User login
Avoiding atopic dermatitis triggers easier said than done
“Guidelines on trigger avoidance are written as if it’s easy to do,” Jonathan I. Silverberg, MD, PhD, MPH, said during the Revolutionizing Atopic Dermatitis virtual symposium. “It turns out that trigger avoidance is really complicated.”
He and his colleagues conducted a study of most common triggers for itch based on a prospective dermatology practice–based study of 587 adults with AD . About two-thirds (65%) reported one or more itch trigger in the past week and 36% had three or more itch triggers in the past week. The two most common triggers were stress (35%) and sweat (31%).
“To me, this is provocative, because this is not how I was trained in residency,” said Dr. Silverberg, director of clinical research in the division of dermatology at George Washington University, Washington. “I was trained that it’s all about excess showering, dry air, or cold temperature. Those are important, but the most common triggers are stress and sweat.”
AD triggers are also commonly linked to seasonality. “If you ask patients when their AD is worse, sometimes it’s winter,” he said. “Sometimes it’s spring. Sometimes it’s summer. It turns out that there is a distinct set of triggers that are associated with AD seasonality.” Wintertime worsening of disease is associated with cold temperature and weather change, he continued, while springtime worsening of disease is often linked to weather change and dry air. Common summertime triggers for flares include hot temperature, heat, sweat, weather change, sunlight, humid air, and dry air. “In the fall, the weather change again comes up as a trigger. Humid air does as well.”
In their prospective study, Dr. Silverberg and colleagues found that 90% of those who had at least three itch triggers reported 3 months or less of AD remission in the past year, “meaning that 90% are reporting persistent disease when they have multiple itch triggers,” he said. In addition, 78% reported two or more flares per year and 61% reported that AD is worse during certain seasons.
Potential mitigation strategies for stress include stress management, biofeedback, meditation, relaxation training, and mindfulness. “These don’t necessarily require expensive psychotherapy,” he said. Freely available iPhone apps can be incorporated into daily practice, such as Calm, Relax with Andrew Johnson, Nature Sounds Relax and Sleep, Breathe2Relax, and Headspace.
Many AD patients are sedentary and avoid vigorous physical activity owing to heat and sweat as triggers. Simple solutions include exercising in a cooler temperature environment, “not just using fans,” he said. “Take a quick shower right after working out and consider pre- and/or post treatment with topical medication.”
High temperature and sweating can be problematic at bedtime, he continued. Even if the indoor temperature is 70° F, that might jump to 85° F or 90° F under a thick blanket. “That heat can trigger itch and may cause sweating, which can trigger itch,” said Dr. Silverberg, who has AD and is director of patch testing at George Washington University. Potential solutions include using a lighter blanket, lowering the indoor temperature, and wearing breathable pajamas.
Dryness, another common AD trigger, can be secondary to a combination of low outdoor and/or indoor humidity. “Lower outdoor humidity is a particular problem in the wintertime, because cold air doesn’t hold moisture as well,” he said. “That’s why the air feels much dryer in the wintertime. There’s also a problem of indoor heating and cooling. Sometimes central air systems can lower humidity to the point where it’s bone dry.”
In an effort to determine the impact of specific climatic factors on the U.S. prevalence of AD, Dr. Silverberg and colleagues conducted a study using a merged analysis of the 2007 National Survey of Children’s Health from a representative sample of 91,642 children aged 0-17 years and 2006-2007 measurements from the National Climate Data Center and Weather Service. They found that childhood AD prevalence was increased in geographical areas that use more indoor heat and cooling and had lower outdoor humidity. “So, we see that there’s a direct correlate of this dryness issue that is leading to more AD throughout the U.S.,” he said.
Practical solutions to mitigate the effect of dry air on AD include opening windows to allow entry of moist air, “which can be particularly helpful in residences that are overheated,” he said. “I deal with this a lot in patients who live in dormitories. Use humidifiers to add moisture back into the air. Aim for 40%-50% indoor humidity to avoid mold and dust mites. It’s better to use demineralized water to reduce bacterial growth. This can be helpful for aeroallergies. Of note, there are really no well-done studies that have examined the efficacy of humidifiers in AD, but based on our anecdotal experience, this is a good way to go.”
Cold temperatures and trigger intense itch, even in the setting of high humidity. “For me personally, this is one of my most brutal triggers,” Dr. Silverberg said. “When I’m in a place with extremes of cold, I get a rapid onset of itch, a mix of itch and pain, particularly on the dorsal hands. For solutions, you can encourage patients to avoid extremely low temperatures, to bundle up, and to potentially use hand warmers or other heating devices.”
Clothing can be a trigger as well, especially tight-fitting clothes, hot and nonbreathable clothes, and large-diameter wool, which has been shown to induce itching and irritation. Mitigation strategies include wearing loose-fitting, lightweight, nonirritating fabric. “Traditional cotton and silk fabrics have mixed evidence in improving AD but are generally safe,” he said. “Ultra- or superfine merino wool has been shown to be nonpruritic. There is sparse evidence to support chemically treated/coated clothing for AD, but this may be an emerging area.”
Dr. Silverberg pointed out variability of cultural perspectives and preferences for bathing practices, including temperature, duration, frequency, optimal bathing products, and the use of loofahs and other scrubbing products. “This stems from different perceptions of what it means to be clean, and how dry our skin should feel after a shower,” he said. “Many clinicians and patients were taught that regular bathing is harmful in AD. It turns out that’s not true.”
In a recently published systematic review and meta-analysis of 13 studies, he and his colleagues examined efficacy outcomes of different bathing/showering regimens in AD. All 13 studies showed numerically reduced AD severity with any bathing regimen in at least one time point. Numerical decreases over time were observed for body surface area (BSA), Eczema Area and Severity Index (EASI), and/or SCORAD measures for daily and less than daily bathing, with or without application of emollients or topical corticosteroids. In random effects regression models, taking baths more than or less than seven times per week were not associated with significant differences of Cohen’s D scores for EASI, SCORAD, or BSA. “The take-home message here is, let your AD patients bathe,” Dr. Silverberg said. “Bathing is good. It can be channeled to help the eczema, but it has to be done the right way.”
Patients should be counseled to use nonirritating cleansers and shampoos, avoid excessively long baths/showers, avoid excessively hot baths/showers, avoid excessive rubbing or scrubbing of skin, and to apply emollients and/or topical corticosteroids immediately after the bath/shower.
PROMIS Itch-Triggers is a simple and feasible checklist to screen for the most common itch triggers in AD in clinical practice (patients are asked to check off which of the following have caused their itch in the previous 7 days: cold temperature, hot temperature, heat, sweat, tight clothing, fragrances, boredom, talking about itch, stress, weather change, sunlight, humid air, dry air). “It takes less than 1 minute to complete,” he said. “Additional testing with skin patch and/or prick testing may be warranted to identify allergenic triggers.”
Dr. Silverberg reported that he is a consultant to and/or an advisory board member for several pharmaceutical companies. He is also a speaker for Regeneron and Sanofi and has received a grant from Galderma.
“Guidelines on trigger avoidance are written as if it’s easy to do,” Jonathan I. Silverberg, MD, PhD, MPH, said during the Revolutionizing Atopic Dermatitis virtual symposium. “It turns out that trigger avoidance is really complicated.”
He and his colleagues conducted a study of most common triggers for itch based on a prospective dermatology practice–based study of 587 adults with AD . About two-thirds (65%) reported one or more itch trigger in the past week and 36% had three or more itch triggers in the past week. The two most common triggers were stress (35%) and sweat (31%).
“To me, this is provocative, because this is not how I was trained in residency,” said Dr. Silverberg, director of clinical research in the division of dermatology at George Washington University, Washington. “I was trained that it’s all about excess showering, dry air, or cold temperature. Those are important, but the most common triggers are stress and sweat.”
AD triggers are also commonly linked to seasonality. “If you ask patients when their AD is worse, sometimes it’s winter,” he said. “Sometimes it’s spring. Sometimes it’s summer. It turns out that there is a distinct set of triggers that are associated with AD seasonality.” Wintertime worsening of disease is associated with cold temperature and weather change, he continued, while springtime worsening of disease is often linked to weather change and dry air. Common summertime triggers for flares include hot temperature, heat, sweat, weather change, sunlight, humid air, and dry air. “In the fall, the weather change again comes up as a trigger. Humid air does as well.”
In their prospective study, Dr. Silverberg and colleagues found that 90% of those who had at least three itch triggers reported 3 months or less of AD remission in the past year, “meaning that 90% are reporting persistent disease when they have multiple itch triggers,” he said. In addition, 78% reported two or more flares per year and 61% reported that AD is worse during certain seasons.
Potential mitigation strategies for stress include stress management, biofeedback, meditation, relaxation training, and mindfulness. “These don’t necessarily require expensive psychotherapy,” he said. Freely available iPhone apps can be incorporated into daily practice, such as Calm, Relax with Andrew Johnson, Nature Sounds Relax and Sleep, Breathe2Relax, and Headspace.
Many AD patients are sedentary and avoid vigorous physical activity owing to heat and sweat as triggers. Simple solutions include exercising in a cooler temperature environment, “not just using fans,” he said. “Take a quick shower right after working out and consider pre- and/or post treatment with topical medication.”
High temperature and sweating can be problematic at bedtime, he continued. Even if the indoor temperature is 70° F, that might jump to 85° F or 90° F under a thick blanket. “That heat can trigger itch and may cause sweating, which can trigger itch,” said Dr. Silverberg, who has AD and is director of patch testing at George Washington University. Potential solutions include using a lighter blanket, lowering the indoor temperature, and wearing breathable pajamas.
Dryness, another common AD trigger, can be secondary to a combination of low outdoor and/or indoor humidity. “Lower outdoor humidity is a particular problem in the wintertime, because cold air doesn’t hold moisture as well,” he said. “That’s why the air feels much dryer in the wintertime. There’s also a problem of indoor heating and cooling. Sometimes central air systems can lower humidity to the point where it’s bone dry.”
In an effort to determine the impact of specific climatic factors on the U.S. prevalence of AD, Dr. Silverberg and colleagues conducted a study using a merged analysis of the 2007 National Survey of Children’s Health from a representative sample of 91,642 children aged 0-17 years and 2006-2007 measurements from the National Climate Data Center and Weather Service. They found that childhood AD prevalence was increased in geographical areas that use more indoor heat and cooling and had lower outdoor humidity. “So, we see that there’s a direct correlate of this dryness issue that is leading to more AD throughout the U.S.,” he said.
Practical solutions to mitigate the effect of dry air on AD include opening windows to allow entry of moist air, “which can be particularly helpful in residences that are overheated,” he said. “I deal with this a lot in patients who live in dormitories. Use humidifiers to add moisture back into the air. Aim for 40%-50% indoor humidity to avoid mold and dust mites. It’s better to use demineralized water to reduce bacterial growth. This can be helpful for aeroallergies. Of note, there are really no well-done studies that have examined the efficacy of humidifiers in AD, but based on our anecdotal experience, this is a good way to go.”
Cold temperatures and trigger intense itch, even in the setting of high humidity. “For me personally, this is one of my most brutal triggers,” Dr. Silverberg said. “When I’m in a place with extremes of cold, I get a rapid onset of itch, a mix of itch and pain, particularly on the dorsal hands. For solutions, you can encourage patients to avoid extremely low temperatures, to bundle up, and to potentially use hand warmers or other heating devices.”
Clothing can be a trigger as well, especially tight-fitting clothes, hot and nonbreathable clothes, and large-diameter wool, which has been shown to induce itching and irritation. Mitigation strategies include wearing loose-fitting, lightweight, nonirritating fabric. “Traditional cotton and silk fabrics have mixed evidence in improving AD but are generally safe,” he said. “Ultra- or superfine merino wool has been shown to be nonpruritic. There is sparse evidence to support chemically treated/coated clothing for AD, but this may be an emerging area.”
Dr. Silverberg pointed out variability of cultural perspectives and preferences for bathing practices, including temperature, duration, frequency, optimal bathing products, and the use of loofahs and other scrubbing products. “This stems from different perceptions of what it means to be clean, and how dry our skin should feel after a shower,” he said. “Many clinicians and patients were taught that regular bathing is harmful in AD. It turns out that’s not true.”
In a recently published systematic review and meta-analysis of 13 studies, he and his colleagues examined efficacy outcomes of different bathing/showering regimens in AD. All 13 studies showed numerically reduced AD severity with any bathing regimen in at least one time point. Numerical decreases over time were observed for body surface area (BSA), Eczema Area and Severity Index (EASI), and/or SCORAD measures for daily and less than daily bathing, with or without application of emollients or topical corticosteroids. In random effects regression models, taking baths more than or less than seven times per week were not associated with significant differences of Cohen’s D scores for EASI, SCORAD, or BSA. “The take-home message here is, let your AD patients bathe,” Dr. Silverberg said. “Bathing is good. It can be channeled to help the eczema, but it has to be done the right way.”
Patients should be counseled to use nonirritating cleansers and shampoos, avoid excessively long baths/showers, avoid excessively hot baths/showers, avoid excessive rubbing or scrubbing of skin, and to apply emollients and/or topical corticosteroids immediately after the bath/shower.
PROMIS Itch-Triggers is a simple and feasible checklist to screen for the most common itch triggers in AD in clinical practice (patients are asked to check off which of the following have caused their itch in the previous 7 days: cold temperature, hot temperature, heat, sweat, tight clothing, fragrances, boredom, talking about itch, stress, weather change, sunlight, humid air, dry air). “It takes less than 1 minute to complete,” he said. “Additional testing with skin patch and/or prick testing may be warranted to identify allergenic triggers.”
Dr. Silverberg reported that he is a consultant to and/or an advisory board member for several pharmaceutical companies. He is also a speaker for Regeneron and Sanofi and has received a grant from Galderma.
“Guidelines on trigger avoidance are written as if it’s easy to do,” Jonathan I. Silverberg, MD, PhD, MPH, said during the Revolutionizing Atopic Dermatitis virtual symposium. “It turns out that trigger avoidance is really complicated.”
He and his colleagues conducted a study of most common triggers for itch based on a prospective dermatology practice–based study of 587 adults with AD . About two-thirds (65%) reported one or more itch trigger in the past week and 36% had three or more itch triggers in the past week. The two most common triggers were stress (35%) and sweat (31%).
“To me, this is provocative, because this is not how I was trained in residency,” said Dr. Silverberg, director of clinical research in the division of dermatology at George Washington University, Washington. “I was trained that it’s all about excess showering, dry air, or cold temperature. Those are important, but the most common triggers are stress and sweat.”
AD triggers are also commonly linked to seasonality. “If you ask patients when their AD is worse, sometimes it’s winter,” he said. “Sometimes it’s spring. Sometimes it’s summer. It turns out that there is a distinct set of triggers that are associated with AD seasonality.” Wintertime worsening of disease is associated with cold temperature and weather change, he continued, while springtime worsening of disease is often linked to weather change and dry air. Common summertime triggers for flares include hot temperature, heat, sweat, weather change, sunlight, humid air, and dry air. “In the fall, the weather change again comes up as a trigger. Humid air does as well.”
In their prospective study, Dr. Silverberg and colleagues found that 90% of those who had at least three itch triggers reported 3 months or less of AD remission in the past year, “meaning that 90% are reporting persistent disease when they have multiple itch triggers,” he said. In addition, 78% reported two or more flares per year and 61% reported that AD is worse during certain seasons.
Potential mitigation strategies for stress include stress management, biofeedback, meditation, relaxation training, and mindfulness. “These don’t necessarily require expensive psychotherapy,” he said. Freely available iPhone apps can be incorporated into daily practice, such as Calm, Relax with Andrew Johnson, Nature Sounds Relax and Sleep, Breathe2Relax, and Headspace.
Many AD patients are sedentary and avoid vigorous physical activity owing to heat and sweat as triggers. Simple solutions include exercising in a cooler temperature environment, “not just using fans,” he said. “Take a quick shower right after working out and consider pre- and/or post treatment with topical medication.”
High temperature and sweating can be problematic at bedtime, he continued. Even if the indoor temperature is 70° F, that might jump to 85° F or 90° F under a thick blanket. “That heat can trigger itch and may cause sweating, which can trigger itch,” said Dr. Silverberg, who has AD and is director of patch testing at George Washington University. Potential solutions include using a lighter blanket, lowering the indoor temperature, and wearing breathable pajamas.
Dryness, another common AD trigger, can be secondary to a combination of low outdoor and/or indoor humidity. “Lower outdoor humidity is a particular problem in the wintertime, because cold air doesn’t hold moisture as well,” he said. “That’s why the air feels much dryer in the wintertime. There’s also a problem of indoor heating and cooling. Sometimes central air systems can lower humidity to the point where it’s bone dry.”
In an effort to determine the impact of specific climatic factors on the U.S. prevalence of AD, Dr. Silverberg and colleagues conducted a study using a merged analysis of the 2007 National Survey of Children’s Health from a representative sample of 91,642 children aged 0-17 years and 2006-2007 measurements from the National Climate Data Center and Weather Service. They found that childhood AD prevalence was increased in geographical areas that use more indoor heat and cooling and had lower outdoor humidity. “So, we see that there’s a direct correlate of this dryness issue that is leading to more AD throughout the U.S.,” he said.
Practical solutions to mitigate the effect of dry air on AD include opening windows to allow entry of moist air, “which can be particularly helpful in residences that are overheated,” he said. “I deal with this a lot in patients who live in dormitories. Use humidifiers to add moisture back into the air. Aim for 40%-50% indoor humidity to avoid mold and dust mites. It’s better to use demineralized water to reduce bacterial growth. This can be helpful for aeroallergies. Of note, there are really no well-done studies that have examined the efficacy of humidifiers in AD, but based on our anecdotal experience, this is a good way to go.”
Cold temperatures and trigger intense itch, even in the setting of high humidity. “For me personally, this is one of my most brutal triggers,” Dr. Silverberg said. “When I’m in a place with extremes of cold, I get a rapid onset of itch, a mix of itch and pain, particularly on the dorsal hands. For solutions, you can encourage patients to avoid extremely low temperatures, to bundle up, and to potentially use hand warmers or other heating devices.”
Clothing can be a trigger as well, especially tight-fitting clothes, hot and nonbreathable clothes, and large-diameter wool, which has been shown to induce itching and irritation. Mitigation strategies include wearing loose-fitting, lightweight, nonirritating fabric. “Traditional cotton and silk fabrics have mixed evidence in improving AD but are generally safe,” he said. “Ultra- or superfine merino wool has been shown to be nonpruritic. There is sparse evidence to support chemically treated/coated clothing for AD, but this may be an emerging area.”
Dr. Silverberg pointed out variability of cultural perspectives and preferences for bathing practices, including temperature, duration, frequency, optimal bathing products, and the use of loofahs and other scrubbing products. “This stems from different perceptions of what it means to be clean, and how dry our skin should feel after a shower,” he said. “Many clinicians and patients were taught that regular bathing is harmful in AD. It turns out that’s not true.”
In a recently published systematic review and meta-analysis of 13 studies, he and his colleagues examined efficacy outcomes of different bathing/showering regimens in AD. All 13 studies showed numerically reduced AD severity with any bathing regimen in at least one time point. Numerical decreases over time were observed for body surface area (BSA), Eczema Area and Severity Index (EASI), and/or SCORAD measures for daily and less than daily bathing, with or without application of emollients or topical corticosteroids. In random effects regression models, taking baths more than or less than seven times per week were not associated with significant differences of Cohen’s D scores for EASI, SCORAD, or BSA. “The take-home message here is, let your AD patients bathe,” Dr. Silverberg said. “Bathing is good. It can be channeled to help the eczema, but it has to be done the right way.”
Patients should be counseled to use nonirritating cleansers and shampoos, avoid excessively long baths/showers, avoid excessively hot baths/showers, avoid excessive rubbing or scrubbing of skin, and to apply emollients and/or topical corticosteroids immediately after the bath/shower.
PROMIS Itch-Triggers is a simple and feasible checklist to screen for the most common itch triggers in AD in clinical practice (patients are asked to check off which of the following have caused their itch in the previous 7 days: cold temperature, hot temperature, heat, sweat, tight clothing, fragrances, boredom, talking about itch, stress, weather change, sunlight, humid air, dry air). “It takes less than 1 minute to complete,” he said. “Additional testing with skin patch and/or prick testing may be warranted to identify allergenic triggers.”
Dr. Silverberg reported that he is a consultant to and/or an advisory board member for several pharmaceutical companies. He is also a speaker for Regeneron and Sanofi and has received a grant from Galderma.
FROM REVOLUTIONIZING AD 2020
Dupilumab curbed itch intensity, frequency in children with severe eczema
.
The findings come from a post hoc analysis of a phase 3 trial known as LIBERTY AD PEDS (NCT03345914) that Gil Yosipovitch, MD, presented during a late-breaking research session at the Revolutionizing Atopic Dermatitis virtual symposium.
“Severe AD is complex, highly symptomatic, multidimensional condition characterized by an intense pruritus that negatively impacts a patient’s life,” said Dr. Yosipovitch, professor of dermatology and director of the Miami Itch Center at the University of Miami. Published data from the double-blind, placebo-controlled, 16-week, LIBERTY AD PEDS trial in children aged 6–11 years with severe AD showed that dupilumab significantly improved AD signs, symptoms, and quality of life, with an acceptable safety profile (J Am Acad Dermatol. 2020;21:119-31).
For the current analysis, Dr. Yosipovitch and colleagues evaluated the time to onset, magnitude, and sustainability of the effect of dupilumab on different measures of itch using data from approved Food and Drug Administration doses studied in the LIBERTY AD PEDS trial. A total of 243 children aged 6-11 years were randomized to dupilumab 300 mg every 4 weeks (300 mg q4w, baseline weight of less than 30 kg; 600-mg loading dose), 200 mg every 2 weeks (200 mg q2w, baseline weight 30 kg or greater; 400-mg loading dose), or placebo. All patients received concomitant medium-potency topical corticosteroids.
The mean age of patients was 8.4 years and those in the 300-mg q4w group were about 2 years younger than those in the 200-mg q2w group. On the Peak Pruritus Numerical Rating Scale (NRS), the researchers observed that treatment with dupilumab was associated with a significant improvement from baseline in daily worst itch score through day 22 in the 300-mg q4w group and the 200-mg q2w group, compared with placebo (–29% vs. –30%, respectively; P less than or equal to .001 and P less than or equal to .05). Treatment with dupilumab was also associated with a significant improvement from baseline in weekly average of daily worst itch score through week 16, compared with placebo (–55% vs. –58%; P less than or equal to .001). Similarly, a higher daily proportion of dupilumab-treated patients achieved a 2-point or more improvement in worst itch score, compared with placebo (51% vs. 49%; P less than or equal to .001 and P less than or equal to .05). The same association held true for the daily proportion of dupilumab-treated patients who achieved a 4-point or more improvement in worst itch score, compared with placebo (21% in both groups; P less than or equal to .05).
By week 16, a higher weekly proportion of dupilumab-treated patients achieved a 2-point or more improvement in worst itch score, compared with placebo (72% in the 300-mg q4w group vs. 74% in the 200-mg q2w group; P less than or equal to .001). The same association held true for the daily proportion of dupilumab-treated patients who achieved a 4-point or more improvement in worst itch score, compared with placebo (54% vs. 61%; P less than or equal to .001).
Next, the researchers evaluated the proportion of patients reporting the number of days with itchy skin over the previous 7 days as assessed from the Patient-Oriented Eczema Measure (POEM) itch item question: “Over the last week, on how many days has your child’s skin been itchy because of their eczema?” By week 16, the majority of children treated with dupilumab achieved a reduction of days experiencing itch from every day at baseline to at most 2 days, with some improvement to zero days per week.
“Overall, in the LIBERTY AD PEDS trial, dupilumab was well tolerated and data were consistent with the known dupilumab safety profile observed in adults and adolescents,” Dr. Yosipovitch said. “Injection site reactions and conjunctivitis were more common with dupilumab. Infections and AD exacerbations were more common with placebo.”
The study was sponsored by Sanofi and Regeneron Pharmaceuticals. Dr. Yosipovitch and coauthors reporting having received financial grants and research grants from numerous pharmaceutical companies.
.
The findings come from a post hoc analysis of a phase 3 trial known as LIBERTY AD PEDS (NCT03345914) that Gil Yosipovitch, MD, presented during a late-breaking research session at the Revolutionizing Atopic Dermatitis virtual symposium.
“Severe AD is complex, highly symptomatic, multidimensional condition characterized by an intense pruritus that negatively impacts a patient’s life,” said Dr. Yosipovitch, professor of dermatology and director of the Miami Itch Center at the University of Miami. Published data from the double-blind, placebo-controlled, 16-week, LIBERTY AD PEDS trial in children aged 6–11 years with severe AD showed that dupilumab significantly improved AD signs, symptoms, and quality of life, with an acceptable safety profile (J Am Acad Dermatol. 2020;21:119-31).
For the current analysis, Dr. Yosipovitch and colleagues evaluated the time to onset, magnitude, and sustainability of the effect of dupilumab on different measures of itch using data from approved Food and Drug Administration doses studied in the LIBERTY AD PEDS trial. A total of 243 children aged 6-11 years were randomized to dupilumab 300 mg every 4 weeks (300 mg q4w, baseline weight of less than 30 kg; 600-mg loading dose), 200 mg every 2 weeks (200 mg q2w, baseline weight 30 kg or greater; 400-mg loading dose), or placebo. All patients received concomitant medium-potency topical corticosteroids.
The mean age of patients was 8.4 years and those in the 300-mg q4w group were about 2 years younger than those in the 200-mg q2w group. On the Peak Pruritus Numerical Rating Scale (NRS), the researchers observed that treatment with dupilumab was associated with a significant improvement from baseline in daily worst itch score through day 22 in the 300-mg q4w group and the 200-mg q2w group, compared with placebo (–29% vs. –30%, respectively; P less than or equal to .001 and P less than or equal to .05). Treatment with dupilumab was also associated with a significant improvement from baseline in weekly average of daily worst itch score through week 16, compared with placebo (–55% vs. –58%; P less than or equal to .001). Similarly, a higher daily proportion of dupilumab-treated patients achieved a 2-point or more improvement in worst itch score, compared with placebo (51% vs. 49%; P less than or equal to .001 and P less than or equal to .05). The same association held true for the daily proportion of dupilumab-treated patients who achieved a 4-point or more improvement in worst itch score, compared with placebo (21% in both groups; P less than or equal to .05).
By week 16, a higher weekly proportion of dupilumab-treated patients achieved a 2-point or more improvement in worst itch score, compared with placebo (72% in the 300-mg q4w group vs. 74% in the 200-mg q2w group; P less than or equal to .001). The same association held true for the daily proportion of dupilumab-treated patients who achieved a 4-point or more improvement in worst itch score, compared with placebo (54% vs. 61%; P less than or equal to .001).
Next, the researchers evaluated the proportion of patients reporting the number of days with itchy skin over the previous 7 days as assessed from the Patient-Oriented Eczema Measure (POEM) itch item question: “Over the last week, on how many days has your child’s skin been itchy because of their eczema?” By week 16, the majority of children treated with dupilumab achieved a reduction of days experiencing itch from every day at baseline to at most 2 days, with some improvement to zero days per week.
“Overall, in the LIBERTY AD PEDS trial, dupilumab was well tolerated and data were consistent with the known dupilumab safety profile observed in adults and adolescents,” Dr. Yosipovitch said. “Injection site reactions and conjunctivitis were more common with dupilumab. Infections and AD exacerbations were more common with placebo.”
The study was sponsored by Sanofi and Regeneron Pharmaceuticals. Dr. Yosipovitch and coauthors reporting having received financial grants and research grants from numerous pharmaceutical companies.
.
The findings come from a post hoc analysis of a phase 3 trial known as LIBERTY AD PEDS (NCT03345914) that Gil Yosipovitch, MD, presented during a late-breaking research session at the Revolutionizing Atopic Dermatitis virtual symposium.
“Severe AD is complex, highly symptomatic, multidimensional condition characterized by an intense pruritus that negatively impacts a patient’s life,” said Dr. Yosipovitch, professor of dermatology and director of the Miami Itch Center at the University of Miami. Published data from the double-blind, placebo-controlled, 16-week, LIBERTY AD PEDS trial in children aged 6–11 years with severe AD showed that dupilumab significantly improved AD signs, symptoms, and quality of life, with an acceptable safety profile (J Am Acad Dermatol. 2020;21:119-31).
For the current analysis, Dr. Yosipovitch and colleagues evaluated the time to onset, magnitude, and sustainability of the effect of dupilumab on different measures of itch using data from approved Food and Drug Administration doses studied in the LIBERTY AD PEDS trial. A total of 243 children aged 6-11 years were randomized to dupilumab 300 mg every 4 weeks (300 mg q4w, baseline weight of less than 30 kg; 600-mg loading dose), 200 mg every 2 weeks (200 mg q2w, baseline weight 30 kg or greater; 400-mg loading dose), or placebo. All patients received concomitant medium-potency topical corticosteroids.
The mean age of patients was 8.4 years and those in the 300-mg q4w group were about 2 years younger than those in the 200-mg q2w group. On the Peak Pruritus Numerical Rating Scale (NRS), the researchers observed that treatment with dupilumab was associated with a significant improvement from baseline in daily worst itch score through day 22 in the 300-mg q4w group and the 200-mg q2w group, compared with placebo (–29% vs. –30%, respectively; P less than or equal to .001 and P less than or equal to .05). Treatment with dupilumab was also associated with a significant improvement from baseline in weekly average of daily worst itch score through week 16, compared with placebo (–55% vs. –58%; P less than or equal to .001). Similarly, a higher daily proportion of dupilumab-treated patients achieved a 2-point or more improvement in worst itch score, compared with placebo (51% vs. 49%; P less than or equal to .001 and P less than or equal to .05). The same association held true for the daily proportion of dupilumab-treated patients who achieved a 4-point or more improvement in worst itch score, compared with placebo (21% in both groups; P less than or equal to .05).
By week 16, a higher weekly proportion of dupilumab-treated patients achieved a 2-point or more improvement in worst itch score, compared with placebo (72% in the 300-mg q4w group vs. 74% in the 200-mg q2w group; P less than or equal to .001). The same association held true for the daily proportion of dupilumab-treated patients who achieved a 4-point or more improvement in worst itch score, compared with placebo (54% vs. 61%; P less than or equal to .001).
Next, the researchers evaluated the proportion of patients reporting the number of days with itchy skin over the previous 7 days as assessed from the Patient-Oriented Eczema Measure (POEM) itch item question: “Over the last week, on how many days has your child’s skin been itchy because of their eczema?” By week 16, the majority of children treated with dupilumab achieved a reduction of days experiencing itch from every day at baseline to at most 2 days, with some improvement to zero days per week.
“Overall, in the LIBERTY AD PEDS trial, dupilumab was well tolerated and data were consistent with the known dupilumab safety profile observed in adults and adolescents,” Dr. Yosipovitch said. “Injection site reactions and conjunctivitis were more common with dupilumab. Infections and AD exacerbations were more common with placebo.”
The study was sponsored by Sanofi and Regeneron Pharmaceuticals. Dr. Yosipovitch and coauthors reporting having received financial grants and research grants from numerous pharmaceutical companies.
REPORTING FROM REVOLUTIONIZING AD 2020
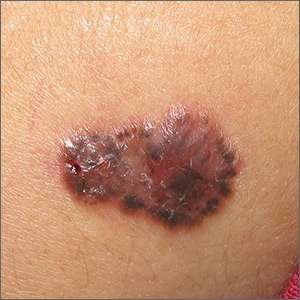
Growing brown and pink plaque
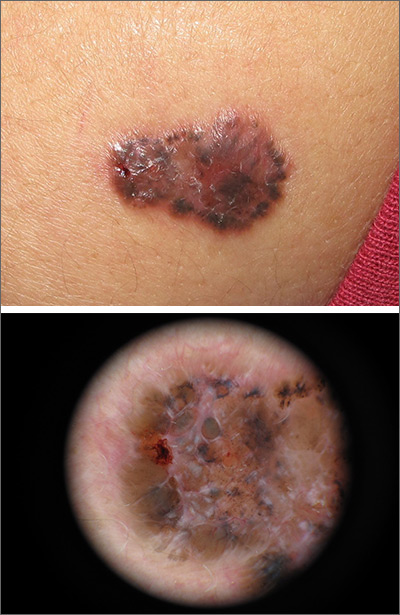
A shave biopsy of the lesion confirmed a nodular and pigmented basal cell carcinoma (BCC). In the United States, BCC is the most common cancer and accounts for 80% of all skin cancer diagnoses. BCC is also the most widespread cancer in White, Hispanic, and Asian patients and the second most common skin cancer in Black patients.
In all patients, BCC presents as a shiny growing macule, papule, or plaque, usually in areas of sun exposure. Much less is known about the role of UV exposure in skin of color because of the lack of high-quality studies in this population. Despite this, BCC in skin of color most often presents on the head and neck, as it does in non-Hispanic White patients. There is a correlation between lighter skin tones in Black patients and increased numbers of BCC diagnoses.
When assessing a suspicious skin lesion in skin of color, be on the lookout for the following visual cues. Pigmentation, clinically and on dermoscopy, is a more common feature of BCCs in non-White patients—occurring in more than half of all BCCs in skin of color.1 While pigmentation in a skin tumor may be mistaken for melanoma, the blue ovoid nests, brown dots in focus, and brown leaf-like areas on dermoscopy are BCC-specific clues that can alert the clinician to the diagnosis. In all patients, telangiectasias are another hallmark of BCC but may be the only feature in White patients and just one of many features in non-White patients.
In small data sets, there is no difference in tumor-related morbidity and prognosis between White and Black patients with BCC. Additionally, BCCs in White, Asian, and Hispanic patients have had no differences in preoperative tumor size, number of Mohs stages, and outcome.1
In this case, the patient underwent a complete excision with a 5-mm margin. She remained free of new or recurrent BCCs over the next 2 years, with surveillance exams twice a year.
Text and photos courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. (Photo copyright retained.)
1. Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526.

A shave biopsy of the lesion confirmed a nodular and pigmented basal cell carcinoma (BCC). In the United States, BCC is the most common cancer and accounts for 80% of all skin cancer diagnoses. BCC is also the most widespread cancer in White, Hispanic, and Asian patients and the second most common skin cancer in Black patients.
In all patients, BCC presents as a shiny growing macule, papule, or plaque, usually in areas of sun exposure. Much less is known about the role of UV exposure in skin of color because of the lack of high-quality studies in this population. Despite this, BCC in skin of color most often presents on the head and neck, as it does in non-Hispanic White patients. There is a correlation between lighter skin tones in Black patients and increased numbers of BCC diagnoses.
When assessing a suspicious skin lesion in skin of color, be on the lookout for the following visual cues. Pigmentation, clinically and on dermoscopy, is a more common feature of BCCs in non-White patients—occurring in more than half of all BCCs in skin of color.1 While pigmentation in a skin tumor may be mistaken for melanoma, the blue ovoid nests, brown dots in focus, and brown leaf-like areas on dermoscopy are BCC-specific clues that can alert the clinician to the diagnosis. In all patients, telangiectasias are another hallmark of BCC but may be the only feature in White patients and just one of many features in non-White patients.
In small data sets, there is no difference in tumor-related morbidity and prognosis between White and Black patients with BCC. Additionally, BCCs in White, Asian, and Hispanic patients have had no differences in preoperative tumor size, number of Mohs stages, and outcome.1
In this case, the patient underwent a complete excision with a 5-mm margin. She remained free of new or recurrent BCCs over the next 2 years, with surveillance exams twice a year.
Text and photos courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. (Photo copyright retained.)

A shave biopsy of the lesion confirmed a nodular and pigmented basal cell carcinoma (BCC). In the United States, BCC is the most common cancer and accounts for 80% of all skin cancer diagnoses. BCC is also the most widespread cancer in White, Hispanic, and Asian patients and the second most common skin cancer in Black patients.
In all patients, BCC presents as a shiny growing macule, papule, or plaque, usually in areas of sun exposure. Much less is known about the role of UV exposure in skin of color because of the lack of high-quality studies in this population. Despite this, BCC in skin of color most often presents on the head and neck, as it does in non-Hispanic White patients. There is a correlation between lighter skin tones in Black patients and increased numbers of BCC diagnoses.
When assessing a suspicious skin lesion in skin of color, be on the lookout for the following visual cues. Pigmentation, clinically and on dermoscopy, is a more common feature of BCCs in non-White patients—occurring in more than half of all BCCs in skin of color.1 While pigmentation in a skin tumor may be mistaken for melanoma, the blue ovoid nests, brown dots in focus, and brown leaf-like areas on dermoscopy are BCC-specific clues that can alert the clinician to the diagnosis. In all patients, telangiectasias are another hallmark of BCC but may be the only feature in White patients and just one of many features in non-White patients.
In small data sets, there is no difference in tumor-related morbidity and prognosis between White and Black patients with BCC. Additionally, BCCs in White, Asian, and Hispanic patients have had no differences in preoperative tumor size, number of Mohs stages, and outcome.1
In this case, the patient underwent a complete excision with a 5-mm margin. She remained free of new or recurrent BCCs over the next 2 years, with surveillance exams twice a year.
Text and photos courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. (Photo copyright retained.)
1. Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526.
1. Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526.
Expert picks top pediatric dermatology studies of 2020
Lawrence F. Eichenfield, MD, said at the annual Coastal Dermatology Symposium, held virtually.
Dr. Eichenfield, professor of dermatology and pediatrics, at the University of California, San Diego, presented a list of studies, some of which resulted in approvals of pediatric indications in 2020, that he believes deserve attention.
Crisaborole
Crisaborole ointment, 2% is now approved for topical treatment of children aged as young as 3 months, based on the results of the CrisADe CARE1 phase 4 study. In this open-label study of infants aged from 3 months to under 2 years with mild to moderate AD, treated with crisaborole twice a day for 28 days, the mean reduction from baseline in the Eczema Area and Severity Index (EASI) score was 49.6% on day 15 and 57.5% on day 29. The most common side effects were erythema and application-site pain, but neither occurred in more than 4% of patients. The discontinuation rate was less than 3%.
When the indication for treatment of young children down to age 3 months (from 24 months) was granted by the Food and Drug Administration in March 2020, crisaborole, a phosphodiesterase-4 inhibitor, became the only nonsteroidal approved for treatment of AD in children aged younger than 2 years, Dr. Eichenfield pointed out.
Tacrolimus
The topical calcineurin inhibitor tacrolimus (Protopic) poses no detectable risk of cancer in children treated for AD, according to a prospective, multinational study that followed nearly 8,000 children with AD who used topical tacrolimus for at least 6 weeks over 10 years. With 44,469 person-years of follow-up in a population with at least 6 weeks of exposure to tacrolimus, there were six confirmed cancers, a rate not different than background rates, and no lymphomas.
“I have always tried to educate my patients about the potential use of the topical calcineurin inhibitors while reassuring them that the data did not support significant risk,” Dr. Eichenfield said. However, a large set of data reconfirming a low risk of cancer, although not definitive, “are really nice to have.”
Ruxolitinib
For treatment of AD in children aged as young as 12 years, a cream formulation of ruxolitinib, a Janus kinase 1/JAK2 inhibitor, met its primary outcomes in the phase 3 TRuE AD1 and TRuE AD2 trials. (These data are not yet published but were presented at the Revolutionizing Atopic Dermatitis virtual symposium in April 2020.) The primary endpoint of 75% EASI clearance (EASI-75) was achieved in approximately 62% of patients treated with the 1.5% dose of ruxolitinib twice daily. This was a highly significant advantage over vehicle in both studies (P < .0001).
The EASI-75 rates at 8 weeks for the 0.75% formulation, at 56% and 51.5% for the TRuE AD1 and TRuE AD2 trials, respectively, were lower but also superior (P < .0001) to the 24.6% and 14.4% response rates on vehicle, respectively.
Emphasizing a consistent benefit on multiple secondary endpoints, including the “really early itch decrease,” Dr. Eichenfield described the phase 3 data as “really excellent results.” The data have not yet led to FDA approval of ruxolitinib for AD, but approval seems likely. Dr. Eichenfield noted that other drugs in the same class, such as abrocitinib and upadacitinib, have also demonstrated promising efficacy in children aged 12 years or older.
Dupilumab
Dupilumab, an interleukin-4 receptor alpha antagonist, was approved in May, 2020, for the treatment of AD in children ages 6-11 years, on the basis of a recently published phase 3, randomized, placebo-controlled trial that enrolled children aged between 6 and 11 years, comparing dupilumab and topical corticosteroids and placebo plus topical corticosteroids. Severe involvement was an entry criterion.
At 16 weeks, an EASI-75 response was achieved by 67% of the group randomized to 200 mg of dupilumab administered every 2 weeks and 70% of the group randomized to 300 mg every 4 weeks versus 27% of those randomized to placebo. More patients in the dupilumab arms developed conjunctivitis (10.8% vs. 4.7%) and had injection-site reactions (8.5% vs. 3.5%), but the monoclonal antibody was otherwise well tolerated and safe.
These data suggest that younger patients with severe disease “do, if anything, better than adults,” Dr. Eichenfield said at the meeting, jointly presented by the University of Louisville and Global Academy for Medical Education. He cautioned that avoiding live vaccines, which is recommended in patients on dupilumab, “is likely more of an issue in children.”
Ixekizumab
Ixekizumab has been approved for pediatric patients aged as young as 6 years who are eligible for systemic therapy on the basis of a phase 3 trial. For the primary endpoint of 75% clearance on the Psoriasis Area and Severity Index, the response rates were 89% for the IL-17 inhibitor administered every 4 weeks and 25% for placebo. The study also associated ixekizumab with a significant improvement in quality of life.
The availability of more targeted therapies for children are likely. In Europe, secukinumab, another IL-17 inhibitor, was approved for treatment in pediatric patients this past summer, Dr. Eichenfield noted. These data are not yet published, but he expects targeted therapies to join a growing list of biologics already approved in children.
For drugs with established efficacy and safety, he advised, “look at your pediatric psoriasis patients and don’t be wimpy.” In children with poorly controlled psoriasis, he concluded these drugs have been associated with improved quality of life.
In November 2019, the American Academy of Dermatology and National Psoriasis Foundation published psoriasis management guidelines for children. Not all of the most recently approved therapies are included in these guidelines, which are the first to provide specific recommendations for children, but Dr. Eichenfield also included this publication among his top picks for important contributions to the pediatric dermatology literature in 2020.
Dr. Eichenfield reported financial relationships with 20 pharmaceutical companies that manufacture dermatologic products, including those for the diseases he discussed.
This publication and Global Academy for Medical Education are owned by the same parent company.
Lawrence F. Eichenfield, MD, said at the annual Coastal Dermatology Symposium, held virtually.
Dr. Eichenfield, professor of dermatology and pediatrics, at the University of California, San Diego, presented a list of studies, some of which resulted in approvals of pediatric indications in 2020, that he believes deserve attention.
Crisaborole
Crisaborole ointment, 2% is now approved for topical treatment of children aged as young as 3 months, based on the results of the CrisADe CARE1 phase 4 study. In this open-label study of infants aged from 3 months to under 2 years with mild to moderate AD, treated with crisaborole twice a day for 28 days, the mean reduction from baseline in the Eczema Area and Severity Index (EASI) score was 49.6% on day 15 and 57.5% on day 29. The most common side effects were erythema and application-site pain, but neither occurred in more than 4% of patients. The discontinuation rate was less than 3%.
When the indication for treatment of young children down to age 3 months (from 24 months) was granted by the Food and Drug Administration in March 2020, crisaborole, a phosphodiesterase-4 inhibitor, became the only nonsteroidal approved for treatment of AD in children aged younger than 2 years, Dr. Eichenfield pointed out.
Tacrolimus
The topical calcineurin inhibitor tacrolimus (Protopic) poses no detectable risk of cancer in children treated for AD, according to a prospective, multinational study that followed nearly 8,000 children with AD who used topical tacrolimus for at least 6 weeks over 10 years. With 44,469 person-years of follow-up in a population with at least 6 weeks of exposure to tacrolimus, there were six confirmed cancers, a rate not different than background rates, and no lymphomas.
“I have always tried to educate my patients about the potential use of the topical calcineurin inhibitors while reassuring them that the data did not support significant risk,” Dr. Eichenfield said. However, a large set of data reconfirming a low risk of cancer, although not definitive, “are really nice to have.”
Ruxolitinib
For treatment of AD in children aged as young as 12 years, a cream formulation of ruxolitinib, a Janus kinase 1/JAK2 inhibitor, met its primary outcomes in the phase 3 TRuE AD1 and TRuE AD2 trials. (These data are not yet published but were presented at the Revolutionizing Atopic Dermatitis virtual symposium in April 2020.) The primary endpoint of 75% EASI clearance (EASI-75) was achieved in approximately 62% of patients treated with the 1.5% dose of ruxolitinib twice daily. This was a highly significant advantage over vehicle in both studies (P < .0001).
The EASI-75 rates at 8 weeks for the 0.75% formulation, at 56% and 51.5% for the TRuE AD1 and TRuE AD2 trials, respectively, were lower but also superior (P < .0001) to the 24.6% and 14.4% response rates on vehicle, respectively.
Emphasizing a consistent benefit on multiple secondary endpoints, including the “really early itch decrease,” Dr. Eichenfield described the phase 3 data as “really excellent results.” The data have not yet led to FDA approval of ruxolitinib for AD, but approval seems likely. Dr. Eichenfield noted that other drugs in the same class, such as abrocitinib and upadacitinib, have also demonstrated promising efficacy in children aged 12 years or older.
Dupilumab
Dupilumab, an interleukin-4 receptor alpha antagonist, was approved in May, 2020, for the treatment of AD in children ages 6-11 years, on the basis of a recently published phase 3, randomized, placebo-controlled trial that enrolled children aged between 6 and 11 years, comparing dupilumab and topical corticosteroids and placebo plus topical corticosteroids. Severe involvement was an entry criterion.
At 16 weeks, an EASI-75 response was achieved by 67% of the group randomized to 200 mg of dupilumab administered every 2 weeks and 70% of the group randomized to 300 mg every 4 weeks versus 27% of those randomized to placebo. More patients in the dupilumab arms developed conjunctivitis (10.8% vs. 4.7%) and had injection-site reactions (8.5% vs. 3.5%), but the monoclonal antibody was otherwise well tolerated and safe.
These data suggest that younger patients with severe disease “do, if anything, better than adults,” Dr. Eichenfield said at the meeting, jointly presented by the University of Louisville and Global Academy for Medical Education. He cautioned that avoiding live vaccines, which is recommended in patients on dupilumab, “is likely more of an issue in children.”
Ixekizumab
Ixekizumab has been approved for pediatric patients aged as young as 6 years who are eligible for systemic therapy on the basis of a phase 3 trial. For the primary endpoint of 75% clearance on the Psoriasis Area and Severity Index, the response rates were 89% for the IL-17 inhibitor administered every 4 weeks and 25% for placebo. The study also associated ixekizumab with a significant improvement in quality of life.
The availability of more targeted therapies for children are likely. In Europe, secukinumab, another IL-17 inhibitor, was approved for treatment in pediatric patients this past summer, Dr. Eichenfield noted. These data are not yet published, but he expects targeted therapies to join a growing list of biologics already approved in children.
For drugs with established efficacy and safety, he advised, “look at your pediatric psoriasis patients and don’t be wimpy.” In children with poorly controlled psoriasis, he concluded these drugs have been associated with improved quality of life.
In November 2019, the American Academy of Dermatology and National Psoriasis Foundation published psoriasis management guidelines for children. Not all of the most recently approved therapies are included in these guidelines, which are the first to provide specific recommendations for children, but Dr. Eichenfield also included this publication among his top picks for important contributions to the pediatric dermatology literature in 2020.
Dr. Eichenfield reported financial relationships with 20 pharmaceutical companies that manufacture dermatologic products, including those for the diseases he discussed.
This publication and Global Academy for Medical Education are owned by the same parent company.
Lawrence F. Eichenfield, MD, said at the annual Coastal Dermatology Symposium, held virtually.
Dr. Eichenfield, professor of dermatology and pediatrics, at the University of California, San Diego, presented a list of studies, some of which resulted in approvals of pediatric indications in 2020, that he believes deserve attention.
Crisaborole
Crisaborole ointment, 2% is now approved for topical treatment of children aged as young as 3 months, based on the results of the CrisADe CARE1 phase 4 study. In this open-label study of infants aged from 3 months to under 2 years with mild to moderate AD, treated with crisaborole twice a day for 28 days, the mean reduction from baseline in the Eczema Area and Severity Index (EASI) score was 49.6% on day 15 and 57.5% on day 29. The most common side effects were erythema and application-site pain, but neither occurred in more than 4% of patients. The discontinuation rate was less than 3%.
When the indication for treatment of young children down to age 3 months (from 24 months) was granted by the Food and Drug Administration in March 2020, crisaborole, a phosphodiesterase-4 inhibitor, became the only nonsteroidal approved for treatment of AD in children aged younger than 2 years, Dr. Eichenfield pointed out.
Tacrolimus
The topical calcineurin inhibitor tacrolimus (Protopic) poses no detectable risk of cancer in children treated for AD, according to a prospective, multinational study that followed nearly 8,000 children with AD who used topical tacrolimus for at least 6 weeks over 10 years. With 44,469 person-years of follow-up in a population with at least 6 weeks of exposure to tacrolimus, there were six confirmed cancers, a rate not different than background rates, and no lymphomas.
“I have always tried to educate my patients about the potential use of the topical calcineurin inhibitors while reassuring them that the data did not support significant risk,” Dr. Eichenfield said. However, a large set of data reconfirming a low risk of cancer, although not definitive, “are really nice to have.”
Ruxolitinib
For treatment of AD in children aged as young as 12 years, a cream formulation of ruxolitinib, a Janus kinase 1/JAK2 inhibitor, met its primary outcomes in the phase 3 TRuE AD1 and TRuE AD2 trials. (These data are not yet published but were presented at the Revolutionizing Atopic Dermatitis virtual symposium in April 2020.) The primary endpoint of 75% EASI clearance (EASI-75) was achieved in approximately 62% of patients treated with the 1.5% dose of ruxolitinib twice daily. This was a highly significant advantage over vehicle in both studies (P < .0001).
The EASI-75 rates at 8 weeks for the 0.75% formulation, at 56% and 51.5% for the TRuE AD1 and TRuE AD2 trials, respectively, were lower but also superior (P < .0001) to the 24.6% and 14.4% response rates on vehicle, respectively.
Emphasizing a consistent benefit on multiple secondary endpoints, including the “really early itch decrease,” Dr. Eichenfield described the phase 3 data as “really excellent results.” The data have not yet led to FDA approval of ruxolitinib for AD, but approval seems likely. Dr. Eichenfield noted that other drugs in the same class, such as abrocitinib and upadacitinib, have also demonstrated promising efficacy in children aged 12 years or older.
Dupilumab
Dupilumab, an interleukin-4 receptor alpha antagonist, was approved in May, 2020, for the treatment of AD in children ages 6-11 years, on the basis of a recently published phase 3, randomized, placebo-controlled trial that enrolled children aged between 6 and 11 years, comparing dupilumab and topical corticosteroids and placebo plus topical corticosteroids. Severe involvement was an entry criterion.
At 16 weeks, an EASI-75 response was achieved by 67% of the group randomized to 200 mg of dupilumab administered every 2 weeks and 70% of the group randomized to 300 mg every 4 weeks versus 27% of those randomized to placebo. More patients in the dupilumab arms developed conjunctivitis (10.8% vs. 4.7%) and had injection-site reactions (8.5% vs. 3.5%), but the monoclonal antibody was otherwise well tolerated and safe.
These data suggest that younger patients with severe disease “do, if anything, better than adults,” Dr. Eichenfield said at the meeting, jointly presented by the University of Louisville and Global Academy for Medical Education. He cautioned that avoiding live vaccines, which is recommended in patients on dupilumab, “is likely more of an issue in children.”
Ixekizumab
Ixekizumab has been approved for pediatric patients aged as young as 6 years who are eligible for systemic therapy on the basis of a phase 3 trial. For the primary endpoint of 75% clearance on the Psoriasis Area and Severity Index, the response rates were 89% for the IL-17 inhibitor administered every 4 weeks and 25% for placebo. The study also associated ixekizumab with a significant improvement in quality of life.
The availability of more targeted therapies for children are likely. In Europe, secukinumab, another IL-17 inhibitor, was approved for treatment in pediatric patients this past summer, Dr. Eichenfield noted. These data are not yet published, but he expects targeted therapies to join a growing list of biologics already approved in children.
For drugs with established efficacy and safety, he advised, “look at your pediatric psoriasis patients and don’t be wimpy.” In children with poorly controlled psoriasis, he concluded these drugs have been associated with improved quality of life.
In November 2019, the American Academy of Dermatology and National Psoriasis Foundation published psoriasis management guidelines for children. Not all of the most recently approved therapies are included in these guidelines, which are the first to provide specific recommendations for children, but Dr. Eichenfield also included this publication among his top picks for important contributions to the pediatric dermatology literature in 2020.
Dr. Eichenfield reported financial relationships with 20 pharmaceutical companies that manufacture dermatologic products, including those for the diseases he discussed.
This publication and Global Academy for Medical Education are owned by the same parent company.
FROM COASTAL DERM
Swedish registry study finds atopic dermatitis significantly associated with autoimmune diseases
in a case control study derived from Swedish national health care registry data.
Atopic dermatitis (AD) is known to be associated with other atopic conditions, and there is increasing evidence it is associated with some nonatopic conditions, including some cancers, cardiovascular disease, and neuropsychiatric disorders, according to Lina U. Ivert, MD, of the dermatology and venereology unit at the Karolinska Institutet, Stockholm, and coauthors. There are also some data indicating that autoimmune diseases, particularly those involving the skin and gastrointestinal tract, are more common in people with AD.
The aim of their study, published in the British Journal of Dermatology, was to investigate a wide spectrum of autoimmune diseases for associations with AD in a large-scale, population-based study using Swedish registers. Findings could lead to better monitoring of comorbidities and deeper understanding of disease burden and AD pathophysiology, they noted.
Large-scale study
With data from the Swedish Board of Health and Welfare’s National Patient Register on inpatient diagnoses since 1964 and specialist outpatient visits since 2001, the investigators included all patients aged 15 years and older with AD diagnoses (104,832) and matched them with controls from the general population (1,022,435). The authors noted that the large number of people included in the analysis allowed for robust estimates, and underscored that 80% of the AD patients included had received their diagnosis in a dermatology department, which reduces the risk of misclassification.
Association with autoimmune disease
The investigators found an association between AD and autoimmune disease, with an adjusted odds ratio) of 1.97 (95% confidence interval, 1.93-2.01). The association was present with several organ systems, particularly the skin and gastrointestinal tract, and with connective tissue diseases. The strongest associations with autoimmune skin diseases were found for dermatitis herpetiformis (aOR, 9.76; 95% CI, 8.10-11.8), alopecia areata (aOR, 5.11; 95% CI, 4.75-5.49), and chronic urticaria (aOR, 4.82; 95% CI, 4.48-5.19).
AD was associated with gastrointestinal diseases, including celiac disease (aOR, 1.96; 95% CI, 1.84-2.09), Crohn disease (aOR 1.83; CI, 1.71-1.96), and ulcerative colitis (aOR 1.58; 95% CI, 1.49-1.68).
Connective tissue diseases significantly associated with AD included systemic lupus erythematosus (aOR, 1.65; 95% CI, 1.42-1.90), ankylosing spondylitis (aOR, 1.46; 95% CI, 1.29-1.66), and RA (aOR, 1.44; 95% CI,1.34-1.54]). Hematologic or hepatic autoimmune disease associations with AD were not observed.
Stronger association with multiple diseases
The association between AD and two or more autoimmune diseases was significantly stronger than the association between AD and having one autoimmune disease. For example, the OR for AD among people with three to five autoimmune diseases was 3.33 (95% CI, 2.86-3.87), and was stronger in men (OR, 3.96; 95% CI, 2.92-5.37) than in women (OR, 3.14; 95% CI, 2.63-3.74).
Sex differences
In the study overall, the association with AD and autoimmune diseases was stronger in men (aOR, 2.18; 95% CI, 2.10-2.25), compared with women (aOR, 1.89; 95% CI, 1.85-1.93), but this “sex difference was only statistically significant between AD and RA and between AD and Celiac disease,” they noted.
Associations between AD and dermatomyositis, systemic scleroderma, systemic lupus erythematosus, Hashimoto’s disease, Graves disease, multiple sclerosis, and polymyalgia rheumatica were found only in women. Dr. Ivert and coauthors observed that “women are in general more likely to develop autoimmune diseases, and 80% of patients with autoimmune diseases are women.”
Provocative questions
Commenting on the findings, Jonathan Silverberg, MD, PhD, MPH, associate professor of dermatology, George Washington University, Washington, said, “At a high level, it is important for clinicians to recognize that atopic dermatitis is a systemic immune-mediated disease. AD is associated with higher rates of comorbid autoimmune disease, similar to psoriasis and other chronic inflammatory skin diseases.”
“At this point, there is nothing immediately actionable about these results,” noted Dr. Silverberg, who was not an author of this study. “That said, in my mind, they raise some provocative questions: What is the difference between AD in adults who do versus those who do not get comorbid autoimmune disease? Does AD then present differently? Does it respond to the same therapies? These will have to be the subject of future research.”
The study was funded by the Swedish Asthma and Allergy Association Research Foundation, Hudfonden (the Welander-Finsen Foundation), and the Swedish Society for Dermatology and Venereology. The authors disclosed no conflicts of interest.
SOURCE: Ivert LU et al. Br J Dermatol. 2020 Oct 22. doi: 10.1111/bjd.19624.
in a case control study derived from Swedish national health care registry data.
Atopic dermatitis (AD) is known to be associated with other atopic conditions, and there is increasing evidence it is associated with some nonatopic conditions, including some cancers, cardiovascular disease, and neuropsychiatric disorders, according to Lina U. Ivert, MD, of the dermatology and venereology unit at the Karolinska Institutet, Stockholm, and coauthors. There are also some data indicating that autoimmune diseases, particularly those involving the skin and gastrointestinal tract, are more common in people with AD.
The aim of their study, published in the British Journal of Dermatology, was to investigate a wide spectrum of autoimmune diseases for associations with AD in a large-scale, population-based study using Swedish registers. Findings could lead to better monitoring of comorbidities and deeper understanding of disease burden and AD pathophysiology, they noted.
Large-scale study
With data from the Swedish Board of Health and Welfare’s National Patient Register on inpatient diagnoses since 1964 and specialist outpatient visits since 2001, the investigators included all patients aged 15 years and older with AD diagnoses (104,832) and matched them with controls from the general population (1,022,435). The authors noted that the large number of people included in the analysis allowed for robust estimates, and underscored that 80% of the AD patients included had received their diagnosis in a dermatology department, which reduces the risk of misclassification.
Association with autoimmune disease
The investigators found an association between AD and autoimmune disease, with an adjusted odds ratio) of 1.97 (95% confidence interval, 1.93-2.01). The association was present with several organ systems, particularly the skin and gastrointestinal tract, and with connective tissue diseases. The strongest associations with autoimmune skin diseases were found for dermatitis herpetiformis (aOR, 9.76; 95% CI, 8.10-11.8), alopecia areata (aOR, 5.11; 95% CI, 4.75-5.49), and chronic urticaria (aOR, 4.82; 95% CI, 4.48-5.19).
AD was associated with gastrointestinal diseases, including celiac disease (aOR, 1.96; 95% CI, 1.84-2.09), Crohn disease (aOR 1.83; CI, 1.71-1.96), and ulcerative colitis (aOR 1.58; 95% CI, 1.49-1.68).
Connective tissue diseases significantly associated with AD included systemic lupus erythematosus (aOR, 1.65; 95% CI, 1.42-1.90), ankylosing spondylitis (aOR, 1.46; 95% CI, 1.29-1.66), and RA (aOR, 1.44; 95% CI,1.34-1.54]). Hematologic or hepatic autoimmune disease associations with AD were not observed.
Stronger association with multiple diseases
The association between AD and two or more autoimmune diseases was significantly stronger than the association between AD and having one autoimmune disease. For example, the OR for AD among people with three to five autoimmune diseases was 3.33 (95% CI, 2.86-3.87), and was stronger in men (OR, 3.96; 95% CI, 2.92-5.37) than in women (OR, 3.14; 95% CI, 2.63-3.74).
Sex differences
In the study overall, the association with AD and autoimmune diseases was stronger in men (aOR, 2.18; 95% CI, 2.10-2.25), compared with women (aOR, 1.89; 95% CI, 1.85-1.93), but this “sex difference was only statistically significant between AD and RA and between AD and Celiac disease,” they noted.
Associations between AD and dermatomyositis, systemic scleroderma, systemic lupus erythematosus, Hashimoto’s disease, Graves disease, multiple sclerosis, and polymyalgia rheumatica were found only in women. Dr. Ivert and coauthors observed that “women are in general more likely to develop autoimmune diseases, and 80% of patients with autoimmune diseases are women.”
Provocative questions
Commenting on the findings, Jonathan Silverberg, MD, PhD, MPH, associate professor of dermatology, George Washington University, Washington, said, “At a high level, it is important for clinicians to recognize that atopic dermatitis is a systemic immune-mediated disease. AD is associated with higher rates of comorbid autoimmune disease, similar to psoriasis and other chronic inflammatory skin diseases.”
“At this point, there is nothing immediately actionable about these results,” noted Dr. Silverberg, who was not an author of this study. “That said, in my mind, they raise some provocative questions: What is the difference between AD in adults who do versus those who do not get comorbid autoimmune disease? Does AD then present differently? Does it respond to the same therapies? These will have to be the subject of future research.”
The study was funded by the Swedish Asthma and Allergy Association Research Foundation, Hudfonden (the Welander-Finsen Foundation), and the Swedish Society for Dermatology and Venereology. The authors disclosed no conflicts of interest.
SOURCE: Ivert LU et al. Br J Dermatol. 2020 Oct 22. doi: 10.1111/bjd.19624.
in a case control study derived from Swedish national health care registry data.
Atopic dermatitis (AD) is known to be associated with other atopic conditions, and there is increasing evidence it is associated with some nonatopic conditions, including some cancers, cardiovascular disease, and neuropsychiatric disorders, according to Lina U. Ivert, MD, of the dermatology and venereology unit at the Karolinska Institutet, Stockholm, and coauthors. There are also some data indicating that autoimmune diseases, particularly those involving the skin and gastrointestinal tract, are more common in people with AD.
The aim of their study, published in the British Journal of Dermatology, was to investigate a wide spectrum of autoimmune diseases for associations with AD in a large-scale, population-based study using Swedish registers. Findings could lead to better monitoring of comorbidities and deeper understanding of disease burden and AD pathophysiology, they noted.
Large-scale study
With data from the Swedish Board of Health and Welfare’s National Patient Register on inpatient diagnoses since 1964 and specialist outpatient visits since 2001, the investigators included all patients aged 15 years and older with AD diagnoses (104,832) and matched them with controls from the general population (1,022,435). The authors noted that the large number of people included in the analysis allowed for robust estimates, and underscored that 80% of the AD patients included had received their diagnosis in a dermatology department, which reduces the risk of misclassification.
Association with autoimmune disease
The investigators found an association between AD and autoimmune disease, with an adjusted odds ratio) of 1.97 (95% confidence interval, 1.93-2.01). The association was present with several organ systems, particularly the skin and gastrointestinal tract, and with connective tissue diseases. The strongest associations with autoimmune skin diseases were found for dermatitis herpetiformis (aOR, 9.76; 95% CI, 8.10-11.8), alopecia areata (aOR, 5.11; 95% CI, 4.75-5.49), and chronic urticaria (aOR, 4.82; 95% CI, 4.48-5.19).
AD was associated with gastrointestinal diseases, including celiac disease (aOR, 1.96; 95% CI, 1.84-2.09), Crohn disease (aOR 1.83; CI, 1.71-1.96), and ulcerative colitis (aOR 1.58; 95% CI, 1.49-1.68).
Connective tissue diseases significantly associated with AD included systemic lupus erythematosus (aOR, 1.65; 95% CI, 1.42-1.90), ankylosing spondylitis (aOR, 1.46; 95% CI, 1.29-1.66), and RA (aOR, 1.44; 95% CI,1.34-1.54]). Hematologic or hepatic autoimmune disease associations with AD were not observed.
Stronger association with multiple diseases
The association between AD and two or more autoimmune diseases was significantly stronger than the association between AD and having one autoimmune disease. For example, the OR for AD among people with three to five autoimmune diseases was 3.33 (95% CI, 2.86-3.87), and was stronger in men (OR, 3.96; 95% CI, 2.92-5.37) than in women (OR, 3.14; 95% CI, 2.63-3.74).
Sex differences
In the study overall, the association with AD and autoimmune diseases was stronger in men (aOR, 2.18; 95% CI, 2.10-2.25), compared with women (aOR, 1.89; 95% CI, 1.85-1.93), but this “sex difference was only statistically significant between AD and RA and between AD and Celiac disease,” they noted.
Associations between AD and dermatomyositis, systemic scleroderma, systemic lupus erythematosus, Hashimoto’s disease, Graves disease, multiple sclerosis, and polymyalgia rheumatica were found only in women. Dr. Ivert and coauthors observed that “women are in general more likely to develop autoimmune diseases, and 80% of patients with autoimmune diseases are women.”
Provocative questions
Commenting on the findings, Jonathan Silverberg, MD, PhD, MPH, associate professor of dermatology, George Washington University, Washington, said, “At a high level, it is important for clinicians to recognize that atopic dermatitis is a systemic immune-mediated disease. AD is associated with higher rates of comorbid autoimmune disease, similar to psoriasis and other chronic inflammatory skin diseases.”
“At this point, there is nothing immediately actionable about these results,” noted Dr. Silverberg, who was not an author of this study. “That said, in my mind, they raise some provocative questions: What is the difference between AD in adults who do versus those who do not get comorbid autoimmune disease? Does AD then present differently? Does it respond to the same therapies? These will have to be the subject of future research.”
The study was funded by the Swedish Asthma and Allergy Association Research Foundation, Hudfonden (the Welander-Finsen Foundation), and the Swedish Society for Dermatology and Venereology. The authors disclosed no conflicts of interest.
SOURCE: Ivert LU et al. Br J Dermatol. 2020 Oct 22. doi: 10.1111/bjd.19624.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Analysis characterizes common wound microbes in epidermolysis bullosa
– in a retrospective analysis of over 700 wound cultures from 158 patients across the United States and Canada.
The findings from the EB Clinical Characterization and Outcomes Database speak to the value of surveillance cultures with routine testing for microbial resistance – including mupirocin resistance – and to the importance of antibiotic stewardship not only for oral antibiotics but for topicals as well, according to Laura E. Levin, MD, and Kimberly D. Morel, MD, of the departments of dermatology and pediatrics, Columbia University Irving Medical Center, New York, the lead and senior authors, respectively, of the paper recently published in Pediatric Dermatology.
Almost all of the 158 patients with at least one wound culture recorded in the database from the period of 2001-2018 had one or more positive culture results. Of 152 patients with positive cultures, 131 (86%) were positive for SA and 56 (37%) and 34 (22%) were positive for PA and GAS, respectively. Other bacteria isolated included Corynebacterium spp and Proteus spp. Nearly half (47%) of patients with SA-positive cultures had methicillin-resistant SA, and 68% had methicillin-susceptible SA. (Some patients grew both MSSA and MRSA at different points in time.)
Mupirocin-susceptibility testing was performed at only some of the 13 participating centers. Of 15 patients whose cultures had recorded SA mupirocin-susceptibility testing, 11 had cultures positive for mupirocin-susceptible SA and 6 (40%) had mupirocin-resistant SA isolates (2 patients grew both). Of these six patients, half had isolates that were also methicillin-resistant.
Mupirocin, a topical antibiotic, has been a cornerstone of decolonization regimens for MSSA and MRSA, but resistance has been demonstrated in other research as well and is not specific to EB, wrote Dr. Levin, Dr. Morel, and coauthors.
“Pediatric dermatologists often rely on topical antimicrobials in the treatment of patients’ open wounds to both prevent and treat infection, depending on the clinical scenario,” and surveillance cultures with routine testing for mupirocin resistance can help guide antibiotic choice and management strategies, Dr. Levin said in an interview.
More broadly, she added, “it’s helpful to know what bacteria are routinely colonizing wounds, not causing infection, versus those that are more likely to be associated with infection, chronic wounds, or the risk of developing skin cancer ... [to know] which wounds need to be treated more aggressively.”
A subset of patients with EB have been known to be at risk for squamous cell carcinoma, and research is implicating certain bacteria “as contributing to wound inflammation,” Dr. Morel said in an interview.
SCC was reported in 23 out of 717 patients in the database – but fewer than half of the patients with SCC had recorded wound cultures. The small numbers precluded the identification of microbes that may confer significant risk.
Correlating particular microbes with clinical features also will take more research. About half (57%) of the patients with recorded wound cultures had wounds with purulent exudate or other features of clinical infection. However, the presence or absence of clinical signs of infection was not temporally correlated with culture results in the database.
The 158 patients with recorded wound cultures had a mean age of 12.8 years and represented a range of EB subtypes.
PA was present in the wounds of patients as young as 1 month old, the authors noted. Investigators are “looking to further study PA and characterize clinical features ... to understand more about this microbe and its impact on patients with EB,” Dr. Morel said.
In the meantime, the analysis reaffirms the importance of antibiotic stewardship. Mupirocin is labeled to be used three times a day for a short period of time, but “tends to be prescribed and used less judiciously than intended,” Dr. Morel said. “It’s important [not to overuse it]. We have seen that patients’ culture results become sensitive to mupirocin again in the future when they avoid it for a period of time.”
The work was supported by the EB Research Partnership and EB Medical Research Foundation, as well as an NIH/NCATS grant. No investigator disclosures were listed.
SOURCE: Pediatr Dermatol. 2020 Nov 28. doi: 10.1111/pde.14444.
– in a retrospective analysis of over 700 wound cultures from 158 patients across the United States and Canada.
The findings from the EB Clinical Characterization and Outcomes Database speak to the value of surveillance cultures with routine testing for microbial resistance – including mupirocin resistance – and to the importance of antibiotic stewardship not only for oral antibiotics but for topicals as well, according to Laura E. Levin, MD, and Kimberly D. Morel, MD, of the departments of dermatology and pediatrics, Columbia University Irving Medical Center, New York, the lead and senior authors, respectively, of the paper recently published in Pediatric Dermatology.
Almost all of the 158 patients with at least one wound culture recorded in the database from the period of 2001-2018 had one or more positive culture results. Of 152 patients with positive cultures, 131 (86%) were positive for SA and 56 (37%) and 34 (22%) were positive for PA and GAS, respectively. Other bacteria isolated included Corynebacterium spp and Proteus spp. Nearly half (47%) of patients with SA-positive cultures had methicillin-resistant SA, and 68% had methicillin-susceptible SA. (Some patients grew both MSSA and MRSA at different points in time.)
Mupirocin-susceptibility testing was performed at only some of the 13 participating centers. Of 15 patients whose cultures had recorded SA mupirocin-susceptibility testing, 11 had cultures positive for mupirocin-susceptible SA and 6 (40%) had mupirocin-resistant SA isolates (2 patients grew both). Of these six patients, half had isolates that were also methicillin-resistant.
Mupirocin, a topical antibiotic, has been a cornerstone of decolonization regimens for MSSA and MRSA, but resistance has been demonstrated in other research as well and is not specific to EB, wrote Dr. Levin, Dr. Morel, and coauthors.
“Pediatric dermatologists often rely on topical antimicrobials in the treatment of patients’ open wounds to both prevent and treat infection, depending on the clinical scenario,” and surveillance cultures with routine testing for mupirocin resistance can help guide antibiotic choice and management strategies, Dr. Levin said in an interview.
More broadly, she added, “it’s helpful to know what bacteria are routinely colonizing wounds, not causing infection, versus those that are more likely to be associated with infection, chronic wounds, or the risk of developing skin cancer ... [to know] which wounds need to be treated more aggressively.”
A subset of patients with EB have been known to be at risk for squamous cell carcinoma, and research is implicating certain bacteria “as contributing to wound inflammation,” Dr. Morel said in an interview.
SCC was reported in 23 out of 717 patients in the database – but fewer than half of the patients with SCC had recorded wound cultures. The small numbers precluded the identification of microbes that may confer significant risk.
Correlating particular microbes with clinical features also will take more research. About half (57%) of the patients with recorded wound cultures had wounds with purulent exudate or other features of clinical infection. However, the presence or absence of clinical signs of infection was not temporally correlated with culture results in the database.
The 158 patients with recorded wound cultures had a mean age of 12.8 years and represented a range of EB subtypes.
PA was present in the wounds of patients as young as 1 month old, the authors noted. Investigators are “looking to further study PA and characterize clinical features ... to understand more about this microbe and its impact on patients with EB,” Dr. Morel said.
In the meantime, the analysis reaffirms the importance of antibiotic stewardship. Mupirocin is labeled to be used three times a day for a short period of time, but “tends to be prescribed and used less judiciously than intended,” Dr. Morel said. “It’s important [not to overuse it]. We have seen that patients’ culture results become sensitive to mupirocin again in the future when they avoid it for a period of time.”
The work was supported by the EB Research Partnership and EB Medical Research Foundation, as well as an NIH/NCATS grant. No investigator disclosures were listed.
SOURCE: Pediatr Dermatol. 2020 Nov 28. doi: 10.1111/pde.14444.
– in a retrospective analysis of over 700 wound cultures from 158 patients across the United States and Canada.
The findings from the EB Clinical Characterization and Outcomes Database speak to the value of surveillance cultures with routine testing for microbial resistance – including mupirocin resistance – and to the importance of antibiotic stewardship not only for oral antibiotics but for topicals as well, according to Laura E. Levin, MD, and Kimberly D. Morel, MD, of the departments of dermatology and pediatrics, Columbia University Irving Medical Center, New York, the lead and senior authors, respectively, of the paper recently published in Pediatric Dermatology.
Almost all of the 158 patients with at least one wound culture recorded in the database from the period of 2001-2018 had one or more positive culture results. Of 152 patients with positive cultures, 131 (86%) were positive for SA and 56 (37%) and 34 (22%) were positive for PA and GAS, respectively. Other bacteria isolated included Corynebacterium spp and Proteus spp. Nearly half (47%) of patients with SA-positive cultures had methicillin-resistant SA, and 68% had methicillin-susceptible SA. (Some patients grew both MSSA and MRSA at different points in time.)
Mupirocin-susceptibility testing was performed at only some of the 13 participating centers. Of 15 patients whose cultures had recorded SA mupirocin-susceptibility testing, 11 had cultures positive for mupirocin-susceptible SA and 6 (40%) had mupirocin-resistant SA isolates (2 patients grew both). Of these six patients, half had isolates that were also methicillin-resistant.
Mupirocin, a topical antibiotic, has been a cornerstone of decolonization regimens for MSSA and MRSA, but resistance has been demonstrated in other research as well and is not specific to EB, wrote Dr. Levin, Dr. Morel, and coauthors.
“Pediatric dermatologists often rely on topical antimicrobials in the treatment of patients’ open wounds to both prevent and treat infection, depending on the clinical scenario,” and surveillance cultures with routine testing for mupirocin resistance can help guide antibiotic choice and management strategies, Dr. Levin said in an interview.
More broadly, she added, “it’s helpful to know what bacteria are routinely colonizing wounds, not causing infection, versus those that are more likely to be associated with infection, chronic wounds, or the risk of developing skin cancer ... [to know] which wounds need to be treated more aggressively.”
A subset of patients with EB have been known to be at risk for squamous cell carcinoma, and research is implicating certain bacteria “as contributing to wound inflammation,” Dr. Morel said in an interview.
SCC was reported in 23 out of 717 patients in the database – but fewer than half of the patients with SCC had recorded wound cultures. The small numbers precluded the identification of microbes that may confer significant risk.
Correlating particular microbes with clinical features also will take more research. About half (57%) of the patients with recorded wound cultures had wounds with purulent exudate or other features of clinical infection. However, the presence or absence of clinical signs of infection was not temporally correlated with culture results in the database.
The 158 patients with recorded wound cultures had a mean age of 12.8 years and represented a range of EB subtypes.
PA was present in the wounds of patients as young as 1 month old, the authors noted. Investigators are “looking to further study PA and characterize clinical features ... to understand more about this microbe and its impact on patients with EB,” Dr. Morel said.
In the meantime, the analysis reaffirms the importance of antibiotic stewardship. Mupirocin is labeled to be used three times a day for a short period of time, but “tends to be prescribed and used less judiciously than intended,” Dr. Morel said. “It’s important [not to overuse it]. We have seen that patients’ culture results become sensitive to mupirocin again in the future when they avoid it for a period of time.”
The work was supported by the EB Research Partnership and EB Medical Research Foundation, as well as an NIH/NCATS grant. No investigator disclosures were listed.
SOURCE: Pediatr Dermatol. 2020 Nov 28. doi: 10.1111/pde.14444.
FROM PEDIATRIC DERMATOLOGY
Light-based technologies emerging as promising acne treatments
such as Fernanda H. Sakamoto, MD, PhD.
“I love treating acne, because it can have a huge impact on our patients’ lives,” Dr. Sakamoto, a dermatologist at the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston, said during a virtual course on laser and aesthetic skin therapy. “Acne is the most common disease in dermatology, affecting about 80% of our patients. Eleven percent of these patients have difficult-to-treat acne, and it is also the No. 1 cause of depression and suicide among teenagers and young adults. And, even though there’s no strong evidence that optical treatments work better than conventional acne treatments, people still spend a lot on those treatments: more than 220 million in 2019.”
Early results from a pilot study suggest that use of a novel laser system known as Accure in patients with mild to moderate acne resulted in an 80% reduction in acne lesions at 12 weeks. The laser prototype, which uses a 1,726 nm wavelength and is being developed by researchers at the Wellman Center for Photomedicine, features a built-in thermal camera in the handpiece that allows the user to monitor the skin’s temperature during treatment.
In initial pilot studies of the device, Dr. Sakamoto and colleagues observed consistent damage of the sebaceous glands, with no damage to the epidermis, surrounding dermis, or other follicular structures. “But because the contrast of absorption of lipids and water is not very high, we needed to create a laser with features that we have never seen before,” she said. “One of them is a robust cooling system. The second prototype features a built-in thermal camera within the handpiece that allows us to see the temperature while we’re treating the patient. It also has built-in software that would shut down the laser if the temperature is too high. “This is the first laser with some safety features that will give the user direct feedback while treating the patient,” she said, noting that its “unique cooling system and real-time monitoring ... makes it different from any of the lasers we see on the market right now.”
Dr. Sakamoto and colleagues (Emil Tanghetti, MD, in San Diego, Roy Geronemus, MD, in New York, and Joel L. Cohen, MD, in Colorado) are conducting a clinical trial of the device, to evaluate whether Accure can selectively target sebaceous glands. As of Oct. 23, 2020, the study enrolled more than 50 patients, who are followed at 4, 8, 12, and 24 weeks post treatment, she said.
To date, 16 patients have completed the study, and the researchers have observed an average lesion reduction of 80% at 12 weeks post treatment, after four treatment sessions. This amounted to more than 12,000 trigger pulls of the device, with no unexpected adverse events. Average visual analogue scale pain scores immediately after treatment have been 1.09 out of 10.
Histologic assessment of skin samples collected from the study participants have revealed selective damage of the sebaceous glands with a normal epidermis and surrounding dermis. “Because this laser is near infrared, it is not absorbed by melanin, making it possible for a safe treatment in darker skin tones,” Dr. Sakamoto said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine.
“We have shown that it is possible to create a selective laser for acne treatment at 1,726 nm. We have proven it mathematically as well as with histological samples,” she said. “Now we are moving on to a larger clinical trial for the FDA clearance.”
Another strategy being developed for acne treatment is to make nonselective lasers selective by adding gold microparticles into the hair follicle and sebaceous glands, to allow the lasers to be absorbed. In a study that used a free electron laser, Dr. Sakamoto and colleagues demonstrated that these microparticles can stay within the sebaceous glands for selective damage of the sebaceous glands. In a subsequent pilot clinical trial they showed that the addition of the gold microparticles followed by a diode laser treatment made it possible to reduce both inflammatory and noninflammatory lesions.
More recently, an open-label European study of acne treatment with light absorbing gold microparticles and optical pulses demonstrated that the treatment led to an 80%-90% reduction of inflammatory lesions at 12 weeks, with a reduction of Investigator’s Global Assessment scale from 2 to 4.
The Food and Drug Administration cleared the treatment, Sebacia Microparticles, for the treatment of mild to moderate acne in September of 2018, but according to Dr. Sakamoto, “the company has struggled, as they were only commercializing the device in California and Washington, DC.”
Photodynamic therapy (PDT) is also being studied as an acne treatment. “PDT uses a photosensitizer that needs to be activated by a light source,” she noted. “The combination of red light and aminolevulinic acid (ALA) or methyl ester ALA has been shown to damage the sebaceous glands”.
In a recent randomized controlled trial that compared PDT to adapalene gel plus oral doxycycline, PDT showed superiority. “Because PDT induces apoptosis of the sebaceous glands, it causes a lot of pain and side effects after treatment,” Dr. Sakamoto said. “However, it can clear 80%-90% of acne in 80%-90% of patients. But because of the side effects, PDT should be limited to those patients who cannot take conventional treatments.”
Dr. Sakamoto reported having received research funding and/or consulting fees from numerous device and pharmaceutical companies.
such as Fernanda H. Sakamoto, MD, PhD.
“I love treating acne, because it can have a huge impact on our patients’ lives,” Dr. Sakamoto, a dermatologist at the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston, said during a virtual course on laser and aesthetic skin therapy. “Acne is the most common disease in dermatology, affecting about 80% of our patients. Eleven percent of these patients have difficult-to-treat acne, and it is also the No. 1 cause of depression and suicide among teenagers and young adults. And, even though there’s no strong evidence that optical treatments work better than conventional acne treatments, people still spend a lot on those treatments: more than 220 million in 2019.”
Early results from a pilot study suggest that use of a novel laser system known as Accure in patients with mild to moderate acne resulted in an 80% reduction in acne lesions at 12 weeks. The laser prototype, which uses a 1,726 nm wavelength and is being developed by researchers at the Wellman Center for Photomedicine, features a built-in thermal camera in the handpiece that allows the user to monitor the skin’s temperature during treatment.
In initial pilot studies of the device, Dr. Sakamoto and colleagues observed consistent damage of the sebaceous glands, with no damage to the epidermis, surrounding dermis, or other follicular structures. “But because the contrast of absorption of lipids and water is not very high, we needed to create a laser with features that we have never seen before,” she said. “One of them is a robust cooling system. The second prototype features a built-in thermal camera within the handpiece that allows us to see the temperature while we’re treating the patient. It also has built-in software that would shut down the laser if the temperature is too high. “This is the first laser with some safety features that will give the user direct feedback while treating the patient,” she said, noting that its “unique cooling system and real-time monitoring ... makes it different from any of the lasers we see on the market right now.”
Dr. Sakamoto and colleagues (Emil Tanghetti, MD, in San Diego, Roy Geronemus, MD, in New York, and Joel L. Cohen, MD, in Colorado) are conducting a clinical trial of the device, to evaluate whether Accure can selectively target sebaceous glands. As of Oct. 23, 2020, the study enrolled more than 50 patients, who are followed at 4, 8, 12, and 24 weeks post treatment, she said.
To date, 16 patients have completed the study, and the researchers have observed an average lesion reduction of 80% at 12 weeks post treatment, after four treatment sessions. This amounted to more than 12,000 trigger pulls of the device, with no unexpected adverse events. Average visual analogue scale pain scores immediately after treatment have been 1.09 out of 10.
Histologic assessment of skin samples collected from the study participants have revealed selective damage of the sebaceous glands with a normal epidermis and surrounding dermis. “Because this laser is near infrared, it is not absorbed by melanin, making it possible for a safe treatment in darker skin tones,” Dr. Sakamoto said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine.
“We have shown that it is possible to create a selective laser for acne treatment at 1,726 nm. We have proven it mathematically as well as with histological samples,” she said. “Now we are moving on to a larger clinical trial for the FDA clearance.”
Another strategy being developed for acne treatment is to make nonselective lasers selective by adding gold microparticles into the hair follicle and sebaceous glands, to allow the lasers to be absorbed. In a study that used a free electron laser, Dr. Sakamoto and colleagues demonstrated that these microparticles can stay within the sebaceous glands for selective damage of the sebaceous glands. In a subsequent pilot clinical trial they showed that the addition of the gold microparticles followed by a diode laser treatment made it possible to reduce both inflammatory and noninflammatory lesions.
More recently, an open-label European study of acne treatment with light absorbing gold microparticles and optical pulses demonstrated that the treatment led to an 80%-90% reduction of inflammatory lesions at 12 weeks, with a reduction of Investigator’s Global Assessment scale from 2 to 4.
The Food and Drug Administration cleared the treatment, Sebacia Microparticles, for the treatment of mild to moderate acne in September of 2018, but according to Dr. Sakamoto, “the company has struggled, as they were only commercializing the device in California and Washington, DC.”
Photodynamic therapy (PDT) is also being studied as an acne treatment. “PDT uses a photosensitizer that needs to be activated by a light source,” she noted. “The combination of red light and aminolevulinic acid (ALA) or methyl ester ALA has been shown to damage the sebaceous glands”.
In a recent randomized controlled trial that compared PDT to adapalene gel plus oral doxycycline, PDT showed superiority. “Because PDT induces apoptosis of the sebaceous glands, it causes a lot of pain and side effects after treatment,” Dr. Sakamoto said. “However, it can clear 80%-90% of acne in 80%-90% of patients. But because of the side effects, PDT should be limited to those patients who cannot take conventional treatments.”
Dr. Sakamoto reported having received research funding and/or consulting fees from numerous device and pharmaceutical companies.
such as Fernanda H. Sakamoto, MD, PhD.
“I love treating acne, because it can have a huge impact on our patients’ lives,” Dr. Sakamoto, a dermatologist at the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston, said during a virtual course on laser and aesthetic skin therapy. “Acne is the most common disease in dermatology, affecting about 80% of our patients. Eleven percent of these patients have difficult-to-treat acne, and it is also the No. 1 cause of depression and suicide among teenagers and young adults. And, even though there’s no strong evidence that optical treatments work better than conventional acne treatments, people still spend a lot on those treatments: more than 220 million in 2019.”
Early results from a pilot study suggest that use of a novel laser system known as Accure in patients with mild to moderate acne resulted in an 80% reduction in acne lesions at 12 weeks. The laser prototype, which uses a 1,726 nm wavelength and is being developed by researchers at the Wellman Center for Photomedicine, features a built-in thermal camera in the handpiece that allows the user to monitor the skin’s temperature during treatment.
In initial pilot studies of the device, Dr. Sakamoto and colleagues observed consistent damage of the sebaceous glands, with no damage to the epidermis, surrounding dermis, or other follicular structures. “But because the contrast of absorption of lipids and water is not very high, we needed to create a laser with features that we have never seen before,” she said. “One of them is a robust cooling system. The second prototype features a built-in thermal camera within the handpiece that allows us to see the temperature while we’re treating the patient. It also has built-in software that would shut down the laser if the temperature is too high. “This is the first laser with some safety features that will give the user direct feedback while treating the patient,” she said, noting that its “unique cooling system and real-time monitoring ... makes it different from any of the lasers we see on the market right now.”
Dr. Sakamoto and colleagues (Emil Tanghetti, MD, in San Diego, Roy Geronemus, MD, in New York, and Joel L. Cohen, MD, in Colorado) are conducting a clinical trial of the device, to evaluate whether Accure can selectively target sebaceous glands. As of Oct. 23, 2020, the study enrolled more than 50 patients, who are followed at 4, 8, 12, and 24 weeks post treatment, she said.
To date, 16 patients have completed the study, and the researchers have observed an average lesion reduction of 80% at 12 weeks post treatment, after four treatment sessions. This amounted to more than 12,000 trigger pulls of the device, with no unexpected adverse events. Average visual analogue scale pain scores immediately after treatment have been 1.09 out of 10.
Histologic assessment of skin samples collected from the study participants have revealed selective damage of the sebaceous glands with a normal epidermis and surrounding dermis. “Because this laser is near infrared, it is not absorbed by melanin, making it possible for a safe treatment in darker skin tones,” Dr. Sakamoto said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine.
“We have shown that it is possible to create a selective laser for acne treatment at 1,726 nm. We have proven it mathematically as well as with histological samples,” she said. “Now we are moving on to a larger clinical trial for the FDA clearance.”
Another strategy being developed for acne treatment is to make nonselective lasers selective by adding gold microparticles into the hair follicle and sebaceous glands, to allow the lasers to be absorbed. In a study that used a free electron laser, Dr. Sakamoto and colleagues demonstrated that these microparticles can stay within the sebaceous glands for selective damage of the sebaceous glands. In a subsequent pilot clinical trial they showed that the addition of the gold microparticles followed by a diode laser treatment made it possible to reduce both inflammatory and noninflammatory lesions.
More recently, an open-label European study of acne treatment with light absorbing gold microparticles and optical pulses demonstrated that the treatment led to an 80%-90% reduction of inflammatory lesions at 12 weeks, with a reduction of Investigator’s Global Assessment scale from 2 to 4.
The Food and Drug Administration cleared the treatment, Sebacia Microparticles, for the treatment of mild to moderate acne in September of 2018, but according to Dr. Sakamoto, “the company has struggled, as they were only commercializing the device in California and Washington, DC.”
Photodynamic therapy (PDT) is also being studied as an acne treatment. “PDT uses a photosensitizer that needs to be activated by a light source,” she noted. “The combination of red light and aminolevulinic acid (ALA) or methyl ester ALA has been shown to damage the sebaceous glands”.
In a recent randomized controlled trial that compared PDT to adapalene gel plus oral doxycycline, PDT showed superiority. “Because PDT induces apoptosis of the sebaceous glands, it causes a lot of pain and side effects after treatment,” Dr. Sakamoto said. “However, it can clear 80%-90% of acne in 80%-90% of patients. But because of the side effects, PDT should be limited to those patients who cannot take conventional treatments.”
Dr. Sakamoto reported having received research funding and/or consulting fees from numerous device and pharmaceutical companies.
EXPERT ANALYSIS FROM A LASER & AESTHETIC SKIN THERAPY COURSE
More severe AD correlates with worse sleep health and attention problems in children
, results from a national survey demonstrated.
“We think it’s important for dermatologists and pediatricians to be monitoring children with AD for sleep and attention dysregulation,” Nina Y. Zhou said during a late-breaking research session at the Revolutionizing Atopic Dermatitis virtual symposium. “It’s also important to highlight sleep hygiene habits to improve sleep health overall.”
In an effort to determine the impact of AD severity on these symptoms in young children with AD and characterize sleep health and attention regulation behaviors, Ms. Zhou, a medical student at Northwestern University, Chicago, and colleagues drew from a national survey distributed via panel company OP4G and the National Eczema Association that was conducted with parents of 60 children with AD aged 1-5 years. Questionnaires included the Patient Reported Outcomes Measurement Information System (PROMIS) Early Childhood Sleep Health Measures to assess sleep health, the Peak Pruritus NRS to measure itch severity, and the Multidimensional Assessment Profile of Attention Regulation (MAPS-AR) to measure attention dysregulation related to inattention and hyperactivity. The researchers performed linear regression to determine the predictors of sleep health and attention dysregulation.
The mean age of 60 children was 3 years, 55% were male, 32% were black, 42% had severe disease, 42% had moderate disease, and 16% had mild disease. Children with more extensive AD were significantly more likely to report worse sleep disturbance. The proportion of children who reported sleep disturbance on at least 5 nights per week was 67% among those with severe AD, 24% among those with moderate AD, and 0% among those with mild AD.
In addition, 72% of parents of children with severe AD reported trouble paying attention at least 3 times per week “no matter what was going on,” compared with 24% of those with moderate AD and none of those with mild AD.
Parents of children with more severe AD reported more itch-related burden and significantly decreased quality of life for their children. For example, 76% of parents with children who had severe AD reported “because of itch, their child was frustrated,” compared to 44% of those with moderate AD and 10% with mild AD.
In fully adjusted linear regression analysis, the strongest predictors of sleep disturbance were AD severity (unstandardized beta value = 0.79, P less than .01) and being Black (unstandardized beta value = 3.89, P = .03). AD severity (unstandardized beta value = 1.22, P less than .01) and being Black (unstandardized beta value = 7.79, P less than .01) also predicted more attention dysregulation.
Household income appeared to differ significantly based on AD severity groups. “If you have mild AD, you are more likely to come from a higher income household,” Ms. Zhou said.
She concluded her presentation by calling for future studies with larger samples sizes to establish causality and directional effects between AD severity, itch, sleep, race, and attention.
The study was funded by the Agency for Healthcare Research and Quality. Ms. Zhou reported having no financial disclosures.
, results from a national survey demonstrated.
“We think it’s important for dermatologists and pediatricians to be monitoring children with AD for sleep and attention dysregulation,” Nina Y. Zhou said during a late-breaking research session at the Revolutionizing Atopic Dermatitis virtual symposium. “It’s also important to highlight sleep hygiene habits to improve sleep health overall.”
In an effort to determine the impact of AD severity on these symptoms in young children with AD and characterize sleep health and attention regulation behaviors, Ms. Zhou, a medical student at Northwestern University, Chicago, and colleagues drew from a national survey distributed via panel company OP4G and the National Eczema Association that was conducted with parents of 60 children with AD aged 1-5 years. Questionnaires included the Patient Reported Outcomes Measurement Information System (PROMIS) Early Childhood Sleep Health Measures to assess sleep health, the Peak Pruritus NRS to measure itch severity, and the Multidimensional Assessment Profile of Attention Regulation (MAPS-AR) to measure attention dysregulation related to inattention and hyperactivity. The researchers performed linear regression to determine the predictors of sleep health and attention dysregulation.
The mean age of 60 children was 3 years, 55% were male, 32% were black, 42% had severe disease, 42% had moderate disease, and 16% had mild disease. Children with more extensive AD were significantly more likely to report worse sleep disturbance. The proportion of children who reported sleep disturbance on at least 5 nights per week was 67% among those with severe AD, 24% among those with moderate AD, and 0% among those with mild AD.
In addition, 72% of parents of children with severe AD reported trouble paying attention at least 3 times per week “no matter what was going on,” compared with 24% of those with moderate AD and none of those with mild AD.
Parents of children with more severe AD reported more itch-related burden and significantly decreased quality of life for their children. For example, 76% of parents with children who had severe AD reported “because of itch, their child was frustrated,” compared to 44% of those with moderate AD and 10% with mild AD.
In fully adjusted linear regression analysis, the strongest predictors of sleep disturbance were AD severity (unstandardized beta value = 0.79, P less than .01) and being Black (unstandardized beta value = 3.89, P = .03). AD severity (unstandardized beta value = 1.22, P less than .01) and being Black (unstandardized beta value = 7.79, P less than .01) also predicted more attention dysregulation.
Household income appeared to differ significantly based on AD severity groups. “If you have mild AD, you are more likely to come from a higher income household,” Ms. Zhou said.
She concluded her presentation by calling for future studies with larger samples sizes to establish causality and directional effects between AD severity, itch, sleep, race, and attention.
The study was funded by the Agency for Healthcare Research and Quality. Ms. Zhou reported having no financial disclosures.
, results from a national survey demonstrated.
“We think it’s important for dermatologists and pediatricians to be monitoring children with AD for sleep and attention dysregulation,” Nina Y. Zhou said during a late-breaking research session at the Revolutionizing Atopic Dermatitis virtual symposium. “It’s also important to highlight sleep hygiene habits to improve sleep health overall.”
In an effort to determine the impact of AD severity on these symptoms in young children with AD and characterize sleep health and attention regulation behaviors, Ms. Zhou, a medical student at Northwestern University, Chicago, and colleagues drew from a national survey distributed via panel company OP4G and the National Eczema Association that was conducted with parents of 60 children with AD aged 1-5 years. Questionnaires included the Patient Reported Outcomes Measurement Information System (PROMIS) Early Childhood Sleep Health Measures to assess sleep health, the Peak Pruritus NRS to measure itch severity, and the Multidimensional Assessment Profile of Attention Regulation (MAPS-AR) to measure attention dysregulation related to inattention and hyperactivity. The researchers performed linear regression to determine the predictors of sleep health and attention dysregulation.
The mean age of 60 children was 3 years, 55% were male, 32% were black, 42% had severe disease, 42% had moderate disease, and 16% had mild disease. Children with more extensive AD were significantly more likely to report worse sleep disturbance. The proportion of children who reported sleep disturbance on at least 5 nights per week was 67% among those with severe AD, 24% among those with moderate AD, and 0% among those with mild AD.
In addition, 72% of parents of children with severe AD reported trouble paying attention at least 3 times per week “no matter what was going on,” compared with 24% of those with moderate AD and none of those with mild AD.
Parents of children with more severe AD reported more itch-related burden and significantly decreased quality of life for their children. For example, 76% of parents with children who had severe AD reported “because of itch, their child was frustrated,” compared to 44% of those with moderate AD and 10% with mild AD.
In fully adjusted linear regression analysis, the strongest predictors of sleep disturbance were AD severity (unstandardized beta value = 0.79, P less than .01) and being Black (unstandardized beta value = 3.89, P = .03). AD severity (unstandardized beta value = 1.22, P less than .01) and being Black (unstandardized beta value = 7.79, P less than .01) also predicted more attention dysregulation.
Household income appeared to differ significantly based on AD severity groups. “If you have mild AD, you are more likely to come from a higher income household,” Ms. Zhou said.
She concluded her presentation by calling for future studies with larger samples sizes to establish causality and directional effects between AD severity, itch, sleep, race, and attention.
The study was funded by the Agency for Healthcare Research and Quality. Ms. Zhou reported having no financial disclosures.
FROM REVOLUTIONIZING AD 2020
Shortcomings identified in study of acne videos on TikTok
, according to an analysis of the top 100 videos using a consumer health validation tool.
The popularity of TikTok among adolescents in particular has implications for the dissemination of acne information, as some teens become “skinfluencers” and receive sponsorship from skin care brands in exchange for social media promotion, wrote David X. Zheng, BA, of the department of dermatology, Case Western Reserve University, Cleveland, and colleagues.
“However, the quality of dermatologic information found on TikTok is largely unknown,” they said.
In a brief report published in Pediatric Dermatology, the researchers identified the top 100 videos on TikTok on May 1, 2020, that were tagged with “#acne.” The information on each video included date of upload, type and gender of the individual uploading the video, physician specialty if applicable, and video category. These top 100 videos had 13,470,501 likes and 64,775 comments over a 7.6-month time period.
The researchers used the DISCERN criteria, a validated 1-5 scale designed to assess consumer health information, to evaluate the video content, with 1 (having “serious” or “extensive shortcomings”) and 5 (having “minimal shortcomings.”)
Overall, the average quality rating of the TikTok acne videos was 2.03. A total of 9 videos were produced by board-certified physicians in the United States, with an average DISCERN score of 2.41.
“Analysis of the DISCERN criteria dimensions suggested that major shortcomings common to both physician and nonphysician uploaders included failure to cite information sources, discuss treatment risks, and provide support for shared decision-making,” the researchers said.
Approximately one-third (34%) of the videos fell into the treatment-product advertisement category, while 26% were personal anecdotes, 20% presented information related to acne, 13% featured home remedy treatments, and 7% were classified as “other.” The researchers also identified the top 200 “#acne” videos on TikTok once a week from May 8, 2020 to June 5, 2020, to determine the evolution of acne content on the app and found a turnover rate of 10.9% per week.
Based on the high turnover and low quality based on DISCERN ratings, the authors suggested that patients seeking acne information should “view acne-related TikTok videos with caution and consult evidence-based resources whenever possible.”
The study findings were limited by several factors including the small sample size of physicians uploading videos, lack of information about the number of nonphysician medical professionals who uploaded videos, and lack of information about the number of video views and country of origin, the researchers noted. However, the results highlight the need for dermatologists to be aware that patients, especially teens, may be using TikTok for acne information that may be of poor quality, they said.
“Conversely, we understand that social media can be a powerful tool for advancing health literacy,” the researchers noted. “Therefore, we also recommend that health care professionals engaging on TikTok create thorough and perhaps standardized educational videos regarding acne, as well as correct any acne-related misinformation that may be present,” they concluded.
The other authors of the study were from the departments of dermatology at Case Western Reserve, University Hospitals Cleveland, and Johns Hopkins University, Baltimore.
The study received no outside funding. The researchers had no financial conflicts to disclose.
SOURCE: Zheng DX et al. Pediatr Dermatol. 2020 Nov 28. doi: 10.1111/pde.14471.
, according to an analysis of the top 100 videos using a consumer health validation tool.
The popularity of TikTok among adolescents in particular has implications for the dissemination of acne information, as some teens become “skinfluencers” and receive sponsorship from skin care brands in exchange for social media promotion, wrote David X. Zheng, BA, of the department of dermatology, Case Western Reserve University, Cleveland, and colleagues.
“However, the quality of dermatologic information found on TikTok is largely unknown,” they said.
In a brief report published in Pediatric Dermatology, the researchers identified the top 100 videos on TikTok on May 1, 2020, that were tagged with “#acne.” The information on each video included date of upload, type and gender of the individual uploading the video, physician specialty if applicable, and video category. These top 100 videos had 13,470,501 likes and 64,775 comments over a 7.6-month time period.
The researchers used the DISCERN criteria, a validated 1-5 scale designed to assess consumer health information, to evaluate the video content, with 1 (having “serious” or “extensive shortcomings”) and 5 (having “minimal shortcomings.”)
Overall, the average quality rating of the TikTok acne videos was 2.03. A total of 9 videos were produced by board-certified physicians in the United States, with an average DISCERN score of 2.41.
“Analysis of the DISCERN criteria dimensions suggested that major shortcomings common to both physician and nonphysician uploaders included failure to cite information sources, discuss treatment risks, and provide support for shared decision-making,” the researchers said.
Approximately one-third (34%) of the videos fell into the treatment-product advertisement category, while 26% were personal anecdotes, 20% presented information related to acne, 13% featured home remedy treatments, and 7% were classified as “other.” The researchers also identified the top 200 “#acne” videos on TikTok once a week from May 8, 2020 to June 5, 2020, to determine the evolution of acne content on the app and found a turnover rate of 10.9% per week.
Based on the high turnover and low quality based on DISCERN ratings, the authors suggested that patients seeking acne information should “view acne-related TikTok videos with caution and consult evidence-based resources whenever possible.”
The study findings were limited by several factors including the small sample size of physicians uploading videos, lack of information about the number of nonphysician medical professionals who uploaded videos, and lack of information about the number of video views and country of origin, the researchers noted. However, the results highlight the need for dermatologists to be aware that patients, especially teens, may be using TikTok for acne information that may be of poor quality, they said.
“Conversely, we understand that social media can be a powerful tool for advancing health literacy,” the researchers noted. “Therefore, we also recommend that health care professionals engaging on TikTok create thorough and perhaps standardized educational videos regarding acne, as well as correct any acne-related misinformation that may be present,” they concluded.
The other authors of the study were from the departments of dermatology at Case Western Reserve, University Hospitals Cleveland, and Johns Hopkins University, Baltimore.
The study received no outside funding. The researchers had no financial conflicts to disclose.
SOURCE: Zheng DX et al. Pediatr Dermatol. 2020 Nov 28. doi: 10.1111/pde.14471.
, according to an analysis of the top 100 videos using a consumer health validation tool.
The popularity of TikTok among adolescents in particular has implications for the dissemination of acne information, as some teens become “skinfluencers” and receive sponsorship from skin care brands in exchange for social media promotion, wrote David X. Zheng, BA, of the department of dermatology, Case Western Reserve University, Cleveland, and colleagues.
“However, the quality of dermatologic information found on TikTok is largely unknown,” they said.
In a brief report published in Pediatric Dermatology, the researchers identified the top 100 videos on TikTok on May 1, 2020, that were tagged with “#acne.” The information on each video included date of upload, type and gender of the individual uploading the video, physician specialty if applicable, and video category. These top 100 videos had 13,470,501 likes and 64,775 comments over a 7.6-month time period.
The researchers used the DISCERN criteria, a validated 1-5 scale designed to assess consumer health information, to evaluate the video content, with 1 (having “serious” or “extensive shortcomings”) and 5 (having “minimal shortcomings.”)
Overall, the average quality rating of the TikTok acne videos was 2.03. A total of 9 videos were produced by board-certified physicians in the United States, with an average DISCERN score of 2.41.
“Analysis of the DISCERN criteria dimensions suggested that major shortcomings common to both physician and nonphysician uploaders included failure to cite information sources, discuss treatment risks, and provide support for shared decision-making,” the researchers said.
Approximately one-third (34%) of the videos fell into the treatment-product advertisement category, while 26% were personal anecdotes, 20% presented information related to acne, 13% featured home remedy treatments, and 7% were classified as “other.” The researchers also identified the top 200 “#acne” videos on TikTok once a week from May 8, 2020 to June 5, 2020, to determine the evolution of acne content on the app and found a turnover rate of 10.9% per week.
Based on the high turnover and low quality based on DISCERN ratings, the authors suggested that patients seeking acne information should “view acne-related TikTok videos with caution and consult evidence-based resources whenever possible.”
The study findings were limited by several factors including the small sample size of physicians uploading videos, lack of information about the number of nonphysician medical professionals who uploaded videos, and lack of information about the number of video views and country of origin, the researchers noted. However, the results highlight the need for dermatologists to be aware that patients, especially teens, may be using TikTok for acne information that may be of poor quality, they said.
“Conversely, we understand that social media can be a powerful tool for advancing health literacy,” the researchers noted. “Therefore, we also recommend that health care professionals engaging on TikTok create thorough and perhaps standardized educational videos regarding acne, as well as correct any acne-related misinformation that may be present,” they concluded.
The other authors of the study were from the departments of dermatology at Case Western Reserve, University Hospitals Cleveland, and Johns Hopkins University, Baltimore.
The study received no outside funding. The researchers had no financial conflicts to disclose.
SOURCE: Zheng DX et al. Pediatr Dermatol. 2020 Nov 28. doi: 10.1111/pde.14471.
FROM PEDIATRIC DERMATOLOGY
Food allergy testing for eczema in kids varies by specialty
Specialists vary on their opinion about the ordering of food allergy tests for children with eczema, a recent survey reveals.
A child with eczema is more likely to be given food allergy tests if seen by an allergist or a pediatrician and less likely to be given these tests if seen by a general practitioner or dermatologist.
“In our survey, we found evidence of variation in practice and a spectrum of opinion on what to do to treat eczema in children,” Matthew Ridd, MD, University of Bristol (England) said in an interview.
His clinician survey was sent to 155 health care providers. Findings were presented at the Food Allergy and Anaphylaxis Meeting–European Consortium on Application of Flow Cytometry in Allergy Congress, held virtually. They revealed big differences in the way physicians follow up on eczema. For a child with eczema with reported reactions to food, 20 of 22 (91%) allergists and 22 of 30 (73%) pediatricians always order food allergy tests.
But only 16 of 65 (25%) general practitioners and 3 of 12 (25%) dermatologists always order tests in the same situation.
A total of 155 health care practitioners responded to the survey, sent by a U.K. research team. Of those, 26 were unable to order allergy tests. Of the remaining 129, 65 (50%) specialized in general practice, 30 (23%) in pediatrics, 22 (17%) in the treatment of allergies, and 12 (9%) in dermatology.
Their opinions varied on when to order food allergy tests. For children with severe eczema who had no prior reaction to food, 8 of 22 (36%) practitioners specializing in allergy said they would order food allergy tests, as did 9 of 30 (30%) in pediatrics.
Of those surveyed, only 6 of 65 in general practice (9%) said they would request an allergy test for severe eczema for a patient with no allergy history, and no dermatologists (0%) would order the tests.
Only if a parent specifically requested a food allergy test would practitioners respond in a similar way. About two-thirds of all respondents said they would sometimes order the test if a parent asked (general practice, 75%; pediatrics, 63%; allergy, 68%; dermatology, 75%).
Dr. Ridd said in an interview that it’s not surprising there’s a wide variation in practice, inasmuch as the guidelines are quite convoluted and complex. “Eczema is a common problem, but we don’t have any good evidence to guide clinicians on when to consider food allergy as a possible cause.”
Current guidelines advise calling for allergy tests only when eczema is difficult to treat. “But this is a complex decision. We know that a third of children with eczema are at higher risk for food allergy,” Dr. Ridd said. A 2014 study published in Clinical and Experimental Allergy showed that infants with eczema are six times more likely to have egg allergy and 11 times more likely to have peanut allergy by 12 months than infants without eczema (Clin Exp Allergy. 2014;45:255-64).
Food allergy is a sticky subject, he said. “So we have to wonder, are general practitioners frightened to raise the question?
“We definitely see uncertainty around it.”
He suspects that parents may also be hesitant to bring it up. “They are likely thinking about it even if they don’t ask,” Dr. Ridd said. “I think it’s important to test for food allergy, to provide reassurance. Once we show it’s not an allergy, we can focus on topical treatment.”
Treating eczema with emollients may increase likelihood of food allergy
In a separate presentation at the FAAM-EUROBAT congress, Maeve Kelleher, MD, Imperial College London, said that, rather than help reduce eczema, emollients in infants probably cause an increase in the risk for skin infection and food allergy. Her research team performed a systematic review of 25,827 participants in randomized controlled trials of the use of skin care interventions in term infants for primary prevention of eczema and food allergy. The study focused especially on topical creams.
Dr. Kelleher reported that skin care interventions “probably don’t prevent eczema. They probably increase local skin infections and may increase food allergy.”
Other interventions need to be explored, she said. “Maybe prevention should be along the line of looking at the microbiome, or exposures on the skin when you’re younger.”
Dr. Ridd and Dr. Kelleher have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Specialists vary on their opinion about the ordering of food allergy tests for children with eczema, a recent survey reveals.
A child with eczema is more likely to be given food allergy tests if seen by an allergist or a pediatrician and less likely to be given these tests if seen by a general practitioner or dermatologist.
“In our survey, we found evidence of variation in practice and a spectrum of opinion on what to do to treat eczema in children,” Matthew Ridd, MD, University of Bristol (England) said in an interview.
His clinician survey was sent to 155 health care providers. Findings were presented at the Food Allergy and Anaphylaxis Meeting–European Consortium on Application of Flow Cytometry in Allergy Congress, held virtually. They revealed big differences in the way physicians follow up on eczema. For a child with eczema with reported reactions to food, 20 of 22 (91%) allergists and 22 of 30 (73%) pediatricians always order food allergy tests.
But only 16 of 65 (25%) general practitioners and 3 of 12 (25%) dermatologists always order tests in the same situation.
A total of 155 health care practitioners responded to the survey, sent by a U.K. research team. Of those, 26 were unable to order allergy tests. Of the remaining 129, 65 (50%) specialized in general practice, 30 (23%) in pediatrics, 22 (17%) in the treatment of allergies, and 12 (9%) in dermatology.
Their opinions varied on when to order food allergy tests. For children with severe eczema who had no prior reaction to food, 8 of 22 (36%) practitioners specializing in allergy said they would order food allergy tests, as did 9 of 30 (30%) in pediatrics.
Of those surveyed, only 6 of 65 in general practice (9%) said they would request an allergy test for severe eczema for a patient with no allergy history, and no dermatologists (0%) would order the tests.
Only if a parent specifically requested a food allergy test would practitioners respond in a similar way. About two-thirds of all respondents said they would sometimes order the test if a parent asked (general practice, 75%; pediatrics, 63%; allergy, 68%; dermatology, 75%).
Dr. Ridd said in an interview that it’s not surprising there’s a wide variation in practice, inasmuch as the guidelines are quite convoluted and complex. “Eczema is a common problem, but we don’t have any good evidence to guide clinicians on when to consider food allergy as a possible cause.”
Current guidelines advise calling for allergy tests only when eczema is difficult to treat. “But this is a complex decision. We know that a third of children with eczema are at higher risk for food allergy,” Dr. Ridd said. A 2014 study published in Clinical and Experimental Allergy showed that infants with eczema are six times more likely to have egg allergy and 11 times more likely to have peanut allergy by 12 months than infants without eczema (Clin Exp Allergy. 2014;45:255-64).
Food allergy is a sticky subject, he said. “So we have to wonder, are general practitioners frightened to raise the question?
“We definitely see uncertainty around it.”
He suspects that parents may also be hesitant to bring it up. “They are likely thinking about it even if they don’t ask,” Dr. Ridd said. “I think it’s important to test for food allergy, to provide reassurance. Once we show it’s not an allergy, we can focus on topical treatment.”
Treating eczema with emollients may increase likelihood of food allergy
In a separate presentation at the FAAM-EUROBAT congress, Maeve Kelleher, MD, Imperial College London, said that, rather than help reduce eczema, emollients in infants probably cause an increase in the risk for skin infection and food allergy. Her research team performed a systematic review of 25,827 participants in randomized controlled trials of the use of skin care interventions in term infants for primary prevention of eczema and food allergy. The study focused especially on topical creams.
Dr. Kelleher reported that skin care interventions “probably don’t prevent eczema. They probably increase local skin infections and may increase food allergy.”
Other interventions need to be explored, she said. “Maybe prevention should be along the line of looking at the microbiome, or exposures on the skin when you’re younger.”
Dr. Ridd and Dr. Kelleher have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Specialists vary on their opinion about the ordering of food allergy tests for children with eczema, a recent survey reveals.
A child with eczema is more likely to be given food allergy tests if seen by an allergist or a pediatrician and less likely to be given these tests if seen by a general practitioner or dermatologist.
“In our survey, we found evidence of variation in practice and a spectrum of opinion on what to do to treat eczema in children,” Matthew Ridd, MD, University of Bristol (England) said in an interview.
His clinician survey was sent to 155 health care providers. Findings were presented at the Food Allergy and Anaphylaxis Meeting–European Consortium on Application of Flow Cytometry in Allergy Congress, held virtually. They revealed big differences in the way physicians follow up on eczema. For a child with eczema with reported reactions to food, 20 of 22 (91%) allergists and 22 of 30 (73%) pediatricians always order food allergy tests.
But only 16 of 65 (25%) general practitioners and 3 of 12 (25%) dermatologists always order tests in the same situation.
A total of 155 health care practitioners responded to the survey, sent by a U.K. research team. Of those, 26 were unable to order allergy tests. Of the remaining 129, 65 (50%) specialized in general practice, 30 (23%) in pediatrics, 22 (17%) in the treatment of allergies, and 12 (9%) in dermatology.
Their opinions varied on when to order food allergy tests. For children with severe eczema who had no prior reaction to food, 8 of 22 (36%) practitioners specializing in allergy said they would order food allergy tests, as did 9 of 30 (30%) in pediatrics.
Of those surveyed, only 6 of 65 in general practice (9%) said they would request an allergy test for severe eczema for a patient with no allergy history, and no dermatologists (0%) would order the tests.
Only if a parent specifically requested a food allergy test would practitioners respond in a similar way. About two-thirds of all respondents said they would sometimes order the test if a parent asked (general practice, 75%; pediatrics, 63%; allergy, 68%; dermatology, 75%).
Dr. Ridd said in an interview that it’s not surprising there’s a wide variation in practice, inasmuch as the guidelines are quite convoluted and complex. “Eczema is a common problem, but we don’t have any good evidence to guide clinicians on when to consider food allergy as a possible cause.”
Current guidelines advise calling for allergy tests only when eczema is difficult to treat. “But this is a complex decision. We know that a third of children with eczema are at higher risk for food allergy,” Dr. Ridd said. A 2014 study published in Clinical and Experimental Allergy showed that infants with eczema are six times more likely to have egg allergy and 11 times more likely to have peanut allergy by 12 months than infants without eczema (Clin Exp Allergy. 2014;45:255-64).
Food allergy is a sticky subject, he said. “So we have to wonder, are general practitioners frightened to raise the question?
“We definitely see uncertainty around it.”
He suspects that parents may also be hesitant to bring it up. “They are likely thinking about it even if they don’t ask,” Dr. Ridd said. “I think it’s important to test for food allergy, to provide reassurance. Once we show it’s not an allergy, we can focus on topical treatment.”
Treating eczema with emollients may increase likelihood of food allergy
In a separate presentation at the FAAM-EUROBAT congress, Maeve Kelleher, MD, Imperial College London, said that, rather than help reduce eczema, emollients in infants probably cause an increase in the risk for skin infection and food allergy. Her research team performed a systematic review of 25,827 participants in randomized controlled trials of the use of skin care interventions in term infants for primary prevention of eczema and food allergy. The study focused especially on topical creams.
Dr. Kelleher reported that skin care interventions “probably don’t prevent eczema. They probably increase local skin infections and may increase food allergy.”
Other interventions need to be explored, she said. “Maybe prevention should be along the line of looking at the microbiome, or exposures on the skin when you’re younger.”
Dr. Ridd and Dr. Kelleher have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.