User login
‘Flakka’: A low-cost, dangerous high
Use of α-pyrrolidinovalerophenone (α-PVP), a psychostimulant related to cathinone derivatives (“bath salts”), has been reported in the United States, especially in Florida.1 Known by the street names “flakka” or “gravel,” α-PVP is inexpensive, with a single dose (typically 100 mg) costing as little as $5.2 Alpha-PVP can be consumed via ingestion, injection, insufflation, or inhalation in vaporized forms, such as E-cigarettes, which deliver the drug quickly into the bloodstream and can make it easy to overdose.1 The low cost of this drug makes it likely to be abused. Here we review the mechanism of action and effects of α-PVP and summarize treatment options.
Mechanism of action
Alpha-PVP is a structural parent of 3,4-methylenedioxypyrovalerone (MDPV)—the first widely abused synthetic cathinone.3 Much like cocaine, α-PVP stimulates the CNS by acting as a potent dopamine and norepinephrine reuptake inhibitor. However, unlike cocaine, it lacks any action on serotonin transporters. The pyrrolidine ring in MDPV and α-PVP is responsible for the highly potent dopamine reuptake inhibitor action of these agents.3
A wide range of adverse effects
Use of α-PVP results in a state of “excited delirium,” with symptoms such as hyperthermia, hallucinations, paranoia, violent aggression, and self-harm.1 Alpha-PVP is known to cause rhabdomyolysis.4 Some studies have reported cardiovascular effects, such as arterial hypertension, palpitations, dyspnea, vasoconstriction, arrhythmia, myocardial infarction (MI), and myocarditis.5 Alpha-PVP also may result in neurologic symptoms, including headache, mydriasis, lightheadedness, paresthesia, seizures, dystonic movements, tremor, amnesia, dysgeusia, cerebral edema, motor automatisms, muscle spasm, nystagmus, parkinsonism, and stroke.5 Death may occur by cardiac arrest, renal damage, or suicide.
Case reports. The effects of α-PVP have been documented in the literature:
- A 17-year-old girl was brought to an emergency department in Florida with acute onset of bizarre behavior, agitation, and altered mental status. It took 6 days and repeated administrations of olanzapine and lorazepam for the patient to become calm, alert, and oriented.2
- ST-elevated MI with several intracardiac thrombi was reported in a 41-year-old woman who used α-PVP.4
- In 2015, 18 deaths related to α-PVP use were reported in South Florida.5
- Deaths related to α-PVP use also have been reported in Japan and Australia.5
Treatment options
There are no treatment guidelines for α-PVP-related psychiatric symptoms. Case reports describe remission of symptoms following aggressive treatment with antipsychotics and benzodiazepines.2 Guidelines for treatment of stimulant-induced behavioral and psychotic symptoms6 may be considered for patients who have used α-PVP.
Reassurance and supportive care are the basic principles of such interventions. A quiet environment and benzodiazepines may provide relief of agitation. Antipsychotics may be helpful if a patient exhibits psychotic symptoms.
Similar drugs may emerge
In 2014, the DEA classified α-PVP as a Schedule I substance. Laws against the import of such substances via the Internet or other means also may help control the spread of this drug. However, chemically similar drugs that may elude drug screens are continually emerging. The lack of evidence-based guidelines on recognizing and managing intoxication, withdrawal, and long-term effects of α-PVP and other “designer drugs” calls for greater research in this emerging area of substance use disorders.
1. National Institute on Drug Abuse. “Flakka” (alpha-PVP). https://www.drugabuse.gov/emerging-trends/flakka-alpha-pvp. Accessed July 26, 2017.
2. Crespi C. Flakka-induced prolonged psychosis. Case Rep Psychiatry. 2016;2016:3460849. doi: 10.1155/2016/3460849.
3. Glennon RA, Young R. Neurobiology of 3,4-methylenedioxypyrovalerone (MDPV) and α-pyrrolidinovalerophenone (α-PVP). Brain Res Bull. 2016;126(pt 1):111-126.
4. Cherry SV, Rodriguez YF. Synthetic stimulant reaching epidemic proportions: flakka-induced ST-elevation myocardial infarction with intracardiac thrombi. J Cardiothorac Vasc Anesth. 2017;31(1):e13-e14.
5. Katselou M, Papoutsis I, Nikolaou P, et al. α-PVP (“flakka”): a new synthetic cathinone invades the drug arena. Forensic Toxicol. 2016;34(1):41-50.
6. Sadock BJ, Sadock VA, Ruiz P. Hallucinogen-related disorders. In: Sadock BJ, Sadock VA, Ruiz P. Kaplan and Sadock’s synopsis of psychiatry: behavioral sciences/clinical psychiatry. 11th ed. Philadelphia, PA: Wolters Kluwer; 2015:648-655.
Use of α-pyrrolidinovalerophenone (α-PVP), a psychostimulant related to cathinone derivatives (“bath salts”), has been reported in the United States, especially in Florida.1 Known by the street names “flakka” or “gravel,” α-PVP is inexpensive, with a single dose (typically 100 mg) costing as little as $5.2 Alpha-PVP can be consumed via ingestion, injection, insufflation, or inhalation in vaporized forms, such as E-cigarettes, which deliver the drug quickly into the bloodstream and can make it easy to overdose.1 The low cost of this drug makes it likely to be abused. Here we review the mechanism of action and effects of α-PVP and summarize treatment options.
Mechanism of action
Alpha-PVP is a structural parent of 3,4-methylenedioxypyrovalerone (MDPV)—the first widely abused synthetic cathinone.3 Much like cocaine, α-PVP stimulates the CNS by acting as a potent dopamine and norepinephrine reuptake inhibitor. However, unlike cocaine, it lacks any action on serotonin transporters. The pyrrolidine ring in MDPV and α-PVP is responsible for the highly potent dopamine reuptake inhibitor action of these agents.3
A wide range of adverse effects
Use of α-PVP results in a state of “excited delirium,” with symptoms such as hyperthermia, hallucinations, paranoia, violent aggression, and self-harm.1 Alpha-PVP is known to cause rhabdomyolysis.4 Some studies have reported cardiovascular effects, such as arterial hypertension, palpitations, dyspnea, vasoconstriction, arrhythmia, myocardial infarction (MI), and myocarditis.5 Alpha-PVP also may result in neurologic symptoms, including headache, mydriasis, lightheadedness, paresthesia, seizures, dystonic movements, tremor, amnesia, dysgeusia, cerebral edema, motor automatisms, muscle spasm, nystagmus, parkinsonism, and stroke.5 Death may occur by cardiac arrest, renal damage, or suicide.
Case reports. The effects of α-PVP have been documented in the literature:
- A 17-year-old girl was brought to an emergency department in Florida with acute onset of bizarre behavior, agitation, and altered mental status. It took 6 days and repeated administrations of olanzapine and lorazepam for the patient to become calm, alert, and oriented.2
- ST-elevated MI with several intracardiac thrombi was reported in a 41-year-old woman who used α-PVP.4
- In 2015, 18 deaths related to α-PVP use were reported in South Florida.5
- Deaths related to α-PVP use also have been reported in Japan and Australia.5
Treatment options
There are no treatment guidelines for α-PVP-related psychiatric symptoms. Case reports describe remission of symptoms following aggressive treatment with antipsychotics and benzodiazepines.2 Guidelines for treatment of stimulant-induced behavioral and psychotic symptoms6 may be considered for patients who have used α-PVP.
Reassurance and supportive care are the basic principles of such interventions. A quiet environment and benzodiazepines may provide relief of agitation. Antipsychotics may be helpful if a patient exhibits psychotic symptoms.
Similar drugs may emerge
In 2014, the DEA classified α-PVP as a Schedule I substance. Laws against the import of such substances via the Internet or other means also may help control the spread of this drug. However, chemically similar drugs that may elude drug screens are continually emerging. The lack of evidence-based guidelines on recognizing and managing intoxication, withdrawal, and long-term effects of α-PVP and other “designer drugs” calls for greater research in this emerging area of substance use disorders.
Use of α-pyrrolidinovalerophenone (α-PVP), a psychostimulant related to cathinone derivatives (“bath salts”), has been reported in the United States, especially in Florida.1 Known by the street names “flakka” or “gravel,” α-PVP is inexpensive, with a single dose (typically 100 mg) costing as little as $5.2 Alpha-PVP can be consumed via ingestion, injection, insufflation, or inhalation in vaporized forms, such as E-cigarettes, which deliver the drug quickly into the bloodstream and can make it easy to overdose.1 The low cost of this drug makes it likely to be abused. Here we review the mechanism of action and effects of α-PVP and summarize treatment options.
Mechanism of action
Alpha-PVP is a structural parent of 3,4-methylenedioxypyrovalerone (MDPV)—the first widely abused synthetic cathinone.3 Much like cocaine, α-PVP stimulates the CNS by acting as a potent dopamine and norepinephrine reuptake inhibitor. However, unlike cocaine, it lacks any action on serotonin transporters. The pyrrolidine ring in MDPV and α-PVP is responsible for the highly potent dopamine reuptake inhibitor action of these agents.3
A wide range of adverse effects
Use of α-PVP results in a state of “excited delirium,” with symptoms such as hyperthermia, hallucinations, paranoia, violent aggression, and self-harm.1 Alpha-PVP is known to cause rhabdomyolysis.4 Some studies have reported cardiovascular effects, such as arterial hypertension, palpitations, dyspnea, vasoconstriction, arrhythmia, myocardial infarction (MI), and myocarditis.5 Alpha-PVP also may result in neurologic symptoms, including headache, mydriasis, lightheadedness, paresthesia, seizures, dystonic movements, tremor, amnesia, dysgeusia, cerebral edema, motor automatisms, muscle spasm, nystagmus, parkinsonism, and stroke.5 Death may occur by cardiac arrest, renal damage, or suicide.
Case reports. The effects of α-PVP have been documented in the literature:
- A 17-year-old girl was brought to an emergency department in Florida with acute onset of bizarre behavior, agitation, and altered mental status. It took 6 days and repeated administrations of olanzapine and lorazepam for the patient to become calm, alert, and oriented.2
- ST-elevated MI with several intracardiac thrombi was reported in a 41-year-old woman who used α-PVP.4
- In 2015, 18 deaths related to α-PVP use were reported in South Florida.5
- Deaths related to α-PVP use also have been reported in Japan and Australia.5
Treatment options
There are no treatment guidelines for α-PVP-related psychiatric symptoms. Case reports describe remission of symptoms following aggressive treatment with antipsychotics and benzodiazepines.2 Guidelines for treatment of stimulant-induced behavioral and psychotic symptoms6 may be considered for patients who have used α-PVP.
Reassurance and supportive care are the basic principles of such interventions. A quiet environment and benzodiazepines may provide relief of agitation. Antipsychotics may be helpful if a patient exhibits psychotic symptoms.
Similar drugs may emerge
In 2014, the DEA classified α-PVP as a Schedule I substance. Laws against the import of such substances via the Internet or other means also may help control the spread of this drug. However, chemically similar drugs that may elude drug screens are continually emerging. The lack of evidence-based guidelines on recognizing and managing intoxication, withdrawal, and long-term effects of α-PVP and other “designer drugs” calls for greater research in this emerging area of substance use disorders.
1. National Institute on Drug Abuse. “Flakka” (alpha-PVP). https://www.drugabuse.gov/emerging-trends/flakka-alpha-pvp. Accessed July 26, 2017.
2. Crespi C. Flakka-induced prolonged psychosis. Case Rep Psychiatry. 2016;2016:3460849. doi: 10.1155/2016/3460849.
3. Glennon RA, Young R. Neurobiology of 3,4-methylenedioxypyrovalerone (MDPV) and α-pyrrolidinovalerophenone (α-PVP). Brain Res Bull. 2016;126(pt 1):111-126.
4. Cherry SV, Rodriguez YF. Synthetic stimulant reaching epidemic proportions: flakka-induced ST-elevation myocardial infarction with intracardiac thrombi. J Cardiothorac Vasc Anesth. 2017;31(1):e13-e14.
5. Katselou M, Papoutsis I, Nikolaou P, et al. α-PVP (“flakka”): a new synthetic cathinone invades the drug arena. Forensic Toxicol. 2016;34(1):41-50.
6. Sadock BJ, Sadock VA, Ruiz P. Hallucinogen-related disorders. In: Sadock BJ, Sadock VA, Ruiz P. Kaplan and Sadock’s synopsis of psychiatry: behavioral sciences/clinical psychiatry. 11th ed. Philadelphia, PA: Wolters Kluwer; 2015:648-655.
1. National Institute on Drug Abuse. “Flakka” (alpha-PVP). https://www.drugabuse.gov/emerging-trends/flakka-alpha-pvp. Accessed July 26, 2017.
2. Crespi C. Flakka-induced prolonged psychosis. Case Rep Psychiatry. 2016;2016:3460849. doi: 10.1155/2016/3460849.
3. Glennon RA, Young R. Neurobiology of 3,4-methylenedioxypyrovalerone (MDPV) and α-pyrrolidinovalerophenone (α-PVP). Brain Res Bull. 2016;126(pt 1):111-126.
4. Cherry SV, Rodriguez YF. Synthetic stimulant reaching epidemic proportions: flakka-induced ST-elevation myocardial infarction with intracardiac thrombi. J Cardiothorac Vasc Anesth. 2017;31(1):e13-e14.
5. Katselou M, Papoutsis I, Nikolaou P, et al. α-PVP (“flakka”): a new synthetic cathinone invades the drug arena. Forensic Toxicol. 2016;34(1):41-50.
6. Sadock BJ, Sadock VA, Ruiz P. Hallucinogen-related disorders. In: Sadock BJ, Sadock VA, Ruiz P. Kaplan and Sadock’s synopsis of psychiatry: behavioral sciences/clinical psychiatry. 11th ed. Philadelphia, PA: Wolters Kluwer; 2015:648-655.
Landmark women’s health care remains law of the land
Starting in 2010 with the Patient Protection and Affordable Care Act (ACA), our patients have had insurance that provides maternity care coverage, no-deductible or copay contraceptives, and access to breast cancer screening. They also have been protected from predatory insurance practices—such as preexisting condition exclusions, arbitrary rescission, and annual or lifetime coverage limits—which had previously and regularly been used to deny coverage. These landmark protections apply to all our patients, regardless of where they live, how much they earn, who their employers are, and which insurance plan they use. They have become part of the fabric of our society.
Between 2008 and 2010, members of the American College of Obstetricians and Gynecologists (ACOG) worked hard to define and help enact these provisions, which we considered the women’s piece of the health care reform puzzle. We also worked with a broad community of clinicians to try to make sure reform would benefit them too. That effort did not go as well, and ACOG ultimately did not endorse the ACA.
Early ACA troubles, misguided solutions
Since the ACA was signed into law 7 years ago, insurers have raised premiums and deductibles and narrowed their provider networks—putting needed care out of the reach of many patients. In addition, skyrocketing prescription drug prices have driven health care costs even higher. Against this backdrop, Congress in 2017 started trying to pass bills that would undo the ACA.
ACOG and our medical colleague organizations stepped up. We brought many ideas to House and Senate Republicans and Democrats and sought opportunities to work with them to improve the ACA for our physicians and patients. Unfortunately, the statute was so polarizing that few in Congress wanted to amend or revise it; most wanted it repealed or left as is.
Throughout these proceedings, ACOG remained committed to ensuring that no one with health insurance coverage would lose it and that Congress would not turn back the clock on women’s health. As long as these 2 principles were assured, we would work with anyone on improving health insurance.
Path to a better way
We delivered our message repeatedly. ACOG President Haywood Brown, MD, often accompanied by his American College of Physicians, American Academy of Pediatrics, American Academy of Family Physicians, American Psychiatric Association, and American Osteopathic Association counterparts, attended high-level meetings with Congressional Republicans and Democrats. Dr. Brown also led fly-ins of our members. In addition, ACOG Past President Tom Gellhaus, MD, together with all 600 ObGyns at the 2017 ACOG Congressional Leadership Conference, spoke out.
Somehow, though, the proposed bills kept getting worse—more patients would be losing coverage, and women’s health protections would be stripped away—and Congress was not seeking or including physician input. None at all.
The ACOG teleconference
In response, ACOG set up a member teleconference headed by Dr. Brown, Dr. Gellhaus, Incoming President Lisa Hollier, MD, Past President and ObGyn PAC Chair Mark DeFrancesco, MD, and Executive Vice President and CEO Hal Lawrence, MD. Discussing our concerns, we focused on the Senate’s Better Care Reconciliation Act (BCRA) and its potential impact on maternity care coverage, preexisting condition coverage, Medicaid, Planned Parenthood (PP), and the opioid epidemic.
BCRA
Dr. Brown led off the teleconference with this assessment: “Without a doubt, the BCRA would not result in better care for our patients. This legislation would pull the rug out from under women and families. The nonpartisan Congressional Budget Office estimated that 22 million Americans, more than half of them women, would lose coverage. More than $770 billion would be cut from Medicaid, the program that covers nearly half of all births nationwide as well as primary and preventive care for low-income patients.”
Coverage for maternity care and preexisting conditions
Dr. Gellhaus discussed how the BCRA would gut maternity care coverage and hurt patients with preexisting conditions. Under this bill, states would be able to drop the requirement for such coverage, thereby creating an enormous hole in patient care. He asked an important question: “If your state opted out and allowed private insurers not to offer maternity care or preventive care, what would this mean for your patients?”
His answer: “It would take us back to a time when only 9 states required insurers cover maternity care, and when only 12% of plans included such coverage; a time when patients had to buy expensive riders, sometimes with 12-month waiting periods, if they wanted maternity coverage; a time when expecting families faced thousands of dollars in out-of-pocket costs. Do we want to go back to that time? It is also important to note that roughly half of all pregnancies are unplanned. Pregnancy should not leave patients fearing bankruptcy and unable to afford the full range of prenatal and postnatal care.
“States that opt out of covering preventive care would discontinue no-copay coverage for women’s preventive services, including contraception. Fifty-five million American women currently have this coverage, and as a result the country’s unintended pregnancy rate is at a 30-year low, and its teen pregnancy rate the lowest in recorded history. We cannot go back.”
Medicaid
Dr. Hollier pointed out that the BCRA would cut $772 billion from Medicaid, ending the program as we know it and shifting costs to states. “This section alone would devastate our patients in every state,” she said.
ACOG is a strong supporter of Medicaid expansion, which increased access to primary and preventive care, including contraception, for low-income women who otherwise would not see a physician until they became pregnant. Thirty-two states and the District of Columbia expanded their Medicaid programs, and other states have expressed interest in doing the same.
Medicaid expansion was a major factor in the almost 50% decrease in the rate of uninsured women since 2010. The BCRA would roll back coverage for essential health benefits beginning in 2020 and end federal expansion funding by 2023.
Dr. Hollier continued, “Regular Medicaid would be threatened, too. The Senate bill would limit, for the first time ever, federal funding for Medicaid services per beneficiary. This would jeopardize the ability of the United States to respond to disasters and public health crises and pose a threat to health care coverage and benefits for tens of millions of Americans.”
“Given that nearly half of US births are covered by Medicaid, cutting this program would have a huge impact on our practices and on our patients with high-risk and expensive pregnancies. What happens when a low-income pregnant patient with hypertension, gestational diabetes, or preeclampsia reaches her Medicaid cap? What happens to a patient with an opioid use disorder or a patient who may have been exposed to the Zika virus? In all likelihood, physicians would have to continue providing care, regardless of coverage, or states would have to reduce physician payments to fill the gap in federal funding. I am sure you are as horrified as I am by these scenarios,” said Dr. Hollier.
Planned Parenthood
Dr. DeFrancesco discussed the threat to PP. First, he explained what defunding the organization would mean. “Planned Parenthood does not just receive a check from the government each year. Like other qualified providers, like us, PP health centers receive federal reimbursement for primary and preventive services provided to patients with Medicaid coverage. Fifty-four percent of these centers are located in rural and medically underserved communities.”
The BCRA would exclude PP health centers from the Medicaid program, which means Medicaid patients would be denied primary and preventive care at these centers. Within the first year, up to 1 million women would find their access to care restricted. In addition, about half of all PP centers would have to close, and most would not reopen. Dr. DeFrancesco asked, “How would this move help our patients? It wouldn’t.”
Two examples shed light on the situation. First, when PP was excluded from a Texas program serving low-income patients, the number of women using the most effective birth control methods decreased by 35%, and the number of Medicaid-covered births increased by 27%. Second, when public health funding cuts forced many Indiana clinics to close, rural areas of the state experienced one of the fastest and largest HIV outbreaks ever to occur in the United States.
Dr. DeFrancesco said, “Excluding Planned Parenthood from the Medicaid program interferes with the patient–physician relationship and sets a dangerous precedent of targeting qualified providers for political purposes.”
Opioid epidemic
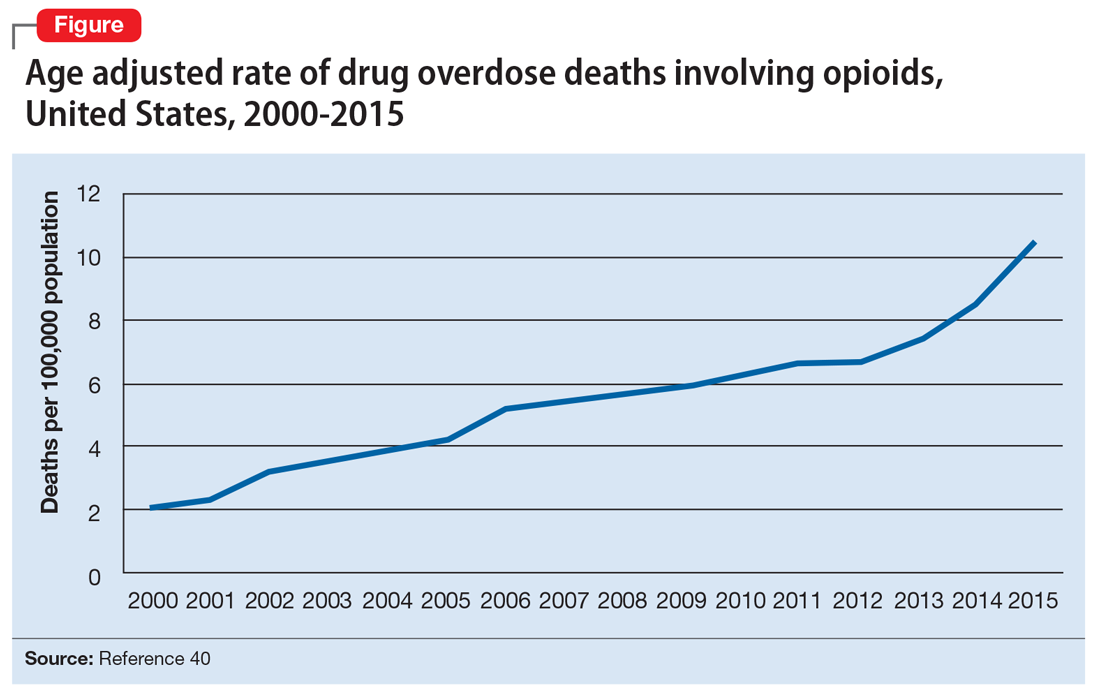
Dr. Brown indicated that the BCRA would cripple attempts to address our very serious national opioid epidemic. The $2 billion the bill would allocate for opioid use disorder treatment for 1 year would replace funding lost by Medicaid and would pay for only a fraction of what is needed. Dr. Brown called this measure a “token, not a commitment, and a big step back in the progress we have made to address this public health crisis.”
The Hippocratic oath
While preparing for the teleconference, I kept thinking about the Hippocratic oath and our deep obligation to our patients. Every physician I know goes beyond the exam room to care for patients. We lose sleep not only when we get up to deliver babies, but when we worry about the ailing mother of four we saw yesterday, or the scared teenager who missed last week’s appointment. We care for our patients because it is the right thing to do, and it is our calling. Well, this year, our patients needed us more than ever. We had to step up, speak out, do everything we could to stop BCRA from passing. The stakes could not have been higher.
The vote, and the road ahead
The morning after Senators Collins, Murkowski, and McCain joined Senate Democrats to end the bill, Dr. Brown wrote the following to ACOG members and the US Congress:
“This was a battle we simply had to win to protect our patients. Thanks to your tireless advocacy, landmark women’s health care protections remain law, and millions of our patients will continue to get the care they need. And our work continues. The ACA is not perfect and needs major reform. ACOG is ready, willing, and able to work with Republicans and Democrats in the US House and Senate to reform the law, through an open and collaborative process. We hope it is clear to everyone in Congress that physicians must be part of the conversation and the solution.”
Starting in 2010 with the Patient Protection and Affordable Care Act (ACA), our patients have had insurance that provides maternity care coverage, no-deductible or copay contraceptives, and access to breast cancer screening. They also have been protected from predatory insurance practices—such as preexisting condition exclusions, arbitrary rescission, and annual or lifetime coverage limits—which had previously and regularly been used to deny coverage. These landmark protections apply to all our patients, regardless of where they live, how much they earn, who their employers are, and which insurance plan they use. They have become part of the fabric of our society.
Between 2008 and 2010, members of the American College of Obstetricians and Gynecologists (ACOG) worked hard to define and help enact these provisions, which we considered the women’s piece of the health care reform puzzle. We also worked with a broad community of clinicians to try to make sure reform would benefit them too. That effort did not go as well, and ACOG ultimately did not endorse the ACA.
Early ACA troubles, misguided solutions
Since the ACA was signed into law 7 years ago, insurers have raised premiums and deductibles and narrowed their provider networks—putting needed care out of the reach of many patients. In addition, skyrocketing prescription drug prices have driven health care costs even higher. Against this backdrop, Congress in 2017 started trying to pass bills that would undo the ACA.
ACOG and our medical colleague organizations stepped up. We brought many ideas to House and Senate Republicans and Democrats and sought opportunities to work with them to improve the ACA for our physicians and patients. Unfortunately, the statute was so polarizing that few in Congress wanted to amend or revise it; most wanted it repealed or left as is.
Throughout these proceedings, ACOG remained committed to ensuring that no one with health insurance coverage would lose it and that Congress would not turn back the clock on women’s health. As long as these 2 principles were assured, we would work with anyone on improving health insurance.
Path to a better way
We delivered our message repeatedly. ACOG President Haywood Brown, MD, often accompanied by his American College of Physicians, American Academy of Pediatrics, American Academy of Family Physicians, American Psychiatric Association, and American Osteopathic Association counterparts, attended high-level meetings with Congressional Republicans and Democrats. Dr. Brown also led fly-ins of our members. In addition, ACOG Past President Tom Gellhaus, MD, together with all 600 ObGyns at the 2017 ACOG Congressional Leadership Conference, spoke out.
Somehow, though, the proposed bills kept getting worse—more patients would be losing coverage, and women’s health protections would be stripped away—and Congress was not seeking or including physician input. None at all.
The ACOG teleconference
In response, ACOG set up a member teleconference headed by Dr. Brown, Dr. Gellhaus, Incoming President Lisa Hollier, MD, Past President and ObGyn PAC Chair Mark DeFrancesco, MD, and Executive Vice President and CEO Hal Lawrence, MD. Discussing our concerns, we focused on the Senate’s Better Care Reconciliation Act (BCRA) and its potential impact on maternity care coverage, preexisting condition coverage, Medicaid, Planned Parenthood (PP), and the opioid epidemic.
BCRA
Dr. Brown led off the teleconference with this assessment: “Without a doubt, the BCRA would not result in better care for our patients. This legislation would pull the rug out from under women and families. The nonpartisan Congressional Budget Office estimated that 22 million Americans, more than half of them women, would lose coverage. More than $770 billion would be cut from Medicaid, the program that covers nearly half of all births nationwide as well as primary and preventive care for low-income patients.”
Coverage for maternity care and preexisting conditions
Dr. Gellhaus discussed how the BCRA would gut maternity care coverage and hurt patients with preexisting conditions. Under this bill, states would be able to drop the requirement for such coverage, thereby creating an enormous hole in patient care. He asked an important question: “If your state opted out and allowed private insurers not to offer maternity care or preventive care, what would this mean for your patients?”
His answer: “It would take us back to a time when only 9 states required insurers cover maternity care, and when only 12% of plans included such coverage; a time when patients had to buy expensive riders, sometimes with 12-month waiting periods, if they wanted maternity coverage; a time when expecting families faced thousands of dollars in out-of-pocket costs. Do we want to go back to that time? It is also important to note that roughly half of all pregnancies are unplanned. Pregnancy should not leave patients fearing bankruptcy and unable to afford the full range of prenatal and postnatal care.
“States that opt out of covering preventive care would discontinue no-copay coverage for women’s preventive services, including contraception. Fifty-five million American women currently have this coverage, and as a result the country’s unintended pregnancy rate is at a 30-year low, and its teen pregnancy rate the lowest in recorded history. We cannot go back.”
Medicaid
Dr. Hollier pointed out that the BCRA would cut $772 billion from Medicaid, ending the program as we know it and shifting costs to states. “This section alone would devastate our patients in every state,” she said.
ACOG is a strong supporter of Medicaid expansion, which increased access to primary and preventive care, including contraception, for low-income women who otherwise would not see a physician until they became pregnant. Thirty-two states and the District of Columbia expanded their Medicaid programs, and other states have expressed interest in doing the same.
Medicaid expansion was a major factor in the almost 50% decrease in the rate of uninsured women since 2010. The BCRA would roll back coverage for essential health benefits beginning in 2020 and end federal expansion funding by 2023.
Dr. Hollier continued, “Regular Medicaid would be threatened, too. The Senate bill would limit, for the first time ever, federal funding for Medicaid services per beneficiary. This would jeopardize the ability of the United States to respond to disasters and public health crises and pose a threat to health care coverage and benefits for tens of millions of Americans.”
“Given that nearly half of US births are covered by Medicaid, cutting this program would have a huge impact on our practices and on our patients with high-risk and expensive pregnancies. What happens when a low-income pregnant patient with hypertension, gestational diabetes, or preeclampsia reaches her Medicaid cap? What happens to a patient with an opioid use disorder or a patient who may have been exposed to the Zika virus? In all likelihood, physicians would have to continue providing care, regardless of coverage, or states would have to reduce physician payments to fill the gap in federal funding. I am sure you are as horrified as I am by these scenarios,” said Dr. Hollier.
Planned Parenthood
Dr. DeFrancesco discussed the threat to PP. First, he explained what defunding the organization would mean. “Planned Parenthood does not just receive a check from the government each year. Like other qualified providers, like us, PP health centers receive federal reimbursement for primary and preventive services provided to patients with Medicaid coverage. Fifty-four percent of these centers are located in rural and medically underserved communities.”
The BCRA would exclude PP health centers from the Medicaid program, which means Medicaid patients would be denied primary and preventive care at these centers. Within the first year, up to 1 million women would find their access to care restricted. In addition, about half of all PP centers would have to close, and most would not reopen. Dr. DeFrancesco asked, “How would this move help our patients? It wouldn’t.”
Two examples shed light on the situation. First, when PP was excluded from a Texas program serving low-income patients, the number of women using the most effective birth control methods decreased by 35%, and the number of Medicaid-covered births increased by 27%. Second, when public health funding cuts forced many Indiana clinics to close, rural areas of the state experienced one of the fastest and largest HIV outbreaks ever to occur in the United States.
Dr. DeFrancesco said, “Excluding Planned Parenthood from the Medicaid program interferes with the patient–physician relationship and sets a dangerous precedent of targeting qualified providers for political purposes.”
Opioid epidemic
Dr. Brown indicated that the BCRA would cripple attempts to address our very serious national opioid epidemic. The $2 billion the bill would allocate for opioid use disorder treatment for 1 year would replace funding lost by Medicaid and would pay for only a fraction of what is needed. Dr. Brown called this measure a “token, not a commitment, and a big step back in the progress we have made to address this public health crisis.”
The Hippocratic oath
While preparing for the teleconference, I kept thinking about the Hippocratic oath and our deep obligation to our patients. Every physician I know goes beyond the exam room to care for patients. We lose sleep not only when we get up to deliver babies, but when we worry about the ailing mother of four we saw yesterday, or the scared teenager who missed last week’s appointment. We care for our patients because it is the right thing to do, and it is our calling. Well, this year, our patients needed us more than ever. We had to step up, speak out, do everything we could to stop BCRA from passing. The stakes could not have been higher.
The vote, and the road ahead
The morning after Senators Collins, Murkowski, and McCain joined Senate Democrats to end the bill, Dr. Brown wrote the following to ACOG members and the US Congress:
“This was a battle we simply had to win to protect our patients. Thanks to your tireless advocacy, landmark women’s health care protections remain law, and millions of our patients will continue to get the care they need. And our work continues. The ACA is not perfect and needs major reform. ACOG is ready, willing, and able to work with Republicans and Democrats in the US House and Senate to reform the law, through an open and collaborative process. We hope it is clear to everyone in Congress that physicians must be part of the conversation and the solution.”
Starting in 2010 with the Patient Protection and Affordable Care Act (ACA), our patients have had insurance that provides maternity care coverage, no-deductible or copay contraceptives, and access to breast cancer screening. They also have been protected from predatory insurance practices—such as preexisting condition exclusions, arbitrary rescission, and annual or lifetime coverage limits—which had previously and regularly been used to deny coverage. These landmark protections apply to all our patients, regardless of where they live, how much they earn, who their employers are, and which insurance plan they use. They have become part of the fabric of our society.
Between 2008 and 2010, members of the American College of Obstetricians and Gynecologists (ACOG) worked hard to define and help enact these provisions, which we considered the women’s piece of the health care reform puzzle. We also worked with a broad community of clinicians to try to make sure reform would benefit them too. That effort did not go as well, and ACOG ultimately did not endorse the ACA.
Early ACA troubles, misguided solutions
Since the ACA was signed into law 7 years ago, insurers have raised premiums and deductibles and narrowed their provider networks—putting needed care out of the reach of many patients. In addition, skyrocketing prescription drug prices have driven health care costs even higher. Against this backdrop, Congress in 2017 started trying to pass bills that would undo the ACA.
ACOG and our medical colleague organizations stepped up. We brought many ideas to House and Senate Republicans and Democrats and sought opportunities to work with them to improve the ACA for our physicians and patients. Unfortunately, the statute was so polarizing that few in Congress wanted to amend or revise it; most wanted it repealed or left as is.
Throughout these proceedings, ACOG remained committed to ensuring that no one with health insurance coverage would lose it and that Congress would not turn back the clock on women’s health. As long as these 2 principles were assured, we would work with anyone on improving health insurance.
Path to a better way
We delivered our message repeatedly. ACOG President Haywood Brown, MD, often accompanied by his American College of Physicians, American Academy of Pediatrics, American Academy of Family Physicians, American Psychiatric Association, and American Osteopathic Association counterparts, attended high-level meetings with Congressional Republicans and Democrats. Dr. Brown also led fly-ins of our members. In addition, ACOG Past President Tom Gellhaus, MD, together with all 600 ObGyns at the 2017 ACOG Congressional Leadership Conference, spoke out.
Somehow, though, the proposed bills kept getting worse—more patients would be losing coverage, and women’s health protections would be stripped away—and Congress was not seeking or including physician input. None at all.
The ACOG teleconference
In response, ACOG set up a member teleconference headed by Dr. Brown, Dr. Gellhaus, Incoming President Lisa Hollier, MD, Past President and ObGyn PAC Chair Mark DeFrancesco, MD, and Executive Vice President and CEO Hal Lawrence, MD. Discussing our concerns, we focused on the Senate’s Better Care Reconciliation Act (BCRA) and its potential impact on maternity care coverage, preexisting condition coverage, Medicaid, Planned Parenthood (PP), and the opioid epidemic.
BCRA
Dr. Brown led off the teleconference with this assessment: “Without a doubt, the BCRA would not result in better care for our patients. This legislation would pull the rug out from under women and families. The nonpartisan Congressional Budget Office estimated that 22 million Americans, more than half of them women, would lose coverage. More than $770 billion would be cut from Medicaid, the program that covers nearly half of all births nationwide as well as primary and preventive care for low-income patients.”
Coverage for maternity care and preexisting conditions
Dr. Gellhaus discussed how the BCRA would gut maternity care coverage and hurt patients with preexisting conditions. Under this bill, states would be able to drop the requirement for such coverage, thereby creating an enormous hole in patient care. He asked an important question: “If your state opted out and allowed private insurers not to offer maternity care or preventive care, what would this mean for your patients?”
His answer: “It would take us back to a time when only 9 states required insurers cover maternity care, and when only 12% of plans included such coverage; a time when patients had to buy expensive riders, sometimes with 12-month waiting periods, if they wanted maternity coverage; a time when expecting families faced thousands of dollars in out-of-pocket costs. Do we want to go back to that time? It is also important to note that roughly half of all pregnancies are unplanned. Pregnancy should not leave patients fearing bankruptcy and unable to afford the full range of prenatal and postnatal care.
“States that opt out of covering preventive care would discontinue no-copay coverage for women’s preventive services, including contraception. Fifty-five million American women currently have this coverage, and as a result the country’s unintended pregnancy rate is at a 30-year low, and its teen pregnancy rate the lowest in recorded history. We cannot go back.”
Medicaid
Dr. Hollier pointed out that the BCRA would cut $772 billion from Medicaid, ending the program as we know it and shifting costs to states. “This section alone would devastate our patients in every state,” she said.
ACOG is a strong supporter of Medicaid expansion, which increased access to primary and preventive care, including contraception, for low-income women who otherwise would not see a physician until they became pregnant. Thirty-two states and the District of Columbia expanded their Medicaid programs, and other states have expressed interest in doing the same.
Medicaid expansion was a major factor in the almost 50% decrease in the rate of uninsured women since 2010. The BCRA would roll back coverage for essential health benefits beginning in 2020 and end federal expansion funding by 2023.
Dr. Hollier continued, “Regular Medicaid would be threatened, too. The Senate bill would limit, for the first time ever, federal funding for Medicaid services per beneficiary. This would jeopardize the ability of the United States to respond to disasters and public health crises and pose a threat to health care coverage and benefits for tens of millions of Americans.”
“Given that nearly half of US births are covered by Medicaid, cutting this program would have a huge impact on our practices and on our patients with high-risk and expensive pregnancies. What happens when a low-income pregnant patient with hypertension, gestational diabetes, or preeclampsia reaches her Medicaid cap? What happens to a patient with an opioid use disorder or a patient who may have been exposed to the Zika virus? In all likelihood, physicians would have to continue providing care, regardless of coverage, or states would have to reduce physician payments to fill the gap in federal funding. I am sure you are as horrified as I am by these scenarios,” said Dr. Hollier.
Planned Parenthood
Dr. DeFrancesco discussed the threat to PP. First, he explained what defunding the organization would mean. “Planned Parenthood does not just receive a check from the government each year. Like other qualified providers, like us, PP health centers receive federal reimbursement for primary and preventive services provided to patients with Medicaid coverage. Fifty-four percent of these centers are located in rural and medically underserved communities.”
The BCRA would exclude PP health centers from the Medicaid program, which means Medicaid patients would be denied primary and preventive care at these centers. Within the first year, up to 1 million women would find their access to care restricted. In addition, about half of all PP centers would have to close, and most would not reopen. Dr. DeFrancesco asked, “How would this move help our patients? It wouldn’t.”
Two examples shed light on the situation. First, when PP was excluded from a Texas program serving low-income patients, the number of women using the most effective birth control methods decreased by 35%, and the number of Medicaid-covered births increased by 27%. Second, when public health funding cuts forced many Indiana clinics to close, rural areas of the state experienced one of the fastest and largest HIV outbreaks ever to occur in the United States.
Dr. DeFrancesco said, “Excluding Planned Parenthood from the Medicaid program interferes with the patient–physician relationship and sets a dangerous precedent of targeting qualified providers for political purposes.”
Opioid epidemic
Dr. Brown indicated that the BCRA would cripple attempts to address our very serious national opioid epidemic. The $2 billion the bill would allocate for opioid use disorder treatment for 1 year would replace funding lost by Medicaid and would pay for only a fraction of what is needed. Dr. Brown called this measure a “token, not a commitment, and a big step back in the progress we have made to address this public health crisis.”
The Hippocratic oath
While preparing for the teleconference, I kept thinking about the Hippocratic oath and our deep obligation to our patients. Every physician I know goes beyond the exam room to care for patients. We lose sleep not only when we get up to deliver babies, but when we worry about the ailing mother of four we saw yesterday, or the scared teenager who missed last week’s appointment. We care for our patients because it is the right thing to do, and it is our calling. Well, this year, our patients needed us more than ever. We had to step up, speak out, do everything we could to stop BCRA from passing. The stakes could not have been higher.
The vote, and the road ahead
The morning after Senators Collins, Murkowski, and McCain joined Senate Democrats to end the bill, Dr. Brown wrote the following to ACOG members and the US Congress:
“This was a battle we simply had to win to protect our patients. Thanks to your tireless advocacy, landmark women’s health care protections remain law, and millions of our patients will continue to get the care they need. And our work continues. The ACA is not perfect and needs major reform. ACOG is ready, willing, and able to work with Republicans and Democrats in the US House and Senate to reform the law, through an open and collaborative process. We hope it is clear to everyone in Congress that physicians must be part of the conversation and the solution.”
Concussion: Evaluation and management
Concussion, also known as mild traumatic brain injury, affects more than 600 adults per 100,000 each year and is commonly treated by nonneurologists.1 Public attention to concussion has been increasing, particularly to concussion sustained during sports. Coincident with this increased attention, the diagnosis of concussion continues to increase in the outpatient setting. Thus, a review of the topic is timely.
ACCELERATION OF THE BRAIN DUE TO TRAUMA
The definition of concussion has changed considerably over the years. It is currently defined as a pathophysiologic process that results from an acceleration or deceleration of the brain induced by trauma.2 It is largely a temporary, functional problem, as opposed to a gross structural injury.2–5
The acceleration of the brain that results in a concussion is usually initiated by a direct blow to the head, although direct impact is not required.6 As the brain rotates, different areas accelerate at different rates, resulting in a shear strain imparted to the parenchyma.
This shear strain causes deformation of axonal membranes and opening of membrane-associated sodium-potassium channels. This in turn leads to release of excitatory neurotransmitters, ultimately culminating in a wave of neuronal depolarization and a spreading depression-like phenomenon that may mediate the loss of consciousness, posttraumatic amnesia, confusion, and many of the other immediate signs and symptoms associated with concussion.
The sudden metabolic demand created by the massive excitatory phenomena triggers an increased utilization of glucose to restore cellular homeostasis. At the same time, cerebral blood flow decreases after concussion, which, in the setting of increased glucose demand, leads to an “energy crisis”: an increased need for adenosine triphosphate with a concomitant decreased delivery of glucose.7 This mismatch between energy demand and supply is thought to underlie the most common signs and symptoms of concussion.
ASSESSMENT
History
The history of present illness is essential to a diagnosis of concussion. In the classic scenario, an otherwise asymptomatic person sustains some trauma to the head that is followed immediately by the signs and symptoms of concussion.
Many of these signs and symptoms are nonspecific and may occur without concussion or other trauma.8,9 Thus, the diagnosis of concussion cannot be made on the basis of symptoms alone, but only in the overall context of history, physical examination, and, at times, additional clinical assessments.
The symptoms of concussion should gradually improve. While they may be exacerbated by certain activities or stimuli, the overall trend should be one of symptom improvement. If symptoms are worsening over time, alternative explanations for the patient’s symptoms should be considered.
Physical examination
A thorough neurologic examination should be conducted in all patients with suspected concussion and include the following.
A mental status examination should include assessment of attention, memory, and recall. Orientation is normal except in the most acute examinations.
Cranial nerve examination must include careful assessment of eye-movement control, including smooth pursuit and saccades. However, even in patients with prominent subjective dizziness, considerable experience may be needed to actually demonstrate abnormalities.
Balance testing. Balance demands careful assessment and, especially for young athletes, this testing should be more difficult than the tandem gait and eyes-closed, feet-together tests.
Standard strength, sensory, reflex, and coordination testing is usually normal.
Any focal neurologic findings should prompt consideration of other causes or of a more serious injury and should lead to further evaluation, including brain imaging.
Diagnostic tests
Current clinical brain imaging cannot diagnose a concussion. The purpose of neuroimaging is to assess for other etiologies or injuries, such as hemorrhage or contusion, that may cause similar symptoms but require different management.
Several guidelines are available to assess the need for imaging in the setting of recent trauma, of which 2 are typically used10–12:
The Canadian CT Head Rule10 states that computed tomography (CT) is indicated in any of the following situations:
- The patient fails to reach a Glasgow Coma Scale score of 15—on a scale of 3 (worst) to 15 (best)—within 2 hours
- There is a suspected open skull fracture
- There is any sign of basal skull fracture
- The patient has 2 or more episodes of vomiting
- The patient is 65 or older
- The patient has retrograde amnesia (ie, cannot remember events that occurred before the injury) for 30 minutes or more
- The mechanism of injury was dangerous (eg, a pedestrian was struck by a motor vehicle, or the patient fell from > 3 feet or > 5 stairs).
The New Orleans Criteria11 state that a patient warrants CT of the head if any of the following is present:
- Severe headache
- Vomiting
- Age over 60
- Drug or alcohol intoxication
- Deficit in short-term memory
- Physical evidence of trauma above the clavicles
- Seizure.
Caveats: these imaging guidelines apply to adults; those for pediatric patients differ.12 Also, because they were designed for use in an emergency department, their utility in clinical practice outside the emergency department is unclear.
Electroencephalography is not necessary in the evaluation of concussion unless a seizure disorder is believed to be the cause of the injury.
Concussion in athletes
Athletes who participate in contact and collision sports are at higher risk of concussion than the nonathletic population. Therefore, specific assessments of symptoms, balance, oculomotor function, cognitive function, and reaction time have been developed for athletes.
Ideally, these measures are taken at preseason baseline, so that they are available for comparison with postinjury assessments after a known or suspected concussion. These assessments can be used to help make the diagnosis of concussion in cases that are unclear and to help monitor recovery. Objective measures of injury are especially useful for athletes who may be reluctant to report symptoms in order to return to play.
Like most medical tests, these assessments need to be properly interpreted in the overall context of the medical history and physical examination by those who know how to administer them. It is important to remember that the natural history of concussion recovery differs between sport-related concussion and concussion that occurs outside of sports.8
MANAGEMENT
The symptoms and signs after concussion are so variable and multidimensional that they make a generally applicable treatment hard to define.
Rest: Physical and cognitive
Treatment depends on the specifics of the injury, but there are common recommendations for the acute days after injury. Lacking hard data, the consensus among experts is that patients should undergo a period of physical and cognitive rest.13,14 Exactly what “rest” means and how long it should last are unknown, leading to a wide variation in its application.
Rest aids recovery but also may have adverse effects: fatigue, diurnal sleep disruption, reactive depression, anxiety, and physiologic deconditioning.15,16 Many guidelines recommend physical and cognitive rest until symptoms resolve,14 but this is likely too cautious. Even without a concussion, inactivity is associated with many of the nonspecific symptoms also associated with concussion. As recovery progresses, the somatic symptoms of concussion improve, while emotional symptoms worsen, likely in part due to prolonged rest.17
We recommend a period of rest lasting 3 to 5 days after injury, followed by a gradual resumption of both physical and cognitive activities as tolerated, remaining below the level at which symptoms are exacerbated.
Not surprisingly, many guidelines for returning to physical activity are focused on athletes. Yet the same principles apply to management of concussion in the general population who exercise: light physical activity (typically walking or stationary bicycling), followed by more vigorous aerobic activity, followed by some resistance activities. Mild aerobic exercise (to below the threshold of symptoms) may speed recovery from refractive postconcussion syndrome, even in those who did not exercise before the injury.18
Athletes require specific and strict instructions to avoid increased trauma to the head during the gradual increase of physical activities. The National Collegiate Athletic Association has published an algorithm for a gradual return to sport-specific training that is echoed in recent consensus statements on concussion.19 Once aerobic reconditioning produces no symptoms, then noncontact, sport-specific activities are begun, followed by contact activities. We have patients return to the clinic once they are symptom-free for repeat evaluation before clearing them for high-risk activities (eg, skiing, bicycling) or contact sports (eg, basketball, soccer, football, ice hockey).
Cognitive rest
While physical rest is fairly straightforward, cognitive rest is more challenging. The concept of cognitive rest is hard to define and even harder to enforce. Patients are often told to minimize any activities that require attention or concentration. This often includes, but is not limited to, avoiding reading, texting, playing video games, and using computers.13
In the modern world, full avoidance of these activities is difficult and can be profoundly socially isolating. Further, complete cognitive rest may be associated with symptoms of its own.15,16,20 Still, some reasonable limitation of cognitive activities, at least initially, is likely beneficial.21 For patients engaged in school or academic work, often the daily schedule needs to be adjusted and accommodations made to help them return to a full academic schedule and level of activity. It is reasonable to have patients return gradually to work or school rather than attempt to immediately return to their preinjury level.
With these interventions, most patients have full resolution of their symptoms and return to preinjury levels of performance.
TREATING SOMATIC SYMPTOMS
Posttraumatic headache
Posttraumatic headache is the most common sequela of concussion.22 Surprisingly, it is more common after concussion than after moderate or severe traumatic brain injury.23 A prior history of headache, particularly migraine, is a known risk factor for development of posttraumatic headache.24
Posttraumatic headache is usually further defined by headache type using the International Classification of Headache Disorders criteria (www.ichd-3.org). Migraine or probable migraine is the most common type of posttraumatic headache; tension headache is less common.25
Analgesics such as nonsteroidal anti-inflammatory drugs (NSAIDs) are often used initially by patients to treat posttraumatic headache. One study found that 70% of patients used acetaminophen or an NSAID.26
Treating early with effective therapy is the most important tenet of posttraumatic headache treatment, since 80% of those who self-treat have incomplete relief, and almost all of them are using over-the-counter products.27 Overuse of over-the-counter abortive medications can lead to medication overuse headache, also known as rebound headache, thus complicating the treatment of posttraumatic headache.26
Earlier treatment with a preventive medication can often limit the need for and overuse of over-the-counter analgesics and can minimize the occurrence of subsequent medication overuse headache. However, in pediatric populations, nonpharmacologic interventions such as rest and sleep hygiene are typically used first, then medications after 4 to 6 weeks if this is ineffective.
A number of medications have been studied for prophylactic treatment of posttraumatic headache, including topiramate, amitriptyline, and divalproex sodium,28–30 but there is little compelling evidence for use of one over the other. If posttraumatic headache is migrainous, beta-blockers, calcium-channel blockers, selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibtors, and gabapentin are other prophylactic medication options under the appropriate circumstances.27,31,32 In adults, we have clinically had success with nortriptyline 20 mg or gabapentin 300 mg at night as an initial prophylactic headache medication, increasing as tolerated or until pain is controlled, though there are no high-quality data to guide this decision.
The ideal prophylactic medication depends on headache type, patient tolerance, comorbidities, allergies, and medication sensitivities. Gabapentin, amitriptyline, and nortriptyline can produce sedation, which can help those suffering from sleep disturbance.
If a provider is not comfortable prescribing these medications or doesn’t prescribe them regularly, the patient should be referred to a concussion or headache specialist more familiar with their use.
In some patients, even some athletes, headache may be related to a cervical strain injury—whiplash—that should be treated with an NSAID (or acetaminophen), perhaps with a short course of a muscle relaxant in adults, and with physical therapy.32
Some patients have chronic headache despite oral medications.26 Therefore, alternatives to oral medications and complementary therapies should be considered. Especially for protracted cases requiring more complicated headache management or injectable treatments, patients should be referred to a pain clinic, headache specialist, or concussion specialist.
Dizziness
Dizziness is also common after concussion. But what the patient means by dizziness requires a little probing. Some have paroxysms of vertigo. This typically represents a peripheral vestibular injury, usually benign paroxysmal positional vertigo. The latter can be elicited with a Hallpike maneuver and treated in the office with the Epley maneuver.33
Usually, dizziness is a subjective sense of poor coordination, gait instability, or dysequilibrium. Patients may also complain of associated nausea and motion sensitivity. This may all be secondary to a mechanism in the middle or inner ear or the brain. Patients should be encouraged to begin movement—gradually and safely—to help the vestibular system accommodate, which it will do with gradual stimulation. It usually resolves spontaneously.
Specific treatment is unfortunately limited. There is no established benefit from vestibular suppressants such as meclizine. Vestibular rehabilitation may accelerate improvement and decrease symptoms.33 Referral for a comprehensive balance assessment or to vestibular therapy (a subset of physical therapy) should be considered and is something we typically undertake in our clinic if there is no recovery from dizziness 4 to 6 weeks after the concussion.
Visual symptoms can contribute to dizziness. Convergence spasm or convergence insufficiency (both related to muscle spasm of the eye) can occur after concussion, with some studies estimating that up to 69% of patients have these symptoms.34 This can interfere with visual tracking and contribute to a feeling of dysequilibrium.34 Referral to a concussion specialist or vestibular rehabilitation physical therapist can be helpful in treating this issue if it does not resolve spontaneously.
Orthostasis and lightheadedness also contribute to dizziness and are associated with cerebrovascular autoregulation. Available data suggest that dysregulation of neurovascular coupling, cerebral vasoreactivity, and cerebral autoregulation contribute to some of the chronic symptoms of concussion, including dizziness. A gradual return to exercise may help regulate cerebral blood flow and improve this type of dizziness.35
Sleep disturbance
Sleep disturbance is common after concussion, but the form is variable: insomnia, excessive daytime somnolence, and alteration of the sleep-wake cycle are all seen and may themselves affect recovery.36
Sleep hygiene education should be the first intervention for postconcussive sleep issues. For example, the patient should be encouraged to do the following:
- Minimize “screen time” an hour before going to bed: cell phone, tablet, and computer screens emit a wavelength of light that suppresses endogenous melatonin release37,38
- Go to bed and wake up at the same time each day
- Minimize or avoid caffeine, nicotine, and alcohol
- Avoid naps.39
Melatonin is a safe and effective treatment that could be added.40 In addition, some studies suggest that melatonin may improve recovery from traumatic brain injury.41,42
Mild exercise (to below the threshold of causing or exacerbating symptoms) may also improve sleep quality.
Amitriptyline or nortriptyline may reduce headache frequency and intensity and also help treat insomnia.
Trazodone is recommended by some as a first-line agent,39 but we usually reserve it for protracted insomnia refractory to the above treatments.
Benzodiazepines should be avoided, as they reduce arousal, impair cognition, and exacerbate motor impairments.43
Emotional symptoms
Acute-onset anxiety or depression often occurs after concussion.44,45 There is abundant evidence that emotional effects of injury may be the most significant factor in recovery.46 A preinjury history of anxiety may be a prognostic factor.9 Patients with a history of anxiety or depression are more likely to develop emotional symptoms after a concussion, but emotional problems may develop in any patient after a concussion.47,48
The circumstances under which an injury is sustained may be traumatic (eg, car accident, assault), leading to an acute stress reaction or disorder and, if untreated, may result in a more chronic condition—posttraumatic stress disorder. Moreover, the injury and subsequent symptoms may have repercussions in many aspects of the patient’s life, leading to further psychologic stress (eg, loss of wages or the inability to handle normal work, school, and family responsibilities).
Referral to a therapist trained in skills-based psychotherapy (eg, cognitive-behavioral therapy, exposure-based treatment) is often helpful.
Pharmacologic treatment can be a useful adjunct. Several studies have shown that selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, and tricyclic antidepressants may improve depression after concussion.49 The prescription of antidepressants, however, is best left to providers with experience in treating anxiety and depression.
As with sleep disorders after concussion, benzodiazepines should be avoided, as they can impair cognition.43
Cognitive problems
Cognitive problems are also common after concussion. Patients complain about everyday experiences of forgetfulness, distractibility, loss of concentration, and mental fatigue. Although patients often subjectively perceive these symptoms as quite limiting, the impairments can be difficult to demonstrate in office testing.
A program of gradual increase in mental activity, parallel to recovery of physical capacity, should be undertaken. Most patients make a gradual recovery within a few weeks.50
When cognitive symptoms cause significant school or vocational problems or become persistent, patients should be referred to a specialty clinic. As with most of the consequences of concussion, there are few established treatments. When cognitive difficulties persist, it is important to consider the complications of concussion mentioned above: headache, pain, sleep disturbance, and anxiety, all of which may cause subjective cognitive problems and are treatable.
If cognitive symptoms are prolonged despite improvement of other issues like headache and sleep disturbance, a low-dose stimulant medication such as amphetamine salts or methylphenidate may be useful for symptoms of poor attention.49 They should be only a temporary measure after concussion to carry the patient through a cognitively challenging period, unless there was a history of attention-deficit disorder before the injury. A variety of other agents, including amantadine,51 have been proposed based on limited studies; all are off-label uses. Before considering these types of interventions, referral to a specialist or a specialty program would be appropriate.
IF SYMPTOMS PERSIST
With the interventions suggested above, most patients with concussion have a resolution of symptoms and can return to preinjury levels of performance. But some have prolonged symptoms and sequelae. Approximately 10% of athletes have persistent signs and symptoms of concussion beyond 2 weeks. If concussion is not sport-related, most patients recover completely within the first 3 months, but up to 33% may have symptoms beyond that.52
Four types of patients have persistent symptoms:
Patients who sustained a high-force mechanism of injury. These patients simply need more time and accommodation.
Patients who sustained multiple concussions. These patients may also need more time and accommodation.
Patients with an underlying neurologic condition, recognized prior to injury or not, may have delayed or incomplete recovery. Even aging may be an “underlying condition” in concussion.
Patients whose symptoms from an apparently single mild concussion do not resolve despite appropriate treatments may have identifiable factors, but intractable pain (usually headache) or significant emotional disturbance or both are common. Once established and persistent, this is difficult to treat. Referral to a specialty practice is appropriate, but even in that setting effective treatment may be elusive.
PATIENT EDUCATION
Most important for patient education is reassurance. Ultimately, concussion is a self-limited phenomenon, and reinforcing this is helpful for patients. If concussion is not sport-related, most patients recover completely within 3 months.
The next important tenet in patient education is that they should rest for 3 to 5 days, then resume gradual physical and cognitive activities. If resuming activities too soon results in symptoms, then they should rest for a day and gradually resume activity. If their recovery is prolonged (ie, longer than 6 weeks), they likely need to be referred to a concussion specialist.
- Cassidy JD, Carroll LJ, Peloso PM, et al; WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. Incidence, risk factors and prevention of mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med 2004; (suppl):28–60.
- Shaw NA. The neurophysiology of concussion. Prog Neurobiol 2002; 67:281–344.
- Denny-Brown DE, Russell WR. Experimental concussion: (section of neurology). Proc R Soc Med 1941; 34:691–692.
- Ommaya AK, Gennarelli TA. Cerebral concussion and traumatic unconsciousness. Correlation of experimental and clinical observations of blunt head injuries. Brain 1974; 97:633–654.
- Houlburn AHS, Edin MA. Mechanics of head injuries. Lancet 1943; 242:438–441.
- Gennarelli TA, Adams JH, Graham DI. Acceleration induced head injury in the monkey. I. The model, its mechanical and physiological correlates. Acta Neuropathol Suppl 1981; 7:23–25.
- Giza CC, Hovda DA. The neurometabolic cascade of concussion. J Athl Train 2001; 36:228–235.
- Meehan WP 3rd, Bachur RG. Sport-related concussion. Pediatrics 2009; 123:114–123.
- Iverson GL, Silverberg ND, Mannix R, et al. Factors associated with concussion-like symptom reporting in high school athletes. JAMA Pediatr 2015; 169:1132–1140.
- Stiell IG, Wells GA, Vandemheen K. et al. The Canadian CT head rule for patients with minor head injury. Lancet 2001; 357:1391–1396.
- Haydel MJ, Preston CA, Mills TJ, Luber S, Blaudeau E, DeBlieux PMC. Indications for computed tomography in patients with minor head injury. N Engl J Med 2000; 343:100–105.
- Kuppermann N, Holmes JF, Dayan PS, et al; Pediatric Emergency Care Applied Research Network (PECARN). Identification of children at very low risk of clinically important brain injuries after head trauma: a prospective cohort study. Lancet 2009; 374:1160–1170.
- McCrory P, Meeuwisse W, Johnston K, et al. Consensus Statement on Concussion in Sport: the 3rd International Conference on Concussion in Sport held in Zurich, November 2008. Br J Sports Med 2009; 43(suppl 1):i76–i90.
- DeMatteo C, Stazyk K, Singh SK, et al; Ontario Neurotrauma Foundation. Development of a conservative protocol to return children and youth to activity following concussive injury. Clin Pediatr (Phila) 2015; 54:152–163.
- Willer B, Leddy JJ. Management of concussion and post-concussion syndrome. Curr Treat Options Neurol 2006; 8:415–426.
- DiFazio M, Silverberg ND, Kirkwood MW, Bernier R, Iverson GL. Prolonged activity restriction after concussion: are we worsening outcomes? Clin Pediatr (Phila) 2016; 55:443–451.
- Thomas DG, Apps JN, Hoffmann RG, McCrea M, Hammeke T. Benefits of strict rest after acute concussion: a randomized controlled trial. Pediatrics 2015; 135:213–223.
- Leddy JJ, Kozlowski K, Donnelly JP, Pendergast DR, Epstein LH, Willer B. A preliminary study of subsymptom threshold exercise training for refractory post-concussion syndrome. Clin J Sport Med 2010; 20:21–27.
- McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med 2013; 47:250–258.
- Buckley TA, Munkasy BA, Clouse BP. Acute cognitive and physical rest may not improve concussion recovery time. J Head Trauma Rehabil 2016; 31:233–241.
- Brown NJ, Mannix RC, O'Brien MJ, Gostine D, Collins MW, Meehan WP 3rd. Effect of cognitive activity level on duration of post-concussion symptoms. Pediatrics 2014; 133:e299–e304.
- Packard RC. Epidemiology and pathogenesis of posttraumatic headache. J Head Trauma Rehabil 1999; 14:9–21.
- Couch JR, Bearss C. Chronic daily headache in the posttrauma syndrome: relation to extent of head injury. Headache 2001; 41:559–564.
- Lucas S, Hoffman JM, Bell KR, Dikmen S. A prospective study of prevalence and characterization of headache following mild traumatic brain injury. Cephalalgia 2014; 34:93–102.
- Lucas S, Hoffman JM, Bell KR, Walker W, Dikmen S. Characterization of headache after traumatic brain injury. Cephalalgia 2012; 32:600–606.
- DiTommaso C, Hoffman JM, Lucas S, Dikmen S, Temkin N, Bell KR. Medication usage patterns for headache treatment after mild traumatic brain injury. Headache 2014; 54:511–519.
- Lucas S. Characterization and management of headache after mild traumatic brain injury. In: Kobeissy FH, ed. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton, FL: CRC Press/Taylor & Franis Group; 2015:145–154.
- Erickson JC. Treatment outcomes of chronic post-traumatic headaches after mild head trauma in US soldiers: an observational study. Headache 2011; 51:932–944.
- Tyler GS, McNeely HE, Dick ML. Treatment of post-traumatic headache with amitriptyline. Headache 1980; 20:213–216.
- Packard RC. Treatment of chronic daily posttraumatic headache with divalproex sodium. Headache 2000; 40:736–739.
- Kacperski J, Arthur T. Management of post-traumatic headaches in children and adolescents. Headache 2016; 56:36–48.
- Lenaerts ME, Couch JR, Couch JR. Posttraumatic headache. Curr Treat Options Neurol 2004; 6:507–517.
- Valovich McLeod TC, Hale TD. Vestibular and balance issues following sport-related concussion. Brain Inj 2015; 29:175–184.
- Master CL, Cheiman M, Gallaway M, et al. Vision diagnoses are common after concussion in adolescents. Clin Pediatr (Phila) 2016; 55:260–267.
- Tan CO, Meehan WP 3rd, Iverson GL, Taylor JA. Cerebrovascular regulation, exercise and mild traumatic brain injury. Neurology 2014; 83:1665–1672.
- Mahmood O, Rapport LJ, Hanks RA, Fichtenberg NL. Neuropsychological performance and sleep disturbance following traumatic brain injury. J Head Trauma Rehabil 2004; 19:378–390.
- Lewy AJ, Wehr TA, Goodwin FK, Newsome DA, Markey SP. Light suppresses melatonin secretion in humans. Science 1980; 210:1267–1269.
- Figueiro MG, Wood B, Plitnick B, Rea MS. The impact of light from computer monitors on melatonin levels in college students. Neuro Endocrinol Lett 2011; 32:158–163.
- Rao V, Rollings P. Sleep disturbances following traumatic brain injury. Curr Treat Options Neurol 2002; 4:77–87.
- Samantaray S, Das A, Thakore NP, et al. Therapeutic potential of melatonin in traumatic central nervous system injury. J Pineal Res 2009; 47:134–142.
- Ding K, Xu J, Wang H, Zhang L, Wu Y, Li T. Melatonin protects the brain from apoptosis by enhancement of autophagy after traumatic brain injury in mice. Neurochem Int 2015; 91:46–54.
- Babaee A, Eftekhar-Vaghefi SH, Asadi-Shekaari M, et al. Melatonin treatment reduces astrogliosis and apoptosis in rats with traumatic brain injury. Iran J Basic Med Sci 2015; 18:867–872.
- Arciniegas DB, Anderson CA, Topkoff J, McAllister TW. Mild traumatic brain injury: a neuropsychiatric approach to diagnosis, evaluation, and treatment. Neuropsychiatr Dis Treat 2005; 1:311–327.
- O’Donnell ML, Creamer M, Pattison P, Atkin C. Psychiatric morbidity following injury. Am J Psychiatry 2004; 161:507–514.
- Dikmen SS, Bombardier CH, Machamer JE, Fann JR, Temkin NR. Natural history of depression in traumatic brain injury. Arch Phys Med Rehabil 2004; 85:1457–1464.
- Massey JS, Meares S, Batchelor J, Bryant RA. An exploratory study of the association of acute posttraumatic stress, depression, and pain to cognitive functioning in mild traumatic brain injury. Neuropsychology 2015; 29:530–542.
- Meares S, Shores EA, Taylor AJ, et al. The prospective course of postconcussion syndrome: the role of mild traumatic brain injury. Neuropsychology 2011; 25:454–465.
- Solomon GS, Kuhn AW, Zuckerman SL. Depression as a modifying factor in sport-related concussion: a critical review of the literature. Phys Sportsmed 2016; 44:14–19.
- Neurobehavioral Guidelines Working Group; Warden DL, Gordon B, McAllister TW, et al. Guidelines for the pharmacologic treatment of neurobehavioral sequelae of traumatic brain injury. J Neurotrauma 2006; 23:1468–1501.
- Dikmen S, McLean A, Temkin N. Neuropsychological and psychosocial consequences of minor head injury. J Neurol Neurosurg Psychiatry 1986; 49:1227–1232.
- Reddy CC, Collins M, Lovell M, Kontos AP. Efficacy of amantadine treatment on symptoms and neurocognitive performance among adolescents following sports-related concussion. J Head Trauma Rehabil 2013; 28:260–265.
- Leddy JJ, Sandhu H, Sodhi V, Baker JG, Willer B. Rehabilitation of concussion and post-concussion syndrome. Sports Health 2012; 4:147–154.
Concussion, also known as mild traumatic brain injury, affects more than 600 adults per 100,000 each year and is commonly treated by nonneurologists.1 Public attention to concussion has been increasing, particularly to concussion sustained during sports. Coincident with this increased attention, the diagnosis of concussion continues to increase in the outpatient setting. Thus, a review of the topic is timely.
ACCELERATION OF THE BRAIN DUE TO TRAUMA
The definition of concussion has changed considerably over the years. It is currently defined as a pathophysiologic process that results from an acceleration or deceleration of the brain induced by trauma.2 It is largely a temporary, functional problem, as opposed to a gross structural injury.2–5
The acceleration of the brain that results in a concussion is usually initiated by a direct blow to the head, although direct impact is not required.6 As the brain rotates, different areas accelerate at different rates, resulting in a shear strain imparted to the parenchyma.
This shear strain causes deformation of axonal membranes and opening of membrane-associated sodium-potassium channels. This in turn leads to release of excitatory neurotransmitters, ultimately culminating in a wave of neuronal depolarization and a spreading depression-like phenomenon that may mediate the loss of consciousness, posttraumatic amnesia, confusion, and many of the other immediate signs and symptoms associated with concussion.
The sudden metabolic demand created by the massive excitatory phenomena triggers an increased utilization of glucose to restore cellular homeostasis. At the same time, cerebral blood flow decreases after concussion, which, in the setting of increased glucose demand, leads to an “energy crisis”: an increased need for adenosine triphosphate with a concomitant decreased delivery of glucose.7 This mismatch between energy demand and supply is thought to underlie the most common signs and symptoms of concussion.
ASSESSMENT
History
The history of present illness is essential to a diagnosis of concussion. In the classic scenario, an otherwise asymptomatic person sustains some trauma to the head that is followed immediately by the signs and symptoms of concussion.
Many of these signs and symptoms are nonspecific and may occur without concussion or other trauma.8,9 Thus, the diagnosis of concussion cannot be made on the basis of symptoms alone, but only in the overall context of history, physical examination, and, at times, additional clinical assessments.
The symptoms of concussion should gradually improve. While they may be exacerbated by certain activities or stimuli, the overall trend should be one of symptom improvement. If symptoms are worsening over time, alternative explanations for the patient’s symptoms should be considered.
Physical examination
A thorough neurologic examination should be conducted in all patients with suspected concussion and include the following.
A mental status examination should include assessment of attention, memory, and recall. Orientation is normal except in the most acute examinations.
Cranial nerve examination must include careful assessment of eye-movement control, including smooth pursuit and saccades. However, even in patients with prominent subjective dizziness, considerable experience may be needed to actually demonstrate abnormalities.
Balance testing. Balance demands careful assessment and, especially for young athletes, this testing should be more difficult than the tandem gait and eyes-closed, feet-together tests.
Standard strength, sensory, reflex, and coordination testing is usually normal.
Any focal neurologic findings should prompt consideration of other causes or of a more serious injury and should lead to further evaluation, including brain imaging.
Diagnostic tests
Current clinical brain imaging cannot diagnose a concussion. The purpose of neuroimaging is to assess for other etiologies or injuries, such as hemorrhage or contusion, that may cause similar symptoms but require different management.
Several guidelines are available to assess the need for imaging in the setting of recent trauma, of which 2 are typically used10–12:
The Canadian CT Head Rule10 states that computed tomography (CT) is indicated in any of the following situations:
- The patient fails to reach a Glasgow Coma Scale score of 15—on a scale of 3 (worst) to 15 (best)—within 2 hours
- There is a suspected open skull fracture
- There is any sign of basal skull fracture
- The patient has 2 or more episodes of vomiting
- The patient is 65 or older
- The patient has retrograde amnesia (ie, cannot remember events that occurred before the injury) for 30 minutes or more
- The mechanism of injury was dangerous (eg, a pedestrian was struck by a motor vehicle, or the patient fell from > 3 feet or > 5 stairs).
The New Orleans Criteria11 state that a patient warrants CT of the head if any of the following is present:
- Severe headache
- Vomiting
- Age over 60
- Drug or alcohol intoxication
- Deficit in short-term memory
- Physical evidence of trauma above the clavicles
- Seizure.
Caveats: these imaging guidelines apply to adults; those for pediatric patients differ.12 Also, because they were designed for use in an emergency department, their utility in clinical practice outside the emergency department is unclear.
Electroencephalography is not necessary in the evaluation of concussion unless a seizure disorder is believed to be the cause of the injury.
Concussion in athletes
Athletes who participate in contact and collision sports are at higher risk of concussion than the nonathletic population. Therefore, specific assessments of symptoms, balance, oculomotor function, cognitive function, and reaction time have been developed for athletes.
Ideally, these measures are taken at preseason baseline, so that they are available for comparison with postinjury assessments after a known or suspected concussion. These assessments can be used to help make the diagnosis of concussion in cases that are unclear and to help monitor recovery. Objective measures of injury are especially useful for athletes who may be reluctant to report symptoms in order to return to play.
Like most medical tests, these assessments need to be properly interpreted in the overall context of the medical history and physical examination by those who know how to administer them. It is important to remember that the natural history of concussion recovery differs between sport-related concussion and concussion that occurs outside of sports.8
MANAGEMENT
The symptoms and signs after concussion are so variable and multidimensional that they make a generally applicable treatment hard to define.
Rest: Physical and cognitive
Treatment depends on the specifics of the injury, but there are common recommendations for the acute days after injury. Lacking hard data, the consensus among experts is that patients should undergo a period of physical and cognitive rest.13,14 Exactly what “rest” means and how long it should last are unknown, leading to a wide variation in its application.
Rest aids recovery but also may have adverse effects: fatigue, diurnal sleep disruption, reactive depression, anxiety, and physiologic deconditioning.15,16 Many guidelines recommend physical and cognitive rest until symptoms resolve,14 but this is likely too cautious. Even without a concussion, inactivity is associated with many of the nonspecific symptoms also associated with concussion. As recovery progresses, the somatic symptoms of concussion improve, while emotional symptoms worsen, likely in part due to prolonged rest.17
We recommend a period of rest lasting 3 to 5 days after injury, followed by a gradual resumption of both physical and cognitive activities as tolerated, remaining below the level at which symptoms are exacerbated.
Not surprisingly, many guidelines for returning to physical activity are focused on athletes. Yet the same principles apply to management of concussion in the general population who exercise: light physical activity (typically walking or stationary bicycling), followed by more vigorous aerobic activity, followed by some resistance activities. Mild aerobic exercise (to below the threshold of symptoms) may speed recovery from refractive postconcussion syndrome, even in those who did not exercise before the injury.18
Athletes require specific and strict instructions to avoid increased trauma to the head during the gradual increase of physical activities. The National Collegiate Athletic Association has published an algorithm for a gradual return to sport-specific training that is echoed in recent consensus statements on concussion.19 Once aerobic reconditioning produces no symptoms, then noncontact, sport-specific activities are begun, followed by contact activities. We have patients return to the clinic once they are symptom-free for repeat evaluation before clearing them for high-risk activities (eg, skiing, bicycling) or contact sports (eg, basketball, soccer, football, ice hockey).
Cognitive rest
While physical rest is fairly straightforward, cognitive rest is more challenging. The concept of cognitive rest is hard to define and even harder to enforce. Patients are often told to minimize any activities that require attention or concentration. This often includes, but is not limited to, avoiding reading, texting, playing video games, and using computers.13
In the modern world, full avoidance of these activities is difficult and can be profoundly socially isolating. Further, complete cognitive rest may be associated with symptoms of its own.15,16,20 Still, some reasonable limitation of cognitive activities, at least initially, is likely beneficial.21 For patients engaged in school or academic work, often the daily schedule needs to be adjusted and accommodations made to help them return to a full academic schedule and level of activity. It is reasonable to have patients return gradually to work or school rather than attempt to immediately return to their preinjury level.
With these interventions, most patients have full resolution of their symptoms and return to preinjury levels of performance.
TREATING SOMATIC SYMPTOMS
Posttraumatic headache
Posttraumatic headache is the most common sequela of concussion.22 Surprisingly, it is more common after concussion than after moderate or severe traumatic brain injury.23 A prior history of headache, particularly migraine, is a known risk factor for development of posttraumatic headache.24
Posttraumatic headache is usually further defined by headache type using the International Classification of Headache Disorders criteria (www.ichd-3.org). Migraine or probable migraine is the most common type of posttraumatic headache; tension headache is less common.25
Analgesics such as nonsteroidal anti-inflammatory drugs (NSAIDs) are often used initially by patients to treat posttraumatic headache. One study found that 70% of patients used acetaminophen or an NSAID.26
Treating early with effective therapy is the most important tenet of posttraumatic headache treatment, since 80% of those who self-treat have incomplete relief, and almost all of them are using over-the-counter products.27 Overuse of over-the-counter abortive medications can lead to medication overuse headache, also known as rebound headache, thus complicating the treatment of posttraumatic headache.26
Earlier treatment with a preventive medication can often limit the need for and overuse of over-the-counter analgesics and can minimize the occurrence of subsequent medication overuse headache. However, in pediatric populations, nonpharmacologic interventions such as rest and sleep hygiene are typically used first, then medications after 4 to 6 weeks if this is ineffective.
A number of medications have been studied for prophylactic treatment of posttraumatic headache, including topiramate, amitriptyline, and divalproex sodium,28–30 but there is little compelling evidence for use of one over the other. If posttraumatic headache is migrainous, beta-blockers, calcium-channel blockers, selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibtors, and gabapentin are other prophylactic medication options under the appropriate circumstances.27,31,32 In adults, we have clinically had success with nortriptyline 20 mg or gabapentin 300 mg at night as an initial prophylactic headache medication, increasing as tolerated or until pain is controlled, though there are no high-quality data to guide this decision.
The ideal prophylactic medication depends on headache type, patient tolerance, comorbidities, allergies, and medication sensitivities. Gabapentin, amitriptyline, and nortriptyline can produce sedation, which can help those suffering from sleep disturbance.
If a provider is not comfortable prescribing these medications or doesn’t prescribe them regularly, the patient should be referred to a concussion or headache specialist more familiar with their use.
In some patients, even some athletes, headache may be related to a cervical strain injury—whiplash—that should be treated with an NSAID (or acetaminophen), perhaps with a short course of a muscle relaxant in adults, and with physical therapy.32
Some patients have chronic headache despite oral medications.26 Therefore, alternatives to oral medications and complementary therapies should be considered. Especially for protracted cases requiring more complicated headache management or injectable treatments, patients should be referred to a pain clinic, headache specialist, or concussion specialist.
Dizziness
Dizziness is also common after concussion. But what the patient means by dizziness requires a little probing. Some have paroxysms of vertigo. This typically represents a peripheral vestibular injury, usually benign paroxysmal positional vertigo. The latter can be elicited with a Hallpike maneuver and treated in the office with the Epley maneuver.33
Usually, dizziness is a subjective sense of poor coordination, gait instability, or dysequilibrium. Patients may also complain of associated nausea and motion sensitivity. This may all be secondary to a mechanism in the middle or inner ear or the brain. Patients should be encouraged to begin movement—gradually and safely—to help the vestibular system accommodate, which it will do with gradual stimulation. It usually resolves spontaneously.
Specific treatment is unfortunately limited. There is no established benefit from vestibular suppressants such as meclizine. Vestibular rehabilitation may accelerate improvement and decrease symptoms.33 Referral for a comprehensive balance assessment or to vestibular therapy (a subset of physical therapy) should be considered and is something we typically undertake in our clinic if there is no recovery from dizziness 4 to 6 weeks after the concussion.
Visual symptoms can contribute to dizziness. Convergence spasm or convergence insufficiency (both related to muscle spasm of the eye) can occur after concussion, with some studies estimating that up to 69% of patients have these symptoms.34 This can interfere with visual tracking and contribute to a feeling of dysequilibrium.34 Referral to a concussion specialist or vestibular rehabilitation physical therapist can be helpful in treating this issue if it does not resolve spontaneously.
Orthostasis and lightheadedness also contribute to dizziness and are associated with cerebrovascular autoregulation. Available data suggest that dysregulation of neurovascular coupling, cerebral vasoreactivity, and cerebral autoregulation contribute to some of the chronic symptoms of concussion, including dizziness. A gradual return to exercise may help regulate cerebral blood flow and improve this type of dizziness.35
Sleep disturbance
Sleep disturbance is common after concussion, but the form is variable: insomnia, excessive daytime somnolence, and alteration of the sleep-wake cycle are all seen and may themselves affect recovery.36
Sleep hygiene education should be the first intervention for postconcussive sleep issues. For example, the patient should be encouraged to do the following:
- Minimize “screen time” an hour before going to bed: cell phone, tablet, and computer screens emit a wavelength of light that suppresses endogenous melatonin release37,38
- Go to bed and wake up at the same time each day
- Minimize or avoid caffeine, nicotine, and alcohol
- Avoid naps.39
Melatonin is a safe and effective treatment that could be added.40 In addition, some studies suggest that melatonin may improve recovery from traumatic brain injury.41,42
Mild exercise (to below the threshold of causing or exacerbating symptoms) may also improve sleep quality.
Amitriptyline or nortriptyline may reduce headache frequency and intensity and also help treat insomnia.
Trazodone is recommended by some as a first-line agent,39 but we usually reserve it for protracted insomnia refractory to the above treatments.
Benzodiazepines should be avoided, as they reduce arousal, impair cognition, and exacerbate motor impairments.43
Emotional symptoms
Acute-onset anxiety or depression often occurs after concussion.44,45 There is abundant evidence that emotional effects of injury may be the most significant factor in recovery.46 A preinjury history of anxiety may be a prognostic factor.9 Patients with a history of anxiety or depression are more likely to develop emotional symptoms after a concussion, but emotional problems may develop in any patient after a concussion.47,48
The circumstances under which an injury is sustained may be traumatic (eg, car accident, assault), leading to an acute stress reaction or disorder and, if untreated, may result in a more chronic condition—posttraumatic stress disorder. Moreover, the injury and subsequent symptoms may have repercussions in many aspects of the patient’s life, leading to further psychologic stress (eg, loss of wages or the inability to handle normal work, school, and family responsibilities).
Referral to a therapist trained in skills-based psychotherapy (eg, cognitive-behavioral therapy, exposure-based treatment) is often helpful.
Pharmacologic treatment can be a useful adjunct. Several studies have shown that selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, and tricyclic antidepressants may improve depression after concussion.49 The prescription of antidepressants, however, is best left to providers with experience in treating anxiety and depression.
As with sleep disorders after concussion, benzodiazepines should be avoided, as they can impair cognition.43
Cognitive problems
Cognitive problems are also common after concussion. Patients complain about everyday experiences of forgetfulness, distractibility, loss of concentration, and mental fatigue. Although patients often subjectively perceive these symptoms as quite limiting, the impairments can be difficult to demonstrate in office testing.
A program of gradual increase in mental activity, parallel to recovery of physical capacity, should be undertaken. Most patients make a gradual recovery within a few weeks.50
When cognitive symptoms cause significant school or vocational problems or become persistent, patients should be referred to a specialty clinic. As with most of the consequences of concussion, there are few established treatments. When cognitive difficulties persist, it is important to consider the complications of concussion mentioned above: headache, pain, sleep disturbance, and anxiety, all of which may cause subjective cognitive problems and are treatable.
If cognitive symptoms are prolonged despite improvement of other issues like headache and sleep disturbance, a low-dose stimulant medication such as amphetamine salts or methylphenidate may be useful for symptoms of poor attention.49 They should be only a temporary measure after concussion to carry the patient through a cognitively challenging period, unless there was a history of attention-deficit disorder before the injury. A variety of other agents, including amantadine,51 have been proposed based on limited studies; all are off-label uses. Before considering these types of interventions, referral to a specialist or a specialty program would be appropriate.
IF SYMPTOMS PERSIST
With the interventions suggested above, most patients with concussion have a resolution of symptoms and can return to preinjury levels of performance. But some have prolonged symptoms and sequelae. Approximately 10% of athletes have persistent signs and symptoms of concussion beyond 2 weeks. If concussion is not sport-related, most patients recover completely within the first 3 months, but up to 33% may have symptoms beyond that.52
Four types of patients have persistent symptoms:
Patients who sustained a high-force mechanism of injury. These patients simply need more time and accommodation.
Patients who sustained multiple concussions. These patients may also need more time and accommodation.
Patients with an underlying neurologic condition, recognized prior to injury or not, may have delayed or incomplete recovery. Even aging may be an “underlying condition” in concussion.
Patients whose symptoms from an apparently single mild concussion do not resolve despite appropriate treatments may have identifiable factors, but intractable pain (usually headache) or significant emotional disturbance or both are common. Once established and persistent, this is difficult to treat. Referral to a specialty practice is appropriate, but even in that setting effective treatment may be elusive.
PATIENT EDUCATION
Most important for patient education is reassurance. Ultimately, concussion is a self-limited phenomenon, and reinforcing this is helpful for patients. If concussion is not sport-related, most patients recover completely within 3 months.
The next important tenet in patient education is that they should rest for 3 to 5 days, then resume gradual physical and cognitive activities. If resuming activities too soon results in symptoms, then they should rest for a day and gradually resume activity. If their recovery is prolonged (ie, longer than 6 weeks), they likely need to be referred to a concussion specialist.
Concussion, also known as mild traumatic brain injury, affects more than 600 adults per 100,000 each year and is commonly treated by nonneurologists.1 Public attention to concussion has been increasing, particularly to concussion sustained during sports. Coincident with this increased attention, the diagnosis of concussion continues to increase in the outpatient setting. Thus, a review of the topic is timely.
ACCELERATION OF THE BRAIN DUE TO TRAUMA
The definition of concussion has changed considerably over the years. It is currently defined as a pathophysiologic process that results from an acceleration or deceleration of the brain induced by trauma.2 It is largely a temporary, functional problem, as opposed to a gross structural injury.2–5
The acceleration of the brain that results in a concussion is usually initiated by a direct blow to the head, although direct impact is not required.6 As the brain rotates, different areas accelerate at different rates, resulting in a shear strain imparted to the parenchyma.
This shear strain causes deformation of axonal membranes and opening of membrane-associated sodium-potassium channels. This in turn leads to release of excitatory neurotransmitters, ultimately culminating in a wave of neuronal depolarization and a spreading depression-like phenomenon that may mediate the loss of consciousness, posttraumatic amnesia, confusion, and many of the other immediate signs and symptoms associated with concussion.
The sudden metabolic demand created by the massive excitatory phenomena triggers an increased utilization of glucose to restore cellular homeostasis. At the same time, cerebral blood flow decreases after concussion, which, in the setting of increased glucose demand, leads to an “energy crisis”: an increased need for adenosine triphosphate with a concomitant decreased delivery of glucose.7 This mismatch between energy demand and supply is thought to underlie the most common signs and symptoms of concussion.
ASSESSMENT
History
The history of present illness is essential to a diagnosis of concussion. In the classic scenario, an otherwise asymptomatic person sustains some trauma to the head that is followed immediately by the signs and symptoms of concussion.
Many of these signs and symptoms are nonspecific and may occur without concussion or other trauma.8,9 Thus, the diagnosis of concussion cannot be made on the basis of symptoms alone, but only in the overall context of history, physical examination, and, at times, additional clinical assessments.
The symptoms of concussion should gradually improve. While they may be exacerbated by certain activities or stimuli, the overall trend should be one of symptom improvement. If symptoms are worsening over time, alternative explanations for the patient’s symptoms should be considered.
Physical examination
A thorough neurologic examination should be conducted in all patients with suspected concussion and include the following.
A mental status examination should include assessment of attention, memory, and recall. Orientation is normal except in the most acute examinations.
Cranial nerve examination must include careful assessment of eye-movement control, including smooth pursuit and saccades. However, even in patients with prominent subjective dizziness, considerable experience may be needed to actually demonstrate abnormalities.
Balance testing. Balance demands careful assessment and, especially for young athletes, this testing should be more difficult than the tandem gait and eyes-closed, feet-together tests.
Standard strength, sensory, reflex, and coordination testing is usually normal.
Any focal neurologic findings should prompt consideration of other causes or of a more serious injury and should lead to further evaluation, including brain imaging.
Diagnostic tests
Current clinical brain imaging cannot diagnose a concussion. The purpose of neuroimaging is to assess for other etiologies or injuries, such as hemorrhage or contusion, that may cause similar symptoms but require different management.
Several guidelines are available to assess the need for imaging in the setting of recent trauma, of which 2 are typically used10–12:
The Canadian CT Head Rule10 states that computed tomography (CT) is indicated in any of the following situations:
- The patient fails to reach a Glasgow Coma Scale score of 15—on a scale of 3 (worst) to 15 (best)—within 2 hours
- There is a suspected open skull fracture
- There is any sign of basal skull fracture
- The patient has 2 or more episodes of vomiting
- The patient is 65 or older
- The patient has retrograde amnesia (ie, cannot remember events that occurred before the injury) for 30 minutes or more
- The mechanism of injury was dangerous (eg, a pedestrian was struck by a motor vehicle, or the patient fell from > 3 feet or > 5 stairs).
The New Orleans Criteria11 state that a patient warrants CT of the head if any of the following is present:
- Severe headache
- Vomiting
- Age over 60
- Drug or alcohol intoxication
- Deficit in short-term memory
- Physical evidence of trauma above the clavicles
- Seizure.
Caveats: these imaging guidelines apply to adults; those for pediatric patients differ.12 Also, because they were designed for use in an emergency department, their utility in clinical practice outside the emergency department is unclear.
Electroencephalography is not necessary in the evaluation of concussion unless a seizure disorder is believed to be the cause of the injury.
Concussion in athletes
Athletes who participate in contact and collision sports are at higher risk of concussion than the nonathletic population. Therefore, specific assessments of symptoms, balance, oculomotor function, cognitive function, and reaction time have been developed for athletes.
Ideally, these measures are taken at preseason baseline, so that they are available for comparison with postinjury assessments after a known or suspected concussion. These assessments can be used to help make the diagnosis of concussion in cases that are unclear and to help monitor recovery. Objective measures of injury are especially useful for athletes who may be reluctant to report symptoms in order to return to play.
Like most medical tests, these assessments need to be properly interpreted in the overall context of the medical history and physical examination by those who know how to administer them. It is important to remember that the natural history of concussion recovery differs between sport-related concussion and concussion that occurs outside of sports.8
MANAGEMENT
The symptoms and signs after concussion are so variable and multidimensional that they make a generally applicable treatment hard to define.
Rest: Physical and cognitive
Treatment depends on the specifics of the injury, but there are common recommendations for the acute days after injury. Lacking hard data, the consensus among experts is that patients should undergo a period of physical and cognitive rest.13,14 Exactly what “rest” means and how long it should last are unknown, leading to a wide variation in its application.
Rest aids recovery but also may have adverse effects: fatigue, diurnal sleep disruption, reactive depression, anxiety, and physiologic deconditioning.15,16 Many guidelines recommend physical and cognitive rest until symptoms resolve,14 but this is likely too cautious. Even without a concussion, inactivity is associated with many of the nonspecific symptoms also associated with concussion. As recovery progresses, the somatic symptoms of concussion improve, while emotional symptoms worsen, likely in part due to prolonged rest.17
We recommend a period of rest lasting 3 to 5 days after injury, followed by a gradual resumption of both physical and cognitive activities as tolerated, remaining below the level at which symptoms are exacerbated.
Not surprisingly, many guidelines for returning to physical activity are focused on athletes. Yet the same principles apply to management of concussion in the general population who exercise: light physical activity (typically walking or stationary bicycling), followed by more vigorous aerobic activity, followed by some resistance activities. Mild aerobic exercise (to below the threshold of symptoms) may speed recovery from refractive postconcussion syndrome, even in those who did not exercise before the injury.18
Athletes require specific and strict instructions to avoid increased trauma to the head during the gradual increase of physical activities. The National Collegiate Athletic Association has published an algorithm for a gradual return to sport-specific training that is echoed in recent consensus statements on concussion.19 Once aerobic reconditioning produces no symptoms, then noncontact, sport-specific activities are begun, followed by contact activities. We have patients return to the clinic once they are symptom-free for repeat evaluation before clearing them for high-risk activities (eg, skiing, bicycling) or contact sports (eg, basketball, soccer, football, ice hockey).
Cognitive rest
While physical rest is fairly straightforward, cognitive rest is more challenging. The concept of cognitive rest is hard to define and even harder to enforce. Patients are often told to minimize any activities that require attention or concentration. This often includes, but is not limited to, avoiding reading, texting, playing video games, and using computers.13
In the modern world, full avoidance of these activities is difficult and can be profoundly socially isolating. Further, complete cognitive rest may be associated with symptoms of its own.15,16,20 Still, some reasonable limitation of cognitive activities, at least initially, is likely beneficial.21 For patients engaged in school or academic work, often the daily schedule needs to be adjusted and accommodations made to help them return to a full academic schedule and level of activity. It is reasonable to have patients return gradually to work or school rather than attempt to immediately return to their preinjury level.
With these interventions, most patients have full resolution of their symptoms and return to preinjury levels of performance.
TREATING SOMATIC SYMPTOMS
Posttraumatic headache
Posttraumatic headache is the most common sequela of concussion.22 Surprisingly, it is more common after concussion than after moderate or severe traumatic brain injury.23 A prior history of headache, particularly migraine, is a known risk factor for development of posttraumatic headache.24
Posttraumatic headache is usually further defined by headache type using the International Classification of Headache Disorders criteria (www.ichd-3.org). Migraine or probable migraine is the most common type of posttraumatic headache; tension headache is less common.25
Analgesics such as nonsteroidal anti-inflammatory drugs (NSAIDs) are often used initially by patients to treat posttraumatic headache. One study found that 70% of patients used acetaminophen or an NSAID.26
Treating early with effective therapy is the most important tenet of posttraumatic headache treatment, since 80% of those who self-treat have incomplete relief, and almost all of them are using over-the-counter products.27 Overuse of over-the-counter abortive medications can lead to medication overuse headache, also known as rebound headache, thus complicating the treatment of posttraumatic headache.26
Earlier treatment with a preventive medication can often limit the need for and overuse of over-the-counter analgesics and can minimize the occurrence of subsequent medication overuse headache. However, in pediatric populations, nonpharmacologic interventions such as rest and sleep hygiene are typically used first, then medications after 4 to 6 weeks if this is ineffective.
A number of medications have been studied for prophylactic treatment of posttraumatic headache, including topiramate, amitriptyline, and divalproex sodium,28–30 but there is little compelling evidence for use of one over the other. If posttraumatic headache is migrainous, beta-blockers, calcium-channel blockers, selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibtors, and gabapentin are other prophylactic medication options under the appropriate circumstances.27,31,32 In adults, we have clinically had success with nortriptyline 20 mg or gabapentin 300 mg at night as an initial prophylactic headache medication, increasing as tolerated or until pain is controlled, though there are no high-quality data to guide this decision.
The ideal prophylactic medication depends on headache type, patient tolerance, comorbidities, allergies, and medication sensitivities. Gabapentin, amitriptyline, and nortriptyline can produce sedation, which can help those suffering from sleep disturbance.
If a provider is not comfortable prescribing these medications or doesn’t prescribe them regularly, the patient should be referred to a concussion or headache specialist more familiar with their use.
In some patients, even some athletes, headache may be related to a cervical strain injury—whiplash—that should be treated with an NSAID (or acetaminophen), perhaps with a short course of a muscle relaxant in adults, and with physical therapy.32
Some patients have chronic headache despite oral medications.26 Therefore, alternatives to oral medications and complementary therapies should be considered. Especially for protracted cases requiring more complicated headache management or injectable treatments, patients should be referred to a pain clinic, headache specialist, or concussion specialist.
Dizziness
Dizziness is also common after concussion. But what the patient means by dizziness requires a little probing. Some have paroxysms of vertigo. This typically represents a peripheral vestibular injury, usually benign paroxysmal positional vertigo. The latter can be elicited with a Hallpike maneuver and treated in the office with the Epley maneuver.33
Usually, dizziness is a subjective sense of poor coordination, gait instability, or dysequilibrium. Patients may also complain of associated nausea and motion sensitivity. This may all be secondary to a mechanism in the middle or inner ear or the brain. Patients should be encouraged to begin movement—gradually and safely—to help the vestibular system accommodate, which it will do with gradual stimulation. It usually resolves spontaneously.
Specific treatment is unfortunately limited. There is no established benefit from vestibular suppressants such as meclizine. Vestibular rehabilitation may accelerate improvement and decrease symptoms.33 Referral for a comprehensive balance assessment or to vestibular therapy (a subset of physical therapy) should be considered and is something we typically undertake in our clinic if there is no recovery from dizziness 4 to 6 weeks after the concussion.
Visual symptoms can contribute to dizziness. Convergence spasm or convergence insufficiency (both related to muscle spasm of the eye) can occur after concussion, with some studies estimating that up to 69% of patients have these symptoms.34 This can interfere with visual tracking and contribute to a feeling of dysequilibrium.34 Referral to a concussion specialist or vestibular rehabilitation physical therapist can be helpful in treating this issue if it does not resolve spontaneously.
Orthostasis and lightheadedness also contribute to dizziness and are associated with cerebrovascular autoregulation. Available data suggest that dysregulation of neurovascular coupling, cerebral vasoreactivity, and cerebral autoregulation contribute to some of the chronic symptoms of concussion, including dizziness. A gradual return to exercise may help regulate cerebral blood flow and improve this type of dizziness.35
Sleep disturbance
Sleep disturbance is common after concussion, but the form is variable: insomnia, excessive daytime somnolence, and alteration of the sleep-wake cycle are all seen and may themselves affect recovery.36
Sleep hygiene education should be the first intervention for postconcussive sleep issues. For example, the patient should be encouraged to do the following:
- Minimize “screen time” an hour before going to bed: cell phone, tablet, and computer screens emit a wavelength of light that suppresses endogenous melatonin release37,38
- Go to bed and wake up at the same time each day
- Minimize or avoid caffeine, nicotine, and alcohol
- Avoid naps.39
Melatonin is a safe and effective treatment that could be added.40 In addition, some studies suggest that melatonin may improve recovery from traumatic brain injury.41,42
Mild exercise (to below the threshold of causing or exacerbating symptoms) may also improve sleep quality.
Amitriptyline or nortriptyline may reduce headache frequency and intensity and also help treat insomnia.
Trazodone is recommended by some as a first-line agent,39 but we usually reserve it for protracted insomnia refractory to the above treatments.
Benzodiazepines should be avoided, as they reduce arousal, impair cognition, and exacerbate motor impairments.43
Emotional symptoms
Acute-onset anxiety or depression often occurs after concussion.44,45 There is abundant evidence that emotional effects of injury may be the most significant factor in recovery.46 A preinjury history of anxiety may be a prognostic factor.9 Patients with a history of anxiety or depression are more likely to develop emotional symptoms after a concussion, but emotional problems may develop in any patient after a concussion.47,48
The circumstances under which an injury is sustained may be traumatic (eg, car accident, assault), leading to an acute stress reaction or disorder and, if untreated, may result in a more chronic condition—posttraumatic stress disorder. Moreover, the injury and subsequent symptoms may have repercussions in many aspects of the patient’s life, leading to further psychologic stress (eg, loss of wages or the inability to handle normal work, school, and family responsibilities).
Referral to a therapist trained in skills-based psychotherapy (eg, cognitive-behavioral therapy, exposure-based treatment) is often helpful.
Pharmacologic treatment can be a useful adjunct. Several studies have shown that selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, and tricyclic antidepressants may improve depression after concussion.49 The prescription of antidepressants, however, is best left to providers with experience in treating anxiety and depression.
As with sleep disorders after concussion, benzodiazepines should be avoided, as they can impair cognition.43
Cognitive problems
Cognitive problems are also common after concussion. Patients complain about everyday experiences of forgetfulness, distractibility, loss of concentration, and mental fatigue. Although patients often subjectively perceive these symptoms as quite limiting, the impairments can be difficult to demonstrate in office testing.
A program of gradual increase in mental activity, parallel to recovery of physical capacity, should be undertaken. Most patients make a gradual recovery within a few weeks.50
When cognitive symptoms cause significant school or vocational problems or become persistent, patients should be referred to a specialty clinic. As with most of the consequences of concussion, there are few established treatments. When cognitive difficulties persist, it is important to consider the complications of concussion mentioned above: headache, pain, sleep disturbance, and anxiety, all of which may cause subjective cognitive problems and are treatable.
If cognitive symptoms are prolonged despite improvement of other issues like headache and sleep disturbance, a low-dose stimulant medication such as amphetamine salts or methylphenidate may be useful for symptoms of poor attention.49 They should be only a temporary measure after concussion to carry the patient through a cognitively challenging period, unless there was a history of attention-deficit disorder before the injury. A variety of other agents, including amantadine,51 have been proposed based on limited studies; all are off-label uses. Before considering these types of interventions, referral to a specialist or a specialty program would be appropriate.
IF SYMPTOMS PERSIST
With the interventions suggested above, most patients with concussion have a resolution of symptoms and can return to preinjury levels of performance. But some have prolonged symptoms and sequelae. Approximately 10% of athletes have persistent signs and symptoms of concussion beyond 2 weeks. If concussion is not sport-related, most patients recover completely within the first 3 months, but up to 33% may have symptoms beyond that.52
Four types of patients have persistent symptoms:
Patients who sustained a high-force mechanism of injury. These patients simply need more time and accommodation.
Patients who sustained multiple concussions. These patients may also need more time and accommodation.
Patients with an underlying neurologic condition, recognized prior to injury or not, may have delayed or incomplete recovery. Even aging may be an “underlying condition” in concussion.
Patients whose symptoms from an apparently single mild concussion do not resolve despite appropriate treatments may have identifiable factors, but intractable pain (usually headache) or significant emotional disturbance or both are common. Once established and persistent, this is difficult to treat. Referral to a specialty practice is appropriate, but even in that setting effective treatment may be elusive.
PATIENT EDUCATION
Most important for patient education is reassurance. Ultimately, concussion is a self-limited phenomenon, and reinforcing this is helpful for patients. If concussion is not sport-related, most patients recover completely within 3 months.
The next important tenet in patient education is that they should rest for 3 to 5 days, then resume gradual physical and cognitive activities. If resuming activities too soon results in symptoms, then they should rest for a day and gradually resume activity. If their recovery is prolonged (ie, longer than 6 weeks), they likely need to be referred to a concussion specialist.
- Cassidy JD, Carroll LJ, Peloso PM, et al; WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. Incidence, risk factors and prevention of mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med 2004; (suppl):28–60.
- Shaw NA. The neurophysiology of concussion. Prog Neurobiol 2002; 67:281–344.
- Denny-Brown DE, Russell WR. Experimental concussion: (section of neurology). Proc R Soc Med 1941; 34:691–692.
- Ommaya AK, Gennarelli TA. Cerebral concussion and traumatic unconsciousness. Correlation of experimental and clinical observations of blunt head injuries. Brain 1974; 97:633–654.
- Houlburn AHS, Edin MA. Mechanics of head injuries. Lancet 1943; 242:438–441.
- Gennarelli TA, Adams JH, Graham DI. Acceleration induced head injury in the monkey. I. The model, its mechanical and physiological correlates. Acta Neuropathol Suppl 1981; 7:23–25.
- Giza CC, Hovda DA. The neurometabolic cascade of concussion. J Athl Train 2001; 36:228–235.
- Meehan WP 3rd, Bachur RG. Sport-related concussion. Pediatrics 2009; 123:114–123.
- Iverson GL, Silverberg ND, Mannix R, et al. Factors associated with concussion-like symptom reporting in high school athletes. JAMA Pediatr 2015; 169:1132–1140.
- Stiell IG, Wells GA, Vandemheen K. et al. The Canadian CT head rule for patients with minor head injury. Lancet 2001; 357:1391–1396.
- Haydel MJ, Preston CA, Mills TJ, Luber S, Blaudeau E, DeBlieux PMC. Indications for computed tomography in patients with minor head injury. N Engl J Med 2000; 343:100–105.
- Kuppermann N, Holmes JF, Dayan PS, et al; Pediatric Emergency Care Applied Research Network (PECARN). Identification of children at very low risk of clinically important brain injuries after head trauma: a prospective cohort study. Lancet 2009; 374:1160–1170.
- McCrory P, Meeuwisse W, Johnston K, et al. Consensus Statement on Concussion in Sport: the 3rd International Conference on Concussion in Sport held in Zurich, November 2008. Br J Sports Med 2009; 43(suppl 1):i76–i90.
- DeMatteo C, Stazyk K, Singh SK, et al; Ontario Neurotrauma Foundation. Development of a conservative protocol to return children and youth to activity following concussive injury. Clin Pediatr (Phila) 2015; 54:152–163.
- Willer B, Leddy JJ. Management of concussion and post-concussion syndrome. Curr Treat Options Neurol 2006; 8:415–426.
- DiFazio M, Silverberg ND, Kirkwood MW, Bernier R, Iverson GL. Prolonged activity restriction after concussion: are we worsening outcomes? Clin Pediatr (Phila) 2016; 55:443–451.
- Thomas DG, Apps JN, Hoffmann RG, McCrea M, Hammeke T. Benefits of strict rest after acute concussion: a randomized controlled trial. Pediatrics 2015; 135:213–223.
- Leddy JJ, Kozlowski K, Donnelly JP, Pendergast DR, Epstein LH, Willer B. A preliminary study of subsymptom threshold exercise training for refractory post-concussion syndrome. Clin J Sport Med 2010; 20:21–27.
- McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med 2013; 47:250–258.
- Buckley TA, Munkasy BA, Clouse BP. Acute cognitive and physical rest may not improve concussion recovery time. J Head Trauma Rehabil 2016; 31:233–241.
- Brown NJ, Mannix RC, O'Brien MJ, Gostine D, Collins MW, Meehan WP 3rd. Effect of cognitive activity level on duration of post-concussion symptoms. Pediatrics 2014; 133:e299–e304.
- Packard RC. Epidemiology and pathogenesis of posttraumatic headache. J Head Trauma Rehabil 1999; 14:9–21.
- Couch JR, Bearss C. Chronic daily headache in the posttrauma syndrome: relation to extent of head injury. Headache 2001; 41:559–564.
- Lucas S, Hoffman JM, Bell KR, Dikmen S. A prospective study of prevalence and characterization of headache following mild traumatic brain injury. Cephalalgia 2014; 34:93–102.
- Lucas S, Hoffman JM, Bell KR, Walker W, Dikmen S. Characterization of headache after traumatic brain injury. Cephalalgia 2012; 32:600–606.
- DiTommaso C, Hoffman JM, Lucas S, Dikmen S, Temkin N, Bell KR. Medication usage patterns for headache treatment after mild traumatic brain injury. Headache 2014; 54:511–519.
- Lucas S. Characterization and management of headache after mild traumatic brain injury. In: Kobeissy FH, ed. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton, FL: CRC Press/Taylor & Franis Group; 2015:145–154.
- Erickson JC. Treatment outcomes of chronic post-traumatic headaches after mild head trauma in US soldiers: an observational study. Headache 2011; 51:932–944.
- Tyler GS, McNeely HE, Dick ML. Treatment of post-traumatic headache with amitriptyline. Headache 1980; 20:213–216.
- Packard RC. Treatment of chronic daily posttraumatic headache with divalproex sodium. Headache 2000; 40:736–739.
- Kacperski J, Arthur T. Management of post-traumatic headaches in children and adolescents. Headache 2016; 56:36–48.
- Lenaerts ME, Couch JR, Couch JR. Posttraumatic headache. Curr Treat Options Neurol 2004; 6:507–517.
- Valovich McLeod TC, Hale TD. Vestibular and balance issues following sport-related concussion. Brain Inj 2015; 29:175–184.
- Master CL, Cheiman M, Gallaway M, et al. Vision diagnoses are common after concussion in adolescents. Clin Pediatr (Phila) 2016; 55:260–267.
- Tan CO, Meehan WP 3rd, Iverson GL, Taylor JA. Cerebrovascular regulation, exercise and mild traumatic brain injury. Neurology 2014; 83:1665–1672.
- Mahmood O, Rapport LJ, Hanks RA, Fichtenberg NL. Neuropsychological performance and sleep disturbance following traumatic brain injury. J Head Trauma Rehabil 2004; 19:378–390.
- Lewy AJ, Wehr TA, Goodwin FK, Newsome DA, Markey SP. Light suppresses melatonin secretion in humans. Science 1980; 210:1267–1269.
- Figueiro MG, Wood B, Plitnick B, Rea MS. The impact of light from computer monitors on melatonin levels in college students. Neuro Endocrinol Lett 2011; 32:158–163.
- Rao V, Rollings P. Sleep disturbances following traumatic brain injury. Curr Treat Options Neurol 2002; 4:77–87.
- Samantaray S, Das A, Thakore NP, et al. Therapeutic potential of melatonin in traumatic central nervous system injury. J Pineal Res 2009; 47:134–142.
- Ding K, Xu J, Wang H, Zhang L, Wu Y, Li T. Melatonin protects the brain from apoptosis by enhancement of autophagy after traumatic brain injury in mice. Neurochem Int 2015; 91:46–54.
- Babaee A, Eftekhar-Vaghefi SH, Asadi-Shekaari M, et al. Melatonin treatment reduces astrogliosis and apoptosis in rats with traumatic brain injury. Iran J Basic Med Sci 2015; 18:867–872.
- Arciniegas DB, Anderson CA, Topkoff J, McAllister TW. Mild traumatic brain injury: a neuropsychiatric approach to diagnosis, evaluation, and treatment. Neuropsychiatr Dis Treat 2005; 1:311–327.
- O’Donnell ML, Creamer M, Pattison P, Atkin C. Psychiatric morbidity following injury. Am J Psychiatry 2004; 161:507–514.
- Dikmen SS, Bombardier CH, Machamer JE, Fann JR, Temkin NR. Natural history of depression in traumatic brain injury. Arch Phys Med Rehabil 2004; 85:1457–1464.
- Massey JS, Meares S, Batchelor J, Bryant RA. An exploratory study of the association of acute posttraumatic stress, depression, and pain to cognitive functioning in mild traumatic brain injury. Neuropsychology 2015; 29:530–542.
- Meares S, Shores EA, Taylor AJ, et al. The prospective course of postconcussion syndrome: the role of mild traumatic brain injury. Neuropsychology 2011; 25:454–465.
- Solomon GS, Kuhn AW, Zuckerman SL. Depression as a modifying factor in sport-related concussion: a critical review of the literature. Phys Sportsmed 2016; 44:14–19.
- Neurobehavioral Guidelines Working Group; Warden DL, Gordon B, McAllister TW, et al. Guidelines for the pharmacologic treatment of neurobehavioral sequelae of traumatic brain injury. J Neurotrauma 2006; 23:1468–1501.
- Dikmen S, McLean A, Temkin N. Neuropsychological and psychosocial consequences of minor head injury. J Neurol Neurosurg Psychiatry 1986; 49:1227–1232.
- Reddy CC, Collins M, Lovell M, Kontos AP. Efficacy of amantadine treatment on symptoms and neurocognitive performance among adolescents following sports-related concussion. J Head Trauma Rehabil 2013; 28:260–265.
- Leddy JJ, Sandhu H, Sodhi V, Baker JG, Willer B. Rehabilitation of concussion and post-concussion syndrome. Sports Health 2012; 4:147–154.
- Cassidy JD, Carroll LJ, Peloso PM, et al; WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. Incidence, risk factors and prevention of mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med 2004; (suppl):28–60.
- Shaw NA. The neurophysiology of concussion. Prog Neurobiol 2002; 67:281–344.
- Denny-Brown DE, Russell WR. Experimental concussion: (section of neurology). Proc R Soc Med 1941; 34:691–692.
- Ommaya AK, Gennarelli TA. Cerebral concussion and traumatic unconsciousness. Correlation of experimental and clinical observations of blunt head injuries. Brain 1974; 97:633–654.
- Houlburn AHS, Edin MA. Mechanics of head injuries. Lancet 1943; 242:438–441.
- Gennarelli TA, Adams JH, Graham DI. Acceleration induced head injury in the monkey. I. The model, its mechanical and physiological correlates. Acta Neuropathol Suppl 1981; 7:23–25.
- Giza CC, Hovda DA. The neurometabolic cascade of concussion. J Athl Train 2001; 36:228–235.
- Meehan WP 3rd, Bachur RG. Sport-related concussion. Pediatrics 2009; 123:114–123.
- Iverson GL, Silverberg ND, Mannix R, et al. Factors associated with concussion-like symptom reporting in high school athletes. JAMA Pediatr 2015; 169:1132–1140.
- Stiell IG, Wells GA, Vandemheen K. et al. The Canadian CT head rule for patients with minor head injury. Lancet 2001; 357:1391–1396.
- Haydel MJ, Preston CA, Mills TJ, Luber S, Blaudeau E, DeBlieux PMC. Indications for computed tomography in patients with minor head injury. N Engl J Med 2000; 343:100–105.
- Kuppermann N, Holmes JF, Dayan PS, et al; Pediatric Emergency Care Applied Research Network (PECARN). Identification of children at very low risk of clinically important brain injuries after head trauma: a prospective cohort study. Lancet 2009; 374:1160–1170.
- McCrory P, Meeuwisse W, Johnston K, et al. Consensus Statement on Concussion in Sport: the 3rd International Conference on Concussion in Sport held in Zurich, November 2008. Br J Sports Med 2009; 43(suppl 1):i76–i90.
- DeMatteo C, Stazyk K, Singh SK, et al; Ontario Neurotrauma Foundation. Development of a conservative protocol to return children and youth to activity following concussive injury. Clin Pediatr (Phila) 2015; 54:152–163.
- Willer B, Leddy JJ. Management of concussion and post-concussion syndrome. Curr Treat Options Neurol 2006; 8:415–426.
- DiFazio M, Silverberg ND, Kirkwood MW, Bernier R, Iverson GL. Prolonged activity restriction after concussion: are we worsening outcomes? Clin Pediatr (Phila) 2016; 55:443–451.
- Thomas DG, Apps JN, Hoffmann RG, McCrea M, Hammeke T. Benefits of strict rest after acute concussion: a randomized controlled trial. Pediatrics 2015; 135:213–223.
- Leddy JJ, Kozlowski K, Donnelly JP, Pendergast DR, Epstein LH, Willer B. A preliminary study of subsymptom threshold exercise training for refractory post-concussion syndrome. Clin J Sport Med 2010; 20:21–27.
- McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med 2013; 47:250–258.
- Buckley TA, Munkasy BA, Clouse BP. Acute cognitive and physical rest may not improve concussion recovery time. J Head Trauma Rehabil 2016; 31:233–241.
- Brown NJ, Mannix RC, O'Brien MJ, Gostine D, Collins MW, Meehan WP 3rd. Effect of cognitive activity level on duration of post-concussion symptoms. Pediatrics 2014; 133:e299–e304.
- Packard RC. Epidemiology and pathogenesis of posttraumatic headache. J Head Trauma Rehabil 1999; 14:9–21.
- Couch JR, Bearss C. Chronic daily headache in the posttrauma syndrome: relation to extent of head injury. Headache 2001; 41:559–564.
- Lucas S, Hoffman JM, Bell KR, Dikmen S. A prospective study of prevalence and characterization of headache following mild traumatic brain injury. Cephalalgia 2014; 34:93–102.
- Lucas S, Hoffman JM, Bell KR, Walker W, Dikmen S. Characterization of headache after traumatic brain injury. Cephalalgia 2012; 32:600–606.
- DiTommaso C, Hoffman JM, Lucas S, Dikmen S, Temkin N, Bell KR. Medication usage patterns for headache treatment after mild traumatic brain injury. Headache 2014; 54:511–519.
- Lucas S. Characterization and management of headache after mild traumatic brain injury. In: Kobeissy FH, ed. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton, FL: CRC Press/Taylor & Franis Group; 2015:145–154.
- Erickson JC. Treatment outcomes of chronic post-traumatic headaches after mild head trauma in US soldiers: an observational study. Headache 2011; 51:932–944.
- Tyler GS, McNeely HE, Dick ML. Treatment of post-traumatic headache with amitriptyline. Headache 1980; 20:213–216.
- Packard RC. Treatment of chronic daily posttraumatic headache with divalproex sodium. Headache 2000; 40:736–739.
- Kacperski J, Arthur T. Management of post-traumatic headaches in children and adolescents. Headache 2016; 56:36–48.
- Lenaerts ME, Couch JR, Couch JR. Posttraumatic headache. Curr Treat Options Neurol 2004; 6:507–517.
- Valovich McLeod TC, Hale TD. Vestibular and balance issues following sport-related concussion. Brain Inj 2015; 29:175–184.
- Master CL, Cheiman M, Gallaway M, et al. Vision diagnoses are common after concussion in adolescents. Clin Pediatr (Phila) 2016; 55:260–267.
- Tan CO, Meehan WP 3rd, Iverson GL, Taylor JA. Cerebrovascular regulation, exercise and mild traumatic brain injury. Neurology 2014; 83:1665–1672.
- Mahmood O, Rapport LJ, Hanks RA, Fichtenberg NL. Neuropsychological performance and sleep disturbance following traumatic brain injury. J Head Trauma Rehabil 2004; 19:378–390.
- Lewy AJ, Wehr TA, Goodwin FK, Newsome DA, Markey SP. Light suppresses melatonin secretion in humans. Science 1980; 210:1267–1269.
- Figueiro MG, Wood B, Plitnick B, Rea MS. The impact of light from computer monitors on melatonin levels in college students. Neuro Endocrinol Lett 2011; 32:158–163.
- Rao V, Rollings P. Sleep disturbances following traumatic brain injury. Curr Treat Options Neurol 2002; 4:77–87.
- Samantaray S, Das A, Thakore NP, et al. Therapeutic potential of melatonin in traumatic central nervous system injury. J Pineal Res 2009; 47:134–142.
- Ding K, Xu J, Wang H, Zhang L, Wu Y, Li T. Melatonin protects the brain from apoptosis by enhancement of autophagy after traumatic brain injury in mice. Neurochem Int 2015; 91:46–54.
- Babaee A, Eftekhar-Vaghefi SH, Asadi-Shekaari M, et al. Melatonin treatment reduces astrogliosis and apoptosis in rats with traumatic brain injury. Iran J Basic Med Sci 2015; 18:867–872.
- Arciniegas DB, Anderson CA, Topkoff J, McAllister TW. Mild traumatic brain injury: a neuropsychiatric approach to diagnosis, evaluation, and treatment. Neuropsychiatr Dis Treat 2005; 1:311–327.
- O’Donnell ML, Creamer M, Pattison P, Atkin C. Psychiatric morbidity following injury. Am J Psychiatry 2004; 161:507–514.
- Dikmen SS, Bombardier CH, Machamer JE, Fann JR, Temkin NR. Natural history of depression in traumatic brain injury. Arch Phys Med Rehabil 2004; 85:1457–1464.
- Massey JS, Meares S, Batchelor J, Bryant RA. An exploratory study of the association of acute posttraumatic stress, depression, and pain to cognitive functioning in mild traumatic brain injury. Neuropsychology 2015; 29:530–542.
- Meares S, Shores EA, Taylor AJ, et al. The prospective course of postconcussion syndrome: the role of mild traumatic brain injury. Neuropsychology 2011; 25:454–465.
- Solomon GS, Kuhn AW, Zuckerman SL. Depression as a modifying factor in sport-related concussion: a critical review of the literature. Phys Sportsmed 2016; 44:14–19.
- Neurobehavioral Guidelines Working Group; Warden DL, Gordon B, McAllister TW, et al. Guidelines for the pharmacologic treatment of neurobehavioral sequelae of traumatic brain injury. J Neurotrauma 2006; 23:1468–1501.
- Dikmen S, McLean A, Temkin N. Neuropsychological and psychosocial consequences of minor head injury. J Neurol Neurosurg Psychiatry 1986; 49:1227–1232.
- Reddy CC, Collins M, Lovell M, Kontos AP. Efficacy of amantadine treatment on symptoms and neurocognitive performance among adolescents following sports-related concussion. J Head Trauma Rehabil 2013; 28:260–265.
- Leddy JJ, Sandhu H, Sodhi V, Baker JG, Willer B. Rehabilitation of concussion and post-concussion syndrome. Sports Health 2012; 4:147–154.
KEY POINTS
- Concussion results from a traumatic acceleration of the brain that leads to a metabolic mismatch, with an increased demand for adenosine triphosphate but decreased blood flow to the brain. This “energy crisis” results in variable signs and symptoms, most commonly headache, dizziness, sleep disturbance, cognitive problems, and emotional difficulties.
- Initial therapy involves several days of cognitive and physical rest, followed by a gradual return to physical and cognitive activities.
- There is no direct treatment for the physiology of concussion, but early treatment of symptoms and education about recovery and accommodations aids functional recovery.
Combined hormonal contraceptives and migraine: An update on the evidence
Combined hormonal contraceptives are contraindicated in women who have migraine with aura because they pose a risk of stroke. But how great is the risk, and how strong is the evidence, particularly with today’s low-dose contraceptives? Can we view migraine with aura as a relative contraindication rather than an absolute one?
This article reviews migraine diagnosis, the effects of estrogen and the menstrual cycle on migraine, the evidence of stroke risk with combined hormonal contraceptive use, and how the frequency of aura may affect risk. It offers practical advice on choosing contraceptive formulations and counseling patients on risks and benefits.
WHAT THE GUIDELINES SAY
Current guidelines restrict the use of combined hormonal contraceptives in the setting of migraine with aura, but not in migraine without aura.
A practice bulletin from the American College of Obstetrics and Gynecology in 2010 noted that extended-cycle or continuous hormonal contraceptives, including oral and parenteral products, might provide relief of migraines by eliminating the drops in estrogen levels that precipitate them.1 However, the bulletin also cautioned that though cerebrovascular accidents in women are rare, the impact of a stroke is so devastating that clinicians should consider intrauterine devices, progestin-only options, and other nonestrogen methods in women who have migraine with focal neurologic signs, women who smoke, and women age 35 or older.1
In 2016, the US Centers for Disease Control and Prevention published updates to its medical eligibility criteria for contraceptive use in various medical conditions. In the case of migraine without aura, the guidelines note no limitation to the use of combined hormonal contraceptives, regardless of the patient’s age. In the case of migraine with aura, the consensus was that the risk associated with combined hormonal contraception typically outweighs its benefits, noting “an unacceptable health risk if the contraceptive method is used.”2
We believe a fresh look at the data is warranted.
EARLY ORAL CONTRACEPTIVES WERE ALL HIGH-DOSE
This issue first surfaced in the decade and a half after the initial launch of oral contraceptives in 1960. The products then were all high-dose pills, containing up to 150 µg of mestranol. In subsequent decades, the dose of estrogen was successively reduced, so that now some pills contain only 10 µg of ethinyl estradiol. High-dose pills—which today contain 50 µg of ethinyl estradiol—account for less than 1% of pills currently sold in the United States and have been eliminated in many countries.
DIAGNOSTIC CRITERIA FOR MIGRAINE
According to the International Classification of Headache Disorders (ICHD),3 the diagnosis of migraine requires 2 of the 4 following criteria:
- Unilateral location
- Pulsating or throbbing pain
- Pain of at least moderate intensity
- Pain aggravated by activity, or causing a preference to avoid activity.
An additional criterion is either nausea or a combination of photophobia and phonophobia with the episode. This criterion can be met if the patient prefers to avoid bright lights and loud noises during an attack.
Headache experts have suggested that patients with a stable pattern of episodic, disabling headache and normal findings on physical examination should be considered to have migraine if there is no contradictory evidence.4,5
Migraine with aura requires at least 2 of the following 4 characteristics3:
- 1 aura symptom, spreading gradually over 5 minutes, or 2 or more aura symptoms occurring in succession, or both
- Each aura symptom lasting 5 to 60 minutes (not “a few seconds,” not “hours”)
- The aura followed by the onset of headache within 60 minutes
- At least 1 aura symptom is unilateral.
Visual blurring, floaters, or split-second flashes before or during a migraine headache do not meet the criteria for aura.
MIGRAINE IS COMMON AND UNDERRECOGNIZED
In a study of 1,203 patients seeking care from a primary care provider for headache,6 94% of the 377 who turned in a diary with enough data to make a diagnosis were diagnosed with a migraine or probable migraine by an expert panel. A quarter of patients who likely had migraine based on an expert review of symptoms did not receive a migraine diagnosis at the time of their office visit.
Similarly, in a large epidemiologic study,7 30,758 adults were asked if they had headaches and, if so, how they named them. Headaches were reported by 23,564 of the participants and were subsequently diagnosed by formal ICHD criteria. Of the 3,074 individuals who met the criteria for migraine, only 53.4% correctly recognized their headaches as migraine. The most common erroneous labels were “sinus headache” and “stress headache.”7
HOW ESTROGEN AFFECTS MIGRAINE
Of note, migraine can be exacerbated during times of cycle irregularity, such as adolescence and perimenopause, the 2 times during a woman’s life associated with the highest risk of unintended pregnancy.10,11
STROKE RISK: ESTROGEN DOSE MATTERS
Shortly after the first combined oral contraceptives were released, reports of adverse events began to appear, although serious events were relatively rare. In response, prescribing guidelines advised against giving oral contraceptives to women with a history of deep vein thrombosis, myocardial infarction, stroke, or hypertension. Also, over the years, the hormonal content of the formulations was successively reduced, and with each reduction in estrogen, a decrease was observed in venous thrombosis and pulmonary embolism.12,13 Current low-dose formulations are considerably safer than high-dose options but are not entirely without risk.14
Stroke risk with combined oral contraceptives was first highlighted in a landmark article in 1975.15 However, the authors were unable to correlate the risk with the estrogen concentration of the pill, since 23 of the 25 women who suffered thrombotic stroke while taking the mestranol-containing formulation took 100-μg pills, and all 20 women who had strokes while taking the ethinyl estradiol formulation took 50-μg pills. Thus, by today’s standards, they were all taking high-dose pills. The risk of thrombotic stroke was 4 to 5 times higher in users than in nonusers.
In 1996, a study from the World Health Organization16 reported an increased risk of stroke with high-dose combined oral contraceptives (odds ratio [OR] 5.30, 95% confidence interval [CI] 2.56–11.0). With preparations containing less than 50 μg of ethinyl estradiol, the risk was not statistically significant (OR 1.53, 95% CI 0.71–3.31). These numbers were for Europe only; in developing countries, the risk was elevated regardless of dose, presumably due to additional risk factors in combined oral contraceptive users. The majority of strokes were in smokers taking 50-μg pills, with an average age greater than 35.
In 2002, a 5-year case-control study in Denmark found that the risk of stroke with combined oral contraceptives correlated directly with the estrogen content, from no increased risk with the newest and lowest-dose formulation (containing ethinyl estradiol 20 µg) to an OR of 4.5 with the older high-dose (50 µg) formulations.17
Reassuringly, a 2012 retrospective review of the Danish national registry13 revealed a low absolute risk of arterial events in users of combined oral contraceptives: 21.4 per 100,000 person-years for thrombotic stroke, and 10.1 per 100,000 person-years for myocardial infarction. Further, these risks were substantially lower with 20-μg ethinyl estradiol products than with those containing 30 to 40 μg.13 An important limitation of this large database review is that it did not control for important stroke risk factors such as obesity and smoking.
Although international studies14,16 continue to show a small but increased risk, more than 30 years have passed since a US study found an increased risk of stroke with combined oral contraceptives.
The discrepancy between US and international studies is possibly explained by the strong relative contraindication in the United States to the use of combined oral contraceptives in smokers over the age of 35 and the more prevalent use of high-dose pills in international studies. High-dose pills had been used in most of the stroke cases in the 1996 World Health Organization study16 but were used by only 0.7% of the women in the case and control groups in 2 pooled US studies from the same time period.18 Similarly, in these US studies, only 17% of the women were smokers on combined oral contraceptives, whereas in the international study, 51% of the women who had strokes and 38% of those in the control groups were smokers.
A large US study19 reviewing 3.6 million woman-years of use found no increased stroke risk (OR 0.96) in current users of low-dose combined oral contraceptives, results similar to those of a pooled analysis of US studies.18 Though this pooled analysis showed an adjusted increased risk of ischemic stroke in women reporting a history of migraine (OR 2.08, 95% CI 1.19–3.65), these conclusions were based on only 4 cases. The prevalence of migraine was identical in women who did or did not have strokes, 7.8% vs 7.7%, respectively, but the risk was judged to be increased after adjusting for other factors. But one important factor was not adjusted for: only 11 of the 1,017 women in the case and control groups were using 50-μg ethinyl estradiol pills, and 4 of the strokes were in this group of 11 women.
STROKE RISK INCREASES WITH FREQUENCY OF MIGRAINE AURA
Use of combined hormonal contraceptives in women who have migraine with aura remains controversial, based on good evidence that aura increases stroke risk20 and good evidence that high-dose oral contraceptives increase stroke risk.15
A cohort study encompassing more than 470,000 person-years with a median follow-up of 26 years found that while migraine without aura conferred no increase in risk of all-cause mortality, migraine with aura did.21
The longitudinal Women’s Health Study analyzed data from 27,798 women over age 45 and found that migraine with aura conferred an increased risk of cardiovascular disease (including stroke) that varied directly with aura frequency.22 Aura frequency less than once a month conferred a risk 2 times higher than in women without migraine, and the risk was more than 4 times higher when aura frequency exceeded once a week.
Similarly, an analysis of the World Health Organization study of stroke in young women found that the adjusted risk of ischemic stroke was significantly and directly associated with aura frequency.20
Potential explanations for this increased risk with greater aura frequency include changes induced during spreading cortical depression, shared genetic predispositions, and common underlying comorbidities such as patent foramen ovale.23–26
Though studies have shown that combined oral contraceptives in continuous regimens27 or in regimens that minimize drops in estrogen levels28 can help improve general headache and menstrual-related migraine, these studies have excluded patients who have migraine with aura.
In a pilot study,29 28 women referred to a tertiary headache clinic who had migraine with aura and intractable menstrual-related migraine were offered combined hormonal contraception in the form of a vaginal ring that releases only 15 μg ethinyl estradiol per 24 hours, thereby reducing peak estrogen exposure to a level lower than those encountered with the native menstrual cycle (with the suppression of ovulation). The women used this continuous ultra-low-dose hormonal contraception without placebo days. After a mean follow-up of 8 months, this regimen reduced aura frequency from a baseline average of 3.2 per month to only 0.2 per month. No woman had an increase in aura frequency, and menstrual-related migraine was eliminated in 21 (91.3%) of the 23 evaluable patients.
CHOOSING THE OPTIMAL CONTRACEPTIVE FORMULATION
Today, ultra-low-dose combined oral contraceptives (containing 10–15 µg of ethinyl estradiol) inhibit ovulation with doses of estrogen that are in a midphysiologic range. Consequently, they expose women to lower peak concentrations of estrogen than they would experience in their natural menstrual cycle (Figure 1). If a combined oral contraceptive is used in women with migraine with aura, lower estrogen doses (≤ 20 µg ethinyl estradiol) are preferred to decrease aura frequency and minimize the risk of stroke associated with high-dose ethinyl estradiol formulations.
Does the progestin matter?
Though there has been debate about whether different types of progestins alter the risk of venous thromboembolism,30,31 the chosen progestin does not seem to affect arterial risks such as stroke and myocardial infarction.14
All current guidelines note that progestin-only pills can be safely offered to women with migraine with aura. However, progestin-only pills have a shorter half-life than combined hormonal contraceptives and must be taken consistently and on time to ensure contraceptive efficacy and minimize abnormal bleeding. Patients who cannot adhere to a strict daily pill regimen may increase their risk of unintended pregnancy. In addition, progestin-only pills do not help with reducing episodes of migraine because they prevent ovulation only about half of the time.2 In contrast, a progestin-only arm implant is not only considered safe to use in women with migraine with aura, it may also prevent ovulation more reliably. Though progestin arm implants have the potential to reduce menstrual migraine and aura, this requires further study to confirm.
For menstrual-related migraine
In clinical practice, providers may offer certain combined hormonal contraceptives to women with debilitating menstrual-related migraine to prevent attacks. Although menstrual-related migraine rarely if ever is accompanied by aura, these patients may still have migraine with aura at other times of the month.
In women with menstrual-related migraine, any decrease in estrogen level greater than 10 µg of ethinyl estradiol may trigger an estrogen-withdrawal migraine. All currently available regimens of combined hormonal contraceptives that follow a 21-days-on, 7-days-off plan entail a drop in ethinyl estradiol of more than 10 µg (Figure 1).
Continuous regimens: Who needs a menstrual cycle anyway?
Of note: ultra-low-estrogen combined hormonal contraceptives that have placebo intervals may not inhibit ovulation consistently in all women.32 Contraceptive efficacy is still maintained, as contraception does not require inhibition of ovulation. Other mechanisms such as thickening of cervical mucus help with pregnancy prevention.
However, if ovulation is not inhibited, the consequent postovulatory decline in estrogen will continue to contribute to estrogen-withdrawal migraine.33,34 Reducing the number of placebo days may help inhibit ovulation. Adding back adequate estrogen during the placebo break (eg, either 0.9 mg conjugated equine estrogen with a 20-µg ethinyl estradiol combined oral contraceptive, or 0.075 mg transdermal 17B estradiol with a 15-µg combined hormonal contraceptive) can prevent these migraines.33,34
Some extended-cycle regimens, which give 4 withdrawal bleeds per year, will likewise prevent estrogen-withdrawal migraine if the decline in estrogen is limited to 10 µg (Table 1). Unfortunately, most extended regimens (Seasonale, Seasonique, and their generics) entail a 20- or 30-µg drop.
Continuous or extended-cycle regimens can be prescribed using any generic 20-µg combined hormonal contraceptive that the patient tolerates, along with specific instructions on the prescription to take the pills in a continuous fashion, eg, “Do not take the placebo pills; start the next pill pack immediately after 21 days.”
Postmenopausal hormone therapy
Neither smoking nor migraine is a contraindication to the use of postmenopausal hormone therapy, which is substantially lower in dosage than combined hormonal contraceptives.
ADVISING PATIENTS ON RISKS VS BENEFITS
It is important to remember that the risks of unintended pregnancy are always greater than the risks of any contraceptive, especially in women with chronic medical conditions, including those who have migraine with aura. Other benefits include the following:
Lower mortality risk. A 2010 analysis demonstrated that in nearly 46,000 women followed since 1968, those taking combined oral contraceptives had statistically significantly lower death rates from any cause and a lower risk of death from cancer and cardiovascular diseases than women who had never taken combined oral contraceptives.36
Stroke. Though the absolute risk of stroke to an individual woman taking a low-dose or ultra-low-dose combined hormonal contraceptive has been shown to be similar to that in women who are not taking combined hormonal contraception, its impact on an otherwise healthy woman could be devastating. Clinicians must remember that current guidelines still caution against prescribing combined hormonal contraceptives in women with migraine with aura and thus should counsel their patients accordingly and document the discussion in the medical record.
Noncontraceptive benefits. Women may be prescribed a combined hormonal contraceptive for benefits beyond contraception. The obvious reasons include beneficial effects on endometriosis, anemia, acne, hirsutism, dysmenorrhea, and prevention of ovarian cysts. But other important major benefits2 include substantial reductions in the risk of ovarian cancer (> 50% decrease after 10 years)37 and endometrial cancer (additional 24% reduction for each 5 years of use),38 and a modest decrease in the risk of colon cancer (37% less risk in ever-users).39 Further, combined oral contraceptive use has been associated with a decrease in mortality rates,40,41 with no increased risk of nonreproductive cancers.41
Ultra-low-dose, continuous formulations may benefit women by decreasing the frequency of migraine with aura and menstrual-related migraine. There is no evidence that reducing aura frequency also reduces stroke risk, but this represents an important area for future research.
WHAT WOULD WE DO?
For a patient who has a history of migraine with aura, if the goal is only to prevent pregnancy, we would recommend another contraceptive option that does not involve estrogen. However, we would consider prescribing a combined hormonal contraceptive in a low-dose regimen if the patient prefers this regimen for other health benefits (eg, acne control), if she has no other risk factors for stroke, and if she gives her informed consent after a discussion of the risks and benefits. Women who have menstrual-related migraine refractory to or who cannot tolerate other migraine therapies are often willing to try a low-dose estrogen-containing contraceptive for control of their migraine, especially if they have tried it in the past and believe that it helped prevent migraine. Patients should have follow-up within 3 months to discuss whether they have benefited from the regimen in terms of headache frequency or severity.
- ACOG Practice Bulletin No. 110: noncontraceptive uses of hormonal contraceptives. Obstet Gynecol 2010; 115:206–218.
- Centers for Disease Control and Prevention. US Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recommendations and reports: Morbidity and mortality weekly report Recommendations and reports/Centers for Disease Control 2016; 65:1–104.
- Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition (beta version). Cephalalgia 2013; 33:629–808.
- Lipton RB, Cady RK, Stewart WF, Wilks K, Hall C. Diagnostic lessons from the Spectrum study. Neurology 2002; 58(suppl 6):S27–S31.
- Lipton RB, Stewart WF, Cady R, et al. 2000 Wolfe Award. Sumatriptan for the range of headaches in migraine sufferers: results of the Spectrum Study. Headache 2000; 40:783–791.
- Tepper SJ, Dahlof CG, Dowson A, et al. Prevalence and diagnosis of migraine in patients consulting their physician with a complaint of headache: data from the Landmark Study. Headache 2004; 44:856–864.
- Lipton RB, Stewart WF, Liberman JN. Self-awareness of migraine: interpreting the labels that headache sufferers apply to their headaches. Neurology 2002; 58(suppl 6):S21–S26.
- Chai NC, Peterlin BL, Calhoun AH. Migraine and estrogen. Curr Opin Neurol 2014; 27:315–324.
- Calhoun AH. Menstrual migraine: update on pathophysiology and approach to therapy and management. Curr Treat Options Neurol 2012; 14:1–14.
- McNamara M, Batur P, DeSapri KT. In the clinic. Perimenopause. Ann Intern Med 2015; 162:ITC1–ITC15.
- O’Brien HL, Cohen JM. Young adults with headaches: the transition from adolescents to adults. Headache 2015; 55:1404–1409.
- Vessey M, Mant D, Smith A, Yeates D. Oral contraceptives and venous thromboembolism: findings in a large prospective study. Br Med J (Clin Res Ed) 1986; 292:526.
- Lidegaard O, Lokkegaard E, Jensen A, Skovlund CW, Keiding N. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med 2012; 366:2257–2266.
- MacGregor EA. Contraception and headache. Headache 2013; 53:247–276.
- Oral contraceptives and stroke in young women. Associated risk factors. JAMA 1975; 231:718–722.
- Ischaemic stroke and combined oral contraceptives: results of an international, multicentre, case-control study. WHO Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Lancet 1996; 348:498–505.
- Lidegaard O, Kreiner S. Contraceptives and cerebral thrombosis: a five-year national case-control study. Contraception 2002; 65:197–205.
- Schwartz SM, Petitti DB, Siscovick DS, et al. Stroke and use of low-dose oral contraceptives in young women: a pooled analysis of two US studies. Stroke 1998; 29:2277–2284.
- Petitti DB, Sidney S, Bernstein A, Wolf S, Quesenberry C, Ziel HK. Stroke in users of low-dose oral contraceptives. N Engl J Med 1996; 335:8–15.
- Donaghy M, Chang CL, Poulter N; European Collaborators of the World Health Organisation Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Duration, frequency, recency, and type of migraine and the risk of ischaemic stroke in women of childbearing age. J Neurol Neurosurg Psychiatry 2002; 73:747–750.
- Gudmundsson LS, Scher AI, Aspelund T, et al. Migraine with aura and risk of cardiovascular and all cause mortality in men and women: prospective cohort study. BMJ 2010; 341:c3966.
- Kurth T, Slomke MA, Kase CS, et al. Migraine, headache, and the risk of stroke in women: a prospective study. Neurology 2005; 64:1020–1026.
- Lee ST, Chu K, Jung KH, et al. Decreased number and function of endothelial progenitor cells in patients with migraine. Neurology 2008; 70:1510–1517.
- Kunz GA, Liang G, Cuculi F, et al. Circulating endothelial progenitor cells predict coronary artery disease severity. Am Heart J 2006; 152:190–195.
- Kurth T, Gaziano JM, Cook NR, Logroscino G, Diener HC, Buring JE. Migraine and risk of cardiovascular disease in women. JAMA 2006; 296:283–291.
- Pezzini A, Del Zotto E, Giossi A, Volonghi I, Grassi M, Padovani A. The migraine-ischemic stroke connection: potential pathogenic mechanisms. Curr Mol Med 2009; 9:215–226.
- Sulak P, Willis S, Kuehl T, Coffee A, Clark J. Headaches and oral contraceptives: impact of eliminating the standard 7-day placebo interval. Headache 2007; 47:27–37.
- Nappi RE, Terreno E, Sances G, et al. Effect of a contraceptive pill containing estradiol valerate and dienogest (E2V/DNG) in women with menstrually-related migraine (MRM). Contraception 2013; 88:369–375.
- Calhoun A, Ford S, Pruitt A. The impact of extended-cycle vaginal ring contraception on migraine aura: a retrospective case series. Headache 2012; 52:1246–1253.
- Wu CQ, Grandi SM, Filion KB, Abenhaim HA, Joseph L, Eisenberg MJ. Drospirenone-containing oral contraceptive pills and the risk of venous and arterial thrombosis: a systematic review. BJOG 2013; 120:801–810.
- Dinger J, Bardenheuer K, Heinemann K. Cardiovascular and general safety of a 24-day regimen of drospirenone-containing combined oral contraceptives: final results from the International Active Surveillance Study of Women Taking Oral Contraceptives. Contraception 2014; 89:253–263.
- Benson LS, Micks EA. Why stop now? Extended and continuous regimens of combined hormonal contraceptive methods. Obstet Gynecol Clin North Am 2015; 42:669–681.
- Mannix LK, Calhoun AH. Menstrual migraine. Curr Treat Options Neurol 2004; 6:489–498.
- Calhoun AH. A novel specific prophylaxis for menstrual-associated migraine. South Med J 2004; 97:819–822.
- Calhoun AH. Current topics and controversies in menstrual migraine. Headache 2012; 52(suppl 1):8–11.
- Hannaford PC, Iversen L, Macfarlane TV, Elliott AM, Angus V, Lee AJ. Mortality among contraceptive pill users: cohort evidence from Royal College of General Practitioners’ Oral Contraception Study. BMJ 2010; 340:c927.
- Havrilesky LJ, Moorman PG, Lowery WJ, et al. Oral contraceptive pills as primary prevention for ovarian cancer: a systematic review and meta-analysis. Obstet Gynecol 2013; 122:139 -147.
- Collaborative Group on Epidemiological Studies on Endometrial Cancer. Endometrial cancer and oral contraceptives: an individual participant meta-analysis of 27,276 women with endometrial cancer from 36 epidemiological studies. Lancet Oncol 2015; 16:1061–1070.
- Fernandez E, La Vecchia C, Franceschi S, et al. Oral contraceptive use and risk of colorectal cancer. Epidemiology 1998; 9:295–300.
- Merritt MA, Riboli E, Murphy N, et al. Reproductive factors and risk of mortality in the European Prospective Investigation into Cancer and Nutrition; a cohort study. BMC Med 2015; 13:252.
- Vessey M, Yeates D. Oral contraceptive use and cancer: final report from the Oxford-Family Planning Association Contraceptive Study. Contraception 2013; 88:678–683.
Combined hormonal contraceptives are contraindicated in women who have migraine with aura because they pose a risk of stroke. But how great is the risk, and how strong is the evidence, particularly with today’s low-dose contraceptives? Can we view migraine with aura as a relative contraindication rather than an absolute one?
This article reviews migraine diagnosis, the effects of estrogen and the menstrual cycle on migraine, the evidence of stroke risk with combined hormonal contraceptive use, and how the frequency of aura may affect risk. It offers practical advice on choosing contraceptive formulations and counseling patients on risks and benefits.
WHAT THE GUIDELINES SAY
Current guidelines restrict the use of combined hormonal contraceptives in the setting of migraine with aura, but not in migraine without aura.
A practice bulletin from the American College of Obstetrics and Gynecology in 2010 noted that extended-cycle or continuous hormonal contraceptives, including oral and parenteral products, might provide relief of migraines by eliminating the drops in estrogen levels that precipitate them.1 However, the bulletin also cautioned that though cerebrovascular accidents in women are rare, the impact of a stroke is so devastating that clinicians should consider intrauterine devices, progestin-only options, and other nonestrogen methods in women who have migraine with focal neurologic signs, women who smoke, and women age 35 or older.1
In 2016, the US Centers for Disease Control and Prevention published updates to its medical eligibility criteria for contraceptive use in various medical conditions. In the case of migraine without aura, the guidelines note no limitation to the use of combined hormonal contraceptives, regardless of the patient’s age. In the case of migraine with aura, the consensus was that the risk associated with combined hormonal contraception typically outweighs its benefits, noting “an unacceptable health risk if the contraceptive method is used.”2
We believe a fresh look at the data is warranted.
EARLY ORAL CONTRACEPTIVES WERE ALL HIGH-DOSE
This issue first surfaced in the decade and a half after the initial launch of oral contraceptives in 1960. The products then were all high-dose pills, containing up to 150 µg of mestranol. In subsequent decades, the dose of estrogen was successively reduced, so that now some pills contain only 10 µg of ethinyl estradiol. High-dose pills—which today contain 50 µg of ethinyl estradiol—account for less than 1% of pills currently sold in the United States and have been eliminated in many countries.
DIAGNOSTIC CRITERIA FOR MIGRAINE
According to the International Classification of Headache Disorders (ICHD),3 the diagnosis of migraine requires 2 of the 4 following criteria:
- Unilateral location
- Pulsating or throbbing pain
- Pain of at least moderate intensity
- Pain aggravated by activity, or causing a preference to avoid activity.
An additional criterion is either nausea or a combination of photophobia and phonophobia with the episode. This criterion can be met if the patient prefers to avoid bright lights and loud noises during an attack.
Headache experts have suggested that patients with a stable pattern of episodic, disabling headache and normal findings on physical examination should be considered to have migraine if there is no contradictory evidence.4,5
Migraine with aura requires at least 2 of the following 4 characteristics3:
- 1 aura symptom, spreading gradually over 5 minutes, or 2 or more aura symptoms occurring in succession, or both
- Each aura symptom lasting 5 to 60 minutes (not “a few seconds,” not “hours”)
- The aura followed by the onset of headache within 60 minutes
- At least 1 aura symptom is unilateral.
Visual blurring, floaters, or split-second flashes before or during a migraine headache do not meet the criteria for aura.
MIGRAINE IS COMMON AND UNDERRECOGNIZED
In a study of 1,203 patients seeking care from a primary care provider for headache,6 94% of the 377 who turned in a diary with enough data to make a diagnosis were diagnosed with a migraine or probable migraine by an expert panel. A quarter of patients who likely had migraine based on an expert review of symptoms did not receive a migraine diagnosis at the time of their office visit.
Similarly, in a large epidemiologic study,7 30,758 adults were asked if they had headaches and, if so, how they named them. Headaches were reported by 23,564 of the participants and were subsequently diagnosed by formal ICHD criteria. Of the 3,074 individuals who met the criteria for migraine, only 53.4% correctly recognized their headaches as migraine. The most common erroneous labels were “sinus headache” and “stress headache.”7
HOW ESTROGEN AFFECTS MIGRAINE
Of note, migraine can be exacerbated during times of cycle irregularity, such as adolescence and perimenopause, the 2 times during a woman’s life associated with the highest risk of unintended pregnancy.10,11
STROKE RISK: ESTROGEN DOSE MATTERS
Shortly after the first combined oral contraceptives were released, reports of adverse events began to appear, although serious events were relatively rare. In response, prescribing guidelines advised against giving oral contraceptives to women with a history of deep vein thrombosis, myocardial infarction, stroke, or hypertension. Also, over the years, the hormonal content of the formulations was successively reduced, and with each reduction in estrogen, a decrease was observed in venous thrombosis and pulmonary embolism.12,13 Current low-dose formulations are considerably safer than high-dose options but are not entirely without risk.14
Stroke risk with combined oral contraceptives was first highlighted in a landmark article in 1975.15 However, the authors were unable to correlate the risk with the estrogen concentration of the pill, since 23 of the 25 women who suffered thrombotic stroke while taking the mestranol-containing formulation took 100-μg pills, and all 20 women who had strokes while taking the ethinyl estradiol formulation took 50-μg pills. Thus, by today’s standards, they were all taking high-dose pills. The risk of thrombotic stroke was 4 to 5 times higher in users than in nonusers.
In 1996, a study from the World Health Organization16 reported an increased risk of stroke with high-dose combined oral contraceptives (odds ratio [OR] 5.30, 95% confidence interval [CI] 2.56–11.0). With preparations containing less than 50 μg of ethinyl estradiol, the risk was not statistically significant (OR 1.53, 95% CI 0.71–3.31). These numbers were for Europe only; in developing countries, the risk was elevated regardless of dose, presumably due to additional risk factors in combined oral contraceptive users. The majority of strokes were in smokers taking 50-μg pills, with an average age greater than 35.
In 2002, a 5-year case-control study in Denmark found that the risk of stroke with combined oral contraceptives correlated directly with the estrogen content, from no increased risk with the newest and lowest-dose formulation (containing ethinyl estradiol 20 µg) to an OR of 4.5 with the older high-dose (50 µg) formulations.17
Reassuringly, a 2012 retrospective review of the Danish national registry13 revealed a low absolute risk of arterial events in users of combined oral contraceptives: 21.4 per 100,000 person-years for thrombotic stroke, and 10.1 per 100,000 person-years for myocardial infarction. Further, these risks were substantially lower with 20-μg ethinyl estradiol products than with those containing 30 to 40 μg.13 An important limitation of this large database review is that it did not control for important stroke risk factors such as obesity and smoking.
Although international studies14,16 continue to show a small but increased risk, more than 30 years have passed since a US study found an increased risk of stroke with combined oral contraceptives.
The discrepancy between US and international studies is possibly explained by the strong relative contraindication in the United States to the use of combined oral contraceptives in smokers over the age of 35 and the more prevalent use of high-dose pills in international studies. High-dose pills had been used in most of the stroke cases in the 1996 World Health Organization study16 but were used by only 0.7% of the women in the case and control groups in 2 pooled US studies from the same time period.18 Similarly, in these US studies, only 17% of the women were smokers on combined oral contraceptives, whereas in the international study, 51% of the women who had strokes and 38% of those in the control groups were smokers.
A large US study19 reviewing 3.6 million woman-years of use found no increased stroke risk (OR 0.96) in current users of low-dose combined oral contraceptives, results similar to those of a pooled analysis of US studies.18 Though this pooled analysis showed an adjusted increased risk of ischemic stroke in women reporting a history of migraine (OR 2.08, 95% CI 1.19–3.65), these conclusions were based on only 4 cases. The prevalence of migraine was identical in women who did or did not have strokes, 7.8% vs 7.7%, respectively, but the risk was judged to be increased after adjusting for other factors. But one important factor was not adjusted for: only 11 of the 1,017 women in the case and control groups were using 50-μg ethinyl estradiol pills, and 4 of the strokes were in this group of 11 women.
STROKE RISK INCREASES WITH FREQUENCY OF MIGRAINE AURA
Use of combined hormonal contraceptives in women who have migraine with aura remains controversial, based on good evidence that aura increases stroke risk20 and good evidence that high-dose oral contraceptives increase stroke risk.15
A cohort study encompassing more than 470,000 person-years with a median follow-up of 26 years found that while migraine without aura conferred no increase in risk of all-cause mortality, migraine with aura did.21
The longitudinal Women’s Health Study analyzed data from 27,798 women over age 45 and found that migraine with aura conferred an increased risk of cardiovascular disease (including stroke) that varied directly with aura frequency.22 Aura frequency less than once a month conferred a risk 2 times higher than in women without migraine, and the risk was more than 4 times higher when aura frequency exceeded once a week.
Similarly, an analysis of the World Health Organization study of stroke in young women found that the adjusted risk of ischemic stroke was significantly and directly associated with aura frequency.20
Potential explanations for this increased risk with greater aura frequency include changes induced during spreading cortical depression, shared genetic predispositions, and common underlying comorbidities such as patent foramen ovale.23–26
Though studies have shown that combined oral contraceptives in continuous regimens27 or in regimens that minimize drops in estrogen levels28 can help improve general headache and menstrual-related migraine, these studies have excluded patients who have migraine with aura.
In a pilot study,29 28 women referred to a tertiary headache clinic who had migraine with aura and intractable menstrual-related migraine were offered combined hormonal contraception in the form of a vaginal ring that releases only 15 μg ethinyl estradiol per 24 hours, thereby reducing peak estrogen exposure to a level lower than those encountered with the native menstrual cycle (with the suppression of ovulation). The women used this continuous ultra-low-dose hormonal contraception without placebo days. After a mean follow-up of 8 months, this regimen reduced aura frequency from a baseline average of 3.2 per month to only 0.2 per month. No woman had an increase in aura frequency, and menstrual-related migraine was eliminated in 21 (91.3%) of the 23 evaluable patients.
CHOOSING THE OPTIMAL CONTRACEPTIVE FORMULATION
Today, ultra-low-dose combined oral contraceptives (containing 10–15 µg of ethinyl estradiol) inhibit ovulation with doses of estrogen that are in a midphysiologic range. Consequently, they expose women to lower peak concentrations of estrogen than they would experience in their natural menstrual cycle (Figure 1). If a combined oral contraceptive is used in women with migraine with aura, lower estrogen doses (≤ 20 µg ethinyl estradiol) are preferred to decrease aura frequency and minimize the risk of stroke associated with high-dose ethinyl estradiol formulations.
Does the progestin matter?
Though there has been debate about whether different types of progestins alter the risk of venous thromboembolism,30,31 the chosen progestin does not seem to affect arterial risks such as stroke and myocardial infarction.14
All current guidelines note that progestin-only pills can be safely offered to women with migraine with aura. However, progestin-only pills have a shorter half-life than combined hormonal contraceptives and must be taken consistently and on time to ensure contraceptive efficacy and minimize abnormal bleeding. Patients who cannot adhere to a strict daily pill regimen may increase their risk of unintended pregnancy. In addition, progestin-only pills do not help with reducing episodes of migraine because they prevent ovulation only about half of the time.2 In contrast, a progestin-only arm implant is not only considered safe to use in women with migraine with aura, it may also prevent ovulation more reliably. Though progestin arm implants have the potential to reduce menstrual migraine and aura, this requires further study to confirm.
For menstrual-related migraine
In clinical practice, providers may offer certain combined hormonal contraceptives to women with debilitating menstrual-related migraine to prevent attacks. Although menstrual-related migraine rarely if ever is accompanied by aura, these patients may still have migraine with aura at other times of the month.
In women with menstrual-related migraine, any decrease in estrogen level greater than 10 µg of ethinyl estradiol may trigger an estrogen-withdrawal migraine. All currently available regimens of combined hormonal contraceptives that follow a 21-days-on, 7-days-off plan entail a drop in ethinyl estradiol of more than 10 µg (Figure 1).
Continuous regimens: Who needs a menstrual cycle anyway?
Of note: ultra-low-estrogen combined hormonal contraceptives that have placebo intervals may not inhibit ovulation consistently in all women.32 Contraceptive efficacy is still maintained, as contraception does not require inhibition of ovulation. Other mechanisms such as thickening of cervical mucus help with pregnancy prevention.
However, if ovulation is not inhibited, the consequent postovulatory decline in estrogen will continue to contribute to estrogen-withdrawal migraine.33,34 Reducing the number of placebo days may help inhibit ovulation. Adding back adequate estrogen during the placebo break (eg, either 0.9 mg conjugated equine estrogen with a 20-µg ethinyl estradiol combined oral contraceptive, or 0.075 mg transdermal 17B estradiol with a 15-µg combined hormonal contraceptive) can prevent these migraines.33,34
Some extended-cycle regimens, which give 4 withdrawal bleeds per year, will likewise prevent estrogen-withdrawal migraine if the decline in estrogen is limited to 10 µg (Table 1). Unfortunately, most extended regimens (Seasonale, Seasonique, and their generics) entail a 20- or 30-µg drop.
Continuous or extended-cycle regimens can be prescribed using any generic 20-µg combined hormonal contraceptive that the patient tolerates, along with specific instructions on the prescription to take the pills in a continuous fashion, eg, “Do not take the placebo pills; start the next pill pack immediately after 21 days.”
Postmenopausal hormone therapy
Neither smoking nor migraine is a contraindication to the use of postmenopausal hormone therapy, which is substantially lower in dosage than combined hormonal contraceptives.
ADVISING PATIENTS ON RISKS VS BENEFITS
It is important to remember that the risks of unintended pregnancy are always greater than the risks of any contraceptive, especially in women with chronic medical conditions, including those who have migraine with aura. Other benefits include the following:
Lower mortality risk. A 2010 analysis demonstrated that in nearly 46,000 women followed since 1968, those taking combined oral contraceptives had statistically significantly lower death rates from any cause and a lower risk of death from cancer and cardiovascular diseases than women who had never taken combined oral contraceptives.36
Stroke. Though the absolute risk of stroke to an individual woman taking a low-dose or ultra-low-dose combined hormonal contraceptive has been shown to be similar to that in women who are not taking combined hormonal contraception, its impact on an otherwise healthy woman could be devastating. Clinicians must remember that current guidelines still caution against prescribing combined hormonal contraceptives in women with migraine with aura and thus should counsel their patients accordingly and document the discussion in the medical record.
Noncontraceptive benefits. Women may be prescribed a combined hormonal contraceptive for benefits beyond contraception. The obvious reasons include beneficial effects on endometriosis, anemia, acne, hirsutism, dysmenorrhea, and prevention of ovarian cysts. But other important major benefits2 include substantial reductions in the risk of ovarian cancer (> 50% decrease after 10 years)37 and endometrial cancer (additional 24% reduction for each 5 years of use),38 and a modest decrease in the risk of colon cancer (37% less risk in ever-users).39 Further, combined oral contraceptive use has been associated with a decrease in mortality rates,40,41 with no increased risk of nonreproductive cancers.41
Ultra-low-dose, continuous formulations may benefit women by decreasing the frequency of migraine with aura and menstrual-related migraine. There is no evidence that reducing aura frequency also reduces stroke risk, but this represents an important area for future research.
WHAT WOULD WE DO?
For a patient who has a history of migraine with aura, if the goal is only to prevent pregnancy, we would recommend another contraceptive option that does not involve estrogen. However, we would consider prescribing a combined hormonal contraceptive in a low-dose regimen if the patient prefers this regimen for other health benefits (eg, acne control), if she has no other risk factors for stroke, and if she gives her informed consent after a discussion of the risks and benefits. Women who have menstrual-related migraine refractory to or who cannot tolerate other migraine therapies are often willing to try a low-dose estrogen-containing contraceptive for control of their migraine, especially if they have tried it in the past and believe that it helped prevent migraine. Patients should have follow-up within 3 months to discuss whether they have benefited from the regimen in terms of headache frequency or severity.
Combined hormonal contraceptives are contraindicated in women who have migraine with aura because they pose a risk of stroke. But how great is the risk, and how strong is the evidence, particularly with today’s low-dose contraceptives? Can we view migraine with aura as a relative contraindication rather than an absolute one?
This article reviews migraine diagnosis, the effects of estrogen and the menstrual cycle on migraine, the evidence of stroke risk with combined hormonal contraceptive use, and how the frequency of aura may affect risk. It offers practical advice on choosing contraceptive formulations and counseling patients on risks and benefits.
WHAT THE GUIDELINES SAY
Current guidelines restrict the use of combined hormonal contraceptives in the setting of migraine with aura, but not in migraine without aura.
A practice bulletin from the American College of Obstetrics and Gynecology in 2010 noted that extended-cycle or continuous hormonal contraceptives, including oral and parenteral products, might provide relief of migraines by eliminating the drops in estrogen levels that precipitate them.1 However, the bulletin also cautioned that though cerebrovascular accidents in women are rare, the impact of a stroke is so devastating that clinicians should consider intrauterine devices, progestin-only options, and other nonestrogen methods in women who have migraine with focal neurologic signs, women who smoke, and women age 35 or older.1
In 2016, the US Centers for Disease Control and Prevention published updates to its medical eligibility criteria for contraceptive use in various medical conditions. In the case of migraine without aura, the guidelines note no limitation to the use of combined hormonal contraceptives, regardless of the patient’s age. In the case of migraine with aura, the consensus was that the risk associated with combined hormonal contraception typically outweighs its benefits, noting “an unacceptable health risk if the contraceptive method is used.”2
We believe a fresh look at the data is warranted.
EARLY ORAL CONTRACEPTIVES WERE ALL HIGH-DOSE
This issue first surfaced in the decade and a half after the initial launch of oral contraceptives in 1960. The products then were all high-dose pills, containing up to 150 µg of mestranol. In subsequent decades, the dose of estrogen was successively reduced, so that now some pills contain only 10 µg of ethinyl estradiol. High-dose pills—which today contain 50 µg of ethinyl estradiol—account for less than 1% of pills currently sold in the United States and have been eliminated in many countries.
DIAGNOSTIC CRITERIA FOR MIGRAINE
According to the International Classification of Headache Disorders (ICHD),3 the diagnosis of migraine requires 2 of the 4 following criteria:
- Unilateral location
- Pulsating or throbbing pain
- Pain of at least moderate intensity
- Pain aggravated by activity, or causing a preference to avoid activity.
An additional criterion is either nausea or a combination of photophobia and phonophobia with the episode. This criterion can be met if the patient prefers to avoid bright lights and loud noises during an attack.
Headache experts have suggested that patients with a stable pattern of episodic, disabling headache and normal findings on physical examination should be considered to have migraine if there is no contradictory evidence.4,5
Migraine with aura requires at least 2 of the following 4 characteristics3:
- 1 aura symptom, spreading gradually over 5 minutes, or 2 or more aura symptoms occurring in succession, or both
- Each aura symptom lasting 5 to 60 minutes (not “a few seconds,” not “hours”)
- The aura followed by the onset of headache within 60 minutes
- At least 1 aura symptom is unilateral.
Visual blurring, floaters, or split-second flashes before or during a migraine headache do not meet the criteria for aura.
MIGRAINE IS COMMON AND UNDERRECOGNIZED
In a study of 1,203 patients seeking care from a primary care provider for headache,6 94% of the 377 who turned in a diary with enough data to make a diagnosis were diagnosed with a migraine or probable migraine by an expert panel. A quarter of patients who likely had migraine based on an expert review of symptoms did not receive a migraine diagnosis at the time of their office visit.
Similarly, in a large epidemiologic study,7 30,758 adults were asked if they had headaches and, if so, how they named them. Headaches were reported by 23,564 of the participants and were subsequently diagnosed by formal ICHD criteria. Of the 3,074 individuals who met the criteria for migraine, only 53.4% correctly recognized their headaches as migraine. The most common erroneous labels were “sinus headache” and “stress headache.”7
HOW ESTROGEN AFFECTS MIGRAINE
Of note, migraine can be exacerbated during times of cycle irregularity, such as adolescence and perimenopause, the 2 times during a woman’s life associated with the highest risk of unintended pregnancy.10,11
STROKE RISK: ESTROGEN DOSE MATTERS
Shortly after the first combined oral contraceptives were released, reports of adverse events began to appear, although serious events were relatively rare. In response, prescribing guidelines advised against giving oral contraceptives to women with a history of deep vein thrombosis, myocardial infarction, stroke, or hypertension. Also, over the years, the hormonal content of the formulations was successively reduced, and with each reduction in estrogen, a decrease was observed in venous thrombosis and pulmonary embolism.12,13 Current low-dose formulations are considerably safer than high-dose options but are not entirely without risk.14
Stroke risk with combined oral contraceptives was first highlighted in a landmark article in 1975.15 However, the authors were unable to correlate the risk with the estrogen concentration of the pill, since 23 of the 25 women who suffered thrombotic stroke while taking the mestranol-containing formulation took 100-μg pills, and all 20 women who had strokes while taking the ethinyl estradiol formulation took 50-μg pills. Thus, by today’s standards, they were all taking high-dose pills. The risk of thrombotic stroke was 4 to 5 times higher in users than in nonusers.
In 1996, a study from the World Health Organization16 reported an increased risk of stroke with high-dose combined oral contraceptives (odds ratio [OR] 5.30, 95% confidence interval [CI] 2.56–11.0). With preparations containing less than 50 μg of ethinyl estradiol, the risk was not statistically significant (OR 1.53, 95% CI 0.71–3.31). These numbers were for Europe only; in developing countries, the risk was elevated regardless of dose, presumably due to additional risk factors in combined oral contraceptive users. The majority of strokes were in smokers taking 50-μg pills, with an average age greater than 35.
In 2002, a 5-year case-control study in Denmark found that the risk of stroke with combined oral contraceptives correlated directly with the estrogen content, from no increased risk with the newest and lowest-dose formulation (containing ethinyl estradiol 20 µg) to an OR of 4.5 with the older high-dose (50 µg) formulations.17
Reassuringly, a 2012 retrospective review of the Danish national registry13 revealed a low absolute risk of arterial events in users of combined oral contraceptives: 21.4 per 100,000 person-years for thrombotic stroke, and 10.1 per 100,000 person-years for myocardial infarction. Further, these risks were substantially lower with 20-μg ethinyl estradiol products than with those containing 30 to 40 μg.13 An important limitation of this large database review is that it did not control for important stroke risk factors such as obesity and smoking.
Although international studies14,16 continue to show a small but increased risk, more than 30 years have passed since a US study found an increased risk of stroke with combined oral contraceptives.
The discrepancy between US and international studies is possibly explained by the strong relative contraindication in the United States to the use of combined oral contraceptives in smokers over the age of 35 and the more prevalent use of high-dose pills in international studies. High-dose pills had been used in most of the stroke cases in the 1996 World Health Organization study16 but were used by only 0.7% of the women in the case and control groups in 2 pooled US studies from the same time period.18 Similarly, in these US studies, only 17% of the women were smokers on combined oral contraceptives, whereas in the international study, 51% of the women who had strokes and 38% of those in the control groups were smokers.
A large US study19 reviewing 3.6 million woman-years of use found no increased stroke risk (OR 0.96) in current users of low-dose combined oral contraceptives, results similar to those of a pooled analysis of US studies.18 Though this pooled analysis showed an adjusted increased risk of ischemic stroke in women reporting a history of migraine (OR 2.08, 95% CI 1.19–3.65), these conclusions were based on only 4 cases. The prevalence of migraine was identical in women who did or did not have strokes, 7.8% vs 7.7%, respectively, but the risk was judged to be increased after adjusting for other factors. But one important factor was not adjusted for: only 11 of the 1,017 women in the case and control groups were using 50-μg ethinyl estradiol pills, and 4 of the strokes were in this group of 11 women.
STROKE RISK INCREASES WITH FREQUENCY OF MIGRAINE AURA
Use of combined hormonal contraceptives in women who have migraine with aura remains controversial, based on good evidence that aura increases stroke risk20 and good evidence that high-dose oral contraceptives increase stroke risk.15
A cohort study encompassing more than 470,000 person-years with a median follow-up of 26 years found that while migraine without aura conferred no increase in risk of all-cause mortality, migraine with aura did.21
The longitudinal Women’s Health Study analyzed data from 27,798 women over age 45 and found that migraine with aura conferred an increased risk of cardiovascular disease (including stroke) that varied directly with aura frequency.22 Aura frequency less than once a month conferred a risk 2 times higher than in women without migraine, and the risk was more than 4 times higher when aura frequency exceeded once a week.
Similarly, an analysis of the World Health Organization study of stroke in young women found that the adjusted risk of ischemic stroke was significantly and directly associated with aura frequency.20
Potential explanations for this increased risk with greater aura frequency include changes induced during spreading cortical depression, shared genetic predispositions, and common underlying comorbidities such as patent foramen ovale.23–26
Though studies have shown that combined oral contraceptives in continuous regimens27 or in regimens that minimize drops in estrogen levels28 can help improve general headache and menstrual-related migraine, these studies have excluded patients who have migraine with aura.
In a pilot study,29 28 women referred to a tertiary headache clinic who had migraine with aura and intractable menstrual-related migraine were offered combined hormonal contraception in the form of a vaginal ring that releases only 15 μg ethinyl estradiol per 24 hours, thereby reducing peak estrogen exposure to a level lower than those encountered with the native menstrual cycle (with the suppression of ovulation). The women used this continuous ultra-low-dose hormonal contraception without placebo days. After a mean follow-up of 8 months, this regimen reduced aura frequency from a baseline average of 3.2 per month to only 0.2 per month. No woman had an increase in aura frequency, and menstrual-related migraine was eliminated in 21 (91.3%) of the 23 evaluable patients.
CHOOSING THE OPTIMAL CONTRACEPTIVE FORMULATION
Today, ultra-low-dose combined oral contraceptives (containing 10–15 µg of ethinyl estradiol) inhibit ovulation with doses of estrogen that are in a midphysiologic range. Consequently, they expose women to lower peak concentrations of estrogen than they would experience in their natural menstrual cycle (Figure 1). If a combined oral contraceptive is used in women with migraine with aura, lower estrogen doses (≤ 20 µg ethinyl estradiol) are preferred to decrease aura frequency and minimize the risk of stroke associated with high-dose ethinyl estradiol formulations.
Does the progestin matter?
Though there has been debate about whether different types of progestins alter the risk of venous thromboembolism,30,31 the chosen progestin does not seem to affect arterial risks such as stroke and myocardial infarction.14
All current guidelines note that progestin-only pills can be safely offered to women with migraine with aura. However, progestin-only pills have a shorter half-life than combined hormonal contraceptives and must be taken consistently and on time to ensure contraceptive efficacy and minimize abnormal bleeding. Patients who cannot adhere to a strict daily pill regimen may increase their risk of unintended pregnancy. In addition, progestin-only pills do not help with reducing episodes of migraine because they prevent ovulation only about half of the time.2 In contrast, a progestin-only arm implant is not only considered safe to use in women with migraine with aura, it may also prevent ovulation more reliably. Though progestin arm implants have the potential to reduce menstrual migraine and aura, this requires further study to confirm.
For menstrual-related migraine
In clinical practice, providers may offer certain combined hormonal contraceptives to women with debilitating menstrual-related migraine to prevent attacks. Although menstrual-related migraine rarely if ever is accompanied by aura, these patients may still have migraine with aura at other times of the month.
In women with menstrual-related migraine, any decrease in estrogen level greater than 10 µg of ethinyl estradiol may trigger an estrogen-withdrawal migraine. All currently available regimens of combined hormonal contraceptives that follow a 21-days-on, 7-days-off plan entail a drop in ethinyl estradiol of more than 10 µg (Figure 1).
Continuous regimens: Who needs a menstrual cycle anyway?
Of note: ultra-low-estrogen combined hormonal contraceptives that have placebo intervals may not inhibit ovulation consistently in all women.32 Contraceptive efficacy is still maintained, as contraception does not require inhibition of ovulation. Other mechanisms such as thickening of cervical mucus help with pregnancy prevention.
However, if ovulation is not inhibited, the consequent postovulatory decline in estrogen will continue to contribute to estrogen-withdrawal migraine.33,34 Reducing the number of placebo days may help inhibit ovulation. Adding back adequate estrogen during the placebo break (eg, either 0.9 mg conjugated equine estrogen with a 20-µg ethinyl estradiol combined oral contraceptive, or 0.075 mg transdermal 17B estradiol with a 15-µg combined hormonal contraceptive) can prevent these migraines.33,34
Some extended-cycle regimens, which give 4 withdrawal bleeds per year, will likewise prevent estrogen-withdrawal migraine if the decline in estrogen is limited to 10 µg (Table 1). Unfortunately, most extended regimens (Seasonale, Seasonique, and their generics) entail a 20- or 30-µg drop.
Continuous or extended-cycle regimens can be prescribed using any generic 20-µg combined hormonal contraceptive that the patient tolerates, along with specific instructions on the prescription to take the pills in a continuous fashion, eg, “Do not take the placebo pills; start the next pill pack immediately after 21 days.”
Postmenopausal hormone therapy
Neither smoking nor migraine is a contraindication to the use of postmenopausal hormone therapy, which is substantially lower in dosage than combined hormonal contraceptives.
ADVISING PATIENTS ON RISKS VS BENEFITS
It is important to remember that the risks of unintended pregnancy are always greater than the risks of any contraceptive, especially in women with chronic medical conditions, including those who have migraine with aura. Other benefits include the following:
Lower mortality risk. A 2010 analysis demonstrated that in nearly 46,000 women followed since 1968, those taking combined oral contraceptives had statistically significantly lower death rates from any cause and a lower risk of death from cancer and cardiovascular diseases than women who had never taken combined oral contraceptives.36
Stroke. Though the absolute risk of stroke to an individual woman taking a low-dose or ultra-low-dose combined hormonal contraceptive has been shown to be similar to that in women who are not taking combined hormonal contraception, its impact on an otherwise healthy woman could be devastating. Clinicians must remember that current guidelines still caution against prescribing combined hormonal contraceptives in women with migraine with aura and thus should counsel their patients accordingly and document the discussion in the medical record.
Noncontraceptive benefits. Women may be prescribed a combined hormonal contraceptive for benefits beyond contraception. The obvious reasons include beneficial effects on endometriosis, anemia, acne, hirsutism, dysmenorrhea, and prevention of ovarian cysts. But other important major benefits2 include substantial reductions in the risk of ovarian cancer (> 50% decrease after 10 years)37 and endometrial cancer (additional 24% reduction for each 5 years of use),38 and a modest decrease in the risk of colon cancer (37% less risk in ever-users).39 Further, combined oral contraceptive use has been associated with a decrease in mortality rates,40,41 with no increased risk of nonreproductive cancers.41
Ultra-low-dose, continuous formulations may benefit women by decreasing the frequency of migraine with aura and menstrual-related migraine. There is no evidence that reducing aura frequency also reduces stroke risk, but this represents an important area for future research.
WHAT WOULD WE DO?
For a patient who has a history of migraine with aura, if the goal is only to prevent pregnancy, we would recommend another contraceptive option that does not involve estrogen. However, we would consider prescribing a combined hormonal contraceptive in a low-dose regimen if the patient prefers this regimen for other health benefits (eg, acne control), if she has no other risk factors for stroke, and if she gives her informed consent after a discussion of the risks and benefits. Women who have menstrual-related migraine refractory to or who cannot tolerate other migraine therapies are often willing to try a low-dose estrogen-containing contraceptive for control of their migraine, especially if they have tried it in the past and believe that it helped prevent migraine. Patients should have follow-up within 3 months to discuss whether they have benefited from the regimen in terms of headache frequency or severity.
- ACOG Practice Bulletin No. 110: noncontraceptive uses of hormonal contraceptives. Obstet Gynecol 2010; 115:206–218.
- Centers for Disease Control and Prevention. US Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recommendations and reports: Morbidity and mortality weekly report Recommendations and reports/Centers for Disease Control 2016; 65:1–104.
- Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition (beta version). Cephalalgia 2013; 33:629–808.
- Lipton RB, Cady RK, Stewart WF, Wilks K, Hall C. Diagnostic lessons from the Spectrum study. Neurology 2002; 58(suppl 6):S27–S31.
- Lipton RB, Stewart WF, Cady R, et al. 2000 Wolfe Award. Sumatriptan for the range of headaches in migraine sufferers: results of the Spectrum Study. Headache 2000; 40:783–791.
- Tepper SJ, Dahlof CG, Dowson A, et al. Prevalence and diagnosis of migraine in patients consulting their physician with a complaint of headache: data from the Landmark Study. Headache 2004; 44:856–864.
- Lipton RB, Stewart WF, Liberman JN. Self-awareness of migraine: interpreting the labels that headache sufferers apply to their headaches. Neurology 2002; 58(suppl 6):S21–S26.
- Chai NC, Peterlin BL, Calhoun AH. Migraine and estrogen. Curr Opin Neurol 2014; 27:315–324.
- Calhoun AH. Menstrual migraine: update on pathophysiology and approach to therapy and management. Curr Treat Options Neurol 2012; 14:1–14.
- McNamara M, Batur P, DeSapri KT. In the clinic. Perimenopause. Ann Intern Med 2015; 162:ITC1–ITC15.
- O’Brien HL, Cohen JM. Young adults with headaches: the transition from adolescents to adults. Headache 2015; 55:1404–1409.
- Vessey M, Mant D, Smith A, Yeates D. Oral contraceptives and venous thromboembolism: findings in a large prospective study. Br Med J (Clin Res Ed) 1986; 292:526.
- Lidegaard O, Lokkegaard E, Jensen A, Skovlund CW, Keiding N. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med 2012; 366:2257–2266.
- MacGregor EA. Contraception and headache. Headache 2013; 53:247–276.
- Oral contraceptives and stroke in young women. Associated risk factors. JAMA 1975; 231:718–722.
- Ischaemic stroke and combined oral contraceptives: results of an international, multicentre, case-control study. WHO Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Lancet 1996; 348:498–505.
- Lidegaard O, Kreiner S. Contraceptives and cerebral thrombosis: a five-year national case-control study. Contraception 2002; 65:197–205.
- Schwartz SM, Petitti DB, Siscovick DS, et al. Stroke and use of low-dose oral contraceptives in young women: a pooled analysis of two US studies. Stroke 1998; 29:2277–2284.
- Petitti DB, Sidney S, Bernstein A, Wolf S, Quesenberry C, Ziel HK. Stroke in users of low-dose oral contraceptives. N Engl J Med 1996; 335:8–15.
- Donaghy M, Chang CL, Poulter N; European Collaborators of the World Health Organisation Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Duration, frequency, recency, and type of migraine and the risk of ischaemic stroke in women of childbearing age. J Neurol Neurosurg Psychiatry 2002; 73:747–750.
- Gudmundsson LS, Scher AI, Aspelund T, et al. Migraine with aura and risk of cardiovascular and all cause mortality in men and women: prospective cohort study. BMJ 2010; 341:c3966.
- Kurth T, Slomke MA, Kase CS, et al. Migraine, headache, and the risk of stroke in women: a prospective study. Neurology 2005; 64:1020–1026.
- Lee ST, Chu K, Jung KH, et al. Decreased number and function of endothelial progenitor cells in patients with migraine. Neurology 2008; 70:1510–1517.
- Kunz GA, Liang G, Cuculi F, et al. Circulating endothelial progenitor cells predict coronary artery disease severity. Am Heart J 2006; 152:190–195.
- Kurth T, Gaziano JM, Cook NR, Logroscino G, Diener HC, Buring JE. Migraine and risk of cardiovascular disease in women. JAMA 2006; 296:283–291.
- Pezzini A, Del Zotto E, Giossi A, Volonghi I, Grassi M, Padovani A. The migraine-ischemic stroke connection: potential pathogenic mechanisms. Curr Mol Med 2009; 9:215–226.
- Sulak P, Willis S, Kuehl T, Coffee A, Clark J. Headaches and oral contraceptives: impact of eliminating the standard 7-day placebo interval. Headache 2007; 47:27–37.
- Nappi RE, Terreno E, Sances G, et al. Effect of a contraceptive pill containing estradiol valerate and dienogest (E2V/DNG) in women with menstrually-related migraine (MRM). Contraception 2013; 88:369–375.
- Calhoun A, Ford S, Pruitt A. The impact of extended-cycle vaginal ring contraception on migraine aura: a retrospective case series. Headache 2012; 52:1246–1253.
- Wu CQ, Grandi SM, Filion KB, Abenhaim HA, Joseph L, Eisenberg MJ. Drospirenone-containing oral contraceptive pills and the risk of venous and arterial thrombosis: a systematic review. BJOG 2013; 120:801–810.
- Dinger J, Bardenheuer K, Heinemann K. Cardiovascular and general safety of a 24-day regimen of drospirenone-containing combined oral contraceptives: final results from the International Active Surveillance Study of Women Taking Oral Contraceptives. Contraception 2014; 89:253–263.
- Benson LS, Micks EA. Why stop now? Extended and continuous regimens of combined hormonal contraceptive methods. Obstet Gynecol Clin North Am 2015; 42:669–681.
- Mannix LK, Calhoun AH. Menstrual migraine. Curr Treat Options Neurol 2004; 6:489–498.
- Calhoun AH. A novel specific prophylaxis for menstrual-associated migraine. South Med J 2004; 97:819–822.
- Calhoun AH. Current topics and controversies in menstrual migraine. Headache 2012; 52(suppl 1):8–11.
- Hannaford PC, Iversen L, Macfarlane TV, Elliott AM, Angus V, Lee AJ. Mortality among contraceptive pill users: cohort evidence from Royal College of General Practitioners’ Oral Contraception Study. BMJ 2010; 340:c927.
- Havrilesky LJ, Moorman PG, Lowery WJ, et al. Oral contraceptive pills as primary prevention for ovarian cancer: a systematic review and meta-analysis. Obstet Gynecol 2013; 122:139 -147.
- Collaborative Group on Epidemiological Studies on Endometrial Cancer. Endometrial cancer and oral contraceptives: an individual participant meta-analysis of 27,276 women with endometrial cancer from 36 epidemiological studies. Lancet Oncol 2015; 16:1061–1070.
- Fernandez E, La Vecchia C, Franceschi S, et al. Oral contraceptive use and risk of colorectal cancer. Epidemiology 1998; 9:295–300.
- Merritt MA, Riboli E, Murphy N, et al. Reproductive factors and risk of mortality in the European Prospective Investigation into Cancer and Nutrition; a cohort study. BMC Med 2015; 13:252.
- Vessey M, Yeates D. Oral contraceptive use and cancer: final report from the Oxford-Family Planning Association Contraceptive Study. Contraception 2013; 88:678–683.
- ACOG Practice Bulletin No. 110: noncontraceptive uses of hormonal contraceptives. Obstet Gynecol 2010; 115:206–218.
- Centers for Disease Control and Prevention. US Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recommendations and reports: Morbidity and mortality weekly report Recommendations and reports/Centers for Disease Control 2016; 65:1–104.
- Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition (beta version). Cephalalgia 2013; 33:629–808.
- Lipton RB, Cady RK, Stewart WF, Wilks K, Hall C. Diagnostic lessons from the Spectrum study. Neurology 2002; 58(suppl 6):S27–S31.
- Lipton RB, Stewart WF, Cady R, et al. 2000 Wolfe Award. Sumatriptan for the range of headaches in migraine sufferers: results of the Spectrum Study. Headache 2000; 40:783–791.
- Tepper SJ, Dahlof CG, Dowson A, et al. Prevalence and diagnosis of migraine in patients consulting their physician with a complaint of headache: data from the Landmark Study. Headache 2004; 44:856–864.
- Lipton RB, Stewart WF, Liberman JN. Self-awareness of migraine: interpreting the labels that headache sufferers apply to their headaches. Neurology 2002; 58(suppl 6):S21–S26.
- Chai NC, Peterlin BL, Calhoun AH. Migraine and estrogen. Curr Opin Neurol 2014; 27:315–324.
- Calhoun AH. Menstrual migraine: update on pathophysiology and approach to therapy and management. Curr Treat Options Neurol 2012; 14:1–14.
- McNamara M, Batur P, DeSapri KT. In the clinic. Perimenopause. Ann Intern Med 2015; 162:ITC1–ITC15.
- O’Brien HL, Cohen JM. Young adults with headaches: the transition from adolescents to adults. Headache 2015; 55:1404–1409.
- Vessey M, Mant D, Smith A, Yeates D. Oral contraceptives and venous thromboembolism: findings in a large prospective study. Br Med J (Clin Res Ed) 1986; 292:526.
- Lidegaard O, Lokkegaard E, Jensen A, Skovlund CW, Keiding N. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med 2012; 366:2257–2266.
- MacGregor EA. Contraception and headache. Headache 2013; 53:247–276.
- Oral contraceptives and stroke in young women. Associated risk factors. JAMA 1975; 231:718–722.
- Ischaemic stroke and combined oral contraceptives: results of an international, multicentre, case-control study. WHO Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Lancet 1996; 348:498–505.
- Lidegaard O, Kreiner S. Contraceptives and cerebral thrombosis: a five-year national case-control study. Contraception 2002; 65:197–205.
- Schwartz SM, Petitti DB, Siscovick DS, et al. Stroke and use of low-dose oral contraceptives in young women: a pooled analysis of two US studies. Stroke 1998; 29:2277–2284.
- Petitti DB, Sidney S, Bernstein A, Wolf S, Quesenberry C, Ziel HK. Stroke in users of low-dose oral contraceptives. N Engl J Med 1996; 335:8–15.
- Donaghy M, Chang CL, Poulter N; European Collaborators of the World Health Organisation Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Duration, frequency, recency, and type of migraine and the risk of ischaemic stroke in women of childbearing age. J Neurol Neurosurg Psychiatry 2002; 73:747–750.
- Gudmundsson LS, Scher AI, Aspelund T, et al. Migraine with aura and risk of cardiovascular and all cause mortality in men and women: prospective cohort study. BMJ 2010; 341:c3966.
- Kurth T, Slomke MA, Kase CS, et al. Migraine, headache, and the risk of stroke in women: a prospective study. Neurology 2005; 64:1020–1026.
- Lee ST, Chu K, Jung KH, et al. Decreased number and function of endothelial progenitor cells in patients with migraine. Neurology 2008; 70:1510–1517.
- Kunz GA, Liang G, Cuculi F, et al. Circulating endothelial progenitor cells predict coronary artery disease severity. Am Heart J 2006; 152:190–195.
- Kurth T, Gaziano JM, Cook NR, Logroscino G, Diener HC, Buring JE. Migraine and risk of cardiovascular disease in women. JAMA 2006; 296:283–291.
- Pezzini A, Del Zotto E, Giossi A, Volonghi I, Grassi M, Padovani A. The migraine-ischemic stroke connection: potential pathogenic mechanisms. Curr Mol Med 2009; 9:215–226.
- Sulak P, Willis S, Kuehl T, Coffee A, Clark J. Headaches and oral contraceptives: impact of eliminating the standard 7-day placebo interval. Headache 2007; 47:27–37.
- Nappi RE, Terreno E, Sances G, et al. Effect of a contraceptive pill containing estradiol valerate and dienogest (E2V/DNG) in women with menstrually-related migraine (MRM). Contraception 2013; 88:369–375.
- Calhoun A, Ford S, Pruitt A. The impact of extended-cycle vaginal ring contraception on migraine aura: a retrospective case series. Headache 2012; 52:1246–1253.
- Wu CQ, Grandi SM, Filion KB, Abenhaim HA, Joseph L, Eisenberg MJ. Drospirenone-containing oral contraceptive pills and the risk of venous and arterial thrombosis: a systematic review. BJOG 2013; 120:801–810.
- Dinger J, Bardenheuer K, Heinemann K. Cardiovascular and general safety of a 24-day regimen of drospirenone-containing combined oral contraceptives: final results from the International Active Surveillance Study of Women Taking Oral Contraceptives. Contraception 2014; 89:253–263.
- Benson LS, Micks EA. Why stop now? Extended and continuous regimens of combined hormonal contraceptive methods. Obstet Gynecol Clin North Am 2015; 42:669–681.
- Mannix LK, Calhoun AH. Menstrual migraine. Curr Treat Options Neurol 2004; 6:489–498.
- Calhoun AH. A novel specific prophylaxis for menstrual-associated migraine. South Med J 2004; 97:819–822.
- Calhoun AH. Current topics and controversies in menstrual migraine. Headache 2012; 52(suppl 1):8–11.
- Hannaford PC, Iversen L, Macfarlane TV, Elliott AM, Angus V, Lee AJ. Mortality among contraceptive pill users: cohort evidence from Royal College of General Practitioners’ Oral Contraception Study. BMJ 2010; 340:c927.
- Havrilesky LJ, Moorman PG, Lowery WJ, et al. Oral contraceptive pills as primary prevention for ovarian cancer: a systematic review and meta-analysis. Obstet Gynecol 2013; 122:139 -147.
- Collaborative Group on Epidemiological Studies on Endometrial Cancer. Endometrial cancer and oral contraceptives: an individual participant meta-analysis of 27,276 women with endometrial cancer from 36 epidemiological studies. Lancet Oncol 2015; 16:1061–1070.
- Fernandez E, La Vecchia C, Franceschi S, et al. Oral contraceptive use and risk of colorectal cancer. Epidemiology 1998; 9:295–300.
- Merritt MA, Riboli E, Murphy N, et al. Reproductive factors and risk of mortality in the European Prospective Investigation into Cancer and Nutrition; a cohort study. BMC Med 2015; 13:252.
- Vessey M, Yeates D. Oral contraceptive use and cancer: final report from the Oxford-Family Planning Association Contraceptive Study. Contraception 2013; 88:678–683.
KEY POINTS
- There is no restriction on the use of combined hormonal contraceptives by women with migraine without aura, and the risk vs benefit for women with aura is debatable.
- Migraine with aura—but not migraine without aura—is associated with a twofold increased risk of ischemic stroke, although the absolute risk is small in healthy women who do not smoke.
- Combined hormonal contraceptives are associated with ischemic stroke, but the risk is dose-dependent. Ultra-low-dose formulations (containing ≤ 20 μg of ethinyl estradiol) do not pose an increased risk of stroke in healthy nonsmokers.
Delirium in hospitalized patients: Risks and benefits of antipsychotics
Delirium is common in hospitalized patients and contributes to healthcare costs and poor patient outcomes, including death. Its diagnosis and management remain clinically challenging. Although consensus panel guidelines recommend antipsychotic medications to treat delirium when conservative measures fail, few head-to-head trials have been done to tell us which antipsychotic drug to select, and antipsychotic use poses risks in the elderly.
Here, we review the risks and benefits of using antipsychotic drugs to manage delirium and describe an approach to selecting and using 5 commonly used antipsychotics.
SCOPE OF THE PROBLEM
Delirium is common and serious, affecting 11% to 42% of patients hospitalized on general medical wards.1 The burden to the public and individual patient is extremely high. Delirium has been found to result in an additional $16,303 to $64,421 per delirious patient per year, with a subsequent total 1-year health-attributable cost between $38 billion and $152 billion in the United States.2 Furthermore, many patients who become delirious in the hospital lose their independence and are placed in long-term care facilities.3
Although delirium was originally thought to be a time-limited neurocognitive disorder, recent evidence shows that it persists much longer4 and that some patients never return to their previous level of function, suggesting that a single episode of delirium can significantly alter the course of an underlying dementia with the dramatic initiation of cognitive decline.3 Most alarmingly, delirium is associated with an increased rate of death.1
DSM-5 DEFINITION
According to the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5),5 delirium is a neurocognitive disorder characterized by the acute onset of disturbance in attention, awareness, and cognition that fluctuates in severity throughout the day and is the direct physiologic consequence of another medical condition. The cognitive impairment seen in delirium is typically global and can affect memory, orientation, language, visuospatial ability, and perception. Other prominent features include psychomotor disturbance, sleep-cycle derangement, and emotional lability.
The pathogenesis of delirium is not clearly delineated but may relate to cholinergic deficiency and dopaminergic excess.
THE FIRST STEPS: NONPHARMACOLOGIC MANAGEMENT
Inouye3 outlined a general 3-part approach to managing delirium:
Identify and address predisposing factors. All patients found to have an acute change in mental status should be evaluated for the underlying cause, with special attention to the most common causes, ie, infection, metabolic derangement, and substance intoxication and withdrawal. A thorough medication reconciliation should also be done to identify medications with psychoactive or anticholinergic effects.
Provide supportive care, eg, addressing volume and nutritional status, mobilizing the patient early, and giving prophylaxis against deep venous thrombosis.
Manage symptoms. Behavioral strategies should be instituted in every delirious patient and should include frequent reorientation, use of observers, encouragement of family involvement, avoidance of physical restraints and Foley catheters, use of vision and hearing aids, and normalizing the sleep-wake cycle.
ANTIPSYCHOTICS: ARE THEY SAFE AND EFFECTIVE?
The US Food and Drug Administration (FDA) has not approved any medications for delirium. However, multiple consensus statements, including those by the American Psychiatric Association,6 the Canadian Coalition for Seniors’ Mental Health,7 and the UK National Institute for Health and Care Excellence,8 advocate for psychopharmacologic management of delirium symptoms in the following situations:
- The patient is in significant distress from his or her symptoms
- The patient poses a safety risk to self or others
- The patient is impeding essential aspects of his or her medical care.
Guidelines from these organizations recommend antipsychotic medications as the first-line drugs for managing delirium symptoms not caused by substance withdrawal. Nevertheless, the use of antipsychotics in the management of delirium remains controversial. While a number of studies suggest these drugs are beneficial,9–11 others do not.12 These consensus panels advocate for the judicious use of antipsychotics, limited to the specific situations outlined above.
The use of antipsychotics in elderly and medically complex patients poses risks. One of the most significant safety concerns is increased risk of death due to adverse cardiac events caused by prolongation of the QT interval.
Antipsychotics, QT prolongation, and torsades de pointes
Most antipsychotics have the potential to prolong the time of ventricular depolarization and repolarization and the QT interval to some extent, which can lead to torsades de pointes.13 Other risk factors for prolonged QT interval and torsades de pointes include:
- Long QT syndrome (a genetic arrhythmia)
- Female sex
- Old age
- Electrolyte abnormalities (hypokalemia, hypocalcemia, hypomagnesemia)
- Preexisting heart conditions such as bradycardia, left ventricular dysfunction, heart failure, mitral valve prolapse, and previous myocardial infarction
- Medical conditions that cause electrolyte derangements
- Medications, including antiarrhythmics, antibiotics (macrolides, quinolones), antifungals, antimalarials, antiemetics, some opioids (methadone), and most antipsychotics.
Haloperidol. Postmarketing analysis in 2007 found 73 cases of haloperidol-related torsades de pointes. However, many of these were confounded by other QT-prolonging medications and medical conditions.14
The QT-prolonging effect of haloperidol administered orally or intramuscularly is actually quite small. The equivalent oral dose of 15 mg of haloperidol (assuming 50% bioavailability) given orally or intramuscularly increases the corrected QT interval (QTc) by only 7 to 8 milliseconds. But intravenous haloperidol can cause much more significant QT prolongation: 8 of the 11 reported cases of fatal torsades de pointes occurred when haloperidol was given intravenously.14 Therefore, the FDA recommends cardiac monitoring for all patients receiving intravenous haloperidol.
Oral olanzapine, risperidone, and quetiapine prolong the QT interval approximately as much as oral haloperidol.
Aripiprazole has not been associated with significant QT prolongation.13
Atypical antipsychotics and stroke
The FDA has issued multiple warnings for prescribing antipsychotic medications in the elderly. In 2003, it warned prescribers of increased cerebrovascular adverse events, including stroke, in elderly patients with dementia who were treated with an atypical antipsychotic (risperidone, olanzapine, or aripiprazole) vs placebo.15
Atypical antipsychotics and risk of death
In 2005, the FDA issued a black-box warning about increased all-cause mortality risk in patients with dementia treated with atypical antipsychotics for behavioral disturbance (relative risk 1.6–1.7).16
This warning was likely based on a meta-analysis by Schneider et al17 of trials in which patients with dementia were randomized to receive either an atypical antipsychotic or placebo. The death rate was 3.5% in patients treated with an atypical antipsychotic vs 2.3% in patients treated with placebo, indicating a number needed to harm of 100. The most common causes of death were cardiovascular disease and pneumonia. However, the trials in this meta-analysis included only patients who were prescribed atypical antipsychotics for ongoing management of behavioral disturbances due to dementia in either the outpatient or nursing home setting. None of the trials looked at patients who were prescribed atypical antipsychotics for a limited time in a closely monitored inpatient setting.
Effectiveness of antipsychotics
While several studies since the FDA black-box warning have shown that antipsychotics are safe, the efficacy of these drugs in delirium management remains controversial.
In a 2016 meta-analysis, Kishi et al18 found that antipsychotics were superior to placebo in terms of response rate (defined as improvement of delirium severity rating scores), with a number needed to treat of 2.
In contrast, a meta-analysis by Neufeld et al12 found that antipsychotic use was not associated with a change in delirium duration, severity, or length of stay in the hospital or intensive care unit. However, the studies in this meta-analysis varied widely in age range, study design, drug comparison, and treatment strategy (with drugs given as both prophylaxis and treatment). Thus, the results are difficult to interpret.
Kishi et al18 found no difference in the incidence of death, extrapyramidal symptoms, akathisia, or QT prolongation between patients treated with antipsychotic drugs vs placebo.
In a prospective observational study, Hatta et al19 followed 2,453 inpatients who became delirious. Only 22 (0.9%) experienced adverse events attributable to antipsychotic use, the most common being aspiration pneumonia (0.7%), followed by cardiovascular events (0.2%). Notably, no patient died of antipsychotic-related events. In this study, the antipsychotic was stopped as soon as the delirium symptoms resolved, in most cases in 3 to 7 days.
Taken together, these studies indicate that despite the risk of QT prolongation with antipsychotic use and increased rates of morbidity with antipsychotic use in dementia, time-limited management of delirium with antipsychotics is effective9–11 and safe.
SELECTING AND USING ANTIPSYCHOTICS TO TREAT DELIRIUM
Identifying a single preferred agent is difficult, since we lack enough evidence from randomized controlled trials that directly compared the various antipsychotics used in delirium management.
Both typical and atypical antipsychotics are used in clinical practice to manage delirium. The typical antipsychotic most often used is haloperidol, while the most commonly used atypical antipsychotics for delirium include olanzapine, quetiapine, risperidone, and (more recently) aripiprazole.
The American Psychiatric Association guidelines6 suggest using haloperidol because it is the antipsychotic that has been most studied for delirium,20 and we have decades of experience with its use. Despite this, recent prospective studies have suggested that the atypical antipsychotics may be better because they have a faster onset of action and lower incidence of extrapyramidal symptoms.18,21
Because we lack enough head-to-head trials comparing the efficacy of the 5 most commonly used antipsychotics for the management of delirium, and because the prospective trials that do exist show equal efficacy across the antipsychotics studied,22 we suggest considering the unique pharmacologic properties of each drug within the patient’s clinical context when selecting which antipsychotic to use.
Table 123–25 summarizes some key characteristics of the 5 most commonly used antipsychotics.
Haloperidol
Haloperidol, a typical antipsychotic, is a potent antagonist of the dopamine D2 receptor.
Haloperidol has the advantage of having the strongest evidence base for use in delirium. In addition, it is available in oral, intravenous, and intramuscular dosage forms, and it has minimal effects on vital signs, negligible anticholinergic activity, and minimal interactions with other medications.21
Intravenous haloperidol poses a significant risk of QT prolongation and so should be used judiciously in patients with preexisting cardiac conditions or other risk factors for QT prolongation as outlined above, and with careful cardiac monitoring. Parenteral haloperidol is approximately twice as potent as oral haloperidol.
Some evidence suggests a higher risk of acute dystonia and other extrapyramidal symptoms with haloperidol than with the atypical antipsychotics.21,26 In contrast, a 2013 prospective study showed that low doses of haloperidol (< 3.5 mg/day) did not result in a greater frequency of extrapyramidal symptoms.22 Nevertheless, if a patient has a history of extrapyramidal symptoms, haloperidol should likely be avoided in favor of an atypical antipsychotic.
Atypical antipsychotics
Olanzapine, quetiapine, and risperidone are atypical antipsychotics that, like haloperidol, antagonize the dopamine D2 receptor, but also have antagonist action at serotonin, histamine, and alpha-2 receptors. This multireceptor antagonism reduces the risk of extrapyramidal symptoms but increases the risk of orthostatic hypotension.
Quetiapine, in particular, imposes an unacceptably high risk of orthostatic hypotension and so is not recommended for use in delirium in the emergency department.27 Additionally, quetiapine is anticholinergic, raising concerns about constipation and urinary retention.
Although the association between fall risk and antipsychotic use remains controversial,28,29 a study found that olanzapine conferred a lower fall risk than quetiapine and risperidone.30
Of these drugs, only olanzapine is available in an intramuscular dosage form. Both risperidone and olanzapine are available in dissolvable tablets; however, they are not sublingually absorbed.
Randomized controlled trials have shown that olanzapine is effective in managing cancer-related nausea, and therefore it may be useful in managing delirium in oncology patients.31,32
Patients with Parkinson disease are exquisitely sensitive to the antidopaminergic effects of antipsychotics but are also vulnerable to delirium, so they present a unique treatment challenge. The agent of choice in patients with Parkinson disease is quetiapine, as multiple trials have shown it has no effect on the motor symptoms of Parkinson disease (reviewed by Desmarais et al in a systematic meta-analysis33).
Aripiprazole is increasingly used to manage delirium. Its mechanism of action differs from that of the other atypical antipsychotics, as it is a partial dopamine agonist. It is available in oral, orally dissolvable, and intramuscular forms. It appears to be slightly less effective than the other atypical antipsychotics,34 but it may be useful for hypoactive delirium as it is less sedating than the other agents.35 Because its effect on the QT interval is negligible, it may also be favored in patients who have a high baseline QTc or other predisposing factors for torsades de pointes.
BALANCING THE RISKS
Antipsychotic drugs have been shown to be effective and generally safe. Antipsychotics do prolong the QT interval. However, other than with intravenous administration of haloperidol, the absolute effect is minimal. Although large meta-analyses have shown a higher rate of all-cause mortality in elderly outpatients with dementia who are prescribed atypical antipsychotics, an increase in death rates has not been borne out by prospective studies focusing on hospitalized patients who receive low doses of antipsychotics for a limited time.
There are no head-to-head randomized controlled trials comparing the efficacy of all of the 5 most commonly used antipsychotics. Therefore, we suggest considering the unique psychopharmacologic properties of each agent within the patient’s clinical setting, specifically taking into account the risk of cardiac arrhythmia, risk of orthostasis and falls, history of extrapyramidal symptoms, other comorbidities such as Parkinson disease and cancer, and the desired route of administration.
At the time the patient is discharged, we recommend a careful medication reconciliation and discontinuation of the antipsychotic drug once delirium has resolved. Studies show that at least 26% of antipsychotics initiated in the hospital are continued after discharge.36,37
Current delirium consensus statements recommend limiting the use of antipsychotics to target patient distress, impediment of care, or safety, because of the putative risks of antipsychotic use in the elderly. However, a growing body of evidence shows that low-dose, time-limited antipsychotic use is safe and effective in the treatment of delirium. In fact, González et al found that delirium is an independent risk factor for death, and each 48-hour increase in delirium is associated with an increased mortality risk of 11%, suggesting that delay in treating delirium may actually increase the risk of death.38
Therefore, we must balance the risks of prescribing antipsychotics in medically vulnerable patients against the increasing burden of evidence supporting the serious risks of morbidity and mortality of delirium, as well as the costs. Much remains to be studied to optimize antipsychotic use in delirium.
- Siddiqi N, House AO, Holmes JD. Occurrence and outcome of delirium in medical in-patients: a systematic literature review. Age Ageing 2006; 35:350–364.
- Leslie DL, Marcantonio ER, Zhang Y, Leo-Summers L, Inouye SK. One-year health care costs associated with delirium in the elderly population. Arch Intern Med 2008; 168:27–32.
- Inouye SK. Delirium in older persons. N Engl J Med 2006; 354:1157–1165.
- Levkoff SE, Evans DA, Liptzin B, et al. Delirium: the occurrence and persistence of symptoms among elderly hospitalized patients. Arch Intern Med 1992; 152:334–340.
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
- Trzepacz P, Breitbart W, Franklin J, Levenson J, Martini DR, Wang P; American Psychiatric Association (APA). Practice guideline for the treatment of patients with delirium. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/delirium.pdf. Accessed July 13, 2017.
- Canadian Coalition for Seniors’ Mental Health. National guidelines for seniors’ mental health: the assessment and treatment of delirium. http://ccsmh.ca/wp-content/uploads/2016/03/NatlGuideline_Delirium.pdf. Accessed July 13, 2017.
- National Institute for Health and Care Excellence (NICE). Delirium: prevention, diagnosis and management. www.nice.org.uk/guidance/cg103. Accessed July 13, 2017.
- Bourne RS, Tahir TA, Borthwick M, Sampson EL. Drug treatment of delirium: past, present and future. J Psychosom Res 2008; 65:273–282.
- Campbell N, Boustani MA, Ayub A, et al. Pharmacological management of delirium in hospitalized adults—a systematic evidence review. J Gen Intern Med 2009; 24:848–853.
- Devlin JW, Skrobik Y. Antipsychotics for the prevention and treatment of delirium in the intensive care unit: what is their role? Harv Rev Psychiatry 2011; 19:59–67.
- Neufeld KJ, Yue J, Robinson TN, Inouye SK, Needham DM. Antipsychotic medication for prevention and treatment of delirium in hospitalized adults: a systematic review and meta-analysis. J Am Geriatr Soc 2016; 64:705–714.
- Beach SR, Celano MC, Noseworthy PA, Januzzi JL, Huffman JC. QTc prolongation, torsades de pointes, and psychotropic medications. Psychosomatics 2013; 54:1–13.
- US Food and Drug Administration (FDA). Information for healthcare professionals: haloperidol (marketed as Haldol, Haldol decanoate and Haldol lactate). www.fda.gov/Drugs/DrugSafety/ucm085203.htm. Accessed July 13, 2017.
- US Food and Drug Administration Center for Drug Evaluation and Research. Approval package for: Application Number: NDA 20-272/S-033, 20-588/S-021 & 21-444/S-004. www.accessdata.fda.gov/drugsatfda_docs/nda/2003/020588_S021_RISPERDAL_TABLETS.pdf. Accessed July 13, 2017.
- US Food and Drug Administration. Public health advisory: deaths with antipsychotics in elderly patients with behavioral disturbances. www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm053171. Accessed July 13, 2017.
- Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA 2005; 294:1934–1943.
- Kishi T, Hirota T, Matsunaga S, Iwata N. Antipsychotic medications for the treatment of delirium: a systematic review and meta-analysis of randomised controlled trials. J Neurol Neurosurg Psychiatry 2016; 87:767–774.
- Hatta K, Kishi Y, Wada K, et al. Antipsychotics for delirium in the general hospital setting in consecutive 2453 inpatients: a prospective observational study. Int J Geriatr Psychiatry 2014; 29;253–262.
- Breitbart W, Marotta R, Platt MM, et al. A double-blind trial of haloperidol, chlorpromazine, and lorazepam in the treatment of delirium in hospitalized AIDS patients. Am J Psychiatry 1996; 153:231–237.
- Wilson MP, Pepper D, Currier GW, Holloman GH Jr, Feifel D. The psychopharmacology of agitation: consensus statement of the American Association For Emergency Psychiatry Project Beta Psychopharmacology Workgroup. West J Emerg Med 2012; 13:26–34.
- Yoon HJ, Park KM, Choi WJ, et al. Efficacy and safety of haloperidol versus atypical antipsychotic medications in the treatment of delirium. BMC Psychiatry 2013; 13:240.
- American Psychiatric Association. Manual of Clinical Psychopharmacology. 8th ed. Arlington, VA: American Psychiatric Publishing; 2015.
- Conley RR, Kelly DL. Pharmacologic Treatment of Schizophrenia. 3rd ed. West Islip, NY: Professional Communications; 2007.
- American Psychiatric Association (APA). The American Psychiatric Publishing Textbook of Psychosomatic Medicine. Psychiatric Care of the Medically Ill, 2nd ed. Arlington, VA: American Psychiatric Publishing; 2011.
- Boettger S, Jenewein J, Breitbart W. Haloperidol, risperidone, olanzapine and aripiprazole in the management of delirium: a comparison of efficacy, safety, and side effects. Palliat Support Care 2015; 13:1079–1085.
- Currier GW, Trenton AJ, Walsh PG, van Wijngaarden E. A pilot, open-label study of quetiapine for treatment of moderate psychotic agitation in the emergency setting. J Psychiatr Pract 2006; 12:223–228.
- Chatterjee S, Chen H, Johnson ML, Aparasu RR. Risk of falls and fractures in older adults using atypical antipsychotic agents: a propensity score-adjusted, retrospective cohort study. Am J Geriatr Pharmacother 2012; 10:84–94.
- Rigler SK, Shireman TI, Cook-Wiens GJ, et al. Fracture risk in nursing home residents initiating antipsychotic medications. J Am Geriatr Soc 2013; 61: 715–722.
- Bozat-Emre S, Doupe M, Kozyrskyj AL, Grymonpre R, Mahmud SM. Atypical antipsychotic drug use and falls among nursing home residents in Winnipeg, Canada. Int J Geriatr Psychiatry 2015; 30:842–850.
- Navari RM, Gray SE, Kerr AC. Olanzapine versus aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a randomized phase III trial. J Support Oncol 2011; 9:188–195.
- Navari RM. Olanzapine for the prevention and treatment of chronic nausea and chemotherapy-induced nausea and vomiting. Eur J Pharmacol 2014; 722:180–186.
- Desmarais P, Massoud F, Filion J, Nguyen QD, Bajsarowicz P. Quetiapine for psychosis in Parkinson disease and neurodegenerative Parkinsonian disorders: a systematic review. J Geriatr Psychiatry Neurol 2016; 29:227–236.
- Citrome L. Comparison of intramuscular ziprasidone, olanzapine, or aripiprazole for agitation: a quantitative review of efficacy and safety. J Clin Psychiatry 2007; 68:1876–1885.
- Marder SR, McQuade RD, Stock E, et al. Aripiprazole in the treatment of schizophrenia: safety and tolerability in short-term, placebo-controlled trials. Schizophr Res 2003; 61:123–136.
- Loh KP, Ramdass S, Garb JL, et al. Long-term outcomes of elders discharged on antipsychotics. J Hosp Med 2016; 11:550–555.
- Herzig SJ, Rothberg MB, Guess JR, et al. Antipsychotic use in hospitalized adults: rates, indications, and predictors. J Am Geriatr Soc 2016; 64:299–305.
- González M, Martínez G, Calderón J, et al. Impact of delirium on short-term mortality in elderly inpatients: a prospective cohort study. Psychosomatics 2009; 50:234–238.
Delirium is common in hospitalized patients and contributes to healthcare costs and poor patient outcomes, including death. Its diagnosis and management remain clinically challenging. Although consensus panel guidelines recommend antipsychotic medications to treat delirium when conservative measures fail, few head-to-head trials have been done to tell us which antipsychotic drug to select, and antipsychotic use poses risks in the elderly.
Here, we review the risks and benefits of using antipsychotic drugs to manage delirium and describe an approach to selecting and using 5 commonly used antipsychotics.
SCOPE OF THE PROBLEM
Delirium is common and serious, affecting 11% to 42% of patients hospitalized on general medical wards.1 The burden to the public and individual patient is extremely high. Delirium has been found to result in an additional $16,303 to $64,421 per delirious patient per year, with a subsequent total 1-year health-attributable cost between $38 billion and $152 billion in the United States.2 Furthermore, many patients who become delirious in the hospital lose their independence and are placed in long-term care facilities.3
Although delirium was originally thought to be a time-limited neurocognitive disorder, recent evidence shows that it persists much longer4 and that some patients never return to their previous level of function, suggesting that a single episode of delirium can significantly alter the course of an underlying dementia with the dramatic initiation of cognitive decline.3 Most alarmingly, delirium is associated with an increased rate of death.1
DSM-5 DEFINITION
According to the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5),5 delirium is a neurocognitive disorder characterized by the acute onset of disturbance in attention, awareness, and cognition that fluctuates in severity throughout the day and is the direct physiologic consequence of another medical condition. The cognitive impairment seen in delirium is typically global and can affect memory, orientation, language, visuospatial ability, and perception. Other prominent features include psychomotor disturbance, sleep-cycle derangement, and emotional lability.
The pathogenesis of delirium is not clearly delineated but may relate to cholinergic deficiency and dopaminergic excess.
THE FIRST STEPS: NONPHARMACOLOGIC MANAGEMENT
Inouye3 outlined a general 3-part approach to managing delirium:
Identify and address predisposing factors. All patients found to have an acute change in mental status should be evaluated for the underlying cause, with special attention to the most common causes, ie, infection, metabolic derangement, and substance intoxication and withdrawal. A thorough medication reconciliation should also be done to identify medications with psychoactive or anticholinergic effects.
Provide supportive care, eg, addressing volume and nutritional status, mobilizing the patient early, and giving prophylaxis against deep venous thrombosis.
Manage symptoms. Behavioral strategies should be instituted in every delirious patient and should include frequent reorientation, use of observers, encouragement of family involvement, avoidance of physical restraints and Foley catheters, use of vision and hearing aids, and normalizing the sleep-wake cycle.
ANTIPSYCHOTICS: ARE THEY SAFE AND EFFECTIVE?
The US Food and Drug Administration (FDA) has not approved any medications for delirium. However, multiple consensus statements, including those by the American Psychiatric Association,6 the Canadian Coalition for Seniors’ Mental Health,7 and the UK National Institute for Health and Care Excellence,8 advocate for psychopharmacologic management of delirium symptoms in the following situations:
- The patient is in significant distress from his or her symptoms
- The patient poses a safety risk to self or others
- The patient is impeding essential aspects of his or her medical care.
Guidelines from these organizations recommend antipsychotic medications as the first-line drugs for managing delirium symptoms not caused by substance withdrawal. Nevertheless, the use of antipsychotics in the management of delirium remains controversial. While a number of studies suggest these drugs are beneficial,9–11 others do not.12 These consensus panels advocate for the judicious use of antipsychotics, limited to the specific situations outlined above.
The use of antipsychotics in elderly and medically complex patients poses risks. One of the most significant safety concerns is increased risk of death due to adverse cardiac events caused by prolongation of the QT interval.
Antipsychotics, QT prolongation, and torsades de pointes
Most antipsychotics have the potential to prolong the time of ventricular depolarization and repolarization and the QT interval to some extent, which can lead to torsades de pointes.13 Other risk factors for prolonged QT interval and torsades de pointes include:
- Long QT syndrome (a genetic arrhythmia)
- Female sex
- Old age
- Electrolyte abnormalities (hypokalemia, hypocalcemia, hypomagnesemia)
- Preexisting heart conditions such as bradycardia, left ventricular dysfunction, heart failure, mitral valve prolapse, and previous myocardial infarction
- Medical conditions that cause electrolyte derangements
- Medications, including antiarrhythmics, antibiotics (macrolides, quinolones), antifungals, antimalarials, antiemetics, some opioids (methadone), and most antipsychotics.
Haloperidol. Postmarketing analysis in 2007 found 73 cases of haloperidol-related torsades de pointes. However, many of these were confounded by other QT-prolonging medications and medical conditions.14
The QT-prolonging effect of haloperidol administered orally or intramuscularly is actually quite small. The equivalent oral dose of 15 mg of haloperidol (assuming 50% bioavailability) given orally or intramuscularly increases the corrected QT interval (QTc) by only 7 to 8 milliseconds. But intravenous haloperidol can cause much more significant QT prolongation: 8 of the 11 reported cases of fatal torsades de pointes occurred when haloperidol was given intravenously.14 Therefore, the FDA recommends cardiac monitoring for all patients receiving intravenous haloperidol.
Oral olanzapine, risperidone, and quetiapine prolong the QT interval approximately as much as oral haloperidol.
Aripiprazole has not been associated with significant QT prolongation.13
Atypical antipsychotics and stroke
The FDA has issued multiple warnings for prescribing antipsychotic medications in the elderly. In 2003, it warned prescribers of increased cerebrovascular adverse events, including stroke, in elderly patients with dementia who were treated with an atypical antipsychotic (risperidone, olanzapine, or aripiprazole) vs placebo.15
Atypical antipsychotics and risk of death
In 2005, the FDA issued a black-box warning about increased all-cause mortality risk in patients with dementia treated with atypical antipsychotics for behavioral disturbance (relative risk 1.6–1.7).16
This warning was likely based on a meta-analysis by Schneider et al17 of trials in which patients with dementia were randomized to receive either an atypical antipsychotic or placebo. The death rate was 3.5% in patients treated with an atypical antipsychotic vs 2.3% in patients treated with placebo, indicating a number needed to harm of 100. The most common causes of death were cardiovascular disease and pneumonia. However, the trials in this meta-analysis included only patients who were prescribed atypical antipsychotics for ongoing management of behavioral disturbances due to dementia in either the outpatient or nursing home setting. None of the trials looked at patients who were prescribed atypical antipsychotics for a limited time in a closely monitored inpatient setting.
Effectiveness of antipsychotics
While several studies since the FDA black-box warning have shown that antipsychotics are safe, the efficacy of these drugs in delirium management remains controversial.
In a 2016 meta-analysis, Kishi et al18 found that antipsychotics were superior to placebo in terms of response rate (defined as improvement of delirium severity rating scores), with a number needed to treat of 2.
In contrast, a meta-analysis by Neufeld et al12 found that antipsychotic use was not associated with a change in delirium duration, severity, or length of stay in the hospital or intensive care unit. However, the studies in this meta-analysis varied widely in age range, study design, drug comparison, and treatment strategy (with drugs given as both prophylaxis and treatment). Thus, the results are difficult to interpret.
Kishi et al18 found no difference in the incidence of death, extrapyramidal symptoms, akathisia, or QT prolongation between patients treated with antipsychotic drugs vs placebo.
In a prospective observational study, Hatta et al19 followed 2,453 inpatients who became delirious. Only 22 (0.9%) experienced adverse events attributable to antipsychotic use, the most common being aspiration pneumonia (0.7%), followed by cardiovascular events (0.2%). Notably, no patient died of antipsychotic-related events. In this study, the antipsychotic was stopped as soon as the delirium symptoms resolved, in most cases in 3 to 7 days.
Taken together, these studies indicate that despite the risk of QT prolongation with antipsychotic use and increased rates of morbidity with antipsychotic use in dementia, time-limited management of delirium with antipsychotics is effective9–11 and safe.
SELECTING AND USING ANTIPSYCHOTICS TO TREAT DELIRIUM
Identifying a single preferred agent is difficult, since we lack enough evidence from randomized controlled trials that directly compared the various antipsychotics used in delirium management.
Both typical and atypical antipsychotics are used in clinical practice to manage delirium. The typical antipsychotic most often used is haloperidol, while the most commonly used atypical antipsychotics for delirium include olanzapine, quetiapine, risperidone, and (more recently) aripiprazole.
The American Psychiatric Association guidelines6 suggest using haloperidol because it is the antipsychotic that has been most studied for delirium,20 and we have decades of experience with its use. Despite this, recent prospective studies have suggested that the atypical antipsychotics may be better because they have a faster onset of action and lower incidence of extrapyramidal symptoms.18,21
Because we lack enough head-to-head trials comparing the efficacy of the 5 most commonly used antipsychotics for the management of delirium, and because the prospective trials that do exist show equal efficacy across the antipsychotics studied,22 we suggest considering the unique pharmacologic properties of each drug within the patient’s clinical context when selecting which antipsychotic to use.
Table 123–25 summarizes some key characteristics of the 5 most commonly used antipsychotics.
Haloperidol
Haloperidol, a typical antipsychotic, is a potent antagonist of the dopamine D2 receptor.
Haloperidol has the advantage of having the strongest evidence base for use in delirium. In addition, it is available in oral, intravenous, and intramuscular dosage forms, and it has minimal effects on vital signs, negligible anticholinergic activity, and minimal interactions with other medications.21
Intravenous haloperidol poses a significant risk of QT prolongation and so should be used judiciously in patients with preexisting cardiac conditions or other risk factors for QT prolongation as outlined above, and with careful cardiac monitoring. Parenteral haloperidol is approximately twice as potent as oral haloperidol.
Some evidence suggests a higher risk of acute dystonia and other extrapyramidal symptoms with haloperidol than with the atypical antipsychotics.21,26 In contrast, a 2013 prospective study showed that low doses of haloperidol (< 3.5 mg/day) did not result in a greater frequency of extrapyramidal symptoms.22 Nevertheless, if a patient has a history of extrapyramidal symptoms, haloperidol should likely be avoided in favor of an atypical antipsychotic.
Atypical antipsychotics
Olanzapine, quetiapine, and risperidone are atypical antipsychotics that, like haloperidol, antagonize the dopamine D2 receptor, but also have antagonist action at serotonin, histamine, and alpha-2 receptors. This multireceptor antagonism reduces the risk of extrapyramidal symptoms but increases the risk of orthostatic hypotension.
Quetiapine, in particular, imposes an unacceptably high risk of orthostatic hypotension and so is not recommended for use in delirium in the emergency department.27 Additionally, quetiapine is anticholinergic, raising concerns about constipation and urinary retention.
Although the association between fall risk and antipsychotic use remains controversial,28,29 a study found that olanzapine conferred a lower fall risk than quetiapine and risperidone.30
Of these drugs, only olanzapine is available in an intramuscular dosage form. Both risperidone and olanzapine are available in dissolvable tablets; however, they are not sublingually absorbed.
Randomized controlled trials have shown that olanzapine is effective in managing cancer-related nausea, and therefore it may be useful in managing delirium in oncology patients.31,32
Patients with Parkinson disease are exquisitely sensitive to the antidopaminergic effects of antipsychotics but are also vulnerable to delirium, so they present a unique treatment challenge. The agent of choice in patients with Parkinson disease is quetiapine, as multiple trials have shown it has no effect on the motor symptoms of Parkinson disease (reviewed by Desmarais et al in a systematic meta-analysis33).
Aripiprazole is increasingly used to manage delirium. Its mechanism of action differs from that of the other atypical antipsychotics, as it is a partial dopamine agonist. It is available in oral, orally dissolvable, and intramuscular forms. It appears to be slightly less effective than the other atypical antipsychotics,34 but it may be useful for hypoactive delirium as it is less sedating than the other agents.35 Because its effect on the QT interval is negligible, it may also be favored in patients who have a high baseline QTc or other predisposing factors for torsades de pointes.
BALANCING THE RISKS
Antipsychotic drugs have been shown to be effective and generally safe. Antipsychotics do prolong the QT interval. However, other than with intravenous administration of haloperidol, the absolute effect is minimal. Although large meta-analyses have shown a higher rate of all-cause mortality in elderly outpatients with dementia who are prescribed atypical antipsychotics, an increase in death rates has not been borne out by prospective studies focusing on hospitalized patients who receive low doses of antipsychotics for a limited time.
There are no head-to-head randomized controlled trials comparing the efficacy of all of the 5 most commonly used antipsychotics. Therefore, we suggest considering the unique psychopharmacologic properties of each agent within the patient’s clinical setting, specifically taking into account the risk of cardiac arrhythmia, risk of orthostasis and falls, history of extrapyramidal symptoms, other comorbidities such as Parkinson disease and cancer, and the desired route of administration.
At the time the patient is discharged, we recommend a careful medication reconciliation and discontinuation of the antipsychotic drug once delirium has resolved. Studies show that at least 26% of antipsychotics initiated in the hospital are continued after discharge.36,37
Current delirium consensus statements recommend limiting the use of antipsychotics to target patient distress, impediment of care, or safety, because of the putative risks of antipsychotic use in the elderly. However, a growing body of evidence shows that low-dose, time-limited antipsychotic use is safe and effective in the treatment of delirium. In fact, González et al found that delirium is an independent risk factor for death, and each 48-hour increase in delirium is associated with an increased mortality risk of 11%, suggesting that delay in treating delirium may actually increase the risk of death.38
Therefore, we must balance the risks of prescribing antipsychotics in medically vulnerable patients against the increasing burden of evidence supporting the serious risks of morbidity and mortality of delirium, as well as the costs. Much remains to be studied to optimize antipsychotic use in delirium.
Delirium is common in hospitalized patients and contributes to healthcare costs and poor patient outcomes, including death. Its diagnosis and management remain clinically challenging. Although consensus panel guidelines recommend antipsychotic medications to treat delirium when conservative measures fail, few head-to-head trials have been done to tell us which antipsychotic drug to select, and antipsychotic use poses risks in the elderly.
Here, we review the risks and benefits of using antipsychotic drugs to manage delirium and describe an approach to selecting and using 5 commonly used antipsychotics.
SCOPE OF THE PROBLEM
Delirium is common and serious, affecting 11% to 42% of patients hospitalized on general medical wards.1 The burden to the public and individual patient is extremely high. Delirium has been found to result in an additional $16,303 to $64,421 per delirious patient per year, with a subsequent total 1-year health-attributable cost between $38 billion and $152 billion in the United States.2 Furthermore, many patients who become delirious in the hospital lose their independence and are placed in long-term care facilities.3
Although delirium was originally thought to be a time-limited neurocognitive disorder, recent evidence shows that it persists much longer4 and that some patients never return to their previous level of function, suggesting that a single episode of delirium can significantly alter the course of an underlying dementia with the dramatic initiation of cognitive decline.3 Most alarmingly, delirium is associated with an increased rate of death.1
DSM-5 DEFINITION
According to the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5),5 delirium is a neurocognitive disorder characterized by the acute onset of disturbance in attention, awareness, and cognition that fluctuates in severity throughout the day and is the direct physiologic consequence of another medical condition. The cognitive impairment seen in delirium is typically global and can affect memory, orientation, language, visuospatial ability, and perception. Other prominent features include psychomotor disturbance, sleep-cycle derangement, and emotional lability.
The pathogenesis of delirium is not clearly delineated but may relate to cholinergic deficiency and dopaminergic excess.
THE FIRST STEPS: NONPHARMACOLOGIC MANAGEMENT
Inouye3 outlined a general 3-part approach to managing delirium:
Identify and address predisposing factors. All patients found to have an acute change in mental status should be evaluated for the underlying cause, with special attention to the most common causes, ie, infection, metabolic derangement, and substance intoxication and withdrawal. A thorough medication reconciliation should also be done to identify medications with psychoactive or anticholinergic effects.
Provide supportive care, eg, addressing volume and nutritional status, mobilizing the patient early, and giving prophylaxis against deep venous thrombosis.
Manage symptoms. Behavioral strategies should be instituted in every delirious patient and should include frequent reorientation, use of observers, encouragement of family involvement, avoidance of physical restraints and Foley catheters, use of vision and hearing aids, and normalizing the sleep-wake cycle.
ANTIPSYCHOTICS: ARE THEY SAFE AND EFFECTIVE?
The US Food and Drug Administration (FDA) has not approved any medications for delirium. However, multiple consensus statements, including those by the American Psychiatric Association,6 the Canadian Coalition for Seniors’ Mental Health,7 and the UK National Institute for Health and Care Excellence,8 advocate for psychopharmacologic management of delirium symptoms in the following situations:
- The patient is in significant distress from his or her symptoms
- The patient poses a safety risk to self or others
- The patient is impeding essential aspects of his or her medical care.
Guidelines from these organizations recommend antipsychotic medications as the first-line drugs for managing delirium symptoms not caused by substance withdrawal. Nevertheless, the use of antipsychotics in the management of delirium remains controversial. While a number of studies suggest these drugs are beneficial,9–11 others do not.12 These consensus panels advocate for the judicious use of antipsychotics, limited to the specific situations outlined above.
The use of antipsychotics in elderly and medically complex patients poses risks. One of the most significant safety concerns is increased risk of death due to adverse cardiac events caused by prolongation of the QT interval.
Antipsychotics, QT prolongation, and torsades de pointes
Most antipsychotics have the potential to prolong the time of ventricular depolarization and repolarization and the QT interval to some extent, which can lead to torsades de pointes.13 Other risk factors for prolonged QT interval and torsades de pointes include:
- Long QT syndrome (a genetic arrhythmia)
- Female sex
- Old age
- Electrolyte abnormalities (hypokalemia, hypocalcemia, hypomagnesemia)
- Preexisting heart conditions such as bradycardia, left ventricular dysfunction, heart failure, mitral valve prolapse, and previous myocardial infarction
- Medical conditions that cause electrolyte derangements
- Medications, including antiarrhythmics, antibiotics (macrolides, quinolones), antifungals, antimalarials, antiemetics, some opioids (methadone), and most antipsychotics.
Haloperidol. Postmarketing analysis in 2007 found 73 cases of haloperidol-related torsades de pointes. However, many of these were confounded by other QT-prolonging medications and medical conditions.14
The QT-prolonging effect of haloperidol administered orally or intramuscularly is actually quite small. The equivalent oral dose of 15 mg of haloperidol (assuming 50% bioavailability) given orally or intramuscularly increases the corrected QT interval (QTc) by only 7 to 8 milliseconds. But intravenous haloperidol can cause much more significant QT prolongation: 8 of the 11 reported cases of fatal torsades de pointes occurred when haloperidol was given intravenously.14 Therefore, the FDA recommends cardiac monitoring for all patients receiving intravenous haloperidol.
Oral olanzapine, risperidone, and quetiapine prolong the QT interval approximately as much as oral haloperidol.
Aripiprazole has not been associated with significant QT prolongation.13
Atypical antipsychotics and stroke
The FDA has issued multiple warnings for prescribing antipsychotic medications in the elderly. In 2003, it warned prescribers of increased cerebrovascular adverse events, including stroke, in elderly patients with dementia who were treated with an atypical antipsychotic (risperidone, olanzapine, or aripiprazole) vs placebo.15
Atypical antipsychotics and risk of death
In 2005, the FDA issued a black-box warning about increased all-cause mortality risk in patients with dementia treated with atypical antipsychotics for behavioral disturbance (relative risk 1.6–1.7).16
This warning was likely based on a meta-analysis by Schneider et al17 of trials in which patients with dementia were randomized to receive either an atypical antipsychotic or placebo. The death rate was 3.5% in patients treated with an atypical antipsychotic vs 2.3% in patients treated with placebo, indicating a number needed to harm of 100. The most common causes of death were cardiovascular disease and pneumonia. However, the trials in this meta-analysis included only patients who were prescribed atypical antipsychotics for ongoing management of behavioral disturbances due to dementia in either the outpatient or nursing home setting. None of the trials looked at patients who were prescribed atypical antipsychotics for a limited time in a closely monitored inpatient setting.
Effectiveness of antipsychotics
While several studies since the FDA black-box warning have shown that antipsychotics are safe, the efficacy of these drugs in delirium management remains controversial.
In a 2016 meta-analysis, Kishi et al18 found that antipsychotics were superior to placebo in terms of response rate (defined as improvement of delirium severity rating scores), with a number needed to treat of 2.
In contrast, a meta-analysis by Neufeld et al12 found that antipsychotic use was not associated with a change in delirium duration, severity, or length of stay in the hospital or intensive care unit. However, the studies in this meta-analysis varied widely in age range, study design, drug comparison, and treatment strategy (with drugs given as both prophylaxis and treatment). Thus, the results are difficult to interpret.
Kishi et al18 found no difference in the incidence of death, extrapyramidal symptoms, akathisia, or QT prolongation between patients treated with antipsychotic drugs vs placebo.
In a prospective observational study, Hatta et al19 followed 2,453 inpatients who became delirious. Only 22 (0.9%) experienced adverse events attributable to antipsychotic use, the most common being aspiration pneumonia (0.7%), followed by cardiovascular events (0.2%). Notably, no patient died of antipsychotic-related events. In this study, the antipsychotic was stopped as soon as the delirium symptoms resolved, in most cases in 3 to 7 days.
Taken together, these studies indicate that despite the risk of QT prolongation with antipsychotic use and increased rates of morbidity with antipsychotic use in dementia, time-limited management of delirium with antipsychotics is effective9–11 and safe.
SELECTING AND USING ANTIPSYCHOTICS TO TREAT DELIRIUM
Identifying a single preferred agent is difficult, since we lack enough evidence from randomized controlled trials that directly compared the various antipsychotics used in delirium management.
Both typical and atypical antipsychotics are used in clinical practice to manage delirium. The typical antipsychotic most often used is haloperidol, while the most commonly used atypical antipsychotics for delirium include olanzapine, quetiapine, risperidone, and (more recently) aripiprazole.
The American Psychiatric Association guidelines6 suggest using haloperidol because it is the antipsychotic that has been most studied for delirium,20 and we have decades of experience with its use. Despite this, recent prospective studies have suggested that the atypical antipsychotics may be better because they have a faster onset of action and lower incidence of extrapyramidal symptoms.18,21
Because we lack enough head-to-head trials comparing the efficacy of the 5 most commonly used antipsychotics for the management of delirium, and because the prospective trials that do exist show equal efficacy across the antipsychotics studied,22 we suggest considering the unique pharmacologic properties of each drug within the patient’s clinical context when selecting which antipsychotic to use.
Table 123–25 summarizes some key characteristics of the 5 most commonly used antipsychotics.
Haloperidol
Haloperidol, a typical antipsychotic, is a potent antagonist of the dopamine D2 receptor.
Haloperidol has the advantage of having the strongest evidence base for use in delirium. In addition, it is available in oral, intravenous, and intramuscular dosage forms, and it has minimal effects on vital signs, negligible anticholinergic activity, and minimal interactions with other medications.21
Intravenous haloperidol poses a significant risk of QT prolongation and so should be used judiciously in patients with preexisting cardiac conditions or other risk factors for QT prolongation as outlined above, and with careful cardiac monitoring. Parenteral haloperidol is approximately twice as potent as oral haloperidol.
Some evidence suggests a higher risk of acute dystonia and other extrapyramidal symptoms with haloperidol than with the atypical antipsychotics.21,26 In contrast, a 2013 prospective study showed that low doses of haloperidol (< 3.5 mg/day) did not result in a greater frequency of extrapyramidal symptoms.22 Nevertheless, if a patient has a history of extrapyramidal symptoms, haloperidol should likely be avoided in favor of an atypical antipsychotic.
Atypical antipsychotics
Olanzapine, quetiapine, and risperidone are atypical antipsychotics that, like haloperidol, antagonize the dopamine D2 receptor, but also have antagonist action at serotonin, histamine, and alpha-2 receptors. This multireceptor antagonism reduces the risk of extrapyramidal symptoms but increases the risk of orthostatic hypotension.
Quetiapine, in particular, imposes an unacceptably high risk of orthostatic hypotension and so is not recommended for use in delirium in the emergency department.27 Additionally, quetiapine is anticholinergic, raising concerns about constipation and urinary retention.
Although the association between fall risk and antipsychotic use remains controversial,28,29 a study found that olanzapine conferred a lower fall risk than quetiapine and risperidone.30
Of these drugs, only olanzapine is available in an intramuscular dosage form. Both risperidone and olanzapine are available in dissolvable tablets; however, they are not sublingually absorbed.
Randomized controlled trials have shown that olanzapine is effective in managing cancer-related nausea, and therefore it may be useful in managing delirium in oncology patients.31,32
Patients with Parkinson disease are exquisitely sensitive to the antidopaminergic effects of antipsychotics but are also vulnerable to delirium, so they present a unique treatment challenge. The agent of choice in patients with Parkinson disease is quetiapine, as multiple trials have shown it has no effect on the motor symptoms of Parkinson disease (reviewed by Desmarais et al in a systematic meta-analysis33).
Aripiprazole is increasingly used to manage delirium. Its mechanism of action differs from that of the other atypical antipsychotics, as it is a partial dopamine agonist. It is available in oral, orally dissolvable, and intramuscular forms. It appears to be slightly less effective than the other atypical antipsychotics,34 but it may be useful for hypoactive delirium as it is less sedating than the other agents.35 Because its effect on the QT interval is negligible, it may also be favored in patients who have a high baseline QTc or other predisposing factors for torsades de pointes.
BALANCING THE RISKS
Antipsychotic drugs have been shown to be effective and generally safe. Antipsychotics do prolong the QT interval. However, other than with intravenous administration of haloperidol, the absolute effect is minimal. Although large meta-analyses have shown a higher rate of all-cause mortality in elderly outpatients with dementia who are prescribed atypical antipsychotics, an increase in death rates has not been borne out by prospective studies focusing on hospitalized patients who receive low doses of antipsychotics for a limited time.
There are no head-to-head randomized controlled trials comparing the efficacy of all of the 5 most commonly used antipsychotics. Therefore, we suggest considering the unique psychopharmacologic properties of each agent within the patient’s clinical setting, specifically taking into account the risk of cardiac arrhythmia, risk of orthostasis and falls, history of extrapyramidal symptoms, other comorbidities such as Parkinson disease and cancer, and the desired route of administration.
At the time the patient is discharged, we recommend a careful medication reconciliation and discontinuation of the antipsychotic drug once delirium has resolved. Studies show that at least 26% of antipsychotics initiated in the hospital are continued after discharge.36,37
Current delirium consensus statements recommend limiting the use of antipsychotics to target patient distress, impediment of care, or safety, because of the putative risks of antipsychotic use in the elderly. However, a growing body of evidence shows that low-dose, time-limited antipsychotic use is safe and effective in the treatment of delirium. In fact, González et al found that delirium is an independent risk factor for death, and each 48-hour increase in delirium is associated with an increased mortality risk of 11%, suggesting that delay in treating delirium may actually increase the risk of death.38
Therefore, we must balance the risks of prescribing antipsychotics in medically vulnerable patients against the increasing burden of evidence supporting the serious risks of morbidity and mortality of delirium, as well as the costs. Much remains to be studied to optimize antipsychotic use in delirium.
- Siddiqi N, House AO, Holmes JD. Occurrence and outcome of delirium in medical in-patients: a systematic literature review. Age Ageing 2006; 35:350–364.
- Leslie DL, Marcantonio ER, Zhang Y, Leo-Summers L, Inouye SK. One-year health care costs associated with delirium in the elderly population. Arch Intern Med 2008; 168:27–32.
- Inouye SK. Delirium in older persons. N Engl J Med 2006; 354:1157–1165.
- Levkoff SE, Evans DA, Liptzin B, et al. Delirium: the occurrence and persistence of symptoms among elderly hospitalized patients. Arch Intern Med 1992; 152:334–340.
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
- Trzepacz P, Breitbart W, Franklin J, Levenson J, Martini DR, Wang P; American Psychiatric Association (APA). Practice guideline for the treatment of patients with delirium. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/delirium.pdf. Accessed July 13, 2017.
- Canadian Coalition for Seniors’ Mental Health. National guidelines for seniors’ mental health: the assessment and treatment of delirium. http://ccsmh.ca/wp-content/uploads/2016/03/NatlGuideline_Delirium.pdf. Accessed July 13, 2017.
- National Institute for Health and Care Excellence (NICE). Delirium: prevention, diagnosis and management. www.nice.org.uk/guidance/cg103. Accessed July 13, 2017.
- Bourne RS, Tahir TA, Borthwick M, Sampson EL. Drug treatment of delirium: past, present and future. J Psychosom Res 2008; 65:273–282.
- Campbell N, Boustani MA, Ayub A, et al. Pharmacological management of delirium in hospitalized adults—a systematic evidence review. J Gen Intern Med 2009; 24:848–853.
- Devlin JW, Skrobik Y. Antipsychotics for the prevention and treatment of delirium in the intensive care unit: what is their role? Harv Rev Psychiatry 2011; 19:59–67.
- Neufeld KJ, Yue J, Robinson TN, Inouye SK, Needham DM. Antipsychotic medication for prevention and treatment of delirium in hospitalized adults: a systematic review and meta-analysis. J Am Geriatr Soc 2016; 64:705–714.
- Beach SR, Celano MC, Noseworthy PA, Januzzi JL, Huffman JC. QTc prolongation, torsades de pointes, and psychotropic medications. Psychosomatics 2013; 54:1–13.
- US Food and Drug Administration (FDA). Information for healthcare professionals: haloperidol (marketed as Haldol, Haldol decanoate and Haldol lactate). www.fda.gov/Drugs/DrugSafety/ucm085203.htm. Accessed July 13, 2017.
- US Food and Drug Administration Center for Drug Evaluation and Research. Approval package for: Application Number: NDA 20-272/S-033, 20-588/S-021 & 21-444/S-004. www.accessdata.fda.gov/drugsatfda_docs/nda/2003/020588_S021_RISPERDAL_TABLETS.pdf. Accessed July 13, 2017.
- US Food and Drug Administration. Public health advisory: deaths with antipsychotics in elderly patients with behavioral disturbances. www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm053171. Accessed July 13, 2017.
- Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA 2005; 294:1934–1943.
- Kishi T, Hirota T, Matsunaga S, Iwata N. Antipsychotic medications for the treatment of delirium: a systematic review and meta-analysis of randomised controlled trials. J Neurol Neurosurg Psychiatry 2016; 87:767–774.
- Hatta K, Kishi Y, Wada K, et al. Antipsychotics for delirium in the general hospital setting in consecutive 2453 inpatients: a prospective observational study. Int J Geriatr Psychiatry 2014; 29;253–262.
- Breitbart W, Marotta R, Platt MM, et al. A double-blind trial of haloperidol, chlorpromazine, and lorazepam in the treatment of delirium in hospitalized AIDS patients. Am J Psychiatry 1996; 153:231–237.
- Wilson MP, Pepper D, Currier GW, Holloman GH Jr, Feifel D. The psychopharmacology of agitation: consensus statement of the American Association For Emergency Psychiatry Project Beta Psychopharmacology Workgroup. West J Emerg Med 2012; 13:26–34.
- Yoon HJ, Park KM, Choi WJ, et al. Efficacy and safety of haloperidol versus atypical antipsychotic medications in the treatment of delirium. BMC Psychiatry 2013; 13:240.
- American Psychiatric Association. Manual of Clinical Psychopharmacology. 8th ed. Arlington, VA: American Psychiatric Publishing; 2015.
- Conley RR, Kelly DL. Pharmacologic Treatment of Schizophrenia. 3rd ed. West Islip, NY: Professional Communications; 2007.
- American Psychiatric Association (APA). The American Psychiatric Publishing Textbook of Psychosomatic Medicine. Psychiatric Care of the Medically Ill, 2nd ed. Arlington, VA: American Psychiatric Publishing; 2011.
- Boettger S, Jenewein J, Breitbart W. Haloperidol, risperidone, olanzapine and aripiprazole in the management of delirium: a comparison of efficacy, safety, and side effects. Palliat Support Care 2015; 13:1079–1085.
- Currier GW, Trenton AJ, Walsh PG, van Wijngaarden E. A pilot, open-label study of quetiapine for treatment of moderate psychotic agitation in the emergency setting. J Psychiatr Pract 2006; 12:223–228.
- Chatterjee S, Chen H, Johnson ML, Aparasu RR. Risk of falls and fractures in older adults using atypical antipsychotic agents: a propensity score-adjusted, retrospective cohort study. Am J Geriatr Pharmacother 2012; 10:84–94.
- Rigler SK, Shireman TI, Cook-Wiens GJ, et al. Fracture risk in nursing home residents initiating antipsychotic medications. J Am Geriatr Soc 2013; 61: 715–722.
- Bozat-Emre S, Doupe M, Kozyrskyj AL, Grymonpre R, Mahmud SM. Atypical antipsychotic drug use and falls among nursing home residents in Winnipeg, Canada. Int J Geriatr Psychiatry 2015; 30:842–850.
- Navari RM, Gray SE, Kerr AC. Olanzapine versus aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a randomized phase III trial. J Support Oncol 2011; 9:188–195.
- Navari RM. Olanzapine for the prevention and treatment of chronic nausea and chemotherapy-induced nausea and vomiting. Eur J Pharmacol 2014; 722:180–186.
- Desmarais P, Massoud F, Filion J, Nguyen QD, Bajsarowicz P. Quetiapine for psychosis in Parkinson disease and neurodegenerative Parkinsonian disorders: a systematic review. J Geriatr Psychiatry Neurol 2016; 29:227–236.
- Citrome L. Comparison of intramuscular ziprasidone, olanzapine, or aripiprazole for agitation: a quantitative review of efficacy and safety. J Clin Psychiatry 2007; 68:1876–1885.
- Marder SR, McQuade RD, Stock E, et al. Aripiprazole in the treatment of schizophrenia: safety and tolerability in short-term, placebo-controlled trials. Schizophr Res 2003; 61:123–136.
- Loh KP, Ramdass S, Garb JL, et al. Long-term outcomes of elders discharged on antipsychotics. J Hosp Med 2016; 11:550–555.
- Herzig SJ, Rothberg MB, Guess JR, et al. Antipsychotic use in hospitalized adults: rates, indications, and predictors. J Am Geriatr Soc 2016; 64:299–305.
- González M, Martínez G, Calderón J, et al. Impact of delirium on short-term mortality in elderly inpatients: a prospective cohort study. Psychosomatics 2009; 50:234–238.
- Siddiqi N, House AO, Holmes JD. Occurrence and outcome of delirium in medical in-patients: a systematic literature review. Age Ageing 2006; 35:350–364.
- Leslie DL, Marcantonio ER, Zhang Y, Leo-Summers L, Inouye SK. One-year health care costs associated with delirium in the elderly population. Arch Intern Med 2008; 168:27–32.
- Inouye SK. Delirium in older persons. N Engl J Med 2006; 354:1157–1165.
- Levkoff SE, Evans DA, Liptzin B, et al. Delirium: the occurrence and persistence of symptoms among elderly hospitalized patients. Arch Intern Med 1992; 152:334–340.
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
- Trzepacz P, Breitbart W, Franklin J, Levenson J, Martini DR, Wang P; American Psychiatric Association (APA). Practice guideline for the treatment of patients with delirium. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/delirium.pdf. Accessed July 13, 2017.
- Canadian Coalition for Seniors’ Mental Health. National guidelines for seniors’ mental health: the assessment and treatment of delirium. http://ccsmh.ca/wp-content/uploads/2016/03/NatlGuideline_Delirium.pdf. Accessed July 13, 2017.
- National Institute for Health and Care Excellence (NICE). Delirium: prevention, diagnosis and management. www.nice.org.uk/guidance/cg103. Accessed July 13, 2017.
- Bourne RS, Tahir TA, Borthwick M, Sampson EL. Drug treatment of delirium: past, present and future. J Psychosom Res 2008; 65:273–282.
- Campbell N, Boustani MA, Ayub A, et al. Pharmacological management of delirium in hospitalized adults—a systematic evidence review. J Gen Intern Med 2009; 24:848–853.
- Devlin JW, Skrobik Y. Antipsychotics for the prevention and treatment of delirium in the intensive care unit: what is their role? Harv Rev Psychiatry 2011; 19:59–67.
- Neufeld KJ, Yue J, Robinson TN, Inouye SK, Needham DM. Antipsychotic medication for prevention and treatment of delirium in hospitalized adults: a systematic review and meta-analysis. J Am Geriatr Soc 2016; 64:705–714.
- Beach SR, Celano MC, Noseworthy PA, Januzzi JL, Huffman JC. QTc prolongation, torsades de pointes, and psychotropic medications. Psychosomatics 2013; 54:1–13.
- US Food and Drug Administration (FDA). Information for healthcare professionals: haloperidol (marketed as Haldol, Haldol decanoate and Haldol lactate). www.fda.gov/Drugs/DrugSafety/ucm085203.htm. Accessed July 13, 2017.
- US Food and Drug Administration Center for Drug Evaluation and Research. Approval package for: Application Number: NDA 20-272/S-033, 20-588/S-021 & 21-444/S-004. www.accessdata.fda.gov/drugsatfda_docs/nda/2003/020588_S021_RISPERDAL_TABLETS.pdf. Accessed July 13, 2017.
- US Food and Drug Administration. Public health advisory: deaths with antipsychotics in elderly patients with behavioral disturbances. www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm053171. Accessed July 13, 2017.
- Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA 2005; 294:1934–1943.
- Kishi T, Hirota T, Matsunaga S, Iwata N. Antipsychotic medications for the treatment of delirium: a systematic review and meta-analysis of randomised controlled trials. J Neurol Neurosurg Psychiatry 2016; 87:767–774.
- Hatta K, Kishi Y, Wada K, et al. Antipsychotics for delirium in the general hospital setting in consecutive 2453 inpatients: a prospective observational study. Int J Geriatr Psychiatry 2014; 29;253–262.
- Breitbart W, Marotta R, Platt MM, et al. A double-blind trial of haloperidol, chlorpromazine, and lorazepam in the treatment of delirium in hospitalized AIDS patients. Am J Psychiatry 1996; 153:231–237.
- Wilson MP, Pepper D, Currier GW, Holloman GH Jr, Feifel D. The psychopharmacology of agitation: consensus statement of the American Association For Emergency Psychiatry Project Beta Psychopharmacology Workgroup. West J Emerg Med 2012; 13:26–34.
- Yoon HJ, Park KM, Choi WJ, et al. Efficacy and safety of haloperidol versus atypical antipsychotic medications in the treatment of delirium. BMC Psychiatry 2013; 13:240.
- American Psychiatric Association. Manual of Clinical Psychopharmacology. 8th ed. Arlington, VA: American Psychiatric Publishing; 2015.
- Conley RR, Kelly DL. Pharmacologic Treatment of Schizophrenia. 3rd ed. West Islip, NY: Professional Communications; 2007.
- American Psychiatric Association (APA). The American Psychiatric Publishing Textbook of Psychosomatic Medicine. Psychiatric Care of the Medically Ill, 2nd ed. Arlington, VA: American Psychiatric Publishing; 2011.
- Boettger S, Jenewein J, Breitbart W. Haloperidol, risperidone, olanzapine and aripiprazole in the management of delirium: a comparison of efficacy, safety, and side effects. Palliat Support Care 2015; 13:1079–1085.
- Currier GW, Trenton AJ, Walsh PG, van Wijngaarden E. A pilot, open-label study of quetiapine for treatment of moderate psychotic agitation in the emergency setting. J Psychiatr Pract 2006; 12:223–228.
- Chatterjee S, Chen H, Johnson ML, Aparasu RR. Risk of falls and fractures in older adults using atypical antipsychotic agents: a propensity score-adjusted, retrospective cohort study. Am J Geriatr Pharmacother 2012; 10:84–94.
- Rigler SK, Shireman TI, Cook-Wiens GJ, et al. Fracture risk in nursing home residents initiating antipsychotic medications. J Am Geriatr Soc 2013; 61: 715–722.
- Bozat-Emre S, Doupe M, Kozyrskyj AL, Grymonpre R, Mahmud SM. Atypical antipsychotic drug use and falls among nursing home residents in Winnipeg, Canada. Int J Geriatr Psychiatry 2015; 30:842–850.
- Navari RM, Gray SE, Kerr AC. Olanzapine versus aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a randomized phase III trial. J Support Oncol 2011; 9:188–195.
- Navari RM. Olanzapine for the prevention and treatment of chronic nausea and chemotherapy-induced nausea and vomiting. Eur J Pharmacol 2014; 722:180–186.
- Desmarais P, Massoud F, Filion J, Nguyen QD, Bajsarowicz P. Quetiapine for psychosis in Parkinson disease and neurodegenerative Parkinsonian disorders: a systematic review. J Geriatr Psychiatry Neurol 2016; 29:227–236.
- Citrome L. Comparison of intramuscular ziprasidone, olanzapine, or aripiprazole for agitation: a quantitative review of efficacy and safety. J Clin Psychiatry 2007; 68:1876–1885.
- Marder SR, McQuade RD, Stock E, et al. Aripiprazole in the treatment of schizophrenia: safety and tolerability in short-term, placebo-controlled trials. Schizophr Res 2003; 61:123–136.
- Loh KP, Ramdass S, Garb JL, et al. Long-term outcomes of elders discharged on antipsychotics. J Hosp Med 2016; 11:550–555.
- Herzig SJ, Rothberg MB, Guess JR, et al. Antipsychotic use in hospitalized adults: rates, indications, and predictors. J Am Geriatr Soc 2016; 64:299–305.
- González M, Martínez G, Calderón J, et al. Impact of delirium on short-term mortality in elderly inpatients: a prospective cohort study. Psychosomatics 2009; 50:234–238.
KEY POINTS
- Delirium is common in hospitalized patients and often leads to loss of independence and nursing-home placement.
- The first-line treatment is to identify and address predisposing factors, provide supportive care, and manage symptoms through behavioral strategies.
- Most antipsychotic medications can prolong the QT interval and thus pose a risk for torsades de pointes. The effect is greatest with intravenous haloperidol and least with aripiprazole.
- Lacking head-to-head trials of antipsychotics, we suggest selecting the drug based on its pharmacologic properties and the patient’s clinical context.
Necrotizing pancreatitis: Diagnose, treat, consult
Acute pancreatitis accounted for more than 300,000 admissions and $2.6 billion in associated healthcare costs in the United States in 2012.1 First-line management is early aggressive fluid resuscitation and analgesics for pain control. Guidelines recommend estimating the clinical severity of each attack using a validated scoring system such as the Bedside Index of Severity in Acute Pancreatitis.2 Clinically severe pancreatitis is associated with necrosis.
Acute pancreatitis results from inappropriate activation of zymogens and subsequent autodigestion of the pancreas by its own enzymes. Though necrotizing pancreatitis is thought to be an ischemic complication, its pathogenesis is not completely understood. Necrosis increases the morbidity and mortality risk of acute pancreatitis because of its association with organ failure and infectious complications. As such, patients with necrotizing pancreatitis may need admission to the intensive care unit, nutritional support, antibiotics, and radiologic, endoscopic, or surgical interventions.
Here, we review current evidence regarding the diagnosis and management of necrotizing pancreatitis.
PROPER TERMINOLOGY HELPS COLLABORATION
Managing necrotizing pancreatitis requires the combined efforts of internists, gastroenterologists, radiologists, and surgeons. This collaboration is aided by proper terminology.
A classification system was devised in Atlanta, GA, in 1992 to facilitate communication and interdisciplinary collaboration.3 Severe pancreatitis was differentiated from mild by the presence of organ failure or the complications of pseudocyst, necrosis, or abscess.
The original Atlanta classification had several limitations. First, the terminology for fluid collections was ambiguous and frequently misused. Second, the assessment of clinical severity required either the Ranson score or the Acute Physiology and Chronic Health Evaluation II score, both of which are complex and have other limitations. Finally, advances in imaging and treatment have rendered the original Atlanta nomenclature obsolete.
In 2012, the Acute Pancreatitis Classification Working Group issued a revised Atlanta classification that modernized the terminology pertaining to natural history, severity, imaging features, and complications. It divides the natural course of acute pancreatitis into early and late phases.4
Early vs late phase
In the early phase, findings on computed tomography (CT) neither correlate with clinical severity nor alter clinical management.6 Thus, early imaging is not indicated unless there is diagnostic uncertainty, lack of response to appropriate treatment, or sudden deterioration.
Moderate pancreatitis describes patients with pancreatic necrosis with or without transient organ failure (organ dysfunction for ≤ 48 hours).
Severe pancreatitis is defined by pancreatic necrosis and persistent organ dysfunction.4 It may be accompanied by pancreatic and peripancreatic fluid collections; bacteremia and sepsis can occur in association with infection of necrotic collections.
Interstitial edematous pancreatitis vs necrotizing pancreatitis
The revised Atlanta classification maintains the original classification of acute pancreatitis into 2 main categories: interstitial edematous pancreatitis and necrotizing pancreatitis.
Necrotizing pancreatitis is further divided into 3 subtypes based on extent and location of necrosis:
- Parenchymal necrosis alone (5% of cases)
- Necrosis of peripancreatic fat alone (20%)
- Necrosis of both parenchyma and peripancreatic fat (75%).
Peripancreatic involvement is commonly found in the mesentery, peripancreatic and distant retroperitoneum, and lesser sac.
Of the three subtypes, peripancreatic necrosis has the best prognosis. However, all of the subtypes of necrotizing pancreatitis are associated with poorer outcomes than interstitial edematous pancreatitis.
Fluid collections
Acute pancreatic fluid collections contain exclusively nonsolid components without an inflammatory wall and are typically found in the peripancreatic fat. These collections often resolve without intervention as the patient recovers. If they persist beyond 4 weeks and develop a nonepithelialized, fibrous wall, they become pseudocysts. Intervention is generally not recommended for pseudocysts unless they are symptomatic.
ROLE OF IMAGING
Radiographic imaging is not usually necessary to diagnose acute pancreatitis. However, it can be a valuable tool to clarify an ambiguous presentation, determine severity, and identify complications.
The timing and appropriate type of imaging are integral to obtaining useful data. Any imaging obtained in acute pancreatitis to evaluate necrosis should be performed at least 3 to 5 days from the initial symptom onset; if imaging is obtained before 72 hours, necrosis cannot be confidently excluded.8
COMPUTED TOMOGRAPHY
CT is the imaging test of choice when evaluating acute pancreatitis. In addition, almost all percutaneous interventions are performed with CT guidance. The Balthazar score is the most well-known CT severity index. It is calculated based on the degree of inflammation, acute fluid collections, and parenchymal necrosis.9 However, a modified severity index incorporates extrapancreatic complications such as ascites and vascular compromise and was found to more strongly correlate with outcomes than the standard Balthazar score.10
Contrast-enhanced CT is performed in 2 phases:
The pancreatic parenchymal phase
The pancreatic parenchymal or late arterial phase is obtained approximately 40 to 45 seconds after the start of the contrast bolus. It is used to detect necrosis in the early phase of acute pancreatitis and to assess the peripancreatic arteries for pseudoaneurysms in the late phase of acute pancreatitis.11
Pancreatic necrosis appears as an area of decreased parenchymal enhancement, either well-defined or heterogeneous. The normal pancreatic parenchyma has a postcontrast enhancement pattern similar to that of the spleen. Parenchyma that does not enhance to the same degree is considered necrotic. The severity of necrosis is graded based on the percentage of the pancreas involved (< 30%, 30%–50%, or > 50%), and a higher percentage correlates with a worse outcome.12,13
Peripancreatic necrosis is harder to detect, as there is no method to assess fat enhancement as there is with pancreatic parenchymal enhancement. In general, radiologists assume that heterogeneous peripancreatic changes, including areas of fat, fluid, and soft tissue attenuation, are consistent with peripancreatic necrosis. After 7 to 10 days, if these changes become more homogeneous and confluent with a more mass-like process, peripancreatic necrosis can be more confidently identified.12,13
The portal venous phase
The later, portal venous phase of the scan is obtained approximately 70 seconds after the start of the contrast bolus. It is used to detect and characterize fluid collections and venous complications of the disease.
Drawbacks of CT
A drawback of CT is the need for iodinated intravenous contrast media, which in severely ill patients may precipitate or worsen pre-existing acute kidney injury.
Further, several studies have shown that findings on CT rarely alter the management of patients in the early phase of acute pancreatitis and in fact may be an overuse of medical resources.14 Unless there are confounding clinical signs or symptoms, CT should be delayed for at least 72 hours.9,10,14,15
MAGNETIC RESONANCE IMAGING
Magnetic resonance imaging (MRI) is not a first-line imaging test in this disease because it is not as available as CT and takes longer to perform—20 to 30 minutes. The patient must be evaluated for candidacy, as it is difficult for acutely ill patients to tolerate an examination that takes this long and requires them to hold their breath multiple times.
MRI is an appropriate alternative in patients who are pregnant or who have severe iodinated-contrast allergy. While contrast is necessary to detect pancreatic necrosis with CT, MRI can detect necrosis without the need for contrast in patients with acute kidney injury or severe chronic kidney disease. Also, MRI may be better in complicated cases requiring repeated imaging because it does not expose the patient to radiation.
On MRI, pancreatic necrosis appears as a heterogeneous area, owing to its liquid and solid components. Liquid components appear hyperintense, and solid components hypointense, on T2 fluid-weighted imaging. This ability to differentiate the components of a walled-off pancreatic necrosis can be useful in determining whether a collection requires drainage or debridement. MRI is also more sensitive for hemorrhagic complications, best seen on T1 fat-weighted images.12,16
Magnetic resonance cholangiopancreatography is an excellent method for ductal evaluation through heavily T2-weighted imaging. It is more sensitive than CT for detecting common bile duct stones and can also detect pancreatic duct strictures or extravasation into fluid collections.16
SUPPORTIVE MANAGEMENT OF EARLY NECROTIZING PANCREATITIS
In the early phase of necrotizing pancreatitis, management is supportive with the primary aim of preventing intravascular volume depletion. Aggressive fluid resuscitation in the first 48 to 72 hours, pain control, and bowel rest are the mainstays of supportive therapy. Intensive care may be necessary if organ failure and hemodynamic instability accompany necrotizing pancreatitis.
Prophylactic antibiotic and antifungal therapy to prevent infected necrosis has been controversial. Recent studies of its utility have not yielded supportive results, and the American College of Gastroenterology and the Infectious Diseases Society of America no longer recommend it.9,17 These medications should not be given unless concomitant cholangitis or extrapancreatic infection is clinically suspected.
Early enteral nutrition is recommended in patients in whom pancreatitis is predicted to be severe and in those not expected to resume oral intake within 5 to 7 days. Enteral nutrition most commonly involves bedside or endoscopic placement of a nasojejunal feeding tube and collaboration with a nutritionist to determine protein-caloric requirements.
Compared with enteral nutrition, total parenteral nutrition is associated with higher rates of infection, multiorgan dysfunction and failure, and death.18
MANAGING COMPLICATIONS OF PANCREATIC NECROSIS
Necrotizing pancreatitis is a defining complication of acute pancreatitis, and its presence alone indicates greater severity. However, superimposed complications may further worsen outcomes.
Infected pancreatic necrosis
Infection occurs in approximately 20% of patients with necrotizing pancreatitis and confers a mortality rate of 20% to 50%.19 Infected pancreatic necrosis occurs when gut organisms translocate into the nearby necrotic pancreatic and peripancreatic tissue. The most commonly identified organisms include Escherichia coli and Enterococcus species.20
This complication usually manifests 2 to 4 weeks after symptom onset; earlier onset is uncommon to rare. It should be considered when the systemic inflammatory response syndrome persists or recurs after 10 days to 2 weeks. Systemic inflammatory response syndrome is also common in sterile necrotizing pancreatitis and sometimes in interstitial pancreatitis, particularly during the first week. However, its sudden appearance or resurgence, high spiking fevers, or worsening organ failure in the later phase (2–4 weeks) of pancreatitis should heighten suspicion of infected pancreatic necrosis.
Imaging may also help diagnose infection, and the presence of gas within a collection or region of necrosis is highly specific. However, the presence of gas is not completely sensitive for infection, as it is seen in only 12% to 22% of infected cases.
Before minimally invasive techniques became available, the diagnosis of infected pancreatic necrosis was confirmed by percutaneous CT-guided aspiration of the necrotic mass or collection for Gram stain and culture.
Antibiotic therapy is indicated in confirmed or suspected cases of infected pancreatic necrosis. Antibiotics with gram-negative coverage and appropriate penetration such as carbapenems, metronidazole, fluoroquinolones, and selected cephalosporins are most commonly used. Meropenem is the antibiotic of choice at our institution.
CT-guided fine-needle aspiration is often done if suspected infected pancreatic necrosis fails to respond to empiric antibiotic therapy.
Debridement or drainage. Generally, the diagnosis or suspicion of infected pancreatic necrosis (suggestive signs are high fever, elevated white blood cell count, and sepsis) warrants an intervention to debride or drain infected pancreatic tissue and control sepsis.21
While source control is integral to the successful treatment of infected pancreatic necrosis, antibiotic therapy may provide a bridge to intervention for critically ill patients by suppressing bacteremia and subsequent sepsis. A 2013 meta-analysis found that 324 of 409 patients with suspected infected pancreatic necrosis were successfully stabilized with antibiotic treatment.21,22 The trend toward conservative management and promising outcomes with antibiotic therapy alone or with minimally invasive techniques has lessened the need for diagnostic CT-guided fine-needle aspiration.
Hemorrhage
Spontaneous hemorrhage into pancreatic necrosis is a rare but life-threatening complication. Because CT is almost always performed with contrast enhancement, this complication is rarely identified with imaging. The diagnosis is made by noting a drop in hemoglobin and hematocrit.
Hemorrhage into the retroperitoneum or the peritoneal cavity, or both, can occur when an inflammatory process erodes into a nearby artery. Luminal gastrointestinal bleeding can occur from gastric varices arising from splenic vein thrombosis and resulting left-sided portal hypertension, or from pseudoaneurysms. These can also bleed into the pancreatic duct (hemosuccus pancreaticus). Pseudoaneurysm is a later complication that occurs when an arterial wall (most commonly the splenic or gastroduodenal artery) is weakened by pancreatic enzymes.23
Prompt recognition of hemorrhagic events and consultation with an interventional radiologist or surgeon are required to prevent death.
Inflammation and abdominal compartment syndrome
Inflammation from necrotizing pancreatitis can cause further complications by blocking nearby structures. Reported complications include jaundice from biliary compression, hydronephrosis from ureteral compression, bowel obstruction, and gastric outlet obstruction.
Abdominal compartment syndrome is an increasingly recognized complication of acute pancreatitis. Abdominal pressure can rise due to a number of factors, including fluid collections, ascites, ileus, and overly aggressive fluid resuscitation.24 Elevated abdominal pressure is associated with complications such as decreased respiratory compliance, increased peak airway pressure, decreased cardiac preload, hypotension, mesenteric and intestinal ischemia, feeding intolerance, and lower-extremity ischemia and thrombosis.
Patients with necrotizing pancreatitis who have abdominal compartment syndrome have a mortality rate 5 times higher than patients without abdominal compartment syndrome.25
Abdominal pressures should be monitored using a bladder pressure sensor in critically ill or ventilated patients with acute pancreatitis. If the abdominal pressure rises above 20 mm Hg, medical and surgical interventions should be offered in a stepwise fashion to decrease it. Interventions include decompression by nasogastric and rectal tube, sedation or paralysis to relax abdominal wall tension, minimization of intravenous fluids, percutaneous drainage of ascites, and (rarely) surgical midline or subcostal laparotomy.
ROLE OF INTERVENTION
The treatment of necrotizing pancreatitis has changed rapidly, thanks to a growing experience with minimally invasive techniques.
Indications for intervention
Infected pancreatic necrosis is the primary indication for surgical, percutaneous, or endoscopic intervention.
In sterile necrosis, the threshold for intervention is less clear, and intervention is often reserved for patients who fail to clinically improve or who have intractable abdominal pain, gastric outlet obstruction, or fistulating disease.26
In asymptomatic cases, intervention is almost never indicated regardless of the location or size of the necrotic area.
In walled-off pancreatic necrosis, less-invasive and less-morbid interventions such as endoscopic or percutaneous drainage or video-assisted retroperitoneal debridement can be done.
Timing of intervention
In the past, delaying intervention was thought to increase the risk of death. However, multiple studies have found that outcomes are often worse if intervention is done early, likely due to the lack of a fully formed fibrous wall or demarcation of the necrotic area.27
If the patient remains clinically stable, it is best to delay intervention until at least 4 weeks after the index event to achieve optimal outcomes. Delay can often be achieved by antibiotic treatment to suppress bacteremia and endoscopic or percutaneous drainage of infected collections to control sepsis.
Open surgery
The gold-standard intervention for infected pancreatic necrosis or symptomatic sterile walled-off pancreatic necrosis is open necrosectomy. This involves exploratory laparotomy with blunt debridement of all visible necrotic pancreatic tissue.
Methods to facilitate later evacuation of residual infected fluid and debris vary widely. Multiple large-caliber drains can be placed to facilitate irrigation and drainage before closure of the abdominal fascia. As infected pancreatic necrosis carries the risk of contaminating the peritoneal cavity, the skin is often left open to heal by secondary intention. An interventional radiologist is frequently enlisted to place, exchange, or downsize drainage catheters.
Infected pancreatic necrosis or symptomatic sterile walled-off pancreatic necrosis often requires more than one operation to achieve satisfactory debridement.
The goals of open necrosectomy are to remove nonviable tissue and infection, preserve viable pancreatic tissue, eliminate fistulous connections, and minimize damage to local organs and vasculature.
Minimally invasive techniques
Video-assisted retroperitoneal debridement has been described as a hybrid between endoscopic and open retroperitoneal debridement.28 This technique requires first placing a percutaneous catheter into the necrotic area through the left flank to create a retroperitoneal tract. A 5-cm incision is made and the necrotic space is entered using the drain for guidance. Necrotic tissue is carefully debrided under direct vision using a combination of forceps, irrigation, and suction. A laparoscopic port can also be introduced into the incision when the procedure can no longer be continued under direct vision.29,30
Although not all patients are candidates for minimal-access surgery, it remains an evolving surgical option.
Endoscopic transmural debridement is another option for infected pancreatic necrosis and symptomatic walled-off pancreatic necrosis. Depending on the location of the necrotic area, an echoendoscope is passed to either the stomach or duodenum. Guided by endoscopic ultrasonography, a needle is passed into the collection, allowing subsequent fistula creation and stenting for internal drainage or debridement. In the past, this process required several steps, multiple devices, fluoroscopic guidance, and considerable time. But newer endoscopic lumen-apposing metal stents have been developed that can be placed in a single step without fluoroscopy. A slimmer endoscope can then be introduced into the necrotic cavity via the stent, and the necrotic debris can be debrided with endoscopic baskets, snares, forceps, and irrigation.9,31
Similar to surgical necrosectomy, satisfactory debridement is not often obtained with a single procedure; 2 to 5 endoscopic procedures may be needed to achieve resolution. However, the luminal approach in endoscopic necrosectomy avoids the significant morbidity of major abdominal surgery and the potential for pancreaticocutaneous fistulae that may occur with drains.
In a randomized trial comparing endoscopic necrosectomy vs surgical necrosectomy (video-assisted retroperitoneal debridement and exploratory laparotomy),32 endoscopic necrosectomy showed less inflammatory response than surgical necrosectomy and had a lower risk of new-onset organ failure, bleeding, fistula formation, and death.32
Selecting the best intervention for the individual patient
Given the multiple available techniques, selecting the best intervention for individual patients can be challenging. A team approach with input from a gastroenterologist, surgeon, and interventional radiologist is best when determining which technique would best suit each patient.
Surgical necrosectomy is still the treatment of choice for unstable patients with infected pancreatic necrosis or multiple, inaccessible collections, but current evidence suggests a different approach in stable infected pancreatic necrosis and symptomatic sterile walled-off pancreatic necrosis.
The Dutch Pancreatitis Group28 randomized 88 patients with infected pancreatic necrosis or symptomatic walled-off pancreatic necrosis to open necrosectomy or a minimally invasive “step-up” approach consisting of up to 2 percutaneous drainage or endoscopic debridement procedures before escalation to video-assisted retroperitoneal debridement. The step-up approach resulted in lower rates of morbidity and death than surgical necrosectomy as first-line treatment. Furthermore, some patients in the step-up group avoided the need for surgery entirely.30
SUMMING UP
Necrosis significantly increases rates of morbidity and mortality in acute pancreatitis. Hospitalists, general internists, and general surgeons are all on the front lines in identifying severe cases and consulting the appropriate specialists for optimal multidisciplinary care. Selective and appropriate timing of radiologic imaging is key, and a vital tool in the management of necrotizing pancreatitis.
While the primary indication for intervention is infected pancreatic necrosis, additional indications are symptomatic walled-off pancreatic necrosis secondary to intractable abdominal pain, bowel obstruction, and failure to thrive. As a result of improving technology and inpatient care, these patients may present with intractable symptoms in the outpatient setting rather than the inpatient setting. The onus is on the primary care physician to maintain a high level of suspicion and refer these patients to subspecialists as appropriate.
Open surgical necrosectomy remains an important approach for care of infected pancreatic necrosis or patients with intractable symptoms. A step-up approach starting with a minimally invasive procedure and escalating if the initial intervention is unsuccessful is gradually becoming the standard of care.
- Peery AF, Crockett SD, Barritt AS, et al. Burden of gastrointestinal, liver, and pancreatic disease in the United States. Gastroenterology 2015; 149:1731–1741e3.
- Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol 2013; 108:1400–1416.
- Bradley EL 3rd. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, GA, September 11 through 13, 1992. Arch Surg 1993; 128:586–590.
- Banks PA, Bollen TL, Dervenis C, et al; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013; 62:102–111.
- Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med 1995; 23:1638–1652.
- Kadiyala V, Suleiman SL, McNabb-Baltar J, Wu BU, Banks PA, Singh VK. The Atlanta classification, revised Atlanta classification, and determinant-based classification of acute pancreatitis: which is best at stratifying outcomes? Pancreas 2016; 45:510–515.
- Singh VK, Bollen TL, Wu BU, et al. An assessment of the severity of interstitial pancreatitis. Clin Gastroenterol Hepatol 2011; 9:1098–1103.
- Kotwal V, Talukdar R, Levy M, Vege SS. Role of endoscopic ultrasound during hospitalization for acute pancreatitis. World J Gastroenterol 2010; 16:4888–4891.
- Balthazar EJ. Acute pancreatitis: assessment of severity with clinical and CT evaluation. Radiology 2002; 223:603–613.
- Mortele KJ, Wiesner W, Intriere L, et al. A modified CT severity index for evaluating acute pancreatitis: improved correlation with patient outcome. AJR Am J Roentgenol 2004; 183:1261–1265.
- Verde F, Fishman EK, Johnson PT. Arterial pseudoaneurysms complicating pancreatitis: literature review. J Comput Assist Tomogr 2015; 39:7–12.
- Shyu JY, Sainani NI, Sahni VA, et al. Necrotizing pancreatitis: diagnosis, imaging, and intervention. Radiographics 2014; 34:1218–1239.
- Thoeni RF. The revised Atlanta classification of acute pancreatitis: its importance for the radiologist and its effect on treatment. Radiology 2012; 262:751–764.
- Morgan DE, Ragheb CM, Lockhart ME, Cary B, Fineberg NS, Berland LL. Acute pancreatitis: computed tomography utilization and radiation exposure are related to severity but not patient age. Clin Gastroenterol Hepatol 2010; 8:303–308.
- Vitellas KM, Paulson EK, Enns RA, Keogan MT, Pappas TN. Pancreatitis complicated by gland necrosis: evolution of findings on contrast-enhanced CT. J Comput Assist Tomogr 1999; 23:898–905.
- Stimac D, Miletic D, Radic M, et al. The role of nonenhanced magnetic resonance imaging in the early assessment of acute pancreatitis. Am J Gastroenterol 2007; 102:997–1004.
- Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Surg Infect (Larchmt) 2010; 11:79–109.
- Petrov MS, Kukosh MV, Emelyanov NV. A randomized controlled trial of enteral versus parenteral feeding in patients with predicted severe acute pancreatitis shows a significant reduction in mortality and in infected pancreatic complications with total enteral nutrition. Dig Surg 2006; 23:336–345.
- Petrov MS, Shanbhag S, Chakraborty M, Phillips AR, Windsor JA. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology 2010; 139:813–820.
- Villatoro E, Bassi C, Larvin M. Antibiotic therapy for prophylaxis against infection of pancreatic necrosis in acute pancreatitis. Cochrane Database Syst Rev 2006; 4:CD002941.
- Baril NB, Ralls PW, Wren SM, et al. Does an infected peripancreatic fluid collection or abscess mandate operation? Ann Surg 2000; 231:361–367.
- Mouli VP, Sreenivas V, Garg PK. Efficacy of conservative treatment, without necrosectomy, for infected pancreatic necrosis: a systematic review and meta-analysis. Gastroenterology 2013; 144:333–340.e2.
- Kirby JM, Vora P, Midia M, Rawlinson J. Vascular complications of pancreatitis: imaging and intervention. Cardiovasc Intervent Radiol 2008; 31:957–970.
- De Waele JJ, Hoste E, Blot SI, Decruyenaere J, Colardyn F. Intra-abdominal hypertension in patients with severe acute pancreatitis. Crit Care 2005; 9:R452–R457.
- van Brunschot S, Schut AJ, Bouwense SA, et al; Dutch Pancreatitis Study Group. Abdominal compartment syndrome in acute pancreatitis: a systematic review. Pancreas 2014; 43:665–674.
- Bugiantella W, Rondelli F, Boni M, et al. Necrotizing pancreatitis: a review of the interventions. Int J Surg 2016; 28(suppl 1):S163–S171.
- Besselink MG, Verwer TJ, Schoenmaeckers EJ, et al. Timing of surgical intervention in necrotizing pancreatitis. Arch Surg 2007; 142:1194–1201.
- van Santvoort HC, Besselink MG, Horvath KD, et al; Dutch Acute Pancreatis Study Group. Videoscopic assisted retroperitoneal debridement in infected necrotizing pancreatitis. HPB (Oxford) 2007; 9:156–159.
- van Santvoort HC, Besselink MG, Bollen TL, Buskens E, van Ramshorst B, Gooszen HG; Dutch Acute Pancreatitis Study Group. Case-matched comparison of the retroperitoneal approach with laparotomy for necrotizing pancreatitis. World J Surg 2007; 31:1635–1642.
- van Santvoort HC, Besselink MG, Bakker OJ, et al; Dutch Pancreatitis Study Group. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med 2010; 362:1491–1502.
- Thompson CC, Kumar N, Slattery J, et al. A standardized method for endoscopic necrosectomy improves complication and mortality rates. Pancreatology 2016; 16:66–72.
- Bakker OJ, van Santvoort HC, van Brunschot S, et al; Dutch Pancreatitis Study Group. Endoscopic transgastric vs surgical necrosectomy for infected necrotizing pancreatitis: a randomized trial. JAMA 2012; 307:1053–1061.
Acute pancreatitis accounted for more than 300,000 admissions and $2.6 billion in associated healthcare costs in the United States in 2012.1 First-line management is early aggressive fluid resuscitation and analgesics for pain control. Guidelines recommend estimating the clinical severity of each attack using a validated scoring system such as the Bedside Index of Severity in Acute Pancreatitis.2 Clinically severe pancreatitis is associated with necrosis.
Acute pancreatitis results from inappropriate activation of zymogens and subsequent autodigestion of the pancreas by its own enzymes. Though necrotizing pancreatitis is thought to be an ischemic complication, its pathogenesis is not completely understood. Necrosis increases the morbidity and mortality risk of acute pancreatitis because of its association with organ failure and infectious complications. As such, patients with necrotizing pancreatitis may need admission to the intensive care unit, nutritional support, antibiotics, and radiologic, endoscopic, or surgical interventions.
Here, we review current evidence regarding the diagnosis and management of necrotizing pancreatitis.
PROPER TERMINOLOGY HELPS COLLABORATION
Managing necrotizing pancreatitis requires the combined efforts of internists, gastroenterologists, radiologists, and surgeons. This collaboration is aided by proper terminology.
A classification system was devised in Atlanta, GA, in 1992 to facilitate communication and interdisciplinary collaboration.3 Severe pancreatitis was differentiated from mild by the presence of organ failure or the complications of pseudocyst, necrosis, or abscess.
The original Atlanta classification had several limitations. First, the terminology for fluid collections was ambiguous and frequently misused. Second, the assessment of clinical severity required either the Ranson score or the Acute Physiology and Chronic Health Evaluation II score, both of which are complex and have other limitations. Finally, advances in imaging and treatment have rendered the original Atlanta nomenclature obsolete.
In 2012, the Acute Pancreatitis Classification Working Group issued a revised Atlanta classification that modernized the terminology pertaining to natural history, severity, imaging features, and complications. It divides the natural course of acute pancreatitis into early and late phases.4
Early vs late phase
In the early phase, findings on computed tomography (CT) neither correlate with clinical severity nor alter clinical management.6 Thus, early imaging is not indicated unless there is diagnostic uncertainty, lack of response to appropriate treatment, or sudden deterioration.
Moderate pancreatitis describes patients with pancreatic necrosis with or without transient organ failure (organ dysfunction for ≤ 48 hours).
Severe pancreatitis is defined by pancreatic necrosis and persistent organ dysfunction.4 It may be accompanied by pancreatic and peripancreatic fluid collections; bacteremia and sepsis can occur in association with infection of necrotic collections.
Interstitial edematous pancreatitis vs necrotizing pancreatitis
The revised Atlanta classification maintains the original classification of acute pancreatitis into 2 main categories: interstitial edematous pancreatitis and necrotizing pancreatitis.
Necrotizing pancreatitis is further divided into 3 subtypes based on extent and location of necrosis:
- Parenchymal necrosis alone (5% of cases)
- Necrosis of peripancreatic fat alone (20%)
- Necrosis of both parenchyma and peripancreatic fat (75%).
Peripancreatic involvement is commonly found in the mesentery, peripancreatic and distant retroperitoneum, and lesser sac.
Of the three subtypes, peripancreatic necrosis has the best prognosis. However, all of the subtypes of necrotizing pancreatitis are associated with poorer outcomes than interstitial edematous pancreatitis.
Fluid collections
Acute pancreatic fluid collections contain exclusively nonsolid components without an inflammatory wall and are typically found in the peripancreatic fat. These collections often resolve without intervention as the patient recovers. If they persist beyond 4 weeks and develop a nonepithelialized, fibrous wall, they become pseudocysts. Intervention is generally not recommended for pseudocysts unless they are symptomatic.
ROLE OF IMAGING
Radiographic imaging is not usually necessary to diagnose acute pancreatitis. However, it can be a valuable tool to clarify an ambiguous presentation, determine severity, and identify complications.
The timing and appropriate type of imaging are integral to obtaining useful data. Any imaging obtained in acute pancreatitis to evaluate necrosis should be performed at least 3 to 5 days from the initial symptom onset; if imaging is obtained before 72 hours, necrosis cannot be confidently excluded.8
COMPUTED TOMOGRAPHY
CT is the imaging test of choice when evaluating acute pancreatitis. In addition, almost all percutaneous interventions are performed with CT guidance. The Balthazar score is the most well-known CT severity index. It is calculated based on the degree of inflammation, acute fluid collections, and parenchymal necrosis.9 However, a modified severity index incorporates extrapancreatic complications such as ascites and vascular compromise and was found to more strongly correlate with outcomes than the standard Balthazar score.10
Contrast-enhanced CT is performed in 2 phases:
The pancreatic parenchymal phase
The pancreatic parenchymal or late arterial phase is obtained approximately 40 to 45 seconds after the start of the contrast bolus. It is used to detect necrosis in the early phase of acute pancreatitis and to assess the peripancreatic arteries for pseudoaneurysms in the late phase of acute pancreatitis.11
Pancreatic necrosis appears as an area of decreased parenchymal enhancement, either well-defined or heterogeneous. The normal pancreatic parenchyma has a postcontrast enhancement pattern similar to that of the spleen. Parenchyma that does not enhance to the same degree is considered necrotic. The severity of necrosis is graded based on the percentage of the pancreas involved (< 30%, 30%–50%, or > 50%), and a higher percentage correlates with a worse outcome.12,13
Peripancreatic necrosis is harder to detect, as there is no method to assess fat enhancement as there is with pancreatic parenchymal enhancement. In general, radiologists assume that heterogeneous peripancreatic changes, including areas of fat, fluid, and soft tissue attenuation, are consistent with peripancreatic necrosis. After 7 to 10 days, if these changes become more homogeneous and confluent with a more mass-like process, peripancreatic necrosis can be more confidently identified.12,13
The portal venous phase
The later, portal venous phase of the scan is obtained approximately 70 seconds after the start of the contrast bolus. It is used to detect and characterize fluid collections and venous complications of the disease.
Drawbacks of CT
A drawback of CT is the need for iodinated intravenous contrast media, which in severely ill patients may precipitate or worsen pre-existing acute kidney injury.
Further, several studies have shown that findings on CT rarely alter the management of patients in the early phase of acute pancreatitis and in fact may be an overuse of medical resources.14 Unless there are confounding clinical signs or symptoms, CT should be delayed for at least 72 hours.9,10,14,15
MAGNETIC RESONANCE IMAGING
Magnetic resonance imaging (MRI) is not a first-line imaging test in this disease because it is not as available as CT and takes longer to perform—20 to 30 minutes. The patient must be evaluated for candidacy, as it is difficult for acutely ill patients to tolerate an examination that takes this long and requires them to hold their breath multiple times.
MRI is an appropriate alternative in patients who are pregnant or who have severe iodinated-contrast allergy. While contrast is necessary to detect pancreatic necrosis with CT, MRI can detect necrosis without the need for contrast in patients with acute kidney injury or severe chronic kidney disease. Also, MRI may be better in complicated cases requiring repeated imaging because it does not expose the patient to radiation.
On MRI, pancreatic necrosis appears as a heterogeneous area, owing to its liquid and solid components. Liquid components appear hyperintense, and solid components hypointense, on T2 fluid-weighted imaging. This ability to differentiate the components of a walled-off pancreatic necrosis can be useful in determining whether a collection requires drainage or debridement. MRI is also more sensitive for hemorrhagic complications, best seen on T1 fat-weighted images.12,16
Magnetic resonance cholangiopancreatography is an excellent method for ductal evaluation through heavily T2-weighted imaging. It is more sensitive than CT for detecting common bile duct stones and can also detect pancreatic duct strictures or extravasation into fluid collections.16
SUPPORTIVE MANAGEMENT OF EARLY NECROTIZING PANCREATITIS
In the early phase of necrotizing pancreatitis, management is supportive with the primary aim of preventing intravascular volume depletion. Aggressive fluid resuscitation in the first 48 to 72 hours, pain control, and bowel rest are the mainstays of supportive therapy. Intensive care may be necessary if organ failure and hemodynamic instability accompany necrotizing pancreatitis.
Prophylactic antibiotic and antifungal therapy to prevent infected necrosis has been controversial. Recent studies of its utility have not yielded supportive results, and the American College of Gastroenterology and the Infectious Diseases Society of America no longer recommend it.9,17 These medications should not be given unless concomitant cholangitis or extrapancreatic infection is clinically suspected.
Early enteral nutrition is recommended in patients in whom pancreatitis is predicted to be severe and in those not expected to resume oral intake within 5 to 7 days. Enteral nutrition most commonly involves bedside or endoscopic placement of a nasojejunal feeding tube and collaboration with a nutritionist to determine protein-caloric requirements.
Compared with enteral nutrition, total parenteral nutrition is associated with higher rates of infection, multiorgan dysfunction and failure, and death.18
MANAGING COMPLICATIONS OF PANCREATIC NECROSIS
Necrotizing pancreatitis is a defining complication of acute pancreatitis, and its presence alone indicates greater severity. However, superimposed complications may further worsen outcomes.
Infected pancreatic necrosis
Infection occurs in approximately 20% of patients with necrotizing pancreatitis and confers a mortality rate of 20% to 50%.19 Infected pancreatic necrosis occurs when gut organisms translocate into the nearby necrotic pancreatic and peripancreatic tissue. The most commonly identified organisms include Escherichia coli and Enterococcus species.20
This complication usually manifests 2 to 4 weeks after symptom onset; earlier onset is uncommon to rare. It should be considered when the systemic inflammatory response syndrome persists or recurs after 10 days to 2 weeks. Systemic inflammatory response syndrome is also common in sterile necrotizing pancreatitis and sometimes in interstitial pancreatitis, particularly during the first week. However, its sudden appearance or resurgence, high spiking fevers, or worsening organ failure in the later phase (2–4 weeks) of pancreatitis should heighten suspicion of infected pancreatic necrosis.
Imaging may also help diagnose infection, and the presence of gas within a collection or region of necrosis is highly specific. However, the presence of gas is not completely sensitive for infection, as it is seen in only 12% to 22% of infected cases.
Before minimally invasive techniques became available, the diagnosis of infected pancreatic necrosis was confirmed by percutaneous CT-guided aspiration of the necrotic mass or collection for Gram stain and culture.
Antibiotic therapy is indicated in confirmed or suspected cases of infected pancreatic necrosis. Antibiotics with gram-negative coverage and appropriate penetration such as carbapenems, metronidazole, fluoroquinolones, and selected cephalosporins are most commonly used. Meropenem is the antibiotic of choice at our institution.
CT-guided fine-needle aspiration is often done if suspected infected pancreatic necrosis fails to respond to empiric antibiotic therapy.
Debridement or drainage. Generally, the diagnosis or suspicion of infected pancreatic necrosis (suggestive signs are high fever, elevated white blood cell count, and sepsis) warrants an intervention to debride or drain infected pancreatic tissue and control sepsis.21
While source control is integral to the successful treatment of infected pancreatic necrosis, antibiotic therapy may provide a bridge to intervention for critically ill patients by suppressing bacteremia and subsequent sepsis. A 2013 meta-analysis found that 324 of 409 patients with suspected infected pancreatic necrosis were successfully stabilized with antibiotic treatment.21,22 The trend toward conservative management and promising outcomes with antibiotic therapy alone or with minimally invasive techniques has lessened the need for diagnostic CT-guided fine-needle aspiration.
Hemorrhage
Spontaneous hemorrhage into pancreatic necrosis is a rare but life-threatening complication. Because CT is almost always performed with contrast enhancement, this complication is rarely identified with imaging. The diagnosis is made by noting a drop in hemoglobin and hematocrit.
Hemorrhage into the retroperitoneum or the peritoneal cavity, or both, can occur when an inflammatory process erodes into a nearby artery. Luminal gastrointestinal bleeding can occur from gastric varices arising from splenic vein thrombosis and resulting left-sided portal hypertension, or from pseudoaneurysms. These can also bleed into the pancreatic duct (hemosuccus pancreaticus). Pseudoaneurysm is a later complication that occurs when an arterial wall (most commonly the splenic or gastroduodenal artery) is weakened by pancreatic enzymes.23
Prompt recognition of hemorrhagic events and consultation with an interventional radiologist or surgeon are required to prevent death.
Inflammation and abdominal compartment syndrome
Inflammation from necrotizing pancreatitis can cause further complications by blocking nearby structures. Reported complications include jaundice from biliary compression, hydronephrosis from ureteral compression, bowel obstruction, and gastric outlet obstruction.
Abdominal compartment syndrome is an increasingly recognized complication of acute pancreatitis. Abdominal pressure can rise due to a number of factors, including fluid collections, ascites, ileus, and overly aggressive fluid resuscitation.24 Elevated abdominal pressure is associated with complications such as decreased respiratory compliance, increased peak airway pressure, decreased cardiac preload, hypotension, mesenteric and intestinal ischemia, feeding intolerance, and lower-extremity ischemia and thrombosis.
Patients with necrotizing pancreatitis who have abdominal compartment syndrome have a mortality rate 5 times higher than patients without abdominal compartment syndrome.25
Abdominal pressures should be monitored using a bladder pressure sensor in critically ill or ventilated patients with acute pancreatitis. If the abdominal pressure rises above 20 mm Hg, medical and surgical interventions should be offered in a stepwise fashion to decrease it. Interventions include decompression by nasogastric and rectal tube, sedation or paralysis to relax abdominal wall tension, minimization of intravenous fluids, percutaneous drainage of ascites, and (rarely) surgical midline or subcostal laparotomy.
ROLE OF INTERVENTION
The treatment of necrotizing pancreatitis has changed rapidly, thanks to a growing experience with minimally invasive techniques.
Indications for intervention
Infected pancreatic necrosis is the primary indication for surgical, percutaneous, or endoscopic intervention.
In sterile necrosis, the threshold for intervention is less clear, and intervention is often reserved for patients who fail to clinically improve or who have intractable abdominal pain, gastric outlet obstruction, or fistulating disease.26
In asymptomatic cases, intervention is almost never indicated regardless of the location or size of the necrotic area.
In walled-off pancreatic necrosis, less-invasive and less-morbid interventions such as endoscopic or percutaneous drainage or video-assisted retroperitoneal debridement can be done.
Timing of intervention
In the past, delaying intervention was thought to increase the risk of death. However, multiple studies have found that outcomes are often worse if intervention is done early, likely due to the lack of a fully formed fibrous wall or demarcation of the necrotic area.27
If the patient remains clinically stable, it is best to delay intervention until at least 4 weeks after the index event to achieve optimal outcomes. Delay can often be achieved by antibiotic treatment to suppress bacteremia and endoscopic or percutaneous drainage of infected collections to control sepsis.
Open surgery
The gold-standard intervention for infected pancreatic necrosis or symptomatic sterile walled-off pancreatic necrosis is open necrosectomy. This involves exploratory laparotomy with blunt debridement of all visible necrotic pancreatic tissue.
Methods to facilitate later evacuation of residual infected fluid and debris vary widely. Multiple large-caliber drains can be placed to facilitate irrigation and drainage before closure of the abdominal fascia. As infected pancreatic necrosis carries the risk of contaminating the peritoneal cavity, the skin is often left open to heal by secondary intention. An interventional radiologist is frequently enlisted to place, exchange, or downsize drainage catheters.
Infected pancreatic necrosis or symptomatic sterile walled-off pancreatic necrosis often requires more than one operation to achieve satisfactory debridement.
The goals of open necrosectomy are to remove nonviable tissue and infection, preserve viable pancreatic tissue, eliminate fistulous connections, and minimize damage to local organs and vasculature.
Minimally invasive techniques
Video-assisted retroperitoneal debridement has been described as a hybrid between endoscopic and open retroperitoneal debridement.28 This technique requires first placing a percutaneous catheter into the necrotic area through the left flank to create a retroperitoneal tract. A 5-cm incision is made and the necrotic space is entered using the drain for guidance. Necrotic tissue is carefully debrided under direct vision using a combination of forceps, irrigation, and suction. A laparoscopic port can also be introduced into the incision when the procedure can no longer be continued under direct vision.29,30
Although not all patients are candidates for minimal-access surgery, it remains an evolving surgical option.
Endoscopic transmural debridement is another option for infected pancreatic necrosis and symptomatic walled-off pancreatic necrosis. Depending on the location of the necrotic area, an echoendoscope is passed to either the stomach or duodenum. Guided by endoscopic ultrasonography, a needle is passed into the collection, allowing subsequent fistula creation and stenting for internal drainage or debridement. In the past, this process required several steps, multiple devices, fluoroscopic guidance, and considerable time. But newer endoscopic lumen-apposing metal stents have been developed that can be placed in a single step without fluoroscopy. A slimmer endoscope can then be introduced into the necrotic cavity via the stent, and the necrotic debris can be debrided with endoscopic baskets, snares, forceps, and irrigation.9,31
Similar to surgical necrosectomy, satisfactory debridement is not often obtained with a single procedure; 2 to 5 endoscopic procedures may be needed to achieve resolution. However, the luminal approach in endoscopic necrosectomy avoids the significant morbidity of major abdominal surgery and the potential for pancreaticocutaneous fistulae that may occur with drains.
In a randomized trial comparing endoscopic necrosectomy vs surgical necrosectomy (video-assisted retroperitoneal debridement and exploratory laparotomy),32 endoscopic necrosectomy showed less inflammatory response than surgical necrosectomy and had a lower risk of new-onset organ failure, bleeding, fistula formation, and death.32
Selecting the best intervention for the individual patient
Given the multiple available techniques, selecting the best intervention for individual patients can be challenging. A team approach with input from a gastroenterologist, surgeon, and interventional radiologist is best when determining which technique would best suit each patient.
Surgical necrosectomy is still the treatment of choice for unstable patients with infected pancreatic necrosis or multiple, inaccessible collections, but current evidence suggests a different approach in stable infected pancreatic necrosis and symptomatic sterile walled-off pancreatic necrosis.
The Dutch Pancreatitis Group28 randomized 88 patients with infected pancreatic necrosis or symptomatic walled-off pancreatic necrosis to open necrosectomy or a minimally invasive “step-up” approach consisting of up to 2 percutaneous drainage or endoscopic debridement procedures before escalation to video-assisted retroperitoneal debridement. The step-up approach resulted in lower rates of morbidity and death than surgical necrosectomy as first-line treatment. Furthermore, some patients in the step-up group avoided the need for surgery entirely.30
SUMMING UP
Necrosis significantly increases rates of morbidity and mortality in acute pancreatitis. Hospitalists, general internists, and general surgeons are all on the front lines in identifying severe cases and consulting the appropriate specialists for optimal multidisciplinary care. Selective and appropriate timing of radiologic imaging is key, and a vital tool in the management of necrotizing pancreatitis.
While the primary indication for intervention is infected pancreatic necrosis, additional indications are symptomatic walled-off pancreatic necrosis secondary to intractable abdominal pain, bowel obstruction, and failure to thrive. As a result of improving technology and inpatient care, these patients may present with intractable symptoms in the outpatient setting rather than the inpatient setting. The onus is on the primary care physician to maintain a high level of suspicion and refer these patients to subspecialists as appropriate.
Open surgical necrosectomy remains an important approach for care of infected pancreatic necrosis or patients with intractable symptoms. A step-up approach starting with a minimally invasive procedure and escalating if the initial intervention is unsuccessful is gradually becoming the standard of care.
Acute pancreatitis accounted for more than 300,000 admissions and $2.6 billion in associated healthcare costs in the United States in 2012.1 First-line management is early aggressive fluid resuscitation and analgesics for pain control. Guidelines recommend estimating the clinical severity of each attack using a validated scoring system such as the Bedside Index of Severity in Acute Pancreatitis.2 Clinically severe pancreatitis is associated with necrosis.
Acute pancreatitis results from inappropriate activation of zymogens and subsequent autodigestion of the pancreas by its own enzymes. Though necrotizing pancreatitis is thought to be an ischemic complication, its pathogenesis is not completely understood. Necrosis increases the morbidity and mortality risk of acute pancreatitis because of its association with organ failure and infectious complications. As such, patients with necrotizing pancreatitis may need admission to the intensive care unit, nutritional support, antibiotics, and radiologic, endoscopic, or surgical interventions.
Here, we review current evidence regarding the diagnosis and management of necrotizing pancreatitis.
PROPER TERMINOLOGY HELPS COLLABORATION
Managing necrotizing pancreatitis requires the combined efforts of internists, gastroenterologists, radiologists, and surgeons. This collaboration is aided by proper terminology.
A classification system was devised in Atlanta, GA, in 1992 to facilitate communication and interdisciplinary collaboration.3 Severe pancreatitis was differentiated from mild by the presence of organ failure or the complications of pseudocyst, necrosis, or abscess.
The original Atlanta classification had several limitations. First, the terminology for fluid collections was ambiguous and frequently misused. Second, the assessment of clinical severity required either the Ranson score or the Acute Physiology and Chronic Health Evaluation II score, both of which are complex and have other limitations. Finally, advances in imaging and treatment have rendered the original Atlanta nomenclature obsolete.
In 2012, the Acute Pancreatitis Classification Working Group issued a revised Atlanta classification that modernized the terminology pertaining to natural history, severity, imaging features, and complications. It divides the natural course of acute pancreatitis into early and late phases.4
Early vs late phase
In the early phase, findings on computed tomography (CT) neither correlate with clinical severity nor alter clinical management.6 Thus, early imaging is not indicated unless there is diagnostic uncertainty, lack of response to appropriate treatment, or sudden deterioration.
Moderate pancreatitis describes patients with pancreatic necrosis with or without transient organ failure (organ dysfunction for ≤ 48 hours).
Severe pancreatitis is defined by pancreatic necrosis and persistent organ dysfunction.4 It may be accompanied by pancreatic and peripancreatic fluid collections; bacteremia and sepsis can occur in association with infection of necrotic collections.
Interstitial edematous pancreatitis vs necrotizing pancreatitis
The revised Atlanta classification maintains the original classification of acute pancreatitis into 2 main categories: interstitial edematous pancreatitis and necrotizing pancreatitis.
Necrotizing pancreatitis is further divided into 3 subtypes based on extent and location of necrosis:
- Parenchymal necrosis alone (5% of cases)
- Necrosis of peripancreatic fat alone (20%)
- Necrosis of both parenchyma and peripancreatic fat (75%).
Peripancreatic involvement is commonly found in the mesentery, peripancreatic and distant retroperitoneum, and lesser sac.
Of the three subtypes, peripancreatic necrosis has the best prognosis. However, all of the subtypes of necrotizing pancreatitis are associated with poorer outcomes than interstitial edematous pancreatitis.
Fluid collections
Acute pancreatic fluid collections contain exclusively nonsolid components without an inflammatory wall and are typically found in the peripancreatic fat. These collections often resolve without intervention as the patient recovers. If they persist beyond 4 weeks and develop a nonepithelialized, fibrous wall, they become pseudocysts. Intervention is generally not recommended for pseudocysts unless they are symptomatic.
ROLE OF IMAGING
Radiographic imaging is not usually necessary to diagnose acute pancreatitis. However, it can be a valuable tool to clarify an ambiguous presentation, determine severity, and identify complications.
The timing and appropriate type of imaging are integral to obtaining useful data. Any imaging obtained in acute pancreatitis to evaluate necrosis should be performed at least 3 to 5 days from the initial symptom onset; if imaging is obtained before 72 hours, necrosis cannot be confidently excluded.8
COMPUTED TOMOGRAPHY
CT is the imaging test of choice when evaluating acute pancreatitis. In addition, almost all percutaneous interventions are performed with CT guidance. The Balthazar score is the most well-known CT severity index. It is calculated based on the degree of inflammation, acute fluid collections, and parenchymal necrosis.9 However, a modified severity index incorporates extrapancreatic complications such as ascites and vascular compromise and was found to more strongly correlate with outcomes than the standard Balthazar score.10
Contrast-enhanced CT is performed in 2 phases:
The pancreatic parenchymal phase
The pancreatic parenchymal or late arterial phase is obtained approximately 40 to 45 seconds after the start of the contrast bolus. It is used to detect necrosis in the early phase of acute pancreatitis and to assess the peripancreatic arteries for pseudoaneurysms in the late phase of acute pancreatitis.11
Pancreatic necrosis appears as an area of decreased parenchymal enhancement, either well-defined or heterogeneous. The normal pancreatic parenchyma has a postcontrast enhancement pattern similar to that of the spleen. Parenchyma that does not enhance to the same degree is considered necrotic. The severity of necrosis is graded based on the percentage of the pancreas involved (< 30%, 30%–50%, or > 50%), and a higher percentage correlates with a worse outcome.12,13
Peripancreatic necrosis is harder to detect, as there is no method to assess fat enhancement as there is with pancreatic parenchymal enhancement. In general, radiologists assume that heterogeneous peripancreatic changes, including areas of fat, fluid, and soft tissue attenuation, are consistent with peripancreatic necrosis. After 7 to 10 days, if these changes become more homogeneous and confluent with a more mass-like process, peripancreatic necrosis can be more confidently identified.12,13
The portal venous phase
The later, portal venous phase of the scan is obtained approximately 70 seconds after the start of the contrast bolus. It is used to detect and characterize fluid collections and venous complications of the disease.
Drawbacks of CT
A drawback of CT is the need for iodinated intravenous contrast media, which in severely ill patients may precipitate or worsen pre-existing acute kidney injury.
Further, several studies have shown that findings on CT rarely alter the management of patients in the early phase of acute pancreatitis and in fact may be an overuse of medical resources.14 Unless there are confounding clinical signs or symptoms, CT should be delayed for at least 72 hours.9,10,14,15
MAGNETIC RESONANCE IMAGING
Magnetic resonance imaging (MRI) is not a first-line imaging test in this disease because it is not as available as CT and takes longer to perform—20 to 30 minutes. The patient must be evaluated for candidacy, as it is difficult for acutely ill patients to tolerate an examination that takes this long and requires them to hold their breath multiple times.
MRI is an appropriate alternative in patients who are pregnant or who have severe iodinated-contrast allergy. While contrast is necessary to detect pancreatic necrosis with CT, MRI can detect necrosis without the need for contrast in patients with acute kidney injury or severe chronic kidney disease. Also, MRI may be better in complicated cases requiring repeated imaging because it does not expose the patient to radiation.
On MRI, pancreatic necrosis appears as a heterogeneous area, owing to its liquid and solid components. Liquid components appear hyperintense, and solid components hypointense, on T2 fluid-weighted imaging. This ability to differentiate the components of a walled-off pancreatic necrosis can be useful in determining whether a collection requires drainage or debridement. MRI is also more sensitive for hemorrhagic complications, best seen on T1 fat-weighted images.12,16
Magnetic resonance cholangiopancreatography is an excellent method for ductal evaluation through heavily T2-weighted imaging. It is more sensitive than CT for detecting common bile duct stones and can also detect pancreatic duct strictures or extravasation into fluid collections.16
SUPPORTIVE MANAGEMENT OF EARLY NECROTIZING PANCREATITIS
In the early phase of necrotizing pancreatitis, management is supportive with the primary aim of preventing intravascular volume depletion. Aggressive fluid resuscitation in the first 48 to 72 hours, pain control, and bowel rest are the mainstays of supportive therapy. Intensive care may be necessary if organ failure and hemodynamic instability accompany necrotizing pancreatitis.
Prophylactic antibiotic and antifungal therapy to prevent infected necrosis has been controversial. Recent studies of its utility have not yielded supportive results, and the American College of Gastroenterology and the Infectious Diseases Society of America no longer recommend it.9,17 These medications should not be given unless concomitant cholangitis or extrapancreatic infection is clinically suspected.
Early enteral nutrition is recommended in patients in whom pancreatitis is predicted to be severe and in those not expected to resume oral intake within 5 to 7 days. Enteral nutrition most commonly involves bedside or endoscopic placement of a nasojejunal feeding tube and collaboration with a nutritionist to determine protein-caloric requirements.
Compared with enteral nutrition, total parenteral nutrition is associated with higher rates of infection, multiorgan dysfunction and failure, and death.18
MANAGING COMPLICATIONS OF PANCREATIC NECROSIS
Necrotizing pancreatitis is a defining complication of acute pancreatitis, and its presence alone indicates greater severity. However, superimposed complications may further worsen outcomes.
Infected pancreatic necrosis
Infection occurs in approximately 20% of patients with necrotizing pancreatitis and confers a mortality rate of 20% to 50%.19 Infected pancreatic necrosis occurs when gut organisms translocate into the nearby necrotic pancreatic and peripancreatic tissue. The most commonly identified organisms include Escherichia coli and Enterococcus species.20
This complication usually manifests 2 to 4 weeks after symptom onset; earlier onset is uncommon to rare. It should be considered when the systemic inflammatory response syndrome persists or recurs after 10 days to 2 weeks. Systemic inflammatory response syndrome is also common in sterile necrotizing pancreatitis and sometimes in interstitial pancreatitis, particularly during the first week. However, its sudden appearance or resurgence, high spiking fevers, or worsening organ failure in the later phase (2–4 weeks) of pancreatitis should heighten suspicion of infected pancreatic necrosis.
Imaging may also help diagnose infection, and the presence of gas within a collection or region of necrosis is highly specific. However, the presence of gas is not completely sensitive for infection, as it is seen in only 12% to 22% of infected cases.
Before minimally invasive techniques became available, the diagnosis of infected pancreatic necrosis was confirmed by percutaneous CT-guided aspiration of the necrotic mass or collection for Gram stain and culture.
Antibiotic therapy is indicated in confirmed or suspected cases of infected pancreatic necrosis. Antibiotics with gram-negative coverage and appropriate penetration such as carbapenems, metronidazole, fluoroquinolones, and selected cephalosporins are most commonly used. Meropenem is the antibiotic of choice at our institution.
CT-guided fine-needle aspiration is often done if suspected infected pancreatic necrosis fails to respond to empiric antibiotic therapy.
Debridement or drainage. Generally, the diagnosis or suspicion of infected pancreatic necrosis (suggestive signs are high fever, elevated white blood cell count, and sepsis) warrants an intervention to debride or drain infected pancreatic tissue and control sepsis.21
While source control is integral to the successful treatment of infected pancreatic necrosis, antibiotic therapy may provide a bridge to intervention for critically ill patients by suppressing bacteremia and subsequent sepsis. A 2013 meta-analysis found that 324 of 409 patients with suspected infected pancreatic necrosis were successfully stabilized with antibiotic treatment.21,22 The trend toward conservative management and promising outcomes with antibiotic therapy alone or with minimally invasive techniques has lessened the need for diagnostic CT-guided fine-needle aspiration.
Hemorrhage
Spontaneous hemorrhage into pancreatic necrosis is a rare but life-threatening complication. Because CT is almost always performed with contrast enhancement, this complication is rarely identified with imaging. The diagnosis is made by noting a drop in hemoglobin and hematocrit.
Hemorrhage into the retroperitoneum or the peritoneal cavity, or both, can occur when an inflammatory process erodes into a nearby artery. Luminal gastrointestinal bleeding can occur from gastric varices arising from splenic vein thrombosis and resulting left-sided portal hypertension, or from pseudoaneurysms. These can also bleed into the pancreatic duct (hemosuccus pancreaticus). Pseudoaneurysm is a later complication that occurs when an arterial wall (most commonly the splenic or gastroduodenal artery) is weakened by pancreatic enzymes.23
Prompt recognition of hemorrhagic events and consultation with an interventional radiologist or surgeon are required to prevent death.
Inflammation and abdominal compartment syndrome
Inflammation from necrotizing pancreatitis can cause further complications by blocking nearby structures. Reported complications include jaundice from biliary compression, hydronephrosis from ureteral compression, bowel obstruction, and gastric outlet obstruction.
Abdominal compartment syndrome is an increasingly recognized complication of acute pancreatitis. Abdominal pressure can rise due to a number of factors, including fluid collections, ascites, ileus, and overly aggressive fluid resuscitation.24 Elevated abdominal pressure is associated with complications such as decreased respiratory compliance, increased peak airway pressure, decreased cardiac preload, hypotension, mesenteric and intestinal ischemia, feeding intolerance, and lower-extremity ischemia and thrombosis.
Patients with necrotizing pancreatitis who have abdominal compartment syndrome have a mortality rate 5 times higher than patients without abdominal compartment syndrome.25
Abdominal pressures should be monitored using a bladder pressure sensor in critically ill or ventilated patients with acute pancreatitis. If the abdominal pressure rises above 20 mm Hg, medical and surgical interventions should be offered in a stepwise fashion to decrease it. Interventions include decompression by nasogastric and rectal tube, sedation or paralysis to relax abdominal wall tension, minimization of intravenous fluids, percutaneous drainage of ascites, and (rarely) surgical midline or subcostal laparotomy.
ROLE OF INTERVENTION
The treatment of necrotizing pancreatitis has changed rapidly, thanks to a growing experience with minimally invasive techniques.
Indications for intervention
Infected pancreatic necrosis is the primary indication for surgical, percutaneous, or endoscopic intervention.
In sterile necrosis, the threshold for intervention is less clear, and intervention is often reserved for patients who fail to clinically improve or who have intractable abdominal pain, gastric outlet obstruction, or fistulating disease.26
In asymptomatic cases, intervention is almost never indicated regardless of the location or size of the necrotic area.
In walled-off pancreatic necrosis, less-invasive and less-morbid interventions such as endoscopic or percutaneous drainage or video-assisted retroperitoneal debridement can be done.
Timing of intervention
In the past, delaying intervention was thought to increase the risk of death. However, multiple studies have found that outcomes are often worse if intervention is done early, likely due to the lack of a fully formed fibrous wall or demarcation of the necrotic area.27
If the patient remains clinically stable, it is best to delay intervention until at least 4 weeks after the index event to achieve optimal outcomes. Delay can often be achieved by antibiotic treatment to suppress bacteremia and endoscopic or percutaneous drainage of infected collections to control sepsis.
Open surgery
The gold-standard intervention for infected pancreatic necrosis or symptomatic sterile walled-off pancreatic necrosis is open necrosectomy. This involves exploratory laparotomy with blunt debridement of all visible necrotic pancreatic tissue.
Methods to facilitate later evacuation of residual infected fluid and debris vary widely. Multiple large-caliber drains can be placed to facilitate irrigation and drainage before closure of the abdominal fascia. As infected pancreatic necrosis carries the risk of contaminating the peritoneal cavity, the skin is often left open to heal by secondary intention. An interventional radiologist is frequently enlisted to place, exchange, or downsize drainage catheters.
Infected pancreatic necrosis or symptomatic sterile walled-off pancreatic necrosis often requires more than one operation to achieve satisfactory debridement.
The goals of open necrosectomy are to remove nonviable tissue and infection, preserve viable pancreatic tissue, eliminate fistulous connections, and minimize damage to local organs and vasculature.
Minimally invasive techniques
Video-assisted retroperitoneal debridement has been described as a hybrid between endoscopic and open retroperitoneal debridement.28 This technique requires first placing a percutaneous catheter into the necrotic area through the left flank to create a retroperitoneal tract. A 5-cm incision is made and the necrotic space is entered using the drain for guidance. Necrotic tissue is carefully debrided under direct vision using a combination of forceps, irrigation, and suction. A laparoscopic port can also be introduced into the incision when the procedure can no longer be continued under direct vision.29,30
Although not all patients are candidates for minimal-access surgery, it remains an evolving surgical option.
Endoscopic transmural debridement is another option for infected pancreatic necrosis and symptomatic walled-off pancreatic necrosis. Depending on the location of the necrotic area, an echoendoscope is passed to either the stomach or duodenum. Guided by endoscopic ultrasonography, a needle is passed into the collection, allowing subsequent fistula creation and stenting for internal drainage or debridement. In the past, this process required several steps, multiple devices, fluoroscopic guidance, and considerable time. But newer endoscopic lumen-apposing metal stents have been developed that can be placed in a single step without fluoroscopy. A slimmer endoscope can then be introduced into the necrotic cavity via the stent, and the necrotic debris can be debrided with endoscopic baskets, snares, forceps, and irrigation.9,31
Similar to surgical necrosectomy, satisfactory debridement is not often obtained with a single procedure; 2 to 5 endoscopic procedures may be needed to achieve resolution. However, the luminal approach in endoscopic necrosectomy avoids the significant morbidity of major abdominal surgery and the potential for pancreaticocutaneous fistulae that may occur with drains.
In a randomized trial comparing endoscopic necrosectomy vs surgical necrosectomy (video-assisted retroperitoneal debridement and exploratory laparotomy),32 endoscopic necrosectomy showed less inflammatory response than surgical necrosectomy and had a lower risk of new-onset organ failure, bleeding, fistula formation, and death.32
Selecting the best intervention for the individual patient
Given the multiple available techniques, selecting the best intervention for individual patients can be challenging. A team approach with input from a gastroenterologist, surgeon, and interventional radiologist is best when determining which technique would best suit each patient.
Surgical necrosectomy is still the treatment of choice for unstable patients with infected pancreatic necrosis or multiple, inaccessible collections, but current evidence suggests a different approach in stable infected pancreatic necrosis and symptomatic sterile walled-off pancreatic necrosis.
The Dutch Pancreatitis Group28 randomized 88 patients with infected pancreatic necrosis or symptomatic walled-off pancreatic necrosis to open necrosectomy or a minimally invasive “step-up” approach consisting of up to 2 percutaneous drainage or endoscopic debridement procedures before escalation to video-assisted retroperitoneal debridement. The step-up approach resulted in lower rates of morbidity and death than surgical necrosectomy as first-line treatment. Furthermore, some patients in the step-up group avoided the need for surgery entirely.30
SUMMING UP
Necrosis significantly increases rates of morbidity and mortality in acute pancreatitis. Hospitalists, general internists, and general surgeons are all on the front lines in identifying severe cases and consulting the appropriate specialists for optimal multidisciplinary care. Selective and appropriate timing of radiologic imaging is key, and a vital tool in the management of necrotizing pancreatitis.
While the primary indication for intervention is infected pancreatic necrosis, additional indications are symptomatic walled-off pancreatic necrosis secondary to intractable abdominal pain, bowel obstruction, and failure to thrive. As a result of improving technology and inpatient care, these patients may present with intractable symptoms in the outpatient setting rather than the inpatient setting. The onus is on the primary care physician to maintain a high level of suspicion and refer these patients to subspecialists as appropriate.
Open surgical necrosectomy remains an important approach for care of infected pancreatic necrosis or patients with intractable symptoms. A step-up approach starting with a minimally invasive procedure and escalating if the initial intervention is unsuccessful is gradually becoming the standard of care.
- Peery AF, Crockett SD, Barritt AS, et al. Burden of gastrointestinal, liver, and pancreatic disease in the United States. Gastroenterology 2015; 149:1731–1741e3.
- Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol 2013; 108:1400–1416.
- Bradley EL 3rd. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, GA, September 11 through 13, 1992. Arch Surg 1993; 128:586–590.
- Banks PA, Bollen TL, Dervenis C, et al; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013; 62:102–111.
- Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med 1995; 23:1638–1652.
- Kadiyala V, Suleiman SL, McNabb-Baltar J, Wu BU, Banks PA, Singh VK. The Atlanta classification, revised Atlanta classification, and determinant-based classification of acute pancreatitis: which is best at stratifying outcomes? Pancreas 2016; 45:510–515.
- Singh VK, Bollen TL, Wu BU, et al. An assessment of the severity of interstitial pancreatitis. Clin Gastroenterol Hepatol 2011; 9:1098–1103.
- Kotwal V, Talukdar R, Levy M, Vege SS. Role of endoscopic ultrasound during hospitalization for acute pancreatitis. World J Gastroenterol 2010; 16:4888–4891.
- Balthazar EJ. Acute pancreatitis: assessment of severity with clinical and CT evaluation. Radiology 2002; 223:603–613.
- Mortele KJ, Wiesner W, Intriere L, et al. A modified CT severity index for evaluating acute pancreatitis: improved correlation with patient outcome. AJR Am J Roentgenol 2004; 183:1261–1265.
- Verde F, Fishman EK, Johnson PT. Arterial pseudoaneurysms complicating pancreatitis: literature review. J Comput Assist Tomogr 2015; 39:7–12.
- Shyu JY, Sainani NI, Sahni VA, et al. Necrotizing pancreatitis: diagnosis, imaging, and intervention. Radiographics 2014; 34:1218–1239.
- Thoeni RF. The revised Atlanta classification of acute pancreatitis: its importance for the radiologist and its effect on treatment. Radiology 2012; 262:751–764.
- Morgan DE, Ragheb CM, Lockhart ME, Cary B, Fineberg NS, Berland LL. Acute pancreatitis: computed tomography utilization and radiation exposure are related to severity but not patient age. Clin Gastroenterol Hepatol 2010; 8:303–308.
- Vitellas KM, Paulson EK, Enns RA, Keogan MT, Pappas TN. Pancreatitis complicated by gland necrosis: evolution of findings on contrast-enhanced CT. J Comput Assist Tomogr 1999; 23:898–905.
- Stimac D, Miletic D, Radic M, et al. The role of nonenhanced magnetic resonance imaging in the early assessment of acute pancreatitis. Am J Gastroenterol 2007; 102:997–1004.
- Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Surg Infect (Larchmt) 2010; 11:79–109.
- Petrov MS, Kukosh MV, Emelyanov NV. A randomized controlled trial of enteral versus parenteral feeding in patients with predicted severe acute pancreatitis shows a significant reduction in mortality and in infected pancreatic complications with total enteral nutrition. Dig Surg 2006; 23:336–345.
- Petrov MS, Shanbhag S, Chakraborty M, Phillips AR, Windsor JA. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology 2010; 139:813–820.
- Villatoro E, Bassi C, Larvin M. Antibiotic therapy for prophylaxis against infection of pancreatic necrosis in acute pancreatitis. Cochrane Database Syst Rev 2006; 4:CD002941.
- Baril NB, Ralls PW, Wren SM, et al. Does an infected peripancreatic fluid collection or abscess mandate operation? Ann Surg 2000; 231:361–367.
- Mouli VP, Sreenivas V, Garg PK. Efficacy of conservative treatment, without necrosectomy, for infected pancreatic necrosis: a systematic review and meta-analysis. Gastroenterology 2013; 144:333–340.e2.
- Kirby JM, Vora P, Midia M, Rawlinson J. Vascular complications of pancreatitis: imaging and intervention. Cardiovasc Intervent Radiol 2008; 31:957–970.
- De Waele JJ, Hoste E, Blot SI, Decruyenaere J, Colardyn F. Intra-abdominal hypertension in patients with severe acute pancreatitis. Crit Care 2005; 9:R452–R457.
- van Brunschot S, Schut AJ, Bouwense SA, et al; Dutch Pancreatitis Study Group. Abdominal compartment syndrome in acute pancreatitis: a systematic review. Pancreas 2014; 43:665–674.
- Bugiantella W, Rondelli F, Boni M, et al. Necrotizing pancreatitis: a review of the interventions. Int J Surg 2016; 28(suppl 1):S163–S171.
- Besselink MG, Verwer TJ, Schoenmaeckers EJ, et al. Timing of surgical intervention in necrotizing pancreatitis. Arch Surg 2007; 142:1194–1201.
- van Santvoort HC, Besselink MG, Horvath KD, et al; Dutch Acute Pancreatis Study Group. Videoscopic assisted retroperitoneal debridement in infected necrotizing pancreatitis. HPB (Oxford) 2007; 9:156–159.
- van Santvoort HC, Besselink MG, Bollen TL, Buskens E, van Ramshorst B, Gooszen HG; Dutch Acute Pancreatitis Study Group. Case-matched comparison of the retroperitoneal approach with laparotomy for necrotizing pancreatitis. World J Surg 2007; 31:1635–1642.
- van Santvoort HC, Besselink MG, Bakker OJ, et al; Dutch Pancreatitis Study Group. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med 2010; 362:1491–1502.
- Thompson CC, Kumar N, Slattery J, et al. A standardized method for endoscopic necrosectomy improves complication and mortality rates. Pancreatology 2016; 16:66–72.
- Bakker OJ, van Santvoort HC, van Brunschot S, et al; Dutch Pancreatitis Study Group. Endoscopic transgastric vs surgical necrosectomy for infected necrotizing pancreatitis: a randomized trial. JAMA 2012; 307:1053–1061.
- Peery AF, Crockett SD, Barritt AS, et al. Burden of gastrointestinal, liver, and pancreatic disease in the United States. Gastroenterology 2015; 149:1731–1741e3.
- Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol 2013; 108:1400–1416.
- Bradley EL 3rd. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, GA, September 11 through 13, 1992. Arch Surg 1993; 128:586–590.
- Banks PA, Bollen TL, Dervenis C, et al; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013; 62:102–111.
- Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med 1995; 23:1638–1652.
- Kadiyala V, Suleiman SL, McNabb-Baltar J, Wu BU, Banks PA, Singh VK. The Atlanta classification, revised Atlanta classification, and determinant-based classification of acute pancreatitis: which is best at stratifying outcomes? Pancreas 2016; 45:510–515.
- Singh VK, Bollen TL, Wu BU, et al. An assessment of the severity of interstitial pancreatitis. Clin Gastroenterol Hepatol 2011; 9:1098–1103.
- Kotwal V, Talukdar R, Levy M, Vege SS. Role of endoscopic ultrasound during hospitalization for acute pancreatitis. World J Gastroenterol 2010; 16:4888–4891.
- Balthazar EJ. Acute pancreatitis: assessment of severity with clinical and CT evaluation. Radiology 2002; 223:603–613.
- Mortele KJ, Wiesner W, Intriere L, et al. A modified CT severity index for evaluating acute pancreatitis: improved correlation with patient outcome. AJR Am J Roentgenol 2004; 183:1261–1265.
- Verde F, Fishman EK, Johnson PT. Arterial pseudoaneurysms complicating pancreatitis: literature review. J Comput Assist Tomogr 2015; 39:7–12.
- Shyu JY, Sainani NI, Sahni VA, et al. Necrotizing pancreatitis: diagnosis, imaging, and intervention. Radiographics 2014; 34:1218–1239.
- Thoeni RF. The revised Atlanta classification of acute pancreatitis: its importance for the radiologist and its effect on treatment. Radiology 2012; 262:751–764.
- Morgan DE, Ragheb CM, Lockhart ME, Cary B, Fineberg NS, Berland LL. Acute pancreatitis: computed tomography utilization and radiation exposure are related to severity but not patient age. Clin Gastroenterol Hepatol 2010; 8:303–308.
- Vitellas KM, Paulson EK, Enns RA, Keogan MT, Pappas TN. Pancreatitis complicated by gland necrosis: evolution of findings on contrast-enhanced CT. J Comput Assist Tomogr 1999; 23:898–905.
- Stimac D, Miletic D, Radic M, et al. The role of nonenhanced magnetic resonance imaging in the early assessment of acute pancreatitis. Am J Gastroenterol 2007; 102:997–1004.
- Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Surg Infect (Larchmt) 2010; 11:79–109.
- Petrov MS, Kukosh MV, Emelyanov NV. A randomized controlled trial of enteral versus parenteral feeding in patients with predicted severe acute pancreatitis shows a significant reduction in mortality and in infected pancreatic complications with total enteral nutrition. Dig Surg 2006; 23:336–345.
- Petrov MS, Shanbhag S, Chakraborty M, Phillips AR, Windsor JA. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology 2010; 139:813–820.
- Villatoro E, Bassi C, Larvin M. Antibiotic therapy for prophylaxis against infection of pancreatic necrosis in acute pancreatitis. Cochrane Database Syst Rev 2006; 4:CD002941.
- Baril NB, Ralls PW, Wren SM, et al. Does an infected peripancreatic fluid collection or abscess mandate operation? Ann Surg 2000; 231:361–367.
- Mouli VP, Sreenivas V, Garg PK. Efficacy of conservative treatment, without necrosectomy, for infected pancreatic necrosis: a systematic review and meta-analysis. Gastroenterology 2013; 144:333–340.e2.
- Kirby JM, Vora P, Midia M, Rawlinson J. Vascular complications of pancreatitis: imaging and intervention. Cardiovasc Intervent Radiol 2008; 31:957–970.
- De Waele JJ, Hoste E, Blot SI, Decruyenaere J, Colardyn F. Intra-abdominal hypertension in patients with severe acute pancreatitis. Crit Care 2005; 9:R452–R457.
- van Brunschot S, Schut AJ, Bouwense SA, et al; Dutch Pancreatitis Study Group. Abdominal compartment syndrome in acute pancreatitis: a systematic review. Pancreas 2014; 43:665–674.
- Bugiantella W, Rondelli F, Boni M, et al. Necrotizing pancreatitis: a review of the interventions. Int J Surg 2016; 28(suppl 1):S163–S171.
- Besselink MG, Verwer TJ, Schoenmaeckers EJ, et al. Timing of surgical intervention in necrotizing pancreatitis. Arch Surg 2007; 142:1194–1201.
- van Santvoort HC, Besselink MG, Horvath KD, et al; Dutch Acute Pancreatis Study Group. Videoscopic assisted retroperitoneal debridement in infected necrotizing pancreatitis. HPB (Oxford) 2007; 9:156–159.
- van Santvoort HC, Besselink MG, Bollen TL, Buskens E, van Ramshorst B, Gooszen HG; Dutch Acute Pancreatitis Study Group. Case-matched comparison of the retroperitoneal approach with laparotomy for necrotizing pancreatitis. World J Surg 2007; 31:1635–1642.
- van Santvoort HC, Besselink MG, Bakker OJ, et al; Dutch Pancreatitis Study Group. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med 2010; 362:1491–1502.
- Thompson CC, Kumar N, Slattery J, et al. A standardized method for endoscopic necrosectomy improves complication and mortality rates. Pancreatology 2016; 16:66–72.
- Bakker OJ, van Santvoort HC, van Brunschot S, et al; Dutch Pancreatitis Study Group. Endoscopic transgastric vs surgical necrosectomy for infected necrotizing pancreatitis: a randomized trial. JAMA 2012; 307:1053–1061.
KEY POINTS
- Selective and appropriate timing of radiologic imaging is vital in managing necrotizing pancreatitis. Protocols are valuable tools.
- While the primary indication for debridement and drainage in necrotizing pancreatitis is infection, other indications are symptomatic walled-off pancreatic necrosis, intractable abdominal pain, bowel obstruction, and failure to thrive.
- Open surgical necrosectomy remains an important treatment for infected pancreatic necrosis or intractable symptoms.
- A “step-up” approach starting with a minimally invasive procedure and escalating if the initial intervention is unsuccessful is gradually becoming the standard of care.
Opioid abuse and overdose: Keep your patients safe
Opioid abuse and overdose are large and growing problems, and in recent years the numbers have been staggering. Overdose deaths related to opioids increased from 28,647 in 2014 to 33,091 in 2015 (Figure).1 More than 2 million individuals in the United States had opioid use disorder in 2015,2 and approximately 80% of them received no treatment,3 even though effective treatment could reduce the scope of abuse.4,5
Although psychiatrists typically are not the primary prescribers of opioid medications, they often treat psychiatric disorders in patients with chronic pain who take prescription opioids. A recent study found that, despite representing only 16% of the adult population, adults with mental health disorders receive more than one-half of all opioid prescriptions distributed each year in the United States.6 Therefore, psychiatrists must be aware of risk assessment strategies for patients receiving opioids.
In this article, we provide recommendations for managing individuals with opioid use disorder, including:
- how to identify risk factors for opioid use disorder and use screening tools
- how to evaluate a patient with suspected opioid use disorder and make the diagnosis
- how to treat a patient with opioid use disorder, including a review of approved pharmaceutical agents.
Risk factors for opioid abuse and overdose
Patients with a history of mental health and/or substance use disorders or at least 3 months of prescribed opioid treatment are at risk for opioid abuse. Those taking a high daily dose of opioids or who have a history of overdose are at risk for overdose from opioid abuse (Table 1).7-12 Standardized tools, such as the Opioid Risk Tool, can be used to screen to assess risk for opioid abuse among individuals prescribed opioids for treatment of chronic pain.12 However, clinicians must be aware that even patients without characteristic risk factors can become dependent on opioids and/or be at risk for an accidental or intentional overdose. For example, opioid therapy following surgical procedures, even in patients who do not have a history of opioid use, increases risk of developing opioid use disorder.13
Evaluation and diagnosis
DSM-5 criteria define 3 degrees of opioid use disorder, depending on how many of the following traits a patient exhibits (mild, 2 to 3; moderate, 4 to 5; and severe, ≥6 )14:
- taking more than the initially intended quantities of opioids or for a longer period of time than intended
- continuous attempts to reduce or otherwise manage opioid use or desires to do so
- a great deal of time using, recovering from, or acquiring opioids
- reports of strong cravings to use opioids
- failing to meet personal objectives at home, work, or school
- continued opioid use even though it causes recurrent social problems
- reduction or elimination of activities the patient once considered important due to opioid use
- opioid use in situations where it is physically dangerous
- continued opioid use despite persistent psychological or physiologic problems despite knowing that continued use is causing or worsening those problems
- tolerance to opioids (not consequential for the diagnosis if the patient is taking opioids under appropriate medical supervision)
- withdrawal or use of opioids (or related substances) to prevent withdrawal (not consequential for the diagnosis if the patient is taking opioids under appropriate medical supervision).
Clinicians should be vigilant for symptoms of opioid use or withdrawal, such as needle marks and weight loss, during the interview (Table 2). High-risk populations that require regular screening include individuals with a history of opioid use disorder, patients taking chronic pain medication, and psychiatric patients.15 During the interview, clinicians should take an nonjudgmental approach and avoid “shame and blame.”
Patients often will withhold information about drug use for various reasons.16 Therefore, collateral information from the patient’s family, close friends, or a referral source is important.
Standardized scales. Various standardized scales can be used to evaluate patients for opioid withdrawal and risk for substance use disorder. Scales for assessing opioid withdrawal include:
- Clinical Opiate Withdrawal Scale
- Subjective Opiate Withdrawal Scale.
Substance use disorder screening tools include:
- Drug Abuse Screen Test-10
- Alcohol Use Disorders Identification Test
- National Institute on Drug Abuse (NIDA) Drug Screening Tool.17
Examination findings. A brief physical examination is necessary to document key findings (Table 2). Patients should undergo a urine drug screen; gas chromatography/mass spectroscopy can confirm positive results. During the examination, clinicians should look for signs and symptoms of co-occurring substance use (eg, benzodiazepines, marijuana, alcohol, cocaine) or mental disorders (mood, anxiety, attention-deficit).18-21 Because nonprescription opioid use is associated with increased risk of suicide attempts and ideation,22 a suicide risk assessment is necessary.
Managing opioid use disorder
Detoxification is a 3-tiered approach that requires judicious prescription of medication, psychosocial support, and supervision to relieve opioid withdrawal symptoms. In both inpatient and outpatient settings, medications used for opioid detoxification include buprenorphine, clonidine, and methadone administered in doses tapered over 5 to 7 days. Appropriate detoxification increases treatment retention for continuing care.23,24
Buprenorphine or buprenorphine/naloxone is the first-line option for outpatient and inpatient detoxification. Short-term detoxification schedules include starting doses between 4 and 16 mg/d, tapered over 5 to 7 days. Compared with methadone, buprenorphine has a lower risk of overdose25 and abuse potential and can be given in an office-based setting. Clonidine, 0.3 to 1.2 mg/d in divided doses, is an alternative to buprenorphine and can be used in inpatient settings.26
Clonidine is not as effective as buprenorphine for detoxification, but it may be used when buprenorphine is contraindicated. Clonidine may require adjuvant symptomatic treatment for insomnia (eg, trazodone, 100 mg at bedtime), anxiety (eg, hydroxyzine, 25 mg, twice a day), or diarrhea (loperamide, 2 mg/d). If a patient needs more structure and monitoring, he (she) should be referred for inpatient detoxification or to a methadone program.27
Medication-assisted therapies
Detoxification alone often is not sufficient treatment. Medication-assisted therapy (MAT) is typically recommended by federal guidelines provided by the Substance Abuse and Mental Health Administration (SAMHSA) for patients with opioid use disorders.3 Patients can be directly transitioned from currently abused opioids to MAT on an outpatient basis. FDA-approved medications for MAT for opioid use disorder include buprenorphine, naltrexone (oral and long-acting injectable), and methadone (Table 3). Choice of MAT depends on several factors, including cost, patient preference, and availability of methadone programs and buprenorphine providers.28
MAT should include psychosocial support29-33 and active monitoring with urine drug screens. Maintenance therapy with medications is usually long-term and has been shown to have better outcomes than detoxification alone or short-term treatment.34 Relapse during MAT should not be cause to discontinue treatment; instead, the patient should be referred to a higher level of care.
Some patients require individualized treatment approaches. For example, the SAMHSA has developed specific treatment improvement protocols to tailor treatments to address specific needs of adolescents.32 The American Academy of Pediatrics recommends MAT with buprenorphine in adolescents with opioid use disorder.33 Although methadone has been approved for pregnant, opioid-dependent patients, recent data indicate buprenorphine is as effective with lower intensity of neonatal abstinence syndrome.34
Buprenorphine. This long-acting (half-life of 24 to 42 hours) opioid partial agonist is approved for treating opioid use disorder in office-based settings according to the Drug Abuse Treatment Act of 2000. Buprenorphine is administered in doses of 8 to 16 mg/d in film or tablet form (sublingual or buccal) and is available in various formulations (Table 4). It is well tolerated; constipation and unpleasant taste are the most common adverse effects. Physicians are required to have a federal waiver to obtain the Drug Enforcement Administration license to prescribe buprenorphine for opioid use disorder in an office setting.
Buprenorphine reduces or eliminates cravings and withdrawal symptoms and helps improve outcomes of abstinence from opioids and retention in treatment.31 Formulations of naloxone combined with buprenorphine reduce the risk of abuse via injection.35 Buprenorphine is safe; however, overdoses can occur when it is combined with benzodiazepines and/or other opiates.
Methadone. This long-acting (half-life 8 to 59 hours), full opioid agonist is approved to treat opioid addiction in federal- and state-regulated opioid treatment programs, also known as methadone maintenance programs. These programs are highly structured and include intensive counseling, monitoring, and dispensing to reduce relapse. Methadone is administered orally either via powder, liquid concentrate, tablet, or solution of diskette. Typically, methadone is dispensed daily in doses of 60 to 100 mg, although higher doses are sometimes necessary. Patients who meet certain criteria for stability may be allowed to take home supplies of methadone.
Methadone has a “black-box” warning for overdose, QT prolongation, and risk for respiratory depression when used in combination with benzodiazepines. Because of its long and unpredictable half-life and tissue accumulation, methadone carries a high overdose risk, particularly with rapidly titrated doses during therapy initiation.35 However, most overdose deaths have occurred with methadone prescribed for pain management. When prescribed and monitored in an opioid treatment program, methadone has shown a high safety profile with respect to overdoses.36
Injectable and oral naltrexone. Used for prevention of relapse to opioid dependence, naltrexone is a pure opioid antagonist that is available as an oral or IM form. Naltrexone has high affinity for the opioid receptors and in therapeutic doses provides an effective blockade for heroin or opioids. Compliance with oral naltrexone has been poor, leading to development of an IM form of naltrexone that can be administered as a single 380-mg dose once every 4 weeks for 6 months or sometimes longer. Naltrexone is also approved for alcohol dependence.
To avoid precipitated withdrawal, patients should be detoxified from opioids for 7 to 10 days before they begin naltrexone, which has no potential for abuse. Common adverse effects include fatigue, nausea, headache, and, for the IM formulation, injection site reactions. There is a “black-box” warning for liver toxicity; therefore, baseline and periodic liver function tests are necessary.
A NIDA review reported poor compliance with oral naltrexone compared with methadone.35 However, naltrexone has been shown to be effective in highly motivated patients (eg, impaired physicians) and the criminal justice population and for preventing relapse following taper from buprenorphine or methadone.37,38
Treatment for opioid overdose
Naloxone is a highly effective treatment to reverse opioid overdose that is delivered via IM or IV injection or by nasal application. Naloxone has no abuse potential. In doses of 0.4 to 2 mg, naloxone reverses overdose within 2 minutes and is effective for 30 to 90 minutes.39 One should call 911 as soon as possible after naloxone is administered. In several states, naloxone is available without a prescription for patients and family members to combat opioid overdoses. The CDC recommends offering naloxone to patients who have risk factors for opioid overdose.40
1. Centers for Disease Control and Prevention. Opioid data analysis. http://www.cdc.gov/drugoverdose/data/analysis.html. Updated February 9, 2017. Accessed June 27, 2017.
2. Substance Abuse and Mental Health Services Administration. Results from the 2015 National Survey on Drug Use and Health: detailed tables. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.pdf.
3. Substance Abuse and Mental Health Services Administration. Medication-assisted treatment of opioid use disorder pocket guide. https://store.samhsa.gov/shin/content//SMA16-4892PG/SMA16-4892PG.pdf. Accessed June 29, 2017.
4. Mutlu C, Demirci AC, Yalcin O, et al. One-year follow-up of heroin-dependent adolescents treated with buprenorphine/naloxone for the first time in a substance treatment unit. J Subst Abuse Treat. 2016;67:1-8.
5. Sharma B, Bruner A, Barnett G, et al. Opioid use disorders. Child Adolesc Psychiatr Clin N Am. 2016;25(3):473-487.
6. Davis MA, Lin LA, Liu H, Sites BD. Prescription Opioid Use among Adults with mental health disorders in the United States. J Am Board Fam Med. 2017;30:42-47.
7. Icahn School of Medicine at Mount Sinai. Substance use: prescription drugs. http://www.mountsinai.org/patient-care/health-library/diseases-and-conditions/opioid-abuse#risk. Accessed June 27, 2017.
8. Boscarino JA, Rukstalis M, Hoffman SN, et al. Risk factors for drug dependence among out-patients on opioid therapy in a large US health-care system. Addiction. 2010;105(10):1776-1782.
9. Edlund M, Steffick D, Hudson T, et al. Risk factors for clinically recognized opioid abuse and dependence among veterans using opioids for chronic non-cancer pain. Pain. 2007;129(3):355-362.
10. Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend. 2006;81(2):103-107.
11. Bohnert AS, Valenstein M, Bair M, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321.
12. Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6(6):432.
13. Sun EC, Darnall BD, Baker LC, et al. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med. 2016;176(9):1286-1293.
14. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
15. Starrels JL, Becker WC, Alford DP, et al. Systematic review: treatment agreements and urine drug testing to reduce opioid misuse in patients with chronic pain. Ann Internal Med. 2010;152(11):712-720.
16. Substance Abuse and Mental Health Services Administration. Clinical guidelines for the use of buprenorphine in the treatment of opioid addiction: a treatment improvement protocol: TIP 40. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2004.
17. NIDA drug screening tool: clinician’s screening tool for drug use in general medical settings. National Institutes of Health. https://www.drugabuse.gov/nmassist. Accessed June 27, 2017.
18. Fareed A, Eilender P, Haber M, et al. Comorbid posttraumatic stress disorder and opiate addiction: a literature review. J Addict Dis. 2013;32(2):168-179.
19. Rosen D, Smith ML, Reynolds CF 3rd. The prevalence of mental and physical health disorders among older methadone patients. Am J Geriatr Psychiatry. 2008;16(6):488-497.
20. Goldner EM, Lusted A, Roerecke M, et al. Prevalence of Axis-1 psychiatric (with focus on depression and anxiety) disorder and symptomatology among non-medical prescription opioid users in substance use treatment: systematic review and meta-analyses. Addict Behav. 2014;39(3):520-531.
21. Barry DT, Cutter CJ, Beitel M, et al. Psychiatric disorders among patients seeking treatment for co-occurring chronic pain and opioid use disorder. J Clin Psychiatry. 2016;77(10):1413-1419.
22. Kuramoto SJ, Chilcoat HD, Ko J, et al. Suicidal ideation and suicide attempt across stages of nonmedical prescription opioid use and presence of prescription opioid disorders among U.S. adults. J Stud Alcohol Drugs. 2012;73(2):178-184.
23. Mattick RP, Breen C, Kimber J, et al. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;(2):CD002207. doi: 10.1002/14651858.CD002207.pub4.
24. Evans E, Li L, Min J, et al. Mortality among individuals accessing pharmacological treatment for opioid dependence in California, 2006-10. Addiction. 2015;110(6):996-1005.
25. Marteu D, McDonald R, Patel K. The relative risk of fatal poisoning by methadone or buprenorphine within the wider population of England and Wales. BMJ Open. 2015;5(5):e007629. doi: 10.1136/bmjopen-2015-007629.
26. Jasinski DR, Johnson RE, Kocher TR. Clonidine in morphine withdrawal. Differential effects on signs and symptoms. Arch Gen Psychiatry. 1985;42(11):1063-1066.
27. Whelan PJ, Remski K. Buprenorphine vs methadone treatment: a review of evidence in both developed and developing worlds. J Neurosci Rural Pract. 2012;3(1):45-50.
28. Schuckit MA. Treatment of opioid-use disorders. N Engl J Med. 2016;375(4):357-368.
29. Dutra L, Stathopoulou G, Basden SL, et al. A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry. 2008;165(2):179-187.
30. Brown HL, Britton KA, Mahaffey D, et al. Methadone maintenance in pregnancy: a reappraisal. Am J Obstet Gynecol. 1998;179(2):459-463.
31. Center for Substance Abuse Treatment. Medication-Assisted Treatment for Opioid Addiction in Opioid Treatment Programs. Treatment Improvement Protocol (TIP) 43.
32. Zimlich R. AAP recommends on medication assisted therapy for adolescent opioid addiction. Contemporary Pediatrics. http://contemporarypediatrics.modernmedicine.com/contemporary-pediatrics/news/aap-recommends-medication-assisted-therapy-adolescent-opioid-addiction. Published September 15, 2016. Accessed June 29, 2017.
33. Patkar A, Lee J, Burgess D. Opioid use disorder. BMJ Publishing Group. http://bestpractice.bmj.com/best-practice/monograph/200.html. Published 2015. Accessed July 6, 2017.
34. Alho H, Sinclair D, Vuori E, et al. Abuse liability of buprenorphine-naloxone tablets in untreated IV drug users. Drug Alcohol Depend. 2007;88(1):75-78.
35. Centers for Disease Control and Prevention (CDC). Vital signs: risk for overdose from methadone used for pain relief - United States, 1999-2010. MMWR Morb Mortal Wkly Rep. 2012;61(26):493-497.
36. Soyka M. New developments in the management of opioid dependence: focus on sublingual buprenorphine-naloxone. Subst Abuse Rehabil. 2015;6:1-14.
37. Lee JD, Friedmann PD, Kinlock TW, et al. Extended-Release Naltrexone to Prevent Opioid Relapse in Criminal Justice Offenders N Engl J Med. 2016;374(13):1232-1242.
38. Vo HT, Robbins E, Westwood M, et al. Relapse prevention medications in community treatment for young adults with opioid addiction. Subst Abus. 2016;37(3):392-397.
39. McDonald R, Campbell ND, Strang J. Twenty years of take-home naloxone for the prevention of overdose deaths from heroin and other opioids-conception and maturation. Drug Alcohol Depend. 2017;178:176-187.
40. Centers for Disease Control and Prevention. Overdose prevention. https://www.cdc.gov/drugoverdose/opioids/odprevention.html. Updated February 9, 2017. Accessed July 6, 2017.
Opioid abuse and overdose are large and growing problems, and in recent years the numbers have been staggering. Overdose deaths related to opioids increased from 28,647 in 2014 to 33,091 in 2015 (Figure).1 More than 2 million individuals in the United States had opioid use disorder in 2015,2 and approximately 80% of them received no treatment,3 even though effective treatment could reduce the scope of abuse.4,5
Although psychiatrists typically are not the primary prescribers of opioid medications, they often treat psychiatric disorders in patients with chronic pain who take prescription opioids. A recent study found that, despite representing only 16% of the adult population, adults with mental health disorders receive more than one-half of all opioid prescriptions distributed each year in the United States.6 Therefore, psychiatrists must be aware of risk assessment strategies for patients receiving opioids.
In this article, we provide recommendations for managing individuals with opioid use disorder, including:
- how to identify risk factors for opioid use disorder and use screening tools
- how to evaluate a patient with suspected opioid use disorder and make the diagnosis
- how to treat a patient with opioid use disorder, including a review of approved pharmaceutical agents.
Risk factors for opioid abuse and overdose
Patients with a history of mental health and/or substance use disorders or at least 3 months of prescribed opioid treatment are at risk for opioid abuse. Those taking a high daily dose of opioids or who have a history of overdose are at risk for overdose from opioid abuse (Table 1).7-12 Standardized tools, such as the Opioid Risk Tool, can be used to screen to assess risk for opioid abuse among individuals prescribed opioids for treatment of chronic pain.12 However, clinicians must be aware that even patients without characteristic risk factors can become dependent on opioids and/or be at risk for an accidental or intentional overdose. For example, opioid therapy following surgical procedures, even in patients who do not have a history of opioid use, increases risk of developing opioid use disorder.13
Evaluation and diagnosis
DSM-5 criteria define 3 degrees of opioid use disorder, depending on how many of the following traits a patient exhibits (mild, 2 to 3; moderate, 4 to 5; and severe, ≥6 )14:
- taking more than the initially intended quantities of opioids or for a longer period of time than intended
- continuous attempts to reduce or otherwise manage opioid use or desires to do so
- a great deal of time using, recovering from, or acquiring opioids
- reports of strong cravings to use opioids
- failing to meet personal objectives at home, work, or school
- continued opioid use even though it causes recurrent social problems
- reduction or elimination of activities the patient once considered important due to opioid use
- opioid use in situations where it is physically dangerous
- continued opioid use despite persistent psychological or physiologic problems despite knowing that continued use is causing or worsening those problems
- tolerance to opioids (not consequential for the diagnosis if the patient is taking opioids under appropriate medical supervision)
- withdrawal or use of opioids (or related substances) to prevent withdrawal (not consequential for the diagnosis if the patient is taking opioids under appropriate medical supervision).
Clinicians should be vigilant for symptoms of opioid use or withdrawal, such as needle marks and weight loss, during the interview (Table 2). High-risk populations that require regular screening include individuals with a history of opioid use disorder, patients taking chronic pain medication, and psychiatric patients.15 During the interview, clinicians should take an nonjudgmental approach and avoid “shame and blame.”
Patients often will withhold information about drug use for various reasons.16 Therefore, collateral information from the patient’s family, close friends, or a referral source is important.
Standardized scales. Various standardized scales can be used to evaluate patients for opioid withdrawal and risk for substance use disorder. Scales for assessing opioid withdrawal include:
- Clinical Opiate Withdrawal Scale
- Subjective Opiate Withdrawal Scale.
Substance use disorder screening tools include:
- Drug Abuse Screen Test-10
- Alcohol Use Disorders Identification Test
- National Institute on Drug Abuse (NIDA) Drug Screening Tool.17
Examination findings. A brief physical examination is necessary to document key findings (Table 2). Patients should undergo a urine drug screen; gas chromatography/mass spectroscopy can confirm positive results. During the examination, clinicians should look for signs and symptoms of co-occurring substance use (eg, benzodiazepines, marijuana, alcohol, cocaine) or mental disorders (mood, anxiety, attention-deficit).18-21 Because nonprescription opioid use is associated with increased risk of suicide attempts and ideation,22 a suicide risk assessment is necessary.
Managing opioid use disorder
Detoxification is a 3-tiered approach that requires judicious prescription of medication, psychosocial support, and supervision to relieve opioid withdrawal symptoms. In both inpatient and outpatient settings, medications used for opioid detoxification include buprenorphine, clonidine, and methadone administered in doses tapered over 5 to 7 days. Appropriate detoxification increases treatment retention for continuing care.23,24
Buprenorphine or buprenorphine/naloxone is the first-line option for outpatient and inpatient detoxification. Short-term detoxification schedules include starting doses between 4 and 16 mg/d, tapered over 5 to 7 days. Compared with methadone, buprenorphine has a lower risk of overdose25 and abuse potential and can be given in an office-based setting. Clonidine, 0.3 to 1.2 mg/d in divided doses, is an alternative to buprenorphine and can be used in inpatient settings.26
Clonidine is not as effective as buprenorphine for detoxification, but it may be used when buprenorphine is contraindicated. Clonidine may require adjuvant symptomatic treatment for insomnia (eg, trazodone, 100 mg at bedtime), anxiety (eg, hydroxyzine, 25 mg, twice a day), or diarrhea (loperamide, 2 mg/d). If a patient needs more structure and monitoring, he (she) should be referred for inpatient detoxification or to a methadone program.27
Medication-assisted therapies
Detoxification alone often is not sufficient treatment. Medication-assisted therapy (MAT) is typically recommended by federal guidelines provided by the Substance Abuse and Mental Health Administration (SAMHSA) for patients with opioid use disorders.3 Patients can be directly transitioned from currently abused opioids to MAT on an outpatient basis. FDA-approved medications for MAT for opioid use disorder include buprenorphine, naltrexone (oral and long-acting injectable), and methadone (Table 3). Choice of MAT depends on several factors, including cost, patient preference, and availability of methadone programs and buprenorphine providers.28
MAT should include psychosocial support29-33 and active monitoring with urine drug screens. Maintenance therapy with medications is usually long-term and has been shown to have better outcomes than detoxification alone or short-term treatment.34 Relapse during MAT should not be cause to discontinue treatment; instead, the patient should be referred to a higher level of care.
Some patients require individualized treatment approaches. For example, the SAMHSA has developed specific treatment improvement protocols to tailor treatments to address specific needs of adolescents.32 The American Academy of Pediatrics recommends MAT with buprenorphine in adolescents with opioid use disorder.33 Although methadone has been approved for pregnant, opioid-dependent patients, recent data indicate buprenorphine is as effective with lower intensity of neonatal abstinence syndrome.34
Buprenorphine. This long-acting (half-life of 24 to 42 hours) opioid partial agonist is approved for treating opioid use disorder in office-based settings according to the Drug Abuse Treatment Act of 2000. Buprenorphine is administered in doses of 8 to 16 mg/d in film or tablet form (sublingual or buccal) and is available in various formulations (Table 4). It is well tolerated; constipation and unpleasant taste are the most common adverse effects. Physicians are required to have a federal waiver to obtain the Drug Enforcement Administration license to prescribe buprenorphine for opioid use disorder in an office setting.
Buprenorphine reduces or eliminates cravings and withdrawal symptoms and helps improve outcomes of abstinence from opioids and retention in treatment.31 Formulations of naloxone combined with buprenorphine reduce the risk of abuse via injection.35 Buprenorphine is safe; however, overdoses can occur when it is combined with benzodiazepines and/or other opiates.
Methadone. This long-acting (half-life 8 to 59 hours), full opioid agonist is approved to treat opioid addiction in federal- and state-regulated opioid treatment programs, also known as methadone maintenance programs. These programs are highly structured and include intensive counseling, monitoring, and dispensing to reduce relapse. Methadone is administered orally either via powder, liquid concentrate, tablet, or solution of diskette. Typically, methadone is dispensed daily in doses of 60 to 100 mg, although higher doses are sometimes necessary. Patients who meet certain criteria for stability may be allowed to take home supplies of methadone.
Methadone has a “black-box” warning for overdose, QT prolongation, and risk for respiratory depression when used in combination with benzodiazepines. Because of its long and unpredictable half-life and tissue accumulation, methadone carries a high overdose risk, particularly with rapidly titrated doses during therapy initiation.35 However, most overdose deaths have occurred with methadone prescribed for pain management. When prescribed and monitored in an opioid treatment program, methadone has shown a high safety profile with respect to overdoses.36
Injectable and oral naltrexone. Used for prevention of relapse to opioid dependence, naltrexone is a pure opioid antagonist that is available as an oral or IM form. Naltrexone has high affinity for the opioid receptors and in therapeutic doses provides an effective blockade for heroin or opioids. Compliance with oral naltrexone has been poor, leading to development of an IM form of naltrexone that can be administered as a single 380-mg dose once every 4 weeks for 6 months or sometimes longer. Naltrexone is also approved for alcohol dependence.
To avoid precipitated withdrawal, patients should be detoxified from opioids for 7 to 10 days before they begin naltrexone, which has no potential for abuse. Common adverse effects include fatigue, nausea, headache, and, for the IM formulation, injection site reactions. There is a “black-box” warning for liver toxicity; therefore, baseline and periodic liver function tests are necessary.
A NIDA review reported poor compliance with oral naltrexone compared with methadone.35 However, naltrexone has been shown to be effective in highly motivated patients (eg, impaired physicians) and the criminal justice population and for preventing relapse following taper from buprenorphine or methadone.37,38
Treatment for opioid overdose
Naloxone is a highly effective treatment to reverse opioid overdose that is delivered via IM or IV injection or by nasal application. Naloxone has no abuse potential. In doses of 0.4 to 2 mg, naloxone reverses overdose within 2 minutes and is effective for 30 to 90 minutes.39 One should call 911 as soon as possible after naloxone is administered. In several states, naloxone is available without a prescription for patients and family members to combat opioid overdoses. The CDC recommends offering naloxone to patients who have risk factors for opioid overdose.40
Opioid abuse and overdose are large and growing problems, and in recent years the numbers have been staggering. Overdose deaths related to opioids increased from 28,647 in 2014 to 33,091 in 2015 (Figure).1 More than 2 million individuals in the United States had opioid use disorder in 2015,2 and approximately 80% of them received no treatment,3 even though effective treatment could reduce the scope of abuse.4,5
Although psychiatrists typically are not the primary prescribers of opioid medications, they often treat psychiatric disorders in patients with chronic pain who take prescription opioids. A recent study found that, despite representing only 16% of the adult population, adults with mental health disorders receive more than one-half of all opioid prescriptions distributed each year in the United States.6 Therefore, psychiatrists must be aware of risk assessment strategies for patients receiving opioids.
In this article, we provide recommendations for managing individuals with opioid use disorder, including:
- how to identify risk factors for opioid use disorder and use screening tools
- how to evaluate a patient with suspected opioid use disorder and make the diagnosis
- how to treat a patient with opioid use disorder, including a review of approved pharmaceutical agents.
Risk factors for opioid abuse and overdose
Patients with a history of mental health and/or substance use disorders or at least 3 months of prescribed opioid treatment are at risk for opioid abuse. Those taking a high daily dose of opioids or who have a history of overdose are at risk for overdose from opioid abuse (Table 1).7-12 Standardized tools, such as the Opioid Risk Tool, can be used to screen to assess risk for opioid abuse among individuals prescribed opioids for treatment of chronic pain.12 However, clinicians must be aware that even patients without characteristic risk factors can become dependent on opioids and/or be at risk for an accidental or intentional overdose. For example, opioid therapy following surgical procedures, even in patients who do not have a history of opioid use, increases risk of developing opioid use disorder.13
Evaluation and diagnosis
DSM-5 criteria define 3 degrees of opioid use disorder, depending on how many of the following traits a patient exhibits (mild, 2 to 3; moderate, 4 to 5; and severe, ≥6 )14:
- taking more than the initially intended quantities of opioids or for a longer period of time than intended
- continuous attempts to reduce or otherwise manage opioid use or desires to do so
- a great deal of time using, recovering from, or acquiring opioids
- reports of strong cravings to use opioids
- failing to meet personal objectives at home, work, or school
- continued opioid use even though it causes recurrent social problems
- reduction or elimination of activities the patient once considered important due to opioid use
- opioid use in situations where it is physically dangerous
- continued opioid use despite persistent psychological or physiologic problems despite knowing that continued use is causing or worsening those problems
- tolerance to opioids (not consequential for the diagnosis if the patient is taking opioids under appropriate medical supervision)
- withdrawal or use of opioids (or related substances) to prevent withdrawal (not consequential for the diagnosis if the patient is taking opioids under appropriate medical supervision).
Clinicians should be vigilant for symptoms of opioid use or withdrawal, such as needle marks and weight loss, during the interview (Table 2). High-risk populations that require regular screening include individuals with a history of opioid use disorder, patients taking chronic pain medication, and psychiatric patients.15 During the interview, clinicians should take an nonjudgmental approach and avoid “shame and blame.”
Patients often will withhold information about drug use for various reasons.16 Therefore, collateral information from the patient’s family, close friends, or a referral source is important.
Standardized scales. Various standardized scales can be used to evaluate patients for opioid withdrawal and risk for substance use disorder. Scales for assessing opioid withdrawal include:
- Clinical Opiate Withdrawal Scale
- Subjective Opiate Withdrawal Scale.
Substance use disorder screening tools include:
- Drug Abuse Screen Test-10
- Alcohol Use Disorders Identification Test
- National Institute on Drug Abuse (NIDA) Drug Screening Tool.17
Examination findings. A brief physical examination is necessary to document key findings (Table 2). Patients should undergo a urine drug screen; gas chromatography/mass spectroscopy can confirm positive results. During the examination, clinicians should look for signs and symptoms of co-occurring substance use (eg, benzodiazepines, marijuana, alcohol, cocaine) or mental disorders (mood, anxiety, attention-deficit).18-21 Because nonprescription opioid use is associated with increased risk of suicide attempts and ideation,22 a suicide risk assessment is necessary.
Managing opioid use disorder
Detoxification is a 3-tiered approach that requires judicious prescription of medication, psychosocial support, and supervision to relieve opioid withdrawal symptoms. In both inpatient and outpatient settings, medications used for opioid detoxification include buprenorphine, clonidine, and methadone administered in doses tapered over 5 to 7 days. Appropriate detoxification increases treatment retention for continuing care.23,24
Buprenorphine or buprenorphine/naloxone is the first-line option for outpatient and inpatient detoxification. Short-term detoxification schedules include starting doses between 4 and 16 mg/d, tapered over 5 to 7 days. Compared with methadone, buprenorphine has a lower risk of overdose25 and abuse potential and can be given in an office-based setting. Clonidine, 0.3 to 1.2 mg/d in divided doses, is an alternative to buprenorphine and can be used in inpatient settings.26
Clonidine is not as effective as buprenorphine for detoxification, but it may be used when buprenorphine is contraindicated. Clonidine may require adjuvant symptomatic treatment for insomnia (eg, trazodone, 100 mg at bedtime), anxiety (eg, hydroxyzine, 25 mg, twice a day), or diarrhea (loperamide, 2 mg/d). If a patient needs more structure and monitoring, he (she) should be referred for inpatient detoxification or to a methadone program.27
Medication-assisted therapies
Detoxification alone often is not sufficient treatment. Medication-assisted therapy (MAT) is typically recommended by federal guidelines provided by the Substance Abuse and Mental Health Administration (SAMHSA) for patients with opioid use disorders.3 Patients can be directly transitioned from currently abused opioids to MAT on an outpatient basis. FDA-approved medications for MAT for opioid use disorder include buprenorphine, naltrexone (oral and long-acting injectable), and methadone (Table 3). Choice of MAT depends on several factors, including cost, patient preference, and availability of methadone programs and buprenorphine providers.28
MAT should include psychosocial support29-33 and active monitoring with urine drug screens. Maintenance therapy with medications is usually long-term and has been shown to have better outcomes than detoxification alone or short-term treatment.34 Relapse during MAT should not be cause to discontinue treatment; instead, the patient should be referred to a higher level of care.
Some patients require individualized treatment approaches. For example, the SAMHSA has developed specific treatment improvement protocols to tailor treatments to address specific needs of adolescents.32 The American Academy of Pediatrics recommends MAT with buprenorphine in adolescents with opioid use disorder.33 Although methadone has been approved for pregnant, opioid-dependent patients, recent data indicate buprenorphine is as effective with lower intensity of neonatal abstinence syndrome.34
Buprenorphine. This long-acting (half-life of 24 to 42 hours) opioid partial agonist is approved for treating opioid use disorder in office-based settings according to the Drug Abuse Treatment Act of 2000. Buprenorphine is administered in doses of 8 to 16 mg/d in film or tablet form (sublingual or buccal) and is available in various formulations (Table 4). It is well tolerated; constipation and unpleasant taste are the most common adverse effects. Physicians are required to have a federal waiver to obtain the Drug Enforcement Administration license to prescribe buprenorphine for opioid use disorder in an office setting.
Buprenorphine reduces or eliminates cravings and withdrawal symptoms and helps improve outcomes of abstinence from opioids and retention in treatment.31 Formulations of naloxone combined with buprenorphine reduce the risk of abuse via injection.35 Buprenorphine is safe; however, overdoses can occur when it is combined with benzodiazepines and/or other opiates.
Methadone. This long-acting (half-life 8 to 59 hours), full opioid agonist is approved to treat opioid addiction in federal- and state-regulated opioid treatment programs, also known as methadone maintenance programs. These programs are highly structured and include intensive counseling, monitoring, and dispensing to reduce relapse. Methadone is administered orally either via powder, liquid concentrate, tablet, or solution of diskette. Typically, methadone is dispensed daily in doses of 60 to 100 mg, although higher doses are sometimes necessary. Patients who meet certain criteria for stability may be allowed to take home supplies of methadone.
Methadone has a “black-box” warning for overdose, QT prolongation, and risk for respiratory depression when used in combination with benzodiazepines. Because of its long and unpredictable half-life and tissue accumulation, methadone carries a high overdose risk, particularly with rapidly titrated doses during therapy initiation.35 However, most overdose deaths have occurred with methadone prescribed for pain management. When prescribed and monitored in an opioid treatment program, methadone has shown a high safety profile with respect to overdoses.36
Injectable and oral naltrexone. Used for prevention of relapse to opioid dependence, naltrexone is a pure opioid antagonist that is available as an oral or IM form. Naltrexone has high affinity for the opioid receptors and in therapeutic doses provides an effective blockade for heroin or opioids. Compliance with oral naltrexone has been poor, leading to development of an IM form of naltrexone that can be administered as a single 380-mg dose once every 4 weeks for 6 months or sometimes longer. Naltrexone is also approved for alcohol dependence.
To avoid precipitated withdrawal, patients should be detoxified from opioids for 7 to 10 days before they begin naltrexone, which has no potential for abuse. Common adverse effects include fatigue, nausea, headache, and, for the IM formulation, injection site reactions. There is a “black-box” warning for liver toxicity; therefore, baseline and periodic liver function tests are necessary.
A NIDA review reported poor compliance with oral naltrexone compared with methadone.35 However, naltrexone has been shown to be effective in highly motivated patients (eg, impaired physicians) and the criminal justice population and for preventing relapse following taper from buprenorphine or methadone.37,38
Treatment for opioid overdose
Naloxone is a highly effective treatment to reverse opioid overdose that is delivered via IM or IV injection or by nasal application. Naloxone has no abuse potential. In doses of 0.4 to 2 mg, naloxone reverses overdose within 2 minutes and is effective for 30 to 90 minutes.39 One should call 911 as soon as possible after naloxone is administered. In several states, naloxone is available without a prescription for patients and family members to combat opioid overdoses. The CDC recommends offering naloxone to patients who have risk factors for opioid overdose.40
1. Centers for Disease Control and Prevention. Opioid data analysis. http://www.cdc.gov/drugoverdose/data/analysis.html. Updated February 9, 2017. Accessed June 27, 2017.
2. Substance Abuse and Mental Health Services Administration. Results from the 2015 National Survey on Drug Use and Health: detailed tables. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.pdf.
3. Substance Abuse and Mental Health Services Administration. Medication-assisted treatment of opioid use disorder pocket guide. https://store.samhsa.gov/shin/content//SMA16-4892PG/SMA16-4892PG.pdf. Accessed June 29, 2017.
4. Mutlu C, Demirci AC, Yalcin O, et al. One-year follow-up of heroin-dependent adolescents treated with buprenorphine/naloxone for the first time in a substance treatment unit. J Subst Abuse Treat. 2016;67:1-8.
5. Sharma B, Bruner A, Barnett G, et al. Opioid use disorders. Child Adolesc Psychiatr Clin N Am. 2016;25(3):473-487.
6. Davis MA, Lin LA, Liu H, Sites BD. Prescription Opioid Use among Adults with mental health disorders in the United States. J Am Board Fam Med. 2017;30:42-47.
7. Icahn School of Medicine at Mount Sinai. Substance use: prescription drugs. http://www.mountsinai.org/patient-care/health-library/diseases-and-conditions/opioid-abuse#risk. Accessed June 27, 2017.
8. Boscarino JA, Rukstalis M, Hoffman SN, et al. Risk factors for drug dependence among out-patients on opioid therapy in a large US health-care system. Addiction. 2010;105(10):1776-1782.
9. Edlund M, Steffick D, Hudson T, et al. Risk factors for clinically recognized opioid abuse and dependence among veterans using opioids for chronic non-cancer pain. Pain. 2007;129(3):355-362.
10. Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend. 2006;81(2):103-107.
11. Bohnert AS, Valenstein M, Bair M, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321.
12. Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6(6):432.
13. Sun EC, Darnall BD, Baker LC, et al. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med. 2016;176(9):1286-1293.
14. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
15. Starrels JL, Becker WC, Alford DP, et al. Systematic review: treatment agreements and urine drug testing to reduce opioid misuse in patients with chronic pain. Ann Internal Med. 2010;152(11):712-720.
16. Substance Abuse and Mental Health Services Administration. Clinical guidelines for the use of buprenorphine in the treatment of opioid addiction: a treatment improvement protocol: TIP 40. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2004.
17. NIDA drug screening tool: clinician’s screening tool for drug use in general medical settings. National Institutes of Health. https://www.drugabuse.gov/nmassist. Accessed June 27, 2017.
18. Fareed A, Eilender P, Haber M, et al. Comorbid posttraumatic stress disorder and opiate addiction: a literature review. J Addict Dis. 2013;32(2):168-179.
19. Rosen D, Smith ML, Reynolds CF 3rd. The prevalence of mental and physical health disorders among older methadone patients. Am J Geriatr Psychiatry. 2008;16(6):488-497.
20. Goldner EM, Lusted A, Roerecke M, et al. Prevalence of Axis-1 psychiatric (with focus on depression and anxiety) disorder and symptomatology among non-medical prescription opioid users in substance use treatment: systematic review and meta-analyses. Addict Behav. 2014;39(3):520-531.
21. Barry DT, Cutter CJ, Beitel M, et al. Psychiatric disorders among patients seeking treatment for co-occurring chronic pain and opioid use disorder. J Clin Psychiatry. 2016;77(10):1413-1419.
22. Kuramoto SJ, Chilcoat HD, Ko J, et al. Suicidal ideation and suicide attempt across stages of nonmedical prescription opioid use and presence of prescription opioid disorders among U.S. adults. J Stud Alcohol Drugs. 2012;73(2):178-184.
23. Mattick RP, Breen C, Kimber J, et al. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;(2):CD002207. doi: 10.1002/14651858.CD002207.pub4.
24. Evans E, Li L, Min J, et al. Mortality among individuals accessing pharmacological treatment for opioid dependence in California, 2006-10. Addiction. 2015;110(6):996-1005.
25. Marteu D, McDonald R, Patel K. The relative risk of fatal poisoning by methadone or buprenorphine within the wider population of England and Wales. BMJ Open. 2015;5(5):e007629. doi: 10.1136/bmjopen-2015-007629.
26. Jasinski DR, Johnson RE, Kocher TR. Clonidine in morphine withdrawal. Differential effects on signs and symptoms. Arch Gen Psychiatry. 1985;42(11):1063-1066.
27. Whelan PJ, Remski K. Buprenorphine vs methadone treatment: a review of evidence in both developed and developing worlds. J Neurosci Rural Pract. 2012;3(1):45-50.
28. Schuckit MA. Treatment of opioid-use disorders. N Engl J Med. 2016;375(4):357-368.
29. Dutra L, Stathopoulou G, Basden SL, et al. A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry. 2008;165(2):179-187.
30. Brown HL, Britton KA, Mahaffey D, et al. Methadone maintenance in pregnancy: a reappraisal. Am J Obstet Gynecol. 1998;179(2):459-463.
31. Center for Substance Abuse Treatment. Medication-Assisted Treatment for Opioid Addiction in Opioid Treatment Programs. Treatment Improvement Protocol (TIP) 43.
32. Zimlich R. AAP recommends on medication assisted therapy for adolescent opioid addiction. Contemporary Pediatrics. http://contemporarypediatrics.modernmedicine.com/contemporary-pediatrics/news/aap-recommends-medication-assisted-therapy-adolescent-opioid-addiction. Published September 15, 2016. Accessed June 29, 2017.
33. Patkar A, Lee J, Burgess D. Opioid use disorder. BMJ Publishing Group. http://bestpractice.bmj.com/best-practice/monograph/200.html. Published 2015. Accessed July 6, 2017.
34. Alho H, Sinclair D, Vuori E, et al. Abuse liability of buprenorphine-naloxone tablets in untreated IV drug users. Drug Alcohol Depend. 2007;88(1):75-78.
35. Centers for Disease Control and Prevention (CDC). Vital signs: risk for overdose from methadone used for pain relief - United States, 1999-2010. MMWR Morb Mortal Wkly Rep. 2012;61(26):493-497.
36. Soyka M. New developments in the management of opioid dependence: focus on sublingual buprenorphine-naloxone. Subst Abuse Rehabil. 2015;6:1-14.
37. Lee JD, Friedmann PD, Kinlock TW, et al. Extended-Release Naltrexone to Prevent Opioid Relapse in Criminal Justice Offenders N Engl J Med. 2016;374(13):1232-1242.
38. Vo HT, Robbins E, Westwood M, et al. Relapse prevention medications in community treatment for young adults with opioid addiction. Subst Abus. 2016;37(3):392-397.
39. McDonald R, Campbell ND, Strang J. Twenty years of take-home naloxone for the prevention of overdose deaths from heroin and other opioids-conception and maturation. Drug Alcohol Depend. 2017;178:176-187.
40. Centers for Disease Control and Prevention. Overdose prevention. https://www.cdc.gov/drugoverdose/opioids/odprevention.html. Updated February 9, 2017. Accessed July 6, 2017.
1. Centers for Disease Control and Prevention. Opioid data analysis. http://www.cdc.gov/drugoverdose/data/analysis.html. Updated February 9, 2017. Accessed June 27, 2017.
2. Substance Abuse and Mental Health Services Administration. Results from the 2015 National Survey on Drug Use and Health: detailed tables. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.pdf.
3. Substance Abuse and Mental Health Services Administration. Medication-assisted treatment of opioid use disorder pocket guide. https://store.samhsa.gov/shin/content//SMA16-4892PG/SMA16-4892PG.pdf. Accessed June 29, 2017.
4. Mutlu C, Demirci AC, Yalcin O, et al. One-year follow-up of heroin-dependent adolescents treated with buprenorphine/naloxone for the first time in a substance treatment unit. J Subst Abuse Treat. 2016;67:1-8.
5. Sharma B, Bruner A, Barnett G, et al. Opioid use disorders. Child Adolesc Psychiatr Clin N Am. 2016;25(3):473-487.
6. Davis MA, Lin LA, Liu H, Sites BD. Prescription Opioid Use among Adults with mental health disorders in the United States. J Am Board Fam Med. 2017;30:42-47.
7. Icahn School of Medicine at Mount Sinai. Substance use: prescription drugs. http://www.mountsinai.org/patient-care/health-library/diseases-and-conditions/opioid-abuse#risk. Accessed June 27, 2017.
8. Boscarino JA, Rukstalis M, Hoffman SN, et al. Risk factors for drug dependence among out-patients on opioid therapy in a large US health-care system. Addiction. 2010;105(10):1776-1782.
9. Edlund M, Steffick D, Hudson T, et al. Risk factors for clinically recognized opioid abuse and dependence among veterans using opioids for chronic non-cancer pain. Pain. 2007;129(3):355-362.
10. Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend. 2006;81(2):103-107.
11. Bohnert AS, Valenstein M, Bair M, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321.
12. Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6(6):432.
13. Sun EC, Darnall BD, Baker LC, et al. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med. 2016;176(9):1286-1293.
14. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
15. Starrels JL, Becker WC, Alford DP, et al. Systematic review: treatment agreements and urine drug testing to reduce opioid misuse in patients with chronic pain. Ann Internal Med. 2010;152(11):712-720.
16. Substance Abuse and Mental Health Services Administration. Clinical guidelines for the use of buprenorphine in the treatment of opioid addiction: a treatment improvement protocol: TIP 40. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2004.
17. NIDA drug screening tool: clinician’s screening tool for drug use in general medical settings. National Institutes of Health. https://www.drugabuse.gov/nmassist. Accessed June 27, 2017.
18. Fareed A, Eilender P, Haber M, et al. Comorbid posttraumatic stress disorder and opiate addiction: a literature review. J Addict Dis. 2013;32(2):168-179.
19. Rosen D, Smith ML, Reynolds CF 3rd. The prevalence of mental and physical health disorders among older methadone patients. Am J Geriatr Psychiatry. 2008;16(6):488-497.
20. Goldner EM, Lusted A, Roerecke M, et al. Prevalence of Axis-1 psychiatric (with focus on depression and anxiety) disorder and symptomatology among non-medical prescription opioid users in substance use treatment: systematic review and meta-analyses. Addict Behav. 2014;39(3):520-531.
21. Barry DT, Cutter CJ, Beitel M, et al. Psychiatric disorders among patients seeking treatment for co-occurring chronic pain and opioid use disorder. J Clin Psychiatry. 2016;77(10):1413-1419.
22. Kuramoto SJ, Chilcoat HD, Ko J, et al. Suicidal ideation and suicide attempt across stages of nonmedical prescription opioid use and presence of prescription opioid disorders among U.S. adults. J Stud Alcohol Drugs. 2012;73(2):178-184.
23. Mattick RP, Breen C, Kimber J, et al. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;(2):CD002207. doi: 10.1002/14651858.CD002207.pub4.
24. Evans E, Li L, Min J, et al. Mortality among individuals accessing pharmacological treatment for opioid dependence in California, 2006-10. Addiction. 2015;110(6):996-1005.
25. Marteu D, McDonald R, Patel K. The relative risk of fatal poisoning by methadone or buprenorphine within the wider population of England and Wales. BMJ Open. 2015;5(5):e007629. doi: 10.1136/bmjopen-2015-007629.
26. Jasinski DR, Johnson RE, Kocher TR. Clonidine in morphine withdrawal. Differential effects on signs and symptoms. Arch Gen Psychiatry. 1985;42(11):1063-1066.
27. Whelan PJ, Remski K. Buprenorphine vs methadone treatment: a review of evidence in both developed and developing worlds. J Neurosci Rural Pract. 2012;3(1):45-50.
28. Schuckit MA. Treatment of opioid-use disorders. N Engl J Med. 2016;375(4):357-368.
29. Dutra L, Stathopoulou G, Basden SL, et al. A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry. 2008;165(2):179-187.
30. Brown HL, Britton KA, Mahaffey D, et al. Methadone maintenance in pregnancy: a reappraisal. Am J Obstet Gynecol. 1998;179(2):459-463.
31. Center for Substance Abuse Treatment. Medication-Assisted Treatment for Opioid Addiction in Opioid Treatment Programs. Treatment Improvement Protocol (TIP) 43.
32. Zimlich R. AAP recommends on medication assisted therapy for adolescent opioid addiction. Contemporary Pediatrics. http://contemporarypediatrics.modernmedicine.com/contemporary-pediatrics/news/aap-recommends-medication-assisted-therapy-adolescent-opioid-addiction. Published September 15, 2016. Accessed June 29, 2017.
33. Patkar A, Lee J, Burgess D. Opioid use disorder. BMJ Publishing Group. http://bestpractice.bmj.com/best-practice/monograph/200.html. Published 2015. Accessed July 6, 2017.
34. Alho H, Sinclair D, Vuori E, et al. Abuse liability of buprenorphine-naloxone tablets in untreated IV drug users. Drug Alcohol Depend. 2007;88(1):75-78.
35. Centers for Disease Control and Prevention (CDC). Vital signs: risk for overdose from methadone used for pain relief - United States, 1999-2010. MMWR Morb Mortal Wkly Rep. 2012;61(26):493-497.
36. Soyka M. New developments in the management of opioid dependence: focus on sublingual buprenorphine-naloxone. Subst Abuse Rehabil. 2015;6:1-14.
37. Lee JD, Friedmann PD, Kinlock TW, et al. Extended-Release Naltrexone to Prevent Opioid Relapse in Criminal Justice Offenders N Engl J Med. 2016;374(13):1232-1242.
38. Vo HT, Robbins E, Westwood M, et al. Relapse prevention medications in community treatment for young adults with opioid addiction. Subst Abus. 2016;37(3):392-397.
39. McDonald R, Campbell ND, Strang J. Twenty years of take-home naloxone for the prevention of overdose deaths from heroin and other opioids-conception and maturation. Drug Alcohol Depend. 2017;178:176-187.
40. Centers for Disease Control and Prevention. Overdose prevention. https://www.cdc.gov/drugoverdose/opioids/odprevention.html. Updated February 9, 2017. Accessed July 6, 2017.
Triple-bead mixed amphetamine salt for ADHD
Stimulants are first-line psychopharmacologic interventions for attention-deficit/hyperactivity disorder (ADHD), and their efficacy is supported by clinical trials and meta-analyses in children and adolescents1 as well as adults.2 Despite decades of tolerability and efficacy data supporting their use, a major drawback of stimulants is that their salutary therapeutic effects wane once the medication is cleared or metabolized. Both mixed amphetamine- and methylphenidate-based preparations have short half-lives, necessitating multiple doses per day (eg, 3 or 4 times a day) when short-acting preparations are used. Over the past 15 years, nearly a dozen formulations were developed that extend the duration of action through delayed release, delayed absorption, or utilizing prodrugs.
The encapsulated preparation contains 3 MAS beads: an immediate-release amphetamine salt bead, a pulsed-delayed release bead, and an extended-release bead (Figure 1), which give rise to a unique pharmacokinetic profile (Figure 2).3
Mechanism of action
Like all MAS, this formulation blocks the reuptake of norepinephrine and dopamine, increasing synaptic concentrations of these monoamine neurotransmitters. Additionally, amphetamine salts may inhibit the activity of monoamine oxidase (MAO), further increasing synaptic levels of monoamines.4 Enhancing noradrenergic, dopaminergic neurotransmission, particularly within the prefrontal cortex, increases attention, working memory, and processing speed in patients with ADHD.4
Pharmacokinetics
Cmax occurs approximately 7 to 10 hours and 8 hours following administration in adolescent and adult patients, respectively (Figure 2).3 In adolescents who were administered a single dose of long-acting, triple-bead MAS, Cmax and area under the curve (AUC) for d- and l-amphetamine were both 21% to 31% higher compared with adults3 and did not appear to be affected by sex or race.3
Half-life is 10 to 11 hours for d-amphetamine and 10 to 13 hours for l-amphetamine and does not statistically differ between pediatric and adult studies.3
Metabolism and elimination. Amphetamines are partially metabolized through cytochrome 450 (CYP) 2D6-dependent mechanisms, and thus in CYP2D6 poor metabolizers medication exposure may be increased, while decreased exposure may occur in ultra-rapid metabolizers; however, there are no guidelines from the Clinical Pharmacogenetics Implementation Consortium regarding alternate dosing strategies for patients based on CYP2D6 genotype or activity phenotype.5 Because amphetamines are renally excreted, dosages should be adjusted in patients with renal impairment.
Drug interactions. Medications that affect gastrointestinal and urinary pH may affect serum concentrations of amphetamine. Specifically, agents that increase gastric pH (eg, proton pump inhibitors) as well as urinary alkalinizing agents (eg, acetazolamide, some thiazide diuretics) increase serum amphetamine concentrations.3 Because amphetamine is a weak MAOI, there is a theoretical risk of serotonin syndrome when amphetamine-based preparations are used concurrently with SSRIs, TCAs, and MAOIs. However, the concurrent use of MAS and SSRIs generally is considered safe and common practice in patients with ADHD and co-occurring anxiety6,7 or depressive disorders.
Dosing
Long-acting, triple-bead MAS is available in 12.5-, 25-, 37.5-, and 50-mg capsules. The capsule may be opened and sprinkled in food for patients who cannot swallow capsules. Opening of the capsule results in similar absorption relative to oral administration of the intact capsule.3
In adults with ADHD, long-acting, triple-bead MAS should be initiated at 12.5 mg in the morning (Table 2). However, in some individuals, long-acting, triple-bead MAS may be initiated at 25 mg each morning. Titration should occur in 12.5-mg weekly increments to a maximum dosage of 50 mg/d.3
In adults with severe renal impairment (glomerular filtrate rate, 15 to 30 mL/min/1.73 m2), the recommended starting dose is 12.5 mg/d, with a maximum dosage of 25 mg/d.3
The efficacy of long-acting, triple-bead MAS in adults with ADHD was demonstrated in 3 studies involving adults ages 18 to 55, and the effectiveness of the medication, with regard to duration of action, was assessed using the Time-Sensitive ADHD Symptom Scale—a self-report scale that consists of items indexed by the ADHD Rating Scale-IV (ADHD-RS-IV) which assesses ADHD symptom severity. Doses up to 75 mg/d were studied; however, there were no significant effects. It should be noted that this maximum daily dose was not determined by any safety parameter.
Study 1 (dose-optimization, triple-bead MAS, n = 137; placebo, n = 135, dosing: 12.5 to 75 mg) and Study 2 (forced dose-titration study, triple-bead MAS, n = 308; placebo, n = 104, dosing: 25 mg, 50 mg, 75 mg) demonstrated efficacy of triple-bead MAS for treating ADHD in adults. Despite differences in study designs, statistically significant and similar clinically relevant improvement was observed with triple-bead MAS (vs placebo) on ADHD-RS-IV total scores in both Study 1 and Study 2.8 An additional study in adults ages 18 to 55 (N = 275) with ADHD (DSM-5 criteria) involved randomization to either 12.5 mg (fixed dose) or forced titration (12.5 to 37.5 mg) or placebo and, as with the first 2 studies, improvement in ADHD symptoms was observed in triple-bead MAS-treated patients relative to those who had received placebo. (See Reference 3 for a summary of the clinical trials of triple-bead MAS in adults with ADHD.)
The tolerability of this medication was evaluated in a 12-month open-label study of adults with ADHD (DSM-IV-TR criteria) in which discontinuation was higher at doses >25 mg/d.7 Treatment-related increases in blood pressure and heart rate were consistent with the known hemodynamic adverse effect profile of stimulants.9
In adolescents with ADHD ages 13 to 17, long-acting, triple-bead MAS should be initiated at 12.5 mg/d and may be increased to 25 mg/d (Table 2). Importantly, in younger patients, including those younger than age 12, triple-bead MAS was associated with an increased risk of adverse events including insomnia and anorexia, and this was thought to be related to increased drug exposure (ie, AUC).
The efficacy of long-acting, triple-bead MAS was evaluated in 2 studies of adolescents ages 13 to 17, including 1 fixed-dose trial (25 mg/d) and 1 flexibly-dosed trial (12.5 to 25 mg/d). These unpublished studies utilized the ADHD-RS-IV score and the Average Permanent Product Measure of Performance, an age-adjusted math test and measure of sustained attention, and revealed statistically significant differences between medication and placebo in the primary outcomes.3
Adverse effects
Long-acting, triple-bead MAS was developed to treat ADHD symptoms throughout the day, and serum concentrations of the medication may be higher with this formulation compared with other long-acting preparations. Therefore, adverse effects that are directly related to plasma exposure (eg, insomnia and appetite suppression) may occur at higher rates with this preparation compared with alternatives. For example, in some of the registration trials, insomnia occurred in more than one-third of patients receiving the active medication (38%).9 Although insomnia was the most frequently reported adverse event in adults with ADHD, most reports of insomnia occurred early in the course of treatment. Of these insomnia-related adverse events, 94% were mild to moderate and resulted in discontinuation of the medication in approximately 2% of patients. Further, 73.9% of treatment-emergent, insomnia–related adverse events resolved during the course of the study. It is also important to note that the Pittsburgh Sleep Quality Index did not differ from placebo in studies of triple-bead MAS in adults with ADHD.10 Similarly, rates of stimulant-induced appetite suppression may be higher with this preparation compared with other long-acting preparations.9
Adverse effects observed in adults with ADHD that occurred in ≥2% of patients receiving triple-bead MAS and at least twice the incidence in patients randomized to placebo included:
- anxiety (7% vs 3%)
- feeling jittery (2% vs 1%)
- agitation (2% vs 0%)
- insomnia (31% vs 8%)
- depression (3% vs 0%)
- decreased appetite (30% vs 4%)
- weight loss (9% vs 0%)
- xerostomia (23% vs 4%)
- diarrhea (3% vs 0%)
- increased heart rate (9% vs 0%)
- palpitations (4% vs 2%)
- dysmenorrhea (4% vs 2%)
- erectile dysfunction (2% vs 1%).
In adolescents receiving triple-bea
1. Punja S, Shamseer L, Hartling L, et al. Amphetamines for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst Rev. 2016;2016(2):CD009996.
2. Castells X, Ramos-Quiroga J, Bosch R, et al. Amphetamines for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database Syst Rev. 2011;(6):CD007813.
3. Mydayis [package insert]. Lexington, MA: Shire; 2017.
4. Heal DJ, Smith SL, Gosden J, et al. Amphetamine, past and present—a pharmacological and clinical perspective. J Psychopharmacol. 2013;27(6):479-496.
5. Hoffman JM, Dunnenberger HM, Kevin Hicks J, et al. Developing knowledge resources to support precision medicine: principles from the Clinical Pharmacogenetics Implementation Consortium (CPIC). J Am Med Inform Assoc. 2016;23(4):766-801.
6. Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359(26):2753-2766.
7. Connolly SD, Bernstein GA; Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2007;46(2):267-283.
8. Goodman DW, Spencer TJ, Adler LA, et al. Clinical evaluation of triple-bead mixed amphetamine salts in adult ADHD. Presented at: 54th Annual Meeting of the American Academy of Child and Adolescent Psychiatry; October 25, 2007; Boston, MA.
9. Adler LA, Frick G, Yan B. A Long-term, open-label, safety study of triple-bead mixed amphetamine salts (SHP465) in adults with ADHD [published online April 1, 2017]. J Atten Disord. doi: 10.1177/1087054717696770.
10. Backhaus J, Junghanns K, Broocks A, et al. test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res. 2002;53(3):737-740.
Stimulants are first-line psychopharmacologic interventions for attention-deficit/hyperactivity disorder (ADHD), and their efficacy is supported by clinical trials and meta-analyses in children and adolescents1 as well as adults.2 Despite decades of tolerability and efficacy data supporting their use, a major drawback of stimulants is that their salutary therapeutic effects wane once the medication is cleared or metabolized. Both mixed amphetamine- and methylphenidate-based preparations have short half-lives, necessitating multiple doses per day (eg, 3 or 4 times a day) when short-acting preparations are used. Over the past 15 years, nearly a dozen formulations were developed that extend the duration of action through delayed release, delayed absorption, or utilizing prodrugs.
The encapsulated preparation contains 3 MAS beads: an immediate-release amphetamine salt bead, a pulsed-delayed release bead, and an extended-release bead (Figure 1), which give rise to a unique pharmacokinetic profile (Figure 2).3
Mechanism of action
Like all MAS, this formulation blocks the reuptake of norepinephrine and dopamine, increasing synaptic concentrations of these monoamine neurotransmitters. Additionally, amphetamine salts may inhibit the activity of monoamine oxidase (MAO), further increasing synaptic levels of monoamines.4 Enhancing noradrenergic, dopaminergic neurotransmission, particularly within the prefrontal cortex, increases attention, working memory, and processing speed in patients with ADHD.4
Pharmacokinetics
Cmax occurs approximately 7 to 10 hours and 8 hours following administration in adolescent and adult patients, respectively (Figure 2).3 In adolescents who were administered a single dose of long-acting, triple-bead MAS, Cmax and area under the curve (AUC) for d- and l-amphetamine were both 21% to 31% higher compared with adults3 and did not appear to be affected by sex or race.3
Half-life is 10 to 11 hours for d-amphetamine and 10 to 13 hours for l-amphetamine and does not statistically differ between pediatric and adult studies.3
Metabolism and elimination. Amphetamines are partially metabolized through cytochrome 450 (CYP) 2D6-dependent mechanisms, and thus in CYP2D6 poor metabolizers medication exposure may be increased, while decreased exposure may occur in ultra-rapid metabolizers; however, there are no guidelines from the Clinical Pharmacogenetics Implementation Consortium regarding alternate dosing strategies for patients based on CYP2D6 genotype or activity phenotype.5 Because amphetamines are renally excreted, dosages should be adjusted in patients with renal impairment.
Drug interactions. Medications that affect gastrointestinal and urinary pH may affect serum concentrations of amphetamine. Specifically, agents that increase gastric pH (eg, proton pump inhibitors) as well as urinary alkalinizing agents (eg, acetazolamide, some thiazide diuretics) increase serum amphetamine concentrations.3 Because amphetamine is a weak MAOI, there is a theoretical risk of serotonin syndrome when amphetamine-based preparations are used concurrently with SSRIs, TCAs, and MAOIs. However, the concurrent use of MAS and SSRIs generally is considered safe and common practice in patients with ADHD and co-occurring anxiety6,7 or depressive disorders.
Dosing
Long-acting, triple-bead MAS is available in 12.5-, 25-, 37.5-, and 50-mg capsules. The capsule may be opened and sprinkled in food for patients who cannot swallow capsules. Opening of the capsule results in similar absorption relative to oral administration of the intact capsule.3
In adults with ADHD, long-acting, triple-bead MAS should be initiated at 12.5 mg in the morning (Table 2). However, in some individuals, long-acting, triple-bead MAS may be initiated at 25 mg each morning. Titration should occur in 12.5-mg weekly increments to a maximum dosage of 50 mg/d.3
In adults with severe renal impairment (glomerular filtrate rate, 15 to 30 mL/min/1.73 m2), the recommended starting dose is 12.5 mg/d, with a maximum dosage of 25 mg/d.3
The efficacy of long-acting, triple-bead MAS in adults with ADHD was demonstrated in 3 studies involving adults ages 18 to 55, and the effectiveness of the medication, with regard to duration of action, was assessed using the Time-Sensitive ADHD Symptom Scale—a self-report scale that consists of items indexed by the ADHD Rating Scale-IV (ADHD-RS-IV) which assesses ADHD symptom severity. Doses up to 75 mg/d were studied; however, there were no significant effects. It should be noted that this maximum daily dose was not determined by any safety parameter.
Study 1 (dose-optimization, triple-bead MAS, n = 137; placebo, n = 135, dosing: 12.5 to 75 mg) and Study 2 (forced dose-titration study, triple-bead MAS, n = 308; placebo, n = 104, dosing: 25 mg, 50 mg, 75 mg) demonstrated efficacy of triple-bead MAS for treating ADHD in adults. Despite differences in study designs, statistically significant and similar clinically relevant improvement was observed with triple-bead MAS (vs placebo) on ADHD-RS-IV total scores in both Study 1 and Study 2.8 An additional study in adults ages 18 to 55 (N = 275) with ADHD (DSM-5 criteria) involved randomization to either 12.5 mg (fixed dose) or forced titration (12.5 to 37.5 mg) or placebo and, as with the first 2 studies, improvement in ADHD symptoms was observed in triple-bead MAS-treated patients relative to those who had received placebo. (See Reference 3 for a summary of the clinical trials of triple-bead MAS in adults with ADHD.)
The tolerability of this medication was evaluated in a 12-month open-label study of adults with ADHD (DSM-IV-TR criteria) in which discontinuation was higher at doses >25 mg/d.7 Treatment-related increases in blood pressure and heart rate were consistent with the known hemodynamic adverse effect profile of stimulants.9
In adolescents with ADHD ages 13 to 17, long-acting, triple-bead MAS should be initiated at 12.5 mg/d and may be increased to 25 mg/d (Table 2). Importantly, in younger patients, including those younger than age 12, triple-bead MAS was associated with an increased risk of adverse events including insomnia and anorexia, and this was thought to be related to increased drug exposure (ie, AUC).
The efficacy of long-acting, triple-bead MAS was evaluated in 2 studies of adolescents ages 13 to 17, including 1 fixed-dose trial (25 mg/d) and 1 flexibly-dosed trial (12.5 to 25 mg/d). These unpublished studies utilized the ADHD-RS-IV score and the Average Permanent Product Measure of Performance, an age-adjusted math test and measure of sustained attention, and revealed statistically significant differences between medication and placebo in the primary outcomes.3
Adverse effects
Long-acting, triple-bead MAS was developed to treat ADHD symptoms throughout the day, and serum concentrations of the medication may be higher with this formulation compared with other long-acting preparations. Therefore, adverse effects that are directly related to plasma exposure (eg, insomnia and appetite suppression) may occur at higher rates with this preparation compared with alternatives. For example, in some of the registration trials, insomnia occurred in more than one-third of patients receiving the active medication (38%).9 Although insomnia was the most frequently reported adverse event in adults with ADHD, most reports of insomnia occurred early in the course of treatment. Of these insomnia-related adverse events, 94% were mild to moderate and resulted in discontinuation of the medication in approximately 2% of patients. Further, 73.9% of treatment-emergent, insomnia–related adverse events resolved during the course of the study. It is also important to note that the Pittsburgh Sleep Quality Index did not differ from placebo in studies of triple-bead MAS in adults with ADHD.10 Similarly, rates of stimulant-induced appetite suppression may be higher with this preparation compared with other long-acting preparations.9
Adverse effects observed in adults with ADHD that occurred in ≥2% of patients receiving triple-bead MAS and at least twice the incidence in patients randomized to placebo included:
- anxiety (7% vs 3%)
- feeling jittery (2% vs 1%)
- agitation (2% vs 0%)
- insomnia (31% vs 8%)
- depression (3% vs 0%)
- decreased appetite (30% vs 4%)
- weight loss (9% vs 0%)
- xerostomia (23% vs 4%)
- diarrhea (3% vs 0%)
- increased heart rate (9% vs 0%)
- palpitations (4% vs 2%)
- dysmenorrhea (4% vs 2%)
- erectile dysfunction (2% vs 1%).
In adolescents receiving triple-bea
Stimulants are first-line psychopharmacologic interventions for attention-deficit/hyperactivity disorder (ADHD), and their efficacy is supported by clinical trials and meta-analyses in children and adolescents1 as well as adults.2 Despite decades of tolerability and efficacy data supporting their use, a major drawback of stimulants is that their salutary therapeutic effects wane once the medication is cleared or metabolized. Both mixed amphetamine- and methylphenidate-based preparations have short half-lives, necessitating multiple doses per day (eg, 3 or 4 times a day) when short-acting preparations are used. Over the past 15 years, nearly a dozen formulations were developed that extend the duration of action through delayed release, delayed absorption, or utilizing prodrugs.
The encapsulated preparation contains 3 MAS beads: an immediate-release amphetamine salt bead, a pulsed-delayed release bead, and an extended-release bead (Figure 1), which give rise to a unique pharmacokinetic profile (Figure 2).3
Mechanism of action
Like all MAS, this formulation blocks the reuptake of norepinephrine and dopamine, increasing synaptic concentrations of these monoamine neurotransmitters. Additionally, amphetamine salts may inhibit the activity of monoamine oxidase (MAO), further increasing synaptic levels of monoamines.4 Enhancing noradrenergic, dopaminergic neurotransmission, particularly within the prefrontal cortex, increases attention, working memory, and processing speed in patients with ADHD.4
Pharmacokinetics
Cmax occurs approximately 7 to 10 hours and 8 hours following administration in adolescent and adult patients, respectively (Figure 2).3 In adolescents who were administered a single dose of long-acting, triple-bead MAS, Cmax and area under the curve (AUC) for d- and l-amphetamine were both 21% to 31% higher compared with adults3 and did not appear to be affected by sex or race.3
Half-life is 10 to 11 hours for d-amphetamine and 10 to 13 hours for l-amphetamine and does not statistically differ between pediatric and adult studies.3
Metabolism and elimination. Amphetamines are partially metabolized through cytochrome 450 (CYP) 2D6-dependent mechanisms, and thus in CYP2D6 poor metabolizers medication exposure may be increased, while decreased exposure may occur in ultra-rapid metabolizers; however, there are no guidelines from the Clinical Pharmacogenetics Implementation Consortium regarding alternate dosing strategies for patients based on CYP2D6 genotype or activity phenotype.5 Because amphetamines are renally excreted, dosages should be adjusted in patients with renal impairment.
Drug interactions. Medications that affect gastrointestinal and urinary pH may affect serum concentrations of amphetamine. Specifically, agents that increase gastric pH (eg, proton pump inhibitors) as well as urinary alkalinizing agents (eg, acetazolamide, some thiazide diuretics) increase serum amphetamine concentrations.3 Because amphetamine is a weak MAOI, there is a theoretical risk of serotonin syndrome when amphetamine-based preparations are used concurrently with SSRIs, TCAs, and MAOIs. However, the concurrent use of MAS and SSRIs generally is considered safe and common practice in patients with ADHD and co-occurring anxiety6,7 or depressive disorders.
Dosing
Long-acting, triple-bead MAS is available in 12.5-, 25-, 37.5-, and 50-mg capsules. The capsule may be opened and sprinkled in food for patients who cannot swallow capsules. Opening of the capsule results in similar absorption relative to oral administration of the intact capsule.3
In adults with ADHD, long-acting, triple-bead MAS should be initiated at 12.5 mg in the morning (Table 2). However, in some individuals, long-acting, triple-bead MAS may be initiated at 25 mg each morning. Titration should occur in 12.5-mg weekly increments to a maximum dosage of 50 mg/d.3
In adults with severe renal impairment (glomerular filtrate rate, 15 to 30 mL/min/1.73 m2), the recommended starting dose is 12.5 mg/d, with a maximum dosage of 25 mg/d.3
The efficacy of long-acting, triple-bead MAS in adults with ADHD was demonstrated in 3 studies involving adults ages 18 to 55, and the effectiveness of the medication, with regard to duration of action, was assessed using the Time-Sensitive ADHD Symptom Scale—a self-report scale that consists of items indexed by the ADHD Rating Scale-IV (ADHD-RS-IV) which assesses ADHD symptom severity. Doses up to 75 mg/d were studied; however, there were no significant effects. It should be noted that this maximum daily dose was not determined by any safety parameter.
Study 1 (dose-optimization, triple-bead MAS, n = 137; placebo, n = 135, dosing: 12.5 to 75 mg) and Study 2 (forced dose-titration study, triple-bead MAS, n = 308; placebo, n = 104, dosing: 25 mg, 50 mg, 75 mg) demonstrated efficacy of triple-bead MAS for treating ADHD in adults. Despite differences in study designs, statistically significant and similar clinically relevant improvement was observed with triple-bead MAS (vs placebo) on ADHD-RS-IV total scores in both Study 1 and Study 2.8 An additional study in adults ages 18 to 55 (N = 275) with ADHD (DSM-5 criteria) involved randomization to either 12.5 mg (fixed dose) or forced titration (12.5 to 37.5 mg) or placebo and, as with the first 2 studies, improvement in ADHD symptoms was observed in triple-bead MAS-treated patients relative to those who had received placebo. (See Reference 3 for a summary of the clinical trials of triple-bead MAS in adults with ADHD.)
The tolerability of this medication was evaluated in a 12-month open-label study of adults with ADHD (DSM-IV-TR criteria) in which discontinuation was higher at doses >25 mg/d.7 Treatment-related increases in blood pressure and heart rate were consistent with the known hemodynamic adverse effect profile of stimulants.9
In adolescents with ADHD ages 13 to 17, long-acting, triple-bead MAS should be initiated at 12.5 mg/d and may be increased to 25 mg/d (Table 2). Importantly, in younger patients, including those younger than age 12, triple-bead MAS was associated with an increased risk of adverse events including insomnia and anorexia, and this was thought to be related to increased drug exposure (ie, AUC).
The efficacy of long-acting, triple-bead MAS was evaluated in 2 studies of adolescents ages 13 to 17, including 1 fixed-dose trial (25 mg/d) and 1 flexibly-dosed trial (12.5 to 25 mg/d). These unpublished studies utilized the ADHD-RS-IV score and the Average Permanent Product Measure of Performance, an age-adjusted math test and measure of sustained attention, and revealed statistically significant differences between medication and placebo in the primary outcomes.3
Adverse effects
Long-acting, triple-bead MAS was developed to treat ADHD symptoms throughout the day, and serum concentrations of the medication may be higher with this formulation compared with other long-acting preparations. Therefore, adverse effects that are directly related to plasma exposure (eg, insomnia and appetite suppression) may occur at higher rates with this preparation compared with alternatives. For example, in some of the registration trials, insomnia occurred in more than one-third of patients receiving the active medication (38%).9 Although insomnia was the most frequently reported adverse event in adults with ADHD, most reports of insomnia occurred early in the course of treatment. Of these insomnia-related adverse events, 94% were mild to moderate and resulted in discontinuation of the medication in approximately 2% of patients. Further, 73.9% of treatment-emergent, insomnia–related adverse events resolved during the course of the study. It is also important to note that the Pittsburgh Sleep Quality Index did not differ from placebo in studies of triple-bead MAS in adults with ADHD.10 Similarly, rates of stimulant-induced appetite suppression may be higher with this preparation compared with other long-acting preparations.9
Adverse effects observed in adults with ADHD that occurred in ≥2% of patients receiving triple-bead MAS and at least twice the incidence in patients randomized to placebo included:
- anxiety (7% vs 3%)
- feeling jittery (2% vs 1%)
- agitation (2% vs 0%)
- insomnia (31% vs 8%)
- depression (3% vs 0%)
- decreased appetite (30% vs 4%)
- weight loss (9% vs 0%)
- xerostomia (23% vs 4%)
- diarrhea (3% vs 0%)
- increased heart rate (9% vs 0%)
- palpitations (4% vs 2%)
- dysmenorrhea (4% vs 2%)
- erectile dysfunction (2% vs 1%).
In adolescents receiving triple-bea
1. Punja S, Shamseer L, Hartling L, et al. Amphetamines for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst Rev. 2016;2016(2):CD009996.
2. Castells X, Ramos-Quiroga J, Bosch R, et al. Amphetamines for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database Syst Rev. 2011;(6):CD007813.
3. Mydayis [package insert]. Lexington, MA: Shire; 2017.
4. Heal DJ, Smith SL, Gosden J, et al. Amphetamine, past and present—a pharmacological and clinical perspective. J Psychopharmacol. 2013;27(6):479-496.
5. Hoffman JM, Dunnenberger HM, Kevin Hicks J, et al. Developing knowledge resources to support precision medicine: principles from the Clinical Pharmacogenetics Implementation Consortium (CPIC). J Am Med Inform Assoc. 2016;23(4):766-801.
6. Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359(26):2753-2766.
7. Connolly SD, Bernstein GA; Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2007;46(2):267-283.
8. Goodman DW, Spencer TJ, Adler LA, et al. Clinical evaluation of triple-bead mixed amphetamine salts in adult ADHD. Presented at: 54th Annual Meeting of the American Academy of Child and Adolescent Psychiatry; October 25, 2007; Boston, MA.
9. Adler LA, Frick G, Yan B. A Long-term, open-label, safety study of triple-bead mixed amphetamine salts (SHP465) in adults with ADHD [published online April 1, 2017]. J Atten Disord. doi: 10.1177/1087054717696770.
10. Backhaus J, Junghanns K, Broocks A, et al. test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res. 2002;53(3):737-740.
1. Punja S, Shamseer L, Hartling L, et al. Amphetamines for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst Rev. 2016;2016(2):CD009996.
2. Castells X, Ramos-Quiroga J, Bosch R, et al. Amphetamines for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database Syst Rev. 2011;(6):CD007813.
3. Mydayis [package insert]. Lexington, MA: Shire; 2017.
4. Heal DJ, Smith SL, Gosden J, et al. Amphetamine, past and present—a pharmacological and clinical perspective. J Psychopharmacol. 2013;27(6):479-496.
5. Hoffman JM, Dunnenberger HM, Kevin Hicks J, et al. Developing knowledge resources to support precision medicine: principles from the Clinical Pharmacogenetics Implementation Consortium (CPIC). J Am Med Inform Assoc. 2016;23(4):766-801.
6. Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359(26):2753-2766.
7. Connolly SD, Bernstein GA; Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2007;46(2):267-283.
8. Goodman DW, Spencer TJ, Adler LA, et al. Clinical evaluation of triple-bead mixed amphetamine salts in adult ADHD. Presented at: 54th Annual Meeting of the American Academy of Child and Adolescent Psychiatry; October 25, 2007; Boston, MA.
9. Adler LA, Frick G, Yan B. A Long-term, open-label, safety study of triple-bead mixed amphetamine salts (SHP465) in adults with ADHD [published online April 1, 2017]. J Atten Disord. doi: 10.1177/1087054717696770.
10. Backhaus J, Junghanns K, Broocks A, et al. test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res. 2002;53(3):737-740.
Caring for medical marijuana patients who request controlled prescriptions
Twenty-eight states and Washington, DC, have legalized marijuana for treating certain medical conditions, but the United States Drug Enforcement Administration (DEA) still classifies marijuana as a Schedule I drug “with no currently accepted medical use and a high potential for abuse.”1 In certain states, clinicians can recommend, but not prescribe, medical marijuana. There is limited guidance in caring for patients who use medical marijuana and request or use DEA-controlled prescription medications, such as benzodiazepines, stimulants, and/or opiates. Physicians can take the following steps to ensure safe care for patients who use medical marijuana and request or take a DEA-controlled prescription medication:
1. Understand your patients’ point of view. Talk with patients who use medical marijuana about the history, frequency, and method of use, and reasons for using medical marijuana. Assess for psychiatric illnesses and any past or active treatment with DEA-controlled prescription medications.
2. Perform screens. Screen for risk factors, past psychiatric history, and prior or current substance use disorders. Treat any existing substance use disorders as appropriate.
3. Provide education. Discuss the risks of marijuana use and its potential adverse effects on the patient’s illness. Explain that marijuana is not currently an FDA-approved treatment and that there often are safer, efficacious alternatives.
4. Set clear boundaries. Be upfront about what is safe clinical practice or the usual standard of medical care and practice within the scope of state and federal laws. Document treatment agreements, utilize prescription drug monitoring programs, and use blood and/or urine toxicology screens as needed. Be aware that a routine drug screen can detect marijuana exposure but may vary in detecting the quantity or length of marijuana use.2
5. Try harm reduction. Any marijuana use, including use that falls short of a Cannabis use disorder, may adversely impact cognition, mood, and/or anxiety.3 Reducing use or abstaining from marijuana use for at least 4 weeks4,5 or reducing or discontinuing the DEA-controlled medication if a patient continues marijuana use are reasonable interventions to see if psychiatric symptoms improve or remit. Polypharmacy with marijuana may place a patient at risk for substance use disorders or additive adverse effects or can hinder the recovery process.
6. Consider alternatives. If a patient feels strongly about continuing medical marijuana use, and you feel that their marijuana use is not clinically harmful and that psychiatric symptoms require treatment, consider medications without a known potential for abuse (eg, antidepressants, buspirone, or hydroxyzine for anxiety; alpha-agonists or atomoxetine for attention-deficit/hyperactivity disorder, etc.). Start such medications at low dosages, titrate slowly, and monitor for benefits and adverse effects.
7. Continue the conversation. Maintain an open and nonjudgmental stance when discussing medical marijuana. Roll with resistance, and frame discussions toward a shared goal of improving the patient’s mental health as safely as possible while using the best medical evidence available.
8. Offer additional support. Refer patients any additional services as appropriate, which may include psychotherapy, a pain specialist, or a substance abuse specialist.
1. United States Drug Enforcement Administration. Drug scheduling. https://www.dea.gov/druginfo/ds.shtml. Accessed June 22, 2017.
2. Verstraete AG. Detection times of drugs of abuse in blood, urine, and oral fluid. Ther Drug Monit. 2004:26(2);200-205.
3. Volkow ND, Baler RD, Compton WM, et al. Adverse health effects of marijuana use. N Engl J Med. 2014:370(23);2219-2227.
4. Schuster RM, Fontaine M, Nip E, et al. Prolonged cannabis withdrawal in young adults with lifetime psychiatric illness [published online February 27, 2017]. Prev Med. pii: S0091-7435(17)30075-0.
5. Bonnet U, Preuss UW. The cannabis withdrawal syndrome: current insights. Subst Abuse Rehabil. 2017:8:9-37.
Twenty-eight states and Washington, DC, have legalized marijuana for treating certain medical conditions, but the United States Drug Enforcement Administration (DEA) still classifies marijuana as a Schedule I drug “with no currently accepted medical use and a high potential for abuse.”1 In certain states, clinicians can recommend, but not prescribe, medical marijuana. There is limited guidance in caring for patients who use medical marijuana and request or use DEA-controlled prescription medications, such as benzodiazepines, stimulants, and/or opiates. Physicians can take the following steps to ensure safe care for patients who use medical marijuana and request or take a DEA-controlled prescription medication:
1. Understand your patients’ point of view. Talk with patients who use medical marijuana about the history, frequency, and method of use, and reasons for using medical marijuana. Assess for psychiatric illnesses and any past or active treatment with DEA-controlled prescription medications.
2. Perform screens. Screen for risk factors, past psychiatric history, and prior or current substance use disorders. Treat any existing substance use disorders as appropriate.
3. Provide education. Discuss the risks of marijuana use and its potential adverse effects on the patient’s illness. Explain that marijuana is not currently an FDA-approved treatment and that there often are safer, efficacious alternatives.
4. Set clear boundaries. Be upfront about what is safe clinical practice or the usual standard of medical care and practice within the scope of state and federal laws. Document treatment agreements, utilize prescription drug monitoring programs, and use blood and/or urine toxicology screens as needed. Be aware that a routine drug screen can detect marijuana exposure but may vary in detecting the quantity or length of marijuana use.2
5. Try harm reduction. Any marijuana use, including use that falls short of a Cannabis use disorder, may adversely impact cognition, mood, and/or anxiety.3 Reducing use or abstaining from marijuana use for at least 4 weeks4,5 or reducing or discontinuing the DEA-controlled medication if a patient continues marijuana use are reasonable interventions to see if psychiatric symptoms improve or remit. Polypharmacy with marijuana may place a patient at risk for substance use disorders or additive adverse effects or can hinder the recovery process.
6. Consider alternatives. If a patient feels strongly about continuing medical marijuana use, and you feel that their marijuana use is not clinically harmful and that psychiatric symptoms require treatment, consider medications without a known potential for abuse (eg, antidepressants, buspirone, or hydroxyzine for anxiety; alpha-agonists or atomoxetine for attention-deficit/hyperactivity disorder, etc.). Start such medications at low dosages, titrate slowly, and monitor for benefits and adverse effects.
7. Continue the conversation. Maintain an open and nonjudgmental stance when discussing medical marijuana. Roll with resistance, and frame discussions toward a shared goal of improving the patient’s mental health as safely as possible while using the best medical evidence available.
8. Offer additional support. Refer patients any additional services as appropriate, which may include psychotherapy, a pain specialist, or a substance abuse specialist.
Twenty-eight states and Washington, DC, have legalized marijuana for treating certain medical conditions, but the United States Drug Enforcement Administration (DEA) still classifies marijuana as a Schedule I drug “with no currently accepted medical use and a high potential for abuse.”1 In certain states, clinicians can recommend, but not prescribe, medical marijuana. There is limited guidance in caring for patients who use medical marijuana and request or use DEA-controlled prescription medications, such as benzodiazepines, stimulants, and/or opiates. Physicians can take the following steps to ensure safe care for patients who use medical marijuana and request or take a DEA-controlled prescription medication:
1. Understand your patients’ point of view. Talk with patients who use medical marijuana about the history, frequency, and method of use, and reasons for using medical marijuana. Assess for psychiatric illnesses and any past or active treatment with DEA-controlled prescription medications.
2. Perform screens. Screen for risk factors, past psychiatric history, and prior or current substance use disorders. Treat any existing substance use disorders as appropriate.
3. Provide education. Discuss the risks of marijuana use and its potential adverse effects on the patient’s illness. Explain that marijuana is not currently an FDA-approved treatment and that there often are safer, efficacious alternatives.
4. Set clear boundaries. Be upfront about what is safe clinical practice or the usual standard of medical care and practice within the scope of state and federal laws. Document treatment agreements, utilize prescription drug monitoring programs, and use blood and/or urine toxicology screens as needed. Be aware that a routine drug screen can detect marijuana exposure but may vary in detecting the quantity or length of marijuana use.2
5. Try harm reduction. Any marijuana use, including use that falls short of a Cannabis use disorder, may adversely impact cognition, mood, and/or anxiety.3 Reducing use or abstaining from marijuana use for at least 4 weeks4,5 or reducing or discontinuing the DEA-controlled medication if a patient continues marijuana use are reasonable interventions to see if psychiatric symptoms improve or remit. Polypharmacy with marijuana may place a patient at risk for substance use disorders or additive adverse effects or can hinder the recovery process.
6. Consider alternatives. If a patient feels strongly about continuing medical marijuana use, and you feel that their marijuana use is not clinically harmful and that psychiatric symptoms require treatment, consider medications without a known potential for abuse (eg, antidepressants, buspirone, or hydroxyzine for anxiety; alpha-agonists or atomoxetine for attention-deficit/hyperactivity disorder, etc.). Start such medications at low dosages, titrate slowly, and monitor for benefits and adverse effects.
7. Continue the conversation. Maintain an open and nonjudgmental stance when discussing medical marijuana. Roll with resistance, and frame discussions toward a shared goal of improving the patient’s mental health as safely as possible while using the best medical evidence available.
8. Offer additional support. Refer patients any additional services as appropriate, which may include psychotherapy, a pain specialist, or a substance abuse specialist.
1. United States Drug Enforcement Administration. Drug scheduling. https://www.dea.gov/druginfo/ds.shtml. Accessed June 22, 2017.
2. Verstraete AG. Detection times of drugs of abuse in blood, urine, and oral fluid. Ther Drug Monit. 2004:26(2);200-205.
3. Volkow ND, Baler RD, Compton WM, et al. Adverse health effects of marijuana use. N Engl J Med. 2014:370(23);2219-2227.
4. Schuster RM, Fontaine M, Nip E, et al. Prolonged cannabis withdrawal in young adults with lifetime psychiatric illness [published online February 27, 2017]. Prev Med. pii: S0091-7435(17)30075-0.
5. Bonnet U, Preuss UW. The cannabis withdrawal syndrome: current insights. Subst Abuse Rehabil. 2017:8:9-37.
1. United States Drug Enforcement Administration. Drug scheduling. https://www.dea.gov/druginfo/ds.shtml. Accessed June 22, 2017.
2. Verstraete AG. Detection times of drugs of abuse in blood, urine, and oral fluid. Ther Drug Monit. 2004:26(2);200-205.
3. Volkow ND, Baler RD, Compton WM, et al. Adverse health effects of marijuana use. N Engl J Med. 2014:370(23);2219-2227.
4. Schuster RM, Fontaine M, Nip E, et al. Prolonged cannabis withdrawal in young adults with lifetime psychiatric illness [published online February 27, 2017]. Prev Med. pii: S0091-7435(17)30075-0.
5. Bonnet U, Preuss UW. The cannabis withdrawal syndrome: current insights. Subst Abuse Rehabil. 2017:8:9-37.