User login
Two states aim to curb diet pill sales to minors
California and New York are on the cusp of going further than the Food and Drug Administration in restricting the sale of nonprescription diet pills to minors as pediatricians and public health advocates try to protect kids from extreme weight-loss gimmicks online.
A bill before Gov. Gavin Newsom would bar anyone under 18 in California from buying over-the-counter weight-loss supplements – whether online or in shops – without a prescription. A similar bill passed by New York lawmakers is on Gov. Kathy Hochul’s desk. Neither Democrat has indicated how he or she will act.
If both bills are signed into law, proponents hope the momentum will build to restrict diet pill sales to children in more states. Massachusetts, New Jersey, and Missouri have introduced similar bills and backers plan to continue their push next year.
Nearly 30 million people in the United States will have an eating disorder in their lifetime; 95% of them are aged between 12 and 25, according to Johns Hopkins All Children’s Hospital. The hospital added that eating disorders pose the highest risk of mortality of any mental health disorder. And it has become easier than ever for minors to get pills that are sold online or on drugstore shelves. All dietary supplements, which include those for weight loss, accounted for nearly 35% of the $63 billion over-the-counter health products industry in 2021, according to Vision Research Reports, a market research firm.
Dietary supplements, which encompass a broad range of vitamins, herbs, and minerals, are classified by the FDA as food and don’t undergo scientific and safety testing as prescription drugs and over-the-counter medicines do.
Public health advocates want to keep weight-loss products – with ads that may promise to “Drop 5 pounds a week!” and pill names like Slim Sense – away from young people, particularly girls, since some research has linked some products to eating disorders. A study in the American Journal of Public Health, which followed more than 10,000 women aged 14-36 over 15 years, found that “those who used diet pills had more than 5 times higher adjusted odds of receiving an eating disorder diagnosis from a health care provider within 1-3 years than those who did not.”
Many pills have been found tainted with banned and dangerous ingredients that may cause cancer, heart attacks, strokes, and other ailments. For example, the FDA advised the public to avoid Slim Sense by Dr. Reade because it contains lorcaserin, which has been found to cause psychiatric disturbances and impairments in attention or memory. The FDA ordered it discontinued and the company couldn’t be reached for comment.
“Unscrupulous manufacturers are willing to take risks with consumers’ health – and they are lacing their products with illegal pharmaceuticals, banned pharmaceuticals, steroids, excessive stimulants, even experimental stimulants,” said S. Bryn Austin, ScD, founding director of the Strategic Training Initiative for the Prevention of Eating Disorders, or STRIPED, which supports the restrictions. “Consumers have no idea that this is what’s in these types of products.”
STRIPED is a public health initiative based at the Harvard School of Public Health, Boston, and Boston Children’s Hospital.
An industry trade group, the Natural Products Association, disputes that diet pills cause eating disorders, citing the lack of consumer complaints to the FDA of adverse events from their members’ products. “According to FDA data, there is no association between the two,” said Kyle Turk, the association’s director of government affairs.
The association contends that its members adhere to safe manufacturing processes, random product testing, and appropriate marketing guidelines. Representatives also worry that if minors can’t buy supplements over the counter, they may buy them from “crooks” on the black market and undermine the integrity of the industry. Under the bills, minors purchasing weight-loss products must show identification along with a prescription.
Not all business groups oppose the ban. The American Herbal Products Association, a trade group representing dietary supplement manufacturers and retailers, dropped its opposition to California’s bill once it was amended to remove ingredient categories that are found in non-diet supplements and vitamins, according to Robert Marriott, director of regulatory affairs.
Children’s advocates have found worrisome trends among young people who envision their ideal body type based on what they see on social media. According to a study commissioned by Fairplay, a nonprofit that seeks to stop harmful marketing practices targeting children, kids as young as 9 were found to be following three or more eating disorder accounts on Instagram, while the median age was 19. The authors called it a “pro–eating disorder bubble.”
Meta, which owns Instagram and Facebook, said the report lacks nuance, such as recognizing the human need to share life’s difficult moments. The company argues that blanket censorship isn’t the answer. “Experts and safety organizations have told us it’s important to strike a balance and allow people to share their personal stories while removing any content that encourages or promotes eating disorders,” Liza Crenshaw, a Meta spokesperson, said in an email.
Jason Nagata, MD, a pediatrician who cares for children and young adults with life-threatening eating disorders, believes that easy access to diet pills contributes to his patients’ conditions at UCSF Benioff Children’s Hospital in San Francisco. That was the case for one of his patients, an emaciated 11-year-old girl.
“She had basically entered a starvation state because she was not getting enough nutrition,” said Dr. Nagata, who provided supporting testimony for the California bill. “She was taking these pills and using other kinds of extreme behaviors to lose weight.”
Dr. Nagata said the number of patients he sees with eating disorders has tripled since the pandemic began. They are desperate to get diet pills, some with modest results. “We’ve had patients who have been so dependent on these products that they will be hospitalized and they’re still ordering these products on Amazon,” he said.
Public health advocates turned to state legislatures in response to the federal government’s limited authority to regulate diet pills. Under a 1994 federal law known as the Dietary Supplement Health and Education Act, the FDA “cannot step in until after there is a clear issue of harm to consumers,” said Dr. Austin.
No match for the supplement industry’s heavy lobbying on Capitol Hill, public health advocates shifted to a state-by-state approach.
There is, however, a push for the FDA to improve oversight of what goes into diet pills. Sen. Dick Durbin (D-Ill.) in April introduced a bill that would require dietary supplement manufacturers to register their products – along with the ingredients – with the regulator.
Proponents say the change is needed because manufacturers have been known to include dangerous ingredients. C. Michael White, PharmD, of the University of Connecticut, Storrs, found 35% of tainted health products came from weight-loss supplements in a review of a health fraud database.
A few ingredients have been banned, including sibutramine, a stimulant. “It was a very commonly used weight-loss supplement that ended up being removed from the U.S. market because of its elevated risk of causing things like heart attacks, strokes, and arrhythmias,” Dr. White said.
Another ingredient was phenolphthalein, which was used in laxatives until it was identified as a suspected carcinogen and banned in 1999. “To think,” he said, “that that product would still be on the U.S. market is just unconscionable.”
This story was produced by KHN, which publishes California Healthline, an editorially independent service of the California Health Care Foundation. KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
California and New York are on the cusp of going further than the Food and Drug Administration in restricting the sale of nonprescription diet pills to minors as pediatricians and public health advocates try to protect kids from extreme weight-loss gimmicks online.
A bill before Gov. Gavin Newsom would bar anyone under 18 in California from buying over-the-counter weight-loss supplements – whether online or in shops – without a prescription. A similar bill passed by New York lawmakers is on Gov. Kathy Hochul’s desk. Neither Democrat has indicated how he or she will act.
If both bills are signed into law, proponents hope the momentum will build to restrict diet pill sales to children in more states. Massachusetts, New Jersey, and Missouri have introduced similar bills and backers plan to continue their push next year.
Nearly 30 million people in the United States will have an eating disorder in their lifetime; 95% of them are aged between 12 and 25, according to Johns Hopkins All Children’s Hospital. The hospital added that eating disorders pose the highest risk of mortality of any mental health disorder. And it has become easier than ever for minors to get pills that are sold online or on drugstore shelves. All dietary supplements, which include those for weight loss, accounted for nearly 35% of the $63 billion over-the-counter health products industry in 2021, according to Vision Research Reports, a market research firm.
Dietary supplements, which encompass a broad range of vitamins, herbs, and minerals, are classified by the FDA as food and don’t undergo scientific and safety testing as prescription drugs and over-the-counter medicines do.
Public health advocates want to keep weight-loss products – with ads that may promise to “Drop 5 pounds a week!” and pill names like Slim Sense – away from young people, particularly girls, since some research has linked some products to eating disorders. A study in the American Journal of Public Health, which followed more than 10,000 women aged 14-36 over 15 years, found that “those who used diet pills had more than 5 times higher adjusted odds of receiving an eating disorder diagnosis from a health care provider within 1-3 years than those who did not.”
Many pills have been found tainted with banned and dangerous ingredients that may cause cancer, heart attacks, strokes, and other ailments. For example, the FDA advised the public to avoid Slim Sense by Dr. Reade because it contains lorcaserin, which has been found to cause psychiatric disturbances and impairments in attention or memory. The FDA ordered it discontinued and the company couldn’t be reached for comment.
“Unscrupulous manufacturers are willing to take risks with consumers’ health – and they are lacing their products with illegal pharmaceuticals, banned pharmaceuticals, steroids, excessive stimulants, even experimental stimulants,” said S. Bryn Austin, ScD, founding director of the Strategic Training Initiative for the Prevention of Eating Disorders, or STRIPED, which supports the restrictions. “Consumers have no idea that this is what’s in these types of products.”
STRIPED is a public health initiative based at the Harvard School of Public Health, Boston, and Boston Children’s Hospital.
An industry trade group, the Natural Products Association, disputes that diet pills cause eating disorders, citing the lack of consumer complaints to the FDA of adverse events from their members’ products. “According to FDA data, there is no association between the two,” said Kyle Turk, the association’s director of government affairs.
The association contends that its members adhere to safe manufacturing processes, random product testing, and appropriate marketing guidelines. Representatives also worry that if minors can’t buy supplements over the counter, they may buy them from “crooks” on the black market and undermine the integrity of the industry. Under the bills, minors purchasing weight-loss products must show identification along with a prescription.
Not all business groups oppose the ban. The American Herbal Products Association, a trade group representing dietary supplement manufacturers and retailers, dropped its opposition to California’s bill once it was amended to remove ingredient categories that are found in non-diet supplements and vitamins, according to Robert Marriott, director of regulatory affairs.
Children’s advocates have found worrisome trends among young people who envision their ideal body type based on what they see on social media. According to a study commissioned by Fairplay, a nonprofit that seeks to stop harmful marketing practices targeting children, kids as young as 9 were found to be following three or more eating disorder accounts on Instagram, while the median age was 19. The authors called it a “pro–eating disorder bubble.”
Meta, which owns Instagram and Facebook, said the report lacks nuance, such as recognizing the human need to share life’s difficult moments. The company argues that blanket censorship isn’t the answer. “Experts and safety organizations have told us it’s important to strike a balance and allow people to share their personal stories while removing any content that encourages or promotes eating disorders,” Liza Crenshaw, a Meta spokesperson, said in an email.
Jason Nagata, MD, a pediatrician who cares for children and young adults with life-threatening eating disorders, believes that easy access to diet pills contributes to his patients’ conditions at UCSF Benioff Children’s Hospital in San Francisco. That was the case for one of his patients, an emaciated 11-year-old girl.
“She had basically entered a starvation state because she was not getting enough nutrition,” said Dr. Nagata, who provided supporting testimony for the California bill. “She was taking these pills and using other kinds of extreme behaviors to lose weight.”
Dr. Nagata said the number of patients he sees with eating disorders has tripled since the pandemic began. They are desperate to get diet pills, some with modest results. “We’ve had patients who have been so dependent on these products that they will be hospitalized and they’re still ordering these products on Amazon,” he said.
Public health advocates turned to state legislatures in response to the federal government’s limited authority to regulate diet pills. Under a 1994 federal law known as the Dietary Supplement Health and Education Act, the FDA “cannot step in until after there is a clear issue of harm to consumers,” said Dr. Austin.
No match for the supplement industry’s heavy lobbying on Capitol Hill, public health advocates shifted to a state-by-state approach.
There is, however, a push for the FDA to improve oversight of what goes into diet pills. Sen. Dick Durbin (D-Ill.) in April introduced a bill that would require dietary supplement manufacturers to register their products – along with the ingredients – with the regulator.
Proponents say the change is needed because manufacturers have been known to include dangerous ingredients. C. Michael White, PharmD, of the University of Connecticut, Storrs, found 35% of tainted health products came from weight-loss supplements in a review of a health fraud database.
A few ingredients have been banned, including sibutramine, a stimulant. “It was a very commonly used weight-loss supplement that ended up being removed from the U.S. market because of its elevated risk of causing things like heart attacks, strokes, and arrhythmias,” Dr. White said.
Another ingredient was phenolphthalein, which was used in laxatives until it was identified as a suspected carcinogen and banned in 1999. “To think,” he said, “that that product would still be on the U.S. market is just unconscionable.”
This story was produced by KHN, which publishes California Healthline, an editorially independent service of the California Health Care Foundation. KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
California and New York are on the cusp of going further than the Food and Drug Administration in restricting the sale of nonprescription diet pills to minors as pediatricians and public health advocates try to protect kids from extreme weight-loss gimmicks online.
A bill before Gov. Gavin Newsom would bar anyone under 18 in California from buying over-the-counter weight-loss supplements – whether online or in shops – without a prescription. A similar bill passed by New York lawmakers is on Gov. Kathy Hochul’s desk. Neither Democrat has indicated how he or she will act.
If both bills are signed into law, proponents hope the momentum will build to restrict diet pill sales to children in more states. Massachusetts, New Jersey, and Missouri have introduced similar bills and backers plan to continue their push next year.
Nearly 30 million people in the United States will have an eating disorder in their lifetime; 95% of them are aged between 12 and 25, according to Johns Hopkins All Children’s Hospital. The hospital added that eating disorders pose the highest risk of mortality of any mental health disorder. And it has become easier than ever for minors to get pills that are sold online or on drugstore shelves. All dietary supplements, which include those for weight loss, accounted for nearly 35% of the $63 billion over-the-counter health products industry in 2021, according to Vision Research Reports, a market research firm.
Dietary supplements, which encompass a broad range of vitamins, herbs, and minerals, are classified by the FDA as food and don’t undergo scientific and safety testing as prescription drugs and over-the-counter medicines do.
Public health advocates want to keep weight-loss products – with ads that may promise to “Drop 5 pounds a week!” and pill names like Slim Sense – away from young people, particularly girls, since some research has linked some products to eating disorders. A study in the American Journal of Public Health, which followed more than 10,000 women aged 14-36 over 15 years, found that “those who used diet pills had more than 5 times higher adjusted odds of receiving an eating disorder diagnosis from a health care provider within 1-3 years than those who did not.”
Many pills have been found tainted with banned and dangerous ingredients that may cause cancer, heart attacks, strokes, and other ailments. For example, the FDA advised the public to avoid Slim Sense by Dr. Reade because it contains lorcaserin, which has been found to cause psychiatric disturbances and impairments in attention or memory. The FDA ordered it discontinued and the company couldn’t be reached for comment.
“Unscrupulous manufacturers are willing to take risks with consumers’ health – and they are lacing their products with illegal pharmaceuticals, banned pharmaceuticals, steroids, excessive stimulants, even experimental stimulants,” said S. Bryn Austin, ScD, founding director of the Strategic Training Initiative for the Prevention of Eating Disorders, or STRIPED, which supports the restrictions. “Consumers have no idea that this is what’s in these types of products.”
STRIPED is a public health initiative based at the Harvard School of Public Health, Boston, and Boston Children’s Hospital.
An industry trade group, the Natural Products Association, disputes that diet pills cause eating disorders, citing the lack of consumer complaints to the FDA of adverse events from their members’ products. “According to FDA data, there is no association between the two,” said Kyle Turk, the association’s director of government affairs.
The association contends that its members adhere to safe manufacturing processes, random product testing, and appropriate marketing guidelines. Representatives also worry that if minors can’t buy supplements over the counter, they may buy them from “crooks” on the black market and undermine the integrity of the industry. Under the bills, minors purchasing weight-loss products must show identification along with a prescription.
Not all business groups oppose the ban. The American Herbal Products Association, a trade group representing dietary supplement manufacturers and retailers, dropped its opposition to California’s bill once it was amended to remove ingredient categories that are found in non-diet supplements and vitamins, according to Robert Marriott, director of regulatory affairs.
Children’s advocates have found worrisome trends among young people who envision their ideal body type based on what they see on social media. According to a study commissioned by Fairplay, a nonprofit that seeks to stop harmful marketing practices targeting children, kids as young as 9 were found to be following three or more eating disorder accounts on Instagram, while the median age was 19. The authors called it a “pro–eating disorder bubble.”
Meta, which owns Instagram and Facebook, said the report lacks nuance, such as recognizing the human need to share life’s difficult moments. The company argues that blanket censorship isn’t the answer. “Experts and safety organizations have told us it’s important to strike a balance and allow people to share their personal stories while removing any content that encourages or promotes eating disorders,” Liza Crenshaw, a Meta spokesperson, said in an email.
Jason Nagata, MD, a pediatrician who cares for children and young adults with life-threatening eating disorders, believes that easy access to diet pills contributes to his patients’ conditions at UCSF Benioff Children’s Hospital in San Francisco. That was the case for one of his patients, an emaciated 11-year-old girl.
“She had basically entered a starvation state because she was not getting enough nutrition,” said Dr. Nagata, who provided supporting testimony for the California bill. “She was taking these pills and using other kinds of extreme behaviors to lose weight.”
Dr. Nagata said the number of patients he sees with eating disorders has tripled since the pandemic began. They are desperate to get diet pills, some with modest results. “We’ve had patients who have been so dependent on these products that they will be hospitalized and they’re still ordering these products on Amazon,” he said.
Public health advocates turned to state legislatures in response to the federal government’s limited authority to regulate diet pills. Under a 1994 federal law known as the Dietary Supplement Health and Education Act, the FDA “cannot step in until after there is a clear issue of harm to consumers,” said Dr. Austin.
No match for the supplement industry’s heavy lobbying on Capitol Hill, public health advocates shifted to a state-by-state approach.
There is, however, a push for the FDA to improve oversight of what goes into diet pills. Sen. Dick Durbin (D-Ill.) in April introduced a bill that would require dietary supplement manufacturers to register their products – along with the ingredients – with the regulator.
Proponents say the change is needed because manufacturers have been known to include dangerous ingredients. C. Michael White, PharmD, of the University of Connecticut, Storrs, found 35% of tainted health products came from weight-loss supplements in a review of a health fraud database.
A few ingredients have been banned, including sibutramine, a stimulant. “It was a very commonly used weight-loss supplement that ended up being removed from the U.S. market because of its elevated risk of causing things like heart attacks, strokes, and arrhythmias,” Dr. White said.
Another ingredient was phenolphthalein, which was used in laxatives until it was identified as a suspected carcinogen and banned in 1999. “To think,” he said, “that that product would still be on the U.S. market is just unconscionable.”
This story was produced by KHN, which publishes California Healthline, an editorially independent service of the California Health Care Foundation. KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
New ESC guidelines for cutting CV risk in noncardiac surgery
The European Society of Cardiology guidelines on cardiovascular assessment and management of patients undergoing noncardiac surgery have seen extensive revision since the 2014 version.
They still have the same aim – to prevent surgery-related bleeding complications, perioperative myocardial infarction/injury (PMI), stent thrombosis, acute heart failure, arrhythmias, pulmonary embolism, ischemic stroke, and cardiovascular (CV) death.
Cochairpersons Sigrun Halvorsen, MD, PhD, and Julinda Mehilli, MD, presented highlights from the guidelines at the annual congress of the European Society of Cardiology and the document was simultaneously published online in the European Heart Journal.
The document classifies noncardiac surgery into three levels of 30-day risk of CV death, MI, or stroke. Low (< 1%) risk includes eye or thyroid surgery; intermediate (1%-5%) risk includes knee or hip replacement or renal transplant; and high (> 5%) risk includes aortic aneurysm, lung transplant, or pancreatic or bladder cancer surgery (see more examples below).
It classifies patients as low risk if they are younger than 65 without CV disease or CV risk factors (smoking, hypertension, diabetes, dyslipidemia, family history); intermediate risk if they are 65 or older or have CV risk factors; and high risk if they have CVD.
In an interview, Dr. Halvorsen, professor in cardiology, University of Oslo, zeroed in on three important revisions:
First, recommendations for preoperative ECG and biomarkers are more specific, he noted.
The guidelines advise that before intermediate- or high-risk noncardiac surgery, in patients who have known CVD, CV risk factors (including age 65 or older), or symptoms suggestive of CVD:
- It is recommended to obtain a preoperative 12-lead ECG (class I).
- It is recommended to measure high-sensitivity cardiac troponin T (hs-cTn T) or high-sensitivity cardiac troponin I (hs-cTn I). It is also recommended to measure these biomarkers at 24 hours and 48 hours post surgery (class I).
- It should be considered to measure B-type natriuretic peptide or N-terminal of the prohormone BNP (NT-proBNP).
However, for low-risk patients undergoing low- and intermediate-risk noncardiac surgery, it is not recommended to routinely obtain preoperative ECG, hs-cTn T/I, or BNP/NT-proBNP concentrations (class III).
Troponins have a stronger class I recommendation, compared with the IIA recommendation for BNP, because they are useful for preoperative risk stratification and for diagnosis of PMI, Dr. Halvorsen explained. “Patients receive painkillers after surgery and may have no pain,” she noted, but they may have PMI, which has a bad prognosis.
Second, the guidelines recommend that “all patients should stop smoking 4 weeks before noncardiac surgery [class I],” she noted. Clinicians should also “measure hemoglobin, and if the patient is anemic, treat the anemia.”
Third, the sections on antithrombotic treatment have been significantly revised. “Bridging – stopping an oral antithrombotic drug and switching to a subcutaneous or IV drug – has been common,” Dr. Halvorsen said, “but recently we have new evidence that in most cases that increases the risk of bleeding.”
“We are [now] much more restrictive with respect to bridging” with unfractionated heparin or low-molecular-weight heparin, she said. “We recommend against bridging in patients with low to moderate thrombotic risk,” and bridging should only be considered in patients with mechanical prosthetic heart valves or with very high thrombotic risk.
More preoperative recommendations
In the guideline overview session at the congress, Dr. Halverson highlighted some of the new recommendations for preoperative risk assessment.
If time allows, it is recommended to optimize guideline-recommended treatment of CVD and control of CV risk factors including blood pressure, dyslipidemia, and diabetes, before noncardiac surgery (class I).
Patients commonly have “murmurs, chest pain, dyspnea, and edema that may suggest severe CVD, but may also be caused by noncardiac disease,” she noted. The guidelines state that “for patients with a newly detected murmur and symptoms or signs of CVD, transthoracic echocardiography is recommended before noncardiac surgery (class I).
“Many studies have been performed to try to find out if initiation of specific drugs before surgery could reduce the risk of complications,” Dr. Halvorsen noted. However, few have shown any benefit and “the question of presurgery initiation of beta-blockers has been greatly debated,” she said. “We have again reviewed the literature and concluded ‘Routine initiation of beta-blockers perioperatively is not recommended (class IIIA).’ “
“We adhere to the guidelines on acute and chronic coronary syndrome recommending 6-12 months of dual antiplatelet treatment as a standard before elective surgery,” she said. “However, in case of time-sensitive surgery, the duration of that treatment can be shortened down to a minimum of 1 month after elective PCI and a minimum of 3 months after PCI and ACS.”
Patients with specific types of CVD
Dr. Mehilli, a professor at Landshut-Achdorf (Germany) Hospital, highlighted some new guideline recommendations for patients who have specific types of cardiovascular disease.
Coronary artery disease (CAD). “For chronic coronary syndrome, a cardiac workup is recommended only for patients undergoing intermediate risk or high-risk noncardiac surgery.”
“Stress imaging should be considered before any high risk, noncardiac surgery in asymptomatic patients with poor functional capacity and prior PCI or coronary artery bypass graft (new recommendation, class IIa).”
Mitral valve regurgitation. For patients undergoing scheduled noncardiac surgery, who remain symptomatic despite guideline-directed medical treatment for mitral valve regurgitation (including resynchronization and myocardial revascularization), consider a valve intervention – either transcatheter or surgical – before noncardiac surgery in eligible patients with acceptable procedural risk (new recommendation).
Cardiac implantable electronic devices (CIED). For high-risk patients with CIEDs undergoing noncardiac surgery with high probability of electromagnetic interference, a CIED checkup and necessary reprogramming immediately before the procedure should be considered (new recommendation).
Arrhythmias. “I want only to stress,” Dr. Mehilli said, “in patients with atrial fibrillation with acute or worsening hemodynamic instability undergoing noncardiac surgery, an emergency electrical cardioversion is recommended (class I).”
Peripheral artery disease (PAD) and abdominal aortic aneurysm. For these patients “we do not recommend a routine referral for a cardiac workup. But we recommend it for patients with poor functional capacity or with significant risk factors or symptoms (new recommendations).”
Chronic arterial hypertension. “We have modified the recommendation, recommending avoidance of large perioperative fluctuations in blood pressure, and we do not recommend deferring noncardiac surgery in patients with stage 1 or 2 hypertension,” she said.
Postoperative cardiovascular complications
The most frequent postoperative cardiovascular complication is PMI, Dr. Mehilli noted.
“In the BASEL-PMI registry, the incidence of this complication around intermediate or high-risk noncardiac surgery was up to 15% among patients older than 65 years or with a history of CAD or PAD, which makes this kind of complication really important to prevent, to assess, and to know how to treat.”
“It is recommended to have a high awareness for perioperative cardiovascular complications, combined with surveillance for PMI in patients undergoing intermediate- or high-risk noncardiac surgery” based on serial measurements of high-sensitivity cardiac troponin.
The guidelines define PMI as “an increase in the delta of high-sensitivity troponin more than the upper level of normal,” Dr. Mehilli said. “It’s different from the one used in a rule-in algorithm for non-STEMI acute coronary syndrome.”
Postoperative atrial fibrillation (AFib) is observed in 2%-30% of noncardiac surgery patients in different registries, particularly in patients undergoing intermediate or high-risk noncardiac surgery, she noted.
“We propose an algorithm on how to prevent and treat this complication. I want to highlight that in patients with hemodynamic unstable postoperative AF[ib], an emergency cardioversion is indicated. For the others, a rate control with the target heart rate of less than 110 beats per minute is indicated.”
In patients with postoperative AFib, long-term oral anticoagulation therapy should be considered in all patients at risk for stroke, considering the anticipated net clinical benefit of oral anticoagulation therapy as well as informed patient preference (new recommendations).
Routine use of beta-blockers to prevent postoperative AFib in patients undergoing noncardiac surgery is not recommended.
The document also covers the management of patients with kidney disease, diabetes, cancer, obesity, and COVID-19. In general, elective noncardiac surgery should be postponed after a patient has COVID-19, until he or she recovers completely, and coexisting conditions are optimized.
The guidelines are available from the ESC website in several formats: pocket guidelines, pocket guidelines smartphone app, guidelines slide set, essential messages, and the European Heart Journal article.
Noncardiac surgery risk categories
The guideline includes a table that classifies noncardiac surgeries into three groups, based on the associated 30-day risk of death, MI, or stroke:
- Low (< 1%): breast, dental, eye, thyroid, and minor gynecologic, orthopedic, and urologic surgery.
- Intermediate (1%-5%): carotid surgery, endovascular aortic aneurysm repair, gallbladder surgery, head or neck surgery, hernia repair, peripheral arterial angioplasty, renal transplant, major gynecologic, orthopedic, or neurologic (hip or spine) surgery, or urologic surgery
- High (> 5%): aortic and major vascular surgery (including aortic aneurysm), bladder removal (usually as a result of cancer), limb amputation, lung or liver transplant, pancreatic surgery, or perforated bowel repair.
The guidelines were endorsed by the European Society of Anaesthesiology and Intensive Care. The guideline authors reported numerous disclosures.
A version of this article first appeared on Medscape.com.
The European Society of Cardiology guidelines on cardiovascular assessment and management of patients undergoing noncardiac surgery have seen extensive revision since the 2014 version.
They still have the same aim – to prevent surgery-related bleeding complications, perioperative myocardial infarction/injury (PMI), stent thrombosis, acute heart failure, arrhythmias, pulmonary embolism, ischemic stroke, and cardiovascular (CV) death.
Cochairpersons Sigrun Halvorsen, MD, PhD, and Julinda Mehilli, MD, presented highlights from the guidelines at the annual congress of the European Society of Cardiology and the document was simultaneously published online in the European Heart Journal.
The document classifies noncardiac surgery into three levels of 30-day risk of CV death, MI, or stroke. Low (< 1%) risk includes eye or thyroid surgery; intermediate (1%-5%) risk includes knee or hip replacement or renal transplant; and high (> 5%) risk includes aortic aneurysm, lung transplant, or pancreatic or bladder cancer surgery (see more examples below).
It classifies patients as low risk if they are younger than 65 without CV disease or CV risk factors (smoking, hypertension, diabetes, dyslipidemia, family history); intermediate risk if they are 65 or older or have CV risk factors; and high risk if they have CVD.
In an interview, Dr. Halvorsen, professor in cardiology, University of Oslo, zeroed in on three important revisions:
First, recommendations for preoperative ECG and biomarkers are more specific, he noted.
The guidelines advise that before intermediate- or high-risk noncardiac surgery, in patients who have known CVD, CV risk factors (including age 65 or older), or symptoms suggestive of CVD:
- It is recommended to obtain a preoperative 12-lead ECG (class I).
- It is recommended to measure high-sensitivity cardiac troponin T (hs-cTn T) or high-sensitivity cardiac troponin I (hs-cTn I). It is also recommended to measure these biomarkers at 24 hours and 48 hours post surgery (class I).
- It should be considered to measure B-type natriuretic peptide or N-terminal of the prohormone BNP (NT-proBNP).
However, for low-risk patients undergoing low- and intermediate-risk noncardiac surgery, it is not recommended to routinely obtain preoperative ECG, hs-cTn T/I, or BNP/NT-proBNP concentrations (class III).
Troponins have a stronger class I recommendation, compared with the IIA recommendation for BNP, because they are useful for preoperative risk stratification and for diagnosis of PMI, Dr. Halvorsen explained. “Patients receive painkillers after surgery and may have no pain,” she noted, but they may have PMI, which has a bad prognosis.
Second, the guidelines recommend that “all patients should stop smoking 4 weeks before noncardiac surgery [class I],” she noted. Clinicians should also “measure hemoglobin, and if the patient is anemic, treat the anemia.”
Third, the sections on antithrombotic treatment have been significantly revised. “Bridging – stopping an oral antithrombotic drug and switching to a subcutaneous or IV drug – has been common,” Dr. Halvorsen said, “but recently we have new evidence that in most cases that increases the risk of bleeding.”
“We are [now] much more restrictive with respect to bridging” with unfractionated heparin or low-molecular-weight heparin, she said. “We recommend against bridging in patients with low to moderate thrombotic risk,” and bridging should only be considered in patients with mechanical prosthetic heart valves or with very high thrombotic risk.
More preoperative recommendations
In the guideline overview session at the congress, Dr. Halverson highlighted some of the new recommendations for preoperative risk assessment.
If time allows, it is recommended to optimize guideline-recommended treatment of CVD and control of CV risk factors including blood pressure, dyslipidemia, and diabetes, before noncardiac surgery (class I).
Patients commonly have “murmurs, chest pain, dyspnea, and edema that may suggest severe CVD, but may also be caused by noncardiac disease,” she noted. The guidelines state that “for patients with a newly detected murmur and symptoms or signs of CVD, transthoracic echocardiography is recommended before noncardiac surgery (class I).
“Many studies have been performed to try to find out if initiation of specific drugs before surgery could reduce the risk of complications,” Dr. Halvorsen noted. However, few have shown any benefit and “the question of presurgery initiation of beta-blockers has been greatly debated,” she said. “We have again reviewed the literature and concluded ‘Routine initiation of beta-blockers perioperatively is not recommended (class IIIA).’ “
“We adhere to the guidelines on acute and chronic coronary syndrome recommending 6-12 months of dual antiplatelet treatment as a standard before elective surgery,” she said. “However, in case of time-sensitive surgery, the duration of that treatment can be shortened down to a minimum of 1 month after elective PCI and a minimum of 3 months after PCI and ACS.”
Patients with specific types of CVD
Dr. Mehilli, a professor at Landshut-Achdorf (Germany) Hospital, highlighted some new guideline recommendations for patients who have specific types of cardiovascular disease.
Coronary artery disease (CAD). “For chronic coronary syndrome, a cardiac workup is recommended only for patients undergoing intermediate risk or high-risk noncardiac surgery.”
“Stress imaging should be considered before any high risk, noncardiac surgery in asymptomatic patients with poor functional capacity and prior PCI or coronary artery bypass graft (new recommendation, class IIa).”
Mitral valve regurgitation. For patients undergoing scheduled noncardiac surgery, who remain symptomatic despite guideline-directed medical treatment for mitral valve regurgitation (including resynchronization and myocardial revascularization), consider a valve intervention – either transcatheter or surgical – before noncardiac surgery in eligible patients with acceptable procedural risk (new recommendation).
Cardiac implantable electronic devices (CIED). For high-risk patients with CIEDs undergoing noncardiac surgery with high probability of electromagnetic interference, a CIED checkup and necessary reprogramming immediately before the procedure should be considered (new recommendation).
Arrhythmias. “I want only to stress,” Dr. Mehilli said, “in patients with atrial fibrillation with acute or worsening hemodynamic instability undergoing noncardiac surgery, an emergency electrical cardioversion is recommended (class I).”
Peripheral artery disease (PAD) and abdominal aortic aneurysm. For these patients “we do not recommend a routine referral for a cardiac workup. But we recommend it for patients with poor functional capacity or with significant risk factors or symptoms (new recommendations).”
Chronic arterial hypertension. “We have modified the recommendation, recommending avoidance of large perioperative fluctuations in blood pressure, and we do not recommend deferring noncardiac surgery in patients with stage 1 or 2 hypertension,” she said.
Postoperative cardiovascular complications
The most frequent postoperative cardiovascular complication is PMI, Dr. Mehilli noted.
“In the BASEL-PMI registry, the incidence of this complication around intermediate or high-risk noncardiac surgery was up to 15% among patients older than 65 years or with a history of CAD or PAD, which makes this kind of complication really important to prevent, to assess, and to know how to treat.”
“It is recommended to have a high awareness for perioperative cardiovascular complications, combined with surveillance for PMI in patients undergoing intermediate- or high-risk noncardiac surgery” based on serial measurements of high-sensitivity cardiac troponin.
The guidelines define PMI as “an increase in the delta of high-sensitivity troponin more than the upper level of normal,” Dr. Mehilli said. “It’s different from the one used in a rule-in algorithm for non-STEMI acute coronary syndrome.”
Postoperative atrial fibrillation (AFib) is observed in 2%-30% of noncardiac surgery patients in different registries, particularly in patients undergoing intermediate or high-risk noncardiac surgery, she noted.
“We propose an algorithm on how to prevent and treat this complication. I want to highlight that in patients with hemodynamic unstable postoperative AF[ib], an emergency cardioversion is indicated. For the others, a rate control with the target heart rate of less than 110 beats per minute is indicated.”
In patients with postoperative AFib, long-term oral anticoagulation therapy should be considered in all patients at risk for stroke, considering the anticipated net clinical benefit of oral anticoagulation therapy as well as informed patient preference (new recommendations).
Routine use of beta-blockers to prevent postoperative AFib in patients undergoing noncardiac surgery is not recommended.
The document also covers the management of patients with kidney disease, diabetes, cancer, obesity, and COVID-19. In general, elective noncardiac surgery should be postponed after a patient has COVID-19, until he or she recovers completely, and coexisting conditions are optimized.
The guidelines are available from the ESC website in several formats: pocket guidelines, pocket guidelines smartphone app, guidelines slide set, essential messages, and the European Heart Journal article.
Noncardiac surgery risk categories
The guideline includes a table that classifies noncardiac surgeries into three groups, based on the associated 30-day risk of death, MI, or stroke:
- Low (< 1%): breast, dental, eye, thyroid, and minor gynecologic, orthopedic, and urologic surgery.
- Intermediate (1%-5%): carotid surgery, endovascular aortic aneurysm repair, gallbladder surgery, head or neck surgery, hernia repair, peripheral arterial angioplasty, renal transplant, major gynecologic, orthopedic, or neurologic (hip or spine) surgery, or urologic surgery
- High (> 5%): aortic and major vascular surgery (including aortic aneurysm), bladder removal (usually as a result of cancer), limb amputation, lung or liver transplant, pancreatic surgery, or perforated bowel repair.
The guidelines were endorsed by the European Society of Anaesthesiology and Intensive Care. The guideline authors reported numerous disclosures.
A version of this article first appeared on Medscape.com.
The European Society of Cardiology guidelines on cardiovascular assessment and management of patients undergoing noncardiac surgery have seen extensive revision since the 2014 version.
They still have the same aim – to prevent surgery-related bleeding complications, perioperative myocardial infarction/injury (PMI), stent thrombosis, acute heart failure, arrhythmias, pulmonary embolism, ischemic stroke, and cardiovascular (CV) death.
Cochairpersons Sigrun Halvorsen, MD, PhD, and Julinda Mehilli, MD, presented highlights from the guidelines at the annual congress of the European Society of Cardiology and the document was simultaneously published online in the European Heart Journal.
The document classifies noncardiac surgery into three levels of 30-day risk of CV death, MI, or stroke. Low (< 1%) risk includes eye or thyroid surgery; intermediate (1%-5%) risk includes knee or hip replacement or renal transplant; and high (> 5%) risk includes aortic aneurysm, lung transplant, or pancreatic or bladder cancer surgery (see more examples below).
It classifies patients as low risk if they are younger than 65 without CV disease or CV risk factors (smoking, hypertension, diabetes, dyslipidemia, family history); intermediate risk if they are 65 or older or have CV risk factors; and high risk if they have CVD.
In an interview, Dr. Halvorsen, professor in cardiology, University of Oslo, zeroed in on three important revisions:
First, recommendations for preoperative ECG and biomarkers are more specific, he noted.
The guidelines advise that before intermediate- or high-risk noncardiac surgery, in patients who have known CVD, CV risk factors (including age 65 or older), or symptoms suggestive of CVD:
- It is recommended to obtain a preoperative 12-lead ECG (class I).
- It is recommended to measure high-sensitivity cardiac troponin T (hs-cTn T) or high-sensitivity cardiac troponin I (hs-cTn I). It is also recommended to measure these biomarkers at 24 hours and 48 hours post surgery (class I).
- It should be considered to measure B-type natriuretic peptide or N-terminal of the prohormone BNP (NT-proBNP).
However, for low-risk patients undergoing low- and intermediate-risk noncardiac surgery, it is not recommended to routinely obtain preoperative ECG, hs-cTn T/I, or BNP/NT-proBNP concentrations (class III).
Troponins have a stronger class I recommendation, compared with the IIA recommendation for BNP, because they are useful for preoperative risk stratification and for diagnosis of PMI, Dr. Halvorsen explained. “Patients receive painkillers after surgery and may have no pain,” she noted, but they may have PMI, which has a bad prognosis.
Second, the guidelines recommend that “all patients should stop smoking 4 weeks before noncardiac surgery [class I],” she noted. Clinicians should also “measure hemoglobin, and if the patient is anemic, treat the anemia.”
Third, the sections on antithrombotic treatment have been significantly revised. “Bridging – stopping an oral antithrombotic drug and switching to a subcutaneous or IV drug – has been common,” Dr. Halvorsen said, “but recently we have new evidence that in most cases that increases the risk of bleeding.”
“We are [now] much more restrictive with respect to bridging” with unfractionated heparin or low-molecular-weight heparin, she said. “We recommend against bridging in patients with low to moderate thrombotic risk,” and bridging should only be considered in patients with mechanical prosthetic heart valves or with very high thrombotic risk.
More preoperative recommendations
In the guideline overview session at the congress, Dr. Halverson highlighted some of the new recommendations for preoperative risk assessment.
If time allows, it is recommended to optimize guideline-recommended treatment of CVD and control of CV risk factors including blood pressure, dyslipidemia, and diabetes, before noncardiac surgery (class I).
Patients commonly have “murmurs, chest pain, dyspnea, and edema that may suggest severe CVD, but may also be caused by noncardiac disease,” she noted. The guidelines state that “for patients with a newly detected murmur and symptoms or signs of CVD, transthoracic echocardiography is recommended before noncardiac surgery (class I).
“Many studies have been performed to try to find out if initiation of specific drugs before surgery could reduce the risk of complications,” Dr. Halvorsen noted. However, few have shown any benefit and “the question of presurgery initiation of beta-blockers has been greatly debated,” she said. “We have again reviewed the literature and concluded ‘Routine initiation of beta-blockers perioperatively is not recommended (class IIIA).’ “
“We adhere to the guidelines on acute and chronic coronary syndrome recommending 6-12 months of dual antiplatelet treatment as a standard before elective surgery,” she said. “However, in case of time-sensitive surgery, the duration of that treatment can be shortened down to a minimum of 1 month after elective PCI and a minimum of 3 months after PCI and ACS.”
Patients with specific types of CVD
Dr. Mehilli, a professor at Landshut-Achdorf (Germany) Hospital, highlighted some new guideline recommendations for patients who have specific types of cardiovascular disease.
Coronary artery disease (CAD). “For chronic coronary syndrome, a cardiac workup is recommended only for patients undergoing intermediate risk or high-risk noncardiac surgery.”
“Stress imaging should be considered before any high risk, noncardiac surgery in asymptomatic patients with poor functional capacity and prior PCI or coronary artery bypass graft (new recommendation, class IIa).”
Mitral valve regurgitation. For patients undergoing scheduled noncardiac surgery, who remain symptomatic despite guideline-directed medical treatment for mitral valve regurgitation (including resynchronization and myocardial revascularization), consider a valve intervention – either transcatheter or surgical – before noncardiac surgery in eligible patients with acceptable procedural risk (new recommendation).
Cardiac implantable electronic devices (CIED). For high-risk patients with CIEDs undergoing noncardiac surgery with high probability of electromagnetic interference, a CIED checkup and necessary reprogramming immediately before the procedure should be considered (new recommendation).
Arrhythmias. “I want only to stress,” Dr. Mehilli said, “in patients with atrial fibrillation with acute or worsening hemodynamic instability undergoing noncardiac surgery, an emergency electrical cardioversion is recommended (class I).”
Peripheral artery disease (PAD) and abdominal aortic aneurysm. For these patients “we do not recommend a routine referral for a cardiac workup. But we recommend it for patients with poor functional capacity or with significant risk factors or symptoms (new recommendations).”
Chronic arterial hypertension. “We have modified the recommendation, recommending avoidance of large perioperative fluctuations in blood pressure, and we do not recommend deferring noncardiac surgery in patients with stage 1 or 2 hypertension,” she said.
Postoperative cardiovascular complications
The most frequent postoperative cardiovascular complication is PMI, Dr. Mehilli noted.
“In the BASEL-PMI registry, the incidence of this complication around intermediate or high-risk noncardiac surgery was up to 15% among patients older than 65 years or with a history of CAD or PAD, which makes this kind of complication really important to prevent, to assess, and to know how to treat.”
“It is recommended to have a high awareness for perioperative cardiovascular complications, combined with surveillance for PMI in patients undergoing intermediate- or high-risk noncardiac surgery” based on serial measurements of high-sensitivity cardiac troponin.
The guidelines define PMI as “an increase in the delta of high-sensitivity troponin more than the upper level of normal,” Dr. Mehilli said. “It’s different from the one used in a rule-in algorithm for non-STEMI acute coronary syndrome.”
Postoperative atrial fibrillation (AFib) is observed in 2%-30% of noncardiac surgery patients in different registries, particularly in patients undergoing intermediate or high-risk noncardiac surgery, she noted.
“We propose an algorithm on how to prevent and treat this complication. I want to highlight that in patients with hemodynamic unstable postoperative AF[ib], an emergency cardioversion is indicated. For the others, a rate control with the target heart rate of less than 110 beats per minute is indicated.”
In patients with postoperative AFib, long-term oral anticoagulation therapy should be considered in all patients at risk for stroke, considering the anticipated net clinical benefit of oral anticoagulation therapy as well as informed patient preference (new recommendations).
Routine use of beta-blockers to prevent postoperative AFib in patients undergoing noncardiac surgery is not recommended.
The document also covers the management of patients with kidney disease, diabetes, cancer, obesity, and COVID-19. In general, elective noncardiac surgery should be postponed after a patient has COVID-19, until he or she recovers completely, and coexisting conditions are optimized.
The guidelines are available from the ESC website in several formats: pocket guidelines, pocket guidelines smartphone app, guidelines slide set, essential messages, and the European Heart Journal article.
Noncardiac surgery risk categories
The guideline includes a table that classifies noncardiac surgeries into three groups, based on the associated 30-day risk of death, MI, or stroke:
- Low (< 1%): breast, dental, eye, thyroid, and minor gynecologic, orthopedic, and urologic surgery.
- Intermediate (1%-5%): carotid surgery, endovascular aortic aneurysm repair, gallbladder surgery, head or neck surgery, hernia repair, peripheral arterial angioplasty, renal transplant, major gynecologic, orthopedic, or neurologic (hip or spine) surgery, or urologic surgery
- High (> 5%): aortic and major vascular surgery (including aortic aneurysm), bladder removal (usually as a result of cancer), limb amputation, lung or liver transplant, pancreatic surgery, or perforated bowel repair.
The guidelines were endorsed by the European Society of Anaesthesiology and Intensive Care. The guideline authors reported numerous disclosures.
A version of this article first appeared on Medscape.com.
FROM ESC CONGRESS 2022
Cumulative blood pressure load: A better predictor of CV events?
Cumulative systolic blood pressure load, which can be calculated from serial blood pressure measurements, may provide better prediction of major cardiovascular events, compared with traditional blood pressure measures, a new study suggests.
“Our results suggest that cumulative blood pressure load is an independent predictor of cardiovascular events and should be used in future cardiovascular risk prediction algorithms,” the authors, led by Nelson Wang, MD, George Institute for Global Health, Sydney, conclude.
The study was published online in the Journal of the American College of Cardiology.
The researchers explain that the management of hypertension has traditionally centered around blood pressure measurements taken at a single timepoint, with adequate control defined as those measurements being below a predefined target threshold.
However, this approach fails to recognize blood pressure as a continuous measure that fluctuates over time and does not acknowledge that the most recently recorded measurement may not reflect previous blood pressure control.
More recently, studies have defined the time a patient spends below blood pressure target, or TIme at TaRgEt (TITRE), as a novel marker of cardiovascular risk that is independent of mean blood pressure.
Although TITRE has the added advantage of incorporating duration of control, it is unable to characterize the magnitude of blood pressure elevation, the researchers note.
They point out that an optimal measure as a risk factor for cardiovascular disease would account for both the magnitude and duration of elevated blood pressure.
Such a measure is cumulative blood pressure load, defined as the area under the curve (AUC) expressed in units of mm Hg by time.
The only prior study of this measure was small and retrospective and calculated cumulative blood pressure load from ambulatory blood pressure monitoring estimated over a short (24-hour) period.
Therefore, the aim of the current study was to estimate the association between cumulative systolic blood pressure load over a longer period (24 months) and subsequent major cardiovascular events.
To do this, the researchers conducted a post-hoc analysis of 9,338 patients with type 2 diabetes in the ADVANCE-ON study.
Cumulative systolic blood pressure load was defined as the AUC for systolic blood pressure values above 130 mm Hg divided by the AUC for all measured systolic blood pressure values over a 24-month exposure period.
Over a median 7.6 years of follow-up, 1,469 major cardiovascular events, 1,615 deaths, and 660 cardiovascular deaths occurred.
Results showed that each one standard deviation increase in cumulative systolic blood pressure load was associated with a 14% increase in major cardiovascular events, a 13% increase in all-cause mortality, and a 21% increase in cardiovascular death.
Cumulative systolic blood pressure load outperformed mean systolic blood pressure, time-below-target, and visit-to-visit systolic blood pressure variability for the prediction of cardiovascular events and death and also discriminated risk and reclassified more patients’ risk correctly than the other measures.
“Small improvements in risk prediction can have a major impact when scaled up across large high-risk populations. Furthermore, cumulative systolic pressure load may also prove useful to inform the design of future clinical trials,” the researchers say.
Although the present study only assessed cumulative systolic blood pressure load over 24 months, clinicians should recognize the importance of this measure over a lifetime, they note.
“This approach emphasizes the importance of early blood pressure–lowering interventions to reduce the cumulative systolic blood pressure load that each individual experiences over their lifetime,” they conclude.
The researchers suggest that, based on these results, cumulative systolic blood pressure load and visit-to-visit systolic blood pressure variability “should be used in conjunction in future cardiovascular risk prediction algorithms.”
In an accompanying editorial, Donald Lloyd-Jones, MD, Northwestern Feinberg School of Medicine, Chicago, says that before routinely adopting these new measures, several additional questions need to be addressed.
He notes that many patients in the current study already had cardiovascular disease, and it is not known whether the benefit was consistent among those with and without cardiovascular disease. In addition, longer term data using blood pressure measurements in the real-world clinical setting would be desirable, as well as information on whether these new measures add incremental value to existing risk prediction equations.
“Certainly, the next guidelines should reconsider all types of blood pressure measures, and other potential predictors, to optimize risk estimation and identification of patients with greatest net benefit from risk-reducing therapies,” Dr. Lloyd-Jones comments.
“Ultimately, clinicians should leverage as much information on their patients as possible to understand their blood pressure–related cardiovascular risk, to identify those who may be more likely have occult or emerging subclinical target organ damage, and to identify those who may have particular net benefit from earlier or more intensive treatment,” he concludes.
“These opportunities are more readily available with integration of data that allow for visualization of longer-term blood pressure patterns and incorporation of home monitoring and ambulatory monitoring data to monitor out-of-office blood pressure levels and control.”
A version of this article first appeared on Medscape.com.
Cumulative systolic blood pressure load, which can be calculated from serial blood pressure measurements, may provide better prediction of major cardiovascular events, compared with traditional blood pressure measures, a new study suggests.
“Our results suggest that cumulative blood pressure load is an independent predictor of cardiovascular events and should be used in future cardiovascular risk prediction algorithms,” the authors, led by Nelson Wang, MD, George Institute for Global Health, Sydney, conclude.
The study was published online in the Journal of the American College of Cardiology.
The researchers explain that the management of hypertension has traditionally centered around blood pressure measurements taken at a single timepoint, with adequate control defined as those measurements being below a predefined target threshold.
However, this approach fails to recognize blood pressure as a continuous measure that fluctuates over time and does not acknowledge that the most recently recorded measurement may not reflect previous blood pressure control.
More recently, studies have defined the time a patient spends below blood pressure target, or TIme at TaRgEt (TITRE), as a novel marker of cardiovascular risk that is independent of mean blood pressure.
Although TITRE has the added advantage of incorporating duration of control, it is unable to characterize the magnitude of blood pressure elevation, the researchers note.
They point out that an optimal measure as a risk factor for cardiovascular disease would account for both the magnitude and duration of elevated blood pressure.
Such a measure is cumulative blood pressure load, defined as the area under the curve (AUC) expressed in units of mm Hg by time.
The only prior study of this measure was small and retrospective and calculated cumulative blood pressure load from ambulatory blood pressure monitoring estimated over a short (24-hour) period.
Therefore, the aim of the current study was to estimate the association between cumulative systolic blood pressure load over a longer period (24 months) and subsequent major cardiovascular events.
To do this, the researchers conducted a post-hoc analysis of 9,338 patients with type 2 diabetes in the ADVANCE-ON study.
Cumulative systolic blood pressure load was defined as the AUC for systolic blood pressure values above 130 mm Hg divided by the AUC for all measured systolic blood pressure values over a 24-month exposure period.
Over a median 7.6 years of follow-up, 1,469 major cardiovascular events, 1,615 deaths, and 660 cardiovascular deaths occurred.
Results showed that each one standard deviation increase in cumulative systolic blood pressure load was associated with a 14% increase in major cardiovascular events, a 13% increase in all-cause mortality, and a 21% increase in cardiovascular death.
Cumulative systolic blood pressure load outperformed mean systolic blood pressure, time-below-target, and visit-to-visit systolic blood pressure variability for the prediction of cardiovascular events and death and also discriminated risk and reclassified more patients’ risk correctly than the other measures.
“Small improvements in risk prediction can have a major impact when scaled up across large high-risk populations. Furthermore, cumulative systolic pressure load may also prove useful to inform the design of future clinical trials,” the researchers say.
Although the present study only assessed cumulative systolic blood pressure load over 24 months, clinicians should recognize the importance of this measure over a lifetime, they note.
“This approach emphasizes the importance of early blood pressure–lowering interventions to reduce the cumulative systolic blood pressure load that each individual experiences over their lifetime,” they conclude.
The researchers suggest that, based on these results, cumulative systolic blood pressure load and visit-to-visit systolic blood pressure variability “should be used in conjunction in future cardiovascular risk prediction algorithms.”
In an accompanying editorial, Donald Lloyd-Jones, MD, Northwestern Feinberg School of Medicine, Chicago, says that before routinely adopting these new measures, several additional questions need to be addressed.
He notes that many patients in the current study already had cardiovascular disease, and it is not known whether the benefit was consistent among those with and without cardiovascular disease. In addition, longer term data using blood pressure measurements in the real-world clinical setting would be desirable, as well as information on whether these new measures add incremental value to existing risk prediction equations.
“Certainly, the next guidelines should reconsider all types of blood pressure measures, and other potential predictors, to optimize risk estimation and identification of patients with greatest net benefit from risk-reducing therapies,” Dr. Lloyd-Jones comments.
“Ultimately, clinicians should leverage as much information on their patients as possible to understand their blood pressure–related cardiovascular risk, to identify those who may be more likely have occult or emerging subclinical target organ damage, and to identify those who may have particular net benefit from earlier or more intensive treatment,” he concludes.
“These opportunities are more readily available with integration of data that allow for visualization of longer-term blood pressure patterns and incorporation of home monitoring and ambulatory monitoring data to monitor out-of-office blood pressure levels and control.”
A version of this article first appeared on Medscape.com.
Cumulative systolic blood pressure load, which can be calculated from serial blood pressure measurements, may provide better prediction of major cardiovascular events, compared with traditional blood pressure measures, a new study suggests.
“Our results suggest that cumulative blood pressure load is an independent predictor of cardiovascular events and should be used in future cardiovascular risk prediction algorithms,” the authors, led by Nelson Wang, MD, George Institute for Global Health, Sydney, conclude.
The study was published online in the Journal of the American College of Cardiology.
The researchers explain that the management of hypertension has traditionally centered around blood pressure measurements taken at a single timepoint, with adequate control defined as those measurements being below a predefined target threshold.
However, this approach fails to recognize blood pressure as a continuous measure that fluctuates over time and does not acknowledge that the most recently recorded measurement may not reflect previous blood pressure control.
More recently, studies have defined the time a patient spends below blood pressure target, or TIme at TaRgEt (TITRE), as a novel marker of cardiovascular risk that is independent of mean blood pressure.
Although TITRE has the added advantage of incorporating duration of control, it is unable to characterize the magnitude of blood pressure elevation, the researchers note.
They point out that an optimal measure as a risk factor for cardiovascular disease would account for both the magnitude and duration of elevated blood pressure.
Such a measure is cumulative blood pressure load, defined as the area under the curve (AUC) expressed in units of mm Hg by time.
The only prior study of this measure was small and retrospective and calculated cumulative blood pressure load from ambulatory blood pressure monitoring estimated over a short (24-hour) period.
Therefore, the aim of the current study was to estimate the association between cumulative systolic blood pressure load over a longer period (24 months) and subsequent major cardiovascular events.
To do this, the researchers conducted a post-hoc analysis of 9,338 patients with type 2 diabetes in the ADVANCE-ON study.
Cumulative systolic blood pressure load was defined as the AUC for systolic blood pressure values above 130 mm Hg divided by the AUC for all measured systolic blood pressure values over a 24-month exposure period.
Over a median 7.6 years of follow-up, 1,469 major cardiovascular events, 1,615 deaths, and 660 cardiovascular deaths occurred.
Results showed that each one standard deviation increase in cumulative systolic blood pressure load was associated with a 14% increase in major cardiovascular events, a 13% increase in all-cause mortality, and a 21% increase in cardiovascular death.
Cumulative systolic blood pressure load outperformed mean systolic blood pressure, time-below-target, and visit-to-visit systolic blood pressure variability for the prediction of cardiovascular events and death and also discriminated risk and reclassified more patients’ risk correctly than the other measures.
“Small improvements in risk prediction can have a major impact when scaled up across large high-risk populations. Furthermore, cumulative systolic pressure load may also prove useful to inform the design of future clinical trials,” the researchers say.
Although the present study only assessed cumulative systolic blood pressure load over 24 months, clinicians should recognize the importance of this measure over a lifetime, they note.
“This approach emphasizes the importance of early blood pressure–lowering interventions to reduce the cumulative systolic blood pressure load that each individual experiences over their lifetime,” they conclude.
The researchers suggest that, based on these results, cumulative systolic blood pressure load and visit-to-visit systolic blood pressure variability “should be used in conjunction in future cardiovascular risk prediction algorithms.”
In an accompanying editorial, Donald Lloyd-Jones, MD, Northwestern Feinberg School of Medicine, Chicago, says that before routinely adopting these new measures, several additional questions need to be addressed.
He notes that many patients in the current study already had cardiovascular disease, and it is not known whether the benefit was consistent among those with and without cardiovascular disease. In addition, longer term data using blood pressure measurements in the real-world clinical setting would be desirable, as well as information on whether these new measures add incremental value to existing risk prediction equations.
“Certainly, the next guidelines should reconsider all types of blood pressure measures, and other potential predictors, to optimize risk estimation and identification of patients with greatest net benefit from risk-reducing therapies,” Dr. Lloyd-Jones comments.
“Ultimately, clinicians should leverage as much information on their patients as possible to understand their blood pressure–related cardiovascular risk, to identify those who may be more likely have occult or emerging subclinical target organ damage, and to identify those who may have particular net benefit from earlier or more intensive treatment,” he concludes.
“These opportunities are more readily available with integration of data that allow for visualization of longer-term blood pressure patterns and incorporation of home monitoring and ambulatory monitoring data to monitor out-of-office blood pressure levels and control.”
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Should patients stand for office BP readings?
a new study suggests.
Combining three standing and three seated BP measurements in the same visit may lead to a “quicker diagnosis and save people a trip back to the office,” Wanpen Vongpatanasin, MD, professor of internal medicine and director of the hypertension section, cardiology division, UT Southwestern Medical Center, Dallas, said in an interview.
The study was presented at the Hypertension Scientific Sessions, sponsored by the American Heart Association.
Practice changing?
Clinical guidelines recommend office BP be taken in a seated position for most patients.
However, research has suggested that the sensitivity of seated office BP in diagnosing hypertension is about 50%, with high specificity around 90% during a single visit, Dr. Vongpatanasin explained.
At the follow-up visit, however, the second office BP yielded higher sensitivity to 80% but specificity fell to 55%. Nevertheless, the accuracy of standing BP measurements for diagnosing hypertension has not been investigated.
In a cross-sectional study, Dr. Vongpatanasin and colleagues determined the accuracy of both seated and standing BP for diagnosing hypertension in a single visit in 125 healthy adults who had not had a previous diagnosis of hypertension and were not taking any BP medications. The cohort had a mean age of 49 years, 62% were women, and 24% were Black.
During each office visit, seated BP was measured three times, then standing BP was measured three times using an automated and validated device.
Average seated BP was 123/76 mm Hg and average standing BP was 126/80 mm Hg.
Of the 125 participants, 42 (34%) had hypertension, defined as 24-hour ambulatory systolic/diastolic BP (SBP/DBP) of ≥ 125/75 mm Hg.
The sensitivity and specificity of seated SBP for hypertension was 43% and 92%, respectively.
“Interestingly, with standing SBP, sensitivity was improved to 74% and specificity dropped to 65% – which is okay; you will have to confirm a positive test anyway and when screening for a common disease you’d rather have a high sensitivity rather than low sensitivity to pick it up in this case,” Dr. Vongpatanasin said.
The area under receiver operating characteristic curve (AUROC) of standing SBP was significantly higher than seated SBP (Bayes factor [BF] = 11.8) when hypertension was defined as 24-hour SBP ≥ 125 mm Hg.
Similarly, when hypertension was defined as 24-hour DBP ≥ 75 mm Hg or daytime DBP ≥ 80 mm Hg, the AUROC of standing DBP was higher than seated DBP (all BF > 3).
The addition of standing to seated BP improved detection of hypertension compared with seated BP alone based on 24-hour SBP/DBP ≥ 125/75 mm Hg or daytime SBP/DBP ≥ 130/80 mm Hg (all BF > 3).
“In our hypertension clinic, we always measure both seated and standing BP in all of our patients,” John Giacona, PA-C, a PhD candidate at UT Southwestern Medical Center and coauthor of the study, told this news organization,
Multiple readings most important
Reached for comment, Johanna Contreras, MD, a cardiologist at Mount Sinai Hospital in New York, noted that diagnosing hypertension is “difficult” and she agrees that multiple readings are important.
“I usually take at least two readings in two different visits before I tell the patient they have high blood pressure,” Dr. Contreras said in an interview.
Dr. Contreras said she takes blood pressure both seated and standing.
“I’m not sure standing versus seated makes a big difference. However, when the patient first comes into the office, it is really important to let them rest and calm down before taking the blood pressure,” she said.
The study had no commercial funding. The authors and Dr. Contreras have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a new study suggests.
Combining three standing and three seated BP measurements in the same visit may lead to a “quicker diagnosis and save people a trip back to the office,” Wanpen Vongpatanasin, MD, professor of internal medicine and director of the hypertension section, cardiology division, UT Southwestern Medical Center, Dallas, said in an interview.
The study was presented at the Hypertension Scientific Sessions, sponsored by the American Heart Association.
Practice changing?
Clinical guidelines recommend office BP be taken in a seated position for most patients.
However, research has suggested that the sensitivity of seated office BP in diagnosing hypertension is about 50%, with high specificity around 90% during a single visit, Dr. Vongpatanasin explained.
At the follow-up visit, however, the second office BP yielded higher sensitivity to 80% but specificity fell to 55%. Nevertheless, the accuracy of standing BP measurements for diagnosing hypertension has not been investigated.
In a cross-sectional study, Dr. Vongpatanasin and colleagues determined the accuracy of both seated and standing BP for diagnosing hypertension in a single visit in 125 healthy adults who had not had a previous diagnosis of hypertension and were not taking any BP medications. The cohort had a mean age of 49 years, 62% were women, and 24% were Black.
During each office visit, seated BP was measured three times, then standing BP was measured three times using an automated and validated device.
Average seated BP was 123/76 mm Hg and average standing BP was 126/80 mm Hg.
Of the 125 participants, 42 (34%) had hypertension, defined as 24-hour ambulatory systolic/diastolic BP (SBP/DBP) of ≥ 125/75 mm Hg.
The sensitivity and specificity of seated SBP for hypertension was 43% and 92%, respectively.
“Interestingly, with standing SBP, sensitivity was improved to 74% and specificity dropped to 65% – which is okay; you will have to confirm a positive test anyway and when screening for a common disease you’d rather have a high sensitivity rather than low sensitivity to pick it up in this case,” Dr. Vongpatanasin said.
The area under receiver operating characteristic curve (AUROC) of standing SBP was significantly higher than seated SBP (Bayes factor [BF] = 11.8) when hypertension was defined as 24-hour SBP ≥ 125 mm Hg.
Similarly, when hypertension was defined as 24-hour DBP ≥ 75 mm Hg or daytime DBP ≥ 80 mm Hg, the AUROC of standing DBP was higher than seated DBP (all BF > 3).
The addition of standing to seated BP improved detection of hypertension compared with seated BP alone based on 24-hour SBP/DBP ≥ 125/75 mm Hg or daytime SBP/DBP ≥ 130/80 mm Hg (all BF > 3).
“In our hypertension clinic, we always measure both seated and standing BP in all of our patients,” John Giacona, PA-C, a PhD candidate at UT Southwestern Medical Center and coauthor of the study, told this news organization,
Multiple readings most important
Reached for comment, Johanna Contreras, MD, a cardiologist at Mount Sinai Hospital in New York, noted that diagnosing hypertension is “difficult” and she agrees that multiple readings are important.
“I usually take at least two readings in two different visits before I tell the patient they have high blood pressure,” Dr. Contreras said in an interview.
Dr. Contreras said she takes blood pressure both seated and standing.
“I’m not sure standing versus seated makes a big difference. However, when the patient first comes into the office, it is really important to let them rest and calm down before taking the blood pressure,” she said.
The study had no commercial funding. The authors and Dr. Contreras have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a new study suggests.
Combining three standing and three seated BP measurements in the same visit may lead to a “quicker diagnosis and save people a trip back to the office,” Wanpen Vongpatanasin, MD, professor of internal medicine and director of the hypertension section, cardiology division, UT Southwestern Medical Center, Dallas, said in an interview.
The study was presented at the Hypertension Scientific Sessions, sponsored by the American Heart Association.
Practice changing?
Clinical guidelines recommend office BP be taken in a seated position for most patients.
However, research has suggested that the sensitivity of seated office BP in diagnosing hypertension is about 50%, with high specificity around 90% during a single visit, Dr. Vongpatanasin explained.
At the follow-up visit, however, the second office BP yielded higher sensitivity to 80% but specificity fell to 55%. Nevertheless, the accuracy of standing BP measurements for diagnosing hypertension has not been investigated.
In a cross-sectional study, Dr. Vongpatanasin and colleagues determined the accuracy of both seated and standing BP for diagnosing hypertension in a single visit in 125 healthy adults who had not had a previous diagnosis of hypertension and were not taking any BP medications. The cohort had a mean age of 49 years, 62% were women, and 24% were Black.
During each office visit, seated BP was measured three times, then standing BP was measured three times using an automated and validated device.
Average seated BP was 123/76 mm Hg and average standing BP was 126/80 mm Hg.
Of the 125 participants, 42 (34%) had hypertension, defined as 24-hour ambulatory systolic/diastolic BP (SBP/DBP) of ≥ 125/75 mm Hg.
The sensitivity and specificity of seated SBP for hypertension was 43% and 92%, respectively.
“Interestingly, with standing SBP, sensitivity was improved to 74% and specificity dropped to 65% – which is okay; you will have to confirm a positive test anyway and when screening for a common disease you’d rather have a high sensitivity rather than low sensitivity to pick it up in this case,” Dr. Vongpatanasin said.
The area under receiver operating characteristic curve (AUROC) of standing SBP was significantly higher than seated SBP (Bayes factor [BF] = 11.8) when hypertension was defined as 24-hour SBP ≥ 125 mm Hg.
Similarly, when hypertension was defined as 24-hour DBP ≥ 75 mm Hg or daytime DBP ≥ 80 mm Hg, the AUROC of standing DBP was higher than seated DBP (all BF > 3).
The addition of standing to seated BP improved detection of hypertension compared with seated BP alone based on 24-hour SBP/DBP ≥ 125/75 mm Hg or daytime SBP/DBP ≥ 130/80 mm Hg (all BF > 3).
“In our hypertension clinic, we always measure both seated and standing BP in all of our patients,” John Giacona, PA-C, a PhD candidate at UT Southwestern Medical Center and coauthor of the study, told this news organization,
Multiple readings most important
Reached for comment, Johanna Contreras, MD, a cardiologist at Mount Sinai Hospital in New York, noted that diagnosing hypertension is “difficult” and she agrees that multiple readings are important.
“I usually take at least two readings in two different visits before I tell the patient they have high blood pressure,” Dr. Contreras said in an interview.
Dr. Contreras said she takes blood pressure both seated and standing.
“I’m not sure standing versus seated makes a big difference. However, when the patient first comes into the office, it is really important to let them rest and calm down before taking the blood pressure,” she said.
The study had no commercial funding. The authors and Dr. Contreras have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM HYPERTENSION 2022
Autoimmune diseases linked to spike in post-MI events
, in a large propensity-matched analysis.
At a median of 2 years after their MI, Medicare beneficiaries with an IMID had adjusted risks that were:
- 15% higher for all-cause death (hazard ratio, 1.15);
- 12% higher for heart failure (HR, 1.12);
- 8% higher for recurrent MI (HR, 1.08); and
- 6% higher risk for coronary reintervention (HR, 1.06; P < .05 for all).
In addition, interventions known to improve outcomes in this context, such as coronary revascularization, were less common in patients with IMID.
“This could be because they usually are sicker and have more risk factors when they present, like kidney disease, so maybe they’re not eligible for the therapy. But by itself, it was surprising they’re not offered these interventions as common[ly] as people who don’t have the disease,” Amgad Mentias, MD, a clinical cardiologist at the Cleveland Clinic, said in an interview.
The study was published Sept. 14 in the Journal of the American Heart Association, with Dr. Mentias as senior author and Heba Wassif, MD, MPH, also with Cleveland Clinic, as first author.
IMIDs, such as rheumatoid arthritis, psoriasis, lupus, and inflammatory bowel disease, are known to be associated with significantly higher cardiovascular disease (CVD) risk due to a greater prevalence of traditional CVD risk factors and chronic systemic inflammation.
Certain disease-modifying agents may also increase patients’ cardiovascular risk. This has been a long-simmering issue for the arthritis and ulcerative colitis drug tofacitinib (Xeljanz, Xeljanz XR), resulting in an updated boxed warning in 2021.
Many of these patients also have joint disease, pain, and fatigue, which can limit physical activity, Dr. Mentias said. “So these small nuances of how to manage these patients, or balance between controlling the inflammation but also improv[ing] cardiac risk factors, is not an easy task.”
Evidence regarding post-MI events has been inconsistent and limited to smaller single-center studies, he said. After propensity-score matching, the present study included 59,820 patients with and 178,547 patients without rheumatic IMIDs followed for a maximum of 6 years.
They were drawn from a cohort of 1.6 million persons aged 65 or older in the Medicare Provider Analysis and Review (MedPAR) file who had been admitted for an MI between 2014 and 2019. Of these, 60,072 had a prior history of rheumatic IMIDs, most commonly rheumatoid arthritis (77.8%), followed by systemic lupus erythematosus (12.2%), psoriasis (5.1%), systemic sclerosis (2.8%), and myositis/dermatomyositis (1.8%).
Patients with an IMID were more often women; had a higher prevalence of valve disease, pulmonary hypertension, hypothyroidism, and anemia; and were more likely to present with non–ST-segment MI (NSTEMI).
Rates of coronary angiography (46.1% vs. 51.5%), percutaneous coronary intervention (31.6% vs. 33.6%), and coronary artery bypass grafting (7.7% vs. 10.7%) were significantly lower in patients with IMIDs who had NSTEMI, compared with patients without an IMID who had NSTEMI. Rates of these interventions were also lower in patients with IMIDs who presented with STEMI versus their peers without an IMID, at 78.2% vs. 80.7%, 70.2% vs. 71.5%, and 4.9% vs. 6.4%, respectively.
Dr. Mentias pointed out that the emerging subspecialty of cardiorheumatology is gaining traction, especially at large hospitals and academic centers, but that less than one-third of persons in the United States with an IMID are likely to be under the care of such specialists.
“It’s important before developing an MI to try and control the different risk factors and improve the risk profile for these patients as much as possible by both specialties, and then, after an unfortunate event like MI happens, it’s important to make sure we offer therapies and treatments that are known to improve outcomes,” he said.
Commenting for this article, Jon Tyler Giles, MD, a clinical researcher who focuses on cardiovascular diseases in rheumatology at Columbia University Vagelos College of Physicians and Surgeons, New York, said that “at least for rheumatoid arthritis, this is something that we already knew. People with rheumatic arthritis, when they have a heart attack, are less likely to get the standard kind of treatments and have worse outcomes. This is a little larger sample, but it’s not a surprise, not a surprise at all.”
He noted that the study could have answered questions regarding potential drivers, but “they didn’t dig down into any of the factors that were associated with the poorer outcomes in the patients with rheumatoid arthritis and lupus and scleroderma.”
Indeed, the investigators acknowledge that the study lacked information on coronary anatomy, duration and severity of the autoimmune disease, imaging data, and medications such as anti-inflammatory or immune-targeted therapies.
Dr. Giles highlighted several factors that can contribute to a poorer post-MI prognosis in patients with rheumatic diseases; these include frailty, being more hypercoaguable, increased rates of myocardial dysfunction and other heart and blood vessel diseases, and chronic treatment with steroids and nonsteroidal anti-inflammatory drugs that often interferes with anticoagulation after a MI or when putting in a stent. “So, there’s lot of moving parts, and not one single thing that is likely the answer.”
In addition, he said, “there’s always going to be a portion of these patients who, despite doing the best that we can with treatment, are going to have very severe disease. That may or may not be the subset of patients that did the worst, but likely they’re overrepresented in those patients.”
Finally, the inability to move the needle may lie with the lack of evidence-based screening and management guidelines for cardiovascular disease in any rheumatic disease, Dr. Giles observed. “There’s no guideline for us to use to decide who needs screening over and above what’s recommended for the general population, and then, even if you do screen, what do you do other than what you would normally?”
Rheumatologists are often reluctant to take up the cardiovascular screening side of things because visits are short, and a lot of that time is spent trying to manage the inflammatory components of a patient’s disease, he said. There’s also a barrier in getting some patients to add a cardiologist to the mix of physicians they already see, especially if they don’t have any symptoms.
“If someone has had an event, it’s a lot easier for people to be convinced to go see the cardiologist, obviously, but prior to having an event, the preventative side of things is something that often gets missed or goes to the wayside,” Dr. Giles said.
The study was partly funded by philanthropic gifts by the Haslam family, Bailey family, and Khouri family to the Cleveland Clinic for coauthor Dr. Milind Desai’s research. Dr. Desai is a consultant for Medtronic and Bristol Myers Squibb and serves on an executive steering committee of a BMS-sponsored trial. The remaining authors report having no relevant disclosures. Dr. Giles is a consultant on drug cardiovascular safety for Pfizer, AbbVie, and Eli Lilly.
A version of this article first appeared on Medscape.com.
, in a large propensity-matched analysis.
At a median of 2 years after their MI, Medicare beneficiaries with an IMID had adjusted risks that were:
- 15% higher for all-cause death (hazard ratio, 1.15);
- 12% higher for heart failure (HR, 1.12);
- 8% higher for recurrent MI (HR, 1.08); and
- 6% higher risk for coronary reintervention (HR, 1.06; P < .05 for all).
In addition, interventions known to improve outcomes in this context, such as coronary revascularization, were less common in patients with IMID.
“This could be because they usually are sicker and have more risk factors when they present, like kidney disease, so maybe they’re not eligible for the therapy. But by itself, it was surprising they’re not offered these interventions as common[ly] as people who don’t have the disease,” Amgad Mentias, MD, a clinical cardiologist at the Cleveland Clinic, said in an interview.
The study was published Sept. 14 in the Journal of the American Heart Association, with Dr. Mentias as senior author and Heba Wassif, MD, MPH, also with Cleveland Clinic, as first author.
IMIDs, such as rheumatoid arthritis, psoriasis, lupus, and inflammatory bowel disease, are known to be associated with significantly higher cardiovascular disease (CVD) risk due to a greater prevalence of traditional CVD risk factors and chronic systemic inflammation.
Certain disease-modifying agents may also increase patients’ cardiovascular risk. This has been a long-simmering issue for the arthritis and ulcerative colitis drug tofacitinib (Xeljanz, Xeljanz XR), resulting in an updated boxed warning in 2021.
Many of these patients also have joint disease, pain, and fatigue, which can limit physical activity, Dr. Mentias said. “So these small nuances of how to manage these patients, or balance between controlling the inflammation but also improv[ing] cardiac risk factors, is not an easy task.”
Evidence regarding post-MI events has been inconsistent and limited to smaller single-center studies, he said. After propensity-score matching, the present study included 59,820 patients with and 178,547 patients without rheumatic IMIDs followed for a maximum of 6 years.
They were drawn from a cohort of 1.6 million persons aged 65 or older in the Medicare Provider Analysis and Review (MedPAR) file who had been admitted for an MI between 2014 and 2019. Of these, 60,072 had a prior history of rheumatic IMIDs, most commonly rheumatoid arthritis (77.8%), followed by systemic lupus erythematosus (12.2%), psoriasis (5.1%), systemic sclerosis (2.8%), and myositis/dermatomyositis (1.8%).
Patients with an IMID were more often women; had a higher prevalence of valve disease, pulmonary hypertension, hypothyroidism, and anemia; and were more likely to present with non–ST-segment MI (NSTEMI).
Rates of coronary angiography (46.1% vs. 51.5%), percutaneous coronary intervention (31.6% vs. 33.6%), and coronary artery bypass grafting (7.7% vs. 10.7%) were significantly lower in patients with IMIDs who had NSTEMI, compared with patients without an IMID who had NSTEMI. Rates of these interventions were also lower in patients with IMIDs who presented with STEMI versus their peers without an IMID, at 78.2% vs. 80.7%, 70.2% vs. 71.5%, and 4.9% vs. 6.4%, respectively.
Dr. Mentias pointed out that the emerging subspecialty of cardiorheumatology is gaining traction, especially at large hospitals and academic centers, but that less than one-third of persons in the United States with an IMID are likely to be under the care of such specialists.
“It’s important before developing an MI to try and control the different risk factors and improve the risk profile for these patients as much as possible by both specialties, and then, after an unfortunate event like MI happens, it’s important to make sure we offer therapies and treatments that are known to improve outcomes,” he said.
Commenting for this article, Jon Tyler Giles, MD, a clinical researcher who focuses on cardiovascular diseases in rheumatology at Columbia University Vagelos College of Physicians and Surgeons, New York, said that “at least for rheumatoid arthritis, this is something that we already knew. People with rheumatic arthritis, when they have a heart attack, are less likely to get the standard kind of treatments and have worse outcomes. This is a little larger sample, but it’s not a surprise, not a surprise at all.”
He noted that the study could have answered questions regarding potential drivers, but “they didn’t dig down into any of the factors that were associated with the poorer outcomes in the patients with rheumatoid arthritis and lupus and scleroderma.”
Indeed, the investigators acknowledge that the study lacked information on coronary anatomy, duration and severity of the autoimmune disease, imaging data, and medications such as anti-inflammatory or immune-targeted therapies.
Dr. Giles highlighted several factors that can contribute to a poorer post-MI prognosis in patients with rheumatic diseases; these include frailty, being more hypercoaguable, increased rates of myocardial dysfunction and other heart and blood vessel diseases, and chronic treatment with steroids and nonsteroidal anti-inflammatory drugs that often interferes with anticoagulation after a MI or when putting in a stent. “So, there’s lot of moving parts, and not one single thing that is likely the answer.”
In addition, he said, “there’s always going to be a portion of these patients who, despite doing the best that we can with treatment, are going to have very severe disease. That may or may not be the subset of patients that did the worst, but likely they’re overrepresented in those patients.”
Finally, the inability to move the needle may lie with the lack of evidence-based screening and management guidelines for cardiovascular disease in any rheumatic disease, Dr. Giles observed. “There’s no guideline for us to use to decide who needs screening over and above what’s recommended for the general population, and then, even if you do screen, what do you do other than what you would normally?”
Rheumatologists are often reluctant to take up the cardiovascular screening side of things because visits are short, and a lot of that time is spent trying to manage the inflammatory components of a patient’s disease, he said. There’s also a barrier in getting some patients to add a cardiologist to the mix of physicians they already see, especially if they don’t have any symptoms.
“If someone has had an event, it’s a lot easier for people to be convinced to go see the cardiologist, obviously, but prior to having an event, the preventative side of things is something that often gets missed or goes to the wayside,” Dr. Giles said.
The study was partly funded by philanthropic gifts by the Haslam family, Bailey family, and Khouri family to the Cleveland Clinic for coauthor Dr. Milind Desai’s research. Dr. Desai is a consultant for Medtronic and Bristol Myers Squibb and serves on an executive steering committee of a BMS-sponsored trial. The remaining authors report having no relevant disclosures. Dr. Giles is a consultant on drug cardiovascular safety for Pfizer, AbbVie, and Eli Lilly.
A version of this article first appeared on Medscape.com.
, in a large propensity-matched analysis.
At a median of 2 years after their MI, Medicare beneficiaries with an IMID had adjusted risks that were:
- 15% higher for all-cause death (hazard ratio, 1.15);
- 12% higher for heart failure (HR, 1.12);
- 8% higher for recurrent MI (HR, 1.08); and
- 6% higher risk for coronary reintervention (HR, 1.06; P < .05 for all).
In addition, interventions known to improve outcomes in this context, such as coronary revascularization, were less common in patients with IMID.
“This could be because they usually are sicker and have more risk factors when they present, like kidney disease, so maybe they’re not eligible for the therapy. But by itself, it was surprising they’re not offered these interventions as common[ly] as people who don’t have the disease,” Amgad Mentias, MD, a clinical cardiologist at the Cleveland Clinic, said in an interview.
The study was published Sept. 14 in the Journal of the American Heart Association, with Dr. Mentias as senior author and Heba Wassif, MD, MPH, also with Cleveland Clinic, as first author.
IMIDs, such as rheumatoid arthritis, psoriasis, lupus, and inflammatory bowel disease, are known to be associated with significantly higher cardiovascular disease (CVD) risk due to a greater prevalence of traditional CVD risk factors and chronic systemic inflammation.
Certain disease-modifying agents may also increase patients’ cardiovascular risk. This has been a long-simmering issue for the arthritis and ulcerative colitis drug tofacitinib (Xeljanz, Xeljanz XR), resulting in an updated boxed warning in 2021.
Many of these patients also have joint disease, pain, and fatigue, which can limit physical activity, Dr. Mentias said. “So these small nuances of how to manage these patients, or balance between controlling the inflammation but also improv[ing] cardiac risk factors, is not an easy task.”
Evidence regarding post-MI events has been inconsistent and limited to smaller single-center studies, he said. After propensity-score matching, the present study included 59,820 patients with and 178,547 patients without rheumatic IMIDs followed for a maximum of 6 years.
They were drawn from a cohort of 1.6 million persons aged 65 or older in the Medicare Provider Analysis and Review (MedPAR) file who had been admitted for an MI between 2014 and 2019. Of these, 60,072 had a prior history of rheumatic IMIDs, most commonly rheumatoid arthritis (77.8%), followed by systemic lupus erythematosus (12.2%), psoriasis (5.1%), systemic sclerosis (2.8%), and myositis/dermatomyositis (1.8%).
Patients with an IMID were more often women; had a higher prevalence of valve disease, pulmonary hypertension, hypothyroidism, and anemia; and were more likely to present with non–ST-segment MI (NSTEMI).
Rates of coronary angiography (46.1% vs. 51.5%), percutaneous coronary intervention (31.6% vs. 33.6%), and coronary artery bypass grafting (7.7% vs. 10.7%) were significantly lower in patients with IMIDs who had NSTEMI, compared with patients without an IMID who had NSTEMI. Rates of these interventions were also lower in patients with IMIDs who presented with STEMI versus their peers without an IMID, at 78.2% vs. 80.7%, 70.2% vs. 71.5%, and 4.9% vs. 6.4%, respectively.
Dr. Mentias pointed out that the emerging subspecialty of cardiorheumatology is gaining traction, especially at large hospitals and academic centers, but that less than one-third of persons in the United States with an IMID are likely to be under the care of such specialists.
“It’s important before developing an MI to try and control the different risk factors and improve the risk profile for these patients as much as possible by both specialties, and then, after an unfortunate event like MI happens, it’s important to make sure we offer therapies and treatments that are known to improve outcomes,” he said.
Commenting for this article, Jon Tyler Giles, MD, a clinical researcher who focuses on cardiovascular diseases in rheumatology at Columbia University Vagelos College of Physicians and Surgeons, New York, said that “at least for rheumatoid arthritis, this is something that we already knew. People with rheumatic arthritis, when they have a heart attack, are less likely to get the standard kind of treatments and have worse outcomes. This is a little larger sample, but it’s not a surprise, not a surprise at all.”
He noted that the study could have answered questions regarding potential drivers, but “they didn’t dig down into any of the factors that were associated with the poorer outcomes in the patients with rheumatoid arthritis and lupus and scleroderma.”
Indeed, the investigators acknowledge that the study lacked information on coronary anatomy, duration and severity of the autoimmune disease, imaging data, and medications such as anti-inflammatory or immune-targeted therapies.
Dr. Giles highlighted several factors that can contribute to a poorer post-MI prognosis in patients with rheumatic diseases; these include frailty, being more hypercoaguable, increased rates of myocardial dysfunction and other heart and blood vessel diseases, and chronic treatment with steroids and nonsteroidal anti-inflammatory drugs that often interferes with anticoagulation after a MI or when putting in a stent. “So, there’s lot of moving parts, and not one single thing that is likely the answer.”
In addition, he said, “there’s always going to be a portion of these patients who, despite doing the best that we can with treatment, are going to have very severe disease. That may or may not be the subset of patients that did the worst, but likely they’re overrepresented in those patients.”
Finally, the inability to move the needle may lie with the lack of evidence-based screening and management guidelines for cardiovascular disease in any rheumatic disease, Dr. Giles observed. “There’s no guideline for us to use to decide who needs screening over and above what’s recommended for the general population, and then, even if you do screen, what do you do other than what you would normally?”
Rheumatologists are often reluctant to take up the cardiovascular screening side of things because visits are short, and a lot of that time is spent trying to manage the inflammatory components of a patient’s disease, he said. There’s also a barrier in getting some patients to add a cardiologist to the mix of physicians they already see, especially if they don’t have any symptoms.
“If someone has had an event, it’s a lot easier for people to be convinced to go see the cardiologist, obviously, but prior to having an event, the preventative side of things is something that often gets missed or goes to the wayside,” Dr. Giles said.
The study was partly funded by philanthropic gifts by the Haslam family, Bailey family, and Khouri family to the Cleveland Clinic for coauthor Dr. Milind Desai’s research. Dr. Desai is a consultant for Medtronic and Bristol Myers Squibb and serves on an executive steering committee of a BMS-sponsored trial. The remaining authors report having no relevant disclosures. Dr. Giles is a consultant on drug cardiovascular safety for Pfizer, AbbVie, and Eli Lilly.
A version of this article first appeared on Medscape.com.
FROM JOURNAL OF THE AMERICAN HEART ASSOCIATION
TBI is an unrecognized risk factor for cardiovascular disease
(CVD). More severe TBI is associated with higher risk of CVD, new research shows.
Given the relatively young age of post-9/11–era veterans with TBI, there may be an increased burden of heart disease in the future as these veterans age and develop traditional risk factors for CVD, the investigators, led by Ian J. Stewart, MD, with Uniformed Services University, Bethesda, Md., wrote.
The study was published online in JAMA Neurology.
Novel data
Since Sept. 11, 2001, 4.5 million people have served in the U.S. military, with their time in service defined by the long-running wars in Iraq and Afghanistan. Estimates suggest that up to 20% of post-9/11 veterans sustained a TBI.
While some evidence suggests that TBI increases the risk of CVD, prior reports have focused mainly on cerebrovascular outcomes. Until now, the potential association of TBI with CVD has not been comprehensively examined in post-9/11–era veterans.
The retrospective cohort study included 1,559,928 predominantly male post-9/11 veterans, including 301,169 (19.3%) with a history of TBI and 1,258,759 (81%) with no TBI history.
In fully adjusted models, compared with veterans with no TBI history, a history of mild, moderate/severe, or penetrating TBI was associated with increased risk of developing the composite CVD endpoint (coronary artery disease, stroke, peripheral artery disease, and CVD death).
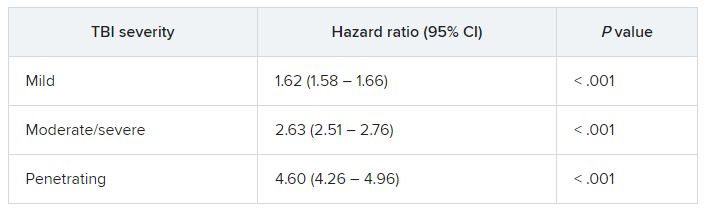
TBIs of all severities were associated with the individual components of the composite outcome, except penetrating TBI and CVD death.
“The association of TBI with subsequent CVD was not attenuated in multivariable models, suggesting that TBI may be accounting for risk that is independent from the other variables,” Dr. Stewart and colleagues wrote.
They noted that the risk was highest shortly after injury, but TBI remained significantly associated with CVD for years after the initial insult.
Why TBI may raise the risk of subsequent CVD remains unclear.
It’s possible that patients with TBI develop more traditional risk factors for CVD through time than do patients without TBI. A study in mice found that TBI led to increased rates of atherosclerosis, the researchers said.
An additional mechanism may be disruption of autonomic regulation, which has been known to occur after TBI.
Another potential pathway is through mental health diagnoses, such as posttraumatic stress disorder; a large body of work has identified associations between PTSD and CVD, including among post-9/11 veterans.
Further work is needed to determine how this risk can be modified to improve outcomes for post-9/11–era veterans, the researchers write.
Unrecognized CVD risk factor?
Reached for comment, Shaheen E. Lakhan, MD, PhD, a neurologist and researcher from Boston who wasn’t involved in the study, said the effects of TBI on heart health are “very underreported, and most clinicians would not make the link.”
“When the brain suffers a traumatic injury, it activates a cascade of neuro-inflammation that goes haywire in an attempt to protect further brain damage. Oftentimes, these inflammatory by-products leak into the body, especially in trauma, when the barriers are broken between brain and body, and can cause systemic body inflammation, which is well associated with heart disease,” Dr. Lakhan said.
In addition, Dr. Lakhan said, “TBI itself localized to just the brain can negatively affect good health habits, leading to worsening heart health, too.”
“Research like this brings light where not much exists and underscores the importance of protecting our brains from physical trauma,” he said.
The study was supported by the assistant secretary of defense for health affairs, endorsed by the Department of Defense through the Psychological Health/Traumatic Brain Injury Research Program Long-Term Impact of Military-Relevant Brain Injury Consortium, and by the U.S. Department of Veterans Affairs. Dr. Stewart and Dr. Lakhan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(CVD). More severe TBI is associated with higher risk of CVD, new research shows.
Given the relatively young age of post-9/11–era veterans with TBI, there may be an increased burden of heart disease in the future as these veterans age and develop traditional risk factors for CVD, the investigators, led by Ian J. Stewart, MD, with Uniformed Services University, Bethesda, Md., wrote.
The study was published online in JAMA Neurology.
Novel data
Since Sept. 11, 2001, 4.5 million people have served in the U.S. military, with their time in service defined by the long-running wars in Iraq and Afghanistan. Estimates suggest that up to 20% of post-9/11 veterans sustained a TBI.
While some evidence suggests that TBI increases the risk of CVD, prior reports have focused mainly on cerebrovascular outcomes. Until now, the potential association of TBI with CVD has not been comprehensively examined in post-9/11–era veterans.
The retrospective cohort study included 1,559,928 predominantly male post-9/11 veterans, including 301,169 (19.3%) with a history of TBI and 1,258,759 (81%) with no TBI history.
In fully adjusted models, compared with veterans with no TBI history, a history of mild, moderate/severe, or penetrating TBI was associated with increased risk of developing the composite CVD endpoint (coronary artery disease, stroke, peripheral artery disease, and CVD death).

TBIs of all severities were associated with the individual components of the composite outcome, except penetrating TBI and CVD death.
“The association of TBI with subsequent CVD was not attenuated in multivariable models, suggesting that TBI may be accounting for risk that is independent from the other variables,” Dr. Stewart and colleagues wrote.
They noted that the risk was highest shortly after injury, but TBI remained significantly associated with CVD for years after the initial insult.
Why TBI may raise the risk of subsequent CVD remains unclear.
It’s possible that patients with TBI develop more traditional risk factors for CVD through time than do patients without TBI. A study in mice found that TBI led to increased rates of atherosclerosis, the researchers said.
An additional mechanism may be disruption of autonomic regulation, which has been known to occur after TBI.
Another potential pathway is through mental health diagnoses, such as posttraumatic stress disorder; a large body of work has identified associations between PTSD and CVD, including among post-9/11 veterans.
Further work is needed to determine how this risk can be modified to improve outcomes for post-9/11–era veterans, the researchers write.
Unrecognized CVD risk factor?
Reached for comment, Shaheen E. Lakhan, MD, PhD, a neurologist and researcher from Boston who wasn’t involved in the study, said the effects of TBI on heart health are “very underreported, and most clinicians would not make the link.”
“When the brain suffers a traumatic injury, it activates a cascade of neuro-inflammation that goes haywire in an attempt to protect further brain damage. Oftentimes, these inflammatory by-products leak into the body, especially in trauma, when the barriers are broken between brain and body, and can cause systemic body inflammation, which is well associated with heart disease,” Dr. Lakhan said.
In addition, Dr. Lakhan said, “TBI itself localized to just the brain can negatively affect good health habits, leading to worsening heart health, too.”
“Research like this brings light where not much exists and underscores the importance of protecting our brains from physical trauma,” he said.
The study was supported by the assistant secretary of defense for health affairs, endorsed by the Department of Defense through the Psychological Health/Traumatic Brain Injury Research Program Long-Term Impact of Military-Relevant Brain Injury Consortium, and by the U.S. Department of Veterans Affairs. Dr. Stewart and Dr. Lakhan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(CVD). More severe TBI is associated with higher risk of CVD, new research shows.
Given the relatively young age of post-9/11–era veterans with TBI, there may be an increased burden of heart disease in the future as these veterans age and develop traditional risk factors for CVD, the investigators, led by Ian J. Stewart, MD, with Uniformed Services University, Bethesda, Md., wrote.
The study was published online in JAMA Neurology.
Novel data
Since Sept. 11, 2001, 4.5 million people have served in the U.S. military, with their time in service defined by the long-running wars in Iraq and Afghanistan. Estimates suggest that up to 20% of post-9/11 veterans sustained a TBI.
While some evidence suggests that TBI increases the risk of CVD, prior reports have focused mainly on cerebrovascular outcomes. Until now, the potential association of TBI with CVD has not been comprehensively examined in post-9/11–era veterans.
The retrospective cohort study included 1,559,928 predominantly male post-9/11 veterans, including 301,169 (19.3%) with a history of TBI and 1,258,759 (81%) with no TBI history.
In fully adjusted models, compared with veterans with no TBI history, a history of mild, moderate/severe, or penetrating TBI was associated with increased risk of developing the composite CVD endpoint (coronary artery disease, stroke, peripheral artery disease, and CVD death).

TBIs of all severities were associated with the individual components of the composite outcome, except penetrating TBI and CVD death.
“The association of TBI with subsequent CVD was not attenuated in multivariable models, suggesting that TBI may be accounting for risk that is independent from the other variables,” Dr. Stewart and colleagues wrote.
They noted that the risk was highest shortly after injury, but TBI remained significantly associated with CVD for years after the initial insult.
Why TBI may raise the risk of subsequent CVD remains unclear.
It’s possible that patients with TBI develop more traditional risk factors for CVD through time than do patients without TBI. A study in mice found that TBI led to increased rates of atherosclerosis, the researchers said.
An additional mechanism may be disruption of autonomic regulation, which has been known to occur after TBI.
Another potential pathway is through mental health diagnoses, such as posttraumatic stress disorder; a large body of work has identified associations between PTSD and CVD, including among post-9/11 veterans.
Further work is needed to determine how this risk can be modified to improve outcomes for post-9/11–era veterans, the researchers write.
Unrecognized CVD risk factor?
Reached for comment, Shaheen E. Lakhan, MD, PhD, a neurologist and researcher from Boston who wasn’t involved in the study, said the effects of TBI on heart health are “very underreported, and most clinicians would not make the link.”
“When the brain suffers a traumatic injury, it activates a cascade of neuro-inflammation that goes haywire in an attempt to protect further brain damage. Oftentimes, these inflammatory by-products leak into the body, especially in trauma, when the barriers are broken between brain and body, and can cause systemic body inflammation, which is well associated with heart disease,” Dr. Lakhan said.
In addition, Dr. Lakhan said, “TBI itself localized to just the brain can negatively affect good health habits, leading to worsening heart health, too.”
“Research like this brings light where not much exists and underscores the importance of protecting our brains from physical trauma,” he said.
The study was supported by the assistant secretary of defense for health affairs, endorsed by the Department of Defense through the Psychological Health/Traumatic Brain Injury Research Program Long-Term Impact of Military-Relevant Brain Injury Consortium, and by the U.S. Department of Veterans Affairs. Dr. Stewart and Dr. Lakhan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Your poop may hold the secret to long life
Lots of things can disrupt your gut health over the years. A high-sugar diet, stress, antibiotics – all are linked to bad changes in the gut microbiome, the microbes that live in your intestinal tract. And this can raise the risk of diseases.
It could be possible, scientists say, by having people take a sample of their own stool when they are young to be put back into their colons when they are older.
While the science to back this up isn’t quite there yet, some researchers are saying we shouldn’t wait. They are calling on existing stool banks to let people start banking their stool now, so it’s there for them to use if the science becomes available.
But how would that work?
First, you’d go to a stool bank and provide a fresh sample of your poop, which would be screened for diseases, washed, processed, and deposited into a long-term storage facility.
Then, down the road, if you get a condition such as inflammatory bowel disease, heart disease, or type 2 diabetes – or if you have a procedure that wipes out your microbiome, like a course of antibiotics or chemotherapy – doctors could use your preserved stool to “re-colonize” your gut, restoring it to its earlier, healthier state, said Scott Weiss, MD, professor of medicine at Harvard Medical School, Boston, and a coauthor of a recent paper on the topic. They would do that using fecal microbiota transplantation, or FMT.
Timing is everything. You’d want a sample from when you’re healthy – say, between the ages of 18 and 35, or before a chronic condition is likely, said Dr. Weiss. But if you’re still healthy into your late 30s, 40s, or even 50s, providing a sample then could still benefit you later in life.
If we could pull off a banking system like this, it could have the potential to treat autoimmune disease, inflammatory bowel disease, diabetes, obesity, and heart disease – or even reverse the effects of aging. How can we make this happen?
Stool banks of today
While stool banks do exist today, the samples inside are destined not for the original donors but rather for sick patients hoping to treat an illness. Using FMT, doctors transfer the fecal material to the patient’s colon, restoring helpful gut microbiota.
Some research shows FMT may help treat inflammatory bowel diseases, such as Crohn’s or ulcerative colitis. Animal studies suggest it could help treat obesity, lengthen lifespan, and reverse some effects of aging, such as age-related decline in brain function. Other clinical trials are looking into its potential as a cancer treatment, said Dr. Weiss.
But outside the lab, FMT is mainly used for one purpose: to treat Clostridioides difficile infection. It works even better than antibiotics, research shows.
But first you need to find a healthy donor, and that’s harder than you might think.
Finding healthy stool samples
Banking our bodily substances is nothing new. Blood banks, for example, are common throughout the United States, and cord blood banking – preserving blood from a baby’s umbilical cord to aid possible future medical needs of the child – is becoming more popular. Sperm donors are highly sought after, and doctors regularly transplant kidneys and bone marrow to patients in need.
So why are we so particular about poop?
Part of the reason may be because feces (like blood, for that matter) can harbor disease – which is why it’s so important to find healthy stool donors. Problem is, this can be surprisingly hard to do.
To donate fecal matter, people must go through a rigorous screening process, said Majdi Osman, MD, chief medical officer for OpenBiome, a nonprofit microbiome research organization.
Until recently, OpenBiome operated a stool donation program, though it has since shifted its focus to research. Potential donors were screened for diseases and mental health conditions, pathogens, and antibiotic resistance. The pass rate was less than 3%.
“We take a very cautious approach because the association between diseases and the microbiome is still being understood,” Dr. Osman said.
FMT also carries risks – though so far, they seem mild. Side effects include mild diarrhea, nausea, belly pain, and fatigue. (The reason? Even the healthiest donor stool may not mix perfectly with your own.)
That’s where the idea of using your own stool comes in, said Yang-Yu Liu, PhD, a Harvard researcher who studies the microbiome and the lead author of the paper mentioned above. It’s not just more appealing but may also be a better “match” for your body.
Should you bank your stool?
While the researchers say we have reason to be optimistic about the future, it’s important to remember that many challenges remain. FMT is early in development, and there’s a lot about the microbiome we still don’t know.
There’s no guarantee, for example, that restoring a person’s microbiome to its formerly disease-free state will keep diseases at bay forever, said Dr. Weiss. If your genes raise your odds of having Crohn’s, for instance, it’s possible the disease could come back.
We also don’t know how long stool samples can be preserved, said Dr. Liu. Stool banks currently store fecal matter for 1 or 2 years, not decades. To protect the proteins and DNA structures for that long, samples would likely need to be stashed at the liquid nitrogen storage temperature of –196° C. (Currently, samples are stored at about –80° C.) Even then, testing would be needed to confirm if the fragile microorganisms in the stool can survive.
This raises another question: Who’s going to regulate all this?
The FDA regulates the use of FMT as a drug for the treatment of C. diff, but as Dr. Liu pointed out, many gastroenterologists consider the gut microbiota an organ. In that case, human fecal matter could be regulated the same way blood, bone, or even egg cells are.
Cord blood banking may be a helpful model, Dr. Liu said.
“We don’t have to start from scratch.”
Then there’s the question of cost. Cord blood banks could be a point of reference for that too, the researchers say. They charge about $1,500 to $2,820 for the first collection and processing, plus a yearly storage fee of $185 to $370.
Despite the unknowns, one thing is for sure: The interest in fecal banking is real – and growing. At least one microbiome firm, Cordlife Group Limited, based in Singapore, announced that it has started to allow people to bank their stool for future use.
“More people should talk about it and think about it,” said Dr. Liu.
A version of this article first appeared on WebMD.com.
Lots of things can disrupt your gut health over the years. A high-sugar diet, stress, antibiotics – all are linked to bad changes in the gut microbiome, the microbes that live in your intestinal tract. And this can raise the risk of diseases.
It could be possible, scientists say, by having people take a sample of their own stool when they are young to be put back into their colons when they are older.
While the science to back this up isn’t quite there yet, some researchers are saying we shouldn’t wait. They are calling on existing stool banks to let people start banking their stool now, so it’s there for them to use if the science becomes available.
But how would that work?
First, you’d go to a stool bank and provide a fresh sample of your poop, which would be screened for diseases, washed, processed, and deposited into a long-term storage facility.
Then, down the road, if you get a condition such as inflammatory bowel disease, heart disease, or type 2 diabetes – or if you have a procedure that wipes out your microbiome, like a course of antibiotics or chemotherapy – doctors could use your preserved stool to “re-colonize” your gut, restoring it to its earlier, healthier state, said Scott Weiss, MD, professor of medicine at Harvard Medical School, Boston, and a coauthor of a recent paper on the topic. They would do that using fecal microbiota transplantation, or FMT.
Timing is everything. You’d want a sample from when you’re healthy – say, between the ages of 18 and 35, or before a chronic condition is likely, said Dr. Weiss. But if you’re still healthy into your late 30s, 40s, or even 50s, providing a sample then could still benefit you later in life.
If we could pull off a banking system like this, it could have the potential to treat autoimmune disease, inflammatory bowel disease, diabetes, obesity, and heart disease – or even reverse the effects of aging. How can we make this happen?
Stool banks of today
While stool banks do exist today, the samples inside are destined not for the original donors but rather for sick patients hoping to treat an illness. Using FMT, doctors transfer the fecal material to the patient’s colon, restoring helpful gut microbiota.
Some research shows FMT may help treat inflammatory bowel diseases, such as Crohn’s or ulcerative colitis. Animal studies suggest it could help treat obesity, lengthen lifespan, and reverse some effects of aging, such as age-related decline in brain function. Other clinical trials are looking into its potential as a cancer treatment, said Dr. Weiss.
But outside the lab, FMT is mainly used for one purpose: to treat Clostridioides difficile infection. It works even better than antibiotics, research shows.
But first you need to find a healthy donor, and that’s harder than you might think.
Finding healthy stool samples
Banking our bodily substances is nothing new. Blood banks, for example, are common throughout the United States, and cord blood banking – preserving blood from a baby’s umbilical cord to aid possible future medical needs of the child – is becoming more popular. Sperm donors are highly sought after, and doctors regularly transplant kidneys and bone marrow to patients in need.
So why are we so particular about poop?
Part of the reason may be because feces (like blood, for that matter) can harbor disease – which is why it’s so important to find healthy stool donors. Problem is, this can be surprisingly hard to do.
To donate fecal matter, people must go through a rigorous screening process, said Majdi Osman, MD, chief medical officer for OpenBiome, a nonprofit microbiome research organization.
Until recently, OpenBiome operated a stool donation program, though it has since shifted its focus to research. Potential donors were screened for diseases and mental health conditions, pathogens, and antibiotic resistance. The pass rate was less than 3%.
“We take a very cautious approach because the association between diseases and the microbiome is still being understood,” Dr. Osman said.
FMT also carries risks – though so far, they seem mild. Side effects include mild diarrhea, nausea, belly pain, and fatigue. (The reason? Even the healthiest donor stool may not mix perfectly with your own.)
That’s where the idea of using your own stool comes in, said Yang-Yu Liu, PhD, a Harvard researcher who studies the microbiome and the lead author of the paper mentioned above. It’s not just more appealing but may also be a better “match” for your body.
Should you bank your stool?
While the researchers say we have reason to be optimistic about the future, it’s important to remember that many challenges remain. FMT is early in development, and there’s a lot about the microbiome we still don’t know.
There’s no guarantee, for example, that restoring a person’s microbiome to its formerly disease-free state will keep diseases at bay forever, said Dr. Weiss. If your genes raise your odds of having Crohn’s, for instance, it’s possible the disease could come back.
We also don’t know how long stool samples can be preserved, said Dr. Liu. Stool banks currently store fecal matter for 1 or 2 years, not decades. To protect the proteins and DNA structures for that long, samples would likely need to be stashed at the liquid nitrogen storage temperature of –196° C. (Currently, samples are stored at about –80° C.) Even then, testing would be needed to confirm if the fragile microorganisms in the stool can survive.
This raises another question: Who’s going to regulate all this?
The FDA regulates the use of FMT as a drug for the treatment of C. diff, but as Dr. Liu pointed out, many gastroenterologists consider the gut microbiota an organ. In that case, human fecal matter could be regulated the same way blood, bone, or even egg cells are.
Cord blood banking may be a helpful model, Dr. Liu said.
“We don’t have to start from scratch.”
Then there’s the question of cost. Cord blood banks could be a point of reference for that too, the researchers say. They charge about $1,500 to $2,820 for the first collection and processing, plus a yearly storage fee of $185 to $370.
Despite the unknowns, one thing is for sure: The interest in fecal banking is real – and growing. At least one microbiome firm, Cordlife Group Limited, based in Singapore, announced that it has started to allow people to bank their stool for future use.
“More people should talk about it and think about it,” said Dr. Liu.
A version of this article first appeared on WebMD.com.
Lots of things can disrupt your gut health over the years. A high-sugar diet, stress, antibiotics – all are linked to bad changes in the gut microbiome, the microbes that live in your intestinal tract. And this can raise the risk of diseases.
It could be possible, scientists say, by having people take a sample of their own stool when they are young to be put back into their colons when they are older.
While the science to back this up isn’t quite there yet, some researchers are saying we shouldn’t wait. They are calling on existing stool banks to let people start banking their stool now, so it’s there for them to use if the science becomes available.
But how would that work?
First, you’d go to a stool bank and provide a fresh sample of your poop, which would be screened for diseases, washed, processed, and deposited into a long-term storage facility.
Then, down the road, if you get a condition such as inflammatory bowel disease, heart disease, or type 2 diabetes – or if you have a procedure that wipes out your microbiome, like a course of antibiotics or chemotherapy – doctors could use your preserved stool to “re-colonize” your gut, restoring it to its earlier, healthier state, said Scott Weiss, MD, professor of medicine at Harvard Medical School, Boston, and a coauthor of a recent paper on the topic. They would do that using fecal microbiota transplantation, or FMT.
Timing is everything. You’d want a sample from when you’re healthy – say, between the ages of 18 and 35, or before a chronic condition is likely, said Dr. Weiss. But if you’re still healthy into your late 30s, 40s, or even 50s, providing a sample then could still benefit you later in life.
If we could pull off a banking system like this, it could have the potential to treat autoimmune disease, inflammatory bowel disease, diabetes, obesity, and heart disease – or even reverse the effects of aging. How can we make this happen?
Stool banks of today
While stool banks do exist today, the samples inside are destined not for the original donors but rather for sick patients hoping to treat an illness. Using FMT, doctors transfer the fecal material to the patient’s colon, restoring helpful gut microbiota.
Some research shows FMT may help treat inflammatory bowel diseases, such as Crohn’s or ulcerative colitis. Animal studies suggest it could help treat obesity, lengthen lifespan, and reverse some effects of aging, such as age-related decline in brain function. Other clinical trials are looking into its potential as a cancer treatment, said Dr. Weiss.
But outside the lab, FMT is mainly used for one purpose: to treat Clostridioides difficile infection. It works even better than antibiotics, research shows.
But first you need to find a healthy donor, and that’s harder than you might think.
Finding healthy stool samples
Banking our bodily substances is nothing new. Blood banks, for example, are common throughout the United States, and cord blood banking – preserving blood from a baby’s umbilical cord to aid possible future medical needs of the child – is becoming more popular. Sperm donors are highly sought after, and doctors regularly transplant kidneys and bone marrow to patients in need.
So why are we so particular about poop?
Part of the reason may be because feces (like blood, for that matter) can harbor disease – which is why it’s so important to find healthy stool donors. Problem is, this can be surprisingly hard to do.
To donate fecal matter, people must go through a rigorous screening process, said Majdi Osman, MD, chief medical officer for OpenBiome, a nonprofit microbiome research organization.
Until recently, OpenBiome operated a stool donation program, though it has since shifted its focus to research. Potential donors were screened for diseases and mental health conditions, pathogens, and antibiotic resistance. The pass rate was less than 3%.
“We take a very cautious approach because the association between diseases and the microbiome is still being understood,” Dr. Osman said.
FMT also carries risks – though so far, they seem mild. Side effects include mild diarrhea, nausea, belly pain, and fatigue. (The reason? Even the healthiest donor stool may not mix perfectly with your own.)
That’s where the idea of using your own stool comes in, said Yang-Yu Liu, PhD, a Harvard researcher who studies the microbiome and the lead author of the paper mentioned above. It’s not just more appealing but may also be a better “match” for your body.
Should you bank your stool?
While the researchers say we have reason to be optimistic about the future, it’s important to remember that many challenges remain. FMT is early in development, and there’s a lot about the microbiome we still don’t know.
There’s no guarantee, for example, that restoring a person’s microbiome to its formerly disease-free state will keep diseases at bay forever, said Dr. Weiss. If your genes raise your odds of having Crohn’s, for instance, it’s possible the disease could come back.
We also don’t know how long stool samples can be preserved, said Dr. Liu. Stool banks currently store fecal matter for 1 or 2 years, not decades. To protect the proteins and DNA structures for that long, samples would likely need to be stashed at the liquid nitrogen storage temperature of –196° C. (Currently, samples are stored at about –80° C.) Even then, testing would be needed to confirm if the fragile microorganisms in the stool can survive.
This raises another question: Who’s going to regulate all this?
The FDA regulates the use of FMT as a drug for the treatment of C. diff, but as Dr. Liu pointed out, many gastroenterologists consider the gut microbiota an organ. In that case, human fecal matter could be regulated the same way blood, bone, or even egg cells are.
Cord blood banking may be a helpful model, Dr. Liu said.
“We don’t have to start from scratch.”
Then there’s the question of cost. Cord blood banks could be a point of reference for that too, the researchers say. They charge about $1,500 to $2,820 for the first collection and processing, plus a yearly storage fee of $185 to $370.
Despite the unknowns, one thing is for sure: The interest in fecal banking is real – and growing. At least one microbiome firm, Cordlife Group Limited, based in Singapore, announced that it has started to allow people to bank their stool for future use.
“More people should talk about it and think about it,” said Dr. Liu.
A version of this article first appeared on WebMD.com.
Heparin pretreatment may safely open arteries before STEMI cath
, suggests a large registry study.
An open infarct-related artery (IRA) at angiography on cath-lab arrival presents STEMI patients an opportunity for earlier reperfusion and a chance, in theory at least, for smaller infarcts and maybe improved clinical outcomes.
In the new analysis, which covers more than 40,000 patients with STEMI in Sweden, the 38% who received heparin before cath-lab arrival were 11% less likely to show IRA occlusion at angiography prior to direct percutaneous coronary intervention (PCI). They also showed a 13% lower 30-day mortality compared with patients who were started on heparin in the cath lab. Importantly, their risk of major bleeding in the hospital did not increase.
The “early reperfusion” associated with IRA patency at angiography “could have long-term benefit due to smaller infarct size,” potentially explaining the observed 30-day survival gain in the pretreatment group, Oskar Love Emilsson, Lund (Sweden) University, said in an interview.
Mr. Emilsson, a third-year medical student, reported the analysis at the annual congress of the European Society of Cardiology, and is lead author on its same-day publication in the journal EuroIntervention.
He mentioned a few cautions in interpreting the study, which is based primarily on data from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR). It included several sensitivity analyses that continued to back pretreatment heparin as a significant predictor of an unoccluded IRA but didn’t consistently support the 30-day mortality benefit seen in the primary analysis.
And, although the pretreatment group overall didn’t have more major bleeds, the risk did go up significantly for those older than 75 or those who weighed less than 60 kg (132 pounds) or underwent catheterization with an access route other than the radial artery. Extra caution should be exercised in such patients who receive heparin before cath-lab arrival for PCI, Mr. Emilsson observed.
“Our results suggest that heparin pretreatment might be a good option to improve patency of infarct related arteries in STEMI,” and potentially clinical outcomes, he said. “However, a definite answer would require a randomized controlled trial.”
Meanwhile, the current study may be the largest yet to look at clinical outcomes after pretreatment with unfractionated heparin before PCI for acute STEMI, the report states. There have been some observational studies, subanalyses of STEMI trials, and even a few limited randomized trials – including the HEAP trial published in 2000 – to weigh in on the subject. Some have supported the strategy, others have not.
“With rapid door-to-balloon times in STEMI, it can be challenging to show a significant difference between a prehospital heparin approach and heparin given in the lab,” observed Sunil V. Rao, MD, NYU Langone Health System, New York, who is not connected with the current study.
Many EDs in the United States have “a STEMI protocol that calls for an IV bolus of heparin. It would be tougher in the U.S. to give it in the ambulance but again, it’s not clear how much advantage that would really provide,” he told this news organization.
Support from randomized trials would be needed before the practice could be formally recommended. “The SCAAR registries have set the standard for how registries should be conducted,” Dr. Rao said. “This is a very well done observational study, but it is observational.”
The priority for STEMI patients, he added, “really should be to get them to the lab as fast as possible. If the ED protocol includes heparin before the cath lab, that’s great, but I don’t think we should delay getting these patients to the lab to accommodate pre–cath-lab heparin.”
The current analysis covered 41,631 patients with STEMI from 2008 through to 2016, of whom 38% were pretreated with heparin in an ambulance or the ED. The remaining 62% initiated heparin in the cath lab.
About one-third of the group had an open IRA at angiography. The adjusted risk ratio (RR) for IRA occlusion at angiography for patients pretreated vs. not pretreated with heparin was 0.89 (95% confidence interval [CI], 0.87-0.90).
The corresponding RR for death within 30 days was 0.87 (95% CI, 0.77-0.99), and for major in-hospital bleeding it was 1.01 (95% CI, 0.86-1.18).
The analysis was adjusted for other medications received before cath-lab arrival, especially a long list of antiplatelets and non-heparin antithrombins. That strengthens the case for heparin pretreatment as an independent predictor of an open IRA at initial angiography, Mr. Emilsson said.
Comparisons of propensity-score–matched subgroups of the total cohort, conducted separately for the IRA-occlusion endpoint and the endpoints of 30-day mortality and major bleeding, produced similar results.
Some observational data suggest that antiplatelet pretreatment with a P2Y12 inhibitor may promote IRA patency on angiography after cath lab arrival, Dr. Rao observed. “This indicates that there probably is a role of earlier antithrombotic therapy in STEMI patients, but the randomized trials have not shown a consistent benefit,” he said, referring in particular to the ATLANTIC trial.
Mr. Emilsson and Dr. Rao disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, suggests a large registry study.
An open infarct-related artery (IRA) at angiography on cath-lab arrival presents STEMI patients an opportunity for earlier reperfusion and a chance, in theory at least, for smaller infarcts and maybe improved clinical outcomes.
In the new analysis, which covers more than 40,000 patients with STEMI in Sweden, the 38% who received heparin before cath-lab arrival were 11% less likely to show IRA occlusion at angiography prior to direct percutaneous coronary intervention (PCI). They also showed a 13% lower 30-day mortality compared with patients who were started on heparin in the cath lab. Importantly, their risk of major bleeding in the hospital did not increase.
The “early reperfusion” associated with IRA patency at angiography “could have long-term benefit due to smaller infarct size,” potentially explaining the observed 30-day survival gain in the pretreatment group, Oskar Love Emilsson, Lund (Sweden) University, said in an interview.
Mr. Emilsson, a third-year medical student, reported the analysis at the annual congress of the European Society of Cardiology, and is lead author on its same-day publication in the journal EuroIntervention.
He mentioned a few cautions in interpreting the study, which is based primarily on data from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR). It included several sensitivity analyses that continued to back pretreatment heparin as a significant predictor of an unoccluded IRA but didn’t consistently support the 30-day mortality benefit seen in the primary analysis.
And, although the pretreatment group overall didn’t have more major bleeds, the risk did go up significantly for those older than 75 or those who weighed less than 60 kg (132 pounds) or underwent catheterization with an access route other than the radial artery. Extra caution should be exercised in such patients who receive heparin before cath-lab arrival for PCI, Mr. Emilsson observed.
“Our results suggest that heparin pretreatment might be a good option to improve patency of infarct related arteries in STEMI,” and potentially clinical outcomes, he said. “However, a definite answer would require a randomized controlled trial.”
Meanwhile, the current study may be the largest yet to look at clinical outcomes after pretreatment with unfractionated heparin before PCI for acute STEMI, the report states. There have been some observational studies, subanalyses of STEMI trials, and even a few limited randomized trials – including the HEAP trial published in 2000 – to weigh in on the subject. Some have supported the strategy, others have not.
“With rapid door-to-balloon times in STEMI, it can be challenging to show a significant difference between a prehospital heparin approach and heparin given in the lab,” observed Sunil V. Rao, MD, NYU Langone Health System, New York, who is not connected with the current study.
Many EDs in the United States have “a STEMI protocol that calls for an IV bolus of heparin. It would be tougher in the U.S. to give it in the ambulance but again, it’s not clear how much advantage that would really provide,” he told this news organization.
Support from randomized trials would be needed before the practice could be formally recommended. “The SCAAR registries have set the standard for how registries should be conducted,” Dr. Rao said. “This is a very well done observational study, but it is observational.”
The priority for STEMI patients, he added, “really should be to get them to the lab as fast as possible. If the ED protocol includes heparin before the cath lab, that’s great, but I don’t think we should delay getting these patients to the lab to accommodate pre–cath-lab heparin.”
The current analysis covered 41,631 patients with STEMI from 2008 through to 2016, of whom 38% were pretreated with heparin in an ambulance or the ED. The remaining 62% initiated heparin in the cath lab.
About one-third of the group had an open IRA at angiography. The adjusted risk ratio (RR) for IRA occlusion at angiography for patients pretreated vs. not pretreated with heparin was 0.89 (95% confidence interval [CI], 0.87-0.90).
The corresponding RR for death within 30 days was 0.87 (95% CI, 0.77-0.99), and for major in-hospital bleeding it was 1.01 (95% CI, 0.86-1.18).
The analysis was adjusted for other medications received before cath-lab arrival, especially a long list of antiplatelets and non-heparin antithrombins. That strengthens the case for heparin pretreatment as an independent predictor of an open IRA at initial angiography, Mr. Emilsson said.
Comparisons of propensity-score–matched subgroups of the total cohort, conducted separately for the IRA-occlusion endpoint and the endpoints of 30-day mortality and major bleeding, produced similar results.
Some observational data suggest that antiplatelet pretreatment with a P2Y12 inhibitor may promote IRA patency on angiography after cath lab arrival, Dr. Rao observed. “This indicates that there probably is a role of earlier antithrombotic therapy in STEMI patients, but the randomized trials have not shown a consistent benefit,” he said, referring in particular to the ATLANTIC trial.
Mr. Emilsson and Dr. Rao disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, suggests a large registry study.
An open infarct-related artery (IRA) at angiography on cath-lab arrival presents STEMI patients an opportunity for earlier reperfusion and a chance, in theory at least, for smaller infarcts and maybe improved clinical outcomes.
In the new analysis, which covers more than 40,000 patients with STEMI in Sweden, the 38% who received heparin before cath-lab arrival were 11% less likely to show IRA occlusion at angiography prior to direct percutaneous coronary intervention (PCI). They also showed a 13% lower 30-day mortality compared with patients who were started on heparin in the cath lab. Importantly, their risk of major bleeding in the hospital did not increase.
The “early reperfusion” associated with IRA patency at angiography “could have long-term benefit due to smaller infarct size,” potentially explaining the observed 30-day survival gain in the pretreatment group, Oskar Love Emilsson, Lund (Sweden) University, said in an interview.
Mr. Emilsson, a third-year medical student, reported the analysis at the annual congress of the European Society of Cardiology, and is lead author on its same-day publication in the journal EuroIntervention.
He mentioned a few cautions in interpreting the study, which is based primarily on data from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR). It included several sensitivity analyses that continued to back pretreatment heparin as a significant predictor of an unoccluded IRA but didn’t consistently support the 30-day mortality benefit seen in the primary analysis.
And, although the pretreatment group overall didn’t have more major bleeds, the risk did go up significantly for those older than 75 or those who weighed less than 60 kg (132 pounds) or underwent catheterization with an access route other than the radial artery. Extra caution should be exercised in such patients who receive heparin before cath-lab arrival for PCI, Mr. Emilsson observed.
“Our results suggest that heparin pretreatment might be a good option to improve patency of infarct related arteries in STEMI,” and potentially clinical outcomes, he said. “However, a definite answer would require a randomized controlled trial.”
Meanwhile, the current study may be the largest yet to look at clinical outcomes after pretreatment with unfractionated heparin before PCI for acute STEMI, the report states. There have been some observational studies, subanalyses of STEMI trials, and even a few limited randomized trials – including the HEAP trial published in 2000 – to weigh in on the subject. Some have supported the strategy, others have not.
“With rapid door-to-balloon times in STEMI, it can be challenging to show a significant difference between a prehospital heparin approach and heparin given in the lab,” observed Sunil V. Rao, MD, NYU Langone Health System, New York, who is not connected with the current study.
Many EDs in the United States have “a STEMI protocol that calls for an IV bolus of heparin. It would be tougher in the U.S. to give it in the ambulance but again, it’s not clear how much advantage that would really provide,” he told this news organization.
Support from randomized trials would be needed before the practice could be formally recommended. “The SCAAR registries have set the standard for how registries should be conducted,” Dr. Rao said. “This is a very well done observational study, but it is observational.”
The priority for STEMI patients, he added, “really should be to get them to the lab as fast as possible. If the ED protocol includes heparin before the cath lab, that’s great, but I don’t think we should delay getting these patients to the lab to accommodate pre–cath-lab heparin.”
The current analysis covered 41,631 patients with STEMI from 2008 through to 2016, of whom 38% were pretreated with heparin in an ambulance or the ED. The remaining 62% initiated heparin in the cath lab.
About one-third of the group had an open IRA at angiography. The adjusted risk ratio (RR) for IRA occlusion at angiography for patients pretreated vs. not pretreated with heparin was 0.89 (95% confidence interval [CI], 0.87-0.90).
The corresponding RR for death within 30 days was 0.87 (95% CI, 0.77-0.99), and for major in-hospital bleeding it was 1.01 (95% CI, 0.86-1.18).
The analysis was adjusted for other medications received before cath-lab arrival, especially a long list of antiplatelets and non-heparin antithrombins. That strengthens the case for heparin pretreatment as an independent predictor of an open IRA at initial angiography, Mr. Emilsson said.
Comparisons of propensity-score–matched subgroups of the total cohort, conducted separately for the IRA-occlusion endpoint and the endpoints of 30-day mortality and major bleeding, produced similar results.
Some observational data suggest that antiplatelet pretreatment with a P2Y12 inhibitor may promote IRA patency on angiography after cath lab arrival, Dr. Rao observed. “This indicates that there probably is a role of earlier antithrombotic therapy in STEMI patients, but the randomized trials have not shown a consistent benefit,” he said, referring in particular to the ATLANTIC trial.
Mr. Emilsson and Dr. Rao disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ESC CONGRESS 2022
PARADISE-MI results obscured as post hoc analysis finds flaws
A post hoc analysis of the PARADISE-MI trial, although not intended to alter the conclusions generated by the published data, suggests that clinically relevant benefits were obscured, providing the basis for recommending different analyses for future studies that are more suited to capture the most clinically significant endpoints.
“What these data show us is that we need clinical trial designs moving towards more pragmatic information that better reflect clinical practice,” reported Otavio Berwanger, MD, PhD, director of the Academic Research Organization at Hospital Israelita Albert Einstein, São Paulo, Brazil.
The reevaluation of the PARADISE-MI data, presented at the annual congress of the European Society of Cardiology in Barcelona, was based on a win ratio analysis and on the inclusion of investigator-reported endpoints, not just adjudicated events. Both appear to reveal clinically meaningful benefits not reflected in the published study, according to Dr. Berwanger.
In PARADISE-MI, which was published in the New England Journal of Medicine last year, more than 5,500 patients were randomized to the angiotensin receptor neprilysin inhibitor (ARNI) sacubitril/valsartan or the ACE inhibitor ramipril after a myocardial infarction. A reduced left ventricular ejection fraction (LVEF), pulmonary congestion, or both were required for enrollment.
For the primary composite outcomes of death from cardiovascular (CV) causes or incident heart failure, the ARNI had a 10% numerical advantage, but it did not reach statistical significance (hazard ratio [HR], 0.90; P = .17).
“PARADISE-MI was a neutral trial. This post hoc analysis will not change that result,” Dr. Berwanger emphasized. However, the post hoc analysis does provide a basis for exploring why conventional trial designs might not be providing answers that are relevant and helpful for clinical practice.
New analysis provides positive trial result
When the data from PARADISE-MI are reevaluated in a hierarchical win ratio analysis with CV death serving as the most severe and important outcome, the principal conclusion changes. Whether events are reevaluated in this format by the clinical events committee (CEC) or by investigators, there is a greater number of total wins than total losses for the ARNI. Combined, sacubitril/valsartan was associated with a win ratio of 1.17 (95% confidence interval, 1.03-1.33; P = 0.015) over ramipril.
Using a sports analogy, Dr. Berwanger explained that the win ratio analysis divides the total number of wins to the total number of losses to provide a much more clinically relevant approach to keeping score. It also used a hierarchical analysis so that the most serious and important events are considered first.
In addition to CV death, this analysis included first hospitalization for heart failure and first outpatient heart failure events. CEC-defined events and events reported by investigators were evaluated separately.
The ARNI had more wins than losses in every category for all outcomes, whether CEC adjudicated or investigator reported, but most of this benefit was generated by the endpoint of CEC-adjudicated CV deaths. This accounted for 36.9% of all events (investigator-documented CV death accounted for 0.7%). This is important because PARADISE-MI, like many standard trials, was conducted on a time-to-primary event basis.
“In this type of analysis, the first event is what counts. Usually time-to-first-event analyses are dominated by nonfatal events,” Dr. Berwanger explained. He believes that placing more weight on the most serious events results in an emphasis on what outcomes are of greatest clinical interest.
In addition, Dr. Berwanger argued that it is important to consider investigator-reported events, not just CEC-adjudicated events. While adjudicated events improve the rigor of the data, Dr. Berwanger suggested it omits outcomes with which clinicians are most concerned.
Investigator, adjudicated outcomes differ
Again, using PARADISE-MI as an example, he reevaluated the primary outcome based on investigator reports. When investigator-reported events are included, the number of events increased in both the ARNI (443 vs. 338) and ramipril (516 vs. 373) arms, but the advantage of the ARNI over the ACE inhibitors now reached statistical significance (HR, 0.85; P = .01).
“The data suggest that maybe we should find definitions for adjudication that are closer to clinical judgment in the real world and clinical practice,” Dr. Berwanger said.
One possible explanation for the neutral result in PARADISE-MI is that benefit of an ARNI over an ACE inhibitor would only be expected in those at risk for progressive left ventricular dysfunction, and it is likely that a substantial proportion of patients enrolled in this trial recovered, according to Johann Bauersachs, MD, PhD, professor and head of cardiology at Hannover (Germany) Medical School.
“You cannot predict which patients with reduced LV function following an MI will go on to chronic remodeling and which will recover,” said Dr. Bauersachs, who was an ESC-invited discussant of Dr. Berwanger’s post hoc analysis.
He agreed that Dr. Berwanger has raised several important issues in standard trial design that might have prevented PARADISE-MI from showing a benefit from an ARNI, but he pointed out that there are other potential issues, such as the low use of mineralocorticoid antagonists in PARADISE-MI, that may have skewed results.
However, he agreed generally with the premise that there is a need for trial design likely to generate more clinically useful information.
“We have now seen the win-ratio approach used in several studies,” said Dr. Bauersachs, citing in particular the EMPULSE trial presented at the 2022 meeting of the American College of Cardiology. “It is a very useful tool, and I think we will be seeing it used more in the future.”
However, he indicated that the issues raised by Dr. Berwanger are not necessarily easily resolved. Dr. Bauersachs endorsed the effort to consider trial designs that generate data that are more immediately clinically applicable but suggested that different types of designs may be required for different types of clinical questions.
Dr. Berwanger reports financial relationships with Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Pfizer, Servier, and Novartis, which provided funding for the PARADISE-MI trial. Dr. Bauersachs reports financial relationships with Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Cardior, Corvia, CVRx, Novartis, Pfizer, Vifor, and Zoll.
A post hoc analysis of the PARADISE-MI trial, although not intended to alter the conclusions generated by the published data, suggests that clinically relevant benefits were obscured, providing the basis for recommending different analyses for future studies that are more suited to capture the most clinically significant endpoints.
“What these data show us is that we need clinical trial designs moving towards more pragmatic information that better reflect clinical practice,” reported Otavio Berwanger, MD, PhD, director of the Academic Research Organization at Hospital Israelita Albert Einstein, São Paulo, Brazil.
The reevaluation of the PARADISE-MI data, presented at the annual congress of the European Society of Cardiology in Barcelona, was based on a win ratio analysis and on the inclusion of investigator-reported endpoints, not just adjudicated events. Both appear to reveal clinically meaningful benefits not reflected in the published study, according to Dr. Berwanger.
In PARADISE-MI, which was published in the New England Journal of Medicine last year, more than 5,500 patients were randomized to the angiotensin receptor neprilysin inhibitor (ARNI) sacubitril/valsartan or the ACE inhibitor ramipril after a myocardial infarction. A reduced left ventricular ejection fraction (LVEF), pulmonary congestion, or both were required for enrollment.
For the primary composite outcomes of death from cardiovascular (CV) causes or incident heart failure, the ARNI had a 10% numerical advantage, but it did not reach statistical significance (hazard ratio [HR], 0.90; P = .17).
“PARADISE-MI was a neutral trial. This post hoc analysis will not change that result,” Dr. Berwanger emphasized. However, the post hoc analysis does provide a basis for exploring why conventional trial designs might not be providing answers that are relevant and helpful for clinical practice.
New analysis provides positive trial result
When the data from PARADISE-MI are reevaluated in a hierarchical win ratio analysis with CV death serving as the most severe and important outcome, the principal conclusion changes. Whether events are reevaluated in this format by the clinical events committee (CEC) or by investigators, there is a greater number of total wins than total losses for the ARNI. Combined, sacubitril/valsartan was associated with a win ratio of 1.17 (95% confidence interval, 1.03-1.33; P = 0.015) over ramipril.
Using a sports analogy, Dr. Berwanger explained that the win ratio analysis divides the total number of wins to the total number of losses to provide a much more clinically relevant approach to keeping score. It also used a hierarchical analysis so that the most serious and important events are considered first.
In addition to CV death, this analysis included first hospitalization for heart failure and first outpatient heart failure events. CEC-defined events and events reported by investigators were evaluated separately.
The ARNI had more wins than losses in every category for all outcomes, whether CEC adjudicated or investigator reported, but most of this benefit was generated by the endpoint of CEC-adjudicated CV deaths. This accounted for 36.9% of all events (investigator-documented CV death accounted for 0.7%). This is important because PARADISE-MI, like many standard trials, was conducted on a time-to-primary event basis.
“In this type of analysis, the first event is what counts. Usually time-to-first-event analyses are dominated by nonfatal events,” Dr. Berwanger explained. He believes that placing more weight on the most serious events results in an emphasis on what outcomes are of greatest clinical interest.
In addition, Dr. Berwanger argued that it is important to consider investigator-reported events, not just CEC-adjudicated events. While adjudicated events improve the rigor of the data, Dr. Berwanger suggested it omits outcomes with which clinicians are most concerned.
Investigator, adjudicated outcomes differ
Again, using PARADISE-MI as an example, he reevaluated the primary outcome based on investigator reports. When investigator-reported events are included, the number of events increased in both the ARNI (443 vs. 338) and ramipril (516 vs. 373) arms, but the advantage of the ARNI over the ACE inhibitors now reached statistical significance (HR, 0.85; P = .01).
“The data suggest that maybe we should find definitions for adjudication that are closer to clinical judgment in the real world and clinical practice,” Dr. Berwanger said.
One possible explanation for the neutral result in PARADISE-MI is that benefit of an ARNI over an ACE inhibitor would only be expected in those at risk for progressive left ventricular dysfunction, and it is likely that a substantial proportion of patients enrolled in this trial recovered, according to Johann Bauersachs, MD, PhD, professor and head of cardiology at Hannover (Germany) Medical School.
“You cannot predict which patients with reduced LV function following an MI will go on to chronic remodeling and which will recover,” said Dr. Bauersachs, who was an ESC-invited discussant of Dr. Berwanger’s post hoc analysis.
He agreed that Dr. Berwanger has raised several important issues in standard trial design that might have prevented PARADISE-MI from showing a benefit from an ARNI, but he pointed out that there are other potential issues, such as the low use of mineralocorticoid antagonists in PARADISE-MI, that may have skewed results.
However, he agreed generally with the premise that there is a need for trial design likely to generate more clinically useful information.
“We have now seen the win-ratio approach used in several studies,” said Dr. Bauersachs, citing in particular the EMPULSE trial presented at the 2022 meeting of the American College of Cardiology. “It is a very useful tool, and I think we will be seeing it used more in the future.”
However, he indicated that the issues raised by Dr. Berwanger are not necessarily easily resolved. Dr. Bauersachs endorsed the effort to consider trial designs that generate data that are more immediately clinically applicable but suggested that different types of designs may be required for different types of clinical questions.
Dr. Berwanger reports financial relationships with Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Pfizer, Servier, and Novartis, which provided funding for the PARADISE-MI trial. Dr. Bauersachs reports financial relationships with Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Cardior, Corvia, CVRx, Novartis, Pfizer, Vifor, and Zoll.
A post hoc analysis of the PARADISE-MI trial, although not intended to alter the conclusions generated by the published data, suggests that clinically relevant benefits were obscured, providing the basis for recommending different analyses for future studies that are more suited to capture the most clinically significant endpoints.
“What these data show us is that we need clinical trial designs moving towards more pragmatic information that better reflect clinical practice,” reported Otavio Berwanger, MD, PhD, director of the Academic Research Organization at Hospital Israelita Albert Einstein, São Paulo, Brazil.
The reevaluation of the PARADISE-MI data, presented at the annual congress of the European Society of Cardiology in Barcelona, was based on a win ratio analysis and on the inclusion of investigator-reported endpoints, not just adjudicated events. Both appear to reveal clinically meaningful benefits not reflected in the published study, according to Dr. Berwanger.
In PARADISE-MI, which was published in the New England Journal of Medicine last year, more than 5,500 patients were randomized to the angiotensin receptor neprilysin inhibitor (ARNI) sacubitril/valsartan or the ACE inhibitor ramipril after a myocardial infarction. A reduced left ventricular ejection fraction (LVEF), pulmonary congestion, or both were required for enrollment.
For the primary composite outcomes of death from cardiovascular (CV) causes or incident heart failure, the ARNI had a 10% numerical advantage, but it did not reach statistical significance (hazard ratio [HR], 0.90; P = .17).
“PARADISE-MI was a neutral trial. This post hoc analysis will not change that result,” Dr. Berwanger emphasized. However, the post hoc analysis does provide a basis for exploring why conventional trial designs might not be providing answers that are relevant and helpful for clinical practice.
New analysis provides positive trial result
When the data from PARADISE-MI are reevaluated in a hierarchical win ratio analysis with CV death serving as the most severe and important outcome, the principal conclusion changes. Whether events are reevaluated in this format by the clinical events committee (CEC) or by investigators, there is a greater number of total wins than total losses for the ARNI. Combined, sacubitril/valsartan was associated with a win ratio of 1.17 (95% confidence interval, 1.03-1.33; P = 0.015) over ramipril.
Using a sports analogy, Dr. Berwanger explained that the win ratio analysis divides the total number of wins to the total number of losses to provide a much more clinically relevant approach to keeping score. It also used a hierarchical analysis so that the most serious and important events are considered first.
In addition to CV death, this analysis included first hospitalization for heart failure and first outpatient heart failure events. CEC-defined events and events reported by investigators were evaluated separately.
The ARNI had more wins than losses in every category for all outcomes, whether CEC adjudicated or investigator reported, but most of this benefit was generated by the endpoint of CEC-adjudicated CV deaths. This accounted for 36.9% of all events (investigator-documented CV death accounted for 0.7%). This is important because PARADISE-MI, like many standard trials, was conducted on a time-to-primary event basis.
“In this type of analysis, the first event is what counts. Usually time-to-first-event analyses are dominated by nonfatal events,” Dr. Berwanger explained. He believes that placing more weight on the most serious events results in an emphasis on what outcomes are of greatest clinical interest.
In addition, Dr. Berwanger argued that it is important to consider investigator-reported events, not just CEC-adjudicated events. While adjudicated events improve the rigor of the data, Dr. Berwanger suggested it omits outcomes with which clinicians are most concerned.
Investigator, adjudicated outcomes differ
Again, using PARADISE-MI as an example, he reevaluated the primary outcome based on investigator reports. When investigator-reported events are included, the number of events increased in both the ARNI (443 vs. 338) and ramipril (516 vs. 373) arms, but the advantage of the ARNI over the ACE inhibitors now reached statistical significance (HR, 0.85; P = .01).
“The data suggest that maybe we should find definitions for adjudication that are closer to clinical judgment in the real world and clinical practice,” Dr. Berwanger said.
One possible explanation for the neutral result in PARADISE-MI is that benefit of an ARNI over an ACE inhibitor would only be expected in those at risk for progressive left ventricular dysfunction, and it is likely that a substantial proportion of patients enrolled in this trial recovered, according to Johann Bauersachs, MD, PhD, professor and head of cardiology at Hannover (Germany) Medical School.
“You cannot predict which patients with reduced LV function following an MI will go on to chronic remodeling and which will recover,” said Dr. Bauersachs, who was an ESC-invited discussant of Dr. Berwanger’s post hoc analysis.
He agreed that Dr. Berwanger has raised several important issues in standard trial design that might have prevented PARADISE-MI from showing a benefit from an ARNI, but he pointed out that there are other potential issues, such as the low use of mineralocorticoid antagonists in PARADISE-MI, that may have skewed results.
However, he agreed generally with the premise that there is a need for trial design likely to generate more clinically useful information.
“We have now seen the win-ratio approach used in several studies,” said Dr. Bauersachs, citing in particular the EMPULSE trial presented at the 2022 meeting of the American College of Cardiology. “It is a very useful tool, and I think we will be seeing it used more in the future.”
However, he indicated that the issues raised by Dr. Berwanger are not necessarily easily resolved. Dr. Bauersachs endorsed the effort to consider trial designs that generate data that are more immediately clinically applicable but suggested that different types of designs may be required for different types of clinical questions.
Dr. Berwanger reports financial relationships with Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Pfizer, Servier, and Novartis, which provided funding for the PARADISE-MI trial. Dr. Bauersachs reports financial relationships with Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Cardior, Corvia, CVRx, Novartis, Pfizer, Vifor, and Zoll.
FROM ESC CONGRESS 2022
Myocardial infarction in women younger than 50: Lessons to learn
Young women (under 50) are increasingly having heart attacks without doctors really knowing why. This is where the Young Women Presenting Acute Myocardial Infarction in France (WAMIF) study comes in, the results of which were presented in an e-poster at the annual congress of the European Society of Cardiology by Stéphane Manzo-Silberman, MD, Institute of Cardiology, Pitié-Salpétrière, Paris. The results (yet to be published) fight several of the preconceived ideas on the topic, Dr. Manzo-Silberman commented in an interview.
Significantly higher hospital death rates in women
“Cardiovascular disease is the main cause of death in women, killing seven times more than breast cancer,” notes Dr. Manzo-Silberman. The hospital death rate is significantly higher in women and, despite going down, is significantly higher than in men (more than double), particularly in women under 50. What’s more, in addition to the typical risk factors, women present specific risk factors related to hormone changes, high-risk inflammatory profiles, and thrombophilia.”
The WAMIF study was designed to determine the clinical, biological, and morphological features linked to hospital mortality after 12 months in women under 50. The prospective, observational study included all women in this age range from 30 sites in France between May 2017 and June 2019.
90% with retrosternal chest pain
The age of the 314 women enrolled was 44.9 years on average. Nearly two-thirds (192) presented with ST-segment elevation myocardial infarction and the other 122 without. In terms of symptoms, 91.6% of these women presented with typical chest pain, and 59.7% had related symptoms.
“With more than 90% having retrosternal pain, the idea that myocardial infarction presents with atypical symptoms in women has been widely challenged, despite the fact that more than half present with related symptoms and it isn’t known in which order these symptoms occur, Dr. Manzo-Silberman said in an interview. But what we can say is that if at any point a young woman mentions chest pain, even when occurring as part of several other symptoms, MI must be deemed a possibility until it has been ruled out.”
The risk profile revealed that 75.5% were smokers, 35% had a family history of heart disease, 33% had pregnancy complications, and 55% had recently experienced a stressful situation. The analysis also showed that cannabis use and oral contraception were primary risk factors in women younger than 35.
“With regard to risk factors, when designing this study we expected that lots of these young women would have largely atypical autoimmune conditions, with high levels of inflammation. We looked for everything, but this was not actually the case. Instead, we found very many women to have classic risk factors; three-quarters were smokers, a modifiable risk factor, which can largely be prevented. The other aspect concerns contraception, and it’s why I insist that gynecologists must be involved insofar as they must inform their patients how to manage their risk factors and tweak their contraception.”
Coronary angiography findings showed that only 1% received a normal result, 29.3% had vessel damage, and 14.6% had aortic dissection. “We were surprised again here because we expected that with young women we would see lots of heart attacks without obstruction, [in other words] normal coronary arteries, atypical forms of MI,” commented Dr. Manzo-Silberman. “In fact, most presented with atheroma, often obstructive lesions, or even triple-vessel disease, in nearly a third of the cohort. So that’s another misconception dispelled – we can’t just think that because a woman is young, nothing will be found. Coronary catheterization should be considered, and the diagnostic process should be completed in full.”
After 1 year, there had been two cancer-related deaths and 25 patients had undergone several angioplasty procedures. Nevertheless, 90.4% had not experienced any type of CV event, and 72% had not even had any symptoms.
“The final surprise was prognosis,” he said. “Previous studies, especially some authored by Viola Vaccarino, MD, PhD, showed an excess hospital rate in women and we had expected this to be the case here, but no hospital deaths were recorded. However, not far off 10% of women attended (at least once) the emergency department in the year following for recurrent chest pain which was not ischemic – ECG normal, troponin normal – so something was missing in their education as a patient.”
“So, there are improvements to be made in terms of secondary prevention, follow-up, and in the education of these young female patients who have experienced the major event that is a myocardial infarction,” concluded Dr. Manzo-Silberman.
This content was originally published on Medscape French edition.
Young women (under 50) are increasingly having heart attacks without doctors really knowing why. This is where the Young Women Presenting Acute Myocardial Infarction in France (WAMIF) study comes in, the results of which were presented in an e-poster at the annual congress of the European Society of Cardiology by Stéphane Manzo-Silberman, MD, Institute of Cardiology, Pitié-Salpétrière, Paris. The results (yet to be published) fight several of the preconceived ideas on the topic, Dr. Manzo-Silberman commented in an interview.
Significantly higher hospital death rates in women
“Cardiovascular disease is the main cause of death in women, killing seven times more than breast cancer,” notes Dr. Manzo-Silberman. The hospital death rate is significantly higher in women and, despite going down, is significantly higher than in men (more than double), particularly in women under 50. What’s more, in addition to the typical risk factors, women present specific risk factors related to hormone changes, high-risk inflammatory profiles, and thrombophilia.”
The WAMIF study was designed to determine the clinical, biological, and morphological features linked to hospital mortality after 12 months in women under 50. The prospective, observational study included all women in this age range from 30 sites in France between May 2017 and June 2019.
90% with retrosternal chest pain
The age of the 314 women enrolled was 44.9 years on average. Nearly two-thirds (192) presented with ST-segment elevation myocardial infarction and the other 122 without. In terms of symptoms, 91.6% of these women presented with typical chest pain, and 59.7% had related symptoms.
“With more than 90% having retrosternal pain, the idea that myocardial infarction presents with atypical symptoms in women has been widely challenged, despite the fact that more than half present with related symptoms and it isn’t known in which order these symptoms occur, Dr. Manzo-Silberman said in an interview. But what we can say is that if at any point a young woman mentions chest pain, even when occurring as part of several other symptoms, MI must be deemed a possibility until it has been ruled out.”
The risk profile revealed that 75.5% were smokers, 35% had a family history of heart disease, 33% had pregnancy complications, and 55% had recently experienced a stressful situation. The analysis also showed that cannabis use and oral contraception were primary risk factors in women younger than 35.
“With regard to risk factors, when designing this study we expected that lots of these young women would have largely atypical autoimmune conditions, with high levels of inflammation. We looked for everything, but this was not actually the case. Instead, we found very many women to have classic risk factors; three-quarters were smokers, a modifiable risk factor, which can largely be prevented. The other aspect concerns contraception, and it’s why I insist that gynecologists must be involved insofar as they must inform their patients how to manage their risk factors and tweak their contraception.”
Coronary angiography findings showed that only 1% received a normal result, 29.3% had vessel damage, and 14.6% had aortic dissection. “We were surprised again here because we expected that with young women we would see lots of heart attacks without obstruction, [in other words] normal coronary arteries, atypical forms of MI,” commented Dr. Manzo-Silberman. “In fact, most presented with atheroma, often obstructive lesions, or even triple-vessel disease, in nearly a third of the cohort. So that’s another misconception dispelled – we can’t just think that because a woman is young, nothing will be found. Coronary catheterization should be considered, and the diagnostic process should be completed in full.”
After 1 year, there had been two cancer-related deaths and 25 patients had undergone several angioplasty procedures. Nevertheless, 90.4% had not experienced any type of CV event, and 72% had not even had any symptoms.
“The final surprise was prognosis,” he said. “Previous studies, especially some authored by Viola Vaccarino, MD, PhD, showed an excess hospital rate in women and we had expected this to be the case here, but no hospital deaths were recorded. However, not far off 10% of women attended (at least once) the emergency department in the year following for recurrent chest pain which was not ischemic – ECG normal, troponin normal – so something was missing in their education as a patient.”
“So, there are improvements to be made in terms of secondary prevention, follow-up, and in the education of these young female patients who have experienced the major event that is a myocardial infarction,” concluded Dr. Manzo-Silberman.
This content was originally published on Medscape French edition.
Young women (under 50) are increasingly having heart attacks without doctors really knowing why. This is where the Young Women Presenting Acute Myocardial Infarction in France (WAMIF) study comes in, the results of which were presented in an e-poster at the annual congress of the European Society of Cardiology by Stéphane Manzo-Silberman, MD, Institute of Cardiology, Pitié-Salpétrière, Paris. The results (yet to be published) fight several of the preconceived ideas on the topic, Dr. Manzo-Silberman commented in an interview.
Significantly higher hospital death rates in women
“Cardiovascular disease is the main cause of death in women, killing seven times more than breast cancer,” notes Dr. Manzo-Silberman. The hospital death rate is significantly higher in women and, despite going down, is significantly higher than in men (more than double), particularly in women under 50. What’s more, in addition to the typical risk factors, women present specific risk factors related to hormone changes, high-risk inflammatory profiles, and thrombophilia.”
The WAMIF study was designed to determine the clinical, biological, and morphological features linked to hospital mortality after 12 months in women under 50. The prospective, observational study included all women in this age range from 30 sites in France between May 2017 and June 2019.
90% with retrosternal chest pain
The age of the 314 women enrolled was 44.9 years on average. Nearly two-thirds (192) presented with ST-segment elevation myocardial infarction and the other 122 without. In terms of symptoms, 91.6% of these women presented with typical chest pain, and 59.7% had related symptoms.
“With more than 90% having retrosternal pain, the idea that myocardial infarction presents with atypical symptoms in women has been widely challenged, despite the fact that more than half present with related symptoms and it isn’t known in which order these symptoms occur, Dr. Manzo-Silberman said in an interview. But what we can say is that if at any point a young woman mentions chest pain, even when occurring as part of several other symptoms, MI must be deemed a possibility until it has been ruled out.”
The risk profile revealed that 75.5% were smokers, 35% had a family history of heart disease, 33% had pregnancy complications, and 55% had recently experienced a stressful situation. The analysis also showed that cannabis use and oral contraception were primary risk factors in women younger than 35.
“With regard to risk factors, when designing this study we expected that lots of these young women would have largely atypical autoimmune conditions, with high levels of inflammation. We looked for everything, but this was not actually the case. Instead, we found very many women to have classic risk factors; three-quarters were smokers, a modifiable risk factor, which can largely be prevented. The other aspect concerns contraception, and it’s why I insist that gynecologists must be involved insofar as they must inform their patients how to manage their risk factors and tweak their contraception.”
Coronary angiography findings showed that only 1% received a normal result, 29.3% had vessel damage, and 14.6% had aortic dissection. “We were surprised again here because we expected that with young women we would see lots of heart attacks without obstruction, [in other words] normal coronary arteries, atypical forms of MI,” commented Dr. Manzo-Silberman. “In fact, most presented with atheroma, often obstructive lesions, or even triple-vessel disease, in nearly a third of the cohort. So that’s another misconception dispelled – we can’t just think that because a woman is young, nothing will be found. Coronary catheterization should be considered, and the diagnostic process should be completed in full.”
After 1 year, there had been two cancer-related deaths and 25 patients had undergone several angioplasty procedures. Nevertheless, 90.4% had not experienced any type of CV event, and 72% had not even had any symptoms.
“The final surprise was prognosis,” he said. “Previous studies, especially some authored by Viola Vaccarino, MD, PhD, showed an excess hospital rate in women and we had expected this to be the case here, but no hospital deaths were recorded. However, not far off 10% of women attended (at least once) the emergency department in the year following for recurrent chest pain which was not ischemic – ECG normal, troponin normal – so something was missing in their education as a patient.”
“So, there are improvements to be made in terms of secondary prevention, follow-up, and in the education of these young female patients who have experienced the major event that is a myocardial infarction,” concluded Dr. Manzo-Silberman.
This content was originally published on Medscape French edition.
FROM ESC CONGRESS 2022