User login
Diffuse Rash With Associated Ulceration
The Diagnosis: Epidermotropic CD8+ T-Cell Lymphoma
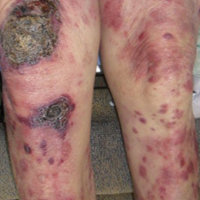
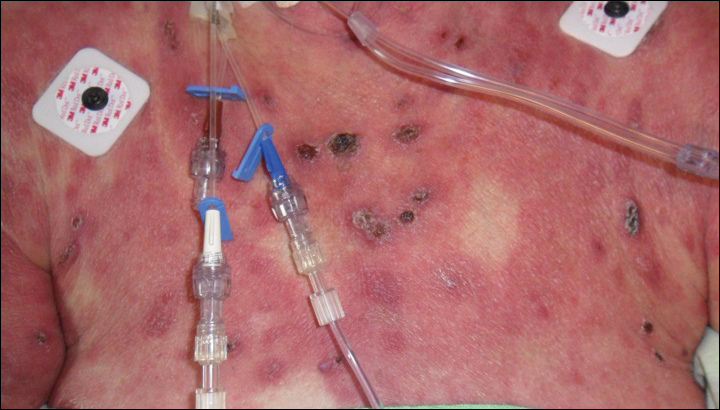
Epidermotropic CD8+ T-cell lymphoma is a rare aggressive form of cutaneous T-cell lymphoma (CTCL), accounting for less than 1% of all cases.1 Since this subtype of CTCL was first described in 1999 by Berti et al,2 approximately 45 cases have been reported in the literature.1 It typically is found in elderly men and presents as disseminated or localized papules, patches, plaques, nodules, and tumors, often with central necrosis, ulceration, crusting, and hemorrhage (Figure 1).1,3 These lesions rapidly progress and can affect any skin site, but acral accentuation and mucosal involvement are common.4 Due to the rapidly progressive nature of this disease, patients typically present with widespread plaque- and tumor-stage disease.3 Frequency of systemic spread is high, with metastasis to the central nervous system, lungs, and testes being most common. Lymph nodes typically are spared, helping to differentiate this form of CTCL from classic mycosis fungoides.
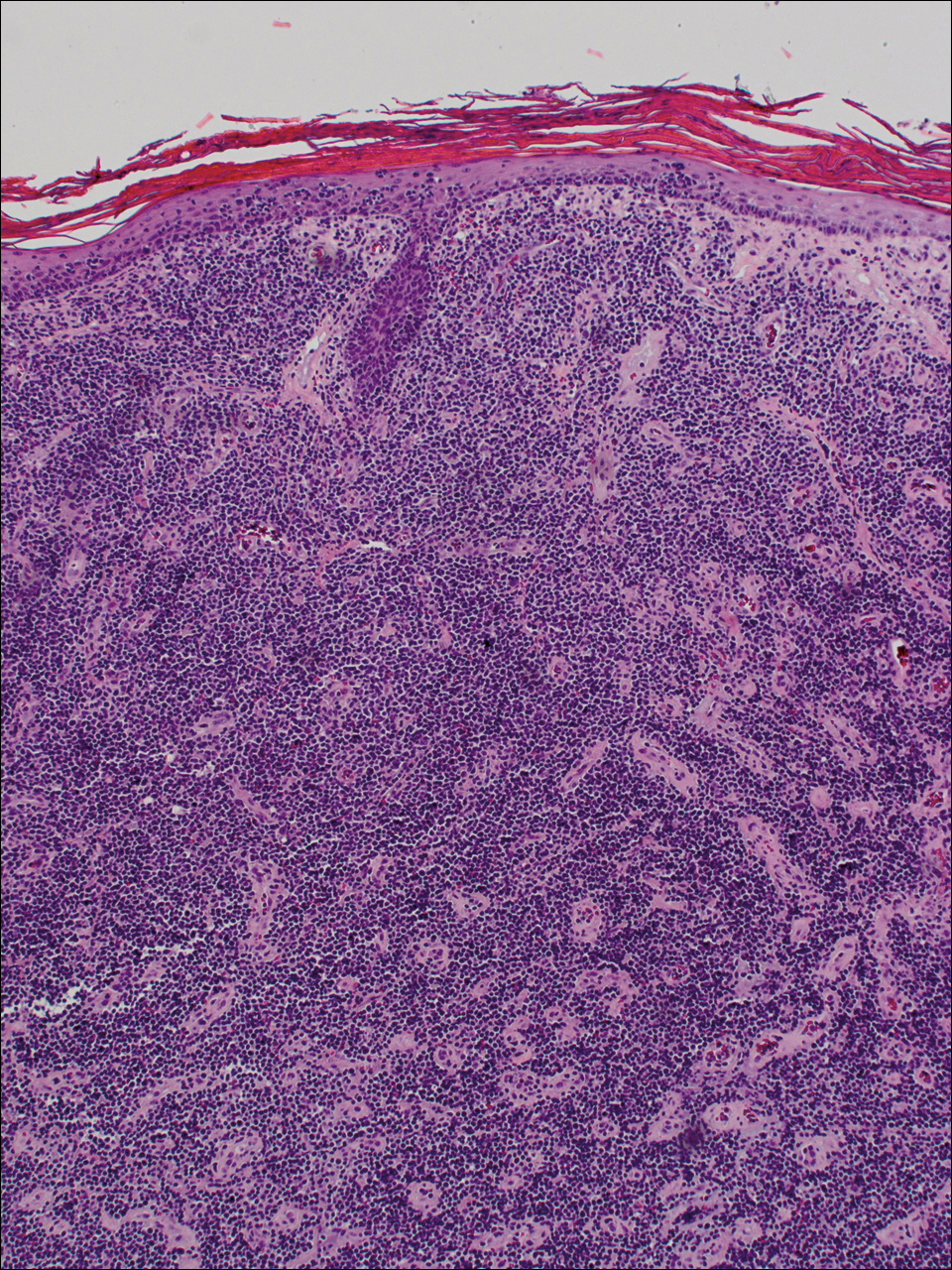
Diagnosis of epidermotropic CD8+ T-cell lymphoma is based on a combination of clinical, histopathologic, and immunohistochemical features. Histopathologic components include epidermotropism, particularly in the basal cell layer, in a pagetoid or linear pattern. A second feature is a dermal infiltrate consisting of a nodular or diffuse pattern of atypical lymphocytes that extend to the subcutaneous fat (Figure 2). All cases of epidermotropic CD8+ T-cell lymphoma express the CD8+ phenotype and most have a high Ki-67 proliferation index and are CD3, CD45RA, and/or T-cell intracellular antigen 1 positive.1
Due to its aggressive nature, epidermotropic CD8+ T-cell lymphoma has a poor prognosis, with an average 5-year survival rate of 18% and median survival of 22.5 months.3 Treatment proves difficult as conventional therapies for CD4+ CTCL have proven ineffective for epidermotropic CD8+ T-cell lymphoma. Partial response has been seen with bexarotene alone and with total skin electron beam therapy combined with oral retinoids.1
- Nofal A, Abdel-Mawla MY, Assaf M, et al. Primary cutaneous aggressive epidermotropic CD8+ T-cell lymphoma: proposed diagnostic criteria and therapeutic evaluation. J Am Acad Dermatol. 2012;67:748-759.
- Berti E, Tomasini D, Vermeer MH, et al. Primary cutaneous CD8-positive epidermotropic cytotoxic T cell lymphomas. a distinct clinicopathological entity with an aggressive clinical behavior. Am J Pathol. 1999;155:483-492.
- Gormley RH, Hess SD, Anand D, et al. Primary cutaneous aggressive epidermotropic CD8+ T-cell lymphoma. J Am Acad Dermatol. 2010;62:300-307.
- Nofal A, Abdel-Mawla MY, Assaf M, et al. Primary cutaneous aggressive epidermotropic CD8+ T cell lymphoma: a diagnostic and therapeutic challenge. Int J Dermatol. 2014;53:76-81.
The Diagnosis: Epidermotropic CD8+ T-Cell Lymphoma
Epidermotropic CD8+ T-cell lymphoma is a rare aggressive form of cutaneous T-cell lymphoma (CTCL), accounting for less than 1% of all cases.1 Since this subtype of CTCL was first described in 1999 by Berti et al,2 approximately 45 cases have been reported in the literature.1 It typically is found in elderly men and presents as disseminated or localized papules, patches, plaques, nodules, and tumors, often with central necrosis, ulceration, crusting, and hemorrhage (Figure 1).1,3 These lesions rapidly progress and can affect any skin site, but acral accentuation and mucosal involvement are common.4 Due to the rapidly progressive nature of this disease, patients typically present with widespread plaque- and tumor-stage disease.3 Frequency of systemic spread is high, with metastasis to the central nervous system, lungs, and testes being most common. Lymph nodes typically are spared, helping to differentiate this form of CTCL from classic mycosis fungoides.

Diagnosis of epidermotropic CD8+ T-cell lymphoma is based on a combination of clinical, histopathologic, and immunohistochemical features. Histopathologic components include epidermotropism, particularly in the basal cell layer, in a pagetoid or linear pattern. A second feature is a dermal infiltrate consisting of a nodular or diffuse pattern of atypical lymphocytes that extend to the subcutaneous fat (Figure 2). All cases of epidermotropic CD8+ T-cell lymphoma express the CD8+ phenotype and most have a high Ki-67 proliferation index and are CD3, CD45RA, and/or T-cell intracellular antigen 1 positive.1

Due to its aggressive nature, epidermotropic CD8+ T-cell lymphoma has a poor prognosis, with an average 5-year survival rate of 18% and median survival of 22.5 months.3 Treatment proves difficult as conventional therapies for CD4+ CTCL have proven ineffective for epidermotropic CD8+ T-cell lymphoma. Partial response has been seen with bexarotene alone and with total skin electron beam therapy combined with oral retinoids.1
The Diagnosis: Epidermotropic CD8+ T-Cell Lymphoma
Epidermotropic CD8+ T-cell lymphoma is a rare aggressive form of cutaneous T-cell lymphoma (CTCL), accounting for less than 1% of all cases.1 Since this subtype of CTCL was first described in 1999 by Berti et al,2 approximately 45 cases have been reported in the literature.1 It typically is found in elderly men and presents as disseminated or localized papules, patches, plaques, nodules, and tumors, often with central necrosis, ulceration, crusting, and hemorrhage (Figure 1).1,3 These lesions rapidly progress and can affect any skin site, but acral accentuation and mucosal involvement are common.4 Due to the rapidly progressive nature of this disease, patients typically present with widespread plaque- and tumor-stage disease.3 Frequency of systemic spread is high, with metastasis to the central nervous system, lungs, and testes being most common. Lymph nodes typically are spared, helping to differentiate this form of CTCL from classic mycosis fungoides.

Diagnosis of epidermotropic CD8+ T-cell lymphoma is based on a combination of clinical, histopathologic, and immunohistochemical features. Histopathologic components include epidermotropism, particularly in the basal cell layer, in a pagetoid or linear pattern. A second feature is a dermal infiltrate consisting of a nodular or diffuse pattern of atypical lymphocytes that extend to the subcutaneous fat (Figure 2). All cases of epidermotropic CD8+ T-cell lymphoma express the CD8+ phenotype and most have a high Ki-67 proliferation index and are CD3, CD45RA, and/or T-cell intracellular antigen 1 positive.1

Due to its aggressive nature, epidermotropic CD8+ T-cell lymphoma has a poor prognosis, with an average 5-year survival rate of 18% and median survival of 22.5 months.3 Treatment proves difficult as conventional therapies for CD4+ CTCL have proven ineffective for epidermotropic CD8+ T-cell lymphoma. Partial response has been seen with bexarotene alone and with total skin electron beam therapy combined with oral retinoids.1
- Nofal A, Abdel-Mawla MY, Assaf M, et al. Primary cutaneous aggressive epidermotropic CD8+ T-cell lymphoma: proposed diagnostic criteria and therapeutic evaluation. J Am Acad Dermatol. 2012;67:748-759.
- Berti E, Tomasini D, Vermeer MH, et al. Primary cutaneous CD8-positive epidermotropic cytotoxic T cell lymphomas. a distinct clinicopathological entity with an aggressive clinical behavior. Am J Pathol. 1999;155:483-492.
- Gormley RH, Hess SD, Anand D, et al. Primary cutaneous aggressive epidermotropic CD8+ T-cell lymphoma. J Am Acad Dermatol. 2010;62:300-307.
- Nofal A, Abdel-Mawla MY, Assaf M, et al. Primary cutaneous aggressive epidermotropic CD8+ T cell lymphoma: a diagnostic and therapeutic challenge. Int J Dermatol. 2014;53:76-81.
- Nofal A, Abdel-Mawla MY, Assaf M, et al. Primary cutaneous aggressive epidermotropic CD8+ T-cell lymphoma: proposed diagnostic criteria and therapeutic evaluation. J Am Acad Dermatol. 2012;67:748-759.
- Berti E, Tomasini D, Vermeer MH, et al. Primary cutaneous CD8-positive epidermotropic cytotoxic T cell lymphomas. a distinct clinicopathological entity with an aggressive clinical behavior. Am J Pathol. 1999;155:483-492.
- Gormley RH, Hess SD, Anand D, et al. Primary cutaneous aggressive epidermotropic CD8+ T-cell lymphoma. J Am Acad Dermatol. 2010;62:300-307.
- Nofal A, Abdel-Mawla MY, Assaf M, et al. Primary cutaneous aggressive epidermotropic CD8+ T cell lymphoma: a diagnostic and therapeutic challenge. Int J Dermatol. 2014;53:76-81.
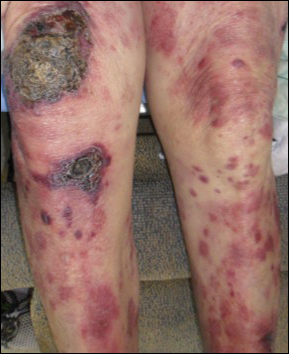
A 72-year-old woman who was admitted for pneumonia and acute hypoxic respiratory failure was seen for an inpatient consultation for a diffuse rash with associated ulceration. She reported a rash of 20 months' duration that began on the legs and then spread to the trunk, arms, head, and neck with minimal pruritus and no pain or photosensitivity. She had been treated with hydroxychloroquine, mycophenolate mofetil, and prednisone without improvement. The patient noted recent ulceration on the rash. Physical examination revealed violaceous patches, plaques, nodules, and tumors with rare ulceration involving the face, trunk, and extremities. Biopsy showed a diffuse infiltration of the dermis with medium-sized atypical lymphocytes with scant cytoplasm and round to irregular hyperchromatic nuclei with clumped chromatin. Epidermotropism with small collections of atypical lymphocytes also was present within the epidermis.
Autoimmune Progesterone Dermatitis Presenting With Purpura
To the Editor:
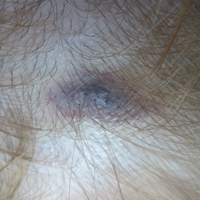
A 32-year-old woman presented with a recurrent painful eruption on the scalp of 1 year's duration. The lesion occurred on the left temporal region 1 week prior to menstruation and spontaneously resolved following menses; it recurred every month for 1 year. She had no notable medical history. She had taken oral contraceptive pills for 4 years and stopped 2 years prior to the development of the lesions. Dermatologic examination revealed a purple-colored, violaceous, centrally elevated, painful plaque that measured 2 cm in diameter in the left temporal region of the scalp (Figure, A). Laboratory test results were within reference range. The lesion spontaneously resolved with mild residual erythema at a follow-up visit after menstruation (Figure, B).
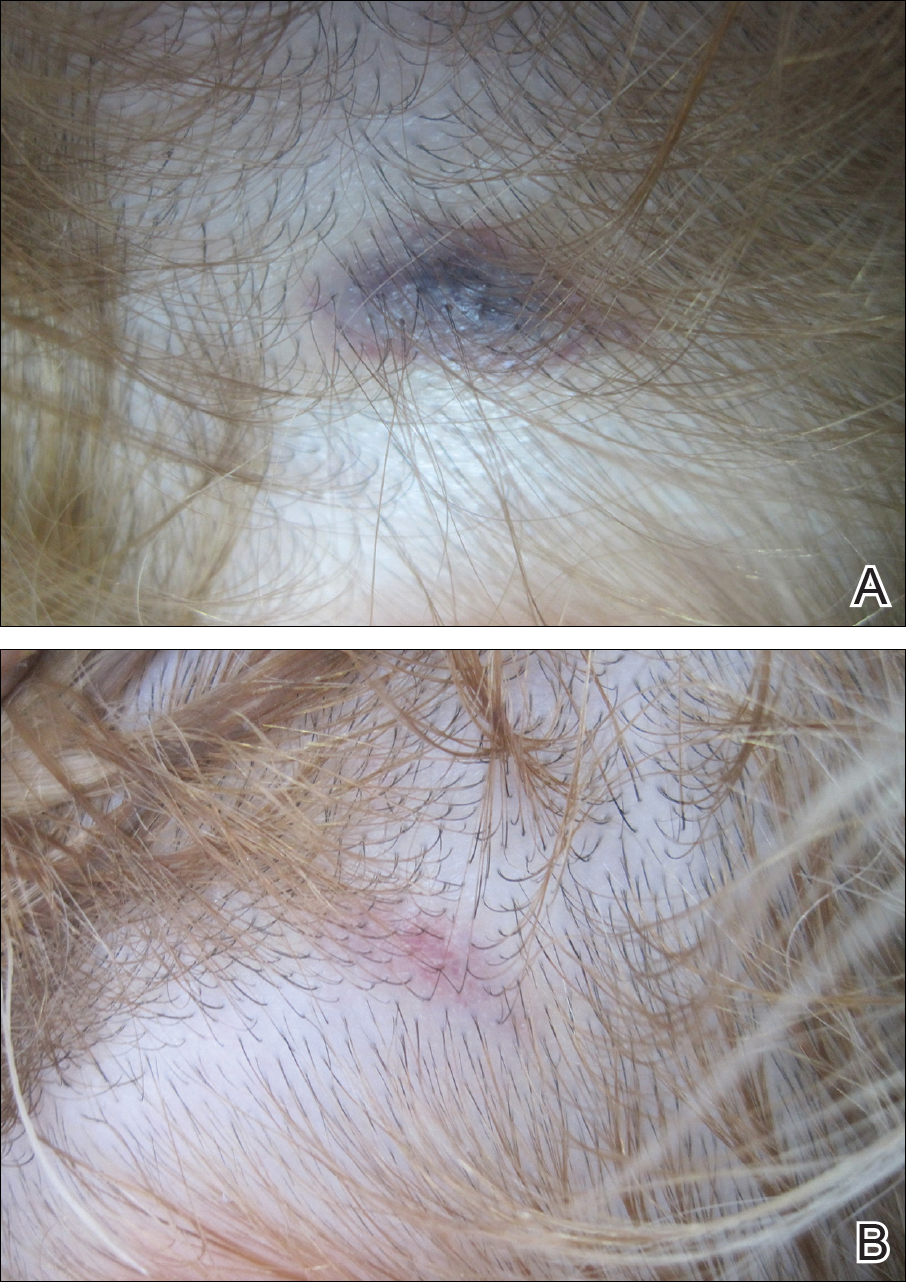
Because the eruption occurred and relapsed with the patient's menstrual cycle, we suspected progesterone hypersensitivity. An intradermal skin test was performed on the forearm with 0.05 mL of medroxyprogesterone acetate, and saline was used as a negative control. An indurated erythematous nodule occurred on the progesterone-treated side within 6 hours. Based on these findings and the patient's history, she was diagnosed with autoimmune progesterone dermatitis (APD). We recommended her to use gonadotropin-releasing hormone agonists as treatment, but the patient refused. At 6-month follow-up she had recurrent lesions but did not report any concerns.
Autoimmune progesterone dermatitis is a rare condition that is characterized by cyclical skin eruptions, typically occurring in the luteal phase of the menstrual cycle with spontaneous resolution after menses.1,2 It was first described by Geber3 in a patient with cyclical urticarial lesions. In 1964, Shelley et al4 characterized APD in a 27-year-old woman with a pruritic vesicular eruption with cyclical premenstrual exacerbations. Although it is believed there is no genetic predisposition to APD, a case series involving 3 sisters demonstrated that genetic susceptibility might play a role in the etiology.5 The etiology of APD is still unknown. It is thought to represent an autoimmune reaction to endogenous or exogenous progesterone.1 Our patient also had used oral contraceptives for 4 years and this exogenous progesterone might have played a role in the sensitization of the patient and the development of this autoimmune reaction.
The clinical features of APD usually begin 3 to 10 days prior to menstruation and end 1 to 2 days after menses. Autoimmune progesterone dermatitis can present in a variety of forms including eczema, erythema multiforme, erythema annulare centrifugum, fixed drug eruption, stomatitis, folliculitis, urticaria, and angioedema.6 A case of APD presenting with petechiae and purpura has been reported.7 There are no specific histologic findings for APD.8 Demonstration of progesterone sensitivity with a progesterone challenge test is the mainstay of diagnosis. Immediate urticaria may occur in some patients, with others experiencing a delayed reaction peaking at 24 to 96 hours.9 The main criteria of APD include the following: recurrent cyclic lesions related to the menstrual cycle; positive intradermal progesterone skin test; and prevention of lesions by inhibiting ovulation.1 Two of these criteria were positive in our patient, but we did not use any medications to prevent ovulation at the patient's request.
Current treatment modalities often attempt to inhibit the secretion of endogenous progesterone by suppressing ovulation. Oral contraceptives and conjugated estrogens have limited efficacy rates.8 Gonadotropin-releasing hormone agonists (ie, buserelin, triptorelin) have been used with success.1,6 Tamoxifen and danazol are other treatment options. For cases refractory to medical treatments, bilateral oophorectomy can be considered a definitive treatment.6
Autoimmune progesterone dermatitis may present in many different clinical forms. It should be considered in the differential diagnosis in patients with recurrent skin lesions related to menstrual cycle both in women of childbearing age and in men taking synthetic progesterone.
- Lee MK, Lee WY, Yong SJ, et al. A case of autoimmune progesterone dermatitis misdiagnosed as allergic contact dermatitis. Allergy Asthma Immunol Res. 2011;3:141-144.
- García-Ortega P, Scorza E. Progesterone autoimmune dermatitis with positive autologous serum skin test result. Obstet Gynecol. 2011;117:495-498.
- Geber J. Desensitization in the treatment of menstrual intoxication and other allergic symptoms. Br J Dermatol. 1930;51:265-268.
- Shelley WB, Preucel RW, Spoont SS. Autoimmune progesterone dermatitis: cure by oophorectomy. JAMA. 1964;190:35-38.
- Chawla SV, Quirk C, Sondheimer SJ, et al. Autoimmune progesterone dermatitis. Arch Dermatol. 2009;145:341-342.
- Medeiros S, Rodrigues-Alves R, Costa M, et al. Autoimmune progesterone dermatitis: treatment with oophorectomy. Clin Exp Dermatol. 2010;35:e12-e13.
- Wintzen M, Goor-van Egmond MB, Noz KC. Autoimmune progesterone dermatitis presenting with purpura and petechiae. Clin Exp Dermatol. 2004;29:316.
- Baptist AP, Baldwin JL. Autoimmune progesterone dermatitis in a patient with endometriosis: case report and review of the literature. Clin Mol Allergy. 2004;2:10.
- Le K, Wood G. A case of autoimmune progesterone dermatitis diagnosed by progesterone pessary. Australas J Dermatol. 2011;52:139-141.
To the Editor:
A 32-year-old woman presented with a recurrent painful eruption on the scalp of 1 year's duration. The lesion occurred on the left temporal region 1 week prior to menstruation and spontaneously resolved following menses; it recurred every month for 1 year. She had no notable medical history. She had taken oral contraceptive pills for 4 years and stopped 2 years prior to the development of the lesions. Dermatologic examination revealed a purple-colored, violaceous, centrally elevated, painful plaque that measured 2 cm in diameter in the left temporal region of the scalp (Figure, A). Laboratory test results were within reference range. The lesion spontaneously resolved with mild residual erythema at a follow-up visit after menstruation (Figure, B).

Because the eruption occurred and relapsed with the patient's menstrual cycle, we suspected progesterone hypersensitivity. An intradermal skin test was performed on the forearm with 0.05 mL of medroxyprogesterone acetate, and saline was used as a negative control. An indurated erythematous nodule occurred on the progesterone-treated side within 6 hours. Based on these findings and the patient's history, she was diagnosed with autoimmune progesterone dermatitis (APD). We recommended her to use gonadotropin-releasing hormone agonists as treatment, but the patient refused. At 6-month follow-up she had recurrent lesions but did not report any concerns.
Autoimmune progesterone dermatitis is a rare condition that is characterized by cyclical skin eruptions, typically occurring in the luteal phase of the menstrual cycle with spontaneous resolution after menses.1,2 It was first described by Geber3 in a patient with cyclical urticarial lesions. In 1964, Shelley et al4 characterized APD in a 27-year-old woman with a pruritic vesicular eruption with cyclical premenstrual exacerbations. Although it is believed there is no genetic predisposition to APD, a case series involving 3 sisters demonstrated that genetic susceptibility might play a role in the etiology.5 The etiology of APD is still unknown. It is thought to represent an autoimmune reaction to endogenous or exogenous progesterone.1 Our patient also had used oral contraceptives for 4 years and this exogenous progesterone might have played a role in the sensitization of the patient and the development of this autoimmune reaction.
The clinical features of APD usually begin 3 to 10 days prior to menstruation and end 1 to 2 days after menses. Autoimmune progesterone dermatitis can present in a variety of forms including eczema, erythema multiforme, erythema annulare centrifugum, fixed drug eruption, stomatitis, folliculitis, urticaria, and angioedema.6 A case of APD presenting with petechiae and purpura has been reported.7 There are no specific histologic findings for APD.8 Demonstration of progesterone sensitivity with a progesterone challenge test is the mainstay of diagnosis. Immediate urticaria may occur in some patients, with others experiencing a delayed reaction peaking at 24 to 96 hours.9 The main criteria of APD include the following: recurrent cyclic lesions related to the menstrual cycle; positive intradermal progesterone skin test; and prevention of lesions by inhibiting ovulation.1 Two of these criteria were positive in our patient, but we did not use any medications to prevent ovulation at the patient's request.
Current treatment modalities often attempt to inhibit the secretion of endogenous progesterone by suppressing ovulation. Oral contraceptives and conjugated estrogens have limited efficacy rates.8 Gonadotropin-releasing hormone agonists (ie, buserelin, triptorelin) have been used with success.1,6 Tamoxifen and danazol are other treatment options. For cases refractory to medical treatments, bilateral oophorectomy can be considered a definitive treatment.6
Autoimmune progesterone dermatitis may present in many different clinical forms. It should be considered in the differential diagnosis in patients with recurrent skin lesions related to menstrual cycle both in women of childbearing age and in men taking synthetic progesterone.
To the Editor:
A 32-year-old woman presented with a recurrent painful eruption on the scalp of 1 year's duration. The lesion occurred on the left temporal region 1 week prior to menstruation and spontaneously resolved following menses; it recurred every month for 1 year. She had no notable medical history. She had taken oral contraceptive pills for 4 years and stopped 2 years prior to the development of the lesions. Dermatologic examination revealed a purple-colored, violaceous, centrally elevated, painful plaque that measured 2 cm in diameter in the left temporal region of the scalp (Figure, A). Laboratory test results were within reference range. The lesion spontaneously resolved with mild residual erythema at a follow-up visit after menstruation (Figure, B).

Because the eruption occurred and relapsed with the patient's menstrual cycle, we suspected progesterone hypersensitivity. An intradermal skin test was performed on the forearm with 0.05 mL of medroxyprogesterone acetate, and saline was used as a negative control. An indurated erythematous nodule occurred on the progesterone-treated side within 6 hours. Based on these findings and the patient's history, she was diagnosed with autoimmune progesterone dermatitis (APD). We recommended her to use gonadotropin-releasing hormone agonists as treatment, but the patient refused. At 6-month follow-up she had recurrent lesions but did not report any concerns.
Autoimmune progesterone dermatitis is a rare condition that is characterized by cyclical skin eruptions, typically occurring in the luteal phase of the menstrual cycle with spontaneous resolution after menses.1,2 It was first described by Geber3 in a patient with cyclical urticarial lesions. In 1964, Shelley et al4 characterized APD in a 27-year-old woman with a pruritic vesicular eruption with cyclical premenstrual exacerbations. Although it is believed there is no genetic predisposition to APD, a case series involving 3 sisters demonstrated that genetic susceptibility might play a role in the etiology.5 The etiology of APD is still unknown. It is thought to represent an autoimmune reaction to endogenous or exogenous progesterone.1 Our patient also had used oral contraceptives for 4 years and this exogenous progesterone might have played a role in the sensitization of the patient and the development of this autoimmune reaction.
The clinical features of APD usually begin 3 to 10 days prior to menstruation and end 1 to 2 days after menses. Autoimmune progesterone dermatitis can present in a variety of forms including eczema, erythema multiforme, erythema annulare centrifugum, fixed drug eruption, stomatitis, folliculitis, urticaria, and angioedema.6 A case of APD presenting with petechiae and purpura has been reported.7 There are no specific histologic findings for APD.8 Demonstration of progesterone sensitivity with a progesterone challenge test is the mainstay of diagnosis. Immediate urticaria may occur in some patients, with others experiencing a delayed reaction peaking at 24 to 96 hours.9 The main criteria of APD include the following: recurrent cyclic lesions related to the menstrual cycle; positive intradermal progesterone skin test; and prevention of lesions by inhibiting ovulation.1 Two of these criteria were positive in our patient, but we did not use any medications to prevent ovulation at the patient's request.
Current treatment modalities often attempt to inhibit the secretion of endogenous progesterone by suppressing ovulation. Oral contraceptives and conjugated estrogens have limited efficacy rates.8 Gonadotropin-releasing hormone agonists (ie, buserelin, triptorelin) have been used with success.1,6 Tamoxifen and danazol are other treatment options. For cases refractory to medical treatments, bilateral oophorectomy can be considered a definitive treatment.6
Autoimmune progesterone dermatitis may present in many different clinical forms. It should be considered in the differential diagnosis in patients with recurrent skin lesions related to menstrual cycle both in women of childbearing age and in men taking synthetic progesterone.
- Lee MK, Lee WY, Yong SJ, et al. A case of autoimmune progesterone dermatitis misdiagnosed as allergic contact dermatitis. Allergy Asthma Immunol Res. 2011;3:141-144.
- García-Ortega P, Scorza E. Progesterone autoimmune dermatitis with positive autologous serum skin test result. Obstet Gynecol. 2011;117:495-498.
- Geber J. Desensitization in the treatment of menstrual intoxication and other allergic symptoms. Br J Dermatol. 1930;51:265-268.
- Shelley WB, Preucel RW, Spoont SS. Autoimmune progesterone dermatitis: cure by oophorectomy. JAMA. 1964;190:35-38.
- Chawla SV, Quirk C, Sondheimer SJ, et al. Autoimmune progesterone dermatitis. Arch Dermatol. 2009;145:341-342.
- Medeiros S, Rodrigues-Alves R, Costa M, et al. Autoimmune progesterone dermatitis: treatment with oophorectomy. Clin Exp Dermatol. 2010;35:e12-e13.
- Wintzen M, Goor-van Egmond MB, Noz KC. Autoimmune progesterone dermatitis presenting with purpura and petechiae. Clin Exp Dermatol. 2004;29:316.
- Baptist AP, Baldwin JL. Autoimmune progesterone dermatitis in a patient with endometriosis: case report and review of the literature. Clin Mol Allergy. 2004;2:10.
- Le K, Wood G. A case of autoimmune progesterone dermatitis diagnosed by progesterone pessary. Australas J Dermatol. 2011;52:139-141.
- Lee MK, Lee WY, Yong SJ, et al. A case of autoimmune progesterone dermatitis misdiagnosed as allergic contact dermatitis. Allergy Asthma Immunol Res. 2011;3:141-144.
- García-Ortega P, Scorza E. Progesterone autoimmune dermatitis with positive autologous serum skin test result. Obstet Gynecol. 2011;117:495-498.
- Geber J. Desensitization in the treatment of menstrual intoxication and other allergic symptoms. Br J Dermatol. 1930;51:265-268.
- Shelley WB, Preucel RW, Spoont SS. Autoimmune progesterone dermatitis: cure by oophorectomy. JAMA. 1964;190:35-38.
- Chawla SV, Quirk C, Sondheimer SJ, et al. Autoimmune progesterone dermatitis. Arch Dermatol. 2009;145:341-342.
- Medeiros S, Rodrigues-Alves R, Costa M, et al. Autoimmune progesterone dermatitis: treatment with oophorectomy. Clin Exp Dermatol. 2010;35:e12-e13.
- Wintzen M, Goor-van Egmond MB, Noz KC. Autoimmune progesterone dermatitis presenting with purpura and petechiae. Clin Exp Dermatol. 2004;29:316.
- Baptist AP, Baldwin JL. Autoimmune progesterone dermatitis in a patient with endometriosis: case report and review of the literature. Clin Mol Allergy. 2004;2:10.
- Le K, Wood G. A case of autoimmune progesterone dermatitis diagnosed by progesterone pessary. Australas J Dermatol. 2011;52:139-141.
Practice Points
- Autoimmune progesterone dermatitis is characterized by cyclical skin eruptions, typically occurring in the second half of the menstrual cycle.
- Autoimmune progesterone dermatitis is thought to be an autoimmune reaction to endogenous or exogenous progesterone.
- This condition should be considered in female patients with recurrent skin lesions related to their menstrual cycle.
Acute Localized Exanthematous Pustulosis Caused by Flurbiprofen
To the Editor:
Acute generalized exanthematous pustulosis (AGEP) is an acute skin reaction that is characterized by generalized, nonfollicular, pinhead-sized, sterile pustules on an erythematous and edematous background. The eruption can be accompanied by fever and neutrophilic leukocytosis. Skin symptoms arise quickly (within a few hours), most commonly following drug administration. The medications most frequently responsible are beta-lactam antibiotics, macrolides, calcium channel blockers, and antimalarials. Pustules spontaneously resolve in 15 days and generalized desquamation occurs approximately 2 weeks later. The estimated incidence rate of AGEP is approximately 1 to 5 cases per million per year. Acute localized exanthematous pustulosis (ALEP) is a less common form of AGEP. We report a case of ALEP localized on the face that was caused by flurbiprofen, a propionic acid derivative from the family of nonsteroidal anti-inflammatory drugs (NSAIDs).
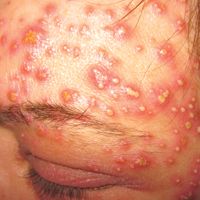
A 40-year-old woman was referred to the dermatology department due to the sudden onset of multiple pustules on the face. One week earlier she started oral flurbiprofen (8.75 mg daily) for a sore throat. After 3 days of therapy, multiple pruritic, erythematous and edematous lesions appeared abruptly on the face with associated multiple small nonfollicular pustules. At presentation the patient was febrile (temperature, 38.2°C) and presented with bilateral ocular edema and superficial small nonfollicular pustules on an erythematous background over the face, scalp, and oral mucosa (Figure 1). The rest of the body was not involved. The patient denied prior adverse reactions to other drugs. The white blood cell count was 15,000/μL (reference range, 4500–11,000/μL), with an increased neutrophil count (12,000/μL [reference range, 1800–7800/μL]). The erythrocyte sedimentation rate and C-reactive protein level was elevated (erythrocyte sedimentation rate, 53 mm/h [reference range, 0–20 mm/h]; C-reactive protein, 98 mg/dL [reference range, 0–5 mg/dL]). Bacterial and fungal cultures of skin lesions were negative. The results of a viral polymerase chain reaction analysis proved the absence of varicella-zoster virus or herpes simplex virus. Histopathology of a skin biopsy specimen showed subcorneal pustules composed of neutrophils and eosinophils, epidermal spongiosis, some necrotic keratinocytes, vacuolization of the basal layer, papillary edema, and a perivascular neutrophil and lymphocyte infiltrate (Figure 2). A leukocytoclastic infiltrate within and around the walls of blood vessels at the superficial level of the dermis and red cell extravasation in the epidermis was present. She discontinued use of flurbiprofen and was treated with a systemic corticosteroid (methylprednisolone 0.5 mg/kg daily). The pustules rapidly resolved within 7 days after discontinuation of flurbiprofen and were followed by transient scaling and discrete residual hyperpigmentation.
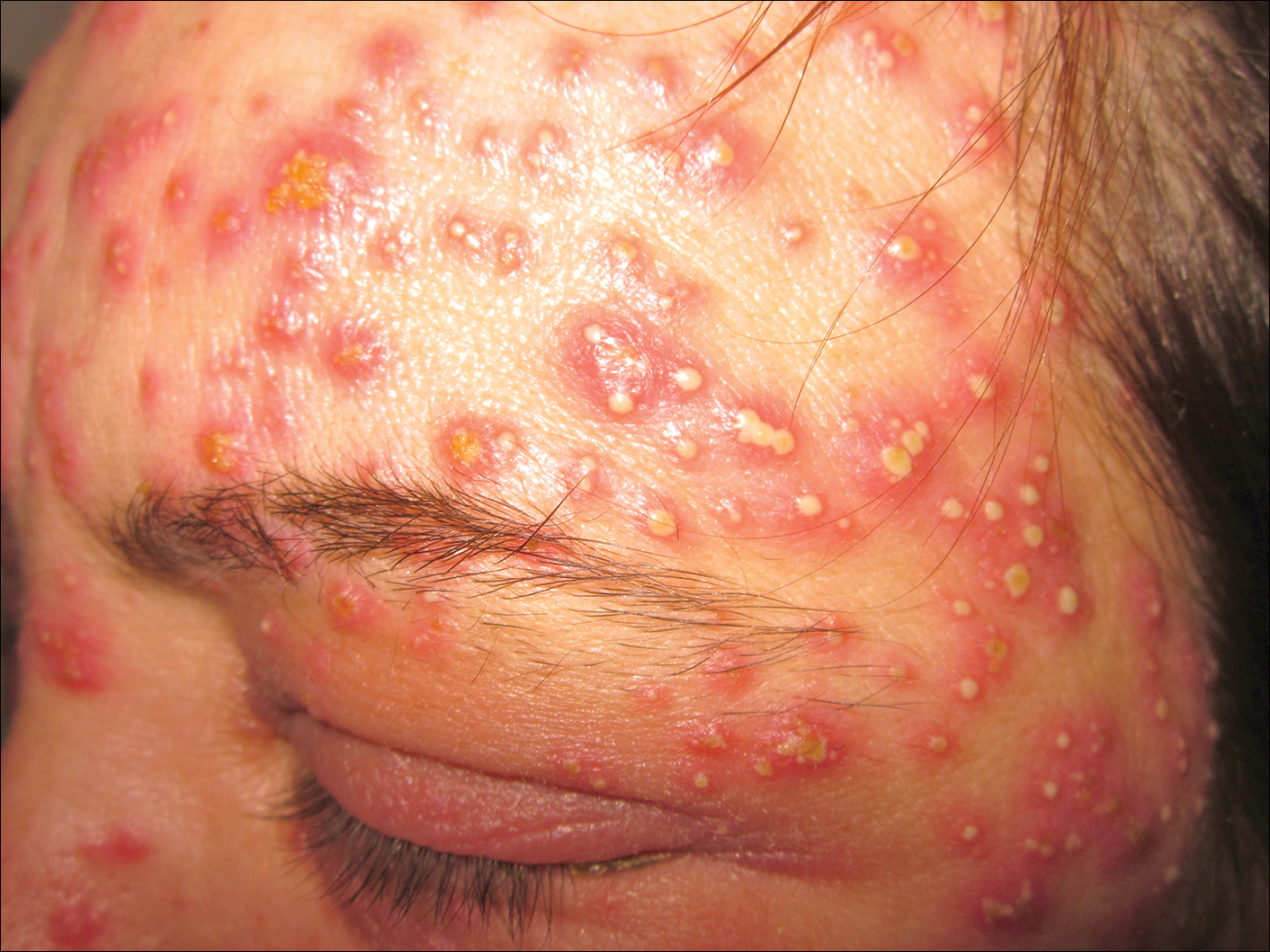
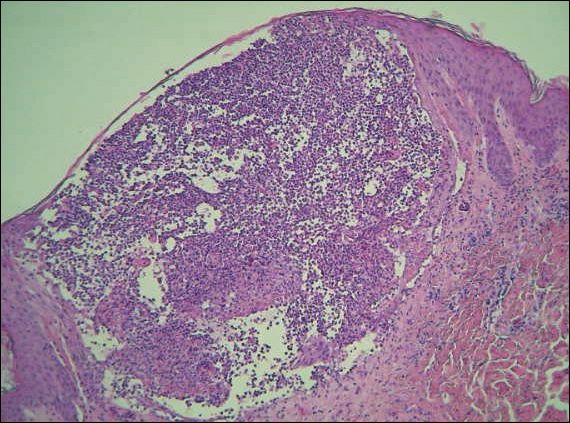
Acute localized exanthematous pustulosis is a less common form of a pustular drug eruption in which lesions are consistent with AGEP but typically are localized to the face, neck, or chest. The definition of ALEP was introduced by Prange et al1 to describe a woman who was diagnosed with a localized pustular eruption on the face without a generalized distribution as in AGEP. In the past, this localized eruption was described under different names (eg, localized pustular eruption, localized toxin follicular pustuloderma, nongeneralized acute exanthematic pustulosis).2-5 According to a PubMed search of articles indexed for MEDLINE using the terms localized pustulosis, localized pustular eruption, and localized pustuloderma, only 16 separate cases of ALEP have been documented since the report by Prange et al.1 The medications most frequently responsible are antibiotics. Three cases developed following administration of amoxicillin2,5,6; 2 cases of amoxicillin–clavulanic acid7,8; 1 of penicillin1; 1 of azithromycin9; 1 of levofloxacin10; and 1 of combination of cephalosporin, sulfamethoxazole-trimethoprim, and vancomycin.11 Other nonantibiotic causative drugs include sulfamethoxazole-trimethoprim,12 infliximab,13 sorafenib,14 docetaxel,15 finasteride,16 ibuprofen,17 and paracetamol.18 In reported cases, the lesions are consistent with the characteristics of AGEP both clinically and histopathologically but are localized typically to the face, neck, or chest. In the majority of patients with ALEP, the absence of fever has been observed, but it does not appear distinctive for diagnosis. Our patient represents another case of ALEP with flurbiprofen as the causative drug. The close relationship between the administration of the drug and the development of the pustules, the rapid acute resolution as soon as treatment was interrupted, and the histologic findings all supported the diagnosis of ALEP following administration of flurbiprofen. This NSAID—2-fluoro-α-methyl-(1,1'-biphenyl)-4-acetic acid—is a prostaglandin synthetase inhibitor with anti-inflammatory activity. It is a propionic acid derivative that is similar to ibuprofen, which was once involved in the occurrence of ALEP.17 In 2009, Rastogi et al17 reported a case of a 64-year-old woman with an acute outbreak of multiple pustular lesions and underlying erythema affecting the cheeks and chin without fever who had been taking ibuprofen for a toothache. The case is similar to ours and confirms that NSAIDs can induce ALEP. Compared with other NSAIDs, propionic acid derivatives are usually well tolerated and serious adverse reactions rarely have been documented.19
The physiopathologic mechanisms of ALEP are unknown but likely are similar to AGEP. The demonstration of drug-specific positive patch test responses and in vitro lymphocyte proliferative responses in patients with a history of AGEP strongly suggests that this adverse cutaneous reaction occurs via a drug-specific T cell–mediated process.20
Further study is needed to understand the etiopathogenesis of the localized form of the disease and to facilitate a correct diagnosis of this rare disorder.
- Prange B, Marini A, Kalke A, et al. Acute localized exanthematous pustulosis (ALEP). J Dtsch Dermatol Ges. 2005;3:210-212.
- Shuttleworth D. A localized, recurrent pustular eruption following amoxycillin administration. Clin Exp Dermatol. 1989;14:367-368.
- De Argila D, Ortiz-Frutos J, Rodriguez-Peralto JL, et al. An atypical case of non-generalized acute exanthematic pustulosis. Actas Dermosifiliogr. 1996;87:475-478.
- Corbalan-Velez R, Peon G, Ara M, et al. Localized toxic follicular pustuloderma. Int J Dermatol. 2000;39:209-211.
- Prieto A, de Barrio M, López-Sáez P, et al. Recurrent localized pustular eruption induced by amoxicillin. Allergy. 1997;52:777-778.
- Vickers JL, Matherne RJ, Mainous EG, et al. Acute localized exanthematous pustulosis: a cutaneous drug reaction in a dental setting. J Am Dent Assoc. 2008;139:1200-1203.
- Betto P, Germi L, Bonoldi E, et al. Acute localized exanthematous pustulosis (ALEP) caused by amoxicillin-clavulanic acid. Int J Dermatol. 2008;47:295-296.
- Ozkaya-Parlakay A, Azkur D, Kara A, et al. Localized acute generalized exanthematous pustulosis with amoxicillin and clavulanic acid. Turk J Pediatr. 2011;53:229-232.
- Zweegers J, Bovenschen HJ. A woman with skin abnormalities around the mouth [in Dutch]. Ned Tijdschr Geneeskd. 2012;156:A4613.
- Corral de la Calle M, Martín Díaz MA, Flores CR, et al. Acute localized exanthematous pustulosis secondary to levofloxacin. Br J Dermatol. 2005;152:1076-1077.
- Sim HS, Seol JE, Chun JS, et al. Acute localized exanthematous pustulosis on the face. Ann Dermatol. 2011;23(suppl 3):S3368-S3370.
- Lee I, Turner M, Lee CC. Acute patchy exanthematous pustulosis caused by sulfamethoxazole-trimethoprim. J Am Acad Dermatol. 2010;63:e41-e43.
- Lee HY, Pelivani N, Beltraminelli H, et al. Amicrobial pustulosis-like rash in a patient with Crohn’s disease under anti-TNF-alpha blocker. Dermatology. 2011;222:304-310.
- Liang CP, Yang CS, Shen JL, et al. Sorafenib-induced acute localized exanthematous pustulosis in a patient with hepatocellular carcinoma. Br J Dermatol. 2011;165:443-445.
- Kim SW, Lee UH, Jang SJ, et al. Acute localized exanthematous pustulosis induced by docetaxel. J Am Acad Dermatol. 2010;63:e44-e46.
- Tresch S, Cozzio A, Kamarashev J, et al. T cell-mediated acute localized exanthematous pustulosis caused by finasteride. J Allergy Clin Immunol. 2012;129:589-594.
- Rastogi S, Modi M, Dhawan V. Acute localized exanthematous pustulosis (ALEP) caused by Ibuprofen. a case report. Br J Oral Maxillofac Surg. 2009;47:132-134.
- Wohl Y, Goldberg I, Sharazi I, et al. A case of paracetamol-induced acute generalized exanthematous pustulosis in a pregnant woman localized in the neck region. Skinmed. 2004;3:47-49.
- Mehra KK, Rupawala AH, Gogtay NJ. Immediate hypersensitivity reaction to a single oral dose of flurbiprofen. J Postgrad Med. 2010;56:36-37.
- Girardi M, Duncan KO, Tigelaar RE, et al. Cross comparison of patch-test and lymphocyte proliferation responses in patients with a history of acute generalized exanthematous pustulosis. Am J Dermatopathol. 2005;27:343-346.
To the Editor:
Acute generalized exanthematous pustulosis (AGEP) is an acute skin reaction that is characterized by generalized, nonfollicular, pinhead-sized, sterile pustules on an erythematous and edematous background. The eruption can be accompanied by fever and neutrophilic leukocytosis. Skin symptoms arise quickly (within a few hours), most commonly following drug administration. The medications most frequently responsible are beta-lactam antibiotics, macrolides, calcium channel blockers, and antimalarials. Pustules spontaneously resolve in 15 days and generalized desquamation occurs approximately 2 weeks later. The estimated incidence rate of AGEP is approximately 1 to 5 cases per million per year. Acute localized exanthematous pustulosis (ALEP) is a less common form of AGEP. We report a case of ALEP localized on the face that was caused by flurbiprofen, a propionic acid derivative from the family of nonsteroidal anti-inflammatory drugs (NSAIDs).
A 40-year-old woman was referred to the dermatology department due to the sudden onset of multiple pustules on the face. One week earlier she started oral flurbiprofen (8.75 mg daily) for a sore throat. After 3 days of therapy, multiple pruritic, erythematous and edematous lesions appeared abruptly on the face with associated multiple small nonfollicular pustules. At presentation the patient was febrile (temperature, 38.2°C) and presented with bilateral ocular edema and superficial small nonfollicular pustules on an erythematous background over the face, scalp, and oral mucosa (Figure 1). The rest of the body was not involved. The patient denied prior adverse reactions to other drugs. The white blood cell count was 15,000/μL (reference range, 4500–11,000/μL), with an increased neutrophil count (12,000/μL [reference range, 1800–7800/μL]). The erythrocyte sedimentation rate and C-reactive protein level was elevated (erythrocyte sedimentation rate, 53 mm/h [reference range, 0–20 mm/h]; C-reactive protein, 98 mg/dL [reference range, 0–5 mg/dL]). Bacterial and fungal cultures of skin lesions were negative. The results of a viral polymerase chain reaction analysis proved the absence of varicella-zoster virus or herpes simplex virus. Histopathology of a skin biopsy specimen showed subcorneal pustules composed of neutrophils and eosinophils, epidermal spongiosis, some necrotic keratinocytes, vacuolization of the basal layer, papillary edema, and a perivascular neutrophil and lymphocyte infiltrate (Figure 2). A leukocytoclastic infiltrate within and around the walls of blood vessels at the superficial level of the dermis and red cell extravasation in the epidermis was present. She discontinued use of flurbiprofen and was treated with a systemic corticosteroid (methylprednisolone 0.5 mg/kg daily). The pustules rapidly resolved within 7 days after discontinuation of flurbiprofen and were followed by transient scaling and discrete residual hyperpigmentation.


Acute localized exanthematous pustulosis is a less common form of a pustular drug eruption in which lesions are consistent with AGEP but typically are localized to the face, neck, or chest. The definition of ALEP was introduced by Prange et al1 to describe a woman who was diagnosed with a localized pustular eruption on the face without a generalized distribution as in AGEP. In the past, this localized eruption was described under different names (eg, localized pustular eruption, localized toxin follicular pustuloderma, nongeneralized acute exanthematic pustulosis).2-5 According to a PubMed search of articles indexed for MEDLINE using the terms localized pustulosis, localized pustular eruption, and localized pustuloderma, only 16 separate cases of ALEP have been documented since the report by Prange et al.1 The medications most frequently responsible are antibiotics. Three cases developed following administration of amoxicillin2,5,6; 2 cases of amoxicillin–clavulanic acid7,8; 1 of penicillin1; 1 of azithromycin9; 1 of levofloxacin10; and 1 of combination of cephalosporin, sulfamethoxazole-trimethoprim, and vancomycin.11 Other nonantibiotic causative drugs include sulfamethoxazole-trimethoprim,12 infliximab,13 sorafenib,14 docetaxel,15 finasteride,16 ibuprofen,17 and paracetamol.18 In reported cases, the lesions are consistent with the characteristics of AGEP both clinically and histopathologically but are localized typically to the face, neck, or chest. In the majority of patients with ALEP, the absence of fever has been observed, but it does not appear distinctive for diagnosis. Our patient represents another case of ALEP with flurbiprofen as the causative drug. The close relationship between the administration of the drug and the development of the pustules, the rapid acute resolution as soon as treatment was interrupted, and the histologic findings all supported the diagnosis of ALEP following administration of flurbiprofen. This NSAID—2-fluoro-α-methyl-(1,1'-biphenyl)-4-acetic acid—is a prostaglandin synthetase inhibitor with anti-inflammatory activity. It is a propionic acid derivative that is similar to ibuprofen, which was once involved in the occurrence of ALEP.17 In 2009, Rastogi et al17 reported a case of a 64-year-old woman with an acute outbreak of multiple pustular lesions and underlying erythema affecting the cheeks and chin without fever who had been taking ibuprofen for a toothache. The case is similar to ours and confirms that NSAIDs can induce ALEP. Compared with other NSAIDs, propionic acid derivatives are usually well tolerated and serious adverse reactions rarely have been documented.19
The physiopathologic mechanisms of ALEP are unknown but likely are similar to AGEP. The demonstration of drug-specific positive patch test responses and in vitro lymphocyte proliferative responses in patients with a history of AGEP strongly suggests that this adverse cutaneous reaction occurs via a drug-specific T cell–mediated process.20
Further study is needed to understand the etiopathogenesis of the localized form of the disease and to facilitate a correct diagnosis of this rare disorder.
To the Editor:
Acute generalized exanthematous pustulosis (AGEP) is an acute skin reaction that is characterized by generalized, nonfollicular, pinhead-sized, sterile pustules on an erythematous and edematous background. The eruption can be accompanied by fever and neutrophilic leukocytosis. Skin symptoms arise quickly (within a few hours), most commonly following drug administration. The medications most frequently responsible are beta-lactam antibiotics, macrolides, calcium channel blockers, and antimalarials. Pustules spontaneously resolve in 15 days and generalized desquamation occurs approximately 2 weeks later. The estimated incidence rate of AGEP is approximately 1 to 5 cases per million per year. Acute localized exanthematous pustulosis (ALEP) is a less common form of AGEP. We report a case of ALEP localized on the face that was caused by flurbiprofen, a propionic acid derivative from the family of nonsteroidal anti-inflammatory drugs (NSAIDs).
A 40-year-old woman was referred to the dermatology department due to the sudden onset of multiple pustules on the face. One week earlier she started oral flurbiprofen (8.75 mg daily) for a sore throat. After 3 days of therapy, multiple pruritic, erythematous and edematous lesions appeared abruptly on the face with associated multiple small nonfollicular pustules. At presentation the patient was febrile (temperature, 38.2°C) and presented with bilateral ocular edema and superficial small nonfollicular pustules on an erythematous background over the face, scalp, and oral mucosa (Figure 1). The rest of the body was not involved. The patient denied prior adverse reactions to other drugs. The white blood cell count was 15,000/μL (reference range, 4500–11,000/μL), with an increased neutrophil count (12,000/μL [reference range, 1800–7800/μL]). The erythrocyte sedimentation rate and C-reactive protein level was elevated (erythrocyte sedimentation rate, 53 mm/h [reference range, 0–20 mm/h]; C-reactive protein, 98 mg/dL [reference range, 0–5 mg/dL]). Bacterial and fungal cultures of skin lesions were negative. The results of a viral polymerase chain reaction analysis proved the absence of varicella-zoster virus or herpes simplex virus. Histopathology of a skin biopsy specimen showed subcorneal pustules composed of neutrophils and eosinophils, epidermal spongiosis, some necrotic keratinocytes, vacuolization of the basal layer, papillary edema, and a perivascular neutrophil and lymphocyte infiltrate (Figure 2). A leukocytoclastic infiltrate within and around the walls of blood vessels at the superficial level of the dermis and red cell extravasation in the epidermis was present. She discontinued use of flurbiprofen and was treated with a systemic corticosteroid (methylprednisolone 0.5 mg/kg daily). The pustules rapidly resolved within 7 days after discontinuation of flurbiprofen and were followed by transient scaling and discrete residual hyperpigmentation.


Acute localized exanthematous pustulosis is a less common form of a pustular drug eruption in which lesions are consistent with AGEP but typically are localized to the face, neck, or chest. The definition of ALEP was introduced by Prange et al1 to describe a woman who was diagnosed with a localized pustular eruption on the face without a generalized distribution as in AGEP. In the past, this localized eruption was described under different names (eg, localized pustular eruption, localized toxin follicular pustuloderma, nongeneralized acute exanthematic pustulosis).2-5 According to a PubMed search of articles indexed for MEDLINE using the terms localized pustulosis, localized pustular eruption, and localized pustuloderma, only 16 separate cases of ALEP have been documented since the report by Prange et al.1 The medications most frequently responsible are antibiotics. Three cases developed following administration of amoxicillin2,5,6; 2 cases of amoxicillin–clavulanic acid7,8; 1 of penicillin1; 1 of azithromycin9; 1 of levofloxacin10; and 1 of combination of cephalosporin, sulfamethoxazole-trimethoprim, and vancomycin.11 Other nonantibiotic causative drugs include sulfamethoxazole-trimethoprim,12 infliximab,13 sorafenib,14 docetaxel,15 finasteride,16 ibuprofen,17 and paracetamol.18 In reported cases, the lesions are consistent with the characteristics of AGEP both clinically and histopathologically but are localized typically to the face, neck, or chest. In the majority of patients with ALEP, the absence of fever has been observed, but it does not appear distinctive for diagnosis. Our patient represents another case of ALEP with flurbiprofen as the causative drug. The close relationship between the administration of the drug and the development of the pustules, the rapid acute resolution as soon as treatment was interrupted, and the histologic findings all supported the diagnosis of ALEP following administration of flurbiprofen. This NSAID—2-fluoro-α-methyl-(1,1'-biphenyl)-4-acetic acid—is a prostaglandin synthetase inhibitor with anti-inflammatory activity. It is a propionic acid derivative that is similar to ibuprofen, which was once involved in the occurrence of ALEP.17 In 2009, Rastogi et al17 reported a case of a 64-year-old woman with an acute outbreak of multiple pustular lesions and underlying erythema affecting the cheeks and chin without fever who had been taking ibuprofen for a toothache. The case is similar to ours and confirms that NSAIDs can induce ALEP. Compared with other NSAIDs, propionic acid derivatives are usually well tolerated and serious adverse reactions rarely have been documented.19
The physiopathologic mechanisms of ALEP are unknown but likely are similar to AGEP. The demonstration of drug-specific positive patch test responses and in vitro lymphocyte proliferative responses in patients with a history of AGEP strongly suggests that this adverse cutaneous reaction occurs via a drug-specific T cell–mediated process.20
Further study is needed to understand the etiopathogenesis of the localized form of the disease and to facilitate a correct diagnosis of this rare disorder.
- Prange B, Marini A, Kalke A, et al. Acute localized exanthematous pustulosis (ALEP). J Dtsch Dermatol Ges. 2005;3:210-212.
- Shuttleworth D. A localized, recurrent pustular eruption following amoxycillin administration. Clin Exp Dermatol. 1989;14:367-368.
- De Argila D, Ortiz-Frutos J, Rodriguez-Peralto JL, et al. An atypical case of non-generalized acute exanthematic pustulosis. Actas Dermosifiliogr. 1996;87:475-478.
- Corbalan-Velez R, Peon G, Ara M, et al. Localized toxic follicular pustuloderma. Int J Dermatol. 2000;39:209-211.
- Prieto A, de Barrio M, López-Sáez P, et al. Recurrent localized pustular eruption induced by amoxicillin. Allergy. 1997;52:777-778.
- Vickers JL, Matherne RJ, Mainous EG, et al. Acute localized exanthematous pustulosis: a cutaneous drug reaction in a dental setting. J Am Dent Assoc. 2008;139:1200-1203.
- Betto P, Germi L, Bonoldi E, et al. Acute localized exanthematous pustulosis (ALEP) caused by amoxicillin-clavulanic acid. Int J Dermatol. 2008;47:295-296.
- Ozkaya-Parlakay A, Azkur D, Kara A, et al. Localized acute generalized exanthematous pustulosis with amoxicillin and clavulanic acid. Turk J Pediatr. 2011;53:229-232.
- Zweegers J, Bovenschen HJ. A woman with skin abnormalities around the mouth [in Dutch]. Ned Tijdschr Geneeskd. 2012;156:A4613.
- Corral de la Calle M, Martín Díaz MA, Flores CR, et al. Acute localized exanthematous pustulosis secondary to levofloxacin. Br J Dermatol. 2005;152:1076-1077.
- Sim HS, Seol JE, Chun JS, et al. Acute localized exanthematous pustulosis on the face. Ann Dermatol. 2011;23(suppl 3):S3368-S3370.
- Lee I, Turner M, Lee CC. Acute patchy exanthematous pustulosis caused by sulfamethoxazole-trimethoprim. J Am Acad Dermatol. 2010;63:e41-e43.
- Lee HY, Pelivani N, Beltraminelli H, et al. Amicrobial pustulosis-like rash in a patient with Crohn’s disease under anti-TNF-alpha blocker. Dermatology. 2011;222:304-310.
- Liang CP, Yang CS, Shen JL, et al. Sorafenib-induced acute localized exanthematous pustulosis in a patient with hepatocellular carcinoma. Br J Dermatol. 2011;165:443-445.
- Kim SW, Lee UH, Jang SJ, et al. Acute localized exanthematous pustulosis induced by docetaxel. J Am Acad Dermatol. 2010;63:e44-e46.
- Tresch S, Cozzio A, Kamarashev J, et al. T cell-mediated acute localized exanthematous pustulosis caused by finasteride. J Allergy Clin Immunol. 2012;129:589-594.
- Rastogi S, Modi M, Dhawan V. Acute localized exanthematous pustulosis (ALEP) caused by Ibuprofen. a case report. Br J Oral Maxillofac Surg. 2009;47:132-134.
- Wohl Y, Goldberg I, Sharazi I, et al. A case of paracetamol-induced acute generalized exanthematous pustulosis in a pregnant woman localized in the neck region. Skinmed. 2004;3:47-49.
- Mehra KK, Rupawala AH, Gogtay NJ. Immediate hypersensitivity reaction to a single oral dose of flurbiprofen. J Postgrad Med. 2010;56:36-37.
- Girardi M, Duncan KO, Tigelaar RE, et al. Cross comparison of patch-test and lymphocyte proliferation responses in patients with a history of acute generalized exanthematous pustulosis. Am J Dermatopathol. 2005;27:343-346.
- Prange B, Marini A, Kalke A, et al. Acute localized exanthematous pustulosis (ALEP). J Dtsch Dermatol Ges. 2005;3:210-212.
- Shuttleworth D. A localized, recurrent pustular eruption following amoxycillin administration. Clin Exp Dermatol. 1989;14:367-368.
- De Argila D, Ortiz-Frutos J, Rodriguez-Peralto JL, et al. An atypical case of non-generalized acute exanthematic pustulosis. Actas Dermosifiliogr. 1996;87:475-478.
- Corbalan-Velez R, Peon G, Ara M, et al. Localized toxic follicular pustuloderma. Int J Dermatol. 2000;39:209-211.
- Prieto A, de Barrio M, López-Sáez P, et al. Recurrent localized pustular eruption induced by amoxicillin. Allergy. 1997;52:777-778.
- Vickers JL, Matherne RJ, Mainous EG, et al. Acute localized exanthematous pustulosis: a cutaneous drug reaction in a dental setting. J Am Dent Assoc. 2008;139:1200-1203.
- Betto P, Germi L, Bonoldi E, et al. Acute localized exanthematous pustulosis (ALEP) caused by amoxicillin-clavulanic acid. Int J Dermatol. 2008;47:295-296.
- Ozkaya-Parlakay A, Azkur D, Kara A, et al. Localized acute generalized exanthematous pustulosis with amoxicillin and clavulanic acid. Turk J Pediatr. 2011;53:229-232.
- Zweegers J, Bovenschen HJ. A woman with skin abnormalities around the mouth [in Dutch]. Ned Tijdschr Geneeskd. 2012;156:A4613.
- Corral de la Calle M, Martín Díaz MA, Flores CR, et al. Acute localized exanthematous pustulosis secondary to levofloxacin. Br J Dermatol. 2005;152:1076-1077.
- Sim HS, Seol JE, Chun JS, et al. Acute localized exanthematous pustulosis on the face. Ann Dermatol. 2011;23(suppl 3):S3368-S3370.
- Lee I, Turner M, Lee CC. Acute patchy exanthematous pustulosis caused by sulfamethoxazole-trimethoprim. J Am Acad Dermatol. 2010;63:e41-e43.
- Lee HY, Pelivani N, Beltraminelli H, et al. Amicrobial pustulosis-like rash in a patient with Crohn’s disease under anti-TNF-alpha blocker. Dermatology. 2011;222:304-310.
- Liang CP, Yang CS, Shen JL, et al. Sorafenib-induced acute localized exanthematous pustulosis in a patient with hepatocellular carcinoma. Br J Dermatol. 2011;165:443-445.
- Kim SW, Lee UH, Jang SJ, et al. Acute localized exanthematous pustulosis induced by docetaxel. J Am Acad Dermatol. 2010;63:e44-e46.
- Tresch S, Cozzio A, Kamarashev J, et al. T cell-mediated acute localized exanthematous pustulosis caused by finasteride. J Allergy Clin Immunol. 2012;129:589-594.
- Rastogi S, Modi M, Dhawan V. Acute localized exanthematous pustulosis (ALEP) caused by Ibuprofen. a case report. Br J Oral Maxillofac Surg. 2009;47:132-134.
- Wohl Y, Goldberg I, Sharazi I, et al. A case of paracetamol-induced acute generalized exanthematous pustulosis in a pregnant woman localized in the neck region. Skinmed. 2004;3:47-49.
- Mehra KK, Rupawala AH, Gogtay NJ. Immediate hypersensitivity reaction to a single oral dose of flurbiprofen. J Postgrad Med. 2010;56:36-37.
- Girardi M, Duncan KO, Tigelaar RE, et al. Cross comparison of patch-test and lymphocyte proliferation responses in patients with a history of acute generalized exanthematous pustulosis. Am J Dermatopathol. 2005;27:343-346.
Practice Points
- Acute localized exanthematous pustulosis is a form of a pustular drug eruption in which lesions are consistent with acute generalized exanthematous pustulosis but typically localized in a single area.
- The medications most frequently responsible are antibiotics. Flurbiprofen, a propionic acid derivative, could be a rare causative agent of this disease.
Contact Allergy to Poliglecaprone 25 Sutures
To the Editor:
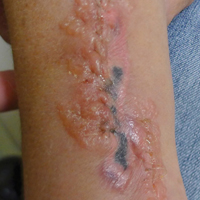
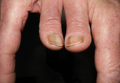
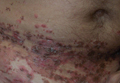
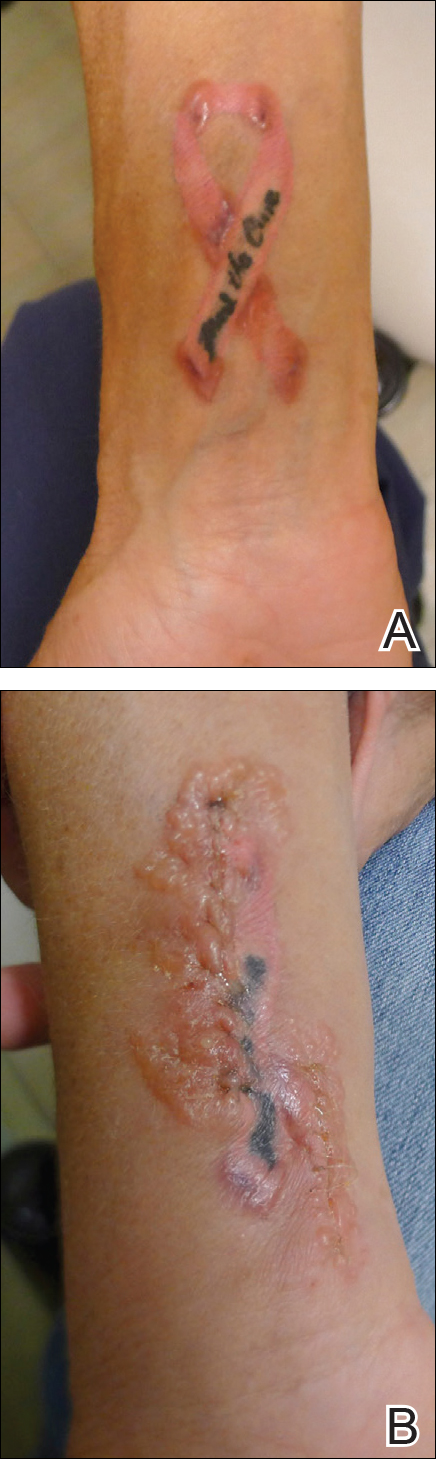
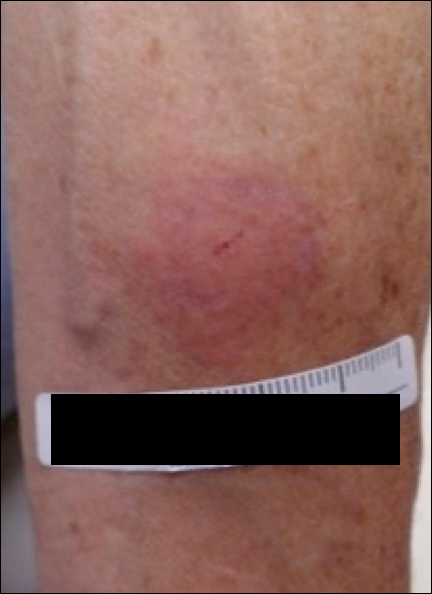
A 42-year-old woman who had a tattoo on the right wrist surgically removed 2 days prior developed severe erythema and swelling at the incision site (Figure 1). Exposure at the incision site was limited to bacitracin, poliglecaprone 25 suture, and plain cotton gauze. Patch testing of bacitracin was performed, which was ++ (moderately positive reaction) at the 96-hour reading, indicating that part of the reaction was due to the topical antibiotic. Testing of the suture was performed by tying the suture to the skin of the forearm and removing it at 48 hours. There was a ++ reaction to the suture prior to removal at 48 hours, which increased to +++ (severely positive reaction) after suture removal at 96 hours (Figure 2). Therefore, it appears that allergy to the suture also was partially responsible for the postsurgical reaction.
Poliglecaprone 25 suture is a monofilament synthetic absorbable material that is a copolymer of glycolide and ε-caprolactone. One case report of oral contact allergy to this suture material resulted in failure of an oral graft; however, no testing was performed to verify the contact allergy.1 Caprolactam ([CH2]5C[O]NH) is a related chemical that can be synthesized by treating caprolactone ([CH2]5CO2) with ammonia at elevated temperatures.2 Contact allergy has been reported to polyamide 6 suture, which is obtained by polymerizing ε-caprolactam. This report stated that contact allergy to ε-caprolactam also has been reported occupationally during manufacture and from its use in fishing nets, socks, gloves, and stockings.3
The package insert for the poliglecaprone 25 suture states that the material is “nonantigenic, nonpyrogenic and elicits only a slight tissue reaction during absorption.”4 We present a case of contact allergy to poliglecaprone 25 suture that was confirmed by allergy testing.
- Mawardi H. Oral contact allergy to suture material results in connective tissue graft failure: a case report. J Periodontol Online. 2014;4:155-160.
- Buntara T, Noel S, Phua PH, et al. Caprolactam from renewable resources: catalytic conversion of 5-hydroxymethylfurfural into caprolactone. Angew Chem Int Ed Engl. 2011;50:7083-7087.
- Hausen BM. Allergic contact dermatitis from colored surgical suture material: contact allergy to epsilon-caprolactam and acid blue 158. Am J Contact Dermat. 2003;14:174-175.
- Monocryl [package insert]. Somerville, NJ: Ethicon, Inc; 1996.
To the Editor:
A 42-year-old woman who had a tattoo on the right wrist surgically removed 2 days prior developed severe erythema and swelling at the incision site (Figure 1). Exposure at the incision site was limited to bacitracin, poliglecaprone 25 suture, and plain cotton gauze. Patch testing of bacitracin was performed, which was ++ (moderately positive reaction) at the 96-hour reading, indicating that part of the reaction was due to the topical antibiotic. Testing of the suture was performed by tying the suture to the skin of the forearm and removing it at 48 hours. There was a ++ reaction to the suture prior to removal at 48 hours, which increased to +++ (severely positive reaction) after suture removal at 96 hours (Figure 2). Therefore, it appears that allergy to the suture also was partially responsible for the postsurgical reaction.


Poliglecaprone 25 suture is a monofilament synthetic absorbable material that is a copolymer of glycolide and ε-caprolactone. One case report of oral contact allergy to this suture material resulted in failure of an oral graft; however, no testing was performed to verify the contact allergy.1 Caprolactam ([CH2]5C[O]NH) is a related chemical that can be synthesized by treating caprolactone ([CH2]5CO2) with ammonia at elevated temperatures.2 Contact allergy has been reported to polyamide 6 suture, which is obtained by polymerizing ε-caprolactam. This report stated that contact allergy to ε-caprolactam also has been reported occupationally during manufacture and from its use in fishing nets, socks, gloves, and stockings.3
The package insert for the poliglecaprone 25 suture states that the material is “nonantigenic, nonpyrogenic and elicits only a slight tissue reaction during absorption.”4 We present a case of contact allergy to poliglecaprone 25 suture that was confirmed by allergy testing.
To the Editor:
A 42-year-old woman who had a tattoo on the right wrist surgically removed 2 days prior developed severe erythema and swelling at the incision site (Figure 1). Exposure at the incision site was limited to bacitracin, poliglecaprone 25 suture, and plain cotton gauze. Patch testing of bacitracin was performed, which was ++ (moderately positive reaction) at the 96-hour reading, indicating that part of the reaction was due to the topical antibiotic. Testing of the suture was performed by tying the suture to the skin of the forearm and removing it at 48 hours. There was a ++ reaction to the suture prior to removal at 48 hours, which increased to +++ (severely positive reaction) after suture removal at 96 hours (Figure 2). Therefore, it appears that allergy to the suture also was partially responsible for the postsurgical reaction.


Poliglecaprone 25 suture is a monofilament synthetic absorbable material that is a copolymer of glycolide and ε-caprolactone. One case report of oral contact allergy to this suture material resulted in failure of an oral graft; however, no testing was performed to verify the contact allergy.1 Caprolactam ([CH2]5C[O]NH) is a related chemical that can be synthesized by treating caprolactone ([CH2]5CO2) with ammonia at elevated temperatures.2 Contact allergy has been reported to polyamide 6 suture, which is obtained by polymerizing ε-caprolactam. This report stated that contact allergy to ε-caprolactam also has been reported occupationally during manufacture and from its use in fishing nets, socks, gloves, and stockings.3
The package insert for the poliglecaprone 25 suture states that the material is “nonantigenic, nonpyrogenic and elicits only a slight tissue reaction during absorption.”4 We present a case of contact allergy to poliglecaprone 25 suture that was confirmed by allergy testing.
- Mawardi H. Oral contact allergy to suture material results in connective tissue graft failure: a case report. J Periodontol Online. 2014;4:155-160.
- Buntara T, Noel S, Phua PH, et al. Caprolactam from renewable resources: catalytic conversion of 5-hydroxymethylfurfural into caprolactone. Angew Chem Int Ed Engl. 2011;50:7083-7087.
- Hausen BM. Allergic contact dermatitis from colored surgical suture material: contact allergy to epsilon-caprolactam and acid blue 158. Am J Contact Dermat. 2003;14:174-175.
- Monocryl [package insert]. Somerville, NJ: Ethicon, Inc; 1996.
- Mawardi H. Oral contact allergy to suture material results in connective tissue graft failure: a case report. J Periodontol Online. 2014;4:155-160.
- Buntara T, Noel S, Phua PH, et al. Caprolactam from renewable resources: catalytic conversion of 5-hydroxymethylfurfural into caprolactone. Angew Chem Int Ed Engl. 2011;50:7083-7087.
- Hausen BM. Allergic contact dermatitis from colored surgical suture material: contact allergy to epsilon-caprolactam and acid blue 158. Am J Contact Dermat. 2003;14:174-175.
- Monocryl [package insert]. Somerville, NJ: Ethicon, Inc; 1996.
Practice Point
- Physicians should be aware that rare contact reactions can occur with certain types of sutures.
Eruptive Seborrheic Keratoses Secondary to Telaprevir-Related Dermatitis
To the Editor:
Telaprevir is a hepatitis C virus (HCV) protease inhibitor used with ribavirin and interferon for the treatment of increased viral load clearance in specific HCV genotypes. We report a case of eruptive seborrheic keratoses (SKs) secondary to telaprevir-related dermatitis.
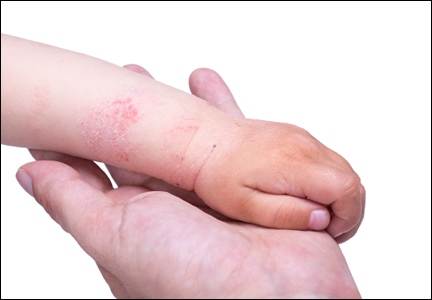
A 65-year-old woman with a history of depression, basal cell carcinoma, and HCV presented 5 months after initiation of antiviral treatment with interferon, ribavirin, and telaprevir. Shortly after initiation of therapy, the patient developed a diffuse itch with a “pricking” sensation. The patient reported that approximately 2 months after starting treatment she developed an erythematous scaling rash that covered 75% of the body, which led to the discontinuation of telaprevir after 10 weeks of therapy; interferon and ribavirin were continued for a total of 6 months. In concert with the eczematous eruption, the patient noticed many new hyperpigmented lesions with enlargement of the few preexisting SKs. She presented to our clinic 6 weeks after the discontinuation of telaprevir for evaluation of these lesions.
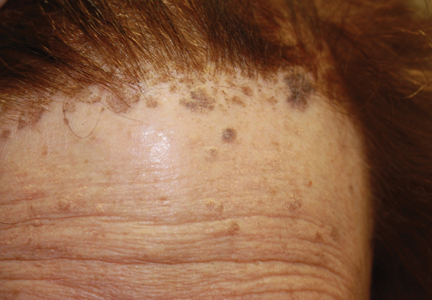
On examination, several brown, hyperpigmented, stuck-on papules and plaques were noted diffusely on the body, most prominently along the frontal hairline (Figure 1). A biopsy of the right side of the forehead showed a reticulated epidermis, horn pseudocysts, and increased basilar pigment diagnostic of an SK (Figure 2).

[[{"attributes":{},"fields":{}}]][[{"attributes":{},"fields":{}}]]
Telaprevir is an HCV protease inhibitor that is given in combination with interferon and ribavirin for increased clearance of genotype 1 HCV infection. Cutaneous reactions to telaprevir are seen in 41% to 61% of treated patients and include Stevens-Johnson syndrome, drug reaction with eosinophilia and systemic symptoms, sarcoidosis, pityriasis rubra pilaris–like drug eruption, and most commonly telaprevir-related dermatitis.1-3 Telaprevir-related dermatitis accounts for up to 95% of cutaneous reactions and presents at a median of 15 days (interquartile range, 4–41 days) after initiation of therapy. Nearly 25% of cases occur in the first 4 days and 46% of cases occur within 4 weeks. It presents as an erythematous eczematous dermatitis commonly associated with pruritus in contrast to the common morbilliform drug eruption. Secondary xerosis, excoriation, and lichenification can be appreciated. With appropriate treatment, resolution occurs in a median of 44 days.1 Treatment of the dermatitis can allow completion of the recommended 12-week course of telaprevir and involves oral antihistamines and topical corticosteroids. Severe cases may require oral corticosteroids and discontinuation of telaprevir. If the cutaneous eruption does not resolve, discontinuation of ribavirin also may be required, as it can cause a similar cutaneous eruption.4
Eruptive SKs may be appreciated in 2 clinical circumstances: associated with an internal malignancy (Leser-Trélat sign), or secondary to an erythrodermic eruption. Flugman et al5 reported 2 cases of eruptive SKs in association with erythroderma. Their first patient developed erythroderma after initiating UVB therapy for psoriasis. The second patient developed an erythrodermic drug hypersensitivity reaction after switching to generic forms of quinidine gluconate and ranitidine. The SKs spontaneously resolved within 6 months and 10 weeks of the resolution of erythroderma, respectively.5 Most of our patient’s eruptive SKs resolved within a few months of their presentation, consistent with the time frame reported in the literature.
Telaprevir-related dermatitis presumably served as the inciting factor for the development of SKs in our patient, as the lesions improved after discontinuation of telaprevir despite continued therapy with ribavirin. As noted by Flugman et al,5 SKs may be seen in erythroderma due to diverse etiologies such as psoriasis, pityriasis rubra pilaris, or allergic contact dermatitis. We hypothesize that the eruption immunologically releases cytokines and/or growth factors that stimulate the production of the SKs. Fibroblast growth factor receptor 3 mutations have been associated with SKs.6 An erythrodermic milieu may incite such mutations in genetically predisposed patients.
We present a case of eruptive SKs related to telaprevir therapy. Our report expands the clinical scenarios in which the clinician can observe eruptive SKs. Although further research is necessary to ascertain the pathogenesis of these lesions, patients may be reassured that most lesions will spontaneously resolve.
- Roujeau J, Mockenhaupt M, Tahan S, et al. Telaprevir-related dermatitis. JAMA Dermatol. 2013;149:152-158.
- Stalling S, Vu J, English J. Telaprevir-induced pityriasis rubra pilaris-like drug eruption. Arch Dermatol. 2012;148:1215-1217.
- Hinds B, Sonnier G, Waldman M. Cutaneous sarcoidosis triggered by immunotherapy for chronic hepatitis C: a case report. J Am Acad Dermatol. 2013;68:AB47.
- Lawitz E. Diagnosis and management of telaprevir-associated rash. Gastroenterol Hepatol. 2011;7:469-471.
- Flugman SL, McClain SA, Clark RA. Transient eruptive seborrheic keratoses associated with erythrodermic psoriasis and erythrodermic drug eruption: report of two cases. J Am Acad Dermatol. 2001;45(6 suppl):S212-S214.
- Hafner C, Hartman A, van Oers JM, et al. FGFR3 mutations in seborrheic keratoses are already present in flat lesions and associated with age and localization. Mod Pathol. 2007;20:895-903.
To the Editor:
Telaprevir is a hepatitis C virus (HCV) protease inhibitor used with ribavirin and interferon for the treatment of increased viral load clearance in specific HCV genotypes. We report a case of eruptive seborrheic keratoses (SKs) secondary to telaprevir-related dermatitis.
A 65-year-old woman with a history of depression, basal cell carcinoma, and HCV presented 5 months after initiation of antiviral treatment with interferon, ribavirin, and telaprevir. Shortly after initiation of therapy, the patient developed a diffuse itch with a “pricking” sensation. The patient reported that approximately 2 months after starting treatment she developed an erythematous scaling rash that covered 75% of the body, which led to the discontinuation of telaprevir after 10 weeks of therapy; interferon and ribavirin were continued for a total of 6 months. In concert with the eczematous eruption, the patient noticed many new hyperpigmented lesions with enlargement of the few preexisting SKs. She presented to our clinic 6 weeks after the discontinuation of telaprevir for evaluation of these lesions.
On examination, several brown, hyperpigmented, stuck-on papules and plaques were noted diffusely on the body, most prominently along the frontal hairline (Figure 1). A biopsy of the right side of the forehead showed a reticulated epidermis, horn pseudocysts, and increased basilar pigment diagnostic of an SK (Figure 2).

[[{"attributes":{},"fields":{}}]][[{"attributes":{},"fields":{}}]]
Telaprevir is an HCV protease inhibitor that is given in combination with interferon and ribavirin for increased clearance of genotype 1 HCV infection. Cutaneous reactions to telaprevir are seen in 41% to 61% of treated patients and include Stevens-Johnson syndrome, drug reaction with eosinophilia and systemic symptoms, sarcoidosis, pityriasis rubra pilaris–like drug eruption, and most commonly telaprevir-related dermatitis.1-3 Telaprevir-related dermatitis accounts for up to 95% of cutaneous reactions and presents at a median of 15 days (interquartile range, 4–41 days) after initiation of therapy. Nearly 25% of cases occur in the first 4 days and 46% of cases occur within 4 weeks. It presents as an erythematous eczematous dermatitis commonly associated with pruritus in contrast to the common morbilliform drug eruption. Secondary xerosis, excoriation, and lichenification can be appreciated. With appropriate treatment, resolution occurs in a median of 44 days.1 Treatment of the dermatitis can allow completion of the recommended 12-week course of telaprevir and involves oral antihistamines and topical corticosteroids. Severe cases may require oral corticosteroids and discontinuation of telaprevir. If the cutaneous eruption does not resolve, discontinuation of ribavirin also may be required, as it can cause a similar cutaneous eruption.4
Eruptive SKs may be appreciated in 2 clinical circumstances: associated with an internal malignancy (Leser-Trélat sign), or secondary to an erythrodermic eruption. Flugman et al5 reported 2 cases of eruptive SKs in association with erythroderma. Their first patient developed erythroderma after initiating UVB therapy for psoriasis. The second patient developed an erythrodermic drug hypersensitivity reaction after switching to generic forms of quinidine gluconate and ranitidine. The SKs spontaneously resolved within 6 months and 10 weeks of the resolution of erythroderma, respectively.5 Most of our patient’s eruptive SKs resolved within a few months of their presentation, consistent with the time frame reported in the literature.
Telaprevir-related dermatitis presumably served as the inciting factor for the development of SKs in our patient, as the lesions improved after discontinuation of telaprevir despite continued therapy with ribavirin. As noted by Flugman et al,5 SKs may be seen in erythroderma due to diverse etiologies such as psoriasis, pityriasis rubra pilaris, or allergic contact dermatitis. We hypothesize that the eruption immunologically releases cytokines and/or growth factors that stimulate the production of the SKs. Fibroblast growth factor receptor 3 mutations have been associated with SKs.6 An erythrodermic milieu may incite such mutations in genetically predisposed patients.
We present a case of eruptive SKs related to telaprevir therapy. Our report expands the clinical scenarios in which the clinician can observe eruptive SKs. Although further research is necessary to ascertain the pathogenesis of these lesions, patients may be reassured that most lesions will spontaneously resolve.
To the Editor:
Telaprevir is a hepatitis C virus (HCV) protease inhibitor used with ribavirin and interferon for the treatment of increased viral load clearance in specific HCV genotypes. We report a case of eruptive seborrheic keratoses (SKs) secondary to telaprevir-related dermatitis.
A 65-year-old woman with a history of depression, basal cell carcinoma, and HCV presented 5 months after initiation of antiviral treatment with interferon, ribavirin, and telaprevir. Shortly after initiation of therapy, the patient developed a diffuse itch with a “pricking” sensation. The patient reported that approximately 2 months after starting treatment she developed an erythematous scaling rash that covered 75% of the body, which led to the discontinuation of telaprevir after 10 weeks of therapy; interferon and ribavirin were continued for a total of 6 months. In concert with the eczematous eruption, the patient noticed many new hyperpigmented lesions with enlargement of the few preexisting SKs. She presented to our clinic 6 weeks after the discontinuation of telaprevir for evaluation of these lesions.
On examination, several brown, hyperpigmented, stuck-on papules and plaques were noted diffusely on the body, most prominently along the frontal hairline (Figure 1). A biopsy of the right side of the forehead showed a reticulated epidermis, horn pseudocysts, and increased basilar pigment diagnostic of an SK (Figure 2).

[[{"attributes":{},"fields":{}}]][[{"attributes":{},"fields":{}}]]
Telaprevir is an HCV protease inhibitor that is given in combination with interferon and ribavirin for increased clearance of genotype 1 HCV infection. Cutaneous reactions to telaprevir are seen in 41% to 61% of treated patients and include Stevens-Johnson syndrome, drug reaction with eosinophilia and systemic symptoms, sarcoidosis, pityriasis rubra pilaris–like drug eruption, and most commonly telaprevir-related dermatitis.1-3 Telaprevir-related dermatitis accounts for up to 95% of cutaneous reactions and presents at a median of 15 days (interquartile range, 4–41 days) after initiation of therapy. Nearly 25% of cases occur in the first 4 days and 46% of cases occur within 4 weeks. It presents as an erythematous eczematous dermatitis commonly associated with pruritus in contrast to the common morbilliform drug eruption. Secondary xerosis, excoriation, and lichenification can be appreciated. With appropriate treatment, resolution occurs in a median of 44 days.1 Treatment of the dermatitis can allow completion of the recommended 12-week course of telaprevir and involves oral antihistamines and topical corticosteroids. Severe cases may require oral corticosteroids and discontinuation of telaprevir. If the cutaneous eruption does not resolve, discontinuation of ribavirin also may be required, as it can cause a similar cutaneous eruption.4
Eruptive SKs may be appreciated in 2 clinical circumstances: associated with an internal malignancy (Leser-Trélat sign), or secondary to an erythrodermic eruption. Flugman et al5 reported 2 cases of eruptive SKs in association with erythroderma. Their first patient developed erythroderma after initiating UVB therapy for psoriasis. The second patient developed an erythrodermic drug hypersensitivity reaction after switching to generic forms of quinidine gluconate and ranitidine. The SKs spontaneously resolved within 6 months and 10 weeks of the resolution of erythroderma, respectively.5 Most of our patient’s eruptive SKs resolved within a few months of their presentation, consistent with the time frame reported in the literature.
Telaprevir-related dermatitis presumably served as the inciting factor for the development of SKs in our patient, as the lesions improved after discontinuation of telaprevir despite continued therapy with ribavirin. As noted by Flugman et al,5 SKs may be seen in erythroderma due to diverse etiologies such as psoriasis, pityriasis rubra pilaris, or allergic contact dermatitis. We hypothesize that the eruption immunologically releases cytokines and/or growth factors that stimulate the production of the SKs. Fibroblast growth factor receptor 3 mutations have been associated with SKs.6 An erythrodermic milieu may incite such mutations in genetically predisposed patients.
We present a case of eruptive SKs related to telaprevir therapy. Our report expands the clinical scenarios in which the clinician can observe eruptive SKs. Although further research is necessary to ascertain the pathogenesis of these lesions, patients may be reassured that most lesions will spontaneously resolve.
- Roujeau J, Mockenhaupt M, Tahan S, et al. Telaprevir-related dermatitis. JAMA Dermatol. 2013;149:152-158.
- Stalling S, Vu J, English J. Telaprevir-induced pityriasis rubra pilaris-like drug eruption. Arch Dermatol. 2012;148:1215-1217.
- Hinds B, Sonnier G, Waldman M. Cutaneous sarcoidosis triggered by immunotherapy for chronic hepatitis C: a case report. J Am Acad Dermatol. 2013;68:AB47.
- Lawitz E. Diagnosis and management of telaprevir-associated rash. Gastroenterol Hepatol. 2011;7:469-471.
- Flugman SL, McClain SA, Clark RA. Transient eruptive seborrheic keratoses associated with erythrodermic psoriasis and erythrodermic drug eruption: report of two cases. J Am Acad Dermatol. 2001;45(6 suppl):S212-S214.
- Hafner C, Hartman A, van Oers JM, et al. FGFR3 mutations in seborrheic keratoses are already present in flat lesions and associated with age and localization. Mod Pathol. 2007;20:895-903.
- Roujeau J, Mockenhaupt M, Tahan S, et al. Telaprevir-related dermatitis. JAMA Dermatol. 2013;149:152-158.
- Stalling S, Vu J, English J. Telaprevir-induced pityriasis rubra pilaris-like drug eruption. Arch Dermatol. 2012;148:1215-1217.
- Hinds B, Sonnier G, Waldman M. Cutaneous sarcoidosis triggered by immunotherapy for chronic hepatitis C: a case report. J Am Acad Dermatol. 2013;68:AB47.
- Lawitz E. Diagnosis and management of telaprevir-associated rash. Gastroenterol Hepatol. 2011;7:469-471.
- Flugman SL, McClain SA, Clark RA. Transient eruptive seborrheic keratoses associated with erythrodermic psoriasis and erythrodermic drug eruption: report of two cases. J Am Acad Dermatol. 2001;45(6 suppl):S212-S214.
- Hafner C, Hartman A, van Oers JM, et al. FGFR3 mutations in seborrheic keratoses are already present in flat lesions and associated with age and localization. Mod Pathol. 2007;20:895-903.
Practice Points
- Cutaneous reactions presenting as eczematous dermatitis are common (41%–61%) during telaprevir treatment.
- Telaprevir-related dermatitis can lead to eruptive seborrheic keratoses that may spontaneously resolve.
Zika Understanding Unfolds
Inundating our popular and academic media circles is information regarding the Zika virus. A recent article by Farahnik et al in the Journal of the American Academy of Dermatology (2016;74:1286-1287) briefly outlines what is known about Zika infection thus far and its dermatologic manifestations. Pairing this article with Centers for Disease Control and Prevention guidelines on the topic, we are presented with an evolving introduction to this new entity. Here’s what we know:
- It is a single-stranded RNA arbovirus in the Flavivirus family transmitted by the bite of Aedes mosquitoes, with cases reported so far in Africa, Asia, and the Americas (particularly southern coastal and island destinations).
- It also is transmitted via transfusion of blood, sexual contact, and mother to fetus.
- There is theoretical risk for fetal microcephaly, intracranial calcifications, and other brain and eye abnormalities.
- Only 1 in 5 affected patients show any systemic manifestations of infection, including self-limited flulike symptoms and nonspecific exanthema, typically sparing acral sites and occurring within 1 to 2 weeks of virus exposure.
- Testing is recommended for pregnant women with possible Zika exposure (ie, travel to an area with active transmission of Zika virus, unprotected sex with a male with this travel history).
- Diagnosis can be made through state health departments, employing real-time reverse transcriptase–polymerase chain reaction (rRT-PCR) or enzyme-linked immunosorbent assay the week after symptom onset using serum, or rRT-PCR 2 weeks after symptom onset using urine. Further antibody testing can be done if a false-negative is suspected, but false-positives also are possible if a patient was exposed to or vaccinated against other flaviviruses (eg, dengue virus, West Nile virus, yellow fever virus)
- Testing is inaccurate if ordered within 7 days or more than 12 weeks following presumed exposure.
- If positive or inconclusive testing arises, serial fetal ultrasonography should be considered; if testing is negative, then a single fetal ultrasound is recommended to detect Zika abnormalities.
- Test results are automatically reported to respective state health departments.
- There is no treatment of this infection aside from supportive care.
What’s the issue?
As with any new outbreak, the applicability to the general population and true risks remain to be seen. Each of our clinics recalls the stark changes in patient intake and screening questions with infections as ubiquitous as methicillin-resistant Staphylococcus aureus to much rarer exposures such as Ebola virus, each with progressive understanding of risk groups, disease manifestations, and eradication and prevention measures.
By mid-June 2016, 30 hits on PubMed addressing Zika had already been cited just within the month, outlining various aspects of the infection, and many specialties, particularly neurology, obstetrics, primary care, infectious disease, and dermatology, are weighing in. Unfortunately, the majority of cases of primary Zika infection do not manifest with skin or systemic symptoms, and even cases that do are nonspecific, exanthematous, and flulike.
Vague as it may be so far, it is nonetheless imperative that clinicians be familiar with what is concretely known about Zika virus and acquaint ourselves with the travel distribution and restrictions, disease risk factors, known sequelae, testing availability and limitations, and reporting guidelines. From personal experience, as I traveled to Belize earlier this year during my first trimester of pregnancy before the travel restrictions were outlined, even obstetricians are not wholly familiar with the manner in which to order testing and the appropriate window to do so. I have been asymptomatic, my blood was drawn in a period of time that exceeded the interval for accurate results (as outlined above) and was therefore inappropriately recommended/ordered, and now serial fetal ultrasonography is being implemented every few weeks.
With lack of ubiquitous knowledge about the infection, clinicians are not universally certain of the appropriate next steps when a patient presents with Zika risk factors, and therefore anxiety remains high for pregnant patients and their contacts. The Centers for Disease Control and Prevention website is the official home base, and we should review it and await their further evolving specific recommendations as more cases unfortunately accumulate.
Have you encountered any patients this year with exposure to or symptoms of Zika infection, and what, if anything, have you outlined for them?
Inundating our popular and academic media circles is information regarding the Zika virus. A recent article by Farahnik et al in the Journal of the American Academy of Dermatology (2016;74:1286-1287) briefly outlines what is known about Zika infection thus far and its dermatologic manifestations. Pairing this article with Centers for Disease Control and Prevention guidelines on the topic, we are presented with an evolving introduction to this new entity. Here’s what we know:
- It is a single-stranded RNA arbovirus in the Flavivirus family transmitted by the bite of Aedes mosquitoes, with cases reported so far in Africa, Asia, and the Americas (particularly southern coastal and island destinations).
- It also is transmitted via transfusion of blood, sexual contact, and mother to fetus.
- There is theoretical risk for fetal microcephaly, intracranial calcifications, and other brain and eye abnormalities.
- Only 1 in 5 affected patients show any systemic manifestations of infection, including self-limited flulike symptoms and nonspecific exanthema, typically sparing acral sites and occurring within 1 to 2 weeks of virus exposure.
- Testing is recommended for pregnant women with possible Zika exposure (ie, travel to an area with active transmission of Zika virus, unprotected sex with a male with this travel history).
- Diagnosis can be made through state health departments, employing real-time reverse transcriptase–polymerase chain reaction (rRT-PCR) or enzyme-linked immunosorbent assay the week after symptom onset using serum, or rRT-PCR 2 weeks after symptom onset using urine. Further antibody testing can be done if a false-negative is suspected, but false-positives also are possible if a patient was exposed to or vaccinated against other flaviviruses (eg, dengue virus, West Nile virus, yellow fever virus)
- Testing is inaccurate if ordered within 7 days or more than 12 weeks following presumed exposure.
- If positive or inconclusive testing arises, serial fetal ultrasonography should be considered; if testing is negative, then a single fetal ultrasound is recommended to detect Zika abnormalities.
- Test results are automatically reported to respective state health departments.
- There is no treatment of this infection aside from supportive care.
What’s the issue?
As with any new outbreak, the applicability to the general population and true risks remain to be seen. Each of our clinics recalls the stark changes in patient intake and screening questions with infections as ubiquitous as methicillin-resistant Staphylococcus aureus to much rarer exposures such as Ebola virus, each with progressive understanding of risk groups, disease manifestations, and eradication and prevention measures.
By mid-June 2016, 30 hits on PubMed addressing Zika had already been cited just within the month, outlining various aspects of the infection, and many specialties, particularly neurology, obstetrics, primary care, infectious disease, and dermatology, are weighing in. Unfortunately, the majority of cases of primary Zika infection do not manifest with skin or systemic symptoms, and even cases that do are nonspecific, exanthematous, and flulike.
Vague as it may be so far, it is nonetheless imperative that clinicians be familiar with what is concretely known about Zika virus and acquaint ourselves with the travel distribution and restrictions, disease risk factors, known sequelae, testing availability and limitations, and reporting guidelines. From personal experience, as I traveled to Belize earlier this year during my first trimester of pregnancy before the travel restrictions were outlined, even obstetricians are not wholly familiar with the manner in which to order testing and the appropriate window to do so. I have been asymptomatic, my blood was drawn in a period of time that exceeded the interval for accurate results (as outlined above) and was therefore inappropriately recommended/ordered, and now serial fetal ultrasonography is being implemented every few weeks.
With lack of ubiquitous knowledge about the infection, clinicians are not universally certain of the appropriate next steps when a patient presents with Zika risk factors, and therefore anxiety remains high for pregnant patients and their contacts. The Centers for Disease Control and Prevention website is the official home base, and we should review it and await their further evolving specific recommendations as more cases unfortunately accumulate.
Have you encountered any patients this year with exposure to or symptoms of Zika infection, and what, if anything, have you outlined for them?
Inundating our popular and academic media circles is information regarding the Zika virus. A recent article by Farahnik et al in the Journal of the American Academy of Dermatology (2016;74:1286-1287) briefly outlines what is known about Zika infection thus far and its dermatologic manifestations. Pairing this article with Centers for Disease Control and Prevention guidelines on the topic, we are presented with an evolving introduction to this new entity. Here’s what we know:
- It is a single-stranded RNA arbovirus in the Flavivirus family transmitted by the bite of Aedes mosquitoes, with cases reported so far in Africa, Asia, and the Americas (particularly southern coastal and island destinations).
- It also is transmitted via transfusion of blood, sexual contact, and mother to fetus.
- There is theoretical risk for fetal microcephaly, intracranial calcifications, and other brain and eye abnormalities.
- Only 1 in 5 affected patients show any systemic manifestations of infection, including self-limited flulike symptoms and nonspecific exanthema, typically sparing acral sites and occurring within 1 to 2 weeks of virus exposure.
- Testing is recommended for pregnant women with possible Zika exposure (ie, travel to an area with active transmission of Zika virus, unprotected sex with a male with this travel history).
- Diagnosis can be made through state health departments, employing real-time reverse transcriptase–polymerase chain reaction (rRT-PCR) or enzyme-linked immunosorbent assay the week after symptom onset using serum, or rRT-PCR 2 weeks after symptom onset using urine. Further antibody testing can be done if a false-negative is suspected, but false-positives also are possible if a patient was exposed to or vaccinated against other flaviviruses (eg, dengue virus, West Nile virus, yellow fever virus)
- Testing is inaccurate if ordered within 7 days or more than 12 weeks following presumed exposure.
- If positive or inconclusive testing arises, serial fetal ultrasonography should be considered; if testing is negative, then a single fetal ultrasound is recommended to detect Zika abnormalities.
- Test results are automatically reported to respective state health departments.
- There is no treatment of this infection aside from supportive care.
What’s the issue?
As with any new outbreak, the applicability to the general population and true risks remain to be seen. Each of our clinics recalls the stark changes in patient intake and screening questions with infections as ubiquitous as methicillin-resistant Staphylococcus aureus to much rarer exposures such as Ebola virus, each with progressive understanding of risk groups, disease manifestations, and eradication and prevention measures.
By mid-June 2016, 30 hits on PubMed addressing Zika had already been cited just within the month, outlining various aspects of the infection, and many specialties, particularly neurology, obstetrics, primary care, infectious disease, and dermatology, are weighing in. Unfortunately, the majority of cases of primary Zika infection do not manifest with skin or systemic symptoms, and even cases that do are nonspecific, exanthematous, and flulike.
Vague as it may be so far, it is nonetheless imperative that clinicians be familiar with what is concretely known about Zika virus and acquaint ourselves with the travel distribution and restrictions, disease risk factors, known sequelae, testing availability and limitations, and reporting guidelines. From personal experience, as I traveled to Belize earlier this year during my first trimester of pregnancy before the travel restrictions were outlined, even obstetricians are not wholly familiar with the manner in which to order testing and the appropriate window to do so. I have been asymptomatic, my blood was drawn in a period of time that exceeded the interval for accurate results (as outlined above) and was therefore inappropriately recommended/ordered, and now serial fetal ultrasonography is being implemented every few weeks.
With lack of ubiquitous knowledge about the infection, clinicians are not universally certain of the appropriate next steps when a patient presents with Zika risk factors, and therefore anxiety remains high for pregnant patients and their contacts. The Centers for Disease Control and Prevention website is the official home base, and we should review it and await their further evolving specific recommendations as more cases unfortunately accumulate.
Have you encountered any patients this year with exposure to or symptoms of Zika infection, and what, if anything, have you outlined for them?
Skin Lesions in Patients Treated With Imatinib Mesylate: A 5-Year Prospective Study
Imatinib mesylate (IM) represents the first-line treatment of chronic myeloid leukemia (CML) and gastrointestinal stromal tumors (GISTs). Its pharmacological activity is related to a specific action on several tyrosine kinases in different tumors, including Bcr-Abl in CML, c-Kit (CD117) in GIST, and platelet-derived growth factor receptor in dermatofibrosarcoma protuberans.1,2
Imatinib mesylate has been shown to improve progression-free survival and overall survival2; however, it also has several side effects. Among the adverse effects (AEs), less than 10% are nonhematologic, such as nausea, vomiting, diarrhea, muscle cramps, and cutaneous reactions.3,4
We followed patients who were treated with IM for 5 years to identify AEs of therapy.
Methods
The aim of this prospective study was to identify and collect data regarding IM cutaneous side effects so that clinicians can detect AEs early and differentiate them from AEs caused by other medications. All patients were subjected to a median of 5 years’ follow-up. We included all the patients treated with IM and excluded patients who had a history of eczematous dermatitis, psoriasis, renal impairment, or dyshidrosis palmoplantar. Before starting IM, all patients presented for a dermatologic visit. They were subsequently evaluated every 3 months.
The incidence rate was defined as the ratio of patients with cutaneous side effects and the total patients treated with IM. Furthermore, we calculated the ratio between each class of patient with a specific cutaneous manifestation and the entire cohort of patients with cutaneous side effects related to IM.
When necessary, microbiological, serological, and histopathological analyses were performed.
Results
In 60 months, we followed 220 patients treated with IM. Among them, 55 (25%) developed cutaneous side effects (35 males; 20 females). The incidence rate of the patients with cutaneous side effects was 1:4. The median age of the entire cohort was 52.5 years. Fifty patients were being treated for CML and 5 for GISTs. All patients received IM at a dosage of 400 mg daily.
The following skin diseases were observed in patients treated with IM (Table): 19 patients with maculopapular rash with pruritus (no maculopapular rash without pruritus was detected), 7 patients with eczematous dermatitis such as stasis dermatitis and seborrheic dermatitis, 6 patients with onychodystrophy melanonychia (Figure 1), 5 patients with psoriasis, 5 patients with skin cancers including basal cell carcinoma (BCC)(Figure 2), 3 patients with periorbital edema (Figure 3), 3 patients with mycosis, 3 patients with dermatofibromas, 2 patients with dyshidrosis palmoplantar, 1 patient with pityriasis rosea–like eruption (Figure 4), and 1 patient with actinic keratoses on the face. No hypopigmentation or hyperpigmentation, excluding the individual case of melanonychia, was observed.
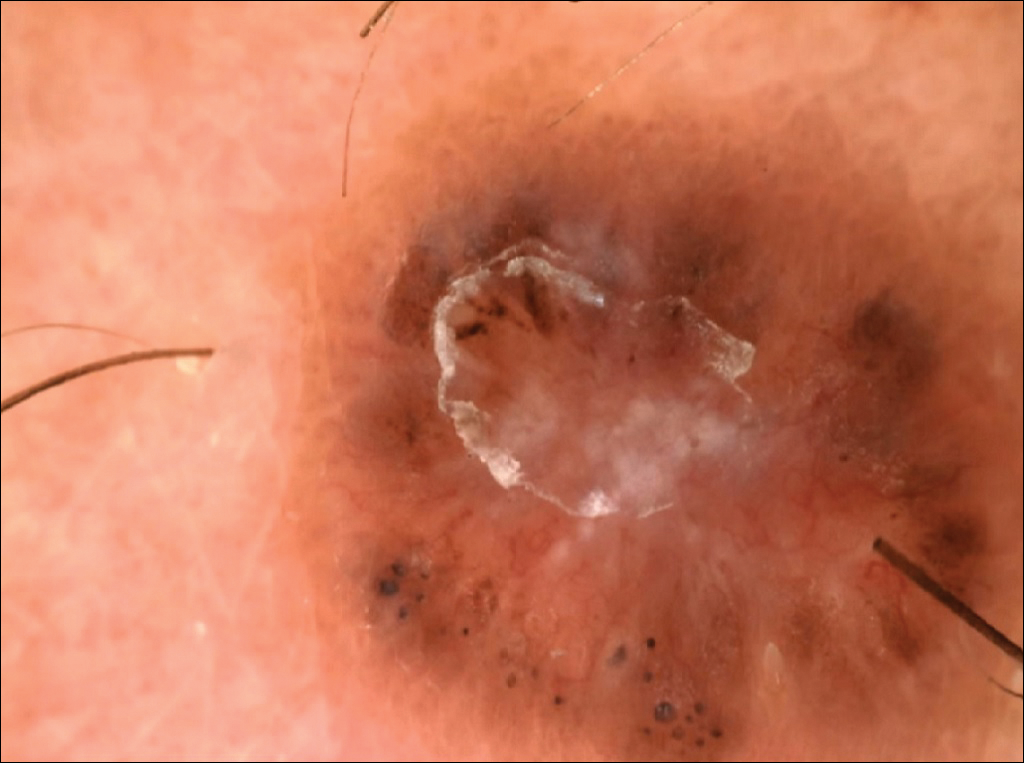
All cutaneous diseases reported in this study appeared after IM therapy (median, 3.8 months). The median time to onset for each cutaneous disorder is reported in the Table. During the first dermatologic visit before starting IM therapy, none of the patients showed any of these cutaneous diseases.
The adverse cutaneous reactions were treated with appropriate drugs. Generally, eczematous dermatitis was treated using topical steroids, emollients, and oral antihistamines. In patients with maculopapular rash with pruritus, oral corticosteroids (eg, betamethasone 3 mg daily or prednisolone 1 mg/kg) in association with antihistamine was necessary. Psoriasis was completely improved with topical betamethasone 0.5 mg and calcipotriol 50 µg. Skin cancers were treated with surgical excision with histologic examination. All treatments are outlined in the Table.
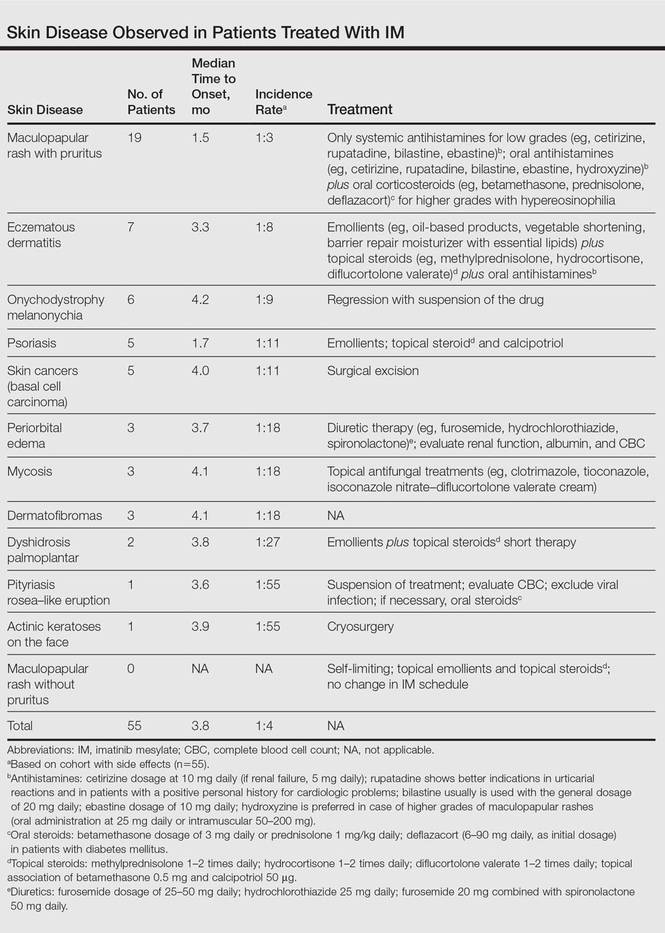
Imatinib mesylate therapy was suspended in 2 patients with maculopapular rash with moderate to severe pruritus; however, despite the temporary suspension of the drug and the appropriate therapies (corticosteroids and antihistamines), cutaneous side effects reappeared 7 to 10 days after therapy resumed. Therefore, the treatment was permanently suspended in these 2 cases and IM was replaced with erlotinib, a second-generation Bcr-Abl tyrosine kinase inhibitor.
Comment
The introduction of IM for the treatment of GIST and CML has changed the history of these diseases. The drug typically is well tolerated and few patients have reported severe AEs. Mild skin reactions are relatively frequent, ranging from 7% to 21% of patients treated.3 In our case, the percentage was relatively higher (25%), likely because of close monitoring of patients, with an increase in the incidence rate.
Imatinib mesylate cutaneous reactions are dose dependent.4 Indeed, in all our cases, the cutaneous reactions arose with an IM dosage of 400 mg daily, which is compatible with the definition of dose-independent cutaneous AEs.
The most common cutaneous AEs reported in the literature were swelling/edema and maculopapular rash. Swelling is the most common AE described during therapy with IM with an incidence of 63% to 84%.5 Swelling often involves the periorbital area and occurs approximately 6 weeks after starting IM. Although its pathogenesis is uncertain, it has been shown that IM blocks the platelet-derived growth factor receptor expressed on blood vessels that regulates the transportation transcapillary. The inhibition of this receptor can lead to increased pore pressure, resulting in edema and erythema. Maculopapular eruptions (50% of cases) often affect the trunk and the limbs and are accompanied by pruritus. Commonly, these rashes arise after 9 weeks of IM therapy. These eruptions are self-limiting and only topical emollients and steroids are required, without any change in IM schedule. To treat maculopapular eruptions with pruritus, oral steroids and antihistamines may be helpful, without suspending IM treatment. When grade 2 or 3 pruriginous maculopapular eruptions arise, the suspension of IM combined with steroids and antihistamines may be necessary. When the readministration of IM is required, it is mandatory to start IM at a lower dose (50–100 mg/d), administering prednisolone 0.5 to 1.0 mg/kg daily. Then, the steroid gradually can be tapered.6 Critical cutaneous AEs that are resistant to supportive measures warrant suspension of IM therapy. However, the incidence of this event is small (<1% of all patients).7
Regarding severe cutaneous AEs from IM therapy, Hsiao et al8 reported the case of Stevens-Johnson syndrome. In this case, IM was immediately stopped and systemic steroids were started. Rarely, erythroderma (grade 4 toxicity) can develop for which a prompt and perpetual suspension of IM is necessary and supportive care therapy with oral and topical steroids is recommended.9
Hyperpigmentation induced by IM, mostly in patients with Fitzpatrick skin types V to VI and with a general prevalence of 16% to 40% in treated patients, often is related to a mutation of c-Kit or other kinases that are activated rather than inhibited by the drug, resulting in overstimulation of melanogenesis.10 The prevalence of Fitzpatrick skin types I to III determined the absence of pigmentation changes in our cohort, excluding melanonychia. Hyperpigmentation was observed in the skin as well as the appendages such as nails, resulting in melanonychia (Figure 1). However, Brazzelli et al11 reported hypopigmentation in 5 white patients treated with IM; furthermore, they found a direct correlation between hypopigmentation and development of skin cancers in these patients. The susceptibility to develop skin cancers may persist, even without a clear manifestation of hypopigmentation, as reported in the current analysis. We documented BCC in 5 patients, 1 patient developed actinic keratoses, and 3 patients developed dermatofibromas. However, these neoplasms probably were not provoked by IM. On the contrary, we did not note squamous cell carcinoma, which was reported by Baskaynak et al12 in 2 CML patients treated with IM.
The administration of IM can be associated with exacerbation of psoriasis. Paradoxically, in genetically predisposed individuals, tumor necrosis factor α (TNF-α) antagonists, such as IM, seem to induce psoriasis, producing IFN-α rather than TNF-α and increasing inflammation.13 In fact, some research shows induction of psoriasis by anti–TNF-α drugs.14-16 Two cases of IM associated with psoriasis have been reported, and both cases represented an exacerbation of previously diagnosed psoriasis.13,17 On the contrary, in our analysis we reported 5 cases of psoriasis vulgaris induced by IM administration. Our patients developed cutaneous psoriatic lesions approximately 1.7 months after the start of IM therapy.
The pityriasis rosea–like eruption (Figure 4) presented as nonpruritic, erythematous, scaly patches on the trunk and extremities, and arose 3.6 months after the start of treatment. This particular cutaneous AE is rare. In 3 case reports, the IM dosage also was 400 mg daily.18-20 The pathophysiology of this rare skin reaction stems from the pharmacological effect of IM rather than a hypersensitivity reaction.18
Deininger et al7 reported that patients with a high basophil count (>20%) rarely show urticarial eruptions after IM due to histamine release from basophils. Premedication with an antihistamine was helpful and the urticarial eruption resolved after normalization in basophil count.7
Given the importance of IM for patients who have limited therapeutic alternatives for their disease and the ability to safely treat the cutaneous AEs, as demonstrated in our analysis, the suspension of IM for dermatological complications is necessary only in rare cases, as shown by the low number of patients (n=2) who had to discontinue therapy. The cutaneous AEs should be diagnosed and treated early with less impact on chemotherapy treatments. The administration of IM should involve a coordinated effort among oncologists and dermatologists to prevent important complications.
- Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031-1037.
- Scheinfeld N. Imatinib mesylate and dermatology part 2: a review of the cutaneous side effects of imatinib mesylate. J Drugs Dermatol. 2006;5:228-231.
- Breccia M, Carmosimo I, Russo E, et al. Early and tardive skin adverse events in chronic myeloid leukaemia patients treated with imatinib. Eur J Haematol. 2005;74:121-123.
- Ugurel S, Hildebrand R, Dippel E, et al. Dose dependent severe cutaneous reactions to imatinib. Br J Cancer. 2003;88:1157-1159.
- Valeyrie L, Bastuji-Garin S, Revuz J, et al. Adverse cutaneous reactions to imatinib (STI571) in Philadelphia chromosome-positive leukaemias: a prospective study of 54 patients. J Am Acad Dermatol. 2003;48:201-206.
- Scott LC, White JD, Reid R, et al. Management of skin toxicity related to the use of imatinibnmesylate (STI571, GlivecTM) for advanced stage gastrointestinal stromal tumors. Sarcoma. 2005;9:157-160.
- Deininger MW, O’Brien SG, Ford JM, et al. Practical management of patients with chronic myeloid leukemia receiving imatinib. J Clin Oncol. 2003;21:1637-1647.
- Hsiao LT, Chung HM, Lin JT, et al. Stevens-Johnson syndrome after treatment with STI571: a case report. Br J Haematol. 2002;117:620-622.
- Sehgal VN, Srivastava G, Sardana K. Erythroderma/exfoliative dermatitis: a synopsis. Int J Dermatol. 2004;43:39-47.
- Pietras K, Pahler J, Bergers G, et al. Functions of paracrine PDGF signaling in the proangiogenic tumor stroma revealed by pharmacological targeting. PLoS Med. 2008;5:e19.
- Brazzelli V, Prestinari F, Barbagallo T, et al. A long-term time course of colorimetric assessment of the effects of imatinib mesylate on skin pigmentation: a study of five patients. J Eur Acad Dermatol Venerol. 2007;21:384-387.
- Baskaynak G, Kreuzer KA, Schwarz M, et al. Squamous cutaneous epithelial cell carcinoma in two CML patients with progressive disease under imatinib treatment. Eur J Haematol. 2003;70:231-234.
- Cheng H, Geist DE, Piperdi M, et al. Management of imatinib-related exacerbation of psoriasis in a patient with a gastrointestinal stromal tumor. Australas J Dermatol. 2009;50:41-43.
- Faillace C, Duarte GV, Cunha RS, et al. Severe infliximab-induced psoriasis treated with adalimumab switching. Int J Dermatol. 2013;52:234-238.
- Iborra M, Beltrán B, Bastida G, et al. Infliximab and adalimumab-induced psoriasis in Crohn’s disease: a aradoxical side effect. J Crohns Colitis. 2011;5:157-161.
- Fernandes IC, Torres T, Sanches M, et al. Psoriasis induced by infliximab. Acta Med Port. 2011;24:709-712.
- Woo SM, Huh CH, Park KC, et al. Exacerbation of psoriasis in a chronic myelogenous leukemia patient treated with imatinib. J Dermatol. 2007;34:724-726.
- Brazzelli V, Prestinari F, Roveda E, et al. Pytiriasis rosea-like eruption during treatment with imatinib mesylate. description of 3 cases. J Am Acad Dermatol. 2005;53:240-243.
- Konstantapoulos K, Papadogianni A, Dimopoulou M, et al. Pytriasis rosea associated with imatinib (STI571, Gleevec). Dermatology. 2002;205:172-173.
- Cho AY, Kim DH, Im M, et al. Pityriasis rosealike drug eruption induced by imatinib mesylate (Gleevec). Ann Dermatol. 2011;23(suppl 3):360-363.
Imatinib mesylate (IM) represents the first-line treatment of chronic myeloid leukemia (CML) and gastrointestinal stromal tumors (GISTs). Its pharmacological activity is related to a specific action on several tyrosine kinases in different tumors, including Bcr-Abl in CML, c-Kit (CD117) in GIST, and platelet-derived growth factor receptor in dermatofibrosarcoma protuberans.1,2
Imatinib mesylate has been shown to improve progression-free survival and overall survival2; however, it also has several side effects. Among the adverse effects (AEs), less than 10% are nonhematologic, such as nausea, vomiting, diarrhea, muscle cramps, and cutaneous reactions.3,4
We followed patients who were treated with IM for 5 years to identify AEs of therapy.
Methods
The aim of this prospective study was to identify and collect data regarding IM cutaneous side effects so that clinicians can detect AEs early and differentiate them from AEs caused by other medications. All patients were subjected to a median of 5 years’ follow-up. We included all the patients treated with IM and excluded patients who had a history of eczematous dermatitis, psoriasis, renal impairment, or dyshidrosis palmoplantar. Before starting IM, all patients presented for a dermatologic visit. They were subsequently evaluated every 3 months.
The incidence rate was defined as the ratio of patients with cutaneous side effects and the total patients treated with IM. Furthermore, we calculated the ratio between each class of patient with a specific cutaneous manifestation and the entire cohort of patients with cutaneous side effects related to IM.
When necessary, microbiological, serological, and histopathological analyses were performed.
Results
In 60 months, we followed 220 patients treated with IM. Among them, 55 (25%) developed cutaneous side effects (35 males; 20 females). The incidence rate of the patients with cutaneous side effects was 1:4. The median age of the entire cohort was 52.5 years. Fifty patients were being treated for CML and 5 for GISTs. All patients received IM at a dosage of 400 mg daily.
The following skin diseases were observed in patients treated with IM (Table): 19 patients with maculopapular rash with pruritus (no maculopapular rash without pruritus was detected), 7 patients with eczematous dermatitis such as stasis dermatitis and seborrheic dermatitis, 6 patients with onychodystrophy melanonychia (Figure 1), 5 patients with psoriasis, 5 patients with skin cancers including basal cell carcinoma (BCC)(Figure 2), 3 patients with periorbital edema (Figure 3), 3 patients with mycosis, 3 patients with dermatofibromas, 2 patients with dyshidrosis palmoplantar, 1 patient with pityriasis rosea–like eruption (Figure 4), and 1 patient with actinic keratoses on the face. No hypopigmentation or hyperpigmentation, excluding the individual case of melanonychia, was observed.




All cutaneous diseases reported in this study appeared after IM therapy (median, 3.8 months). The median time to onset for each cutaneous disorder is reported in the Table. During the first dermatologic visit before starting IM therapy, none of the patients showed any of these cutaneous diseases.
The adverse cutaneous reactions were treated with appropriate drugs. Generally, eczematous dermatitis was treated using topical steroids, emollients, and oral antihistamines. In patients with maculopapular rash with pruritus, oral corticosteroids (eg, betamethasone 3 mg daily or prednisolone 1 mg/kg) in association with antihistamine was necessary. Psoriasis was completely improved with topical betamethasone 0.5 mg and calcipotriol 50 µg. Skin cancers were treated with surgical excision with histologic examination. All treatments are outlined in the Table.

Imatinib mesylate therapy was suspended in 2 patients with maculopapular rash with moderate to severe pruritus; however, despite the temporary suspension of the drug and the appropriate therapies (corticosteroids and antihistamines), cutaneous side effects reappeared 7 to 10 days after therapy resumed. Therefore, the treatment was permanently suspended in these 2 cases and IM was replaced with erlotinib, a second-generation Bcr-Abl tyrosine kinase inhibitor.
Comment
The introduction of IM for the treatment of GIST and CML has changed the history of these diseases. The drug typically is well tolerated and few patients have reported severe AEs. Mild skin reactions are relatively frequent, ranging from 7% to 21% of patients treated.3 In our case, the percentage was relatively higher (25%), likely because of close monitoring of patients, with an increase in the incidence rate.
Imatinib mesylate cutaneous reactions are dose dependent.4 Indeed, in all our cases, the cutaneous reactions arose with an IM dosage of 400 mg daily, which is compatible with the definition of dose-independent cutaneous AEs.
The most common cutaneous AEs reported in the literature were swelling/edema and maculopapular rash. Swelling is the most common AE described during therapy with IM with an incidence of 63% to 84%.5 Swelling often involves the periorbital area and occurs approximately 6 weeks after starting IM. Although its pathogenesis is uncertain, it has been shown that IM blocks the platelet-derived growth factor receptor expressed on blood vessels that regulates the transportation transcapillary. The inhibition of this receptor can lead to increased pore pressure, resulting in edema and erythema. Maculopapular eruptions (50% of cases) often affect the trunk and the limbs and are accompanied by pruritus. Commonly, these rashes arise after 9 weeks of IM therapy. These eruptions are self-limiting and only topical emollients and steroids are required, without any change in IM schedule. To treat maculopapular eruptions with pruritus, oral steroids and antihistamines may be helpful, without suspending IM treatment. When grade 2 or 3 pruriginous maculopapular eruptions arise, the suspension of IM combined with steroids and antihistamines may be necessary. When the readministration of IM is required, it is mandatory to start IM at a lower dose (50–100 mg/d), administering prednisolone 0.5 to 1.0 mg/kg daily. Then, the steroid gradually can be tapered.6 Critical cutaneous AEs that are resistant to supportive measures warrant suspension of IM therapy. However, the incidence of this event is small (<1% of all patients).7
Regarding severe cutaneous AEs from IM therapy, Hsiao et al8 reported the case of Stevens-Johnson syndrome. In this case, IM was immediately stopped and systemic steroids were started. Rarely, erythroderma (grade 4 toxicity) can develop for which a prompt and perpetual suspension of IM is necessary and supportive care therapy with oral and topical steroids is recommended.9
Hyperpigmentation induced by IM, mostly in patients with Fitzpatrick skin types V to VI and with a general prevalence of 16% to 40% in treated patients, often is related to a mutation of c-Kit or other kinases that are activated rather than inhibited by the drug, resulting in overstimulation of melanogenesis.10 The prevalence of Fitzpatrick skin types I to III determined the absence of pigmentation changes in our cohort, excluding melanonychia. Hyperpigmentation was observed in the skin as well as the appendages such as nails, resulting in melanonychia (Figure 1). However, Brazzelli et al11 reported hypopigmentation in 5 white patients treated with IM; furthermore, they found a direct correlation between hypopigmentation and development of skin cancers in these patients. The susceptibility to develop skin cancers may persist, even without a clear manifestation of hypopigmentation, as reported in the current analysis. We documented BCC in 5 patients, 1 patient developed actinic keratoses, and 3 patients developed dermatofibromas. However, these neoplasms probably were not provoked by IM. On the contrary, we did not note squamous cell carcinoma, which was reported by Baskaynak et al12 in 2 CML patients treated with IM.
The administration of IM can be associated with exacerbation of psoriasis. Paradoxically, in genetically predisposed individuals, tumor necrosis factor α (TNF-α) antagonists, such as IM, seem to induce psoriasis, producing IFN-α rather than TNF-α and increasing inflammation.13 In fact, some research shows induction of psoriasis by anti–TNF-α drugs.14-16 Two cases of IM associated with psoriasis have been reported, and both cases represented an exacerbation of previously diagnosed psoriasis.13,17 On the contrary, in our analysis we reported 5 cases of psoriasis vulgaris induced by IM administration. Our patients developed cutaneous psoriatic lesions approximately 1.7 months after the start of IM therapy.
The pityriasis rosea–like eruption (Figure 4) presented as nonpruritic, erythematous, scaly patches on the trunk and extremities, and arose 3.6 months after the start of treatment. This particular cutaneous AE is rare. In 3 case reports, the IM dosage also was 400 mg daily.18-20 The pathophysiology of this rare skin reaction stems from the pharmacological effect of IM rather than a hypersensitivity reaction.18
Deininger et al7 reported that patients with a high basophil count (>20%) rarely show urticarial eruptions after IM due to histamine release from basophils. Premedication with an antihistamine was helpful and the urticarial eruption resolved after normalization in basophil count.7
Given the importance of IM for patients who have limited therapeutic alternatives for their disease and the ability to safely treat the cutaneous AEs, as demonstrated in our analysis, the suspension of IM for dermatological complications is necessary only in rare cases, as shown by the low number of patients (n=2) who had to discontinue therapy. The cutaneous AEs should be diagnosed and treated early with less impact on chemotherapy treatments. The administration of IM should involve a coordinated effort among oncologists and dermatologists to prevent important complications.
Imatinib mesylate (IM) represents the first-line treatment of chronic myeloid leukemia (CML) and gastrointestinal stromal tumors (GISTs). Its pharmacological activity is related to a specific action on several tyrosine kinases in different tumors, including Bcr-Abl in CML, c-Kit (CD117) in GIST, and platelet-derived growth factor receptor in dermatofibrosarcoma protuberans.1,2
Imatinib mesylate has been shown to improve progression-free survival and overall survival2; however, it also has several side effects. Among the adverse effects (AEs), less than 10% are nonhematologic, such as nausea, vomiting, diarrhea, muscle cramps, and cutaneous reactions.3,4
We followed patients who were treated with IM for 5 years to identify AEs of therapy.
Methods
The aim of this prospective study was to identify and collect data regarding IM cutaneous side effects so that clinicians can detect AEs early and differentiate them from AEs caused by other medications. All patients were subjected to a median of 5 years’ follow-up. We included all the patients treated with IM and excluded patients who had a history of eczematous dermatitis, psoriasis, renal impairment, or dyshidrosis palmoplantar. Before starting IM, all patients presented for a dermatologic visit. They were subsequently evaluated every 3 months.
The incidence rate was defined as the ratio of patients with cutaneous side effects and the total patients treated with IM. Furthermore, we calculated the ratio between each class of patient with a specific cutaneous manifestation and the entire cohort of patients with cutaneous side effects related to IM.
When necessary, microbiological, serological, and histopathological analyses were performed.
Results
In 60 months, we followed 220 patients treated with IM. Among them, 55 (25%) developed cutaneous side effects (35 males; 20 females). The incidence rate of the patients with cutaneous side effects was 1:4. The median age of the entire cohort was 52.5 years. Fifty patients were being treated for CML and 5 for GISTs. All patients received IM at a dosage of 400 mg daily.
The following skin diseases were observed in patients treated with IM (Table): 19 patients with maculopapular rash with pruritus (no maculopapular rash without pruritus was detected), 7 patients with eczematous dermatitis such as stasis dermatitis and seborrheic dermatitis, 6 patients with onychodystrophy melanonychia (Figure 1), 5 patients with psoriasis, 5 patients with skin cancers including basal cell carcinoma (BCC)(Figure 2), 3 patients with periorbital edema (Figure 3), 3 patients with mycosis, 3 patients with dermatofibromas, 2 patients with dyshidrosis palmoplantar, 1 patient with pityriasis rosea–like eruption (Figure 4), and 1 patient with actinic keratoses on the face. No hypopigmentation or hyperpigmentation, excluding the individual case of melanonychia, was observed.




All cutaneous diseases reported in this study appeared after IM therapy (median, 3.8 months). The median time to onset for each cutaneous disorder is reported in the Table. During the first dermatologic visit before starting IM therapy, none of the patients showed any of these cutaneous diseases.
The adverse cutaneous reactions were treated with appropriate drugs. Generally, eczematous dermatitis was treated using topical steroids, emollients, and oral antihistamines. In patients with maculopapular rash with pruritus, oral corticosteroids (eg, betamethasone 3 mg daily or prednisolone 1 mg/kg) in association with antihistamine was necessary. Psoriasis was completely improved with topical betamethasone 0.5 mg and calcipotriol 50 µg. Skin cancers were treated with surgical excision with histologic examination. All treatments are outlined in the Table.

Imatinib mesylate therapy was suspended in 2 patients with maculopapular rash with moderate to severe pruritus; however, despite the temporary suspension of the drug and the appropriate therapies (corticosteroids and antihistamines), cutaneous side effects reappeared 7 to 10 days after therapy resumed. Therefore, the treatment was permanently suspended in these 2 cases and IM was replaced with erlotinib, a second-generation Bcr-Abl tyrosine kinase inhibitor.
Comment
The introduction of IM for the treatment of GIST and CML has changed the history of these diseases. The drug typically is well tolerated and few patients have reported severe AEs. Mild skin reactions are relatively frequent, ranging from 7% to 21% of patients treated.3 In our case, the percentage was relatively higher (25%), likely because of close monitoring of patients, with an increase in the incidence rate.
Imatinib mesylate cutaneous reactions are dose dependent.4 Indeed, in all our cases, the cutaneous reactions arose with an IM dosage of 400 mg daily, which is compatible with the definition of dose-independent cutaneous AEs.
The most common cutaneous AEs reported in the literature were swelling/edema and maculopapular rash. Swelling is the most common AE described during therapy with IM with an incidence of 63% to 84%.5 Swelling often involves the periorbital area and occurs approximately 6 weeks after starting IM. Although its pathogenesis is uncertain, it has been shown that IM blocks the platelet-derived growth factor receptor expressed on blood vessels that regulates the transportation transcapillary. The inhibition of this receptor can lead to increased pore pressure, resulting in edema and erythema. Maculopapular eruptions (50% of cases) often affect the trunk and the limbs and are accompanied by pruritus. Commonly, these rashes arise after 9 weeks of IM therapy. These eruptions are self-limiting and only topical emollients and steroids are required, without any change in IM schedule. To treat maculopapular eruptions with pruritus, oral steroids and antihistamines may be helpful, without suspending IM treatment. When grade 2 or 3 pruriginous maculopapular eruptions arise, the suspension of IM combined with steroids and antihistamines may be necessary. When the readministration of IM is required, it is mandatory to start IM at a lower dose (50–100 mg/d), administering prednisolone 0.5 to 1.0 mg/kg daily. Then, the steroid gradually can be tapered.6 Critical cutaneous AEs that are resistant to supportive measures warrant suspension of IM therapy. However, the incidence of this event is small (<1% of all patients).7
Regarding severe cutaneous AEs from IM therapy, Hsiao et al8 reported the case of Stevens-Johnson syndrome. In this case, IM was immediately stopped and systemic steroids were started. Rarely, erythroderma (grade 4 toxicity) can develop for which a prompt and perpetual suspension of IM is necessary and supportive care therapy with oral and topical steroids is recommended.9
Hyperpigmentation induced by IM, mostly in patients with Fitzpatrick skin types V to VI and with a general prevalence of 16% to 40% in treated patients, often is related to a mutation of c-Kit or other kinases that are activated rather than inhibited by the drug, resulting in overstimulation of melanogenesis.10 The prevalence of Fitzpatrick skin types I to III determined the absence of pigmentation changes in our cohort, excluding melanonychia. Hyperpigmentation was observed in the skin as well as the appendages such as nails, resulting in melanonychia (Figure 1). However, Brazzelli et al11 reported hypopigmentation in 5 white patients treated with IM; furthermore, they found a direct correlation between hypopigmentation and development of skin cancers in these patients. The susceptibility to develop skin cancers may persist, even without a clear manifestation of hypopigmentation, as reported in the current analysis. We documented BCC in 5 patients, 1 patient developed actinic keratoses, and 3 patients developed dermatofibromas. However, these neoplasms probably were not provoked by IM. On the contrary, we did not note squamous cell carcinoma, which was reported by Baskaynak et al12 in 2 CML patients treated with IM.
The administration of IM can be associated with exacerbation of psoriasis. Paradoxically, in genetically predisposed individuals, tumor necrosis factor α (TNF-α) antagonists, such as IM, seem to induce psoriasis, producing IFN-α rather than TNF-α and increasing inflammation.13 In fact, some research shows induction of psoriasis by anti–TNF-α drugs.14-16 Two cases of IM associated with psoriasis have been reported, and both cases represented an exacerbation of previously diagnosed psoriasis.13,17 On the contrary, in our analysis we reported 5 cases of psoriasis vulgaris induced by IM administration. Our patients developed cutaneous psoriatic lesions approximately 1.7 months after the start of IM therapy.
The pityriasis rosea–like eruption (Figure 4) presented as nonpruritic, erythematous, scaly patches on the trunk and extremities, and arose 3.6 months after the start of treatment. This particular cutaneous AE is rare. In 3 case reports, the IM dosage also was 400 mg daily.18-20 The pathophysiology of this rare skin reaction stems from the pharmacological effect of IM rather than a hypersensitivity reaction.18
Deininger et al7 reported that patients with a high basophil count (>20%) rarely show urticarial eruptions after IM due to histamine release from basophils. Premedication with an antihistamine was helpful and the urticarial eruption resolved after normalization in basophil count.7
Given the importance of IM for patients who have limited therapeutic alternatives for their disease and the ability to safely treat the cutaneous AEs, as demonstrated in our analysis, the suspension of IM for dermatological complications is necessary only in rare cases, as shown by the low number of patients (n=2) who had to discontinue therapy. The cutaneous AEs should be diagnosed and treated early with less impact on chemotherapy treatments. The administration of IM should involve a coordinated effort among oncologists and dermatologists to prevent important complications.
- Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031-1037.
- Scheinfeld N. Imatinib mesylate and dermatology part 2: a review of the cutaneous side effects of imatinib mesylate. J Drugs Dermatol. 2006;5:228-231.
- Breccia M, Carmosimo I, Russo E, et al. Early and tardive skin adverse events in chronic myeloid leukaemia patients treated with imatinib. Eur J Haematol. 2005;74:121-123.
- Ugurel S, Hildebrand R, Dippel E, et al. Dose dependent severe cutaneous reactions to imatinib. Br J Cancer. 2003;88:1157-1159.
- Valeyrie L, Bastuji-Garin S, Revuz J, et al. Adverse cutaneous reactions to imatinib (STI571) in Philadelphia chromosome-positive leukaemias: a prospective study of 54 patients. J Am Acad Dermatol. 2003;48:201-206.
- Scott LC, White JD, Reid R, et al. Management of skin toxicity related to the use of imatinibnmesylate (STI571, GlivecTM) for advanced stage gastrointestinal stromal tumors. Sarcoma. 2005;9:157-160.
- Deininger MW, O’Brien SG, Ford JM, et al. Practical management of patients with chronic myeloid leukemia receiving imatinib. J Clin Oncol. 2003;21:1637-1647.
- Hsiao LT, Chung HM, Lin JT, et al. Stevens-Johnson syndrome after treatment with STI571: a case report. Br J Haematol. 2002;117:620-622.
- Sehgal VN, Srivastava G, Sardana K. Erythroderma/exfoliative dermatitis: a synopsis. Int J Dermatol. 2004;43:39-47.
- Pietras K, Pahler J, Bergers G, et al. Functions of paracrine PDGF signaling in the proangiogenic tumor stroma revealed by pharmacological targeting. PLoS Med. 2008;5:e19.
- Brazzelli V, Prestinari F, Barbagallo T, et al. A long-term time course of colorimetric assessment of the effects of imatinib mesylate on skin pigmentation: a study of five patients. J Eur Acad Dermatol Venerol. 2007;21:384-387.
- Baskaynak G, Kreuzer KA, Schwarz M, et al. Squamous cutaneous epithelial cell carcinoma in two CML patients with progressive disease under imatinib treatment. Eur J Haematol. 2003;70:231-234.
- Cheng H, Geist DE, Piperdi M, et al. Management of imatinib-related exacerbation of psoriasis in a patient with a gastrointestinal stromal tumor. Australas J Dermatol. 2009;50:41-43.
- Faillace C, Duarte GV, Cunha RS, et al. Severe infliximab-induced psoriasis treated with adalimumab switching. Int J Dermatol. 2013;52:234-238.
- Iborra M, Beltrán B, Bastida G, et al. Infliximab and adalimumab-induced psoriasis in Crohn’s disease: a aradoxical side effect. J Crohns Colitis. 2011;5:157-161.
- Fernandes IC, Torres T, Sanches M, et al. Psoriasis induced by infliximab. Acta Med Port. 2011;24:709-712.
- Woo SM, Huh CH, Park KC, et al. Exacerbation of psoriasis in a chronic myelogenous leukemia patient treated with imatinib. J Dermatol. 2007;34:724-726.
- Brazzelli V, Prestinari F, Roveda E, et al. Pytiriasis rosea-like eruption during treatment with imatinib mesylate. description of 3 cases. J Am Acad Dermatol. 2005;53:240-243.
- Konstantapoulos K, Papadogianni A, Dimopoulou M, et al. Pytriasis rosea associated with imatinib (STI571, Gleevec). Dermatology. 2002;205:172-173.
- Cho AY, Kim DH, Im M, et al. Pityriasis rosealike drug eruption induced by imatinib mesylate (Gleevec). Ann Dermatol. 2011;23(suppl 3):360-363.
- Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031-1037.
- Scheinfeld N. Imatinib mesylate and dermatology part 2: a review of the cutaneous side effects of imatinib mesylate. J Drugs Dermatol. 2006;5:228-231.
- Breccia M, Carmosimo I, Russo E, et al. Early and tardive skin adverse events in chronic myeloid leukaemia patients treated with imatinib. Eur J Haematol. 2005;74:121-123.
- Ugurel S, Hildebrand R, Dippel E, et al. Dose dependent severe cutaneous reactions to imatinib. Br J Cancer. 2003;88:1157-1159.
- Valeyrie L, Bastuji-Garin S, Revuz J, et al. Adverse cutaneous reactions to imatinib (STI571) in Philadelphia chromosome-positive leukaemias: a prospective study of 54 patients. J Am Acad Dermatol. 2003;48:201-206.
- Scott LC, White JD, Reid R, et al. Management of skin toxicity related to the use of imatinibnmesylate (STI571, GlivecTM) for advanced stage gastrointestinal stromal tumors. Sarcoma. 2005;9:157-160.
- Deininger MW, O’Brien SG, Ford JM, et al. Practical management of patients with chronic myeloid leukemia receiving imatinib. J Clin Oncol. 2003;21:1637-1647.
- Hsiao LT, Chung HM, Lin JT, et al. Stevens-Johnson syndrome after treatment with STI571: a case report. Br J Haematol. 2002;117:620-622.
- Sehgal VN, Srivastava G, Sardana K. Erythroderma/exfoliative dermatitis: a synopsis. Int J Dermatol. 2004;43:39-47.
- Pietras K, Pahler J, Bergers G, et al. Functions of paracrine PDGF signaling in the proangiogenic tumor stroma revealed by pharmacological targeting. PLoS Med. 2008;5:e19.
- Brazzelli V, Prestinari F, Barbagallo T, et al. A long-term time course of colorimetric assessment of the effects of imatinib mesylate on skin pigmentation: a study of five patients. J Eur Acad Dermatol Venerol. 2007;21:384-387.
- Baskaynak G, Kreuzer KA, Schwarz M, et al. Squamous cutaneous epithelial cell carcinoma in two CML patients with progressive disease under imatinib treatment. Eur J Haematol. 2003;70:231-234.
- Cheng H, Geist DE, Piperdi M, et al. Management of imatinib-related exacerbation of psoriasis in a patient with a gastrointestinal stromal tumor. Australas J Dermatol. 2009;50:41-43.
- Faillace C, Duarte GV, Cunha RS, et al. Severe infliximab-induced psoriasis treated with adalimumab switching. Int J Dermatol. 2013;52:234-238.
- Iborra M, Beltrán B, Bastida G, et al. Infliximab and adalimumab-induced psoriasis in Crohn’s disease: a aradoxical side effect. J Crohns Colitis. 2011;5:157-161.
- Fernandes IC, Torres T, Sanches M, et al. Psoriasis induced by infliximab. Acta Med Port. 2011;24:709-712.
- Woo SM, Huh CH, Park KC, et al. Exacerbation of psoriasis in a chronic myelogenous leukemia patient treated with imatinib. J Dermatol. 2007;34:724-726.
- Brazzelli V, Prestinari F, Roveda E, et al. Pytiriasis rosea-like eruption during treatment with imatinib mesylate. description of 3 cases. J Am Acad Dermatol. 2005;53:240-243.
- Konstantapoulos K, Papadogianni A, Dimopoulou M, et al. Pytriasis rosea associated with imatinib (STI571, Gleevec). Dermatology. 2002;205:172-173.
- Cho AY, Kim DH, Im M, et al. Pityriasis rosealike drug eruption induced by imatinib mesylate (Gleevec). Ann Dermatol. 2011;23(suppl 3):360-363.
Practice Points
- The most common cutaneous adverse reactions from imatinib mesylate (IM) are swelling and edema.
- Maculopapular rash with pruritus is one of the most common side effects from IM and can be effectively treated with oral or systemic antihistamines.
- The onset of periorbital edema requires a complete evaluation of renal function.
Necrolytic Migratory Erythema With Recalcitrant Dermatitis as the Only Presenting Symptom
To the Editor:
A 52-year-old man presented with recalcitrant dermatitis of 6 years’ duration. He was otherwise in excellent health. On initial presentation, physical examination revealed symmetrical, erythematous, blanching plaques with areas of erosions and overlying hemorrhagic crust on the eyebrows, scalp, back, dorsal aspects of the hands, axillae, abdomen (Figure), buttocks, groin, scrotum, pubis, and lower legs. Some areas showed slight necrosis. He denied any fevers, chills, night sweats, cough, chest pain, shortness of breath, dizziness, lightheadedness, weight loss, or appetite change.
Throughout the disease course the patient had visited numerous dermatologists seeking treatment. He had response to higher doses of oral prednisone (80 mg taper), but the condition would recur at the end of an extended taper. Treatment with narrowband UVB, mycophenolate mofetil, methotrexate, acitretin, topical clobetasol, and topical pimecrolimus provided no relief. Eventually he was placed on azathioprine 100 mg twice daily, which led to near-complete resolution. Outbreaks continued every few months and required courses of prednisone.
Multiple biopsies over the years revealed subacute spongiotic or psoriasiform dermatitis. At multiple visits it was noted that during flares there were areas of crusting and mild necrosis, which led to an extensive biochemical investigation. The glucagon level was markedly elevated at 630 ng/L (reference range, 40–130 ng/L), as was insulin at 71 μIU/mL (reference range, 6–27 μIU/mL). Complete blood cell counts over the disease course showed mild normochromic normocytic anemia. The abnormal laboratory findings led to computed tomography of the abdomen, which revealed a mass in the body of the pancreas measuring 3×3.8 cm. After computed tomography, the patient underwent a laparoscopic distal pancreatectomy and splenectomy. Histologic examination revealed a well-differentiated pancreatic endocrine tumor (glucagonoma) confined to the pancreas. After the surgery, the patient’s rash resolved within a few days and he discontinued all medications.
Diagnosis of glucagonomas often is delayed due to their rarity and lack of classical signs and symptoms. The distribution of the lesions seen in necrolytic migratory erythema (NME) usually involves the inguinal crease, perineum, lower extremities, buttocks, and other intertriginous areas.1 Our patient had involvement in the typical distribution but also had involvement of the scalp, face, and upper body. The typical histology for NME is crusted psoriasiform dermatitis with a tendency for the upper epidermis to have necrosis and a vacuolated pale epidermis.2 Our patient’s histologic findings were less specific showing epidermal spongiosis with a scant lymphocytic infiltrate and at times acanthosis. The lack of classical skin findings and histology delayed diagnosis. In more than 50% of patients, metastasis has already occurred by the time the patient is diagnosed.3 Treatment is aimed at complete removal of the pancreatic tumor, which typically leads to a rapid improvement in symptoms. For patients unable to undergo surgery, chemotherapy agents and octreotide are used; unfortunately, symptoms may persist.4 The response to azathioprine in our patient suggests it is a possible alternate therapy for those with persistent NME.
This patient highlights the difficulty of diagnosing a glucagonoma when the only clinical manifestation may be NME. Moreover, skin biopsies that can sometimes be diagnostic may be nonspecific. This patient also shows a potential benefit of azathioprine in the treatment of NME.
- Shi W, Liao W, Mei X, et al. Necrolytic migratory erythema associated with glucagonoma syndrome [published online June 7, 2010]. J Clin Oncol. 2010;28:e329-e331.
- Rapini RP. Practical Dermatopathology. London, England: Elsevier Mosby; 2005.
- Oberg K, Eriksson B. Endocrine tumors of the pancreas. Best Pract Res Clin Gastroenterol. 2005;19:753-781.
- Wermers RA, Fatourechi V, Wynne AG, et al. The glucagonoma syndrome: clinical and pathologic features in 21 patients. Medicine (Baltimore). 1996;72:53-63.
To the Editor:
A 52-year-old man presented with recalcitrant dermatitis of 6 years’ duration. He was otherwise in excellent health. On initial presentation, physical examination revealed symmetrical, erythematous, blanching plaques with areas of erosions and overlying hemorrhagic crust on the eyebrows, scalp, back, dorsal aspects of the hands, axillae, abdomen (Figure), buttocks, groin, scrotum, pubis, and lower legs. Some areas showed slight necrosis. He denied any fevers, chills, night sweats, cough, chest pain, shortness of breath, dizziness, lightheadedness, weight loss, or appetite change.

Throughout the disease course the patient had visited numerous dermatologists seeking treatment. He had response to higher doses of oral prednisone (80 mg taper), but the condition would recur at the end of an extended taper. Treatment with narrowband UVB, mycophenolate mofetil, methotrexate, acitretin, topical clobetasol, and topical pimecrolimus provided no relief. Eventually he was placed on azathioprine 100 mg twice daily, which led to near-complete resolution. Outbreaks continued every few months and required courses of prednisone.
Multiple biopsies over the years revealed subacute spongiotic or psoriasiform dermatitis. At multiple visits it was noted that during flares there were areas of crusting and mild necrosis, which led to an extensive biochemical investigation. The glucagon level was markedly elevated at 630 ng/L (reference range, 40–130 ng/L), as was insulin at 71 μIU/mL (reference range, 6–27 μIU/mL). Complete blood cell counts over the disease course showed mild normochromic normocytic anemia. The abnormal laboratory findings led to computed tomography of the abdomen, which revealed a mass in the body of the pancreas measuring 3×3.8 cm. After computed tomography, the patient underwent a laparoscopic distal pancreatectomy and splenectomy. Histologic examination revealed a well-differentiated pancreatic endocrine tumor (glucagonoma) confined to the pancreas. After the surgery, the patient’s rash resolved within a few days and he discontinued all medications.
Diagnosis of glucagonomas often is delayed due to their rarity and lack of classical signs and symptoms. The distribution of the lesions seen in necrolytic migratory erythema (NME) usually involves the inguinal crease, perineum, lower extremities, buttocks, and other intertriginous areas.1 Our patient had involvement in the typical distribution but also had involvement of the scalp, face, and upper body. The typical histology for NME is crusted psoriasiform dermatitis with a tendency for the upper epidermis to have necrosis and a vacuolated pale epidermis.2 Our patient’s histologic findings were less specific showing epidermal spongiosis with a scant lymphocytic infiltrate and at times acanthosis. The lack of classical skin findings and histology delayed diagnosis. In more than 50% of patients, metastasis has already occurred by the time the patient is diagnosed.3 Treatment is aimed at complete removal of the pancreatic tumor, which typically leads to a rapid improvement in symptoms. For patients unable to undergo surgery, chemotherapy agents and octreotide are used; unfortunately, symptoms may persist.4 The response to azathioprine in our patient suggests it is a possible alternate therapy for those with persistent NME.
This patient highlights the difficulty of diagnosing a glucagonoma when the only clinical manifestation may be NME. Moreover, skin biopsies that can sometimes be diagnostic may be nonspecific. This patient also shows a potential benefit of azathioprine in the treatment of NME.
To the Editor:
A 52-year-old man presented with recalcitrant dermatitis of 6 years’ duration. He was otherwise in excellent health. On initial presentation, physical examination revealed symmetrical, erythematous, blanching plaques with areas of erosions and overlying hemorrhagic crust on the eyebrows, scalp, back, dorsal aspects of the hands, axillae, abdomen (Figure), buttocks, groin, scrotum, pubis, and lower legs. Some areas showed slight necrosis. He denied any fevers, chills, night sweats, cough, chest pain, shortness of breath, dizziness, lightheadedness, weight loss, or appetite change.

Throughout the disease course the patient had visited numerous dermatologists seeking treatment. He had response to higher doses of oral prednisone (80 mg taper), but the condition would recur at the end of an extended taper. Treatment with narrowband UVB, mycophenolate mofetil, methotrexate, acitretin, topical clobetasol, and topical pimecrolimus provided no relief. Eventually he was placed on azathioprine 100 mg twice daily, which led to near-complete resolution. Outbreaks continued every few months and required courses of prednisone.
Multiple biopsies over the years revealed subacute spongiotic or psoriasiform dermatitis. At multiple visits it was noted that during flares there were areas of crusting and mild necrosis, which led to an extensive biochemical investigation. The glucagon level was markedly elevated at 630 ng/L (reference range, 40–130 ng/L), as was insulin at 71 μIU/mL (reference range, 6–27 μIU/mL). Complete blood cell counts over the disease course showed mild normochromic normocytic anemia. The abnormal laboratory findings led to computed tomography of the abdomen, which revealed a mass in the body of the pancreas measuring 3×3.8 cm. After computed tomography, the patient underwent a laparoscopic distal pancreatectomy and splenectomy. Histologic examination revealed a well-differentiated pancreatic endocrine tumor (glucagonoma) confined to the pancreas. After the surgery, the patient’s rash resolved within a few days and he discontinued all medications.
Diagnosis of glucagonomas often is delayed due to their rarity and lack of classical signs and symptoms. The distribution of the lesions seen in necrolytic migratory erythema (NME) usually involves the inguinal crease, perineum, lower extremities, buttocks, and other intertriginous areas.1 Our patient had involvement in the typical distribution but also had involvement of the scalp, face, and upper body. The typical histology for NME is crusted psoriasiform dermatitis with a tendency for the upper epidermis to have necrosis and a vacuolated pale epidermis.2 Our patient’s histologic findings were less specific showing epidermal spongiosis with a scant lymphocytic infiltrate and at times acanthosis. The lack of classical skin findings and histology delayed diagnosis. In more than 50% of patients, metastasis has already occurred by the time the patient is diagnosed.3 Treatment is aimed at complete removal of the pancreatic tumor, which typically leads to a rapid improvement in symptoms. For patients unable to undergo surgery, chemotherapy agents and octreotide are used; unfortunately, symptoms may persist.4 The response to azathioprine in our patient suggests it is a possible alternate therapy for those with persistent NME.
This patient highlights the difficulty of diagnosing a glucagonoma when the only clinical manifestation may be NME. Moreover, skin biopsies that can sometimes be diagnostic may be nonspecific. This patient also shows a potential benefit of azathioprine in the treatment of NME.
- Shi W, Liao W, Mei X, et al. Necrolytic migratory erythema associated with glucagonoma syndrome [published online June 7, 2010]. J Clin Oncol. 2010;28:e329-e331.
- Rapini RP. Practical Dermatopathology. London, England: Elsevier Mosby; 2005.
- Oberg K, Eriksson B. Endocrine tumors of the pancreas. Best Pract Res Clin Gastroenterol. 2005;19:753-781.
- Wermers RA, Fatourechi V, Wynne AG, et al. The glucagonoma syndrome: clinical and pathologic features in 21 patients. Medicine (Baltimore). 1996;72:53-63.
- Shi W, Liao W, Mei X, et al. Necrolytic migratory erythema associated with glucagonoma syndrome [published online June 7, 2010]. J Clin Oncol. 2010;28:e329-e331.
- Rapini RP. Practical Dermatopathology. London, England: Elsevier Mosby; 2005.
- Oberg K, Eriksson B. Endocrine tumors of the pancreas. Best Pract Res Clin Gastroenterol. 2005;19:753-781.
- Wermers RA, Fatourechi V, Wynne AG, et al. The glucagonoma syndrome: clinical and pathologic features in 21 patients. Medicine (Baltimore). 1996;72:53-63.
Practice Points
- Recalcitrant dermatitis may be a symptom of internal malignancy.
- Glucagon levels are helpful in identifying glucagonomas of the pancreas.
- Although surgical excision is the preferred treatment of glucagonomas, azathioprine also can control dermatitis associated with necrolytic migratory erythema.
A Practical Overview of Pediatric Atopic Dermatitis, Part 3: Differential Diagnosis, Comorbidities, and Measurement of Disease Burden
In parts 1 and 2 of this series on atopic dermatitis (AD),1,2 the current putative pathogenesis, scoring systems for severity grading, and epidemiology were reviewed. Part 3 reviews the differential diagnosis, with an emphasis on the difficulty of differentiation from some rare but notable illnesses, as well as the recently expanding data on comorbidities that identify AD as a multisystem disorder with widespread health implications for the patient.
Differential Diagnosis for Pediatric AD
The differential diagnosis for pediatric AD includes chronic dermatoses (eg, seborrheic dermatitis, psoriasis), congenital disorders (eg, Netherton syndrome), malignant diseases (eg, cutaneous T-cell lymphoma [CTCL]), immunodeficiencies, infections, and metabolic disorders.3 Netherton syndrome must be ruled out to prevent extensive drug absorption when treating with topical calcineurin inhibitors (TCIs).4 Due to the presence of bamboo hairs in these patients, a hair mount may aid in the diagnosis of Netherton syndrome. Misdiagnosis of CTCL as AD may complicate the analysis of safety data on TCIs.4,5 Multiple skin biopsies are essential in cases of suspected CTCL to provide an accurate diagnosis. Biopsy can be considered in AD cases with changing and/or unusual morphology, erythrodermic skin changes, and disease that is poorly responsive to multiple therapeutic modalities.
Comorbidities in Pediatric AD
Psychosocial Comorbidities
Pediatric AD often takes a psychological toll on patients as well as household members. Almost half of children with AD are reported to have a severely impaired quality of life (QOL).6 Contributing factors include fatigue, sleep disturbance, activity restriction (eg, inability to participate in sports), and depression.7
Chamlin et al8 developed the Childhood Atopic Dermatitis Impact Scale (CADIS), a 45-item instrument (refined from a 62-item prototype), to measure QOL in young children with AD and their family members. Responses were evaluated with consideration of 5 domains: symptoms and activity limitations/behaviors in children, as well as family/social function, sleep, and emotions in parents. The top 12 factors that parents found most bothersome about AD included itching/scratching, child’s pain/discomfort, sleep issues, embarrassment or worry about appearance, child’s fussiness/irritability/crying/unhappiness, helplessness/can’t control it/predict it, worry about skin infection, dryness of skin/nonsmooth skin, skin bleeding, worry about damage/scars, stares/comments of strangers and other children, and rashes/redness of skin/discoloration. Parents were asked to respond to items about their emotional health and social functioning, such as “My child’s skin condition has strained my relationship with my spouse or partner,” “My child’s skin condition makes me feel sad or depressed,” and “I am bothered by the reaction of strangers to this skin condition.”8
Kiebert et al9 found that AD patients had lower scores on the Short Form-36 Health Survey’s vitality, social functioning, and mental health subscales compared to individuals in the general population. The authors noted that anxiety in AD patients is of particular concern, as stress has been found to trigger the itch-scratch cycle, potentially setting off AD flare-ups.9 Family impact of AD is aggravated by disease severity. Sleeplessness, relationship stress, and time management can all cause family problems in patients with AD.8
In a survey of 3775 older teenagers aged 18 to 19 years (80% response rate out of 4774 prospective participants), 9.7% of participants reported having current AD.10 Suicidal ideation was higher in those with current AD than those without AD (15.5% vs 9.1%). The prevalence of suicidal ideation rose to 23.8% in those with both AD and itch. Diagnosis of AD (as determined through participant responses to the question, ‘‘Do you have, or have you had eczema?’’) was associated with mental health problems in 16.0% of those with AD compared to 10.1% of those without AD, with an especially reduced likelihood of romantic relationships for adolescent boys with AD, as measured using the Strength and Difficulties Questionnaire, which measures 4 problem domains and assesses presence of mental health issues in the past 6 months, and the Hopkins Symptom Checklist 10, which uses 10 questions to measure anxiety and depression symptoms in the past week.10
Dalgard et al11 assessed whether the psychological burden of AD persists in adulthood in an international, multicenter, observational, cross-sectional study conducted in 13 European countries. Each dermatology clinic recruited 250 consecutive adult outpatients to complete a questionnaire along with a control group of 125 hospital employees without skin disease from the same institution but from different departments. The study included a total of 4994 participants (3635 patients and 1359 controls). Clinical depression and anxiety were present in 10.1% and 17.6% of patients, respectively, versus 4.3% and 11.1% of controls, respectively. The prevalence of depression and anxiety was highest in patients with leg ulcers, hand eczema, psoriasis, and AD.11 This study demonstrated that the psychological comorbidities of childhood conditions such as AD may persist into adulthood.
Lymphoma
In a systematic review of the literature and a separate meta-analysis, Legendre et al12 identified a slight increase in lymphoma among AD patients, with an uncertain but potential increase associated with topical corticosteroid application. This finding is similar to trends seen in other systemic inflammatory conditions that involve the skin, such as psoriasis, and is felt to relate to long-term inflammation.
Obesity
Obesity has been associated with a greater risk for moderate to severe AD in children.13,14
Infections
Children with AD are at a higher risk for cutaneous infections and generalization of these infections. The leading infections would be with Staphylococcus aureus, but group A streptococci infections do occur. Herpes simplex virus, vaccinia virus or Kaposi varicelliform eruption (KVE), molluscum with or without dermatitis, and fungal infections occur less commonly but with greater morbidity, largely due to the impaired barrier and some innate reduction in cutaneous immunity.15
Atopic dermatitis in children also is associated with a higher prevalence of extracutaneous infections such as influenza, pneumonia, urinary tract infections, varicella-zoster virus, recurrent ear infections, sinus infections, sore throat, and head or chest colds.16 Children with AD and warts (human papillomavirus infection) have an even greater risk for these comorbidities.17 Warts and molluscum infections may become more extensive in children with AD.18 Generalization of herpetic infections occurs more easily in AD patients due to the impaired skin barrier, which includes generalized skin surface extension of herpes simplex virus type 1, varicella-zoster virus, and historically smallpox. A similar clinical appearance of generalized vesiculopustular lesions with fever can be seen when coxsackievirus A6 infections occur in AD patients; these conditions are called eczema herpeticum due to herpes simplex virus, KVE due to varicella-zoster virus and smallpox, and eczema coxsackium due to coxsackievirus A6,19 though some authors refer to all of these as KVE.20 These generalized viral illnesses overlying AD often result in fever, malaise, pain, and life-threatening skin denudation with risk for dehydration and superinfection with S aureus.7,18 It has been shown that the occurrence of eczema herpeticum in AD is associated with and may be caused by an inability to induce human β-defensin 2 and 3 as well as cathelicidin.21
Staphylococcus aureus colonization has been noted in 90% to 100% of AD cases, which can be associated with a higher eczema area and severity index score.22-24 The role of S aureus in AD includes flare triggering through release of superantigens, leading to IL-31–induced pruritis.25 Recurrent infection with either methicillin-sensitive or methicillin-resistant S aureus has been noted in AD.18,26 Skin infections also occur in AD and appear as erosions and pustules, and coinfection with Streptococcus and Staphylococcus does occur; therefore, cultures often are needed to determine the type of bacteria present on the skin in severe cases and when infection is suspected.27 Perianal bacterial dermatitis is a variant of infected AD occurring in the anal/groin area that is associated with S aureus and/or streptococcal superinfection in which topical corticosteroids and topical anti-infectives can be used. In some severe cases, oral antibiotics may be needed.28
Injury/Hyperactivity
Children aged 0 to 5 years with AD carry an increased risk for injuries requiring medical attention, with association in part due to attention deficit disorder, depression, and anxiety. Antihistamines are believed to aggravate this issue by promoting daytime somnolence29; however, pruritus-induced sleep disturbances in AD also may be responsible for daytime somnolence.30
Contact Allergy and Sensitization
Children with AD may become sensitized to environmental allergens through delayed-type hypersensitivity. The presumed mechanism is that these agents include ingredients added into applied medicaments and application occurs over an impaired skin barrier allowing for absorption and greater risk of antigen presentation. Approximately 50% of children with difficult-to-control AD will react to 1 or more epicutaneous allergens, and patch testing can be performed to identify relevant allergens that can improve skin severity.7 Severe dermatitis and id generalized hypersensitivity reactions in patients with AD and nickel allergic contact dermatitis have been described and may aggravate underlying AD.31
Family Burden of AD
Parents or caregivers of children with moderate and severe AD spend nearly 3 hours a day caring for their child’s skin and experience QOL impairments including lack of sleep and/or privacy, often due to cosleeping; treatment-related financial expenditures; and feelings of hopelessness, guilt, and depression.7
Steroid Phobia
Steroid phobia is the fear of topical application of corticosteroids resulting in systemic side effects including unrealistic fears (eg, fear that the child will develop muscles such as an anabolic steroid user) as well as realistic but statistically low-risk fears (eg, fear of systemic absorption). These fears often result in underutilization of prescribed topical corticosteroid therapies and undertreatment of children with AD.32,33
Financial Burden
The cost of AD can be high in the United States, with adult data demonstrating costs ranging from $371 to $489 per person.34 The last published cost data for pediatric AD was from 2003, with an average cost of $219 per year.35 Costs include time lost from work, household purchases (eg, skin care products), and co-pays for visits and medication, with an estimated average expenditure per person (SE) of $601.06 ($137.26) annually in 2012.36 The cost of ambulatory care and emergency department visits for AD in children in the United States in 1993 was estimated at $364 million.37-39 In 2002, Ellis et al40 estimated the overall cost of AD to be between $900 million and $3.8 billion in the United States (1997-1998) based on projections from claims, prescriptions, and comorbidities reported to a private insurer and Medicaid. Ellis et al41 further determined that topical tacrolimus was similar in cost to high-potency corticosteroids.
Pediatric AD often progresses to adult hand eczema and leads to further morbidity, especially in health care workers.42 Kemp43 reviewed the cost of AD in children and concluded that AD was a condition with major handicap with personal, financial, and social effects. A cost review of studies conducted in 163,700 children with AD showed that costs related to AD totaled $316.7 million per year. The author concluded that there were substantial psychosocial and financial stresses associated with pediatric AD but no clear path to potential reduction in related costs.43
Sleep Disturbances
Sleep disturbances are common in pediatric AD patients. Pruritus usually is exacerbated at bedtime due to reduced humidity and lack of distractions to prevent scratching. Sleep deprivation has a substantial impact on both the patient and his/her household. Parental frustration increases with sleep disturbance.18,44 Sleep deprivation is associated with greater severity, both because it is one of the most difficult aspects of illness and because the associated pruritus makes for greater damage done to the skin through injurious scratching.
Sleep disturbances also may interfere with growth and overnight release of growth hormones.18,44 This latter issue can result in reduced linear growth velocity. Furthermore, sleep deprivation can cause increased risk of accidents and poor school performance.18,44,45
Many children do not outgrow AD. In adults, AD-associated sleep deprivation has been shown to have an association with fatigue, regular daytime sleepiness, and regular insomnia, correlating to number of sick days, doctor visits, and poorer overall health status.45
Inadequate Disease Control
Inadequate disease control has been described by Eichenfeld46 as an important issue in AD at this time. Untreated, undertreated, and improperly treated AD are important issues affecting long-term AD care. He further cited steroid phobia as a contributor to undertreatment.46 Fleischer47 has cited the black box warning present on TCIs as a further deterrent to adequate therapeutic control in our current therapeutic paradigm. Undertreatment may result in uncontrolled disease activity, impaired QOL, infections, and sleep disturbances. The role of undertreatment as a driver of the atopic march is unknown.
Conclusion
Atopic dermatitis is a multisystem disorder that has wide-reaching comorbidities and may mimic a variety of skin conditions. The topic of comorbidities is new and emerging and bears further review to define risk factors, prevention strategies, and long-term monitoring requirements.
- Silverberg NB. A practical overview of pediatric atopic dermatitis, part 1: epidemiology and pathogenesis. Cutis. 2016;97:267-271.
- Silverberg NB. A practical overview of pediatric atopic dermatitis, part 2: triggers and grading. Cutis. 2016;97:326-329.
- Siegfried EC, Hebert AA. Diagnosis of atopic dermatitis: mimics, overlaps, and complications. J Clin Med. 2015;4:884-917.
- Allen A, Siegfried E, Silverman R, et al. Significant absorption of topical tacrolimus in 3 patients with Netherton syndrome. Arch Dermatol. 2001;137:747-750.
- Carr WW. Topical calcineurin inhibitors for atopic dermatitis: review and treatment recommendations. Paediatr Drugs. 2013;15:303-310.
- Chamlin SL, Lai JS, Cella D, et al. Childhood Atopic Dermatitis Impact Scale: reliability, discriminative and concurrent validity, and responsiveness. Arch Dermatol. 2007;143:768-772.
- Tollefson MM, Bruckner AL. Atopic dermatitis: skin-directed management. Pediatrics. 2014;134:E1735-E1744.
- Chamlin SL, Cella D, Frieden IJ, et al. Development of the Childhood Atopic Dermatitis Impact Scale: initial validation of a quality-of-life measure for young children with atopic dermatitis and their families. J Invest Dermatol. 2005;125:1106-1111.
- Kiebert G, Sorensen SV, Revicki D, et al. Atopic dermatitis is associated with a decrement in health-related quality of life. Int J Dermatol. 2002;41:151-158.
- Halvorsen JA, Lien L, Dalgard F, et al. Suicidal ideation, mental health problems, and social function in adolescents with eczema: a population-based study. J Invest Dermatol. 2014;134:1847-1854.
- Dalgard FJ, Gieler U, Tomas-Aragones L, et al. The psychological burden of skin diseases: a cross-sectional multicenter study among dermatological out-patients in 13 European countries. J Invest Dermatol. 2015;135:984-991.
- Legendre L, Barnetche T, Mazereeuw-Hautier J, et al. Risk of lymphoma in patients with atopic dermatitis and the role of topical treatment: a systematic review and meta-analysis. J Am Acad Dermatol. 2015;72:992-1002.
- Koutroulis I, Magnelli L, Gaughan J, et al. Atopic dermatitis is more severe in children over the age of two who have an increased body mass index. Acta Paediatr. 2015;104:713-717.
- Silverberg JI, Becker L, Kwasny M, et al. Central obesity and high blood pressure in pediatric patients with atopic dermatitis. JAMA Dermatol. 2015;151:144-152.
- De D, Kanwar AJ, Handa S. Comparative efficacy of Hanifin and Rajka’s criteria and the UK working party’s diagnostic criteria in diagnosis of atopic dermatitis in a hospital setting in North India. J Eur Acad Dermatol Venereol. 2006;20:853-859.
- Silverberg JI, Silverberg NB. Childhood atopic dermatitis and warts are associated with increased risk of infection: a US population-based study [published online October 4, 2013]. J Allergy Clin Immunol. 2014;133:1041-1047.
- Silverberg J, Garg N, Silverberg NB. New developments in comorbidities of atopic dermatitis. Cutis. 2014;93:222-224.
- Kiken DA, Silverberg NB. Atopic dermatitis in children, part 1: epidemiology, clinical features, and complications. Cutis. 2006;78:241-247.
- Mathes EF, Oza V, Frieden IJ, et al. “Eczema coxsackium” and unusual cutaneous findings in an enterovirus outbreak. Pediatrics. 2013;132:E149-E157.
- Vora RV, Pilani AP, Jivani NB, et al. Kaposi varicelliform eruption. Indian Dermatol Online J. 2015;6:364-366.
- Hata TR, Kotol P, Boguniewicz M, et al. History of eczema herpeticum is associated with the inability to induce human β-defensin (HBD)-2, HBD-3 and cathelicidin in the skin of patients with atopic dermatitis. Br J Dermatol. 2010;163:659-661.
- Abeck D, Mempel M. Staphylococcus aureus colonization in atopic dermatitis and its therapeutic implications. Br J Dermatol. 1998;139:13-16.
- Leyden JJ, Marples RR, Kligman AM. Staphylococcus aureus in the lesions of atopic dermatitis. Br J Dermatol. 1974;90:525-530.
- Lipnharski C, d’Azevedo PA, Quinto VP, et al. Colonization by S. aureus increases the EASI and the number of appointments by patients with atopic dermatitis: cohort with 93 patients. An Bras Dermatol. 2013;88:518-521.
- Paller AS. Latest approaches to treating atopic dermatitis. Chem Immunol Allergy. 2012;96:132-140.
- Groner A, Laing-Grayman D, Silverberg NB. Outpatient pediatric community-acquired methicillin-resistant Staphylococcus aureus: a polymorphous clinical disease. Cutis. 2008;81:115-122.
- Sugarman JL, Hersh AL, Okamura T, et al. A retrospective review of streptococcal infections in pediatric atopic dermatitis. Pediatr Dermatol. 2011;28:230-234.
- Heath C, Desai N, Silverberg NB. Recent microbiological shifts in perianal bacterial dermatitis: Staphylococcus aureus predominance. Pediatr Dermatol. 2009;26:696-700.
- Garg N, Silverberg JI. Association between childhood allergic disease, psychological comorbidity, and injury requiring medical attention. Ann Allergy Asthma Immunol. 2014;112:525-532.
- Lavery MJ, Stull C, Kinney MO, et al. Nocturnal pruritus: the battle for a peaceful night’s sleep. Int J Mol Sci. 2016;17:E425.
- Silverberg NB, Licht J, Friedler S, et al. Nickel contact hypersensitivity in children. Pediatr Dermatol. 2002;19:110-113.
- Aubert-Wastiaux H, Moret L, Le Rhun A, et al. Topical corticosteroid phobia in atopic dermatitis: a study of its nature, origins and frequency. Br J Dermatol. 2011;165:808-814.
- Kojima R, Fujiwara T, Matsuda A, et al. Factors associated with steroid phobia in caregivers of children with atopic dermatitis. Pediatr Dermatol. 2013;30:29-35.
- Silverberg JI. Health care utilization, patient costs, and access to care in US adults with eczema: a population-based study. JAMA Dermatol. 2015;151:743-752.
- Weinmann S, Kamtsiuris P, Henke KD, et al. The costs of atopy and asthma in children: assessment of direct costs and their determinants in a birth cohort. Pediatr Allergy Immunol. 2003;14:18-26.
- Fivenson D, Arnold RJ, Kaniecki DJ, et al. The effect of atopic dermatitis on total burden of illness and quality of life on adults and children in a large managed care organization. J Manag Care Pharm. 2002;8:333-342.
- Verboom P, Hakkaart-Van L, Sturkenboom M, et al. The cost of atopic dermatitis in the Netherlands: an international comparison. Br J Dermatol. 2002;147:716-724.
- Lapidus CS, Schwarz DF, Honig PJ. Atopic dermatitis in children: who cares? who pays? J Am Acad Dermatol. 1993;28:699-703.
- Carroll CL, Balkrishnan R, Feldman SR, et al. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol. 2005;22:192-199.
- Ellis CN, Drake LA, Prendergast MM, et al. Cost of atopic dermatitis and eczema in the United States. J Am Acad Dermatol. 2002;46:361-370.
- Ellis CN, Prendergast MM, Tokar M, et al. Quantifying costs associated with atopic dermatitis. J Manag Care Pharm. 2003;9:278.
- Lee SW, Cheong SH, Byun JY, et al. Occupational hand eczema among nursing staffs in Korea: self-reported hand eczema and contact sensitization of hospital nursing staffs. J Dermatol. 2013;40:182-187.
- Kemp AS. Cost of illness of atopic dermatitis in children: a societal perspective. Pharmacoeconomics. 2003;21:105-113.
- Munro DD. Topical corticosteroid therapy and its effect on the hypothalamic-pituitary-adrenal axis. Dermatologica. 1976;152:173-180.
- Silverberg JI, Garg NK, Paller AS, et al. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. J Invest Dermatol. 2015;135:56-66.
- Eichenfield LF. Improving outcomes in atopic dermatitis. for advances in dermatology. Dermatology Focus. 2015;34:1-6.
- Fleischer AB Jr. Black box warning for topical calcineurin inhibitors and the death of common sense. Dermatol Online J. 2006;12:2.
In parts 1 and 2 of this series on atopic dermatitis (AD),1,2 the current putative pathogenesis, scoring systems for severity grading, and epidemiology were reviewed. Part 3 reviews the differential diagnosis, with an emphasis on the difficulty of differentiation from some rare but notable illnesses, as well as the recently expanding data on comorbidities that identify AD as a multisystem disorder with widespread health implications for the patient.
Differential Diagnosis for Pediatric AD
The differential diagnosis for pediatric AD includes chronic dermatoses (eg, seborrheic dermatitis, psoriasis), congenital disorders (eg, Netherton syndrome), malignant diseases (eg, cutaneous T-cell lymphoma [CTCL]), immunodeficiencies, infections, and metabolic disorders.3 Netherton syndrome must be ruled out to prevent extensive drug absorption when treating with topical calcineurin inhibitors (TCIs).4 Due to the presence of bamboo hairs in these patients, a hair mount may aid in the diagnosis of Netherton syndrome. Misdiagnosis of CTCL as AD may complicate the analysis of safety data on TCIs.4,5 Multiple skin biopsies are essential in cases of suspected CTCL to provide an accurate diagnosis. Biopsy can be considered in AD cases with changing and/or unusual morphology, erythrodermic skin changes, and disease that is poorly responsive to multiple therapeutic modalities.
Comorbidities in Pediatric AD
Psychosocial Comorbidities
Pediatric AD often takes a psychological toll on patients as well as household members. Almost half of children with AD are reported to have a severely impaired quality of life (QOL).6 Contributing factors include fatigue, sleep disturbance, activity restriction (eg, inability to participate in sports), and depression.7
Chamlin et al8 developed the Childhood Atopic Dermatitis Impact Scale (CADIS), a 45-item instrument (refined from a 62-item prototype), to measure QOL in young children with AD and their family members. Responses were evaluated with consideration of 5 domains: symptoms and activity limitations/behaviors in children, as well as family/social function, sleep, and emotions in parents. The top 12 factors that parents found most bothersome about AD included itching/scratching, child’s pain/discomfort, sleep issues, embarrassment or worry about appearance, child’s fussiness/irritability/crying/unhappiness, helplessness/can’t control it/predict it, worry about skin infection, dryness of skin/nonsmooth skin, skin bleeding, worry about damage/scars, stares/comments of strangers and other children, and rashes/redness of skin/discoloration. Parents were asked to respond to items about their emotional health and social functioning, such as “My child’s skin condition has strained my relationship with my spouse or partner,” “My child’s skin condition makes me feel sad or depressed,” and “I am bothered by the reaction of strangers to this skin condition.”8
Kiebert et al9 found that AD patients had lower scores on the Short Form-36 Health Survey’s vitality, social functioning, and mental health subscales compared to individuals in the general population. The authors noted that anxiety in AD patients is of particular concern, as stress has been found to trigger the itch-scratch cycle, potentially setting off AD flare-ups.9 Family impact of AD is aggravated by disease severity. Sleeplessness, relationship stress, and time management can all cause family problems in patients with AD.8
In a survey of 3775 older teenagers aged 18 to 19 years (80% response rate out of 4774 prospective participants), 9.7% of participants reported having current AD.10 Suicidal ideation was higher in those with current AD than those without AD (15.5% vs 9.1%). The prevalence of suicidal ideation rose to 23.8% in those with both AD and itch. Diagnosis of AD (as determined through participant responses to the question, ‘‘Do you have, or have you had eczema?’’) was associated with mental health problems in 16.0% of those with AD compared to 10.1% of those without AD, with an especially reduced likelihood of romantic relationships for adolescent boys with AD, as measured using the Strength and Difficulties Questionnaire, which measures 4 problem domains and assesses presence of mental health issues in the past 6 months, and the Hopkins Symptom Checklist 10, which uses 10 questions to measure anxiety and depression symptoms in the past week.10
Dalgard et al11 assessed whether the psychological burden of AD persists in adulthood in an international, multicenter, observational, cross-sectional study conducted in 13 European countries. Each dermatology clinic recruited 250 consecutive adult outpatients to complete a questionnaire along with a control group of 125 hospital employees without skin disease from the same institution but from different departments. The study included a total of 4994 participants (3635 patients and 1359 controls). Clinical depression and anxiety were present in 10.1% and 17.6% of patients, respectively, versus 4.3% and 11.1% of controls, respectively. The prevalence of depression and anxiety was highest in patients with leg ulcers, hand eczema, psoriasis, and AD.11 This study demonstrated that the psychological comorbidities of childhood conditions such as AD may persist into adulthood.
Lymphoma
In a systematic review of the literature and a separate meta-analysis, Legendre et al12 identified a slight increase in lymphoma among AD patients, with an uncertain but potential increase associated with topical corticosteroid application. This finding is similar to trends seen in other systemic inflammatory conditions that involve the skin, such as psoriasis, and is felt to relate to long-term inflammation.
Obesity
Obesity has been associated with a greater risk for moderate to severe AD in children.13,14
Infections
Children with AD are at a higher risk for cutaneous infections and generalization of these infections. The leading infections would be with Staphylococcus aureus, but group A streptococci infections do occur. Herpes simplex virus, vaccinia virus or Kaposi varicelliform eruption (KVE), molluscum with or without dermatitis, and fungal infections occur less commonly but with greater morbidity, largely due to the impaired barrier and some innate reduction in cutaneous immunity.15
Atopic dermatitis in children also is associated with a higher prevalence of extracutaneous infections such as influenza, pneumonia, urinary tract infections, varicella-zoster virus, recurrent ear infections, sinus infections, sore throat, and head or chest colds.16 Children with AD and warts (human papillomavirus infection) have an even greater risk for these comorbidities.17 Warts and molluscum infections may become more extensive in children with AD.18 Generalization of herpetic infections occurs more easily in AD patients due to the impaired skin barrier, which includes generalized skin surface extension of herpes simplex virus type 1, varicella-zoster virus, and historically smallpox. A similar clinical appearance of generalized vesiculopustular lesions with fever can be seen when coxsackievirus A6 infections occur in AD patients; these conditions are called eczema herpeticum due to herpes simplex virus, KVE due to varicella-zoster virus and smallpox, and eczema coxsackium due to coxsackievirus A6,19 though some authors refer to all of these as KVE.20 These generalized viral illnesses overlying AD often result in fever, malaise, pain, and life-threatening skin denudation with risk for dehydration and superinfection with S aureus.7,18 It has been shown that the occurrence of eczema herpeticum in AD is associated with and may be caused by an inability to induce human β-defensin 2 and 3 as well as cathelicidin.21
Staphylococcus aureus colonization has been noted in 90% to 100% of AD cases, which can be associated with a higher eczema area and severity index score.22-24 The role of S aureus in AD includes flare triggering through release of superantigens, leading to IL-31–induced pruritis.25 Recurrent infection with either methicillin-sensitive or methicillin-resistant S aureus has been noted in AD.18,26 Skin infections also occur in AD and appear as erosions and pustules, and coinfection with Streptococcus and Staphylococcus does occur; therefore, cultures often are needed to determine the type of bacteria present on the skin in severe cases and when infection is suspected.27 Perianal bacterial dermatitis is a variant of infected AD occurring in the anal/groin area that is associated with S aureus and/or streptococcal superinfection in which topical corticosteroids and topical anti-infectives can be used. In some severe cases, oral antibiotics may be needed.28
Injury/Hyperactivity
Children aged 0 to 5 years with AD carry an increased risk for injuries requiring medical attention, with association in part due to attention deficit disorder, depression, and anxiety. Antihistamines are believed to aggravate this issue by promoting daytime somnolence29; however, pruritus-induced sleep disturbances in AD also may be responsible for daytime somnolence.30
Contact Allergy and Sensitization
Children with AD may become sensitized to environmental allergens through delayed-type hypersensitivity. The presumed mechanism is that these agents include ingredients added into applied medicaments and application occurs over an impaired skin barrier allowing for absorption and greater risk of antigen presentation. Approximately 50% of children with difficult-to-control AD will react to 1 or more epicutaneous allergens, and patch testing can be performed to identify relevant allergens that can improve skin severity.7 Severe dermatitis and id generalized hypersensitivity reactions in patients with AD and nickel allergic contact dermatitis have been described and may aggravate underlying AD.31
Family Burden of AD
Parents or caregivers of children with moderate and severe AD spend nearly 3 hours a day caring for their child’s skin and experience QOL impairments including lack of sleep and/or privacy, often due to cosleeping; treatment-related financial expenditures; and feelings of hopelessness, guilt, and depression.7
Steroid Phobia
Steroid phobia is the fear of topical application of corticosteroids resulting in systemic side effects including unrealistic fears (eg, fear that the child will develop muscles such as an anabolic steroid user) as well as realistic but statistically low-risk fears (eg, fear of systemic absorption). These fears often result in underutilization of prescribed topical corticosteroid therapies and undertreatment of children with AD.32,33
Financial Burden
The cost of AD can be high in the United States, with adult data demonstrating costs ranging from $371 to $489 per person.34 The last published cost data for pediatric AD was from 2003, with an average cost of $219 per year.35 Costs include time lost from work, household purchases (eg, skin care products), and co-pays for visits and medication, with an estimated average expenditure per person (SE) of $601.06 ($137.26) annually in 2012.36 The cost of ambulatory care and emergency department visits for AD in children in the United States in 1993 was estimated at $364 million.37-39 In 2002, Ellis et al40 estimated the overall cost of AD to be between $900 million and $3.8 billion in the United States (1997-1998) based on projections from claims, prescriptions, and comorbidities reported to a private insurer and Medicaid. Ellis et al41 further determined that topical tacrolimus was similar in cost to high-potency corticosteroids.
Pediatric AD often progresses to adult hand eczema and leads to further morbidity, especially in health care workers.42 Kemp43 reviewed the cost of AD in children and concluded that AD was a condition with major handicap with personal, financial, and social effects. A cost review of studies conducted in 163,700 children with AD showed that costs related to AD totaled $316.7 million per year. The author concluded that there were substantial psychosocial and financial stresses associated with pediatric AD but no clear path to potential reduction in related costs.43
Sleep Disturbances
Sleep disturbances are common in pediatric AD patients. Pruritus usually is exacerbated at bedtime due to reduced humidity and lack of distractions to prevent scratching. Sleep deprivation has a substantial impact on both the patient and his/her household. Parental frustration increases with sleep disturbance.18,44 Sleep deprivation is associated with greater severity, both because it is one of the most difficult aspects of illness and because the associated pruritus makes for greater damage done to the skin through injurious scratching.
Sleep disturbances also may interfere with growth and overnight release of growth hormones.18,44 This latter issue can result in reduced linear growth velocity. Furthermore, sleep deprivation can cause increased risk of accidents and poor school performance.18,44,45
Many children do not outgrow AD. In adults, AD-associated sleep deprivation has been shown to have an association with fatigue, regular daytime sleepiness, and regular insomnia, correlating to number of sick days, doctor visits, and poorer overall health status.45
Inadequate Disease Control
Inadequate disease control has been described by Eichenfeld46 as an important issue in AD at this time. Untreated, undertreated, and improperly treated AD are important issues affecting long-term AD care. He further cited steroid phobia as a contributor to undertreatment.46 Fleischer47 has cited the black box warning present on TCIs as a further deterrent to adequate therapeutic control in our current therapeutic paradigm. Undertreatment may result in uncontrolled disease activity, impaired QOL, infections, and sleep disturbances. The role of undertreatment as a driver of the atopic march is unknown.
Conclusion
Atopic dermatitis is a multisystem disorder that has wide-reaching comorbidities and may mimic a variety of skin conditions. The topic of comorbidities is new and emerging and bears further review to define risk factors, prevention strategies, and long-term monitoring requirements.
In parts 1 and 2 of this series on atopic dermatitis (AD),1,2 the current putative pathogenesis, scoring systems for severity grading, and epidemiology were reviewed. Part 3 reviews the differential diagnosis, with an emphasis on the difficulty of differentiation from some rare but notable illnesses, as well as the recently expanding data on comorbidities that identify AD as a multisystem disorder with widespread health implications for the patient.
Differential Diagnosis for Pediatric AD
The differential diagnosis for pediatric AD includes chronic dermatoses (eg, seborrheic dermatitis, psoriasis), congenital disorders (eg, Netherton syndrome), malignant diseases (eg, cutaneous T-cell lymphoma [CTCL]), immunodeficiencies, infections, and metabolic disorders.3 Netherton syndrome must be ruled out to prevent extensive drug absorption when treating with topical calcineurin inhibitors (TCIs).4 Due to the presence of bamboo hairs in these patients, a hair mount may aid in the diagnosis of Netherton syndrome. Misdiagnosis of CTCL as AD may complicate the analysis of safety data on TCIs.4,5 Multiple skin biopsies are essential in cases of suspected CTCL to provide an accurate diagnosis. Biopsy can be considered in AD cases with changing and/or unusual morphology, erythrodermic skin changes, and disease that is poorly responsive to multiple therapeutic modalities.
Comorbidities in Pediatric AD
Psychosocial Comorbidities
Pediatric AD often takes a psychological toll on patients as well as household members. Almost half of children with AD are reported to have a severely impaired quality of life (QOL).6 Contributing factors include fatigue, sleep disturbance, activity restriction (eg, inability to participate in sports), and depression.7
Chamlin et al8 developed the Childhood Atopic Dermatitis Impact Scale (CADIS), a 45-item instrument (refined from a 62-item prototype), to measure QOL in young children with AD and their family members. Responses were evaluated with consideration of 5 domains: symptoms and activity limitations/behaviors in children, as well as family/social function, sleep, and emotions in parents. The top 12 factors that parents found most bothersome about AD included itching/scratching, child’s pain/discomfort, sleep issues, embarrassment or worry about appearance, child’s fussiness/irritability/crying/unhappiness, helplessness/can’t control it/predict it, worry about skin infection, dryness of skin/nonsmooth skin, skin bleeding, worry about damage/scars, stares/comments of strangers and other children, and rashes/redness of skin/discoloration. Parents were asked to respond to items about their emotional health and social functioning, such as “My child’s skin condition has strained my relationship with my spouse or partner,” “My child’s skin condition makes me feel sad or depressed,” and “I am bothered by the reaction of strangers to this skin condition.”8
Kiebert et al9 found that AD patients had lower scores on the Short Form-36 Health Survey’s vitality, social functioning, and mental health subscales compared to individuals in the general population. The authors noted that anxiety in AD patients is of particular concern, as stress has been found to trigger the itch-scratch cycle, potentially setting off AD flare-ups.9 Family impact of AD is aggravated by disease severity. Sleeplessness, relationship stress, and time management can all cause family problems in patients with AD.8
In a survey of 3775 older teenagers aged 18 to 19 years (80% response rate out of 4774 prospective participants), 9.7% of participants reported having current AD.10 Suicidal ideation was higher in those with current AD than those without AD (15.5% vs 9.1%). The prevalence of suicidal ideation rose to 23.8% in those with both AD and itch. Diagnosis of AD (as determined through participant responses to the question, ‘‘Do you have, or have you had eczema?’’) was associated with mental health problems in 16.0% of those with AD compared to 10.1% of those without AD, with an especially reduced likelihood of romantic relationships for adolescent boys with AD, as measured using the Strength and Difficulties Questionnaire, which measures 4 problem domains and assesses presence of mental health issues in the past 6 months, and the Hopkins Symptom Checklist 10, which uses 10 questions to measure anxiety and depression symptoms in the past week.10
Dalgard et al11 assessed whether the psychological burden of AD persists in adulthood in an international, multicenter, observational, cross-sectional study conducted in 13 European countries. Each dermatology clinic recruited 250 consecutive adult outpatients to complete a questionnaire along with a control group of 125 hospital employees without skin disease from the same institution but from different departments. The study included a total of 4994 participants (3635 patients and 1359 controls). Clinical depression and anxiety were present in 10.1% and 17.6% of patients, respectively, versus 4.3% and 11.1% of controls, respectively. The prevalence of depression and anxiety was highest in patients with leg ulcers, hand eczema, psoriasis, and AD.11 This study demonstrated that the psychological comorbidities of childhood conditions such as AD may persist into adulthood.
Lymphoma
In a systematic review of the literature and a separate meta-analysis, Legendre et al12 identified a slight increase in lymphoma among AD patients, with an uncertain but potential increase associated with topical corticosteroid application. This finding is similar to trends seen in other systemic inflammatory conditions that involve the skin, such as psoriasis, and is felt to relate to long-term inflammation.
Obesity
Obesity has been associated with a greater risk for moderate to severe AD in children.13,14
Infections
Children with AD are at a higher risk for cutaneous infections and generalization of these infections. The leading infections would be with Staphylococcus aureus, but group A streptococci infections do occur. Herpes simplex virus, vaccinia virus or Kaposi varicelliform eruption (KVE), molluscum with or without dermatitis, and fungal infections occur less commonly but with greater morbidity, largely due to the impaired barrier and some innate reduction in cutaneous immunity.15
Atopic dermatitis in children also is associated with a higher prevalence of extracutaneous infections such as influenza, pneumonia, urinary tract infections, varicella-zoster virus, recurrent ear infections, sinus infections, sore throat, and head or chest colds.16 Children with AD and warts (human papillomavirus infection) have an even greater risk for these comorbidities.17 Warts and molluscum infections may become more extensive in children with AD.18 Generalization of herpetic infections occurs more easily in AD patients due to the impaired skin barrier, which includes generalized skin surface extension of herpes simplex virus type 1, varicella-zoster virus, and historically smallpox. A similar clinical appearance of generalized vesiculopustular lesions with fever can be seen when coxsackievirus A6 infections occur in AD patients; these conditions are called eczema herpeticum due to herpes simplex virus, KVE due to varicella-zoster virus and smallpox, and eczema coxsackium due to coxsackievirus A6,19 though some authors refer to all of these as KVE.20 These generalized viral illnesses overlying AD often result in fever, malaise, pain, and life-threatening skin denudation with risk for dehydration and superinfection with S aureus.7,18 It has been shown that the occurrence of eczema herpeticum in AD is associated with and may be caused by an inability to induce human β-defensin 2 and 3 as well as cathelicidin.21
Staphylococcus aureus colonization has been noted in 90% to 100% of AD cases, which can be associated with a higher eczema area and severity index score.22-24 The role of S aureus in AD includes flare triggering through release of superantigens, leading to IL-31–induced pruritis.25 Recurrent infection with either methicillin-sensitive or methicillin-resistant S aureus has been noted in AD.18,26 Skin infections also occur in AD and appear as erosions and pustules, and coinfection with Streptococcus and Staphylococcus does occur; therefore, cultures often are needed to determine the type of bacteria present on the skin in severe cases and when infection is suspected.27 Perianal bacterial dermatitis is a variant of infected AD occurring in the anal/groin area that is associated with S aureus and/or streptococcal superinfection in which topical corticosteroids and topical anti-infectives can be used. In some severe cases, oral antibiotics may be needed.28
Injury/Hyperactivity
Children aged 0 to 5 years with AD carry an increased risk for injuries requiring medical attention, with association in part due to attention deficit disorder, depression, and anxiety. Antihistamines are believed to aggravate this issue by promoting daytime somnolence29; however, pruritus-induced sleep disturbances in AD also may be responsible for daytime somnolence.30
Contact Allergy and Sensitization
Children with AD may become sensitized to environmental allergens through delayed-type hypersensitivity. The presumed mechanism is that these agents include ingredients added into applied medicaments and application occurs over an impaired skin barrier allowing for absorption and greater risk of antigen presentation. Approximately 50% of children with difficult-to-control AD will react to 1 or more epicutaneous allergens, and patch testing can be performed to identify relevant allergens that can improve skin severity.7 Severe dermatitis and id generalized hypersensitivity reactions in patients with AD and nickel allergic contact dermatitis have been described and may aggravate underlying AD.31
Family Burden of AD
Parents or caregivers of children with moderate and severe AD spend nearly 3 hours a day caring for their child’s skin and experience QOL impairments including lack of sleep and/or privacy, often due to cosleeping; treatment-related financial expenditures; and feelings of hopelessness, guilt, and depression.7
Steroid Phobia
Steroid phobia is the fear of topical application of corticosteroids resulting in systemic side effects including unrealistic fears (eg, fear that the child will develop muscles such as an anabolic steroid user) as well as realistic but statistically low-risk fears (eg, fear of systemic absorption). These fears often result in underutilization of prescribed topical corticosteroid therapies and undertreatment of children with AD.32,33
Financial Burden
The cost of AD can be high in the United States, with adult data demonstrating costs ranging from $371 to $489 per person.34 The last published cost data for pediatric AD was from 2003, with an average cost of $219 per year.35 Costs include time lost from work, household purchases (eg, skin care products), and co-pays for visits and medication, with an estimated average expenditure per person (SE) of $601.06 ($137.26) annually in 2012.36 The cost of ambulatory care and emergency department visits for AD in children in the United States in 1993 was estimated at $364 million.37-39 In 2002, Ellis et al40 estimated the overall cost of AD to be between $900 million and $3.8 billion in the United States (1997-1998) based on projections from claims, prescriptions, and comorbidities reported to a private insurer and Medicaid. Ellis et al41 further determined that topical tacrolimus was similar in cost to high-potency corticosteroids.
Pediatric AD often progresses to adult hand eczema and leads to further morbidity, especially in health care workers.42 Kemp43 reviewed the cost of AD in children and concluded that AD was a condition with major handicap with personal, financial, and social effects. A cost review of studies conducted in 163,700 children with AD showed that costs related to AD totaled $316.7 million per year. The author concluded that there were substantial psychosocial and financial stresses associated with pediatric AD but no clear path to potential reduction in related costs.43
Sleep Disturbances
Sleep disturbances are common in pediatric AD patients. Pruritus usually is exacerbated at bedtime due to reduced humidity and lack of distractions to prevent scratching. Sleep deprivation has a substantial impact on both the patient and his/her household. Parental frustration increases with sleep disturbance.18,44 Sleep deprivation is associated with greater severity, both because it is one of the most difficult aspects of illness and because the associated pruritus makes for greater damage done to the skin through injurious scratching.
Sleep disturbances also may interfere with growth and overnight release of growth hormones.18,44 This latter issue can result in reduced linear growth velocity. Furthermore, sleep deprivation can cause increased risk of accidents and poor school performance.18,44,45
Many children do not outgrow AD. In adults, AD-associated sleep deprivation has been shown to have an association with fatigue, regular daytime sleepiness, and regular insomnia, correlating to number of sick days, doctor visits, and poorer overall health status.45
Inadequate Disease Control
Inadequate disease control has been described by Eichenfeld46 as an important issue in AD at this time. Untreated, undertreated, and improperly treated AD are important issues affecting long-term AD care. He further cited steroid phobia as a contributor to undertreatment.46 Fleischer47 has cited the black box warning present on TCIs as a further deterrent to adequate therapeutic control in our current therapeutic paradigm. Undertreatment may result in uncontrolled disease activity, impaired QOL, infections, and sleep disturbances. The role of undertreatment as a driver of the atopic march is unknown.
Conclusion
Atopic dermatitis is a multisystem disorder that has wide-reaching comorbidities and may mimic a variety of skin conditions. The topic of comorbidities is new and emerging and bears further review to define risk factors, prevention strategies, and long-term monitoring requirements.
- Silverberg NB. A practical overview of pediatric atopic dermatitis, part 1: epidemiology and pathogenesis. Cutis. 2016;97:267-271.
- Silverberg NB. A practical overview of pediatric atopic dermatitis, part 2: triggers and grading. Cutis. 2016;97:326-329.
- Siegfried EC, Hebert AA. Diagnosis of atopic dermatitis: mimics, overlaps, and complications. J Clin Med. 2015;4:884-917.
- Allen A, Siegfried E, Silverman R, et al. Significant absorption of topical tacrolimus in 3 patients with Netherton syndrome. Arch Dermatol. 2001;137:747-750.
- Carr WW. Topical calcineurin inhibitors for atopic dermatitis: review and treatment recommendations. Paediatr Drugs. 2013;15:303-310.
- Chamlin SL, Lai JS, Cella D, et al. Childhood Atopic Dermatitis Impact Scale: reliability, discriminative and concurrent validity, and responsiveness. Arch Dermatol. 2007;143:768-772.
- Tollefson MM, Bruckner AL. Atopic dermatitis: skin-directed management. Pediatrics. 2014;134:E1735-E1744.
- Chamlin SL, Cella D, Frieden IJ, et al. Development of the Childhood Atopic Dermatitis Impact Scale: initial validation of a quality-of-life measure for young children with atopic dermatitis and their families. J Invest Dermatol. 2005;125:1106-1111.
- Kiebert G, Sorensen SV, Revicki D, et al. Atopic dermatitis is associated with a decrement in health-related quality of life. Int J Dermatol. 2002;41:151-158.
- Halvorsen JA, Lien L, Dalgard F, et al. Suicidal ideation, mental health problems, and social function in adolescents with eczema: a population-based study. J Invest Dermatol. 2014;134:1847-1854.
- Dalgard FJ, Gieler U, Tomas-Aragones L, et al. The psychological burden of skin diseases: a cross-sectional multicenter study among dermatological out-patients in 13 European countries. J Invest Dermatol. 2015;135:984-991.
- Legendre L, Barnetche T, Mazereeuw-Hautier J, et al. Risk of lymphoma in patients with atopic dermatitis and the role of topical treatment: a systematic review and meta-analysis. J Am Acad Dermatol. 2015;72:992-1002.
- Koutroulis I, Magnelli L, Gaughan J, et al. Atopic dermatitis is more severe in children over the age of two who have an increased body mass index. Acta Paediatr. 2015;104:713-717.
- Silverberg JI, Becker L, Kwasny M, et al. Central obesity and high blood pressure in pediatric patients with atopic dermatitis. JAMA Dermatol. 2015;151:144-152.
- De D, Kanwar AJ, Handa S. Comparative efficacy of Hanifin and Rajka’s criteria and the UK working party’s diagnostic criteria in diagnosis of atopic dermatitis in a hospital setting in North India. J Eur Acad Dermatol Venereol. 2006;20:853-859.
- Silverberg JI, Silverberg NB. Childhood atopic dermatitis and warts are associated with increased risk of infection: a US population-based study [published online October 4, 2013]. J Allergy Clin Immunol. 2014;133:1041-1047.
- Silverberg J, Garg N, Silverberg NB. New developments in comorbidities of atopic dermatitis. Cutis. 2014;93:222-224.
- Kiken DA, Silverberg NB. Atopic dermatitis in children, part 1: epidemiology, clinical features, and complications. Cutis. 2006;78:241-247.
- Mathes EF, Oza V, Frieden IJ, et al. “Eczema coxsackium” and unusual cutaneous findings in an enterovirus outbreak. Pediatrics. 2013;132:E149-E157.
- Vora RV, Pilani AP, Jivani NB, et al. Kaposi varicelliform eruption. Indian Dermatol Online J. 2015;6:364-366.
- Hata TR, Kotol P, Boguniewicz M, et al. History of eczema herpeticum is associated with the inability to induce human β-defensin (HBD)-2, HBD-3 and cathelicidin in the skin of patients with atopic dermatitis. Br J Dermatol. 2010;163:659-661.
- Abeck D, Mempel M. Staphylococcus aureus colonization in atopic dermatitis and its therapeutic implications. Br J Dermatol. 1998;139:13-16.
- Leyden JJ, Marples RR, Kligman AM. Staphylococcus aureus in the lesions of atopic dermatitis. Br J Dermatol. 1974;90:525-530.
- Lipnharski C, d’Azevedo PA, Quinto VP, et al. Colonization by S. aureus increases the EASI and the number of appointments by patients with atopic dermatitis: cohort with 93 patients. An Bras Dermatol. 2013;88:518-521.
- Paller AS. Latest approaches to treating atopic dermatitis. Chem Immunol Allergy. 2012;96:132-140.
- Groner A, Laing-Grayman D, Silverberg NB. Outpatient pediatric community-acquired methicillin-resistant Staphylococcus aureus: a polymorphous clinical disease. Cutis. 2008;81:115-122.
- Sugarman JL, Hersh AL, Okamura T, et al. A retrospective review of streptococcal infections in pediatric atopic dermatitis. Pediatr Dermatol. 2011;28:230-234.
- Heath C, Desai N, Silverberg NB. Recent microbiological shifts in perianal bacterial dermatitis: Staphylococcus aureus predominance. Pediatr Dermatol. 2009;26:696-700.
- Garg N, Silverberg JI. Association between childhood allergic disease, psychological comorbidity, and injury requiring medical attention. Ann Allergy Asthma Immunol. 2014;112:525-532.
- Lavery MJ, Stull C, Kinney MO, et al. Nocturnal pruritus: the battle for a peaceful night’s sleep. Int J Mol Sci. 2016;17:E425.
- Silverberg NB, Licht J, Friedler S, et al. Nickel contact hypersensitivity in children. Pediatr Dermatol. 2002;19:110-113.
- Aubert-Wastiaux H, Moret L, Le Rhun A, et al. Topical corticosteroid phobia in atopic dermatitis: a study of its nature, origins and frequency. Br J Dermatol. 2011;165:808-814.
- Kojima R, Fujiwara T, Matsuda A, et al. Factors associated with steroid phobia in caregivers of children with atopic dermatitis. Pediatr Dermatol. 2013;30:29-35.
- Silverberg JI. Health care utilization, patient costs, and access to care in US adults with eczema: a population-based study. JAMA Dermatol. 2015;151:743-752.
- Weinmann S, Kamtsiuris P, Henke KD, et al. The costs of atopy and asthma in children: assessment of direct costs and their determinants in a birth cohort. Pediatr Allergy Immunol. 2003;14:18-26.
- Fivenson D, Arnold RJ, Kaniecki DJ, et al. The effect of atopic dermatitis on total burden of illness and quality of life on adults and children in a large managed care organization. J Manag Care Pharm. 2002;8:333-342.
- Verboom P, Hakkaart-Van L, Sturkenboom M, et al. The cost of atopic dermatitis in the Netherlands: an international comparison. Br J Dermatol. 2002;147:716-724.
- Lapidus CS, Schwarz DF, Honig PJ. Atopic dermatitis in children: who cares? who pays? J Am Acad Dermatol. 1993;28:699-703.
- Carroll CL, Balkrishnan R, Feldman SR, et al. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol. 2005;22:192-199.
- Ellis CN, Drake LA, Prendergast MM, et al. Cost of atopic dermatitis and eczema in the United States. J Am Acad Dermatol. 2002;46:361-370.
- Ellis CN, Prendergast MM, Tokar M, et al. Quantifying costs associated with atopic dermatitis. J Manag Care Pharm. 2003;9:278.
- Lee SW, Cheong SH, Byun JY, et al. Occupational hand eczema among nursing staffs in Korea: self-reported hand eczema and contact sensitization of hospital nursing staffs. J Dermatol. 2013;40:182-187.
- Kemp AS. Cost of illness of atopic dermatitis in children: a societal perspective. Pharmacoeconomics. 2003;21:105-113.
- Munro DD. Topical corticosteroid therapy and its effect on the hypothalamic-pituitary-adrenal axis. Dermatologica. 1976;152:173-180.
- Silverberg JI, Garg NK, Paller AS, et al. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. J Invest Dermatol. 2015;135:56-66.
- Eichenfield LF. Improving outcomes in atopic dermatitis. for advances in dermatology. Dermatology Focus. 2015;34:1-6.
- Fleischer AB Jr. Black box warning for topical calcineurin inhibitors and the death of common sense. Dermatol Online J. 2006;12:2.
- Silverberg NB. A practical overview of pediatric atopic dermatitis, part 1: epidemiology and pathogenesis. Cutis. 2016;97:267-271.
- Silverberg NB. A practical overview of pediatric atopic dermatitis, part 2: triggers and grading. Cutis. 2016;97:326-329.
- Siegfried EC, Hebert AA. Diagnosis of atopic dermatitis: mimics, overlaps, and complications. J Clin Med. 2015;4:884-917.
- Allen A, Siegfried E, Silverman R, et al. Significant absorption of topical tacrolimus in 3 patients with Netherton syndrome. Arch Dermatol. 2001;137:747-750.
- Carr WW. Topical calcineurin inhibitors for atopic dermatitis: review and treatment recommendations. Paediatr Drugs. 2013;15:303-310.
- Chamlin SL, Lai JS, Cella D, et al. Childhood Atopic Dermatitis Impact Scale: reliability, discriminative and concurrent validity, and responsiveness. Arch Dermatol. 2007;143:768-772.
- Tollefson MM, Bruckner AL. Atopic dermatitis: skin-directed management. Pediatrics. 2014;134:E1735-E1744.
- Chamlin SL, Cella D, Frieden IJ, et al. Development of the Childhood Atopic Dermatitis Impact Scale: initial validation of a quality-of-life measure for young children with atopic dermatitis and their families. J Invest Dermatol. 2005;125:1106-1111.
- Kiebert G, Sorensen SV, Revicki D, et al. Atopic dermatitis is associated with a decrement in health-related quality of life. Int J Dermatol. 2002;41:151-158.
- Halvorsen JA, Lien L, Dalgard F, et al. Suicidal ideation, mental health problems, and social function in adolescents with eczema: a population-based study. J Invest Dermatol. 2014;134:1847-1854.
- Dalgard FJ, Gieler U, Tomas-Aragones L, et al. The psychological burden of skin diseases: a cross-sectional multicenter study among dermatological out-patients in 13 European countries. J Invest Dermatol. 2015;135:984-991.
- Legendre L, Barnetche T, Mazereeuw-Hautier J, et al. Risk of lymphoma in patients with atopic dermatitis and the role of topical treatment: a systematic review and meta-analysis. J Am Acad Dermatol. 2015;72:992-1002.
- Koutroulis I, Magnelli L, Gaughan J, et al. Atopic dermatitis is more severe in children over the age of two who have an increased body mass index. Acta Paediatr. 2015;104:713-717.
- Silverberg JI, Becker L, Kwasny M, et al. Central obesity and high blood pressure in pediatric patients with atopic dermatitis. JAMA Dermatol. 2015;151:144-152.
- De D, Kanwar AJ, Handa S. Comparative efficacy of Hanifin and Rajka’s criteria and the UK working party’s diagnostic criteria in diagnosis of atopic dermatitis in a hospital setting in North India. J Eur Acad Dermatol Venereol. 2006;20:853-859.
- Silverberg JI, Silverberg NB. Childhood atopic dermatitis and warts are associated with increased risk of infection: a US population-based study [published online October 4, 2013]. J Allergy Clin Immunol. 2014;133:1041-1047.
- Silverberg J, Garg N, Silverberg NB. New developments in comorbidities of atopic dermatitis. Cutis. 2014;93:222-224.
- Kiken DA, Silverberg NB. Atopic dermatitis in children, part 1: epidemiology, clinical features, and complications. Cutis. 2006;78:241-247.
- Mathes EF, Oza V, Frieden IJ, et al. “Eczema coxsackium” and unusual cutaneous findings in an enterovirus outbreak. Pediatrics. 2013;132:E149-E157.
- Vora RV, Pilani AP, Jivani NB, et al. Kaposi varicelliform eruption. Indian Dermatol Online J. 2015;6:364-366.
- Hata TR, Kotol P, Boguniewicz M, et al. History of eczema herpeticum is associated with the inability to induce human β-defensin (HBD)-2, HBD-3 and cathelicidin in the skin of patients with atopic dermatitis. Br J Dermatol. 2010;163:659-661.
- Abeck D, Mempel M. Staphylococcus aureus colonization in atopic dermatitis and its therapeutic implications. Br J Dermatol. 1998;139:13-16.
- Leyden JJ, Marples RR, Kligman AM. Staphylococcus aureus in the lesions of atopic dermatitis. Br J Dermatol. 1974;90:525-530.
- Lipnharski C, d’Azevedo PA, Quinto VP, et al. Colonization by S. aureus increases the EASI and the number of appointments by patients with atopic dermatitis: cohort with 93 patients. An Bras Dermatol. 2013;88:518-521.
- Paller AS. Latest approaches to treating atopic dermatitis. Chem Immunol Allergy. 2012;96:132-140.
- Groner A, Laing-Grayman D, Silverberg NB. Outpatient pediatric community-acquired methicillin-resistant Staphylococcus aureus: a polymorphous clinical disease. Cutis. 2008;81:115-122.
- Sugarman JL, Hersh AL, Okamura T, et al. A retrospective review of streptococcal infections in pediatric atopic dermatitis. Pediatr Dermatol. 2011;28:230-234.
- Heath C, Desai N, Silverberg NB. Recent microbiological shifts in perianal bacterial dermatitis: Staphylococcus aureus predominance. Pediatr Dermatol. 2009;26:696-700.
- Garg N, Silverberg JI. Association between childhood allergic disease, psychological comorbidity, and injury requiring medical attention. Ann Allergy Asthma Immunol. 2014;112:525-532.
- Lavery MJ, Stull C, Kinney MO, et al. Nocturnal pruritus: the battle for a peaceful night’s sleep. Int J Mol Sci. 2016;17:E425.
- Silverberg NB, Licht J, Friedler S, et al. Nickel contact hypersensitivity in children. Pediatr Dermatol. 2002;19:110-113.
- Aubert-Wastiaux H, Moret L, Le Rhun A, et al. Topical corticosteroid phobia in atopic dermatitis: a study of its nature, origins and frequency. Br J Dermatol. 2011;165:808-814.
- Kojima R, Fujiwara T, Matsuda A, et al. Factors associated with steroid phobia in caregivers of children with atopic dermatitis. Pediatr Dermatol. 2013;30:29-35.
- Silverberg JI. Health care utilization, patient costs, and access to care in US adults with eczema: a population-based study. JAMA Dermatol. 2015;151:743-752.
- Weinmann S, Kamtsiuris P, Henke KD, et al. The costs of atopy and asthma in children: assessment of direct costs and their determinants in a birth cohort. Pediatr Allergy Immunol. 2003;14:18-26.
- Fivenson D, Arnold RJ, Kaniecki DJ, et al. The effect of atopic dermatitis on total burden of illness and quality of life on adults and children in a large managed care organization. J Manag Care Pharm. 2002;8:333-342.
- Verboom P, Hakkaart-Van L, Sturkenboom M, et al. The cost of atopic dermatitis in the Netherlands: an international comparison. Br J Dermatol. 2002;147:716-724.
- Lapidus CS, Schwarz DF, Honig PJ. Atopic dermatitis in children: who cares? who pays? J Am Acad Dermatol. 1993;28:699-703.
- Carroll CL, Balkrishnan R, Feldman SR, et al. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol. 2005;22:192-199.
- Ellis CN, Drake LA, Prendergast MM, et al. Cost of atopic dermatitis and eczema in the United States. J Am Acad Dermatol. 2002;46:361-370.
- Ellis CN, Prendergast MM, Tokar M, et al. Quantifying costs associated with atopic dermatitis. J Manag Care Pharm. 2003;9:278.
- Lee SW, Cheong SH, Byun JY, et al. Occupational hand eczema among nursing staffs in Korea: self-reported hand eczema and contact sensitization of hospital nursing staffs. J Dermatol. 2013;40:182-187.
- Kemp AS. Cost of illness of atopic dermatitis in children: a societal perspective. Pharmacoeconomics. 2003;21:105-113.
- Munro DD. Topical corticosteroid therapy and its effect on the hypothalamic-pituitary-adrenal axis. Dermatologica. 1976;152:173-180.
- Silverberg JI, Garg NK, Paller AS, et al. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. J Invest Dermatol. 2015;135:56-66.
- Eichenfield LF. Improving outcomes in atopic dermatitis. for advances in dermatology. Dermatology Focus. 2015;34:1-6.
- Fleischer AB Jr. Black box warning for topical calcineurin inhibitors and the death of common sense. Dermatol Online J. 2006;12:2.
Practice Points
- Atopic dermatitis (AD) has a variety of comorbidities including psychosocial disorders, obesity, and infection.
- A variety of skin conditions can mimic AD.
- Atopic dermatitis can be complicated by coinfections.
A Practical Overview of Pediatric Atopic Dermatitis, Part 2: Triggers and Grading
Atopic dermatitis (AD) may be triggered by viral infections, food allergens, weather, and other causes, and it may trigger an inflammatory progression known as the atopic march. This article reviews research on triggers of pediatric AD so that dermatologists may discuss trigger avoidance with patients and guardians. Other factors affecting AD development include genetics and hygiene. Grading of AD also is discussed.
The Atopic March
The persistence of AD in untreated skin can trigger an inflammatory progression called the atopic march in which food and environmental allergies as well as asthma may occur progressively due to ongoing inflammatory triggering.1 In a study of asthma and food allergy reporting and management in public schools in Chicago, Illinois, food allergies were seen in 9.3% of asthmatic students (n=18,000), and 40.1% of food allergic students (n=4000) had asthma.2 An observational study by Flohr et al3 in London, England, included 619 exclusively breastfed infants who were recruited at 3 months of age. The investigators determined that food sensitization was unrelated to the presence of filaggrin mutations, type of eczema (flexural vs nonflexural), and transepidermal water loss but was associated with AD severity as determined by SCORAD (SCORing Atopic Dermatitis), a composite score of AD that includes pruritus as a factor in severity. Other AD associations included 3 leading food allergens: eggs, milk, and peanuts. No association with cod, wheat, or sesame allergy was noted. The investigators concluded that AD and AD severity were the leading skin-related risk factors for food allergies and therefore food allergy development in breastfed infants was probably mediated by cutaneous antigen-presenting cells.3
The skin has been documented to react to contact with known food allergens4 and is known to be a route of allergic sensitization to allergens such as fragrance in patients with AD.5,6 Two phenotypes of eczema that have been associated with asthma development are severe AD disease and multiple environmental allergies, supporting the theory of the atopic march.7 There also is evidence that release of danger-associated proteins from an impaired barrier also may trigger asthma.8 An analysis of the 2007 National Survey of Children’s Health, a population-based study of91,642 children aged 0 to 17 years, showed that children with AD had a higher prevalence of comorbid asthma (25.1% vs 12.3%), hay fever (34.4% vs 14.3%), and food allergies (15.1% vs 3.6%) compared to children without AD.9 A recent article provided detailed information on how food and diet interplay with AD.10
Triggers of Disease Flares
Triggers are the leading source of AD flare initiation, and avoidance of triggers is an important mechanism by which patients can control disease activity. Despite the best skin care and trigger avoidance, disease flares occur, sometimes due to ongoing inflammation and other times due to inability to prevent flares such as heat and humidity. A survey of patients with AD in Spain identified the following triggers: cosmetic products, clothing, mites, detergents/soaps, and temperature changes.11 In childhood, wool also is a known trigger of AD.12 Viral infections including respiratory syncytial virus may trigger the first onset of AD.13 Patients with AD may become allergic to fragrance and metals causing disease exacerbation on exposure.14,15 Food allergens contribute to approximately 40% of cases of AD in infancy but are not the cause of AD. The best evidence for improvement of AD with food allergen avoidance exists for egg white allergy.16 Food avoidance programs should be developed in conjunction with an allergist, as it is no longer advised in many cases to completely withdraw foods; therefore, an allergist has to assess the level of allergic severity and the risk-benefit ratio of food avoidance or introduction.17 Emotional stressors, heat, and humidity, as well as indoor heating in the winter months, can cause AD flares.18
A study by Silverberg et al19 provided evidence of climate influences on the US prevalence of childhood eczema using a merged analysis of the 2007 National Survey of Children’s Health and the 2006-2007 National Climate Data Center and Weather Service. Results showed that eczema prevalence was significantly lower when associated with higher annual relative humidity (P=.01), UV index (P<.0001), and highest-quartile air temperature (P=.002).19 The Pediatric Eczema Elective Registry also showed that warm, humid, and high-sun-exposure climates are associated with poorly controlled eczema in affected patients.20 The association of eczema with latitude as well as its negative association with mean annual outdoor temperature has been described by Weiland et al21 in the ISAAC (International Study of Asthma and Allergies in Childhood) study. Long airplane flights in low humidity can trigger eczema in adults. Climate has been postulated to affect eczema through alterations in filaggrin and skin barrier function.22 Indoor temperature and humidity regulation may be used adjunctively for daily flare prevention.
Genetics and AD
Of 762 infants in a birth cohort with a parent with atopy in Cincinnati, Ohio, 39% developed eczema by the age of 3 years. Single nucleotide polymorphisms of IL-4Rα 175 V and CD14-159 C/T were linked to greater eczema risk at 2 to 3 years of age.23 Monozygotic twins have a concordance rate of 0.72 to 0.86 versus 0.21 to 0.23 in dizygotic twins, demonstrating a strong genetic component in the development of AD.24 Linkage to AD has been positively made to the epidermal differentiation complex on human chromosome 1q21, which contains the genes for filaggrin and other proteins such as loricrin. Other genes linked to AD include the serine protease inhibitor SPINK5 (serine peptidase inhibitor, Kazal type 5) implicated in Netherton syndrome (triad of ichthyosis linearis circumflexa, bamboo hair, and atopic disorders); RANTES (regulated on activation, normal T-expressed, and secreted), which has been associated with severity of AD; IL-4; and IL-13.5,25,26
The Hygiene Hypothesis
Atopic dermatitis is more common in wealthy developed countries, leading some to believe that hygiene and relative reduction in illness via vaccination have contributed to the rise of AD prevalence in developed nations.13,27 There currently is evidence demonstrating that wild-type varicella infection confers long-standing protection against AD and mediates reduced total IgE and peripheral blood lymphocytes.27
Grading of AD
Grading of AD is a subject of controversy, as there currently are no uniform grading scales.28 A recent outcomes group attempted to determine the best scale for disease monitoring. Schmitt et al29 presented the Harmonizing Outcome Measures for Eczema (HOME) roadmap, which was intended to determine a core outcome set for eczema; however, because these outcome measurements have not yet been standardized, only the eczema assessment and severity index (EASI) scoring system meets criteria for standardization. In clinical practice, physicians often assign mild, moderate, or severe labeling based on their general sense of the disease extent using an investigator global assessment score.28
The EASI score is a well-validated composite score of AD severity based on 4 body regions: (1) head and neck, (2) trunk (including genital area), (3) upper limbs, and (4) lower limbs (including buttocks). The total area of involvement in each region is graded on a scale of 0 to 6, and AD severity is graded as a composite of 4 parameters (ranked on a scale of 0–3), including redness (erythema, inflammation), thickness (induration, papulation, swelling [acute eczema]), scratching (excoriation), and lichenification (prurigo nodules [chronic eczema]). The surface area of each region relative to body size is used as a multiplying factor, resulting in the following severity strata: 0=clear; 0.1–1.0=almost clear; 1.1–7.0=mild; 7.1–21.0=moderate; 21.1–50.0=severe; 50.1–72.0=very severe (κ=0.75).30-32 The six area, six sign AD (SASSAD) score32,33 is a similar score without adjustment for body surface area by region.34
An older, now less frequently used eczema score is the SCORAD, which addressed surface area by rule of nines and severity of 6 features—redness, swelling, oozing/crusting, scratch marks, skin thickening (lichenification), dryness (assessed in an area with no inflammation)—by region on a scale of 0 to 3. A subjective symptom parameter for itching and sleeplessness helped highlight that these comorbidities are important in gauging disease activity and impact on a child’s life.35
Natural History of AD
The clinical dogma has been that AD would improve with age, with reduction at grade school entry and perhaps full disappearance in adulthood; however, 3 recent surveys have suggested otherwise. The ISAAC group has found prevalence of AD in wealthy developed countries among children aged 6 to 7 years to be at a consistent increase.36 A US-based survey from the National Health Interview Survey showed a 1-year prevalence of 10.2% of active AD in adults and 9.8% when occupational dermatitis was excluded.37 Halvorsen et al38 demonstrated that eczema prevalence is 9.7% in individuals aged 18 to 19 years.
A prospective trial of eighth graders followed from 1995 to 2010 demonstrated that AD persisted in 50% at school age. Persistent eczema into adulthood was associated with early-onset childhood allergic rhinitis and hand eczema.39 In a cohort of hand eczema patients (N=368), 28% had AD and 39% had an atopic illness.40 An association with allergic contact dermatitis and increased IgE to Malassezia furfur was further associated.41
Conclusion
The role of triggers and allergens in disease activity in AD is an important consideration in children with AD and requires ongoing consideration with age and varied exposures. Understanding the grading of AD is important in evaluating clinical trial data. The natural history of AD has changed, which is important for the practitioner to note when counseling patients and guardians.
- Li M. Current evidence of epidermal barrier dysfunction and thymic stromal lymphopoietin in the atopic march. Eur Respir Rev. 2014;23:292-298.
- Gupta RS, Rivkina V, DeSantiago-Cardenas L, et al. Asthma and food allergy management in Chicago public schools. Pediatrics. 2014;134:729-736.
- Flohr C, Perkin M, Logan K, et al. Atopic dermatitis and disease severity are the main risk factors for food sensitization in exclusively breastfed infants. J Invest Dermatol. 2014;134:345-350.
- Silverberg NB. Food, glorious food. Cutis. 2011;87:267-268.
- De Benedetto A, Kubo A, Beck LA. Skin barrier disruption: a requirement for allergen sensitization? J Invest Dermatol. 2012;132:949-963.
- Thyssen JP, McFadden JP, Kimber I. The multiple factors affecting the association between atopic dermatitis and contact sensitization. Allergy. 2014;69:28-36.
- Amat F, Saint-Pierre P, Bourrat E, et al. Early-onset atopic dermatitis in children: which are the phenotypes at risk of asthma? results from the ORCA Cohort. PLoS One. 2015;10:e0131369.
- Demehri S, Morimoto M, Holtzman MJ, et al. Skin-derived TSLP triggers progression from epidermal-barrier defects to asthma. PLoS Biol. 2009;7:e1000067.
- Silverberg JI, Simpson EL. Association between severe eczema in children and multiple comorbid conditions and increased healthcare utilization. Pediatr Allergy Immunol. 2013;24:476-486.
- Silverberg NB, Lee-Wong M, Yosipovitch G. Diet and atopic dermatitis. Cutis. 2016;97:227-232.
- Ortiz de Frutos FJ, Torrelo A, de Lucas R, et al. Patient perspectives on triggers, adherence to medical recommendations, and disease control in atopic dermatitis: the DATOP study. Actas Dermosifiliogr. 2014;105:487-496.
- Ricci G, Patrizi A, Bellini F, et al. Use of textiles in atopic dermatitis: care of atopic dermatitis. Curr Probl Dermatol. 2006;33:127-143.
- Welliver RC, Wong DT, Sun M, et al. The development of respiratory syncytial virus-specific IgE and the release of histamine in nasopharyngeal secretions after infection. N Engl J Med. 1981;305:841-846.
- Aquino M, Fonacier L. The role of contact dermatitis in patients with atopic dermatitis. J Allergy Clin Immunol Pract. 2014;2:382-387.
- Brod BA, Treat JR, Rothe MJ, et al. Allergic contact dermatitis: kids are not just little people. Clin Dermatol. 2015;33:605-612.
- Martorell A, Alonso E, Boné J, et al. Position document: IgE-mediated allergy to egg protein. Allergol Immunopathol (Madr). 2013;41:320-336.
- Sicherer SH. Early introduction of peanut to infants at high allergic risk can reduce peanut allergy at age 5 years [published online September 17, 2015]. Evid Based Med. 2015;20:204.
- Kiken DA, Silverberg NB. Atopic dermatitis in children, part 1: epidemiology, clinical features, and complications. Cutis. 2006;78:241-247.
- Silverberg JI, Hanifin J, Simpson EL. Climatic factors are associated with childhood eczema prevalence in the United States. J Invest Dermatol. 2013;133:1752-1759.
- Sargen MR, Hoffstad O, Margolis DJ. Warm, humid, and high sun exposure climates are associated with poorly controlled eczema: PEER (Pediatric Eczema Elective Registry) cohort, 2004-2012. J Invest Dermatol. 2014;134:51-57.
- Weiland SK, Hüsing A, Strachan DP, et al. Climate and the prevalence of symptoms of asthma, allergic rhinitis, and atopic eczema in children. Occup Environ Med. 2004;61:609-615.
- Langan SM, Irvine AD. Childhood eczema and the importance of the physical environment. J Invest Dermatol. 2013;133:1706-1709.
- Biagini Myers JM, Wang N, LeMasters GK, et al. Genetic and environmental risk factors for childhood eczema development and allergic sensitization in the CCAAPS cohort. J Invest Dermatol. 2010;130:430-437.
- Brown SJ, McLean WH. Eczema genetics: current state of knowledge and future goals. J Invest Dermatol. 2009;129:543-552.
- Hanifin JM. Evolving concepts of pathogenesis in atopic dermatitis and other eczemas. J Invest Dermatol. 2009;129:320-322.
- Paller AS. Latest approaches to treating atopic dermatitis. Chem Immunol Allergy. 2012;96:132-140.
- Silverberg JI, Norowitz KB, Kleiman E, et al. Association between varicella zoster virus infection and atopic dermatitis in early and late childhood: a case-control study. J Allergy Clin Immunol. 2010;126:300-305.
- Futamura M, Leshem YA, Thomas KS, et al. A systematic review of Investigator Global Assessment (IGA) in atopic dermatitis (AD) trials: many options, no standards. J Am Acad Dermatol. 2016;74:288-294.
- Schmitt J, Apfelbacher C, Spuls PI, et al. The Harmonizing Outcome Measures for Eczema (HOME) roadmap: a methodological framework to develop core sets of outcome measurements in dermatology. J Invest Dermatol. 2015;135:24-30.
- Hanifin JM, Thurston M, Omoto M, et al. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp Dermatol. 2001;10:11-18.
- Leshem YA, Hajar T, Hanifin JM, et al. What the Eczema Area and Severity Index score tells us about the severity of atopic dermatitis: an interpretability study. Br J Dermatol. 2015;172:1353-1357.
- Barbier N, Paul C, Luger T, et al. Validation of the Eczema Area and Severity Index for atopic dermatitis in a cohort of 1550 patients from the pimecrolimus cream 1% randomized controlled clinical trials programme. Br J Dermatol. 2004;150:96-102.
- Berth-Jones J. Six area, six sign atopic dermatitis (SASSAD) severity score: a simple system for monitoring disease activity in atopic dermatitis. Br J Dermatol. 1996;135(suppl 48):25-30.
- Zhao CY, Tran AQ, Lazo-Dizon JP, et al. A pilot comparison study of four clinician-rated atopic dermatitis severity scales. Br J Dermatol. 2015;173:488-497.
- Kunz B, Oranje AP, Labrèze L, et al. Clinical validation and guidelines for the SCORAD index: consensus report of the European Task Force on Atopic Dermatitis. Dermatology. 1997;195:10-19.
- Williams H, Stewart A, von Mutius E, et al. Is eczema really on the increase worldwide? J Allergy Clin Immunol. 2008;121:947-954.
- Silverberg JI, Hanifin JM. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population-based study. J Allergy Clin Immunol. 2013;132:1132-1138.
- Halvorsen JA, Lien L, Dalgard F, et al. Suicidal ideation, mental health problems, and social function in adolescents with eczema: a population-based study. J Invest Dermatol. 2014;134:1847-1854.
- Mortz CG, Andersen KE, Dellgren C, et al. Atopic dermatitis from adolescence to adulthood in the TOACS cohort: prevalence, persistence, and comorbidities. Allergy. 2015;70:836-845.
- Rystedt I. Atopic background in patients with occupational hand eczema. Contact Dermatitis. 1985;12:247-254.
- Mortz CG, Andersen KE, Dellgren C, et al. Atopic dermatitis from adolescence to adulthood in the TOACS cohort: prevalence, persistence and comorbidities. Allergy. 2015;70:836-845.
Atopic dermatitis (AD) may be triggered by viral infections, food allergens, weather, and other causes, and it may trigger an inflammatory progression known as the atopic march. This article reviews research on triggers of pediatric AD so that dermatologists may discuss trigger avoidance with patients and guardians. Other factors affecting AD development include genetics and hygiene. Grading of AD also is discussed.
The Atopic March
The persistence of AD in untreated skin can trigger an inflammatory progression called the atopic march in which food and environmental allergies as well as asthma may occur progressively due to ongoing inflammatory triggering.1 In a study of asthma and food allergy reporting and management in public schools in Chicago, Illinois, food allergies were seen in 9.3% of asthmatic students (n=18,000), and 40.1% of food allergic students (n=4000) had asthma.2 An observational study by Flohr et al3 in London, England, included 619 exclusively breastfed infants who were recruited at 3 months of age. The investigators determined that food sensitization was unrelated to the presence of filaggrin mutations, type of eczema (flexural vs nonflexural), and transepidermal water loss but was associated with AD severity as determined by SCORAD (SCORing Atopic Dermatitis), a composite score of AD that includes pruritus as a factor in severity. Other AD associations included 3 leading food allergens: eggs, milk, and peanuts. No association with cod, wheat, or sesame allergy was noted. The investigators concluded that AD and AD severity were the leading skin-related risk factors for food allergies and therefore food allergy development in breastfed infants was probably mediated by cutaneous antigen-presenting cells.3
The skin has been documented to react to contact with known food allergens4 and is known to be a route of allergic sensitization to allergens such as fragrance in patients with AD.5,6 Two phenotypes of eczema that have been associated with asthma development are severe AD disease and multiple environmental allergies, supporting the theory of the atopic march.7 There also is evidence that release of danger-associated proteins from an impaired barrier also may trigger asthma.8 An analysis of the 2007 National Survey of Children’s Health, a population-based study of91,642 children aged 0 to 17 years, showed that children with AD had a higher prevalence of comorbid asthma (25.1% vs 12.3%), hay fever (34.4% vs 14.3%), and food allergies (15.1% vs 3.6%) compared to children without AD.9 A recent article provided detailed information on how food and diet interplay with AD.10
Triggers of Disease Flares
Triggers are the leading source of AD flare initiation, and avoidance of triggers is an important mechanism by which patients can control disease activity. Despite the best skin care and trigger avoidance, disease flares occur, sometimes due to ongoing inflammation and other times due to inability to prevent flares such as heat and humidity. A survey of patients with AD in Spain identified the following triggers: cosmetic products, clothing, mites, detergents/soaps, and temperature changes.11 In childhood, wool also is a known trigger of AD.12 Viral infections including respiratory syncytial virus may trigger the first onset of AD.13 Patients with AD may become allergic to fragrance and metals causing disease exacerbation on exposure.14,15 Food allergens contribute to approximately 40% of cases of AD in infancy but are not the cause of AD. The best evidence for improvement of AD with food allergen avoidance exists for egg white allergy.16 Food avoidance programs should be developed in conjunction with an allergist, as it is no longer advised in many cases to completely withdraw foods; therefore, an allergist has to assess the level of allergic severity and the risk-benefit ratio of food avoidance or introduction.17 Emotional stressors, heat, and humidity, as well as indoor heating in the winter months, can cause AD flares.18
A study by Silverberg et al19 provided evidence of climate influences on the US prevalence of childhood eczema using a merged analysis of the 2007 National Survey of Children’s Health and the 2006-2007 National Climate Data Center and Weather Service. Results showed that eczema prevalence was significantly lower when associated with higher annual relative humidity (P=.01), UV index (P<.0001), and highest-quartile air temperature (P=.002).19 The Pediatric Eczema Elective Registry also showed that warm, humid, and high-sun-exposure climates are associated with poorly controlled eczema in affected patients.20 The association of eczema with latitude as well as its negative association with mean annual outdoor temperature has been described by Weiland et al21 in the ISAAC (International Study of Asthma and Allergies in Childhood) study. Long airplane flights in low humidity can trigger eczema in adults. Climate has been postulated to affect eczema through alterations in filaggrin and skin barrier function.22 Indoor temperature and humidity regulation may be used adjunctively for daily flare prevention.
Genetics and AD
Of 762 infants in a birth cohort with a parent with atopy in Cincinnati, Ohio, 39% developed eczema by the age of 3 years. Single nucleotide polymorphisms of IL-4Rα 175 V and CD14-159 C/T were linked to greater eczema risk at 2 to 3 years of age.23 Monozygotic twins have a concordance rate of 0.72 to 0.86 versus 0.21 to 0.23 in dizygotic twins, demonstrating a strong genetic component in the development of AD.24 Linkage to AD has been positively made to the epidermal differentiation complex on human chromosome 1q21, which contains the genes for filaggrin and other proteins such as loricrin. Other genes linked to AD include the serine protease inhibitor SPINK5 (serine peptidase inhibitor, Kazal type 5) implicated in Netherton syndrome (triad of ichthyosis linearis circumflexa, bamboo hair, and atopic disorders); RANTES (regulated on activation, normal T-expressed, and secreted), which has been associated with severity of AD; IL-4; and IL-13.5,25,26
The Hygiene Hypothesis
Atopic dermatitis is more common in wealthy developed countries, leading some to believe that hygiene and relative reduction in illness via vaccination have contributed to the rise of AD prevalence in developed nations.13,27 There currently is evidence demonstrating that wild-type varicella infection confers long-standing protection against AD and mediates reduced total IgE and peripheral blood lymphocytes.27
Grading of AD
Grading of AD is a subject of controversy, as there currently are no uniform grading scales.28 A recent outcomes group attempted to determine the best scale for disease monitoring. Schmitt et al29 presented the Harmonizing Outcome Measures for Eczema (HOME) roadmap, which was intended to determine a core outcome set for eczema; however, because these outcome measurements have not yet been standardized, only the eczema assessment and severity index (EASI) scoring system meets criteria for standardization. In clinical practice, physicians often assign mild, moderate, or severe labeling based on their general sense of the disease extent using an investigator global assessment score.28
The EASI score is a well-validated composite score of AD severity based on 4 body regions: (1) head and neck, (2) trunk (including genital area), (3) upper limbs, and (4) lower limbs (including buttocks). The total area of involvement in each region is graded on a scale of 0 to 6, and AD severity is graded as a composite of 4 parameters (ranked on a scale of 0–3), including redness (erythema, inflammation), thickness (induration, papulation, swelling [acute eczema]), scratching (excoriation), and lichenification (prurigo nodules [chronic eczema]). The surface area of each region relative to body size is used as a multiplying factor, resulting in the following severity strata: 0=clear; 0.1–1.0=almost clear; 1.1–7.0=mild; 7.1–21.0=moderate; 21.1–50.0=severe; 50.1–72.0=very severe (κ=0.75).30-32 The six area, six sign AD (SASSAD) score32,33 is a similar score without adjustment for body surface area by region.34
An older, now less frequently used eczema score is the SCORAD, which addressed surface area by rule of nines and severity of 6 features—redness, swelling, oozing/crusting, scratch marks, skin thickening (lichenification), dryness (assessed in an area with no inflammation)—by region on a scale of 0 to 3. A subjective symptom parameter for itching and sleeplessness helped highlight that these comorbidities are important in gauging disease activity and impact on a child’s life.35
Natural History of AD
The clinical dogma has been that AD would improve with age, with reduction at grade school entry and perhaps full disappearance in adulthood; however, 3 recent surveys have suggested otherwise. The ISAAC group has found prevalence of AD in wealthy developed countries among children aged 6 to 7 years to be at a consistent increase.36 A US-based survey from the National Health Interview Survey showed a 1-year prevalence of 10.2% of active AD in adults and 9.8% when occupational dermatitis was excluded.37 Halvorsen et al38 demonstrated that eczema prevalence is 9.7% in individuals aged 18 to 19 years.
A prospective trial of eighth graders followed from 1995 to 2010 demonstrated that AD persisted in 50% at school age. Persistent eczema into adulthood was associated with early-onset childhood allergic rhinitis and hand eczema.39 In a cohort of hand eczema patients (N=368), 28% had AD and 39% had an atopic illness.40 An association with allergic contact dermatitis and increased IgE to Malassezia furfur was further associated.41
Conclusion
The role of triggers and allergens in disease activity in AD is an important consideration in children with AD and requires ongoing consideration with age and varied exposures. Understanding the grading of AD is important in evaluating clinical trial data. The natural history of AD has changed, which is important for the practitioner to note when counseling patients and guardians.
Atopic dermatitis (AD) may be triggered by viral infections, food allergens, weather, and other causes, and it may trigger an inflammatory progression known as the atopic march. This article reviews research on triggers of pediatric AD so that dermatologists may discuss trigger avoidance with patients and guardians. Other factors affecting AD development include genetics and hygiene. Grading of AD also is discussed.
The Atopic March
The persistence of AD in untreated skin can trigger an inflammatory progression called the atopic march in which food and environmental allergies as well as asthma may occur progressively due to ongoing inflammatory triggering.1 In a study of asthma and food allergy reporting and management in public schools in Chicago, Illinois, food allergies were seen in 9.3% of asthmatic students (n=18,000), and 40.1% of food allergic students (n=4000) had asthma.2 An observational study by Flohr et al3 in London, England, included 619 exclusively breastfed infants who were recruited at 3 months of age. The investigators determined that food sensitization was unrelated to the presence of filaggrin mutations, type of eczema (flexural vs nonflexural), and transepidermal water loss but was associated with AD severity as determined by SCORAD (SCORing Atopic Dermatitis), a composite score of AD that includes pruritus as a factor in severity. Other AD associations included 3 leading food allergens: eggs, milk, and peanuts. No association with cod, wheat, or sesame allergy was noted. The investigators concluded that AD and AD severity were the leading skin-related risk factors for food allergies and therefore food allergy development in breastfed infants was probably mediated by cutaneous antigen-presenting cells.3
The skin has been documented to react to contact with known food allergens4 and is known to be a route of allergic sensitization to allergens such as fragrance in patients with AD.5,6 Two phenotypes of eczema that have been associated with asthma development are severe AD disease and multiple environmental allergies, supporting the theory of the atopic march.7 There also is evidence that release of danger-associated proteins from an impaired barrier also may trigger asthma.8 An analysis of the 2007 National Survey of Children’s Health, a population-based study of91,642 children aged 0 to 17 years, showed that children with AD had a higher prevalence of comorbid asthma (25.1% vs 12.3%), hay fever (34.4% vs 14.3%), and food allergies (15.1% vs 3.6%) compared to children without AD.9 A recent article provided detailed information on how food and diet interplay with AD.10
Triggers of Disease Flares
Triggers are the leading source of AD flare initiation, and avoidance of triggers is an important mechanism by which patients can control disease activity. Despite the best skin care and trigger avoidance, disease flares occur, sometimes due to ongoing inflammation and other times due to inability to prevent flares such as heat and humidity. A survey of patients with AD in Spain identified the following triggers: cosmetic products, clothing, mites, detergents/soaps, and temperature changes.11 In childhood, wool also is a known trigger of AD.12 Viral infections including respiratory syncytial virus may trigger the first onset of AD.13 Patients with AD may become allergic to fragrance and metals causing disease exacerbation on exposure.14,15 Food allergens contribute to approximately 40% of cases of AD in infancy but are not the cause of AD. The best evidence for improvement of AD with food allergen avoidance exists for egg white allergy.16 Food avoidance programs should be developed in conjunction with an allergist, as it is no longer advised in many cases to completely withdraw foods; therefore, an allergist has to assess the level of allergic severity and the risk-benefit ratio of food avoidance or introduction.17 Emotional stressors, heat, and humidity, as well as indoor heating in the winter months, can cause AD flares.18
A study by Silverberg et al19 provided evidence of climate influences on the US prevalence of childhood eczema using a merged analysis of the 2007 National Survey of Children’s Health and the 2006-2007 National Climate Data Center and Weather Service. Results showed that eczema prevalence was significantly lower when associated with higher annual relative humidity (P=.01), UV index (P<.0001), and highest-quartile air temperature (P=.002).19 The Pediatric Eczema Elective Registry also showed that warm, humid, and high-sun-exposure climates are associated with poorly controlled eczema in affected patients.20 The association of eczema with latitude as well as its negative association with mean annual outdoor temperature has been described by Weiland et al21 in the ISAAC (International Study of Asthma and Allergies in Childhood) study. Long airplane flights in low humidity can trigger eczema in adults. Climate has been postulated to affect eczema through alterations in filaggrin and skin barrier function.22 Indoor temperature and humidity regulation may be used adjunctively for daily flare prevention.
Genetics and AD
Of 762 infants in a birth cohort with a parent with atopy in Cincinnati, Ohio, 39% developed eczema by the age of 3 years. Single nucleotide polymorphisms of IL-4Rα 175 V and CD14-159 C/T were linked to greater eczema risk at 2 to 3 years of age.23 Monozygotic twins have a concordance rate of 0.72 to 0.86 versus 0.21 to 0.23 in dizygotic twins, demonstrating a strong genetic component in the development of AD.24 Linkage to AD has been positively made to the epidermal differentiation complex on human chromosome 1q21, which contains the genes for filaggrin and other proteins such as loricrin. Other genes linked to AD include the serine protease inhibitor SPINK5 (serine peptidase inhibitor, Kazal type 5) implicated in Netherton syndrome (triad of ichthyosis linearis circumflexa, bamboo hair, and atopic disorders); RANTES (regulated on activation, normal T-expressed, and secreted), which has been associated with severity of AD; IL-4; and IL-13.5,25,26
The Hygiene Hypothesis
Atopic dermatitis is more common in wealthy developed countries, leading some to believe that hygiene and relative reduction in illness via vaccination have contributed to the rise of AD prevalence in developed nations.13,27 There currently is evidence demonstrating that wild-type varicella infection confers long-standing protection against AD and mediates reduced total IgE and peripheral blood lymphocytes.27
Grading of AD
Grading of AD is a subject of controversy, as there currently are no uniform grading scales.28 A recent outcomes group attempted to determine the best scale for disease monitoring. Schmitt et al29 presented the Harmonizing Outcome Measures for Eczema (HOME) roadmap, which was intended to determine a core outcome set for eczema; however, because these outcome measurements have not yet been standardized, only the eczema assessment and severity index (EASI) scoring system meets criteria for standardization. In clinical practice, physicians often assign mild, moderate, or severe labeling based on their general sense of the disease extent using an investigator global assessment score.28
The EASI score is a well-validated composite score of AD severity based on 4 body regions: (1) head and neck, (2) trunk (including genital area), (3) upper limbs, and (4) lower limbs (including buttocks). The total area of involvement in each region is graded on a scale of 0 to 6, and AD severity is graded as a composite of 4 parameters (ranked on a scale of 0–3), including redness (erythema, inflammation), thickness (induration, papulation, swelling [acute eczema]), scratching (excoriation), and lichenification (prurigo nodules [chronic eczema]). The surface area of each region relative to body size is used as a multiplying factor, resulting in the following severity strata: 0=clear; 0.1–1.0=almost clear; 1.1–7.0=mild; 7.1–21.0=moderate; 21.1–50.0=severe; 50.1–72.0=very severe (κ=0.75).30-32 The six area, six sign AD (SASSAD) score32,33 is a similar score without adjustment for body surface area by region.34
An older, now less frequently used eczema score is the SCORAD, which addressed surface area by rule of nines and severity of 6 features—redness, swelling, oozing/crusting, scratch marks, skin thickening (lichenification), dryness (assessed in an area with no inflammation)—by region on a scale of 0 to 3. A subjective symptom parameter for itching and sleeplessness helped highlight that these comorbidities are important in gauging disease activity and impact on a child’s life.35
Natural History of AD
The clinical dogma has been that AD would improve with age, with reduction at grade school entry and perhaps full disappearance in adulthood; however, 3 recent surveys have suggested otherwise. The ISAAC group has found prevalence of AD in wealthy developed countries among children aged 6 to 7 years to be at a consistent increase.36 A US-based survey from the National Health Interview Survey showed a 1-year prevalence of 10.2% of active AD in adults and 9.8% when occupational dermatitis was excluded.37 Halvorsen et al38 demonstrated that eczema prevalence is 9.7% in individuals aged 18 to 19 years.
A prospective trial of eighth graders followed from 1995 to 2010 demonstrated that AD persisted in 50% at school age. Persistent eczema into adulthood was associated with early-onset childhood allergic rhinitis and hand eczema.39 In a cohort of hand eczema patients (N=368), 28% had AD and 39% had an atopic illness.40 An association with allergic contact dermatitis and increased IgE to Malassezia furfur was further associated.41
Conclusion
The role of triggers and allergens in disease activity in AD is an important consideration in children with AD and requires ongoing consideration with age and varied exposures. Understanding the grading of AD is important in evaluating clinical trial data. The natural history of AD has changed, which is important for the practitioner to note when counseling patients and guardians.
- Li M. Current evidence of epidermal barrier dysfunction and thymic stromal lymphopoietin in the atopic march. Eur Respir Rev. 2014;23:292-298.
- Gupta RS, Rivkina V, DeSantiago-Cardenas L, et al. Asthma and food allergy management in Chicago public schools. Pediatrics. 2014;134:729-736.
- Flohr C, Perkin M, Logan K, et al. Atopic dermatitis and disease severity are the main risk factors for food sensitization in exclusively breastfed infants. J Invest Dermatol. 2014;134:345-350.
- Silverberg NB. Food, glorious food. Cutis. 2011;87:267-268.
- De Benedetto A, Kubo A, Beck LA. Skin barrier disruption: a requirement for allergen sensitization? J Invest Dermatol. 2012;132:949-963.
- Thyssen JP, McFadden JP, Kimber I. The multiple factors affecting the association between atopic dermatitis and contact sensitization. Allergy. 2014;69:28-36.
- Amat F, Saint-Pierre P, Bourrat E, et al. Early-onset atopic dermatitis in children: which are the phenotypes at risk of asthma? results from the ORCA Cohort. PLoS One. 2015;10:e0131369.
- Demehri S, Morimoto M, Holtzman MJ, et al. Skin-derived TSLP triggers progression from epidermal-barrier defects to asthma. PLoS Biol. 2009;7:e1000067.
- Silverberg JI, Simpson EL. Association between severe eczema in children and multiple comorbid conditions and increased healthcare utilization. Pediatr Allergy Immunol. 2013;24:476-486.
- Silverberg NB, Lee-Wong M, Yosipovitch G. Diet and atopic dermatitis. Cutis. 2016;97:227-232.
- Ortiz de Frutos FJ, Torrelo A, de Lucas R, et al. Patient perspectives on triggers, adherence to medical recommendations, and disease control in atopic dermatitis: the DATOP study. Actas Dermosifiliogr. 2014;105:487-496.
- Ricci G, Patrizi A, Bellini F, et al. Use of textiles in atopic dermatitis: care of atopic dermatitis. Curr Probl Dermatol. 2006;33:127-143.
- Welliver RC, Wong DT, Sun M, et al. The development of respiratory syncytial virus-specific IgE and the release of histamine in nasopharyngeal secretions after infection. N Engl J Med. 1981;305:841-846.
- Aquino M, Fonacier L. The role of contact dermatitis in patients with atopic dermatitis. J Allergy Clin Immunol Pract. 2014;2:382-387.
- Brod BA, Treat JR, Rothe MJ, et al. Allergic contact dermatitis: kids are not just little people. Clin Dermatol. 2015;33:605-612.
- Martorell A, Alonso E, Boné J, et al. Position document: IgE-mediated allergy to egg protein. Allergol Immunopathol (Madr). 2013;41:320-336.
- Sicherer SH. Early introduction of peanut to infants at high allergic risk can reduce peanut allergy at age 5 years [published online September 17, 2015]. Evid Based Med. 2015;20:204.
- Kiken DA, Silverberg NB. Atopic dermatitis in children, part 1: epidemiology, clinical features, and complications. Cutis. 2006;78:241-247.
- Silverberg JI, Hanifin J, Simpson EL. Climatic factors are associated with childhood eczema prevalence in the United States. J Invest Dermatol. 2013;133:1752-1759.
- Sargen MR, Hoffstad O, Margolis DJ. Warm, humid, and high sun exposure climates are associated with poorly controlled eczema: PEER (Pediatric Eczema Elective Registry) cohort, 2004-2012. J Invest Dermatol. 2014;134:51-57.
- Weiland SK, Hüsing A, Strachan DP, et al. Climate and the prevalence of symptoms of asthma, allergic rhinitis, and atopic eczema in children. Occup Environ Med. 2004;61:609-615.
- Langan SM, Irvine AD. Childhood eczema and the importance of the physical environment. J Invest Dermatol. 2013;133:1706-1709.
- Biagini Myers JM, Wang N, LeMasters GK, et al. Genetic and environmental risk factors for childhood eczema development and allergic sensitization in the CCAAPS cohort. J Invest Dermatol. 2010;130:430-437.
- Brown SJ, McLean WH. Eczema genetics: current state of knowledge and future goals. J Invest Dermatol. 2009;129:543-552.
- Hanifin JM. Evolving concepts of pathogenesis in atopic dermatitis and other eczemas. J Invest Dermatol. 2009;129:320-322.
- Paller AS. Latest approaches to treating atopic dermatitis. Chem Immunol Allergy. 2012;96:132-140.
- Silverberg JI, Norowitz KB, Kleiman E, et al. Association between varicella zoster virus infection and atopic dermatitis in early and late childhood: a case-control study. J Allergy Clin Immunol. 2010;126:300-305.
- Futamura M, Leshem YA, Thomas KS, et al. A systematic review of Investigator Global Assessment (IGA) in atopic dermatitis (AD) trials: many options, no standards. J Am Acad Dermatol. 2016;74:288-294.
- Schmitt J, Apfelbacher C, Spuls PI, et al. The Harmonizing Outcome Measures for Eczema (HOME) roadmap: a methodological framework to develop core sets of outcome measurements in dermatology. J Invest Dermatol. 2015;135:24-30.
- Hanifin JM, Thurston M, Omoto M, et al. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp Dermatol. 2001;10:11-18.
- Leshem YA, Hajar T, Hanifin JM, et al. What the Eczema Area and Severity Index score tells us about the severity of atopic dermatitis: an interpretability study. Br J Dermatol. 2015;172:1353-1357.
- Barbier N, Paul C, Luger T, et al. Validation of the Eczema Area and Severity Index for atopic dermatitis in a cohort of 1550 patients from the pimecrolimus cream 1% randomized controlled clinical trials programme. Br J Dermatol. 2004;150:96-102.
- Berth-Jones J. Six area, six sign atopic dermatitis (SASSAD) severity score: a simple system for monitoring disease activity in atopic dermatitis. Br J Dermatol. 1996;135(suppl 48):25-30.
- Zhao CY, Tran AQ, Lazo-Dizon JP, et al. A pilot comparison study of four clinician-rated atopic dermatitis severity scales. Br J Dermatol. 2015;173:488-497.
- Kunz B, Oranje AP, Labrèze L, et al. Clinical validation and guidelines for the SCORAD index: consensus report of the European Task Force on Atopic Dermatitis. Dermatology. 1997;195:10-19.
- Williams H, Stewart A, von Mutius E, et al. Is eczema really on the increase worldwide? J Allergy Clin Immunol. 2008;121:947-954.
- Silverberg JI, Hanifin JM. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population-based study. J Allergy Clin Immunol. 2013;132:1132-1138.
- Halvorsen JA, Lien L, Dalgard F, et al. Suicidal ideation, mental health problems, and social function in adolescents with eczema: a population-based study. J Invest Dermatol. 2014;134:1847-1854.
- Mortz CG, Andersen KE, Dellgren C, et al. Atopic dermatitis from adolescence to adulthood in the TOACS cohort: prevalence, persistence, and comorbidities. Allergy. 2015;70:836-845.
- Rystedt I. Atopic background in patients with occupational hand eczema. Contact Dermatitis. 1985;12:247-254.
- Mortz CG, Andersen KE, Dellgren C, et al. Atopic dermatitis from adolescence to adulthood in the TOACS cohort: prevalence, persistence and comorbidities. Allergy. 2015;70:836-845.
- Li M. Current evidence of epidermal barrier dysfunction and thymic stromal lymphopoietin in the atopic march. Eur Respir Rev. 2014;23:292-298.
- Gupta RS, Rivkina V, DeSantiago-Cardenas L, et al. Asthma and food allergy management in Chicago public schools. Pediatrics. 2014;134:729-736.
- Flohr C, Perkin M, Logan K, et al. Atopic dermatitis and disease severity are the main risk factors for food sensitization in exclusively breastfed infants. J Invest Dermatol. 2014;134:345-350.
- Silverberg NB. Food, glorious food. Cutis. 2011;87:267-268.
- De Benedetto A, Kubo A, Beck LA. Skin barrier disruption: a requirement for allergen sensitization? J Invest Dermatol. 2012;132:949-963.
- Thyssen JP, McFadden JP, Kimber I. The multiple factors affecting the association between atopic dermatitis and contact sensitization. Allergy. 2014;69:28-36.
- Amat F, Saint-Pierre P, Bourrat E, et al. Early-onset atopic dermatitis in children: which are the phenotypes at risk of asthma? results from the ORCA Cohort. PLoS One. 2015;10:e0131369.
- Demehri S, Morimoto M, Holtzman MJ, et al. Skin-derived TSLP triggers progression from epidermal-barrier defects to asthma. PLoS Biol. 2009;7:e1000067.
- Silverberg JI, Simpson EL. Association between severe eczema in children and multiple comorbid conditions and increased healthcare utilization. Pediatr Allergy Immunol. 2013;24:476-486.
- Silverberg NB, Lee-Wong M, Yosipovitch G. Diet and atopic dermatitis. Cutis. 2016;97:227-232.
- Ortiz de Frutos FJ, Torrelo A, de Lucas R, et al. Patient perspectives on triggers, adherence to medical recommendations, and disease control in atopic dermatitis: the DATOP study. Actas Dermosifiliogr. 2014;105:487-496.
- Ricci G, Patrizi A, Bellini F, et al. Use of textiles in atopic dermatitis: care of atopic dermatitis. Curr Probl Dermatol. 2006;33:127-143.
- Welliver RC, Wong DT, Sun M, et al. The development of respiratory syncytial virus-specific IgE and the release of histamine in nasopharyngeal secretions after infection. N Engl J Med. 1981;305:841-846.
- Aquino M, Fonacier L. The role of contact dermatitis in patients with atopic dermatitis. J Allergy Clin Immunol Pract. 2014;2:382-387.
- Brod BA, Treat JR, Rothe MJ, et al. Allergic contact dermatitis: kids are not just little people. Clin Dermatol. 2015;33:605-612.
- Martorell A, Alonso E, Boné J, et al. Position document: IgE-mediated allergy to egg protein. Allergol Immunopathol (Madr). 2013;41:320-336.
- Sicherer SH. Early introduction of peanut to infants at high allergic risk can reduce peanut allergy at age 5 years [published online September 17, 2015]. Evid Based Med. 2015;20:204.
- Kiken DA, Silverberg NB. Atopic dermatitis in children, part 1: epidemiology, clinical features, and complications. Cutis. 2006;78:241-247.
- Silverberg JI, Hanifin J, Simpson EL. Climatic factors are associated with childhood eczema prevalence in the United States. J Invest Dermatol. 2013;133:1752-1759.
- Sargen MR, Hoffstad O, Margolis DJ. Warm, humid, and high sun exposure climates are associated with poorly controlled eczema: PEER (Pediatric Eczema Elective Registry) cohort, 2004-2012. J Invest Dermatol. 2014;134:51-57.
- Weiland SK, Hüsing A, Strachan DP, et al. Climate and the prevalence of symptoms of asthma, allergic rhinitis, and atopic eczema in children. Occup Environ Med. 2004;61:609-615.
- Langan SM, Irvine AD. Childhood eczema and the importance of the physical environment. J Invest Dermatol. 2013;133:1706-1709.
- Biagini Myers JM, Wang N, LeMasters GK, et al. Genetic and environmental risk factors for childhood eczema development and allergic sensitization in the CCAAPS cohort. J Invest Dermatol. 2010;130:430-437.
- Brown SJ, McLean WH. Eczema genetics: current state of knowledge and future goals. J Invest Dermatol. 2009;129:543-552.
- Hanifin JM. Evolving concepts of pathogenesis in atopic dermatitis and other eczemas. J Invest Dermatol. 2009;129:320-322.
- Paller AS. Latest approaches to treating atopic dermatitis. Chem Immunol Allergy. 2012;96:132-140.
- Silverberg JI, Norowitz KB, Kleiman E, et al. Association between varicella zoster virus infection and atopic dermatitis in early and late childhood: a case-control study. J Allergy Clin Immunol. 2010;126:300-305.
- Futamura M, Leshem YA, Thomas KS, et al. A systematic review of Investigator Global Assessment (IGA) in atopic dermatitis (AD) trials: many options, no standards. J Am Acad Dermatol. 2016;74:288-294.
- Schmitt J, Apfelbacher C, Spuls PI, et al. The Harmonizing Outcome Measures for Eczema (HOME) roadmap: a methodological framework to develop core sets of outcome measurements in dermatology. J Invest Dermatol. 2015;135:24-30.
- Hanifin JM, Thurston M, Omoto M, et al. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp Dermatol. 2001;10:11-18.
- Leshem YA, Hajar T, Hanifin JM, et al. What the Eczema Area and Severity Index score tells us about the severity of atopic dermatitis: an interpretability study. Br J Dermatol. 2015;172:1353-1357.
- Barbier N, Paul C, Luger T, et al. Validation of the Eczema Area and Severity Index for atopic dermatitis in a cohort of 1550 patients from the pimecrolimus cream 1% randomized controlled clinical trials programme. Br J Dermatol. 2004;150:96-102.
- Berth-Jones J. Six area, six sign atopic dermatitis (SASSAD) severity score: a simple system for monitoring disease activity in atopic dermatitis. Br J Dermatol. 1996;135(suppl 48):25-30.
- Zhao CY, Tran AQ, Lazo-Dizon JP, et al. A pilot comparison study of four clinician-rated atopic dermatitis severity scales. Br J Dermatol. 2015;173:488-497.
- Kunz B, Oranje AP, Labrèze L, et al. Clinical validation and guidelines for the SCORAD index: consensus report of the European Task Force on Atopic Dermatitis. Dermatology. 1997;195:10-19.
- Williams H, Stewart A, von Mutius E, et al. Is eczema really on the increase worldwide? J Allergy Clin Immunol. 2008;121:947-954.
- Silverberg JI, Hanifin JM. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population-based study. J Allergy Clin Immunol. 2013;132:1132-1138.
- Halvorsen JA, Lien L, Dalgard F, et al. Suicidal ideation, mental health problems, and social function in adolescents with eczema: a population-based study. J Invest Dermatol. 2014;134:1847-1854.
- Mortz CG, Andersen KE, Dellgren C, et al. Atopic dermatitis from adolescence to adulthood in the TOACS cohort: prevalence, persistence, and comorbidities. Allergy. 2015;70:836-845.
- Rystedt I. Atopic background in patients with occupational hand eczema. Contact Dermatitis. 1985;12:247-254.
- Mortz CG, Andersen KE, Dellgren C, et al. Atopic dermatitis from adolescence to adulthood in the TOACS cohort: prevalence, persistence and comorbidities. Allergy. 2015;70:836-845.
Practice Points
- Atopic dermatitis (AD) can be triggered by viral infections, weather, and food allergens.
- The scoring of AD is largely used experimentally and includes the eczema assessment and severity index; the SCORAD (SCORing Atopic Dermatitis); and the six area, six sign AD (SASSAD) scores.
- There is a strong genetic contribution to the development of AD.
- Children with AD may have persistent disease into adulthood in half of cases.