User login
Once-weekly topical therapy shows promise for moderate to severe acne
TOPLINE:
METHODOLOGY:
- Poor patient compliance with topical acne therapies is a common clinical challenge.
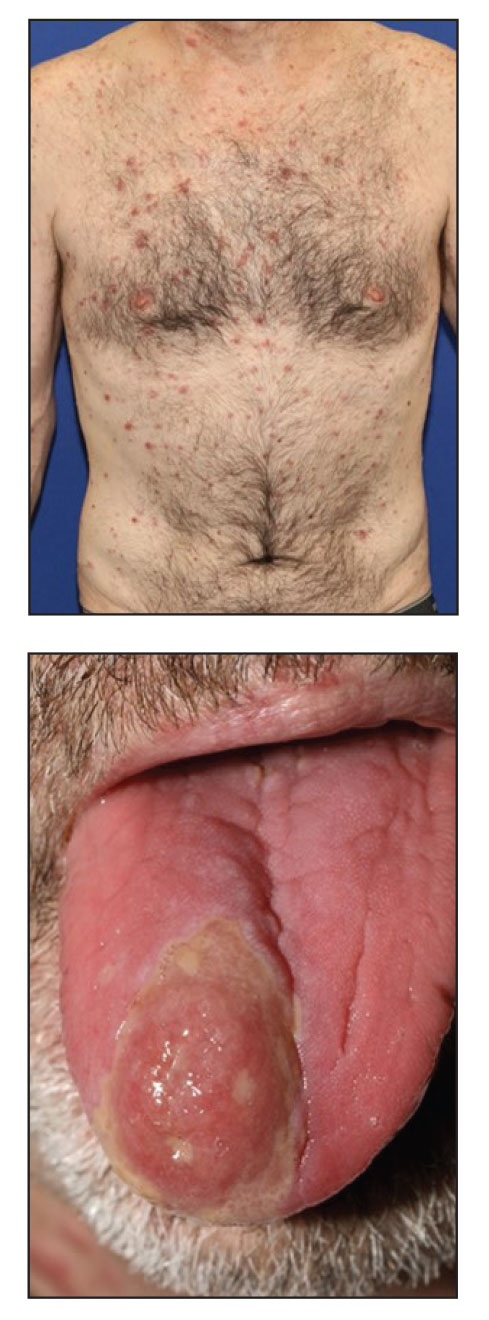
- In a 12-week, randomized, controlled, phase 2b trial of 181 patients 12 years of age and older, researchers investigated the safety, tolerability, and efficacy of DMT310, a powdered mixture of Spongilla lacustris for treating moderate to severe acne. (In vitro studies have found that components of S. lacustris, a freshwater sponge, have effects that include antimicrobial activity against Cutibacterium acnes and anti-inflammatory activity in human keratinocytes).
- The study’s primary efficacy endpoint was the absolute change in inflammatory lesion count from baseline to week 12.
- Endpoint success was defined as an Investigator Global Assessment (IGA) score of 0 or 1 and at least a two-grade improvement from baseline at week 12.
TAKEAWAY:
- Of the 181 patients, 91 received DMT310 (applied once a week to the face and washed off after 10-15 minutes), and 90 received placebo.
- Patients in the DMT310 arm showed a significantly greater mean reduction in the number of inflammatory lesions at week 12, compared with those in the placebo arm (–15.64 vs. –10.84, respectively; P < .001).
- Similarly, patients in the DMT310 arm showed a significantly greater mean reduction in the number of noninflammatory lesions at week 12, compared with those in the placebo arm (–18.26 vs. –12.41, respectively; P < .001).
- At week 12, endpoint success based on IGA scores also significantly favored patients in the DMT310 arm, compared with those in the placebo arm (44.40% vs. 17.78%; P < .001).
IN PRACTICE:
This study is too preliminary to have practice application. The researchers concluded that the findings “support further study of DMT310 in larger, confirmatory phase 3 trials.”
SOURCE:
Lawrence F. Eichenfield, MD, professor of dermatology and pediatrics at the University of California, San Diego, led the research. The study was published online June 7 in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The analysis did not include an active comparator group and it enrolled a limited number of Asian patients.
DISCLOSURES:
Dr. Eichenfield disclosed that he is a consultant to Dermata, which is developing DMT310, as were three other authors of the study. One author is a company employee. The remaining authors disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Poor patient compliance with topical acne therapies is a common clinical challenge.
- In a 12-week, randomized, controlled, phase 2b trial of 181 patients 12 years of age and older, researchers investigated the safety, tolerability, and efficacy of DMT310, a powdered mixture of Spongilla lacustris for treating moderate to severe acne. (In vitro studies have found that components of S. lacustris, a freshwater sponge, have effects that include antimicrobial activity against Cutibacterium acnes and anti-inflammatory activity in human keratinocytes).
- The study’s primary efficacy endpoint was the absolute change in inflammatory lesion count from baseline to week 12.
- Endpoint success was defined as an Investigator Global Assessment (IGA) score of 0 or 1 and at least a two-grade improvement from baseline at week 12.
TAKEAWAY:
- Of the 181 patients, 91 received DMT310 (applied once a week to the face and washed off after 10-15 minutes), and 90 received placebo.
- Patients in the DMT310 arm showed a significantly greater mean reduction in the number of inflammatory lesions at week 12, compared with those in the placebo arm (–15.64 vs. –10.84, respectively; P < .001).
- Similarly, patients in the DMT310 arm showed a significantly greater mean reduction in the number of noninflammatory lesions at week 12, compared with those in the placebo arm (–18.26 vs. –12.41, respectively; P < .001).
- At week 12, endpoint success based on IGA scores also significantly favored patients in the DMT310 arm, compared with those in the placebo arm (44.40% vs. 17.78%; P < .001).
IN PRACTICE:
This study is too preliminary to have practice application. The researchers concluded that the findings “support further study of DMT310 in larger, confirmatory phase 3 trials.”
SOURCE:
Lawrence F. Eichenfield, MD, professor of dermatology and pediatrics at the University of California, San Diego, led the research. The study was published online June 7 in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The analysis did not include an active comparator group and it enrolled a limited number of Asian patients.
DISCLOSURES:
Dr. Eichenfield disclosed that he is a consultant to Dermata, which is developing DMT310, as were three other authors of the study. One author is a company employee. The remaining authors disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Poor patient compliance with topical acne therapies is a common clinical challenge.
- In a 12-week, randomized, controlled, phase 2b trial of 181 patients 12 years of age and older, researchers investigated the safety, tolerability, and efficacy of DMT310, a powdered mixture of Spongilla lacustris for treating moderate to severe acne. (In vitro studies have found that components of S. lacustris, a freshwater sponge, have effects that include antimicrobial activity against Cutibacterium acnes and anti-inflammatory activity in human keratinocytes).
- The study’s primary efficacy endpoint was the absolute change in inflammatory lesion count from baseline to week 12.
- Endpoint success was defined as an Investigator Global Assessment (IGA) score of 0 or 1 and at least a two-grade improvement from baseline at week 12.
TAKEAWAY:
- Of the 181 patients, 91 received DMT310 (applied once a week to the face and washed off after 10-15 minutes), and 90 received placebo.
- Patients in the DMT310 arm showed a significantly greater mean reduction in the number of inflammatory lesions at week 12, compared with those in the placebo arm (–15.64 vs. –10.84, respectively; P < .001).
- Similarly, patients in the DMT310 arm showed a significantly greater mean reduction in the number of noninflammatory lesions at week 12, compared with those in the placebo arm (–18.26 vs. –12.41, respectively; P < .001).
- At week 12, endpoint success based on IGA scores also significantly favored patients in the DMT310 arm, compared with those in the placebo arm (44.40% vs. 17.78%; P < .001).
IN PRACTICE:
This study is too preliminary to have practice application. The researchers concluded that the findings “support further study of DMT310 in larger, confirmatory phase 3 trials.”
SOURCE:
Lawrence F. Eichenfield, MD, professor of dermatology and pediatrics at the University of California, San Diego, led the research. The study was published online June 7 in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The analysis did not include an active comparator group and it enrolled a limited number of Asian patients.
DISCLOSURES:
Dr. Eichenfield disclosed that he is a consultant to Dermata, which is developing DMT310, as were three other authors of the study. One author is a company employee. The remaining authors disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Novel triple-threat approach to acne beats placebo
TOPLINE:
A topical fixed-dose combination of three approved acne treatments significantly improves moderate to severe acne with a strong safety profile.
METHODOLOGY:
- The two multicenter studies included 363 individuals aged 9 years and older with moderate to severe acne from 30 centers, including 15 in North America.
- Moderate to severe acne was defined as having 30-100 inflammatory lesions (papules, pustules, or nodules), 35-150 noninflammatory lesions (open or closed comedones), and at least two nodules.
- Participants were randomly assigned to receive treatment with a combination gel containing phosphate 1.2%, 0.15%, and 3.1% (known as IDP-126) or a vehicle gel for once-daily application for 12 weeks.
- Treatment success was defined as a reduction of at least two grades from baseline on the Evaluator’s Global Severity Score (EGSS) and lesion counts of clear (0) or almost clear (1) at weeks 2, 4, 8, and 12.
TAKEAWAY:
- Treatment success occurred in 49.6% of the IDP-126 group, vs 24.9% of the vehicle group in study 1, and in 50.5% of the IDP-126 group, vs 20.5% of the vehicle group in study 2. Overall treatment compliance was 93.7% and 91.3% for studies 1 and 2, respectively (P < .01 for both).
- Patients in the IDP-126 groups for both studies 1 and 2 had significantly greater absolute mean reductions in both inflammatory and noninflammatory lesions from baseline to week 12 compared to the vehicle patients (P ≤ .001 for all).
- Significantly more patients in the IDP-126 group achieved a grade reduction of 2 or more in EGSS compared with those who received the vehicle, with treatment differences of approximately 32% in both studies. Changes in lesion reductions between the treatment and the vehicle groups were significantly greater as early as week 4.
- The most common treatment-related adverse events among patients treated with IDP-126 were erythema, application-site pain, dryness, irritation, and exfoliation. Discontinuation of the study drug as a result of adverse events occurred in 2.5% and 3.3% of these patients in studies 1 and 2, respectively.
IN PRACTICE:
“With its simple treatment regimen containing 3 recommended acne treatments (benzoyl peroxide, a topical retinoid, and a topical antibiotic), IDP-126 is a potential new treatment option for acne,” the researchers concluded.
SOURCE:
The study was led by Linda Stein Gold, MD, of Henry Ford Hospital, Detroit. The study was published online in the Journal of the American Academy of Dermatology.
LIMITATIONS:
In both studies, treatment duration was short, and the studies may not reflect patients’ real-world experiences. The results may be affected by interobserver bias or variation in assessment of acne severity.
DISCLOSURES:
Gold has served as investigator/consultant or speaker for Ortho Dermatologics, LEO Pharma, Dermavant, Incyte, Novartis, AbbVie, Pfizer, Sun Pharma, UCB, Arcutis, and Lilly. Other study coauthors have relationships with multiple companies, including Ortho Dermatologics, which provided medical writing support for the study.
A version of this article first appeared on Medscape.com.
TOPLINE:
A topical fixed-dose combination of three approved acne treatments significantly improves moderate to severe acne with a strong safety profile.
METHODOLOGY:
- The two multicenter studies included 363 individuals aged 9 years and older with moderate to severe acne from 30 centers, including 15 in North America.
- Moderate to severe acne was defined as having 30-100 inflammatory lesions (papules, pustules, or nodules), 35-150 noninflammatory lesions (open or closed comedones), and at least two nodules.
- Participants were randomly assigned to receive treatment with a combination gel containing phosphate 1.2%, 0.15%, and 3.1% (known as IDP-126) or a vehicle gel for once-daily application for 12 weeks.
- Treatment success was defined as a reduction of at least two grades from baseline on the Evaluator’s Global Severity Score (EGSS) and lesion counts of clear (0) or almost clear (1) at weeks 2, 4, 8, and 12.
TAKEAWAY:
- Treatment success occurred in 49.6% of the IDP-126 group, vs 24.9% of the vehicle group in study 1, and in 50.5% of the IDP-126 group, vs 20.5% of the vehicle group in study 2. Overall treatment compliance was 93.7% and 91.3% for studies 1 and 2, respectively (P < .01 for both).
- Patients in the IDP-126 groups for both studies 1 and 2 had significantly greater absolute mean reductions in both inflammatory and noninflammatory lesions from baseline to week 12 compared to the vehicle patients (P ≤ .001 for all).
- Significantly more patients in the IDP-126 group achieved a grade reduction of 2 or more in EGSS compared with those who received the vehicle, with treatment differences of approximately 32% in both studies. Changes in lesion reductions between the treatment and the vehicle groups were significantly greater as early as week 4.
- The most common treatment-related adverse events among patients treated with IDP-126 were erythema, application-site pain, dryness, irritation, and exfoliation. Discontinuation of the study drug as a result of adverse events occurred in 2.5% and 3.3% of these patients in studies 1 and 2, respectively.
IN PRACTICE:
“With its simple treatment regimen containing 3 recommended acne treatments (benzoyl peroxide, a topical retinoid, and a topical antibiotic), IDP-126 is a potential new treatment option for acne,” the researchers concluded.
SOURCE:
The study was led by Linda Stein Gold, MD, of Henry Ford Hospital, Detroit. The study was published online in the Journal of the American Academy of Dermatology.
LIMITATIONS:
In both studies, treatment duration was short, and the studies may not reflect patients’ real-world experiences. The results may be affected by interobserver bias or variation in assessment of acne severity.
DISCLOSURES:
Gold has served as investigator/consultant or speaker for Ortho Dermatologics, LEO Pharma, Dermavant, Incyte, Novartis, AbbVie, Pfizer, Sun Pharma, UCB, Arcutis, and Lilly. Other study coauthors have relationships with multiple companies, including Ortho Dermatologics, which provided medical writing support for the study.
A version of this article first appeared on Medscape.com.
TOPLINE:
A topical fixed-dose combination of three approved acne treatments significantly improves moderate to severe acne with a strong safety profile.
METHODOLOGY:
- The two multicenter studies included 363 individuals aged 9 years and older with moderate to severe acne from 30 centers, including 15 in North America.
- Moderate to severe acne was defined as having 30-100 inflammatory lesions (papules, pustules, or nodules), 35-150 noninflammatory lesions (open or closed comedones), and at least two nodules.
- Participants were randomly assigned to receive treatment with a combination gel containing phosphate 1.2%, 0.15%, and 3.1% (known as IDP-126) or a vehicle gel for once-daily application for 12 weeks.
- Treatment success was defined as a reduction of at least two grades from baseline on the Evaluator’s Global Severity Score (EGSS) and lesion counts of clear (0) or almost clear (1) at weeks 2, 4, 8, and 12.
TAKEAWAY:
- Treatment success occurred in 49.6% of the IDP-126 group, vs 24.9% of the vehicle group in study 1, and in 50.5% of the IDP-126 group, vs 20.5% of the vehicle group in study 2. Overall treatment compliance was 93.7% and 91.3% for studies 1 and 2, respectively (P < .01 for both).
- Patients in the IDP-126 groups for both studies 1 and 2 had significantly greater absolute mean reductions in both inflammatory and noninflammatory lesions from baseline to week 12 compared to the vehicle patients (P ≤ .001 for all).
- Significantly more patients in the IDP-126 group achieved a grade reduction of 2 or more in EGSS compared with those who received the vehicle, with treatment differences of approximately 32% in both studies. Changes in lesion reductions between the treatment and the vehicle groups were significantly greater as early as week 4.
- The most common treatment-related adverse events among patients treated with IDP-126 were erythema, application-site pain, dryness, irritation, and exfoliation. Discontinuation of the study drug as a result of adverse events occurred in 2.5% and 3.3% of these patients in studies 1 and 2, respectively.
IN PRACTICE:
“With its simple treatment regimen containing 3 recommended acne treatments (benzoyl peroxide, a topical retinoid, and a topical antibiotic), IDP-126 is a potential new treatment option for acne,” the researchers concluded.
SOURCE:
The study was led by Linda Stein Gold, MD, of Henry Ford Hospital, Detroit. The study was published online in the Journal of the American Academy of Dermatology.
LIMITATIONS:
In both studies, treatment duration was short, and the studies may not reflect patients’ real-world experiences. The results may be affected by interobserver bias or variation in assessment of acne severity.
DISCLOSURES:
Gold has served as investigator/consultant or speaker for Ortho Dermatologics, LEO Pharma, Dermavant, Incyte, Novartis, AbbVie, Pfizer, Sun Pharma, UCB, Arcutis, and Lilly. Other study coauthors have relationships with multiple companies, including Ortho Dermatologics, which provided medical writing support for the study.
A version of this article first appeared on Medscape.com.
Youth Exposure to Spironolactone in TikTok Videos
The short-form video hosting service TikTok has become a mainstream platform for individuals to share their ideas and educate the public regarding dermatologic diseases such as atopic dermatitis, alopecia, and acne. Users can create and post videos, leave comments, and indicate their interest in or approval of certain content by “liking” videos. In 2022, according to a Pew Research Center survey, approximately 67% of American teenagers aged 13 to 17 years reported using TikTok at least once.1 This population, along with the rest of its users, are increasing their use of TikTok to share information on dermatologic topics such as acne and isotretinoin.2,3 Spironolactone is an effective medication for acne but is not as widely known to the public as other acne medications such as retinoids, salicylic acid, and benzoyl peroxide. Being aware of youth exposure to media related to acne and spironolactone can help dermatologists understand gaps in education and refine their interactions with this patient population.
To gain insight into youth exposure to spironolactone, we conducted a search of TikTok on July 26, 2022, using the term #spironolactone to retrieve the top 50 videos identified by TikTok under the “Top” tab on spironolactone. Search results and the top 10 comments for each video were reviewed. The total number of views and likes for the top 50 videos were 6,735,992 and 851,856, respectively.
Videos were subdivided into educational information related to spironolactone and/or skin care (32% [16/50]), discussion of side effects of spironolactone (26% [13/50]), those with noticeable improvement of acne following treatment with spironolactone (20% [10/50]), recommendations to see a physician or dermatologist to treat acne (10% [5/50]), and other (12% [6/50]). Other takeaways from the top 50 videos included the following:
- Common side effects: irregular periods (10% [5/50]), frequent urination (8% [4/50]), dizziness/lightheadedness (8% [4/50]), and breast tenderness (6% [3/50])
- Longest reported use of spironolactone: 4 years, with complete acne resolution
- Average treatment length prior to noticeable results: 4 to 6 months, with the shortest being 1 month
- Reported dosages of spironolactone: ranged from 50 to 200 mg/d. The most common dosage was 100 mg/d (10% [5/50]). The lowest reported dosage was 50 mg/d (4% [2/50]), while the highest reported dosage was 200 mg/d (2% [1/50])
- Self-reported concurrent use of spironolactone with a combined oral contraceptive: drospirenoneTimes New Roman–ethinyl estradiol (4% [2/50]), norethindrone acetateTimes New Roman–ethinyl estradiol/ferrous fumarate (2% [1/50]), and norgestimateTimes New Roman–ethinyl estradiol (2% [1/50])
- Negative experiences with side effects and lack of acne improvement that led to treatment cessation: 8% (4/50).
Even though spironolactone is not as well-known as other treatments for acne, we found many TikTok users posting about, commenting on, and highlighting the relevance of this therapeutic option. There was no suggestion in any of the videos that spironolactone could be obtained without physician care and/or prescription. A prior report discussing youth sentiment of isotretinoin use on TikTok found that popular videos and videos with the most likes focused on the drug’s positive impact on acne improvement, while comments displayed heightened desires to learn more about isotretinoin and its side effects.3 Our analysis showed a similar response to spironolactone. In all videos showcasing the skin before and after treatment, there were noticeable improvements in the poster’s acne. Most of the video comments displayed a desire to learn more about spironolactone and its side effects. There also were many questions about time to noticeable results. In contrast to the study on isotretinoin,3 the most-liked spironolactone videos contained educational information about spironolactone and/or skin care rather than focusing solely on the impact of the drug on acne. Additionally, the study on isotretinoin found no videos mentioning the importance of seeing a dermatologist or other health care professional,3 while our search found multiple videos (10% [5/50]) on spironolactone that advised seeking physician help. In fact, several popular videos (8% [4/50]) were created by board-certified dermatologists who mainly focused on providing educational information. This difference in educational content may be attributed to spironolactone’s lesser-known function in treating acne. Furthermore, the comments suggested a growing interest in learning more about spironolactone as a treatment option for acne, specifically its mechanism of action and side effects.
With nearly 2 billion monthly active users globally and 94.1 million monthly active users in the United States (as of March 2023),4 TikTok is a popular social media platform that allows dermatologists to better understand youth sentiment on acne treatments such as spironolactone and isotretinoin and also provides an opportunity for medical education to reach a larger audience. This increased youth insight from TikTok can be utilized by dermatologists to make more informed decisions in developing patient-centered care that appeals to the adolescent population.
- Vogels EA, Gelles-Watnick R, Massarat N. Teens, social media and technology 2022. Published August 10, 2022. Accessed September 16, 2023. https://www.pewresearch.org/internet/2022/08/10/teens-social-media-and-technology-2022/
- Szeto MD, Mamo A, Afrin A, et al. Social media in dermatology and an overview of popular social media platforms. Curr Dermatol Rep. 2021;10:97-104. doi:10.1007/s13671-021-00343-4
- Galamgam J, Jia JL. “Accutane check”: insights into youth sentiment toward isotretinoin from a TikTok trend. Pediatr Dermatol. 2021;38:980-981. doi:10.1111/pde.14660
- Aslam S. TikTok by the numbers: stats, demographics & fun facts. Omnicore website. February 27, 2023. Accessed September 14, 2023. https://www.omnicoreagency.com/tiktok-statistics/
The short-form video hosting service TikTok has become a mainstream platform for individuals to share their ideas and educate the public regarding dermatologic diseases such as atopic dermatitis, alopecia, and acne. Users can create and post videos, leave comments, and indicate their interest in or approval of certain content by “liking” videos. In 2022, according to a Pew Research Center survey, approximately 67% of American teenagers aged 13 to 17 years reported using TikTok at least once.1 This population, along with the rest of its users, are increasing their use of TikTok to share information on dermatologic topics such as acne and isotretinoin.2,3 Spironolactone is an effective medication for acne but is not as widely known to the public as other acne medications such as retinoids, salicylic acid, and benzoyl peroxide. Being aware of youth exposure to media related to acne and spironolactone can help dermatologists understand gaps in education and refine their interactions with this patient population.
To gain insight into youth exposure to spironolactone, we conducted a search of TikTok on July 26, 2022, using the term #spironolactone to retrieve the top 50 videos identified by TikTok under the “Top” tab on spironolactone. Search results and the top 10 comments for each video were reviewed. The total number of views and likes for the top 50 videos were 6,735,992 and 851,856, respectively.
Videos were subdivided into educational information related to spironolactone and/or skin care (32% [16/50]), discussion of side effects of spironolactone (26% [13/50]), those with noticeable improvement of acne following treatment with spironolactone (20% [10/50]), recommendations to see a physician or dermatologist to treat acne (10% [5/50]), and other (12% [6/50]). Other takeaways from the top 50 videos included the following:
- Common side effects: irregular periods (10% [5/50]), frequent urination (8% [4/50]), dizziness/lightheadedness (8% [4/50]), and breast tenderness (6% [3/50])
- Longest reported use of spironolactone: 4 years, with complete acne resolution
- Average treatment length prior to noticeable results: 4 to 6 months, with the shortest being 1 month
- Reported dosages of spironolactone: ranged from 50 to 200 mg/d. The most common dosage was 100 mg/d (10% [5/50]). The lowest reported dosage was 50 mg/d (4% [2/50]), while the highest reported dosage was 200 mg/d (2% [1/50])
- Self-reported concurrent use of spironolactone with a combined oral contraceptive: drospirenoneTimes New Roman–ethinyl estradiol (4% [2/50]), norethindrone acetateTimes New Roman–ethinyl estradiol/ferrous fumarate (2% [1/50]), and norgestimateTimes New Roman–ethinyl estradiol (2% [1/50])
- Negative experiences with side effects and lack of acne improvement that led to treatment cessation: 8% (4/50).
Even though spironolactone is not as well-known as other treatments for acne, we found many TikTok users posting about, commenting on, and highlighting the relevance of this therapeutic option. There was no suggestion in any of the videos that spironolactone could be obtained without physician care and/or prescription. A prior report discussing youth sentiment of isotretinoin use on TikTok found that popular videos and videos with the most likes focused on the drug’s positive impact on acne improvement, while comments displayed heightened desires to learn more about isotretinoin and its side effects.3 Our analysis showed a similar response to spironolactone. In all videos showcasing the skin before and after treatment, there were noticeable improvements in the poster’s acne. Most of the video comments displayed a desire to learn more about spironolactone and its side effects. There also were many questions about time to noticeable results. In contrast to the study on isotretinoin,3 the most-liked spironolactone videos contained educational information about spironolactone and/or skin care rather than focusing solely on the impact of the drug on acne. Additionally, the study on isotretinoin found no videos mentioning the importance of seeing a dermatologist or other health care professional,3 while our search found multiple videos (10% [5/50]) on spironolactone that advised seeking physician help. In fact, several popular videos (8% [4/50]) were created by board-certified dermatologists who mainly focused on providing educational information. This difference in educational content may be attributed to spironolactone’s lesser-known function in treating acne. Furthermore, the comments suggested a growing interest in learning more about spironolactone as a treatment option for acne, specifically its mechanism of action and side effects.
With nearly 2 billion monthly active users globally and 94.1 million monthly active users in the United States (as of March 2023),4 TikTok is a popular social media platform that allows dermatologists to better understand youth sentiment on acne treatments such as spironolactone and isotretinoin and also provides an opportunity for medical education to reach a larger audience. This increased youth insight from TikTok can be utilized by dermatologists to make more informed decisions in developing patient-centered care that appeals to the adolescent population.
The short-form video hosting service TikTok has become a mainstream platform for individuals to share their ideas and educate the public regarding dermatologic diseases such as atopic dermatitis, alopecia, and acne. Users can create and post videos, leave comments, and indicate their interest in or approval of certain content by “liking” videos. In 2022, according to a Pew Research Center survey, approximately 67% of American teenagers aged 13 to 17 years reported using TikTok at least once.1 This population, along with the rest of its users, are increasing their use of TikTok to share information on dermatologic topics such as acne and isotretinoin.2,3 Spironolactone is an effective medication for acne but is not as widely known to the public as other acne medications such as retinoids, salicylic acid, and benzoyl peroxide. Being aware of youth exposure to media related to acne and spironolactone can help dermatologists understand gaps in education and refine their interactions with this patient population.
To gain insight into youth exposure to spironolactone, we conducted a search of TikTok on July 26, 2022, using the term #spironolactone to retrieve the top 50 videos identified by TikTok under the “Top” tab on spironolactone. Search results and the top 10 comments for each video were reviewed. The total number of views and likes for the top 50 videos were 6,735,992 and 851,856, respectively.
Videos were subdivided into educational information related to spironolactone and/or skin care (32% [16/50]), discussion of side effects of spironolactone (26% [13/50]), those with noticeable improvement of acne following treatment with spironolactone (20% [10/50]), recommendations to see a physician or dermatologist to treat acne (10% [5/50]), and other (12% [6/50]). Other takeaways from the top 50 videos included the following:
- Common side effects: irregular periods (10% [5/50]), frequent urination (8% [4/50]), dizziness/lightheadedness (8% [4/50]), and breast tenderness (6% [3/50])
- Longest reported use of spironolactone: 4 years, with complete acne resolution
- Average treatment length prior to noticeable results: 4 to 6 months, with the shortest being 1 month
- Reported dosages of spironolactone: ranged from 50 to 200 mg/d. The most common dosage was 100 mg/d (10% [5/50]). The lowest reported dosage was 50 mg/d (4% [2/50]), while the highest reported dosage was 200 mg/d (2% [1/50])
- Self-reported concurrent use of spironolactone with a combined oral contraceptive: drospirenoneTimes New Roman–ethinyl estradiol (4% [2/50]), norethindrone acetateTimes New Roman–ethinyl estradiol/ferrous fumarate (2% [1/50]), and norgestimateTimes New Roman–ethinyl estradiol (2% [1/50])
- Negative experiences with side effects and lack of acne improvement that led to treatment cessation: 8% (4/50).
Even though spironolactone is not as well-known as other treatments for acne, we found many TikTok users posting about, commenting on, and highlighting the relevance of this therapeutic option. There was no suggestion in any of the videos that spironolactone could be obtained without physician care and/or prescription. A prior report discussing youth sentiment of isotretinoin use on TikTok found that popular videos and videos with the most likes focused on the drug’s positive impact on acne improvement, while comments displayed heightened desires to learn more about isotretinoin and its side effects.3 Our analysis showed a similar response to spironolactone. In all videos showcasing the skin before and after treatment, there were noticeable improvements in the poster’s acne. Most of the video comments displayed a desire to learn more about spironolactone and its side effects. There also were many questions about time to noticeable results. In contrast to the study on isotretinoin,3 the most-liked spironolactone videos contained educational information about spironolactone and/or skin care rather than focusing solely on the impact of the drug on acne. Additionally, the study on isotretinoin found no videos mentioning the importance of seeing a dermatologist or other health care professional,3 while our search found multiple videos (10% [5/50]) on spironolactone that advised seeking physician help. In fact, several popular videos (8% [4/50]) were created by board-certified dermatologists who mainly focused on providing educational information. This difference in educational content may be attributed to spironolactone’s lesser-known function in treating acne. Furthermore, the comments suggested a growing interest in learning more about spironolactone as a treatment option for acne, specifically its mechanism of action and side effects.
With nearly 2 billion monthly active users globally and 94.1 million monthly active users in the United States (as of March 2023),4 TikTok is a popular social media platform that allows dermatologists to better understand youth sentiment on acne treatments such as spironolactone and isotretinoin and also provides an opportunity for medical education to reach a larger audience. This increased youth insight from TikTok can be utilized by dermatologists to make more informed decisions in developing patient-centered care that appeals to the adolescent population.
- Vogels EA, Gelles-Watnick R, Massarat N. Teens, social media and technology 2022. Published August 10, 2022. Accessed September 16, 2023. https://www.pewresearch.org/internet/2022/08/10/teens-social-media-and-technology-2022/
- Szeto MD, Mamo A, Afrin A, et al. Social media in dermatology and an overview of popular social media platforms. Curr Dermatol Rep. 2021;10:97-104. doi:10.1007/s13671-021-00343-4
- Galamgam J, Jia JL. “Accutane check”: insights into youth sentiment toward isotretinoin from a TikTok trend. Pediatr Dermatol. 2021;38:980-981. doi:10.1111/pde.14660
- Aslam S. TikTok by the numbers: stats, demographics & fun facts. Omnicore website. February 27, 2023. Accessed September 14, 2023. https://www.omnicoreagency.com/tiktok-statistics/
- Vogels EA, Gelles-Watnick R, Massarat N. Teens, social media and technology 2022. Published August 10, 2022. Accessed September 16, 2023. https://www.pewresearch.org/internet/2022/08/10/teens-social-media-and-technology-2022/
- Szeto MD, Mamo A, Afrin A, et al. Social media in dermatology and an overview of popular social media platforms. Curr Dermatol Rep. 2021;10:97-104. doi:10.1007/s13671-021-00343-4
- Galamgam J, Jia JL. “Accutane check”: insights into youth sentiment toward isotretinoin from a TikTok trend. Pediatr Dermatol. 2021;38:980-981. doi:10.1111/pde.14660
- Aslam S. TikTok by the numbers: stats, demographics & fun facts. Omnicore website. February 27, 2023. Accessed September 14, 2023. https://www.omnicoreagency.com/tiktok-statistics/
From Breakouts to Bargains: Strategies for Patient-Centered, Cost-effective Acne Care
In the United States, acne affects 85% of adolescents and can persist into adulthood at a prevalence of 30% to 50% in adult women. 1,2 The pathogenesis of acne is multifactorial and involves hyperkeratinization of the follicle, bacterial colonization with Cutibacterium acnes , and increased androgen-induced sebum production, which together lead to inflammation. 3,4 A wide range of treatment guideline–recommended options are available, including benzoyl peroxide (BPO), topical retinoids, topical and oral antibiotics, antiandrogens, and isotretinoin. 5 However, these options vary widely in their clinical uses, effectiveness, and costs.
Why Cost-effective Acne Care Matters
Out-of-pocket spending by patients on acne treatments can be substantial, with surveys finding that acne patients often spend hundreds to thousands of dollars per year.6,7 In a poll conducted in 2019 by the Kaiser Family Foundation, 3 in 10 patients said they had not taken their medicine as prescribed because of costs.8 A mixed methods study by Ryskina et al9 found that 65% (17/26) of participants who reported primary nonadherence—intended to fill prescriptions but were unable to do so—cited cost or coverage-related barriers as the reason. With the continued rise of dermatologic drug prices and increased prevalence of high-deductible health plans, cost-effective treatment continues to grow in importance. Failure to consider cost-effective, patient-centered care may lead to increased financial toxicity, reduced adherence, and ultimately worse outcomes and patient satisfaction. We aim to review the cost-effectiveness of current prescription therapies for acne management and highlight the most cost-effective approaches to patients with mild to moderate acne as well as moderate to severe acne.
In this review, we will take a value-oriented framework.10 Value can be defined as the cost per outcome of interest. Therefore, a treatment does not necessarily need to be inexpensive to provide high value if it delivers outstanding clinical outcomes. In addition, we will focus on incremental cost-effectiveness relative to common alternatives (eg, a retinoid could deliver high value relative to a vehicle but still provide limited value compared to other available retinoids if it is more expensive but not more efficacious). When possible, we present data from cost-effectiveness studies.11,12 We also use recent available price data obtained from GoodRx on August 11, 2023, to guide this discussion.13 However, as comparative-effectiveness and cost-effectiveness studies rarely are performed for acne medications, much of this discussion will be based on expert opinion.
Treatment Categories
Topical Retinoids—There currently are 4 topical retinoids that are approved by the US Food and Drug Administration (FDA) for the treatment of acne: tretinoin, tazarotene, trifarotene, and adapalene. These drugs are vitamin A derivatives that bind retinoic acid receptors and function as comedolytic and anti-inflammatory agents.5 In general, generic tretinoin and adapalene products have the lowest cost (Table).
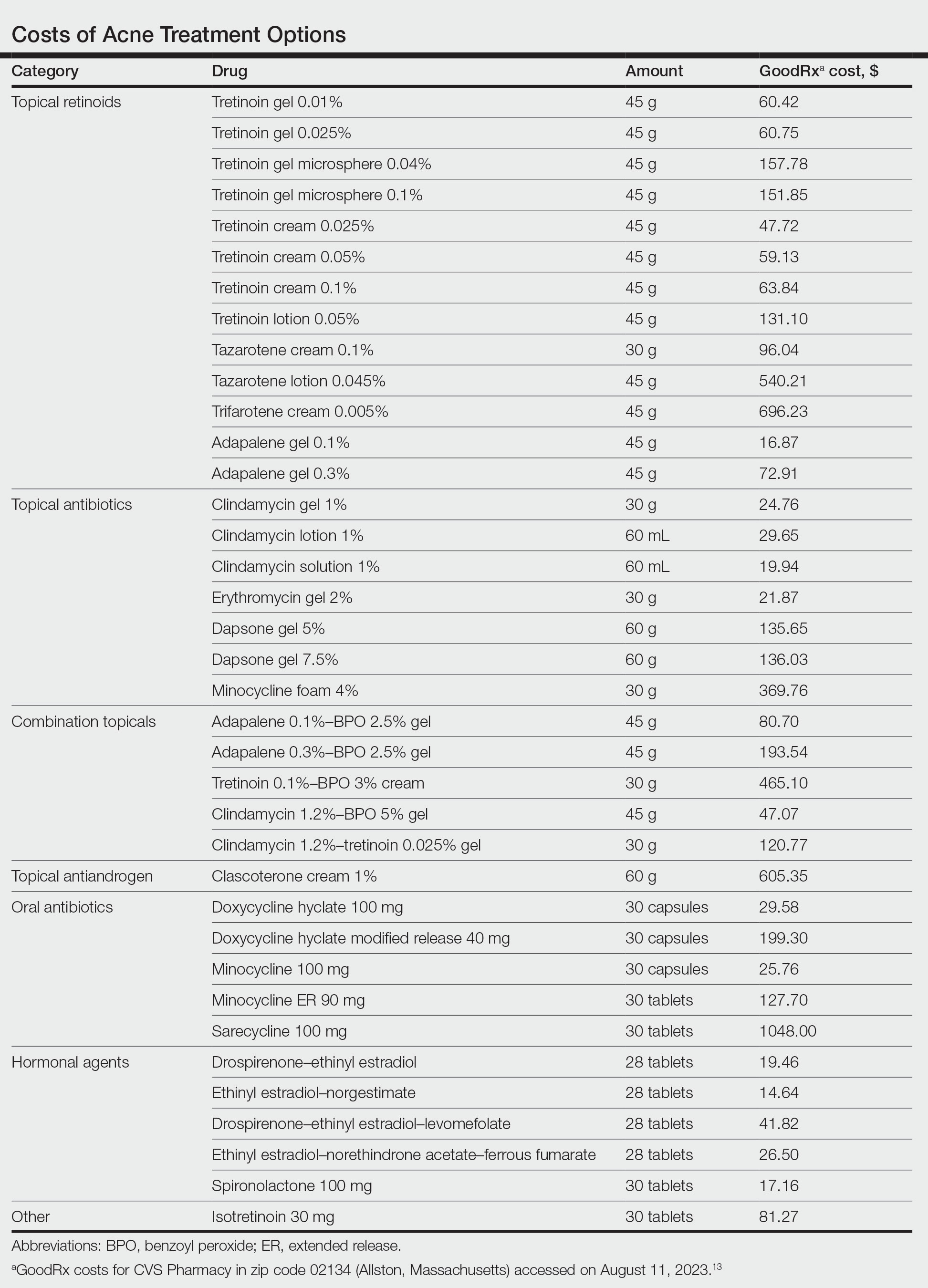
In network meta-analyses, tretinoin and adapalene often are highly ranked topical treatment options with respect to efficacy.14 Combined with their low cost, generic tretinoin and adapalene likely are excellent initial options for topical therapy from the standpoint of cost-effectiveness.15 Adapalene may be preferred in many situations because of its better photostability and compatibility with BPO.
Due to the importance of the vehicle in determining retinoid tolerability, efforts have been made to use encapsulation and polymeric emulsion technology to improve tolerability. Recently, polymeric lotion formulations of tretinoin and tazarotene have become available. In a phase 2 study, tazarotene lotion 0.045% was found to have equivalent efficacy and superior tolerability to tazarotene cream 0.1%.16 Although head-to-head data are not available, it is likely that tretinoin lotion may offer similar tolerability improvements.17 Although these formulations currently are more costly, this improved tolerability may be critical for some patients to be able to use topical retinoids, and the additional cost may be worthwhile. In addition, as these products lose market exclusivity, they may become more affordable and similarly priced to other topical retinoids. It is important to keep in mind that in clinical trials of tretinoin and adapalene, rates of dropout due to adverse events typically were 1% to 2%; therefore, because many patients can tolerate generic tretinoin and adapalene, at current prices the lotion formulations of retinoids may not be cost-effective relative to these generics.14
Trifarotene cream 0.005%, a fourth-generation topical retinoid that is highly sensitive for retinoic acid receptor γ, recently was FDA approved for the treatment of acne. Although trifarotene is efficacious for both facial and truncal acne, there is a lack of active comparator data compared to other topical retinoids.18 In a 2023 network meta-analysis, trifarotene was found to be both less efficacious and less tolerable compared to other topical retinoids.19 Thus, it is unclear if trifarotene offers any improved efficacy compared to other options, and it comes at a much higher cost (Table). In a tolerability study, trifarotene was found to be significantly more irritating than tazarotene lotion 0.045% and adapalene gel 0.3% (P<.05).20 Therefore, trifarotene cream 0.005% is unlikely to be a cost-effective option; in fact, it may be overall inferior to other topical retinoids, given its potentially lower tolerability.
Topical Antibiotics—There are 4 commonly prescribed topical antibiotics that are approved by the FDA for the treatment of acne: clindamycin, erythromycin, dapsone, and minocycline. The American Academy of Dermatology guidelines for the treatment of acne recommend concomitant use of BPO to prevent antibiotic resistance.5 Clindamycin is favored over erythromycin because of increasing antibiotic resistance to erythromycin.21 Inexpensive generic options in multiple vehicles (eg, solution, foam, gel) make clindamycin a highly cost-effective option when antibiotic therapy is desired as part of a topical regimen (Table).
The cost-effectiveness of dapsone gel and minocycline foam relative to clindamycin are less certain. Rates of resistance to minocycline are lower than clindamycin, and minocycline foam may be a reasonable alternative in patients who have not had success with other topical antibiotics, such as clindamycin.22 However, given the absence of comparative effectiveness data to suggest minocycline is more effective than clindamycin, it is difficult to justify the substantially higher cost for the typical patient. Although dapsone gel has been suggested as an option for adult women with acne, there are no data to support that it is any more effective than other topical antibiotics in this patient population.23 As generic dapsone prices decrease, it may become a reasonable alternative to clindamycin. In addition, the antineutrophil properties of dapsone may be useful in other acneform and inflammatory eruptions, such as scalp folliculitis and folliculitis decalvans.24
Combination Topicals—Current combination topical products include antibiotic and BPO, antibiotic and retinoid, and retinoid and BPO. Use of combination agents is recommended to reduce the risk for resistance and to enhance effectiveness. Combination products offer improved convenience, which is associated with better adherence and outcomes.25 Generic fixed-dose adapalene-BPO can be a highly cost-effective option that can sometimes be less expensive than the individual component products (Table). Similarly, fixed-dose clindamycin-BPO also is likely to be highly cost-effective. A network meta-analysis found fixed-dose adapalene-BPO to be the most efficacious topical treatment, though it also was found to be the most irritating—more so than fixed-dose clindamycin-BPO, which may have similar efficacy.14,26,27 Generic fixed-dose tretinoin-clindamycin offers improved convenience and adherence compared to the individual components, but it is more expensive, and its cost-effectiveness may be influenced by the importance of convenience for the patient.25 An encapsulated, fixed-dose tretinoin 0.1%–BPO 3% cream is FDA approved for acne, but the cost is high and there is a lack of comparative effectiveness data demonstrating advantages over generic fixed-dose adapalene-BPO products.
Topical Antiandrogen—Clascoterone was introduced in 2020 as the first FDA-approved topical medication to target the hormonal pathogenesis of acne, inhibiting the androgen receptors in the sebaceous gland.28 Because it is rapidly metabolized to cortexolone and does not have systemic antiandrogen effects, clascoterone can be used in both men and women with acne. In clinical trials, it had minimal side effects, including no evidence of irritability, which is an advantage over topical retinoids and BPO.29 In addition, a phase 2 study found that clascoterone may have similar to superior efficacy to tretinoin cream 0.05%.30 Although clascoterone has several strengths, including its efficacy, tolerability, and unique mechanism of action, its cost-effectiveness is limited due to its high cost (Table) and the need for twice-daily application, which reduces convenience. Clascoterone likely is best reserved for patients with a strong hormonal pathogenesis of their acne or difficulty tolerating other topicals, or as an additional therapy to complement other topicals.
Oral Antibiotics—Oral antibiotics are the most commonly prescribed systemic treatments for acne, particularly tetracyclines such as doxycycline, minocycline, and sarecycline.31-34 Doxycycline and minocycline are considered first-line oral antibiotic therapy in the United States and are inexpensive and easily accessible.5 Doxycycline generally is recommended over minocycline given lack of evidence of superior efficacy of minocycline and concerns about severe adverse cutaneous reactions and drug-induced lupus with minocycline.35
In recent years, there has been growing concern of the development of antibiotic resistance.5 Sarecycline is a narrow-spectrum tetracycline that was FDA approved for acne in 2018. In vitro studies demonstrate sarecycline maintains high efficacy against C acnes with less activity against other bacteria, particularly gram-negative enterobes.36 The selectivity of sarecycline may lessen alterations of the gut microbiome seen with other oral antibiotics and reduce gastrointestinal tract side effects. Although comparative effectiveness studies are lacking, sarecycline was efficacious in phase 3 trials with few side effects compared with placebo.37 However, at this time, given the absence of comparative effectiveness data and its high cost (Table), sarecycline likely is best reserved for patients with comorbidities (eg, gastrointestinal disease), those requiring long-term antibiotic therapy, or those with acne that has failed to respond to other oral antibiotics.
Hormonal Treatments—Hormonal treatments such as combined oral contraceptives (COCs) and spironolactone often are considered second-line options, though they may represent cost-effective and safe alternatives to oral antibiotics for women with moderate to severe acne.38-41 There currently are 4 COCs approved by the FDA for the treatment of moderate acne in postmenarcheal females: drospirenone-ethinyl estradiol (Yaz [Bayer HealthCare Pharmaceuticals, Inc]), ethinyl estradiol-norgestimate (Ortho Tri-Cyclen [Ortho-McNeil Pharmaceuticals, Inc]), drospirenone-ethinyl estradiol-levomefolate (Beyaz [Bayer HealthCare Pharmaceuticals, Inc]), and ethinyl estradiol-norethindrone acetate-ferrous fumarate (Estrostep Fe [Allergan USA, Inc]).5 Treatment with COCs has been shown to cause substantial reductions in lesion counts across all lesion types compared to placebo, and a meta-analysis of 24 randomized trials conducted by Arowojolu et al42 demonstrated no consistent differences in acne reduction among different COCs.43,44 Although oral antibiotics are associated with faster improvement than COCs, there is some evidence that they have similar efficacy at 6 months of therapy.45 Combined oral contraceptives are inexpensive and likely reflect a highly cost-effective option (Table).
Spironolactone is an aldosterone inhibitor and androgen receptor blocker that is used off label to treat acne. It is one of the least expensive systemic medications for acne (Table). Although randomized controlled trials are lacking, several large case series support the effectiveness of spironolactone for women with acne.38,46 In addition, observational data suggest spironolactone may have similar effectiveness to oral antibiotics.41 Spironolactone generally is well tolerated, with the most common adverse effects being menstrual irregularities, breast tenderness, and diuresis.47,48 Many of these adverse effects are dose dependent and less likely with the dosing used in acne care. Additionally, menstrual irregularities can be reduced by concomitant use of a COC.48
Although frequent potassium monitoring remains common among patients being treated with spironolactone, there is growing evidence to suggest that potassium monitoring is of low value in young healthy women with acne.49-51 Reducing this laboratory monitoring likely represents an opportunity to provide higher-value care to patients being treated with spironolactone. However, laboratory monitoring should be considered if risk factors for hyperkalemia are present (eg, older age, comorbidities, medications).51
Isotretinoin—Isotretinoin is the most efficacious treatment available for acne and has the unique property of being able to induce a remission of acne activity for many patients.5 Although it remains modestly expensive (Table), it may be less costly overall relative to other treatments that may need continued use over many years because it can induce a remission of acne activity. As with spironolactone, frequent laboratory monitoring remains common among patients being treated with isotretinoin. There is no evidence to support checking complete blood cell counts.52 Several observational studies and a Delphi consensus support reduced monitoring, such as checking lipids and alanine aminotransferase at baseline and peak dose in otherwise young healthy patients.53,54 A recent critically appraised topic published in the British Journal of Dermatology has proposed eliminating laboratory monitoring entirely.55 Reducing laboratory monitoring for patients being treated with isotretinoin has been estimated to potentially save $100 million to $200 million per year in the United States.52-54
Other Strategies to Reduce Patient Costs
Although choosing a cost-effective treatment approach is critical to preventing financial toxicity given poor coverage for acne care and the growth of high-deductible insurance plans, some patients may still experience high treatment costs.56 Because pharmacy costs often are inflated, potentially related to practices of pharmacy benefit managers, it often is possible to find better prices than the presented list price, either by using platforms such as GoodRx or through direct-to-patient mail-order pharmacies such as Cost Plus Drug.57 For branded medications, some patients may be eligible for patient-assistance programs, though they typically are not available for those with public insurance such as Medicare or Medicaid. Compounding pharmacies offer another approach to reduce cost and improve convenience for patients, but because the vehicle can influence the efficacy and tolerability of some topical medications, it is possible that these compounded formulations may not perform similarly to the original FDA-approved products.
Conclusion
For mild to moderate acne, multimodal topical therapy often is required. Fixed-dose combination adapalene-BPO and clindamycin-BPO are highly cost-effective options for most patients. Lotion formulations of topical retinoids may be useful in patients with difficulty tolerating other formulations. Clascoterone is a novel topical antiandrogen that is more expensive than other topical therapies but can complement other topical therapies and is well tolerated.
For moderate to severe acne, doxycycline or hormonal therapy (ie, COCs, spironolactone) are highly cost-effective options. Isotretinoin is recommended for severe or scarring acne. Reduced laboratory monitoring for spironolactone and isotretinoin is an opportunity to provide higher-value care.
- Bhate K, Williams HC. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168:474-485. doi:10.1111/bjd.12149
- Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59. doi:10.1016/j.jaad.2007.06.045
- Webster GF. The pathophysiology of acne. Cutis. 2005;76(2 suppl):4-7.
- Degitz K, Placzek M, Borelli C, et al. Pathophysiology of acne. J Dtsch Dermatol Ges. 2007;5:316-323. doi:10.1111/j.1610-0387.2007.06274.x
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33. doi:10.1016/j.jaad.2015.12.037
- Felmingham C, Kerr A, Veysey E. Costs incurred by patients with acne prior to dermatological consultation and their relation to patient income. Australas J Dermatol. 2020;61:384-386. doi:10.1111/ajd.13324
- Perche P, Singh R, Feldman S. Patient preferences for acne vulgaris treatment and barriers to care: a survey study. J Drugs Dermatol. 2022;21:1191-1195. doi:10.36849/JDD.6940
- KFF Health Tracking Poll—February 2019. Accessed August 9, 2023. https://files.kff.org/attachment/Topline-KFF-Health-Tracking-Poll-February-2019
- Ryskina KL, Goldberg E, Lott B, et al. The role of the physician in patient perceptions of barriers to primary adherence with acne medications. JAMA Dermatol. 2018;154:456-459. doi:10.1001/jamadermatol.2017.6144
- Porter ME. What is value in health care? N Engl J Med. 2010;363:2477-2481. doi:10.1056/NEJMp1011024
- Barbieri JS, Tan JKL, Adamson AS. Active comparator trial designs used to promote development of innovative new medications. Cutis. 2020;106:E4-E6. doi:10.12788/cutis.0067
- Miller J, Ly S, Mostaghimi A, et al. Use of active comparator trials for topical medications in dermatology. JAMA Dermatol. 2021;157:597-599. doi:10.1001/jamadermatol.2021.0356
- GoodRx. Accessed August 11, 2023. https://www.goodrx.com
- Stuart B, Maund E, Wilcox C, et al. Topical preparations for the treatment of mild‐to‐moderate acne vulgaris: systematic review and network meta‐analysis. Br J Dermatol. 2021;185:512-525. doi:10.1111/bjd.20080
- Mavranezouli I, Welton NJ, Daly CH, et al. Cost-effectiveness of topical pharmacological, oral pharmacological, physical and combined treatments for acne vulgaris. Clin Exp Dermatol. 2022;47:2176-2187. doi:10.1111/ced.15356
- Tanghetti E, Werschler W, Lain T, et al. Tazarotene 0.045% lotion for once-daily treatment of moderate-to-severe acne vulgaris: results from two phase 3 trials. J Drugs Dermatol. 2020;19:70-77. doi:10.36849/JDD.2020.3977
- Tyring SK, Kircik LH, Pariser DM, et al. Novel tretinoin 0.05% lotion for the once-daily treatment of moderate-to-severe acne vulgaris: assessment of efficacy and safety in patients aged 9 years and older. J Drugs Dermatol. 2018;17:1084-1091.
- Tan J, Thiboutot D, Popp G, et al. Randomized phase 3 evaluation of trifarotene 50 μg/g cream treatment of moderate facial and truncal acne. J Am Acad Dermatol. 2019;80:1691-1699. doi:10.1016/j.jaad.2019.02.044
- Huang CY, Chang IJ, Bolick N, et al. Comparative efficacy of pharmacological treatments for acne vulgaris: a network meta-analysis of 221 randomized controlled trials. Ann Fam Med. 2023;21:358-369. doi:10.1370/afm.2995
- Draelos ZD. Low irritation potential of tazarotene 0.045% lotion: head-to-head comparison to adapalene 0.3% gel and trifarotene 0.005% cream in two studies. J Dermatolog Treat. 2023;34:2166346. doi:10.1080/09546634.2023.2166346
- Dessinioti C, Katsambas A. Antibiotics and antimicrobial resistance in acne: epidemiological trends and clinical practice considerations. Yale J Biol Med. 2022;95:429-443.
- Gold LS, Dhawan S, Weiss J, et al. A novel topical minocycline foam for the treatment of moderate-to-severe acne vulgaris: results of 2 randomized, double-blind, phase 3 studies. J Am Acad Dermatol. 2019;80:168-177. doi:10.1016/j.jaad.2018.08.020
- Wang X, Wang Z, Sun L, et al. Efficacy and safety of dapsone gel for acne: a systematic review and meta-analysis. Ann Palliat Med. 2022;11:611-620. doi:10.21037/apm-21-3935
- Melián-Olivera A, Burgos-Blasco P, Selda-Enríquez G, et al. Topical dapsone for folliculitis decalvans: a retrospective cohort study. J Am Acad Dermatol. 2022;87:150-151. doi:10.1016/j.jaad.2021.07.004
- Yentzer BA, Ade RA, Fountain JM, et al. Simplifying regimens promotes greater adherence and outcomes with topical acne medications: a randomized controlled trial. Cutis. 2010;86:103-108.
- Ting W. Randomized, observer-blind, split-face study to compare the irritation potential of 2 topical acne formulations over a 14-day treatment period. Cutis. 2012;90:91-96.
- Aschoff R, Möller S, Haase R, et al. Tolerability and efficacy ofclindamycin/tretinoin versus adapalene/benzoyl peroxide in the treatment of acne vulgaris. J Drugs Dermatol. 2021;20:295-301. doi:10.36849/JDD.2021.5641
- Rosette C, Agan FJ, Mazzetti A, et al. Cortexolone 17α-propionate (clascoterone) is a novel androgen receptor antagonist that inhibits production of lipids and inflammatory cytokines from sebocytes in vitro. J Drugs Dermatol. 2019;18:412-418.
- Hebert A, Thiboutot D, Stein Gold L, et al. Efficacy and safety of topical clascoterone cream, 1%, for treatment in patients with facial acne: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156:621-630. doi:10.1001/jamadermatol.2020.0465
- Trifu V, Tiplica GS, Naumescu E, et al. Cortexolone 17α-propionate 1% cream, a new potent antiandrogen for topical treatment of acne vulgaris. a pilot randomized, double-blind comparative study vs. placebo and tretinoin 0·05% cream. Br J Dermatol. 2011;165:177-183. doi:10.1111/j.1365-2133.2011.10332.x
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319. doi:10.1001/jamadermatol.2019.4818
- Guzman AK, Barbieri JS. Comparative analysis of prescribing patterns of tetracycline class antibiotics and spironolactone between advanced practice providers and physicians in the treatment of acne vulgaris. J Am Acad Dermatol. 2021;84:1119-1121. doi:10.1016/j.jaad.2020.06.044
- Barbieri JS, James WD, Margolis DJ. Trends in prescribing behavior of systemic agents used in the treatment of acne among dermatologists and nondermatologists: a retrospective analysis, 2004-2013. J Am Acad Dermatol. 2017;77:456-463.e4. doi:10.1016/j.jaad.2017.04.016
- Barbieri JS, Bhate K, Hartnett KP, et al. Trends in oral antibiotic prescription in dermatology, 2008 to 2016. JAMA Dermatol. 2019;155:290-297. doi:10.1001/jamadermatol.2018.4944
- Garner SE, Eady A, Bennett C, et al. Minocycline for acne vulgaris: efficacy and safety. Cochrane Database Syst Rev. 2012;2012:CD002086. doi:10.1002/14651858.CD002086.pub2
- Zhanel G, Critchley I, Lin LY, et al. Microbiological profile of sarecycline, a novel targeted spectrum tetracycline for the treatment of acne vulgaris. Antimicrob Agents Chemother. 2018;63:e01297-18. doi:10.1128/AAC.01297-18
- Moore A, Green LJ, Bruce S, et al. Once-daily oral sarecycline 1.5 mg/kg/day is effective for moderate to severe acne vulgaris: results from two identically designed, phase 3, randomized, double-blind clinical trials. J Drugs Dermatol. 2018;17:987-996.
- Garg V, Choi JK, James WD, et al. Long-term use of spironolactone for acne in women: a case series of 403 patients. J Am Acad Dermatol. 2021;84:1348-1355. doi:10.1016/j.jaad.2020.12.071
- Barbieri JS, Choi JK, James WD, et al. Real-world drug usage survival of spironolactone versus oral antibiotics for the management of female patients with acne. J Am Acad Dermatol. 2019;81:848-851. doi:10.1016/j.jaad.2019.03.036
- Barbieri JS, Spaccarelli N, Margolis DJ, et al. Approaches to limit systemic antibiotic use in acne: systemic alternatives, emerging topical therapies, dietary modification, and laser and light-based treatments. J Am Acad Dermatol. 2019;80:538-549. doi:10.1016/j.jaad.2018.09.055
- Barbieri JS, Choi JK, Mitra N, et al. Frequency of treatment switching for spironolactone compared to oral tetracycline-class antibiotics for women with acne: a retrospective cohort study 2010-2016. J Drugs Dermatol. 2018;17:632-638.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012;7:CD004425. doi:10.1002/14651858.CD004425.pub6
- Maloney JM, Dietze P, Watson D, et al. Treatment of acne using a 3-milligram drospirenone/20-microgram ethinyl estradiol oral contraceptive administered in a 24/4 regimen. Obstet Gynecol. 2008;112:773-781. doi:10.1097/AOG.0b013e318187e1c5
- Lucky AW, Koltun W, Thiboutot D, et al. A combined oral contraceptive containing 3-mg drospirenone/20-microg ethinyl estradiol in the treatment of acne vulgaris: a randomized, double-blind, placebo-controlled study evaluating lesion counts and participant self-assessment. Cutis. 2008;82:143-150.
- Koo EB, Petersen TD, Kimball AB. Meta-analysis comparing efficacy of antibiotics versus oral contraceptives in acne vulgaris. J Am Acad Dermatol. 2014;71:450-459. doi:10.1016/j.jaad.2014.03.051
- Roberts EE, Nowsheen S, Davis DMR, et al. Use of spironolactone to treat acne in adolescent females. Pediatr Dermatol. 2021;38:72-76. doi:10.1111/pde.14391
- Shaw JC. Low-dose adjunctive spironolactone in the treatment of acne in women: a retrospective analysis of 85 consecutively treated patients. J Am Acad Dermatol. 2000;43:498-502. doi:10.1067/mjd.2000.105557
- Layton AM, Eady EA, Whitehouse H, et al. Oral spironolactone for acne vulgaris in adult females: a hybrid systematic review. Am J Clin Dermatol. 2017;18:169-191. doi:10.1007/s40257-016-0245-x
- Barbieri JS, Margolis DJ, Mostaghimi A. Temporal trends and clinician variability in potassium monitoring of healthy young women treated for acne with spironolactone. JAMA Dermatol. 2021;157:296-300. doi:10.1001/jamadermatol.2020.5468
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944. doi:10.1001/jamadermatol.2015.34
- Thiede RM, Rastogi S, Nardone B, et al. Hyperkalemia in women with acne exposed to oral spironolactone: a retrospective study from the RADAR (Research on Adverse Drug Events and Reports) program. Int J Womens Dermatol. 2019;5:155-157. doi:10.1016/j.ijwd.2019.04.024
- Barbieri JS, Shin DB, Wang S, et al. The clinical utility of laboratory monitoring during isotretinoin therapy for acne and changes to monitoring practices over time. J Am Acad Dermatol. 2020;82:72-79. doi:10.1016/j.jaad.2019.06.025
- Lee YH, Scharnitz TP, Muscat J, et al. Laboratory monitoring during isotretinoin therapy for acne: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:35-44. doi:10.1001/jamadermatol.2015.3091
- Xia E, Han J, Faletsky A, et al. Isotretinoin laboratory monitoring in acne treatment: a Delphi consensus study. JAMA Dermatol. 2022;158:942-948. doi:10.1001/jamadermatol.2022.2044
- Affleck A, Jackson D, Williams HC, et al. Is routine laboratory testing in healthy young patients taking isotretinoin necessary: a critically appraised topic. Br J Dermatol. 2022;187:857-865. doi:10.1111/bjd.21840
- Barbieri JS, LaChance A, Albrecht J. Double standards and inconsistencies in access to care-what constitutes a cosmetic treatment? JAMA Dermatol. 2023;159:245-246. doi:10.1001/jamadermatol.2022.6322
- Trish E, Van Nuys K, Popovian R. US consumers overpay for generic drugs. Schaeffer Center White Paper Series. May 31, 2022. doi:10.25549/m589-2268
In the United States, acne affects 85% of adolescents and can persist into adulthood at a prevalence of 30% to 50% in adult women. 1,2 The pathogenesis of acne is multifactorial and involves hyperkeratinization of the follicle, bacterial colonization with Cutibacterium acnes , and increased androgen-induced sebum production, which together lead to inflammation. 3,4 A wide range of treatment guideline–recommended options are available, including benzoyl peroxide (BPO), topical retinoids, topical and oral antibiotics, antiandrogens, and isotretinoin. 5 However, these options vary widely in their clinical uses, effectiveness, and costs.
Why Cost-effective Acne Care Matters
Out-of-pocket spending by patients on acne treatments can be substantial, with surveys finding that acne patients often spend hundreds to thousands of dollars per year.6,7 In a poll conducted in 2019 by the Kaiser Family Foundation, 3 in 10 patients said they had not taken their medicine as prescribed because of costs.8 A mixed methods study by Ryskina et al9 found that 65% (17/26) of participants who reported primary nonadherence—intended to fill prescriptions but were unable to do so—cited cost or coverage-related barriers as the reason. With the continued rise of dermatologic drug prices and increased prevalence of high-deductible health plans, cost-effective treatment continues to grow in importance. Failure to consider cost-effective, patient-centered care may lead to increased financial toxicity, reduced adherence, and ultimately worse outcomes and patient satisfaction. We aim to review the cost-effectiveness of current prescription therapies for acne management and highlight the most cost-effective approaches to patients with mild to moderate acne as well as moderate to severe acne.
In this review, we will take a value-oriented framework.10 Value can be defined as the cost per outcome of interest. Therefore, a treatment does not necessarily need to be inexpensive to provide high value if it delivers outstanding clinical outcomes. In addition, we will focus on incremental cost-effectiveness relative to common alternatives (eg, a retinoid could deliver high value relative to a vehicle but still provide limited value compared to other available retinoids if it is more expensive but not more efficacious). When possible, we present data from cost-effectiveness studies.11,12 We also use recent available price data obtained from GoodRx on August 11, 2023, to guide this discussion.13 However, as comparative-effectiveness and cost-effectiveness studies rarely are performed for acne medications, much of this discussion will be based on expert opinion.
Treatment Categories
Topical Retinoids—There currently are 4 topical retinoids that are approved by the US Food and Drug Administration (FDA) for the treatment of acne: tretinoin, tazarotene, trifarotene, and adapalene. These drugs are vitamin A derivatives that bind retinoic acid receptors and function as comedolytic and anti-inflammatory agents.5 In general, generic tretinoin and adapalene products have the lowest cost (Table).

In network meta-analyses, tretinoin and adapalene often are highly ranked topical treatment options with respect to efficacy.14 Combined with their low cost, generic tretinoin and adapalene likely are excellent initial options for topical therapy from the standpoint of cost-effectiveness.15 Adapalene may be preferred in many situations because of its better photostability and compatibility with BPO.
Due to the importance of the vehicle in determining retinoid tolerability, efforts have been made to use encapsulation and polymeric emulsion technology to improve tolerability. Recently, polymeric lotion formulations of tretinoin and tazarotene have become available. In a phase 2 study, tazarotene lotion 0.045% was found to have equivalent efficacy and superior tolerability to tazarotene cream 0.1%.16 Although head-to-head data are not available, it is likely that tretinoin lotion may offer similar tolerability improvements.17 Although these formulations currently are more costly, this improved tolerability may be critical for some patients to be able to use topical retinoids, and the additional cost may be worthwhile. In addition, as these products lose market exclusivity, they may become more affordable and similarly priced to other topical retinoids. It is important to keep in mind that in clinical trials of tretinoin and adapalene, rates of dropout due to adverse events typically were 1% to 2%; therefore, because many patients can tolerate generic tretinoin and adapalene, at current prices the lotion formulations of retinoids may not be cost-effective relative to these generics.14
Trifarotene cream 0.005%, a fourth-generation topical retinoid that is highly sensitive for retinoic acid receptor γ, recently was FDA approved for the treatment of acne. Although trifarotene is efficacious for both facial and truncal acne, there is a lack of active comparator data compared to other topical retinoids.18 In a 2023 network meta-analysis, trifarotene was found to be both less efficacious and less tolerable compared to other topical retinoids.19 Thus, it is unclear if trifarotene offers any improved efficacy compared to other options, and it comes at a much higher cost (Table). In a tolerability study, trifarotene was found to be significantly more irritating than tazarotene lotion 0.045% and adapalene gel 0.3% (P<.05).20 Therefore, trifarotene cream 0.005% is unlikely to be a cost-effective option; in fact, it may be overall inferior to other topical retinoids, given its potentially lower tolerability.
Topical Antibiotics—There are 4 commonly prescribed topical antibiotics that are approved by the FDA for the treatment of acne: clindamycin, erythromycin, dapsone, and minocycline. The American Academy of Dermatology guidelines for the treatment of acne recommend concomitant use of BPO to prevent antibiotic resistance.5 Clindamycin is favored over erythromycin because of increasing antibiotic resistance to erythromycin.21 Inexpensive generic options in multiple vehicles (eg, solution, foam, gel) make clindamycin a highly cost-effective option when antibiotic therapy is desired as part of a topical regimen (Table).
The cost-effectiveness of dapsone gel and minocycline foam relative to clindamycin are less certain. Rates of resistance to minocycline are lower than clindamycin, and minocycline foam may be a reasonable alternative in patients who have not had success with other topical antibiotics, such as clindamycin.22 However, given the absence of comparative effectiveness data to suggest minocycline is more effective than clindamycin, it is difficult to justify the substantially higher cost for the typical patient. Although dapsone gel has been suggested as an option for adult women with acne, there are no data to support that it is any more effective than other topical antibiotics in this patient population.23 As generic dapsone prices decrease, it may become a reasonable alternative to clindamycin. In addition, the antineutrophil properties of dapsone may be useful in other acneform and inflammatory eruptions, such as scalp folliculitis and folliculitis decalvans.24
Combination Topicals—Current combination topical products include antibiotic and BPO, antibiotic and retinoid, and retinoid and BPO. Use of combination agents is recommended to reduce the risk for resistance and to enhance effectiveness. Combination products offer improved convenience, which is associated with better adherence and outcomes.25 Generic fixed-dose adapalene-BPO can be a highly cost-effective option that can sometimes be less expensive than the individual component products (Table). Similarly, fixed-dose clindamycin-BPO also is likely to be highly cost-effective. A network meta-analysis found fixed-dose adapalene-BPO to be the most efficacious topical treatment, though it also was found to be the most irritating—more so than fixed-dose clindamycin-BPO, which may have similar efficacy.14,26,27 Generic fixed-dose tretinoin-clindamycin offers improved convenience and adherence compared to the individual components, but it is more expensive, and its cost-effectiveness may be influenced by the importance of convenience for the patient.25 An encapsulated, fixed-dose tretinoin 0.1%–BPO 3% cream is FDA approved for acne, but the cost is high and there is a lack of comparative effectiveness data demonstrating advantages over generic fixed-dose adapalene-BPO products.
Topical Antiandrogen—Clascoterone was introduced in 2020 as the first FDA-approved topical medication to target the hormonal pathogenesis of acne, inhibiting the androgen receptors in the sebaceous gland.28 Because it is rapidly metabolized to cortexolone and does not have systemic antiandrogen effects, clascoterone can be used in both men and women with acne. In clinical trials, it had minimal side effects, including no evidence of irritability, which is an advantage over topical retinoids and BPO.29 In addition, a phase 2 study found that clascoterone may have similar to superior efficacy to tretinoin cream 0.05%.30 Although clascoterone has several strengths, including its efficacy, tolerability, and unique mechanism of action, its cost-effectiveness is limited due to its high cost (Table) and the need for twice-daily application, which reduces convenience. Clascoterone likely is best reserved for patients with a strong hormonal pathogenesis of their acne or difficulty tolerating other topicals, or as an additional therapy to complement other topicals.
Oral Antibiotics—Oral antibiotics are the most commonly prescribed systemic treatments for acne, particularly tetracyclines such as doxycycline, minocycline, and sarecycline.31-34 Doxycycline and minocycline are considered first-line oral antibiotic therapy in the United States and are inexpensive and easily accessible.5 Doxycycline generally is recommended over minocycline given lack of evidence of superior efficacy of minocycline and concerns about severe adverse cutaneous reactions and drug-induced lupus with minocycline.35
In recent years, there has been growing concern of the development of antibiotic resistance.5 Sarecycline is a narrow-spectrum tetracycline that was FDA approved for acne in 2018. In vitro studies demonstrate sarecycline maintains high efficacy against C acnes with less activity against other bacteria, particularly gram-negative enterobes.36 The selectivity of sarecycline may lessen alterations of the gut microbiome seen with other oral antibiotics and reduce gastrointestinal tract side effects. Although comparative effectiveness studies are lacking, sarecycline was efficacious in phase 3 trials with few side effects compared with placebo.37 However, at this time, given the absence of comparative effectiveness data and its high cost (Table), sarecycline likely is best reserved for patients with comorbidities (eg, gastrointestinal disease), those requiring long-term antibiotic therapy, or those with acne that has failed to respond to other oral antibiotics.
Hormonal Treatments—Hormonal treatments such as combined oral contraceptives (COCs) and spironolactone often are considered second-line options, though they may represent cost-effective and safe alternatives to oral antibiotics for women with moderate to severe acne.38-41 There currently are 4 COCs approved by the FDA for the treatment of moderate acne in postmenarcheal females: drospirenone-ethinyl estradiol (Yaz [Bayer HealthCare Pharmaceuticals, Inc]), ethinyl estradiol-norgestimate (Ortho Tri-Cyclen [Ortho-McNeil Pharmaceuticals, Inc]), drospirenone-ethinyl estradiol-levomefolate (Beyaz [Bayer HealthCare Pharmaceuticals, Inc]), and ethinyl estradiol-norethindrone acetate-ferrous fumarate (Estrostep Fe [Allergan USA, Inc]).5 Treatment with COCs has been shown to cause substantial reductions in lesion counts across all lesion types compared to placebo, and a meta-analysis of 24 randomized trials conducted by Arowojolu et al42 demonstrated no consistent differences in acne reduction among different COCs.43,44 Although oral antibiotics are associated with faster improvement than COCs, there is some evidence that they have similar efficacy at 6 months of therapy.45 Combined oral contraceptives are inexpensive and likely reflect a highly cost-effective option (Table).
Spironolactone is an aldosterone inhibitor and androgen receptor blocker that is used off label to treat acne. It is one of the least expensive systemic medications for acne (Table). Although randomized controlled trials are lacking, several large case series support the effectiveness of spironolactone for women with acne.38,46 In addition, observational data suggest spironolactone may have similar effectiveness to oral antibiotics.41 Spironolactone generally is well tolerated, with the most common adverse effects being menstrual irregularities, breast tenderness, and diuresis.47,48 Many of these adverse effects are dose dependent and less likely with the dosing used in acne care. Additionally, menstrual irregularities can be reduced by concomitant use of a COC.48
Although frequent potassium monitoring remains common among patients being treated with spironolactone, there is growing evidence to suggest that potassium monitoring is of low value in young healthy women with acne.49-51 Reducing this laboratory monitoring likely represents an opportunity to provide higher-value care to patients being treated with spironolactone. However, laboratory monitoring should be considered if risk factors for hyperkalemia are present (eg, older age, comorbidities, medications).51
Isotretinoin—Isotretinoin is the most efficacious treatment available for acne and has the unique property of being able to induce a remission of acne activity for many patients.5 Although it remains modestly expensive (Table), it may be less costly overall relative to other treatments that may need continued use over many years because it can induce a remission of acne activity. As with spironolactone, frequent laboratory monitoring remains common among patients being treated with isotretinoin. There is no evidence to support checking complete blood cell counts.52 Several observational studies and a Delphi consensus support reduced monitoring, such as checking lipids and alanine aminotransferase at baseline and peak dose in otherwise young healthy patients.53,54 A recent critically appraised topic published in the British Journal of Dermatology has proposed eliminating laboratory monitoring entirely.55 Reducing laboratory monitoring for patients being treated with isotretinoin has been estimated to potentially save $100 million to $200 million per year in the United States.52-54
Other Strategies to Reduce Patient Costs
Although choosing a cost-effective treatment approach is critical to preventing financial toxicity given poor coverage for acne care and the growth of high-deductible insurance plans, some patients may still experience high treatment costs.56 Because pharmacy costs often are inflated, potentially related to practices of pharmacy benefit managers, it often is possible to find better prices than the presented list price, either by using platforms such as GoodRx or through direct-to-patient mail-order pharmacies such as Cost Plus Drug.57 For branded medications, some patients may be eligible for patient-assistance programs, though they typically are not available for those with public insurance such as Medicare or Medicaid. Compounding pharmacies offer another approach to reduce cost and improve convenience for patients, but because the vehicle can influence the efficacy and tolerability of some topical medications, it is possible that these compounded formulations may not perform similarly to the original FDA-approved products.
Conclusion
For mild to moderate acne, multimodal topical therapy often is required. Fixed-dose combination adapalene-BPO and clindamycin-BPO are highly cost-effective options for most patients. Lotion formulations of topical retinoids may be useful in patients with difficulty tolerating other formulations. Clascoterone is a novel topical antiandrogen that is more expensive than other topical therapies but can complement other topical therapies and is well tolerated.
For moderate to severe acne, doxycycline or hormonal therapy (ie, COCs, spironolactone) are highly cost-effective options. Isotretinoin is recommended for severe or scarring acne. Reduced laboratory monitoring for spironolactone and isotretinoin is an opportunity to provide higher-value care.
In the United States, acne affects 85% of adolescents and can persist into adulthood at a prevalence of 30% to 50% in adult women. 1,2 The pathogenesis of acne is multifactorial and involves hyperkeratinization of the follicle, bacterial colonization with Cutibacterium acnes , and increased androgen-induced sebum production, which together lead to inflammation. 3,4 A wide range of treatment guideline–recommended options are available, including benzoyl peroxide (BPO), topical retinoids, topical and oral antibiotics, antiandrogens, and isotretinoin. 5 However, these options vary widely in their clinical uses, effectiveness, and costs.
Why Cost-effective Acne Care Matters
Out-of-pocket spending by patients on acne treatments can be substantial, with surveys finding that acne patients often spend hundreds to thousands of dollars per year.6,7 In a poll conducted in 2019 by the Kaiser Family Foundation, 3 in 10 patients said they had not taken their medicine as prescribed because of costs.8 A mixed methods study by Ryskina et al9 found that 65% (17/26) of participants who reported primary nonadherence—intended to fill prescriptions but were unable to do so—cited cost or coverage-related barriers as the reason. With the continued rise of dermatologic drug prices and increased prevalence of high-deductible health plans, cost-effective treatment continues to grow in importance. Failure to consider cost-effective, patient-centered care may lead to increased financial toxicity, reduced adherence, and ultimately worse outcomes and patient satisfaction. We aim to review the cost-effectiveness of current prescription therapies for acne management and highlight the most cost-effective approaches to patients with mild to moderate acne as well as moderate to severe acne.
In this review, we will take a value-oriented framework.10 Value can be defined as the cost per outcome of interest. Therefore, a treatment does not necessarily need to be inexpensive to provide high value if it delivers outstanding clinical outcomes. In addition, we will focus on incremental cost-effectiveness relative to common alternatives (eg, a retinoid could deliver high value relative to a vehicle but still provide limited value compared to other available retinoids if it is more expensive but not more efficacious). When possible, we present data from cost-effectiveness studies.11,12 We also use recent available price data obtained from GoodRx on August 11, 2023, to guide this discussion.13 However, as comparative-effectiveness and cost-effectiveness studies rarely are performed for acne medications, much of this discussion will be based on expert opinion.
Treatment Categories
Topical Retinoids—There currently are 4 topical retinoids that are approved by the US Food and Drug Administration (FDA) for the treatment of acne: tretinoin, tazarotene, trifarotene, and adapalene. These drugs are vitamin A derivatives that bind retinoic acid receptors and function as comedolytic and anti-inflammatory agents.5 In general, generic tretinoin and adapalene products have the lowest cost (Table).

In network meta-analyses, tretinoin and adapalene often are highly ranked topical treatment options with respect to efficacy.14 Combined with their low cost, generic tretinoin and adapalene likely are excellent initial options for topical therapy from the standpoint of cost-effectiveness.15 Adapalene may be preferred in many situations because of its better photostability and compatibility with BPO.
Due to the importance of the vehicle in determining retinoid tolerability, efforts have been made to use encapsulation and polymeric emulsion technology to improve tolerability. Recently, polymeric lotion formulations of tretinoin and tazarotene have become available. In a phase 2 study, tazarotene lotion 0.045% was found to have equivalent efficacy and superior tolerability to tazarotene cream 0.1%.16 Although head-to-head data are not available, it is likely that tretinoin lotion may offer similar tolerability improvements.17 Although these formulations currently are more costly, this improved tolerability may be critical for some patients to be able to use topical retinoids, and the additional cost may be worthwhile. In addition, as these products lose market exclusivity, they may become more affordable and similarly priced to other topical retinoids. It is important to keep in mind that in clinical trials of tretinoin and adapalene, rates of dropout due to adverse events typically were 1% to 2%; therefore, because many patients can tolerate generic tretinoin and adapalene, at current prices the lotion formulations of retinoids may not be cost-effective relative to these generics.14
Trifarotene cream 0.005%, a fourth-generation topical retinoid that is highly sensitive for retinoic acid receptor γ, recently was FDA approved for the treatment of acne. Although trifarotene is efficacious for both facial and truncal acne, there is a lack of active comparator data compared to other topical retinoids.18 In a 2023 network meta-analysis, trifarotene was found to be both less efficacious and less tolerable compared to other topical retinoids.19 Thus, it is unclear if trifarotene offers any improved efficacy compared to other options, and it comes at a much higher cost (Table). In a tolerability study, trifarotene was found to be significantly more irritating than tazarotene lotion 0.045% and adapalene gel 0.3% (P<.05).20 Therefore, trifarotene cream 0.005% is unlikely to be a cost-effective option; in fact, it may be overall inferior to other topical retinoids, given its potentially lower tolerability.
Topical Antibiotics—There are 4 commonly prescribed topical antibiotics that are approved by the FDA for the treatment of acne: clindamycin, erythromycin, dapsone, and minocycline. The American Academy of Dermatology guidelines for the treatment of acne recommend concomitant use of BPO to prevent antibiotic resistance.5 Clindamycin is favored over erythromycin because of increasing antibiotic resistance to erythromycin.21 Inexpensive generic options in multiple vehicles (eg, solution, foam, gel) make clindamycin a highly cost-effective option when antibiotic therapy is desired as part of a topical regimen (Table).
The cost-effectiveness of dapsone gel and minocycline foam relative to clindamycin are less certain. Rates of resistance to minocycline are lower than clindamycin, and minocycline foam may be a reasonable alternative in patients who have not had success with other topical antibiotics, such as clindamycin.22 However, given the absence of comparative effectiveness data to suggest minocycline is more effective than clindamycin, it is difficult to justify the substantially higher cost for the typical patient. Although dapsone gel has been suggested as an option for adult women with acne, there are no data to support that it is any more effective than other topical antibiotics in this patient population.23 As generic dapsone prices decrease, it may become a reasonable alternative to clindamycin. In addition, the antineutrophil properties of dapsone may be useful in other acneform and inflammatory eruptions, such as scalp folliculitis and folliculitis decalvans.24
Combination Topicals—Current combination topical products include antibiotic and BPO, antibiotic and retinoid, and retinoid and BPO. Use of combination agents is recommended to reduce the risk for resistance and to enhance effectiveness. Combination products offer improved convenience, which is associated with better adherence and outcomes.25 Generic fixed-dose adapalene-BPO can be a highly cost-effective option that can sometimes be less expensive than the individual component products (Table). Similarly, fixed-dose clindamycin-BPO also is likely to be highly cost-effective. A network meta-analysis found fixed-dose adapalene-BPO to be the most efficacious topical treatment, though it also was found to be the most irritating—more so than fixed-dose clindamycin-BPO, which may have similar efficacy.14,26,27 Generic fixed-dose tretinoin-clindamycin offers improved convenience and adherence compared to the individual components, but it is more expensive, and its cost-effectiveness may be influenced by the importance of convenience for the patient.25 An encapsulated, fixed-dose tretinoin 0.1%–BPO 3% cream is FDA approved for acne, but the cost is high and there is a lack of comparative effectiveness data demonstrating advantages over generic fixed-dose adapalene-BPO products.
Topical Antiandrogen—Clascoterone was introduced in 2020 as the first FDA-approved topical medication to target the hormonal pathogenesis of acne, inhibiting the androgen receptors in the sebaceous gland.28 Because it is rapidly metabolized to cortexolone and does not have systemic antiandrogen effects, clascoterone can be used in both men and women with acne. In clinical trials, it had minimal side effects, including no evidence of irritability, which is an advantage over topical retinoids and BPO.29 In addition, a phase 2 study found that clascoterone may have similar to superior efficacy to tretinoin cream 0.05%.30 Although clascoterone has several strengths, including its efficacy, tolerability, and unique mechanism of action, its cost-effectiveness is limited due to its high cost (Table) and the need for twice-daily application, which reduces convenience. Clascoterone likely is best reserved for patients with a strong hormonal pathogenesis of their acne or difficulty tolerating other topicals, or as an additional therapy to complement other topicals.
Oral Antibiotics—Oral antibiotics are the most commonly prescribed systemic treatments for acne, particularly tetracyclines such as doxycycline, minocycline, and sarecycline.31-34 Doxycycline and minocycline are considered first-line oral antibiotic therapy in the United States and are inexpensive and easily accessible.5 Doxycycline generally is recommended over minocycline given lack of evidence of superior efficacy of minocycline and concerns about severe adverse cutaneous reactions and drug-induced lupus with minocycline.35
In recent years, there has been growing concern of the development of antibiotic resistance.5 Sarecycline is a narrow-spectrum tetracycline that was FDA approved for acne in 2018. In vitro studies demonstrate sarecycline maintains high efficacy against C acnes with less activity against other bacteria, particularly gram-negative enterobes.36 The selectivity of sarecycline may lessen alterations of the gut microbiome seen with other oral antibiotics and reduce gastrointestinal tract side effects. Although comparative effectiveness studies are lacking, sarecycline was efficacious in phase 3 trials with few side effects compared with placebo.37 However, at this time, given the absence of comparative effectiveness data and its high cost (Table), sarecycline likely is best reserved for patients with comorbidities (eg, gastrointestinal disease), those requiring long-term antibiotic therapy, or those with acne that has failed to respond to other oral antibiotics.
Hormonal Treatments—Hormonal treatments such as combined oral contraceptives (COCs) and spironolactone often are considered second-line options, though they may represent cost-effective and safe alternatives to oral antibiotics for women with moderate to severe acne.38-41 There currently are 4 COCs approved by the FDA for the treatment of moderate acne in postmenarcheal females: drospirenone-ethinyl estradiol (Yaz [Bayer HealthCare Pharmaceuticals, Inc]), ethinyl estradiol-norgestimate (Ortho Tri-Cyclen [Ortho-McNeil Pharmaceuticals, Inc]), drospirenone-ethinyl estradiol-levomefolate (Beyaz [Bayer HealthCare Pharmaceuticals, Inc]), and ethinyl estradiol-norethindrone acetate-ferrous fumarate (Estrostep Fe [Allergan USA, Inc]).5 Treatment with COCs has been shown to cause substantial reductions in lesion counts across all lesion types compared to placebo, and a meta-analysis of 24 randomized trials conducted by Arowojolu et al42 demonstrated no consistent differences in acne reduction among different COCs.43,44 Although oral antibiotics are associated with faster improvement than COCs, there is some evidence that they have similar efficacy at 6 months of therapy.45 Combined oral contraceptives are inexpensive and likely reflect a highly cost-effective option (Table).
Spironolactone is an aldosterone inhibitor and androgen receptor blocker that is used off label to treat acne. It is one of the least expensive systemic medications for acne (Table). Although randomized controlled trials are lacking, several large case series support the effectiveness of spironolactone for women with acne.38,46 In addition, observational data suggest spironolactone may have similar effectiveness to oral antibiotics.41 Spironolactone generally is well tolerated, with the most common adverse effects being menstrual irregularities, breast tenderness, and diuresis.47,48 Many of these adverse effects are dose dependent and less likely with the dosing used in acne care. Additionally, menstrual irregularities can be reduced by concomitant use of a COC.48
Although frequent potassium monitoring remains common among patients being treated with spironolactone, there is growing evidence to suggest that potassium monitoring is of low value in young healthy women with acne.49-51 Reducing this laboratory monitoring likely represents an opportunity to provide higher-value care to patients being treated with spironolactone. However, laboratory monitoring should be considered if risk factors for hyperkalemia are present (eg, older age, comorbidities, medications).51
Isotretinoin—Isotretinoin is the most efficacious treatment available for acne and has the unique property of being able to induce a remission of acne activity for many patients.5 Although it remains modestly expensive (Table), it may be less costly overall relative to other treatments that may need continued use over many years because it can induce a remission of acne activity. As with spironolactone, frequent laboratory monitoring remains common among patients being treated with isotretinoin. There is no evidence to support checking complete blood cell counts.52 Several observational studies and a Delphi consensus support reduced monitoring, such as checking lipids and alanine aminotransferase at baseline and peak dose in otherwise young healthy patients.53,54 A recent critically appraised topic published in the British Journal of Dermatology has proposed eliminating laboratory monitoring entirely.55 Reducing laboratory monitoring for patients being treated with isotretinoin has been estimated to potentially save $100 million to $200 million per year in the United States.52-54
Other Strategies to Reduce Patient Costs
Although choosing a cost-effective treatment approach is critical to preventing financial toxicity given poor coverage for acne care and the growth of high-deductible insurance plans, some patients may still experience high treatment costs.56 Because pharmacy costs often are inflated, potentially related to practices of pharmacy benefit managers, it often is possible to find better prices than the presented list price, either by using platforms such as GoodRx or through direct-to-patient mail-order pharmacies such as Cost Plus Drug.57 For branded medications, some patients may be eligible for patient-assistance programs, though they typically are not available for those with public insurance such as Medicare or Medicaid. Compounding pharmacies offer another approach to reduce cost and improve convenience for patients, but because the vehicle can influence the efficacy and tolerability of some topical medications, it is possible that these compounded formulations may not perform similarly to the original FDA-approved products.
Conclusion
For mild to moderate acne, multimodal topical therapy often is required. Fixed-dose combination adapalene-BPO and clindamycin-BPO are highly cost-effective options for most patients. Lotion formulations of topical retinoids may be useful in patients with difficulty tolerating other formulations. Clascoterone is a novel topical antiandrogen that is more expensive than other topical therapies but can complement other topical therapies and is well tolerated.
For moderate to severe acne, doxycycline or hormonal therapy (ie, COCs, spironolactone) are highly cost-effective options. Isotretinoin is recommended for severe or scarring acne. Reduced laboratory monitoring for spironolactone and isotretinoin is an opportunity to provide higher-value care.
- Bhate K, Williams HC. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168:474-485. doi:10.1111/bjd.12149
- Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59. doi:10.1016/j.jaad.2007.06.045
- Webster GF. The pathophysiology of acne. Cutis. 2005;76(2 suppl):4-7.
- Degitz K, Placzek M, Borelli C, et al. Pathophysiology of acne. J Dtsch Dermatol Ges. 2007;5:316-323. doi:10.1111/j.1610-0387.2007.06274.x
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33. doi:10.1016/j.jaad.2015.12.037
- Felmingham C, Kerr A, Veysey E. Costs incurred by patients with acne prior to dermatological consultation and their relation to patient income. Australas J Dermatol. 2020;61:384-386. doi:10.1111/ajd.13324
- Perche P, Singh R, Feldman S. Patient preferences for acne vulgaris treatment and barriers to care: a survey study. J Drugs Dermatol. 2022;21:1191-1195. doi:10.36849/JDD.6940
- KFF Health Tracking Poll—February 2019. Accessed August 9, 2023. https://files.kff.org/attachment/Topline-KFF-Health-Tracking-Poll-February-2019
- Ryskina KL, Goldberg E, Lott B, et al. The role of the physician in patient perceptions of barriers to primary adherence with acne medications. JAMA Dermatol. 2018;154:456-459. doi:10.1001/jamadermatol.2017.6144
- Porter ME. What is value in health care? N Engl J Med. 2010;363:2477-2481. doi:10.1056/NEJMp1011024
- Barbieri JS, Tan JKL, Adamson AS. Active comparator trial designs used to promote development of innovative new medications. Cutis. 2020;106:E4-E6. doi:10.12788/cutis.0067
- Miller J, Ly S, Mostaghimi A, et al. Use of active comparator trials for topical medications in dermatology. JAMA Dermatol. 2021;157:597-599. doi:10.1001/jamadermatol.2021.0356
- GoodRx. Accessed August 11, 2023. https://www.goodrx.com
- Stuart B, Maund E, Wilcox C, et al. Topical preparations for the treatment of mild‐to‐moderate acne vulgaris: systematic review and network meta‐analysis. Br J Dermatol. 2021;185:512-525. doi:10.1111/bjd.20080
- Mavranezouli I, Welton NJ, Daly CH, et al. Cost-effectiveness of topical pharmacological, oral pharmacological, physical and combined treatments for acne vulgaris. Clin Exp Dermatol. 2022;47:2176-2187. doi:10.1111/ced.15356
- Tanghetti E, Werschler W, Lain T, et al. Tazarotene 0.045% lotion for once-daily treatment of moderate-to-severe acne vulgaris: results from two phase 3 trials. J Drugs Dermatol. 2020;19:70-77. doi:10.36849/JDD.2020.3977
- Tyring SK, Kircik LH, Pariser DM, et al. Novel tretinoin 0.05% lotion for the once-daily treatment of moderate-to-severe acne vulgaris: assessment of efficacy and safety in patients aged 9 years and older. J Drugs Dermatol. 2018;17:1084-1091.
- Tan J, Thiboutot D, Popp G, et al. Randomized phase 3 evaluation of trifarotene 50 μg/g cream treatment of moderate facial and truncal acne. J Am Acad Dermatol. 2019;80:1691-1699. doi:10.1016/j.jaad.2019.02.044
- Huang CY, Chang IJ, Bolick N, et al. Comparative efficacy of pharmacological treatments for acne vulgaris: a network meta-analysis of 221 randomized controlled trials. Ann Fam Med. 2023;21:358-369. doi:10.1370/afm.2995
- Draelos ZD. Low irritation potential of tazarotene 0.045% lotion: head-to-head comparison to adapalene 0.3% gel and trifarotene 0.005% cream in two studies. J Dermatolog Treat. 2023;34:2166346. doi:10.1080/09546634.2023.2166346
- Dessinioti C, Katsambas A. Antibiotics and antimicrobial resistance in acne: epidemiological trends and clinical practice considerations. Yale J Biol Med. 2022;95:429-443.
- Gold LS, Dhawan S, Weiss J, et al. A novel topical minocycline foam for the treatment of moderate-to-severe acne vulgaris: results of 2 randomized, double-blind, phase 3 studies. J Am Acad Dermatol. 2019;80:168-177. doi:10.1016/j.jaad.2018.08.020
- Wang X, Wang Z, Sun L, et al. Efficacy and safety of dapsone gel for acne: a systematic review and meta-analysis. Ann Palliat Med. 2022;11:611-620. doi:10.21037/apm-21-3935
- Melián-Olivera A, Burgos-Blasco P, Selda-Enríquez G, et al. Topical dapsone for folliculitis decalvans: a retrospective cohort study. J Am Acad Dermatol. 2022;87:150-151. doi:10.1016/j.jaad.2021.07.004
- Yentzer BA, Ade RA, Fountain JM, et al. Simplifying regimens promotes greater adherence and outcomes with topical acne medications: a randomized controlled trial. Cutis. 2010;86:103-108.
- Ting W. Randomized, observer-blind, split-face study to compare the irritation potential of 2 topical acne formulations over a 14-day treatment period. Cutis. 2012;90:91-96.
- Aschoff R, Möller S, Haase R, et al. Tolerability and efficacy ofclindamycin/tretinoin versus adapalene/benzoyl peroxide in the treatment of acne vulgaris. J Drugs Dermatol. 2021;20:295-301. doi:10.36849/JDD.2021.5641
- Rosette C, Agan FJ, Mazzetti A, et al. Cortexolone 17α-propionate (clascoterone) is a novel androgen receptor antagonist that inhibits production of lipids and inflammatory cytokines from sebocytes in vitro. J Drugs Dermatol. 2019;18:412-418.
- Hebert A, Thiboutot D, Stein Gold L, et al. Efficacy and safety of topical clascoterone cream, 1%, for treatment in patients with facial acne: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156:621-630. doi:10.1001/jamadermatol.2020.0465
- Trifu V, Tiplica GS, Naumescu E, et al. Cortexolone 17α-propionate 1% cream, a new potent antiandrogen for topical treatment of acne vulgaris. a pilot randomized, double-blind comparative study vs. placebo and tretinoin 0·05% cream. Br J Dermatol. 2011;165:177-183. doi:10.1111/j.1365-2133.2011.10332.x
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319. doi:10.1001/jamadermatol.2019.4818
- Guzman AK, Barbieri JS. Comparative analysis of prescribing patterns of tetracycline class antibiotics and spironolactone between advanced practice providers and physicians in the treatment of acne vulgaris. J Am Acad Dermatol. 2021;84:1119-1121. doi:10.1016/j.jaad.2020.06.044
- Barbieri JS, James WD, Margolis DJ. Trends in prescribing behavior of systemic agents used in the treatment of acne among dermatologists and nondermatologists: a retrospective analysis, 2004-2013. J Am Acad Dermatol. 2017;77:456-463.e4. doi:10.1016/j.jaad.2017.04.016
- Barbieri JS, Bhate K, Hartnett KP, et al. Trends in oral antibiotic prescription in dermatology, 2008 to 2016. JAMA Dermatol. 2019;155:290-297. doi:10.1001/jamadermatol.2018.4944
- Garner SE, Eady A, Bennett C, et al. Minocycline for acne vulgaris: efficacy and safety. Cochrane Database Syst Rev. 2012;2012:CD002086. doi:10.1002/14651858.CD002086.pub2
- Zhanel G, Critchley I, Lin LY, et al. Microbiological profile of sarecycline, a novel targeted spectrum tetracycline for the treatment of acne vulgaris. Antimicrob Agents Chemother. 2018;63:e01297-18. doi:10.1128/AAC.01297-18
- Moore A, Green LJ, Bruce S, et al. Once-daily oral sarecycline 1.5 mg/kg/day is effective for moderate to severe acne vulgaris: results from two identically designed, phase 3, randomized, double-blind clinical trials. J Drugs Dermatol. 2018;17:987-996.
- Garg V, Choi JK, James WD, et al. Long-term use of spironolactone for acne in women: a case series of 403 patients. J Am Acad Dermatol. 2021;84:1348-1355. doi:10.1016/j.jaad.2020.12.071
- Barbieri JS, Choi JK, James WD, et al. Real-world drug usage survival of spironolactone versus oral antibiotics for the management of female patients with acne. J Am Acad Dermatol. 2019;81:848-851. doi:10.1016/j.jaad.2019.03.036
- Barbieri JS, Spaccarelli N, Margolis DJ, et al. Approaches to limit systemic antibiotic use in acne: systemic alternatives, emerging topical therapies, dietary modification, and laser and light-based treatments. J Am Acad Dermatol. 2019;80:538-549. doi:10.1016/j.jaad.2018.09.055
- Barbieri JS, Choi JK, Mitra N, et al. Frequency of treatment switching for spironolactone compared to oral tetracycline-class antibiotics for women with acne: a retrospective cohort study 2010-2016. J Drugs Dermatol. 2018;17:632-638.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012;7:CD004425. doi:10.1002/14651858.CD004425.pub6
- Maloney JM, Dietze P, Watson D, et al. Treatment of acne using a 3-milligram drospirenone/20-microgram ethinyl estradiol oral contraceptive administered in a 24/4 regimen. Obstet Gynecol. 2008;112:773-781. doi:10.1097/AOG.0b013e318187e1c5
- Lucky AW, Koltun W, Thiboutot D, et al. A combined oral contraceptive containing 3-mg drospirenone/20-microg ethinyl estradiol in the treatment of acne vulgaris: a randomized, double-blind, placebo-controlled study evaluating lesion counts and participant self-assessment. Cutis. 2008;82:143-150.
- Koo EB, Petersen TD, Kimball AB. Meta-analysis comparing efficacy of antibiotics versus oral contraceptives in acne vulgaris. J Am Acad Dermatol. 2014;71:450-459. doi:10.1016/j.jaad.2014.03.051
- Roberts EE, Nowsheen S, Davis DMR, et al. Use of spironolactone to treat acne in adolescent females. Pediatr Dermatol. 2021;38:72-76. doi:10.1111/pde.14391
- Shaw JC. Low-dose adjunctive spironolactone in the treatment of acne in women: a retrospective analysis of 85 consecutively treated patients. J Am Acad Dermatol. 2000;43:498-502. doi:10.1067/mjd.2000.105557
- Layton AM, Eady EA, Whitehouse H, et al. Oral spironolactone for acne vulgaris in adult females: a hybrid systematic review. Am J Clin Dermatol. 2017;18:169-191. doi:10.1007/s40257-016-0245-x
- Barbieri JS, Margolis DJ, Mostaghimi A. Temporal trends and clinician variability in potassium monitoring of healthy young women treated for acne with spironolactone. JAMA Dermatol. 2021;157:296-300. doi:10.1001/jamadermatol.2020.5468
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944. doi:10.1001/jamadermatol.2015.34
- Thiede RM, Rastogi S, Nardone B, et al. Hyperkalemia in women with acne exposed to oral spironolactone: a retrospective study from the RADAR (Research on Adverse Drug Events and Reports) program. Int J Womens Dermatol. 2019;5:155-157. doi:10.1016/j.ijwd.2019.04.024
- Barbieri JS, Shin DB, Wang S, et al. The clinical utility of laboratory monitoring during isotretinoin therapy for acne and changes to monitoring practices over time. J Am Acad Dermatol. 2020;82:72-79. doi:10.1016/j.jaad.2019.06.025
- Lee YH, Scharnitz TP, Muscat J, et al. Laboratory monitoring during isotretinoin therapy for acne: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:35-44. doi:10.1001/jamadermatol.2015.3091
- Xia E, Han J, Faletsky A, et al. Isotretinoin laboratory monitoring in acne treatment: a Delphi consensus study. JAMA Dermatol. 2022;158:942-948. doi:10.1001/jamadermatol.2022.2044
- Affleck A, Jackson D, Williams HC, et al. Is routine laboratory testing in healthy young patients taking isotretinoin necessary: a critically appraised topic. Br J Dermatol. 2022;187:857-865. doi:10.1111/bjd.21840
- Barbieri JS, LaChance A, Albrecht J. Double standards and inconsistencies in access to care-what constitutes a cosmetic treatment? JAMA Dermatol. 2023;159:245-246. doi:10.1001/jamadermatol.2022.6322
- Trish E, Van Nuys K, Popovian R. US consumers overpay for generic drugs. Schaeffer Center White Paper Series. May 31, 2022. doi:10.25549/m589-2268
- Bhate K, Williams HC. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168:474-485. doi:10.1111/bjd.12149
- Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59. doi:10.1016/j.jaad.2007.06.045
- Webster GF. The pathophysiology of acne. Cutis. 2005;76(2 suppl):4-7.
- Degitz K, Placzek M, Borelli C, et al. Pathophysiology of acne. J Dtsch Dermatol Ges. 2007;5:316-323. doi:10.1111/j.1610-0387.2007.06274.x
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33. doi:10.1016/j.jaad.2015.12.037
- Felmingham C, Kerr A, Veysey E. Costs incurred by patients with acne prior to dermatological consultation and their relation to patient income. Australas J Dermatol. 2020;61:384-386. doi:10.1111/ajd.13324
- Perche P, Singh R, Feldman S. Patient preferences for acne vulgaris treatment and barriers to care: a survey study. J Drugs Dermatol. 2022;21:1191-1195. doi:10.36849/JDD.6940
- KFF Health Tracking Poll—February 2019. Accessed August 9, 2023. https://files.kff.org/attachment/Topline-KFF-Health-Tracking-Poll-February-2019
- Ryskina KL, Goldberg E, Lott B, et al. The role of the physician in patient perceptions of barriers to primary adherence with acne medications. JAMA Dermatol. 2018;154:456-459. doi:10.1001/jamadermatol.2017.6144
- Porter ME. What is value in health care? N Engl J Med. 2010;363:2477-2481. doi:10.1056/NEJMp1011024
- Barbieri JS, Tan JKL, Adamson AS. Active comparator trial designs used to promote development of innovative new medications. Cutis. 2020;106:E4-E6. doi:10.12788/cutis.0067
- Miller J, Ly S, Mostaghimi A, et al. Use of active comparator trials for topical medications in dermatology. JAMA Dermatol. 2021;157:597-599. doi:10.1001/jamadermatol.2021.0356
- GoodRx. Accessed August 11, 2023. https://www.goodrx.com
- Stuart B, Maund E, Wilcox C, et al. Topical preparations for the treatment of mild‐to‐moderate acne vulgaris: systematic review and network meta‐analysis. Br J Dermatol. 2021;185:512-525. doi:10.1111/bjd.20080
- Mavranezouli I, Welton NJ, Daly CH, et al. Cost-effectiveness of topical pharmacological, oral pharmacological, physical and combined treatments for acne vulgaris. Clin Exp Dermatol. 2022;47:2176-2187. doi:10.1111/ced.15356
- Tanghetti E, Werschler W, Lain T, et al. Tazarotene 0.045% lotion for once-daily treatment of moderate-to-severe acne vulgaris: results from two phase 3 trials. J Drugs Dermatol. 2020;19:70-77. doi:10.36849/JDD.2020.3977
- Tyring SK, Kircik LH, Pariser DM, et al. Novel tretinoin 0.05% lotion for the once-daily treatment of moderate-to-severe acne vulgaris: assessment of efficacy and safety in patients aged 9 years and older. J Drugs Dermatol. 2018;17:1084-1091.
- Tan J, Thiboutot D, Popp G, et al. Randomized phase 3 evaluation of trifarotene 50 μg/g cream treatment of moderate facial and truncal acne. J Am Acad Dermatol. 2019;80:1691-1699. doi:10.1016/j.jaad.2019.02.044
- Huang CY, Chang IJ, Bolick N, et al. Comparative efficacy of pharmacological treatments for acne vulgaris: a network meta-analysis of 221 randomized controlled trials. Ann Fam Med. 2023;21:358-369. doi:10.1370/afm.2995
- Draelos ZD. Low irritation potential of tazarotene 0.045% lotion: head-to-head comparison to adapalene 0.3% gel and trifarotene 0.005% cream in two studies. J Dermatolog Treat. 2023;34:2166346. doi:10.1080/09546634.2023.2166346
- Dessinioti C, Katsambas A. Antibiotics and antimicrobial resistance in acne: epidemiological trends and clinical practice considerations. Yale J Biol Med. 2022;95:429-443.
- Gold LS, Dhawan S, Weiss J, et al. A novel topical minocycline foam for the treatment of moderate-to-severe acne vulgaris: results of 2 randomized, double-blind, phase 3 studies. J Am Acad Dermatol. 2019;80:168-177. doi:10.1016/j.jaad.2018.08.020
- Wang X, Wang Z, Sun L, et al. Efficacy and safety of dapsone gel for acne: a systematic review and meta-analysis. Ann Palliat Med. 2022;11:611-620. doi:10.21037/apm-21-3935
- Melián-Olivera A, Burgos-Blasco P, Selda-Enríquez G, et al. Topical dapsone for folliculitis decalvans: a retrospective cohort study. J Am Acad Dermatol. 2022;87:150-151. doi:10.1016/j.jaad.2021.07.004
- Yentzer BA, Ade RA, Fountain JM, et al. Simplifying regimens promotes greater adherence and outcomes with topical acne medications: a randomized controlled trial. Cutis. 2010;86:103-108.
- Ting W. Randomized, observer-blind, split-face study to compare the irritation potential of 2 topical acne formulations over a 14-day treatment period. Cutis. 2012;90:91-96.
- Aschoff R, Möller S, Haase R, et al. Tolerability and efficacy ofclindamycin/tretinoin versus adapalene/benzoyl peroxide in the treatment of acne vulgaris. J Drugs Dermatol. 2021;20:295-301. doi:10.36849/JDD.2021.5641
- Rosette C, Agan FJ, Mazzetti A, et al. Cortexolone 17α-propionate (clascoterone) is a novel androgen receptor antagonist that inhibits production of lipids and inflammatory cytokines from sebocytes in vitro. J Drugs Dermatol. 2019;18:412-418.
- Hebert A, Thiboutot D, Stein Gold L, et al. Efficacy and safety of topical clascoterone cream, 1%, for treatment in patients with facial acne: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156:621-630. doi:10.1001/jamadermatol.2020.0465
- Trifu V, Tiplica GS, Naumescu E, et al. Cortexolone 17α-propionate 1% cream, a new potent antiandrogen for topical treatment of acne vulgaris. a pilot randomized, double-blind comparative study vs. placebo and tretinoin 0·05% cream. Br J Dermatol. 2011;165:177-183. doi:10.1111/j.1365-2133.2011.10332.x
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319. doi:10.1001/jamadermatol.2019.4818
- Guzman AK, Barbieri JS. Comparative analysis of prescribing patterns of tetracycline class antibiotics and spironolactone between advanced practice providers and physicians in the treatment of acne vulgaris. J Am Acad Dermatol. 2021;84:1119-1121. doi:10.1016/j.jaad.2020.06.044
- Barbieri JS, James WD, Margolis DJ. Trends in prescribing behavior of systemic agents used in the treatment of acne among dermatologists and nondermatologists: a retrospective analysis, 2004-2013. J Am Acad Dermatol. 2017;77:456-463.e4. doi:10.1016/j.jaad.2017.04.016
- Barbieri JS, Bhate K, Hartnett KP, et al. Trends in oral antibiotic prescription in dermatology, 2008 to 2016. JAMA Dermatol. 2019;155:290-297. doi:10.1001/jamadermatol.2018.4944
- Garner SE, Eady A, Bennett C, et al. Minocycline for acne vulgaris: efficacy and safety. Cochrane Database Syst Rev. 2012;2012:CD002086. doi:10.1002/14651858.CD002086.pub2
- Zhanel G, Critchley I, Lin LY, et al. Microbiological profile of sarecycline, a novel targeted spectrum tetracycline for the treatment of acne vulgaris. Antimicrob Agents Chemother. 2018;63:e01297-18. doi:10.1128/AAC.01297-18
- Moore A, Green LJ, Bruce S, et al. Once-daily oral sarecycline 1.5 mg/kg/day is effective for moderate to severe acne vulgaris: results from two identically designed, phase 3, randomized, double-blind clinical trials. J Drugs Dermatol. 2018;17:987-996.
- Garg V, Choi JK, James WD, et al. Long-term use of spironolactone for acne in women: a case series of 403 patients. J Am Acad Dermatol. 2021;84:1348-1355. doi:10.1016/j.jaad.2020.12.071
- Barbieri JS, Choi JK, James WD, et al. Real-world drug usage survival of spironolactone versus oral antibiotics for the management of female patients with acne. J Am Acad Dermatol. 2019;81:848-851. doi:10.1016/j.jaad.2019.03.036
- Barbieri JS, Spaccarelli N, Margolis DJ, et al. Approaches to limit systemic antibiotic use in acne: systemic alternatives, emerging topical therapies, dietary modification, and laser and light-based treatments. J Am Acad Dermatol. 2019;80:538-549. doi:10.1016/j.jaad.2018.09.055
- Barbieri JS, Choi JK, Mitra N, et al. Frequency of treatment switching for spironolactone compared to oral tetracycline-class antibiotics for women with acne: a retrospective cohort study 2010-2016. J Drugs Dermatol. 2018;17:632-638.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012;7:CD004425. doi:10.1002/14651858.CD004425.pub6
- Maloney JM, Dietze P, Watson D, et al. Treatment of acne using a 3-milligram drospirenone/20-microgram ethinyl estradiol oral contraceptive administered in a 24/4 regimen. Obstet Gynecol. 2008;112:773-781. doi:10.1097/AOG.0b013e318187e1c5
- Lucky AW, Koltun W, Thiboutot D, et al. A combined oral contraceptive containing 3-mg drospirenone/20-microg ethinyl estradiol in the treatment of acne vulgaris: a randomized, double-blind, placebo-controlled study evaluating lesion counts and participant self-assessment. Cutis. 2008;82:143-150.
- Koo EB, Petersen TD, Kimball AB. Meta-analysis comparing efficacy of antibiotics versus oral contraceptives in acne vulgaris. J Am Acad Dermatol. 2014;71:450-459. doi:10.1016/j.jaad.2014.03.051
- Roberts EE, Nowsheen S, Davis DMR, et al. Use of spironolactone to treat acne in adolescent females. Pediatr Dermatol. 2021;38:72-76. doi:10.1111/pde.14391
- Shaw JC. Low-dose adjunctive spironolactone in the treatment of acne in women: a retrospective analysis of 85 consecutively treated patients. J Am Acad Dermatol. 2000;43:498-502. doi:10.1067/mjd.2000.105557
- Layton AM, Eady EA, Whitehouse H, et al. Oral spironolactone for acne vulgaris in adult females: a hybrid systematic review. Am J Clin Dermatol. 2017;18:169-191. doi:10.1007/s40257-016-0245-x
- Barbieri JS, Margolis DJ, Mostaghimi A. Temporal trends and clinician variability in potassium monitoring of healthy young women treated for acne with spironolactone. JAMA Dermatol. 2021;157:296-300. doi:10.1001/jamadermatol.2020.5468
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944. doi:10.1001/jamadermatol.2015.34
- Thiede RM, Rastogi S, Nardone B, et al. Hyperkalemia in women with acne exposed to oral spironolactone: a retrospective study from the RADAR (Research on Adverse Drug Events and Reports) program. Int J Womens Dermatol. 2019;5:155-157. doi:10.1016/j.ijwd.2019.04.024
- Barbieri JS, Shin DB, Wang S, et al. The clinical utility of laboratory monitoring during isotretinoin therapy for acne and changes to monitoring practices over time. J Am Acad Dermatol. 2020;82:72-79. doi:10.1016/j.jaad.2019.06.025
- Lee YH, Scharnitz TP, Muscat J, et al. Laboratory monitoring during isotretinoin therapy for acne: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:35-44. doi:10.1001/jamadermatol.2015.3091
- Xia E, Han J, Faletsky A, et al. Isotretinoin laboratory monitoring in acne treatment: a Delphi consensus study. JAMA Dermatol. 2022;158:942-948. doi:10.1001/jamadermatol.2022.2044
- Affleck A, Jackson D, Williams HC, et al. Is routine laboratory testing in healthy young patients taking isotretinoin necessary: a critically appraised topic. Br J Dermatol. 2022;187:857-865. doi:10.1111/bjd.21840
- Barbieri JS, LaChance A, Albrecht J. Double standards and inconsistencies in access to care-what constitutes a cosmetic treatment? JAMA Dermatol. 2023;159:245-246. doi:10.1001/jamadermatol.2022.6322
- Trish E, Van Nuys K, Popovian R. US consumers overpay for generic drugs. Schaeffer Center White Paper Series. May 31, 2022. doi:10.25549/m589-2268
Practice Points
- For mild to moderate acne, fixed-dose combination adapalene–benzoyl peroxide and clindamycin–benzoyl peroxide are highly cost-effective options for most patients.
- For moderate to severe acne, doxycycline or hormonal therapy (ie, combined oral contraceptives, spironolactone) are highly cost-effective options.
- Reduction of laboratory monitoring for spironolactone and isotretinoin is an opportunity to provide higher-value care.
Analysis reveals recent acne prescribing trends
While.
Notably, isotretinoin prescribing among men and women decreased slightly during the study period, “which may reflect ongoing administrative burdens associated with iPLEDGE,” study author John S. Barbieri, MD, MBA, of the department of dermatology, at Brigham and Women’s Hospital, Boston, told this news organization.
For the cross-sectional study, which was published online as a research letter in JAMA Dermatology, Dr. Barbieri drew from the Truven Health MarketScan Commercial Claims Database from Jan. 1, 2017, to Dec. 31, 2020, to identify individuals with an encounter for acne, prescriptions for oral tetracycline antibiotics (doxycycline, minocycline), other commonly prescribed oral antibiotics (trimethoprim-sulfamethoxazole, amoxicillin, cephalexin), spironolactone, and isotretinoin. Only drug courses greater than 28 days were included in the analysis, and Dr. Barbieri stratified them according to clinician type (dermatologist, nondermatology physician, and nurse-practitioner or physician assistant). To normalize prescribing rates (to address possible changes in the number of patients treated for acne over time), the number of treatment courses prescribed each year was standardized to the number of encounters for acne with that clinician type during the same calendar year.
The study period included a mean of 1.9 million acne encounters per year.
Dr. Barbieri found that dermatologists prescribed more oral antibiotics per clinician for acne than any other major medical specialty and that oral antibiotics remained frequently prescribed for treating acne by both dermatologists and nondermatologists. “Among oral antibiotics, minocycline and trimethoprim-sulfamethoxazole remain relatively commonly prescribed, despite potential safety concerns and a lack of evidence that they are any more effective than doxycycline,” he said in an interview.
“Patient outcomes could likely be improved by reducing use of minocycline and particularly trimethoprim-sulfamethoxazole given its high risk of serious side effects such as SJS/TEN [Stevens-Johnson syndrome/toxic epidermal necrolysis] and acute respiratory failure,” he added.
Dr. Barbieri noted that there are likely opportunities to consider nonantibiotic alternatives such as hormonal therapy (spironolactone, combined oral contraceptives) and isotretinoin. “There is also a need for continued research to identify nonantibiotic treatment options for patients with acne,” he said.
The analysis revealed that for women with acne prescriptions for spironolactone increased about three- to fourfold during the study period among all clinician types. In 2017, oral antibiotics were prescribed about two- to threefold more often than spironolactone, but by 2020 they were being prescribed at about the same frequency. “Given spironolactone may have similar effectiveness to oral antibiotics in the treatment of acne, this shift in practice has the potential to improve outcomes for patients by reducing the risk of antibiotic-associated complications,” Dr. Barbieri wrote. Still, in 2020, oral antibiotics were still slightly more commonly prescribed than spironolactone by nondermatology physicians and NP or PAs.
In other findings, isotretinoin prescribing decreased slightly among male and female patients during the study period. Among antibiotic prescriptions, prescribing for doxycycline increased at a higher rate than prescribing for minocycline, especially among dermatologists and NPs or PAs.
In the interview, Dr. Barbieri acknowledged certain limitations of the study, including the fact that the dataset “does not allow for evaluation of severity of acne and it is not possible to directly link prescriptions to diagnoses, so some prescriptions might not be for acne and others that are for acne might not have been included.”
Lawrence J. Green, MD, of the department of dermatology at George Washington University, Washington, who was asked to comment on the results, said that, while a course of antibiotic therapy was tied to an office visit in the analysis, the duration of each course of therapy was unclear. It would be interesting to see if antibiotic courses became shorter during the time period analyzed, such as 1-3 months versus 4 or more months, he added, “as this should reduce risks associated with long-term use of oral antibiotics.”
Dr. Barbieri reported personal fees from Dexcel Pharma for consulting outside the submitted work. Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
While.
Notably, isotretinoin prescribing among men and women decreased slightly during the study period, “which may reflect ongoing administrative burdens associated with iPLEDGE,” study author John S. Barbieri, MD, MBA, of the department of dermatology, at Brigham and Women’s Hospital, Boston, told this news organization.
For the cross-sectional study, which was published online as a research letter in JAMA Dermatology, Dr. Barbieri drew from the Truven Health MarketScan Commercial Claims Database from Jan. 1, 2017, to Dec. 31, 2020, to identify individuals with an encounter for acne, prescriptions for oral tetracycline antibiotics (doxycycline, minocycline), other commonly prescribed oral antibiotics (trimethoprim-sulfamethoxazole, amoxicillin, cephalexin), spironolactone, and isotretinoin. Only drug courses greater than 28 days were included in the analysis, and Dr. Barbieri stratified them according to clinician type (dermatologist, nondermatology physician, and nurse-practitioner or physician assistant). To normalize prescribing rates (to address possible changes in the number of patients treated for acne over time), the number of treatment courses prescribed each year was standardized to the number of encounters for acne with that clinician type during the same calendar year.
The study period included a mean of 1.9 million acne encounters per year.
Dr. Barbieri found that dermatologists prescribed more oral antibiotics per clinician for acne than any other major medical specialty and that oral antibiotics remained frequently prescribed for treating acne by both dermatologists and nondermatologists. “Among oral antibiotics, minocycline and trimethoprim-sulfamethoxazole remain relatively commonly prescribed, despite potential safety concerns and a lack of evidence that they are any more effective than doxycycline,” he said in an interview.
“Patient outcomes could likely be improved by reducing use of minocycline and particularly trimethoprim-sulfamethoxazole given its high risk of serious side effects such as SJS/TEN [Stevens-Johnson syndrome/toxic epidermal necrolysis] and acute respiratory failure,” he added.
Dr. Barbieri noted that there are likely opportunities to consider nonantibiotic alternatives such as hormonal therapy (spironolactone, combined oral contraceptives) and isotretinoin. “There is also a need for continued research to identify nonantibiotic treatment options for patients with acne,” he said.
The analysis revealed that for women with acne prescriptions for spironolactone increased about three- to fourfold during the study period among all clinician types. In 2017, oral antibiotics were prescribed about two- to threefold more often than spironolactone, but by 2020 they were being prescribed at about the same frequency. “Given spironolactone may have similar effectiveness to oral antibiotics in the treatment of acne, this shift in practice has the potential to improve outcomes for patients by reducing the risk of antibiotic-associated complications,” Dr. Barbieri wrote. Still, in 2020, oral antibiotics were still slightly more commonly prescribed than spironolactone by nondermatology physicians and NP or PAs.
In other findings, isotretinoin prescribing decreased slightly among male and female patients during the study period. Among antibiotic prescriptions, prescribing for doxycycline increased at a higher rate than prescribing for minocycline, especially among dermatologists and NPs or PAs.
In the interview, Dr. Barbieri acknowledged certain limitations of the study, including the fact that the dataset “does not allow for evaluation of severity of acne and it is not possible to directly link prescriptions to diagnoses, so some prescriptions might not be for acne and others that are for acne might not have been included.”
Lawrence J. Green, MD, of the department of dermatology at George Washington University, Washington, who was asked to comment on the results, said that, while a course of antibiotic therapy was tied to an office visit in the analysis, the duration of each course of therapy was unclear. It would be interesting to see if antibiotic courses became shorter during the time period analyzed, such as 1-3 months versus 4 or more months, he added, “as this should reduce risks associated with long-term use of oral antibiotics.”
Dr. Barbieri reported personal fees from Dexcel Pharma for consulting outside the submitted work. Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
While.
Notably, isotretinoin prescribing among men and women decreased slightly during the study period, “which may reflect ongoing administrative burdens associated with iPLEDGE,” study author John S. Barbieri, MD, MBA, of the department of dermatology, at Brigham and Women’s Hospital, Boston, told this news organization.
For the cross-sectional study, which was published online as a research letter in JAMA Dermatology, Dr. Barbieri drew from the Truven Health MarketScan Commercial Claims Database from Jan. 1, 2017, to Dec. 31, 2020, to identify individuals with an encounter for acne, prescriptions for oral tetracycline antibiotics (doxycycline, minocycline), other commonly prescribed oral antibiotics (trimethoprim-sulfamethoxazole, amoxicillin, cephalexin), spironolactone, and isotretinoin. Only drug courses greater than 28 days were included in the analysis, and Dr. Barbieri stratified them according to clinician type (dermatologist, nondermatology physician, and nurse-practitioner or physician assistant). To normalize prescribing rates (to address possible changes in the number of patients treated for acne over time), the number of treatment courses prescribed each year was standardized to the number of encounters for acne with that clinician type during the same calendar year.
The study period included a mean of 1.9 million acne encounters per year.
Dr. Barbieri found that dermatologists prescribed more oral antibiotics per clinician for acne than any other major medical specialty and that oral antibiotics remained frequently prescribed for treating acne by both dermatologists and nondermatologists. “Among oral antibiotics, minocycline and trimethoprim-sulfamethoxazole remain relatively commonly prescribed, despite potential safety concerns and a lack of evidence that they are any more effective than doxycycline,” he said in an interview.
“Patient outcomes could likely be improved by reducing use of minocycline and particularly trimethoprim-sulfamethoxazole given its high risk of serious side effects such as SJS/TEN [Stevens-Johnson syndrome/toxic epidermal necrolysis] and acute respiratory failure,” he added.
Dr. Barbieri noted that there are likely opportunities to consider nonantibiotic alternatives such as hormonal therapy (spironolactone, combined oral contraceptives) and isotretinoin. “There is also a need for continued research to identify nonantibiotic treatment options for patients with acne,” he said.
The analysis revealed that for women with acne prescriptions for spironolactone increased about three- to fourfold during the study period among all clinician types. In 2017, oral antibiotics were prescribed about two- to threefold more often than spironolactone, but by 2020 they were being prescribed at about the same frequency. “Given spironolactone may have similar effectiveness to oral antibiotics in the treatment of acne, this shift in practice has the potential to improve outcomes for patients by reducing the risk of antibiotic-associated complications,” Dr. Barbieri wrote. Still, in 2020, oral antibiotics were still slightly more commonly prescribed than spironolactone by nondermatology physicians and NP or PAs.
In other findings, isotretinoin prescribing decreased slightly among male and female patients during the study period. Among antibiotic prescriptions, prescribing for doxycycline increased at a higher rate than prescribing for minocycline, especially among dermatologists and NPs or PAs.
In the interview, Dr. Barbieri acknowledged certain limitations of the study, including the fact that the dataset “does not allow for evaluation of severity of acne and it is not possible to directly link prescriptions to diagnoses, so some prescriptions might not be for acne and others that are for acne might not have been included.”
Lawrence J. Green, MD, of the department of dermatology at George Washington University, Washington, who was asked to comment on the results, said that, while a course of antibiotic therapy was tied to an office visit in the analysis, the duration of each course of therapy was unclear. It would be interesting to see if antibiotic courses became shorter during the time period analyzed, such as 1-3 months versus 4 or more months, he added, “as this should reduce risks associated with long-term use of oral antibiotics.”
Dr. Barbieri reported personal fees from Dexcel Pharma for consulting outside the submitted work. Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
FROM JAMA DERMATOLOGY
Understanding Medical Standards for Entrance Into Military Service and Disqualifying Dermatologic Conditions
Purpose of Medical Standards in the US Military
Young adults in the United States traditionally have viewed military service as a viable career given its stable salary, career training, opportunities for progression, comprehensive health care coverage, tuition assistance, and other benefits; however, not all who desire to serve in the US Military are eligible to join. The Department of Defense (DoD) maintains fitness and health requirements (ie, accession standards), which are codified in DoD Instruction 6130.03, Volume 1,1 that help ensure potential recruits can safely and fully perform their military duties. These accession standards change over time with the evolving understanding of diseases, medical advances, and accrued experience conducting operations in various environments. Accession standards serve to both preserve the health of the applicant and to ensure military mission success.
Dermatologic diseases have been prevalent in conflicts throughout US military history, representing a considerable source of morbidity to service members, inability of service members to remain on active duty, and costly use of resources. Hospitalizations of US Army soldiers for skin conditions led to the loss of more than 2 million days of service in World War I.2 In World War II, skin diseases made up 25% and 75% of all temperate and tropical climate visits, respectively. Cutaneous diseases were the most frequently addressed category for US service members in Vietnam, representing more than 1.5 million visits and nearly 10% of disease-related evacuations.2 Skin disease remains vital in 21st-century conflict. At a military hospital in Afghanistan, a review of 2421 outpatient medical records from June through July 2007 identified that dermatologic conditions resulted in 20% of military patient evaluations, 7% of nontraumatic hospital admissions, and 2% of total patient evacuations, at an estimated cost of $80,000 per evacuee.3 Between 2003 and 2006, 918 service members were evacuated for dermatologic reasons from combat zones in Afghanistan and Iraq.4
Unpredictable military environments may result in flares of a previously controlled condition, new skin diseases, or infection with endemic diseases. Mild cases of common conditions such as psoriasis or atopic dermatitis can present an unacceptable risk for severe flare in the setting of deployed military operations.5 Personnel may face extremes in temperature and humidity and work long hours under stress with limited or nonexistent opportunities for hygiene or self-care. Shared equipment and close living quarters permit the spread of infectious diseases and complicate the treatment of infestations. Military equipment and supplies such as gas masks and insect repellents can contain compounds that act as irritants or sensitizing agents, leading to contact dermatitis or urticaria. When dermatologic conditions develop or flare, further challenges are associated with evaluation and management. Health care resources vary considerably by location, with potential limitations in the availability of medications; supplies; refrigeration capabilities; and laboratory, microbiology, and histology services. Furthermore, dermatology referrals and services typically are not feasible in most deployed settings,3 though teledermatology has been available in the armed forces since 2002.
Deployed environments compound the consequences of dermatologic conditions and can impact the military mission. Military units deploy with the number of personnel needed to complete a mission and cannot replace members who become ill or injured or are medically evacuated. Something seemingly trivial, such as poor sleep due to pruritic dermatitis, may impair daytime alertness with potentially grave consequences in critical tasks such as guard or flying duties. The evacuation of a service member can compromise those left behind, and losing a service member with a unique required skill set may jeopardize a unit’s chance of success. Additionally, the impact of an evacuation itself extends beyond its direct cost and effects on the service member’s unit. The military does not maintain dedicated medical evacuation aircraft, instead repurposing aircraft in the deployed setting as needed.6 Evacuations can delay flights initially scheduled to move troops, ammunition, food, or other supplies and equipment elsewhere.
Disqualifying Skin and Soft Tissue Conditions
Current accession standards, which are listed in a publicly released document (DoD Instruction 6130.03, Volume 1), are updated based on medical, societal, and technical advances.1 These standards differ from retention standards, which apply to members actively serving in the military. Although the DoD creates a minimum standard for the entire military, the US Army, Navy, and Air Force adopt these standards and adjust as required for each branch’s needs. An updated copy can be found on the DoD Directives Division website (https://www.esd.whs.mil/dd/) or Med Standards, a third-party mobile application (app) available as a free download for Apple iOS and Android devices (https://www.doc-apps.com/). The app also includes each military branch’s interpretation of the requirements.
The accession standards outline medical conditions that, if present or verified in an applicant’s medical history, preclude joining the military (eTable). These standards are organized into general systems, with a section dedicated to dermatologic (skin and soft tissue) conditions.1 When a candidate has a potentially disqualifying medical condition identified by a screening questionnaire, medical record review, or military entrance physical examination, a referral for a determination of fitness for duty may be required. Medical accession standards are not solely driven by the diagnosis but also by the extent, nature, and timing of medical management. Procedures or prescriptions requiring frequent clinical monitoring, special handling, or severe dietary restrictions may deem the applicant’s condition potentially unsuitable. The need for immunosuppressive, anticoagulant, or refrigerated medications can impact a patient’s eligibility due to future deployment requirements and suitability for prolonged service, especially if treated for any substantial length of time. Chronic dermatologic conditions that are unresponsive to treatment, are susceptible to exacerbation despite treatment, require regular follow-up care, or interfere with the wear of military gear may be inconsistent with future deployment standards. Although the dermatologist should primarily focus on the skin and soft tissue conditions section of the accession standards, some dermatologic conditions can overlap with other medical systems and be located in a different section; for example, the section on lower extremity conditions includes a disqualifying condition of “[c]urrent ingrown toenails, if infected or symptomatic.”1
Waiver Process
Medical conditions listed in the accession standards are deemed ineligible for military service; however, applicants can apply for a waiver.1 The goal is for service members to be well controlled without treatment or with treatment widely available at military clinics and hospitals. Waivers ensure that service members are “[m]edically capable of performing duties without aggravating physical defects or medical conditions,” are “[m]edically adaptable to the military environment without geographical area limitations,” and are “free of medical conditions or physical defects that may reasonably be expected to require excessive time lost from duty for necessary treatment or hospitalization, or may result in separation from the Military Service for unfitness.”1 The waiver process requires an evaluation from specialists with verification and documentation but does not guarantee approval. Although each military branch follows the same guidelines for disqualifying medical conditions, the evaluation and waiver process varies.
Considerations for Civilian Dermatologists
For several reasons, accurate and detailed medical documentation is essential for patients who pursue military service. Applicants must complete detailed health questionnaires and may need to provide copies of health records. The military electronic health record connects to large civilian health information exchanges and pulls primary documentation from records at many hospitals and clinics. Although applicants may request supportive clarification from their dermatologists, the military relies on primary medical documentation throughout the recruitment process. Accurate diagnostic codes reduce ambiguity, as accession standards are organized by diagnosis; for example, an unspecified history of psoriasis disqualifies applicants unless documentation supports nonrecurrent childhood guttate psoriasis.1 Clear documentation of symptom severity, response to treatment, or resolution of a condition may elucidate suitability for service when matching a potentially disqualifying condition to a standard is not straightforward. Correct documentation will ensure that potential service members achieve a waiver when it is appropriate. If they are found to be unfit, it may save a patient from a bad outcome or a military unit from mission failure.
Dermatologists in the United States can reference current military medical accession standards to guide patients when needed. For example, a prospective recruit may be hesitant to start isotretinoin for severe nodulocystic acne, concerned that this medication may preclude them from joining the military. The current standards state that “[a]pplicants under treatment with systemic retinoids . . . do not meet the standard until 4 weeks after completing therapy,” while active severe nodulocystic acne is a disqualifying condition.1 Therefore, the patient could proceed with isotretinoin therapy and, pending clinical response, meet accession standards as soon as 4 weeks after treatment. A clear understanding of the purpose of these standards, including protecting the applicant’s health and maximizing the chance of combat mission accomplishment, helps to reinforce responsibilities when caring for patients who wish to serve.


- US Department of Defense. DoD Instruction 6130.03, Volume 1. Medical Standards for Military Service: Appointment, Enlistment, or Induction. Updated November 16, 2022. Accessed May 22, 2023. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003_vol1.PDF?ver=7fhqacc0jGX_R9_1iexudA%3D%3D
- Becker LE, James WD. Historical overview and principles of diagnosis. In: Becker LE, James WD. Military Dermatology. Office of the Surgeon General, US Department of the Army; 1994: 1-20.
- Arnold JG, Michener MD. Evaluation of dermatologic conditions by primary care providers in deployed military settings. Mil Med. 2008;173:882-888. doi:10.7205/MILMED.173.9.882
- McGraw TA, Norton SA. Military aeromedical evacuations from central and southwest Asia for ill-defined dermatologic diseases. Arch Dermatol. 2009;145:165-170.
- Gelman AB, Norton SA, Valdes-Rodriguez R, et al. A review of skin conditions in modern warfare and peacekeeping operations. Mil Med. 2015;180:32-37.
- Fang R, Dorlac GR, Allan PF, et al. Intercontinental aeromedical evacuation of patients with traumatic brain injuries during Operations Iraqi Freedom and Enduring Freedom. Neurosurg Focus. 2010;28:E11.
Purpose of Medical Standards in the US Military
Young adults in the United States traditionally have viewed military service as a viable career given its stable salary, career training, opportunities for progression, comprehensive health care coverage, tuition assistance, and other benefits; however, not all who desire to serve in the US Military are eligible to join. The Department of Defense (DoD) maintains fitness and health requirements (ie, accession standards), which are codified in DoD Instruction 6130.03, Volume 1,1 that help ensure potential recruits can safely and fully perform their military duties. These accession standards change over time with the evolving understanding of diseases, medical advances, and accrued experience conducting operations in various environments. Accession standards serve to both preserve the health of the applicant and to ensure military mission success.
Dermatologic diseases have been prevalent in conflicts throughout US military history, representing a considerable source of morbidity to service members, inability of service members to remain on active duty, and costly use of resources. Hospitalizations of US Army soldiers for skin conditions led to the loss of more than 2 million days of service in World War I.2 In World War II, skin diseases made up 25% and 75% of all temperate and tropical climate visits, respectively. Cutaneous diseases were the most frequently addressed category for US service members in Vietnam, representing more than 1.5 million visits and nearly 10% of disease-related evacuations.2 Skin disease remains vital in 21st-century conflict. At a military hospital in Afghanistan, a review of 2421 outpatient medical records from June through July 2007 identified that dermatologic conditions resulted in 20% of military patient evaluations, 7% of nontraumatic hospital admissions, and 2% of total patient evacuations, at an estimated cost of $80,000 per evacuee.3 Between 2003 and 2006, 918 service members were evacuated for dermatologic reasons from combat zones in Afghanistan and Iraq.4
Unpredictable military environments may result in flares of a previously controlled condition, new skin diseases, or infection with endemic diseases. Mild cases of common conditions such as psoriasis or atopic dermatitis can present an unacceptable risk for severe flare in the setting of deployed military operations.5 Personnel may face extremes in temperature and humidity and work long hours under stress with limited or nonexistent opportunities for hygiene or self-care. Shared equipment and close living quarters permit the spread of infectious diseases and complicate the treatment of infestations. Military equipment and supplies such as gas masks and insect repellents can contain compounds that act as irritants or sensitizing agents, leading to contact dermatitis or urticaria. When dermatologic conditions develop or flare, further challenges are associated with evaluation and management. Health care resources vary considerably by location, with potential limitations in the availability of medications; supplies; refrigeration capabilities; and laboratory, microbiology, and histology services. Furthermore, dermatology referrals and services typically are not feasible in most deployed settings,3 though teledermatology has been available in the armed forces since 2002.
Deployed environments compound the consequences of dermatologic conditions and can impact the military mission. Military units deploy with the number of personnel needed to complete a mission and cannot replace members who become ill or injured or are medically evacuated. Something seemingly trivial, such as poor sleep due to pruritic dermatitis, may impair daytime alertness with potentially grave consequences in critical tasks such as guard or flying duties. The evacuation of a service member can compromise those left behind, and losing a service member with a unique required skill set may jeopardize a unit’s chance of success. Additionally, the impact of an evacuation itself extends beyond its direct cost and effects on the service member’s unit. The military does not maintain dedicated medical evacuation aircraft, instead repurposing aircraft in the deployed setting as needed.6 Evacuations can delay flights initially scheduled to move troops, ammunition, food, or other supplies and equipment elsewhere.
Disqualifying Skin and Soft Tissue Conditions
Current accession standards, which are listed in a publicly released document (DoD Instruction 6130.03, Volume 1), are updated based on medical, societal, and technical advances.1 These standards differ from retention standards, which apply to members actively serving in the military. Although the DoD creates a minimum standard for the entire military, the US Army, Navy, and Air Force adopt these standards and adjust as required for each branch’s needs. An updated copy can be found on the DoD Directives Division website (https://www.esd.whs.mil/dd/) or Med Standards, a third-party mobile application (app) available as a free download for Apple iOS and Android devices (https://www.doc-apps.com/). The app also includes each military branch’s interpretation of the requirements.
The accession standards outline medical conditions that, if present or verified in an applicant’s medical history, preclude joining the military (eTable). These standards are organized into general systems, with a section dedicated to dermatologic (skin and soft tissue) conditions.1 When a candidate has a potentially disqualifying medical condition identified by a screening questionnaire, medical record review, or military entrance physical examination, a referral for a determination of fitness for duty may be required. Medical accession standards are not solely driven by the diagnosis but also by the extent, nature, and timing of medical management. Procedures or prescriptions requiring frequent clinical monitoring, special handling, or severe dietary restrictions may deem the applicant’s condition potentially unsuitable. The need for immunosuppressive, anticoagulant, or refrigerated medications can impact a patient’s eligibility due to future deployment requirements and suitability for prolonged service, especially if treated for any substantial length of time. Chronic dermatologic conditions that are unresponsive to treatment, are susceptible to exacerbation despite treatment, require regular follow-up care, or interfere with the wear of military gear may be inconsistent with future deployment standards. Although the dermatologist should primarily focus on the skin and soft tissue conditions section of the accession standards, some dermatologic conditions can overlap with other medical systems and be located in a different section; for example, the section on lower extremity conditions includes a disqualifying condition of “[c]urrent ingrown toenails, if infected or symptomatic.”1
Waiver Process
Medical conditions listed in the accession standards are deemed ineligible for military service; however, applicants can apply for a waiver.1 The goal is for service members to be well controlled without treatment or with treatment widely available at military clinics and hospitals. Waivers ensure that service members are “[m]edically capable of performing duties without aggravating physical defects or medical conditions,” are “[m]edically adaptable to the military environment without geographical area limitations,” and are “free of medical conditions or physical defects that may reasonably be expected to require excessive time lost from duty for necessary treatment or hospitalization, or may result in separation from the Military Service for unfitness.”1 The waiver process requires an evaluation from specialists with verification and documentation but does not guarantee approval. Although each military branch follows the same guidelines for disqualifying medical conditions, the evaluation and waiver process varies.
Considerations for Civilian Dermatologists
For several reasons, accurate and detailed medical documentation is essential for patients who pursue military service. Applicants must complete detailed health questionnaires and may need to provide copies of health records. The military electronic health record connects to large civilian health information exchanges and pulls primary documentation from records at many hospitals and clinics. Although applicants may request supportive clarification from their dermatologists, the military relies on primary medical documentation throughout the recruitment process. Accurate diagnostic codes reduce ambiguity, as accession standards are organized by diagnosis; for example, an unspecified history of psoriasis disqualifies applicants unless documentation supports nonrecurrent childhood guttate psoriasis.1 Clear documentation of symptom severity, response to treatment, or resolution of a condition may elucidate suitability for service when matching a potentially disqualifying condition to a standard is not straightforward. Correct documentation will ensure that potential service members achieve a waiver when it is appropriate. If they are found to be unfit, it may save a patient from a bad outcome or a military unit from mission failure.
Dermatologists in the United States can reference current military medical accession standards to guide patients when needed. For example, a prospective recruit may be hesitant to start isotretinoin for severe nodulocystic acne, concerned that this medication may preclude them from joining the military. The current standards state that “[a]pplicants under treatment with systemic retinoids . . . do not meet the standard until 4 weeks after completing therapy,” while active severe nodulocystic acne is a disqualifying condition.1 Therefore, the patient could proceed with isotretinoin therapy and, pending clinical response, meet accession standards as soon as 4 weeks after treatment. A clear understanding of the purpose of these standards, including protecting the applicant’s health and maximizing the chance of combat mission accomplishment, helps to reinforce responsibilities when caring for patients who wish to serve.


Purpose of Medical Standards in the US Military
Young adults in the United States traditionally have viewed military service as a viable career given its stable salary, career training, opportunities for progression, comprehensive health care coverage, tuition assistance, and other benefits; however, not all who desire to serve in the US Military are eligible to join. The Department of Defense (DoD) maintains fitness and health requirements (ie, accession standards), which are codified in DoD Instruction 6130.03, Volume 1,1 that help ensure potential recruits can safely and fully perform their military duties. These accession standards change over time with the evolving understanding of diseases, medical advances, and accrued experience conducting operations in various environments. Accession standards serve to both preserve the health of the applicant and to ensure military mission success.
Dermatologic diseases have been prevalent in conflicts throughout US military history, representing a considerable source of morbidity to service members, inability of service members to remain on active duty, and costly use of resources. Hospitalizations of US Army soldiers for skin conditions led to the loss of more than 2 million days of service in World War I.2 In World War II, skin diseases made up 25% and 75% of all temperate and tropical climate visits, respectively. Cutaneous diseases were the most frequently addressed category for US service members in Vietnam, representing more than 1.5 million visits and nearly 10% of disease-related evacuations.2 Skin disease remains vital in 21st-century conflict. At a military hospital in Afghanistan, a review of 2421 outpatient medical records from June through July 2007 identified that dermatologic conditions resulted in 20% of military patient evaluations, 7% of nontraumatic hospital admissions, and 2% of total patient evacuations, at an estimated cost of $80,000 per evacuee.3 Between 2003 and 2006, 918 service members were evacuated for dermatologic reasons from combat zones in Afghanistan and Iraq.4
Unpredictable military environments may result in flares of a previously controlled condition, new skin diseases, or infection with endemic diseases. Mild cases of common conditions such as psoriasis or atopic dermatitis can present an unacceptable risk for severe flare in the setting of deployed military operations.5 Personnel may face extremes in temperature and humidity and work long hours under stress with limited or nonexistent opportunities for hygiene or self-care. Shared equipment and close living quarters permit the spread of infectious diseases and complicate the treatment of infestations. Military equipment and supplies such as gas masks and insect repellents can contain compounds that act as irritants or sensitizing agents, leading to contact dermatitis or urticaria. When dermatologic conditions develop or flare, further challenges are associated with evaluation and management. Health care resources vary considerably by location, with potential limitations in the availability of medications; supplies; refrigeration capabilities; and laboratory, microbiology, and histology services. Furthermore, dermatology referrals and services typically are not feasible in most deployed settings,3 though teledermatology has been available in the armed forces since 2002.
Deployed environments compound the consequences of dermatologic conditions and can impact the military mission. Military units deploy with the number of personnel needed to complete a mission and cannot replace members who become ill or injured or are medically evacuated. Something seemingly trivial, such as poor sleep due to pruritic dermatitis, may impair daytime alertness with potentially grave consequences in critical tasks such as guard or flying duties. The evacuation of a service member can compromise those left behind, and losing a service member with a unique required skill set may jeopardize a unit’s chance of success. Additionally, the impact of an evacuation itself extends beyond its direct cost and effects on the service member’s unit. The military does not maintain dedicated medical evacuation aircraft, instead repurposing aircraft in the deployed setting as needed.6 Evacuations can delay flights initially scheduled to move troops, ammunition, food, or other supplies and equipment elsewhere.
Disqualifying Skin and Soft Tissue Conditions
Current accession standards, which are listed in a publicly released document (DoD Instruction 6130.03, Volume 1), are updated based on medical, societal, and technical advances.1 These standards differ from retention standards, which apply to members actively serving in the military. Although the DoD creates a minimum standard for the entire military, the US Army, Navy, and Air Force adopt these standards and adjust as required for each branch’s needs. An updated copy can be found on the DoD Directives Division website (https://www.esd.whs.mil/dd/) or Med Standards, a third-party mobile application (app) available as a free download for Apple iOS and Android devices (https://www.doc-apps.com/). The app also includes each military branch’s interpretation of the requirements.
The accession standards outline medical conditions that, if present or verified in an applicant’s medical history, preclude joining the military (eTable). These standards are organized into general systems, with a section dedicated to dermatologic (skin and soft tissue) conditions.1 When a candidate has a potentially disqualifying medical condition identified by a screening questionnaire, medical record review, or military entrance physical examination, a referral for a determination of fitness for duty may be required. Medical accession standards are not solely driven by the diagnosis but also by the extent, nature, and timing of medical management. Procedures or prescriptions requiring frequent clinical monitoring, special handling, or severe dietary restrictions may deem the applicant’s condition potentially unsuitable. The need for immunosuppressive, anticoagulant, or refrigerated medications can impact a patient’s eligibility due to future deployment requirements and suitability for prolonged service, especially if treated for any substantial length of time. Chronic dermatologic conditions that are unresponsive to treatment, are susceptible to exacerbation despite treatment, require regular follow-up care, or interfere with the wear of military gear may be inconsistent with future deployment standards. Although the dermatologist should primarily focus on the skin and soft tissue conditions section of the accession standards, some dermatologic conditions can overlap with other medical systems and be located in a different section; for example, the section on lower extremity conditions includes a disqualifying condition of “[c]urrent ingrown toenails, if infected or symptomatic.”1
Waiver Process
Medical conditions listed in the accession standards are deemed ineligible for military service; however, applicants can apply for a waiver.1 The goal is for service members to be well controlled without treatment or with treatment widely available at military clinics and hospitals. Waivers ensure that service members are “[m]edically capable of performing duties without aggravating physical defects or medical conditions,” are “[m]edically adaptable to the military environment without geographical area limitations,” and are “free of medical conditions or physical defects that may reasonably be expected to require excessive time lost from duty for necessary treatment or hospitalization, or may result in separation from the Military Service for unfitness.”1 The waiver process requires an evaluation from specialists with verification and documentation but does not guarantee approval. Although each military branch follows the same guidelines for disqualifying medical conditions, the evaluation and waiver process varies.
Considerations for Civilian Dermatologists
For several reasons, accurate and detailed medical documentation is essential for patients who pursue military service. Applicants must complete detailed health questionnaires and may need to provide copies of health records. The military electronic health record connects to large civilian health information exchanges and pulls primary documentation from records at many hospitals and clinics. Although applicants may request supportive clarification from their dermatologists, the military relies on primary medical documentation throughout the recruitment process. Accurate diagnostic codes reduce ambiguity, as accession standards are organized by diagnosis; for example, an unspecified history of psoriasis disqualifies applicants unless documentation supports nonrecurrent childhood guttate psoriasis.1 Clear documentation of symptom severity, response to treatment, or resolution of a condition may elucidate suitability for service when matching a potentially disqualifying condition to a standard is not straightforward. Correct documentation will ensure that potential service members achieve a waiver when it is appropriate. If they are found to be unfit, it may save a patient from a bad outcome or a military unit from mission failure.
Dermatologists in the United States can reference current military medical accession standards to guide patients when needed. For example, a prospective recruit may be hesitant to start isotretinoin for severe nodulocystic acne, concerned that this medication may preclude them from joining the military. The current standards state that “[a]pplicants under treatment with systemic retinoids . . . do not meet the standard until 4 weeks after completing therapy,” while active severe nodulocystic acne is a disqualifying condition.1 Therefore, the patient could proceed with isotretinoin therapy and, pending clinical response, meet accession standards as soon as 4 weeks after treatment. A clear understanding of the purpose of these standards, including protecting the applicant’s health and maximizing the chance of combat mission accomplishment, helps to reinforce responsibilities when caring for patients who wish to serve.


- US Department of Defense. DoD Instruction 6130.03, Volume 1. Medical Standards for Military Service: Appointment, Enlistment, or Induction. Updated November 16, 2022. Accessed May 22, 2023. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003_vol1.PDF?ver=7fhqacc0jGX_R9_1iexudA%3D%3D
- Becker LE, James WD. Historical overview and principles of diagnosis. In: Becker LE, James WD. Military Dermatology. Office of the Surgeon General, US Department of the Army; 1994: 1-20.
- Arnold JG, Michener MD. Evaluation of dermatologic conditions by primary care providers in deployed military settings. Mil Med. 2008;173:882-888. doi:10.7205/MILMED.173.9.882
- McGraw TA, Norton SA. Military aeromedical evacuations from central and southwest Asia for ill-defined dermatologic diseases. Arch Dermatol. 2009;145:165-170.
- Gelman AB, Norton SA, Valdes-Rodriguez R, et al. A review of skin conditions in modern warfare and peacekeeping operations. Mil Med. 2015;180:32-37.
- Fang R, Dorlac GR, Allan PF, et al. Intercontinental aeromedical evacuation of patients with traumatic brain injuries during Operations Iraqi Freedom and Enduring Freedom. Neurosurg Focus. 2010;28:E11.
- US Department of Defense. DoD Instruction 6130.03, Volume 1. Medical Standards for Military Service: Appointment, Enlistment, or Induction. Updated November 16, 2022. Accessed May 22, 2023. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003_vol1.PDF?ver=7fhqacc0jGX_R9_1iexudA%3D%3D
- Becker LE, James WD. Historical overview and principles of diagnosis. In: Becker LE, James WD. Military Dermatology. Office of the Surgeon General, US Department of the Army; 1994: 1-20.
- Arnold JG, Michener MD. Evaluation of dermatologic conditions by primary care providers in deployed military settings. Mil Med. 2008;173:882-888. doi:10.7205/MILMED.173.9.882
- McGraw TA, Norton SA. Military aeromedical evacuations from central and southwest Asia for ill-defined dermatologic diseases. Arch Dermatol. 2009;145:165-170.
- Gelman AB, Norton SA, Valdes-Rodriguez R, et al. A review of skin conditions in modern warfare and peacekeeping operations. Mil Med. 2015;180:32-37.
- Fang R, Dorlac GR, Allan PF, et al. Intercontinental aeromedical evacuation of patients with traumatic brain injuries during Operations Iraqi Freedom and Enduring Freedom. Neurosurg Focus. 2010;28:E11.
Practice Points
- Dermatologic diseases have played a substantial role in conflicts throughout US military history, representing a considerable source of morbidity to service members, loss of active-duty service members trained with necessary skills, and costly use of resources.
- The strict standards are designed to protect the health of the individual and maximize mission success.
- The Department of Defense has a publicly available document (DoD Instruction 6130.03, Volume 1) that details conditions that are disqualifying for entrance into the military. Dermatologists can reference this to provide guidance to adolescents and young adults interested in joining the military.
Evaluation of Laboratory Follow-up in Acne Patients Treated With Isotretinoin
Isotretinoin is used in the treatment of nodulocystic and severe papulopustular acne. During the treatment period, laboratory monitoring is recommended to identify the risk for complications such as hepatotoxicity, teratogenicity, rhabdomyolysis, hyperlipidemia, and pancreatitis.1 There is a lack of consensus of the frequency of follow-up of laboratory parameters during isotretinoin treatment. This study evaluated the changes in laboratory parameters used in daily practice for patients with acne who were treated with isotretinoin to determine the optimum test repetition frequency.
Materials and Methods
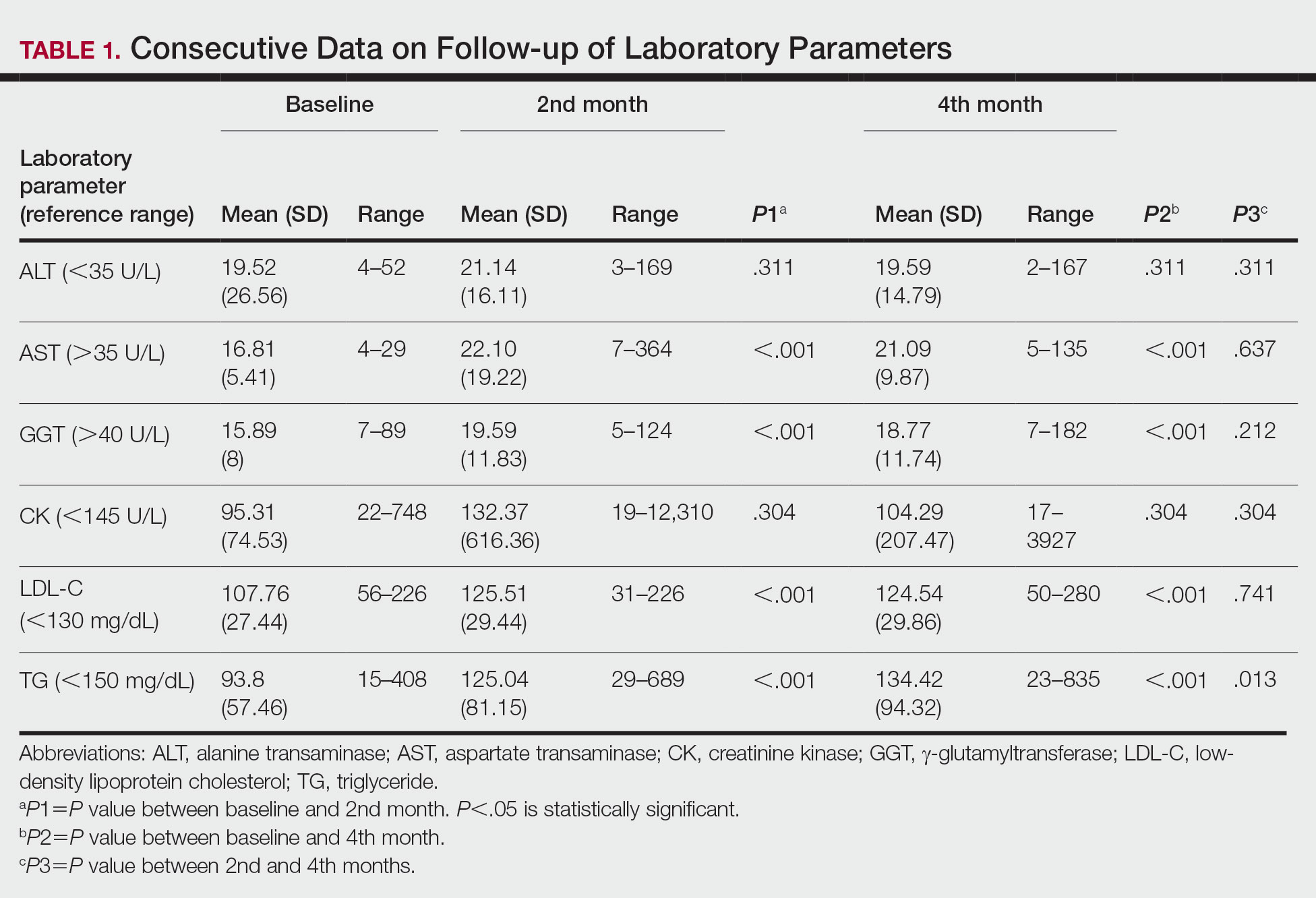
Statistical Analysis—The descriptive statistics of the measurements were presented as means, standard deviations, or medians (first and third quartiles). With respect to the normal distribution, the consistency of the measurements was evaluated with the Kolmogorov-Smirnov test, and small deviations from the normal distribution were observed. Changes in laboratory measurements were evaluated with simple repeated-measures analysis of variance, and changes that differed significantly were determined by a Holm-Sidak post hoc test. Relationships between total cumulative doses and laboratory measurements at second visits were evaluated by the Pearson correlation analysis. The statistical significance level was P<.05. SPSS Statistics 23 (IBM) was used in the calculations.
Results
Consecutive Data at Baseline and Follow-up—A total of 415 patients with a mean age (SD) of 21.49 (7.25) years (range, 12–53 years) were included in our study. The mean total cumulative dose (SD) of the patients was 7267.27 (1878.4) mg. The consecutive data of the means of the laboratory parameters are shown in Table 1 and Figure 1. There was no significant change in the ALT levels between baseline and the fourth month as well as between the second- and fourth-month assessments (both P=.311).
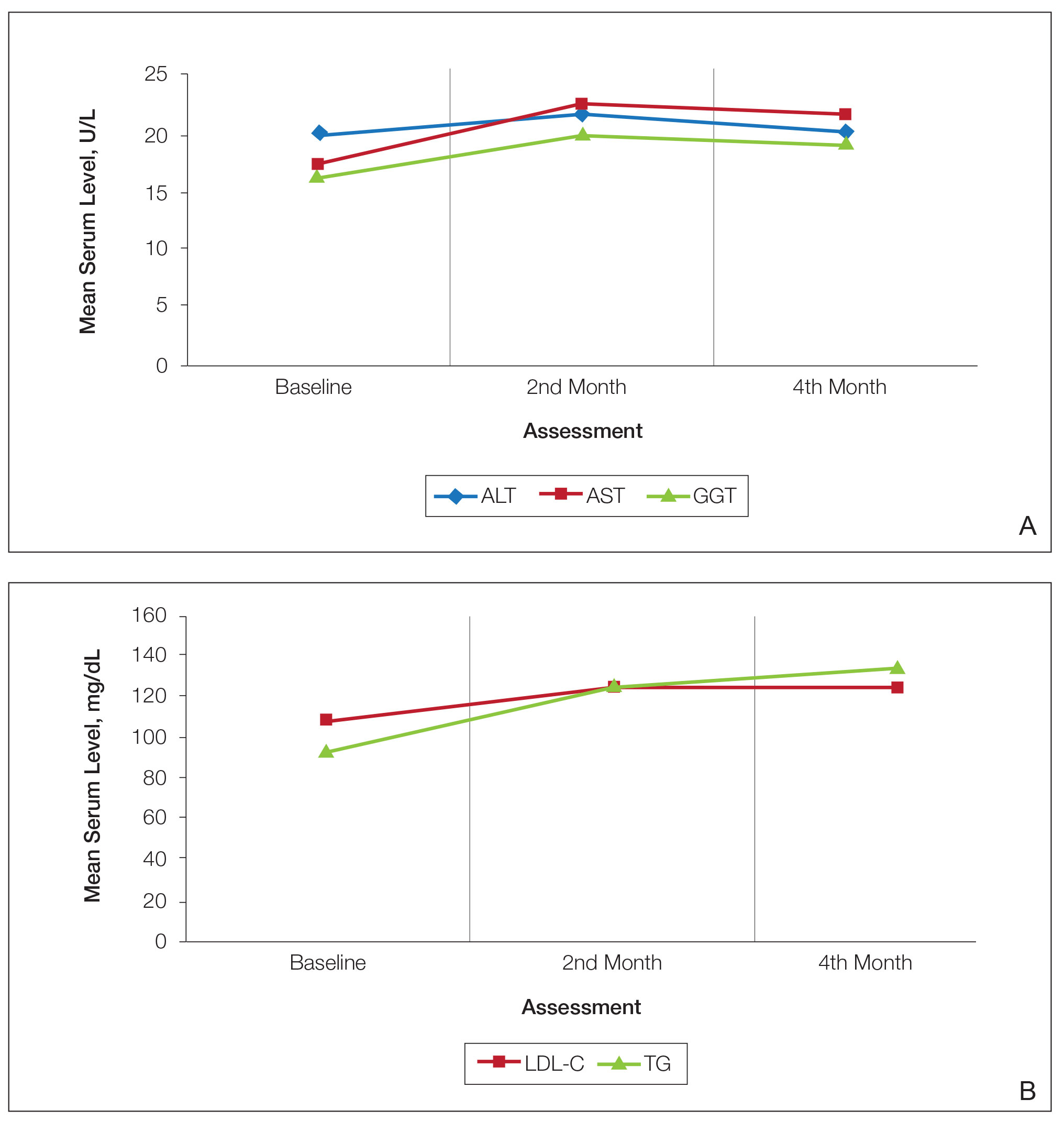
Abnormal Laboratory Measurements—The distribution of abnormal laboratory measurements during treatment is shown in Table 2 and Figure 2. Grade 3 or higher elevations of liver transaminases (ALT, AST) and GGT were observed in fewer than 2% of patients during treatment compared with baseline (grade 3 elevations of ALT and AST together in 2 patients; grade 4 AST elevation in 1 patient; grade 3 elevations of ALT, AST, and GGT combined in 1 patient; isolated grade 3 GGT elevation in 1 patient). All of the patients who developed grade 3 liver transaminases and isolated grade 3 GGT elevation had improved values when these were rechecked within 2 weeks.
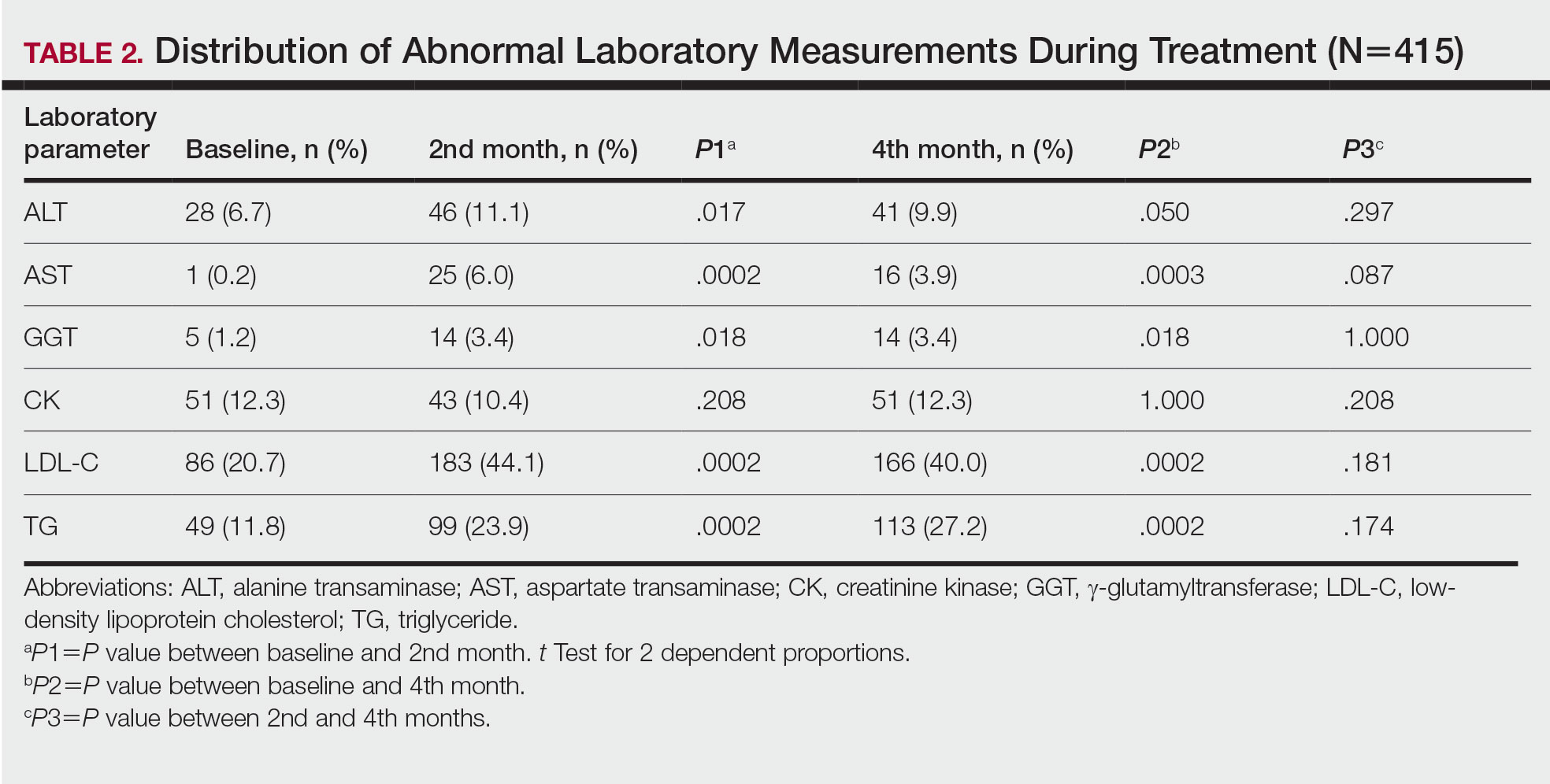
In the patient who developed hepatotoxicity in the second month, the ALT level rose from a baseline of 19 U/L to 169 U/L, the AST level from a baseline of 19 U/L to 61 U/L, and the GGT level from a baseline of 24 U/L to 124 U/L. The patient was asymptomatic. Liver function test levels returned to reference range 4 weeks after discontinuation of therapy. Hepatotoxicity did not recur after treatment was re-administered.
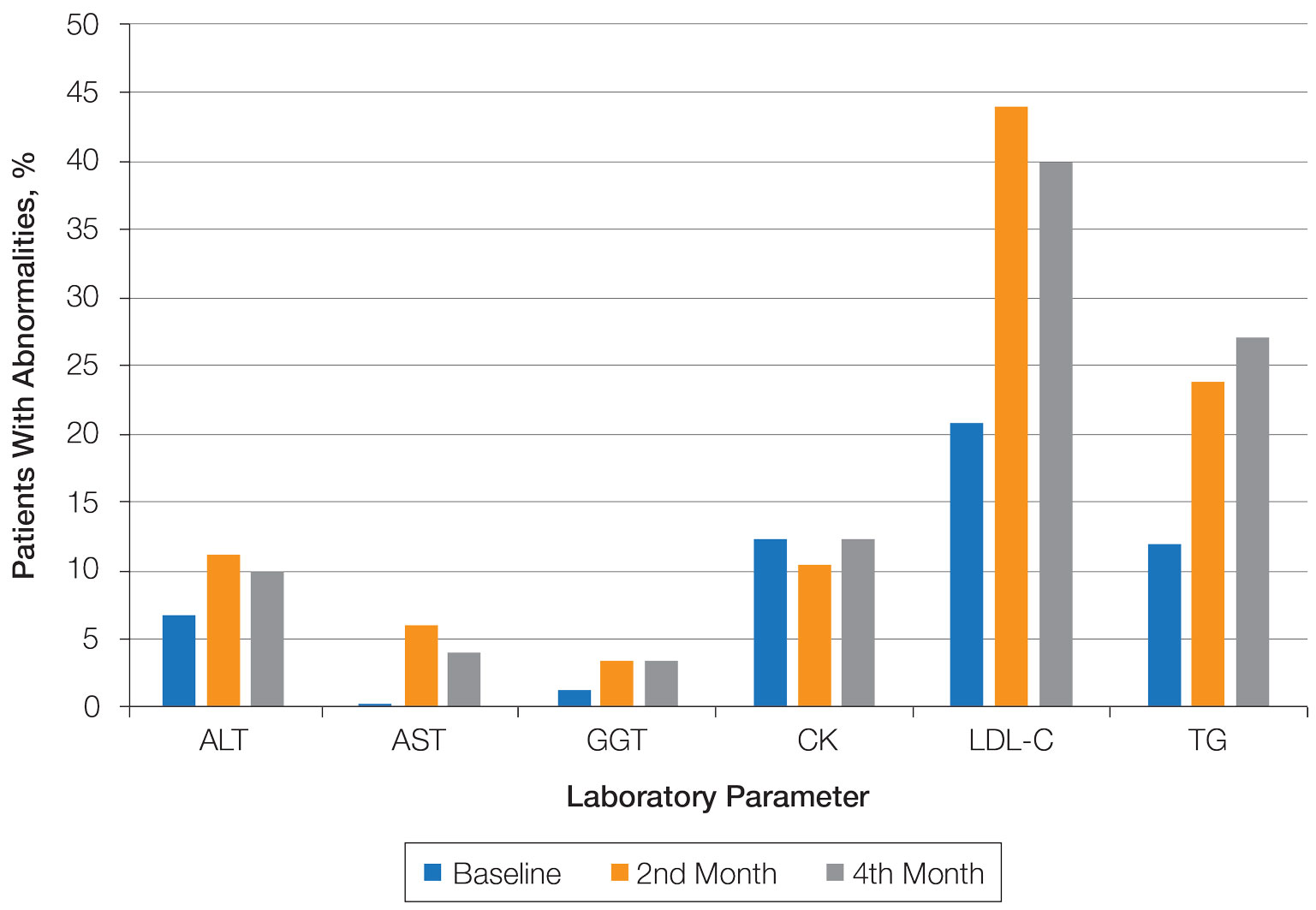
The patient who developed grade 4 AST elevation (364 U/L) experienced fatigue and myalgia. He had done vigorous exercise up to 2 days before the test and also had a grade 4 CK elevation (12,310 U/L). He was thought to have isotretinoin-related rhabdomyolysis. His treatment was discontinued, and he was advised to hydrate and rest. Treatment was re-started after 2 weeks. With frequent laboratory monitoring and avoidance of vigorous physical activity, the patient completed the remaining course of isotretinoin without any laboratory abnormalities or symptoms.
Creatinine kinase abnormalities in the second and fourth months compared with baseline were not statistically significant. The patients with grade 3 or higher CK elevations, except for the case with rhabdomyolysis, had no clinical signs or other characteristic laboratory findings of rhabdomyolysis.
Hypercholesterolemia (LDL-C ≥130 mg/dL) occurred most frequently, with a maximum of 280 mg/dL in 1 patient (in the fourth month) and less than 250 mg/dL in all other patients. Hypercholesterolemia occurred in 183 (44.1%) patients in the second month and in 166 (40.0%) patients in the fourth month. However, baseline abnormalities also were frequent (86 [20.7%]), and hypercholesterolemia persisted in the second and fourth months in all of these patients.
It was observed that the patients with TG abnormalities increased continuously in the second (99 [23.9%]) and fourth (113 [27.2%]) months compared with baseline (49 [11.8%]). Grade 3 TG elevations were observed in 2.2% of patients (n=9; 5 patients in the second month, 4 patients in the fourth month) during treatment compared with baseline, and all patients had grade 1 or 2 hypertriglyceridemia at baseline. Of the patients with grade 3 TG elevation, 3 patients in the second month and 2 patients in the fourth month were obese at baseline. No grade 4 TG elevations were observed. Complications related to hyperlipidemia, such as pancreatitis, were observed in 1 patient. No patient terminated treatment because of lipid abnormalities. The treatment of our patients with major hypercholesterolemia and/or grade 3 hypertriglyceridemia was interrupted. The hyperlipidemia of these patients was controlled by a low-fat diet and a short-term dose reduction.
Relationship Between Total Cumulative Dose and Laboratory Parameters—The relationships between the total cumulative dose and changes up to the fourth month are presented in Table 3. As the total dose increased, the changes in TG and LDL-C levels significantly increased in the fourth month (both P=.001). However, the degree of these relationships was weak. No significant correlation was found between the periodic changes of other laboratory parameters and the total dose.
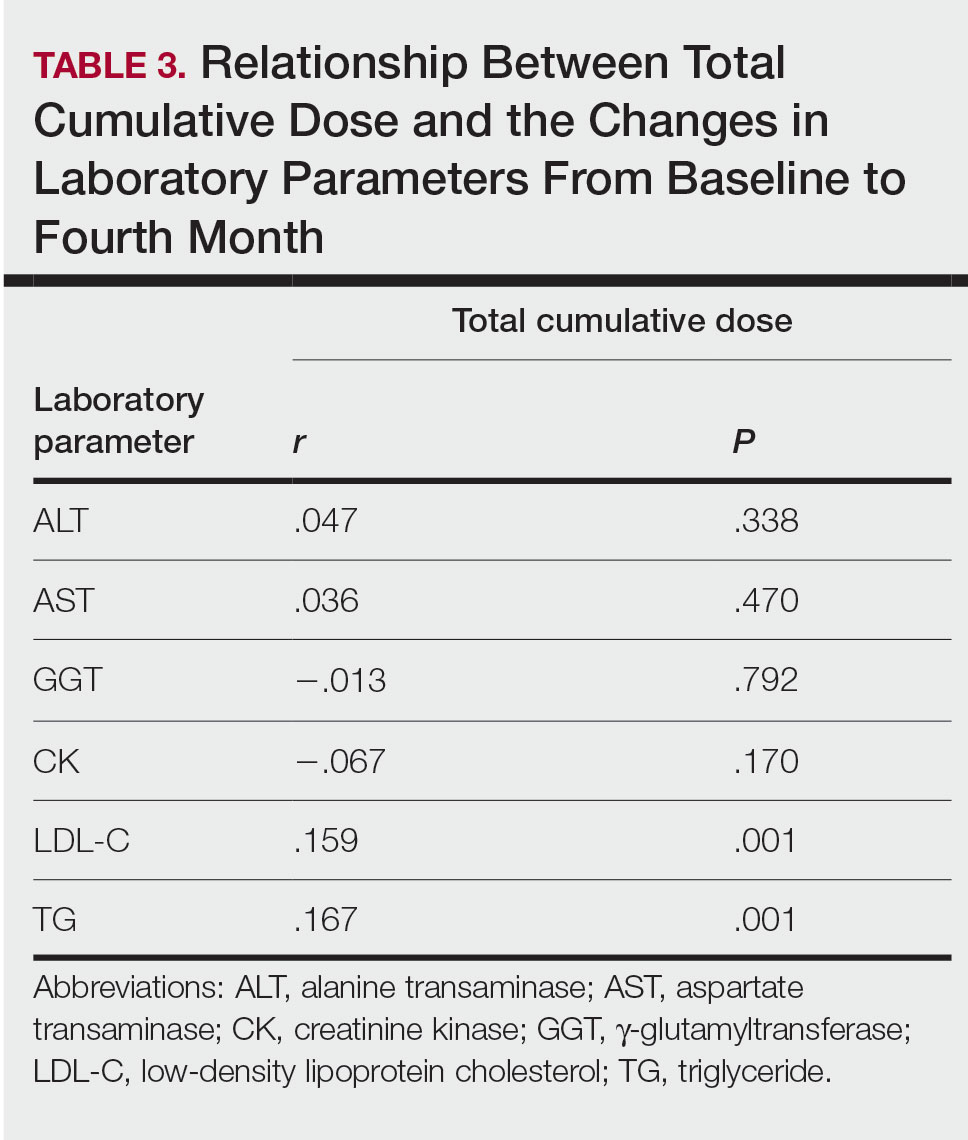
Comment
The parameters followed in our study show that TG levels tend to increase continuously from baseline during isotretinoin treatment, while ALT, AST, GGT, and LDL-C levels increase in the second month and decrease at 4 months. Although this same trend occurs with CK levels, the change was not statistically significant. The most common laboratory abnormality in our study was hyperlipidemia. Levels of LDL-C and TG were both found to be statistically elevated in the second and fourth months of treatment compared with baseline. Parthasarathy et al3 reported that obesity had an important role in the increase of lipid levels in patients using isotretinoin at baseline. In our study, 5 of 9 patients (55.6%) with grade 3 TG elevation were obese, which supports the theory that obesity plays an important role in the increase in lipid levels. Up-to-date laboratory follow-up of lipids suggests that there is no need to follow up serum lipids after the second month of treatment. Patients with risk factors for hyperlipidemia, such as abdominal obesity and familial hyperlipidemia, do not require further follow-up if there is no increase in serum lipids in the first month of treatment.1 The presence of grade 1 or 2 hypertriglyceridemia at baseline in all our patients with grade 3 TG elevation may suggest that periodic laboratory follow-up during isotretinoin treatment is necessary to detect patients with grade 3 and higher TG levels.
The lack of knowledge of other risk factors (eg, familial hyperlipidemia, insulin resistance) for hyperlipidemia in all patients at baseline may be a limitation of our study. Although hypercholesterolemia persisted in the follow-up of our patients with initial LDL-C abnormalities, hypercholesterolemia over 250 mg/dL was very rare (1 patient). Possible complications associated with serum lipid abnormalities are pancreatitis and metabolic syndrome.4 In our study, none of the patients with lipid abnormalities had any relevant clinical sequelae. The dose-dependent elevation of the changes in LDL-C and TG (Table 3) may be important to predict the significant elevation of lipids and the associated complications in patients with a high total cumulative dose target that may require a long treatment duration. However, considering the short follow-up periods in our patients, the absence of clinical sequelae may be misleading. There are differences in recommendations between the US and European guidelines for isotretinoin dosage. Although the US guidelines recommend a total cumulative dose target, the European guidelines recommend low-dose isotretinoin daily for at least 6 months instead of a cumulative dose.
Most liver transaminase abnormalities were detected in the second month. Abnormalities in GGT were seen in the second month and remained elevated at the next follow-up. However, clinically important grade 3 transaminase and GGT elevations were rare. It has been reported that GGT levels are more specific than transaminases in measuring hepatotoxicity.7 The fact that our patient with hepatotoxicity had a grade 3 GGT elevation in addition to grade 3 transaminase elevations supports that GGT elevation is more specific than transaminase levels in measuring hepatotoxicity. When these parameters were rechecked in our patients with grade 3 transaminase elevations, except in the case of hepatotoxicity, transaminase elevations did not recur, and GGT elevations did not accompany elevated transaminases, which suggested that transaminases may be elevated due to an extrahepatic origin (eg, hemolysis, exercise).
Rhabdomyolysis secondary to isotretinoin is rare in the literature of acne studies. In addition to clinical findings such as myalgia and fatigue, increased CK and abnormal liver enzymes, specifically AST, suggest the development of rhabdomyolysis.8 Our patient who developed rhabdomyolysis also had a recent history of vigorous exercise, grade 4 CK, and AST elevations. Other patients with isolated grade 3 CK elevations were informed about possible clinical signs of rhabdomyolysis, and they were able to complete their courses without any incident. According to a study by Landau et al,9 isotretinoin-associated hyperCKemia has been reported as benign. Similarly, our study found that isolated CK elevation during isotretinoin treatment may be misleading as a sign of rhabdomyolysis. Instead, CK monitoring may be more appropriate and cost-effective in patients with suspected clinical signs of rhabdomyolysis or in those with major elevations in transaminases, especially AST.
Conclusion
According to our study, hyperlipidemia was the most common complication in acne patients using isotretinoin. It may be appropriate to monitor the TG level at 2-month intervals in patients with grade 1 or 2 TG elevation at baseline to detect the possible risk for developing grade 3 hyperlipidemia. Periodic monitoring of LDL-C and TG levels may be appropriate, especially in patients who require a high total cumulative dose of isotretinoin. Clinically important liver enzyme abnormalities were rare in our study. Our findings support the idea that routine monthly monitoring of normal laboratory parameters is unnecessary and wasteful. Additionally, periodic monitoring of abnormal laboratory parameters should be considered on an individual basis.
- Affleck A, Jackson D, Williams HC, et al. Is routine laboratory testing in healthy young patients taking isotretinoin necessary: a critically appraised topic. Br J Dermatol. 2022;187:857-865.
- National Cancer Institute. Common Terminology Criteria for Adverse Events v3.0 (CTCAE). August 9, 2006. Accessed June 12, 2023. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf
- Parthasarathy V, Shah N, Kirkorian AY. The utility of laboratory testing for pediatric patients undergoing isotretinoin treatment. Pediatr Dermatol. 2022;39:731-733.
- Sarkar T, Sarkar S, Patra A. Low-dose isotretinoin therapy and blood lipid abnormality: a case series with sixty patients. J Family Med Prim Care. 2018;7:171-174.
- Nast A, Dréno B, Bettoli V, et al. European evidence-based (S3) guideline for the treatment of acne - update 2016 - short version. J Eur Acad Dermatol Venereol. 2016;30:1261-1268.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.
- Webster GF, Webster TG, Grimes LR. Laboratory tests in patients treated with isotretinoin: occurrence of liver and muscle abnormalities and failure of AST and ALT to predict liver abnormality. Dermatol Online J. 2017;23:13030/qt7rv7j80p.
- Raneses E, Schmidgal EC. Rhabdomyolysis caused by isotretinoin and exercise in an otherwise healthy female patient. Cureus. 2022;14:E25981.
- Landau M, Mesterman R, Ophir J, et al. Clinical significance of markedly elevated serum creatine kinase levels in patients with acne on isotretinoin. Acta Derm Venereol. 2001;81:350-352.
Isotretinoin is used in the treatment of nodulocystic and severe papulopustular acne. During the treatment period, laboratory monitoring is recommended to identify the risk for complications such as hepatotoxicity, teratogenicity, rhabdomyolysis, hyperlipidemia, and pancreatitis.1 There is a lack of consensus of the frequency of follow-up of laboratory parameters during isotretinoin treatment. This study evaluated the changes in laboratory parameters used in daily practice for patients with acne who were treated with isotretinoin to determine the optimum test repetition frequency.
Materials and Methods

Statistical Analysis—The descriptive statistics of the measurements were presented as means, standard deviations, or medians (first and third quartiles). With respect to the normal distribution, the consistency of the measurements was evaluated with the Kolmogorov-Smirnov test, and small deviations from the normal distribution were observed. Changes in laboratory measurements were evaluated with simple repeated-measures analysis of variance, and changes that differed significantly were determined by a Holm-Sidak post hoc test. Relationships between total cumulative doses and laboratory measurements at second visits were evaluated by the Pearson correlation analysis. The statistical significance level was P<.05. SPSS Statistics 23 (IBM) was used in the calculations.
Results
Consecutive Data at Baseline and Follow-up—A total of 415 patients with a mean age (SD) of 21.49 (7.25) years (range, 12–53 years) were included in our study. The mean total cumulative dose (SD) of the patients was 7267.27 (1878.4) mg. The consecutive data of the means of the laboratory parameters are shown in Table 1 and Figure 1. There was no significant change in the ALT levels between baseline and the fourth month as well as between the second- and fourth-month assessments (both P=.311).

Abnormal Laboratory Measurements—The distribution of abnormal laboratory measurements during treatment is shown in Table 2 and Figure 2. Grade 3 or higher elevations of liver transaminases (ALT, AST) and GGT were observed in fewer than 2% of patients during treatment compared with baseline (grade 3 elevations of ALT and AST together in 2 patients; grade 4 AST elevation in 1 patient; grade 3 elevations of ALT, AST, and GGT combined in 1 patient; isolated grade 3 GGT elevation in 1 patient). All of the patients who developed grade 3 liver transaminases and isolated grade 3 GGT elevation had improved values when these were rechecked within 2 weeks.

In the patient who developed hepatotoxicity in the second month, the ALT level rose from a baseline of 19 U/L to 169 U/L, the AST level from a baseline of 19 U/L to 61 U/L, and the GGT level from a baseline of 24 U/L to 124 U/L. The patient was asymptomatic. Liver function test levels returned to reference range 4 weeks after discontinuation of therapy. Hepatotoxicity did not recur after treatment was re-administered.

The patient who developed grade 4 AST elevation (364 U/L) experienced fatigue and myalgia. He had done vigorous exercise up to 2 days before the test and also had a grade 4 CK elevation (12,310 U/L). He was thought to have isotretinoin-related rhabdomyolysis. His treatment was discontinued, and he was advised to hydrate and rest. Treatment was re-started after 2 weeks. With frequent laboratory monitoring and avoidance of vigorous physical activity, the patient completed the remaining course of isotretinoin without any laboratory abnormalities or symptoms.
Creatinine kinase abnormalities in the second and fourth months compared with baseline were not statistically significant. The patients with grade 3 or higher CK elevations, except for the case with rhabdomyolysis, had no clinical signs or other characteristic laboratory findings of rhabdomyolysis.
Hypercholesterolemia (LDL-C ≥130 mg/dL) occurred most frequently, with a maximum of 280 mg/dL in 1 patient (in the fourth month) and less than 250 mg/dL in all other patients. Hypercholesterolemia occurred in 183 (44.1%) patients in the second month and in 166 (40.0%) patients in the fourth month. However, baseline abnormalities also were frequent (86 [20.7%]), and hypercholesterolemia persisted in the second and fourth months in all of these patients.
It was observed that the patients with TG abnormalities increased continuously in the second (99 [23.9%]) and fourth (113 [27.2%]) months compared with baseline (49 [11.8%]). Grade 3 TG elevations were observed in 2.2% of patients (n=9; 5 patients in the second month, 4 patients in the fourth month) during treatment compared with baseline, and all patients had grade 1 or 2 hypertriglyceridemia at baseline. Of the patients with grade 3 TG elevation, 3 patients in the second month and 2 patients in the fourth month were obese at baseline. No grade 4 TG elevations were observed. Complications related to hyperlipidemia, such as pancreatitis, were observed in 1 patient. No patient terminated treatment because of lipid abnormalities. The treatment of our patients with major hypercholesterolemia and/or grade 3 hypertriglyceridemia was interrupted. The hyperlipidemia of these patients was controlled by a low-fat diet and a short-term dose reduction.
Relationship Between Total Cumulative Dose and Laboratory Parameters—The relationships between the total cumulative dose and changes up to the fourth month are presented in Table 3. As the total dose increased, the changes in TG and LDL-C levels significantly increased in the fourth month (both P=.001). However, the degree of these relationships was weak. No significant correlation was found between the periodic changes of other laboratory parameters and the total dose.

Comment
The parameters followed in our study show that TG levels tend to increase continuously from baseline during isotretinoin treatment, while ALT, AST, GGT, and LDL-C levels increase in the second month and decrease at 4 months. Although this same trend occurs with CK levels, the change was not statistically significant. The most common laboratory abnormality in our study was hyperlipidemia. Levels of LDL-C and TG were both found to be statistically elevated in the second and fourth months of treatment compared with baseline. Parthasarathy et al3 reported that obesity had an important role in the increase of lipid levels in patients using isotretinoin at baseline. In our study, 5 of 9 patients (55.6%) with grade 3 TG elevation were obese, which supports the theory that obesity plays an important role in the increase in lipid levels. Up-to-date laboratory follow-up of lipids suggests that there is no need to follow up serum lipids after the second month of treatment. Patients with risk factors for hyperlipidemia, such as abdominal obesity and familial hyperlipidemia, do not require further follow-up if there is no increase in serum lipids in the first month of treatment.1 The presence of grade 1 or 2 hypertriglyceridemia at baseline in all our patients with grade 3 TG elevation may suggest that periodic laboratory follow-up during isotretinoin treatment is necessary to detect patients with grade 3 and higher TG levels.
The lack of knowledge of other risk factors (eg, familial hyperlipidemia, insulin resistance) for hyperlipidemia in all patients at baseline may be a limitation of our study. Although hypercholesterolemia persisted in the follow-up of our patients with initial LDL-C abnormalities, hypercholesterolemia over 250 mg/dL was very rare (1 patient). Possible complications associated with serum lipid abnormalities are pancreatitis and metabolic syndrome.4 In our study, none of the patients with lipid abnormalities had any relevant clinical sequelae. The dose-dependent elevation of the changes in LDL-C and TG (Table 3) may be important to predict the significant elevation of lipids and the associated complications in patients with a high total cumulative dose target that may require a long treatment duration. However, considering the short follow-up periods in our patients, the absence of clinical sequelae may be misleading. There are differences in recommendations between the US and European guidelines for isotretinoin dosage. Although the US guidelines recommend a total cumulative dose target, the European guidelines recommend low-dose isotretinoin daily for at least 6 months instead of a cumulative dose.
Most liver transaminase abnormalities were detected in the second month. Abnormalities in GGT were seen in the second month and remained elevated at the next follow-up. However, clinically important grade 3 transaminase and GGT elevations were rare. It has been reported that GGT levels are more specific than transaminases in measuring hepatotoxicity.7 The fact that our patient with hepatotoxicity had a grade 3 GGT elevation in addition to grade 3 transaminase elevations supports that GGT elevation is more specific than transaminase levels in measuring hepatotoxicity. When these parameters were rechecked in our patients with grade 3 transaminase elevations, except in the case of hepatotoxicity, transaminase elevations did not recur, and GGT elevations did not accompany elevated transaminases, which suggested that transaminases may be elevated due to an extrahepatic origin (eg, hemolysis, exercise).
Rhabdomyolysis secondary to isotretinoin is rare in the literature of acne studies. In addition to clinical findings such as myalgia and fatigue, increased CK and abnormal liver enzymes, specifically AST, suggest the development of rhabdomyolysis.8 Our patient who developed rhabdomyolysis also had a recent history of vigorous exercise, grade 4 CK, and AST elevations. Other patients with isolated grade 3 CK elevations were informed about possible clinical signs of rhabdomyolysis, and they were able to complete their courses without any incident. According to a study by Landau et al,9 isotretinoin-associated hyperCKemia has been reported as benign. Similarly, our study found that isolated CK elevation during isotretinoin treatment may be misleading as a sign of rhabdomyolysis. Instead, CK monitoring may be more appropriate and cost-effective in patients with suspected clinical signs of rhabdomyolysis or in those with major elevations in transaminases, especially AST.
Conclusion
According to our study, hyperlipidemia was the most common complication in acne patients using isotretinoin. It may be appropriate to monitor the TG level at 2-month intervals in patients with grade 1 or 2 TG elevation at baseline to detect the possible risk for developing grade 3 hyperlipidemia. Periodic monitoring of LDL-C and TG levels may be appropriate, especially in patients who require a high total cumulative dose of isotretinoin. Clinically important liver enzyme abnormalities were rare in our study. Our findings support the idea that routine monthly monitoring of normal laboratory parameters is unnecessary and wasteful. Additionally, periodic monitoring of abnormal laboratory parameters should be considered on an individual basis.
Isotretinoin is used in the treatment of nodulocystic and severe papulopustular acne. During the treatment period, laboratory monitoring is recommended to identify the risk for complications such as hepatotoxicity, teratogenicity, rhabdomyolysis, hyperlipidemia, and pancreatitis.1 There is a lack of consensus of the frequency of follow-up of laboratory parameters during isotretinoin treatment. This study evaluated the changes in laboratory parameters used in daily practice for patients with acne who were treated with isotretinoin to determine the optimum test repetition frequency.
Materials and Methods

Statistical Analysis—The descriptive statistics of the measurements were presented as means, standard deviations, or medians (first and third quartiles). With respect to the normal distribution, the consistency of the measurements was evaluated with the Kolmogorov-Smirnov test, and small deviations from the normal distribution were observed. Changes in laboratory measurements were evaluated with simple repeated-measures analysis of variance, and changes that differed significantly were determined by a Holm-Sidak post hoc test. Relationships between total cumulative doses and laboratory measurements at second visits were evaluated by the Pearson correlation analysis. The statistical significance level was P<.05. SPSS Statistics 23 (IBM) was used in the calculations.
Results
Consecutive Data at Baseline and Follow-up—A total of 415 patients with a mean age (SD) of 21.49 (7.25) years (range, 12–53 years) were included in our study. The mean total cumulative dose (SD) of the patients was 7267.27 (1878.4) mg. The consecutive data of the means of the laboratory parameters are shown in Table 1 and Figure 1. There was no significant change in the ALT levels between baseline and the fourth month as well as between the second- and fourth-month assessments (both P=.311).

Abnormal Laboratory Measurements—The distribution of abnormal laboratory measurements during treatment is shown in Table 2 and Figure 2. Grade 3 or higher elevations of liver transaminases (ALT, AST) and GGT were observed in fewer than 2% of patients during treatment compared with baseline (grade 3 elevations of ALT and AST together in 2 patients; grade 4 AST elevation in 1 patient; grade 3 elevations of ALT, AST, and GGT combined in 1 patient; isolated grade 3 GGT elevation in 1 patient). All of the patients who developed grade 3 liver transaminases and isolated grade 3 GGT elevation had improved values when these were rechecked within 2 weeks.

In the patient who developed hepatotoxicity in the second month, the ALT level rose from a baseline of 19 U/L to 169 U/L, the AST level from a baseline of 19 U/L to 61 U/L, and the GGT level from a baseline of 24 U/L to 124 U/L. The patient was asymptomatic. Liver function test levels returned to reference range 4 weeks after discontinuation of therapy. Hepatotoxicity did not recur after treatment was re-administered.

The patient who developed grade 4 AST elevation (364 U/L) experienced fatigue and myalgia. He had done vigorous exercise up to 2 days before the test and also had a grade 4 CK elevation (12,310 U/L). He was thought to have isotretinoin-related rhabdomyolysis. His treatment was discontinued, and he was advised to hydrate and rest. Treatment was re-started after 2 weeks. With frequent laboratory monitoring and avoidance of vigorous physical activity, the patient completed the remaining course of isotretinoin without any laboratory abnormalities or symptoms.
Creatinine kinase abnormalities in the second and fourth months compared with baseline were not statistically significant. The patients with grade 3 or higher CK elevations, except for the case with rhabdomyolysis, had no clinical signs or other characteristic laboratory findings of rhabdomyolysis.
Hypercholesterolemia (LDL-C ≥130 mg/dL) occurred most frequently, with a maximum of 280 mg/dL in 1 patient (in the fourth month) and less than 250 mg/dL in all other patients. Hypercholesterolemia occurred in 183 (44.1%) patients in the second month and in 166 (40.0%) patients in the fourth month. However, baseline abnormalities also were frequent (86 [20.7%]), and hypercholesterolemia persisted in the second and fourth months in all of these patients.
It was observed that the patients with TG abnormalities increased continuously in the second (99 [23.9%]) and fourth (113 [27.2%]) months compared with baseline (49 [11.8%]). Grade 3 TG elevations were observed in 2.2% of patients (n=9; 5 patients in the second month, 4 patients in the fourth month) during treatment compared with baseline, and all patients had grade 1 or 2 hypertriglyceridemia at baseline. Of the patients with grade 3 TG elevation, 3 patients in the second month and 2 patients in the fourth month were obese at baseline. No grade 4 TG elevations were observed. Complications related to hyperlipidemia, such as pancreatitis, were observed in 1 patient. No patient terminated treatment because of lipid abnormalities. The treatment of our patients with major hypercholesterolemia and/or grade 3 hypertriglyceridemia was interrupted. The hyperlipidemia of these patients was controlled by a low-fat diet and a short-term dose reduction.
Relationship Between Total Cumulative Dose and Laboratory Parameters—The relationships between the total cumulative dose and changes up to the fourth month are presented in Table 3. As the total dose increased, the changes in TG and LDL-C levels significantly increased in the fourth month (both P=.001). However, the degree of these relationships was weak. No significant correlation was found between the periodic changes of other laboratory parameters and the total dose.

Comment
The parameters followed in our study show that TG levels tend to increase continuously from baseline during isotretinoin treatment, while ALT, AST, GGT, and LDL-C levels increase in the second month and decrease at 4 months. Although this same trend occurs with CK levels, the change was not statistically significant. The most common laboratory abnormality in our study was hyperlipidemia. Levels of LDL-C and TG were both found to be statistically elevated in the second and fourth months of treatment compared with baseline. Parthasarathy et al3 reported that obesity had an important role in the increase of lipid levels in patients using isotretinoin at baseline. In our study, 5 of 9 patients (55.6%) with grade 3 TG elevation were obese, which supports the theory that obesity plays an important role in the increase in lipid levels. Up-to-date laboratory follow-up of lipids suggests that there is no need to follow up serum lipids after the second month of treatment. Patients with risk factors for hyperlipidemia, such as abdominal obesity and familial hyperlipidemia, do not require further follow-up if there is no increase in serum lipids in the first month of treatment.1 The presence of grade 1 or 2 hypertriglyceridemia at baseline in all our patients with grade 3 TG elevation may suggest that periodic laboratory follow-up during isotretinoin treatment is necessary to detect patients with grade 3 and higher TG levels.
The lack of knowledge of other risk factors (eg, familial hyperlipidemia, insulin resistance) for hyperlipidemia in all patients at baseline may be a limitation of our study. Although hypercholesterolemia persisted in the follow-up of our patients with initial LDL-C abnormalities, hypercholesterolemia over 250 mg/dL was very rare (1 patient). Possible complications associated with serum lipid abnormalities are pancreatitis and metabolic syndrome.4 In our study, none of the patients with lipid abnormalities had any relevant clinical sequelae. The dose-dependent elevation of the changes in LDL-C and TG (Table 3) may be important to predict the significant elevation of lipids and the associated complications in patients with a high total cumulative dose target that may require a long treatment duration. However, considering the short follow-up periods in our patients, the absence of clinical sequelae may be misleading. There are differences in recommendations between the US and European guidelines for isotretinoin dosage. Although the US guidelines recommend a total cumulative dose target, the European guidelines recommend low-dose isotretinoin daily for at least 6 months instead of a cumulative dose.
Most liver transaminase abnormalities were detected in the second month. Abnormalities in GGT were seen in the second month and remained elevated at the next follow-up. However, clinically important grade 3 transaminase and GGT elevations were rare. It has been reported that GGT levels are more specific than transaminases in measuring hepatotoxicity.7 The fact that our patient with hepatotoxicity had a grade 3 GGT elevation in addition to grade 3 transaminase elevations supports that GGT elevation is more specific than transaminase levels in measuring hepatotoxicity. When these parameters were rechecked in our patients with grade 3 transaminase elevations, except in the case of hepatotoxicity, transaminase elevations did not recur, and GGT elevations did not accompany elevated transaminases, which suggested that transaminases may be elevated due to an extrahepatic origin (eg, hemolysis, exercise).
Rhabdomyolysis secondary to isotretinoin is rare in the literature of acne studies. In addition to clinical findings such as myalgia and fatigue, increased CK and abnormal liver enzymes, specifically AST, suggest the development of rhabdomyolysis.8 Our patient who developed rhabdomyolysis also had a recent history of vigorous exercise, grade 4 CK, and AST elevations. Other patients with isolated grade 3 CK elevations were informed about possible clinical signs of rhabdomyolysis, and they were able to complete their courses without any incident. According to a study by Landau et al,9 isotretinoin-associated hyperCKemia has been reported as benign. Similarly, our study found that isolated CK elevation during isotretinoin treatment may be misleading as a sign of rhabdomyolysis. Instead, CK monitoring may be more appropriate and cost-effective in patients with suspected clinical signs of rhabdomyolysis or in those with major elevations in transaminases, especially AST.
Conclusion
According to our study, hyperlipidemia was the most common complication in acne patients using isotretinoin. It may be appropriate to monitor the TG level at 2-month intervals in patients with grade 1 or 2 TG elevation at baseline to detect the possible risk for developing grade 3 hyperlipidemia. Periodic monitoring of LDL-C and TG levels may be appropriate, especially in patients who require a high total cumulative dose of isotretinoin. Clinically important liver enzyme abnormalities were rare in our study. Our findings support the idea that routine monthly monitoring of normal laboratory parameters is unnecessary and wasteful. Additionally, periodic monitoring of abnormal laboratory parameters should be considered on an individual basis.
- Affleck A, Jackson D, Williams HC, et al. Is routine laboratory testing in healthy young patients taking isotretinoin necessary: a critically appraised topic. Br J Dermatol. 2022;187:857-865.
- National Cancer Institute. Common Terminology Criteria for Adverse Events v3.0 (CTCAE). August 9, 2006. Accessed June 12, 2023. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf
- Parthasarathy V, Shah N, Kirkorian AY. The utility of laboratory testing for pediatric patients undergoing isotretinoin treatment. Pediatr Dermatol. 2022;39:731-733.
- Sarkar T, Sarkar S, Patra A. Low-dose isotretinoin therapy and blood lipid abnormality: a case series with sixty patients. J Family Med Prim Care. 2018;7:171-174.
- Nast A, Dréno B, Bettoli V, et al. European evidence-based (S3) guideline for the treatment of acne - update 2016 - short version. J Eur Acad Dermatol Venereol. 2016;30:1261-1268.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.
- Webster GF, Webster TG, Grimes LR. Laboratory tests in patients treated with isotretinoin: occurrence of liver and muscle abnormalities and failure of AST and ALT to predict liver abnormality. Dermatol Online J. 2017;23:13030/qt7rv7j80p.
- Raneses E, Schmidgal EC. Rhabdomyolysis caused by isotretinoin and exercise in an otherwise healthy female patient. Cureus. 2022;14:E25981.
- Landau M, Mesterman R, Ophir J, et al. Clinical significance of markedly elevated serum creatine kinase levels in patients with acne on isotretinoin. Acta Derm Venereol. 2001;81:350-352.
- Affleck A, Jackson D, Williams HC, et al. Is routine laboratory testing in healthy young patients taking isotretinoin necessary: a critically appraised topic. Br J Dermatol. 2022;187:857-865.
- National Cancer Institute. Common Terminology Criteria for Adverse Events v3.0 (CTCAE). August 9, 2006. Accessed June 12, 2023. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf
- Parthasarathy V, Shah N, Kirkorian AY. The utility of laboratory testing for pediatric patients undergoing isotretinoin treatment. Pediatr Dermatol. 2022;39:731-733.
- Sarkar T, Sarkar S, Patra A. Low-dose isotretinoin therapy and blood lipid abnormality: a case series with sixty patients. J Family Med Prim Care. 2018;7:171-174.
- Nast A, Dréno B, Bettoli V, et al. European evidence-based (S3) guideline for the treatment of acne - update 2016 - short version. J Eur Acad Dermatol Venereol. 2016;30:1261-1268.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.
- Webster GF, Webster TG, Grimes LR. Laboratory tests in patients treated with isotretinoin: occurrence of liver and muscle abnormalities and failure of AST and ALT to predict liver abnormality. Dermatol Online J. 2017;23:13030/qt7rv7j80p.
- Raneses E, Schmidgal EC. Rhabdomyolysis caused by isotretinoin and exercise in an otherwise healthy female patient. Cureus. 2022;14:E25981.
- Landau M, Mesterman R, Ophir J, et al. Clinical significance of markedly elevated serum creatine kinase levels in patients with acne on isotretinoin. Acta Derm Venereol. 2001;81:350-352.
Practice Points
- Hyperlipidemia was the most common complication in patients with acne using isotretinoin.
- It may be appropriate to monitor triglyceride levels at 2-month intervals in patients with grade 1 or 2 triglyceride elevation at baseline to detect the possible risk for developing grade 3 hyperlipidemia.
- Routine monthly monitoring of normal laboratory parameters is unnecessary and wasteful. Periodic monitoring of abnormal laboratory parameters should be considered on an individual basis.
The Growing Pains of Changing Times for Acne and Rosacea Pathophysiology: Where Will It All End Up?
It is interesting to observe the changes in dermatology that have occurred over the last 1 to 2 decades, especially as major advances in basic science research techniques have rapidly expanded our current understanding of the pathophysiology of many disease states—psoriasis, psoriatic arthritis, atopic dermatitis, alopecia areata, vitiligo, hidradenitis suppurativa, and lichen planus.1 Although acne vulgaris (AV) and rosacea do not make front-page news quite as often as some of these other aforementioned disease states in the pathophysiology arena, advances still have been made in understanding the pathophysiology, albeit slower and often less popularized in dermatology publications and other forms of media.2-4
If one looks at our fundamental understanding of AV, most of the discussion over multiple decades has been driven by new treatments and in some cases new formulations and packaging differences with topical agents. Although we understood that adrenarche, a subsequent increase in androgen synthesis, and the ensuing sebocyte development with formation of sebum were prerequisites for the development of AV, the absence of therapeutic options to address these vital components of AV—especially US Food and Drug Administration (FDA)–approved therapies—resulted in limited discussion about this specific area.5 Rather, the discussion was dominated by the notable role of Propionibacterium acnes (now called Cutibacterium acnes) in AV pathophysiology, as we had therapies such as benzoyl peroxide and antibiotics that improved AV in direct correlation with reductions in P acnes.6 This was soon coupled with an advanced understanding of how to reduce follicular hyperkeratinization with the development of topical tretinoin, followed by 3 other topical retinoids over time—adapalene, tazarotene, and trifarotene. Over subsequent years, slowly emerging basic science developments and collective data reviews added to our understanding of AV and how different therapies appear to work, including the role of toll-like receptors, anti-inflammatory properties of tetracyclines, and inflammasomes.7-9 Without a doubt, the availability of oral isotretinoin revolutionized AV therapy, especially in patients with severe refractory disease, with advanced formulations allowing for optimization of sustained remission without the need for high dietary fat intake.10-12
Progress in the pathophysiology of rosacea has been slower to develop, with the first true discussion of specific clinical presentations published after the new millennium.13 This was followed by more advanced basic science and clinical research, which led to an improved ability to understand modes of action of various therapies and to correlate treatment selection with specific visible manifestations of rosacea, including incorporation of physical devices.14-16 A newer perspective on evaluation and management of rosacea moved away from the “buckets” of rosacea subtypes to phenotypes observed at the time of clinical presentation.17,18
I could elaborate on research advancements with both diseases, but the bottom line is that information, developments, and current perspectives change over time. Keeping up is a challenge for all who study and practice dermatology. It is human nature to revert to what we already believe and do, which sometimes remains valid and other times is quite outdated and truly replaced by more optimal approaches. With AV and rosacea, progress is much slower in availability of newer agents. With AV, new agents have included topical dapsone, oral sarecycline, and topical clascoterone, with the latter being the first FDA-approved topical agent to mitigate the effects of androgens and sebum in both males and females. For rosacea, the 2 most recent FDA-approved therapies are minocycline foam and microencapsulated benzoyl peroxide. All of these therapies are proven to be effective for the modes of action and skin manifestations they specifically manage. Over the upcoming year, we are hoping to see the first triple-combination topical product come to market for AV, which will prompt our minds to consider if and how 3 established agents can work together to further augment treatment efficacy with favorable tolerability and safety.
Where will all of this end up? It is hard to say. We still have several other areas to tackle with both disease states, including establishing a well-substantiated understanding of the pathophysiologic role of the microbiome, sorting out the role of antibiotic use due to concerns about bacterial resistance, integration of FDA-approved physical devices in AV, and data on both diet and optimized skin care, to name a few.19-21
There is a lot on the plate to accomplish and digest. I have remained very involved in this subject matter for almost 3 decades and am still feeling the growing pains. Fortunately, the satisfaction of being part of a process so important to the lives of millions of patients makes this worth every moment. Stay tuned—more valuable information is to come.
- Wu J, Fang Z, Liu T, et al. Maximizing the utility of transcriptomics data in inflammatory skin diseases. Front Immunol. 2021;12:761890.
- Firlej E, Kowalska W, Szymaszek K, et al. The role of skin immune system in acne. J Clin Med. 2022;11:1579.
- Mias C, Mengeaud V, Bessou-Touya S, et al. Recent advances in understanding inflammatory acne: deciphering the relationship between Cutibacterium acnes and Th17 inflammatory pathway. J Eur Acad Dermatol Venereol. 2023;(37 suppl 2):3-11.
- Buddenkotte J, Steinhoff M. Recent advances in understanding and managing rosacea. F1000Res. 2018;7:F1000 Faculty Rev-1885. doi:10.12688/f1000research.16537.1
- Platsidaki E, Dessinioti C. Recent advances in understanding Propionibacterium acnes (Cutibacterium acnes) in acne. F1000Res. 2018;7:F1000 Faculty Rev-1953. doi:10.12688/f1000research.15659.1
- Leyden JJ. The evolving role of Propionibacterium acnes in acne. Semin Cutan Med Surg. 2001;20:139-143.
- Kim J. Review of the innate immune response in acne vulgaris: activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. Dermatology. 2005;211:193-198.
- Del Rosso JQ, Webster G, Weiss JS, et al. Nonantibiotic properties of tetracyclines in rosacea and their clinical implications. J Clin Aesthet Dermatol. 2021;14:14-21.
- Zhu W, Wang HL, Bu XL, et al. A narrative review of research progress on the role of NLRP3 inflammasome in acne vulgaris. Ann Transl Med. 2022;10:645.
- Leyden JJ, Del Rosso JQ, Baum EW. The use of isotretinoin in the treatment of acne vulgaris: clinical considerations and future directions. J Clin Aesthet Dermatol. 2014;7(2 suppl):S3-S21.
- Webster GF, Leyden JJ, Gross JA. Comparative pharmacokinetic profiles of a novel isotretinoin formulation (isotretinoin-Lidose) and the innovator isotretinoin formulation: a randomized, treatment, crossover study. J Am Acad Dermatol. 2013;69:762-767.
- Del Rosso JQ, Stein Gold L, Seagal J, et al. An open-label, phase IV study evaluating Lidose-isotretinoin administered without food in patients with severe recalcitrant nodular acne: low relapse rates observed over the 104-week post-treatment period. J Clin Aesthet Dermatol. 2019;12:13-18.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Steinhoff M, Buddenkotte J, Aubert J, et al. Clinical, cellular, and molecular aspects in the pathophysiology of rosacea. J Investig Dermatol Symp Proc. 2011;15:2-11.
- Yamasaki K, Gallo RL. The molecular pathology of rosacea. J Dermatol Sci. 2009;55:77-81.
- Tanghetti E, Del Rosso JQ, Thiboutot D, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 4: a status report on physical modalities and devices. Cutis. 2014;93:71-76.
- Del Rosso JQ, Gallo RL, Tanghetti E, et al. An evaluation of potential correlations between pathophysiologic mechanisms, clinical manifestations, and management of rosacea. Cutis. 2013;91(3 suppl):1-8.
- Schaller M, Almeida LMC, Bewley A, et al. Recommendations for rosacea diagnosis, classification and management: update from the global ROSacea COnsensus 2019 panel. Br J Dermatol. 2020;182:1269-1276.
- Xu H, Li H. Acne, the skin microbiome, and antibiotic treatment. Am J Clin Dermatol. 2019;20:335-344.
- Daou H, Paradiso M, Hennessy K. Rosacea and the microbiome: a systematic review. Dermatol Ther (Heidelb). 2021;11:1-12.
- Kayiran MA, Karadag AS, Al-Khuzaei S, et al. Antibiotic resistance in acne: mechanisms, complications and management. Am J Clin Dermatol. 2020;21:813-819.
It is interesting to observe the changes in dermatology that have occurred over the last 1 to 2 decades, especially as major advances in basic science research techniques have rapidly expanded our current understanding of the pathophysiology of many disease states—psoriasis, psoriatic arthritis, atopic dermatitis, alopecia areata, vitiligo, hidradenitis suppurativa, and lichen planus.1 Although acne vulgaris (AV) and rosacea do not make front-page news quite as often as some of these other aforementioned disease states in the pathophysiology arena, advances still have been made in understanding the pathophysiology, albeit slower and often less popularized in dermatology publications and other forms of media.2-4
If one looks at our fundamental understanding of AV, most of the discussion over multiple decades has been driven by new treatments and in some cases new formulations and packaging differences with topical agents. Although we understood that adrenarche, a subsequent increase in androgen synthesis, and the ensuing sebocyte development with formation of sebum were prerequisites for the development of AV, the absence of therapeutic options to address these vital components of AV—especially US Food and Drug Administration (FDA)–approved therapies—resulted in limited discussion about this specific area.5 Rather, the discussion was dominated by the notable role of Propionibacterium acnes (now called Cutibacterium acnes) in AV pathophysiology, as we had therapies such as benzoyl peroxide and antibiotics that improved AV in direct correlation with reductions in P acnes.6 This was soon coupled with an advanced understanding of how to reduce follicular hyperkeratinization with the development of topical tretinoin, followed by 3 other topical retinoids over time—adapalene, tazarotene, and trifarotene. Over subsequent years, slowly emerging basic science developments and collective data reviews added to our understanding of AV and how different therapies appear to work, including the role of toll-like receptors, anti-inflammatory properties of tetracyclines, and inflammasomes.7-9 Without a doubt, the availability of oral isotretinoin revolutionized AV therapy, especially in patients with severe refractory disease, with advanced formulations allowing for optimization of sustained remission without the need for high dietary fat intake.10-12
Progress in the pathophysiology of rosacea has been slower to develop, with the first true discussion of specific clinical presentations published after the new millennium.13 This was followed by more advanced basic science and clinical research, which led to an improved ability to understand modes of action of various therapies and to correlate treatment selection with specific visible manifestations of rosacea, including incorporation of physical devices.14-16 A newer perspective on evaluation and management of rosacea moved away from the “buckets” of rosacea subtypes to phenotypes observed at the time of clinical presentation.17,18
I could elaborate on research advancements with both diseases, but the bottom line is that information, developments, and current perspectives change over time. Keeping up is a challenge for all who study and practice dermatology. It is human nature to revert to what we already believe and do, which sometimes remains valid and other times is quite outdated and truly replaced by more optimal approaches. With AV and rosacea, progress is much slower in availability of newer agents. With AV, new agents have included topical dapsone, oral sarecycline, and topical clascoterone, with the latter being the first FDA-approved topical agent to mitigate the effects of androgens and sebum in both males and females. For rosacea, the 2 most recent FDA-approved therapies are minocycline foam and microencapsulated benzoyl peroxide. All of these therapies are proven to be effective for the modes of action and skin manifestations they specifically manage. Over the upcoming year, we are hoping to see the first triple-combination topical product come to market for AV, which will prompt our minds to consider if and how 3 established agents can work together to further augment treatment efficacy with favorable tolerability and safety.
Where will all of this end up? It is hard to say. We still have several other areas to tackle with both disease states, including establishing a well-substantiated understanding of the pathophysiologic role of the microbiome, sorting out the role of antibiotic use due to concerns about bacterial resistance, integration of FDA-approved physical devices in AV, and data on both diet and optimized skin care, to name a few.19-21
There is a lot on the plate to accomplish and digest. I have remained very involved in this subject matter for almost 3 decades and am still feeling the growing pains. Fortunately, the satisfaction of being part of a process so important to the lives of millions of patients makes this worth every moment. Stay tuned—more valuable information is to come.
It is interesting to observe the changes in dermatology that have occurred over the last 1 to 2 decades, especially as major advances in basic science research techniques have rapidly expanded our current understanding of the pathophysiology of many disease states—psoriasis, psoriatic arthritis, atopic dermatitis, alopecia areata, vitiligo, hidradenitis suppurativa, and lichen planus.1 Although acne vulgaris (AV) and rosacea do not make front-page news quite as often as some of these other aforementioned disease states in the pathophysiology arena, advances still have been made in understanding the pathophysiology, albeit slower and often less popularized in dermatology publications and other forms of media.2-4
If one looks at our fundamental understanding of AV, most of the discussion over multiple decades has been driven by new treatments and in some cases new formulations and packaging differences with topical agents. Although we understood that adrenarche, a subsequent increase in androgen synthesis, and the ensuing sebocyte development with formation of sebum were prerequisites for the development of AV, the absence of therapeutic options to address these vital components of AV—especially US Food and Drug Administration (FDA)–approved therapies—resulted in limited discussion about this specific area.5 Rather, the discussion was dominated by the notable role of Propionibacterium acnes (now called Cutibacterium acnes) in AV pathophysiology, as we had therapies such as benzoyl peroxide and antibiotics that improved AV in direct correlation with reductions in P acnes.6 This was soon coupled with an advanced understanding of how to reduce follicular hyperkeratinization with the development of topical tretinoin, followed by 3 other topical retinoids over time—adapalene, tazarotene, and trifarotene. Over subsequent years, slowly emerging basic science developments and collective data reviews added to our understanding of AV and how different therapies appear to work, including the role of toll-like receptors, anti-inflammatory properties of tetracyclines, and inflammasomes.7-9 Without a doubt, the availability of oral isotretinoin revolutionized AV therapy, especially in patients with severe refractory disease, with advanced formulations allowing for optimization of sustained remission without the need for high dietary fat intake.10-12
Progress in the pathophysiology of rosacea has been slower to develop, with the first true discussion of specific clinical presentations published after the new millennium.13 This was followed by more advanced basic science and clinical research, which led to an improved ability to understand modes of action of various therapies and to correlate treatment selection with specific visible manifestations of rosacea, including incorporation of physical devices.14-16 A newer perspective on evaluation and management of rosacea moved away from the “buckets” of rosacea subtypes to phenotypes observed at the time of clinical presentation.17,18
I could elaborate on research advancements with both diseases, but the bottom line is that information, developments, and current perspectives change over time. Keeping up is a challenge for all who study and practice dermatology. It is human nature to revert to what we already believe and do, which sometimes remains valid and other times is quite outdated and truly replaced by more optimal approaches. With AV and rosacea, progress is much slower in availability of newer agents. With AV, new agents have included topical dapsone, oral sarecycline, and topical clascoterone, with the latter being the first FDA-approved topical agent to mitigate the effects of androgens and sebum in both males and females. For rosacea, the 2 most recent FDA-approved therapies are minocycline foam and microencapsulated benzoyl peroxide. All of these therapies are proven to be effective for the modes of action and skin manifestations they specifically manage. Over the upcoming year, we are hoping to see the first triple-combination topical product come to market for AV, which will prompt our minds to consider if and how 3 established agents can work together to further augment treatment efficacy with favorable tolerability and safety.
Where will all of this end up? It is hard to say. We still have several other areas to tackle with both disease states, including establishing a well-substantiated understanding of the pathophysiologic role of the microbiome, sorting out the role of antibiotic use due to concerns about bacterial resistance, integration of FDA-approved physical devices in AV, and data on both diet and optimized skin care, to name a few.19-21
There is a lot on the plate to accomplish and digest. I have remained very involved in this subject matter for almost 3 decades and am still feeling the growing pains. Fortunately, the satisfaction of being part of a process so important to the lives of millions of patients makes this worth every moment. Stay tuned—more valuable information is to come.
- Wu J, Fang Z, Liu T, et al. Maximizing the utility of transcriptomics data in inflammatory skin diseases. Front Immunol. 2021;12:761890.
- Firlej E, Kowalska W, Szymaszek K, et al. The role of skin immune system in acne. J Clin Med. 2022;11:1579.
- Mias C, Mengeaud V, Bessou-Touya S, et al. Recent advances in understanding inflammatory acne: deciphering the relationship between Cutibacterium acnes and Th17 inflammatory pathway. J Eur Acad Dermatol Venereol. 2023;(37 suppl 2):3-11.
- Buddenkotte J, Steinhoff M. Recent advances in understanding and managing rosacea. F1000Res. 2018;7:F1000 Faculty Rev-1885. doi:10.12688/f1000research.16537.1
- Platsidaki E, Dessinioti C. Recent advances in understanding Propionibacterium acnes (Cutibacterium acnes) in acne. F1000Res. 2018;7:F1000 Faculty Rev-1953. doi:10.12688/f1000research.15659.1
- Leyden JJ. The evolving role of Propionibacterium acnes in acne. Semin Cutan Med Surg. 2001;20:139-143.
- Kim J. Review of the innate immune response in acne vulgaris: activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. Dermatology. 2005;211:193-198.
- Del Rosso JQ, Webster G, Weiss JS, et al. Nonantibiotic properties of tetracyclines in rosacea and their clinical implications. J Clin Aesthet Dermatol. 2021;14:14-21.
- Zhu W, Wang HL, Bu XL, et al. A narrative review of research progress on the role of NLRP3 inflammasome in acne vulgaris. Ann Transl Med. 2022;10:645.
- Leyden JJ, Del Rosso JQ, Baum EW. The use of isotretinoin in the treatment of acne vulgaris: clinical considerations and future directions. J Clin Aesthet Dermatol. 2014;7(2 suppl):S3-S21.
- Webster GF, Leyden JJ, Gross JA. Comparative pharmacokinetic profiles of a novel isotretinoin formulation (isotretinoin-Lidose) and the innovator isotretinoin formulation: a randomized, treatment, crossover study. J Am Acad Dermatol. 2013;69:762-767.
- Del Rosso JQ, Stein Gold L, Seagal J, et al. An open-label, phase IV study evaluating Lidose-isotretinoin administered without food in patients with severe recalcitrant nodular acne: low relapse rates observed over the 104-week post-treatment period. J Clin Aesthet Dermatol. 2019;12:13-18.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Steinhoff M, Buddenkotte J, Aubert J, et al. Clinical, cellular, and molecular aspects in the pathophysiology of rosacea. J Investig Dermatol Symp Proc. 2011;15:2-11.
- Yamasaki K, Gallo RL. The molecular pathology of rosacea. J Dermatol Sci. 2009;55:77-81.
- Tanghetti E, Del Rosso JQ, Thiboutot D, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 4: a status report on physical modalities and devices. Cutis. 2014;93:71-76.
- Del Rosso JQ, Gallo RL, Tanghetti E, et al. An evaluation of potential correlations between pathophysiologic mechanisms, clinical manifestations, and management of rosacea. Cutis. 2013;91(3 suppl):1-8.
- Schaller M, Almeida LMC, Bewley A, et al. Recommendations for rosacea diagnosis, classification and management: update from the global ROSacea COnsensus 2019 panel. Br J Dermatol. 2020;182:1269-1276.
- Xu H, Li H. Acne, the skin microbiome, and antibiotic treatment. Am J Clin Dermatol. 2019;20:335-344.
- Daou H, Paradiso M, Hennessy K. Rosacea and the microbiome: a systematic review. Dermatol Ther (Heidelb). 2021;11:1-12.
- Kayiran MA, Karadag AS, Al-Khuzaei S, et al. Antibiotic resistance in acne: mechanisms, complications and management. Am J Clin Dermatol. 2020;21:813-819.
- Wu J, Fang Z, Liu T, et al. Maximizing the utility of transcriptomics data in inflammatory skin diseases. Front Immunol. 2021;12:761890.
- Firlej E, Kowalska W, Szymaszek K, et al. The role of skin immune system in acne. J Clin Med. 2022;11:1579.
- Mias C, Mengeaud V, Bessou-Touya S, et al. Recent advances in understanding inflammatory acne: deciphering the relationship between Cutibacterium acnes and Th17 inflammatory pathway. J Eur Acad Dermatol Venereol. 2023;(37 suppl 2):3-11.
- Buddenkotte J, Steinhoff M. Recent advances in understanding and managing rosacea. F1000Res. 2018;7:F1000 Faculty Rev-1885. doi:10.12688/f1000research.16537.1
- Platsidaki E, Dessinioti C. Recent advances in understanding Propionibacterium acnes (Cutibacterium acnes) in acne. F1000Res. 2018;7:F1000 Faculty Rev-1953. doi:10.12688/f1000research.15659.1
- Leyden JJ. The evolving role of Propionibacterium acnes in acne. Semin Cutan Med Surg. 2001;20:139-143.
- Kim J. Review of the innate immune response in acne vulgaris: activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. Dermatology. 2005;211:193-198.
- Del Rosso JQ, Webster G, Weiss JS, et al. Nonantibiotic properties of tetracyclines in rosacea and their clinical implications. J Clin Aesthet Dermatol. 2021;14:14-21.
- Zhu W, Wang HL, Bu XL, et al. A narrative review of research progress on the role of NLRP3 inflammasome in acne vulgaris. Ann Transl Med. 2022;10:645.
- Leyden JJ, Del Rosso JQ, Baum EW. The use of isotretinoin in the treatment of acne vulgaris: clinical considerations and future directions. J Clin Aesthet Dermatol. 2014;7(2 suppl):S3-S21.
- Webster GF, Leyden JJ, Gross JA. Comparative pharmacokinetic profiles of a novel isotretinoin formulation (isotretinoin-Lidose) and the innovator isotretinoin formulation: a randomized, treatment, crossover study. J Am Acad Dermatol. 2013;69:762-767.
- Del Rosso JQ, Stein Gold L, Seagal J, et al. An open-label, phase IV study evaluating Lidose-isotretinoin administered without food in patients with severe recalcitrant nodular acne: low relapse rates observed over the 104-week post-treatment period. J Clin Aesthet Dermatol. 2019;12:13-18.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Steinhoff M, Buddenkotte J, Aubert J, et al. Clinical, cellular, and molecular aspects in the pathophysiology of rosacea. J Investig Dermatol Symp Proc. 2011;15:2-11.
- Yamasaki K, Gallo RL. The molecular pathology of rosacea. J Dermatol Sci. 2009;55:77-81.
- Tanghetti E, Del Rosso JQ, Thiboutot D, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 4: a status report on physical modalities and devices. Cutis. 2014;93:71-76.
- Del Rosso JQ, Gallo RL, Tanghetti E, et al. An evaluation of potential correlations between pathophysiologic mechanisms, clinical manifestations, and management of rosacea. Cutis. 2013;91(3 suppl):1-8.
- Schaller M, Almeida LMC, Bewley A, et al. Recommendations for rosacea diagnosis, classification and management: update from the global ROSacea COnsensus 2019 panel. Br J Dermatol. 2020;182:1269-1276.
- Xu H, Li H. Acne, the skin microbiome, and antibiotic treatment. Am J Clin Dermatol. 2019;20:335-344.
- Daou H, Paradiso M, Hennessy K. Rosacea and the microbiome: a systematic review. Dermatol Ther (Heidelb). 2021;11:1-12.
- Kayiran MA, Karadag AS, Al-Khuzaei S, et al. Antibiotic resistance in acne: mechanisms, complications and management. Am J Clin Dermatol. 2020;21:813-819.
Long-term Remission of Pyoderma Gangrenosum, Acne, and Hidradenitis Suppurativa Syndrome
Pyoderma gangrenosum (PG), acne, and hidradenitis suppurativa (HS)(PASH) syndrome is a recently identified disease process within the spectrum of autoinflammatory diseases (AIDs), which are distinct from autoimmune, infectious, and allergic syndromes and are gaining increasing interest given their complex pathophysiology and therapeutic resistance.1 Autoinflammatory diseases are defined by a dysregulation of the innate immune system in the absence of typical autoimmune features, including autoantibodies and antigen-specific T lymphocytes.2 Mutations affecting proteins of the inflammasome or proteins involved in regulating inflammasome function have been associated with these AIDs.2
Many AIDs have cutaneous involvement, as seen in PASH syndrome. Pyoderma gangrenosum is a neutrophilic dermatosis presenting as skin ulcers with undermined, erythematous, violaceous borders. It can be isolated, syndromic, or associated with inflammatory conditions (eg, inflammatory bowel disease, rheumatologic disorders, hematologic disorders).1 Acne vulgaris develops because of chronic obstruction of hair follicles as a result of disordered keratinization and abnormal sebaceous stem cell differentiation.2 Propionibacterium acnes can reside and replicate within the biofilm community of the hair follicle and activate the inflammasome.2,3 Hidradenitis suppurativa, a chronic relapsing neutrophilic dermatosis, is a debilitating inflammatory disease of the hair follicles involving apocrine gland–bearing skin (ie, the axillary, inguinal, and anogenital regions).2 Onset often occurs between the ages of 20 and 40 years, with a 3-fold higher incidence in women compared to men.3 Patients experience painful, deep-seated nodules that drain into sinus tracts and abscesses. The condition can be isolated or associated with inflammatory conditions, such as inflammatory bowel disease.4
PASH syndrome has been described as a polygenic autoinflammatory condition that most commonly presents in young adults, with onset of acne beginning years prior to other manifestations. A study analyzing 5 patients with PASH syndrome reported an average age of 32.2 years at diagnosis with a disease duration of 3 to 7 years.5 Pathophysiology of this condition is not well understood, with many hypotheses calling upon dysregulation of the innate immune system, a commonality this syndrome may share with other AIDs. Given its poorly understood pathophysiology, treating PASH syndrome can be especially difficult. We report a novel case of disease remission lasting more than 4 years using adalimumab and cyclosporine. We also discuss prior treatment successes and hypotheses regarding etiologic factors in PASH syndrome.
Case Report
A 36-year-old woman presented for evaluation of open draining ulcerations on the back of 18 months’ duration. She had a 16-year history of scarring cystic acne of the face and HS of the groin. The patient’s family history was remarkable for severe cystic acne in her brother and son as well as HS in her mother and another brother. Her treatment history included isotretinoin, doxycycline, and topical steroids.
Physical examination revealed 2 ulcerations with violaceous borders involving the left upper back (greatest diameter, 5×7 cm)(Figure 1). Evidence of papular and cystic acne with residual scarring was noted on the cheeks. Scarring from HS was noted in the axillae and right groin. A biopsy from the edge of an ulceration on the back demonstrated epidermal spongiosis with acute and chronic inflammation and fibrosis (Figure 2). The clinicopathologic findings were most consistent with PG, and the patient was diagnosed with PASH syndrome, given the constellation of cutaneous lesions.
After treatment with topical and systemic antibiotics for acne and HS for more than 1 year failed, the patient was started on adalimumab. The initial dose was 160 mg subcutaneously, then 80 mg 2 weeks later, then 40 mg weekly thereafter. Doxycycline was continued for treatment of the acne and HS. After 6 weeks of adalimumab, the PG worsened and prednisone was added. She developed tender furuncles on the back, and cultures grew Pseudomonas aeruginosa and methicillin-sensitive Staphylococcus aureus that responded to ciprofloxacin and cephalexin.
Due to progression of PG on adalimumab, switching to an infliximab infusion or anakinra was considered, but these options were not covered by the patient’s health insurance. Three months after the initial presentation, the patient was started on cyclosporine 100 mg 3 times daily (5 mg/kg/d) while adalimumab was continued; the ulcers started to improve within 2.5 weeks. After 3 months (Figure 3), the cyclosporine was reduced to 100 mg twice daily, and adalimumab was continued. She had a slight flare of PG after 8 months of treatment when adalimumab was unavailable to her for 2 months. After 8 months on cyclosporine, the dosage was tapered to 100 mg/d and then completely discontinued after 12 months.
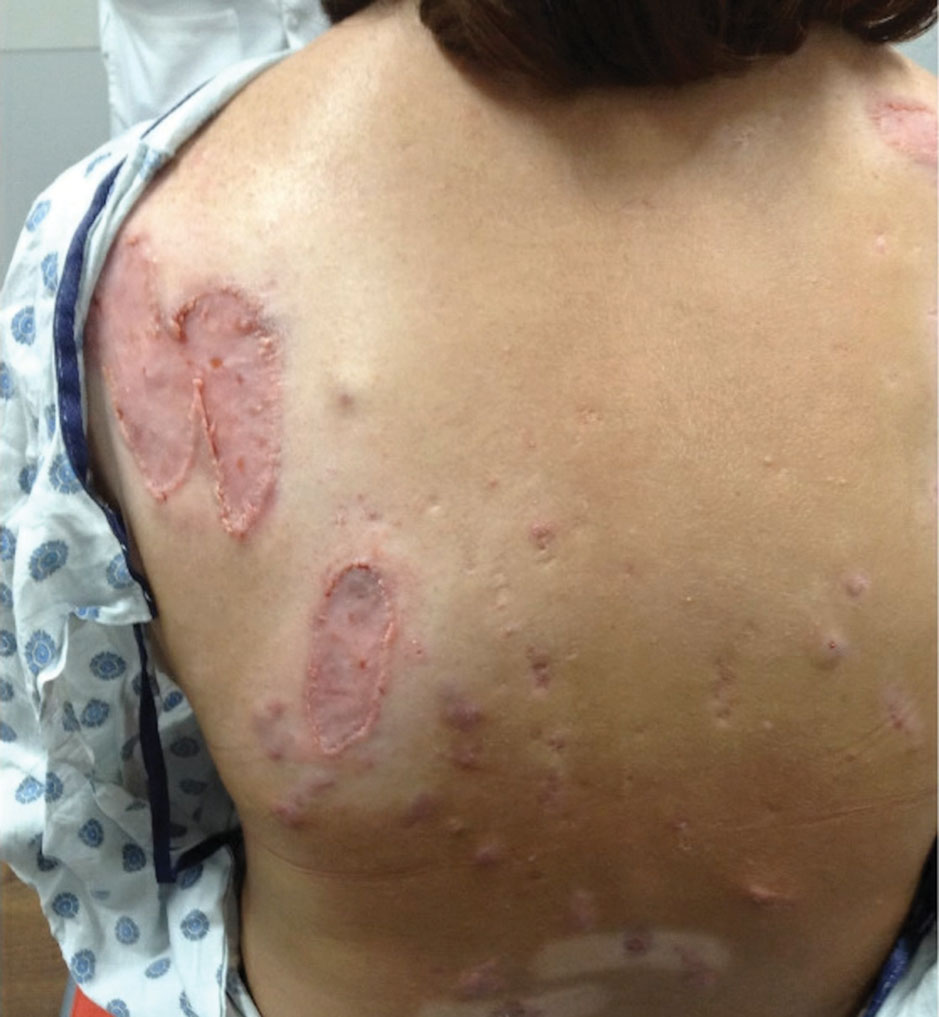
The patient has continued on adalimumab 40 mg weekly with excellent control of the PG (Figure 4), although she did have one HS flare in the left axilla 11 months after the initial treatment. The patient’s cystic acne has intermittently flared and has been managed with spironolactone 100 mg/d for 3 years. After 4 years of management, the patient’s PG and HS remain well controlled on adalimumab.
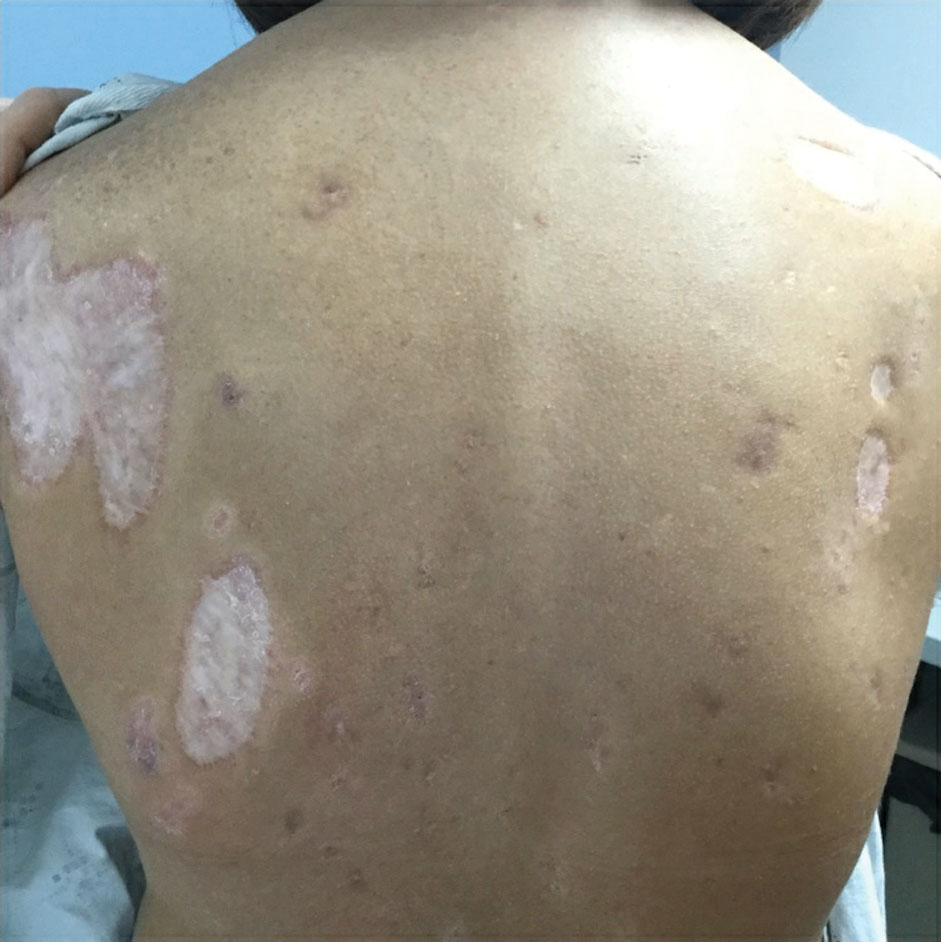
Comment
Our case represents a major step in refining long-term treatment approaches for PASH syndrome due to the 4-year remission. Prior cases have reported use of anakinra, anakinra-cyclosporine combination, prednisone, azathioprine, topical tacrolimus, etanercept, and dapsone without sustainable success.1-6 The case studies discussed below have achieved remission via alternative drug combinations.
Staub et al4 found greatest success with a combination of infliximab, dapsone, and cyclosporine, and their patient had been in remission for 20 months at time of publication. Their hypothesis proposed that multiple inflammatory signaling pathways are involved in PASH syndrome, and this is why combination therapy is required for remission.4 In 2018, Lamiaux et al7 demonstrated successful treatment with rifampicin and clindamycin. Their patient had been in remission for 22 months at the time of publication—this time frame included 12 months of combination therapy and 10 months without medication. The authors hypothesized that, because of the autoinflammatory nature of these antibiotics, this pharmacologic combination could eradicate pathogenic bacteria from host microbiota while also inhibiting neutrophil function and synthesis of chemokines and cytokines.7
More recently, reports have been published regarding the success of tildrakizumab, an IL-23 antagonist, and ixekizumab, an IL-17 antagonist, in the treatment of PASH syndrome.6,8 Ixekizumab was used in combination with doxycycline, and remission was achieved in 12 months.8 However, tildrakizumab was used alone and achieved greater than 75% improvement in disease manifestations within 2 months.
Marzano et al5 conducted protein arrays and enzyme-linked immunosorbent assay to analyze the expression of cytokine, chemokine, and effector molecule profiles in PASH syndrome. It was determined that serum analysis displayed a normal cytokine/chemokine profile, with the only abnormalities being anemia and elevated C-reactive protein. There were no statistically significant differences in serum levels of IL-1β, tumor necrosis factor (TNF) α, or IL-17 between PASH syndrome and healthy controls. However, cutaneous analysis revealed extensive cytokine and chemokine hyperactivity for IL-1β and IL-1β receptor; TNF-α; C-X-C motif ligands 1, 2, and 3; C-X-C motif ligand 16;
Ead et al3 presented a unique perspective focusing on cutaneous biofilm involvement in PASH syndrome. Microbes within these biofilms induce the migration and proliferation of inflammatory cells that consume factors normally utilized for tissue catabolism. These organisms deplete necessary biochemical cofactors used during healing. This lack of nutrients needed for healing not only slows the process but also promotes favorable conditions for the growth of anerobic species. In conjunction, biofilm formation restricts bacterial access to oxygen and nutrients, thus decreasing the bacterial metabolic rate and preventing the effects of antibiotic therapy. These features of biofilm communities contribute to inflammation and possibly the troubling resistance to many therapeutic options for PASH syndrome.
Each component of PASH syndrome has been associated with biofilm formation. As previously described, PG manifests in the skin as painful ulcerations, often with slough. This slough is hypothesized to be a consequence of increased vascular permeability and exudative byproducts that accompany the inflammatory nature of biofilms.3 Acne vulgaris has well-described associations with P acnes. Ead et al3 described P acnes as a component of the biofilm community within the microcomedone of hair follicles. This biofilm allows for antibiotic resistance occasionally seen in the treatment of acne and is potentially the pathogenic factor that both impedes healing and enhances the inflammatory state. Hidradenitis suppurativa has been associated with biofilm formation.3
In further pursuit of PASH syndrome pathophysiology, many experts have sought to uncover the relationship between PASH syndrome and the previously described pyogenic arthritis, PG, and acne (PAPA) syndrome, another entity within the AIDs spectrum (Table). This condition was first recognized in 1997 in a 3-generation family with 10 affected members.1 It is characterized by PG and acne, similar to PASH; however, PAPA syndrome includes PG arthritis and lacks HS. Pyogenic arthritis manifests as recurrent aseptic inflammation of the joints, mainly the elbows, knees, and ankles. Pyogenic arthritis commonly is the presenting symptom of PAPA syndrome, with onset in childhood.2 As patients age, the arthritic symptoms decrease, and skin manifestations become more prominent.
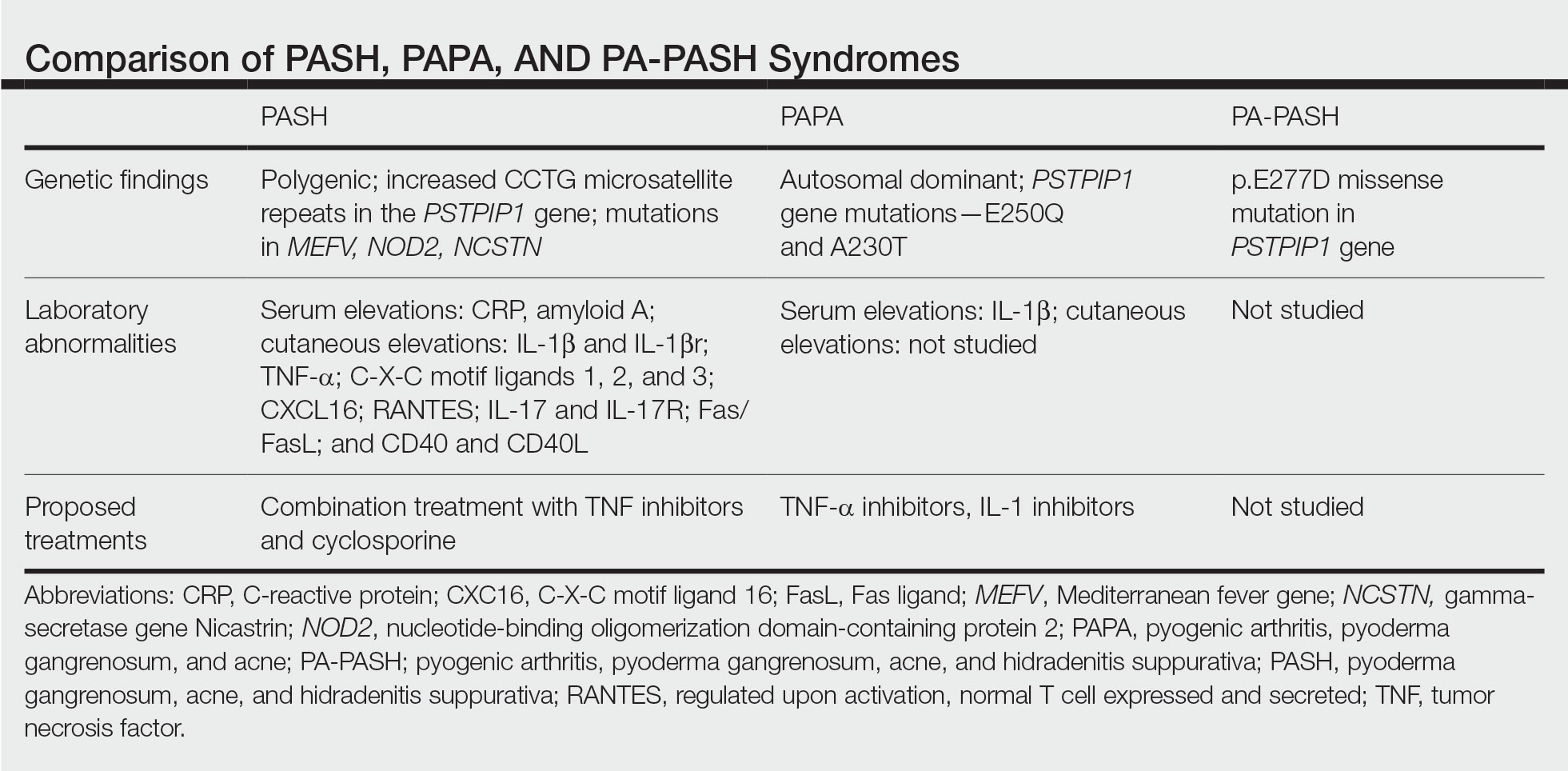
PAPA syndrome has autosomal-dominant inheritance with mutations on chromosome 15 in the proline-serine-threonine phosphatase interacting protein 1 (PSTPIP1) gene.1 This mutation induces hyperphosphorylation of PSTPIP1, allowing for increased binding affinity to pyrin. Both PSTPIP1 and pyrin are co-expressed as parts of the NLRP3 inflammasome in granulocytes and monocytes.1 As a result, pyrin is more highly bound and loses its inhibitory effect on the NLRP3 inflammasome pathway. This lack of inhibition allows for uninhibited cleavage of pro–IL-1β to active IL-1β by the inflammasome.1
Elevated concentrations of IL-1β in patients with PAPA syndrome result in a dysregulation of the innate immune system. IL-1β induces the release of proinflammatory cytokines, namely TNF-α; interferon γ; IL-8; and regulated on activation, normal T cell expressed and secreted (RANTES), all of which activate neutrophils and induce neutrophilic inflammation.2 IL-1β not only initiates this entire cascade but also acts as an antiapoptotic signal for neutrophils.2 When IL-1β reaches a critical threshold, it induces enough inflammation to cause severe tissue damage, thus causing joint and cutaneous disease in PAPA syndrome. IL-1 inhibitors (anakinra) or TNF-α inhibitors (etanercept, adalimumab, infliximab) have been used many times to successfully treat PAPA syndrome, with TNF-α inhibitors providing the most consistent results.
Another AIDs entity with similarities to both PAPA syndrome and PASH syndrome is pyogenic arthritis, PG, acne, and HS (PA-PASH) syndrome. First identified in 2012 by Bruzzese,9 genetic analyses revealed a p.E277D missense mutation in PSTPIP1 in PA-PASH syndrome. Research has suggested that the key molecular feature is neutrophil activation by TH17 cells and the TNF-α axis.9 This syndrome has not been further characterized, and little is known regarding adequate treatment for PA-PASH syndrome.
Although it is similar in phenotype to aspects of PAPA and PA-PASH syndromes, PASH syndrome has distinct genotypic and immunologic abnormalities. Genetic analysis of this condition has shown an increased number of CCTG repeats in proximity to the PSTPIP1 promoter. It is hypothesized that these additional repeats predispose patients to neutrophilic inflammation in a similar manner to a condition described in France, termed aseptic abscess syndrome.1,5 Other mutations have been identified, including those in IL-1N, PSMB8, MEFV, NOD2, NCSTN, and more.2,7 However, it has been determined that the majority of these variants have already been filed in the Single Nucleotide Polymorphism Database or in the Registry of Hereditary Auto-inflammatory Disorders Mutations.2 The question remains regarding the origin of inflammation seen in PASH syndrome; the potential role of biofilms; and the relationship between PASH, PAPA, and PA-PASH syndromes. Much work remains to be done in refining therapeutic options for PASH syndrome. Continued biochemical research is necessary, as well as collaboration among dermatologists worldwide who find success in treating this condition.
Conclusion
There are genotypic and phenotypic similarities between PASH, PAPA, and PA-PASH syndromes, with various mutations within or near the PSTPIP1 gene; however, their genetic discrepancies seem to play a major role in the pathophysiology of each syndrome. Much work remains to be done in PA-PASH syndrome, which has not yet been well described. Meanwhile, PAPA syndrome has been well characterized with mutations affecting proteins of the NLRP3 inflammasome, resulting in elevated IL-1β and excess neutrophilic inflammation. In PASH syndrome, the importance of increased repeats near the PSTPIP1 promoter is yet to be elucidated. It has been shown that these abnormalities predispose individuals to neutrophilic inflammation, but the mechanism by which they do so is unknown. In addition, consideration of biofilms and their predisposition to inflammation within the pathophysiology of PASH syndrome is a possibility that must be considered when discussing therapeutic options. Based on our case study and previous successes in treating PASH syndrome, it is clear that a multidrug approach is necessary for remission. It is likely that the etiology of PASH syndrome is multifaceted and involves hyperactivity in multiple arms of the innate immune system.
Patients with PASH syndrome have severely impaired quality of life and often experience social withdrawal due to the disfiguring sequelae and limited treatment options available. To improve patient outcomes, it is essential for physicians and scientists to report on successful treatment strategies and advances in immunologic understanding. Improved understanding of PASH syndrome calls for further genetic exploration into the role of additional genomic repeats and how these affect the PSTPIP1 gene and inflammasome activity. As medical advances improve understanding of the pathophysiology of this disease entity, it will likely become clear which mechanisms are most important in disease progression and how clinicians can best optimize treatment.
- Braun-Falco M, Kovnerystyy O, Lohse P, et al. Pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH)—a new autoinflammatory syndrome distinct from PAPA syndrome. J Am Acad Dermatol. 2012;66:409-415.
- Cugno M, Borghi A, Marzano AV. PAPA, PASH and PAPASH syndromes: pathophysiology, presentation and treatment. Am J Clin Dermatol. 2017;18:555-562.
- Ead JK, Snyder RJ, Wise J, et al. Is PASH syndrome a biofilm disease?: a case series and review of the literature. Wounds. 2018;30:216-223.
- Staub J, Pfannschmidt N, Strohal R, et al. Successful treatment of PASH syndrome with infliximab, cyclosporine and dapsone. J Eur Acad Dermatol Venereol. 2015;29:2243-2247.
- Marzano AV, Ceccherini I, Gattorno M, et al. Association of pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH) shares genetic and cytokine profiles with other autoinflammatory diseases. Medicine (Baltimore). 2014;93:E187.
- Kok Y, Nicolopoulos J, Varigos G, et al. Tildrakizumab in the treatment of PASH syndrome: a potential novel therapeutic target. Australas J Dermatol. 2020;61:E373-E374.
- Lamiaux M, Dabouz F, Wantz M, et al. Successful combined antibiotic therapy with oral clindamycin and oral rifampicin for pyoderma gangrenosum in patient with PASH syndrome. JAAD Case Rep. 2018;4:17-21.
- Gul MI, Singam V, Hanson C, et al. Remission of refractory PASH syndrome using ixekizumab and doxycycline. J Drugs Dermatol. 2020;19:1123.
- Bruzzese V. Pyoderma gangrenosum, acne conglobata, suppurative hidradenitis, and axial spondyloarthritis: efficacy of anti-tumor necrosis factor α therapy. J Clin Rheumatol. 2012;18:413-415.
Pyoderma gangrenosum (PG), acne, and hidradenitis suppurativa (HS)(PASH) syndrome is a recently identified disease process within the spectrum of autoinflammatory diseases (AIDs), which are distinct from autoimmune, infectious, and allergic syndromes and are gaining increasing interest given their complex pathophysiology and therapeutic resistance.1 Autoinflammatory diseases are defined by a dysregulation of the innate immune system in the absence of typical autoimmune features, including autoantibodies and antigen-specific T lymphocytes.2 Mutations affecting proteins of the inflammasome or proteins involved in regulating inflammasome function have been associated with these AIDs.2
Many AIDs have cutaneous involvement, as seen in PASH syndrome. Pyoderma gangrenosum is a neutrophilic dermatosis presenting as skin ulcers with undermined, erythematous, violaceous borders. It can be isolated, syndromic, or associated with inflammatory conditions (eg, inflammatory bowel disease, rheumatologic disorders, hematologic disorders).1 Acne vulgaris develops because of chronic obstruction of hair follicles as a result of disordered keratinization and abnormal sebaceous stem cell differentiation.2 Propionibacterium acnes can reside and replicate within the biofilm community of the hair follicle and activate the inflammasome.2,3 Hidradenitis suppurativa, a chronic relapsing neutrophilic dermatosis, is a debilitating inflammatory disease of the hair follicles involving apocrine gland–bearing skin (ie, the axillary, inguinal, and anogenital regions).2 Onset often occurs between the ages of 20 and 40 years, with a 3-fold higher incidence in women compared to men.3 Patients experience painful, deep-seated nodules that drain into sinus tracts and abscesses. The condition can be isolated or associated with inflammatory conditions, such as inflammatory bowel disease.4
PASH syndrome has been described as a polygenic autoinflammatory condition that most commonly presents in young adults, with onset of acne beginning years prior to other manifestations. A study analyzing 5 patients with PASH syndrome reported an average age of 32.2 years at diagnosis with a disease duration of 3 to 7 years.5 Pathophysiology of this condition is not well understood, with many hypotheses calling upon dysregulation of the innate immune system, a commonality this syndrome may share with other AIDs. Given its poorly understood pathophysiology, treating PASH syndrome can be especially difficult. We report a novel case of disease remission lasting more than 4 years using adalimumab and cyclosporine. We also discuss prior treatment successes and hypotheses regarding etiologic factors in PASH syndrome.
Case Report
A 36-year-old woman presented for evaluation of open draining ulcerations on the back of 18 months’ duration. She had a 16-year history of scarring cystic acne of the face and HS of the groin. The patient’s family history was remarkable for severe cystic acne in her brother and son as well as HS in her mother and another brother. Her treatment history included isotretinoin, doxycycline, and topical steroids.

Physical examination revealed 2 ulcerations with violaceous borders involving the left upper back (greatest diameter, 5×7 cm)(Figure 1). Evidence of papular and cystic acne with residual scarring was noted on the cheeks. Scarring from HS was noted in the axillae and right groin. A biopsy from the edge of an ulceration on the back demonstrated epidermal spongiosis with acute and chronic inflammation and fibrosis (Figure 2). The clinicopathologic findings were most consistent with PG, and the patient was diagnosed with PASH syndrome, given the constellation of cutaneous lesions.

After treatment with topical and systemic antibiotics for acne and HS for more than 1 year failed, the patient was started on adalimumab. The initial dose was 160 mg subcutaneously, then 80 mg 2 weeks later, then 40 mg weekly thereafter. Doxycycline was continued for treatment of the acne and HS. After 6 weeks of adalimumab, the PG worsened and prednisone was added. She developed tender furuncles on the back, and cultures grew Pseudomonas aeruginosa and methicillin-sensitive Staphylococcus aureus that responded to ciprofloxacin and cephalexin.
Due to progression of PG on adalimumab, switching to an infliximab infusion or anakinra was considered, but these options were not covered by the patient’s health insurance. Three months after the initial presentation, the patient was started on cyclosporine 100 mg 3 times daily (5 mg/kg/d) while adalimumab was continued; the ulcers started to improve within 2.5 weeks. After 3 months (Figure 3), the cyclosporine was reduced to 100 mg twice daily, and adalimumab was continued. She had a slight flare of PG after 8 months of treatment when adalimumab was unavailable to her for 2 months. After 8 months on cyclosporine, the dosage was tapered to 100 mg/d and then completely discontinued after 12 months.

The patient has continued on adalimumab 40 mg weekly with excellent control of the PG (Figure 4), although she did have one HS flare in the left axilla 11 months after the initial treatment. The patient’s cystic acne has intermittently flared and has been managed with spironolactone 100 mg/d for 3 years. After 4 years of management, the patient’s PG and HS remain well controlled on adalimumab.

Comment
Our case represents a major step in refining long-term treatment approaches for PASH syndrome due to the 4-year remission. Prior cases have reported use of anakinra, anakinra-cyclosporine combination, prednisone, azathioprine, topical tacrolimus, etanercept, and dapsone without sustainable success.1-6 The case studies discussed below have achieved remission via alternative drug combinations.
Staub et al4 found greatest success with a combination of infliximab, dapsone, and cyclosporine, and their patient had been in remission for 20 months at time of publication. Their hypothesis proposed that multiple inflammatory signaling pathways are involved in PASH syndrome, and this is why combination therapy is required for remission.4 In 2018, Lamiaux et al7 demonstrated successful treatment with rifampicin and clindamycin. Their patient had been in remission for 22 months at the time of publication—this time frame included 12 months of combination therapy and 10 months without medication. The authors hypothesized that, because of the autoinflammatory nature of these antibiotics, this pharmacologic combination could eradicate pathogenic bacteria from host microbiota while also inhibiting neutrophil function and synthesis of chemokines and cytokines.7
More recently, reports have been published regarding the success of tildrakizumab, an IL-23 antagonist, and ixekizumab, an IL-17 antagonist, in the treatment of PASH syndrome.6,8 Ixekizumab was used in combination with doxycycline, and remission was achieved in 12 months.8 However, tildrakizumab was used alone and achieved greater than 75% improvement in disease manifestations within 2 months.
Marzano et al5 conducted protein arrays and enzyme-linked immunosorbent assay to analyze the expression of cytokine, chemokine, and effector molecule profiles in PASH syndrome. It was determined that serum analysis displayed a normal cytokine/chemokine profile, with the only abnormalities being anemia and elevated C-reactive protein. There were no statistically significant differences in serum levels of IL-1β, tumor necrosis factor (TNF) α, or IL-17 between PASH syndrome and healthy controls. However, cutaneous analysis revealed extensive cytokine and chemokine hyperactivity for IL-1β and IL-1β receptor; TNF-α; C-X-C motif ligands 1, 2, and 3; C-X-C motif ligand 16;
Ead et al3 presented a unique perspective focusing on cutaneous biofilm involvement in PASH syndrome. Microbes within these biofilms induce the migration and proliferation of inflammatory cells that consume factors normally utilized for tissue catabolism. These organisms deplete necessary biochemical cofactors used during healing. This lack of nutrients needed for healing not only slows the process but also promotes favorable conditions for the growth of anerobic species. In conjunction, biofilm formation restricts bacterial access to oxygen and nutrients, thus decreasing the bacterial metabolic rate and preventing the effects of antibiotic therapy. These features of biofilm communities contribute to inflammation and possibly the troubling resistance to many therapeutic options for PASH syndrome.
Each component of PASH syndrome has been associated with biofilm formation. As previously described, PG manifests in the skin as painful ulcerations, often with slough. This slough is hypothesized to be a consequence of increased vascular permeability and exudative byproducts that accompany the inflammatory nature of biofilms.3 Acne vulgaris has well-described associations with P acnes. Ead et al3 described P acnes as a component of the biofilm community within the microcomedone of hair follicles. This biofilm allows for antibiotic resistance occasionally seen in the treatment of acne and is potentially the pathogenic factor that both impedes healing and enhances the inflammatory state. Hidradenitis suppurativa has been associated with biofilm formation.3
In further pursuit of PASH syndrome pathophysiology, many experts have sought to uncover the relationship between PASH syndrome and the previously described pyogenic arthritis, PG, and acne (PAPA) syndrome, another entity within the AIDs spectrum (Table). This condition was first recognized in 1997 in a 3-generation family with 10 affected members.1 It is characterized by PG and acne, similar to PASH; however, PAPA syndrome includes PG arthritis and lacks HS. Pyogenic arthritis manifests as recurrent aseptic inflammation of the joints, mainly the elbows, knees, and ankles. Pyogenic arthritis commonly is the presenting symptom of PAPA syndrome, with onset in childhood.2 As patients age, the arthritic symptoms decrease, and skin manifestations become more prominent.

PAPA syndrome has autosomal-dominant inheritance with mutations on chromosome 15 in the proline-serine-threonine phosphatase interacting protein 1 (PSTPIP1) gene.1 This mutation induces hyperphosphorylation of PSTPIP1, allowing for increased binding affinity to pyrin. Both PSTPIP1 and pyrin are co-expressed as parts of the NLRP3 inflammasome in granulocytes and monocytes.1 As a result, pyrin is more highly bound and loses its inhibitory effect on the NLRP3 inflammasome pathway. This lack of inhibition allows for uninhibited cleavage of pro–IL-1β to active IL-1β by the inflammasome.1
Elevated concentrations of IL-1β in patients with PAPA syndrome result in a dysregulation of the innate immune system. IL-1β induces the release of proinflammatory cytokines, namely TNF-α; interferon γ; IL-8; and regulated on activation, normal T cell expressed and secreted (RANTES), all of which activate neutrophils and induce neutrophilic inflammation.2 IL-1β not only initiates this entire cascade but also acts as an antiapoptotic signal for neutrophils.2 When IL-1β reaches a critical threshold, it induces enough inflammation to cause severe tissue damage, thus causing joint and cutaneous disease in PAPA syndrome. IL-1 inhibitors (anakinra) or TNF-α inhibitors (etanercept, adalimumab, infliximab) have been used many times to successfully treat PAPA syndrome, with TNF-α inhibitors providing the most consistent results.
Another AIDs entity with similarities to both PAPA syndrome and PASH syndrome is pyogenic arthritis, PG, acne, and HS (PA-PASH) syndrome. First identified in 2012 by Bruzzese,9 genetic analyses revealed a p.E277D missense mutation in PSTPIP1 in PA-PASH syndrome. Research has suggested that the key molecular feature is neutrophil activation by TH17 cells and the TNF-α axis.9 This syndrome has not been further characterized, and little is known regarding adequate treatment for PA-PASH syndrome.
Although it is similar in phenotype to aspects of PAPA and PA-PASH syndromes, PASH syndrome has distinct genotypic and immunologic abnormalities. Genetic analysis of this condition has shown an increased number of CCTG repeats in proximity to the PSTPIP1 promoter. It is hypothesized that these additional repeats predispose patients to neutrophilic inflammation in a similar manner to a condition described in France, termed aseptic abscess syndrome.1,5 Other mutations have been identified, including those in IL-1N, PSMB8, MEFV, NOD2, NCSTN, and more.2,7 However, it has been determined that the majority of these variants have already been filed in the Single Nucleotide Polymorphism Database or in the Registry of Hereditary Auto-inflammatory Disorders Mutations.2 The question remains regarding the origin of inflammation seen in PASH syndrome; the potential role of biofilms; and the relationship between PASH, PAPA, and PA-PASH syndromes. Much work remains to be done in refining therapeutic options for PASH syndrome. Continued biochemical research is necessary, as well as collaboration among dermatologists worldwide who find success in treating this condition.
Conclusion
There are genotypic and phenotypic similarities between PASH, PAPA, and PA-PASH syndromes, with various mutations within or near the PSTPIP1 gene; however, their genetic discrepancies seem to play a major role in the pathophysiology of each syndrome. Much work remains to be done in PA-PASH syndrome, which has not yet been well described. Meanwhile, PAPA syndrome has been well characterized with mutations affecting proteins of the NLRP3 inflammasome, resulting in elevated IL-1β and excess neutrophilic inflammation. In PASH syndrome, the importance of increased repeats near the PSTPIP1 promoter is yet to be elucidated. It has been shown that these abnormalities predispose individuals to neutrophilic inflammation, but the mechanism by which they do so is unknown. In addition, consideration of biofilms and their predisposition to inflammation within the pathophysiology of PASH syndrome is a possibility that must be considered when discussing therapeutic options. Based on our case study and previous successes in treating PASH syndrome, it is clear that a multidrug approach is necessary for remission. It is likely that the etiology of PASH syndrome is multifaceted and involves hyperactivity in multiple arms of the innate immune system.
Patients with PASH syndrome have severely impaired quality of life and often experience social withdrawal due to the disfiguring sequelae and limited treatment options available. To improve patient outcomes, it is essential for physicians and scientists to report on successful treatment strategies and advances in immunologic understanding. Improved understanding of PASH syndrome calls for further genetic exploration into the role of additional genomic repeats and how these affect the PSTPIP1 gene and inflammasome activity. As medical advances improve understanding of the pathophysiology of this disease entity, it will likely become clear which mechanisms are most important in disease progression and how clinicians can best optimize treatment.
Pyoderma gangrenosum (PG), acne, and hidradenitis suppurativa (HS)(PASH) syndrome is a recently identified disease process within the spectrum of autoinflammatory diseases (AIDs), which are distinct from autoimmune, infectious, and allergic syndromes and are gaining increasing interest given their complex pathophysiology and therapeutic resistance.1 Autoinflammatory diseases are defined by a dysregulation of the innate immune system in the absence of typical autoimmune features, including autoantibodies and antigen-specific T lymphocytes.2 Mutations affecting proteins of the inflammasome or proteins involved in regulating inflammasome function have been associated with these AIDs.2
Many AIDs have cutaneous involvement, as seen in PASH syndrome. Pyoderma gangrenosum is a neutrophilic dermatosis presenting as skin ulcers with undermined, erythematous, violaceous borders. It can be isolated, syndromic, or associated with inflammatory conditions (eg, inflammatory bowel disease, rheumatologic disorders, hematologic disorders).1 Acne vulgaris develops because of chronic obstruction of hair follicles as a result of disordered keratinization and abnormal sebaceous stem cell differentiation.2 Propionibacterium acnes can reside and replicate within the biofilm community of the hair follicle and activate the inflammasome.2,3 Hidradenitis suppurativa, a chronic relapsing neutrophilic dermatosis, is a debilitating inflammatory disease of the hair follicles involving apocrine gland–bearing skin (ie, the axillary, inguinal, and anogenital regions).2 Onset often occurs between the ages of 20 and 40 years, with a 3-fold higher incidence in women compared to men.3 Patients experience painful, deep-seated nodules that drain into sinus tracts and abscesses. The condition can be isolated or associated with inflammatory conditions, such as inflammatory bowel disease.4
PASH syndrome has been described as a polygenic autoinflammatory condition that most commonly presents in young adults, with onset of acne beginning years prior to other manifestations. A study analyzing 5 patients with PASH syndrome reported an average age of 32.2 years at diagnosis with a disease duration of 3 to 7 years.5 Pathophysiology of this condition is not well understood, with many hypotheses calling upon dysregulation of the innate immune system, a commonality this syndrome may share with other AIDs. Given its poorly understood pathophysiology, treating PASH syndrome can be especially difficult. We report a novel case of disease remission lasting more than 4 years using adalimumab and cyclosporine. We also discuss prior treatment successes and hypotheses regarding etiologic factors in PASH syndrome.
Case Report
A 36-year-old woman presented for evaluation of open draining ulcerations on the back of 18 months’ duration. She had a 16-year history of scarring cystic acne of the face and HS of the groin. The patient’s family history was remarkable for severe cystic acne in her brother and son as well as HS in her mother and another brother. Her treatment history included isotretinoin, doxycycline, and topical steroids.

Physical examination revealed 2 ulcerations with violaceous borders involving the left upper back (greatest diameter, 5×7 cm)(Figure 1). Evidence of papular and cystic acne with residual scarring was noted on the cheeks. Scarring from HS was noted in the axillae and right groin. A biopsy from the edge of an ulceration on the back demonstrated epidermal spongiosis with acute and chronic inflammation and fibrosis (Figure 2). The clinicopathologic findings were most consistent with PG, and the patient was diagnosed with PASH syndrome, given the constellation of cutaneous lesions.

After treatment with topical and systemic antibiotics for acne and HS for more than 1 year failed, the patient was started on adalimumab. The initial dose was 160 mg subcutaneously, then 80 mg 2 weeks later, then 40 mg weekly thereafter. Doxycycline was continued for treatment of the acne and HS. After 6 weeks of adalimumab, the PG worsened and prednisone was added. She developed tender furuncles on the back, and cultures grew Pseudomonas aeruginosa and methicillin-sensitive Staphylococcus aureus that responded to ciprofloxacin and cephalexin.
Due to progression of PG on adalimumab, switching to an infliximab infusion or anakinra was considered, but these options were not covered by the patient’s health insurance. Three months after the initial presentation, the patient was started on cyclosporine 100 mg 3 times daily (5 mg/kg/d) while adalimumab was continued; the ulcers started to improve within 2.5 weeks. After 3 months (Figure 3), the cyclosporine was reduced to 100 mg twice daily, and adalimumab was continued. She had a slight flare of PG after 8 months of treatment when adalimumab was unavailable to her for 2 months. After 8 months on cyclosporine, the dosage was tapered to 100 mg/d and then completely discontinued after 12 months.

The patient has continued on adalimumab 40 mg weekly with excellent control of the PG (Figure 4), although she did have one HS flare in the left axilla 11 months after the initial treatment. The patient’s cystic acne has intermittently flared and has been managed with spironolactone 100 mg/d for 3 years. After 4 years of management, the patient’s PG and HS remain well controlled on adalimumab.

Comment
Our case represents a major step in refining long-term treatment approaches for PASH syndrome due to the 4-year remission. Prior cases have reported use of anakinra, anakinra-cyclosporine combination, prednisone, azathioprine, topical tacrolimus, etanercept, and dapsone without sustainable success.1-6 The case studies discussed below have achieved remission via alternative drug combinations.
Staub et al4 found greatest success with a combination of infliximab, dapsone, and cyclosporine, and their patient had been in remission for 20 months at time of publication. Their hypothesis proposed that multiple inflammatory signaling pathways are involved in PASH syndrome, and this is why combination therapy is required for remission.4 In 2018, Lamiaux et al7 demonstrated successful treatment with rifampicin and clindamycin. Their patient had been in remission for 22 months at the time of publication—this time frame included 12 months of combination therapy and 10 months without medication. The authors hypothesized that, because of the autoinflammatory nature of these antibiotics, this pharmacologic combination could eradicate pathogenic bacteria from host microbiota while also inhibiting neutrophil function and synthesis of chemokines and cytokines.7
More recently, reports have been published regarding the success of tildrakizumab, an IL-23 antagonist, and ixekizumab, an IL-17 antagonist, in the treatment of PASH syndrome.6,8 Ixekizumab was used in combination with doxycycline, and remission was achieved in 12 months.8 However, tildrakizumab was used alone and achieved greater than 75% improvement in disease manifestations within 2 months.
Marzano et al5 conducted protein arrays and enzyme-linked immunosorbent assay to analyze the expression of cytokine, chemokine, and effector molecule profiles in PASH syndrome. It was determined that serum analysis displayed a normal cytokine/chemokine profile, with the only abnormalities being anemia and elevated C-reactive protein. There were no statistically significant differences in serum levels of IL-1β, tumor necrosis factor (TNF) α, or IL-17 between PASH syndrome and healthy controls. However, cutaneous analysis revealed extensive cytokine and chemokine hyperactivity for IL-1β and IL-1β receptor; TNF-α; C-X-C motif ligands 1, 2, and 3; C-X-C motif ligand 16;
Ead et al3 presented a unique perspective focusing on cutaneous biofilm involvement in PASH syndrome. Microbes within these biofilms induce the migration and proliferation of inflammatory cells that consume factors normally utilized for tissue catabolism. These organisms deplete necessary biochemical cofactors used during healing. This lack of nutrients needed for healing not only slows the process but also promotes favorable conditions for the growth of anerobic species. In conjunction, biofilm formation restricts bacterial access to oxygen and nutrients, thus decreasing the bacterial metabolic rate and preventing the effects of antibiotic therapy. These features of biofilm communities contribute to inflammation and possibly the troubling resistance to many therapeutic options for PASH syndrome.
Each component of PASH syndrome has been associated with biofilm formation. As previously described, PG manifests in the skin as painful ulcerations, often with slough. This slough is hypothesized to be a consequence of increased vascular permeability and exudative byproducts that accompany the inflammatory nature of biofilms.3 Acne vulgaris has well-described associations with P acnes. Ead et al3 described P acnes as a component of the biofilm community within the microcomedone of hair follicles. This biofilm allows for antibiotic resistance occasionally seen in the treatment of acne and is potentially the pathogenic factor that both impedes healing and enhances the inflammatory state. Hidradenitis suppurativa has been associated with biofilm formation.3
In further pursuit of PASH syndrome pathophysiology, many experts have sought to uncover the relationship between PASH syndrome and the previously described pyogenic arthritis, PG, and acne (PAPA) syndrome, another entity within the AIDs spectrum (Table). This condition was first recognized in 1997 in a 3-generation family with 10 affected members.1 It is characterized by PG and acne, similar to PASH; however, PAPA syndrome includes PG arthritis and lacks HS. Pyogenic arthritis manifests as recurrent aseptic inflammation of the joints, mainly the elbows, knees, and ankles. Pyogenic arthritis commonly is the presenting symptom of PAPA syndrome, with onset in childhood.2 As patients age, the arthritic symptoms decrease, and skin manifestations become more prominent.

PAPA syndrome has autosomal-dominant inheritance with mutations on chromosome 15 in the proline-serine-threonine phosphatase interacting protein 1 (PSTPIP1) gene.1 This mutation induces hyperphosphorylation of PSTPIP1, allowing for increased binding affinity to pyrin. Both PSTPIP1 and pyrin are co-expressed as parts of the NLRP3 inflammasome in granulocytes and monocytes.1 As a result, pyrin is more highly bound and loses its inhibitory effect on the NLRP3 inflammasome pathway. This lack of inhibition allows for uninhibited cleavage of pro–IL-1β to active IL-1β by the inflammasome.1
Elevated concentrations of IL-1β in patients with PAPA syndrome result in a dysregulation of the innate immune system. IL-1β induces the release of proinflammatory cytokines, namely TNF-α; interferon γ; IL-8; and regulated on activation, normal T cell expressed and secreted (RANTES), all of which activate neutrophils and induce neutrophilic inflammation.2 IL-1β not only initiates this entire cascade but also acts as an antiapoptotic signal for neutrophils.2 When IL-1β reaches a critical threshold, it induces enough inflammation to cause severe tissue damage, thus causing joint and cutaneous disease in PAPA syndrome. IL-1 inhibitors (anakinra) or TNF-α inhibitors (etanercept, adalimumab, infliximab) have been used many times to successfully treat PAPA syndrome, with TNF-α inhibitors providing the most consistent results.
Another AIDs entity with similarities to both PAPA syndrome and PASH syndrome is pyogenic arthritis, PG, acne, and HS (PA-PASH) syndrome. First identified in 2012 by Bruzzese,9 genetic analyses revealed a p.E277D missense mutation in PSTPIP1 in PA-PASH syndrome. Research has suggested that the key molecular feature is neutrophil activation by TH17 cells and the TNF-α axis.9 This syndrome has not been further characterized, and little is known regarding adequate treatment for PA-PASH syndrome.
Although it is similar in phenotype to aspects of PAPA and PA-PASH syndromes, PASH syndrome has distinct genotypic and immunologic abnormalities. Genetic analysis of this condition has shown an increased number of CCTG repeats in proximity to the PSTPIP1 promoter. It is hypothesized that these additional repeats predispose patients to neutrophilic inflammation in a similar manner to a condition described in France, termed aseptic abscess syndrome.1,5 Other mutations have been identified, including those in IL-1N, PSMB8, MEFV, NOD2, NCSTN, and more.2,7 However, it has been determined that the majority of these variants have already been filed in the Single Nucleotide Polymorphism Database or in the Registry of Hereditary Auto-inflammatory Disorders Mutations.2 The question remains regarding the origin of inflammation seen in PASH syndrome; the potential role of biofilms; and the relationship between PASH, PAPA, and PA-PASH syndromes. Much work remains to be done in refining therapeutic options for PASH syndrome. Continued biochemical research is necessary, as well as collaboration among dermatologists worldwide who find success in treating this condition.
Conclusion
There are genotypic and phenotypic similarities between PASH, PAPA, and PA-PASH syndromes, with various mutations within or near the PSTPIP1 gene; however, their genetic discrepancies seem to play a major role in the pathophysiology of each syndrome. Much work remains to be done in PA-PASH syndrome, which has not yet been well described. Meanwhile, PAPA syndrome has been well characterized with mutations affecting proteins of the NLRP3 inflammasome, resulting in elevated IL-1β and excess neutrophilic inflammation. In PASH syndrome, the importance of increased repeats near the PSTPIP1 promoter is yet to be elucidated. It has been shown that these abnormalities predispose individuals to neutrophilic inflammation, but the mechanism by which they do so is unknown. In addition, consideration of biofilms and their predisposition to inflammation within the pathophysiology of PASH syndrome is a possibility that must be considered when discussing therapeutic options. Based on our case study and previous successes in treating PASH syndrome, it is clear that a multidrug approach is necessary for remission. It is likely that the etiology of PASH syndrome is multifaceted and involves hyperactivity in multiple arms of the innate immune system.
Patients with PASH syndrome have severely impaired quality of life and often experience social withdrawal due to the disfiguring sequelae and limited treatment options available. To improve patient outcomes, it is essential for physicians and scientists to report on successful treatment strategies and advances in immunologic understanding. Improved understanding of PASH syndrome calls for further genetic exploration into the role of additional genomic repeats and how these affect the PSTPIP1 gene and inflammasome activity. As medical advances improve understanding of the pathophysiology of this disease entity, it will likely become clear which mechanisms are most important in disease progression and how clinicians can best optimize treatment.
- Braun-Falco M, Kovnerystyy O, Lohse P, et al. Pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH)—a new autoinflammatory syndrome distinct from PAPA syndrome. J Am Acad Dermatol. 2012;66:409-415.
- Cugno M, Borghi A, Marzano AV. PAPA, PASH and PAPASH syndromes: pathophysiology, presentation and treatment. Am J Clin Dermatol. 2017;18:555-562.
- Ead JK, Snyder RJ, Wise J, et al. Is PASH syndrome a biofilm disease?: a case series and review of the literature. Wounds. 2018;30:216-223.
- Staub J, Pfannschmidt N, Strohal R, et al. Successful treatment of PASH syndrome with infliximab, cyclosporine and dapsone. J Eur Acad Dermatol Venereol. 2015;29:2243-2247.
- Marzano AV, Ceccherini I, Gattorno M, et al. Association of pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH) shares genetic and cytokine profiles with other autoinflammatory diseases. Medicine (Baltimore). 2014;93:E187.
- Kok Y, Nicolopoulos J, Varigos G, et al. Tildrakizumab in the treatment of PASH syndrome: a potential novel therapeutic target. Australas J Dermatol. 2020;61:E373-E374.
- Lamiaux M, Dabouz F, Wantz M, et al. Successful combined antibiotic therapy with oral clindamycin and oral rifampicin for pyoderma gangrenosum in patient with PASH syndrome. JAAD Case Rep. 2018;4:17-21.
- Gul MI, Singam V, Hanson C, et al. Remission of refractory PASH syndrome using ixekizumab and doxycycline. J Drugs Dermatol. 2020;19:1123.
- Bruzzese V. Pyoderma gangrenosum, acne conglobata, suppurative hidradenitis, and axial spondyloarthritis: efficacy of anti-tumor necrosis factor α therapy. J Clin Rheumatol. 2012;18:413-415.
- Braun-Falco M, Kovnerystyy O, Lohse P, et al. Pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH)—a new autoinflammatory syndrome distinct from PAPA syndrome. J Am Acad Dermatol. 2012;66:409-415.
- Cugno M, Borghi A, Marzano AV. PAPA, PASH and PAPASH syndromes: pathophysiology, presentation and treatment. Am J Clin Dermatol. 2017;18:555-562.
- Ead JK, Snyder RJ, Wise J, et al. Is PASH syndrome a biofilm disease?: a case series and review of the literature. Wounds. 2018;30:216-223.
- Staub J, Pfannschmidt N, Strohal R, et al. Successful treatment of PASH syndrome with infliximab, cyclosporine and dapsone. J Eur Acad Dermatol Venereol. 2015;29:2243-2247.
- Marzano AV, Ceccherini I, Gattorno M, et al. Association of pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH) shares genetic and cytokine profiles with other autoinflammatory diseases. Medicine (Baltimore). 2014;93:E187.
- Kok Y, Nicolopoulos J, Varigos G, et al. Tildrakizumab in the treatment of PASH syndrome: a potential novel therapeutic target. Australas J Dermatol. 2020;61:E373-E374.
- Lamiaux M, Dabouz F, Wantz M, et al. Successful combined antibiotic therapy with oral clindamycin and oral rifampicin for pyoderma gangrenosum in patient with PASH syndrome. JAAD Case Rep. 2018;4:17-21.
- Gul MI, Singam V, Hanson C, et al. Remission of refractory PASH syndrome using ixekizumab and doxycycline. J Drugs Dermatol. 2020;19:1123.
- Bruzzese V. Pyoderma gangrenosum, acne conglobata, suppurative hidradenitis, and axial spondyloarthritis: efficacy of anti-tumor necrosis factor α therapy. J Clin Rheumatol. 2012;18:413-415.
Practice Points
- Despite phenotypic similarities among pyoderma gangrenosum (PG), acne, and hidradenitis suppurativa (PASH) syndrome; pyogenic arthritis, PG, and acne syndrome; and pyogenic arthritis–PASH syndrome, there are genotypic differences that contribute to unique inflammatory cytokine patterns and the need for distinct pharmacologic considerations within each entity.
- When formulating therapeutic regimens for patients with PASH syndrome, it is essential for dermatologists to consider the likelihood of hyperactivity in multiple pathways of the innate immune system and utilize a combination of multimodal antiinflammatory therapies.
Papular Acneform Eruption With Mucositis
The Diagnosis: Syphilis
Histopathology revealed psoriasiform hyperplasia, endothelial cell swelling, and a brisk lichenoid inflammation with plasma cells (Figure, A). There also was pustular folliculitis in association with well-formed granulomatous inflammation and a prominent number of plasma cells (Figure, B). Treponema pallidum immunostaining showed numerous organisms in the epidermal and follicular epithelium. Rapid plasma reagin was found to be positive with a titer of 1:128. Evaluation for neurosyphilis through lumbar puncture was negative; the patient also was HIV negative. All of our patient’s skin lesions cleared after a 3-week course of weekly intramuscular benzathine G injections. Due to his substantial clinical improvement, the patient was subsequently lost to follow-up.
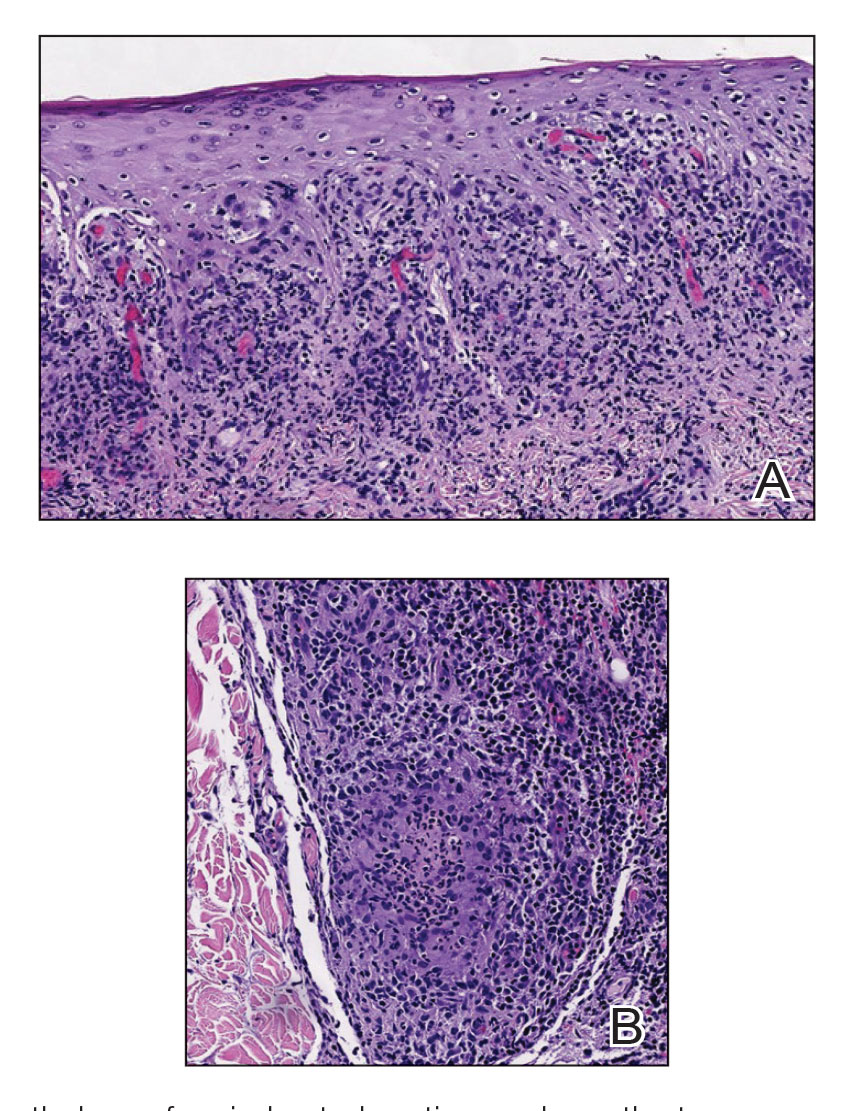
Syphilis, an infectious disease caused by the spirochete bacterium T pallidum, has a well-known natural history defined by various stages classically categorized as primary, secondary, latent, or late (tertiary).1 The classic lesion in primary syphilis is the chancre, a painless ulcer with raised borders that develops within approximately 3 weeks following the initial inoculation.2 Secondary syphilis manifests with mucocutaneous findings in up to 97% of patients, and untreated patients develop secondary syphilis at a rate of approximately 25%.3 Although mucocutaneous findings in secondary syphilis can vary widely, patients most commonly develop a diffuse maculopapular exanthem, and 40% develop mucosal findings including genital ulcers, mucous patches, and condylomata lata.1 In latent syphilis, there is seroreactivity, but otherwise there are no clinical symptoms. A clear symptomatic history of prior primary or secondary syphilis may be known or unknown. Latent syphilis is divided into early and late phases, and the World Health Organization designates 2 years after the first suspected exposure as the cutoff point for early and late latency.4 During the first 4 years of latent syphilis, patients may exhibit mucocutaneous relapses. Our patient denied any sexual activity for more than 3 years prior to presentation. Because of the start of iatrogenic immunosuppression during this period, this case was classified as late latent syphilis with mucocutaneous reactivation.
Behçet disease was included within the differential diagnosis but is characterized by multiorgan systemic vasculitis that causes various mucocutaneous findings including aphthous ulcers, papulopustular lesions, and genital ulcers.5 Histopathologic features are nonspecific, and the clinical finding of recurrent genital and oral ulceration should be present for diagnosis. This disease predominantly occurs in East Asian or Mediterranean populations and is otherwise rare in White individuals.
SAPHO (synovitis, acne, pustulosis, hyperostosis, osteitis) syndrome is a rare disorder consisting of skin, joint, and bone manifestations.6 Severe acne generally is accompanied by palmoplantar pustulosis along with pain and joint tenderness involving the anterior chest and axial skeleton, both of which were absent in our patient.
Pustular psoriasis can be localized or generalized. Localized presentations frequently are acral and may be associated with a variable degree of nail dystrophy and arthritis. Generalized presentations are characterized by hyperemic, well-defined patches with variable numbers of pustules.7 The pustules are the consequence of exuberate neutrophilic exocytosis into the epidermis and are nonfollicular.
Steroid-induced acne may be considered in the proper clinical setting of an acneform eruption with a prior history of systemic steroid treatment. However, additional findings of mucositis would not be expected, and although our patient was prescribed prednisone from his primary care physician prior to presentation to our clinic, this medication was given after the onset of the cutaneous eruption.
Syphilis commonly is referred to as the great mimicker due to its potential diverse morphologic presentations, which can involve acneform eruptions, though rare.8 In the setting of mucositis, generalized acneform eruptions should raise suspicion for the possibility of syphilis, even in the absence of other more classic cutaneous features.
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: historical aspects, microbiology, epidemiology, and clinical manifestations. J Am Acad Dermatol. 2020;82:1-14.
- Sparling PF. Natural history of syphilis. In: Holmes KK, Mardh PA, Sparling PF, et al, eds. Sexually Transmitted Diseases. McGraw Hill; 1990:213.
- Clark EG, Danbolt N. The Oslo study of the natural course of untreated syphilis: an epidemiologic investigation based on a re-study of the Boeck-Bruusgaard material. Med Clin North Am. 1964;48:613.
- Sule RR, Deshpande SG, Dharmadhikari NJ, et al. Late cutaneous syphilis. Cutis. 1997;59:135-137.
- Wilder EG, Frieder J, Sulhan S, et al. Spectrum of orocutaneous disease associations: genodermatoses and inflammatory conditions. J Am Acad Dermatol. 2017;77:809-830.
- Carneiro S, Sampaio-Barros PD. SAPHO syndrome. Rheum Dis Clin North Am. 2013;39:401-418.
- Bachelez H. Pustular psoriasis and related pustular skin diseases. Br J Dermatol. 2018;178:614-618.
- Domantay-Apostol GP, Handog EB, Gabriel MT. Syphilis: the international challenge of the great imitator. Dermatol Clin. 2008; 26:191-202, v. doi:10.1016/j.det.2007.12.001
The Diagnosis: Syphilis
Histopathology revealed psoriasiform hyperplasia, endothelial cell swelling, and a brisk lichenoid inflammation with plasma cells (Figure, A). There also was pustular folliculitis in association with well-formed granulomatous inflammation and a prominent number of plasma cells (Figure, B). Treponema pallidum immunostaining showed numerous organisms in the epidermal and follicular epithelium. Rapid plasma reagin was found to be positive with a titer of 1:128. Evaluation for neurosyphilis through lumbar puncture was negative; the patient also was HIV negative. All of our patient’s skin lesions cleared after a 3-week course of weekly intramuscular benzathine G injections. Due to his substantial clinical improvement, the patient was subsequently lost to follow-up.

Syphilis, an infectious disease caused by the spirochete bacterium T pallidum, has a well-known natural history defined by various stages classically categorized as primary, secondary, latent, or late (tertiary).1 The classic lesion in primary syphilis is the chancre, a painless ulcer with raised borders that develops within approximately 3 weeks following the initial inoculation.2 Secondary syphilis manifests with mucocutaneous findings in up to 97% of patients, and untreated patients develop secondary syphilis at a rate of approximately 25%.3 Although mucocutaneous findings in secondary syphilis can vary widely, patients most commonly develop a diffuse maculopapular exanthem, and 40% develop mucosal findings including genital ulcers, mucous patches, and condylomata lata.1 In latent syphilis, there is seroreactivity, but otherwise there are no clinical symptoms. A clear symptomatic history of prior primary or secondary syphilis may be known or unknown. Latent syphilis is divided into early and late phases, and the World Health Organization designates 2 years after the first suspected exposure as the cutoff point for early and late latency.4 During the first 4 years of latent syphilis, patients may exhibit mucocutaneous relapses. Our patient denied any sexual activity for more than 3 years prior to presentation. Because of the start of iatrogenic immunosuppression during this period, this case was classified as late latent syphilis with mucocutaneous reactivation.
Behçet disease was included within the differential diagnosis but is characterized by multiorgan systemic vasculitis that causes various mucocutaneous findings including aphthous ulcers, papulopustular lesions, and genital ulcers.5 Histopathologic features are nonspecific, and the clinical finding of recurrent genital and oral ulceration should be present for diagnosis. This disease predominantly occurs in East Asian or Mediterranean populations and is otherwise rare in White individuals.
SAPHO (synovitis, acne, pustulosis, hyperostosis, osteitis) syndrome is a rare disorder consisting of skin, joint, and bone manifestations.6 Severe acne generally is accompanied by palmoplantar pustulosis along with pain and joint tenderness involving the anterior chest and axial skeleton, both of which were absent in our patient.
Pustular psoriasis can be localized or generalized. Localized presentations frequently are acral and may be associated with a variable degree of nail dystrophy and arthritis. Generalized presentations are characterized by hyperemic, well-defined patches with variable numbers of pustules.7 The pustules are the consequence of exuberate neutrophilic exocytosis into the epidermis and are nonfollicular.
Steroid-induced acne may be considered in the proper clinical setting of an acneform eruption with a prior history of systemic steroid treatment. However, additional findings of mucositis would not be expected, and although our patient was prescribed prednisone from his primary care physician prior to presentation to our clinic, this medication was given after the onset of the cutaneous eruption.
Syphilis commonly is referred to as the great mimicker due to its potential diverse morphologic presentations, which can involve acneform eruptions, though rare.8 In the setting of mucositis, generalized acneform eruptions should raise suspicion for the possibility of syphilis, even in the absence of other more classic cutaneous features.
The Diagnosis: Syphilis
Histopathology revealed psoriasiform hyperplasia, endothelial cell swelling, and a brisk lichenoid inflammation with plasma cells (Figure, A). There also was pustular folliculitis in association with well-formed granulomatous inflammation and a prominent number of plasma cells (Figure, B). Treponema pallidum immunostaining showed numerous organisms in the epidermal and follicular epithelium. Rapid plasma reagin was found to be positive with a titer of 1:128. Evaluation for neurosyphilis through lumbar puncture was negative; the patient also was HIV negative. All of our patient’s skin lesions cleared after a 3-week course of weekly intramuscular benzathine G injections. Due to his substantial clinical improvement, the patient was subsequently lost to follow-up.

Syphilis, an infectious disease caused by the spirochete bacterium T pallidum, has a well-known natural history defined by various stages classically categorized as primary, secondary, latent, or late (tertiary).1 The classic lesion in primary syphilis is the chancre, a painless ulcer with raised borders that develops within approximately 3 weeks following the initial inoculation.2 Secondary syphilis manifests with mucocutaneous findings in up to 97% of patients, and untreated patients develop secondary syphilis at a rate of approximately 25%.3 Although mucocutaneous findings in secondary syphilis can vary widely, patients most commonly develop a diffuse maculopapular exanthem, and 40% develop mucosal findings including genital ulcers, mucous patches, and condylomata lata.1 In latent syphilis, there is seroreactivity, but otherwise there are no clinical symptoms. A clear symptomatic history of prior primary or secondary syphilis may be known or unknown. Latent syphilis is divided into early and late phases, and the World Health Organization designates 2 years after the first suspected exposure as the cutoff point for early and late latency.4 During the first 4 years of latent syphilis, patients may exhibit mucocutaneous relapses. Our patient denied any sexual activity for more than 3 years prior to presentation. Because of the start of iatrogenic immunosuppression during this period, this case was classified as late latent syphilis with mucocutaneous reactivation.
Behçet disease was included within the differential diagnosis but is characterized by multiorgan systemic vasculitis that causes various mucocutaneous findings including aphthous ulcers, papulopustular lesions, and genital ulcers.5 Histopathologic features are nonspecific, and the clinical finding of recurrent genital and oral ulceration should be present for diagnosis. This disease predominantly occurs in East Asian or Mediterranean populations and is otherwise rare in White individuals.
SAPHO (synovitis, acne, pustulosis, hyperostosis, osteitis) syndrome is a rare disorder consisting of skin, joint, and bone manifestations.6 Severe acne generally is accompanied by palmoplantar pustulosis along with pain and joint tenderness involving the anterior chest and axial skeleton, both of which were absent in our patient.
Pustular psoriasis can be localized or generalized. Localized presentations frequently are acral and may be associated with a variable degree of nail dystrophy and arthritis. Generalized presentations are characterized by hyperemic, well-defined patches with variable numbers of pustules.7 The pustules are the consequence of exuberate neutrophilic exocytosis into the epidermis and are nonfollicular.
Steroid-induced acne may be considered in the proper clinical setting of an acneform eruption with a prior history of systemic steroid treatment. However, additional findings of mucositis would not be expected, and although our patient was prescribed prednisone from his primary care physician prior to presentation to our clinic, this medication was given after the onset of the cutaneous eruption.
Syphilis commonly is referred to as the great mimicker due to its potential diverse morphologic presentations, which can involve acneform eruptions, though rare.8 In the setting of mucositis, generalized acneform eruptions should raise suspicion for the possibility of syphilis, even in the absence of other more classic cutaneous features.
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: historical aspects, microbiology, epidemiology, and clinical manifestations. J Am Acad Dermatol. 2020;82:1-14.
- Sparling PF. Natural history of syphilis. In: Holmes KK, Mardh PA, Sparling PF, et al, eds. Sexually Transmitted Diseases. McGraw Hill; 1990:213.
- Clark EG, Danbolt N. The Oslo study of the natural course of untreated syphilis: an epidemiologic investigation based on a re-study of the Boeck-Bruusgaard material. Med Clin North Am. 1964;48:613.
- Sule RR, Deshpande SG, Dharmadhikari NJ, et al. Late cutaneous syphilis. Cutis. 1997;59:135-137.
- Wilder EG, Frieder J, Sulhan S, et al. Spectrum of orocutaneous disease associations: genodermatoses and inflammatory conditions. J Am Acad Dermatol. 2017;77:809-830.
- Carneiro S, Sampaio-Barros PD. SAPHO syndrome. Rheum Dis Clin North Am. 2013;39:401-418.
- Bachelez H. Pustular psoriasis and related pustular skin diseases. Br J Dermatol. 2018;178:614-618.
- Domantay-Apostol GP, Handog EB, Gabriel MT. Syphilis: the international challenge of the great imitator. Dermatol Clin. 2008; 26:191-202, v. doi:10.1016/j.det.2007.12.001
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: historical aspects, microbiology, epidemiology, and clinical manifestations. J Am Acad Dermatol. 2020;82:1-14.
- Sparling PF. Natural history of syphilis. In: Holmes KK, Mardh PA, Sparling PF, et al, eds. Sexually Transmitted Diseases. McGraw Hill; 1990:213.
- Clark EG, Danbolt N. The Oslo study of the natural course of untreated syphilis: an epidemiologic investigation based on a re-study of the Boeck-Bruusgaard material. Med Clin North Am. 1964;48:613.
- Sule RR, Deshpande SG, Dharmadhikari NJ, et al. Late cutaneous syphilis. Cutis. 1997;59:135-137.
- Wilder EG, Frieder J, Sulhan S, et al. Spectrum of orocutaneous disease associations: genodermatoses and inflammatory conditions. J Am Acad Dermatol. 2017;77:809-830.
- Carneiro S, Sampaio-Barros PD. SAPHO syndrome. Rheum Dis Clin North Am. 2013;39:401-418.
- Bachelez H. Pustular psoriasis and related pustular skin diseases. Br J Dermatol. 2018;178:614-618.
- Domantay-Apostol GP, Handog EB, Gabriel MT. Syphilis: the international challenge of the great imitator. Dermatol Clin. 2008; 26:191-202, v. doi:10.1016/j.det.2007.12.001
A 48-year-old man with a history of ulcerative colitis that was well-controlled with adalimumab presented with a generalized acneform eruption involving the face, chest (top) and back, as well as a well-defined ovoid ulcer on the anterior aspect of the tongue (bottom) of 2 months’ duration. Prior treatment with prednisone 60 mg daily for 14 days resulted in no improvement. He denied unintentional weight loss, cyclic fever, or arthritis. A complete blood cell count with differential showed mild anemia (hemoglobin, 11.6 g/dL [reference range, 13.2–16.6 g/dL]) with a differential cell count that was within reference range for each cell type. The erythrocyte sedimentation rate was elevated at 44 mm/h (reference range, 0–22 mm/h). A 4-mm punch biopsy specimen of an indurated cystic papule on the torso was obtained.