User login
ESC: Lead-free pacemaker shows good safety, efficacy at 6 months
LONDON – A wireless pacemaker that is secured within the right ventricle of the heart proved both effective and safe in a prospective, nonrandomized, multicenter study.
Interim results obtained for 300 patients at 6 months’ follow-up in the LEADLESS II study showed that the primary efficacy endpoint of both an acceptable pacing threshold and an acceptable sensing amplitude was achieved in 90% of patients, and the primary safety endpoint of freedom from device-related serious adverse events was achieved in 93.3%.
Both of these findings exceeded the performance goal of 85% set in the study, said principal study investigator Dr. Vivek Reddy at the annual congress of the European Society of Cardiology.
“Regular pacemakers are very reliable devices; they’ve been around for half a century,” said Dr. Reddy, who is professor of medicine at Mount Sinai Hospital in New York. “Having said that, they are not perfect,” he observed.
Conventional pacemakers require surgical implantation in the chest, and the wires or leads that go from the device need to be embedded in the vasculature of the heart itself. While the risk of complications is low (around 4% of all implanted devices), when they do happen they usually occur around the site where the pacemaker is implanted or involve placement of the leads in veins.
In contrast, the small cylindrical Nanostim device used in the study is a fully self-contained leadless pacemaker that can be nonsurgically implanted and removed via a catheter threaded through the femoral vein and into the patient’s right ventricle. It is about the size of an AAA battery and, based on the study’s findings, has a potential battery life of 15 years. This is comparable to similar standard single-chamber ventricular pacemakers, according to the manufacturer, St. Jude Medical.
The feasibility of using the device was first shown in the LEADLESS study (Circulation 2014;129:1466-71) and are now confirmed in the LEADLESS II study, which has enrolled 526 patients to date, with successful implantation of the device in 504 patients.
The mean age of the mostly male (61.8%) study cohort was 76 years, with the primary indication for pacemaker placement being atrial fibrillation with atrioventricular block in 55.9%.
The study was performed in 56 centers in the United States, Canada, and Australia and involved 100 operators, only one of whom had prior experience with leadless pacing, Dr. Reddy observed. On average the leadless device was placed within a half hour, and the majority (70.2%) did not require repositioning of the device during the original procedure.
The rate of serious device-related adverse events, such as dislodgement warranting percutaneous retrieval or cardiac perforation, was low, at a rate of 6.7% overall and 1.7% and 1.3%, respectively, with just 1.3% of patients needing to have the device removed because of an increased pacing threshold. There were no device-related deaths, but two deaths occurred that were thought to be procedure related. This is comparable to traditional pacemakers, Dr. Reddy said, although he noted that he was referring to historical controls as this was not a randomized trial.
“The device was shown to be retrievable in a subgroup of seven patients who needed a replacement at a mean of 160 days after implantation,” he noted. Due to the relatively short duration of follow-up, however, “we really can’t talk about what happens with extended follow-up and what happens 5, 10, or 15 years down the line whether we retrieve the devices or simply abandon them and put in a second device.”
The study included patients who had an indication for a single-chamber pacemaker only. Dr. Reddy noted that one of the study limitations is that the device is not suitable for patients needing dual-chamber pacing. The observational nature of the trial also limits the conclusions that can be drawn, and the device does not provide electrocardiogram data.
The Nanostim device is one of two self-contained, miniature wireless pacemakers given marketing authorization in Europe, but both have yet to be approved by the Food and Drug Administration. The other device, Medtronic’s Micra Transcatheter Pacing System, is also placed in the right ventricle and is being studied in the Micra Transcatheter Pacing Study, with promising first-in-human results presented recently at Heart Rhythm 2015.
St. Jude Medical funded the study. Dr. Reddy has received grant support, acted as a consultant to, and received stock options in Nanostim from the company.
LONDON – A wireless pacemaker that is secured within the right ventricle of the heart proved both effective and safe in a prospective, nonrandomized, multicenter study.
Interim results obtained for 300 patients at 6 months’ follow-up in the LEADLESS II study showed that the primary efficacy endpoint of both an acceptable pacing threshold and an acceptable sensing amplitude was achieved in 90% of patients, and the primary safety endpoint of freedom from device-related serious adverse events was achieved in 93.3%.
Both of these findings exceeded the performance goal of 85% set in the study, said principal study investigator Dr. Vivek Reddy at the annual congress of the European Society of Cardiology.
“Regular pacemakers are very reliable devices; they’ve been around for half a century,” said Dr. Reddy, who is professor of medicine at Mount Sinai Hospital in New York. “Having said that, they are not perfect,” he observed.
Conventional pacemakers require surgical implantation in the chest, and the wires or leads that go from the device need to be embedded in the vasculature of the heart itself. While the risk of complications is low (around 4% of all implanted devices), when they do happen they usually occur around the site where the pacemaker is implanted or involve placement of the leads in veins.
In contrast, the small cylindrical Nanostim device used in the study is a fully self-contained leadless pacemaker that can be nonsurgically implanted and removed via a catheter threaded through the femoral vein and into the patient’s right ventricle. It is about the size of an AAA battery and, based on the study’s findings, has a potential battery life of 15 years. This is comparable to similar standard single-chamber ventricular pacemakers, according to the manufacturer, St. Jude Medical.
The feasibility of using the device was first shown in the LEADLESS study (Circulation 2014;129:1466-71) and are now confirmed in the LEADLESS II study, which has enrolled 526 patients to date, with successful implantation of the device in 504 patients.
The mean age of the mostly male (61.8%) study cohort was 76 years, with the primary indication for pacemaker placement being atrial fibrillation with atrioventricular block in 55.9%.
The study was performed in 56 centers in the United States, Canada, and Australia and involved 100 operators, only one of whom had prior experience with leadless pacing, Dr. Reddy observed. On average the leadless device was placed within a half hour, and the majority (70.2%) did not require repositioning of the device during the original procedure.
The rate of serious device-related adverse events, such as dislodgement warranting percutaneous retrieval or cardiac perforation, was low, at a rate of 6.7% overall and 1.7% and 1.3%, respectively, with just 1.3% of patients needing to have the device removed because of an increased pacing threshold. There were no device-related deaths, but two deaths occurred that were thought to be procedure related. This is comparable to traditional pacemakers, Dr. Reddy said, although he noted that he was referring to historical controls as this was not a randomized trial.
“The device was shown to be retrievable in a subgroup of seven patients who needed a replacement at a mean of 160 days after implantation,” he noted. Due to the relatively short duration of follow-up, however, “we really can’t talk about what happens with extended follow-up and what happens 5, 10, or 15 years down the line whether we retrieve the devices or simply abandon them and put in a second device.”
The study included patients who had an indication for a single-chamber pacemaker only. Dr. Reddy noted that one of the study limitations is that the device is not suitable for patients needing dual-chamber pacing. The observational nature of the trial also limits the conclusions that can be drawn, and the device does not provide electrocardiogram data.
The Nanostim device is one of two self-contained, miniature wireless pacemakers given marketing authorization in Europe, but both have yet to be approved by the Food and Drug Administration. The other device, Medtronic’s Micra Transcatheter Pacing System, is also placed in the right ventricle and is being studied in the Micra Transcatheter Pacing Study, with promising first-in-human results presented recently at Heart Rhythm 2015.
St. Jude Medical funded the study. Dr. Reddy has received grant support, acted as a consultant to, and received stock options in Nanostim from the company.
LONDON – A wireless pacemaker that is secured within the right ventricle of the heart proved both effective and safe in a prospective, nonrandomized, multicenter study.
Interim results obtained for 300 patients at 6 months’ follow-up in the LEADLESS II study showed that the primary efficacy endpoint of both an acceptable pacing threshold and an acceptable sensing amplitude was achieved in 90% of patients, and the primary safety endpoint of freedom from device-related serious adverse events was achieved in 93.3%.
Both of these findings exceeded the performance goal of 85% set in the study, said principal study investigator Dr. Vivek Reddy at the annual congress of the European Society of Cardiology.
“Regular pacemakers are very reliable devices; they’ve been around for half a century,” said Dr. Reddy, who is professor of medicine at Mount Sinai Hospital in New York. “Having said that, they are not perfect,” he observed.
Conventional pacemakers require surgical implantation in the chest, and the wires or leads that go from the device need to be embedded in the vasculature of the heart itself. While the risk of complications is low (around 4% of all implanted devices), when they do happen they usually occur around the site where the pacemaker is implanted or involve placement of the leads in veins.
In contrast, the small cylindrical Nanostim device used in the study is a fully self-contained leadless pacemaker that can be nonsurgically implanted and removed via a catheter threaded through the femoral vein and into the patient’s right ventricle. It is about the size of an AAA battery and, based on the study’s findings, has a potential battery life of 15 years. This is comparable to similar standard single-chamber ventricular pacemakers, according to the manufacturer, St. Jude Medical.
The feasibility of using the device was first shown in the LEADLESS study (Circulation 2014;129:1466-71) and are now confirmed in the LEADLESS II study, which has enrolled 526 patients to date, with successful implantation of the device in 504 patients.
The mean age of the mostly male (61.8%) study cohort was 76 years, with the primary indication for pacemaker placement being atrial fibrillation with atrioventricular block in 55.9%.
The study was performed in 56 centers in the United States, Canada, and Australia and involved 100 operators, only one of whom had prior experience with leadless pacing, Dr. Reddy observed. On average the leadless device was placed within a half hour, and the majority (70.2%) did not require repositioning of the device during the original procedure.
The rate of serious device-related adverse events, such as dislodgement warranting percutaneous retrieval or cardiac perforation, was low, at a rate of 6.7% overall and 1.7% and 1.3%, respectively, with just 1.3% of patients needing to have the device removed because of an increased pacing threshold. There were no device-related deaths, but two deaths occurred that were thought to be procedure related. This is comparable to traditional pacemakers, Dr. Reddy said, although he noted that he was referring to historical controls as this was not a randomized trial.
“The device was shown to be retrievable in a subgroup of seven patients who needed a replacement at a mean of 160 days after implantation,” he noted. Due to the relatively short duration of follow-up, however, “we really can’t talk about what happens with extended follow-up and what happens 5, 10, or 15 years down the line whether we retrieve the devices or simply abandon them and put in a second device.”
The study included patients who had an indication for a single-chamber pacemaker only. Dr. Reddy noted that one of the study limitations is that the device is not suitable for patients needing dual-chamber pacing. The observational nature of the trial also limits the conclusions that can be drawn, and the device does not provide electrocardiogram data.
The Nanostim device is one of two self-contained, miniature wireless pacemakers given marketing authorization in Europe, but both have yet to be approved by the Food and Drug Administration. The other device, Medtronic’s Micra Transcatheter Pacing System, is also placed in the right ventricle and is being studied in the Micra Transcatheter Pacing Study, with promising first-in-human results presented recently at Heart Rhythm 2015.
St. Jude Medical funded the study. Dr. Reddy has received grant support, acted as a consultant to, and received stock options in Nanostim from the company.
Key clinical point: The Nanostim leadless pacemaker showed good efficacy and safety in this observational study.
Major finding: At 6 months, the primary efficacy and safety endpoints were met in 90% and 93.5% of 300 patients, respectively.
Data source: Interim results of an ongoing, prospective, nonrandomized, multicenter study, in 565 patients in need of single-chamber ventricular pacing.
Disclosures: St. Jude Medical funded the study. Dr. Reddy has received grant support, acted as a consultant to, and received stock options in Nanostim from the company.
3-D–printed devices mitigate pediatric tracheobronchomalacia
Immediate and continued life-sustaining improvement was seen in three pediatric patients implanted with 3-D–printed tracheobronchial splints as a treatment for terminal tracheobronchomalacia (TBM), a condition of excessive collapse of the airways during respiration leading to cardiopulmonary arrest.
The particular value of such 3-D–printable biomaterials to pediatric surgery is their ability to adopt a 4-D modality – to exhibit specifically engineered shape changes in response to surrounding tissue growth over a defined time period. In addition to their malleability, these devices also are designed to biodegrade. These features have proven especially useful as seen in this medical device emergency use exemption study performed at the University of Michigan, according to a report published in Science Translational Medicine (2015 Apr 29. [doi: 10.1126/scitranslmed.3010825]).
“Our multidisciplinary team designed an archetype device to allow radial expansion of the affected airway over the critical growth period while resisting external compression and intrinsic collapse,” wrote Dr. Robert J. Morrison of the University of Michigan, Ann Arbor, and his colleagues.
The study population involved three infant boys, aged 3 months, 5 months, and 16 months at time of treatment. In each patient, a sternotomy exposed their affected airways. The 3-D–printed splint, consisting of conjoined rib-like C-shaped arches was placed around the affected airway and secured with polypropylene sutures. The splint counters external pressure on the airway and holds it open. Because the splint is malleable, with an expandable opening placed opposite to the main collapsing pressure, it is capable of expanding as the airway grows.
Examination of the airway immediately after placement demonstrated patency, which was confirmed 1 month later. Results showed the benefit of the splints for all three patients, although total results were complicated by additional comorbidities:
• Patient 1: Blood gases returned to normal immediately after implantation and remained normal at 3 months’ follow-up. A week after implantation, weaning from mechanical ventilation was initiated and, 3 weeks after the procedure, the child was discharged to home. Repeat imaging at 1, 3, 6, 12, and 39 months postoperatively demonstrated continued resolution of the TBM, with evidence of fragmentation and degradation of the splint at 39 months.
• Patient 2: Immediately after implantation of the device, blood gases improved greatly and the left lung perfused. The patient had opioid and benzodiazepine dependence from long-term ventilator support, requiring a longer controlled wean from the ventilator. Four weeks after surgery, the patient was transitioned to a portable ventilator system, completely weaned at 15 weeks, and discharged from the hospital to home for the first time in his life.
• Patient 3: After implantation, the patient ceased experiencing life-threatening desaturation episodes and showed sustained improvement in blood gases. Imaging showed continued patency of the left main bronchus with resolution of left-lung air trapping. However, at 14 months post implantation, he remained on permanent ventilator support, “presumably because of distal left segmental bronchomalacia beyond what the splint was designed to address,” according to Dr. Morrison and his colleagues.
“We report successful implantation of patient-specific bioresorbable airway splints for the treatment of severe TBM. The personalized splints conformed to the patients’ individual geometries and expanded with airway growth (in the ‘fourth dimension’),” the researchers summarized.
“The three pediatric patients implanted with these 3-D–printed airway splints had a terminal form of TBM. The clinical improvement in each case was immediate and sustained, suggesting that improvement is not attributable to the natural history of the disease alone,” they concluded.
The study was funded by the National Institutes of Health. Two of the study authors were coinventors of the device for which they have filed a patent. There were no other disclosures.
As illustrated in this article, the investigators demonstrate the potential for the future of 3-D–printing in medicine. While there have been numerous reports of utilizing 3-D–printing for a generation of personalized prostheses, these have been used in static circumstances in which the prosthesis is not required to change over time. In pediatric applications, as the child grows, the prosthesis also needs to adapt to this growth, thereby necessitating “4-D” printing.

|
Dr. Sai Yendamuri |
In three patients, the authors have applied this 4-D–printing paradigm to devise external bronchial splints to alleviate life-threatening tracheobronchomalacia using a novel design and a bioresorbable material, resulting in superb medium-term outcomes for patients with an otherwise dire prognosis.
Dr. Sai Yendamuri is an attending surgeon at the department of thoracic surgery, and director, Thoracic Surgery Research Laboratory, and an associate professor of oncology at Roswell Park Cancer Institute, Buffalo, N.Y. He is also an associate medical editor for Thoracic Surgery News.
As illustrated in this article, the investigators demonstrate the potential for the future of 3-D–printing in medicine. While there have been numerous reports of utilizing 3-D–printing for a generation of personalized prostheses, these have been used in static circumstances in which the prosthesis is not required to change over time. In pediatric applications, as the child grows, the prosthesis also needs to adapt to this growth, thereby necessitating “4-D” printing.

|
Dr. Sai Yendamuri |
In three patients, the authors have applied this 4-D–printing paradigm to devise external bronchial splints to alleviate life-threatening tracheobronchomalacia using a novel design and a bioresorbable material, resulting in superb medium-term outcomes for patients with an otherwise dire prognosis.
Dr. Sai Yendamuri is an attending surgeon at the department of thoracic surgery, and director, Thoracic Surgery Research Laboratory, and an associate professor of oncology at Roswell Park Cancer Institute, Buffalo, N.Y. He is also an associate medical editor for Thoracic Surgery News.
As illustrated in this article, the investigators demonstrate the potential for the future of 3-D–printing in medicine. While there have been numerous reports of utilizing 3-D–printing for a generation of personalized prostheses, these have been used in static circumstances in which the prosthesis is not required to change over time. In pediatric applications, as the child grows, the prosthesis also needs to adapt to this growth, thereby necessitating “4-D” printing.

|
Dr. Sai Yendamuri |
In three patients, the authors have applied this 4-D–printing paradigm to devise external bronchial splints to alleviate life-threatening tracheobronchomalacia using a novel design and a bioresorbable material, resulting in superb medium-term outcomes for patients with an otherwise dire prognosis.
Dr. Sai Yendamuri is an attending surgeon at the department of thoracic surgery, and director, Thoracic Surgery Research Laboratory, and an associate professor of oncology at Roswell Park Cancer Institute, Buffalo, N.Y. He is also an associate medical editor for Thoracic Surgery News.
Immediate and continued life-sustaining improvement was seen in three pediatric patients implanted with 3-D–printed tracheobronchial splints as a treatment for terminal tracheobronchomalacia (TBM), a condition of excessive collapse of the airways during respiration leading to cardiopulmonary arrest.
The particular value of such 3-D–printable biomaterials to pediatric surgery is their ability to adopt a 4-D modality – to exhibit specifically engineered shape changes in response to surrounding tissue growth over a defined time period. In addition to their malleability, these devices also are designed to biodegrade. These features have proven especially useful as seen in this medical device emergency use exemption study performed at the University of Michigan, according to a report published in Science Translational Medicine (2015 Apr 29. [doi: 10.1126/scitranslmed.3010825]).
“Our multidisciplinary team designed an archetype device to allow radial expansion of the affected airway over the critical growth period while resisting external compression and intrinsic collapse,” wrote Dr. Robert J. Morrison of the University of Michigan, Ann Arbor, and his colleagues.
The study population involved three infant boys, aged 3 months, 5 months, and 16 months at time of treatment. In each patient, a sternotomy exposed their affected airways. The 3-D–printed splint, consisting of conjoined rib-like C-shaped arches was placed around the affected airway and secured with polypropylene sutures. The splint counters external pressure on the airway and holds it open. Because the splint is malleable, with an expandable opening placed opposite to the main collapsing pressure, it is capable of expanding as the airway grows.
Examination of the airway immediately after placement demonstrated patency, which was confirmed 1 month later. Results showed the benefit of the splints for all three patients, although total results were complicated by additional comorbidities:
• Patient 1: Blood gases returned to normal immediately after implantation and remained normal at 3 months’ follow-up. A week after implantation, weaning from mechanical ventilation was initiated and, 3 weeks after the procedure, the child was discharged to home. Repeat imaging at 1, 3, 6, 12, and 39 months postoperatively demonstrated continued resolution of the TBM, with evidence of fragmentation and degradation of the splint at 39 months.
• Patient 2: Immediately after implantation of the device, blood gases improved greatly and the left lung perfused. The patient had opioid and benzodiazepine dependence from long-term ventilator support, requiring a longer controlled wean from the ventilator. Four weeks after surgery, the patient was transitioned to a portable ventilator system, completely weaned at 15 weeks, and discharged from the hospital to home for the first time in his life.
• Patient 3: After implantation, the patient ceased experiencing life-threatening desaturation episodes and showed sustained improvement in blood gases. Imaging showed continued patency of the left main bronchus with resolution of left-lung air trapping. However, at 14 months post implantation, he remained on permanent ventilator support, “presumably because of distal left segmental bronchomalacia beyond what the splint was designed to address,” according to Dr. Morrison and his colleagues.
“We report successful implantation of patient-specific bioresorbable airway splints for the treatment of severe TBM. The personalized splints conformed to the patients’ individual geometries and expanded with airway growth (in the ‘fourth dimension’),” the researchers summarized.
“The three pediatric patients implanted with these 3-D–printed airway splints had a terminal form of TBM. The clinical improvement in each case was immediate and sustained, suggesting that improvement is not attributable to the natural history of the disease alone,” they concluded.
The study was funded by the National Institutes of Health. Two of the study authors were coinventors of the device for which they have filed a patent. There were no other disclosures.
Immediate and continued life-sustaining improvement was seen in three pediatric patients implanted with 3-D–printed tracheobronchial splints as a treatment for terminal tracheobronchomalacia (TBM), a condition of excessive collapse of the airways during respiration leading to cardiopulmonary arrest.
The particular value of such 3-D–printable biomaterials to pediatric surgery is their ability to adopt a 4-D modality – to exhibit specifically engineered shape changes in response to surrounding tissue growth over a defined time period. In addition to their malleability, these devices also are designed to biodegrade. These features have proven especially useful as seen in this medical device emergency use exemption study performed at the University of Michigan, according to a report published in Science Translational Medicine (2015 Apr 29. [doi: 10.1126/scitranslmed.3010825]).
“Our multidisciplinary team designed an archetype device to allow radial expansion of the affected airway over the critical growth period while resisting external compression and intrinsic collapse,” wrote Dr. Robert J. Morrison of the University of Michigan, Ann Arbor, and his colleagues.
The study population involved three infant boys, aged 3 months, 5 months, and 16 months at time of treatment. In each patient, a sternotomy exposed their affected airways. The 3-D–printed splint, consisting of conjoined rib-like C-shaped arches was placed around the affected airway and secured with polypropylene sutures. The splint counters external pressure on the airway and holds it open. Because the splint is malleable, with an expandable opening placed opposite to the main collapsing pressure, it is capable of expanding as the airway grows.
Examination of the airway immediately after placement demonstrated patency, which was confirmed 1 month later. Results showed the benefit of the splints for all three patients, although total results were complicated by additional comorbidities:
• Patient 1: Blood gases returned to normal immediately after implantation and remained normal at 3 months’ follow-up. A week after implantation, weaning from mechanical ventilation was initiated and, 3 weeks after the procedure, the child was discharged to home. Repeat imaging at 1, 3, 6, 12, and 39 months postoperatively demonstrated continued resolution of the TBM, with evidence of fragmentation and degradation of the splint at 39 months.
• Patient 2: Immediately after implantation of the device, blood gases improved greatly and the left lung perfused. The patient had opioid and benzodiazepine dependence from long-term ventilator support, requiring a longer controlled wean from the ventilator. Four weeks after surgery, the patient was transitioned to a portable ventilator system, completely weaned at 15 weeks, and discharged from the hospital to home for the first time in his life.
• Patient 3: After implantation, the patient ceased experiencing life-threatening desaturation episodes and showed sustained improvement in blood gases. Imaging showed continued patency of the left main bronchus with resolution of left-lung air trapping. However, at 14 months post implantation, he remained on permanent ventilator support, “presumably because of distal left segmental bronchomalacia beyond what the splint was designed to address,” according to Dr. Morrison and his colleagues.
“We report successful implantation of patient-specific bioresorbable airway splints for the treatment of severe TBM. The personalized splints conformed to the patients’ individual geometries and expanded with airway growth (in the ‘fourth dimension’),” the researchers summarized.
“The three pediatric patients implanted with these 3-D–printed airway splints had a terminal form of TBM. The clinical improvement in each case was immediate and sustained, suggesting that improvement is not attributable to the natural history of the disease alone,” they concluded.
The study was funded by the National Institutes of Health. Two of the study authors were coinventors of the device for which they have filed a patent. There were no other disclosures.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: The use of 3-D–printed airway implants mitigated life-threatening tracheobronchomalacia (TBM) in three infants.
Major finding: Three infants with a terminal form of TBM ceased exhibiting life-threatening airway disease and showed continued growth of pulmonary airways after 3-D tracheal implants.
Data source: A study performed at the University of Michigan, Ann Arbor, of three infants with terminal TBM who received a medical device emergency use exemption for a 3-D tracheal implant.
Disclosures: The study was funded by the National Institutes of Health. Two of the study authors were coinventors of the device for which they have filed a patent. There were no other disclosures.
Surgeon volume may affect choice of surgery type for NSCLC
A surgeon’s comfort level with a favored operation for non–small cell lung cancer can strongly influence whether the patient will have that operation, which, in turn, can affect the patient’s outcome and long-term survival, according to an analysis of a population-linked database. For patients whose surgeons have lower levels of experience, that could mean a greater chance they will have more invasive total lung removal rather than more difficult operations that spare part of the affected lung, according to investigators at McMaster University in Hamilton, Ontario.
“If a surgeon with high surgical volumes is less likely to perform higher-risk pneumonectomy procedures than one with lower volumes, this may translate to a significant reduction in adverse events,” said lead author Dr. Christian Finley and coauthors online in the Journal of Thoracic and Cardiovascular Surgery (2015 Jun 30 [doi: 10.1016/j.jtcvs.2015.04.060]). “Surgeon volume should be considered an important component in how care is delivered in this population.”
The McMaster investigators evaluated 8,070 patients in an Ontario population-based linked database who underwent surgical resection for non–small cell lung cancer during 2004-2011, including pneumonectomy, or total lung removal (842 patients), lobectomy (6,212 patients), and wedge resection (1,002 patients). Over the years of the study, the proportion of patients who underwent pneumonectomy fell by more than half, from 14.8% in 2004 to 7.6% in 2011.
Of the three procedures, pneumonectomy carries a threefold greater mortality and while the procedure is often avoidable, there may be cases where it’s necessary because of the location of the tumor, Dr. Finley and his colleagues said. Lobectomy is desirable because it spares the parenchyma and has lower recurrence rates than laser resections.
The study investigators aimed to explore the hypothesis that surgeons with less expertise are more inclined to perform the higher-risk pneumonectomy or sublobar resections such as a segmentectomy or a wedge resection than a lobectomy, the rationale being that these procedures can be less challenging than a standard or sleeve lobectomy. The study analyzed results from 124 different physicians at 45 institutions in Ontario.
Data analysis showed that physician volume, age, year of procedure, sex, and comorbidities were predictive of the surgeon performing a pneumonectomy. “Adjusting for these variables, the results indicated that for each 10-unit increase in physician volume, the relative risk of performing a pneumonectomy decreased by 9.1%,” Dr. Finley and his colleagues wrote. They also found no significant difference in stage distribution among low-, medium-, and high-volume surgeons.
“This is meaningful as pneumonectomy is known to have the highest mortality rate of lung cancer resection, found in this study to be 12.6%, demonstrating a potentially large impact on patient survival,” Dr. Finley and his colleagues said.
This analysis cites an earlier study that surgeon volume for many procedures was a key determinant in the link between hospital volume and operative mortality (N Engl J Med. 2003 Nov 27;349[2]:2117-27.). “This study suggests that a patient may improve their chance of survival substantially, even at high-volume institutions, by selecting surgeons who perform operations more frequently,” Dr. Finley and his colleagues said.
They said that despite their study’s limitations, the findings on how surgeon experience can influence the choice of lung resection for cancer warrant further study.
McMaster University, Division of Thoracic Surgery, provided funding for the study. The study authors had no disclosures.
Because the McMaster University study derived the reported outcomes from registry data, determining the reasons that influenced surgeons’ choices of lung resection is impossible, Dr. Eric Lim of the Imperial College of Medicine, London, said in his invited commentary (J Thorac Cardiovasc Surg. 2015 May 21 [doi:10.1016/j.jtcvs.2015.05.048]).
The study authors noted that lower-volume surgeons were more inclined to perform pneumonectomy, and, Dr. Lim noted, previous studies have found that higher-volume centers tended to see more patients with advanced-stage cancers and increased morbidities. “An alternative explanation is that higher-volume surgeons have better skill sets to undertake procedures such as sleeve lobectomies that would lower the pneumonectomy rates and possibly more segmentectomies to lower the wedge-resection rate,” Dr. Lim said.
Until better evidence exists on what procedure is best for central and peripheral tumors, “surgeons can argue either way,” Dr. Lim said. The questions that follow from the study should concentrate on the relative harm of each procedure and the level of practice variation that’s unacceptable.
“As a surgical community, it is incumbent on us to continue to evaluate surgical treatments generating the highest levels of evidence possible (randomized trials) and have sufficient humility to cross refer to colleagues when appropriate to ensure the best care for our patients,” Dr. Lim concluded.
Because the McMaster University study derived the reported outcomes from registry data, determining the reasons that influenced surgeons’ choices of lung resection is impossible, Dr. Eric Lim of the Imperial College of Medicine, London, said in his invited commentary (J Thorac Cardiovasc Surg. 2015 May 21 [doi:10.1016/j.jtcvs.2015.05.048]).
The study authors noted that lower-volume surgeons were more inclined to perform pneumonectomy, and, Dr. Lim noted, previous studies have found that higher-volume centers tended to see more patients with advanced-stage cancers and increased morbidities. “An alternative explanation is that higher-volume surgeons have better skill sets to undertake procedures such as sleeve lobectomies that would lower the pneumonectomy rates and possibly more segmentectomies to lower the wedge-resection rate,” Dr. Lim said.
Until better evidence exists on what procedure is best for central and peripheral tumors, “surgeons can argue either way,” Dr. Lim said. The questions that follow from the study should concentrate on the relative harm of each procedure and the level of practice variation that’s unacceptable.
“As a surgical community, it is incumbent on us to continue to evaluate surgical treatments generating the highest levels of evidence possible (randomized trials) and have sufficient humility to cross refer to colleagues when appropriate to ensure the best care for our patients,” Dr. Lim concluded.
Because the McMaster University study derived the reported outcomes from registry data, determining the reasons that influenced surgeons’ choices of lung resection is impossible, Dr. Eric Lim of the Imperial College of Medicine, London, said in his invited commentary (J Thorac Cardiovasc Surg. 2015 May 21 [doi:10.1016/j.jtcvs.2015.05.048]).
The study authors noted that lower-volume surgeons were more inclined to perform pneumonectomy, and, Dr. Lim noted, previous studies have found that higher-volume centers tended to see more patients with advanced-stage cancers and increased morbidities. “An alternative explanation is that higher-volume surgeons have better skill sets to undertake procedures such as sleeve lobectomies that would lower the pneumonectomy rates and possibly more segmentectomies to lower the wedge-resection rate,” Dr. Lim said.
Until better evidence exists on what procedure is best for central and peripheral tumors, “surgeons can argue either way,” Dr. Lim said. The questions that follow from the study should concentrate on the relative harm of each procedure and the level of practice variation that’s unacceptable.
“As a surgical community, it is incumbent on us to continue to evaluate surgical treatments generating the highest levels of evidence possible (randomized trials) and have sufficient humility to cross refer to colleagues when appropriate to ensure the best care for our patients,” Dr. Lim concluded.
A surgeon’s comfort level with a favored operation for non–small cell lung cancer can strongly influence whether the patient will have that operation, which, in turn, can affect the patient’s outcome and long-term survival, according to an analysis of a population-linked database. For patients whose surgeons have lower levels of experience, that could mean a greater chance they will have more invasive total lung removal rather than more difficult operations that spare part of the affected lung, according to investigators at McMaster University in Hamilton, Ontario.
“If a surgeon with high surgical volumes is less likely to perform higher-risk pneumonectomy procedures than one with lower volumes, this may translate to a significant reduction in adverse events,” said lead author Dr. Christian Finley and coauthors online in the Journal of Thoracic and Cardiovascular Surgery (2015 Jun 30 [doi: 10.1016/j.jtcvs.2015.04.060]). “Surgeon volume should be considered an important component in how care is delivered in this population.”
The McMaster investigators evaluated 8,070 patients in an Ontario population-based linked database who underwent surgical resection for non–small cell lung cancer during 2004-2011, including pneumonectomy, or total lung removal (842 patients), lobectomy (6,212 patients), and wedge resection (1,002 patients). Over the years of the study, the proportion of patients who underwent pneumonectomy fell by more than half, from 14.8% in 2004 to 7.6% in 2011.
Of the three procedures, pneumonectomy carries a threefold greater mortality and while the procedure is often avoidable, there may be cases where it’s necessary because of the location of the tumor, Dr. Finley and his colleagues said. Lobectomy is desirable because it spares the parenchyma and has lower recurrence rates than laser resections.
The study investigators aimed to explore the hypothesis that surgeons with less expertise are more inclined to perform the higher-risk pneumonectomy or sublobar resections such as a segmentectomy or a wedge resection than a lobectomy, the rationale being that these procedures can be less challenging than a standard or sleeve lobectomy. The study analyzed results from 124 different physicians at 45 institutions in Ontario.
Data analysis showed that physician volume, age, year of procedure, sex, and comorbidities were predictive of the surgeon performing a pneumonectomy. “Adjusting for these variables, the results indicated that for each 10-unit increase in physician volume, the relative risk of performing a pneumonectomy decreased by 9.1%,” Dr. Finley and his colleagues wrote. They also found no significant difference in stage distribution among low-, medium-, and high-volume surgeons.
“This is meaningful as pneumonectomy is known to have the highest mortality rate of lung cancer resection, found in this study to be 12.6%, demonstrating a potentially large impact on patient survival,” Dr. Finley and his colleagues said.
This analysis cites an earlier study that surgeon volume for many procedures was a key determinant in the link between hospital volume and operative mortality (N Engl J Med. 2003 Nov 27;349[2]:2117-27.). “This study suggests that a patient may improve their chance of survival substantially, even at high-volume institutions, by selecting surgeons who perform operations more frequently,” Dr. Finley and his colleagues said.
They said that despite their study’s limitations, the findings on how surgeon experience can influence the choice of lung resection for cancer warrant further study.
McMaster University, Division of Thoracic Surgery, provided funding for the study. The study authors had no disclosures.
A surgeon’s comfort level with a favored operation for non–small cell lung cancer can strongly influence whether the patient will have that operation, which, in turn, can affect the patient’s outcome and long-term survival, according to an analysis of a population-linked database. For patients whose surgeons have lower levels of experience, that could mean a greater chance they will have more invasive total lung removal rather than more difficult operations that spare part of the affected lung, according to investigators at McMaster University in Hamilton, Ontario.
“If a surgeon with high surgical volumes is less likely to perform higher-risk pneumonectomy procedures than one with lower volumes, this may translate to a significant reduction in adverse events,” said lead author Dr. Christian Finley and coauthors online in the Journal of Thoracic and Cardiovascular Surgery (2015 Jun 30 [doi: 10.1016/j.jtcvs.2015.04.060]). “Surgeon volume should be considered an important component in how care is delivered in this population.”
The McMaster investigators evaluated 8,070 patients in an Ontario population-based linked database who underwent surgical resection for non–small cell lung cancer during 2004-2011, including pneumonectomy, or total lung removal (842 patients), lobectomy (6,212 patients), and wedge resection (1,002 patients). Over the years of the study, the proportion of patients who underwent pneumonectomy fell by more than half, from 14.8% in 2004 to 7.6% in 2011.
Of the three procedures, pneumonectomy carries a threefold greater mortality and while the procedure is often avoidable, there may be cases where it’s necessary because of the location of the tumor, Dr. Finley and his colleagues said. Lobectomy is desirable because it spares the parenchyma and has lower recurrence rates than laser resections.
The study investigators aimed to explore the hypothesis that surgeons with less expertise are more inclined to perform the higher-risk pneumonectomy or sublobar resections such as a segmentectomy or a wedge resection than a lobectomy, the rationale being that these procedures can be less challenging than a standard or sleeve lobectomy. The study analyzed results from 124 different physicians at 45 institutions in Ontario.
Data analysis showed that physician volume, age, year of procedure, sex, and comorbidities were predictive of the surgeon performing a pneumonectomy. “Adjusting for these variables, the results indicated that for each 10-unit increase in physician volume, the relative risk of performing a pneumonectomy decreased by 9.1%,” Dr. Finley and his colleagues wrote. They also found no significant difference in stage distribution among low-, medium-, and high-volume surgeons.
“This is meaningful as pneumonectomy is known to have the highest mortality rate of lung cancer resection, found in this study to be 12.6%, demonstrating a potentially large impact on patient survival,” Dr. Finley and his colleagues said.
This analysis cites an earlier study that surgeon volume for many procedures was a key determinant in the link between hospital volume and operative mortality (N Engl J Med. 2003 Nov 27;349[2]:2117-27.). “This study suggests that a patient may improve their chance of survival substantially, even at high-volume institutions, by selecting surgeons who perform operations more frequently,” Dr. Finley and his colleagues said.
They said that despite their study’s limitations, the findings on how surgeon experience can influence the choice of lung resection for cancer warrant further study.
McMaster University, Division of Thoracic Surgery, provided funding for the study. The study authors had no disclosures.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Surgeon volume is a predictor of procedure selection for lung cancer surgery and has implications on outcomes.
Major finding: For each 10 unit increase in physician volume, the relative risk of performing a pneumonectomy decreased by 9.1%.
Data source: Dataset of 8,070 patients constructed from Ontario population-based linked databases accessed via the Institute for Clinical Evaluate Sciences.
Disclosures: McMaster University, Division of Thoracic Surgery, provided study funding. The authors had no disclosures.
How patient-reported outcomes may alter care
As Medicare and commercial payers rely increasingly on quality outcomes to determine reimbursement levels, clinicians focused on traditional clinical outcomes like length of hospital stay and readmission may be missing the point if they don’t take into consideration more patient-centric outcome. A team of investigators at MD Anderson Cancer Center in Houston recently evaluated that institution’s tool for measuring patient-reported outcomes in tracking results after thoracic surgery for lung cancer.
Christopher P. Fagundes, Ph.D., led the research team that reported their findings in the September issue of the Journal of Thoracic and Cardiovascular Surgery (2015 [doi:10.1016/j.jtcvs.2015.05.057]).
“We used the MD Anderson Symptom Inventory (MDASI) to elicit patient reports of the worst symptoms experienced after thoracic surgery,” Dr. Fagundes and his colleagues said in what may be the first study to use patient-reported outcomes to chart a recovery course after surgery. “This study demonstrates that the MDASI is a sensitive tool for detecting symptomatic recovery with an expected relationship among surgery type, preoperative performance status, and comorbid conditions,” the authors stated.
The MDASI measures the severity of 13 common cancer-related symptoms over the previous 24 hours and rates each on a scale from 0-10, with 10 as most severe. Patients in the study completed a written MDASI questionnaire, 60 upon enrollment, and 77 patients 3 and 5 days after surgery in the hospital. After discharge and for 3 months after surgery, patients received weekly calls from a computer/telephone interactive voice-response system that asked them to rate their symptoms.
All patients were newly diagnosed and treatment naive with early-stage non–small cell lung cancer (NSCLC) who had either standard open thoracotomy or video-assisted thoracoscopic surgery (VATS) lobectomy.
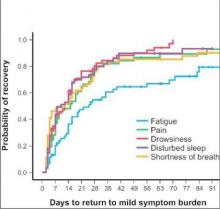
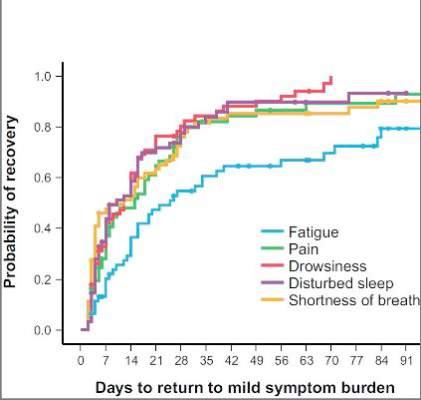
The investigators used a two-pronged approach to define recovery after surgery: symptoms returning to their baseline levels or to a mild level of severity. In the first week after surgery, pain, fatigue, and shortness of breath were “highly prevalent,” perhaps a combined effect from surgical insult and care during and after surgery. After a month, ratings for most symptoms returned to baseline, but fatigue remained the most-persistent symptom during the 3-month study.
NSCLC patients typically have a host of symptoms after major surgery, including inflammation, organ stress, and reaction to medications. In addition, up to a quarter have postsurgical complications that can amplify their symptoms and even delay their course of cancer treatment. The five most-severe postoperative symptoms patients in the study reported were fatigue, pain, shortness of breath, disturbed sleep, and drowsiness.
The median time to return to mild symptom severity for these five symptoms was shorter than return to baseline severity, with fatigue taking longer. Pain recovered significantly faster for patients who underwent VATS lobectomy vs. standard open thoracotomy (8 vs. 18 days, respectively; P = .022), according to the researchers. In addition, the researchers found that patients who had poor preoperative performance status or comorbidities reported significantly higher postoperative pain (P less than .05).
Having the ability to measure patient-reported outcomes allows physicians to identify patients at greatest risk for symptoms after surgery and to help caregivers develop pathways to speed up recovery and get patients to chemotherapy or other cancer treatments on schedule, Dr. Fagundes and his coauthors said.
“Using a straightforward, concise tool like the MDASI to obtain the patient’s perspective on how well he or she is recovering is a clinically relevant and user-friendly method for optimizing perioperative care,” the authors added.
Minimally invasive surgical techniques along with opioid-sparing analgesia have been incorporated into enhanced recovery programs to aim for better objective postoperative outcomes like fewer complications and shorter hospital stays. “Missing from these metrics is the voice of the patient, who is arguably the best source of information about what ‘recovery’ from surgery means,” Dr. Fagundes and colleagues said. “Lack of research on how to define and measure symptomatic and functional recovery after major cancer surgery from the patent’s perspective is an important gap in comprehensive postoperative care; it also compromises any comparison of ERP [enhanced recovery program] innovations against standard care.”
Study coauthors received funding from the National Cancer Institute of the National Institutes of Health, the MD Anderson Cancer Center Support Grant program and the American Cancer Society Research Scholar Grant program. The coauthors reported no conflicts of interest in this work.
“Whether ‘subjective’ or not, the measurements of five common postoperative symptoms appear to reflect the real world experience of many,” Dr. Robert B. Cameron of the University of California, Los Angeles, said in his invited commentary (J. Thorac. Cardiovasc. Surg. 2015 [doi: 10.1016/j.jtcvs.2015.06.017]).
What’s more, because the study investigators collected patient data after discharge, they eliminated opportunities to “game the system,” in Dr. Cameron’s words. One example of gaming the outcome measures would be to discharge patients early after surgery with a Heimlich chest tube, subjecting them to problems at home and requiring physicians visits and even emergency department care that can go unrecorded.
“Subjective” patient experiences are “real issues,” Dr. Cameron said. “After all, patients are the customers that the health care system serves.”
Patient-reported outcome measures could dramatically influence how physicians and health systems report patient outcomes. “While length of stay (LOS) and hospital complication rates appear to be valid outcome measures to report, in reality, they are outcomes only in a world when hospitals are the ‘customers,’ ” Dr. Cameron said. “In a patient-centric system, when patient outcomes matter, [patient-reported outcomes], such as those proposed by the MD Anderson investigators, are what truly matter.”
“Whether ‘subjective’ or not, the measurements of five common postoperative symptoms appear to reflect the real world experience of many,” Dr. Robert B. Cameron of the University of California, Los Angeles, said in his invited commentary (J. Thorac. Cardiovasc. Surg. 2015 [doi: 10.1016/j.jtcvs.2015.06.017]).
What’s more, because the study investigators collected patient data after discharge, they eliminated opportunities to “game the system,” in Dr. Cameron’s words. One example of gaming the outcome measures would be to discharge patients early after surgery with a Heimlich chest tube, subjecting them to problems at home and requiring physicians visits and even emergency department care that can go unrecorded.
“Subjective” patient experiences are “real issues,” Dr. Cameron said. “After all, patients are the customers that the health care system serves.”
Patient-reported outcome measures could dramatically influence how physicians and health systems report patient outcomes. “While length of stay (LOS) and hospital complication rates appear to be valid outcome measures to report, in reality, they are outcomes only in a world when hospitals are the ‘customers,’ ” Dr. Cameron said. “In a patient-centric system, when patient outcomes matter, [patient-reported outcomes], such as those proposed by the MD Anderson investigators, are what truly matter.”
“Whether ‘subjective’ or not, the measurements of five common postoperative symptoms appear to reflect the real world experience of many,” Dr. Robert B. Cameron of the University of California, Los Angeles, said in his invited commentary (J. Thorac. Cardiovasc. Surg. 2015 [doi: 10.1016/j.jtcvs.2015.06.017]).
What’s more, because the study investigators collected patient data after discharge, they eliminated opportunities to “game the system,” in Dr. Cameron’s words. One example of gaming the outcome measures would be to discharge patients early after surgery with a Heimlich chest tube, subjecting them to problems at home and requiring physicians visits and even emergency department care that can go unrecorded.
“Subjective” patient experiences are “real issues,” Dr. Cameron said. “After all, patients are the customers that the health care system serves.”
Patient-reported outcome measures could dramatically influence how physicians and health systems report patient outcomes. “While length of stay (LOS) and hospital complication rates appear to be valid outcome measures to report, in reality, they are outcomes only in a world when hospitals are the ‘customers,’ ” Dr. Cameron said. “In a patient-centric system, when patient outcomes matter, [patient-reported outcomes], such as those proposed by the MD Anderson investigators, are what truly matter.”
As Medicare and commercial payers rely increasingly on quality outcomes to determine reimbursement levels, clinicians focused on traditional clinical outcomes like length of hospital stay and readmission may be missing the point if they don’t take into consideration more patient-centric outcome. A team of investigators at MD Anderson Cancer Center in Houston recently evaluated that institution’s tool for measuring patient-reported outcomes in tracking results after thoracic surgery for lung cancer.
Christopher P. Fagundes, Ph.D., led the research team that reported their findings in the September issue of the Journal of Thoracic and Cardiovascular Surgery (2015 [doi:10.1016/j.jtcvs.2015.05.057]).
“We used the MD Anderson Symptom Inventory (MDASI) to elicit patient reports of the worst symptoms experienced after thoracic surgery,” Dr. Fagundes and his colleagues said in what may be the first study to use patient-reported outcomes to chart a recovery course after surgery. “This study demonstrates that the MDASI is a sensitive tool for detecting symptomatic recovery with an expected relationship among surgery type, preoperative performance status, and comorbid conditions,” the authors stated.
The MDASI measures the severity of 13 common cancer-related symptoms over the previous 24 hours and rates each on a scale from 0-10, with 10 as most severe. Patients in the study completed a written MDASI questionnaire, 60 upon enrollment, and 77 patients 3 and 5 days after surgery in the hospital. After discharge and for 3 months after surgery, patients received weekly calls from a computer/telephone interactive voice-response system that asked them to rate their symptoms.
All patients were newly diagnosed and treatment naive with early-stage non–small cell lung cancer (NSCLC) who had either standard open thoracotomy or video-assisted thoracoscopic surgery (VATS) lobectomy.
The investigators used a two-pronged approach to define recovery after surgery: symptoms returning to their baseline levels or to a mild level of severity. In the first week after surgery, pain, fatigue, and shortness of breath were “highly prevalent,” perhaps a combined effect from surgical insult and care during and after surgery. After a month, ratings for most symptoms returned to baseline, but fatigue remained the most-persistent symptom during the 3-month study.
NSCLC patients typically have a host of symptoms after major surgery, including inflammation, organ stress, and reaction to medications. In addition, up to a quarter have postsurgical complications that can amplify their symptoms and even delay their course of cancer treatment. The five most-severe postoperative symptoms patients in the study reported were fatigue, pain, shortness of breath, disturbed sleep, and drowsiness.
The median time to return to mild symptom severity for these five symptoms was shorter than return to baseline severity, with fatigue taking longer. Pain recovered significantly faster for patients who underwent VATS lobectomy vs. standard open thoracotomy (8 vs. 18 days, respectively; P = .022), according to the researchers. In addition, the researchers found that patients who had poor preoperative performance status or comorbidities reported significantly higher postoperative pain (P less than .05).
Having the ability to measure patient-reported outcomes allows physicians to identify patients at greatest risk for symptoms after surgery and to help caregivers develop pathways to speed up recovery and get patients to chemotherapy or other cancer treatments on schedule, Dr. Fagundes and his coauthors said.
“Using a straightforward, concise tool like the MDASI to obtain the patient’s perspective on how well he or she is recovering is a clinically relevant and user-friendly method for optimizing perioperative care,” the authors added.
Minimally invasive surgical techniques along with opioid-sparing analgesia have been incorporated into enhanced recovery programs to aim for better objective postoperative outcomes like fewer complications and shorter hospital stays. “Missing from these metrics is the voice of the patient, who is arguably the best source of information about what ‘recovery’ from surgery means,” Dr. Fagundes and colleagues said. “Lack of research on how to define and measure symptomatic and functional recovery after major cancer surgery from the patent’s perspective is an important gap in comprehensive postoperative care; it also compromises any comparison of ERP [enhanced recovery program] innovations against standard care.”
Study coauthors received funding from the National Cancer Institute of the National Institutes of Health, the MD Anderson Cancer Center Support Grant program and the American Cancer Society Research Scholar Grant program. The coauthors reported no conflicts of interest in this work.
As Medicare and commercial payers rely increasingly on quality outcomes to determine reimbursement levels, clinicians focused on traditional clinical outcomes like length of hospital stay and readmission may be missing the point if they don’t take into consideration more patient-centric outcome. A team of investigators at MD Anderson Cancer Center in Houston recently evaluated that institution’s tool for measuring patient-reported outcomes in tracking results after thoracic surgery for lung cancer.
Christopher P. Fagundes, Ph.D., led the research team that reported their findings in the September issue of the Journal of Thoracic and Cardiovascular Surgery (2015 [doi:10.1016/j.jtcvs.2015.05.057]).
“We used the MD Anderson Symptom Inventory (MDASI) to elicit patient reports of the worst symptoms experienced after thoracic surgery,” Dr. Fagundes and his colleagues said in what may be the first study to use patient-reported outcomes to chart a recovery course after surgery. “This study demonstrates that the MDASI is a sensitive tool for detecting symptomatic recovery with an expected relationship among surgery type, preoperative performance status, and comorbid conditions,” the authors stated.
The MDASI measures the severity of 13 common cancer-related symptoms over the previous 24 hours and rates each on a scale from 0-10, with 10 as most severe. Patients in the study completed a written MDASI questionnaire, 60 upon enrollment, and 77 patients 3 and 5 days after surgery in the hospital. After discharge and for 3 months after surgery, patients received weekly calls from a computer/telephone interactive voice-response system that asked them to rate their symptoms.
All patients were newly diagnosed and treatment naive with early-stage non–small cell lung cancer (NSCLC) who had either standard open thoracotomy or video-assisted thoracoscopic surgery (VATS) lobectomy.
The investigators used a two-pronged approach to define recovery after surgery: symptoms returning to their baseline levels or to a mild level of severity. In the first week after surgery, pain, fatigue, and shortness of breath were “highly prevalent,” perhaps a combined effect from surgical insult and care during and after surgery. After a month, ratings for most symptoms returned to baseline, but fatigue remained the most-persistent symptom during the 3-month study.
NSCLC patients typically have a host of symptoms after major surgery, including inflammation, organ stress, and reaction to medications. In addition, up to a quarter have postsurgical complications that can amplify their symptoms and even delay their course of cancer treatment. The five most-severe postoperative symptoms patients in the study reported were fatigue, pain, shortness of breath, disturbed sleep, and drowsiness.
The median time to return to mild symptom severity for these five symptoms was shorter than return to baseline severity, with fatigue taking longer. Pain recovered significantly faster for patients who underwent VATS lobectomy vs. standard open thoracotomy (8 vs. 18 days, respectively; P = .022), according to the researchers. In addition, the researchers found that patients who had poor preoperative performance status or comorbidities reported significantly higher postoperative pain (P less than .05).
Having the ability to measure patient-reported outcomes allows physicians to identify patients at greatest risk for symptoms after surgery and to help caregivers develop pathways to speed up recovery and get patients to chemotherapy or other cancer treatments on schedule, Dr. Fagundes and his coauthors said.
“Using a straightforward, concise tool like the MDASI to obtain the patient’s perspective on how well he or she is recovering is a clinically relevant and user-friendly method for optimizing perioperative care,” the authors added.
Minimally invasive surgical techniques along with opioid-sparing analgesia have been incorporated into enhanced recovery programs to aim for better objective postoperative outcomes like fewer complications and shorter hospital stays. “Missing from these metrics is the voice of the patient, who is arguably the best source of information about what ‘recovery’ from surgery means,” Dr. Fagundes and colleagues said. “Lack of research on how to define and measure symptomatic and functional recovery after major cancer surgery from the patent’s perspective is an important gap in comprehensive postoperative care; it also compromises any comparison of ERP [enhanced recovery program] innovations against standard care.”
Study coauthors received funding from the National Cancer Institute of the National Institutes of Health, the MD Anderson Cancer Center Support Grant program and the American Cancer Society Research Scholar Grant program. The coauthors reported no conflicts of interest in this work.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Using the MD Anderson Symptom Inventory to elicit patient-reported symptom burden is a simple, clinically relevant way to optimize care after thoracic surgery for lung cancer.
Major finding: Assessing symptoms from the patient’s perspective throughout the postoperative recovery period is an effective strategy for evaluating perioperative care.
Data source: Sixty newly diagnosed patients with early-stage non–small cell lung cancer scheduled for thoracic surgery prospectively were recruited for evaluation.
Disclosures: Study coauthors received funding from the National Cancer Institute of the National Institutes of Health, the MD Anderson Cancer Center Support Grant program, and the American Cancer Society Research Scholar Grant program. The coauthors reported no conflicts of interest in this work.
CTS Innovation: The promise of tissue engineering for heart valve replacement and repair
Bioengineered tissues are showing promise in the treatment of valvular heart disease, according to the results of several early clinical and preclinical studies demonstrating the benefits of using heterologous substrates as scaffolding capable of promoting in vivo cell infiltration and remodeling.
Ultimately, cardiac tissue engineering seeks to develop the ideal material for replacement and repair of a variety of heart components – a material able to integrate, function, and, when necessary, grow and develop in a manner undifferentiated from normal cellular structures.
One of the most important areas of focus for such efforts is the development of heart valves and valve patches suitable for use in replacement or repair. The need is obvious: “The fact that literature continues to be published debating the best type of valve prosthesis is proof that, to date, the ideal valve substitute has not been found,” according to Cristian Rosu, Ph.D. and Dr. Edward G. Soltesz of the Cleveland Clinic (Semin Thorac Cardiovasc Surg. 2015 June 30 [doi: 10.1053/j.semtcvs.2015.06.007]).
Dr. Roşu and Dr. Soltesz go on to say that this lack of an ideal prosthetic heart valve leaves surgeons and their patients with a difficult choice at the time of valve surgery. The focus of their concern relates to how current bioprostheses are being used in younger and younger patients, and how these devices have a finite lifespan – requiring eventual reoperation and replacement, perhaps multiple times. In addition, calcification is a prominent and well-known risk of bioprosthetic valves, especially in children (Circulation. 2014 Jul 1;130[1]:51-60).
As for mechanical valves, although durable, they require a lifetime of anticoagulation therapy to prevent thrombosis. And the lack of growth potential found in both mechanical and current bioprosthetic devices is obviously a bane to pediatric valve therapy.
The latest efforts in tissue engineering therefore seek to develop valves and valve components that may be more permanent solutions by better mimicking natural valves.
But better mimicking of a natural valve is not an easy task, when such valves exist in “a near-perfect correlation of structure and function, enabling the valve to avoid excess stress on the cusps while simultaneously withstanding the wear and tear of 40-million repetitive deformations per year, equivalent to some 3 billion over a 75-year lifetime.” (“Principles of Tissue Engineering,” 4th ed. [London: Academic Press 2014, p. 813]).
The “holy grail” of tissue engineering, therefore, is to provide a completely in vitro–developed, autologous, fully cellularized, functional scaffolding for implantation that can live up to these requirements. In order to do this, extensive research into the search for the best cell sources and growth matrices and methods is underway, as illustrated by several recent reviews (Front Cell Dev Biol. 30 June 2015 [doi.org/10.3389/fcell.2015.00039] and Mater Sci Eng C. 2015 March 1;48:556-65).
Candidate cell types include a variety of embryonic stem cells, as well as adult cell types that have proven amenable to rejuvenation and redifferentiation (Adv Drug Deliv Rev. 2014;69-70:254-69).
In one example of the quest for completely in vitro human-tissue designed valves, Dr. Jean Dubé of Laval University, Quebec, and his colleagues reported research on a human tissue–engineered trileaflet heart valve assembled in vitro using human fibroblasts. These cells self-assembled into living tissue sheets when cultured in the presence of sodium ascorbate. These sheets could be layered together to create a thick construct, with the ultimate goal of replacing the use of bovine pericardium tissue implants with ones made of autologous cells from the patient (Acta Biomater. 2014 Aug;10[8]:3563-70).
Currently, however, many of the preclinical studies of fully tissue engineered heart valves have shown retraction of the heart valve leaflets as a major mechanism of functional failure. This retraction is caused by both passive and active cell stress and passive matrix stress, according to a review by Inge A.E.W. van Loosdregt, Ph.D., and her colleagues at the Eindhoven (the Netherlands) University of Technology (J Biomech. 2014 Jun 27;47[9]:2064-9).
While all of these developmental issues regarding the use of fully tissue-engineered valves are being worked out, early clinical applications are already being found for a new generation of valve prostheses that take an intermediate approach, one that uses an implanted scaffolding material that allows autologous cell infiltration and replacement in vivo.
Two of the most prominent examples of these scaffoldings currently in investigation and in early clinical use for heart valve repair or replacement are the bovine pericardium-derived CardioCel (Admedus, Brisbane, Australia) and the porcine intestinal submucosa-derived CorMatrix ECM(CorMatrix Cardiovascular, Roswell, Ga.). CorMatrix ECM was approved by the Food and Drug Administration in 2005 for pericardial repair and reconstruction, and in 2007 for cardiac tissue repair. The FDA approved CardioCel in 2014 for use in the United States in pericardial closure and for the repair of cardiac and vascular defects in both adults and children.
Both technologies rely on the concept of matrix infiltration by autologous cells after implantation in order to create a living mimic of the patient’s own tissues.
CardioCel is a highly treated, bovine pericardium-derived, decellularized collagen matrix. It showed significant resistance to calcification in mitral and pulmonary implants in a juvenile sheep model as reported by Dr. Christian P. Brizard at Royal Children’s Hospital, Melbourne, and his colleagues (J Thorac Cardiovasc Surg. 2014 Dec;148[6]:3194-201). These investigators replaced the posterior leaflet of the mitral valve and one of the pulmonary valve cusps with patches in 10-month-old ewes. They compared the use of CardioCel in six ewes to a control group of four ewes repaired with autologous pericardium that was treated intraoperatively with glutaraldehyde, which is the standard default used at their institution for more than 2 decades as the best material for valve repair in their pediatric patients.
The primary end points of the study were thickening and calcium content. They found that all animals survived with normal valve echocardiography until sacrifice at 7 months. They reported that the bovine pericardium patches allowed accurate valve repair at both systemic and pulmonary pressure with preserved mechanical properties and more-controlled healing and without calcification as compared with the controls. (Calcification is a known major risk factor for the eventual failure of bioprosthetic valves.) Additionally, the bovine pericardium patched valves showed the in vivo development of dense but thin cellularized outer layers of mature collagen I, compared with the controls, which had outer layers that were much less dense and showed the presence of immature collagen III.
In another case of valvular use of the CardioCel material, at the recent American Association for Thoracic Surgery Mitral Conclave 2015, M. Bonnie Ghosh-Dastidar, Ph.D., and her colleagues from the Royal Brompton Hospital, London, reported on a severely ill patient with significant mitral regurgitation and an infected mitral valve with large vegetations on the anterior leaflets. The infected tissue was resected and a large patch of CardioCel bovine pericardium was used to reconstruct the leaflets. Postoperative assessment showed a competent mitral valve with good area of leaflet coaptation, according to Dr. Ghosh-Dastidar.
In his invited commentary on the animal-model research by Dr. Brizard, Dr. Niv Ad, director of cardiac surgery research at Inova Heart and Vascular Institute, Falls Church, Va., said that despite the promise of the CardioCel patches, there were a number of alternative approaches being investigated. “Other engineered materials currently being study include processes such as lyophilization, which has shown promising results in reducing inflammation. Another proposed approach is the use of decellularized vessels and patches with the promise of normal remodeling and growth, such as extracellular matrix and its potential for tissue regeneration.”
Dr. Ad also stated that, “the key bioengineering challenge is to determine how biologic, structural, and mechanical factors interact and function in vivo. The understanding of these factors will prove critical to the development of a clinically viable tissue-engineered heart valve,” (J Thorac Cardiovasc Surg. 2014 Dec;148[6]:3202-3).
An example of the use of extracellular matrix material referred to by Dr. Ad is the CorMatrix ECM, which is an extracellular matrix material derived from porcine small-intestinal submucosa, processed and decellularized.
Dr. Marc W. Gerdisch and his colleagues from the Franciscan St. Francis Heart Center, Indianapolis, reported on the results of treating 19 patients with mitral valve disease using the CorMatrix patch material for partial or subtotal leaflet repair or extension. There were three deaths unrelated to the repair and no instances of perioperative or late stroke. Two patients with a history of cancer and cancer therapy experienced failure of the initial repair, requiring reintervention. However, the other mitral valve repair patients continued to show good valvular function and no calcification on echocardiographic follow-up of 4 days to 48 months (Thorac Cardiovasc Surg. 2014 Oct;148[4]:1370-8).
And in 2015, CorMatrix Cardiovascular announced FDA approval of an investigational device exemption for an early feasibility study of their CorMatrix ECM Tricuspid Heart Valve in up to 15 patients at 5 U.S. centers (NCT02397668). Indications are for the surgical management of tricuspid valve disease not amenable to annuloplasty or repair, including tricuspid valve disease secondary to congenital heart disease in pediatric patients and adult endocarditis patients. The CorMatrix ECM Tricuspid Valve is a flexible, unstented valve acting as a 3‐D scaffold designed to function immediately as a prosthetic, but one constructed to permit native cellular infiltration and remodeling.
Ultimately, both the CardioCel and the CorMatrix materials are just the tip of an iceberg of research into tissue engineering as a clinical tool. And success and the wider adoption of any current or developing technologies will require the results of far-more-extensive studies and long-term clinical results.
At some future date, engineered total organ substitutes or the routine injection of genetically engineered stem cells may provide a practical alternative for treating a diseased or damaged heart. But for now, the most-likely scenario is the continued exploration of tissue engineering to develop the most durable, growth-, stress-, and biologically compatible patches, valves, and vasculatures possible – defined tools that can be adapted to current surgical techniques, designed to obtain the best repair of congenital or acquired heart disease conditions.
As with any transformative technology in medicine, the path from laboratory success and optimistic short-term clinical results to durable, long-term postoperative benefits may be a rocky one. But given the current enthusiasm, it certainly looks as if it will be a well-traveled road.
Dr. Brizard reported consulting and lecturing fees from Admedus. His research was supported with the assistance of a grant from Admedus. Dr. Gerdisch reported consulting fees and equity ownership in CorMatrix Cardiovascular.
Bioengineered tissues are showing promise in the treatment of valvular heart disease, according to the results of several early clinical and preclinical studies demonstrating the benefits of using heterologous substrates as scaffolding capable of promoting in vivo cell infiltration and remodeling.
Ultimately, cardiac tissue engineering seeks to develop the ideal material for replacement and repair of a variety of heart components – a material able to integrate, function, and, when necessary, grow and develop in a manner undifferentiated from normal cellular structures.
One of the most important areas of focus for such efforts is the development of heart valves and valve patches suitable for use in replacement or repair. The need is obvious: “The fact that literature continues to be published debating the best type of valve prosthesis is proof that, to date, the ideal valve substitute has not been found,” according to Cristian Rosu, Ph.D. and Dr. Edward G. Soltesz of the Cleveland Clinic (Semin Thorac Cardiovasc Surg. 2015 June 30 [doi: 10.1053/j.semtcvs.2015.06.007]).
Dr. Roşu and Dr. Soltesz go on to say that this lack of an ideal prosthetic heart valve leaves surgeons and their patients with a difficult choice at the time of valve surgery. The focus of their concern relates to how current bioprostheses are being used in younger and younger patients, and how these devices have a finite lifespan – requiring eventual reoperation and replacement, perhaps multiple times. In addition, calcification is a prominent and well-known risk of bioprosthetic valves, especially in children (Circulation. 2014 Jul 1;130[1]:51-60).
As for mechanical valves, although durable, they require a lifetime of anticoagulation therapy to prevent thrombosis. And the lack of growth potential found in both mechanical and current bioprosthetic devices is obviously a bane to pediatric valve therapy.
The latest efforts in tissue engineering therefore seek to develop valves and valve components that may be more permanent solutions by better mimicking natural valves.
But better mimicking of a natural valve is not an easy task, when such valves exist in “a near-perfect correlation of structure and function, enabling the valve to avoid excess stress on the cusps while simultaneously withstanding the wear and tear of 40-million repetitive deformations per year, equivalent to some 3 billion over a 75-year lifetime.” (“Principles of Tissue Engineering,” 4th ed. [London: Academic Press 2014, p. 813]).
The “holy grail” of tissue engineering, therefore, is to provide a completely in vitro–developed, autologous, fully cellularized, functional scaffolding for implantation that can live up to these requirements. In order to do this, extensive research into the search for the best cell sources and growth matrices and methods is underway, as illustrated by several recent reviews (Front Cell Dev Biol. 30 June 2015 [doi.org/10.3389/fcell.2015.00039] and Mater Sci Eng C. 2015 March 1;48:556-65).
Candidate cell types include a variety of embryonic stem cells, as well as adult cell types that have proven amenable to rejuvenation and redifferentiation (Adv Drug Deliv Rev. 2014;69-70:254-69).
In one example of the quest for completely in vitro human-tissue designed valves, Dr. Jean Dubé of Laval University, Quebec, and his colleagues reported research on a human tissue–engineered trileaflet heart valve assembled in vitro using human fibroblasts. These cells self-assembled into living tissue sheets when cultured in the presence of sodium ascorbate. These sheets could be layered together to create a thick construct, with the ultimate goal of replacing the use of bovine pericardium tissue implants with ones made of autologous cells from the patient (Acta Biomater. 2014 Aug;10[8]:3563-70).
Currently, however, many of the preclinical studies of fully tissue engineered heart valves have shown retraction of the heart valve leaflets as a major mechanism of functional failure. This retraction is caused by both passive and active cell stress and passive matrix stress, according to a review by Inge A.E.W. van Loosdregt, Ph.D., and her colleagues at the Eindhoven (the Netherlands) University of Technology (J Biomech. 2014 Jun 27;47[9]:2064-9).
While all of these developmental issues regarding the use of fully tissue-engineered valves are being worked out, early clinical applications are already being found for a new generation of valve prostheses that take an intermediate approach, one that uses an implanted scaffolding material that allows autologous cell infiltration and replacement in vivo.
Two of the most prominent examples of these scaffoldings currently in investigation and in early clinical use for heart valve repair or replacement are the bovine pericardium-derived CardioCel (Admedus, Brisbane, Australia) and the porcine intestinal submucosa-derived CorMatrix ECM(CorMatrix Cardiovascular, Roswell, Ga.). CorMatrix ECM was approved by the Food and Drug Administration in 2005 for pericardial repair and reconstruction, and in 2007 for cardiac tissue repair. The FDA approved CardioCel in 2014 for use in the United States in pericardial closure and for the repair of cardiac and vascular defects in both adults and children.
Both technologies rely on the concept of matrix infiltration by autologous cells after implantation in order to create a living mimic of the patient’s own tissues.
CardioCel is a highly treated, bovine pericardium-derived, decellularized collagen matrix. It showed significant resistance to calcification in mitral and pulmonary implants in a juvenile sheep model as reported by Dr. Christian P. Brizard at Royal Children’s Hospital, Melbourne, and his colleagues (J Thorac Cardiovasc Surg. 2014 Dec;148[6]:3194-201). These investigators replaced the posterior leaflet of the mitral valve and one of the pulmonary valve cusps with patches in 10-month-old ewes. They compared the use of CardioCel in six ewes to a control group of four ewes repaired with autologous pericardium that was treated intraoperatively with glutaraldehyde, which is the standard default used at their institution for more than 2 decades as the best material for valve repair in their pediatric patients.
The primary end points of the study were thickening and calcium content. They found that all animals survived with normal valve echocardiography until sacrifice at 7 months. They reported that the bovine pericardium patches allowed accurate valve repair at both systemic and pulmonary pressure with preserved mechanical properties and more-controlled healing and without calcification as compared with the controls. (Calcification is a known major risk factor for the eventual failure of bioprosthetic valves.) Additionally, the bovine pericardium patched valves showed the in vivo development of dense but thin cellularized outer layers of mature collagen I, compared with the controls, which had outer layers that were much less dense and showed the presence of immature collagen III.
In another case of valvular use of the CardioCel material, at the recent American Association for Thoracic Surgery Mitral Conclave 2015, M. Bonnie Ghosh-Dastidar, Ph.D., and her colleagues from the Royal Brompton Hospital, London, reported on a severely ill patient with significant mitral regurgitation and an infected mitral valve with large vegetations on the anterior leaflets. The infected tissue was resected and a large patch of CardioCel bovine pericardium was used to reconstruct the leaflets. Postoperative assessment showed a competent mitral valve with good area of leaflet coaptation, according to Dr. Ghosh-Dastidar.
In his invited commentary on the animal-model research by Dr. Brizard, Dr. Niv Ad, director of cardiac surgery research at Inova Heart and Vascular Institute, Falls Church, Va., said that despite the promise of the CardioCel patches, there were a number of alternative approaches being investigated. “Other engineered materials currently being study include processes such as lyophilization, which has shown promising results in reducing inflammation. Another proposed approach is the use of decellularized vessels and patches with the promise of normal remodeling and growth, such as extracellular matrix and its potential for tissue regeneration.”
Dr. Ad also stated that, “the key bioengineering challenge is to determine how biologic, structural, and mechanical factors interact and function in vivo. The understanding of these factors will prove critical to the development of a clinically viable tissue-engineered heart valve,” (J Thorac Cardiovasc Surg. 2014 Dec;148[6]:3202-3).
An example of the use of extracellular matrix material referred to by Dr. Ad is the CorMatrix ECM, which is an extracellular matrix material derived from porcine small-intestinal submucosa, processed and decellularized.
Dr. Marc W. Gerdisch and his colleagues from the Franciscan St. Francis Heart Center, Indianapolis, reported on the results of treating 19 patients with mitral valve disease using the CorMatrix patch material for partial or subtotal leaflet repair or extension. There were three deaths unrelated to the repair and no instances of perioperative or late stroke. Two patients with a history of cancer and cancer therapy experienced failure of the initial repair, requiring reintervention. However, the other mitral valve repair patients continued to show good valvular function and no calcification on echocardiographic follow-up of 4 days to 48 months (Thorac Cardiovasc Surg. 2014 Oct;148[4]:1370-8).
And in 2015, CorMatrix Cardiovascular announced FDA approval of an investigational device exemption for an early feasibility study of their CorMatrix ECM Tricuspid Heart Valve in up to 15 patients at 5 U.S. centers (NCT02397668). Indications are for the surgical management of tricuspid valve disease not amenable to annuloplasty or repair, including tricuspid valve disease secondary to congenital heart disease in pediatric patients and adult endocarditis patients. The CorMatrix ECM Tricuspid Valve is a flexible, unstented valve acting as a 3‐D scaffold designed to function immediately as a prosthetic, but one constructed to permit native cellular infiltration and remodeling.
Ultimately, both the CardioCel and the CorMatrix materials are just the tip of an iceberg of research into tissue engineering as a clinical tool. And success and the wider adoption of any current or developing technologies will require the results of far-more-extensive studies and long-term clinical results.
At some future date, engineered total organ substitutes or the routine injection of genetically engineered stem cells may provide a practical alternative for treating a diseased or damaged heart. But for now, the most-likely scenario is the continued exploration of tissue engineering to develop the most durable, growth-, stress-, and biologically compatible patches, valves, and vasculatures possible – defined tools that can be adapted to current surgical techniques, designed to obtain the best repair of congenital or acquired heart disease conditions.
As with any transformative technology in medicine, the path from laboratory success and optimistic short-term clinical results to durable, long-term postoperative benefits may be a rocky one. But given the current enthusiasm, it certainly looks as if it will be a well-traveled road.
Dr. Brizard reported consulting and lecturing fees from Admedus. His research was supported with the assistance of a grant from Admedus. Dr. Gerdisch reported consulting fees and equity ownership in CorMatrix Cardiovascular.
Bioengineered tissues are showing promise in the treatment of valvular heart disease, according to the results of several early clinical and preclinical studies demonstrating the benefits of using heterologous substrates as scaffolding capable of promoting in vivo cell infiltration and remodeling.
Ultimately, cardiac tissue engineering seeks to develop the ideal material for replacement and repair of a variety of heart components – a material able to integrate, function, and, when necessary, grow and develop in a manner undifferentiated from normal cellular structures.
One of the most important areas of focus for such efforts is the development of heart valves and valve patches suitable for use in replacement or repair. The need is obvious: “The fact that literature continues to be published debating the best type of valve prosthesis is proof that, to date, the ideal valve substitute has not been found,” according to Cristian Rosu, Ph.D. and Dr. Edward G. Soltesz of the Cleveland Clinic (Semin Thorac Cardiovasc Surg. 2015 June 30 [doi: 10.1053/j.semtcvs.2015.06.007]).
Dr. Roşu and Dr. Soltesz go on to say that this lack of an ideal prosthetic heart valve leaves surgeons and their patients with a difficult choice at the time of valve surgery. The focus of their concern relates to how current bioprostheses are being used in younger and younger patients, and how these devices have a finite lifespan – requiring eventual reoperation and replacement, perhaps multiple times. In addition, calcification is a prominent and well-known risk of bioprosthetic valves, especially in children (Circulation. 2014 Jul 1;130[1]:51-60).
As for mechanical valves, although durable, they require a lifetime of anticoagulation therapy to prevent thrombosis. And the lack of growth potential found in both mechanical and current bioprosthetic devices is obviously a bane to pediatric valve therapy.
The latest efforts in tissue engineering therefore seek to develop valves and valve components that may be more permanent solutions by better mimicking natural valves.
But better mimicking of a natural valve is not an easy task, when such valves exist in “a near-perfect correlation of structure and function, enabling the valve to avoid excess stress on the cusps while simultaneously withstanding the wear and tear of 40-million repetitive deformations per year, equivalent to some 3 billion over a 75-year lifetime.” (“Principles of Tissue Engineering,” 4th ed. [London: Academic Press 2014, p. 813]).
The “holy grail” of tissue engineering, therefore, is to provide a completely in vitro–developed, autologous, fully cellularized, functional scaffolding for implantation that can live up to these requirements. In order to do this, extensive research into the search for the best cell sources and growth matrices and methods is underway, as illustrated by several recent reviews (Front Cell Dev Biol. 30 June 2015 [doi.org/10.3389/fcell.2015.00039] and Mater Sci Eng C. 2015 March 1;48:556-65).
Candidate cell types include a variety of embryonic stem cells, as well as adult cell types that have proven amenable to rejuvenation and redifferentiation (Adv Drug Deliv Rev. 2014;69-70:254-69).
In one example of the quest for completely in vitro human-tissue designed valves, Dr. Jean Dubé of Laval University, Quebec, and his colleagues reported research on a human tissue–engineered trileaflet heart valve assembled in vitro using human fibroblasts. These cells self-assembled into living tissue sheets when cultured in the presence of sodium ascorbate. These sheets could be layered together to create a thick construct, with the ultimate goal of replacing the use of bovine pericardium tissue implants with ones made of autologous cells from the patient (Acta Biomater. 2014 Aug;10[8]:3563-70).
Currently, however, many of the preclinical studies of fully tissue engineered heart valves have shown retraction of the heart valve leaflets as a major mechanism of functional failure. This retraction is caused by both passive and active cell stress and passive matrix stress, according to a review by Inge A.E.W. van Loosdregt, Ph.D., and her colleagues at the Eindhoven (the Netherlands) University of Technology (J Biomech. 2014 Jun 27;47[9]:2064-9).
While all of these developmental issues regarding the use of fully tissue-engineered valves are being worked out, early clinical applications are already being found for a new generation of valve prostheses that take an intermediate approach, one that uses an implanted scaffolding material that allows autologous cell infiltration and replacement in vivo.
Two of the most prominent examples of these scaffoldings currently in investigation and in early clinical use for heart valve repair or replacement are the bovine pericardium-derived CardioCel (Admedus, Brisbane, Australia) and the porcine intestinal submucosa-derived CorMatrix ECM(CorMatrix Cardiovascular, Roswell, Ga.). CorMatrix ECM was approved by the Food and Drug Administration in 2005 for pericardial repair and reconstruction, and in 2007 for cardiac tissue repair. The FDA approved CardioCel in 2014 for use in the United States in pericardial closure and for the repair of cardiac and vascular defects in both adults and children.
Both technologies rely on the concept of matrix infiltration by autologous cells after implantation in order to create a living mimic of the patient’s own tissues.
CardioCel is a highly treated, bovine pericardium-derived, decellularized collagen matrix. It showed significant resistance to calcification in mitral and pulmonary implants in a juvenile sheep model as reported by Dr. Christian P. Brizard at Royal Children’s Hospital, Melbourne, and his colleagues (J Thorac Cardiovasc Surg. 2014 Dec;148[6]:3194-201). These investigators replaced the posterior leaflet of the mitral valve and one of the pulmonary valve cusps with patches in 10-month-old ewes. They compared the use of CardioCel in six ewes to a control group of four ewes repaired with autologous pericardium that was treated intraoperatively with glutaraldehyde, which is the standard default used at their institution for more than 2 decades as the best material for valve repair in their pediatric patients.
The primary end points of the study were thickening and calcium content. They found that all animals survived with normal valve echocardiography until sacrifice at 7 months. They reported that the bovine pericardium patches allowed accurate valve repair at both systemic and pulmonary pressure with preserved mechanical properties and more-controlled healing and without calcification as compared with the controls. (Calcification is a known major risk factor for the eventual failure of bioprosthetic valves.) Additionally, the bovine pericardium patched valves showed the in vivo development of dense but thin cellularized outer layers of mature collagen I, compared with the controls, which had outer layers that were much less dense and showed the presence of immature collagen III.
In another case of valvular use of the CardioCel material, at the recent American Association for Thoracic Surgery Mitral Conclave 2015, M. Bonnie Ghosh-Dastidar, Ph.D., and her colleagues from the Royal Brompton Hospital, London, reported on a severely ill patient with significant mitral regurgitation and an infected mitral valve with large vegetations on the anterior leaflets. The infected tissue was resected and a large patch of CardioCel bovine pericardium was used to reconstruct the leaflets. Postoperative assessment showed a competent mitral valve with good area of leaflet coaptation, according to Dr. Ghosh-Dastidar.
In his invited commentary on the animal-model research by Dr. Brizard, Dr. Niv Ad, director of cardiac surgery research at Inova Heart and Vascular Institute, Falls Church, Va., said that despite the promise of the CardioCel patches, there were a number of alternative approaches being investigated. “Other engineered materials currently being study include processes such as lyophilization, which has shown promising results in reducing inflammation. Another proposed approach is the use of decellularized vessels and patches with the promise of normal remodeling and growth, such as extracellular matrix and its potential for tissue regeneration.”
Dr. Ad also stated that, “the key bioengineering challenge is to determine how biologic, structural, and mechanical factors interact and function in vivo. The understanding of these factors will prove critical to the development of a clinically viable tissue-engineered heart valve,” (J Thorac Cardiovasc Surg. 2014 Dec;148[6]:3202-3).
An example of the use of extracellular matrix material referred to by Dr. Ad is the CorMatrix ECM, which is an extracellular matrix material derived from porcine small-intestinal submucosa, processed and decellularized.
Dr. Marc W. Gerdisch and his colleagues from the Franciscan St. Francis Heart Center, Indianapolis, reported on the results of treating 19 patients with mitral valve disease using the CorMatrix patch material for partial or subtotal leaflet repair or extension. There were three deaths unrelated to the repair and no instances of perioperative or late stroke. Two patients with a history of cancer and cancer therapy experienced failure of the initial repair, requiring reintervention. However, the other mitral valve repair patients continued to show good valvular function and no calcification on echocardiographic follow-up of 4 days to 48 months (Thorac Cardiovasc Surg. 2014 Oct;148[4]:1370-8).
And in 2015, CorMatrix Cardiovascular announced FDA approval of an investigational device exemption for an early feasibility study of their CorMatrix ECM Tricuspid Heart Valve in up to 15 patients at 5 U.S. centers (NCT02397668). Indications are for the surgical management of tricuspid valve disease not amenable to annuloplasty or repair, including tricuspid valve disease secondary to congenital heart disease in pediatric patients and adult endocarditis patients. The CorMatrix ECM Tricuspid Valve is a flexible, unstented valve acting as a 3‐D scaffold designed to function immediately as a prosthetic, but one constructed to permit native cellular infiltration and remodeling.
Ultimately, both the CardioCel and the CorMatrix materials are just the tip of an iceberg of research into tissue engineering as a clinical tool. And success and the wider adoption of any current or developing technologies will require the results of far-more-extensive studies and long-term clinical results.
At some future date, engineered total organ substitutes or the routine injection of genetically engineered stem cells may provide a practical alternative for treating a diseased or damaged heart. But for now, the most-likely scenario is the continued exploration of tissue engineering to develop the most durable, growth-, stress-, and biologically compatible patches, valves, and vasculatures possible – defined tools that can be adapted to current surgical techniques, designed to obtain the best repair of congenital or acquired heart disease conditions.
As with any transformative technology in medicine, the path from laboratory success and optimistic short-term clinical results to durable, long-term postoperative benefits may be a rocky one. But given the current enthusiasm, it certainly looks as if it will be a well-traveled road.
Dr. Brizard reported consulting and lecturing fees from Admedus. His research was supported with the assistance of a grant from Admedus. Dr. Gerdisch reported consulting fees and equity ownership in CorMatrix Cardiovascular.
Perioperative cardiovascular assessment guidelines pose new challenges
ESTES PARK, COLO. – The latest American College of Cardiology/American Heart Association guidelines on perioperative cardiovascular evaluation in patients undergoing noncardiac surgery create several major new responsibilities for physicians doing the evaluations.
Some primary care physicians will be uncomfortable with several of the new recommendations. Perhaps that shouldn’t come as a surprise, since not a single primary care physician was included among the 17 members of the writing committee or the 38-member review committee. Cardiologists of all subspecialties, surgeons, anesthesiologists, hospitalists, a patient representative – all had a voice. But not primary care, Dr. Robert E. Burke noted at a conference on internal medicine sponsored by the University of Colorado.
He was quick to add, however, that the document (Circulation. 2014 Dec 9;130[24]:e278-333) is an impressive, thoughtful piece of work built upon 490 reference citations, and it deserves to be incorporated into daily clinical practice, albeit with a few caveats.
“I think the writing panel did a really good job, but the recommendations are only as good as the evidence – and there are a couple of areas where the evidence is not very strong ... It’s up to you and your patient to decide what to do in the controversial areas,” said Dr. Burke, assistant chief of hospital medicine at the Denver VA Medical Center and an internist at the university.
A big change from the previous 2007 ACC/AHA guidelines that Dr. Burke fully supports is that it’s no longer sufficient for the evaluating physician to formally consider only the level of risk posed by the patient’s planned noncardiac surgery, which can vary from a low-risk operation such as cataract surgery to a far-higher-risk procedure addressing, for example, an expanding abdominal aortic aneurysm.
Under the 2014 guidelines, it’s also important to incorporate the surgical risk with an estimate of the individual’s personalized risk of perioperative major adverse cardiovascular events. If the estimated risk is less than 1%, it’s appropriate to proceed straightaway with surgery. In contrast, a 1% or greater perioperative risk is considered high, and it’s then recommended to continue along a lengthy management algorithm. This involves sending the patient for cardiac stress testing provided two conditions are met: the surgeon has to be unwilling to operate without the stress test results and the patient must be willing to undergo CABG before the noncardiac surgery, should the stress test and follow-up cardiac catheterization prove positive.
The guidelines recommend using either of two validated risk-prediction tools for calculating patient risk: the Revised Cardiac Risk Index (Circulation 1999 Sep 7;100[10]:1043-9) or the American College of Surgeons’ National Surgical Quality Improvement Project (NSQIP) predictor. Dr. Burke prefers the NSQIP tool. It predicts multiple cardiac and noncardiac outcomes, including all-cause mortality, within 30 days of surgery, and it has been validated in 525 U.S. hospitals with more than 1 million operations. It’s also quite user-friendly, according to Dr. Burke.
In his view, the guidelines contain two major areas of controvery. One involves the much more aggressive approach to revascularization prior to noncardiac surgery in high-cardiovascular-risk patients compared with the 2007 guidelines. The current guidelines give a class I recommendation to revascularization before noncardiac surgery in patients with left main or triple-vessel disease, as well as two-vessel disease that includes the left anterior descending coronary artery. He said this is a leap that’s not supported by the best available evidence, which comes from the older CARP (Coronary-Artery Revascularization before Elective Major Vascular Surgery) trial.
In CARP, 510 military veterans with documented coronary artery disease and planned vascular surgery were randomized to preoperative revascularization or not. The only exclusions were left main disease, severe aortic stenosis, or a left ventricular ejection fraction below 20%. After 2.8 years of follow-up post surgery, preoperative revascularization wasn’t associated with any mortality benefit in any patient subgroup (N Engl J Med. 2004 Dec 30;351[27]:2795-804).
“I want to restate that in this well-done randomized controlled trial, they could not find any benefit in any outcome in any group within this very-high-risk group of patients. This is really the main trial that exists in perioperative care, and I’m very impressed with the results. The guidelines extrapolate from studies in other areas. This is one area where I think the cardiologists are more interventional than I would like. If I had a patient in my office I could not tell him with a straight face that revascularization before their planned surgery is going to offer them any benefit during up to nearly 3 years of follow-up unless they have left main disease, which was an exclusion in CARP,” Dr. Burke said.
The other main controversy in his view is the ACC/AHA stance on postoperative troponin measurement. It gets a class III rating – meaning no benefit, don’t do it – for routine screening in unselected patients without signs or symptoms suggestive of myocardial ischemia. This recommendation is based upon the absence to date of a prospective trial showing that acting on an elevated postoperative troponin in such patients improves outcomes. True enough, Dr. Burke conceded, but he finds persuasive the results of VISION (Vascular Events in Noncardiac Surgery Patients Cohort Evaluation), a prospective international study in which more than 15,000 patients undergoing noncardiac surgery underwent screening troponin measurements on the first 3 days after surgery.
Thirty-day mortality in the VISION study was 1% in those with a peak fourth-generation troponin of 0.01 ng/mL or less. Mortality was significantly increased in the nearly 12% of patients with a troponin of at least 0.02 ng/mL, climbing to a peak mortality rate of 16.9% in those with a postoperative troponin of 0.30 ng/mL or more (JAMA. 2012 Jun 6;307[21]:2295-304).
“I don’t know of any other test in the perioperative setting that’s as good as this in terms of risk-stratifying patients. We can’t just sit by and let this sort of 30-day mortality rate happen. We should do something. I think that if you treat these asymptomatic patients with an elevated postop troponin as if they had an MI – get them on optimal medical therapy and really push smoking cessation – it’s likely to have a benefit on outcomes,” said Dr. Burke.
He reported having no financial conflicts regarding his presentation.
ESTES PARK, COLO. – The latest American College of Cardiology/American Heart Association guidelines on perioperative cardiovascular evaluation in patients undergoing noncardiac surgery create several major new responsibilities for physicians doing the evaluations.
Some primary care physicians will be uncomfortable with several of the new recommendations. Perhaps that shouldn’t come as a surprise, since not a single primary care physician was included among the 17 members of the writing committee or the 38-member review committee. Cardiologists of all subspecialties, surgeons, anesthesiologists, hospitalists, a patient representative – all had a voice. But not primary care, Dr. Robert E. Burke noted at a conference on internal medicine sponsored by the University of Colorado.
He was quick to add, however, that the document (Circulation. 2014 Dec 9;130[24]:e278-333) is an impressive, thoughtful piece of work built upon 490 reference citations, and it deserves to be incorporated into daily clinical practice, albeit with a few caveats.
“I think the writing panel did a really good job, but the recommendations are only as good as the evidence – and there are a couple of areas where the evidence is not very strong ... It’s up to you and your patient to decide what to do in the controversial areas,” said Dr. Burke, assistant chief of hospital medicine at the Denver VA Medical Center and an internist at the university.
A big change from the previous 2007 ACC/AHA guidelines that Dr. Burke fully supports is that it’s no longer sufficient for the evaluating physician to formally consider only the level of risk posed by the patient’s planned noncardiac surgery, which can vary from a low-risk operation such as cataract surgery to a far-higher-risk procedure addressing, for example, an expanding abdominal aortic aneurysm.
Under the 2014 guidelines, it’s also important to incorporate the surgical risk with an estimate of the individual’s personalized risk of perioperative major adverse cardiovascular events. If the estimated risk is less than 1%, it’s appropriate to proceed straightaway with surgery. In contrast, a 1% or greater perioperative risk is considered high, and it’s then recommended to continue along a lengthy management algorithm. This involves sending the patient for cardiac stress testing provided two conditions are met: the surgeon has to be unwilling to operate without the stress test results and the patient must be willing to undergo CABG before the noncardiac surgery, should the stress test and follow-up cardiac catheterization prove positive.
The guidelines recommend using either of two validated risk-prediction tools for calculating patient risk: the Revised Cardiac Risk Index (Circulation 1999 Sep 7;100[10]:1043-9) or the American College of Surgeons’ National Surgical Quality Improvement Project (NSQIP) predictor. Dr. Burke prefers the NSQIP tool. It predicts multiple cardiac and noncardiac outcomes, including all-cause mortality, within 30 days of surgery, and it has been validated in 525 U.S. hospitals with more than 1 million operations. It’s also quite user-friendly, according to Dr. Burke.
In his view, the guidelines contain two major areas of controvery. One involves the much more aggressive approach to revascularization prior to noncardiac surgery in high-cardiovascular-risk patients compared with the 2007 guidelines. The current guidelines give a class I recommendation to revascularization before noncardiac surgery in patients with left main or triple-vessel disease, as well as two-vessel disease that includes the left anterior descending coronary artery. He said this is a leap that’s not supported by the best available evidence, which comes from the older CARP (Coronary-Artery Revascularization before Elective Major Vascular Surgery) trial.
In CARP, 510 military veterans with documented coronary artery disease and planned vascular surgery were randomized to preoperative revascularization or not. The only exclusions were left main disease, severe aortic stenosis, or a left ventricular ejection fraction below 20%. After 2.8 years of follow-up post surgery, preoperative revascularization wasn’t associated with any mortality benefit in any patient subgroup (N Engl J Med. 2004 Dec 30;351[27]:2795-804).
“I want to restate that in this well-done randomized controlled trial, they could not find any benefit in any outcome in any group within this very-high-risk group of patients. This is really the main trial that exists in perioperative care, and I’m very impressed with the results. The guidelines extrapolate from studies in other areas. This is one area where I think the cardiologists are more interventional than I would like. If I had a patient in my office I could not tell him with a straight face that revascularization before their planned surgery is going to offer them any benefit during up to nearly 3 years of follow-up unless they have left main disease, which was an exclusion in CARP,” Dr. Burke said.
The other main controversy in his view is the ACC/AHA stance on postoperative troponin measurement. It gets a class III rating – meaning no benefit, don’t do it – for routine screening in unselected patients without signs or symptoms suggestive of myocardial ischemia. This recommendation is based upon the absence to date of a prospective trial showing that acting on an elevated postoperative troponin in such patients improves outcomes. True enough, Dr. Burke conceded, but he finds persuasive the results of VISION (Vascular Events in Noncardiac Surgery Patients Cohort Evaluation), a prospective international study in which more than 15,000 patients undergoing noncardiac surgery underwent screening troponin measurements on the first 3 days after surgery.
Thirty-day mortality in the VISION study was 1% in those with a peak fourth-generation troponin of 0.01 ng/mL or less. Mortality was significantly increased in the nearly 12% of patients with a troponin of at least 0.02 ng/mL, climbing to a peak mortality rate of 16.9% in those with a postoperative troponin of 0.30 ng/mL or more (JAMA. 2012 Jun 6;307[21]:2295-304).
“I don’t know of any other test in the perioperative setting that’s as good as this in terms of risk-stratifying patients. We can’t just sit by and let this sort of 30-day mortality rate happen. We should do something. I think that if you treat these asymptomatic patients with an elevated postop troponin as if they had an MI – get them on optimal medical therapy and really push smoking cessation – it’s likely to have a benefit on outcomes,” said Dr. Burke.
He reported having no financial conflicts regarding his presentation.
ESTES PARK, COLO. – The latest American College of Cardiology/American Heart Association guidelines on perioperative cardiovascular evaluation in patients undergoing noncardiac surgery create several major new responsibilities for physicians doing the evaluations.
Some primary care physicians will be uncomfortable with several of the new recommendations. Perhaps that shouldn’t come as a surprise, since not a single primary care physician was included among the 17 members of the writing committee or the 38-member review committee. Cardiologists of all subspecialties, surgeons, anesthesiologists, hospitalists, a patient representative – all had a voice. But not primary care, Dr. Robert E. Burke noted at a conference on internal medicine sponsored by the University of Colorado.
He was quick to add, however, that the document (Circulation. 2014 Dec 9;130[24]:e278-333) is an impressive, thoughtful piece of work built upon 490 reference citations, and it deserves to be incorporated into daily clinical practice, albeit with a few caveats.
“I think the writing panel did a really good job, but the recommendations are only as good as the evidence – and there are a couple of areas where the evidence is not very strong ... It’s up to you and your patient to decide what to do in the controversial areas,” said Dr. Burke, assistant chief of hospital medicine at the Denver VA Medical Center and an internist at the university.
A big change from the previous 2007 ACC/AHA guidelines that Dr. Burke fully supports is that it’s no longer sufficient for the evaluating physician to formally consider only the level of risk posed by the patient’s planned noncardiac surgery, which can vary from a low-risk operation such as cataract surgery to a far-higher-risk procedure addressing, for example, an expanding abdominal aortic aneurysm.
Under the 2014 guidelines, it’s also important to incorporate the surgical risk with an estimate of the individual’s personalized risk of perioperative major adverse cardiovascular events. If the estimated risk is less than 1%, it’s appropriate to proceed straightaway with surgery. In contrast, a 1% or greater perioperative risk is considered high, and it’s then recommended to continue along a lengthy management algorithm. This involves sending the patient for cardiac stress testing provided two conditions are met: the surgeon has to be unwilling to operate without the stress test results and the patient must be willing to undergo CABG before the noncardiac surgery, should the stress test and follow-up cardiac catheterization prove positive.
The guidelines recommend using either of two validated risk-prediction tools for calculating patient risk: the Revised Cardiac Risk Index (Circulation 1999 Sep 7;100[10]:1043-9) or the American College of Surgeons’ National Surgical Quality Improvement Project (NSQIP) predictor. Dr. Burke prefers the NSQIP tool. It predicts multiple cardiac and noncardiac outcomes, including all-cause mortality, within 30 days of surgery, and it has been validated in 525 U.S. hospitals with more than 1 million operations. It’s also quite user-friendly, according to Dr. Burke.
In his view, the guidelines contain two major areas of controvery. One involves the much more aggressive approach to revascularization prior to noncardiac surgery in high-cardiovascular-risk patients compared with the 2007 guidelines. The current guidelines give a class I recommendation to revascularization before noncardiac surgery in patients with left main or triple-vessel disease, as well as two-vessel disease that includes the left anterior descending coronary artery. He said this is a leap that’s not supported by the best available evidence, which comes from the older CARP (Coronary-Artery Revascularization before Elective Major Vascular Surgery) trial.
In CARP, 510 military veterans with documented coronary artery disease and planned vascular surgery were randomized to preoperative revascularization or not. The only exclusions were left main disease, severe aortic stenosis, or a left ventricular ejection fraction below 20%. After 2.8 years of follow-up post surgery, preoperative revascularization wasn’t associated with any mortality benefit in any patient subgroup (N Engl J Med. 2004 Dec 30;351[27]:2795-804).
“I want to restate that in this well-done randomized controlled trial, they could not find any benefit in any outcome in any group within this very-high-risk group of patients. This is really the main trial that exists in perioperative care, and I’m very impressed with the results. The guidelines extrapolate from studies in other areas. This is one area where I think the cardiologists are more interventional than I would like. If I had a patient in my office I could not tell him with a straight face that revascularization before their planned surgery is going to offer them any benefit during up to nearly 3 years of follow-up unless they have left main disease, which was an exclusion in CARP,” Dr. Burke said.
The other main controversy in his view is the ACC/AHA stance on postoperative troponin measurement. It gets a class III rating – meaning no benefit, don’t do it – for routine screening in unselected patients without signs or symptoms suggestive of myocardial ischemia. This recommendation is based upon the absence to date of a prospective trial showing that acting on an elevated postoperative troponin in such patients improves outcomes. True enough, Dr. Burke conceded, but he finds persuasive the results of VISION (Vascular Events in Noncardiac Surgery Patients Cohort Evaluation), a prospective international study in which more than 15,000 patients undergoing noncardiac surgery underwent screening troponin measurements on the first 3 days after surgery.
Thirty-day mortality in the VISION study was 1% in those with a peak fourth-generation troponin of 0.01 ng/mL or less. Mortality was significantly increased in the nearly 12% of patients with a troponin of at least 0.02 ng/mL, climbing to a peak mortality rate of 16.9% in those with a postoperative troponin of 0.30 ng/mL or more (JAMA. 2012 Jun 6;307[21]:2295-304).
“I don’t know of any other test in the perioperative setting that’s as good as this in terms of risk-stratifying patients. We can’t just sit by and let this sort of 30-day mortality rate happen. We should do something. I think that if you treat these asymptomatic patients with an elevated postop troponin as if they had an MI – get them on optimal medical therapy and really push smoking cessation – it’s likely to have a benefit on outcomes,” said Dr. Burke.
He reported having no financial conflicts regarding his presentation.
EXPERT ANALYSIS FROM THE ANNUAL INTERNAL MEDICINE PROGRAM
Inpatient mortality down for high-volume conditions
Inpatient mortality for pneumonia, acute MI, heart failure, and stroke each fell significantly from 2002 to 2012, the Agency for Healthcare Research and Quality reported.
Over that period, mortality among adults hospitalized with pneumonia went from 65 per 1,000 admissions to 35.8 per 1,000 for a drop of 45% – largest of the four high-volume conditions. Corresponding declines for the others were 41% for acute MI, 29% for heart failure, and 27% for stroke, the AHRQ noted.

Since “death following discharge from a hospital is not reflected in these data,” the report said, measures of inpatient mortality “can reflect both improvements in health care and shifts in where end-of-life care takes place over time.”
The estimates in the report are based on data from the Nationwide Inpatient Sample (2002-2011) and State Inpatient Databases (2012).
Inpatient mortality for pneumonia, acute MI, heart failure, and stroke each fell significantly from 2002 to 2012, the Agency for Healthcare Research and Quality reported.
Over that period, mortality among adults hospitalized with pneumonia went from 65 per 1,000 admissions to 35.8 per 1,000 for a drop of 45% – largest of the four high-volume conditions. Corresponding declines for the others were 41% for acute MI, 29% for heart failure, and 27% for stroke, the AHRQ noted.

Since “death following discharge from a hospital is not reflected in these data,” the report said, measures of inpatient mortality “can reflect both improvements in health care and shifts in where end-of-life care takes place over time.”
The estimates in the report are based on data from the Nationwide Inpatient Sample (2002-2011) and State Inpatient Databases (2012).
Inpatient mortality for pneumonia, acute MI, heart failure, and stroke each fell significantly from 2002 to 2012, the Agency for Healthcare Research and Quality reported.
Over that period, mortality among adults hospitalized with pneumonia went from 65 per 1,000 admissions to 35.8 per 1,000 for a drop of 45% – largest of the four high-volume conditions. Corresponding declines for the others were 41% for acute MI, 29% for heart failure, and 27% for stroke, the AHRQ noted.

Since “death following discharge from a hospital is not reflected in these data,” the report said, measures of inpatient mortality “can reflect both improvements in health care and shifts in where end-of-life care takes place over time.”
The estimates in the report are based on data from the Nationwide Inpatient Sample (2002-2011) and State Inpatient Databases (2012).
Solid histology predicts poor survival in resected lung adenocarcinoma
For patients with lung adenocarcinoma, a predominantly solid tumor subtype was associated with earlier recurrence and shorter postrecurrence survival (PRS). These patients were more likely to have more extrathoracic and multisite recurrences, according to a single-center retrospective record review of stage I lung adenocarcinoma patients who received surgical resection.
“Despite curative-intent surgical resection, tumor recurrence and spread remain the primary causes of cancer-related death among patients with early-stage lung cancer,” Dr. Hideki Ujiie of Memorial Sloan Kettering Cancer Center (New York), wrote in a study published online in Journal of Clinical Oncology.
To identify which tumor subtypes place patients at higher risk for recurrence after surgery, Dr. Ujiie and his colleagues reviewed records of 1,120 patients undergoing complete surgical resection for stage I lung adenocarcinoma from 1999 to 2009 at Memorial-Sloan Kettering Cancer Center.
The 2-year PRS in the patients studied was 45%, with median PRS of just over 26 months; median follow-up was 60 months. On multivariable analysis, Dr. Ujiie and coauthors found that predominantly solid tumor type was the variable most strongly associated with worse PRS (HR 1.76, 95% CI 1.11-2.77, P = .016).
“The risk of recurrence for tumors with solid predominant histologic subtype peaked within 12 months and ... these tumors were associated with a higher incidence of extrathoracic, multiple-site recurrences in patients with stage I lung adenocarcinoma,” the investigators said (J Clin Oncol. 2015 Aug 10. doi:10.1200/JCO2015.60.9818).
The study was prompted, in part, by the new International Association for the Study of Lung Cancer, American Thoracic Society, and European Respiratory Society lung cancer classification system. The new classification schema recognizes the histologic heterogeneity of lung adenocarcinoma subtypes. Dr. Ujiie and his coauthors sought to use these characterizations to develop prognostic factors for recurrence risk and PRS. Their findings have clinical implications: “[R]egular surveillance is even more important when tumors of aggressive predominant subtypes (e.g., solid predominant histologic pattern) are present,” said Dr. Ujiie and his coauthors.
The National Institutes of Health and the U.S. Department of Defense supported the study. Dr. Chaft reported ties with DAVA Oncology, Myriad Genetics, Biodesix, Otsuka, and Eli Lilly. Dr. Sima is employed by Genentech/Roche. Dr. Huang received funding from Bristol-Myers Squibb. Dr. Rudin reported ties with AbbVia, Aveo Pharmaceuticals, Boehringer Ingelheim, GlaxoSmithKlin, Merck, Celgene, and BioMarin Pharmaceutical. Dr. Adusumilli received funding from Myriad Genetics. The other authors reported no disclosures.
On Twitter: @karioakes
For patients with lung adenocarcinoma, a predominantly solid tumor subtype was associated with earlier recurrence and shorter postrecurrence survival (PRS). These patients were more likely to have more extrathoracic and multisite recurrences, according to a single-center retrospective record review of stage I lung adenocarcinoma patients who received surgical resection.
“Despite curative-intent surgical resection, tumor recurrence and spread remain the primary causes of cancer-related death among patients with early-stage lung cancer,” Dr. Hideki Ujiie of Memorial Sloan Kettering Cancer Center (New York), wrote in a study published online in Journal of Clinical Oncology.
To identify which tumor subtypes place patients at higher risk for recurrence after surgery, Dr. Ujiie and his colleagues reviewed records of 1,120 patients undergoing complete surgical resection for stage I lung adenocarcinoma from 1999 to 2009 at Memorial-Sloan Kettering Cancer Center.
The 2-year PRS in the patients studied was 45%, with median PRS of just over 26 months; median follow-up was 60 months. On multivariable analysis, Dr. Ujiie and coauthors found that predominantly solid tumor type was the variable most strongly associated with worse PRS (HR 1.76, 95% CI 1.11-2.77, P = .016).
“The risk of recurrence for tumors with solid predominant histologic subtype peaked within 12 months and ... these tumors were associated with a higher incidence of extrathoracic, multiple-site recurrences in patients with stage I lung adenocarcinoma,” the investigators said (J Clin Oncol. 2015 Aug 10. doi:10.1200/JCO2015.60.9818).
The study was prompted, in part, by the new International Association for the Study of Lung Cancer, American Thoracic Society, and European Respiratory Society lung cancer classification system. The new classification schema recognizes the histologic heterogeneity of lung adenocarcinoma subtypes. Dr. Ujiie and his coauthors sought to use these characterizations to develop prognostic factors for recurrence risk and PRS. Their findings have clinical implications: “[R]egular surveillance is even more important when tumors of aggressive predominant subtypes (e.g., solid predominant histologic pattern) are present,” said Dr. Ujiie and his coauthors.
The National Institutes of Health and the U.S. Department of Defense supported the study. Dr. Chaft reported ties with DAVA Oncology, Myriad Genetics, Biodesix, Otsuka, and Eli Lilly. Dr. Sima is employed by Genentech/Roche. Dr. Huang received funding from Bristol-Myers Squibb. Dr. Rudin reported ties with AbbVia, Aveo Pharmaceuticals, Boehringer Ingelheim, GlaxoSmithKlin, Merck, Celgene, and BioMarin Pharmaceutical. Dr. Adusumilli received funding from Myriad Genetics. The other authors reported no disclosures.
On Twitter: @karioakes
For patients with lung adenocarcinoma, a predominantly solid tumor subtype was associated with earlier recurrence and shorter postrecurrence survival (PRS). These patients were more likely to have more extrathoracic and multisite recurrences, according to a single-center retrospective record review of stage I lung adenocarcinoma patients who received surgical resection.
“Despite curative-intent surgical resection, tumor recurrence and spread remain the primary causes of cancer-related death among patients with early-stage lung cancer,” Dr. Hideki Ujiie of Memorial Sloan Kettering Cancer Center (New York), wrote in a study published online in Journal of Clinical Oncology.
To identify which tumor subtypes place patients at higher risk for recurrence after surgery, Dr. Ujiie and his colleagues reviewed records of 1,120 patients undergoing complete surgical resection for stage I lung adenocarcinoma from 1999 to 2009 at Memorial-Sloan Kettering Cancer Center.
The 2-year PRS in the patients studied was 45%, with median PRS of just over 26 months; median follow-up was 60 months. On multivariable analysis, Dr. Ujiie and coauthors found that predominantly solid tumor type was the variable most strongly associated with worse PRS (HR 1.76, 95% CI 1.11-2.77, P = .016).
“The risk of recurrence for tumors with solid predominant histologic subtype peaked within 12 months and ... these tumors were associated with a higher incidence of extrathoracic, multiple-site recurrences in patients with stage I lung adenocarcinoma,” the investigators said (J Clin Oncol. 2015 Aug 10. doi:10.1200/JCO2015.60.9818).
The study was prompted, in part, by the new International Association for the Study of Lung Cancer, American Thoracic Society, and European Respiratory Society lung cancer classification system. The new classification schema recognizes the histologic heterogeneity of lung adenocarcinoma subtypes. Dr. Ujiie and his coauthors sought to use these characterizations to develop prognostic factors for recurrence risk and PRS. Their findings have clinical implications: “[R]egular surveillance is even more important when tumors of aggressive predominant subtypes (e.g., solid predominant histologic pattern) are present,” said Dr. Ujiie and his coauthors.
The National Institutes of Health and the U.S. Department of Defense supported the study. Dr. Chaft reported ties with DAVA Oncology, Myriad Genetics, Biodesix, Otsuka, and Eli Lilly. Dr. Sima is employed by Genentech/Roche. Dr. Huang received funding from Bristol-Myers Squibb. Dr. Rudin reported ties with AbbVia, Aveo Pharmaceuticals, Boehringer Ingelheim, GlaxoSmithKlin, Merck, Celgene, and BioMarin Pharmaceutical. Dr. Adusumilli received funding from Myriad Genetics. The other authors reported no disclosures.
On Twitter: @karioakes
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Predominantly solid tumor histology predicted earlier recurrence and poorer postrecurrence survival (PRS) in surgically resected stage I lung adenocarcinoma.
Major finding: Lung adenocarcinoma patients with predominantly solid tumor subtype had worse PRS than did those with other subtypes after surgical resection (HR, 1.76, P = .016).
Data source: Retrospective, single-site medical record review of 1,120 patients undergoing complete resection for stage I lung adenocarcinoma from 1999 to 2009.
Disclosures: The National Institutes of Health and the U.S. Department of Defense supported the study. Dr. Chaft reported ties with DAVA Oncology, Myriad Genetics, Biodesix, Otsuka, and Eli Lilly. Dr. Sima is employed by Genentech/Roche. Dr. Huang received funding from Bristol-Myers Squibb. Dr. Rudin reported ties with AbbVia, Aveo Pharmaceuticals, Boehringer Ingelheim, GlaxoSmithKlin, Merck, Celgene, and BioMarin Pharmaceutical. Dr. Adusumilli received funding from Myriad Genetics. The other authors reported no disclosures.
Outpatient venography can be performed safely
Venoplasties and stenting carried out in an office-based setting have the same therapeutic results and carry no greater risk as the same procedure done in an inpatient setting, researchers reported.
Dr. Arkady Ganelin and researchers from the Total Vascular Center in Brooklyn, N.Y. evaluated 245 patients who had undergone venography for the correction of suspected iliac vein stenosis at their office-based center. Overall, 90 women and 47 men underwent unilateral intervention and 23 women and 14 men underwent bilateral intervention.
There was a low incidence of complications such as thrombosis (2%), a figure that was similar to an inpatient setting, the researchers reported (J Vasc Surg: Venous and Lym Dis. 2015 doi: 10.1016/j.jvsv.2015.03.007).
One patient had a retroperitoneal hematoma, which occurred more than 30 days after the procedure. The average pain score was 2 out of 10 on the Likert scale.
“Our initial experience with conducting office-based procedures that were formerly only inpatient procedures has demonstrated that an office-based procedure can be safely performed with minimal complications,” the study authors wrote.
The financial burden of U.S. health care has been continuously increasing and the shift of endovascular procedures from the hospital to an office-based setting is the natural next step, they said.
If the results are sustained over the long term, office-based iliac venography and stent placement may replace the need of performing these procedures in the hospital, they concluded.
This conclusion, however, poses the question of which option would be chosen by a patient, they added.
The researchers reported having no financial disclosures.
There are more than 500 office-based labs. Complicated endovascular procedures are performed in this setting with results comparable or better than hospital-based procedures with extremely high patient satisfaction. Experience with complicated venous procedures in the office has been limited because there is no reimbursement for use of intravascular ultrasound (IVUS) in the office. This may change in January as the Centers for Medicare & Medicaid Services may start reimbursing the use of IVUS in office. IVUS is an important element in endovascular management of venous obstruction.
Researchers from Brooklyn, N.Y., performed 285 venous angioplasties and stent placements in an office setting. There was a 2% incidence of thrombosis that occurred in patients with a previous history of deep venous thrombosis. This subset of patients would naturally be at a higher risk for thrombosis. There was one bleeding complication after 30 days, which was successfully managed by nonoperative means. The complication rate was comparable to the procedures done in the hospital setting. One would expect similar complication rates when the same operator is doing the procedure at two different sites. However, the indications for these procedures are not well defined in the literature and there are very few studies showing long-term results. Accordingly, there is a real need for a prospective randomized study to determine the indications and efficacy of these procedures.
Dr. Krishna Jain is clinical associate professor of surgery, Western Michigan University School of Medicine, Kalamazoo. He is an associate medical editor of Vascular Specialist.
There are more than 500 office-based labs. Complicated endovascular procedures are performed in this setting with results comparable or better than hospital-based procedures with extremely high patient satisfaction. Experience with complicated venous procedures in the office has been limited because there is no reimbursement for use of intravascular ultrasound (IVUS) in the office. This may change in January as the Centers for Medicare & Medicaid Services may start reimbursing the use of IVUS in office. IVUS is an important element in endovascular management of venous obstruction.
Researchers from Brooklyn, N.Y., performed 285 venous angioplasties and stent placements in an office setting. There was a 2% incidence of thrombosis that occurred in patients with a previous history of deep venous thrombosis. This subset of patients would naturally be at a higher risk for thrombosis. There was one bleeding complication after 30 days, which was successfully managed by nonoperative means. The complication rate was comparable to the procedures done in the hospital setting. One would expect similar complication rates when the same operator is doing the procedure at two different sites. However, the indications for these procedures are not well defined in the literature and there are very few studies showing long-term results. Accordingly, there is a real need for a prospective randomized study to determine the indications and efficacy of these procedures.
Dr. Krishna Jain is clinical associate professor of surgery, Western Michigan University School of Medicine, Kalamazoo. He is an associate medical editor of Vascular Specialist.
There are more than 500 office-based labs. Complicated endovascular procedures are performed in this setting with results comparable or better than hospital-based procedures with extremely high patient satisfaction. Experience with complicated venous procedures in the office has been limited because there is no reimbursement for use of intravascular ultrasound (IVUS) in the office. This may change in January as the Centers for Medicare & Medicaid Services may start reimbursing the use of IVUS in office. IVUS is an important element in endovascular management of venous obstruction.
Researchers from Brooklyn, N.Y., performed 285 venous angioplasties and stent placements in an office setting. There was a 2% incidence of thrombosis that occurred in patients with a previous history of deep venous thrombosis. This subset of patients would naturally be at a higher risk for thrombosis. There was one bleeding complication after 30 days, which was successfully managed by nonoperative means. The complication rate was comparable to the procedures done in the hospital setting. One would expect similar complication rates when the same operator is doing the procedure at two different sites. However, the indications for these procedures are not well defined in the literature and there are very few studies showing long-term results. Accordingly, there is a real need for a prospective randomized study to determine the indications and efficacy of these procedures.
Dr. Krishna Jain is clinical associate professor of surgery, Western Michigan University School of Medicine, Kalamazoo. He is an associate medical editor of Vascular Specialist.
Venoplasties and stenting carried out in an office-based setting have the same therapeutic results and carry no greater risk as the same procedure done in an inpatient setting, researchers reported.
Dr. Arkady Ganelin and researchers from the Total Vascular Center in Brooklyn, N.Y. evaluated 245 patients who had undergone venography for the correction of suspected iliac vein stenosis at their office-based center. Overall, 90 women and 47 men underwent unilateral intervention and 23 women and 14 men underwent bilateral intervention.
There was a low incidence of complications such as thrombosis (2%), a figure that was similar to an inpatient setting, the researchers reported (J Vasc Surg: Venous and Lym Dis. 2015 doi: 10.1016/j.jvsv.2015.03.007).
One patient had a retroperitoneal hematoma, which occurred more than 30 days after the procedure. The average pain score was 2 out of 10 on the Likert scale.
“Our initial experience with conducting office-based procedures that were formerly only inpatient procedures has demonstrated that an office-based procedure can be safely performed with minimal complications,” the study authors wrote.
The financial burden of U.S. health care has been continuously increasing and the shift of endovascular procedures from the hospital to an office-based setting is the natural next step, they said.
If the results are sustained over the long term, office-based iliac venography and stent placement may replace the need of performing these procedures in the hospital, they concluded.
This conclusion, however, poses the question of which option would be chosen by a patient, they added.
The researchers reported having no financial disclosures.
Venoplasties and stenting carried out in an office-based setting have the same therapeutic results and carry no greater risk as the same procedure done in an inpatient setting, researchers reported.
Dr. Arkady Ganelin and researchers from the Total Vascular Center in Brooklyn, N.Y. evaluated 245 patients who had undergone venography for the correction of suspected iliac vein stenosis at their office-based center. Overall, 90 women and 47 men underwent unilateral intervention and 23 women and 14 men underwent bilateral intervention.
There was a low incidence of complications such as thrombosis (2%), a figure that was similar to an inpatient setting, the researchers reported (J Vasc Surg: Venous and Lym Dis. 2015 doi: 10.1016/j.jvsv.2015.03.007).
One patient had a retroperitoneal hematoma, which occurred more than 30 days after the procedure. The average pain score was 2 out of 10 on the Likert scale.
“Our initial experience with conducting office-based procedures that were formerly only inpatient procedures has demonstrated that an office-based procedure can be safely performed with minimal complications,” the study authors wrote.
The financial burden of U.S. health care has been continuously increasing and the shift of endovascular procedures from the hospital to an office-based setting is the natural next step, they said.
If the results are sustained over the long term, office-based iliac venography and stent placement may replace the need of performing these procedures in the hospital, they concluded.
This conclusion, however, poses the question of which option would be chosen by a patient, they added.
The researchers reported having no financial disclosures.
FROM THE JOURNAL OF VASCULAR SURGERY: VENOUS AND LYMPHATIC DISORDERS
Key clinical point: Office-based iliac venography and stent placement may replace the need to perform these procedures in the hospital.
Major finding: Outpatient venography had the same therapeutic results and carried no greater risk as the same procedure done in an inpatient setting.
Data source: 245 patients who had undergone venography for the correction of suspected iliac vein stenosis in an office-based setting.
Disclosures: The researchers reported having no financial disclosures.
‘David technique’ may enhance aortic repair
Many techniques for repair of aortic dissection have evolved, but no trials have compared those techniques to determine which is the best. However, a study team has attempted to evaluate a surgical approach (the “David technique”) that includes three specific steps – no aortic cross clamp, the use of deep hypothermic circulatory arrest (DHCA), and the antegrade resumption of cardiopulmonary bypass. They found that this approach yielded significantly better long-term outcomes than did other approaches tried.
The study investigators, led by Dr. Jennifer S. Lawton of Washington University in St. Louis, reported their findings in the Journal of Thoracic and Cardiovascular Surgery (J. Thorac. Cardiovasc. Surg. 2015 [doi:10.1016/j.jtcvs.2015.03.023]). “We hypothesized that a surgical strategy to prevent cross-clamp injury or false lumen pressurization would be associated with reduced morbidity, mortality, persistent false lumen patency, and improved survival,” Dr. Lawton and her coauthors wrote. “This study was designed to determine the differences in outcomes between operative techniques.”
The study evaluated 196 patients who had surgery for acute type A aortic dissection over 17 years. Group 1, which comprised 49 patients, had the operation according to the protocol that involved the three specific steps, as Dr. Tirone David of the University of Toronto first reported in 1999 (Ann. Thorac. Surg. 1999;67:1999-2001) — the “David technique,” as the study authors called it. Group 2 consisted of patients whose repair involved a variety of techniques, including one or two steps of the David technique but not all three.
Study endpoints were 30-day mortality rate, postoperative adverse events, presence of a false aortic lumen, and overall survival, the latter defined as the time from the date of surgery to the date or death or last follow-up. The evaluation included examination of patients’ latest CT scan or MRI that was at least 6 months after the operation for false lumen, but only 78 patients had imaging at that interval.
Patients in Group 1 had a higher rate of persistent false lumen – 74% vs. 68% in Group 2. Thirty-day mortality was 6.1% in Group 1 and 15.7% in Group 2, but Dr. Lawton and her coauthors said this difference was not statistically significant.
Survival rates at 1, 5 and 10 years among both groups were “consistent with published ranges,” the authors said. At 5 years, the predicted survival was 86% for Group 1 and 56% for Group 2; and at 10 years, 72% and 37% respectively.
The study authors acknowledged the controversy that surrounds the use of retrograde resumption of cardiopulmonary bypass after replacement of the ascending aorta and that there’s no consensus on which method is best for resuming cardiopulmonary bypass after repair of a type A aortic dissection.
The study also found no difference in the incidence of false lumen between the two groups, but again, this is a source of controversy. “Persistence of a false lumen following repair for type A aortic dissection has been reported to be associated with poor prognosis and reduced long-term survival,” Dr. Lawton and her colleagues said.
“Others have reported a patent false lumen was not an independent predictor of late reoperation, but was a predictor of aortic growth following repair of type A aortic dissection.”
The study authors said one limit of their findings is its retrospective nature, but they also said that a prospective, randomized trial would be difficult to conduct.
None of the study coauthors had any relationships to disclose. They presented their original data at the American Association for Thoracic Surgery Aortic Symposium, April 24-25, 2014, in New York.
Whether or not to use a cross-clamp in type A aortic dissection repair is a critical question, but a major concern of this study was the wide variability of techniques used in the comparison group, Dr. Richard J. Shemin said in his invited commentary (J. Thorac. Cardiovasc. Surg. 2015 [doi:10.1016/j.jtcvs.2015.04.038]). “The variety of approaches attests to the lack of institutional agreement on the surgical principles tested in the study,” he said. “The large variety of techniques in the control group makes the comparison and interpretation of this study difficult.”
“There are more questions to consider from this study than answers derived from the data about the clamp strategy,” he said
But, Dr. Shemin said, using the cross-clamp with axillary antegrade perfusion is “not a major issue.” And the use of clamping during the cooling period can save overall cardiac arrest time during the operation.
“If one does use femoral cannulation, then not applying the cross-clamp until achieving circulatory arrest is prudent,” he said. “With axillary cannulation, one achieves antegrade perfusion so early cross-clamping can be safely performed with the advantages of saving operative time.”
The clamp site must be inspected during circulatory arrest. Antegrade cerebral perfusion is proven to be an excellent technique and is facilitated by right axillary cannulation, Dr. Shemin said. “Most importantly, establishing antegrade CPB [cardiopulmonary bypass] perfusion after circulatory arrest is mandatory in all cases to minimize distal aorta trauma,” he said.
Dr. Richard J. Shemin is a cardiothoracic surgeon at UCLA Medical Center, Santa Monica, Calif.
Whether or not to use a cross-clamp in type A aortic dissection repair is a critical question, but a major concern of this study was the wide variability of techniques used in the comparison group, Dr. Richard J. Shemin said in his invited commentary (J. Thorac. Cardiovasc. Surg. 2015 [doi:10.1016/j.jtcvs.2015.04.038]). “The variety of approaches attests to the lack of institutional agreement on the surgical principles tested in the study,” he said. “The large variety of techniques in the control group makes the comparison and interpretation of this study difficult.”
“There are more questions to consider from this study than answers derived from the data about the clamp strategy,” he said
But, Dr. Shemin said, using the cross-clamp with axillary antegrade perfusion is “not a major issue.” And the use of clamping during the cooling period can save overall cardiac arrest time during the operation.
“If one does use femoral cannulation, then not applying the cross-clamp until achieving circulatory arrest is prudent,” he said. “With axillary cannulation, one achieves antegrade perfusion so early cross-clamping can be safely performed with the advantages of saving operative time.”
The clamp site must be inspected during circulatory arrest. Antegrade cerebral perfusion is proven to be an excellent technique and is facilitated by right axillary cannulation, Dr. Shemin said. “Most importantly, establishing antegrade CPB [cardiopulmonary bypass] perfusion after circulatory arrest is mandatory in all cases to minimize distal aorta trauma,” he said.
Dr. Richard J. Shemin is a cardiothoracic surgeon at UCLA Medical Center, Santa Monica, Calif.
Whether or not to use a cross-clamp in type A aortic dissection repair is a critical question, but a major concern of this study was the wide variability of techniques used in the comparison group, Dr. Richard J. Shemin said in his invited commentary (J. Thorac. Cardiovasc. Surg. 2015 [doi:10.1016/j.jtcvs.2015.04.038]). “The variety of approaches attests to the lack of institutional agreement on the surgical principles tested in the study,” he said. “The large variety of techniques in the control group makes the comparison and interpretation of this study difficult.”
“There are more questions to consider from this study than answers derived from the data about the clamp strategy,” he said
But, Dr. Shemin said, using the cross-clamp with axillary antegrade perfusion is “not a major issue.” And the use of clamping during the cooling period can save overall cardiac arrest time during the operation.
“If one does use femoral cannulation, then not applying the cross-clamp until achieving circulatory arrest is prudent,” he said. “With axillary cannulation, one achieves antegrade perfusion so early cross-clamping can be safely performed with the advantages of saving operative time.”
The clamp site must be inspected during circulatory arrest. Antegrade cerebral perfusion is proven to be an excellent technique and is facilitated by right axillary cannulation, Dr. Shemin said. “Most importantly, establishing antegrade CPB [cardiopulmonary bypass] perfusion after circulatory arrest is mandatory in all cases to minimize distal aorta trauma,” he said.
Dr. Richard J. Shemin is a cardiothoracic surgeon at UCLA Medical Center, Santa Monica, Calif.
Many techniques for repair of aortic dissection have evolved, but no trials have compared those techniques to determine which is the best. However, a study team has attempted to evaluate a surgical approach (the “David technique”) that includes three specific steps – no aortic cross clamp, the use of deep hypothermic circulatory arrest (DHCA), and the antegrade resumption of cardiopulmonary bypass. They found that this approach yielded significantly better long-term outcomes than did other approaches tried.
The study investigators, led by Dr. Jennifer S. Lawton of Washington University in St. Louis, reported their findings in the Journal of Thoracic and Cardiovascular Surgery (J. Thorac. Cardiovasc. Surg. 2015 [doi:10.1016/j.jtcvs.2015.03.023]). “We hypothesized that a surgical strategy to prevent cross-clamp injury or false lumen pressurization would be associated with reduced morbidity, mortality, persistent false lumen patency, and improved survival,” Dr. Lawton and her coauthors wrote. “This study was designed to determine the differences in outcomes between operative techniques.”
The study evaluated 196 patients who had surgery for acute type A aortic dissection over 17 years. Group 1, which comprised 49 patients, had the operation according to the protocol that involved the three specific steps, as Dr. Tirone David of the University of Toronto first reported in 1999 (Ann. Thorac. Surg. 1999;67:1999-2001) — the “David technique,” as the study authors called it. Group 2 consisted of patients whose repair involved a variety of techniques, including one or two steps of the David technique but not all three.
Study endpoints were 30-day mortality rate, postoperative adverse events, presence of a false aortic lumen, and overall survival, the latter defined as the time from the date of surgery to the date or death or last follow-up. The evaluation included examination of patients’ latest CT scan or MRI that was at least 6 months after the operation for false lumen, but only 78 patients had imaging at that interval.
Patients in Group 1 had a higher rate of persistent false lumen – 74% vs. 68% in Group 2. Thirty-day mortality was 6.1% in Group 1 and 15.7% in Group 2, but Dr. Lawton and her coauthors said this difference was not statistically significant.
Survival rates at 1, 5 and 10 years among both groups were “consistent with published ranges,” the authors said. At 5 years, the predicted survival was 86% for Group 1 and 56% for Group 2; and at 10 years, 72% and 37% respectively.
The study authors acknowledged the controversy that surrounds the use of retrograde resumption of cardiopulmonary bypass after replacement of the ascending aorta and that there’s no consensus on which method is best for resuming cardiopulmonary bypass after repair of a type A aortic dissection.
The study also found no difference in the incidence of false lumen between the two groups, but again, this is a source of controversy. “Persistence of a false lumen following repair for type A aortic dissection has been reported to be associated with poor prognosis and reduced long-term survival,” Dr. Lawton and her colleagues said.
“Others have reported a patent false lumen was not an independent predictor of late reoperation, but was a predictor of aortic growth following repair of type A aortic dissection.”
The study authors said one limit of their findings is its retrospective nature, but they also said that a prospective, randomized trial would be difficult to conduct.
None of the study coauthors had any relationships to disclose. They presented their original data at the American Association for Thoracic Surgery Aortic Symposium, April 24-25, 2014, in New York.
Many techniques for repair of aortic dissection have evolved, but no trials have compared those techniques to determine which is the best. However, a study team has attempted to evaluate a surgical approach (the “David technique”) that includes three specific steps – no aortic cross clamp, the use of deep hypothermic circulatory arrest (DHCA), and the antegrade resumption of cardiopulmonary bypass. They found that this approach yielded significantly better long-term outcomes than did other approaches tried.
The study investigators, led by Dr. Jennifer S. Lawton of Washington University in St. Louis, reported their findings in the Journal of Thoracic and Cardiovascular Surgery (J. Thorac. Cardiovasc. Surg. 2015 [doi:10.1016/j.jtcvs.2015.03.023]). “We hypothesized that a surgical strategy to prevent cross-clamp injury or false lumen pressurization would be associated with reduced morbidity, mortality, persistent false lumen patency, and improved survival,” Dr. Lawton and her coauthors wrote. “This study was designed to determine the differences in outcomes between operative techniques.”
The study evaluated 196 patients who had surgery for acute type A aortic dissection over 17 years. Group 1, which comprised 49 patients, had the operation according to the protocol that involved the three specific steps, as Dr. Tirone David of the University of Toronto first reported in 1999 (Ann. Thorac. Surg. 1999;67:1999-2001) — the “David technique,” as the study authors called it. Group 2 consisted of patients whose repair involved a variety of techniques, including one or two steps of the David technique but not all three.
Study endpoints were 30-day mortality rate, postoperative adverse events, presence of a false aortic lumen, and overall survival, the latter defined as the time from the date of surgery to the date or death or last follow-up. The evaluation included examination of patients’ latest CT scan or MRI that was at least 6 months after the operation for false lumen, but only 78 patients had imaging at that interval.
Patients in Group 1 had a higher rate of persistent false lumen – 74% vs. 68% in Group 2. Thirty-day mortality was 6.1% in Group 1 and 15.7% in Group 2, but Dr. Lawton and her coauthors said this difference was not statistically significant.
Survival rates at 1, 5 and 10 years among both groups were “consistent with published ranges,” the authors said. At 5 years, the predicted survival was 86% for Group 1 and 56% for Group 2; and at 10 years, 72% and 37% respectively.
The study authors acknowledged the controversy that surrounds the use of retrograde resumption of cardiopulmonary bypass after replacement of the ascending aorta and that there’s no consensus on which method is best for resuming cardiopulmonary bypass after repair of a type A aortic dissection.
The study also found no difference in the incidence of false lumen between the two groups, but again, this is a source of controversy. “Persistence of a false lumen following repair for type A aortic dissection has been reported to be associated with poor prognosis and reduced long-term survival,” Dr. Lawton and her colleagues said.
“Others have reported a patent false lumen was not an independent predictor of late reoperation, but was a predictor of aortic growth following repair of type A aortic dissection.”
The study authors said one limit of their findings is its retrospective nature, but they also said that a prospective, randomized trial would be difficult to conduct.
None of the study coauthors had any relationships to disclose. They presented their original data at the American Association for Thoracic Surgery Aortic Symposium, April 24-25, 2014, in New York.
Key clinical point: An operation to repair type A aortic dissection that involves three specific steps achieves better outcomes than do other surgical approaches.
Major finding: Survival rates at 5 years were 86% for the group that had operations in which the surgeons used the three specific steps vs. 56% for the other group.
Data source: Retrospective analysis of single-center population of 146 patients who had repairs for type A aortic dissection.
Disclosures: None of the study coauthors had any relationships to disclose.