation should be avoided to lower the risk of perforation.33
In colorectal ESD the bleeding rate has been reported to be 2.2%; applying coagulation to an area where a blood vessel is suspected before cutting (precoagulation) may prevent subsequent bleeding.21
Perforation
For gastric ESD, perforation rates range from 1.2% to 5.2%.69 Esophageal perforation rates can be up to 4%.49 In colorectal ESD, perforation rates have been reported to be 1.6% to 6.6%.60,72
Although most of the cases were successfully managed with conservative treatment, some required emergency surgery.60,73
Strictures
In a case series of 532 patients undergoing gastric ESD, stricture was reported in 5 patients, all of whom presented with obstructive symptoms.74 Risk factors for post-ESD gastric stenosis are a mucosal defect with a circumferential extent of more than three-fourths or a longitudinal extent of more than 5 cm.75
Strictures are common after esophageal ESD, with rates ranging from 2% to 26%. The risk is higher when longer segments are removed or circumferential resection is performed. As previously mentioned, this complication may be reduced with ingestion or injection of steroids after the procedure.53,54
Surprisingly, ESD of large colorectal lesions involving more than three-fourths of the circumference of the rectum is rarely complicated by stenosis.76
LIMITATIONS OF ESD
ESD requires a high level of technical skill, is time-consuming, and has a higher rate of complications than conventional endoscopic resection. A standardized ESD training system is needed, as the procedure is more difficult than EMR. Training in porcine models has been shown to confer competency in ESD in a Western setting.13,16,33
Colorectal ESD is an even more challenging procedure, given the potential for complications related to its anatomy. Training centers in Japan usually have their trainees first master gastric ESD, then assist in more than 20 colorectal ESDs conducted by experienced endoscopists, and accomplish 30 cases before performing the procedure safely and independently.
As the incidence of gastric cancer is low in Western countries, trainees may also begin with lower rectal lesions, which are easier to remove.77 Incorporation of ESD in the West would require a clear treatment algorithm. It is a complex procedure, with higher rates of complications, a prolonged learning curve, and prolonged procedure time. Therefore, it should be performed in specialized centers and under the special situations discussed here to ensure that the benefits for the patients outweigh the risks.
VALUE OF ENDOSCOPIC SUBMUCOSAL DISSECTION
The optimal method for resecting gastrointestinal neoplasms should be safe, cost-effective, and quick and should also completely remove the lesion. The best treatment strategy takes into account the characteristics of the lesion and the comorbidities and wishes of the patient. Internists should be aware of the multiple options available to achieve the best outcome for the patient.1
Endoscopic resection of superficial gastrointestinal neoplasms, including EMR and ESD, has been a subject of increasing interest due to its minimally invasive and potentially curative character. However, cancer can recur after endoscopic resection because the procedure is organ-sparing.
ESD allows resection of early gastrointestinal tumors with a minimally invasive technique. It can achieve higher curative resection rates and lower recurrence rates compared with EMR. Compared with surgery, ESD leads to less morbidity, fewer procedure-related complications, and lower medical costs. Indications should be rigorously followed to achieve successful treatments in selected patients.
Multiple variables have to be taken into account when deciding which treatment is best, such as tumor characteristics, the patient’s baseline condition, physician expertise, and hospital resources.48 Less-invasive treatments may improve the prognosis of patients. No matter the approach, patients should be treated in specialized treatment centers.
Internal medicine physicians should be aware of the advances in treatments for early gastrointestinal cancer so appropriate options can be considered.
- Burgess NG, Bourke MJ. Endoscopic resection of colorectal lesions: the narrowing divide between East and West. Dig Endosc 2016; 28:296–305.
- Kim DH, Jung HY, Gong EJ, et al. Endoscopic and oncologic outcomes of endoscopic resection for superficial esophageal neoplasm. Gut Liver 2015; 9:470–477.
- Draganov PV, Gotoda T, Chavalitdhamrong D, Wallace MB. Techniques of endoscopic submucosal dissection: application for the Western endoscopist? Gastrointest Endosc 2013; 78:677–688.
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011; 14:101–112.
- Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2015; 47:829–854.
- Farhat S, Chaussade S, Ponchon T, et al; SFED ESD Study Group. Endoscopic submucosal dissection in a European setting. A multi-institutional report of a technique in development. Endoscopy 2011; 43:664–670.
- Gotoda T, Jung H. Endoscopic resection (endoscopic mucosal resection/endoscopic submucosal dissection) for early gastric cancer. Dig Endosc 2013; 25(suppl 1):55–63.
- Chung IK, Lee JH, Lee SH, et al. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc 2009; 69:1228–1235.
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011; 14:113–123.
- Ono H. Endoscopic submucosal dissection for early gastric cancer. Chin J Dig Dis 2005; 6:119–121.
- Watanabe T, Itabashi M, Shimada Y, et al; Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol 2015; 20:207–239.
- Oyama T, Yahagi N, Ponchon T, Kiesslich T, Berr F. How to establish endoscopic submucosal dissection in Western countries. World J Gastroenterol 2015; 21:11209–11220.
- Bhatt A, Abe S, Kumaravel A, et al. SU1575 Western skill training in endoscopic submucosal dissection (ESD)—an international remote video based study—the WEST ESD Study. Gastrointest Endosc 2015; 81(suppl):AB335–AB336.
- Sano T, Sasako M, Kinoshita T, Maruyama K. Recurrence of early gastric cancer follow-up of 1475 patients and review of the Japanese literature. Cancer 1993; 72:3174–3178.
- Japan Esophageal Society. Japanese classification of esophageal cancer, tenth edition: part I. Esophagus 2009; 6:1–25.
- Bhatt A, Abe S, Kumaravel A, Vargo J, Saito Y. Indications and techniques for endoscopic submucosal dissection. Am J Gastroenterol 2015; 110:784–791.
- Eleftheriadis N, Inoue H, Ikeda H, et al. Definition and staging of early esophageal, gastric and colorectal cancer. J Tumor 2014; 2:161–178.
- Yoshinaga S, Oda I, Nonaka S, Kushima R, Saito Y. Endoscopic ultrasound using ultrasound probes for the diagnosis of early esophageal and gastric cancers. World J Gastrointest Endosc 2012; 4:218–226.
- Stahl M, Mariette C, Haustermans K, Cervantes A, Arnold D; ESMO Guidelines Working Group. Oesophageal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013; 24(suppl 6):vi51–vi56.
- Higuchi K, Tanabe S, Azuma M, et al. A phase II study of endoscopic submucosal dissection for superficial esophageal neoplasms (KDOG 0901). Gastrointest Endosc 2013; 78:704–710.
- Sakamoto T, Mori G, Yamada M, et al. Endoscopic submucosal dissection for colorectal neoplasms: a review. World J Gastroenterol 2014; 20:16153–16158.
- Ohta A, Tominaga K, Sakai Y. Efficacy of magnifying colonoscopy for the diagnosis of colorectal neoplasia: comparison with histopathological findings. Dig Endosc 2004; 16:308–314.
- Katagiri A, Fu K, Sano Y, et al. Narrow band imaging with magnifying colonoscopy as diagnostic tool for predicting histology of early colorectal neoplasia. Aliment Pharmacol Ther 2008; 27:1269–1274.
- Fu KI, Kato S, Sano Y, et al. Staging of early colorectal cancers: magnifying colonoscopy versus endoscopic ultrasonography for estimation of depth of invasion. Dig Dis Sci 2008; 53:1886–1892.
- Uraoka T, Saito Y, Ikematsu H, Yamamoto K, Sano Y. Sano’s capillary pattern classification for narrow-band imaging of early colorectal lesions. Dig Endosc 2011; 23(suppl 1):112–115.
- Ikematsu H, Matsuda T, Emura F, et al. Efficacy of capillary pattern type IIIA/IIIB by magnifying narrow band imaging for estimating depth of invasion of early colorectal neoplasms. BMC Gastroenterol 2010;10:33.
- Matsuda T, Fujii T, Saito Y, et al. Efficacy of the invasive/non-invasive pattern by magnifying chromoendoscopy to estimate the depth of invasion of early colorectal neoplasms. Am J Gastroenterol 2008; 103:2700–2706.
- Sato H, Inoue H, Ikeda H, et al. Utility of intrapapillary capillary loops seen on magnifying narrow-band imaging in estimating invasive depth of esophageal squamous cell carcinoma. Endoscopy 2015; 8:122–128.
- Muto M, Yao K, Kaise M, et al. Magnifying endoscopy simple diagnostic algorithm for early gastric cancer (MESDA-G). Dig Endosc 2016; 28:379–393.
- Waddell T, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D; European Society for Medical Oncology (ESMO); European Society of Surgical Oncology (ESSO); European Society of Radiotherapy and Oncology (ESTRO). Gastric cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013; 24(suppl 6):vi57–vi63.
- Kuwano H, Nishimura Y, Ohtsu A, et al. Guidelines for diagnosis and treatment of carcinoma of the esophagus. April 2007 edition: part I - Edited by the Japan Esophageal Society. Esophagus 2008; 5:61–73.
- Tanaka S, Kashida H, Saito Y, et al. JGES guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc 2015; 27:417–434.
- Gotoda T, Ho KY, Soetikno R, Kaltenbach T, Draganov P. Gastric ESD: current status and future directions of devices and training. Gastrointest Endosc Clin North Am 2014; 24:213–233.
- Saito Y, Sakamoto T, Nakajima T, Matsuda T. Colorectal ESD: current indications and latest technical advances. Gastrointest Endosc Clin N Am 2014; 24:245–255.
- Saito Y, Otake Y, Sakamoto T, et al. Indications for and technical aspects of colorectal endoscopic submucosal dissection. Gut Liver 2013; 7:263–269.
- Saragoni L. Upgrading the definition of early gastric cancer: better staging means more appropriate treatment. Cancer Biol Med 2015; 12:355–361.
- Tsujitani S, Oka S, Saito H, et al. Less invasive surgery for early gastric cancer based on the low probability of lymph node metastasis. Surgery 1999; 125:148–154.
- Soetikno RM, Gotoda T, Nakanishi Y, Soehendra N. Endoscopic mucosal resection. Gastrointest Endosc 2003; 57:567–579.
- Hirasawa T, Gotoda T, Miyata S, et al. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer 2009; 12:148–152.
- Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer 2000; 3:219–225.
- Suzuki H, Oda I, Abe S, et al. High rate of 5-year survival among patients with early gastric cancer undergoing curative endoscopic submucosal dissection. Gastric Cancer 2016; 19:198–205.
- Matsuda T, Ajiki W, Marugame T, Ioka A, Tsukuma H, Sobue T; Research Group of Population-Based Cancer Registries of Japan. Population-based survival of cancer patients diagnosed between 1993 and 1999 in Japan: a chronological and international comparative study. Jpn J Clin Oncol 2011; 41:40–51.
- Ahn JY, Jung HY, Choi KD, et al. Endoscopic and oncologic outcomes after endoscopic resection for early gastric cancer: 1370 cases of absolute and extended indications. Gastrointest Endosc 2011; 74:485–493.
- Kim Y, Kim YW, Choi IJ, et al. Cost comparison between surgical treatments and endoscopic submucosal dissection in patients with early gastric cancer in Korea. Gut Liver 2015; 9:174–180.
- Abe S, Oda I, Nakajima T, et al. A case of local recurrence and distant metastasis following curative endoscopic submucosal dissection of early gastric cancer. Gastric Cancer 2015; 18:188–192.
- Hahn KY, Park JC, Kim EH, et al. Incidence and impact of scheduled endoscopic surveillance on recurrence after curative endoscopic resection for early gastric cancer. Gastrointest Endosc 2016; 84:628–638.e1.
- Wang S, Zhang Z, Liu M, Li S, Jiang C. Endoscopic resection compared with gastrectomy to treat early gastric cancer: a systematic review and meta-analysis. PLoS One 2015; 10:e0144774.
- Kondo A, de Moura EG, Bernardo WM, et al. Endoscopy vs surgery in the treatment of early gastric cancer: systematic review. World J Gastroenterol 2015; 21:13177–13187.
- Kothari S, Kaul V. Endoscopic mucosal resection and endoscopic submucosal dissection for endoscopic therapy of Barrett’s esophagus-related neoplasia. Gastroenterol Clin North Am 2015; 44:317–335.
- Yamashita T, Zeniya A, Ishii H, et al. Endoscopic mucosal resection using a cap-fitted panendoscope and endoscopic submucosal dissection as optimal endoscopic procedures for superficial esophageal carcinoma. Surg Endosc 2011; 25:2541–2546.
- Kagemoto K, Oka S, Tanaka S, et al. Clinical outcomes of endoscopic submucosal dissection for superficial Barrett’s adenocarcinoma. Gastrointest Endosc 2014; 80:239–245.
- Katada C, Muto M, Manabe T, Boku N, Ohtsu A, Yoshida S. Esophageal stenosis after endoscopic mucosal resection of superficial esophageal lesions. Gastrointest Endosc 2003; 57:165–169.
- Hanaoka N, Ishihara R, Takeuchi Y, et al. 1139: A single session of intralesional steroid injection to prevent esophageal stricture after endoscopic submucosal dissection for esophageal squamous cell carcinoma. Gastrointest Endosc 2012; 75(suppl):AB175.
- Yamaguchi N, Isomoto H, Nakayama T, et al. Usefulness of oral prednisolone in the treatment of esophageal stricture after endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. Gastrointest Endosc 2011; 73:1115–1121.
- Ono S, Fujishiro M, Niimi K, et al. Long-term outcomes of endoscopic submucosal dissection for superficial esophageal squamous cell neoplasms. Gastrointest Endosc 2009; 70:860–866.
- Katada C, Muto M, Manabe T, Ohtsu A, Yoshida S. Local recurrence of squamous-cell carcinoma of the esophagus after EMR. Gastrointest Endosc 2005; 61:219–225.
- Hirasawa K, Kokawa A, Oka H, et al. Superficial adenocarcinoma of the esophagogastric junction: long-term results of endoscopic submucosal dissection. Gastrointest Endosc 2010; 72:960–966.
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.
- Nakajima T, Saito Y, Tanaka S, et al. Current status of endoscopic resection strategy for large, early colorectal neoplasia in Japan. Surg Endosc 2013; 27:3262–3770.
- Saito Y, Uraoka T, Yamaguchi Y, et al. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointest Endosc 2010; 72:1217–1225.
- Tanaka S, Saitoh Y, Matsuda T, et al; Japanese Society of Gastroenterology. Evidence-based clinical practice guidelines for management of colorectal polyps. J Gastroenterol 2015; 50:252–260.
- Oka S, Tanaka S, Saito Y, et al; Colorectal Endoscopic Resection Standardization Implementation Working Group of the Japanese Society for Cancer of the Colon and Rectum, Tokyo, Japan. Local recurrence after endoscopic resection for large colorectal neoplasia: a multicenter prospective study in Japan. Am J Gastroenterol 2015; 110:697–707.
- Saito Y, Fukuzawa M, Matsuda T, et al. Clinical outcome of endoscopic submucosal dissection versus endoscopic mucosal resection of large colorectal tumors as determined by curative resection. Surg Endosc 2010; 24:343–352.
- Makazu M, Sakamoto T, So E, et al. Relationship between indeterminate or positive lateral margin and local recurrence after endoscopic resection of colorectal polyps. Endosc Int Open 2015; 3:E252–E257.
- Belderbos TD, Leenders M, Moons LM, Siersema PD. Local recurrence after endoscopic mucosal resection of nonpedunculated colorectal lesions: systematic review and meta-analysis. Endoscopy 2014; 46:388–402.
- Fujiya M, Tanaka K, Dokoshi T, et al. Efficacy and adverse events of EMR and endoscopic submucosal dissection for the treatment of colon neoplasms: a meta-analysis of studies comparing EMR and endoscopic submucosal dissection. Gastrointest Endosc 2015; 81:583–595.
- Rahmi G, Tanaka S, Ohara Y, et al. Efficacy of endoscopic submucosal dissection for residual or recurrent superficial colorectal tumors after endoscopic mucosal resection. J Dig Dis 2015; 16:14–21.
- Abe S, Oda I, Suzuki H, et al. Long-term surveillance and treatment outcomes of metachronous gastric cancer occurring after curative endoscopic submucosal dissection. Endoscopy 2015; 47:1113–1118.
- Oda I, Suzuki H, Nonaka S, Yoshinaga S. Complications of gastric endoscopic submucosal dissection. Dig Endosc 2013; 25(suppl 1):71–78.
- Takizawa K, Oda I, Gotoda T, et al. Routine coagulation of visible vessels may prevent delayed bleeding after endoscopic submucosal dissection—an analysis of risk factors. Endoscopy 2008; 40:179–183.
- Uedo N, Takeuchi Y, Yamada T, et al. Effect of a proton pump inhibitor or an H2-receptor antagonist on prevention of bleeding from ulcer after endoscopic submucosal dissection of early gastric cancer: a prospective randomized controlled trial. Am J Gastroenterol 2007; 102:1610–1616.
- Hayashi N, Tanaka S, Nishiyama S, et al. Predictors of incomplete resection and perforation associated with endoscopic submucosal dissection for colorectal tumors. Gastrointest Endosc 2014; 79:427–435.
- Suzuki H, Oda I, Sekiguchi M, et al. Management and associated factors of delayed perforation after gastric endoscopic submucosal dissection. World J Gastroenterol 2015; 21:12635–12643.
- Tsunada S, Ogata S, Mannen K, et al. Case series of endoscopic balloon dilation to treat a stricture caused by circumferential resection of the gastric antrum by endoscopic submucosal dissection. Gastrointest Endosc 2008; 67:979–983.
- Coda S, Oda I, Gotoda T, Yokoi C, Kikuchi T, Ono H. Risk factors for cardiac and pyloric stenosis after endoscopic submucosal dissection, and efficacy of endoscopic balloon dilation treatment. Endoscopy 2009; 41:421–426.
- Abe S, Sakamoto T, Takamaru H, et al. Stenosis rates after endoscopic submucosal dissection of large rectal tumors involving greater than three quarters of the luminal circumference. Surg Endosc 2016; 30:5459–5464.
- Sakamoto T, Saito Y, Fukunaga S, Nakajima T, Matsuda T. Learning curve associated with colorectal endoscopic submucosal dissection for endoscopists experienced in gastric endoscopic submucosal dissection. Dis Colon Rectum 2011; 54:1307–1312.
The treatment of early esophageal, gastric, and colorectal cancer is changing.1 For many years, surgery was the mainstay of treatment for early-stage gastrointestinal cancer. Unfortunately, surgery leads to significant loss of function of the organ, resulting in increased morbidity and decreased quality of life.2
Endoscopic techniques, particularly endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD), have been developed and are widely used in Japan, where gastrointestinal cancer is more common than in the West. This article reviews the indications, complications, and outcomes of ESD for early gastrointestinal neoplasms, so that readers will recognize the subset of patients who would benefit from ESD in a Western setting.
ENDOSCOPIC MUCOSAL RESECTION AND SUBMUCOSAL DISSECTION
Since the first therapeutic polypectomy was performed in Japan in 1974, several endoscopic techniques for tumor resection have been developed.3
EMR, one of the most successful and widely used techniques, involves elevating the lesion either with submucosal injection of a solution or with cap suction, and then removing it with a snare.4 Most lesions smaller than 20 mm can be removed in one piece (en bloc).5 Larger lesions are removed in multiple pieces (ie, piecemeal). Unfortunately, some fibrotic lesions, which are usually difficult to lift, cannot be completely removed by EMR.
ESD was first performed in the late 1990s with the aim of overcoming the limitations of EMR in resecting large or fibrotic tumors en bloc.6,7 Since then, ESD technique has been standardized and training centers have been created, especially in Asia, where it is widely used for treatment of early gastric cancer.3,8–10 Since 2012 it has been covered by the Japanese National Health Insurance for treatment of early gastric cancer, and since 2014 for treatment of colorectal malignant tumors measuring 2 to 5 cm.11
Adoption of ESD has been slow in Western countries, where many patients are still referred for surgery or undergo EMR for removal of superficial neoplasms. Reasons for this slow adoption are that gastric cancer is much less common in Western countries, and also that ESD demands a high level of technical skill, is difficult to learn, and is expensive.3,12,13 However, small groups of Western endoscopists have become interested and are advocating it, first studying it on their own and then training in a Japanese center and learning from experts performing the procedure.
Therefore, in a Western setting, ESD should be performed in specialized endoscopy centers and offered to selected patients.1
CANDIDATES SHOULD HAVE EARLY-STAGE, SUPERFICIAL TUMORS
Ideal candidates for endoscopic resection are patients who have early cancer with a negligible risk of lymph node metastasis, such as cancer limited to the mucosa (stage T1a).7 Therefore, to determine the best treatment for a patient with a newly diagnosed gastrointestinal neoplasm, it is mandatory to estimate the depth of invasion.
The depth of invasion is directly correlated with lymph node involvement, which is ultimately the main predictive factor for long-term adverse outcomes of gastrointestinal tumors.4,14–17 Accurate multidisciplinary preprocedure estimations are mandatory, as incorrect evaluations may result in inappropriate therapy and residual cancer.18
Other factors that have been used to predict lymph node involvement include tumor size, macroscopic appearance, histologic differentiation, and lymphatic and vascular involvement.19 Some of these factors can be assessed by special endoscopic techniques (chromoendoscopy and narrow-band imaging with magnifying endoscopy) that allow accurate real-time estimation of the depth of invasion of the lesion.5,17,20–27 Evaluation of microsurface and microvascular arrangements is especially useful for determining the feasibility of ESD in gastric tumors, evaluation of intracapillary loops is useful in esophageal lesions, and assessment of mucosal pit patterns is useful for colorectal lesions.21–29
Endoscopic ultrasonography is another tool that has been used to estimate the depth of the tumor. Although it can differentiate between definite intramucosal and definite submucosal invasive cancers, its ability to confirm minute submucosal invasion is limited. Its use as the sole tumor staging modality is not encouraged, and it should always be used in conjunction with endoscopic evaluation.18
Though the aforementioned factors help stratify patients, pathologic staging is the best predictor of lymph node metastasis. ESD provides adequate specimens for accurate pathologic evaluation, as it removes lesions en bloc.30
All patients found to have risk factors for lymph node metastasis on endoscopic, ultrasonographic, or pathologic analysis should be referred for surgical evaluation.9,19,31,32
ENDOSCOPIC SUBMUCOSAL DISSECTION
Before the procedure, the patient’s physicians need to do the following:
Determine the best type of intervention (EMR, ESD, ablation, surgery) for the specific lesion.3 A multidisciplinary approach is encouraged, with involvement of the internist, gastroenterologist, and surgeon.
Plan for anesthesia, additional consultations, pre- and postprocedural hospital admission, and need for special equipment.33
During the procedure
Define the lateral extent of the lesion using magnification chromoendoscopy or narrow-band imaging. In the stomach, a biopsy sample should be taken from the worst-looking segment and from normal-looking mucosa. Multiple biopsies should be avoided to prevent subsequent fibrosis.33 In the colon, biopsy should be avoided.34
Identify and circumferentially mark the target lesion. Cautery or argon plasma coagulation can be used for making markings at a distance of 5 to 10 mm from the edges.33 This is done to recognize the borders of the lesion, because they can become distorted after submucosal injection.14 This step is unnecessary in colorectal cases, as tumor margins can be adequately visualized after chromoendoscopy.16,35
Lift the lesion by injecting saline, 0.5% hyaluronate, or glycerin to create a submucosal fluid cushion.19,33
Perform a circumferential incision lateral to the mucosal margins to allow for a normal tissue margin.33 Partial incision is performed for esophageal and colorectal ESD to avoid fluid leakage from the submucosal layer, achieving a sustained submucosal lift and safer dissection.16
Submucosal dissection. The submucosal layer is dissected with an electrocautery knife until the lesion is completely removed. Dissection should be done carefully to keep the submucosal plane.33 Hemoclips or hemostat forceps can be used to control visible bleeding. The resected specimen is then stretched and fixed to a board using small pins for further histopathologic evaluation.35
Postprocedural monitoring. All patients should be admitted for overnight observation. Those who undergo gastric ESD should receive high-dose acid suppression, and the next day they can be started on a liquid diet.19
STOMACH CANCER
Indications for ESD for stomach cancer in the East
The incidence of gastric cancer is higher in Japan and Korea, where widespread screening programs have led to early identification and early treatment of this disease.36
Pathology studies37 of samples from patients with gastric cancer identified the following as risk factors for lymph node metastasis, which would make ESD unsuitable:
- Undifferentiated type
- Tumors larger than 2 cm
- Lymphatic or venous involvement
- Submucosal invasion
- Ulcerative change.
Based on these findings, the situations in which there was no risk of lymph node involvement (ie, when none of the above factors are present) were accepted as absolute indications for endoscopic resection of early gastric cancer.38 Further histologic studies identified a subset of patients with lesions with very low risk of lymph node metastasis, which outweighed the risk of surgery. Based on these findings, expanded criteria for gastric ESD were proposed,39,40 and the Japanese gastric cancer treatment guidelines now include these expanded preoperative indications9,17 (Table 1).
The Japanese Gastric Cancer Association has proposed a treatment algorithm based on the histopathologic evaluation after resection (Figure 2).9
Outcomes
In the largest series of patients who underwent curative ESD for early gastric cancer, the 5-year survival rate was 92.6%, the 5-year disease-specific survival rate was 99.9%, and the 5-year relative survival rate was 105%.41
Similarly, in a Japanese population-based survival analysis, the relative 5-year survival rate for localized gastric cancer was 94.4%.42 Rates of en bloc resection and complete resection with ESD are higher than those with EMR, resulting in a lower risk of local recurrence in selected patients who undergo ESD.8,43,44
Although rare, local recurrence after curative gastric ESD has been reported.45 The annual incidence of local recurrence has been estimated to be 0.84%.46
ESD entails a shorter hospital stay and requires fewer resources than surgery, resulting in lower medical costs (Table 2).44 Additionally, as endoscopic resection is associated with less morbidity, fewer procedure-related adverse events, and fewer complications, ESD could be used as the standard treatment for early gastric cancer.47,48
The Western perspective on endoscopic submucosal dissection for gastric cancer
Since the prevalence of gastric cancer in Western countries is significantly lower than in Japan and Korea, local data and experience are scarce. However, experts performing ESD in the West have adopted the indications of the Japan Gastroenterological Endoscopy Society. The European Society of Gastrointestinal Endoscopy recommends ESD for excision of most superficial gastric neoplasms, with EMR being preferred only in lesions smaller than 15 mm, Paris classification 0 or IIA.5,32
Patients with gastric lesions measuring 15 mm or larger should undergo high-quality endoscopy, preferably chromoendoscopy, to evaluate the mucosal patterns and determine the depth of invasion. If superficial involvement is confirmed, other imaging techniques are not routinely recommended.5 A surgery consult is also recommended.
ESOPHAGEAL CANCER
Indications for ESD for esophageal cancer in the East
Due to the success of ESD for early gastric cancer, this technique is now also used for superficial esophageal neoplasms.19,49 It should be done in a specialized center, as it is more technically difficult than gastric ESD: the esophageal lumen is narrow, the wall is thin, and the esophagus moves with respiration and heartbeat.50 A multidisciplinary approach including an endoscopist, a surgeon, and a pathologist is highly recommended for evaluation and treatment.
EMR is preferred for removal of mucosal cancer, in view of its safety profile and success rates. ESD can be considered in cases of lesions larger than 15 mm, poorly lifting tumors, and those with the possibility of submucosal invasion (Table 3).5,45,49,51
Circumference involvement is critical when determining eligible candidates, as a defect involving more than three-fourths of the esophageal circumference can lead to esophageal strictures.52 Controlled prospective studies have shown promising results from giving intralesional and oral steroids to prevent stricture after ESD, which could potentially overcome this size limitation.53,54
Outcomes for esophageal cancer
ESD has been shown to be safe and effective, achieving en bloc resection in 85% to 100% of patients.19,51 Its advantages over EMR include en bloc resection, complete resection, and high curative rates, resulting in higher recurrence-free survival.2,55,56 Although the incidence of complications such as bleeding, perforation, and stricture formation are higher with ESD, patients usually recover uneventfully.2,19,20
ESD in the esophagus: The Western perspective
As data on the efficacy of EMR vs ESD for the treatment of Barrett esophagus with adenocarcinoma are limited, EMR is the gold standard endoscopic technique for removal of visible esophageal dysplastic lesions.5,51,57 ESD can be considered for tumors larger than 15 mm, for poorly lifting lesions, and if there is suspicion of submucosal invasion.5
Patients should be evaluated by an experienced endoscopist, using an advanced imaging technique such as narrow-band imaging or chromoendoscopy. If suspicious features are found, endoscopic ultrasonography should be considered to confirm submucosal invasion or lymph node involvement.5
COLORECTAL CANCER
Indications for ESD for colorectal cancer in the East
Colon cancer is one of the leading causes of cancer-related deaths worldwide.58 Since ESD has been found to be effective and safe in treating gastric cancer, it has also been used to remove large colorectal tumors.59 However, ESD is not universally accepted in the treatment of colorectal neoplasms due to its greater technical difficulty, longer procedural time, and higher risk of perforating the thinner colonic wall compared with EMR.21,60
Outcomes for colorectal cancer
Tumor size of 50 mm or larger is a risk factor for complications, while a high procedure volume at the center is a protective factor.60
Endoscopic treatment of colorectal cancer: The Western perspective
EMR is the gold standard for removal of superficial colorectal lesions. However, ESD can be considered if there is suspicion of superficial submucosal invasion, especially for lesions larger than 20 mm that cannot be resected en bloc by EMR.32 ESD can also be used for fibrotic lesions not amenable to complete EMR removal, or as a salvage procedure after recurrence after EMR.67 Proper selection of cases is critical.1
Patients who have a superficial colonic lesion should be evaluated by means of high-definition endoscopy and chromoendoscopy to assess the mucosal pattern and establish feasibility of endoscopic resection. If submucosal invasion is suspected, staging with endoscopic ultrasonography or magnetic resonance imaging should be considered.5
FOLLOW-UP AFTER ESD
Endoscopic surveillance after the procedure is recommended, given the persistent risk of metachronous cancer after curative ESD due to its organ-sparing quality.68 Surveillance endoscopy aims to achieve early detection and subsequent endoscopic resection of metachronous lesions.
Histopathologic evaluation assessing the presence of malignant cells in the margins of a resected sample is mandatory for determining the next step in treatment. If margins are negative, follow-up endoscopy can be done every 6 to 12 months. If margins are positive, the approach includes surgery, reattempting ESD or endoscopic surveillance in 3 or 6 months.3,32 Although the surveillance strategy varies according to individual risk of metachronous cancer, it should be continued indefinitely.68
COMPLICATIONS OF ESD
The most common procedure-related complications of ESD are bleeding, perforation, and stricture. Most intraprocedural adverse events can be managed endoscopically.69
Bleeding
Most bleeding occurs during the procedure or early after it and can be controlled with electrocautery.49,69 No episodes of massive bleeding, defined as causing clinical symptoms and requiring transfusion or surgery, have been reported.20,43,55
In gastric ESD, delayed bleeding rates have ranged from 0 to 15.6%.69 Bleeding may be prevented with endoscopic coagulation of visible vessels after dissection has been completed and by proton pump inhibitor therapy.70,71 Excessive coagulation should be avoided to lower the risk of perforation.33
In colorectal ESD the bleeding rate has been reported to be 2.2%; applying coagulation to an area where a blood vessel is suspected before cutting (precoagulation) may prevent subsequent bleeding.21
Perforation
For gastric ESD, perforation rates range from 1.2% to 5.2%.69 Esophageal perforation rates can be up to 4%.49 In colorectal ESD, perforation rates have been reported to be 1.6% to 6.6%.60,72
Although most of the cases were successfully managed with conservative treatment, some required emergency surgery.60,73
Strictures
In a case series of 532 patients undergoing gastric ESD, stricture was reported in 5 patients, all of whom presented with obstructive symptoms.74 Risk factors for post-ESD gastric stenosis are a mucosal defect with a circumferential extent of more than three-fourths or a longitudinal extent of more than 5 cm.75
Strictures are common after esophageal ESD, with rates ranging from 2% to 26%. The risk is higher when longer segments are removed or circumferential resection is performed. As previously mentioned, this complication may be reduced with ingestion or injection of steroids after the procedure.53,54
Surprisingly, ESD of large colorectal lesions involving more than three-fourths of the circumference of the rectum is rarely complicated by stenosis.76
LIMITATIONS OF ESD
ESD requires a high level of technical skill, is time-consuming, and has a higher rate of complications than conventional endoscopic resection. A standardized ESD training system is needed, as the procedure is more difficult than EMR. Training in porcine models has been shown to confer competency in ESD in a Western setting.13,16,33
Colorectal ESD is an even more challenging procedure, given the potential for complications related to its anatomy. Training centers in Japan usually have their trainees first master gastric ESD, then assist in more than 20 colorectal ESDs conducted by experienced endoscopists, and accomplish 30 cases before performing the procedure safely and independently.
As the incidence of gastric cancer is low in Western countries, trainees may also begin with lower rectal lesions, which are easier to remove.77 Incorporation of ESD in the West would require a clear treatment algorithm. It is a complex procedure, with higher rates of complications, a prolonged learning curve, and prolonged procedure time. Therefore, it should be performed in specialized centers and under the special situations discussed here to ensure that the benefits for the patients outweigh the risks.
VALUE OF ENDOSCOPIC SUBMUCOSAL DISSECTION
The optimal method for resecting gastrointestinal neoplasms should be safe, cost-effective, and quick and should also completely remove the lesion. The best treatment strategy takes into account the characteristics of the lesion and the comorbidities and wishes of the patient. Internists should be aware of the multiple options available to achieve the best outcome for the patient.1
Endoscopic resection of superficial gastrointestinal neoplasms, including EMR and ESD, has been a subject of increasing interest due to its minimally invasive and potentially curative character. However, cancer can recur after endoscopic resection because the procedure is organ-sparing.
ESD allows resection of early gastrointestinal tumors with a minimally invasive technique. It can achieve higher curative resection rates and lower recurrence rates compared with EMR. Compared with surgery, ESD leads to less morbidity, fewer procedure-related complications, and lower medical costs. Indications should be rigorously followed to achieve successful treatments in selected patients.
Multiple variables have to be taken into account when deciding which treatment is best, such as tumor characteristics, the patient’s baseline condition, physician expertise, and hospital resources.48 Less-invasive treatments may improve the prognosis of patients. No matter the approach, patients should be treated in specialized treatment centers.
Internal medicine physicians should be aware of the advances in treatments for early gastrointestinal cancer so appropriate options can be considered.
The treatment of early esophageal, gastric, and colorectal cancer is changing.1 For many years, surgery was the mainstay of treatment for early-stage gastrointestinal cancer. Unfortunately, surgery leads to significant loss of function of the organ, resulting in increased morbidity and decreased quality of life.2
Endoscopic techniques, particularly endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD), have been developed and are widely used in Japan, where gastrointestinal cancer is more common than in the West. This article reviews the indications, complications, and outcomes of ESD for early gastrointestinal neoplasms, so that readers will recognize the subset of patients who would benefit from ESD in a Western setting.
ENDOSCOPIC MUCOSAL RESECTION AND SUBMUCOSAL DISSECTION
Since the first therapeutic polypectomy was performed in Japan in 1974, several endoscopic techniques for tumor resection have been developed.3
EMR, one of the most successful and widely used techniques, involves elevating the lesion either with submucosal injection of a solution or with cap suction, and then removing it with a snare.4 Most lesions smaller than 20 mm can be removed in one piece (en bloc).5 Larger lesions are removed in multiple pieces (ie, piecemeal). Unfortunately, some fibrotic lesions, which are usually difficult to lift, cannot be completely removed by EMR.
ESD was first performed in the late 1990s with the aim of overcoming the limitations of EMR in resecting large or fibrotic tumors en bloc.6,7 Since then, ESD technique has been standardized and training centers have been created, especially in Asia, where it is widely used for treatment of early gastric cancer.3,8–10 Since 2012 it has been covered by the Japanese National Health Insurance for treatment of early gastric cancer, and since 2014 for treatment of colorectal malignant tumors measuring 2 to 5 cm.11
Adoption of ESD has been slow in Western countries, where many patients are still referred for surgery or undergo EMR for removal of superficial neoplasms. Reasons for this slow adoption are that gastric cancer is much less common in Western countries, and also that ESD demands a high level of technical skill, is difficult to learn, and is expensive.3,12,13 However, small groups of Western endoscopists have become interested and are advocating it, first studying it on their own and then training in a Japanese center and learning from experts performing the procedure.
Therefore, in a Western setting, ESD should be performed in specialized endoscopy centers and offered to selected patients.1
CANDIDATES SHOULD HAVE EARLY-STAGE, SUPERFICIAL TUMORS
Ideal candidates for endoscopic resection are patients who have early cancer with a negligible risk of lymph node metastasis, such as cancer limited to the mucosa (stage T1a).7 Therefore, to determine the best treatment for a patient with a newly diagnosed gastrointestinal neoplasm, it is mandatory to estimate the depth of invasion.
The depth of invasion is directly correlated with lymph node involvement, which is ultimately the main predictive factor for long-term adverse outcomes of gastrointestinal tumors.4,14–17 Accurate multidisciplinary preprocedure estimations are mandatory, as incorrect evaluations may result in inappropriate therapy and residual cancer.18
Other factors that have been used to predict lymph node involvement include tumor size, macroscopic appearance, histologic differentiation, and lymphatic and vascular involvement.19 Some of these factors can be assessed by special endoscopic techniques (chromoendoscopy and narrow-band imaging with magnifying endoscopy) that allow accurate real-time estimation of the depth of invasion of the lesion.5,17,20–27 Evaluation of microsurface and microvascular arrangements is especially useful for determining the feasibility of ESD in gastric tumors, evaluation of intracapillary loops is useful in esophageal lesions, and assessment of mucosal pit patterns is useful for colorectal lesions.21–29
Endoscopic ultrasonography is another tool that has been used to estimate the depth of the tumor. Although it can differentiate between definite intramucosal and definite submucosal invasive cancers, its ability to confirm minute submucosal invasion is limited. Its use as the sole tumor staging modality is not encouraged, and it should always be used in conjunction with endoscopic evaluation.18
Though the aforementioned factors help stratify patients, pathologic staging is the best predictor of lymph node metastasis. ESD provides adequate specimens for accurate pathologic evaluation, as it removes lesions en bloc.30
All patients found to have risk factors for lymph node metastasis on endoscopic, ultrasonographic, or pathologic analysis should be referred for surgical evaluation.9,19,31,32
ENDOSCOPIC SUBMUCOSAL DISSECTION
Before the procedure, the patient’s physicians need to do the following:
Determine the best type of intervention (EMR, ESD, ablation, surgery) for the specific lesion.3 A multidisciplinary approach is encouraged, with involvement of the internist, gastroenterologist, and surgeon.
Plan for anesthesia, additional consultations, pre- and postprocedural hospital admission, and need for special equipment.33
During the procedure
Define the lateral extent of the lesion using magnification chromoendoscopy or narrow-band imaging. In the stomach, a biopsy sample should be taken from the worst-looking segment and from normal-looking mucosa. Multiple biopsies should be avoided to prevent subsequent fibrosis.33 In the colon, biopsy should be avoided.34
Identify and circumferentially mark the target lesion. Cautery or argon plasma coagulation can be used for making markings at a distance of 5 to 10 mm from the edges.33 This is done to recognize the borders of the lesion, because they can become distorted after submucosal injection.14 This step is unnecessary in colorectal cases, as tumor margins can be adequately visualized after chromoendoscopy.16,35
Lift the lesion by injecting saline, 0.5% hyaluronate, or glycerin to create a submucosal fluid cushion.19,33
Perform a circumferential incision lateral to the mucosal margins to allow for a normal tissue margin.33 Partial incision is performed for esophageal and colorectal ESD to avoid fluid leakage from the submucosal layer, achieving a sustained submucosal lift and safer dissection.16
Submucosal dissection. The submucosal layer is dissected with an electrocautery knife until the lesion is completely removed. Dissection should be done carefully to keep the submucosal plane.33 Hemoclips or hemostat forceps can be used to control visible bleeding. The resected specimen is then stretched and fixed to a board using small pins for further histopathologic evaluation.35
Postprocedural monitoring. All patients should be admitted for overnight observation. Those who undergo gastric ESD should receive high-dose acid suppression, and the next day they can be started on a liquid diet.19
STOMACH CANCER
Indications for ESD for stomach cancer in the East
The incidence of gastric cancer is higher in Japan and Korea, where widespread screening programs have led to early identification and early treatment of this disease.36
Pathology studies37 of samples from patients with gastric cancer identified the following as risk factors for lymph node metastasis, which would make ESD unsuitable:
- Undifferentiated type
- Tumors larger than 2 cm
- Lymphatic or venous involvement
- Submucosal invasion
- Ulcerative change.
Based on these findings, the situations in which there was no risk of lymph node involvement (ie, when none of the above factors are present) were accepted as absolute indications for endoscopic resection of early gastric cancer.38 Further histologic studies identified a subset of patients with lesions with very low risk of lymph node metastasis, which outweighed the risk of surgery. Based on these findings, expanded criteria for gastric ESD were proposed,39,40 and the Japanese gastric cancer treatment guidelines now include these expanded preoperative indications9,17 (Table 1).
The Japanese Gastric Cancer Association has proposed a treatment algorithm based on the histopathologic evaluation after resection (Figure 2).9
Outcomes
In the largest series of patients who underwent curative ESD for early gastric cancer, the 5-year survival rate was 92.6%, the 5-year disease-specific survival rate was 99.9%, and the 5-year relative survival rate was 105%.41
Similarly, in a Japanese population-based survival analysis, the relative 5-year survival rate for localized gastric cancer was 94.4%.42 Rates of en bloc resection and complete resection with ESD are higher than those with EMR, resulting in a lower risk of local recurrence in selected patients who undergo ESD.8,43,44
Although rare, local recurrence after curative gastric ESD has been reported.45 The annual incidence of local recurrence has been estimated to be 0.84%.46
ESD entails a shorter hospital stay and requires fewer resources than surgery, resulting in lower medical costs (Table 2).44 Additionally, as endoscopic resection is associated with less morbidity, fewer procedure-related adverse events, and fewer complications, ESD could be used as the standard treatment for early gastric cancer.47,48
The Western perspective on endoscopic submucosal dissection for gastric cancer
Since the prevalence of gastric cancer in Western countries is significantly lower than in Japan and Korea, local data and experience are scarce. However, experts performing ESD in the West have adopted the indications of the Japan Gastroenterological Endoscopy Society. The European Society of Gastrointestinal Endoscopy recommends ESD for excision of most superficial gastric neoplasms, with EMR being preferred only in lesions smaller than 15 mm, Paris classification 0 or IIA.5,32
Patients with gastric lesions measuring 15 mm or larger should undergo high-quality endoscopy, preferably chromoendoscopy, to evaluate the mucosal patterns and determine the depth of invasion. If superficial involvement is confirmed, other imaging techniques are not routinely recommended.5 A surgery consult is also recommended.
ESOPHAGEAL CANCER
Indications for ESD for esophageal cancer in the East
Due to the success of ESD for early gastric cancer, this technique is now also used for superficial esophageal neoplasms.19,49 It should be done in a specialized center, as it is more technically difficult than gastric ESD: the esophageal lumen is narrow, the wall is thin, and the esophagus moves with respiration and heartbeat.50 A multidisciplinary approach including an endoscopist, a surgeon, and a pathologist is highly recommended for evaluation and treatment.
EMR is preferred for removal of mucosal cancer, in view of its safety profile and success rates. ESD can be considered in cases of lesions larger than 15 mm, poorly lifting tumors, and those with the possibility of submucosal invasion (Table 3).5,45,49,51
Circumference involvement is critical when determining eligible candidates, as a defect involving more than three-fourths of the esophageal circumference can lead to esophageal strictures.52 Controlled prospective studies have shown promising results from giving intralesional and oral steroids to prevent stricture after ESD, which could potentially overcome this size limitation.53,54
Outcomes for esophageal cancer
ESD has been shown to be safe and effective, achieving en bloc resection in 85% to 100% of patients.19,51 Its advantages over EMR include en bloc resection, complete resection, and high curative rates, resulting in higher recurrence-free survival.2,55,56 Although the incidence of complications such as bleeding, perforation, and stricture formation are higher with ESD, patients usually recover uneventfully.2,19,20
ESD in the esophagus: The Western perspective
As data on the efficacy of EMR vs ESD for the treatment of Barrett esophagus with adenocarcinoma are limited, EMR is the gold standard endoscopic technique for removal of visible esophageal dysplastic lesions.5,51,57 ESD can be considered for tumors larger than 15 mm, for poorly lifting lesions, and if there is suspicion of submucosal invasion.5
Patients should be evaluated by an experienced endoscopist, using an advanced imaging technique such as narrow-band imaging or chromoendoscopy. If suspicious features are found, endoscopic ultrasonography should be considered to confirm submucosal invasion or lymph node involvement.5
COLORECTAL CANCER
Indications for ESD for colorectal cancer in the East
Colon cancer is one of the leading causes of cancer-related deaths worldwide.58 Since ESD has been found to be effective and safe in treating gastric cancer, it has also been used to remove large colorectal tumors.59 However, ESD is not universally accepted in the treatment of colorectal neoplasms due to its greater technical difficulty, longer procedural time, and higher risk of perforating the thinner colonic wall compared with EMR.21,60
Outcomes for colorectal cancer
Tumor size of 50 mm or larger is a risk factor for complications, while a high procedure volume at the center is a protective factor.60
Endoscopic treatment of colorectal cancer: The Western perspective
EMR is the gold standard for removal of superficial colorectal lesions. However, ESD can be considered if there is suspicion of superficial submucosal invasion, especially for lesions larger than 20 mm that cannot be resected en bloc by EMR.32 ESD can also be used for fibrotic lesions not amenable to complete EMR removal, or as a salvage procedure after recurrence after EMR.67 Proper selection of cases is critical.1
Patients who have a superficial colonic lesion should be evaluated by means of high-definition endoscopy and chromoendoscopy to assess the mucosal pattern and establish feasibility of endoscopic resection. If submucosal invasion is suspected, staging with endoscopic ultrasonography or magnetic resonance imaging should be considered.5
FOLLOW-UP AFTER ESD
Endoscopic surveillance after the procedure is recommended, given the persistent risk of metachronous cancer after curative ESD due to its organ-sparing quality.68 Surveillance endoscopy aims to achieve early detection and subsequent endoscopic resection of metachronous lesions.
Histopathologic evaluation assessing the presence of malignant cells in the margins of a resected sample is mandatory for determining the next step in treatment. If margins are negative, follow-up endoscopy can be done every 6 to 12 months. If margins are positive, the approach includes surgery, reattempting ESD or endoscopic surveillance in 3 or 6 months.3,32 Although the surveillance strategy varies according to individual risk of metachronous cancer, it should be continued indefinitely.68
COMPLICATIONS OF ESD
The most common procedure-related complications of ESD are bleeding, perforation, and stricture. Most intraprocedural adverse events can be managed endoscopically.69
Bleeding
Most bleeding occurs during the procedure or early after it and can be controlled with electrocautery.49,69 No episodes of massive bleeding, defined as causing clinical symptoms and requiring transfusion or surgery, have been reported.20,43,55
In gastric ESD, delayed bleeding rates have ranged from 0 to 15.6%.69 Bleeding may be prevented with endoscopic coagulation of visible vessels after dissection has been completed and by proton pump inhibitor therapy.70,71 Excessive coagulation should be avoided to lower the risk of perforation.33
In colorectal ESD the bleeding rate has been reported to be 2.2%; applying coagulation to an area where a blood vessel is suspected before cutting (precoagulation) may prevent subsequent bleeding.21
Perforation
For gastric ESD, perforation rates range from 1.2% to 5.2%.69 Esophageal perforation rates can be up to 4%.49 In colorectal ESD, perforation rates have been reported to be 1.6% to 6.6%.60,72
Although most of the cases were successfully managed with conservative treatment, some required emergency surgery.60,73
Strictures
In a case series of 532 patients undergoing gastric ESD, stricture was reported in 5 patients, all of whom presented with obstructive symptoms.74 Risk factors for post-ESD gastric stenosis are a mucosal defect with a circumferential extent of more than three-fourths or a longitudinal extent of more than 5 cm.75
Strictures are common after esophageal ESD, with rates ranging from 2% to 26%. The risk is higher when longer segments are removed or circumferential resection is performed. As previously mentioned, this complication may be reduced with ingestion or injection of steroids after the procedure.53,54
Surprisingly, ESD of large colorectal lesions involving more than three-fourths of the circumference of the rectum is rarely complicated by stenosis.76
LIMITATIONS OF ESD
ESD requires a high level of technical skill, is time-consuming, and has a higher rate of complications than conventional endoscopic resection. A standardized ESD training system is needed, as the procedure is more difficult than EMR. Training in porcine models has been shown to confer competency in ESD in a Western setting.13,16,33
Colorectal ESD is an even more challenging procedure, given the potential for complications related to its anatomy. Training centers in Japan usually have their trainees first master gastric ESD, then assist in more than 20 colorectal ESDs conducted by experienced endoscopists, and accomplish 30 cases before performing the procedure safely and independently.
As the incidence of gastric cancer is low in Western countries, trainees may also begin with lower rectal lesions, which are easier to remove.77 Incorporation of ESD in the West would require a clear treatment algorithm. It is a complex procedure, with higher rates of complications, a prolonged learning curve, and prolonged procedure time. Therefore, it should be performed in specialized centers and under the special situations discussed here to ensure that the benefits for the patients outweigh the risks.
VALUE OF ENDOSCOPIC SUBMUCOSAL DISSECTION
The optimal method for resecting gastrointestinal neoplasms should be safe, cost-effective, and quick and should also completely remove the lesion. The best treatment strategy takes into account the characteristics of the lesion and the comorbidities and wishes of the patient. Internists should be aware of the multiple options available to achieve the best outcome for the patient.1
Endoscopic resection of superficial gastrointestinal neoplasms, including EMR and ESD, has been a subject of increasing interest due to its minimally invasive and potentially curative character. However, cancer can recur after endoscopic resection because the procedure is organ-sparing.
ESD allows resection of early gastrointestinal tumors with a minimally invasive technique. It can achieve higher curative resection rates and lower recurrence rates compared with EMR. Compared with surgery, ESD leads to less morbidity, fewer procedure-related complications, and lower medical costs. Indications should be rigorously followed to achieve successful treatments in selected patients.
Multiple variables have to be taken into account when deciding which treatment is best, such as tumor characteristics, the patient’s baseline condition, physician expertise, and hospital resources.48 Less-invasive treatments may improve the prognosis of patients. No matter the approach, patients should be treated in specialized treatment centers.
Internal medicine physicians should be aware of the advances in treatments for early gastrointestinal cancer so appropriate options can be considered.
- Burgess NG, Bourke MJ. Endoscopic resection of colorectal lesions: the narrowing divide between East and West. Dig Endosc 2016; 28:296–305.
- Kim DH, Jung HY, Gong EJ, et al. Endoscopic and oncologic outcomes of endoscopic resection for superficial esophageal neoplasm. Gut Liver 2015; 9:470–477.
- Draganov PV, Gotoda T, Chavalitdhamrong D, Wallace MB. Techniques of endoscopic submucosal dissection: application for the Western endoscopist? Gastrointest Endosc 2013; 78:677–688.
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011; 14:101–112.
- Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2015; 47:829–854.
- Farhat S, Chaussade S, Ponchon T, et al; SFED ESD Study Group. Endoscopic submucosal dissection in a European setting. A multi-institutional report of a technique in development. Endoscopy 2011; 43:664–670.
- Gotoda T, Jung H. Endoscopic resection (endoscopic mucosal resection/endoscopic submucosal dissection) for early gastric cancer. Dig Endosc 2013; 25(suppl 1):55–63.
- Chung IK, Lee JH, Lee SH, et al. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc 2009; 69:1228–1235.
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011; 14:113–123.
- Ono H. Endoscopic submucosal dissection for early gastric cancer. Chin J Dig Dis 2005; 6:119–121.
- Watanabe T, Itabashi M, Shimada Y, et al; Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol 2015; 20:207–239.
- Oyama T, Yahagi N, Ponchon T, Kiesslich T, Berr F. How to establish endoscopic submucosal dissection in Western countries. World J Gastroenterol 2015; 21:11209–11220.
- Bhatt A, Abe S, Kumaravel A, et al. SU1575 Western skill training in endoscopic submucosal dissection (ESD)—an international remote video based study—the WEST ESD Study. Gastrointest Endosc 2015; 81(suppl):AB335–AB336.
- Sano T, Sasako M, Kinoshita T, Maruyama K. Recurrence of early gastric cancer follow-up of 1475 patients and review of the Japanese literature. Cancer 1993; 72:3174–3178.
- Japan Esophageal Society. Japanese classification of esophageal cancer, tenth edition: part I. Esophagus 2009; 6:1–25.
- Bhatt A, Abe S, Kumaravel A, Vargo J, Saito Y. Indications and techniques for endoscopic submucosal dissection. Am J Gastroenterol 2015; 110:784–791.
- Eleftheriadis N, Inoue H, Ikeda H, et al. Definition and staging of early esophageal, gastric and colorectal cancer. J Tumor 2014; 2:161–178.
- Yoshinaga S, Oda I, Nonaka S, Kushima R, Saito Y. Endoscopic ultrasound using ultrasound probes for the diagnosis of early esophageal and gastric cancers. World J Gastrointest Endosc 2012; 4:218–226.
- Stahl M, Mariette C, Haustermans K, Cervantes A, Arnold D; ESMO Guidelines Working Group. Oesophageal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013; 24(suppl 6):vi51–vi56.
- Higuchi K, Tanabe S, Azuma M, et al. A phase II study of endoscopic submucosal dissection for superficial esophageal neoplasms (KDOG 0901). Gastrointest Endosc 2013; 78:704–710.
- Sakamoto T, Mori G, Yamada M, et al. Endoscopic submucosal dissection for colorectal neoplasms: a review. World J Gastroenterol 2014; 20:16153–16158.
- Ohta A, Tominaga K, Sakai Y. Efficacy of magnifying colonoscopy for the diagnosis of colorectal neoplasia: comparison with histopathological findings. Dig Endosc 2004; 16:308–314.
- Katagiri A, Fu K, Sano Y, et al. Narrow band imaging with magnifying colonoscopy as diagnostic tool for predicting histology of early colorectal neoplasia. Aliment Pharmacol Ther 2008; 27:1269–1274.
- Fu KI, Kato S, Sano Y, et al. Staging of early colorectal cancers: magnifying colonoscopy versus endoscopic ultrasonography for estimation of depth of invasion. Dig Dis Sci 2008; 53:1886–1892.
- Uraoka T, Saito Y, Ikematsu H, Yamamoto K, Sano Y. Sano’s capillary pattern classification for narrow-band imaging of early colorectal lesions. Dig Endosc 2011; 23(suppl 1):112–115.
- Ikematsu H, Matsuda T, Emura F, et al. Efficacy of capillary pattern type IIIA/IIIB by magnifying narrow band imaging for estimating depth of invasion of early colorectal neoplasms. BMC Gastroenterol 2010;10:33.
- Matsuda T, Fujii T, Saito Y, et al. Efficacy of the invasive/non-invasive pattern by magnifying chromoendoscopy to estimate the depth of invasion of early colorectal neoplasms. Am J Gastroenterol 2008; 103:2700–2706.
- Sato H, Inoue H, Ikeda H, et al. Utility of intrapapillary capillary loops seen on magnifying narrow-band imaging in estimating invasive depth of esophageal squamous cell carcinoma. Endoscopy 2015; 8:122–128.
- Muto M, Yao K, Kaise M, et al. Magnifying endoscopy simple diagnostic algorithm for early gastric cancer (MESDA-G). Dig Endosc 2016; 28:379–393.
- Waddell T, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D; European Society for Medical Oncology (ESMO); European Society of Surgical Oncology (ESSO); European Society of Radiotherapy and Oncology (ESTRO). Gastric cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013; 24(suppl 6):vi57–vi63.
- Kuwano H, Nishimura Y, Ohtsu A, et al. Guidelines for diagnosis and treatment of carcinoma of the esophagus. April 2007 edition: part I - Edited by the Japan Esophageal Society. Esophagus 2008; 5:61–73.
- Tanaka S, Kashida H, Saito Y, et al. JGES guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc 2015; 27:417–434.
- Gotoda T, Ho KY, Soetikno R, Kaltenbach T, Draganov P. Gastric ESD: current status and future directions of devices and training. Gastrointest Endosc Clin North Am 2014; 24:213–233.
- Saito Y, Sakamoto T, Nakajima T, Matsuda T. Colorectal ESD: current indications and latest technical advances. Gastrointest Endosc Clin N Am 2014; 24:245–255.
- Saito Y, Otake Y, Sakamoto T, et al. Indications for and technical aspects of colorectal endoscopic submucosal dissection. Gut Liver 2013; 7:263–269.
- Saragoni L. Upgrading the definition of early gastric cancer: better staging means more appropriate treatment. Cancer Biol Med 2015; 12:355–361.
- Tsujitani S, Oka S, Saito H, et al. Less invasive surgery for early gastric cancer based on the low probability of lymph node metastasis. Surgery 1999; 125:148–154.
- Soetikno RM, Gotoda T, Nakanishi Y, Soehendra N. Endoscopic mucosal resection. Gastrointest Endosc 2003; 57:567–579.
- Hirasawa T, Gotoda T, Miyata S, et al. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer 2009; 12:148–152.
- Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer 2000; 3:219–225.
- Suzuki H, Oda I, Abe S, et al. High rate of 5-year survival among patients with early gastric cancer undergoing curative endoscopic submucosal dissection. Gastric Cancer 2016; 19:198–205.
- Matsuda T, Ajiki W, Marugame T, Ioka A, Tsukuma H, Sobue T; Research Group of Population-Based Cancer Registries of Japan. Population-based survival of cancer patients diagnosed between 1993 and 1999 in Japan: a chronological and international comparative study. Jpn J Clin Oncol 2011; 41:40–51.
- Ahn JY, Jung HY, Choi KD, et al. Endoscopic and oncologic outcomes after endoscopic resection for early gastric cancer: 1370 cases of absolute and extended indications. Gastrointest Endosc 2011; 74:485–493.
- Kim Y, Kim YW, Choi IJ, et al. Cost comparison between surgical treatments and endoscopic submucosal dissection in patients with early gastric cancer in Korea. Gut Liver 2015; 9:174–180.
- Abe S, Oda I, Nakajima T, et al. A case of local recurrence and distant metastasis following curative endoscopic submucosal dissection of early gastric cancer. Gastric Cancer 2015; 18:188–192.
- Hahn KY, Park JC, Kim EH, et al. Incidence and impact of scheduled endoscopic surveillance on recurrence after curative endoscopic resection for early gastric cancer. Gastrointest Endosc 2016; 84:628–638.e1.
- Wang S, Zhang Z, Liu M, Li S, Jiang C. Endoscopic resection compared with gastrectomy to treat early gastric cancer: a systematic review and meta-analysis. PLoS One 2015; 10:e0144774.
- Kondo A, de Moura EG, Bernardo WM, et al. Endoscopy vs surgery in the treatment of early gastric cancer: systematic review. World J Gastroenterol 2015; 21:13177–13187.
- Kothari S, Kaul V. Endoscopic mucosal resection and endoscopic submucosal dissection for endoscopic therapy of Barrett’s esophagus-related neoplasia. Gastroenterol Clin North Am 2015; 44:317–335.
- Yamashita T, Zeniya A, Ishii H, et al. Endoscopic mucosal resection using a cap-fitted panendoscope and endoscopic submucosal dissection as optimal endoscopic procedures for superficial esophageal carcinoma. Surg Endosc 2011; 25:2541–2546.
- Kagemoto K, Oka S, Tanaka S, et al. Clinical outcomes of endoscopic submucosal dissection for superficial Barrett’s adenocarcinoma. Gastrointest Endosc 2014; 80:239–245.
- Katada C, Muto M, Manabe T, Boku N, Ohtsu A, Yoshida S. Esophageal stenosis after endoscopic mucosal resection of superficial esophageal lesions. Gastrointest Endosc 2003; 57:165–169.
- Hanaoka N, Ishihara R, Takeuchi Y, et al. 1139: A single session of intralesional steroid injection to prevent esophageal stricture after endoscopic submucosal dissection for esophageal squamous cell carcinoma. Gastrointest Endosc 2012; 75(suppl):AB175.
- Yamaguchi N, Isomoto H, Nakayama T, et al. Usefulness of oral prednisolone in the treatment of esophageal stricture after endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. Gastrointest Endosc 2011; 73:1115–1121.
- Ono S, Fujishiro M, Niimi K, et al. Long-term outcomes of endoscopic submucosal dissection for superficial esophageal squamous cell neoplasms. Gastrointest Endosc 2009; 70:860–866.
- Katada C, Muto M, Manabe T, Ohtsu A, Yoshida S. Local recurrence of squamous-cell carcinoma of the esophagus after EMR. Gastrointest Endosc 2005; 61:219–225.
- Hirasawa K, Kokawa A, Oka H, et al. Superficial adenocarcinoma of the esophagogastric junction: long-term results of endoscopic submucosal dissection. Gastrointest Endosc 2010; 72:960–966.
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.
- Nakajima T, Saito Y, Tanaka S, et al. Current status of endoscopic resection strategy for large, early colorectal neoplasia in Japan. Surg Endosc 2013; 27:3262–3770.
- Saito Y, Uraoka T, Yamaguchi Y, et al. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointest Endosc 2010; 72:1217–1225.
- Tanaka S, Saitoh Y, Matsuda T, et al; Japanese Society of Gastroenterology. Evidence-based clinical practice guidelines for management of colorectal polyps. J Gastroenterol 2015; 50:252–260.
- Oka S, Tanaka S, Saito Y, et al; Colorectal Endoscopic Resection Standardization Implementation Working Group of the Japanese Society for Cancer of the Colon and Rectum, Tokyo, Japan. Local recurrence after endoscopic resection for large colorectal neoplasia: a multicenter prospective study in Japan. Am J Gastroenterol 2015; 110:697–707.
- Saito Y, Fukuzawa M, Matsuda T, et al. Clinical outcome of endoscopic submucosal dissection versus endoscopic mucosal resection of large colorectal tumors as determined by curative resection. Surg Endosc 2010; 24:343–352.
- Makazu M, Sakamoto T, So E, et al. Relationship between indeterminate or positive lateral margin and local recurrence after endoscopic resection of colorectal polyps. Endosc Int Open 2015; 3:E252–E257.
- Belderbos TD, Leenders M, Moons LM, Siersema PD. Local recurrence after endoscopic mucosal resection of nonpedunculated colorectal lesions: systematic review and meta-analysis. Endoscopy 2014; 46:388–402.
- Fujiya M, Tanaka K, Dokoshi T, et al. Efficacy and adverse events of EMR and endoscopic submucosal dissection for the treatment of colon neoplasms: a meta-analysis of studies comparing EMR and endoscopic submucosal dissection. Gastrointest Endosc 2015; 81:583–595.
- Rahmi G, Tanaka S, Ohara Y, et al. Efficacy of endoscopic submucosal dissection for residual or recurrent superficial colorectal tumors after endoscopic mucosal resection. J Dig Dis 2015; 16:14–21.
- Abe S, Oda I, Suzuki H, et al. Long-term surveillance and treatment outcomes of metachronous gastric cancer occurring after curative endoscopic submucosal dissection. Endoscopy 2015; 47:1113–1118.
- Oda I, Suzuki H, Nonaka S, Yoshinaga S. Complications of gastric endoscopic submucosal dissection. Dig Endosc 2013; 25(suppl 1):71–78.
- Takizawa K, Oda I, Gotoda T, et al. Routine coagulation of visible vessels may prevent delayed bleeding after endoscopic submucosal dissection—an analysis of risk factors. Endoscopy 2008; 40:179–183.
- Uedo N, Takeuchi Y, Yamada T, et al. Effect of a proton pump inhibitor or an H2-receptor antagonist on prevention of bleeding from ulcer after endoscopic submucosal dissection of early gastric cancer: a prospective randomized controlled trial. Am J Gastroenterol 2007; 102:1610–1616.
- Hayashi N, Tanaka S, Nishiyama S, et al. Predictors of incomplete resection and perforation associated with endoscopic submucosal dissection for colorectal tumors. Gastrointest Endosc 2014; 79:427–435.
- Suzuki H, Oda I, Sekiguchi M, et al. Management and associated factors of delayed perforation after gastric endoscopic submucosal dissection. World J Gastroenterol 2015; 21:12635–12643.
- Tsunada S, Ogata S, Mannen K, et al. Case series of endoscopic balloon dilation to treat a stricture caused by circumferential resection of the gastric antrum by endoscopic submucosal dissection. Gastrointest Endosc 2008; 67:979–983.
- Coda S, Oda I, Gotoda T, Yokoi C, Kikuchi T, Ono H. Risk factors for cardiac and pyloric stenosis after endoscopic submucosal dissection, and efficacy of endoscopic balloon dilation treatment. Endoscopy 2009; 41:421–426.
- Abe S, Sakamoto T, Takamaru H, et al. Stenosis rates after endoscopic submucosal dissection of large rectal tumors involving greater than three quarters of the luminal circumference. Surg Endosc 2016; 30:5459–5464.
- Sakamoto T, Saito Y, Fukunaga S, Nakajima T, Matsuda T. Learning curve associated with colorectal endoscopic submucosal dissection for endoscopists experienced in gastric endoscopic submucosal dissection. Dis Colon Rectum 2011; 54:1307–1312.
- Burgess NG, Bourke MJ. Endoscopic resection of colorectal lesions: the narrowing divide between East and West. Dig Endosc 2016; 28:296–305.
- Kim DH, Jung HY, Gong EJ, et al. Endoscopic and oncologic outcomes of endoscopic resection for superficial esophageal neoplasm. Gut Liver 2015; 9:470–477.
- Draganov PV, Gotoda T, Chavalitdhamrong D, Wallace MB. Techniques of endoscopic submucosal dissection: application for the Western endoscopist? Gastrointest Endosc 2013; 78:677–688.
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011; 14:101–112.
- Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2015; 47:829–854.
- Farhat S, Chaussade S, Ponchon T, et al; SFED ESD Study Group. Endoscopic submucosal dissection in a European setting. A multi-institutional report of a technique in development. Endoscopy 2011; 43:664–670.
- Gotoda T, Jung H. Endoscopic resection (endoscopic mucosal resection/endoscopic submucosal dissection) for early gastric cancer. Dig Endosc 2013; 25(suppl 1):55–63.
- Chung IK, Lee JH, Lee SH, et al. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc 2009; 69:1228–1235.
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011; 14:113–123.
- Ono H. Endoscopic submucosal dissection for early gastric cancer. Chin J Dig Dis 2005; 6:119–121.
- Watanabe T, Itabashi M, Shimada Y, et al; Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol 2015; 20:207–239.
- Oyama T, Yahagi N, Ponchon T, Kiesslich T, Berr F. How to establish endoscopic submucosal dissection in Western countries. World J Gastroenterol 2015; 21:11209–11220.
- Bhatt A, Abe S, Kumaravel A, et al. SU1575 Western skill training in endoscopic submucosal dissection (ESD)—an international remote video based study—the WEST ESD Study. Gastrointest Endosc 2015; 81(suppl):AB335–AB336.
- Sano T, Sasako M, Kinoshita T, Maruyama K. Recurrence of early gastric cancer follow-up of 1475 patients and review of the Japanese literature. Cancer 1993; 72:3174–3178.
- Japan Esophageal Society. Japanese classification of esophageal cancer, tenth edition: part I. Esophagus 2009; 6:1–25.
- Bhatt A, Abe S, Kumaravel A, Vargo J, Saito Y. Indications and techniques for endoscopic submucosal dissection. Am J Gastroenterol 2015; 110:784–791.
- Eleftheriadis N, Inoue H, Ikeda H, et al. Definition and staging of early esophageal, gastric and colorectal cancer. J Tumor 2014; 2:161–178.
- Yoshinaga S, Oda I, Nonaka S, Kushima R, Saito Y. Endoscopic ultrasound using ultrasound probes for the diagnosis of early esophageal and gastric cancers. World J Gastrointest Endosc 2012; 4:218–226.
- Stahl M, Mariette C, Haustermans K, Cervantes A, Arnold D; ESMO Guidelines Working Group. Oesophageal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013; 24(suppl 6):vi51–vi56.
- Higuchi K, Tanabe S, Azuma M, et al. A phase II study of endoscopic submucosal dissection for superficial esophageal neoplasms (KDOG 0901). Gastrointest Endosc 2013; 78:704–710.
- Sakamoto T, Mori G, Yamada M, et al. Endoscopic submucosal dissection for colorectal neoplasms: a review. World J Gastroenterol 2014; 20:16153–16158.
- Ohta A, Tominaga K, Sakai Y. Efficacy of magnifying colonoscopy for the diagnosis of colorectal neoplasia: comparison with histopathological findings. Dig Endosc 2004; 16:308–314.
- Katagiri A, Fu K, Sano Y, et al. Narrow band imaging with magnifying colonoscopy as diagnostic tool for predicting histology of early colorectal neoplasia. Aliment Pharmacol Ther 2008; 27:1269–1274.
- Fu KI, Kato S, Sano Y, et al. Staging of early colorectal cancers: magnifying colonoscopy versus endoscopic ultrasonography for estimation of depth of invasion. Dig Dis Sci 2008; 53:1886–1892.
- Uraoka T, Saito Y, Ikematsu H, Yamamoto K, Sano Y. Sano’s capillary pattern classification for narrow-band imaging of early colorectal lesions. Dig Endosc 2011; 23(suppl 1):112–115.
- Ikematsu H, Matsuda T, Emura F, et al. Efficacy of capillary pattern type IIIA/IIIB by magnifying narrow band imaging for estimating depth of invasion of early colorectal neoplasms. BMC Gastroenterol 2010;10:33.
- Matsuda T, Fujii T, Saito Y, et al. Efficacy of the invasive/non-invasive pattern by magnifying chromoendoscopy to estimate the depth of invasion of early colorectal neoplasms. Am J Gastroenterol 2008; 103:2700–2706.
- Sato H, Inoue H, Ikeda H, et al. Utility of intrapapillary capillary loops seen on magnifying narrow-band imaging in estimating invasive depth of esophageal squamous cell carcinoma. Endoscopy 2015; 8:122–128.
- Muto M, Yao K, Kaise M, et al. Magnifying endoscopy simple diagnostic algorithm for early gastric cancer (MESDA-G). Dig Endosc 2016; 28:379–393.
- Waddell T, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D; European Society for Medical Oncology (ESMO); European Society of Surgical Oncology (ESSO); European Society of Radiotherapy and Oncology (ESTRO). Gastric cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013; 24(suppl 6):vi57–vi63.
- Kuwano H, Nishimura Y, Ohtsu A, et al. Guidelines for diagnosis and treatment of carcinoma of the esophagus. April 2007 edition: part I - Edited by the Japan Esophageal Society. Esophagus 2008; 5:61–73.
- Tanaka S, Kashida H, Saito Y, et al. JGES guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc 2015; 27:417–434.
- Gotoda T, Ho KY, Soetikno R, Kaltenbach T, Draganov P. Gastric ESD: current status and future directions of devices and training. Gastrointest Endosc Clin North Am 2014; 24:213–233.
- Saito Y, Sakamoto T, Nakajima T, Matsuda T. Colorectal ESD: current indications and latest technical advances. Gastrointest Endosc Clin N Am 2014; 24:245–255.
- Saito Y, Otake Y, Sakamoto T, et al. Indications for and technical aspects of colorectal endoscopic submucosal dissection. Gut Liver 2013; 7:263–269.
- Saragoni L. Upgrading the definition of early gastric cancer: better staging means more appropriate treatment. Cancer Biol Med 2015; 12:355–361.
- Tsujitani S, Oka S, Saito H, et al. Less invasive surgery for early gastric cancer based on the low probability of lymph node metastasis. Surgery 1999; 125:148–154.
- Soetikno RM, Gotoda T, Nakanishi Y, Soehendra N. Endoscopic mucosal resection. Gastrointest Endosc 2003; 57:567–579.
- Hirasawa T, Gotoda T, Miyata S, et al. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer 2009; 12:148–152.
- Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer 2000; 3:219–225.
- Suzuki H, Oda I, Abe S, et al. High rate of 5-year survival among patients with early gastric cancer undergoing curative endoscopic submucosal dissection. Gastric Cancer 2016; 19:198–205.
- Matsuda T, Ajiki W, Marugame T, Ioka A, Tsukuma H, Sobue T; Research Group of Population-Based Cancer Registries of Japan. Population-based survival of cancer patients diagnosed between 1993 and 1999 in Japan: a chronological and international comparative study. Jpn J Clin Oncol 2011; 41:40–51.
- Ahn JY, Jung HY, Choi KD, et al. Endoscopic and oncologic outcomes after endoscopic resection for early gastric cancer: 1370 cases of absolute and extended indications. Gastrointest Endosc 2011; 74:485–493.
- Kim Y, Kim YW, Choi IJ, et al. Cost comparison between surgical treatments and endoscopic submucosal dissection in patients with early gastric cancer in Korea. Gut Liver 2015; 9:174–180.
- Abe S, Oda I, Nakajima T, et al. A case of local recurrence and distant metastasis following curative endoscopic submucosal dissection of early gastric cancer. Gastric Cancer 2015; 18:188–192.
- Hahn KY, Park JC, Kim EH, et al. Incidence and impact of scheduled endoscopic surveillance on recurrence after curative endoscopic resection for early gastric cancer. Gastrointest Endosc 2016; 84:628–638.e1.
- Wang S, Zhang Z, Liu M, Li S, Jiang C. Endoscopic resection compared with gastrectomy to treat early gastric cancer: a systematic review and meta-analysis. PLoS One 2015; 10:e0144774.
- Kondo A, de Moura EG, Bernardo WM, et al. Endoscopy vs surgery in the treatment of early gastric cancer: systematic review. World J Gastroenterol 2015; 21:13177–13187.
- Kothari S, Kaul V. Endoscopic mucosal resection and endoscopic submucosal dissection for endoscopic therapy of Barrett’s esophagus-related neoplasia. Gastroenterol Clin North Am 2015; 44:317–335.
- Yamashita T, Zeniya A, Ishii H, et al. Endoscopic mucosal resection using a cap-fitted panendoscope and endoscopic submucosal dissection as optimal endoscopic procedures for superficial esophageal carcinoma. Surg Endosc 2011; 25:2541–2546.
- Kagemoto K, Oka S, Tanaka S, et al. Clinical outcomes of endoscopic submucosal dissection for superficial Barrett’s adenocarcinoma. Gastrointest Endosc 2014; 80:239–245.
- Katada C, Muto M, Manabe T, Boku N, Ohtsu A, Yoshida S. Esophageal stenosis after endoscopic mucosal resection of superficial esophageal lesions. Gastrointest Endosc 2003; 57:165–169.
- Hanaoka N, Ishihara R, Takeuchi Y, et al. 1139: A single session of intralesional steroid injection to prevent esophageal stricture after endoscopic submucosal dissection for esophageal squamous cell carcinoma. Gastrointest Endosc 2012; 75(suppl):AB175.
- Yamaguchi N, Isomoto H, Nakayama T, et al. Usefulness of oral prednisolone in the treatment of esophageal stricture after endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. Gastrointest Endosc 2011; 73:1115–1121.
- Ono S, Fujishiro M, Niimi K, et al. Long-term outcomes of endoscopic submucosal dissection for superficial esophageal squamous cell neoplasms. Gastrointest Endosc 2009; 70:860–866.
- Katada C, Muto M, Manabe T, Ohtsu A, Yoshida S. Local recurrence of squamous-cell carcinoma of the esophagus after EMR. Gastrointest Endosc 2005; 61:219–225.
- Hirasawa K, Kokawa A, Oka H, et al. Superficial adenocarcinoma of the esophagogastric junction: long-term results of endoscopic submucosal dissection. Gastrointest Endosc 2010; 72:960–966.
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.
- Nakajima T, Saito Y, Tanaka S, et al. Current status of endoscopic resection strategy for large, early colorectal neoplasia in Japan. Surg Endosc 2013; 27:3262–3770.
- Saito Y, Uraoka T, Yamaguchi Y, et al. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointest Endosc 2010; 72:1217–1225.
- Tanaka S, Saitoh Y, Matsuda T, et al; Japanese Society of Gastroenterology. Evidence-based clinical practice guidelines for management of colorectal polyps. J Gastroenterol 2015; 50:252–260.
- Oka S, Tanaka S, Saito Y, et al; Colorectal Endoscopic Resection Standardization Implementation Working Group of the Japanese Society for Cancer of the Colon and Rectum, Tokyo, Japan. Local recurrence after endoscopic resection for large colorectal neoplasia: a multicenter prospective study in Japan. Am J Gastroenterol 2015; 110:697–707.
- Saito Y, Fukuzawa M, Matsuda T, et al. Clinical outcome of endoscopic submucosal dissection versus endoscopic mucosal resection of large colorectal tumors as determined by curative resection. Surg Endosc 2010; 24:343–352.
- Makazu M, Sakamoto T, So E, et al. Relationship between indeterminate or positive lateral margin and local recurrence after endoscopic resection of colorectal polyps. Endosc Int Open 2015; 3:E252–E257.
- Belderbos TD, Leenders M, Moons LM, Siersema PD. Local recurrence after endoscopic mucosal resection of nonpedunculated colorectal lesions: systematic review and meta-analysis. Endoscopy 2014; 46:388–402.
- Fujiya M, Tanaka K, Dokoshi T, et al. Efficacy and adverse events of EMR and endoscopic submucosal dissection for the treatment of colon neoplasms: a meta-analysis of studies comparing EMR and endoscopic submucosal dissection. Gastrointest Endosc 2015; 81:583–595.
- Rahmi G, Tanaka S, Ohara Y, et al. Efficacy of endoscopic submucosal dissection for residual or recurrent superficial colorectal tumors after endoscopic mucosal resection. J Dig Dis 2015; 16:14–21.
- Abe S, Oda I, Suzuki H, et al. Long-term surveillance and treatment outcomes of metachronous gastric cancer occurring after curative endoscopic submucosal dissection. Endoscopy 2015; 47:1113–1118.
- Oda I, Suzuki H, Nonaka S, Yoshinaga S. Complications of gastric endoscopic submucosal dissection. Dig Endosc 2013; 25(suppl 1):71–78.
- Takizawa K, Oda I, Gotoda T, et al. Routine coagulation of visible vessels may prevent delayed bleeding after endoscopic submucosal dissection—an analysis of risk factors. Endoscopy 2008; 40:179–183.
- Uedo N, Takeuchi Y, Yamada T, et al. Effect of a proton pump inhibitor or an H2-receptor antagonist on prevention of bleeding from ulcer after endoscopic submucosal dissection of early gastric cancer: a prospective randomized controlled trial. Am J Gastroenterol 2007; 102:1610–1616.
- Hayashi N, Tanaka S, Nishiyama S, et al. Predictors of incomplete resection and perforation associated with endoscopic submucosal dissection for colorectal tumors. Gastrointest Endosc 2014; 79:427–435.
- Suzuki H, Oda I, Sekiguchi M, et al. Management and associated factors of delayed perforation after gastric endoscopic submucosal dissection. World J Gastroenterol 2015; 21:12635–12643.
- Tsunada S, Ogata S, Mannen K, et al. Case series of endoscopic balloon dilation to treat a stricture caused by circumferential resection of the gastric antrum by endoscopic submucosal dissection. Gastrointest Endosc 2008; 67:979–983.
- Coda S, Oda I, Gotoda T, Yokoi C, Kikuchi T, Ono H. Risk factors for cardiac and pyloric stenosis after endoscopic submucosal dissection, and efficacy of endoscopic balloon dilation treatment. Endoscopy 2009; 41:421–426.
- Abe S, Sakamoto T, Takamaru H, et al. Stenosis rates after endoscopic submucosal dissection of large rectal tumors involving greater than three quarters of the luminal circumference. Surg Endosc 2016; 30:5459–5464.
- Sakamoto T, Saito Y, Fukunaga S, Nakajima T, Matsuda T. Learning curve associated with colorectal endoscopic submucosal dissection for endoscopists experienced in gastric endoscopic submucosal dissection. Dis Colon Rectum 2011; 54:1307–1312.
KEY POINTS
- ESD is a minimally invasive endoscopic technique with curative potential for patients with superficial GI neoplasia.
- ESD preserves the integrity of the organ while achieving curative resection of large neoplasms.
- ESD is indicated rather than surgery in patients with early GI lesions with a negligible risk of lymph node metastasis.
- Complications of the procedure include bleeding, perforation, and stenosis. Most of these respond to endoscopic treatment.
- Successful ESD requires supportive teamwork among internists, gastroenterologists, pathologists, and surgeons.
Reproductive planning for women after solid-organ transplant
Increasing numbers of women of childbearing age are receiving solid-organ transplants. All need counseling on how to prevent pregnancy while they are taking immunosuppressive agents. Some want to become pregnant after their transplant and thus require counseling and follow-up to maintain good health during pregnancy (Table 1).1
Primary care physicians can assist with basic contraception counseling and pregnancy planning for their patients who have had solid-organ transplants. In this review, we describe contraceptive options and pregnancy planning for these women.
TRANSPLANTS IN WOMEN ARE INCREASING
Over the past 20 years, the number of solid-organ transplants in US women has increased steadily. Since 1988, 38% of the 634,000 transplants performed were in women, and 47% of these women were of childbearing age (ages 18 to 49).2 Kidneys accounted for 60% of solid-organ transplants,2 and kidney transplant is now commonly performed in women of childbearing age. In 2012, of 176,000 patients with a functioning renal graft, 40.5% were women, and recipients between ages 20 and 44 composed the second-largest age group.3
FERTILITY IN WOMEN WITH END-STAGE RENAL DISEASE
Women in their reproductive years who have end-stage renal disease have lower fertility rates than women in the general population. In women undergoing peritoneal dialysis or hemodialysis, conception rates decrease to around 0.5% per year.4 This lower rate is most likely related to hypothalamic-pituitary-gonadal dysfunction, leading to reduced or total impairment of ovulation, menstrual irregularities, and infertility.5
Fertility often returns within a few months after transplant,1,6 and reported posttransplant pregnancy rates range from 3.3% to 18%,7–9 with up to one-third of pregnancies being unintended.6,10 These numbers are likely an underestimate because they do not reflect all pregnancies that are terminated, as many women do not voluntarily report having had an abortion.
Fertility is also severely diminished in women with end-stage liver disease. After liver transplant, sex hormone levels return to normal for many women, and menses soon resume.11
In 2005, the National Transplantation Pregnancy Registry reported 1,418 pregnancies in 919 female recipients of solid-organ transplants. In 2010, this number had increased to 1,940 pregnancies in 1,185 recipients, of whom 75% were kidney transplant recipients.12
A successful pregnancy outcome is most likely when a minimum of 1 year intervenes between transplant and conception.12,13
TERATOGENICITY OF IMMUNOSUPPRESSANTS
Immunosuppressant drugs commonly used for maintenance therapy after solid-organ transplant include the following:
- Calcineurin inhibitors (eg, cyclosporine, tacrolimus)
- Antiproliferative and antimetabolite agents (eg, mycophenolate mofetil, azathioprine)
- Corticosteroids
- Mammalian target of rapamycin inhibitors (eg, sirolimus, everolimus)
- T-cell costimulation blockers (eg, belatacept).14
The US Food and Drug Administration (FDA) previously classified mycophenolate mofetil and azathioprine in pregnancy risk category D (positive evidence of human fetal risk). The teratogenic risk of mycophenolate mofetil is well established in studies documenting specific congenital malformations and fetal loss in the first trimester.13,15 The teratogenic risk of azathioprine, on the other hand, is estimated to be minimal to small.16 Many of the associated fetal abnormalities may be related to the complexity of the underlying medical condition of the mother rather than to the medication.16
In June 2015, the FDA’s new Pregnancy and Lactation Labeling Rule went into effect, which removes the pregnancy letter categories A, B, C, D, and X from labeling.17 This rule was designed to help providers counsel their patients regarding the specific risks and benefits of a drug when used by pregnant or nursing women. However, the ABCDX categories are still commonly used. Table 2 shows information about the risks during pregnancy and lactation posed by the immunosuppressive drugs commonly used by posttransplant patients.18
CRITERIA FOR A SUCCESSFUL PREGNANCY
To ensure a safe and successful pregnancy with the fewest fetal and maternal complications, women are generally advised to avoid pregnancy for at least 1 year after transplant.19,20
In addition, women should meet certain clinical prerequisites after transplant before they conceive, as outlined by the American Society of Transplantation.19,20 These include:
- No rejection within the previous year
- Adequate and stable graft function (eg, serum creatinine < 1.5 mg/dL and urinary protein excretion < 500 mg/24 hours)
- No acute infection that might affect the fetus
- Maintenance immunosuppression at stable dosages.
Other circumstances to consider include episodes of rejection in the first year after transplant (as evidenced by biopsy results or glomerular filtration rate), the woman’s age (advanced maternal age is unfavorable), or any history of noncompliance.
Every pregnancy in a transplant recipient must be carefully planned. Primary care providers should encourage patients to meet with their transplant team and obstetricians early and often to allow time for the care team to adjust the type and dosing of immunosuppressant drugs, to ensure stable graft function, and to optimize any current chronic medical conditions such as diabetes mellitus or hypertension before conception.
CONTRACEPTIVE COUNSELING AFTER TRANSPLANT
Pregnancy should be avoided while transplant patients are taking FDA category D immunosuppressant drugs and, as already mentioned, during the first year after transplant. Unintended pregnancy can have serious health consequences for the mother and the fetus, as well as poor pregnancy outcomes. The US Centers for Disease Control and Prevention (CDC) lists solid-organ transplant within the past 2 years as a condition that can lead to adverse events as a result of pregnancy.21 After a transplant, a woman’s risks from an unintended pregnancy are always greater than the risks from any contraceptive, and this is important to reinforce in counseling.
Two forms of reliable contraception should be used at all times, and consistent condom use should be encouraged as one of the methods. Condoms are not reliable when used as the sole contraceptive method because they have an 18% typical-use failure rate. However, they are an excellent adjunct to other contraceptive methods because they have the additional benefit of protecting against sexually transmitted disease.
Choosing the appropriate contraceptive method for recipients of solid-organ transplants can be challenging because of several factors, including the recipient’s preexisting medical problems and drug interactions of immunosuppressant medications.
CDC criteria and categories for contraceptive use
In 2010, the CDC released the US version of the Medical Eligibility Criteria (US MEC) for contraceptive use, which was based on the 2009 World Health Organization Medical Eligibility Criteria (WHO MEC); these criteria were revised in August 2016.21
- Category 1: A condition for which there is no restriction for the use of the contraceptive method
- Category 2: A condition for which the advantages of using the method generally outweigh the theoretical or proven risks
- Category 3: A condition for which the theoretical or proven risks usually outweigh the advantages of using the method
- Category 4: A condition that represents an unacceptable health risk if the contraceptive method is used.
These recommendations aimed to improve family planning options by clarifying the possible safe and effective contraceptive options available while considering the patient’s medical condition. The CDC added solid-organ transplant recipients to this document because of the prevalence of this group in the United States.
The CDC categorizes a patient’s medical condition after transplant as either complicated or uncomplicated. Complicated conditions include acute or chronic graft failure, graft rejection, and cardiac allograft vasculopathy.21
Effectiveness of contraceptive methods
Contraceptive methods can be divided into 4 categories based on estimated effectiveness, ie, the pregnancy rate with “typical use” of that particular method in 1 year21–23:
- Very effective (0%–0.9%)
- Effective (1%–9%)
- Moderately effective (10%–25%)
- Less effective (26%–32%).
Typical use refers to failure rates for women and men whose use is not consistent nor always correct. Correct use, also described in the sections that follow, refers to failure rates for those whose use is consistent and always correct.
Women should be counseled regarding all available contraceptive options that are medically suitable for them, so they can choose the method that best fits their needs and lifestyle. They should receive counseling on emergency contraception, barrier protection against sexually transmitted disease, and the correct use of the contraceptive method they choose. They should be advised that if their chosen contraceptive method is unsatisfactory for any reason, they can switch to another method. Most importantly, providers need to impress on their patients that the risks associated with unintended pregnancy are far greater than the risks from any of the contraceptive methods.
VERY EFFECTIVE CONTRACEPTIVES (UNINTENDED PREGNANCY RATE 0%–0.9%)
This tier of contraception is the most effective regardless of the patient’s adherence; it includes long-acting, reversible contraceptives and permanent sterilization (both male and female) (Table 3).21–23
Long-acting reversible contraceptives include intrauterine devices (IUDs) and the subdermal etonogestrel implant. Given their efficacy and favorable safety profile, long-acting reversible contraceptives are being promoted for use in women who have chronic medical conditions, such as transplants.24
Intrauterine devices
IUDs are long-acting and reversible. They can be used by women who are nulliparous and those of all ages, including adolescents.22
Two types of IUDs are available in the United States: nonhormonal (copper) and hormonal (levonorgestrel). The copper IUD is effective for at least 10 years, whereas the levonorgestrel IUDs last for 3 to 5 years.22
Four levonorgestrel IUDs are currently available in the United States. Their sizes and doses vary: Mirena (52 µg), Skyla (13.5 µg), Liletta (52 µg), and Kyleena (19.5 µg).
Fewer than 1% of women become pregnant in the first year of IUD use.22,23 IUDs are an ideal option for women with solid-organ transplants because they are so effective and because the patient does not have to do anything once the IUD is in.22–24 The levonorgestrel IUD Mirena has the additional advantage of reducing heavy menstrual bleeding and is currently the only hormonal IUD with FDA approval for the management of menorrhagia.
About 12% of women in the general population use IUDs as their contraceptive method of choice,25 whereas after solid-organ transplantation about 15% to 20% of women do.26
Two historic concerns regarding IUDs may explain their low rate of use in transplant recipients.
First, IUDs were believed to be less effective in women on immunosuppressive drugs because IUDs act by inducing a local inflammatory reaction. However, IUDs involve macrophage activation, which is independent of the immune processes modified by immunosuppressants (primarily T-cell function).27 A recent pilot study showed a strong inflammatory reaction in the endometrium of transplant recipients after levonorgestrel IUD insertion.28
Second, there was concern about the increased risk of pelvic inflammatory disease with IUDs, but studies have shown levonorgestrel IUDs to be safe in transplant patients.29,30
The CDC21 lists copper and levonorgestrel IUDs in MEC category 3 (the risks generally outweigh the advantages) for initiation in patients with complicated transplants and in category 2 (advantages generally outweigh the risks) in patients with uncomplicated organ transplants. The devices are in category 2 for both complicated and uncomplicated cases if the IUD is already in place.
Subdermal implant
A subdermal implant consisting of a single rod containing 68 mg of etonogestrel is commercially available in the United States. It is one of the most effective contraceptive methods, with the lowest rates of pregnancy—less than 1% per year, with protection lasting at least 3 years.22,23 This low risk makes the subdermal implant a suitable method of contraception after transplant. Daily compliance is not required, and there are no hepatic first-pass effects, which results in higher bioavailability and less chance of drug interactions.
The main disadvantage of the subdermal implant and IUDs is unscheduled bleeding. An important benefit is prolonged amenorrhea, not only for patient convenience, but for reduction of endometrial cancer risk. Insertion and removal of the implant are considered minor office procedures. The implants are classified as US MEC category 2 in uncomplicated cases; initiation in complicated cases is considered category 3 but continuation is considered category 2.21
Permanent sterilization
Permanent sterilization is another option for women and men. In women, the fallopian tubes can be occluded with a coil system implanted vaginally through a hysteroscope, or they can be severed, tied, or clamped in a laparoscopic procedure or during cesarean delivery. Pregnancy rates after tubal ligation are less than 1%,23,31 although concern exists for high failure rates with the hysteroscopic method.
Because younger patients are more likely than older patients to subsequently regret having the procedure done, all available contraceptive options should be discussed with them.31
For men, permanent sterilization is done by vasectomy, which has less associated risk and cost compared with sterilization for women.
EFFECTIVE CONTRACEPTIVE METHODS (UNINTENDED PREGNANCY RATE 1%–9%)
Effective contraceptive methods, the next tier down from very effective methods, include injectable contraceptives, combined hormonal contraceptives, and progestin-only contraceptives (Table 4).
Injectable contraceptives
Depot medroxyprogesterone acetate is an injectable progestin-only contraceptive that carries a pregnancy risk of 6% with typical use and less than 1% with correct use.23 Thus, some failures are due to patients not returning for follow-up, but in some patients this method is not effective. Injections are given intramuscularly once every 3 months, avoiding the need for daily use.
A valid concern for transplant patients is that medroxyprogesterone acetate reduces bone mineral density. Although the bone effects are reversible in healthy adult women, caution is needed when prescribing this option to transplant patients who are already at increased risk of bone disease attributable to renal osteodystrophy and chronic corticosteroid use. 32,33
Recently, a subcutaneous formulation of depot medroxyprogesterone acetate (104 mg)was added to the WHO MEC for contraceptive use.34,35 The recommendations for the subcutaneous form are similar to those for the intramuscular form. In healthy women, the subcutaneous formulation is as safe and effective as the intramuscular form,36 but its efficacy after solid-organ transplant has not been determined. Both forms of depot medroxyprogesterone acetate are category 2 in the US MEC for both complicated and uncomplicated transplant cases.21
Combined hormonal contraceptives
Combined hormonal contraceptives contain both estrogen and progesterone and are available as pills, patches, or rings. Each product has an unintended pregnancy risk of 9% with typical use and less than 1% with correct use.23 They require strict patient adherence to regular daily use, which likely explains their high failure rate with typical use.
Combined hormonal contraceptives reduce mortality risk in women in the general population,37 but their effect on mortality risk after transplant is unknown and needs further study. In women who received liver transplants, low-dose combined hormonal contraceptives have been found to be effective and well tolerated, but initiation should be delayed at least 6 months until postoperative organ stability is demonstrated.11
Combined oral contraceptives are the most widely prescribed because they are convenient and familiar and have an acceptable safety profile in transplant patients,11,33,37 despite their high failure rate with typical use. They regulate the menstrual cycle and reduce anemia associated with menstruation.
The transdermal contraceptive patch has a mechanism of action similar to that of the combined oral contraceptives, but it delivers estrogen and progesterone transdermally through the abdominal wall, thus avoiding first-pass metabolism in the liver and enzymatic degradation in the gut. It delivers 35 µg of ethinyl estradiol and 150 µg of norelgestromin (an active metabolite of norgestimate) daily.38 It may cause higher circulating levels of estrogen than a combined oral contraceptive and may be associated with a higher risk of venous thromboembolism, but the evidence is conflicting.39–42
The vaginal ring, made of Silastic, delivers ethinyl estradiol in a low dose (15 µg/day) and etonorgestrel 0.12 mg/day. Like the patch, it has the advantage of bypassing first-pass metabolism in the liver, making it a good option for transplant patients who are taking antirejection drugs, thus avoiding drug interactions.41
Both the transdermal patch and vaginal ring were studied in transplant patients and had favorable results.24,43 The combined hormonal oral contraceptive pills, patch, and ring are in category 4 (unacceptable health risk) in the US MEC in patients with complicated cases, but they are in category 2 in uncomplicated cases.21
Combined hormonal contraceptives should not be considered first-line options by themselves for transplant patients because of their high failure rate with typical use.24
Progestin-only pills
Although progestin-only pills have not been studied specifically in transplant patients, they can be considered for women who have contraindications to estrogen use. Estrogen use is contraindicated in women with a history of venous thromboembolism, thrombogenic mutations, estrogen-dependent neoplasia, hepatocellular adenoma, severe hypertension, vascular disease, and Budd-Chiari syndrome.
Progestin-only pills inhibit ovulation in only about half of a woman’s cycles, but they prevent conception by other mechanisms as well, such as causing thickening of the cervical mucus. They also alter the endometrium to make it unfavorable for implantation and reduce the ciliary activity of the fallopian tube.
Strict adherence is important for effectiveness because progestin-only pills have a shorter half-life than combined hormonal contraceptives and also suppress ovulation less effectively.22 Failure rates are similar or somewhat higher than with combined hormonal contraceptives; with typical use, about 9 in 100 women can become pregnant in the first year.23 According to the US MEC,21 progestin-only pills are classified as category 2 for patients after both complicated and uncomplicated transplants.
MODERATELY EFFECTIVE METHODS (PREGNANCY RATE 10%–25%)
This tier of contraceptives includes all barrier methods, ie, male and female condoms, vaginal diaphragms, cervical caps, and sponges (Table 5).
Condoms (male and female)
When male condoms are used as the only birth control method, pregnancy occurs less often (18% with typical use and 2% with correct use) than with female condoms (21% with typical use and 5% with correct use).23 Male and female condoms are the only contraceptive methods that also prevent transmission of sexually transmitted disease.24
Caps, sponges, diaphragms
Cervical caps, vaginal sponges, and vaginal diaphragms are other forms of barrier contraceptives. All barrier methods should be combined with another contraceptive method to provide reliable protection against pregnancy. These methods are considered category 1 according to the US MEC.
LESS-EFFECTIVE METHODS
Fertility awareness-based methods such as the rhythm method have an associated pregnancy rate of about 25% with typical use and 3% to 5% with correct use23 and cannot be relied on for use by transplant recipients.24
Withdrawal and spermicides are considered least effective and unreliable for pregnancy prevention.
KNOW YOUR OPTIONS
With the growing number of women in their reproductive years receiving solid-organ transplants in the United States, it is increasingly important for healthcare providers to be aware of contraceptive options and reproductive life planning for this high-risk population.
Safe and effective forms of contraception are available, and additional information to guide the choice can be found in the Summary Chart of US MEC for Contraceptive Use, which is also available in a free smart phone app through the CDC.44
Pregnancy after transplant carries high risks, requiring these patients to have special counseling and monitoring. Fortunately, planned pregnancy at least 1 year after transplant can lead to successful outcomes in these women.
- McKay DB, Josephson MA. Pregnancy in recipients of solid organs: effects on mother and child. N Engl J Med 2006; 354:1281–1293.
- US Department of Health and Human Services. Organ procurement and transplantation network. https://optn.transplant.hrsa.gov/. Accessed July 17, 2017.
- United States Renal Data System. 2014 annual data report. https://www.usrds.org/2014/view/Default.aspx. Accessed July 17, 2017.
- Hou S. Pregnancy in chronic renal insufficiency and end-stage renal disease. Am J Kidney Dis 1999; 33:235–252.
- Josephson MA, McKay DB. Women and transplantation: fertility, sexuality, pregnancy, contraception. Adv Chronic Kidney Dis 2013; 20:433–440.
- Gill JS, Zalunardo N, Rose C, Tonelli M. The pregnancy rate and live birth rate in kidney transplant recipients. Am J Transplant 2009; 9:1541–1549.
- Mohapatra A, Basu G. Pregnancy in kidney disease. Health Sciences 2012; 1(2). http://healthsciences.ac.in/july-sep-12/downloads/pregnancy_in_kidney_disease.pdf. Accessed July 25, 2017.
- Potluri K, Moldenhauer J, Karlman R, Hou S. Beta HCG levels in a pregnant dialysis patient: a cautionary tale. NDT Plus 2011; 4:42–43.
- Kennedy C, Hussein W, Spencer S, et al. Reproductive health in Irish female renal transplant recipients. Ir J Med Sci 2012; 181:59–63.
- Ghazizadeh S, Lessan-Pezeshki M, Khatami M, et al. Unwanted pregnancy among kidney transplant recipients in Iran. Transplant Proc 2005; 37:3085–3086.
- Jabiry-Zieniewicz Z, Bobrowska K, Kaminski P, Wielgos M, Zieniewicz K, Krawczyk M. Low-dose hormonal contraception after liver transplantation. Transplant Proc 2007; 39:1530–1532.
- Coscia LA, Constantinescu S, Moritz MJ, et al. Report from the National Transplantation Pregnancy Registry (NTPR): outcomes of pregnancy after transplantation. Clin Transpl 2010: 24:65–85.
- Mohamed-Ahmed O, Nelson-Piercy C, Bramham K, et al. Pregnancy outcomes in liver and cardiothoracic transplant recipients: a UK national cohort study. PLoS One 2014; 9:e89151.
- Enderby C, Keller CA. An overview of immunosuppression in solid organ transplantation. Am J Manag Care 2015; 21(suppl 1):s12–s23.
- Hoeltzenbein M, Elefant E, Vial T, et al. Teratogenicity of mycophenolate confirmed in a prospective study of the European Network of Teratology Information Services. Am J Med Genet A 2012; 158A:588–596.
- Polifka JE, Friedman JM. Teratogen update: azathioprine and 6-mercaptopurine. Teratology 2002; 65:240–261.
- Dinatale M. The pregnancy and lactation labeling rule (PLLR). US Food and Drug Administration, 2016. https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/PediatricAdvisoryCommittee/UCM520454.pdf. Accessed July 25, 2017.
- Lexicomp. http://online.lexi.com/lco/action/api/find/globalid/6612?utd=1. Accessed July 27, 2017.
- Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 2009; 9(suppl 3):S1–S155.
- Deshpande NA, Coscia LA, Gomez-Lobo V, Moritz MJ, Armenti VT. Pregnancy after solid organ transplantation: a guide for obstetric management. Rev Obstet Gynecol 2013; 6:116–125.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. US medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep 2016; 65:1–103.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 121: Long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol 2011; 118:184–196.
- Trussell J. Contraceptive failure in the United States. Contraception 2011; 83:397–404.
- Krajewski CM, Geetha D, Gomez-Lobo V. Contraceptive options for women with a history of solid-organ transplantation. Transplantation 2013; 95:1183–1186.
- Stern LF, Simons HR, Kohn JE, Debevec EJ, Morfesis JM, Patel AA. Differences in contraceptive use between family planning providers and the U.S. population: results of a nationwide survey. Contraception 2015; 91:464–469.
- Rafie S, Lai S, Garcia JE, Mody SK. Contraceptive use in female recipients of a solid-organ transplant. Prog Transplant 2014; 24:344–348.
- Labied S, Galant C, Nisolle M, et al. Differential elevation of matrix metalloproteinase expression in women exposed to levonorgestrel-releasing intrauterine system for a short or prolonged period of time. Hum Reprod 2009; 24:113–121.
- Kim CR, Martinez-Maza O, Magpantay L, et al. Immunologic evaluation of the endometrium with a levonorgestrel intrauterine device in solid organ transplant women and healthy controls. Contraception 2016; 94:534–540.
- Ramhendar T, Byrne P. Use of the levonorgestrel-releasing intrauterine system in renal transplant recipients: a retrospective case review. Contraception 2012; 86:288–289.
- Huguelet PS, Sheehan C, Spitzer RF, Scott S. Use of the levonorgestrel 52-mg intrauterine system in adolescent and young adult solid organ transplant recipients: a case series. Contraception 2017; 95:378–381.
- Peterson HB, Xia Z, Hughes JM, Wilcox LS, Tylor LR, Trussell J. The risk of pregnancy after tubal sterilization: findings from the US Collaborative Review of Sterilization. Am J Obstet Gynecol 1996; 174:1161–1168.
- Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int 2007; 18:1319–1328.
- Krajewski C, Sucato G. Reproductive health care after transplantation. Best Pract Res Clin Obstet Gynaecol 2014; 28:1222–1234.
- World Health Organization. Medical eligibility criteria for contraceptive use. Fifth edition 2015. http://apps.who.int/iris/bitstream/10665/172915/1/WHO_RHR_15.07_eng.pdf. Accessed July 27, 2017.
- Pietrzak B, Bobrowska K, Jabiry-Zieniewicz Z, et al. Oral and transdermal hormonal contraception in women after kidney transplantation. Transplant Proc 2007; 39:2759–2762.
- Jain J, Jakimiuk AJ, Bode FR, Ross D, Kaunitz AM. Contraceptive efficacy and safety of DMPA-SC. Contraception 2004; 70:269–275.
- Vessey M, Painter R, Yeates D. Mortality in relation to oral contraceptive use and cigarette smoking. Lancet 2003; 362:185–191.
- van den Heuvel MW, van Bragt AJ, Alnabawy AK, Kaptein MC. Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptive formulations: the vaginal ring, the transdermal patch and an oral contraceptive. Contraception 2005; 72:168–174.
- Jick SS, Kaye JA, Russmann S, Jick H. Risk of nonfatal venous thromboembolism in women using a contraceptive transdermal patch and oral contraceptives containing norgestimate and 35 microg of ethinyl estradiol. Contraception 2006; 73:223–228.
- Jick S, Kaye JA, Li L, Jick H. Further results on the risk of nonfatal venous thromboembolism in users of the contraceptive transdermal patch compared to users of oral contraceptives containing norgestimate and 35 microg of ethinyl estradiol. Contraception 2007; 76:4–7.
- Estes CM, Westhoff C. Contraception for the transplant patient. Semin Perinatol 2007; 31:372–377.
- Cole JA, Norman H, Doherty M, Walker AM. Venous thromboembolism, myocardial infarction, and stroke among transdermal contraceptive system users. Obstet Gynecol 2007; 109:339–346.
- Paternoster DM, Riboni F, Bertolino M, et al. The contraceptive vaginal ring in women with renal and liver transplantation: analysis of preliminary results. Transplant Proc 2010; 42:1162–1165.
- Centers for Disease Control and Prevention (CDC). Summary chart of US medical eligibility criteria for contraceptive use. https://www.cdc.gov/reproductivehealth/unintendedpregnancy/pdf/legal_summary-chart_english_final_tag508.pdf. Accessed July 17, 2017.
Increasing numbers of women of childbearing age are receiving solid-organ transplants. All need counseling on how to prevent pregnancy while they are taking immunosuppressive agents. Some want to become pregnant after their transplant and thus require counseling and follow-up to maintain good health during pregnancy (Table 1).1
Primary care physicians can assist with basic contraception counseling and pregnancy planning for their patients who have had solid-organ transplants. In this review, we describe contraceptive options and pregnancy planning for these women.
TRANSPLANTS IN WOMEN ARE INCREASING
Over the past 20 years, the number of solid-organ transplants in US women has increased steadily. Since 1988, 38% of the 634,000 transplants performed were in women, and 47% of these women were of childbearing age (ages 18 to 49).2 Kidneys accounted for 60% of solid-organ transplants,2 and kidney transplant is now commonly performed in women of childbearing age. In 2012, of 176,000 patients with a functioning renal graft, 40.5% were women, and recipients between ages 20 and 44 composed the second-largest age group.3
FERTILITY IN WOMEN WITH END-STAGE RENAL DISEASE
Women in their reproductive years who have end-stage renal disease have lower fertility rates than women in the general population. In women undergoing peritoneal dialysis or hemodialysis, conception rates decrease to around 0.5% per year.4 This lower rate is most likely related to hypothalamic-pituitary-gonadal dysfunction, leading to reduced or total impairment of ovulation, menstrual irregularities, and infertility.5
Fertility often returns within a few months after transplant,1,6 and reported posttransplant pregnancy rates range from 3.3% to 18%,7–9 with up to one-third of pregnancies being unintended.6,10 These numbers are likely an underestimate because they do not reflect all pregnancies that are terminated, as many women do not voluntarily report having had an abortion.
Fertility is also severely diminished in women with end-stage liver disease. After liver transplant, sex hormone levels return to normal for many women, and menses soon resume.11
In 2005, the National Transplantation Pregnancy Registry reported 1,418 pregnancies in 919 female recipients of solid-organ transplants. In 2010, this number had increased to 1,940 pregnancies in 1,185 recipients, of whom 75% were kidney transplant recipients.12
A successful pregnancy outcome is most likely when a minimum of 1 year intervenes between transplant and conception.12,13
TERATOGENICITY OF IMMUNOSUPPRESSANTS
Immunosuppressant drugs commonly used for maintenance therapy after solid-organ transplant include the following:
- Calcineurin inhibitors (eg, cyclosporine, tacrolimus)
- Antiproliferative and antimetabolite agents (eg, mycophenolate mofetil, azathioprine)
- Corticosteroids
- Mammalian target of rapamycin inhibitors (eg, sirolimus, everolimus)
- T-cell costimulation blockers (eg, belatacept).14
The US Food and Drug Administration (FDA) previously classified mycophenolate mofetil and azathioprine in pregnancy risk category D (positive evidence of human fetal risk). The teratogenic risk of mycophenolate mofetil is well established in studies documenting specific congenital malformations and fetal loss in the first trimester.13,15 The teratogenic risk of azathioprine, on the other hand, is estimated to be minimal to small.16 Many of the associated fetal abnormalities may be related to the complexity of the underlying medical condition of the mother rather than to the medication.16
In June 2015, the FDA’s new Pregnancy and Lactation Labeling Rule went into effect, which removes the pregnancy letter categories A, B, C, D, and X from labeling.17 This rule was designed to help providers counsel their patients regarding the specific risks and benefits of a drug when used by pregnant or nursing women. However, the ABCDX categories are still commonly used. Table 2 shows information about the risks during pregnancy and lactation posed by the immunosuppressive drugs commonly used by posttransplant patients.18
CRITERIA FOR A SUCCESSFUL PREGNANCY
To ensure a safe and successful pregnancy with the fewest fetal and maternal complications, women are generally advised to avoid pregnancy for at least 1 year after transplant.19,20
In addition, women should meet certain clinical prerequisites after transplant before they conceive, as outlined by the American Society of Transplantation.19,20 These include:
- No rejection within the previous year
- Adequate and stable graft function (eg, serum creatinine < 1.5 mg/dL and urinary protein excretion < 500 mg/24 hours)
- No acute infection that might affect the fetus
- Maintenance immunosuppression at stable dosages.
Other circumstances to consider include episodes of rejection in the first year after transplant (as evidenced by biopsy results or glomerular filtration rate), the woman’s age (advanced maternal age is unfavorable), or any history of noncompliance.
Every pregnancy in a transplant recipient must be carefully planned. Primary care providers should encourage patients to meet with their transplant team and obstetricians early and often to allow time for the care team to adjust the type and dosing of immunosuppressant drugs, to ensure stable graft function, and to optimize any current chronic medical conditions such as diabetes mellitus or hypertension before conception.
CONTRACEPTIVE COUNSELING AFTER TRANSPLANT
Pregnancy should be avoided while transplant patients are taking FDA category D immunosuppressant drugs and, as already mentioned, during the first year after transplant. Unintended pregnancy can have serious health consequences for the mother and the fetus, as well as poor pregnancy outcomes. The US Centers for Disease Control and Prevention (CDC) lists solid-organ transplant within the past 2 years as a condition that can lead to adverse events as a result of pregnancy.21 After a transplant, a woman’s risks from an unintended pregnancy are always greater than the risks from any contraceptive, and this is important to reinforce in counseling.
Two forms of reliable contraception should be used at all times, and consistent condom use should be encouraged as one of the methods. Condoms are not reliable when used as the sole contraceptive method because they have an 18% typical-use failure rate. However, they are an excellent adjunct to other contraceptive methods because they have the additional benefit of protecting against sexually transmitted disease.
Choosing the appropriate contraceptive method for recipients of solid-organ transplants can be challenging because of several factors, including the recipient’s preexisting medical problems and drug interactions of immunosuppressant medications.
CDC criteria and categories for contraceptive use
In 2010, the CDC released the US version of the Medical Eligibility Criteria (US MEC) for contraceptive use, which was based on the 2009 World Health Organization Medical Eligibility Criteria (WHO MEC); these criteria were revised in August 2016.21
- Category 1: A condition for which there is no restriction for the use of the contraceptive method
- Category 2: A condition for which the advantages of using the method generally outweigh the theoretical or proven risks
- Category 3: A condition for which the theoretical or proven risks usually outweigh the advantages of using the method
- Category 4: A condition that represents an unacceptable health risk if the contraceptive method is used.
These recommendations aimed to improve family planning options by clarifying the possible safe and effective contraceptive options available while considering the patient’s medical condition. The CDC added solid-organ transplant recipients to this document because of the prevalence of this group in the United States.
The CDC categorizes a patient’s medical condition after transplant as either complicated or uncomplicated. Complicated conditions include acute or chronic graft failure, graft rejection, and cardiac allograft vasculopathy.21
Effectiveness of contraceptive methods
Contraceptive methods can be divided into 4 categories based on estimated effectiveness, ie, the pregnancy rate with “typical use” of that particular method in 1 year21–23:
- Very effective (0%–0.9%)
- Effective (1%–9%)
- Moderately effective (10%–25%)
- Less effective (26%–32%).
Typical use refers to failure rates for women and men whose use is not consistent nor always correct. Correct use, also described in the sections that follow, refers to failure rates for those whose use is consistent and always correct.
Women should be counseled regarding all available contraceptive options that are medically suitable for them, so they can choose the method that best fits their needs and lifestyle. They should receive counseling on emergency contraception, barrier protection against sexually transmitted disease, and the correct use of the contraceptive method they choose. They should be advised that if their chosen contraceptive method is unsatisfactory for any reason, they can switch to another method. Most importantly, providers need to impress on their patients that the risks associated with unintended pregnancy are far greater than the risks from any of the contraceptive methods.
VERY EFFECTIVE CONTRACEPTIVES (UNINTENDED PREGNANCY RATE 0%–0.9%)
This tier of contraception is the most effective regardless of the patient’s adherence; it includes long-acting, reversible contraceptives and permanent sterilization (both male and female) (Table 3).21–23
Long-acting reversible contraceptives include intrauterine devices (IUDs) and the subdermal etonogestrel implant. Given their efficacy and favorable safety profile, long-acting reversible contraceptives are being promoted for use in women who have chronic medical conditions, such as transplants.24
Intrauterine devices
IUDs are long-acting and reversible. They can be used by women who are nulliparous and those of all ages, including adolescents.22
Two types of IUDs are available in the United States: nonhormonal (copper) and hormonal (levonorgestrel). The copper IUD is effective for at least 10 years, whereas the levonorgestrel IUDs last for 3 to 5 years.22
Four levonorgestrel IUDs are currently available in the United States. Their sizes and doses vary: Mirena (52 µg), Skyla (13.5 µg), Liletta (52 µg), and Kyleena (19.5 µg).
Fewer than 1% of women become pregnant in the first year of IUD use.22,23 IUDs are an ideal option for women with solid-organ transplants because they are so effective and because the patient does not have to do anything once the IUD is in.22–24 The levonorgestrel IUD Mirena has the additional advantage of reducing heavy menstrual bleeding and is currently the only hormonal IUD with FDA approval for the management of menorrhagia.
About 12% of women in the general population use IUDs as their contraceptive method of choice,25 whereas after solid-organ transplantation about 15% to 20% of women do.26
Two historic concerns regarding IUDs may explain their low rate of use in transplant recipients.
First, IUDs were believed to be less effective in women on immunosuppressive drugs because IUDs act by inducing a local inflammatory reaction. However, IUDs involve macrophage activation, which is independent of the immune processes modified by immunosuppressants (primarily T-cell function).27 A recent pilot study showed a strong inflammatory reaction in the endometrium of transplant recipients after levonorgestrel IUD insertion.28
Second, there was concern about the increased risk of pelvic inflammatory disease with IUDs, but studies have shown levonorgestrel IUDs to be safe in transplant patients.29,30
The CDC21 lists copper and levonorgestrel IUDs in MEC category 3 (the risks generally outweigh the advantages) for initiation in patients with complicated transplants and in category 2 (advantages generally outweigh the risks) in patients with uncomplicated organ transplants. The devices are in category 2 for both complicated and uncomplicated cases if the IUD is already in place.
Subdermal implant
A subdermal implant consisting of a single rod containing 68 mg of etonogestrel is commercially available in the United States. It is one of the most effective contraceptive methods, with the lowest rates of pregnancy—less than 1% per year, with protection lasting at least 3 years.22,23 This low risk makes the subdermal implant a suitable method of contraception after transplant. Daily compliance is not required, and there are no hepatic first-pass effects, which results in higher bioavailability and less chance of drug interactions.
The main disadvantage of the subdermal implant and IUDs is unscheduled bleeding. An important benefit is prolonged amenorrhea, not only for patient convenience, but for reduction of endometrial cancer risk. Insertion and removal of the implant are considered minor office procedures. The implants are classified as US MEC category 2 in uncomplicated cases; initiation in complicated cases is considered category 3 but continuation is considered category 2.21
Permanent sterilization
Permanent sterilization is another option for women and men. In women, the fallopian tubes can be occluded with a coil system implanted vaginally through a hysteroscope, or they can be severed, tied, or clamped in a laparoscopic procedure or during cesarean delivery. Pregnancy rates after tubal ligation are less than 1%,23,31 although concern exists for high failure rates with the hysteroscopic method.
Because younger patients are more likely than older patients to subsequently regret having the procedure done, all available contraceptive options should be discussed with them.31
For men, permanent sterilization is done by vasectomy, which has less associated risk and cost compared with sterilization for women.
EFFECTIVE CONTRACEPTIVE METHODS (UNINTENDED PREGNANCY RATE 1%–9%)
Effective contraceptive methods, the next tier down from very effective methods, include injectable contraceptives, combined hormonal contraceptives, and progestin-only contraceptives (Table 4).
Injectable contraceptives
Depot medroxyprogesterone acetate is an injectable progestin-only contraceptive that carries a pregnancy risk of 6% with typical use and less than 1% with correct use.23 Thus, some failures are due to patients not returning for follow-up, but in some patients this method is not effective. Injections are given intramuscularly once every 3 months, avoiding the need for daily use.
A valid concern for transplant patients is that medroxyprogesterone acetate reduces bone mineral density. Although the bone effects are reversible in healthy adult women, caution is needed when prescribing this option to transplant patients who are already at increased risk of bone disease attributable to renal osteodystrophy and chronic corticosteroid use. 32,33
Recently, a subcutaneous formulation of depot medroxyprogesterone acetate (104 mg)was added to the WHO MEC for contraceptive use.34,35 The recommendations for the subcutaneous form are similar to those for the intramuscular form. In healthy women, the subcutaneous formulation is as safe and effective as the intramuscular form,36 but its efficacy after solid-organ transplant has not been determined. Both forms of depot medroxyprogesterone acetate are category 2 in the US MEC for both complicated and uncomplicated transplant cases.21
Combined hormonal contraceptives
Combined hormonal contraceptives contain both estrogen and progesterone and are available as pills, patches, or rings. Each product has an unintended pregnancy risk of 9% with typical use and less than 1% with correct use.23 They require strict patient adherence to regular daily use, which likely explains their high failure rate with typical use.
Combined hormonal contraceptives reduce mortality risk in women in the general population,37 but their effect on mortality risk after transplant is unknown and needs further study. In women who received liver transplants, low-dose combined hormonal contraceptives have been found to be effective and well tolerated, but initiation should be delayed at least 6 months until postoperative organ stability is demonstrated.11
Combined oral contraceptives are the most widely prescribed because they are convenient and familiar and have an acceptable safety profile in transplant patients,11,33,37 despite their high failure rate with typical use. They regulate the menstrual cycle and reduce anemia associated with menstruation.
The transdermal contraceptive patch has a mechanism of action similar to that of the combined oral contraceptives, but it delivers estrogen and progesterone transdermally through the abdominal wall, thus avoiding first-pass metabolism in the liver and enzymatic degradation in the gut. It delivers 35 µg of ethinyl estradiol and 150 µg of norelgestromin (an active metabolite of norgestimate) daily.38 It may cause higher circulating levels of estrogen than a combined oral contraceptive and may be associated with a higher risk of venous thromboembolism, but the evidence is conflicting.39–42
The vaginal ring, made of Silastic, delivers ethinyl estradiol in a low dose (15 µg/day) and etonorgestrel 0.12 mg/day. Like the patch, it has the advantage of bypassing first-pass metabolism in the liver, making it a good option for transplant patients who are taking antirejection drugs, thus avoiding drug interactions.41
Both the transdermal patch and vaginal ring were studied in transplant patients and had favorable results.24,43 The combined hormonal oral contraceptive pills, patch, and ring are in category 4 (unacceptable health risk) in the US MEC in patients with complicated cases, but they are in category 2 in uncomplicated cases.21
Combined hormonal contraceptives should not be considered first-line options by themselves for transplant patients because of their high failure rate with typical use.24
Progestin-only pills
Although progestin-only pills have not been studied specifically in transplant patients, they can be considered for women who have contraindications to estrogen use. Estrogen use is contraindicated in women with a history of venous thromboembolism, thrombogenic mutations, estrogen-dependent neoplasia, hepatocellular adenoma, severe hypertension, vascular disease, and Budd-Chiari syndrome.
Progestin-only pills inhibit ovulation in only about half of a woman’s cycles, but they prevent conception by other mechanisms as well, such as causing thickening of the cervical mucus. They also alter the endometrium to make it unfavorable for implantation and reduce the ciliary activity of the fallopian tube.
Strict adherence is important for effectiveness because progestin-only pills have a shorter half-life than combined hormonal contraceptives and also suppress ovulation less effectively.22 Failure rates are similar or somewhat higher than with combined hormonal contraceptives; with typical use, about 9 in 100 women can become pregnant in the first year.23 According to the US MEC,21 progestin-only pills are classified as category 2 for patients after both complicated and uncomplicated transplants.
MODERATELY EFFECTIVE METHODS (PREGNANCY RATE 10%–25%)
This tier of contraceptives includes all barrier methods, ie, male and female condoms, vaginal diaphragms, cervical caps, and sponges (Table 5).
Condoms (male and female)
When male condoms are used as the only birth control method, pregnancy occurs less often (18% with typical use and 2% with correct use) than with female condoms (21% with typical use and 5% with correct use).23 Male and female condoms are the only contraceptive methods that also prevent transmission of sexually transmitted disease.24
Caps, sponges, diaphragms
Cervical caps, vaginal sponges, and vaginal diaphragms are other forms of barrier contraceptives. All barrier methods should be combined with another contraceptive method to provide reliable protection against pregnancy. These methods are considered category 1 according to the US MEC.
LESS-EFFECTIVE METHODS
Fertility awareness-based methods such as the rhythm method have an associated pregnancy rate of about 25% with typical use and 3% to 5% with correct use23 and cannot be relied on for use by transplant recipients.24
Withdrawal and spermicides are considered least effective and unreliable for pregnancy prevention.
KNOW YOUR OPTIONS
With the growing number of women in their reproductive years receiving solid-organ transplants in the United States, it is increasingly important for healthcare providers to be aware of contraceptive options and reproductive life planning for this high-risk population.
Safe and effective forms of contraception are available, and additional information to guide the choice can be found in the Summary Chart of US MEC for Contraceptive Use, which is also available in a free smart phone app through the CDC.44
Pregnancy after transplant carries high risks, requiring these patients to have special counseling and monitoring. Fortunately, planned pregnancy at least 1 year after transplant can lead to successful outcomes in these women.
Increasing numbers of women of childbearing age are receiving solid-organ transplants. All need counseling on how to prevent pregnancy while they are taking immunosuppressive agents. Some want to become pregnant after their transplant and thus require counseling and follow-up to maintain good health during pregnancy (Table 1).1
Primary care physicians can assist with basic contraception counseling and pregnancy planning for their patients who have had solid-organ transplants. In this review, we describe contraceptive options and pregnancy planning for these women.
TRANSPLANTS IN WOMEN ARE INCREASING
Over the past 20 years, the number of solid-organ transplants in US women has increased steadily. Since 1988, 38% of the 634,000 transplants performed were in women, and 47% of these women were of childbearing age (ages 18 to 49).2 Kidneys accounted for 60% of solid-organ transplants,2 and kidney transplant is now commonly performed in women of childbearing age. In 2012, of 176,000 patients with a functioning renal graft, 40.5% were women, and recipients between ages 20 and 44 composed the second-largest age group.3
FERTILITY IN WOMEN WITH END-STAGE RENAL DISEASE
Women in their reproductive years who have end-stage renal disease have lower fertility rates than women in the general population. In women undergoing peritoneal dialysis or hemodialysis, conception rates decrease to around 0.5% per year.4 This lower rate is most likely related to hypothalamic-pituitary-gonadal dysfunction, leading to reduced or total impairment of ovulation, menstrual irregularities, and infertility.5
Fertility often returns within a few months after transplant,1,6 and reported posttransplant pregnancy rates range from 3.3% to 18%,7–9 with up to one-third of pregnancies being unintended.6,10 These numbers are likely an underestimate because they do not reflect all pregnancies that are terminated, as many women do not voluntarily report having had an abortion.
Fertility is also severely diminished in women with end-stage liver disease. After liver transplant, sex hormone levels return to normal for many women, and menses soon resume.11
In 2005, the National Transplantation Pregnancy Registry reported 1,418 pregnancies in 919 female recipients of solid-organ transplants. In 2010, this number had increased to 1,940 pregnancies in 1,185 recipients, of whom 75% were kidney transplant recipients.12
A successful pregnancy outcome is most likely when a minimum of 1 year intervenes between transplant and conception.12,13
TERATOGENICITY OF IMMUNOSUPPRESSANTS
Immunosuppressant drugs commonly used for maintenance therapy after solid-organ transplant include the following:
- Calcineurin inhibitors (eg, cyclosporine, tacrolimus)
- Antiproliferative and antimetabolite agents (eg, mycophenolate mofetil, azathioprine)
- Corticosteroids
- Mammalian target of rapamycin inhibitors (eg, sirolimus, everolimus)
- T-cell costimulation blockers (eg, belatacept).14
The US Food and Drug Administration (FDA) previously classified mycophenolate mofetil and azathioprine in pregnancy risk category D (positive evidence of human fetal risk). The teratogenic risk of mycophenolate mofetil is well established in studies documenting specific congenital malformations and fetal loss in the first trimester.13,15 The teratogenic risk of azathioprine, on the other hand, is estimated to be minimal to small.16 Many of the associated fetal abnormalities may be related to the complexity of the underlying medical condition of the mother rather than to the medication.16
In June 2015, the FDA’s new Pregnancy and Lactation Labeling Rule went into effect, which removes the pregnancy letter categories A, B, C, D, and X from labeling.17 This rule was designed to help providers counsel their patients regarding the specific risks and benefits of a drug when used by pregnant or nursing women. However, the ABCDX categories are still commonly used. Table 2 shows information about the risks during pregnancy and lactation posed by the immunosuppressive drugs commonly used by posttransplant patients.18
CRITERIA FOR A SUCCESSFUL PREGNANCY
To ensure a safe and successful pregnancy with the fewest fetal and maternal complications, women are generally advised to avoid pregnancy for at least 1 year after transplant.19,20
In addition, women should meet certain clinical prerequisites after transplant before they conceive, as outlined by the American Society of Transplantation.19,20 These include:
- No rejection within the previous year
- Adequate and stable graft function (eg, serum creatinine < 1.5 mg/dL and urinary protein excretion < 500 mg/24 hours)
- No acute infection that might affect the fetus
- Maintenance immunosuppression at stable dosages.
Other circumstances to consider include episodes of rejection in the first year after transplant (as evidenced by biopsy results or glomerular filtration rate), the woman’s age (advanced maternal age is unfavorable), or any history of noncompliance.
Every pregnancy in a transplant recipient must be carefully planned. Primary care providers should encourage patients to meet with their transplant team and obstetricians early and often to allow time for the care team to adjust the type and dosing of immunosuppressant drugs, to ensure stable graft function, and to optimize any current chronic medical conditions such as diabetes mellitus or hypertension before conception.
CONTRACEPTIVE COUNSELING AFTER TRANSPLANT
Pregnancy should be avoided while transplant patients are taking FDA category D immunosuppressant drugs and, as already mentioned, during the first year after transplant. Unintended pregnancy can have serious health consequences for the mother and the fetus, as well as poor pregnancy outcomes. The US Centers for Disease Control and Prevention (CDC) lists solid-organ transplant within the past 2 years as a condition that can lead to adverse events as a result of pregnancy.21 After a transplant, a woman’s risks from an unintended pregnancy are always greater than the risks from any contraceptive, and this is important to reinforce in counseling.
Two forms of reliable contraception should be used at all times, and consistent condom use should be encouraged as one of the methods. Condoms are not reliable when used as the sole contraceptive method because they have an 18% typical-use failure rate. However, they are an excellent adjunct to other contraceptive methods because they have the additional benefit of protecting against sexually transmitted disease.
Choosing the appropriate contraceptive method for recipients of solid-organ transplants can be challenging because of several factors, including the recipient’s preexisting medical problems and drug interactions of immunosuppressant medications.
CDC criteria and categories for contraceptive use
In 2010, the CDC released the US version of the Medical Eligibility Criteria (US MEC) for contraceptive use, which was based on the 2009 World Health Organization Medical Eligibility Criteria (WHO MEC); these criteria were revised in August 2016.21
- Category 1: A condition for which there is no restriction for the use of the contraceptive method
- Category 2: A condition for which the advantages of using the method generally outweigh the theoretical or proven risks
- Category 3: A condition for which the theoretical or proven risks usually outweigh the advantages of using the method
- Category 4: A condition that represents an unacceptable health risk if the contraceptive method is used.
These recommendations aimed to improve family planning options by clarifying the possible safe and effective contraceptive options available while considering the patient’s medical condition. The CDC added solid-organ transplant recipients to this document because of the prevalence of this group in the United States.
The CDC categorizes a patient’s medical condition after transplant as either complicated or uncomplicated. Complicated conditions include acute or chronic graft failure, graft rejection, and cardiac allograft vasculopathy.21
Effectiveness of contraceptive methods
Contraceptive methods can be divided into 4 categories based on estimated effectiveness, ie, the pregnancy rate with “typical use” of that particular method in 1 year21–23:
- Very effective (0%–0.9%)
- Effective (1%–9%)
- Moderately effective (10%–25%)
- Less effective (26%–32%).
Typical use refers to failure rates for women and men whose use is not consistent nor always correct. Correct use, also described in the sections that follow, refers to failure rates for those whose use is consistent and always correct.
Women should be counseled regarding all available contraceptive options that are medically suitable for them, so they can choose the method that best fits their needs and lifestyle. They should receive counseling on emergency contraception, barrier protection against sexually transmitted disease, and the correct use of the contraceptive method they choose. They should be advised that if their chosen contraceptive method is unsatisfactory for any reason, they can switch to another method. Most importantly, providers need to impress on their patients that the risks associated with unintended pregnancy are far greater than the risks from any of the contraceptive methods.
VERY EFFECTIVE CONTRACEPTIVES (UNINTENDED PREGNANCY RATE 0%–0.9%)
This tier of contraception is the most effective regardless of the patient’s adherence; it includes long-acting, reversible contraceptives and permanent sterilization (both male and female) (Table 3).21–23
Long-acting reversible contraceptives include intrauterine devices (IUDs) and the subdermal etonogestrel implant. Given their efficacy and favorable safety profile, long-acting reversible contraceptives are being promoted for use in women who have chronic medical conditions, such as transplants.24
Intrauterine devices
IUDs are long-acting and reversible. They can be used by women who are nulliparous and those of all ages, including adolescents.22
Two types of IUDs are available in the United States: nonhormonal (copper) and hormonal (levonorgestrel). The copper IUD is effective for at least 10 years, whereas the levonorgestrel IUDs last for 3 to 5 years.22
Four levonorgestrel IUDs are currently available in the United States. Their sizes and doses vary: Mirena (52 µg), Skyla (13.5 µg), Liletta (52 µg), and Kyleena (19.5 µg).
Fewer than 1% of women become pregnant in the first year of IUD use.22,23 IUDs are an ideal option for women with solid-organ transplants because they are so effective and because the patient does not have to do anything once the IUD is in.22–24 The levonorgestrel IUD Mirena has the additional advantage of reducing heavy menstrual bleeding and is currently the only hormonal IUD with FDA approval for the management of menorrhagia.
About 12% of women in the general population use IUDs as their contraceptive method of choice,25 whereas after solid-organ transplantation about 15% to 20% of women do.26
Two historic concerns regarding IUDs may explain their low rate of use in transplant recipients.
First, IUDs were believed to be less effective in women on immunosuppressive drugs because IUDs act by inducing a local inflammatory reaction. However, IUDs involve macrophage activation, which is independent of the immune processes modified by immunosuppressants (primarily T-cell function).27 A recent pilot study showed a strong inflammatory reaction in the endometrium of transplant recipients after levonorgestrel IUD insertion.28
Second, there was concern about the increased risk of pelvic inflammatory disease with IUDs, but studies have shown levonorgestrel IUDs to be safe in transplant patients.29,30
The CDC21 lists copper and levonorgestrel IUDs in MEC category 3 (the risks generally outweigh the advantages) for initiation in patients with complicated transplants and in category 2 (advantages generally outweigh the risks) in patients with uncomplicated organ transplants. The devices are in category 2 for both complicated and uncomplicated cases if the IUD is already in place.
Subdermal implant
A subdermal implant consisting of a single rod containing 68 mg of etonogestrel is commercially available in the United States. It is one of the most effective contraceptive methods, with the lowest rates of pregnancy—less than 1% per year, with protection lasting at least 3 years.22,23 This low risk makes the subdermal implant a suitable method of contraception after transplant. Daily compliance is not required, and there are no hepatic first-pass effects, which results in higher bioavailability and less chance of drug interactions.
The main disadvantage of the subdermal implant and IUDs is unscheduled bleeding. An important benefit is prolonged amenorrhea, not only for patient convenience, but for reduction of endometrial cancer risk. Insertion and removal of the implant are considered minor office procedures. The implants are classified as US MEC category 2 in uncomplicated cases; initiation in complicated cases is considered category 3 but continuation is considered category 2.21
Permanent sterilization
Permanent sterilization is another option for women and men. In women, the fallopian tubes can be occluded with a coil system implanted vaginally through a hysteroscope, or they can be severed, tied, or clamped in a laparoscopic procedure or during cesarean delivery. Pregnancy rates after tubal ligation are less than 1%,23,31 although concern exists for high failure rates with the hysteroscopic method.
Because younger patients are more likely than older patients to subsequently regret having the procedure done, all available contraceptive options should be discussed with them.31
For men, permanent sterilization is done by vasectomy, which has less associated risk and cost compared with sterilization for women.
EFFECTIVE CONTRACEPTIVE METHODS (UNINTENDED PREGNANCY RATE 1%–9%)
Effective contraceptive methods, the next tier down from very effective methods, include injectable contraceptives, combined hormonal contraceptives, and progestin-only contraceptives (Table 4).
Injectable contraceptives
Depot medroxyprogesterone acetate is an injectable progestin-only contraceptive that carries a pregnancy risk of 6% with typical use and less than 1% with correct use.23 Thus, some failures are due to patients not returning for follow-up, but in some patients this method is not effective. Injections are given intramuscularly once every 3 months, avoiding the need for daily use.
A valid concern for transplant patients is that medroxyprogesterone acetate reduces bone mineral density. Although the bone effects are reversible in healthy adult women, caution is needed when prescribing this option to transplant patients who are already at increased risk of bone disease attributable to renal osteodystrophy and chronic corticosteroid use. 32,33
Recently, a subcutaneous formulation of depot medroxyprogesterone acetate (104 mg)was added to the WHO MEC for contraceptive use.34,35 The recommendations for the subcutaneous form are similar to those for the intramuscular form. In healthy women, the subcutaneous formulation is as safe and effective as the intramuscular form,36 but its efficacy after solid-organ transplant has not been determined. Both forms of depot medroxyprogesterone acetate are category 2 in the US MEC for both complicated and uncomplicated transplant cases.21
Combined hormonal contraceptives
Combined hormonal contraceptives contain both estrogen and progesterone and are available as pills, patches, or rings. Each product has an unintended pregnancy risk of 9% with typical use and less than 1% with correct use.23 They require strict patient adherence to regular daily use, which likely explains their high failure rate with typical use.
Combined hormonal contraceptives reduce mortality risk in women in the general population,37 but their effect on mortality risk after transplant is unknown and needs further study. In women who received liver transplants, low-dose combined hormonal contraceptives have been found to be effective and well tolerated, but initiation should be delayed at least 6 months until postoperative organ stability is demonstrated.11
Combined oral contraceptives are the most widely prescribed because they are convenient and familiar and have an acceptable safety profile in transplant patients,11,33,37 despite their high failure rate with typical use. They regulate the menstrual cycle and reduce anemia associated with menstruation.
The transdermal contraceptive patch has a mechanism of action similar to that of the combined oral contraceptives, but it delivers estrogen and progesterone transdermally through the abdominal wall, thus avoiding first-pass metabolism in the liver and enzymatic degradation in the gut. It delivers 35 µg of ethinyl estradiol and 150 µg of norelgestromin (an active metabolite of norgestimate) daily.38 It may cause higher circulating levels of estrogen than a combined oral contraceptive and may be associated with a higher risk of venous thromboembolism, but the evidence is conflicting.39–42
The vaginal ring, made of Silastic, delivers ethinyl estradiol in a low dose (15 µg/day) and etonorgestrel 0.12 mg/day. Like the patch, it has the advantage of bypassing first-pass metabolism in the liver, making it a good option for transplant patients who are taking antirejection drugs, thus avoiding drug interactions.41
Both the transdermal patch and vaginal ring were studied in transplant patients and had favorable results.24,43 The combined hormonal oral contraceptive pills, patch, and ring are in category 4 (unacceptable health risk) in the US MEC in patients with complicated cases, but they are in category 2 in uncomplicated cases.21
Combined hormonal contraceptives should not be considered first-line options by themselves for transplant patients because of their high failure rate with typical use.24
Progestin-only pills
Although progestin-only pills have not been studied specifically in transplant patients, they can be considered for women who have contraindications to estrogen use. Estrogen use is contraindicated in women with a history of venous thromboembolism, thrombogenic mutations, estrogen-dependent neoplasia, hepatocellular adenoma, severe hypertension, vascular disease, and Budd-Chiari syndrome.
Progestin-only pills inhibit ovulation in only about half of a woman’s cycles, but they prevent conception by other mechanisms as well, such as causing thickening of the cervical mucus. They also alter the endometrium to make it unfavorable for implantation and reduce the ciliary activity of the fallopian tube.
Strict adherence is important for effectiveness because progestin-only pills have a shorter half-life than combined hormonal contraceptives and also suppress ovulation less effectively.22 Failure rates are similar or somewhat higher than with combined hormonal contraceptives; with typical use, about 9 in 100 women can become pregnant in the first year.23 According to the US MEC,21 progestin-only pills are classified as category 2 for patients after both complicated and uncomplicated transplants.
MODERATELY EFFECTIVE METHODS (PREGNANCY RATE 10%–25%)
This tier of contraceptives includes all barrier methods, ie, male and female condoms, vaginal diaphragms, cervical caps, and sponges (Table 5).
Condoms (male and female)
When male condoms are used as the only birth control method, pregnancy occurs less often (18% with typical use and 2% with correct use) than with female condoms (21% with typical use and 5% with correct use).23 Male and female condoms are the only contraceptive methods that also prevent transmission of sexually transmitted disease.24
Caps, sponges, diaphragms
Cervical caps, vaginal sponges, and vaginal diaphragms are other forms of barrier contraceptives. All barrier methods should be combined with another contraceptive method to provide reliable protection against pregnancy. These methods are considered category 1 according to the US MEC.
LESS-EFFECTIVE METHODS
Fertility awareness-based methods such as the rhythm method have an associated pregnancy rate of about 25% with typical use and 3% to 5% with correct use23 and cannot be relied on for use by transplant recipients.24
Withdrawal and spermicides are considered least effective and unreliable for pregnancy prevention.
KNOW YOUR OPTIONS
With the growing number of women in their reproductive years receiving solid-organ transplants in the United States, it is increasingly important for healthcare providers to be aware of contraceptive options and reproductive life planning for this high-risk population.
Safe and effective forms of contraception are available, and additional information to guide the choice can be found in the Summary Chart of US MEC for Contraceptive Use, which is also available in a free smart phone app through the CDC.44
Pregnancy after transplant carries high risks, requiring these patients to have special counseling and monitoring. Fortunately, planned pregnancy at least 1 year after transplant can lead to successful outcomes in these women.
- McKay DB, Josephson MA. Pregnancy in recipients of solid organs: effects on mother and child. N Engl J Med 2006; 354:1281–1293.
- US Department of Health and Human Services. Organ procurement and transplantation network. https://optn.transplant.hrsa.gov/. Accessed July 17, 2017.
- United States Renal Data System. 2014 annual data report. https://www.usrds.org/2014/view/Default.aspx. Accessed July 17, 2017.
- Hou S. Pregnancy in chronic renal insufficiency and end-stage renal disease. Am J Kidney Dis 1999; 33:235–252.
- Josephson MA, McKay DB. Women and transplantation: fertility, sexuality, pregnancy, contraception. Adv Chronic Kidney Dis 2013; 20:433–440.
- Gill JS, Zalunardo N, Rose C, Tonelli M. The pregnancy rate and live birth rate in kidney transplant recipients. Am J Transplant 2009; 9:1541–1549.
- Mohapatra A, Basu G. Pregnancy in kidney disease. Health Sciences 2012; 1(2). http://healthsciences.ac.in/july-sep-12/downloads/pregnancy_in_kidney_disease.pdf. Accessed July 25, 2017.
- Potluri K, Moldenhauer J, Karlman R, Hou S. Beta HCG levels in a pregnant dialysis patient: a cautionary tale. NDT Plus 2011; 4:42–43.
- Kennedy C, Hussein W, Spencer S, et al. Reproductive health in Irish female renal transplant recipients. Ir J Med Sci 2012; 181:59–63.
- Ghazizadeh S, Lessan-Pezeshki M, Khatami M, et al. Unwanted pregnancy among kidney transplant recipients in Iran. Transplant Proc 2005; 37:3085–3086.
- Jabiry-Zieniewicz Z, Bobrowska K, Kaminski P, Wielgos M, Zieniewicz K, Krawczyk M. Low-dose hormonal contraception after liver transplantation. Transplant Proc 2007; 39:1530–1532.
- Coscia LA, Constantinescu S, Moritz MJ, et al. Report from the National Transplantation Pregnancy Registry (NTPR): outcomes of pregnancy after transplantation. Clin Transpl 2010: 24:65–85.
- Mohamed-Ahmed O, Nelson-Piercy C, Bramham K, et al. Pregnancy outcomes in liver and cardiothoracic transplant recipients: a UK national cohort study. PLoS One 2014; 9:e89151.
- Enderby C, Keller CA. An overview of immunosuppression in solid organ transplantation. Am J Manag Care 2015; 21(suppl 1):s12–s23.
- Hoeltzenbein M, Elefant E, Vial T, et al. Teratogenicity of mycophenolate confirmed in a prospective study of the European Network of Teratology Information Services. Am J Med Genet A 2012; 158A:588–596.
- Polifka JE, Friedman JM. Teratogen update: azathioprine and 6-mercaptopurine. Teratology 2002; 65:240–261.
- Dinatale M. The pregnancy and lactation labeling rule (PLLR). US Food and Drug Administration, 2016. https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/PediatricAdvisoryCommittee/UCM520454.pdf. Accessed July 25, 2017.
- Lexicomp. http://online.lexi.com/lco/action/api/find/globalid/6612?utd=1. Accessed July 27, 2017.
- Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 2009; 9(suppl 3):S1–S155.
- Deshpande NA, Coscia LA, Gomez-Lobo V, Moritz MJ, Armenti VT. Pregnancy after solid organ transplantation: a guide for obstetric management. Rev Obstet Gynecol 2013; 6:116–125.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. US medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep 2016; 65:1–103.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 121: Long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol 2011; 118:184–196.
- Trussell J. Contraceptive failure in the United States. Contraception 2011; 83:397–404.
- Krajewski CM, Geetha D, Gomez-Lobo V. Contraceptive options for women with a history of solid-organ transplantation. Transplantation 2013; 95:1183–1186.
- Stern LF, Simons HR, Kohn JE, Debevec EJ, Morfesis JM, Patel AA. Differences in contraceptive use between family planning providers and the U.S. population: results of a nationwide survey. Contraception 2015; 91:464–469.
- Rafie S, Lai S, Garcia JE, Mody SK. Contraceptive use in female recipients of a solid-organ transplant. Prog Transplant 2014; 24:344–348.
- Labied S, Galant C, Nisolle M, et al. Differential elevation of matrix metalloproteinase expression in women exposed to levonorgestrel-releasing intrauterine system for a short or prolonged period of time. Hum Reprod 2009; 24:113–121.
- Kim CR, Martinez-Maza O, Magpantay L, et al. Immunologic evaluation of the endometrium with a levonorgestrel intrauterine device in solid organ transplant women and healthy controls. Contraception 2016; 94:534–540.
- Ramhendar T, Byrne P. Use of the levonorgestrel-releasing intrauterine system in renal transplant recipients: a retrospective case review. Contraception 2012; 86:288–289.
- Huguelet PS, Sheehan C, Spitzer RF, Scott S. Use of the levonorgestrel 52-mg intrauterine system in adolescent and young adult solid organ transplant recipients: a case series. Contraception 2017; 95:378–381.
- Peterson HB, Xia Z, Hughes JM, Wilcox LS, Tylor LR, Trussell J. The risk of pregnancy after tubal sterilization: findings from the US Collaborative Review of Sterilization. Am J Obstet Gynecol 1996; 174:1161–1168.
- Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int 2007; 18:1319–1328.
- Krajewski C, Sucato G. Reproductive health care after transplantation. Best Pract Res Clin Obstet Gynaecol 2014; 28:1222–1234.
- World Health Organization. Medical eligibility criteria for contraceptive use. Fifth edition 2015. http://apps.who.int/iris/bitstream/10665/172915/1/WHO_RHR_15.07_eng.pdf. Accessed July 27, 2017.
- Pietrzak B, Bobrowska K, Jabiry-Zieniewicz Z, et al. Oral and transdermal hormonal contraception in women after kidney transplantation. Transplant Proc 2007; 39:2759–2762.
- Jain J, Jakimiuk AJ, Bode FR, Ross D, Kaunitz AM. Contraceptive efficacy and safety of DMPA-SC. Contraception 2004; 70:269–275.
- Vessey M, Painter R, Yeates D. Mortality in relation to oral contraceptive use and cigarette smoking. Lancet 2003; 362:185–191.
- van den Heuvel MW, van Bragt AJ, Alnabawy AK, Kaptein MC. Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptive formulations: the vaginal ring, the transdermal patch and an oral contraceptive. Contraception 2005; 72:168–174.
- Jick SS, Kaye JA, Russmann S, Jick H. Risk of nonfatal venous thromboembolism in women using a contraceptive transdermal patch and oral contraceptives containing norgestimate and 35 microg of ethinyl estradiol. Contraception 2006; 73:223–228.
- Jick S, Kaye JA, Li L, Jick H. Further results on the risk of nonfatal venous thromboembolism in users of the contraceptive transdermal patch compared to users of oral contraceptives containing norgestimate and 35 microg of ethinyl estradiol. Contraception 2007; 76:4–7.
- Estes CM, Westhoff C. Contraception for the transplant patient. Semin Perinatol 2007; 31:372–377.
- Cole JA, Norman H, Doherty M, Walker AM. Venous thromboembolism, myocardial infarction, and stroke among transdermal contraceptive system users. Obstet Gynecol 2007; 109:339–346.
- Paternoster DM, Riboni F, Bertolino M, et al. The contraceptive vaginal ring in women with renal and liver transplantation: analysis of preliminary results. Transplant Proc 2010; 42:1162–1165.
- Centers for Disease Control and Prevention (CDC). Summary chart of US medical eligibility criteria for contraceptive use. https://www.cdc.gov/reproductivehealth/unintendedpregnancy/pdf/legal_summary-chart_english_final_tag508.pdf. Accessed July 17, 2017.
- McKay DB, Josephson MA. Pregnancy in recipients of solid organs: effects on mother and child. N Engl J Med 2006; 354:1281–1293.
- US Department of Health and Human Services. Organ procurement and transplantation network. https://optn.transplant.hrsa.gov/. Accessed July 17, 2017.
- United States Renal Data System. 2014 annual data report. https://www.usrds.org/2014/view/Default.aspx. Accessed July 17, 2017.
- Hou S. Pregnancy in chronic renal insufficiency and end-stage renal disease. Am J Kidney Dis 1999; 33:235–252.
- Josephson MA, McKay DB. Women and transplantation: fertility, sexuality, pregnancy, contraception. Adv Chronic Kidney Dis 2013; 20:433–440.
- Gill JS, Zalunardo N, Rose C, Tonelli M. The pregnancy rate and live birth rate in kidney transplant recipients. Am J Transplant 2009; 9:1541–1549.
- Mohapatra A, Basu G. Pregnancy in kidney disease. Health Sciences 2012; 1(2). http://healthsciences.ac.in/july-sep-12/downloads/pregnancy_in_kidney_disease.pdf. Accessed July 25, 2017.
- Potluri K, Moldenhauer J, Karlman R, Hou S. Beta HCG levels in a pregnant dialysis patient: a cautionary tale. NDT Plus 2011; 4:42–43.
- Kennedy C, Hussein W, Spencer S, et al. Reproductive health in Irish female renal transplant recipients. Ir J Med Sci 2012; 181:59–63.
- Ghazizadeh S, Lessan-Pezeshki M, Khatami M, et al. Unwanted pregnancy among kidney transplant recipients in Iran. Transplant Proc 2005; 37:3085–3086.
- Jabiry-Zieniewicz Z, Bobrowska K, Kaminski P, Wielgos M, Zieniewicz K, Krawczyk M. Low-dose hormonal contraception after liver transplantation. Transplant Proc 2007; 39:1530–1532.
- Coscia LA, Constantinescu S, Moritz MJ, et al. Report from the National Transplantation Pregnancy Registry (NTPR): outcomes of pregnancy after transplantation. Clin Transpl 2010: 24:65–85.
- Mohamed-Ahmed O, Nelson-Piercy C, Bramham K, et al. Pregnancy outcomes in liver and cardiothoracic transplant recipients: a UK national cohort study. PLoS One 2014; 9:e89151.
- Enderby C, Keller CA. An overview of immunosuppression in solid organ transplantation. Am J Manag Care 2015; 21(suppl 1):s12–s23.
- Hoeltzenbein M, Elefant E, Vial T, et al. Teratogenicity of mycophenolate confirmed in a prospective study of the European Network of Teratology Information Services. Am J Med Genet A 2012; 158A:588–596.
- Polifka JE, Friedman JM. Teratogen update: azathioprine and 6-mercaptopurine. Teratology 2002; 65:240–261.
- Dinatale M. The pregnancy and lactation labeling rule (PLLR). US Food and Drug Administration, 2016. https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/PediatricAdvisoryCommittee/UCM520454.pdf. Accessed July 25, 2017.
- Lexicomp. http://online.lexi.com/lco/action/api/find/globalid/6612?utd=1. Accessed July 27, 2017.
- Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 2009; 9(suppl 3):S1–S155.
- Deshpande NA, Coscia LA, Gomez-Lobo V, Moritz MJ, Armenti VT. Pregnancy after solid organ transplantation: a guide for obstetric management. Rev Obstet Gynecol 2013; 6:116–125.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. US medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep 2016; 65:1–103.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 121: Long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol 2011; 118:184–196.
- Trussell J. Contraceptive failure in the United States. Contraception 2011; 83:397–404.
- Krajewski CM, Geetha D, Gomez-Lobo V. Contraceptive options for women with a history of solid-organ transplantation. Transplantation 2013; 95:1183–1186.
- Stern LF, Simons HR, Kohn JE, Debevec EJ, Morfesis JM, Patel AA. Differences in contraceptive use between family planning providers and the U.S. population: results of a nationwide survey. Contraception 2015; 91:464–469.
- Rafie S, Lai S, Garcia JE, Mody SK. Contraceptive use in female recipients of a solid-organ transplant. Prog Transplant 2014; 24:344–348.
- Labied S, Galant C, Nisolle M, et al. Differential elevation of matrix metalloproteinase expression in women exposed to levonorgestrel-releasing intrauterine system for a short or prolonged period of time. Hum Reprod 2009; 24:113–121.
- Kim CR, Martinez-Maza O, Magpantay L, et al. Immunologic evaluation of the endometrium with a levonorgestrel intrauterine device in solid organ transplant women and healthy controls. Contraception 2016; 94:534–540.
- Ramhendar T, Byrne P. Use of the levonorgestrel-releasing intrauterine system in renal transplant recipients: a retrospective case review. Contraception 2012; 86:288–289.
- Huguelet PS, Sheehan C, Spitzer RF, Scott S. Use of the levonorgestrel 52-mg intrauterine system in adolescent and young adult solid organ transplant recipients: a case series. Contraception 2017; 95:378–381.
- Peterson HB, Xia Z, Hughes JM, Wilcox LS, Tylor LR, Trussell J. The risk of pregnancy after tubal sterilization: findings from the US Collaborative Review of Sterilization. Am J Obstet Gynecol 1996; 174:1161–1168.
- Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int 2007; 18:1319–1328.
- Krajewski C, Sucato G. Reproductive health care after transplantation. Best Pract Res Clin Obstet Gynaecol 2014; 28:1222–1234.
- World Health Organization. Medical eligibility criteria for contraceptive use. Fifth edition 2015. http://apps.who.int/iris/bitstream/10665/172915/1/WHO_RHR_15.07_eng.pdf. Accessed July 27, 2017.
- Pietrzak B, Bobrowska K, Jabiry-Zieniewicz Z, et al. Oral and transdermal hormonal contraception in women after kidney transplantation. Transplant Proc 2007; 39:2759–2762.
- Jain J, Jakimiuk AJ, Bode FR, Ross D, Kaunitz AM. Contraceptive efficacy and safety of DMPA-SC. Contraception 2004; 70:269–275.
- Vessey M, Painter R, Yeates D. Mortality in relation to oral contraceptive use and cigarette smoking. Lancet 2003; 362:185–191.
- van den Heuvel MW, van Bragt AJ, Alnabawy AK, Kaptein MC. Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptive formulations: the vaginal ring, the transdermal patch and an oral contraceptive. Contraception 2005; 72:168–174.
- Jick SS, Kaye JA, Russmann S, Jick H. Risk of nonfatal venous thromboembolism in women using a contraceptive transdermal patch and oral contraceptives containing norgestimate and 35 microg of ethinyl estradiol. Contraception 2006; 73:223–228.
- Jick S, Kaye JA, Li L, Jick H. Further results on the risk of nonfatal venous thromboembolism in users of the contraceptive transdermal patch compared to users of oral contraceptives containing norgestimate and 35 microg of ethinyl estradiol. Contraception 2007; 76:4–7.
- Estes CM, Westhoff C. Contraception for the transplant patient. Semin Perinatol 2007; 31:372–377.
- Cole JA, Norman H, Doherty M, Walker AM. Venous thromboembolism, myocardial infarction, and stroke among transdermal contraceptive system users. Obstet Gynecol 2007; 109:339–346.
- Paternoster DM, Riboni F, Bertolino M, et al. The contraceptive vaginal ring in women with renal and liver transplantation: analysis of preliminary results. Transplant Proc 2010; 42:1162–1165.
- Centers for Disease Control and Prevention (CDC). Summary chart of US medical eligibility criteria for contraceptive use. https://www.cdc.gov/reproductivehealth/unintendedpregnancy/pdf/legal_summary-chart_english_final_tag508.pdf. Accessed July 17, 2017.
KEY POINTS
- The number of solid-organ transplants in US women of childbearing age has increased over the past 20 years.
- Women should wait at least 1 year after receiving a solid-organ transplant before attempting to become pregnant, and then should do so only when cleared by the transplant team and obstetrician, with close monitoring.
- The various types of contraception can be grouped by their effectiveness and by the medical eligibility criteria set by the US Centers for Disease Control and Prevention.
- Transplant recipients of childbearing age should use 2 contraceptive methods concurrently, one of which should be condoms.
Create an effective social media campaign to market your practice: Here’s how
Developing an effective social media marketing campaign can expand your practice to bring you more of the type of patient you want to treat. Although ObGyns are often not trained in marketing, we can bring our practices to the attention of women who need our services with a few simple processes.
The American Marketing Association defines marketing as “the activity, set of institutions, and processes for creating, communicating, delivering, and exchanging offerings that have value for customers, clients, partners, and society at large.”1 Social media is described as various forms of online and mobile electronic communication with user-generated content.2 Social media marketing is the application of traditional marketing strategies to a social media platform. Delivering an effective social media marketing campaign requires focused targeting of a particular community to match the needs of those patients with the value of services and products your practice provides.
By communicating and connecting with the spoken and unspoken needs and desires of potential patients, you will generate greater enthusiasm for your medical services. Social media marketing benefits include: accessibility, low cost, the ability to build brand recognition and social capital, and the availability of analytics that provide large amounts of data to measure the effectiveness of the campaign.3
Though social media is pervasive, the medical community has not rapidly embraced it for marketing.4,5 Creating a social media strategy, rather than randomly or impulsively posting on social media, allows for more effective marketing. The discussion here focuses on Facebook, which has 2 billion monthly users,6 but these strategies and tactics can be applied to any social media platform, including YouTube, Instagram, and Twitter.7
Use Facebook to create a business page
Your medical practice needs to have a Facebook account and a Facebook page, separate from your personal account. A business-related Facebook page is similar to a personal Facebook profile except that pages are designed for organizations, brands, businesses, and public figures to share photos, stories, and events with the public.
If you do not have a Facebook account, you can create a new account and profile at http://www.facebook.com. After creating a profile, click on the “create a Facebook page” link. Follow the instructions and select the page category you would like to create; most physicians would select the “Company,” “Organization,” or “Institution” category. Next, follow the instructions to complete the registration.8 Once your Facebook page is created, build an audience asking others to “like” your page. Start posting content and use hashtags in your posts to make them discoverable to others (ie, #fibroids #noscar #singlesitesurgery).9
Related article:
Using the Internet in your practice. Part 2: Generating new patients using social media
One benefit to having a practice-based Facebook page is the automated visible analytics that come with the page, which are not available for personal profiles. When you write a post or upload a photo or video, Facebook provides the demographics of those engaged with your posts plus analytics on that post, including the number of people who viewed the post, clicked on a photo, and viewed the video for more than 3 seconds.
Read how to get patients interested in your practice
Develop a social media marketing strategy
There are several key factors to consider when planning a strategy. First, know the mission of your organization and the specific service, value, or benefit you would like to provide to the targeted community.8
Segment, target, and position (STP)
It is tempting to try to reach out to all women because your ObGyn practice entails pre‑natal care, family planning, and gynecologic surgery, but by narrowing your target audience, your campaign will be better focused. A very specific target audience can reduce the costs for “boosting” (paid promotion of your posts on Facebook to a chosen audience based on demographics, interests, and behaviors) your posts and improve your return on investment (ROI).
Create different marketing campaigns, but focus on one at a time. Decide on the ideal patient you want to serve in your practice. The more detailed and focused you are about the demographics and type of medical needs to be served, the better you can target this patient.10
Segment. Divide the communities you are considering into different segments. For instance, even though you may do obstetrics and gynecologic surgery, consider breaking up the campaign to focus on 1 specific group, such as those interested in fibroid management.
Target. Identify the kinds of communities where you might find this patient. For example, if you want to focus on laparoscopic hysterectomies or myomectomies, start looking on Facebook for groups, pages, or website discussion boards or blogs that discuss abnormal uterine bleeding or fibroids and follow those pages.
Also, think about what other characteristics are associated with these ideal patients. For example, you might narrow it down to perimenopausal women with fibroids. A potential targeted group could be 40- to 50-year-old women who participate in yoga or running who have concerns about fibroids interfering in their active lifestyle. Perhaps this type of patient would want a minimally invasive surgical approach. A holistic health activist might be interested in nonsurgical management of fibroids.
Position. Once you have identified the specific community to target, position your practice within the community with the value proposition you are offering. For example, as an ObGyn who is focused on surgery, your position might be that your practice will provide the best experience for those medical services, with specific counseling to patients about resuming their active lifestyle.
Related article:
Four pillars of a successful practice: 2. Attract new patients
Get your potential patient to “raise her hand.” In the campaign, you are not trying to convince everyone up front to schedule an appointment from one post. First, try to get people who may be interested in your service(s) to “raise their hands.” Once your target market has expressed interest, either by their likes of your post, likes of your page, or other engagement, reach out to them with links for more information, such as free fibroid surgery education materials located on your website. On your website, create an opt-in page asking them to register their email address; once you have a compiled email list, send out monthly newsletters on your practice.11
Read how to guide patients to your office
Understand that marketing is a process
Think of marketing as an overall process in which you are guiding potential patients to come to your office. Your campaign has several steps; recognize that just one post will not make a huge difference. Use Facebook analytics to measure cost per engagement to calculate your return on investment and the campaign’s effectiveness, and revise as necessary.
Rather than just considering social media as a soap box to advertise your practice, break up the marketing process into 3 units: the before unit, the during unit, and the after unit.11 The word “unit” denotes the service, benefit, or product you are providing.
The before unit refers to the initial marketing that identifies potential patients—initially getting them to raise their hands and ultimately building an audience. (Once a potential patient provides her email address, you can send her a monthly newsletter or updates about your practice to continue the engagement.) Statistics show that an ObGyn needs to have 7 contacts, on average, with a patient over 18 months to “penetrate” her consciousness in a given market.12 Of course if there is an urgent or emergent need to see a physician, that timeline would be much shorter.
The during unit occurs when the patient comes to your practice and service is being provided. Since you know what she is coming for, you can create informational packets focused on her particular needs, perhaps about different management options for fibroids.
The after unit includes following up with the patient in some automated way. For those being treated for fibroids, it may be a reminder email that discusses the value of follow-up ultrasonography or the various kinds of surgical interventions for fibroids.
In order to continue your campaign, it is helpful to have a designated social media manager who will continue the social media posts and engagement.
When creating the posts, consider developing prescheduled assets (posts that are already produced with photos or links to articles), which can be done through Facebook or Hootsuite (http://www.hootsuite.com).
Manage the risks of social media interaction
There are risks associated with social media. Some things to consider are:
- Policy. Develop a policy for your practice; if you work for an institution, align your policy with the institution’s.
- Postings. Supervise content being posted. Never allow social media to be placed by someone without supervision. Either you should do this or assign a manager to be accountable to check on social media interactions so that any inappropriate comments can be addressed immediately.
- Privacy. Never mention patients’ private health information or use the platform to publicly engage with a patient or future patient about their care. Do not post any references to patients or their photos without written consent.
- Images. Use photographs and other images properly: obtain releases and obey copyright laws.
Related article:
Your patients are talking: Isn’t it time you take responsibility for your online reputation?
Bottom line
Social media is a powerful platform. Combined with good marketing strategies, social media campaigns can have a significant impact on expanding your practice to offer the kind of medical services you want to provide.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Definition of Marketing. American Marketing Association website. https://www.ama.org/AboutAMA/Pages/Definition-of-Marketing.aspx. Published July 2013. Accessed August 8, 2017.
- Kaplan AH, Haenlein M. Users of the world, unite! The challenges and opportunities of social media. Business Horiz. 2010;53(1):59–68.
- Lin KY, Lu HP. Intention to continue using Facebook fan pages from the perspective of social capital theory. Cyberpsychol Behav Soc Netw. 2011;14(10):565–570.
- Hawn C. Take two aspirin and tweet me in the morning: how Twitter, Facebook, and other social media are reshaping health care. Health Aff (Millwood). 2009;28(2):361–368.
- Wheeler CK, Said H, Prucz R, Rodrich RJ, Mathes DW. Social media in plastic surgery practices: emerging trends in North America. Aesthet Surg J. 2011;31(4):435–441.
- Nowak M, Spiller G. Two billion people coming together on Facebook. Facebook Newsroom. https://newsroom.fb.com/news/2017/06/two-billion-people-coming-together-on-facebook/. Published June 27, 2017. Accessed August 8, 2017.
- Adamson A. No contest: Twitter and Facebook can both play a role in branding. Forbes. http://www.forbes.com/2009/05/06/twitter-facebook-branding-leadership-cmo-network-adamson.html. Published May 6, 2009. Accessed August 8, 2017.
- Kim DS. Harness social media, enhance your practice. Contemp Obstet Gynecol. 2012;57(7):40–42,44–46.
- Wolf J. Social Media: Master, Manipulate, And Dominate Social Media Marketing Facebook, Twitter, YouTube, Instagram And LinkedIn. Createspace Independent Publishing Platform; 2015:129–143.
- Kotler PT, Keller KL. Marketing Management. 12th ed. Upper Saddle River, NJ: Prentice Hall; 2006:239–268.
- Jackson DP. Sunday marketing matinee: I love marketing live–Before, during, and after unit thinking. http://ilovemarketing.com/sunday-marketing-matineei-love-marketing-live-before-during-and-after-unit-thinking/. Accessed July 24, 2017.
- Payne D. How many contacts does it take before someone buys your product? Business Insider website. http://www.businessinsider.com/how-many-contacts-does-it-take-before-someone-buys-your-product-2011-7. Published July 12, 2011. Accessed August 8, 2017.
Developing an effective social media marketing campaign can expand your practice to bring you more of the type of patient you want to treat. Although ObGyns are often not trained in marketing, we can bring our practices to the attention of women who need our services with a few simple processes.
The American Marketing Association defines marketing as “the activity, set of institutions, and processes for creating, communicating, delivering, and exchanging offerings that have value for customers, clients, partners, and society at large.”1 Social media is described as various forms of online and mobile electronic communication with user-generated content.2 Social media marketing is the application of traditional marketing strategies to a social media platform. Delivering an effective social media marketing campaign requires focused targeting of a particular community to match the needs of those patients with the value of services and products your practice provides.
By communicating and connecting with the spoken and unspoken needs and desires of potential patients, you will generate greater enthusiasm for your medical services. Social media marketing benefits include: accessibility, low cost, the ability to build brand recognition and social capital, and the availability of analytics that provide large amounts of data to measure the effectiveness of the campaign.3
Though social media is pervasive, the medical community has not rapidly embraced it for marketing.4,5 Creating a social media strategy, rather than randomly or impulsively posting on social media, allows for more effective marketing. The discussion here focuses on Facebook, which has 2 billion monthly users,6 but these strategies and tactics can be applied to any social media platform, including YouTube, Instagram, and Twitter.7
Use Facebook to create a business page
Your medical practice needs to have a Facebook account and a Facebook page, separate from your personal account. A business-related Facebook page is similar to a personal Facebook profile except that pages are designed for organizations, brands, businesses, and public figures to share photos, stories, and events with the public.
If you do not have a Facebook account, you can create a new account and profile at http://www.facebook.com. After creating a profile, click on the “create a Facebook page” link. Follow the instructions and select the page category you would like to create; most physicians would select the “Company,” “Organization,” or “Institution” category. Next, follow the instructions to complete the registration.8 Once your Facebook page is created, build an audience asking others to “like” your page. Start posting content and use hashtags in your posts to make them discoverable to others (ie, #fibroids #noscar #singlesitesurgery).9
Related article:
Using the Internet in your practice. Part 2: Generating new patients using social media
One benefit to having a practice-based Facebook page is the automated visible analytics that come with the page, which are not available for personal profiles. When you write a post or upload a photo or video, Facebook provides the demographics of those engaged with your posts plus analytics on that post, including the number of people who viewed the post, clicked on a photo, and viewed the video for more than 3 seconds.
Read how to get patients interested in your practice
Develop a social media marketing strategy
There are several key factors to consider when planning a strategy. First, know the mission of your organization and the specific service, value, or benefit you would like to provide to the targeted community.8
Segment, target, and position (STP)
It is tempting to try to reach out to all women because your ObGyn practice entails pre‑natal care, family planning, and gynecologic surgery, but by narrowing your target audience, your campaign will be better focused. A very specific target audience can reduce the costs for “boosting” (paid promotion of your posts on Facebook to a chosen audience based on demographics, interests, and behaviors) your posts and improve your return on investment (ROI).
Create different marketing campaigns, but focus on one at a time. Decide on the ideal patient you want to serve in your practice. The more detailed and focused you are about the demographics and type of medical needs to be served, the better you can target this patient.10
Segment. Divide the communities you are considering into different segments. For instance, even though you may do obstetrics and gynecologic surgery, consider breaking up the campaign to focus on 1 specific group, such as those interested in fibroid management.
Target. Identify the kinds of communities where you might find this patient. For example, if you want to focus on laparoscopic hysterectomies or myomectomies, start looking on Facebook for groups, pages, or website discussion boards or blogs that discuss abnormal uterine bleeding or fibroids and follow those pages.
Also, think about what other characteristics are associated with these ideal patients. For example, you might narrow it down to perimenopausal women with fibroids. A potential targeted group could be 40- to 50-year-old women who participate in yoga or running who have concerns about fibroids interfering in their active lifestyle. Perhaps this type of patient would want a minimally invasive surgical approach. A holistic health activist might be interested in nonsurgical management of fibroids.
Position. Once you have identified the specific community to target, position your practice within the community with the value proposition you are offering. For example, as an ObGyn who is focused on surgery, your position might be that your practice will provide the best experience for those medical services, with specific counseling to patients about resuming their active lifestyle.
Related article:
Four pillars of a successful practice: 2. Attract new patients
Get your potential patient to “raise her hand.” In the campaign, you are not trying to convince everyone up front to schedule an appointment from one post. First, try to get people who may be interested in your service(s) to “raise their hands.” Once your target market has expressed interest, either by their likes of your post, likes of your page, or other engagement, reach out to them with links for more information, such as free fibroid surgery education materials located on your website. On your website, create an opt-in page asking them to register their email address; once you have a compiled email list, send out monthly newsletters on your practice.11
Read how to guide patients to your office
Understand that marketing is a process
Think of marketing as an overall process in which you are guiding potential patients to come to your office. Your campaign has several steps; recognize that just one post will not make a huge difference. Use Facebook analytics to measure cost per engagement to calculate your return on investment and the campaign’s effectiveness, and revise as necessary.
Rather than just considering social media as a soap box to advertise your practice, break up the marketing process into 3 units: the before unit, the during unit, and the after unit.11 The word “unit” denotes the service, benefit, or product you are providing.
The before unit refers to the initial marketing that identifies potential patients—initially getting them to raise their hands and ultimately building an audience. (Once a potential patient provides her email address, you can send her a monthly newsletter or updates about your practice to continue the engagement.) Statistics show that an ObGyn needs to have 7 contacts, on average, with a patient over 18 months to “penetrate” her consciousness in a given market.12 Of course if there is an urgent or emergent need to see a physician, that timeline would be much shorter.
The during unit occurs when the patient comes to your practice and service is being provided. Since you know what she is coming for, you can create informational packets focused on her particular needs, perhaps about different management options for fibroids.
The after unit includes following up with the patient in some automated way. For those being treated for fibroids, it may be a reminder email that discusses the value of follow-up ultrasonography or the various kinds of surgical interventions for fibroids.
In order to continue your campaign, it is helpful to have a designated social media manager who will continue the social media posts and engagement.
When creating the posts, consider developing prescheduled assets (posts that are already produced with photos or links to articles), which can be done through Facebook or Hootsuite (http://www.hootsuite.com).
Manage the risks of social media interaction
There are risks associated with social media. Some things to consider are:
- Policy. Develop a policy for your practice; if you work for an institution, align your policy with the institution’s.
- Postings. Supervise content being posted. Never allow social media to be placed by someone without supervision. Either you should do this or assign a manager to be accountable to check on social media interactions so that any inappropriate comments can be addressed immediately.
- Privacy. Never mention patients’ private health information or use the platform to publicly engage with a patient or future patient about their care. Do not post any references to patients or their photos without written consent.
- Images. Use photographs and other images properly: obtain releases and obey copyright laws.
Related article:
Your patients are talking: Isn’t it time you take responsibility for your online reputation?
Bottom line
Social media is a powerful platform. Combined with good marketing strategies, social media campaigns can have a significant impact on expanding your practice to offer the kind of medical services you want to provide.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Developing an effective social media marketing campaign can expand your practice to bring you more of the type of patient you want to treat. Although ObGyns are often not trained in marketing, we can bring our practices to the attention of women who need our services with a few simple processes.
The American Marketing Association defines marketing as “the activity, set of institutions, and processes for creating, communicating, delivering, and exchanging offerings that have value for customers, clients, partners, and society at large.”1 Social media is described as various forms of online and mobile electronic communication with user-generated content.2 Social media marketing is the application of traditional marketing strategies to a social media platform. Delivering an effective social media marketing campaign requires focused targeting of a particular community to match the needs of those patients with the value of services and products your practice provides.
By communicating and connecting with the spoken and unspoken needs and desires of potential patients, you will generate greater enthusiasm for your medical services. Social media marketing benefits include: accessibility, low cost, the ability to build brand recognition and social capital, and the availability of analytics that provide large amounts of data to measure the effectiveness of the campaign.3
Though social media is pervasive, the medical community has not rapidly embraced it for marketing.4,5 Creating a social media strategy, rather than randomly or impulsively posting on social media, allows for more effective marketing. The discussion here focuses on Facebook, which has 2 billion monthly users,6 but these strategies and tactics can be applied to any social media platform, including YouTube, Instagram, and Twitter.7
Use Facebook to create a business page
Your medical practice needs to have a Facebook account and a Facebook page, separate from your personal account. A business-related Facebook page is similar to a personal Facebook profile except that pages are designed for organizations, brands, businesses, and public figures to share photos, stories, and events with the public.
If you do not have a Facebook account, you can create a new account and profile at http://www.facebook.com. After creating a profile, click on the “create a Facebook page” link. Follow the instructions and select the page category you would like to create; most physicians would select the “Company,” “Organization,” or “Institution” category. Next, follow the instructions to complete the registration.8 Once your Facebook page is created, build an audience asking others to “like” your page. Start posting content and use hashtags in your posts to make them discoverable to others (ie, #fibroids #noscar #singlesitesurgery).9
Related article:
Using the Internet in your practice. Part 2: Generating new patients using social media
One benefit to having a practice-based Facebook page is the automated visible analytics that come with the page, which are not available for personal profiles. When you write a post or upload a photo or video, Facebook provides the demographics of those engaged with your posts plus analytics on that post, including the number of people who viewed the post, clicked on a photo, and viewed the video for more than 3 seconds.
Read how to get patients interested in your practice
Develop a social media marketing strategy
There are several key factors to consider when planning a strategy. First, know the mission of your organization and the specific service, value, or benefit you would like to provide to the targeted community.8
Segment, target, and position (STP)
It is tempting to try to reach out to all women because your ObGyn practice entails pre‑natal care, family planning, and gynecologic surgery, but by narrowing your target audience, your campaign will be better focused. A very specific target audience can reduce the costs for “boosting” (paid promotion of your posts on Facebook to a chosen audience based on demographics, interests, and behaviors) your posts and improve your return on investment (ROI).
Create different marketing campaigns, but focus on one at a time. Decide on the ideal patient you want to serve in your practice. The more detailed and focused you are about the demographics and type of medical needs to be served, the better you can target this patient.10
Segment. Divide the communities you are considering into different segments. For instance, even though you may do obstetrics and gynecologic surgery, consider breaking up the campaign to focus on 1 specific group, such as those interested in fibroid management.
Target. Identify the kinds of communities where you might find this patient. For example, if you want to focus on laparoscopic hysterectomies or myomectomies, start looking on Facebook for groups, pages, or website discussion boards or blogs that discuss abnormal uterine bleeding or fibroids and follow those pages.
Also, think about what other characteristics are associated with these ideal patients. For example, you might narrow it down to perimenopausal women with fibroids. A potential targeted group could be 40- to 50-year-old women who participate in yoga or running who have concerns about fibroids interfering in their active lifestyle. Perhaps this type of patient would want a minimally invasive surgical approach. A holistic health activist might be interested in nonsurgical management of fibroids.
Position. Once you have identified the specific community to target, position your practice within the community with the value proposition you are offering. For example, as an ObGyn who is focused on surgery, your position might be that your practice will provide the best experience for those medical services, with specific counseling to patients about resuming their active lifestyle.
Related article:
Four pillars of a successful practice: 2. Attract new patients
Get your potential patient to “raise her hand.” In the campaign, you are not trying to convince everyone up front to schedule an appointment from one post. First, try to get people who may be interested in your service(s) to “raise their hands.” Once your target market has expressed interest, either by their likes of your post, likes of your page, or other engagement, reach out to them with links for more information, such as free fibroid surgery education materials located on your website. On your website, create an opt-in page asking them to register their email address; once you have a compiled email list, send out monthly newsletters on your practice.11
Read how to guide patients to your office
Understand that marketing is a process
Think of marketing as an overall process in which you are guiding potential patients to come to your office. Your campaign has several steps; recognize that just one post will not make a huge difference. Use Facebook analytics to measure cost per engagement to calculate your return on investment and the campaign’s effectiveness, and revise as necessary.
Rather than just considering social media as a soap box to advertise your practice, break up the marketing process into 3 units: the before unit, the during unit, and the after unit.11 The word “unit” denotes the service, benefit, or product you are providing.
The before unit refers to the initial marketing that identifies potential patients—initially getting them to raise their hands and ultimately building an audience. (Once a potential patient provides her email address, you can send her a monthly newsletter or updates about your practice to continue the engagement.) Statistics show that an ObGyn needs to have 7 contacts, on average, with a patient over 18 months to “penetrate” her consciousness in a given market.12 Of course if there is an urgent or emergent need to see a physician, that timeline would be much shorter.
The during unit occurs when the patient comes to your practice and service is being provided. Since you know what she is coming for, you can create informational packets focused on her particular needs, perhaps about different management options for fibroids.
The after unit includes following up with the patient in some automated way. For those being treated for fibroids, it may be a reminder email that discusses the value of follow-up ultrasonography or the various kinds of surgical interventions for fibroids.
In order to continue your campaign, it is helpful to have a designated social media manager who will continue the social media posts and engagement.
When creating the posts, consider developing prescheduled assets (posts that are already produced with photos or links to articles), which can be done through Facebook or Hootsuite (http://www.hootsuite.com).
Manage the risks of social media interaction
There are risks associated with social media. Some things to consider are:
- Policy. Develop a policy for your practice; if you work for an institution, align your policy with the institution’s.
- Postings. Supervise content being posted. Never allow social media to be placed by someone without supervision. Either you should do this or assign a manager to be accountable to check on social media interactions so that any inappropriate comments can be addressed immediately.
- Privacy. Never mention patients’ private health information or use the platform to publicly engage with a patient or future patient about their care. Do not post any references to patients or their photos without written consent.
- Images. Use photographs and other images properly: obtain releases and obey copyright laws.
Related article:
Your patients are talking: Isn’t it time you take responsibility for your online reputation?
Bottom line
Social media is a powerful platform. Combined with good marketing strategies, social media campaigns can have a significant impact on expanding your practice to offer the kind of medical services you want to provide.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Definition of Marketing. American Marketing Association website. https://www.ama.org/AboutAMA/Pages/Definition-of-Marketing.aspx. Published July 2013. Accessed August 8, 2017.
- Kaplan AH, Haenlein M. Users of the world, unite! The challenges and opportunities of social media. Business Horiz. 2010;53(1):59–68.
- Lin KY, Lu HP. Intention to continue using Facebook fan pages from the perspective of social capital theory. Cyberpsychol Behav Soc Netw. 2011;14(10):565–570.
- Hawn C. Take two aspirin and tweet me in the morning: how Twitter, Facebook, and other social media are reshaping health care. Health Aff (Millwood). 2009;28(2):361–368.
- Wheeler CK, Said H, Prucz R, Rodrich RJ, Mathes DW. Social media in plastic surgery practices: emerging trends in North America. Aesthet Surg J. 2011;31(4):435–441.
- Nowak M, Spiller G. Two billion people coming together on Facebook. Facebook Newsroom. https://newsroom.fb.com/news/2017/06/two-billion-people-coming-together-on-facebook/. Published June 27, 2017. Accessed August 8, 2017.
- Adamson A. No contest: Twitter and Facebook can both play a role in branding. Forbes. http://www.forbes.com/2009/05/06/twitter-facebook-branding-leadership-cmo-network-adamson.html. Published May 6, 2009. Accessed August 8, 2017.
- Kim DS. Harness social media, enhance your practice. Contemp Obstet Gynecol. 2012;57(7):40–42,44–46.
- Wolf J. Social Media: Master, Manipulate, And Dominate Social Media Marketing Facebook, Twitter, YouTube, Instagram And LinkedIn. Createspace Independent Publishing Platform; 2015:129–143.
- Kotler PT, Keller KL. Marketing Management. 12th ed. Upper Saddle River, NJ: Prentice Hall; 2006:239–268.
- Jackson DP. Sunday marketing matinee: I love marketing live–Before, during, and after unit thinking. http://ilovemarketing.com/sunday-marketing-matineei-love-marketing-live-before-during-and-after-unit-thinking/. Accessed July 24, 2017.
- Payne D. How many contacts does it take before someone buys your product? Business Insider website. http://www.businessinsider.com/how-many-contacts-does-it-take-before-someone-buys-your-product-2011-7. Published July 12, 2011. Accessed August 8, 2017.
- Definition of Marketing. American Marketing Association website. https://www.ama.org/AboutAMA/Pages/Definition-of-Marketing.aspx. Published July 2013. Accessed August 8, 2017.
- Kaplan AH, Haenlein M. Users of the world, unite! The challenges and opportunities of social media. Business Horiz. 2010;53(1):59–68.
- Lin KY, Lu HP. Intention to continue using Facebook fan pages from the perspective of social capital theory. Cyberpsychol Behav Soc Netw. 2011;14(10):565–570.
- Hawn C. Take two aspirin and tweet me in the morning: how Twitter, Facebook, and other social media are reshaping health care. Health Aff (Millwood). 2009;28(2):361–368.
- Wheeler CK, Said H, Prucz R, Rodrich RJ, Mathes DW. Social media in plastic surgery practices: emerging trends in North America. Aesthet Surg J. 2011;31(4):435–441.
- Nowak M, Spiller G. Two billion people coming together on Facebook. Facebook Newsroom. https://newsroom.fb.com/news/2017/06/two-billion-people-coming-together-on-facebook/. Published June 27, 2017. Accessed August 8, 2017.
- Adamson A. No contest: Twitter and Facebook can both play a role in branding. Forbes. http://www.forbes.com/2009/05/06/twitter-facebook-branding-leadership-cmo-network-adamson.html. Published May 6, 2009. Accessed August 8, 2017.
- Kim DS. Harness social media, enhance your practice. Contemp Obstet Gynecol. 2012;57(7):40–42,44–46.
- Wolf J. Social Media: Master, Manipulate, And Dominate Social Media Marketing Facebook, Twitter, YouTube, Instagram And LinkedIn. Createspace Independent Publishing Platform; 2015:129–143.
- Kotler PT, Keller KL. Marketing Management. 12th ed. Upper Saddle River, NJ: Prentice Hall; 2006:239–268.
- Jackson DP. Sunday marketing matinee: I love marketing live–Before, during, and after unit thinking. http://ilovemarketing.com/sunday-marketing-matineei-love-marketing-live-before-during-and-after-unit-thinking/. Accessed July 24, 2017.
- Payne D. How many contacts does it take before someone buys your product? Business Insider website. http://www.businessinsider.com/how-many-contacts-does-it-take-before-someone-buys-your-product-2011-7. Published July 12, 2011. Accessed August 8, 2017.
Fast Tracks
- Open a business Facebook page, compile an email list from those who like your postings, and send out useful information and updates on your practice
- Develop an office policy for social media, supervise postings, ensure patient privacy, and obey copyright laws
The etiology of premenstrual dysphoric disorder: 5 interwoven pieces
In an age when psychiatry strives to identify the biologic causes of disease, studying endocrine-related mood disorders is particularly intriguing. DSM-5 defines premenstrual dysphoric disorder (PMDD) as a depressive disorder, with a 12-month prevalence ranging from 1.8% to 5.8% among women who menstruate.1-3 Factors that differentiate PMDD from other affective disorders include etiology, duration, and temporal relationship with the menstrual cycle.
PMDD is a disorder of consistent yet intermittent change in mental health and functionality. Therefore, it may be underdiagnosed and consequently undertreated if a psychiatric evaluation does not coincide with symptom occurrence or if patients do not understand that intermittent symptoms are treatable.
This article summarizes what is known about the etiology of PMDD. Although there are several treatments for PMDD, many women experience adverse effects or incomplete effectiveness. Further understanding of this disorder may lead to more efficacious treatments. Additionally, understanding the pathophysiology of PMDD might shed a light on the etiology of other disorders that are temporally related to reproductive life changes, such as pregnancy-, postpartum-, or menopause-related affective dysregulation.
Making the diagnosis
The diagnosis of PMDD is made when a patient has at least 5 of 11 specific symptoms that occur during the week before onset of menses, improve within a few days after the onset of menses (shown as the “PMDD Hazard Zone” in Figure 1), and are minimal or absent post-menses.3 Symptoms should be tracked prospectively for at least 2 menstrual cycles in order to confirm the diagnosis (one must be an affective symptom and another must be a behavioral/cognitive symptom).3
The affective symptoms are:
- lability of affect (eg, sudden sadness, tearfulness, or sensitivity to rejection)
- irritability, anger, or increased interpersonal conflicts
- depressed mood, hopelessness, or self- deprecating thoughts
- anxiety or tension, feeling “keyed up” or “on edge.”
The behavioral/cognitive symptoms are:
- decreased interest in usual activities (eg, work, hobbies, friends, school)
- difficulty concentrating
- lethargy, low energy, easy fatigability
- change in appetite, overeating, food cravings
- hypersomnia or insomnia
- feeling overwhelmed or out of control
- physical symptoms (breast tenderness or swelling, headache, joint or muscle pain, bloating, weight gain).
Ruling out premenstrual exacerbation (PME). Perhaps the most common cause for misdiagnosis of PMDD is failing to rule out PME of another underlying or comorbid condition (Figure 2). In many women who have a primary mood or anxiety disorder, the late luteal phase is a vulnerable time. A patient might be coping with untreated anxiety, for example, but the symptoms become unbearable the week before menstruation begins, which is likely when she seeks help. At this stage, a diagnosis of PMDD should be provisional at best. Often, PME is treated by treating the underlying condition. Therefore, a full diagnostic psychiatric interview is important to first rule out other underlying psychiatric disorders. PMDD is diagnosed if the premenstrual symptoms persist for 2 consecutive months after treating the suspected mood or anxiety disorder. Patients can use one of many PMDD daily symptom charts available online. Alternatively, they can use a cycle-tracking mobile phone application to correlate their symptoms with their cycle and share this information with their providers.
Consider these 5 interwoven pieces
The many variables that contribute to the pathophysiology of PMDD overlap and should be considered connecting pieces in the puzzle that is the etiology of this disorder (Figure 3). In reviewing the literature, we have identified 5 topics likely to be major contributors to this disorder:
- genetic susceptibility
- progesterone and allopregnanolone (ALLO)
- estrogen, serotonin, and brain-derived neurotrophic factor (BDNF)
- putative brain structural and functional differences
- further involvement of the hypothalamic–pituitary–adrenal (HPA) axis and hypothalamic–pituitary–gonadal (HPG) axis: trauma, resiliency, and inflammation.
Genetic susceptibility. PMDD is thought to have a heritability range between 30% to 80%.3 This is demonstrated by family and twin studies4-7 and specific genetic studies.8 The involvement of genetics means an underlying neurobiologic pathophysiology is in place.
Estrogen receptor alpha (ESR1) gene. Huo et al8 found an associated variation in ESR1 in women with PMDD compared with controls. They speculated that because ESR1 is important for arousal, if dysfunctional, this gene could be implicated in somatic as well as affective and cognitive deficits in PMDD patients. In another study, investigators reported a relationship between PMDD and heritable personality traits, as well as a link between these traits and ESR1 polymorphic variants.1 They suggested that personality traits (independent of affective state) might be used to distinguish patients with PMDD from controls.1
Studies on serotonin gene polymorphism and serotonin transporter genotype. Although a study of serotonin gene polymorphism did not find an association between serotonin1A gene polymorphism and PMDD, it did show that the presence of at least 1 C allele was associated with a 2.5-fold increased risk of PMDD.9 Another study did not find an association between the serotonin transporter genotype 5-HTTLPR and PMDD.10 However, it showed lower frontocingulate cortex activation during the luteal phase of PMDD patients compared with controls, suggesting that PMDD is linked to impaired frontocingulate cortex activation induced by emotions during the luteal phase.10
Seasonal affective disorder (SAD) and PMDD have shared clinical features. A polymorphism in the serotonin transporter promoter gene 5-HTTLPR has been associated with SAD. One study found that patients with comorbid SAD and PMDD are genetically more vulnerable to comorbid affective disorders compared with patients who have SAD only.11
Progesterone and ALLO. Chronic exposure to progesterone and ALLO (a main progesterone metabolite) and rapid withdrawal from ovarian hormones may play a role in the etiology of PMDD. Much like alcohol or benzodiazepines, ALLO is a potent positive allosteric modulator of GABAA receptors and has sedative, anesthetic, and anxiolytic properties. In times of acute stress, increased ALLO is known to provide relief.12,13 However, in women with PMDD, this typical ALLO increase might not occur.14
Patients with PMDD have been reported to have decreased levels of ALLO in the luteal phase.15-17 In one study, women with highly symptomatic PMDD had lower levels of ALLO compared with women with less symptomatic PMDD.14 A gonadotropin-releasing hormone challenge study showed the increase in ALLO response was less in PMDD patients compared with controls.17 Luteal-phase ALLO concentrations are reported to be lower in women with premenstrual syndrome (PMS), a milder form of PMDD.14,17
The efficacy of selective serotonin reuptake inhibitors (SSRIs) for treating PMDD could be the result of the interaction of these medications with neuroactive steroids,18 possibly because SSRIs enhance the sensitivity of GABAA receptors or promote the formation of more ALLO (Figure 4).19-21
Estrogen, serotonin, and BDNF. Estrogen affects multiple neurotransmitter systems that regulate mood, cognition, sleep, and eating.22 Studying estrogen in context of PMDD is important because women with PMDD can have low mood, specific food cravings, and impaired cognitive function.
Estrogen–serotonin interactions are thought to be involved in hormone-related mood disorders such as perimenopausal depression and PMDD.23,24 However, the nature of their relationship is not yet fully understood. Ovariectomized animals have shown estrogen-induced changes related to serotonin metabolism, binding, and transmission in the regions of the brain involved in regulation of affect and cognition. Research in menopausal women also has provided some support for this interaction.24
Positron emission tomography studies in humans have found increased cortical serotonin binding modulated by levels of estrogen, similar to those previously seen in rat studies.24-27 One study showed an increased binding potential of serotonin in the cerebral cortex with estrogen treatment. This study further showed an even greater binding potential with estrogen plus progesterone, signaling a synergistic effect of the 2 hormones.28
SSRIs are an effective treatment for the irritability, anxiety, and mood swings of PMDD.29-30 Although the exact mechanism of action is unknown, the serotonergic properties are certainly of primary attention. For some PMDD patients, SSRIs work within hours to days, as opposed to days or weeks for patients with depression or anxiety, which suggests a separate or co-occurring mechanism of action is in place. In a double-blind, placebo-controlled crossover study, researchers administered the serotonin receptor antagonist metergoline to women with PMDD whose symptoms had remitted during treatment with fluoxetine and a group of healthy controls who were not receiving any medication.31 The women with PMDD experienced a return of symptoms 24 hours after treatment with metergoline but not with placebo; the controls experienced no mood changes.31
BDNF is a neurotransmitter linked to estrogen and likely related to PMDD. BDNF is critical for neurogenesis and is expressed in brain regions involved in learning and memory and also affects regulation.32 BDNF levels are increased by serotonergic antidepressants, affected by estradiol, and have cyclicity throughout the menstrual cycle.33-35
Putative brain structural and functional differences. Imaging studies have suggested differences in brain structure in women with PMDD, with a focus on the amygdala and the prefrontal cortex. Women with PMDD have greater gray matter volume in the posterior cerebellum,36 greater gray matter density of hippocampal cortex, and lower gray matter density in the parahippocampal cortex.37
Some studies have shown a functional variability of the amygdala’s response to stress in women with PMDD vs healthy controls.38,39 A proton magnetic resonance spectroscopy (1H-MRS) study of the displays the possibility of an altered GABAergic function in patients with PMDD.40
Patients will PMDD have enhanced dorsolateral prefrontal cortex reactivity when anticipating negative stimuli (but not to the actual exposure) during the luteal phase. A positive correlation between this reactivity and progesterone levels also was observed.41 Some researchers have suggested that prefrontal cortex dysfunction may be a risk factor for PMDD.42
HPA axis and HPG axis: Trauma, resiliency, inflammation. Altered cortisol levels (higher during the luteal phase43 and lower during times of stress14,44) suggest a possibly altered HPA axis in some women with PMDD. However, studies on this topic have been few and inconsistent.
Dysregulation of the HPG axis could cause vasomotor symptoms, sleep dysregulation, and mood symptoms during menopause; women with PMDD can also experience these symptoms. The influence of estrogen and progesterone on mood is also highly dependent on this axis.
Ultimately, the interplay between the HPA axis and the HPG axis is important. One study found that women with PMDD who had high serum ALLO levels (HPG-related) had blunted nocturnal cortisol levels (HPA-related) compared with healthy controls who had low ALLO levels.45
Significant stress and trauma exposure have been associated with PMDD. A study of 3,968 women found a history of trauma and PTSD were independently associated with PMDD.46 Another study of approximately 3,000 women found a strong correlation between abuse and PMS.47 However, a third study found no correlations between PMDD and trauma.48
Patients with a predisposition to PMDD may be more vulnerable to develop a posttraumatic stress-related disorder, perhaps due to decreased biologic resiliency. For example, the startle response (hypervigilance) has been shown to be different in women with PMDD. One study suggested that suboptimal production of premenstrual ALLO may lead to increased arousal and increased stress reactivity to psychosocial or environmental triggers.49
The possible role of inflammation in PMDD deserves further investigation. The luteal phase entails an increase in the production of proinflammatory markers.50,51 A 10-fold increase in progesterone is correlated with a 20% to 23% increase in C-reactive protein levels.52,53 Women with inflammatory diseases (eg, gingivitis or irritable bowel syndrome) show worsening of symptoms prior to menstruation.54-56 One study found increased levels of proinflammatory markers in women with PMDD compared with controls.57
Putting together the 5 pieces of the puzzle
Because PMDD is heritable, it must have an underlying neurobiologic pathophysiology. Brain imaging studies show differences in structure and function in women with PMDD across the menstrual cycle. Conversion of progesterone to ALLO and the GABAergic influence of this metabolite is a topic of interest in current research. Similarly, the role of estrogen and its connection to serotonin and other neurotransmitters such as BDNF have been implicated.
The link between a history of stress, trauma, and PMDD raises the question of biologic resiliency and illness in these patients, as it connects to the HPA and HPG axis and production of inflammatory stress hormones and steroid hormones and their metabolites. PMDD can be conceptualized as variable sensitivity to hormonal response to stress,58 thus contextualizing biochemical and psychological resiliency.
Further research is needed to clarify the possibility of a shared pathophysiology between endocrine-related mood disorders such as postpartum depression (PPD) and PMDD because current research is controversial.59,60 In PPD, women who are exposed to high levels of progesterone and estrogen during pregnancy (just like in the mid-luteal phase) have a sudden drop in these hormones postpartum.
The ‘withdrawal theory.’ The affective symptoms of PMDD resolve almost instantaneously after the start of menstruation. Perhaps this type of immediate relief is akin to substance use disorders and symptoms of withdrawal. It could be that reinstatement of a certain amount of gonadal steroids in the follicular phase of the cycle diminishes a withdrawal-like response to these steroids.
Currently, the main leading theory is that PMDD is a result of “an abnormal response to normal hormonal changes.”61 A new study also has shown that the change in estradiol/progesterone levels (vs the steady state) was associated with PMDD symptoms.62 Thinking of PMDD as a disorder of withdrawal offers an alternative (yet complementary) perspective to the current theory: PMDD may be caused by the absence or diminishing of the above-named hormones and their metabolites in the late luteal phase (in the context of developed “tolerance” during the early- to mid-luteal phase).
Considering the interplay between neurotransmitters and neurosteroids, both a “serotonin withdrawal theory” (caused by a drop in steroid hormones) and a “GABAergic withdrawal theory” (due to the decline in progesterone) could be proposed. This theory would be supported by the fact that SSRIs seem to mitigate symptoms of PMDD as well as the genetic association between PMDD and ESR1. It is more than likely that the “withdrawal” is caused by the interactions between estrogen-serotonin, progesterone-ALLO, and GABA receptors, and the complementary fashion in which progesterone and estrogen influence each other.
1. Miller A, Vo H, Huo L, et al. Estrogen receptor alpha (ESR-1) associations with psychological traits in women with PMDD and controls. J Psychiatr Res. 2010;44(12):788-794.
2. Epperson CN, Steiner M, Hartlage SA, et al. Premenstrual dysphoric disorder: evidence for a new category for DSM-5. Am J Psychiatry. 2012;169(5):465-475.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Wilson CA, Turner CW, Keye WR Jr. Firstborn adolescent daughters and mothers with and without premenstrual syndrome: a comparison. J Adolesc Health. 1991;12(2):130-137.
5. Kendler KS, Silberg JL, Neale MC, et al. Genetic and environmental factors in the aetiology of menstrual, premenstrual and neurotic symptoms: a population-based twin study. Psychol Med. 1992;22(1):85-100.
6. Condon JT. The premenstrual syndrome: a twin study. Br J Psychiatry. 1993;162:481-486.
7. Kendler KS, Karkowski LM, Corey LA, et al. Longitudinal population-based twin study of retrospectively reported premenstrual symptoms and lifetime major depression. Am J Psychiatry. 1998;155(9):1234-1240.
8. Huo L, Straub RE, Roca C, et al. Risk for premenstrual dysphoric disorder is associated with genetic variation in ESR1, the estrogen receptor alpha gene. Biol Psychiatry. 2007;62(8):925-933.
9. Dhingra V, Magnay JL, O’Brien PM, et al. Serotonin receptor 1A C(-1019)G polymorphism associated with premenstrual dysphoric disorder. Obstet Gynecol. 2007;110(4):788-792.
10. Comasco E, Hahn A, Ganger S, et al. Emotional fronto-cingulate cortex activation and brain derived neurotrophic factor polymorphism in premenstrual dysphoric disorder. Hum Brain Mapp. 2014;35(9):4450-4458.
11. Praschak-Rieder N, Willeit M, Winkler D, et al. Role of family history and 5-HTTLPR polymorphism in female seasonal affective disorder patients with and without premenstrual dysphoric disorder. Eur Neuropsychopharmacol. 2002;12(2):129-134.
12. Klatzkin RR, Morrow AL, Light KC, et al. Associations of histories of depression and PMDD diagnosis with allopregnanolone concentrations following the oral administration of micronized progesterone. Psychoneuroendocrinology. 2006;31(10):1208-1219.
13. Crowley SK, Girdler SS. Neurosteroid, GABAergic and hypothalamic pituitary adrenal (HPA) axis regulation: what is the current state of knowledge in humans? Psychopharmacology (Berl). 2014;231(17):3619-3634.
14. Girdler SS, Straneva PA, Light KC, et al. Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biol Psychiatry. 2001;49(9):788-797.
15. Rapkin AJ, Morgan M, Goldman L, et al. Progesterone metabolite allopregnanolone in women with premenstrual syndrome. Obstet Gynecol. 1997;90(5):709-714.
16. Bicíková M, Dibbelt L, Hill M, et al. Allopregnanolone in women with premenstrual syndrome. Horm Metab Res. 1998;30(4):227-230.
17. Monteleone P, Luisi S, Tonetti A, et al. Allopregnanolone concentrations and premenstrual syndrome. Eur J Endocrinol. 2000;142(3):269-273.
18. Steiner M, Steinberg S, Stewart D, et al. Fluoxetine in the treatment of premenstrual dysphoria. Canadian Fluoxetine/Premenstrual Dysphoria Collaborative Study Group. N Engl J Med. 1995;332(23):1529-1534.
19. Sundström I, Bäckström T. Citalopram increases pregnanolone sensitivity in patients with premenstrual syndrome: an open trial. Psychoneuroendocrinology. 1998;23(1):73-88.
20. Griffin LD, Mellon SH. Selective serotonin reuptake inhibitors directly alter activity of neurosteroidogenic enzymes. Proc Natl Acad Sci U S A. 1999;96(23):13512-13517.
21. Trauger JW, Jiang A, Stearns BA, et al. Kinetics of allopregnanolone formation catalyzed by human 3 alpha-hydroxysteroid dehydrogenase type III (AKR1C2). Biochemistry. 2002;41(45):13451-13459.
22. Shanmugan S, Epperson CN. Estrogen and the prefrontal cortex: towards a new understanding of estrogen’s effects on executive functions in the menopause transition. Hum Brain Mapp. 2014;35(3):847-865.
23. Rubinow DR, Schmidt PJ, Roca CA. Estrogen-serotonin interactions: implications for affective regulation. Biol Psychiatry. 1998;44(9):839-850.
24. Amin Z, Canli T, Epperson CN. Effect of estrogen-serotonin interactions on mood and cognition. Behav Cogn Neurosci Rev. 2005;4(1):43-58.
25. Cyr M, Bossé R, Di Paolo T. Gonadal hormones modulate 5-hydroxytryptamine2A receptors: emphasis on the rat frontal cortex. Neuroscience. 1998;83(3):829-836.
26. Fink G, Sumner BE, Rosie R, et al. Estrogen control of central neurotransmission: effect on mood, mental state, and memory. Cell Mol Neurobiol. 1996;16(3):325-344.
27. Sumner BE, Grant KE, Rosie R, et al. Effects of tamoxifen on serotonin transporter and 5-hydroxytryptamine(2A) receptor binding sites and mRNA levels in the brain of ovariectomized rats with or without acute estradiol replacement. Brain Res Mol Brain Res. 1999;73(1-2):119-128.
28. Moses-Kolko EL, Berga SL, Greer PJ, et al. Widespread increases of cortical serotonin type 2A receptor availability after hormone therapy in euthymic postmenopausal women. Fertil Steril. 2003;80(3):554-559.
29. Su TP, Schmidt PJ, Danaceau MA, et al. Fluoxetine in the treatment of premenstrual dysphoria. Neuropsychopharmacology. 1997;16(5):346-356.
30. Steinberg EM, Cardoso GM, Martinez PE, et al. Rapid response to fluoxetine in women with premenstrual dysphoric disorder. Depress Anxiety. 2012;29(6):531-540.
31. Roca CA, Schmidt PJ, Smith MJ, et al. Effects of metergoline on symptoms in women with premenstrual dysphoric disorder. Am J Psychiatry. 2002;159(11):1876-1881.
32. Gray JD, Milner TA, McEwen BS. Dynamic plasticity: the role of glucocorticoids, brain-derived neurotrophic factor and other trophic factors. Neuroscience. 2013;239:214-227.
33. Carbone DL, Handa RJ. Sex and stress hormone influences on the expression and activity of brain-derived neurotrophic factor. Neuroscience. 2013;239:295-303.
34. Pilar-Cuéllar F, Vidal R, Pazos A. Subchronic treatment with fluoxetine and ketanserin increases hippocampal brain-derived neurotrophic factor, β-catenin and antidepressant-like effects. Br J Pharmacol. 2012;165(4b):1046-1057.
35. Deuschle M, Gilles M, Scharnholz B, et al. Changes of serum concentrations of brain-derived neurotrophic factor (BDNF) during treatment with venlafaxine and mirtazapine: role of medication and response to treatment. Pharmacopsychiatry. 2013;46(2):54-58.
36. Berman SM, London ED, Morgan M, et al. Elevated gray matter volume of the emotional cerebellum in women with premenstrual dysphoric disorder. J Affect Disord. 2013;146(2):266-271.
37. Jeong HG, Ham BJ, Yeo HB, et al. Gray matter abnormalities in patients with premenstrual dysphoric disorder: an optimized voxel-based morphometry. J Affect Disord. 2012;140(3):260-267.
38. Protopopescu X, Tuescher O, Pan H, et al. Toward a functional neuroanatomy of premenstrual dysphoric disorder. J Affect Disord. 2008;108(1-2):87-94.
39. Gingnell M, Morell A, Bannbers E, et al. Menstrual cycle effects on amygdala reactivity to emotional stimulation in premenstrual dysphoric disorder. Horm Behav. 2012;62(4):400-406.
40. Epperson CN, Haga K, Mason GF, et al. Cortical gamma-aminobutyric acid levels across the menstrual cycle in healthy women and those with premenstrual dysphoric disorder: a proton magnetic resonance spectroscopy study. Arch Gen Psychiatry. 2002;59(9):851-858.
41. Gingnell M, Bannbers E, Wikström J, et al. Premenstrual dysphoric disorder and prefrontal reactivity during anticipation of emotional stimuli. Eur Neuropsychopharmacol. 2013;23(11):1474-1483.
42. Baller EB, Wei SM, Kohn PD, et al. Abnormalities of dorsolateral prefrontal function in women with premenstrual dysphoric disorder: a multimodal neuroimaging study. Am J Psychiatry. 2013;170(3):305-314.
43. Rasgon N, McGuire M, Tanavoli S, et al. Neuroendocrine response to an intravenous L-tryptophan challenge in women with premenstrual syndrome. Fertil Steril. 2000;73(1):144-149.
44. Huang Y, Zhou R, Wu M, et al. Premenstrual syndrome is associated with blunted cortisol reactivity to the TSST. Stress. 2015;18(2):160-168.
45. Segebladh B, Bannbers E, Moby L, et al. Allopregnanolone serum concentrations and diurnal cortisol secretion in women with premenstrual dysphoric disorder. Arch Womens Ment Health. 2013;16(2):131-137.
46. Pilver CE, Levy BR, Libby DJ, et al. Posttraumatic stress disorder and trauma characteristics are correlates of premenstrual dysphoric disorder. Arch Womens Ment Health. 2011;14(5):383-393.
47. Bertone-Johnson ER, Whitcomb BW, Missmer SA, et al. Early life emotional, physical, and sexual abuse and the development of premenstrual syndrome: a longitudinal study. J Womens Health (Larchmt). 2014;23(9):729-739.
48. Segebladh B, Bannbers E, Kask K, et al. Prevalence of violence exposure in women with premenstrual dysphoric disorder in comparison with other gynecological patients and asymptomatic controls. Acta Obstet Gynecol Scand. 2011;90(7):746-752.
49. Kask K, Gulinello M, Bäckström T, et al. Patients with premenstrual dysphoric disorder have increased startle response across both cycle phases and lower levels of prepulse inhibition during the late luteal phase of the menstrual cycle. Neuropsychopharmacology. 2008;33(9):2283-2290.
50. O’Brien SM, Fitzgerald P, Scully P, et al. Impact of gender and menstrual cycle phase on plasma cytokine concentrations. Neuroimmunomodulation. 2007;14(2):84-90.
51. Northoff H, Symons S, Zieker D, et al. Gender- and menstrual phase dependent regulation of inflammatory gene expression in response to aerobic exercise. Exerc Immunol Rev. 2008;14:86-103.
52. Gaskins AJ, Wilchesky M, Mumford SL, et al. Endogenous reproductive hormones and C-reactive protein across the menstrual cycle: the BioCycle Study. Am J Epidemiol. 2012;175(5):423-431.
53. Wander K, Brindle E, O’Connor KA. C-reactive protein across the menstrual cycle. Am J Phys Anthropol. 2008;136(2):138-146.
54. Jane ZY, Chang CC, Lin HK, et al. The association between the exacerbation of irritable bowel syndrome and menstrual symptoms in young Taiwanese women. Gastroenterol Nurs. 2011;34(4):277-286.
55. Kane SV, Sable K, Hanauer SB. The menstrual cycle and its effect on inflammatory bowel disease and irritable bowel syndrome: a prevalence study. Am J Gastroenterol. 1998;93(10):1867-1872.
56. Shourie V, Dwarakanath CD, Prashanth GV, et al. The effect of menstrual cycle on periodontal health - a clinical and microbiological study. Oral Health Prev Dent. 2012;10(2):185-192.
57. Hantsoo L, Epperson CN. Premenstrual dysphoric disorder: epidemiology and treatment. Curr Psychiatry Rep. 2015;17(11):87.
58. Maeng LY, Milad MR. Sex differences in anxiety disorders: Interactions between fear, stress, and gonadal hormones. Horm Behav. 2015;76:106-117.
59. Lee YJ, Yi SW, Ju DH, et al. Correlation between postpartum depression and premenstrual dysphoric disorder: single center study. Obstet Gynecol Sci. 2015;58(5):353-358.
60. Kepple AL, Lee EE, Haq N, et al. History of postpartum depression in a clinic-based sample of women with premenstrual dysphoric disorder. J Clin Psychiatry. 2016;77(4):e415-e420.
61. Schmidt PJ, Nieman LK, Danaceau MA, et al. Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. N Engl J Med. 1998;338(4):209-216.
62. Schmidt PJ, Martinez PE, Nieman LK, et al. Premenstrual dysphoric disorder symptoms following ovarian suppression: Triggered by change in ovarian steroid levels but not continuous stable levels. Am J Psychiatry. [published online April 21, 2017]. doi: 10.1176/appi.ajp.2017.16101113.
In an age when psychiatry strives to identify the biologic causes of disease, studying endocrine-related mood disorders is particularly intriguing. DSM-5 defines premenstrual dysphoric disorder (PMDD) as a depressive disorder, with a 12-month prevalence ranging from 1.8% to 5.8% among women who menstruate.1-3 Factors that differentiate PMDD from other affective disorders include etiology, duration, and temporal relationship with the menstrual cycle.
PMDD is a disorder of consistent yet intermittent change in mental health and functionality. Therefore, it may be underdiagnosed and consequently undertreated if a psychiatric evaluation does not coincide with symptom occurrence or if patients do not understand that intermittent symptoms are treatable.
This article summarizes what is known about the etiology of PMDD. Although there are several treatments for PMDD, many women experience adverse effects or incomplete effectiveness. Further understanding of this disorder may lead to more efficacious treatments. Additionally, understanding the pathophysiology of PMDD might shed a light on the etiology of other disorders that are temporally related to reproductive life changes, such as pregnancy-, postpartum-, or menopause-related affective dysregulation.
Making the diagnosis
The diagnosis of PMDD is made when a patient has at least 5 of 11 specific symptoms that occur during the week before onset of menses, improve within a few days after the onset of menses (shown as the “PMDD Hazard Zone” in Figure 1), and are minimal or absent post-menses.3 Symptoms should be tracked prospectively for at least 2 menstrual cycles in order to confirm the diagnosis (one must be an affective symptom and another must be a behavioral/cognitive symptom).3
The affective symptoms are:
- lability of affect (eg, sudden sadness, tearfulness, or sensitivity to rejection)
- irritability, anger, or increased interpersonal conflicts
- depressed mood, hopelessness, or self- deprecating thoughts
- anxiety or tension, feeling “keyed up” or “on edge.”
The behavioral/cognitive symptoms are:
- decreased interest in usual activities (eg, work, hobbies, friends, school)
- difficulty concentrating
- lethargy, low energy, easy fatigability
- change in appetite, overeating, food cravings
- hypersomnia or insomnia
- feeling overwhelmed or out of control
- physical symptoms (breast tenderness or swelling, headache, joint or muscle pain, bloating, weight gain).
Ruling out premenstrual exacerbation (PME). Perhaps the most common cause for misdiagnosis of PMDD is failing to rule out PME of another underlying or comorbid condition (Figure 2). In many women who have a primary mood or anxiety disorder, the late luteal phase is a vulnerable time. A patient might be coping with untreated anxiety, for example, but the symptoms become unbearable the week before menstruation begins, which is likely when she seeks help. At this stage, a diagnosis of PMDD should be provisional at best. Often, PME is treated by treating the underlying condition. Therefore, a full diagnostic psychiatric interview is important to first rule out other underlying psychiatric disorders. PMDD is diagnosed if the premenstrual symptoms persist for 2 consecutive months after treating the suspected mood or anxiety disorder. Patients can use one of many PMDD daily symptom charts available online. Alternatively, they can use a cycle-tracking mobile phone application to correlate their symptoms with their cycle and share this information with their providers.
Consider these 5 interwoven pieces
The many variables that contribute to the pathophysiology of PMDD overlap and should be considered connecting pieces in the puzzle that is the etiology of this disorder (Figure 3). In reviewing the literature, we have identified 5 topics likely to be major contributors to this disorder:
- genetic susceptibility
- progesterone and allopregnanolone (ALLO)
- estrogen, serotonin, and brain-derived neurotrophic factor (BDNF)
- putative brain structural and functional differences
- further involvement of the hypothalamic–pituitary–adrenal (HPA) axis and hypothalamic–pituitary–gonadal (HPG) axis: trauma, resiliency, and inflammation.
Genetic susceptibility. PMDD is thought to have a heritability range between 30% to 80%.3 This is demonstrated by family and twin studies4-7 and specific genetic studies.8 The involvement of genetics means an underlying neurobiologic pathophysiology is in place.
Estrogen receptor alpha (ESR1) gene. Huo et al8 found an associated variation in ESR1 in women with PMDD compared with controls. They speculated that because ESR1 is important for arousal, if dysfunctional, this gene could be implicated in somatic as well as affective and cognitive deficits in PMDD patients. In another study, investigators reported a relationship between PMDD and heritable personality traits, as well as a link between these traits and ESR1 polymorphic variants.1 They suggested that personality traits (independent of affective state) might be used to distinguish patients with PMDD from controls.1
Studies on serotonin gene polymorphism and serotonin transporter genotype. Although a study of serotonin gene polymorphism did not find an association between serotonin1A gene polymorphism and PMDD, it did show that the presence of at least 1 C allele was associated with a 2.5-fold increased risk of PMDD.9 Another study did not find an association between the serotonin transporter genotype 5-HTTLPR and PMDD.10 However, it showed lower frontocingulate cortex activation during the luteal phase of PMDD patients compared with controls, suggesting that PMDD is linked to impaired frontocingulate cortex activation induced by emotions during the luteal phase.10
Seasonal affective disorder (SAD) and PMDD have shared clinical features. A polymorphism in the serotonin transporter promoter gene 5-HTTLPR has been associated with SAD. One study found that patients with comorbid SAD and PMDD are genetically more vulnerable to comorbid affective disorders compared with patients who have SAD only.11
Progesterone and ALLO. Chronic exposure to progesterone and ALLO (a main progesterone metabolite) and rapid withdrawal from ovarian hormones may play a role in the etiology of PMDD. Much like alcohol or benzodiazepines, ALLO is a potent positive allosteric modulator of GABAA receptors and has sedative, anesthetic, and anxiolytic properties. In times of acute stress, increased ALLO is known to provide relief.12,13 However, in women with PMDD, this typical ALLO increase might not occur.14
Patients with PMDD have been reported to have decreased levels of ALLO in the luteal phase.15-17 In one study, women with highly symptomatic PMDD had lower levels of ALLO compared with women with less symptomatic PMDD.14 A gonadotropin-releasing hormone challenge study showed the increase in ALLO response was less in PMDD patients compared with controls.17 Luteal-phase ALLO concentrations are reported to be lower in women with premenstrual syndrome (PMS), a milder form of PMDD.14,17
The efficacy of selective serotonin reuptake inhibitors (SSRIs) for treating PMDD could be the result of the interaction of these medications with neuroactive steroids,18 possibly because SSRIs enhance the sensitivity of GABAA receptors or promote the formation of more ALLO (Figure 4).19-21
Estrogen, serotonin, and BDNF. Estrogen affects multiple neurotransmitter systems that regulate mood, cognition, sleep, and eating.22 Studying estrogen in context of PMDD is important because women with PMDD can have low mood, specific food cravings, and impaired cognitive function.
Estrogen–serotonin interactions are thought to be involved in hormone-related mood disorders such as perimenopausal depression and PMDD.23,24 However, the nature of their relationship is not yet fully understood. Ovariectomized animals have shown estrogen-induced changes related to serotonin metabolism, binding, and transmission in the regions of the brain involved in regulation of affect and cognition. Research in menopausal women also has provided some support for this interaction.24
Positron emission tomography studies in humans have found increased cortical serotonin binding modulated by levels of estrogen, similar to those previously seen in rat studies.24-27 One study showed an increased binding potential of serotonin in the cerebral cortex with estrogen treatment. This study further showed an even greater binding potential with estrogen plus progesterone, signaling a synergistic effect of the 2 hormones.28
SSRIs are an effective treatment for the irritability, anxiety, and mood swings of PMDD.29-30 Although the exact mechanism of action is unknown, the serotonergic properties are certainly of primary attention. For some PMDD patients, SSRIs work within hours to days, as opposed to days or weeks for patients with depression or anxiety, which suggests a separate or co-occurring mechanism of action is in place. In a double-blind, placebo-controlled crossover study, researchers administered the serotonin receptor antagonist metergoline to women with PMDD whose symptoms had remitted during treatment with fluoxetine and a group of healthy controls who were not receiving any medication.31 The women with PMDD experienced a return of symptoms 24 hours after treatment with metergoline but not with placebo; the controls experienced no mood changes.31
BDNF is a neurotransmitter linked to estrogen and likely related to PMDD. BDNF is critical for neurogenesis and is expressed in brain regions involved in learning and memory and also affects regulation.32 BDNF levels are increased by serotonergic antidepressants, affected by estradiol, and have cyclicity throughout the menstrual cycle.33-35
Putative brain structural and functional differences. Imaging studies have suggested differences in brain structure in women with PMDD, with a focus on the amygdala and the prefrontal cortex. Women with PMDD have greater gray matter volume in the posterior cerebellum,36 greater gray matter density of hippocampal cortex, and lower gray matter density in the parahippocampal cortex.37
Some studies have shown a functional variability of the amygdala’s response to stress in women with PMDD vs healthy controls.38,39 A proton magnetic resonance spectroscopy (1H-MRS) study of the displays the possibility of an altered GABAergic function in patients with PMDD.40
Patients will PMDD have enhanced dorsolateral prefrontal cortex reactivity when anticipating negative stimuli (but not to the actual exposure) during the luteal phase. A positive correlation between this reactivity and progesterone levels also was observed.41 Some researchers have suggested that prefrontal cortex dysfunction may be a risk factor for PMDD.42
HPA axis and HPG axis: Trauma, resiliency, inflammation. Altered cortisol levels (higher during the luteal phase43 and lower during times of stress14,44) suggest a possibly altered HPA axis in some women with PMDD. However, studies on this topic have been few and inconsistent.
Dysregulation of the HPG axis could cause vasomotor symptoms, sleep dysregulation, and mood symptoms during menopause; women with PMDD can also experience these symptoms. The influence of estrogen and progesterone on mood is also highly dependent on this axis.
Ultimately, the interplay between the HPA axis and the HPG axis is important. One study found that women with PMDD who had high serum ALLO levels (HPG-related) had blunted nocturnal cortisol levels (HPA-related) compared with healthy controls who had low ALLO levels.45
Significant stress and trauma exposure have been associated with PMDD. A study of 3,968 women found a history of trauma and PTSD were independently associated with PMDD.46 Another study of approximately 3,000 women found a strong correlation between abuse and PMS.47 However, a third study found no correlations between PMDD and trauma.48
Patients with a predisposition to PMDD may be more vulnerable to develop a posttraumatic stress-related disorder, perhaps due to decreased biologic resiliency. For example, the startle response (hypervigilance) has been shown to be different in women with PMDD. One study suggested that suboptimal production of premenstrual ALLO may lead to increased arousal and increased stress reactivity to psychosocial or environmental triggers.49
The possible role of inflammation in PMDD deserves further investigation. The luteal phase entails an increase in the production of proinflammatory markers.50,51 A 10-fold increase in progesterone is correlated with a 20% to 23% increase in C-reactive protein levels.52,53 Women with inflammatory diseases (eg, gingivitis or irritable bowel syndrome) show worsening of symptoms prior to menstruation.54-56 One study found increased levels of proinflammatory markers in women with PMDD compared with controls.57
Putting together the 5 pieces of the puzzle
Because PMDD is heritable, it must have an underlying neurobiologic pathophysiology. Brain imaging studies show differences in structure and function in women with PMDD across the menstrual cycle. Conversion of progesterone to ALLO and the GABAergic influence of this metabolite is a topic of interest in current research. Similarly, the role of estrogen and its connection to serotonin and other neurotransmitters such as BDNF have been implicated.
The link between a history of stress, trauma, and PMDD raises the question of biologic resiliency and illness in these patients, as it connects to the HPA and HPG axis and production of inflammatory stress hormones and steroid hormones and their metabolites. PMDD can be conceptualized as variable sensitivity to hormonal response to stress,58 thus contextualizing biochemical and psychological resiliency.
Further research is needed to clarify the possibility of a shared pathophysiology between endocrine-related mood disorders such as postpartum depression (PPD) and PMDD because current research is controversial.59,60 In PPD, women who are exposed to high levels of progesterone and estrogen during pregnancy (just like in the mid-luteal phase) have a sudden drop in these hormones postpartum.
The ‘withdrawal theory.’ The affective symptoms of PMDD resolve almost instantaneously after the start of menstruation. Perhaps this type of immediate relief is akin to substance use disorders and symptoms of withdrawal. It could be that reinstatement of a certain amount of gonadal steroids in the follicular phase of the cycle diminishes a withdrawal-like response to these steroids.
Currently, the main leading theory is that PMDD is a result of “an abnormal response to normal hormonal changes.”61 A new study also has shown that the change in estradiol/progesterone levels (vs the steady state) was associated with PMDD symptoms.62 Thinking of PMDD as a disorder of withdrawal offers an alternative (yet complementary) perspective to the current theory: PMDD may be caused by the absence or diminishing of the above-named hormones and their metabolites in the late luteal phase (in the context of developed “tolerance” during the early- to mid-luteal phase).
Considering the interplay between neurotransmitters and neurosteroids, both a “serotonin withdrawal theory” (caused by a drop in steroid hormones) and a “GABAergic withdrawal theory” (due to the decline in progesterone) could be proposed. This theory would be supported by the fact that SSRIs seem to mitigate symptoms of PMDD as well as the genetic association between PMDD and ESR1. It is more than likely that the “withdrawal” is caused by the interactions between estrogen-serotonin, progesterone-ALLO, and GABA receptors, and the complementary fashion in which progesterone and estrogen influence each other.
In an age when psychiatry strives to identify the biologic causes of disease, studying endocrine-related mood disorders is particularly intriguing. DSM-5 defines premenstrual dysphoric disorder (PMDD) as a depressive disorder, with a 12-month prevalence ranging from 1.8% to 5.8% among women who menstruate.1-3 Factors that differentiate PMDD from other affective disorders include etiology, duration, and temporal relationship with the menstrual cycle.
PMDD is a disorder of consistent yet intermittent change in mental health and functionality. Therefore, it may be underdiagnosed and consequently undertreated if a psychiatric evaluation does not coincide with symptom occurrence or if patients do not understand that intermittent symptoms are treatable.
This article summarizes what is known about the etiology of PMDD. Although there are several treatments for PMDD, many women experience adverse effects or incomplete effectiveness. Further understanding of this disorder may lead to more efficacious treatments. Additionally, understanding the pathophysiology of PMDD might shed a light on the etiology of other disorders that are temporally related to reproductive life changes, such as pregnancy-, postpartum-, or menopause-related affective dysregulation.
Making the diagnosis
The diagnosis of PMDD is made when a patient has at least 5 of 11 specific symptoms that occur during the week before onset of menses, improve within a few days after the onset of menses (shown as the “PMDD Hazard Zone” in Figure 1), and are minimal or absent post-menses.3 Symptoms should be tracked prospectively for at least 2 menstrual cycles in order to confirm the diagnosis (one must be an affective symptom and another must be a behavioral/cognitive symptom).3
The affective symptoms are:
- lability of affect (eg, sudden sadness, tearfulness, or sensitivity to rejection)
- irritability, anger, or increased interpersonal conflicts
- depressed mood, hopelessness, or self- deprecating thoughts
- anxiety or tension, feeling “keyed up” or “on edge.”
The behavioral/cognitive symptoms are:
- decreased interest in usual activities (eg, work, hobbies, friends, school)
- difficulty concentrating
- lethargy, low energy, easy fatigability
- change in appetite, overeating, food cravings
- hypersomnia or insomnia
- feeling overwhelmed or out of control
- physical symptoms (breast tenderness or swelling, headache, joint or muscle pain, bloating, weight gain).
Ruling out premenstrual exacerbation (PME). Perhaps the most common cause for misdiagnosis of PMDD is failing to rule out PME of another underlying or comorbid condition (Figure 2). In many women who have a primary mood or anxiety disorder, the late luteal phase is a vulnerable time. A patient might be coping with untreated anxiety, for example, but the symptoms become unbearable the week before menstruation begins, which is likely when she seeks help. At this stage, a diagnosis of PMDD should be provisional at best. Often, PME is treated by treating the underlying condition. Therefore, a full diagnostic psychiatric interview is important to first rule out other underlying psychiatric disorders. PMDD is diagnosed if the premenstrual symptoms persist for 2 consecutive months after treating the suspected mood or anxiety disorder. Patients can use one of many PMDD daily symptom charts available online. Alternatively, they can use a cycle-tracking mobile phone application to correlate their symptoms with their cycle and share this information with their providers.
Consider these 5 interwoven pieces
The many variables that contribute to the pathophysiology of PMDD overlap and should be considered connecting pieces in the puzzle that is the etiology of this disorder (Figure 3). In reviewing the literature, we have identified 5 topics likely to be major contributors to this disorder:
- genetic susceptibility
- progesterone and allopregnanolone (ALLO)
- estrogen, serotonin, and brain-derived neurotrophic factor (BDNF)
- putative brain structural and functional differences
- further involvement of the hypothalamic–pituitary–adrenal (HPA) axis and hypothalamic–pituitary–gonadal (HPG) axis: trauma, resiliency, and inflammation.
Genetic susceptibility. PMDD is thought to have a heritability range between 30% to 80%.3 This is demonstrated by family and twin studies4-7 and specific genetic studies.8 The involvement of genetics means an underlying neurobiologic pathophysiology is in place.
Estrogen receptor alpha (ESR1) gene. Huo et al8 found an associated variation in ESR1 in women with PMDD compared with controls. They speculated that because ESR1 is important for arousal, if dysfunctional, this gene could be implicated in somatic as well as affective and cognitive deficits in PMDD patients. In another study, investigators reported a relationship between PMDD and heritable personality traits, as well as a link between these traits and ESR1 polymorphic variants.1 They suggested that personality traits (independent of affective state) might be used to distinguish patients with PMDD from controls.1
Studies on serotonin gene polymorphism and serotonin transporter genotype. Although a study of serotonin gene polymorphism did not find an association between serotonin1A gene polymorphism and PMDD, it did show that the presence of at least 1 C allele was associated with a 2.5-fold increased risk of PMDD.9 Another study did not find an association between the serotonin transporter genotype 5-HTTLPR and PMDD.10 However, it showed lower frontocingulate cortex activation during the luteal phase of PMDD patients compared with controls, suggesting that PMDD is linked to impaired frontocingulate cortex activation induced by emotions during the luteal phase.10
Seasonal affective disorder (SAD) and PMDD have shared clinical features. A polymorphism in the serotonin transporter promoter gene 5-HTTLPR has been associated with SAD. One study found that patients with comorbid SAD and PMDD are genetically more vulnerable to comorbid affective disorders compared with patients who have SAD only.11
Progesterone and ALLO. Chronic exposure to progesterone and ALLO (a main progesterone metabolite) and rapid withdrawal from ovarian hormones may play a role in the etiology of PMDD. Much like alcohol or benzodiazepines, ALLO is a potent positive allosteric modulator of GABAA receptors and has sedative, anesthetic, and anxiolytic properties. In times of acute stress, increased ALLO is known to provide relief.12,13 However, in women with PMDD, this typical ALLO increase might not occur.14
Patients with PMDD have been reported to have decreased levels of ALLO in the luteal phase.15-17 In one study, women with highly symptomatic PMDD had lower levels of ALLO compared with women with less symptomatic PMDD.14 A gonadotropin-releasing hormone challenge study showed the increase in ALLO response was less in PMDD patients compared with controls.17 Luteal-phase ALLO concentrations are reported to be lower in women with premenstrual syndrome (PMS), a milder form of PMDD.14,17
The efficacy of selective serotonin reuptake inhibitors (SSRIs) for treating PMDD could be the result of the interaction of these medications with neuroactive steroids,18 possibly because SSRIs enhance the sensitivity of GABAA receptors or promote the formation of more ALLO (Figure 4).19-21
Estrogen, serotonin, and BDNF. Estrogen affects multiple neurotransmitter systems that regulate mood, cognition, sleep, and eating.22 Studying estrogen in context of PMDD is important because women with PMDD can have low mood, specific food cravings, and impaired cognitive function.
Estrogen–serotonin interactions are thought to be involved in hormone-related mood disorders such as perimenopausal depression and PMDD.23,24 However, the nature of their relationship is not yet fully understood. Ovariectomized animals have shown estrogen-induced changes related to serotonin metabolism, binding, and transmission in the regions of the brain involved in regulation of affect and cognition. Research in menopausal women also has provided some support for this interaction.24
Positron emission tomography studies in humans have found increased cortical serotonin binding modulated by levels of estrogen, similar to those previously seen in rat studies.24-27 One study showed an increased binding potential of serotonin in the cerebral cortex with estrogen treatment. This study further showed an even greater binding potential with estrogen plus progesterone, signaling a synergistic effect of the 2 hormones.28
SSRIs are an effective treatment for the irritability, anxiety, and mood swings of PMDD.29-30 Although the exact mechanism of action is unknown, the serotonergic properties are certainly of primary attention. For some PMDD patients, SSRIs work within hours to days, as opposed to days or weeks for patients with depression or anxiety, which suggests a separate or co-occurring mechanism of action is in place. In a double-blind, placebo-controlled crossover study, researchers administered the serotonin receptor antagonist metergoline to women with PMDD whose symptoms had remitted during treatment with fluoxetine and a group of healthy controls who were not receiving any medication.31 The women with PMDD experienced a return of symptoms 24 hours after treatment with metergoline but not with placebo; the controls experienced no mood changes.31
BDNF is a neurotransmitter linked to estrogen and likely related to PMDD. BDNF is critical for neurogenesis and is expressed in brain regions involved in learning and memory and also affects regulation.32 BDNF levels are increased by serotonergic antidepressants, affected by estradiol, and have cyclicity throughout the menstrual cycle.33-35
Putative brain structural and functional differences. Imaging studies have suggested differences in brain structure in women with PMDD, with a focus on the amygdala and the prefrontal cortex. Women with PMDD have greater gray matter volume in the posterior cerebellum,36 greater gray matter density of hippocampal cortex, and lower gray matter density in the parahippocampal cortex.37
Some studies have shown a functional variability of the amygdala’s response to stress in women with PMDD vs healthy controls.38,39 A proton magnetic resonance spectroscopy (1H-MRS) study of the displays the possibility of an altered GABAergic function in patients with PMDD.40
Patients will PMDD have enhanced dorsolateral prefrontal cortex reactivity when anticipating negative stimuli (but not to the actual exposure) during the luteal phase. A positive correlation between this reactivity and progesterone levels also was observed.41 Some researchers have suggested that prefrontal cortex dysfunction may be a risk factor for PMDD.42
HPA axis and HPG axis: Trauma, resiliency, inflammation. Altered cortisol levels (higher during the luteal phase43 and lower during times of stress14,44) suggest a possibly altered HPA axis in some women with PMDD. However, studies on this topic have been few and inconsistent.
Dysregulation of the HPG axis could cause vasomotor symptoms, sleep dysregulation, and mood symptoms during menopause; women with PMDD can also experience these symptoms. The influence of estrogen and progesterone on mood is also highly dependent on this axis.
Ultimately, the interplay between the HPA axis and the HPG axis is important. One study found that women with PMDD who had high serum ALLO levels (HPG-related) had blunted nocturnal cortisol levels (HPA-related) compared with healthy controls who had low ALLO levels.45
Significant stress and trauma exposure have been associated with PMDD. A study of 3,968 women found a history of trauma and PTSD were independently associated with PMDD.46 Another study of approximately 3,000 women found a strong correlation between abuse and PMS.47 However, a third study found no correlations between PMDD and trauma.48
Patients with a predisposition to PMDD may be more vulnerable to develop a posttraumatic stress-related disorder, perhaps due to decreased biologic resiliency. For example, the startle response (hypervigilance) has been shown to be different in women with PMDD. One study suggested that suboptimal production of premenstrual ALLO may lead to increased arousal and increased stress reactivity to psychosocial or environmental triggers.49
The possible role of inflammation in PMDD deserves further investigation. The luteal phase entails an increase in the production of proinflammatory markers.50,51 A 10-fold increase in progesterone is correlated with a 20% to 23% increase in C-reactive protein levels.52,53 Women with inflammatory diseases (eg, gingivitis or irritable bowel syndrome) show worsening of symptoms prior to menstruation.54-56 One study found increased levels of proinflammatory markers in women with PMDD compared with controls.57
Putting together the 5 pieces of the puzzle
Because PMDD is heritable, it must have an underlying neurobiologic pathophysiology. Brain imaging studies show differences in structure and function in women with PMDD across the menstrual cycle. Conversion of progesterone to ALLO and the GABAergic influence of this metabolite is a topic of interest in current research. Similarly, the role of estrogen and its connection to serotonin and other neurotransmitters such as BDNF have been implicated.
The link between a history of stress, trauma, and PMDD raises the question of biologic resiliency and illness in these patients, as it connects to the HPA and HPG axis and production of inflammatory stress hormones and steroid hormones and their metabolites. PMDD can be conceptualized as variable sensitivity to hormonal response to stress,58 thus contextualizing biochemical and psychological resiliency.
Further research is needed to clarify the possibility of a shared pathophysiology between endocrine-related mood disorders such as postpartum depression (PPD) and PMDD because current research is controversial.59,60 In PPD, women who are exposed to high levels of progesterone and estrogen during pregnancy (just like in the mid-luteal phase) have a sudden drop in these hormones postpartum.
The ‘withdrawal theory.’ The affective symptoms of PMDD resolve almost instantaneously after the start of menstruation. Perhaps this type of immediate relief is akin to substance use disorders and symptoms of withdrawal. It could be that reinstatement of a certain amount of gonadal steroids in the follicular phase of the cycle diminishes a withdrawal-like response to these steroids.
Currently, the main leading theory is that PMDD is a result of “an abnormal response to normal hormonal changes.”61 A new study also has shown that the change in estradiol/progesterone levels (vs the steady state) was associated with PMDD symptoms.62 Thinking of PMDD as a disorder of withdrawal offers an alternative (yet complementary) perspective to the current theory: PMDD may be caused by the absence or diminishing of the above-named hormones and their metabolites in the late luteal phase (in the context of developed “tolerance” during the early- to mid-luteal phase).
Considering the interplay between neurotransmitters and neurosteroids, both a “serotonin withdrawal theory” (caused by a drop in steroid hormones) and a “GABAergic withdrawal theory” (due to the decline in progesterone) could be proposed. This theory would be supported by the fact that SSRIs seem to mitigate symptoms of PMDD as well as the genetic association between PMDD and ESR1. It is more than likely that the “withdrawal” is caused by the interactions between estrogen-serotonin, progesterone-ALLO, and GABA receptors, and the complementary fashion in which progesterone and estrogen influence each other.
1. Miller A, Vo H, Huo L, et al. Estrogen receptor alpha (ESR-1) associations with psychological traits in women with PMDD and controls. J Psychiatr Res. 2010;44(12):788-794.
2. Epperson CN, Steiner M, Hartlage SA, et al. Premenstrual dysphoric disorder: evidence for a new category for DSM-5. Am J Psychiatry. 2012;169(5):465-475.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Wilson CA, Turner CW, Keye WR Jr. Firstborn adolescent daughters and mothers with and without premenstrual syndrome: a comparison. J Adolesc Health. 1991;12(2):130-137.
5. Kendler KS, Silberg JL, Neale MC, et al. Genetic and environmental factors in the aetiology of menstrual, premenstrual and neurotic symptoms: a population-based twin study. Psychol Med. 1992;22(1):85-100.
6. Condon JT. The premenstrual syndrome: a twin study. Br J Psychiatry. 1993;162:481-486.
7. Kendler KS, Karkowski LM, Corey LA, et al. Longitudinal population-based twin study of retrospectively reported premenstrual symptoms and lifetime major depression. Am J Psychiatry. 1998;155(9):1234-1240.
8. Huo L, Straub RE, Roca C, et al. Risk for premenstrual dysphoric disorder is associated with genetic variation in ESR1, the estrogen receptor alpha gene. Biol Psychiatry. 2007;62(8):925-933.
9. Dhingra V, Magnay JL, O’Brien PM, et al. Serotonin receptor 1A C(-1019)G polymorphism associated with premenstrual dysphoric disorder. Obstet Gynecol. 2007;110(4):788-792.
10. Comasco E, Hahn A, Ganger S, et al. Emotional fronto-cingulate cortex activation and brain derived neurotrophic factor polymorphism in premenstrual dysphoric disorder. Hum Brain Mapp. 2014;35(9):4450-4458.
11. Praschak-Rieder N, Willeit M, Winkler D, et al. Role of family history and 5-HTTLPR polymorphism in female seasonal affective disorder patients with and without premenstrual dysphoric disorder. Eur Neuropsychopharmacol. 2002;12(2):129-134.
12. Klatzkin RR, Morrow AL, Light KC, et al. Associations of histories of depression and PMDD diagnosis with allopregnanolone concentrations following the oral administration of micronized progesterone. Psychoneuroendocrinology. 2006;31(10):1208-1219.
13. Crowley SK, Girdler SS. Neurosteroid, GABAergic and hypothalamic pituitary adrenal (HPA) axis regulation: what is the current state of knowledge in humans? Psychopharmacology (Berl). 2014;231(17):3619-3634.
14. Girdler SS, Straneva PA, Light KC, et al. Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biol Psychiatry. 2001;49(9):788-797.
15. Rapkin AJ, Morgan M, Goldman L, et al. Progesterone metabolite allopregnanolone in women with premenstrual syndrome. Obstet Gynecol. 1997;90(5):709-714.
16. Bicíková M, Dibbelt L, Hill M, et al. Allopregnanolone in women with premenstrual syndrome. Horm Metab Res. 1998;30(4):227-230.
17. Monteleone P, Luisi S, Tonetti A, et al. Allopregnanolone concentrations and premenstrual syndrome. Eur J Endocrinol. 2000;142(3):269-273.
18. Steiner M, Steinberg S, Stewart D, et al. Fluoxetine in the treatment of premenstrual dysphoria. Canadian Fluoxetine/Premenstrual Dysphoria Collaborative Study Group. N Engl J Med. 1995;332(23):1529-1534.
19. Sundström I, Bäckström T. Citalopram increases pregnanolone sensitivity in patients with premenstrual syndrome: an open trial. Psychoneuroendocrinology. 1998;23(1):73-88.
20. Griffin LD, Mellon SH. Selective serotonin reuptake inhibitors directly alter activity of neurosteroidogenic enzymes. Proc Natl Acad Sci U S A. 1999;96(23):13512-13517.
21. Trauger JW, Jiang A, Stearns BA, et al. Kinetics of allopregnanolone formation catalyzed by human 3 alpha-hydroxysteroid dehydrogenase type III (AKR1C2). Biochemistry. 2002;41(45):13451-13459.
22. Shanmugan S, Epperson CN. Estrogen and the prefrontal cortex: towards a new understanding of estrogen’s effects on executive functions in the menopause transition. Hum Brain Mapp. 2014;35(3):847-865.
23. Rubinow DR, Schmidt PJ, Roca CA. Estrogen-serotonin interactions: implications for affective regulation. Biol Psychiatry. 1998;44(9):839-850.
24. Amin Z, Canli T, Epperson CN. Effect of estrogen-serotonin interactions on mood and cognition. Behav Cogn Neurosci Rev. 2005;4(1):43-58.
25. Cyr M, Bossé R, Di Paolo T. Gonadal hormones modulate 5-hydroxytryptamine2A receptors: emphasis on the rat frontal cortex. Neuroscience. 1998;83(3):829-836.
26. Fink G, Sumner BE, Rosie R, et al. Estrogen control of central neurotransmission: effect on mood, mental state, and memory. Cell Mol Neurobiol. 1996;16(3):325-344.
27. Sumner BE, Grant KE, Rosie R, et al. Effects of tamoxifen on serotonin transporter and 5-hydroxytryptamine(2A) receptor binding sites and mRNA levels in the brain of ovariectomized rats with or without acute estradiol replacement. Brain Res Mol Brain Res. 1999;73(1-2):119-128.
28. Moses-Kolko EL, Berga SL, Greer PJ, et al. Widespread increases of cortical serotonin type 2A receptor availability after hormone therapy in euthymic postmenopausal women. Fertil Steril. 2003;80(3):554-559.
29. Su TP, Schmidt PJ, Danaceau MA, et al. Fluoxetine in the treatment of premenstrual dysphoria. Neuropsychopharmacology. 1997;16(5):346-356.
30. Steinberg EM, Cardoso GM, Martinez PE, et al. Rapid response to fluoxetine in women with premenstrual dysphoric disorder. Depress Anxiety. 2012;29(6):531-540.
31. Roca CA, Schmidt PJ, Smith MJ, et al. Effects of metergoline on symptoms in women with premenstrual dysphoric disorder. Am J Psychiatry. 2002;159(11):1876-1881.
32. Gray JD, Milner TA, McEwen BS. Dynamic plasticity: the role of glucocorticoids, brain-derived neurotrophic factor and other trophic factors. Neuroscience. 2013;239:214-227.
33. Carbone DL, Handa RJ. Sex and stress hormone influences on the expression and activity of brain-derived neurotrophic factor. Neuroscience. 2013;239:295-303.
34. Pilar-Cuéllar F, Vidal R, Pazos A. Subchronic treatment with fluoxetine and ketanserin increases hippocampal brain-derived neurotrophic factor, β-catenin and antidepressant-like effects. Br J Pharmacol. 2012;165(4b):1046-1057.
35. Deuschle M, Gilles M, Scharnholz B, et al. Changes of serum concentrations of brain-derived neurotrophic factor (BDNF) during treatment with venlafaxine and mirtazapine: role of medication and response to treatment. Pharmacopsychiatry. 2013;46(2):54-58.
36. Berman SM, London ED, Morgan M, et al. Elevated gray matter volume of the emotional cerebellum in women with premenstrual dysphoric disorder. J Affect Disord. 2013;146(2):266-271.
37. Jeong HG, Ham BJ, Yeo HB, et al. Gray matter abnormalities in patients with premenstrual dysphoric disorder: an optimized voxel-based morphometry. J Affect Disord. 2012;140(3):260-267.
38. Protopopescu X, Tuescher O, Pan H, et al. Toward a functional neuroanatomy of premenstrual dysphoric disorder. J Affect Disord. 2008;108(1-2):87-94.
39. Gingnell M, Morell A, Bannbers E, et al. Menstrual cycle effects on amygdala reactivity to emotional stimulation in premenstrual dysphoric disorder. Horm Behav. 2012;62(4):400-406.
40. Epperson CN, Haga K, Mason GF, et al. Cortical gamma-aminobutyric acid levels across the menstrual cycle in healthy women and those with premenstrual dysphoric disorder: a proton magnetic resonance spectroscopy study. Arch Gen Psychiatry. 2002;59(9):851-858.
41. Gingnell M, Bannbers E, Wikström J, et al. Premenstrual dysphoric disorder and prefrontal reactivity during anticipation of emotional stimuli. Eur Neuropsychopharmacol. 2013;23(11):1474-1483.
42. Baller EB, Wei SM, Kohn PD, et al. Abnormalities of dorsolateral prefrontal function in women with premenstrual dysphoric disorder: a multimodal neuroimaging study. Am J Psychiatry. 2013;170(3):305-314.
43. Rasgon N, McGuire M, Tanavoli S, et al. Neuroendocrine response to an intravenous L-tryptophan challenge in women with premenstrual syndrome. Fertil Steril. 2000;73(1):144-149.
44. Huang Y, Zhou R, Wu M, et al. Premenstrual syndrome is associated with blunted cortisol reactivity to the TSST. Stress. 2015;18(2):160-168.
45. Segebladh B, Bannbers E, Moby L, et al. Allopregnanolone serum concentrations and diurnal cortisol secretion in women with premenstrual dysphoric disorder. Arch Womens Ment Health. 2013;16(2):131-137.
46. Pilver CE, Levy BR, Libby DJ, et al. Posttraumatic stress disorder and trauma characteristics are correlates of premenstrual dysphoric disorder. Arch Womens Ment Health. 2011;14(5):383-393.
47. Bertone-Johnson ER, Whitcomb BW, Missmer SA, et al. Early life emotional, physical, and sexual abuse and the development of premenstrual syndrome: a longitudinal study. J Womens Health (Larchmt). 2014;23(9):729-739.
48. Segebladh B, Bannbers E, Kask K, et al. Prevalence of violence exposure in women with premenstrual dysphoric disorder in comparison with other gynecological patients and asymptomatic controls. Acta Obstet Gynecol Scand. 2011;90(7):746-752.
49. Kask K, Gulinello M, Bäckström T, et al. Patients with premenstrual dysphoric disorder have increased startle response across both cycle phases and lower levels of prepulse inhibition during the late luteal phase of the menstrual cycle. Neuropsychopharmacology. 2008;33(9):2283-2290.
50. O’Brien SM, Fitzgerald P, Scully P, et al. Impact of gender and menstrual cycle phase on plasma cytokine concentrations. Neuroimmunomodulation. 2007;14(2):84-90.
51. Northoff H, Symons S, Zieker D, et al. Gender- and menstrual phase dependent regulation of inflammatory gene expression in response to aerobic exercise. Exerc Immunol Rev. 2008;14:86-103.
52. Gaskins AJ, Wilchesky M, Mumford SL, et al. Endogenous reproductive hormones and C-reactive protein across the menstrual cycle: the BioCycle Study. Am J Epidemiol. 2012;175(5):423-431.
53. Wander K, Brindle E, O’Connor KA. C-reactive protein across the menstrual cycle. Am J Phys Anthropol. 2008;136(2):138-146.
54. Jane ZY, Chang CC, Lin HK, et al. The association between the exacerbation of irritable bowel syndrome and menstrual symptoms in young Taiwanese women. Gastroenterol Nurs. 2011;34(4):277-286.
55. Kane SV, Sable K, Hanauer SB. The menstrual cycle and its effect on inflammatory bowel disease and irritable bowel syndrome: a prevalence study. Am J Gastroenterol. 1998;93(10):1867-1872.
56. Shourie V, Dwarakanath CD, Prashanth GV, et al. The effect of menstrual cycle on periodontal health - a clinical and microbiological study. Oral Health Prev Dent. 2012;10(2):185-192.
57. Hantsoo L, Epperson CN. Premenstrual dysphoric disorder: epidemiology and treatment. Curr Psychiatry Rep. 2015;17(11):87.
58. Maeng LY, Milad MR. Sex differences in anxiety disorders: Interactions between fear, stress, and gonadal hormones. Horm Behav. 2015;76:106-117.
59. Lee YJ, Yi SW, Ju DH, et al. Correlation between postpartum depression and premenstrual dysphoric disorder: single center study. Obstet Gynecol Sci. 2015;58(5):353-358.
60. Kepple AL, Lee EE, Haq N, et al. History of postpartum depression in a clinic-based sample of women with premenstrual dysphoric disorder. J Clin Psychiatry. 2016;77(4):e415-e420.
61. Schmidt PJ, Nieman LK, Danaceau MA, et al. Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. N Engl J Med. 1998;338(4):209-216.
62. Schmidt PJ, Martinez PE, Nieman LK, et al. Premenstrual dysphoric disorder symptoms following ovarian suppression: Triggered by change in ovarian steroid levels but not continuous stable levels. Am J Psychiatry. [published online April 21, 2017]. doi: 10.1176/appi.ajp.2017.16101113.
1. Miller A, Vo H, Huo L, et al. Estrogen receptor alpha (ESR-1) associations with psychological traits in women with PMDD and controls. J Psychiatr Res. 2010;44(12):788-794.
2. Epperson CN, Steiner M, Hartlage SA, et al. Premenstrual dysphoric disorder: evidence for a new category for DSM-5. Am J Psychiatry. 2012;169(5):465-475.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Wilson CA, Turner CW, Keye WR Jr. Firstborn adolescent daughters and mothers with and without premenstrual syndrome: a comparison. J Adolesc Health. 1991;12(2):130-137.
5. Kendler KS, Silberg JL, Neale MC, et al. Genetic and environmental factors in the aetiology of menstrual, premenstrual and neurotic symptoms: a population-based twin study. Psychol Med. 1992;22(1):85-100.
6. Condon JT. The premenstrual syndrome: a twin study. Br J Psychiatry. 1993;162:481-486.
7. Kendler KS, Karkowski LM, Corey LA, et al. Longitudinal population-based twin study of retrospectively reported premenstrual symptoms and lifetime major depression. Am J Psychiatry. 1998;155(9):1234-1240.
8. Huo L, Straub RE, Roca C, et al. Risk for premenstrual dysphoric disorder is associated with genetic variation in ESR1, the estrogen receptor alpha gene. Biol Psychiatry. 2007;62(8):925-933.
9. Dhingra V, Magnay JL, O’Brien PM, et al. Serotonin receptor 1A C(-1019)G polymorphism associated with premenstrual dysphoric disorder. Obstet Gynecol. 2007;110(4):788-792.
10. Comasco E, Hahn A, Ganger S, et al. Emotional fronto-cingulate cortex activation and brain derived neurotrophic factor polymorphism in premenstrual dysphoric disorder. Hum Brain Mapp. 2014;35(9):4450-4458.
11. Praschak-Rieder N, Willeit M, Winkler D, et al. Role of family history and 5-HTTLPR polymorphism in female seasonal affective disorder patients with and without premenstrual dysphoric disorder. Eur Neuropsychopharmacol. 2002;12(2):129-134.
12. Klatzkin RR, Morrow AL, Light KC, et al. Associations of histories of depression and PMDD diagnosis with allopregnanolone concentrations following the oral administration of micronized progesterone. Psychoneuroendocrinology. 2006;31(10):1208-1219.
13. Crowley SK, Girdler SS. Neurosteroid, GABAergic and hypothalamic pituitary adrenal (HPA) axis regulation: what is the current state of knowledge in humans? Psychopharmacology (Berl). 2014;231(17):3619-3634.
14. Girdler SS, Straneva PA, Light KC, et al. Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biol Psychiatry. 2001;49(9):788-797.
15. Rapkin AJ, Morgan M, Goldman L, et al. Progesterone metabolite allopregnanolone in women with premenstrual syndrome. Obstet Gynecol. 1997;90(5):709-714.
16. Bicíková M, Dibbelt L, Hill M, et al. Allopregnanolone in women with premenstrual syndrome. Horm Metab Res. 1998;30(4):227-230.
17. Monteleone P, Luisi S, Tonetti A, et al. Allopregnanolone concentrations and premenstrual syndrome. Eur J Endocrinol. 2000;142(3):269-273.
18. Steiner M, Steinberg S, Stewart D, et al. Fluoxetine in the treatment of premenstrual dysphoria. Canadian Fluoxetine/Premenstrual Dysphoria Collaborative Study Group. N Engl J Med. 1995;332(23):1529-1534.
19. Sundström I, Bäckström T. Citalopram increases pregnanolone sensitivity in patients with premenstrual syndrome: an open trial. Psychoneuroendocrinology. 1998;23(1):73-88.
20. Griffin LD, Mellon SH. Selective serotonin reuptake inhibitors directly alter activity of neurosteroidogenic enzymes. Proc Natl Acad Sci U S A. 1999;96(23):13512-13517.
21. Trauger JW, Jiang A, Stearns BA, et al. Kinetics of allopregnanolone formation catalyzed by human 3 alpha-hydroxysteroid dehydrogenase type III (AKR1C2). Biochemistry. 2002;41(45):13451-13459.
22. Shanmugan S, Epperson CN. Estrogen and the prefrontal cortex: towards a new understanding of estrogen’s effects on executive functions in the menopause transition. Hum Brain Mapp. 2014;35(3):847-865.
23. Rubinow DR, Schmidt PJ, Roca CA. Estrogen-serotonin interactions: implications for affective regulation. Biol Psychiatry. 1998;44(9):839-850.
24. Amin Z, Canli T, Epperson CN. Effect of estrogen-serotonin interactions on mood and cognition. Behav Cogn Neurosci Rev. 2005;4(1):43-58.
25. Cyr M, Bossé R, Di Paolo T. Gonadal hormones modulate 5-hydroxytryptamine2A receptors: emphasis on the rat frontal cortex. Neuroscience. 1998;83(3):829-836.
26. Fink G, Sumner BE, Rosie R, et al. Estrogen control of central neurotransmission: effect on mood, mental state, and memory. Cell Mol Neurobiol. 1996;16(3):325-344.
27. Sumner BE, Grant KE, Rosie R, et al. Effects of tamoxifen on serotonin transporter and 5-hydroxytryptamine(2A) receptor binding sites and mRNA levels in the brain of ovariectomized rats with or without acute estradiol replacement. Brain Res Mol Brain Res. 1999;73(1-2):119-128.
28. Moses-Kolko EL, Berga SL, Greer PJ, et al. Widespread increases of cortical serotonin type 2A receptor availability after hormone therapy in euthymic postmenopausal women. Fertil Steril. 2003;80(3):554-559.
29. Su TP, Schmidt PJ, Danaceau MA, et al. Fluoxetine in the treatment of premenstrual dysphoria. Neuropsychopharmacology. 1997;16(5):346-356.
30. Steinberg EM, Cardoso GM, Martinez PE, et al. Rapid response to fluoxetine in women with premenstrual dysphoric disorder. Depress Anxiety. 2012;29(6):531-540.
31. Roca CA, Schmidt PJ, Smith MJ, et al. Effects of metergoline on symptoms in women with premenstrual dysphoric disorder. Am J Psychiatry. 2002;159(11):1876-1881.
32. Gray JD, Milner TA, McEwen BS. Dynamic plasticity: the role of glucocorticoids, brain-derived neurotrophic factor and other trophic factors. Neuroscience. 2013;239:214-227.
33. Carbone DL, Handa RJ. Sex and stress hormone influences on the expression and activity of brain-derived neurotrophic factor. Neuroscience. 2013;239:295-303.
34. Pilar-Cuéllar F, Vidal R, Pazos A. Subchronic treatment with fluoxetine and ketanserin increases hippocampal brain-derived neurotrophic factor, β-catenin and antidepressant-like effects. Br J Pharmacol. 2012;165(4b):1046-1057.
35. Deuschle M, Gilles M, Scharnholz B, et al. Changes of serum concentrations of brain-derived neurotrophic factor (BDNF) during treatment with venlafaxine and mirtazapine: role of medication and response to treatment. Pharmacopsychiatry. 2013;46(2):54-58.
36. Berman SM, London ED, Morgan M, et al. Elevated gray matter volume of the emotional cerebellum in women with premenstrual dysphoric disorder. J Affect Disord. 2013;146(2):266-271.
37. Jeong HG, Ham BJ, Yeo HB, et al. Gray matter abnormalities in patients with premenstrual dysphoric disorder: an optimized voxel-based morphometry. J Affect Disord. 2012;140(3):260-267.
38. Protopopescu X, Tuescher O, Pan H, et al. Toward a functional neuroanatomy of premenstrual dysphoric disorder. J Affect Disord. 2008;108(1-2):87-94.
39. Gingnell M, Morell A, Bannbers E, et al. Menstrual cycle effects on amygdala reactivity to emotional stimulation in premenstrual dysphoric disorder. Horm Behav. 2012;62(4):400-406.
40. Epperson CN, Haga K, Mason GF, et al. Cortical gamma-aminobutyric acid levels across the menstrual cycle in healthy women and those with premenstrual dysphoric disorder: a proton magnetic resonance spectroscopy study. Arch Gen Psychiatry. 2002;59(9):851-858.
41. Gingnell M, Bannbers E, Wikström J, et al. Premenstrual dysphoric disorder and prefrontal reactivity during anticipation of emotional stimuli. Eur Neuropsychopharmacol. 2013;23(11):1474-1483.
42. Baller EB, Wei SM, Kohn PD, et al. Abnormalities of dorsolateral prefrontal function in women with premenstrual dysphoric disorder: a multimodal neuroimaging study. Am J Psychiatry. 2013;170(3):305-314.
43. Rasgon N, McGuire M, Tanavoli S, et al. Neuroendocrine response to an intravenous L-tryptophan challenge in women with premenstrual syndrome. Fertil Steril. 2000;73(1):144-149.
44. Huang Y, Zhou R, Wu M, et al. Premenstrual syndrome is associated with blunted cortisol reactivity to the TSST. Stress. 2015;18(2):160-168.
45. Segebladh B, Bannbers E, Moby L, et al. Allopregnanolone serum concentrations and diurnal cortisol secretion in women with premenstrual dysphoric disorder. Arch Womens Ment Health. 2013;16(2):131-137.
46. Pilver CE, Levy BR, Libby DJ, et al. Posttraumatic stress disorder and trauma characteristics are correlates of premenstrual dysphoric disorder. Arch Womens Ment Health. 2011;14(5):383-393.
47. Bertone-Johnson ER, Whitcomb BW, Missmer SA, et al. Early life emotional, physical, and sexual abuse and the development of premenstrual syndrome: a longitudinal study. J Womens Health (Larchmt). 2014;23(9):729-739.
48. Segebladh B, Bannbers E, Kask K, et al. Prevalence of violence exposure in women with premenstrual dysphoric disorder in comparison with other gynecological patients and asymptomatic controls. Acta Obstet Gynecol Scand. 2011;90(7):746-752.
49. Kask K, Gulinello M, Bäckström T, et al. Patients with premenstrual dysphoric disorder have increased startle response across both cycle phases and lower levels of prepulse inhibition during the late luteal phase of the menstrual cycle. Neuropsychopharmacology. 2008;33(9):2283-2290.
50. O’Brien SM, Fitzgerald P, Scully P, et al. Impact of gender and menstrual cycle phase on plasma cytokine concentrations. Neuroimmunomodulation. 2007;14(2):84-90.
51. Northoff H, Symons S, Zieker D, et al. Gender- and menstrual phase dependent regulation of inflammatory gene expression in response to aerobic exercise. Exerc Immunol Rev. 2008;14:86-103.
52. Gaskins AJ, Wilchesky M, Mumford SL, et al. Endogenous reproductive hormones and C-reactive protein across the menstrual cycle: the BioCycle Study. Am J Epidemiol. 2012;175(5):423-431.
53. Wander K, Brindle E, O’Connor KA. C-reactive protein across the menstrual cycle. Am J Phys Anthropol. 2008;136(2):138-146.
54. Jane ZY, Chang CC, Lin HK, et al. The association between the exacerbation of irritable bowel syndrome and menstrual symptoms in young Taiwanese women. Gastroenterol Nurs. 2011;34(4):277-286.
55. Kane SV, Sable K, Hanauer SB. The menstrual cycle and its effect on inflammatory bowel disease and irritable bowel syndrome: a prevalence study. Am J Gastroenterol. 1998;93(10):1867-1872.
56. Shourie V, Dwarakanath CD, Prashanth GV, et al. The effect of menstrual cycle on periodontal health - a clinical and microbiological study. Oral Health Prev Dent. 2012;10(2):185-192.
57. Hantsoo L, Epperson CN. Premenstrual dysphoric disorder: epidemiology and treatment. Curr Psychiatry Rep. 2015;17(11):87.
58. Maeng LY, Milad MR. Sex differences in anxiety disorders: Interactions between fear, stress, and gonadal hormones. Horm Behav. 2015;76:106-117.
59. Lee YJ, Yi SW, Ju DH, et al. Correlation between postpartum depression and premenstrual dysphoric disorder: single center study. Obstet Gynecol Sci. 2015;58(5):353-358.
60. Kepple AL, Lee EE, Haq N, et al. History of postpartum depression in a clinic-based sample of women with premenstrual dysphoric disorder. J Clin Psychiatry. 2016;77(4):e415-e420.
61. Schmidt PJ, Nieman LK, Danaceau MA, et al. Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. N Engl J Med. 1998;338(4):209-216.
62. Schmidt PJ, Martinez PE, Nieman LK, et al. Premenstrual dysphoric disorder symptoms following ovarian suppression: Triggered by change in ovarian steroid levels but not continuous stable levels. Am J Psychiatry. [published online April 21, 2017]. doi: 10.1176/appi.ajp.2017.16101113.
How to preserve your own well-being in a challenging medical environment
Like all physicians, psychiatrists practice in an increasingly complex health care environment, with escalating demands for productivity, rising threats of malpractice, expanding clinical oversight, and growing concerns about income. Additionally, psychiatric practice presents its own challenges, including limited resources and concerns about patient violence and suicide. These concerns can make it difficult to establish a healthy work–life balance.
Physicians, including psychiatrists, are at risk for alcohol or substance abuse/dependency, burnout, and suicide. As psychiatrists, we need to attend to our own personal and professional health so that we can best help our patients. This review focuses on the challenges psychiatrists face that can adversely affect their well-being and offers strategies to reduce the risk of burnout and enhance wellness.
The challenges of medicine and their impact on psychiatrists
The practice of medicine is inherently challenging. It requires hard work, discipline, dedication, and faithfulness to high ethical standards. Additional challenges include declining autonomy and opportunities for social support, increasing accountability, and a growing interest in reducing the cost of care by employing more non-physician health professionals—which in psychiatry typically include psychologists, nurse practitioners, and social workers. The uncertainty of the Affordable Care Act, declining income, and concerns about the nature of future medical practice are also stressors.1,2
Factors that contribute to psychiatrists’ stress include:
- limited resources
- concerns about patient violence and suicide
- crowded inpatient units
- changing culture in mental health services
- high work demands
- poorly defined roles of consultants
- declining authority
- frustration with the inability to impact systemic change
- conflict between responsibility toward employers vs the patient
- isolation.3
Concern about patient suicide is a significant stressor.4,5 Some evidence suggests that the impact of a patient’s suicide on a physician is more severe when it occurs during training than after graduation and is inversely correlated with the clinician’s perceived social integration into their professional network.5
Impediments to a physician’s well-being
Alcohol abuse/dependence. Approximately 13% of male physicians and 21% of female physicians meet Alcohol Use Disorders Identification Test Version C criteria for alcohol abuse or dependence, according to a study of approximately 7,300 U.S. physicians from all specialties.6 (In this study, prescription drug abuse and use of illicit drugs were rare.) Age, hours worked, male sex, being married or partnered, having children, and being in a specialty other than internal medicine were independently associated with alcohol abuse or dependence.
Fortunately, psychiatrists were among the specialties with below average likelihood to meet diagnostic criteria for alcohol abuse/dependency.6 However, alcohol abuse or dependency was associated with burnout, depression, suicidal ideation, lower quality of life, lower career satisfaction, and medical errors.
Burnout is a long-term stress reaction consisting of:
- physical and emotional exhaustion (feeling depleted)
- depersonalization (cynicism, lack of engagement with or negative attitudes toward patients)
- reduced sense of personal accomplishment (lack of a sense of purpose).7
In a 2017 survey of >14,000 U.S. physicians from 27 specialties, 42% of psychiatrists reported burnout.8 In another survey of approximately 300 resident physicians across all specialties in a tertiary academic hospital, 69% met criteria for burnout.9 This condition affects resident physicians as well as those in practice. Residents and program directors cited a lack of work–life balance and feeling unappreciated as factors contributing to burnout.
Among physicians, factors that contribute to burnout include loss of autonomy, diminished status as physicians, and increased work pressures. Burnout has a negative impact on both patients and health care systems. It is associated with an increased risk of depression and can contribute to:
- broken relationships
- alcohol abuse
- physician suicide
- decreased quality of care, including patient safety and satisfaction
- increased risk of malpractice suits
- reduced patient adherence to medical recommendations.5,10-12
Physicians who embrace medicine as a calling (ie, committing one’s life to personally meaningful work that serves a prosocial purpose) experience less burnout. According to a survey of approximately 900 primary care physicians and 300 psychiatrists, 42% of psychiatrists strongly agreed that medicine is a calling.13 Overall, physicians with a high sense of calling reported less burnout than those with a lower sense of calling (17% vs 31%, respectively).13
Depression and suicide. Gold et al12 analyzed a database that included information on approximately 31,600 adult suicide victims, and 203 of these victims were physicians. Compared with others, physicians were more likely to have a diagnosed mental illness or an occupation-related problem that contributed to suicide. Toxicology results also showed that physician suicide victims were significantly more likely than non-physician victims to test positive for benzodiazepines and barbiturates, but not antidepressants, which suggests that physicians with depression may not have been receiving adequate treatment.12
Although occupation-related stress and inadequate mental health treatment may be modifiable risk factors to reduce suicide deaths among physicians, stigma and fear of medical staff and licensure issues may deter physicians from seeking treatment.14
Steps to avoid burnout
Evidence-based interventions. There is limited evidence-based data regarding specific interventions for preventing burnout and reducing stress among physicians, particularly among psychiatrists.4
A randomized controlled trial of 74 practicing physicians at the Mayo Clinic in Rochester, Minnesota, evaluated the effectiveness of 19 biweekly physician-facilitated discussion groups.15 The groups covered topics such as elements of mindfulness, reflection, shared experience, and small-group learning. The institution provided 1 hour of paid time every other week for physicians to participate in this program. Physicians in the control group could schedule and use this time as they chose. Researchers also collected data on 350 non-trial participants.
The proportion of participants who strongly agreed that their work was meaningful increased 6.3% in the intervention group but decreased 6.3% in the control group and 13.4% among non-trial participants (P = .04).15 Rates of depersonalization, emotional exhaustion, and overall burnout decreased substantially in the intervention group, decreased slightly in the control group, and increased in the non-trial cohort. Results were sustained at 12 months after the study. There were no statistically significant differences in stress, symptoms of depression, overall quality of life, or job satisfaction.15
Preliminary evidence suggests that residents and fellows would find a wellness or suicide prevention program helpful. One study found that the use of one such program, which provided individual counseling, psychiatric evaluation, and wellness workshops for residents, fellows, and faculty in an academic health center, increased from 5% to 25% of eligible participants, and participants reported high levels of satisfaction with the program.16 Such programs would require institutional support for space and clinical staff.15
Empathy. As psychiatrists, we are taught to be empathetic. Yet, with the numerous challenges we face, it is not always easy. Stressors such as an increased workload or burnout can adversely affect a psychiatrist’s ability to provide empathetic care.17 However, empathetic treatment has clear benefits for both physicians and patients. Empathic skills can lead to more professional satisfaction and outcomes, which are important components of accountability, and can:
- promote patient satisfaction
- establish trust
- reduce anxiety
- increase adherence to treatment regimens
- improve health outcomes
- decrease the likelihood of malpractice suits.17
Mindfulness is a “flexible state of mind in which we are actively engaged in the present, noticing new things and sensitive to context.”18,19 It may sound mundane to cling to phrases such as “living in the present,” but mindfulness can be a valuable tool for psychiatrists who struggle to maintain well-being in medicine’s challenging milieu. The process of mindfulness—actively drawing distinctions and noticing new things, “seeing the familiar in the novel and the novel in the familiar”—can ensure that we have active minds, that we are involved, and that we are capturing the joy of living in the stimulating present.18
Focus on issues you can control
Many of the factors that negatively influence professional satisfaction and well-being, such as loss of autonomy, demand for increased patient care volume, and increasing scrutiny on the quality of care, are beyond a psychiatrist’s control. Medical administrators can help reduce some of these issues by increasing physician autonomy, offering physicians the opportunity to work part-time, offering medical staff workshops to enhance positive communication, or addressing leadership problems. However, psychiatrists may benefit most by identifying modifiable issues under their own control, such as prioritizing a work–life balance, applying the fundamentals of a health prevention strategy to their own lives (Box20,21), approaching medicine as a calling, embracing an empathetic approach to patient care, and bringing mindfulness to medical practice.
1. Goitein L. Physician well-being: addressing downstream effects, but looking upstream. JAMA Intern Med. 2014;174(4):533-534.
2. Dunn PM, Arnetz BB, Christensen JF, et al. Meeting the imperative to improve physician well-being: assessment of an innovative program. J Gen Intern Med. 2007;22(11):1544-1552.
3. Kumar S. Burnout in psychiatrists. World Psychiatry. 2007;6(3):186-189.
4. Fothergill A, Edwards D, Burnard P. Stress, burnout, coping and stress management in psychiatrists: findings from a systematic review. Int J Soc Psychiatry. 2004;50(1):54-65.
5. Ruskin R, Sakinofsky I, Bagby RM, et al. Impact of patient suicide on psychiatrists and psychiatric trainees. Acad Psychiatry. 2004;28(2):104-110.
6. Oreskovich MR, Shanafelt T, Dyrbye LN, et al. The prevalence of substance use disorders in American physicians. Am J Addict. 2015;24(1):30-38.
7. Maslach C, Jackson SE. The measurement of experienced burnout. J Occup Behav. 1981;2:99-113.
8. Peckham C. Medscape Psychiatrist Lifestyle Report 2017: race and ethnicity, bias and burnout. http://www.medscape.com/features/slideshow/lifestyle/2017/psychiatry#page=1. Published January 11, 2017. Accessed July 25, 2017.
9. Holmes EG, Connolly A, Putnam KT, et al. Taking care of our own: a multispecialty study of resident and program director perspectives on contributors to burnout and potential interventions. Acad Psychiatry. 2017;41(2):159-166.
10. Shanafelt TD, Noseworthy JH. Executive leadership and physician well-being: nine organizational strategies to promote engagement and reduce burnout. Mayo Clin Proc. 2017;92(1):129-146.
11. Gold KJ, Sen A, Schwenk TL. Details on suicide among US physicians: data from the National Violent Death Reporting System. Gen Hosp Psychiatry. 2013;35(1):45-49.
12. Gold MS, Frost-Pineda K, Melker RJ. Physician suicide and drug abuse. Am J Psychiatry. 2005;162:1390; author reply 1390.
13. Yoon JD, Daley BM, Curlin FA. The association between a sense of calling and physician well-being: a national study of primary care physicians and psychiatrists. Acad Psychiatry. 2017;41(2):167-173.
14. Gold KJ, Andrew LB, Goldman EB, et al. “I would never want to have a mental health diagnosis on my record”: a survey of female physicians on mental health diagnosis, treatment, and reporting. Gen Hosp Psychiatry. 2016;43:51-57.
15. West CP, Dyrbye LN, Rabatin JT, et al. Intervention to promote physician well-being, job satisfaction, and professionalism: a randomized clinical trial. JAMA Intern Med. 2014;174(4):527-533.
16. Ey S, Moffit M, Kinzie JM, et al. Feasibility of a comprehensive wellness and suicide prevention program: a decade of caring for physicians in training and practice. J Grad Med Educ. 2016;8(5):747-753.
17. Newton BW. Walking a fine line: is it possible to remain an empathic physician and have a hardened heart? Front Hum Neurosci. 2013;7:233.
18. Langer EJ. Mindful learning: current directions in psychological science. Am Psychological Society. 2000(6);9:220-223.
19. Crum AJ, Langer EJ. Mind-set matters: exercise and the placebo effect. Psychol Sci. 2007;18(2):165-171.
20. U.S. Department of Health & Human Services, Office of the Surgeon General. National Prevention Strategy. https://www.surgeongeneral.gov/priorities/prevention/strategy/report.pdf. Published June 2011. Accessed July 26, 2017.
21. Benjamin RM. The national prevention strategy: shifting the nation’s health-care system. Public Health Rep. 2011;126(6):774-776.
Like all physicians, psychiatrists practice in an increasingly complex health care environment, with escalating demands for productivity, rising threats of malpractice, expanding clinical oversight, and growing concerns about income. Additionally, psychiatric practice presents its own challenges, including limited resources and concerns about patient violence and suicide. These concerns can make it difficult to establish a healthy work–life balance.
Physicians, including psychiatrists, are at risk for alcohol or substance abuse/dependency, burnout, and suicide. As psychiatrists, we need to attend to our own personal and professional health so that we can best help our patients. This review focuses on the challenges psychiatrists face that can adversely affect their well-being and offers strategies to reduce the risk of burnout and enhance wellness.
The challenges of medicine and their impact on psychiatrists
The practice of medicine is inherently challenging. It requires hard work, discipline, dedication, and faithfulness to high ethical standards. Additional challenges include declining autonomy and opportunities for social support, increasing accountability, and a growing interest in reducing the cost of care by employing more non-physician health professionals—which in psychiatry typically include psychologists, nurse practitioners, and social workers. The uncertainty of the Affordable Care Act, declining income, and concerns about the nature of future medical practice are also stressors.1,2
Factors that contribute to psychiatrists’ stress include:
- limited resources
- concerns about patient violence and suicide
- crowded inpatient units
- changing culture in mental health services
- high work demands
- poorly defined roles of consultants
- declining authority
- frustration with the inability to impact systemic change
- conflict between responsibility toward employers vs the patient
- isolation.3
Concern about patient suicide is a significant stressor.4,5 Some evidence suggests that the impact of a patient’s suicide on a physician is more severe when it occurs during training than after graduation and is inversely correlated with the clinician’s perceived social integration into their professional network.5
Impediments to a physician’s well-being
Alcohol abuse/dependence. Approximately 13% of male physicians and 21% of female physicians meet Alcohol Use Disorders Identification Test Version C criteria for alcohol abuse or dependence, according to a study of approximately 7,300 U.S. physicians from all specialties.6 (In this study, prescription drug abuse and use of illicit drugs were rare.) Age, hours worked, male sex, being married or partnered, having children, and being in a specialty other than internal medicine were independently associated with alcohol abuse or dependence.
Fortunately, psychiatrists were among the specialties with below average likelihood to meet diagnostic criteria for alcohol abuse/dependency.6 However, alcohol abuse or dependency was associated with burnout, depression, suicidal ideation, lower quality of life, lower career satisfaction, and medical errors.
Burnout is a long-term stress reaction consisting of:
- physical and emotional exhaustion (feeling depleted)
- depersonalization (cynicism, lack of engagement with or negative attitudes toward patients)
- reduced sense of personal accomplishment (lack of a sense of purpose).7
In a 2017 survey of >14,000 U.S. physicians from 27 specialties, 42% of psychiatrists reported burnout.8 In another survey of approximately 300 resident physicians across all specialties in a tertiary academic hospital, 69% met criteria for burnout.9 This condition affects resident physicians as well as those in practice. Residents and program directors cited a lack of work–life balance and feeling unappreciated as factors contributing to burnout.
Among physicians, factors that contribute to burnout include loss of autonomy, diminished status as physicians, and increased work pressures. Burnout has a negative impact on both patients and health care systems. It is associated with an increased risk of depression and can contribute to:
- broken relationships
- alcohol abuse
- physician suicide
- decreased quality of care, including patient safety and satisfaction
- increased risk of malpractice suits
- reduced patient adherence to medical recommendations.5,10-12
Physicians who embrace medicine as a calling (ie, committing one’s life to personally meaningful work that serves a prosocial purpose) experience less burnout. According to a survey of approximately 900 primary care physicians and 300 psychiatrists, 42% of psychiatrists strongly agreed that medicine is a calling.13 Overall, physicians with a high sense of calling reported less burnout than those with a lower sense of calling (17% vs 31%, respectively).13
Depression and suicide. Gold et al12 analyzed a database that included information on approximately 31,600 adult suicide victims, and 203 of these victims were physicians. Compared with others, physicians were more likely to have a diagnosed mental illness or an occupation-related problem that contributed to suicide. Toxicology results also showed that physician suicide victims were significantly more likely than non-physician victims to test positive for benzodiazepines and barbiturates, but not antidepressants, which suggests that physicians with depression may not have been receiving adequate treatment.12
Although occupation-related stress and inadequate mental health treatment may be modifiable risk factors to reduce suicide deaths among physicians, stigma and fear of medical staff and licensure issues may deter physicians from seeking treatment.14
Steps to avoid burnout
Evidence-based interventions. There is limited evidence-based data regarding specific interventions for preventing burnout and reducing stress among physicians, particularly among psychiatrists.4
A randomized controlled trial of 74 practicing physicians at the Mayo Clinic in Rochester, Minnesota, evaluated the effectiveness of 19 biweekly physician-facilitated discussion groups.15 The groups covered topics such as elements of mindfulness, reflection, shared experience, and small-group learning. The institution provided 1 hour of paid time every other week for physicians to participate in this program. Physicians in the control group could schedule and use this time as they chose. Researchers also collected data on 350 non-trial participants.
The proportion of participants who strongly agreed that their work was meaningful increased 6.3% in the intervention group but decreased 6.3% in the control group and 13.4% among non-trial participants (P = .04).15 Rates of depersonalization, emotional exhaustion, and overall burnout decreased substantially in the intervention group, decreased slightly in the control group, and increased in the non-trial cohort. Results were sustained at 12 months after the study. There were no statistically significant differences in stress, symptoms of depression, overall quality of life, or job satisfaction.15
Preliminary evidence suggests that residents and fellows would find a wellness or suicide prevention program helpful. One study found that the use of one such program, which provided individual counseling, psychiatric evaluation, and wellness workshops for residents, fellows, and faculty in an academic health center, increased from 5% to 25% of eligible participants, and participants reported high levels of satisfaction with the program.16 Such programs would require institutional support for space and clinical staff.15
Empathy. As psychiatrists, we are taught to be empathetic. Yet, with the numerous challenges we face, it is not always easy. Stressors such as an increased workload or burnout can adversely affect a psychiatrist’s ability to provide empathetic care.17 However, empathetic treatment has clear benefits for both physicians and patients. Empathic skills can lead to more professional satisfaction and outcomes, which are important components of accountability, and can:
- promote patient satisfaction
- establish trust
- reduce anxiety
- increase adherence to treatment regimens
- improve health outcomes
- decrease the likelihood of malpractice suits.17
Mindfulness is a “flexible state of mind in which we are actively engaged in the present, noticing new things and sensitive to context.”18,19 It may sound mundane to cling to phrases such as “living in the present,” but mindfulness can be a valuable tool for psychiatrists who struggle to maintain well-being in medicine’s challenging milieu. The process of mindfulness—actively drawing distinctions and noticing new things, “seeing the familiar in the novel and the novel in the familiar”—can ensure that we have active minds, that we are involved, and that we are capturing the joy of living in the stimulating present.18
Focus on issues you can control
Many of the factors that negatively influence professional satisfaction and well-being, such as loss of autonomy, demand for increased patient care volume, and increasing scrutiny on the quality of care, are beyond a psychiatrist’s control. Medical administrators can help reduce some of these issues by increasing physician autonomy, offering physicians the opportunity to work part-time, offering medical staff workshops to enhance positive communication, or addressing leadership problems. However, psychiatrists may benefit most by identifying modifiable issues under their own control, such as prioritizing a work–life balance, applying the fundamentals of a health prevention strategy to their own lives (Box20,21), approaching medicine as a calling, embracing an empathetic approach to patient care, and bringing mindfulness to medical practice.
Like all physicians, psychiatrists practice in an increasingly complex health care environment, with escalating demands for productivity, rising threats of malpractice, expanding clinical oversight, and growing concerns about income. Additionally, psychiatric practice presents its own challenges, including limited resources and concerns about patient violence and suicide. These concerns can make it difficult to establish a healthy work–life balance.
Physicians, including psychiatrists, are at risk for alcohol or substance abuse/dependency, burnout, and suicide. As psychiatrists, we need to attend to our own personal and professional health so that we can best help our patients. This review focuses on the challenges psychiatrists face that can adversely affect their well-being and offers strategies to reduce the risk of burnout and enhance wellness.
The challenges of medicine and their impact on psychiatrists
The practice of medicine is inherently challenging. It requires hard work, discipline, dedication, and faithfulness to high ethical standards. Additional challenges include declining autonomy and opportunities for social support, increasing accountability, and a growing interest in reducing the cost of care by employing more non-physician health professionals—which in psychiatry typically include psychologists, nurse practitioners, and social workers. The uncertainty of the Affordable Care Act, declining income, and concerns about the nature of future medical practice are also stressors.1,2
Factors that contribute to psychiatrists’ stress include:
- limited resources
- concerns about patient violence and suicide
- crowded inpatient units
- changing culture in mental health services
- high work demands
- poorly defined roles of consultants
- declining authority
- frustration with the inability to impact systemic change
- conflict between responsibility toward employers vs the patient
- isolation.3
Concern about patient suicide is a significant stressor.4,5 Some evidence suggests that the impact of a patient’s suicide on a physician is more severe when it occurs during training than after graduation and is inversely correlated with the clinician’s perceived social integration into their professional network.5
Impediments to a physician’s well-being
Alcohol abuse/dependence. Approximately 13% of male physicians and 21% of female physicians meet Alcohol Use Disorders Identification Test Version C criteria for alcohol abuse or dependence, according to a study of approximately 7,300 U.S. physicians from all specialties.6 (In this study, prescription drug abuse and use of illicit drugs were rare.) Age, hours worked, male sex, being married or partnered, having children, and being in a specialty other than internal medicine were independently associated with alcohol abuse or dependence.
Fortunately, psychiatrists were among the specialties with below average likelihood to meet diagnostic criteria for alcohol abuse/dependency.6 However, alcohol abuse or dependency was associated with burnout, depression, suicidal ideation, lower quality of life, lower career satisfaction, and medical errors.
Burnout is a long-term stress reaction consisting of:
- physical and emotional exhaustion (feeling depleted)
- depersonalization (cynicism, lack of engagement with or negative attitudes toward patients)
- reduced sense of personal accomplishment (lack of a sense of purpose).7
In a 2017 survey of >14,000 U.S. physicians from 27 specialties, 42% of psychiatrists reported burnout.8 In another survey of approximately 300 resident physicians across all specialties in a tertiary academic hospital, 69% met criteria for burnout.9 This condition affects resident physicians as well as those in practice. Residents and program directors cited a lack of work–life balance and feeling unappreciated as factors contributing to burnout.
Among physicians, factors that contribute to burnout include loss of autonomy, diminished status as physicians, and increased work pressures. Burnout has a negative impact on both patients and health care systems. It is associated with an increased risk of depression and can contribute to:
- broken relationships
- alcohol abuse
- physician suicide
- decreased quality of care, including patient safety and satisfaction
- increased risk of malpractice suits
- reduced patient adherence to medical recommendations.5,10-12
Physicians who embrace medicine as a calling (ie, committing one’s life to personally meaningful work that serves a prosocial purpose) experience less burnout. According to a survey of approximately 900 primary care physicians and 300 psychiatrists, 42% of psychiatrists strongly agreed that medicine is a calling.13 Overall, physicians with a high sense of calling reported less burnout than those with a lower sense of calling (17% vs 31%, respectively).13
Depression and suicide. Gold et al12 analyzed a database that included information on approximately 31,600 adult suicide victims, and 203 of these victims were physicians. Compared with others, physicians were more likely to have a diagnosed mental illness or an occupation-related problem that contributed to suicide. Toxicology results also showed that physician suicide victims were significantly more likely than non-physician victims to test positive for benzodiazepines and barbiturates, but not antidepressants, which suggests that physicians with depression may not have been receiving adequate treatment.12
Although occupation-related stress and inadequate mental health treatment may be modifiable risk factors to reduce suicide deaths among physicians, stigma and fear of medical staff and licensure issues may deter physicians from seeking treatment.14
Steps to avoid burnout
Evidence-based interventions. There is limited evidence-based data regarding specific interventions for preventing burnout and reducing stress among physicians, particularly among psychiatrists.4
A randomized controlled trial of 74 practicing physicians at the Mayo Clinic in Rochester, Minnesota, evaluated the effectiveness of 19 biweekly physician-facilitated discussion groups.15 The groups covered topics such as elements of mindfulness, reflection, shared experience, and small-group learning. The institution provided 1 hour of paid time every other week for physicians to participate in this program. Physicians in the control group could schedule and use this time as they chose. Researchers also collected data on 350 non-trial participants.
The proportion of participants who strongly agreed that their work was meaningful increased 6.3% in the intervention group but decreased 6.3% in the control group and 13.4% among non-trial participants (P = .04).15 Rates of depersonalization, emotional exhaustion, and overall burnout decreased substantially in the intervention group, decreased slightly in the control group, and increased in the non-trial cohort. Results were sustained at 12 months after the study. There were no statistically significant differences in stress, symptoms of depression, overall quality of life, or job satisfaction.15
Preliminary evidence suggests that residents and fellows would find a wellness or suicide prevention program helpful. One study found that the use of one such program, which provided individual counseling, psychiatric evaluation, and wellness workshops for residents, fellows, and faculty in an academic health center, increased from 5% to 25% of eligible participants, and participants reported high levels of satisfaction with the program.16 Such programs would require institutional support for space and clinical staff.15
Empathy. As psychiatrists, we are taught to be empathetic. Yet, with the numerous challenges we face, it is not always easy. Stressors such as an increased workload or burnout can adversely affect a psychiatrist’s ability to provide empathetic care.17 However, empathetic treatment has clear benefits for both physicians and patients. Empathic skills can lead to more professional satisfaction and outcomes, which are important components of accountability, and can:
- promote patient satisfaction
- establish trust
- reduce anxiety
- increase adherence to treatment regimens
- improve health outcomes
- decrease the likelihood of malpractice suits.17
Mindfulness is a “flexible state of mind in which we are actively engaged in the present, noticing new things and sensitive to context.”18,19 It may sound mundane to cling to phrases such as “living in the present,” but mindfulness can be a valuable tool for psychiatrists who struggle to maintain well-being in medicine’s challenging milieu. The process of mindfulness—actively drawing distinctions and noticing new things, “seeing the familiar in the novel and the novel in the familiar”—can ensure that we have active minds, that we are involved, and that we are capturing the joy of living in the stimulating present.18
Focus on issues you can control
Many of the factors that negatively influence professional satisfaction and well-being, such as loss of autonomy, demand for increased patient care volume, and increasing scrutiny on the quality of care, are beyond a psychiatrist’s control. Medical administrators can help reduce some of these issues by increasing physician autonomy, offering physicians the opportunity to work part-time, offering medical staff workshops to enhance positive communication, or addressing leadership problems. However, psychiatrists may benefit most by identifying modifiable issues under their own control, such as prioritizing a work–life balance, applying the fundamentals of a health prevention strategy to their own lives (Box20,21), approaching medicine as a calling, embracing an empathetic approach to patient care, and bringing mindfulness to medical practice.
1. Goitein L. Physician well-being: addressing downstream effects, but looking upstream. JAMA Intern Med. 2014;174(4):533-534.
2. Dunn PM, Arnetz BB, Christensen JF, et al. Meeting the imperative to improve physician well-being: assessment of an innovative program. J Gen Intern Med. 2007;22(11):1544-1552.
3. Kumar S. Burnout in psychiatrists. World Psychiatry. 2007;6(3):186-189.
4. Fothergill A, Edwards D, Burnard P. Stress, burnout, coping and stress management in psychiatrists: findings from a systematic review. Int J Soc Psychiatry. 2004;50(1):54-65.
5. Ruskin R, Sakinofsky I, Bagby RM, et al. Impact of patient suicide on psychiatrists and psychiatric trainees. Acad Psychiatry. 2004;28(2):104-110.
6. Oreskovich MR, Shanafelt T, Dyrbye LN, et al. The prevalence of substance use disorders in American physicians. Am J Addict. 2015;24(1):30-38.
7. Maslach C, Jackson SE. The measurement of experienced burnout. J Occup Behav. 1981;2:99-113.
8. Peckham C. Medscape Psychiatrist Lifestyle Report 2017: race and ethnicity, bias and burnout. http://www.medscape.com/features/slideshow/lifestyle/2017/psychiatry#page=1. Published January 11, 2017. Accessed July 25, 2017.
9. Holmes EG, Connolly A, Putnam KT, et al. Taking care of our own: a multispecialty study of resident and program director perspectives on contributors to burnout and potential interventions. Acad Psychiatry. 2017;41(2):159-166.
10. Shanafelt TD, Noseworthy JH. Executive leadership and physician well-being: nine organizational strategies to promote engagement and reduce burnout. Mayo Clin Proc. 2017;92(1):129-146.
11. Gold KJ, Sen A, Schwenk TL. Details on suicide among US physicians: data from the National Violent Death Reporting System. Gen Hosp Psychiatry. 2013;35(1):45-49.
12. Gold MS, Frost-Pineda K, Melker RJ. Physician suicide and drug abuse. Am J Psychiatry. 2005;162:1390; author reply 1390.
13. Yoon JD, Daley BM, Curlin FA. The association between a sense of calling and physician well-being: a national study of primary care physicians and psychiatrists. Acad Psychiatry. 2017;41(2):167-173.
14. Gold KJ, Andrew LB, Goldman EB, et al. “I would never want to have a mental health diagnosis on my record”: a survey of female physicians on mental health diagnosis, treatment, and reporting. Gen Hosp Psychiatry. 2016;43:51-57.
15. West CP, Dyrbye LN, Rabatin JT, et al. Intervention to promote physician well-being, job satisfaction, and professionalism: a randomized clinical trial. JAMA Intern Med. 2014;174(4):527-533.
16. Ey S, Moffit M, Kinzie JM, et al. Feasibility of a comprehensive wellness and suicide prevention program: a decade of caring for physicians in training and practice. J Grad Med Educ. 2016;8(5):747-753.
17. Newton BW. Walking a fine line: is it possible to remain an empathic physician and have a hardened heart? Front Hum Neurosci. 2013;7:233.
18. Langer EJ. Mindful learning: current directions in psychological science. Am Psychological Society. 2000(6);9:220-223.
19. Crum AJ, Langer EJ. Mind-set matters: exercise and the placebo effect. Psychol Sci. 2007;18(2):165-171.
20. U.S. Department of Health & Human Services, Office of the Surgeon General. National Prevention Strategy. https://www.surgeongeneral.gov/priorities/prevention/strategy/report.pdf. Published June 2011. Accessed July 26, 2017.
21. Benjamin RM. The national prevention strategy: shifting the nation’s health-care system. Public Health Rep. 2011;126(6):774-776.
1. Goitein L. Physician well-being: addressing downstream effects, but looking upstream. JAMA Intern Med. 2014;174(4):533-534.
2. Dunn PM, Arnetz BB, Christensen JF, et al. Meeting the imperative to improve physician well-being: assessment of an innovative program. J Gen Intern Med. 2007;22(11):1544-1552.
3. Kumar S. Burnout in psychiatrists. World Psychiatry. 2007;6(3):186-189.
4. Fothergill A, Edwards D, Burnard P. Stress, burnout, coping and stress management in psychiatrists: findings from a systematic review. Int J Soc Psychiatry. 2004;50(1):54-65.
5. Ruskin R, Sakinofsky I, Bagby RM, et al. Impact of patient suicide on psychiatrists and psychiatric trainees. Acad Psychiatry. 2004;28(2):104-110.
6. Oreskovich MR, Shanafelt T, Dyrbye LN, et al. The prevalence of substance use disorders in American physicians. Am J Addict. 2015;24(1):30-38.
7. Maslach C, Jackson SE. The measurement of experienced burnout. J Occup Behav. 1981;2:99-113.
8. Peckham C. Medscape Psychiatrist Lifestyle Report 2017: race and ethnicity, bias and burnout. http://www.medscape.com/features/slideshow/lifestyle/2017/psychiatry#page=1. Published January 11, 2017. Accessed July 25, 2017.
9. Holmes EG, Connolly A, Putnam KT, et al. Taking care of our own: a multispecialty study of resident and program director perspectives on contributors to burnout and potential interventions. Acad Psychiatry. 2017;41(2):159-166.
10. Shanafelt TD, Noseworthy JH. Executive leadership and physician well-being: nine organizational strategies to promote engagement and reduce burnout. Mayo Clin Proc. 2017;92(1):129-146.
11. Gold KJ, Sen A, Schwenk TL. Details on suicide among US physicians: data from the National Violent Death Reporting System. Gen Hosp Psychiatry. 2013;35(1):45-49.
12. Gold MS, Frost-Pineda K, Melker RJ. Physician suicide and drug abuse. Am J Psychiatry. 2005;162:1390; author reply 1390.
13. Yoon JD, Daley BM, Curlin FA. The association between a sense of calling and physician well-being: a national study of primary care physicians and psychiatrists. Acad Psychiatry. 2017;41(2):167-173.
14. Gold KJ, Andrew LB, Goldman EB, et al. “I would never want to have a mental health diagnosis on my record”: a survey of female physicians on mental health diagnosis, treatment, and reporting. Gen Hosp Psychiatry. 2016;43:51-57.
15. West CP, Dyrbye LN, Rabatin JT, et al. Intervention to promote physician well-being, job satisfaction, and professionalism: a randomized clinical trial. JAMA Intern Med. 2014;174(4):527-533.
16. Ey S, Moffit M, Kinzie JM, et al. Feasibility of a comprehensive wellness and suicide prevention program: a decade of caring for physicians in training and practice. J Grad Med Educ. 2016;8(5):747-753.
17. Newton BW. Walking a fine line: is it possible to remain an empathic physician and have a hardened heart? Front Hum Neurosci. 2013;7:233.
18. Langer EJ. Mindful learning: current directions in psychological science. Am Psychological Society. 2000(6);9:220-223.
19. Crum AJ, Langer EJ. Mind-set matters: exercise and the placebo effect. Psychol Sci. 2007;18(2):165-171.
20. U.S. Department of Health & Human Services, Office of the Surgeon General. National Prevention Strategy. https://www.surgeongeneral.gov/priorities/prevention/strategy/report.pdf. Published June 2011. Accessed July 26, 2017.
21. Benjamin RM. The national prevention strategy: shifting the nation’s health-care system. Public Health Rep. 2011;126(6):774-776.
Can melatonin alleviate antipsychotic-induced weight gain?
Second-generation antipsychotics (SGAs) have been a remarkably effective innovation in psychotropic therapy. Unfortunately, the metabolic effects of these medications
Modabbernia et al1 demonstrated positive results from melatonin augmentation in an 8-week, randomized, double-blind, placebo-controlled study of 48 patients with first-episode schizophrenia. Compared with patients who received olanzapine and placebo, those taking olanzapine and melatonin, 3 mg/d, had significantly less weight gain, smaller increases in abdominal obesity, and lower triglycerides. Patients who were given melatonin also had a significantly greater reduction on the Positive and Negative Symptom Scale score.1
Romo-Nava et al2 had similar findings in an 8-week, randomized, double-blind, placebo-controlled trial. Forty-four patients (24 with schizophrenia, 20 with bipolar disorder) who were taking clozapine, quetiapine, risperidone, or olanzapine received adjunctive melatonin, 5 mg/d, or placebo. Patients receiving melatonin had significantly less weight gain (P = .04) and significantly reduced diastolic blood pressure (5.1 vs 1.1 mm Hg; P = .03).
In both studies, researchers hypothesized that melatonin exerted its effect through the suprachiasmatic nucleus—the part of the hypothalamus that regulates body weight, energy balance, and metabolism. Exogenous melatonin suppresses intra-abdominal fat and restores serum leptin and insulin levels in middle-aged rats, partly due to correcting the age-related decline in melatonin production.3
Wang et al4 conducted a systematic review of using melatonin in patients taking SGAs. In addition to preventing metabolic adverse effects of antipsychotics, melatonin also reduced weight gain from lithium.
Early evidence suggests that this inexpensive and relatively safe augmenting agent can minimize metabolic effects of SGAs. It is surprising that scheduled melatonin has eluded popular use in psychiatry.
1. Modabbernia A, Heidari P, Soleimani R, et al. Melatonin for prevention of metabolic side-effects of olanzapine in patients with first-episode schizophrenia: randomized double-blind placebo-controlled study. J Psychiatr Res. 2014;53:133-140.
2. Romo-Nava F, Alvarez-Icaza González D, Fresán-Orellana A, et al. Melatonin attenuates antipsychotic metabolic effects: an eight-week randomized, double-blind, parallel-group, placebo-controlled clinical trial. Bipolar Disord. 2014;16(4):410-421.
3. Rasmussen DD, Marck BT, Boldt BM, et al. Suppression of hypothalamic pro-opiomelanocortin (POMC) gene expression by daily melatonin supplementation in aging rats. J Pineal Res. 2003;34(2):127-133.
4. Wang HR, Woo YS, Bahk WM. The role of melatonin and melatonin agonists in counteracting antipsychotic-induced metabolic side effects: a systematic review. Int Clin Psychopharmacol. 2016;31(6):301-306.
Second-generation antipsychotics (SGAs) have been a remarkably effective innovation in psychotropic therapy. Unfortunately, the metabolic effects of these medications
Modabbernia et al1 demonstrated positive results from melatonin augmentation in an 8-week, randomized, double-blind, placebo-controlled study of 48 patients with first-episode schizophrenia. Compared with patients who received olanzapine and placebo, those taking olanzapine and melatonin, 3 mg/d, had significantly less weight gain, smaller increases in abdominal obesity, and lower triglycerides. Patients who were given melatonin also had a significantly greater reduction on the Positive and Negative Symptom Scale score.1
Romo-Nava et al2 had similar findings in an 8-week, randomized, double-blind, placebo-controlled trial. Forty-four patients (24 with schizophrenia, 20 with bipolar disorder) who were taking clozapine, quetiapine, risperidone, or olanzapine received adjunctive melatonin, 5 mg/d, or placebo. Patients receiving melatonin had significantly less weight gain (P = .04) and significantly reduced diastolic blood pressure (5.1 vs 1.1 mm Hg; P = .03).
In both studies, researchers hypothesized that melatonin exerted its effect through the suprachiasmatic nucleus—the part of the hypothalamus that regulates body weight, energy balance, and metabolism. Exogenous melatonin suppresses intra-abdominal fat and restores serum leptin and insulin levels in middle-aged rats, partly due to correcting the age-related decline in melatonin production.3
Wang et al4 conducted a systematic review of using melatonin in patients taking SGAs. In addition to preventing metabolic adverse effects of antipsychotics, melatonin also reduced weight gain from lithium.
Early evidence suggests that this inexpensive and relatively safe augmenting agent can minimize metabolic effects of SGAs. It is surprising that scheduled melatonin has eluded popular use in psychiatry.
Second-generation antipsychotics (SGAs) have been a remarkably effective innovation in psychotropic therapy. Unfortunately, the metabolic effects of these medications
Modabbernia et al1 demonstrated positive results from melatonin augmentation in an 8-week, randomized, double-blind, placebo-controlled study of 48 patients with first-episode schizophrenia. Compared with patients who received olanzapine and placebo, those taking olanzapine and melatonin, 3 mg/d, had significantly less weight gain, smaller increases in abdominal obesity, and lower triglycerides. Patients who were given melatonin also had a significantly greater reduction on the Positive and Negative Symptom Scale score.1
Romo-Nava et al2 had similar findings in an 8-week, randomized, double-blind, placebo-controlled trial. Forty-four patients (24 with schizophrenia, 20 with bipolar disorder) who were taking clozapine, quetiapine, risperidone, or olanzapine received adjunctive melatonin, 5 mg/d, or placebo. Patients receiving melatonin had significantly less weight gain (P = .04) and significantly reduced diastolic blood pressure (5.1 vs 1.1 mm Hg; P = .03).
In both studies, researchers hypothesized that melatonin exerted its effect through the suprachiasmatic nucleus—the part of the hypothalamus that regulates body weight, energy balance, and metabolism. Exogenous melatonin suppresses intra-abdominal fat and restores serum leptin and insulin levels in middle-aged rats, partly due to correcting the age-related decline in melatonin production.3
Wang et al4 conducted a systematic review of using melatonin in patients taking SGAs. In addition to preventing metabolic adverse effects of antipsychotics, melatonin also reduced weight gain from lithium.
Early evidence suggests that this inexpensive and relatively safe augmenting agent can minimize metabolic effects of SGAs. It is surprising that scheduled melatonin has eluded popular use in psychiatry.
1. Modabbernia A, Heidari P, Soleimani R, et al. Melatonin for prevention of metabolic side-effects of olanzapine in patients with first-episode schizophrenia: randomized double-blind placebo-controlled study. J Psychiatr Res. 2014;53:133-140.
2. Romo-Nava F, Alvarez-Icaza González D, Fresán-Orellana A, et al. Melatonin attenuates antipsychotic metabolic effects: an eight-week randomized, double-blind, parallel-group, placebo-controlled clinical trial. Bipolar Disord. 2014;16(4):410-421.
3. Rasmussen DD, Marck BT, Boldt BM, et al. Suppression of hypothalamic pro-opiomelanocortin (POMC) gene expression by daily melatonin supplementation in aging rats. J Pineal Res. 2003;34(2):127-133.
4. Wang HR, Woo YS, Bahk WM. The role of melatonin and melatonin agonists in counteracting antipsychotic-induced metabolic side effects: a systematic review. Int Clin Psychopharmacol. 2016;31(6):301-306.
1. Modabbernia A, Heidari P, Soleimani R, et al. Melatonin for prevention of metabolic side-effects of olanzapine in patients with first-episode schizophrenia: randomized double-blind placebo-controlled study. J Psychiatr Res. 2014;53:133-140.
2. Romo-Nava F, Alvarez-Icaza González D, Fresán-Orellana A, et al. Melatonin attenuates antipsychotic metabolic effects: an eight-week randomized, double-blind, parallel-group, placebo-controlled clinical trial. Bipolar Disord. 2014;16(4):410-421.
3. Rasmussen DD, Marck BT, Boldt BM, et al. Suppression of hypothalamic pro-opiomelanocortin (POMC) gene expression by daily melatonin supplementation in aging rats. J Pineal Res. 2003;34(2):127-133.
4. Wang HR, Woo YS, Bahk WM. The role of melatonin and melatonin agonists in counteracting antipsychotic-induced metabolic side effects: a systematic review. Int Clin Psychopharmacol. 2016;31(6):301-306.
Latest recommendations for the 2017-2018 flu season
The Centers for Disease Control and Prevention (CDC) recently reported details of the 2016-2017 influenza season in Morbidity and Mortality Weekly Report1 and at the June meeting of the Advisory Committee on Immunization Practices. The CDC monitors influenza activity using several systems, and last flu season was shown to be moderately severe, starting in December in the Western United States, moving east, and peaking in February.
During the peak, 5.1% of outpatient visits were attributed to influenza-like illnesses, and 8.2% of reported deaths were due to pneumonia and influenza. For the whole influenza season, there were more than 18,000 confirmed influenza-related hospitalizations, with 60% of these occurring among those ≥65 years.1 Confirmed influenza-associated pediatric deaths totaled 98.1
The predominant influenza strain last year was type A (H3N2), accounting for about 76% of positive tests in public health laboratories (FIGURE).1 This was followed by influenza B (all lineages) at 22%, and influenza A (H1N1), accounting for only 2%. However, in early April, the predominant strain changed from A (H3N2) to influenza B. Importantly, all viruses tested last year were sensitive to oseltamivir, zanamivir, and peramivir. No antiviral resistance was detected to these neuraminidase inhibitors.
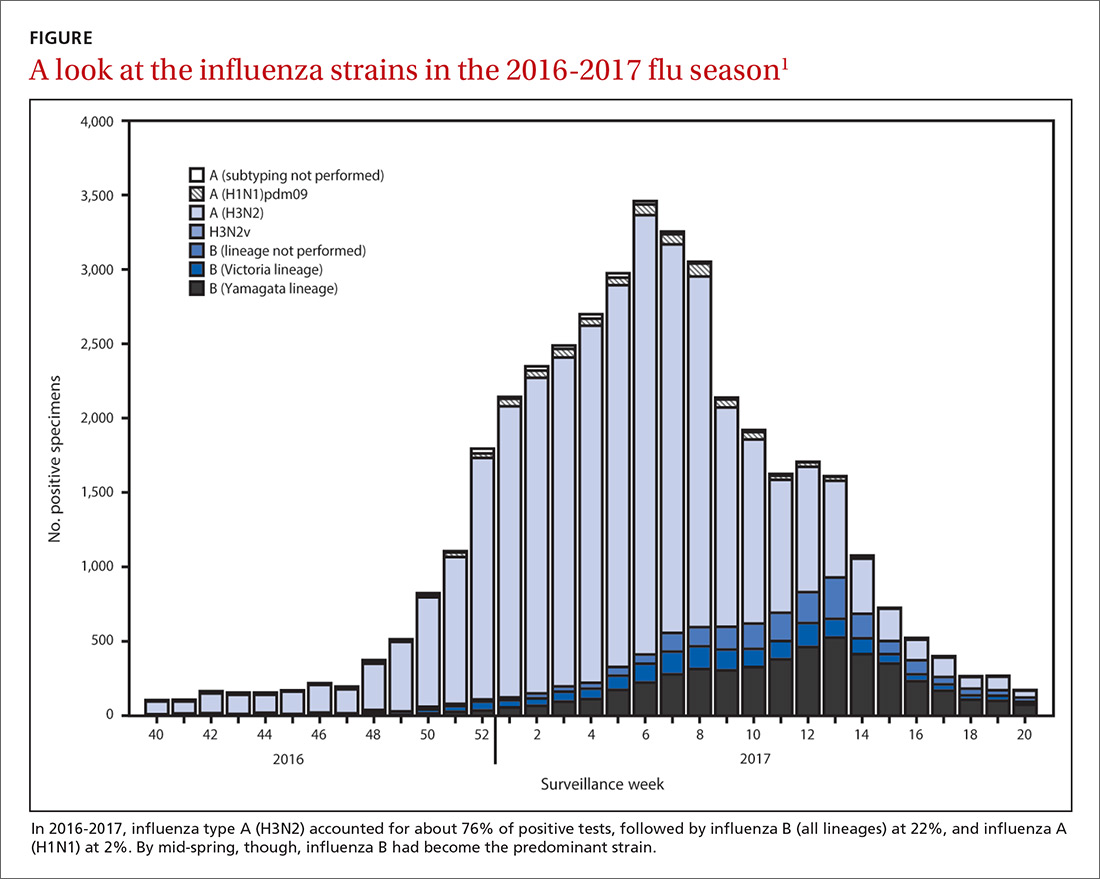
Good news and bad news on vaccine effectiveness. The good news: Circulating viruses were a close match to those contained in the vaccine. The bad news: Vaccine effectiveness at preventing illness was estimated to be just 34% against A (H3N2) and 56% against influenza B viruses.1 There has been no analysis of the relative effectiveness of different vaccines and vaccine types.
The past 6 influenza seasons have revealed a pattern of lower vaccine effectiveness against A (H3N2) compared with effectiveness against A (H1N1) and influenza B viruses. While vaccine effectiveness is not optimal, routine universal use still prevents a great deal of mortality and morbidity. It’s estimated that in 2012-2013, vaccine effectiveness (comparable to that in 2016-2017) prevented 5.6 million illnesses, 2.7 million medical visits, 61,500 hospitalizations, and 1800 deaths.1
More good news: Vaccine safety studies are reassuring
The CDC monitors influenza vaccine safety by using several sources, including the Vaccine Adverse Event Reporting System and the Vaccine Safety Datalink.2
Changes for the 2017-2018 influenza season
The composition of influenza vaccine products for the 2017-2018 season will differ slightly from last year’s formulation in the H1N1 component. Viral antigens to be included in the trivalent products are A/Michigan (H1N1), A/Hong Kong (H3N2), and B/Brisbane.3 Quadrivalent products will add B/Phuket to the other 3 antigens.3 A wide array of influenza vaccine products is available. Each one is described on the CDC Web site.4
Two minor changes in the recommendations were made at the June ACIP meeting.5 Afluria is approved by the FDA for use in children starting at age 5 years. ACIP had recommended that its use be reserved for children 9 years and older because previous influenza seasons had raised concerns about increased rates of febrile seizures in children younger than age 9. These concerns have been resolved, however, and the ACIP recommendations are now in concert with those of the FDA for this product.
Influenza immunization with an inactivated influenza vaccine product has been recommended for all pregnant women. Safety data are increasingly available for other product options as well, and ACIP now recommends vaccination in pregnancy with any age-appropriate product except for live attenuated influenza vaccine. 5
Antivirals: Give as needed, even before lab confirmation
The CDC recommends antiviral medication for individuals with confirmed or suspected influenza who have severe, complicated, or progressive illness, who require hospitalization, or who are at high risk of complications from influenza (TABLE6). Start treatment without waiting for laboratory confirmation for those with suspected influenza who are seriously ill. Outcomes are best when antivirals are started within 48 hours of illness onset, but they can be started even after this “window” has passed.
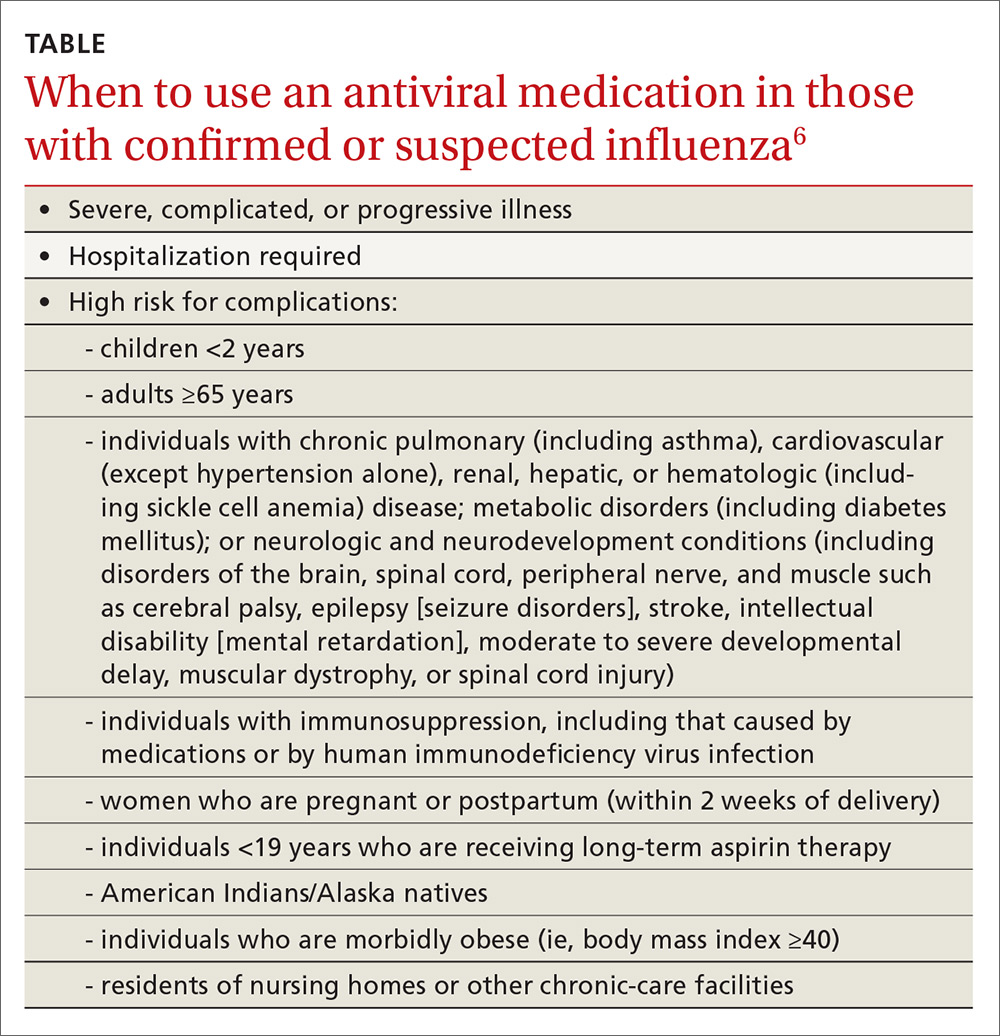
Once antiviral treatment has begun, make sure the full 5-day course is completed regardless of culture or rapid-test results.6 Use only neuraminidase inhibitors, as there is widespread resistance to adamantanes among influenza A viruses.
Influenza can occur year round
Rates of influenza infection are low in the summer, but cases do occur. Be especially alert if patients with influenza-like illness have been exposed to swine or poultry; they may have contracted a novel influenza A virus. Report such cases to the state or local health department so that staff can facilitate laboratory testing of viral subtypes. Follow the same protocol for patients with influenza symptoms who have traveled to areas where avian influenza viruses have been detected. The CDC is interested in detecting novel influenza viruses, which can start a pandemic.
Prepare for the 2017-2018 influenza season
Family physicians can help prevent influenza and its associated morbidity and mortality in several ways. Offer immunization to all patients, and immunize all health care personnel in your offices and clinics. Treat with antivirals those for whom they are recommended. Prepare office triage policies that prevent patients with flu symptoms from mixing with other patients, ensure that clinic infection control practices are enforced, and advise ill patients to avoid exposing others.7 Finally, stay current on influenza epidemiology and changes in recommendations for treatment and vaccination.
1. Blanton L, Alabi N, Mustaquim D, et al. Update: Influenza activity in the United States during the 2016-2017 season and composition of the 2017-2018 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2017;66:668-676.
2. Shimabukuro T. End-of-season update: 2016-2017 influenza vaccine safety monitoring. Presented at: meeting of the Advisory Committee on Immunization Practices; June 21, 2017; Atlanta, Ga. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-06/flu-04-shimabukuro.pdf. Accessed August 1, 2017.
3. CDC. Frequently asked flu questions 2017-2018 influenza season. Available at: https://www.cdc.gov/flu/about/season/flu-season-2017-2018.htm. Accessed July 17, 2017.
4. CDC. Influenza vaccines — United States, 2016-17 influenza season. Available at: https://www.cdc.gov/flu/protect/vaccine/vaccines.htm. Accessed July 17, 2017.
5. Grohskopf L. Influenza WG considerations and proposed recommendations. Presented at: meeting of the Advisory Committee on Immunization Practices; June 21, 2017; Atlanta, Ga. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-06/flu-06-grohskopf.pdf. Accessed August 1, 2017.
6. CDC. Use of antivirals. Available at: https://www.cdc.gov/flu/professionals/antivirals/antiviral-use-influenza.htm#Box. Accessed July 17, 2017.
7. CDC. Prevention strategies for seasonal influenza in healthcare settings. Available at: https://www.cdc.gov/flu/professionals/infectioncontrol/healthcaresettings.htm. Accessed July 17, 2017.
The Centers for Disease Control and Prevention (CDC) recently reported details of the 2016-2017 influenza season in Morbidity and Mortality Weekly Report1 and at the June meeting of the Advisory Committee on Immunization Practices. The CDC monitors influenza activity using several systems, and last flu season was shown to be moderately severe, starting in December in the Western United States, moving east, and peaking in February.
During the peak, 5.1% of outpatient visits were attributed to influenza-like illnesses, and 8.2% of reported deaths were due to pneumonia and influenza. For the whole influenza season, there were more than 18,000 confirmed influenza-related hospitalizations, with 60% of these occurring among those ≥65 years.1 Confirmed influenza-associated pediatric deaths totaled 98.1
The predominant influenza strain last year was type A (H3N2), accounting for about 76% of positive tests in public health laboratories (FIGURE).1 This was followed by influenza B (all lineages) at 22%, and influenza A (H1N1), accounting for only 2%. However, in early April, the predominant strain changed from A (H3N2) to influenza B. Importantly, all viruses tested last year were sensitive to oseltamivir, zanamivir, and peramivir. No antiviral resistance was detected to these neuraminidase inhibitors.

Good news and bad news on vaccine effectiveness. The good news: Circulating viruses were a close match to those contained in the vaccine. The bad news: Vaccine effectiveness at preventing illness was estimated to be just 34% against A (H3N2) and 56% against influenza B viruses.1 There has been no analysis of the relative effectiveness of different vaccines and vaccine types.
The past 6 influenza seasons have revealed a pattern of lower vaccine effectiveness against A (H3N2) compared with effectiveness against A (H1N1) and influenza B viruses. While vaccine effectiveness is not optimal, routine universal use still prevents a great deal of mortality and morbidity. It’s estimated that in 2012-2013, vaccine effectiveness (comparable to that in 2016-2017) prevented 5.6 million illnesses, 2.7 million medical visits, 61,500 hospitalizations, and 1800 deaths.1
More good news: Vaccine safety studies are reassuring
The CDC monitors influenza vaccine safety by using several sources, including the Vaccine Adverse Event Reporting System and the Vaccine Safety Datalink.2
Changes for the 2017-2018 influenza season
The composition of influenza vaccine products for the 2017-2018 season will differ slightly from last year’s formulation in the H1N1 component. Viral antigens to be included in the trivalent products are A/Michigan (H1N1), A/Hong Kong (H3N2), and B/Brisbane.3 Quadrivalent products will add B/Phuket to the other 3 antigens.3 A wide array of influenza vaccine products is available. Each one is described on the CDC Web site.4
Two minor changes in the recommendations were made at the June ACIP meeting.5 Afluria is approved by the FDA for use in children starting at age 5 years. ACIP had recommended that its use be reserved for children 9 years and older because previous influenza seasons had raised concerns about increased rates of febrile seizures in children younger than age 9. These concerns have been resolved, however, and the ACIP recommendations are now in concert with those of the FDA for this product.
Influenza immunization with an inactivated influenza vaccine product has been recommended for all pregnant women. Safety data are increasingly available for other product options as well, and ACIP now recommends vaccination in pregnancy with any age-appropriate product except for live attenuated influenza vaccine. 5
Antivirals: Give as needed, even before lab confirmation
The CDC recommends antiviral medication for individuals with confirmed or suspected influenza who have severe, complicated, or progressive illness, who require hospitalization, or who are at high risk of complications from influenza (TABLE6). Start treatment without waiting for laboratory confirmation for those with suspected influenza who are seriously ill. Outcomes are best when antivirals are started within 48 hours of illness onset, but they can be started even after this “window” has passed.

Once antiviral treatment has begun, make sure the full 5-day course is completed regardless of culture or rapid-test results.6 Use only neuraminidase inhibitors, as there is widespread resistance to adamantanes among influenza A viruses.
Influenza can occur year round
Rates of influenza infection are low in the summer, but cases do occur. Be especially alert if patients with influenza-like illness have been exposed to swine or poultry; they may have contracted a novel influenza A virus. Report such cases to the state or local health department so that staff can facilitate laboratory testing of viral subtypes. Follow the same protocol for patients with influenza symptoms who have traveled to areas where avian influenza viruses have been detected. The CDC is interested in detecting novel influenza viruses, which can start a pandemic.
Prepare for the 2017-2018 influenza season
Family physicians can help prevent influenza and its associated morbidity and mortality in several ways. Offer immunization to all patients, and immunize all health care personnel in your offices and clinics. Treat with antivirals those for whom they are recommended. Prepare office triage policies that prevent patients with flu symptoms from mixing with other patients, ensure that clinic infection control practices are enforced, and advise ill patients to avoid exposing others.7 Finally, stay current on influenza epidemiology and changes in recommendations for treatment and vaccination.
The Centers for Disease Control and Prevention (CDC) recently reported details of the 2016-2017 influenza season in Morbidity and Mortality Weekly Report1 and at the June meeting of the Advisory Committee on Immunization Practices. The CDC monitors influenza activity using several systems, and last flu season was shown to be moderately severe, starting in December in the Western United States, moving east, and peaking in February.
During the peak, 5.1% of outpatient visits were attributed to influenza-like illnesses, and 8.2% of reported deaths were due to pneumonia and influenza. For the whole influenza season, there were more than 18,000 confirmed influenza-related hospitalizations, with 60% of these occurring among those ≥65 years.1 Confirmed influenza-associated pediatric deaths totaled 98.1
The predominant influenza strain last year was type A (H3N2), accounting for about 76% of positive tests in public health laboratories (FIGURE).1 This was followed by influenza B (all lineages) at 22%, and influenza A (H1N1), accounting for only 2%. However, in early April, the predominant strain changed from A (H3N2) to influenza B. Importantly, all viruses tested last year were sensitive to oseltamivir, zanamivir, and peramivir. No antiviral resistance was detected to these neuraminidase inhibitors.

Good news and bad news on vaccine effectiveness. The good news: Circulating viruses were a close match to those contained in the vaccine. The bad news: Vaccine effectiveness at preventing illness was estimated to be just 34% against A (H3N2) and 56% against influenza B viruses.1 There has been no analysis of the relative effectiveness of different vaccines and vaccine types.
The past 6 influenza seasons have revealed a pattern of lower vaccine effectiveness against A (H3N2) compared with effectiveness against A (H1N1) and influenza B viruses. While vaccine effectiveness is not optimal, routine universal use still prevents a great deal of mortality and morbidity. It’s estimated that in 2012-2013, vaccine effectiveness (comparable to that in 2016-2017) prevented 5.6 million illnesses, 2.7 million medical visits, 61,500 hospitalizations, and 1800 deaths.1
More good news: Vaccine safety studies are reassuring
The CDC monitors influenza vaccine safety by using several sources, including the Vaccine Adverse Event Reporting System and the Vaccine Safety Datalink.2
Changes for the 2017-2018 influenza season
The composition of influenza vaccine products for the 2017-2018 season will differ slightly from last year’s formulation in the H1N1 component. Viral antigens to be included in the trivalent products are A/Michigan (H1N1), A/Hong Kong (H3N2), and B/Brisbane.3 Quadrivalent products will add B/Phuket to the other 3 antigens.3 A wide array of influenza vaccine products is available. Each one is described on the CDC Web site.4
Two minor changes in the recommendations were made at the June ACIP meeting.5 Afluria is approved by the FDA for use in children starting at age 5 years. ACIP had recommended that its use be reserved for children 9 years and older because previous influenza seasons had raised concerns about increased rates of febrile seizures in children younger than age 9. These concerns have been resolved, however, and the ACIP recommendations are now in concert with those of the FDA for this product.
Influenza immunization with an inactivated influenza vaccine product has been recommended for all pregnant women. Safety data are increasingly available for other product options as well, and ACIP now recommends vaccination in pregnancy with any age-appropriate product except for live attenuated influenza vaccine. 5
Antivirals: Give as needed, even before lab confirmation
The CDC recommends antiviral medication for individuals with confirmed or suspected influenza who have severe, complicated, or progressive illness, who require hospitalization, or who are at high risk of complications from influenza (TABLE6). Start treatment without waiting for laboratory confirmation for those with suspected influenza who are seriously ill. Outcomes are best when antivirals are started within 48 hours of illness onset, but they can be started even after this “window” has passed.

Once antiviral treatment has begun, make sure the full 5-day course is completed regardless of culture or rapid-test results.6 Use only neuraminidase inhibitors, as there is widespread resistance to adamantanes among influenza A viruses.
Influenza can occur year round
Rates of influenza infection are low in the summer, but cases do occur. Be especially alert if patients with influenza-like illness have been exposed to swine or poultry; they may have contracted a novel influenza A virus. Report such cases to the state or local health department so that staff can facilitate laboratory testing of viral subtypes. Follow the same protocol for patients with influenza symptoms who have traveled to areas where avian influenza viruses have been detected. The CDC is interested in detecting novel influenza viruses, which can start a pandemic.
Prepare for the 2017-2018 influenza season
Family physicians can help prevent influenza and its associated morbidity and mortality in several ways. Offer immunization to all patients, and immunize all health care personnel in your offices and clinics. Treat with antivirals those for whom they are recommended. Prepare office triage policies that prevent patients with flu symptoms from mixing with other patients, ensure that clinic infection control practices are enforced, and advise ill patients to avoid exposing others.7 Finally, stay current on influenza epidemiology and changes in recommendations for treatment and vaccination.
1. Blanton L, Alabi N, Mustaquim D, et al. Update: Influenza activity in the United States during the 2016-2017 season and composition of the 2017-2018 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2017;66:668-676.
2. Shimabukuro T. End-of-season update: 2016-2017 influenza vaccine safety monitoring. Presented at: meeting of the Advisory Committee on Immunization Practices; June 21, 2017; Atlanta, Ga. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-06/flu-04-shimabukuro.pdf. Accessed August 1, 2017.
3. CDC. Frequently asked flu questions 2017-2018 influenza season. Available at: https://www.cdc.gov/flu/about/season/flu-season-2017-2018.htm. Accessed July 17, 2017.
4. CDC. Influenza vaccines — United States, 2016-17 influenza season. Available at: https://www.cdc.gov/flu/protect/vaccine/vaccines.htm. Accessed July 17, 2017.
5. Grohskopf L. Influenza WG considerations and proposed recommendations. Presented at: meeting of the Advisory Committee on Immunization Practices; June 21, 2017; Atlanta, Ga. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-06/flu-06-grohskopf.pdf. Accessed August 1, 2017.
6. CDC. Use of antivirals. Available at: https://www.cdc.gov/flu/professionals/antivirals/antiviral-use-influenza.htm#Box. Accessed July 17, 2017.
7. CDC. Prevention strategies for seasonal influenza in healthcare settings. Available at: https://www.cdc.gov/flu/professionals/infectioncontrol/healthcaresettings.htm. Accessed July 17, 2017.
1. Blanton L, Alabi N, Mustaquim D, et al. Update: Influenza activity in the United States during the 2016-2017 season and composition of the 2017-2018 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2017;66:668-676.
2. Shimabukuro T. End-of-season update: 2016-2017 influenza vaccine safety monitoring. Presented at: meeting of the Advisory Committee on Immunization Practices; June 21, 2017; Atlanta, Ga. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-06/flu-04-shimabukuro.pdf. Accessed August 1, 2017.
3. CDC. Frequently asked flu questions 2017-2018 influenza season. Available at: https://www.cdc.gov/flu/about/season/flu-season-2017-2018.htm. Accessed July 17, 2017.
4. CDC. Influenza vaccines — United States, 2016-17 influenza season. Available at: https://www.cdc.gov/flu/protect/vaccine/vaccines.htm. Accessed July 17, 2017.
5. Grohskopf L. Influenza WG considerations and proposed recommendations. Presented at: meeting of the Advisory Committee on Immunization Practices; June 21, 2017; Atlanta, Ga. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-06/flu-06-grohskopf.pdf. Accessed August 1, 2017.
6. CDC. Use of antivirals. Available at: https://www.cdc.gov/flu/professionals/antivirals/antiviral-use-influenza.htm#Box. Accessed July 17, 2017.
7. CDC. Prevention strategies for seasonal influenza in healthcare settings. Available at: https://www.cdc.gov/flu/professionals/infectioncontrol/healthcaresettings.htm. Accessed July 17, 2017.
The Weekend Effect in Hospitalized Patients: A Meta-Analysis
The presence of a “weekend effect” (increased mortality rate during Saturday and/or Sunday admissions) for hospitalized inpatients is uncertain. Several observational studies1-3 suggested a positive correlation between weekend admission and increased mortality, whereas other studies demonstrated no correlation4-6 or mixed results.7,8 The majority of studies have been published only within the last decade.
Several possible reasons are cited to explain the weekend effect. Decreased and presence of inexperienced staffing on weekends may contribute to a deficit in care.7,9,10 Patients admitted during the weekend may be less likely to undergo procedures or have significant delays before receiving needed intervention.11-13 Another possibility is that there may be differences in severity of illness or comorbidities in patients admitted during the weekend compared with those admitted during the remainder of the week. Due to inconsistency between studies regarding the existence of such an effect, we performed a meta-analysis in hospitalized inpatients to delineate whether or not there is a weekend effect on mortality.
METHODS
Data Sources and Searches
This study was exempt from institutional review board review, and we utilized the recommendations from the Meta-analysis of Observational Studies in Epidemiology statement. We examined the mortality rate for hospital inpatients admitted during the weekend (weekend death) compared with the mortality rate for those admitted during the workweek (workweek death). We performed a literature search (January 1966−April 2013) of multiple databases, including PubMed, EMBASE, SCOPUS, and the Cochrane library (see Appendix). Two reviewers (LP, RJP) independently evaluated the full article of each abstract. Any disputes were resolved by a third reviewer (CW). Bibliographic references were hand searched for additional literature.
Study Selection
To be included in the systematic review, the study had to provide discrete mortality data on the weekends (including holidays) versus weekdays, include patients who were admitted as inpatients over the weekend, and be published in the English language. We excluded studies that combined weekend with weekday “off hours” (eg, weekday night shift) data, which could not be extracted or analyzed separately.
Data Extraction and Quality Assessment
Once an article was accepted to be included for the systematic review, the authors extracted relevant data if available, including study location, number and type of patients studied, patient comorbidity data, procedure-related data (type of procedure, difference in rate of procedure and time to procedure performed for both weekday and weekends), any stated and/or implied differences in staffing patterns between weekend and weekdays, and definition of mortality. We used the Newcastle-Ottawa Quality Assessment Scale to assess the quality of methodological reporting of the study.14 The definition of weekend and extraction and classification of data (weekend versus weekday) was based on the original study definition. We made no attempt to impose a universal definition of “weekend” on all studies. Similarly, the definition of mortality (eg, 3-/7-/30-day) was based according to the original study definition. Death from a patient admitted on the weekend was defined as a “weekend death” (regardless of ultimate time of death) and similarly, death from a patient admitted on a weekday was defined as a “weekday death.” Although some articles provided specific information on healthcare worker staffing patterns between weekends and weekdays, differences in weekend versus weekday staffing were implied in many articles. In these studies, staffing paradigms were considered to be different between weekend and weekdays if there were specific descriptions of the type of hospitals (urban versus rural, teaching versus nonteaching, large versus small) in the database, which would imply a typical routine staffing pattern as currently occurs in most hospitals (ie, generally less healthcare worker staff on weekends). We only included data that provided times (mean minutes/hours) from admission to the specific intervention and that provided actual rates of intervention performed for both weekend and weekday patients. We only included data that provided an actual rate of intervention performed for both weekend and weekday patients. With regard to patient comorbidities or illness severity index, we used the original studies classification (defined by the original manuscripts), which might include widely accepted global indices or a listing of specific comorbidities and/or physiologic parameters present on admission.
Data Synthesis and Analysis
We used a random effects meta-analysis approach for estimating an overall relative risk (RR) and risk differences of mortality for weekends versus weekdays, as well as subgroup specific estimates, and for computing confidence limits. The DerSimonian and Laird approach was used to estimate the random effects. Within each of the 4 subgroups (weekend staffing, procedure rates and delays, illness severity), we grouped each qualified individual study by the presence of a difference (ie, difference, no difference, or mixed) and then pooled the mortality rates for all of the studies in that group. For instance, in the subgroup of staffing, we sorted available studies by whether weekend staffing was the same or decreased versus weekday staffing, then pooled the mortality rates for studies where staffing levels were the same (versus weekday) and also separately pooled studies where staffing levels were decreased (versus weekday). Data were managed with Stata 13 (Stata Statistical Software: Release 13; StataCorp. 2013, College Station, TX) and R, and all meta-analyses were performed with the metafor package in R.15 Pooled estimated are presented as RR (95% confidence intervals [CI]).
RESULTS
A literature search retrieved a total of 594 unique citations. A review of the bibliographic references yielded an additional 20 articles. Upon evaluation, 97 studies (N = 51,114,109 patients) met inclusion criteria (Figure 1). The articles were published between 2001–2012; the kappa statistic comparing interrater reliability in the selection of articles was 0.86. Supplementary Tables 1 and 2 present a summary of study characteristics and outcomes of the accepted articles. A summary of accepted studies is in Supplementary Table 1. When summing the total number of subjects across all 97 articles, 76% were classified as weekday and 24% were weekend patients.
Weekend Admission/Inpatient Status and Mortality
The definition of the weekend varied among the included studies. The weekend time period was delineated as Friday midnight to Sunday midnight in 66% (65/99) of the studies. The remaining studies typically defined the weekend to be between Friday evening and Monday morning although studies from the Middle East generally defined the weekend as Wednesday/Thursday through Saturday. The definition of mortality also varied among researchers with most studies describing death rate as hospital inpatient mortality although some studies also examined multiple definitions of mortality (eg, 30-day all-cause mortality and hospital inpatient mortality). Not all studies provided a specific timeframe for mortality.
Fifty studies did not report a specific time frame for deaths. When a specific time frame for death was reported, the most common reported time frame was 30 days (n = 15 studies) and risk of mortality at 30 days still was higher for weekends (RR = 1.07; 95% CI,1.03-1.12; I2 = 90%). When we restricted the analysis to the studies that specified any timeframe for mortality (n = 49 studies), the risk of mortality was still significantly higher for weekends (RR = 1.12; 95% CI,1.09-1.15; I2 = 95%).
Weekend Effect Factors
We also performed subgroup analyses to investigate the overall weekend effect by hospital level factors (weekend staffing, procedure rates and delays, illness severity). Complete data were not available for all studies (staffing levels = 73 studies, time to intervention = 18 studies, rate of intervention = 30 studies, illness severity = 64 studies). Patients admitted on the weekends consistently had higher mortality than those admitted during the week, regardless of the levels of weekend/weekday differences in staffing, procedure rates and delays, illness severity (Figure 3). Analysis of studies that included staffing data for weekends revealed that decreased staffing levels on the weekends was associated with a higher mortality for weekend patients (RR = 1.16; 95% CI, 1.12-1.20; I2 = 99%; Figure 3). There was no difference in mortality for weekend patients when staffing was similar to that for the weekdays (RR = 1.21; 95% CI, 0.91-1.63; I2 = 99%).
Analysis for weekend data revealed that longer times to interventions on weekends were associated with significantly higher mortality rates (RR = 1.11; 95% CI, 1.08-1.15; I2 = 0%; Figure 3). When there were no delays to weekend procedure/interventions, there was no difference in mortality between weekend and weekday procedures/interventions (RR = 1.04; 95% CI, 0.96-1.13; I2 = 55%; Figure 3). Some articles included several procedures with “mixed” results (some procedures were “positive,” while other were “negative” for increased mortality). In studies that showed a mixed result for time to intervention, there was a significant increase in mortality (RR = 1.16; 95% CI, 1.06-1.27; I2 = 42%) for weekend patients (Figure 3).
Analyses showed a higher mortality rate on the weekends regardless of whether the rate of intervention/procedures was lower (RR=1.12; 95% CI, 1.07-1.17; I2 = 79%) or the same between weekend and weekdays (RR = 1.08; 95% CI, 1.01-1.16; I2 = 90%; Figure 3). Analyses showed a higher mortality rate on the weekends regardless of whether the illness severity was higher on the weekends (RR = 1.21; 95% CI, 1.07-1.38; I2 = 99%) or the same (RR = 1.21; 95% CI, 1.14-1.28; I2 = 99%) versus that for weekday patients (Figure 3). An inverse funnel plot for publication bias is shown in Figure 4.
DISCUSSION
We have presented one of the first meta-analyses to examine the mortality rate for hospital inpatients admitted during the weekend compared with those admitted during the workweek. We found that patients admitted on the weekends had a significantly higher overall mortality (RR = 1.19; 95% CI, 1.14-1.23; risk difference = 0.014; 95% CI, 0.013-0.016). This association was not modified by differences in weekday and weekend staffing patterns, and other hospital characteristics. Previous systematic reviews have been exclusive to the intensive care unit setting16 or did not specifically examine weekend mortality, which was a component of “off-shift” and/or “after-hours” care.17
These findings should be placed in the context of the recently published literature.18,19 A meta-analysis of cohort studies found that off-hour admission was associated with increased mortality for 28 diseases although the associations varied considerably for different diseases.18 Likewise, a meta-analysis of 21 cohort studies noted that off-hour presentation for patients with acute ischemic stroke was associated with significantly higher short-term mortality.19 Our results of increased weekend mortality corroborate that found in these two meta-analyses. However, our study differs in that we specifically examined only weekend mortality and did not include after-hours care on weekdays, which was included in the off-hour mortality in the other meta-analyses.18,19
Differences in healthcare worker staffing between weekends and weekdays have been proposed to contribute to the observed increase in mortality.7,16,20 Data indicate that lower levels of nursing are associated with increased mortality.10,21-23 The presence of less experienced and/or fewer physician specialists may contribute to increases in mortality.24-26 Fewer or less experienced staff during weekends may contribute to inadequacies in patient handovers and/or handoffs, delays in patient assessment and/or interventions, and overall continuity of care for newly admitted patients.27-33
Our data show little conclusive evidence that the weekend mortality versus weekday mortality vary by staffing level differences. While the estimated RR of mortality differs in magnitude for facilities with no difference in weekend and weekday staffing versus those that have a difference in staffing levels, both estimate an increased mortality on weekends, and the difference in these effects is not statistically significant. It should be noted that there was no difference in mortality for weekend (versus weekday) patients where there was no difference between weekend and weekday staffing; these studies were typically in high acuity units or centers where the general expectation is for 24/7/365 uniform staffing coverage.
A decrease in the use of interventions and/or procedures on weekends has been suggested to contribute to increases in mortality for patients admitted on the weekends.34 Several studies have associated lower weekend rates to higher mortality for a variety of interventions,13,35-37 although some other studies have suggested that lower procedure rates on weekends have no effect on mortality.38-40 Lower diagnostic procedure weekend rates linked to higher mortality rates may exacerbate underlying healthcare disparities.41 Our results do not conclusively show that a decrease rate of intervention and/or procedures for weekends patients is associated with a higher risk of mortality for weekends compared to weekdays.
Delays in intervention and/or procedure on weekends have also been suggested to contribute to increases in mortality.34,42 Similar to that seen with lower rates of diagnostic or therapeutic intervention and/or procedure performed on weekends, delays in potentially critical intervention and/or procedures might ultimately manifest as an increase in mortality.43 Patients admitted to the hospital on weekends and requiring an early procedure were less likely to receive it within 2 days of admission.42 Several studies have shown an association between delays in diagnostic or therapeutic intervention and/or procedure on weekends to a higher hospital inpatient mortality35,42,44,45; however, some data suggested that a delay in time to procedure on weekends may not always be associated with increased mortality.46 Depending on the procedure, there may be a threshold below which the effect of reducing delay times will have no effect on mortality rates.47,48
Patients admitted on the weekends may be different (in the severity of illness and/or comorbidities) than those admitted during the workweek and these potential differences may be a factor for increases in mortality for weekend patients. Whether there is a selection bias for weekend versus weekday patients is not clear.34 This is a complex issue as there is significant heterogeneity in patient case mix depending on the specific disease or condition studied. For instance, one would expect that weekend trauma patients would be different than those seen during the regular workweek.49 Some large scale studies suggest that weekend patients may not be more sick than weekday patients and that any increase in weekend mortality is probably not due to factors such as severity of illness.1,7 Although we were unable to determine if there was an overall difference in illness severity between weekend and weekday patients due to the wide variety of assessments used for illness severity, our results showed statistically comparable higher mortality rate on the weekends regardless of whether the illness severity was higher, the same, or mixed between weekend and weekday patients, suggesting that general illness severity per se may not be as important as the weekend effect on mortality; however, illness severity may still have an important effect on mortality for more specific subgroups (eg, trauma).49
There are several implications of our results. We found a mean increased RR mortality of approximately 19% for patients admitted on the weekends, a number similar to one of the largest published observational studies containing almost 5 million subjects.2 Even if we took a more conservative estimate of 10% increased risk of weekend mortality, this would be equivalent to an excess of 25,000 preventable deaths per year. If the weekend effect were to be placed in context of a public health issue, the weekend effect would be the number 8 cause of death below the 29,000 deaths due to gun violence, but above the 20,000 deaths resulting from sexual behavior (sexual transmitted diseases) in 2000.3, 50,51 Although our data suggest that staffing shortfalls and decreases or delays for procedures on weekends may be associated with an increased mortality for patients admitted on the weekends, further large-scale studies are needed to confirm these findings. Increasing nurse and physician staffing levels and skill mix to cover any potential shortfall on weekends may be expensive, although theoretically, there may be savings accrued from reduced adverse events and shorter length of stay.26,52 Changes to weekend care might only benefit daytime hospitalizations because some studies have shown increased mortality during nighttime regardless of weekend or weekday admission.53
Several methodologic points in our study need to be clarified. We excluded many studies which examined the relationship of off-hours or after-hours admissions and mortality as off-hours studies typically combined weekend and after-hours weekday data. Some studies suggest that off-hour admission may be associated with increased mortality and delays in time for critical procedures during off-hours.18,19 This is a complex topic, but it is clear that the risks of hospitalization vary not just by the day of the week but also by time of the day.54 The use of meta-analyses of nonrandomized trials has been somewhat controversial,55,56 and there may be significant bias or confounding in the pooling of highly varied studies. It is important to keep in mind that there are very different definitions of weekends, populations studied, and measures of mortality rates, even as the pooled statistic suggests a homogeneity among the studies that does not exist.
There are several limitations to our study. Our systematic review may be seen as limited as we included only English language papers. In addition, we did not search nontraditional sources and abstracts. We accepted the definition of a weekend as defined by the original study, which resulted in varied definitions of weekend time period and mortality. There was a lack of specific data on staffing patterns and procedures in many studies, particularly those using databases. We were not able to further subdivide our analysis by admitting service. We were not able to undertake a subgroup analysis by country or continent, which may have implications on the effect of different healthcare systems on healthcare quality. It is unclear whether correlations in our study are a direct consequence of poorer weekend care or are the result of other unknown or unexamined differences between weekend and weekday patient populations.34,57 For instance, there may be other global factors (higher rates of medical errors, higher hospital volumes) which may not be specifically related to weekend care and therefore not been accounted for in many of the studies we examined.10,27,58-61 There may be potential bias of patient phenotypes (are weekend patients different than weekday patients?) admitted on the weekend. Holidays were included in the weekend data and it is not clear how this would affect our findings as some data suggest that there is a significantly higher mortality rate on holidays (versus weekends or weekdays),61 while other data do not.62 There was no universal definition for the timeframe for a weekend and as such, we had to rely on the original article for their determination and definition of weekend versus weekday death.
In summary, our meta-analysis suggests that hospital inpatients admitted during the weekend have a significantly increased mortality compared with those admitted on weekday. While none of our subgroup analyses showed strong evidence on effect modification, the interpretation of these results is hampered by the relatively small number of studies. Further research should be directed to determine the presence of causality between various factors purported to affect mortality and it is possible that we ultimately find that the weekend effect may exist for some but not all patients.
Acknowledgments
The authors would like to acknowledge Jaime Blanck, MLIS, MPA, AHIP, Clinical Informationist, Welch Medical Library, for her invaluable assistance in undertaking the literature searches for this manuscript.
Disclosure
This manuscript has been supported by the Department of Anesthesiology and Critical Care Medicine; The Johns Hopkins School of Medicine; Baltimore, Maryland. There are no relevant conflicts of interests.
1. Aylin P, Yunus A, Bottle A, Majeed A, Bell D. Weekend mortality for emergency
admissions. A large, multicentre study. Qual Saf Health Care. 2010;19(3):213-217. PubMed
2. Handel AE, Patel SV, Skingsley A, Bramley K, Sobieski R, Ramagopalan SV.
Weekend admissions as an independent predictor of mortality: an analysis of
Scottish hospital admissions. BMJ Open. 2012;2(6): pii: e001789. PubMed
3. Ricciardi R, Roberts PL, Read TE, Baxter NN, Marcello PW, Schoetz DJ. Mortality
rate after nonelective hospital admission. Arch Surg. 2011;146(5):545-551. PubMed
4. Fonarow GC, Abraham WT, Albert NM, et al. Day of admission and clinical
outcomes for patients hospitalized for heart failure: findings from the Organized
Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart
Failure (OPTIMIZE-HF). Circ Heart Fail. 2008;1(1):50-57. PubMed
5. Hoh BL, Chi YY, Waters MF, Mocco J, Barker FG 2nd. Effect of weekend compared
with weekday stroke admission on thrombolytic use, in-hospital mortality,
discharge disposition, hospital charges, and length of stay in the Nationwide Inpatient
Sample Database, 2002 to 2007. Stroke. 2010;41(10):2323-2328. PubMed
6. Koike S, Tanabe S, Ogawa T, et al. Effect of time and day of admission on 1-month
survival and neurologically favourable 1-month survival in out-of-hospital cardiopulmonary
arrest patients. Resuscitation. 2011;82(7):863-868. PubMed
7. Bell CM, Redelmeier DA. Mortality among patients admitted to hospitals on
weekends as compared with weekdays. N Engl J Med. 2001;345(9):663-668. PubMed
8. Freemantle N, Richardson M, Wood J, et al. Weekend hospitalization and additional
risk of death: an analysis of inpatient data. J R Soc Med. 2012;105(2):74-84. PubMed
9. Schilling PL, Campbell DA Jr, Englesbe MJ, Davis MM. A comparison of in-hospital
mortality risk conferred by high hospital occupancy, differences in nurse
staffing levels, weekend admission, and seasonal influenza. Med Care. 2010;48(3):
224-232. PubMed
10. Wong HJ, Morra D. Excellent hospital care for all: open and operating 24/7. J Gen
Intern Med. 2011;26(9):1050-1052. PubMed
11. Dorn SD, Shah ND, Berg BP, Naessens JM. Effect of weekend hospital admission
on gastrointestinal hemorrhage outcomes. Dig Dis Sci. 2010;55(6):1658-1666. PubMed
12. Kostis WJ, Demissie K, Marcella SW, et al. Weekend versus weekday admission
and mortality from myocardial infarction. N Engl J Med. 2007;356(11):1099-1109. PubMed
13. McKinney JS, Deng Y, Kasner SE, Kostis JB; Myocardial Infarction Data Acquisition
System (MIDAS 15) Study Group. Comprehensive stroke centers overcome
the weekend versus weekday gap in stroke treatment and mortality. Stroke.
2011;42(9):2403-2409. PubMed
14. Margulis AV, Pladevall M, Riera-Guardia N, et al. Quality assessment of observational
studies in a drug-safety systematic review, comparison of two tools: the
Newcastle-Ottawa Scale and the RTI item bank. Clin Epidemiol. 2014;6:359-368. PubMed
15. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat
Softw. 2010;36(3):1-48.
16. Cavallazzi R, Marik PE, Hirani A, Pachinburavan M, Vasu TS, Leiby BE. Association
between time of admission to the ICU and mortality: a systematic review and
metaanalysis. Chest. 2010;138(1):68-75. PubMed
17. de Cordova PB, Phibbs CS, Bartel AP, Stone PW. Twenty-four/seven: a
mixed-method systematic review of the off-shift literature. J Adv Nurs.
2012;68(7):1454-1468. PubMed
18. Zhou Y, Li W, Herath C, Xia J, Hu B, Song F, Cao S, Lu Z. Off-hour admission and
mortality risk for 28 specific diseases: a systematic review and meta-analysis of 251
cohorts. J Am Heart Assoc. 2016;5(3):e003102. PubMed
19. Sorita A, Ahmed A, Starr SR, et al. Off-hour presentation and outcomes in
patients with acute myocardial infarction: systematic review and meta-analysis.
BMJ. 2014;348:f7393. PubMed
20. Ricciardi R, Nelson J, Roberts PL, Marcello PW, Read TE, Schoetz DJ. Is the
presence of medical trainees associated with increased mortality with weekend
admission? BMC Med Educ. 2014;14(1):4. PubMed
21. Needleman J, Buerhaus P, Pankratz VS, Leibson CL, Stevens SR, Harris M. Nurse
staffing and inpatient hospital mortality. N Engl J Med. 2011;364(11):1037-1045. PubMed
22. Aiken LH, Clarke SP, Sloane DM, Sochalski J, Silber JH. Hospital nurse
staffing and patient mortality, nurse burnout, and job dissatisfaction. JAMA.
2002;288(16):1987-1993. PubMed
23. Hamilton KE, Redshaw ME, Tarnow-Mordi W. Nurse staffing in relation to
risk-adjusted mortality in neonatal care. Arch Dis Child Fetal Neonatal Ed.
2007;92(2):F99-F103. PubMed
766 An Official Publication of the Society of Hospital Medicine Journal of Hospital Medicine Vol 12 | No 9 | September 2017
Pauls et al | The Weekend Effect: A Meta-Analysis
24. Haut ER, Chang DC, Efron DT, Cornwell EE 3rd. Injured patients have lower
mortality when treated by “full-time” trauma surgeons vs. surgeons who cover
trauma “part-time”. J Trauma. 2006;61(2):272-278. PubMed
25. Wallace DJ, Angus DC, Barnato AE, Kramer AA, Kahn JM. Nighttime intensivist
staffing and mortality among critically ill patients. N Engl J Med.
2012;366(22):2093-2101. PubMed
26. Pronovost PJ, Angus DC, Dorman T, Robinson KA, Dremsizov TT, Young TL.
Physician staffing patterns and clinical outcomes in critically ill patients: a systematic
review. JAMA. 2002;288(17):2151-2162. PubMed
27. Weissman JS, Rothschild JM, Bendavid E, et al. Hospital workload and adverse
events. Med Care. 2007;45(5):448-455. PubMed
28. Hamilton P, Eschiti VS, Hernandez K, Neill D. Differences between weekend and
weekday nurse work environments and patient outcomes: a focus group approach
to model testing. J Perinat Neonatal Nurs. 2007;21(4):331-341. PubMed
29. Johner AM, Merchant S, Aslani N, et al Acute general surgery in Canada: a survey
of current handover practices. Can J Surg. 2013;56(3):E24-E28. PubMed
30. de Cordova PB, Phibbs CS, Stone PW. Perceptions and observations of off-shift
nursing. J Nurs Manag. 2013;21(2):283-292. PubMed
31. Pfeffer PE, Nazareth D, Main N, Hardoon S, Choudhury AB. Are weekend
handovers of adequate quality for the on-call general medical team? Clin Med.
2011;11(6):536-540. PubMed
32. Eschiti V, Hamilton P. Off-peak nurse staffing: critical-care nurses speak. Dimens
Crit Care Nurs. 2011;30(1):62-69. PubMed
33. Button LA, Roberts SE, Evans PA, et al. Hospitalized incidence and case fatality
for upper gastrointestinal bleeding from 1999 to 2007: a record linkage study. Aliment
Pharmacol Ther. 2011;33(1):64-76. PubMed
34. Becker DJ. Weekend hospitalization and mortality: a critical review. Expert Rev
Pharmacoecon Outcomes Res. 2008;8(1):23-26. PubMed
35. Deshmukh A, Pant S, Kumar G, Bursac Z, Paydak H, Mehta JL. Comparison of
outcomes of weekend versus weekday admissions for atrial fibrillation. Am J Cardiol.
2012;110(2):208-211. PubMed
36. Nanchal R, Kumar G, Taneja A, et al. Pulmonary embolism: the weekend effect.
Chest. 2012;142(3):690-696. PubMed
37. Palmer WL, Bottle A, Davie C, Vincent CA, Aylin P. Dying for the weekend: a
retrospective cohort study on the association between day of hospital presentation
and the quality and safety of stroke care. Arch Neurol. 2012;69(10):1296-1302. PubMed
38. Dasenbrock HH, Pradilla G, Witham TF, Gokaslan ZL, Bydon A. The impact
of weekend hospital admission on the timing of intervention and outcomes after
surgery for spinal metastases. Neurosurgery. 2012;70(3):586-593. PubMed
39. Jairath V, Kahan BC, Logan RF, et al. Mortality from acute upper gastrointestinal
bleeding in the United Kingdom: does it display a “weekend effect”? Am J Gastroenterol.
2011;106(9):1621-1628. PubMed
40. Myers RP, Kaplan GG, Shaheen AM. The effect of weekend versus weekday
admission on outcomes of esophageal variceal hemorrhage. Can J Gastroenterol.
2009;23(7):495-501. PubMed
41. Rudd AG, Hoffman A, Down C, Pearson M, Lowe D. Access to stroke care in
England, Wales and Northern Ireland: the effect of age, gender and weekend admission.
Age Ageing. 2007;36(3):247-255. PubMed
42. Lapointe-Shaw L, Abushomar H, Chen XK, et al. Care and outcomes of patients
with cancer admitted to the hospital on weekends and holidays: a retrospective
cohort study. J Natl Compr Canc Netw. 2016;14(7):867-874. PubMed
43. Chan PS, Krumholz HM, Nichol G, Nallamothu BK; American Heart Association
National Registry of Cardiopulmonary Resuscitation Investigators. Delayed time to
defibrillation after in-hospital cardiac arrest. N Engl J Med. 2008;358(1):9-17. PubMed
44. McGuire KJ, Bernstein J, Polsky D, Silber JH. The 2004 Marshall Urist Award:
Delays until surgery after hip fracture increases mortality. Clin Orthop Relat Res.
2004;(428):294-301. PubMed
45. Krüth P, Zeymer U, Gitt A, et al. Influence of presentation at the weekend on
treatment and outcome in ST-elevation myocardial infarction in hospitals with
catheterization laboratories. Clin Res Cardiol. 2008;97(10):742-747. PubMed
46. Jneid H, Fonarow GC, Cannon CP, et al. Impact of time of presentation on the care
and outcomes of acute myocardial infarction. Circulation. 2008;117(19):2502-2509. PubMed
47. Menees DS, Peterson ED, Wang Y, et al. Door-to-balloon time and mortality
among patients undergoing primary PCI. N Engl J Med. 2013;369(10):901-909. PubMed
48. Bates ER, Jacobs AK. Time to treatment in patients with STEMI. N Engl J Med.
2013;369(10):889-892. PubMed
49. Carmody IC, Romero J, Velmahos GC. Day for night: should we staff a trauma
center like a nightclub? Am Surg. 2002;68(12):1048-1051. PubMed
50. Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the
United States, 2000. JAMA. 2004;291(10):1238-1245. PubMed
51. McCook A. More hospital deaths on weekends. http://www.reuters.com/article/
2011/05/20/us-more-hospital-deaths-weekends-idUSTRE74J5RM20110520.
Accessed March 7, 2017.
52. Mourad M, Adler J. Safe, high quality care around the clock: what will it take to
get us there? J Gen Intern Med. 2011;26(9):948-950. PubMed
53. Magid DJ, Wang Y, Herrin J, et al. Relationship between time of day, day of week,
timeliness of reperfusion, and in-hospital mortality for patients with acute ST-segment
elevation myocardial infarction. JAMA. 2005;294(7):803-812. PubMed
54. Coiera E, Wang Y, Magrabi F, Concha OP, Gallego B, Runciman W. Predicting
the cumulative risk of death during hospitalization by modeling weekend, weekday
and diurnal mortality risks. BMC Health Serv Res. 2014;14:226. PubMed
55. Greenland S. Can meta-analysis be salvaged? Am J Epidemiol. 1994;140(9):783-787. PubMed
56. Shapiro S. Meta-analysis/Shmeta-analysis. Am J Epidemiol. 1994;140(9):771-778. PubMed
57. Halm EA, Chassin MR. Why do hospital death rates vary? N Engl J Med.
2001;345(9):692-694. PubMed
58. Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality
in the United States. N Engl J Med. 2002;346(15):1128-1137. PubMed
59. Kaier K, Mutters NT, Frank U. Bed occupancy rates and hospital-acquired infections
– should beds be kept empty? Clin Microbiol Infect. 2012;18(10):941-945. PubMed
60. Chrusch CA, Olafson KP, McMillian PM, Roberts DE, Gray PR. High occupancy
increases the risk of early death or readmission after transfer from intensive care.
Crit Care Med. 2009;37(10):2753-2758. PubMed
61. Foss NB, Kehlet H. Short-term mortality in hip fracture patients admitted during
weekends and holidays. Br J Anaesth. 2006;96(4):450-454. PubMed
62. Daugaard CL, Jørgensen HL, Riis T, Lauritzen JB, Duus BR, van der Mark S. Is
mortality after hip fracture associated with surgical delay or admission during
weekends and public holidays? A retrospective study of 38,020 patients. Acta Orthop.
2012;83(6):609-613. PubMed
The presence of a “weekend effect” (increased mortality rate during Saturday and/or Sunday admissions) for hospitalized inpatients is uncertain. Several observational studies1-3 suggested a positive correlation between weekend admission and increased mortality, whereas other studies demonstrated no correlation4-6 or mixed results.7,8 The majority of studies have been published only within the last decade.
Several possible reasons are cited to explain the weekend effect. Decreased and presence of inexperienced staffing on weekends may contribute to a deficit in care.7,9,10 Patients admitted during the weekend may be less likely to undergo procedures or have significant delays before receiving needed intervention.11-13 Another possibility is that there may be differences in severity of illness or comorbidities in patients admitted during the weekend compared with those admitted during the remainder of the week. Due to inconsistency between studies regarding the existence of such an effect, we performed a meta-analysis in hospitalized inpatients to delineate whether or not there is a weekend effect on mortality.
METHODS
Data Sources and Searches
This study was exempt from institutional review board review, and we utilized the recommendations from the Meta-analysis of Observational Studies in Epidemiology statement. We examined the mortality rate for hospital inpatients admitted during the weekend (weekend death) compared with the mortality rate for those admitted during the workweek (workweek death). We performed a literature search (January 1966−April 2013) of multiple databases, including PubMed, EMBASE, SCOPUS, and the Cochrane library (see Appendix). Two reviewers (LP, RJP) independently evaluated the full article of each abstract. Any disputes were resolved by a third reviewer (CW). Bibliographic references were hand searched for additional literature.
Study Selection
To be included in the systematic review, the study had to provide discrete mortality data on the weekends (including holidays) versus weekdays, include patients who were admitted as inpatients over the weekend, and be published in the English language. We excluded studies that combined weekend with weekday “off hours” (eg, weekday night shift) data, which could not be extracted or analyzed separately.
Data Extraction and Quality Assessment
Once an article was accepted to be included for the systematic review, the authors extracted relevant data if available, including study location, number and type of patients studied, patient comorbidity data, procedure-related data (type of procedure, difference in rate of procedure and time to procedure performed for both weekday and weekends), any stated and/or implied differences in staffing patterns between weekend and weekdays, and definition of mortality. We used the Newcastle-Ottawa Quality Assessment Scale to assess the quality of methodological reporting of the study.14 The definition of weekend and extraction and classification of data (weekend versus weekday) was based on the original study definition. We made no attempt to impose a universal definition of “weekend” on all studies. Similarly, the definition of mortality (eg, 3-/7-/30-day) was based according to the original study definition. Death from a patient admitted on the weekend was defined as a “weekend death” (regardless of ultimate time of death) and similarly, death from a patient admitted on a weekday was defined as a “weekday death.” Although some articles provided specific information on healthcare worker staffing patterns between weekends and weekdays, differences in weekend versus weekday staffing were implied in many articles. In these studies, staffing paradigms were considered to be different between weekend and weekdays if there were specific descriptions of the type of hospitals (urban versus rural, teaching versus nonteaching, large versus small) in the database, which would imply a typical routine staffing pattern as currently occurs in most hospitals (ie, generally less healthcare worker staff on weekends). We only included data that provided times (mean minutes/hours) from admission to the specific intervention and that provided actual rates of intervention performed for both weekend and weekday patients. We only included data that provided an actual rate of intervention performed for both weekend and weekday patients. With regard to patient comorbidities or illness severity index, we used the original studies classification (defined by the original manuscripts), which might include widely accepted global indices or a listing of specific comorbidities and/or physiologic parameters present on admission.
Data Synthesis and Analysis
We used a random effects meta-analysis approach for estimating an overall relative risk (RR) and risk differences of mortality for weekends versus weekdays, as well as subgroup specific estimates, and for computing confidence limits. The DerSimonian and Laird approach was used to estimate the random effects. Within each of the 4 subgroups (weekend staffing, procedure rates and delays, illness severity), we grouped each qualified individual study by the presence of a difference (ie, difference, no difference, or mixed) and then pooled the mortality rates for all of the studies in that group. For instance, in the subgroup of staffing, we sorted available studies by whether weekend staffing was the same or decreased versus weekday staffing, then pooled the mortality rates for studies where staffing levels were the same (versus weekday) and also separately pooled studies where staffing levels were decreased (versus weekday). Data were managed with Stata 13 (Stata Statistical Software: Release 13; StataCorp. 2013, College Station, TX) and R, and all meta-analyses were performed with the metafor package in R.15 Pooled estimated are presented as RR (95% confidence intervals [CI]).
RESULTS
A literature search retrieved a total of 594 unique citations. A review of the bibliographic references yielded an additional 20 articles. Upon evaluation, 97 studies (N = 51,114,109 patients) met inclusion criteria (Figure 1). The articles were published between 2001–2012; the kappa statistic comparing interrater reliability in the selection of articles was 0.86. Supplementary Tables 1 and 2 present a summary of study characteristics and outcomes of the accepted articles. A summary of accepted studies is in Supplementary Table 1. When summing the total number of subjects across all 97 articles, 76% were classified as weekday and 24% were weekend patients.
Weekend Admission/Inpatient Status and Mortality
The definition of the weekend varied among the included studies. The weekend time period was delineated as Friday midnight to Sunday midnight in 66% (65/99) of the studies. The remaining studies typically defined the weekend to be between Friday evening and Monday morning although studies from the Middle East generally defined the weekend as Wednesday/Thursday through Saturday. The definition of mortality also varied among researchers with most studies describing death rate as hospital inpatient mortality although some studies also examined multiple definitions of mortality (eg, 30-day all-cause mortality and hospital inpatient mortality). Not all studies provided a specific timeframe for mortality.
Fifty studies did not report a specific time frame for deaths. When a specific time frame for death was reported, the most common reported time frame was 30 days (n = 15 studies) and risk of mortality at 30 days still was higher for weekends (RR = 1.07; 95% CI,1.03-1.12; I2 = 90%). When we restricted the analysis to the studies that specified any timeframe for mortality (n = 49 studies), the risk of mortality was still significantly higher for weekends (RR = 1.12; 95% CI,1.09-1.15; I2 = 95%).
Weekend Effect Factors
We also performed subgroup analyses to investigate the overall weekend effect by hospital level factors (weekend staffing, procedure rates and delays, illness severity). Complete data were not available for all studies (staffing levels = 73 studies, time to intervention = 18 studies, rate of intervention = 30 studies, illness severity = 64 studies). Patients admitted on the weekends consistently had higher mortality than those admitted during the week, regardless of the levels of weekend/weekday differences in staffing, procedure rates and delays, illness severity (Figure 3). Analysis of studies that included staffing data for weekends revealed that decreased staffing levels on the weekends was associated with a higher mortality for weekend patients (RR = 1.16; 95% CI, 1.12-1.20; I2 = 99%; Figure 3). There was no difference in mortality for weekend patients when staffing was similar to that for the weekdays (RR = 1.21; 95% CI, 0.91-1.63; I2 = 99%).
Analysis for weekend data revealed that longer times to interventions on weekends were associated with significantly higher mortality rates (RR = 1.11; 95% CI, 1.08-1.15; I2 = 0%; Figure 3). When there were no delays to weekend procedure/interventions, there was no difference in mortality between weekend and weekday procedures/interventions (RR = 1.04; 95% CI, 0.96-1.13; I2 = 55%; Figure 3). Some articles included several procedures with “mixed” results (some procedures were “positive,” while other were “negative” for increased mortality). In studies that showed a mixed result for time to intervention, there was a significant increase in mortality (RR = 1.16; 95% CI, 1.06-1.27; I2 = 42%) for weekend patients (Figure 3).
Analyses showed a higher mortality rate on the weekends regardless of whether the rate of intervention/procedures was lower (RR=1.12; 95% CI, 1.07-1.17; I2 = 79%) or the same between weekend and weekdays (RR = 1.08; 95% CI, 1.01-1.16; I2 = 90%; Figure 3). Analyses showed a higher mortality rate on the weekends regardless of whether the illness severity was higher on the weekends (RR = 1.21; 95% CI, 1.07-1.38; I2 = 99%) or the same (RR = 1.21; 95% CI, 1.14-1.28; I2 = 99%) versus that for weekday patients (Figure 3). An inverse funnel plot for publication bias is shown in Figure 4.
DISCUSSION
We have presented one of the first meta-analyses to examine the mortality rate for hospital inpatients admitted during the weekend compared with those admitted during the workweek. We found that patients admitted on the weekends had a significantly higher overall mortality (RR = 1.19; 95% CI, 1.14-1.23; risk difference = 0.014; 95% CI, 0.013-0.016). This association was not modified by differences in weekday and weekend staffing patterns, and other hospital characteristics. Previous systematic reviews have been exclusive to the intensive care unit setting16 or did not specifically examine weekend mortality, which was a component of “off-shift” and/or “after-hours” care.17
These findings should be placed in the context of the recently published literature.18,19 A meta-analysis of cohort studies found that off-hour admission was associated with increased mortality for 28 diseases although the associations varied considerably for different diseases.18 Likewise, a meta-analysis of 21 cohort studies noted that off-hour presentation for patients with acute ischemic stroke was associated with significantly higher short-term mortality.19 Our results of increased weekend mortality corroborate that found in these two meta-analyses. However, our study differs in that we specifically examined only weekend mortality and did not include after-hours care on weekdays, which was included in the off-hour mortality in the other meta-analyses.18,19
Differences in healthcare worker staffing between weekends and weekdays have been proposed to contribute to the observed increase in mortality.7,16,20 Data indicate that lower levels of nursing are associated with increased mortality.10,21-23 The presence of less experienced and/or fewer physician specialists may contribute to increases in mortality.24-26 Fewer or less experienced staff during weekends may contribute to inadequacies in patient handovers and/or handoffs, delays in patient assessment and/or interventions, and overall continuity of care for newly admitted patients.27-33
Our data show little conclusive evidence that the weekend mortality versus weekday mortality vary by staffing level differences. While the estimated RR of mortality differs in magnitude for facilities with no difference in weekend and weekday staffing versus those that have a difference in staffing levels, both estimate an increased mortality on weekends, and the difference in these effects is not statistically significant. It should be noted that there was no difference in mortality for weekend (versus weekday) patients where there was no difference between weekend and weekday staffing; these studies were typically in high acuity units or centers where the general expectation is for 24/7/365 uniform staffing coverage.
A decrease in the use of interventions and/or procedures on weekends has been suggested to contribute to increases in mortality for patients admitted on the weekends.34 Several studies have associated lower weekend rates to higher mortality for a variety of interventions,13,35-37 although some other studies have suggested that lower procedure rates on weekends have no effect on mortality.38-40 Lower diagnostic procedure weekend rates linked to higher mortality rates may exacerbate underlying healthcare disparities.41 Our results do not conclusively show that a decrease rate of intervention and/or procedures for weekends patients is associated with a higher risk of mortality for weekends compared to weekdays.
Delays in intervention and/or procedure on weekends have also been suggested to contribute to increases in mortality.34,42 Similar to that seen with lower rates of diagnostic or therapeutic intervention and/or procedure performed on weekends, delays in potentially critical intervention and/or procedures might ultimately manifest as an increase in mortality.43 Patients admitted to the hospital on weekends and requiring an early procedure were less likely to receive it within 2 days of admission.42 Several studies have shown an association between delays in diagnostic or therapeutic intervention and/or procedure on weekends to a higher hospital inpatient mortality35,42,44,45; however, some data suggested that a delay in time to procedure on weekends may not always be associated with increased mortality.46 Depending on the procedure, there may be a threshold below which the effect of reducing delay times will have no effect on mortality rates.47,48
Patients admitted on the weekends may be different (in the severity of illness and/or comorbidities) than those admitted during the workweek and these potential differences may be a factor for increases in mortality for weekend patients. Whether there is a selection bias for weekend versus weekday patients is not clear.34 This is a complex issue as there is significant heterogeneity in patient case mix depending on the specific disease or condition studied. For instance, one would expect that weekend trauma patients would be different than those seen during the regular workweek.49 Some large scale studies suggest that weekend patients may not be more sick than weekday patients and that any increase in weekend mortality is probably not due to factors such as severity of illness.1,7 Although we were unable to determine if there was an overall difference in illness severity between weekend and weekday patients due to the wide variety of assessments used for illness severity, our results showed statistically comparable higher mortality rate on the weekends regardless of whether the illness severity was higher, the same, or mixed between weekend and weekday patients, suggesting that general illness severity per se may not be as important as the weekend effect on mortality; however, illness severity may still have an important effect on mortality for more specific subgroups (eg, trauma).49
There are several implications of our results. We found a mean increased RR mortality of approximately 19% for patients admitted on the weekends, a number similar to one of the largest published observational studies containing almost 5 million subjects.2 Even if we took a more conservative estimate of 10% increased risk of weekend mortality, this would be equivalent to an excess of 25,000 preventable deaths per year. If the weekend effect were to be placed in context of a public health issue, the weekend effect would be the number 8 cause of death below the 29,000 deaths due to gun violence, but above the 20,000 deaths resulting from sexual behavior (sexual transmitted diseases) in 2000.3, 50,51 Although our data suggest that staffing shortfalls and decreases or delays for procedures on weekends may be associated with an increased mortality for patients admitted on the weekends, further large-scale studies are needed to confirm these findings. Increasing nurse and physician staffing levels and skill mix to cover any potential shortfall on weekends may be expensive, although theoretically, there may be savings accrued from reduced adverse events and shorter length of stay.26,52 Changes to weekend care might only benefit daytime hospitalizations because some studies have shown increased mortality during nighttime regardless of weekend or weekday admission.53
Several methodologic points in our study need to be clarified. We excluded many studies which examined the relationship of off-hours or after-hours admissions and mortality as off-hours studies typically combined weekend and after-hours weekday data. Some studies suggest that off-hour admission may be associated with increased mortality and delays in time for critical procedures during off-hours.18,19 This is a complex topic, but it is clear that the risks of hospitalization vary not just by the day of the week but also by time of the day.54 The use of meta-analyses of nonrandomized trials has been somewhat controversial,55,56 and there may be significant bias or confounding in the pooling of highly varied studies. It is important to keep in mind that there are very different definitions of weekends, populations studied, and measures of mortality rates, even as the pooled statistic suggests a homogeneity among the studies that does not exist.
There are several limitations to our study. Our systematic review may be seen as limited as we included only English language papers. In addition, we did not search nontraditional sources and abstracts. We accepted the definition of a weekend as defined by the original study, which resulted in varied definitions of weekend time period and mortality. There was a lack of specific data on staffing patterns and procedures in many studies, particularly those using databases. We were not able to further subdivide our analysis by admitting service. We were not able to undertake a subgroup analysis by country or continent, which may have implications on the effect of different healthcare systems on healthcare quality. It is unclear whether correlations in our study are a direct consequence of poorer weekend care or are the result of other unknown or unexamined differences between weekend and weekday patient populations.34,57 For instance, there may be other global factors (higher rates of medical errors, higher hospital volumes) which may not be specifically related to weekend care and therefore not been accounted for in many of the studies we examined.10,27,58-61 There may be potential bias of patient phenotypes (are weekend patients different than weekday patients?) admitted on the weekend. Holidays were included in the weekend data and it is not clear how this would affect our findings as some data suggest that there is a significantly higher mortality rate on holidays (versus weekends or weekdays),61 while other data do not.62 There was no universal definition for the timeframe for a weekend and as such, we had to rely on the original article for their determination and definition of weekend versus weekday death.
In summary, our meta-analysis suggests that hospital inpatients admitted during the weekend have a significantly increased mortality compared with those admitted on weekday. While none of our subgroup analyses showed strong evidence on effect modification, the interpretation of these results is hampered by the relatively small number of studies. Further research should be directed to determine the presence of causality between various factors purported to affect mortality and it is possible that we ultimately find that the weekend effect may exist for some but not all patients.
Acknowledgments
The authors would like to acknowledge Jaime Blanck, MLIS, MPA, AHIP, Clinical Informationist, Welch Medical Library, for her invaluable assistance in undertaking the literature searches for this manuscript.
Disclosure
This manuscript has been supported by the Department of Anesthesiology and Critical Care Medicine; The Johns Hopkins School of Medicine; Baltimore, Maryland. There are no relevant conflicts of interests.
The presence of a “weekend effect” (increased mortality rate during Saturday and/or Sunday admissions) for hospitalized inpatients is uncertain. Several observational studies1-3 suggested a positive correlation between weekend admission and increased mortality, whereas other studies demonstrated no correlation4-6 or mixed results.7,8 The majority of studies have been published only within the last decade.
Several possible reasons are cited to explain the weekend effect. Decreased and presence of inexperienced staffing on weekends may contribute to a deficit in care.7,9,10 Patients admitted during the weekend may be less likely to undergo procedures or have significant delays before receiving needed intervention.11-13 Another possibility is that there may be differences in severity of illness or comorbidities in patients admitted during the weekend compared with those admitted during the remainder of the week. Due to inconsistency between studies regarding the existence of such an effect, we performed a meta-analysis in hospitalized inpatients to delineate whether or not there is a weekend effect on mortality.
METHODS
Data Sources and Searches
This study was exempt from institutional review board review, and we utilized the recommendations from the Meta-analysis of Observational Studies in Epidemiology statement. We examined the mortality rate for hospital inpatients admitted during the weekend (weekend death) compared with the mortality rate for those admitted during the workweek (workweek death). We performed a literature search (January 1966−April 2013) of multiple databases, including PubMed, EMBASE, SCOPUS, and the Cochrane library (see Appendix). Two reviewers (LP, RJP) independently evaluated the full article of each abstract. Any disputes were resolved by a third reviewer (CW). Bibliographic references were hand searched for additional literature.
Study Selection
To be included in the systematic review, the study had to provide discrete mortality data on the weekends (including holidays) versus weekdays, include patients who were admitted as inpatients over the weekend, and be published in the English language. We excluded studies that combined weekend with weekday “off hours” (eg, weekday night shift) data, which could not be extracted or analyzed separately.
Data Extraction and Quality Assessment
Once an article was accepted to be included for the systematic review, the authors extracted relevant data if available, including study location, number and type of patients studied, patient comorbidity data, procedure-related data (type of procedure, difference in rate of procedure and time to procedure performed for both weekday and weekends), any stated and/or implied differences in staffing patterns between weekend and weekdays, and definition of mortality. We used the Newcastle-Ottawa Quality Assessment Scale to assess the quality of methodological reporting of the study.14 The definition of weekend and extraction and classification of data (weekend versus weekday) was based on the original study definition. We made no attempt to impose a universal definition of “weekend” on all studies. Similarly, the definition of mortality (eg, 3-/7-/30-day) was based according to the original study definition. Death from a patient admitted on the weekend was defined as a “weekend death” (regardless of ultimate time of death) and similarly, death from a patient admitted on a weekday was defined as a “weekday death.” Although some articles provided specific information on healthcare worker staffing patterns between weekends and weekdays, differences in weekend versus weekday staffing were implied in many articles. In these studies, staffing paradigms were considered to be different between weekend and weekdays if there were specific descriptions of the type of hospitals (urban versus rural, teaching versus nonteaching, large versus small) in the database, which would imply a typical routine staffing pattern as currently occurs in most hospitals (ie, generally less healthcare worker staff on weekends). We only included data that provided times (mean minutes/hours) from admission to the specific intervention and that provided actual rates of intervention performed for both weekend and weekday patients. We only included data that provided an actual rate of intervention performed for both weekend and weekday patients. With regard to patient comorbidities or illness severity index, we used the original studies classification (defined by the original manuscripts), which might include widely accepted global indices or a listing of specific comorbidities and/or physiologic parameters present on admission.
Data Synthesis and Analysis
We used a random effects meta-analysis approach for estimating an overall relative risk (RR) and risk differences of mortality for weekends versus weekdays, as well as subgroup specific estimates, and for computing confidence limits. The DerSimonian and Laird approach was used to estimate the random effects. Within each of the 4 subgroups (weekend staffing, procedure rates and delays, illness severity), we grouped each qualified individual study by the presence of a difference (ie, difference, no difference, or mixed) and then pooled the mortality rates for all of the studies in that group. For instance, in the subgroup of staffing, we sorted available studies by whether weekend staffing was the same or decreased versus weekday staffing, then pooled the mortality rates for studies where staffing levels were the same (versus weekday) and also separately pooled studies where staffing levels were decreased (versus weekday). Data were managed with Stata 13 (Stata Statistical Software: Release 13; StataCorp. 2013, College Station, TX) and R, and all meta-analyses were performed with the metafor package in R.15 Pooled estimated are presented as RR (95% confidence intervals [CI]).
RESULTS
A literature search retrieved a total of 594 unique citations. A review of the bibliographic references yielded an additional 20 articles. Upon evaluation, 97 studies (N = 51,114,109 patients) met inclusion criteria (Figure 1). The articles were published between 2001–2012; the kappa statistic comparing interrater reliability in the selection of articles was 0.86. Supplementary Tables 1 and 2 present a summary of study characteristics and outcomes of the accepted articles. A summary of accepted studies is in Supplementary Table 1. When summing the total number of subjects across all 97 articles, 76% were classified as weekday and 24% were weekend patients.
Weekend Admission/Inpatient Status and Mortality
The definition of the weekend varied among the included studies. The weekend time period was delineated as Friday midnight to Sunday midnight in 66% (65/99) of the studies. The remaining studies typically defined the weekend to be between Friday evening and Monday morning although studies from the Middle East generally defined the weekend as Wednesday/Thursday through Saturday. The definition of mortality also varied among researchers with most studies describing death rate as hospital inpatient mortality although some studies also examined multiple definitions of mortality (eg, 30-day all-cause mortality and hospital inpatient mortality). Not all studies provided a specific timeframe for mortality.
Fifty studies did not report a specific time frame for deaths. When a specific time frame for death was reported, the most common reported time frame was 30 days (n = 15 studies) and risk of mortality at 30 days still was higher for weekends (RR = 1.07; 95% CI,1.03-1.12; I2 = 90%). When we restricted the analysis to the studies that specified any timeframe for mortality (n = 49 studies), the risk of mortality was still significantly higher for weekends (RR = 1.12; 95% CI,1.09-1.15; I2 = 95%).
Weekend Effect Factors
We also performed subgroup analyses to investigate the overall weekend effect by hospital level factors (weekend staffing, procedure rates and delays, illness severity). Complete data were not available for all studies (staffing levels = 73 studies, time to intervention = 18 studies, rate of intervention = 30 studies, illness severity = 64 studies). Patients admitted on the weekends consistently had higher mortality than those admitted during the week, regardless of the levels of weekend/weekday differences in staffing, procedure rates and delays, illness severity (Figure 3). Analysis of studies that included staffing data for weekends revealed that decreased staffing levels on the weekends was associated with a higher mortality for weekend patients (RR = 1.16; 95% CI, 1.12-1.20; I2 = 99%; Figure 3). There was no difference in mortality for weekend patients when staffing was similar to that for the weekdays (RR = 1.21; 95% CI, 0.91-1.63; I2 = 99%).
Analysis for weekend data revealed that longer times to interventions on weekends were associated with significantly higher mortality rates (RR = 1.11; 95% CI, 1.08-1.15; I2 = 0%; Figure 3). When there were no delays to weekend procedure/interventions, there was no difference in mortality between weekend and weekday procedures/interventions (RR = 1.04; 95% CI, 0.96-1.13; I2 = 55%; Figure 3). Some articles included several procedures with “mixed” results (some procedures were “positive,” while other were “negative” for increased mortality). In studies that showed a mixed result for time to intervention, there was a significant increase in mortality (RR = 1.16; 95% CI, 1.06-1.27; I2 = 42%) for weekend patients (Figure 3).
Analyses showed a higher mortality rate on the weekends regardless of whether the rate of intervention/procedures was lower (RR=1.12; 95% CI, 1.07-1.17; I2 = 79%) or the same between weekend and weekdays (RR = 1.08; 95% CI, 1.01-1.16; I2 = 90%; Figure 3). Analyses showed a higher mortality rate on the weekends regardless of whether the illness severity was higher on the weekends (RR = 1.21; 95% CI, 1.07-1.38; I2 = 99%) or the same (RR = 1.21; 95% CI, 1.14-1.28; I2 = 99%) versus that for weekday patients (Figure 3). An inverse funnel plot for publication bias is shown in Figure 4.
DISCUSSION
We have presented one of the first meta-analyses to examine the mortality rate for hospital inpatients admitted during the weekend compared with those admitted during the workweek. We found that patients admitted on the weekends had a significantly higher overall mortality (RR = 1.19; 95% CI, 1.14-1.23; risk difference = 0.014; 95% CI, 0.013-0.016). This association was not modified by differences in weekday and weekend staffing patterns, and other hospital characteristics. Previous systematic reviews have been exclusive to the intensive care unit setting16 or did not specifically examine weekend mortality, which was a component of “off-shift” and/or “after-hours” care.17
These findings should be placed in the context of the recently published literature.18,19 A meta-analysis of cohort studies found that off-hour admission was associated with increased mortality for 28 diseases although the associations varied considerably for different diseases.18 Likewise, a meta-analysis of 21 cohort studies noted that off-hour presentation for patients with acute ischemic stroke was associated with significantly higher short-term mortality.19 Our results of increased weekend mortality corroborate that found in these two meta-analyses. However, our study differs in that we specifically examined only weekend mortality and did not include after-hours care on weekdays, which was included in the off-hour mortality in the other meta-analyses.18,19
Differences in healthcare worker staffing between weekends and weekdays have been proposed to contribute to the observed increase in mortality.7,16,20 Data indicate that lower levels of nursing are associated with increased mortality.10,21-23 The presence of less experienced and/or fewer physician specialists may contribute to increases in mortality.24-26 Fewer or less experienced staff during weekends may contribute to inadequacies in patient handovers and/or handoffs, delays in patient assessment and/or interventions, and overall continuity of care for newly admitted patients.27-33
Our data show little conclusive evidence that the weekend mortality versus weekday mortality vary by staffing level differences. While the estimated RR of mortality differs in magnitude for facilities with no difference in weekend and weekday staffing versus those that have a difference in staffing levels, both estimate an increased mortality on weekends, and the difference in these effects is not statistically significant. It should be noted that there was no difference in mortality for weekend (versus weekday) patients where there was no difference between weekend and weekday staffing; these studies were typically in high acuity units or centers where the general expectation is for 24/7/365 uniform staffing coverage.
A decrease in the use of interventions and/or procedures on weekends has been suggested to contribute to increases in mortality for patients admitted on the weekends.34 Several studies have associated lower weekend rates to higher mortality for a variety of interventions,13,35-37 although some other studies have suggested that lower procedure rates on weekends have no effect on mortality.38-40 Lower diagnostic procedure weekend rates linked to higher mortality rates may exacerbate underlying healthcare disparities.41 Our results do not conclusively show that a decrease rate of intervention and/or procedures for weekends patients is associated with a higher risk of mortality for weekends compared to weekdays.
Delays in intervention and/or procedure on weekends have also been suggested to contribute to increases in mortality.34,42 Similar to that seen with lower rates of diagnostic or therapeutic intervention and/or procedure performed on weekends, delays in potentially critical intervention and/or procedures might ultimately manifest as an increase in mortality.43 Patients admitted to the hospital on weekends and requiring an early procedure were less likely to receive it within 2 days of admission.42 Several studies have shown an association between delays in diagnostic or therapeutic intervention and/or procedure on weekends to a higher hospital inpatient mortality35,42,44,45; however, some data suggested that a delay in time to procedure on weekends may not always be associated with increased mortality.46 Depending on the procedure, there may be a threshold below which the effect of reducing delay times will have no effect on mortality rates.47,48
Patients admitted on the weekends may be different (in the severity of illness and/or comorbidities) than those admitted during the workweek and these potential differences may be a factor for increases in mortality for weekend patients. Whether there is a selection bias for weekend versus weekday patients is not clear.34 This is a complex issue as there is significant heterogeneity in patient case mix depending on the specific disease or condition studied. For instance, one would expect that weekend trauma patients would be different than those seen during the regular workweek.49 Some large scale studies suggest that weekend patients may not be more sick than weekday patients and that any increase in weekend mortality is probably not due to factors such as severity of illness.1,7 Although we were unable to determine if there was an overall difference in illness severity between weekend and weekday patients due to the wide variety of assessments used for illness severity, our results showed statistically comparable higher mortality rate on the weekends regardless of whether the illness severity was higher, the same, or mixed between weekend and weekday patients, suggesting that general illness severity per se may not be as important as the weekend effect on mortality; however, illness severity may still have an important effect on mortality for more specific subgroups (eg, trauma).49
There are several implications of our results. We found a mean increased RR mortality of approximately 19% for patients admitted on the weekends, a number similar to one of the largest published observational studies containing almost 5 million subjects.2 Even if we took a more conservative estimate of 10% increased risk of weekend mortality, this would be equivalent to an excess of 25,000 preventable deaths per year. If the weekend effect were to be placed in context of a public health issue, the weekend effect would be the number 8 cause of death below the 29,000 deaths due to gun violence, but above the 20,000 deaths resulting from sexual behavior (sexual transmitted diseases) in 2000.3, 50,51 Although our data suggest that staffing shortfalls and decreases or delays for procedures on weekends may be associated with an increased mortality for patients admitted on the weekends, further large-scale studies are needed to confirm these findings. Increasing nurse and physician staffing levels and skill mix to cover any potential shortfall on weekends may be expensive, although theoretically, there may be savings accrued from reduced adverse events and shorter length of stay.26,52 Changes to weekend care might only benefit daytime hospitalizations because some studies have shown increased mortality during nighttime regardless of weekend or weekday admission.53
Several methodologic points in our study need to be clarified. We excluded many studies which examined the relationship of off-hours or after-hours admissions and mortality as off-hours studies typically combined weekend and after-hours weekday data. Some studies suggest that off-hour admission may be associated with increased mortality and delays in time for critical procedures during off-hours.18,19 This is a complex topic, but it is clear that the risks of hospitalization vary not just by the day of the week but also by time of the day.54 The use of meta-analyses of nonrandomized trials has been somewhat controversial,55,56 and there may be significant bias or confounding in the pooling of highly varied studies. It is important to keep in mind that there are very different definitions of weekends, populations studied, and measures of mortality rates, even as the pooled statistic suggests a homogeneity among the studies that does not exist.
There are several limitations to our study. Our systematic review may be seen as limited as we included only English language papers. In addition, we did not search nontraditional sources and abstracts. We accepted the definition of a weekend as defined by the original study, which resulted in varied definitions of weekend time period and mortality. There was a lack of specific data on staffing patterns and procedures in many studies, particularly those using databases. We were not able to further subdivide our analysis by admitting service. We were not able to undertake a subgroup analysis by country or continent, which may have implications on the effect of different healthcare systems on healthcare quality. It is unclear whether correlations in our study are a direct consequence of poorer weekend care or are the result of other unknown or unexamined differences between weekend and weekday patient populations.34,57 For instance, there may be other global factors (higher rates of medical errors, higher hospital volumes) which may not be specifically related to weekend care and therefore not been accounted for in many of the studies we examined.10,27,58-61 There may be potential bias of patient phenotypes (are weekend patients different than weekday patients?) admitted on the weekend. Holidays were included in the weekend data and it is not clear how this would affect our findings as some data suggest that there is a significantly higher mortality rate on holidays (versus weekends or weekdays),61 while other data do not.62 There was no universal definition for the timeframe for a weekend and as such, we had to rely on the original article for their determination and definition of weekend versus weekday death.
In summary, our meta-analysis suggests that hospital inpatients admitted during the weekend have a significantly increased mortality compared with those admitted on weekday. While none of our subgroup analyses showed strong evidence on effect modification, the interpretation of these results is hampered by the relatively small number of studies. Further research should be directed to determine the presence of causality between various factors purported to affect mortality and it is possible that we ultimately find that the weekend effect may exist for some but not all patients.
Acknowledgments
The authors would like to acknowledge Jaime Blanck, MLIS, MPA, AHIP, Clinical Informationist, Welch Medical Library, for her invaluable assistance in undertaking the literature searches for this manuscript.
Disclosure
This manuscript has been supported by the Department of Anesthesiology and Critical Care Medicine; The Johns Hopkins School of Medicine; Baltimore, Maryland. There are no relevant conflicts of interests.
1. Aylin P, Yunus A, Bottle A, Majeed A, Bell D. Weekend mortality for emergency
admissions. A large, multicentre study. Qual Saf Health Care. 2010;19(3):213-217. PubMed
2. Handel AE, Patel SV, Skingsley A, Bramley K, Sobieski R, Ramagopalan SV.
Weekend admissions as an independent predictor of mortality: an analysis of
Scottish hospital admissions. BMJ Open. 2012;2(6): pii: e001789. PubMed
3. Ricciardi R, Roberts PL, Read TE, Baxter NN, Marcello PW, Schoetz DJ. Mortality
rate after nonelective hospital admission. Arch Surg. 2011;146(5):545-551. PubMed
4. Fonarow GC, Abraham WT, Albert NM, et al. Day of admission and clinical
outcomes for patients hospitalized for heart failure: findings from the Organized
Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart
Failure (OPTIMIZE-HF). Circ Heart Fail. 2008;1(1):50-57. PubMed
5. Hoh BL, Chi YY, Waters MF, Mocco J, Barker FG 2nd. Effect of weekend compared
with weekday stroke admission on thrombolytic use, in-hospital mortality,
discharge disposition, hospital charges, and length of stay in the Nationwide Inpatient
Sample Database, 2002 to 2007. Stroke. 2010;41(10):2323-2328. PubMed
6. Koike S, Tanabe S, Ogawa T, et al. Effect of time and day of admission on 1-month
survival and neurologically favourable 1-month survival in out-of-hospital cardiopulmonary
arrest patients. Resuscitation. 2011;82(7):863-868. PubMed
7. Bell CM, Redelmeier DA. Mortality among patients admitted to hospitals on
weekends as compared with weekdays. N Engl J Med. 2001;345(9):663-668. PubMed
8. Freemantle N, Richardson M, Wood J, et al. Weekend hospitalization and additional
risk of death: an analysis of inpatient data. J R Soc Med. 2012;105(2):74-84. PubMed
9. Schilling PL, Campbell DA Jr, Englesbe MJ, Davis MM. A comparison of in-hospital
mortality risk conferred by high hospital occupancy, differences in nurse
staffing levels, weekend admission, and seasonal influenza. Med Care. 2010;48(3):
224-232. PubMed
10. Wong HJ, Morra D. Excellent hospital care for all: open and operating 24/7. J Gen
Intern Med. 2011;26(9):1050-1052. PubMed
11. Dorn SD, Shah ND, Berg BP, Naessens JM. Effect of weekend hospital admission
on gastrointestinal hemorrhage outcomes. Dig Dis Sci. 2010;55(6):1658-1666. PubMed
12. Kostis WJ, Demissie K, Marcella SW, et al. Weekend versus weekday admission
and mortality from myocardial infarction. N Engl J Med. 2007;356(11):1099-1109. PubMed
13. McKinney JS, Deng Y, Kasner SE, Kostis JB; Myocardial Infarction Data Acquisition
System (MIDAS 15) Study Group. Comprehensive stroke centers overcome
the weekend versus weekday gap in stroke treatment and mortality. Stroke.
2011;42(9):2403-2409. PubMed
14. Margulis AV, Pladevall M, Riera-Guardia N, et al. Quality assessment of observational
studies in a drug-safety systematic review, comparison of two tools: the
Newcastle-Ottawa Scale and the RTI item bank. Clin Epidemiol. 2014;6:359-368. PubMed
15. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat
Softw. 2010;36(3):1-48.
16. Cavallazzi R, Marik PE, Hirani A, Pachinburavan M, Vasu TS, Leiby BE. Association
between time of admission to the ICU and mortality: a systematic review and
metaanalysis. Chest. 2010;138(1):68-75. PubMed
17. de Cordova PB, Phibbs CS, Bartel AP, Stone PW. Twenty-four/seven: a
mixed-method systematic review of the off-shift literature. J Adv Nurs.
2012;68(7):1454-1468. PubMed
18. Zhou Y, Li W, Herath C, Xia J, Hu B, Song F, Cao S, Lu Z. Off-hour admission and
mortality risk for 28 specific diseases: a systematic review and meta-analysis of 251
cohorts. J Am Heart Assoc. 2016;5(3):e003102. PubMed
19. Sorita A, Ahmed A, Starr SR, et al. Off-hour presentation and outcomes in
patients with acute myocardial infarction: systematic review and meta-analysis.
BMJ. 2014;348:f7393. PubMed
20. Ricciardi R, Nelson J, Roberts PL, Marcello PW, Read TE, Schoetz DJ. Is the
presence of medical trainees associated with increased mortality with weekend
admission? BMC Med Educ. 2014;14(1):4. PubMed
21. Needleman J, Buerhaus P, Pankratz VS, Leibson CL, Stevens SR, Harris M. Nurse
staffing and inpatient hospital mortality. N Engl J Med. 2011;364(11):1037-1045. PubMed
22. Aiken LH, Clarke SP, Sloane DM, Sochalski J, Silber JH. Hospital nurse
staffing and patient mortality, nurse burnout, and job dissatisfaction. JAMA.
2002;288(16):1987-1993. PubMed
23. Hamilton KE, Redshaw ME, Tarnow-Mordi W. Nurse staffing in relation to
risk-adjusted mortality in neonatal care. Arch Dis Child Fetal Neonatal Ed.
2007;92(2):F99-F103. PubMed
766 An Official Publication of the Society of Hospital Medicine Journal of Hospital Medicine Vol 12 | No 9 | September 2017
Pauls et al | The Weekend Effect: A Meta-Analysis
24. Haut ER, Chang DC, Efron DT, Cornwell EE 3rd. Injured patients have lower
mortality when treated by “full-time” trauma surgeons vs. surgeons who cover
trauma “part-time”. J Trauma. 2006;61(2):272-278. PubMed
25. Wallace DJ, Angus DC, Barnato AE, Kramer AA, Kahn JM. Nighttime intensivist
staffing and mortality among critically ill patients. N Engl J Med.
2012;366(22):2093-2101. PubMed
26. Pronovost PJ, Angus DC, Dorman T, Robinson KA, Dremsizov TT, Young TL.
Physician staffing patterns and clinical outcomes in critically ill patients: a systematic
review. JAMA. 2002;288(17):2151-2162. PubMed
27. Weissman JS, Rothschild JM, Bendavid E, et al. Hospital workload and adverse
events. Med Care. 2007;45(5):448-455. PubMed
28. Hamilton P, Eschiti VS, Hernandez K, Neill D. Differences between weekend and
weekday nurse work environments and patient outcomes: a focus group approach
to model testing. J Perinat Neonatal Nurs. 2007;21(4):331-341. PubMed
29. Johner AM, Merchant S, Aslani N, et al Acute general surgery in Canada: a survey
of current handover practices. Can J Surg. 2013;56(3):E24-E28. PubMed
30. de Cordova PB, Phibbs CS, Stone PW. Perceptions and observations of off-shift
nursing. J Nurs Manag. 2013;21(2):283-292. PubMed
31. Pfeffer PE, Nazareth D, Main N, Hardoon S, Choudhury AB. Are weekend
handovers of adequate quality for the on-call general medical team? Clin Med.
2011;11(6):536-540. PubMed
32. Eschiti V, Hamilton P. Off-peak nurse staffing: critical-care nurses speak. Dimens
Crit Care Nurs. 2011;30(1):62-69. PubMed
33. Button LA, Roberts SE, Evans PA, et al. Hospitalized incidence and case fatality
for upper gastrointestinal bleeding from 1999 to 2007: a record linkage study. Aliment
Pharmacol Ther. 2011;33(1):64-76. PubMed
34. Becker DJ. Weekend hospitalization and mortality: a critical review. Expert Rev
Pharmacoecon Outcomes Res. 2008;8(1):23-26. PubMed
35. Deshmukh A, Pant S, Kumar G, Bursac Z, Paydak H, Mehta JL. Comparison of
outcomes of weekend versus weekday admissions for atrial fibrillation. Am J Cardiol.
2012;110(2):208-211. PubMed
36. Nanchal R, Kumar G, Taneja A, et al. Pulmonary embolism: the weekend effect.
Chest. 2012;142(3):690-696. PubMed
37. Palmer WL, Bottle A, Davie C, Vincent CA, Aylin P. Dying for the weekend: a
retrospective cohort study on the association between day of hospital presentation
and the quality and safety of stroke care. Arch Neurol. 2012;69(10):1296-1302. PubMed
38. Dasenbrock HH, Pradilla G, Witham TF, Gokaslan ZL, Bydon A. The impact
of weekend hospital admission on the timing of intervention and outcomes after
surgery for spinal metastases. Neurosurgery. 2012;70(3):586-593. PubMed
39. Jairath V, Kahan BC, Logan RF, et al. Mortality from acute upper gastrointestinal
bleeding in the United Kingdom: does it display a “weekend effect”? Am J Gastroenterol.
2011;106(9):1621-1628. PubMed
40. Myers RP, Kaplan GG, Shaheen AM. The effect of weekend versus weekday
admission on outcomes of esophageal variceal hemorrhage. Can J Gastroenterol.
2009;23(7):495-501. PubMed
41. Rudd AG, Hoffman A, Down C, Pearson M, Lowe D. Access to stroke care in
England, Wales and Northern Ireland: the effect of age, gender and weekend admission.
Age Ageing. 2007;36(3):247-255. PubMed
42. Lapointe-Shaw L, Abushomar H, Chen XK, et al. Care and outcomes of patients
with cancer admitted to the hospital on weekends and holidays: a retrospective
cohort study. J Natl Compr Canc Netw. 2016;14(7):867-874. PubMed
43. Chan PS, Krumholz HM, Nichol G, Nallamothu BK; American Heart Association
National Registry of Cardiopulmonary Resuscitation Investigators. Delayed time to
defibrillation after in-hospital cardiac arrest. N Engl J Med. 2008;358(1):9-17. PubMed
44. McGuire KJ, Bernstein J, Polsky D, Silber JH. The 2004 Marshall Urist Award:
Delays until surgery after hip fracture increases mortality. Clin Orthop Relat Res.
2004;(428):294-301. PubMed
45. Krüth P, Zeymer U, Gitt A, et al. Influence of presentation at the weekend on
treatment and outcome in ST-elevation myocardial infarction in hospitals with
catheterization laboratories. Clin Res Cardiol. 2008;97(10):742-747. PubMed
46. Jneid H, Fonarow GC, Cannon CP, et al. Impact of time of presentation on the care
and outcomes of acute myocardial infarction. Circulation. 2008;117(19):2502-2509. PubMed
47. Menees DS, Peterson ED, Wang Y, et al. Door-to-balloon time and mortality
among patients undergoing primary PCI. N Engl J Med. 2013;369(10):901-909. PubMed
48. Bates ER, Jacobs AK. Time to treatment in patients with STEMI. N Engl J Med.
2013;369(10):889-892. PubMed
49. Carmody IC, Romero J, Velmahos GC. Day for night: should we staff a trauma
center like a nightclub? Am Surg. 2002;68(12):1048-1051. PubMed
50. Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the
United States, 2000. JAMA. 2004;291(10):1238-1245. PubMed
51. McCook A. More hospital deaths on weekends. http://www.reuters.com/article/
2011/05/20/us-more-hospital-deaths-weekends-idUSTRE74J5RM20110520.
Accessed March 7, 2017.
52. Mourad M, Adler J. Safe, high quality care around the clock: what will it take to
get us there? J Gen Intern Med. 2011;26(9):948-950. PubMed
53. Magid DJ, Wang Y, Herrin J, et al. Relationship between time of day, day of week,
timeliness of reperfusion, and in-hospital mortality for patients with acute ST-segment
elevation myocardial infarction. JAMA. 2005;294(7):803-812. PubMed
54. Coiera E, Wang Y, Magrabi F, Concha OP, Gallego B, Runciman W. Predicting
the cumulative risk of death during hospitalization by modeling weekend, weekday
and diurnal mortality risks. BMC Health Serv Res. 2014;14:226. PubMed
55. Greenland S. Can meta-analysis be salvaged? Am J Epidemiol. 1994;140(9):783-787. PubMed
56. Shapiro S. Meta-analysis/Shmeta-analysis. Am J Epidemiol. 1994;140(9):771-778. PubMed
57. Halm EA, Chassin MR. Why do hospital death rates vary? N Engl J Med.
2001;345(9):692-694. PubMed
58. Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality
in the United States. N Engl J Med. 2002;346(15):1128-1137. PubMed
59. Kaier K, Mutters NT, Frank U. Bed occupancy rates and hospital-acquired infections
– should beds be kept empty? Clin Microbiol Infect. 2012;18(10):941-945. PubMed
60. Chrusch CA, Olafson KP, McMillian PM, Roberts DE, Gray PR. High occupancy
increases the risk of early death or readmission after transfer from intensive care.
Crit Care Med. 2009;37(10):2753-2758. PubMed
61. Foss NB, Kehlet H. Short-term mortality in hip fracture patients admitted during
weekends and holidays. Br J Anaesth. 2006;96(4):450-454. PubMed
62. Daugaard CL, Jørgensen HL, Riis T, Lauritzen JB, Duus BR, van der Mark S. Is
mortality after hip fracture associated with surgical delay or admission during
weekends and public holidays? A retrospective study of 38,020 patients. Acta Orthop.
2012;83(6):609-613. PubMed
1. Aylin P, Yunus A, Bottle A, Majeed A, Bell D. Weekend mortality for emergency
admissions. A large, multicentre study. Qual Saf Health Care. 2010;19(3):213-217. PubMed
2. Handel AE, Patel SV, Skingsley A, Bramley K, Sobieski R, Ramagopalan SV.
Weekend admissions as an independent predictor of mortality: an analysis of
Scottish hospital admissions. BMJ Open. 2012;2(6): pii: e001789. PubMed
3. Ricciardi R, Roberts PL, Read TE, Baxter NN, Marcello PW, Schoetz DJ. Mortality
rate after nonelective hospital admission. Arch Surg. 2011;146(5):545-551. PubMed
4. Fonarow GC, Abraham WT, Albert NM, et al. Day of admission and clinical
outcomes for patients hospitalized for heart failure: findings from the Organized
Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart
Failure (OPTIMIZE-HF). Circ Heart Fail. 2008;1(1):50-57. PubMed
5. Hoh BL, Chi YY, Waters MF, Mocco J, Barker FG 2nd. Effect of weekend compared
with weekday stroke admission on thrombolytic use, in-hospital mortality,
discharge disposition, hospital charges, and length of stay in the Nationwide Inpatient
Sample Database, 2002 to 2007. Stroke. 2010;41(10):2323-2328. PubMed
6. Koike S, Tanabe S, Ogawa T, et al. Effect of time and day of admission on 1-month
survival and neurologically favourable 1-month survival in out-of-hospital cardiopulmonary
arrest patients. Resuscitation. 2011;82(7):863-868. PubMed
7. Bell CM, Redelmeier DA. Mortality among patients admitted to hospitals on
weekends as compared with weekdays. N Engl J Med. 2001;345(9):663-668. PubMed
8. Freemantle N, Richardson M, Wood J, et al. Weekend hospitalization and additional
risk of death: an analysis of inpatient data. J R Soc Med. 2012;105(2):74-84. PubMed
9. Schilling PL, Campbell DA Jr, Englesbe MJ, Davis MM. A comparison of in-hospital
mortality risk conferred by high hospital occupancy, differences in nurse
staffing levels, weekend admission, and seasonal influenza. Med Care. 2010;48(3):
224-232. PubMed
10. Wong HJ, Morra D. Excellent hospital care for all: open and operating 24/7. J Gen
Intern Med. 2011;26(9):1050-1052. PubMed
11. Dorn SD, Shah ND, Berg BP, Naessens JM. Effect of weekend hospital admission
on gastrointestinal hemorrhage outcomes. Dig Dis Sci. 2010;55(6):1658-1666. PubMed
12. Kostis WJ, Demissie K, Marcella SW, et al. Weekend versus weekday admission
and mortality from myocardial infarction. N Engl J Med. 2007;356(11):1099-1109. PubMed
13. McKinney JS, Deng Y, Kasner SE, Kostis JB; Myocardial Infarction Data Acquisition
System (MIDAS 15) Study Group. Comprehensive stroke centers overcome
the weekend versus weekday gap in stroke treatment and mortality. Stroke.
2011;42(9):2403-2409. PubMed
14. Margulis AV, Pladevall M, Riera-Guardia N, et al. Quality assessment of observational
studies in a drug-safety systematic review, comparison of two tools: the
Newcastle-Ottawa Scale and the RTI item bank. Clin Epidemiol. 2014;6:359-368. PubMed
15. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat
Softw. 2010;36(3):1-48.
16. Cavallazzi R, Marik PE, Hirani A, Pachinburavan M, Vasu TS, Leiby BE. Association
between time of admission to the ICU and mortality: a systematic review and
metaanalysis. Chest. 2010;138(1):68-75. PubMed
17. de Cordova PB, Phibbs CS, Bartel AP, Stone PW. Twenty-four/seven: a
mixed-method systematic review of the off-shift literature. J Adv Nurs.
2012;68(7):1454-1468. PubMed
18. Zhou Y, Li W, Herath C, Xia J, Hu B, Song F, Cao S, Lu Z. Off-hour admission and
mortality risk for 28 specific diseases: a systematic review and meta-analysis of 251
cohorts. J Am Heart Assoc. 2016;5(3):e003102. PubMed
19. Sorita A, Ahmed A, Starr SR, et al. Off-hour presentation and outcomes in
patients with acute myocardial infarction: systematic review and meta-analysis.
BMJ. 2014;348:f7393. PubMed
20. Ricciardi R, Nelson J, Roberts PL, Marcello PW, Read TE, Schoetz DJ. Is the
presence of medical trainees associated with increased mortality with weekend
admission? BMC Med Educ. 2014;14(1):4. PubMed
21. Needleman J, Buerhaus P, Pankratz VS, Leibson CL, Stevens SR, Harris M. Nurse
staffing and inpatient hospital mortality. N Engl J Med. 2011;364(11):1037-1045. PubMed
22. Aiken LH, Clarke SP, Sloane DM, Sochalski J, Silber JH. Hospital nurse
staffing and patient mortality, nurse burnout, and job dissatisfaction. JAMA.
2002;288(16):1987-1993. PubMed
23. Hamilton KE, Redshaw ME, Tarnow-Mordi W. Nurse staffing in relation to
risk-adjusted mortality in neonatal care. Arch Dis Child Fetal Neonatal Ed.
2007;92(2):F99-F103. PubMed
766 An Official Publication of the Society of Hospital Medicine Journal of Hospital Medicine Vol 12 | No 9 | September 2017
Pauls et al | The Weekend Effect: A Meta-Analysis
24. Haut ER, Chang DC, Efron DT, Cornwell EE 3rd. Injured patients have lower
mortality when treated by “full-time” trauma surgeons vs. surgeons who cover
trauma “part-time”. J Trauma. 2006;61(2):272-278. PubMed
25. Wallace DJ, Angus DC, Barnato AE, Kramer AA, Kahn JM. Nighttime intensivist
staffing and mortality among critically ill patients. N Engl J Med.
2012;366(22):2093-2101. PubMed
26. Pronovost PJ, Angus DC, Dorman T, Robinson KA, Dremsizov TT, Young TL.
Physician staffing patterns and clinical outcomes in critically ill patients: a systematic
review. JAMA. 2002;288(17):2151-2162. PubMed
27. Weissman JS, Rothschild JM, Bendavid E, et al. Hospital workload and adverse
events. Med Care. 2007;45(5):448-455. PubMed
28. Hamilton P, Eschiti VS, Hernandez K, Neill D. Differences between weekend and
weekday nurse work environments and patient outcomes: a focus group approach
to model testing. J Perinat Neonatal Nurs. 2007;21(4):331-341. PubMed
29. Johner AM, Merchant S, Aslani N, et al Acute general surgery in Canada: a survey
of current handover practices. Can J Surg. 2013;56(3):E24-E28. PubMed
30. de Cordova PB, Phibbs CS, Stone PW. Perceptions and observations of off-shift
nursing. J Nurs Manag. 2013;21(2):283-292. PubMed
31. Pfeffer PE, Nazareth D, Main N, Hardoon S, Choudhury AB. Are weekend
handovers of adequate quality for the on-call general medical team? Clin Med.
2011;11(6):536-540. PubMed
32. Eschiti V, Hamilton P. Off-peak nurse staffing: critical-care nurses speak. Dimens
Crit Care Nurs. 2011;30(1):62-69. PubMed
33. Button LA, Roberts SE, Evans PA, et al. Hospitalized incidence and case fatality
for upper gastrointestinal bleeding from 1999 to 2007: a record linkage study. Aliment
Pharmacol Ther. 2011;33(1):64-76. PubMed
34. Becker DJ. Weekend hospitalization and mortality: a critical review. Expert Rev
Pharmacoecon Outcomes Res. 2008;8(1):23-26. PubMed
35. Deshmukh A, Pant S, Kumar G, Bursac Z, Paydak H, Mehta JL. Comparison of
outcomes of weekend versus weekday admissions for atrial fibrillation. Am J Cardiol.
2012;110(2):208-211. PubMed
36. Nanchal R, Kumar G, Taneja A, et al. Pulmonary embolism: the weekend effect.
Chest. 2012;142(3):690-696. PubMed
37. Palmer WL, Bottle A, Davie C, Vincent CA, Aylin P. Dying for the weekend: a
retrospective cohort study on the association between day of hospital presentation
and the quality and safety of stroke care. Arch Neurol. 2012;69(10):1296-1302. PubMed
38. Dasenbrock HH, Pradilla G, Witham TF, Gokaslan ZL, Bydon A. The impact
of weekend hospital admission on the timing of intervention and outcomes after
surgery for spinal metastases. Neurosurgery. 2012;70(3):586-593. PubMed
39. Jairath V, Kahan BC, Logan RF, et al. Mortality from acute upper gastrointestinal
bleeding in the United Kingdom: does it display a “weekend effect”? Am J Gastroenterol.
2011;106(9):1621-1628. PubMed
40. Myers RP, Kaplan GG, Shaheen AM. The effect of weekend versus weekday
admission on outcomes of esophageal variceal hemorrhage. Can J Gastroenterol.
2009;23(7):495-501. PubMed
41. Rudd AG, Hoffman A, Down C, Pearson M, Lowe D. Access to stroke care in
England, Wales and Northern Ireland: the effect of age, gender and weekend admission.
Age Ageing. 2007;36(3):247-255. PubMed
42. Lapointe-Shaw L, Abushomar H, Chen XK, et al. Care and outcomes of patients
with cancer admitted to the hospital on weekends and holidays: a retrospective
cohort study. J Natl Compr Canc Netw. 2016;14(7):867-874. PubMed
43. Chan PS, Krumholz HM, Nichol G, Nallamothu BK; American Heart Association
National Registry of Cardiopulmonary Resuscitation Investigators. Delayed time to
defibrillation after in-hospital cardiac arrest. N Engl J Med. 2008;358(1):9-17. PubMed
44. McGuire KJ, Bernstein J, Polsky D, Silber JH. The 2004 Marshall Urist Award:
Delays until surgery after hip fracture increases mortality. Clin Orthop Relat Res.
2004;(428):294-301. PubMed
45. Krüth P, Zeymer U, Gitt A, et al. Influence of presentation at the weekend on
treatment and outcome in ST-elevation myocardial infarction in hospitals with
catheterization laboratories. Clin Res Cardiol. 2008;97(10):742-747. PubMed
46. Jneid H, Fonarow GC, Cannon CP, et al. Impact of time of presentation on the care
and outcomes of acute myocardial infarction. Circulation. 2008;117(19):2502-2509. PubMed
47. Menees DS, Peterson ED, Wang Y, et al. Door-to-balloon time and mortality
among patients undergoing primary PCI. N Engl J Med. 2013;369(10):901-909. PubMed
48. Bates ER, Jacobs AK. Time to treatment in patients with STEMI. N Engl J Med.
2013;369(10):889-892. PubMed
49. Carmody IC, Romero J, Velmahos GC. Day for night: should we staff a trauma
center like a nightclub? Am Surg. 2002;68(12):1048-1051. PubMed
50. Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the
United States, 2000. JAMA. 2004;291(10):1238-1245. PubMed
51. McCook A. More hospital deaths on weekends. http://www.reuters.com/article/
2011/05/20/us-more-hospital-deaths-weekends-idUSTRE74J5RM20110520.
Accessed March 7, 2017.
52. Mourad M, Adler J. Safe, high quality care around the clock: what will it take to
get us there? J Gen Intern Med. 2011;26(9):948-950. PubMed
53. Magid DJ, Wang Y, Herrin J, et al. Relationship between time of day, day of week,
timeliness of reperfusion, and in-hospital mortality for patients with acute ST-segment
elevation myocardial infarction. JAMA. 2005;294(7):803-812. PubMed
54. Coiera E, Wang Y, Magrabi F, Concha OP, Gallego B, Runciman W. Predicting
the cumulative risk of death during hospitalization by modeling weekend, weekday
and diurnal mortality risks. BMC Health Serv Res. 2014;14:226. PubMed
55. Greenland S. Can meta-analysis be salvaged? Am J Epidemiol. 1994;140(9):783-787. PubMed
56. Shapiro S. Meta-analysis/Shmeta-analysis. Am J Epidemiol. 1994;140(9):771-778. PubMed
57. Halm EA, Chassin MR. Why do hospital death rates vary? N Engl J Med.
2001;345(9):692-694. PubMed
58. Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality
in the United States. N Engl J Med. 2002;346(15):1128-1137. PubMed
59. Kaier K, Mutters NT, Frank U. Bed occupancy rates and hospital-acquired infections
– should beds be kept empty? Clin Microbiol Infect. 2012;18(10):941-945. PubMed
60. Chrusch CA, Olafson KP, McMillian PM, Roberts DE, Gray PR. High occupancy
increases the risk of early death or readmission after transfer from intensive care.
Crit Care Med. 2009;37(10):2753-2758. PubMed
61. Foss NB, Kehlet H. Short-term mortality in hip fracture patients admitted during
weekends and holidays. Br J Anaesth. 2006;96(4):450-454. PubMed
62. Daugaard CL, Jørgensen HL, Riis T, Lauritzen JB, Duus BR, van der Mark S. Is
mortality after hip fracture associated with surgical delay or admission during
weekends and public holidays? A retrospective study of 38,020 patients. Acta Orthop.
2012;83(6):609-613. PubMed
© 2017 Society of Hospital Medicine