User login
New AHA statement on managing ACS in older adults
Age-related changes in general and cardiovascular health likely require modifications in how acute coronary syndrome (ACS) is diagnosed and managed in adults aged 75 and older, the American Heart Association says in a new scientific statement.
The statement outlines a framework to integrate geriatric risks into the management of ACS, including the diagnostic approach, pharmacotherapy, revascularization strategies, prevention of adverse events, and transition care planning.
The 31-page statement was published online in the AHA journal Circulation (2022 Dec 12. doi: 10.1161/CIR.0000000000001112). It updates a 2007 AHA statement on treatment of ACS in the elderly.
Complex patient group
Adults aged 75 and older make up roughly 30%-40% of all hospitalized patients with ACS and the majority of ACS-related deaths occur in this group, the writing group notes.
“Older patients have more pronounced anatomical changes and more severe functional impairment, and they are more likely to have additional health conditions,” writing group chair Abdulla A. Damluji, MD, PhD, director of the Inova Center of Outcomes Research in Fairfax, Va., notes in a news release.
“These include frailty, other chronic disorders (treated with multiple medications), physical dysfunction, cognitive decline and/or urinary incontinence – and these are not regularly studied in the context of ACS,” Dr. Damluji explained.
The writing group notes that the presence of one or more geriatric syndromes may substantially affect ACS clinical presentation, clinical course and prognosis, therapeutic decision-making, and response to treatment.
“It is therefore fundamental that clinicians caring for older patients with ACS be alert to the presence of geriatric syndromes and be able to integrate them into the care plan when appropriate,” they say.
They recommend a holistic, individualized, and patient-centered approach to ACS care in the elderly, taking into consideration coexisting and overlapping health issues.
Considerations for clinical care
The AHA statement offers several “considerations for clinical practice” with regard to ACS diagnosis and management in elderly adults. They include:
- ACS presentations without chest pain, such as shortness of breath, syncope, or sudden confusion, are more common in older adults.
- Many older adults have persistent elevations in cardiac troponin levels from myocardial fibrosis and kidney disease that diminish the positive predictive value of high-sensitivity cardiac troponin (hs-cTn) assays for identifying acute and chronic myocardial injury. For this reason, evaluating patterns of rise and fall is essential.
- Age-related changes in metabolism, weight, and muscle mass may require different choices in anticoagulant medications to lower bleeding risk.
- Clopidogrel (Plavix) is the preferred P2Y12 inhibitor because of a significantly lower bleeding profile than ticagrelor (Brilinta) or prasugrel (Effient). For patients with ST-segment myocardial infarction (STEMI) or complex anatomy, the use of ticagrelor is “reasonable.”
- Poor kidney function can increase the risk for contrast-induced acute kidney injury.
- Although the risks are greater, percutaneous coronary intervention or bypass surgery are beneficial in select older adults with ACS.
- Post-MI care should include cardiac rehabilitation tailored to address each patient’s circumstances and personal goals of care.
- For patients with cognitive difficulties and limited mobility, consider simplified medication plans with fewer doses per day and 90-day supplies to prevent the need for frequent refills.
- Patient care plans should be individualized, with input from a multidisciplinary team that may include cardiologists, surgeons, geriatricians, primary care clinicians, nutritionists, social workers, and family members.
- Determine a priori goals of care in older patients to help avoid an unwanted or futile intervention.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Cardiovascular Diseases in Older Populations Committee of the Council on Clinical Cardiology; the Council on Cardiovascular and Stroke Nursing; the Council on Cardiovascular Radiology and Intervention; and the Council on Lifestyle and Cardiometabolic Health.
A version of this article first appeared on Medscape.com.
Age-related changes in general and cardiovascular health likely require modifications in how acute coronary syndrome (ACS) is diagnosed and managed in adults aged 75 and older, the American Heart Association says in a new scientific statement.
The statement outlines a framework to integrate geriatric risks into the management of ACS, including the diagnostic approach, pharmacotherapy, revascularization strategies, prevention of adverse events, and transition care planning.
The 31-page statement was published online in the AHA journal Circulation (2022 Dec 12. doi: 10.1161/CIR.0000000000001112). It updates a 2007 AHA statement on treatment of ACS in the elderly.
Complex patient group
Adults aged 75 and older make up roughly 30%-40% of all hospitalized patients with ACS and the majority of ACS-related deaths occur in this group, the writing group notes.
“Older patients have more pronounced anatomical changes and more severe functional impairment, and they are more likely to have additional health conditions,” writing group chair Abdulla A. Damluji, MD, PhD, director of the Inova Center of Outcomes Research in Fairfax, Va., notes in a news release.
“These include frailty, other chronic disorders (treated with multiple medications), physical dysfunction, cognitive decline and/or urinary incontinence – and these are not regularly studied in the context of ACS,” Dr. Damluji explained.
The writing group notes that the presence of one or more geriatric syndromes may substantially affect ACS clinical presentation, clinical course and prognosis, therapeutic decision-making, and response to treatment.
“It is therefore fundamental that clinicians caring for older patients with ACS be alert to the presence of geriatric syndromes and be able to integrate them into the care plan when appropriate,” they say.
They recommend a holistic, individualized, and patient-centered approach to ACS care in the elderly, taking into consideration coexisting and overlapping health issues.
Considerations for clinical care
The AHA statement offers several “considerations for clinical practice” with regard to ACS diagnosis and management in elderly adults. They include:
- ACS presentations without chest pain, such as shortness of breath, syncope, or sudden confusion, are more common in older adults.
- Many older adults have persistent elevations in cardiac troponin levels from myocardial fibrosis and kidney disease that diminish the positive predictive value of high-sensitivity cardiac troponin (hs-cTn) assays for identifying acute and chronic myocardial injury. For this reason, evaluating patterns of rise and fall is essential.
- Age-related changes in metabolism, weight, and muscle mass may require different choices in anticoagulant medications to lower bleeding risk.
- Clopidogrel (Plavix) is the preferred P2Y12 inhibitor because of a significantly lower bleeding profile than ticagrelor (Brilinta) or prasugrel (Effient). For patients with ST-segment myocardial infarction (STEMI) or complex anatomy, the use of ticagrelor is “reasonable.”
- Poor kidney function can increase the risk for contrast-induced acute kidney injury.
- Although the risks are greater, percutaneous coronary intervention or bypass surgery are beneficial in select older adults with ACS.
- Post-MI care should include cardiac rehabilitation tailored to address each patient’s circumstances and personal goals of care.
- For patients with cognitive difficulties and limited mobility, consider simplified medication plans with fewer doses per day and 90-day supplies to prevent the need for frequent refills.
- Patient care plans should be individualized, with input from a multidisciplinary team that may include cardiologists, surgeons, geriatricians, primary care clinicians, nutritionists, social workers, and family members.
- Determine a priori goals of care in older patients to help avoid an unwanted or futile intervention.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Cardiovascular Diseases in Older Populations Committee of the Council on Clinical Cardiology; the Council on Cardiovascular and Stroke Nursing; the Council on Cardiovascular Radiology and Intervention; and the Council on Lifestyle and Cardiometabolic Health.
A version of this article first appeared on Medscape.com.
Age-related changes in general and cardiovascular health likely require modifications in how acute coronary syndrome (ACS) is diagnosed and managed in adults aged 75 and older, the American Heart Association says in a new scientific statement.
The statement outlines a framework to integrate geriatric risks into the management of ACS, including the diagnostic approach, pharmacotherapy, revascularization strategies, prevention of adverse events, and transition care planning.
The 31-page statement was published online in the AHA journal Circulation (2022 Dec 12. doi: 10.1161/CIR.0000000000001112). It updates a 2007 AHA statement on treatment of ACS in the elderly.
Complex patient group
Adults aged 75 and older make up roughly 30%-40% of all hospitalized patients with ACS and the majority of ACS-related deaths occur in this group, the writing group notes.
“Older patients have more pronounced anatomical changes and more severe functional impairment, and they are more likely to have additional health conditions,” writing group chair Abdulla A. Damluji, MD, PhD, director of the Inova Center of Outcomes Research in Fairfax, Va., notes in a news release.
“These include frailty, other chronic disorders (treated with multiple medications), physical dysfunction, cognitive decline and/or urinary incontinence – and these are not regularly studied in the context of ACS,” Dr. Damluji explained.
The writing group notes that the presence of one or more geriatric syndromes may substantially affect ACS clinical presentation, clinical course and prognosis, therapeutic decision-making, and response to treatment.
“It is therefore fundamental that clinicians caring for older patients with ACS be alert to the presence of geriatric syndromes and be able to integrate them into the care plan when appropriate,” they say.
They recommend a holistic, individualized, and patient-centered approach to ACS care in the elderly, taking into consideration coexisting and overlapping health issues.
Considerations for clinical care
The AHA statement offers several “considerations for clinical practice” with regard to ACS diagnosis and management in elderly adults. They include:
- ACS presentations without chest pain, such as shortness of breath, syncope, or sudden confusion, are more common in older adults.
- Many older adults have persistent elevations in cardiac troponin levels from myocardial fibrosis and kidney disease that diminish the positive predictive value of high-sensitivity cardiac troponin (hs-cTn) assays for identifying acute and chronic myocardial injury. For this reason, evaluating patterns of rise and fall is essential.
- Age-related changes in metabolism, weight, and muscle mass may require different choices in anticoagulant medications to lower bleeding risk.
- Clopidogrel (Plavix) is the preferred P2Y12 inhibitor because of a significantly lower bleeding profile than ticagrelor (Brilinta) or prasugrel (Effient). For patients with ST-segment myocardial infarction (STEMI) or complex anatomy, the use of ticagrelor is “reasonable.”
- Poor kidney function can increase the risk for contrast-induced acute kidney injury.
- Although the risks are greater, percutaneous coronary intervention or bypass surgery are beneficial in select older adults with ACS.
- Post-MI care should include cardiac rehabilitation tailored to address each patient’s circumstances and personal goals of care.
- For patients with cognitive difficulties and limited mobility, consider simplified medication plans with fewer doses per day and 90-day supplies to prevent the need for frequent refills.
- Patient care plans should be individualized, with input from a multidisciplinary team that may include cardiologists, surgeons, geriatricians, primary care clinicians, nutritionists, social workers, and family members.
- Determine a priori goals of care in older patients to help avoid an unwanted or futile intervention.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Cardiovascular Diseases in Older Populations Committee of the Council on Clinical Cardiology; the Council on Cardiovascular and Stroke Nursing; the Council on Cardiovascular Radiology and Intervention; and the Council on Lifestyle and Cardiometabolic Health.
A version of this article first appeared on Medscape.com.
Consider this SGLT2 inhibitor for patients with HF with preserved ejection fraction
ILLUSTRATIVE CASE
A 72-year-old man with a history of hypertension, permanent atrial fibrillation, and heart failure (HF) comes into your clinic for follow-up. He was hospitalized a few months ago for HF requiring diuresis. His echocardiogram at that time showed an EF of 50% and no significant valvular disease. He does not have a history of diabetes or tobacco use. His medication regimen includes metoprolol,
HFpEF was first defined as HF in patients with a left ventricular ejection fraction (LVEF) > 40%. However, HF with an LVEF between 41% and 49% has been reclassified as its own category:
In comparison with HF with reduced ejection fraction (HFrEF), there are limited proven treatment options with cardiovascular (CV) benefit in HFpEF.
STUDY SUMMARY
Confirmation of benefit of empagliflozin for patients with HFpEF
The EMPEROR-Preserved study was a double-blind, placebo-controlled trial that randomized adult patients with HFpEF (defined by an LVEF > 40%) to either placebo or empagliflozin 10 mg/d, in addition to usual therapy. Patients were randomized in a 1:1 ratio stratified by geographic region, diabetes status, renal function (estimated glomerular filtration rate [eGFR] either < 60 or ≥ 60 mL/min/1.73 m2), and LVEF > 40% to < 50% or LVEF ≥ 50%.
Included patients were 18 years or older and had an NT-proBNP level > 300 pg/mL (or > 900 pg/mL if the patient had atrial fibrillation at baseline
The primary outcome was a composite of CV death or first hospitalization for HF. The secondary outcomes were all hospitalizations for HF and the rate of decline in eGFR.
Of the 5988 patients in the trial, 2997 were randomized to receive empagliflozin and 2991 were randomized to placebo. The average age was 72 years in each group, 45% of patients were women, about 76% were White, and 12% were from North America. About 81% of patients were classified as NYHA class II, nearly half had diabetes, and half had an eGFR < 60 mL/min/1.73 m2. The median body mass index (BMI) was 30, and the median LVEF was 54%. At baseline, the groups were similar in BMI, history of HF hospitalization in the past 12 months, history of common risk factors for HFpEF (atrial fibrillation, diabetes, and hypertension), and prescribed CV medications (ACE inhibitor or ARB with or without a neprilysin inhibitor, spironolactone, beta-blocker, digitalis glycosides, aspirin, and statins). Patients were followed for a median of 26.2 months.
Continue to: The primary composite...
The primary composite outcome of death from CV causes or HF-related hospitalization occurred in 415 patients (13.8%) in the empagliflozin group and in 511 patients (17.1%) in the placebo group (hazard ratio [HR] = 0.79; 95% CI, 0.69-0.90; P < .001). The number needed to treat to prevent 1 primary outcome event was 31 (95% CI, 20-69). Hospitalization for HF occurred in 259 patients (8.6%) with empagliflozin vs 352 patients (11.8%) with placebo (HR = 0.71; 95% CI, 0.60-0.83), and CV death occurred in 219 patients (7.3%) with empagliflozin vs 244 patients (8.2%) with placebo (HR = 0.91; 95% CI, 0.76-1.09). The effect was consistent in patients with or without diabetes at baseline; however, the largest reduction in the primary composite outcome was seen in those with an LVEF < 50%, age ≥ 70 years old, BMI < 30, and NYHA class II status.
The secondary outcome of total number of hospitalizations for HF was 407 with empagliflozin vs 541 with placebo (HR = 0.73; 95% CI, 0.61-0.88; P < .001). The rate of decline in the eGFR per year was –1.25 in the empagliflozin group vs –2.62 in the placebo group (P < .001), indicating that those taking empagliflozin had preserved renal function compared with those taking placebo.
Death from any cause occurred in 422 patients (14.1%) in the empagliflozin group and 427 patients (14.3%) in the placebo group (HR = 1.00; 95% CI, 0.87-1.15). Empagliflozin treatment was associated with higher rates of genital infections (2.2% vs 0.7%; P value not provided), urinary tract infections (9.9% vs 8.1%; P value not provided), and hypotension (10.4% vs 8.6%; P value not provided), compared to placebo.
WHAT’S NEW
Risk of hospitalization significantly reduced for patients with HFpEF
In the EMPEROR-Preserved study, empagliflozin led to a lower incidence of hospitalization for HF in patients with HFpEF but did not significantly reduce the number of deaths from CV disease or other causes. In comparison, in the similarly designed EMPEROR-Reduced trial, treatment with empagliflozin reduced CV and all-cause mortality in individuals with HFrEF.8
CAVEATS
HF criteria, study population may limit generalizability
The reduction in the primary outcome of CV death or first hospitalization was most pronounced in patients with an LVEF > 40% to < 50%, typically defined as HFmrEF, who often have clinical features similar to those with HFrEF. This raises the question of how generalizable these results are for all patients with HFpEF.
Continue to: The study's generalizability...
The study’s generalizability was further limited by its significant exclusion criteria, which included elevated blood pressure, chronic obstructive pulmonary disease on home oxygen, liver disease, renal disease with an eGFR < 20 mL/min/1.73 m2 or requiring dialysis, and BMI ≥ 45.
Finally, only 12% of patients were from North America, and results were not significant for this subgroup (HR = 0.72; 95% CI, 0.52-1.00), which may challenge its external validity. The authors noted that 23% of patients discontinued treatment for reasons other than death, which may have driven the null effect.
CHALLENGES TO IMPLEMENTATION
Empagliflozin is expensive,but coverage may improve
Cost could be a major barrier to implementation. Retail pricing for empagliflozin is estimated to be more than $550 per month, which may be prohibitive for patients with no insurance or with higher-deductible plans.11 However, the US Food and Drug Administration has approved empagliflozin to reduce the risk of CV death and hospitalization for HF in adults,12 which may help to improve insurance coverage.
1. Anker SD, Butler J, Filippatos G, et al; EMPEROR-Preserved Trial Investigators. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451-1461. doi: 10.1056/NEJMoa2107038
2. Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e895-e1032. doi: 10.1161/CIR.0000000000001063
3. Gevaert AB, Kataria R, Zannad F, et al. Heart failure with preserved ejection fraction: recent concepts in diagnosis, mechanisms and management. Heart. 2022;108:1342-1350. doi: 10.1136/heartjnl-2021-319605
4. Vaduganathan M, Claggett BL, Jhund PS, et al. Estimating lifetime benefits of comprehensive disease-modifying pharmacological therapies in patients with heart failure with reduced ejection fraction: a comparative analysis of three randomised controlled trials. Lancet. 2020;396:121-128. doi: 10.1016/S0140-6736(20)30748-0
5. Solomon SD, Claggett B, Lewis EF, et al; TOPCAT Investigators. Influence of ejection fraction on outcomes and efficacy of spironolactone in patients with heart failure with preserved ejection fraction. Eur Heart J. 2016;37:455-462. doi: 10.1093/eurheartj/ehv464
6. Martin N, Manoharan K, Thomas J, et al. Beta-blockers and inhibitors of the renin-angiotensin aldosterone system for chronic heart failure with preserved ejection fraction. Cochrane Database Syst Rev. 2018;6:CD012721. doi: 10.1002/14651858.CD012721.pub2
7. Solomon SD, McMurray JJV, Anand IS, et al; PARAGON-HF Investigators and Committees. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381:1609-1620. doi: 10.1056/NEJMoa1908655
8. Zannad F, Ferreira JP, Pocock SJ, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet. 2020;396:819-829. doi: 10.1016/S0140-6736(20)31824-9
9. Kato ET, Silverman MG, Mosenzon O, et al. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation. 2019;139:2528-2536. doi: 10.1161/CIRCULATIONAHA. 119.040130
10. Bhatt DL, Szarek M, Steg PG, et al; SOLOIST-WHF Trial Investigators. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2021;384:117-128. doi: 10.1056/NEJM oa2030183
11. Empagliflozin. GoodRx.com. Accessed June 3, 2022. www.goodrx.com/empagliflozin
12. FDA approves treatment for wider range of patients with heart failure. News release. US Food and Drug Administration; February 24, 2022. Accessed June 3, 2022. www.fda.gov/news-events/press-announcements/fda-approves-treatment-wider-range-patients-heart-failure
ILLUSTRATIVE CASE
A 72-year-old man with a history of hypertension, permanent atrial fibrillation, and heart failure (HF) comes into your clinic for follow-up. He was hospitalized a few months ago for HF requiring diuresis. His echocardiogram at that time showed an EF of 50% and no significant valvular disease. He does not have a history of diabetes or tobacco use. His medication regimen includes metoprolol,
HFpEF was first defined as HF in patients with a left ventricular ejection fraction (LVEF) > 40%. However, HF with an LVEF between 41% and 49% has been reclassified as its own category:
In comparison with HF with reduced ejection fraction (HFrEF), there are limited proven treatment options with cardiovascular (CV) benefit in HFpEF.
STUDY SUMMARY
Confirmation of benefit of empagliflozin for patients with HFpEF
The EMPEROR-Preserved study was a double-blind, placebo-controlled trial that randomized adult patients with HFpEF (defined by an LVEF > 40%) to either placebo or empagliflozin 10 mg/d, in addition to usual therapy. Patients were randomized in a 1:1 ratio stratified by geographic region, diabetes status, renal function (estimated glomerular filtration rate [eGFR] either < 60 or ≥ 60 mL/min/1.73 m2), and LVEF > 40% to < 50% or LVEF ≥ 50%.
Included patients were 18 years or older and had an NT-proBNP level > 300 pg/mL (or > 900 pg/mL if the patient had atrial fibrillation at baseline
The primary outcome was a composite of CV death or first hospitalization for HF. The secondary outcomes were all hospitalizations for HF and the rate of decline in eGFR.
Of the 5988 patients in the trial, 2997 were randomized to receive empagliflozin and 2991 were randomized to placebo. The average age was 72 years in each group, 45% of patients were women, about 76% were White, and 12% were from North America. About 81% of patients were classified as NYHA class II, nearly half had diabetes, and half had an eGFR < 60 mL/min/1.73 m2. The median body mass index (BMI) was 30, and the median LVEF was 54%. At baseline, the groups were similar in BMI, history of HF hospitalization in the past 12 months, history of common risk factors for HFpEF (atrial fibrillation, diabetes, and hypertension), and prescribed CV medications (ACE inhibitor or ARB with or without a neprilysin inhibitor, spironolactone, beta-blocker, digitalis glycosides, aspirin, and statins). Patients were followed for a median of 26.2 months.
Continue to: The primary composite...
The primary composite outcome of death from CV causes or HF-related hospitalization occurred in 415 patients (13.8%) in the empagliflozin group and in 511 patients (17.1%) in the placebo group (hazard ratio [HR] = 0.79; 95% CI, 0.69-0.90; P < .001). The number needed to treat to prevent 1 primary outcome event was 31 (95% CI, 20-69). Hospitalization for HF occurred in 259 patients (8.6%) with empagliflozin vs 352 patients (11.8%) with placebo (HR = 0.71; 95% CI, 0.60-0.83), and CV death occurred in 219 patients (7.3%) with empagliflozin vs 244 patients (8.2%) with placebo (HR = 0.91; 95% CI, 0.76-1.09). The effect was consistent in patients with or without diabetes at baseline; however, the largest reduction in the primary composite outcome was seen in those with an LVEF < 50%, age ≥ 70 years old, BMI < 30, and NYHA class II status.
The secondary outcome of total number of hospitalizations for HF was 407 with empagliflozin vs 541 with placebo (HR = 0.73; 95% CI, 0.61-0.88; P < .001). The rate of decline in the eGFR per year was –1.25 in the empagliflozin group vs –2.62 in the placebo group (P < .001), indicating that those taking empagliflozin had preserved renal function compared with those taking placebo.
Death from any cause occurred in 422 patients (14.1%) in the empagliflozin group and 427 patients (14.3%) in the placebo group (HR = 1.00; 95% CI, 0.87-1.15). Empagliflozin treatment was associated with higher rates of genital infections (2.2% vs 0.7%; P value not provided), urinary tract infections (9.9% vs 8.1%; P value not provided), and hypotension (10.4% vs 8.6%; P value not provided), compared to placebo.
WHAT’S NEW
Risk of hospitalization significantly reduced for patients with HFpEF
In the EMPEROR-Preserved study, empagliflozin led to a lower incidence of hospitalization for HF in patients with HFpEF but did not significantly reduce the number of deaths from CV disease or other causes. In comparison, in the similarly designed EMPEROR-Reduced trial, treatment with empagliflozin reduced CV and all-cause mortality in individuals with HFrEF.8
CAVEATS
HF criteria, study population may limit generalizability
The reduction in the primary outcome of CV death or first hospitalization was most pronounced in patients with an LVEF > 40% to < 50%, typically defined as HFmrEF, who often have clinical features similar to those with HFrEF. This raises the question of how generalizable these results are for all patients with HFpEF.
Continue to: The study's generalizability...
The study’s generalizability was further limited by its significant exclusion criteria, which included elevated blood pressure, chronic obstructive pulmonary disease on home oxygen, liver disease, renal disease with an eGFR < 20 mL/min/1.73 m2 or requiring dialysis, and BMI ≥ 45.
Finally, only 12% of patients were from North America, and results were not significant for this subgroup (HR = 0.72; 95% CI, 0.52-1.00), which may challenge its external validity. The authors noted that 23% of patients discontinued treatment for reasons other than death, which may have driven the null effect.
CHALLENGES TO IMPLEMENTATION
Empagliflozin is expensive,but coverage may improve
Cost could be a major barrier to implementation. Retail pricing for empagliflozin is estimated to be more than $550 per month, which may be prohibitive for patients with no insurance or with higher-deductible plans.11 However, the US Food and Drug Administration has approved empagliflozin to reduce the risk of CV death and hospitalization for HF in adults,12 which may help to improve insurance coverage.
ILLUSTRATIVE CASE
A 72-year-old man with a history of hypertension, permanent atrial fibrillation, and heart failure (HF) comes into your clinic for follow-up. He was hospitalized a few months ago for HF requiring diuresis. His echocardiogram at that time showed an EF of 50% and no significant valvular disease. He does not have a history of diabetes or tobacco use. His medication regimen includes metoprolol,
HFpEF was first defined as HF in patients with a left ventricular ejection fraction (LVEF) > 40%. However, HF with an LVEF between 41% and 49% has been reclassified as its own category:
In comparison with HF with reduced ejection fraction (HFrEF), there are limited proven treatment options with cardiovascular (CV) benefit in HFpEF.
STUDY SUMMARY
Confirmation of benefit of empagliflozin for patients with HFpEF
The EMPEROR-Preserved study was a double-blind, placebo-controlled trial that randomized adult patients with HFpEF (defined by an LVEF > 40%) to either placebo or empagliflozin 10 mg/d, in addition to usual therapy. Patients were randomized in a 1:1 ratio stratified by geographic region, diabetes status, renal function (estimated glomerular filtration rate [eGFR] either < 60 or ≥ 60 mL/min/1.73 m2), and LVEF > 40% to < 50% or LVEF ≥ 50%.
Included patients were 18 years or older and had an NT-proBNP level > 300 pg/mL (or > 900 pg/mL if the patient had atrial fibrillation at baseline
The primary outcome was a composite of CV death or first hospitalization for HF. The secondary outcomes were all hospitalizations for HF and the rate of decline in eGFR.
Of the 5988 patients in the trial, 2997 were randomized to receive empagliflozin and 2991 were randomized to placebo. The average age was 72 years in each group, 45% of patients were women, about 76% were White, and 12% were from North America. About 81% of patients were classified as NYHA class II, nearly half had diabetes, and half had an eGFR < 60 mL/min/1.73 m2. The median body mass index (BMI) was 30, and the median LVEF was 54%. At baseline, the groups were similar in BMI, history of HF hospitalization in the past 12 months, history of common risk factors for HFpEF (atrial fibrillation, diabetes, and hypertension), and prescribed CV medications (ACE inhibitor or ARB with or without a neprilysin inhibitor, spironolactone, beta-blocker, digitalis glycosides, aspirin, and statins). Patients were followed for a median of 26.2 months.
Continue to: The primary composite...
The primary composite outcome of death from CV causes or HF-related hospitalization occurred in 415 patients (13.8%) in the empagliflozin group and in 511 patients (17.1%) in the placebo group (hazard ratio [HR] = 0.79; 95% CI, 0.69-0.90; P < .001). The number needed to treat to prevent 1 primary outcome event was 31 (95% CI, 20-69). Hospitalization for HF occurred in 259 patients (8.6%) with empagliflozin vs 352 patients (11.8%) with placebo (HR = 0.71; 95% CI, 0.60-0.83), and CV death occurred in 219 patients (7.3%) with empagliflozin vs 244 patients (8.2%) with placebo (HR = 0.91; 95% CI, 0.76-1.09). The effect was consistent in patients with or without diabetes at baseline; however, the largest reduction in the primary composite outcome was seen in those with an LVEF < 50%, age ≥ 70 years old, BMI < 30, and NYHA class II status.
The secondary outcome of total number of hospitalizations for HF was 407 with empagliflozin vs 541 with placebo (HR = 0.73; 95% CI, 0.61-0.88; P < .001). The rate of decline in the eGFR per year was –1.25 in the empagliflozin group vs –2.62 in the placebo group (P < .001), indicating that those taking empagliflozin had preserved renal function compared with those taking placebo.
Death from any cause occurred in 422 patients (14.1%) in the empagliflozin group and 427 patients (14.3%) in the placebo group (HR = 1.00; 95% CI, 0.87-1.15). Empagliflozin treatment was associated with higher rates of genital infections (2.2% vs 0.7%; P value not provided), urinary tract infections (9.9% vs 8.1%; P value not provided), and hypotension (10.4% vs 8.6%; P value not provided), compared to placebo.
WHAT’S NEW
Risk of hospitalization significantly reduced for patients with HFpEF
In the EMPEROR-Preserved study, empagliflozin led to a lower incidence of hospitalization for HF in patients with HFpEF but did not significantly reduce the number of deaths from CV disease or other causes. In comparison, in the similarly designed EMPEROR-Reduced trial, treatment with empagliflozin reduced CV and all-cause mortality in individuals with HFrEF.8
CAVEATS
HF criteria, study population may limit generalizability
The reduction in the primary outcome of CV death or first hospitalization was most pronounced in patients with an LVEF > 40% to < 50%, typically defined as HFmrEF, who often have clinical features similar to those with HFrEF. This raises the question of how generalizable these results are for all patients with HFpEF.
Continue to: The study's generalizability...
The study’s generalizability was further limited by its significant exclusion criteria, which included elevated blood pressure, chronic obstructive pulmonary disease on home oxygen, liver disease, renal disease with an eGFR < 20 mL/min/1.73 m2 or requiring dialysis, and BMI ≥ 45.
Finally, only 12% of patients were from North America, and results were not significant for this subgroup (HR = 0.72; 95% CI, 0.52-1.00), which may challenge its external validity. The authors noted that 23% of patients discontinued treatment for reasons other than death, which may have driven the null effect.
CHALLENGES TO IMPLEMENTATION
Empagliflozin is expensive,but coverage may improve
Cost could be a major barrier to implementation. Retail pricing for empagliflozin is estimated to be more than $550 per month, which may be prohibitive for patients with no insurance or with higher-deductible plans.11 However, the US Food and Drug Administration has approved empagliflozin to reduce the risk of CV death and hospitalization for HF in adults,12 which may help to improve insurance coverage.
1. Anker SD, Butler J, Filippatos G, et al; EMPEROR-Preserved Trial Investigators. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451-1461. doi: 10.1056/NEJMoa2107038
2. Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e895-e1032. doi: 10.1161/CIR.0000000000001063
3. Gevaert AB, Kataria R, Zannad F, et al. Heart failure with preserved ejection fraction: recent concepts in diagnosis, mechanisms and management. Heart. 2022;108:1342-1350. doi: 10.1136/heartjnl-2021-319605
4. Vaduganathan M, Claggett BL, Jhund PS, et al. Estimating lifetime benefits of comprehensive disease-modifying pharmacological therapies in patients with heart failure with reduced ejection fraction: a comparative analysis of three randomised controlled trials. Lancet. 2020;396:121-128. doi: 10.1016/S0140-6736(20)30748-0
5. Solomon SD, Claggett B, Lewis EF, et al; TOPCAT Investigators. Influence of ejection fraction on outcomes and efficacy of spironolactone in patients with heart failure with preserved ejection fraction. Eur Heart J. 2016;37:455-462. doi: 10.1093/eurheartj/ehv464
6. Martin N, Manoharan K, Thomas J, et al. Beta-blockers and inhibitors of the renin-angiotensin aldosterone system for chronic heart failure with preserved ejection fraction. Cochrane Database Syst Rev. 2018;6:CD012721. doi: 10.1002/14651858.CD012721.pub2
7. Solomon SD, McMurray JJV, Anand IS, et al; PARAGON-HF Investigators and Committees. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381:1609-1620. doi: 10.1056/NEJMoa1908655
8. Zannad F, Ferreira JP, Pocock SJ, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet. 2020;396:819-829. doi: 10.1016/S0140-6736(20)31824-9
9. Kato ET, Silverman MG, Mosenzon O, et al. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation. 2019;139:2528-2536. doi: 10.1161/CIRCULATIONAHA. 119.040130
10. Bhatt DL, Szarek M, Steg PG, et al; SOLOIST-WHF Trial Investigators. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2021;384:117-128. doi: 10.1056/NEJM oa2030183
11. Empagliflozin. GoodRx.com. Accessed June 3, 2022. www.goodrx.com/empagliflozin
12. FDA approves treatment for wider range of patients with heart failure. News release. US Food and Drug Administration; February 24, 2022. Accessed June 3, 2022. www.fda.gov/news-events/press-announcements/fda-approves-treatment-wider-range-patients-heart-failure
1. Anker SD, Butler J, Filippatos G, et al; EMPEROR-Preserved Trial Investigators. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451-1461. doi: 10.1056/NEJMoa2107038
2. Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e895-e1032. doi: 10.1161/CIR.0000000000001063
3. Gevaert AB, Kataria R, Zannad F, et al. Heart failure with preserved ejection fraction: recent concepts in diagnosis, mechanisms and management. Heart. 2022;108:1342-1350. doi: 10.1136/heartjnl-2021-319605
4. Vaduganathan M, Claggett BL, Jhund PS, et al. Estimating lifetime benefits of comprehensive disease-modifying pharmacological therapies in patients with heart failure with reduced ejection fraction: a comparative analysis of three randomised controlled trials. Lancet. 2020;396:121-128. doi: 10.1016/S0140-6736(20)30748-0
5. Solomon SD, Claggett B, Lewis EF, et al; TOPCAT Investigators. Influence of ejection fraction on outcomes and efficacy of spironolactone in patients with heart failure with preserved ejection fraction. Eur Heart J. 2016;37:455-462. doi: 10.1093/eurheartj/ehv464
6. Martin N, Manoharan K, Thomas J, et al. Beta-blockers and inhibitors of the renin-angiotensin aldosterone system for chronic heart failure with preserved ejection fraction. Cochrane Database Syst Rev. 2018;6:CD012721. doi: 10.1002/14651858.CD012721.pub2
7. Solomon SD, McMurray JJV, Anand IS, et al; PARAGON-HF Investigators and Committees. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381:1609-1620. doi: 10.1056/NEJMoa1908655
8. Zannad F, Ferreira JP, Pocock SJ, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet. 2020;396:819-829. doi: 10.1016/S0140-6736(20)31824-9
9. Kato ET, Silverman MG, Mosenzon O, et al. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation. 2019;139:2528-2536. doi: 10.1161/CIRCULATIONAHA. 119.040130
10. Bhatt DL, Szarek M, Steg PG, et al; SOLOIST-WHF Trial Investigators. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2021;384:117-128. doi: 10.1056/NEJM oa2030183
11. Empagliflozin. GoodRx.com. Accessed June 3, 2022. www.goodrx.com/empagliflozin
12. FDA approves treatment for wider range of patients with heart failure. News release. US Food and Drug Administration; February 24, 2022. Accessed June 3, 2022. www.fda.gov/news-events/press-announcements/fda-approves-treatment-wider-range-patients-heart-failure
PRACTICE CHANGER
Consider adding empagliflozin 10 mg to usual therapy to reduce hospitalization of symptomatic patients with
STRENGTH OF RECOMMENDATION
B: Based on a single, good-quality, multicenter, randomized controlled trial.1
Anker SD, Butler J, Filippatos G, et al; EMPEROR-Preserved Trial Investigators. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451-1461. doi: 10.1056/NEJM oa2107038
40-year-old woman • fever • rash • arthralgia • Dx?
THE CASE
A 40-year-old woman with no significant medical history sought care at the emergency department for a fever, rash, and arthralgia. On admission, she had worsening bilateral ankle pain and was having difficulty walking. During the previous 3 months, she’d had 3 episodes of tonsillitis, all of which were presumed to be caused by Streptococcus, although no swabs were obtained. Her primary care physician treated her with antibiotics each time: 1 round of amoxicillin 500 mg twice daily for 10 days and 2 rounds of amoxicillin/clavulanate 875 mg twice daily for 7 to 10 days. During the previous month, she’d experienced intermittent fevers ranging from 100.2 °F to 100.8
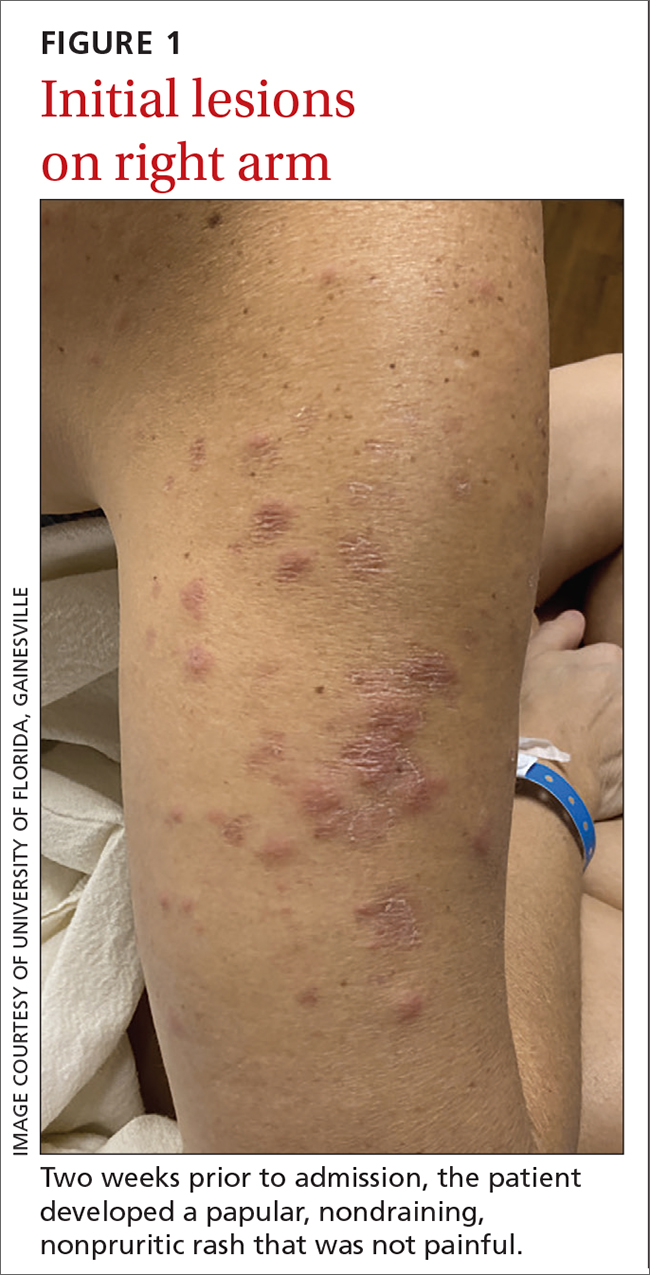
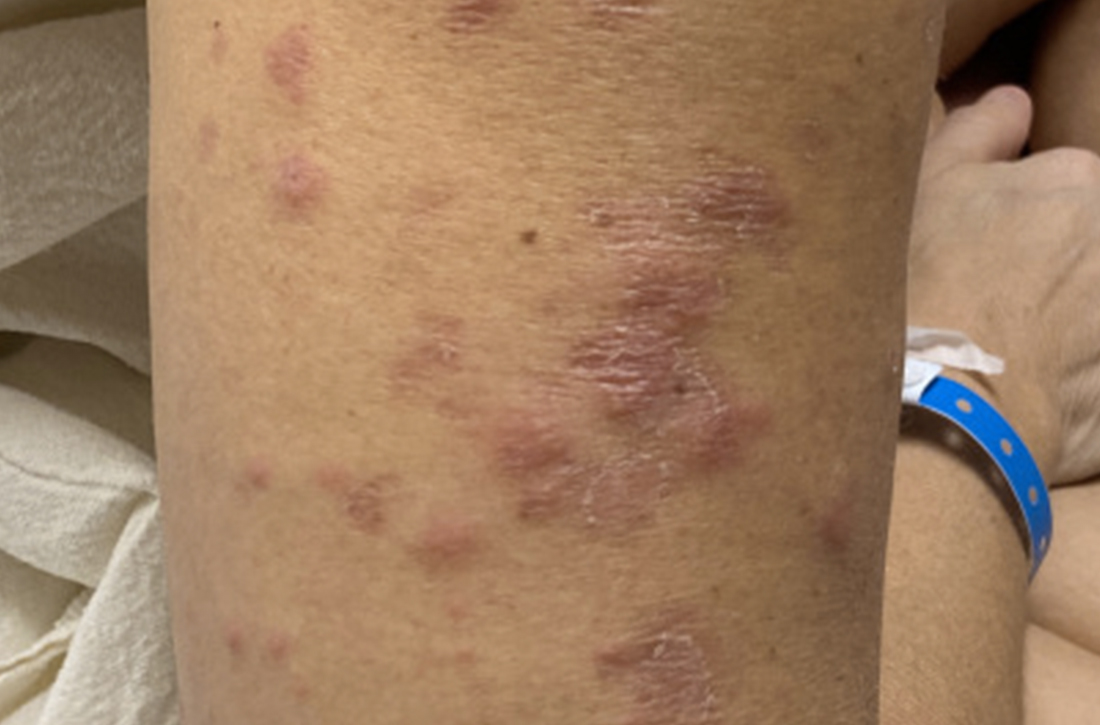
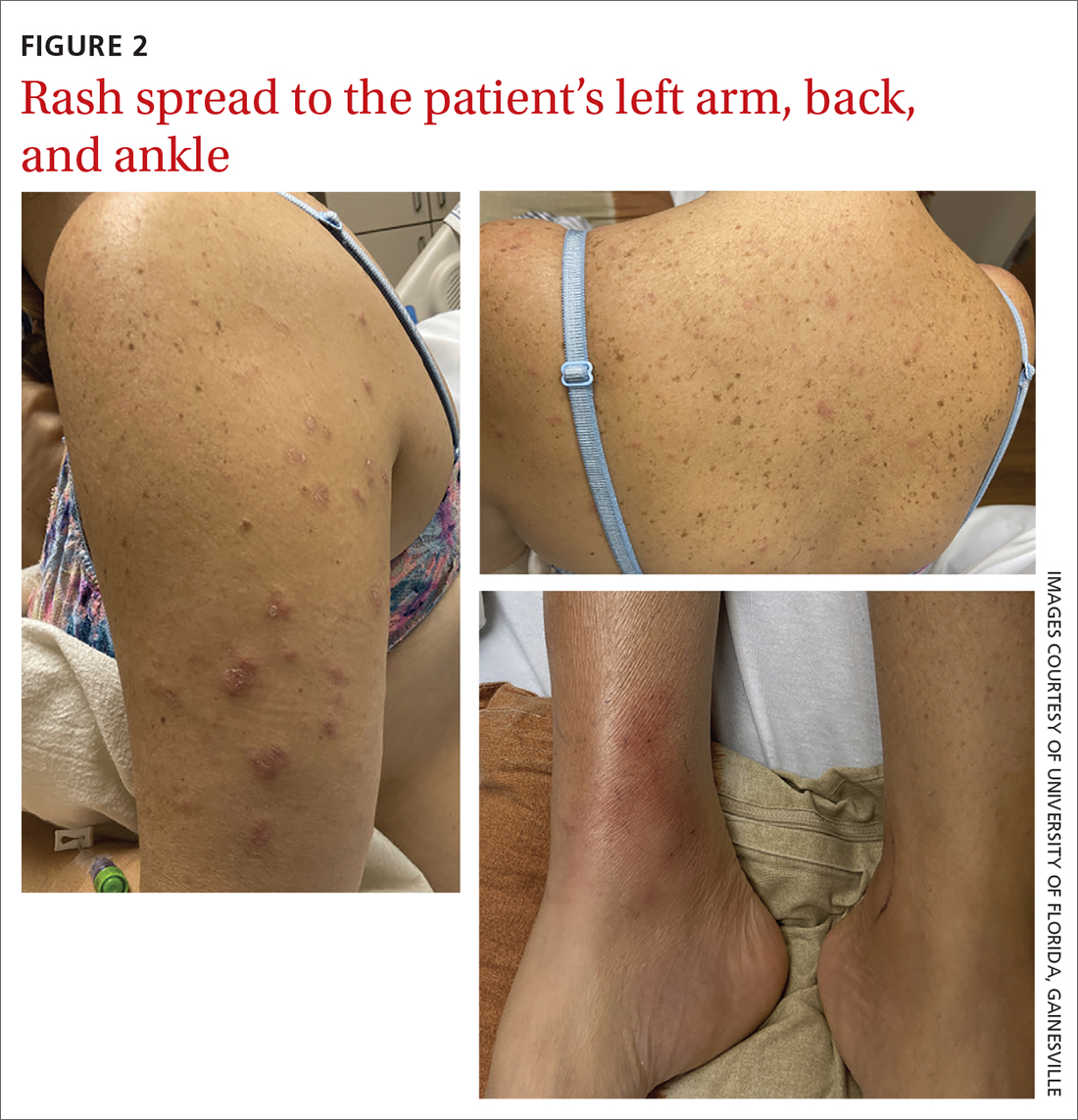
The patient said that 2 weeks prior to her admission to the hospital, she’d developed a rash on her right arm, which was papular, nondraining, nonpruritic, and not painful (FIGURE 1). Six days later, the rash spread to her left arm, chest, and back, with a few lesions on her legs (FIGURE 2). A few days later, she developed arthralgias in her hips, knees, and ankles. These were associated with the appearance of large, flat, erythematous lesions on her anterior lower extremities (FIGURE 2). About 5 days before she was admitted to our hospital, the patient was seen at another hospital and treated for possible cellulitis with cephalexin (500 mg 4 times daily for 5-7 days), but her symptoms persisted.
At this point, she sought care at our hospital for her worsening lower extremity arthralgia, difficulty walking, and the persistent rash. An initial lab report showed a white blood cell (WBC) count of 12.6 × 103/µL (normal range, 4.0-10.0 × 103/µL) with an absolute neutrophil count of 9.7 × 103/µL (normal, 1.7-7.0 × 103/µL). Her C-reactive protein (CRP) level was elevated (194.7 mg/L; normal, 0.0-5.0 mg/L), as was her erythrocyte sedimentation rate (ESR) (102.0 mm/h; normal, 0.0-20.0 mm/h). A rapid pharyngeal strep test was negative. Her anti-streptolysin O (ASO) titer was elevated (2092.0 IU/mL; normal, < 250.0 IU/mL), and her rheumatic factor was mildly elevated (19.0 IU/mL; normal, 0.0-14.0 IU/mL). An antinuclear antibody panel was positive at 1:80. Further testing was performed, and the patient was found to be negative for Sjögren syndrome A, Sjögren syndrome B, anti-Smith, scleroderma-70, double-stranded DNA, and chromatin AB—making an autoimmune disease unlikely.
THE DIAGNOSIS
The patient met the American Heart Association’s revised Jones criteria for the diagnosis of rheumatic fever: She had a positive ASO titer; polyarthritis and subcutaneous nodules (2 major criteria); and ESR > 60 mm/h and CRP > 3 mg/L (1 minor criterion).1 She started taking naproxen 500 mg twice per day and was given a penicillin G 1.5-million-unit injection. A transthoracic echocardiogram also was performed during her admission to rule out endocarditis; no abnormalities were found.
A few days after starting treatment for rheumatic fever, the patient’s WBC count returned to within normal limits and her joint swelling and pain improved; however, her rash did not go away, leading us to wonder if there was a second disease at work. Dermatology was consulted, and a punch biopsy was obtained. The results showed acute febrile neutrophilic dermatosis, or Sweet syndrome.
DISCUSSION
Sweet syndrome is considered rare, and incidence numbers are elusive.2 It has a worldwide distribution and no racial bias.3 Sweet syndrome usually occurs in women ages 30 to 50 years, although it may also occur in younger adults and children.
Three subtypes have been defined based on etiology: (1) classical (or idiopathic) Sweet syndrome; (2) malignancy-associated Sweet syndrome, which is most often related to acute myelogenous leukemia; and (3) drug-induced Sweet syndrome, which is usually associated with granulocyte colony–stimulating factor treatment.4 Our patient had the most common subtype: classical Sweet syndrome.
Continue to: What you'll see
What you’ll see.
Corticosteroid therapy is the gold standard for treatment of classical Sweet syndrome. Dosing usually starts with prednisone 1 mg/kg/d, which can be tapered to 10 mg/d within 4 to 6 weeks.5 If steroid treatment is contraindicated in the patient, alternative treatments are colchicine 0.5 mg 3 times daily for 10 to 21 days or enteric-coated potassium iodide 300 mg 3 times daily until the rash subsides.5 Without treatment, symptoms may resolve within weeks to months; with treatment, the rash usually resolves within 2 to 5 days. Some resistant forms may require 2 to 3 months of treatment.
There is a risk of recurrence in approximately one-third of patients after successful treatment of classical Sweet syndrome.5 Recurrence can be caused by another inciting factor (ie, irritable bowel disease, upper respiratory tract infection, malignancy, or a new medication), making a new investigation necessary. However, treatment would entail the same medications.5
The patient was placed on penicillin V 250 mg twice daily for 5 years due to the significant risk of carditis in the setting of rheumatic fever. She started an oral steroid regimen of a prednisone weekly taper, starting with 60 mg/d, for 4 to 6 weeks. Her papular rash improved soon after initiation of steroid therapy.
THE TAKEAWAY
On presentation, this patient’s symptoms met the Jones criteria for rheumatic fever, but she did not respond to treatment. This led us to revisit her case, order additional tests, and identify a second diagnosis—Sweet syndrome—that responded positively to treatment. This case is a reminder that sometimes the signs and symptoms we are looking at are the result of 2 underlying illnesses, with 1 possibly triggering the other. That was likely what occurred in this case.
Farah Leclercq, DO, Department of Family Medicine, University of Florida, 12041 Southwest 1 Lane, Gainesville, FL 32607; [email protected]
1. Gewitz MH, Baltimore SR, Tani LY, et al. Revision of the Jones Criteria for the diagnosis of acute rheumatic fever in the era of doppler echocardiography: a scientific statement from the American Heart Association. Circulation. 2015;131:1806-1818. doi: 10.1161/CIR.0000000000000205
2. Joshi TP, Friske SK, Hsiou DA, Duvic M. New practical aspects of Sweet syndrome. Am J Clin Dermatol. 2022;23:301-318. doi: 10.1007/s40257-022-00673-4
3. Cohen PR, Kurzrock R. Sweets syndrome revisited: a review of disease concepts. Int J Dermatol. 2003;42:761-778. doi: 10.1046/j.1365-4362.2003.01891.x
4. Merola JF. Sweet syndrome (acute febrile neutrophilic dermatosis): pathogenesis, clinical manifestations, and diagnosis. UpToDate. August 9, 2020. Accessed October 27, 2022. www.uptodate.com/contents/sweet-syndrome-acute-febrile-neutrophilic-dermatosis-pathogenesis-clinical-manifestations-and-diagnosis
5. Cohen PR. Sweets syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34. doi: 10.1186/1750-1172-2-34
THE CASE
A 40-year-old woman with no significant medical history sought care at the emergency department for a fever, rash, and arthralgia. On admission, she had worsening bilateral ankle pain and was having difficulty walking. During the previous 3 months, she’d had 3 episodes of tonsillitis, all of which were presumed to be caused by Streptococcus, although no swabs were obtained. Her primary care physician treated her with antibiotics each time: 1 round of amoxicillin 500 mg twice daily for 10 days and 2 rounds of amoxicillin/clavulanate 875 mg twice daily for 7 to 10 days. During the previous month, she’d experienced intermittent fevers ranging from 100.2 °F to 100.8

The patient said that 2 weeks prior to her admission to the hospital, she’d developed a rash on her right arm, which was papular, nondraining, nonpruritic, and not painful (FIGURE 1). Six days later, the rash spread to her left arm, chest, and back, with a few lesions on her legs (FIGURE 2). A few days later, she developed arthralgias in her hips, knees, and ankles. These were associated with the appearance of large, flat, erythematous lesions on her anterior lower extremities (FIGURE 2). About 5 days before she was admitted to our hospital, the patient was seen at another hospital and treated for possible cellulitis with cephalexin (500 mg 4 times daily for 5-7 days), but her symptoms persisted.

At this point, she sought care at our hospital for her worsening lower extremity arthralgia, difficulty walking, and the persistent rash. An initial lab report showed a white blood cell (WBC) count of 12.6 × 103/µL (normal range, 4.0-10.0 × 103/µL) with an absolute neutrophil count of 9.7 × 103/µL (normal, 1.7-7.0 × 103/µL). Her C-reactive protein (CRP) level was elevated (194.7 mg/L; normal, 0.0-5.0 mg/L), as was her erythrocyte sedimentation rate (ESR) (102.0 mm/h; normal, 0.0-20.0 mm/h). A rapid pharyngeal strep test was negative. Her anti-streptolysin O (ASO) titer was elevated (2092.0 IU/mL; normal, < 250.0 IU/mL), and her rheumatic factor was mildly elevated (19.0 IU/mL; normal, 0.0-14.0 IU/mL). An antinuclear antibody panel was positive at 1:80. Further testing was performed, and the patient was found to be negative for Sjögren syndrome A, Sjögren syndrome B, anti-Smith, scleroderma-70, double-stranded DNA, and chromatin AB—making an autoimmune disease unlikely.
THE DIAGNOSIS
The patient met the American Heart Association’s revised Jones criteria for the diagnosis of rheumatic fever: She had a positive ASO titer; polyarthritis and subcutaneous nodules (2 major criteria); and ESR > 60 mm/h and CRP > 3 mg/L (1 minor criterion).1 She started taking naproxen 500 mg twice per day and was given a penicillin G 1.5-million-unit injection. A transthoracic echocardiogram also was performed during her admission to rule out endocarditis; no abnormalities were found.
A few days after starting treatment for rheumatic fever, the patient’s WBC count returned to within normal limits and her joint swelling and pain improved; however, her rash did not go away, leading us to wonder if there was a second disease at work. Dermatology was consulted, and a punch biopsy was obtained. The results showed acute febrile neutrophilic dermatosis, or Sweet syndrome.
DISCUSSION
Sweet syndrome is considered rare, and incidence numbers are elusive.2 It has a worldwide distribution and no racial bias.3 Sweet syndrome usually occurs in women ages 30 to 50 years, although it may also occur in younger adults and children.
Three subtypes have been defined based on etiology: (1) classical (or idiopathic) Sweet syndrome; (2) malignancy-associated Sweet syndrome, which is most often related to acute myelogenous leukemia; and (3) drug-induced Sweet syndrome, which is usually associated with granulocyte colony–stimulating factor treatment.4 Our patient had the most common subtype: classical Sweet syndrome.
Continue to: What you'll see
What you’ll see.
Corticosteroid therapy is the gold standard for treatment of classical Sweet syndrome. Dosing usually starts with prednisone 1 mg/kg/d, which can be tapered to 10 mg/d within 4 to 6 weeks.5 If steroid treatment is contraindicated in the patient, alternative treatments are colchicine 0.5 mg 3 times daily for 10 to 21 days or enteric-coated potassium iodide 300 mg 3 times daily until the rash subsides.5 Without treatment, symptoms may resolve within weeks to months; with treatment, the rash usually resolves within 2 to 5 days. Some resistant forms may require 2 to 3 months of treatment.
There is a risk of recurrence in approximately one-third of patients after successful treatment of classical Sweet syndrome.5 Recurrence can be caused by another inciting factor (ie, irritable bowel disease, upper respiratory tract infection, malignancy, or a new medication), making a new investigation necessary. However, treatment would entail the same medications.5
The patient was placed on penicillin V 250 mg twice daily for 5 years due to the significant risk of carditis in the setting of rheumatic fever. She started an oral steroid regimen of a prednisone weekly taper, starting with 60 mg/d, for 4 to 6 weeks. Her papular rash improved soon after initiation of steroid therapy.
THE TAKEAWAY
On presentation, this patient’s symptoms met the Jones criteria for rheumatic fever, but she did not respond to treatment. This led us to revisit her case, order additional tests, and identify a second diagnosis—Sweet syndrome—that responded positively to treatment. This case is a reminder that sometimes the signs and symptoms we are looking at are the result of 2 underlying illnesses, with 1 possibly triggering the other. That was likely what occurred in this case.
Farah Leclercq, DO, Department of Family Medicine, University of Florida, 12041 Southwest 1 Lane, Gainesville, FL 32607; [email protected]
THE CASE
A 40-year-old woman with no significant medical history sought care at the emergency department for a fever, rash, and arthralgia. On admission, she had worsening bilateral ankle pain and was having difficulty walking. During the previous 3 months, she’d had 3 episodes of tonsillitis, all of which were presumed to be caused by Streptococcus, although no swabs were obtained. Her primary care physician treated her with antibiotics each time: 1 round of amoxicillin 500 mg twice daily for 10 days and 2 rounds of amoxicillin/clavulanate 875 mg twice daily for 7 to 10 days. During the previous month, she’d experienced intermittent fevers ranging from 100.2 °F to 100.8

The patient said that 2 weeks prior to her admission to the hospital, she’d developed a rash on her right arm, which was papular, nondraining, nonpruritic, and not painful (FIGURE 1). Six days later, the rash spread to her left arm, chest, and back, with a few lesions on her legs (FIGURE 2). A few days later, she developed arthralgias in her hips, knees, and ankles. These were associated with the appearance of large, flat, erythematous lesions on her anterior lower extremities (FIGURE 2). About 5 days before she was admitted to our hospital, the patient was seen at another hospital and treated for possible cellulitis with cephalexin (500 mg 4 times daily for 5-7 days), but her symptoms persisted.

At this point, she sought care at our hospital for her worsening lower extremity arthralgia, difficulty walking, and the persistent rash. An initial lab report showed a white blood cell (WBC) count of 12.6 × 103/µL (normal range, 4.0-10.0 × 103/µL) with an absolute neutrophil count of 9.7 × 103/µL (normal, 1.7-7.0 × 103/µL). Her C-reactive protein (CRP) level was elevated (194.7 mg/L; normal, 0.0-5.0 mg/L), as was her erythrocyte sedimentation rate (ESR) (102.0 mm/h; normal, 0.0-20.0 mm/h). A rapid pharyngeal strep test was negative. Her anti-streptolysin O (ASO) titer was elevated (2092.0 IU/mL; normal, < 250.0 IU/mL), and her rheumatic factor was mildly elevated (19.0 IU/mL; normal, 0.0-14.0 IU/mL). An antinuclear antibody panel was positive at 1:80. Further testing was performed, and the patient was found to be negative for Sjögren syndrome A, Sjögren syndrome B, anti-Smith, scleroderma-70, double-stranded DNA, and chromatin AB—making an autoimmune disease unlikely.
THE DIAGNOSIS
The patient met the American Heart Association’s revised Jones criteria for the diagnosis of rheumatic fever: She had a positive ASO titer; polyarthritis and subcutaneous nodules (2 major criteria); and ESR > 60 mm/h and CRP > 3 mg/L (1 minor criterion).1 She started taking naproxen 500 mg twice per day and was given a penicillin G 1.5-million-unit injection. A transthoracic echocardiogram also was performed during her admission to rule out endocarditis; no abnormalities were found.
A few days after starting treatment for rheumatic fever, the patient’s WBC count returned to within normal limits and her joint swelling and pain improved; however, her rash did not go away, leading us to wonder if there was a second disease at work. Dermatology was consulted, and a punch biopsy was obtained. The results showed acute febrile neutrophilic dermatosis, or Sweet syndrome.
DISCUSSION
Sweet syndrome is considered rare, and incidence numbers are elusive.2 It has a worldwide distribution and no racial bias.3 Sweet syndrome usually occurs in women ages 30 to 50 years, although it may also occur in younger adults and children.
Three subtypes have been defined based on etiology: (1) classical (or idiopathic) Sweet syndrome; (2) malignancy-associated Sweet syndrome, which is most often related to acute myelogenous leukemia; and (3) drug-induced Sweet syndrome, which is usually associated with granulocyte colony–stimulating factor treatment.4 Our patient had the most common subtype: classical Sweet syndrome.
Continue to: What you'll see
What you’ll see.
Corticosteroid therapy is the gold standard for treatment of classical Sweet syndrome. Dosing usually starts with prednisone 1 mg/kg/d, which can be tapered to 10 mg/d within 4 to 6 weeks.5 If steroid treatment is contraindicated in the patient, alternative treatments are colchicine 0.5 mg 3 times daily for 10 to 21 days or enteric-coated potassium iodide 300 mg 3 times daily until the rash subsides.5 Without treatment, symptoms may resolve within weeks to months; with treatment, the rash usually resolves within 2 to 5 days. Some resistant forms may require 2 to 3 months of treatment.
There is a risk of recurrence in approximately one-third of patients after successful treatment of classical Sweet syndrome.5 Recurrence can be caused by another inciting factor (ie, irritable bowel disease, upper respiratory tract infection, malignancy, or a new medication), making a new investigation necessary. However, treatment would entail the same medications.5
The patient was placed on penicillin V 250 mg twice daily for 5 years due to the significant risk of carditis in the setting of rheumatic fever. She started an oral steroid regimen of a prednisone weekly taper, starting with 60 mg/d, for 4 to 6 weeks. Her papular rash improved soon after initiation of steroid therapy.
THE TAKEAWAY
On presentation, this patient’s symptoms met the Jones criteria for rheumatic fever, but she did not respond to treatment. This led us to revisit her case, order additional tests, and identify a second diagnosis—Sweet syndrome—that responded positively to treatment. This case is a reminder that sometimes the signs and symptoms we are looking at are the result of 2 underlying illnesses, with 1 possibly triggering the other. That was likely what occurred in this case.
Farah Leclercq, DO, Department of Family Medicine, University of Florida, 12041 Southwest 1 Lane, Gainesville, FL 32607; [email protected]
1. Gewitz MH, Baltimore SR, Tani LY, et al. Revision of the Jones Criteria for the diagnosis of acute rheumatic fever in the era of doppler echocardiography: a scientific statement from the American Heart Association. Circulation. 2015;131:1806-1818. doi: 10.1161/CIR.0000000000000205
2. Joshi TP, Friske SK, Hsiou DA, Duvic M. New practical aspects of Sweet syndrome. Am J Clin Dermatol. 2022;23:301-318. doi: 10.1007/s40257-022-00673-4
3. Cohen PR, Kurzrock R. Sweets syndrome revisited: a review of disease concepts. Int J Dermatol. 2003;42:761-778. doi: 10.1046/j.1365-4362.2003.01891.x
4. Merola JF. Sweet syndrome (acute febrile neutrophilic dermatosis): pathogenesis, clinical manifestations, and diagnosis. UpToDate. August 9, 2020. Accessed October 27, 2022. www.uptodate.com/contents/sweet-syndrome-acute-febrile-neutrophilic-dermatosis-pathogenesis-clinical-manifestations-and-diagnosis
5. Cohen PR. Sweets syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34. doi: 10.1186/1750-1172-2-34
1. Gewitz MH, Baltimore SR, Tani LY, et al. Revision of the Jones Criteria for the diagnosis of acute rheumatic fever in the era of doppler echocardiography: a scientific statement from the American Heart Association. Circulation. 2015;131:1806-1818. doi: 10.1161/CIR.0000000000000205
2. Joshi TP, Friske SK, Hsiou DA, Duvic M. New practical aspects of Sweet syndrome. Am J Clin Dermatol. 2022;23:301-318. doi: 10.1007/s40257-022-00673-4
3. Cohen PR, Kurzrock R. Sweets syndrome revisited: a review of disease concepts. Int J Dermatol. 2003;42:761-778. doi: 10.1046/j.1365-4362.2003.01891.x
4. Merola JF. Sweet syndrome (acute febrile neutrophilic dermatosis): pathogenesis, clinical manifestations, and diagnosis. UpToDate. August 9, 2020. Accessed October 27, 2022. www.uptodate.com/contents/sweet-syndrome-acute-febrile-neutrophilic-dermatosis-pathogenesis-clinical-manifestations-and-diagnosis
5. Cohen PR. Sweets syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34. doi: 10.1186/1750-1172-2-34
Bite-sized bouts of exercise: Why they are valuable and what they are missing
Short bursts of activity are approximately as effective for general health as longer sessions, especially for those who are mainly sedentary, according to several recently published studies.
If your fitness goals are greater, and you want to build muscle strength and endurance, compete in a 5K, or just look better in your swimsuit, you will need to do more. But for basic health, it appears that short bursts can help, the new research papers and experts suggest.
“Whether you accumulate activity in many short bouts versus one extended bout, the general health benefits tend to be similar,” Amanda Paluch, PhD, a physical activity epidemiologist at the University of Massachusetts, Amherst, said in an interview.
Current public health recommendations from the Centers for Disease Control and Prevention suggest doing at least 150 minutes of moderate intensity physical activity per week for health benefits, but this activity can be accumulated in any way over the week, she noted. Previous versions of the CDC guidelines on exercise suggested that physical activity bouts should be at least 10 minutes each, but the latest version of the guidelines acknowledges that bursts of less than 10 minutes may be beneficial.
However, “the activity or fitness level at which someone starts and the specific health goals matter,” Dr. Paluch continued. “Short bouts may be particularly beneficial for those least active to get moving more to improve their general wellness.”
The current federal physical activity guidelines are still worth striving for, and patients can work their way to this goal, accumulating 150 or more minutes in a way that works best for them, she added.
“There is a lack of research directly comparing individuals who consistently accumulate their activity in many short bouts versus single bouts over an extended period of time,” Dr. Paluch noted. From a public health perspective, since both short and long bouts have health benefits, the best physical activity is what fits into your life and helps build a lifelong habit.
The benefits of exercise for cardiovascular health are well documented. A review from Circulation published in 2003 summarized the benefits of regular physical activity on measures of cardiovascular health including reduction in body weight, blood pressure, and bad cholesterol, while increasing insulin sensitivity, good cholesterol, and muscular strength and function. In that review, author Jonathan N. Myers, PhD, now of Stanford (Calif.) University, noted that “one need not be a marathon runner or an elite athlete to derive significant benefits from physical activity.” In fact, “the greatest gains in terms of mortality are achieved when an individual goes from being sedentary to becoming moderately active.”
A recent large, population-based study showed the value of short bursts of exercise for those previously sedentary. In this study, published in Nature Medicine, a team in Australia used wearable fitness trackers to measure the health benefits of what researchers have named “vigorous intermittent lifestyle physical activity” or VILPA.
Some examples of VILPA include power walking on the way to work, climbing stairs, or even running around with your kids on the playground.
Specifically, individuals who engaged in the median VILPA frequency of three bursts of vigorous activity lasting 1-2 minutes showed a 38%-40% reduction in all-cause mortality risk and cancer mortality risk, and a 48%-49% reduction in cardiovascular mortality risk.
The researchers repeated their analysis for a group of 62,344 adults from the UK Biobank who reported regular vigorous physical activity (VPA). They found similar effects on mortality, based on 1,552 deaths reported.
These results suggest that VILPA may be a reasonable physical activity target, especially for people not able or willing to exercise more formally or intensely, the researchers noted.
“We have known for a long time that leisure-time exercise often reaches vigorous intensity and has many health benefits, but we understand less about the health potential of daily movement, especially activities done as part of daily living that reach vigorous intensity,” lead author Emmanuel Stamatakis, PhD, professor of physical activity, lifestyle and population health at the University of Sydney’s Charles Perkins Centre, said in an interview.
“As long as the heart rate goes up for a minute or 2 it will likely be vigorous activity,” Dr. Stamatakis said in an interview. “It is also important that clinicians effectively communicate how patients can know that they are reaching vigorous intensity,” he said.
Signs of vigorous intensity include increased heart rate and getting out of breath after about 20-40 seconds from the start of the VILPA burst. After about a minute of VILPA, the person doing it should be too out of breath to speak more than a few words comfortably, he said.
Data support value of any and all exercise
The Nature Medicine study supports other recent research showing the value of short, intense bursts of physical activity. A pair of recent studies also used fitness trackers to measure activity in adults and assess the benefits on outcomes including death and heart disease.
One of these studies, which was published in the European Heart Journal, also used fitness trackers to measure physical activity at moderate and vigorous levels. The researchers found that individuals who performed at least 20% of their physical activity at a moderate to high level, such as by doing brisk walking in lieu of strolling had a significantly lower risk of heart disease than those whose daily activity included less than 20% at a moderate or intense level.
In another study from the European Heart Journal, researchers found that short bursts of vigorous physical activity of 2 minutes or less adding up to 15-20 minutes per week was enough to reduce mortality by as much as 40%.
Plus, a meta-analysis published in the Lancet showed a decrease in all-cause mortality with an increase in the number of daily steps, although the impact of stepping rate on mortality was inconsistent.
“Many studies have investigated the health benefits of physical activity, but not the importance of these difficult-to-capture VILPA bouts that accrue during the course of normal activities of daily living,” Lee Stoner, PhD, an exercise physiologist and director of the Cardiometabolic Lab at the University of North Carolina at Chapel Hill, said in an interview.
Dr. Stoner, who was not involved in the Nature Medicine study, said he was not surprised by the overall finding that doing short bursts of activity impacted mortality and cardiovascular disease, but was slightly surprised by the strength of the evidence.
“The referent group in the Nature Medicine study were those accruing no VILPA”, likely meaning they were very inactive,” Dr. Stoner said and added that he thinks this demonstrates the value of VILPA.
Even without immediately meeting the specific numbers recommended by the CDC, “any physical activity is better than none, especially if vigorous, and VILPA can be built into normal daily routines,” Dr. Stoner added.
What’s missing in short bursts?
Short bursts of activity do have their limits when it comes to overall fitness, said Dr. Stoner.
“Endurance will not be improved as much through short bursts, because such activities are unlikely to be as effective at empowering the mitochondria – the batteries keeping our cells running, including skeletal muscle cells,” he said. “Additionally, the vigorous bouts are unlikely to be as effective at improving muscular strength and endurance. For this, it is recommended that we engage each muscle group in strengthening exercises two times per week.”
However, Dr. Stoner agreed that prescribing short bursts of intense activity as part of daily living may be a great way to get people started with exercise.
“The key is to remove barriers to physical activity pursuit, then focusing on long-term routine rather than short-term gain,” he said. “Individuals are better served if they focus on goals other than weight loss, for which physical activity or exercise may not be the solution. Rather, being physically active can improve vigor, make daily activities simpler, and improve cognitive abilities,” and any physical activity is one of the most effective solutions for regulating blood glucose levels and improving cardiovascular risk factors.
Make it routine – and fun
To benefit from physical activity, cultivating and sustaining a long-term routine is key, said Dr. Stoner, whose research has focused on sedentary behavior and cardiovascular disease. Whatever the activity is, shorter bursts, or longer bouts or both, it is essential that individuals figure out activities that they enjoy if they want to create sustained behavior, and thus health change, Gabriel Zieff, MA, a doctoral candidate in Dr. Stoner’s Cardiometabolic Lab, who conducts studies on exercise, noted in an interview.
“We exercise enthusiasts and researchers are often hyperfocused on whether this duration or that duration is better, whether this intensity or that intensity is better,” but at the end of the day, it is the enjoyment factor that often predicts sustained behavior change, and should be part of discussions with patients to help reduce sedentary behavior and promote activity, Mr. Zieff said.
Short bouts can encourage hesitant exercisers
“To best support health, clinicians should consider taking a few seconds to ask patients about their physical activity levels,” said Dr. Paluch, who was the lead author on the Lancet meta-analysis of daily steps. In that study, Dr. Paluch and colleagues found that taking more steps each day was associated with a progressively lower risk of all-cause mortality. However, that study did not measure step rate.
Clinicians can emphasize that health benefits do not require an hour-long exercise routine and special equipment, and moving more, even in shorts bursts of activity can have meaningful associations with health, particularly for those who are less active, she said.
The recent studies on short bursts of activity agree that “some physical activity is better than none and adults should move more throughout the day in whatever way makes sense to them and fits best into their lives,” said Dr. Paluch. “For example, opting for the stairs instead of the elevator, a brisk walk to the bus stop, a short game of hide and seek with the children or grandchildren – anything that gets your body moving more, even if briefly. Making simple lifestyle changes is often easier in small bites. In time, this can grow into long-term habits, ultimately leading to an overall active lifestyle that supports living healthier for longer.”
The Nature Medicine study was supported by the Australian National Health and Medical Research Council. Several coauthors were supported by the Wellcome Trust, the National Institute for Health Research Oxford Biomedical Research Centre, Novo Nordisk, the British Heart Foundation Centre of Research Excellence, the Alan Turing Institute, the British Heart Foundation, and Health Data Research UK, an initiative funded by UK Research and Innovation. Dr. Paluch and Dr. Stoner had no financial conflicts to disclose.
Short bursts of activity are approximately as effective for general health as longer sessions, especially for those who are mainly sedentary, according to several recently published studies.
If your fitness goals are greater, and you want to build muscle strength and endurance, compete in a 5K, or just look better in your swimsuit, you will need to do more. But for basic health, it appears that short bursts can help, the new research papers and experts suggest.
“Whether you accumulate activity in many short bouts versus one extended bout, the general health benefits tend to be similar,” Amanda Paluch, PhD, a physical activity epidemiologist at the University of Massachusetts, Amherst, said in an interview.
Current public health recommendations from the Centers for Disease Control and Prevention suggest doing at least 150 minutes of moderate intensity physical activity per week for health benefits, but this activity can be accumulated in any way over the week, she noted. Previous versions of the CDC guidelines on exercise suggested that physical activity bouts should be at least 10 minutes each, but the latest version of the guidelines acknowledges that bursts of less than 10 minutes may be beneficial.
However, “the activity or fitness level at which someone starts and the specific health goals matter,” Dr. Paluch continued. “Short bouts may be particularly beneficial for those least active to get moving more to improve their general wellness.”
The current federal physical activity guidelines are still worth striving for, and patients can work their way to this goal, accumulating 150 or more minutes in a way that works best for them, she added.
“There is a lack of research directly comparing individuals who consistently accumulate their activity in many short bouts versus single bouts over an extended period of time,” Dr. Paluch noted. From a public health perspective, since both short and long bouts have health benefits, the best physical activity is what fits into your life and helps build a lifelong habit.
The benefits of exercise for cardiovascular health are well documented. A review from Circulation published in 2003 summarized the benefits of regular physical activity on measures of cardiovascular health including reduction in body weight, blood pressure, and bad cholesterol, while increasing insulin sensitivity, good cholesterol, and muscular strength and function. In that review, author Jonathan N. Myers, PhD, now of Stanford (Calif.) University, noted that “one need not be a marathon runner or an elite athlete to derive significant benefits from physical activity.” In fact, “the greatest gains in terms of mortality are achieved when an individual goes from being sedentary to becoming moderately active.”
A recent large, population-based study showed the value of short bursts of exercise for those previously sedentary. In this study, published in Nature Medicine, a team in Australia used wearable fitness trackers to measure the health benefits of what researchers have named “vigorous intermittent lifestyle physical activity” or VILPA.
Some examples of VILPA include power walking on the way to work, climbing stairs, or even running around with your kids on the playground.
Specifically, individuals who engaged in the median VILPA frequency of three bursts of vigorous activity lasting 1-2 minutes showed a 38%-40% reduction in all-cause mortality risk and cancer mortality risk, and a 48%-49% reduction in cardiovascular mortality risk.
The researchers repeated their analysis for a group of 62,344 adults from the UK Biobank who reported regular vigorous physical activity (VPA). They found similar effects on mortality, based on 1,552 deaths reported.
These results suggest that VILPA may be a reasonable physical activity target, especially for people not able or willing to exercise more formally or intensely, the researchers noted.
“We have known for a long time that leisure-time exercise often reaches vigorous intensity and has many health benefits, but we understand less about the health potential of daily movement, especially activities done as part of daily living that reach vigorous intensity,” lead author Emmanuel Stamatakis, PhD, professor of physical activity, lifestyle and population health at the University of Sydney’s Charles Perkins Centre, said in an interview.
“As long as the heart rate goes up for a minute or 2 it will likely be vigorous activity,” Dr. Stamatakis said in an interview. “It is also important that clinicians effectively communicate how patients can know that they are reaching vigorous intensity,” he said.
Signs of vigorous intensity include increased heart rate and getting out of breath after about 20-40 seconds from the start of the VILPA burst. After about a minute of VILPA, the person doing it should be too out of breath to speak more than a few words comfortably, he said.
Data support value of any and all exercise
The Nature Medicine study supports other recent research showing the value of short, intense bursts of physical activity. A pair of recent studies also used fitness trackers to measure activity in adults and assess the benefits on outcomes including death and heart disease.
One of these studies, which was published in the European Heart Journal, also used fitness trackers to measure physical activity at moderate and vigorous levels. The researchers found that individuals who performed at least 20% of their physical activity at a moderate to high level, such as by doing brisk walking in lieu of strolling had a significantly lower risk of heart disease than those whose daily activity included less than 20% at a moderate or intense level.
In another study from the European Heart Journal, researchers found that short bursts of vigorous physical activity of 2 minutes or less adding up to 15-20 minutes per week was enough to reduce mortality by as much as 40%.
Plus, a meta-analysis published in the Lancet showed a decrease in all-cause mortality with an increase in the number of daily steps, although the impact of stepping rate on mortality was inconsistent.
“Many studies have investigated the health benefits of physical activity, but not the importance of these difficult-to-capture VILPA bouts that accrue during the course of normal activities of daily living,” Lee Stoner, PhD, an exercise physiologist and director of the Cardiometabolic Lab at the University of North Carolina at Chapel Hill, said in an interview.
Dr. Stoner, who was not involved in the Nature Medicine study, said he was not surprised by the overall finding that doing short bursts of activity impacted mortality and cardiovascular disease, but was slightly surprised by the strength of the evidence.
“The referent group in the Nature Medicine study were those accruing no VILPA”, likely meaning they were very inactive,” Dr. Stoner said and added that he thinks this demonstrates the value of VILPA.
Even without immediately meeting the specific numbers recommended by the CDC, “any physical activity is better than none, especially if vigorous, and VILPA can be built into normal daily routines,” Dr. Stoner added.
What’s missing in short bursts?
Short bursts of activity do have their limits when it comes to overall fitness, said Dr. Stoner.
“Endurance will not be improved as much through short bursts, because such activities are unlikely to be as effective at empowering the mitochondria – the batteries keeping our cells running, including skeletal muscle cells,” he said. “Additionally, the vigorous bouts are unlikely to be as effective at improving muscular strength and endurance. For this, it is recommended that we engage each muscle group in strengthening exercises two times per week.”
However, Dr. Stoner agreed that prescribing short bursts of intense activity as part of daily living may be a great way to get people started with exercise.
“The key is to remove barriers to physical activity pursuit, then focusing on long-term routine rather than short-term gain,” he said. “Individuals are better served if they focus on goals other than weight loss, for which physical activity or exercise may not be the solution. Rather, being physically active can improve vigor, make daily activities simpler, and improve cognitive abilities,” and any physical activity is one of the most effective solutions for regulating blood glucose levels and improving cardiovascular risk factors.
Make it routine – and fun
To benefit from physical activity, cultivating and sustaining a long-term routine is key, said Dr. Stoner, whose research has focused on sedentary behavior and cardiovascular disease. Whatever the activity is, shorter bursts, or longer bouts or both, it is essential that individuals figure out activities that they enjoy if they want to create sustained behavior, and thus health change, Gabriel Zieff, MA, a doctoral candidate in Dr. Stoner’s Cardiometabolic Lab, who conducts studies on exercise, noted in an interview.
“We exercise enthusiasts and researchers are often hyperfocused on whether this duration or that duration is better, whether this intensity or that intensity is better,” but at the end of the day, it is the enjoyment factor that often predicts sustained behavior change, and should be part of discussions with patients to help reduce sedentary behavior and promote activity, Mr. Zieff said.
Short bouts can encourage hesitant exercisers
“To best support health, clinicians should consider taking a few seconds to ask patients about their physical activity levels,” said Dr. Paluch, who was the lead author on the Lancet meta-analysis of daily steps. In that study, Dr. Paluch and colleagues found that taking more steps each day was associated with a progressively lower risk of all-cause mortality. However, that study did not measure step rate.
Clinicians can emphasize that health benefits do not require an hour-long exercise routine and special equipment, and moving more, even in shorts bursts of activity can have meaningful associations with health, particularly for those who are less active, she said.
The recent studies on short bursts of activity agree that “some physical activity is better than none and adults should move more throughout the day in whatever way makes sense to them and fits best into their lives,” said Dr. Paluch. “For example, opting for the stairs instead of the elevator, a brisk walk to the bus stop, a short game of hide and seek with the children or grandchildren – anything that gets your body moving more, even if briefly. Making simple lifestyle changes is often easier in small bites. In time, this can grow into long-term habits, ultimately leading to an overall active lifestyle that supports living healthier for longer.”
The Nature Medicine study was supported by the Australian National Health and Medical Research Council. Several coauthors were supported by the Wellcome Trust, the National Institute for Health Research Oxford Biomedical Research Centre, Novo Nordisk, the British Heart Foundation Centre of Research Excellence, the Alan Turing Institute, the British Heart Foundation, and Health Data Research UK, an initiative funded by UK Research and Innovation. Dr. Paluch and Dr. Stoner had no financial conflicts to disclose.
Short bursts of activity are approximately as effective for general health as longer sessions, especially for those who are mainly sedentary, according to several recently published studies.
If your fitness goals are greater, and you want to build muscle strength and endurance, compete in a 5K, or just look better in your swimsuit, you will need to do more. But for basic health, it appears that short bursts can help, the new research papers and experts suggest.
“Whether you accumulate activity in many short bouts versus one extended bout, the general health benefits tend to be similar,” Amanda Paluch, PhD, a physical activity epidemiologist at the University of Massachusetts, Amherst, said in an interview.
Current public health recommendations from the Centers for Disease Control and Prevention suggest doing at least 150 minutes of moderate intensity physical activity per week for health benefits, but this activity can be accumulated in any way over the week, she noted. Previous versions of the CDC guidelines on exercise suggested that physical activity bouts should be at least 10 minutes each, but the latest version of the guidelines acknowledges that bursts of less than 10 minutes may be beneficial.
However, “the activity or fitness level at which someone starts and the specific health goals matter,” Dr. Paluch continued. “Short bouts may be particularly beneficial for those least active to get moving more to improve their general wellness.”
The current federal physical activity guidelines are still worth striving for, and patients can work their way to this goal, accumulating 150 or more minutes in a way that works best for them, she added.
“There is a lack of research directly comparing individuals who consistently accumulate their activity in many short bouts versus single bouts over an extended period of time,” Dr. Paluch noted. From a public health perspective, since both short and long bouts have health benefits, the best physical activity is what fits into your life and helps build a lifelong habit.
The benefits of exercise for cardiovascular health are well documented. A review from Circulation published in 2003 summarized the benefits of regular physical activity on measures of cardiovascular health including reduction in body weight, blood pressure, and bad cholesterol, while increasing insulin sensitivity, good cholesterol, and muscular strength and function. In that review, author Jonathan N. Myers, PhD, now of Stanford (Calif.) University, noted that “one need not be a marathon runner or an elite athlete to derive significant benefits from physical activity.” In fact, “the greatest gains in terms of mortality are achieved when an individual goes from being sedentary to becoming moderately active.”
A recent large, population-based study showed the value of short bursts of exercise for those previously sedentary. In this study, published in Nature Medicine, a team in Australia used wearable fitness trackers to measure the health benefits of what researchers have named “vigorous intermittent lifestyle physical activity” or VILPA.
Some examples of VILPA include power walking on the way to work, climbing stairs, or even running around with your kids on the playground.
Specifically, individuals who engaged in the median VILPA frequency of three bursts of vigorous activity lasting 1-2 minutes showed a 38%-40% reduction in all-cause mortality risk and cancer mortality risk, and a 48%-49% reduction in cardiovascular mortality risk.
The researchers repeated their analysis for a group of 62,344 adults from the UK Biobank who reported regular vigorous physical activity (VPA). They found similar effects on mortality, based on 1,552 deaths reported.
These results suggest that VILPA may be a reasonable physical activity target, especially for people not able or willing to exercise more formally or intensely, the researchers noted.
“We have known for a long time that leisure-time exercise often reaches vigorous intensity and has many health benefits, but we understand less about the health potential of daily movement, especially activities done as part of daily living that reach vigorous intensity,” lead author Emmanuel Stamatakis, PhD, professor of physical activity, lifestyle and population health at the University of Sydney’s Charles Perkins Centre, said in an interview.
“As long as the heart rate goes up for a minute or 2 it will likely be vigorous activity,” Dr. Stamatakis said in an interview. “It is also important that clinicians effectively communicate how patients can know that they are reaching vigorous intensity,” he said.
Signs of vigorous intensity include increased heart rate and getting out of breath after about 20-40 seconds from the start of the VILPA burst. After about a minute of VILPA, the person doing it should be too out of breath to speak more than a few words comfortably, he said.
Data support value of any and all exercise
The Nature Medicine study supports other recent research showing the value of short, intense bursts of physical activity. A pair of recent studies also used fitness trackers to measure activity in adults and assess the benefits on outcomes including death and heart disease.
One of these studies, which was published in the European Heart Journal, also used fitness trackers to measure physical activity at moderate and vigorous levels. The researchers found that individuals who performed at least 20% of their physical activity at a moderate to high level, such as by doing brisk walking in lieu of strolling had a significantly lower risk of heart disease than those whose daily activity included less than 20% at a moderate or intense level.
In another study from the European Heart Journal, researchers found that short bursts of vigorous physical activity of 2 minutes or less adding up to 15-20 minutes per week was enough to reduce mortality by as much as 40%.
Plus, a meta-analysis published in the Lancet showed a decrease in all-cause mortality with an increase in the number of daily steps, although the impact of stepping rate on mortality was inconsistent.
“Many studies have investigated the health benefits of physical activity, but not the importance of these difficult-to-capture VILPA bouts that accrue during the course of normal activities of daily living,” Lee Stoner, PhD, an exercise physiologist and director of the Cardiometabolic Lab at the University of North Carolina at Chapel Hill, said in an interview.
Dr. Stoner, who was not involved in the Nature Medicine study, said he was not surprised by the overall finding that doing short bursts of activity impacted mortality and cardiovascular disease, but was slightly surprised by the strength of the evidence.
“The referent group in the Nature Medicine study were those accruing no VILPA”, likely meaning they were very inactive,” Dr. Stoner said and added that he thinks this demonstrates the value of VILPA.
Even without immediately meeting the specific numbers recommended by the CDC, “any physical activity is better than none, especially if vigorous, and VILPA can be built into normal daily routines,” Dr. Stoner added.
What’s missing in short bursts?
Short bursts of activity do have their limits when it comes to overall fitness, said Dr. Stoner.
“Endurance will not be improved as much through short bursts, because such activities are unlikely to be as effective at empowering the mitochondria – the batteries keeping our cells running, including skeletal muscle cells,” he said. “Additionally, the vigorous bouts are unlikely to be as effective at improving muscular strength and endurance. For this, it is recommended that we engage each muscle group in strengthening exercises two times per week.”
However, Dr. Stoner agreed that prescribing short bursts of intense activity as part of daily living may be a great way to get people started with exercise.
“The key is to remove barriers to physical activity pursuit, then focusing on long-term routine rather than short-term gain,” he said. “Individuals are better served if they focus on goals other than weight loss, for which physical activity or exercise may not be the solution. Rather, being physically active can improve vigor, make daily activities simpler, and improve cognitive abilities,” and any physical activity is one of the most effective solutions for regulating blood glucose levels and improving cardiovascular risk factors.
Make it routine – and fun
To benefit from physical activity, cultivating and sustaining a long-term routine is key, said Dr. Stoner, whose research has focused on sedentary behavior and cardiovascular disease. Whatever the activity is, shorter bursts, or longer bouts or both, it is essential that individuals figure out activities that they enjoy if they want to create sustained behavior, and thus health change, Gabriel Zieff, MA, a doctoral candidate in Dr. Stoner’s Cardiometabolic Lab, who conducts studies on exercise, noted in an interview.
“We exercise enthusiasts and researchers are often hyperfocused on whether this duration or that duration is better, whether this intensity or that intensity is better,” but at the end of the day, it is the enjoyment factor that often predicts sustained behavior change, and should be part of discussions with patients to help reduce sedentary behavior and promote activity, Mr. Zieff said.
Short bouts can encourage hesitant exercisers
“To best support health, clinicians should consider taking a few seconds to ask patients about their physical activity levels,” said Dr. Paluch, who was the lead author on the Lancet meta-analysis of daily steps. In that study, Dr. Paluch and colleagues found that taking more steps each day was associated with a progressively lower risk of all-cause mortality. However, that study did not measure step rate.
Clinicians can emphasize that health benefits do not require an hour-long exercise routine and special equipment, and moving more, even in shorts bursts of activity can have meaningful associations with health, particularly for those who are less active, she said.
The recent studies on short bursts of activity agree that “some physical activity is better than none and adults should move more throughout the day in whatever way makes sense to them and fits best into their lives,” said Dr. Paluch. “For example, opting for the stairs instead of the elevator, a brisk walk to the bus stop, a short game of hide and seek with the children or grandchildren – anything that gets your body moving more, even if briefly. Making simple lifestyle changes is often easier in small bites. In time, this can grow into long-term habits, ultimately leading to an overall active lifestyle that supports living healthier for longer.”
The Nature Medicine study was supported by the Australian National Health and Medical Research Council. Several coauthors were supported by the Wellcome Trust, the National Institute for Health Research Oxford Biomedical Research Centre, Novo Nordisk, the British Heart Foundation Centre of Research Excellence, the Alan Turing Institute, the British Heart Foundation, and Health Data Research UK, an initiative funded by UK Research and Innovation. Dr. Paluch and Dr. Stoner had no financial conflicts to disclose.
Chlorthalidone, HCTZ equally effective in hypertension: DCP published
The Diuretic Comparison Project (DCP) trial, showing no difference in reduction of clinical events between the thiazide diuretics chlorthalidone and hydrochlorothiazide when used for the treatment of hypertension, has now been published.
The trial was first presented at the 2022 annual scientific sessions of the American Heart Association.
In the current paper, published online in the New England Journal of Medicine, the authors, led by Areef Ishani, MD, Minneapolis Veterans Affairs Health Care System, explained that early studies suggested that chlorthalidone was superior to hydrochlorothiazide in patients with hypertension, but more recent observational studies have shown that the two drugs reduced cardiovascular events at a similar rate. Chlorthalidone may be associated with an increased risk of adverse events, including hypokalemia.
They noted that, in 2020, Part D Medicare expenditures showed that approximately 1.5 million persons received prescriptions for chlorthalidone, compared with 11.5 million who received prescriptions for hydrochlorothiazide, despite guidelines that recommended chlorthalidone as the preferred agent. The discrepancy between guideline recommendation and real-world use is possibly related to the belief that chlorthalidone has a greater risk for adverse effects without clear evidence for differences in cardiovascular outcomes, the authors suggested.
They conducted the current study to directly compare the effect of the two agents on cardiovascular outcomes in patients with hypertension.
The pragmatic DCP trial was carried out within the VA Healthcare System, and randomly assigned 13,523 patients (mean age, 72.5 years) with hypertension who were receiving hydrochlorothiazide at baseline (25 or 50 mg per day) to continue hydrochlorothiazide at their baseline dose or to switch to chlorthalidone (12.5 or 25 mg per day).
The mean baseline systolic blood pressure was 139 mm Hg in both trial groups and did not change substantially during the trial.
Over a median follow-up of 2.4 years, there was no difference in the primary outcome – a composite of MI, stroke, hospitalization for heart failure, urgent coronary revascularization for unstable angina, and non–cancer-related death – between the chlorthalidone group (10.4%) and the hydrochlorothiazide group (10.0%), giving a hazard ratio of 1.04 (95% CI, 0.94-1.16; P = .45).
In addition, there were no treatment differences between the two groups in any primary outcome component. Hypokalemia and potassium supplement use were more common in the chlorthalidone group than in the hydrochlorothiazide group.
‘Importance lies in the design’
In an accompanying editorial, Julie R. Ingelfinger, MD, deputy editor of the New England Journal of Medicine, said the results are not surprising and may not change clinical practice. But she suggested that the importance of the trial lies in its design, which shows that a high-quality pragmatic comparative effectiveness trial can be accomplished in a cost-effective manner within a health care system with little disruption in patient care.
Dr. Ingelfinger pointed out several limitations of the trial. These include a lower-than-expected occurrence of primary outcome events, and the stipulation that patients were eligible to participate only if they continued to have hypertension while receiving hydrochlorothiazide.
In addition, 95% of the participants were receiving 25 mg of hydrochlorothiazide and only 5% were receiving 50 mg, which limited comparisons of the dose generally used in practice. Also, only approximately 13% of the patients were receiving hydrochlorothiazide alone for the treatment of hypertension at baseline.
She noted that the DCP is the first head-to-head comparison of hydrochlorothiazide and chlorthalidone in a randomized, prospective outcome trial.
“Without an apparent difference in the hazard ratios for the primary outcome in the two groups over the median follow-up of 2.4 years, results suggest that chlorthalidone therapy remains a good choice for hypertension despite the secondary observation that hypokalemia was more common with chlorthalidone than with hydrochlorothiazide,” Dr. Ingelfinger said.
“Although a subgroup analysis suggested that chlorthalidone was better than hydrochlorothiazide for participants with a history of myocardial infarction or stroke, that result may have been by chance,” she added.
As clinicians generally prefer using hydrochlorothiazide, she suggested that these DCP results will not provide any impetus for change.
“Furthermore, combined therapy and polypills may alter therapy beyond the results of this well-done, highly anticipated trial. Thus, its major effect may be as a model for other pragmatic study programs, which are greatly needed,” she concluded.
This study was supported by the Veterans Affairs Cooperative Studies Program through a grant to the Diuretic Comparison Project. Dr. Ishani reported no relevant financial relationships. Dr. Ingelfinger reported book royalties from Springer and from St. Martin’s Press, outside the submitted work, and that she is employed by the New England Journal of Medicine as deputy editor.
A version of this article first appeared on Medscape.com.
The Diuretic Comparison Project (DCP) trial, showing no difference in reduction of clinical events between the thiazide diuretics chlorthalidone and hydrochlorothiazide when used for the treatment of hypertension, has now been published.
The trial was first presented at the 2022 annual scientific sessions of the American Heart Association.
In the current paper, published online in the New England Journal of Medicine, the authors, led by Areef Ishani, MD, Minneapolis Veterans Affairs Health Care System, explained that early studies suggested that chlorthalidone was superior to hydrochlorothiazide in patients with hypertension, but more recent observational studies have shown that the two drugs reduced cardiovascular events at a similar rate. Chlorthalidone may be associated with an increased risk of adverse events, including hypokalemia.
They noted that, in 2020, Part D Medicare expenditures showed that approximately 1.5 million persons received prescriptions for chlorthalidone, compared with 11.5 million who received prescriptions for hydrochlorothiazide, despite guidelines that recommended chlorthalidone as the preferred agent. The discrepancy between guideline recommendation and real-world use is possibly related to the belief that chlorthalidone has a greater risk for adverse effects without clear evidence for differences in cardiovascular outcomes, the authors suggested.
They conducted the current study to directly compare the effect of the two agents on cardiovascular outcomes in patients with hypertension.
The pragmatic DCP trial was carried out within the VA Healthcare System, and randomly assigned 13,523 patients (mean age, 72.5 years) with hypertension who were receiving hydrochlorothiazide at baseline (25 or 50 mg per day) to continue hydrochlorothiazide at their baseline dose or to switch to chlorthalidone (12.5 or 25 mg per day).
The mean baseline systolic blood pressure was 139 mm Hg in both trial groups and did not change substantially during the trial.
Over a median follow-up of 2.4 years, there was no difference in the primary outcome – a composite of MI, stroke, hospitalization for heart failure, urgent coronary revascularization for unstable angina, and non–cancer-related death – between the chlorthalidone group (10.4%) and the hydrochlorothiazide group (10.0%), giving a hazard ratio of 1.04 (95% CI, 0.94-1.16; P = .45).
In addition, there were no treatment differences between the two groups in any primary outcome component. Hypokalemia and potassium supplement use were more common in the chlorthalidone group than in the hydrochlorothiazide group.
‘Importance lies in the design’
In an accompanying editorial, Julie R. Ingelfinger, MD, deputy editor of the New England Journal of Medicine, said the results are not surprising and may not change clinical practice. But she suggested that the importance of the trial lies in its design, which shows that a high-quality pragmatic comparative effectiveness trial can be accomplished in a cost-effective manner within a health care system with little disruption in patient care.
Dr. Ingelfinger pointed out several limitations of the trial. These include a lower-than-expected occurrence of primary outcome events, and the stipulation that patients were eligible to participate only if they continued to have hypertension while receiving hydrochlorothiazide.
In addition, 95% of the participants were receiving 25 mg of hydrochlorothiazide and only 5% were receiving 50 mg, which limited comparisons of the dose generally used in practice. Also, only approximately 13% of the patients were receiving hydrochlorothiazide alone for the treatment of hypertension at baseline.
She noted that the DCP is the first head-to-head comparison of hydrochlorothiazide and chlorthalidone in a randomized, prospective outcome trial.
“Without an apparent difference in the hazard ratios for the primary outcome in the two groups over the median follow-up of 2.4 years, results suggest that chlorthalidone therapy remains a good choice for hypertension despite the secondary observation that hypokalemia was more common with chlorthalidone than with hydrochlorothiazide,” Dr. Ingelfinger said.
“Although a subgroup analysis suggested that chlorthalidone was better than hydrochlorothiazide for participants with a history of myocardial infarction or stroke, that result may have been by chance,” she added.
As clinicians generally prefer using hydrochlorothiazide, she suggested that these DCP results will not provide any impetus for change.
“Furthermore, combined therapy and polypills may alter therapy beyond the results of this well-done, highly anticipated trial. Thus, its major effect may be as a model for other pragmatic study programs, which are greatly needed,” she concluded.
This study was supported by the Veterans Affairs Cooperative Studies Program through a grant to the Diuretic Comparison Project. Dr. Ishani reported no relevant financial relationships. Dr. Ingelfinger reported book royalties from Springer and from St. Martin’s Press, outside the submitted work, and that she is employed by the New England Journal of Medicine as deputy editor.
A version of this article first appeared on Medscape.com.
The Diuretic Comparison Project (DCP) trial, showing no difference in reduction of clinical events between the thiazide diuretics chlorthalidone and hydrochlorothiazide when used for the treatment of hypertension, has now been published.
The trial was first presented at the 2022 annual scientific sessions of the American Heart Association.
In the current paper, published online in the New England Journal of Medicine, the authors, led by Areef Ishani, MD, Minneapolis Veterans Affairs Health Care System, explained that early studies suggested that chlorthalidone was superior to hydrochlorothiazide in patients with hypertension, but more recent observational studies have shown that the two drugs reduced cardiovascular events at a similar rate. Chlorthalidone may be associated with an increased risk of adverse events, including hypokalemia.
They noted that, in 2020, Part D Medicare expenditures showed that approximately 1.5 million persons received prescriptions for chlorthalidone, compared with 11.5 million who received prescriptions for hydrochlorothiazide, despite guidelines that recommended chlorthalidone as the preferred agent. The discrepancy between guideline recommendation and real-world use is possibly related to the belief that chlorthalidone has a greater risk for adverse effects without clear evidence for differences in cardiovascular outcomes, the authors suggested.
They conducted the current study to directly compare the effect of the two agents on cardiovascular outcomes in patients with hypertension.
The pragmatic DCP trial was carried out within the VA Healthcare System, and randomly assigned 13,523 patients (mean age, 72.5 years) with hypertension who were receiving hydrochlorothiazide at baseline (25 or 50 mg per day) to continue hydrochlorothiazide at their baseline dose or to switch to chlorthalidone (12.5 or 25 mg per day).
The mean baseline systolic blood pressure was 139 mm Hg in both trial groups and did not change substantially during the trial.
Over a median follow-up of 2.4 years, there was no difference in the primary outcome – a composite of MI, stroke, hospitalization for heart failure, urgent coronary revascularization for unstable angina, and non–cancer-related death – between the chlorthalidone group (10.4%) and the hydrochlorothiazide group (10.0%), giving a hazard ratio of 1.04 (95% CI, 0.94-1.16; P = .45).
In addition, there were no treatment differences between the two groups in any primary outcome component. Hypokalemia and potassium supplement use were more common in the chlorthalidone group than in the hydrochlorothiazide group.
‘Importance lies in the design’
In an accompanying editorial, Julie R. Ingelfinger, MD, deputy editor of the New England Journal of Medicine, said the results are not surprising and may not change clinical practice. But she suggested that the importance of the trial lies in its design, which shows that a high-quality pragmatic comparative effectiveness trial can be accomplished in a cost-effective manner within a health care system with little disruption in patient care.
Dr. Ingelfinger pointed out several limitations of the trial. These include a lower-than-expected occurrence of primary outcome events, and the stipulation that patients were eligible to participate only if they continued to have hypertension while receiving hydrochlorothiazide.
In addition, 95% of the participants were receiving 25 mg of hydrochlorothiazide and only 5% were receiving 50 mg, which limited comparisons of the dose generally used in practice. Also, only approximately 13% of the patients were receiving hydrochlorothiazide alone for the treatment of hypertension at baseline.
She noted that the DCP is the first head-to-head comparison of hydrochlorothiazide and chlorthalidone in a randomized, prospective outcome trial.
“Without an apparent difference in the hazard ratios for the primary outcome in the two groups over the median follow-up of 2.4 years, results suggest that chlorthalidone therapy remains a good choice for hypertension despite the secondary observation that hypokalemia was more common with chlorthalidone than with hydrochlorothiazide,” Dr. Ingelfinger said.
“Although a subgroup analysis suggested that chlorthalidone was better than hydrochlorothiazide for participants with a history of myocardial infarction or stroke, that result may have been by chance,” she added.
As clinicians generally prefer using hydrochlorothiazide, she suggested that these DCP results will not provide any impetus for change.
“Furthermore, combined therapy and polypills may alter therapy beyond the results of this well-done, highly anticipated trial. Thus, its major effect may be as a model for other pragmatic study programs, which are greatly needed,” she concluded.
This study was supported by the Veterans Affairs Cooperative Studies Program through a grant to the Diuretic Comparison Project. Dr. Ishani reported no relevant financial relationships. Dr. Ingelfinger reported book royalties from Springer and from St. Martin’s Press, outside the submitted work, and that she is employed by the New England Journal of Medicine as deputy editor.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Nitroglycerin’s safety and value examined
He has stable angina, having chest pain with exercise. He uses sublingual nitroglycerin (SL NTG prn) about three times a month. His blood pressure is 140/70 mm Hg. His pulse is 60 beats per minute. His current medications are lisinopril, atorvastatin, aspirin, and SL NTG tablets as needed.
What would you recommend?
A. No sildenafil; refer to urologist for other ED options.
B. Okay to use sildenafil if greater than 6 hours from NTG use.
C. Recommend tadalafil.
Is coprescribing nitrates and phosphodiesterase inhibitors safe?
The FDA warns against the use of phosphodiesterase inhibitors in patients taking nitrates. Combining nitrates with phosphodiesterase type 5 (PDE5) inhibitors is contraindicated because of a synergistic blood pressure lowering effect.1 This warning/contraindication was based on theoretical concerns, as well as concern that of the first 130 deaths reported in patients who took sildenafil, 16 of the patients also were taking nitrates.2
Parker and colleagues studied the safety of giving IV nitroglycerin to patients with coronary artery disease (CAD) who have taken sildenafil.3 The study was a randomized, placebo-controlled, crossover trial. Participants received sildenafil 100 mg or placebo, then received intravenous NTG. Patients who received sildenafil had a 4-6 mm Hg systolic BP drop compared with those who took the placebo. There was no difference in severe events between the sildenafil and placebo groups. The blood levels of nitroglycerin in this study were very likely much higher than the levels that occur with SL NTG.
A recent study by Holt et al. looked at overall cardiovascular outcomes with coprescribing nitrates and phosphodiesterase inhibitors.4 The study was a case crossover design, using a nationwide Danish health registry over the period of 2000-2018. In 2000, the rate of coprescribing of phosphodiesterase inhibitors in ischemic heart disease patients on nitrates was .9 per 100 persons/year and rose to 19.5 prescriptions per 100 persons/year in 2018. During this same time, no statistically significant association was found between the coprescription of nitrates with PDE5 inhibitors and the risk for MI, cardiac arrest, syncope, stroke, or an adverse drug event.
Does nitroglycerin response help determine cause of chest pain?
Nitroglycerin response has long been used as a clinical indicator on whether a patient’s chest pain is cardiac or not. Eric A. Shry, MD, and his colleagues looked at the usefulness of nitroglycerin response in the treatment of chest pain as a predictor of ischemic chest pain in an emergency department setting.5
The study was a retrospective review of 223 patients who presented to the emergency department over a 5-month period with ongoing chest pain. They looked at patients who had ongoing chest pain in the emergency department, received nitroglycerin, and did not receive any therapy other than aspirin within 10 minutes of receiving nitroglycerin. Response to the drug was compared with the final diagnosis of cardiac versus noncardiac chest pain.
Of the patients with a final determination of cardiac chest pain, 88% had a nitroglycerin response, whereas 92% of the patients with noncardiac chest pain had a nitroglycerin response (P = .50).
Deborah B. Diercks, MD, and her colleagues looked at improvement in chest pain scores in the emergency department in patients treated with nitroglycerin and whether it correlated with a cardiac etiology of chest pain.6 The study was a prospective, observational study of 664 patients in an urban tertiary care emergency department over a 16-month period. An 11-point numeric chest pain scale was assessed and recorded by research assistants before and 5 minutes after receiving nitroglycerin. The scale ranged from 0 (no pain) to 10 (worst pain imaginable).
A final diagnosis of a cardiac etiology for chest pain was found in 18% of the patients in the study. Of the patients who had cardiac-related chest pain, 20% had no reduction in pain with nitroglycerin, compared with 19% of the patients without cardiac-related chest pain.
A complete or significant reduction in chest pain occurred with nitroglycerin in 31% of patients with cardiac chest pain and 27% of the patients without cardiac chest pain (P = .76).
Nitroglycerin response does not appear to be helpful in distinguishing cardiac from noncardiac chest pain, but a study by His and colleagues offers an interesting twist.7
The authors of this research studied 118 patients looking to see if the side effect of headache with nitroglycerin was more common in patients who did not have CAD than in those who did. All the patients had a varying degree of relief of chest pain with NTG administration within 10 minutes. In patients with normal coronary arteries or minimal CAD, 73% had headache caused by NTG, whereas in patients with obstructive CAD, only 23% had headache after NTG use.
Take-home messages
- Short acting nitroglycerin may not be a contraindication for phosphodiesterase inhibitor use.
- More data are still needed.
- Nitroglycerin response does not help distinguish chest pain from CAD from noncardiac causes.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Schwartz BG, Kloner RA. Drug interactions with phosphodiesterase-5 inhibitors used for the treatment of erectile dysfunction or pulmonary hypertension. Circulation. 2010;122:88-95.
2. Kloner RA, Zusman RM. Cardiovascular effects of sildenafil citrate and recommendations for its use. Am J Cardiol. 1999 Sep 9;84(5B):11N-17N.
3. Parker JD et al. Safety of intravenous nitroglycerin after administration of sildenafil citrate to men with coronary artery disease: A double-blind, placebo-controlled, randomized, crossover trial. Crit Care Med. 2007;35:1863-8.
4. Holt A et al. Adverse events associated with coprescription of phosphodiesterase type inhibitors and oral organic nitrates in male patients with ischemic heart disease. Ann Intern Med. 2022 Jun;175(6):774-82.
5. Shry EA et al. Usefulness of the response to sublingual nitroglycerin as a predictor of ischemic chest pain in the emergency department. Am J Cardiol. 2002 Dec 1;90(11):1264-6.
6. Diercks DB et al. Changes in the numeric descriptive scale for pain after sublingual nitroglycerin do not predict cardiac etiology of chest pain. Ann Emerg Med. 2005 Jun;45(6):581-5.
7. His DH et al. Headache response to glyceryl trinitrate in patients with and without obstructive coronary artery disease. Heart 2005;91:1164-6.
He has stable angina, having chest pain with exercise. He uses sublingual nitroglycerin (SL NTG prn) about three times a month. His blood pressure is 140/70 mm Hg. His pulse is 60 beats per minute. His current medications are lisinopril, atorvastatin, aspirin, and SL NTG tablets as needed.
What would you recommend?
A. No sildenafil; refer to urologist for other ED options.
B. Okay to use sildenafil if greater than 6 hours from NTG use.
C. Recommend tadalafil.
Is coprescribing nitrates and phosphodiesterase inhibitors safe?
The FDA warns against the use of phosphodiesterase inhibitors in patients taking nitrates. Combining nitrates with phosphodiesterase type 5 (PDE5) inhibitors is contraindicated because of a synergistic blood pressure lowering effect.1 This warning/contraindication was based on theoretical concerns, as well as concern that of the first 130 deaths reported in patients who took sildenafil, 16 of the patients also were taking nitrates.2
Parker and colleagues studied the safety of giving IV nitroglycerin to patients with coronary artery disease (CAD) who have taken sildenafil.3 The study was a randomized, placebo-controlled, crossover trial. Participants received sildenafil 100 mg or placebo, then received intravenous NTG. Patients who received sildenafil had a 4-6 mm Hg systolic BP drop compared with those who took the placebo. There was no difference in severe events between the sildenafil and placebo groups. The blood levels of nitroglycerin in this study were very likely much higher than the levels that occur with SL NTG.
A recent study by Holt et al. looked at overall cardiovascular outcomes with coprescribing nitrates and phosphodiesterase inhibitors.4 The study was a case crossover design, using a nationwide Danish health registry over the period of 2000-2018. In 2000, the rate of coprescribing of phosphodiesterase inhibitors in ischemic heart disease patients on nitrates was .9 per 100 persons/year and rose to 19.5 prescriptions per 100 persons/year in 2018. During this same time, no statistically significant association was found between the coprescription of nitrates with PDE5 inhibitors and the risk for MI, cardiac arrest, syncope, stroke, or an adverse drug event.
Does nitroglycerin response help determine cause of chest pain?
Nitroglycerin response has long been used as a clinical indicator on whether a patient’s chest pain is cardiac or not. Eric A. Shry, MD, and his colleagues looked at the usefulness of nitroglycerin response in the treatment of chest pain as a predictor of ischemic chest pain in an emergency department setting.5
The study was a retrospective review of 223 patients who presented to the emergency department over a 5-month period with ongoing chest pain. They looked at patients who had ongoing chest pain in the emergency department, received nitroglycerin, and did not receive any therapy other than aspirin within 10 minutes of receiving nitroglycerin. Response to the drug was compared with the final diagnosis of cardiac versus noncardiac chest pain.
Of the patients with a final determination of cardiac chest pain, 88% had a nitroglycerin response, whereas 92% of the patients with noncardiac chest pain had a nitroglycerin response (P = .50).
Deborah B. Diercks, MD, and her colleagues looked at improvement in chest pain scores in the emergency department in patients treated with nitroglycerin and whether it correlated with a cardiac etiology of chest pain.6 The study was a prospective, observational study of 664 patients in an urban tertiary care emergency department over a 16-month period. An 11-point numeric chest pain scale was assessed and recorded by research assistants before and 5 minutes after receiving nitroglycerin. The scale ranged from 0 (no pain) to 10 (worst pain imaginable).
A final diagnosis of a cardiac etiology for chest pain was found in 18% of the patients in the study. Of the patients who had cardiac-related chest pain, 20% had no reduction in pain with nitroglycerin, compared with 19% of the patients without cardiac-related chest pain.
A complete or significant reduction in chest pain occurred with nitroglycerin in 31% of patients with cardiac chest pain and 27% of the patients without cardiac chest pain (P = .76).
Nitroglycerin response does not appear to be helpful in distinguishing cardiac from noncardiac chest pain, but a study by His and colleagues offers an interesting twist.7
The authors of this research studied 118 patients looking to see if the side effect of headache with nitroglycerin was more common in patients who did not have CAD than in those who did. All the patients had a varying degree of relief of chest pain with NTG administration within 10 minutes. In patients with normal coronary arteries or minimal CAD, 73% had headache caused by NTG, whereas in patients with obstructive CAD, only 23% had headache after NTG use.
Take-home messages
- Short acting nitroglycerin may not be a contraindication for phosphodiesterase inhibitor use.
- More data are still needed.
- Nitroglycerin response does not help distinguish chest pain from CAD from noncardiac causes.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Schwartz BG, Kloner RA. Drug interactions with phosphodiesterase-5 inhibitors used for the treatment of erectile dysfunction or pulmonary hypertension. Circulation. 2010;122:88-95.
2. Kloner RA, Zusman RM. Cardiovascular effects of sildenafil citrate and recommendations for its use. Am J Cardiol. 1999 Sep 9;84(5B):11N-17N.
3. Parker JD et al. Safety of intravenous nitroglycerin after administration of sildenafil citrate to men with coronary artery disease: A double-blind, placebo-controlled, randomized, crossover trial. Crit Care Med. 2007;35:1863-8.
4. Holt A et al. Adverse events associated with coprescription of phosphodiesterase type inhibitors and oral organic nitrates in male patients with ischemic heart disease. Ann Intern Med. 2022 Jun;175(6):774-82.
5. Shry EA et al. Usefulness of the response to sublingual nitroglycerin as a predictor of ischemic chest pain in the emergency department. Am J Cardiol. 2002 Dec 1;90(11):1264-6.
6. Diercks DB et al. Changes in the numeric descriptive scale for pain after sublingual nitroglycerin do not predict cardiac etiology of chest pain. Ann Emerg Med. 2005 Jun;45(6):581-5.
7. His DH et al. Headache response to glyceryl trinitrate in patients with and without obstructive coronary artery disease. Heart 2005;91:1164-6.
He has stable angina, having chest pain with exercise. He uses sublingual nitroglycerin (SL NTG prn) about three times a month. His blood pressure is 140/70 mm Hg. His pulse is 60 beats per minute. His current medications are lisinopril, atorvastatin, aspirin, and SL NTG tablets as needed.
What would you recommend?
A. No sildenafil; refer to urologist for other ED options.
B. Okay to use sildenafil if greater than 6 hours from NTG use.
C. Recommend tadalafil.
Is coprescribing nitrates and phosphodiesterase inhibitors safe?
The FDA warns against the use of phosphodiesterase inhibitors in patients taking nitrates. Combining nitrates with phosphodiesterase type 5 (PDE5) inhibitors is contraindicated because of a synergistic blood pressure lowering effect.1 This warning/contraindication was based on theoretical concerns, as well as concern that of the first 130 deaths reported in patients who took sildenafil, 16 of the patients also were taking nitrates.2
Parker and colleagues studied the safety of giving IV nitroglycerin to patients with coronary artery disease (CAD) who have taken sildenafil.3 The study was a randomized, placebo-controlled, crossover trial. Participants received sildenafil 100 mg or placebo, then received intravenous NTG. Patients who received sildenafil had a 4-6 mm Hg systolic BP drop compared with those who took the placebo. There was no difference in severe events between the sildenafil and placebo groups. The blood levels of nitroglycerin in this study were very likely much higher than the levels that occur with SL NTG.
A recent study by Holt et al. looked at overall cardiovascular outcomes with coprescribing nitrates and phosphodiesterase inhibitors.4 The study was a case crossover design, using a nationwide Danish health registry over the period of 2000-2018. In 2000, the rate of coprescribing of phosphodiesterase inhibitors in ischemic heart disease patients on nitrates was .9 per 100 persons/year and rose to 19.5 prescriptions per 100 persons/year in 2018. During this same time, no statistically significant association was found between the coprescription of nitrates with PDE5 inhibitors and the risk for MI, cardiac arrest, syncope, stroke, or an adverse drug event.
Does nitroglycerin response help determine cause of chest pain?
Nitroglycerin response has long been used as a clinical indicator on whether a patient’s chest pain is cardiac or not. Eric A. Shry, MD, and his colleagues looked at the usefulness of nitroglycerin response in the treatment of chest pain as a predictor of ischemic chest pain in an emergency department setting.5
The study was a retrospective review of 223 patients who presented to the emergency department over a 5-month period with ongoing chest pain. They looked at patients who had ongoing chest pain in the emergency department, received nitroglycerin, and did not receive any therapy other than aspirin within 10 minutes of receiving nitroglycerin. Response to the drug was compared with the final diagnosis of cardiac versus noncardiac chest pain.
Of the patients with a final determination of cardiac chest pain, 88% had a nitroglycerin response, whereas 92% of the patients with noncardiac chest pain had a nitroglycerin response (P = .50).
Deborah B. Diercks, MD, and her colleagues looked at improvement in chest pain scores in the emergency department in patients treated with nitroglycerin and whether it correlated with a cardiac etiology of chest pain.6 The study was a prospective, observational study of 664 patients in an urban tertiary care emergency department over a 16-month period. An 11-point numeric chest pain scale was assessed and recorded by research assistants before and 5 minutes after receiving nitroglycerin. The scale ranged from 0 (no pain) to 10 (worst pain imaginable).
A final diagnosis of a cardiac etiology for chest pain was found in 18% of the patients in the study. Of the patients who had cardiac-related chest pain, 20% had no reduction in pain with nitroglycerin, compared with 19% of the patients without cardiac-related chest pain.
A complete or significant reduction in chest pain occurred with nitroglycerin in 31% of patients with cardiac chest pain and 27% of the patients without cardiac chest pain (P = .76).
Nitroglycerin response does not appear to be helpful in distinguishing cardiac from noncardiac chest pain, but a study by His and colleagues offers an interesting twist.7
The authors of this research studied 118 patients looking to see if the side effect of headache with nitroglycerin was more common in patients who did not have CAD than in those who did. All the patients had a varying degree of relief of chest pain with NTG administration within 10 minutes. In patients with normal coronary arteries or minimal CAD, 73% had headache caused by NTG, whereas in patients with obstructive CAD, only 23% had headache after NTG use.
Take-home messages
- Short acting nitroglycerin may not be a contraindication for phosphodiesterase inhibitor use.
- More data are still needed.
- Nitroglycerin response does not help distinguish chest pain from CAD from noncardiac causes.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Schwartz BG, Kloner RA. Drug interactions with phosphodiesterase-5 inhibitors used for the treatment of erectile dysfunction or pulmonary hypertension. Circulation. 2010;122:88-95.
2. Kloner RA, Zusman RM. Cardiovascular effects of sildenafil citrate and recommendations for its use. Am J Cardiol. 1999 Sep 9;84(5B):11N-17N.
3. Parker JD et al. Safety of intravenous nitroglycerin after administration of sildenafil citrate to men with coronary artery disease: A double-blind, placebo-controlled, randomized, crossover trial. Crit Care Med. 2007;35:1863-8.
4. Holt A et al. Adverse events associated with coprescription of phosphodiesterase type inhibitors and oral organic nitrates in male patients with ischemic heart disease. Ann Intern Med. 2022 Jun;175(6):774-82.
5. Shry EA et al. Usefulness of the response to sublingual nitroglycerin as a predictor of ischemic chest pain in the emergency department. Am J Cardiol. 2002 Dec 1;90(11):1264-6.
6. Diercks DB et al. Changes in the numeric descriptive scale for pain after sublingual nitroglycerin do not predict cardiac etiology of chest pain. Ann Emerg Med. 2005 Jun;45(6):581-5.
7. His DH et al. Headache response to glyceryl trinitrate in patients with and without obstructive coronary artery disease. Heart 2005;91:1164-6.
DELIVER subanalysis ‘seals deal’ for dapagliflozin in HF
A prespecified analysis of a large global trial of patients with symptomatic heart failure with mildly reduced and preserved ejection fraction “seals the deal” on the efficacy of sodium-glucose cotransporter 2 (SGLT2) inhibitors to manage and improve their symptoms.
The prespecified analysis of the DELIVER (Dapagliflozin Evaluation to Improve the Lives of Patients with Preserved Ejection Fraction Heart Failure) trial included 5,795 patients with mildly reduced and preserved ejection fraction who completed the Kansas City Cardiomyopathy Questionnaire (KCCQ) after taking the SGLT2 inhibitor dapagliflozin or placebo. The results were published online in the Journal of the American College of Cardiology.
“We’ve known from studies prior to DELIVER that SGLT2 inhibitors have been shown to improve health status, patient symptoms and quality of life among those that are living with heart failure and mildly reduced [HFmrEF] and preserved [HFpEF] ejection fraction,” lead author Mikhail N. Kosiborod, MD, vice president for research at Saint Luke’s Health System, and codirector of the St. Luke’s Michael and Marly Haverty Cardiometabolic Center of Excellence at St. Luke’s Mid America Heart Institute, Kansas City, Mo., said in an interview. “But the picture was incomplete for a number of different reasons, partly because the previous studies were either relatively modest in size, geographically limited, or suggested potential attenuation of these benefits in patients with completely normal ejection fraction.”
Specifically, the study authors noted the EMPEROR-Preserved trial of the SGLT2 inhibitor empagliflozin showed improvement in health status vs. placebo across the range of EF except in those with normal EF of 65% or greater. The PRESERVED-HF trial of dapagliflozin demonstrated a more robust response than EMPEROR-Preserved or DELIVER, but PRESERVED-HF patients were recruited only in the United States and had more debilitating HF symptoms at baseline.
“Because of the results of the DELIVER trial and because of how large, extensive, and inclusive the trial was, it really seals the deal on the value of SGLT2 inhibitors in patients with heart failure,” said Dr. Kosiborod, who is also a professor of medicine at the University of Missouri–Kansas City.
The DELIVER analysis found that the effects of dapagliflozin on reducing cardiovascular death and worsening HF were greatest in patients who had the most debilitating symptoms at baseline, measured as KCCQ total symptom score (TSS) as 63 or less, the lowest of three tertiles used in the analysis. At baseline, these patients had the highest rates of CV death or worsening HF than those in the other two tertiles: KCCQ-TSS of 63-84, and greater than 84.
Compared with placebo, treated patients in the lowest KCCQ-TSS quartile had a 30% reduction in risk for the primary composite outcome, which consisted of time to first CV death or HF event (hazard ratio, 0.70; 95% confidence interval, 0.58-0.84; P < .001). In the second tertile, the relative risk reduction was 19% (HR, 0.81; 95% CI, 0.65-1.01; P < .006), and the highest quartile showed no significant difference between treatment and placebo (HR, 1.07; 95% CI, 0.83-1.37; P < .62).
“The most important take home message is that the SGLT2 inhibitor dapagliflozin significantly improved patient symptoms as measured by the Kansas City Cardiomyopathy Questionnaire symptom score,” Dr. Kosiborod said. “It improved those symptoms within 1 month and those benefits were sustained out to 8 months.”
DELIVER patients also showed improvement in all other key KCCQ domains across the board, he added. “In addition, dapagliflozin also improved the proportion of patients who had small, moderate, and large improvements in a responder analysis. So really, by every measure that we had, dapagliflozin had a significant beneficial effect.”
The DELIVER results taken collectively with the EMPEROR-Preserved and PRESERVED-HF trials cinch the deal for SGLT2 inhibitors, Dr. Kosiborod said. “They deliver on the triple goal of care in patients with heart failure. They reduce the risk of cardiovascular death and worsening heart failure and they improve patient symptoms, function and quality of life – and they accomplish that across the entire continuum of heart failure regardless of ejection fraction, regardless of whether patients are hospitalized or in an ambulatory setting, regardless of age or background therapies or other comorbidities.”
He added: “It’s a pretty historic development because we haven’t had that before.”
AstraZeneca funded the DELIVER trial. Dr. Kosiborod disclosed financial relationships with Alnylam, Amgen, Applied Therapeutics, Bayer, Boehringer Ingelheim, Cytokinetics, Dexcom, Eli Lilly, Esperion Therapeutics, Janssen, Lexicon, Merck (Diabetes and Cardiovascular), Novo Nordisk, Sanofi, Pharmacosmos and Vifor Pharma.
A prespecified analysis of a large global trial of patients with symptomatic heart failure with mildly reduced and preserved ejection fraction “seals the deal” on the efficacy of sodium-glucose cotransporter 2 (SGLT2) inhibitors to manage and improve their symptoms.
The prespecified analysis of the DELIVER (Dapagliflozin Evaluation to Improve the Lives of Patients with Preserved Ejection Fraction Heart Failure) trial included 5,795 patients with mildly reduced and preserved ejection fraction who completed the Kansas City Cardiomyopathy Questionnaire (KCCQ) after taking the SGLT2 inhibitor dapagliflozin or placebo. The results were published online in the Journal of the American College of Cardiology.
“We’ve known from studies prior to DELIVER that SGLT2 inhibitors have been shown to improve health status, patient symptoms and quality of life among those that are living with heart failure and mildly reduced [HFmrEF] and preserved [HFpEF] ejection fraction,” lead author Mikhail N. Kosiborod, MD, vice president for research at Saint Luke’s Health System, and codirector of the St. Luke’s Michael and Marly Haverty Cardiometabolic Center of Excellence at St. Luke’s Mid America Heart Institute, Kansas City, Mo., said in an interview. “But the picture was incomplete for a number of different reasons, partly because the previous studies were either relatively modest in size, geographically limited, or suggested potential attenuation of these benefits in patients with completely normal ejection fraction.”
Specifically, the study authors noted the EMPEROR-Preserved trial of the SGLT2 inhibitor empagliflozin showed improvement in health status vs. placebo across the range of EF except in those with normal EF of 65% or greater. The PRESERVED-HF trial of dapagliflozin demonstrated a more robust response than EMPEROR-Preserved or DELIVER, but PRESERVED-HF patients were recruited only in the United States and had more debilitating HF symptoms at baseline.
“Because of the results of the DELIVER trial and because of how large, extensive, and inclusive the trial was, it really seals the deal on the value of SGLT2 inhibitors in patients with heart failure,” said Dr. Kosiborod, who is also a professor of medicine at the University of Missouri–Kansas City.
The DELIVER analysis found that the effects of dapagliflozin on reducing cardiovascular death and worsening HF were greatest in patients who had the most debilitating symptoms at baseline, measured as KCCQ total symptom score (TSS) as 63 or less, the lowest of three tertiles used in the analysis. At baseline, these patients had the highest rates of CV death or worsening HF than those in the other two tertiles: KCCQ-TSS of 63-84, and greater than 84.
Compared with placebo, treated patients in the lowest KCCQ-TSS quartile had a 30% reduction in risk for the primary composite outcome, which consisted of time to first CV death or HF event (hazard ratio, 0.70; 95% confidence interval, 0.58-0.84; P < .001). In the second tertile, the relative risk reduction was 19% (HR, 0.81; 95% CI, 0.65-1.01; P < .006), and the highest quartile showed no significant difference between treatment and placebo (HR, 1.07; 95% CI, 0.83-1.37; P < .62).
“The most important take home message is that the SGLT2 inhibitor dapagliflozin significantly improved patient symptoms as measured by the Kansas City Cardiomyopathy Questionnaire symptom score,” Dr. Kosiborod said. “It improved those symptoms within 1 month and those benefits were sustained out to 8 months.”
DELIVER patients also showed improvement in all other key KCCQ domains across the board, he added. “In addition, dapagliflozin also improved the proportion of patients who had small, moderate, and large improvements in a responder analysis. So really, by every measure that we had, dapagliflozin had a significant beneficial effect.”
The DELIVER results taken collectively with the EMPEROR-Preserved and PRESERVED-HF trials cinch the deal for SGLT2 inhibitors, Dr. Kosiborod said. “They deliver on the triple goal of care in patients with heart failure. They reduce the risk of cardiovascular death and worsening heart failure and they improve patient symptoms, function and quality of life – and they accomplish that across the entire continuum of heart failure regardless of ejection fraction, regardless of whether patients are hospitalized or in an ambulatory setting, regardless of age or background therapies or other comorbidities.”
He added: “It’s a pretty historic development because we haven’t had that before.”
AstraZeneca funded the DELIVER trial. Dr. Kosiborod disclosed financial relationships with Alnylam, Amgen, Applied Therapeutics, Bayer, Boehringer Ingelheim, Cytokinetics, Dexcom, Eli Lilly, Esperion Therapeutics, Janssen, Lexicon, Merck (Diabetes and Cardiovascular), Novo Nordisk, Sanofi, Pharmacosmos and Vifor Pharma.
A prespecified analysis of a large global trial of patients with symptomatic heart failure with mildly reduced and preserved ejection fraction “seals the deal” on the efficacy of sodium-glucose cotransporter 2 (SGLT2) inhibitors to manage and improve their symptoms.
The prespecified analysis of the DELIVER (Dapagliflozin Evaluation to Improve the Lives of Patients with Preserved Ejection Fraction Heart Failure) trial included 5,795 patients with mildly reduced and preserved ejection fraction who completed the Kansas City Cardiomyopathy Questionnaire (KCCQ) after taking the SGLT2 inhibitor dapagliflozin or placebo. The results were published online in the Journal of the American College of Cardiology.
“We’ve known from studies prior to DELIVER that SGLT2 inhibitors have been shown to improve health status, patient symptoms and quality of life among those that are living with heart failure and mildly reduced [HFmrEF] and preserved [HFpEF] ejection fraction,” lead author Mikhail N. Kosiborod, MD, vice president for research at Saint Luke’s Health System, and codirector of the St. Luke’s Michael and Marly Haverty Cardiometabolic Center of Excellence at St. Luke’s Mid America Heart Institute, Kansas City, Mo., said in an interview. “But the picture was incomplete for a number of different reasons, partly because the previous studies were either relatively modest in size, geographically limited, or suggested potential attenuation of these benefits in patients with completely normal ejection fraction.”
Specifically, the study authors noted the EMPEROR-Preserved trial of the SGLT2 inhibitor empagliflozin showed improvement in health status vs. placebo across the range of EF except in those with normal EF of 65% or greater. The PRESERVED-HF trial of dapagliflozin demonstrated a more robust response than EMPEROR-Preserved or DELIVER, but PRESERVED-HF patients were recruited only in the United States and had more debilitating HF symptoms at baseline.
“Because of the results of the DELIVER trial and because of how large, extensive, and inclusive the trial was, it really seals the deal on the value of SGLT2 inhibitors in patients with heart failure,” said Dr. Kosiborod, who is also a professor of medicine at the University of Missouri–Kansas City.
The DELIVER analysis found that the effects of dapagliflozin on reducing cardiovascular death and worsening HF were greatest in patients who had the most debilitating symptoms at baseline, measured as KCCQ total symptom score (TSS) as 63 or less, the lowest of three tertiles used in the analysis. At baseline, these patients had the highest rates of CV death or worsening HF than those in the other two tertiles: KCCQ-TSS of 63-84, and greater than 84.
Compared with placebo, treated patients in the lowest KCCQ-TSS quartile had a 30% reduction in risk for the primary composite outcome, which consisted of time to first CV death or HF event (hazard ratio, 0.70; 95% confidence interval, 0.58-0.84; P < .001). In the second tertile, the relative risk reduction was 19% (HR, 0.81; 95% CI, 0.65-1.01; P < .006), and the highest quartile showed no significant difference between treatment and placebo (HR, 1.07; 95% CI, 0.83-1.37; P < .62).
“The most important take home message is that the SGLT2 inhibitor dapagliflozin significantly improved patient symptoms as measured by the Kansas City Cardiomyopathy Questionnaire symptom score,” Dr. Kosiborod said. “It improved those symptoms within 1 month and those benefits were sustained out to 8 months.”
DELIVER patients also showed improvement in all other key KCCQ domains across the board, he added. “In addition, dapagliflozin also improved the proportion of patients who had small, moderate, and large improvements in a responder analysis. So really, by every measure that we had, dapagliflozin had a significant beneficial effect.”
The DELIVER results taken collectively with the EMPEROR-Preserved and PRESERVED-HF trials cinch the deal for SGLT2 inhibitors, Dr. Kosiborod said. “They deliver on the triple goal of care in patients with heart failure. They reduce the risk of cardiovascular death and worsening heart failure and they improve patient symptoms, function and quality of life – and they accomplish that across the entire continuum of heart failure regardless of ejection fraction, regardless of whether patients are hospitalized or in an ambulatory setting, regardless of age or background therapies or other comorbidities.”
He added: “It’s a pretty historic development because we haven’t had that before.”
AstraZeneca funded the DELIVER trial. Dr. Kosiborod disclosed financial relationships with Alnylam, Amgen, Applied Therapeutics, Bayer, Boehringer Ingelheim, Cytokinetics, Dexcom, Eli Lilly, Esperion Therapeutics, Janssen, Lexicon, Merck (Diabetes and Cardiovascular), Novo Nordisk, Sanofi, Pharmacosmos and Vifor Pharma.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
FDA panel votes no on omecamtiv mecarbil for heart failure
A panel of advisers to the Food and Drug Administration has recommended against approval of omecamtiv mecarbil (Cytokinetics) for the treatment of heart failure with reduced ejection fraction (HFrEF).
Omecamtiv mecarbil is a first-in-class, selective cardiac myosin activator designed to improve cardiac performance.
The FDA Cardiovascular and Renal Drugs Advisory Committee on Dec. 13 voted 8 to 3 (with no abstentions) that the benefits of omecamtiv mecarbil do not outweigh the risks for HFrEF.
Those who voted in favor of the drug cited the clinical benefit (albeit small) and good safety profile of the drug as well as the unmet need for new treatments.
C. Noel Bairey Merz, MD, Cedars-Sinai Medical Center, Los Angeles, said she voted yes “on the basis of need,” and her personal experience, as well as the data presented, that “up to half of severe heart failure patients are intolerant of guidelines directed medical therapy.”
Christopher M. O’Connor, MD, with Inova Heart and Vascular Institute, Falls Church, Va., who also voted in favor of approval for the drug, cited the “important unmet need,” and said he believes “a path was constructed in which one could go forward safely and with enhanced efficacy.
“It may be a narrow path, but I think it’s a path that would afford a lot of benefit to this high-risk patient population,” said Dr. O’Connor.
Those who voted against approval generally felt the benefit was not large enough and that more data are needed, given this is a first-in-class agent.
Julia B. Lewis, MD, Vanderbilt Medical Center, Nashville, Tenn., who voted no, said she was concerned that, despite the large size of the trial, “a more positive effect could not have been found.” She was also concerned that there was no benefit on quality of life or any other secondary outcomes.
David J. Moliterno, MD, University of Kentucky Medical Center, Lexington, who also voted no, felt the benefits were “more singular and that being a modest reduction primarily limited to fewer outpatient visits.” Dr. Moliterno, like many of the committee members who voted no, called for more study.
The committee’s decision was based on results from the phase 3 GALACTIC-HF trial, which enrolled 8,256 patients with HFrEF who were at risk of hospitalization and death, despite standard-of-care therapy.
As previously reported by this news organization, omecamtiv mecarbil produced a positive result for the study’s primary endpoint, with a 2.1% absolute reduction in the combined rate of cardiovascular (CV) death, first HF hospitalization, or first urgent visit for HF, compared with placebo during a median follow-up of about 22 months.
This represented an 8% relative risk reduction and broke down as a 0.6% absolute drop in CV death, compared with placebo, a 0.7% cut in HF hospitalization, and a 0.8% drop in urgent outpatient HF visits.
The results were presented at the American Heart Association 2020 scientific sessions and simultaneously published in the New England Journal of Medicine.
In a statement, Robert I. Blum, president and CEO of Cytokinetics, said, “We are disappointed there was not a greater consensus amongst committee members relating to the benefit-risk of omecamtiv mecarbil, and we maintain our conviction in the strength of evidence supporting its potential benefit for patients suffering from HFrEF.”
He added that the company plans to engage constructively with the FDA as it completes its review of the application for omecamtiv mecarbil.
The drug has a Prescription Drug User Fee Act target date of Feb. 28, 2023.
A version of this article first appeared on Medscape.com.
A panel of advisers to the Food and Drug Administration has recommended against approval of omecamtiv mecarbil (Cytokinetics) for the treatment of heart failure with reduced ejection fraction (HFrEF).
Omecamtiv mecarbil is a first-in-class, selective cardiac myosin activator designed to improve cardiac performance.
The FDA Cardiovascular and Renal Drugs Advisory Committee on Dec. 13 voted 8 to 3 (with no abstentions) that the benefits of omecamtiv mecarbil do not outweigh the risks for HFrEF.
Those who voted in favor of the drug cited the clinical benefit (albeit small) and good safety profile of the drug as well as the unmet need for new treatments.
C. Noel Bairey Merz, MD, Cedars-Sinai Medical Center, Los Angeles, said she voted yes “on the basis of need,” and her personal experience, as well as the data presented, that “up to half of severe heart failure patients are intolerant of guidelines directed medical therapy.”
Christopher M. O’Connor, MD, with Inova Heart and Vascular Institute, Falls Church, Va., who also voted in favor of approval for the drug, cited the “important unmet need,” and said he believes “a path was constructed in which one could go forward safely and with enhanced efficacy.
“It may be a narrow path, but I think it’s a path that would afford a lot of benefit to this high-risk patient population,” said Dr. O’Connor.
Those who voted against approval generally felt the benefit was not large enough and that more data are needed, given this is a first-in-class agent.
Julia B. Lewis, MD, Vanderbilt Medical Center, Nashville, Tenn., who voted no, said she was concerned that, despite the large size of the trial, “a more positive effect could not have been found.” She was also concerned that there was no benefit on quality of life or any other secondary outcomes.
David J. Moliterno, MD, University of Kentucky Medical Center, Lexington, who also voted no, felt the benefits were “more singular and that being a modest reduction primarily limited to fewer outpatient visits.” Dr. Moliterno, like many of the committee members who voted no, called for more study.
The committee’s decision was based on results from the phase 3 GALACTIC-HF trial, which enrolled 8,256 patients with HFrEF who were at risk of hospitalization and death, despite standard-of-care therapy.
As previously reported by this news organization, omecamtiv mecarbil produced a positive result for the study’s primary endpoint, with a 2.1% absolute reduction in the combined rate of cardiovascular (CV) death, first HF hospitalization, or first urgent visit for HF, compared with placebo during a median follow-up of about 22 months.
This represented an 8% relative risk reduction and broke down as a 0.6% absolute drop in CV death, compared with placebo, a 0.7% cut in HF hospitalization, and a 0.8% drop in urgent outpatient HF visits.
The results were presented at the American Heart Association 2020 scientific sessions and simultaneously published in the New England Journal of Medicine.
In a statement, Robert I. Blum, president and CEO of Cytokinetics, said, “We are disappointed there was not a greater consensus amongst committee members relating to the benefit-risk of omecamtiv mecarbil, and we maintain our conviction in the strength of evidence supporting its potential benefit for patients suffering from HFrEF.”
He added that the company plans to engage constructively with the FDA as it completes its review of the application for omecamtiv mecarbil.
The drug has a Prescription Drug User Fee Act target date of Feb. 28, 2023.
A version of this article first appeared on Medscape.com.
A panel of advisers to the Food and Drug Administration has recommended against approval of omecamtiv mecarbil (Cytokinetics) for the treatment of heart failure with reduced ejection fraction (HFrEF).
Omecamtiv mecarbil is a first-in-class, selective cardiac myosin activator designed to improve cardiac performance.
The FDA Cardiovascular and Renal Drugs Advisory Committee on Dec. 13 voted 8 to 3 (with no abstentions) that the benefits of omecamtiv mecarbil do not outweigh the risks for HFrEF.
Those who voted in favor of the drug cited the clinical benefit (albeit small) and good safety profile of the drug as well as the unmet need for new treatments.
C. Noel Bairey Merz, MD, Cedars-Sinai Medical Center, Los Angeles, said she voted yes “on the basis of need,” and her personal experience, as well as the data presented, that “up to half of severe heart failure patients are intolerant of guidelines directed medical therapy.”
Christopher M. O’Connor, MD, with Inova Heart and Vascular Institute, Falls Church, Va., who also voted in favor of approval for the drug, cited the “important unmet need,” and said he believes “a path was constructed in which one could go forward safely and with enhanced efficacy.
“It may be a narrow path, but I think it’s a path that would afford a lot of benefit to this high-risk patient population,” said Dr. O’Connor.
Those who voted against approval generally felt the benefit was not large enough and that more data are needed, given this is a first-in-class agent.
Julia B. Lewis, MD, Vanderbilt Medical Center, Nashville, Tenn., who voted no, said she was concerned that, despite the large size of the trial, “a more positive effect could not have been found.” She was also concerned that there was no benefit on quality of life or any other secondary outcomes.
David J. Moliterno, MD, University of Kentucky Medical Center, Lexington, who also voted no, felt the benefits were “more singular and that being a modest reduction primarily limited to fewer outpatient visits.” Dr. Moliterno, like many of the committee members who voted no, called for more study.
The committee’s decision was based on results from the phase 3 GALACTIC-HF trial, which enrolled 8,256 patients with HFrEF who were at risk of hospitalization and death, despite standard-of-care therapy.
As previously reported by this news organization, omecamtiv mecarbil produced a positive result for the study’s primary endpoint, with a 2.1% absolute reduction in the combined rate of cardiovascular (CV) death, first HF hospitalization, or first urgent visit for HF, compared with placebo during a median follow-up of about 22 months.
This represented an 8% relative risk reduction and broke down as a 0.6% absolute drop in CV death, compared with placebo, a 0.7% cut in HF hospitalization, and a 0.8% drop in urgent outpatient HF visits.
The results were presented at the American Heart Association 2020 scientific sessions and simultaneously published in the New England Journal of Medicine.
In a statement, Robert I. Blum, president and CEO of Cytokinetics, said, “We are disappointed there was not a greater consensus amongst committee members relating to the benefit-risk of omecamtiv mecarbil, and we maintain our conviction in the strength of evidence supporting its potential benefit for patients suffering from HFrEF.”
He added that the company plans to engage constructively with the FDA as it completes its review of the application for omecamtiv mecarbil.
The drug has a Prescription Drug User Fee Act target date of Feb. 28, 2023.
A version of this article first appeared on Medscape.com.
Cardiologist sues hospital, claims he was fired in retaliation
alleging that he was fired and maligned after raising concerns about poorly performed surgeries and poor ethical practices at the hospital.
Dr. Zelman, from Barnstable, Mass., has been affiliated with Cape Cod Hospital in Hyannis, Mass., for more than 30 years. He helped found the hospital’s Heart and Vascular Institute and has served as its medical director since 2018.
In his lawsuit filed Dec. 6, Dr. Zelman alleges that the defendants, under Mr. Lauf’s leadership, “placed profit above all else, including by prioritizing revenue generation over patient safety and public health.”
Dr. Zelman says the defendants supported him “to the extent his actions were profitable.”
Yet, when he raised patient safety concerns that harmed that bottom line, Dr. Zelman says the defendants retaliated against him, including by threatening his career and reputation and unlawfully terminating his employment with the hospital.
The complaint notes Dr. Zelman is bringing this action “to recover damages for violations of the Massachusetts Healthcare Provider Whistleblower Statute ... as well as for breach of contract and common law claims.”
Dr. Zelman’s complaint alleges the defendants refused to adequately address the “dangerous care and violations of the professional standards of practice” that he reported, “resulting in harmful and tragic consequences.”
It also alleges Mr. Lauf restricted the use of a cerebral protection device used in patients undergoing transcatheter aortic-valve replacement (TAVR) deemed to be at high risk for periprocedural stroke to only those patients whose insurance reimbursed at higher rates.
Dr. Zelman says he objected to this prohibition “in accordance with his contractual and ethical obligations to ensure treatment of patients without regard to their ability to pay.”
Dr. Zelman’s lawsuit further alleges that Mr. Lauf launched a “trumped-up” and “baseless, biased, and retaliatory sham” investigation against him.
In a statement sent to the Boston Globe, Cape Cod Hospital denied Dr. Zelman’s claims that the cardiologist was retaliated against for raising patient safety issues, or that the hospital didn’t take action to improve cardiac care at the facility.
Voiced concerns
In a statement sent to this news organization, Dr. Zelman, now in private practice, said, “Over the past 25 years, I have been instrumental in bringing advanced cardiac care to Cape Cod. My commitment has always been to delivering the same quality outcomes and safety as the academic centers in Boston.
“Unfortunately, over the past 5 years, there has been inadequate oversight by the hospital administration and problems have occurred that in my opinion have led to serious patient consequences,” Dr. Zelman stated.
He said he has “voiced concerns over several years and they have been ignored.”
He added that Cape Cod Hospital offered him a million-dollar contract as long as he agreed to immediately issue a written statement endorsing the quality and safety of the cardiac surgical program that no longer exists.
“No amount of money was going to buy my silence,” Dr. Zelman told this news organization.
In his lawsuit, Dr. Zelman is seeking an undisclosed amount in damages, including back and front pay, lost benefits, physical and emotional distress, and attorneys’ fees.
This news organization reached out to Cape Cod Hospital for comment but has not yet received a response.
A version of this article first appeared on Medscape.com.
alleging that he was fired and maligned after raising concerns about poorly performed surgeries and poor ethical practices at the hospital.
Dr. Zelman, from Barnstable, Mass., has been affiliated with Cape Cod Hospital in Hyannis, Mass., for more than 30 years. He helped found the hospital’s Heart and Vascular Institute and has served as its medical director since 2018.
In his lawsuit filed Dec. 6, Dr. Zelman alleges that the defendants, under Mr. Lauf’s leadership, “placed profit above all else, including by prioritizing revenue generation over patient safety and public health.”
Dr. Zelman says the defendants supported him “to the extent his actions were profitable.”
Yet, when he raised patient safety concerns that harmed that bottom line, Dr. Zelman says the defendants retaliated against him, including by threatening his career and reputation and unlawfully terminating his employment with the hospital.
The complaint notes Dr. Zelman is bringing this action “to recover damages for violations of the Massachusetts Healthcare Provider Whistleblower Statute ... as well as for breach of contract and common law claims.”
Dr. Zelman’s complaint alleges the defendants refused to adequately address the “dangerous care and violations of the professional standards of practice” that he reported, “resulting in harmful and tragic consequences.”
It also alleges Mr. Lauf restricted the use of a cerebral protection device used in patients undergoing transcatheter aortic-valve replacement (TAVR) deemed to be at high risk for periprocedural stroke to only those patients whose insurance reimbursed at higher rates.
Dr. Zelman says he objected to this prohibition “in accordance with his contractual and ethical obligations to ensure treatment of patients without regard to their ability to pay.”
Dr. Zelman’s lawsuit further alleges that Mr. Lauf launched a “trumped-up” and “baseless, biased, and retaliatory sham” investigation against him.
In a statement sent to the Boston Globe, Cape Cod Hospital denied Dr. Zelman’s claims that the cardiologist was retaliated against for raising patient safety issues, or that the hospital didn’t take action to improve cardiac care at the facility.
Voiced concerns
In a statement sent to this news organization, Dr. Zelman, now in private practice, said, “Over the past 25 years, I have been instrumental in bringing advanced cardiac care to Cape Cod. My commitment has always been to delivering the same quality outcomes and safety as the academic centers in Boston.
“Unfortunately, over the past 5 years, there has been inadequate oversight by the hospital administration and problems have occurred that in my opinion have led to serious patient consequences,” Dr. Zelman stated.
He said he has “voiced concerns over several years and they have been ignored.”
He added that Cape Cod Hospital offered him a million-dollar contract as long as he agreed to immediately issue a written statement endorsing the quality and safety of the cardiac surgical program that no longer exists.
“No amount of money was going to buy my silence,” Dr. Zelman told this news organization.
In his lawsuit, Dr. Zelman is seeking an undisclosed amount in damages, including back and front pay, lost benefits, physical and emotional distress, and attorneys’ fees.
This news organization reached out to Cape Cod Hospital for comment but has not yet received a response.
A version of this article first appeared on Medscape.com.
alleging that he was fired and maligned after raising concerns about poorly performed surgeries and poor ethical practices at the hospital.
Dr. Zelman, from Barnstable, Mass., has been affiliated with Cape Cod Hospital in Hyannis, Mass., for more than 30 years. He helped found the hospital’s Heart and Vascular Institute and has served as its medical director since 2018.
In his lawsuit filed Dec. 6, Dr. Zelman alleges that the defendants, under Mr. Lauf’s leadership, “placed profit above all else, including by prioritizing revenue generation over patient safety and public health.”
Dr. Zelman says the defendants supported him “to the extent his actions were profitable.”
Yet, when he raised patient safety concerns that harmed that bottom line, Dr. Zelman says the defendants retaliated against him, including by threatening his career and reputation and unlawfully terminating his employment with the hospital.
The complaint notes Dr. Zelman is bringing this action “to recover damages for violations of the Massachusetts Healthcare Provider Whistleblower Statute ... as well as for breach of contract and common law claims.”
Dr. Zelman’s complaint alleges the defendants refused to adequately address the “dangerous care and violations of the professional standards of practice” that he reported, “resulting in harmful and tragic consequences.”
It also alleges Mr. Lauf restricted the use of a cerebral protection device used in patients undergoing transcatheter aortic-valve replacement (TAVR) deemed to be at high risk for periprocedural stroke to only those patients whose insurance reimbursed at higher rates.
Dr. Zelman says he objected to this prohibition “in accordance with his contractual and ethical obligations to ensure treatment of patients without regard to their ability to pay.”
Dr. Zelman’s lawsuit further alleges that Mr. Lauf launched a “trumped-up” and “baseless, biased, and retaliatory sham” investigation against him.
In a statement sent to the Boston Globe, Cape Cod Hospital denied Dr. Zelman’s claims that the cardiologist was retaliated against for raising patient safety issues, or that the hospital didn’t take action to improve cardiac care at the facility.
Voiced concerns
In a statement sent to this news organization, Dr. Zelman, now in private practice, said, “Over the past 25 years, I have been instrumental in bringing advanced cardiac care to Cape Cod. My commitment has always been to delivering the same quality outcomes and safety as the academic centers in Boston.
“Unfortunately, over the past 5 years, there has been inadequate oversight by the hospital administration and problems have occurred that in my opinion have led to serious patient consequences,” Dr. Zelman stated.
He said he has “voiced concerns over several years and they have been ignored.”
He added that Cape Cod Hospital offered him a million-dollar contract as long as he agreed to immediately issue a written statement endorsing the quality and safety of the cardiac surgical program that no longer exists.
“No amount of money was going to buy my silence,” Dr. Zelman told this news organization.
In his lawsuit, Dr. Zelman is seeking an undisclosed amount in damages, including back and front pay, lost benefits, physical and emotional distress, and attorneys’ fees.
This news organization reached out to Cape Cod Hospital for comment but has not yet received a response.
A version of this article first appeared on Medscape.com.
Bempedoic acid cuts CV risk in the statin-intolerant: CLEAR top-line results
The randomized, placebo-controlled CLEAR Outcomes trial has shown a significant reduction in risk for a composite cardiovascular (CV) endpoint among its patients treated with the lipid-lowering agent bempedoic acid (Nexletol), the drug’s owner, Esperion, announced today.
The trial marks the first time an ATP-citrate lyase inhibitor has shown significant and “clinically meaningful” benefit for patients not adequately managed with standard lipid-modifying agents, Esperion president and CEO Sheldon Koenig said in a press release.
The brief statement provided only top-line results, without P values or other evidence of the magnitude of benefit in the active-therapy group. The company expects to present more complete results “at a key medical conference in the first quarter of 2023.”
CLEAR Outcomes had entered 14,014 patients with a history of or at high risk for CV disease events, elevated low-density lipoprotein cholesterol (LDL-C) levels, and demonstrated intolerance to at least two statins.
They were randomly assigned to bempedoic acid 180 mg once daily or placebo and followed for the primary endpoint of CV death, nonfatal myocardial infarction, nonfatal stroke, or coronary revascularization. The trial, conducted in 32 countries, launched in December 2016.
Bempedoic acid is currently approved for adults with heterozygous familial hypercholesterolemia or established atherosclerotic cardiovascular disease on maximally tolerated statins who require additional LDL-C lowering, the company states.
Concomitant use of bempedoic acid with simvastatin or pravastatin, the press release says, may lead to increased statin concentrations and risk for “simvastatin- or pravastatin-related myopathy.” Therefore, “use with greater than 20 mg of simvastatin or 40 mg of pravastatin should be avoided.”
A version of this article first appeared on Medscape.com.
The randomized, placebo-controlled CLEAR Outcomes trial has shown a significant reduction in risk for a composite cardiovascular (CV) endpoint among its patients treated with the lipid-lowering agent bempedoic acid (Nexletol), the drug’s owner, Esperion, announced today.
The trial marks the first time an ATP-citrate lyase inhibitor has shown significant and “clinically meaningful” benefit for patients not adequately managed with standard lipid-modifying agents, Esperion president and CEO Sheldon Koenig said in a press release.
The brief statement provided only top-line results, without P values or other evidence of the magnitude of benefit in the active-therapy group. The company expects to present more complete results “at a key medical conference in the first quarter of 2023.”
CLEAR Outcomes had entered 14,014 patients with a history of or at high risk for CV disease events, elevated low-density lipoprotein cholesterol (LDL-C) levels, and demonstrated intolerance to at least two statins.
They were randomly assigned to bempedoic acid 180 mg once daily or placebo and followed for the primary endpoint of CV death, nonfatal myocardial infarction, nonfatal stroke, or coronary revascularization. The trial, conducted in 32 countries, launched in December 2016.
Bempedoic acid is currently approved for adults with heterozygous familial hypercholesterolemia or established atherosclerotic cardiovascular disease on maximally tolerated statins who require additional LDL-C lowering, the company states.
Concomitant use of bempedoic acid with simvastatin or pravastatin, the press release says, may lead to increased statin concentrations and risk for “simvastatin- or pravastatin-related myopathy.” Therefore, “use with greater than 20 mg of simvastatin or 40 mg of pravastatin should be avoided.”
A version of this article first appeared on Medscape.com.
The randomized, placebo-controlled CLEAR Outcomes trial has shown a significant reduction in risk for a composite cardiovascular (CV) endpoint among its patients treated with the lipid-lowering agent bempedoic acid (Nexletol), the drug’s owner, Esperion, announced today.
The trial marks the first time an ATP-citrate lyase inhibitor has shown significant and “clinically meaningful” benefit for patients not adequately managed with standard lipid-modifying agents, Esperion president and CEO Sheldon Koenig said in a press release.
The brief statement provided only top-line results, without P values or other evidence of the magnitude of benefit in the active-therapy group. The company expects to present more complete results “at a key medical conference in the first quarter of 2023.”
CLEAR Outcomes had entered 14,014 patients with a history of or at high risk for CV disease events, elevated low-density lipoprotein cholesterol (LDL-C) levels, and demonstrated intolerance to at least two statins.
They were randomly assigned to bempedoic acid 180 mg once daily or placebo and followed for the primary endpoint of CV death, nonfatal myocardial infarction, nonfatal stroke, or coronary revascularization. The trial, conducted in 32 countries, launched in December 2016.
Bempedoic acid is currently approved for adults with heterozygous familial hypercholesterolemia or established atherosclerotic cardiovascular disease on maximally tolerated statins who require additional LDL-C lowering, the company states.
Concomitant use of bempedoic acid with simvastatin or pravastatin, the press release says, may lead to increased statin concentrations and risk for “simvastatin- or pravastatin-related myopathy.” Therefore, “use with greater than 20 mg of simvastatin or 40 mg of pravastatin should be avoided.”
A version of this article first appeared on Medscape.com.