User login
Belimumab may improve skin in scleroderma
MAUI, HAWAII – Belimumab shows promise as a novel biologic treatment for skin involvement in early diffuse cutaneous systemic sclerosis, Janet E. Pope, MD, said at the 2020 Rheumatology Winter Clinical Symposium.
She highlighted a single-center, double-blind, placebo-controlled, New York pilot study including 20 patients with early diffuse cutaneous systemic sclerosis and moderate skin involvement. Participants had recently started on background mycophenolate mofetil (MMF) at 1,000 mg twice daily and were then randomized to add-on belimumab (Benlysta) at the dosing approved for systemic lupus erythematosus or to infusions of normal saline.
At 52 weeks, the modified Rodnan skin thickness score (mRSS) decreased by a median of 10 points from a baseline of 27 in the belimumab group, compared with just a 3-point reduction in controls on MMF plus placebo.
This small study raises several key points. It definitely warrants confirmation in a large phase 3 trial, according to Dr. Pope, professor of medicine at the University of Western Ontario and head of the division of rheumatology at St. Joseph’s Health Care, both in London.
For one thing, the pilot study makes a good case for multidrug therapy in scleroderma. “In rheumatoid arthritis, if in general one drug is not as good as two, why would we ever think, in our most difficult-to-treat disease, one drug would be okay?” the rheumatologist observed.
The belimumab study also highlights the role of abnormalities in B-cell function in the pathogenesis of skin involvement in early diffuse cutaneous systemic sclerosis. Belimumab is a fully human monoclonal antibody which binds to soluble B-lymphocyte stimulator and inhibits autoantibody production.
Belimumab’s mechanism of benefit was as expected: The improvement in skin scores in the belimumab group was accompanied by decreased expression of profibrotic genes and B-cell signaling, changes that didn’t occur in the controls on MMF alone.
The belimumab study makes another important point: MMF, despite its growing popularity for treatment of skin manifestations of scleroderma, is actually a wimpy drug for that purpose, achieving a mere 3-point reduction in mRSS.
“To be quite honest, mycophenolate mofetil is not all that great on skin,” Dr. Pope said.
Nonetheless, when she and her coworkers recently polled 170 scleroderma experts as to their favored treatments directed at various target organs impaired by the disease, as she had previously done in 2012, a clear trend was evident. “There’s a shift in that mycophenolate mofetil is moving to first-line treatment across the board for skin,” Dr. Pope observed.
Indeed, in the more recent survey, 71% of the experts agreed upon a scleroderma skin involvement treatment algorithm in which the first-line treatment for severe skin disease as defined by an mRSS of 32 was MMF, with methotrexate as second line, intravenous cyclophosphamide third, and autologous stem cell transplantation as fourth line for the small number of patients who qualify for it.
For moderate skin involvement, with an mRSS of 24, methotrexate was endorsed as first line, although by the narrowest of margins, over MMF, with intravenous cyclophosphamide as third line. For mild disease, with an mRSS of 10, methotrexate again narrowly beat out MMF by expert consensus as the preferred first-line therapy.
When asked about concomitant use of corticosteroids for treatment of skin involvement, 35% of experts said they never prescribe them for that indication, 33% do so occasionally, 19% sometimes, and 13% routinely. There was an even split on dosing among those who prescribe steroids: 49% suggested using prednisone at less than 7.5 mg/day, and 51% recommended 7.5-20 mg/day.
The purpose in polling the experts, who were drawn from the Scleroderma Clinical Trials Consortium and the Canadian Scleroderma Research Group, was to provide treatment guidance to general rheumatologists and dermatologists who may not see many patients with scleroderma. In contrast, the great majority of the polled experts see more than 50 scleroderma patients per year. And they had a high level of total agreement for treatment algorithms addressing not only skin disease, but also pulmonary arterial hypertension, interstitial lung disease, Raynaud’s phenomenon, renal crisis, digital ulcers, inflammatory arthritis, cardiac involvement, and gastrointestinal disease, Dr. Pope noted.
She attributed the experts’ rising enthusiasm for MMF for scleroderma skin involvement to the results of the Scleroderma Lung Study II, the first randomized, controlled trial to compare MMF and cyclophosphamide for the treatment of symptomatic scleroderma interstitial lung disease. Two years of MMF improved forced vital capacity as much as 1 year of oral cyclophosphamide. At 2 years of follow-up, the mRSS dropped modestly from baseline by an average of 6.1 points in the cyclophosphamide group and 2.9 points with MMF, a nonsignificant difference. But the incidence of serious adverse events was roughly three times higher and deaths were twice as frequent in the cyclophosphamide group.
“I think mycophenolate mofetil is surging for treatment of skin because of the lung protection and it was safer, but it’s hard for me to know if the deaths were more common in the cyclophosphamide group because of the cyclophosphamide or because of no treatment in year 2,” Dr. Pope commented.
She reported receiving research grants from Bristol-Myers Squibb, Merck, Roche, Seattle Genetics, and UCB, and serving as a consultant to more than a dozen pharmaceutical companies.
MAUI, HAWAII – Belimumab shows promise as a novel biologic treatment for skin involvement in early diffuse cutaneous systemic sclerosis, Janet E. Pope, MD, said at the 2020 Rheumatology Winter Clinical Symposium.
She highlighted a single-center, double-blind, placebo-controlled, New York pilot study including 20 patients with early diffuse cutaneous systemic sclerosis and moderate skin involvement. Participants had recently started on background mycophenolate mofetil (MMF) at 1,000 mg twice daily and were then randomized to add-on belimumab (Benlysta) at the dosing approved for systemic lupus erythematosus or to infusions of normal saline.
At 52 weeks, the modified Rodnan skin thickness score (mRSS) decreased by a median of 10 points from a baseline of 27 in the belimumab group, compared with just a 3-point reduction in controls on MMF plus placebo.
This small study raises several key points. It definitely warrants confirmation in a large phase 3 trial, according to Dr. Pope, professor of medicine at the University of Western Ontario and head of the division of rheumatology at St. Joseph’s Health Care, both in London.
For one thing, the pilot study makes a good case for multidrug therapy in scleroderma. “In rheumatoid arthritis, if in general one drug is not as good as two, why would we ever think, in our most difficult-to-treat disease, one drug would be okay?” the rheumatologist observed.
The belimumab study also highlights the role of abnormalities in B-cell function in the pathogenesis of skin involvement in early diffuse cutaneous systemic sclerosis. Belimumab is a fully human monoclonal antibody which binds to soluble B-lymphocyte stimulator and inhibits autoantibody production.
Belimumab’s mechanism of benefit was as expected: The improvement in skin scores in the belimumab group was accompanied by decreased expression of profibrotic genes and B-cell signaling, changes that didn’t occur in the controls on MMF alone.
The belimumab study makes another important point: MMF, despite its growing popularity for treatment of skin manifestations of scleroderma, is actually a wimpy drug for that purpose, achieving a mere 3-point reduction in mRSS.
“To be quite honest, mycophenolate mofetil is not all that great on skin,” Dr. Pope said.
Nonetheless, when she and her coworkers recently polled 170 scleroderma experts as to their favored treatments directed at various target organs impaired by the disease, as she had previously done in 2012, a clear trend was evident. “There’s a shift in that mycophenolate mofetil is moving to first-line treatment across the board for skin,” Dr. Pope observed.
Indeed, in the more recent survey, 71% of the experts agreed upon a scleroderma skin involvement treatment algorithm in which the first-line treatment for severe skin disease as defined by an mRSS of 32 was MMF, with methotrexate as second line, intravenous cyclophosphamide third, and autologous stem cell transplantation as fourth line for the small number of patients who qualify for it.
For moderate skin involvement, with an mRSS of 24, methotrexate was endorsed as first line, although by the narrowest of margins, over MMF, with intravenous cyclophosphamide as third line. For mild disease, with an mRSS of 10, methotrexate again narrowly beat out MMF by expert consensus as the preferred first-line therapy.
When asked about concomitant use of corticosteroids for treatment of skin involvement, 35% of experts said they never prescribe them for that indication, 33% do so occasionally, 19% sometimes, and 13% routinely. There was an even split on dosing among those who prescribe steroids: 49% suggested using prednisone at less than 7.5 mg/day, and 51% recommended 7.5-20 mg/day.
The purpose in polling the experts, who were drawn from the Scleroderma Clinical Trials Consortium and the Canadian Scleroderma Research Group, was to provide treatment guidance to general rheumatologists and dermatologists who may not see many patients with scleroderma. In contrast, the great majority of the polled experts see more than 50 scleroderma patients per year. And they had a high level of total agreement for treatment algorithms addressing not only skin disease, but also pulmonary arterial hypertension, interstitial lung disease, Raynaud’s phenomenon, renal crisis, digital ulcers, inflammatory arthritis, cardiac involvement, and gastrointestinal disease, Dr. Pope noted.
She attributed the experts’ rising enthusiasm for MMF for scleroderma skin involvement to the results of the Scleroderma Lung Study II, the first randomized, controlled trial to compare MMF and cyclophosphamide for the treatment of symptomatic scleroderma interstitial lung disease. Two years of MMF improved forced vital capacity as much as 1 year of oral cyclophosphamide. At 2 years of follow-up, the mRSS dropped modestly from baseline by an average of 6.1 points in the cyclophosphamide group and 2.9 points with MMF, a nonsignificant difference. But the incidence of serious adverse events was roughly three times higher and deaths were twice as frequent in the cyclophosphamide group.
“I think mycophenolate mofetil is surging for treatment of skin because of the lung protection and it was safer, but it’s hard for me to know if the deaths were more common in the cyclophosphamide group because of the cyclophosphamide or because of no treatment in year 2,” Dr. Pope commented.
She reported receiving research grants from Bristol-Myers Squibb, Merck, Roche, Seattle Genetics, and UCB, and serving as a consultant to more than a dozen pharmaceutical companies.
MAUI, HAWAII – Belimumab shows promise as a novel biologic treatment for skin involvement in early diffuse cutaneous systemic sclerosis, Janet E. Pope, MD, said at the 2020 Rheumatology Winter Clinical Symposium.
She highlighted a single-center, double-blind, placebo-controlled, New York pilot study including 20 patients with early diffuse cutaneous systemic sclerosis and moderate skin involvement. Participants had recently started on background mycophenolate mofetil (MMF) at 1,000 mg twice daily and were then randomized to add-on belimumab (Benlysta) at the dosing approved for systemic lupus erythematosus or to infusions of normal saline.
At 52 weeks, the modified Rodnan skin thickness score (mRSS) decreased by a median of 10 points from a baseline of 27 in the belimumab group, compared with just a 3-point reduction in controls on MMF plus placebo.
This small study raises several key points. It definitely warrants confirmation in a large phase 3 trial, according to Dr. Pope, professor of medicine at the University of Western Ontario and head of the division of rheumatology at St. Joseph’s Health Care, both in London.
For one thing, the pilot study makes a good case for multidrug therapy in scleroderma. “In rheumatoid arthritis, if in general one drug is not as good as two, why would we ever think, in our most difficult-to-treat disease, one drug would be okay?” the rheumatologist observed.
The belimumab study also highlights the role of abnormalities in B-cell function in the pathogenesis of skin involvement in early diffuse cutaneous systemic sclerosis. Belimumab is a fully human monoclonal antibody which binds to soluble B-lymphocyte stimulator and inhibits autoantibody production.
Belimumab’s mechanism of benefit was as expected: The improvement in skin scores in the belimumab group was accompanied by decreased expression of profibrotic genes and B-cell signaling, changes that didn’t occur in the controls on MMF alone.
The belimumab study makes another important point: MMF, despite its growing popularity for treatment of skin manifestations of scleroderma, is actually a wimpy drug for that purpose, achieving a mere 3-point reduction in mRSS.
“To be quite honest, mycophenolate mofetil is not all that great on skin,” Dr. Pope said.
Nonetheless, when she and her coworkers recently polled 170 scleroderma experts as to their favored treatments directed at various target organs impaired by the disease, as she had previously done in 2012, a clear trend was evident. “There’s a shift in that mycophenolate mofetil is moving to first-line treatment across the board for skin,” Dr. Pope observed.
Indeed, in the more recent survey, 71% of the experts agreed upon a scleroderma skin involvement treatment algorithm in which the first-line treatment for severe skin disease as defined by an mRSS of 32 was MMF, with methotrexate as second line, intravenous cyclophosphamide third, and autologous stem cell transplantation as fourth line for the small number of patients who qualify for it.
For moderate skin involvement, with an mRSS of 24, methotrexate was endorsed as first line, although by the narrowest of margins, over MMF, with intravenous cyclophosphamide as third line. For mild disease, with an mRSS of 10, methotrexate again narrowly beat out MMF by expert consensus as the preferred first-line therapy.
When asked about concomitant use of corticosteroids for treatment of skin involvement, 35% of experts said they never prescribe them for that indication, 33% do so occasionally, 19% sometimes, and 13% routinely. There was an even split on dosing among those who prescribe steroids: 49% suggested using prednisone at less than 7.5 mg/day, and 51% recommended 7.5-20 mg/day.
The purpose in polling the experts, who were drawn from the Scleroderma Clinical Trials Consortium and the Canadian Scleroderma Research Group, was to provide treatment guidance to general rheumatologists and dermatologists who may not see many patients with scleroderma. In contrast, the great majority of the polled experts see more than 50 scleroderma patients per year. And they had a high level of total agreement for treatment algorithms addressing not only skin disease, but also pulmonary arterial hypertension, interstitial lung disease, Raynaud’s phenomenon, renal crisis, digital ulcers, inflammatory arthritis, cardiac involvement, and gastrointestinal disease, Dr. Pope noted.
She attributed the experts’ rising enthusiasm for MMF for scleroderma skin involvement to the results of the Scleroderma Lung Study II, the first randomized, controlled trial to compare MMF and cyclophosphamide for the treatment of symptomatic scleroderma interstitial lung disease. Two years of MMF improved forced vital capacity as much as 1 year of oral cyclophosphamide. At 2 years of follow-up, the mRSS dropped modestly from baseline by an average of 6.1 points in the cyclophosphamide group and 2.9 points with MMF, a nonsignificant difference. But the incidence of serious adverse events was roughly three times higher and deaths were twice as frequent in the cyclophosphamide group.
“I think mycophenolate mofetil is surging for treatment of skin because of the lung protection and it was safer, but it’s hard for me to know if the deaths were more common in the cyclophosphamide group because of the cyclophosphamide or because of no treatment in year 2,” Dr. Pope commented.
She reported receiving research grants from Bristol-Myers Squibb, Merck, Roche, Seattle Genetics, and UCB, and serving as a consultant to more than a dozen pharmaceutical companies.
REPORTING FROM RWCS 2020
Red painful nodules in a hospitalized patient
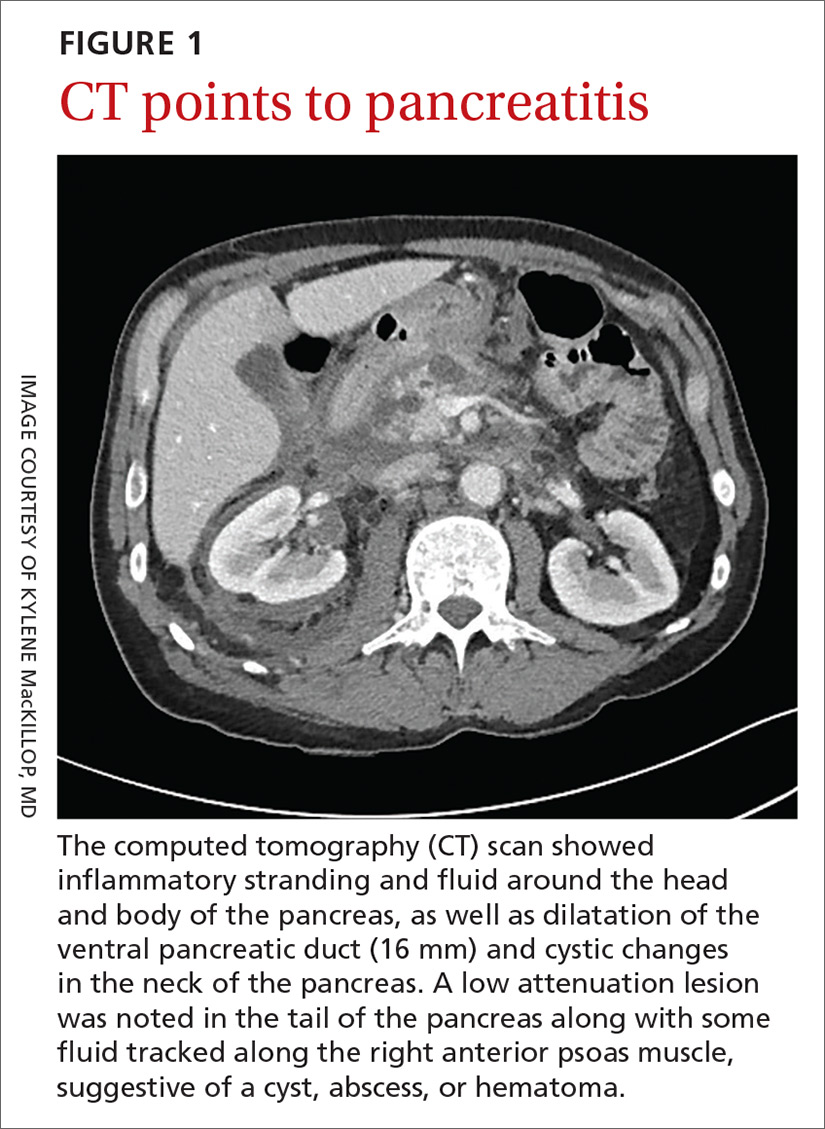
A 58-year-old white man with a history of alcoholism presented to the emergency department with epigastric and right upper quadrant pain radiating to the back, as well as emesis and anorexia. An elevated lipase of 16,609 U/L (reference range, 31–186 U/L) and pathognomonic abdominal computed tomography (CT) findings (FIGURE 1) led to the diagnosis of acute pancreatitis, for which he was admitted.
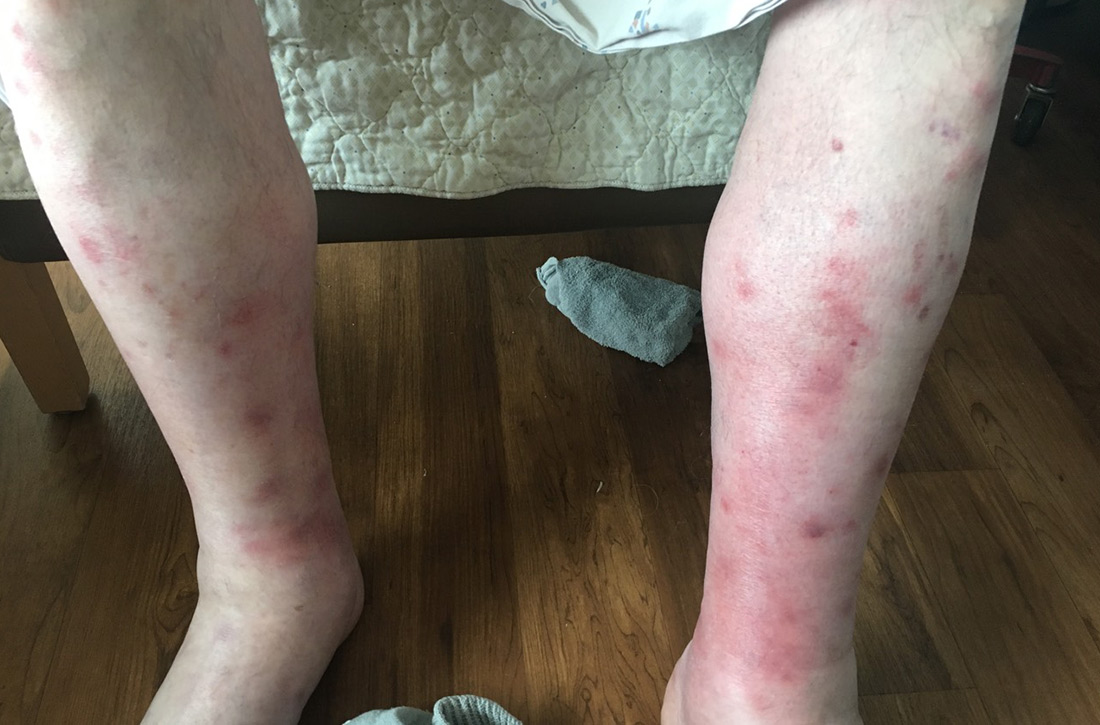
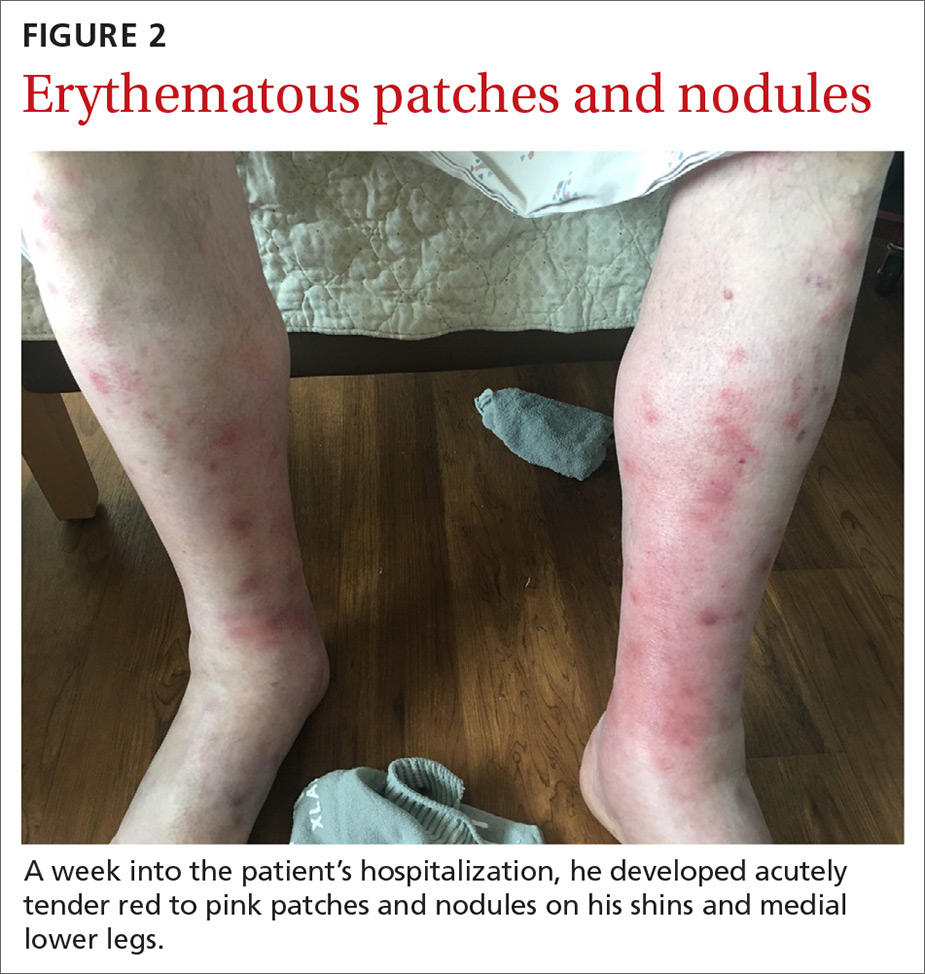
Fluid resuscitation and pain management were implemented, and over 3 days his diet was advanced from NPO to clear fluids to a full diet. On the sixth day of hospitalization, the patient developed increasing abdominal pain and worsening leukocytosis (white blood cell count, 16.6–22 K/mcL [reference range, 4.5–11 K/mcL]). Repeat CT and blood cultures were obtained, and the patient was started on intravenous meropenem 1 g every 8 hours for presumed necrotizing pancreatitis. The next day he developed acutely tender red to pink patches and nodules on his shins and medial lower legs (FIGURE 2).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Pancreatic panniculitis
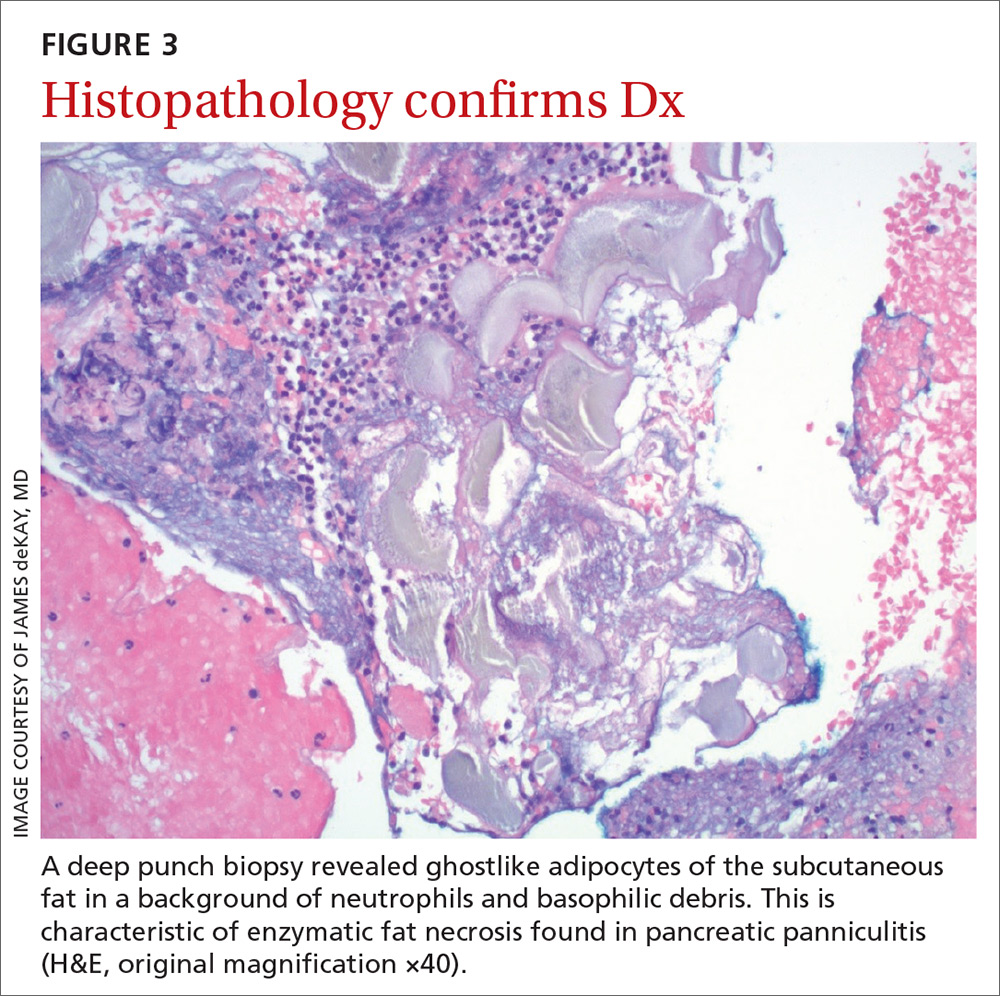
It’s theorized that the systemic release of trypsin from pancreatic cell destruction causes increased capillary permeability and subsequent escape of lipase from the circulation into the subcutaneous fat. This causes fat necrosis, saponification, and inflammation.3,4 Pancreatic panniculitis is demonstrated histologically as hollowed-out adipocytes with granular basophilic cytoplasm and displaced or absent nuclei—aptly named “ghostlike” adipocytes.3-6
Painful, erythematous nodules most commonly present on the distal lower extremities. Nodules may be found over the shins, posterior calves, and periarticular skin. Rarely, nodules may occur on the buttocks, abdomen, or intramedullary bone.7 In severe cases, nodules spontaneously may ulcerate and drain an oily brown, viscous material formed from necrotic adipocytes.1
Timing of the eruption of skin lesions is varied and may even precede abdominal pain. Lesions can involute and regress several weeks after the underlying etiology improves. With pancreatic carcinoma, there is a greater likelihood of persistence, atypical locations of involvement, ulcerations, and recurrences.7
The histologic features of pancreatic panniculitis and the assessment of the subcutaneous fat are paramount in diagnosis. A deep punch biopsy or incisional biopsy is necessary to reliably reach the depth of the subcutaneous tissue. In our patient, a deep punch biopsy from the lateral calf was performed at the suggestion of Dermatology, and histopathology revealed necrosis of fat lobules with calcium soap around necrotic lipocytes, consistent with pancreatic panniculitis (FIGURE 3).
Continue to: Differential was complicated by antibiotic use
Differential was complicated by antibiotic use
The differential diagnosis was broad due to the confounding factors of recent antibiotic use and worsening pancreatitis.
Cellulitis may present as a red patch and is common on the lower legs; it often is associated with skin pathogens including Staphylococcus and Streptococcus. Usually, symptoms are unilateral and associated with warmth to the touch, expanding borders, leukocytosis, and systemic symptoms.
Vasculitis, which is an inflammation of various sized vessels through immunologic or infectious processes, often manifests on the lower legs. The characteristic sign of small vessel vasculitis is nonblanching purpura or petechiae. There often is a preceding illness or medication that triggers immunoglobulin proliferation and off-target inflammation of the vessels. Associated symptoms include pain and pruritus.
Drug eruptions may present as red patches on the skin. Often the patches are scaly and red and have more widespread distribution than the lower legs. A history of exposure is important, but common inciting drugs include nonsteroidal anti-inflammatory drugs that may be used only occasionally and are challenging to elicit in the history. Our patient did have known drug changes (ie, the introduction of meropenem) while hospitalized, but the morphology was not consistent with this diagnosis.
Treatment is directed to underlying disease
Treatment of pancreatic panniculitis primarily is supportive and directed toward treating the underlying pancreatic disease. Depending upon the underlying pancreatic diagnosis, surgical correction of anatomic or ductal anomalies or pseudocysts may lead to resolution of panniculitis.3,7,8
Continue to: In this case
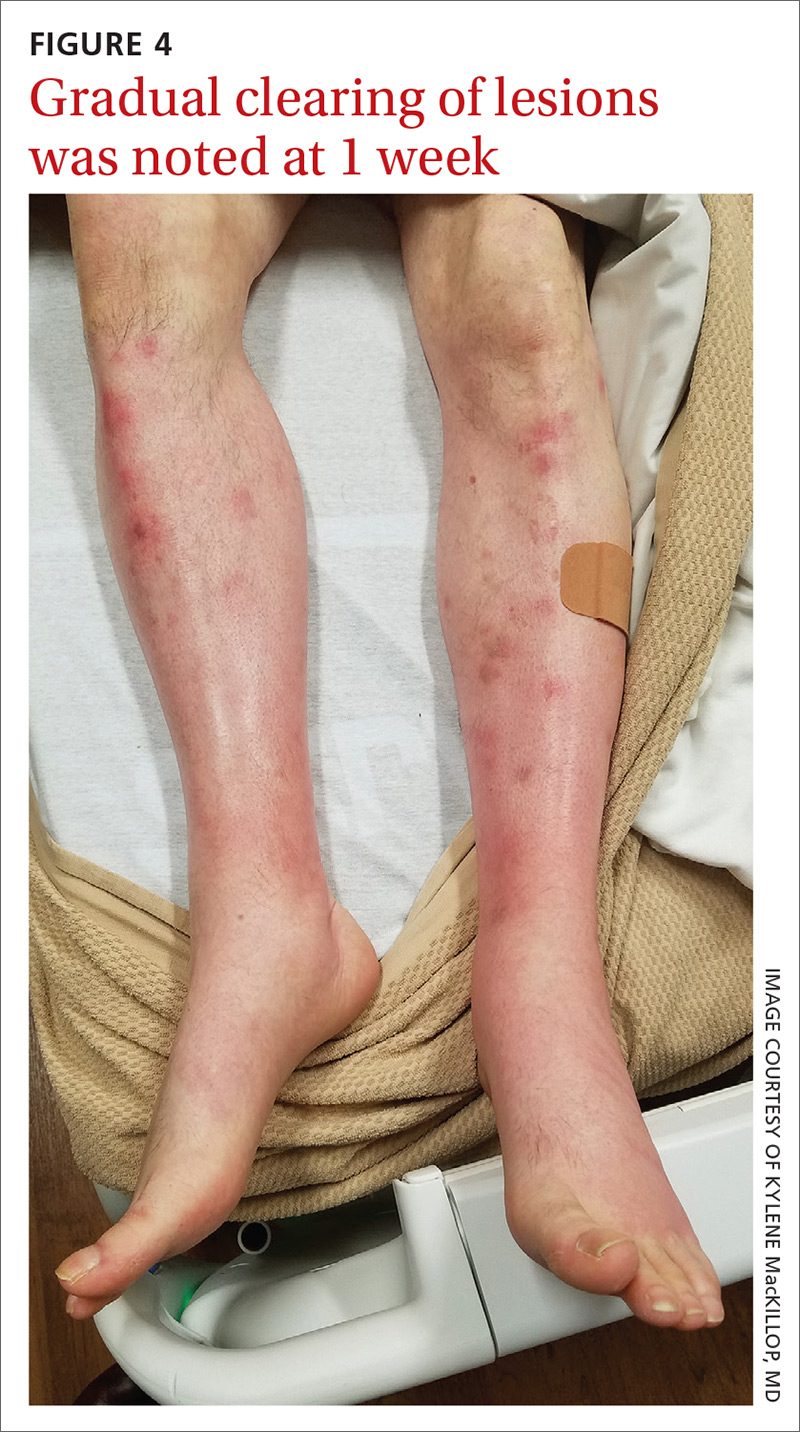
In this case, our patient had already received fluid resuscitation and pain management, and his diet had been advanced. In addition, his antibiotics were changed to exclude drug eruption as a cause. Over the course of a week, our patient saw a reduction in his pain level and an improvement in the appearance of his legs (FIGURE 4).
His pancreatitis, however, continued to persist and resist increases in his diet. He ultimately required transfer to a tertiary care center for consideration of interventional options including stenting. The patient ultimately recovered, after stenting of the main pancreatic duct, and was discharged home.
CORRESPONDENCE
Jonathan Karnes, MD, 6 East Chestnut Street, Augusta, ME 04330; [email protected]
1. Madarasingha NP, Satgurunathan K, Fernando R. Pancreatic panniculitis: a rare form of panniculitis. Dermatol Online J. 2009;15:17.
2. Haber RM, Assaad DM. Panniculitis associated with a pancreas divisum. J Am Acad Dermatol. 1986;14(2 pt 2):331-334.
3. Requena L, Sánchez Yus E. Panniculitis. part II. mostly lobular panniculitis. J Am Acad Dermatol. 2001;45:325-361.
4. Rongioletti F, Caputo V. Pancreatic panniculitis. G Ital Dermatol Venereol. 2013;148:419-425.
5. Förström TL, Winkelmann RK. Acute, generalized panniculitis with amylase and lipase in skin. Arch Dermatol. 1975;111:497-502.
6. Hughes SH, Apisarnthanarax P, Mullins F. Subcutaneous fat necrosis associated with pancreatic disease. Arch Dermatol. 1975;111:506-510.
7. Dahl PR, Su WP, Cullimore KC, et al. Pancreatic panniculitis. J Am Acad Dermatol. 1995;33:413-417.
8. Lambiase P, Seery JP, Taylor-Robinson SD, et al. Resolution of panniculitis after placement of pancreatic duct stent in chro nic pancreatitis. Am J Gastroenterol. 1996;91:1835-1837.
A 58-year-old white man with a history of alcoholism presented to the emergency department with epigastric and right upper quadrant pain radiating to the back, as well as emesis and anorexia. An elevated lipase of 16,609 U/L (reference range, 31–186 U/L) and pathognomonic abdominal computed tomography (CT) findings (FIGURE 1) led to the diagnosis of acute pancreatitis, for which he was admitted.

Fluid resuscitation and pain management were implemented, and over 3 days his diet was advanced from NPO to clear fluids to a full diet. On the sixth day of hospitalization, the patient developed increasing abdominal pain and worsening leukocytosis (white blood cell count, 16.6–22 K/mcL [reference range, 4.5–11 K/mcL]). Repeat CT and blood cultures were obtained, and the patient was started on intravenous meropenem 1 g every 8 hours for presumed necrotizing pancreatitis. The next day he developed acutely tender red to pink patches and nodules on his shins and medial lower legs (FIGURE 2).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Pancreatic panniculitis
It’s theorized that the systemic release of trypsin from pancreatic cell destruction causes increased capillary permeability and subsequent escape of lipase from the circulation into the subcutaneous fat. This causes fat necrosis, saponification, and inflammation.3,4 Pancreatic panniculitis is demonstrated histologically as hollowed-out adipocytes with granular basophilic cytoplasm and displaced or absent nuclei—aptly named “ghostlike” adipocytes.3-6
Painful, erythematous nodules most commonly present on the distal lower extremities. Nodules may be found over the shins, posterior calves, and periarticular skin. Rarely, nodules may occur on the buttocks, abdomen, or intramedullary bone.7 In severe cases, nodules spontaneously may ulcerate and drain an oily brown, viscous material formed from necrotic adipocytes.1
Timing of the eruption of skin lesions is varied and may even precede abdominal pain. Lesions can involute and regress several weeks after the underlying etiology improves. With pancreatic carcinoma, there is a greater likelihood of persistence, atypical locations of involvement, ulcerations, and recurrences.7
The histologic features of pancreatic panniculitis and the assessment of the subcutaneous fat are paramount in diagnosis. A deep punch biopsy or incisional biopsy is necessary to reliably reach the depth of the subcutaneous tissue. In our patient, a deep punch biopsy from the lateral calf was performed at the suggestion of Dermatology, and histopathology revealed necrosis of fat lobules with calcium soap around necrotic lipocytes, consistent with pancreatic panniculitis (FIGURE 3).

Continue to: Differential was complicated by antibiotic use
Differential was complicated by antibiotic use
The differential diagnosis was broad due to the confounding factors of recent antibiotic use and worsening pancreatitis.
Cellulitis may present as a red patch and is common on the lower legs; it often is associated with skin pathogens including Staphylococcus and Streptococcus. Usually, symptoms are unilateral and associated with warmth to the touch, expanding borders, leukocytosis, and systemic symptoms.
Vasculitis, which is an inflammation of various sized vessels through immunologic or infectious processes, often manifests on the lower legs. The characteristic sign of small vessel vasculitis is nonblanching purpura or petechiae. There often is a preceding illness or medication that triggers immunoglobulin proliferation and off-target inflammation of the vessels. Associated symptoms include pain and pruritus.
Drug eruptions may present as red patches on the skin. Often the patches are scaly and red and have more widespread distribution than the lower legs. A history of exposure is important, but common inciting drugs include nonsteroidal anti-inflammatory drugs that may be used only occasionally and are challenging to elicit in the history. Our patient did have known drug changes (ie, the introduction of meropenem) while hospitalized, but the morphology was not consistent with this diagnosis.
Treatment is directed to underlying disease
Treatment of pancreatic panniculitis primarily is supportive and directed toward treating the underlying pancreatic disease. Depending upon the underlying pancreatic diagnosis, surgical correction of anatomic or ductal anomalies or pseudocysts may lead to resolution of panniculitis.3,7,8
Continue to: In this case
In this case, our patient had already received fluid resuscitation and pain management, and his diet had been advanced. In addition, his antibiotics were changed to exclude drug eruption as a cause. Over the course of a week, our patient saw a reduction in his pain level and an improvement in the appearance of his legs (FIGURE 4).

His pancreatitis, however, continued to persist and resist increases in his diet. He ultimately required transfer to a tertiary care center for consideration of interventional options including stenting. The patient ultimately recovered, after stenting of the main pancreatic duct, and was discharged home.
CORRESPONDENCE
Jonathan Karnes, MD, 6 East Chestnut Street, Augusta, ME 04330; [email protected]
A 58-year-old white man with a history of alcoholism presented to the emergency department with epigastric and right upper quadrant pain radiating to the back, as well as emesis and anorexia. An elevated lipase of 16,609 U/L (reference range, 31–186 U/L) and pathognomonic abdominal computed tomography (CT) findings (FIGURE 1) led to the diagnosis of acute pancreatitis, for which he was admitted.

Fluid resuscitation and pain management were implemented, and over 3 days his diet was advanced from NPO to clear fluids to a full diet. On the sixth day of hospitalization, the patient developed increasing abdominal pain and worsening leukocytosis (white blood cell count, 16.6–22 K/mcL [reference range, 4.5–11 K/mcL]). Repeat CT and blood cultures were obtained, and the patient was started on intravenous meropenem 1 g every 8 hours for presumed necrotizing pancreatitis. The next day he developed acutely tender red to pink patches and nodules on his shins and medial lower legs (FIGURE 2).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Pancreatic panniculitis
It’s theorized that the systemic release of trypsin from pancreatic cell destruction causes increased capillary permeability and subsequent escape of lipase from the circulation into the subcutaneous fat. This causes fat necrosis, saponification, and inflammation.3,4 Pancreatic panniculitis is demonstrated histologically as hollowed-out adipocytes with granular basophilic cytoplasm and displaced or absent nuclei—aptly named “ghostlike” adipocytes.3-6
Painful, erythematous nodules most commonly present on the distal lower extremities. Nodules may be found over the shins, posterior calves, and periarticular skin. Rarely, nodules may occur on the buttocks, abdomen, or intramedullary bone.7 In severe cases, nodules spontaneously may ulcerate and drain an oily brown, viscous material formed from necrotic adipocytes.1
Timing of the eruption of skin lesions is varied and may even precede abdominal pain. Lesions can involute and regress several weeks after the underlying etiology improves. With pancreatic carcinoma, there is a greater likelihood of persistence, atypical locations of involvement, ulcerations, and recurrences.7
The histologic features of pancreatic panniculitis and the assessment of the subcutaneous fat are paramount in diagnosis. A deep punch biopsy or incisional biopsy is necessary to reliably reach the depth of the subcutaneous tissue. In our patient, a deep punch biopsy from the lateral calf was performed at the suggestion of Dermatology, and histopathology revealed necrosis of fat lobules with calcium soap around necrotic lipocytes, consistent with pancreatic panniculitis (FIGURE 3).

Continue to: Differential was complicated by antibiotic use
Differential was complicated by antibiotic use
The differential diagnosis was broad due to the confounding factors of recent antibiotic use and worsening pancreatitis.
Cellulitis may present as a red patch and is common on the lower legs; it often is associated with skin pathogens including Staphylococcus and Streptococcus. Usually, symptoms are unilateral and associated with warmth to the touch, expanding borders, leukocytosis, and systemic symptoms.
Vasculitis, which is an inflammation of various sized vessels through immunologic or infectious processes, often manifests on the lower legs. The characteristic sign of small vessel vasculitis is nonblanching purpura or petechiae. There often is a preceding illness or medication that triggers immunoglobulin proliferation and off-target inflammation of the vessels. Associated symptoms include pain and pruritus.
Drug eruptions may present as red patches on the skin. Often the patches are scaly and red and have more widespread distribution than the lower legs. A history of exposure is important, but common inciting drugs include nonsteroidal anti-inflammatory drugs that may be used only occasionally and are challenging to elicit in the history. Our patient did have known drug changes (ie, the introduction of meropenem) while hospitalized, but the morphology was not consistent with this diagnosis.
Treatment is directed to underlying disease
Treatment of pancreatic panniculitis primarily is supportive and directed toward treating the underlying pancreatic disease. Depending upon the underlying pancreatic diagnosis, surgical correction of anatomic or ductal anomalies or pseudocysts may lead to resolution of panniculitis.3,7,8
Continue to: In this case
In this case, our patient had already received fluid resuscitation and pain management, and his diet had been advanced. In addition, his antibiotics were changed to exclude drug eruption as a cause. Over the course of a week, our patient saw a reduction in his pain level and an improvement in the appearance of his legs (FIGURE 4).

His pancreatitis, however, continued to persist and resist increases in his diet. He ultimately required transfer to a tertiary care center for consideration of interventional options including stenting. The patient ultimately recovered, after stenting of the main pancreatic duct, and was discharged home.
CORRESPONDENCE
Jonathan Karnes, MD, 6 East Chestnut Street, Augusta, ME 04330; [email protected]
1. Madarasingha NP, Satgurunathan K, Fernando R. Pancreatic panniculitis: a rare form of panniculitis. Dermatol Online J. 2009;15:17.
2. Haber RM, Assaad DM. Panniculitis associated with a pancreas divisum. J Am Acad Dermatol. 1986;14(2 pt 2):331-334.
3. Requena L, Sánchez Yus E. Panniculitis. part II. mostly lobular panniculitis. J Am Acad Dermatol. 2001;45:325-361.
4. Rongioletti F, Caputo V. Pancreatic panniculitis. G Ital Dermatol Venereol. 2013;148:419-425.
5. Förström TL, Winkelmann RK. Acute, generalized panniculitis with amylase and lipase in skin. Arch Dermatol. 1975;111:497-502.
6. Hughes SH, Apisarnthanarax P, Mullins F. Subcutaneous fat necrosis associated with pancreatic disease. Arch Dermatol. 1975;111:506-510.
7. Dahl PR, Su WP, Cullimore KC, et al. Pancreatic panniculitis. J Am Acad Dermatol. 1995;33:413-417.
8. Lambiase P, Seery JP, Taylor-Robinson SD, et al. Resolution of panniculitis after placement of pancreatic duct stent in chro nic pancreatitis. Am J Gastroenterol. 1996;91:1835-1837.
1. Madarasingha NP, Satgurunathan K, Fernando R. Pancreatic panniculitis: a rare form of panniculitis. Dermatol Online J. 2009;15:17.
2. Haber RM, Assaad DM. Panniculitis associated with a pancreas divisum. J Am Acad Dermatol. 1986;14(2 pt 2):331-334.
3. Requena L, Sánchez Yus E. Panniculitis. part II. mostly lobular panniculitis. J Am Acad Dermatol. 2001;45:325-361.
4. Rongioletti F, Caputo V. Pancreatic panniculitis. G Ital Dermatol Venereol. 2013;148:419-425.
5. Förström TL, Winkelmann RK. Acute, generalized panniculitis with amylase and lipase in skin. Arch Dermatol. 1975;111:497-502.
6. Hughes SH, Apisarnthanarax P, Mullins F. Subcutaneous fat necrosis associated with pancreatic disease. Arch Dermatol. 1975;111:506-510.
7. Dahl PR, Su WP, Cullimore KC, et al. Pancreatic panniculitis. J Am Acad Dermatol. 1995;33:413-417.
8. Lambiase P, Seery JP, Taylor-Robinson SD, et al. Resolution of panniculitis after placement of pancreatic duct stent in chro nic pancreatitis. Am J Gastroenterol. 1996;91:1835-1837.
A decade of telemedicine policy has advanced in just 2 weeks
The rapid spread of , which he’d never used.
But as soon as he learned that telehealth regulations had been relaxed by the Centers for Medicare & Medicaid Services and that reimbursement had been broadened, Dr. Desai, a dermatologist in private practice and his staff began to mobilize.
“Kaboom! We made the decision to start doing it,” he said in an interview. “We drafted a consent form, uploaded it to our website, called patients, changed our voice greeting, and got clarity on insurance coverage. We’ve been flying by the seat of our pants.”
“I’m doing it because I don’t have a choice at this point,” said Dr. Desai, who is a member of the American Academy of Dermatology board of directors and its coronavirus task force. “I’m very worried about continuing to be able to meet our payroll expenses for staff and overhead to keep the office open.”
“Flying by the seat of our pants” to see patients virtually
Dermatologists have long been considered pioneers in telemedicine. They have, since the 1990s, capitalized on the visual nature of the specialty to diagnose and treat skin diseases by incorporating photos, videos, and virtual-patient visits. But the pandemic has forced the hands of even holdouts like Dr. Desai, who clung to in-person consults because of confusion related to HIPAA compliance issues and the sense that teledermatology “really dehumanizes patient interaction” for him.
In fact, as of 2017, only 15% of the nation’s 11,000 or so dermatologists had implemented telehealth into their practices, according to an AAD practice survey. In the wake of COVID-19, however, that percentage has likely more than tripled, experts estimate.
Now, dermatologists are assuming the mantle of educators for other specialists who never considered telehealth before in-person visits became fraught with concerns about the spread of the virus. And some are publishing guidelines for colleagues on how to prioritize teledermatology to stem transmission and conserve personal protective equipment (PPE) and hospital beds.
User-friendly technology and the relaxed telehealth restrictions have made it fairly simple for patients and physicians to connect. Facetime and other once-prohibited platforms are all currently permissible, although physicians are encouraged to notify patients about potential privacy risks, according to an AAD teledermatology tool kit.
Teledermatology innovators
“We’ve moved 10 years in telemedicine policy in 2 weeks,” said Karen Edison, MD, of the University of Missouri, Columbia. “The federal government has really loosened the reins.”
At least half of all dermatologists in the United States have adopted telehealth since the pandemic emerged, she estimated. And most, like Dr. Desai, have done so in just the last several weeks.
“You can do about 90% of what you need to do as a dermatologist using the technology,” said Dr. Edison, who launched the first dermatology Extension for Community Healthcare Outcomes, or ECHO, program in the Midwest. That telehealth model was originally developed to connect rural general practitioners with specialists at academic medical centers or large health systems.
“People are used to taking pictures with their phones. In some ways, this crisis may change the face of our specialty,” she said in an interview.
“As we’re all practicing social distancing, I think physicians and patients are rethinking how we can access healthcare without pursuing traditional face-to-face interactions,” said Ivy Lee, MD, from the University of California, San Francisco, who is past chair of the AAD telemedicine task force and current chair of the teledermatology committee at the American Telemedicine Association. “Virtual health and telemedicine fit perfectly with that.”
Even before the pandemic, the innovative ways dermatologists were using telehealth were garnering increasing acclaim. All four clinical groups short-listed for dermatology team of the year at the BMJ Awards 2020 employed telehealth to improve patient services in the United Kingdom.
In the United States, dermatologists are joining forces to boost understanding of how telehealth can protect patients and clinicians from some of the ravages of the virus.
The Society of Dermatology Hospitalists has developed an algorithm – built on experiences its members have had caring for hospitalized patients with acute dermatologic conditions – to provide a “logical way” to triage telemedicine consults in multiple hospital settings during the coronavirus crisis, said President-Elect Daniela Kroshinsky, MD, from Massachusetts General Hospital in Boston.
Telemedicine consultation is prioritized and patients at high risk for COVID-19 exposure are identified so that exposure time and resource use are limited and patient and staff safety are maximized.
“We want to empower our colleagues in community hospitals to play a role in safely providing care for patients in need but to be mindful about preserving resources,” said Dr. Kroshinsky, who reported that the algorithm will be published imminently.
“If you don’t have to see a patient in person and can offer recommendations through telederm, you don’t need to put on a gown, gloves, mask, or goggles,” she said in an interview. “If you’re unable to assess photos, then of course you’ll use the appropriate protective wear, but it will be better if you can obtain the same result” without having to do so.
Sharing expertise
After the first week of tracking data to gauge the effectiveness of the algorithm at Massachusetts General, Dr. Kroshinsky said she is buoyed.
Of the 35 patients assessed electronically – all of whom would previously have been seen in person – only 4 ended up needing a subsequent in-person consult, she reported.
“It’s worked out great,” said Dr. Kroshinsky, who noted that the pandemic is a “nice opportunity” to test different telehealth platforms and improve quality down the line. “We never had to use any excessive PPE, beyond what was routine, and the majority of patients were able to be staffed remotely. All patients had successful outcomes.”
With telehealth more firmly established in dermatology than in most other specialties, dermatologists are now helping clinicians in other fields who are rapidly ramping up their own telemedicine offerings.
These might include obstetrics and gynecology or “any medical specialty where they need to do checkups with their patients and don’t want them coming in for nonemergent visits,” said Carrie L. Kovarik, MD, of the University of Pennsylvania, Philadelphia.
In addition to fielding many recent calls and emails from physicians seeking guidance on telehealth, Dr. Kovarik, Dr. Lee, and colleagues have published the steps required to integrate the technology into outpatient practices.
“Now that there’s a time for broad implementation, our colleagues are looking to us for help and troubleshooting advice,” said Dr. Kovarik, who is also a member of the AAD COVID-19 response task force.
Various specialties “lend themselves to telehealth, depending on how image- or data-dependent they are,” Dr. Lee said in an interview. “But all specialists thinking of limiting or shutting down their practices are thinking about how they can provide continuity of care without exposing patients or staff to the risk of contracting the coronavirus.”
After-COVID goals
In his first week of virtual patient consults, Dr. Desai said he saw about 50 patients, which is still far fewer than the 160-180 he sees in person during a normal week.
“The problem is that patients don’t really want to do telehealth. You’d think it would be a good option,” he said, “but patients hesitate because they don’t really know how to use their device.” Some have instead rescheduled in-person appointments for months down the line.
Although telehealth has enabled Dr. Desai to readily assess patients with acne, hair loss, psoriasis, rashes, warts, and eczema, he’s concerned that necessary procedures, such as biopsies and dermoscopies, could be dangerously delayed. It’s also hard to assess the texture and thickness of certain skin lesions in photos or videos, he said.
“I’m trying to stay optimistic that this will get better and we’re able to move back to taking care of patients the way we need to,” he said.
Like Dr. Desai, other dermatologists who’ve implemented telemedicine during the pandemic have largely been swayed by the relaxed CMS regulations. “It’s made all the difference,” Dr. Kovarik said. “It has brought down the anxiety level and decreased questions about platforms and concentrated them on how to code the visits.”
And although it’s difficult to envision post-COVID medical practice in the thick of the pandemic, dermatologists expect the current strides in telemedicine will stick.
“I’m hoping that telehealth use isn’t dialed back all the way to baseline” after the pandemic eases, Dr. Kovarik said. “The cat’s out of the bag, and now that it is, hopefully it won’t be put back in.”
“If there’s a silver lining to this,” Dr. Kroshinsky said, “I hope it’s that we’ll be able to innovate around health care in a fashion we wouldn’t have seen otherwise.”
A version of this article originally appeared on Medscape.com.
The rapid spread of , which he’d never used.
But as soon as he learned that telehealth regulations had been relaxed by the Centers for Medicare & Medicaid Services and that reimbursement had been broadened, Dr. Desai, a dermatologist in private practice and his staff began to mobilize.
“Kaboom! We made the decision to start doing it,” he said in an interview. “We drafted a consent form, uploaded it to our website, called patients, changed our voice greeting, and got clarity on insurance coverage. We’ve been flying by the seat of our pants.”
“I’m doing it because I don’t have a choice at this point,” said Dr. Desai, who is a member of the American Academy of Dermatology board of directors and its coronavirus task force. “I’m very worried about continuing to be able to meet our payroll expenses for staff and overhead to keep the office open.”
“Flying by the seat of our pants” to see patients virtually
Dermatologists have long been considered pioneers in telemedicine. They have, since the 1990s, capitalized on the visual nature of the specialty to diagnose and treat skin diseases by incorporating photos, videos, and virtual-patient visits. But the pandemic has forced the hands of even holdouts like Dr. Desai, who clung to in-person consults because of confusion related to HIPAA compliance issues and the sense that teledermatology “really dehumanizes patient interaction” for him.
In fact, as of 2017, only 15% of the nation’s 11,000 or so dermatologists had implemented telehealth into their practices, according to an AAD practice survey. In the wake of COVID-19, however, that percentage has likely more than tripled, experts estimate.
Now, dermatologists are assuming the mantle of educators for other specialists who never considered telehealth before in-person visits became fraught with concerns about the spread of the virus. And some are publishing guidelines for colleagues on how to prioritize teledermatology to stem transmission and conserve personal protective equipment (PPE) and hospital beds.
User-friendly technology and the relaxed telehealth restrictions have made it fairly simple for patients and physicians to connect. Facetime and other once-prohibited platforms are all currently permissible, although physicians are encouraged to notify patients about potential privacy risks, according to an AAD teledermatology tool kit.
Teledermatology innovators
“We’ve moved 10 years in telemedicine policy in 2 weeks,” said Karen Edison, MD, of the University of Missouri, Columbia. “The federal government has really loosened the reins.”
At least half of all dermatologists in the United States have adopted telehealth since the pandemic emerged, she estimated. And most, like Dr. Desai, have done so in just the last several weeks.
“You can do about 90% of what you need to do as a dermatologist using the technology,” said Dr. Edison, who launched the first dermatology Extension for Community Healthcare Outcomes, or ECHO, program in the Midwest. That telehealth model was originally developed to connect rural general practitioners with specialists at academic medical centers or large health systems.
“People are used to taking pictures with their phones. In some ways, this crisis may change the face of our specialty,” she said in an interview.
“As we’re all practicing social distancing, I think physicians and patients are rethinking how we can access healthcare without pursuing traditional face-to-face interactions,” said Ivy Lee, MD, from the University of California, San Francisco, who is past chair of the AAD telemedicine task force and current chair of the teledermatology committee at the American Telemedicine Association. “Virtual health and telemedicine fit perfectly with that.”
Even before the pandemic, the innovative ways dermatologists were using telehealth were garnering increasing acclaim. All four clinical groups short-listed for dermatology team of the year at the BMJ Awards 2020 employed telehealth to improve patient services in the United Kingdom.
In the United States, dermatologists are joining forces to boost understanding of how telehealth can protect patients and clinicians from some of the ravages of the virus.
The Society of Dermatology Hospitalists has developed an algorithm – built on experiences its members have had caring for hospitalized patients with acute dermatologic conditions – to provide a “logical way” to triage telemedicine consults in multiple hospital settings during the coronavirus crisis, said President-Elect Daniela Kroshinsky, MD, from Massachusetts General Hospital in Boston.
Telemedicine consultation is prioritized and patients at high risk for COVID-19 exposure are identified so that exposure time and resource use are limited and patient and staff safety are maximized.
“We want to empower our colleagues in community hospitals to play a role in safely providing care for patients in need but to be mindful about preserving resources,” said Dr. Kroshinsky, who reported that the algorithm will be published imminently.
“If you don’t have to see a patient in person and can offer recommendations through telederm, you don’t need to put on a gown, gloves, mask, or goggles,” she said in an interview. “If you’re unable to assess photos, then of course you’ll use the appropriate protective wear, but it will be better if you can obtain the same result” without having to do so.
Sharing expertise
After the first week of tracking data to gauge the effectiveness of the algorithm at Massachusetts General, Dr. Kroshinsky said she is buoyed.
Of the 35 patients assessed electronically – all of whom would previously have been seen in person – only 4 ended up needing a subsequent in-person consult, she reported.
“It’s worked out great,” said Dr. Kroshinsky, who noted that the pandemic is a “nice opportunity” to test different telehealth platforms and improve quality down the line. “We never had to use any excessive PPE, beyond what was routine, and the majority of patients were able to be staffed remotely. All patients had successful outcomes.”
With telehealth more firmly established in dermatology than in most other specialties, dermatologists are now helping clinicians in other fields who are rapidly ramping up their own telemedicine offerings.
These might include obstetrics and gynecology or “any medical specialty where they need to do checkups with their patients and don’t want them coming in for nonemergent visits,” said Carrie L. Kovarik, MD, of the University of Pennsylvania, Philadelphia.
In addition to fielding many recent calls and emails from physicians seeking guidance on telehealth, Dr. Kovarik, Dr. Lee, and colleagues have published the steps required to integrate the technology into outpatient practices.
“Now that there’s a time for broad implementation, our colleagues are looking to us for help and troubleshooting advice,” said Dr. Kovarik, who is also a member of the AAD COVID-19 response task force.
Various specialties “lend themselves to telehealth, depending on how image- or data-dependent they are,” Dr. Lee said in an interview. “But all specialists thinking of limiting or shutting down their practices are thinking about how they can provide continuity of care without exposing patients or staff to the risk of contracting the coronavirus.”
After-COVID goals
In his first week of virtual patient consults, Dr. Desai said he saw about 50 patients, which is still far fewer than the 160-180 he sees in person during a normal week.
“The problem is that patients don’t really want to do telehealth. You’d think it would be a good option,” he said, “but patients hesitate because they don’t really know how to use their device.” Some have instead rescheduled in-person appointments for months down the line.
Although telehealth has enabled Dr. Desai to readily assess patients with acne, hair loss, psoriasis, rashes, warts, and eczema, he’s concerned that necessary procedures, such as biopsies and dermoscopies, could be dangerously delayed. It’s also hard to assess the texture and thickness of certain skin lesions in photos or videos, he said.
“I’m trying to stay optimistic that this will get better and we’re able to move back to taking care of patients the way we need to,” he said.
Like Dr. Desai, other dermatologists who’ve implemented telemedicine during the pandemic have largely been swayed by the relaxed CMS regulations. “It’s made all the difference,” Dr. Kovarik said. “It has brought down the anxiety level and decreased questions about platforms and concentrated them on how to code the visits.”
And although it’s difficult to envision post-COVID medical practice in the thick of the pandemic, dermatologists expect the current strides in telemedicine will stick.
“I’m hoping that telehealth use isn’t dialed back all the way to baseline” after the pandemic eases, Dr. Kovarik said. “The cat’s out of the bag, and now that it is, hopefully it won’t be put back in.”
“If there’s a silver lining to this,” Dr. Kroshinsky said, “I hope it’s that we’ll be able to innovate around health care in a fashion we wouldn’t have seen otherwise.”
A version of this article originally appeared on Medscape.com.
The rapid spread of , which he’d never used.
But as soon as he learned that telehealth regulations had been relaxed by the Centers for Medicare & Medicaid Services and that reimbursement had been broadened, Dr. Desai, a dermatologist in private practice and his staff began to mobilize.
“Kaboom! We made the decision to start doing it,” he said in an interview. “We drafted a consent form, uploaded it to our website, called patients, changed our voice greeting, and got clarity on insurance coverage. We’ve been flying by the seat of our pants.”
“I’m doing it because I don’t have a choice at this point,” said Dr. Desai, who is a member of the American Academy of Dermatology board of directors and its coronavirus task force. “I’m very worried about continuing to be able to meet our payroll expenses for staff and overhead to keep the office open.”
“Flying by the seat of our pants” to see patients virtually
Dermatologists have long been considered pioneers in telemedicine. They have, since the 1990s, capitalized on the visual nature of the specialty to diagnose and treat skin diseases by incorporating photos, videos, and virtual-patient visits. But the pandemic has forced the hands of even holdouts like Dr. Desai, who clung to in-person consults because of confusion related to HIPAA compliance issues and the sense that teledermatology “really dehumanizes patient interaction” for him.
In fact, as of 2017, only 15% of the nation’s 11,000 or so dermatologists had implemented telehealth into their practices, according to an AAD practice survey. In the wake of COVID-19, however, that percentage has likely more than tripled, experts estimate.
Now, dermatologists are assuming the mantle of educators for other specialists who never considered telehealth before in-person visits became fraught with concerns about the spread of the virus. And some are publishing guidelines for colleagues on how to prioritize teledermatology to stem transmission and conserve personal protective equipment (PPE) and hospital beds.
User-friendly technology and the relaxed telehealth restrictions have made it fairly simple for patients and physicians to connect. Facetime and other once-prohibited platforms are all currently permissible, although physicians are encouraged to notify patients about potential privacy risks, according to an AAD teledermatology tool kit.
Teledermatology innovators
“We’ve moved 10 years in telemedicine policy in 2 weeks,” said Karen Edison, MD, of the University of Missouri, Columbia. “The federal government has really loosened the reins.”
At least half of all dermatologists in the United States have adopted telehealth since the pandemic emerged, she estimated. And most, like Dr. Desai, have done so in just the last several weeks.
“You can do about 90% of what you need to do as a dermatologist using the technology,” said Dr. Edison, who launched the first dermatology Extension for Community Healthcare Outcomes, or ECHO, program in the Midwest. That telehealth model was originally developed to connect rural general practitioners with specialists at academic medical centers or large health systems.
“People are used to taking pictures with their phones. In some ways, this crisis may change the face of our specialty,” she said in an interview.
“As we’re all practicing social distancing, I think physicians and patients are rethinking how we can access healthcare without pursuing traditional face-to-face interactions,” said Ivy Lee, MD, from the University of California, San Francisco, who is past chair of the AAD telemedicine task force and current chair of the teledermatology committee at the American Telemedicine Association. “Virtual health and telemedicine fit perfectly with that.”
Even before the pandemic, the innovative ways dermatologists were using telehealth were garnering increasing acclaim. All four clinical groups short-listed for dermatology team of the year at the BMJ Awards 2020 employed telehealth to improve patient services in the United Kingdom.
In the United States, dermatologists are joining forces to boost understanding of how telehealth can protect patients and clinicians from some of the ravages of the virus.
The Society of Dermatology Hospitalists has developed an algorithm – built on experiences its members have had caring for hospitalized patients with acute dermatologic conditions – to provide a “logical way” to triage telemedicine consults in multiple hospital settings during the coronavirus crisis, said President-Elect Daniela Kroshinsky, MD, from Massachusetts General Hospital in Boston.
Telemedicine consultation is prioritized and patients at high risk for COVID-19 exposure are identified so that exposure time and resource use are limited and patient and staff safety are maximized.
“We want to empower our colleagues in community hospitals to play a role in safely providing care for patients in need but to be mindful about preserving resources,” said Dr. Kroshinsky, who reported that the algorithm will be published imminently.
“If you don’t have to see a patient in person and can offer recommendations through telederm, you don’t need to put on a gown, gloves, mask, or goggles,” she said in an interview. “If you’re unable to assess photos, then of course you’ll use the appropriate protective wear, but it will be better if you can obtain the same result” without having to do so.
Sharing expertise
After the first week of tracking data to gauge the effectiveness of the algorithm at Massachusetts General, Dr. Kroshinsky said she is buoyed.
Of the 35 patients assessed electronically – all of whom would previously have been seen in person – only 4 ended up needing a subsequent in-person consult, she reported.
“It’s worked out great,” said Dr. Kroshinsky, who noted that the pandemic is a “nice opportunity” to test different telehealth platforms and improve quality down the line. “We never had to use any excessive PPE, beyond what was routine, and the majority of patients were able to be staffed remotely. All patients had successful outcomes.”
With telehealth more firmly established in dermatology than in most other specialties, dermatologists are now helping clinicians in other fields who are rapidly ramping up their own telemedicine offerings.
These might include obstetrics and gynecology or “any medical specialty where they need to do checkups with their patients and don’t want them coming in for nonemergent visits,” said Carrie L. Kovarik, MD, of the University of Pennsylvania, Philadelphia.
In addition to fielding many recent calls and emails from physicians seeking guidance on telehealth, Dr. Kovarik, Dr. Lee, and colleagues have published the steps required to integrate the technology into outpatient practices.
“Now that there’s a time for broad implementation, our colleagues are looking to us for help and troubleshooting advice,” said Dr. Kovarik, who is also a member of the AAD COVID-19 response task force.
Various specialties “lend themselves to telehealth, depending on how image- or data-dependent they are,” Dr. Lee said in an interview. “But all specialists thinking of limiting or shutting down their practices are thinking about how they can provide continuity of care without exposing patients or staff to the risk of contracting the coronavirus.”
After-COVID goals
In his first week of virtual patient consults, Dr. Desai said he saw about 50 patients, which is still far fewer than the 160-180 he sees in person during a normal week.
“The problem is that patients don’t really want to do telehealth. You’d think it would be a good option,” he said, “but patients hesitate because they don’t really know how to use their device.” Some have instead rescheduled in-person appointments for months down the line.
Although telehealth has enabled Dr. Desai to readily assess patients with acne, hair loss, psoriasis, rashes, warts, and eczema, he’s concerned that necessary procedures, such as biopsies and dermoscopies, could be dangerously delayed. It’s also hard to assess the texture and thickness of certain skin lesions in photos or videos, he said.
“I’m trying to stay optimistic that this will get better and we’re able to move back to taking care of patients the way we need to,” he said.
Like Dr. Desai, other dermatologists who’ve implemented telemedicine during the pandemic have largely been swayed by the relaxed CMS regulations. “It’s made all the difference,” Dr. Kovarik said. “It has brought down the anxiety level and decreased questions about platforms and concentrated them on how to code the visits.”
And although it’s difficult to envision post-COVID medical practice in the thick of the pandemic, dermatologists expect the current strides in telemedicine will stick.
“I’m hoping that telehealth use isn’t dialed back all the way to baseline” after the pandemic eases, Dr. Kovarik said. “The cat’s out of the bag, and now that it is, hopefully it won’t be put back in.”
“If there’s a silver lining to this,” Dr. Kroshinsky said, “I hope it’s that we’ll be able to innovate around health care in a fashion we wouldn’t have seen otherwise.”
A version of this article originally appeared on Medscape.com.
Novel acne drug now under review at the FDA
LAHAINA, HAWAII – by the Food and Drug Administration, is already generating considerable buzz in the patient-advocacy community even though the agency won’t issue its decision until August.
“I’ve actually had a lot of interest in this already from parents, especially regarding girls who have very hormonal acne but the parents are really not interested in starting them on a systemic hormonal therapy at their age,” Jessica Sprague, MD, said at the SDEF Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Clascoterone targets androgen receptors in the skin in order to reduce cutaneous 5-alpha dihydrotestosterone.
“It’s being developed for use in both males and females, which is great because at this point there’s no hormonal treatment for males,” noted Dr. Sprague, a pediatric dermatologist at Rady Children’s Hospital and the University of California, both in San Diego.
The manufacturer’s application for marketing approval of clascoterone cream 1% under FDA review includes evidence from two identical phase-3, double-blind, vehicle-controlled, 12-week, randomized trials. The two studies included a total of 1,440 patients aged 9 years through adulthood with moderate to severe facial acne vulgaris who were randomized to twice-daily application of clascoterone or its vehicle.
The primary outcome was the reduction in inflammatory lesions at week 12: a 46.2% decline from baseline with clascoterone 1% cream, which was a significantly greater improvement than the 32.7% reduction for vehicle. The secondary outcome – change in noninflammatory lesion counts at week 12 – was also positive for the topical androgen receptor inhibitor, which achieved a 29.8% reduction, compared with 18.9% for vehicle. Clascoterone exhibited a favorable safety and tolerability profile, with numerically fewer treatment-emergent adverse events than in the vehicle control group. A stronger formulation of the topical agent is in advanced clinical trials for the treatment of androgenetic alopecia in both males and females.
Dr. Sprague reported having no financial conflicts regarding her presentation.
The SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – by the Food and Drug Administration, is already generating considerable buzz in the patient-advocacy community even though the agency won’t issue its decision until August.
“I’ve actually had a lot of interest in this already from parents, especially regarding girls who have very hormonal acne but the parents are really not interested in starting them on a systemic hormonal therapy at their age,” Jessica Sprague, MD, said at the SDEF Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Clascoterone targets androgen receptors in the skin in order to reduce cutaneous 5-alpha dihydrotestosterone.
“It’s being developed for use in both males and females, which is great because at this point there’s no hormonal treatment for males,” noted Dr. Sprague, a pediatric dermatologist at Rady Children’s Hospital and the University of California, both in San Diego.
The manufacturer’s application for marketing approval of clascoterone cream 1% under FDA review includes evidence from two identical phase-3, double-blind, vehicle-controlled, 12-week, randomized trials. The two studies included a total of 1,440 patients aged 9 years through adulthood with moderate to severe facial acne vulgaris who were randomized to twice-daily application of clascoterone or its vehicle.
The primary outcome was the reduction in inflammatory lesions at week 12: a 46.2% decline from baseline with clascoterone 1% cream, which was a significantly greater improvement than the 32.7% reduction for vehicle. The secondary outcome – change in noninflammatory lesion counts at week 12 – was also positive for the topical androgen receptor inhibitor, which achieved a 29.8% reduction, compared with 18.9% for vehicle. Clascoterone exhibited a favorable safety and tolerability profile, with numerically fewer treatment-emergent adverse events than in the vehicle control group. A stronger formulation of the topical agent is in advanced clinical trials for the treatment of androgenetic alopecia in both males and females.
Dr. Sprague reported having no financial conflicts regarding her presentation.
The SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – by the Food and Drug Administration, is already generating considerable buzz in the patient-advocacy community even though the agency won’t issue its decision until August.
“I’ve actually had a lot of interest in this already from parents, especially regarding girls who have very hormonal acne but the parents are really not interested in starting them on a systemic hormonal therapy at their age,” Jessica Sprague, MD, said at the SDEF Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Clascoterone targets androgen receptors in the skin in order to reduce cutaneous 5-alpha dihydrotestosterone.
“It’s being developed for use in both males and females, which is great because at this point there’s no hormonal treatment for males,” noted Dr. Sprague, a pediatric dermatologist at Rady Children’s Hospital and the University of California, both in San Diego.
The manufacturer’s application for marketing approval of clascoterone cream 1% under FDA review includes evidence from two identical phase-3, double-blind, vehicle-controlled, 12-week, randomized trials. The two studies included a total of 1,440 patients aged 9 years through adulthood with moderate to severe facial acne vulgaris who were randomized to twice-daily application of clascoterone or its vehicle.
The primary outcome was the reduction in inflammatory lesions at week 12: a 46.2% decline from baseline with clascoterone 1% cream, which was a significantly greater improvement than the 32.7% reduction for vehicle. The secondary outcome – change in noninflammatory lesion counts at week 12 – was also positive for the topical androgen receptor inhibitor, which achieved a 29.8% reduction, compared with 18.9% for vehicle. Clascoterone exhibited a favorable safety and tolerability profile, with numerically fewer treatment-emergent adverse events than in the vehicle control group. A stronger formulation of the topical agent is in advanced clinical trials for the treatment of androgenetic alopecia in both males and females.
Dr. Sprague reported having no financial conflicts regarding her presentation.
The SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM THE SDEF HAWAII DERMATOLOGY SEMINAR
iPLEDGE allows at-home pregnancy tests during pandemic
tests to comply with the requirements of the iPLEDGE program during the COVID-19 pandemic, according to an update program posted on the iPLEDGE website.
The program’s other requirements – the prescription window and two forms of birth control – remain unchanged.
The change follows recent guidance from the Department of Health & Human Services and the Food and Drug Administration regarding accommodations for medical care and drugs subject to Risk Evaluation and Mitigation Strategies (REMS) in the midst of a public health emergency that requires most people to remain in their homes except for essential services.
Allowing females to take at-home pregnancy tests and communicate the results to physician according to their preference is “a game changer for the middle of a pandemic, obviously,” Neil Goldberg, MD, a dermatologist in Westchester County, New York, said in an interview. “These are patients who don’t need to spend time outside just to get pregnancy tests done. It makes it a lot easier.”
Dr. Goldberg is frustrated, however, that the accommodations have not been more widely publicized; he discovered the change incidentally when speaking to an iPLEDGE program representative to request a waiver for a patient who had taken her pregnancy test too early. The program had denied a similar request for a 15-year-old patient of his the previous week, despite the patient being abstinent and having been in shelter-in-place for several weeks.
“The size of your notice [on the website] should be proportionate to how important it is,” Dr. Goldberg said, and the small red box on the site is easy to miss. By contrast, asking anyone to leave their homes to go to a lab for a pregnancy test in the midst of a global pandemic so they can continue their medication would be putting patients at risk, he added.
The iPLEDGE program is designed in part to ensure unplanned pregnancies do not occur in females while taking the teratogenic acne drug. But the rules are onerous and difficult even during normal times, pointed out Hilary Baldwin, MD, medical director of the Acne Treatment and Research Center in New York City and past president of the American Acne and Rosacea Society.
Male patients taking isotretinoin must visit their physician every month to get a new no-refills prescription, but females must get a pregnancy test at a Clinical Laboratory Improvement Amendments–certified lab, which must then provide physical results to the prescribing physician. The doctor enters the negative pregnancy test and the two forms of birth control the patient is taking in the iPLEDGE program site.
Then the patient must take an online test at home to acknowledge they understand what it means to not get pregnant and enter the two forms of birth control they are using – which must match what the doctor enters – before the pharmacy can dispense the drug. The entire process must occur within 7 days or else the patient has to wait 19 days before starting the process over.
“We run a very tight schedule for girls. And every month, we would worry that something would interfere, a snow storm or something else, and that they wouldn’t be able to complete their objectives within the 7-day period,” Dr Baldwin said in an interview. “It was always difficult, and now with us not being able to see the patient and the patient not wanting to go to the lab, this became completely impossible.”
Until this change, some patients may not have been able to get their prescription for severe nodulocystic acne, which can cause physical and psychological scarring, and “postponing treatment increases the likelihood of scarring,” Dr. Baldwin pointed out.

Dr. Goldberg’s patients now take a pregnancy test at home and send him a photo of the negative test that he then inserts into their EMR.
According to a March 17 statement from HHS, potential penalties for HIPAA violations are waived for good-faith use of “everyday communication technologies,” such as Skype or FaceTime, for telehealth treatment or diagnostics. The change was intended to allow telehealth services to continue healthcare for practices that had not previously had secure telehealth technology established.
Despite the changes for at-home pregnancy tests for females and in-person visits for all patients, the program has not altered the 7-day prescription window or the requirement to have two forms of birth control.
With reports of a global condom shortage, Dr Baldwin said she has more concerns about her adult patients being able to find a required barrier method of birth control than about her adolescent patients.
“This is a unique opportunity for us to trust our teenage patients because they can’t leave the house,” Dr. Baldwin said. “I’m actually more worried about my adult women on the drug who are bored and cooped up in a house with their significant other.”
Dr. Baldwin and Dr. Goldberg had no relevant disclosures. Dr. Goldberg is a Dermatology News board member.
tests to comply with the requirements of the iPLEDGE program during the COVID-19 pandemic, according to an update program posted on the iPLEDGE website.
The program’s other requirements – the prescription window and two forms of birth control – remain unchanged.
The change follows recent guidance from the Department of Health & Human Services and the Food and Drug Administration regarding accommodations for medical care and drugs subject to Risk Evaluation and Mitigation Strategies (REMS) in the midst of a public health emergency that requires most people to remain in their homes except for essential services.
Allowing females to take at-home pregnancy tests and communicate the results to physician according to their preference is “a game changer for the middle of a pandemic, obviously,” Neil Goldberg, MD, a dermatologist in Westchester County, New York, said in an interview. “These are patients who don’t need to spend time outside just to get pregnancy tests done. It makes it a lot easier.”
Dr. Goldberg is frustrated, however, that the accommodations have not been more widely publicized; he discovered the change incidentally when speaking to an iPLEDGE program representative to request a waiver for a patient who had taken her pregnancy test too early. The program had denied a similar request for a 15-year-old patient of his the previous week, despite the patient being abstinent and having been in shelter-in-place for several weeks.
“The size of your notice [on the website] should be proportionate to how important it is,” Dr. Goldberg said, and the small red box on the site is easy to miss. By contrast, asking anyone to leave their homes to go to a lab for a pregnancy test in the midst of a global pandemic so they can continue their medication would be putting patients at risk, he added.
The iPLEDGE program is designed in part to ensure unplanned pregnancies do not occur in females while taking the teratogenic acne drug. But the rules are onerous and difficult even during normal times, pointed out Hilary Baldwin, MD, medical director of the Acne Treatment and Research Center in New York City and past president of the American Acne and Rosacea Society.
Male patients taking isotretinoin must visit their physician every month to get a new no-refills prescription, but females must get a pregnancy test at a Clinical Laboratory Improvement Amendments–certified lab, which must then provide physical results to the prescribing physician. The doctor enters the negative pregnancy test and the two forms of birth control the patient is taking in the iPLEDGE program site.
Then the patient must take an online test at home to acknowledge they understand what it means to not get pregnant and enter the two forms of birth control they are using – which must match what the doctor enters – before the pharmacy can dispense the drug. The entire process must occur within 7 days or else the patient has to wait 19 days before starting the process over.
“We run a very tight schedule for girls. And every month, we would worry that something would interfere, a snow storm or something else, and that they wouldn’t be able to complete their objectives within the 7-day period,” Dr Baldwin said in an interview. “It was always difficult, and now with us not being able to see the patient and the patient not wanting to go to the lab, this became completely impossible.”
Until this change, some patients may not have been able to get their prescription for severe nodulocystic acne, which can cause physical and psychological scarring, and “postponing treatment increases the likelihood of scarring,” Dr. Baldwin pointed out.

Dr. Goldberg’s patients now take a pregnancy test at home and send him a photo of the negative test that he then inserts into their EMR.
According to a March 17 statement from HHS, potential penalties for HIPAA violations are waived for good-faith use of “everyday communication technologies,” such as Skype or FaceTime, for telehealth treatment or diagnostics. The change was intended to allow telehealth services to continue healthcare for practices that had not previously had secure telehealth technology established.
Despite the changes for at-home pregnancy tests for females and in-person visits for all patients, the program has not altered the 7-day prescription window or the requirement to have two forms of birth control.
With reports of a global condom shortage, Dr Baldwin said she has more concerns about her adult patients being able to find a required barrier method of birth control than about her adolescent patients.
“This is a unique opportunity for us to trust our teenage patients because they can’t leave the house,” Dr. Baldwin said. “I’m actually more worried about my adult women on the drug who are bored and cooped up in a house with their significant other.”
Dr. Baldwin and Dr. Goldberg had no relevant disclosures. Dr. Goldberg is a Dermatology News board member.
tests to comply with the requirements of the iPLEDGE program during the COVID-19 pandemic, according to an update program posted on the iPLEDGE website.
The program’s other requirements – the prescription window and two forms of birth control – remain unchanged.
The change follows recent guidance from the Department of Health & Human Services and the Food and Drug Administration regarding accommodations for medical care and drugs subject to Risk Evaluation and Mitigation Strategies (REMS) in the midst of a public health emergency that requires most people to remain in their homes except for essential services.
Allowing females to take at-home pregnancy tests and communicate the results to physician according to their preference is “a game changer for the middle of a pandemic, obviously,” Neil Goldberg, MD, a dermatologist in Westchester County, New York, said in an interview. “These are patients who don’t need to spend time outside just to get pregnancy tests done. It makes it a lot easier.”
Dr. Goldberg is frustrated, however, that the accommodations have not been more widely publicized; he discovered the change incidentally when speaking to an iPLEDGE program representative to request a waiver for a patient who had taken her pregnancy test too early. The program had denied a similar request for a 15-year-old patient of his the previous week, despite the patient being abstinent and having been in shelter-in-place for several weeks.
“The size of your notice [on the website] should be proportionate to how important it is,” Dr. Goldberg said, and the small red box on the site is easy to miss. By contrast, asking anyone to leave their homes to go to a lab for a pregnancy test in the midst of a global pandemic so they can continue their medication would be putting patients at risk, he added.
The iPLEDGE program is designed in part to ensure unplanned pregnancies do not occur in females while taking the teratogenic acne drug. But the rules are onerous and difficult even during normal times, pointed out Hilary Baldwin, MD, medical director of the Acne Treatment and Research Center in New York City and past president of the American Acne and Rosacea Society.
Male patients taking isotretinoin must visit their physician every month to get a new no-refills prescription, but females must get a pregnancy test at a Clinical Laboratory Improvement Amendments–certified lab, which must then provide physical results to the prescribing physician. The doctor enters the negative pregnancy test and the two forms of birth control the patient is taking in the iPLEDGE program site.
Then the patient must take an online test at home to acknowledge they understand what it means to not get pregnant and enter the two forms of birth control they are using – which must match what the doctor enters – before the pharmacy can dispense the drug. The entire process must occur within 7 days or else the patient has to wait 19 days before starting the process over.
“We run a very tight schedule for girls. And every month, we would worry that something would interfere, a snow storm or something else, and that they wouldn’t be able to complete their objectives within the 7-day period,” Dr Baldwin said in an interview. “It was always difficult, and now with us not being able to see the patient and the patient not wanting to go to the lab, this became completely impossible.”
Until this change, some patients may not have been able to get their prescription for severe nodulocystic acne, which can cause physical and psychological scarring, and “postponing treatment increases the likelihood of scarring,” Dr. Baldwin pointed out.

Dr. Goldberg’s patients now take a pregnancy test at home and send him a photo of the negative test that he then inserts into their EMR.
According to a March 17 statement from HHS, potential penalties for HIPAA violations are waived for good-faith use of “everyday communication technologies,” such as Skype or FaceTime, for telehealth treatment or diagnostics. The change was intended to allow telehealth services to continue healthcare for practices that had not previously had secure telehealth technology established.
Despite the changes for at-home pregnancy tests for females and in-person visits for all patients, the program has not altered the 7-day prescription window or the requirement to have two forms of birth control.
With reports of a global condom shortage, Dr Baldwin said she has more concerns about her adult patients being able to find a required barrier method of birth control than about her adolescent patients.
“This is a unique opportunity for us to trust our teenage patients because they can’t leave the house,” Dr. Baldwin said. “I’m actually more worried about my adult women on the drug who are bored and cooped up in a house with their significant other.”
Dr. Baldwin and Dr. Goldberg had no relevant disclosures. Dr. Goldberg is a Dermatology News board member.
Routinely screen for depression in atopic dermatitis
Jonathan I. Silverberg, MD, PhD, declared in a video presentation during a virtual meeting held by the George Washington University department of dermatology.
The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic.
Dr. Silverberg presented highlights of his recent study of depression screening rates in the National Ambulatory Medical Care Survey, an annual population-based survey by the National Center for Health Statistics. He and his coinvestigator analyzed 9,345 office visits for atopic dermatitis (AD) and 2,085 for psoriasis (Br J Dermatol. 2019 Oct 24. doi: 10.1111/bjd.18629.). The picture that emerged showed that there is much room for improvement.
“We found that depression screening rates were abysmally low in atopic dermatitis patients, with less than 2% patients being screened. There was very little difference in screening rates between patients on an advanced therapy, like systemic phototherapy or a biologic, compared to those who were just on topical therapy alone, meaning even the more severe patients aren’t being asked these questions. And no difference between dermatologists and primary care physicians,” said Dr. Silverberg, director of clinical research and contact dermatitis in the department of dermatology at George Washington University, Washington.
For Dr. Silverberg, known for his pioneering work documenting the marked yet often-underappreciated negative impact of AD on quality of life and mental health, these rock-bottom screening rates were particularly galling.
“There are very high rates of anxiety and depression amongst our patients with atopic dermatitis,” the dermatologist emphasized. “Mental health symptoms are an incredibly important domain in atopic dermatitis that we need to ask our patients about. We don’t ask enough.
“This to me is actually a very important symptom to measure. It’s not just a theoretical construct involved in understanding the burden of the disease, it’s something that’s actionable because most of these cases of mental health symptoms are reversible or modifiable with improved control of the atopic dermatitis,” he continued. “I use this as an indication to step up therapy. If a patient is clinically depressed and we believe that’s secondary to their chronic atopic dermatitis, this is a reason to step up therapy to something stronger.”
If the depressive symptoms don’t improve after stepping up the intensity of the dermatologic therapy, it’s probably time for the patient to see a mental health professional, Dr. Silverberg advised, adding, “I’m not telling every dermatology resident out there to become a psychiatrist.”
Depression and anxiety in AD: How common?
In an analysis of multiyear data from the Medical Expenditure Panel Surveys, an annual population-based project conducted by the Agency for Healthcare Research and Quality, Dr. Silverberg and a coinvestigator found that adults with AD were an adjusted 186% more likely than those without AD to screen positive for depressive symptoms on the two-item Patient Health Questionnaire (PHQ-2), with rates of 44.3% and 21.9%, respectively. The AD patients were also 500% more likely to screen positive for severe psychological distress, with a 25.9% rate of having a Kessler-6 index score of 13 or more, compared with 5.5% in adults without AD.
The rate of severe psychological distress was higher in adults with AD than in those with asthma, diabetes, hypertension, urticaria, or psoriasis, and was comparable with the rate in individuals with autoimmune disease (Ann Allergy Asthma Immunol. 2019 Aug;123[2]:179-85).
“It’s surprising when you think that the majority of the cases of atopic dermatitis in the population are mild and yet when you look at a population-based sample such as this you see a strong signal come up. It means that, with all the dilution of mild disease, the signal is still there. It emphasizes that even patients with mild disease get these depressive symptoms and psychosocial distress,” Dr. Silverberg observed.
In a separate analysis of the same national database, this time looking at Short Form-6D health utility scores – a measure of overall quality of life encompassing key domains including vitality, physical function, mental health, fatigue – adults with AD scored markedly worse than individuals with no chronic health disorders. Health utility scores were particularly low in adults with AD and comorbid symptoms of anxiety or depression, suggesting that those affective symptoms are major drivers of the demonstrably poor quality of life in adult AD (Ann Allergy Asthma Immunol. 2020 Jan;124[1]:88-9).
In the Atopic Dermatitis in America Study, Dr. Silverberg and coinvestigators cross-sectionally surveyed 2,893 adults using the seven-item Hospital Anxiety and Depression Scale anxiety (HADS-A) and depression (HADS-D) assessment instruments. Individuals with AD as determined using the modified U.K. Diagnostic Criteria had dramatically higher rates of both depression and anxiety. For example, the prevalence of a HADS-A score of 11 or more, which is considered to be case finding for clinically important anxiety, was 28.6% in adults with AD, nearly twice the 15.5% prevalence in those without the dermatologic disease. A HADS-D score of 11 or greater was present in 13.5% of subjects with AD and 9% of those without.
HADS-A and -D scores were higher in adults with moderate AD, compared with mild disease, and higher still in those with severe AD. Indeed, virtually all individuals with moderate to severe AD had symptoms of anxiety and depression, which in a large proportion had gone undiagnosed. A multivariate analysis strongly suggested that AD severity was the major driver of anxiety and depression in adults with AD (Br J Dermatol. 2019 Sep;181[3]:554-65).
An important finding was that 100% of adults with AD who had scores in the severe range on three validated measures of itch, frequency of symptoms, and lesion severity had borderline or abnormal scores on the HADS-A and -D.
“Of course, if you don’t ask, you’re not going to know about it,” Dr. Silverberg noted.
Dr. Silverberg reported receiving research grants from Galderma and GlaxoSmithKline and serving as a consultant to those pharmaceutical companies and more than a dozen others.
Jonathan I. Silverberg, MD, PhD, declared in a video presentation during a virtual meeting held by the George Washington University department of dermatology.
The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic.
Dr. Silverberg presented highlights of his recent study of depression screening rates in the National Ambulatory Medical Care Survey, an annual population-based survey by the National Center for Health Statistics. He and his coinvestigator analyzed 9,345 office visits for atopic dermatitis (AD) and 2,085 for psoriasis (Br J Dermatol. 2019 Oct 24. doi: 10.1111/bjd.18629.). The picture that emerged showed that there is much room for improvement.
“We found that depression screening rates were abysmally low in atopic dermatitis patients, with less than 2% patients being screened. There was very little difference in screening rates between patients on an advanced therapy, like systemic phototherapy or a biologic, compared to those who were just on topical therapy alone, meaning even the more severe patients aren’t being asked these questions. And no difference between dermatologists and primary care physicians,” said Dr. Silverberg, director of clinical research and contact dermatitis in the department of dermatology at George Washington University, Washington.
For Dr. Silverberg, known for his pioneering work documenting the marked yet often-underappreciated negative impact of AD on quality of life and mental health, these rock-bottom screening rates were particularly galling.
“There are very high rates of anxiety and depression amongst our patients with atopic dermatitis,” the dermatologist emphasized. “Mental health symptoms are an incredibly important domain in atopic dermatitis that we need to ask our patients about. We don’t ask enough.
“This to me is actually a very important symptom to measure. It’s not just a theoretical construct involved in understanding the burden of the disease, it’s something that’s actionable because most of these cases of mental health symptoms are reversible or modifiable with improved control of the atopic dermatitis,” he continued. “I use this as an indication to step up therapy. If a patient is clinically depressed and we believe that’s secondary to their chronic atopic dermatitis, this is a reason to step up therapy to something stronger.”
If the depressive symptoms don’t improve after stepping up the intensity of the dermatologic therapy, it’s probably time for the patient to see a mental health professional, Dr. Silverberg advised, adding, “I’m not telling every dermatology resident out there to become a psychiatrist.”
Depression and anxiety in AD: How common?
In an analysis of multiyear data from the Medical Expenditure Panel Surveys, an annual population-based project conducted by the Agency for Healthcare Research and Quality, Dr. Silverberg and a coinvestigator found that adults with AD were an adjusted 186% more likely than those without AD to screen positive for depressive symptoms on the two-item Patient Health Questionnaire (PHQ-2), with rates of 44.3% and 21.9%, respectively. The AD patients were also 500% more likely to screen positive for severe psychological distress, with a 25.9% rate of having a Kessler-6 index score of 13 or more, compared with 5.5% in adults without AD.
The rate of severe psychological distress was higher in adults with AD than in those with asthma, diabetes, hypertension, urticaria, or psoriasis, and was comparable with the rate in individuals with autoimmune disease (Ann Allergy Asthma Immunol. 2019 Aug;123[2]:179-85).
“It’s surprising when you think that the majority of the cases of atopic dermatitis in the population are mild and yet when you look at a population-based sample such as this you see a strong signal come up. It means that, with all the dilution of mild disease, the signal is still there. It emphasizes that even patients with mild disease get these depressive symptoms and psychosocial distress,” Dr. Silverberg observed.
In a separate analysis of the same national database, this time looking at Short Form-6D health utility scores – a measure of overall quality of life encompassing key domains including vitality, physical function, mental health, fatigue – adults with AD scored markedly worse than individuals with no chronic health disorders. Health utility scores were particularly low in adults with AD and comorbid symptoms of anxiety or depression, suggesting that those affective symptoms are major drivers of the demonstrably poor quality of life in adult AD (Ann Allergy Asthma Immunol. 2020 Jan;124[1]:88-9).
In the Atopic Dermatitis in America Study, Dr. Silverberg and coinvestigators cross-sectionally surveyed 2,893 adults using the seven-item Hospital Anxiety and Depression Scale anxiety (HADS-A) and depression (HADS-D) assessment instruments. Individuals with AD as determined using the modified U.K. Diagnostic Criteria had dramatically higher rates of both depression and anxiety. For example, the prevalence of a HADS-A score of 11 or more, which is considered to be case finding for clinically important anxiety, was 28.6% in adults with AD, nearly twice the 15.5% prevalence in those without the dermatologic disease. A HADS-D score of 11 or greater was present in 13.5% of subjects with AD and 9% of those without.
HADS-A and -D scores were higher in adults with moderate AD, compared with mild disease, and higher still in those with severe AD. Indeed, virtually all individuals with moderate to severe AD had symptoms of anxiety and depression, which in a large proportion had gone undiagnosed. A multivariate analysis strongly suggested that AD severity was the major driver of anxiety and depression in adults with AD (Br J Dermatol. 2019 Sep;181[3]:554-65).
An important finding was that 100% of adults with AD who had scores in the severe range on three validated measures of itch, frequency of symptoms, and lesion severity had borderline or abnormal scores on the HADS-A and -D.
“Of course, if you don’t ask, you’re not going to know about it,” Dr. Silverberg noted.
Dr. Silverberg reported receiving research grants from Galderma and GlaxoSmithKline and serving as a consultant to those pharmaceutical companies and more than a dozen others.
Jonathan I. Silverberg, MD, PhD, declared in a video presentation during a virtual meeting held by the George Washington University department of dermatology.
The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic.
Dr. Silverberg presented highlights of his recent study of depression screening rates in the National Ambulatory Medical Care Survey, an annual population-based survey by the National Center for Health Statistics. He and his coinvestigator analyzed 9,345 office visits for atopic dermatitis (AD) and 2,085 for psoriasis (Br J Dermatol. 2019 Oct 24. doi: 10.1111/bjd.18629.). The picture that emerged showed that there is much room for improvement.
“We found that depression screening rates were abysmally low in atopic dermatitis patients, with less than 2% patients being screened. There was very little difference in screening rates between patients on an advanced therapy, like systemic phototherapy or a biologic, compared to those who were just on topical therapy alone, meaning even the more severe patients aren’t being asked these questions. And no difference between dermatologists and primary care physicians,” said Dr. Silverberg, director of clinical research and contact dermatitis in the department of dermatology at George Washington University, Washington.
For Dr. Silverberg, known for his pioneering work documenting the marked yet often-underappreciated negative impact of AD on quality of life and mental health, these rock-bottom screening rates were particularly galling.
“There are very high rates of anxiety and depression amongst our patients with atopic dermatitis,” the dermatologist emphasized. “Mental health symptoms are an incredibly important domain in atopic dermatitis that we need to ask our patients about. We don’t ask enough.
“This to me is actually a very important symptom to measure. It’s not just a theoretical construct involved in understanding the burden of the disease, it’s something that’s actionable because most of these cases of mental health symptoms are reversible or modifiable with improved control of the atopic dermatitis,” he continued. “I use this as an indication to step up therapy. If a patient is clinically depressed and we believe that’s secondary to their chronic atopic dermatitis, this is a reason to step up therapy to something stronger.”
If the depressive symptoms don’t improve after stepping up the intensity of the dermatologic therapy, it’s probably time for the patient to see a mental health professional, Dr. Silverberg advised, adding, “I’m not telling every dermatology resident out there to become a psychiatrist.”
Depression and anxiety in AD: How common?
In an analysis of multiyear data from the Medical Expenditure Panel Surveys, an annual population-based project conducted by the Agency for Healthcare Research and Quality, Dr. Silverberg and a coinvestigator found that adults with AD were an adjusted 186% more likely than those without AD to screen positive for depressive symptoms on the two-item Patient Health Questionnaire (PHQ-2), with rates of 44.3% and 21.9%, respectively. The AD patients were also 500% more likely to screen positive for severe psychological distress, with a 25.9% rate of having a Kessler-6 index score of 13 or more, compared with 5.5% in adults without AD.
The rate of severe psychological distress was higher in adults with AD than in those with asthma, diabetes, hypertension, urticaria, or psoriasis, and was comparable with the rate in individuals with autoimmune disease (Ann Allergy Asthma Immunol. 2019 Aug;123[2]:179-85).
“It’s surprising when you think that the majority of the cases of atopic dermatitis in the population are mild and yet when you look at a population-based sample such as this you see a strong signal come up. It means that, with all the dilution of mild disease, the signal is still there. It emphasizes that even patients with mild disease get these depressive symptoms and psychosocial distress,” Dr. Silverberg observed.
In a separate analysis of the same national database, this time looking at Short Form-6D health utility scores – a measure of overall quality of life encompassing key domains including vitality, physical function, mental health, fatigue – adults with AD scored markedly worse than individuals with no chronic health disorders. Health utility scores were particularly low in adults with AD and comorbid symptoms of anxiety or depression, suggesting that those affective symptoms are major drivers of the demonstrably poor quality of life in adult AD (Ann Allergy Asthma Immunol. 2020 Jan;124[1]:88-9).
In the Atopic Dermatitis in America Study, Dr. Silverberg and coinvestigators cross-sectionally surveyed 2,893 adults using the seven-item Hospital Anxiety and Depression Scale anxiety (HADS-A) and depression (HADS-D) assessment instruments. Individuals with AD as determined using the modified U.K. Diagnostic Criteria had dramatically higher rates of both depression and anxiety. For example, the prevalence of a HADS-A score of 11 or more, which is considered to be case finding for clinically important anxiety, was 28.6% in adults with AD, nearly twice the 15.5% prevalence in those without the dermatologic disease. A HADS-D score of 11 or greater was present in 13.5% of subjects with AD and 9% of those without.
HADS-A and -D scores were higher in adults with moderate AD, compared with mild disease, and higher still in those with severe AD. Indeed, virtually all individuals with moderate to severe AD had symptoms of anxiety and depression, which in a large proportion had gone undiagnosed. A multivariate analysis strongly suggested that AD severity was the major driver of anxiety and depression in adults with AD (Br J Dermatol. 2019 Sep;181[3]:554-65).
An important finding was that 100% of adults with AD who had scores in the severe range on three validated measures of itch, frequency of symptoms, and lesion severity had borderline or abnormal scores on the HADS-A and -D.
“Of course, if you don’t ask, you’re not going to know about it,” Dr. Silverberg noted.
Dr. Silverberg reported receiving research grants from Galderma and GlaxoSmithKline and serving as a consultant to those pharmaceutical companies and more than a dozen others.
Thickening of tattoo

A punch biopsy to rule out other causes of the patient’s signs and symptoms confirmed tattoo granulomas with sarcoidal nodules.
Tattoo granulomas can occur years after the initial placement of the tattoo. They appear, as in this case, as a thickening of the tattooed skin due to a hypersensitivity reaction to the foreign body. It has been more commonly reported in yellow and red inks but also has been reported in black ink. Sarcoidosis has been diagnosed based on occurrence in tattoos, but in this case, the patient’s calcium level was normal, chest X-ray showed no signs of adenopathy, and she had no symptoms of sarcoidosis such as fevers, chills, night sweats, or fatigue. Thus, no further work-up for systemic sarcoidosis was performed.
During the patient’s biopsy visit, she was started on oral montelukast 10 mg/d for a possible allergic reaction to the tattoo ink. By the time pathology results returned 1 week later, her itching and puffiness had resolved. Montelukast is a leukotriene antagonist that is approved by the US Food and Drug Administration (FDA) for use in asthma and allergic rhinitis; it is also used off label for chronic urticaria.
Usual therapies for tattoo granulomas include intralesional steroid injections or laser therapy to destroy the pigment. This patient had a large number of tattoos that would have required extensive laser therapy destruction of the pigment or numerous intralesional injections. Since she was no longer symptomatic, she elected to continue on the montelukast. She was cautioned about the possible development of suicidal ideation on this medication and was instructed to seek care if any such thoughts occurred.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Tittelbach J, Peckruhn M, Schliemann S, et al. Sarcoidal foreign body reaction as a severe side-effect to permanent makeup: successful treatment with intralesional triamcinolone. Acta Derm Venereol. 2018;98:458-459.

A punch biopsy to rule out other causes of the patient’s signs and symptoms confirmed tattoo granulomas with sarcoidal nodules.
Tattoo granulomas can occur years after the initial placement of the tattoo. They appear, as in this case, as a thickening of the tattooed skin due to a hypersensitivity reaction to the foreign body. It has been more commonly reported in yellow and red inks but also has been reported in black ink. Sarcoidosis has been diagnosed based on occurrence in tattoos, but in this case, the patient’s calcium level was normal, chest X-ray showed no signs of adenopathy, and she had no symptoms of sarcoidosis such as fevers, chills, night sweats, or fatigue. Thus, no further work-up for systemic sarcoidosis was performed.
During the patient’s biopsy visit, she was started on oral montelukast 10 mg/d for a possible allergic reaction to the tattoo ink. By the time pathology results returned 1 week later, her itching and puffiness had resolved. Montelukast is a leukotriene antagonist that is approved by the US Food and Drug Administration (FDA) for use in asthma and allergic rhinitis; it is also used off label for chronic urticaria.
Usual therapies for tattoo granulomas include intralesional steroid injections or laser therapy to destroy the pigment. This patient had a large number of tattoos that would have required extensive laser therapy destruction of the pigment or numerous intralesional injections. Since she was no longer symptomatic, she elected to continue on the montelukast. She was cautioned about the possible development of suicidal ideation on this medication and was instructed to seek care if any such thoughts occurred.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

A punch biopsy to rule out other causes of the patient’s signs and symptoms confirmed tattoo granulomas with sarcoidal nodules.
Tattoo granulomas can occur years after the initial placement of the tattoo. They appear, as in this case, as a thickening of the tattooed skin due to a hypersensitivity reaction to the foreign body. It has been more commonly reported in yellow and red inks but also has been reported in black ink. Sarcoidosis has been diagnosed based on occurrence in tattoos, but in this case, the patient’s calcium level was normal, chest X-ray showed no signs of adenopathy, and she had no symptoms of sarcoidosis such as fevers, chills, night sweats, or fatigue. Thus, no further work-up for systemic sarcoidosis was performed.
During the patient’s biopsy visit, she was started on oral montelukast 10 mg/d for a possible allergic reaction to the tattoo ink. By the time pathology results returned 1 week later, her itching and puffiness had resolved. Montelukast is a leukotriene antagonist that is approved by the US Food and Drug Administration (FDA) for use in asthma and allergic rhinitis; it is also used off label for chronic urticaria.
Usual therapies for tattoo granulomas include intralesional steroid injections or laser therapy to destroy the pigment. This patient had a large number of tattoos that would have required extensive laser therapy destruction of the pigment or numerous intralesional injections. Since she was no longer symptomatic, she elected to continue on the montelukast. She was cautioned about the possible development of suicidal ideation on this medication and was instructed to seek care if any such thoughts occurred.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Tittelbach J, Peckruhn M, Schliemann S, et al. Sarcoidal foreign body reaction as a severe side-effect to permanent makeup: successful treatment with intralesional triamcinolone. Acta Derm Venereol. 2018;98:458-459.
Tittelbach J, Peckruhn M, Schliemann S, et al. Sarcoidal foreign body reaction as a severe side-effect to permanent makeup: successful treatment with intralesional triamcinolone. Acta Derm Venereol. 2018;98:458-459.
Low-income DC communities have restricted access to iPLEDGE pharmacies
Residents of , results from a survey demonstrated.
Prescription of isotretinoin is regulated by the iPLEDGE program, which strives to ensure that no female patient starts isotretinoin therapy if pregnant and that no female patient on isotretinoin therapy becomes pregnant. “Over the years, many studies have criticized the program by demonstrating that iPLEDGE has promoted health care disparities,” Nidhi Shah said during a virtual meeting held by the George Washington University department of dermatology. “For example, racial minorities and women are more likely to be underprescribed isotretinoin, as well as face more delays in treatment.”
In an effort to evaluate the geographic distribution of iPLEDGE pharmacies in Washington DC, and its correlation with sociodemographic factors, Ms. Shah, a third-year medical student at the George Washington University, Washington, and colleagues obtained a list of active pharmacies in Washington from the local government. They also surveyed each outpatient pharmacy in the District of Columbia to verify their iPLEDGE registration status, for a total of 146 pharmacies.
Ms. Shah reported that 82% of all outpatient pharmacies were enrolled in iPLEDGE. However, enrollment significantly varied by the type of pharmacy. For example, 100% of chain pharmacies were enrolled, compared with 46% of independent pharmacies and 60% of hospital-based pharmacies.
When the researchers evaluated the number and type of iPLEDGE pharmacy by each of the eight wards in Washington, they observed a high density of pharmacies in wards 1 and 2, communities with a generally low proportion of residents who live in poverty, and low density of pharmacies in wards 7 and 8, communities with a higher proportion of residents who live in poverty. In addition, there were more independent than chain pharmacies in wards 7 and 8, and residents in those wards had a greater distance to travel to reach an iPLEDGE pharmacy, compared with residents who live in the other wards.
When Ms. Shah and colleagues examined the correlation between pharmacies per 10,000 residents and specific sociodemographic factors, they observed a strong, positive correlation between iPLEDGE pharmacy density and median household income (P = .0003). On the other hand, there was a strong negative correlation between iPLEDGE pharmacy density and the percentage of individuals with public insurance (P less than .0001), as well as the percentage of nonwhite individuals (P = .0009).
“Our study highlights the lack of isotretinoin-dispensing pharmacies in low-income communities,” Ms. Shah concluded. “Not only are there fewer such pharmacies available in low income communities, but the residents must also travel further to reach them. The spatial heterogeneity of iPLEDGE pharmacies may be an important patient barrier to timely access of isotretinoin, especially for female patients who have a strict 7-day window to collect their medication. We hope that future public health reform works to close this gap.”
The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic. Ms. Shah reported having no disclosures.
Residents of , results from a survey demonstrated.
Prescription of isotretinoin is regulated by the iPLEDGE program, which strives to ensure that no female patient starts isotretinoin therapy if pregnant and that no female patient on isotretinoin therapy becomes pregnant. “Over the years, many studies have criticized the program by demonstrating that iPLEDGE has promoted health care disparities,” Nidhi Shah said during a virtual meeting held by the George Washington University department of dermatology. “For example, racial minorities and women are more likely to be underprescribed isotretinoin, as well as face more delays in treatment.”
In an effort to evaluate the geographic distribution of iPLEDGE pharmacies in Washington DC, and its correlation with sociodemographic factors, Ms. Shah, a third-year medical student at the George Washington University, Washington, and colleagues obtained a list of active pharmacies in Washington from the local government. They also surveyed each outpatient pharmacy in the District of Columbia to verify their iPLEDGE registration status, for a total of 146 pharmacies.
Ms. Shah reported that 82% of all outpatient pharmacies were enrolled in iPLEDGE. However, enrollment significantly varied by the type of pharmacy. For example, 100% of chain pharmacies were enrolled, compared with 46% of independent pharmacies and 60% of hospital-based pharmacies.
When the researchers evaluated the number and type of iPLEDGE pharmacy by each of the eight wards in Washington, they observed a high density of pharmacies in wards 1 and 2, communities with a generally low proportion of residents who live in poverty, and low density of pharmacies in wards 7 and 8, communities with a higher proportion of residents who live in poverty. In addition, there were more independent than chain pharmacies in wards 7 and 8, and residents in those wards had a greater distance to travel to reach an iPLEDGE pharmacy, compared with residents who live in the other wards.
When Ms. Shah and colleagues examined the correlation between pharmacies per 10,000 residents and specific sociodemographic factors, they observed a strong, positive correlation between iPLEDGE pharmacy density and median household income (P = .0003). On the other hand, there was a strong negative correlation between iPLEDGE pharmacy density and the percentage of individuals with public insurance (P less than .0001), as well as the percentage of nonwhite individuals (P = .0009).
“Our study highlights the lack of isotretinoin-dispensing pharmacies in low-income communities,” Ms. Shah concluded. “Not only are there fewer such pharmacies available in low income communities, but the residents must also travel further to reach them. The spatial heterogeneity of iPLEDGE pharmacies may be an important patient barrier to timely access of isotretinoin, especially for female patients who have a strict 7-day window to collect their medication. We hope that future public health reform works to close this gap.”
The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic. Ms. Shah reported having no disclosures.
Residents of , results from a survey demonstrated.
Prescription of isotretinoin is regulated by the iPLEDGE program, which strives to ensure that no female patient starts isotretinoin therapy if pregnant and that no female patient on isotretinoin therapy becomes pregnant. “Over the years, many studies have criticized the program by demonstrating that iPLEDGE has promoted health care disparities,” Nidhi Shah said during a virtual meeting held by the George Washington University department of dermatology. “For example, racial minorities and women are more likely to be underprescribed isotretinoin, as well as face more delays in treatment.”
In an effort to evaluate the geographic distribution of iPLEDGE pharmacies in Washington DC, and its correlation with sociodemographic factors, Ms. Shah, a third-year medical student at the George Washington University, Washington, and colleagues obtained a list of active pharmacies in Washington from the local government. They also surveyed each outpatient pharmacy in the District of Columbia to verify their iPLEDGE registration status, for a total of 146 pharmacies.
Ms. Shah reported that 82% of all outpatient pharmacies were enrolled in iPLEDGE. However, enrollment significantly varied by the type of pharmacy. For example, 100% of chain pharmacies were enrolled, compared with 46% of independent pharmacies and 60% of hospital-based pharmacies.
When the researchers evaluated the number and type of iPLEDGE pharmacy by each of the eight wards in Washington, they observed a high density of pharmacies in wards 1 and 2, communities with a generally low proportion of residents who live in poverty, and low density of pharmacies in wards 7 and 8, communities with a higher proportion of residents who live in poverty. In addition, there were more independent than chain pharmacies in wards 7 and 8, and residents in those wards had a greater distance to travel to reach an iPLEDGE pharmacy, compared with residents who live in the other wards.
When Ms. Shah and colleagues examined the correlation between pharmacies per 10,000 residents and specific sociodemographic factors, they observed a strong, positive correlation between iPLEDGE pharmacy density and median household income (P = .0003). On the other hand, there was a strong negative correlation between iPLEDGE pharmacy density and the percentage of individuals with public insurance (P less than .0001), as well as the percentage of nonwhite individuals (P = .0009).
“Our study highlights the lack of isotretinoin-dispensing pharmacies in low-income communities,” Ms. Shah concluded. “Not only are there fewer such pharmacies available in low income communities, but the residents must also travel further to reach them. The spatial heterogeneity of iPLEDGE pharmacies may be an important patient barrier to timely access of isotretinoin, especially for female patients who have a strict 7-day window to collect their medication. We hope that future public health reform works to close this gap.”
The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic. Ms. Shah reported having no disclosures.
Rapid shift to adalimumab biosimilars in Denmark contrasts with U.S. experience
Adalimumab biosimilars are years away from entering the marketplace in the United States because of patent disputes, but they already have led to substantial discounts in Denmark, researchers wrote in JAMA Internal Medicine.
The Danish health care system switched almost entirely to adalimumab biosimilars after the patent on the original adalimumab product, Humira, expired there in October 2018. The switch to biosimilars led to an 82% decrease in costs for the medication, wrote Thomas Bo Jensen, MD, and colleagues in a research letter.
Denmark did not automatically substitute biosimilars, but the Danish Medicines Council recommended adalimumab biosimilars for all indications following Humira’s patent expiration. The recommendations “included switching patients to a biosimilar who were already well treated with the originator,” the researchers wrote.
To study the shift to adalimumab biosimilars across all indications in Denmark and calculate cost reductions, Dr. Jensen, of the department of clinical pharmacology at Copenhagen University Hospital Bispebjerg, and coinvestigators examined monthly data on drug sales from Amgros, which purchases all hospital drugs in the country.
“The proportion of adalimumab biosimilars increased from 71.6% (7,040 of 9,829 pens) in November 2018 to 95.1% (8,974 of 9,438 pens) in December 2018,” the researchers wrote. “Costs of adalimumab decreased by 82.8% from September 2018 to December 2018 (September: 8,197 pens at $5.13 million; December: 9,438 pens at $1.01 million).” The results were similar in rheumatology, dermatology, and gastroenterology.
The Food and Drug Administration has approved five adalimumab biosimilars in the United States, but “they will not enter the market until 2023 owing to patent disputes with AbbVie, the manufacturer of Humira,” wrote Jennifer D. Claytor, MD, of the department of internal medicine at University of California, San Francisco, and Walid Gellad, MD, of the division of general internal medicine at University of Pittsburgh, in an accompanying editorial.
The annual postrebate price of Humira doubled between 2013 and 2018, from $19,000 to $38,000, and these price increases may influence the price of biosimilars, “which will be priced using Humira’s price as an anchor,” Dr. Claytor and Dr. Gellad wrote.
A rapid shift to adalimumab biosimilars across the United States when they become available is “unlikely,” they wrote. Nonetheless, “some health care systems of comparable size to Denmark (e.g., the Veterans Affairs system) and others that are larger (e.g., Kaiser Permanente) ... have the ability to switch products quickly through use of formularies and a prescriber workforce. For example, Kaiser Permanente has successfully replaced Remicade (infliximab) with biosimilars in 80% of patients.”
Given the many biologics in development and increasing health care spending, “we need to take seriously the substantial savings offered by biosimilars and the feasibility, as evidenced by Denmark, of switching to biosimilars quickly once they are available on the market,” Dr. Claytor and Dr. Gellad concluded.
The research was supported by an unrestricted grant from Helsefonden. One author disclosed receiving grants from Pfizer, AbbVie, Roche, and Bristol-Myers Squibb outside the current study. The editorial authors had no disclosures.
SOURCE: Jensen TB et al. JAMA Intern Med. 2020 Mar 30. doi: 10.1001/jamainternmed.2020.0338.
Adalimumab biosimilars are years away from entering the marketplace in the United States because of patent disputes, but they already have led to substantial discounts in Denmark, researchers wrote in JAMA Internal Medicine.
The Danish health care system switched almost entirely to adalimumab biosimilars after the patent on the original adalimumab product, Humira, expired there in October 2018. The switch to biosimilars led to an 82% decrease in costs for the medication, wrote Thomas Bo Jensen, MD, and colleagues in a research letter.
Denmark did not automatically substitute biosimilars, but the Danish Medicines Council recommended adalimumab biosimilars for all indications following Humira’s patent expiration. The recommendations “included switching patients to a biosimilar who were already well treated with the originator,” the researchers wrote.
To study the shift to adalimumab biosimilars across all indications in Denmark and calculate cost reductions, Dr. Jensen, of the department of clinical pharmacology at Copenhagen University Hospital Bispebjerg, and coinvestigators examined monthly data on drug sales from Amgros, which purchases all hospital drugs in the country.
“The proportion of adalimumab biosimilars increased from 71.6% (7,040 of 9,829 pens) in November 2018 to 95.1% (8,974 of 9,438 pens) in December 2018,” the researchers wrote. “Costs of adalimumab decreased by 82.8% from September 2018 to December 2018 (September: 8,197 pens at $5.13 million; December: 9,438 pens at $1.01 million).” The results were similar in rheumatology, dermatology, and gastroenterology.
The Food and Drug Administration has approved five adalimumab biosimilars in the United States, but “they will not enter the market until 2023 owing to patent disputes with AbbVie, the manufacturer of Humira,” wrote Jennifer D. Claytor, MD, of the department of internal medicine at University of California, San Francisco, and Walid Gellad, MD, of the division of general internal medicine at University of Pittsburgh, in an accompanying editorial.
The annual postrebate price of Humira doubled between 2013 and 2018, from $19,000 to $38,000, and these price increases may influence the price of biosimilars, “which will be priced using Humira’s price as an anchor,” Dr. Claytor and Dr. Gellad wrote.
A rapid shift to adalimumab biosimilars across the United States when they become available is “unlikely,” they wrote. Nonetheless, “some health care systems of comparable size to Denmark (e.g., the Veterans Affairs system) and others that are larger (e.g., Kaiser Permanente) ... have the ability to switch products quickly through use of formularies and a prescriber workforce. For example, Kaiser Permanente has successfully replaced Remicade (infliximab) with biosimilars in 80% of patients.”
Given the many biologics in development and increasing health care spending, “we need to take seriously the substantial savings offered by biosimilars and the feasibility, as evidenced by Denmark, of switching to biosimilars quickly once they are available on the market,” Dr. Claytor and Dr. Gellad concluded.
The research was supported by an unrestricted grant from Helsefonden. One author disclosed receiving grants from Pfizer, AbbVie, Roche, and Bristol-Myers Squibb outside the current study. The editorial authors had no disclosures.
SOURCE: Jensen TB et al. JAMA Intern Med. 2020 Mar 30. doi: 10.1001/jamainternmed.2020.0338.
Adalimumab biosimilars are years away from entering the marketplace in the United States because of patent disputes, but they already have led to substantial discounts in Denmark, researchers wrote in JAMA Internal Medicine.
The Danish health care system switched almost entirely to adalimumab biosimilars after the patent on the original adalimumab product, Humira, expired there in October 2018. The switch to biosimilars led to an 82% decrease in costs for the medication, wrote Thomas Bo Jensen, MD, and colleagues in a research letter.
Denmark did not automatically substitute biosimilars, but the Danish Medicines Council recommended adalimumab biosimilars for all indications following Humira’s patent expiration. The recommendations “included switching patients to a biosimilar who were already well treated with the originator,” the researchers wrote.
To study the shift to adalimumab biosimilars across all indications in Denmark and calculate cost reductions, Dr. Jensen, of the department of clinical pharmacology at Copenhagen University Hospital Bispebjerg, and coinvestigators examined monthly data on drug sales from Amgros, which purchases all hospital drugs in the country.
“The proportion of adalimumab biosimilars increased from 71.6% (7,040 of 9,829 pens) in November 2018 to 95.1% (8,974 of 9,438 pens) in December 2018,” the researchers wrote. “Costs of adalimumab decreased by 82.8% from September 2018 to December 2018 (September: 8,197 pens at $5.13 million; December: 9,438 pens at $1.01 million).” The results were similar in rheumatology, dermatology, and gastroenterology.
The Food and Drug Administration has approved five adalimumab biosimilars in the United States, but “they will not enter the market until 2023 owing to patent disputes with AbbVie, the manufacturer of Humira,” wrote Jennifer D. Claytor, MD, of the department of internal medicine at University of California, San Francisco, and Walid Gellad, MD, of the division of general internal medicine at University of Pittsburgh, in an accompanying editorial.
The annual postrebate price of Humira doubled between 2013 and 2018, from $19,000 to $38,000, and these price increases may influence the price of biosimilars, “which will be priced using Humira’s price as an anchor,” Dr. Claytor and Dr. Gellad wrote.
A rapid shift to adalimumab biosimilars across the United States when they become available is “unlikely,” they wrote. Nonetheless, “some health care systems of comparable size to Denmark (e.g., the Veterans Affairs system) and others that are larger (e.g., Kaiser Permanente) ... have the ability to switch products quickly through use of formularies and a prescriber workforce. For example, Kaiser Permanente has successfully replaced Remicade (infliximab) with biosimilars in 80% of patients.”
Given the many biologics in development and increasing health care spending, “we need to take seriously the substantial savings offered by biosimilars and the feasibility, as evidenced by Denmark, of switching to biosimilars quickly once they are available on the market,” Dr. Claytor and Dr. Gellad concluded.
The research was supported by an unrestricted grant from Helsefonden. One author disclosed receiving grants from Pfizer, AbbVie, Roche, and Bristol-Myers Squibb outside the current study. The editorial authors had no disclosures.
SOURCE: Jensen TB et al. JAMA Intern Med. 2020 Mar 30. doi: 10.1001/jamainternmed.2020.0338.
FROM JAMA INTERNAL MEDICINE
FDA approves ixekizumab for pediatric plaque psoriasis
The according to an announcement from Lilly.
Patients need to be candidates for systemic therapy or phototherapy and have no known hypersensitivity to the biologic.
The safety, tolerability, and efficacy of the interleukin-17a antagonist were demonstrated in a phase 3 study that included 171 patients aged 6-17 years with moderate to severe plaque psoriasis. At 12 weeks, 89% those on ixekizumab achieved a 75% improvement on Psoriasis Area and Severity Index score, compared with 25% of those on placebo, and 81% achieved a static Physician’s Global Assessment of clear or almost clear, compared with 11% of those on placebo, according to the Lilly statement.
The safety profile seen with ixekizumab (Taltz) among the pediatric patients with plaque psoriasis is consistent with what has been observed among adult patients, although there were higher rates of conjunctivitis, influenza, and urticaria among the pediatric patients, the statement noted. The biologic may increase the risk of infection, and patients should be evaluated for tuberculosis, hypersensitivity, and inflammatory bowel disease. It is also recommended that routine immunizations be completed before initiating treatment.
Ixekizumab was initially approved for treating adults with moderate to severe plaque psoriasis in 2016, followed by approvals for treatment of adults with active psoriatic arthritis in 2017, and for adults with ankylosing spondylitis in August 2019.
The biologic therapies – etanercept, a tumor necrosis factor blocker, and ustekinumab (Stelara), an IL-12/23 antagonist – were previously approved by the FDA for pediatric psoriasis, in children ages 4 years and older and 12 years and older, respectively.
Updated prescribing information for ixekizumab can be found on the Lilly website.
[email protected]
The according to an announcement from Lilly.
Patients need to be candidates for systemic therapy or phototherapy and have no known hypersensitivity to the biologic.
The safety, tolerability, and efficacy of the interleukin-17a antagonist were demonstrated in a phase 3 study that included 171 patients aged 6-17 years with moderate to severe plaque psoriasis. At 12 weeks, 89% those on ixekizumab achieved a 75% improvement on Psoriasis Area and Severity Index score, compared with 25% of those on placebo, and 81% achieved a static Physician’s Global Assessment of clear or almost clear, compared with 11% of those on placebo, according to the Lilly statement.
The safety profile seen with ixekizumab (Taltz) among the pediatric patients with plaque psoriasis is consistent with what has been observed among adult patients, although there were higher rates of conjunctivitis, influenza, and urticaria among the pediatric patients, the statement noted. The biologic may increase the risk of infection, and patients should be evaluated for tuberculosis, hypersensitivity, and inflammatory bowel disease. It is also recommended that routine immunizations be completed before initiating treatment.
Ixekizumab was initially approved for treating adults with moderate to severe plaque psoriasis in 2016, followed by approvals for treatment of adults with active psoriatic arthritis in 2017, and for adults with ankylosing spondylitis in August 2019.
The biologic therapies – etanercept, a tumor necrosis factor blocker, and ustekinumab (Stelara), an IL-12/23 antagonist – were previously approved by the FDA for pediatric psoriasis, in children ages 4 years and older and 12 years and older, respectively.
Updated prescribing information for ixekizumab can be found on the Lilly website.
[email protected]
The according to an announcement from Lilly.
Patients need to be candidates for systemic therapy or phototherapy and have no known hypersensitivity to the biologic.
The safety, tolerability, and efficacy of the interleukin-17a antagonist were demonstrated in a phase 3 study that included 171 patients aged 6-17 years with moderate to severe plaque psoriasis. At 12 weeks, 89% those on ixekizumab achieved a 75% improvement on Psoriasis Area and Severity Index score, compared with 25% of those on placebo, and 81% achieved a static Physician’s Global Assessment of clear or almost clear, compared with 11% of those on placebo, according to the Lilly statement.
The safety profile seen with ixekizumab (Taltz) among the pediatric patients with plaque psoriasis is consistent with what has been observed among adult patients, although there were higher rates of conjunctivitis, influenza, and urticaria among the pediatric patients, the statement noted. The biologic may increase the risk of infection, and patients should be evaluated for tuberculosis, hypersensitivity, and inflammatory bowel disease. It is also recommended that routine immunizations be completed before initiating treatment.
Ixekizumab was initially approved for treating adults with moderate to severe plaque psoriasis in 2016, followed by approvals for treatment of adults with active psoriatic arthritis in 2017, and for adults with ankylosing spondylitis in August 2019.
The biologic therapies – etanercept, a tumor necrosis factor blocker, and ustekinumab (Stelara), an IL-12/23 antagonist – were previously approved by the FDA for pediatric psoriasis, in children ages 4 years and older and 12 years and older, respectively.
Updated prescribing information for ixekizumab can be found on the Lilly website.
[email protected]