User login
How Safe is Anti–IL-6 Therapy During Pregnancy?
TOPLINE:
The maternal and neonatal outcomes in pregnant women treated with anti–interleukin (IL)-6 therapy for COVID-19 are largely favorable, with transient neonatal cytopenia observed in around one third of the babies being the only possible adverse outcome that could be related to anti–IL-6 therapy.
METHODOLOGY:
- Despite guidance, very few pregnant women with COVID-19 are offered evidence-based therapies such as anti–IL-6 due to concerns regarding fetal safety in later pregnancy.
- In this retrospective study, researchers evaluated maternal and neonatal outcomes in 25 pregnant women with COVID-19 (mean age at admission, 33 years) treated with anti–IL-6 (tocilizumab or sarilumab) at two tertiary hospitals in London.
- Most women (n = 16) received anti–IL-6 in the third trimester of pregnancy, whereas nine received it during the second trimester.
- Maternal and neonatal outcomes were assessed through medical record reviews and maternal medicine networks, with follow-up for 12 months.
- The women included in the study constituted a high-risk population with severe COVID-19; 24 required level two or three critical care. All women were receiving at least three concomitant medications due to their critical illness.
TAKEAWAY:
- Overall, 24 of 25 women treated with IL-6 receptor antibodies survived until hospital discharge.
- The sole death occurred in a woman with severe COVID-19 pneumonitis who later developed myocarditis and cardiac arrest. The physicians believed that these complications were more likely due to severe COVID-19 rather than anti–IL-6 therapy.
- All pregnancies resulted in live births; however, 16 babies had to be delivered preterm due to COVID-19 complications.
- Transient cytopenia was observed in 6 of 19 babies in whom a full blood count was performed. All the six babies were premature, with cytopenia resolving within 7 days in four babies; one baby died from complications associated with extreme prematurity.
IN PRACTICE:
“Although the authors found mild, transitory cytopenia in some (6 of 19) exposed infants, most had been delivered prematurely due to progressive COVID-19–related morbidity, and distinguishing drug effects from similar prematurity-related effects is difficult,” wrote Steven L. Clark, MD, from the Department of Obstetrics and Gynecology, Baylor College of Medicine, Houston, in an accompanying editorial.
SOURCE:
The study was led by Melanie Nana, MRCP, from the Department of Obstetric Medicine, St Thomas’ Hospital, London, England. It was published online in The Lancet Rheumatology.
LIMITATIONS:
The study was retrospective in design, which may have introduced bias. The small sample size of 25 women may have limited the generalizability of the findings. Additionally, the study did not include a control group, which made it difficult to attribute outcomes solely to anti–IL-6 therapy. The lack of long-term follow-up data on the neonates also limited the understanding of potential long-term effects.
DISCLOSURES:
This study did not receive any funding. Some authors, including the lead author, received speaker fees, grants, or consultancy fees from academic institutions or pharmaceutical companies or had other ties with various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
The maternal and neonatal outcomes in pregnant women treated with anti–interleukin (IL)-6 therapy for COVID-19 are largely favorable, with transient neonatal cytopenia observed in around one third of the babies being the only possible adverse outcome that could be related to anti–IL-6 therapy.
METHODOLOGY:
- Despite guidance, very few pregnant women with COVID-19 are offered evidence-based therapies such as anti–IL-6 due to concerns regarding fetal safety in later pregnancy.
- In this retrospective study, researchers evaluated maternal and neonatal outcomes in 25 pregnant women with COVID-19 (mean age at admission, 33 years) treated with anti–IL-6 (tocilizumab or sarilumab) at two tertiary hospitals in London.
- Most women (n = 16) received anti–IL-6 in the third trimester of pregnancy, whereas nine received it during the second trimester.
- Maternal and neonatal outcomes were assessed through medical record reviews and maternal medicine networks, with follow-up for 12 months.
- The women included in the study constituted a high-risk population with severe COVID-19; 24 required level two or three critical care. All women were receiving at least three concomitant medications due to their critical illness.
TAKEAWAY:
- Overall, 24 of 25 women treated with IL-6 receptor antibodies survived until hospital discharge.
- The sole death occurred in a woman with severe COVID-19 pneumonitis who later developed myocarditis and cardiac arrest. The physicians believed that these complications were more likely due to severe COVID-19 rather than anti–IL-6 therapy.
- All pregnancies resulted in live births; however, 16 babies had to be delivered preterm due to COVID-19 complications.
- Transient cytopenia was observed in 6 of 19 babies in whom a full blood count was performed. All the six babies were premature, with cytopenia resolving within 7 days in four babies; one baby died from complications associated with extreme prematurity.
IN PRACTICE:
“Although the authors found mild, transitory cytopenia in some (6 of 19) exposed infants, most had been delivered prematurely due to progressive COVID-19–related morbidity, and distinguishing drug effects from similar prematurity-related effects is difficult,” wrote Steven L. Clark, MD, from the Department of Obstetrics and Gynecology, Baylor College of Medicine, Houston, in an accompanying editorial.
SOURCE:
The study was led by Melanie Nana, MRCP, from the Department of Obstetric Medicine, St Thomas’ Hospital, London, England. It was published online in The Lancet Rheumatology.
LIMITATIONS:
The study was retrospective in design, which may have introduced bias. The small sample size of 25 women may have limited the generalizability of the findings. Additionally, the study did not include a control group, which made it difficult to attribute outcomes solely to anti–IL-6 therapy. The lack of long-term follow-up data on the neonates also limited the understanding of potential long-term effects.
DISCLOSURES:
This study did not receive any funding. Some authors, including the lead author, received speaker fees, grants, or consultancy fees from academic institutions or pharmaceutical companies or had other ties with various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
The maternal and neonatal outcomes in pregnant women treated with anti–interleukin (IL)-6 therapy for COVID-19 are largely favorable, with transient neonatal cytopenia observed in around one third of the babies being the only possible adverse outcome that could be related to anti–IL-6 therapy.
METHODOLOGY:
- Despite guidance, very few pregnant women with COVID-19 are offered evidence-based therapies such as anti–IL-6 due to concerns regarding fetal safety in later pregnancy.
- In this retrospective study, researchers evaluated maternal and neonatal outcomes in 25 pregnant women with COVID-19 (mean age at admission, 33 years) treated with anti–IL-6 (tocilizumab or sarilumab) at two tertiary hospitals in London.
- Most women (n = 16) received anti–IL-6 in the third trimester of pregnancy, whereas nine received it during the second trimester.
- Maternal and neonatal outcomes were assessed through medical record reviews and maternal medicine networks, with follow-up for 12 months.
- The women included in the study constituted a high-risk population with severe COVID-19; 24 required level two or three critical care. All women were receiving at least three concomitant medications due to their critical illness.
TAKEAWAY:
- Overall, 24 of 25 women treated with IL-6 receptor antibodies survived until hospital discharge.
- The sole death occurred in a woman with severe COVID-19 pneumonitis who later developed myocarditis and cardiac arrest. The physicians believed that these complications were more likely due to severe COVID-19 rather than anti–IL-6 therapy.
- All pregnancies resulted in live births; however, 16 babies had to be delivered preterm due to COVID-19 complications.
- Transient cytopenia was observed in 6 of 19 babies in whom a full blood count was performed. All the six babies were premature, with cytopenia resolving within 7 days in four babies; one baby died from complications associated with extreme prematurity.
IN PRACTICE:
“Although the authors found mild, transitory cytopenia in some (6 of 19) exposed infants, most had been delivered prematurely due to progressive COVID-19–related morbidity, and distinguishing drug effects from similar prematurity-related effects is difficult,” wrote Steven L. Clark, MD, from the Department of Obstetrics and Gynecology, Baylor College of Medicine, Houston, in an accompanying editorial.
SOURCE:
The study was led by Melanie Nana, MRCP, from the Department of Obstetric Medicine, St Thomas’ Hospital, London, England. It was published online in The Lancet Rheumatology.
LIMITATIONS:
The study was retrospective in design, which may have introduced bias. The small sample size of 25 women may have limited the generalizability of the findings. Additionally, the study did not include a control group, which made it difficult to attribute outcomes solely to anti–IL-6 therapy. The lack of long-term follow-up data on the neonates also limited the understanding of potential long-term effects.
DISCLOSURES:
This study did not receive any funding. Some authors, including the lead author, received speaker fees, grants, or consultancy fees from academic institutions or pharmaceutical companies or had other ties with various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Anti-Smith and Anti–Double-Stranded DNA Antibodies in a Patient With Henoch-Schönlein Purpura Following COVID-19 Vaccination
To the Editor:
Henoch-Schönlein purpura (HSP)(also known as IgA vasculitis) is a small vessel vasculitis characterized by deposition of IgA in small vessels, resulting in the development of purpura on the legs. Based on the European Alliance of Associations for Rheumatology criteria,1 the patient also must have at least 1 of the following: arthritis, arthralgia, abdominal pain, leukocytoclastic vasculitis with IgA deposition, or kidney involvement. The disease can be triggered by infection—with more than 75% of patients reporting an antecedent upper respiratory tract infection2—as well as medications, circulating immune complexes, certain foods, vaccines, and rarely cancer.3,4 The disease more commonly occurs in children but also can affect adults.
Several cases of HSP have been reported following COVID-19 vaccination.5 We report a case of HSP developing days after the messenger RNA Pfizer-BioNTech COVID-19 vaccine booster that was associated with anti-Smith and anti–double-stranded DNA (dsDNA) antibodies as well as antineutrophil cytoplasmic antibodies (ANCAs).
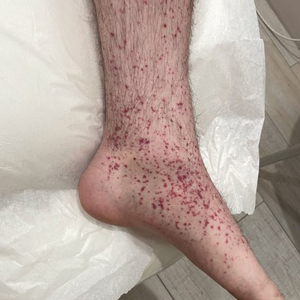
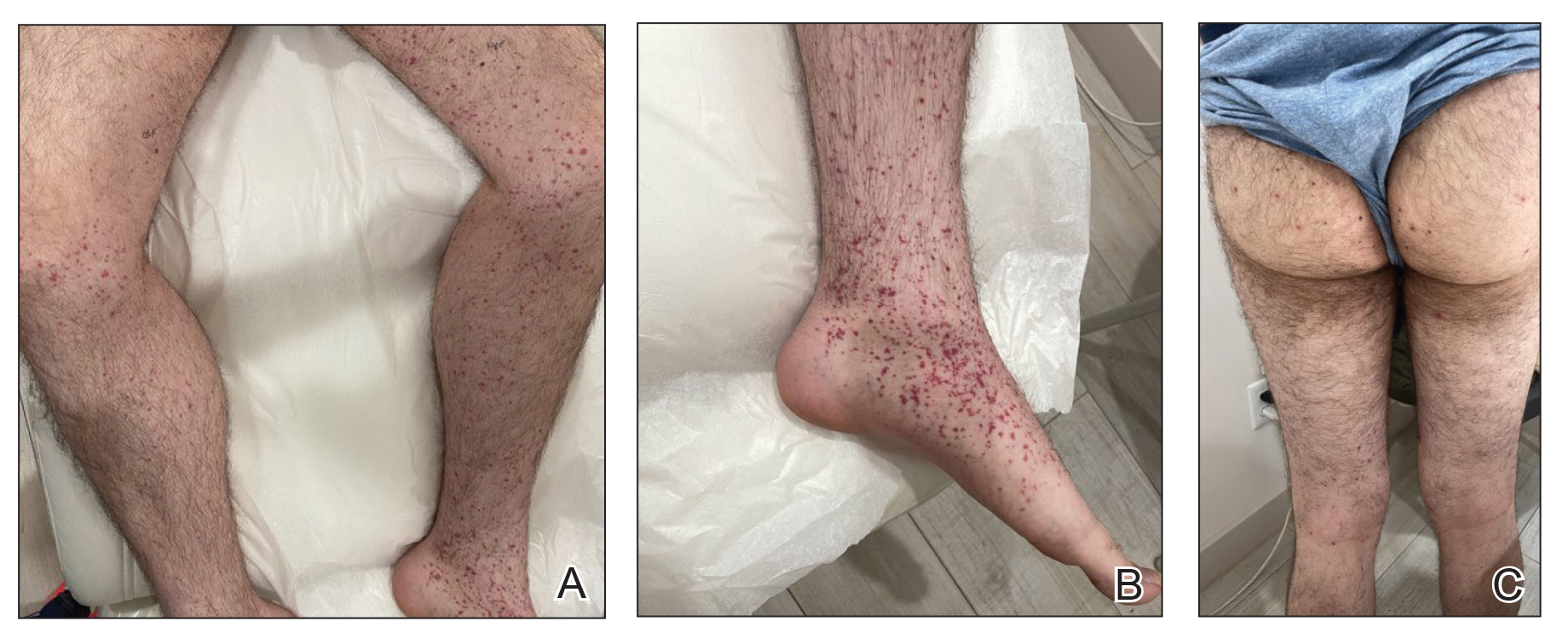
A 24-year-old man presented to dermatology with a rash of 3 weeks’ duration that first appeared 1 week after receiving his second booster of the messenger RNA Pfizer-BioNTech COVID-19 vaccine. Physical examination revealed petechiae with nonblanching erythematous macules and papules covering the legs below the knees (Figure 1) as well as the back of the right arm. A few days later, he developed arthralgia in the knees, hands, and feet. The patient denied any recent infections as well as respiratory and urinary tract symptoms. Approximately 10 days after the rash appeared, he developed epigastric abdominal pain that gradually worsened and sought care from his primary care physician, who ordered computed tomography and referred him for endoscopy. Computed tomography with and without contrast was suspicious for colitis. Colonoscopy and endoscopy were unremarkable. Laboratory tests were notable for elevated white blood cell count (17.08×103/µL [reference range, 3.66–10.60×103/µL]), serum IgA (437 mg/dL [reference range, 70–400 mg/dL]), C-reactive protein (1.5 mg/dL [reference range, <0.5 mg/dL]), anti-Smith antibody (28.1 CU [reference range, <20 CU), positive antinuclear antibody with titer (1:160 [reference range, <1:80]), anti-dsDNA (40.4 IU/mL [reference range, <27 IU/mL]), and cytoplasmic ANCA (c-ANCA) titer (1:320 [reference range, <1:20]). Blood urea nitrogen, creatinine, and estimated glomerular filtration rate were all within reference range. Urinalysis with microscopic examination was notable for 2 to 5 red blood cells per high-power field (reference range, 0) and proteinuria of 1+ (reference range, negative for protein).
The patient’s rash progressively worsened over the next few weeks, spreading proximally on the legs to the buttocks and the back of both elbows. A repeat complete blood cell count showed resolution of the leukocytosis. Two biopsies were taken from a lesion on the left proximal thigh: 1 for hematoxylin and eosin stain for histopathologic examination and 1 for direct immunofluorescence examination.
The patient was preliminarily diagnosed with HSP, and dermatology prescribed oral tofacitinib 5 mg twice daily for 5 days, which was supposed to be increased to 10 mg twice daily on the sixth day of treatment; however, the patient discontinued the medication after 4 days based on his primary care physician’s recommendation due to clotting concerns. The rash and arthralgia temporarily improved for 1 week, then relapsed.
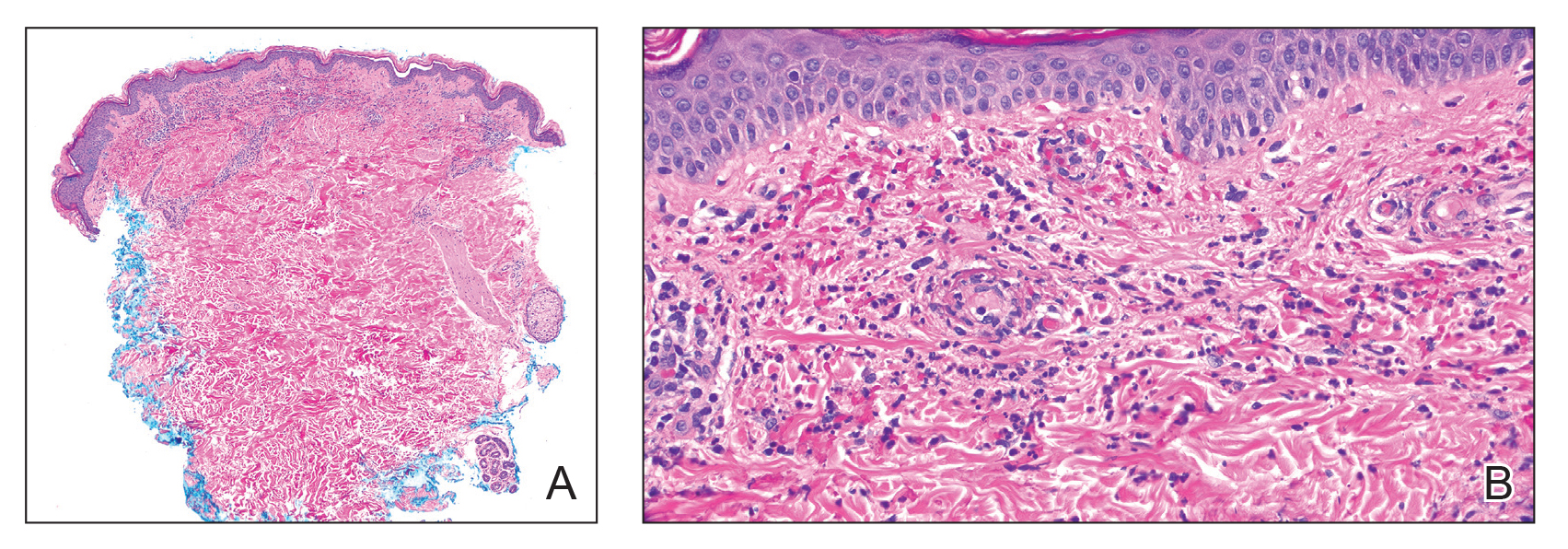
Histopathology revealed neutrophils surrounding and infiltrating small dermal blood vessel walls as well as associated neutrophilic debris and erythrocytes, consistent with leukocytoclastic vasculitis (Figure 2). Direct immunofluorescence was negative for IgA antibodies. His primary care physician, in consultation with his dermatologist, then started the patient on oral prednisone 70 mg once daily for 7 days with a plan to taper. Three days after prednisone was started, the arthralgia and abdominal pain resolved, and the rash became lighter in color. After 1 week, the rash resolved completely.
Due to the unusual antibodies, the patient was referred to a rheumatologist, who repeated the blood tests approximately 1 week after the patient started prednisone. The tests were negative for anti-Smith, anti-dsDNA, and c-ANCA but showed an elevated atypical perinuclear ANCA (p-ANCA) titer of 1:80 (reference range [negative], <1:20). A repeat urinalysis was unremarkable. The patient slowly tapered the prednisone over the course of 3 months and was subsequently lost to follow-up. The rash and other symptoms had not recurred as of the patient’s last physician contact. The most recent laboratory results showed a white blood cell count of 14.0×103/µL (reference range, 3.4–10.8×103/µL), likely due to the prednisone; blood urea nitrogen, creatinine, and estimated glomerular filtration rate were within reference range. The urinalysis was notable for occult blood and was negative for protein. C-reactive protein was 1 mg/dL (reference range, 0–10 mg/dL); p-ANCA, c-ANCA, and atypical p-ANCA, as well as antinuclear antibody, were negative. As of his last follow-up, the patient felt well.
The major differential diagnoses for our patient included HSP, ANCA vasculitis, and systemic lupus erythematosus. Although ANCA vasculitis has been reported after SARS-CoV-2 infection,6 the lack of pulmonary symptoms made this diagnosis unlikely.7 Although our patient initially had elevated anti-Smith and anti-dsDNA antibodies as well as mild renal involvement, he fulfilled at most only 2 of the 11 criteria necessary for diagnosing lupus: malar rash, discoid rash (includes alopecia), photosensitivity, ocular ulcers, nonerosive arthritis, serositis, renal disorder (protein >500 mg/24 h, red blood cells, casts), neurologic disorder (seizures, psychosis), hematologic disorders (hemolytic anemia, leukopenia), ANA, and immunologic disorder (anti-Smith). Four of the 11 criteria are necessary for the diagnosis of lupus.8
Torraca et al7 reported a case of HSP with positive c-ANCA (1:640) in a patient lacking pulmonary symptoms who was diagnosed with HSP. Cytoplasmic ANCA is not a typical finding in HSP. However, the additional findings of anti-Smith, anti-dsDNA, and mildly elevated atypical p-ANCA antibodies in our patient were unexpected and could be explained by the proposed pathogenesis of HSP—an overzealous immune response resulting in aberrant antibody complex deposition with ensuing complement activation.5,9 Production of these additional antibodies could be part of the overzealous response to COVID-19 vaccination.
Of all the COVID-19 vaccines, messenger RNA–based vaccines have been associated with the majority of cutaneous reactions, including local injection-site reactions (most common), delayed local reactions, urticaria, angioedema, morbilliform eruption, herpes zoster eruption, bullous eruptions, dermal filler reactions, chilblains, and pityriasis rosea. Less common reactions have included acute generalized exanthematous pustulosis, Stevens-Johnson syndrome, erythema multiforme, Sweet Syndrome, lichen planus, papulovesicular eruptions, pityriasis rosea–like eruptions, generalized annular lesions, facial pustular neutrophilic eruptions, and flares of underlying autoimmune skin conditions.10 Multiple cases of HSP have been reported following COVID-19 vaccination from all the major vaccine companies.5
In our patient, laboratory tests were repeated by a rheumatologist and were negative for anti-Smith and anti-dsDNA antibodies as well as c-ANCA, most likely because he started taking prednisone approximately 1 week prior, which may have resulted in decreased antibodies. Also, the patient’s symptoms resolved after 1 week of steroid therapy. Therefore, the diagnosis is most consistent with HSP associated with COVID-19 vaccination. The clinical presentation, microscopic hematuria and proteinuria, and histopathology were consistent with the European Alliance of Associations for Rheumatology criteria for HSP.1
Although direct immunofluorescence typically is positive for IgA deposition on biopsies, it can be negative for IgA, especially in lesions that are biopsied more than 7 days after their appearance, as shown in our case; a negative IgA on immunofluorescence does not rule out HSP.4 Elevated serum IgA is seen in more than 50% of cases of HSP.11 Although the disease typically is self-limited, glucocorticoids are used if the disease course is prolonged or if there is evidence of kidney involvement.9 The unique combination of anti-Smith and anti-dsDNA antibodies as well as ANCAs associated with HSP with negative IgA on direct immunofluorescence has been reported with lupus.12 Clinicians should be aware of COVID-19 vaccine–associated HSP that is negative for IgA deposition and positive for anti-Smith and anti-dsDNA antibodies as well as ANCAs.
Acknowledgment—We thank our patient for granting permission to publish this information.
- Ozen S, Ruperto N, Dillon MJ, et al. EULAR/PReS endorsed consensus criteria for the classification of childhood vasculitides. Ann Rheum Dis. 2006;65:936-941. doi:10.1136/ard.2005.046300
- Rai A, Nast C, Adler S. Henoch–Schönlein purpura nephritis. J Am Soc Nephrol. 1999;10:2637-2644.
- Casini F, Magenes VC, De Sanctis M, et al. Henoch-Schönlein purpura following COVID-19 vaccine in a child: a case report. Ital J Pediatr. 2022;48:158. doi:10.1186/s13052-022-01351-1
- Poudel P, Adams SH, Mirchia K, et al. IgA negative immunofluorescence in diagnoses of adult-onset Henoch-Schönlein purpura. Proc (Bayl Univ Med Cent). 2020;33:436-437. doi:10.1080/08998280.2020.1770526
- Maronese CA, Zelin E, Avallone G, et al. Cutaneous vasculitis and vasculopathy in the era of COVID-19 pandemic. Front Med (Lausanne). 2022;9:996288. doi:10.3389/fmed.2022.996288
- Bryant MC, Spencer LT, Yalcindag A. A case of ANCA-associated vasculitis in a 16-year-old female following SARS-COV-2 infection and a systematic review of the literature. Pediatr Rheumatol Online J. 2022;20:65. doi:10.1186/s12969-022-00727-1
- Torraca PFS, Castro BC, Hans Filho G. Henoch-Schönlein purpura with c-ANCA antibody in adult. An Bras Dermatol. 2016;91:667-669. doi:10.1590/abd1806-4841.20164181
- Agabegi SS, Agabegi ED. Step-Up to Medicine. 4th ed. Wolters Kluwer; 2015.
- Ball-Burack MR, Kosowsky JM. A Case of leukocytoclastic vasculitis following SARS-CoV-2 vaccination. J Emerg Med. 2022;63:E62-E65. doi:10.1016/j.jemermed.2021.10.005
- Tan SW, Tam YC, Pang SM. Cutaneous reactions to COVID-19 vaccines: a review. JAAD Int. 2022;7:178-186. doi:10.1016/j.jdin.2022.01.011
- Calviño MC, Llorca J, García-Porrúa C, et al. Henoch-Schönlein purpura in children from northwestern Spain: a 20-year epidemiologic and clinical study. Medicine (Baltimore). 2001;80:279-290.
- Hu P, Huang BY, Zhang DD, et al. Henoch-Schönlein purpura in a pediatric patient with lupus. Arch Med Sci. 2017;13:689-690. doi:10.5114/aoms.2017.67288
To the Editor:
Henoch-Schönlein purpura (HSP)(also known as IgA vasculitis) is a small vessel vasculitis characterized by deposition of IgA in small vessels, resulting in the development of purpura on the legs. Based on the European Alliance of Associations for Rheumatology criteria,1 the patient also must have at least 1 of the following: arthritis, arthralgia, abdominal pain, leukocytoclastic vasculitis with IgA deposition, or kidney involvement. The disease can be triggered by infection—with more than 75% of patients reporting an antecedent upper respiratory tract infection2—as well as medications, circulating immune complexes, certain foods, vaccines, and rarely cancer.3,4 The disease more commonly occurs in children but also can affect adults.
Several cases of HSP have been reported following COVID-19 vaccination.5 We report a case of HSP developing days after the messenger RNA Pfizer-BioNTech COVID-19 vaccine booster that was associated with anti-Smith and anti–double-stranded DNA (dsDNA) antibodies as well as antineutrophil cytoplasmic antibodies (ANCAs).
A 24-year-old man presented to dermatology with a rash of 3 weeks’ duration that first appeared 1 week after receiving his second booster of the messenger RNA Pfizer-BioNTech COVID-19 vaccine. Physical examination revealed petechiae with nonblanching erythematous macules and papules covering the legs below the knees (Figure 1) as well as the back of the right arm. A few days later, he developed arthralgia in the knees, hands, and feet. The patient denied any recent infections as well as respiratory and urinary tract symptoms. Approximately 10 days after the rash appeared, he developed epigastric abdominal pain that gradually worsened and sought care from his primary care physician, who ordered computed tomography and referred him for endoscopy. Computed tomography with and without contrast was suspicious for colitis. Colonoscopy and endoscopy were unremarkable. Laboratory tests were notable for elevated white blood cell count (17.08×103/µL [reference range, 3.66–10.60×103/µL]), serum IgA (437 mg/dL [reference range, 70–400 mg/dL]), C-reactive protein (1.5 mg/dL [reference range, <0.5 mg/dL]), anti-Smith antibody (28.1 CU [reference range, <20 CU), positive antinuclear antibody with titer (1:160 [reference range, <1:80]), anti-dsDNA (40.4 IU/mL [reference range, <27 IU/mL]), and cytoplasmic ANCA (c-ANCA) titer (1:320 [reference range, <1:20]). Blood urea nitrogen, creatinine, and estimated glomerular filtration rate were all within reference range. Urinalysis with microscopic examination was notable for 2 to 5 red blood cells per high-power field (reference range, 0) and proteinuria of 1+ (reference range, negative for protein).
The patient’s rash progressively worsened over the next few weeks, spreading proximally on the legs to the buttocks and the back of both elbows. A repeat complete blood cell count showed resolution of the leukocytosis. Two biopsies were taken from a lesion on the left proximal thigh: 1 for hematoxylin and eosin stain for histopathologic examination and 1 for direct immunofluorescence examination.
The patient was preliminarily diagnosed with HSP, and dermatology prescribed oral tofacitinib 5 mg twice daily for 5 days, which was supposed to be increased to 10 mg twice daily on the sixth day of treatment; however, the patient discontinued the medication after 4 days based on his primary care physician’s recommendation due to clotting concerns. The rash and arthralgia temporarily improved for 1 week, then relapsed.
Histopathology revealed neutrophils surrounding and infiltrating small dermal blood vessel walls as well as associated neutrophilic debris and erythrocytes, consistent with leukocytoclastic vasculitis (Figure 2). Direct immunofluorescence was negative for IgA antibodies. His primary care physician, in consultation with his dermatologist, then started the patient on oral prednisone 70 mg once daily for 7 days with a plan to taper. Three days after prednisone was started, the arthralgia and abdominal pain resolved, and the rash became lighter in color. After 1 week, the rash resolved completely.
Due to the unusual antibodies, the patient was referred to a rheumatologist, who repeated the blood tests approximately 1 week after the patient started prednisone. The tests were negative for anti-Smith, anti-dsDNA, and c-ANCA but showed an elevated atypical perinuclear ANCA (p-ANCA) titer of 1:80 (reference range [negative], <1:20). A repeat urinalysis was unremarkable. The patient slowly tapered the prednisone over the course of 3 months and was subsequently lost to follow-up. The rash and other symptoms had not recurred as of the patient’s last physician contact. The most recent laboratory results showed a white blood cell count of 14.0×103/µL (reference range, 3.4–10.8×103/µL), likely due to the prednisone; blood urea nitrogen, creatinine, and estimated glomerular filtration rate were within reference range. The urinalysis was notable for occult blood and was negative for protein. C-reactive protein was 1 mg/dL (reference range, 0–10 mg/dL); p-ANCA, c-ANCA, and atypical p-ANCA, as well as antinuclear antibody, were negative. As of his last follow-up, the patient felt well.
The major differential diagnoses for our patient included HSP, ANCA vasculitis, and systemic lupus erythematosus. Although ANCA vasculitis has been reported after SARS-CoV-2 infection,6 the lack of pulmonary symptoms made this diagnosis unlikely.7 Although our patient initially had elevated anti-Smith and anti-dsDNA antibodies as well as mild renal involvement, he fulfilled at most only 2 of the 11 criteria necessary for diagnosing lupus: malar rash, discoid rash (includes alopecia), photosensitivity, ocular ulcers, nonerosive arthritis, serositis, renal disorder (protein >500 mg/24 h, red blood cells, casts), neurologic disorder (seizures, psychosis), hematologic disorders (hemolytic anemia, leukopenia), ANA, and immunologic disorder (anti-Smith). Four of the 11 criteria are necessary for the diagnosis of lupus.8
Torraca et al7 reported a case of HSP with positive c-ANCA (1:640) in a patient lacking pulmonary symptoms who was diagnosed with HSP. Cytoplasmic ANCA is not a typical finding in HSP. However, the additional findings of anti-Smith, anti-dsDNA, and mildly elevated atypical p-ANCA antibodies in our patient were unexpected and could be explained by the proposed pathogenesis of HSP—an overzealous immune response resulting in aberrant antibody complex deposition with ensuing complement activation.5,9 Production of these additional antibodies could be part of the overzealous response to COVID-19 vaccination.


Of all the COVID-19 vaccines, messenger RNA–based vaccines have been associated with the majority of cutaneous reactions, including local injection-site reactions (most common), delayed local reactions, urticaria, angioedema, morbilliform eruption, herpes zoster eruption, bullous eruptions, dermal filler reactions, chilblains, and pityriasis rosea. Less common reactions have included acute generalized exanthematous pustulosis, Stevens-Johnson syndrome, erythema multiforme, Sweet Syndrome, lichen planus, papulovesicular eruptions, pityriasis rosea–like eruptions, generalized annular lesions, facial pustular neutrophilic eruptions, and flares of underlying autoimmune skin conditions.10 Multiple cases of HSP have been reported following COVID-19 vaccination from all the major vaccine companies.5
In our patient, laboratory tests were repeated by a rheumatologist and were negative for anti-Smith and anti-dsDNA antibodies as well as c-ANCA, most likely because he started taking prednisone approximately 1 week prior, which may have resulted in decreased antibodies. Also, the patient’s symptoms resolved after 1 week of steroid therapy. Therefore, the diagnosis is most consistent with HSP associated with COVID-19 vaccination. The clinical presentation, microscopic hematuria and proteinuria, and histopathology were consistent with the European Alliance of Associations for Rheumatology criteria for HSP.1
Although direct immunofluorescence typically is positive for IgA deposition on biopsies, it can be negative for IgA, especially in lesions that are biopsied more than 7 days after their appearance, as shown in our case; a negative IgA on immunofluorescence does not rule out HSP.4 Elevated serum IgA is seen in more than 50% of cases of HSP.11 Although the disease typically is self-limited, glucocorticoids are used if the disease course is prolonged or if there is evidence of kidney involvement.9 The unique combination of anti-Smith and anti-dsDNA antibodies as well as ANCAs associated with HSP with negative IgA on direct immunofluorescence has been reported with lupus.12 Clinicians should be aware of COVID-19 vaccine–associated HSP that is negative for IgA deposition and positive for anti-Smith and anti-dsDNA antibodies as well as ANCAs.
Acknowledgment—We thank our patient for granting permission to publish this information.
To the Editor:
Henoch-Schönlein purpura (HSP)(also known as IgA vasculitis) is a small vessel vasculitis characterized by deposition of IgA in small vessels, resulting in the development of purpura on the legs. Based on the European Alliance of Associations for Rheumatology criteria,1 the patient also must have at least 1 of the following: arthritis, arthralgia, abdominal pain, leukocytoclastic vasculitis with IgA deposition, or kidney involvement. The disease can be triggered by infection—with more than 75% of patients reporting an antecedent upper respiratory tract infection2—as well as medications, circulating immune complexes, certain foods, vaccines, and rarely cancer.3,4 The disease more commonly occurs in children but also can affect adults.
Several cases of HSP have been reported following COVID-19 vaccination.5 We report a case of HSP developing days after the messenger RNA Pfizer-BioNTech COVID-19 vaccine booster that was associated with anti-Smith and anti–double-stranded DNA (dsDNA) antibodies as well as antineutrophil cytoplasmic antibodies (ANCAs).
A 24-year-old man presented to dermatology with a rash of 3 weeks’ duration that first appeared 1 week after receiving his second booster of the messenger RNA Pfizer-BioNTech COVID-19 vaccine. Physical examination revealed petechiae with nonblanching erythematous macules and papules covering the legs below the knees (Figure 1) as well as the back of the right arm. A few days later, he developed arthralgia in the knees, hands, and feet. The patient denied any recent infections as well as respiratory and urinary tract symptoms. Approximately 10 days after the rash appeared, he developed epigastric abdominal pain that gradually worsened and sought care from his primary care physician, who ordered computed tomography and referred him for endoscopy. Computed tomography with and without contrast was suspicious for colitis. Colonoscopy and endoscopy were unremarkable. Laboratory tests were notable for elevated white blood cell count (17.08×103/µL [reference range, 3.66–10.60×103/µL]), serum IgA (437 mg/dL [reference range, 70–400 mg/dL]), C-reactive protein (1.5 mg/dL [reference range, <0.5 mg/dL]), anti-Smith antibody (28.1 CU [reference range, <20 CU), positive antinuclear antibody with titer (1:160 [reference range, <1:80]), anti-dsDNA (40.4 IU/mL [reference range, <27 IU/mL]), and cytoplasmic ANCA (c-ANCA) titer (1:320 [reference range, <1:20]). Blood urea nitrogen, creatinine, and estimated glomerular filtration rate were all within reference range. Urinalysis with microscopic examination was notable for 2 to 5 red blood cells per high-power field (reference range, 0) and proteinuria of 1+ (reference range, negative for protein).
The patient’s rash progressively worsened over the next few weeks, spreading proximally on the legs to the buttocks and the back of both elbows. A repeat complete blood cell count showed resolution of the leukocytosis. Two biopsies were taken from a lesion on the left proximal thigh: 1 for hematoxylin and eosin stain for histopathologic examination and 1 for direct immunofluorescence examination.
The patient was preliminarily diagnosed with HSP, and dermatology prescribed oral tofacitinib 5 mg twice daily for 5 days, which was supposed to be increased to 10 mg twice daily on the sixth day of treatment; however, the patient discontinued the medication after 4 days based on his primary care physician’s recommendation due to clotting concerns. The rash and arthralgia temporarily improved for 1 week, then relapsed.
Histopathology revealed neutrophils surrounding and infiltrating small dermal blood vessel walls as well as associated neutrophilic debris and erythrocytes, consistent with leukocytoclastic vasculitis (Figure 2). Direct immunofluorescence was negative for IgA antibodies. His primary care physician, in consultation with his dermatologist, then started the patient on oral prednisone 70 mg once daily for 7 days with a plan to taper. Three days after prednisone was started, the arthralgia and abdominal pain resolved, and the rash became lighter in color. After 1 week, the rash resolved completely.
Due to the unusual antibodies, the patient was referred to a rheumatologist, who repeated the blood tests approximately 1 week after the patient started prednisone. The tests were negative for anti-Smith, anti-dsDNA, and c-ANCA but showed an elevated atypical perinuclear ANCA (p-ANCA) titer of 1:80 (reference range [negative], <1:20). A repeat urinalysis was unremarkable. The patient slowly tapered the prednisone over the course of 3 months and was subsequently lost to follow-up. The rash and other symptoms had not recurred as of the patient’s last physician contact. The most recent laboratory results showed a white blood cell count of 14.0×103/µL (reference range, 3.4–10.8×103/µL), likely due to the prednisone; blood urea nitrogen, creatinine, and estimated glomerular filtration rate were within reference range. The urinalysis was notable for occult blood and was negative for protein. C-reactive protein was 1 mg/dL (reference range, 0–10 mg/dL); p-ANCA, c-ANCA, and atypical p-ANCA, as well as antinuclear antibody, were negative. As of his last follow-up, the patient felt well.
The major differential diagnoses for our patient included HSP, ANCA vasculitis, and systemic lupus erythematosus. Although ANCA vasculitis has been reported after SARS-CoV-2 infection,6 the lack of pulmonary symptoms made this diagnosis unlikely.7 Although our patient initially had elevated anti-Smith and anti-dsDNA antibodies as well as mild renal involvement, he fulfilled at most only 2 of the 11 criteria necessary for diagnosing lupus: malar rash, discoid rash (includes alopecia), photosensitivity, ocular ulcers, nonerosive arthritis, serositis, renal disorder (protein >500 mg/24 h, red blood cells, casts), neurologic disorder (seizures, psychosis), hematologic disorders (hemolytic anemia, leukopenia), ANA, and immunologic disorder (anti-Smith). Four of the 11 criteria are necessary for the diagnosis of lupus.8
Torraca et al7 reported a case of HSP with positive c-ANCA (1:640) in a patient lacking pulmonary symptoms who was diagnosed with HSP. Cytoplasmic ANCA is not a typical finding in HSP. However, the additional findings of anti-Smith, anti-dsDNA, and mildly elevated atypical p-ANCA antibodies in our patient were unexpected and could be explained by the proposed pathogenesis of HSP—an overzealous immune response resulting in aberrant antibody complex deposition with ensuing complement activation.5,9 Production of these additional antibodies could be part of the overzealous response to COVID-19 vaccination.


Of all the COVID-19 vaccines, messenger RNA–based vaccines have been associated with the majority of cutaneous reactions, including local injection-site reactions (most common), delayed local reactions, urticaria, angioedema, morbilliform eruption, herpes zoster eruption, bullous eruptions, dermal filler reactions, chilblains, and pityriasis rosea. Less common reactions have included acute generalized exanthematous pustulosis, Stevens-Johnson syndrome, erythema multiforme, Sweet Syndrome, lichen planus, papulovesicular eruptions, pityriasis rosea–like eruptions, generalized annular lesions, facial pustular neutrophilic eruptions, and flares of underlying autoimmune skin conditions.10 Multiple cases of HSP have been reported following COVID-19 vaccination from all the major vaccine companies.5
In our patient, laboratory tests were repeated by a rheumatologist and were negative for anti-Smith and anti-dsDNA antibodies as well as c-ANCA, most likely because he started taking prednisone approximately 1 week prior, which may have resulted in decreased antibodies. Also, the patient’s symptoms resolved after 1 week of steroid therapy. Therefore, the diagnosis is most consistent with HSP associated with COVID-19 vaccination. The clinical presentation, microscopic hematuria and proteinuria, and histopathology were consistent with the European Alliance of Associations for Rheumatology criteria for HSP.1
Although direct immunofluorescence typically is positive for IgA deposition on biopsies, it can be negative for IgA, especially in lesions that are biopsied more than 7 days after their appearance, as shown in our case; a negative IgA on immunofluorescence does not rule out HSP.4 Elevated serum IgA is seen in more than 50% of cases of HSP.11 Although the disease typically is self-limited, glucocorticoids are used if the disease course is prolonged or if there is evidence of kidney involvement.9 The unique combination of anti-Smith and anti-dsDNA antibodies as well as ANCAs associated with HSP with negative IgA on direct immunofluorescence has been reported with lupus.12 Clinicians should be aware of COVID-19 vaccine–associated HSP that is negative for IgA deposition and positive for anti-Smith and anti-dsDNA antibodies as well as ANCAs.
Acknowledgment—We thank our patient for granting permission to publish this information.
- Ozen S, Ruperto N, Dillon MJ, et al. EULAR/PReS endorsed consensus criteria for the classification of childhood vasculitides. Ann Rheum Dis. 2006;65:936-941. doi:10.1136/ard.2005.046300
- Rai A, Nast C, Adler S. Henoch–Schönlein purpura nephritis. J Am Soc Nephrol. 1999;10:2637-2644.
- Casini F, Magenes VC, De Sanctis M, et al. Henoch-Schönlein purpura following COVID-19 vaccine in a child: a case report. Ital J Pediatr. 2022;48:158. doi:10.1186/s13052-022-01351-1
- Poudel P, Adams SH, Mirchia K, et al. IgA negative immunofluorescence in diagnoses of adult-onset Henoch-Schönlein purpura. Proc (Bayl Univ Med Cent). 2020;33:436-437. doi:10.1080/08998280.2020.1770526
- Maronese CA, Zelin E, Avallone G, et al. Cutaneous vasculitis and vasculopathy in the era of COVID-19 pandemic. Front Med (Lausanne). 2022;9:996288. doi:10.3389/fmed.2022.996288
- Bryant MC, Spencer LT, Yalcindag A. A case of ANCA-associated vasculitis in a 16-year-old female following SARS-COV-2 infection and a systematic review of the literature. Pediatr Rheumatol Online J. 2022;20:65. doi:10.1186/s12969-022-00727-1
- Torraca PFS, Castro BC, Hans Filho G. Henoch-Schönlein purpura with c-ANCA antibody in adult. An Bras Dermatol. 2016;91:667-669. doi:10.1590/abd1806-4841.20164181
- Agabegi SS, Agabegi ED. Step-Up to Medicine. 4th ed. Wolters Kluwer; 2015.
- Ball-Burack MR, Kosowsky JM. A Case of leukocytoclastic vasculitis following SARS-CoV-2 vaccination. J Emerg Med. 2022;63:E62-E65. doi:10.1016/j.jemermed.2021.10.005
- Tan SW, Tam YC, Pang SM. Cutaneous reactions to COVID-19 vaccines: a review. JAAD Int. 2022;7:178-186. doi:10.1016/j.jdin.2022.01.011
- Calviño MC, Llorca J, García-Porrúa C, et al. Henoch-Schönlein purpura in children from northwestern Spain: a 20-year epidemiologic and clinical study. Medicine (Baltimore). 2001;80:279-290.
- Hu P, Huang BY, Zhang DD, et al. Henoch-Schönlein purpura in a pediatric patient with lupus. Arch Med Sci. 2017;13:689-690. doi:10.5114/aoms.2017.67288
- Ozen S, Ruperto N, Dillon MJ, et al. EULAR/PReS endorsed consensus criteria for the classification of childhood vasculitides. Ann Rheum Dis. 2006;65:936-941. doi:10.1136/ard.2005.046300
- Rai A, Nast C, Adler S. Henoch–Schönlein purpura nephritis. J Am Soc Nephrol. 1999;10:2637-2644.
- Casini F, Magenes VC, De Sanctis M, et al. Henoch-Schönlein purpura following COVID-19 vaccine in a child: a case report. Ital J Pediatr. 2022;48:158. doi:10.1186/s13052-022-01351-1
- Poudel P, Adams SH, Mirchia K, et al. IgA negative immunofluorescence in diagnoses of adult-onset Henoch-Schönlein purpura. Proc (Bayl Univ Med Cent). 2020;33:436-437. doi:10.1080/08998280.2020.1770526
- Maronese CA, Zelin E, Avallone G, et al. Cutaneous vasculitis and vasculopathy in the era of COVID-19 pandemic. Front Med (Lausanne). 2022;9:996288. doi:10.3389/fmed.2022.996288
- Bryant MC, Spencer LT, Yalcindag A. A case of ANCA-associated vasculitis in a 16-year-old female following SARS-COV-2 infection and a systematic review of the literature. Pediatr Rheumatol Online J. 2022;20:65. doi:10.1186/s12969-022-00727-1
- Torraca PFS, Castro BC, Hans Filho G. Henoch-Schönlein purpura with c-ANCA antibody in adult. An Bras Dermatol. 2016;91:667-669. doi:10.1590/abd1806-4841.20164181
- Agabegi SS, Agabegi ED. Step-Up to Medicine. 4th ed. Wolters Kluwer; 2015.
- Ball-Burack MR, Kosowsky JM. A Case of leukocytoclastic vasculitis following SARS-CoV-2 vaccination. J Emerg Med. 2022;63:E62-E65. doi:10.1016/j.jemermed.2021.10.005
- Tan SW, Tam YC, Pang SM. Cutaneous reactions to COVID-19 vaccines: a review. JAAD Int. 2022;7:178-186. doi:10.1016/j.jdin.2022.01.011
- Calviño MC, Llorca J, García-Porrúa C, et al. Henoch-Schönlein purpura in children from northwestern Spain: a 20-year epidemiologic and clinical study. Medicine (Baltimore). 2001;80:279-290.
- Hu P, Huang BY, Zhang DD, et al. Henoch-Schönlein purpura in a pediatric patient with lupus. Arch Med Sci. 2017;13:689-690. doi:10.5114/aoms.2017.67288
Practice Points
- Dermatologists should be vigilant for Henoch-Schönlein purpura (HSP) despite negative direct immunofluorescence of IgA deposition and unusual antibodies.
- Messenger RNA–based COVID-19 vaccines are associated with various cutaneous reactions, including HSP.
- Anti-Smith and anti–double-stranded DNA antibodies typically are not associated with HSP but may be seen in patients with coexisting systemic lupus erythematosus.
Pediatric Studies Produce Mixed Messages on Relationship Between COVID and Asthma
In one of several recently published studies on the relationship between COVID-19 infection and asthma, according to data drawn from the National Survey of Children’s Health (NSCH).
The inverse correlation between symptoms and vaccination was strong and statistically significant, according to investigators led by Matthew M. Davis, MD, Physician in Chief and Chief Scientific Officer, Nemours Children’s Health, Wilmington, Delaware.
“With each increase of 10 percentage points in COVID-19 vaccination coverage, the parent-reported child asthma symptoms prevalence decreased by 0.36 percentage points (P < .05),” Dr. Davis and his coinvestigators reported in a research letter published in JAMA Network Open.
Studies Explore Relationship of COVID and Asthma
The reduced risk of asthma symptoms with COVID-19 vaccination in children at the population level is just one of several recently published studies exploring the interaction between COVID-19 infection and asthma, but two studies that posed the same question did not reach the same conclusion.
In one, COVID-19 infection in children was not found to be a trigger for new-onset asthma, but the second found that it was. In a third study, the preponderance of evidence from a meta-analysis found that patients with asthma – whether children or adults – did not necessarily experience a more severe course of COVID-19 infection than in those without asthma.
The NSCH database study calculated state-level change in scores for patient-reported childhood asthma symptoms in the years in the years 2018-2019, which preceded the pandemic and the years 2020-2021, when the pandemic began. The hypothesis was that the proportion of the population 5 years of age or older who completed the COVID-19 primary vaccination would be inversely related to asthma symptom prevalence.
Relative to the 2018-2019 years, the mean rate of parent-reported asthma symptoms was 0.85% lower (6.93% vs 7.77%; P < .001) in 2020-2021, when the mean primary series COVID-19 vaccination rate was 72.3%.
The study was not able to evaluate the impact of COVID-19 vaccination specifically in children with asthma, because history of asthma is not captured in the NSCH data, but Dr. Davis contended that the reduction in symptomatic asthma among children with increased vaccination offers validation for the state-level findings.
“Moreover, the absence of an association of COVID-19 vaccination administered predominantly in 2021 with population-level COVID-19 mortality in 2020 serves as a negative control,” he and his colleagues wrote in their research letter.
Protection from Respiratory Viruses Seen for Asthma Patients
In an interview, Dr. Davis reported that these data are consistent with previous evidence that immunization against influenza also reduces risk of asthma symptoms. In a meta-analysis published in 2017, it was estimated that live vaccines reduced risk of influenza by 81% and prevented 59%-72% of asthma attacks leading to hospitalizations or emergency room visits.
“The similarity of our findings regarding COVID-19 vaccination to prior data regarding influenza vaccination underscores the importance of preventing viral illnesses in individuals with a history of asthma,” Dr. Davis said. It is not yet clear if this is true of respiratory syncytial virus (RSV). Because of the short time that the RSV vaccine has been available, it is too soon to conduct an analysis.
One message from this study is that “clinicians should continue to encourage COVID-19 vaccination for children because of its general benefits in preventing coronavirus-related illness and the apparent specific benefits for children with a history of asthma,” he said.
While vaccination appears to reduce asthmatic symptoms related to COVID-19 infection, one study suggests that COVID-19 does not trigger new-onset asthma. In a retrospective study published in Pediatrics, no association between COVID-19 infection and new-onset asthma could be made in an analysis of 27,423 children (ages, 1-16 years) from the Children’s Hospital of Philadelphia (CHOP) Care Network.
Across all the pediatric age groups evaluated, the consistent finding was “SARS-CoV-2 positivity does not confer an additional risk for asthma diagnosis at least within the first 18 months after a [polymerase chain reaction] test,” concluded the investigators, led by David A. Hill, MD, PhD, Division of Allergy and Immunology, CHOP, Philadelphia, Pennsylvania.
Risk of Asthma Doubled After COVID-19 Infection
However, the opposite conclusion was reached by investigators evaluating data from two cohorts of children ages 5-18 drawn from the TriNetX database, a global health research network with data on more than 250 million individuals. Cohort 1 included more than 250,000 children. These children had never received COVID-19 vaccination. The 50,000 patients in cohort 2 had all received COVID19 vaccination.
To compare the impact of COVID-19 infection on new-onset asthma, the patients who were infected with COVID-19 were compared with those who were not infected after propensity score matching over 18 months of follow-up.
In cohort 1, the rate of new onset asthma was more than twofold greater among those with COVID-19 infection (4.7% vs 2.0%). The hazard ratio (HR) of 2.25 had tight confidence intervals (95% CI, 2.158-2.367).
In cohort 2, the risk of new-onset asthma at 18 months among those who had a COVID-19 infection relative to those without was even greater (8.3% vs 3.1%). The relative risk approached a 3-fold increase (HR 2.745; 95% CI, 2.521-2.99).
The conclusion of these investigators, led by Chia-Chi Lung, PhD, Department of Public Health, Chung Shan Medical University, Taichung City, Taiwan, was that there is “a critical need for ongoing monitoring and customized healthcare strategies to mitigate the long-term respiratory impacts of COVID-19 in children.”
These health risks might not be as significant as once feared. In the recently published study from Environmental Health Insights, the goal of a meta-analysis was to determine if patients with asthma relative to those without asthma face a higher risk of serious disease from COVID-19 infection. The meta-analysis included studies of children and adults. The answer, according an in-depth analysis of 21 articles in a “scoping review,” was a qualified no.
Of the 21 articles, 4 concluded that asthma is a risk factor for serious COVID-19 infection, but 17 did not, according to Chukwudi S. Ubah, PhD, Department of Public Health, Brody School of Medicine, East Caroline University, Greenville, North Carolina.
None of These Questions are Fully Resolved
However, given the disparity in the results and the fact that many of the studies included in this analysis had small sample sizes, Dr. Ubah called for larger studies and studies with better controls. He noted, for example, that the studies did not consistently evaluate mitigating factors, such as used of inhaled or oral corticosteroids, which might affect risk of the severity of a COVID-19 infection.
Rather, “our findings pointed out that the type of medication prescribed for asthma may have implications for the severity of COVID-19 infection in these patients,” Dr. Ubah said in an interview.
Overall, the data do not support a major interaction between asthma and COVID-19, even if the data are not conclusive. Each of the senior authors of these studies called for larger and better investigations to further explore whether COVID-19 infection and preexisting asthma interact. So far, the data indicate that if COVID-19 infection poses a risk of precipitating new-onset asthma or inducing a more severe infection in children with asthma, it is low, but the degree of risk, if any, remains unresolved in subgroups defined by asthma treatment or asthma severity.
Dr. Davis, Dr. Hill, Dr. Lung, and Dr. Ubah reported no potential conflicts of interest. None of these studies received funding from commercial interests.
In one of several recently published studies on the relationship between COVID-19 infection and asthma, according to data drawn from the National Survey of Children’s Health (NSCH).
The inverse correlation between symptoms and vaccination was strong and statistically significant, according to investigators led by Matthew M. Davis, MD, Physician in Chief and Chief Scientific Officer, Nemours Children’s Health, Wilmington, Delaware.
“With each increase of 10 percentage points in COVID-19 vaccination coverage, the parent-reported child asthma symptoms prevalence decreased by 0.36 percentage points (P < .05),” Dr. Davis and his coinvestigators reported in a research letter published in JAMA Network Open.
Studies Explore Relationship of COVID and Asthma
The reduced risk of asthma symptoms with COVID-19 vaccination in children at the population level is just one of several recently published studies exploring the interaction between COVID-19 infection and asthma, but two studies that posed the same question did not reach the same conclusion.
In one, COVID-19 infection in children was not found to be a trigger for new-onset asthma, but the second found that it was. In a third study, the preponderance of evidence from a meta-analysis found that patients with asthma – whether children or adults – did not necessarily experience a more severe course of COVID-19 infection than in those without asthma.
The NSCH database study calculated state-level change in scores for patient-reported childhood asthma symptoms in the years in the years 2018-2019, which preceded the pandemic and the years 2020-2021, when the pandemic began. The hypothesis was that the proportion of the population 5 years of age or older who completed the COVID-19 primary vaccination would be inversely related to asthma symptom prevalence.
Relative to the 2018-2019 years, the mean rate of parent-reported asthma symptoms was 0.85% lower (6.93% vs 7.77%; P < .001) in 2020-2021, when the mean primary series COVID-19 vaccination rate was 72.3%.
The study was not able to evaluate the impact of COVID-19 vaccination specifically in children with asthma, because history of asthma is not captured in the NSCH data, but Dr. Davis contended that the reduction in symptomatic asthma among children with increased vaccination offers validation for the state-level findings.
“Moreover, the absence of an association of COVID-19 vaccination administered predominantly in 2021 with population-level COVID-19 mortality in 2020 serves as a negative control,” he and his colleagues wrote in their research letter.
Protection from Respiratory Viruses Seen for Asthma Patients
In an interview, Dr. Davis reported that these data are consistent with previous evidence that immunization against influenza also reduces risk of asthma symptoms. In a meta-analysis published in 2017, it was estimated that live vaccines reduced risk of influenza by 81% and prevented 59%-72% of asthma attacks leading to hospitalizations or emergency room visits.
“The similarity of our findings regarding COVID-19 vaccination to prior data regarding influenza vaccination underscores the importance of preventing viral illnesses in individuals with a history of asthma,” Dr. Davis said. It is not yet clear if this is true of respiratory syncytial virus (RSV). Because of the short time that the RSV vaccine has been available, it is too soon to conduct an analysis.
One message from this study is that “clinicians should continue to encourage COVID-19 vaccination for children because of its general benefits in preventing coronavirus-related illness and the apparent specific benefits for children with a history of asthma,” he said.
While vaccination appears to reduce asthmatic symptoms related to COVID-19 infection, one study suggests that COVID-19 does not trigger new-onset asthma. In a retrospective study published in Pediatrics, no association between COVID-19 infection and new-onset asthma could be made in an analysis of 27,423 children (ages, 1-16 years) from the Children’s Hospital of Philadelphia (CHOP) Care Network.
Across all the pediatric age groups evaluated, the consistent finding was “SARS-CoV-2 positivity does not confer an additional risk for asthma diagnosis at least within the first 18 months after a [polymerase chain reaction] test,” concluded the investigators, led by David A. Hill, MD, PhD, Division of Allergy and Immunology, CHOP, Philadelphia, Pennsylvania.
Risk of Asthma Doubled After COVID-19 Infection
However, the opposite conclusion was reached by investigators evaluating data from two cohorts of children ages 5-18 drawn from the TriNetX database, a global health research network with data on more than 250 million individuals. Cohort 1 included more than 250,000 children. These children had never received COVID-19 vaccination. The 50,000 patients in cohort 2 had all received COVID19 vaccination.
To compare the impact of COVID-19 infection on new-onset asthma, the patients who were infected with COVID-19 were compared with those who were not infected after propensity score matching over 18 months of follow-up.
In cohort 1, the rate of new onset asthma was more than twofold greater among those with COVID-19 infection (4.7% vs 2.0%). The hazard ratio (HR) of 2.25 had tight confidence intervals (95% CI, 2.158-2.367).
In cohort 2, the risk of new-onset asthma at 18 months among those who had a COVID-19 infection relative to those without was even greater (8.3% vs 3.1%). The relative risk approached a 3-fold increase (HR 2.745; 95% CI, 2.521-2.99).
The conclusion of these investigators, led by Chia-Chi Lung, PhD, Department of Public Health, Chung Shan Medical University, Taichung City, Taiwan, was that there is “a critical need for ongoing monitoring and customized healthcare strategies to mitigate the long-term respiratory impacts of COVID-19 in children.”
These health risks might not be as significant as once feared. In the recently published study from Environmental Health Insights, the goal of a meta-analysis was to determine if patients with asthma relative to those without asthma face a higher risk of serious disease from COVID-19 infection. The meta-analysis included studies of children and adults. The answer, according an in-depth analysis of 21 articles in a “scoping review,” was a qualified no.
Of the 21 articles, 4 concluded that asthma is a risk factor for serious COVID-19 infection, but 17 did not, according to Chukwudi S. Ubah, PhD, Department of Public Health, Brody School of Medicine, East Caroline University, Greenville, North Carolina.
None of These Questions are Fully Resolved
However, given the disparity in the results and the fact that many of the studies included in this analysis had small sample sizes, Dr. Ubah called for larger studies and studies with better controls. He noted, for example, that the studies did not consistently evaluate mitigating factors, such as used of inhaled or oral corticosteroids, which might affect risk of the severity of a COVID-19 infection.
Rather, “our findings pointed out that the type of medication prescribed for asthma may have implications for the severity of COVID-19 infection in these patients,” Dr. Ubah said in an interview.
Overall, the data do not support a major interaction between asthma and COVID-19, even if the data are not conclusive. Each of the senior authors of these studies called for larger and better investigations to further explore whether COVID-19 infection and preexisting asthma interact. So far, the data indicate that if COVID-19 infection poses a risk of precipitating new-onset asthma or inducing a more severe infection in children with asthma, it is low, but the degree of risk, if any, remains unresolved in subgroups defined by asthma treatment or asthma severity.
Dr. Davis, Dr. Hill, Dr. Lung, and Dr. Ubah reported no potential conflicts of interest. None of these studies received funding from commercial interests.
In one of several recently published studies on the relationship between COVID-19 infection and asthma, according to data drawn from the National Survey of Children’s Health (NSCH).
The inverse correlation between symptoms and vaccination was strong and statistically significant, according to investigators led by Matthew M. Davis, MD, Physician in Chief and Chief Scientific Officer, Nemours Children’s Health, Wilmington, Delaware.
“With each increase of 10 percentage points in COVID-19 vaccination coverage, the parent-reported child asthma symptoms prevalence decreased by 0.36 percentage points (P < .05),” Dr. Davis and his coinvestigators reported in a research letter published in JAMA Network Open.
Studies Explore Relationship of COVID and Asthma
The reduced risk of asthma symptoms with COVID-19 vaccination in children at the population level is just one of several recently published studies exploring the interaction between COVID-19 infection and asthma, but two studies that posed the same question did not reach the same conclusion.
In one, COVID-19 infection in children was not found to be a trigger for new-onset asthma, but the second found that it was. In a third study, the preponderance of evidence from a meta-analysis found that patients with asthma – whether children or adults – did not necessarily experience a more severe course of COVID-19 infection than in those without asthma.
The NSCH database study calculated state-level change in scores for patient-reported childhood asthma symptoms in the years in the years 2018-2019, which preceded the pandemic and the years 2020-2021, when the pandemic began. The hypothesis was that the proportion of the population 5 years of age or older who completed the COVID-19 primary vaccination would be inversely related to asthma symptom prevalence.
Relative to the 2018-2019 years, the mean rate of parent-reported asthma symptoms was 0.85% lower (6.93% vs 7.77%; P < .001) in 2020-2021, when the mean primary series COVID-19 vaccination rate was 72.3%.
The study was not able to evaluate the impact of COVID-19 vaccination specifically in children with asthma, because history of asthma is not captured in the NSCH data, but Dr. Davis contended that the reduction in symptomatic asthma among children with increased vaccination offers validation for the state-level findings.
“Moreover, the absence of an association of COVID-19 vaccination administered predominantly in 2021 with population-level COVID-19 mortality in 2020 serves as a negative control,” he and his colleagues wrote in their research letter.
Protection from Respiratory Viruses Seen for Asthma Patients
In an interview, Dr. Davis reported that these data are consistent with previous evidence that immunization against influenza also reduces risk of asthma symptoms. In a meta-analysis published in 2017, it was estimated that live vaccines reduced risk of influenza by 81% and prevented 59%-72% of asthma attacks leading to hospitalizations or emergency room visits.
“The similarity of our findings regarding COVID-19 vaccination to prior data regarding influenza vaccination underscores the importance of preventing viral illnesses in individuals with a history of asthma,” Dr. Davis said. It is not yet clear if this is true of respiratory syncytial virus (RSV). Because of the short time that the RSV vaccine has been available, it is too soon to conduct an analysis.
One message from this study is that “clinicians should continue to encourage COVID-19 vaccination for children because of its general benefits in preventing coronavirus-related illness and the apparent specific benefits for children with a history of asthma,” he said.
While vaccination appears to reduce asthmatic symptoms related to COVID-19 infection, one study suggests that COVID-19 does not trigger new-onset asthma. In a retrospective study published in Pediatrics, no association between COVID-19 infection and new-onset asthma could be made in an analysis of 27,423 children (ages, 1-16 years) from the Children’s Hospital of Philadelphia (CHOP) Care Network.
Across all the pediatric age groups evaluated, the consistent finding was “SARS-CoV-2 positivity does not confer an additional risk for asthma diagnosis at least within the first 18 months after a [polymerase chain reaction] test,” concluded the investigators, led by David A. Hill, MD, PhD, Division of Allergy and Immunology, CHOP, Philadelphia, Pennsylvania.
Risk of Asthma Doubled After COVID-19 Infection
However, the opposite conclusion was reached by investigators evaluating data from two cohorts of children ages 5-18 drawn from the TriNetX database, a global health research network with data on more than 250 million individuals. Cohort 1 included more than 250,000 children. These children had never received COVID-19 vaccination. The 50,000 patients in cohort 2 had all received COVID19 vaccination.
To compare the impact of COVID-19 infection on new-onset asthma, the patients who were infected with COVID-19 were compared with those who were not infected after propensity score matching over 18 months of follow-up.
In cohort 1, the rate of new onset asthma was more than twofold greater among those with COVID-19 infection (4.7% vs 2.0%). The hazard ratio (HR) of 2.25 had tight confidence intervals (95% CI, 2.158-2.367).
In cohort 2, the risk of new-onset asthma at 18 months among those who had a COVID-19 infection relative to those without was even greater (8.3% vs 3.1%). The relative risk approached a 3-fold increase (HR 2.745; 95% CI, 2.521-2.99).
The conclusion of these investigators, led by Chia-Chi Lung, PhD, Department of Public Health, Chung Shan Medical University, Taichung City, Taiwan, was that there is “a critical need for ongoing monitoring and customized healthcare strategies to mitigate the long-term respiratory impacts of COVID-19 in children.”
These health risks might not be as significant as once feared. In the recently published study from Environmental Health Insights, the goal of a meta-analysis was to determine if patients with asthma relative to those without asthma face a higher risk of serious disease from COVID-19 infection. The meta-analysis included studies of children and adults. The answer, according an in-depth analysis of 21 articles in a “scoping review,” was a qualified no.
Of the 21 articles, 4 concluded that asthma is a risk factor for serious COVID-19 infection, but 17 did not, according to Chukwudi S. Ubah, PhD, Department of Public Health, Brody School of Medicine, East Caroline University, Greenville, North Carolina.
None of These Questions are Fully Resolved
However, given the disparity in the results and the fact that many of the studies included in this analysis had small sample sizes, Dr. Ubah called for larger studies and studies with better controls. He noted, for example, that the studies did not consistently evaluate mitigating factors, such as used of inhaled or oral corticosteroids, which might affect risk of the severity of a COVID-19 infection.
Rather, “our findings pointed out that the type of medication prescribed for asthma may have implications for the severity of COVID-19 infection in these patients,” Dr. Ubah said in an interview.
Overall, the data do not support a major interaction between asthma and COVID-19, even if the data are not conclusive. Each of the senior authors of these studies called for larger and better investigations to further explore whether COVID-19 infection and preexisting asthma interact. So far, the data indicate that if COVID-19 infection poses a risk of precipitating new-onset asthma or inducing a more severe infection in children with asthma, it is low, but the degree of risk, if any, remains unresolved in subgroups defined by asthma treatment or asthma severity.
Dr. Davis, Dr. Hill, Dr. Lung, and Dr. Ubah reported no potential conflicts of interest. None of these studies received funding from commercial interests.
FROM JAMA NETWORK OPEN
Chronic Absenteeism
Among the more unheralded examples of collateral damage of the COVID epidemic is chronic absenteeism. A recent NPR/Ipsos poll found that parents ranked chronic absenteeism last in a list of 12 school-related concerns. Only 5% listed it first.
This is surprising and concerning, given that In fact, in some states the chronic absenteeism rate is 40%. In 2020 8 million students were chronically absent. This number is now over 14 million. Chronic absenteeism is a metric defined as a student absent for 15 days or more, which comes out to around 10% of the school year. Chronic absenteeism has been used as a predictor of the student dropout rate.
The initial contribution of the pandemic is easily explained, as parents were understandably concerned about sending their children into an environment that might cause disease, or at least bring the disease home to a more vulnerable family member. The reasons behind the trend’s persistence are a bit more complicated.
Family schedules initially disrupted by the pandemic have settled back into a pattern that may make it more difficult for a child to get to school. Day care and work schedules may have changed, but not yet readjusted to sync with the school schedule.
In the simplest terms, children and their families may have simply fallen out of the habit of going to school. For children (and maybe their parents) who had always struggled with an unresolved separation anxiety, the time at home — or at least not in school — came as a relief. Which, in turn, meant that any gains in dealing with the anxiety have been undone. The child who was already struggling academically or socially found being at home much less challenging. It’s not surprising that he/she might resist climbing back in the academic saddle.
It is very likely that a significant contributor to the persistent trend in chronic absenteeism is what social scientists call “norm erosion.” Not just children, but families may have developed an attitude that time spent in school just isn’t as valuable as they once believed, or were at least told that it was. There seems to be more parents questioning what their children are being taught in school. The home schooling movement existed before the pandemic. Its roots may be growing under the surface in the form of general skepticism about the importance of school in the bigger scheme of things. The home schooling movement was ready to blossom when the COVID pandemic triggered school closures. We hoped and dreamed that remote learning would be just as good as in-person school. We now realize that, in most cases, that was wishful thinking.
It feels as though a “Perfect Attendance Record” may have lost the cachet it once had. During the pandemic anyone claiming to have never missed a day at school lost that gold star. Did opening your computer every day to watch a remote learning session count for anything?
The threshold for allowing a child to stay home from school may be reaching a historic low. Families seem to regard the school schedule as a guideline that can easily be ignored when planning a vacation. Take little brother out of school to attend big brother’s lacrosse playoff game, not to worry if the youngster misses school days for a trip.
Who is responsible for reversing the trend? Teachers already know it is a serious problem. They view attendance as important. Maybe educators could make school more appealing. But to whom? Sounds like this message should be targeted at the parents. Would stiff penalties for parents whose children are chronically absent help? Would demanding a note from a physician after a certain number of absences help? It might. But, are pediatricians and educators ready to take on one more task in which parents have dropped the ball?
An unknown percentage of chronically absent children are missing school because of a previously unrecognized or inadequately treated mental health condition or learning disability. Involving physicians in a community’s response to chronic absenteeism may be the first step in getting a child back on track. If socioeconomic factors are contributing to a child’s truancy, the involvement of social service agencies may be the answer.
I have a friend who is often asked to address graduating classes at both the high school and college level. One of his standard pieces of advice, whether it be about school or a workplace you may not be in love with, is to at least “show up.” The family that treats school attendance as optional is likely to produce adults who take a similarly nonchalant attitude toward their employment opportunities — with unfortunate results.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Among the more unheralded examples of collateral damage of the COVID epidemic is chronic absenteeism. A recent NPR/Ipsos poll found that parents ranked chronic absenteeism last in a list of 12 school-related concerns. Only 5% listed it first.
This is surprising and concerning, given that In fact, in some states the chronic absenteeism rate is 40%. In 2020 8 million students were chronically absent. This number is now over 14 million. Chronic absenteeism is a metric defined as a student absent for 15 days or more, which comes out to around 10% of the school year. Chronic absenteeism has been used as a predictor of the student dropout rate.
The initial contribution of the pandemic is easily explained, as parents were understandably concerned about sending their children into an environment that might cause disease, or at least bring the disease home to a more vulnerable family member. The reasons behind the trend’s persistence are a bit more complicated.
Family schedules initially disrupted by the pandemic have settled back into a pattern that may make it more difficult for a child to get to school. Day care and work schedules may have changed, but not yet readjusted to sync with the school schedule.
In the simplest terms, children and their families may have simply fallen out of the habit of going to school. For children (and maybe their parents) who had always struggled with an unresolved separation anxiety, the time at home — or at least not in school — came as a relief. Which, in turn, meant that any gains in dealing with the anxiety have been undone. The child who was already struggling academically or socially found being at home much less challenging. It’s not surprising that he/she might resist climbing back in the academic saddle.
It is very likely that a significant contributor to the persistent trend in chronic absenteeism is what social scientists call “norm erosion.” Not just children, but families may have developed an attitude that time spent in school just isn’t as valuable as they once believed, or were at least told that it was. There seems to be more parents questioning what their children are being taught in school. The home schooling movement existed before the pandemic. Its roots may be growing under the surface in the form of general skepticism about the importance of school in the bigger scheme of things. The home schooling movement was ready to blossom when the COVID pandemic triggered school closures. We hoped and dreamed that remote learning would be just as good as in-person school. We now realize that, in most cases, that was wishful thinking.
It feels as though a “Perfect Attendance Record” may have lost the cachet it once had. During the pandemic anyone claiming to have never missed a day at school lost that gold star. Did opening your computer every day to watch a remote learning session count for anything?
The threshold for allowing a child to stay home from school may be reaching a historic low. Families seem to regard the school schedule as a guideline that can easily be ignored when planning a vacation. Take little brother out of school to attend big brother’s lacrosse playoff game, not to worry if the youngster misses school days for a trip.
Who is responsible for reversing the trend? Teachers already know it is a serious problem. They view attendance as important. Maybe educators could make school more appealing. But to whom? Sounds like this message should be targeted at the parents. Would stiff penalties for parents whose children are chronically absent help? Would demanding a note from a physician after a certain number of absences help? It might. But, are pediatricians and educators ready to take on one more task in which parents have dropped the ball?
An unknown percentage of chronically absent children are missing school because of a previously unrecognized or inadequately treated mental health condition or learning disability. Involving physicians in a community’s response to chronic absenteeism may be the first step in getting a child back on track. If socioeconomic factors are contributing to a child’s truancy, the involvement of social service agencies may be the answer.
I have a friend who is often asked to address graduating classes at both the high school and college level. One of his standard pieces of advice, whether it be about school or a workplace you may not be in love with, is to at least “show up.” The family that treats school attendance as optional is likely to produce adults who take a similarly nonchalant attitude toward their employment opportunities — with unfortunate results.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Among the more unheralded examples of collateral damage of the COVID epidemic is chronic absenteeism. A recent NPR/Ipsos poll found that parents ranked chronic absenteeism last in a list of 12 school-related concerns. Only 5% listed it first.
This is surprising and concerning, given that In fact, in some states the chronic absenteeism rate is 40%. In 2020 8 million students were chronically absent. This number is now over 14 million. Chronic absenteeism is a metric defined as a student absent for 15 days or more, which comes out to around 10% of the school year. Chronic absenteeism has been used as a predictor of the student dropout rate.
The initial contribution of the pandemic is easily explained, as parents were understandably concerned about sending their children into an environment that might cause disease, or at least bring the disease home to a more vulnerable family member. The reasons behind the trend’s persistence are a bit more complicated.
Family schedules initially disrupted by the pandemic have settled back into a pattern that may make it more difficult for a child to get to school. Day care and work schedules may have changed, but not yet readjusted to sync with the school schedule.
In the simplest terms, children and their families may have simply fallen out of the habit of going to school. For children (and maybe their parents) who had always struggled with an unresolved separation anxiety, the time at home — or at least not in school — came as a relief. Which, in turn, meant that any gains in dealing with the anxiety have been undone. The child who was already struggling academically or socially found being at home much less challenging. It’s not surprising that he/she might resist climbing back in the academic saddle.
It is very likely that a significant contributor to the persistent trend in chronic absenteeism is what social scientists call “norm erosion.” Not just children, but families may have developed an attitude that time spent in school just isn’t as valuable as they once believed, or were at least told that it was. There seems to be more parents questioning what their children are being taught in school. The home schooling movement existed before the pandemic. Its roots may be growing under the surface in the form of general skepticism about the importance of school in the bigger scheme of things. The home schooling movement was ready to blossom when the COVID pandemic triggered school closures. We hoped and dreamed that remote learning would be just as good as in-person school. We now realize that, in most cases, that was wishful thinking.
It feels as though a “Perfect Attendance Record” may have lost the cachet it once had. During the pandemic anyone claiming to have never missed a day at school lost that gold star. Did opening your computer every day to watch a remote learning session count for anything?
The threshold for allowing a child to stay home from school may be reaching a historic low. Families seem to regard the school schedule as a guideline that can easily be ignored when planning a vacation. Take little brother out of school to attend big brother’s lacrosse playoff game, not to worry if the youngster misses school days for a trip.
Who is responsible for reversing the trend? Teachers already know it is a serious problem. They view attendance as important. Maybe educators could make school more appealing. But to whom? Sounds like this message should be targeted at the parents. Would stiff penalties for parents whose children are chronically absent help? Would demanding a note from a physician after a certain number of absences help? It might. But, are pediatricians and educators ready to take on one more task in which parents have dropped the ball?
An unknown percentage of chronically absent children are missing school because of a previously unrecognized or inadequately treated mental health condition or learning disability. Involving physicians in a community’s response to chronic absenteeism may be the first step in getting a child back on track. If socioeconomic factors are contributing to a child’s truancy, the involvement of social service agencies may be the answer.
I have a friend who is often asked to address graduating classes at both the high school and college level. One of his standard pieces of advice, whether it be about school or a workplace you may not be in love with, is to at least “show up.” The family that treats school attendance as optional is likely to produce adults who take a similarly nonchalant attitude toward their employment opportunities — with unfortunate results.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
The Push to Get More People Into Long COVID Studies
When Ezra Spier was diagnosed with long COVID in late 2022, his main symptom, postexertional malaise, caused fatigue so severe that it forced him to quit his job as a technology entrepreneur. Since then, it’s been a tough road for Spier, 37, who said he wouldn’t wish his hellish condition on anyone.
Last spring, he enrolled in a clinical trial of a new long COVID therapy at Stanford University, and he’s about to start another at the University of California, San Francisco.
For Spier, who lives in Oakland, California, being part of the clinical trials connected him with people dealing with similar health issues while also moving the needle toward better treatments for everyone. Yet many potential participants are unaware that these clinical trials exist. Clinical trial researchers also express frustration over the challenge of enrolling participants.
That’s why Spier created a new website to help match long COVID patients with clinical trials that can help.
“I wanted a way to make long COVID clinical trials more accessible to the general public,” he said. Spier’s website, aptly named Long Covid Studies, launched in March. The site already includes details from about 550 trials globally and, in the future, will include many more.
It’s Not the Number of Studies, It’s Navigating Them
In all, nearly 9300 long COVID trials are listed on ClinicalTrials.gov. But many patients find the site difficult to navigate, said David F. Putrino, PhD, who runs the long COVID clinic at Mount Sinai Health System in New York City. He said Spier’s website helps make trials easier for patients to manage in ways that remove the enrollment challenges.
“Ezra’s platform pulls data from ClinicalTrials.gov and puts it into a space that’s much easier for patients to manage,” said Dr. Putrino. The site only includes the most relevant information, such as the study location, eligibility, and purpose and how to sign up.
Another of Spier’s goals is to make the process easier for patients who are already marginalized and often excluded from the healthcare system. Long COVID disproportionately impacts people in minority ethnic groups and women, as well as those who are impoverished or live in rural areas.
According to the National Institutes of Health (NIH), 1 in 4 patients with severe long COVID-19 are Black or Hispanic whereas only 1 in 7 are White. Yet participation by White persons in clinical trials is much higher overall: 77% of participants are White, compared with only 14% for Black persons and 15% for Hispanic persons. Without more balanced representation, research becomes skewed and less accurate, said Grace McComsey, MD, who leads one of the 15 nationwide long COVID centers funded by the federal RECOVER (Researching COVID to Enhance Recovery) Initiative in Cleveland.
Websites that are easier for the layperson to access would allow for wider participation, said McComsey.
Too Many Barriers to Entry
A study published in the Journal of Applied Gerontology found that transportation plays an outsized role in influencing study participation, which may also lead to less diverse participation.
Decentralized trials — in which participants receive therapy at home — also make enrolling in clinical trials easier for marginalized patients and those too sick to make it to a research center, said Dr. Putrino. Research published recently in The American Journal of Medicine demonstrated that for many patients, remote studies are the future of COVID research. The study, focusing on the efficacy of Paxlovid, recruited patients living in the 48 contiguous US states. Participation was entirely remote.
“We need to have more consideration for bedbound and housebound patients in our research,” said Dr. Putrino. “Some people don’t have the ability to show up to a prestigious university to take part in an academic trial.”
Dr. Putrino and colleagues at Yale School of Medicine’s Yale COVID Recovery Study plan to release a paper in the near future on the methodology for running decentralized or remote studies that could provide guidance for researchers elsewhere.
Decentralized studies serve a larger audience, but they’re also more expensive and cost has plagued long COVID research from the start, said Michael Peluso, MD, an assistant research professor of infectious medicine at UCSF School of Medicine, University of California, San Francisco.
“You need to have a staff in place that’s trained to do home visits in order to conduct remote trials,” Dr. Peluso said, adding that his biggest challenge has been connecting patients to appropriate clinical trials.
Individual eligibility has been an ongoing issue. For example, Dr. Peluso’s current trials are testing monoclonal antibodies — antibodies produced by cloning unique white blood cells to target viral persistence, which is thought to be a cause of long COVID. Only patients who were infected with certain variants of acute COVID are eligible because of the antibodies needed to target SARS-CoV-2 spike proteins.
“This can lead to a lot of frustration among patients who might think they can participate, but aren’t eligible,” said Dr. Peluso.
Long Fight for Better Long COVID Research
For Spier, one of the hardest parts of his health issues and lack of energy is that they have sharply curtailed his social interactions with friends and colleagues.
He has channeled his energies into researching new treatments that could potentially improve his symptoms. That research is partly what drove him to create the Long Covid Studies website.
His goal is still to help others with long COVID find trials that can improve their symptoms as well. The more people who participate, the closer scientists will come to providing effective treatments for everyone, he said.
“For all my frustrations, we’re still at the forefront of science globally,” he said. “And if we have the level of funding the NIH is equipped to provide, we can show the world what’s possible with long COVID research.”
A version of this article first appeared on Medscape.com.
When Ezra Spier was diagnosed with long COVID in late 2022, his main symptom, postexertional malaise, caused fatigue so severe that it forced him to quit his job as a technology entrepreneur. Since then, it’s been a tough road for Spier, 37, who said he wouldn’t wish his hellish condition on anyone.
Last spring, he enrolled in a clinical trial of a new long COVID therapy at Stanford University, and he’s about to start another at the University of California, San Francisco.
For Spier, who lives in Oakland, California, being part of the clinical trials connected him with people dealing with similar health issues while also moving the needle toward better treatments for everyone. Yet many potential participants are unaware that these clinical trials exist. Clinical trial researchers also express frustration over the challenge of enrolling participants.
That’s why Spier created a new website to help match long COVID patients with clinical trials that can help.
“I wanted a way to make long COVID clinical trials more accessible to the general public,” he said. Spier’s website, aptly named Long Covid Studies, launched in March. The site already includes details from about 550 trials globally and, in the future, will include many more.
It’s Not the Number of Studies, It’s Navigating Them
In all, nearly 9300 long COVID trials are listed on ClinicalTrials.gov. But many patients find the site difficult to navigate, said David F. Putrino, PhD, who runs the long COVID clinic at Mount Sinai Health System in New York City. He said Spier’s website helps make trials easier for patients to manage in ways that remove the enrollment challenges.
“Ezra’s platform pulls data from ClinicalTrials.gov and puts it into a space that’s much easier for patients to manage,” said Dr. Putrino. The site only includes the most relevant information, such as the study location, eligibility, and purpose and how to sign up.
Another of Spier’s goals is to make the process easier for patients who are already marginalized and often excluded from the healthcare system. Long COVID disproportionately impacts people in minority ethnic groups and women, as well as those who are impoverished or live in rural areas.
According to the National Institutes of Health (NIH), 1 in 4 patients with severe long COVID-19 are Black or Hispanic whereas only 1 in 7 are White. Yet participation by White persons in clinical trials is much higher overall: 77% of participants are White, compared with only 14% for Black persons and 15% for Hispanic persons. Without more balanced representation, research becomes skewed and less accurate, said Grace McComsey, MD, who leads one of the 15 nationwide long COVID centers funded by the federal RECOVER (Researching COVID to Enhance Recovery) Initiative in Cleveland.
Websites that are easier for the layperson to access would allow for wider participation, said McComsey.
Too Many Barriers to Entry
A study published in the Journal of Applied Gerontology found that transportation plays an outsized role in influencing study participation, which may also lead to less diverse participation.
Decentralized trials — in which participants receive therapy at home — also make enrolling in clinical trials easier for marginalized patients and those too sick to make it to a research center, said Dr. Putrino. Research published recently in The American Journal of Medicine demonstrated that for many patients, remote studies are the future of COVID research. The study, focusing on the efficacy of Paxlovid, recruited patients living in the 48 contiguous US states. Participation was entirely remote.
“We need to have more consideration for bedbound and housebound patients in our research,” said Dr. Putrino. “Some people don’t have the ability to show up to a prestigious university to take part in an academic trial.”
Dr. Putrino and colleagues at Yale School of Medicine’s Yale COVID Recovery Study plan to release a paper in the near future on the methodology for running decentralized or remote studies that could provide guidance for researchers elsewhere.
Decentralized studies serve a larger audience, but they’re also more expensive and cost has plagued long COVID research from the start, said Michael Peluso, MD, an assistant research professor of infectious medicine at UCSF School of Medicine, University of California, San Francisco.
“You need to have a staff in place that’s trained to do home visits in order to conduct remote trials,” Dr. Peluso said, adding that his biggest challenge has been connecting patients to appropriate clinical trials.
Individual eligibility has been an ongoing issue. For example, Dr. Peluso’s current trials are testing monoclonal antibodies — antibodies produced by cloning unique white blood cells to target viral persistence, which is thought to be a cause of long COVID. Only patients who were infected with certain variants of acute COVID are eligible because of the antibodies needed to target SARS-CoV-2 spike proteins.
“This can lead to a lot of frustration among patients who might think they can participate, but aren’t eligible,” said Dr. Peluso.
Long Fight for Better Long COVID Research
For Spier, one of the hardest parts of his health issues and lack of energy is that they have sharply curtailed his social interactions with friends and colleagues.
He has channeled his energies into researching new treatments that could potentially improve his symptoms. That research is partly what drove him to create the Long Covid Studies website.
His goal is still to help others with long COVID find trials that can improve their symptoms as well. The more people who participate, the closer scientists will come to providing effective treatments for everyone, he said.
“For all my frustrations, we’re still at the forefront of science globally,” he said. “And if we have the level of funding the NIH is equipped to provide, we can show the world what’s possible with long COVID research.”
A version of this article first appeared on Medscape.com.
When Ezra Spier was diagnosed with long COVID in late 2022, his main symptom, postexertional malaise, caused fatigue so severe that it forced him to quit his job as a technology entrepreneur. Since then, it’s been a tough road for Spier, 37, who said he wouldn’t wish his hellish condition on anyone.
Last spring, he enrolled in a clinical trial of a new long COVID therapy at Stanford University, and he’s about to start another at the University of California, San Francisco.
For Spier, who lives in Oakland, California, being part of the clinical trials connected him with people dealing with similar health issues while also moving the needle toward better treatments for everyone. Yet many potential participants are unaware that these clinical trials exist. Clinical trial researchers also express frustration over the challenge of enrolling participants.
That’s why Spier created a new website to help match long COVID patients with clinical trials that can help.
“I wanted a way to make long COVID clinical trials more accessible to the general public,” he said. Spier’s website, aptly named Long Covid Studies, launched in March. The site already includes details from about 550 trials globally and, in the future, will include many more.
It’s Not the Number of Studies, It’s Navigating Them
In all, nearly 9300 long COVID trials are listed on ClinicalTrials.gov. But many patients find the site difficult to navigate, said David F. Putrino, PhD, who runs the long COVID clinic at Mount Sinai Health System in New York City. He said Spier’s website helps make trials easier for patients to manage in ways that remove the enrollment challenges.
“Ezra’s platform pulls data from ClinicalTrials.gov and puts it into a space that’s much easier for patients to manage,” said Dr. Putrino. The site only includes the most relevant information, such as the study location, eligibility, and purpose and how to sign up.
Another of Spier’s goals is to make the process easier for patients who are already marginalized and often excluded from the healthcare system. Long COVID disproportionately impacts people in minority ethnic groups and women, as well as those who are impoverished or live in rural areas.
According to the National Institutes of Health (NIH), 1 in 4 patients with severe long COVID-19 are Black or Hispanic whereas only 1 in 7 are White. Yet participation by White persons in clinical trials is much higher overall: 77% of participants are White, compared with only 14% for Black persons and 15% for Hispanic persons. Without more balanced representation, research becomes skewed and less accurate, said Grace McComsey, MD, who leads one of the 15 nationwide long COVID centers funded by the federal RECOVER (Researching COVID to Enhance Recovery) Initiative in Cleveland.
Websites that are easier for the layperson to access would allow for wider participation, said McComsey.
Too Many Barriers to Entry
A study published in the Journal of Applied Gerontology found that transportation plays an outsized role in influencing study participation, which may also lead to less diverse participation.
Decentralized trials — in which participants receive therapy at home — also make enrolling in clinical trials easier for marginalized patients and those too sick to make it to a research center, said Dr. Putrino. Research published recently in The American Journal of Medicine demonstrated that for many patients, remote studies are the future of COVID research. The study, focusing on the efficacy of Paxlovid, recruited patients living in the 48 contiguous US states. Participation was entirely remote.
“We need to have more consideration for bedbound and housebound patients in our research,” said Dr. Putrino. “Some people don’t have the ability to show up to a prestigious university to take part in an academic trial.”
Dr. Putrino and colleagues at Yale School of Medicine’s Yale COVID Recovery Study plan to release a paper in the near future on the methodology for running decentralized or remote studies that could provide guidance for researchers elsewhere.
Decentralized studies serve a larger audience, but they’re also more expensive and cost has plagued long COVID research from the start, said Michael Peluso, MD, an assistant research professor of infectious medicine at UCSF School of Medicine, University of California, San Francisco.
“You need to have a staff in place that’s trained to do home visits in order to conduct remote trials,” Dr. Peluso said, adding that his biggest challenge has been connecting patients to appropriate clinical trials.
Individual eligibility has been an ongoing issue. For example, Dr. Peluso’s current trials are testing monoclonal antibodies — antibodies produced by cloning unique white blood cells to target viral persistence, which is thought to be a cause of long COVID. Only patients who were infected with certain variants of acute COVID are eligible because of the antibodies needed to target SARS-CoV-2 spike proteins.
“This can lead to a lot of frustration among patients who might think they can participate, but aren’t eligible,” said Dr. Peluso.
Long Fight for Better Long COVID Research
For Spier, one of the hardest parts of his health issues and lack of energy is that they have sharply curtailed his social interactions with friends and colleagues.
He has channeled his energies into researching new treatments that could potentially improve his symptoms. That research is partly what drove him to create the Long Covid Studies website.
His goal is still to help others with long COVID find trials that can improve their symptoms as well. The more people who participate, the closer scientists will come to providing effective treatments for everyone, he said.
“For all my frustrations, we’re still at the forefront of science globally,” he said. “And if we have the level of funding the NIH is equipped to provide, we can show the world what’s possible with long COVID research.”
A version of this article first appeared on Medscape.com.
Post-COVID Mental Health Risks Linger for Veterans
Not surprisingly, anxiety, depression, posttraumatic stress disorder, and other mental health issues became more prevalent during the COVID-19 pandemic—and after. Studies have found that neurologic and psychiatric sequelae may last up to 6 months following COVID-19 infection.
It appears that COVID-19 infection—even past the acute stage—could put hospitalized patients at risk of exacerbating existing mental health conditions or even developing new conditions. Researchers from Salem Veterans Affairs Health Care System conducted a retrospective observational study from January 1, 2020, through January 1, 2022, of 50,805 veterans hospitalized with COVID-19 and 50,805 patients hospitalized for other reasons.
The researchers found that veterans with COVID-19 group had significantly higher rates of psychiatry-related hospitalization at both 90 and 180 days, as well as a significant increase in the incidence of outpatient mental health visits at 180 days. They also noted a significantly higher risk of new-onset depression and new-onset dementia in the COVID-19 patients at 180 days compared with the non-COVID-19 cohort.
The exact mechanism of the impact of COVID-19 hospitalization on new or worsening depression has yet to be uncovered, the researchers say, but it is known to be complex and interrelated. They point to post-COVID-19 follow-up studies that have found that even mild and asymptomatic infection may lead to cognitive impairment, delirium, extreme fatigue, and clinically relevant mood symptoms. The residual effects of COVID-19 appear to span multiple organ systems.
The researchers also cite current hypotheses about the psychiatric sequelae of COVID-19 that suggest sustained neuroinflammatory processes disrupt the blood-brain barrier, leading to damaged neurons and glia in the brain. In a systematic review, roughly one-third of patients developed neurologic symptoms in the acute phase of the disease, with brain abnormalities “suggestive of COVID-19 etiology.” What’s more, multiple studies have found that anxiety and depression worsen the clinical course of chronic disease, indicating that this mechanism is bidirectional.
Future studies should, among other things include outcomes assessed by COVID-19 disease severity, as well as various psychiatric adverse effects, to enhance provider vigilance and promote closer monitoring.
Not surprisingly, anxiety, depression, posttraumatic stress disorder, and other mental health issues became more prevalent during the COVID-19 pandemic—and after. Studies have found that neurologic and psychiatric sequelae may last up to 6 months following COVID-19 infection.
It appears that COVID-19 infection—even past the acute stage—could put hospitalized patients at risk of exacerbating existing mental health conditions or even developing new conditions. Researchers from Salem Veterans Affairs Health Care System conducted a retrospective observational study from January 1, 2020, through January 1, 2022, of 50,805 veterans hospitalized with COVID-19 and 50,805 patients hospitalized for other reasons.
The researchers found that veterans with COVID-19 group had significantly higher rates of psychiatry-related hospitalization at both 90 and 180 days, as well as a significant increase in the incidence of outpatient mental health visits at 180 days. They also noted a significantly higher risk of new-onset depression and new-onset dementia in the COVID-19 patients at 180 days compared with the non-COVID-19 cohort.
The exact mechanism of the impact of COVID-19 hospitalization on new or worsening depression has yet to be uncovered, the researchers say, but it is known to be complex and interrelated. They point to post-COVID-19 follow-up studies that have found that even mild and asymptomatic infection may lead to cognitive impairment, delirium, extreme fatigue, and clinically relevant mood symptoms. The residual effects of COVID-19 appear to span multiple organ systems.
The researchers also cite current hypotheses about the psychiatric sequelae of COVID-19 that suggest sustained neuroinflammatory processes disrupt the blood-brain barrier, leading to damaged neurons and glia in the brain. In a systematic review, roughly one-third of patients developed neurologic symptoms in the acute phase of the disease, with brain abnormalities “suggestive of COVID-19 etiology.” What’s more, multiple studies have found that anxiety and depression worsen the clinical course of chronic disease, indicating that this mechanism is bidirectional.
Future studies should, among other things include outcomes assessed by COVID-19 disease severity, as well as various psychiatric adverse effects, to enhance provider vigilance and promote closer monitoring.
Not surprisingly, anxiety, depression, posttraumatic stress disorder, and other mental health issues became more prevalent during the COVID-19 pandemic—and after. Studies have found that neurologic and psychiatric sequelae may last up to 6 months following COVID-19 infection.
It appears that COVID-19 infection—even past the acute stage—could put hospitalized patients at risk of exacerbating existing mental health conditions or even developing new conditions. Researchers from Salem Veterans Affairs Health Care System conducted a retrospective observational study from January 1, 2020, through January 1, 2022, of 50,805 veterans hospitalized with COVID-19 and 50,805 patients hospitalized for other reasons.
The researchers found that veterans with COVID-19 group had significantly higher rates of psychiatry-related hospitalization at both 90 and 180 days, as well as a significant increase in the incidence of outpatient mental health visits at 180 days. They also noted a significantly higher risk of new-onset depression and new-onset dementia in the COVID-19 patients at 180 days compared with the non-COVID-19 cohort.
The exact mechanism of the impact of COVID-19 hospitalization on new or worsening depression has yet to be uncovered, the researchers say, but it is known to be complex and interrelated. They point to post-COVID-19 follow-up studies that have found that even mild and asymptomatic infection may lead to cognitive impairment, delirium, extreme fatigue, and clinically relevant mood symptoms. The residual effects of COVID-19 appear to span multiple organ systems.
The researchers also cite current hypotheses about the psychiatric sequelae of COVID-19 that suggest sustained neuroinflammatory processes disrupt the blood-brain barrier, leading to damaged neurons and glia in the brain. In a systematic review, roughly one-third of patients developed neurologic symptoms in the acute phase of the disease, with brain abnormalities “suggestive of COVID-19 etiology.” What’s more, multiple studies have found that anxiety and depression worsen the clinical course of chronic disease, indicating that this mechanism is bidirectional.
Future studies should, among other things include outcomes assessed by COVID-19 disease severity, as well as various psychiatric adverse effects, to enhance provider vigilance and promote closer monitoring.
New mRNA Vaccines in Development for Cancer and Infections
Martina Prelog, MD, a pediatric and adolescent medicine specialist at the University Hospital of Würzburg in Germany, reported on the principles, research status, and perspectives for these vaccines at the 25th Travel and Health Forum of the Center for Travel Medicine in Berlin.
To understand the future, the immunologist first examined the past. “The induction of cellular and humoral immune responses by externally injected mRNA was discovered in the 1990s,” she said.
Instability Challenge
Significant hurdles in mRNA vaccinations included the instability of mRNA and the immune system’s ability to identify foreign mRNA as a threat and destroy mRNA fragments. “The breakthrough toward vaccination came through Dr. Katalin Karikó, who, along with Dr. Drew Weissman, both of the University of Pennsylvania School of Medicine, discovered in 2005 that modifications of mRNA (replacing the nucleoside uridine with pseudouridine) enable better stability of mRNA, reduced immunogenicity, and higher translational capacity at the ribosomes,” said Dr. Prelog.
With this discovery, the two researchers paved the way for the development of mRNA vaccines against COVID-19 and other diseases. They were awarded the Nobel Prize in medicine for their discovery last year.
Improved Scalability
“Since 2009, mRNA vaccines have been studied as a treatment option for cancer,” said Dr. Prelog. “Since 2012, they have been studied for the influenza virus and respiratory syncytial virus [RSV].” Consequently, several mRNA vaccines are currently in development or in approval studies. “The mRNA technology offers the advantage of quickly and flexibly responding to new variants of pathogens and the ability to scale up production when there is high demand for a particular vaccine.”
Different forms and designations of mRNA vaccines are used, depending on the application and desired effect, said Dr. Prelog.
In nucleoside-modified mRNA vaccines, modifications in the mRNA sequence enable the mRNA to remain in the body longer and to induce protein synthesis more effectively.
Lipid nanoparticle (LNP)–encapsulated mRNA vaccines protect the coding mRNA sequences against degradation by the body’s enzymes and facilitate the uptake of mRNA into cells, where it then triggers the production of the desired protein. In addition, LNPs are involved in cell stimulation and support the self-adjuvant effect of mRNA vaccines, thus eliminating the need for adjuvants.
Self-amplifying mRNA vaccines include a special mRNA that replicates itself in the cell and contains a sequence for RNA replicase, in addition to the coding sequence for the protein. This composition enables increased production of the target protein without the need for a high amount of external mRNA administration. Such vaccines could trigger a longer and stronger immune response because the immune system has more time to interact with the protein.
Cancer Immunotherapy
Dr. Prelog also discussed personalized vaccines for cancer immunotherapy. Personalized mRNA vaccines are tailored to the patient’s genetic characteristics and antigens. They could be used in cancer immunotherapy to activate the immune system selectively against tumor cells.
Multivalent mRNA vaccines contain mRNA that codes for multiple antigens rather than just one protein to generate an immune response. These vaccines could be particularly useful in fighting pathogens with variable or changing surface structures or in eliciting protection against multiple pathogens simultaneously.
The technology of mRNA-encoded antibodies involves introducing mRNA into the cell, which creates light and heavy chains of antibodies. This step leads to the formation of antibodies targeted against toxins (eg, diphtheria and tetanus), animal venoms, infectious agents, or tumor cells.
Genetic Engineering
Dr. Prelog also reviewed genetic engineering techniques. In regenerative therapy or protein replacement therapy, skin fibroblasts or other cells are transfected with mRNA to enable conversion into induced pluripotent stem cells. This approach avoids the risk for DNA integration into the genome and associated mutation risks.
Another approach is making post-transcriptional modifications through RNA interference. For example, RNA structures can be used to inhibit the translation of disease-causing proteins. This technique is currently being tested against HIV and tumors such as melanoma.
In addition, mRNA technologies can be combined with CRISPR/Cas9 technology (“gene scissors”) to influence the creation of gene products even more precisely. The advantage of this technique is that mRNA is only transiently expressed, thus preventing unwanted side effects. Furthermore, mRNA is translated directly in the cytoplasm, leading to a faster initiation of gene editing.
Of the numerous ongoing clinical mRNA vaccine studies, around 70% focus on infections, about 12% on cancer, and the rest on autoimmune diseases and neurodegenerative disorders, said Dr. Prelog.
Research in Infections
Research in the fields of infectious diseases and oncology is the most advanced: mRNA vaccines against influenza and RSV are already in advanced clinical trials, Dr. Prelog told this news organization.
“Conventional influenza vaccines contain immunogenic surface molecules against hemagglutinin and neuraminidase in various combinations of influenza strains A and B and are produced in egg or cell cultures,” she said. “This is a time-consuming manufacturing process that takes months and, particularly with the egg-based process, bears the risk of changing the vaccine strain.”
“Additionally, influenza viruses undergo antigenic shift and drift through recombination, thus requiring annual adjustments to the vaccines. Thus, these influenza vaccines often lose accuracy in targeting circulating seasonal influenza strains.”
Several mRNA vaccines being tested contain not only coding sequences against hemagglutinin and neuraminidase but also for structural proteins of influenza viruses. “These are more conserved and mutate less easily, meaning they could serve as the basis for universal pandemic influenza vaccines,” said Dr. Prelog.
An advantage of mRNA vaccines, she added, is the strong cellular immune response that they elicit. This response is intended to provide additional protection alongside specific antibodies. An mRNA vaccine with coding sequences for the pre-fusion protein of RSV is in phase 3 trials for approval for vaccination in patients aged 60 years and older. It shows high effectiveness even in older patients and those with comorbidities.
Elaborate Purification Process
Bacterial origin plasmid DNA is used to produce mRNA vaccines. The mRNA vaccines for COVID-19 raised concerns that production-related DNA residues could pose a safety risk and cause autoimmune diseases.
These vaccines “typically undergo a very elaborate purification process,” said Dr. Prelog. “This involves enzymatic digestion with DNase to fragment and deplete plasmid DNA, followed by purification using chromatography columns, so that no safety-relevant DNA fragments should remain afterward.”
Thus, the Paul-Ehrlich-Institut also pointed out the very small, fragmented plasmid DNA residues of bacterial origin in mRNA COVID-19 vaccines pose no risk, unlike residual DNA from animal cell culture might pose in other vaccines.
Prevention and Therapy
In addition to the numerous advantages of mRNA vaccines (such as rapid adaptability to new or mutated pathogens, scalability, rapid production capability, self-adjuvant effect, strong induction of cellular immune responses, and safety), there are also challenges in RNA technology as a preventive and therapeutic measure, according to Dr. Prelog.
“Stability and storability, as well as the costs of new vaccine developments, play a role, as do the long-term effects regarding the persistence of antibody and cellular responses,” she said. The COVID-19 mRNA vaccines, for example, showed a well-maintained cellular immune response despite a tendency toward a rapid decline in humoral immune response.
“The experience with COVID-19 mRNA vaccines and the new vaccine developments based on mRNA technology give hope for an efficient and safe preventive and therapeutic use, particularly in the fields of infectious diseases and oncology,” Dr. Prelog concluded.
This story was translated from the Medscape German edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Martina Prelog, MD, a pediatric and adolescent medicine specialist at the University Hospital of Würzburg in Germany, reported on the principles, research status, and perspectives for these vaccines at the 25th Travel and Health Forum of the Center for Travel Medicine in Berlin.
To understand the future, the immunologist first examined the past. “The induction of cellular and humoral immune responses by externally injected mRNA was discovered in the 1990s,” she said.
Instability Challenge
Significant hurdles in mRNA vaccinations included the instability of mRNA and the immune system’s ability to identify foreign mRNA as a threat and destroy mRNA fragments. “The breakthrough toward vaccination came through Dr. Katalin Karikó, who, along with Dr. Drew Weissman, both of the University of Pennsylvania School of Medicine, discovered in 2005 that modifications of mRNA (replacing the nucleoside uridine with pseudouridine) enable better stability of mRNA, reduced immunogenicity, and higher translational capacity at the ribosomes,” said Dr. Prelog.
With this discovery, the two researchers paved the way for the development of mRNA vaccines against COVID-19 and other diseases. They were awarded the Nobel Prize in medicine for their discovery last year.
Improved Scalability
“Since 2009, mRNA vaccines have been studied as a treatment option for cancer,” said Dr. Prelog. “Since 2012, they have been studied for the influenza virus and respiratory syncytial virus [RSV].” Consequently, several mRNA vaccines are currently in development or in approval studies. “The mRNA technology offers the advantage of quickly and flexibly responding to new variants of pathogens and the ability to scale up production when there is high demand for a particular vaccine.”
Different forms and designations of mRNA vaccines are used, depending on the application and desired effect, said Dr. Prelog.
In nucleoside-modified mRNA vaccines, modifications in the mRNA sequence enable the mRNA to remain in the body longer and to induce protein synthesis more effectively.
Lipid nanoparticle (LNP)–encapsulated mRNA vaccines protect the coding mRNA sequences against degradation by the body’s enzymes and facilitate the uptake of mRNA into cells, where it then triggers the production of the desired protein. In addition, LNPs are involved in cell stimulation and support the self-adjuvant effect of mRNA vaccines, thus eliminating the need for adjuvants.
Self-amplifying mRNA vaccines include a special mRNA that replicates itself in the cell and contains a sequence for RNA replicase, in addition to the coding sequence for the protein. This composition enables increased production of the target protein without the need for a high amount of external mRNA administration. Such vaccines could trigger a longer and stronger immune response because the immune system has more time to interact with the protein.
Cancer Immunotherapy
Dr. Prelog also discussed personalized vaccines for cancer immunotherapy. Personalized mRNA vaccines are tailored to the patient’s genetic characteristics and antigens. They could be used in cancer immunotherapy to activate the immune system selectively against tumor cells.
Multivalent mRNA vaccines contain mRNA that codes for multiple antigens rather than just one protein to generate an immune response. These vaccines could be particularly useful in fighting pathogens with variable or changing surface structures or in eliciting protection against multiple pathogens simultaneously.
The technology of mRNA-encoded antibodies involves introducing mRNA into the cell, which creates light and heavy chains of antibodies. This step leads to the formation of antibodies targeted against toxins (eg, diphtheria and tetanus), animal venoms, infectious agents, or tumor cells.
Genetic Engineering
Dr. Prelog also reviewed genetic engineering techniques. In regenerative therapy or protein replacement therapy, skin fibroblasts or other cells are transfected with mRNA to enable conversion into induced pluripotent stem cells. This approach avoids the risk for DNA integration into the genome and associated mutation risks.
Another approach is making post-transcriptional modifications through RNA interference. For example, RNA structures can be used to inhibit the translation of disease-causing proteins. This technique is currently being tested against HIV and tumors such as melanoma.
In addition, mRNA technologies can be combined with CRISPR/Cas9 technology (“gene scissors”) to influence the creation of gene products even more precisely. The advantage of this technique is that mRNA is only transiently expressed, thus preventing unwanted side effects. Furthermore, mRNA is translated directly in the cytoplasm, leading to a faster initiation of gene editing.
Of the numerous ongoing clinical mRNA vaccine studies, around 70% focus on infections, about 12% on cancer, and the rest on autoimmune diseases and neurodegenerative disorders, said Dr. Prelog.
Research in Infections
Research in the fields of infectious diseases and oncology is the most advanced: mRNA vaccines against influenza and RSV are already in advanced clinical trials, Dr. Prelog told this news organization.
“Conventional influenza vaccines contain immunogenic surface molecules against hemagglutinin and neuraminidase in various combinations of influenza strains A and B and are produced in egg or cell cultures,” she said. “This is a time-consuming manufacturing process that takes months and, particularly with the egg-based process, bears the risk of changing the vaccine strain.”
“Additionally, influenza viruses undergo antigenic shift and drift through recombination, thus requiring annual adjustments to the vaccines. Thus, these influenza vaccines often lose accuracy in targeting circulating seasonal influenza strains.”
Several mRNA vaccines being tested contain not only coding sequences against hemagglutinin and neuraminidase but also for structural proteins of influenza viruses. “These are more conserved and mutate less easily, meaning they could serve as the basis for universal pandemic influenza vaccines,” said Dr. Prelog.
An advantage of mRNA vaccines, she added, is the strong cellular immune response that they elicit. This response is intended to provide additional protection alongside specific antibodies. An mRNA vaccine with coding sequences for the pre-fusion protein of RSV is in phase 3 trials for approval for vaccination in patients aged 60 years and older. It shows high effectiveness even in older patients and those with comorbidities.
Elaborate Purification Process
Bacterial origin plasmid DNA is used to produce mRNA vaccines. The mRNA vaccines for COVID-19 raised concerns that production-related DNA residues could pose a safety risk and cause autoimmune diseases.
These vaccines “typically undergo a very elaborate purification process,” said Dr. Prelog. “This involves enzymatic digestion with DNase to fragment and deplete plasmid DNA, followed by purification using chromatography columns, so that no safety-relevant DNA fragments should remain afterward.”
Thus, the Paul-Ehrlich-Institut also pointed out the very small, fragmented plasmid DNA residues of bacterial origin in mRNA COVID-19 vaccines pose no risk, unlike residual DNA from animal cell culture might pose in other vaccines.
Prevention and Therapy
In addition to the numerous advantages of mRNA vaccines (such as rapid adaptability to new or mutated pathogens, scalability, rapid production capability, self-adjuvant effect, strong induction of cellular immune responses, and safety), there are also challenges in RNA technology as a preventive and therapeutic measure, according to Dr. Prelog.
“Stability and storability, as well as the costs of new vaccine developments, play a role, as do the long-term effects regarding the persistence of antibody and cellular responses,” she said. The COVID-19 mRNA vaccines, for example, showed a well-maintained cellular immune response despite a tendency toward a rapid decline in humoral immune response.
“The experience with COVID-19 mRNA vaccines and the new vaccine developments based on mRNA technology give hope for an efficient and safe preventive and therapeutic use, particularly in the fields of infectious diseases and oncology,” Dr. Prelog concluded.
This story was translated from the Medscape German edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Martina Prelog, MD, a pediatric and adolescent medicine specialist at the University Hospital of Würzburg in Germany, reported on the principles, research status, and perspectives for these vaccines at the 25th Travel and Health Forum of the Center for Travel Medicine in Berlin.
To understand the future, the immunologist first examined the past. “The induction of cellular and humoral immune responses by externally injected mRNA was discovered in the 1990s,” she said.
Instability Challenge
Significant hurdles in mRNA vaccinations included the instability of mRNA and the immune system’s ability to identify foreign mRNA as a threat and destroy mRNA fragments. “The breakthrough toward vaccination came through Dr. Katalin Karikó, who, along with Dr. Drew Weissman, both of the University of Pennsylvania School of Medicine, discovered in 2005 that modifications of mRNA (replacing the nucleoside uridine with pseudouridine) enable better stability of mRNA, reduced immunogenicity, and higher translational capacity at the ribosomes,” said Dr. Prelog.
With this discovery, the two researchers paved the way for the development of mRNA vaccines against COVID-19 and other diseases. They were awarded the Nobel Prize in medicine for their discovery last year.
Improved Scalability
“Since 2009, mRNA vaccines have been studied as a treatment option for cancer,” said Dr. Prelog. “Since 2012, they have been studied for the influenza virus and respiratory syncytial virus [RSV].” Consequently, several mRNA vaccines are currently in development or in approval studies. “The mRNA technology offers the advantage of quickly and flexibly responding to new variants of pathogens and the ability to scale up production when there is high demand for a particular vaccine.”
Different forms and designations of mRNA vaccines are used, depending on the application and desired effect, said Dr. Prelog.
In nucleoside-modified mRNA vaccines, modifications in the mRNA sequence enable the mRNA to remain in the body longer and to induce protein synthesis more effectively.
Lipid nanoparticle (LNP)–encapsulated mRNA vaccines protect the coding mRNA sequences against degradation by the body’s enzymes and facilitate the uptake of mRNA into cells, where it then triggers the production of the desired protein. In addition, LNPs are involved in cell stimulation and support the self-adjuvant effect of mRNA vaccines, thus eliminating the need for adjuvants.
Self-amplifying mRNA vaccines include a special mRNA that replicates itself in the cell and contains a sequence for RNA replicase, in addition to the coding sequence for the protein. This composition enables increased production of the target protein without the need for a high amount of external mRNA administration. Such vaccines could trigger a longer and stronger immune response because the immune system has more time to interact with the protein.
Cancer Immunotherapy
Dr. Prelog also discussed personalized vaccines for cancer immunotherapy. Personalized mRNA vaccines are tailored to the patient’s genetic characteristics and antigens. They could be used in cancer immunotherapy to activate the immune system selectively against tumor cells.
Multivalent mRNA vaccines contain mRNA that codes for multiple antigens rather than just one protein to generate an immune response. These vaccines could be particularly useful in fighting pathogens with variable or changing surface structures or in eliciting protection against multiple pathogens simultaneously.
The technology of mRNA-encoded antibodies involves introducing mRNA into the cell, which creates light and heavy chains of antibodies. This step leads to the formation of antibodies targeted against toxins (eg, diphtheria and tetanus), animal venoms, infectious agents, or tumor cells.
Genetic Engineering
Dr. Prelog also reviewed genetic engineering techniques. In regenerative therapy or protein replacement therapy, skin fibroblasts or other cells are transfected with mRNA to enable conversion into induced pluripotent stem cells. This approach avoids the risk for DNA integration into the genome and associated mutation risks.
Another approach is making post-transcriptional modifications through RNA interference. For example, RNA structures can be used to inhibit the translation of disease-causing proteins. This technique is currently being tested against HIV and tumors such as melanoma.
In addition, mRNA technologies can be combined with CRISPR/Cas9 technology (“gene scissors”) to influence the creation of gene products even more precisely. The advantage of this technique is that mRNA is only transiently expressed, thus preventing unwanted side effects. Furthermore, mRNA is translated directly in the cytoplasm, leading to a faster initiation of gene editing.
Of the numerous ongoing clinical mRNA vaccine studies, around 70% focus on infections, about 12% on cancer, and the rest on autoimmune diseases and neurodegenerative disorders, said Dr. Prelog.
Research in Infections
Research in the fields of infectious diseases and oncology is the most advanced: mRNA vaccines against influenza and RSV are already in advanced clinical trials, Dr. Prelog told this news organization.
“Conventional influenza vaccines contain immunogenic surface molecules against hemagglutinin and neuraminidase in various combinations of influenza strains A and B and are produced in egg or cell cultures,” she said. “This is a time-consuming manufacturing process that takes months and, particularly with the egg-based process, bears the risk of changing the vaccine strain.”
“Additionally, influenza viruses undergo antigenic shift and drift through recombination, thus requiring annual adjustments to the vaccines. Thus, these influenza vaccines often lose accuracy in targeting circulating seasonal influenza strains.”
Several mRNA vaccines being tested contain not only coding sequences against hemagglutinin and neuraminidase but also for structural proteins of influenza viruses. “These are more conserved and mutate less easily, meaning they could serve as the basis for universal pandemic influenza vaccines,” said Dr. Prelog.
An advantage of mRNA vaccines, she added, is the strong cellular immune response that they elicit. This response is intended to provide additional protection alongside specific antibodies. An mRNA vaccine with coding sequences for the pre-fusion protein of RSV is in phase 3 trials for approval for vaccination in patients aged 60 years and older. It shows high effectiveness even in older patients and those with comorbidities.
Elaborate Purification Process
Bacterial origin plasmid DNA is used to produce mRNA vaccines. The mRNA vaccines for COVID-19 raised concerns that production-related DNA residues could pose a safety risk and cause autoimmune diseases.
These vaccines “typically undergo a very elaborate purification process,” said Dr. Prelog. “This involves enzymatic digestion with DNase to fragment and deplete plasmid DNA, followed by purification using chromatography columns, so that no safety-relevant DNA fragments should remain afterward.”
Thus, the Paul-Ehrlich-Institut also pointed out the very small, fragmented plasmid DNA residues of bacterial origin in mRNA COVID-19 vaccines pose no risk, unlike residual DNA from animal cell culture might pose in other vaccines.
Prevention and Therapy
In addition to the numerous advantages of mRNA vaccines (such as rapid adaptability to new or mutated pathogens, scalability, rapid production capability, self-adjuvant effect, strong induction of cellular immune responses, and safety), there are also challenges in RNA technology as a preventive and therapeutic measure, according to Dr. Prelog.
“Stability and storability, as well as the costs of new vaccine developments, play a role, as do the long-term effects regarding the persistence of antibody and cellular responses,” she said. The COVID-19 mRNA vaccines, for example, showed a well-maintained cellular immune response despite a tendency toward a rapid decline in humoral immune response.
“The experience with COVID-19 mRNA vaccines and the new vaccine developments based on mRNA technology give hope for an efficient and safe preventive and therapeutic use, particularly in the fields of infectious diseases and oncology,” Dr. Prelog concluded.
This story was translated from the Medscape German edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
COVID Vaccines and New-Onset Seizures: New Data
There is no association between the SARS-CoV-2 vaccine and the risk for new-onset seizure, data from a new meta-analysis of six randomized, placebo-controlled clinical trials (RCTs) showed.
Results of the pooled analysis that included 63,500 individuals vaccinated with SARS-CoV-2 and 55,000 who received a placebo vaccine showed there was no significant difference between the two groups with respect to new-onset seizures at 28- or 43-day follow-up.
Regarding new-onset seizures in the general population, there was no statistically significant difference in risk for seizure incidence among vaccinated individuals vs placebo recipients, according to our meta-analysis, wrote the investigators, led by Ali Rafati, MD, MPH, Iran University of Medical Sciences in Tehran.
The findings were published online in JAMA Neurology.
Mixed Results
Results from previous research have been mixed regarding the link between the SARS-CoV-2 vaccination and new-onset seizures, with some showing an association.
To learn more about the possible association between the vaccines and new-onset seizures, the researchers conducted a literature review and identified six RCTs that measured adverse events following SARS-CoV-2 vaccinations (including messenger RNA, viral vector, and inactivated virus) vs placebo or other vaccines.
While five of the studies defined new-onset seizures according to the Medical Dictionary for Regulatory Activities, trial investigators in the sixth RCT assessed and determined new-onset seizures in participants.
Participants received two vaccinations 28 days apart in five RCTs and only one vaccine in the sixth trial.
The research team searched the data for new-onset seizure in the 28 days following one or both COVID vaccinations.
No Link Found
After comparing the incidence of new-onset seizure between the 63,500 vaccine (nine new-onset seizures, 0.014%) and 55,000 placebo recipients (one new-onset seizure, 0.002%), investigators found no significant difference between the two groups (odds ratio [OR], 2.70; 95% CI, 0.76-9.57; P = .12)
Investigators also sliced the data several ways to see if it would yield different results. When they analyzed data by vaccine platform (viral vector) and age group (children), they didn’t observe significant differences in new-onset data.
The researchers also searched for data beyond the month following the injection to encompass the entire blinded phase, so they analyzed the results of three RCTs that reported adverse events up to 162 days after the vaccine.
After pooling the results from the three studies, investigators found no statistical difference between the vaccine and placebo groups in terms of the new-onset seizure (OR, 2.31; 95% CI, 0.86%-3.23; P > .99)
Study limitations included the missing information on vaccine doses or risk factors for the development of seizures. Also, the RCTs included in the meta-analysis were conducted at different times, so the SARS-CoV-2 vaccines may have differed in their composition and efficacy.
“The global vaccination drive against SARS-CoV-2 has been a monumental effort in combating the pandemic. SARS-CoV-2 vaccinations that are now available appear safe and appropriate,” the authors wrote.
There were no study funding sources or disclosures reported.
A version of this article appeared on Medscape.com.
There is no association between the SARS-CoV-2 vaccine and the risk for new-onset seizure, data from a new meta-analysis of six randomized, placebo-controlled clinical trials (RCTs) showed.
Results of the pooled analysis that included 63,500 individuals vaccinated with SARS-CoV-2 and 55,000 who received a placebo vaccine showed there was no significant difference between the two groups with respect to new-onset seizures at 28- or 43-day follow-up.
Regarding new-onset seizures in the general population, there was no statistically significant difference in risk for seizure incidence among vaccinated individuals vs placebo recipients, according to our meta-analysis, wrote the investigators, led by Ali Rafati, MD, MPH, Iran University of Medical Sciences in Tehran.
The findings were published online in JAMA Neurology.
Mixed Results
Results from previous research have been mixed regarding the link between the SARS-CoV-2 vaccination and new-onset seizures, with some showing an association.
To learn more about the possible association between the vaccines and new-onset seizures, the researchers conducted a literature review and identified six RCTs that measured adverse events following SARS-CoV-2 vaccinations (including messenger RNA, viral vector, and inactivated virus) vs placebo or other vaccines.
While five of the studies defined new-onset seizures according to the Medical Dictionary for Regulatory Activities, trial investigators in the sixth RCT assessed and determined new-onset seizures in participants.
Participants received two vaccinations 28 days apart in five RCTs and only one vaccine in the sixth trial.
The research team searched the data for new-onset seizure in the 28 days following one or both COVID vaccinations.
No Link Found
After comparing the incidence of new-onset seizure between the 63,500 vaccine (nine new-onset seizures, 0.014%) and 55,000 placebo recipients (one new-onset seizure, 0.002%), investigators found no significant difference between the two groups (odds ratio [OR], 2.70; 95% CI, 0.76-9.57; P = .12)
Investigators also sliced the data several ways to see if it would yield different results. When they analyzed data by vaccine platform (viral vector) and age group (children), they didn’t observe significant differences in new-onset data.
The researchers also searched for data beyond the month following the injection to encompass the entire blinded phase, so they analyzed the results of three RCTs that reported adverse events up to 162 days after the vaccine.
After pooling the results from the three studies, investigators found no statistical difference between the vaccine and placebo groups in terms of the new-onset seizure (OR, 2.31; 95% CI, 0.86%-3.23; P > .99)
Study limitations included the missing information on vaccine doses or risk factors for the development of seizures. Also, the RCTs included in the meta-analysis were conducted at different times, so the SARS-CoV-2 vaccines may have differed in their composition and efficacy.
“The global vaccination drive against SARS-CoV-2 has been a monumental effort in combating the pandemic. SARS-CoV-2 vaccinations that are now available appear safe and appropriate,” the authors wrote.
There were no study funding sources or disclosures reported.
A version of this article appeared on Medscape.com.
There is no association between the SARS-CoV-2 vaccine and the risk for new-onset seizure, data from a new meta-analysis of six randomized, placebo-controlled clinical trials (RCTs) showed.
Results of the pooled analysis that included 63,500 individuals vaccinated with SARS-CoV-2 and 55,000 who received a placebo vaccine showed there was no significant difference between the two groups with respect to new-onset seizures at 28- or 43-day follow-up.
Regarding new-onset seizures in the general population, there was no statistically significant difference in risk for seizure incidence among vaccinated individuals vs placebo recipients, according to our meta-analysis, wrote the investigators, led by Ali Rafati, MD, MPH, Iran University of Medical Sciences in Tehran.
The findings were published online in JAMA Neurology.
Mixed Results
Results from previous research have been mixed regarding the link between the SARS-CoV-2 vaccination and new-onset seizures, with some showing an association.
To learn more about the possible association between the vaccines and new-onset seizures, the researchers conducted a literature review and identified six RCTs that measured adverse events following SARS-CoV-2 vaccinations (including messenger RNA, viral vector, and inactivated virus) vs placebo or other vaccines.
While five of the studies defined new-onset seizures according to the Medical Dictionary for Regulatory Activities, trial investigators in the sixth RCT assessed and determined new-onset seizures in participants.
Participants received two vaccinations 28 days apart in five RCTs and only one vaccine in the sixth trial.
The research team searched the data for new-onset seizure in the 28 days following one or both COVID vaccinations.
No Link Found
After comparing the incidence of new-onset seizure between the 63,500 vaccine (nine new-onset seizures, 0.014%) and 55,000 placebo recipients (one new-onset seizure, 0.002%), investigators found no significant difference between the two groups (odds ratio [OR], 2.70; 95% CI, 0.76-9.57; P = .12)
Investigators also sliced the data several ways to see if it would yield different results. When they analyzed data by vaccine platform (viral vector) and age group (children), they didn’t observe significant differences in new-onset data.
The researchers also searched for data beyond the month following the injection to encompass the entire blinded phase, so they analyzed the results of three RCTs that reported adverse events up to 162 days after the vaccine.
After pooling the results from the three studies, investigators found no statistical difference between the vaccine and placebo groups in terms of the new-onset seizure (OR, 2.31; 95% CI, 0.86%-3.23; P > .99)
Study limitations included the missing information on vaccine doses or risk factors for the development of seizures. Also, the RCTs included in the meta-analysis were conducted at different times, so the SARS-CoV-2 vaccines may have differed in their composition and efficacy.
“The global vaccination drive against SARS-CoV-2 has been a monumental effort in combating the pandemic. SARS-CoV-2 vaccinations that are now available appear safe and appropriate,” the authors wrote.
There were no study funding sources or disclosures reported.
A version of this article appeared on Medscape.com.
Risk for COVID-19 Infection in Patients With Vitiligo
To the Editor:
Vitiligo is a depigmentation disorder that results from the loss of melanocytes in the epidermis.1 The most widely accepted pathophysiology for melanocyte destruction in vitiligo is an autoimmune process involving dysregulated cytokine production and autoreactive T-cell activation.1 Individuals with cutaneous autoinflammatory conditions currently are vital patient populations warranting research, as their susceptibility to COVID-19 infection may differ from the general population. We previously found a small increased risk for COVID-19 infection in patients with psoriasis,2 which suggests that other dermatologic conditions also may impact COVID-19 risk. The risk for COVID-19 infection in patients with vitiligo remains largely unknown. In this retrospective cohort study, we investigated the risk for COVID-19 infection in patients with vitiligo compared with those without vitiligo utilizing claims data from the COVID-19 Research Database (https://covid19researchdatabase.org/).
Claims were evaluated for patients aged 3 years and older with a vitiligo diagnosis (International Classification of Diseases, Tenth Revision [ICD-10] code L80) that was made between January 1, 2016, and January 1, 2020. Individuals without a vitiligo diagnosis during the same period were placed (4:1 ratio) in the control group and were matched with study group patients for age and sex. All comorbidity variables and vitiligo diagnoses were extracted from ICD-10 codes that were given prior to a diagnosis of COVID-19. We then constructed multivariable logistic regression models adjusting for measured confounders to evaluate if vitiligo was associated with higher risk for COVID-19 infection after January 1, 2020.
The vitiligo and nonvitiligo cohorts included 40,363 and 161,452 patients, respectively (Table 1). Logistic regression analysis with adjustment for confounding variables, including high comorbid risk factors (Table 2) revealed that patients with a diagnosis of vitiligo had significantly increased odds of COVID-19 infection compared with patients without vitiligo (adjusted odds ratio [AOR], 1.47; 95% CI, 1.37-1.57; P<.001)(Table 3). Additionally, subgroup logistic analyses for sex, age, and exclusion of patients who were HIV positive revealed that females with vitiligo had higher odds of contracting COVID-19 than males with vitiligo (Table 3).
Our results showed that patients with vitiligo had a higher relative risk for contracting COVID-19 than individuals without vitiligo. It has been reported that the prevalence of COVID-19 is higher among patients with autoimmune diseases compared to the general population.3 Additionally, a handful of vitiligo patients are managed with immunosuppressive agents that may further weaken their immune response.1 Moreover, survey results from dermatologists managing vitiligo patients revealed that physicians were fairly comfortable prescribing immunosuppressants and encouraging in-office phototherapy during the COVID-19 pandemic.4 As a result, more patients may have been attending in-office visits for their phototherapy, which may have increased their risk for COVID-19. Although these factors play a role in COVID-19 infection rates, the underlying immune dysregulation in vitiligo in relation to COVID-19 remains unknown and should be further explored.
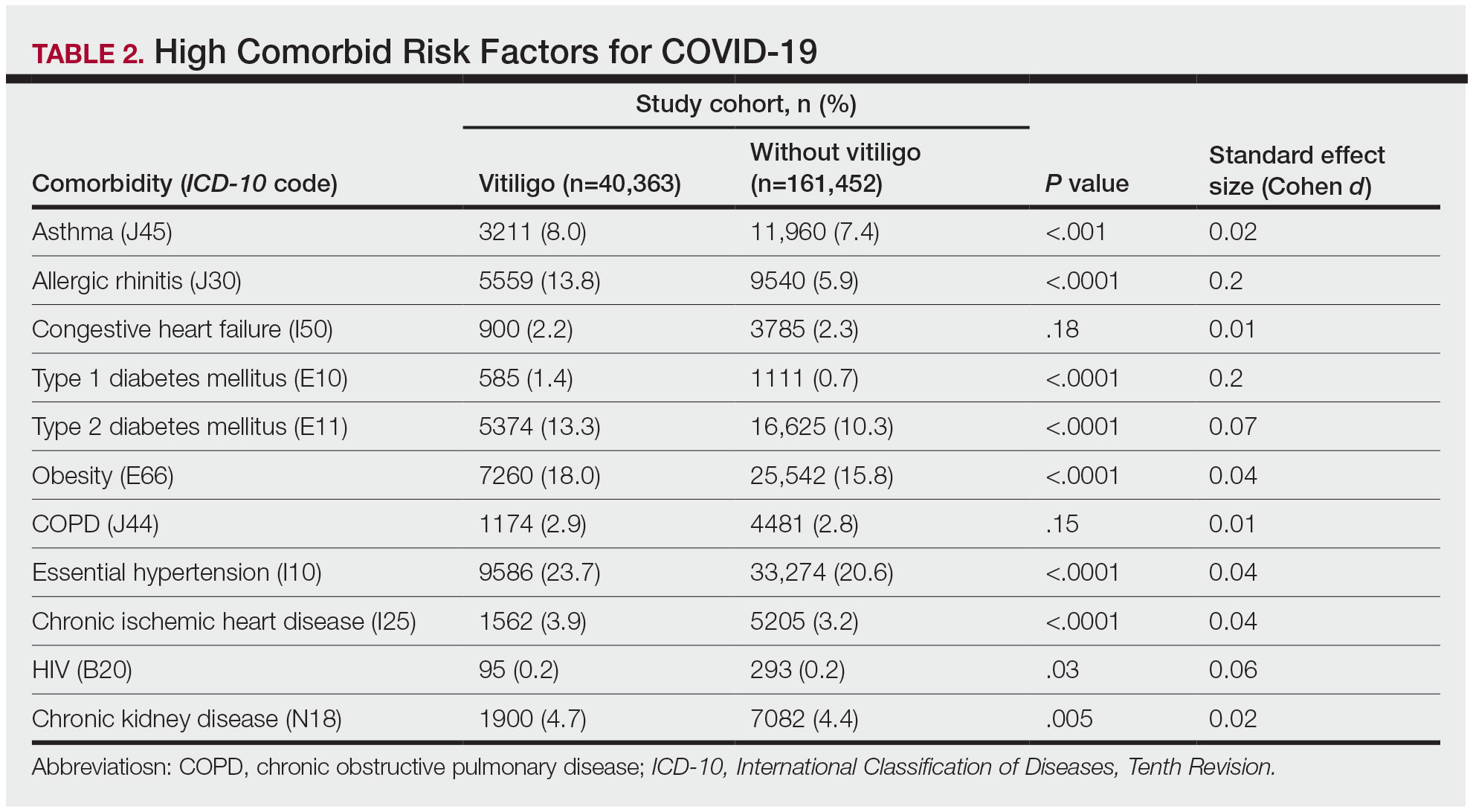
Our findings are limited by the use of ICD-10 codes, the inability to control for all potential confounding variables, the lack of data regarding the stage of vitiligo, and the absence of data for undiagnosed COVID-19 infections. In addition, patients with vitiligo may be more likely to seek care, potentially increasing their rates of COVID-19 testing. The inability to identify the stage of vitiligo during enrollment in the database may have altered our results, as individuals with active disease have increased levels of IFN-γ. Increased secretion of IFN-γ also potentially helps in the clearance of COVID-19 infection.1 Future studies should investigate this relationship via planned COVID-19 testing, identification of vitiligo stage, and controlling for other associated comorbidities.
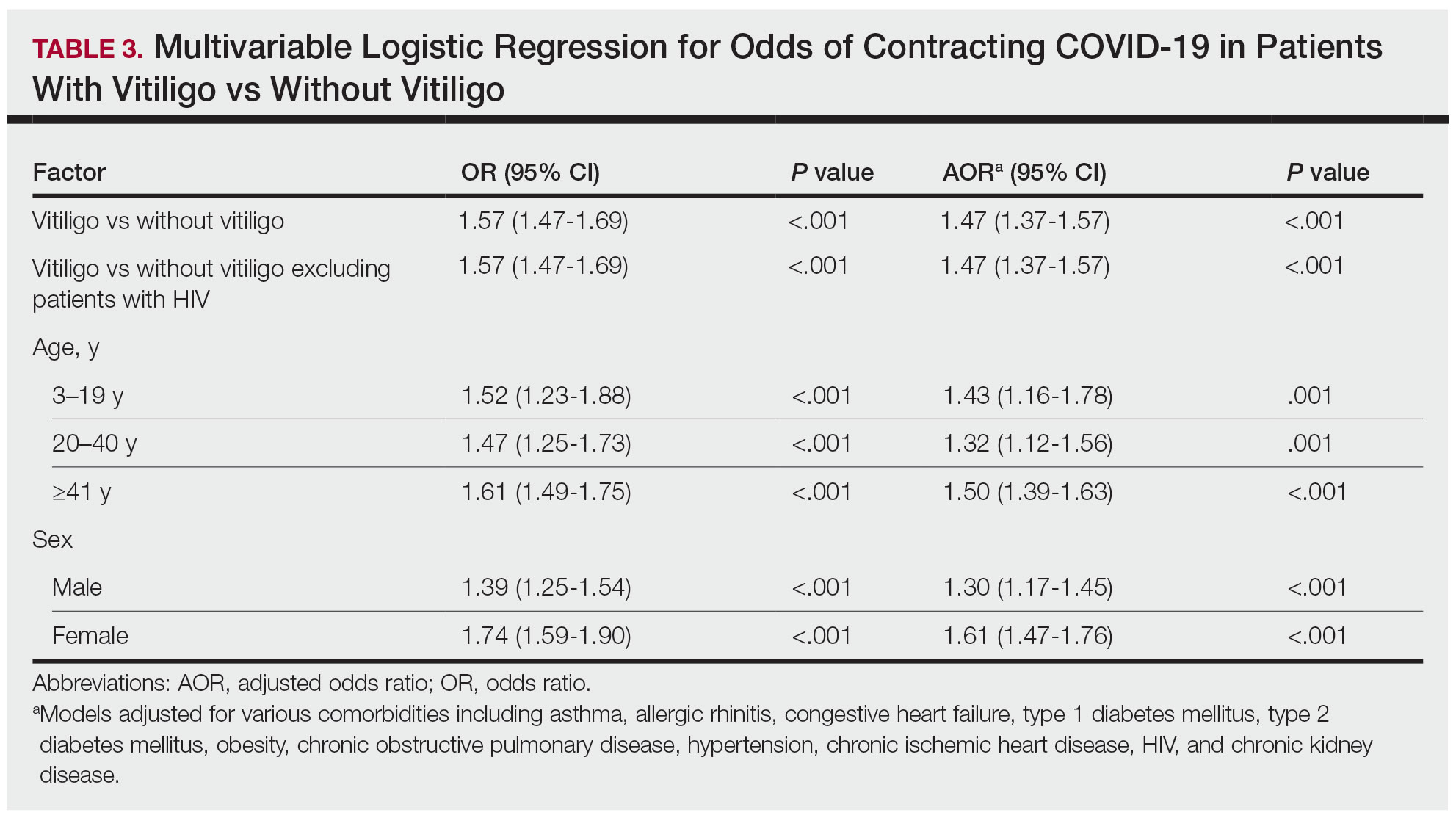
- Rashighi M, Harris JE. Vitiligo pathogenesis and emerging treatments. Dermatol Clin. 2017;35:257-265. doi:10.1016/j.det.2016.11.014
- Wu JJ, Liu J, Thatiparthi A, et al. The risk of COVID-19 in patients with psoriasis—a retrospective cohort study [published online September 20, 2022]. J Am Acad Dermatol. doi:10.1016/j.jaad.2022.07.040
- Zhong J, Shen G, Yang H, et al. COVID-19 in patients with rheumatic disease in Hubei province, China: a multicentre retrospective observational study. Lancet Rheumatol. 2020;2:E557-E564. doi:10.1016/S2665-9913(20)30227-7
- Chatterjee M, Das A. Management of vitiligo amidst the COVID-19 pandemic: a survey and resulting consensus. Indian J Dermatol. 2021;66:479-483. doi:10.4103/ijd.ijd_859_20
To the Editor:
Vitiligo is a depigmentation disorder that results from the loss of melanocytes in the epidermis.1 The most widely accepted pathophysiology for melanocyte destruction in vitiligo is an autoimmune process involving dysregulated cytokine production and autoreactive T-cell activation.1 Individuals with cutaneous autoinflammatory conditions currently are vital patient populations warranting research, as their susceptibility to COVID-19 infection may differ from the general population. We previously found a small increased risk for COVID-19 infection in patients with psoriasis,2 which suggests that other dermatologic conditions also may impact COVID-19 risk. The risk for COVID-19 infection in patients with vitiligo remains largely unknown. In this retrospective cohort study, we investigated the risk for COVID-19 infection in patients with vitiligo compared with those without vitiligo utilizing claims data from the COVID-19 Research Database (https://covid19researchdatabase.org/).
Claims were evaluated for patients aged 3 years and older with a vitiligo diagnosis (International Classification of Diseases, Tenth Revision [ICD-10] code L80) that was made between January 1, 2016, and January 1, 2020. Individuals without a vitiligo diagnosis during the same period were placed (4:1 ratio) in the control group and were matched with study group patients for age and sex. All comorbidity variables and vitiligo diagnoses were extracted from ICD-10 codes that were given prior to a diagnosis of COVID-19. We then constructed multivariable logistic regression models adjusting for measured confounders to evaluate if vitiligo was associated with higher risk for COVID-19 infection after January 1, 2020.
The vitiligo and nonvitiligo cohorts included 40,363 and 161,452 patients, respectively (Table 1). Logistic regression analysis with adjustment for confounding variables, including high comorbid risk factors (Table 2) revealed that patients with a diagnosis of vitiligo had significantly increased odds of COVID-19 infection compared with patients without vitiligo (adjusted odds ratio [AOR], 1.47; 95% CI, 1.37-1.57; P<.001)(Table 3). Additionally, subgroup logistic analyses for sex, age, and exclusion of patients who were HIV positive revealed that females with vitiligo had higher odds of contracting COVID-19 than males with vitiligo (Table 3).

Our results showed that patients with vitiligo had a higher relative risk for contracting COVID-19 than individuals without vitiligo. It has been reported that the prevalence of COVID-19 is higher among patients with autoimmune diseases compared to the general population.3 Additionally, a handful of vitiligo patients are managed with immunosuppressive agents that may further weaken their immune response.1 Moreover, survey results from dermatologists managing vitiligo patients revealed that physicians were fairly comfortable prescribing immunosuppressants and encouraging in-office phototherapy during the COVID-19 pandemic.4 As a result, more patients may have been attending in-office visits for their phototherapy, which may have increased their risk for COVID-19. Although these factors play a role in COVID-19 infection rates, the underlying immune dysregulation in vitiligo in relation to COVID-19 remains unknown and should be further explored.

Our findings are limited by the use of ICD-10 codes, the inability to control for all potential confounding variables, the lack of data regarding the stage of vitiligo, and the absence of data for undiagnosed COVID-19 infections. In addition, patients with vitiligo may be more likely to seek care, potentially increasing their rates of COVID-19 testing. The inability to identify the stage of vitiligo during enrollment in the database may have altered our results, as individuals with active disease have increased levels of IFN-γ. Increased secretion of IFN-γ also potentially helps in the clearance of COVID-19 infection.1 Future studies should investigate this relationship via planned COVID-19 testing, identification of vitiligo stage, and controlling for other associated comorbidities.

To the Editor:
Vitiligo is a depigmentation disorder that results from the loss of melanocytes in the epidermis.1 The most widely accepted pathophysiology for melanocyte destruction in vitiligo is an autoimmune process involving dysregulated cytokine production and autoreactive T-cell activation.1 Individuals with cutaneous autoinflammatory conditions currently are vital patient populations warranting research, as their susceptibility to COVID-19 infection may differ from the general population. We previously found a small increased risk for COVID-19 infection in patients with psoriasis,2 which suggests that other dermatologic conditions also may impact COVID-19 risk. The risk for COVID-19 infection in patients with vitiligo remains largely unknown. In this retrospective cohort study, we investigated the risk for COVID-19 infection in patients with vitiligo compared with those without vitiligo utilizing claims data from the COVID-19 Research Database (https://covid19researchdatabase.org/).
Claims were evaluated for patients aged 3 years and older with a vitiligo diagnosis (International Classification of Diseases, Tenth Revision [ICD-10] code L80) that was made between January 1, 2016, and January 1, 2020. Individuals without a vitiligo diagnosis during the same period were placed (4:1 ratio) in the control group and were matched with study group patients for age and sex. All comorbidity variables and vitiligo diagnoses were extracted from ICD-10 codes that were given prior to a diagnosis of COVID-19. We then constructed multivariable logistic regression models adjusting for measured confounders to evaluate if vitiligo was associated with higher risk for COVID-19 infection after January 1, 2020.
The vitiligo and nonvitiligo cohorts included 40,363 and 161,452 patients, respectively (Table 1). Logistic regression analysis with adjustment for confounding variables, including high comorbid risk factors (Table 2) revealed that patients with a diagnosis of vitiligo had significantly increased odds of COVID-19 infection compared with patients without vitiligo (adjusted odds ratio [AOR], 1.47; 95% CI, 1.37-1.57; P<.001)(Table 3). Additionally, subgroup logistic analyses for sex, age, and exclusion of patients who were HIV positive revealed that females with vitiligo had higher odds of contracting COVID-19 than males with vitiligo (Table 3).

Our results showed that patients with vitiligo had a higher relative risk for contracting COVID-19 than individuals without vitiligo. It has been reported that the prevalence of COVID-19 is higher among patients with autoimmune diseases compared to the general population.3 Additionally, a handful of vitiligo patients are managed with immunosuppressive agents that may further weaken their immune response.1 Moreover, survey results from dermatologists managing vitiligo patients revealed that physicians were fairly comfortable prescribing immunosuppressants and encouraging in-office phototherapy during the COVID-19 pandemic.4 As a result, more patients may have been attending in-office visits for their phototherapy, which may have increased their risk for COVID-19. Although these factors play a role in COVID-19 infection rates, the underlying immune dysregulation in vitiligo in relation to COVID-19 remains unknown and should be further explored.

Our findings are limited by the use of ICD-10 codes, the inability to control for all potential confounding variables, the lack of data regarding the stage of vitiligo, and the absence of data for undiagnosed COVID-19 infections. In addition, patients with vitiligo may be more likely to seek care, potentially increasing their rates of COVID-19 testing. The inability to identify the stage of vitiligo during enrollment in the database may have altered our results, as individuals with active disease have increased levels of IFN-γ. Increased secretion of IFN-γ also potentially helps in the clearance of COVID-19 infection.1 Future studies should investigate this relationship via planned COVID-19 testing, identification of vitiligo stage, and controlling for other associated comorbidities.

- Rashighi M, Harris JE. Vitiligo pathogenesis and emerging treatments. Dermatol Clin. 2017;35:257-265. doi:10.1016/j.det.2016.11.014
- Wu JJ, Liu J, Thatiparthi A, et al. The risk of COVID-19 in patients with psoriasis—a retrospective cohort study [published online September 20, 2022]. J Am Acad Dermatol. doi:10.1016/j.jaad.2022.07.040
- Zhong J, Shen G, Yang H, et al. COVID-19 in patients with rheumatic disease in Hubei province, China: a multicentre retrospective observational study. Lancet Rheumatol. 2020;2:E557-E564. doi:10.1016/S2665-9913(20)30227-7
- Chatterjee M, Das A. Management of vitiligo amidst the COVID-19 pandemic: a survey and resulting consensus. Indian J Dermatol. 2021;66:479-483. doi:10.4103/ijd.ijd_859_20
- Rashighi M, Harris JE. Vitiligo pathogenesis and emerging treatments. Dermatol Clin. 2017;35:257-265. doi:10.1016/j.det.2016.11.014
- Wu JJ, Liu J, Thatiparthi A, et al. The risk of COVID-19 in patients with psoriasis—a retrospective cohort study [published online September 20, 2022]. J Am Acad Dermatol. doi:10.1016/j.jaad.2022.07.040
- Zhong J, Shen G, Yang H, et al. COVID-19 in patients with rheumatic disease in Hubei province, China: a multicentre retrospective observational study. Lancet Rheumatol. 2020;2:E557-E564. doi:10.1016/S2665-9913(20)30227-7
- Chatterjee M, Das A. Management of vitiligo amidst the COVID-19 pandemic: a survey and resulting consensus. Indian J Dermatol. 2021;66:479-483. doi:10.4103/ijd.ijd_859_20
Practice Points
- The underlying autoimmune process in vitiligo can result in various changes to the immune system.
- A diagnosis of vitiligo may alter the body’s immune response to COVID-19 infection.
Federal Trade Commission Bans Noncompete Agreements, Urges More Protections for Healthcare Workers
But business groups have vowed to challenge the decision in court.
The proposed final rule passed on a 3-2 vote, with the dissenting commissioners disputing the FTC’s authority to broadly ban noncompetes.
Tensions around noncompetes have been building for years. In 2021, President Biden issued an executive order supporting measures to improve economic competition, in which he urged the FTC to consider its rulemaking authority to address noncompete clauses that unfairly limit workers’ mobility. In January 2023, per that directive, the agency proposed ending the restrictive covenants.
While the FTC estimates that the final rule will reduce healthcare costs by up to $194 billion over the next decade and increase worker earnings by $300 million annually, the ruling faces legal hurdles.
US Chamber of Commerce president and CEO Suzanne P. Clark said in a statement that the move is a “blatant power grab” that will undermine competitive business practices, adding that the Chamber will sue to block the measure.
The FTC received more than 26,000 comments on noncompetes during the public feedback period, with about 25,000 supporting the measure, said Benjamin Cady, JD, an FTC attorney.
Mr. Cady called the feedback “compelling,” citing instances of workers who were forced to commute long distances, uproot their families, or risk expensive litigation for wanting to pursue job opportunities.
For example, a comment from a physician working in Appalachia highlights the potential real-life implications of the agreements. “With hospital systems merging, providers with aggressive noncompetes must abandon the community that they serve if they [choose] to leave their employer. Healthcare providers feel trapped in their current employment situation, leading to significant burnout that can shorten their [career] longevity.”
Commissioner Alvaro Bedoya said physicians have had their lives upended by cumbersome noncompetes, often having to move out of state to practice. “A pandemic killed a million people in this country, and there are doctors who cannot work because of a noncompete,” he said.
It’s unclear whether physicians and others who work for nonprofit healthcare groups or hospitals will be covered by the new ban. FTC Commissioner Rebecca Slaughter acknowledged that the agency’s jurisdictional limitations mean that employees of “certain nonprofit organizations” may not benefit from the rule.
“We want to be transparent about the limitation and recognize there are workers, especially healthcare workers, who are bound by anticompetitive and unfair noncompete clauses, that our rule will struggle to reach,” she said. To cover nonprofit healthcare employees, Ms. Slaughter urged Congress to pass legislation banning noncompetes, such as the Workforce Mobility Act of 2021 and the Freedom to Compete Act of 2023.
The FTC final rule will take effect 120 days after it is published in the federal register, and new noncompete agreements will be banned as of this date. However, existing contracts for senior executives will remain in effect because these individuals are less likely to experience “acute harm” due to their ability to negotiate accordingly, said Mr. Cady.
States, AMA Take Aim at Noncompetes
Before the federal ban, several states had already passed legislation limiting the reach of noncompetes. According to a recent article in the Journal of the American College of Cardiology, 12 states prohibit noncompete clauses for physicians: Alabama, California, Colorado, Delaware, Massachusetts, Montana, New Hampshire, New Mexico, North Dakota, Oklahoma, Rhode Island, and South Dakota.
The remaining states allow noncompetes in some form, often excluding them for employees earning below a certain threshold. For example, in Oregon, noncompete agreements may apply to employees earning more than $113,241. Most states have provisions to adjust the threshold annually. The District of Columbia permits 2-year noncompetes for “medical specialists” earning over $250,000 annually.
Indiana employers can no longer enter into noncompete agreements with primary care providers. Other specialties may be subject to the clauses, except when the physician terminates the contract for cause or when an employer terminates the contract without cause.
Rachel Marcus, MD, a cardiologist in Washington, DC, found out how limiting her employment contract’s noncompete clause was when she wanted to leave a former position. Due to the restrictions, she told this news organization that she couldn’t work locally for a competitor for 2 years. The closest location she could seek employment without violating the agreement was Baltimore, approximately 40 miles away.
Dr. Marcus ultimately moved to another position within the same organization because of the company’s reputation for being “aggressive” in their enforcement actions.
Although the American Medical Association (AMA) does not support a total ban, its House of Delegates adopted policies last year to support the prohibition of noncompete contracts for physicians employed by for-profit or nonprofit hospitals, hospital systems, or staffing companies.
Challenges Await
The American Hospital Association, which opposed the proposed rule, called it “bad policy.” The decision “will likely be short-lived, with courts almost certain to stop it before it can do damage to hospitals’ ability to care for their patients and communities,” the association said in a statement.
To ease the transition to the new rule, the FTC also released a model language for employers to use when discussing the changes with their employees. “All employers need to do to comply with the rule is to stop enforcing existing noncompetes with workers other than senior executives and provide notice to such workers,” he said.
Dr. Marcus hopes the ban improves doctors’ lives. “Your employer is going to have to treat you better because they know that you can easily go across town to a place that has a higher salary, and your patient can go with you.”
A version of this article appeared on Medscape.com.
But business groups have vowed to challenge the decision in court.
The proposed final rule passed on a 3-2 vote, with the dissenting commissioners disputing the FTC’s authority to broadly ban noncompetes.
Tensions around noncompetes have been building for years. In 2021, President Biden issued an executive order supporting measures to improve economic competition, in which he urged the FTC to consider its rulemaking authority to address noncompete clauses that unfairly limit workers’ mobility. In January 2023, per that directive, the agency proposed ending the restrictive covenants.
While the FTC estimates that the final rule will reduce healthcare costs by up to $194 billion over the next decade and increase worker earnings by $300 million annually, the ruling faces legal hurdles.
US Chamber of Commerce president and CEO Suzanne P. Clark said in a statement that the move is a “blatant power grab” that will undermine competitive business practices, adding that the Chamber will sue to block the measure.
The FTC received more than 26,000 comments on noncompetes during the public feedback period, with about 25,000 supporting the measure, said Benjamin Cady, JD, an FTC attorney.
Mr. Cady called the feedback “compelling,” citing instances of workers who were forced to commute long distances, uproot their families, or risk expensive litigation for wanting to pursue job opportunities.
For example, a comment from a physician working in Appalachia highlights the potential real-life implications of the agreements. “With hospital systems merging, providers with aggressive noncompetes must abandon the community that they serve if they [choose] to leave their employer. Healthcare providers feel trapped in their current employment situation, leading to significant burnout that can shorten their [career] longevity.”
Commissioner Alvaro Bedoya said physicians have had their lives upended by cumbersome noncompetes, often having to move out of state to practice. “A pandemic killed a million people in this country, and there are doctors who cannot work because of a noncompete,” he said.
It’s unclear whether physicians and others who work for nonprofit healthcare groups or hospitals will be covered by the new ban. FTC Commissioner Rebecca Slaughter acknowledged that the agency’s jurisdictional limitations mean that employees of “certain nonprofit organizations” may not benefit from the rule.
“We want to be transparent about the limitation and recognize there are workers, especially healthcare workers, who are bound by anticompetitive and unfair noncompete clauses, that our rule will struggle to reach,” she said. To cover nonprofit healthcare employees, Ms. Slaughter urged Congress to pass legislation banning noncompetes, such as the Workforce Mobility Act of 2021 and the Freedom to Compete Act of 2023.
The FTC final rule will take effect 120 days after it is published in the federal register, and new noncompete agreements will be banned as of this date. However, existing contracts for senior executives will remain in effect because these individuals are less likely to experience “acute harm” due to their ability to negotiate accordingly, said Mr. Cady.
States, AMA Take Aim at Noncompetes
Before the federal ban, several states had already passed legislation limiting the reach of noncompetes. According to a recent article in the Journal of the American College of Cardiology, 12 states prohibit noncompete clauses for physicians: Alabama, California, Colorado, Delaware, Massachusetts, Montana, New Hampshire, New Mexico, North Dakota, Oklahoma, Rhode Island, and South Dakota.
The remaining states allow noncompetes in some form, often excluding them for employees earning below a certain threshold. For example, in Oregon, noncompete agreements may apply to employees earning more than $113,241. Most states have provisions to adjust the threshold annually. The District of Columbia permits 2-year noncompetes for “medical specialists” earning over $250,000 annually.
Indiana employers can no longer enter into noncompete agreements with primary care providers. Other specialties may be subject to the clauses, except when the physician terminates the contract for cause or when an employer terminates the contract without cause.
Rachel Marcus, MD, a cardiologist in Washington, DC, found out how limiting her employment contract’s noncompete clause was when she wanted to leave a former position. Due to the restrictions, she told this news organization that she couldn’t work locally for a competitor for 2 years. The closest location she could seek employment without violating the agreement was Baltimore, approximately 40 miles away.
Dr. Marcus ultimately moved to another position within the same organization because of the company’s reputation for being “aggressive” in their enforcement actions.
Although the American Medical Association (AMA) does not support a total ban, its House of Delegates adopted policies last year to support the prohibition of noncompete contracts for physicians employed by for-profit or nonprofit hospitals, hospital systems, or staffing companies.
Challenges Await
The American Hospital Association, which opposed the proposed rule, called it “bad policy.” The decision “will likely be short-lived, with courts almost certain to stop it before it can do damage to hospitals’ ability to care for their patients and communities,” the association said in a statement.
To ease the transition to the new rule, the FTC also released a model language for employers to use when discussing the changes with their employees. “All employers need to do to comply with the rule is to stop enforcing existing noncompetes with workers other than senior executives and provide notice to such workers,” he said.
Dr. Marcus hopes the ban improves doctors’ lives. “Your employer is going to have to treat you better because they know that you can easily go across town to a place that has a higher salary, and your patient can go with you.”
A version of this article appeared on Medscape.com.
But business groups have vowed to challenge the decision in court.
The proposed final rule passed on a 3-2 vote, with the dissenting commissioners disputing the FTC’s authority to broadly ban noncompetes.
Tensions around noncompetes have been building for years. In 2021, President Biden issued an executive order supporting measures to improve economic competition, in which he urged the FTC to consider its rulemaking authority to address noncompete clauses that unfairly limit workers’ mobility. In January 2023, per that directive, the agency proposed ending the restrictive covenants.
While the FTC estimates that the final rule will reduce healthcare costs by up to $194 billion over the next decade and increase worker earnings by $300 million annually, the ruling faces legal hurdles.
US Chamber of Commerce president and CEO Suzanne P. Clark said in a statement that the move is a “blatant power grab” that will undermine competitive business practices, adding that the Chamber will sue to block the measure.
The FTC received more than 26,000 comments on noncompetes during the public feedback period, with about 25,000 supporting the measure, said Benjamin Cady, JD, an FTC attorney.
Mr. Cady called the feedback “compelling,” citing instances of workers who were forced to commute long distances, uproot their families, or risk expensive litigation for wanting to pursue job opportunities.
For example, a comment from a physician working in Appalachia highlights the potential real-life implications of the agreements. “With hospital systems merging, providers with aggressive noncompetes must abandon the community that they serve if they [choose] to leave their employer. Healthcare providers feel trapped in their current employment situation, leading to significant burnout that can shorten their [career] longevity.”
Commissioner Alvaro Bedoya said physicians have had their lives upended by cumbersome noncompetes, often having to move out of state to practice. “A pandemic killed a million people in this country, and there are doctors who cannot work because of a noncompete,” he said.
It’s unclear whether physicians and others who work for nonprofit healthcare groups or hospitals will be covered by the new ban. FTC Commissioner Rebecca Slaughter acknowledged that the agency’s jurisdictional limitations mean that employees of “certain nonprofit organizations” may not benefit from the rule.
“We want to be transparent about the limitation and recognize there are workers, especially healthcare workers, who are bound by anticompetitive and unfair noncompete clauses, that our rule will struggle to reach,” she said. To cover nonprofit healthcare employees, Ms. Slaughter urged Congress to pass legislation banning noncompetes, such as the Workforce Mobility Act of 2021 and the Freedom to Compete Act of 2023.
The FTC final rule will take effect 120 days after it is published in the federal register, and new noncompete agreements will be banned as of this date. However, existing contracts for senior executives will remain in effect because these individuals are less likely to experience “acute harm” due to their ability to negotiate accordingly, said Mr. Cady.
States, AMA Take Aim at Noncompetes
Before the federal ban, several states had already passed legislation limiting the reach of noncompetes. According to a recent article in the Journal of the American College of Cardiology, 12 states prohibit noncompete clauses for physicians: Alabama, California, Colorado, Delaware, Massachusetts, Montana, New Hampshire, New Mexico, North Dakota, Oklahoma, Rhode Island, and South Dakota.
The remaining states allow noncompetes in some form, often excluding them for employees earning below a certain threshold. For example, in Oregon, noncompete agreements may apply to employees earning more than $113,241. Most states have provisions to adjust the threshold annually. The District of Columbia permits 2-year noncompetes for “medical specialists” earning over $250,000 annually.
Indiana employers can no longer enter into noncompete agreements with primary care providers. Other specialties may be subject to the clauses, except when the physician terminates the contract for cause or when an employer terminates the contract without cause.
Rachel Marcus, MD, a cardiologist in Washington, DC, found out how limiting her employment contract’s noncompete clause was when she wanted to leave a former position. Due to the restrictions, she told this news organization that she couldn’t work locally for a competitor for 2 years. The closest location she could seek employment without violating the agreement was Baltimore, approximately 40 miles away.
Dr. Marcus ultimately moved to another position within the same organization because of the company’s reputation for being “aggressive” in their enforcement actions.
Although the American Medical Association (AMA) does not support a total ban, its House of Delegates adopted policies last year to support the prohibition of noncompete contracts for physicians employed by for-profit or nonprofit hospitals, hospital systems, or staffing companies.
Challenges Await
The American Hospital Association, which opposed the proposed rule, called it “bad policy.” The decision “will likely be short-lived, with courts almost certain to stop it before it can do damage to hospitals’ ability to care for their patients and communities,” the association said in a statement.
To ease the transition to the new rule, the FTC also released a model language for employers to use when discussing the changes with their employees. “All employers need to do to comply with the rule is to stop enforcing existing noncompetes with workers other than senior executives and provide notice to such workers,” he said.
Dr. Marcus hopes the ban improves doctors’ lives. “Your employer is going to have to treat you better because they know that you can easily go across town to a place that has a higher salary, and your patient can go with you.”
A version of this article appeared on Medscape.com.