User login
Chest pain and difficulty swallowing
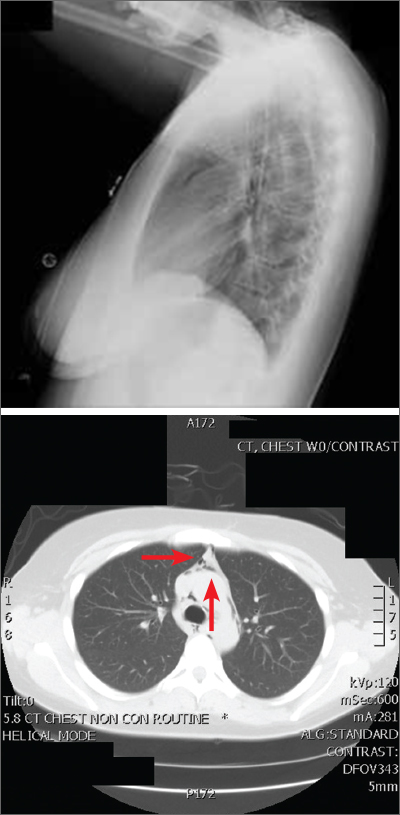
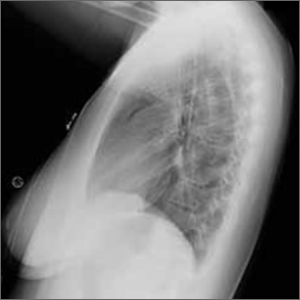
The posteroanterior (PA) and lateral view CXRs, as well as the CT scan, revealed the presence of retrosternal air and confirmed the patient’s diagnosis of pneumomediastinum.
Pneumomediastinum—the presence of free air in the mediastinum—can develop spontaneously (as was the case with our patient) or in response to trauma. Common causes include respiratory diseases such as asthma, and trauma to the esophagus secondary to mechanical ventilation, endoscopy, and excessive vomiting.1 Other possible causes include respiratory infections, foreign body aspiration, recent dental extraction, diabetic ketoacidosis, esophageal perforation, barotrauma (due to activities such as flying or scuba diving), and use of illicit drugs.1
Patients with pneumomediastinum often complain of retrosternal, pleuritic pain that radiates to their back, shoulders, and arms. They may also have difficulty swallowing (globus pharyngeus), a nasal voice, and/or dyspnea. Physical findings can include subcutaneous emphysema in the neck and supraclavicular fossa as manifested by the Hamman sign (a precordial “crunching” sound heard during systole), a fever, and distended neck veins.1
Diagnosis is made by CXR and/or chest CT. On a CXR, retrosternal air is best seen in the lateral projection. Small amounts of air can appear as linear lucencies outlining mediastinal contours. This air can be seen under the skin, surrounding the pericardium, around the pulmonary and/or aortic vasculature, and/or between the parietal pleura and diaphragm.2 A pleural effusion—particularly on the patient’s left side—should raise concern for esophageal perforation.
Pneumomediastinum is generally a self-limiting condition. Thus, patients who don’t have severe symptoms, such as respiratory distress or signs of inflammation, should be observed for 2 days, managed with rest and pain control, and discharged home. If severe symptoms or inflammatory signs are present, a Gastrografin swallow study is recommended to rule out esophageal perforation.3 If the result of this test is abnormal, a follow-up study with barium is recommended.3
The patient underwent both swallow studies, and they were negative. This patient received subsequent serial CXRs that showed improvement in the pneumomediastinum. Once the patient’s pain was well controlled with oral nonsteroidal anti-inflammatory drugs, she was discharged (after a 3-day hospitalization) with close follow-up. One week later, her pain had almost entirely resolved.
This case was adapted from: Gawrys B, Shaha D. Pleuritic chest pain and globus pharyngeus. J Fam Pract. 2015;64:305-307.
1. Park DE, Vallieres E. Pneumomediastinum and mediastinitis. In: Mason R, Broaddus V, Murray J, et al, eds. Murray and Nadel’s Textbook of Respiratory Medicine. 4th ed. Elsevier Health Sciences; 2005:2039-2068.
2. Zylak CM, Standen JR, Barnes GR, et al. Pneumomediastinum revisited. Radiographics. 2000;20:1043-1057. doi: 10.1148/radiographics.20.4.g00jl13104
3. Takada K, Matsumoto S, Hiramatsu T, et al. Management of spontaneous pneumomediastinum based on clinical experience of 25 cases. Respir Med. 2008;102:1329-1334. doi: 10.1016/j.rmed.2008.03.023

The posteroanterior (PA) and lateral view CXRs, as well as the CT scan, revealed the presence of retrosternal air and confirmed the patient’s diagnosis of pneumomediastinum.
Pneumomediastinum—the presence of free air in the mediastinum—can develop spontaneously (as was the case with our patient) or in response to trauma. Common causes include respiratory diseases such as asthma, and trauma to the esophagus secondary to mechanical ventilation, endoscopy, and excessive vomiting.1 Other possible causes include respiratory infections, foreign body aspiration, recent dental extraction, diabetic ketoacidosis, esophageal perforation, barotrauma (due to activities such as flying or scuba diving), and use of illicit drugs.1
Patients with pneumomediastinum often complain of retrosternal, pleuritic pain that radiates to their back, shoulders, and arms. They may also have difficulty swallowing (globus pharyngeus), a nasal voice, and/or dyspnea. Physical findings can include subcutaneous emphysema in the neck and supraclavicular fossa as manifested by the Hamman sign (a precordial “crunching” sound heard during systole), a fever, and distended neck veins.1
Diagnosis is made by CXR and/or chest CT. On a CXR, retrosternal air is best seen in the lateral projection. Small amounts of air can appear as linear lucencies outlining mediastinal contours. This air can be seen under the skin, surrounding the pericardium, around the pulmonary and/or aortic vasculature, and/or between the parietal pleura and diaphragm.2 A pleural effusion—particularly on the patient’s left side—should raise concern for esophageal perforation.
Pneumomediastinum is generally a self-limiting condition. Thus, patients who don’t have severe symptoms, such as respiratory distress or signs of inflammation, should be observed for 2 days, managed with rest and pain control, and discharged home. If severe symptoms or inflammatory signs are present, a Gastrografin swallow study is recommended to rule out esophageal perforation.3 If the result of this test is abnormal, a follow-up study with barium is recommended.3
The patient underwent both swallow studies, and they were negative. This patient received subsequent serial CXRs that showed improvement in the pneumomediastinum. Once the patient’s pain was well controlled with oral nonsteroidal anti-inflammatory drugs, she was discharged (after a 3-day hospitalization) with close follow-up. One week later, her pain had almost entirely resolved.
This case was adapted from: Gawrys B, Shaha D. Pleuritic chest pain and globus pharyngeus. J Fam Pract. 2015;64:305-307.

The posteroanterior (PA) and lateral view CXRs, as well as the CT scan, revealed the presence of retrosternal air and confirmed the patient’s diagnosis of pneumomediastinum.
Pneumomediastinum—the presence of free air in the mediastinum—can develop spontaneously (as was the case with our patient) or in response to trauma. Common causes include respiratory diseases such as asthma, and trauma to the esophagus secondary to mechanical ventilation, endoscopy, and excessive vomiting.1 Other possible causes include respiratory infections, foreign body aspiration, recent dental extraction, diabetic ketoacidosis, esophageal perforation, barotrauma (due to activities such as flying or scuba diving), and use of illicit drugs.1
Patients with pneumomediastinum often complain of retrosternal, pleuritic pain that radiates to their back, shoulders, and arms. They may also have difficulty swallowing (globus pharyngeus), a nasal voice, and/or dyspnea. Physical findings can include subcutaneous emphysema in the neck and supraclavicular fossa as manifested by the Hamman sign (a precordial “crunching” sound heard during systole), a fever, and distended neck veins.1
Diagnosis is made by CXR and/or chest CT. On a CXR, retrosternal air is best seen in the lateral projection. Small amounts of air can appear as linear lucencies outlining mediastinal contours. This air can be seen under the skin, surrounding the pericardium, around the pulmonary and/or aortic vasculature, and/or between the parietal pleura and diaphragm.2 A pleural effusion—particularly on the patient’s left side—should raise concern for esophageal perforation.
Pneumomediastinum is generally a self-limiting condition. Thus, patients who don’t have severe symptoms, such as respiratory distress or signs of inflammation, should be observed for 2 days, managed with rest and pain control, and discharged home. If severe symptoms or inflammatory signs are present, a Gastrografin swallow study is recommended to rule out esophageal perforation.3 If the result of this test is abnormal, a follow-up study with barium is recommended.3
The patient underwent both swallow studies, and they were negative. This patient received subsequent serial CXRs that showed improvement in the pneumomediastinum. Once the patient’s pain was well controlled with oral nonsteroidal anti-inflammatory drugs, she was discharged (after a 3-day hospitalization) with close follow-up. One week later, her pain had almost entirely resolved.
This case was adapted from: Gawrys B, Shaha D. Pleuritic chest pain and globus pharyngeus. J Fam Pract. 2015;64:305-307.
1. Park DE, Vallieres E. Pneumomediastinum and mediastinitis. In: Mason R, Broaddus V, Murray J, et al, eds. Murray and Nadel’s Textbook of Respiratory Medicine. 4th ed. Elsevier Health Sciences; 2005:2039-2068.
2. Zylak CM, Standen JR, Barnes GR, et al. Pneumomediastinum revisited. Radiographics. 2000;20:1043-1057. doi: 10.1148/radiographics.20.4.g00jl13104
3. Takada K, Matsumoto S, Hiramatsu T, et al. Management of spontaneous pneumomediastinum based on clinical experience of 25 cases. Respir Med. 2008;102:1329-1334. doi: 10.1016/j.rmed.2008.03.023
1. Park DE, Vallieres E. Pneumomediastinum and mediastinitis. In: Mason R, Broaddus V, Murray J, et al, eds. Murray and Nadel’s Textbook of Respiratory Medicine. 4th ed. Elsevier Health Sciences; 2005:2039-2068.
2. Zylak CM, Standen JR, Barnes GR, et al. Pneumomediastinum revisited. Radiographics. 2000;20:1043-1057. doi: 10.1148/radiographics.20.4.g00jl13104
3. Takada K, Matsumoto S, Hiramatsu T, et al. Management of spontaneous pneumomediastinum based on clinical experience of 25 cases. Respir Med. 2008;102:1329-1334. doi: 10.1016/j.rmed.2008.03.023
Topical gel for epidermolysis bullosa shows ongoing benefit
GLASGOW, Scotland – the phase 3 safety and efficacy study of the treatment.
Over 200 patients from the trial, including 105 who began treatment with a control gel, continued taking oleogel-S10 after 90 days. The current interim analysis at 12 months indicates there was a 55% reduction in the proportion of the body affected, compared with baseline.
Moreover, reductions in skin activity scores seen in the double-blind phase of the trial were maintained during the open-label extension. About 6% of patients experienced adverse events that led to withdrawal from the study.
The results show that oleogel-S10 was associated with “accelerated wound healing,” said study presenter Tracey Cunningham, MD, chief medical officer, Amryt Pharmaceuticals DAC, Dublin, which is developing the topical agent. “There were no new safety signals with this longer exposure to oleogel-S10, and patients had sustained improvement in wound burden,” she added.
The research was presented at the British Association of Dermatologists (BAD) 2022 Annual Meeting on July 6.
In April, European Medicines Agency recommended approval of oleogel-S10 for the treatment of partial-thickness skin wounds associated with dystrophic and junctional EB for patients aged 6 months and older.
However, just a month earlier, the U.S. Food and Drug Administration declined to approve the topical agent for use in EB, even after it extended its review by 3 months to include additional analyses of data previously submitted by the company.
In the post-presentation discussion, Dr. Cunningham said that the FDA had “not been satisfied at this point with the information that we have given them,” adding, “We don’t agree with the decision, and we will be appealing.”
Raman K. Madan, MD, a dermatologist at Northwell Health, Huntington, New York, who was not involved in the study, said that the reductions in wound healing seen in the study are “meaningful” and that the numbers represent a “big breakthrough.”
He told this news organization that there are “very few products on the market” for EB and that having an option for patients “would be amazing.”
“The big issue here would be cost and coverage for patients,” he said. If approved, “hopefully” it will be affordable, he added.
Dr. Madan noted that from his perspective, the majority of the reactions to the topical gel were “mild,” and there are “a lot of confounding factors” underlying the number of serious adverse events. “These patients with epidermolysis are prone to some of these issues regardless of treatment,” he said.
During her presentation, Dr. Cunningham noted that EB is a rare, debilitating condition that is characterized by varying degrees of skin fragility, blisters, and impaired wound healing that in turn lead to serious complications that affect quality of life.
While wound management is a “fundamental priority” for patients living with EB, she said, there is a “high, unmet” clinical need.
To those ends, EASE was the largest randomized controlled phase 3 efficacy and safety study in EB. In the study, 252 patients were allocated to receive oleogel-S10 or control gel plus standard-of-care nonadhesive wound dressing.
The double-blind phase of the trial met its primary endpoint: A higher proportion of patients who were given oleogel-S10 achieved first complete closure of the EB target wound by day 45, compared with patients who were given control gel, at 41.3% versus 28.9%. This equated to a relative risk of wound closure by day 45 of 1.44, or an odds ratio of 1.84 (P = .013).
However, as reported at the time by this news organization, the difference in time to wound healing by day 90 between the two patient groups was not statistically significant (P = .302), with 50.5% of oleogel-S10 patients achieving wound closure, versus 43.9% of those in the control group.
Dr. Cunningham discussed the open-label extension, which involved 205 patients from the double-blind phase (mean age, of 16.3 years) treated with oleogel-S10 or control gel plus standard-of-care nonadhesive wound dressing for 24 months.
In presenting the results of the first 12 months of the open-label extension, she said that oleogel-S10 led to “consistent” reductions in the body surface area percentage (BSAP) affected by EB. The overall reduction from baseline was 55% after receiving treatment for 15 months.
Between day 90 and month 12 of the open-label extension, the absolute BSAP was reduced from 7.4% to 5.4% for patients who had received oleogel-S10 from the start of the study. For those who started in the control group and then switched to the oleogel-S10 arm during the open-label extension, the reduction was from 8.3% to 6.4%.
Dr. Cunningham pointed out that a 1% reduction in BSAP equates approximately to the palmar surface of the hand.
Scores on the Epidermolysis Bullosa Disease Activity and Scarring Index (EBDASI) Skin activity subscale indicated that the reductions achieved in the double-blind phase of the trial were maintained.
Among patients who received oleogel-S10 from the start of the trial, EBDASI Skin scores were reduced from 19.6 at baseline to 13.5 at 12 months’ follow-up in the open-label extension. The reduction was from 19.6 to 13.5 for those who began the trial taking control gel.
Dr. Cunningham showed that adverse events of any grade were seen in 72.0% of patients who began taking oleogel-S10 at the start of the trial and in 69.5% of those who began the trial taking control gel.
Serious adverse events were recorded in 23.0% and 20.0% of patients, respectively, while 6.0% of those who initially received oleogel-S10 and 6.7% of those initially assigned to control gel experienced adverse events that led to study withdrawal during the open-label phase.
The most frequently reported adverse events in the open-label extension were wound complications, seen in 39.5% of patients; anemia, seen in 14.1%; wound infection, seen in 9.3%; pyrexia, seen in 8.3%; and pruritus, seen in 5.9%. No more details regarding adverse events were provided.
The study was funded by Amryt Pharmaceuticals DAC. Dr. Cunningham is an employee of Amryt Pharmaceuticals. No other relevant financial relationships have been disclosed.
A version of this article first appeared on Medscape.com.
GLASGOW, Scotland – the phase 3 safety and efficacy study of the treatment.
Over 200 patients from the trial, including 105 who began treatment with a control gel, continued taking oleogel-S10 after 90 days. The current interim analysis at 12 months indicates there was a 55% reduction in the proportion of the body affected, compared with baseline.
Moreover, reductions in skin activity scores seen in the double-blind phase of the trial were maintained during the open-label extension. About 6% of patients experienced adverse events that led to withdrawal from the study.
The results show that oleogel-S10 was associated with “accelerated wound healing,” said study presenter Tracey Cunningham, MD, chief medical officer, Amryt Pharmaceuticals DAC, Dublin, which is developing the topical agent. “There were no new safety signals with this longer exposure to oleogel-S10, and patients had sustained improvement in wound burden,” she added.
The research was presented at the British Association of Dermatologists (BAD) 2022 Annual Meeting on July 6.
In April, European Medicines Agency recommended approval of oleogel-S10 for the treatment of partial-thickness skin wounds associated with dystrophic and junctional EB for patients aged 6 months and older.
However, just a month earlier, the U.S. Food and Drug Administration declined to approve the topical agent for use in EB, even after it extended its review by 3 months to include additional analyses of data previously submitted by the company.
In the post-presentation discussion, Dr. Cunningham said that the FDA had “not been satisfied at this point with the information that we have given them,” adding, “We don’t agree with the decision, and we will be appealing.”
Raman K. Madan, MD, a dermatologist at Northwell Health, Huntington, New York, who was not involved in the study, said that the reductions in wound healing seen in the study are “meaningful” and that the numbers represent a “big breakthrough.”
He told this news organization that there are “very few products on the market” for EB and that having an option for patients “would be amazing.”
“The big issue here would be cost and coverage for patients,” he said. If approved, “hopefully” it will be affordable, he added.
Dr. Madan noted that from his perspective, the majority of the reactions to the topical gel were “mild,” and there are “a lot of confounding factors” underlying the number of serious adverse events. “These patients with epidermolysis are prone to some of these issues regardless of treatment,” he said.
During her presentation, Dr. Cunningham noted that EB is a rare, debilitating condition that is characterized by varying degrees of skin fragility, blisters, and impaired wound healing that in turn lead to serious complications that affect quality of life.
While wound management is a “fundamental priority” for patients living with EB, she said, there is a “high, unmet” clinical need.
To those ends, EASE was the largest randomized controlled phase 3 efficacy and safety study in EB. In the study, 252 patients were allocated to receive oleogel-S10 or control gel plus standard-of-care nonadhesive wound dressing.
The double-blind phase of the trial met its primary endpoint: A higher proportion of patients who were given oleogel-S10 achieved first complete closure of the EB target wound by day 45, compared with patients who were given control gel, at 41.3% versus 28.9%. This equated to a relative risk of wound closure by day 45 of 1.44, or an odds ratio of 1.84 (P = .013).
However, as reported at the time by this news organization, the difference in time to wound healing by day 90 between the two patient groups was not statistically significant (P = .302), with 50.5% of oleogel-S10 patients achieving wound closure, versus 43.9% of those in the control group.
Dr. Cunningham discussed the open-label extension, which involved 205 patients from the double-blind phase (mean age, of 16.3 years) treated with oleogel-S10 or control gel plus standard-of-care nonadhesive wound dressing for 24 months.
In presenting the results of the first 12 months of the open-label extension, she said that oleogel-S10 led to “consistent” reductions in the body surface area percentage (BSAP) affected by EB. The overall reduction from baseline was 55% after receiving treatment for 15 months.
Between day 90 and month 12 of the open-label extension, the absolute BSAP was reduced from 7.4% to 5.4% for patients who had received oleogel-S10 from the start of the study. For those who started in the control group and then switched to the oleogel-S10 arm during the open-label extension, the reduction was from 8.3% to 6.4%.
Dr. Cunningham pointed out that a 1% reduction in BSAP equates approximately to the palmar surface of the hand.
Scores on the Epidermolysis Bullosa Disease Activity and Scarring Index (EBDASI) Skin activity subscale indicated that the reductions achieved in the double-blind phase of the trial were maintained.
Among patients who received oleogel-S10 from the start of the trial, EBDASI Skin scores were reduced from 19.6 at baseline to 13.5 at 12 months’ follow-up in the open-label extension. The reduction was from 19.6 to 13.5 for those who began the trial taking control gel.
Dr. Cunningham showed that adverse events of any grade were seen in 72.0% of patients who began taking oleogel-S10 at the start of the trial and in 69.5% of those who began the trial taking control gel.
Serious adverse events were recorded in 23.0% and 20.0% of patients, respectively, while 6.0% of those who initially received oleogel-S10 and 6.7% of those initially assigned to control gel experienced adverse events that led to study withdrawal during the open-label phase.
The most frequently reported adverse events in the open-label extension were wound complications, seen in 39.5% of patients; anemia, seen in 14.1%; wound infection, seen in 9.3%; pyrexia, seen in 8.3%; and pruritus, seen in 5.9%. No more details regarding adverse events were provided.
The study was funded by Amryt Pharmaceuticals DAC. Dr. Cunningham is an employee of Amryt Pharmaceuticals. No other relevant financial relationships have been disclosed.
A version of this article first appeared on Medscape.com.
GLASGOW, Scotland – the phase 3 safety and efficacy study of the treatment.
Over 200 patients from the trial, including 105 who began treatment with a control gel, continued taking oleogel-S10 after 90 days. The current interim analysis at 12 months indicates there was a 55% reduction in the proportion of the body affected, compared with baseline.
Moreover, reductions in skin activity scores seen in the double-blind phase of the trial were maintained during the open-label extension. About 6% of patients experienced adverse events that led to withdrawal from the study.
The results show that oleogel-S10 was associated with “accelerated wound healing,” said study presenter Tracey Cunningham, MD, chief medical officer, Amryt Pharmaceuticals DAC, Dublin, which is developing the topical agent. “There were no new safety signals with this longer exposure to oleogel-S10, and patients had sustained improvement in wound burden,” she added.
The research was presented at the British Association of Dermatologists (BAD) 2022 Annual Meeting on July 6.
In April, European Medicines Agency recommended approval of oleogel-S10 for the treatment of partial-thickness skin wounds associated with dystrophic and junctional EB for patients aged 6 months and older.
However, just a month earlier, the U.S. Food and Drug Administration declined to approve the topical agent for use in EB, even after it extended its review by 3 months to include additional analyses of data previously submitted by the company.
In the post-presentation discussion, Dr. Cunningham said that the FDA had “not been satisfied at this point with the information that we have given them,” adding, “We don’t agree with the decision, and we will be appealing.”
Raman K. Madan, MD, a dermatologist at Northwell Health, Huntington, New York, who was not involved in the study, said that the reductions in wound healing seen in the study are “meaningful” and that the numbers represent a “big breakthrough.”
He told this news organization that there are “very few products on the market” for EB and that having an option for patients “would be amazing.”
“The big issue here would be cost and coverage for patients,” he said. If approved, “hopefully” it will be affordable, he added.
Dr. Madan noted that from his perspective, the majority of the reactions to the topical gel were “mild,” and there are “a lot of confounding factors” underlying the number of serious adverse events. “These patients with epidermolysis are prone to some of these issues regardless of treatment,” he said.
During her presentation, Dr. Cunningham noted that EB is a rare, debilitating condition that is characterized by varying degrees of skin fragility, blisters, and impaired wound healing that in turn lead to serious complications that affect quality of life.
While wound management is a “fundamental priority” for patients living with EB, she said, there is a “high, unmet” clinical need.
To those ends, EASE was the largest randomized controlled phase 3 efficacy and safety study in EB. In the study, 252 patients were allocated to receive oleogel-S10 or control gel plus standard-of-care nonadhesive wound dressing.
The double-blind phase of the trial met its primary endpoint: A higher proportion of patients who were given oleogel-S10 achieved first complete closure of the EB target wound by day 45, compared with patients who were given control gel, at 41.3% versus 28.9%. This equated to a relative risk of wound closure by day 45 of 1.44, or an odds ratio of 1.84 (P = .013).
However, as reported at the time by this news organization, the difference in time to wound healing by day 90 between the two patient groups was not statistically significant (P = .302), with 50.5% of oleogel-S10 patients achieving wound closure, versus 43.9% of those in the control group.
Dr. Cunningham discussed the open-label extension, which involved 205 patients from the double-blind phase (mean age, of 16.3 years) treated with oleogel-S10 or control gel plus standard-of-care nonadhesive wound dressing for 24 months.
In presenting the results of the first 12 months of the open-label extension, she said that oleogel-S10 led to “consistent” reductions in the body surface area percentage (BSAP) affected by EB. The overall reduction from baseline was 55% after receiving treatment for 15 months.
Between day 90 and month 12 of the open-label extension, the absolute BSAP was reduced from 7.4% to 5.4% for patients who had received oleogel-S10 from the start of the study. For those who started in the control group and then switched to the oleogel-S10 arm during the open-label extension, the reduction was from 8.3% to 6.4%.
Dr. Cunningham pointed out that a 1% reduction in BSAP equates approximately to the palmar surface of the hand.
Scores on the Epidermolysis Bullosa Disease Activity and Scarring Index (EBDASI) Skin activity subscale indicated that the reductions achieved in the double-blind phase of the trial were maintained.
Among patients who received oleogel-S10 from the start of the trial, EBDASI Skin scores were reduced from 19.6 at baseline to 13.5 at 12 months’ follow-up in the open-label extension. The reduction was from 19.6 to 13.5 for those who began the trial taking control gel.
Dr. Cunningham showed that adverse events of any grade were seen in 72.0% of patients who began taking oleogel-S10 at the start of the trial and in 69.5% of those who began the trial taking control gel.
Serious adverse events were recorded in 23.0% and 20.0% of patients, respectively, while 6.0% of those who initially received oleogel-S10 and 6.7% of those initially assigned to control gel experienced adverse events that led to study withdrawal during the open-label phase.
The most frequently reported adverse events in the open-label extension were wound complications, seen in 39.5% of patients; anemia, seen in 14.1%; wound infection, seen in 9.3%; pyrexia, seen in 8.3%; and pruritus, seen in 5.9%. No more details regarding adverse events were provided.
The study was funded by Amryt Pharmaceuticals DAC. Dr. Cunningham is an employee of Amryt Pharmaceuticals. No other relevant financial relationships have been disclosed.
A version of this article first appeared on Medscape.com.
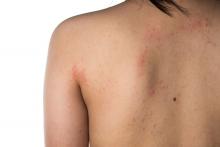
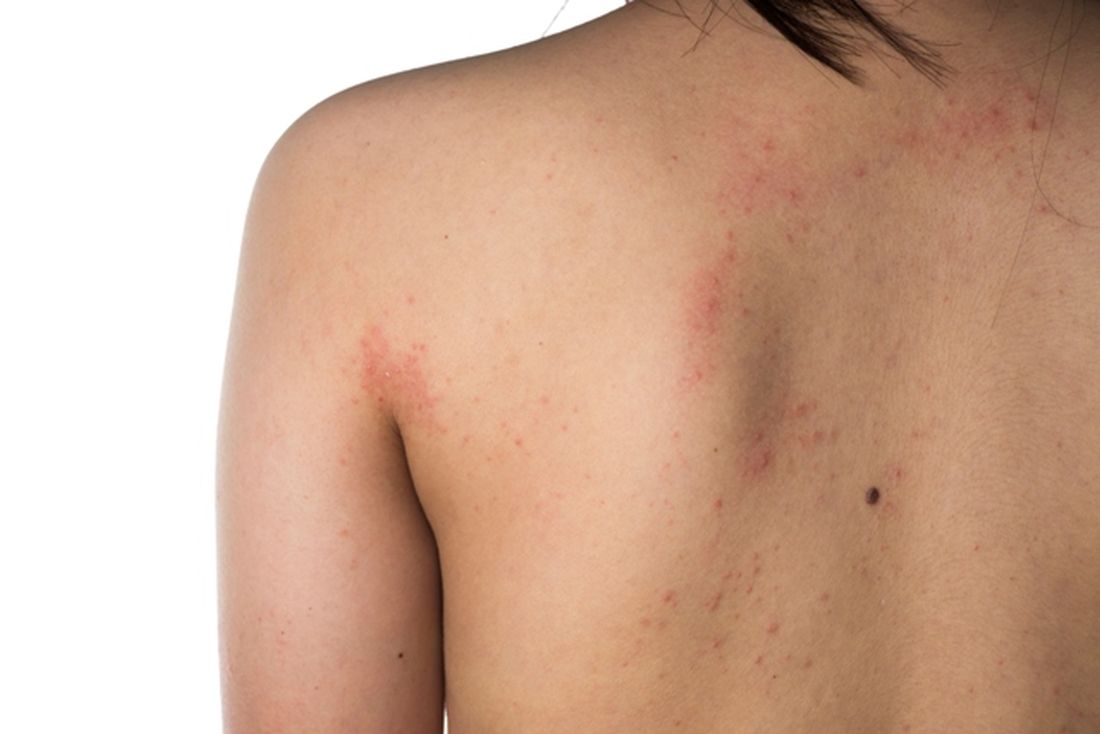
Eczema causes substantial burden for many infants and preschoolers
INDIANAPOLIS – . Those are key findings from a large international web-based survey that was presented during a poster session at the annual meeting of the Society for Pediatric Dermatology.
“Improved knowledge of the AD-related burden may help reinforce the medical need in the pediatric population and contribute to better and earlier adequate management of the disease,” authors led by Stephan Weidinger , MD, PhD, vice head of the department of dermatology at University Hospital Schleswig-Holstein, Kiel, Germany, wrote in the abstract.
For the study, Dr. Weidinger and colleagues evaluated 1,486 infants and preschoolers with AD aged 6 months to under 6 years, who participated in the Epidemiology of Children with Atopic Dermatitis Reporting on their Experience (EPI-CARE), an international, cross-sectional, web-based survey of children and adolescents. The study population resided in 18 countries from five regions of the world, including North America, Latin America, Europe, Middle East/Eurasia, and East Asia. Parents or guardians answered all questions for infants/preschoolers younger than 4 years of age, while preschoolers aged 4 to younger than 6 years were asked to answer questions related to the impact of AD on their health-related quality of life.
AD severity was assessed using Patient Global Assessment (PtGA), where parents or guardians described their child’s eczema severity over the last week as mild, moderate, or severe. The researchers stratified outcomes by geographic region and AD severity, which included the following atopic comorbidities: worst itch, worst skin pain, and overall sleep disturbance in the past 24 hours as measured by the 0-10 numeric rating scale, where higher scores indicate worse severity; eczema-related hospitalization in the past 12 months; and frequency and average duration of flares over the past month.
The mean age of the study participants was 3 years and 61.6% had mild disease. The most common atopic comorbidities were hay fever, asthma, and seasonal allergies, and the incidence of atopic comorbidities increased with increasing AD severity. One or more atopic comorbidities was reported in 88.3% of patients with mild AD, compared with 92.1% of those with moderate disease and 95.8% of those with severe disease. In addition, infants and preschoolers with moderate or severe AD had worse itch, skin pain, and sleep disturbances over the past 24 hours, compared with those who had mild AD.
More than half of infants and preschoolers with severe AD (54.1%) were reported to have been hospitalized in the past 12 months (this ranged from 30.2% to 71.3% across regions), as did 35% of patients with moderate AD and 32.1% of those with mild AD. In addition, 50.6% of infants and preschoolers with severe AD had more than two flares in the past month, compared with 18.1% of those with moderate AD and 6.3% of those with mild disease.
In other findings, 50.7% of infants and preschoolers with severe AD had flares than lasted an average of 2 or more weeks, compared with 20.8% of those with moderate disease and 10% of those with mild disease. Also, 78.3% of preschoolers aged 4 to less than 6 years had missed one or more days of school in the previous 4 weeks: a mean of 5.1 days among those with mild AD, a mean of 7.3 days among those with moderate AD, and a mean of 12.1 days among those with severe disease.
Raj J. Chovatiya MD, PhD, of the department of dermatology at Northwestern University, Chicago, who was asked to comment on the study, said that infants and preschoolers remain an understudied group despite the high prevalence of AD in this age range. “The results of this study demonstrate a substantial burden of disease in this population, particularly among those with more severe disease,” said Dr. Chovatiya, who also directs the university’s Center for Eczema and Itch. “This includes longer and more frequent AD flares as well as high rates of inpatient hospitalization. These findings suggest that additional research is needed to better characterize disease burden and optimize outcomes for young children with AD.”
The study was funded by Regeneron Pharmaceuticals and Sanofi. Dr. Weidinger and other coauthors reported having received institutional research grants and consulting fees from many pharmaceutical companies that manufacture drugs used for the treatment of psoriasis and eczema.
Dr. Chovatiya disclosed that he has served as an advisory board member, consultant, speaker, and/or investigator for AbbVie, Arcutis, Arena, Beiersdorf, Bristol Myers Squibb, Dermavant, Eli Lilly, EPI Health, Incyte, L’Oréal, the National Eczema Association, Pfizer, Regeneron, Sanofi, and UCB.
INDIANAPOLIS – . Those are key findings from a large international web-based survey that was presented during a poster session at the annual meeting of the Society for Pediatric Dermatology.
“Improved knowledge of the AD-related burden may help reinforce the medical need in the pediatric population and contribute to better and earlier adequate management of the disease,” authors led by Stephan Weidinger , MD, PhD, vice head of the department of dermatology at University Hospital Schleswig-Holstein, Kiel, Germany, wrote in the abstract.
For the study, Dr. Weidinger and colleagues evaluated 1,486 infants and preschoolers with AD aged 6 months to under 6 years, who participated in the Epidemiology of Children with Atopic Dermatitis Reporting on their Experience (EPI-CARE), an international, cross-sectional, web-based survey of children and adolescents. The study population resided in 18 countries from five regions of the world, including North America, Latin America, Europe, Middle East/Eurasia, and East Asia. Parents or guardians answered all questions for infants/preschoolers younger than 4 years of age, while preschoolers aged 4 to younger than 6 years were asked to answer questions related to the impact of AD on their health-related quality of life.
AD severity was assessed using Patient Global Assessment (PtGA), where parents or guardians described their child’s eczema severity over the last week as mild, moderate, or severe. The researchers stratified outcomes by geographic region and AD severity, which included the following atopic comorbidities: worst itch, worst skin pain, and overall sleep disturbance in the past 24 hours as measured by the 0-10 numeric rating scale, where higher scores indicate worse severity; eczema-related hospitalization in the past 12 months; and frequency and average duration of flares over the past month.
The mean age of the study participants was 3 years and 61.6% had mild disease. The most common atopic comorbidities were hay fever, asthma, and seasonal allergies, and the incidence of atopic comorbidities increased with increasing AD severity. One or more atopic comorbidities was reported in 88.3% of patients with mild AD, compared with 92.1% of those with moderate disease and 95.8% of those with severe disease. In addition, infants and preschoolers with moderate or severe AD had worse itch, skin pain, and sleep disturbances over the past 24 hours, compared with those who had mild AD.
More than half of infants and preschoolers with severe AD (54.1%) were reported to have been hospitalized in the past 12 months (this ranged from 30.2% to 71.3% across regions), as did 35% of patients with moderate AD and 32.1% of those with mild AD. In addition, 50.6% of infants and preschoolers with severe AD had more than two flares in the past month, compared with 18.1% of those with moderate AD and 6.3% of those with mild disease.
In other findings, 50.7% of infants and preschoolers with severe AD had flares than lasted an average of 2 or more weeks, compared with 20.8% of those with moderate disease and 10% of those with mild disease. Also, 78.3% of preschoolers aged 4 to less than 6 years had missed one or more days of school in the previous 4 weeks: a mean of 5.1 days among those with mild AD, a mean of 7.3 days among those with moderate AD, and a mean of 12.1 days among those with severe disease.
Raj J. Chovatiya MD, PhD, of the department of dermatology at Northwestern University, Chicago, who was asked to comment on the study, said that infants and preschoolers remain an understudied group despite the high prevalence of AD in this age range. “The results of this study demonstrate a substantial burden of disease in this population, particularly among those with more severe disease,” said Dr. Chovatiya, who also directs the university’s Center for Eczema and Itch. “This includes longer and more frequent AD flares as well as high rates of inpatient hospitalization. These findings suggest that additional research is needed to better characterize disease burden and optimize outcomes for young children with AD.”
The study was funded by Regeneron Pharmaceuticals and Sanofi. Dr. Weidinger and other coauthors reported having received institutional research grants and consulting fees from many pharmaceutical companies that manufacture drugs used for the treatment of psoriasis and eczema.
Dr. Chovatiya disclosed that he has served as an advisory board member, consultant, speaker, and/or investigator for AbbVie, Arcutis, Arena, Beiersdorf, Bristol Myers Squibb, Dermavant, Eli Lilly, EPI Health, Incyte, L’Oréal, the National Eczema Association, Pfizer, Regeneron, Sanofi, and UCB.
INDIANAPOLIS – . Those are key findings from a large international web-based survey that was presented during a poster session at the annual meeting of the Society for Pediatric Dermatology.
“Improved knowledge of the AD-related burden may help reinforce the medical need in the pediatric population and contribute to better and earlier adequate management of the disease,” authors led by Stephan Weidinger , MD, PhD, vice head of the department of dermatology at University Hospital Schleswig-Holstein, Kiel, Germany, wrote in the abstract.
For the study, Dr. Weidinger and colleagues evaluated 1,486 infants and preschoolers with AD aged 6 months to under 6 years, who participated in the Epidemiology of Children with Atopic Dermatitis Reporting on their Experience (EPI-CARE), an international, cross-sectional, web-based survey of children and adolescents. The study population resided in 18 countries from five regions of the world, including North America, Latin America, Europe, Middle East/Eurasia, and East Asia. Parents or guardians answered all questions for infants/preschoolers younger than 4 years of age, while preschoolers aged 4 to younger than 6 years were asked to answer questions related to the impact of AD on their health-related quality of life.
AD severity was assessed using Patient Global Assessment (PtGA), where parents or guardians described their child’s eczema severity over the last week as mild, moderate, or severe. The researchers stratified outcomes by geographic region and AD severity, which included the following atopic comorbidities: worst itch, worst skin pain, and overall sleep disturbance in the past 24 hours as measured by the 0-10 numeric rating scale, where higher scores indicate worse severity; eczema-related hospitalization in the past 12 months; and frequency and average duration of flares over the past month.
The mean age of the study participants was 3 years and 61.6% had mild disease. The most common atopic comorbidities were hay fever, asthma, and seasonal allergies, and the incidence of atopic comorbidities increased with increasing AD severity. One or more atopic comorbidities was reported in 88.3% of patients with mild AD, compared with 92.1% of those with moderate disease and 95.8% of those with severe disease. In addition, infants and preschoolers with moderate or severe AD had worse itch, skin pain, and sleep disturbances over the past 24 hours, compared with those who had mild AD.
More than half of infants and preschoolers with severe AD (54.1%) were reported to have been hospitalized in the past 12 months (this ranged from 30.2% to 71.3% across regions), as did 35% of patients with moderate AD and 32.1% of those with mild AD. In addition, 50.6% of infants and preschoolers with severe AD had more than two flares in the past month, compared with 18.1% of those with moderate AD and 6.3% of those with mild disease.
In other findings, 50.7% of infants and preschoolers with severe AD had flares than lasted an average of 2 or more weeks, compared with 20.8% of those with moderate disease and 10% of those with mild disease. Also, 78.3% of preschoolers aged 4 to less than 6 years had missed one or more days of school in the previous 4 weeks: a mean of 5.1 days among those with mild AD, a mean of 7.3 days among those with moderate AD, and a mean of 12.1 days among those with severe disease.
Raj J. Chovatiya MD, PhD, of the department of dermatology at Northwestern University, Chicago, who was asked to comment on the study, said that infants and preschoolers remain an understudied group despite the high prevalence of AD in this age range. “The results of this study demonstrate a substantial burden of disease in this population, particularly among those with more severe disease,” said Dr. Chovatiya, who also directs the university’s Center for Eczema and Itch. “This includes longer and more frequent AD flares as well as high rates of inpatient hospitalization. These findings suggest that additional research is needed to better characterize disease burden and optimize outcomes for young children with AD.”
The study was funded by Regeneron Pharmaceuticals and Sanofi. Dr. Weidinger and other coauthors reported having received institutional research grants and consulting fees from many pharmaceutical companies that manufacture drugs used for the treatment of psoriasis and eczema.
Dr. Chovatiya disclosed that he has served as an advisory board member, consultant, speaker, and/or investigator for AbbVie, Arcutis, Arena, Beiersdorf, Bristol Myers Squibb, Dermavant, Eli Lilly, EPI Health, Incyte, L’Oréal, the National Eczema Association, Pfizer, Regeneron, Sanofi, and UCB.
AT SPD 2022
European survey finds wide variations in the use of phototherapy for atopic eczema
GLASGOW, Scotland – , which points to the need for management guidelines.
Over 140 phototherapy practitioners from 27 European countries responded to the survey. Of the practitioners surveyed, 96% used narrow-band ultraviolet B (NB-UVB), and about 50% prescribed psoralen and ultraviolet A (PUVA) for adults. Fewer than 10% did so for children.
There was considerable variation in prescribing practices, “especially when it comes to dosing and treatment duration,” said study presenter Mia Steyn, MD, dermatology registrar, St. John’s Institute of Dermatology, Guy’s and St. Thomas’s Hospital, London.
These results, she said, demonstrate that “an optimal treatment modality either is not known or agreed upon” and that studies are required to determine treatment efficacy, cost, and safety “in a range of skin types.”
Dr. Steyn said that what is needed first is a set of consensus treatment guidelines, “hopefully leading to a randomized controlled trial” that would compare the various treatment options.
The research was presented at the British Association of Dermatologists (BAD) 2022 Annual Meeting on July 7.
Session co-chair Adam Fityan, MD, a consultant dermatologist at University Hospital Southampton NHS Foundation Trust, U.K., commented that the study was “fascinating” and “really helpful.”
Dr. Fityan, who was not involved with the survey, told this news organization that, “clearly, what we’ve seen is that there is a huge variation in the way everyone uses the different modalities of phototherapy.”
“Having that sort of knowledge will hopefully help us to think a bit more clearly about the regimens and protocols that we use and to maybe find the evidence that everyone needs to have the most effective protocol.”
The data from the study are also useful on an individual level, Dr. Fityan continued, as “you have no idea what anyone is doing” and whether “you are an outlier.”
Dr. Steyn said that phototherapy is commonly used for the treatment of atopic eczema, but the evidence for its efficacy, its impact on quality on life, its cost-effectiveness, and short- and long-term safety is “weak,” particularly in relation to real-life use.
Electronic survey
In lieu of a well-designed randomized controlled trial to answer these questions, the researchers set up a task force to assess how phototherapy is currently being used to treat atopic eczema across the United Kingdom and Europe so as to guide further research.
An electronic survey was devised, and 144 members of phototherapy groups from 27 European countries submitted their responses during 2020. Most responses came from the Netherlands (20), Italy (16), the United Kingdom (14), France (11), and Germany (10).
The results showed that NB-UVB was the most widely used modality of phototherapy, chosen by 96% of respondents. In addition, 17% of respondents said they also prescribed home-based NB-UVB, which was available in eight of the 27 countries.
When asked how they used NB-UVB, the majority (68%) of respondents said they had an age cutoff for use in children, which was set at an average age of 9 years and older, although the range was age 2 years to 16 years.
NBUVB was used as a second-line therapy instead of systemic treatments in up to 93% of adults and in 69% of children. It was used concomitantly with systemic treatment in up to 58% of adults and 11% of children, according to the survey responses.
For about 70% of respondents, the use of NB-UVB was determined by assessing the Fitzpatrick skin type, although almost 40% relied on clinical experience.
Frequency of treatment
NB-UVB was prescribed three times a week by 59% of respondents; 31% of respondents prescribed it twice a week; 7%, five times per week; and 2%, four times a week. The typical number of treatments was 21-30 for 53% of respondents, 0-20 treatments for 24%, and 31-40 treatments for 20%.
The dose was typically increased in 10% increments, although there were wide variations in how the treatment was stepped up. Dose was increased after each treatment by almost 50% of respondents, after every two treatments by almost 25%, and after every three treatments by approximately 15%.
For the majority (53%) of respondents, response to NBUVB was assessed after 7-15 treatments, while 43% waited until after 16-30 treatments. Success was defined as a 75% reduction in eczema from baseline by 56% of respondents, while 54% looked to patient satisfaction, and 47% relied on quality of life to determine success of treatment.
Maintenance NB-UVB was never used by 54% of respondents, but 44% said they used it occasionally, and 83% said they did not follow a weaning schedule at the end of treatment.
The most commonly reported adverse effects of NB-UVB were significant erythema, hyperpigmentation, and eczema flare, while the most commonly cited absolute contraindications included a history of melanoma, a history of squamous cell carcinoma, the use of photosensitizing medications, and claustrophobia.
Use of PUVA, UVA1
The next most commonly used phototherapy for atopic eczema was PUVA. Although it was available to 83% of respondents, only 52% of respondents had personally prescribed the treatment for adults, and only 7% prescribed it for children.
Of the respondents, 71% said they would switch from NB-UVB to PUVA if desired treatment outcomes were not achieved with the former, and 44% said they would “sometimes consider” PUVA as second-line therapy instead of systemic treatments. Only 13% said they would use it concomitantly with systemic treatment.
Ultraviolet A1 (UVA1) phototherapy was not widely available, with 66% of respondents declaring that they did not have access to this option and just 29% saying they prescribed it.
But when it was used, UVA1 was cited as being used often in adults by 24% of respondents, while 33% used it was used sometimes, and 43% said it was used rarely. It was used for children by 26% of respondents. In addition, 29% said they favored using UVA1 for chronic atopic eczema, and 33% favored using it for acute eczema while 38% had no preference over whether to use it for chronic versus acute atopic eczema.
Similarly to NB-UVB, there were wide variations in the use of PUVA and UVA1 by respondents in terms of dosing schedules, duration of treatment, and how response to treatment was measured.
No funding for the study has been reported. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
GLASGOW, Scotland – , which points to the need for management guidelines.
Over 140 phototherapy practitioners from 27 European countries responded to the survey. Of the practitioners surveyed, 96% used narrow-band ultraviolet B (NB-UVB), and about 50% prescribed psoralen and ultraviolet A (PUVA) for adults. Fewer than 10% did so for children.
There was considerable variation in prescribing practices, “especially when it comes to dosing and treatment duration,” said study presenter Mia Steyn, MD, dermatology registrar, St. John’s Institute of Dermatology, Guy’s and St. Thomas’s Hospital, London.
These results, she said, demonstrate that “an optimal treatment modality either is not known or agreed upon” and that studies are required to determine treatment efficacy, cost, and safety “in a range of skin types.”
Dr. Steyn said that what is needed first is a set of consensus treatment guidelines, “hopefully leading to a randomized controlled trial” that would compare the various treatment options.
The research was presented at the British Association of Dermatologists (BAD) 2022 Annual Meeting on July 7.
Session co-chair Adam Fityan, MD, a consultant dermatologist at University Hospital Southampton NHS Foundation Trust, U.K., commented that the study was “fascinating” and “really helpful.”
Dr. Fityan, who was not involved with the survey, told this news organization that, “clearly, what we’ve seen is that there is a huge variation in the way everyone uses the different modalities of phototherapy.”
“Having that sort of knowledge will hopefully help us to think a bit more clearly about the regimens and protocols that we use and to maybe find the evidence that everyone needs to have the most effective protocol.”
The data from the study are also useful on an individual level, Dr. Fityan continued, as “you have no idea what anyone is doing” and whether “you are an outlier.”
Dr. Steyn said that phototherapy is commonly used for the treatment of atopic eczema, but the evidence for its efficacy, its impact on quality on life, its cost-effectiveness, and short- and long-term safety is “weak,” particularly in relation to real-life use.
Electronic survey
In lieu of a well-designed randomized controlled trial to answer these questions, the researchers set up a task force to assess how phototherapy is currently being used to treat atopic eczema across the United Kingdom and Europe so as to guide further research.
An electronic survey was devised, and 144 members of phototherapy groups from 27 European countries submitted their responses during 2020. Most responses came from the Netherlands (20), Italy (16), the United Kingdom (14), France (11), and Germany (10).
The results showed that NB-UVB was the most widely used modality of phototherapy, chosen by 96% of respondents. In addition, 17% of respondents said they also prescribed home-based NB-UVB, which was available in eight of the 27 countries.
When asked how they used NB-UVB, the majority (68%) of respondents said they had an age cutoff for use in children, which was set at an average age of 9 years and older, although the range was age 2 years to 16 years.
NBUVB was used as a second-line therapy instead of systemic treatments in up to 93% of adults and in 69% of children. It was used concomitantly with systemic treatment in up to 58% of adults and 11% of children, according to the survey responses.
For about 70% of respondents, the use of NB-UVB was determined by assessing the Fitzpatrick skin type, although almost 40% relied on clinical experience.
Frequency of treatment
NB-UVB was prescribed three times a week by 59% of respondents; 31% of respondents prescribed it twice a week; 7%, five times per week; and 2%, four times a week. The typical number of treatments was 21-30 for 53% of respondents, 0-20 treatments for 24%, and 31-40 treatments for 20%.
The dose was typically increased in 10% increments, although there were wide variations in how the treatment was stepped up. Dose was increased after each treatment by almost 50% of respondents, after every two treatments by almost 25%, and after every three treatments by approximately 15%.
For the majority (53%) of respondents, response to NBUVB was assessed after 7-15 treatments, while 43% waited until after 16-30 treatments. Success was defined as a 75% reduction in eczema from baseline by 56% of respondents, while 54% looked to patient satisfaction, and 47% relied on quality of life to determine success of treatment.
Maintenance NB-UVB was never used by 54% of respondents, but 44% said they used it occasionally, and 83% said they did not follow a weaning schedule at the end of treatment.
The most commonly reported adverse effects of NB-UVB were significant erythema, hyperpigmentation, and eczema flare, while the most commonly cited absolute contraindications included a history of melanoma, a history of squamous cell carcinoma, the use of photosensitizing medications, and claustrophobia.
Use of PUVA, UVA1
The next most commonly used phototherapy for atopic eczema was PUVA. Although it was available to 83% of respondents, only 52% of respondents had personally prescribed the treatment for adults, and only 7% prescribed it for children.
Of the respondents, 71% said they would switch from NB-UVB to PUVA if desired treatment outcomes were not achieved with the former, and 44% said they would “sometimes consider” PUVA as second-line therapy instead of systemic treatments. Only 13% said they would use it concomitantly with systemic treatment.
Ultraviolet A1 (UVA1) phototherapy was not widely available, with 66% of respondents declaring that they did not have access to this option and just 29% saying they prescribed it.
But when it was used, UVA1 was cited as being used often in adults by 24% of respondents, while 33% used it was used sometimes, and 43% said it was used rarely. It was used for children by 26% of respondents. In addition, 29% said they favored using UVA1 for chronic atopic eczema, and 33% favored using it for acute eczema while 38% had no preference over whether to use it for chronic versus acute atopic eczema.
Similarly to NB-UVB, there were wide variations in the use of PUVA and UVA1 by respondents in terms of dosing schedules, duration of treatment, and how response to treatment was measured.
No funding for the study has been reported. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
GLASGOW, Scotland – , which points to the need for management guidelines.
Over 140 phototherapy practitioners from 27 European countries responded to the survey. Of the practitioners surveyed, 96% used narrow-band ultraviolet B (NB-UVB), and about 50% prescribed psoralen and ultraviolet A (PUVA) for adults. Fewer than 10% did so for children.
There was considerable variation in prescribing practices, “especially when it comes to dosing and treatment duration,” said study presenter Mia Steyn, MD, dermatology registrar, St. John’s Institute of Dermatology, Guy’s and St. Thomas’s Hospital, London.
These results, she said, demonstrate that “an optimal treatment modality either is not known or agreed upon” and that studies are required to determine treatment efficacy, cost, and safety “in a range of skin types.”
Dr. Steyn said that what is needed first is a set of consensus treatment guidelines, “hopefully leading to a randomized controlled trial” that would compare the various treatment options.
The research was presented at the British Association of Dermatologists (BAD) 2022 Annual Meeting on July 7.
Session co-chair Adam Fityan, MD, a consultant dermatologist at University Hospital Southampton NHS Foundation Trust, U.K., commented that the study was “fascinating” and “really helpful.”
Dr. Fityan, who was not involved with the survey, told this news organization that, “clearly, what we’ve seen is that there is a huge variation in the way everyone uses the different modalities of phototherapy.”
“Having that sort of knowledge will hopefully help us to think a bit more clearly about the regimens and protocols that we use and to maybe find the evidence that everyone needs to have the most effective protocol.”
The data from the study are also useful on an individual level, Dr. Fityan continued, as “you have no idea what anyone is doing” and whether “you are an outlier.”
Dr. Steyn said that phototherapy is commonly used for the treatment of atopic eczema, but the evidence for its efficacy, its impact on quality on life, its cost-effectiveness, and short- and long-term safety is “weak,” particularly in relation to real-life use.
Electronic survey
In lieu of a well-designed randomized controlled trial to answer these questions, the researchers set up a task force to assess how phototherapy is currently being used to treat atopic eczema across the United Kingdom and Europe so as to guide further research.
An electronic survey was devised, and 144 members of phototherapy groups from 27 European countries submitted their responses during 2020. Most responses came from the Netherlands (20), Italy (16), the United Kingdom (14), France (11), and Germany (10).
The results showed that NB-UVB was the most widely used modality of phototherapy, chosen by 96% of respondents. In addition, 17% of respondents said they also prescribed home-based NB-UVB, which was available in eight of the 27 countries.
When asked how they used NB-UVB, the majority (68%) of respondents said they had an age cutoff for use in children, which was set at an average age of 9 years and older, although the range was age 2 years to 16 years.
NBUVB was used as a second-line therapy instead of systemic treatments in up to 93% of adults and in 69% of children. It was used concomitantly with systemic treatment in up to 58% of adults and 11% of children, according to the survey responses.
For about 70% of respondents, the use of NB-UVB was determined by assessing the Fitzpatrick skin type, although almost 40% relied on clinical experience.
Frequency of treatment
NB-UVB was prescribed three times a week by 59% of respondents; 31% of respondents prescribed it twice a week; 7%, five times per week; and 2%, four times a week. The typical number of treatments was 21-30 for 53% of respondents, 0-20 treatments for 24%, and 31-40 treatments for 20%.
The dose was typically increased in 10% increments, although there were wide variations in how the treatment was stepped up. Dose was increased after each treatment by almost 50% of respondents, after every two treatments by almost 25%, and after every three treatments by approximately 15%.
For the majority (53%) of respondents, response to NBUVB was assessed after 7-15 treatments, while 43% waited until after 16-30 treatments. Success was defined as a 75% reduction in eczema from baseline by 56% of respondents, while 54% looked to patient satisfaction, and 47% relied on quality of life to determine success of treatment.
Maintenance NB-UVB was never used by 54% of respondents, but 44% said they used it occasionally, and 83% said they did not follow a weaning schedule at the end of treatment.
The most commonly reported adverse effects of NB-UVB were significant erythema, hyperpigmentation, and eczema flare, while the most commonly cited absolute contraindications included a history of melanoma, a history of squamous cell carcinoma, the use of photosensitizing medications, and claustrophobia.
Use of PUVA, UVA1
The next most commonly used phototherapy for atopic eczema was PUVA. Although it was available to 83% of respondents, only 52% of respondents had personally prescribed the treatment for adults, and only 7% prescribed it for children.
Of the respondents, 71% said they would switch from NB-UVB to PUVA if desired treatment outcomes were not achieved with the former, and 44% said they would “sometimes consider” PUVA as second-line therapy instead of systemic treatments. Only 13% said they would use it concomitantly with systemic treatment.
Ultraviolet A1 (UVA1) phototherapy was not widely available, with 66% of respondents declaring that they did not have access to this option and just 29% saying they prescribed it.
But when it was used, UVA1 was cited as being used often in adults by 24% of respondents, while 33% used it was used sometimes, and 43% said it was used rarely. It was used for children by 26% of respondents. In addition, 29% said they favored using UVA1 for chronic atopic eczema, and 33% favored using it for acute eczema while 38% had no preference over whether to use it for chronic versus acute atopic eczema.
Similarly to NB-UVB, there were wide variations in the use of PUVA and UVA1 by respondents in terms of dosing schedules, duration of treatment, and how response to treatment was measured.
No funding for the study has been reported. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Zoster vaccination does not appear to increase flare risk in patients with immune-mediated inflammatory disease
, according to research published in Arthritis & Rheumatology.
The authors of the study noted that individuals with IMIDs are at increased risk for herpes zoster and related complications, including postherpetic neuralgia, and that vaccination has been recommended for certain groups of patients with rheumatoid arthritis, inflammatory bowel disease, and psoriasis, by the American College of Rheumatology and other professional organizations for individuals aged 50 and older.
The study investigators used medical claims from IBM MarketScan, which provided data on patients aged 50-64 years, and data from the Centers for Medicare and Medicaid Services’ Medicare on patients aged 65 and older.
They defined presumed flares in three ways: hospitalization/emergency department visits for IMIDs, steroid treatment with a short-acting oral glucocorticoid, or treatment with a parenteral glucocorticoid injection. The investigators conducted a self-controlled case series (SCCS) analysis to examine any temporal link between the RZV and disease flares.
Among enrollees with IMIDs, 14.8% of the 55,654 patients in the MarketScan database and 43.2% of the 160,545 patients in the Medicare database received at least one dose of RZV during 2018-2019. The two-dose series completion within 6 months was 76.6% in the MarketScan group (age range, 50-64 years) and 85.4% among Medicare enrollees (age range, 65 years and older). In the SCCS analysis, 10% and 13% of patients developed flares in the control group as compared to 9%, and 11%-12% in the risk window following one or two doses of RZV among MarketScan and Medicare enrollees, respectively.
Based on these findings, the investigators concluded there was no statistically significant increase in flares subsequent to RZV administration for any IMID in either patients aged 50-64 years or patients aged 65 years and older following the first dose or second dose.
Nilanjana Bose, MD, a rheumatologist with Lonestar Rheumatology, Houston, Texas, who was not involved with the study, said that the research addresses a topic where there is uneasiness, namely vaccination in patients with IMIDs.
“Anytime you are vaccinating a patient with an autoimmune disease, especially one on a biologic, you always worry about the risk of flares,” said Dr. Bose. “Any time you tamper with the immune system, there is a risk of flares.”
The study serves as a clarification for the primary care setting, said Dr. Bose. “A lot of the time, the shingles vaccine is administered not by rheumatology but by primary care or through the pharmacy,” she said. “This study puts them [primary care physicians] at ease.”
Findings from the study reflect that most RZV vaccinations were administered in pharmacies.
One of the weaknesses of the study is that the investigators did not include patients younger than 50 years old, said Dr. Bose. “It would have been nice if they could have looked at younger patients,” she said. “We try to vaccinate all our [immunocompromised] adult patients, even the younger ones, because they are also at risk for shingles.”
Given that there are increasing options of medical therapies in rheumatology that are immunomodulatory, the subject of vaccination for patients is often one of discussion, added Dr. Bose.
Arthur Kavanaugh, MD, professor of medicine, University of California San Diego (UCSD), La Jolla, Calif., and director of the Center for Innovative Therapy in the UCSD Division of Rheumatology, Allergy, and Immunology, told this news organization that a strength of the study is its large numbers of patients but noted the shortcoming of using claims data. “Claims data has inherent limitations, such as the lack of detailed granular data on the patients,” wrote Dr. Kavanaugh, who was not involved with the study. He described this investigation as “really about the first evidence that I am aware of addressing this issue.”
No funding source was listed. One author disclosed having received research grants and consulting fees received from Pfizer and GSK for unrelated work; the other authors had no disclosures. Dr. Bose and Dr. Kavanaugh had no relevant disclosures.
, according to research published in Arthritis & Rheumatology.
The authors of the study noted that individuals with IMIDs are at increased risk for herpes zoster and related complications, including postherpetic neuralgia, and that vaccination has been recommended for certain groups of patients with rheumatoid arthritis, inflammatory bowel disease, and psoriasis, by the American College of Rheumatology and other professional organizations for individuals aged 50 and older.
The study investigators used medical claims from IBM MarketScan, which provided data on patients aged 50-64 years, and data from the Centers for Medicare and Medicaid Services’ Medicare on patients aged 65 and older.
They defined presumed flares in three ways: hospitalization/emergency department visits for IMIDs, steroid treatment with a short-acting oral glucocorticoid, or treatment with a parenteral glucocorticoid injection. The investigators conducted a self-controlled case series (SCCS) analysis to examine any temporal link between the RZV and disease flares.
Among enrollees with IMIDs, 14.8% of the 55,654 patients in the MarketScan database and 43.2% of the 160,545 patients in the Medicare database received at least one dose of RZV during 2018-2019. The two-dose series completion within 6 months was 76.6% in the MarketScan group (age range, 50-64 years) and 85.4% among Medicare enrollees (age range, 65 years and older). In the SCCS analysis, 10% and 13% of patients developed flares in the control group as compared to 9%, and 11%-12% in the risk window following one or two doses of RZV among MarketScan and Medicare enrollees, respectively.
Based on these findings, the investigators concluded there was no statistically significant increase in flares subsequent to RZV administration for any IMID in either patients aged 50-64 years or patients aged 65 years and older following the first dose or second dose.
Nilanjana Bose, MD, a rheumatologist with Lonestar Rheumatology, Houston, Texas, who was not involved with the study, said that the research addresses a topic where there is uneasiness, namely vaccination in patients with IMIDs.
“Anytime you are vaccinating a patient with an autoimmune disease, especially one on a biologic, you always worry about the risk of flares,” said Dr. Bose. “Any time you tamper with the immune system, there is a risk of flares.”
The study serves as a clarification for the primary care setting, said Dr. Bose. “A lot of the time, the shingles vaccine is administered not by rheumatology but by primary care or through the pharmacy,” she said. “This study puts them [primary care physicians] at ease.”
Findings from the study reflect that most RZV vaccinations were administered in pharmacies.
One of the weaknesses of the study is that the investigators did not include patients younger than 50 years old, said Dr. Bose. “It would have been nice if they could have looked at younger patients,” she said. “We try to vaccinate all our [immunocompromised] adult patients, even the younger ones, because they are also at risk for shingles.”
Given that there are increasing options of medical therapies in rheumatology that are immunomodulatory, the subject of vaccination for patients is often one of discussion, added Dr. Bose.
Arthur Kavanaugh, MD, professor of medicine, University of California San Diego (UCSD), La Jolla, Calif., and director of the Center for Innovative Therapy in the UCSD Division of Rheumatology, Allergy, and Immunology, told this news organization that a strength of the study is its large numbers of patients but noted the shortcoming of using claims data. “Claims data has inherent limitations, such as the lack of detailed granular data on the patients,” wrote Dr. Kavanaugh, who was not involved with the study. He described this investigation as “really about the first evidence that I am aware of addressing this issue.”
No funding source was listed. One author disclosed having received research grants and consulting fees received from Pfizer and GSK for unrelated work; the other authors had no disclosures. Dr. Bose and Dr. Kavanaugh had no relevant disclosures.
, according to research published in Arthritis & Rheumatology.
The authors of the study noted that individuals with IMIDs are at increased risk for herpes zoster and related complications, including postherpetic neuralgia, and that vaccination has been recommended for certain groups of patients with rheumatoid arthritis, inflammatory bowel disease, and psoriasis, by the American College of Rheumatology and other professional organizations for individuals aged 50 and older.
The study investigators used medical claims from IBM MarketScan, which provided data on patients aged 50-64 years, and data from the Centers for Medicare and Medicaid Services’ Medicare on patients aged 65 and older.
They defined presumed flares in three ways: hospitalization/emergency department visits for IMIDs, steroid treatment with a short-acting oral glucocorticoid, or treatment with a parenteral glucocorticoid injection. The investigators conducted a self-controlled case series (SCCS) analysis to examine any temporal link between the RZV and disease flares.
Among enrollees with IMIDs, 14.8% of the 55,654 patients in the MarketScan database and 43.2% of the 160,545 patients in the Medicare database received at least one dose of RZV during 2018-2019. The two-dose series completion within 6 months was 76.6% in the MarketScan group (age range, 50-64 years) and 85.4% among Medicare enrollees (age range, 65 years and older). In the SCCS analysis, 10% and 13% of patients developed flares in the control group as compared to 9%, and 11%-12% in the risk window following one or two doses of RZV among MarketScan and Medicare enrollees, respectively.
Based on these findings, the investigators concluded there was no statistically significant increase in flares subsequent to RZV administration for any IMID in either patients aged 50-64 years or patients aged 65 years and older following the first dose or second dose.
Nilanjana Bose, MD, a rheumatologist with Lonestar Rheumatology, Houston, Texas, who was not involved with the study, said that the research addresses a topic where there is uneasiness, namely vaccination in patients with IMIDs.
“Anytime you are vaccinating a patient with an autoimmune disease, especially one on a biologic, you always worry about the risk of flares,” said Dr. Bose. “Any time you tamper with the immune system, there is a risk of flares.”
The study serves as a clarification for the primary care setting, said Dr. Bose. “A lot of the time, the shingles vaccine is administered not by rheumatology but by primary care or through the pharmacy,” she said. “This study puts them [primary care physicians] at ease.”
Findings from the study reflect that most RZV vaccinations were administered in pharmacies.
One of the weaknesses of the study is that the investigators did not include patients younger than 50 years old, said Dr. Bose. “It would have been nice if they could have looked at younger patients,” she said. “We try to vaccinate all our [immunocompromised] adult patients, even the younger ones, because they are also at risk for shingles.”
Given that there are increasing options of medical therapies in rheumatology that are immunomodulatory, the subject of vaccination for patients is often one of discussion, added Dr. Bose.
Arthur Kavanaugh, MD, professor of medicine, University of California San Diego (UCSD), La Jolla, Calif., and director of the Center for Innovative Therapy in the UCSD Division of Rheumatology, Allergy, and Immunology, told this news organization that a strength of the study is its large numbers of patients but noted the shortcoming of using claims data. “Claims data has inherent limitations, such as the lack of detailed granular data on the patients,” wrote Dr. Kavanaugh, who was not involved with the study. He described this investigation as “really about the first evidence that I am aware of addressing this issue.”
No funding source was listed. One author disclosed having received research grants and consulting fees received from Pfizer and GSK for unrelated work; the other authors had no disclosures. Dr. Bose and Dr. Kavanaugh had no relevant disclosures.
Large study reaffirms rare risk of TNF inhibitor–induced psoriasis in patients with RA, IBD
according to a new study published in JAMA Dermatology.
Despite this finding, the authors of the large Danish nationwide cohort study noted that TNFi-induced psoriasis is still a rare adverse event. “Practitioners and patients should be aware and observant of the potential for TNFi-associated psoriasis during TNFi treatment but keep in mind that the absolute risk appears to be low,” David Thein, MB, of the department of dermatology at Bispebjerg Hospital, University of Copenhagen, and colleagues wrote in the study.
They analyzed 109,085 patients with RA and IBD enrolled in Danish national registries between 1995 and 2018 without a previous diagnosis of psoriasis, who received either TNFi (20,910 patients) or conventional treatments (108,024 patients) and were followed for 5 years. They were a mean of 50 years old when they started treatment, 62% were women, with 87.8% of patients in the TNFi group receiving prior conventional therapy and 1% of patients in the conventional therapy group receiving prior TNFi treatment.
The investigators assessed the risk of developing any psoriasis, nonpustular psoriasis, and pustular psoriasis in the two groups using ICD-10 codes as well as a record of two consecutive prescriptions for topical vitamin D analogs.
Overall, 1,471 patients (1.4%) developed psoriasis of any type; 1,332 had non-pustular psoriasis, 127 had palmoplantar pustulosis, and 12 had generalized pustulosis.
The incidence rate of developing any psoriasis was 3.0 per 1,000 patient-years (95% confidence interval, 2.9-3.2) for patients receiving conventional therapy and 7.8 per 1,000 patient-years (95% CI, 7.5-8.9) for patients receiving TNFi treatment. Compared with conventional treatment, the risk of developing nonpustular psoriasis was twofold higher among patients receiving TNFi treatment (hazard ratio, 2.12; 95% CI, 1.87-2.40; P < .001). The risk of developing pustular psoriasis was more than sixfold higher among those on a TNFi (HR, 6.50; 95% CI, 4.60-9.23; P < .001).
Dr. Thein and colleagues estimated that the exposure needed to harm 1 additional patient was 241 patient-years for any psoriasis type, 342 patient-years for nonpustular psoriasis, and 909 patient-years for pustular psoriasis, with an estimated absolute risk difference of 5 per 1,000 patient-years.
Best evidence to date on risk
Asked to comment on the study findings, Anthony Fernandez, MD, PhD, director of medical dermatology at the Cleveland Clinic, said that he applauded the researchers for performing this well-designed study to determine the risk of TNF inhibitor–induced psoriasis in patients with RA and IBD.
The strengths of the study include excluding patients with a history of psoriasis to rule out disease recurrence and having a large comparator group of patients with IBD and RA who were taking medications other than TNF inhibitors, while one limitation was the potential accuracy of the ICD-10 codes used as the basis for diagnosing psoriasis. “It’s probably closer to the truth of what the true risk is compared to studies done in the past,” he said in an interview.
Dr. Fernandez noted that the results aren’t likely to change how dermatologists, rheumatologists, or gastroenterologists practice, but the message to stay the course in initially treating TNFi-induced psoriasis also holds value. “We don’t need to change anything in our clinical practice when it comes to TNF-alpha inhibitors.”
For patients with RA or IBD who develop TNFi-induced psoriasis with disease that is well controlled with TNFi treatment, keeping them on that treatment is a priority, Dr. Fernandez explained. “The first and foremost goal is, if the TNF inhibitor is working very well to control the disease that it was prescribed for, then you exhaust your efforts to try to control the psoriasis and allow those patients to stay on the TNF inhibitor.”
In his experience, most patients with RA and IBD who develop TNFi-induced psoriasis are controlled with topical medications. Switching to another TNFi is not recommended, he noted, as patients are “likely to have that reaction with any TNF inhibitor.”
However, Dr. Fernandez said that won’t be an option for all patients with RA and IBD. “In some patients you do simply have to stop the TNF inhibitor” and try an alternative treatment with a different mechanism of action.
The cause of TNFi-induced psoriasis is still not well understood. “There certainly is evidence to support that interferon alpha production by plasmacytoid dendritic cells is playing some role in this phenomenon,” but there is “more to the story” and unanswered questions remain, Dr. Fernandez said.
What’s most interesting about this phenomenon, he added, is that “patients can develop it at any time when exposed to a TNF inhibitor.” For instance, most patients develop drug reactions within 2-3 weeks of starting a treatment, but TNFi-induced psoriasis can appear after a single dose or several years after initiating treatment.
“Why so few patients, and why is there such variability in terms of how long they’re on the TNF inhibitor before the reaction occurs?” he asked. “That really points to ... some other trigger besides exposure to the TNF inhibitor needed for the initiation of this reaction.”
He noted that it would be valuable to identify triggers – or the most likely triggers – which would be challenging, but could “potentially impact clinical practice.”
The authors reported personal and institutional relationships in the form of personal and institutional research grants, honoraria, personal fees, investigator fees paid to university, consultancies, and speaker’s bureau positions for a variety of pharmaceutical companies, data companies, hospitals, and foundations. Dr. Fernandez reported he has nonbranded speaking, consulting, and research relationships with AbbVie and Novartis; and is a consultant for UCB, Bristol-Myers Squibb, and Boehringer Ingelheim on related products.
according to a new study published in JAMA Dermatology.
Despite this finding, the authors of the large Danish nationwide cohort study noted that TNFi-induced psoriasis is still a rare adverse event. “Practitioners and patients should be aware and observant of the potential for TNFi-associated psoriasis during TNFi treatment but keep in mind that the absolute risk appears to be low,” David Thein, MB, of the department of dermatology at Bispebjerg Hospital, University of Copenhagen, and colleagues wrote in the study.
They analyzed 109,085 patients with RA and IBD enrolled in Danish national registries between 1995 and 2018 without a previous diagnosis of psoriasis, who received either TNFi (20,910 patients) or conventional treatments (108,024 patients) and were followed for 5 years. They were a mean of 50 years old when they started treatment, 62% were women, with 87.8% of patients in the TNFi group receiving prior conventional therapy and 1% of patients in the conventional therapy group receiving prior TNFi treatment.
The investigators assessed the risk of developing any psoriasis, nonpustular psoriasis, and pustular psoriasis in the two groups using ICD-10 codes as well as a record of two consecutive prescriptions for topical vitamin D analogs.
Overall, 1,471 patients (1.4%) developed psoriasis of any type; 1,332 had non-pustular psoriasis, 127 had palmoplantar pustulosis, and 12 had generalized pustulosis.
The incidence rate of developing any psoriasis was 3.0 per 1,000 patient-years (95% confidence interval, 2.9-3.2) for patients receiving conventional therapy and 7.8 per 1,000 patient-years (95% CI, 7.5-8.9) for patients receiving TNFi treatment. Compared with conventional treatment, the risk of developing nonpustular psoriasis was twofold higher among patients receiving TNFi treatment (hazard ratio, 2.12; 95% CI, 1.87-2.40; P < .001). The risk of developing pustular psoriasis was more than sixfold higher among those on a TNFi (HR, 6.50; 95% CI, 4.60-9.23; P < .001).
Dr. Thein and colleagues estimated that the exposure needed to harm 1 additional patient was 241 patient-years for any psoriasis type, 342 patient-years for nonpustular psoriasis, and 909 patient-years for pustular psoriasis, with an estimated absolute risk difference of 5 per 1,000 patient-years.
Best evidence to date on risk
Asked to comment on the study findings, Anthony Fernandez, MD, PhD, director of medical dermatology at the Cleveland Clinic, said that he applauded the researchers for performing this well-designed study to determine the risk of TNF inhibitor–induced psoriasis in patients with RA and IBD.
The strengths of the study include excluding patients with a history of psoriasis to rule out disease recurrence and having a large comparator group of patients with IBD and RA who were taking medications other than TNF inhibitors, while one limitation was the potential accuracy of the ICD-10 codes used as the basis for diagnosing psoriasis. “It’s probably closer to the truth of what the true risk is compared to studies done in the past,” he said in an interview.
Dr. Fernandez noted that the results aren’t likely to change how dermatologists, rheumatologists, or gastroenterologists practice, but the message to stay the course in initially treating TNFi-induced psoriasis also holds value. “We don’t need to change anything in our clinical practice when it comes to TNF-alpha inhibitors.”
For patients with RA or IBD who develop TNFi-induced psoriasis with disease that is well controlled with TNFi treatment, keeping them on that treatment is a priority, Dr. Fernandez explained. “The first and foremost goal is, if the TNF inhibitor is working very well to control the disease that it was prescribed for, then you exhaust your efforts to try to control the psoriasis and allow those patients to stay on the TNF inhibitor.”
In his experience, most patients with RA and IBD who develop TNFi-induced psoriasis are controlled with topical medications. Switching to another TNFi is not recommended, he noted, as patients are “likely to have that reaction with any TNF inhibitor.”
However, Dr. Fernandez said that won’t be an option for all patients with RA and IBD. “In some patients you do simply have to stop the TNF inhibitor” and try an alternative treatment with a different mechanism of action.
The cause of TNFi-induced psoriasis is still not well understood. “There certainly is evidence to support that interferon alpha production by plasmacytoid dendritic cells is playing some role in this phenomenon,” but there is “more to the story” and unanswered questions remain, Dr. Fernandez said.
What’s most interesting about this phenomenon, he added, is that “patients can develop it at any time when exposed to a TNF inhibitor.” For instance, most patients develop drug reactions within 2-3 weeks of starting a treatment, but TNFi-induced psoriasis can appear after a single dose or several years after initiating treatment.
“Why so few patients, and why is there such variability in terms of how long they’re on the TNF inhibitor before the reaction occurs?” he asked. “That really points to ... some other trigger besides exposure to the TNF inhibitor needed for the initiation of this reaction.”
He noted that it would be valuable to identify triggers – or the most likely triggers – which would be challenging, but could “potentially impact clinical practice.”
The authors reported personal and institutional relationships in the form of personal and institutional research grants, honoraria, personal fees, investigator fees paid to university, consultancies, and speaker’s bureau positions for a variety of pharmaceutical companies, data companies, hospitals, and foundations. Dr. Fernandez reported he has nonbranded speaking, consulting, and research relationships with AbbVie and Novartis; and is a consultant for UCB, Bristol-Myers Squibb, and Boehringer Ingelheim on related products.
according to a new study published in JAMA Dermatology.
Despite this finding, the authors of the large Danish nationwide cohort study noted that TNFi-induced psoriasis is still a rare adverse event. “Practitioners and patients should be aware and observant of the potential for TNFi-associated psoriasis during TNFi treatment but keep in mind that the absolute risk appears to be low,” David Thein, MB, of the department of dermatology at Bispebjerg Hospital, University of Copenhagen, and colleagues wrote in the study.
They analyzed 109,085 patients with RA and IBD enrolled in Danish national registries between 1995 and 2018 without a previous diagnosis of psoriasis, who received either TNFi (20,910 patients) or conventional treatments (108,024 patients) and were followed for 5 years. They were a mean of 50 years old when they started treatment, 62% were women, with 87.8% of patients in the TNFi group receiving prior conventional therapy and 1% of patients in the conventional therapy group receiving prior TNFi treatment.
The investigators assessed the risk of developing any psoriasis, nonpustular psoriasis, and pustular psoriasis in the two groups using ICD-10 codes as well as a record of two consecutive prescriptions for topical vitamin D analogs.
Overall, 1,471 patients (1.4%) developed psoriasis of any type; 1,332 had non-pustular psoriasis, 127 had palmoplantar pustulosis, and 12 had generalized pustulosis.
The incidence rate of developing any psoriasis was 3.0 per 1,000 patient-years (95% confidence interval, 2.9-3.2) for patients receiving conventional therapy and 7.8 per 1,000 patient-years (95% CI, 7.5-8.9) for patients receiving TNFi treatment. Compared with conventional treatment, the risk of developing nonpustular psoriasis was twofold higher among patients receiving TNFi treatment (hazard ratio, 2.12; 95% CI, 1.87-2.40; P < .001). The risk of developing pustular psoriasis was more than sixfold higher among those on a TNFi (HR, 6.50; 95% CI, 4.60-9.23; P < .001).
Dr. Thein and colleagues estimated that the exposure needed to harm 1 additional patient was 241 patient-years for any psoriasis type, 342 patient-years for nonpustular psoriasis, and 909 patient-years for pustular psoriasis, with an estimated absolute risk difference of 5 per 1,000 patient-years.
Best evidence to date on risk
Asked to comment on the study findings, Anthony Fernandez, MD, PhD, director of medical dermatology at the Cleveland Clinic, said that he applauded the researchers for performing this well-designed study to determine the risk of TNF inhibitor–induced psoriasis in patients with RA and IBD.
The strengths of the study include excluding patients with a history of psoriasis to rule out disease recurrence and having a large comparator group of patients with IBD and RA who were taking medications other than TNF inhibitors, while one limitation was the potential accuracy of the ICD-10 codes used as the basis for diagnosing psoriasis. “It’s probably closer to the truth of what the true risk is compared to studies done in the past,” he said in an interview.
Dr. Fernandez noted that the results aren’t likely to change how dermatologists, rheumatologists, or gastroenterologists practice, but the message to stay the course in initially treating TNFi-induced psoriasis also holds value. “We don’t need to change anything in our clinical practice when it comes to TNF-alpha inhibitors.”
For patients with RA or IBD who develop TNFi-induced psoriasis with disease that is well controlled with TNFi treatment, keeping them on that treatment is a priority, Dr. Fernandez explained. “The first and foremost goal is, if the TNF inhibitor is working very well to control the disease that it was prescribed for, then you exhaust your efforts to try to control the psoriasis and allow those patients to stay on the TNF inhibitor.”
In his experience, most patients with RA and IBD who develop TNFi-induced psoriasis are controlled with topical medications. Switching to another TNFi is not recommended, he noted, as patients are “likely to have that reaction with any TNF inhibitor.”
However, Dr. Fernandez said that won’t be an option for all patients with RA and IBD. “In some patients you do simply have to stop the TNF inhibitor” and try an alternative treatment with a different mechanism of action.
The cause of TNFi-induced psoriasis is still not well understood. “There certainly is evidence to support that interferon alpha production by plasmacytoid dendritic cells is playing some role in this phenomenon,” but there is “more to the story” and unanswered questions remain, Dr. Fernandez said.
What’s most interesting about this phenomenon, he added, is that “patients can develop it at any time when exposed to a TNF inhibitor.” For instance, most patients develop drug reactions within 2-3 weeks of starting a treatment, but TNFi-induced psoriasis can appear after a single dose or several years after initiating treatment.
“Why so few patients, and why is there such variability in terms of how long they’re on the TNF inhibitor before the reaction occurs?” he asked. “That really points to ... some other trigger besides exposure to the TNF inhibitor needed for the initiation of this reaction.”
He noted that it would be valuable to identify triggers – or the most likely triggers – which would be challenging, but could “potentially impact clinical practice.”
The authors reported personal and institutional relationships in the form of personal and institutional research grants, honoraria, personal fees, investigator fees paid to university, consultancies, and speaker’s bureau positions for a variety of pharmaceutical companies, data companies, hospitals, and foundations. Dr. Fernandez reported he has nonbranded speaking, consulting, and research relationships with AbbVie and Novartis; and is a consultant for UCB, Bristol-Myers Squibb, and Boehringer Ingelheim on related products.
FROM JAMA DERMATOLOGY
Compulsivity contributes to poor outcomes in body-focused repetitive behaviors
Although body-focused repetitive behaviors (BFRBs), specifically trichotillomania and skin-picking disorder, are similar in clinical presentation to aspects of obsessive-compulsive disorder (OCD), the role of compulsivity in TTM and SPD has not been well studied, wrote Jon E. Grant, MD, of the University of Chicago and colleagues.
In a study published in the Journal of Psychiatric Research, the authors recruited 69 women and 22 men who met DSM-5 criteria for TTM and SPD. Participants completed diagnostic interviews, symptom inventories, and measures of disability/functioning. Compulsivity was measured using the 15-item Cambridge-Chicago Compulsivity Trait Scale (CHI-T). The average age of the participants was 30.9 years; 48 had TTM, 37 had SPD, and 2 had both conditions.
Overall, total CHI-T scores were significantly correlated with worse disability and quality of life, based on the Quality of Life Inventory (P = .0278) and the Sheehan Disability Scale (P = .0085) but not with severity of TTM or SPD symptoms. TTM and SPD symptoms were assessed using the Massachusetts General Hospital Hair Pulling Scale and the Skin Picking Symptom Symptom Assessment Scale.
“In the current study, we did not find a link between conventional symptom severity measures for BFRBs and disability or quality of life, whereas trans-diagnostic compulsivity did correlate with these clinically important parameters,” the researchers wrote in their discussion. “These findings might suggest the current symptom measures for BFRBs are not including an important aspect of the disease and that a fuller understanding of these symptoms requires measurement of compulsivity. Including validated measures of compulsivity in clinical trials of therapy or medication would also seem to be important for future work,” they said.
The study findings were limited by several factors including the use of a community sample that may not generalize to a clinical setting, the researchers noted. Other limitations include the cross-sectional design, which prevents conclusions about causality, the lack of a control group, and the relatively small sample size, they said.
However, the study is the first known to use a validated compulsivity measure to assess BFRBs, and the results suggest a clinically relevant impact of compulsivity on both psychosocial dysfunction and poor quality of life in this patient population, with possible implications for treatment, the researchers wrote.
The study received no outside funding. Lead author Dr. Grant disclosed research grants from Otsuka and Biohaven Pharmaceuticals, yearly compensation from Springer Publishing for acting as editor in chief of the Journal of Gambling Studies, and royalties from Oxford University Press, American Psychiatric Publishing, Norton Press, and McGraw Hill.
Although body-focused repetitive behaviors (BFRBs), specifically trichotillomania and skin-picking disorder, are similar in clinical presentation to aspects of obsessive-compulsive disorder (OCD), the role of compulsivity in TTM and SPD has not been well studied, wrote Jon E. Grant, MD, of the University of Chicago and colleagues.
In a study published in the Journal of Psychiatric Research, the authors recruited 69 women and 22 men who met DSM-5 criteria for TTM and SPD. Participants completed diagnostic interviews, symptom inventories, and measures of disability/functioning. Compulsivity was measured using the 15-item Cambridge-Chicago Compulsivity Trait Scale (CHI-T). The average age of the participants was 30.9 years; 48 had TTM, 37 had SPD, and 2 had both conditions.
Overall, total CHI-T scores were significantly correlated with worse disability and quality of life, based on the Quality of Life Inventory (P = .0278) and the Sheehan Disability Scale (P = .0085) but not with severity of TTM or SPD symptoms. TTM and SPD symptoms were assessed using the Massachusetts General Hospital Hair Pulling Scale and the Skin Picking Symptom Symptom Assessment Scale.
“In the current study, we did not find a link between conventional symptom severity measures for BFRBs and disability or quality of life, whereas trans-diagnostic compulsivity did correlate with these clinically important parameters,” the researchers wrote in their discussion. “These findings might suggest the current symptom measures for BFRBs are not including an important aspect of the disease and that a fuller understanding of these symptoms requires measurement of compulsivity. Including validated measures of compulsivity in clinical trials of therapy or medication would also seem to be important for future work,” they said.
The study findings were limited by several factors including the use of a community sample that may not generalize to a clinical setting, the researchers noted. Other limitations include the cross-sectional design, which prevents conclusions about causality, the lack of a control group, and the relatively small sample size, they said.
However, the study is the first known to use a validated compulsivity measure to assess BFRBs, and the results suggest a clinically relevant impact of compulsivity on both psychosocial dysfunction and poor quality of life in this patient population, with possible implications for treatment, the researchers wrote.
The study received no outside funding. Lead author Dr. Grant disclosed research grants from Otsuka and Biohaven Pharmaceuticals, yearly compensation from Springer Publishing for acting as editor in chief of the Journal of Gambling Studies, and royalties from Oxford University Press, American Psychiatric Publishing, Norton Press, and McGraw Hill.
Although body-focused repetitive behaviors (BFRBs), specifically trichotillomania and skin-picking disorder, are similar in clinical presentation to aspects of obsessive-compulsive disorder (OCD), the role of compulsivity in TTM and SPD has not been well studied, wrote Jon E. Grant, MD, of the University of Chicago and colleagues.
In a study published in the Journal of Psychiatric Research, the authors recruited 69 women and 22 men who met DSM-5 criteria for TTM and SPD. Participants completed diagnostic interviews, symptom inventories, and measures of disability/functioning. Compulsivity was measured using the 15-item Cambridge-Chicago Compulsivity Trait Scale (CHI-T). The average age of the participants was 30.9 years; 48 had TTM, 37 had SPD, and 2 had both conditions.
Overall, total CHI-T scores were significantly correlated with worse disability and quality of life, based on the Quality of Life Inventory (P = .0278) and the Sheehan Disability Scale (P = .0085) but not with severity of TTM or SPD symptoms. TTM and SPD symptoms were assessed using the Massachusetts General Hospital Hair Pulling Scale and the Skin Picking Symptom Symptom Assessment Scale.
“In the current study, we did not find a link between conventional symptom severity measures for BFRBs and disability or quality of life, whereas trans-diagnostic compulsivity did correlate with these clinically important parameters,” the researchers wrote in their discussion. “These findings might suggest the current symptom measures for BFRBs are not including an important aspect of the disease and that a fuller understanding of these symptoms requires measurement of compulsivity. Including validated measures of compulsivity in clinical trials of therapy or medication would also seem to be important for future work,” they said.
The study findings were limited by several factors including the use of a community sample that may not generalize to a clinical setting, the researchers noted. Other limitations include the cross-sectional design, which prevents conclusions about causality, the lack of a control group, and the relatively small sample size, they said.
However, the study is the first known to use a validated compulsivity measure to assess BFRBs, and the results suggest a clinically relevant impact of compulsivity on both psychosocial dysfunction and poor quality of life in this patient population, with possible implications for treatment, the researchers wrote.
The study received no outside funding. Lead author Dr. Grant disclosed research grants from Otsuka and Biohaven Pharmaceuticals, yearly compensation from Springer Publishing for acting as editor in chief of the Journal of Gambling Studies, and royalties from Oxford University Press, American Psychiatric Publishing, Norton Press, and McGraw Hill.
FROM THE JOURNAL OF PSYCHIATRIC RESEARCH
U.K. survey: Dermatologists want training in prescribing antipsychotics for delusional infestation
GLASGOW – that also indicated there is a clear demand for training in prescribing these drugs.
Delusional infestation is a rare disorder characterized by an individual’s belief that his or her skin, body, or immediate environment is infested by small, living pathogens, despite a lack of any medical evidence. Most of these patients require antipsychotic medication to alleviate symptoms.
The survey of almost 80 dermatologists found that almost 90% had not prescribed antipsychotics in the previous month for patients with psychodermatology conditions and that the most common barrier to prescribing was lack of experience with the drugs.
This was reflected in only 10% of survey respondents who said they were “happy to” prescribe antipsychotics without consulting either dermatology or psychiatric colleagues, and less than half having attended a related course.
Yet the research, presented at the annual meeting of the British Association of Dermatologists, indicated that more than 75% of respondents would attend such a course to increase their confidence.
This finding, said study presenter Ling Li, MD, Churchill Hospital, Oxford (England) University Hospitals NHS Foundation Trust, shows that there is a “clear demand for training, particularly among all the registrars [residents] who we surveyed.”
Dr. Li noted that the UK’s Joint Royal Colleges of Physicians Training Board’s latest curriculum for dermatology training highlights psychocutaneous medicine as a key area, and “that will include antipsychotic medication.”
The BAD also recently published guidelines for the management of adults with delusional infestation, which includes a recommendation to conduct a survey on attitudes toward antipsychotic prescribing for the condition among U.K. dermatologists.
Heeding that call, Dr. Li and colleagues sent an email containing a 10-question online survey to members of the BAD and the British Society for Medical Dermatology. Questions covered familiarity with antipsychotics and frequency of prescribing, confidence around antipsychotics, and current training and future needs. Responses were received between February through April 2021.
Among the 79 respondents, 51 (65%) were consultants and 20 (25%) were dermatology registrars, with the remainder dermatology clinical fellows, foundation doctors, or other doctors. A total of 31 respondents had an average of more than 50 visits with patients per week, 18 had an average of 41-50 patient visits, and 13 had an average of 31-40 visits per week; the remainder had an average of 11-30 visits per week.
Most of the respondents (39) said they had seen 2-5 patients with psychodermatology conditions in the last 6 months, while 17 said they had seen 1 patient, 13 said they had seen more than 10 patients, and 6 said they had seen 6-10 patients (4 had seen none and 1 could not remember).
The most commonly prescribed antipsychotics for psychodermatology patients in the past 6 months were risperidone (Risperdal; prescribed by five respondents), followed by olanzapine (Zyprexa; by four respondents). Seventy respondents had not prescribed any antipsychotics.
Asked about how confident they felt about prescribing antipsychotic medication for patients with delusional infestation, 8 (10%) said they were happy to prescribe independently, while 42 (54%) said they were not at all confident. Another 10 (13%) respondents said they would be happy to prescribe the medications after liaising with a dermatology colleague, while 17 (22%) said they would prefer to consult with the psychiatry team.
The most common barrier to prescribing antipsychotic medications was a lack of experience with the drugs, cited by 66 respondents, followed by concerns over drug monitoring, cited by 43 respondents.
In addition, 42 respondents highlighted concerns over adverse effects, 36 cited lack of experience in psychodermatology clinics, and 19 cited lack of experience in discussing psychodermatologic conditions with patients. Other barriers mentioned by the respondents included difficulties with patient acceptance of a psychiatric medication prescribed by a dermatologist.
An audience member went further, saying that clinicians have been told not to “confront” such patients and that the temptation is therefore to cloak the discussion of antipsychotics in nonthreatening language so that it is more acceptable to the patient.
However, under the U.K. system, a letter with the results of the consultation, including information that an antipsychotic has been prescribed, must be sent to the patient’s family doctor along with a copy that goes to the patient. “The situation is almost impossible,” the audience member said, adding that there “must be some arrangement where in certain circumstances dermatologists could be allowed not to write to the patient” or alternatively, “write an entirely different letter” to the family doctor.
Session cochair Susannah Baron, MD, a consultant dermatologist at St. John’s Institute of Dermatology, Guy’s and St. Thomas’ Hospital, London, said that, in these situations, it is “really helpful to talk about doses” with patients.
She explained that she uses the analogy of aspirin, which has different effects depending on the dose given, giving pain relief at high doses but primarily an antiplatelet effect at low doses.
In the case of an antipsychotic, it is helpful to explain to the patient that “you don’t think they’re psychotic, and you’re prescribing it in a very low dose, because what it can do is help with their symptoms,” Dr. Baron added. “You have to be very open because if you’re not, they go to the pharmacy, and the pharmacist says: ‘Why are you on an antipsychotic?’ ”
Further results from the survey revealed that 56 (71%) respondents did not have access to a specialist psychodermatology clinic, whereas 36 (46%) had not yet attended a psychodermatology course.
Despite these responses, 60 (77%) respondents said they would be interested in attending a training course for prescribing antipsychotics, which included all 20 of the registrars who took part in the survey. a psychodermatologist at Frimley Health Foundation Trust, Windsor, England, and lead author of the BAD guidelines, commented from the audience that the survey results were “sort of what we expected.”
She explained that the intention of the authors when developing the guidelines “was to be able to help our junior colleagues and our peers to be able to feel competent to discuss antipsychotics with patients with delusional infestation and also initiate management.”
Dr. Ahmed added: “Why we’re encouraging our colleagues to prescribe antipsychotics is the longer you leave this type of psychotic illness untreated, the worse the prognosis.”
No funding or relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
GLASGOW – that also indicated there is a clear demand for training in prescribing these drugs.
Delusional infestation is a rare disorder characterized by an individual’s belief that his or her skin, body, or immediate environment is infested by small, living pathogens, despite a lack of any medical evidence. Most of these patients require antipsychotic medication to alleviate symptoms.
The survey of almost 80 dermatologists found that almost 90% had not prescribed antipsychotics in the previous month for patients with psychodermatology conditions and that the most common barrier to prescribing was lack of experience with the drugs.
This was reflected in only 10% of survey respondents who said they were “happy to” prescribe antipsychotics without consulting either dermatology or psychiatric colleagues, and less than half having attended a related course.
Yet the research, presented at the annual meeting of the British Association of Dermatologists, indicated that more than 75% of respondents would attend such a course to increase their confidence.
This finding, said study presenter Ling Li, MD, Churchill Hospital, Oxford (England) University Hospitals NHS Foundation Trust, shows that there is a “clear demand for training, particularly among all the registrars [residents] who we surveyed.”
Dr. Li noted that the UK’s Joint Royal Colleges of Physicians Training Board’s latest curriculum for dermatology training highlights psychocutaneous medicine as a key area, and “that will include antipsychotic medication.”
The BAD also recently published guidelines for the management of adults with delusional infestation, which includes a recommendation to conduct a survey on attitudes toward antipsychotic prescribing for the condition among U.K. dermatologists.
Heeding that call, Dr. Li and colleagues sent an email containing a 10-question online survey to members of the BAD and the British Society for Medical Dermatology. Questions covered familiarity with antipsychotics and frequency of prescribing, confidence around antipsychotics, and current training and future needs. Responses were received between February through April 2021.
Among the 79 respondents, 51 (65%) were consultants and 20 (25%) were dermatology registrars, with the remainder dermatology clinical fellows, foundation doctors, or other doctors. A total of 31 respondents had an average of more than 50 visits with patients per week, 18 had an average of 41-50 patient visits, and 13 had an average of 31-40 visits per week; the remainder had an average of 11-30 visits per week.
Most of the respondents (39) said they had seen 2-5 patients with psychodermatology conditions in the last 6 months, while 17 said they had seen 1 patient, 13 said they had seen more than 10 patients, and 6 said they had seen 6-10 patients (4 had seen none and 1 could not remember).
The most commonly prescribed antipsychotics for psychodermatology patients in the past 6 months were risperidone (Risperdal; prescribed by five respondents), followed by olanzapine (Zyprexa; by four respondents). Seventy respondents had not prescribed any antipsychotics.
Asked about how confident they felt about prescribing antipsychotic medication for patients with delusional infestation, 8 (10%) said they were happy to prescribe independently, while 42 (54%) said they were not at all confident. Another 10 (13%) respondents said they would be happy to prescribe the medications after liaising with a dermatology colleague, while 17 (22%) said they would prefer to consult with the psychiatry team.
The most common barrier to prescribing antipsychotic medications was a lack of experience with the drugs, cited by 66 respondents, followed by concerns over drug monitoring, cited by 43 respondents.
In addition, 42 respondents highlighted concerns over adverse effects, 36 cited lack of experience in psychodermatology clinics, and 19 cited lack of experience in discussing psychodermatologic conditions with patients. Other barriers mentioned by the respondents included difficulties with patient acceptance of a psychiatric medication prescribed by a dermatologist.
An audience member went further, saying that clinicians have been told not to “confront” such patients and that the temptation is therefore to cloak the discussion of antipsychotics in nonthreatening language so that it is more acceptable to the patient.
However, under the U.K. system, a letter with the results of the consultation, including information that an antipsychotic has been prescribed, must be sent to the patient’s family doctor along with a copy that goes to the patient. “The situation is almost impossible,” the audience member said, adding that there “must be some arrangement where in certain circumstances dermatologists could be allowed not to write to the patient” or alternatively, “write an entirely different letter” to the family doctor.
Session cochair Susannah Baron, MD, a consultant dermatologist at St. John’s Institute of Dermatology, Guy’s and St. Thomas’ Hospital, London, said that, in these situations, it is “really helpful to talk about doses” with patients.
She explained that she uses the analogy of aspirin, which has different effects depending on the dose given, giving pain relief at high doses but primarily an antiplatelet effect at low doses.
In the case of an antipsychotic, it is helpful to explain to the patient that “you don’t think they’re psychotic, and you’re prescribing it in a very low dose, because what it can do is help with their symptoms,” Dr. Baron added. “You have to be very open because if you’re not, they go to the pharmacy, and the pharmacist says: ‘Why are you on an antipsychotic?’ ”
Further results from the survey revealed that 56 (71%) respondents did not have access to a specialist psychodermatology clinic, whereas 36 (46%) had not yet attended a psychodermatology course.
Despite these responses, 60 (77%) respondents said they would be interested in attending a training course for prescribing antipsychotics, which included all 20 of the registrars who took part in the survey. a psychodermatologist at Frimley Health Foundation Trust, Windsor, England, and lead author of the BAD guidelines, commented from the audience that the survey results were “sort of what we expected.”
She explained that the intention of the authors when developing the guidelines “was to be able to help our junior colleagues and our peers to be able to feel competent to discuss antipsychotics with patients with delusional infestation and also initiate management.”
Dr. Ahmed added: “Why we’re encouraging our colleagues to prescribe antipsychotics is the longer you leave this type of psychotic illness untreated, the worse the prognosis.”
No funding or relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
GLASGOW – that also indicated there is a clear demand for training in prescribing these drugs.
Delusional infestation is a rare disorder characterized by an individual’s belief that his or her skin, body, or immediate environment is infested by small, living pathogens, despite a lack of any medical evidence. Most of these patients require antipsychotic medication to alleviate symptoms.
The survey of almost 80 dermatologists found that almost 90% had not prescribed antipsychotics in the previous month for patients with psychodermatology conditions and that the most common barrier to prescribing was lack of experience with the drugs.
This was reflected in only 10% of survey respondents who said they were “happy to” prescribe antipsychotics without consulting either dermatology or psychiatric colleagues, and less than half having attended a related course.
Yet the research, presented at the annual meeting of the British Association of Dermatologists, indicated that more than 75% of respondents would attend such a course to increase their confidence.
This finding, said study presenter Ling Li, MD, Churchill Hospital, Oxford (England) University Hospitals NHS Foundation Trust, shows that there is a “clear demand for training, particularly among all the registrars [residents] who we surveyed.”
Dr. Li noted that the UK’s Joint Royal Colleges of Physicians Training Board’s latest curriculum for dermatology training highlights psychocutaneous medicine as a key area, and “that will include antipsychotic medication.”
The BAD also recently published guidelines for the management of adults with delusional infestation, which includes a recommendation to conduct a survey on attitudes toward antipsychotic prescribing for the condition among U.K. dermatologists.
Heeding that call, Dr. Li and colleagues sent an email containing a 10-question online survey to members of the BAD and the British Society for Medical Dermatology. Questions covered familiarity with antipsychotics and frequency of prescribing, confidence around antipsychotics, and current training and future needs. Responses were received between February through April 2021.
Among the 79 respondents, 51 (65%) were consultants and 20 (25%) were dermatology registrars, with the remainder dermatology clinical fellows, foundation doctors, or other doctors. A total of 31 respondents had an average of more than 50 visits with patients per week, 18 had an average of 41-50 patient visits, and 13 had an average of 31-40 visits per week; the remainder had an average of 11-30 visits per week.
Most of the respondents (39) said they had seen 2-5 patients with psychodermatology conditions in the last 6 months, while 17 said they had seen 1 patient, 13 said they had seen more than 10 patients, and 6 said they had seen 6-10 patients (4 had seen none and 1 could not remember).
The most commonly prescribed antipsychotics for psychodermatology patients in the past 6 months were risperidone (Risperdal; prescribed by five respondents), followed by olanzapine (Zyprexa; by four respondents). Seventy respondents had not prescribed any antipsychotics.
Asked about how confident they felt about prescribing antipsychotic medication for patients with delusional infestation, 8 (10%) said they were happy to prescribe independently, while 42 (54%) said they were not at all confident. Another 10 (13%) respondents said they would be happy to prescribe the medications after liaising with a dermatology colleague, while 17 (22%) said they would prefer to consult with the psychiatry team.
The most common barrier to prescribing antipsychotic medications was a lack of experience with the drugs, cited by 66 respondents, followed by concerns over drug monitoring, cited by 43 respondents.
In addition, 42 respondents highlighted concerns over adverse effects, 36 cited lack of experience in psychodermatology clinics, and 19 cited lack of experience in discussing psychodermatologic conditions with patients. Other barriers mentioned by the respondents included difficulties with patient acceptance of a psychiatric medication prescribed by a dermatologist.
An audience member went further, saying that clinicians have been told not to “confront” such patients and that the temptation is therefore to cloak the discussion of antipsychotics in nonthreatening language so that it is more acceptable to the patient.
However, under the U.K. system, a letter with the results of the consultation, including information that an antipsychotic has been prescribed, must be sent to the patient’s family doctor along with a copy that goes to the patient. “The situation is almost impossible,” the audience member said, adding that there “must be some arrangement where in certain circumstances dermatologists could be allowed not to write to the patient” or alternatively, “write an entirely different letter” to the family doctor.
Session cochair Susannah Baron, MD, a consultant dermatologist at St. John’s Institute of Dermatology, Guy’s and St. Thomas’ Hospital, London, said that, in these situations, it is “really helpful to talk about doses” with patients.
She explained that she uses the analogy of aspirin, which has different effects depending on the dose given, giving pain relief at high doses but primarily an antiplatelet effect at low doses.
In the case of an antipsychotic, it is helpful to explain to the patient that “you don’t think they’re psychotic, and you’re prescribing it in a very low dose, because what it can do is help with their symptoms,” Dr. Baron added. “You have to be very open because if you’re not, they go to the pharmacy, and the pharmacist says: ‘Why are you on an antipsychotic?’ ”
Further results from the survey revealed that 56 (71%) respondents did not have access to a specialist psychodermatology clinic, whereas 36 (46%) had not yet attended a psychodermatology course.
Despite these responses, 60 (77%) respondents said they would be interested in attending a training course for prescribing antipsychotics, which included all 20 of the registrars who took part in the survey. a psychodermatologist at Frimley Health Foundation Trust, Windsor, England, and lead author of the BAD guidelines, commented from the audience that the survey results were “sort of what we expected.”
She explained that the intention of the authors when developing the guidelines “was to be able to help our junior colleagues and our peers to be able to feel competent to discuss antipsychotics with patients with delusional infestation and also initiate management.”
Dr. Ahmed added: “Why we’re encouraging our colleagues to prescribe antipsychotics is the longer you leave this type of psychotic illness untreated, the worse the prognosis.”
No funding or relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
AT BAD 2022
Eczema severity, time spent on management strongly associated with overall disease burden
. However, AD severity and spending 11 hours or more per week managing the condition did correlate with higher overall disease burden.
“Research has documented the disease burden of AD, including its visible nature and the effect on itch and sleep, but knowledge gaps remain,” Aaron M. Drucker, MD, of the division of dermatology at the University of Toronto, and colleagues wrote in the study published online in JAMA Dermatology. “Gaps include a poor understanding of symptoms other than itch, patients’ treatment experience, and how different elements of burden of disease interact.”
Dr. Drucker and colleagues collected data from an externally led patient-focused drug development survey on AD, a 32-item questionnaire that was administered electronically between Aug. 1, 2019, and Oct. 11, 2019. Respondents were asked to rate the overall impact of their AD in the past months and the specific elements of disease burden on a 1-5 scale, with 1 meaning no impact, and 5 meaning a significant impact. They were also asked to rate current mood changes and mood changes at the worst point of AD on a 4-point scale that ranged from “not present” to “severe.” The researchers used multivariable ordinal regression to examine associations between demographic and clinical variables and patient-reported overall AD impact scores.
Survey results
Of the 1,065 respondents, 33% were aged 18-34 years, 50% were aged 35-50 years, 17% were aged 65 years or older, and 83% were female. Nearly half (45%) reported having moderate AD, while 28% had severe AD. When asked about the overall disease burden of AD symptoms in the past month, 30% reported a significant impact on life, 28% reported a moderate impact score, 21% reported a high impact score, 18% reported a low impact score, and 3% of respondents reported no impact.
In the multivariable proportional odds analysis, moderate AD (odds ratio [OR], 4.13) and severe AD (OR, 13.63) were both associated with greater disease burden compared with mild AD. Also, spending 11 or more hours per week managing AD symptoms was associated with greater disease burden compared with 0 to 4 hours (an OR of 2.67 for 11-20 hours per week spent managing AD and OR of 5.34 for 21 or more hours per week spent managing AD).
Correlations between specific impact domains such as sleep, cognitive thinking, and physical activity and overall AD impact scores ranged from weak to moderate, and no individual aspect of disease burden correlated strongly with overall impact scores. The researchers observed similar results after they stratified the analysis by age, current severity, and time spent managing AD.
In other findings, 40% of study participants reported mild changes in mood related to their AD, 30% reported moderate changes, 9% reported severe changes, while the remainder reported no changes in mood. The variable most strongly associated with current mood changes was having severe AD at the time of the survey (OR 5.29).
Understanding of disease burden ‘limited’
“Atopic dermatitis is associated with an immense clinical burden,” said Raj Chovatiya, MD, PhD, assistant professor in the department of dermatology at Northwestern University, Chicago, who was asked to comment on the study. “However, our understanding of disease burden from the patient perspective is limited,” he added.
“Interestingly, no single specific element of disease burden was strongly correlated with overall burden, further supporting the complex, multidimensional nature” of the impact of AD, he said, noting that the study “highlights the need for clinicians to look beyond the skin when it comes to AD and underscores the need for additional research to better understand the patient and caregiver perspective.”
Zelma Chiesa Fuxench, MD, MSCE, assistant professor of dermatology at the University of Pennsylvania, Philadelphia, who was also asked to comment on the study, noted that aside from the well discussed impact and burden of itch and its impact on sleep loss, much remains to be learned about the full impact of AD, particularly among adults.
“For example, it is commonly accepted and expected that patients with more severe AD likely experience higher disease burden, but are there other factors that can influence this risk?” she asked. “Can we explain the high impact of AD disease aside from the level of disease severity, particularly among adults with AD?”
The study, she added, “is important because it provides additional insights into those possible factors, including ‘time spent managing their disease’ and ‘associated depression.’ In particular, understanding the association between ‘time spent managing their disease’ and higher disease burden is critical because, in my opinion, it emphasizes the need to develop better strategies for improving the care of patients with AD including the development of more efficacious and safer treatment strategies.”
Dr. Drucker and colleagues acknowledged certain limitations of the analysis, including its cross-sectional design, the potential for selection bias, and the fact that it did not use the patient-oriented outcome measure or the dermatology life quality index. “Further work to address the complex burden of AD, including strategies to reduce time spent managing AD, and understanding the fullness of the patient experience is needed,” they concluded.
The work was supported in part by a grant from the National Eczema Association (NEA). Dr. Drucker reported that he receives compensation from the British Journal of Dermatology (as reviewer and section editor), American Academy of Dermatology (guidelines writer), and NEA (grant reviewer). Coauthors representing the NEA and other patient organizations including the Allergy & Asthma Network, Asthma and Allergy Foundation of America, Global Parents for Eczema Research, and International Topical Steroid Awareness Network received organizational grants (Pfizer) and sponsorship funding for these analyses from AbbVie, Eli Lilly, Incyte, LEO Pharma, Regeneron Pharmaceuticals, and Sanofi Genzyme.
Dr. Chovatiya disclosed that he has served as an advisory board member, consultant, speaker, and/or investigator for AbbVie, Arcutis, Arena, Beiersdorf, Bristol Myers Squibb, Dermavant, Eli Lilly and Company, EPI Health, Incyte, L’Oréal, the NEA, Pfizer, Regeneron, Sanofi, and UCB.
Dr. Chiesa Fuxench disclosed that she has received research grants from Lilly, LEO Pharma, Regeneron, Sanofi, Tioga, and Vanda for work related to AD She has served as consultant for the Asthma and Allergy Foundation of America, NEA, AbbVie, Incyte Corporation, and Pfizer; and received honoraria for CME work in AD sponsored by education grants from Regeneron/Sanofi and Pfizer.
. However, AD severity and spending 11 hours or more per week managing the condition did correlate with higher overall disease burden.
“Research has documented the disease burden of AD, including its visible nature and the effect on itch and sleep, but knowledge gaps remain,” Aaron M. Drucker, MD, of the division of dermatology at the University of Toronto, and colleagues wrote in the study published online in JAMA Dermatology. “Gaps include a poor understanding of symptoms other than itch, patients’ treatment experience, and how different elements of burden of disease interact.”
Dr. Drucker and colleagues collected data from an externally led patient-focused drug development survey on AD, a 32-item questionnaire that was administered electronically between Aug. 1, 2019, and Oct. 11, 2019. Respondents were asked to rate the overall impact of their AD in the past months and the specific elements of disease burden on a 1-5 scale, with 1 meaning no impact, and 5 meaning a significant impact. They were also asked to rate current mood changes and mood changes at the worst point of AD on a 4-point scale that ranged from “not present” to “severe.” The researchers used multivariable ordinal regression to examine associations between demographic and clinical variables and patient-reported overall AD impact scores.
Survey results
Of the 1,065 respondents, 33% were aged 18-34 years, 50% were aged 35-50 years, 17% were aged 65 years or older, and 83% were female. Nearly half (45%) reported having moderate AD, while 28% had severe AD. When asked about the overall disease burden of AD symptoms in the past month, 30% reported a significant impact on life, 28% reported a moderate impact score, 21% reported a high impact score, 18% reported a low impact score, and 3% of respondents reported no impact.
In the multivariable proportional odds analysis, moderate AD (odds ratio [OR], 4.13) and severe AD (OR, 13.63) were both associated with greater disease burden compared with mild AD. Also, spending 11 or more hours per week managing AD symptoms was associated with greater disease burden compared with 0 to 4 hours (an OR of 2.67 for 11-20 hours per week spent managing AD and OR of 5.34 for 21 or more hours per week spent managing AD).
Correlations between specific impact domains such as sleep, cognitive thinking, and physical activity and overall AD impact scores ranged from weak to moderate, and no individual aspect of disease burden correlated strongly with overall impact scores. The researchers observed similar results after they stratified the analysis by age, current severity, and time spent managing AD.
In other findings, 40% of study participants reported mild changes in mood related to their AD, 30% reported moderate changes, 9% reported severe changes, while the remainder reported no changes in mood. The variable most strongly associated with current mood changes was having severe AD at the time of the survey (OR 5.29).
Understanding of disease burden ‘limited’
“Atopic dermatitis is associated with an immense clinical burden,” said Raj Chovatiya, MD, PhD, assistant professor in the department of dermatology at Northwestern University, Chicago, who was asked to comment on the study. “However, our understanding of disease burden from the patient perspective is limited,” he added.
“Interestingly, no single specific element of disease burden was strongly correlated with overall burden, further supporting the complex, multidimensional nature” of the impact of AD, he said, noting that the study “highlights the need for clinicians to look beyond the skin when it comes to AD and underscores the need for additional research to better understand the patient and caregiver perspective.”
Zelma Chiesa Fuxench, MD, MSCE, assistant professor of dermatology at the University of Pennsylvania, Philadelphia, who was also asked to comment on the study, noted that aside from the well discussed impact and burden of itch and its impact on sleep loss, much remains to be learned about the full impact of AD, particularly among adults.
“For example, it is commonly accepted and expected that patients with more severe AD likely experience higher disease burden, but are there other factors that can influence this risk?” she asked. “Can we explain the high impact of AD disease aside from the level of disease severity, particularly among adults with AD?”
The study, she added, “is important because it provides additional insights into those possible factors, including ‘time spent managing their disease’ and ‘associated depression.’ In particular, understanding the association between ‘time spent managing their disease’ and higher disease burden is critical because, in my opinion, it emphasizes the need to develop better strategies for improving the care of patients with AD including the development of more efficacious and safer treatment strategies.”
Dr. Drucker and colleagues acknowledged certain limitations of the analysis, including its cross-sectional design, the potential for selection bias, and the fact that it did not use the patient-oriented outcome measure or the dermatology life quality index. “Further work to address the complex burden of AD, including strategies to reduce time spent managing AD, and understanding the fullness of the patient experience is needed,” they concluded.
The work was supported in part by a grant from the National Eczema Association (NEA). Dr. Drucker reported that he receives compensation from the British Journal of Dermatology (as reviewer and section editor), American Academy of Dermatology (guidelines writer), and NEA (grant reviewer). Coauthors representing the NEA and other patient organizations including the Allergy & Asthma Network, Asthma and Allergy Foundation of America, Global Parents for Eczema Research, and International Topical Steroid Awareness Network received organizational grants (Pfizer) and sponsorship funding for these analyses from AbbVie, Eli Lilly, Incyte, LEO Pharma, Regeneron Pharmaceuticals, and Sanofi Genzyme.
Dr. Chovatiya disclosed that he has served as an advisory board member, consultant, speaker, and/or investigator for AbbVie, Arcutis, Arena, Beiersdorf, Bristol Myers Squibb, Dermavant, Eli Lilly and Company, EPI Health, Incyte, L’Oréal, the NEA, Pfizer, Regeneron, Sanofi, and UCB.
Dr. Chiesa Fuxench disclosed that she has received research grants from Lilly, LEO Pharma, Regeneron, Sanofi, Tioga, and Vanda for work related to AD She has served as consultant for the Asthma and Allergy Foundation of America, NEA, AbbVie, Incyte Corporation, and Pfizer; and received honoraria for CME work in AD sponsored by education grants from Regeneron/Sanofi and Pfizer.
. However, AD severity and spending 11 hours or more per week managing the condition did correlate with higher overall disease burden.
“Research has documented the disease burden of AD, including its visible nature and the effect on itch and sleep, but knowledge gaps remain,” Aaron M. Drucker, MD, of the division of dermatology at the University of Toronto, and colleagues wrote in the study published online in JAMA Dermatology. “Gaps include a poor understanding of symptoms other than itch, patients’ treatment experience, and how different elements of burden of disease interact.”
Dr. Drucker and colleagues collected data from an externally led patient-focused drug development survey on AD, a 32-item questionnaire that was administered electronically between Aug. 1, 2019, and Oct. 11, 2019. Respondents were asked to rate the overall impact of their AD in the past months and the specific elements of disease burden on a 1-5 scale, with 1 meaning no impact, and 5 meaning a significant impact. They were also asked to rate current mood changes and mood changes at the worst point of AD on a 4-point scale that ranged from “not present” to “severe.” The researchers used multivariable ordinal regression to examine associations between demographic and clinical variables and patient-reported overall AD impact scores.
Survey results
Of the 1,065 respondents, 33% were aged 18-34 years, 50% were aged 35-50 years, 17% were aged 65 years or older, and 83% were female. Nearly half (45%) reported having moderate AD, while 28% had severe AD. When asked about the overall disease burden of AD symptoms in the past month, 30% reported a significant impact on life, 28% reported a moderate impact score, 21% reported a high impact score, 18% reported a low impact score, and 3% of respondents reported no impact.
In the multivariable proportional odds analysis, moderate AD (odds ratio [OR], 4.13) and severe AD (OR, 13.63) were both associated with greater disease burden compared with mild AD. Also, spending 11 or more hours per week managing AD symptoms was associated with greater disease burden compared with 0 to 4 hours (an OR of 2.67 for 11-20 hours per week spent managing AD and OR of 5.34 for 21 or more hours per week spent managing AD).
Correlations between specific impact domains such as sleep, cognitive thinking, and physical activity and overall AD impact scores ranged from weak to moderate, and no individual aspect of disease burden correlated strongly with overall impact scores. The researchers observed similar results after they stratified the analysis by age, current severity, and time spent managing AD.
In other findings, 40% of study participants reported mild changes in mood related to their AD, 30% reported moderate changes, 9% reported severe changes, while the remainder reported no changes in mood. The variable most strongly associated with current mood changes was having severe AD at the time of the survey (OR 5.29).
Understanding of disease burden ‘limited’
“Atopic dermatitis is associated with an immense clinical burden,” said Raj Chovatiya, MD, PhD, assistant professor in the department of dermatology at Northwestern University, Chicago, who was asked to comment on the study. “However, our understanding of disease burden from the patient perspective is limited,” he added.
“Interestingly, no single specific element of disease burden was strongly correlated with overall burden, further supporting the complex, multidimensional nature” of the impact of AD, he said, noting that the study “highlights the need for clinicians to look beyond the skin when it comes to AD and underscores the need for additional research to better understand the patient and caregiver perspective.”
Zelma Chiesa Fuxench, MD, MSCE, assistant professor of dermatology at the University of Pennsylvania, Philadelphia, who was also asked to comment on the study, noted that aside from the well discussed impact and burden of itch and its impact on sleep loss, much remains to be learned about the full impact of AD, particularly among adults.
“For example, it is commonly accepted and expected that patients with more severe AD likely experience higher disease burden, but are there other factors that can influence this risk?” she asked. “Can we explain the high impact of AD disease aside from the level of disease severity, particularly among adults with AD?”
The study, she added, “is important because it provides additional insights into those possible factors, including ‘time spent managing their disease’ and ‘associated depression.’ In particular, understanding the association between ‘time spent managing their disease’ and higher disease burden is critical because, in my opinion, it emphasizes the need to develop better strategies for improving the care of patients with AD including the development of more efficacious and safer treatment strategies.”
Dr. Drucker and colleagues acknowledged certain limitations of the analysis, including its cross-sectional design, the potential for selection bias, and the fact that it did not use the patient-oriented outcome measure or the dermatology life quality index. “Further work to address the complex burden of AD, including strategies to reduce time spent managing AD, and understanding the fullness of the patient experience is needed,” they concluded.
The work was supported in part by a grant from the National Eczema Association (NEA). Dr. Drucker reported that he receives compensation from the British Journal of Dermatology (as reviewer and section editor), American Academy of Dermatology (guidelines writer), and NEA (grant reviewer). Coauthors representing the NEA and other patient organizations including the Allergy & Asthma Network, Asthma and Allergy Foundation of America, Global Parents for Eczema Research, and International Topical Steroid Awareness Network received organizational grants (Pfizer) and sponsorship funding for these analyses from AbbVie, Eli Lilly, Incyte, LEO Pharma, Regeneron Pharmaceuticals, and Sanofi Genzyme.
Dr. Chovatiya disclosed that he has served as an advisory board member, consultant, speaker, and/or investigator for AbbVie, Arcutis, Arena, Beiersdorf, Bristol Myers Squibb, Dermavant, Eli Lilly and Company, EPI Health, Incyte, L’Oréal, the NEA, Pfizer, Regeneron, Sanofi, and UCB.
Dr. Chiesa Fuxench disclosed that she has received research grants from Lilly, LEO Pharma, Regeneron, Sanofi, Tioga, and Vanda for work related to AD She has served as consultant for the Asthma and Allergy Foundation of America, NEA, AbbVie, Incyte Corporation, and Pfizer; and received honoraria for CME work in AD sponsored by education grants from Regeneron/Sanofi and Pfizer.
FROM JAMA DERMATOLOGY
Study explores gender differences in pediatric melanoma
INDIANAPOLIS – .
In addition, male gender was independently associated with increased mortality, but age was not.
Those are key findings from a retrospective cohort analysis of nearly 5,000 records from the National Cancer Database.
“There are multiple studies from primarily adult populations showing females with melanoma have a different presentation and better outcomes than males,” co-first author Rebecca M. Thiede, MD, a dermatologist at the University of Arizona, Tucson, said in an interview with this news organization in advance of the annual meeting of the Society for Pediatric Dermatology, where the abstract was presented during a poster session. “However, because melanoma is so rare in younger patients, little is known about gender differences in presentation and survival in pediatric and adolescent patients. To our knowledge, this is one of the largest studies to date in this population, and the first to explore gender differences in detail in pediatric and adolescent patients with melanoma.”
Working with co-first author Sabrina Dahak, a fourth-year medical student at the University of Arizona, Phoenix, Dr. Thiede and colleagues retrospectively analyzed the National Cancer Database to identify biopsy-confirmed invasive primary cutaneous melanoma cases diagnosed in patients 0-21 years of age between 2004 and 2018. The search yielded 4,645 cases, and the researchers used American Academy of Pediatrics definitions to categorize the patients by age, from infancy (birth to 2 years), to childhood (3-10 years), early adolescence (11-14 years), middle adolescence (15-17 years), and late adolescence (18-21 years). They used the Kaplan Meier analysis to determine overall survival and multivariate Cox regression to determine independent survival predictors.
Of the 4,645 pediatric melanoma cases, 63.4% were in females and 36.6% were in males, a difference that was significant (P < .001). Dr. Thiede and colleagues also observed a significant relationship between primary site and gender (P < .001). Primary sites included the trunk (34.3% of females vs. 32.9% of males, respectively), head and neck (16.4% vs. 30.9%), upper extremities (19.5% vs. 16%), lower extremities (27.9% vs. 16.5%), and “unspecified” (1.9% vs. 3.7%).
Females had higher rates of superficial spreading melanoma while males were affected by nodular melanoma more often. For example, the median Breslow depth was higher for males (1.05 mm; interquartile range [IQR] 0.50-2.31) than for females (0.80 mm; IQR, 0.40-1.67; P < .001).
Although females accounted for a higher percentage of cases than males overall, from birth to 17 years, a higher percentage of males than females were found to have later stage of melanoma at time of diagnosis: Females were more likely to be diagnosed with stage I disease (67.8%) than were males (53.6%), and males were more likely than were females to be diagnosed with stages II (15.9% vs. 12.3%), III (27.1% vs. 18.3%), and IV disease (3.3% vs. 1.6%; P < .001 for all).
In other findings, the 5- and 10-year overall survival rates were higher for females (95.9% and 93.9%, respectively) than for males (92.0% vs. 86.7%, respectively; P < .001). However, by age group, overall survival rates were similar between females and males among infants, children, and those in early adolescence – but not for those in middle adolescence (96.7% vs. 91.9%; P < .001) or late adolescence (95.7% vs. 90.4%; P < .001).
When the researchers adjusted for confounding variables, male gender was independently associated with an increased risk of death (adjusted hazard ratio 1.37; P < .001), but age was not.
“It was particularly surprising to see that even at such a young age, there is a significant difference in overall survival between males and females, where females have better outcomes than males,” Dr. Thiede said. “When examining pediatric and adolescent patients, it is essential to maintain cutaneous melanoma on the differential,” she advised. “It is important for clinicians to perform a thorough exam at annual visits particularly for those at high risk for melanoma to catch this rare but potentially devastating diagnosis.”
She acknowledged certain limitations of the study, including its reliance on one database, “as comparing multiple databases would strengthen the conclusions,” she said. “There was some missing data present in our dataset, and a large percentage of the histologic subtypes were unspecified, both of which are common issues with cancer registries. An additional limitation is related to the low death rates in adolescent and pediatric patients, which may impact the analysis related to survival and independent predictors of survival.”
Asked to comment on the study results, Carrie C. Coughlin, MD, who directs the section of pediatric dermatology Washington University/St. Louis Children’s Hospital, said that the finding that males were more likely to present with stage II or higher disease compared with females “could be related to their finding that females had more superficial spreading melanomas, whereas males had more nodular melanoma.” Those differences “could influence how providers evaluate melanocytic lesions in children,” she added.
Dr. Coughlin, who directs the pediatric dermatology fellowship at Washington University/St. Louis Children’s Hospital, said it was “interesting” that the authors found no association between older age and an increased risk of death. “It would be helpful to have more data about melanoma subtype, including information about Spitz or Spitzoid melanomas,” she said. “Also, knowing the distribution of melanoma across the age categories could provide more insight into their data.”
Ms. Dahak received an award from the National Cancer Institute to fund travel for presentation of this study at the SPD meeting. No other financial conflicts were reported by the researchers. Dr. Coughlin is on the board of the Pediatric Dermatology Research Alliance (PeDRA) and the International Immunosuppression and Transplant Skin Cancer Collaborative.
INDIANAPOLIS – .
In addition, male gender was independently associated with increased mortality, but age was not.
Those are key findings from a retrospective cohort analysis of nearly 5,000 records from the National Cancer Database.
“There are multiple studies from primarily adult populations showing females with melanoma have a different presentation and better outcomes than males,” co-first author Rebecca M. Thiede, MD, a dermatologist at the University of Arizona, Tucson, said in an interview with this news organization in advance of the annual meeting of the Society for Pediatric Dermatology, where the abstract was presented during a poster session. “However, because melanoma is so rare in younger patients, little is known about gender differences in presentation and survival in pediatric and adolescent patients. To our knowledge, this is one of the largest studies to date in this population, and the first to explore gender differences in detail in pediatric and adolescent patients with melanoma.”
Working with co-first author Sabrina Dahak, a fourth-year medical student at the University of Arizona, Phoenix, Dr. Thiede and colleagues retrospectively analyzed the National Cancer Database to identify biopsy-confirmed invasive primary cutaneous melanoma cases diagnosed in patients 0-21 years of age between 2004 and 2018. The search yielded 4,645 cases, and the researchers used American Academy of Pediatrics definitions to categorize the patients by age, from infancy (birth to 2 years), to childhood (3-10 years), early adolescence (11-14 years), middle adolescence (15-17 years), and late adolescence (18-21 years). They used the Kaplan Meier analysis to determine overall survival and multivariate Cox regression to determine independent survival predictors.
Of the 4,645 pediatric melanoma cases, 63.4% were in females and 36.6% were in males, a difference that was significant (P < .001). Dr. Thiede and colleagues also observed a significant relationship between primary site and gender (P < .001). Primary sites included the trunk (34.3% of females vs. 32.9% of males, respectively), head and neck (16.4% vs. 30.9%), upper extremities (19.5% vs. 16%), lower extremities (27.9% vs. 16.5%), and “unspecified” (1.9% vs. 3.7%).
Females had higher rates of superficial spreading melanoma while males were affected by nodular melanoma more often. For example, the median Breslow depth was higher for males (1.05 mm; interquartile range [IQR] 0.50-2.31) than for females (0.80 mm; IQR, 0.40-1.67; P < .001).
Although females accounted for a higher percentage of cases than males overall, from birth to 17 years, a higher percentage of males than females were found to have later stage of melanoma at time of diagnosis: Females were more likely to be diagnosed with stage I disease (67.8%) than were males (53.6%), and males were more likely than were females to be diagnosed with stages II (15.9% vs. 12.3%), III (27.1% vs. 18.3%), and IV disease (3.3% vs. 1.6%; P < .001 for all).
In other findings, the 5- and 10-year overall survival rates were higher for females (95.9% and 93.9%, respectively) than for males (92.0% vs. 86.7%, respectively; P < .001). However, by age group, overall survival rates were similar between females and males among infants, children, and those in early adolescence – but not for those in middle adolescence (96.7% vs. 91.9%; P < .001) or late adolescence (95.7% vs. 90.4%; P < .001).
When the researchers adjusted for confounding variables, male gender was independently associated with an increased risk of death (adjusted hazard ratio 1.37; P < .001), but age was not.
“It was particularly surprising to see that even at such a young age, there is a significant difference in overall survival between males and females, where females have better outcomes than males,” Dr. Thiede said. “When examining pediatric and adolescent patients, it is essential to maintain cutaneous melanoma on the differential,” she advised. “It is important for clinicians to perform a thorough exam at annual visits particularly for those at high risk for melanoma to catch this rare but potentially devastating diagnosis.”
She acknowledged certain limitations of the study, including its reliance on one database, “as comparing multiple databases would strengthen the conclusions,” she said. “There was some missing data present in our dataset, and a large percentage of the histologic subtypes were unspecified, both of which are common issues with cancer registries. An additional limitation is related to the low death rates in adolescent and pediatric patients, which may impact the analysis related to survival and independent predictors of survival.”
Asked to comment on the study results, Carrie C. Coughlin, MD, who directs the section of pediatric dermatology Washington University/St. Louis Children’s Hospital, said that the finding that males were more likely to present with stage II or higher disease compared with females “could be related to their finding that females had more superficial spreading melanomas, whereas males had more nodular melanoma.” Those differences “could influence how providers evaluate melanocytic lesions in children,” she added.
Dr. Coughlin, who directs the pediatric dermatology fellowship at Washington University/St. Louis Children’s Hospital, said it was “interesting” that the authors found no association between older age and an increased risk of death. “It would be helpful to have more data about melanoma subtype, including information about Spitz or Spitzoid melanomas,” she said. “Also, knowing the distribution of melanoma across the age categories could provide more insight into their data.”
Ms. Dahak received an award from the National Cancer Institute to fund travel for presentation of this study at the SPD meeting. No other financial conflicts were reported by the researchers. Dr. Coughlin is on the board of the Pediatric Dermatology Research Alliance (PeDRA) and the International Immunosuppression and Transplant Skin Cancer Collaborative.
INDIANAPOLIS – .
In addition, male gender was independently associated with increased mortality, but age was not.
Those are key findings from a retrospective cohort analysis of nearly 5,000 records from the National Cancer Database.
“There are multiple studies from primarily adult populations showing females with melanoma have a different presentation and better outcomes than males,” co-first author Rebecca M. Thiede, MD, a dermatologist at the University of Arizona, Tucson, said in an interview with this news organization in advance of the annual meeting of the Society for Pediatric Dermatology, where the abstract was presented during a poster session. “However, because melanoma is so rare in younger patients, little is known about gender differences in presentation and survival in pediatric and adolescent patients. To our knowledge, this is one of the largest studies to date in this population, and the first to explore gender differences in detail in pediatric and adolescent patients with melanoma.”
Working with co-first author Sabrina Dahak, a fourth-year medical student at the University of Arizona, Phoenix, Dr. Thiede and colleagues retrospectively analyzed the National Cancer Database to identify biopsy-confirmed invasive primary cutaneous melanoma cases diagnosed in patients 0-21 years of age between 2004 and 2018. The search yielded 4,645 cases, and the researchers used American Academy of Pediatrics definitions to categorize the patients by age, from infancy (birth to 2 years), to childhood (3-10 years), early adolescence (11-14 years), middle adolescence (15-17 years), and late adolescence (18-21 years). They used the Kaplan Meier analysis to determine overall survival and multivariate Cox regression to determine independent survival predictors.
Of the 4,645 pediatric melanoma cases, 63.4% were in females and 36.6% were in males, a difference that was significant (P < .001). Dr. Thiede and colleagues also observed a significant relationship between primary site and gender (P < .001). Primary sites included the trunk (34.3% of females vs. 32.9% of males, respectively), head and neck (16.4% vs. 30.9%), upper extremities (19.5% vs. 16%), lower extremities (27.9% vs. 16.5%), and “unspecified” (1.9% vs. 3.7%).
Females had higher rates of superficial spreading melanoma while males were affected by nodular melanoma more often. For example, the median Breslow depth was higher for males (1.05 mm; interquartile range [IQR] 0.50-2.31) than for females (0.80 mm; IQR, 0.40-1.67; P < .001).
Although females accounted for a higher percentage of cases than males overall, from birth to 17 years, a higher percentage of males than females were found to have later stage of melanoma at time of diagnosis: Females were more likely to be diagnosed with stage I disease (67.8%) than were males (53.6%), and males were more likely than were females to be diagnosed with stages II (15.9% vs. 12.3%), III (27.1% vs. 18.3%), and IV disease (3.3% vs. 1.6%; P < .001 for all).
In other findings, the 5- and 10-year overall survival rates were higher for females (95.9% and 93.9%, respectively) than for males (92.0% vs. 86.7%, respectively; P < .001). However, by age group, overall survival rates were similar between females and males among infants, children, and those in early adolescence – but not for those in middle adolescence (96.7% vs. 91.9%; P < .001) or late adolescence (95.7% vs. 90.4%; P < .001).
When the researchers adjusted for confounding variables, male gender was independently associated with an increased risk of death (adjusted hazard ratio 1.37; P < .001), but age was not.
“It was particularly surprising to see that even at such a young age, there is a significant difference in overall survival between males and females, where females have better outcomes than males,” Dr. Thiede said. “When examining pediatric and adolescent patients, it is essential to maintain cutaneous melanoma on the differential,” she advised. “It is important for clinicians to perform a thorough exam at annual visits particularly for those at high risk for melanoma to catch this rare but potentially devastating diagnosis.”
She acknowledged certain limitations of the study, including its reliance on one database, “as comparing multiple databases would strengthen the conclusions,” she said. “There was some missing data present in our dataset, and a large percentage of the histologic subtypes were unspecified, both of which are common issues with cancer registries. An additional limitation is related to the low death rates in adolescent and pediatric patients, which may impact the analysis related to survival and independent predictors of survival.”
Asked to comment on the study results, Carrie C. Coughlin, MD, who directs the section of pediatric dermatology Washington University/St. Louis Children’s Hospital, said that the finding that males were more likely to present with stage II or higher disease compared with females “could be related to their finding that females had more superficial spreading melanomas, whereas males had more nodular melanoma.” Those differences “could influence how providers evaluate melanocytic lesions in children,” she added.
Dr. Coughlin, who directs the pediatric dermatology fellowship at Washington University/St. Louis Children’s Hospital, said it was “interesting” that the authors found no association between older age and an increased risk of death. “It would be helpful to have more data about melanoma subtype, including information about Spitz or Spitzoid melanomas,” she said. “Also, knowing the distribution of melanoma across the age categories could provide more insight into their data.”
Ms. Dahak received an award from the National Cancer Institute to fund travel for presentation of this study at the SPD meeting. No other financial conflicts were reported by the researchers. Dr. Coughlin is on the board of the Pediatric Dermatology Research Alliance (PeDRA) and the International Immunosuppression and Transplant Skin Cancer Collaborative.
AT SPD 2022