User login
Ruxolitinib found to benefit adolescents with vitiligo up to one year
INDIANAPOLIS – and a higher proportion responded at week 52, results from a pooled analysis of phase 3 data showed.
Currently, there is no treatment approved by the Food and Drug Administration to repigment patients with vitiligo, but the cream formulation of the Janus kinase inhibitor ruxolitinib was shown to be effective and have a favorable safety profile in patients aged 12 years and up in the phase 3 clinical trials, TRuE-V1 and TruE-V2. “We know that about half of patients will develop vitiligo by the age of 20, so there is a significant need to have treatments available for the pediatric population,” lead study author David Rosmarin, MD, told this news organization in advance of the annual meeting of the Society for Pediatric Dermatology.
In September 2021, topical ruxolitinib (Opzelura) was approved by the FDA for treating atopic dermatitis in nonimmunocompromised patients aged 12 years and older. The manufacturer, Incyte, has submitted an application for approval to the agency for treating vitiligo in patients ages 12 years and older based on 24-week results; the FDA is expected to make a decision by July 18.
For the current study, presented during a poster session at the meeting, Dr. Rosmarin, of the department of dermatology at Tufts Medical Center, Boston, and colleagues pooled efficacy and safety data for adolescent patients aged 12-17 years from the TRuE-V studies, which enrolled patients 12 years of age and older diagnosed with nonsegmental vitiligo with depigmentation covering up to 10% of total body surface area (BSA), including facial and total Vitiligo Area Scoring Index (F-VASI/T-VASI) scores of ≥ 0.5/≥ 3. Investigators randomized patients 2:1 to twice-daily 1.5% ruxolitinib cream or vehicle for 24 weeks, after which all patients could apply 1.5% ruxolitinib cream through week 52. Efficacy endpoints included the proportions of patients who achieved at least 75%, 50%, and 90% improvement from baseline in F-VASI scores (F-VASI75, F-VASI50, F-VASI90); the proportion of patients who achieved at least a 50% improvement from baseline in T-VASI (T-VASI50); the proportion of patients who achieved a Vitiligo Noticeability Scale (VNS) rating of 4 or 5; and percentage change from baseline in facial BSA (F-BSA). Safety and tolerability were also assessed.
For the pooled analysis, Dr. Rosmarin and colleagues reported results on 72 adolescents: 55 who received ruxolitinib cream and 17 who received vehicle. At week 24, 32.1% of adolescents treated with ruxolitinib cream achieved F-VASI75, compared with none of those in the vehicle group. Further, response rates at week 52 for patients who applied ruxolitinib cream from day 1 were as follows: F-VASI75, 48.0%; F-VASI50, 70.0%; F-VASI90, 24.0%; T-VASI50, 60.0%; VNS score of 4/5, 56.0%; and F-BSA mean percentage change from baseline, –41.9%.
Efficacy at week 52 among crossover patients (after 28 weeks of ruxolitinib cream) was consistent with week 24 data in patients who applied ruxolitinib cream from day 1.
“As we know that repigmentation takes time, about half of the patients achieved the F-VASI75 at the 52-week endpoint,” said Dr. Rosmarin, who is also vice-chair for research and education at Tufts Medical Center, Boston. “Particularly remarkable is that 60% of adolescents achieved a T-VASI50 [50% or more repigmentation of the whole body at the year mark] and over half the patients described their vitiligo as a lot less noticeable or no longer noticeable at the year mark.”
In terms of safety, treatment-related adverse events occurred in 12.9% of patients treated with ruxolitinib (no information was available on the specific events). Serious adverse events occurred in 1.4% of patients; none were considered related to treatment.
“Overall, these results are quite impressive,” Dr. Rosmarin said. “While it can be very challenging to repigment patients with vitiligo, ruxolitinib cream provides an effective option which can help many of my patients.” He acknowledged certain limitations of the analysis, including the fact that the TRuE-V studies were conducted during the COVID-19 pandemic, “which may have contributed to patients being lost to follow-up. Also, the majority of the patients had skin phototypes 1-3.”
Carrie C. Coughlin, MD, who was asked to comment on the study, said that patients with vitiligo need treatment options that are well-studied and covered by insurance. “This study is a great step forward in developing medications for this underserved patient population,” said Dr. Coughlin, who directs the section of pediatric dermatology at Washington University/St. Louis Children’s Hospital.
However, she continued, “the authors mention approximately 13% of patients had a treatment-related adverse reaction, but the abstract does not delineate these reactions.” In addition, the study was limited to children who had less than or equal to 10% body surface area involvement of vitiligo, she noted, adding that “more work is needed to learn about safety of application to larger surface areas.”
Going forward, “it will be important to learn the durability of response,” said Dr. Coughlin, who is also assistant professor of dermatology at Washington University in St. Louis. “Does the vitiligo return if patients stop applying the ruxolitinib cream?”
Dr. Rosmarin disclosed that he has received honoraria as a consultant for Incyte, AbbVie, Abcuro, AltruBio, Arena, Boehringer Ingelheim, Bristol Meyers Squibb, Celgene, Concert, CSL Behring, Dermavant, Dermira, Janssen, Kyowa Kirin, Lilly, Novartis, Pfizer, Regeneron, Revolo Biotherapeutics, Sanofi, Sun Pharmaceuticals, UCB, and VielaBio. He has also received research support from Incyte, AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Dermira, Galderma, Janssen, Lilly, Merck, Novartis, Pfizer, and Regeneron; and has served as a paid speaker for Incyte, AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Incyte, Janssen, Lilly, Novartis, Pfizer, Regeneron, and Sanofi. Dr. Coughlin is on the board of the Pediatric Dermatology Research Alliance and the International Immunosuppression and Transplant Skin Cancer Collaborative.
INDIANAPOLIS – and a higher proportion responded at week 52, results from a pooled analysis of phase 3 data showed.
Currently, there is no treatment approved by the Food and Drug Administration to repigment patients with vitiligo, but the cream formulation of the Janus kinase inhibitor ruxolitinib was shown to be effective and have a favorable safety profile in patients aged 12 years and up in the phase 3 clinical trials, TRuE-V1 and TruE-V2. “We know that about half of patients will develop vitiligo by the age of 20, so there is a significant need to have treatments available for the pediatric population,” lead study author David Rosmarin, MD, told this news organization in advance of the annual meeting of the Society for Pediatric Dermatology.
In September 2021, topical ruxolitinib (Opzelura) was approved by the FDA for treating atopic dermatitis in nonimmunocompromised patients aged 12 years and older. The manufacturer, Incyte, has submitted an application for approval to the agency for treating vitiligo in patients ages 12 years and older based on 24-week results; the FDA is expected to make a decision by July 18.
For the current study, presented during a poster session at the meeting, Dr. Rosmarin, of the department of dermatology at Tufts Medical Center, Boston, and colleagues pooled efficacy and safety data for adolescent patients aged 12-17 years from the TRuE-V studies, which enrolled patients 12 years of age and older diagnosed with nonsegmental vitiligo with depigmentation covering up to 10% of total body surface area (BSA), including facial and total Vitiligo Area Scoring Index (F-VASI/T-VASI) scores of ≥ 0.5/≥ 3. Investigators randomized patients 2:1 to twice-daily 1.5% ruxolitinib cream or vehicle for 24 weeks, after which all patients could apply 1.5% ruxolitinib cream through week 52. Efficacy endpoints included the proportions of patients who achieved at least 75%, 50%, and 90% improvement from baseline in F-VASI scores (F-VASI75, F-VASI50, F-VASI90); the proportion of patients who achieved at least a 50% improvement from baseline in T-VASI (T-VASI50); the proportion of patients who achieved a Vitiligo Noticeability Scale (VNS) rating of 4 or 5; and percentage change from baseline in facial BSA (F-BSA). Safety and tolerability were also assessed.
For the pooled analysis, Dr. Rosmarin and colleagues reported results on 72 adolescents: 55 who received ruxolitinib cream and 17 who received vehicle. At week 24, 32.1% of adolescents treated with ruxolitinib cream achieved F-VASI75, compared with none of those in the vehicle group. Further, response rates at week 52 for patients who applied ruxolitinib cream from day 1 were as follows: F-VASI75, 48.0%; F-VASI50, 70.0%; F-VASI90, 24.0%; T-VASI50, 60.0%; VNS score of 4/5, 56.0%; and F-BSA mean percentage change from baseline, –41.9%.
Efficacy at week 52 among crossover patients (after 28 weeks of ruxolitinib cream) was consistent with week 24 data in patients who applied ruxolitinib cream from day 1.
“As we know that repigmentation takes time, about half of the patients achieved the F-VASI75 at the 52-week endpoint,” said Dr. Rosmarin, who is also vice-chair for research and education at Tufts Medical Center, Boston. “Particularly remarkable is that 60% of adolescents achieved a T-VASI50 [50% or more repigmentation of the whole body at the year mark] and over half the patients described their vitiligo as a lot less noticeable or no longer noticeable at the year mark.”
In terms of safety, treatment-related adverse events occurred in 12.9% of patients treated with ruxolitinib (no information was available on the specific events). Serious adverse events occurred in 1.4% of patients; none were considered related to treatment.
“Overall, these results are quite impressive,” Dr. Rosmarin said. “While it can be very challenging to repigment patients with vitiligo, ruxolitinib cream provides an effective option which can help many of my patients.” He acknowledged certain limitations of the analysis, including the fact that the TRuE-V studies were conducted during the COVID-19 pandemic, “which may have contributed to patients being lost to follow-up. Also, the majority of the patients had skin phototypes 1-3.”
Carrie C. Coughlin, MD, who was asked to comment on the study, said that patients with vitiligo need treatment options that are well-studied and covered by insurance. “This study is a great step forward in developing medications for this underserved patient population,” said Dr. Coughlin, who directs the section of pediatric dermatology at Washington University/St. Louis Children’s Hospital.
However, she continued, “the authors mention approximately 13% of patients had a treatment-related adverse reaction, but the abstract does not delineate these reactions.” In addition, the study was limited to children who had less than or equal to 10% body surface area involvement of vitiligo, she noted, adding that “more work is needed to learn about safety of application to larger surface areas.”
Going forward, “it will be important to learn the durability of response,” said Dr. Coughlin, who is also assistant professor of dermatology at Washington University in St. Louis. “Does the vitiligo return if patients stop applying the ruxolitinib cream?”
Dr. Rosmarin disclosed that he has received honoraria as a consultant for Incyte, AbbVie, Abcuro, AltruBio, Arena, Boehringer Ingelheim, Bristol Meyers Squibb, Celgene, Concert, CSL Behring, Dermavant, Dermira, Janssen, Kyowa Kirin, Lilly, Novartis, Pfizer, Regeneron, Revolo Biotherapeutics, Sanofi, Sun Pharmaceuticals, UCB, and VielaBio. He has also received research support from Incyte, AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Dermira, Galderma, Janssen, Lilly, Merck, Novartis, Pfizer, and Regeneron; and has served as a paid speaker for Incyte, AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Incyte, Janssen, Lilly, Novartis, Pfizer, Regeneron, and Sanofi. Dr. Coughlin is on the board of the Pediatric Dermatology Research Alliance and the International Immunosuppression and Transplant Skin Cancer Collaborative.
INDIANAPOLIS – and a higher proportion responded at week 52, results from a pooled analysis of phase 3 data showed.
Currently, there is no treatment approved by the Food and Drug Administration to repigment patients with vitiligo, but the cream formulation of the Janus kinase inhibitor ruxolitinib was shown to be effective and have a favorable safety profile in patients aged 12 years and up in the phase 3 clinical trials, TRuE-V1 and TruE-V2. “We know that about half of patients will develop vitiligo by the age of 20, so there is a significant need to have treatments available for the pediatric population,” lead study author David Rosmarin, MD, told this news organization in advance of the annual meeting of the Society for Pediatric Dermatology.
In September 2021, topical ruxolitinib (Opzelura) was approved by the FDA for treating atopic dermatitis in nonimmunocompromised patients aged 12 years and older. The manufacturer, Incyte, has submitted an application for approval to the agency for treating vitiligo in patients ages 12 years and older based on 24-week results; the FDA is expected to make a decision by July 18.
For the current study, presented during a poster session at the meeting, Dr. Rosmarin, of the department of dermatology at Tufts Medical Center, Boston, and colleagues pooled efficacy and safety data for adolescent patients aged 12-17 years from the TRuE-V studies, which enrolled patients 12 years of age and older diagnosed with nonsegmental vitiligo with depigmentation covering up to 10% of total body surface area (BSA), including facial and total Vitiligo Area Scoring Index (F-VASI/T-VASI) scores of ≥ 0.5/≥ 3. Investigators randomized patients 2:1 to twice-daily 1.5% ruxolitinib cream or vehicle for 24 weeks, after which all patients could apply 1.5% ruxolitinib cream through week 52. Efficacy endpoints included the proportions of patients who achieved at least 75%, 50%, and 90% improvement from baseline in F-VASI scores (F-VASI75, F-VASI50, F-VASI90); the proportion of patients who achieved at least a 50% improvement from baseline in T-VASI (T-VASI50); the proportion of patients who achieved a Vitiligo Noticeability Scale (VNS) rating of 4 or 5; and percentage change from baseline in facial BSA (F-BSA). Safety and tolerability were also assessed.
For the pooled analysis, Dr. Rosmarin and colleagues reported results on 72 adolescents: 55 who received ruxolitinib cream and 17 who received vehicle. At week 24, 32.1% of adolescents treated with ruxolitinib cream achieved F-VASI75, compared with none of those in the vehicle group. Further, response rates at week 52 for patients who applied ruxolitinib cream from day 1 were as follows: F-VASI75, 48.0%; F-VASI50, 70.0%; F-VASI90, 24.0%; T-VASI50, 60.0%; VNS score of 4/5, 56.0%; and F-BSA mean percentage change from baseline, –41.9%.
Efficacy at week 52 among crossover patients (after 28 weeks of ruxolitinib cream) was consistent with week 24 data in patients who applied ruxolitinib cream from day 1.
“As we know that repigmentation takes time, about half of the patients achieved the F-VASI75 at the 52-week endpoint,” said Dr. Rosmarin, who is also vice-chair for research and education at Tufts Medical Center, Boston. “Particularly remarkable is that 60% of adolescents achieved a T-VASI50 [50% or more repigmentation of the whole body at the year mark] and over half the patients described their vitiligo as a lot less noticeable or no longer noticeable at the year mark.”
In terms of safety, treatment-related adverse events occurred in 12.9% of patients treated with ruxolitinib (no information was available on the specific events). Serious adverse events occurred in 1.4% of patients; none were considered related to treatment.
“Overall, these results are quite impressive,” Dr. Rosmarin said. “While it can be very challenging to repigment patients with vitiligo, ruxolitinib cream provides an effective option which can help many of my patients.” He acknowledged certain limitations of the analysis, including the fact that the TRuE-V studies were conducted during the COVID-19 pandemic, “which may have contributed to patients being lost to follow-up. Also, the majority of the patients had skin phototypes 1-3.”
Carrie C. Coughlin, MD, who was asked to comment on the study, said that patients with vitiligo need treatment options that are well-studied and covered by insurance. “This study is a great step forward in developing medications for this underserved patient population,” said Dr. Coughlin, who directs the section of pediatric dermatology at Washington University/St. Louis Children’s Hospital.
However, she continued, “the authors mention approximately 13% of patients had a treatment-related adverse reaction, but the abstract does not delineate these reactions.” In addition, the study was limited to children who had less than or equal to 10% body surface area involvement of vitiligo, she noted, adding that “more work is needed to learn about safety of application to larger surface areas.”
Going forward, “it will be important to learn the durability of response,” said Dr. Coughlin, who is also assistant professor of dermatology at Washington University in St. Louis. “Does the vitiligo return if patients stop applying the ruxolitinib cream?”
Dr. Rosmarin disclosed that he has received honoraria as a consultant for Incyte, AbbVie, Abcuro, AltruBio, Arena, Boehringer Ingelheim, Bristol Meyers Squibb, Celgene, Concert, CSL Behring, Dermavant, Dermira, Janssen, Kyowa Kirin, Lilly, Novartis, Pfizer, Regeneron, Revolo Biotherapeutics, Sanofi, Sun Pharmaceuticals, UCB, and VielaBio. He has also received research support from Incyte, AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Dermira, Galderma, Janssen, Lilly, Merck, Novartis, Pfizer, and Regeneron; and has served as a paid speaker for Incyte, AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Incyte, Janssen, Lilly, Novartis, Pfizer, Regeneron, and Sanofi. Dr. Coughlin is on the board of the Pediatric Dermatology Research Alliance and the International Immunosuppression and Transplant Skin Cancer Collaborative.
AT SPD 2022
Baricitinib’s approval for alopecia areata: Considerations for starting patients on treatment
On June 13, the FDA approved baricitinib, a Janus kinase inhibitor (Olumiant, Lilly), for severe AA, and two other options may not be far behind. Pfizer and Concert Pharmaceuticals have JAK inhibitors in late-stage development for AA. JAK inhibitors, including baricitinib, are already on the market for treating rheumatoid arthritis and other autoimmune diseases.
Meanwhile, dermatologists have been fielding calls from hopeful patients and sorting out who should get the treatment, how to advise patients on risks and benefits, and what tests should be used before and after starting treatment.
Uptake for new systemic drugs, such as biologics, can be slow in dermatology, noted Adam Friedman, MD, professor and chair of dermatology, George Washington University, Washington, as some doctors like to stick with what they know.
He told this news organization that he hopes that uptake for baricitinib is quicker, as it is the only approved oral systemic treatment for patients with severe alopecia areata, which affects about 300,000 people a year in the United States. Other treatments, including steroid injections in the scalp, have lacked efficacy and convenience.
Beyond the physical effects, the mental toll of patchy hair clumps and missing brows and lashes can be devastating for patients with alopecia areata.
Fielding patient inquiries
Word of the FDA approval spread fast, and calls and emails are coming into dermatologists’ offices and clinics from interested patients.
Physicians should be ready for patients with any kind of hair loss, not just severe alopecia areata, to ask about the drug, Dr. Friedman said. Some patients contacting him don’t fit the indication, which “highlights how disabling hair loss” is for people, considering that, in general, “people see this and think it is for them.”
Baricitinib is not a new drug, but a drug with a new indication. It had already been approved for treating moderate to severe RA in patients who have had an inadequate response to one or more tumor necrosis factor blockers, and for treating COVID-19 in certain hospitalized adults.
Boxed warning
Patients may ask about the boxed warning in the baricitinib label about the increased risk for serious infections, mortality, malignancy, major adverse cardiovascular events, and thrombosis.
Natasha A. Mesinkovska, MD, PhD, an investigator in the clinical trials that led to FDA approval of baricitinib and the chief scientific officer at the National Alopecia Areata Foundation, told this news organization that several aspects of the label are important to point out.
One is that the warning is for all the JAK inhibitors used to treat RA and other inflammatory conditions, not just baricitinib. Also, the warning is based mostly on data on patients with RA who, she noted, have substantial comorbidities and have been taking toxic immunosuppressive medications. The RA population is also typically many years older than the alopecia areata population.
“Whether the warnings apply to the alopecia areata patients is as yet unclear,” said Dr. Mesinkovska, who is also an associate professor of dermatology at the University of California, Irvine.
Patients are also asking about how well it works.
In one of the two trials that led up to the FDA approval, which enrolled patients with at least 50% scalp hair loss for over 6 months, 22% of the patients who received 2 mg of baricitinib and 35% of those who received 4 mg saw adequate hair coverage (at least 80%) at week 36, compared with 5% on placebo. In the second trial, 17% of those who received 2 mg and 32% who received 4 mg saw adequate hair coverage, compared with 3% on placebo.
Common side effects associated with baricitinib, according to the FDA, are lower respiratory tract infections, headache, acne, high cholesterol, increased creatinine phosphokinase, urinary tract infection, liver enzyme elevations, folliculitis, fatigue, nausea, genital yeast infections, anemia, neutropenia, abdominal pain, herpes zoster (shingles), and weight gain.
The risk-benefit discussions with patients should also include potential benefits beyond hair regrowth on the scalp. Loss of hair in the ears and nose can affect hearing and allergies, Dr. Mesinkovska said.
“About 30%-50% with alopecia areata, depending on age group or part of the world, will have allergies,” she said.
Patients should also know that baricitinib will need to be taken “for a very long time,” Dr. Mesinkovska noted. It’s possible that could be forever and that stopping the medication at any point may result in hair falling out again, she says, but duration will vary from case to case.
The good news is that it has been well tolerated. “We give a lot of medications for acne like doxycycline and other antibiotics and people have more stomach problems and angst with those than with [baricitinib],” she said.
Regrowth takes time
Benjamin Ungar, MD, a dermatologist at the Alopecia Center of Excellence at Mount Sinai, New York, told this news organization that an important message for patients is that hair regrowth takes time. For some other skin conditions, patients start treatment and see almost instant improvement.
“That is not the case for alopecia areata,” he said. “The expectation is that it will take months for regrowth in general.”
He said he hasn’t started prescribing baricitinib yet, but plans to do so soon.
“Obviously, I’ll have conversations with patients about it, but it’s a medication I’m going to be using, definitely. I have no reservations,” Dr. Ungar said.
After initial testing, physicians may find that some patients might not be ideal candidates, he added. People with liver disease, a history of blood clots, abnormal blood counts, or low neutrophils are among those who may not be the best candidates for baricitinib.
For most with severe alopecia areata, though, baricitinib provides hope.
“Treatment options have been not readily available, often inaccessible, ineffective, often dangerous,” he said. “There’s a treatment now that can be accessed, generally is safe and is effective for many people.”
Be up front with patients about the unknown
Additionally, it’s important to tell patients what is not yet known, the experts interviewed say.
“Alopecia areata is a chronic disease. We don’t have long-term data on the patient population yet,” Dr. Friedman said.
Also unknown is how easy it will be for physicians to get insurance to reimburse for baricitinib, which, at the end of June, was priced at about $5,000 a month for the 4-mg dose. FDA approval was important in that regard. Previously, some claims had been rejected for drugs used off label for AA.
“We dermatologists know how much it affects patients,” Dr. Mesinkovska said. “As long as we stick by what we know and convey to insurers how much it affects people’s lives, they should cover it.”
Another unknown is what other drugs can be taken with baricitinib. In clinical trials, it was used alone, she said. Currently, concomitant use of other immune suppressants – such as methotrexate or prednisone – is not recommended. But it remains to be seen what other medications will be safe to use at the same time as more long-term data are available.
Lynne J. Goldberg, MD, professor of dermatology, pathology, and laboratory medicine, Boston University, and director of the Hair Clinic at Boston Medical Center, said that she received a slew of emails from patients asking about baricitinib, but most of them did not have alopecia areata and were not candidates for this treatment.
She said that nurses in her clinic have been instructed on what to tell patients about which patients the drug is meant to treat, side effects, and benefits.
Access won’t be immediate
Dr. Goldberg said the drug’s approval does not mean immediate access. The patient has to come in, discuss the treatment, and get lab tests first. “It’s not a casual drug. This is a potent immunosuppressant drug. You need lab tests and once you start it you need blood tests every 3 months to stay on it.”
Those tests may vary by physician, but people will generally need a standard blood count and a comprehensive metabolic panel and lipid panel. “There’s nothing esoteric,” she said.
She added that physicians will need to check for presence of infections including tuberculosis and hepatitis B and C before prescribing, just as they would before they start prescribing a biologic.
“You don’t want to reactivate something,” she noted.
But, Dr. Goldberg added, the benefits for all who have been either living with only patches of hair or no hair or who put on a wig or hat every day are “life changing.”
Dr. Mesinkovska is on the advisory boards and runs trials for Eli Lilly, Pfizer, and Concert Pharmaceuticals. Dr. Friedman, Dr. Goldberg, and Dr. Ungar reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
On June 13, the FDA approved baricitinib, a Janus kinase inhibitor (Olumiant, Lilly), for severe AA, and two other options may not be far behind. Pfizer and Concert Pharmaceuticals have JAK inhibitors in late-stage development for AA. JAK inhibitors, including baricitinib, are already on the market for treating rheumatoid arthritis and other autoimmune diseases.
Meanwhile, dermatologists have been fielding calls from hopeful patients and sorting out who should get the treatment, how to advise patients on risks and benefits, and what tests should be used before and after starting treatment.
Uptake for new systemic drugs, such as biologics, can be slow in dermatology, noted Adam Friedman, MD, professor and chair of dermatology, George Washington University, Washington, as some doctors like to stick with what they know.
He told this news organization that he hopes that uptake for baricitinib is quicker, as it is the only approved oral systemic treatment for patients with severe alopecia areata, which affects about 300,000 people a year in the United States. Other treatments, including steroid injections in the scalp, have lacked efficacy and convenience.
Beyond the physical effects, the mental toll of patchy hair clumps and missing brows and lashes can be devastating for patients with alopecia areata.
Fielding patient inquiries
Word of the FDA approval spread fast, and calls and emails are coming into dermatologists’ offices and clinics from interested patients.
Physicians should be ready for patients with any kind of hair loss, not just severe alopecia areata, to ask about the drug, Dr. Friedman said. Some patients contacting him don’t fit the indication, which “highlights how disabling hair loss” is for people, considering that, in general, “people see this and think it is for them.”
Baricitinib is not a new drug, but a drug with a new indication. It had already been approved for treating moderate to severe RA in patients who have had an inadequate response to one or more tumor necrosis factor blockers, and for treating COVID-19 in certain hospitalized adults.
Boxed warning
Patients may ask about the boxed warning in the baricitinib label about the increased risk for serious infections, mortality, malignancy, major adverse cardiovascular events, and thrombosis.
Natasha A. Mesinkovska, MD, PhD, an investigator in the clinical trials that led to FDA approval of baricitinib and the chief scientific officer at the National Alopecia Areata Foundation, told this news organization that several aspects of the label are important to point out.
One is that the warning is for all the JAK inhibitors used to treat RA and other inflammatory conditions, not just baricitinib. Also, the warning is based mostly on data on patients with RA who, she noted, have substantial comorbidities and have been taking toxic immunosuppressive medications. The RA population is also typically many years older than the alopecia areata population.
“Whether the warnings apply to the alopecia areata patients is as yet unclear,” said Dr. Mesinkovska, who is also an associate professor of dermatology at the University of California, Irvine.
Patients are also asking about how well it works.
In one of the two trials that led up to the FDA approval, which enrolled patients with at least 50% scalp hair loss for over 6 months, 22% of the patients who received 2 mg of baricitinib and 35% of those who received 4 mg saw adequate hair coverage (at least 80%) at week 36, compared with 5% on placebo. In the second trial, 17% of those who received 2 mg and 32% who received 4 mg saw adequate hair coverage, compared with 3% on placebo.
Common side effects associated with baricitinib, according to the FDA, are lower respiratory tract infections, headache, acne, high cholesterol, increased creatinine phosphokinase, urinary tract infection, liver enzyme elevations, folliculitis, fatigue, nausea, genital yeast infections, anemia, neutropenia, abdominal pain, herpes zoster (shingles), and weight gain.
The risk-benefit discussions with patients should also include potential benefits beyond hair regrowth on the scalp. Loss of hair in the ears and nose can affect hearing and allergies, Dr. Mesinkovska said.
“About 30%-50% with alopecia areata, depending on age group or part of the world, will have allergies,” she said.
Patients should also know that baricitinib will need to be taken “for a very long time,” Dr. Mesinkovska noted. It’s possible that could be forever and that stopping the medication at any point may result in hair falling out again, she says, but duration will vary from case to case.
The good news is that it has been well tolerated. “We give a lot of medications for acne like doxycycline and other antibiotics and people have more stomach problems and angst with those than with [baricitinib],” she said.
Regrowth takes time
Benjamin Ungar, MD, a dermatologist at the Alopecia Center of Excellence at Mount Sinai, New York, told this news organization that an important message for patients is that hair regrowth takes time. For some other skin conditions, patients start treatment and see almost instant improvement.
“That is not the case for alopecia areata,” he said. “The expectation is that it will take months for regrowth in general.”
He said he hasn’t started prescribing baricitinib yet, but plans to do so soon.
“Obviously, I’ll have conversations with patients about it, but it’s a medication I’m going to be using, definitely. I have no reservations,” Dr. Ungar said.
After initial testing, physicians may find that some patients might not be ideal candidates, he added. People with liver disease, a history of blood clots, abnormal blood counts, or low neutrophils are among those who may not be the best candidates for baricitinib.
For most with severe alopecia areata, though, baricitinib provides hope.
“Treatment options have been not readily available, often inaccessible, ineffective, often dangerous,” he said. “There’s a treatment now that can be accessed, generally is safe and is effective for many people.”
Be up front with patients about the unknown
Additionally, it’s important to tell patients what is not yet known, the experts interviewed say.
“Alopecia areata is a chronic disease. We don’t have long-term data on the patient population yet,” Dr. Friedman said.
Also unknown is how easy it will be for physicians to get insurance to reimburse for baricitinib, which, at the end of June, was priced at about $5,000 a month for the 4-mg dose. FDA approval was important in that regard. Previously, some claims had been rejected for drugs used off label for AA.
“We dermatologists know how much it affects patients,” Dr. Mesinkovska said. “As long as we stick by what we know and convey to insurers how much it affects people’s lives, they should cover it.”
Another unknown is what other drugs can be taken with baricitinib. In clinical trials, it was used alone, she said. Currently, concomitant use of other immune suppressants – such as methotrexate or prednisone – is not recommended. But it remains to be seen what other medications will be safe to use at the same time as more long-term data are available.
Lynne J. Goldberg, MD, professor of dermatology, pathology, and laboratory medicine, Boston University, and director of the Hair Clinic at Boston Medical Center, said that she received a slew of emails from patients asking about baricitinib, but most of them did not have alopecia areata and were not candidates for this treatment.
She said that nurses in her clinic have been instructed on what to tell patients about which patients the drug is meant to treat, side effects, and benefits.
Access won’t be immediate
Dr. Goldberg said the drug’s approval does not mean immediate access. The patient has to come in, discuss the treatment, and get lab tests first. “It’s not a casual drug. This is a potent immunosuppressant drug. You need lab tests and once you start it you need blood tests every 3 months to stay on it.”
Those tests may vary by physician, but people will generally need a standard blood count and a comprehensive metabolic panel and lipid panel. “There’s nothing esoteric,” she said.
She added that physicians will need to check for presence of infections including tuberculosis and hepatitis B and C before prescribing, just as they would before they start prescribing a biologic.
“You don’t want to reactivate something,” she noted.
But, Dr. Goldberg added, the benefits for all who have been either living with only patches of hair or no hair or who put on a wig or hat every day are “life changing.”
Dr. Mesinkovska is on the advisory boards and runs trials for Eli Lilly, Pfizer, and Concert Pharmaceuticals. Dr. Friedman, Dr. Goldberg, and Dr. Ungar reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
On June 13, the FDA approved baricitinib, a Janus kinase inhibitor (Olumiant, Lilly), for severe AA, and two other options may not be far behind. Pfizer and Concert Pharmaceuticals have JAK inhibitors in late-stage development for AA. JAK inhibitors, including baricitinib, are already on the market for treating rheumatoid arthritis and other autoimmune diseases.
Meanwhile, dermatologists have been fielding calls from hopeful patients and sorting out who should get the treatment, how to advise patients on risks and benefits, and what tests should be used before and after starting treatment.
Uptake for new systemic drugs, such as biologics, can be slow in dermatology, noted Adam Friedman, MD, professor and chair of dermatology, George Washington University, Washington, as some doctors like to stick with what they know.
He told this news organization that he hopes that uptake for baricitinib is quicker, as it is the only approved oral systemic treatment for patients with severe alopecia areata, which affects about 300,000 people a year in the United States. Other treatments, including steroid injections in the scalp, have lacked efficacy and convenience.
Beyond the physical effects, the mental toll of patchy hair clumps and missing brows and lashes can be devastating for patients with alopecia areata.
Fielding patient inquiries
Word of the FDA approval spread fast, and calls and emails are coming into dermatologists’ offices and clinics from interested patients.
Physicians should be ready for patients with any kind of hair loss, not just severe alopecia areata, to ask about the drug, Dr. Friedman said. Some patients contacting him don’t fit the indication, which “highlights how disabling hair loss” is for people, considering that, in general, “people see this and think it is for them.”
Baricitinib is not a new drug, but a drug with a new indication. It had already been approved for treating moderate to severe RA in patients who have had an inadequate response to one or more tumor necrosis factor blockers, and for treating COVID-19 in certain hospitalized adults.
Boxed warning
Patients may ask about the boxed warning in the baricitinib label about the increased risk for serious infections, mortality, malignancy, major adverse cardiovascular events, and thrombosis.
Natasha A. Mesinkovska, MD, PhD, an investigator in the clinical trials that led to FDA approval of baricitinib and the chief scientific officer at the National Alopecia Areata Foundation, told this news organization that several aspects of the label are important to point out.
One is that the warning is for all the JAK inhibitors used to treat RA and other inflammatory conditions, not just baricitinib. Also, the warning is based mostly on data on patients with RA who, she noted, have substantial comorbidities and have been taking toxic immunosuppressive medications. The RA population is also typically many years older than the alopecia areata population.
“Whether the warnings apply to the alopecia areata patients is as yet unclear,” said Dr. Mesinkovska, who is also an associate professor of dermatology at the University of California, Irvine.
Patients are also asking about how well it works.
In one of the two trials that led up to the FDA approval, which enrolled patients with at least 50% scalp hair loss for over 6 months, 22% of the patients who received 2 mg of baricitinib and 35% of those who received 4 mg saw adequate hair coverage (at least 80%) at week 36, compared with 5% on placebo. In the second trial, 17% of those who received 2 mg and 32% who received 4 mg saw adequate hair coverage, compared with 3% on placebo.
Common side effects associated with baricitinib, according to the FDA, are lower respiratory tract infections, headache, acne, high cholesterol, increased creatinine phosphokinase, urinary tract infection, liver enzyme elevations, folliculitis, fatigue, nausea, genital yeast infections, anemia, neutropenia, abdominal pain, herpes zoster (shingles), and weight gain.
The risk-benefit discussions with patients should also include potential benefits beyond hair regrowth on the scalp. Loss of hair in the ears and nose can affect hearing and allergies, Dr. Mesinkovska said.
“About 30%-50% with alopecia areata, depending on age group or part of the world, will have allergies,” she said.
Patients should also know that baricitinib will need to be taken “for a very long time,” Dr. Mesinkovska noted. It’s possible that could be forever and that stopping the medication at any point may result in hair falling out again, she says, but duration will vary from case to case.
The good news is that it has been well tolerated. “We give a lot of medications for acne like doxycycline and other antibiotics and people have more stomach problems and angst with those than with [baricitinib],” she said.
Regrowth takes time
Benjamin Ungar, MD, a dermatologist at the Alopecia Center of Excellence at Mount Sinai, New York, told this news organization that an important message for patients is that hair regrowth takes time. For some other skin conditions, patients start treatment and see almost instant improvement.
“That is not the case for alopecia areata,” he said. “The expectation is that it will take months for regrowth in general.”
He said he hasn’t started prescribing baricitinib yet, but plans to do so soon.
“Obviously, I’ll have conversations with patients about it, but it’s a medication I’m going to be using, definitely. I have no reservations,” Dr. Ungar said.
After initial testing, physicians may find that some patients might not be ideal candidates, he added. People with liver disease, a history of blood clots, abnormal blood counts, or low neutrophils are among those who may not be the best candidates for baricitinib.
For most with severe alopecia areata, though, baricitinib provides hope.
“Treatment options have been not readily available, often inaccessible, ineffective, often dangerous,” he said. “There’s a treatment now that can be accessed, generally is safe and is effective for many people.”
Be up front with patients about the unknown
Additionally, it’s important to tell patients what is not yet known, the experts interviewed say.
“Alopecia areata is a chronic disease. We don’t have long-term data on the patient population yet,” Dr. Friedman said.
Also unknown is how easy it will be for physicians to get insurance to reimburse for baricitinib, which, at the end of June, was priced at about $5,000 a month for the 4-mg dose. FDA approval was important in that regard. Previously, some claims had been rejected for drugs used off label for AA.
“We dermatologists know how much it affects patients,” Dr. Mesinkovska said. “As long as we stick by what we know and convey to insurers how much it affects people’s lives, they should cover it.”
Another unknown is what other drugs can be taken with baricitinib. In clinical trials, it was used alone, she said. Currently, concomitant use of other immune suppressants – such as methotrexate or prednisone – is not recommended. But it remains to be seen what other medications will be safe to use at the same time as more long-term data are available.
Lynne J. Goldberg, MD, professor of dermatology, pathology, and laboratory medicine, Boston University, and director of the Hair Clinic at Boston Medical Center, said that she received a slew of emails from patients asking about baricitinib, but most of them did not have alopecia areata and were not candidates for this treatment.
She said that nurses in her clinic have been instructed on what to tell patients about which patients the drug is meant to treat, side effects, and benefits.
Access won’t be immediate
Dr. Goldberg said the drug’s approval does not mean immediate access. The patient has to come in, discuss the treatment, and get lab tests first. “It’s not a casual drug. This is a potent immunosuppressant drug. You need lab tests and once you start it you need blood tests every 3 months to stay on it.”
Those tests may vary by physician, but people will generally need a standard blood count and a comprehensive metabolic panel and lipid panel. “There’s nothing esoteric,” she said.
She added that physicians will need to check for presence of infections including tuberculosis and hepatitis B and C before prescribing, just as they would before they start prescribing a biologic.
“You don’t want to reactivate something,” she noted.
But, Dr. Goldberg added, the benefits for all who have been either living with only patches of hair or no hair or who put on a wig or hat every day are “life changing.”
Dr. Mesinkovska is on the advisory boards and runs trials for Eli Lilly, Pfizer, and Concert Pharmaceuticals. Dr. Friedman, Dr. Goldberg, and Dr. Ungar reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
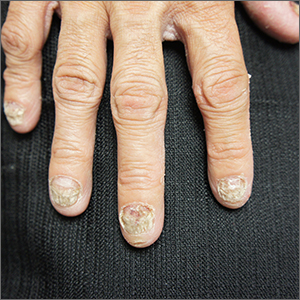
Nail dystrophy and foot pain
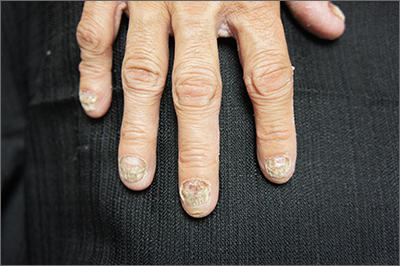
These findings are consistent with a type of heritable keratoderma called pachyonychia congenita (also called twenty-nails dystrophy). It is easy to mistake this unusual cause of thickening nails with a more common cause: onychomycosis.
Pachyonychia congenita describes a set of disorders driven by heritable defects in 1 of 5 keratin genes. The disorder is often transmitted in an autosomal dominant fashion, although a third of patients are thought to have a spontaneous mutation.1 These gene changes can cause 1 or multiple dystrophic nails, thickened nail beds, natal teeth, thick plantar or palmar nodules or plaques, and hearing difficulties. Some patients may have symptoms at birth, while other patients do not develop symptoms until later in life.1
There is currently no cure for pachyonychia congenita. Patients with suspected heritable keratoderma benefit from referral to Medical Genetics and a dermatologist who is comfortable treating keratodermas. Patients can obtain free genetic testing, educational material, and additional resources through pachyonychia.org.
This patient was prescribed topical urea 40% cream that was to be applied to the feet nightly, until the nodules became less painful. He was also evaluated for pressure-offloading orthotics. Nails may be treated with topical urea lacquer nightly until patients are satisfied with the appearance, although this patient chose to forgo the lacquer.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Smith FJD, Hansen CD, Hull PR, et al. Pachyonychia congenita. In: Adam MP, Mirzaa GM, Pagon RA, et al., eds. GeneReviews. Seattle (WA): University of Washington, Seattle; 2006. Updated November 30, 2017. Accessed June 27, 2022. https://www.ncbi.nlm.nih.gov/books/NBK1280/

These findings are consistent with a type of heritable keratoderma called pachyonychia congenita (also called twenty-nails dystrophy). It is easy to mistake this unusual cause of thickening nails with a more common cause: onychomycosis.
Pachyonychia congenita describes a set of disorders driven by heritable defects in 1 of 5 keratin genes. The disorder is often transmitted in an autosomal dominant fashion, although a third of patients are thought to have a spontaneous mutation.1 These gene changes can cause 1 or multiple dystrophic nails, thickened nail beds, natal teeth, thick plantar or palmar nodules or plaques, and hearing difficulties. Some patients may have symptoms at birth, while other patients do not develop symptoms until later in life.1
There is currently no cure for pachyonychia congenita. Patients with suspected heritable keratoderma benefit from referral to Medical Genetics and a dermatologist who is comfortable treating keratodermas. Patients can obtain free genetic testing, educational material, and additional resources through pachyonychia.org.
This patient was prescribed topical urea 40% cream that was to be applied to the feet nightly, until the nodules became less painful. He was also evaluated for pressure-offloading orthotics. Nails may be treated with topical urea lacquer nightly until patients are satisfied with the appearance, although this patient chose to forgo the lacquer.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).

These findings are consistent with a type of heritable keratoderma called pachyonychia congenita (also called twenty-nails dystrophy). It is easy to mistake this unusual cause of thickening nails with a more common cause: onychomycosis.
Pachyonychia congenita describes a set of disorders driven by heritable defects in 1 of 5 keratin genes. The disorder is often transmitted in an autosomal dominant fashion, although a third of patients are thought to have a spontaneous mutation.1 These gene changes can cause 1 or multiple dystrophic nails, thickened nail beds, natal teeth, thick plantar or palmar nodules or plaques, and hearing difficulties. Some patients may have symptoms at birth, while other patients do not develop symptoms until later in life.1
There is currently no cure for pachyonychia congenita. Patients with suspected heritable keratoderma benefit from referral to Medical Genetics and a dermatologist who is comfortable treating keratodermas. Patients can obtain free genetic testing, educational material, and additional resources through pachyonychia.org.
This patient was prescribed topical urea 40% cream that was to be applied to the feet nightly, until the nodules became less painful. He was also evaluated for pressure-offloading orthotics. Nails may be treated with topical urea lacquer nightly until patients are satisfied with the appearance, although this patient chose to forgo the lacquer.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Smith FJD, Hansen CD, Hull PR, et al. Pachyonychia congenita. In: Adam MP, Mirzaa GM, Pagon RA, et al., eds. GeneReviews. Seattle (WA): University of Washington, Seattle; 2006. Updated November 30, 2017. Accessed June 27, 2022. https://www.ncbi.nlm.nih.gov/books/NBK1280/
1. Smith FJD, Hansen CD, Hull PR, et al. Pachyonychia congenita. In: Adam MP, Mirzaa GM, Pagon RA, et al., eds. GeneReviews. Seattle (WA): University of Washington, Seattle; 2006. Updated November 30, 2017. Accessed June 27, 2022. https://www.ncbi.nlm.nih.gov/books/NBK1280/
Skin reactions after COVID-19 vaccination have six patterns
Skin manifestations of COVID-19 were among the topics presented in several sessions at the 49th Congress of the Spanish Academy of Dermatology and Venereology. Specialists agreed that fewer skin changes associated with this virus have been seen with the latest variants of SARS-CoV-2. They highlighted the results of the most remarkable research on this topic that were presented in this forum.
In the study, which was carried out by Spanish dermatologists with the support of the AEDV, researchers analyzed skin reactions associated with the COVID-19 vaccine.
Study author Cristina Galván, MD, a dermatologist at the University Hospital of Móstoles, Madrid, said, of the dermatological manifestations caused as a reaction to these vaccines.”
The study was carried out during the first months of COVID-19 vaccination, Dr. Galván told this news organization. It was proposed as a continuation of a COVID skin study that was published in the British Journal of Dermatology. That study documented the first classification of skin lesions associated with COVID-19. Dr. Galván is the lead author of the latter study.
“The objectives of this study were to characterize and classify skin reactions after vaccination, identify their chronology, and analyze the associations with a series of antecedents: dermatological and allergic diseases, previous SARS-CoV-2 infection, and skin reactions associated with COVID-19,” said Dr. Galván. The study was a team effort, she added.
“It was conducted between Feb. 15 and May 12, 2021, and information was gathered on 405 reactions that appeared during the 21 days after any dose of the COVID-19 vaccines approved at that time in Spain: the Pfizer/BioNTech, Moderna, and University of Oxford/AstraZeneca vaccines,” she added.
Dr. Galván explained that the study shows very clear patterns and investigators reached conclusions that match those of other groups that have investigated this topic. “Six reaction patterns were described according to their frequency. The first is the ‘COVID-19 arm,’ which consists of a local reaction at the injection site and occurs almost exclusively in women and in 70% of cases after inoculation with the Moderna serum. It is a manifestation that resolves well and does not always recur in subsequent doses. More than half are of delayed onset: biopsied patients show signs of a delayed hypersensitivity reaction. In line with all the publications in this regard, it was found that this reaction is not a reason to skip or delay a dose.”
Herpes zoster reactivation
The second pattern is urticarial, which, according to the specialist, occurs with equal frequency after the administration of all vaccines and is well controlled with antihistamines. “This is a very nonspecific pattern, which does not prevent it from still being frequent. It was not associated with drug intake.
“The morbilliform pattern is more frequent after the Pfizer/BioNTech and AstraZeneca vaccines. It affects the trunk and extremities, and up to a quarter of the cases required systemic corticosteroids. The papulovesicular and pityriasis rosea–like patterns are equally frequent in all vaccines. The latter is found in a younger age group. Finally, there is the purpuric pattern, more localized in the extremities and more frequent after the Pfizer/BioNTech and AstraZeneca vaccines. On biopsy, this pattern showed small-vessel vasculitis.”
Less frequently, reactivations or de novo onset of different dermatologic diseases were found. “Varicella-zoster virus reactivations were observed with a frequency of 13.8%, being more common after the Pfizer/BioNTech vaccine,” said Dr. Galván. “Other studies have corroborated this increase in herpes zoster, although it has been seen that the absolute number is low, so the benefits of the vaccine outweigh this eventual complication. At the same time and along the same lines, vaccination against herpes zoster is recommended for those over 50 years of age.”
Another fact revealed by the study is that these reactions were not significantly more severe in people with dermatologic diseases, those with previous infection, or those with skin manifestation associated with COVID-19.
Dr. Galván highlighted that, except for the COVID-19 arm, these patterns were among those associated with the disease, “which supports [the idea] that it does not demonstrate that the host’s immune reaction to the infection was playing a role.”
Women and young people
“As for pseudoperniosis, it is poorly represented in our series: 0.7% compared to 2% in the American registry. Although neither the SARS-CoV-2–pseudoperniosis association nor its pathophysiology is clear, the idea is that if this manifestation is related to the host’s immune response during infection, pseudoperniosis after vaccination could also be linked to the immune response to the vaccine,” said Dr. Galván.
Many of these reactions are more intense in women. “Before starting to use these vaccines, we already knew that messenger RNA vaccines (a powerful activator of innate immunity) induce frequent reactions, that adjuvants and excipients (polyethylene glycol and polysorbate) also generate them, and that other factors influence reactogenicity, among those of us of the same age and sex, reactions being more frequent in younger people and in women,” said Dr. Galván. “This may be one of the reasons why the COVID-19 arm is so much more prevalent in the female population and that 80% of all reactions that were collected were in women.”
In relation to the fact that manifestations differed, depending on the type of inoculated serum, Dr. Galván said, “Some reactions are just as common after any of the vaccines. However, others are not, as is the case with the COVID-19 arm for the Moderna vaccine or reactivations of the herpes virus, more frequent after the Pfizer/BioNTech vaccine.
“Undoubtedly, behind these differences are particularities in the immune reaction caused by each of the vaccines and their composition, including the excipients,” she said.
Regarding the fact that these reactions were the same throughout the vaccine regimen or that they varied in intensity, depending on the dose, Dr. Galván said, “In our study, as in those carried out by other groups, there were no significant differences in terms of frequency after the first and second doses. One thing to keep in mind is that, due to the temporary design of our study and the time at which it was conducted, it was not possible to collect reactions after second doses of AstraZeneca.
“Manifestations have generally been mild and well controlled. Many of them did not recur after the second dose, and the vast majority did not prevent completion of the vaccination scheme, but we must not lose sight of the fact that 20% of these manifestations were assessed by the dermatologist as serious or very serious,” Dr. Galván added.
Regarding the next steps planned for this line of research, Dr. Galván commented, “We are awaiting the evolution of the reported cases and the reactions that may arise, although for now, our group does not have any open studies. The most important thing now is to be alert and report the data observed in the pharmacovigilance systems, in open registries, and in scientific literature to generate evidence.”
Dr. Galván has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Skin manifestations of COVID-19 were among the topics presented in several sessions at the 49th Congress of the Spanish Academy of Dermatology and Venereology. Specialists agreed that fewer skin changes associated with this virus have been seen with the latest variants of SARS-CoV-2. They highlighted the results of the most remarkable research on this topic that were presented in this forum.
In the study, which was carried out by Spanish dermatologists with the support of the AEDV, researchers analyzed skin reactions associated with the COVID-19 vaccine.
Study author Cristina Galván, MD, a dermatologist at the University Hospital of Móstoles, Madrid, said, of the dermatological manifestations caused as a reaction to these vaccines.”
The study was carried out during the first months of COVID-19 vaccination, Dr. Galván told this news organization. It was proposed as a continuation of a COVID skin study that was published in the British Journal of Dermatology. That study documented the first classification of skin lesions associated with COVID-19. Dr. Galván is the lead author of the latter study.
“The objectives of this study were to characterize and classify skin reactions after vaccination, identify their chronology, and analyze the associations with a series of antecedents: dermatological and allergic diseases, previous SARS-CoV-2 infection, and skin reactions associated with COVID-19,” said Dr. Galván. The study was a team effort, she added.
“It was conducted between Feb. 15 and May 12, 2021, and information was gathered on 405 reactions that appeared during the 21 days after any dose of the COVID-19 vaccines approved at that time in Spain: the Pfizer/BioNTech, Moderna, and University of Oxford/AstraZeneca vaccines,” she added.
Dr. Galván explained that the study shows very clear patterns and investigators reached conclusions that match those of other groups that have investigated this topic. “Six reaction patterns were described according to their frequency. The first is the ‘COVID-19 arm,’ which consists of a local reaction at the injection site and occurs almost exclusively in women and in 70% of cases after inoculation with the Moderna serum. It is a manifestation that resolves well and does not always recur in subsequent doses. More than half are of delayed onset: biopsied patients show signs of a delayed hypersensitivity reaction. In line with all the publications in this regard, it was found that this reaction is not a reason to skip or delay a dose.”
Herpes zoster reactivation
The second pattern is urticarial, which, according to the specialist, occurs with equal frequency after the administration of all vaccines and is well controlled with antihistamines. “This is a very nonspecific pattern, which does not prevent it from still being frequent. It was not associated with drug intake.
“The morbilliform pattern is more frequent after the Pfizer/BioNTech and AstraZeneca vaccines. It affects the trunk and extremities, and up to a quarter of the cases required systemic corticosteroids. The papulovesicular and pityriasis rosea–like patterns are equally frequent in all vaccines. The latter is found in a younger age group. Finally, there is the purpuric pattern, more localized in the extremities and more frequent after the Pfizer/BioNTech and AstraZeneca vaccines. On biopsy, this pattern showed small-vessel vasculitis.”
Less frequently, reactivations or de novo onset of different dermatologic diseases were found. “Varicella-zoster virus reactivations were observed with a frequency of 13.8%, being more common after the Pfizer/BioNTech vaccine,” said Dr. Galván. “Other studies have corroborated this increase in herpes zoster, although it has been seen that the absolute number is low, so the benefits of the vaccine outweigh this eventual complication. At the same time and along the same lines, vaccination against herpes zoster is recommended for those over 50 years of age.”
Another fact revealed by the study is that these reactions were not significantly more severe in people with dermatologic diseases, those with previous infection, or those with skin manifestation associated with COVID-19.
Dr. Galván highlighted that, except for the COVID-19 arm, these patterns were among those associated with the disease, “which supports [the idea] that it does not demonstrate that the host’s immune reaction to the infection was playing a role.”
Women and young people
“As for pseudoperniosis, it is poorly represented in our series: 0.7% compared to 2% in the American registry. Although neither the SARS-CoV-2–pseudoperniosis association nor its pathophysiology is clear, the idea is that if this manifestation is related to the host’s immune response during infection, pseudoperniosis after vaccination could also be linked to the immune response to the vaccine,” said Dr. Galván.
Many of these reactions are more intense in women. “Before starting to use these vaccines, we already knew that messenger RNA vaccines (a powerful activator of innate immunity) induce frequent reactions, that adjuvants and excipients (polyethylene glycol and polysorbate) also generate them, and that other factors influence reactogenicity, among those of us of the same age and sex, reactions being more frequent in younger people and in women,” said Dr. Galván. “This may be one of the reasons why the COVID-19 arm is so much more prevalent in the female population and that 80% of all reactions that were collected were in women.”
In relation to the fact that manifestations differed, depending on the type of inoculated serum, Dr. Galván said, “Some reactions are just as common after any of the vaccines. However, others are not, as is the case with the COVID-19 arm for the Moderna vaccine or reactivations of the herpes virus, more frequent after the Pfizer/BioNTech vaccine.
“Undoubtedly, behind these differences are particularities in the immune reaction caused by each of the vaccines and their composition, including the excipients,” she said.
Regarding the fact that these reactions were the same throughout the vaccine regimen or that they varied in intensity, depending on the dose, Dr. Galván said, “In our study, as in those carried out by other groups, there were no significant differences in terms of frequency after the first and second doses. One thing to keep in mind is that, due to the temporary design of our study and the time at which it was conducted, it was not possible to collect reactions after second doses of AstraZeneca.
“Manifestations have generally been mild and well controlled. Many of them did not recur after the second dose, and the vast majority did not prevent completion of the vaccination scheme, but we must not lose sight of the fact that 20% of these manifestations were assessed by the dermatologist as serious or very serious,” Dr. Galván added.
Regarding the next steps planned for this line of research, Dr. Galván commented, “We are awaiting the evolution of the reported cases and the reactions that may arise, although for now, our group does not have any open studies. The most important thing now is to be alert and report the data observed in the pharmacovigilance systems, in open registries, and in scientific literature to generate evidence.”
Dr. Galván has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Skin manifestations of COVID-19 were among the topics presented in several sessions at the 49th Congress of the Spanish Academy of Dermatology and Venereology. Specialists agreed that fewer skin changes associated with this virus have been seen with the latest variants of SARS-CoV-2. They highlighted the results of the most remarkable research on this topic that were presented in this forum.
In the study, which was carried out by Spanish dermatologists with the support of the AEDV, researchers analyzed skin reactions associated with the COVID-19 vaccine.
Study author Cristina Galván, MD, a dermatologist at the University Hospital of Móstoles, Madrid, said, of the dermatological manifestations caused as a reaction to these vaccines.”
The study was carried out during the first months of COVID-19 vaccination, Dr. Galván told this news organization. It was proposed as a continuation of a COVID skin study that was published in the British Journal of Dermatology. That study documented the first classification of skin lesions associated with COVID-19. Dr. Galván is the lead author of the latter study.
“The objectives of this study were to characterize and classify skin reactions after vaccination, identify their chronology, and analyze the associations with a series of antecedents: dermatological and allergic diseases, previous SARS-CoV-2 infection, and skin reactions associated with COVID-19,” said Dr. Galván. The study was a team effort, she added.
“It was conducted between Feb. 15 and May 12, 2021, and information was gathered on 405 reactions that appeared during the 21 days after any dose of the COVID-19 vaccines approved at that time in Spain: the Pfizer/BioNTech, Moderna, and University of Oxford/AstraZeneca vaccines,” she added.
Dr. Galván explained that the study shows very clear patterns and investigators reached conclusions that match those of other groups that have investigated this topic. “Six reaction patterns were described according to their frequency. The first is the ‘COVID-19 arm,’ which consists of a local reaction at the injection site and occurs almost exclusively in women and in 70% of cases after inoculation with the Moderna serum. It is a manifestation that resolves well and does not always recur in subsequent doses. More than half are of delayed onset: biopsied patients show signs of a delayed hypersensitivity reaction. In line with all the publications in this regard, it was found that this reaction is not a reason to skip or delay a dose.”
Herpes zoster reactivation
The second pattern is urticarial, which, according to the specialist, occurs with equal frequency after the administration of all vaccines and is well controlled with antihistamines. “This is a very nonspecific pattern, which does not prevent it from still being frequent. It was not associated with drug intake.
“The morbilliform pattern is more frequent after the Pfizer/BioNTech and AstraZeneca vaccines. It affects the trunk and extremities, and up to a quarter of the cases required systemic corticosteroids. The papulovesicular and pityriasis rosea–like patterns are equally frequent in all vaccines. The latter is found in a younger age group. Finally, there is the purpuric pattern, more localized in the extremities and more frequent after the Pfizer/BioNTech and AstraZeneca vaccines. On biopsy, this pattern showed small-vessel vasculitis.”
Less frequently, reactivations or de novo onset of different dermatologic diseases were found. “Varicella-zoster virus reactivations were observed with a frequency of 13.8%, being more common after the Pfizer/BioNTech vaccine,” said Dr. Galván. “Other studies have corroborated this increase in herpes zoster, although it has been seen that the absolute number is low, so the benefits of the vaccine outweigh this eventual complication. At the same time and along the same lines, vaccination against herpes zoster is recommended for those over 50 years of age.”
Another fact revealed by the study is that these reactions were not significantly more severe in people with dermatologic diseases, those with previous infection, or those with skin manifestation associated with COVID-19.
Dr. Galván highlighted that, except for the COVID-19 arm, these patterns were among those associated with the disease, “which supports [the idea] that it does not demonstrate that the host’s immune reaction to the infection was playing a role.”
Women and young people
“As for pseudoperniosis, it is poorly represented in our series: 0.7% compared to 2% in the American registry. Although neither the SARS-CoV-2–pseudoperniosis association nor its pathophysiology is clear, the idea is that if this manifestation is related to the host’s immune response during infection, pseudoperniosis after vaccination could also be linked to the immune response to the vaccine,” said Dr. Galván.
Many of these reactions are more intense in women. “Before starting to use these vaccines, we already knew that messenger RNA vaccines (a powerful activator of innate immunity) induce frequent reactions, that adjuvants and excipients (polyethylene glycol and polysorbate) also generate them, and that other factors influence reactogenicity, among those of us of the same age and sex, reactions being more frequent in younger people and in women,” said Dr. Galván. “This may be one of the reasons why the COVID-19 arm is so much more prevalent in the female population and that 80% of all reactions that were collected were in women.”
In relation to the fact that manifestations differed, depending on the type of inoculated serum, Dr. Galván said, “Some reactions are just as common after any of the vaccines. However, others are not, as is the case with the COVID-19 arm for the Moderna vaccine or reactivations of the herpes virus, more frequent after the Pfizer/BioNTech vaccine.
“Undoubtedly, behind these differences are particularities in the immune reaction caused by each of the vaccines and their composition, including the excipients,” she said.
Regarding the fact that these reactions were the same throughout the vaccine regimen or that they varied in intensity, depending on the dose, Dr. Galván said, “In our study, as in those carried out by other groups, there were no significant differences in terms of frequency after the first and second doses. One thing to keep in mind is that, due to the temporary design of our study and the time at which it was conducted, it was not possible to collect reactions after second doses of AstraZeneca.
“Manifestations have generally been mild and well controlled. Many of them did not recur after the second dose, and the vast majority did not prevent completion of the vaccination scheme, but we must not lose sight of the fact that 20% of these manifestations were assessed by the dermatologist as serious or very serious,” Dr. Galván added.
Regarding the next steps planned for this line of research, Dr. Galván commented, “We are awaiting the evolution of the reported cases and the reactions that may arise, although for now, our group does not have any open studies. The most important thing now is to be alert and report the data observed in the pharmacovigilance systems, in open registries, and in scientific literature to generate evidence.”
Dr. Galván has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Can dietary tweaks improve some skin diseases?
Since 1950, the terms “diet and skin” in the medical literature have markedly increased, said Vivian Shi, MD associate professor of dermatology at the University of Arkansas for Medical Sciences, Little Rock, who talked about nutritional approaches for select skin diseases at MedscapeLive’s Women’s and Pediatric Dermatology Seminar.
Myths abound, but some associations of diet with skin diseases hold water, and she said.
Acne
What’s known, Dr. Shi said, is that the prevalence of acne is substantially lower in non-Westernized countries, and that diets in those countries generally have a low glycemic load, which decreases IGF-1 insulinlike growth factor 1 (IGF-1) concentrations, an accepted risk factor for acne. The Western diet also includes the hormonal effects of cow’s milk products.
Whey protein, which is popular as a supplement, isn’t good for acne, Dr. Shi said. It takes a couple of hours to digest, while casein protein digests more slowly, over 5-7 hours. If casein protein isn’t acceptable, good alternatives to whey protein are hemp seed, plant protein blends (peas, seeds, berries), egg white, brown rice isolate, and soy isolate protein.
Dairy products increase IGF-1 levels, hormonal mediators that can make acne worse. In addition, industrial cow’s milk can contain anabolic steroids and growth factor, leading to sebogenesis, Dr. Shi said. As for the type of milk, skim milk tends to be the most acnegenic and associated with the highest blood levels of IGF-1.
Supplementing with omega-3 fatty acids and gamma-linolenic acid improved mild to moderate acne in a double-blind, controlled study. Researchers randomized 45 patients with mild to moderate acne to an omega-3 fatty acid group (2,000 mg of eicosapentaenoic acid and docosahexaenoic acid), a gamma-linolenic acid group (borage oil with 400 mg gamma-linolenic acid) or a control group. After 10 weeks in both treatment groups, there was a significant reduction in inflammatory and noninflammatory lesions.
Those with acne are more likely to be deficient in Vitamin D, research suggests. Researchers also found that among those who had vitamin D deficiency, supplementing with 1,000 IU daily for 2 months reduced inflammatory lesions by 35% after 8 weeks, compared with a 6% reduction in the control group.
Other research has found that those with a low serum zinc level had more severe acne and that 30-200 mg of zinc orally for 2-4 months reduced inflammatory acne. However, Dr. Shi cautioned that those taking zinc for more than 2 months also need a copper supplement, as zinc reduces the amount of copper absorbed by the body.
Dr. Shi’s “do’s” diet list for acne patients is a follows: Paleolithic and Mediterranean diets, omega-3 fatty acids, gamma-linolenic acids, Vitamin D, zinc, tubers, legumes, vegetables, fruits, and fish.
Unknowns, she said, include chocolate, caffeine, green tea, and high salt.
Hidradenitis suppurativa
Patents with HS who follow a Mediterranean diet most closely have less severe disease, research has found. In this study, those patients with HS with the lowest adherence had a Sartorius HS score of 59.38, while those who followed it the most closely had a score of 39 (of 80).
In another study, patients with HS reported the following foods as exacerbating HS: sweets, bread/pasta/rice, dairy, and high-fat foods. Alleviating foods included vegetables, fruit, chicken, and fish.
Dr. Shi’s dietary recommendations for patients with HS: Follow a Mediterranean diet, avoid high fat foods and highly processed foods, and focus on eating more vegetables, fresh fruit, corn-based cereal, white meat, and fish.
A retrospective study of patients with Hurley stage 1 and 2 found that oral zinc gluconate, 90 mg a day, combined with 2% topical triclosan twice a day, resulted in significantly decreased HS scores and nodules and improved quality of life after 3 months. Expect vitamin D deficiency, she added.
Lastly, Dr. Shi recommended, if necessary, “weight loss to reduce the inflammatory burden.”
Rosacea
Dietary triggers for rosacea are thought to include high-fat foods, dairy foods, spicy foods, hot drinks, cinnamon, and vanilla.
A population-based case-control study in China, which evaluated 1,347 rosacea patients and 1,290 healthy controls, found that a high intake of fatty foods positively correlated with erythematotelangiectatic rosacea (ETR) and phymatous rosacea. High-frequency dairy intake negatively correlated with ETR and papulopustular rosacea, which was a surprise, she said. And in this study, no significant correlations were found between sweets, coffee, and spicy foods. That goes against the traditional thinking, she said, but this was a Chinese cohort and their diet is probably vastly different than those in the United States.
Other rosacea triggers, Dr. Shi said, are niacin-containing foods such as turkey, chicken breast, crustaceans, dried Shiitake mushrooms, peanuts, tuna, and liver, as well as cold drinks, and formalin-containing foods (fish, squid, tofu, wet noodles).
As the field of nutrigenics – how genes affect how the body responds to food – evolves, more answers about the impact of diet on these diseases will be forthcoming, Dr. Shi said.
In an interactive panel discussion, she was asked if she talks about diet with all her patients with acne, rosacea, and HS, or just those not responding to traditional therapy.
“I think it’s an important conversation to have,” Dr. Shi responded. “When I’m done with the medication [instructions], I say: ‘There is something else you can do to augment what I just told you.’ ” That’s when she explains the dietary information. She also has a handout on diet and routinely refers patients for dietary counseling.
MedscapeLive and this news organization are owned by the same parent company. Dr. Shi disclosed consulting, investigative and research funding from several sources, but not directly related to the content of her talk.
Since 1950, the terms “diet and skin” in the medical literature have markedly increased, said Vivian Shi, MD associate professor of dermatology at the University of Arkansas for Medical Sciences, Little Rock, who talked about nutritional approaches for select skin diseases at MedscapeLive’s Women’s and Pediatric Dermatology Seminar.
Myths abound, but some associations of diet with skin diseases hold water, and she said.
Acne
What’s known, Dr. Shi said, is that the prevalence of acne is substantially lower in non-Westernized countries, and that diets in those countries generally have a low glycemic load, which decreases IGF-1 insulinlike growth factor 1 (IGF-1) concentrations, an accepted risk factor for acne. The Western diet also includes the hormonal effects of cow’s milk products.
Whey protein, which is popular as a supplement, isn’t good for acne, Dr. Shi said. It takes a couple of hours to digest, while casein protein digests more slowly, over 5-7 hours. If casein protein isn’t acceptable, good alternatives to whey protein are hemp seed, plant protein blends (peas, seeds, berries), egg white, brown rice isolate, and soy isolate protein.
Dairy products increase IGF-1 levels, hormonal mediators that can make acne worse. In addition, industrial cow’s milk can contain anabolic steroids and growth factor, leading to sebogenesis, Dr. Shi said. As for the type of milk, skim milk tends to be the most acnegenic and associated with the highest blood levels of IGF-1.
Supplementing with omega-3 fatty acids and gamma-linolenic acid improved mild to moderate acne in a double-blind, controlled study. Researchers randomized 45 patients with mild to moderate acne to an omega-3 fatty acid group (2,000 mg of eicosapentaenoic acid and docosahexaenoic acid), a gamma-linolenic acid group (borage oil with 400 mg gamma-linolenic acid) or a control group. After 10 weeks in both treatment groups, there was a significant reduction in inflammatory and noninflammatory lesions.
Those with acne are more likely to be deficient in Vitamin D, research suggests. Researchers also found that among those who had vitamin D deficiency, supplementing with 1,000 IU daily for 2 months reduced inflammatory lesions by 35% after 8 weeks, compared with a 6% reduction in the control group.
Other research has found that those with a low serum zinc level had more severe acne and that 30-200 mg of zinc orally for 2-4 months reduced inflammatory acne. However, Dr. Shi cautioned that those taking zinc for more than 2 months also need a copper supplement, as zinc reduces the amount of copper absorbed by the body.
Dr. Shi’s “do’s” diet list for acne patients is a follows: Paleolithic and Mediterranean diets, omega-3 fatty acids, gamma-linolenic acids, Vitamin D, zinc, tubers, legumes, vegetables, fruits, and fish.
Unknowns, she said, include chocolate, caffeine, green tea, and high salt.
Hidradenitis suppurativa
Patents with HS who follow a Mediterranean diet most closely have less severe disease, research has found. In this study, those patients with HS with the lowest adherence had a Sartorius HS score of 59.38, while those who followed it the most closely had a score of 39 (of 80).
In another study, patients with HS reported the following foods as exacerbating HS: sweets, bread/pasta/rice, dairy, and high-fat foods. Alleviating foods included vegetables, fruit, chicken, and fish.
Dr. Shi’s dietary recommendations for patients with HS: Follow a Mediterranean diet, avoid high fat foods and highly processed foods, and focus on eating more vegetables, fresh fruit, corn-based cereal, white meat, and fish.
A retrospective study of patients with Hurley stage 1 and 2 found that oral zinc gluconate, 90 mg a day, combined with 2% topical triclosan twice a day, resulted in significantly decreased HS scores and nodules and improved quality of life after 3 months. Expect vitamin D deficiency, she added.
Lastly, Dr. Shi recommended, if necessary, “weight loss to reduce the inflammatory burden.”
Rosacea
Dietary triggers for rosacea are thought to include high-fat foods, dairy foods, spicy foods, hot drinks, cinnamon, and vanilla.
A population-based case-control study in China, which evaluated 1,347 rosacea patients and 1,290 healthy controls, found that a high intake of fatty foods positively correlated with erythematotelangiectatic rosacea (ETR) and phymatous rosacea. High-frequency dairy intake negatively correlated with ETR and papulopustular rosacea, which was a surprise, she said. And in this study, no significant correlations were found between sweets, coffee, and spicy foods. That goes against the traditional thinking, she said, but this was a Chinese cohort and their diet is probably vastly different than those in the United States.
Other rosacea triggers, Dr. Shi said, are niacin-containing foods such as turkey, chicken breast, crustaceans, dried Shiitake mushrooms, peanuts, tuna, and liver, as well as cold drinks, and formalin-containing foods (fish, squid, tofu, wet noodles).
As the field of nutrigenics – how genes affect how the body responds to food – evolves, more answers about the impact of diet on these diseases will be forthcoming, Dr. Shi said.
In an interactive panel discussion, she was asked if she talks about diet with all her patients with acne, rosacea, and HS, or just those not responding to traditional therapy.
“I think it’s an important conversation to have,” Dr. Shi responded. “When I’m done with the medication [instructions], I say: ‘There is something else you can do to augment what I just told you.’ ” That’s when she explains the dietary information. She also has a handout on diet and routinely refers patients for dietary counseling.
MedscapeLive and this news organization are owned by the same parent company. Dr. Shi disclosed consulting, investigative and research funding from several sources, but not directly related to the content of her talk.
Since 1950, the terms “diet and skin” in the medical literature have markedly increased, said Vivian Shi, MD associate professor of dermatology at the University of Arkansas for Medical Sciences, Little Rock, who talked about nutritional approaches for select skin diseases at MedscapeLive’s Women’s and Pediatric Dermatology Seminar.
Myths abound, but some associations of diet with skin diseases hold water, and she said.
Acne
What’s known, Dr. Shi said, is that the prevalence of acne is substantially lower in non-Westernized countries, and that diets in those countries generally have a low glycemic load, which decreases IGF-1 insulinlike growth factor 1 (IGF-1) concentrations, an accepted risk factor for acne. The Western diet also includes the hormonal effects of cow’s milk products.
Whey protein, which is popular as a supplement, isn’t good for acne, Dr. Shi said. It takes a couple of hours to digest, while casein protein digests more slowly, over 5-7 hours. If casein protein isn’t acceptable, good alternatives to whey protein are hemp seed, plant protein blends (peas, seeds, berries), egg white, brown rice isolate, and soy isolate protein.
Dairy products increase IGF-1 levels, hormonal mediators that can make acne worse. In addition, industrial cow’s milk can contain anabolic steroids and growth factor, leading to sebogenesis, Dr. Shi said. As for the type of milk, skim milk tends to be the most acnegenic and associated with the highest blood levels of IGF-1.
Supplementing with omega-3 fatty acids and gamma-linolenic acid improved mild to moderate acne in a double-blind, controlled study. Researchers randomized 45 patients with mild to moderate acne to an omega-3 fatty acid group (2,000 mg of eicosapentaenoic acid and docosahexaenoic acid), a gamma-linolenic acid group (borage oil with 400 mg gamma-linolenic acid) or a control group. After 10 weeks in both treatment groups, there was a significant reduction in inflammatory and noninflammatory lesions.
Those with acne are more likely to be deficient in Vitamin D, research suggests. Researchers also found that among those who had vitamin D deficiency, supplementing with 1,000 IU daily for 2 months reduced inflammatory lesions by 35% after 8 weeks, compared with a 6% reduction in the control group.
Other research has found that those with a low serum zinc level had more severe acne and that 30-200 mg of zinc orally for 2-4 months reduced inflammatory acne. However, Dr. Shi cautioned that those taking zinc for more than 2 months also need a copper supplement, as zinc reduces the amount of copper absorbed by the body.
Dr. Shi’s “do’s” diet list for acne patients is a follows: Paleolithic and Mediterranean diets, omega-3 fatty acids, gamma-linolenic acids, Vitamin D, zinc, tubers, legumes, vegetables, fruits, and fish.
Unknowns, she said, include chocolate, caffeine, green tea, and high salt.
Hidradenitis suppurativa
Patents with HS who follow a Mediterranean diet most closely have less severe disease, research has found. In this study, those patients with HS with the lowest adherence had a Sartorius HS score of 59.38, while those who followed it the most closely had a score of 39 (of 80).
In another study, patients with HS reported the following foods as exacerbating HS: sweets, bread/pasta/rice, dairy, and high-fat foods. Alleviating foods included vegetables, fruit, chicken, and fish.
Dr. Shi’s dietary recommendations for patients with HS: Follow a Mediterranean diet, avoid high fat foods and highly processed foods, and focus on eating more vegetables, fresh fruit, corn-based cereal, white meat, and fish.
A retrospective study of patients with Hurley stage 1 and 2 found that oral zinc gluconate, 90 mg a day, combined with 2% topical triclosan twice a day, resulted in significantly decreased HS scores and nodules and improved quality of life after 3 months. Expect vitamin D deficiency, she added.
Lastly, Dr. Shi recommended, if necessary, “weight loss to reduce the inflammatory burden.”
Rosacea
Dietary triggers for rosacea are thought to include high-fat foods, dairy foods, spicy foods, hot drinks, cinnamon, and vanilla.
A population-based case-control study in China, which evaluated 1,347 rosacea patients and 1,290 healthy controls, found that a high intake of fatty foods positively correlated with erythematotelangiectatic rosacea (ETR) and phymatous rosacea. High-frequency dairy intake negatively correlated with ETR and papulopustular rosacea, which was a surprise, she said. And in this study, no significant correlations were found between sweets, coffee, and spicy foods. That goes against the traditional thinking, she said, but this was a Chinese cohort and their diet is probably vastly different than those in the United States.
Other rosacea triggers, Dr. Shi said, are niacin-containing foods such as turkey, chicken breast, crustaceans, dried Shiitake mushrooms, peanuts, tuna, and liver, as well as cold drinks, and formalin-containing foods (fish, squid, tofu, wet noodles).
As the field of nutrigenics – how genes affect how the body responds to food – evolves, more answers about the impact of diet on these diseases will be forthcoming, Dr. Shi said.
In an interactive panel discussion, she was asked if she talks about diet with all her patients with acne, rosacea, and HS, or just those not responding to traditional therapy.
“I think it’s an important conversation to have,” Dr. Shi responded. “When I’m done with the medication [instructions], I say: ‘There is something else you can do to augment what I just told you.’ ” That’s when she explains the dietary information. She also has a handout on diet and routinely refers patients for dietary counseling.
MedscapeLive and this news organization are owned by the same parent company. Dr. Shi disclosed consulting, investigative and research funding from several sources, but not directly related to the content of her talk.
FROM MEDSCAPELIVE WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR
Diabetes devices may give children contact dermatitis
Devices that help children control their diabetes and lead fuller lives may also give them contact dermatitis, report the authors of a new study that calls for mandatory labeling of ingredients for allergy patch testing.
“A high share of patients showed positive reactions to isobornyl acrylate adhesive (IBOA) and/or their medical devices (insulin pumps or glucose devices),” the study authors write in Contact Dermatitis. “A third of patients showed positive reactions to benzoyl peroxide (BP),” used in adhesives.
“The presence of additional unidentified allergens cannot be excluded,” they add. “Overall, our experience once more highlights the importance of having access to a full description of the chemical composition of diabetes devices and related medical devices to efficiently manage patients (including children) who experience adverse skin reactions from such devices.”
Lead study author Catarina Alves da Silva, MD, of the department of dermatology and venereology of Aarhus (Denmark) University Hospital, and her colleagues conducted a retrospective study of 15 referred patients younger than 18 years who had type 1 diabetes. The children were patch tested in the university’s dermatology clinic between 2018 and 2020 in a study of skin reactions linked with diabetes devices.
Contact dermatitis from device-related allergens may be common
Many children in the study reacted to chemical compounds related to their devices.
- Of the 15 patients, seven showed positive patch test reactions to IBOA, and five showed positive reactions to BP.
- Ten children had positive patch test reactions to materials from glucose sensors and insulin pumps.
- Three showed positive reactions to adhesive remover wipes.
- Five reacted to .
Marcia Hogeling, MD, a pediatric dermatologist at UCLA Health in Santa Monica, Calif., told this news organization that she expected acrylates to cause problems but was surprised that BP caused positive patch test reactions.
BP is known to be a strong irritant but a weak allergen, the authors wrote.
“It was important to identify the allergens in these devices. Hopefully, this information will be used by manufacturers to create safer products for patients,” Dr. Hogeling, who was not involved in the study, said in an email.
Dr. Hogeling acknowledged that the small sample size is a weakness of the study, although she added that the findings may help providers select devices that do not contain their patients’ contact allergens.
Ryan J. McDonough, DO, a pediatric endocrinologist and the codirector of the Diabetes Center at Children’s Mercy Kansas City (Mo.), said in an email that, despite the small sample size, the study “highlights important device-related experiences of those living with type 1 diabetes that clinicians often encounter.
“We often spend considerable time aiding patients and their families in finding ways to mitigate the reactions,” he explained. “Having a broader understanding of these chemical compositions would help clinicians choose the right devices for their patients and prevent and treat these types of reactions.”
Dr. McDonough, who was not involved in the study, noted that the patients were in Denmark, and they were able to easily transition between insulin pumps and glucose monitoring devices.
“In the U.S., it is often more challenging to switch between devices, due to insurance-related concerns.
“The true rates of reaction in the broad type 1 diabetes population are difficult to assess,” Dr. McDonough said. “The study participants were drawn from patients referred to a dermatology clinic for evaluation of reaction. Many patients either don’t develop reactions or are treated for mild symptoms locally by their endocrinologists.
“This study should serve as a call to action for continued improvements in the transparency of the components that make up the devices and adhesives, and it can provide an opportunity to develop additional interventions to prevent these reactions,” he advised.
No information regarding funding for the study was provided. The authors, Dr. Hogeling, and Dr. McDonough reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Devices that help children control their diabetes and lead fuller lives may also give them contact dermatitis, report the authors of a new study that calls for mandatory labeling of ingredients for allergy patch testing.
“A high share of patients showed positive reactions to isobornyl acrylate adhesive (IBOA) and/or their medical devices (insulin pumps or glucose devices),” the study authors write in Contact Dermatitis. “A third of patients showed positive reactions to benzoyl peroxide (BP),” used in adhesives.
“The presence of additional unidentified allergens cannot be excluded,” they add. “Overall, our experience once more highlights the importance of having access to a full description of the chemical composition of diabetes devices and related medical devices to efficiently manage patients (including children) who experience adverse skin reactions from such devices.”
Lead study author Catarina Alves da Silva, MD, of the department of dermatology and venereology of Aarhus (Denmark) University Hospital, and her colleagues conducted a retrospective study of 15 referred patients younger than 18 years who had type 1 diabetes. The children were patch tested in the university’s dermatology clinic between 2018 and 2020 in a study of skin reactions linked with diabetes devices.
Contact dermatitis from device-related allergens may be common
Many children in the study reacted to chemical compounds related to their devices.
- Of the 15 patients, seven showed positive patch test reactions to IBOA, and five showed positive reactions to BP.
- Ten children had positive patch test reactions to materials from glucose sensors and insulin pumps.
- Three showed positive reactions to adhesive remover wipes.
- Five reacted to .
Marcia Hogeling, MD, a pediatric dermatologist at UCLA Health in Santa Monica, Calif., told this news organization that she expected acrylates to cause problems but was surprised that BP caused positive patch test reactions.
BP is known to be a strong irritant but a weak allergen, the authors wrote.
“It was important to identify the allergens in these devices. Hopefully, this information will be used by manufacturers to create safer products for patients,” Dr. Hogeling, who was not involved in the study, said in an email.
Dr. Hogeling acknowledged that the small sample size is a weakness of the study, although she added that the findings may help providers select devices that do not contain their patients’ contact allergens.
Ryan J. McDonough, DO, a pediatric endocrinologist and the codirector of the Diabetes Center at Children’s Mercy Kansas City (Mo.), said in an email that, despite the small sample size, the study “highlights important device-related experiences of those living with type 1 diabetes that clinicians often encounter.
“We often spend considerable time aiding patients and their families in finding ways to mitigate the reactions,” he explained. “Having a broader understanding of these chemical compositions would help clinicians choose the right devices for their patients and prevent and treat these types of reactions.”
Dr. McDonough, who was not involved in the study, noted that the patients were in Denmark, and they were able to easily transition between insulin pumps and glucose monitoring devices.
“In the U.S., it is often more challenging to switch between devices, due to insurance-related concerns.
“The true rates of reaction in the broad type 1 diabetes population are difficult to assess,” Dr. McDonough said. “The study participants were drawn from patients referred to a dermatology clinic for evaluation of reaction. Many patients either don’t develop reactions or are treated for mild symptoms locally by their endocrinologists.
“This study should serve as a call to action for continued improvements in the transparency of the components that make up the devices and adhesives, and it can provide an opportunity to develop additional interventions to prevent these reactions,” he advised.
No information regarding funding for the study was provided. The authors, Dr. Hogeling, and Dr. McDonough reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Devices that help children control their diabetes and lead fuller lives may also give them contact dermatitis, report the authors of a new study that calls for mandatory labeling of ingredients for allergy patch testing.
“A high share of patients showed positive reactions to isobornyl acrylate adhesive (IBOA) and/or their medical devices (insulin pumps or glucose devices),” the study authors write in Contact Dermatitis. “A third of patients showed positive reactions to benzoyl peroxide (BP),” used in adhesives.
“The presence of additional unidentified allergens cannot be excluded,” they add. “Overall, our experience once more highlights the importance of having access to a full description of the chemical composition of diabetes devices and related medical devices to efficiently manage patients (including children) who experience adverse skin reactions from such devices.”
Lead study author Catarina Alves da Silva, MD, of the department of dermatology and venereology of Aarhus (Denmark) University Hospital, and her colleagues conducted a retrospective study of 15 referred patients younger than 18 years who had type 1 diabetes. The children were patch tested in the university’s dermatology clinic between 2018 and 2020 in a study of skin reactions linked with diabetes devices.
Contact dermatitis from device-related allergens may be common
Many children in the study reacted to chemical compounds related to their devices.
- Of the 15 patients, seven showed positive patch test reactions to IBOA, and five showed positive reactions to BP.
- Ten children had positive patch test reactions to materials from glucose sensors and insulin pumps.
- Three showed positive reactions to adhesive remover wipes.
- Five reacted to .
Marcia Hogeling, MD, a pediatric dermatologist at UCLA Health in Santa Monica, Calif., told this news organization that she expected acrylates to cause problems but was surprised that BP caused positive patch test reactions.
BP is known to be a strong irritant but a weak allergen, the authors wrote.
“It was important to identify the allergens in these devices. Hopefully, this information will be used by manufacturers to create safer products for patients,” Dr. Hogeling, who was not involved in the study, said in an email.
Dr. Hogeling acknowledged that the small sample size is a weakness of the study, although she added that the findings may help providers select devices that do not contain their patients’ contact allergens.
Ryan J. McDonough, DO, a pediatric endocrinologist and the codirector of the Diabetes Center at Children’s Mercy Kansas City (Mo.), said in an email that, despite the small sample size, the study “highlights important device-related experiences of those living with type 1 diabetes that clinicians often encounter.
“We often spend considerable time aiding patients and their families in finding ways to mitigate the reactions,” he explained. “Having a broader understanding of these chemical compositions would help clinicians choose the right devices for their patients and prevent and treat these types of reactions.”
Dr. McDonough, who was not involved in the study, noted that the patients were in Denmark, and they were able to easily transition between insulin pumps and glucose monitoring devices.
“In the U.S., it is often more challenging to switch between devices, due to insurance-related concerns.
“The true rates of reaction in the broad type 1 diabetes population are difficult to assess,” Dr. McDonough said. “The study participants were drawn from patients referred to a dermatology clinic for evaluation of reaction. Many patients either don’t develop reactions or are treated for mild symptoms locally by their endocrinologists.
“This study should serve as a call to action for continued improvements in the transparency of the components that make up the devices and adhesives, and it can provide an opportunity to develop additional interventions to prevent these reactions,” he advised.
No information regarding funding for the study was provided. The authors, Dr. Hogeling, and Dr. McDonough reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Widespread rash in toddler
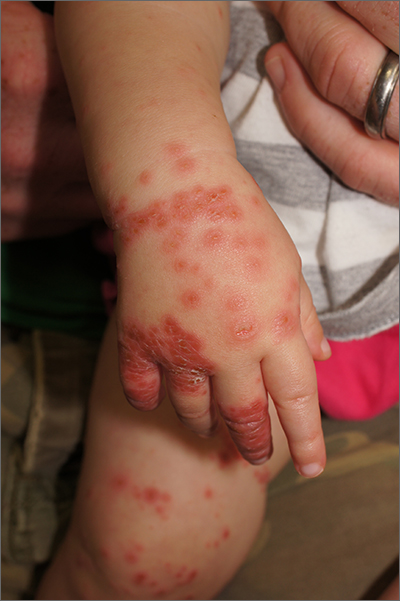
This patient was given a diagnosis of Gianotti Crosti syndrome (GCS; also called infantile acrodermatitis of childhood), which is a self-resolving (often dramatic) dermatosis triggered by a viral infection or immunization. Patients with this syndrome develop papules, vesicles, and plaques on their face, hands, feet, and extremities a week (or more) after having a viral illness or receiving an immunization. In patients with darker skin types, lesions may appear purple to brown rather than bright red to red/orange. The syndrome typically occurs in children between the ages of 1 to 4 years, but almost all patients are under the age of 15.1 Scratching and sleep disturbance are common. The condition typically resolves on its own after 3 or 4 weeks.
Globally, the hepatitis B virus (HBV) is the most common cause of GCS.1 Other reported triggering viruses include hepatitis A and C, cytomegalovirus, Epstein-Barr virus, enteroviruses, HIV, parainfluenza viruses, parvoviruses, rubella, and COVID-19.2
Since the cause of this patient’s case of GCS was likely linked to a viral infection that produced the loose stools in a population with low-HBV risk, no further serologic testing was performed. Serologic testing may have been necessary if other infections, disease risks, or symptoms were identified. To relieve itching, topical triamcinolone 0.1% cream was prescribed for use once to twice daily on the extremities and hydrocortisone 1% cream was prescribed once to twice daily for use on the child’s face. At the 6-week follow-up visit, the lesions had resolved; light pink discoloration remained but was expected to further fade. In patients with darker skin, post-inflammatory hyperpigmentation may take several months to resolve.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Brandt O, Abeck D, Gianotti R, et al. Gianotti-Crosti syndrome. J Am Acad Dermatol. 2006;54:136-45. doi: 10.1016/j.jaad.2005.09.033
2. Berná-Rico ED, Álvarez-Pinheiro C, Burgos-Blasco P, et al. A Gianotti-Crosti-like eruption in the setting of SARS-CoV-2 infection. Dermatol Ther. 2021;34:e15071. Doi:10.1111/dth.15071

This patient was given a diagnosis of Gianotti Crosti syndrome (GCS; also called infantile acrodermatitis of childhood), which is a self-resolving (often dramatic) dermatosis triggered by a viral infection or immunization. Patients with this syndrome develop papules, vesicles, and plaques on their face, hands, feet, and extremities a week (or more) after having a viral illness or receiving an immunization. In patients with darker skin types, lesions may appear purple to brown rather than bright red to red/orange. The syndrome typically occurs in children between the ages of 1 to 4 years, but almost all patients are under the age of 15.1 Scratching and sleep disturbance are common. The condition typically resolves on its own after 3 or 4 weeks.
Globally, the hepatitis B virus (HBV) is the most common cause of GCS.1 Other reported triggering viruses include hepatitis A and C, cytomegalovirus, Epstein-Barr virus, enteroviruses, HIV, parainfluenza viruses, parvoviruses, rubella, and COVID-19.2
Since the cause of this patient’s case of GCS was likely linked to a viral infection that produced the loose stools in a population with low-HBV risk, no further serologic testing was performed. Serologic testing may have been necessary if other infections, disease risks, or symptoms were identified. To relieve itching, topical triamcinolone 0.1% cream was prescribed for use once to twice daily on the extremities and hydrocortisone 1% cream was prescribed once to twice daily for use on the child’s face. At the 6-week follow-up visit, the lesions had resolved; light pink discoloration remained but was expected to further fade. In patients with darker skin, post-inflammatory hyperpigmentation may take several months to resolve.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).

This patient was given a diagnosis of Gianotti Crosti syndrome (GCS; also called infantile acrodermatitis of childhood), which is a self-resolving (often dramatic) dermatosis triggered by a viral infection or immunization. Patients with this syndrome develop papules, vesicles, and plaques on their face, hands, feet, and extremities a week (or more) after having a viral illness or receiving an immunization. In patients with darker skin types, lesions may appear purple to brown rather than bright red to red/orange. The syndrome typically occurs in children between the ages of 1 to 4 years, but almost all patients are under the age of 15.1 Scratching and sleep disturbance are common. The condition typically resolves on its own after 3 or 4 weeks.
Globally, the hepatitis B virus (HBV) is the most common cause of GCS.1 Other reported triggering viruses include hepatitis A and C, cytomegalovirus, Epstein-Barr virus, enteroviruses, HIV, parainfluenza viruses, parvoviruses, rubella, and COVID-19.2
Since the cause of this patient’s case of GCS was likely linked to a viral infection that produced the loose stools in a population with low-HBV risk, no further serologic testing was performed. Serologic testing may have been necessary if other infections, disease risks, or symptoms were identified. To relieve itching, topical triamcinolone 0.1% cream was prescribed for use once to twice daily on the extremities and hydrocortisone 1% cream was prescribed once to twice daily for use on the child’s face. At the 6-week follow-up visit, the lesions had resolved; light pink discoloration remained but was expected to further fade. In patients with darker skin, post-inflammatory hyperpigmentation may take several months to resolve.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Brandt O, Abeck D, Gianotti R, et al. Gianotti-Crosti syndrome. J Am Acad Dermatol. 2006;54:136-45. doi: 10.1016/j.jaad.2005.09.033
2. Berná-Rico ED, Álvarez-Pinheiro C, Burgos-Blasco P, et al. A Gianotti-Crosti-like eruption in the setting of SARS-CoV-2 infection. Dermatol Ther. 2021;34:e15071. Doi:10.1111/dth.15071
1. Brandt O, Abeck D, Gianotti R, et al. Gianotti-Crosti syndrome. J Am Acad Dermatol. 2006;54:136-45. doi: 10.1016/j.jaad.2005.09.033
2. Berná-Rico ED, Álvarez-Pinheiro C, Burgos-Blasco P, et al. A Gianotti-Crosti-like eruption in the setting of SARS-CoV-2 infection. Dermatol Ther. 2021;34:e15071. Doi:10.1111/dth.15071
No more ‘escape hatch’: Post Roe, new worries about meds linked to birth defects
As states ban or limit abortion in the wake of the demise of Roe v. Wade, physicians are turning their attention to widely-used drugs that can cause birth defects. At issue: Should these drugs still be prescribed to women of childbearing age if they don’t have the option of terminating their pregnancies?
“Doctors are going to understandably be terrified that a patient may become pregnant using a teratogen that they have prescribed,” said University of Pittsburgh rheumatologist Mehret Birru Talabi, MD, PhD, who works in a state where the future of abortion rights is uncertain. “While this was a feared outcome before Roe v. Wade was overturned, abortion provided an escape hatch by which women could avoid having to continue a pregnancy and potentially raise a child with congenital anomalies. I believe that prescribing is going to become much more defensive and conservative. Some clinicians may choose not to prescribe these medications to patients who have childbearing potential, even if they don’t have much risk for pregnancy.”
Other physicians expressed similar concerns in interviews. Duke University, Durham, N.C., rheumatologist Megan E. B. Clowse, MD, MPH, fears that physicians will be wary of prescribing a variety of medications – including new ones for which there are few pregnancy data – if abortion is unavailable. “Women who receive these new or teratogenic medications will likely lose their reproductive autonomy and be forced to choose between having sexual relationships with men, obtaining procedures that make them permanently sterile, or using contraception that may cause intolerable side effects,” she said. “I am very concerned that young women with rheumatic disease will now be left with active disease resulting in joint damage and renal failure.”
Abortion is now banned in at least six states, according to The New York Times. That number may rise to 16 as more restrictions become law. Another five states aren’t expected to ban abortion soon but have implemented gestational age limits on abortion or are expected to adopt them. In another nine states, courts or lawmakers will decide whether abortion remains legal.
Only 20 states and the District of Columbia have firm abortion protections in place.
Numerous drugs are considered teratogens, which means they may cause birth defects. Thalidomide is the most infamous, but there are many more, including several used in rheumatology, dermatology, and gastroenterology. Among the most widely used teratogenic medications are the acne drugs isotretinoin and methotrexate, which are used to treat a variety of conditions, such as cancer, rheumatoid arthritis, and psoriasis.
Dr. Clowse, who helps manage an industry-supported website devoted to reproductive care for women with lupus (www.LupusPregnancy.org), noted that several drugs linked to birth defects and pregnancy loss are commonly prescribed in rheumatology.
“Methotrexate is the most common medication and has been the cornerstone of rheumatoid arthritis [treatment] for at least two decades,” she said. “Mycophenolate is our best medication to treat lupus nephritis, which is inflammation in the kidneys caused by lupus. This is a common complication for young women with lupus, and all of our guideline-recommended treatment regimens include a medication that causes pregnancy loss and birth defects, either mycophenolate or cyclophosphamide.”
Rheumatologists also prescribe a large number of new drugs for which there are few data about pregnancy risks. “It typically takes about two decades to have sufficient data about the safety of our medications,” she said.
Reflecting the sensitivity of the topic, Dr. Clowse made clear that her opinions don’t represent the views of her institution. She works in North Carolina, where the fate of abortion rights is uncertain, according to The New York Times.
What about alternatives? “The short answer is that some of these medications work really well and sometimes much better than the nonteratogenic alternatives,” said Dr. Birru Talabi. “I’m worried about methotrexate. It has been used to induce abortions but is primarily used in the United States as a highly effective treatment for cancer as well as a myriad of rheumatic diseases. If legislators try to restrict access to methotrexate, we may see increasing disability and even death among people who need this medication but cannot access it.”
Rheumatologists aren’t the only physicians who are worrying about the fates of their patients in a new era of abortion restrictions. Gastroenterologist Sunanda Kane, MD, MSPH, of the Mayo Clinic, Rochester, Minn., said several teratogenic medications are used in her field to treat constipation, viral hepatitis, and inflammatory bowel disease.
“When treating women of childbearing age, there are usually alternatives. If we do prescribe a medication with a high teratogenic potential, we counsel and document that we have discussed two forms of birth control to avoid pregnancy. We usually do not prescribe a drug with teratogenic potential with the ‘out’ being an abortion if a pregnancy does occur,” she said. However, “if abortion is not even on the table as an option, we may be much less likely to prescribe these medications. This will be particularly true in patients who clearly do not have the means to travel to have an abortion in any situation.”
Abortion is expected to remain legal in Minnesota, where Dr. Kane practices, but it may be restricted or banned in nearby Wisconsin, depending on the state legislature. None of her patients have had abortions after becoming pregnant while taking the medications, she said, although she “did have a patient who because of her religious faith did not have an abortion after exposure and ended up with a stillbirth.”
The crackdown on abortion won’t just pose risks to patients who take potentially dangerous medications, physicians said. Dr. Kane said pregnancy itself is a significant risk for patients with “very active, uncontrolled gastrointestinal conditions where a pregnancy could be harmful to the mother’s health or result in offspring that are very unhealthy.” These include decompensated cirrhosis, uncontrolled Crohn’s disease or ulcerative colitis, refractory gastroparesis, uncontrolled celiac sprue, and chronic pancreatitis, she said.
“There have been times when after shared decisionmaking, a patient with very active inflammatory bowel disease has decided to terminate the pregnancy because of her own ongoing health issues,” she said. “Not having this option will potentially lead to disastrous results.”
Dr. Clowse, the Duke University rheumatologist, echoed Dr. Kane’s concerns about women who are too sick to bear children. “The removal of abortion rights puts the lives and quality of life for women with rheumatic disease at risk. For patients with lupus and other systemic rheumatic disease, pregnancy can be medically catastrophic, leading to permanent harm and even death to the woman and her offspring. I am worried that women in these conditions will die without lifesaving pregnancy terminations, due to worries about the legal consequences for their physicians.”
The U.S. Supreme Court’s ruling that overturned Roe v. Wade has also raised the prospect that the court could ultimately allow birth control to be restricted or outlawed.
While the ruling states that “nothing in this opinion should be understood to cast doubt on precedents that do not concern abortion,” Justice Clarence Thomas wrote a concurrence in which he said that the court should reconsider a 1960s ruling that forbids the banning of contraceptives. Republicans have dismissed concerns about bans being allowed, although Democrats, including the president and vice president, starkly warn that they could happen.
“If we as providers have to be concerned that there will be an unplanned pregnancy because of the lack of access to contraception,” Dr. Kane said, “this will have significant downstream consequences to the kind of care we can provide and might just drive some providers to not give care to female patients at all given this concern.”
The physicians quoted in this article report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
As states ban or limit abortion in the wake of the demise of Roe v. Wade, physicians are turning their attention to widely-used drugs that can cause birth defects. At issue: Should these drugs still be prescribed to women of childbearing age if they don’t have the option of terminating their pregnancies?
“Doctors are going to understandably be terrified that a patient may become pregnant using a teratogen that they have prescribed,” said University of Pittsburgh rheumatologist Mehret Birru Talabi, MD, PhD, who works in a state where the future of abortion rights is uncertain. “While this was a feared outcome before Roe v. Wade was overturned, abortion provided an escape hatch by which women could avoid having to continue a pregnancy and potentially raise a child with congenital anomalies. I believe that prescribing is going to become much more defensive and conservative. Some clinicians may choose not to prescribe these medications to patients who have childbearing potential, even if they don’t have much risk for pregnancy.”
Other physicians expressed similar concerns in interviews. Duke University, Durham, N.C., rheumatologist Megan E. B. Clowse, MD, MPH, fears that physicians will be wary of prescribing a variety of medications – including new ones for which there are few pregnancy data – if abortion is unavailable. “Women who receive these new or teratogenic medications will likely lose their reproductive autonomy and be forced to choose between having sexual relationships with men, obtaining procedures that make them permanently sterile, or using contraception that may cause intolerable side effects,” she said. “I am very concerned that young women with rheumatic disease will now be left with active disease resulting in joint damage and renal failure.”
Abortion is now banned in at least six states, according to The New York Times. That number may rise to 16 as more restrictions become law. Another five states aren’t expected to ban abortion soon but have implemented gestational age limits on abortion or are expected to adopt them. In another nine states, courts or lawmakers will decide whether abortion remains legal.
Only 20 states and the District of Columbia have firm abortion protections in place.
Numerous drugs are considered teratogens, which means they may cause birth defects. Thalidomide is the most infamous, but there are many more, including several used in rheumatology, dermatology, and gastroenterology. Among the most widely used teratogenic medications are the acne drugs isotretinoin and methotrexate, which are used to treat a variety of conditions, such as cancer, rheumatoid arthritis, and psoriasis.
Dr. Clowse, who helps manage an industry-supported website devoted to reproductive care for women with lupus (www.LupusPregnancy.org), noted that several drugs linked to birth defects and pregnancy loss are commonly prescribed in rheumatology.
“Methotrexate is the most common medication and has been the cornerstone of rheumatoid arthritis [treatment] for at least two decades,” she said. “Mycophenolate is our best medication to treat lupus nephritis, which is inflammation in the kidneys caused by lupus. This is a common complication for young women with lupus, and all of our guideline-recommended treatment regimens include a medication that causes pregnancy loss and birth defects, either mycophenolate or cyclophosphamide.”
Rheumatologists also prescribe a large number of new drugs for which there are few data about pregnancy risks. “It typically takes about two decades to have sufficient data about the safety of our medications,” she said.
Reflecting the sensitivity of the topic, Dr. Clowse made clear that her opinions don’t represent the views of her institution. She works in North Carolina, where the fate of abortion rights is uncertain, according to The New York Times.
What about alternatives? “The short answer is that some of these medications work really well and sometimes much better than the nonteratogenic alternatives,” said Dr. Birru Talabi. “I’m worried about methotrexate. It has been used to induce abortions but is primarily used in the United States as a highly effective treatment for cancer as well as a myriad of rheumatic diseases. If legislators try to restrict access to methotrexate, we may see increasing disability and even death among people who need this medication but cannot access it.”
Rheumatologists aren’t the only physicians who are worrying about the fates of their patients in a new era of abortion restrictions. Gastroenterologist Sunanda Kane, MD, MSPH, of the Mayo Clinic, Rochester, Minn., said several teratogenic medications are used in her field to treat constipation, viral hepatitis, and inflammatory bowel disease.
“When treating women of childbearing age, there are usually alternatives. If we do prescribe a medication with a high teratogenic potential, we counsel and document that we have discussed two forms of birth control to avoid pregnancy. We usually do not prescribe a drug with teratogenic potential with the ‘out’ being an abortion if a pregnancy does occur,” she said. However, “if abortion is not even on the table as an option, we may be much less likely to prescribe these medications. This will be particularly true in patients who clearly do not have the means to travel to have an abortion in any situation.”
Abortion is expected to remain legal in Minnesota, where Dr. Kane practices, but it may be restricted or banned in nearby Wisconsin, depending on the state legislature. None of her patients have had abortions after becoming pregnant while taking the medications, she said, although she “did have a patient who because of her religious faith did not have an abortion after exposure and ended up with a stillbirth.”
The crackdown on abortion won’t just pose risks to patients who take potentially dangerous medications, physicians said. Dr. Kane said pregnancy itself is a significant risk for patients with “very active, uncontrolled gastrointestinal conditions where a pregnancy could be harmful to the mother’s health or result in offspring that are very unhealthy.” These include decompensated cirrhosis, uncontrolled Crohn’s disease or ulcerative colitis, refractory gastroparesis, uncontrolled celiac sprue, and chronic pancreatitis, she said.
“There have been times when after shared decisionmaking, a patient with very active inflammatory bowel disease has decided to terminate the pregnancy because of her own ongoing health issues,” she said. “Not having this option will potentially lead to disastrous results.”
Dr. Clowse, the Duke University rheumatologist, echoed Dr. Kane’s concerns about women who are too sick to bear children. “The removal of abortion rights puts the lives and quality of life for women with rheumatic disease at risk. For patients with lupus and other systemic rheumatic disease, pregnancy can be medically catastrophic, leading to permanent harm and even death to the woman and her offspring. I am worried that women in these conditions will die without lifesaving pregnancy terminations, due to worries about the legal consequences for their physicians.”
The U.S. Supreme Court’s ruling that overturned Roe v. Wade has also raised the prospect that the court could ultimately allow birth control to be restricted or outlawed.
While the ruling states that “nothing in this opinion should be understood to cast doubt on precedents that do not concern abortion,” Justice Clarence Thomas wrote a concurrence in which he said that the court should reconsider a 1960s ruling that forbids the banning of contraceptives. Republicans have dismissed concerns about bans being allowed, although Democrats, including the president and vice president, starkly warn that they could happen.
“If we as providers have to be concerned that there will be an unplanned pregnancy because of the lack of access to contraception,” Dr. Kane said, “this will have significant downstream consequences to the kind of care we can provide and might just drive some providers to not give care to female patients at all given this concern.”
The physicians quoted in this article report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
As states ban or limit abortion in the wake of the demise of Roe v. Wade, physicians are turning their attention to widely-used drugs that can cause birth defects. At issue: Should these drugs still be prescribed to women of childbearing age if they don’t have the option of terminating their pregnancies?
“Doctors are going to understandably be terrified that a patient may become pregnant using a teratogen that they have prescribed,” said University of Pittsburgh rheumatologist Mehret Birru Talabi, MD, PhD, who works in a state where the future of abortion rights is uncertain. “While this was a feared outcome before Roe v. Wade was overturned, abortion provided an escape hatch by which women could avoid having to continue a pregnancy and potentially raise a child with congenital anomalies. I believe that prescribing is going to become much more defensive and conservative. Some clinicians may choose not to prescribe these medications to patients who have childbearing potential, even if they don’t have much risk for pregnancy.”
Other physicians expressed similar concerns in interviews. Duke University, Durham, N.C., rheumatologist Megan E. B. Clowse, MD, MPH, fears that physicians will be wary of prescribing a variety of medications – including new ones for which there are few pregnancy data – if abortion is unavailable. “Women who receive these new or teratogenic medications will likely lose their reproductive autonomy and be forced to choose between having sexual relationships with men, obtaining procedures that make them permanently sterile, or using contraception that may cause intolerable side effects,” she said. “I am very concerned that young women with rheumatic disease will now be left with active disease resulting in joint damage and renal failure.”
Abortion is now banned in at least six states, according to The New York Times. That number may rise to 16 as more restrictions become law. Another five states aren’t expected to ban abortion soon but have implemented gestational age limits on abortion or are expected to adopt them. In another nine states, courts or lawmakers will decide whether abortion remains legal.
Only 20 states and the District of Columbia have firm abortion protections in place.
Numerous drugs are considered teratogens, which means they may cause birth defects. Thalidomide is the most infamous, but there are many more, including several used in rheumatology, dermatology, and gastroenterology. Among the most widely used teratogenic medications are the acne drugs isotretinoin and methotrexate, which are used to treat a variety of conditions, such as cancer, rheumatoid arthritis, and psoriasis.
Dr. Clowse, who helps manage an industry-supported website devoted to reproductive care for women with lupus (www.LupusPregnancy.org), noted that several drugs linked to birth defects and pregnancy loss are commonly prescribed in rheumatology.
“Methotrexate is the most common medication and has been the cornerstone of rheumatoid arthritis [treatment] for at least two decades,” she said. “Mycophenolate is our best medication to treat lupus nephritis, which is inflammation in the kidneys caused by lupus. This is a common complication for young women with lupus, and all of our guideline-recommended treatment regimens include a medication that causes pregnancy loss and birth defects, either mycophenolate or cyclophosphamide.”
Rheumatologists also prescribe a large number of new drugs for which there are few data about pregnancy risks. “It typically takes about two decades to have sufficient data about the safety of our medications,” she said.
Reflecting the sensitivity of the topic, Dr. Clowse made clear that her opinions don’t represent the views of her institution. She works in North Carolina, where the fate of abortion rights is uncertain, according to The New York Times.
What about alternatives? “The short answer is that some of these medications work really well and sometimes much better than the nonteratogenic alternatives,” said Dr. Birru Talabi. “I’m worried about methotrexate. It has been used to induce abortions but is primarily used in the United States as a highly effective treatment for cancer as well as a myriad of rheumatic diseases. If legislators try to restrict access to methotrexate, we may see increasing disability and even death among people who need this medication but cannot access it.”
Rheumatologists aren’t the only physicians who are worrying about the fates of their patients in a new era of abortion restrictions. Gastroenterologist Sunanda Kane, MD, MSPH, of the Mayo Clinic, Rochester, Minn., said several teratogenic medications are used in her field to treat constipation, viral hepatitis, and inflammatory bowel disease.
“When treating women of childbearing age, there are usually alternatives. If we do prescribe a medication with a high teratogenic potential, we counsel and document that we have discussed two forms of birth control to avoid pregnancy. We usually do not prescribe a drug with teratogenic potential with the ‘out’ being an abortion if a pregnancy does occur,” she said. However, “if abortion is not even on the table as an option, we may be much less likely to prescribe these medications. This will be particularly true in patients who clearly do not have the means to travel to have an abortion in any situation.”
Abortion is expected to remain legal in Minnesota, where Dr. Kane practices, but it may be restricted or banned in nearby Wisconsin, depending on the state legislature. None of her patients have had abortions after becoming pregnant while taking the medications, she said, although she “did have a patient who because of her religious faith did not have an abortion after exposure and ended up with a stillbirth.”
The crackdown on abortion won’t just pose risks to patients who take potentially dangerous medications, physicians said. Dr. Kane said pregnancy itself is a significant risk for patients with “very active, uncontrolled gastrointestinal conditions where a pregnancy could be harmful to the mother’s health or result in offspring that are very unhealthy.” These include decompensated cirrhosis, uncontrolled Crohn’s disease or ulcerative colitis, refractory gastroparesis, uncontrolled celiac sprue, and chronic pancreatitis, she said.
“There have been times when after shared decisionmaking, a patient with very active inflammatory bowel disease has decided to terminate the pregnancy because of her own ongoing health issues,” she said. “Not having this option will potentially lead to disastrous results.”
Dr. Clowse, the Duke University rheumatologist, echoed Dr. Kane’s concerns about women who are too sick to bear children. “The removal of abortion rights puts the lives and quality of life for women with rheumatic disease at risk. For patients with lupus and other systemic rheumatic disease, pregnancy can be medically catastrophic, leading to permanent harm and even death to the woman and her offspring. I am worried that women in these conditions will die without lifesaving pregnancy terminations, due to worries about the legal consequences for their physicians.”
The U.S. Supreme Court’s ruling that overturned Roe v. Wade has also raised the prospect that the court could ultimately allow birth control to be restricted or outlawed.
While the ruling states that “nothing in this opinion should be understood to cast doubt on precedents that do not concern abortion,” Justice Clarence Thomas wrote a concurrence in which he said that the court should reconsider a 1960s ruling that forbids the banning of contraceptives. Republicans have dismissed concerns about bans being allowed, although Democrats, including the president and vice president, starkly warn that they could happen.
“If we as providers have to be concerned that there will be an unplanned pregnancy because of the lack of access to contraception,” Dr. Kane said, “this will have significant downstream consequences to the kind of care we can provide and might just drive some providers to not give care to female patients at all given this concern.”
The physicians quoted in this article report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Study finds higher risk of skin cancer after childhood organ transplant
A large study showing an increased risk of keratinocyte carcinoma (KC) in children who receive a solid-organ transplant highlights the need for early education about risk reduction and more research to determine optimal timing for screening, say an investigator and two dermatologists with expertise in transplant-related skin issues.
The increased incidence of KC in pediatric transplant recipients is “really high, so we definitely know there’s risk there,” just as there is for adult recipients of solid-organ transplants, said Cathryn Sibbald, MD, MSc, a dermatologist at the Hospital for Sick Children in Toronto and coauthor of a research letter published in June in JAMA Dermatology.
For their study, Dr. Sibbald and her coinvestigators turned to the Ontario Health Insurance plan database, which covers health care for Canadian citizens and qualified residents in the province. They identified 951 patients younger than the age of 18 who received a solid-organ transplant between 1991 and 2004 at an Ontario hospital
They then used a validated health insurance claims–based algorithm to identify diagnoses of KC for the transplant recipients and for more than 5 million age-matched controls. KC, including squamous and basal cell carcinoma, is the most prevalent skin cancer for people who have had a solid-organ transplant.
Fifteen posttransplant KCs (10 patients, 1.1%) were reported a mean of 13.1 years after transplant, with none reported in the first 4 years. The mean age at transplant was 7.8 years, and the mean age at KC diagnosis was 25.2 years. Kidney transplants were the most common (42.1% of transplantations). Most of the transplants recipients (eight patients) who developed KC had kidney transplantation, and most of them had functional graft at the time of KC diagnosis.
Researchers found an increased incidence of KC compared with that of the general population (standardized incidence ratio, 9.09; 95% confidence interval, 5.48-15.08). And the risk for KC increased with time since transplant, with adjusted hazard ratios for KC of 3.63 (95% CI, 0.51-25.77) for 1-5 years, 5.14 (95% CI, 1.28-20.55) for 5-10 years, and 4.80 (95% CI, 2.29-10.08) for 10 years or more, compared with the control population.
Several years ago, another research team performed a similar population-based cohort study of adult transplant recipients in Ontario and found a 6.6-times increased risk of KC in transplant recipients compared with the general population.
Sun protection and skin cancer screening
In commenting on the study, Sarah Arron, MD, PhD, a San Francisco Bay area dermatologist and immediate past president of the International Immunosuppression and Transplant Skin Cancer Collaborative (www.itscc.org), said she feels “reassured” that young transplant patients tend not to develop the skin cancer until young adulthood.
A ”large study like this is important because the overall rate of KC is low in this age group,” she noted.
The findings “suggest that we can focus our efforts on prevention during childhood, with sun protection and skin cancer education,” she said. “Then, as these children move into adulthood, we can begin screening with skin examinations. Of course, [any child] with a skin lesion or mole that concerns their parents or transplant team should be referred to dermatology for evaluation.”
Pediatric transplant recipients and their parents are most interested in learning about skin cancer prevention either before or immediately after transplantation, according to a survey by other researchers.
Intervention studies needed
The increased risk of KC probably stems largely from immunosuppression, said Dr. Sibbald in an interview. “We know [this is the case] in the older population, and it’s likely true in the younger population as well that it’s one of the primary drivers,” she said.
More research to extensively analyze risk factors should come next, she said. This includes “the granularity of what [immunosuppressants and other] medications are received, and at what dose and for what periods of time, so we can calculate cumulative exposure and its relation to risk,” she said.
Kristin Bibee, MD, PhD, assistant professor of dermatology at Johns Hopkins University in Baltimore, said she’d like to see further studies “evaluate appropriate interventions, like sun-protective behavior in childhood and adolescence or immunosuppression modulation, to prevent malignancy development.”
The optimal time and intensity of screening for young transplant recipients must still be determined, both Dr. Bibee and Dr. Arron said. Patients deemed through further research to be at higher risk may need earlier and/or more intensive surveillance.
The role of race in skin cancer risk in this population is “one question the study leaves open,” said Dr. Arron. U.S. studies have shown that among adult transplant recipients White patients are “at highest risk for the ultraviolet-associated melanoma and squamous cell carcinoma, followed by Asian and Latino patients. African Americans have had the lowest risk, but some still developed skin cancer after transplant,” she said.
Prior studies of cancer in pediatric transplant recipients have reported primarily on internal malignant neoplasms, with limited data on KC, Dr. Sibbald and coauthors wrote. It is possible the incidence of KS is underestimated in the new study because of “undiagnosed or unreported KCs,” they noted.
The new study was funded by a grant from the Pediatric Dermatology Research Alliance and a Hospital for Sick Children grant. In disclosures, Dr. Sibbald reported to JAMA Dermatology receiving grants from the alliance and from Paediatric Consultants Partnership during the conduct of the study. Dr. Arron and Dr. Bibee both said they have no disclosures relevant to the study and its content.
A large study showing an increased risk of keratinocyte carcinoma (KC) in children who receive a solid-organ transplant highlights the need for early education about risk reduction and more research to determine optimal timing for screening, say an investigator and two dermatologists with expertise in transplant-related skin issues.
The increased incidence of KC in pediatric transplant recipients is “really high, so we definitely know there’s risk there,” just as there is for adult recipients of solid-organ transplants, said Cathryn Sibbald, MD, MSc, a dermatologist at the Hospital for Sick Children in Toronto and coauthor of a research letter published in June in JAMA Dermatology.
For their study, Dr. Sibbald and her coinvestigators turned to the Ontario Health Insurance plan database, which covers health care for Canadian citizens and qualified residents in the province. They identified 951 patients younger than the age of 18 who received a solid-organ transplant between 1991 and 2004 at an Ontario hospital
They then used a validated health insurance claims–based algorithm to identify diagnoses of KC for the transplant recipients and for more than 5 million age-matched controls. KC, including squamous and basal cell carcinoma, is the most prevalent skin cancer for people who have had a solid-organ transplant.
Fifteen posttransplant KCs (10 patients, 1.1%) were reported a mean of 13.1 years after transplant, with none reported in the first 4 years. The mean age at transplant was 7.8 years, and the mean age at KC diagnosis was 25.2 years. Kidney transplants were the most common (42.1% of transplantations). Most of the transplants recipients (eight patients) who developed KC had kidney transplantation, and most of them had functional graft at the time of KC diagnosis.
Researchers found an increased incidence of KC compared with that of the general population (standardized incidence ratio, 9.09; 95% confidence interval, 5.48-15.08). And the risk for KC increased with time since transplant, with adjusted hazard ratios for KC of 3.63 (95% CI, 0.51-25.77) for 1-5 years, 5.14 (95% CI, 1.28-20.55) for 5-10 years, and 4.80 (95% CI, 2.29-10.08) for 10 years or more, compared with the control population.
Several years ago, another research team performed a similar population-based cohort study of adult transplant recipients in Ontario and found a 6.6-times increased risk of KC in transplant recipients compared with the general population.
Sun protection and skin cancer screening
In commenting on the study, Sarah Arron, MD, PhD, a San Francisco Bay area dermatologist and immediate past president of the International Immunosuppression and Transplant Skin Cancer Collaborative (www.itscc.org), said she feels “reassured” that young transplant patients tend not to develop the skin cancer until young adulthood.
A ”large study like this is important because the overall rate of KC is low in this age group,” she noted.
The findings “suggest that we can focus our efforts on prevention during childhood, with sun protection and skin cancer education,” she said. “Then, as these children move into adulthood, we can begin screening with skin examinations. Of course, [any child] with a skin lesion or mole that concerns their parents or transplant team should be referred to dermatology for evaluation.”
Pediatric transplant recipients and their parents are most interested in learning about skin cancer prevention either before or immediately after transplantation, according to a survey by other researchers.
Intervention studies needed
The increased risk of KC probably stems largely from immunosuppression, said Dr. Sibbald in an interview. “We know [this is the case] in the older population, and it’s likely true in the younger population as well that it’s one of the primary drivers,” she said.
More research to extensively analyze risk factors should come next, she said. This includes “the granularity of what [immunosuppressants and other] medications are received, and at what dose and for what periods of time, so we can calculate cumulative exposure and its relation to risk,” she said.
Kristin Bibee, MD, PhD, assistant professor of dermatology at Johns Hopkins University in Baltimore, said she’d like to see further studies “evaluate appropriate interventions, like sun-protective behavior in childhood and adolescence or immunosuppression modulation, to prevent malignancy development.”
The optimal time and intensity of screening for young transplant recipients must still be determined, both Dr. Bibee and Dr. Arron said. Patients deemed through further research to be at higher risk may need earlier and/or more intensive surveillance.
The role of race in skin cancer risk in this population is “one question the study leaves open,” said Dr. Arron. U.S. studies have shown that among adult transplant recipients White patients are “at highest risk for the ultraviolet-associated melanoma and squamous cell carcinoma, followed by Asian and Latino patients. African Americans have had the lowest risk, but some still developed skin cancer after transplant,” she said.
Prior studies of cancer in pediatric transplant recipients have reported primarily on internal malignant neoplasms, with limited data on KC, Dr. Sibbald and coauthors wrote. It is possible the incidence of KS is underestimated in the new study because of “undiagnosed or unreported KCs,” they noted.
The new study was funded by a grant from the Pediatric Dermatology Research Alliance and a Hospital for Sick Children grant. In disclosures, Dr. Sibbald reported to JAMA Dermatology receiving grants from the alliance and from Paediatric Consultants Partnership during the conduct of the study. Dr. Arron and Dr. Bibee both said they have no disclosures relevant to the study and its content.
A large study showing an increased risk of keratinocyte carcinoma (KC) in children who receive a solid-organ transplant highlights the need for early education about risk reduction and more research to determine optimal timing for screening, say an investigator and two dermatologists with expertise in transplant-related skin issues.
The increased incidence of KC in pediatric transplant recipients is “really high, so we definitely know there’s risk there,” just as there is for adult recipients of solid-organ transplants, said Cathryn Sibbald, MD, MSc, a dermatologist at the Hospital for Sick Children in Toronto and coauthor of a research letter published in June in JAMA Dermatology.
For their study, Dr. Sibbald and her coinvestigators turned to the Ontario Health Insurance plan database, which covers health care for Canadian citizens and qualified residents in the province. They identified 951 patients younger than the age of 18 who received a solid-organ transplant between 1991 and 2004 at an Ontario hospital
They then used a validated health insurance claims–based algorithm to identify diagnoses of KC for the transplant recipients and for more than 5 million age-matched controls. KC, including squamous and basal cell carcinoma, is the most prevalent skin cancer for people who have had a solid-organ transplant.
Fifteen posttransplant KCs (10 patients, 1.1%) were reported a mean of 13.1 years after transplant, with none reported in the first 4 years. The mean age at transplant was 7.8 years, and the mean age at KC diagnosis was 25.2 years. Kidney transplants were the most common (42.1% of transplantations). Most of the transplants recipients (eight patients) who developed KC had kidney transplantation, and most of them had functional graft at the time of KC diagnosis.
Researchers found an increased incidence of KC compared with that of the general population (standardized incidence ratio, 9.09; 95% confidence interval, 5.48-15.08). And the risk for KC increased with time since transplant, with adjusted hazard ratios for KC of 3.63 (95% CI, 0.51-25.77) for 1-5 years, 5.14 (95% CI, 1.28-20.55) for 5-10 years, and 4.80 (95% CI, 2.29-10.08) for 10 years or more, compared with the control population.
Several years ago, another research team performed a similar population-based cohort study of adult transplant recipients in Ontario and found a 6.6-times increased risk of KC in transplant recipients compared with the general population.
Sun protection and skin cancer screening
In commenting on the study, Sarah Arron, MD, PhD, a San Francisco Bay area dermatologist and immediate past president of the International Immunosuppression and Transplant Skin Cancer Collaborative (www.itscc.org), said she feels “reassured” that young transplant patients tend not to develop the skin cancer until young adulthood.
A ”large study like this is important because the overall rate of KC is low in this age group,” she noted.
The findings “suggest that we can focus our efforts on prevention during childhood, with sun protection and skin cancer education,” she said. “Then, as these children move into adulthood, we can begin screening with skin examinations. Of course, [any child] with a skin lesion or mole that concerns their parents or transplant team should be referred to dermatology for evaluation.”
Pediatric transplant recipients and their parents are most interested in learning about skin cancer prevention either before or immediately after transplantation, according to a survey by other researchers.
Intervention studies needed
The increased risk of KC probably stems largely from immunosuppression, said Dr. Sibbald in an interview. “We know [this is the case] in the older population, and it’s likely true in the younger population as well that it’s one of the primary drivers,” she said.
More research to extensively analyze risk factors should come next, she said. This includes “the granularity of what [immunosuppressants and other] medications are received, and at what dose and for what periods of time, so we can calculate cumulative exposure and its relation to risk,” she said.
Kristin Bibee, MD, PhD, assistant professor of dermatology at Johns Hopkins University in Baltimore, said she’d like to see further studies “evaluate appropriate interventions, like sun-protective behavior in childhood and adolescence or immunosuppression modulation, to prevent malignancy development.”
The optimal time and intensity of screening for young transplant recipients must still be determined, both Dr. Bibee and Dr. Arron said. Patients deemed through further research to be at higher risk may need earlier and/or more intensive surveillance.
The role of race in skin cancer risk in this population is “one question the study leaves open,” said Dr. Arron. U.S. studies have shown that among adult transplant recipients White patients are “at highest risk for the ultraviolet-associated melanoma and squamous cell carcinoma, followed by Asian and Latino patients. African Americans have had the lowest risk, but some still developed skin cancer after transplant,” she said.
Prior studies of cancer in pediatric transplant recipients have reported primarily on internal malignant neoplasms, with limited data on KC, Dr. Sibbald and coauthors wrote. It is possible the incidence of KS is underestimated in the new study because of “undiagnosed or unreported KCs,” they noted.
The new study was funded by a grant from the Pediatric Dermatology Research Alliance and a Hospital for Sick Children grant. In disclosures, Dr. Sibbald reported to JAMA Dermatology receiving grants from the alliance and from Paediatric Consultants Partnership during the conduct of the study. Dr. Arron and Dr. Bibee both said they have no disclosures relevant to the study and its content.
FROM JAMA DERMATOLOGY
Introduce allergens early, say French allergists
Although in many cases, food-allergen tolerance can be achieved with oral immunotherapy, primary prevention of food allergies remains crucial, according to the French Society of Allergology. In new recommendations that were presented at a session of the Congress of French Pediatric Societies, the academic society advocated early introduction of allergens for all children, starting at 4 months of age.
The latest prevention data from two major studies, LEAP and EAT, have prompted European and French experts to rethink their stance on food diversification. The new French proposals were recently published under the coordination of Dominique Sabouraud-Leclerc, MD, pediatrics department, Reims (France) University Hospital, on behalf of the Food Allergy Working Group of the French Society of Allergology.
For all newborns, regardless of whether they have a history of atopic or nonatopic dermatitis, food diversification is now recommended from 4 months of age instead of 6 months, as was previously recommended. If the child does not develop atopic dermatitis or develops only a mild form, peanuts, eggs, and nuts may be introduced at home.
However, if the child experiences severe atopic dermatitis, an allergy testing panel for peanuts, nuts, eggs, and cow’s milk proteins should be performed. An oral food challenge may be conducted at the allergist’s discretion.
Regarding peanuts, the working group proposed introducing a purée in the form of either a mixture of peanuts/hazelnuts/cashew nuts (1 level teaspoon five times a week; 2 g of protein/food per week) or a 100% peanut paste (1 scant teaspoon four times a week; 2 g of peanut protein/week). If the family is worried, the allergist can suggest monitoring the child in the clinic waiting room for 30 minutes after the first dose.
“We shouldn’t delay the introduction of the primary allergens anymore, regardless of whether children are at risk for a food allergy, and particularly a peanut allergy,” explained Stéphanie Lejeune, MD, pediatric pulmonologist and allergist at Lille (France) Regional University Hospital, who presented these new findings at the congress. “In fact, if we only target at-risk children, we overlook children with no family history who will nevertheless develop food allergies. The idea is to introduce everything, especially peanuts, between 4 and 6 months of age and to no longer do so gradually, one food after another, as was being done until now, beginning at 6 months and over. We must give priority to regularity over quantity.”
Although this approach is based on clinical trials, no real-life data are currently available.
LEAP and EAT studies support early introduction of peanuts
A study from 2021 summed up the risk factors for peanut allergy. About 61% of infants (4-11 months) had atopic dermatitis, 18% had a food allergy, 62% had a first-degree relative with a peanut allergy, and 11% had a confirmed peanut allergy. The risk of peanut allergy increased with age and severe eczema.
In 2015, the LEAP study, which was conducted in the United Kingdom with 640 infants aged 4-11 months who had risk factors for peanut allergy, revolutionized peanut-allergy primary prevention. Regardless of whether the children were sensitized or not, the number of children who developed a peanut allergy was systematically lower in the group that ingested the allergen in comparison with the “avoidance” group.
Additionally, the LEAP-ON study showed that protection against peanut allergy persisted for 12 months after cessation of consumption between ages 5 and 6 years among children who had consumed peanuts previously.
Early diversification in the general population was investigated in the EAT study, which involved 1303 breastfed infants. Of these infants, 24% had atopic dermatitis (median SCORAD score, 7.5). They were divided into two arms: avoidance and breast feeding until 6 months (standard introduction) or early introduction at 3 months (boiled egg, milk, peanuts, sesame, white fish, wheat, 2 g of protein twice a week). In the per-protocol analysis, there were 13 cases of peanut allergy in the standard introduction group; there were no cases in the early introduction group.
A version of this article first appeared on Medscape.com.
Although in many cases, food-allergen tolerance can be achieved with oral immunotherapy, primary prevention of food allergies remains crucial, according to the French Society of Allergology. In new recommendations that were presented at a session of the Congress of French Pediatric Societies, the academic society advocated early introduction of allergens for all children, starting at 4 months of age.
The latest prevention data from two major studies, LEAP and EAT, have prompted European and French experts to rethink their stance on food diversification. The new French proposals were recently published under the coordination of Dominique Sabouraud-Leclerc, MD, pediatrics department, Reims (France) University Hospital, on behalf of the Food Allergy Working Group of the French Society of Allergology.
For all newborns, regardless of whether they have a history of atopic or nonatopic dermatitis, food diversification is now recommended from 4 months of age instead of 6 months, as was previously recommended. If the child does not develop atopic dermatitis or develops only a mild form, peanuts, eggs, and nuts may be introduced at home.
However, if the child experiences severe atopic dermatitis, an allergy testing panel for peanuts, nuts, eggs, and cow’s milk proteins should be performed. An oral food challenge may be conducted at the allergist’s discretion.
Regarding peanuts, the working group proposed introducing a purée in the form of either a mixture of peanuts/hazelnuts/cashew nuts (1 level teaspoon five times a week; 2 g of protein/food per week) or a 100% peanut paste (1 scant teaspoon four times a week; 2 g of peanut protein/week). If the family is worried, the allergist can suggest monitoring the child in the clinic waiting room for 30 minutes after the first dose.
“We shouldn’t delay the introduction of the primary allergens anymore, regardless of whether children are at risk for a food allergy, and particularly a peanut allergy,” explained Stéphanie Lejeune, MD, pediatric pulmonologist and allergist at Lille (France) Regional University Hospital, who presented these new findings at the congress. “In fact, if we only target at-risk children, we overlook children with no family history who will nevertheless develop food allergies. The idea is to introduce everything, especially peanuts, between 4 and 6 months of age and to no longer do so gradually, one food after another, as was being done until now, beginning at 6 months and over. We must give priority to regularity over quantity.”
Although this approach is based on clinical trials, no real-life data are currently available.
LEAP and EAT studies support early introduction of peanuts
A study from 2021 summed up the risk factors for peanut allergy. About 61% of infants (4-11 months) had atopic dermatitis, 18% had a food allergy, 62% had a first-degree relative with a peanut allergy, and 11% had a confirmed peanut allergy. The risk of peanut allergy increased with age and severe eczema.
In 2015, the LEAP study, which was conducted in the United Kingdom with 640 infants aged 4-11 months who had risk factors for peanut allergy, revolutionized peanut-allergy primary prevention. Regardless of whether the children were sensitized or not, the number of children who developed a peanut allergy was systematically lower in the group that ingested the allergen in comparison with the “avoidance” group.
Additionally, the LEAP-ON study showed that protection against peanut allergy persisted for 12 months after cessation of consumption between ages 5 and 6 years among children who had consumed peanuts previously.
Early diversification in the general population was investigated in the EAT study, which involved 1303 breastfed infants. Of these infants, 24% had atopic dermatitis (median SCORAD score, 7.5). They were divided into two arms: avoidance and breast feeding until 6 months (standard introduction) or early introduction at 3 months (boiled egg, milk, peanuts, sesame, white fish, wheat, 2 g of protein twice a week). In the per-protocol analysis, there were 13 cases of peanut allergy in the standard introduction group; there were no cases in the early introduction group.
A version of this article first appeared on Medscape.com.
Although in many cases, food-allergen tolerance can be achieved with oral immunotherapy, primary prevention of food allergies remains crucial, according to the French Society of Allergology. In new recommendations that were presented at a session of the Congress of French Pediatric Societies, the academic society advocated early introduction of allergens for all children, starting at 4 months of age.
The latest prevention data from two major studies, LEAP and EAT, have prompted European and French experts to rethink their stance on food diversification. The new French proposals were recently published under the coordination of Dominique Sabouraud-Leclerc, MD, pediatrics department, Reims (France) University Hospital, on behalf of the Food Allergy Working Group of the French Society of Allergology.
For all newborns, regardless of whether they have a history of atopic or nonatopic dermatitis, food diversification is now recommended from 4 months of age instead of 6 months, as was previously recommended. If the child does not develop atopic dermatitis or develops only a mild form, peanuts, eggs, and nuts may be introduced at home.
However, if the child experiences severe atopic dermatitis, an allergy testing panel for peanuts, nuts, eggs, and cow’s milk proteins should be performed. An oral food challenge may be conducted at the allergist’s discretion.
Regarding peanuts, the working group proposed introducing a purée in the form of either a mixture of peanuts/hazelnuts/cashew nuts (1 level teaspoon five times a week; 2 g of protein/food per week) or a 100% peanut paste (1 scant teaspoon four times a week; 2 g of peanut protein/week). If the family is worried, the allergist can suggest monitoring the child in the clinic waiting room for 30 minutes after the first dose.
“We shouldn’t delay the introduction of the primary allergens anymore, regardless of whether children are at risk for a food allergy, and particularly a peanut allergy,” explained Stéphanie Lejeune, MD, pediatric pulmonologist and allergist at Lille (France) Regional University Hospital, who presented these new findings at the congress. “In fact, if we only target at-risk children, we overlook children with no family history who will nevertheless develop food allergies. The idea is to introduce everything, especially peanuts, between 4 and 6 months of age and to no longer do so gradually, one food after another, as was being done until now, beginning at 6 months and over. We must give priority to regularity over quantity.”
Although this approach is based on clinical trials, no real-life data are currently available.
LEAP and EAT studies support early introduction of peanuts
A study from 2021 summed up the risk factors for peanut allergy. About 61% of infants (4-11 months) had atopic dermatitis, 18% had a food allergy, 62% had a first-degree relative with a peanut allergy, and 11% had a confirmed peanut allergy. The risk of peanut allergy increased with age and severe eczema.
In 2015, the LEAP study, which was conducted in the United Kingdom with 640 infants aged 4-11 months who had risk factors for peanut allergy, revolutionized peanut-allergy primary prevention. Regardless of whether the children were sensitized or not, the number of children who developed a peanut allergy was systematically lower in the group that ingested the allergen in comparison with the “avoidance” group.
Additionally, the LEAP-ON study showed that protection against peanut allergy persisted for 12 months after cessation of consumption between ages 5 and 6 years among children who had consumed peanuts previously.
Early diversification in the general population was investigated in the EAT study, which involved 1303 breastfed infants. Of these infants, 24% had atopic dermatitis (median SCORAD score, 7.5). They were divided into two arms: avoidance and breast feeding until 6 months (standard introduction) or early introduction at 3 months (boiled egg, milk, peanuts, sesame, white fish, wheat, 2 g of protein twice a week). In the per-protocol analysis, there were 13 cases of peanut allergy in the standard introduction group; there were no cases in the early introduction group.
A version of this article first appeared on Medscape.com.