User login
Physician-assisted suicide for mental illness – right or wrong?
Until recently, this contentious issue applied only to patients with physical illnesses who want to end their suffering and who seek out a physician willing to assist with that objective. In the United States and other countries, this is the patient population who may be able to avail themselves of this option.
However, newly proposed legislation in Canada – which has the largest number of physician-assisted deaths worldwide – would expand the indication for MAID to include serious mental illness. Originally set to be passed in March 2023, the law has been deferred for final decision until March 2024.
A recent commentary by psychiatrist Dinah Miller, MD, explored the ethics of this proposed legislation for mental health professionals. “To offer the option of death facilitated by the very person who is trying to get [patients with serious mental illness] better seems so counter to everything I have learned and contradicts our role as psychiatrists who work so hard to prevent suicide,” Dr. Miller wrote. “As psychiatrists, do we offer hope to our most vulnerable patients, or do we offer death? Do we rail against suicide, or do we facilitate it?”
Dr. Miller’s piece garnered a huge amount of reader response that included many laudatory comments: a “nuanced and open-ended inquiry here,” “timely and honest,” and “beautifully written.” One reader thanked the author for “this thoughtful, questioning, and open reflection on what it means to be a psychiatrist facing a thorny and deeply personal practice and philosophical question.”
Cognitive distortion or objective reality?
Many readers were opposed to any type of physician involvement in hastening a patient’s death, regardless of whether the condition is medical or psychiatric. “Let others who wish to die make their own arrangement without the aid of the medical profession,” one reader wrote.
But others felt that for those with terminal physical illnesses or intractable pain, it is justified for medical professionals to either facilitate death or, at the very least, withhold life-prolonging treatments.
A critical-care physician described responding to families’ accusations that withdrawal of life-sustaining measures means “playing God.” On the contrary, the physician wrote, “there is a limit to our abilities; and withdrawing those life-prolonging interventions allows nature or God or whatever to play a role and take its course.”
Another U.S. reader pointed out that “multiple polls in this country have shown that the majority of the general public, physicians in general and psychiatrists in particular, support the option of MAID for the terminally ill. They do not find it at variance with their calling as physicians.”
“I and many of my elderly friends don’t fear death but fear prolonged dementia with its dependency and lack of quality of life,” one reader wrote. “We would be much more at peace if we could put in our advanced directive that we request MAID once some point of dependency has been reached.”
Another wrote that in the event of entering a state of “degrading dependency and hardship on the family, please let me go peacefully, without burdening others, into that good night [of death].”
Many felt that not only physical illness but also the prospect of cognitive degeneration – specifically dementia – also justifies assisting patient death.
“Enormous suffering”
One Canadian reader noted that provisions for advance consent are now in place in Canada for those facing the prospect of neurodegenerative disease and who are still mentally competent to make such decisions.
But therein lies the rub – the concept of an advanced directive goes to the question of decisional capacity. Dr. Miller noted that “depression distorts cognition and leads many patients to believe that they would be better off dead and that their loves ones would be better off without them.”
The concept of “cognitive distortion” implies that the illness itself may impede the decisional capacity required for an advanced directive or a decision made in the moment, in the absence of such a directive. Dr. Miller asked, “How do we determine whether patients with serious mental illness are competent to make such a decision or whether it is mental illness that is driving their perception of a future without hope?”
One reader attested to this from personal experience. “As both a physician and a sufferer of severe, often profound depression for 50 years, I can confidently say that ... the pursuit of death is the result of an impaired mental state, which simultaneously prevents a rational decision.”
Another agreed. “As a psychiatrist, I treated suicidal patients almost every day of my 27-year career. I believe in making every effort to prevent the suicide of a healthy depressed person and I do not support MAID for psychiatric conditions. But I do support MAID for the terminally ill.”
Other readers disagreed. In the words of one commentator, “I think that if we are going to help mentally ill people, we have to consider their choices. The stress imposed by a severe/intractable mental illness is as bad as any other devastating medical illness. If no one is going to hold their hand in life (as support services are limited and psychiatric treatments often fail), allow a medical professional to hold their hand through their final moments.”
A psychiatrist described very ill patients with treatment-resistant bipolar disorder who had not only received medications, including ketamine, but had also received electroconvulsive therapy and still did not respond.
“Their suffering is enormous and the truth is that there is no improvement for more than a few days or weeks and later they return to their hell. Appreciating that they would be better off dead for themselves and their families is not always a cognitive distortion but an objective evaluation of their reality.”
However, as another reader pointed out, an issue with the argument presented here is that it presupposes that MAID requires “clinical justification.” But in Canada, “MAID ... is understood as an expression of personal autonomy. Rooted in liberal political philosophy of individualism ... approval for death need not be based on a clinical assessment.” Instead, “death is seen as a ‘right’ that the state must therefore provide.”
Another form of eugenics?
Dr. Miller raised the ethical implications of racial and socioeconomic factors playing a role in the consideration of MAID for those with psychiatric illness.
“Might those who are poor, who have less access to expensive treatment options and social support, be more likely to request facilitated death?” she asks. “Do we risk facilitating a patient’s demise when other options are unavailable because of a lack of access to treatment or when social and financial struggles exacerbate a person’s hopelessness? Should we worry that psychiatric euthanasia will turn into a form of eugenics where those who can’t contribute are made to feel that they should bow out?”
Several readers agreed. “Looking at the disproportionate burden of illness, rates of imprisonment, and application of the death penalty indicates to me that race and socioeconomic status will be an immediate factor in how, where, and with whom MAID for mental illness would be practice in much, if not all, of the U.S.,” wrote one physician.
A Canadian reader expressed similar concerns. “I’ve seen a handful of people, including someone I considered a good friend, opt for MAID because it was easier than living as a disabled person in poverty without adequate mental health care.”
Another Canadian clinician noted that offering people good care can make all the difference. “As a Canadian mental health clinician who served youth with severe mental illness for over 20 years through our socialized medical network, I can attest to the difference good care usually makes in shifting clients from despair and a commitment to death to embracing life once again. Let’s not, as clinicians, embrace easing a government/state-sanctioned pathway to death.”
Some readers believe that these types of issues can’t be solved with a blanket approach, noting that each case is different. “Real life is always more complicated than academic discussions. Having served on a hospital ethics committee, I know that each case is unique,” one reader noted.
Another reader added, “I think all we can do as physicians is to let people decide for themselves and participate only if our conscience allows.”
A version of this article first appeared on Medscape.com.
Until recently, this contentious issue applied only to patients with physical illnesses who want to end their suffering and who seek out a physician willing to assist with that objective. In the United States and other countries, this is the patient population who may be able to avail themselves of this option.
However, newly proposed legislation in Canada – which has the largest number of physician-assisted deaths worldwide – would expand the indication for MAID to include serious mental illness. Originally set to be passed in March 2023, the law has been deferred for final decision until March 2024.
A recent commentary by psychiatrist Dinah Miller, MD, explored the ethics of this proposed legislation for mental health professionals. “To offer the option of death facilitated by the very person who is trying to get [patients with serious mental illness] better seems so counter to everything I have learned and contradicts our role as psychiatrists who work so hard to prevent suicide,” Dr. Miller wrote. “As psychiatrists, do we offer hope to our most vulnerable patients, or do we offer death? Do we rail against suicide, or do we facilitate it?”
Dr. Miller’s piece garnered a huge amount of reader response that included many laudatory comments: a “nuanced and open-ended inquiry here,” “timely and honest,” and “beautifully written.” One reader thanked the author for “this thoughtful, questioning, and open reflection on what it means to be a psychiatrist facing a thorny and deeply personal practice and philosophical question.”
Cognitive distortion or objective reality?
Many readers were opposed to any type of physician involvement in hastening a patient’s death, regardless of whether the condition is medical or psychiatric. “Let others who wish to die make their own arrangement without the aid of the medical profession,” one reader wrote.
But others felt that for those with terminal physical illnesses or intractable pain, it is justified for medical professionals to either facilitate death or, at the very least, withhold life-prolonging treatments.
A critical-care physician described responding to families’ accusations that withdrawal of life-sustaining measures means “playing God.” On the contrary, the physician wrote, “there is a limit to our abilities; and withdrawing those life-prolonging interventions allows nature or God or whatever to play a role and take its course.”
Another U.S. reader pointed out that “multiple polls in this country have shown that the majority of the general public, physicians in general and psychiatrists in particular, support the option of MAID for the terminally ill. They do not find it at variance with their calling as physicians.”
“I and many of my elderly friends don’t fear death but fear prolonged dementia with its dependency and lack of quality of life,” one reader wrote. “We would be much more at peace if we could put in our advanced directive that we request MAID once some point of dependency has been reached.”
Another wrote that in the event of entering a state of “degrading dependency and hardship on the family, please let me go peacefully, without burdening others, into that good night [of death].”
Many felt that not only physical illness but also the prospect of cognitive degeneration – specifically dementia – also justifies assisting patient death.
“Enormous suffering”
One Canadian reader noted that provisions for advance consent are now in place in Canada for those facing the prospect of neurodegenerative disease and who are still mentally competent to make such decisions.
But therein lies the rub – the concept of an advanced directive goes to the question of decisional capacity. Dr. Miller noted that “depression distorts cognition and leads many patients to believe that they would be better off dead and that their loves ones would be better off without them.”
The concept of “cognitive distortion” implies that the illness itself may impede the decisional capacity required for an advanced directive or a decision made in the moment, in the absence of such a directive. Dr. Miller asked, “How do we determine whether patients with serious mental illness are competent to make such a decision or whether it is mental illness that is driving their perception of a future without hope?”
One reader attested to this from personal experience. “As both a physician and a sufferer of severe, often profound depression for 50 years, I can confidently say that ... the pursuit of death is the result of an impaired mental state, which simultaneously prevents a rational decision.”
Another agreed. “As a psychiatrist, I treated suicidal patients almost every day of my 27-year career. I believe in making every effort to prevent the suicide of a healthy depressed person and I do not support MAID for psychiatric conditions. But I do support MAID for the terminally ill.”
Other readers disagreed. In the words of one commentator, “I think that if we are going to help mentally ill people, we have to consider their choices. The stress imposed by a severe/intractable mental illness is as bad as any other devastating medical illness. If no one is going to hold their hand in life (as support services are limited and psychiatric treatments often fail), allow a medical professional to hold their hand through their final moments.”
A psychiatrist described very ill patients with treatment-resistant bipolar disorder who had not only received medications, including ketamine, but had also received electroconvulsive therapy and still did not respond.
“Their suffering is enormous and the truth is that there is no improvement for more than a few days or weeks and later they return to their hell. Appreciating that they would be better off dead for themselves and their families is not always a cognitive distortion but an objective evaluation of their reality.”
However, as another reader pointed out, an issue with the argument presented here is that it presupposes that MAID requires “clinical justification.” But in Canada, “MAID ... is understood as an expression of personal autonomy. Rooted in liberal political philosophy of individualism ... approval for death need not be based on a clinical assessment.” Instead, “death is seen as a ‘right’ that the state must therefore provide.”
Another form of eugenics?
Dr. Miller raised the ethical implications of racial and socioeconomic factors playing a role in the consideration of MAID for those with psychiatric illness.
“Might those who are poor, who have less access to expensive treatment options and social support, be more likely to request facilitated death?” she asks. “Do we risk facilitating a patient’s demise when other options are unavailable because of a lack of access to treatment or when social and financial struggles exacerbate a person’s hopelessness? Should we worry that psychiatric euthanasia will turn into a form of eugenics where those who can’t contribute are made to feel that they should bow out?”
Several readers agreed. “Looking at the disproportionate burden of illness, rates of imprisonment, and application of the death penalty indicates to me that race and socioeconomic status will be an immediate factor in how, where, and with whom MAID for mental illness would be practice in much, if not all, of the U.S.,” wrote one physician.
A Canadian reader expressed similar concerns. “I’ve seen a handful of people, including someone I considered a good friend, opt for MAID because it was easier than living as a disabled person in poverty without adequate mental health care.”
Another Canadian clinician noted that offering people good care can make all the difference. “As a Canadian mental health clinician who served youth with severe mental illness for over 20 years through our socialized medical network, I can attest to the difference good care usually makes in shifting clients from despair and a commitment to death to embracing life once again. Let’s not, as clinicians, embrace easing a government/state-sanctioned pathway to death.”
Some readers believe that these types of issues can’t be solved with a blanket approach, noting that each case is different. “Real life is always more complicated than academic discussions. Having served on a hospital ethics committee, I know that each case is unique,” one reader noted.
Another reader added, “I think all we can do as physicians is to let people decide for themselves and participate only if our conscience allows.”
A version of this article first appeared on Medscape.com.
Until recently, this contentious issue applied only to patients with physical illnesses who want to end their suffering and who seek out a physician willing to assist with that objective. In the United States and other countries, this is the patient population who may be able to avail themselves of this option.
However, newly proposed legislation in Canada – which has the largest number of physician-assisted deaths worldwide – would expand the indication for MAID to include serious mental illness. Originally set to be passed in March 2023, the law has been deferred for final decision until March 2024.
A recent commentary by psychiatrist Dinah Miller, MD, explored the ethics of this proposed legislation for mental health professionals. “To offer the option of death facilitated by the very person who is trying to get [patients with serious mental illness] better seems so counter to everything I have learned and contradicts our role as psychiatrists who work so hard to prevent suicide,” Dr. Miller wrote. “As psychiatrists, do we offer hope to our most vulnerable patients, or do we offer death? Do we rail against suicide, or do we facilitate it?”
Dr. Miller’s piece garnered a huge amount of reader response that included many laudatory comments: a “nuanced and open-ended inquiry here,” “timely and honest,” and “beautifully written.” One reader thanked the author for “this thoughtful, questioning, and open reflection on what it means to be a psychiatrist facing a thorny and deeply personal practice and philosophical question.”
Cognitive distortion or objective reality?
Many readers were opposed to any type of physician involvement in hastening a patient’s death, regardless of whether the condition is medical or psychiatric. “Let others who wish to die make their own arrangement without the aid of the medical profession,” one reader wrote.
But others felt that for those with terminal physical illnesses or intractable pain, it is justified for medical professionals to either facilitate death or, at the very least, withhold life-prolonging treatments.
A critical-care physician described responding to families’ accusations that withdrawal of life-sustaining measures means “playing God.” On the contrary, the physician wrote, “there is a limit to our abilities; and withdrawing those life-prolonging interventions allows nature or God or whatever to play a role and take its course.”
Another U.S. reader pointed out that “multiple polls in this country have shown that the majority of the general public, physicians in general and psychiatrists in particular, support the option of MAID for the terminally ill. They do not find it at variance with their calling as physicians.”
“I and many of my elderly friends don’t fear death but fear prolonged dementia with its dependency and lack of quality of life,” one reader wrote. “We would be much more at peace if we could put in our advanced directive that we request MAID once some point of dependency has been reached.”
Another wrote that in the event of entering a state of “degrading dependency and hardship on the family, please let me go peacefully, without burdening others, into that good night [of death].”
Many felt that not only physical illness but also the prospect of cognitive degeneration – specifically dementia – also justifies assisting patient death.
“Enormous suffering”
One Canadian reader noted that provisions for advance consent are now in place in Canada for those facing the prospect of neurodegenerative disease and who are still mentally competent to make such decisions.
But therein lies the rub – the concept of an advanced directive goes to the question of decisional capacity. Dr. Miller noted that “depression distorts cognition and leads many patients to believe that they would be better off dead and that their loves ones would be better off without them.”
The concept of “cognitive distortion” implies that the illness itself may impede the decisional capacity required for an advanced directive or a decision made in the moment, in the absence of such a directive. Dr. Miller asked, “How do we determine whether patients with serious mental illness are competent to make such a decision or whether it is mental illness that is driving their perception of a future without hope?”
One reader attested to this from personal experience. “As both a physician and a sufferer of severe, often profound depression for 50 years, I can confidently say that ... the pursuit of death is the result of an impaired mental state, which simultaneously prevents a rational decision.”
Another agreed. “As a psychiatrist, I treated suicidal patients almost every day of my 27-year career. I believe in making every effort to prevent the suicide of a healthy depressed person and I do not support MAID for psychiatric conditions. But I do support MAID for the terminally ill.”
Other readers disagreed. In the words of one commentator, “I think that if we are going to help mentally ill people, we have to consider their choices. The stress imposed by a severe/intractable mental illness is as bad as any other devastating medical illness. If no one is going to hold their hand in life (as support services are limited and psychiatric treatments often fail), allow a medical professional to hold their hand through their final moments.”
A psychiatrist described very ill patients with treatment-resistant bipolar disorder who had not only received medications, including ketamine, but had also received electroconvulsive therapy and still did not respond.
“Their suffering is enormous and the truth is that there is no improvement for more than a few days or weeks and later they return to their hell. Appreciating that they would be better off dead for themselves and their families is not always a cognitive distortion but an objective evaluation of their reality.”
However, as another reader pointed out, an issue with the argument presented here is that it presupposes that MAID requires “clinical justification.” But in Canada, “MAID ... is understood as an expression of personal autonomy. Rooted in liberal political philosophy of individualism ... approval for death need not be based on a clinical assessment.” Instead, “death is seen as a ‘right’ that the state must therefore provide.”
Another form of eugenics?
Dr. Miller raised the ethical implications of racial and socioeconomic factors playing a role in the consideration of MAID for those with psychiatric illness.
“Might those who are poor, who have less access to expensive treatment options and social support, be more likely to request facilitated death?” she asks. “Do we risk facilitating a patient’s demise when other options are unavailable because of a lack of access to treatment or when social and financial struggles exacerbate a person’s hopelessness? Should we worry that psychiatric euthanasia will turn into a form of eugenics where those who can’t contribute are made to feel that they should bow out?”
Several readers agreed. “Looking at the disproportionate burden of illness, rates of imprisonment, and application of the death penalty indicates to me that race and socioeconomic status will be an immediate factor in how, where, and with whom MAID for mental illness would be practice in much, if not all, of the U.S.,” wrote one physician.
A Canadian reader expressed similar concerns. “I’ve seen a handful of people, including someone I considered a good friend, opt for MAID because it was easier than living as a disabled person in poverty without adequate mental health care.”
Another Canadian clinician noted that offering people good care can make all the difference. “As a Canadian mental health clinician who served youth with severe mental illness for over 20 years through our socialized medical network, I can attest to the difference good care usually makes in shifting clients from despair and a commitment to death to embracing life once again. Let’s not, as clinicians, embrace easing a government/state-sanctioned pathway to death.”
Some readers believe that these types of issues can’t be solved with a blanket approach, noting that each case is different. “Real life is always more complicated than academic discussions. Having served on a hospital ethics committee, I know that each case is unique,” one reader noted.
Another reader added, “I think all we can do as physicians is to let people decide for themselves and participate only if our conscience allows.”
A version of this article first appeared on Medscape.com.
TBI tied to increased mental health diagnoses, time to suicide
Investigators also found that increases in new mental health diagnoses are significantly higher in soldiers with a history of TBI – in some cases, strikingly higher. For example, cases of substance use disorder rose by 100% among veterans with TBI compared to just 14.5% in those with no brain injury.
“We had had pieces of these findings for a long time but to be able to lay out this longitudinal story over time is the part that’s new and important to really switch the focus to people’s whole lives and things that happen over time, both psychological and physical,” lead author Lisa Brenner, PhD, director of the Veterans Health Administration (VHA) Rocky Mountain Mental Illness Research Education and Clinical Center, Aurora, Colo., said in an interview.
“If we take that life-course view, it’s a very different way about thinking about conceptualizing exposures and conceptualizing risk and it’s a different way of thinking about treatment and prevention,” added Dr. Brenner, professor of physical medicine and rehabilitation, psychiatry, and neurology at the University of Colorado, Aurora. “I think that definitely applies to civilian populations.”
The findings were published online in JAMA Network Open.
Largest, longest study to date
Researchers have long suspected that TBI and a higher rate of new mental illness and a shorter time to suicide are all somehow linked. But this study examined all three components longitudinally, in what is thought to be the largest and longest study on the topic to date, including more than 860,000 people who were followed for up to a decade.
Investigators studied health data from the Substance Use and Psychological Injury Combat Study database on 860,892 U.S. Army soldiers who returned from deployment in Iraq or Afghanistan between 2008 and 2014 and were 18-24 years old at the end of that deployment. They then examined new mental health diagnoses and suicide trends over time.
Nearly 109,000 (12.6%) experienced a TBI during deployment, and 2,695 had died by suicide through the end of 2018.
New-onset diagnoses of anxiety, mood disorders, posttraumatic stress disorder, alcohol use, and substance use disorder (SUD) after deployment were all more common in soldiers who experienced PTSD while serving compared with those with no history of TBI.
There was a 67.7% increase in mood disorders in participants with TBI compared with a 37.5% increase in those without TBI. The increase in new cases of alcohol use disorder was also greater in the TBI group (a 31.9% increase vs. a 10.3% increase).
But the sharpest difference was the increase in substance use disorder among those with TBI, which rose 100% compared with a 14.5% increase in solders with no history of TBI.
Sharp differences in time to suicide
Death by suicide was only slightly more common in those with TBI compared with those without (0.4% vs. 0.3%, respectively). But those with a brain injury committed suicide 21.3% sooner than did those without a head injury, after the researchers controlled for sex, age, race, ethnicity, and fiscal year of return from deployment.
Time to suicide was faster in those with a TBI and two or more new mental health diagnoses and fastest among those with TBI and a new SUD diagnosis, who took their own lives 62.8% faster than did those without a TBI.
The findings offer an important message to medical professionals in many different specialties, Dr. Brenner said.
“Folks in mental health probably have a lot of patients who have brain injury in their practice, and they don’t know it and that’s an important thing to know,” she said, adding that “neurologists should screen for depression and other mental health conditions and make sure those people have evidence-based treatments for those mental health conditions while they’re addressing the TBI-related symptoms.”
Applicable to civilians?
“The complex interplay between TBI, its potential effects on mental health, and risk of suicide remains a vexing focus of ongoing investigations and academic inquiry,” Ross Zafonte, DO, president of Spaulding Rehabilitation Hospital Network and professor and chair of physical medicine and rehabilitation at Harvard Medical School, Boston, and colleagues, wrote in an accompanying editorial.
The study builds on earlier work, they added, and praised the study’s longitudinal design and large cohort as key to the findings. The data on increased rates of new-onset substance use disorder, which was also associated with a faster time to suicide in the TBI group, were of particular interest.
“In this work, Brenner and colleagues identified substance use disorder as a key factor in faster time to suicide for active-duty service members with a history of TBI compared with those without TBI and theorized that a multiple stress or exposure burden may enhance risk,” they wrote. “This theory is reasonable and has been postulated among individuals with medical sequelae linked to TBI.”
However, the authors caution against applying these findings in military veterans to civilians.
“While this work is critical in the military population, caution should be given to avoid direct generalization to other populations, such as athletes, for whom the linkage to suicidal ideation is less understood,” they wrote.
The study was funded by National Institute of Mental Health and Office of the Director at National Institutes of Health. Dr. Brenner has received personal fees from Wolters Kluwer, Rand, American Psychological Association, and Oxford University Press and serves as a consultant to sports leagues via her university affiliation. Dr. Zafonte reported receiving royalties from Springer/Demos; serving as a member of the editorial boards of Journal of Neurotrauma and Frontiers in Neurology and scientific advisory boards of Myomo, Nanodiagnostics, Onecare.ai, and Kisbee; and evaluating patients in the MGH Brain and Body-TRUST Program, which is funded by the National Football League Players Association.
A version of this article first appeared on Medscape.com.
Investigators also found that increases in new mental health diagnoses are significantly higher in soldiers with a history of TBI – in some cases, strikingly higher. For example, cases of substance use disorder rose by 100% among veterans with TBI compared to just 14.5% in those with no brain injury.
“We had had pieces of these findings for a long time but to be able to lay out this longitudinal story over time is the part that’s new and important to really switch the focus to people’s whole lives and things that happen over time, both psychological and physical,” lead author Lisa Brenner, PhD, director of the Veterans Health Administration (VHA) Rocky Mountain Mental Illness Research Education and Clinical Center, Aurora, Colo., said in an interview.
“If we take that life-course view, it’s a very different way about thinking about conceptualizing exposures and conceptualizing risk and it’s a different way of thinking about treatment and prevention,” added Dr. Brenner, professor of physical medicine and rehabilitation, psychiatry, and neurology at the University of Colorado, Aurora. “I think that definitely applies to civilian populations.”
The findings were published online in JAMA Network Open.
Largest, longest study to date
Researchers have long suspected that TBI and a higher rate of new mental illness and a shorter time to suicide are all somehow linked. But this study examined all three components longitudinally, in what is thought to be the largest and longest study on the topic to date, including more than 860,000 people who were followed for up to a decade.
Investigators studied health data from the Substance Use and Psychological Injury Combat Study database on 860,892 U.S. Army soldiers who returned from deployment in Iraq or Afghanistan between 2008 and 2014 and were 18-24 years old at the end of that deployment. They then examined new mental health diagnoses and suicide trends over time.
Nearly 109,000 (12.6%) experienced a TBI during deployment, and 2,695 had died by suicide through the end of 2018.
New-onset diagnoses of anxiety, mood disorders, posttraumatic stress disorder, alcohol use, and substance use disorder (SUD) after deployment were all more common in soldiers who experienced PTSD while serving compared with those with no history of TBI.
There was a 67.7% increase in mood disorders in participants with TBI compared with a 37.5% increase in those without TBI. The increase in new cases of alcohol use disorder was also greater in the TBI group (a 31.9% increase vs. a 10.3% increase).
But the sharpest difference was the increase in substance use disorder among those with TBI, which rose 100% compared with a 14.5% increase in solders with no history of TBI.
Sharp differences in time to suicide
Death by suicide was only slightly more common in those with TBI compared with those without (0.4% vs. 0.3%, respectively). But those with a brain injury committed suicide 21.3% sooner than did those without a head injury, after the researchers controlled for sex, age, race, ethnicity, and fiscal year of return from deployment.
Time to suicide was faster in those with a TBI and two or more new mental health diagnoses and fastest among those with TBI and a new SUD diagnosis, who took their own lives 62.8% faster than did those without a TBI.
The findings offer an important message to medical professionals in many different specialties, Dr. Brenner said.
“Folks in mental health probably have a lot of patients who have brain injury in their practice, and they don’t know it and that’s an important thing to know,” she said, adding that “neurologists should screen for depression and other mental health conditions and make sure those people have evidence-based treatments for those mental health conditions while they’re addressing the TBI-related symptoms.”
Applicable to civilians?
“The complex interplay between TBI, its potential effects on mental health, and risk of suicide remains a vexing focus of ongoing investigations and academic inquiry,” Ross Zafonte, DO, president of Spaulding Rehabilitation Hospital Network and professor and chair of physical medicine and rehabilitation at Harvard Medical School, Boston, and colleagues, wrote in an accompanying editorial.
The study builds on earlier work, they added, and praised the study’s longitudinal design and large cohort as key to the findings. The data on increased rates of new-onset substance use disorder, which was also associated with a faster time to suicide in the TBI group, were of particular interest.
“In this work, Brenner and colleagues identified substance use disorder as a key factor in faster time to suicide for active-duty service members with a history of TBI compared with those without TBI and theorized that a multiple stress or exposure burden may enhance risk,” they wrote. “This theory is reasonable and has been postulated among individuals with medical sequelae linked to TBI.”
However, the authors caution against applying these findings in military veterans to civilians.
“While this work is critical in the military population, caution should be given to avoid direct generalization to other populations, such as athletes, for whom the linkage to suicidal ideation is less understood,” they wrote.
The study was funded by National Institute of Mental Health and Office of the Director at National Institutes of Health. Dr. Brenner has received personal fees from Wolters Kluwer, Rand, American Psychological Association, and Oxford University Press and serves as a consultant to sports leagues via her university affiliation. Dr. Zafonte reported receiving royalties from Springer/Demos; serving as a member of the editorial boards of Journal of Neurotrauma and Frontiers in Neurology and scientific advisory boards of Myomo, Nanodiagnostics, Onecare.ai, and Kisbee; and evaluating patients in the MGH Brain and Body-TRUST Program, which is funded by the National Football League Players Association.
A version of this article first appeared on Medscape.com.
Investigators also found that increases in new mental health diagnoses are significantly higher in soldiers with a history of TBI – in some cases, strikingly higher. For example, cases of substance use disorder rose by 100% among veterans with TBI compared to just 14.5% in those with no brain injury.
“We had had pieces of these findings for a long time but to be able to lay out this longitudinal story over time is the part that’s new and important to really switch the focus to people’s whole lives and things that happen over time, both psychological and physical,” lead author Lisa Brenner, PhD, director of the Veterans Health Administration (VHA) Rocky Mountain Mental Illness Research Education and Clinical Center, Aurora, Colo., said in an interview.
“If we take that life-course view, it’s a very different way about thinking about conceptualizing exposures and conceptualizing risk and it’s a different way of thinking about treatment and prevention,” added Dr. Brenner, professor of physical medicine and rehabilitation, psychiatry, and neurology at the University of Colorado, Aurora. “I think that definitely applies to civilian populations.”
The findings were published online in JAMA Network Open.
Largest, longest study to date
Researchers have long suspected that TBI and a higher rate of new mental illness and a shorter time to suicide are all somehow linked. But this study examined all three components longitudinally, in what is thought to be the largest and longest study on the topic to date, including more than 860,000 people who were followed for up to a decade.
Investigators studied health data from the Substance Use and Psychological Injury Combat Study database on 860,892 U.S. Army soldiers who returned from deployment in Iraq or Afghanistan between 2008 and 2014 and were 18-24 years old at the end of that deployment. They then examined new mental health diagnoses and suicide trends over time.
Nearly 109,000 (12.6%) experienced a TBI during deployment, and 2,695 had died by suicide through the end of 2018.
New-onset diagnoses of anxiety, mood disorders, posttraumatic stress disorder, alcohol use, and substance use disorder (SUD) after deployment were all more common in soldiers who experienced PTSD while serving compared with those with no history of TBI.
There was a 67.7% increase in mood disorders in participants with TBI compared with a 37.5% increase in those without TBI. The increase in new cases of alcohol use disorder was also greater in the TBI group (a 31.9% increase vs. a 10.3% increase).
But the sharpest difference was the increase in substance use disorder among those with TBI, which rose 100% compared with a 14.5% increase in solders with no history of TBI.
Sharp differences in time to suicide
Death by suicide was only slightly more common in those with TBI compared with those without (0.4% vs. 0.3%, respectively). But those with a brain injury committed suicide 21.3% sooner than did those without a head injury, after the researchers controlled for sex, age, race, ethnicity, and fiscal year of return from deployment.
Time to suicide was faster in those with a TBI and two or more new mental health diagnoses and fastest among those with TBI and a new SUD diagnosis, who took their own lives 62.8% faster than did those without a TBI.
The findings offer an important message to medical professionals in many different specialties, Dr. Brenner said.
“Folks in mental health probably have a lot of patients who have brain injury in their practice, and they don’t know it and that’s an important thing to know,” she said, adding that “neurologists should screen for depression and other mental health conditions and make sure those people have evidence-based treatments for those mental health conditions while they’re addressing the TBI-related symptoms.”
Applicable to civilians?
“The complex interplay between TBI, its potential effects on mental health, and risk of suicide remains a vexing focus of ongoing investigations and academic inquiry,” Ross Zafonte, DO, president of Spaulding Rehabilitation Hospital Network and professor and chair of physical medicine and rehabilitation at Harvard Medical School, Boston, and colleagues, wrote in an accompanying editorial.
The study builds on earlier work, they added, and praised the study’s longitudinal design and large cohort as key to the findings. The data on increased rates of new-onset substance use disorder, which was also associated with a faster time to suicide in the TBI group, were of particular interest.
“In this work, Brenner and colleagues identified substance use disorder as a key factor in faster time to suicide for active-duty service members with a history of TBI compared with those without TBI and theorized that a multiple stress or exposure burden may enhance risk,” they wrote. “This theory is reasonable and has been postulated among individuals with medical sequelae linked to TBI.”
However, the authors caution against applying these findings in military veterans to civilians.
“While this work is critical in the military population, caution should be given to avoid direct generalization to other populations, such as athletes, for whom the linkage to suicidal ideation is less understood,” they wrote.
The study was funded by National Institute of Mental Health and Office of the Director at National Institutes of Health. Dr. Brenner has received personal fees from Wolters Kluwer, Rand, American Psychological Association, and Oxford University Press and serves as a consultant to sports leagues via her university affiliation. Dr. Zafonte reported receiving royalties from Springer/Demos; serving as a member of the editorial boards of Journal of Neurotrauma and Frontiers in Neurology and scientific advisory boards of Myomo, Nanodiagnostics, Onecare.ai, and Kisbee; and evaluating patients in the MGH Brain and Body-TRUST Program, which is funded by the National Football League Players Association.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Experts highlight benefits and offer caveats for first postpartum depression pill
For the first time, the Food and Drug Administration approved a pill taken once daily for 14 days to help women manage the often strong, sometimes overpowering symptoms of postpartum depression.
for a condition that affects an estimated 1 in 8 women in the United States. What will it mean for easing symptoms such as hopelessness, crankiness, and lack of interest in bonding with the baby or, in the case of multiples, babies – and in some cases, thoughts of death or suicide?
A fast-acting option
“We don’t have many oral medications that are fast-acting antidepressants, so this is incredibly exciting,” said Sarah Oreck, MD, a psychiatrist in private practice in Los Angeles who specializes in reproductive psychiatry. The rapid response is likely because the medication targets the hormonal mechanism underlying postpartum depression, she added.
Zuranolone (Zurzuvae, Biogen/Sage) is different from most other antidepressants – it is designed to be taken for a shorter period. Also, Because zuranolone is a pill, it is more convenient to take than the other FDA-approved treatment, the IV infusion brexanolone (Zulresso, Sage).
“It’s obviously game changing to have something in pill form. The infusion has to be done at an infusion center to monitor people for any complications,” said Kimberly Yonkers, MD, a psychiatrist specializing in women’s health, a Distinguished Life Fellow of the American Psychiatric Association (APA), and the Katz Family Chair of Psychiatry at the University of Massachusetts Chan Medical School/UMass Memorial Medical Center in Worcester.
Women may experience improvement in postpartum depression in as soon as 3 days after starting the medication. In contrast, “typical antidepressants can take up to 2 weeks before patients notice a difference and 4 to 8 weeks to see a full response. A fast-acting pill that can be taken orally could be an ideal option for the 15% to 20% of women who experience postpartum depression,” said Priya Gopalan, MD, a psychiatrist with UPMC Western Psychiatric Hospital and Magee-Womens Hospital in Pittsburgh.
The medical community, and reproductive psychiatrists in particular, has always suspected differences in the biological underpinnings of postpartum depression and major depressive disorder, Dr. Oreck said. “We know that postpartum depression looks different from major depressive disorder and that hormonal shifts during pregnancy and postpartum are a huge risk factor for postpartum depression,” she said.
Although selective serotonin reuptake inhibitors (SSRIs) are helpful and currently the standard of care for treating moderate to severe postpartum depression in combination with therapy, Dr. Oreck added, early studies suggest that zuranolone may work faster and potentially be more effective than SSRIs in treating the condition.
Zuranolone is a version of a naturally occurring hormone called allopregnanolone, a metabolite of progesterone. Concentrations of allopregnanolone rise dramatically during pregnancy and then drop precipitously after childbirth. Zuranolone works through modulating GABA-A, a neurotransmitter implicated in the development of depression.
“It is encouraging that postpartum individuals may now have more options to manage a debilitating condition that affects them and their families,” said Christopher Zahn, MD, interim CEO and chief of clinical practice and health equity and quality for the American College of Obstetricians and Gynecologists (ACOG).
ACOG recommends women be screened for depression at least three times – during early pregnancy, later in pregnancy, and again after delivery. A decision to start this or any other medicine should be individualized and based on shared decision-making between a patient and doctor, Dr. Zahn added.
The cost of zuranolone is not yet known. Dr. Yonkers said cost of the infusion can serve as a cautionary tale for the manufacturer. Some reports put the infusion cost at $34,000. “Cost is going to be an important component to this. The previous intervention was priced so high that it was not affordable to many people and it was difficult to access.”
Beyond ‘baby blues’
The APA has changed the name from “postpartum depression” to “peripartum depression” because evidence suggests feelings and symptoms also can start late in pregnancy. “It means you don’t have to wait until somebody delivers to screen for depression. We have to recognize that depression can occur during pregnancy,” Dr. Yonkers said. “In fact it is not uncommon during the third trimester.”
No matter when it starts, the condition can be “very serious,” particularly if the person already experiences depression, including bipolar disorder, Dr. Yonkers added.
Postpartum depression “is more than just ‘baby blues.’ It is a potentially debilitating illness that causes feelings of intense sadness and worthlessness, making it difficult to care for and bond with your newborn,” Dr. Gopalan said.
Can be a medical emergency
Severe postpartum depression requires immediate attention and treatment.
“One of the things we have to be cautious about is for people with previous predisposition to hurt themselves,” Dr. Yonkers said. “It is therefore important to consider somebody’s medical and behavioral health history as well.
“For an individual with recurring depression or severe episodes of depression, this may not be sufficient, because they are just going to get these 14 days of therapy,” Dr. Yonkers said. “They may need ongoing antidepressants.
“It may not be the right pill for everybody,” Dr. Yonkers added. She recommended everyone be followed closely during and after treatment “to make sure they are responding and to monitor for relapse.”
The science that led to approval
The clinical trials showed early response in patients with severe postpartum depression. Researchers conducted two studies of women who developed a major depressive episode in the third trimester of pregnancy or within 4 weeks of delivery. They found women who took zuranolone once in the evening for 14 days “showed significantly more improvement in their symptoms compared to those in the placebo group.”
The antidepressant effect lasted at least 4 weeks after stopping the medication.
Drowsiness, dizziness, diarrhea, fatigue, nasopharyngitis, and urinary tract infection were the most common side effects. The label has a boxed warning noting that the medication can affect a person’s ability to drive and perform other potentially hazardous activities. Use of zuranolone may also cause suicidal thoughts and behavior, according to an FDA news release announcing the approval.
The start of more help for mothers?
Zuranolone is not a cure-all. As with most psychiatric prescriptions, the medication likely will work best in conjunction with behavioral health treatments such as psychotherapy, use of other medications, behavioral management, support groups, and self-care tools such as meditation, exercise, and yoga, Dr. Gopalan said.
Dr. Oreck said she hopes this first pill approval will lead to more discoveries. “I hope this is the beginning of more innovation and development of novel treatments that can target women’s mental health issues specifically – female reproductive hormones impact mental health in unique ways and it’s exciting to finally see research and development dollars dedicated to them,” she said. “The FDA approval of this pill provides the potential to improve the lives of millions of Americans suffering from postpartum depression.”
Dr. Oreck, Dr. Yonkers, Dr. Gopalan, and Dr. Zahn have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For the first time, the Food and Drug Administration approved a pill taken once daily for 14 days to help women manage the often strong, sometimes overpowering symptoms of postpartum depression.
for a condition that affects an estimated 1 in 8 women in the United States. What will it mean for easing symptoms such as hopelessness, crankiness, and lack of interest in bonding with the baby or, in the case of multiples, babies – and in some cases, thoughts of death or suicide?
A fast-acting option
“We don’t have many oral medications that are fast-acting antidepressants, so this is incredibly exciting,” said Sarah Oreck, MD, a psychiatrist in private practice in Los Angeles who specializes in reproductive psychiatry. The rapid response is likely because the medication targets the hormonal mechanism underlying postpartum depression, she added.
Zuranolone (Zurzuvae, Biogen/Sage) is different from most other antidepressants – it is designed to be taken for a shorter period. Also, Because zuranolone is a pill, it is more convenient to take than the other FDA-approved treatment, the IV infusion brexanolone (Zulresso, Sage).
“It’s obviously game changing to have something in pill form. The infusion has to be done at an infusion center to monitor people for any complications,” said Kimberly Yonkers, MD, a psychiatrist specializing in women’s health, a Distinguished Life Fellow of the American Psychiatric Association (APA), and the Katz Family Chair of Psychiatry at the University of Massachusetts Chan Medical School/UMass Memorial Medical Center in Worcester.
Women may experience improvement in postpartum depression in as soon as 3 days after starting the medication. In contrast, “typical antidepressants can take up to 2 weeks before patients notice a difference and 4 to 8 weeks to see a full response. A fast-acting pill that can be taken orally could be an ideal option for the 15% to 20% of women who experience postpartum depression,” said Priya Gopalan, MD, a psychiatrist with UPMC Western Psychiatric Hospital and Magee-Womens Hospital in Pittsburgh.
The medical community, and reproductive psychiatrists in particular, has always suspected differences in the biological underpinnings of postpartum depression and major depressive disorder, Dr. Oreck said. “We know that postpartum depression looks different from major depressive disorder and that hormonal shifts during pregnancy and postpartum are a huge risk factor for postpartum depression,” she said.
Although selective serotonin reuptake inhibitors (SSRIs) are helpful and currently the standard of care for treating moderate to severe postpartum depression in combination with therapy, Dr. Oreck added, early studies suggest that zuranolone may work faster and potentially be more effective than SSRIs in treating the condition.
Zuranolone is a version of a naturally occurring hormone called allopregnanolone, a metabolite of progesterone. Concentrations of allopregnanolone rise dramatically during pregnancy and then drop precipitously after childbirth. Zuranolone works through modulating GABA-A, a neurotransmitter implicated in the development of depression.
“It is encouraging that postpartum individuals may now have more options to manage a debilitating condition that affects them and their families,” said Christopher Zahn, MD, interim CEO and chief of clinical practice and health equity and quality for the American College of Obstetricians and Gynecologists (ACOG).
ACOG recommends women be screened for depression at least three times – during early pregnancy, later in pregnancy, and again after delivery. A decision to start this or any other medicine should be individualized and based on shared decision-making between a patient and doctor, Dr. Zahn added.
The cost of zuranolone is not yet known. Dr. Yonkers said cost of the infusion can serve as a cautionary tale for the manufacturer. Some reports put the infusion cost at $34,000. “Cost is going to be an important component to this. The previous intervention was priced so high that it was not affordable to many people and it was difficult to access.”
Beyond ‘baby blues’
The APA has changed the name from “postpartum depression” to “peripartum depression” because evidence suggests feelings and symptoms also can start late in pregnancy. “It means you don’t have to wait until somebody delivers to screen for depression. We have to recognize that depression can occur during pregnancy,” Dr. Yonkers said. “In fact it is not uncommon during the third trimester.”
No matter when it starts, the condition can be “very serious,” particularly if the person already experiences depression, including bipolar disorder, Dr. Yonkers added.
Postpartum depression “is more than just ‘baby blues.’ It is a potentially debilitating illness that causes feelings of intense sadness and worthlessness, making it difficult to care for and bond with your newborn,” Dr. Gopalan said.
Can be a medical emergency
Severe postpartum depression requires immediate attention and treatment.
“One of the things we have to be cautious about is for people with previous predisposition to hurt themselves,” Dr. Yonkers said. “It is therefore important to consider somebody’s medical and behavioral health history as well.
“For an individual with recurring depression or severe episodes of depression, this may not be sufficient, because they are just going to get these 14 days of therapy,” Dr. Yonkers said. “They may need ongoing antidepressants.
“It may not be the right pill for everybody,” Dr. Yonkers added. She recommended everyone be followed closely during and after treatment “to make sure they are responding and to monitor for relapse.”
The science that led to approval
The clinical trials showed early response in patients with severe postpartum depression. Researchers conducted two studies of women who developed a major depressive episode in the third trimester of pregnancy or within 4 weeks of delivery. They found women who took zuranolone once in the evening for 14 days “showed significantly more improvement in their symptoms compared to those in the placebo group.”
The antidepressant effect lasted at least 4 weeks after stopping the medication.
Drowsiness, dizziness, diarrhea, fatigue, nasopharyngitis, and urinary tract infection were the most common side effects. The label has a boxed warning noting that the medication can affect a person’s ability to drive and perform other potentially hazardous activities. Use of zuranolone may also cause suicidal thoughts and behavior, according to an FDA news release announcing the approval.
The start of more help for mothers?
Zuranolone is not a cure-all. As with most psychiatric prescriptions, the medication likely will work best in conjunction with behavioral health treatments such as psychotherapy, use of other medications, behavioral management, support groups, and self-care tools such as meditation, exercise, and yoga, Dr. Gopalan said.
Dr. Oreck said she hopes this first pill approval will lead to more discoveries. “I hope this is the beginning of more innovation and development of novel treatments that can target women’s mental health issues specifically – female reproductive hormones impact mental health in unique ways and it’s exciting to finally see research and development dollars dedicated to them,” she said. “The FDA approval of this pill provides the potential to improve the lives of millions of Americans suffering from postpartum depression.”
Dr. Oreck, Dr. Yonkers, Dr. Gopalan, and Dr. Zahn have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For the first time, the Food and Drug Administration approved a pill taken once daily for 14 days to help women manage the often strong, sometimes overpowering symptoms of postpartum depression.
for a condition that affects an estimated 1 in 8 women in the United States. What will it mean for easing symptoms such as hopelessness, crankiness, and lack of interest in bonding with the baby or, in the case of multiples, babies – and in some cases, thoughts of death or suicide?
A fast-acting option
“We don’t have many oral medications that are fast-acting antidepressants, so this is incredibly exciting,” said Sarah Oreck, MD, a psychiatrist in private practice in Los Angeles who specializes in reproductive psychiatry. The rapid response is likely because the medication targets the hormonal mechanism underlying postpartum depression, she added.
Zuranolone (Zurzuvae, Biogen/Sage) is different from most other antidepressants – it is designed to be taken for a shorter period. Also, Because zuranolone is a pill, it is more convenient to take than the other FDA-approved treatment, the IV infusion brexanolone (Zulresso, Sage).
“It’s obviously game changing to have something in pill form. The infusion has to be done at an infusion center to monitor people for any complications,” said Kimberly Yonkers, MD, a psychiatrist specializing in women’s health, a Distinguished Life Fellow of the American Psychiatric Association (APA), and the Katz Family Chair of Psychiatry at the University of Massachusetts Chan Medical School/UMass Memorial Medical Center in Worcester.
Women may experience improvement in postpartum depression in as soon as 3 days after starting the medication. In contrast, “typical antidepressants can take up to 2 weeks before patients notice a difference and 4 to 8 weeks to see a full response. A fast-acting pill that can be taken orally could be an ideal option for the 15% to 20% of women who experience postpartum depression,” said Priya Gopalan, MD, a psychiatrist with UPMC Western Psychiatric Hospital and Magee-Womens Hospital in Pittsburgh.
The medical community, and reproductive psychiatrists in particular, has always suspected differences in the biological underpinnings of postpartum depression and major depressive disorder, Dr. Oreck said. “We know that postpartum depression looks different from major depressive disorder and that hormonal shifts during pregnancy and postpartum are a huge risk factor for postpartum depression,” she said.
Although selective serotonin reuptake inhibitors (SSRIs) are helpful and currently the standard of care for treating moderate to severe postpartum depression in combination with therapy, Dr. Oreck added, early studies suggest that zuranolone may work faster and potentially be more effective than SSRIs in treating the condition.
Zuranolone is a version of a naturally occurring hormone called allopregnanolone, a metabolite of progesterone. Concentrations of allopregnanolone rise dramatically during pregnancy and then drop precipitously after childbirth. Zuranolone works through modulating GABA-A, a neurotransmitter implicated in the development of depression.
“It is encouraging that postpartum individuals may now have more options to manage a debilitating condition that affects them and their families,” said Christopher Zahn, MD, interim CEO and chief of clinical practice and health equity and quality for the American College of Obstetricians and Gynecologists (ACOG).
ACOG recommends women be screened for depression at least three times – during early pregnancy, later in pregnancy, and again after delivery. A decision to start this or any other medicine should be individualized and based on shared decision-making between a patient and doctor, Dr. Zahn added.
The cost of zuranolone is not yet known. Dr. Yonkers said cost of the infusion can serve as a cautionary tale for the manufacturer. Some reports put the infusion cost at $34,000. “Cost is going to be an important component to this. The previous intervention was priced so high that it was not affordable to many people and it was difficult to access.”
Beyond ‘baby blues’
The APA has changed the name from “postpartum depression” to “peripartum depression” because evidence suggests feelings and symptoms also can start late in pregnancy. “It means you don’t have to wait until somebody delivers to screen for depression. We have to recognize that depression can occur during pregnancy,” Dr. Yonkers said. “In fact it is not uncommon during the third trimester.”
No matter when it starts, the condition can be “very serious,” particularly if the person already experiences depression, including bipolar disorder, Dr. Yonkers added.
Postpartum depression “is more than just ‘baby blues.’ It is a potentially debilitating illness that causes feelings of intense sadness and worthlessness, making it difficult to care for and bond with your newborn,” Dr. Gopalan said.
Can be a medical emergency
Severe postpartum depression requires immediate attention and treatment.
“One of the things we have to be cautious about is for people with previous predisposition to hurt themselves,” Dr. Yonkers said. “It is therefore important to consider somebody’s medical and behavioral health history as well.
“For an individual with recurring depression or severe episodes of depression, this may not be sufficient, because they are just going to get these 14 days of therapy,” Dr. Yonkers said. “They may need ongoing antidepressants.
“It may not be the right pill for everybody,” Dr. Yonkers added. She recommended everyone be followed closely during and after treatment “to make sure they are responding and to monitor for relapse.”
The science that led to approval
The clinical trials showed early response in patients with severe postpartum depression. Researchers conducted two studies of women who developed a major depressive episode in the third trimester of pregnancy or within 4 weeks of delivery. They found women who took zuranolone once in the evening for 14 days “showed significantly more improvement in their symptoms compared to those in the placebo group.”
The antidepressant effect lasted at least 4 weeks after stopping the medication.
Drowsiness, dizziness, diarrhea, fatigue, nasopharyngitis, and urinary tract infection were the most common side effects. The label has a boxed warning noting that the medication can affect a person’s ability to drive and perform other potentially hazardous activities. Use of zuranolone may also cause suicidal thoughts and behavior, according to an FDA news release announcing the approval.
The start of more help for mothers?
Zuranolone is not a cure-all. As with most psychiatric prescriptions, the medication likely will work best in conjunction with behavioral health treatments such as psychotherapy, use of other medications, behavioral management, support groups, and self-care tools such as meditation, exercise, and yoga, Dr. Gopalan said.
Dr. Oreck said she hopes this first pill approval will lead to more discoveries. “I hope this is the beginning of more innovation and development of novel treatments that can target women’s mental health issues specifically – female reproductive hormones impact mental health in unique ways and it’s exciting to finally see research and development dollars dedicated to them,” she said. “The FDA approval of this pill provides the potential to improve the lives of millions of Americans suffering from postpartum depression.”
Dr. Oreck, Dr. Yonkers, Dr. Gopalan, and Dr. Zahn have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA approves first pill for postpartum depression
a condition that affects an estimated one in seven mothers in the United States.
The pill, zuranolone (Zurzuvae), is a neuroactive steroid that acts on GABAA receptors in the brain responsible for regulating mood, arousal, behavior, and cognition, according to Biogen, which, along with Sage Therapeutics, developed the product. The recommended dose for Zurzuvae is 50 mg taken once daily for 14 days, in the evening with a fatty meal, according to the FDA.
Postpartum depression often goes undiagnosed and untreated. Many mothers are hesitant to reveal their symptoms to family and clinicians, fearing they’ll be judged on their parenting. A 2017 study found that suicide accounted for roughly 5% of perinatal deaths among women in Canada, with most of those deaths occurring in the first 3 months in the year after giving birth.
“Postpartum depression is a serious and potentially life-threatening condition in which women experience sadness, guilt, worthlessness – even, in severe cases, thoughts of harming themselves or their child. And, because postpartum depression can disrupt the maternal-infant bond, it can also have consequences for the child’s physical and emotional development,” Tiffany R. Farchione, MD, director of the division of psychiatry at the FDA’s Center for Drug Evaluation and Research, said in a statement about the approval. “Having access to an oral medication will be a beneficial option for many of these women coping with extreme, and sometimes life-threatening, feelings.”
The other approved therapy for postpartum depression is the intravenous agent brexanolone (Zulresso; Sage). But the product requires prolonged infusions in hospital settings and costs $34,000.
FDA approval of Zurzuvae was based in part on data reported in a 2023 study in the American Journal of Psychiatry, which showed that the drug led to significantly greater improvement in depressive symptoms at 15 days compared with the placebo group. Improvements were observed on day 3, the earliest assessment, and were sustained at all subsequent visits during the treatment and follow-up period (through day 42).
Patients with anxiety who received the active drug experienced improvement in related symptoms compared with the patients who received a placebo.
The most common adverse events reported in the trial were somnolence and headaches. Weight gain, sexual dysfunction, withdrawal symptoms, and increased suicidal ideation or behavior were not observed.
The packaging for Zurzuvae will include a boxed warning noting that the drug can affect a user’s ability to drive and perform other potentially hazardous activities, possibly without their knowledge of the impairment, the FDA said. As a result, people who use Zurzuvae should not drive or operate heavy machinery for at least 12 hours after taking the pill.
A version of this article first appeared on Medscape.com.
a condition that affects an estimated one in seven mothers in the United States.
The pill, zuranolone (Zurzuvae), is a neuroactive steroid that acts on GABAA receptors in the brain responsible for regulating mood, arousal, behavior, and cognition, according to Biogen, which, along with Sage Therapeutics, developed the product. The recommended dose for Zurzuvae is 50 mg taken once daily for 14 days, in the evening with a fatty meal, according to the FDA.
Postpartum depression often goes undiagnosed and untreated. Many mothers are hesitant to reveal their symptoms to family and clinicians, fearing they’ll be judged on their parenting. A 2017 study found that suicide accounted for roughly 5% of perinatal deaths among women in Canada, with most of those deaths occurring in the first 3 months in the year after giving birth.
“Postpartum depression is a serious and potentially life-threatening condition in which women experience sadness, guilt, worthlessness – even, in severe cases, thoughts of harming themselves or their child. And, because postpartum depression can disrupt the maternal-infant bond, it can also have consequences for the child’s physical and emotional development,” Tiffany R. Farchione, MD, director of the division of psychiatry at the FDA’s Center for Drug Evaluation and Research, said in a statement about the approval. “Having access to an oral medication will be a beneficial option for many of these women coping with extreme, and sometimes life-threatening, feelings.”
The other approved therapy for postpartum depression is the intravenous agent brexanolone (Zulresso; Sage). But the product requires prolonged infusions in hospital settings and costs $34,000.
FDA approval of Zurzuvae was based in part on data reported in a 2023 study in the American Journal of Psychiatry, which showed that the drug led to significantly greater improvement in depressive symptoms at 15 days compared with the placebo group. Improvements were observed on day 3, the earliest assessment, and were sustained at all subsequent visits during the treatment and follow-up period (through day 42).
Patients with anxiety who received the active drug experienced improvement in related symptoms compared with the patients who received a placebo.
The most common adverse events reported in the trial were somnolence and headaches. Weight gain, sexual dysfunction, withdrawal symptoms, and increased suicidal ideation or behavior were not observed.
The packaging for Zurzuvae will include a boxed warning noting that the drug can affect a user’s ability to drive and perform other potentially hazardous activities, possibly without their knowledge of the impairment, the FDA said. As a result, people who use Zurzuvae should not drive or operate heavy machinery for at least 12 hours after taking the pill.
A version of this article first appeared on Medscape.com.
a condition that affects an estimated one in seven mothers in the United States.
The pill, zuranolone (Zurzuvae), is a neuroactive steroid that acts on GABAA receptors in the brain responsible for regulating mood, arousal, behavior, and cognition, according to Biogen, which, along with Sage Therapeutics, developed the product. The recommended dose for Zurzuvae is 50 mg taken once daily for 14 days, in the evening with a fatty meal, according to the FDA.
Postpartum depression often goes undiagnosed and untreated. Many mothers are hesitant to reveal their symptoms to family and clinicians, fearing they’ll be judged on their parenting. A 2017 study found that suicide accounted for roughly 5% of perinatal deaths among women in Canada, with most of those deaths occurring in the first 3 months in the year after giving birth.
“Postpartum depression is a serious and potentially life-threatening condition in which women experience sadness, guilt, worthlessness – even, in severe cases, thoughts of harming themselves or their child. And, because postpartum depression can disrupt the maternal-infant bond, it can also have consequences for the child’s physical and emotional development,” Tiffany R. Farchione, MD, director of the division of psychiatry at the FDA’s Center for Drug Evaluation and Research, said in a statement about the approval. “Having access to an oral medication will be a beneficial option for many of these women coping with extreme, and sometimes life-threatening, feelings.”
The other approved therapy for postpartum depression is the intravenous agent brexanolone (Zulresso; Sage). But the product requires prolonged infusions in hospital settings and costs $34,000.
FDA approval of Zurzuvae was based in part on data reported in a 2023 study in the American Journal of Psychiatry, which showed that the drug led to significantly greater improvement in depressive symptoms at 15 days compared with the placebo group. Improvements were observed on day 3, the earliest assessment, and were sustained at all subsequent visits during the treatment and follow-up period (through day 42).
Patients with anxiety who received the active drug experienced improvement in related symptoms compared with the patients who received a placebo.
The most common adverse events reported in the trial were somnolence and headaches. Weight gain, sexual dysfunction, withdrawal symptoms, and increased suicidal ideation or behavior were not observed.
The packaging for Zurzuvae will include a boxed warning noting that the drug can affect a user’s ability to drive and perform other potentially hazardous activities, possibly without their knowledge of the impairment, the FDA said. As a result, people who use Zurzuvae should not drive or operate heavy machinery for at least 12 hours after taking the pill.
A version of this article first appeared on Medscape.com.
Depression at any stage of life tied to increased dementia risk
Adults with depression have more than double the risk of developing dementia and the risk persists regardless of when in life depression is diagnosed, a large population-based study shows.
That the association between depression and dementia persisted even among individuals first diagnosed with depression in early or mid-life provides “strong evidence that depression is not only an early symptom of dementia, but also that depression increases dementia risk,” study investigator Holly Elser, MD, PhD, epidemiologist and resident physician, University of Pennsylvania, Philadelphia, told this news organization.
The study was published online in JAMA Neurology.
Double the risk
Several prior studies that have examined the relationship between depression and dementia over the life course have consistently shown depression later in life is associated with subsequent dementia.
“Late-life depression is generally thought to be an early symptom of dementia or a reaction to subclinical cognitive decline,” said Dr. Elser.
The investigators wanted to examine whether the association between depression and dementia persists even when depression is diagnosed earlier in life, which may suggest it increases the risk of dementia.
“To my knowledge, ours is the largest study on this topic to date, leveraging routinely and prospectively collected data from more than 1.4 million Danish citizens followed from 1977 to 2018,” Dr. Elser noted.
The cohort included 246,499 individuals diagnosed with depression and 1,190,302 individuals without depression.
In both groups, the median age was 50 years and 65% were women. Roughly two-thirds (68%) of those diagnosed with depression were diagnosed before age 60 years.
In Cox proportional hazards regression models, the overall hazard of dementia was more than doubled in those diagnosed with depression (hazard ratio [HR] 2.41). The risk of dementia with depression was more pronounced for men (HR, 2.98) than in women (HR, 2.21).
This association persisted even when the time elapsed from depression diagnosis was between 20 and 39 years (HR, 1.79) and whether depression was diagnosed in early life (18-44 years: HR, 3.08), mid-life (45-59 years: HR, 2.95), or late life (≥ 60 years: HR, 2.31).
It remains unclear whether effective treatment of depression modifies the risk of dementia, as the current study explored the role of antidepressants in a “very limited fashion,” Dr. Elser said.
Specifically, the researchers considered whether an individual was treated with an antidepressant within 6 months of the initial depression diagnosis and found no evidence of a difference in dementia risk between the treated and untreated groups.
“Research that explores implications of the timing and duration of treatment with antidepressants for dementia, treatment with cognitive behavioral therapy, and is able to evaluate the effectiveness of those treatments will be extremely important,” Dr. Elser said.
‘An assault on the brain’
Reached for comment, John Showalter, MD, chief product officer at Linus Health, said one of the most “intriguing” findings of the study is that a depression diagnosis earlier in adulthood conferred a greater risk of developing vascular dementia (HR, 3.28) than did dementia due to Alzheimer’s disease (HR, 1.73).
“The difference in risk for subtypes of dementia is a meaningful addition to our understanding of depression’s connection to dementia,” said Dr. Showalter, who was not involved in the study.
Also weighing in, Shaheen Lakhan, MD, PhD, a neurologist and researcher in Boston, said the findings from this “far-reaching investigation leave little room for doubt – depression unleashes a devastating storm within the brain, wreaking havoc on the lives of those ensnared by its grip.
“This massive, multi-decade, and high-data quality registry study adds another brick to the growing edifice of evidence attesting to the profound connection between psychiatric health and the very essence of brain health,” said Dr. Lakhan, who was not involved in the study.
“In a resounding declaration, this research underscores that psychiatric health should be perceived as an integral component of overall health – a paradigm shift that challenges long-standing misconceptions and stigmas surrounding mental disorders. Depression, once marginalized, now claims its rightful place on the pedestal of health concerns that must be addressed with unwavering resolve,” said Dr. Lakhan.
He noted that depression is “not just a mental battle, it’s a profound assault on the very fabric of the brain, leaving lives in turmoil and hearts in search of hope. No longer shrouded in silence, depression demands society’s attention.”
The study had no specific funding. Dr. Elser, Dr. Showalter, and Dr. Lakhan have reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Adults with depression have more than double the risk of developing dementia and the risk persists regardless of when in life depression is diagnosed, a large population-based study shows.
That the association between depression and dementia persisted even among individuals first diagnosed with depression in early or mid-life provides “strong evidence that depression is not only an early symptom of dementia, but also that depression increases dementia risk,” study investigator Holly Elser, MD, PhD, epidemiologist and resident physician, University of Pennsylvania, Philadelphia, told this news organization.
The study was published online in JAMA Neurology.
Double the risk
Several prior studies that have examined the relationship between depression and dementia over the life course have consistently shown depression later in life is associated with subsequent dementia.
“Late-life depression is generally thought to be an early symptom of dementia or a reaction to subclinical cognitive decline,” said Dr. Elser.
The investigators wanted to examine whether the association between depression and dementia persists even when depression is diagnosed earlier in life, which may suggest it increases the risk of dementia.
“To my knowledge, ours is the largest study on this topic to date, leveraging routinely and prospectively collected data from more than 1.4 million Danish citizens followed from 1977 to 2018,” Dr. Elser noted.
The cohort included 246,499 individuals diagnosed with depression and 1,190,302 individuals without depression.
In both groups, the median age was 50 years and 65% were women. Roughly two-thirds (68%) of those diagnosed with depression were diagnosed before age 60 years.
In Cox proportional hazards regression models, the overall hazard of dementia was more than doubled in those diagnosed with depression (hazard ratio [HR] 2.41). The risk of dementia with depression was more pronounced for men (HR, 2.98) than in women (HR, 2.21).
This association persisted even when the time elapsed from depression diagnosis was between 20 and 39 years (HR, 1.79) and whether depression was diagnosed in early life (18-44 years: HR, 3.08), mid-life (45-59 years: HR, 2.95), or late life (≥ 60 years: HR, 2.31).
It remains unclear whether effective treatment of depression modifies the risk of dementia, as the current study explored the role of antidepressants in a “very limited fashion,” Dr. Elser said.
Specifically, the researchers considered whether an individual was treated with an antidepressant within 6 months of the initial depression diagnosis and found no evidence of a difference in dementia risk between the treated and untreated groups.
“Research that explores implications of the timing and duration of treatment with antidepressants for dementia, treatment with cognitive behavioral therapy, and is able to evaluate the effectiveness of those treatments will be extremely important,” Dr. Elser said.
‘An assault on the brain’
Reached for comment, John Showalter, MD, chief product officer at Linus Health, said one of the most “intriguing” findings of the study is that a depression diagnosis earlier in adulthood conferred a greater risk of developing vascular dementia (HR, 3.28) than did dementia due to Alzheimer’s disease (HR, 1.73).
“The difference in risk for subtypes of dementia is a meaningful addition to our understanding of depression’s connection to dementia,” said Dr. Showalter, who was not involved in the study.
Also weighing in, Shaheen Lakhan, MD, PhD, a neurologist and researcher in Boston, said the findings from this “far-reaching investigation leave little room for doubt – depression unleashes a devastating storm within the brain, wreaking havoc on the lives of those ensnared by its grip.
“This massive, multi-decade, and high-data quality registry study adds another brick to the growing edifice of evidence attesting to the profound connection between psychiatric health and the very essence of brain health,” said Dr. Lakhan, who was not involved in the study.
“In a resounding declaration, this research underscores that psychiatric health should be perceived as an integral component of overall health – a paradigm shift that challenges long-standing misconceptions and stigmas surrounding mental disorders. Depression, once marginalized, now claims its rightful place on the pedestal of health concerns that must be addressed with unwavering resolve,” said Dr. Lakhan.
He noted that depression is “not just a mental battle, it’s a profound assault on the very fabric of the brain, leaving lives in turmoil and hearts in search of hope. No longer shrouded in silence, depression demands society’s attention.”
The study had no specific funding. Dr. Elser, Dr. Showalter, and Dr. Lakhan have reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Adults with depression have more than double the risk of developing dementia and the risk persists regardless of when in life depression is diagnosed, a large population-based study shows.
That the association between depression and dementia persisted even among individuals first diagnosed with depression in early or mid-life provides “strong evidence that depression is not only an early symptom of dementia, but also that depression increases dementia risk,” study investigator Holly Elser, MD, PhD, epidemiologist and resident physician, University of Pennsylvania, Philadelphia, told this news organization.
The study was published online in JAMA Neurology.
Double the risk
Several prior studies that have examined the relationship between depression and dementia over the life course have consistently shown depression later in life is associated with subsequent dementia.
“Late-life depression is generally thought to be an early symptom of dementia or a reaction to subclinical cognitive decline,” said Dr. Elser.
The investigators wanted to examine whether the association between depression and dementia persists even when depression is diagnosed earlier in life, which may suggest it increases the risk of dementia.
“To my knowledge, ours is the largest study on this topic to date, leveraging routinely and prospectively collected data from more than 1.4 million Danish citizens followed from 1977 to 2018,” Dr. Elser noted.
The cohort included 246,499 individuals diagnosed with depression and 1,190,302 individuals without depression.
In both groups, the median age was 50 years and 65% were women. Roughly two-thirds (68%) of those diagnosed with depression were diagnosed before age 60 years.
In Cox proportional hazards regression models, the overall hazard of dementia was more than doubled in those diagnosed with depression (hazard ratio [HR] 2.41). The risk of dementia with depression was more pronounced for men (HR, 2.98) than in women (HR, 2.21).
This association persisted even when the time elapsed from depression diagnosis was between 20 and 39 years (HR, 1.79) and whether depression was diagnosed in early life (18-44 years: HR, 3.08), mid-life (45-59 years: HR, 2.95), or late life (≥ 60 years: HR, 2.31).
It remains unclear whether effective treatment of depression modifies the risk of dementia, as the current study explored the role of antidepressants in a “very limited fashion,” Dr. Elser said.
Specifically, the researchers considered whether an individual was treated with an antidepressant within 6 months of the initial depression diagnosis and found no evidence of a difference in dementia risk between the treated and untreated groups.
“Research that explores implications of the timing and duration of treatment with antidepressants for dementia, treatment with cognitive behavioral therapy, and is able to evaluate the effectiveness of those treatments will be extremely important,” Dr. Elser said.
‘An assault on the brain’
Reached for comment, John Showalter, MD, chief product officer at Linus Health, said one of the most “intriguing” findings of the study is that a depression diagnosis earlier in adulthood conferred a greater risk of developing vascular dementia (HR, 3.28) than did dementia due to Alzheimer’s disease (HR, 1.73).
“The difference in risk for subtypes of dementia is a meaningful addition to our understanding of depression’s connection to dementia,” said Dr. Showalter, who was not involved in the study.
Also weighing in, Shaheen Lakhan, MD, PhD, a neurologist and researcher in Boston, said the findings from this “far-reaching investigation leave little room for doubt – depression unleashes a devastating storm within the brain, wreaking havoc on the lives of those ensnared by its grip.
“This massive, multi-decade, and high-data quality registry study adds another brick to the growing edifice of evidence attesting to the profound connection between psychiatric health and the very essence of brain health,” said Dr. Lakhan, who was not involved in the study.
“In a resounding declaration, this research underscores that psychiatric health should be perceived as an integral component of overall health – a paradigm shift that challenges long-standing misconceptions and stigmas surrounding mental disorders. Depression, once marginalized, now claims its rightful place on the pedestal of health concerns that must be addressed with unwavering resolve,” said Dr. Lakhan.
He noted that depression is “not just a mental battle, it’s a profound assault on the very fabric of the brain, leaving lives in turmoil and hearts in search of hope. No longer shrouded in silence, depression demands society’s attention.”
The study had no specific funding. Dr. Elser, Dr. Showalter, and Dr. Lakhan have reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Prescribing lifestyle changes: When medicine isn’t enough
In psychiatry, patients come to us with their list of symptoms, often a diagnosis they’ve made themselves, and the expectation that they will be given medication to fix their problem. Their diagnoses are often right on target – people often know if they are depressed or anxious, and Doctor Google may provide useful information.
Sometimes they want a specific medication, one they saw in a TV ad, or one that helped them in the past or has helped someone they know. As psychiatrists have focused more on their strengths as psychopharmacologists and less on psychotherapy, it gets easy for both the patient and the doctor to look to medication, cocktails, and titration as the only thing we do.
“My medicine stopped working,” is a line I commonly hear. Often the patient is on a complicated regimen that has been serving them well, and it seems unlikely that the five psychotropic medications they are taking have suddenly “stopped working.” An obvious exception is the SSRI “poop out” that can occur 6-12 months or more after beginning treatment. In addition, it’s important to make sure patients are taking their medications as prescribed, and that the generic formulations have not changed.
But as rates of mental illness increase, some of it spurred on by difficult times,
This is not to devalue our medications, but to help the patient see symptoms as having multiple factors and give them some means to intervene, in addition to medications. At the beginning of therapy, it is important to “prescribe” lifestyle changes that will facilitate the best possible outcomes.
Nonpharmaceutical prescriptions
Early in my career, people with alcohol use problems were told they needed to be substance free before they were candidates for antidepressants. While we no longer do that, it is still important to emphasize abstinence from addictive substances, and to recommend specific treatment when necessary.
Patients are often reluctant to see their use of alcohol, marijuana (it’s medical! It’s part of wellness!), or their pain medications as part of the problem, and this can be difficult. There have been times, after multiple medications have failed to help their symptoms, when I have said, “If you don’t get treatment for this problem, I am not going to be able to help you feel better” and that has been motivating for the patient.
There are other “prescriptions” to write. Regular sleep is essential for people with mood disorders, and this can be difficult for many patients, especially those who do shift work, or who have regular disruptions to their sleep from noise, pets, and children. Exercise is wonderful for the cardiovascular system, calms anxiety, and maintains strength, endurance, mobility, and quality of life as people age. But it can be a hard sell to people in a mental health crisis.
Nature is healing, and sunshine helps with maintaining circadian rhythms. For those who don’t exercise, I often “prescribe” 20 to 30 minutes a day of walking, preferably outside, during daylight hours, in a park or natural setting. For people with anxiety, it is important to check their caffeine consumption and to suggest ways to moderate it – moving to decaffeinated beverages or titrating down by mixing decaf with caffeinated.
Meditation is something that many people find helpful. For anxious people, it can be very difficult, and I will prescribe a specific instructional video course that I like on the well-being app InsightTimer – Sarah Blondin’s Learn How to Meditate in Seven Days. The sessions are approximately 10 minutes long, and that seems like the right amount of time for a beginner.
When people are very ill and don’t want to go into the hospital, I talk with them about things that happen in the hospital that are helpful, things they can try to mimic at home. In the hospital, patients don’t go to work, they don’t spend hours a day on the computer, and they are given a pass from dealing with the routine stresses of daily life.
I ask them to take time off work, to avoid as much stress as possible, to spend time with loved ones who give them comfort, and to avoid the people who leave them feeling drained or distressed. I ask them to engage in activities they find healing, to eat well, exercise, and avoid social media. In the hospital, I emphasize, they wake patients up in the morning, ask them to get out of bed and engage in therapeutic activities. They are fed and kept from intoxicants.
When it comes to nutrition, we know so little about how food affects mental health. I feel like it can’t hurt to ask people to avoid fast foods, soft drinks, and processed foods, and so I do.
And what about compliance? Of course, not everyone complies; not everyone is interested in making changes and these can be hard changes. I’ve recently started to recommend the book Atomic Habits by James Clear. Sometimes a bit of motivational interviewing can also be helpful in getting people to look at slowly moving toward making changes.
In prescribing lifestyle changes, it is important to offer most of these changes as suggestions, not as things we insist on, or that will leave the patient feeling ashamed if he doesn’t follow through. They should be discussed early in treatment so that patients don’t feel blamed for their illness or relapses. As with all the things we prescribe, some of these behavior changes help some of the people some of the time. Suggesting them, however, makes the strong statement that treating psychiatric disorders can be about more than passively swallowing a pill.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is an assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore. She disclosed no relevant conflicts of interest.
In psychiatry, patients come to us with their list of symptoms, often a diagnosis they’ve made themselves, and the expectation that they will be given medication to fix their problem. Their diagnoses are often right on target – people often know if they are depressed or anxious, and Doctor Google may provide useful information.
Sometimes they want a specific medication, one they saw in a TV ad, or one that helped them in the past or has helped someone they know. As psychiatrists have focused more on their strengths as psychopharmacologists and less on psychotherapy, it gets easy for both the patient and the doctor to look to medication, cocktails, and titration as the only thing we do.
“My medicine stopped working,” is a line I commonly hear. Often the patient is on a complicated regimen that has been serving them well, and it seems unlikely that the five psychotropic medications they are taking have suddenly “stopped working.” An obvious exception is the SSRI “poop out” that can occur 6-12 months or more after beginning treatment. In addition, it’s important to make sure patients are taking their medications as prescribed, and that the generic formulations have not changed.
But as rates of mental illness increase, some of it spurred on by difficult times,
This is not to devalue our medications, but to help the patient see symptoms as having multiple factors and give them some means to intervene, in addition to medications. At the beginning of therapy, it is important to “prescribe” lifestyle changes that will facilitate the best possible outcomes.
Nonpharmaceutical prescriptions
Early in my career, people with alcohol use problems were told they needed to be substance free before they were candidates for antidepressants. While we no longer do that, it is still important to emphasize abstinence from addictive substances, and to recommend specific treatment when necessary.
Patients are often reluctant to see their use of alcohol, marijuana (it’s medical! It’s part of wellness!), or their pain medications as part of the problem, and this can be difficult. There have been times, after multiple medications have failed to help their symptoms, when I have said, “If you don’t get treatment for this problem, I am not going to be able to help you feel better” and that has been motivating for the patient.
There are other “prescriptions” to write. Regular sleep is essential for people with mood disorders, and this can be difficult for many patients, especially those who do shift work, or who have regular disruptions to their sleep from noise, pets, and children. Exercise is wonderful for the cardiovascular system, calms anxiety, and maintains strength, endurance, mobility, and quality of life as people age. But it can be a hard sell to people in a mental health crisis.
Nature is healing, and sunshine helps with maintaining circadian rhythms. For those who don’t exercise, I often “prescribe” 20 to 30 minutes a day of walking, preferably outside, during daylight hours, in a park or natural setting. For people with anxiety, it is important to check their caffeine consumption and to suggest ways to moderate it – moving to decaffeinated beverages or titrating down by mixing decaf with caffeinated.
Meditation is something that many people find helpful. For anxious people, it can be very difficult, and I will prescribe a specific instructional video course that I like on the well-being app InsightTimer – Sarah Blondin’s Learn How to Meditate in Seven Days. The sessions are approximately 10 minutes long, and that seems like the right amount of time for a beginner.
When people are very ill and don’t want to go into the hospital, I talk with them about things that happen in the hospital that are helpful, things they can try to mimic at home. In the hospital, patients don’t go to work, they don’t spend hours a day on the computer, and they are given a pass from dealing with the routine stresses of daily life.
I ask them to take time off work, to avoid as much stress as possible, to spend time with loved ones who give them comfort, and to avoid the people who leave them feeling drained or distressed. I ask them to engage in activities they find healing, to eat well, exercise, and avoid social media. In the hospital, I emphasize, they wake patients up in the morning, ask them to get out of bed and engage in therapeutic activities. They are fed and kept from intoxicants.
When it comes to nutrition, we know so little about how food affects mental health. I feel like it can’t hurt to ask people to avoid fast foods, soft drinks, and processed foods, and so I do.
And what about compliance? Of course, not everyone complies; not everyone is interested in making changes and these can be hard changes. I’ve recently started to recommend the book Atomic Habits by James Clear. Sometimes a bit of motivational interviewing can also be helpful in getting people to look at slowly moving toward making changes.
In prescribing lifestyle changes, it is important to offer most of these changes as suggestions, not as things we insist on, or that will leave the patient feeling ashamed if he doesn’t follow through. They should be discussed early in treatment so that patients don’t feel blamed for their illness or relapses. As with all the things we prescribe, some of these behavior changes help some of the people some of the time. Suggesting them, however, makes the strong statement that treating psychiatric disorders can be about more than passively swallowing a pill.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is an assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore. She disclosed no relevant conflicts of interest.
In psychiatry, patients come to us with their list of symptoms, often a diagnosis they’ve made themselves, and the expectation that they will be given medication to fix their problem. Their diagnoses are often right on target – people often know if they are depressed or anxious, and Doctor Google may provide useful information.
Sometimes they want a specific medication, one they saw in a TV ad, or one that helped them in the past or has helped someone they know. As psychiatrists have focused more on their strengths as psychopharmacologists and less on psychotherapy, it gets easy for both the patient and the doctor to look to medication, cocktails, and titration as the only thing we do.
“My medicine stopped working,” is a line I commonly hear. Often the patient is on a complicated regimen that has been serving them well, and it seems unlikely that the five psychotropic medications they are taking have suddenly “stopped working.” An obvious exception is the SSRI “poop out” that can occur 6-12 months or more after beginning treatment. In addition, it’s important to make sure patients are taking their medications as prescribed, and that the generic formulations have not changed.
But as rates of mental illness increase, some of it spurred on by difficult times,
This is not to devalue our medications, but to help the patient see symptoms as having multiple factors and give them some means to intervene, in addition to medications. At the beginning of therapy, it is important to “prescribe” lifestyle changes that will facilitate the best possible outcomes.
Nonpharmaceutical prescriptions
Early in my career, people with alcohol use problems were told they needed to be substance free before they were candidates for antidepressants. While we no longer do that, it is still important to emphasize abstinence from addictive substances, and to recommend specific treatment when necessary.
Patients are often reluctant to see their use of alcohol, marijuana (it’s medical! It’s part of wellness!), or their pain medications as part of the problem, and this can be difficult. There have been times, after multiple medications have failed to help their symptoms, when I have said, “If you don’t get treatment for this problem, I am not going to be able to help you feel better” and that has been motivating for the patient.
There are other “prescriptions” to write. Regular sleep is essential for people with mood disorders, and this can be difficult for many patients, especially those who do shift work, or who have regular disruptions to their sleep from noise, pets, and children. Exercise is wonderful for the cardiovascular system, calms anxiety, and maintains strength, endurance, mobility, and quality of life as people age. But it can be a hard sell to people in a mental health crisis.
Nature is healing, and sunshine helps with maintaining circadian rhythms. For those who don’t exercise, I often “prescribe” 20 to 30 minutes a day of walking, preferably outside, during daylight hours, in a park or natural setting. For people with anxiety, it is important to check their caffeine consumption and to suggest ways to moderate it – moving to decaffeinated beverages or titrating down by mixing decaf with caffeinated.
Meditation is something that many people find helpful. For anxious people, it can be very difficult, and I will prescribe a specific instructional video course that I like on the well-being app InsightTimer – Sarah Blondin’s Learn How to Meditate in Seven Days. The sessions are approximately 10 minutes long, and that seems like the right amount of time for a beginner.
When people are very ill and don’t want to go into the hospital, I talk with them about things that happen in the hospital that are helpful, things they can try to mimic at home. In the hospital, patients don’t go to work, they don’t spend hours a day on the computer, and they are given a pass from dealing with the routine stresses of daily life.
I ask them to take time off work, to avoid as much stress as possible, to spend time with loved ones who give them comfort, and to avoid the people who leave them feeling drained or distressed. I ask them to engage in activities they find healing, to eat well, exercise, and avoid social media. In the hospital, I emphasize, they wake patients up in the morning, ask them to get out of bed and engage in therapeutic activities. They are fed and kept from intoxicants.
When it comes to nutrition, we know so little about how food affects mental health. I feel like it can’t hurt to ask people to avoid fast foods, soft drinks, and processed foods, and so I do.
And what about compliance? Of course, not everyone complies; not everyone is interested in making changes and these can be hard changes. I’ve recently started to recommend the book Atomic Habits by James Clear. Sometimes a bit of motivational interviewing can also be helpful in getting people to look at slowly moving toward making changes.
In prescribing lifestyle changes, it is important to offer most of these changes as suggestions, not as things we insist on, or that will leave the patient feeling ashamed if he doesn’t follow through. They should be discussed early in treatment so that patients don’t feel blamed for their illness or relapses. As with all the things we prescribe, some of these behavior changes help some of the people some of the time. Suggesting them, however, makes the strong statement that treating psychiatric disorders can be about more than passively swallowing a pill.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is an assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore. She disclosed no relevant conflicts of interest.
Burnout among surgeons: Lessons for psychiatrists
Burnout is an occupational phenomenon and a syndrome resulting from unsuccessfully managed chronic workplace stress. The characteristic features of burnout include feelings of exhaustion, cynicism, and reduced professional efficacy.1 A career in surgery is associated with demanding and unpredictable work hours in a high-stress environment.2-8 Research indicates that surgeons are at an elevated risk for developing burnout and mental health problems that can compromise patient care. A survey of the fellows of the American College of Surgeons found that 40% of surgeons experience burnout, 30% experience symptoms of depression, and 28% have a mental quality of life (QOL) score greater than one-half an SD below the population norm.9,10 Surgeon burnout was also found to compromise the delivery of medical care.9,10
To prevent serious harm to surgeons and patients, it is critical to understand the causative factors of burnout among surgeons and how they can be addressed. We conducted this systematic review to identify factors linked to burnout across surgical specialties and to suggest ways to mitigate these risk factors.
Methods
To identify studies of burnout among surgeons, we conducted an electronic search of Ovid MEDLINE, Ovid PsycInfo, SCOPUS, Cochrane Database of Systematic Reviews, and Cochrane Central Register of Controlled Trials. The headings and keywords used are listed in Supplemental Table 1. Studies met the inclusion criteria if they evaluated residents or attendings, used a tool to measure burnout, and examined any surgical specialty. Studies were excluded if they were published before 2010; were conducted outside the United States; were review articles, commentaries, or abstracts without full text articles; evaluated medical school students; were published in a language other than English; did not use a tool to measure burnout; or examined a nonsurgical specialty. Our analysis was guided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)11 and is outlined in the Supplemental Figure.
Results
Surgical specialties and burnout
We identified 56 studies2-10,12-58 that focused on specific surgical specialties in relation to burnout. Supplemental Table 22-10,12-58 lists these studies and the surgical specialties they evaluated.
Work/life balance factors
Fifteen studies
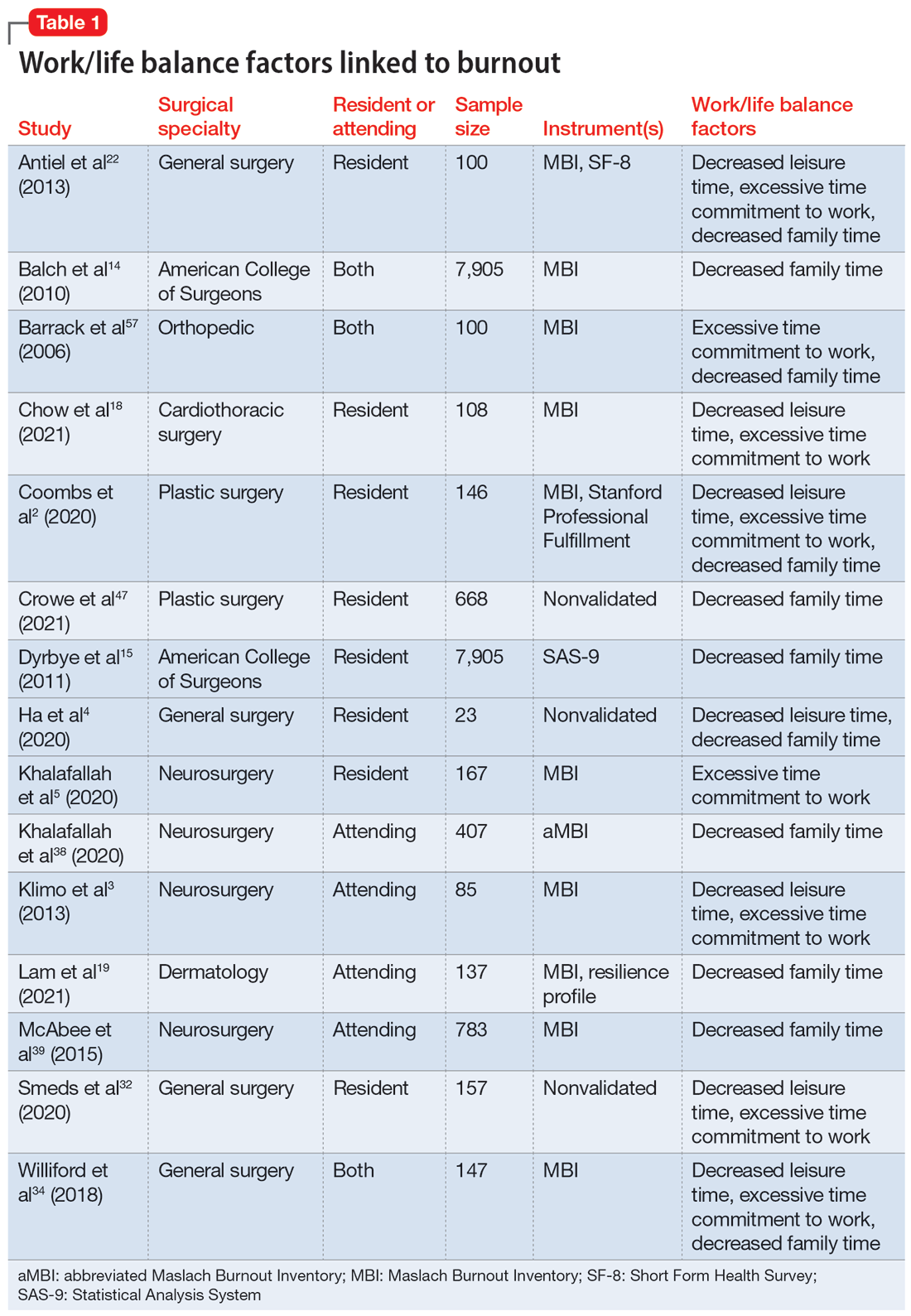
Work hours
Fifteen studies2,7,14,20,21,30,34,41,42,44-46,50,52,56 examined work hours and burnout. Of these, 142,7,14,20,21,30,34,42,44-46,50,52,56 found a correlation between increased work hours and burnout, while only 1 study41 found no correlation between these factors.
Medical errors
Six studies2,14,18,43,49,52 discussed the role of burnout in medical errors. Of these, 52,14,43,49,52 reported a correlation between burnout and medical errors, while 1 study18 found no link between burnout and medical errors. The medical errors were self-reported.14,49 They included actions that resulted in patient harm, sample collection error, and errors in medication orders and laboratory test orders.2
Continue to: Institutional and organizational factors
Institutional and organizational factors
Eighteen studies3,13,14,18,20,22,23,29,30,36-38,44,45,47,54,56,57 examined how different organizational factors play a role in burnout. Four studies3,13,20,37 discussed administrative/bureaucratic work, 420,45,54,57 mentioned electronic medical documentation, 222,30 covered duty hour regulations, 318,45,57 discussed mistreatment of physicians, and 613,18,23,44,47,56 described the importance of workplace support in addressing burnout.
Physical and mental health factors
Eighteen studies6,7,14,15,17,20,26,27,29,34,43,44,48,52,54,57-59 discussed aspects of physical and mental health linked to burnout. Among these, 334,43,59 discussed the importance of physical health and focused on how improving physical health can reduce stress and burnout. Three studies6,17,58 noted the prevalence of suicidal ideation in both residents and attendings experiencing prolonged burnout. Five studies26,29,43,44,48 described the systematic barriers that inhibit physicians from getting professional help. Two studies7,27 reported marital status as a factor for burnout; participants who were single reported higher levels of depression and suicidal ideation. Five studies6,14,15,54,57 outlined how depression is associated with burnout.
Strategies to mitigate burnout
Fifteen studies
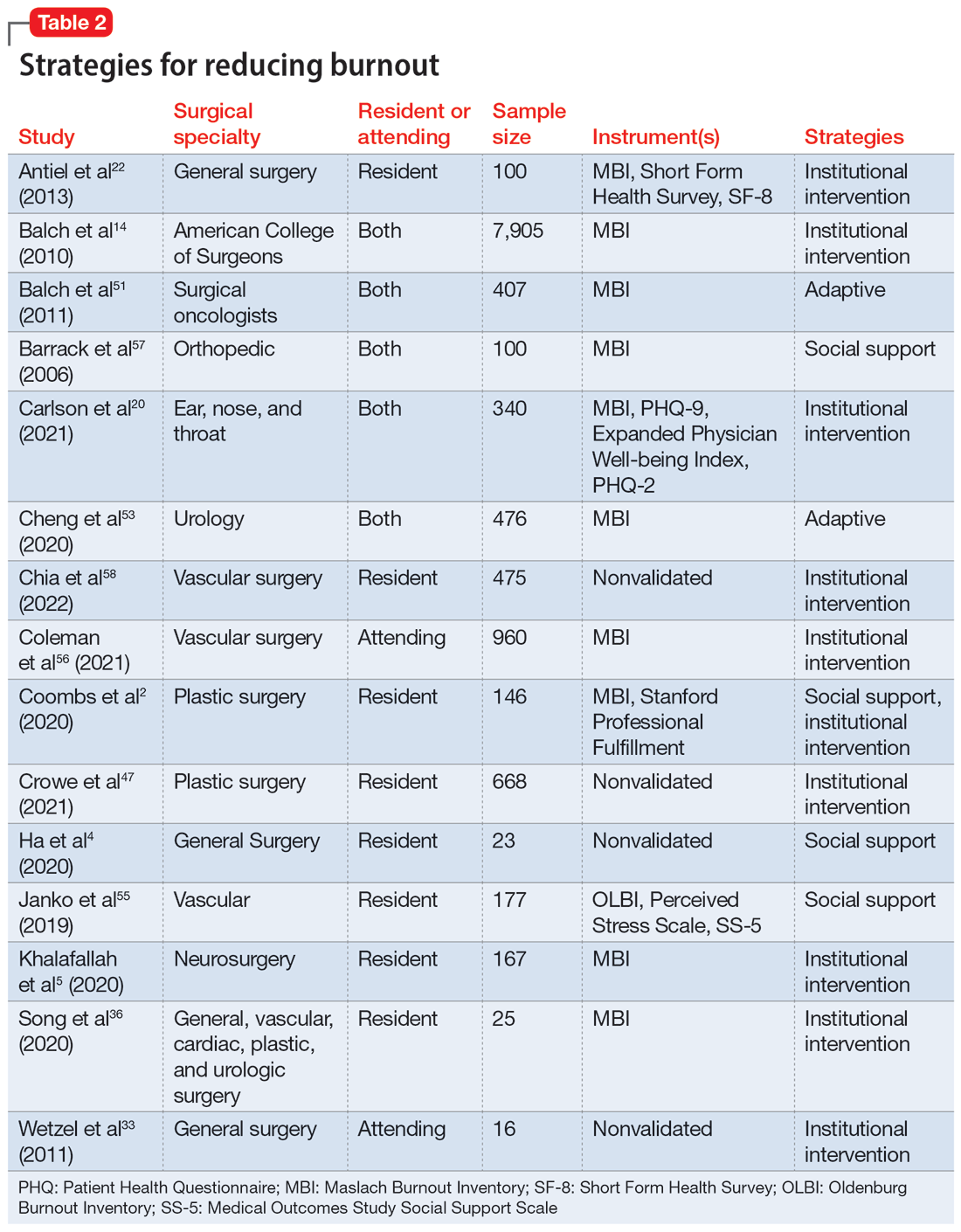
Take-home points
Research that focused on work/life balance and burnout found excessive time commitment to work is a major factor associated with poor work/life balance. Residents who worked >80 hours a week had a significantly higher burnout rate.56 One study found that 70% of residents reported not getting enough sleep, 30% reported not having enough energy for relationships, and 39% reported that they were not eating or exercising due to time constraints.4 A high correlation was found between the number of hours worked per week and rates of burnout, emotional exhaustion, and depersonalization. Emotional exhaustion and depersonalization are aspects of burnout measured by the Maslach Burnout Inventory (MBI).24 The excessive time commitment to work not only contributes to burnout but also prevents physicians from getting professional help. In 1 study, both residents (56%) and attendings (24%) reported that 1 of the biggest barriers to getting help for their burnout symptoms was the inability to take time off.34 Research indicates that the hours worked per week and work/home conflicts were independently associated with burnout and career satisfaction.15 A decrease of weekly work hours may give physicians time to meet their responsibilities at work and home, allowing for a decrease in burnout and an increase in career satisfaction.
Increased work hours have also been found to be correlated with medical errors. One study found that those who worked 60 hours per week were significantly less likely to report any major medical errors in the previous 3 months compared with those who worked 80 hours per week.9 The risk for the number of medical errors has been reported as being 2-fold if surgeons are unable to combat the burnout.49 On the other hand, a positive and supportive environment with easy access to resources to combat burnout and burnout prevention programs can reduce the frequency of medical errors, which also can reduce the risk of malpractice, thus further reducing stress and burnout.43
Continue to: In response to resident complaints...
In response to resident complaints about long duty hours, a new rule has been implemented that states residents cannot work >16 hours per shift.30 This rule has been found to increase quality of life and prevent burnout.30
The amount of time spent on electronic medical records and documentation has been a major complaint from doctors and was identified as a factor contributing to burnout.45 It can act as a time drain that impedes the physician from providing optimal patient care and cause additional stress. This suggests the need for organizations to find solutions to minimize this strain.
A concerning issue reported as an institutional factor and associated with burnout is mistreatment through discrimination, harassment, and physical or verbal abuse. A recent study found 45% of general and vascular surgeons reported being mistreated in some fashion.57 The strategies reported as helpful for institutions to combat mistreatment include resilience training, improved mentorship, and implicit bias training.57
Burnout has been positively correlated with anxiety and depression.6 A recent study reported that 13% of orthopedic surgery residents screened positive for depression.44 Higher levels of burnout and depersonalization have been found to be closely associated with increased rates of suicidal ideation.17 In a study of vascular surgeons, 8% were found to report suicidal ideation, and this increased to 15% among vascular surgeons who had higher levels of depersonalization and emotional exhaustion,58 both of which are associated with burnout. In another study, surgery residents and fellows were found to have lower levels of personal achievement and higher levels of depersonalization, depressive symptoms, alcohol abuse, and suicidal ideation compared to attending physicians and the general population.54 These findings spell out the association between burnout and depressive symptoms among surgeons and emphasize the need for institutions to create a culture that supports the mental health needs of their physicians. Without access to supportive resources, residents resort to alternative methods that may be detrimental in the long run. In a recent study, 17% of residents admitted to using alcohol, including binge drinking, to cope with their stress.4
Burnout and depression are linked to physical health risks such as cardiovascular disease, diabetes, substance abuse, and male infertility.6 Exercise has been shown to be beneficial for stress reduction, which can lead to changes in metabolism, inflammation, coagulation, and autonomic function.6 One study of surgeons found aerobic exercise and strength training were associated with lower rates of burnout and a higher quality of life.59
Continue to: The amount of burnout physicians...
The amount of burnout physicians experience can be determined by how they respond to adversities. Adaptive behaviors such as socializing, mindfulness, volunteering, and exercising have been found to be protective against burnout.6,37,54 Resilience training and maintaining low stress at work can decrease burnout.37 These findings highlight the need for physicians to be trained in the appropriate ways to combat their burnout symptoms.
Unfortunately, seeking help by health care professionals to improve mental health has been stigmatized, causing physicians to not seek help and instead resort to other ways to cope with their distress.26,34 While some of these coping methods may be positive, others—such as substance abuse or stress eating—can be maladaptive, leading to a poor quality of life, and in some cases, suicide.54 It is vital that effective mental health services become more accessible and for health care professionals to become aware of their maladaptive behaviors.34
Institutions finding ways to ease the path for their physicians to seek professional help to combat burnout may mitigate its negative impact. One strategy is to embed access to mental health services within regular wellness checks. Institutions can use wellness checks to provide resources to physicians who need it. These interventions have been found to be effective because they give physicians a safe space to seek help and become aware of any factors that could lead to burnout.18 Apart from these direct attempts to combat burnout, program-sponsored social events would also promote social connectedness with colleagues and contribute to a sense of well-being that could help decrease levels of burnout and depression.13 Mentorship has been shown to play a crucial role in decreasing burnout among residents. One study that examined the role of mentorship reported that 55% of residents felt supported, and of these, 96% felt mentorship was critical to their success.18 The role of institutions in helping to improve the well-being of surgeons is highlighted by the finding that increasing workplace support results in psychological resilience that can mitigate burnout at its roots.29
Bottom Line
Surgeons are at risk for burnout, which can impact their mental health and reduce their professional efficacy. Both institutions and surgeons themselves can take action to prevent burnout and treat burnout early when it occurs.
Related Resources
- Pahal P, Lippmann S. Building a better work/life balance. Current Psychiatry. 2021;20(8):e1-e2. doi:10.12788/cp.0158
- Gibson R, Hategan A. Physician burnout vs depression: recognize the signs. Current Psychiatry. 2019;18(10):41-42.
1. World Health Organization. International Statistical Classification of Diseases and Related Health Problems (ICD). 11th ed. World Health Organization; 2019.
2. Coombs DM, Lanni MA, Fosnot J, et al. Professional burnout in United States plastic surgery residents: is it a legitimate concern? Aesthet Surg J. 2020;40(7):802-810.
3. Klimo P Jr, DeCuypere M, Ragel BT, et al. Career satisfaction and burnout among U.S. neurosurgeons: a feasibility and pilot study. World Neurosurg. 2013;80(5):e59-e68.
4. Ha GQ, Go JT, Murayama KM, et al. Identifying sources of stress across years of general surgery residency. Hawaii J Health Soc Welf. 2020;79(3):75-81.
5. Khalafallah AM, Lam S, Gami A, et al. A national survey on the impact of the COVID-19 pandemic upon burnout and career satisfaction among neurosurgery residents. J Clin Neurosci. 2020;80:137-142.
6. Al-Humadi SM, Cáceda R, Bronson B, et al. Orthopaedic surgeon mental health during the COVID-19 pandemic. Geriatric Orthop Surg Rehabil. 2021;12:21514593211035230.
7. Larson DP, Carlson ML, Lohse CM, et al. Prevalence of and associations with distress and professional burnout among otolaryngologists: part I, trainees. Otolaryngol Head Neck Surg. 2021;164(5):1019-1029.
8. Streu R, Hawley S, Gay A, et al. Satisfaction with career choice among U.S. plastic surgeons: results from a national survey. Plast Reconstr Surg. 2010;126(2):636-642.
9. Shanafelt TD, Balch CM, Bechamps GJ, et al. Burnout and career satisfaction among American surgeons. Ann Surg. 2009;250(3):463-471.
10. Shanafelt TD, Balch CM, Bechamps G, et al. Burnout and medical errors among American surgeons. Ann Surg. 2010;251(6):995-1000.
11. Moher D, Liberati A, Tetzlaff J, et al; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336-341.
12. Yesantharao P, Lee E, Kraenzlin F, et al. Surgical block time satisfaction: a multi-institutional experience across twelve surgical disciplines. Perioperative Care Operating Room Manage. 2020;21:100128.
13. Nituica C, Bota OA, Blebea J. Specialty differences in resident resilience and burnout - a national survey. Am J Surg. 2021;222(2):319-328.
14. Balch CM, Shanafelt TD, Dyrbye L, et al. Surgeon distress as calibrated by hours worked and nights on call. J Am Coll Surg. 2010;211(5):609-619.
15. Dyrbye LN, Shanafelt TD, Balch CM, Satele D, Sloan J, Freischlag J. Relationship between work-home conflicts and burnout among American surgeons: a comparison by sex. Arch Surg. 2011;146(2):211-217.
16. Mahoney ST, Irish W, Strassle PD, et al. Practice characteristics and job satisfaction of private practice and academic surgeons. JAMA Surg. 2021;156(3):247-254.
17. Shanafelt TD, Balch CM, Dyrbye L, et al. Special report: suicidal ideation among American surgeons. Arch Surg. 2011;146(1):54-62.
18. Chow OS, Sudarshan M, Maxfield MW, et al. National survey of burnout and distress among cardiothoracic surgery trainees. Ann Thorac Surg. 2021;111(6):2066-2071.
19. Lam C, Kim Y, Cruz M, et al. Burnout and resiliency in Mohs surgeons: a survey study. Int J Womens Dermatol. 2021;7(3):319-322.
20. Carlson ML, Larson DP, O’Brien EK, et al. Prevalence of and associations with distress and professional burnout among otolaryngologists: part II, attending physicians. Otolaryngol Head Neck Surg. 2021;164(5):1030-1039.
21. Nida AM, Googe BJ, Lewis AF, et al. Resident fatigue in otolaryngology residents: a Web based survey. Am J Otolaryngol. 2016;37(3):210-216.
22. Antiel RM, Reed DA, Van Arendonk KJ, et al. Effects of duty hour restrictions on core competencies, education, quality of life, and burnout among general surgery interns. JAMA Surg. 2013;148(5):448-455.
23. Appelbaum NP, Lee N, Amendola M, et al. Surgical resident burnout and job satisfaction: the role of workplace climate and perceived support. J Surg Res. 2019;234:20-25.
24. Elmore LC, Jeffe DB, Jin L, et al. National survey of burnout among US general surgery residents. J Am Coll Surg. 2016;223(3):440-451.
25. Garcia DI, Pannuccio A, Gallegos J, et al. Resident-driven wellness initiatives improve resident wellness and perception of work environment. J Surg Res. 2021;258:8-16.
26. Hochberg MS, Berman RS, Kalet AL, et al. The stress of residency: recognizing the signs of depression and suicide in you and your fellow residents. Am J Surg. 2013;205(2):141-146.
27. Kurbatov V, Shaughnessy M, Baratta V, et al. Application of advanced bioinformatics to understand and predict burnout among surgical trainees. J Surg Educ. 2020;77(3):499-507.
28. Leach PK, Nygaard RM, Chipman JG, et al. Impostor phenomenon and burnout in general surgeons and general surgery residents. J Surg Educ. 2019;76(1):99-106.
29. Lebares CC, Greenberg AL, Ascher NL, et al. Exploration of individual and system-level well-being initiatives at an academic surgical residency program: a mixed-methods study. JAMA Netw Open. 2021;4(1):e2032676.
30. Lindeman BM, Sacks BC, Hirose K, et al. Multifaceted longitudinal study of surgical resident education, quality of life, and patient care before and after July 2011. J Surg Educ. 2013;70(6):769-776.
31. Rasmussen JM, Najarian MM, Ties JS, et al. Career satisfaction, gender bias, and work-life balance: a contemporary assessment of general surgeons. J Surg Educ. 2021;78(1):119-125.
32. Smeds MR, Janko MR, Allen S, et al. Burnout and its relationship with perceived stress, self-efficacy, depression, social support, and programmatic factors in general surgery residents. Am J Surg. 2020;219(6):907-912.
33. Wetzel CM, George A, Hanna GB, et al. Stress management training for surgeons--a randomized, controlled, intervention study. Ann Surg. 2011;253(3):488-494.
34. Williford ML, Scarlet S, Meyers MO, et al. Multiple-institution comparison of resident and faculty perceptions of burnout and depression during surgical training. JAMA Surg. 2018;153(8):705-711.
35. Zubair MH, Hussain LR, Williams KN, et al. Work-related quality of life of US general surgery residents: is it really so bad? J Surg Educ. 2017;74(6):e138-e146.
36. Song Y, Swendiman RA, Shannon AB, et al. Can we coach resilience? An evaluation of professional resilience coaching as a well-being initiative for surgical interns. J Surg Educ. 2020;77(6):1481-1489.
37. Morrell NT, Sears ED, Desai MJ, et al. A survey of burnout among members of the American Society for Surgery of the Hand. J Hand Surg Am. 2020;45(7):573-581.e516.
38. Khalafallah AM, Lam S, Gami A, et al. Burnout and career satisfaction among attending neurosurgeons during the COVID-19 pandemic. Clin Neurol Neurosurg. 2020;198:106193.
39. McAbee JH, Ragel BT, McCartney S, et al. Factors associated with career satisfaction and burnout among US neurosurgeons: results of a nationwide survey. J Neurosurg. 2015;123(1):161-173.
40. Shakir HJ, McPheeters MJ, Shallwani H, et al. The prevalence of burnout among US neurosurgery residents. Neurosurgery. 2018;83(3):582-590.
41. Govardhan LM, Pinelli V, Schnatz PF. Burnout, depression and job satisfaction in obstetrics and gynecology residents. Conn Med. 2012;76(7):389-395.
42. Driesman AS, Strauss EJ, Konda SR, et al. Factors associated with orthopaedic resident burnout: a pilot study. J Am Acad Orthop Surg. 2020;28(21):900-906.
43. Lichstein PM, He JK, Estok D, et al. What is the prevalence of burnout, depression, and substance use among orthopaedic surgery residents and what are the risk factors? A collaborative orthopaedic educational research group survey study. Clin Orthop Relat Res. 2020;478(8):1709-1718.
44. Somerson JS, Patton A, Ahmed AA, et al. Burnout among United States orthopaedic surgery residents. J Surg Educ. 2020;77(4):961-968.
45. Verret CI, Nguyen J, Verret C, et al. How do areas of work life drive burnout in orthopaedic attending surgeons, fellows, and residents? Clin Orthop Relat Res. 2021;479(2):251-262.
46. Sarosi A, Coakley BA, Berman L, et al. A cross-sectional analysis of compassion fatigue, burnout, and compassion satisfaction in pediatric surgeons in the U.S. J Pediatr Surg. 2021;56(8):1276-1284.
47. Crowe CS, Lopez J, Morrison SD, et al. The effects of the COVID-19 pandemic on resident education and wellness: a national survey of plastic surgery residents. Plast Reconstr Surg. 2021;148(3):462e-474e.
48. Qureshi HA, Rawlani R, Mioton LM, et al. Burnout phenomenon in U.S. plastic surgeons: risk factors and impact on quality of life. Plast Reconstr Surg. 2015;135(2):619-626.
49. Streu R, Hansen J, Abrahamse P, et al. Professional burnout among US plastic surgeons: results of a national survey. Ann Plast Surg. 2014;72(3):346-350.
50. Zhang JQ, Riba L, Magrini L, ET AL. Assessing burnout and professional fulfillment in breast surgery: results from a national survey of the American Society of Breast Surgeons. Ann Surg Oncol. 2019;26(10):3089-3098.
51. Balch CM, Shanafelt TD, Sloan J, et al. Burnout and career satisfaction among surgical oncologists compared with other surgical specialties. Ann Surg Oncol. 2011;18(1):16-25.
52. Wu D, Gross B, Rittenhouse K, et al. A preliminary analysis of compassion fatigue in a surgeon population: are female surgeons at heightened risk? Am Surg. 2017;83(11):1302-1307.
53. Cheng JW, Wagner H, Hernandez BC, et al. Stressors and coping mechanisms related to burnout within urology. Urology. 2020;139:27-36.
54. Koo K, Javier-DesLoges JF, Fang R, ET AL. Professional burnout, career choice regret, and unmet needs for well-being among urology residents. Urology. 2021;157:57-63.
55. Janko MR, Smeds MR. Burnout, depression, perceived stress, and self-efficacy in vascular surgery trainees. J Vasc Surg. 2019;69(4):1233-1242.
56. Coleman DM, Money SR, Meltzer AJ, et al. Vascular surgeon wellness and burnout: a report from the Society for Vascular Surgery Wellness Task Force. J Vasc Surg. 2021;73(6):1841-1850.e3.
57. Barrack RL, Miller LS, Sotile WM, et al. Effect of duty hour standards on burnout among orthopaedic surgery residents. Clin Orthop Relat Res. 2006;449:134-137.
58. Chia MC, Hu YY, Li RD, et al. Prevalence and risk factors for burnout in U.S. vascular surgery trainees. J Vasc Surg. 2022;75(1):308-315.e4.
59. Shanafelt TD, Oreskovich MR, Dyrbye LN, et al. Avoiding burnout: the personal health habits and wellness practices of US surgeons. Ann Surg. 2012;255(4):625-633.
Burnout is an occupational phenomenon and a syndrome resulting from unsuccessfully managed chronic workplace stress. The characteristic features of burnout include feelings of exhaustion, cynicism, and reduced professional efficacy.1 A career in surgery is associated with demanding and unpredictable work hours in a high-stress environment.2-8 Research indicates that surgeons are at an elevated risk for developing burnout and mental health problems that can compromise patient care. A survey of the fellows of the American College of Surgeons found that 40% of surgeons experience burnout, 30% experience symptoms of depression, and 28% have a mental quality of life (QOL) score greater than one-half an SD below the population norm.9,10 Surgeon burnout was also found to compromise the delivery of medical care.9,10
To prevent serious harm to surgeons and patients, it is critical to understand the causative factors of burnout among surgeons and how they can be addressed. We conducted this systematic review to identify factors linked to burnout across surgical specialties and to suggest ways to mitigate these risk factors.

Methods
To identify studies of burnout among surgeons, we conducted an electronic search of Ovid MEDLINE, Ovid PsycInfo, SCOPUS, Cochrane Database of Systematic Reviews, and Cochrane Central Register of Controlled Trials. The headings and keywords used are listed in Supplemental Table 1. Studies met the inclusion criteria if they evaluated residents or attendings, used a tool to measure burnout, and examined any surgical specialty. Studies were excluded if they were published before 2010; were conducted outside the United States; were review articles, commentaries, or abstracts without full text articles; evaluated medical school students; were published in a language other than English; did not use a tool to measure burnout; or examined a nonsurgical specialty. Our analysis was guided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)11 and is outlined in the Supplemental Figure.

Results
Surgical specialties and burnout
We identified 56 studies2-10,12-58 that focused on specific surgical specialties in relation to burnout. Supplemental Table 22-10,12-58 lists these studies and the surgical specialties they evaluated.

Work/life balance factors
Fifteen studies

Work hours
Fifteen studies2,7,14,20,21,30,34,41,42,44-46,50,52,56 examined work hours and burnout. Of these, 142,7,14,20,21,30,34,42,44-46,50,52,56 found a correlation between increased work hours and burnout, while only 1 study41 found no correlation between these factors.
Medical errors
Six studies2,14,18,43,49,52 discussed the role of burnout in medical errors. Of these, 52,14,43,49,52 reported a correlation between burnout and medical errors, while 1 study18 found no link between burnout and medical errors. The medical errors were self-reported.14,49 They included actions that resulted in patient harm, sample collection error, and errors in medication orders and laboratory test orders.2
Continue to: Institutional and organizational factors
Institutional and organizational factors
Eighteen studies3,13,14,18,20,22,23,29,30,36-38,44,45,47,54,56,57 examined how different organizational factors play a role in burnout. Four studies3,13,20,37 discussed administrative/bureaucratic work, 420,45,54,57 mentioned electronic medical documentation, 222,30 covered duty hour regulations, 318,45,57 discussed mistreatment of physicians, and 613,18,23,44,47,56 described the importance of workplace support in addressing burnout.
Physical and mental health factors
Eighteen studies6,7,14,15,17,20,26,27,29,34,43,44,48,52,54,57-59 discussed aspects of physical and mental health linked to burnout. Among these, 334,43,59 discussed the importance of physical health and focused on how improving physical health can reduce stress and burnout. Three studies6,17,58 noted the prevalence of suicidal ideation in both residents and attendings experiencing prolonged burnout. Five studies26,29,43,44,48 described the systematic barriers that inhibit physicians from getting professional help. Two studies7,27 reported marital status as a factor for burnout; participants who were single reported higher levels of depression and suicidal ideation. Five studies6,14,15,54,57 outlined how depression is associated with burnout.
Strategies to mitigate burnout
Fifteen studies

Take-home points
Research that focused on work/life balance and burnout found excessive time commitment to work is a major factor associated with poor work/life balance. Residents who worked >80 hours a week had a significantly higher burnout rate.56 One study found that 70% of residents reported not getting enough sleep, 30% reported not having enough energy for relationships, and 39% reported that they were not eating or exercising due to time constraints.4 A high correlation was found between the number of hours worked per week and rates of burnout, emotional exhaustion, and depersonalization. Emotional exhaustion and depersonalization are aspects of burnout measured by the Maslach Burnout Inventory (MBI).24 The excessive time commitment to work not only contributes to burnout but also prevents physicians from getting professional help. In 1 study, both residents (56%) and attendings (24%) reported that 1 of the biggest barriers to getting help for their burnout symptoms was the inability to take time off.34 Research indicates that the hours worked per week and work/home conflicts were independently associated with burnout and career satisfaction.15 A decrease of weekly work hours may give physicians time to meet their responsibilities at work and home, allowing for a decrease in burnout and an increase in career satisfaction.
Increased work hours have also been found to be correlated with medical errors. One study found that those who worked 60 hours per week were significantly less likely to report any major medical errors in the previous 3 months compared with those who worked 80 hours per week.9 The risk for the number of medical errors has been reported as being 2-fold if surgeons are unable to combat the burnout.49 On the other hand, a positive and supportive environment with easy access to resources to combat burnout and burnout prevention programs can reduce the frequency of medical errors, which also can reduce the risk of malpractice, thus further reducing stress and burnout.43
Continue to: In response to resident complaints...
In response to resident complaints about long duty hours, a new rule has been implemented that states residents cannot work >16 hours per shift.30 This rule has been found to increase quality of life and prevent burnout.30
The amount of time spent on electronic medical records and documentation has been a major complaint from doctors and was identified as a factor contributing to burnout.45 It can act as a time drain that impedes the physician from providing optimal patient care and cause additional stress. This suggests the need for organizations to find solutions to minimize this strain.
A concerning issue reported as an institutional factor and associated with burnout is mistreatment through discrimination, harassment, and physical or verbal abuse. A recent study found 45% of general and vascular surgeons reported being mistreated in some fashion.57 The strategies reported as helpful for institutions to combat mistreatment include resilience training, improved mentorship, and implicit bias training.57
Burnout has been positively correlated with anxiety and depression.6 A recent study reported that 13% of orthopedic surgery residents screened positive for depression.44 Higher levels of burnout and depersonalization have been found to be closely associated with increased rates of suicidal ideation.17 In a study of vascular surgeons, 8% were found to report suicidal ideation, and this increased to 15% among vascular surgeons who had higher levels of depersonalization and emotional exhaustion,58 both of which are associated with burnout. In another study, surgery residents and fellows were found to have lower levels of personal achievement and higher levels of depersonalization, depressive symptoms, alcohol abuse, and suicidal ideation compared to attending physicians and the general population.54 These findings spell out the association between burnout and depressive symptoms among surgeons and emphasize the need for institutions to create a culture that supports the mental health needs of their physicians. Without access to supportive resources, residents resort to alternative methods that may be detrimental in the long run. In a recent study, 17% of residents admitted to using alcohol, including binge drinking, to cope with their stress.4
Burnout and depression are linked to physical health risks such as cardiovascular disease, diabetes, substance abuse, and male infertility.6 Exercise has been shown to be beneficial for stress reduction, which can lead to changes in metabolism, inflammation, coagulation, and autonomic function.6 One study of surgeons found aerobic exercise and strength training were associated with lower rates of burnout and a higher quality of life.59
Continue to: The amount of burnout physicians...
The amount of burnout physicians experience can be determined by how they respond to adversities. Adaptive behaviors such as socializing, mindfulness, volunteering, and exercising have been found to be protective against burnout.6,37,54 Resilience training and maintaining low stress at work can decrease burnout.37 These findings highlight the need for physicians to be trained in the appropriate ways to combat their burnout symptoms.
Unfortunately, seeking help by health care professionals to improve mental health has been stigmatized, causing physicians to not seek help and instead resort to other ways to cope with their distress.26,34 While some of these coping methods may be positive, others—such as substance abuse or stress eating—can be maladaptive, leading to a poor quality of life, and in some cases, suicide.54 It is vital that effective mental health services become more accessible and for health care professionals to become aware of their maladaptive behaviors.34
Institutions finding ways to ease the path for their physicians to seek professional help to combat burnout may mitigate its negative impact. One strategy is to embed access to mental health services within regular wellness checks. Institutions can use wellness checks to provide resources to physicians who need it. These interventions have been found to be effective because they give physicians a safe space to seek help and become aware of any factors that could lead to burnout.18 Apart from these direct attempts to combat burnout, program-sponsored social events would also promote social connectedness with colleagues and contribute to a sense of well-being that could help decrease levels of burnout and depression.13 Mentorship has been shown to play a crucial role in decreasing burnout among residents. One study that examined the role of mentorship reported that 55% of residents felt supported, and of these, 96% felt mentorship was critical to their success.18 The role of institutions in helping to improve the well-being of surgeons is highlighted by the finding that increasing workplace support results in psychological resilience that can mitigate burnout at its roots.29
Bottom Line
Surgeons are at risk for burnout, which can impact their mental health and reduce their professional efficacy. Both institutions and surgeons themselves can take action to prevent burnout and treat burnout early when it occurs.
Related Resources
- Pahal P, Lippmann S. Building a better work/life balance. Current Psychiatry. 2021;20(8):e1-e2. doi:10.12788/cp.0158
- Gibson R, Hategan A. Physician burnout vs depression: recognize the signs. Current Psychiatry. 2019;18(10):41-42.
Burnout is an occupational phenomenon and a syndrome resulting from unsuccessfully managed chronic workplace stress. The characteristic features of burnout include feelings of exhaustion, cynicism, and reduced professional efficacy.1 A career in surgery is associated with demanding and unpredictable work hours in a high-stress environment.2-8 Research indicates that surgeons are at an elevated risk for developing burnout and mental health problems that can compromise patient care. A survey of the fellows of the American College of Surgeons found that 40% of surgeons experience burnout, 30% experience symptoms of depression, and 28% have a mental quality of life (QOL) score greater than one-half an SD below the population norm.9,10 Surgeon burnout was also found to compromise the delivery of medical care.9,10
To prevent serious harm to surgeons and patients, it is critical to understand the causative factors of burnout among surgeons and how they can be addressed. We conducted this systematic review to identify factors linked to burnout across surgical specialties and to suggest ways to mitigate these risk factors.

Methods
To identify studies of burnout among surgeons, we conducted an electronic search of Ovid MEDLINE, Ovid PsycInfo, SCOPUS, Cochrane Database of Systematic Reviews, and Cochrane Central Register of Controlled Trials. The headings and keywords used are listed in Supplemental Table 1. Studies met the inclusion criteria if they evaluated residents or attendings, used a tool to measure burnout, and examined any surgical specialty. Studies were excluded if they were published before 2010; were conducted outside the United States; were review articles, commentaries, or abstracts without full text articles; evaluated medical school students; were published in a language other than English; did not use a tool to measure burnout; or examined a nonsurgical specialty. Our analysis was guided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)11 and is outlined in the Supplemental Figure.

Results
Surgical specialties and burnout
We identified 56 studies2-10,12-58 that focused on specific surgical specialties in relation to burnout. Supplemental Table 22-10,12-58 lists these studies and the surgical specialties they evaluated.

Work/life balance factors
Fifteen studies

Work hours
Fifteen studies2,7,14,20,21,30,34,41,42,44-46,50,52,56 examined work hours and burnout. Of these, 142,7,14,20,21,30,34,42,44-46,50,52,56 found a correlation between increased work hours and burnout, while only 1 study41 found no correlation between these factors.
Medical errors
Six studies2,14,18,43,49,52 discussed the role of burnout in medical errors. Of these, 52,14,43,49,52 reported a correlation between burnout and medical errors, while 1 study18 found no link between burnout and medical errors. The medical errors were self-reported.14,49 They included actions that resulted in patient harm, sample collection error, and errors in medication orders and laboratory test orders.2
Continue to: Institutional and organizational factors
Institutional and organizational factors
Eighteen studies3,13,14,18,20,22,23,29,30,36-38,44,45,47,54,56,57 examined how different organizational factors play a role in burnout. Four studies3,13,20,37 discussed administrative/bureaucratic work, 420,45,54,57 mentioned electronic medical documentation, 222,30 covered duty hour regulations, 318,45,57 discussed mistreatment of physicians, and 613,18,23,44,47,56 described the importance of workplace support in addressing burnout.
Physical and mental health factors
Eighteen studies6,7,14,15,17,20,26,27,29,34,43,44,48,52,54,57-59 discussed aspects of physical and mental health linked to burnout. Among these, 334,43,59 discussed the importance of physical health and focused on how improving physical health can reduce stress and burnout. Three studies6,17,58 noted the prevalence of suicidal ideation in both residents and attendings experiencing prolonged burnout. Five studies26,29,43,44,48 described the systematic barriers that inhibit physicians from getting professional help. Two studies7,27 reported marital status as a factor for burnout; participants who were single reported higher levels of depression and suicidal ideation. Five studies6,14,15,54,57 outlined how depression is associated with burnout.
Strategies to mitigate burnout
Fifteen studies

Take-home points
Research that focused on work/life balance and burnout found excessive time commitment to work is a major factor associated with poor work/life balance. Residents who worked >80 hours a week had a significantly higher burnout rate.56 One study found that 70% of residents reported not getting enough sleep, 30% reported not having enough energy for relationships, and 39% reported that they were not eating or exercising due to time constraints.4 A high correlation was found between the number of hours worked per week and rates of burnout, emotional exhaustion, and depersonalization. Emotional exhaustion and depersonalization are aspects of burnout measured by the Maslach Burnout Inventory (MBI).24 The excessive time commitment to work not only contributes to burnout but also prevents physicians from getting professional help. In 1 study, both residents (56%) and attendings (24%) reported that 1 of the biggest barriers to getting help for their burnout symptoms was the inability to take time off.34 Research indicates that the hours worked per week and work/home conflicts were independently associated with burnout and career satisfaction.15 A decrease of weekly work hours may give physicians time to meet their responsibilities at work and home, allowing for a decrease in burnout and an increase in career satisfaction.
Increased work hours have also been found to be correlated with medical errors. One study found that those who worked 60 hours per week were significantly less likely to report any major medical errors in the previous 3 months compared with those who worked 80 hours per week.9 The risk for the number of medical errors has been reported as being 2-fold if surgeons are unable to combat the burnout.49 On the other hand, a positive and supportive environment with easy access to resources to combat burnout and burnout prevention programs can reduce the frequency of medical errors, which also can reduce the risk of malpractice, thus further reducing stress and burnout.43
Continue to: In response to resident complaints...
In response to resident complaints about long duty hours, a new rule has been implemented that states residents cannot work >16 hours per shift.30 This rule has been found to increase quality of life and prevent burnout.30
The amount of time spent on electronic medical records and documentation has been a major complaint from doctors and was identified as a factor contributing to burnout.45 It can act as a time drain that impedes the physician from providing optimal patient care and cause additional stress. This suggests the need for organizations to find solutions to minimize this strain.
A concerning issue reported as an institutional factor and associated with burnout is mistreatment through discrimination, harassment, and physical or verbal abuse. A recent study found 45% of general and vascular surgeons reported being mistreated in some fashion.57 The strategies reported as helpful for institutions to combat mistreatment include resilience training, improved mentorship, and implicit bias training.57
Burnout has been positively correlated with anxiety and depression.6 A recent study reported that 13% of orthopedic surgery residents screened positive for depression.44 Higher levels of burnout and depersonalization have been found to be closely associated with increased rates of suicidal ideation.17 In a study of vascular surgeons, 8% were found to report suicidal ideation, and this increased to 15% among vascular surgeons who had higher levels of depersonalization and emotional exhaustion,58 both of which are associated with burnout. In another study, surgery residents and fellows were found to have lower levels of personal achievement and higher levels of depersonalization, depressive symptoms, alcohol abuse, and suicidal ideation compared to attending physicians and the general population.54 These findings spell out the association between burnout and depressive symptoms among surgeons and emphasize the need for institutions to create a culture that supports the mental health needs of their physicians. Without access to supportive resources, residents resort to alternative methods that may be detrimental in the long run. In a recent study, 17% of residents admitted to using alcohol, including binge drinking, to cope with their stress.4
Burnout and depression are linked to physical health risks such as cardiovascular disease, diabetes, substance abuse, and male infertility.6 Exercise has been shown to be beneficial for stress reduction, which can lead to changes in metabolism, inflammation, coagulation, and autonomic function.6 One study of surgeons found aerobic exercise and strength training were associated with lower rates of burnout and a higher quality of life.59
Continue to: The amount of burnout physicians...
The amount of burnout physicians experience can be determined by how they respond to adversities. Adaptive behaviors such as socializing, mindfulness, volunteering, and exercising have been found to be protective against burnout.6,37,54 Resilience training and maintaining low stress at work can decrease burnout.37 These findings highlight the need for physicians to be trained in the appropriate ways to combat their burnout symptoms.
Unfortunately, seeking help by health care professionals to improve mental health has been stigmatized, causing physicians to not seek help and instead resort to other ways to cope with their distress.26,34 While some of these coping methods may be positive, others—such as substance abuse or stress eating—can be maladaptive, leading to a poor quality of life, and in some cases, suicide.54 It is vital that effective mental health services become more accessible and for health care professionals to become aware of their maladaptive behaviors.34
Institutions finding ways to ease the path for their physicians to seek professional help to combat burnout may mitigate its negative impact. One strategy is to embed access to mental health services within regular wellness checks. Institutions can use wellness checks to provide resources to physicians who need it. These interventions have been found to be effective because they give physicians a safe space to seek help and become aware of any factors that could lead to burnout.18 Apart from these direct attempts to combat burnout, program-sponsored social events would also promote social connectedness with colleagues and contribute to a sense of well-being that could help decrease levels of burnout and depression.13 Mentorship has been shown to play a crucial role in decreasing burnout among residents. One study that examined the role of mentorship reported that 55% of residents felt supported, and of these, 96% felt mentorship was critical to their success.18 The role of institutions in helping to improve the well-being of surgeons is highlighted by the finding that increasing workplace support results in psychological resilience that can mitigate burnout at its roots.29
Bottom Line
Surgeons are at risk for burnout, which can impact their mental health and reduce their professional efficacy. Both institutions and surgeons themselves can take action to prevent burnout and treat burnout early when it occurs.
Related Resources
- Pahal P, Lippmann S. Building a better work/life balance. Current Psychiatry. 2021;20(8):e1-e2. doi:10.12788/cp.0158
- Gibson R, Hategan A. Physician burnout vs depression: recognize the signs. Current Psychiatry. 2019;18(10):41-42.
1. World Health Organization. International Statistical Classification of Diseases and Related Health Problems (ICD). 11th ed. World Health Organization; 2019.
2. Coombs DM, Lanni MA, Fosnot J, et al. Professional burnout in United States plastic surgery residents: is it a legitimate concern? Aesthet Surg J. 2020;40(7):802-810.
3. Klimo P Jr, DeCuypere M, Ragel BT, et al. Career satisfaction and burnout among U.S. neurosurgeons: a feasibility and pilot study. World Neurosurg. 2013;80(5):e59-e68.
4. Ha GQ, Go JT, Murayama KM, et al. Identifying sources of stress across years of general surgery residency. Hawaii J Health Soc Welf. 2020;79(3):75-81.
5. Khalafallah AM, Lam S, Gami A, et al. A national survey on the impact of the COVID-19 pandemic upon burnout and career satisfaction among neurosurgery residents. J Clin Neurosci. 2020;80:137-142.
6. Al-Humadi SM, Cáceda R, Bronson B, et al. Orthopaedic surgeon mental health during the COVID-19 pandemic. Geriatric Orthop Surg Rehabil. 2021;12:21514593211035230.
7. Larson DP, Carlson ML, Lohse CM, et al. Prevalence of and associations with distress and professional burnout among otolaryngologists: part I, trainees. Otolaryngol Head Neck Surg. 2021;164(5):1019-1029.
8. Streu R, Hawley S, Gay A, et al. Satisfaction with career choice among U.S. plastic surgeons: results from a national survey. Plast Reconstr Surg. 2010;126(2):636-642.
9. Shanafelt TD, Balch CM, Bechamps GJ, et al. Burnout and career satisfaction among American surgeons. Ann Surg. 2009;250(3):463-471.
10. Shanafelt TD, Balch CM, Bechamps G, et al. Burnout and medical errors among American surgeons. Ann Surg. 2010;251(6):995-1000.
11. Moher D, Liberati A, Tetzlaff J, et al; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336-341.
12. Yesantharao P, Lee E, Kraenzlin F, et al. Surgical block time satisfaction: a multi-institutional experience across twelve surgical disciplines. Perioperative Care Operating Room Manage. 2020;21:100128.
13. Nituica C, Bota OA, Blebea J. Specialty differences in resident resilience and burnout - a national survey. Am J Surg. 2021;222(2):319-328.
14. Balch CM, Shanafelt TD, Dyrbye L, et al. Surgeon distress as calibrated by hours worked and nights on call. J Am Coll Surg. 2010;211(5):609-619.
15. Dyrbye LN, Shanafelt TD, Balch CM, Satele D, Sloan J, Freischlag J. Relationship between work-home conflicts and burnout among American surgeons: a comparison by sex. Arch Surg. 2011;146(2):211-217.
16. Mahoney ST, Irish W, Strassle PD, et al. Practice characteristics and job satisfaction of private practice and academic surgeons. JAMA Surg. 2021;156(3):247-254.
17. Shanafelt TD, Balch CM, Dyrbye L, et al. Special report: suicidal ideation among American surgeons. Arch Surg. 2011;146(1):54-62.
18. Chow OS, Sudarshan M, Maxfield MW, et al. National survey of burnout and distress among cardiothoracic surgery trainees. Ann Thorac Surg. 2021;111(6):2066-2071.
19. Lam C, Kim Y, Cruz M, et al. Burnout and resiliency in Mohs surgeons: a survey study. Int J Womens Dermatol. 2021;7(3):319-322.
20. Carlson ML, Larson DP, O’Brien EK, et al. Prevalence of and associations with distress and professional burnout among otolaryngologists: part II, attending physicians. Otolaryngol Head Neck Surg. 2021;164(5):1030-1039.
21. Nida AM, Googe BJ, Lewis AF, et al. Resident fatigue in otolaryngology residents: a Web based survey. Am J Otolaryngol. 2016;37(3):210-216.
22. Antiel RM, Reed DA, Van Arendonk KJ, et al. Effects of duty hour restrictions on core competencies, education, quality of life, and burnout among general surgery interns. JAMA Surg. 2013;148(5):448-455.
23. Appelbaum NP, Lee N, Amendola M, et al. Surgical resident burnout and job satisfaction: the role of workplace climate and perceived support. J Surg Res. 2019;234:20-25.
24. Elmore LC, Jeffe DB, Jin L, et al. National survey of burnout among US general surgery residents. J Am Coll Surg. 2016;223(3):440-451.
25. Garcia DI, Pannuccio A, Gallegos J, et al. Resident-driven wellness initiatives improve resident wellness and perception of work environment. J Surg Res. 2021;258:8-16.
26. Hochberg MS, Berman RS, Kalet AL, et al. The stress of residency: recognizing the signs of depression and suicide in you and your fellow residents. Am J Surg. 2013;205(2):141-146.
27. Kurbatov V, Shaughnessy M, Baratta V, et al. Application of advanced bioinformatics to understand and predict burnout among surgical trainees. J Surg Educ. 2020;77(3):499-507.
28. Leach PK, Nygaard RM, Chipman JG, et al. Impostor phenomenon and burnout in general surgeons and general surgery residents. J Surg Educ. 2019;76(1):99-106.
29. Lebares CC, Greenberg AL, Ascher NL, et al. Exploration of individual and system-level well-being initiatives at an academic surgical residency program: a mixed-methods study. JAMA Netw Open. 2021;4(1):e2032676.
30. Lindeman BM, Sacks BC, Hirose K, et al. Multifaceted longitudinal study of surgical resident education, quality of life, and patient care before and after July 2011. J Surg Educ. 2013;70(6):769-776.
31. Rasmussen JM, Najarian MM, Ties JS, et al. Career satisfaction, gender bias, and work-life balance: a contemporary assessment of general surgeons. J Surg Educ. 2021;78(1):119-125.
32. Smeds MR, Janko MR, Allen S, et al. Burnout and its relationship with perceived stress, self-efficacy, depression, social support, and programmatic factors in general surgery residents. Am J Surg. 2020;219(6):907-912.
33. Wetzel CM, George A, Hanna GB, et al. Stress management training for surgeons--a randomized, controlled, intervention study. Ann Surg. 2011;253(3):488-494.
34. Williford ML, Scarlet S, Meyers MO, et al. Multiple-institution comparison of resident and faculty perceptions of burnout and depression during surgical training. JAMA Surg. 2018;153(8):705-711.
35. Zubair MH, Hussain LR, Williams KN, et al. Work-related quality of life of US general surgery residents: is it really so bad? J Surg Educ. 2017;74(6):e138-e146.
36. Song Y, Swendiman RA, Shannon AB, et al. Can we coach resilience? An evaluation of professional resilience coaching as a well-being initiative for surgical interns. J Surg Educ. 2020;77(6):1481-1489.
37. Morrell NT, Sears ED, Desai MJ, et al. A survey of burnout among members of the American Society for Surgery of the Hand. J Hand Surg Am. 2020;45(7):573-581.e516.
38. Khalafallah AM, Lam S, Gami A, et al. Burnout and career satisfaction among attending neurosurgeons during the COVID-19 pandemic. Clin Neurol Neurosurg. 2020;198:106193.
39. McAbee JH, Ragel BT, McCartney S, et al. Factors associated with career satisfaction and burnout among US neurosurgeons: results of a nationwide survey. J Neurosurg. 2015;123(1):161-173.
40. Shakir HJ, McPheeters MJ, Shallwani H, et al. The prevalence of burnout among US neurosurgery residents. Neurosurgery. 2018;83(3):582-590.
41. Govardhan LM, Pinelli V, Schnatz PF. Burnout, depression and job satisfaction in obstetrics and gynecology residents. Conn Med. 2012;76(7):389-395.
42. Driesman AS, Strauss EJ, Konda SR, et al. Factors associated with orthopaedic resident burnout: a pilot study. J Am Acad Orthop Surg. 2020;28(21):900-906.
43. Lichstein PM, He JK, Estok D, et al. What is the prevalence of burnout, depression, and substance use among orthopaedic surgery residents and what are the risk factors? A collaborative orthopaedic educational research group survey study. Clin Orthop Relat Res. 2020;478(8):1709-1718.
44. Somerson JS, Patton A, Ahmed AA, et al. Burnout among United States orthopaedic surgery residents. J Surg Educ. 2020;77(4):961-968.
45. Verret CI, Nguyen J, Verret C, et al. How do areas of work life drive burnout in orthopaedic attending surgeons, fellows, and residents? Clin Orthop Relat Res. 2021;479(2):251-262.
46. Sarosi A, Coakley BA, Berman L, et al. A cross-sectional analysis of compassion fatigue, burnout, and compassion satisfaction in pediatric surgeons in the U.S. J Pediatr Surg. 2021;56(8):1276-1284.
47. Crowe CS, Lopez J, Morrison SD, et al. The effects of the COVID-19 pandemic on resident education and wellness: a national survey of plastic surgery residents. Plast Reconstr Surg. 2021;148(3):462e-474e.
48. Qureshi HA, Rawlani R, Mioton LM, et al. Burnout phenomenon in U.S. plastic surgeons: risk factors and impact on quality of life. Plast Reconstr Surg. 2015;135(2):619-626.
49. Streu R, Hansen J, Abrahamse P, et al. Professional burnout among US plastic surgeons: results of a national survey. Ann Plast Surg. 2014;72(3):346-350.
50. Zhang JQ, Riba L, Magrini L, ET AL. Assessing burnout and professional fulfillment in breast surgery: results from a national survey of the American Society of Breast Surgeons. Ann Surg Oncol. 2019;26(10):3089-3098.
51. Balch CM, Shanafelt TD, Sloan J, et al. Burnout and career satisfaction among surgical oncologists compared with other surgical specialties. Ann Surg Oncol. 2011;18(1):16-25.
52. Wu D, Gross B, Rittenhouse K, et al. A preliminary analysis of compassion fatigue in a surgeon population: are female surgeons at heightened risk? Am Surg. 2017;83(11):1302-1307.
53. Cheng JW, Wagner H, Hernandez BC, et al. Stressors and coping mechanisms related to burnout within urology. Urology. 2020;139:27-36.
54. Koo K, Javier-DesLoges JF, Fang R, ET AL. Professional burnout, career choice regret, and unmet needs for well-being among urology residents. Urology. 2021;157:57-63.
55. Janko MR, Smeds MR. Burnout, depression, perceived stress, and self-efficacy in vascular surgery trainees. J Vasc Surg. 2019;69(4):1233-1242.
56. Coleman DM, Money SR, Meltzer AJ, et al. Vascular surgeon wellness and burnout: a report from the Society for Vascular Surgery Wellness Task Force. J Vasc Surg. 2021;73(6):1841-1850.e3.
57. Barrack RL, Miller LS, Sotile WM, et al. Effect of duty hour standards on burnout among orthopaedic surgery residents. Clin Orthop Relat Res. 2006;449:134-137.
58. Chia MC, Hu YY, Li RD, et al. Prevalence and risk factors for burnout in U.S. vascular surgery trainees. J Vasc Surg. 2022;75(1):308-315.e4.
59. Shanafelt TD, Oreskovich MR, Dyrbye LN, et al. Avoiding burnout: the personal health habits and wellness practices of US surgeons. Ann Surg. 2012;255(4):625-633.
1. World Health Organization. International Statistical Classification of Diseases and Related Health Problems (ICD). 11th ed. World Health Organization; 2019.
2. Coombs DM, Lanni MA, Fosnot J, et al. Professional burnout in United States plastic surgery residents: is it a legitimate concern? Aesthet Surg J. 2020;40(7):802-810.
3. Klimo P Jr, DeCuypere M, Ragel BT, et al. Career satisfaction and burnout among U.S. neurosurgeons: a feasibility and pilot study. World Neurosurg. 2013;80(5):e59-e68.
4. Ha GQ, Go JT, Murayama KM, et al. Identifying sources of stress across years of general surgery residency. Hawaii J Health Soc Welf. 2020;79(3):75-81.
5. Khalafallah AM, Lam S, Gami A, et al. A national survey on the impact of the COVID-19 pandemic upon burnout and career satisfaction among neurosurgery residents. J Clin Neurosci. 2020;80:137-142.
6. Al-Humadi SM, Cáceda R, Bronson B, et al. Orthopaedic surgeon mental health during the COVID-19 pandemic. Geriatric Orthop Surg Rehabil. 2021;12:21514593211035230.
7. Larson DP, Carlson ML, Lohse CM, et al. Prevalence of and associations with distress and professional burnout among otolaryngologists: part I, trainees. Otolaryngol Head Neck Surg. 2021;164(5):1019-1029.
8. Streu R, Hawley S, Gay A, et al. Satisfaction with career choice among U.S. plastic surgeons: results from a national survey. Plast Reconstr Surg. 2010;126(2):636-642.
9. Shanafelt TD, Balch CM, Bechamps GJ, et al. Burnout and career satisfaction among American surgeons. Ann Surg. 2009;250(3):463-471.
10. Shanafelt TD, Balch CM, Bechamps G, et al. Burnout and medical errors among American surgeons. Ann Surg. 2010;251(6):995-1000.
11. Moher D, Liberati A, Tetzlaff J, et al; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336-341.
12. Yesantharao P, Lee E, Kraenzlin F, et al. Surgical block time satisfaction: a multi-institutional experience across twelve surgical disciplines. Perioperative Care Operating Room Manage. 2020;21:100128.
13. Nituica C, Bota OA, Blebea J. Specialty differences in resident resilience and burnout - a national survey. Am J Surg. 2021;222(2):319-328.
14. Balch CM, Shanafelt TD, Dyrbye L, et al. Surgeon distress as calibrated by hours worked and nights on call. J Am Coll Surg. 2010;211(5):609-619.
15. Dyrbye LN, Shanafelt TD, Balch CM, Satele D, Sloan J, Freischlag J. Relationship between work-home conflicts and burnout among American surgeons: a comparison by sex. Arch Surg. 2011;146(2):211-217.
16. Mahoney ST, Irish W, Strassle PD, et al. Practice characteristics and job satisfaction of private practice and academic surgeons. JAMA Surg. 2021;156(3):247-254.
17. Shanafelt TD, Balch CM, Dyrbye L, et al. Special report: suicidal ideation among American surgeons. Arch Surg. 2011;146(1):54-62.
18. Chow OS, Sudarshan M, Maxfield MW, et al. National survey of burnout and distress among cardiothoracic surgery trainees. Ann Thorac Surg. 2021;111(6):2066-2071.
19. Lam C, Kim Y, Cruz M, et al. Burnout and resiliency in Mohs surgeons: a survey study. Int J Womens Dermatol. 2021;7(3):319-322.
20. Carlson ML, Larson DP, O’Brien EK, et al. Prevalence of and associations with distress and professional burnout among otolaryngologists: part II, attending physicians. Otolaryngol Head Neck Surg. 2021;164(5):1030-1039.
21. Nida AM, Googe BJ, Lewis AF, et al. Resident fatigue in otolaryngology residents: a Web based survey. Am J Otolaryngol. 2016;37(3):210-216.
22. Antiel RM, Reed DA, Van Arendonk KJ, et al. Effects of duty hour restrictions on core competencies, education, quality of life, and burnout among general surgery interns. JAMA Surg. 2013;148(5):448-455.
23. Appelbaum NP, Lee N, Amendola M, et al. Surgical resident burnout and job satisfaction: the role of workplace climate and perceived support. J Surg Res. 2019;234:20-25.
24. Elmore LC, Jeffe DB, Jin L, et al. National survey of burnout among US general surgery residents. J Am Coll Surg. 2016;223(3):440-451.
25. Garcia DI, Pannuccio A, Gallegos J, et al. Resident-driven wellness initiatives improve resident wellness and perception of work environment. J Surg Res. 2021;258:8-16.
26. Hochberg MS, Berman RS, Kalet AL, et al. The stress of residency: recognizing the signs of depression and suicide in you and your fellow residents. Am J Surg. 2013;205(2):141-146.
27. Kurbatov V, Shaughnessy M, Baratta V, et al. Application of advanced bioinformatics to understand and predict burnout among surgical trainees. J Surg Educ. 2020;77(3):499-507.
28. Leach PK, Nygaard RM, Chipman JG, et al. Impostor phenomenon and burnout in general surgeons and general surgery residents. J Surg Educ. 2019;76(1):99-106.
29. Lebares CC, Greenberg AL, Ascher NL, et al. Exploration of individual and system-level well-being initiatives at an academic surgical residency program: a mixed-methods study. JAMA Netw Open. 2021;4(1):e2032676.
30. Lindeman BM, Sacks BC, Hirose K, et al. Multifaceted longitudinal study of surgical resident education, quality of life, and patient care before and after July 2011. J Surg Educ. 2013;70(6):769-776.
31. Rasmussen JM, Najarian MM, Ties JS, et al. Career satisfaction, gender bias, and work-life balance: a contemporary assessment of general surgeons. J Surg Educ. 2021;78(1):119-125.
32. Smeds MR, Janko MR, Allen S, et al. Burnout and its relationship with perceived stress, self-efficacy, depression, social support, and programmatic factors in general surgery residents. Am J Surg. 2020;219(6):907-912.
33. Wetzel CM, George A, Hanna GB, et al. Stress management training for surgeons--a randomized, controlled, intervention study. Ann Surg. 2011;253(3):488-494.
34. Williford ML, Scarlet S, Meyers MO, et al. Multiple-institution comparison of resident and faculty perceptions of burnout and depression during surgical training. JAMA Surg. 2018;153(8):705-711.
35. Zubair MH, Hussain LR, Williams KN, et al. Work-related quality of life of US general surgery residents: is it really so bad? J Surg Educ. 2017;74(6):e138-e146.
36. Song Y, Swendiman RA, Shannon AB, et al. Can we coach resilience? An evaluation of professional resilience coaching as a well-being initiative for surgical interns. J Surg Educ. 2020;77(6):1481-1489.
37. Morrell NT, Sears ED, Desai MJ, et al. A survey of burnout among members of the American Society for Surgery of the Hand. J Hand Surg Am. 2020;45(7):573-581.e516.
38. Khalafallah AM, Lam S, Gami A, et al. Burnout and career satisfaction among attending neurosurgeons during the COVID-19 pandemic. Clin Neurol Neurosurg. 2020;198:106193.
39. McAbee JH, Ragel BT, McCartney S, et al. Factors associated with career satisfaction and burnout among US neurosurgeons: results of a nationwide survey. J Neurosurg. 2015;123(1):161-173.
40. Shakir HJ, McPheeters MJ, Shallwani H, et al. The prevalence of burnout among US neurosurgery residents. Neurosurgery. 2018;83(3):582-590.
41. Govardhan LM, Pinelli V, Schnatz PF. Burnout, depression and job satisfaction in obstetrics and gynecology residents. Conn Med. 2012;76(7):389-395.
42. Driesman AS, Strauss EJ, Konda SR, et al. Factors associated with orthopaedic resident burnout: a pilot study. J Am Acad Orthop Surg. 2020;28(21):900-906.
43. Lichstein PM, He JK, Estok D, et al. What is the prevalence of burnout, depression, and substance use among orthopaedic surgery residents and what are the risk factors? A collaborative orthopaedic educational research group survey study. Clin Orthop Relat Res. 2020;478(8):1709-1718.
44. Somerson JS, Patton A, Ahmed AA, et al. Burnout among United States orthopaedic surgery residents. J Surg Educ. 2020;77(4):961-968.
45. Verret CI, Nguyen J, Verret C, et al. How do areas of work life drive burnout in orthopaedic attending surgeons, fellows, and residents? Clin Orthop Relat Res. 2021;479(2):251-262.
46. Sarosi A, Coakley BA, Berman L, et al. A cross-sectional analysis of compassion fatigue, burnout, and compassion satisfaction in pediatric surgeons in the U.S. J Pediatr Surg. 2021;56(8):1276-1284.
47. Crowe CS, Lopez J, Morrison SD, et al. The effects of the COVID-19 pandemic on resident education and wellness: a national survey of plastic surgery residents. Plast Reconstr Surg. 2021;148(3):462e-474e.
48. Qureshi HA, Rawlani R, Mioton LM, et al. Burnout phenomenon in U.S. plastic surgeons: risk factors and impact on quality of life. Plast Reconstr Surg. 2015;135(2):619-626.
49. Streu R, Hansen J, Abrahamse P, et al. Professional burnout among US plastic surgeons: results of a national survey. Ann Plast Surg. 2014;72(3):346-350.
50. Zhang JQ, Riba L, Magrini L, ET AL. Assessing burnout and professional fulfillment in breast surgery: results from a national survey of the American Society of Breast Surgeons. Ann Surg Oncol. 2019;26(10):3089-3098.
51. Balch CM, Shanafelt TD, Sloan J, et al. Burnout and career satisfaction among surgical oncologists compared with other surgical specialties. Ann Surg Oncol. 2011;18(1):16-25.
52. Wu D, Gross B, Rittenhouse K, et al. A preliminary analysis of compassion fatigue in a surgeon population: are female surgeons at heightened risk? Am Surg. 2017;83(11):1302-1307.
53. Cheng JW, Wagner H, Hernandez BC, et al. Stressors and coping mechanisms related to burnout within urology. Urology. 2020;139:27-36.
54. Koo K, Javier-DesLoges JF, Fang R, ET AL. Professional burnout, career choice regret, and unmet needs for well-being among urology residents. Urology. 2021;157:57-63.
55. Janko MR, Smeds MR. Burnout, depression, perceived stress, and self-efficacy in vascular surgery trainees. J Vasc Surg. 2019;69(4):1233-1242.
56. Coleman DM, Money SR, Meltzer AJ, et al. Vascular surgeon wellness and burnout: a report from the Society for Vascular Surgery Wellness Task Force. J Vasc Surg. 2021;73(6):1841-1850.e3.
57. Barrack RL, Miller LS, Sotile WM, et al. Effect of duty hour standards on burnout among orthopaedic surgery residents. Clin Orthop Relat Res. 2006;449:134-137.
58. Chia MC, Hu YY, Li RD, et al. Prevalence and risk factors for burnout in U.S. vascular surgery trainees. J Vasc Surg. 2022;75(1):308-315.e4.
59. Shanafelt TD, Oreskovich MR, Dyrbye LN, et al. Avoiding burnout: the personal health habits and wellness practices of US surgeons. Ann Surg. 2012;255(4):625-633.
Fast-acting postpartum depression drug is effective
The Food and Drug Administration is considering approving a postpartum depression medication that can start working rapidly – in as little as 3 days. Promising results for the drug, zuranolone, were published recently in The American Journal of Psychiatry.
Approximately 17% of women are affected by postpartum depression (PPD) during pregnancy or after birth, study authors noted. The condition often results in reduced breastfeeding, poor maternal-infant bonding, and hindering behavioral, emotional and brain development of the baby. Severe PPD can lead to suicide of the mother, which accounts for 20% of all postpartum deaths, they wrote.
The study included 196 people who had given birth in the past year, and were between the ages of 18 and 45 years old. Participants had major depression that began in the 3rd trimester of pregnancy or during the first 4 weeks of the postpartum period. Among participants, 22% were Black and 38% were Hispanic.
The average time it took for symptoms to significantly decline was 9 days. Most people who saw improvements had them continue for the entire 45-day follow-up period. The most common side effects were drowsiness, dizziness, and sleepiness.
Currently, PPD treatment includes taking antidepressants, which can take up to 12 weeks to work.
Researchers noted that the limitations of the study were that it only included women with severe PPD, and that women with a history of bipolar or psychotic disorders were excluded. Women in the study were not allowed to breastfeed, so the effect of zuranolone on lactation is unknown, they wrote.
A February news release from drugmaker Biogen indicated the FDA may decide whether to approve the medicine by Aug. 5.
A version of this article first appeared on WebMD.com.
The Food and Drug Administration is considering approving a postpartum depression medication that can start working rapidly – in as little as 3 days. Promising results for the drug, zuranolone, were published recently in The American Journal of Psychiatry.
Approximately 17% of women are affected by postpartum depression (PPD) during pregnancy or after birth, study authors noted. The condition often results in reduced breastfeeding, poor maternal-infant bonding, and hindering behavioral, emotional and brain development of the baby. Severe PPD can lead to suicide of the mother, which accounts for 20% of all postpartum deaths, they wrote.
The study included 196 people who had given birth in the past year, and were between the ages of 18 and 45 years old. Participants had major depression that began in the 3rd trimester of pregnancy or during the first 4 weeks of the postpartum period. Among participants, 22% were Black and 38% were Hispanic.
The average time it took for symptoms to significantly decline was 9 days. Most people who saw improvements had them continue for the entire 45-day follow-up period. The most common side effects were drowsiness, dizziness, and sleepiness.
Currently, PPD treatment includes taking antidepressants, which can take up to 12 weeks to work.
Researchers noted that the limitations of the study were that it only included women with severe PPD, and that women with a history of bipolar or psychotic disorders were excluded. Women in the study were not allowed to breastfeed, so the effect of zuranolone on lactation is unknown, they wrote.
A February news release from drugmaker Biogen indicated the FDA may decide whether to approve the medicine by Aug. 5.
A version of this article first appeared on WebMD.com.
The Food and Drug Administration is considering approving a postpartum depression medication that can start working rapidly – in as little as 3 days. Promising results for the drug, zuranolone, were published recently in The American Journal of Psychiatry.
Approximately 17% of women are affected by postpartum depression (PPD) during pregnancy or after birth, study authors noted. The condition often results in reduced breastfeeding, poor maternal-infant bonding, and hindering behavioral, emotional and brain development of the baby. Severe PPD can lead to suicide of the mother, which accounts for 20% of all postpartum deaths, they wrote.
The study included 196 people who had given birth in the past year, and were between the ages of 18 and 45 years old. Participants had major depression that began in the 3rd trimester of pregnancy or during the first 4 weeks of the postpartum period. Among participants, 22% were Black and 38% were Hispanic.
The average time it took for symptoms to significantly decline was 9 days. Most people who saw improvements had them continue for the entire 45-day follow-up period. The most common side effects were drowsiness, dizziness, and sleepiness.
Currently, PPD treatment includes taking antidepressants, which can take up to 12 weeks to work.
Researchers noted that the limitations of the study were that it only included women with severe PPD, and that women with a history of bipolar or psychotic disorders were excluded. Women in the study were not allowed to breastfeed, so the effect of zuranolone on lactation is unknown, they wrote.
A February news release from drugmaker Biogen indicated the FDA may decide whether to approve the medicine by Aug. 5.
A version of this article first appeared on WebMD.com.
FROM THE AMERICAN JOURNAL OF PSYCHIATRY
Subcutaneous ketamine for TRD practical, safe, and highly effective
“In this severely treatment-resistant population, of which 24% had failed to respond to treatment with electroconvulsive therapy, adequately dosed racemic ketamine produced benefits that were large, being both clinically and statistically superior to midazolam,” report the researchers, led by Colleen Loo, MD, MBBS, with Black Dog Institute, University of New South Wales, Sydney.
The study was published online in the British Journal of Psychiatry.
Individualized dosing
“Previously, most studies of racemic ketamine [administered] it by intravenous infusion over half an hour to several hours, which is a much more medically complex and expensive procedure,” Dr. Loo said in an interview.
The fact that subcutaneously administered ketamine was “highly effective” given by this practical and feasible route is a “major contribution to the field,” said Dr. Loo.
The Ketamine for Adult Depression trial assessed the acute efficacy and safety of a 4-week course of twice-weekly subcutaneous injections of racemic ketamine or midazolam (active control) in 174 adults with TRD.
Initially, the trial tested a fixed dose of 0.5 mg/kg ketamine vs. 0.025 mg/kg midazolam (cohort 1; 68 patients). Dosing was subsequently revised, after the data safety monitoring board recommended flexible-dose ketamine (0.5-0.9 mg/kg) or midazolam (0.025-0.045 mg/kg) with response-guided dosing increments (cohort 2; 106 patients).
The primary outcome was remission defined as Montgomery-Åsberg Rating Scale for Depression (MADRS) score ≤ 10 at week 4.
On this outcome, in the fixed-dose cohort, there was no statistically significant difference in remission rates between ketamine and midazolam (6.3% and 8.8%; odds ratio, 1.34; 95% CI, 0.22-8.21; P = .76).
However, there was a significant difference in remission in the flexible-dose cohort, with remission rates 19.6% for ketamine vs. just 2% for midazolam (OR, 12.11; 95% CI, 2.12-69.17; P = .005).
“The study showed that individualized dose adjustment, based on clinical response, was very important in optimizing the benefit of ketamine,” said Dr. Loo.
“It meant that one, you are more likely to respond as you receive a higher dose if needed, and two, you don’t receive a higher dose than needed, given that side effects are also dose-related,” she said.
Results also favored flexible-dose ketamine over midazolam for the secondary outcomes of response (≥ 50% reduction in MADRS: 29% vs. 4%; P = .001) and remission defined by a less rigid definition (MADRS ≤ 12: 22% vs. 4%; P = .007).
The results also confirm that the antidepressant effects of ketamine are not sustained when treatment stops.
“The study included careful follow-up for 4 weeks after the end of treatment. This is an important contribution to the literature, as it shows that ongoing treatment beyond the 4 weeks will be necessary for most people to maintain the benefits of ketamine treatment if you respond to the treatment. This study provided clear evidence of this, for racemic ketamine,” said Dr. Loo.
Overall, ketamine was well-tolerated, with the well-established acute effects of ketamine observed in both cohorts. The acute effects resolved or returned to pretreatment levels within the 2-hour observation period. No one required medical intervention, and there was no evidence of cognitive impairment.
Rigorous research, compelling data
Reached for comment, Roger S. McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the mood disorders psychopharmacology unit, said the data are “compelling with respect to efficacy and safety of subcutaneous ketamine in adults with major depression.”
Dr. McIntyre said the data are “highly relevant” for several reasons. “First, it is the most rigorous study conducted to date with subcutaneous administration of ketamine for adults living with treatment-resistant depression.”
Second, it “demonstrates the efficacy and safety of this route of delivery, which until now has not been studied with this level of rigor and which is a more scalable and accessible approach to administer ketamine to suitable candidates,” Dr. McIntyre said.
The study was funded by a competitive research grant from the Australian National Health and Medical Research Council. Dr. Loo has disclosed relationships with Douglas Pharmaceuticals and Janssen Cilag and is the medical director of neurostimulation and interventional psychiatry at Ramsay Health Care. Dr. McIntyre has received speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, AbbVie, and Atai Life Sciences. Dr. McIntyre is a CEO of Braxia Scientific Corp.
A version of this article first appeared on Medscape.com.
“In this severely treatment-resistant population, of which 24% had failed to respond to treatment with electroconvulsive therapy, adequately dosed racemic ketamine produced benefits that were large, being both clinically and statistically superior to midazolam,” report the researchers, led by Colleen Loo, MD, MBBS, with Black Dog Institute, University of New South Wales, Sydney.
The study was published online in the British Journal of Psychiatry.
Individualized dosing
“Previously, most studies of racemic ketamine [administered] it by intravenous infusion over half an hour to several hours, which is a much more medically complex and expensive procedure,” Dr. Loo said in an interview.
The fact that subcutaneously administered ketamine was “highly effective” given by this practical and feasible route is a “major contribution to the field,” said Dr. Loo.
The Ketamine for Adult Depression trial assessed the acute efficacy and safety of a 4-week course of twice-weekly subcutaneous injections of racemic ketamine or midazolam (active control) in 174 adults with TRD.
Initially, the trial tested a fixed dose of 0.5 mg/kg ketamine vs. 0.025 mg/kg midazolam (cohort 1; 68 patients). Dosing was subsequently revised, after the data safety monitoring board recommended flexible-dose ketamine (0.5-0.9 mg/kg) or midazolam (0.025-0.045 mg/kg) with response-guided dosing increments (cohort 2; 106 patients).
The primary outcome was remission defined as Montgomery-Åsberg Rating Scale for Depression (MADRS) score ≤ 10 at week 4.
On this outcome, in the fixed-dose cohort, there was no statistically significant difference in remission rates between ketamine and midazolam (6.3% and 8.8%; odds ratio, 1.34; 95% CI, 0.22-8.21; P = .76).
However, there was a significant difference in remission in the flexible-dose cohort, with remission rates 19.6% for ketamine vs. just 2% for midazolam (OR, 12.11; 95% CI, 2.12-69.17; P = .005).
“The study showed that individualized dose adjustment, based on clinical response, was very important in optimizing the benefit of ketamine,” said Dr. Loo.
“It meant that one, you are more likely to respond as you receive a higher dose if needed, and two, you don’t receive a higher dose than needed, given that side effects are also dose-related,” she said.
Results also favored flexible-dose ketamine over midazolam for the secondary outcomes of response (≥ 50% reduction in MADRS: 29% vs. 4%; P = .001) and remission defined by a less rigid definition (MADRS ≤ 12: 22% vs. 4%; P = .007).
The results also confirm that the antidepressant effects of ketamine are not sustained when treatment stops.
“The study included careful follow-up for 4 weeks after the end of treatment. This is an important contribution to the literature, as it shows that ongoing treatment beyond the 4 weeks will be necessary for most people to maintain the benefits of ketamine treatment if you respond to the treatment. This study provided clear evidence of this, for racemic ketamine,” said Dr. Loo.
Overall, ketamine was well-tolerated, with the well-established acute effects of ketamine observed in both cohorts. The acute effects resolved or returned to pretreatment levels within the 2-hour observation period. No one required medical intervention, and there was no evidence of cognitive impairment.
Rigorous research, compelling data
Reached for comment, Roger S. McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the mood disorders psychopharmacology unit, said the data are “compelling with respect to efficacy and safety of subcutaneous ketamine in adults with major depression.”
Dr. McIntyre said the data are “highly relevant” for several reasons. “First, it is the most rigorous study conducted to date with subcutaneous administration of ketamine for adults living with treatment-resistant depression.”
Second, it “demonstrates the efficacy and safety of this route of delivery, which until now has not been studied with this level of rigor and which is a more scalable and accessible approach to administer ketamine to suitable candidates,” Dr. McIntyre said.
The study was funded by a competitive research grant from the Australian National Health and Medical Research Council. Dr. Loo has disclosed relationships with Douglas Pharmaceuticals and Janssen Cilag and is the medical director of neurostimulation and interventional psychiatry at Ramsay Health Care. Dr. McIntyre has received speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, AbbVie, and Atai Life Sciences. Dr. McIntyre is a CEO of Braxia Scientific Corp.
A version of this article first appeared on Medscape.com.
“In this severely treatment-resistant population, of which 24% had failed to respond to treatment with electroconvulsive therapy, adequately dosed racemic ketamine produced benefits that were large, being both clinically and statistically superior to midazolam,” report the researchers, led by Colleen Loo, MD, MBBS, with Black Dog Institute, University of New South Wales, Sydney.
The study was published online in the British Journal of Psychiatry.
Individualized dosing
“Previously, most studies of racemic ketamine [administered] it by intravenous infusion over half an hour to several hours, which is a much more medically complex and expensive procedure,” Dr. Loo said in an interview.
The fact that subcutaneously administered ketamine was “highly effective” given by this practical and feasible route is a “major contribution to the field,” said Dr. Loo.
The Ketamine for Adult Depression trial assessed the acute efficacy and safety of a 4-week course of twice-weekly subcutaneous injections of racemic ketamine or midazolam (active control) in 174 adults with TRD.
Initially, the trial tested a fixed dose of 0.5 mg/kg ketamine vs. 0.025 mg/kg midazolam (cohort 1; 68 patients). Dosing was subsequently revised, after the data safety monitoring board recommended flexible-dose ketamine (0.5-0.9 mg/kg) or midazolam (0.025-0.045 mg/kg) with response-guided dosing increments (cohort 2; 106 patients).
The primary outcome was remission defined as Montgomery-Åsberg Rating Scale for Depression (MADRS) score ≤ 10 at week 4.
On this outcome, in the fixed-dose cohort, there was no statistically significant difference in remission rates between ketamine and midazolam (6.3% and 8.8%; odds ratio, 1.34; 95% CI, 0.22-8.21; P = .76).
However, there was a significant difference in remission in the flexible-dose cohort, with remission rates 19.6% for ketamine vs. just 2% for midazolam (OR, 12.11; 95% CI, 2.12-69.17; P = .005).
“The study showed that individualized dose adjustment, based on clinical response, was very important in optimizing the benefit of ketamine,” said Dr. Loo.
“It meant that one, you are more likely to respond as you receive a higher dose if needed, and two, you don’t receive a higher dose than needed, given that side effects are also dose-related,” she said.
Results also favored flexible-dose ketamine over midazolam for the secondary outcomes of response (≥ 50% reduction in MADRS: 29% vs. 4%; P = .001) and remission defined by a less rigid definition (MADRS ≤ 12: 22% vs. 4%; P = .007).
The results also confirm that the antidepressant effects of ketamine are not sustained when treatment stops.
“The study included careful follow-up for 4 weeks after the end of treatment. This is an important contribution to the literature, as it shows that ongoing treatment beyond the 4 weeks will be necessary for most people to maintain the benefits of ketamine treatment if you respond to the treatment. This study provided clear evidence of this, for racemic ketamine,” said Dr. Loo.
Overall, ketamine was well-tolerated, with the well-established acute effects of ketamine observed in both cohorts. The acute effects resolved or returned to pretreatment levels within the 2-hour observation period. No one required medical intervention, and there was no evidence of cognitive impairment.
Rigorous research, compelling data
Reached for comment, Roger S. McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the mood disorders psychopharmacology unit, said the data are “compelling with respect to efficacy and safety of subcutaneous ketamine in adults with major depression.”
Dr. McIntyre said the data are “highly relevant” for several reasons. “First, it is the most rigorous study conducted to date with subcutaneous administration of ketamine for adults living with treatment-resistant depression.”
Second, it “demonstrates the efficacy and safety of this route of delivery, which until now has not been studied with this level of rigor and which is a more scalable and accessible approach to administer ketamine to suitable candidates,” Dr. McIntyre said.
The study was funded by a competitive research grant from the Australian National Health and Medical Research Council. Dr. Loo has disclosed relationships with Douglas Pharmaceuticals and Janssen Cilag and is the medical director of neurostimulation and interventional psychiatry at Ramsay Health Care. Dr. McIntyre has received speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, AbbVie, and Atai Life Sciences. Dr. McIntyre is a CEO of Braxia Scientific Corp.
A version of this article first appeared on Medscape.com.
FROM THE BRITISH JOURNAL OF PSYCHIATRY
Novel talk therapy challenges CBT for treating anhedonic depression
While the trial was not adequately powered to test if augmented depression therapy (ADepT) is superior to CBT, “results nevertheless were encouraging,” lead author Barnaby Dunn, PhD, professor of clinical psychology, University of Exeter (England), said in an interview.
The trial showed that ADepT is feasible, acceptable, and “not worse than CBT,” also showing the “potential to be better than CBT in clinical outcomes,” Dr. Dunn said.
“By building positive mood, ADepT may help individuals stay well for longer in the future,” he noted.
The early results were published online in eClinicalMedicine.
Dual approach
“There are two sides to the depression coin – heightened negative mood and reduced positive mood. Classic CBT focuses mainly on repairing negative mood and pays less attention to building positive mood,” Dr. Dunn explained.
He said when he speaks to clients about what is key to recovery from depression, they often mention the importance of reconnecting to the positive.
“ADepT pays equal attention to building the positives as it does reducing the negatives, giving clients new skills to ‘act opposite’ to old ways of thinking and feeling that can stop them making the most of opportunities and being able to experience well-being,” Dr. Dunn said.
ADepT is an individual therapy delivered over 15 acute and 5 booster sessions, which is similar in “dose” to classic CBT, Dr. Dunn said.
The primary focus of ADepT is building well-being (capacity to experience pleasure, meaning, and social connection in life) and functional recovery, with depression conceptualized as patterns of thinking, feeling, and behaving that serve as barriers to achieving this goal.
Patients work with trained therapists to overcome barriers to being resilient (managing challenges to reduce negative affect) and thriving (taking opportunities to maximize positive affect).
A total of 82 adults with a moderate to severe current major depressive episode with features of anhedonia took part in the pilot trial. They were randomly allocated 1:1 to either 20 individual sessions of ADepT or CBT, delivered in the University of Exeter Accessing Evidence Based Psychological Therapies outpatient clinic.
Researcher-blinded assessments were completed at intake and after 6, 12, and 18 months. Coprimary outcomes were depression, measured via the Patient Health Questionnaire and well-being, gauged with the Warwick Edinburgh Mental Wellbeing Scale at 6 months.
Within-group analyses showed that both ADepT and CBT led to clinically meaningful improvements in depression, well-being, and all other secondary outcomes, including measures of anhedonia, Dr. Dunn said.
Between-group effects favored ADepT over CBT for depression and well-being. “For example, about 80% of clients no longer met diagnostic criteria for depression after ADepT, compared to around 56% of clients in CBT,” Dr. Dunn said.
“There were also numerically bigger gains in well-being and reductions in anhedonia in ADepT relative to CBT. A greater number of clients who recovered at the end of therapy stayed well over the longer term in ADepT relative to CBT,” he noted.
ADepT costs the same amount to deliver as CBT “but resulted in greater gains in quality of life, meaning it showed a high probability of being cost effective,” Dr. Dunn said.
ADepT has also been designed so that trained CBT therapists will be able to deliver it with minimal additional training.
“The next step,” said Dr. Dunn, “is a bigger definitive trial, which will formally test if ADepT is clinically superior to and better value for money than CBT when delivering ADepT in more routine care settings [U.K. NHS clinics rather than specialist university mood disorder center].”
The trial was funded by a Career Development Fellowship awarded to Dr. Dunn by the National Institute for Health and Care Research. Dr. Dunn has a book contract with Guilford Press to write the ADepT treatment manual and receives occasional payment or honoraria (including support for attending meetings) for delivering workshops and talks on ADepT.
A version of this article first appeared on Medscape.com.
While the trial was not adequately powered to test if augmented depression therapy (ADepT) is superior to CBT, “results nevertheless were encouraging,” lead author Barnaby Dunn, PhD, professor of clinical psychology, University of Exeter (England), said in an interview.
The trial showed that ADepT is feasible, acceptable, and “not worse than CBT,” also showing the “potential to be better than CBT in clinical outcomes,” Dr. Dunn said.
“By building positive mood, ADepT may help individuals stay well for longer in the future,” he noted.
The early results were published online in eClinicalMedicine.
Dual approach
“There are two sides to the depression coin – heightened negative mood and reduced positive mood. Classic CBT focuses mainly on repairing negative mood and pays less attention to building positive mood,” Dr. Dunn explained.
He said when he speaks to clients about what is key to recovery from depression, they often mention the importance of reconnecting to the positive.
“ADepT pays equal attention to building the positives as it does reducing the negatives, giving clients new skills to ‘act opposite’ to old ways of thinking and feeling that can stop them making the most of opportunities and being able to experience well-being,” Dr. Dunn said.
ADepT is an individual therapy delivered over 15 acute and 5 booster sessions, which is similar in “dose” to classic CBT, Dr. Dunn said.
The primary focus of ADepT is building well-being (capacity to experience pleasure, meaning, and social connection in life) and functional recovery, with depression conceptualized as patterns of thinking, feeling, and behaving that serve as barriers to achieving this goal.
Patients work with trained therapists to overcome barriers to being resilient (managing challenges to reduce negative affect) and thriving (taking opportunities to maximize positive affect).
A total of 82 adults with a moderate to severe current major depressive episode with features of anhedonia took part in the pilot trial. They were randomly allocated 1:1 to either 20 individual sessions of ADepT or CBT, delivered in the University of Exeter Accessing Evidence Based Psychological Therapies outpatient clinic.
Researcher-blinded assessments were completed at intake and after 6, 12, and 18 months. Coprimary outcomes were depression, measured via the Patient Health Questionnaire and well-being, gauged with the Warwick Edinburgh Mental Wellbeing Scale at 6 months.
Within-group analyses showed that both ADepT and CBT led to clinically meaningful improvements in depression, well-being, and all other secondary outcomes, including measures of anhedonia, Dr. Dunn said.
Between-group effects favored ADepT over CBT for depression and well-being. “For example, about 80% of clients no longer met diagnostic criteria for depression after ADepT, compared to around 56% of clients in CBT,” Dr. Dunn said.
“There were also numerically bigger gains in well-being and reductions in anhedonia in ADepT relative to CBT. A greater number of clients who recovered at the end of therapy stayed well over the longer term in ADepT relative to CBT,” he noted.
ADepT costs the same amount to deliver as CBT “but resulted in greater gains in quality of life, meaning it showed a high probability of being cost effective,” Dr. Dunn said.
ADepT has also been designed so that trained CBT therapists will be able to deliver it with minimal additional training.
“The next step,” said Dr. Dunn, “is a bigger definitive trial, which will formally test if ADepT is clinically superior to and better value for money than CBT when delivering ADepT in more routine care settings [U.K. NHS clinics rather than specialist university mood disorder center].”
The trial was funded by a Career Development Fellowship awarded to Dr. Dunn by the National Institute for Health and Care Research. Dr. Dunn has a book contract with Guilford Press to write the ADepT treatment manual and receives occasional payment or honoraria (including support for attending meetings) for delivering workshops and talks on ADepT.
A version of this article first appeared on Medscape.com.
While the trial was not adequately powered to test if augmented depression therapy (ADepT) is superior to CBT, “results nevertheless were encouraging,” lead author Barnaby Dunn, PhD, professor of clinical psychology, University of Exeter (England), said in an interview.
The trial showed that ADepT is feasible, acceptable, and “not worse than CBT,” also showing the “potential to be better than CBT in clinical outcomes,” Dr. Dunn said.
“By building positive mood, ADepT may help individuals stay well for longer in the future,” he noted.
The early results were published online in eClinicalMedicine.
Dual approach
“There are two sides to the depression coin – heightened negative mood and reduced positive mood. Classic CBT focuses mainly on repairing negative mood and pays less attention to building positive mood,” Dr. Dunn explained.
He said when he speaks to clients about what is key to recovery from depression, they often mention the importance of reconnecting to the positive.
“ADepT pays equal attention to building the positives as it does reducing the negatives, giving clients new skills to ‘act opposite’ to old ways of thinking and feeling that can stop them making the most of opportunities and being able to experience well-being,” Dr. Dunn said.
ADepT is an individual therapy delivered over 15 acute and 5 booster sessions, which is similar in “dose” to classic CBT, Dr. Dunn said.
The primary focus of ADepT is building well-being (capacity to experience pleasure, meaning, and social connection in life) and functional recovery, with depression conceptualized as patterns of thinking, feeling, and behaving that serve as barriers to achieving this goal.
Patients work with trained therapists to overcome barriers to being resilient (managing challenges to reduce negative affect) and thriving (taking opportunities to maximize positive affect).
A total of 82 adults with a moderate to severe current major depressive episode with features of anhedonia took part in the pilot trial. They were randomly allocated 1:1 to either 20 individual sessions of ADepT or CBT, delivered in the University of Exeter Accessing Evidence Based Psychological Therapies outpatient clinic.
Researcher-blinded assessments were completed at intake and after 6, 12, and 18 months. Coprimary outcomes were depression, measured via the Patient Health Questionnaire and well-being, gauged with the Warwick Edinburgh Mental Wellbeing Scale at 6 months.
Within-group analyses showed that both ADepT and CBT led to clinically meaningful improvements in depression, well-being, and all other secondary outcomes, including measures of anhedonia, Dr. Dunn said.
Between-group effects favored ADepT over CBT for depression and well-being. “For example, about 80% of clients no longer met diagnostic criteria for depression after ADepT, compared to around 56% of clients in CBT,” Dr. Dunn said.
“There were also numerically bigger gains in well-being and reductions in anhedonia in ADepT relative to CBT. A greater number of clients who recovered at the end of therapy stayed well over the longer term in ADepT relative to CBT,” he noted.
ADepT costs the same amount to deliver as CBT “but resulted in greater gains in quality of life, meaning it showed a high probability of being cost effective,” Dr. Dunn said.
ADepT has also been designed so that trained CBT therapists will be able to deliver it with minimal additional training.
“The next step,” said Dr. Dunn, “is a bigger definitive trial, which will formally test if ADepT is clinically superior to and better value for money than CBT when delivering ADepT in more routine care settings [U.K. NHS clinics rather than specialist university mood disorder center].”
The trial was funded by a Career Development Fellowship awarded to Dr. Dunn by the National Institute for Health and Care Research. Dr. Dunn has a book contract with Guilford Press to write the ADepT treatment manual and receives occasional payment or honoraria (including support for attending meetings) for delivering workshops and talks on ADepT.
A version of this article first appeared on Medscape.com.
FROM ECLINICALMEDICINE