User login
Doctor’s illegal opioid prescriptions lead to five deaths
According to court documents, between January 2014 and October 2019, family physician David Chisholm, MD, 64, of Wasilla, Alaska, wrote more than 20,000 prescriptions to approximately 350 patients for oxycodone, methadone, and hydrocodone, often prescribing the pills using variations of patients’ names in an attempt to avoid being red-flagged by payers.
When Walmart refused to continue filling the prescriptions, Dr. Chisholm told his staff to advise the patients to use other pharmacies. In addition, he often prescribed combinations of medications, such as concurrent opioids, benzodiazepines, sedatives, and carisoprodol, thus increasing the chances that his patients would become addicted to or overdose on the drugs. Chisholm, who pleaded guilty in June, acknowledged to federal officials that his prescriptions were a significant contributing factor to the overdose deaths of five of his patients during this time, according to a statement by the U.S. Attorney’s Office for the District of Alaska.
According to the Anchorage Daily News, Dr. Chisholm, who was not board certified in pain medicine, said his reason for prescribing the drugs was not to make money but to help patients suffering from chronic pain and because he enjoyed the challenge.
Dr. Chisholm’s attorney, Nick Oberheiden, told CNN his client “sacrificed his reputation as a patient advocate and his years of service to the Alaskan community” in overprescribing opioids. “He expressed his sincere remorse in open court and he accepts the consequences of his misconduct. He hopes that his case serves as a warning to other physicians facing the same dilemma when treating chronic pain.”
He surrendered his medical license in November 2020 before being formally charged in April 2021.
Texas hospital CEO, seven doctors settle kickback
A hospital executive and seven physicians have agreed to pay a total of $1.1 million to settle allegations that they violated the Anti-Kickback Statute and Stark Law. The eight have also agreed to cooperate in investigations and litigation involving other parties.
The Texas physicians involved in the settlement are internist Jaspaul Bhangoo, MD, of Denton; family physician Robert Megna, DO, of Ferris; cardiologist Baxter Montgomery, MD, of Houston; internist Murtaza Mussaji, DO, of Houston; family physician David Sneed, DO, of Austin; family physician Kevin Lewis, DO, of Houston; and family physician Angela Mosley-Nunnery, MD, of Kingwood.
Also settling was Richard DeFoore, former CEO of Jones County Regional Healthcare (dba Stamford Memorial Hospital).
The physicians were accused of accepting payments from organizations in exchange for ordering lab tests from True Health Diagnostics, Little River Healthcare, and Boston Heart. The payments to the physicians were disguised as investment returns but, according to the allegations, were in fact offered in exchange for the doctors’ referrals. Mr. DeFoore, the hospital executive involved in the settlement, allegedly oversaw a similar scheme that benefited the now-defunct Stamford Memorial.
“Paying kickbacks to physicians distorts the medical decision-making process, corrupts our health care system, and increases the cost of healthcare funded by the taxpayer,” Brit Featherston, U.S. attorney for the Eastern District of Texas, said in a statement announcing the agreement. “Laboratories, marketers, and physicians cannot immunize their conduct by attempting to disguise the kickbacks as some sort of investment arrangement.”
Practice administrative assistant sentenced for fraudulent prescriptions
An administrative assistant at an Illinois orthopedics office was sentenced to a year and a day in federal prison for writing fraudulent prescriptions for opioids.
Amanda Biesiada, 39, of Alsip, Ill., who worked as an administrative assistant at Hinsdale Orthopaedics in Westmont, Ill., was not a licensed physician and could not legally write the prescriptions unless instructed to do so and supervised by licensed doctors.
According to a statement by the U.S. Attorney’s Office for the Northern District of Illinois, the prescriptions for hydrocodone, oxycodone, and other controlled substances – 85 prescriptions in all from 2017 to 2019 – were made out to an acquaintance of Biesiada’s and written without the knowledge or approval of the providers in whose names she wrote them.
Federal officials said Ms. Biesiada attempted to conceal the fraudulent prescriptions by marking them “filed in error” in the practice’s prescription system.
Lab owner pleads guilty to $6.9 million testing fraud scheme
A Florida lab owner has pleaded guilty to conspiracy to defraud Medicare through false and fraudulent claims totaling more than $6.9 million.
According to court documents, Christopher Licata, 45, of Delray Beach, Fla., admitted to bribing patient brokers to refer orders for medically unnecessary genetic testing to his lab. The tests were then billed to Medicare.
Mr. Licata and the patient brokers entered sham agreements meant to disguise the true purpose of the payments, according to a statement from the Department of Justice. The 45-year-old owner of Boca Toxicology (dba Lab Dynamics) pleaded guilty to one count of conspiring to commit health care fraud.
The scheme began in 2018; however, once the pandemic began, Mr. Licata shifted strategies, playing on patients’ fears of COVID-19 to bundle inexpensive COVID tests with more expensive medically unnecessary tests. These tests included respiratory pathogen panels and genetic testing for cardiovascular disease, cancer, diabetes, Alzheimer’s disease, and other illnesses. In all, Mr. Licata’s laboratory submitted over $6.9 million in false and fraudulent claims to Medicare for these unnecessary tests, according to the DOJ statement.
The case is a part of the U.S. Attorney General’s COVID-19 Fraud Enforcement Task Force that was established to enhance the efforts of agencies and governments across the country to combat and prevent pandemic-related fraud.
Mr. Licata faces a maximum penalty of 10 years in prison. His sentencing is scheduled for March 24.
A version of this article first appeared on Medscape.com.
According to court documents, between January 2014 and October 2019, family physician David Chisholm, MD, 64, of Wasilla, Alaska, wrote more than 20,000 prescriptions to approximately 350 patients for oxycodone, methadone, and hydrocodone, often prescribing the pills using variations of patients’ names in an attempt to avoid being red-flagged by payers.
When Walmart refused to continue filling the prescriptions, Dr. Chisholm told his staff to advise the patients to use other pharmacies. In addition, he often prescribed combinations of medications, such as concurrent opioids, benzodiazepines, sedatives, and carisoprodol, thus increasing the chances that his patients would become addicted to or overdose on the drugs. Chisholm, who pleaded guilty in June, acknowledged to federal officials that his prescriptions were a significant contributing factor to the overdose deaths of five of his patients during this time, according to a statement by the U.S. Attorney’s Office for the District of Alaska.
According to the Anchorage Daily News, Dr. Chisholm, who was not board certified in pain medicine, said his reason for prescribing the drugs was not to make money but to help patients suffering from chronic pain and because he enjoyed the challenge.
Dr. Chisholm’s attorney, Nick Oberheiden, told CNN his client “sacrificed his reputation as a patient advocate and his years of service to the Alaskan community” in overprescribing opioids. “He expressed his sincere remorse in open court and he accepts the consequences of his misconduct. He hopes that his case serves as a warning to other physicians facing the same dilemma when treating chronic pain.”
He surrendered his medical license in November 2020 before being formally charged in April 2021.
Texas hospital CEO, seven doctors settle kickback
A hospital executive and seven physicians have agreed to pay a total of $1.1 million to settle allegations that they violated the Anti-Kickback Statute and Stark Law. The eight have also agreed to cooperate in investigations and litigation involving other parties.
The Texas physicians involved in the settlement are internist Jaspaul Bhangoo, MD, of Denton; family physician Robert Megna, DO, of Ferris; cardiologist Baxter Montgomery, MD, of Houston; internist Murtaza Mussaji, DO, of Houston; family physician David Sneed, DO, of Austin; family physician Kevin Lewis, DO, of Houston; and family physician Angela Mosley-Nunnery, MD, of Kingwood.
Also settling was Richard DeFoore, former CEO of Jones County Regional Healthcare (dba Stamford Memorial Hospital).
The physicians were accused of accepting payments from organizations in exchange for ordering lab tests from True Health Diagnostics, Little River Healthcare, and Boston Heart. The payments to the physicians were disguised as investment returns but, according to the allegations, were in fact offered in exchange for the doctors’ referrals. Mr. DeFoore, the hospital executive involved in the settlement, allegedly oversaw a similar scheme that benefited the now-defunct Stamford Memorial.
“Paying kickbacks to physicians distorts the medical decision-making process, corrupts our health care system, and increases the cost of healthcare funded by the taxpayer,” Brit Featherston, U.S. attorney for the Eastern District of Texas, said in a statement announcing the agreement. “Laboratories, marketers, and physicians cannot immunize their conduct by attempting to disguise the kickbacks as some sort of investment arrangement.”
Practice administrative assistant sentenced for fraudulent prescriptions
An administrative assistant at an Illinois orthopedics office was sentenced to a year and a day in federal prison for writing fraudulent prescriptions for opioids.
Amanda Biesiada, 39, of Alsip, Ill., who worked as an administrative assistant at Hinsdale Orthopaedics in Westmont, Ill., was not a licensed physician and could not legally write the prescriptions unless instructed to do so and supervised by licensed doctors.
According to a statement by the U.S. Attorney’s Office for the Northern District of Illinois, the prescriptions for hydrocodone, oxycodone, and other controlled substances – 85 prescriptions in all from 2017 to 2019 – were made out to an acquaintance of Biesiada’s and written without the knowledge or approval of the providers in whose names she wrote them.
Federal officials said Ms. Biesiada attempted to conceal the fraudulent prescriptions by marking them “filed in error” in the practice’s prescription system.
Lab owner pleads guilty to $6.9 million testing fraud scheme
A Florida lab owner has pleaded guilty to conspiracy to defraud Medicare through false and fraudulent claims totaling more than $6.9 million.
According to court documents, Christopher Licata, 45, of Delray Beach, Fla., admitted to bribing patient brokers to refer orders for medically unnecessary genetic testing to his lab. The tests were then billed to Medicare.
Mr. Licata and the patient brokers entered sham agreements meant to disguise the true purpose of the payments, according to a statement from the Department of Justice. The 45-year-old owner of Boca Toxicology (dba Lab Dynamics) pleaded guilty to one count of conspiring to commit health care fraud.
The scheme began in 2018; however, once the pandemic began, Mr. Licata shifted strategies, playing on patients’ fears of COVID-19 to bundle inexpensive COVID tests with more expensive medically unnecessary tests. These tests included respiratory pathogen panels and genetic testing for cardiovascular disease, cancer, diabetes, Alzheimer’s disease, and other illnesses. In all, Mr. Licata’s laboratory submitted over $6.9 million in false and fraudulent claims to Medicare for these unnecessary tests, according to the DOJ statement.
The case is a part of the U.S. Attorney General’s COVID-19 Fraud Enforcement Task Force that was established to enhance the efforts of agencies and governments across the country to combat and prevent pandemic-related fraud.
Mr. Licata faces a maximum penalty of 10 years in prison. His sentencing is scheduled for March 24.
A version of this article first appeared on Medscape.com.
According to court documents, between January 2014 and October 2019, family physician David Chisholm, MD, 64, of Wasilla, Alaska, wrote more than 20,000 prescriptions to approximately 350 patients for oxycodone, methadone, and hydrocodone, often prescribing the pills using variations of patients’ names in an attempt to avoid being red-flagged by payers.
When Walmart refused to continue filling the prescriptions, Dr. Chisholm told his staff to advise the patients to use other pharmacies. In addition, he often prescribed combinations of medications, such as concurrent opioids, benzodiazepines, sedatives, and carisoprodol, thus increasing the chances that his patients would become addicted to or overdose on the drugs. Chisholm, who pleaded guilty in June, acknowledged to federal officials that his prescriptions were a significant contributing factor to the overdose deaths of five of his patients during this time, according to a statement by the U.S. Attorney’s Office for the District of Alaska.
According to the Anchorage Daily News, Dr. Chisholm, who was not board certified in pain medicine, said his reason for prescribing the drugs was not to make money but to help patients suffering from chronic pain and because he enjoyed the challenge.
Dr. Chisholm’s attorney, Nick Oberheiden, told CNN his client “sacrificed his reputation as a patient advocate and his years of service to the Alaskan community” in overprescribing opioids. “He expressed his sincere remorse in open court and he accepts the consequences of his misconduct. He hopes that his case serves as a warning to other physicians facing the same dilemma when treating chronic pain.”
He surrendered his medical license in November 2020 before being formally charged in April 2021.
Texas hospital CEO, seven doctors settle kickback
A hospital executive and seven physicians have agreed to pay a total of $1.1 million to settle allegations that they violated the Anti-Kickback Statute and Stark Law. The eight have also agreed to cooperate in investigations and litigation involving other parties.
The Texas physicians involved in the settlement are internist Jaspaul Bhangoo, MD, of Denton; family physician Robert Megna, DO, of Ferris; cardiologist Baxter Montgomery, MD, of Houston; internist Murtaza Mussaji, DO, of Houston; family physician David Sneed, DO, of Austin; family physician Kevin Lewis, DO, of Houston; and family physician Angela Mosley-Nunnery, MD, of Kingwood.
Also settling was Richard DeFoore, former CEO of Jones County Regional Healthcare (dba Stamford Memorial Hospital).
The physicians were accused of accepting payments from organizations in exchange for ordering lab tests from True Health Diagnostics, Little River Healthcare, and Boston Heart. The payments to the physicians were disguised as investment returns but, according to the allegations, were in fact offered in exchange for the doctors’ referrals. Mr. DeFoore, the hospital executive involved in the settlement, allegedly oversaw a similar scheme that benefited the now-defunct Stamford Memorial.
“Paying kickbacks to physicians distorts the medical decision-making process, corrupts our health care system, and increases the cost of healthcare funded by the taxpayer,” Brit Featherston, U.S. attorney for the Eastern District of Texas, said in a statement announcing the agreement. “Laboratories, marketers, and physicians cannot immunize their conduct by attempting to disguise the kickbacks as some sort of investment arrangement.”
Practice administrative assistant sentenced for fraudulent prescriptions
An administrative assistant at an Illinois orthopedics office was sentenced to a year and a day in federal prison for writing fraudulent prescriptions for opioids.
Amanda Biesiada, 39, of Alsip, Ill., who worked as an administrative assistant at Hinsdale Orthopaedics in Westmont, Ill., was not a licensed physician and could not legally write the prescriptions unless instructed to do so and supervised by licensed doctors.
According to a statement by the U.S. Attorney’s Office for the Northern District of Illinois, the prescriptions for hydrocodone, oxycodone, and other controlled substances – 85 prescriptions in all from 2017 to 2019 – were made out to an acquaintance of Biesiada’s and written without the knowledge or approval of the providers in whose names she wrote them.
Federal officials said Ms. Biesiada attempted to conceal the fraudulent prescriptions by marking them “filed in error” in the practice’s prescription system.
Lab owner pleads guilty to $6.9 million testing fraud scheme
A Florida lab owner has pleaded guilty to conspiracy to defraud Medicare through false and fraudulent claims totaling more than $6.9 million.
According to court documents, Christopher Licata, 45, of Delray Beach, Fla., admitted to bribing patient brokers to refer orders for medically unnecessary genetic testing to his lab. The tests were then billed to Medicare.
Mr. Licata and the patient brokers entered sham agreements meant to disguise the true purpose of the payments, according to a statement from the Department of Justice. The 45-year-old owner of Boca Toxicology (dba Lab Dynamics) pleaded guilty to one count of conspiring to commit health care fraud.
The scheme began in 2018; however, once the pandemic began, Mr. Licata shifted strategies, playing on patients’ fears of COVID-19 to bundle inexpensive COVID tests with more expensive medically unnecessary tests. These tests included respiratory pathogen panels and genetic testing for cardiovascular disease, cancer, diabetes, Alzheimer’s disease, and other illnesses. In all, Mr. Licata’s laboratory submitted over $6.9 million in false and fraudulent claims to Medicare for these unnecessary tests, according to the DOJ statement.
The case is a part of the U.S. Attorney General’s COVID-19 Fraud Enforcement Task Force that was established to enhance the efforts of agencies and governments across the country to combat and prevent pandemic-related fraud.
Mr. Licata faces a maximum penalty of 10 years in prison. His sentencing is scheduled for March 24.
A version of this article first appeared on Medscape.com.
Ketamine an ‘intriguing new therapy’ for alcoholism
Three weekly infusions of the dissociative anesthetic ketamine coupled with mindfulness-based relapse prevention therapy may help adults with alcohol use disorder (AUD) maintain abstinence, new research suggests.
Preliminary results from a phase 2, double-blind, placebo-controlled trial show ketamine was well tolerated and, compared with placebo, associated with more days of abstinence from alcohol at 6 months.
The results suggest ketamine plus psychological therapy may be a “new, relatively brief treatment that has long lasting effects in AUD,” Celia Morgan, PhD, professor of psychopharmacology, University of Exeter, United Kingdom, told this news organization.
The study was published online Jan. 11 in the American Journal of Psychiatry.
Target depression
Depressive symptoms are common in patients under treatment for AUD and increase relapse risk.
“Ketamine may support alcohol abstinence by temporarily alleviating depressive symptoms during the high-risk relapse period in the weeks after detoxification,” the investigators note.
Ketamine may also provide a “temporary boost to synaptogenesis and neurogenesis, which may allow psychological therapies and new strategies for managing addiction to embed more readily,” they add.
To test these theories, the researchers recruited 96 adults (mean age, 44 years, 35 women) with severe AUD to participate in the trial.
All participants had to abstain from alcohol for at least 24 hours before the trial started and have a reading of 0.0 on a breath alcohol test at the baseline visit.
Participants were randomly allocated to one of four groups:
1. three weekly ketamine infusions of 0.8 mg/kg IV over 40 minutes plus psychological therapy
2. three saline infusions plus psychological therapy
3. three ketamine infusions plus alcohol education
4. three saline infusions plus alcohol education
The primary outcome was self-reported percentage of days abstinent, as well as confirmed alcohol relapse at 6-month follow-up.
(mean difference, 10.1%; 95% confidence interval, 1.1-19.0), “although confidence intervals were wide, consistent with a proof-of-concept study,” the authors note.
The greatest reduction in total days off alcohol occurred in the ketamine plus relapse-prevention therapy group compared with the saline plus alcohol education group (mean difference, 15.9%; 95% CI, 3.8-28.1).
There was no significant difference in relapse rate between the ketamine and placebo groups. No serious adverse effects were reported in any participant.
Growing evidence
These findings support some other studies that have also suggested a benefit of ketamine in AUD.
As reported by this news organization, one recent study found a single infusion of ketamine combined with counseling may help alcohol-dependent patients curb their drinking.
A separate study showed that a single dose of ketamine plus therapy that focused on reactivating drinking-related “maladaptive reward memories” reduced drinking urges and alcohol intake more than just ketamine or a placebo infusion alone.
“That ketamine can reduce both alcohol use and depression in AUD is encouraging therapeutically,” the researchers write.
“While a clear link between depression and AUD is acknowledged, alcohol and mental health services still struggle to meet the needs of dual-diagnosis patients, so ketamine may represent a solution to this long-standing comorbidity,” they add.
Dr. Morgan said in an interview that adjunctive ketamine with relapse-prevention therapy is “currently being delivered in Awakn Clinics in the U.K. and Norway, but we need to conduct the phase 3 trial in order to make the treatment more widely accessible.”
An ‘Intriguing new therapy’
Reached for comment, Timothy Brennan, MD, MPH, chief of clinical services, Addiction Institute of Mount Sinai, New York, said ketamine “continues to be an intriguing new therapy for a variety of mental health conditions.”
“Unfortunately, the study did not show any difference in rates of relapse to alcohol, though an improvement in days of abstinence is certainly noteworthy,” Dr. Brennan said in an interview.
“Because this was just a proof-of-concept study and did not compare ketamine to any FDA-approved pharmacotherapy for alcohol, it remains too early to recommend ketamine infusions to those suffering from alcohol use disorder,” he cautioned.
The study was supported by the Medical Research Council. Dr. Morgan has received royalties for KARE (Ketamine for Reduction of Alcoholic Relapse) therapy license distribution. KARE therapy is licensed from University of Exeter to Awakn Life Sciences. Dr. Morgan has received research funding from Awakn Life Sciences and has served as a consultant for Janssen Pharmaceuticals. Other coauthors have disclosed relationships with industry; the full list can be found with the original article. Dr. Brennan has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Three weekly infusions of the dissociative anesthetic ketamine coupled with mindfulness-based relapse prevention therapy may help adults with alcohol use disorder (AUD) maintain abstinence, new research suggests.
Preliminary results from a phase 2, double-blind, placebo-controlled trial show ketamine was well tolerated and, compared with placebo, associated with more days of abstinence from alcohol at 6 months.
The results suggest ketamine plus psychological therapy may be a “new, relatively brief treatment that has long lasting effects in AUD,” Celia Morgan, PhD, professor of psychopharmacology, University of Exeter, United Kingdom, told this news organization.
The study was published online Jan. 11 in the American Journal of Psychiatry.
Target depression
Depressive symptoms are common in patients under treatment for AUD and increase relapse risk.
“Ketamine may support alcohol abstinence by temporarily alleviating depressive symptoms during the high-risk relapse period in the weeks after detoxification,” the investigators note.
Ketamine may also provide a “temporary boost to synaptogenesis and neurogenesis, which may allow psychological therapies and new strategies for managing addiction to embed more readily,” they add.
To test these theories, the researchers recruited 96 adults (mean age, 44 years, 35 women) with severe AUD to participate in the trial.
All participants had to abstain from alcohol for at least 24 hours before the trial started and have a reading of 0.0 on a breath alcohol test at the baseline visit.
Participants were randomly allocated to one of four groups:
1. three weekly ketamine infusions of 0.8 mg/kg IV over 40 minutes plus psychological therapy
2. three saline infusions plus psychological therapy
3. three ketamine infusions plus alcohol education
4. three saline infusions plus alcohol education
The primary outcome was self-reported percentage of days abstinent, as well as confirmed alcohol relapse at 6-month follow-up.
(mean difference, 10.1%; 95% confidence interval, 1.1-19.0), “although confidence intervals were wide, consistent with a proof-of-concept study,” the authors note.
The greatest reduction in total days off alcohol occurred in the ketamine plus relapse-prevention therapy group compared with the saline plus alcohol education group (mean difference, 15.9%; 95% CI, 3.8-28.1).
There was no significant difference in relapse rate between the ketamine and placebo groups. No serious adverse effects were reported in any participant.
Growing evidence
These findings support some other studies that have also suggested a benefit of ketamine in AUD.
As reported by this news organization, one recent study found a single infusion of ketamine combined with counseling may help alcohol-dependent patients curb their drinking.
A separate study showed that a single dose of ketamine plus therapy that focused on reactivating drinking-related “maladaptive reward memories” reduced drinking urges and alcohol intake more than just ketamine or a placebo infusion alone.
“That ketamine can reduce both alcohol use and depression in AUD is encouraging therapeutically,” the researchers write.
“While a clear link between depression and AUD is acknowledged, alcohol and mental health services still struggle to meet the needs of dual-diagnosis patients, so ketamine may represent a solution to this long-standing comorbidity,” they add.
Dr. Morgan said in an interview that adjunctive ketamine with relapse-prevention therapy is “currently being delivered in Awakn Clinics in the U.K. and Norway, but we need to conduct the phase 3 trial in order to make the treatment more widely accessible.”
An ‘Intriguing new therapy’
Reached for comment, Timothy Brennan, MD, MPH, chief of clinical services, Addiction Institute of Mount Sinai, New York, said ketamine “continues to be an intriguing new therapy for a variety of mental health conditions.”
“Unfortunately, the study did not show any difference in rates of relapse to alcohol, though an improvement in days of abstinence is certainly noteworthy,” Dr. Brennan said in an interview.
“Because this was just a proof-of-concept study and did not compare ketamine to any FDA-approved pharmacotherapy for alcohol, it remains too early to recommend ketamine infusions to those suffering from alcohol use disorder,” he cautioned.
The study was supported by the Medical Research Council. Dr. Morgan has received royalties for KARE (Ketamine for Reduction of Alcoholic Relapse) therapy license distribution. KARE therapy is licensed from University of Exeter to Awakn Life Sciences. Dr. Morgan has received research funding from Awakn Life Sciences and has served as a consultant for Janssen Pharmaceuticals. Other coauthors have disclosed relationships with industry; the full list can be found with the original article. Dr. Brennan has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Three weekly infusions of the dissociative anesthetic ketamine coupled with mindfulness-based relapse prevention therapy may help adults with alcohol use disorder (AUD) maintain abstinence, new research suggests.
Preliminary results from a phase 2, double-blind, placebo-controlled trial show ketamine was well tolerated and, compared with placebo, associated with more days of abstinence from alcohol at 6 months.
The results suggest ketamine plus psychological therapy may be a “new, relatively brief treatment that has long lasting effects in AUD,” Celia Morgan, PhD, professor of psychopharmacology, University of Exeter, United Kingdom, told this news organization.
The study was published online Jan. 11 in the American Journal of Psychiatry.
Target depression
Depressive symptoms are common in patients under treatment for AUD and increase relapse risk.
“Ketamine may support alcohol abstinence by temporarily alleviating depressive symptoms during the high-risk relapse period in the weeks after detoxification,” the investigators note.
Ketamine may also provide a “temporary boost to synaptogenesis and neurogenesis, which may allow psychological therapies and new strategies for managing addiction to embed more readily,” they add.
To test these theories, the researchers recruited 96 adults (mean age, 44 years, 35 women) with severe AUD to participate in the trial.
All participants had to abstain from alcohol for at least 24 hours before the trial started and have a reading of 0.0 on a breath alcohol test at the baseline visit.
Participants were randomly allocated to one of four groups:
1. three weekly ketamine infusions of 0.8 mg/kg IV over 40 minutes plus psychological therapy
2. three saline infusions plus psychological therapy
3. three ketamine infusions plus alcohol education
4. three saline infusions plus alcohol education
The primary outcome was self-reported percentage of days abstinent, as well as confirmed alcohol relapse at 6-month follow-up.
(mean difference, 10.1%; 95% confidence interval, 1.1-19.0), “although confidence intervals were wide, consistent with a proof-of-concept study,” the authors note.
The greatest reduction in total days off alcohol occurred in the ketamine plus relapse-prevention therapy group compared with the saline plus alcohol education group (mean difference, 15.9%; 95% CI, 3.8-28.1).
There was no significant difference in relapse rate between the ketamine and placebo groups. No serious adverse effects were reported in any participant.
Growing evidence
These findings support some other studies that have also suggested a benefit of ketamine in AUD.
As reported by this news organization, one recent study found a single infusion of ketamine combined with counseling may help alcohol-dependent patients curb their drinking.
A separate study showed that a single dose of ketamine plus therapy that focused on reactivating drinking-related “maladaptive reward memories” reduced drinking urges and alcohol intake more than just ketamine or a placebo infusion alone.
“That ketamine can reduce both alcohol use and depression in AUD is encouraging therapeutically,” the researchers write.
“While a clear link between depression and AUD is acknowledged, alcohol and mental health services still struggle to meet the needs of dual-diagnosis patients, so ketamine may represent a solution to this long-standing comorbidity,” they add.
Dr. Morgan said in an interview that adjunctive ketamine with relapse-prevention therapy is “currently being delivered in Awakn Clinics in the U.K. and Norway, but we need to conduct the phase 3 trial in order to make the treatment more widely accessible.”
An ‘Intriguing new therapy’
Reached for comment, Timothy Brennan, MD, MPH, chief of clinical services, Addiction Institute of Mount Sinai, New York, said ketamine “continues to be an intriguing new therapy for a variety of mental health conditions.”
“Unfortunately, the study did not show any difference in rates of relapse to alcohol, though an improvement in days of abstinence is certainly noteworthy,” Dr. Brennan said in an interview.
“Because this was just a proof-of-concept study and did not compare ketamine to any FDA-approved pharmacotherapy for alcohol, it remains too early to recommend ketamine infusions to those suffering from alcohol use disorder,” he cautioned.
The study was supported by the Medical Research Council. Dr. Morgan has received royalties for KARE (Ketamine for Reduction of Alcoholic Relapse) therapy license distribution. KARE therapy is licensed from University of Exeter to Awakn Life Sciences. Dr. Morgan has received research funding from Awakn Life Sciences and has served as a consultant for Janssen Pharmaceuticals. Other coauthors have disclosed relationships with industry; the full list can be found with the original article. Dr. Brennan has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
How to screen for and treat teen alcohol use
THE CASE
Paul F* is a 16-year-old White boy who lives with his mother and spends some weekends with his father who has shared custody. He recently presented to the clinic for treatment due to an arrest for disorderly conduct at school. He and a friend were found drinking liquor outside the school building when they were scheduled to be in class. Paul reported that he and his friends often drink at school and at extracurricular functions. He has been using alcohol for the past 2 years, with escalating consumption (5 or more drinks per episode) in the past year. Paul has been drinking most days of the week and has even driven under the influence at times. He said, “I just feel happier when I am drinking.” An accomplished soccer player recruited by colleges, Paul recently was suspended from the team due to his poor grades. His response was, “It’s stupid anyway. What’s the point of playing?”
●
* The patient’s name and some personal details have been changed to protect his identity.
Alcohol is the number 1 substance of abuse for adolescents, used more than tobacco or drugs.1-3 In 2007 and again in 2016, the Surgeon General of the United States issued reports to highlight this important topic,1,2 noting that early and repeated exposure to alcohol during this crucial time of brain development increases the risk for future problems, including addiction.2
Adolescent alcohol use is often underestimated by parents and physicians, including misjudging how much, how often, and how young children are when they begin to drink.1 Boys and girls tend to start drinking at similar ages (13.9 and 14.4 years, respectively),3 but as girls age, they tend to drink more and binge more.4 In 2019, 1 in 4 adolescents reported drinking and more than 4 million reported at least 1 episode of binge drinking in the prior month.4 These numbers have further ramifications: early drinking is associated with alcohol dependence, relapse, use of other substances, risky sexual behaviors, injurious behaviors, suicide, motor vehicle accidents, and dating violence.4-6
Diagnosing alcohol use disorder
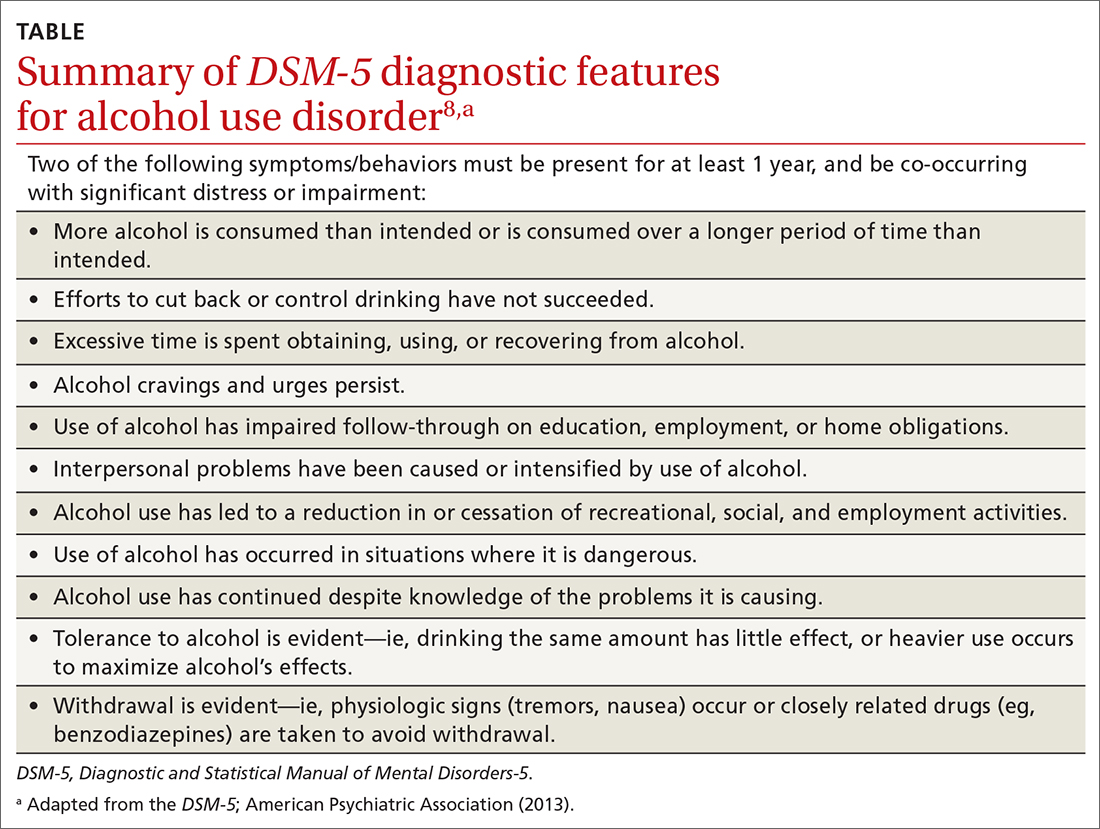
The range of alcohol use includes consumption, bingeing, abuse, and dependence.7,8 Consumption is defined as the drinking of alcoholic beverages. Bingeing is the consumption of more than 5 drinks for men or 4 drinks for women in 2 hours, according to the National Institute on Alcohol Abuse and Alcoholism.7 However, the criterion is slightly different for the Substance Abuse and Mental Health Services Administration, which broadens the timeframe to “on the same occasion.”9 While previously known as separate disorders, alcohol abuse (or misuse) and alcohol dependence are now diagnostically classified together as alcohol use disorders (AUDs), per the Diagnostic and Statistical Manual of Mental Disorders-5 (DSM-5).8 AUD is further stratified as mild, moderate, or severe, depending on the number of criteria that are met by the patient (TABLE).8,10
Alcohol screening
Currently, the US Preventive Services Task Force (USPSTF) does not recommend screening adolescents ages 12 to 17 for AUD, and has instead issued an “I” statement (insufficient evidence).11 While the USPSTF recognizes the potential burdens of adolescent alcohol use, the potential harms of screening include “stigma, anxiety, labeling, discrimination, privacy concerns, and interference with the patient–clinician relationship.”11 The USPSTF also notes that it “did not find any evidence that specifically examined the harms of screening for alcohol use in adolescents.”11
This is at odds with recommendations from the American Academy of Pediatrics (AAP), which in 2011 released a policy statement advocating screening, brief intervention, and referral to treatment for adolescent substance use.12 In the United States, even though 83% of adolescents see a physician at least once each year,12,13 alcohol misuse screening still varies, occurring in the range of 50% to 86% of office visits.12 When screening does occur, it is often based on clinical impression only.12 Studies have shown that when a screening tool is not used, up to two-thirds of substance use disorders may be missed.12-15
Continue to: A full and complete biopsychosocial interview
A full and complete biopsychosocial interview with adolescents is a necessity, and should include queries about alcohol, drugs, and other substances. Acknowledgment of use should trigger further investigation into the substance use areas. Interviews may start with open-ended questions about alcohol use at home or at school before moving to more personalized and detailed questioning and use of screening tools.16
While various screening instruments exist, for the sake of brevity we provide as an example the Screening to Brief Intervention (S2BI) tool. It is an efficient, single-page tool that can help clinicians in their routine care of adolescents to quickly stratify the patient risk of substance use disorder as none/low, moderate, or severe.12 It can be found here: www.mcpap.com/pdf/S2Bi%20Toolkit.pdf (see page 10).
For all patients, but particularly for adolescents, confidentiality is important, and many specialty societies have created language to address this issue.12 Discuss confidentiality with both the adolescent patient and the patient’s caregiver simultaneously, with dialogue that includes: (a) the need to speak with adolescents alone during the office visit, (b) the benefits of confidentiality in the physician–patient relationship, and (c) the need to disclose selected information to keep patients safe.12 Describing the process for required disclosures is essential. Benefits of disclosure include further support for the adolescent patient as well as appropriate parental participation and support for possible referrals.12
Treating AUD
Treatment for AUD should be multifaceted. Screen for comorbid mood disorders, such as generalized anxiety,17,18 social anxiety,18 and depression,19 as well as for insomnia.18 Studies have demonstrated a strong link between insomnia and anxiety, and again between anxiety and AUD.17-19 Finally, screen for adverse childhood events such as trauma, victimization, and abuse.20 Addressing issues discovered in screening allows for more targeted and personalized treatment of AUD.
The National Institute on Drug Abuse categorizes evidence-based treatment into 3 areas: behavioral therapies, family therapies, and medications.21
Continue to: Behavioral therapies
Behavioral therapies can include group therapy, cognitive behavioral therapy (CBT), motivational enhancement therapy, 12-Step facilitation, and contingency management, in which small rewards or incentives are given for participation in treatment to reinforce positive behaviors.21
Family-based therapies, such as brief strategic family therapy, functional family therapy, and multisystem therapy recognize that adolescents exist in systems of families in communities, and that the patient’s success in treatment may be supported by these relationships.21
Some medications may achieve modest benefit for treatment of adolescents with AUD. Naltrexone, acamprosate, and disulfiram have all been used successfully to treat AUD in adults21; some physicians may choose to use these medications “off label” in adolescents. Bupropion has been used successfully in the treatment of nicotine use disorder,21 and a small study in 2005 showed some success with bupropion in treating adolescents with attention-deficit/hyperactivity disorder, comorbid depression, and substance use disorder.22 Naltrexone has also been studied in adolescents with opioid use disorder, although these were not large studies.23
Adolescents with serious, sustained issues with AUD may require more in-depth treatments such as an intensive outpatient program, a partial hospitalization program, or a residential treatment program.15 The least-restrictive environment is preferable.15 Families are generally included as part of the treatment and recovery process in those settings.21 Some patients may require detoxification prior to referral to residential treatment settings; the American Society of Addiction Medicine has published a comprehensive guideline on alcohol withdrawal.24
Paul’s family physician diagnosed his condition as AUD and referred him for CBT with a psychologist, who treated him for both the AUD and an underlying depressive disorder that was later identified. CBT focused on cognitive restructuring of depressive thoughts as well as support for continued abstinence from alcohol. The patient, with family support, declined antidepressant medication.
After 6 months of treatment, Paul and his parents were pleased with his progress. His grades improved to the point that he was permitted to play soccer again, and he was seriously looking at his future college options.
CORRESPONDENCE
Scott A. Fields, PhD, 3200 MacCorkle Avenue Southeast, 5th Floor, Robert C. Byrd Clinical Teaching Center, Department of Family Medicine, Charleston, WV 25304; [email protected]
1. US Department of Health and Human Services. The Surgeon General’s Call to Action to Prevent and Reduce Underage Drinking. Washington, DC; US Department of Health and Human Services, Office of the Surgeon General. 2007.
2. US Department of Health and Human Services. Facing Addiction in America: The Surgeon General’s Report on Alcohol, Drugs, and Health. Washington, DC; US Department of Health and Human Services, Office of the Surgeon General. 2016.
3. Hingson R, White A. New research findings since the 2007 Surgeon General’s Call to Action to Prevent and Reduce Underage Drinking: A review. J Stud Alcohol Drugs Suppl. 2014; 75:158-169.
4. National Institute on Alcohol Abuse and Alcoholism. Underage drinking. National Institute of Health. Accessed December 22, 2021. www.niaaa.nih.gov/publications/brochures-and-fact-sheets/underage-drinking.
5. Hingson R, Zha W, Iannotti R, et al. Physician advice to adolescents about drinking and other health behaviors. Pediatrics. 2013;131:249-257.
6. Schaus JF, Sole ML, McCoy TP, et al. Screening for high-risk drinking in a college student health center: characterizing students based on quantity, frequency, and harms. J Stud Alcohol Drugs Suppl. 2009;16:34-44.
7. National Institute on Alcohol Abuse and Alcoholism. Drinking levels defined. Accessed December 27, 2021. www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking
8. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). Arlington, VA; American Psychiatric Association. 2013.
9. Substance Abuse and Mental Health Services Administration. Bringing down binge drinking. Accessed December 27, 2021. www.samhsa.gov/sites/default/files/programs_campaigns/nation_prevention_week/data-binge-drinking.pdf
10. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 Alcohol Use Disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757-766.
11. USPSTF. Screening and behavioral counseling interventions to reduce unhealthy alcohol use in adolescents and adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320:1899-1909.
12. Levy SJ, Williams JF, Committee on Substance Use and Prevention. Substance use screening, brief intervention, and referral to treatment. Pediatrics. 2016;138:e20161211.
13. MacKay AP, Duran CP. Adolescent Health in the United States. National Center for Health Statistics, Centers for Disease Control and Prevention. 2007.
14. Haller DM, Meynard A, Lefebvre D, et al. Effectiveness of training family physicians to deliver a brief intervention to address excessive substance use among young patients: a cluster randomized controlled trial. CMAJ. 2014;186:E263-E272.
15. Borus J, Parhami I, Levy S. Screening, brief intervention, and referral to treatment. Child Adolesc Psychiatric Clin N Am. 2016;25:579-601.
16. Knight J, Roberts T, Gabrielli J, et al. Adolescent alcohol and substance use and abuse. Performing preventive services: A bright futures handbook. Accessed December 22, 2021. American Academy of Pediatrics. https://ocfcpacourts.us/wp-content/uploads/2020/06/Adolescent_Alcohol_and_Substance_Abuse_001005.pdf
17. Dyer ML, Heron J, Hickman M, et al. Alcohol use in late adolescence and early adulthood: the role of generalized anxiety disorder and drinking to cope motives. Drug Alcohol Depend. 2019;204:107480.
18. Blumenthal H, Taylor DJ, Cloutier RM, et al. The links between social anxiety disorder, insomnia symptoms, and alcohol use disorders: findings from a large sample of adolescents in the United States. Behav Ther. 2019;50:50-59.
19. Pedrelli P, Shapero B, Archibald A, et al. Alcohol use and depression during adolescence and young adulthood: a summary and interpretation of mixed findings. Curr Addict Rep. 2016;3:91-97.
20. Davis JP, Dworkin ER, Helton J, et al. Extending poly-victimization theory: differential effects of adolescents’ experiences of victimization on substance use disorder diagnoses upon treatment entry. Child Abuse Negl. 2019; 89:165-177.
21. NIDA. Principles of adolescent substance use disorder treatment: a research-based guide. Accessed December 22, 2021. www.drugabuse.gov/publications/principles-adolescent-substance-use-disorder-treatment-research-based-guide
22. Solhkhah R, Wilens TE, Daly J, et al. Bupropion SR for the treatment of substance-abusing outpatient adolescents with attention-deficit/hyperactivity disorder and mood disorders. J Child Adolesc Psychopharmacol. 2005:15:777-786.
23. Camenga DR, Colon-Rivera HA, Muvvala SB. Medications for maintenance treatment of opioid use disorder in adolescents. J Stud Alcohol Drugs. 2019;80:393-402.
24. American Society of Addiction Medicine. The ASAM clinical practice guideline on alcohol withdrawal management. Accessed December 22, 2021. www.asam.org/quality-care/clinical-guidelines/alcohol-withdrawal-management-guideline
THE CASE
Paul F* is a 16-year-old White boy who lives with his mother and spends some weekends with his father who has shared custody. He recently presented to the clinic for treatment due to an arrest for disorderly conduct at school. He and a friend were found drinking liquor outside the school building when they were scheduled to be in class. Paul reported that he and his friends often drink at school and at extracurricular functions. He has been using alcohol for the past 2 years, with escalating consumption (5 or more drinks per episode) in the past year. Paul has been drinking most days of the week and has even driven under the influence at times. He said, “I just feel happier when I am drinking.” An accomplished soccer player recruited by colleges, Paul recently was suspended from the team due to his poor grades. His response was, “It’s stupid anyway. What’s the point of playing?”
●
* The patient’s name and some personal details have been changed to protect his identity.
Alcohol is the number 1 substance of abuse for adolescents, used more than tobacco or drugs.1-3 In 2007 and again in 2016, the Surgeon General of the United States issued reports to highlight this important topic,1,2 noting that early and repeated exposure to alcohol during this crucial time of brain development increases the risk for future problems, including addiction.2
Adolescent alcohol use is often underestimated by parents and physicians, including misjudging how much, how often, and how young children are when they begin to drink.1 Boys and girls tend to start drinking at similar ages (13.9 and 14.4 years, respectively),3 but as girls age, they tend to drink more and binge more.4 In 2019, 1 in 4 adolescents reported drinking and more than 4 million reported at least 1 episode of binge drinking in the prior month.4 These numbers have further ramifications: early drinking is associated with alcohol dependence, relapse, use of other substances, risky sexual behaviors, injurious behaviors, suicide, motor vehicle accidents, and dating violence.4-6
Diagnosing alcohol use disorder
The range of alcohol use includes consumption, bingeing, abuse, and dependence.7,8 Consumption is defined as the drinking of alcoholic beverages. Bingeing is the consumption of more than 5 drinks for men or 4 drinks for women in 2 hours, according to the National Institute on Alcohol Abuse and Alcoholism.7 However, the criterion is slightly different for the Substance Abuse and Mental Health Services Administration, which broadens the timeframe to “on the same occasion.”9 While previously known as separate disorders, alcohol abuse (or misuse) and alcohol dependence are now diagnostically classified together as alcohol use disorders (AUDs), per the Diagnostic and Statistical Manual of Mental Disorders-5 (DSM-5).8 AUD is further stratified as mild, moderate, or severe, depending on the number of criteria that are met by the patient (TABLE).8,10

Alcohol screening
Currently, the US Preventive Services Task Force (USPSTF) does not recommend screening adolescents ages 12 to 17 for AUD, and has instead issued an “I” statement (insufficient evidence).11 While the USPSTF recognizes the potential burdens of adolescent alcohol use, the potential harms of screening include “stigma, anxiety, labeling, discrimination, privacy concerns, and interference with the patient–clinician relationship.”11 The USPSTF also notes that it “did not find any evidence that specifically examined the harms of screening for alcohol use in adolescents.”11
This is at odds with recommendations from the American Academy of Pediatrics (AAP), which in 2011 released a policy statement advocating screening, brief intervention, and referral to treatment for adolescent substance use.12 In the United States, even though 83% of adolescents see a physician at least once each year,12,13 alcohol misuse screening still varies, occurring in the range of 50% to 86% of office visits.12 When screening does occur, it is often based on clinical impression only.12 Studies have shown that when a screening tool is not used, up to two-thirds of substance use disorders may be missed.12-15
Continue to: A full and complete biopsychosocial interview
A full and complete biopsychosocial interview with adolescents is a necessity, and should include queries about alcohol, drugs, and other substances. Acknowledgment of use should trigger further investigation into the substance use areas. Interviews may start with open-ended questions about alcohol use at home or at school before moving to more personalized and detailed questioning and use of screening tools.16
While various screening instruments exist, for the sake of brevity we provide as an example the Screening to Brief Intervention (S2BI) tool. It is an efficient, single-page tool that can help clinicians in their routine care of adolescents to quickly stratify the patient risk of substance use disorder as none/low, moderate, or severe.12 It can be found here: www.mcpap.com/pdf/S2Bi%20Toolkit.pdf (see page 10).
For all patients, but particularly for adolescents, confidentiality is important, and many specialty societies have created language to address this issue.12 Discuss confidentiality with both the adolescent patient and the patient’s caregiver simultaneously, with dialogue that includes: (a) the need to speak with adolescents alone during the office visit, (b) the benefits of confidentiality in the physician–patient relationship, and (c) the need to disclose selected information to keep patients safe.12 Describing the process for required disclosures is essential. Benefits of disclosure include further support for the adolescent patient as well as appropriate parental participation and support for possible referrals.12
Treating AUD
Treatment for AUD should be multifaceted. Screen for comorbid mood disorders, such as generalized anxiety,17,18 social anxiety,18 and depression,19 as well as for insomnia.18 Studies have demonstrated a strong link between insomnia and anxiety, and again between anxiety and AUD.17-19 Finally, screen for adverse childhood events such as trauma, victimization, and abuse.20 Addressing issues discovered in screening allows for more targeted and personalized treatment of AUD.
The National Institute on Drug Abuse categorizes evidence-based treatment into 3 areas: behavioral therapies, family therapies, and medications.21
Continue to: Behavioral therapies
Behavioral therapies can include group therapy, cognitive behavioral therapy (CBT), motivational enhancement therapy, 12-Step facilitation, and contingency management, in which small rewards or incentives are given for participation in treatment to reinforce positive behaviors.21
Family-based therapies, such as brief strategic family therapy, functional family therapy, and multisystem therapy recognize that adolescents exist in systems of families in communities, and that the patient’s success in treatment may be supported by these relationships.21
Some medications may achieve modest benefit for treatment of adolescents with AUD. Naltrexone, acamprosate, and disulfiram have all been used successfully to treat AUD in adults21; some physicians may choose to use these medications “off label” in adolescents. Bupropion has been used successfully in the treatment of nicotine use disorder,21 and a small study in 2005 showed some success with bupropion in treating adolescents with attention-deficit/hyperactivity disorder, comorbid depression, and substance use disorder.22 Naltrexone has also been studied in adolescents with opioid use disorder, although these were not large studies.23
Adolescents with serious, sustained issues with AUD may require more in-depth treatments such as an intensive outpatient program, a partial hospitalization program, or a residential treatment program.15 The least-restrictive environment is preferable.15 Families are generally included as part of the treatment and recovery process in those settings.21 Some patients may require detoxification prior to referral to residential treatment settings; the American Society of Addiction Medicine has published a comprehensive guideline on alcohol withdrawal.24
Paul’s family physician diagnosed his condition as AUD and referred him for CBT with a psychologist, who treated him for both the AUD and an underlying depressive disorder that was later identified. CBT focused on cognitive restructuring of depressive thoughts as well as support for continued abstinence from alcohol. The patient, with family support, declined antidepressant medication.
After 6 months of treatment, Paul and his parents were pleased with his progress. His grades improved to the point that he was permitted to play soccer again, and he was seriously looking at his future college options.
CORRESPONDENCE
Scott A. Fields, PhD, 3200 MacCorkle Avenue Southeast, 5th Floor, Robert C. Byrd Clinical Teaching Center, Department of Family Medicine, Charleston, WV 25304; [email protected]
THE CASE
Paul F* is a 16-year-old White boy who lives with his mother and spends some weekends with his father who has shared custody. He recently presented to the clinic for treatment due to an arrest for disorderly conduct at school. He and a friend were found drinking liquor outside the school building when they were scheduled to be in class. Paul reported that he and his friends often drink at school and at extracurricular functions. He has been using alcohol for the past 2 years, with escalating consumption (5 or more drinks per episode) in the past year. Paul has been drinking most days of the week and has even driven under the influence at times. He said, “I just feel happier when I am drinking.” An accomplished soccer player recruited by colleges, Paul recently was suspended from the team due to his poor grades. His response was, “It’s stupid anyway. What’s the point of playing?”
●
* The patient’s name and some personal details have been changed to protect his identity.
Alcohol is the number 1 substance of abuse for adolescents, used more than tobacco or drugs.1-3 In 2007 and again in 2016, the Surgeon General of the United States issued reports to highlight this important topic,1,2 noting that early and repeated exposure to alcohol during this crucial time of brain development increases the risk for future problems, including addiction.2
Adolescent alcohol use is often underestimated by parents and physicians, including misjudging how much, how often, and how young children are when they begin to drink.1 Boys and girls tend to start drinking at similar ages (13.9 and 14.4 years, respectively),3 but as girls age, they tend to drink more and binge more.4 In 2019, 1 in 4 adolescents reported drinking and more than 4 million reported at least 1 episode of binge drinking in the prior month.4 These numbers have further ramifications: early drinking is associated with alcohol dependence, relapse, use of other substances, risky sexual behaviors, injurious behaviors, suicide, motor vehicle accidents, and dating violence.4-6
Diagnosing alcohol use disorder
The range of alcohol use includes consumption, bingeing, abuse, and dependence.7,8 Consumption is defined as the drinking of alcoholic beverages. Bingeing is the consumption of more than 5 drinks for men or 4 drinks for women in 2 hours, according to the National Institute on Alcohol Abuse and Alcoholism.7 However, the criterion is slightly different for the Substance Abuse and Mental Health Services Administration, which broadens the timeframe to “on the same occasion.”9 While previously known as separate disorders, alcohol abuse (or misuse) and alcohol dependence are now diagnostically classified together as alcohol use disorders (AUDs), per the Diagnostic and Statistical Manual of Mental Disorders-5 (DSM-5).8 AUD is further stratified as mild, moderate, or severe, depending on the number of criteria that are met by the patient (TABLE).8,10

Alcohol screening
Currently, the US Preventive Services Task Force (USPSTF) does not recommend screening adolescents ages 12 to 17 for AUD, and has instead issued an “I” statement (insufficient evidence).11 While the USPSTF recognizes the potential burdens of adolescent alcohol use, the potential harms of screening include “stigma, anxiety, labeling, discrimination, privacy concerns, and interference with the patient–clinician relationship.”11 The USPSTF also notes that it “did not find any evidence that specifically examined the harms of screening for alcohol use in adolescents.”11
This is at odds with recommendations from the American Academy of Pediatrics (AAP), which in 2011 released a policy statement advocating screening, brief intervention, and referral to treatment for adolescent substance use.12 In the United States, even though 83% of adolescents see a physician at least once each year,12,13 alcohol misuse screening still varies, occurring in the range of 50% to 86% of office visits.12 When screening does occur, it is often based on clinical impression only.12 Studies have shown that when a screening tool is not used, up to two-thirds of substance use disorders may be missed.12-15
Continue to: A full and complete biopsychosocial interview
A full and complete biopsychosocial interview with adolescents is a necessity, and should include queries about alcohol, drugs, and other substances. Acknowledgment of use should trigger further investigation into the substance use areas. Interviews may start with open-ended questions about alcohol use at home or at school before moving to more personalized and detailed questioning and use of screening tools.16
While various screening instruments exist, for the sake of brevity we provide as an example the Screening to Brief Intervention (S2BI) tool. It is an efficient, single-page tool that can help clinicians in their routine care of adolescents to quickly stratify the patient risk of substance use disorder as none/low, moderate, or severe.12 It can be found here: www.mcpap.com/pdf/S2Bi%20Toolkit.pdf (see page 10).
For all patients, but particularly for adolescents, confidentiality is important, and many specialty societies have created language to address this issue.12 Discuss confidentiality with both the adolescent patient and the patient’s caregiver simultaneously, with dialogue that includes: (a) the need to speak with adolescents alone during the office visit, (b) the benefits of confidentiality in the physician–patient relationship, and (c) the need to disclose selected information to keep patients safe.12 Describing the process for required disclosures is essential. Benefits of disclosure include further support for the adolescent patient as well as appropriate parental participation and support for possible referrals.12
Treating AUD
Treatment for AUD should be multifaceted. Screen for comorbid mood disorders, such as generalized anxiety,17,18 social anxiety,18 and depression,19 as well as for insomnia.18 Studies have demonstrated a strong link between insomnia and anxiety, and again between anxiety and AUD.17-19 Finally, screen for adverse childhood events such as trauma, victimization, and abuse.20 Addressing issues discovered in screening allows for more targeted and personalized treatment of AUD.
The National Institute on Drug Abuse categorizes evidence-based treatment into 3 areas: behavioral therapies, family therapies, and medications.21
Continue to: Behavioral therapies
Behavioral therapies can include group therapy, cognitive behavioral therapy (CBT), motivational enhancement therapy, 12-Step facilitation, and contingency management, in which small rewards or incentives are given for participation in treatment to reinforce positive behaviors.21
Family-based therapies, such as brief strategic family therapy, functional family therapy, and multisystem therapy recognize that adolescents exist in systems of families in communities, and that the patient’s success in treatment may be supported by these relationships.21
Some medications may achieve modest benefit for treatment of adolescents with AUD. Naltrexone, acamprosate, and disulfiram have all been used successfully to treat AUD in adults21; some physicians may choose to use these medications “off label” in adolescents. Bupropion has been used successfully in the treatment of nicotine use disorder,21 and a small study in 2005 showed some success with bupropion in treating adolescents with attention-deficit/hyperactivity disorder, comorbid depression, and substance use disorder.22 Naltrexone has also been studied in adolescents with opioid use disorder, although these were not large studies.23
Adolescents with serious, sustained issues with AUD may require more in-depth treatments such as an intensive outpatient program, a partial hospitalization program, or a residential treatment program.15 The least-restrictive environment is preferable.15 Families are generally included as part of the treatment and recovery process in those settings.21 Some patients may require detoxification prior to referral to residential treatment settings; the American Society of Addiction Medicine has published a comprehensive guideline on alcohol withdrawal.24
Paul’s family physician diagnosed his condition as AUD and referred him for CBT with a psychologist, who treated him for both the AUD and an underlying depressive disorder that was later identified. CBT focused on cognitive restructuring of depressive thoughts as well as support for continued abstinence from alcohol. The patient, with family support, declined antidepressant medication.
After 6 months of treatment, Paul and his parents were pleased with his progress. His grades improved to the point that he was permitted to play soccer again, and he was seriously looking at his future college options.
CORRESPONDENCE
Scott A. Fields, PhD, 3200 MacCorkle Avenue Southeast, 5th Floor, Robert C. Byrd Clinical Teaching Center, Department of Family Medicine, Charleston, WV 25304; [email protected]
1. US Department of Health and Human Services. The Surgeon General’s Call to Action to Prevent and Reduce Underage Drinking. Washington, DC; US Department of Health and Human Services, Office of the Surgeon General. 2007.
2. US Department of Health and Human Services. Facing Addiction in America: The Surgeon General’s Report on Alcohol, Drugs, and Health. Washington, DC; US Department of Health and Human Services, Office of the Surgeon General. 2016.
3. Hingson R, White A. New research findings since the 2007 Surgeon General’s Call to Action to Prevent and Reduce Underage Drinking: A review. J Stud Alcohol Drugs Suppl. 2014; 75:158-169.
4. National Institute on Alcohol Abuse and Alcoholism. Underage drinking. National Institute of Health. Accessed December 22, 2021. www.niaaa.nih.gov/publications/brochures-and-fact-sheets/underage-drinking.
5. Hingson R, Zha W, Iannotti R, et al. Physician advice to adolescents about drinking and other health behaviors. Pediatrics. 2013;131:249-257.
6. Schaus JF, Sole ML, McCoy TP, et al. Screening for high-risk drinking in a college student health center: characterizing students based on quantity, frequency, and harms. J Stud Alcohol Drugs Suppl. 2009;16:34-44.
7. National Institute on Alcohol Abuse and Alcoholism. Drinking levels defined. Accessed December 27, 2021. www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking
8. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). Arlington, VA; American Psychiatric Association. 2013.
9. Substance Abuse and Mental Health Services Administration. Bringing down binge drinking. Accessed December 27, 2021. www.samhsa.gov/sites/default/files/programs_campaigns/nation_prevention_week/data-binge-drinking.pdf
10. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 Alcohol Use Disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757-766.
11. USPSTF. Screening and behavioral counseling interventions to reduce unhealthy alcohol use in adolescents and adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320:1899-1909.
12. Levy SJ, Williams JF, Committee on Substance Use and Prevention. Substance use screening, brief intervention, and referral to treatment. Pediatrics. 2016;138:e20161211.
13. MacKay AP, Duran CP. Adolescent Health in the United States. National Center for Health Statistics, Centers for Disease Control and Prevention. 2007.
14. Haller DM, Meynard A, Lefebvre D, et al. Effectiveness of training family physicians to deliver a brief intervention to address excessive substance use among young patients: a cluster randomized controlled trial. CMAJ. 2014;186:E263-E272.
15. Borus J, Parhami I, Levy S. Screening, brief intervention, and referral to treatment. Child Adolesc Psychiatric Clin N Am. 2016;25:579-601.
16. Knight J, Roberts T, Gabrielli J, et al. Adolescent alcohol and substance use and abuse. Performing preventive services: A bright futures handbook. Accessed December 22, 2021. American Academy of Pediatrics. https://ocfcpacourts.us/wp-content/uploads/2020/06/Adolescent_Alcohol_and_Substance_Abuse_001005.pdf
17. Dyer ML, Heron J, Hickman M, et al. Alcohol use in late adolescence and early adulthood: the role of generalized anxiety disorder and drinking to cope motives. Drug Alcohol Depend. 2019;204:107480.
18. Blumenthal H, Taylor DJ, Cloutier RM, et al. The links between social anxiety disorder, insomnia symptoms, and alcohol use disorders: findings from a large sample of adolescents in the United States. Behav Ther. 2019;50:50-59.
19. Pedrelli P, Shapero B, Archibald A, et al. Alcohol use and depression during adolescence and young adulthood: a summary and interpretation of mixed findings. Curr Addict Rep. 2016;3:91-97.
20. Davis JP, Dworkin ER, Helton J, et al. Extending poly-victimization theory: differential effects of adolescents’ experiences of victimization on substance use disorder diagnoses upon treatment entry. Child Abuse Negl. 2019; 89:165-177.
21. NIDA. Principles of adolescent substance use disorder treatment: a research-based guide. Accessed December 22, 2021. www.drugabuse.gov/publications/principles-adolescent-substance-use-disorder-treatment-research-based-guide
22. Solhkhah R, Wilens TE, Daly J, et al. Bupropion SR for the treatment of substance-abusing outpatient adolescents with attention-deficit/hyperactivity disorder and mood disorders. J Child Adolesc Psychopharmacol. 2005:15:777-786.
23. Camenga DR, Colon-Rivera HA, Muvvala SB. Medications for maintenance treatment of opioid use disorder in adolescents. J Stud Alcohol Drugs. 2019;80:393-402.
24. American Society of Addiction Medicine. The ASAM clinical practice guideline on alcohol withdrawal management. Accessed December 22, 2021. www.asam.org/quality-care/clinical-guidelines/alcohol-withdrawal-management-guideline
1. US Department of Health and Human Services. The Surgeon General’s Call to Action to Prevent and Reduce Underage Drinking. Washington, DC; US Department of Health and Human Services, Office of the Surgeon General. 2007.
2. US Department of Health and Human Services. Facing Addiction in America: The Surgeon General’s Report on Alcohol, Drugs, and Health. Washington, DC; US Department of Health and Human Services, Office of the Surgeon General. 2016.
3. Hingson R, White A. New research findings since the 2007 Surgeon General’s Call to Action to Prevent and Reduce Underage Drinking: A review. J Stud Alcohol Drugs Suppl. 2014; 75:158-169.
4. National Institute on Alcohol Abuse and Alcoholism. Underage drinking. National Institute of Health. Accessed December 22, 2021. www.niaaa.nih.gov/publications/brochures-and-fact-sheets/underage-drinking.
5. Hingson R, Zha W, Iannotti R, et al. Physician advice to adolescents about drinking and other health behaviors. Pediatrics. 2013;131:249-257.
6. Schaus JF, Sole ML, McCoy TP, et al. Screening for high-risk drinking in a college student health center: characterizing students based on quantity, frequency, and harms. J Stud Alcohol Drugs Suppl. 2009;16:34-44.
7. National Institute on Alcohol Abuse and Alcoholism. Drinking levels defined. Accessed December 27, 2021. www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking
8. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). Arlington, VA; American Psychiatric Association. 2013.
9. Substance Abuse and Mental Health Services Administration. Bringing down binge drinking. Accessed December 27, 2021. www.samhsa.gov/sites/default/files/programs_campaigns/nation_prevention_week/data-binge-drinking.pdf
10. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 Alcohol Use Disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757-766.
11. USPSTF. Screening and behavioral counseling interventions to reduce unhealthy alcohol use in adolescents and adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320:1899-1909.
12. Levy SJ, Williams JF, Committee on Substance Use and Prevention. Substance use screening, brief intervention, and referral to treatment. Pediatrics. 2016;138:e20161211.
13. MacKay AP, Duran CP. Adolescent Health in the United States. National Center for Health Statistics, Centers for Disease Control and Prevention. 2007.
14. Haller DM, Meynard A, Lefebvre D, et al. Effectiveness of training family physicians to deliver a brief intervention to address excessive substance use among young patients: a cluster randomized controlled trial. CMAJ. 2014;186:E263-E272.
15. Borus J, Parhami I, Levy S. Screening, brief intervention, and referral to treatment. Child Adolesc Psychiatric Clin N Am. 2016;25:579-601.
16. Knight J, Roberts T, Gabrielli J, et al. Adolescent alcohol and substance use and abuse. Performing preventive services: A bright futures handbook. Accessed December 22, 2021. American Academy of Pediatrics. https://ocfcpacourts.us/wp-content/uploads/2020/06/Adolescent_Alcohol_and_Substance_Abuse_001005.pdf
17. Dyer ML, Heron J, Hickman M, et al. Alcohol use in late adolescence and early adulthood: the role of generalized anxiety disorder and drinking to cope motives. Drug Alcohol Depend. 2019;204:107480.
18. Blumenthal H, Taylor DJ, Cloutier RM, et al. The links between social anxiety disorder, insomnia symptoms, and alcohol use disorders: findings from a large sample of adolescents in the United States. Behav Ther. 2019;50:50-59.
19. Pedrelli P, Shapero B, Archibald A, et al. Alcohol use and depression during adolescence and young adulthood: a summary and interpretation of mixed findings. Curr Addict Rep. 2016;3:91-97.
20. Davis JP, Dworkin ER, Helton J, et al. Extending poly-victimization theory: differential effects of adolescents’ experiences of victimization on substance use disorder diagnoses upon treatment entry. Child Abuse Negl. 2019; 89:165-177.
21. NIDA. Principles of adolescent substance use disorder treatment: a research-based guide. Accessed December 22, 2021. www.drugabuse.gov/publications/principles-adolescent-substance-use-disorder-treatment-research-based-guide
22. Solhkhah R, Wilens TE, Daly J, et al. Bupropion SR for the treatment of substance-abusing outpatient adolescents with attention-deficit/hyperactivity disorder and mood disorders. J Child Adolesc Psychopharmacol. 2005:15:777-786.
23. Camenga DR, Colon-Rivera HA, Muvvala SB. Medications for maintenance treatment of opioid use disorder in adolescents. J Stud Alcohol Drugs. 2019;80:393-402.
24. American Society of Addiction Medicine. The ASAM clinical practice guideline on alcohol withdrawal management. Accessed December 22, 2021. www.asam.org/quality-care/clinical-guidelines/alcohol-withdrawal-management-guideline
Dramatic increase in driving high after cannabis legislation
Since Canada legalized marijuana in 2018, there has been a dramatic increase in the number of individuals driving while high, new research shows.
Investigators studied over 4,000 drivers treated after a motor vehicle collision in British Columbia trauma centers and found that, before cannabis was legalized, a THC level greater than 0 ng/mL in the blood was present in roughly 10% of drivers. After the drug was legalized this percentage increased to 18%. The percentages of injured drivers with at least 2 ng/mL, the Canadian legal limit, and at least 5 ng/mL more than doubled.
“It’s concerning that we’re seeing such a dramatic increase,” study investigator Jeffrey Brubacher, MD, associate professor, department of emergency medicine, University of British Columbia, Vancouver, said in a press release.
“There are serious risks associated with driving after cannabis use and our findings suggest more [work] is needed to deter this dangerous behavior in light of legalization,” he said.
The study was published online Jan. 12 in the New England Journal of Medicine.
Impact of legalization?
The investigators note that the Canadian government introduced a law aiming to prevent cannabis-impaired driving by establishing penalties and criminal charges for drivers found with a whole-blood THC level of 2 ng/mL, with more severe penalties for those with a THC level of greater than 5 ng/mL or greater than 2.5 ng/mL combined with a blood alcohol level of .05%.
Cannabis use is “associated with cognitive deficits and psychomotor impairment, and there is evidence that it increases the risk of motor vehicle crashes, especially at higher THC levels,” they noted.
“I’m an emergency physician at Vancouver General Hospital’s trauma center. We’ve been measuring drug levels in injured drivers since 2013 here in British Columbia and, in particular, we’ve been measuring THC levels,” Dr. Brubacher said in an interview. “We thought it would be interesting and important to see what would happen after legalization.”
The investigators studied 4,339 drivers – 3,550 whose accident took place before legalization of cannabis, and 789 after legalization – who had been moderately injured in a motor vehicle collision and presented to four British Columbia trauma centers between January 2013 and March 2020.
said Dr. Brubacher. Drivers included in the study had excess blood remaining after the clinical testing had been completed, which was then used for drug analysis.
Insufficient laws
After legalization there was an increased prevalence of drivers with a THC level greater than 0 ng/mL, a TCH level of at least 2 ng/mL, and a THC level of at least 5 ng/mL.
The largest increases in a THC level of at least 2 ng/mL were in drivers 50 years of age or older and among male drivers (adjusted prevalence ratio, 5.18; 95% confidence interval, 2.49-10.78 and aPR, 2.44; 95% CI, 1.60-3.74, respectively).
“There were no significant changes in the prevalence of drivers testing positive for alcohol,” the authors reported.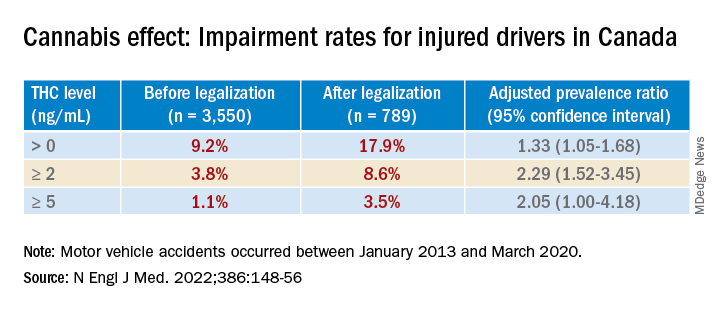
Dr. Brubacher said the evidence suggests these new laws “are not enough to stop everyone from driving after using cannabis.”
The findings have implications for clinicians and patients and for policymakers, he said. “My moderately conservative recommendations are that, if you are going to smoke cannabis, wait at least 4 hours after smoking before you drive. Edibles last longer, and patients should wait least 8 hours after ingesting [edibles] before driving. And of course, if you continue to feel the effects of the THC, you should avoid driving altogether until the time has elapsed and you no longer feel any effects.”
Dr. Brubacher hopes policy makers will use the study’s findings to “design public information campaigns and enforcement measures that encourage drivers, especially older drivers, to separate cannabis use from driving.”
Additionally, “policy makers shouldn’t lose sight of drinking and driving because that’s an even bigger problem than the risk of driving under the influence of cannabis.”
Focus on older adults
In a comment, Anees Bahji, MD, an International Collaborative Addiction Medicine research fellow at the British Columbia Centre on Substance Use, called the study “interesting and relevant.”
He raised several questions regarding the “correlation between the level of a substance in a person’s system and the degree of impairment.” For example, “does the same level of THC in the blood affect us all the same way? And to what extent do the levels detected at the time of the analysis correlate with the level in the person’s system at the time of driving?”
An additional consideration “is for individuals with cannabis use disorder and for those who have developed tolerance to the psychoactive effects of THC: Does it affect their driving skills in the same way as someone who is cannabis naive?” asked Dr. Bahji, a clinical assistant professor at the University of Calgary (Alta.) who was not involved with the study.
Also commenting, Eric Sevigny, PhD, associate professor of criminal justice and criminology at Georgia State University, Atlanta, described it as a “well-designed study that adds yet another data point for considering appropriate road safety policy responses alongside ongoing cannabis liberalization.”
However, the findings “cannot say much about whether cannabis legalization leads to an increase in cannabis-impaired driving, because current research finds little correlation between biological THC concentrations and driving performance,” said Dr. Sevigny, who was not involved with the study.
The finding of “higher THC prevalence among older adults is also relevant for road safety, as this population has a number of concomitant risk factors, such as cognitive decline and prescription drug use,” Dr. Sevigny added.
The study was supported by the Canadian Institutes of Health Research. Dr. Brubacher and Dr. Sevigny disclosed no relevant financial relationships. Dr. Bahji reported receiving research funding from the Canadian Institutes of Health Research, the Calgary Health Trust, the American Psychiatric Association, NIDA, and the University of Calgary.
A version of this article first appeared on Medscape.com.
Since Canada legalized marijuana in 2018, there has been a dramatic increase in the number of individuals driving while high, new research shows.
Investigators studied over 4,000 drivers treated after a motor vehicle collision in British Columbia trauma centers and found that, before cannabis was legalized, a THC level greater than 0 ng/mL in the blood was present in roughly 10% of drivers. After the drug was legalized this percentage increased to 18%. The percentages of injured drivers with at least 2 ng/mL, the Canadian legal limit, and at least 5 ng/mL more than doubled.
“It’s concerning that we’re seeing such a dramatic increase,” study investigator Jeffrey Brubacher, MD, associate professor, department of emergency medicine, University of British Columbia, Vancouver, said in a press release.
“There are serious risks associated with driving after cannabis use and our findings suggest more [work] is needed to deter this dangerous behavior in light of legalization,” he said.
The study was published online Jan. 12 in the New England Journal of Medicine.
Impact of legalization?
The investigators note that the Canadian government introduced a law aiming to prevent cannabis-impaired driving by establishing penalties and criminal charges for drivers found with a whole-blood THC level of 2 ng/mL, with more severe penalties for those with a THC level of greater than 5 ng/mL or greater than 2.5 ng/mL combined with a blood alcohol level of .05%.
Cannabis use is “associated with cognitive deficits and psychomotor impairment, and there is evidence that it increases the risk of motor vehicle crashes, especially at higher THC levels,” they noted.
“I’m an emergency physician at Vancouver General Hospital’s trauma center. We’ve been measuring drug levels in injured drivers since 2013 here in British Columbia and, in particular, we’ve been measuring THC levels,” Dr. Brubacher said in an interview. “We thought it would be interesting and important to see what would happen after legalization.”
The investigators studied 4,339 drivers – 3,550 whose accident took place before legalization of cannabis, and 789 after legalization – who had been moderately injured in a motor vehicle collision and presented to four British Columbia trauma centers between January 2013 and March 2020.
said Dr. Brubacher. Drivers included in the study had excess blood remaining after the clinical testing had been completed, which was then used for drug analysis.
Insufficient laws
After legalization there was an increased prevalence of drivers with a THC level greater than 0 ng/mL, a TCH level of at least 2 ng/mL, and a THC level of at least 5 ng/mL.
The largest increases in a THC level of at least 2 ng/mL were in drivers 50 years of age or older and among male drivers (adjusted prevalence ratio, 5.18; 95% confidence interval, 2.49-10.78 and aPR, 2.44; 95% CI, 1.60-3.74, respectively).
“There were no significant changes in the prevalence of drivers testing positive for alcohol,” the authors reported.
Dr. Brubacher said the evidence suggests these new laws “are not enough to stop everyone from driving after using cannabis.”
The findings have implications for clinicians and patients and for policymakers, he said. “My moderately conservative recommendations are that, if you are going to smoke cannabis, wait at least 4 hours after smoking before you drive. Edibles last longer, and patients should wait least 8 hours after ingesting [edibles] before driving. And of course, if you continue to feel the effects of the THC, you should avoid driving altogether until the time has elapsed and you no longer feel any effects.”
Dr. Brubacher hopes policy makers will use the study’s findings to “design public information campaigns and enforcement measures that encourage drivers, especially older drivers, to separate cannabis use from driving.”
Additionally, “policy makers shouldn’t lose sight of drinking and driving because that’s an even bigger problem than the risk of driving under the influence of cannabis.”
Focus on older adults
In a comment, Anees Bahji, MD, an International Collaborative Addiction Medicine research fellow at the British Columbia Centre on Substance Use, called the study “interesting and relevant.”
He raised several questions regarding the “correlation between the level of a substance in a person’s system and the degree of impairment.” For example, “does the same level of THC in the blood affect us all the same way? And to what extent do the levels detected at the time of the analysis correlate with the level in the person’s system at the time of driving?”
An additional consideration “is for individuals with cannabis use disorder and for those who have developed tolerance to the psychoactive effects of THC: Does it affect their driving skills in the same way as someone who is cannabis naive?” asked Dr. Bahji, a clinical assistant professor at the University of Calgary (Alta.) who was not involved with the study.
Also commenting, Eric Sevigny, PhD, associate professor of criminal justice and criminology at Georgia State University, Atlanta, described it as a “well-designed study that adds yet another data point for considering appropriate road safety policy responses alongside ongoing cannabis liberalization.”
However, the findings “cannot say much about whether cannabis legalization leads to an increase in cannabis-impaired driving, because current research finds little correlation between biological THC concentrations and driving performance,” said Dr. Sevigny, who was not involved with the study.
The finding of “higher THC prevalence among older adults is also relevant for road safety, as this population has a number of concomitant risk factors, such as cognitive decline and prescription drug use,” Dr. Sevigny added.
The study was supported by the Canadian Institutes of Health Research. Dr. Brubacher and Dr. Sevigny disclosed no relevant financial relationships. Dr. Bahji reported receiving research funding from the Canadian Institutes of Health Research, the Calgary Health Trust, the American Psychiatric Association, NIDA, and the University of Calgary.
A version of this article first appeared on Medscape.com.
Since Canada legalized marijuana in 2018, there has been a dramatic increase in the number of individuals driving while high, new research shows.
Investigators studied over 4,000 drivers treated after a motor vehicle collision in British Columbia trauma centers and found that, before cannabis was legalized, a THC level greater than 0 ng/mL in the blood was present in roughly 10% of drivers. After the drug was legalized this percentage increased to 18%. The percentages of injured drivers with at least 2 ng/mL, the Canadian legal limit, and at least 5 ng/mL more than doubled.
“It’s concerning that we’re seeing such a dramatic increase,” study investigator Jeffrey Brubacher, MD, associate professor, department of emergency medicine, University of British Columbia, Vancouver, said in a press release.
“There are serious risks associated with driving after cannabis use and our findings suggest more [work] is needed to deter this dangerous behavior in light of legalization,” he said.
The study was published online Jan. 12 in the New England Journal of Medicine.
Impact of legalization?
The investigators note that the Canadian government introduced a law aiming to prevent cannabis-impaired driving by establishing penalties and criminal charges for drivers found with a whole-blood THC level of 2 ng/mL, with more severe penalties for those with a THC level of greater than 5 ng/mL or greater than 2.5 ng/mL combined with a blood alcohol level of .05%.
Cannabis use is “associated with cognitive deficits and psychomotor impairment, and there is evidence that it increases the risk of motor vehicle crashes, especially at higher THC levels,” they noted.
“I’m an emergency physician at Vancouver General Hospital’s trauma center. We’ve been measuring drug levels in injured drivers since 2013 here in British Columbia and, in particular, we’ve been measuring THC levels,” Dr. Brubacher said in an interview. “We thought it would be interesting and important to see what would happen after legalization.”
The investigators studied 4,339 drivers – 3,550 whose accident took place before legalization of cannabis, and 789 after legalization – who had been moderately injured in a motor vehicle collision and presented to four British Columbia trauma centers between January 2013 and March 2020.
said Dr. Brubacher. Drivers included in the study had excess blood remaining after the clinical testing had been completed, which was then used for drug analysis.
Insufficient laws
After legalization there was an increased prevalence of drivers with a THC level greater than 0 ng/mL, a TCH level of at least 2 ng/mL, and a THC level of at least 5 ng/mL.
The largest increases in a THC level of at least 2 ng/mL were in drivers 50 years of age or older and among male drivers (adjusted prevalence ratio, 5.18; 95% confidence interval, 2.49-10.78 and aPR, 2.44; 95% CI, 1.60-3.74, respectively).
“There were no significant changes in the prevalence of drivers testing positive for alcohol,” the authors reported.
Dr. Brubacher said the evidence suggests these new laws “are not enough to stop everyone from driving after using cannabis.”
The findings have implications for clinicians and patients and for policymakers, he said. “My moderately conservative recommendations are that, if you are going to smoke cannabis, wait at least 4 hours after smoking before you drive. Edibles last longer, and patients should wait least 8 hours after ingesting [edibles] before driving. And of course, if you continue to feel the effects of the THC, you should avoid driving altogether until the time has elapsed and you no longer feel any effects.”
Dr. Brubacher hopes policy makers will use the study’s findings to “design public information campaigns and enforcement measures that encourage drivers, especially older drivers, to separate cannabis use from driving.”
Additionally, “policy makers shouldn’t lose sight of drinking and driving because that’s an even bigger problem than the risk of driving under the influence of cannabis.”
Focus on older adults
In a comment, Anees Bahji, MD, an International Collaborative Addiction Medicine research fellow at the British Columbia Centre on Substance Use, called the study “interesting and relevant.”
He raised several questions regarding the “correlation between the level of a substance in a person’s system and the degree of impairment.” For example, “does the same level of THC in the blood affect us all the same way? And to what extent do the levels detected at the time of the analysis correlate with the level in the person’s system at the time of driving?”
An additional consideration “is for individuals with cannabis use disorder and for those who have developed tolerance to the psychoactive effects of THC: Does it affect their driving skills in the same way as someone who is cannabis naive?” asked Dr. Bahji, a clinical assistant professor at the University of Calgary (Alta.) who was not involved with the study.
Also commenting, Eric Sevigny, PhD, associate professor of criminal justice and criminology at Georgia State University, Atlanta, described it as a “well-designed study that adds yet another data point for considering appropriate road safety policy responses alongside ongoing cannabis liberalization.”
However, the findings “cannot say much about whether cannabis legalization leads to an increase in cannabis-impaired driving, because current research finds little correlation between biological THC concentrations and driving performance,” said Dr. Sevigny, who was not involved with the study.
The finding of “higher THC prevalence among older adults is also relevant for road safety, as this population has a number of concomitant risk factors, such as cognitive decline and prescription drug use,” Dr. Sevigny added.
The study was supported by the Canadian Institutes of Health Research. Dr. Brubacher and Dr. Sevigny disclosed no relevant financial relationships. Dr. Bahji reported receiving research funding from the Canadian Institutes of Health Research, the Calgary Health Trust, the American Psychiatric Association, NIDA, and the University of Calgary.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Orally dissolving buprenorphine tied to severe tooth decay, FDA warns
Orally dissolving medications containing buprenorphine are linked to severe dental problems, including total tooth loss, the U.S. Food and Drug Administration warns in a safety communication.
The oral side effects of these medications, which are used to treat opioid use disorder (OUD) and pain, include cavities/tooth decay, including rampant caries; dental abscesses/infection; tooth erosion; fillings falling out; and, in some cases, total tooth loss.
Multiple cases have been reported even in patients with no history of dental problems.
The FDA is adding a warning about the risk of dental problems to the prescribing information and the patient medication guide for all buprenorphine-containing medicines dissolved in the mouth.
The FDA emphasizes, however, that buprenorphine remains “an important treatment option for OUD and pain, and the benefits of these medicines clearly outweigh the risks.”
More than 300 reported cases
Buprenorphine was approved in 2002 as a sublingual tablet, and in 2015 as a film to be placed inside the cheek to treat pain. Both delivery methods have been associated with dental problems.
Since buprenorphine was approved, the FDA has identified 305 cases of dental problems associated with orally dissolving buprenorphine, including 131 classified as serious.
There may be other cases, the FDA says, as this represents only cases reported to the FDA or published in the medical literature.
, but those as young as 18 years old were also affected.
Most cases occurred in patients using the medicines for OUD; however, 28 cases of dental problems occurred in patients using it to treat pain.
In 26 cases, patients had no prior history of dental problems. Some dental problems developed as soon as 2 weeks after treatment began; the median time to diagnosis was about 2 years after starting treatment.
Among all 305 cases reported, 113 involved two or more teeth.
The most common treatment for the dental problems was tooth extraction/removal, which was reported in 71 cases. Other cases required root canals, dental surgery, and other procedures such as crowns and implants.
Recommendations
The FDA says health care providers should counsel patients that severe and extensive tooth decay, tooth loss, and tooth fracture have been reported with the use of transmucosal buprenorphine-containing medicines and emphasize the importance of visiting their dentist to closely monitor their teeth.
Patients should be counseled to continue taking buprenorphine medications as prescribed and not stop suddenly without first talking to their health care provider, as this could lead to serious consequences, including relapse, misuse or abuse of other opioids, overdose, and death.
Patients are also being advised to take extra steps to help lessen the risk of serious dental problems.
Patients should also be educated on strategies to maintain or improve oral health while taking transmucosal buprenorphine medicines.
Counsel them that after the medicine is completely dissolved, the patient should take a large sip of water, swish it gently around the teeth and gums, swallow, and wait at least 1 hour before brushing their teeth, as the FDA advises. This will allow time for the mouth to gradually return to oral homeostasis and avoid any mechanical damage that may occur due to brushing.
The FDA also advises that patients tell their provider about any history of tooth problems, including cavities, and schedule a dentist visit soon after starting the medicine.
Dental problems related to transmucosal buprenorphine-containing medicines should be reported to the FDA’s MedWatch program.
A version of this article first appeared on Medscape.com.
Orally dissolving medications containing buprenorphine are linked to severe dental problems, including total tooth loss, the U.S. Food and Drug Administration warns in a safety communication.
The oral side effects of these medications, which are used to treat opioid use disorder (OUD) and pain, include cavities/tooth decay, including rampant caries; dental abscesses/infection; tooth erosion; fillings falling out; and, in some cases, total tooth loss.
Multiple cases have been reported even in patients with no history of dental problems.
The FDA is adding a warning about the risk of dental problems to the prescribing information and the patient medication guide for all buprenorphine-containing medicines dissolved in the mouth.
The FDA emphasizes, however, that buprenorphine remains “an important treatment option for OUD and pain, and the benefits of these medicines clearly outweigh the risks.”
More than 300 reported cases
Buprenorphine was approved in 2002 as a sublingual tablet, and in 2015 as a film to be placed inside the cheek to treat pain. Both delivery methods have been associated with dental problems.
Since buprenorphine was approved, the FDA has identified 305 cases of dental problems associated with orally dissolving buprenorphine, including 131 classified as serious.
There may be other cases, the FDA says, as this represents only cases reported to the FDA or published in the medical literature.
, but those as young as 18 years old were also affected.
Most cases occurred in patients using the medicines for OUD; however, 28 cases of dental problems occurred in patients using it to treat pain.
In 26 cases, patients had no prior history of dental problems. Some dental problems developed as soon as 2 weeks after treatment began; the median time to diagnosis was about 2 years after starting treatment.
Among all 305 cases reported, 113 involved two or more teeth.
The most common treatment for the dental problems was tooth extraction/removal, which was reported in 71 cases. Other cases required root canals, dental surgery, and other procedures such as crowns and implants.
Recommendations
The FDA says health care providers should counsel patients that severe and extensive tooth decay, tooth loss, and tooth fracture have been reported with the use of transmucosal buprenorphine-containing medicines and emphasize the importance of visiting their dentist to closely monitor their teeth.
Patients should be counseled to continue taking buprenorphine medications as prescribed and not stop suddenly without first talking to their health care provider, as this could lead to serious consequences, including relapse, misuse or abuse of other opioids, overdose, and death.
Patients are also being advised to take extra steps to help lessen the risk of serious dental problems.
Patients should also be educated on strategies to maintain or improve oral health while taking transmucosal buprenorphine medicines.
Counsel them that after the medicine is completely dissolved, the patient should take a large sip of water, swish it gently around the teeth and gums, swallow, and wait at least 1 hour before brushing their teeth, as the FDA advises. This will allow time for the mouth to gradually return to oral homeostasis and avoid any mechanical damage that may occur due to brushing.
The FDA also advises that patients tell their provider about any history of tooth problems, including cavities, and schedule a dentist visit soon after starting the medicine.
Dental problems related to transmucosal buprenorphine-containing medicines should be reported to the FDA’s MedWatch program.
A version of this article first appeared on Medscape.com.
Orally dissolving medications containing buprenorphine are linked to severe dental problems, including total tooth loss, the U.S. Food and Drug Administration warns in a safety communication.
The oral side effects of these medications, which are used to treat opioid use disorder (OUD) and pain, include cavities/tooth decay, including rampant caries; dental abscesses/infection; tooth erosion; fillings falling out; and, in some cases, total tooth loss.
Multiple cases have been reported even in patients with no history of dental problems.
The FDA is adding a warning about the risk of dental problems to the prescribing information and the patient medication guide for all buprenorphine-containing medicines dissolved in the mouth.
The FDA emphasizes, however, that buprenorphine remains “an important treatment option for OUD and pain, and the benefits of these medicines clearly outweigh the risks.”
More than 300 reported cases
Buprenorphine was approved in 2002 as a sublingual tablet, and in 2015 as a film to be placed inside the cheek to treat pain. Both delivery methods have been associated with dental problems.
Since buprenorphine was approved, the FDA has identified 305 cases of dental problems associated with orally dissolving buprenorphine, including 131 classified as serious.
There may be other cases, the FDA says, as this represents only cases reported to the FDA or published in the medical literature.
, but those as young as 18 years old were also affected.
Most cases occurred in patients using the medicines for OUD; however, 28 cases of dental problems occurred in patients using it to treat pain.
In 26 cases, patients had no prior history of dental problems. Some dental problems developed as soon as 2 weeks after treatment began; the median time to diagnosis was about 2 years after starting treatment.
Among all 305 cases reported, 113 involved two or more teeth.
The most common treatment for the dental problems was tooth extraction/removal, which was reported in 71 cases. Other cases required root canals, dental surgery, and other procedures such as crowns and implants.
Recommendations
The FDA says health care providers should counsel patients that severe and extensive tooth decay, tooth loss, and tooth fracture have been reported with the use of transmucosal buprenorphine-containing medicines and emphasize the importance of visiting their dentist to closely monitor their teeth.
Patients should be counseled to continue taking buprenorphine medications as prescribed and not stop suddenly without first talking to their health care provider, as this could lead to serious consequences, including relapse, misuse or abuse of other opioids, overdose, and death.
Patients are also being advised to take extra steps to help lessen the risk of serious dental problems.
Patients should also be educated on strategies to maintain or improve oral health while taking transmucosal buprenorphine medicines.
Counsel them that after the medicine is completely dissolved, the patient should take a large sip of water, swish it gently around the teeth and gums, swallow, and wait at least 1 hour before brushing their teeth, as the FDA advises. This will allow time for the mouth to gradually return to oral homeostasis and avoid any mechanical damage that may occur due to brushing.
The FDA also advises that patients tell their provider about any history of tooth problems, including cavities, and schedule a dentist visit soon after starting the medicine.
Dental problems related to transmucosal buprenorphine-containing medicines should be reported to the FDA’s MedWatch program.
A version of this article first appeared on Medscape.com.
Should psychiatry categorize ‘substance-induced paraphilia?’
‘substance-induced paraphilia?’
The dopamine receptors of the brain get their fair share amid the didactics we receive in residency. From discussions of antipsychotics and schizophrenia to stimulants and ADHD, dopamine plays a key role. Depending on the program and interest of faculty, methamphetamine may get its own lecture or be mixed in with other stimulants of abuse. During that discussion, a comment might be made in passing on the impact of methamphetamine on sexual desire and activity.
Experiences in the emergency department caring for patients who are intoxicated from methamphetamine then effectively make up for any gaps in trainees’ knowledge base. From patients engaging in self-pleasing pursuits in the emergency room to unfiltered reports of sexual exploits and desires, the impact of methamphetamine on sexual behavior quickly becomes apparent. Those experiences are later reinforced when residents are exposed to more long-term rehabilitation programs and have more in-depth conversations with patients about the sex-culture surrounding methamphetamine.
It is common to hear that, under the influence of methamphetamine, any available body will become an acceptable sexual partner – at times resulting in significant regrets, dangerous sexual activity, and complicated questions surrounding consent. Some early studies have found up to 72% increase in risky sexual behavior in methamphetamine users.1 This is particularly problematic as society has recently taken on the difficult and important work to re-examine the role and nature of consent in sexual activities. This falls within the larger #MeToo movement and has led to advocating for harsher sentencing of sexual offenders.
Yet simultaneously, society has also reconsidered its approach to apportioning blame on drug users.2 This shift to a more compassionate stance has resulted in a desire to treat and care for a disorder, rather than punish and condemn a poor choice. As forensic psychiatrists, we have noted this significant change. Where substance use disorders were once considered a risk factor for recidivism, they are now considered a disability that not only warrants treatment but can also diminish the share of blame one may be responsible for.
The convergence of those two societal movements often plays out in the courtroom, and in our experience when faced with those two opposing viewpoints, triers of fact (judges and juries) often favor punishing sexual offense over empathizing with an addictive disorder. While certainly not implying methamphetamine use condones sexual offense, we do posit the particular relationship between methamphetamine use and sexual activity should be explained to those entrusted with deciding guilt.
Examples of such problems are extremely common. A routine case involves IK,3 a 48-year-old male without significant history of legal problems, arrested for indecent exposure. His history of mental illness is closely intertwined with a history of substance use, leading to many psychiatric hospitalizations for methamphetamine-induced psychosis. After many hospitalizations he was placed in an assertive community treatment (ACT) team.
One day, IK is approached by an industrious drug dealer who frequents multiple board-and-cares in search for customers interested in relapsing. IK uses methamphetamine and within hours finds himself having walked miles away, naked, in the middle of an RV park. He subsequently describes the experience of unrelenting sexual desire, accompanied by ideas of reference involving billboards encouraging him to demonstrate his sexual prowess, as well as auditory hallucinations of women cheering him on. This leads to him pleasing himself publicly and his subsequent arrest.
Interviewing IK, 3 months later, he is embarrassed and apologetic. He is cognizant of the inappropriate nature of the incident and the foolishness of his actions. However, when asked whether he considers himself a sexual offender, he protests that he would never act in such a manner if not under the influence of methamphetamine. He points to his lack of significant sexual urges when sober, his lack of prior sexual offense, his lack of sexually violent offense, and his lack of unusual sexual interests.
It is unclear to us how society will or should adjudicate on such a case. It is not under the purview of forensic psychiatry to become a trier of fact. However, psychiatry should have a better working framework of how to discuss and conceptualize such situations, especially considering the dire consequences for those involved.
While any criminal conviction already has the potential to destroy a person’s life, sexual crimes bring particularly serious consequences. Entry into the national sex offender registry, in addition to carrying an unshakable stigma, comes with additional degrees of lost freedom. These individuals are prohibited from living or working in areas that have children in proximity, subjecting them to the outskirts of society and greatly restricting any chance of economic escape from poverty. Parks, libraries, and shopping malls can become off limits. Privacy for these individuals is nonexistent; from websites they visit to where they travel physically can be monitored. Even where they live and a detailed physical description are often easily accessible by members of their community.
When should it be permissible to consider sex offender status for someone on the grounds of a mental illness? A patient with obsessive-compulsive disorder might have sadistic obsessions and compulsions to commit violent sexual acts, which, along with being repugnant to society, are entirely ego-dystonic to the suffering patient. Psychosis is often characterized as involving a loss of insight and impaired reality testing. If society accepts insanity as grounds to mitigate sentencing, then why not permit it for grounds to wave the designation of sex offender to those with certain disorders, including substance use disorder? Wherever we come down on this issue,
Should IK have to register as a sex offender? Regardless of the circumstances, he did publicly masturbate. Society has determined that public sexual displays are a crime worth carrying the pariah status of sex offender – why should an exception be made for methamphetamine use? On the other hand, it is difficult to claim that IK’s behavior was entirely of his own free will. Most triers of fact will have never experienced that amount of dopamine reward. They can’t attest to the remaining free will after experiencing more pleasurable salience and positive reinforcement than ever naturally possible.
How we deal with the behavioral consequences, and other sequelae, of methamphetamine use is a growing problem. Access to and use of methamphetamine is no longer reserved for soldiers patrolling the jungles of Vietnam. Once thought to be a scourge of the West Coast, methamphetamine is now widely available throughout the United States.4 The use of methamphetamine is likely to continue to expand as society keeps pursuing the decriminalizing of drug use. Psychiatrists practicing in areas heavily affected by methamphetamine see firsthand the burden it places on community resources in the form of increased psychosis, emergency room utilization, medical resource strain, and encounters with police.5
The presence of mental illness is tied to a small but statistically significant risk of violence. However, substance use is a well-established risk factor for violence.6 What is often missed is that many sexual offenders have not committed a violent offense. However, like IK, they have been charged with indecent exposure or other nonviolent sexual offenses, such as prostitution and solicitation. Those nonviolent offenses are driven by poor judgment and impulsivity, the trademarks of substance use. The answer cannot be to incarcerate, and eventually add to the sex offender registry, the growing number of these individuals.
Yet, as psychiatrists, we seem at a loss for how to treat these patients. The prescription of allowing them to spend a night in the ED with a complementary sandwich garnished with olanzapine often feels like enabling. Substance use treatment programs are too limited, and the wait list is rarely shorter than the time it takes our patient to purchase their next hit.
There are no effective pharmacologic treatments for methamphetamine use disorder.7 The recommendations of cognitive-behavioral therapy, family and group therapy, contingency management, and a 12-step program may be sufficient for the most motivated and well-supported patients but are inadequate for the vast majority.8 As much as we want to laud the merits of community psychiatry and the ACT [assertive community treatment] model of care, it is hard to carry that banner while confronted with the reality these patients face on a day-to-day basis during any shift in the emergency room. Eventually the countless encounters with homeless, helplessly meth-addicted patients ending in discharge back to the streets begins to tarnish the bright rhetoric surrounding community care, which starts to sound more and more like abandonment of patients to suffer in futility.9
It is not up to forensic psychiatrists, or even psychiatry as a whole, to fix the myriad of inadequacies surrounding how society handles those suffering from methamphetamine addiction. However, it is essential for psychiatry to study and educate society on the interaction of methamphetamine use and sexual behavior. There has been some exploration into other risk factors for paraphilic behavior while under the influence of substances, but there is a dearth of information on this topic. Establishing a nomenclature called “substance-induced paraphilia” might be a way to bring clarity to such instances in both a forensic and general psychiatric setting.
Dr. Compton is a psychiatry resident at University of California, San Diego. His background includes medical education, mental health advocacy, work with underserved populations, and brain cancer research. Dr. Compton has no conflicts of interest. Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. He has no conflicts of interest.
References
1. Psychol Addict Behav. 2016;30(2)147-57.
2. Monitor Psychol. 2019;50(6).
3. IK’s case has been modified in certain ways to maintain confidentiality.
4. J Psychoactive Drugs. 2000;(2):137-41.
5. Acad Emerg Med. 2020 Nov;27(11):1116-25.
6. Swanson JW. Mental disorder, substance abuse, and community violence: An epidemiological approach, in: Monahan J and Steadman HJ, eds. “Violence and Mental Disorder: Developments in Risk Assessment” (Chicago: University of Chicago Press, 1994, pp. 101-36).
7. Addiction. 2004 Jun;99(6)708-17.
8. Am Fam Physician. 2007 Oct 15;76(8):1169-74.
9. Perspect Biol Med. 2021;64(1)70-81.
The dopamine receptors of the brain get their fair share amid the didactics we receive in residency. From discussions of antipsychotics and schizophrenia to stimulants and ADHD, dopamine plays a key role. Depending on the program and interest of faculty, methamphetamine may get its own lecture or be mixed in with other stimulants of abuse. During that discussion, a comment might be made in passing on the impact of methamphetamine on sexual desire and activity.
Experiences in the emergency department caring for patients who are intoxicated from methamphetamine then effectively make up for any gaps in trainees’ knowledge base. From patients engaging in self-pleasing pursuits in the emergency room to unfiltered reports of sexual exploits and desires, the impact of methamphetamine on sexual behavior quickly becomes apparent. Those experiences are later reinforced when residents are exposed to more long-term rehabilitation programs and have more in-depth conversations with patients about the sex-culture surrounding methamphetamine.
It is common to hear that, under the influence of methamphetamine, any available body will become an acceptable sexual partner – at times resulting in significant regrets, dangerous sexual activity, and complicated questions surrounding consent. Some early studies have found up to 72% increase in risky sexual behavior in methamphetamine users.1 This is particularly problematic as society has recently taken on the difficult and important work to re-examine the role and nature of consent in sexual activities. This falls within the larger #MeToo movement and has led to advocating for harsher sentencing of sexual offenders.
Yet simultaneously, society has also reconsidered its approach to apportioning blame on drug users.2 This shift to a more compassionate stance has resulted in a desire to treat and care for a disorder, rather than punish and condemn a poor choice. As forensic psychiatrists, we have noted this significant change. Where substance use disorders were once considered a risk factor for recidivism, they are now considered a disability that not only warrants treatment but can also diminish the share of blame one may be responsible for.
The convergence of those two societal movements often plays out in the courtroom, and in our experience when faced with those two opposing viewpoints, triers of fact (judges and juries) often favor punishing sexual offense over empathizing with an addictive disorder. While certainly not implying methamphetamine use condones sexual offense, we do posit the particular relationship between methamphetamine use and sexual activity should be explained to those entrusted with deciding guilt.
Examples of such problems are extremely common. A routine case involves IK,3 a 48-year-old male without significant history of legal problems, arrested for indecent exposure. His history of mental illness is closely intertwined with a history of substance use, leading to many psychiatric hospitalizations for methamphetamine-induced psychosis. After many hospitalizations he was placed in an assertive community treatment (ACT) team.
One day, IK is approached by an industrious drug dealer who frequents multiple board-and-cares in search for customers interested in relapsing. IK uses methamphetamine and within hours finds himself having walked miles away, naked, in the middle of an RV park. He subsequently describes the experience of unrelenting sexual desire, accompanied by ideas of reference involving billboards encouraging him to demonstrate his sexual prowess, as well as auditory hallucinations of women cheering him on. This leads to him pleasing himself publicly and his subsequent arrest.
Interviewing IK, 3 months later, he is embarrassed and apologetic. He is cognizant of the inappropriate nature of the incident and the foolishness of his actions. However, when asked whether he considers himself a sexual offender, he protests that he would never act in such a manner if not under the influence of methamphetamine. He points to his lack of significant sexual urges when sober, his lack of prior sexual offense, his lack of sexually violent offense, and his lack of unusual sexual interests.
It is unclear to us how society will or should adjudicate on such a case. It is not under the purview of forensic psychiatry to become a trier of fact. However, psychiatry should have a better working framework of how to discuss and conceptualize such situations, especially considering the dire consequences for those involved.
While any criminal conviction already has the potential to destroy a person’s life, sexual crimes bring particularly serious consequences. Entry into the national sex offender registry, in addition to carrying an unshakable stigma, comes with additional degrees of lost freedom. These individuals are prohibited from living or working in areas that have children in proximity, subjecting them to the outskirts of society and greatly restricting any chance of economic escape from poverty. Parks, libraries, and shopping malls can become off limits. Privacy for these individuals is nonexistent; from websites they visit to where they travel physically can be monitored. Even where they live and a detailed physical description are often easily accessible by members of their community.
When should it be permissible to consider sex offender status for someone on the grounds of a mental illness? A patient with obsessive-compulsive disorder might have sadistic obsessions and compulsions to commit violent sexual acts, which, along with being repugnant to society, are entirely ego-dystonic to the suffering patient. Psychosis is often characterized as involving a loss of insight and impaired reality testing. If society accepts insanity as grounds to mitigate sentencing, then why not permit it for grounds to wave the designation of sex offender to those with certain disorders, including substance use disorder? Wherever we come down on this issue,
Should IK have to register as a sex offender? Regardless of the circumstances, he did publicly masturbate. Society has determined that public sexual displays are a crime worth carrying the pariah status of sex offender – why should an exception be made for methamphetamine use? On the other hand, it is difficult to claim that IK’s behavior was entirely of his own free will. Most triers of fact will have never experienced that amount of dopamine reward. They can’t attest to the remaining free will after experiencing more pleasurable salience and positive reinforcement than ever naturally possible.
How we deal with the behavioral consequences, and other sequelae, of methamphetamine use is a growing problem. Access to and use of methamphetamine is no longer reserved for soldiers patrolling the jungles of Vietnam. Once thought to be a scourge of the West Coast, methamphetamine is now widely available throughout the United States.4 The use of methamphetamine is likely to continue to expand as society keeps pursuing the decriminalizing of drug use. Psychiatrists practicing in areas heavily affected by methamphetamine see firsthand the burden it places on community resources in the form of increased psychosis, emergency room utilization, medical resource strain, and encounters with police.5
The presence of mental illness is tied to a small but statistically significant risk of violence. However, substance use is a well-established risk factor for violence.6 What is often missed is that many sexual offenders have not committed a violent offense. However, like IK, they have been charged with indecent exposure or other nonviolent sexual offenses, such as prostitution and solicitation. Those nonviolent offenses are driven by poor judgment and impulsivity, the trademarks of substance use. The answer cannot be to incarcerate, and eventually add to the sex offender registry, the growing number of these individuals.
Yet, as psychiatrists, we seem at a loss for how to treat these patients. The prescription of allowing them to spend a night in the ED with a complementary sandwich garnished with olanzapine often feels like enabling. Substance use treatment programs are too limited, and the wait list is rarely shorter than the time it takes our patient to purchase their next hit.
There are no effective pharmacologic treatments for methamphetamine use disorder.7 The recommendations of cognitive-behavioral therapy, family and group therapy, contingency management, and a 12-step program may be sufficient for the most motivated and well-supported patients but are inadequate for the vast majority.8 As much as we want to laud the merits of community psychiatry and the ACT [assertive community treatment] model of care, it is hard to carry that banner while confronted with the reality these patients face on a day-to-day basis during any shift in the emergency room. Eventually the countless encounters with homeless, helplessly meth-addicted patients ending in discharge back to the streets begins to tarnish the bright rhetoric surrounding community care, which starts to sound more and more like abandonment of patients to suffer in futility.9
It is not up to forensic psychiatrists, or even psychiatry as a whole, to fix the myriad of inadequacies surrounding how society handles those suffering from methamphetamine addiction. However, it is essential for psychiatry to study and educate society on the interaction of methamphetamine use and sexual behavior. There has been some exploration into other risk factors for paraphilic behavior while under the influence of substances, but there is a dearth of information on this topic. Establishing a nomenclature called “substance-induced paraphilia” might be a way to bring clarity to such instances in both a forensic and general psychiatric setting.
Dr. Compton is a psychiatry resident at University of California, San Diego. His background includes medical education, mental health advocacy, work with underserved populations, and brain cancer research. Dr. Compton has no conflicts of interest. Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. He has no conflicts of interest.
References
1. Psychol Addict Behav. 2016;30(2)147-57.
2. Monitor Psychol. 2019;50(6).
3. IK’s case has been modified in certain ways to maintain confidentiality.
4. J Psychoactive Drugs. 2000;(2):137-41.
5. Acad Emerg Med. 2020 Nov;27(11):1116-25.
6. Swanson JW. Mental disorder, substance abuse, and community violence: An epidemiological approach, in: Monahan J and Steadman HJ, eds. “Violence and Mental Disorder: Developments in Risk Assessment” (Chicago: University of Chicago Press, 1994, pp. 101-36).
7. Addiction. 2004 Jun;99(6)708-17.
8. Am Fam Physician. 2007 Oct 15;76(8):1169-74.
9. Perspect Biol Med. 2021;64(1)70-81.
The dopamine receptors of the brain get their fair share amid the didactics we receive in residency. From discussions of antipsychotics and schizophrenia to stimulants and ADHD, dopamine plays a key role. Depending on the program and interest of faculty, methamphetamine may get its own lecture or be mixed in with other stimulants of abuse. During that discussion, a comment might be made in passing on the impact of methamphetamine on sexual desire and activity.
Experiences in the emergency department caring for patients who are intoxicated from methamphetamine then effectively make up for any gaps in trainees’ knowledge base. From patients engaging in self-pleasing pursuits in the emergency room to unfiltered reports of sexual exploits and desires, the impact of methamphetamine on sexual behavior quickly becomes apparent. Those experiences are later reinforced when residents are exposed to more long-term rehabilitation programs and have more in-depth conversations with patients about the sex-culture surrounding methamphetamine.
It is common to hear that, under the influence of methamphetamine, any available body will become an acceptable sexual partner – at times resulting in significant regrets, dangerous sexual activity, and complicated questions surrounding consent. Some early studies have found up to 72% increase in risky sexual behavior in methamphetamine users.1 This is particularly problematic as society has recently taken on the difficult and important work to re-examine the role and nature of consent in sexual activities. This falls within the larger #MeToo movement and has led to advocating for harsher sentencing of sexual offenders.
Yet simultaneously, society has also reconsidered its approach to apportioning blame on drug users.2 This shift to a more compassionate stance has resulted in a desire to treat and care for a disorder, rather than punish and condemn a poor choice. As forensic psychiatrists, we have noted this significant change. Where substance use disorders were once considered a risk factor for recidivism, they are now considered a disability that not only warrants treatment but can also diminish the share of blame one may be responsible for.
The convergence of those two societal movements often plays out in the courtroom, and in our experience when faced with those two opposing viewpoints, triers of fact (judges and juries) often favor punishing sexual offense over empathizing with an addictive disorder. While certainly not implying methamphetamine use condones sexual offense, we do posit the particular relationship between methamphetamine use and sexual activity should be explained to those entrusted with deciding guilt.
Examples of such problems are extremely common. A routine case involves IK,3 a 48-year-old male without significant history of legal problems, arrested for indecent exposure. His history of mental illness is closely intertwined with a history of substance use, leading to many psychiatric hospitalizations for methamphetamine-induced psychosis. After many hospitalizations he was placed in an assertive community treatment (ACT) team.
One day, IK is approached by an industrious drug dealer who frequents multiple board-and-cares in search for customers interested in relapsing. IK uses methamphetamine and within hours finds himself having walked miles away, naked, in the middle of an RV park. He subsequently describes the experience of unrelenting sexual desire, accompanied by ideas of reference involving billboards encouraging him to demonstrate his sexual prowess, as well as auditory hallucinations of women cheering him on. This leads to him pleasing himself publicly and his subsequent arrest.
Interviewing IK, 3 months later, he is embarrassed and apologetic. He is cognizant of the inappropriate nature of the incident and the foolishness of his actions. However, when asked whether he considers himself a sexual offender, he protests that he would never act in such a manner if not under the influence of methamphetamine. He points to his lack of significant sexual urges when sober, his lack of prior sexual offense, his lack of sexually violent offense, and his lack of unusual sexual interests.
It is unclear to us how society will or should adjudicate on such a case. It is not under the purview of forensic psychiatry to become a trier of fact. However, psychiatry should have a better working framework of how to discuss and conceptualize such situations, especially considering the dire consequences for those involved.
While any criminal conviction already has the potential to destroy a person’s life, sexual crimes bring particularly serious consequences. Entry into the national sex offender registry, in addition to carrying an unshakable stigma, comes with additional degrees of lost freedom. These individuals are prohibited from living or working in areas that have children in proximity, subjecting them to the outskirts of society and greatly restricting any chance of economic escape from poverty. Parks, libraries, and shopping malls can become off limits. Privacy for these individuals is nonexistent; from websites they visit to where they travel physically can be monitored. Even where they live and a detailed physical description are often easily accessible by members of their community.
When should it be permissible to consider sex offender status for someone on the grounds of a mental illness? A patient with obsessive-compulsive disorder might have sadistic obsessions and compulsions to commit violent sexual acts, which, along with being repugnant to society, are entirely ego-dystonic to the suffering patient. Psychosis is often characterized as involving a loss of insight and impaired reality testing. If society accepts insanity as grounds to mitigate sentencing, then why not permit it for grounds to wave the designation of sex offender to those with certain disorders, including substance use disorder? Wherever we come down on this issue,
Should IK have to register as a sex offender? Regardless of the circumstances, he did publicly masturbate. Society has determined that public sexual displays are a crime worth carrying the pariah status of sex offender – why should an exception be made for methamphetamine use? On the other hand, it is difficult to claim that IK’s behavior was entirely of his own free will. Most triers of fact will have never experienced that amount of dopamine reward. They can’t attest to the remaining free will after experiencing more pleasurable salience and positive reinforcement than ever naturally possible.
How we deal with the behavioral consequences, and other sequelae, of methamphetamine use is a growing problem. Access to and use of methamphetamine is no longer reserved for soldiers patrolling the jungles of Vietnam. Once thought to be a scourge of the West Coast, methamphetamine is now widely available throughout the United States.4 The use of methamphetamine is likely to continue to expand as society keeps pursuing the decriminalizing of drug use. Psychiatrists practicing in areas heavily affected by methamphetamine see firsthand the burden it places on community resources in the form of increased psychosis, emergency room utilization, medical resource strain, and encounters with police.5
The presence of mental illness is tied to a small but statistically significant risk of violence. However, substance use is a well-established risk factor for violence.6 What is often missed is that many sexual offenders have not committed a violent offense. However, like IK, they have been charged with indecent exposure or other nonviolent sexual offenses, such as prostitution and solicitation. Those nonviolent offenses are driven by poor judgment and impulsivity, the trademarks of substance use. The answer cannot be to incarcerate, and eventually add to the sex offender registry, the growing number of these individuals.
Yet, as psychiatrists, we seem at a loss for how to treat these patients. The prescription of allowing them to spend a night in the ED with a complementary sandwich garnished with olanzapine often feels like enabling. Substance use treatment programs are too limited, and the wait list is rarely shorter than the time it takes our patient to purchase their next hit.
There are no effective pharmacologic treatments for methamphetamine use disorder.7 The recommendations of cognitive-behavioral therapy, family and group therapy, contingency management, and a 12-step program may be sufficient for the most motivated and well-supported patients but are inadequate for the vast majority.8 As much as we want to laud the merits of community psychiatry and the ACT [assertive community treatment] model of care, it is hard to carry that banner while confronted with the reality these patients face on a day-to-day basis during any shift in the emergency room. Eventually the countless encounters with homeless, helplessly meth-addicted patients ending in discharge back to the streets begins to tarnish the bright rhetoric surrounding community care, which starts to sound more and more like abandonment of patients to suffer in futility.9
It is not up to forensic psychiatrists, or even psychiatry as a whole, to fix the myriad of inadequacies surrounding how society handles those suffering from methamphetamine addiction. However, it is essential for psychiatry to study and educate society on the interaction of methamphetamine use and sexual behavior. There has been some exploration into other risk factors for paraphilic behavior while under the influence of substances, but there is a dearth of information on this topic. Establishing a nomenclature called “substance-induced paraphilia” might be a way to bring clarity to such instances in both a forensic and general psychiatric setting.
Dr. Compton is a psychiatry resident at University of California, San Diego. His background includes medical education, mental health advocacy, work with underserved populations, and brain cancer research. Dr. Compton has no conflicts of interest. Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. He has no conflicts of interest.
References
1. Psychol Addict Behav. 2016;30(2)147-57.
2. Monitor Psychol. 2019;50(6).
3. IK’s case has been modified in certain ways to maintain confidentiality.
4. J Psychoactive Drugs. 2000;(2):137-41.
5. Acad Emerg Med. 2020 Nov;27(11):1116-25.
6. Swanson JW. Mental disorder, substance abuse, and community violence: An epidemiological approach, in: Monahan J and Steadman HJ, eds. “Violence and Mental Disorder: Developments in Risk Assessment” (Chicago: University of Chicago Press, 1994, pp. 101-36).
7. Addiction. 2004 Jun;99(6)708-17.
8. Am Fam Physician. 2007 Oct 15;76(8):1169-74.
9. Perspect Biol Med. 2021;64(1)70-81.
‘substance-induced paraphilia?’
‘substance-induced paraphilia?’
Scheduled Acetaminophen to Minimize Neuropsychiatric Symptoms in Wernicke-Korsakoff Syndrome
To manage the physical, cognitive, and emotional symptoms of a veteran hospitalized for Wernicke-Korsakoff syndrome secondary to chronic alcohol overuse, acetaminophen was administered in place of psychoactive medications.
Alcohol is the most common substance misused by veterans. 1 Veterans may m isuse alcohol as a result of mental illness or posttraumatic stress disorder (PTSD), having difficulties adjusting to civilian life, or because of heavy drinking habits acquired before leaving active duty. 2 One potential long-term effect of chronic alcohol misuse is Wernicke-Korsakoff syndrome (WKS), a neuropsychiatric condition secondary to a deficiency of thiamine. 3 The disease is characterized by altered mental status, oculomotor findings, and ataxia. 3 Patients with WKS may exhibit challenging behaviors, including aggression, disinhibition, and lack of awareness of their illness. 4 Due to long-standing cognitive and physical deficits, many patients require lifelong care with a focus on a palliative approach. 3
The mainstay of pharmacologic management for the neuropsychiatric symptoms of WKS continues to be psychoactive medications, such as antipsychotics, benzodiazepines, antidepressants, and anticonvulsant medications.4-6 Though atypical antipsychotic medications remain the most widely used, they have a high adverse effect (AE) profile.5,6 Among the potential AEs are metabolic syndrome, anticholinergic effects, QTc prolongation, orthostatic hypotension, extrapyramidal effects, sedation, and falls. There also is a US Food and Drug Administration boxed warning for increased risk of mortality.7 With the goal of improving and maintaining patient safety, pharmacologic interventions with lower AEs may be beneficial in the management of the neuropsychiatric symptoms of WKS.
This case describes a veteran who was initially hospitalized due to confusion, ataxia, and nystagmus secondary to chronic alcohol overuse. The aim of the case was to consider the use of acetaminophen in place of psychoactive medications as a way to manage neuropsychiatric symptoms of WKS even when pain was not present.
Case Presentation
A veteran presented to the local US Department of Veterans Affairs (VA) emergency department (ED) due to their spouse’s concern of acute onset confusion and ambulatory difficulties. The veteran’s medical history included extensive alcohol misuse, mild asthma, and diet-controlled hyperlipidemia. On initial evaluation, the veteran displayed symptoms of ataxia and confusion. When asked why the veteran was at the ED, the response was, “I just came to the hospital to find my sister.” Based on their medical history, clinical evaluation, and altered mental status, the veteran was admitted to the acute care medical service with a presumptive diagnosis of WKS.
On admission, the laboratory evaluation revealed normal alanine transaminase (ALT) and aspartate transaminase (AST) levels but markedly elevated γ-glutamyl transferase (GGT) consistent with alcohol toxicity. COVID-19 testing was negative. Magnetic resonance imaging (MRI) of the brain revealed evidence of alterations in the mammillary bodies and moderately severe cortical and cerebellar volume loss suggestive of long-standing alcohol use.
The veteran was hospitalized for 12 days and treated with high-dose IV thiamine, which resulted in improvement of their ophthalmic disorder (nystagmus) and ataxia. However, they continued to exhibit poor recall, confusion, and occasional agitation characterized by verbal outbursts and aggression toward the staff.
The veteran’s spouse worked full time and did not feel capable of providing the necessary follow-up care at home. The safest discharge plan found was to transfer the veteran to the local VA community living center (CLC) for physical therapy and further support of their marked cognitive decline and agitation.
Following admission to the CLC, the veteran was placed in a secured memory unit with staff trained specifically on management of veterans with cognitive impairment and behavioral concerns. As the veteran did not have decisional capacity on admission, the staff arranged a meeting with the spouse. Based on that conversation, the goals of care were to focus on a palliative approach and the hope that the veteran would one day be able to return home to their spouse.
At the CLC, the veteran was initially treated with thiamine 200 mg orally once daily and albuterol inhaler as needed. A clinical psychologist performed a comprehensive psychological evaluation on admission, which confirmed evidence of WKS with symptoms, including confusion, disorientation, and confabulation. There was no evidence of cultural diversity factors regarding the veteran’s delusional beliefs.
After the first full day in the CLC, the nursing staff observed anger and agitation that seemed to start midafternoon and continued until around dinnertime. The veteran displayed verbal outbursts, refusal to cooperate with the staff, and multiple attempts to leave the CLC. With the guidance of a geriatric psychiatrist, risperidone 1 mg once daily as needed was initiated, and staff continued with verbal redirection, both with limited efficacy. After 3 days, due to safety concerns for the veteran, other CLC patients, and CLC staff, risperidone dosing was increased to 1 mg twice daily, which had limited efficacy. Lorazepam 1 mg once daily also was added. A careful medication review was performed to minimize any potential AEs or interactions that might have contributed to the veteran’s behavior, but no pharmacologic interventions were found to fully abate their behavioral issues.
After 5 weeks of ongoing intermittent behavioral issues, the medical team again met to discuss new treatment options.A case reported by Husebo and colleagues used scheduled acetaminophen to help relieve neuropsychiatric symptoms of dementia in a patient who exhibited similar behavioral issues and did not respond well to antipsychotics or benzodiazepines.8 Although our veteran did not express or exhibit obvious pain, the medical team chose to trial this intervention, and the veteran was started on acetaminophen 650 mg orally 3 times daily. A comprehensive metabolic panel, including GGT and thyroid-stimulating hormone, was performed before starting acetaminophen; no abnormalities were noted. The clinical examination did not reveal physical abnormalities other than ataxia.
After 5 days of therapy with the scheduled acetaminophen, the veteran’s clinical behavior dramatically improved. The veteran exhibited infrequent agitated behavior and became cooperative with staff. Three days later, the scheduled lorazepam was discontinued, and eventually they were tapered off risperidone. One month after starting scheduled acetaminophen, the veteran had improved to a point where the staff determined a safe discharge plan could be initiated. The veteran’s nystagmus resolved and behavioral issues improved, although cognitive impairment persisted.
Due to COVID-19, a teleconference was scheduled with the veteran’s spouse to discuss a discharge plan. The spouse was pleased that the veteran had progressed adequately both functionally and behaviorally to make a safe discharge home possible. The spouse arranged to take family leave from their job to help support the veteran after discharge. The veteran was able to return home with a safe discharge plan 1 week later. The acetaminophen was continued with twice-daily dosing and was continued because there were no new behavioral issues. This was done to enhance postfacility adherence and minimize the risk of drug-drug interactions. Attempts to follow up with the veteran postdischarge were unfortunately unsuccessful as the family lived out of the local area.
Discussion
Alcohol misuse is a common finding in many US veterans, as well as in the general population.1,3 As a result, it is not uncommon to see patients with physical and psychological symptoms related to this abuse. Many of these patients will become verbally and physically abusive, thus having appropriate pharmacologic and nonpharmacologic interventions is important.
In this case study, the veteran was diagnosed with WKS and exhibited physical, cognitive, and emotional symptoms consistent with this disease. Although the physical symptoms improved with thiamine and abstinence from alcohol, their cognitive impairment, verbal outbursts, and aggressive demeanor persisted.
After using antipsychotic and anxiolytic medications with minimal clinical improvement, a trial of acetaminophen 650 mg 3 times daily was instituted. The patient’s behavior improved; demeanor became calmer, and they were easily redirected by the nursing staff. Psychological support was again employed, which enhanced and supported the veteran’s calmer demeanor. Although there is limited medical literature on the use of acetaminophen in clinical situations not related to pain, there has been research documenting its effect on social interaction.9,10
Acetaminophen is an analgesic medication that acts through central neural mechanisms. It has been hypothesized that social and physical pain rely on shared neurochemical underpinnings, and some of the regions of the brain involved in affective experience of physical pain also have been found to be involved in the experience of social pain.11 Acetaminophen may impact an individual’s social well-being as social pain processes.11 It has been shown to blunt reactivity to both physical pain as well as negative stimuli.11
Conclusions
A 2019 survey on alcohol and drug use found 5.6% of adults aged ≥ 18 have an alcohol use disorder.12 In severe cases, this can result in WKS. Although replacement of thiamine is critical for physical improvement, psychological deficits may persist. Small studies have advanced the concept of using scheduled acetaminophen even when the patient is not verbalizing or displaying pain.13 Although more research needs to be done on this topic, this palliative approach may be worth considering, especially if the risks of antipsychotics and anxiolytics outweigh the benefits.
1. National Institute on Drug Abuse. Substance use and military life drug facts. Published October 2019. Accessed November 10, 2021. https://www.drugabuse.gov/publications/drugfacts/substance-use-military-life
2. National Veterans Foundation. What statistics show about veteran substance abuse and why proper treatment is important. Published March 30, 2016. Accessed November 10, 2021. https://nvf.org/veteran-substance-abuse-statistics
3. National Center for Biotechnology Information. Korsakoff syndrome. Updated July 10, 2020. Accessed November 10, 2021. https://www.ncbi.nlm.nih.gov/books/NBK539854
4. Gerridzen IJ, Goossensen MA. Patients with Korsakoff syndrome in nursing homes: characteristics, comorbidity, and use of psychotropic drugs. Int Psychogeriatr. 2014;26(1):115-121. doi:10.1017/S1041610213001543
5. Press D, Alexander M. Management of neuropsychiatric symptoms of dementia. Updated October 2021. Accessed November 10, 2021. https://www.uptodate.com/contents/management-of-neuropsychiatric-symptoms-of-dementia
6. Steinberg M, Lyketsos CG. Atypical antipsychotic use in patients with dementia: Managing safety concerns. Am J Psychiatry. 2012;169(9):900-906. doi:10.1176/appi.ajp.2012.12030342
7. Jibson MD. Second-generation antipsychotic medications: pharmacology, administration, and side effects. https://www.uptodate.com/contents/second-generation-antipsychotic-medications-pharmacology-administration-and-side-effects
8. Husebo BS, Ballard C, Sandvik R, Nilsen OB, Aarsland D. Efficacy of treating pain to reduce behavioural disturbances in residents of nursing homes with dementia: cluster randomised clinical trial. BMJ. 2011;343:d4065. doi:10.1136/bmj.d4065
9. Fung K, Alden LE. Once hurt, twice shy: social pain contributes to social anxiety. Emotion. 2017;(2):231-239. doi:10.1037/emo0000223
10. Roberts ID, Krajbich I, Cheavens JS, Campo JV, Way BM. Acetaminophen Reduces Distrust in Individuals with Borderline Personality Disorder Features. Clin Psychol Sci. 2018;6(1):145-154. doi:10.1177/2167702617731374
11. Dewall CN, Macdonald G, Webster GD, et al. Acetaminophen reduces social pain: behavioral and neural evidence. Psychol Sci. 2010;21(7):931-937. doi:10.1177/0956797610374741
12. National Institute on Alcohol Abuse and Alcoholism. Alcohol facts and statistics. Updated June 2021. Accessed November 2, 202November 10, 2021. https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/alcohol-facts-and-statistics
13. Chibnall JT, Tait RC, Harman B, Luebbert RA. Effect of acetaminophen on behavior, well-being, and psychotropic medication use in nursing home residents with moderate-to-severe dementia. J Am Geriatrics Soc. 2005;53(11):1921-9. doi:10.1111/j.1532-5415.2005.53572.x
To manage the physical, cognitive, and emotional symptoms of a veteran hospitalized for Wernicke-Korsakoff syndrome secondary to chronic alcohol overuse, acetaminophen was administered in place of psychoactive medications.
To manage the physical, cognitive, and emotional symptoms of a veteran hospitalized for Wernicke-Korsakoff syndrome secondary to chronic alcohol overuse, acetaminophen was administered in place of psychoactive medications.
Alcohol is the most common substance misused by veterans. 1 Veterans may m isuse alcohol as a result of mental illness or posttraumatic stress disorder (PTSD), having difficulties adjusting to civilian life, or because of heavy drinking habits acquired before leaving active duty. 2 One potential long-term effect of chronic alcohol misuse is Wernicke-Korsakoff syndrome (WKS), a neuropsychiatric condition secondary to a deficiency of thiamine. 3 The disease is characterized by altered mental status, oculomotor findings, and ataxia. 3 Patients with WKS may exhibit challenging behaviors, including aggression, disinhibition, and lack of awareness of their illness. 4 Due to long-standing cognitive and physical deficits, many patients require lifelong care with a focus on a palliative approach. 3
The mainstay of pharmacologic management for the neuropsychiatric symptoms of WKS continues to be psychoactive medications, such as antipsychotics, benzodiazepines, antidepressants, and anticonvulsant medications.4-6 Though atypical antipsychotic medications remain the most widely used, they have a high adverse effect (AE) profile.5,6 Among the potential AEs are metabolic syndrome, anticholinergic effects, QTc prolongation, orthostatic hypotension, extrapyramidal effects, sedation, and falls. There also is a US Food and Drug Administration boxed warning for increased risk of mortality.7 With the goal of improving and maintaining patient safety, pharmacologic interventions with lower AEs may be beneficial in the management of the neuropsychiatric symptoms of WKS.
This case describes a veteran who was initially hospitalized due to confusion, ataxia, and nystagmus secondary to chronic alcohol overuse. The aim of the case was to consider the use of acetaminophen in place of psychoactive medications as a way to manage neuropsychiatric symptoms of WKS even when pain was not present.
Case Presentation
A veteran presented to the local US Department of Veterans Affairs (VA) emergency department (ED) due to their spouse’s concern of acute onset confusion and ambulatory difficulties. The veteran’s medical history included extensive alcohol misuse, mild asthma, and diet-controlled hyperlipidemia. On initial evaluation, the veteran displayed symptoms of ataxia and confusion. When asked why the veteran was at the ED, the response was, “I just came to the hospital to find my sister.” Based on their medical history, clinical evaluation, and altered mental status, the veteran was admitted to the acute care medical service with a presumptive diagnosis of WKS.
On admission, the laboratory evaluation revealed normal alanine transaminase (ALT) and aspartate transaminase (AST) levels but markedly elevated γ-glutamyl transferase (GGT) consistent with alcohol toxicity. COVID-19 testing was negative. Magnetic resonance imaging (MRI) of the brain revealed evidence of alterations in the mammillary bodies and moderately severe cortical and cerebellar volume loss suggestive of long-standing alcohol use.
The veteran was hospitalized for 12 days and treated with high-dose IV thiamine, which resulted in improvement of their ophthalmic disorder (nystagmus) and ataxia. However, they continued to exhibit poor recall, confusion, and occasional agitation characterized by verbal outbursts and aggression toward the staff.
The veteran’s spouse worked full time and did not feel capable of providing the necessary follow-up care at home. The safest discharge plan found was to transfer the veteran to the local VA community living center (CLC) for physical therapy and further support of their marked cognitive decline and agitation.
Following admission to the CLC, the veteran was placed in a secured memory unit with staff trained specifically on management of veterans with cognitive impairment and behavioral concerns. As the veteran did not have decisional capacity on admission, the staff arranged a meeting with the spouse. Based on that conversation, the goals of care were to focus on a palliative approach and the hope that the veteran would one day be able to return home to their spouse.
At the CLC, the veteran was initially treated with thiamine 200 mg orally once daily and albuterol inhaler as needed. A clinical psychologist performed a comprehensive psychological evaluation on admission, which confirmed evidence of WKS with symptoms, including confusion, disorientation, and confabulation. There was no evidence of cultural diversity factors regarding the veteran’s delusional beliefs.
After the first full day in the CLC, the nursing staff observed anger and agitation that seemed to start midafternoon and continued until around dinnertime. The veteran displayed verbal outbursts, refusal to cooperate with the staff, and multiple attempts to leave the CLC. With the guidance of a geriatric psychiatrist, risperidone 1 mg once daily as needed was initiated, and staff continued with verbal redirection, both with limited efficacy. After 3 days, due to safety concerns for the veteran, other CLC patients, and CLC staff, risperidone dosing was increased to 1 mg twice daily, which had limited efficacy. Lorazepam 1 mg once daily also was added. A careful medication review was performed to minimize any potential AEs or interactions that might have contributed to the veteran’s behavior, but no pharmacologic interventions were found to fully abate their behavioral issues.
After 5 weeks of ongoing intermittent behavioral issues, the medical team again met to discuss new treatment options.A case reported by Husebo and colleagues used scheduled acetaminophen to help relieve neuropsychiatric symptoms of dementia in a patient who exhibited similar behavioral issues and did not respond well to antipsychotics or benzodiazepines.8 Although our veteran did not express or exhibit obvious pain, the medical team chose to trial this intervention, and the veteran was started on acetaminophen 650 mg orally 3 times daily. A comprehensive metabolic panel, including GGT and thyroid-stimulating hormone, was performed before starting acetaminophen; no abnormalities were noted. The clinical examination did not reveal physical abnormalities other than ataxia.
After 5 days of therapy with the scheduled acetaminophen, the veteran’s clinical behavior dramatically improved. The veteran exhibited infrequent agitated behavior and became cooperative with staff. Three days later, the scheduled lorazepam was discontinued, and eventually they were tapered off risperidone. One month after starting scheduled acetaminophen, the veteran had improved to a point where the staff determined a safe discharge plan could be initiated. The veteran’s nystagmus resolved and behavioral issues improved, although cognitive impairment persisted.
Due to COVID-19, a teleconference was scheduled with the veteran’s spouse to discuss a discharge plan. The spouse was pleased that the veteran had progressed adequately both functionally and behaviorally to make a safe discharge home possible. The spouse arranged to take family leave from their job to help support the veteran after discharge. The veteran was able to return home with a safe discharge plan 1 week later. The acetaminophen was continued with twice-daily dosing and was continued because there were no new behavioral issues. This was done to enhance postfacility adherence and minimize the risk of drug-drug interactions. Attempts to follow up with the veteran postdischarge were unfortunately unsuccessful as the family lived out of the local area.
Discussion
Alcohol misuse is a common finding in many US veterans, as well as in the general population.1,3 As a result, it is not uncommon to see patients with physical and psychological symptoms related to this abuse. Many of these patients will become verbally and physically abusive, thus having appropriate pharmacologic and nonpharmacologic interventions is important.
In this case study, the veteran was diagnosed with WKS and exhibited physical, cognitive, and emotional symptoms consistent with this disease. Although the physical symptoms improved with thiamine and abstinence from alcohol, their cognitive impairment, verbal outbursts, and aggressive demeanor persisted.
After using antipsychotic and anxiolytic medications with minimal clinical improvement, a trial of acetaminophen 650 mg 3 times daily was instituted. The patient’s behavior improved; demeanor became calmer, and they were easily redirected by the nursing staff. Psychological support was again employed, which enhanced and supported the veteran’s calmer demeanor. Although there is limited medical literature on the use of acetaminophen in clinical situations not related to pain, there has been research documenting its effect on social interaction.9,10
Acetaminophen is an analgesic medication that acts through central neural mechanisms. It has been hypothesized that social and physical pain rely on shared neurochemical underpinnings, and some of the regions of the brain involved in affective experience of physical pain also have been found to be involved in the experience of social pain.11 Acetaminophen may impact an individual’s social well-being as social pain processes.11 It has been shown to blunt reactivity to both physical pain as well as negative stimuli.11
Conclusions
A 2019 survey on alcohol and drug use found 5.6% of adults aged ≥ 18 have an alcohol use disorder.12 In severe cases, this can result in WKS. Although replacement of thiamine is critical for physical improvement, psychological deficits may persist. Small studies have advanced the concept of using scheduled acetaminophen even when the patient is not verbalizing or displaying pain.13 Although more research needs to be done on this topic, this palliative approach may be worth considering, especially if the risks of antipsychotics and anxiolytics outweigh the benefits.
Alcohol is the most common substance misused by veterans. 1 Veterans may m isuse alcohol as a result of mental illness or posttraumatic stress disorder (PTSD), having difficulties adjusting to civilian life, or because of heavy drinking habits acquired before leaving active duty. 2 One potential long-term effect of chronic alcohol misuse is Wernicke-Korsakoff syndrome (WKS), a neuropsychiatric condition secondary to a deficiency of thiamine. 3 The disease is characterized by altered mental status, oculomotor findings, and ataxia. 3 Patients with WKS may exhibit challenging behaviors, including aggression, disinhibition, and lack of awareness of their illness. 4 Due to long-standing cognitive and physical deficits, many patients require lifelong care with a focus on a palliative approach. 3
The mainstay of pharmacologic management for the neuropsychiatric symptoms of WKS continues to be psychoactive medications, such as antipsychotics, benzodiazepines, antidepressants, and anticonvulsant medications.4-6 Though atypical antipsychotic medications remain the most widely used, they have a high adverse effect (AE) profile.5,6 Among the potential AEs are metabolic syndrome, anticholinergic effects, QTc prolongation, orthostatic hypotension, extrapyramidal effects, sedation, and falls. There also is a US Food and Drug Administration boxed warning for increased risk of mortality.7 With the goal of improving and maintaining patient safety, pharmacologic interventions with lower AEs may be beneficial in the management of the neuropsychiatric symptoms of WKS.
This case describes a veteran who was initially hospitalized due to confusion, ataxia, and nystagmus secondary to chronic alcohol overuse. The aim of the case was to consider the use of acetaminophen in place of psychoactive medications as a way to manage neuropsychiatric symptoms of WKS even when pain was not present.
Case Presentation
A veteran presented to the local US Department of Veterans Affairs (VA) emergency department (ED) due to their spouse’s concern of acute onset confusion and ambulatory difficulties. The veteran’s medical history included extensive alcohol misuse, mild asthma, and diet-controlled hyperlipidemia. On initial evaluation, the veteran displayed symptoms of ataxia and confusion. When asked why the veteran was at the ED, the response was, “I just came to the hospital to find my sister.” Based on their medical history, clinical evaluation, and altered mental status, the veteran was admitted to the acute care medical service with a presumptive diagnosis of WKS.
On admission, the laboratory evaluation revealed normal alanine transaminase (ALT) and aspartate transaminase (AST) levels but markedly elevated γ-glutamyl transferase (GGT) consistent with alcohol toxicity. COVID-19 testing was negative. Magnetic resonance imaging (MRI) of the brain revealed evidence of alterations in the mammillary bodies and moderately severe cortical and cerebellar volume loss suggestive of long-standing alcohol use.
The veteran was hospitalized for 12 days and treated with high-dose IV thiamine, which resulted in improvement of their ophthalmic disorder (nystagmus) and ataxia. However, they continued to exhibit poor recall, confusion, and occasional agitation characterized by verbal outbursts and aggression toward the staff.
The veteran’s spouse worked full time and did not feel capable of providing the necessary follow-up care at home. The safest discharge plan found was to transfer the veteran to the local VA community living center (CLC) for physical therapy and further support of their marked cognitive decline and agitation.
Following admission to the CLC, the veteran was placed in a secured memory unit with staff trained specifically on management of veterans with cognitive impairment and behavioral concerns. As the veteran did not have decisional capacity on admission, the staff arranged a meeting with the spouse. Based on that conversation, the goals of care were to focus on a palliative approach and the hope that the veteran would one day be able to return home to their spouse.
At the CLC, the veteran was initially treated with thiamine 200 mg orally once daily and albuterol inhaler as needed. A clinical psychologist performed a comprehensive psychological evaluation on admission, which confirmed evidence of WKS with symptoms, including confusion, disorientation, and confabulation. There was no evidence of cultural diversity factors regarding the veteran’s delusional beliefs.
After the first full day in the CLC, the nursing staff observed anger and agitation that seemed to start midafternoon and continued until around dinnertime. The veteran displayed verbal outbursts, refusal to cooperate with the staff, and multiple attempts to leave the CLC. With the guidance of a geriatric psychiatrist, risperidone 1 mg once daily as needed was initiated, and staff continued with verbal redirection, both with limited efficacy. After 3 days, due to safety concerns for the veteran, other CLC patients, and CLC staff, risperidone dosing was increased to 1 mg twice daily, which had limited efficacy. Lorazepam 1 mg once daily also was added. A careful medication review was performed to minimize any potential AEs or interactions that might have contributed to the veteran’s behavior, but no pharmacologic interventions were found to fully abate their behavioral issues.
After 5 weeks of ongoing intermittent behavioral issues, the medical team again met to discuss new treatment options.A case reported by Husebo and colleagues used scheduled acetaminophen to help relieve neuropsychiatric symptoms of dementia in a patient who exhibited similar behavioral issues and did not respond well to antipsychotics or benzodiazepines.8 Although our veteran did not express or exhibit obvious pain, the medical team chose to trial this intervention, and the veteran was started on acetaminophen 650 mg orally 3 times daily. A comprehensive metabolic panel, including GGT and thyroid-stimulating hormone, was performed before starting acetaminophen; no abnormalities were noted. The clinical examination did not reveal physical abnormalities other than ataxia.
After 5 days of therapy with the scheduled acetaminophen, the veteran’s clinical behavior dramatically improved. The veteran exhibited infrequent agitated behavior and became cooperative with staff. Three days later, the scheduled lorazepam was discontinued, and eventually they were tapered off risperidone. One month after starting scheduled acetaminophen, the veteran had improved to a point where the staff determined a safe discharge plan could be initiated. The veteran’s nystagmus resolved and behavioral issues improved, although cognitive impairment persisted.
Due to COVID-19, a teleconference was scheduled with the veteran’s spouse to discuss a discharge plan. The spouse was pleased that the veteran had progressed adequately both functionally and behaviorally to make a safe discharge home possible. The spouse arranged to take family leave from their job to help support the veteran after discharge. The veteran was able to return home with a safe discharge plan 1 week later. The acetaminophen was continued with twice-daily dosing and was continued because there were no new behavioral issues. This was done to enhance postfacility adherence and minimize the risk of drug-drug interactions. Attempts to follow up with the veteran postdischarge were unfortunately unsuccessful as the family lived out of the local area.
Discussion
Alcohol misuse is a common finding in many US veterans, as well as in the general population.1,3 As a result, it is not uncommon to see patients with physical and psychological symptoms related to this abuse. Many of these patients will become verbally and physically abusive, thus having appropriate pharmacologic and nonpharmacologic interventions is important.
In this case study, the veteran was diagnosed with WKS and exhibited physical, cognitive, and emotional symptoms consistent with this disease. Although the physical symptoms improved with thiamine and abstinence from alcohol, their cognitive impairment, verbal outbursts, and aggressive demeanor persisted.
After using antipsychotic and anxiolytic medications with minimal clinical improvement, a trial of acetaminophen 650 mg 3 times daily was instituted. The patient’s behavior improved; demeanor became calmer, and they were easily redirected by the nursing staff. Psychological support was again employed, which enhanced and supported the veteran’s calmer demeanor. Although there is limited medical literature on the use of acetaminophen in clinical situations not related to pain, there has been research documenting its effect on social interaction.9,10
Acetaminophen is an analgesic medication that acts through central neural mechanisms. It has been hypothesized that social and physical pain rely on shared neurochemical underpinnings, and some of the regions of the brain involved in affective experience of physical pain also have been found to be involved in the experience of social pain.11 Acetaminophen may impact an individual’s social well-being as social pain processes.11 It has been shown to blunt reactivity to both physical pain as well as negative stimuli.11
Conclusions
A 2019 survey on alcohol and drug use found 5.6% of adults aged ≥ 18 have an alcohol use disorder.12 In severe cases, this can result in WKS. Although replacement of thiamine is critical for physical improvement, psychological deficits may persist. Small studies have advanced the concept of using scheduled acetaminophen even when the patient is not verbalizing or displaying pain.13 Although more research needs to be done on this topic, this palliative approach may be worth considering, especially if the risks of antipsychotics and anxiolytics outweigh the benefits.
1. National Institute on Drug Abuse. Substance use and military life drug facts. Published October 2019. Accessed November 10, 2021. https://www.drugabuse.gov/publications/drugfacts/substance-use-military-life
2. National Veterans Foundation. What statistics show about veteran substance abuse and why proper treatment is important. Published March 30, 2016. Accessed November 10, 2021. https://nvf.org/veteran-substance-abuse-statistics
3. National Center for Biotechnology Information. Korsakoff syndrome. Updated July 10, 2020. Accessed November 10, 2021. https://www.ncbi.nlm.nih.gov/books/NBK539854
4. Gerridzen IJ, Goossensen MA. Patients with Korsakoff syndrome in nursing homes: characteristics, comorbidity, and use of psychotropic drugs. Int Psychogeriatr. 2014;26(1):115-121. doi:10.1017/S1041610213001543
5. Press D, Alexander M. Management of neuropsychiatric symptoms of dementia. Updated October 2021. Accessed November 10, 2021. https://www.uptodate.com/contents/management-of-neuropsychiatric-symptoms-of-dementia
6. Steinberg M, Lyketsos CG. Atypical antipsychotic use in patients with dementia: Managing safety concerns. Am J Psychiatry. 2012;169(9):900-906. doi:10.1176/appi.ajp.2012.12030342
7. Jibson MD. Second-generation antipsychotic medications: pharmacology, administration, and side effects. https://www.uptodate.com/contents/second-generation-antipsychotic-medications-pharmacology-administration-and-side-effects
8. Husebo BS, Ballard C, Sandvik R, Nilsen OB, Aarsland D. Efficacy of treating pain to reduce behavioural disturbances in residents of nursing homes with dementia: cluster randomised clinical trial. BMJ. 2011;343:d4065. doi:10.1136/bmj.d4065
9. Fung K, Alden LE. Once hurt, twice shy: social pain contributes to social anxiety. Emotion. 2017;(2):231-239. doi:10.1037/emo0000223
10. Roberts ID, Krajbich I, Cheavens JS, Campo JV, Way BM. Acetaminophen Reduces Distrust in Individuals with Borderline Personality Disorder Features. Clin Psychol Sci. 2018;6(1):145-154. doi:10.1177/2167702617731374
11. Dewall CN, Macdonald G, Webster GD, et al. Acetaminophen reduces social pain: behavioral and neural evidence. Psychol Sci. 2010;21(7):931-937. doi:10.1177/0956797610374741
12. National Institute on Alcohol Abuse and Alcoholism. Alcohol facts and statistics. Updated June 2021. Accessed November 2, 202November 10, 2021. https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/alcohol-facts-and-statistics
13. Chibnall JT, Tait RC, Harman B, Luebbert RA. Effect of acetaminophen on behavior, well-being, and psychotropic medication use in nursing home residents with moderate-to-severe dementia. J Am Geriatrics Soc. 2005;53(11):1921-9. doi:10.1111/j.1532-5415.2005.53572.x
1. National Institute on Drug Abuse. Substance use and military life drug facts. Published October 2019. Accessed November 10, 2021. https://www.drugabuse.gov/publications/drugfacts/substance-use-military-life
2. National Veterans Foundation. What statistics show about veteran substance abuse and why proper treatment is important. Published March 30, 2016. Accessed November 10, 2021. https://nvf.org/veteran-substance-abuse-statistics
3. National Center for Biotechnology Information. Korsakoff syndrome. Updated July 10, 2020. Accessed November 10, 2021. https://www.ncbi.nlm.nih.gov/books/NBK539854
4. Gerridzen IJ, Goossensen MA. Patients with Korsakoff syndrome in nursing homes: characteristics, comorbidity, and use of psychotropic drugs. Int Psychogeriatr. 2014;26(1):115-121. doi:10.1017/S1041610213001543
5. Press D, Alexander M. Management of neuropsychiatric symptoms of dementia. Updated October 2021. Accessed November 10, 2021. https://www.uptodate.com/contents/management-of-neuropsychiatric-symptoms-of-dementia
6. Steinberg M, Lyketsos CG. Atypical antipsychotic use in patients with dementia: Managing safety concerns. Am J Psychiatry. 2012;169(9):900-906. doi:10.1176/appi.ajp.2012.12030342
7. Jibson MD. Second-generation antipsychotic medications: pharmacology, administration, and side effects. https://www.uptodate.com/contents/second-generation-antipsychotic-medications-pharmacology-administration-and-side-effects
8. Husebo BS, Ballard C, Sandvik R, Nilsen OB, Aarsland D. Efficacy of treating pain to reduce behavioural disturbances in residents of nursing homes with dementia: cluster randomised clinical trial. BMJ. 2011;343:d4065. doi:10.1136/bmj.d4065
9. Fung K, Alden LE. Once hurt, twice shy: social pain contributes to social anxiety. Emotion. 2017;(2):231-239. doi:10.1037/emo0000223
10. Roberts ID, Krajbich I, Cheavens JS, Campo JV, Way BM. Acetaminophen Reduces Distrust in Individuals with Borderline Personality Disorder Features. Clin Psychol Sci. 2018;6(1):145-154. doi:10.1177/2167702617731374
11. Dewall CN, Macdonald G, Webster GD, et al. Acetaminophen reduces social pain: behavioral and neural evidence. Psychol Sci. 2010;21(7):931-937. doi:10.1177/0956797610374741
12. National Institute on Alcohol Abuse and Alcoholism. Alcohol facts and statistics. Updated June 2021. Accessed November 2, 202November 10, 2021. https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/alcohol-facts-and-statistics
13. Chibnall JT, Tait RC, Harman B, Luebbert RA. Effect of acetaminophen on behavior, well-being, and psychotropic medication use in nursing home residents with moderate-to-severe dementia. J Am Geriatrics Soc. 2005;53(11):1921-9. doi:10.1111/j.1532-5415.2005.53572.x
Nicotine and Nicotine Replacement Therapy Use During Myocardial Perfusion Imaging
Chest pain is one of the most common concerns in patients presenting to the emergency department in the United States, accounting for approximately 7.6 million visits annually.1 Given the high mortality rate associated with acute coronary syndromes, prompt evaluation of chest pain is essential.2 Even in mild cases, recognition of newly onset or worsening coronary artery disease (CAD) is crucial to ensure that patients receive optimal medication therapy.
In symptomatic patients with risk factors for CAD, such as advanced age, hypertension, hyperlipidemia, obesity, and diabetes mellitus, myocardial perfusion imaging (MPI) is frequently used as a modality to assess the presence, location, and severity of ischemic or infarcted myocardium.2 MPI requires administration of a radiopharmaceutical before and after the patient undergoes a form of stress.2 This radiopharmaceutical is then detected in the myocardium with a nuclear camera, and images are obtained of the heart to assess myocardial blood flow.2
MPI can be performed using exercise-induced stress via a treadmill, or medication-induced stress (Table 1). In both strategies, healthy coronary arteries dilate to provide the myocardium with more blood flow to meet the increasing myocardial oxygen demand during this period of stress. While healthy vessels are able to dilate appropriately, coronary arteries with flow-limiting stenoses are unable to dilate to the same extent in response to stress.2 Because radioactive isotope uptake by the myocardium is directly related to arterial blood flow, MPI is able to demonstrate a mismatch in coronary blood flow between healthy and diseased coronary arteries indicated by differences in radioisotope uptake.2 The presence of such a mismatch, in conjunction with clinical history, potentially suggests the presence of CAD.
Prior to conducting MPI with a medication, certain substances should be avoided. For instance, methylxanthines, such as caffeine, aminophylline, and theophylline, antagonize adenosine receptors and can have major drug interactions with regadenoson, adenosine, and dipyridamole. Therefore, it is advised that these substances be stopped for at least 12 hours before testing.3 In some cases, other medications that can affect coronary blood flow, such as long-acting nitrates, β-blockers, and calcium channel blockers, are recommended to be avoided for 12 to 48 hours in order to obtain the most accurate depiction of underlying coronary disease.4
Because nicotine and nicotine replacement therapy (NRT) may have substantial effects on coronary circulation, a current area of controversy is whether these should be stopped prior to the use of a stress-inducing medication during MPI. To date, no formal drug interaction studies have been conducted between nicotine and regadenoson.5 Similarly, the ADVANCE MPI 2 Trial, which led to the US Food and Drug Administration approval of regadenoson, did not specify restrictions on the use of nicotine prior to stress testing in the protocol.6 However, as this trial was multicenter, investigators admit that individual study sites could have had their own restrictions on the use of nicotine prior to stress testing with regadenoson, but this information was not collected.6 The current review focuses on how the simultaneous use of nicotine or NRT during MPI with pharmacologic agents, such as regadenoson, may affect the accuracy of imaging results and the clinical impact of this interaction.
Nicotine Coronary Artery Effect
It is well documented that long-term cigarette smoking is a major risk factor for CAD.7 Compared with nonsmokers, cigarette smokers experience 2 times greater risk of morbidity and mortality from ischemic heart disease.7 There are several mechanisms by which nicotine induces damage to the myocardium (Figure). Nicotine has direct effects on both the sympathetic nervous system (SNS) and myocardial endothelium.8 Together, these factors result in reduced coronary blood flow, leading to less oxygen supply to meet an increased oxygen demand, resulting in myocardial ischemia.
Nicotine’s effect on coronary vasomotor tone occurs primarily through noradrenergic stimulation of α and β receptors associated with coronary vasoconstriction or vasodilation, respectively.9,10 These competing influences on coronary blood flow appear to manifest differently based on whether patients are at rest or in a stressed state. A study by Czerin and colleagues demonstrated that in healthy patients with relatively short smoking histories and in a healthy nonsmoker control group, coronary blood flow increased by 25% and 40%, respectively, with nicotine use at rest.9 However, when these patients were stressed with dipyramidole and while smoking during the examination, myocardial blood flow was reduced by 11% in the study group and 14% in the control group.9 This is likely because the patients studied had relatively healthy coronary arteries that were able to maximally dilate when stressed. In this scenario, nicotine’s dilatory effects are offset by nicotine’s α-receptor–mediated vasoconstriction effects.9 Of note, patients in the study group experienced a somewhat diminished increase in coronary blood flow at rest with nicotine use, suggesting that even a short smoking history may damage the myocardial endothelium, rendering it less responsive to nicotine’s vasodilatory effects.9
These principles similarly apply to patients with underlying moderate-to-severe cardiovascular disease (CVD). With nicotine use at rest, patients with significant CAD do not experience as dramatic of an increase in coronary blood flow, which typically decreases or remains the same despite increased myocardial work.10 This may be because patients with moderate-to-severe CAD often have flow-limiting stenoses and damaged endothelium that do not allow vessels to respond as efficiently to increased myocardial demand or to nicotine’s β-receptor–mediated vasodilatory effects.10,11 Moreover, when stressed, diseased coronary arteries are not able to further dilate and nicotine’s α-receptor–mediated vasoconstriction effects dominate.10,11
In a study by Quillen and colleagues of patients with moderate-to-severe CAD, the mean diameter of proximal coronary artery segments decreased by 5%, the distal coronary diameter decreased 8%, and the coronary vascular resistance increased by 21% while smoking at rest.12 The investigators did not analyze how parameters changed when these diseased coronary arteries were stressed using a medication during MPI. However, it can be predicted that coronary arteries would have constricted to a similar or greater degree than observed in Czerin and colleagues’ study, given that the underlying myocardium was diseased and more susceptible to nicotine’s vasoconstriction effects.9 Importantly, these studies have several limitations, most notably that they are older and have small sample sizes. Additionally, while statistically significant differences were found in the degree of changes in coronary circulation with nicotine use at rest and during stress, it is unclear whether this translates to a clinically significant and impactful finding.9-12
Nicotine Replacement Therapy and Stress Testing
Given the association between cigarette smoking and CAD, medical practitioners strongly encourage patients to quit smoking to reduce their risk of adverse cardiovascular outcomes. Various smoking cessation treatments are available for patients. Common, readily accessible forms of therapy include nicotine replacement products (Table 2).
Early studies of NRT in patients with underlying CVD found an increased risk of cardiovascular events, such as myocardial infarction, presumably due to the nicotine content of these products.13,14 However, the concentration of nicotine in NRT is substantially lower than that found in cigarettes and in some formulations, such as transdermal patches, nicotine is delivered over a prolonged period of time.15 For this reason, NRT is thought to be safe in patients with underlying CVD and stable ischemic heart disease. A recent systematic review and meta-analysis found that while NRT may be associated with tachycardia, it did not increase the risk of more serious cardiovascular adverse effects (AEs).16,17
Given the lower nicotine concentration in NRT products, the associated hemodynamic effect of nicotine also is thought to be less pronounced. In a study conducted by Tzivoni and colleagues in patients with CAD using transdermal nicotine patches, no differences in blood pressure, heart rate, ischemia, or arrhythmias were found from baseline to 2 weeks.18 These findings were further confirmed in a small study by Lucini and colleagues, which found that nicotine patches produced slight hemodynamic effects, but to a lesser extent than cigarette smoking.19 For the NRT gum formulation, while a small study found that 4 mg produced coronary vasoconstriction in patients with underlying CAD, a study by Nitenberg and Antony demonstrated that healthy and diseased coronary arteries did not significantly constrict while patients were using nicotine gum both before and after a cold pressor test, suggesting a lesser degree of coronary vasoconstriction than nicotine from cigarette smoking.20,21 Similar findings have been described with the nicotine intranasal spray in a study by Keeley and colleagues, which showed no additional AEs on myocardial demand or vasoconstriction when an intranasal nicotine spray was added to cigarette smoking.22 Importantly, a review of the transdermal and gum formulations found that these less pronounced hemodynamic effects were observed across different doses of NRT; however, further studies are needed to clarify the relationship between NRT dose and cardiovascular effects.23
Overall, NRT does not seem to activate the SNS to the same degree as nicotine obtained via cigarette smoking and likely does not increase the myocardial oxygen demand as much. Additionally, by containing a lower concentration of nicotine, NRT may not impair the myocardium’s ability to supply oxygen to coronary arteries to the same extent as nicotine from cigarette smoking. Therefore, the effects of NRT on MPI using a stress-inducing medication may not be as pronounced. However, due to study limitations, results should be interpreted cautiously.18-23
Conclusions
Because of the close relationship between cigarette smoking and CAD, many patients with underlying CVD are either current smokers or may be using NRT for smoking cessation. Therefore, the question of whether to refrain from nicotine use prior to MPI is clinically relevant. Currently, there is a lack of high-quality studies demonstrating the effects of nicotine and NRT on coronary perfusion. Because of this, the impact of nicotine and NRT use on the accuracy of MPI using stress-inducing medications remains uncertain. Nevertheless, given that nicotine and NRT may largely affect the accuracy of imaging results, several institutions have adopted protocols that prohibit patients from using these drugs on the day of nuclear stress testing.
There are currently no data specifying the number of hours to hold nicotine products prior to cardiac stress testing. It is generally recommended that other medications that affect coronary blood flow be held for 5 half-lives before conducting MPI.4 Following the same guidance for nicotine and NRT may present a reasonable approach to ensure accurate imaging results. Based on the discussed literature, patients should be instructed to refrain from cigarette smoking for at least 5 to 10 hours prior to MPI, given nicotine’s half-life of about 1 to 2 hours.24
The data for NRT are less clear. While use of NRT may not be an absolute contraindication to conducting MPI, it is important to consider that this may affect the accuracy of results. Given this uncertainty, it is likely ideal to hold NRT prior to MPI, based on the specific formulation of NRT and that product's half-life. Further robust studies are needed to analyze the impact of nicotine and NRT on the accuracy of nuclear stress testing using a medication.
1. Rui P, Kang K, Ashman JJ. National Hospital Ambulatory Medical Care Survey: 2016 emergency department summary tables. Published 2016. Accessed March 30, 2020. https://www.cdc.gov/nchs/data/nhamcs/web_tables/2016_ed_web_tables.pdf
2. Lange RA. Cardiovascular testing. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey L. eds. Pharmacotherapy: A Pathophysiologic Approach, 10th ed. McGraw Hill; 2017.
3. Mace S. Observation Medicine: Principles and Protocols. Cambridge University Press; 2017.
4. Currie GM. Pharmacology, part 4: nuclear cardiology. J Nucl Med Technol. 2019;47(2):97-110. doi:10.2967/jnmt.118.219675
5. Regadenoson; Package insert. Astellas Pharma US Inc; 2008.
6. Iskandrian AE, Bateman TM, Belardinelli L, et al. Adenosine versus regadenoson comparative evaluation in myocardial perfusion imaging: results of the ADVANCE phase 3 multicenter international trial. J Nucl Cardiol. 2007;14(5):645-658. doi:10.1016/j.nuclcard.2007.06.114
7. Hajar R. Risk factors for coronary artery disease: historical perspectives. Heart Views. 2017;18(3):109-114. doi:10.4103/HEARTVIEWS.HEARTVIEWS_106_17
8. Benowitz NL, Burbank AD. Cardiovascular toxicity of nicotine: implications for electronic cigarette use. Trends Cardiovasc Med. 2016;26(6):515-523. doi:10.1016/j.tcm.2016.03.001
9. Czernin J, Sun K, Brunken R, Böttcher M, Phelps M, Schelbert H. Effect of acute and long-term smoking on myocardial blood flow and flow reserve. Circulation. 1995;91:2891-2897. doi:10.1161/01.CIR.91.12.2891
10. Winniford MD, Wheelan KR, Kremers MS, et al. Smoking-induced coronary vasoconstriction in patients with atherosclerotic coronary artery disease: evidence for adrenergically mediated alterations in coronary artery tone. Circulation. 1986;73(4):662-667. doi:10.1161/01.cir.73.4.662
11. Klein LW, Ambrose J, Pichard A, Holt J, Gorlin R, Teichholz LE. Acute coronary hemodynamic response to cigarette smoking in patients with coronary artery disease. J Am Coll Cardiol. 1984;3(4):879-886. doi:10.1016/s0735-1097(84)80344-7
12. Quillen JE, Rossen JD, Oskarsson HJ, Minor RL Jr, Lopez AG, Winniford MD. Acute effect of cigarette smoking on the coronary circulation: constriction of epicardial and resistance vessels. J Am Coll Cardiol. 1993;22(3):642-647. doi:10.1016/0735-1097(93)90170-6
13. Dacosta A, Guy JM, Tardy B, et al. Myocardial infarction and nicotine patch: a contributing or causative factor?. Eur Heart J. 1993;14(12):1709-1711. doi:10.1093/eurheartj/14.12.1709
14. Ottervanger JP, Festen JM, de Vries AG, Stricker BH. Acute myocardial infarction while using the nicotine patch. Chest. 1995;107(6):1765-1766. doi:10.1378/chest.107.6.1765
15. Dollerup J, Vestbo J, Murray-Thomas T, et al. Cardiovascular risks in smokers treated with nicotine replacement therapy: a historical cohort study. Clin Epidemiol. 2017;9:231-243. Published 2017 Apr 26. doi:10.2147/CLEP.S127775
16. Mills EJ, Wu P, Lockhart I, Wilson K, Ebbert JO. Adverse events associated with nicotine replacement therapy (NRT) for smoking cessation. A systematic review and meta-analysis of one hundred and twenty studies involving 177,390 individuals. Tob Induc Dis. 2010;8(1):8. Published 2010 Jul 13. doi:10.1186/1617-9625-8-8
17. Mills EJ, Thorlund K, Eapen S, Wu P, Prochaska JJ. Cardiovascular events associated with smoking cessation pharmacotherapies: a network meta-analysis. Circulation. 2014;129(1):28-41. doi:10.1161/CIRCULATIONAHA.113.003961
18. Tzivoni D, Keren A, Meyler S, Khoury Z, Lerer T, Brunel P. Cardiovascular safety of transdermal nicotine patches in patients with coronary artery disease who try to quit smoking. Cardiovasc Drugs Ther. 1998;12(3):239-244. doi:10.1023/a:1007757530765
19. Lucini D, Bertocchi F, Malliani A, Pagani M. Autonomic effects of nicotine patch administration in habitual cigarette smokers: a double-blind, placebo-controlled study using spectral analysis of RR interval and systolic arterial pressure variabilities. J Cardiovasc Pharmacol. 1998;31(5):714-720. doi:10.1097/00005344-199805000-00010
20. Kaijser L, Berglund B. Effect of nicotine on coronary blood-flow in man. Clin Physiol. 1985;5(6):541-552. doi:10.1111/j.1475-097x.1985.tb00767.x
21. Nitenberg A, Antony I. Effects of nicotine gum on coronary vasomotor responses during sympathetic stimulation in patients with coronary artery stenosis. J Cardiovasc Pharmacol. 1999;34(5):694-699. doi:10.1097/00005344-199911000-00011
22. Keeley EC, Pirwitz MJ, Landau C, et al. Intranasal nicotine spray does not augment the adverse effects of cigarette smoking on myocardial oxygen demand or coronary arterial dimensions. Am J Med. 1996;101(4):357-363. doi:10.1016/s0002-9343(96)00237-9
23. Benowitz NL, Gourlay SG. Cardiovascular toxicity of nicotine: implications for nicotine replacement therapy. J Am Coll Cardiol. 1997;29(7):1422-1431. doi:10.1016/s0735-1097(97)00079-x
24. Flowers L. Nicotine replacement therapy. Amer J Psych. 2017;11(6):4-7.
25. Adenosine; Package insert. Astellas Pharma US Inc; 1989.
26. Dipyridamole; Package insert. Boehringer Ingelheim Pharmaceuticals Inc; 2019.
27. Dobutamine; Package insert. Baxter Healthcare Corporation; 2012.
Chest pain is one of the most common concerns in patients presenting to the emergency department in the United States, accounting for approximately 7.6 million visits annually.1 Given the high mortality rate associated with acute coronary syndromes, prompt evaluation of chest pain is essential.2 Even in mild cases, recognition of newly onset or worsening coronary artery disease (CAD) is crucial to ensure that patients receive optimal medication therapy.
In symptomatic patients with risk factors for CAD, such as advanced age, hypertension, hyperlipidemia, obesity, and diabetes mellitus, myocardial perfusion imaging (MPI) is frequently used as a modality to assess the presence, location, and severity of ischemic or infarcted myocardium.2 MPI requires administration of a radiopharmaceutical before and after the patient undergoes a form of stress.2 This radiopharmaceutical is then detected in the myocardium with a nuclear camera, and images are obtained of the heart to assess myocardial blood flow.2
MPI can be performed using exercise-induced stress via a treadmill, or medication-induced stress (Table 1). In both strategies, healthy coronary arteries dilate to provide the myocardium with more blood flow to meet the increasing myocardial oxygen demand during this period of stress. While healthy vessels are able to dilate appropriately, coronary arteries with flow-limiting stenoses are unable to dilate to the same extent in response to stress.2 Because radioactive isotope uptake by the myocardium is directly related to arterial blood flow, MPI is able to demonstrate a mismatch in coronary blood flow between healthy and diseased coronary arteries indicated by differences in radioisotope uptake.2 The presence of such a mismatch, in conjunction with clinical history, potentially suggests the presence of CAD.
Prior to conducting MPI with a medication, certain substances should be avoided. For instance, methylxanthines, such as caffeine, aminophylline, and theophylline, antagonize adenosine receptors and can have major drug interactions with regadenoson, adenosine, and dipyridamole. Therefore, it is advised that these substances be stopped for at least 12 hours before testing.3 In some cases, other medications that can affect coronary blood flow, such as long-acting nitrates, β-blockers, and calcium channel blockers, are recommended to be avoided for 12 to 48 hours in order to obtain the most accurate depiction of underlying coronary disease.4
Because nicotine and nicotine replacement therapy (NRT) may have substantial effects on coronary circulation, a current area of controversy is whether these should be stopped prior to the use of a stress-inducing medication during MPI. To date, no formal drug interaction studies have been conducted between nicotine and regadenoson.5 Similarly, the ADVANCE MPI 2 Trial, which led to the US Food and Drug Administration approval of regadenoson, did not specify restrictions on the use of nicotine prior to stress testing in the protocol.6 However, as this trial was multicenter, investigators admit that individual study sites could have had their own restrictions on the use of nicotine prior to stress testing with regadenoson, but this information was not collected.6 The current review focuses on how the simultaneous use of nicotine or NRT during MPI with pharmacologic agents, such as regadenoson, may affect the accuracy of imaging results and the clinical impact of this interaction.
Nicotine Coronary Artery Effect
It is well documented that long-term cigarette smoking is a major risk factor for CAD.7 Compared with nonsmokers, cigarette smokers experience 2 times greater risk of morbidity and mortality from ischemic heart disease.7 There are several mechanisms by which nicotine induces damage to the myocardium (Figure). Nicotine has direct effects on both the sympathetic nervous system (SNS) and myocardial endothelium.8 Together, these factors result in reduced coronary blood flow, leading to less oxygen supply to meet an increased oxygen demand, resulting in myocardial ischemia.
Nicotine’s effect on coronary vasomotor tone occurs primarily through noradrenergic stimulation of α and β receptors associated with coronary vasoconstriction or vasodilation, respectively.9,10 These competing influences on coronary blood flow appear to manifest differently based on whether patients are at rest or in a stressed state. A study by Czerin and colleagues demonstrated that in healthy patients with relatively short smoking histories and in a healthy nonsmoker control group, coronary blood flow increased by 25% and 40%, respectively, with nicotine use at rest.9 However, when these patients were stressed with dipyramidole and while smoking during the examination, myocardial blood flow was reduced by 11% in the study group and 14% in the control group.9 This is likely because the patients studied had relatively healthy coronary arteries that were able to maximally dilate when stressed. In this scenario, nicotine’s dilatory effects are offset by nicotine’s α-receptor–mediated vasoconstriction effects.9 Of note, patients in the study group experienced a somewhat diminished increase in coronary blood flow at rest with nicotine use, suggesting that even a short smoking history may damage the myocardial endothelium, rendering it less responsive to nicotine’s vasodilatory effects.9
These principles similarly apply to patients with underlying moderate-to-severe cardiovascular disease (CVD). With nicotine use at rest, patients with significant CAD do not experience as dramatic of an increase in coronary blood flow, which typically decreases or remains the same despite increased myocardial work.10 This may be because patients with moderate-to-severe CAD often have flow-limiting stenoses and damaged endothelium that do not allow vessels to respond as efficiently to increased myocardial demand or to nicotine’s β-receptor–mediated vasodilatory effects.10,11 Moreover, when stressed, diseased coronary arteries are not able to further dilate and nicotine’s α-receptor–mediated vasoconstriction effects dominate.10,11
In a study by Quillen and colleagues of patients with moderate-to-severe CAD, the mean diameter of proximal coronary artery segments decreased by 5%, the distal coronary diameter decreased 8%, and the coronary vascular resistance increased by 21% while smoking at rest.12 The investigators did not analyze how parameters changed when these diseased coronary arteries were stressed using a medication during MPI. However, it can be predicted that coronary arteries would have constricted to a similar or greater degree than observed in Czerin and colleagues’ study, given that the underlying myocardium was diseased and more susceptible to nicotine’s vasoconstriction effects.9 Importantly, these studies have several limitations, most notably that they are older and have small sample sizes. Additionally, while statistically significant differences were found in the degree of changes in coronary circulation with nicotine use at rest and during stress, it is unclear whether this translates to a clinically significant and impactful finding.9-12
Nicotine Replacement Therapy and Stress Testing
Given the association between cigarette smoking and CAD, medical practitioners strongly encourage patients to quit smoking to reduce their risk of adverse cardiovascular outcomes. Various smoking cessation treatments are available for patients. Common, readily accessible forms of therapy include nicotine replacement products (Table 2).
Early studies of NRT in patients with underlying CVD found an increased risk of cardiovascular events, such as myocardial infarction, presumably due to the nicotine content of these products.13,14 However, the concentration of nicotine in NRT is substantially lower than that found in cigarettes and in some formulations, such as transdermal patches, nicotine is delivered over a prolonged period of time.15 For this reason, NRT is thought to be safe in patients with underlying CVD and stable ischemic heart disease. A recent systematic review and meta-analysis found that while NRT may be associated with tachycardia, it did not increase the risk of more serious cardiovascular adverse effects (AEs).16,17
Given the lower nicotine concentration in NRT products, the associated hemodynamic effect of nicotine also is thought to be less pronounced. In a study conducted by Tzivoni and colleagues in patients with CAD using transdermal nicotine patches, no differences in blood pressure, heart rate, ischemia, or arrhythmias were found from baseline to 2 weeks.18 These findings were further confirmed in a small study by Lucini and colleagues, which found that nicotine patches produced slight hemodynamic effects, but to a lesser extent than cigarette smoking.19 For the NRT gum formulation, while a small study found that 4 mg produced coronary vasoconstriction in patients with underlying CAD, a study by Nitenberg and Antony demonstrated that healthy and diseased coronary arteries did not significantly constrict while patients were using nicotine gum both before and after a cold pressor test, suggesting a lesser degree of coronary vasoconstriction than nicotine from cigarette smoking.20,21 Similar findings have been described with the nicotine intranasal spray in a study by Keeley and colleagues, which showed no additional AEs on myocardial demand or vasoconstriction when an intranasal nicotine spray was added to cigarette smoking.22 Importantly, a review of the transdermal and gum formulations found that these less pronounced hemodynamic effects were observed across different doses of NRT; however, further studies are needed to clarify the relationship between NRT dose and cardiovascular effects.23
Overall, NRT does not seem to activate the SNS to the same degree as nicotine obtained via cigarette smoking and likely does not increase the myocardial oxygen demand as much. Additionally, by containing a lower concentration of nicotine, NRT may not impair the myocardium’s ability to supply oxygen to coronary arteries to the same extent as nicotine from cigarette smoking. Therefore, the effects of NRT on MPI using a stress-inducing medication may not be as pronounced. However, due to study limitations, results should be interpreted cautiously.18-23
Conclusions
Because of the close relationship between cigarette smoking and CAD, many patients with underlying CVD are either current smokers or may be using NRT for smoking cessation. Therefore, the question of whether to refrain from nicotine use prior to MPI is clinically relevant. Currently, there is a lack of high-quality studies demonstrating the effects of nicotine and NRT on coronary perfusion. Because of this, the impact of nicotine and NRT use on the accuracy of MPI using stress-inducing medications remains uncertain. Nevertheless, given that nicotine and NRT may largely affect the accuracy of imaging results, several institutions have adopted protocols that prohibit patients from using these drugs on the day of nuclear stress testing.
There are currently no data specifying the number of hours to hold nicotine products prior to cardiac stress testing. It is generally recommended that other medications that affect coronary blood flow be held for 5 half-lives before conducting MPI.4 Following the same guidance for nicotine and NRT may present a reasonable approach to ensure accurate imaging results. Based on the discussed literature, patients should be instructed to refrain from cigarette smoking for at least 5 to 10 hours prior to MPI, given nicotine’s half-life of about 1 to 2 hours.24
The data for NRT are less clear. While use of NRT may not be an absolute contraindication to conducting MPI, it is important to consider that this may affect the accuracy of results. Given this uncertainty, it is likely ideal to hold NRT prior to MPI, based on the specific formulation of NRT and that product's half-life. Further robust studies are needed to analyze the impact of nicotine and NRT on the accuracy of nuclear stress testing using a medication.
Chest pain is one of the most common concerns in patients presenting to the emergency department in the United States, accounting for approximately 7.6 million visits annually.1 Given the high mortality rate associated with acute coronary syndromes, prompt evaluation of chest pain is essential.2 Even in mild cases, recognition of newly onset or worsening coronary artery disease (CAD) is crucial to ensure that patients receive optimal medication therapy.
In symptomatic patients with risk factors for CAD, such as advanced age, hypertension, hyperlipidemia, obesity, and diabetes mellitus, myocardial perfusion imaging (MPI) is frequently used as a modality to assess the presence, location, and severity of ischemic or infarcted myocardium.2 MPI requires administration of a radiopharmaceutical before and after the patient undergoes a form of stress.2 This radiopharmaceutical is then detected in the myocardium with a nuclear camera, and images are obtained of the heart to assess myocardial blood flow.2
MPI can be performed using exercise-induced stress via a treadmill, or medication-induced stress (Table 1). In both strategies, healthy coronary arteries dilate to provide the myocardium with more blood flow to meet the increasing myocardial oxygen demand during this period of stress. While healthy vessels are able to dilate appropriately, coronary arteries with flow-limiting stenoses are unable to dilate to the same extent in response to stress.2 Because radioactive isotope uptake by the myocardium is directly related to arterial blood flow, MPI is able to demonstrate a mismatch in coronary blood flow between healthy and diseased coronary arteries indicated by differences in radioisotope uptake.2 The presence of such a mismatch, in conjunction with clinical history, potentially suggests the presence of CAD.
Prior to conducting MPI with a medication, certain substances should be avoided. For instance, methylxanthines, such as caffeine, aminophylline, and theophylline, antagonize adenosine receptors and can have major drug interactions with regadenoson, adenosine, and dipyridamole. Therefore, it is advised that these substances be stopped for at least 12 hours before testing.3 In some cases, other medications that can affect coronary blood flow, such as long-acting nitrates, β-blockers, and calcium channel blockers, are recommended to be avoided for 12 to 48 hours in order to obtain the most accurate depiction of underlying coronary disease.4
Because nicotine and nicotine replacement therapy (NRT) may have substantial effects on coronary circulation, a current area of controversy is whether these should be stopped prior to the use of a stress-inducing medication during MPI. To date, no formal drug interaction studies have been conducted between nicotine and regadenoson.5 Similarly, the ADVANCE MPI 2 Trial, which led to the US Food and Drug Administration approval of regadenoson, did not specify restrictions on the use of nicotine prior to stress testing in the protocol.6 However, as this trial was multicenter, investigators admit that individual study sites could have had their own restrictions on the use of nicotine prior to stress testing with regadenoson, but this information was not collected.6 The current review focuses on how the simultaneous use of nicotine or NRT during MPI with pharmacologic agents, such as regadenoson, may affect the accuracy of imaging results and the clinical impact of this interaction.
Nicotine Coronary Artery Effect
It is well documented that long-term cigarette smoking is a major risk factor for CAD.7 Compared with nonsmokers, cigarette smokers experience 2 times greater risk of morbidity and mortality from ischemic heart disease.7 There are several mechanisms by which nicotine induces damage to the myocardium (Figure). Nicotine has direct effects on both the sympathetic nervous system (SNS) and myocardial endothelium.8 Together, these factors result in reduced coronary blood flow, leading to less oxygen supply to meet an increased oxygen demand, resulting in myocardial ischemia.
Nicotine’s effect on coronary vasomotor tone occurs primarily through noradrenergic stimulation of α and β receptors associated with coronary vasoconstriction or vasodilation, respectively.9,10 These competing influences on coronary blood flow appear to manifest differently based on whether patients are at rest or in a stressed state. A study by Czerin and colleagues demonstrated that in healthy patients with relatively short smoking histories and in a healthy nonsmoker control group, coronary blood flow increased by 25% and 40%, respectively, with nicotine use at rest.9 However, when these patients were stressed with dipyramidole and while smoking during the examination, myocardial blood flow was reduced by 11% in the study group and 14% in the control group.9 This is likely because the patients studied had relatively healthy coronary arteries that were able to maximally dilate when stressed. In this scenario, nicotine’s dilatory effects are offset by nicotine’s α-receptor–mediated vasoconstriction effects.9 Of note, patients in the study group experienced a somewhat diminished increase in coronary blood flow at rest with nicotine use, suggesting that even a short smoking history may damage the myocardial endothelium, rendering it less responsive to nicotine’s vasodilatory effects.9
These principles similarly apply to patients with underlying moderate-to-severe cardiovascular disease (CVD). With nicotine use at rest, patients with significant CAD do not experience as dramatic of an increase in coronary blood flow, which typically decreases or remains the same despite increased myocardial work.10 This may be because patients with moderate-to-severe CAD often have flow-limiting stenoses and damaged endothelium that do not allow vessels to respond as efficiently to increased myocardial demand or to nicotine’s β-receptor–mediated vasodilatory effects.10,11 Moreover, when stressed, diseased coronary arteries are not able to further dilate and nicotine’s α-receptor–mediated vasoconstriction effects dominate.10,11
In a study by Quillen and colleagues of patients with moderate-to-severe CAD, the mean diameter of proximal coronary artery segments decreased by 5%, the distal coronary diameter decreased 8%, and the coronary vascular resistance increased by 21% while smoking at rest.12 The investigators did not analyze how parameters changed when these diseased coronary arteries were stressed using a medication during MPI. However, it can be predicted that coronary arteries would have constricted to a similar or greater degree than observed in Czerin and colleagues’ study, given that the underlying myocardium was diseased and more susceptible to nicotine’s vasoconstriction effects.9 Importantly, these studies have several limitations, most notably that they are older and have small sample sizes. Additionally, while statistically significant differences were found in the degree of changes in coronary circulation with nicotine use at rest and during stress, it is unclear whether this translates to a clinically significant and impactful finding.9-12
Nicotine Replacement Therapy and Stress Testing
Given the association between cigarette smoking and CAD, medical practitioners strongly encourage patients to quit smoking to reduce their risk of adverse cardiovascular outcomes. Various smoking cessation treatments are available for patients. Common, readily accessible forms of therapy include nicotine replacement products (Table 2).
Early studies of NRT in patients with underlying CVD found an increased risk of cardiovascular events, such as myocardial infarction, presumably due to the nicotine content of these products.13,14 However, the concentration of nicotine in NRT is substantially lower than that found in cigarettes and in some formulations, such as transdermal patches, nicotine is delivered over a prolonged period of time.15 For this reason, NRT is thought to be safe in patients with underlying CVD and stable ischemic heart disease. A recent systematic review and meta-analysis found that while NRT may be associated with tachycardia, it did not increase the risk of more serious cardiovascular adverse effects (AEs).16,17
Given the lower nicotine concentration in NRT products, the associated hemodynamic effect of nicotine also is thought to be less pronounced. In a study conducted by Tzivoni and colleagues in patients with CAD using transdermal nicotine patches, no differences in blood pressure, heart rate, ischemia, or arrhythmias were found from baseline to 2 weeks.18 These findings were further confirmed in a small study by Lucini and colleagues, which found that nicotine patches produced slight hemodynamic effects, but to a lesser extent than cigarette smoking.19 For the NRT gum formulation, while a small study found that 4 mg produced coronary vasoconstriction in patients with underlying CAD, a study by Nitenberg and Antony demonstrated that healthy and diseased coronary arteries did not significantly constrict while patients were using nicotine gum both before and after a cold pressor test, suggesting a lesser degree of coronary vasoconstriction than nicotine from cigarette smoking.20,21 Similar findings have been described with the nicotine intranasal spray in a study by Keeley and colleagues, which showed no additional AEs on myocardial demand or vasoconstriction when an intranasal nicotine spray was added to cigarette smoking.22 Importantly, a review of the transdermal and gum formulations found that these less pronounced hemodynamic effects were observed across different doses of NRT; however, further studies are needed to clarify the relationship between NRT dose and cardiovascular effects.23
Overall, NRT does not seem to activate the SNS to the same degree as nicotine obtained via cigarette smoking and likely does not increase the myocardial oxygen demand as much. Additionally, by containing a lower concentration of nicotine, NRT may not impair the myocardium’s ability to supply oxygen to coronary arteries to the same extent as nicotine from cigarette smoking. Therefore, the effects of NRT on MPI using a stress-inducing medication may not be as pronounced. However, due to study limitations, results should be interpreted cautiously.18-23
Conclusions
Because of the close relationship between cigarette smoking and CAD, many patients with underlying CVD are either current smokers or may be using NRT for smoking cessation. Therefore, the question of whether to refrain from nicotine use prior to MPI is clinically relevant. Currently, there is a lack of high-quality studies demonstrating the effects of nicotine and NRT on coronary perfusion. Because of this, the impact of nicotine and NRT use on the accuracy of MPI using stress-inducing medications remains uncertain. Nevertheless, given that nicotine and NRT may largely affect the accuracy of imaging results, several institutions have adopted protocols that prohibit patients from using these drugs on the day of nuclear stress testing.
There are currently no data specifying the number of hours to hold nicotine products prior to cardiac stress testing. It is generally recommended that other medications that affect coronary blood flow be held for 5 half-lives before conducting MPI.4 Following the same guidance for nicotine and NRT may present a reasonable approach to ensure accurate imaging results. Based on the discussed literature, patients should be instructed to refrain from cigarette smoking for at least 5 to 10 hours prior to MPI, given nicotine’s half-life of about 1 to 2 hours.24
The data for NRT are less clear. While use of NRT may not be an absolute contraindication to conducting MPI, it is important to consider that this may affect the accuracy of results. Given this uncertainty, it is likely ideal to hold NRT prior to MPI, based on the specific formulation of NRT and that product's half-life. Further robust studies are needed to analyze the impact of nicotine and NRT on the accuracy of nuclear stress testing using a medication.
1. Rui P, Kang K, Ashman JJ. National Hospital Ambulatory Medical Care Survey: 2016 emergency department summary tables. Published 2016. Accessed March 30, 2020. https://www.cdc.gov/nchs/data/nhamcs/web_tables/2016_ed_web_tables.pdf
2. Lange RA. Cardiovascular testing. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey L. eds. Pharmacotherapy: A Pathophysiologic Approach, 10th ed. McGraw Hill; 2017.
3. Mace S. Observation Medicine: Principles and Protocols. Cambridge University Press; 2017.
4. Currie GM. Pharmacology, part 4: nuclear cardiology. J Nucl Med Technol. 2019;47(2):97-110. doi:10.2967/jnmt.118.219675
5. Regadenoson; Package insert. Astellas Pharma US Inc; 2008.
6. Iskandrian AE, Bateman TM, Belardinelli L, et al. Adenosine versus regadenoson comparative evaluation in myocardial perfusion imaging: results of the ADVANCE phase 3 multicenter international trial. J Nucl Cardiol. 2007;14(5):645-658. doi:10.1016/j.nuclcard.2007.06.114
7. Hajar R. Risk factors for coronary artery disease: historical perspectives. Heart Views. 2017;18(3):109-114. doi:10.4103/HEARTVIEWS.HEARTVIEWS_106_17
8. Benowitz NL, Burbank AD. Cardiovascular toxicity of nicotine: implications for electronic cigarette use. Trends Cardiovasc Med. 2016;26(6):515-523. doi:10.1016/j.tcm.2016.03.001
9. Czernin J, Sun K, Brunken R, Böttcher M, Phelps M, Schelbert H. Effect of acute and long-term smoking on myocardial blood flow and flow reserve. Circulation. 1995;91:2891-2897. doi:10.1161/01.CIR.91.12.2891
10. Winniford MD, Wheelan KR, Kremers MS, et al. Smoking-induced coronary vasoconstriction in patients with atherosclerotic coronary artery disease: evidence for adrenergically mediated alterations in coronary artery tone. Circulation. 1986;73(4):662-667. doi:10.1161/01.cir.73.4.662
11. Klein LW, Ambrose J, Pichard A, Holt J, Gorlin R, Teichholz LE. Acute coronary hemodynamic response to cigarette smoking in patients with coronary artery disease. J Am Coll Cardiol. 1984;3(4):879-886. doi:10.1016/s0735-1097(84)80344-7
12. Quillen JE, Rossen JD, Oskarsson HJ, Minor RL Jr, Lopez AG, Winniford MD. Acute effect of cigarette smoking on the coronary circulation: constriction of epicardial and resistance vessels. J Am Coll Cardiol. 1993;22(3):642-647. doi:10.1016/0735-1097(93)90170-6
13. Dacosta A, Guy JM, Tardy B, et al. Myocardial infarction and nicotine patch: a contributing or causative factor?. Eur Heart J. 1993;14(12):1709-1711. doi:10.1093/eurheartj/14.12.1709
14. Ottervanger JP, Festen JM, de Vries AG, Stricker BH. Acute myocardial infarction while using the nicotine patch. Chest. 1995;107(6):1765-1766. doi:10.1378/chest.107.6.1765
15. Dollerup J, Vestbo J, Murray-Thomas T, et al. Cardiovascular risks in smokers treated with nicotine replacement therapy: a historical cohort study. Clin Epidemiol. 2017;9:231-243. Published 2017 Apr 26. doi:10.2147/CLEP.S127775
16. Mills EJ, Wu P, Lockhart I, Wilson K, Ebbert JO. Adverse events associated with nicotine replacement therapy (NRT) for smoking cessation. A systematic review and meta-analysis of one hundred and twenty studies involving 177,390 individuals. Tob Induc Dis. 2010;8(1):8. Published 2010 Jul 13. doi:10.1186/1617-9625-8-8
17. Mills EJ, Thorlund K, Eapen S, Wu P, Prochaska JJ. Cardiovascular events associated with smoking cessation pharmacotherapies: a network meta-analysis. Circulation. 2014;129(1):28-41. doi:10.1161/CIRCULATIONAHA.113.003961
18. Tzivoni D, Keren A, Meyler S, Khoury Z, Lerer T, Brunel P. Cardiovascular safety of transdermal nicotine patches in patients with coronary artery disease who try to quit smoking. Cardiovasc Drugs Ther. 1998;12(3):239-244. doi:10.1023/a:1007757530765
19. Lucini D, Bertocchi F, Malliani A, Pagani M. Autonomic effects of nicotine patch administration in habitual cigarette smokers: a double-blind, placebo-controlled study using spectral analysis of RR interval and systolic arterial pressure variabilities. J Cardiovasc Pharmacol. 1998;31(5):714-720. doi:10.1097/00005344-199805000-00010
20. Kaijser L, Berglund B. Effect of nicotine on coronary blood-flow in man. Clin Physiol. 1985;5(6):541-552. doi:10.1111/j.1475-097x.1985.tb00767.x
21. Nitenberg A, Antony I. Effects of nicotine gum on coronary vasomotor responses during sympathetic stimulation in patients with coronary artery stenosis. J Cardiovasc Pharmacol. 1999;34(5):694-699. doi:10.1097/00005344-199911000-00011
22. Keeley EC, Pirwitz MJ, Landau C, et al. Intranasal nicotine spray does not augment the adverse effects of cigarette smoking on myocardial oxygen demand or coronary arterial dimensions. Am J Med. 1996;101(4):357-363. doi:10.1016/s0002-9343(96)00237-9
23. Benowitz NL, Gourlay SG. Cardiovascular toxicity of nicotine: implications for nicotine replacement therapy. J Am Coll Cardiol. 1997;29(7):1422-1431. doi:10.1016/s0735-1097(97)00079-x
24. Flowers L. Nicotine replacement therapy. Amer J Psych. 2017;11(6):4-7.
25. Adenosine; Package insert. Astellas Pharma US Inc; 1989.
26. Dipyridamole; Package insert. Boehringer Ingelheim Pharmaceuticals Inc; 2019.
27. Dobutamine; Package insert. Baxter Healthcare Corporation; 2012.
1. Rui P, Kang K, Ashman JJ. National Hospital Ambulatory Medical Care Survey: 2016 emergency department summary tables. Published 2016. Accessed March 30, 2020. https://www.cdc.gov/nchs/data/nhamcs/web_tables/2016_ed_web_tables.pdf
2. Lange RA. Cardiovascular testing. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey L. eds. Pharmacotherapy: A Pathophysiologic Approach, 10th ed. McGraw Hill; 2017.
3. Mace S. Observation Medicine: Principles and Protocols. Cambridge University Press; 2017.
4. Currie GM. Pharmacology, part 4: nuclear cardiology. J Nucl Med Technol. 2019;47(2):97-110. doi:10.2967/jnmt.118.219675
5. Regadenoson; Package insert. Astellas Pharma US Inc; 2008.
6. Iskandrian AE, Bateman TM, Belardinelli L, et al. Adenosine versus regadenoson comparative evaluation in myocardial perfusion imaging: results of the ADVANCE phase 3 multicenter international trial. J Nucl Cardiol. 2007;14(5):645-658. doi:10.1016/j.nuclcard.2007.06.114
7. Hajar R. Risk factors for coronary artery disease: historical perspectives. Heart Views. 2017;18(3):109-114. doi:10.4103/HEARTVIEWS.HEARTVIEWS_106_17
8. Benowitz NL, Burbank AD. Cardiovascular toxicity of nicotine: implications for electronic cigarette use. Trends Cardiovasc Med. 2016;26(6):515-523. doi:10.1016/j.tcm.2016.03.001
9. Czernin J, Sun K, Brunken R, Böttcher M, Phelps M, Schelbert H. Effect of acute and long-term smoking on myocardial blood flow and flow reserve. Circulation. 1995;91:2891-2897. doi:10.1161/01.CIR.91.12.2891
10. Winniford MD, Wheelan KR, Kremers MS, et al. Smoking-induced coronary vasoconstriction in patients with atherosclerotic coronary artery disease: evidence for adrenergically mediated alterations in coronary artery tone. Circulation. 1986;73(4):662-667. doi:10.1161/01.cir.73.4.662
11. Klein LW, Ambrose J, Pichard A, Holt J, Gorlin R, Teichholz LE. Acute coronary hemodynamic response to cigarette smoking in patients with coronary artery disease. J Am Coll Cardiol. 1984;3(4):879-886. doi:10.1016/s0735-1097(84)80344-7
12. Quillen JE, Rossen JD, Oskarsson HJ, Minor RL Jr, Lopez AG, Winniford MD. Acute effect of cigarette smoking on the coronary circulation: constriction of epicardial and resistance vessels. J Am Coll Cardiol. 1993;22(3):642-647. doi:10.1016/0735-1097(93)90170-6
13. Dacosta A, Guy JM, Tardy B, et al. Myocardial infarction and nicotine patch: a contributing or causative factor?. Eur Heart J. 1993;14(12):1709-1711. doi:10.1093/eurheartj/14.12.1709
14. Ottervanger JP, Festen JM, de Vries AG, Stricker BH. Acute myocardial infarction while using the nicotine patch. Chest. 1995;107(6):1765-1766. doi:10.1378/chest.107.6.1765
15. Dollerup J, Vestbo J, Murray-Thomas T, et al. Cardiovascular risks in smokers treated with nicotine replacement therapy: a historical cohort study. Clin Epidemiol. 2017;9:231-243. Published 2017 Apr 26. doi:10.2147/CLEP.S127775
16. Mills EJ, Wu P, Lockhart I, Wilson K, Ebbert JO. Adverse events associated with nicotine replacement therapy (NRT) for smoking cessation. A systematic review and meta-analysis of one hundred and twenty studies involving 177,390 individuals. Tob Induc Dis. 2010;8(1):8. Published 2010 Jul 13. doi:10.1186/1617-9625-8-8
17. Mills EJ, Thorlund K, Eapen S, Wu P, Prochaska JJ. Cardiovascular events associated with smoking cessation pharmacotherapies: a network meta-analysis. Circulation. 2014;129(1):28-41. doi:10.1161/CIRCULATIONAHA.113.003961
18. Tzivoni D, Keren A, Meyler S, Khoury Z, Lerer T, Brunel P. Cardiovascular safety of transdermal nicotine patches in patients with coronary artery disease who try to quit smoking. Cardiovasc Drugs Ther. 1998;12(3):239-244. doi:10.1023/a:1007757530765
19. Lucini D, Bertocchi F, Malliani A, Pagani M. Autonomic effects of nicotine patch administration in habitual cigarette smokers: a double-blind, placebo-controlled study using spectral analysis of RR interval and systolic arterial pressure variabilities. J Cardiovasc Pharmacol. 1998;31(5):714-720. doi:10.1097/00005344-199805000-00010
20. Kaijser L, Berglund B. Effect of nicotine on coronary blood-flow in man. Clin Physiol. 1985;5(6):541-552. doi:10.1111/j.1475-097x.1985.tb00767.x
21. Nitenberg A, Antony I. Effects of nicotine gum on coronary vasomotor responses during sympathetic stimulation in patients with coronary artery stenosis. J Cardiovasc Pharmacol. 1999;34(5):694-699. doi:10.1097/00005344-199911000-00011
22. Keeley EC, Pirwitz MJ, Landau C, et al. Intranasal nicotine spray does not augment the adverse effects of cigarette smoking on myocardial oxygen demand or coronary arterial dimensions. Am J Med. 1996;101(4):357-363. doi:10.1016/s0002-9343(96)00237-9
23. Benowitz NL, Gourlay SG. Cardiovascular toxicity of nicotine: implications for nicotine replacement therapy. J Am Coll Cardiol. 1997;29(7):1422-1431. doi:10.1016/s0735-1097(97)00079-x
24. Flowers L. Nicotine replacement therapy. Amer J Psych. 2017;11(6):4-7.
25. Adenosine; Package insert. Astellas Pharma US Inc; 1989.
26. Dipyridamole; Package insert. Boehringer Ingelheim Pharmaceuticals Inc; 2019.
27. Dobutamine; Package insert. Baxter Healthcare Corporation; 2012.
Effective alternatives to psychotherapy for borderline personality disorder
Early interventions that focus on clinical case management and psychiatric care, and not necessarily on individual psychotherapy, are effective for young patients with borderline personality disorder (BPD), new research suggests.
Findings from the Monitoring Outcomes of Borderline Personality Disorder in Youth (MOBY) trial also showed improved psychosocial functioning and reduced suicide ideation with these therapies.
The results suggest that, contrary to common belief, psychotherapy is not the only effective approach for early BPD, lead author Andrew M. Chanen, PhD, director of clinical programs and services and head of personality disorder research at Orygen, Melbourne, told this news organization.
“We can say that early diagnosis and early treatment is effective, and the treatment doesn’t need to involve individual psychotherapy but does need to involve clinical case management and psychiatric care,” said Dr. Chanen, a professorial fellow at the Centre for Youth Mental Health, University of Melbourne.
The findings were published online in JAMA Psychiatry.
Extreme sensitivity
Patients with BPD have “extreme sensitivity to interpersonal slights” and often exhibit intense and volatile emotions and impulsive behavior, Dr. Chanen noted. Many will self-harm, abuse drugs, or attempt suicide; the suicide rate among patients with BPD is 8%-10%.
The condition is typically diagnosed in puberty or early adulthood, affecting about 3% of young people and a little more than 1% of adults.
Because of their aggression and interpersonal difficulties, patients with BPD are often discriminated against by health professionals and end up not getting treated, said Dr. Chanen.
Those who are treated often receive individual psychotherapy, such as dialectical behavior therapy (DBT). That type of therapy, which teaches healthy ways to cope with stress and regulate emotions, is very effective, Dr. Chanen said.
The MOBY trial examined three treatment approaches: the Helping Young People Early (HYPE) model, HYPE combined with weekly “befriending,” and a general youth mental health service (YMHS) model combined with befriending.
A key element of HYPE is cognitive analytic therapy, a psychotherapy program focused on understanding problematic self-management and interpersonal relationship patterns. The model includes clinical case management, such as attending to housing, vocational and educational issues, other mental health needs, and physical health needs.
In the second model, the psychotherapy of the HYPE program was replaced with befriending, which involves chatting with a patient about neutral topics such as sports and avoiding emotionally loaded topics such as interpersonal problems.
For YMHS plus befriending, experts trained in treating young people, but not specialized in treating BPD, were involved in managing patients.
‘High satisfaction’
Researchers randomly assigned 139 participants aged 15-25 years (80.6% women; mean age, 19.1 years) with BPD to one of the treatment arms. Of these, 128 (92.1%) were included in the intent-to-treat analysis.
The primary endpoint was psychosocial functioning, as measured by the Inventory of Interpersonal Problems Circumplex Version and the Social Adjustment Scale–Self-Report. Secondary endpoints included suicidal ideation, suicide attempts, nonsuicidal self-injury, depression, substance use, and treatment satisfaction.
The investigators reported group averages, but the study’s noninferiority design did not allow for determining if one treatment had superior efficacy.
All groups improved significantly on the primary endpoint. At 12 months, there was a mean 28.91-point (23.8%) drop in interpersonal problems and a mean 0.55-point (19.3%) drop in social adjustment scores.
For secondary outcomes, mean improvements at 12 months ranged from 40.7% (17.64 points) on the depression scale to 52.7% (6.22 points) for suicide ideation.
“The only area where the treatment didn’t really have an impact was substance use,” said Dr. Chanen. “Satisfaction was high for all three interventions throughout the study, and it’s hard to improve on high satisfaction.”
‘Turns things upside down’
That patients across all groups had marked and sustained improvements “in ways you wouldn’t expect for BPD” supports the conclusion that the interventions had a true effect, Dr. Chanen said.
he added.
They also imply there are effective alternatives to psychotherapy, which many individuals in the field insist is the only way to treat BPD. “This study turns things upside down and says actually it’s not. It’s the basics of treatment that are important,” Dr. Chanen said.
When a patient presents at the emergency department following a severe overdose, “it’s a reflex” for clinicians to refer that person to a psychotherapy program. “The problem is, these programs are not plentiful enough to be able to service the needs of this group,” Dr. Chanen noted.
On the other hand, the skills for clinical case management and psychiatric care “are available throughout the mental health systems,” he added.
The researchers are planning another analysis to determine whether age and sex predict better outcomes in these patients with BPD.
Unique contribution
Commenting for this news organization, John M. Oldham, MD, distinguished emeritus professor, Baylor College of Medicine, Houston, said a “unique and important contribution” of the study is the focus on early intervention.
“The general standard approach in psychiatry and the diagnostic world has been to not even consider anything until after somebody is 18 years of age, which is a mistake because these kids can become quite impaired earlier than that,” he said.
Dr. Oldham, who was not involved with the research, chaired the American Psychiatric Association workgroup that developed the 2001 evidence-based practice guideline for treating BPD, which recommended psychotherapy as the primary treatment. The guideline was last updated in 2005 – and another update is currently being developed, he noted.
There is an emerging trend toward “good psychiatric management” that focuses on level of functioning rather than on a specific strategy requiring a certificate of training that “not many people out there have,” said Dr. Oldham.
“You’re not going to make much headway with these kids if you’re going to be searching around for a DBT-certified therapist. What you need is to bring them in, get them to trust you, and in a sense be a kind of overall behavioral medicine navigator for them,” he added.
Dr. Oldham noted that, although the primary study outcome improved between 19% and 24%, “that means three-quarters of the people didn’t improve.”
He also pointed out this was only a 1-year trial. “Sometimes treatment for people with a personality disorder such as borderline takes a lot longer than that,” Dr. Oldham concluded.
The trial was funded by the National Health and Medical Research Council. Dr. Chanen reports receiving grants from the Australian government’s National Health and Medical Research Council during the conduct of the study and other support from the Helping Young People Early (HYPE) translational program outside the submitted work. He and another investigator cofounded and lead the HYPE clinical program, a government-funded program with continuous support, and the HYPE translational program, a not-for-profit training program. Dr. Oldham reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Early interventions that focus on clinical case management and psychiatric care, and not necessarily on individual psychotherapy, are effective for young patients with borderline personality disorder (BPD), new research suggests.
Findings from the Monitoring Outcomes of Borderline Personality Disorder in Youth (MOBY) trial also showed improved psychosocial functioning and reduced suicide ideation with these therapies.
The results suggest that, contrary to common belief, psychotherapy is not the only effective approach for early BPD, lead author Andrew M. Chanen, PhD, director of clinical programs and services and head of personality disorder research at Orygen, Melbourne, told this news organization.
“We can say that early diagnosis and early treatment is effective, and the treatment doesn’t need to involve individual psychotherapy but does need to involve clinical case management and psychiatric care,” said Dr. Chanen, a professorial fellow at the Centre for Youth Mental Health, University of Melbourne.
The findings were published online in JAMA Psychiatry.
Extreme sensitivity
Patients with BPD have “extreme sensitivity to interpersonal slights” and often exhibit intense and volatile emotions and impulsive behavior, Dr. Chanen noted. Many will self-harm, abuse drugs, or attempt suicide; the suicide rate among patients with BPD is 8%-10%.
The condition is typically diagnosed in puberty or early adulthood, affecting about 3% of young people and a little more than 1% of adults.
Because of their aggression and interpersonal difficulties, patients with BPD are often discriminated against by health professionals and end up not getting treated, said Dr. Chanen.
Those who are treated often receive individual psychotherapy, such as dialectical behavior therapy (DBT). That type of therapy, which teaches healthy ways to cope with stress and regulate emotions, is very effective, Dr. Chanen said.
The MOBY trial examined three treatment approaches: the Helping Young People Early (HYPE) model, HYPE combined with weekly “befriending,” and a general youth mental health service (YMHS) model combined with befriending.
A key element of HYPE is cognitive analytic therapy, a psychotherapy program focused on understanding problematic self-management and interpersonal relationship patterns. The model includes clinical case management, such as attending to housing, vocational and educational issues, other mental health needs, and physical health needs.
In the second model, the psychotherapy of the HYPE program was replaced with befriending, which involves chatting with a patient about neutral topics such as sports and avoiding emotionally loaded topics such as interpersonal problems.
For YMHS plus befriending, experts trained in treating young people, but not specialized in treating BPD, were involved in managing patients.
‘High satisfaction’
Researchers randomly assigned 139 participants aged 15-25 years (80.6% women; mean age, 19.1 years) with BPD to one of the treatment arms. Of these, 128 (92.1%) were included in the intent-to-treat analysis.
The primary endpoint was psychosocial functioning, as measured by the Inventory of Interpersonal Problems Circumplex Version and the Social Adjustment Scale–Self-Report. Secondary endpoints included suicidal ideation, suicide attempts, nonsuicidal self-injury, depression, substance use, and treatment satisfaction.
The investigators reported group averages, but the study’s noninferiority design did not allow for determining if one treatment had superior efficacy.
All groups improved significantly on the primary endpoint. At 12 months, there was a mean 28.91-point (23.8%) drop in interpersonal problems and a mean 0.55-point (19.3%) drop in social adjustment scores.
For secondary outcomes, mean improvements at 12 months ranged from 40.7% (17.64 points) on the depression scale to 52.7% (6.22 points) for suicide ideation.
“The only area where the treatment didn’t really have an impact was substance use,” said Dr. Chanen. “Satisfaction was high for all three interventions throughout the study, and it’s hard to improve on high satisfaction.”
‘Turns things upside down’
That patients across all groups had marked and sustained improvements “in ways you wouldn’t expect for BPD” supports the conclusion that the interventions had a true effect, Dr. Chanen said.
he added.
They also imply there are effective alternatives to psychotherapy, which many individuals in the field insist is the only way to treat BPD. “This study turns things upside down and says actually it’s not. It’s the basics of treatment that are important,” Dr. Chanen said.
When a patient presents at the emergency department following a severe overdose, “it’s a reflex” for clinicians to refer that person to a psychotherapy program. “The problem is, these programs are not plentiful enough to be able to service the needs of this group,” Dr. Chanen noted.
On the other hand, the skills for clinical case management and psychiatric care “are available throughout the mental health systems,” he added.
The researchers are planning another analysis to determine whether age and sex predict better outcomes in these patients with BPD.
Unique contribution
Commenting for this news organization, John M. Oldham, MD, distinguished emeritus professor, Baylor College of Medicine, Houston, said a “unique and important contribution” of the study is the focus on early intervention.
“The general standard approach in psychiatry and the diagnostic world has been to not even consider anything until after somebody is 18 years of age, which is a mistake because these kids can become quite impaired earlier than that,” he said.
Dr. Oldham, who was not involved with the research, chaired the American Psychiatric Association workgroup that developed the 2001 evidence-based practice guideline for treating BPD, which recommended psychotherapy as the primary treatment. The guideline was last updated in 2005 – and another update is currently being developed, he noted.
There is an emerging trend toward “good psychiatric management” that focuses on level of functioning rather than on a specific strategy requiring a certificate of training that “not many people out there have,” said Dr. Oldham.
“You’re not going to make much headway with these kids if you’re going to be searching around for a DBT-certified therapist. What you need is to bring them in, get them to trust you, and in a sense be a kind of overall behavioral medicine navigator for them,” he added.
Dr. Oldham noted that, although the primary study outcome improved between 19% and 24%, “that means three-quarters of the people didn’t improve.”
He also pointed out this was only a 1-year trial. “Sometimes treatment for people with a personality disorder such as borderline takes a lot longer than that,” Dr. Oldham concluded.
The trial was funded by the National Health and Medical Research Council. Dr. Chanen reports receiving grants from the Australian government’s National Health and Medical Research Council during the conduct of the study and other support from the Helping Young People Early (HYPE) translational program outside the submitted work. He and another investigator cofounded and lead the HYPE clinical program, a government-funded program with continuous support, and the HYPE translational program, a not-for-profit training program. Dr. Oldham reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Early interventions that focus on clinical case management and psychiatric care, and not necessarily on individual psychotherapy, are effective for young patients with borderline personality disorder (BPD), new research suggests.
Findings from the Monitoring Outcomes of Borderline Personality Disorder in Youth (MOBY) trial also showed improved psychosocial functioning and reduced suicide ideation with these therapies.
The results suggest that, contrary to common belief, psychotherapy is not the only effective approach for early BPD, lead author Andrew M. Chanen, PhD, director of clinical programs and services and head of personality disorder research at Orygen, Melbourne, told this news organization.
“We can say that early diagnosis and early treatment is effective, and the treatment doesn’t need to involve individual psychotherapy but does need to involve clinical case management and psychiatric care,” said Dr. Chanen, a professorial fellow at the Centre for Youth Mental Health, University of Melbourne.
The findings were published online in JAMA Psychiatry.
Extreme sensitivity
Patients with BPD have “extreme sensitivity to interpersonal slights” and often exhibit intense and volatile emotions and impulsive behavior, Dr. Chanen noted. Many will self-harm, abuse drugs, or attempt suicide; the suicide rate among patients with BPD is 8%-10%.
The condition is typically diagnosed in puberty or early adulthood, affecting about 3% of young people and a little more than 1% of adults.
Because of their aggression and interpersonal difficulties, patients with BPD are often discriminated against by health professionals and end up not getting treated, said Dr. Chanen.
Those who are treated often receive individual psychotherapy, such as dialectical behavior therapy (DBT). That type of therapy, which teaches healthy ways to cope with stress and regulate emotions, is very effective, Dr. Chanen said.
The MOBY trial examined three treatment approaches: the Helping Young People Early (HYPE) model, HYPE combined with weekly “befriending,” and a general youth mental health service (YMHS) model combined with befriending.
A key element of HYPE is cognitive analytic therapy, a psychotherapy program focused on understanding problematic self-management and interpersonal relationship patterns. The model includes clinical case management, such as attending to housing, vocational and educational issues, other mental health needs, and physical health needs.
In the second model, the psychotherapy of the HYPE program was replaced with befriending, which involves chatting with a patient about neutral topics such as sports and avoiding emotionally loaded topics such as interpersonal problems.
For YMHS plus befriending, experts trained in treating young people, but not specialized in treating BPD, were involved in managing patients.
‘High satisfaction’
Researchers randomly assigned 139 participants aged 15-25 years (80.6% women; mean age, 19.1 years) with BPD to one of the treatment arms. Of these, 128 (92.1%) were included in the intent-to-treat analysis.
The primary endpoint was psychosocial functioning, as measured by the Inventory of Interpersonal Problems Circumplex Version and the Social Adjustment Scale–Self-Report. Secondary endpoints included suicidal ideation, suicide attempts, nonsuicidal self-injury, depression, substance use, and treatment satisfaction.
The investigators reported group averages, but the study’s noninferiority design did not allow for determining if one treatment had superior efficacy.
All groups improved significantly on the primary endpoint. At 12 months, there was a mean 28.91-point (23.8%) drop in interpersonal problems and a mean 0.55-point (19.3%) drop in social adjustment scores.
For secondary outcomes, mean improvements at 12 months ranged from 40.7% (17.64 points) on the depression scale to 52.7% (6.22 points) for suicide ideation.
“The only area where the treatment didn’t really have an impact was substance use,” said Dr. Chanen. “Satisfaction was high for all three interventions throughout the study, and it’s hard to improve on high satisfaction.”
‘Turns things upside down’
That patients across all groups had marked and sustained improvements “in ways you wouldn’t expect for BPD” supports the conclusion that the interventions had a true effect, Dr. Chanen said.
he added.
They also imply there are effective alternatives to psychotherapy, which many individuals in the field insist is the only way to treat BPD. “This study turns things upside down and says actually it’s not. It’s the basics of treatment that are important,” Dr. Chanen said.
When a patient presents at the emergency department following a severe overdose, “it’s a reflex” for clinicians to refer that person to a psychotherapy program. “The problem is, these programs are not plentiful enough to be able to service the needs of this group,” Dr. Chanen noted.
On the other hand, the skills for clinical case management and psychiatric care “are available throughout the mental health systems,” he added.
The researchers are planning another analysis to determine whether age and sex predict better outcomes in these patients with BPD.
Unique contribution
Commenting for this news organization, John M. Oldham, MD, distinguished emeritus professor, Baylor College of Medicine, Houston, said a “unique and important contribution” of the study is the focus on early intervention.
“The general standard approach in psychiatry and the diagnostic world has been to not even consider anything until after somebody is 18 years of age, which is a mistake because these kids can become quite impaired earlier than that,” he said.
Dr. Oldham, who was not involved with the research, chaired the American Psychiatric Association workgroup that developed the 2001 evidence-based practice guideline for treating BPD, which recommended psychotherapy as the primary treatment. The guideline was last updated in 2005 – and another update is currently being developed, he noted.
There is an emerging trend toward “good psychiatric management” that focuses on level of functioning rather than on a specific strategy requiring a certificate of training that “not many people out there have,” said Dr. Oldham.
“You’re not going to make much headway with these kids if you’re going to be searching around for a DBT-certified therapist. What you need is to bring them in, get them to trust you, and in a sense be a kind of overall behavioral medicine navigator for them,” he added.
Dr. Oldham noted that, although the primary study outcome improved between 19% and 24%, “that means three-quarters of the people didn’t improve.”
He also pointed out this was only a 1-year trial. “Sometimes treatment for people with a personality disorder such as borderline takes a lot longer than that,” Dr. Oldham concluded.
The trial was funded by the National Health and Medical Research Council. Dr. Chanen reports receiving grants from the Australian government’s National Health and Medical Research Council during the conduct of the study and other support from the Helping Young People Early (HYPE) translational program outside the submitted work. He and another investigator cofounded and lead the HYPE clinical program, a government-funded program with continuous support, and the HYPE translational program, a not-for-profit training program. Dr. Oldham reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA PSYCHIATRY
Study finds sharp drop in opioid scripts among most specialties
The volume of prescription opioids dispensed at retail pharmacies in the United States dropped by 21% in recent years amid efforts to reduce unnecessary use of the painkillers, but the rate of decline varied greatly among types of patients and by type of clinician, a study found.
In a brief report published by Annals of Internal Medicine, researchers from the nonprofit RAND Corp reported an analysis of opioid prescriptions from two periods, 2008-2009 and 2017-2018.
The researchers sought to assess total opioid use rather than simply track the number of pills dispensed. So they used days’ supply and total daily dose to calculate per capita morphine milligram equivalents (MME) for opioid prescriptions, write Bradley D. Stein, MD, PhD, MPH, the study’s lead author and a senior physician researcher at RAND Corp, and his coauthors in their paper.
For the study, the researchers used data from the consulting firm IQVIA, which they say covers about 90% of U.S. prescriptions. Total opioid volume per capita by prescriptions filled in retail pharmacies decreased from 951.4 MME in 2008-2009 to 749.3 MME in 2017-2018, Dr. Stein’s group found.
(In 2020, IQVIA separately said that prescription opioid use per adult in this country rose from an average of 16 pills, or 134 MMEs, in 1992 to a peak of about 55 pills a person, or 790 MMEs, in 2011. By 2019, opioid use per adult had declined to 29 pills and 366 MMEs per capita.)
The RAND report found substantial variation in opioid volume by type of insurance, including a 41.5% decline (636.5 MME to 372.6 MME) among people covered by commercial health plans. That exceeded the 27.7% drop seen for people enrolled in Medicaid (646.8 MME to 467.7 MME). The decline was smaller (17.5%; 2,780.2 MME to 2,294.2 MME) for those on Medicare, who as a group used the most opioids.
‘Almost functions as a Rorschach test’
The causes of the decline are easy to guess, although definitive conclusions are impossible, Dr. Stein told this news organization.
Significant work has been done in recent years to change attitudes about opioid prescriptions by physicians, researchers, and lawmakers. Aggressive promotion of prescription painkillers, particularly Purdue Pharma’s OxyContin, in the 1990s, is widely cited as the triggering event for the national opioid crisis.
In response, states created databases known as prescription drug monitoring programs. The Centers for Disease Control and Prevention in 2016 issued guidelines intended to curb unnecessary use of opioids. The guidelines noted that other medicines could treat chronic pain without raising the risk of addiction. The Choosing Wisely campaign, run by a foundation of the American Board of Internal Medicine, also offered recommendations about limiting use of opioids. And insurers have restricted access to opioids through the prior authorization process. As a result, researchers will make their own guesses at the causes of the decline in opioid prescriptions, based on their own experiences and research interests, Dr. Stein said.
“It almost functions as a Rorschach test,” he said.
Dr. Stein’s group also looked at trends among medical specialties. They found the largest reduction between 2008-2009 and 2017-2018 among emergency physicians (70.5% drop from 99,254.5 MME to 29,234.3 MME), psychiatrists (67.2% drop from 50,464.3 MME to 16,533.0 MME) and oncologists (59.5% drop from 51,731.2 MME to 20,941.4).
Among surgeons, the RAND researchers found a drop of 49.3% from 220,764.6 to 111,904.4. Among dentists, they found a drop of 41.3% from 22,345.3 to 13,126.1.
Among pain specialists, they found a drop of 15.4% from 1,020,808.4 MME to 863,140.7 MME.
Among adult primary care clinicians, Dr. Stein and his colleagues found a drop of 40% from 651,489.4 MME in 2008-2009 to 390,841.0 MME in 2017-2018.
However, one of the groups tracked in the study increased the volume of opioid prescriptions written: advanced practice providers, among whom scripts for the drugs rose 22.7%, from 112,873.9 MME to 138,459.3 MME.
Dr. Stein said he suspects that this gain reflects a change in the nature of the practice of primary care, with nurse practitioners and physician assistants taking more active roles in treatment of patients. Some of the reduction seen among primary care clinicians who treat adults may reflect a shift in which medical personnel in a practice write the opioid prescriptions.
Still, the trends in general seen by Dr. Stein and coauthors are encouraging, even if further study of these patterns is needed, he said.
“This is one of those papers that I think potentially raises as many questions as it provides answers for,” he said.
What’s missing
Maya Hambright, MD, a family medicine physician in New York’s Hudson Valley, who has been working mainly in addiction in response to the opioid overdose crisis, observed that the drop in total prescribed volume of prescription painkillers does not necessarily translate into a reduction in use of opioids
“No one is taking fewer opioids,” Dr. Hambright told this news organization. “I can say that comfortably. They are just getting them from other sources.”
CDC data support Dr. Hambright’s view.
An estimated 100,306 people in the United States died of a drug overdose in the 12 months that ended in April 2021, an increase of 28.5% from the 78,056 deaths during the same period the year before, according to the CDC.
Dr. Hambright said more physicians need to be involved in prescribing medication-assisted treatment (MAT).
The federal government has in the past year loosened restrictions on a requirement, known as an X waiver. Certain clinicians have been exempted from training requirements, as explained in the frequently asked questions page on the Substance Abuse and Mental Health Services Administration website.
SAMHSA says legislation is required to eliminate the waiver. As of Dec. 30, 2021, more than half of the members of the U.S. House of Representatives were listed as sponsors of the Mainstreaming Addiction Treatment (MAT) Act (HR 1384), which would end the need for X waivers. The bill has the backing of 187 Democrats and 43 Republicans.
At this time, too many physicians shy away from offering MAT, Dr. Hambright said.
“People are still scared of it,” she said. “People don’t want to deal with addicts.”
But Dr. Hambright said it’s well worth the initial time invested in having the needed conversations with patients about MAT.
“Afterwards, it’s so straightforward. People feel better. They’re healthier. It’s amazing,” she said. “You’re changing lives.”
The research was supported by grants from the National Institutes of Health. Dr. Stein and coauthors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The volume of prescription opioids dispensed at retail pharmacies in the United States dropped by 21% in recent years amid efforts to reduce unnecessary use of the painkillers, but the rate of decline varied greatly among types of patients and by type of clinician, a study found.
In a brief report published by Annals of Internal Medicine, researchers from the nonprofit RAND Corp reported an analysis of opioid prescriptions from two periods, 2008-2009 and 2017-2018.
The researchers sought to assess total opioid use rather than simply track the number of pills dispensed. So they used days’ supply and total daily dose to calculate per capita morphine milligram equivalents (MME) for opioid prescriptions, write Bradley D. Stein, MD, PhD, MPH, the study’s lead author and a senior physician researcher at RAND Corp, and his coauthors in their paper.
For the study, the researchers used data from the consulting firm IQVIA, which they say covers about 90% of U.S. prescriptions. Total opioid volume per capita by prescriptions filled in retail pharmacies decreased from 951.4 MME in 2008-2009 to 749.3 MME in 2017-2018, Dr. Stein’s group found.
(In 2020, IQVIA separately said that prescription opioid use per adult in this country rose from an average of 16 pills, or 134 MMEs, in 1992 to a peak of about 55 pills a person, or 790 MMEs, in 2011. By 2019, opioid use per adult had declined to 29 pills and 366 MMEs per capita.)
The RAND report found substantial variation in opioid volume by type of insurance, including a 41.5% decline (636.5 MME to 372.6 MME) among people covered by commercial health plans. That exceeded the 27.7% drop seen for people enrolled in Medicaid (646.8 MME to 467.7 MME). The decline was smaller (17.5%; 2,780.2 MME to 2,294.2 MME) for those on Medicare, who as a group used the most opioids.
‘Almost functions as a Rorschach test’
The causes of the decline are easy to guess, although definitive conclusions are impossible, Dr. Stein told this news organization.
Significant work has been done in recent years to change attitudes about opioid prescriptions by physicians, researchers, and lawmakers. Aggressive promotion of prescription painkillers, particularly Purdue Pharma’s OxyContin, in the 1990s, is widely cited as the triggering event for the national opioid crisis.
In response, states created databases known as prescription drug monitoring programs. The Centers for Disease Control and Prevention in 2016 issued guidelines intended to curb unnecessary use of opioids. The guidelines noted that other medicines could treat chronic pain without raising the risk of addiction. The Choosing Wisely campaign, run by a foundation of the American Board of Internal Medicine, also offered recommendations about limiting use of opioids. And insurers have restricted access to opioids through the prior authorization process. As a result, researchers will make their own guesses at the causes of the decline in opioid prescriptions, based on their own experiences and research interests, Dr. Stein said.
“It almost functions as a Rorschach test,” he said.
Dr. Stein’s group also looked at trends among medical specialties. They found the largest reduction between 2008-2009 and 2017-2018 among emergency physicians (70.5% drop from 99,254.5 MME to 29,234.3 MME), psychiatrists (67.2% drop from 50,464.3 MME to 16,533.0 MME) and oncologists (59.5% drop from 51,731.2 MME to 20,941.4).
Among surgeons, the RAND researchers found a drop of 49.3% from 220,764.6 to 111,904.4. Among dentists, they found a drop of 41.3% from 22,345.3 to 13,126.1.
Among pain specialists, they found a drop of 15.4% from 1,020,808.4 MME to 863,140.7 MME.
Among adult primary care clinicians, Dr. Stein and his colleagues found a drop of 40% from 651,489.4 MME in 2008-2009 to 390,841.0 MME in 2017-2018.
However, one of the groups tracked in the study increased the volume of opioid prescriptions written: advanced practice providers, among whom scripts for the drugs rose 22.7%, from 112,873.9 MME to 138,459.3 MME.
Dr. Stein said he suspects that this gain reflects a change in the nature of the practice of primary care, with nurse practitioners and physician assistants taking more active roles in treatment of patients. Some of the reduction seen among primary care clinicians who treat adults may reflect a shift in which medical personnel in a practice write the opioid prescriptions.
Still, the trends in general seen by Dr. Stein and coauthors are encouraging, even if further study of these patterns is needed, he said.
“This is one of those papers that I think potentially raises as many questions as it provides answers for,” he said.
What’s missing
Maya Hambright, MD, a family medicine physician in New York’s Hudson Valley, who has been working mainly in addiction in response to the opioid overdose crisis, observed that the drop in total prescribed volume of prescription painkillers does not necessarily translate into a reduction in use of opioids
“No one is taking fewer opioids,” Dr. Hambright told this news organization. “I can say that comfortably. They are just getting them from other sources.”
CDC data support Dr. Hambright’s view.
An estimated 100,306 people in the United States died of a drug overdose in the 12 months that ended in April 2021, an increase of 28.5% from the 78,056 deaths during the same period the year before, according to the CDC.
Dr. Hambright said more physicians need to be involved in prescribing medication-assisted treatment (MAT).
The federal government has in the past year loosened restrictions on a requirement, known as an X waiver. Certain clinicians have been exempted from training requirements, as explained in the frequently asked questions page on the Substance Abuse and Mental Health Services Administration website.
SAMHSA says legislation is required to eliminate the waiver. As of Dec. 30, 2021, more than half of the members of the U.S. House of Representatives were listed as sponsors of the Mainstreaming Addiction Treatment (MAT) Act (HR 1384), which would end the need for X waivers. The bill has the backing of 187 Democrats and 43 Republicans.
At this time, too many physicians shy away from offering MAT, Dr. Hambright said.
“People are still scared of it,” she said. “People don’t want to deal with addicts.”
But Dr. Hambright said it’s well worth the initial time invested in having the needed conversations with patients about MAT.
“Afterwards, it’s so straightforward. People feel better. They’re healthier. It’s amazing,” she said. “You’re changing lives.”
The research was supported by grants from the National Institutes of Health. Dr. Stein and coauthors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The volume of prescription opioids dispensed at retail pharmacies in the United States dropped by 21% in recent years amid efforts to reduce unnecessary use of the painkillers, but the rate of decline varied greatly among types of patients and by type of clinician, a study found.
In a brief report published by Annals of Internal Medicine, researchers from the nonprofit RAND Corp reported an analysis of opioid prescriptions from two periods, 2008-2009 and 2017-2018.
The researchers sought to assess total opioid use rather than simply track the number of pills dispensed. So they used days’ supply and total daily dose to calculate per capita morphine milligram equivalents (MME) for opioid prescriptions, write Bradley D. Stein, MD, PhD, MPH, the study’s lead author and a senior physician researcher at RAND Corp, and his coauthors in their paper.
For the study, the researchers used data from the consulting firm IQVIA, which they say covers about 90% of U.S. prescriptions. Total opioid volume per capita by prescriptions filled in retail pharmacies decreased from 951.4 MME in 2008-2009 to 749.3 MME in 2017-2018, Dr. Stein’s group found.
(In 2020, IQVIA separately said that prescription opioid use per adult in this country rose from an average of 16 pills, or 134 MMEs, in 1992 to a peak of about 55 pills a person, or 790 MMEs, in 2011. By 2019, opioid use per adult had declined to 29 pills and 366 MMEs per capita.)
The RAND report found substantial variation in opioid volume by type of insurance, including a 41.5% decline (636.5 MME to 372.6 MME) among people covered by commercial health plans. That exceeded the 27.7% drop seen for people enrolled in Medicaid (646.8 MME to 467.7 MME). The decline was smaller (17.5%; 2,780.2 MME to 2,294.2 MME) for those on Medicare, who as a group used the most opioids.
‘Almost functions as a Rorschach test’
The causes of the decline are easy to guess, although definitive conclusions are impossible, Dr. Stein told this news organization.
Significant work has been done in recent years to change attitudes about opioid prescriptions by physicians, researchers, and lawmakers. Aggressive promotion of prescription painkillers, particularly Purdue Pharma’s OxyContin, in the 1990s, is widely cited as the triggering event for the national opioid crisis.
In response, states created databases known as prescription drug monitoring programs. The Centers for Disease Control and Prevention in 2016 issued guidelines intended to curb unnecessary use of opioids. The guidelines noted that other medicines could treat chronic pain without raising the risk of addiction. The Choosing Wisely campaign, run by a foundation of the American Board of Internal Medicine, also offered recommendations about limiting use of opioids. And insurers have restricted access to opioids through the prior authorization process. As a result, researchers will make their own guesses at the causes of the decline in opioid prescriptions, based on their own experiences and research interests, Dr. Stein said.
“It almost functions as a Rorschach test,” he said.
Dr. Stein’s group also looked at trends among medical specialties. They found the largest reduction between 2008-2009 and 2017-2018 among emergency physicians (70.5% drop from 99,254.5 MME to 29,234.3 MME), psychiatrists (67.2% drop from 50,464.3 MME to 16,533.0 MME) and oncologists (59.5% drop from 51,731.2 MME to 20,941.4).
Among surgeons, the RAND researchers found a drop of 49.3% from 220,764.6 to 111,904.4. Among dentists, they found a drop of 41.3% from 22,345.3 to 13,126.1.
Among pain specialists, they found a drop of 15.4% from 1,020,808.4 MME to 863,140.7 MME.
Among adult primary care clinicians, Dr. Stein and his colleagues found a drop of 40% from 651,489.4 MME in 2008-2009 to 390,841.0 MME in 2017-2018.
However, one of the groups tracked in the study increased the volume of opioid prescriptions written: advanced practice providers, among whom scripts for the drugs rose 22.7%, from 112,873.9 MME to 138,459.3 MME.
Dr. Stein said he suspects that this gain reflects a change in the nature of the practice of primary care, with nurse practitioners and physician assistants taking more active roles in treatment of patients. Some of the reduction seen among primary care clinicians who treat adults may reflect a shift in which medical personnel in a practice write the opioid prescriptions.
Still, the trends in general seen by Dr. Stein and coauthors are encouraging, even if further study of these patterns is needed, he said.
“This is one of those papers that I think potentially raises as many questions as it provides answers for,” he said.
What’s missing
Maya Hambright, MD, a family medicine physician in New York’s Hudson Valley, who has been working mainly in addiction in response to the opioid overdose crisis, observed that the drop in total prescribed volume of prescription painkillers does not necessarily translate into a reduction in use of opioids
“No one is taking fewer opioids,” Dr. Hambright told this news organization. “I can say that comfortably. They are just getting them from other sources.”
CDC data support Dr. Hambright’s view.
An estimated 100,306 people in the United States died of a drug overdose in the 12 months that ended in April 2021, an increase of 28.5% from the 78,056 deaths during the same period the year before, according to the CDC.
Dr. Hambright said more physicians need to be involved in prescribing medication-assisted treatment (MAT).
The federal government has in the past year loosened restrictions on a requirement, known as an X waiver. Certain clinicians have been exempted from training requirements, as explained in the frequently asked questions page on the Substance Abuse and Mental Health Services Administration website.
SAMHSA says legislation is required to eliminate the waiver. As of Dec. 30, 2021, more than half of the members of the U.S. House of Representatives were listed as sponsors of the Mainstreaming Addiction Treatment (MAT) Act (HR 1384), which would end the need for X waivers. The bill has the backing of 187 Democrats and 43 Republicans.
At this time, too many physicians shy away from offering MAT, Dr. Hambright said.
“People are still scared of it,” she said. “People don’t want to deal with addicts.”
But Dr. Hambright said it’s well worth the initial time invested in having the needed conversations with patients about MAT.
“Afterwards, it’s so straightforward. People feel better. They’re healthier. It’s amazing,” she said. “You’re changing lives.”
The research was supported by grants from the National Institutes of Health. Dr. Stein and coauthors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.