User login
These images of diabetic retinopathy tell the story better
I read, with great interest, Dr. Farford’s thorough review article “Diabetic retinopathy: the FP’s role in preserving vision” (J Fam Pract. 2020;69:120-126). I am a family physician with ophthalmology training. For more than 20 years, I have regularly performed dilated eye exams and reviewed nonmydriatic fundus photos for uninsured patients with diabetic retinopathy (DR) at the community health clinic where I work. The burden of visual loss from poorly controlled diabetes is staggering.
I do, however, want to point out some inaccuracies in the labeling of 2 of the photos included in Table 1.
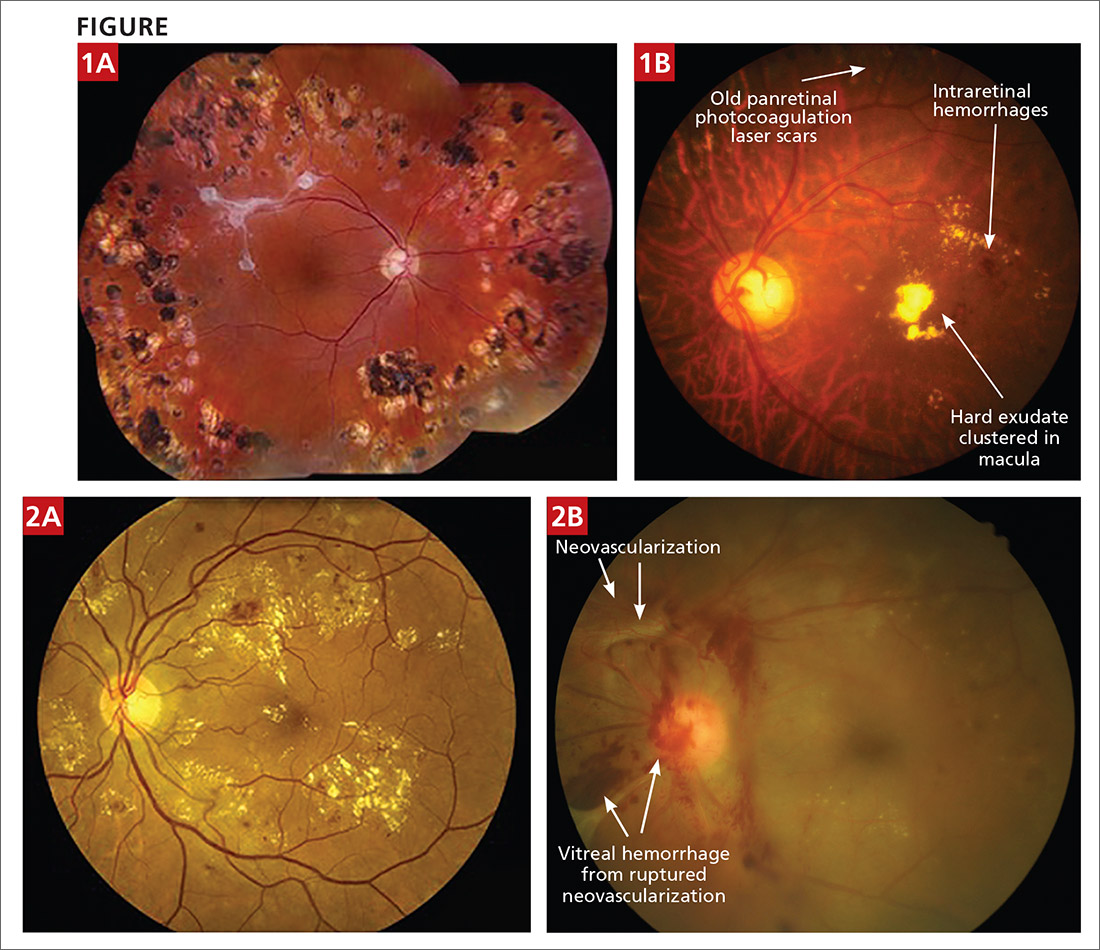
- The photo labeled “Severe NPDR [nonproliferative DR]”—Figure 1A—actually shows an eye that has been treated with panretinal photocoagulation (multiple laser scars present in all quadrants) with nice regression of DR. Along the superior temporal arcade there is fibrosis, which likely represents regression of vitreal neovascularization or resolution of vitreal hemorrhage. There is little apparent active DR in this photo. The caption indicated the presence of intraretinal microvascular abnormalities; however, while these abnormalities may be present, they are not evident due to the photo resolution.
- The photo labeled “Proliferative diabetic retinopathy”—Figure 2a—does not show evidence of neovascularization of the disc or the retina. This photo would be more accurately labeled “severe DR with likely clinically significant macular edema.”
The 2 photos shown here, from my photo collection, are perhaps more instructive:
- FIGURE 1B is an example of severe NPDR and maculopathy (this eye has undergone previous panretinal photocoagulation, a treatment option for severe NPDR and proliferative DR [defined as new vessel growth or neovascularization]).
- FIGURE 2b is an example of proliferative DR with vitreal hemorrhage that can lead to irreversible visual loss via traction retinal detachment.
I appreciate your efforts in publishing Dr. Farford’s article. DR is a broad, complicated topic, and this informative article will help many FPs.
Kenneth Libre, MD
Central City Community Health Center
Salt Lake City, UT
I read, with great interest, Dr. Farford’s thorough review article “Diabetic retinopathy: the FP’s role in preserving vision” (J Fam Pract. 2020;69:120-126). I am a family physician with ophthalmology training. For more than 20 years, I have regularly performed dilated eye exams and reviewed nonmydriatic fundus photos for uninsured patients with diabetic retinopathy (DR) at the community health clinic where I work. The burden of visual loss from poorly controlled diabetes is staggering.
I do, however, want to point out some inaccuracies in the labeling of 2 of the photos included in Table 1.
- The photo labeled “Severe NPDR [nonproliferative DR]”—Figure 1A—actually shows an eye that has been treated with panretinal photocoagulation (multiple laser scars present in all quadrants) with nice regression of DR. Along the superior temporal arcade there is fibrosis, which likely represents regression of vitreal neovascularization or resolution of vitreal hemorrhage. There is little apparent active DR in this photo. The caption indicated the presence of intraretinal microvascular abnormalities; however, while these abnormalities may be present, they are not evident due to the photo resolution.
- The photo labeled “Proliferative diabetic retinopathy”—Figure 2a—does not show evidence of neovascularization of the disc or the retina. This photo would be more accurately labeled “severe DR with likely clinically significant macular edema.”
The 2 photos shown here, from my photo collection, are perhaps more instructive:
- FIGURE 1B is an example of severe NPDR and maculopathy (this eye has undergone previous panretinal photocoagulation, a treatment option for severe NPDR and proliferative DR [defined as new vessel growth or neovascularization]).
- FIGURE 2b is an example of proliferative DR with vitreal hemorrhage that can lead to irreversible visual loss via traction retinal detachment.

I appreciate your efforts in publishing Dr. Farford’s article. DR is a broad, complicated topic, and this informative article will help many FPs.
Kenneth Libre, MD
Central City Community Health Center
Salt Lake City, UT
I read, with great interest, Dr. Farford’s thorough review article “Diabetic retinopathy: the FP’s role in preserving vision” (J Fam Pract. 2020;69:120-126). I am a family physician with ophthalmology training. For more than 20 years, I have regularly performed dilated eye exams and reviewed nonmydriatic fundus photos for uninsured patients with diabetic retinopathy (DR) at the community health clinic where I work. The burden of visual loss from poorly controlled diabetes is staggering.
I do, however, want to point out some inaccuracies in the labeling of 2 of the photos included in Table 1.
- The photo labeled “Severe NPDR [nonproliferative DR]”—Figure 1A—actually shows an eye that has been treated with panretinal photocoagulation (multiple laser scars present in all quadrants) with nice regression of DR. Along the superior temporal arcade there is fibrosis, which likely represents regression of vitreal neovascularization or resolution of vitreal hemorrhage. There is little apparent active DR in this photo. The caption indicated the presence of intraretinal microvascular abnormalities; however, while these abnormalities may be present, they are not evident due to the photo resolution.
- The photo labeled “Proliferative diabetic retinopathy”—Figure 2a—does not show evidence of neovascularization of the disc or the retina. This photo would be more accurately labeled “severe DR with likely clinically significant macular edema.”
The 2 photos shown here, from my photo collection, are perhaps more instructive:
- FIGURE 1B is an example of severe NPDR and maculopathy (this eye has undergone previous panretinal photocoagulation, a treatment option for severe NPDR and proliferative DR [defined as new vessel growth or neovascularization]).
- FIGURE 2b is an example of proliferative DR with vitreal hemorrhage that can lead to irreversible visual loss via traction retinal detachment.

I appreciate your efforts in publishing Dr. Farford’s article. DR is a broad, complicated topic, and this informative article will help many FPs.
Kenneth Libre, MD
Central City Community Health Center
Salt Lake City, UT
T2D treatments create tension between glycemic and cardiovascular goals
It was no surprise that updated guidelines recently published by the European Society of Cardiology for managing cardiovascular disease in patients with diabetes highlighted optimized treatment from a cardiovascular disease perspective, while a nearly concurrent update from two major diabetes societies saw the same issue from a more glycemic point of view.
This difference led to divergent approaches to managing hyperglycemia in patients with type 2 diabetes (T2D). The two diabetes societies that wrote one set of recommendations, the American Diabetes Association and the European Association for the Study of Diabetes, put metformin at the pinnacle of their drug hierarchy. Patients with T2D and established atherosclerotic cardiovascular disease (CVD), chronic kidney disease, or heart failure should all receive metformin first unless contraindicated or not tolerated, their updated consensus report said.
Once metformin is on board, a clinician can then add a second diabetes agent from among the two drug classes recently proven to also reduce cardiovascular and renal events, either the SGLT2 (sodium-glucose transporter 2) inhibitors, or GLP-1 (glucagonlike peptide–1) receptor agonists, they advised.
Cardiovascular disease focus represents a ‘major paradigm shift’
In contrast, the ESC guidelines called for upfront, systematic assessment of CVD risk in patients with T2D before treatment starts, and for patients in high- or very high–risk strata, the guidelines recommended starting the patient first on an SGLT2 inhibitor or a GLP-1 receptor agonist, and only adding metformin in patients who need additional glycemic control.
The guidelines also recommended starting treatment-naive patients with moderate CVD risk on metformin. For patients already on metformin, the new ESC guidelines called for adding an agent from at least one of these two drug classes with proven CVD benefits for those at high or very high CVD risk. The guidelines also note that the CVD benefits of the two newer drug classes differ and hence require further individualization depending on the risks faced by each patient, such as the risk for heart failure hospitalizations.
It’s an approach “driven by data from the cardiovascular outcome trials,” that showed several drugs from both the SGLT2 inhibitor and GLP-1 receptor agonist classes have substantial benefit for preventing cardiovascular events, renal events, hospitalizations for heart failure, and in some studies all-cause mortality, said Francesco Cosentino, MD, during a discussion of the guideline differences at the virtual annual meeting of the European Association for the Study of Diabetes.
The ESC approach also represents “a major paradigm shift,” a “change from a glucose-centric approach to an approach driven by cardiovascular disease events,” summed up Dr. Cosentino, professor of cardiology at the Karolinska Institute in Stockholm and chair of the task force that wrote the ESC’s 2019 updated guidelines. The ESC approach advocates initiating drugs for treating patients with T2D “based on cardiovascular disease risk classification,” he highlighted. Results from some SGLT2 inhibitor cardiovascular outcome trials showed that the CVD benefit was similar regardless of whether or not patients also received metformin.
ADA, EASD call for ‘a different emphasis’
“There is a different emphasis” in the statement issued by the diabetologists of the ADA and EASD, admitted Peter J. Grant, MD, a professor of diabetes and endocrinology at the University of Leeds (England) and cochair of the ESC guidelines task force. Dr. Grant represented the EASD on the task force, and the Association collaborated with the ESC in producing its guidelines.
“The ADA and EASD recommendations “look primarily at glucose control, with cardiovascular disease management as secondary.” In contrast, the ESC guidelines “are primarily cardiovascular disease risk guidelines, with a glucose interest,” Dr. Grant declared.
Despite his involvement in writing the ESC guidelines, Dr. Grant tilted toward the ADA/EASD statement as more globally relevant.
“There is much more to vasculopathy in diabetes than just macrovascular disease. Many patients with type 2 diabetes without macrovascular complications have microvascular disease,” including the potential for retinopathy, nephropathy, and neuropathy, he said. These complications can also have a strong impact on psychological well being and treatment satisfaction.
“It’s important that we’re not glucocentric any more, but it’s equally important that we treat glucose because it has such a benefit for microvascular disease.” Dr. Grant also cited metformin’s long history of safety and good tolerance, clinician comfort prescribing it, and its low price. Heavier reliance on SGLT2 inhibitors and GLP-1 receptor agonists will be expensive for the short term while the cost of these drugs remains high, which places a higher burden on “knowing we’re doing it right,” said Dr. Grant.
Dr. Cosentino pointed out that the higher cost of the drugs in the two classes shown to exert important cardiovascular and renal effects needs to be considered in a cost-effectiveness context, not just by cost alone.
‘Clinical inertia’ could be a danger
Dr. Cosentino played down a major disagreement between the two guidelines, suggesting that “focusing on the differences leads to clinical inertia” by the practicing community when they are unsure how to reconcile the two positions.
Dr. Grant agreed that adding a second drug to metformin right away made sense in at least selected patients. “Look at each patient and decide whether they need glycemic control. If so, and if they also have cardiovascular disease, use both drugs,” metformin, plus one agent from one of the two newer classes.
Something both experts agreed on is that it’s time to generally steer clear of sulfonylurea drugs. “We have evidence for harmful effects from sulfonylureas,” Dr. Cosentino said.
“I’d dump sulfonylureas,” was Dr. Grant’s assessment, but he added that they still have a role for patients who need additional glycemic control but can’t afford the newer drugs.
Dr. Cosentino has had financial relationships with Abbott, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Merck, Mundipharma, Novo Nordisk, and Pfizer, Dr. Grant has lectured on behalf of AstraZeneca, GlaxoSmithKline, Merck, Novo Nordisk, the Medicines Company, and Takeda, and he has been an adviser to Amgen, AstraZeneca, Novartis, Novo Nordisk, and Synexus.
It was no surprise that updated guidelines recently published by the European Society of Cardiology for managing cardiovascular disease in patients with diabetes highlighted optimized treatment from a cardiovascular disease perspective, while a nearly concurrent update from two major diabetes societies saw the same issue from a more glycemic point of view.
This difference led to divergent approaches to managing hyperglycemia in patients with type 2 diabetes (T2D). The two diabetes societies that wrote one set of recommendations, the American Diabetes Association and the European Association for the Study of Diabetes, put metformin at the pinnacle of their drug hierarchy. Patients with T2D and established atherosclerotic cardiovascular disease (CVD), chronic kidney disease, or heart failure should all receive metformin first unless contraindicated or not tolerated, their updated consensus report said.
Once metformin is on board, a clinician can then add a second diabetes agent from among the two drug classes recently proven to also reduce cardiovascular and renal events, either the SGLT2 (sodium-glucose transporter 2) inhibitors, or GLP-1 (glucagonlike peptide–1) receptor agonists, they advised.
Cardiovascular disease focus represents a ‘major paradigm shift’
In contrast, the ESC guidelines called for upfront, systematic assessment of CVD risk in patients with T2D before treatment starts, and for patients in high- or very high–risk strata, the guidelines recommended starting the patient first on an SGLT2 inhibitor or a GLP-1 receptor agonist, and only adding metformin in patients who need additional glycemic control.
The guidelines also recommended starting treatment-naive patients with moderate CVD risk on metformin. For patients already on metformin, the new ESC guidelines called for adding an agent from at least one of these two drug classes with proven CVD benefits for those at high or very high CVD risk. The guidelines also note that the CVD benefits of the two newer drug classes differ and hence require further individualization depending on the risks faced by each patient, such as the risk for heart failure hospitalizations.
It’s an approach “driven by data from the cardiovascular outcome trials,” that showed several drugs from both the SGLT2 inhibitor and GLP-1 receptor agonist classes have substantial benefit for preventing cardiovascular events, renal events, hospitalizations for heart failure, and in some studies all-cause mortality, said Francesco Cosentino, MD, during a discussion of the guideline differences at the virtual annual meeting of the European Association for the Study of Diabetes.
The ESC approach also represents “a major paradigm shift,” a “change from a glucose-centric approach to an approach driven by cardiovascular disease events,” summed up Dr. Cosentino, professor of cardiology at the Karolinska Institute in Stockholm and chair of the task force that wrote the ESC’s 2019 updated guidelines. The ESC approach advocates initiating drugs for treating patients with T2D “based on cardiovascular disease risk classification,” he highlighted. Results from some SGLT2 inhibitor cardiovascular outcome trials showed that the CVD benefit was similar regardless of whether or not patients also received metformin.
ADA, EASD call for ‘a different emphasis’
“There is a different emphasis” in the statement issued by the diabetologists of the ADA and EASD, admitted Peter J. Grant, MD, a professor of diabetes and endocrinology at the University of Leeds (England) and cochair of the ESC guidelines task force. Dr. Grant represented the EASD on the task force, and the Association collaborated with the ESC in producing its guidelines.
“The ADA and EASD recommendations “look primarily at glucose control, with cardiovascular disease management as secondary.” In contrast, the ESC guidelines “are primarily cardiovascular disease risk guidelines, with a glucose interest,” Dr. Grant declared.
Despite his involvement in writing the ESC guidelines, Dr. Grant tilted toward the ADA/EASD statement as more globally relevant.
“There is much more to vasculopathy in diabetes than just macrovascular disease. Many patients with type 2 diabetes without macrovascular complications have microvascular disease,” including the potential for retinopathy, nephropathy, and neuropathy, he said. These complications can also have a strong impact on psychological well being and treatment satisfaction.
“It’s important that we’re not glucocentric any more, but it’s equally important that we treat glucose because it has such a benefit for microvascular disease.” Dr. Grant also cited metformin’s long history of safety and good tolerance, clinician comfort prescribing it, and its low price. Heavier reliance on SGLT2 inhibitors and GLP-1 receptor agonists will be expensive for the short term while the cost of these drugs remains high, which places a higher burden on “knowing we’re doing it right,” said Dr. Grant.
Dr. Cosentino pointed out that the higher cost of the drugs in the two classes shown to exert important cardiovascular and renal effects needs to be considered in a cost-effectiveness context, not just by cost alone.
‘Clinical inertia’ could be a danger
Dr. Cosentino played down a major disagreement between the two guidelines, suggesting that “focusing on the differences leads to clinical inertia” by the practicing community when they are unsure how to reconcile the two positions.
Dr. Grant agreed that adding a second drug to metformin right away made sense in at least selected patients. “Look at each patient and decide whether they need glycemic control. If so, and if they also have cardiovascular disease, use both drugs,” metformin, plus one agent from one of the two newer classes.
Something both experts agreed on is that it’s time to generally steer clear of sulfonylurea drugs. “We have evidence for harmful effects from sulfonylureas,” Dr. Cosentino said.
“I’d dump sulfonylureas,” was Dr. Grant’s assessment, but he added that they still have a role for patients who need additional glycemic control but can’t afford the newer drugs.
Dr. Cosentino has had financial relationships with Abbott, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Merck, Mundipharma, Novo Nordisk, and Pfizer, Dr. Grant has lectured on behalf of AstraZeneca, GlaxoSmithKline, Merck, Novo Nordisk, the Medicines Company, and Takeda, and he has been an adviser to Amgen, AstraZeneca, Novartis, Novo Nordisk, and Synexus.
It was no surprise that updated guidelines recently published by the European Society of Cardiology for managing cardiovascular disease in patients with diabetes highlighted optimized treatment from a cardiovascular disease perspective, while a nearly concurrent update from two major diabetes societies saw the same issue from a more glycemic point of view.
This difference led to divergent approaches to managing hyperglycemia in patients with type 2 diabetes (T2D). The two diabetes societies that wrote one set of recommendations, the American Diabetes Association and the European Association for the Study of Diabetes, put metformin at the pinnacle of their drug hierarchy. Patients with T2D and established atherosclerotic cardiovascular disease (CVD), chronic kidney disease, or heart failure should all receive metformin first unless contraindicated or not tolerated, their updated consensus report said.
Once metformin is on board, a clinician can then add a second diabetes agent from among the two drug classes recently proven to also reduce cardiovascular and renal events, either the SGLT2 (sodium-glucose transporter 2) inhibitors, or GLP-1 (glucagonlike peptide–1) receptor agonists, they advised.
Cardiovascular disease focus represents a ‘major paradigm shift’
In contrast, the ESC guidelines called for upfront, systematic assessment of CVD risk in patients with T2D before treatment starts, and for patients in high- or very high–risk strata, the guidelines recommended starting the patient first on an SGLT2 inhibitor or a GLP-1 receptor agonist, and only adding metformin in patients who need additional glycemic control.
The guidelines also recommended starting treatment-naive patients with moderate CVD risk on metformin. For patients already on metformin, the new ESC guidelines called for adding an agent from at least one of these two drug classes with proven CVD benefits for those at high or very high CVD risk. The guidelines also note that the CVD benefits of the two newer drug classes differ and hence require further individualization depending on the risks faced by each patient, such as the risk for heart failure hospitalizations.
It’s an approach “driven by data from the cardiovascular outcome trials,” that showed several drugs from both the SGLT2 inhibitor and GLP-1 receptor agonist classes have substantial benefit for preventing cardiovascular events, renal events, hospitalizations for heart failure, and in some studies all-cause mortality, said Francesco Cosentino, MD, during a discussion of the guideline differences at the virtual annual meeting of the European Association for the Study of Diabetes.
The ESC approach also represents “a major paradigm shift,” a “change from a glucose-centric approach to an approach driven by cardiovascular disease events,” summed up Dr. Cosentino, professor of cardiology at the Karolinska Institute in Stockholm and chair of the task force that wrote the ESC’s 2019 updated guidelines. The ESC approach advocates initiating drugs for treating patients with T2D “based on cardiovascular disease risk classification,” he highlighted. Results from some SGLT2 inhibitor cardiovascular outcome trials showed that the CVD benefit was similar regardless of whether or not patients also received metformin.
ADA, EASD call for ‘a different emphasis’
“There is a different emphasis” in the statement issued by the diabetologists of the ADA and EASD, admitted Peter J. Grant, MD, a professor of diabetes and endocrinology at the University of Leeds (England) and cochair of the ESC guidelines task force. Dr. Grant represented the EASD on the task force, and the Association collaborated with the ESC in producing its guidelines.
“The ADA and EASD recommendations “look primarily at glucose control, with cardiovascular disease management as secondary.” In contrast, the ESC guidelines “are primarily cardiovascular disease risk guidelines, with a glucose interest,” Dr. Grant declared.
Despite his involvement in writing the ESC guidelines, Dr. Grant tilted toward the ADA/EASD statement as more globally relevant.
“There is much more to vasculopathy in diabetes than just macrovascular disease. Many patients with type 2 diabetes without macrovascular complications have microvascular disease,” including the potential for retinopathy, nephropathy, and neuropathy, he said. These complications can also have a strong impact on psychological well being and treatment satisfaction.
“It’s important that we’re not glucocentric any more, but it’s equally important that we treat glucose because it has such a benefit for microvascular disease.” Dr. Grant also cited metformin’s long history of safety and good tolerance, clinician comfort prescribing it, and its low price. Heavier reliance on SGLT2 inhibitors and GLP-1 receptor agonists will be expensive for the short term while the cost of these drugs remains high, which places a higher burden on “knowing we’re doing it right,” said Dr. Grant.
Dr. Cosentino pointed out that the higher cost of the drugs in the two classes shown to exert important cardiovascular and renal effects needs to be considered in a cost-effectiveness context, not just by cost alone.
‘Clinical inertia’ could be a danger
Dr. Cosentino played down a major disagreement between the two guidelines, suggesting that “focusing on the differences leads to clinical inertia” by the practicing community when they are unsure how to reconcile the two positions.
Dr. Grant agreed that adding a second drug to metformin right away made sense in at least selected patients. “Look at each patient and decide whether they need glycemic control. If so, and if they also have cardiovascular disease, use both drugs,” metformin, plus one agent from one of the two newer classes.
Something both experts agreed on is that it’s time to generally steer clear of sulfonylurea drugs. “We have evidence for harmful effects from sulfonylureas,” Dr. Cosentino said.
“I’d dump sulfonylureas,” was Dr. Grant’s assessment, but he added that they still have a role for patients who need additional glycemic control but can’t afford the newer drugs.
Dr. Cosentino has had financial relationships with Abbott, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Merck, Mundipharma, Novo Nordisk, and Pfizer, Dr. Grant has lectured on behalf of AstraZeneca, GlaxoSmithKline, Merck, Novo Nordisk, the Medicines Company, and Takeda, and he has been an adviser to Amgen, AstraZeneca, Novartis, Novo Nordisk, and Synexus.
FROM EASD 2020
Empagliflozin cut PA pressures in heart failure patients
Elevated pulmonary artery diastolic pressure is “perhaps the best predictor of bad outcomes in patients with heart failure, including hospitalization and death,” and new evidence clearly showed that the sodium-glucose cotransporter 2 (SGLT2) inhibitor empagliflozin cuts this metric in patients by a clinically significant amount, Mikhail Kosiborod, MD, said at the virtual annual scientific meeting of the Heart Failure Society of America.
The evidence he collected from a total of 65 heart failure patients with either reduced or preserved ejection fraction is the first documentation from a randomized, controlled study to show a direct effect by a SGLT2 inhibitor on pulmonary artery (PA) pressures.
Other key findings were that the drop in PA diastolic pressure with empagliflozin treatment compared with placebo became discernible early (within the first 4 weeks on treatment), that the pressure-lowering effect steadily grew over time, and that it showed no link to the intensity of loop diuretic treatment, which held steady during 12 weeks on treatment and 13 weeks of overall monitoring.
The study’s primary endpoint was the change from baseline in PA diastolic pressure after 12 weeks on treatment. The 31 patients who completed the full 12-week course had an average drop in their PA diastolic pressure of about 1.5 mm Hg, compared with 28 patients who completed 12 weeks on placebo. Average PA diastolic pressure at baseline was about 21 mm Hg in both treatment arms, and on treatment this fell by more than 0.5 mm Hg among those who received empagliflozin and rose by close to 1 mm Hg among control patients.
“There appears to be a direct effect of empagliflozin on pulmonary artery pressure that’s not been previously demonstrated” by an SGLT2 inhibitor, Dr. Kosiborod said. “I think this is one mechanism of action” for this drug class. “If you control pulmonary artery filling pressures you can prevent hospitalizations and deaths.”
Small reductions matter
“Small pressure differences are particularly important for pulmonary hypertension,” commented Lynne W. Stevenson, MD, professor of medicine at Vanderbilt University Medical Center in Nashville, Tenn., and the report’s designated discussant.
“In the Vanderbilt heart failure database, patients with a pulmonary artery mean pressure of 20-24 mm Hg had 30% higher mortality than patients with lower pressures,” Dr. Stevenson noted. “This has led to a new definition of pulmonary hypertension, a mean pulmonary artery pressure above at or above 20 mm Hg.”
In Dr. Kosiborod’s study, patients began with an average PA mean pressure of about 30 mm Hg, and empagliflozin treatment led to a reduction in this metric with about the same magnitude as its effect on PA diastolic pressure. Empagliflozin also produced a similar reduction in average PA systolic pressure.
A study built on ambulatory PA monitoring
The results “also provide more proof for the concept of ambulatory hemodynamic monitoring” in patients with heart failure to monitor their status, she added. The study enrolled only patients who had already received a CardioMEMS implant as part of their routine care. This device allows for frequent, noninvasive monitoring of PA pressures. Researchers collected PA pressure data from patients twice daily for the entire 13-week study.
The EMBRACE HF (Empagliflozin Impact on Hemodynamics in Patients With Heart Failure) study enrolled patients with established heart failure, a CardioMEMS implant, and New York Heart Association class II-IV symptoms at any of eight U.S. centers. Patients averaged about 65 years old, and slightly more than half had class III disease, which denotes marked limitation of physical activity.
Despite the brief treatment period, patients who received empagliflozin showed other evidence of benefit including a trend toward improved quality of life scores, reduced levels of two different forms of brain natriuretic peptide, and significant weight loss, compared with controls, that averaged 2.4 kg.
The mechanism by which empagliflozin and other drugs in its class might lower PA filling pressures is unclear, but Dr. Kosiborod stressed that the consistent level of loop diuretic use during the study seems to rule out a diuretic effect from the SGLT2 inhibitor as having a role. A pulmonary vasculature effect is “much more likely,” perhaps mediated through modified endothelial function and vasodilation, he suggested.
EMBRACE HF was funded by Boehringer Ingelheim, the company that markets empagliflozin (Jardiance) along with Eli Lilly. Dr. Kosiborod has received research support and honoraria from Boehringer Ingelheim, and he has received honoraria from several other companies. Dr. Stevenson had no disclosures.
Elevated pulmonary artery diastolic pressure is “perhaps the best predictor of bad outcomes in patients with heart failure, including hospitalization and death,” and new evidence clearly showed that the sodium-glucose cotransporter 2 (SGLT2) inhibitor empagliflozin cuts this metric in patients by a clinically significant amount, Mikhail Kosiborod, MD, said at the virtual annual scientific meeting of the Heart Failure Society of America.
The evidence he collected from a total of 65 heart failure patients with either reduced or preserved ejection fraction is the first documentation from a randomized, controlled study to show a direct effect by a SGLT2 inhibitor on pulmonary artery (PA) pressures.
Other key findings were that the drop in PA diastolic pressure with empagliflozin treatment compared with placebo became discernible early (within the first 4 weeks on treatment), that the pressure-lowering effect steadily grew over time, and that it showed no link to the intensity of loop diuretic treatment, which held steady during 12 weeks on treatment and 13 weeks of overall monitoring.
The study’s primary endpoint was the change from baseline in PA diastolic pressure after 12 weeks on treatment. The 31 patients who completed the full 12-week course had an average drop in their PA diastolic pressure of about 1.5 mm Hg, compared with 28 patients who completed 12 weeks on placebo. Average PA diastolic pressure at baseline was about 21 mm Hg in both treatment arms, and on treatment this fell by more than 0.5 mm Hg among those who received empagliflozin and rose by close to 1 mm Hg among control patients.
“There appears to be a direct effect of empagliflozin on pulmonary artery pressure that’s not been previously demonstrated” by an SGLT2 inhibitor, Dr. Kosiborod said. “I think this is one mechanism of action” for this drug class. “If you control pulmonary artery filling pressures you can prevent hospitalizations and deaths.”
Small reductions matter
“Small pressure differences are particularly important for pulmonary hypertension,” commented Lynne W. Stevenson, MD, professor of medicine at Vanderbilt University Medical Center in Nashville, Tenn., and the report’s designated discussant.
“In the Vanderbilt heart failure database, patients with a pulmonary artery mean pressure of 20-24 mm Hg had 30% higher mortality than patients with lower pressures,” Dr. Stevenson noted. “This has led to a new definition of pulmonary hypertension, a mean pulmonary artery pressure above at or above 20 mm Hg.”
In Dr. Kosiborod’s study, patients began with an average PA mean pressure of about 30 mm Hg, and empagliflozin treatment led to a reduction in this metric with about the same magnitude as its effect on PA diastolic pressure. Empagliflozin also produced a similar reduction in average PA systolic pressure.
A study built on ambulatory PA monitoring
The results “also provide more proof for the concept of ambulatory hemodynamic monitoring” in patients with heart failure to monitor their status, she added. The study enrolled only patients who had already received a CardioMEMS implant as part of their routine care. This device allows for frequent, noninvasive monitoring of PA pressures. Researchers collected PA pressure data from patients twice daily for the entire 13-week study.
The EMBRACE HF (Empagliflozin Impact on Hemodynamics in Patients With Heart Failure) study enrolled patients with established heart failure, a CardioMEMS implant, and New York Heart Association class II-IV symptoms at any of eight U.S. centers. Patients averaged about 65 years old, and slightly more than half had class III disease, which denotes marked limitation of physical activity.
Despite the brief treatment period, patients who received empagliflozin showed other evidence of benefit including a trend toward improved quality of life scores, reduced levels of two different forms of brain natriuretic peptide, and significant weight loss, compared with controls, that averaged 2.4 kg.
The mechanism by which empagliflozin and other drugs in its class might lower PA filling pressures is unclear, but Dr. Kosiborod stressed that the consistent level of loop diuretic use during the study seems to rule out a diuretic effect from the SGLT2 inhibitor as having a role. A pulmonary vasculature effect is “much more likely,” perhaps mediated through modified endothelial function and vasodilation, he suggested.
EMBRACE HF was funded by Boehringer Ingelheim, the company that markets empagliflozin (Jardiance) along with Eli Lilly. Dr. Kosiborod has received research support and honoraria from Boehringer Ingelheim, and he has received honoraria from several other companies. Dr. Stevenson had no disclosures.
Elevated pulmonary artery diastolic pressure is “perhaps the best predictor of bad outcomes in patients with heart failure, including hospitalization and death,” and new evidence clearly showed that the sodium-glucose cotransporter 2 (SGLT2) inhibitor empagliflozin cuts this metric in patients by a clinically significant amount, Mikhail Kosiborod, MD, said at the virtual annual scientific meeting of the Heart Failure Society of America.
The evidence he collected from a total of 65 heart failure patients with either reduced or preserved ejection fraction is the first documentation from a randomized, controlled study to show a direct effect by a SGLT2 inhibitor on pulmonary artery (PA) pressures.
Other key findings were that the drop in PA diastolic pressure with empagliflozin treatment compared with placebo became discernible early (within the first 4 weeks on treatment), that the pressure-lowering effect steadily grew over time, and that it showed no link to the intensity of loop diuretic treatment, which held steady during 12 weeks on treatment and 13 weeks of overall monitoring.
The study’s primary endpoint was the change from baseline in PA diastolic pressure after 12 weeks on treatment. The 31 patients who completed the full 12-week course had an average drop in their PA diastolic pressure of about 1.5 mm Hg, compared with 28 patients who completed 12 weeks on placebo. Average PA diastolic pressure at baseline was about 21 mm Hg in both treatment arms, and on treatment this fell by more than 0.5 mm Hg among those who received empagliflozin and rose by close to 1 mm Hg among control patients.
“There appears to be a direct effect of empagliflozin on pulmonary artery pressure that’s not been previously demonstrated” by an SGLT2 inhibitor, Dr. Kosiborod said. “I think this is one mechanism of action” for this drug class. “If you control pulmonary artery filling pressures you can prevent hospitalizations and deaths.”
Small reductions matter
“Small pressure differences are particularly important for pulmonary hypertension,” commented Lynne W. Stevenson, MD, professor of medicine at Vanderbilt University Medical Center in Nashville, Tenn., and the report’s designated discussant.
“In the Vanderbilt heart failure database, patients with a pulmonary artery mean pressure of 20-24 mm Hg had 30% higher mortality than patients with lower pressures,” Dr. Stevenson noted. “This has led to a new definition of pulmonary hypertension, a mean pulmonary artery pressure above at or above 20 mm Hg.”
In Dr. Kosiborod’s study, patients began with an average PA mean pressure of about 30 mm Hg, and empagliflozin treatment led to a reduction in this metric with about the same magnitude as its effect on PA diastolic pressure. Empagliflozin also produced a similar reduction in average PA systolic pressure.
A study built on ambulatory PA monitoring
The results “also provide more proof for the concept of ambulatory hemodynamic monitoring” in patients with heart failure to monitor their status, she added. The study enrolled only patients who had already received a CardioMEMS implant as part of their routine care. This device allows for frequent, noninvasive monitoring of PA pressures. Researchers collected PA pressure data from patients twice daily for the entire 13-week study.
The EMBRACE HF (Empagliflozin Impact on Hemodynamics in Patients With Heart Failure) study enrolled patients with established heart failure, a CardioMEMS implant, and New York Heart Association class II-IV symptoms at any of eight U.S. centers. Patients averaged about 65 years old, and slightly more than half had class III disease, which denotes marked limitation of physical activity.
Despite the brief treatment period, patients who received empagliflozin showed other evidence of benefit including a trend toward improved quality of life scores, reduced levels of two different forms of brain natriuretic peptide, and significant weight loss, compared with controls, that averaged 2.4 kg.
The mechanism by which empagliflozin and other drugs in its class might lower PA filling pressures is unclear, but Dr. Kosiborod stressed that the consistent level of loop diuretic use during the study seems to rule out a diuretic effect from the SGLT2 inhibitor as having a role. A pulmonary vasculature effect is “much more likely,” perhaps mediated through modified endothelial function and vasodilation, he suggested.
EMBRACE HF was funded by Boehringer Ingelheim, the company that markets empagliflozin (Jardiance) along with Eli Lilly. Dr. Kosiborod has received research support and honoraria from Boehringer Ingelheim, and he has received honoraria from several other companies. Dr. Stevenson had no disclosures.
FROM HFSA 2020
Chronic, nonhealing leg ulcer
An 80-year-old woman with a history of hypertension, hyperlipidemia, psoriasis vulgaris with associated pruritus, and well-controlled type 2 diabetes mellitus presented with a slowly enlarging ulceration on her left leg of 1 year’s duration. She noted that this lesion healed less rapidly than previous stasis leg ulcerations, despite using the same treatment approach that included dressings, elevation, and diuretics to decrease pedal edema.
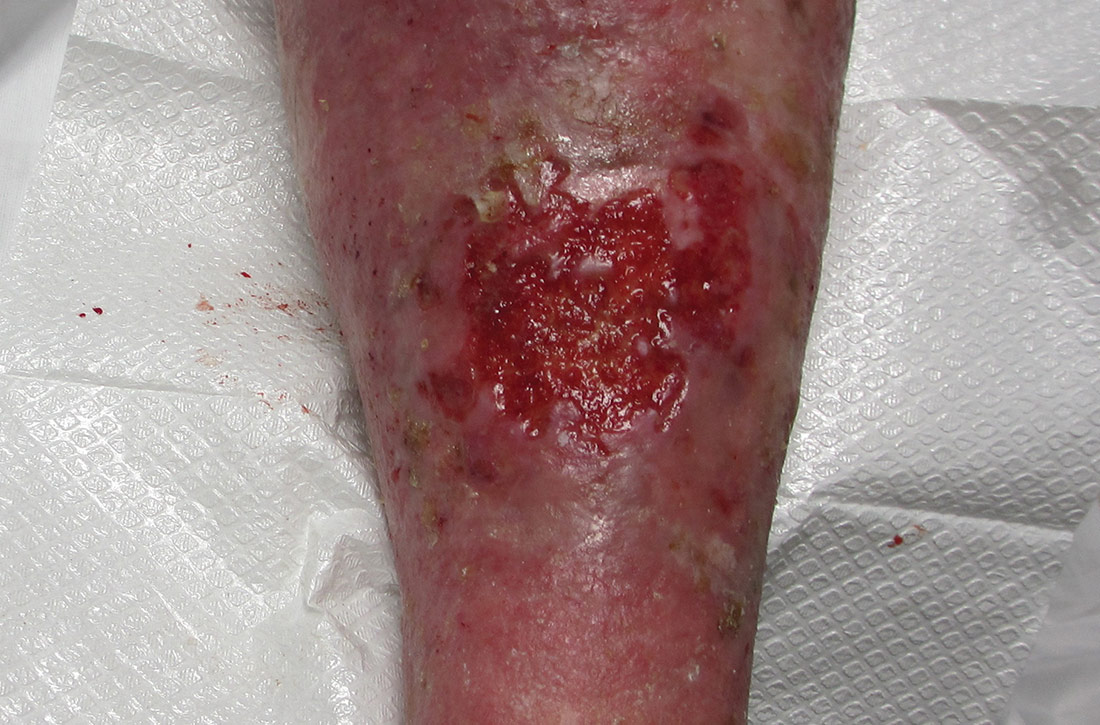
Physical examination revealed plaques with white micaceous scaling over her extensor surfaces and scalp, as well as guttate lesions on the trunk, typical of psoriasis vulgaris. A 5.8 × 7.2-cm malodorous ulceration was superimposed on a large psoriatic plaque on her left anterior lower leg (FIGURE 1). A 4-mm punch biopsy was obtained from the peripheral margin.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Basal cell carcinoma
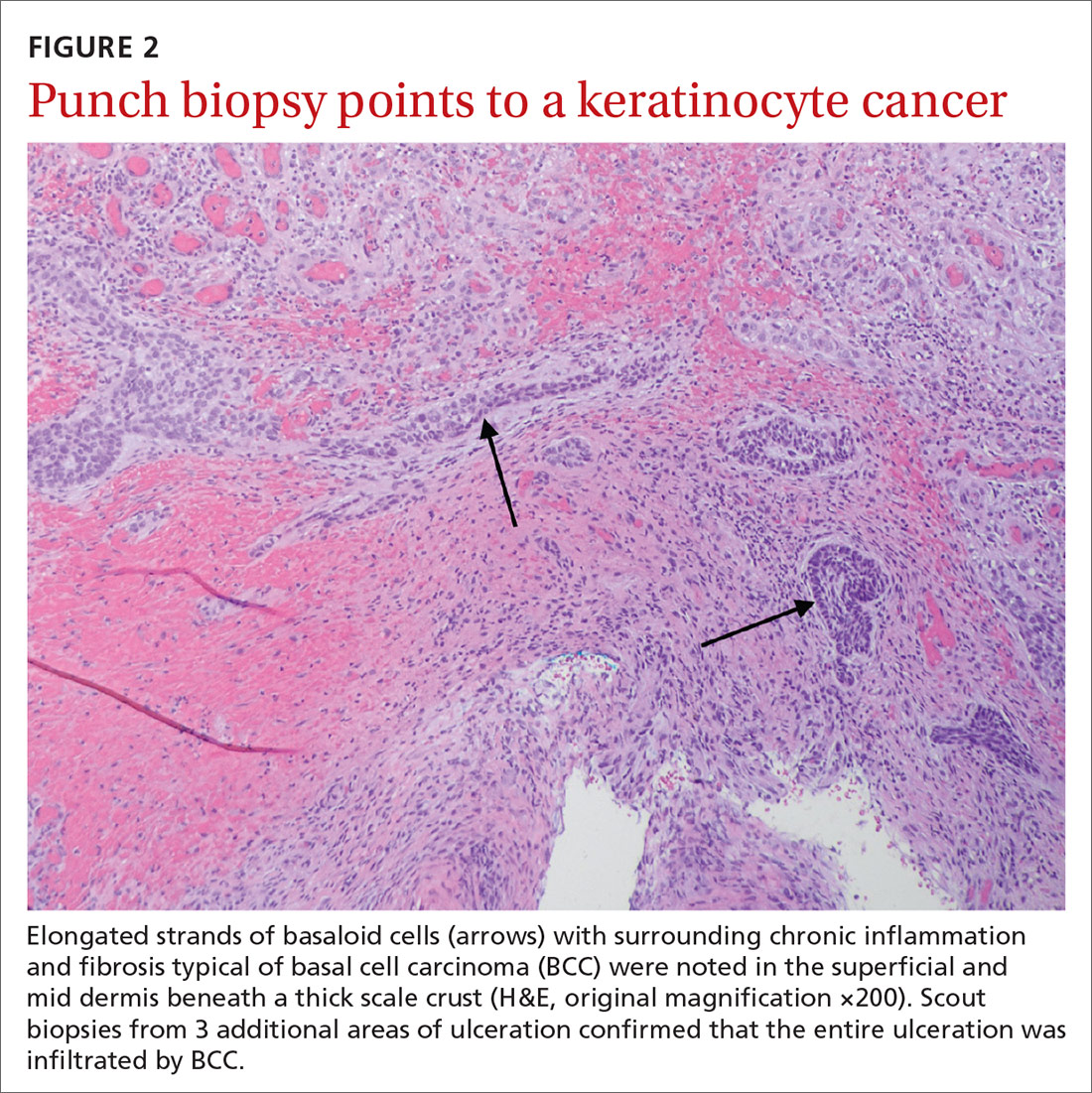
Histopathological examination revealed elongated strands of closely packed basaloid cells embedded in a dense fibrous stroma with overlying ulceration and crusting (FIGURE 2). Immunohistochemical staining with cytokeratin (CK) 5/6 decorated the cytoplasm of the tumor cells, which confirmed that the tumor was a keratinocyte cancer. CK 20 was negative, excluding the possibility of a Merkel cell carcinoma. Scout biopsies from 3 additional areas of ulceration confirmed that the entire ulceration was infiltrated by basal cell carcinoma (BCC).
A surprise hidden in a chronic ulcer
More than 6 million Americans have chronic ulcers and most occur on the legs.1 The majority of these chronic ulcerations are etiologically related to venous stasis, arterial insufficiency, or neuropathy.2
Bacterial pyoderma, chronic infection caused by atypical acid-fast bacilli or deep fungal infection, pyoderma gangrenosum, cutaneous vasculitis, calciphylaxis, and venous ulceration were all considered to explain this patient’s nonhealing wound. A biopsy was required to fully assess these possibilities.
Don’t overlook the possibility of malignancy. In a cross-sectional, multicenter study by Senet et al,3 144 patients with 154 total chronic leg ulcers were evaluated in tertiary care centers for malignancy, which was found to occur at a rate of 10.4%. Similarly, Ghasemi et al4 demonstrated a malignancy rate of 16.1% in 124 patients who underwent biopsy; the anterior shin was determined to be the most frequent location for malignancy. The most common skin cancer identified within the setting of chronic ulcers is squamous cell carcinoma.3 Although rare, there are reports of BCC identified in chronic wounds.3-7
Morphological signs suggestive of malignancy in chronic ulcerations include hyperkeratosis, granulation tissue surrounded by a raised border, unusual pain or bleeding, and increased tissue friability. Our patient had none of these signs and symptoms. However, it is possible that she had a tumor that ulcerated and would not heal.
Continue to: Which came first?
Which came first? It’s difficult to know in this case whether a persistent BCC ulcerated, forming this lesion, or if scarring associated with a chronic ulceration led to the development of the BCC.6 Based on biopsies taken at an earlier date, Schnirring-Judge and Belpedio7 concluded that a chronic leg ulcer could, indeed, transform into a BCC; however, pre-existing BCC more commonly ulcerates and then does not heal.
Treatment options
While smaller, superficial BCCs can be treated with topical imiquimod, photodynamic therapy, or electrodesiccation and curettage, larger lesions should be treated with Mohs micrographic surgery and excisional surgery with grafting. Inoperable tumors may be treated with radiation therapy and vismodegib.
Our patient. Once the diagnosis of BCC was established, treatment options were discussed, including excision, local radiation therapy, and oral hedgehog inhibitor drug therapy.8 Our patient opted to undergo a wide local excision of the lesion followed by negative-pressure wound therapy, which led to complete healing.
CORRESPONDENCE
David Crasto, DO, William Carey University College of Osteopathic Medicine, 498 Tuscan Avenue, Hattiesburg, MS 39401; [email protected]
1. Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17:763-771.
2. Fox JD, Baquerizo Nole KL, Berriman SJ, et al. Chronic wounds: the need for greater emphasis in medical schools, post-graduate training and public health discussions. Ann Surg. 2016;264:241-243.
3. Senet P, Combemale P, Debure C, et al. Malignancy and chronic leg ulcers. Arch Dermatol. 2012;148:704-708.
4. Ghasemi F, Anooshirvani N, Sibbald RG, et al. The point prevalence of malignancy in a wound clinic. Int J Low Extrem Wounds. 2016;15:58-62.
5. Labropoulos N, Manalo D, Patel N, et al. Uncommon leg ulcers in the lower extremity. J Vasc Surg. 2007;45:568-573.
6. Tchanque-Fossuo CN, Millsop J, Johnson MA, et al. Ulcerated basal cell carcinomas masquerading as venous leg ulcers. Adv Skin Wound Care. 2018;31:130-134.
7. Schnirring-Judge M, Belpedio D. Malignant transformation of a chronic venous stasis ulcer to basal cell carcinoma in a diabetic patient: case and review of the pathophysiology. J Foot Ankle Surg. 2010;49:75-79.
8. Puig S, Berrocal A. Management of high-risk and advanced basal cell carcinoma. Clin Transl Oncol. 2015;17:497-503.
An 80-year-old woman with a history of hypertension, hyperlipidemia, psoriasis vulgaris with associated pruritus, and well-controlled type 2 diabetes mellitus presented with a slowly enlarging ulceration on her left leg of 1 year’s duration. She noted that this lesion healed less rapidly than previous stasis leg ulcerations, despite using the same treatment approach that included dressings, elevation, and diuretics to decrease pedal edema.
Physical examination revealed plaques with white micaceous scaling over her extensor surfaces and scalp, as well as guttate lesions on the trunk, typical of psoriasis vulgaris. A 5.8 × 7.2-cm malodorous ulceration was superimposed on a large psoriatic plaque on her left anterior lower leg (FIGURE 1). A 4-mm punch biopsy was obtained from the peripheral margin.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Basal cell carcinoma
Histopathological examination revealed elongated strands of closely packed basaloid cells embedded in a dense fibrous stroma with overlying ulceration and crusting (FIGURE 2). Immunohistochemical staining with cytokeratin (CK) 5/6 decorated the cytoplasm of the tumor cells, which confirmed that the tumor was a keratinocyte cancer. CK 20 was negative, excluding the possibility of a Merkel cell carcinoma. Scout biopsies from 3 additional areas of ulceration confirmed that the entire ulceration was infiltrated by basal cell carcinoma (BCC).

A surprise hidden in a chronic ulcer
More than 6 million Americans have chronic ulcers and most occur on the legs.1 The majority of these chronic ulcerations are etiologically related to venous stasis, arterial insufficiency, or neuropathy.2
Bacterial pyoderma, chronic infection caused by atypical acid-fast bacilli or deep fungal infection, pyoderma gangrenosum, cutaneous vasculitis, calciphylaxis, and venous ulceration were all considered to explain this patient’s nonhealing wound. A biopsy was required to fully assess these possibilities.
Don’t overlook the possibility of malignancy. In a cross-sectional, multicenter study by Senet et al,3 144 patients with 154 total chronic leg ulcers were evaluated in tertiary care centers for malignancy, which was found to occur at a rate of 10.4%. Similarly, Ghasemi et al4 demonstrated a malignancy rate of 16.1% in 124 patients who underwent biopsy; the anterior shin was determined to be the most frequent location for malignancy. The most common skin cancer identified within the setting of chronic ulcers is squamous cell carcinoma.3 Although rare, there are reports of BCC identified in chronic wounds.3-7
Morphological signs suggestive of malignancy in chronic ulcerations include hyperkeratosis, granulation tissue surrounded by a raised border, unusual pain or bleeding, and increased tissue friability. Our patient had none of these signs and symptoms. However, it is possible that she had a tumor that ulcerated and would not heal.
Continue to: Which came first?
Which came first? It’s difficult to know in this case whether a persistent BCC ulcerated, forming this lesion, or if scarring associated with a chronic ulceration led to the development of the BCC.6 Based on biopsies taken at an earlier date, Schnirring-Judge and Belpedio7 concluded that a chronic leg ulcer could, indeed, transform into a BCC; however, pre-existing BCC more commonly ulcerates and then does not heal.
Treatment options
While smaller, superficial BCCs can be treated with topical imiquimod, photodynamic therapy, or electrodesiccation and curettage, larger lesions should be treated with Mohs micrographic surgery and excisional surgery with grafting. Inoperable tumors may be treated with radiation therapy and vismodegib.
Our patient. Once the diagnosis of BCC was established, treatment options were discussed, including excision, local radiation therapy, and oral hedgehog inhibitor drug therapy.8 Our patient opted to undergo a wide local excision of the lesion followed by negative-pressure wound therapy, which led to complete healing.
CORRESPONDENCE
David Crasto, DO, William Carey University College of Osteopathic Medicine, 498 Tuscan Avenue, Hattiesburg, MS 39401; [email protected]
An 80-year-old woman with a history of hypertension, hyperlipidemia, psoriasis vulgaris with associated pruritus, and well-controlled type 2 diabetes mellitus presented with a slowly enlarging ulceration on her left leg of 1 year’s duration. She noted that this lesion healed less rapidly than previous stasis leg ulcerations, despite using the same treatment approach that included dressings, elevation, and diuretics to decrease pedal edema.
Physical examination revealed plaques with white micaceous scaling over her extensor surfaces and scalp, as well as guttate lesions on the trunk, typical of psoriasis vulgaris. A 5.8 × 7.2-cm malodorous ulceration was superimposed on a large psoriatic plaque on her left anterior lower leg (FIGURE 1). A 4-mm punch biopsy was obtained from the peripheral margin.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Basal cell carcinoma
Histopathological examination revealed elongated strands of closely packed basaloid cells embedded in a dense fibrous stroma with overlying ulceration and crusting (FIGURE 2). Immunohistochemical staining with cytokeratin (CK) 5/6 decorated the cytoplasm of the tumor cells, which confirmed that the tumor was a keratinocyte cancer. CK 20 was negative, excluding the possibility of a Merkel cell carcinoma. Scout biopsies from 3 additional areas of ulceration confirmed that the entire ulceration was infiltrated by basal cell carcinoma (BCC).

A surprise hidden in a chronic ulcer
More than 6 million Americans have chronic ulcers and most occur on the legs.1 The majority of these chronic ulcerations are etiologically related to venous stasis, arterial insufficiency, or neuropathy.2
Bacterial pyoderma, chronic infection caused by atypical acid-fast bacilli or deep fungal infection, pyoderma gangrenosum, cutaneous vasculitis, calciphylaxis, and venous ulceration were all considered to explain this patient’s nonhealing wound. A biopsy was required to fully assess these possibilities.
Don’t overlook the possibility of malignancy. In a cross-sectional, multicenter study by Senet et al,3 144 patients with 154 total chronic leg ulcers were evaluated in tertiary care centers for malignancy, which was found to occur at a rate of 10.4%. Similarly, Ghasemi et al4 demonstrated a malignancy rate of 16.1% in 124 patients who underwent biopsy; the anterior shin was determined to be the most frequent location for malignancy. The most common skin cancer identified within the setting of chronic ulcers is squamous cell carcinoma.3 Although rare, there are reports of BCC identified in chronic wounds.3-7
Morphological signs suggestive of malignancy in chronic ulcerations include hyperkeratosis, granulation tissue surrounded by a raised border, unusual pain or bleeding, and increased tissue friability. Our patient had none of these signs and symptoms. However, it is possible that she had a tumor that ulcerated and would not heal.
Continue to: Which came first?
Which came first? It’s difficult to know in this case whether a persistent BCC ulcerated, forming this lesion, or if scarring associated with a chronic ulceration led to the development of the BCC.6 Based on biopsies taken at an earlier date, Schnirring-Judge and Belpedio7 concluded that a chronic leg ulcer could, indeed, transform into a BCC; however, pre-existing BCC more commonly ulcerates and then does not heal.
Treatment options
While smaller, superficial BCCs can be treated with topical imiquimod, photodynamic therapy, or electrodesiccation and curettage, larger lesions should be treated with Mohs micrographic surgery and excisional surgery with grafting. Inoperable tumors may be treated with radiation therapy and vismodegib.
Our patient. Once the diagnosis of BCC was established, treatment options were discussed, including excision, local radiation therapy, and oral hedgehog inhibitor drug therapy.8 Our patient opted to undergo a wide local excision of the lesion followed by negative-pressure wound therapy, which led to complete healing.
CORRESPONDENCE
David Crasto, DO, William Carey University College of Osteopathic Medicine, 498 Tuscan Avenue, Hattiesburg, MS 39401; [email protected]
1. Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17:763-771.
2. Fox JD, Baquerizo Nole KL, Berriman SJ, et al. Chronic wounds: the need for greater emphasis in medical schools, post-graduate training and public health discussions. Ann Surg. 2016;264:241-243.
3. Senet P, Combemale P, Debure C, et al. Malignancy and chronic leg ulcers. Arch Dermatol. 2012;148:704-708.
4. Ghasemi F, Anooshirvani N, Sibbald RG, et al. The point prevalence of malignancy in a wound clinic. Int J Low Extrem Wounds. 2016;15:58-62.
5. Labropoulos N, Manalo D, Patel N, et al. Uncommon leg ulcers in the lower extremity. J Vasc Surg. 2007;45:568-573.
6. Tchanque-Fossuo CN, Millsop J, Johnson MA, et al. Ulcerated basal cell carcinomas masquerading as venous leg ulcers. Adv Skin Wound Care. 2018;31:130-134.
7. Schnirring-Judge M, Belpedio D. Malignant transformation of a chronic venous stasis ulcer to basal cell carcinoma in a diabetic patient: case and review of the pathophysiology. J Foot Ankle Surg. 2010;49:75-79.
8. Puig S, Berrocal A. Management of high-risk and advanced basal cell carcinoma. Clin Transl Oncol. 2015;17:497-503.
1. Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17:763-771.
2. Fox JD, Baquerizo Nole KL, Berriman SJ, et al. Chronic wounds: the need for greater emphasis in medical schools, post-graduate training and public health discussions. Ann Surg. 2016;264:241-243.
3. Senet P, Combemale P, Debure C, et al. Malignancy and chronic leg ulcers. Arch Dermatol. 2012;148:704-708.
4. Ghasemi F, Anooshirvani N, Sibbald RG, et al. The point prevalence of malignancy in a wound clinic. Int J Low Extrem Wounds. 2016;15:58-62.
5. Labropoulos N, Manalo D, Patel N, et al. Uncommon leg ulcers in the lower extremity. J Vasc Surg. 2007;45:568-573.
6. Tchanque-Fossuo CN, Millsop J, Johnson MA, et al. Ulcerated basal cell carcinomas masquerading as venous leg ulcers. Adv Skin Wound Care. 2018;31:130-134.
7. Schnirring-Judge M, Belpedio D. Malignant transformation of a chronic venous stasis ulcer to basal cell carcinoma in a diabetic patient: case and review of the pathophysiology. J Foot Ankle Surg. 2010;49:75-79.
8. Puig S, Berrocal A. Management of high-risk and advanced basal cell carcinoma. Clin Transl Oncol. 2015;17:497-503.
Choosing Wisely: 10 practices to stop—or adopt—to reduce overuse in health care
When medical care is based on consistent, good-quality evidence, most physicians adopt it. However, not all care is well supported by the literature and may, in fact, be overused without offering benefit to patients. Choosing Wisely, at www.choosingwisely.org, is a health care initiative that highlights screening and testing recommendations from specialty societies in an effort to encourage patients and clinicians to talk about how to make high-value, effective health care decisions and avoid overuse. (See “Test and Tx overutilization: A bigger problem than you might think"1-3).
SIDEBAR
Test and Tx overutilization: A bigger problem than you might think
Care that isn’t backed up by the medical literature is adopted by some physicians and not adopted by others, leading to practice variations. Some variation is to be expected, since no 2 patients require exactly the same care, but substantial variations may be a clue to overuse.
A 2006 analysis of inpatient lab studies found that doctors ordered an average of 2.96 studies per patient per day, but only 29% of these tests (0.95 test/patient/day) contributed to management.1 A 2016 systematic review found more than 800 studies on overuse were published in a single year.2 One study of thyroid nodules followed almost 1000 patients with nodules as they underwent routine follow-up imaging. At the end of the study, 7 were found to have cancer, but of those, only 3 had enlarging or changing nodules that would have been detected with the follow-up imaging being studied. Three of the cancers were stable in size and 1 was found incidentally.3
Enabling physician and patient dialogue. The initiative began in 2010 when the American Board of Internal Medicine convened a panel of experts to identify low-value tests and therapies. Their list took the form of a “Top Five Things” that may not be high value in patient care, and it used language tailored to patients and physicians so that they could converse meaningfully. Physicians could use the evidence to make a clinical decision, and patients could feel empowered to ask informed questions about recommendations they received. The initiative has now expanded to include ways that health care systems can reduce low-value interventions.
Scope of participation. Since the first Choosing Wisely recommendations were published in 2013, more than 80 professional associations have contributed lists of their own. Professional societies participate voluntarily. The American Academy of Family Physicians (AAFP), Society of General Internal Medicine, and American Academy of Pediatrics (AAP) have contributed lists relevant to primary care. All Choosing Wisely recommendations can be searched or sorted by specialty organization. Recommendations are reviewed and revised regularly. If the evidence becomes conflicted or contradictory, recommendations are withdrawn.
Making meaningful improvements by Choosing Wisely
Several studies have shown that health care systems can implement Choosing Wisely recommendations to reduce overuse of unnecessary tests. A 2015 study examined the effect of applying a Choosing Wisely recommendation to reduce the use of continuous pulse oximetry in pediatric inpatients with asthma, wheezing, or bronchiolitis. The recommendation, from the Society of Hospital Medicine–Pediatric Hospital Medicine, advises against continuous pulse oximetry in children with acute respiratory illnesses unless the child is using supplemental oxygen.4 This study, done at the Cincinnati Children’s Hospital Medical Center, found that within 3 months of initiating a protocol on all general pediatrics floors, the average time on pulse oximetry after meeting clinical goals decreased from 10.7 hours to 3.1 hours. In addition, the percentage of patients who had their continuous pulse oximetry stopped within 2 hours of clinical stability (a goal time) increased from 25% to 46%.5
Patients are important drivers of health care utilization. A 2003 study showed that physicians are more likely to order referrals, tests, and prescriptions when patients ask for them, and that nearly 1 in 4 patients did so.6 A 2002 study found that physicians granted all but 3% of patient’s requests for orders or tests, and that fulfilling requests correlated with patient satisfaction in the specialty office studied (cardiology) but not in the primary care (internal medicine) office.7
From its inception, Choosing Wisely has considered patients as full partners in conversations about health care utilization. Choosing Wisely partners with Consumer Reports to create and disseminate plain-language summaries of recommendations. Community groups and physician organizations have also participated in implementation efforts. In 2018, Choosing Wisely secured a grant to expand outreach to diverse or underserved communities.
Choosing Wisely recommendations are not guidelines or mandates. They are intended to be evidence-based advice from a specialty society to its members and to patients about care that is often unnecessary. The goal is to create a conversation and not to eliminate these services from ever being offered or used.
Continue to: Improve your practice with these 10 primary care recommendations
Improve your practice with these 10 primary care recommendations
1 Avoid imaging studies in early acute low back pain without red flags.
Both the AAFP and the American Society of Anesthesiologists recommend against routine X-rays, magnetic resonance imaging, and computed tomography (CT) scans in the first 6 weeks of acute low back pain (LBP).8,9 The American College of Emergency Physicians (ACEP) recommends against routine lumbar spine imaging for emergency department (ED) patients.10 In all cases, imaging is indicated if the patient has any signs or symptoms of neurologic deficits or other indications, such as signs of spinal infection or fracture. However, as ACEP notes, diagnostic imaging does not typically help identify the cause of acute LBP, and when it does, it does not reduce the time to symptom improvement.10
2 Prescribe oral contraceptives on the basis of a medical history and a blood pressure measurement. No routine pelvic exam or other physical exam is necessary.
This AAFP recommendation11 is based on clinical practice guidelines from the American College of Obstetricians and Gynecologists (ACOG) and other research.12 The ACOG practice guideline supports provision of hormonal contraception without a pelvic exam, cervical cancer (Pap) testing, urine pregnancy testing, or testing for sexually transmitted infections. ACOG guidelines also support over-the-counter provision of hormonal contraceptives, including combined oral contraceptives.12
3 Stop recommending daily self-glucose monitoring for patients with diabetes who are not using insulin.
Both the AAFP and the Society for General Internal Medicine recommend against daily blood sugar checks for people who do not use insulin.13,14 A Cochrane review of 9 trials (3300 patients) found that after 6 months, hemoglobin A1C was reduced by 0.3% in people who checked their sugar daily compared with those who did not, but this difference was not significant after a year.15 Hypoglycemic episodes were more common in the “checking” group, and there were no differences in quality of life. A qualitative study found that blood sugar results had little impact on patients’ motivation to change behavior.16
4 Don’t screen for herpes simplex virus (HSV) infection in asymptomatic adults, even those who are pregnant.
This AAFP recommendation17 comes from a US Preventive Services Task Force (USPSTF) Grade D recommendation.18 Most people with positive HSV-2 serology have had an outbreak; even those who do not think they have had one will realize that they had the symptoms once they hear them described.18 With available tests, 1 in 2 positive results for HSV-2 among asymptomatic people will be a false-positive.18
There is no known cure, intervention, or reduction in transmission for infected patients who do not have symptoms.18 Also, serologically detected HSV-2 does not reliably predict genital herpes; and HSV-1 has been found to cause an increasing percentage of genital infection cases.18
Continue to: 5 Don't screen for testicular cancer in asymptomatic individuals
5 Don’t screen for testicular cancer in asymptomatic individuals.
This AAFP recommendation19 also comes from a USPSTF Grade D recommendation.20 A 2010 systematic review found no evidence to support screening of asymptomatic people with a physical exam or ultrasound. All available studies involved symptomatic patients.20
6 Stop recommending cough and cold medicines for children younger than 4 years.
The AAP recommends that clinicians discourage the use of any cough or cold medicine for children in this age-group.21 A 2008 study found that more than 7000 children annually presented to EDs for adverse events from cough and cold medicines.22 Previous studies found no benefit in reducing symptoms.23 In children older than 12 months, a Cochrane review found that honey has a modest benefit for cough in single-night trials.24
7 Avoid performing serum allergy panels.
The American Academy of Allergy, Asthma, and Immunology discourages the use of serum panel testing when patients present with allergy symptoms.25 A patient can have a strong positive immunoglobulin E (IgE) serum result to an allergen and have no clinical allergic symptoms or can have a weak positive serum result and a strong clinical reaction. Targeted skin or serum IgE testing—for example, testing for cashew allergy in a patient known to have had a reaction after eating one—is reasonable.26
8 Avoid routine electroencephalography (EEG), head CT, and carotid ultrasound as initial work-up for simple syncope in adults.
These recommendations, from the American Epilepsy Society,27 ACEP,28 American College of Physicians,29 and American Academy of Neurology (AAN),30 emphasize the low yield of routine work-ups for patients with simple syncope. The AAN notes that 40% of people will experience syncope during adulthood and most will not have carotid disease, which generally manifests with stroke-like symptoms rather than syncope. One study found that approximately 1 in 8 patients referred to an epilepsy clinic had neurocardiogenic syncope rather than epilepsy.31
EEGs have high false-negative and false-positive rates, and history-taking is a better tool with which to make a diagnosis. CT scans performed in the ED were found to contribute to the diagnosis of simple syncope in fewer than 2% of cases of syncope, compared with orthostatic blood pressure (25% of cases).32
Continue to: 9 Wait to refer children with umbilical hernias to pediatric surgery until they are 4 to 5 years of age
9 Wait to refer children with umbilical hernias to pediatric surgery until they are 4 to 5 years of age.
The AAP Section on Surgery offers evidence that the risk-benefit analysis strongly favors waiting on intervention.33 About 1 in 4 children will have an umbilical hernia, and about 85% of cases will resolve by age 5. The strangulation rate with umbilical hernias is very low, and although the risk of infection with surgery is likewise low, the risk of recurrence following surgery before the age of 4 is as high as 2.4%.34 The AAP Section on Surgery recommends against strapping or restraining the hernia, as well.
10 Avoid using appetite stimulants, such as megesterol, and high-calorie nutritional supplements to treat anorexia and cachexia in older adults.
Instead, the American Geriatrics Society recommends that physicians encourage caregivers to serve appealing food, provide support with eating, and remove barriers to appetite and nutrition.35 A Cochrane review showed that high-calorie supplements, such as Boost or Ensure, are associated with very modest weight gain—about 2% of weight—but are not associated with an increased life expectancy or improved quality of life.36
Prescription appetite stimulants are associated with adverse effects and yield inconsistent benefits in older adults. Megesterol, for example, was associated with headache, gastrointestinal adverse effects, insomnia, weakness, and fatigue. Mirtazapine is associated with sedation and fatigue.37
CORRESPONDENCE
Kathleen Rowland, MD, MS, Rush Copley Family Medicine Residency, Rush Medical College, 600 South Paulina, Kidston House Room 605, Chicago IL 60612; [email protected].
1. Miyakis S, Karamanof G, Liontos M, et al. Factors contributing to inappropriate ordering of tests in an academic medical department and the effect of an educational feedback strategy. Postgrad Med J. 2006;82:823-829.
2. Morgan DJ, Dhruva SS, Wright SM, et al. Update on medical overuse: a systematic review. JAMA Intern Med. 2016;176:1687-1692.
3. Durante C, Costante G, Lucisano G, et al. The natural history of benign thyroid nodules. JAMA. 2015;313:926-935.
4. Choosing Wisely. Society of Hospital Medicine—Pediatric hospital medicine. Don’t use continuous pulse oximetry routinely in children with acute respiratory illness unless they are on supplemental oxygen. www.choosingwisely.org/clinician-lists/society-hospital-medicine-pediatric-continuous-pulse-oximetry-in-children-with-acute-respiratory-illness/. Accessed September 28, 2020.
5. Schondelmeyer AC, Simmons JM, Statile AM, et al. Using quality improvement to reduce continuous pulse oximetry use in children with wheezing. Pediatrics. 2015;135:e1044-e1051.
6. Kravitz RL, Bell RA, Azari R, et al. Direct observation of requests for clinical services in office practice: what do patients want and do they get it? Arch Intern Med. 2003;163:1673-1681.
7. Kravitz RL, Bell RA, Franz CE, et al. Characterizing patient requests and physician responses in office practice. Health Serv Res. 2002;37:217-238.
8. Choosing Wisely. American Academy of Family Physicians. Don’t do imaging for low back pain within the first six weeks, unless red flags are present. www.choosingwisely.org/clinician-lists/american-academy-family-physicians-imaging-low-back-pain/. Accessed September 28, 2020.
9. Choosing Wisely. American Society of Anesthesiologists–Pain Medicine. Avoid imaging studies (MRI, CT or X-rays) for acute low back pain without specific indications. www.choosingwisely.org/clinician-lists/american-society-anesthesiologists-imaging-studies-for-acute-low-back-pain/. Accessed September 28, 2020.
10. Choosing Wisely. American College of Emergency Physicians. Avoid lumbar spine imaging in the emergency department for adults with non-traumatic back pain unless the patient has severe or progressive neurologic deficits or is suspected of having a serious underlying condition (such as vertebral infection, cauda equina syndrome, or cancer with bony metastasis). www.choosingwisely.org/clinician-lists/acep-lumbar-spine-imaging-in-the-ed/. Accessed September 28, 2020.
11. Choosing Wisely. American Academy of Family Physicians. Don’t require a pelvic exam or other physical exam to prescribe oral contraceptive medications. www.choosingwisely.org/clinician-lists/american-academy-family-physicians-pelvic-or-physical-exams-to-prescribe-oral-contraceptives/. Accessed September 28, 2020.
12. Over-the-counter access to hormonal contraception. ACOG Committee Opinion, Number 788. Obstet Gynecol. 2019;134:e96-e105. https://journals.lww.com/greenjournal/Fulltext/2019/10000/Over_the_Counter_Access_to_Hormonal_Contraception_.46.aspx. Accessed September 28, 2020.
13. Choosing Wisely. American Academy of Family Physicians. Don’t routinely recommend daily home glucose monitoring for patients who have Type 2 diabetes mellitus and are not using insulin. www.choosingwisely.org/clinician-lists/aafp-daily-home-glucose-monitoring-for-patients-with-type-2-diabetes. Accessed September 28, 2020.
14. Choosing Wisely. Society of General Internal Medicine. Don’t recommend daily home finger glucose testing in patients with Type 2 diabetes mellitus not using insulin. www.choosingwisely.org/clinician-lists/society-general-internal-medicine-daily-home-finger-glucose-testing-type-2-diabetes-mellitus/. Accessed September 28, 2020.
15. Malanda UL, Welschen LM, Riphagen II, et al. Self‐monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin. Cochrane Database Syst Rev. 2012(1):CD005060.
16. Peel E, Douglas M, Lawton J. Self monitoring of blood glucose in type 2 diabetes: longitudinal qualitative study of patients’ perspectives. BMJ. 2007;335:493.
17. Choosing Wisely. American Academy of Family Physicians. Don’t screen for genital herpes simplex virus infection (HSV) in asymptomatic adults, including pregnant women. www.choosingwisely.org/clinician-lists/aafp-genital-herpes-screening-in-asymptomatic-adults/. Accessed September 28, 2020.
18. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Serologic screening for genital herpes infection: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:2525-2530.
19. Choosing Wisely. American Academy of Family Physicians. Don’t screen for testicular cancer in asymptomatic adolescent and adult males. www.choosingwisely.org/clinician-lists/aafp-testicular-cancer-screening-in-asymptomatic-adolescent-and-adult-men/. Accessed September 28, 2020.
20. Lin K, Sharangpani R. Screening for testicular cancer: an evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2010;153:396-399.
21. Choosing Wisely. American Academy of Pediatrics. Cough and cold medicines should not be prescribed, recommended or used for respiratory illnesses in young children. www.choosingwisely.org/clinician-lists/american-academy-pediatrics-cough-and-cold-medicines-for-children-under-four/. Accessed September 28, 2020.
22. Schaefer MK, Shehab N, Cohen AL, et al. Adverse events from cough and cold medications in children. Pediatrics. 2008;121:783-787.
23. Carr BC. Efficacy, abuse, and toxicity of over-the-counter cough and cold medicines in the pediatric population. Curr Opin Pediatr. 2006;18:184-188.
24. Oduwole O, Udoh EE, Oyo‐Ita A, et al. Honey for acute cough in children. Cochrane Database Syst Rev. 2018(4):CD007094.
25. Choosing Wisely. American Academy of Allergy, Asthma & Immunology. Don’t perform unproven diagnostic tests, such as immunoglobulin G(lgG) testing or an indiscriminate battery of immunoglobulin E(lgE) tests, in the evaluation of allergy. www.choosingwisely.org/clinician-lists/american-academy-allergy-asthma-immunology-diagnostic-tests-for-allergy-evaluation/. Accessed September 28, 2020.
26. Cox L, Williams B, Sicherer S, et al. Pearls and pitfalls of allergy diagnostic testing: report from the American College of Allergy, Asthma and Immunology Specific IgE Test Task Force. Ann Allergy Asthma Immunol. 2008;101:580-592.
27. Choosing Wisely. American Epilepsy Society. Do not routinely order electroencephalogram (EEG) as part of initial syncope work-up. www.choosingwisely.org/clinician-lists/aes-eeg-as-part-of-initial-syncope-work-up/. Accessed September 28, 2020.
28. Choosing Wisely. American College of Emergency Physicians. Avoid CT of the head in asymptomatic adult patients in the emergency department with syncope, insignificant trauma and a normal neurological evaluation. www.choosingwisely.org/clinician-lists/acep-avoid-head-ct-for-asymptomatic-adults-with-syncope/. Accessed September 28, 2020.
29. Choosing Wisely. American College of Physicians. In the evaluation of simple syncope and a normal neurological examination, don’t obtain brain imaging studies (CT or MRI). www.choosingwisely.org/clinician-lists/american-college-physicians-brain-imaging-to-evaluate-simple-syncope/. Accessed September 28, 2020.
30. Choosing Wisely. American Academy of Neurology. Don’t perform imaging of the carotid arteries for simple syncope without other neurologic symptoms. www.choosingwisely.org/clinician-lists/american-academy-neurology-carotid-artery-imaging-for-simple-syncope/. Accessed September 28, 2020.
31. Josephson CB, Rahey S, Sadler RM. Neurocardiogenic syncope: frequency and consequences of its misdiagnosis as epilepsy. Can J Neurol Sci. 2007;34:221-224.
32. Mendu ML, McAvay G, Lampert R, et al. Yield of diagnostic tests in evaluating syncopal episodes in older patients. Arch Intern Med. 2009;169:1299-1305.
33. Choosing Wisely. American Academy of Pediatrics–Section on Surgery. Avoid referring most children with umbilical hernias to a pediatric surgeon until around age 4-5 years. www.choosingwisely.org/clinician-lists/aap-sosu-avoid-surgery-referral-for-umbilical-hernias-until-age-4-5/. Accessed September 28, 2020.
34. Antonoff MB, Kreykes NS, Saltzman DA, et al. American Academy of Pediatrics Section on Surgery hernia survey revisited. J Pediatr Surg. 2005;40:1009-1014.
35. Choosing Wisely. American Geriatrics Society. Avoid using prescription appetite stimulants or high-calorie supplements for treatment of anorexia or cachexia in older adults; instead, optimize social supports, discontinue medications that may interfere with eating, provide appealing food and feeding assistance, and clarify patient goals and expectations. www.choosingwisely.org/clinician-lists/american-geriatrics-society-prescription-appetite-stimulants-to-treat-anorexia-cachexia-in-elderly/. Accessed September 28, 2020.
36. Milne AC, Potter J, Vivanti A, et al. Protein and energy supplementation in elderly people at risk from malnutrition. Cochrane Database Sys Rev. 2009(2):CD003288.
37. Fox CB, Treadway AK, Blaszczyk AT, et al. Megestrol acetate and mirtazapine for the treatment of unplanned weight loss in the elderly. Pharmacotherapy. 2009;29:383-397.
When medical care is based on consistent, good-quality evidence, most physicians adopt it. However, not all care is well supported by the literature and may, in fact, be overused without offering benefit to patients. Choosing Wisely, at www.choosingwisely.org, is a health care initiative that highlights screening and testing recommendations from specialty societies in an effort to encourage patients and clinicians to talk about how to make high-value, effective health care decisions and avoid overuse. (See “Test and Tx overutilization: A bigger problem than you might think"1-3).
SIDEBAR
Test and Tx overutilization: A bigger problem than you might think
Care that isn’t backed up by the medical literature is adopted by some physicians and not adopted by others, leading to practice variations. Some variation is to be expected, since no 2 patients require exactly the same care, but substantial variations may be a clue to overuse.
A 2006 analysis of inpatient lab studies found that doctors ordered an average of 2.96 studies per patient per day, but only 29% of these tests (0.95 test/patient/day) contributed to management.1 A 2016 systematic review found more than 800 studies on overuse were published in a single year.2 One study of thyroid nodules followed almost 1000 patients with nodules as they underwent routine follow-up imaging. At the end of the study, 7 were found to have cancer, but of those, only 3 had enlarging or changing nodules that would have been detected with the follow-up imaging being studied. Three of the cancers were stable in size and 1 was found incidentally.3
Enabling physician and patient dialogue. The initiative began in 2010 when the American Board of Internal Medicine convened a panel of experts to identify low-value tests and therapies. Their list took the form of a “Top Five Things” that may not be high value in patient care, and it used language tailored to patients and physicians so that they could converse meaningfully. Physicians could use the evidence to make a clinical decision, and patients could feel empowered to ask informed questions about recommendations they received. The initiative has now expanded to include ways that health care systems can reduce low-value interventions.

Scope of participation. Since the first Choosing Wisely recommendations were published in 2013, more than 80 professional associations have contributed lists of their own. Professional societies participate voluntarily. The American Academy of Family Physicians (AAFP), Society of General Internal Medicine, and American Academy of Pediatrics (AAP) have contributed lists relevant to primary care. All Choosing Wisely recommendations can be searched or sorted by specialty organization. Recommendations are reviewed and revised regularly. If the evidence becomes conflicted or contradictory, recommendations are withdrawn.
Making meaningful improvements by Choosing Wisely
Several studies have shown that health care systems can implement Choosing Wisely recommendations to reduce overuse of unnecessary tests. A 2015 study examined the effect of applying a Choosing Wisely recommendation to reduce the use of continuous pulse oximetry in pediatric inpatients with asthma, wheezing, or bronchiolitis. The recommendation, from the Society of Hospital Medicine–Pediatric Hospital Medicine, advises against continuous pulse oximetry in children with acute respiratory illnesses unless the child is using supplemental oxygen.4 This study, done at the Cincinnati Children’s Hospital Medical Center, found that within 3 months of initiating a protocol on all general pediatrics floors, the average time on pulse oximetry after meeting clinical goals decreased from 10.7 hours to 3.1 hours. In addition, the percentage of patients who had their continuous pulse oximetry stopped within 2 hours of clinical stability (a goal time) increased from 25% to 46%.5
Patients are important drivers of health care utilization. A 2003 study showed that physicians are more likely to order referrals, tests, and prescriptions when patients ask for them, and that nearly 1 in 4 patients did so.6 A 2002 study found that physicians granted all but 3% of patient’s requests for orders or tests, and that fulfilling requests correlated with patient satisfaction in the specialty office studied (cardiology) but not in the primary care (internal medicine) office.7
From its inception, Choosing Wisely has considered patients as full partners in conversations about health care utilization. Choosing Wisely partners with Consumer Reports to create and disseminate plain-language summaries of recommendations. Community groups and physician organizations have also participated in implementation efforts. In 2018, Choosing Wisely secured a grant to expand outreach to diverse or underserved communities.
Choosing Wisely recommendations are not guidelines or mandates. They are intended to be evidence-based advice from a specialty society to its members and to patients about care that is often unnecessary. The goal is to create a conversation and not to eliminate these services from ever being offered or used.
Continue to: Improve your practice with these 10 primary care recommendations
Improve your practice with these 10 primary care recommendations
1 Avoid imaging studies in early acute low back pain without red flags.
Both the AAFP and the American Society of Anesthesiologists recommend against routine X-rays, magnetic resonance imaging, and computed tomography (CT) scans in the first 6 weeks of acute low back pain (LBP).8,9 The American College of Emergency Physicians (ACEP) recommends against routine lumbar spine imaging for emergency department (ED) patients.10 In all cases, imaging is indicated if the patient has any signs or symptoms of neurologic deficits or other indications, such as signs of spinal infection or fracture. However, as ACEP notes, diagnostic imaging does not typically help identify the cause of acute LBP, and when it does, it does not reduce the time to symptom improvement.10
2 Prescribe oral contraceptives on the basis of a medical history and a blood pressure measurement. No routine pelvic exam or other physical exam is necessary.
This AAFP recommendation11 is based on clinical practice guidelines from the American College of Obstetricians and Gynecologists (ACOG) and other research.12 The ACOG practice guideline supports provision of hormonal contraception without a pelvic exam, cervical cancer (Pap) testing, urine pregnancy testing, or testing for sexually transmitted infections. ACOG guidelines also support over-the-counter provision of hormonal contraceptives, including combined oral contraceptives.12
3 Stop recommending daily self-glucose monitoring for patients with diabetes who are not using insulin.
Both the AAFP and the Society for General Internal Medicine recommend against daily blood sugar checks for people who do not use insulin.13,14 A Cochrane review of 9 trials (3300 patients) found that after 6 months, hemoglobin A1C was reduced by 0.3% in people who checked their sugar daily compared with those who did not, but this difference was not significant after a year.15 Hypoglycemic episodes were more common in the “checking” group, and there were no differences in quality of life. A qualitative study found that blood sugar results had little impact on patients’ motivation to change behavior.16
4 Don’t screen for herpes simplex virus (HSV) infection in asymptomatic adults, even those who are pregnant.
This AAFP recommendation17 comes from a US Preventive Services Task Force (USPSTF) Grade D recommendation.18 Most people with positive HSV-2 serology have had an outbreak; even those who do not think they have had one will realize that they had the symptoms once they hear them described.18 With available tests, 1 in 2 positive results for HSV-2 among asymptomatic people will be a false-positive.18
There is no known cure, intervention, or reduction in transmission for infected patients who do not have symptoms.18 Also, serologically detected HSV-2 does not reliably predict genital herpes; and HSV-1 has been found to cause an increasing percentage of genital infection cases.18
Continue to: 5 Don't screen for testicular cancer in asymptomatic individuals
5 Don’t screen for testicular cancer in asymptomatic individuals.
This AAFP recommendation19 also comes from a USPSTF Grade D recommendation.20 A 2010 systematic review found no evidence to support screening of asymptomatic people with a physical exam or ultrasound. All available studies involved symptomatic patients.20
6 Stop recommending cough and cold medicines for children younger than 4 years.
The AAP recommends that clinicians discourage the use of any cough or cold medicine for children in this age-group.21 A 2008 study found that more than 7000 children annually presented to EDs for adverse events from cough and cold medicines.22 Previous studies found no benefit in reducing symptoms.23 In children older than 12 months, a Cochrane review found that honey has a modest benefit for cough in single-night trials.24
7 Avoid performing serum allergy panels.
The American Academy of Allergy, Asthma, and Immunology discourages the use of serum panel testing when patients present with allergy symptoms.25 A patient can have a strong positive immunoglobulin E (IgE) serum result to an allergen and have no clinical allergic symptoms or can have a weak positive serum result and a strong clinical reaction. Targeted skin or serum IgE testing—for example, testing for cashew allergy in a patient known to have had a reaction after eating one—is reasonable.26
8 Avoid routine electroencephalography (EEG), head CT, and carotid ultrasound as initial work-up for simple syncope in adults.
These recommendations, from the American Epilepsy Society,27 ACEP,28 American College of Physicians,29 and American Academy of Neurology (AAN),30 emphasize the low yield of routine work-ups for patients with simple syncope. The AAN notes that 40% of people will experience syncope during adulthood and most will not have carotid disease, which generally manifests with stroke-like symptoms rather than syncope. One study found that approximately 1 in 8 patients referred to an epilepsy clinic had neurocardiogenic syncope rather than epilepsy.31
EEGs have high false-negative and false-positive rates, and history-taking is a better tool with which to make a diagnosis. CT scans performed in the ED were found to contribute to the diagnosis of simple syncope in fewer than 2% of cases of syncope, compared with orthostatic blood pressure (25% of cases).32
Continue to: 9 Wait to refer children with umbilical hernias to pediatric surgery until they are 4 to 5 years of age
9 Wait to refer children with umbilical hernias to pediatric surgery until they are 4 to 5 years of age.
The AAP Section on Surgery offers evidence that the risk-benefit analysis strongly favors waiting on intervention.33 About 1 in 4 children will have an umbilical hernia, and about 85% of cases will resolve by age 5. The strangulation rate with umbilical hernias is very low, and although the risk of infection with surgery is likewise low, the risk of recurrence following surgery before the age of 4 is as high as 2.4%.34 The AAP Section on Surgery recommends against strapping or restraining the hernia, as well.
10 Avoid using appetite stimulants, such as megesterol, and high-calorie nutritional supplements to treat anorexia and cachexia in older adults.
Instead, the American Geriatrics Society recommends that physicians encourage caregivers to serve appealing food, provide support with eating, and remove barriers to appetite and nutrition.35 A Cochrane review showed that high-calorie supplements, such as Boost or Ensure, are associated with very modest weight gain—about 2% of weight—but are not associated with an increased life expectancy or improved quality of life.36
Prescription appetite stimulants are associated with adverse effects and yield inconsistent benefits in older adults. Megesterol, for example, was associated with headache, gastrointestinal adverse effects, insomnia, weakness, and fatigue. Mirtazapine is associated with sedation and fatigue.37
CORRESPONDENCE
Kathleen Rowland, MD, MS, Rush Copley Family Medicine Residency, Rush Medical College, 600 South Paulina, Kidston House Room 605, Chicago IL 60612; [email protected].
When medical care is based on consistent, good-quality evidence, most physicians adopt it. However, not all care is well supported by the literature and may, in fact, be overused without offering benefit to patients. Choosing Wisely, at www.choosingwisely.org, is a health care initiative that highlights screening and testing recommendations from specialty societies in an effort to encourage patients and clinicians to talk about how to make high-value, effective health care decisions and avoid overuse. (See “Test and Tx overutilization: A bigger problem than you might think"1-3).
SIDEBAR
Test and Tx overutilization: A bigger problem than you might think
Care that isn’t backed up by the medical literature is adopted by some physicians and not adopted by others, leading to practice variations. Some variation is to be expected, since no 2 patients require exactly the same care, but substantial variations may be a clue to overuse.
A 2006 analysis of inpatient lab studies found that doctors ordered an average of 2.96 studies per patient per day, but only 29% of these tests (0.95 test/patient/day) contributed to management.1 A 2016 systematic review found more than 800 studies on overuse were published in a single year.2 One study of thyroid nodules followed almost 1000 patients with nodules as they underwent routine follow-up imaging. At the end of the study, 7 were found to have cancer, but of those, only 3 had enlarging or changing nodules that would have been detected with the follow-up imaging being studied. Three of the cancers were stable in size and 1 was found incidentally.3
Enabling physician and patient dialogue. The initiative began in 2010 when the American Board of Internal Medicine convened a panel of experts to identify low-value tests and therapies. Their list took the form of a “Top Five Things” that may not be high value in patient care, and it used language tailored to patients and physicians so that they could converse meaningfully. Physicians could use the evidence to make a clinical decision, and patients could feel empowered to ask informed questions about recommendations they received. The initiative has now expanded to include ways that health care systems can reduce low-value interventions.

Scope of participation. Since the first Choosing Wisely recommendations were published in 2013, more than 80 professional associations have contributed lists of their own. Professional societies participate voluntarily. The American Academy of Family Physicians (AAFP), Society of General Internal Medicine, and American Academy of Pediatrics (AAP) have contributed lists relevant to primary care. All Choosing Wisely recommendations can be searched or sorted by specialty organization. Recommendations are reviewed and revised regularly. If the evidence becomes conflicted or contradictory, recommendations are withdrawn.
Making meaningful improvements by Choosing Wisely
Several studies have shown that health care systems can implement Choosing Wisely recommendations to reduce overuse of unnecessary tests. A 2015 study examined the effect of applying a Choosing Wisely recommendation to reduce the use of continuous pulse oximetry in pediatric inpatients with asthma, wheezing, or bronchiolitis. The recommendation, from the Society of Hospital Medicine–Pediatric Hospital Medicine, advises against continuous pulse oximetry in children with acute respiratory illnesses unless the child is using supplemental oxygen.4 This study, done at the Cincinnati Children’s Hospital Medical Center, found that within 3 months of initiating a protocol on all general pediatrics floors, the average time on pulse oximetry after meeting clinical goals decreased from 10.7 hours to 3.1 hours. In addition, the percentage of patients who had their continuous pulse oximetry stopped within 2 hours of clinical stability (a goal time) increased from 25% to 46%.5
Patients are important drivers of health care utilization. A 2003 study showed that physicians are more likely to order referrals, tests, and prescriptions when patients ask for them, and that nearly 1 in 4 patients did so.6 A 2002 study found that physicians granted all but 3% of patient’s requests for orders or tests, and that fulfilling requests correlated with patient satisfaction in the specialty office studied (cardiology) but not in the primary care (internal medicine) office.7
From its inception, Choosing Wisely has considered patients as full partners in conversations about health care utilization. Choosing Wisely partners with Consumer Reports to create and disseminate plain-language summaries of recommendations. Community groups and physician organizations have also participated in implementation efforts. In 2018, Choosing Wisely secured a grant to expand outreach to diverse or underserved communities.
Choosing Wisely recommendations are not guidelines or mandates. They are intended to be evidence-based advice from a specialty society to its members and to patients about care that is often unnecessary. The goal is to create a conversation and not to eliminate these services from ever being offered or used.
Continue to: Improve your practice with these 10 primary care recommendations
Improve your practice with these 10 primary care recommendations
1 Avoid imaging studies in early acute low back pain without red flags.
Both the AAFP and the American Society of Anesthesiologists recommend against routine X-rays, magnetic resonance imaging, and computed tomography (CT) scans in the first 6 weeks of acute low back pain (LBP).8,9 The American College of Emergency Physicians (ACEP) recommends against routine lumbar spine imaging for emergency department (ED) patients.10 In all cases, imaging is indicated if the patient has any signs or symptoms of neurologic deficits or other indications, such as signs of spinal infection or fracture. However, as ACEP notes, diagnostic imaging does not typically help identify the cause of acute LBP, and when it does, it does not reduce the time to symptom improvement.10
2 Prescribe oral contraceptives on the basis of a medical history and a blood pressure measurement. No routine pelvic exam or other physical exam is necessary.
This AAFP recommendation11 is based on clinical practice guidelines from the American College of Obstetricians and Gynecologists (ACOG) and other research.12 The ACOG practice guideline supports provision of hormonal contraception without a pelvic exam, cervical cancer (Pap) testing, urine pregnancy testing, or testing for sexually transmitted infections. ACOG guidelines also support over-the-counter provision of hormonal contraceptives, including combined oral contraceptives.12
3 Stop recommending daily self-glucose monitoring for patients with diabetes who are not using insulin.
Both the AAFP and the Society for General Internal Medicine recommend against daily blood sugar checks for people who do not use insulin.13,14 A Cochrane review of 9 trials (3300 patients) found that after 6 months, hemoglobin A1C was reduced by 0.3% in people who checked their sugar daily compared with those who did not, but this difference was not significant after a year.15 Hypoglycemic episodes were more common in the “checking” group, and there were no differences in quality of life. A qualitative study found that blood sugar results had little impact on patients’ motivation to change behavior.16
4 Don’t screen for herpes simplex virus (HSV) infection in asymptomatic adults, even those who are pregnant.
This AAFP recommendation17 comes from a US Preventive Services Task Force (USPSTF) Grade D recommendation.18 Most people with positive HSV-2 serology have had an outbreak; even those who do not think they have had one will realize that they had the symptoms once they hear them described.18 With available tests, 1 in 2 positive results for HSV-2 among asymptomatic people will be a false-positive.18
There is no known cure, intervention, or reduction in transmission for infected patients who do not have symptoms.18 Also, serologically detected HSV-2 does not reliably predict genital herpes; and HSV-1 has been found to cause an increasing percentage of genital infection cases.18
Continue to: 5 Don't screen for testicular cancer in asymptomatic individuals
5 Don’t screen for testicular cancer in asymptomatic individuals.
This AAFP recommendation19 also comes from a USPSTF Grade D recommendation.20 A 2010 systematic review found no evidence to support screening of asymptomatic people with a physical exam or ultrasound. All available studies involved symptomatic patients.20
6 Stop recommending cough and cold medicines for children younger than 4 years.
The AAP recommends that clinicians discourage the use of any cough or cold medicine for children in this age-group.21 A 2008 study found that more than 7000 children annually presented to EDs for adverse events from cough and cold medicines.22 Previous studies found no benefit in reducing symptoms.23 In children older than 12 months, a Cochrane review found that honey has a modest benefit for cough in single-night trials.24
7 Avoid performing serum allergy panels.
The American Academy of Allergy, Asthma, and Immunology discourages the use of serum panel testing when patients present with allergy symptoms.25 A patient can have a strong positive immunoglobulin E (IgE) serum result to an allergen and have no clinical allergic symptoms or can have a weak positive serum result and a strong clinical reaction. Targeted skin or serum IgE testing—for example, testing for cashew allergy in a patient known to have had a reaction after eating one—is reasonable.26
8 Avoid routine electroencephalography (EEG), head CT, and carotid ultrasound as initial work-up for simple syncope in adults.
These recommendations, from the American Epilepsy Society,27 ACEP,28 American College of Physicians,29 and American Academy of Neurology (AAN),30 emphasize the low yield of routine work-ups for patients with simple syncope. The AAN notes that 40% of people will experience syncope during adulthood and most will not have carotid disease, which generally manifests with stroke-like symptoms rather than syncope. One study found that approximately 1 in 8 patients referred to an epilepsy clinic had neurocardiogenic syncope rather than epilepsy.31
EEGs have high false-negative and false-positive rates, and history-taking is a better tool with which to make a diagnosis. CT scans performed in the ED were found to contribute to the diagnosis of simple syncope in fewer than 2% of cases of syncope, compared with orthostatic blood pressure (25% of cases).32
Continue to: 9 Wait to refer children with umbilical hernias to pediatric surgery until they are 4 to 5 years of age
9 Wait to refer children with umbilical hernias to pediatric surgery until they are 4 to 5 years of age.
The AAP Section on Surgery offers evidence that the risk-benefit analysis strongly favors waiting on intervention.33 About 1 in 4 children will have an umbilical hernia, and about 85% of cases will resolve by age 5. The strangulation rate with umbilical hernias is very low, and although the risk of infection with surgery is likewise low, the risk of recurrence following surgery before the age of 4 is as high as 2.4%.34 The AAP Section on Surgery recommends against strapping or restraining the hernia, as well.
10 Avoid using appetite stimulants, such as megesterol, and high-calorie nutritional supplements to treat anorexia and cachexia in older adults.
Instead, the American Geriatrics Society recommends that physicians encourage caregivers to serve appealing food, provide support with eating, and remove barriers to appetite and nutrition.35 A Cochrane review showed that high-calorie supplements, such as Boost or Ensure, are associated with very modest weight gain—about 2% of weight—but are not associated with an increased life expectancy or improved quality of life.36
Prescription appetite stimulants are associated with adverse effects and yield inconsistent benefits in older adults. Megesterol, for example, was associated with headache, gastrointestinal adverse effects, insomnia, weakness, and fatigue. Mirtazapine is associated with sedation and fatigue.37
CORRESPONDENCE
Kathleen Rowland, MD, MS, Rush Copley Family Medicine Residency, Rush Medical College, 600 South Paulina, Kidston House Room 605, Chicago IL 60612; [email protected].
1. Miyakis S, Karamanof G, Liontos M, et al. Factors contributing to inappropriate ordering of tests in an academic medical department and the effect of an educational feedback strategy. Postgrad Med J. 2006;82:823-829.
2. Morgan DJ, Dhruva SS, Wright SM, et al. Update on medical overuse: a systematic review. JAMA Intern Med. 2016;176:1687-1692.
3. Durante C, Costante G, Lucisano G, et al. The natural history of benign thyroid nodules. JAMA. 2015;313:926-935.
4. Choosing Wisely. Society of Hospital Medicine—Pediatric hospital medicine. Don’t use continuous pulse oximetry routinely in children with acute respiratory illness unless they are on supplemental oxygen. www.choosingwisely.org/clinician-lists/society-hospital-medicine-pediatric-continuous-pulse-oximetry-in-children-with-acute-respiratory-illness/. Accessed September 28, 2020.
5. Schondelmeyer AC, Simmons JM, Statile AM, et al. Using quality improvement to reduce continuous pulse oximetry use in children with wheezing. Pediatrics. 2015;135:e1044-e1051.
6. Kravitz RL, Bell RA, Azari R, et al. Direct observation of requests for clinical services in office practice: what do patients want and do they get it? Arch Intern Med. 2003;163:1673-1681.
7. Kravitz RL, Bell RA, Franz CE, et al. Characterizing patient requests and physician responses in office practice. Health Serv Res. 2002;37:217-238.
8. Choosing Wisely. American Academy of Family Physicians. Don’t do imaging for low back pain within the first six weeks, unless red flags are present. www.choosingwisely.org/clinician-lists/american-academy-family-physicians-imaging-low-back-pain/. Accessed September 28, 2020.
9. Choosing Wisely. American Society of Anesthesiologists–Pain Medicine. Avoid imaging studies (MRI, CT or X-rays) for acute low back pain without specific indications. www.choosingwisely.org/clinician-lists/american-society-anesthesiologists-imaging-studies-for-acute-low-back-pain/. Accessed September 28, 2020.
10. Choosing Wisely. American College of Emergency Physicians. Avoid lumbar spine imaging in the emergency department for adults with non-traumatic back pain unless the patient has severe or progressive neurologic deficits or is suspected of having a serious underlying condition (such as vertebral infection, cauda equina syndrome, or cancer with bony metastasis). www.choosingwisely.org/clinician-lists/acep-lumbar-spine-imaging-in-the-ed/. Accessed September 28, 2020.
11. Choosing Wisely. American Academy of Family Physicians. Don’t require a pelvic exam or other physical exam to prescribe oral contraceptive medications. www.choosingwisely.org/clinician-lists/american-academy-family-physicians-pelvic-or-physical-exams-to-prescribe-oral-contraceptives/. Accessed September 28, 2020.
12. Over-the-counter access to hormonal contraception. ACOG Committee Opinion, Number 788. Obstet Gynecol. 2019;134:e96-e105. https://journals.lww.com/greenjournal/Fulltext/2019/10000/Over_the_Counter_Access_to_Hormonal_Contraception_.46.aspx. Accessed September 28, 2020.
13. Choosing Wisely. American Academy of Family Physicians. Don’t routinely recommend daily home glucose monitoring for patients who have Type 2 diabetes mellitus and are not using insulin. www.choosingwisely.org/clinician-lists/aafp-daily-home-glucose-monitoring-for-patients-with-type-2-diabetes. Accessed September 28, 2020.
14. Choosing Wisely. Society of General Internal Medicine. Don’t recommend daily home finger glucose testing in patients with Type 2 diabetes mellitus not using insulin. www.choosingwisely.org/clinician-lists/society-general-internal-medicine-daily-home-finger-glucose-testing-type-2-diabetes-mellitus/. Accessed September 28, 2020.
15. Malanda UL, Welschen LM, Riphagen II, et al. Self‐monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin. Cochrane Database Syst Rev. 2012(1):CD005060.
16. Peel E, Douglas M, Lawton J. Self monitoring of blood glucose in type 2 diabetes: longitudinal qualitative study of patients’ perspectives. BMJ. 2007;335:493.
17. Choosing Wisely. American Academy of Family Physicians. Don’t screen for genital herpes simplex virus infection (HSV) in asymptomatic adults, including pregnant women. www.choosingwisely.org/clinician-lists/aafp-genital-herpes-screening-in-asymptomatic-adults/. Accessed September 28, 2020.
18. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Serologic screening for genital herpes infection: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:2525-2530.
19. Choosing Wisely. American Academy of Family Physicians. Don’t screen for testicular cancer in asymptomatic adolescent and adult males. www.choosingwisely.org/clinician-lists/aafp-testicular-cancer-screening-in-asymptomatic-adolescent-and-adult-men/. Accessed September 28, 2020.
20. Lin K, Sharangpani R. Screening for testicular cancer: an evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2010;153:396-399.
21. Choosing Wisely. American Academy of Pediatrics. Cough and cold medicines should not be prescribed, recommended or used for respiratory illnesses in young children. www.choosingwisely.org/clinician-lists/american-academy-pediatrics-cough-and-cold-medicines-for-children-under-four/. Accessed September 28, 2020.
22. Schaefer MK, Shehab N, Cohen AL, et al. Adverse events from cough and cold medications in children. Pediatrics. 2008;121:783-787.
23. Carr BC. Efficacy, abuse, and toxicity of over-the-counter cough and cold medicines in the pediatric population. Curr Opin Pediatr. 2006;18:184-188.
24. Oduwole O, Udoh EE, Oyo‐Ita A, et al. Honey for acute cough in children. Cochrane Database Syst Rev. 2018(4):CD007094.
25. Choosing Wisely. American Academy of Allergy, Asthma & Immunology. Don’t perform unproven diagnostic tests, such as immunoglobulin G(lgG) testing or an indiscriminate battery of immunoglobulin E(lgE) tests, in the evaluation of allergy. www.choosingwisely.org/clinician-lists/american-academy-allergy-asthma-immunology-diagnostic-tests-for-allergy-evaluation/. Accessed September 28, 2020.
26. Cox L, Williams B, Sicherer S, et al. Pearls and pitfalls of allergy diagnostic testing: report from the American College of Allergy, Asthma and Immunology Specific IgE Test Task Force. Ann Allergy Asthma Immunol. 2008;101:580-592.
27. Choosing Wisely. American Epilepsy Society. Do not routinely order electroencephalogram (EEG) as part of initial syncope work-up. www.choosingwisely.org/clinician-lists/aes-eeg-as-part-of-initial-syncope-work-up/. Accessed September 28, 2020.
28. Choosing Wisely. American College of Emergency Physicians. Avoid CT of the head in asymptomatic adult patients in the emergency department with syncope, insignificant trauma and a normal neurological evaluation. www.choosingwisely.org/clinician-lists/acep-avoid-head-ct-for-asymptomatic-adults-with-syncope/. Accessed September 28, 2020.
29. Choosing Wisely. American College of Physicians. In the evaluation of simple syncope and a normal neurological examination, don’t obtain brain imaging studies (CT or MRI). www.choosingwisely.org/clinician-lists/american-college-physicians-brain-imaging-to-evaluate-simple-syncope/. Accessed September 28, 2020.
30. Choosing Wisely. American Academy of Neurology. Don’t perform imaging of the carotid arteries for simple syncope without other neurologic symptoms. www.choosingwisely.org/clinician-lists/american-academy-neurology-carotid-artery-imaging-for-simple-syncope/. Accessed September 28, 2020.
31. Josephson CB, Rahey S, Sadler RM. Neurocardiogenic syncope: frequency and consequences of its misdiagnosis as epilepsy. Can J Neurol Sci. 2007;34:221-224.
32. Mendu ML, McAvay G, Lampert R, et al. Yield of diagnostic tests in evaluating syncopal episodes in older patients. Arch Intern Med. 2009;169:1299-1305.
33. Choosing Wisely. American Academy of Pediatrics–Section on Surgery. Avoid referring most children with umbilical hernias to a pediatric surgeon until around age 4-5 years. www.choosingwisely.org/clinician-lists/aap-sosu-avoid-surgery-referral-for-umbilical-hernias-until-age-4-5/. Accessed September 28, 2020.
34. Antonoff MB, Kreykes NS, Saltzman DA, et al. American Academy of Pediatrics Section on Surgery hernia survey revisited. J Pediatr Surg. 2005;40:1009-1014.
35. Choosing Wisely. American Geriatrics Society. Avoid using prescription appetite stimulants or high-calorie supplements for treatment of anorexia or cachexia in older adults; instead, optimize social supports, discontinue medications that may interfere with eating, provide appealing food and feeding assistance, and clarify patient goals and expectations. www.choosingwisely.org/clinician-lists/american-geriatrics-society-prescription-appetite-stimulants-to-treat-anorexia-cachexia-in-elderly/. Accessed September 28, 2020.
36. Milne AC, Potter J, Vivanti A, et al. Protein and energy supplementation in elderly people at risk from malnutrition. Cochrane Database Sys Rev. 2009(2):CD003288.
37. Fox CB, Treadway AK, Blaszczyk AT, et al. Megestrol acetate and mirtazapine for the treatment of unplanned weight loss in the elderly. Pharmacotherapy. 2009;29:383-397.
1. Miyakis S, Karamanof G, Liontos M, et al. Factors contributing to inappropriate ordering of tests in an academic medical department and the effect of an educational feedback strategy. Postgrad Med J. 2006;82:823-829.
2. Morgan DJ, Dhruva SS, Wright SM, et al. Update on medical overuse: a systematic review. JAMA Intern Med. 2016;176:1687-1692.
3. Durante C, Costante G, Lucisano G, et al. The natural history of benign thyroid nodules. JAMA. 2015;313:926-935.
4. Choosing Wisely. Society of Hospital Medicine—Pediatric hospital medicine. Don’t use continuous pulse oximetry routinely in children with acute respiratory illness unless they are on supplemental oxygen. www.choosingwisely.org/clinician-lists/society-hospital-medicine-pediatric-continuous-pulse-oximetry-in-children-with-acute-respiratory-illness/. Accessed September 28, 2020.
5. Schondelmeyer AC, Simmons JM, Statile AM, et al. Using quality improvement to reduce continuous pulse oximetry use in children with wheezing. Pediatrics. 2015;135:e1044-e1051.
6. Kravitz RL, Bell RA, Azari R, et al. Direct observation of requests for clinical services in office practice: what do patients want and do they get it? Arch Intern Med. 2003;163:1673-1681.
7. Kravitz RL, Bell RA, Franz CE, et al. Characterizing patient requests and physician responses in office practice. Health Serv Res. 2002;37:217-238.
8. Choosing Wisely. American Academy of Family Physicians. Don’t do imaging for low back pain within the first six weeks, unless red flags are present. www.choosingwisely.org/clinician-lists/american-academy-family-physicians-imaging-low-back-pain/. Accessed September 28, 2020.
9. Choosing Wisely. American Society of Anesthesiologists–Pain Medicine. Avoid imaging studies (MRI, CT or X-rays) for acute low back pain without specific indications. www.choosingwisely.org/clinician-lists/american-society-anesthesiologists-imaging-studies-for-acute-low-back-pain/. Accessed September 28, 2020.
10. Choosing Wisely. American College of Emergency Physicians. Avoid lumbar spine imaging in the emergency department for adults with non-traumatic back pain unless the patient has severe or progressive neurologic deficits or is suspected of having a serious underlying condition (such as vertebral infection, cauda equina syndrome, or cancer with bony metastasis). www.choosingwisely.org/clinician-lists/acep-lumbar-spine-imaging-in-the-ed/. Accessed September 28, 2020.
11. Choosing Wisely. American Academy of Family Physicians. Don’t require a pelvic exam or other physical exam to prescribe oral contraceptive medications. www.choosingwisely.org/clinician-lists/american-academy-family-physicians-pelvic-or-physical-exams-to-prescribe-oral-contraceptives/. Accessed September 28, 2020.
12. Over-the-counter access to hormonal contraception. ACOG Committee Opinion, Number 788. Obstet Gynecol. 2019;134:e96-e105. https://journals.lww.com/greenjournal/Fulltext/2019/10000/Over_the_Counter_Access_to_Hormonal_Contraception_.46.aspx. Accessed September 28, 2020.
13. Choosing Wisely. American Academy of Family Physicians. Don’t routinely recommend daily home glucose monitoring for patients who have Type 2 diabetes mellitus and are not using insulin. www.choosingwisely.org/clinician-lists/aafp-daily-home-glucose-monitoring-for-patients-with-type-2-diabetes. Accessed September 28, 2020.
14. Choosing Wisely. Society of General Internal Medicine. Don’t recommend daily home finger glucose testing in patients with Type 2 diabetes mellitus not using insulin. www.choosingwisely.org/clinician-lists/society-general-internal-medicine-daily-home-finger-glucose-testing-type-2-diabetes-mellitus/. Accessed September 28, 2020.
15. Malanda UL, Welschen LM, Riphagen II, et al. Self‐monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin. Cochrane Database Syst Rev. 2012(1):CD005060.
16. Peel E, Douglas M, Lawton J. Self monitoring of blood glucose in type 2 diabetes: longitudinal qualitative study of patients’ perspectives. BMJ. 2007;335:493.
17. Choosing Wisely. American Academy of Family Physicians. Don’t screen for genital herpes simplex virus infection (HSV) in asymptomatic adults, including pregnant women. www.choosingwisely.org/clinician-lists/aafp-genital-herpes-screening-in-asymptomatic-adults/. Accessed September 28, 2020.
18. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Serologic screening for genital herpes infection: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:2525-2530.
19. Choosing Wisely. American Academy of Family Physicians. Don’t screen for testicular cancer in asymptomatic adolescent and adult males. www.choosingwisely.org/clinician-lists/aafp-testicular-cancer-screening-in-asymptomatic-adolescent-and-adult-men/. Accessed September 28, 2020.
20. Lin K, Sharangpani R. Screening for testicular cancer: an evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2010;153:396-399.
21. Choosing Wisely. American Academy of Pediatrics. Cough and cold medicines should not be prescribed, recommended or used for respiratory illnesses in young children. www.choosingwisely.org/clinician-lists/american-academy-pediatrics-cough-and-cold-medicines-for-children-under-four/. Accessed September 28, 2020.
22. Schaefer MK, Shehab N, Cohen AL, et al. Adverse events from cough and cold medications in children. Pediatrics. 2008;121:783-787.
23. Carr BC. Efficacy, abuse, and toxicity of over-the-counter cough and cold medicines in the pediatric population. Curr Opin Pediatr. 2006;18:184-188.
24. Oduwole O, Udoh EE, Oyo‐Ita A, et al. Honey for acute cough in children. Cochrane Database Syst Rev. 2018(4):CD007094.
25. Choosing Wisely. American Academy of Allergy, Asthma & Immunology. Don’t perform unproven diagnostic tests, such as immunoglobulin G(lgG) testing or an indiscriminate battery of immunoglobulin E(lgE) tests, in the evaluation of allergy. www.choosingwisely.org/clinician-lists/american-academy-allergy-asthma-immunology-diagnostic-tests-for-allergy-evaluation/. Accessed September 28, 2020.
26. Cox L, Williams B, Sicherer S, et al. Pearls and pitfalls of allergy diagnostic testing: report from the American College of Allergy, Asthma and Immunology Specific IgE Test Task Force. Ann Allergy Asthma Immunol. 2008;101:580-592.
27. Choosing Wisely. American Epilepsy Society. Do not routinely order electroencephalogram (EEG) as part of initial syncope work-up. www.choosingwisely.org/clinician-lists/aes-eeg-as-part-of-initial-syncope-work-up/. Accessed September 28, 2020.
28. Choosing Wisely. American College of Emergency Physicians. Avoid CT of the head in asymptomatic adult patients in the emergency department with syncope, insignificant trauma and a normal neurological evaluation. www.choosingwisely.org/clinician-lists/acep-avoid-head-ct-for-asymptomatic-adults-with-syncope/. Accessed September 28, 2020.
29. Choosing Wisely. American College of Physicians. In the evaluation of simple syncope and a normal neurological examination, don’t obtain brain imaging studies (CT or MRI). www.choosingwisely.org/clinician-lists/american-college-physicians-brain-imaging-to-evaluate-simple-syncope/. Accessed September 28, 2020.
30. Choosing Wisely. American Academy of Neurology. Don’t perform imaging of the carotid arteries for simple syncope without other neurologic symptoms. www.choosingwisely.org/clinician-lists/american-academy-neurology-carotid-artery-imaging-for-simple-syncope/. Accessed September 28, 2020.
31. Josephson CB, Rahey S, Sadler RM. Neurocardiogenic syncope: frequency and consequences of its misdiagnosis as epilepsy. Can J Neurol Sci. 2007;34:221-224.
32. Mendu ML, McAvay G, Lampert R, et al. Yield of diagnostic tests in evaluating syncopal episodes in older patients. Arch Intern Med. 2009;169:1299-1305.
33. Choosing Wisely. American Academy of Pediatrics–Section on Surgery. Avoid referring most children with umbilical hernias to a pediatric surgeon until around age 4-5 years. www.choosingwisely.org/clinician-lists/aap-sosu-avoid-surgery-referral-for-umbilical-hernias-until-age-4-5/. Accessed September 28, 2020.
34. Antonoff MB, Kreykes NS, Saltzman DA, et al. American Academy of Pediatrics Section on Surgery hernia survey revisited. J Pediatr Surg. 2005;40:1009-1014.
35. Choosing Wisely. American Geriatrics Society. Avoid using prescription appetite stimulants or high-calorie supplements for treatment of anorexia or cachexia in older adults; instead, optimize social supports, discontinue medications that may interfere with eating, provide appealing food and feeding assistance, and clarify patient goals and expectations. www.choosingwisely.org/clinician-lists/american-geriatrics-society-prescription-appetite-stimulants-to-treat-anorexia-cachexia-in-elderly/. Accessed September 28, 2020.
36. Milne AC, Potter J, Vivanti A, et al. Protein and energy supplementation in elderly people at risk from malnutrition. Cochrane Database Sys Rev. 2009(2):CD003288.
37. Fox CB, Treadway AK, Blaszczyk AT, et al. Megestrol acetate and mirtazapine for the treatment of unplanned weight loss in the elderly. Pharmacotherapy. 2009;29:383-397.
Evaluation of Glycemic Control and Cost Savings Associated With Liraglutide Dose Reduction at a Veterans Affairs Hospital
Glucagon-like peptide 1 receptor agonists (GLP-1 RAs) are injectable incretin hormones approved for the treatment of type 2 diabetes mellitus (T2DM). They are highly efficacious agents with hemoglobin A1c (HbA1c) reduction potential of approximately 0.8 to 1.6% and mechanisms of action that result in an average weight loss of 1 to 3 kg.1,2 Published in 2016, The LEADER (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results) trial established cardiovascular benefits associated with liraglutide, making it a preferred GLP-1 RA.3
In addition to HbA1c reduction, weight loss, and cardiovascular benefits, liraglutide also has shown insulin-sparing effects when used in combination with insulin. A trial by Lane and colleagues revealed a 34% decrease in total daily insulin dose 6 months after the addition of liraglutide to insulin in patients with T2DM receiving > 100 units of insulin daily.4 When used in combination with basal insulin analogues (glargine or detemir) similar findings also were shown.5
The Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) in Houston, Texas, selected liraglutide as its preferred GLP-1 RA because of its favorable glycemic and cardiovascular outcomes. In addition, as part of a cost-savings initiative for fiscal year 2018, liraglutide 6 mg/mL injection 2-count pen packs was selected as the preferred liraglutide product. Before the availability of the 2-count pen packs, veterans previously received 3-count pen packs, which allowed for up to a 30-day supply of liraglutide 1.8 mg daily dosing. However, the cost-efficient 2-count pen packs allow for up to 1.2 mg daily dose of liraglutide for a 30-day supply. Due to these changes, veterans at MEDVAMC were converted from liraglutide 1.8 mg daily to 1.2 mg daily between May 2018 and August 2018.
The primary objective of this study was to assess sustained glycemic control and cost savings that resulted from this change. The secondary objectives were to assess sustained weight loss and adverse effects (AEs).
Methods
This study was approved by the MEDVAMC Quality Assurance and Regulatory Affairs committee. In this single-center study, a retrospective chart review was conducted on veterans with T2DM who underwent a liraglutide dose reduction from 1.8 mg daily to 1.2 mg daily between May 2018 and August 2018. Patients were included if they were aged ≥ 18 years with an active prescription for liraglutide 1.8 mg daily and insulin (with or without other antihyperglycemic agents) at the time of conversion. In addition, patients must have had ≥ 1 HbA1c reading within 3 months of the dose conversion and a follow-up HbA1c within 6 months after the dose conversion. To assess the primary objective of glycemic control that resulted from the liraglutide dose reduction, mean change of HbA1c at time of dose conversion was compared with mean HbA1c 6 months postconversion. To assess savings, cost information was obtained from the US Department of Veterans Affairs (VA) Drug Price Database and monthly and annual costs of liraglutide 6 mg/mL injection 2-count pen pack were compared with that of the 3-count pen pack. A chart review of patients’ electronic health records assessed secondary outcomes. The VA Computerized Patient Record System (CPRS) was used to collect patient data.
Patients and Characteristics
The following patient information was obtained from patients’ records: age, sex, race/ethnicity, diabetic medications (at time of conversion and 6 months after conversion), cardiovascular history and risk factors (hypertension, coronary artery disease, heart failure, arrhythmias, peripheral artery disease, obesity, etc), prescriber type (physician, nurse practitioner/physician assistant, pharmacist, etc), weight (at baseline, at time of conversion, and 6 months after conversion), HbA1c (at baseline, at time of conversion, and 6 months after conversion), average blood glucose (at baseline, at time of conversion, and 6 months after conversion), insulin dose (at time of conversion and 6 months after conversion), and reported AEs.
Statistical Analysis
The 2-tailed, paired t test was used to assess changes in HbA1c, average blood glucose, and body weight. Demographic data and other outcomes were assessed using descriptive statistics.
Results
Prior to the dose reduction, 312 veterans had active prescriptions for liraglutide 1.8 mg daily. Due to lack of glycemic control benefit (failing to achieve a HbA1c reduction of at least 0.5% after at least 3 to 6 months following initiation of therapy) or nonadherence (assessed by medication refill history), 126 veterans did not meet the criteria for the dose conversion. As a result, liraglutide was discontinued, and veterans were sent patient letter notifications and health care providers were notified via medication review notes in the patient electronic health record “to make medication adjustments if warranted. A total of 186 veterans underwent a liraglutide dose reduction between May and August 2018. Thirty-two veterans were without active insulin prescriptions, 53 were without HbA1c results, and 4 veterans died; resulting in 97 veterans who were included in the study (Figure 1).
Most of the patients included in the study were male (90.7%) and White (63.9%) with an average (SD) age of 65.9 years (7.9) and a mean (SD) HbA1c at baseline of 8.4% (1.2). About 56.7% received concurrent T2DM treatment with metformin, and 8.3% received concurrent treatment with empagliflozin. The most common cardiovascular disease/risk factors included hypertension (93.8%), hyperlipidemia (85.6%), and obesity (85.6%) (Table 1).
Glycemic Control and Weight Loss
At the time of conversion, the average (SD) HbA1c was 8.2% (1.4) and increased to an average (SD) of 8.7% (1.8) (P =.0005) 6 months after the dose reduction (Table 2). The average (SD) body weight was 116.2 kg (23.2) at time of conversion and increased to 116.5 (24.6) 6 months following the dose reduction; however, the difference was not statistically significant (P = .8).
As a result of the HbA1c change, 41.2% of veterans underwent an insulin dose increase with dose increase of 5 to 200 units of total daily insulin during the 6-month period. Antihyperglycemic regimen remained unchanged for 40.2% of veterans, while additional glucose lowering agents were initiated in 6 veterans. Medications initiated included empagliflozin in 4 veterans and saxagliptin in 2 veterans.
HbA1c reduction was noted in 33% of veterans (Figure 2) mostly due to improved diet and exercise habits. A majority of veterans, 62%, experienced an increase in HbA1c, whereas 5.2% of veterans maintained the same HbA1c. Of 60 veterans with HbA1c increases, 15 had an increase between 0.1% and 0.5%, another 15 with an increase between 0.5 to 0.9%, and half had HbA1c increases of at least 1% with a maximum increase of 5.1% (Figure 3).
Cost Savings
Cost information was obtained from the VA Drug Price Database. The estimated monthly cost savings per patient associated with the conversion from 3-count to 2-count injection pen packs of liraglutide 6 mg/mL was $103.46. With 186 veterans converted to the 2-count pen packs, MEDVAMC saved $115,461.36 in a 6-month period. The estimated annualized cost savings was estimated to be about $231,000 (Figure 4).
Adverse Effects During the 6-month period following the dose conversion, no major AEs associated with liraglutide were documented. Documented AEs included 3 cases of diarrhea, resulting in the discontinuation of metformin. Metformin also was discontinued in a veteran with worsened renal function and eGFR < 30 mL/min/1.73 m2.
Discussion
According to previous clinical trials, when used in combination with insulin, 1.2 mg and 1.8 mg daily liraglutide showed significant improvement in glycemic control and body weight and was associated with decreased insulin requirements.4-6 However, subgroup analyses were not performed to show differences in benefit between the liraglutide 1.8 mg and 1.2 mg groups.4-6 Similarly, cardiovascular benefit was observed in patients receiving liraglutide 1.2 mg daily and liraglutide 1.8 mg daily in the LEADER trial with no subgroup analysis or distinction between treatment doses.3 With this information and approval by the Veterans Integrated Services Network, the pharmacoeconomics team at MEDVAMC made the decision to select a more cost-efficient preparation and, hence, lower dose of liraglutide.
To ensure that patients only taking liraglutide for glycemic control were captured, patients without insulin therapies at baseline were excluded. Due to concerns of potential off-label use of liraglutide for weight loss, patients without active prescriptions for insulin at baseline were excluded.
A mean HbA1c increase of 0.5% was observed over the 6-month period, supporting findings of a dose-dependent HbA1c decrease observed in clinical trials. In the LEAD-3 MONO trial when used as monotherapy, liraglutide 1.8 mg was associated with significantly greater HbA1c reduction than liraglutide 1.2 mg (–0·29%; –0·50 to –0.09, P = .005) after 52 weeks of treatment.7 Liraglutide 1.8 mg was also associated with higher rates of AEs; particularly gastrointestinal. 7 To minimize these AEs, it is recommended to initiate liraglutide at 0.6 mg daily for a week then increase to 1.2 mg daily. If tolerated, liraglutide can be further titrated to 1.8 mg daily to optimize glycemic control.8 Unsurprisingly, no major AEs were noted in this study, as AEs are typically noted with increased doses.
Despite the observed trend of increased HbA1c, no changes were made to glucoselowering agents in 39 veterans. This group of veterans consisted primarily of those whose HbA1c remained unchanged during the 6-month period, those whose HbA1c improved (with no documented hypoglycemia), and older veterans with less stringent HbA1c goals. As a result, doses of glucose lowering agents were maintained as appropriate.
No significant difference was noted in body weight during the 6-month period. The slight weight gain observed may have been due to several factors. Lack of exercise and dietary changes may have contributed to weight gain. In addition, insulin doses were increased in 40 veterans, which may have contributed to the observed weight gain.
As expected, significant cost savings were achieved as a result of the liraglutide dose reduction. Of note, liraglutide was discontinued in 126 veterans (prior to the dose reduction) due to nonadherence or inadequate response to therapy, which also resulted in additional savings. Although cost savings was achieved, the long-term benefit of this initiative still remains unknown. The worsened glycemic control that was detected may increase the risk of microvascular and macrovascular complications, thereby negating cost savings achieved. To assess this effect, longterm prospective studies are warranted.
Limitations
A number of issues limit these finding, including its retrospective data review, small sample size, additional factors contributing to HbA1c increase, and missing documentation in some patient records. Only 97 patients were included in the study, reflecting less than half of the charts reviewed (52% exclusion rate). In addition, several confounding factors may have contributed to the increased HbA1c observed. Medication changes and lifestyle factors may have contributed to the observed change in HbA1c levels. Exclusion of patients without active prescriptions for insulin may have contributed to a selection bias, as most patients included in the study were veterans with uncontrolled T2DM requiring insulin. Finally, as a retrospective study involving patient records, investigators relied heavily on information provided in patients’ charts (HbA1c, body weight, insulin doses, adverse effects, etc), which may not entirely be accurate and may have been missing other pertinent information.
Conclusions
The daily dose reduction of liraglutide from 1.8 mg to 1.2 mg due to a cost-savings initiative resulted in a HbA1c increase of 0.5% in a 6-month period. Due to HbA1c increases, 41.2% of veterans underwent an insulin dose increase, negating the insulin-sparing role of liraglutide. Although this study further confirms the dose-dependent HbA1c reduction with liraglutide that has been noted in previous trials, long-term prospective studies and cost-effectiveness analyses are warranted to assess the overall clinical significance and other benefits of the change, including its effects on cardiovascular outcomes.
1. American Diabetes Association. Pharmacologic approaches to glycemic treatment. Diabetes Care. 2019;42(suppl 1):S90-S102. doi:10.2337/dc19-S009
2. Hinnen D. Glucagon-like peptide 1 receptor agonists for type 2 diabetes. Diabetes Spectr. 2017;30(3):202-210. doi:10.2337/ds16-0026
3. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311-322. doi:10.1056/NEJMoa1603827
4. Lane W, Weinrib S, Rappaport J, Hale C. The effect of addition of liraglutide to high-dose intensive insulin therapy: a randomized prospective trial. Diabetes Obes Metab. 2014;16(9):827-832. doi:10.1111/dom.12286
5. Ahmann A, Rodbard HW, Rosenstock J, et al. Efficacy and safety of liraglutide versus placebo added to basal insulin analogues (with or without metformin) in patients with type 2 diabetes: a randomized, placebo-controlled trial. Diabetes Obes Metab. 2015;17(11):1056-1064. doi:10.1111/dom.12539
6. Lane W, Weinrib S, Rappaport J. The effect of liraglutide added to U-500 insulin in patients with type 2 diabetes and high insulin requirements. Diabetes Technol Ther. 2011;13(5):592-595. doi:10.1089/dia.2010.0221
7. Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373(9662):473-481. doi:10.1016/S0140-6736(08)61246-5.
8. Victoza [package insert]. Princeton: Novo Nordisk Inc; 2020.
Glucagon-like peptide 1 receptor agonists (GLP-1 RAs) are injectable incretin hormones approved for the treatment of type 2 diabetes mellitus (T2DM). They are highly efficacious agents with hemoglobin A1c (HbA1c) reduction potential of approximately 0.8 to 1.6% and mechanisms of action that result in an average weight loss of 1 to 3 kg.1,2 Published in 2016, The LEADER (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results) trial established cardiovascular benefits associated with liraglutide, making it a preferred GLP-1 RA.3
In addition to HbA1c reduction, weight loss, and cardiovascular benefits, liraglutide also has shown insulin-sparing effects when used in combination with insulin. A trial by Lane and colleagues revealed a 34% decrease in total daily insulin dose 6 months after the addition of liraglutide to insulin in patients with T2DM receiving > 100 units of insulin daily.4 When used in combination with basal insulin analogues (glargine or detemir) similar findings also were shown.5
The Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) in Houston, Texas, selected liraglutide as its preferred GLP-1 RA because of its favorable glycemic and cardiovascular outcomes. In addition, as part of a cost-savings initiative for fiscal year 2018, liraglutide 6 mg/mL injection 2-count pen packs was selected as the preferred liraglutide product. Before the availability of the 2-count pen packs, veterans previously received 3-count pen packs, which allowed for up to a 30-day supply of liraglutide 1.8 mg daily dosing. However, the cost-efficient 2-count pen packs allow for up to 1.2 mg daily dose of liraglutide for a 30-day supply. Due to these changes, veterans at MEDVAMC were converted from liraglutide 1.8 mg daily to 1.2 mg daily between May 2018 and August 2018.
The primary objective of this study was to assess sustained glycemic control and cost savings that resulted from this change. The secondary objectives were to assess sustained weight loss and adverse effects (AEs).
Methods
This study was approved by the MEDVAMC Quality Assurance and Regulatory Affairs committee. In this single-center study, a retrospective chart review was conducted on veterans with T2DM who underwent a liraglutide dose reduction from 1.8 mg daily to 1.2 mg daily between May 2018 and August 2018. Patients were included if they were aged ≥ 18 years with an active prescription for liraglutide 1.8 mg daily and insulin (with or without other antihyperglycemic agents) at the time of conversion. In addition, patients must have had ≥ 1 HbA1c reading within 3 months of the dose conversion and a follow-up HbA1c within 6 months after the dose conversion. To assess the primary objective of glycemic control that resulted from the liraglutide dose reduction, mean change of HbA1c at time of dose conversion was compared with mean HbA1c 6 months postconversion. To assess savings, cost information was obtained from the US Department of Veterans Affairs (VA) Drug Price Database and monthly and annual costs of liraglutide 6 mg/mL injection 2-count pen pack were compared with that of the 3-count pen pack. A chart review of patients’ electronic health records assessed secondary outcomes. The VA Computerized Patient Record System (CPRS) was used to collect patient data.
Patients and Characteristics
The following patient information was obtained from patients’ records: age, sex, race/ethnicity, diabetic medications (at time of conversion and 6 months after conversion), cardiovascular history and risk factors (hypertension, coronary artery disease, heart failure, arrhythmias, peripheral artery disease, obesity, etc), prescriber type (physician, nurse practitioner/physician assistant, pharmacist, etc), weight (at baseline, at time of conversion, and 6 months after conversion), HbA1c (at baseline, at time of conversion, and 6 months after conversion), average blood glucose (at baseline, at time of conversion, and 6 months after conversion), insulin dose (at time of conversion and 6 months after conversion), and reported AEs.
Statistical Analysis
The 2-tailed, paired t test was used to assess changes in HbA1c, average blood glucose, and body weight. Demographic data and other outcomes were assessed using descriptive statistics.
Results
Prior to the dose reduction, 312 veterans had active prescriptions for liraglutide 1.8 mg daily. Due to lack of glycemic control benefit (failing to achieve a HbA1c reduction of at least 0.5% after at least 3 to 6 months following initiation of therapy) or nonadherence (assessed by medication refill history), 126 veterans did not meet the criteria for the dose conversion. As a result, liraglutide was discontinued, and veterans were sent patient letter notifications and health care providers were notified via medication review notes in the patient electronic health record “to make medication adjustments if warranted. A total of 186 veterans underwent a liraglutide dose reduction between May and August 2018. Thirty-two veterans were without active insulin prescriptions, 53 were without HbA1c results, and 4 veterans died; resulting in 97 veterans who were included in the study (Figure 1).
Most of the patients included in the study were male (90.7%) and White (63.9%) with an average (SD) age of 65.9 years (7.9) and a mean (SD) HbA1c at baseline of 8.4% (1.2). About 56.7% received concurrent T2DM treatment with metformin, and 8.3% received concurrent treatment with empagliflozin. The most common cardiovascular disease/risk factors included hypertension (93.8%), hyperlipidemia (85.6%), and obesity (85.6%) (Table 1).
Glycemic Control and Weight Loss
At the time of conversion, the average (SD) HbA1c was 8.2% (1.4) and increased to an average (SD) of 8.7% (1.8) (P =.0005) 6 months after the dose reduction (Table 2). The average (SD) body weight was 116.2 kg (23.2) at time of conversion and increased to 116.5 (24.6) 6 months following the dose reduction; however, the difference was not statistically significant (P = .8).
As a result of the HbA1c change, 41.2% of veterans underwent an insulin dose increase with dose increase of 5 to 200 units of total daily insulin during the 6-month period. Antihyperglycemic regimen remained unchanged for 40.2% of veterans, while additional glucose lowering agents were initiated in 6 veterans. Medications initiated included empagliflozin in 4 veterans and saxagliptin in 2 veterans.
HbA1c reduction was noted in 33% of veterans (Figure 2) mostly due to improved diet and exercise habits. A majority of veterans, 62%, experienced an increase in HbA1c, whereas 5.2% of veterans maintained the same HbA1c. Of 60 veterans with HbA1c increases, 15 had an increase between 0.1% and 0.5%, another 15 with an increase between 0.5 to 0.9%, and half had HbA1c increases of at least 1% with a maximum increase of 5.1% (Figure 3).
Cost Savings
Cost information was obtained from the VA Drug Price Database. The estimated monthly cost savings per patient associated with the conversion from 3-count to 2-count injection pen packs of liraglutide 6 mg/mL was $103.46. With 186 veterans converted to the 2-count pen packs, MEDVAMC saved $115,461.36 in a 6-month period. The estimated annualized cost savings was estimated to be about $231,000 (Figure 4).
Adverse Effects During the 6-month period following the dose conversion, no major AEs associated with liraglutide were documented. Documented AEs included 3 cases of diarrhea, resulting in the discontinuation of metformin. Metformin also was discontinued in a veteran with worsened renal function and eGFR < 30 mL/min/1.73 m2.
Discussion
According to previous clinical trials, when used in combination with insulin, 1.2 mg and 1.8 mg daily liraglutide showed significant improvement in glycemic control and body weight and was associated with decreased insulin requirements.4-6 However, subgroup analyses were not performed to show differences in benefit between the liraglutide 1.8 mg and 1.2 mg groups.4-6 Similarly, cardiovascular benefit was observed in patients receiving liraglutide 1.2 mg daily and liraglutide 1.8 mg daily in the LEADER trial with no subgroup analysis or distinction between treatment doses.3 With this information and approval by the Veterans Integrated Services Network, the pharmacoeconomics team at MEDVAMC made the decision to select a more cost-efficient preparation and, hence, lower dose of liraglutide.
To ensure that patients only taking liraglutide for glycemic control were captured, patients without insulin therapies at baseline were excluded. Due to concerns of potential off-label use of liraglutide for weight loss, patients without active prescriptions for insulin at baseline were excluded.
A mean HbA1c increase of 0.5% was observed over the 6-month period, supporting findings of a dose-dependent HbA1c decrease observed in clinical trials. In the LEAD-3 MONO trial when used as monotherapy, liraglutide 1.8 mg was associated with significantly greater HbA1c reduction than liraglutide 1.2 mg (–0·29%; –0·50 to –0.09, P = .005) after 52 weeks of treatment.7 Liraglutide 1.8 mg was also associated with higher rates of AEs; particularly gastrointestinal. 7 To minimize these AEs, it is recommended to initiate liraglutide at 0.6 mg daily for a week then increase to 1.2 mg daily. If tolerated, liraglutide can be further titrated to 1.8 mg daily to optimize glycemic control.8 Unsurprisingly, no major AEs were noted in this study, as AEs are typically noted with increased doses.
Despite the observed trend of increased HbA1c, no changes were made to glucoselowering agents in 39 veterans. This group of veterans consisted primarily of those whose HbA1c remained unchanged during the 6-month period, those whose HbA1c improved (with no documented hypoglycemia), and older veterans with less stringent HbA1c goals. As a result, doses of glucose lowering agents were maintained as appropriate.
No significant difference was noted in body weight during the 6-month period. The slight weight gain observed may have been due to several factors. Lack of exercise and dietary changes may have contributed to weight gain. In addition, insulin doses were increased in 40 veterans, which may have contributed to the observed weight gain.
As expected, significant cost savings were achieved as a result of the liraglutide dose reduction. Of note, liraglutide was discontinued in 126 veterans (prior to the dose reduction) due to nonadherence or inadequate response to therapy, which also resulted in additional savings. Although cost savings was achieved, the long-term benefit of this initiative still remains unknown. The worsened glycemic control that was detected may increase the risk of microvascular and macrovascular complications, thereby negating cost savings achieved. To assess this effect, longterm prospective studies are warranted.
Limitations
A number of issues limit these finding, including its retrospective data review, small sample size, additional factors contributing to HbA1c increase, and missing documentation in some patient records. Only 97 patients were included in the study, reflecting less than half of the charts reviewed (52% exclusion rate). In addition, several confounding factors may have contributed to the increased HbA1c observed. Medication changes and lifestyle factors may have contributed to the observed change in HbA1c levels. Exclusion of patients without active prescriptions for insulin may have contributed to a selection bias, as most patients included in the study were veterans with uncontrolled T2DM requiring insulin. Finally, as a retrospective study involving patient records, investigators relied heavily on information provided in patients’ charts (HbA1c, body weight, insulin doses, adverse effects, etc), which may not entirely be accurate and may have been missing other pertinent information.
Conclusions
The daily dose reduction of liraglutide from 1.8 mg to 1.2 mg due to a cost-savings initiative resulted in a HbA1c increase of 0.5% in a 6-month period. Due to HbA1c increases, 41.2% of veterans underwent an insulin dose increase, negating the insulin-sparing role of liraglutide. Although this study further confirms the dose-dependent HbA1c reduction with liraglutide that has been noted in previous trials, long-term prospective studies and cost-effectiveness analyses are warranted to assess the overall clinical significance and other benefits of the change, including its effects on cardiovascular outcomes.
Glucagon-like peptide 1 receptor agonists (GLP-1 RAs) are injectable incretin hormones approved for the treatment of type 2 diabetes mellitus (T2DM). They are highly efficacious agents with hemoglobin A1c (HbA1c) reduction potential of approximately 0.8 to 1.6% and mechanisms of action that result in an average weight loss of 1 to 3 kg.1,2 Published in 2016, The LEADER (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results) trial established cardiovascular benefits associated with liraglutide, making it a preferred GLP-1 RA.3
In addition to HbA1c reduction, weight loss, and cardiovascular benefits, liraglutide also has shown insulin-sparing effects when used in combination with insulin. A trial by Lane and colleagues revealed a 34% decrease in total daily insulin dose 6 months after the addition of liraglutide to insulin in patients with T2DM receiving > 100 units of insulin daily.4 When used in combination with basal insulin analogues (glargine or detemir) similar findings also were shown.5
The Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) in Houston, Texas, selected liraglutide as its preferred GLP-1 RA because of its favorable glycemic and cardiovascular outcomes. In addition, as part of a cost-savings initiative for fiscal year 2018, liraglutide 6 mg/mL injection 2-count pen packs was selected as the preferred liraglutide product. Before the availability of the 2-count pen packs, veterans previously received 3-count pen packs, which allowed for up to a 30-day supply of liraglutide 1.8 mg daily dosing. However, the cost-efficient 2-count pen packs allow for up to 1.2 mg daily dose of liraglutide for a 30-day supply. Due to these changes, veterans at MEDVAMC were converted from liraglutide 1.8 mg daily to 1.2 mg daily between May 2018 and August 2018.
The primary objective of this study was to assess sustained glycemic control and cost savings that resulted from this change. The secondary objectives were to assess sustained weight loss and adverse effects (AEs).
Methods
This study was approved by the MEDVAMC Quality Assurance and Regulatory Affairs committee. In this single-center study, a retrospective chart review was conducted on veterans with T2DM who underwent a liraglutide dose reduction from 1.8 mg daily to 1.2 mg daily between May 2018 and August 2018. Patients were included if they were aged ≥ 18 years with an active prescription for liraglutide 1.8 mg daily and insulin (with or without other antihyperglycemic agents) at the time of conversion. In addition, patients must have had ≥ 1 HbA1c reading within 3 months of the dose conversion and a follow-up HbA1c within 6 months after the dose conversion. To assess the primary objective of glycemic control that resulted from the liraglutide dose reduction, mean change of HbA1c at time of dose conversion was compared with mean HbA1c 6 months postconversion. To assess savings, cost information was obtained from the US Department of Veterans Affairs (VA) Drug Price Database and monthly and annual costs of liraglutide 6 mg/mL injection 2-count pen pack were compared with that of the 3-count pen pack. A chart review of patients’ electronic health records assessed secondary outcomes. The VA Computerized Patient Record System (CPRS) was used to collect patient data.
Patients and Characteristics
The following patient information was obtained from patients’ records: age, sex, race/ethnicity, diabetic medications (at time of conversion and 6 months after conversion), cardiovascular history and risk factors (hypertension, coronary artery disease, heart failure, arrhythmias, peripheral artery disease, obesity, etc), prescriber type (physician, nurse practitioner/physician assistant, pharmacist, etc), weight (at baseline, at time of conversion, and 6 months after conversion), HbA1c (at baseline, at time of conversion, and 6 months after conversion), average blood glucose (at baseline, at time of conversion, and 6 months after conversion), insulin dose (at time of conversion and 6 months after conversion), and reported AEs.
Statistical Analysis
The 2-tailed, paired t test was used to assess changes in HbA1c, average blood glucose, and body weight. Demographic data and other outcomes were assessed using descriptive statistics.
Results
Prior to the dose reduction, 312 veterans had active prescriptions for liraglutide 1.8 mg daily. Due to lack of glycemic control benefit (failing to achieve a HbA1c reduction of at least 0.5% after at least 3 to 6 months following initiation of therapy) or nonadherence (assessed by medication refill history), 126 veterans did not meet the criteria for the dose conversion. As a result, liraglutide was discontinued, and veterans were sent patient letter notifications and health care providers were notified via medication review notes in the patient electronic health record “to make medication adjustments if warranted. A total of 186 veterans underwent a liraglutide dose reduction between May and August 2018. Thirty-two veterans were without active insulin prescriptions, 53 were without HbA1c results, and 4 veterans died; resulting in 97 veterans who were included in the study (Figure 1).
Most of the patients included in the study were male (90.7%) and White (63.9%) with an average (SD) age of 65.9 years (7.9) and a mean (SD) HbA1c at baseline of 8.4% (1.2). About 56.7% received concurrent T2DM treatment with metformin, and 8.3% received concurrent treatment with empagliflozin. The most common cardiovascular disease/risk factors included hypertension (93.8%), hyperlipidemia (85.6%), and obesity (85.6%) (Table 1).
Glycemic Control and Weight Loss
At the time of conversion, the average (SD) HbA1c was 8.2% (1.4) and increased to an average (SD) of 8.7% (1.8) (P =.0005) 6 months after the dose reduction (Table 2). The average (SD) body weight was 116.2 kg (23.2) at time of conversion and increased to 116.5 (24.6) 6 months following the dose reduction; however, the difference was not statistically significant (P = .8).
As a result of the HbA1c change, 41.2% of veterans underwent an insulin dose increase with dose increase of 5 to 200 units of total daily insulin during the 6-month period. Antihyperglycemic regimen remained unchanged for 40.2% of veterans, while additional glucose lowering agents were initiated in 6 veterans. Medications initiated included empagliflozin in 4 veterans and saxagliptin in 2 veterans.
HbA1c reduction was noted in 33% of veterans (Figure 2) mostly due to improved diet and exercise habits. A majority of veterans, 62%, experienced an increase in HbA1c, whereas 5.2% of veterans maintained the same HbA1c. Of 60 veterans with HbA1c increases, 15 had an increase between 0.1% and 0.5%, another 15 with an increase between 0.5 to 0.9%, and half had HbA1c increases of at least 1% with a maximum increase of 5.1% (Figure 3).
Cost Savings
Cost information was obtained from the VA Drug Price Database. The estimated monthly cost savings per patient associated with the conversion from 3-count to 2-count injection pen packs of liraglutide 6 mg/mL was $103.46. With 186 veterans converted to the 2-count pen packs, MEDVAMC saved $115,461.36 in a 6-month period. The estimated annualized cost savings was estimated to be about $231,000 (Figure 4).
Adverse Effects During the 6-month period following the dose conversion, no major AEs associated with liraglutide were documented. Documented AEs included 3 cases of diarrhea, resulting in the discontinuation of metformin. Metformin also was discontinued in a veteran with worsened renal function and eGFR < 30 mL/min/1.73 m2.
Discussion
According to previous clinical trials, when used in combination with insulin, 1.2 mg and 1.8 mg daily liraglutide showed significant improvement in glycemic control and body weight and was associated with decreased insulin requirements.4-6 However, subgroup analyses were not performed to show differences in benefit between the liraglutide 1.8 mg and 1.2 mg groups.4-6 Similarly, cardiovascular benefit was observed in patients receiving liraglutide 1.2 mg daily and liraglutide 1.8 mg daily in the LEADER trial with no subgroup analysis or distinction between treatment doses.3 With this information and approval by the Veterans Integrated Services Network, the pharmacoeconomics team at MEDVAMC made the decision to select a more cost-efficient preparation and, hence, lower dose of liraglutide.
To ensure that patients only taking liraglutide for glycemic control were captured, patients without insulin therapies at baseline were excluded. Due to concerns of potential off-label use of liraglutide for weight loss, patients without active prescriptions for insulin at baseline were excluded.
A mean HbA1c increase of 0.5% was observed over the 6-month period, supporting findings of a dose-dependent HbA1c decrease observed in clinical trials. In the LEAD-3 MONO trial when used as monotherapy, liraglutide 1.8 mg was associated with significantly greater HbA1c reduction than liraglutide 1.2 mg (–0·29%; –0·50 to –0.09, P = .005) after 52 weeks of treatment.7 Liraglutide 1.8 mg was also associated with higher rates of AEs; particularly gastrointestinal. 7 To minimize these AEs, it is recommended to initiate liraglutide at 0.6 mg daily for a week then increase to 1.2 mg daily. If tolerated, liraglutide can be further titrated to 1.8 mg daily to optimize glycemic control.8 Unsurprisingly, no major AEs were noted in this study, as AEs are typically noted with increased doses.
Despite the observed trend of increased HbA1c, no changes were made to glucoselowering agents in 39 veterans. This group of veterans consisted primarily of those whose HbA1c remained unchanged during the 6-month period, those whose HbA1c improved (with no documented hypoglycemia), and older veterans with less stringent HbA1c goals. As a result, doses of glucose lowering agents were maintained as appropriate.
No significant difference was noted in body weight during the 6-month period. The slight weight gain observed may have been due to several factors. Lack of exercise and dietary changes may have contributed to weight gain. In addition, insulin doses were increased in 40 veterans, which may have contributed to the observed weight gain.
As expected, significant cost savings were achieved as a result of the liraglutide dose reduction. Of note, liraglutide was discontinued in 126 veterans (prior to the dose reduction) due to nonadherence or inadequate response to therapy, which also resulted in additional savings. Although cost savings was achieved, the long-term benefit of this initiative still remains unknown. The worsened glycemic control that was detected may increase the risk of microvascular and macrovascular complications, thereby negating cost savings achieved. To assess this effect, longterm prospective studies are warranted.
Limitations
A number of issues limit these finding, including its retrospective data review, small sample size, additional factors contributing to HbA1c increase, and missing documentation in some patient records. Only 97 patients were included in the study, reflecting less than half of the charts reviewed (52% exclusion rate). In addition, several confounding factors may have contributed to the increased HbA1c observed. Medication changes and lifestyle factors may have contributed to the observed change in HbA1c levels. Exclusion of patients without active prescriptions for insulin may have contributed to a selection bias, as most patients included in the study were veterans with uncontrolled T2DM requiring insulin. Finally, as a retrospective study involving patient records, investigators relied heavily on information provided in patients’ charts (HbA1c, body weight, insulin doses, adverse effects, etc), which may not entirely be accurate and may have been missing other pertinent information.
Conclusions
The daily dose reduction of liraglutide from 1.8 mg to 1.2 mg due to a cost-savings initiative resulted in a HbA1c increase of 0.5% in a 6-month period. Due to HbA1c increases, 41.2% of veterans underwent an insulin dose increase, negating the insulin-sparing role of liraglutide. Although this study further confirms the dose-dependent HbA1c reduction with liraglutide that has been noted in previous trials, long-term prospective studies and cost-effectiveness analyses are warranted to assess the overall clinical significance and other benefits of the change, including its effects on cardiovascular outcomes.
1. American Diabetes Association. Pharmacologic approaches to glycemic treatment. Diabetes Care. 2019;42(suppl 1):S90-S102. doi:10.2337/dc19-S009
2. Hinnen D. Glucagon-like peptide 1 receptor agonists for type 2 diabetes. Diabetes Spectr. 2017;30(3):202-210. doi:10.2337/ds16-0026
3. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311-322. doi:10.1056/NEJMoa1603827
4. Lane W, Weinrib S, Rappaport J, Hale C. The effect of addition of liraglutide to high-dose intensive insulin therapy: a randomized prospective trial. Diabetes Obes Metab. 2014;16(9):827-832. doi:10.1111/dom.12286
5. Ahmann A, Rodbard HW, Rosenstock J, et al. Efficacy and safety of liraglutide versus placebo added to basal insulin analogues (with or without metformin) in patients with type 2 diabetes: a randomized, placebo-controlled trial. Diabetes Obes Metab. 2015;17(11):1056-1064. doi:10.1111/dom.12539
6. Lane W, Weinrib S, Rappaport J. The effect of liraglutide added to U-500 insulin in patients with type 2 diabetes and high insulin requirements. Diabetes Technol Ther. 2011;13(5):592-595. doi:10.1089/dia.2010.0221
7. Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373(9662):473-481. doi:10.1016/S0140-6736(08)61246-5.
8. Victoza [package insert]. Princeton: Novo Nordisk Inc; 2020.
1. American Diabetes Association. Pharmacologic approaches to glycemic treatment. Diabetes Care. 2019;42(suppl 1):S90-S102. doi:10.2337/dc19-S009
2. Hinnen D. Glucagon-like peptide 1 receptor agonists for type 2 diabetes. Diabetes Spectr. 2017;30(3):202-210. doi:10.2337/ds16-0026
3. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311-322. doi:10.1056/NEJMoa1603827
4. Lane W, Weinrib S, Rappaport J, Hale C. The effect of addition of liraglutide to high-dose intensive insulin therapy: a randomized prospective trial. Diabetes Obes Metab. 2014;16(9):827-832. doi:10.1111/dom.12286
5. Ahmann A, Rodbard HW, Rosenstock J, et al. Efficacy and safety of liraglutide versus placebo added to basal insulin analogues (with or without metformin) in patients with type 2 diabetes: a randomized, placebo-controlled trial. Diabetes Obes Metab. 2015;17(11):1056-1064. doi:10.1111/dom.12539
6. Lane W, Weinrib S, Rappaport J. The effect of liraglutide added to U-500 insulin in patients with type 2 diabetes and high insulin requirements. Diabetes Technol Ther. 2011;13(5):592-595. doi:10.1089/dia.2010.0221
7. Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373(9662):473-481. doi:10.1016/S0140-6736(08)61246-5.
8. Victoza [package insert]. Princeton: Novo Nordisk Inc; 2020.
EMPEROR-Reduced: Empagliflozin’s HFrEF benefit holds steady on top of sacubitril/valsartan
The latest drug shown to benefit patients with heart failure with reduced ejection fraction, the SGLT2 inhibitor empagliflozin, works just as well when added on top of a second major agent used to treat these patients, the renin-angiotensin system–inhibiting combination of sacubitril/valsartan, based on a post-hoc analysis of data from the EMPEROR-Reduced trial.
“When there are two very effective treatments, it’s common for people to ask: Which should I use?’ The goal of my presentation was to emphasize that the answer is both. We shouldn’t choose between neprilysin inhibition [sacubitril inhibits the enzyme neprilysin] and SGLT2 [sodium-glucose transporter 2] inhibition; we should use both,” said Milton Packer, MD at the virtual annual meeting of the Heart Failure Society of America.
EMPEROR-Reduced had the primary goal of testing the safety and efficacy of the SGLT2 inhibitor empagliflozin (Jardiance) in patients with heart failure with reduced ejection fraction (HFrEF). The results showed that adding this drug on top of standard treatments led to a 25% relative cut in the study’s primary efficacy endpoint, compared with placebo, and had this effect regardless of whether or not patients also had type 2 diabetes (N Engl J Med. 2020 Aug 29. doi: 10.1056/NEJMoa2022190).
Among the 3,730 patients enrolled in the trial, 727 (19%) were on sacubitril/valsartan (Entresto) at entry, which gave Dr. Packer the data to perform the analysis he reported. He presented the study’s three major endpoints as well as a quality of life analysis that compared the performance of empagliflozin in patients who were on sacubitril/valsartan at baseline with the other study patients, who were either on a different type of renin-angiotensin system (RAS) blocker (roughly 70% of study patients) or on no RAS inhibition (about 10% of patients).
The results showed no statistically significant indication of an interaction, suggesting that patients with sacubitril/valsartan on board had just as good response to empagliflozin as patients who were not on this combination. The landmark PARADIGM-HF trial proved several years ago that treatment of HFrEF patients with sacubitril/valsartan led to significantly better outcomes than did treatment with another form of RAS inhibition (N Engl J Med. 2014 Sep 11;371[11]:993-1004).
For example, EMPEROR-Reduced’s primary endpoint, the combined rate of cardiovascular death or hospitalization for heart failure, fell by 36% relative to placebo in patients who received empagliflozin on top of sacubitril/valsartan, and by 23% relative to placebo among the remaining patients who received empagliflozin on top of a different type of RAS inhibitor drug or no RAS inhibition.
“Background treatment with sacubitril/valsartan did not diminish, and may have enhanced the efficacy of empagliflozin,” concluded Dr. Packer. Further analyses also showed that concurrent sacubitril/valsartan had no statistically significant impact on empagliflozin’s ability to reduce the rate of total heart failure hospitalizations, or to slow progressive loss of renal function, compared with placebo. The fourth efficacy analysis Dr. Packer presented showed that empagliflozin was also as effective for improving a quality-of-life measure in patients compared with placebo regardless of the type of RAS inhibition used. For all four outcomes, the point-estimate of empagliflozin’s benefit was higher when used along with sacubitril/valsartan.
Brian L. Claggett, PhD, a biostatistician at Brigham and Women’s Hospital and Harvard Medical School in Boston, designated discussant for the report, disagreed with Dr. Packer’s suggestion that the efficacy of empagliflozin may have been greater when administered against a background of sacubitril/valsartan. From a statistical perspective, there is no basis to suggest that patients did better when they were on both drugs, he cautioned. But Dr. Claggett acknowledged that the new analyses suggested that empagliflozin’s benefit wasn’t compromised by concurrent sacubitril/valsartan use. He also highlighted the value of more fully documenting the safety and efficacy of a new drug when used as part of “comprehensive therapy” with the established drugs that a patient may concurrently receive.
Dr. Packer also presented several measures of treatment safety that all showed similar rates of adverse effects between the empagliflozin and placebo recipients regardless of background RAS inhibition. A notable finding was that the incidence of hypokalemia was 5.9% in patients on empagliflozin and sacubitril/valsartan and 7.5% among patients on empagliflozin and a different type of RAS inhibition.
EMPEROR-Reduced was funded by Boehringer Ingelheim and Eli Lilly, the companies that market empagliflozin. Dr. Packer has received personal fees from Boehringer Ingelheim and Eli Lilly and from several other companies. Dr. Claggett has been a consultant to Amgen, AO Biome, Biogen, Corvia, Myokardia, and Novartis.
The latest drug shown to benefit patients with heart failure with reduced ejection fraction, the SGLT2 inhibitor empagliflozin, works just as well when added on top of a second major agent used to treat these patients, the renin-angiotensin system–inhibiting combination of sacubitril/valsartan, based on a post-hoc analysis of data from the EMPEROR-Reduced trial.
“When there are two very effective treatments, it’s common for people to ask: Which should I use?’ The goal of my presentation was to emphasize that the answer is both. We shouldn’t choose between neprilysin inhibition [sacubitril inhibits the enzyme neprilysin] and SGLT2 [sodium-glucose transporter 2] inhibition; we should use both,” said Milton Packer, MD at the virtual annual meeting of the Heart Failure Society of America.
EMPEROR-Reduced had the primary goal of testing the safety and efficacy of the SGLT2 inhibitor empagliflozin (Jardiance) in patients with heart failure with reduced ejection fraction (HFrEF). The results showed that adding this drug on top of standard treatments led to a 25% relative cut in the study’s primary efficacy endpoint, compared with placebo, and had this effect regardless of whether or not patients also had type 2 diabetes (N Engl J Med. 2020 Aug 29. doi: 10.1056/NEJMoa2022190).
Among the 3,730 patients enrolled in the trial, 727 (19%) were on sacubitril/valsartan (Entresto) at entry, which gave Dr. Packer the data to perform the analysis he reported. He presented the study’s three major endpoints as well as a quality of life analysis that compared the performance of empagliflozin in patients who were on sacubitril/valsartan at baseline with the other study patients, who were either on a different type of renin-angiotensin system (RAS) blocker (roughly 70% of study patients) or on no RAS inhibition (about 10% of patients).
The results showed no statistically significant indication of an interaction, suggesting that patients with sacubitril/valsartan on board had just as good response to empagliflozin as patients who were not on this combination. The landmark PARADIGM-HF trial proved several years ago that treatment of HFrEF patients with sacubitril/valsartan led to significantly better outcomes than did treatment with another form of RAS inhibition (N Engl J Med. 2014 Sep 11;371[11]:993-1004).
For example, EMPEROR-Reduced’s primary endpoint, the combined rate of cardiovascular death or hospitalization for heart failure, fell by 36% relative to placebo in patients who received empagliflozin on top of sacubitril/valsartan, and by 23% relative to placebo among the remaining patients who received empagliflozin on top of a different type of RAS inhibitor drug or no RAS inhibition.
“Background treatment with sacubitril/valsartan did not diminish, and may have enhanced the efficacy of empagliflozin,” concluded Dr. Packer. Further analyses also showed that concurrent sacubitril/valsartan had no statistically significant impact on empagliflozin’s ability to reduce the rate of total heart failure hospitalizations, or to slow progressive loss of renal function, compared with placebo. The fourth efficacy analysis Dr. Packer presented showed that empagliflozin was also as effective for improving a quality-of-life measure in patients compared with placebo regardless of the type of RAS inhibition used. For all four outcomes, the point-estimate of empagliflozin’s benefit was higher when used along with sacubitril/valsartan.
Brian L. Claggett, PhD, a biostatistician at Brigham and Women’s Hospital and Harvard Medical School in Boston, designated discussant for the report, disagreed with Dr. Packer’s suggestion that the efficacy of empagliflozin may have been greater when administered against a background of sacubitril/valsartan. From a statistical perspective, there is no basis to suggest that patients did better when they were on both drugs, he cautioned. But Dr. Claggett acknowledged that the new analyses suggested that empagliflozin’s benefit wasn’t compromised by concurrent sacubitril/valsartan use. He also highlighted the value of more fully documenting the safety and efficacy of a new drug when used as part of “comprehensive therapy” with the established drugs that a patient may concurrently receive.
Dr. Packer also presented several measures of treatment safety that all showed similar rates of adverse effects between the empagliflozin and placebo recipients regardless of background RAS inhibition. A notable finding was that the incidence of hypokalemia was 5.9% in patients on empagliflozin and sacubitril/valsartan and 7.5% among patients on empagliflozin and a different type of RAS inhibition.
EMPEROR-Reduced was funded by Boehringer Ingelheim and Eli Lilly, the companies that market empagliflozin. Dr. Packer has received personal fees from Boehringer Ingelheim and Eli Lilly and from several other companies. Dr. Claggett has been a consultant to Amgen, AO Biome, Biogen, Corvia, Myokardia, and Novartis.
The latest drug shown to benefit patients with heart failure with reduced ejection fraction, the SGLT2 inhibitor empagliflozin, works just as well when added on top of a second major agent used to treat these patients, the renin-angiotensin system–inhibiting combination of sacubitril/valsartan, based on a post-hoc analysis of data from the EMPEROR-Reduced trial.
“When there are two very effective treatments, it’s common for people to ask: Which should I use?’ The goal of my presentation was to emphasize that the answer is both. We shouldn’t choose between neprilysin inhibition [sacubitril inhibits the enzyme neprilysin] and SGLT2 [sodium-glucose transporter 2] inhibition; we should use both,” said Milton Packer, MD at the virtual annual meeting of the Heart Failure Society of America.
EMPEROR-Reduced had the primary goal of testing the safety and efficacy of the SGLT2 inhibitor empagliflozin (Jardiance) in patients with heart failure with reduced ejection fraction (HFrEF). The results showed that adding this drug on top of standard treatments led to a 25% relative cut in the study’s primary efficacy endpoint, compared with placebo, and had this effect regardless of whether or not patients also had type 2 diabetes (N Engl J Med. 2020 Aug 29. doi: 10.1056/NEJMoa2022190).
Among the 3,730 patients enrolled in the trial, 727 (19%) were on sacubitril/valsartan (Entresto) at entry, which gave Dr. Packer the data to perform the analysis he reported. He presented the study’s three major endpoints as well as a quality of life analysis that compared the performance of empagliflozin in patients who were on sacubitril/valsartan at baseline with the other study patients, who were either on a different type of renin-angiotensin system (RAS) blocker (roughly 70% of study patients) or on no RAS inhibition (about 10% of patients).
The results showed no statistically significant indication of an interaction, suggesting that patients with sacubitril/valsartan on board had just as good response to empagliflozin as patients who were not on this combination. The landmark PARADIGM-HF trial proved several years ago that treatment of HFrEF patients with sacubitril/valsartan led to significantly better outcomes than did treatment with another form of RAS inhibition (N Engl J Med. 2014 Sep 11;371[11]:993-1004).
For example, EMPEROR-Reduced’s primary endpoint, the combined rate of cardiovascular death or hospitalization for heart failure, fell by 36% relative to placebo in patients who received empagliflozin on top of sacubitril/valsartan, and by 23% relative to placebo among the remaining patients who received empagliflozin on top of a different type of RAS inhibitor drug or no RAS inhibition.
“Background treatment with sacubitril/valsartan did not diminish, and may have enhanced the efficacy of empagliflozin,” concluded Dr. Packer. Further analyses also showed that concurrent sacubitril/valsartan had no statistically significant impact on empagliflozin’s ability to reduce the rate of total heart failure hospitalizations, or to slow progressive loss of renal function, compared with placebo. The fourth efficacy analysis Dr. Packer presented showed that empagliflozin was also as effective for improving a quality-of-life measure in patients compared with placebo regardless of the type of RAS inhibition used. For all four outcomes, the point-estimate of empagliflozin’s benefit was higher when used along with sacubitril/valsartan.
Brian L. Claggett, PhD, a biostatistician at Brigham and Women’s Hospital and Harvard Medical School in Boston, designated discussant for the report, disagreed with Dr. Packer’s suggestion that the efficacy of empagliflozin may have been greater when administered against a background of sacubitril/valsartan. From a statistical perspective, there is no basis to suggest that patients did better when they were on both drugs, he cautioned. But Dr. Claggett acknowledged that the new analyses suggested that empagliflozin’s benefit wasn’t compromised by concurrent sacubitril/valsartan use. He also highlighted the value of more fully documenting the safety and efficacy of a new drug when used as part of “comprehensive therapy” with the established drugs that a patient may concurrently receive.
Dr. Packer also presented several measures of treatment safety that all showed similar rates of adverse effects between the empagliflozin and placebo recipients regardless of background RAS inhibition. A notable finding was that the incidence of hypokalemia was 5.9% in patients on empagliflozin and sacubitril/valsartan and 7.5% among patients on empagliflozin and a different type of RAS inhibition.
EMPEROR-Reduced was funded by Boehringer Ingelheim and Eli Lilly, the companies that market empagliflozin. Dr. Packer has received personal fees from Boehringer Ingelheim and Eli Lilly and from several other companies. Dr. Claggett has been a consultant to Amgen, AO Biome, Biogen, Corvia, Myokardia, and Novartis.
FROM HFSA 2020
PPIs associated with diabetes risk, but questions remain
Regular use of proton pump inhibitors (PPIs) is associated with an increased risk of type 2 diabetes, according to a large prospective analysis of the Nurses’ Health Study. The results follow on other studies suggesting other potential adverse effects of PPIs such as dementia, kidney damage, and micronutrient deficiencies.
The authors, led by Jinqiu Yuan and Changhua Zhang of Sun Yat-sen University (Guangdong, China), call for regular blood glucose testing and diabetes screening for patients on long-term PPIs. But not all are convinced. “I think that’s a strong recommendation from the available data and it’s unclear how that would be put into practice. I think instead practitioners should adhere to best practices, which emphasize using the lowest effective dose of PPIs for patients with appropriate indications,” David Leiman, MD, MSHP, assistant professor of medicine at Duke University, Durham, N.C. said in an interview.
“Overall, the data from the study can be classified as provocative results that I think may warrant further study,” he added. Randomized, controlled trials or many more observational studies would be required to establish causality between PPI use and diabetes risk, and in any case the findings of the current study don’t warrant a change in practice, Dr. Leiman said, noting that the study’s design makes it likely that much or all of the observed associations were due to confounding.
The study appeared online Sept. 28 in Gut.
The researchers analyzed data from 80,500 women from the Nurses’ Health Study, 95,550 women from the Nurses’ Health Study II, and 28,639 men from the Health Professionals Follow-up Study (HPFS), with a median follow-up time of 12 years in NHS and NHS2 and 9.8 years in HPFS.
The absolute risk of diabetes was 7.44 per 1,000 person-years in PPI users versus 4.32 among nonusers. After adjustment for lagging PPI use for 2 years and stratification by age and study period, PPI use was associated with a 74% increased risk of diabetes (hazard ratio , 1.74; 95% confidence interval, 1.37-2.20). Multivariable adjustment for demographic factors, lifestyle habits, comorbidities, and use of other medications and clinical indications for PPI use attenuated the association but did not eliminate it (HR, 1.24; 95% CI, 1.17-1.31).
There was no statistically significant association in the HPFS group (HR, 1.12; 95% CI, 0.91-1.38), possibly because of the smaller sample size.
At 1 year, the number needed to harm with PPIs was 318.9 (95% CI, 285.2-385.0). At 2 years it was 170.8 (95% CI, 150.8-209.7) and at 3 years it was 77.3 (95% CI, 66.8-97.0).
At 0-2 years, PPI use was associated with a 5% increase in diabetes risk (HR, 1.05; 95% CI, 0.93-1.19). More than 2 years of use was associated with higher risk (HR, 1.26; 95% CI, 1.18-1.35).
There was also an association between stopping PPI use and a decreased risk of diabetes: Compared with current PPI users, those who had stopped within the past 2 years had a 17% reduction in risk (HR, 0.83; 95% CI, 0.70-0.98), and those who had stopped more than 2 years previously had a 19% reduction (HR, 0.81; 95% CI, 0.76-0.86).
The researchers also examined diabetes risk associated with use of H2 receptor agonists (H2RAs), since the drugs share clinical indications with PPIs. H2RA use was also associated with a higher risk of diabetes (adjusted HR, 1.14; 95% CI, 1.07-1.23).
The researchers suggested that the fact that the less potent H2RA inhibitors had a less pronounced association with diabetes risk supports the idea that acid suppression may be related to diabetes pathogenesis.
The authors also suggest that changes to the gut microbiota may underlie increased risk. PPI use has been shown to reduce gut microbiome diversity and alter its phenotype. Such changes could lead to weight gain, metabolic syndrome, and chronic liver disease, which could in turn heighten risk.
The study is limited by its observational nature, and lacked detailed information on dosage, frequency, and indications for PPI use.
SOURCE: Yuan J et al. Gut. 2020 Sep 28. doi: 10.1136/gutjnl-2020-322557.
Regular use of proton pump inhibitors (PPIs) is associated with an increased risk of type 2 diabetes, according to a large prospective analysis of the Nurses’ Health Study. The results follow on other studies suggesting other potential adverse effects of PPIs such as dementia, kidney damage, and micronutrient deficiencies.
The authors, led by Jinqiu Yuan and Changhua Zhang of Sun Yat-sen University (Guangdong, China), call for regular blood glucose testing and diabetes screening for patients on long-term PPIs. But not all are convinced. “I think that’s a strong recommendation from the available data and it’s unclear how that would be put into practice. I think instead practitioners should adhere to best practices, which emphasize using the lowest effective dose of PPIs for patients with appropriate indications,” David Leiman, MD, MSHP, assistant professor of medicine at Duke University, Durham, N.C. said in an interview.
“Overall, the data from the study can be classified as provocative results that I think may warrant further study,” he added. Randomized, controlled trials or many more observational studies would be required to establish causality between PPI use and diabetes risk, and in any case the findings of the current study don’t warrant a change in practice, Dr. Leiman said, noting that the study’s design makes it likely that much or all of the observed associations were due to confounding.
The study appeared online Sept. 28 in Gut.
The researchers analyzed data from 80,500 women from the Nurses’ Health Study, 95,550 women from the Nurses’ Health Study II, and 28,639 men from the Health Professionals Follow-up Study (HPFS), with a median follow-up time of 12 years in NHS and NHS2 and 9.8 years in HPFS.
The absolute risk of diabetes was 7.44 per 1,000 person-years in PPI users versus 4.32 among nonusers. After adjustment for lagging PPI use for 2 years and stratification by age and study period, PPI use was associated with a 74% increased risk of diabetes (hazard ratio , 1.74; 95% confidence interval, 1.37-2.20). Multivariable adjustment for demographic factors, lifestyle habits, comorbidities, and use of other medications and clinical indications for PPI use attenuated the association but did not eliminate it (HR, 1.24; 95% CI, 1.17-1.31).
There was no statistically significant association in the HPFS group (HR, 1.12; 95% CI, 0.91-1.38), possibly because of the smaller sample size.
At 1 year, the number needed to harm with PPIs was 318.9 (95% CI, 285.2-385.0). At 2 years it was 170.8 (95% CI, 150.8-209.7) and at 3 years it was 77.3 (95% CI, 66.8-97.0).
At 0-2 years, PPI use was associated with a 5% increase in diabetes risk (HR, 1.05; 95% CI, 0.93-1.19). More than 2 years of use was associated with higher risk (HR, 1.26; 95% CI, 1.18-1.35).
There was also an association between stopping PPI use and a decreased risk of diabetes: Compared with current PPI users, those who had stopped within the past 2 years had a 17% reduction in risk (HR, 0.83; 95% CI, 0.70-0.98), and those who had stopped more than 2 years previously had a 19% reduction (HR, 0.81; 95% CI, 0.76-0.86).
The researchers also examined diabetes risk associated with use of H2 receptor agonists (H2RAs), since the drugs share clinical indications with PPIs. H2RA use was also associated with a higher risk of diabetes (adjusted HR, 1.14; 95% CI, 1.07-1.23).
The researchers suggested that the fact that the less potent H2RA inhibitors had a less pronounced association with diabetes risk supports the idea that acid suppression may be related to diabetes pathogenesis.
The authors also suggest that changes to the gut microbiota may underlie increased risk. PPI use has been shown to reduce gut microbiome diversity and alter its phenotype. Such changes could lead to weight gain, metabolic syndrome, and chronic liver disease, which could in turn heighten risk.
The study is limited by its observational nature, and lacked detailed information on dosage, frequency, and indications for PPI use.
SOURCE: Yuan J et al. Gut. 2020 Sep 28. doi: 10.1136/gutjnl-2020-322557.
Regular use of proton pump inhibitors (PPIs) is associated with an increased risk of type 2 diabetes, according to a large prospective analysis of the Nurses’ Health Study. The results follow on other studies suggesting other potential adverse effects of PPIs such as dementia, kidney damage, and micronutrient deficiencies.
The authors, led by Jinqiu Yuan and Changhua Zhang of Sun Yat-sen University (Guangdong, China), call for regular blood glucose testing and diabetes screening for patients on long-term PPIs. But not all are convinced. “I think that’s a strong recommendation from the available data and it’s unclear how that would be put into practice. I think instead practitioners should adhere to best practices, which emphasize using the lowest effective dose of PPIs for patients with appropriate indications,” David Leiman, MD, MSHP, assistant professor of medicine at Duke University, Durham, N.C. said in an interview.
“Overall, the data from the study can be classified as provocative results that I think may warrant further study,” he added. Randomized, controlled trials or many more observational studies would be required to establish causality between PPI use and diabetes risk, and in any case the findings of the current study don’t warrant a change in practice, Dr. Leiman said, noting that the study’s design makes it likely that much or all of the observed associations were due to confounding.
The study appeared online Sept. 28 in Gut.
The researchers analyzed data from 80,500 women from the Nurses’ Health Study, 95,550 women from the Nurses’ Health Study II, and 28,639 men from the Health Professionals Follow-up Study (HPFS), with a median follow-up time of 12 years in NHS and NHS2 and 9.8 years in HPFS.
The absolute risk of diabetes was 7.44 per 1,000 person-years in PPI users versus 4.32 among nonusers. After adjustment for lagging PPI use for 2 years and stratification by age and study period, PPI use was associated with a 74% increased risk of diabetes (hazard ratio , 1.74; 95% confidence interval, 1.37-2.20). Multivariable adjustment for demographic factors, lifestyle habits, comorbidities, and use of other medications and clinical indications for PPI use attenuated the association but did not eliminate it (HR, 1.24; 95% CI, 1.17-1.31).
There was no statistically significant association in the HPFS group (HR, 1.12; 95% CI, 0.91-1.38), possibly because of the smaller sample size.
At 1 year, the number needed to harm with PPIs was 318.9 (95% CI, 285.2-385.0). At 2 years it was 170.8 (95% CI, 150.8-209.7) and at 3 years it was 77.3 (95% CI, 66.8-97.0).
At 0-2 years, PPI use was associated with a 5% increase in diabetes risk (HR, 1.05; 95% CI, 0.93-1.19). More than 2 years of use was associated with higher risk (HR, 1.26; 95% CI, 1.18-1.35).
There was also an association between stopping PPI use and a decreased risk of diabetes: Compared with current PPI users, those who had stopped within the past 2 years had a 17% reduction in risk (HR, 0.83; 95% CI, 0.70-0.98), and those who had stopped more than 2 years previously had a 19% reduction (HR, 0.81; 95% CI, 0.76-0.86).
The researchers also examined diabetes risk associated with use of H2 receptor agonists (H2RAs), since the drugs share clinical indications with PPIs. H2RA use was also associated with a higher risk of diabetes (adjusted HR, 1.14; 95% CI, 1.07-1.23).
The researchers suggested that the fact that the less potent H2RA inhibitors had a less pronounced association with diabetes risk supports the idea that acid suppression may be related to diabetes pathogenesis.
The authors also suggest that changes to the gut microbiota may underlie increased risk. PPI use has been shown to reduce gut microbiome diversity and alter its phenotype. Such changes could lead to weight gain, metabolic syndrome, and chronic liver disease, which could in turn heighten risk.
The study is limited by its observational nature, and lacked detailed information on dosage, frequency, and indications for PPI use.
SOURCE: Yuan J et al. Gut. 2020 Sep 28. doi: 10.1136/gutjnl-2020-322557.
FROM GUT
Time to screen for liver disease in type 2 diabetes?
With high rates of fatty liver disease known to occur among people with type 2 diabetes, is it time to introduce routine liver screening into daily diabetes practice? The answer depends on whom you ask, and then there are still some important caveats.
From the hepatologist’s perspective, there is no excuse not to consider liver surveillance now that noninvasive screening methods are available, suggested Michael Trauner, MD, of the Medical University of Vienna.
“From a practical standpoint, I think every type 2 diabetic over 50 years of age is at high risk,” and consequently should be screened at diagnosis, Dr. Trauner said during a debate at the virtual annual meeting of the European Association for the Study of Diabetes. “I would screen at diagnosis and then decide on recall depending on noninvasive fibrosis markers.”
“It’s a rising problem that we are facing these days,” observed Michael Roden, MD, chair and professor of internal medicine, endocrinology and metabolic diseases at Heinrich-Heine University in Düsseldorf, Germany, and who cochaired the session. Not only do people with type 2 diabetes have an increased risk for developing liver diseases, but also there’s a higher risk for those with fatty liver diseases developing type 2 diabetes.
A meta-analysis published in Gut in just last week illustrates just how big a problem this is – nonalcoholic fatty liver disease (NAFLD) “doubled the risk of type 2 diabetes,” said Dr Rosen, who is also the director of the division of endocrinology and diabetology at University Clinics Düsseldorf. That analysis was based on more than 500,000 people, almost 28,000 of whom had incident diabetes over a 5-year period.
Screening tools scarce
This makes liver screening in type 2 diabetes patients “a formidable challenge,” cautioned Gianluca Perseghin, MD, professor of endocrinology at the Monza (Italy) Polyclinic and the University of Milano-Bicocca in Milan.
“Hepatologists generally see only the most severe cases,” Dr. Perseghin said. Diabetologists and endocrinologists would be likely to see huge numbers of patients that could potentially be at risk for liver disease and following the recommendations set out in the joint European Association for the Study of the Liver/EASD/European Association for the Study of Obesity guidelines would result in a huge number of patients being identified and potentially needing referral, he argued.
“At this stage, we need to build friendly, reliable and cost-effective screening process to be applied in the health systems,” Dr. Perseghin suggested. He proposed that liver surveillance would need to be not only personalized on a patient level, but also at the infrastructure level. Measuring liver enzymes, for example, was going to be less accurate in picking up liver disease but blood tests were widely available, whereas imaging methods were not going to be something all diabetes clinics would have immediate access to.
“There are clearly a lot of provocative decisions still to be made,” acknowledged Philip Newsome, PhD, FRCPE, an honorary consultant hepatologist at the University of Birmingham (England) and who cochaired the debate.
“We need to demonstrate that looking for the presence of liver disease in this cohort changes their outcomes in a way that is cost effective,” Dr. Newsome, who is also the secretary general of EASL.
“Tests are evolving, but more importantly, treatments are evolving. So, the decision around cost effectiveness will clearly change,” he added.
NAFLD therapies unclar
“There are still a lot of questions,” Dr. Newsome said during a Novo-Nordisk–sponsored “Meet the Expert” session discussing EASL-EASD-EASO guidelines. “We don’t have any licensed therapies at the moment. But there’s been a huge amount of investment, looking at all sorts of different approaches.”
Dr. Newsome added: “We also don’t know how to monitor these patients. Most of the noninvasive are very useful for staging patients, but we don’t really understand how useful they are for monitoring changes in fibrosis.”
Diabetologist Hannele Yki-Järvinen, MD, PhD, of the University of Helsinki, gave her thoughts on the topic during the same session.
“We should add FIB-4 [Fibrosis-4 index] to the annual exam and ask the lab to calculate FIB-4 automatically,” Dr. Yki-Järvinen said. FIB-4is calculated using the patients age and the results of readily available blood tests that measure the AST/ALT ratio and the platelet count.
Dr. Trauner has received advisory fees and grant support from various companies with an interest in developing liver-directed therapies, and is also a coinventor of 24-norursodeoxycholic acid under development for cholestatic liver disease and potentially NAFLD. Dr. Perseghin has received honoraria and grant support from various pharmaceutical companies with an interest in diabetes care. Dr. Roden did not provide any disclosures. Dr. Newsome has received research grants from Boehringer Ingelheim and Novo Nordisk and acted as a consultant to many pharmaceutical companies. Dr. Yki-Järvinen disclosed receiving consultancy fees from Eli Lilly, MSD, and Novo Nordisk.
SOURCE: Trauner M; Persghin G. EASD 2020, Session S27.
With high rates of fatty liver disease known to occur among people with type 2 diabetes, is it time to introduce routine liver screening into daily diabetes practice? The answer depends on whom you ask, and then there are still some important caveats.
From the hepatologist’s perspective, there is no excuse not to consider liver surveillance now that noninvasive screening methods are available, suggested Michael Trauner, MD, of the Medical University of Vienna.
“From a practical standpoint, I think every type 2 diabetic over 50 years of age is at high risk,” and consequently should be screened at diagnosis, Dr. Trauner said during a debate at the virtual annual meeting of the European Association for the Study of Diabetes. “I would screen at diagnosis and then decide on recall depending on noninvasive fibrosis markers.”
“It’s a rising problem that we are facing these days,” observed Michael Roden, MD, chair and professor of internal medicine, endocrinology and metabolic diseases at Heinrich-Heine University in Düsseldorf, Germany, and who cochaired the session. Not only do people with type 2 diabetes have an increased risk for developing liver diseases, but also there’s a higher risk for those with fatty liver diseases developing type 2 diabetes.
A meta-analysis published in Gut in just last week illustrates just how big a problem this is – nonalcoholic fatty liver disease (NAFLD) “doubled the risk of type 2 diabetes,” said Dr Rosen, who is also the director of the division of endocrinology and diabetology at University Clinics Düsseldorf. That analysis was based on more than 500,000 people, almost 28,000 of whom had incident diabetes over a 5-year period.
Screening tools scarce
This makes liver screening in type 2 diabetes patients “a formidable challenge,” cautioned Gianluca Perseghin, MD, professor of endocrinology at the Monza (Italy) Polyclinic and the University of Milano-Bicocca in Milan.
“Hepatologists generally see only the most severe cases,” Dr. Perseghin said. Diabetologists and endocrinologists would be likely to see huge numbers of patients that could potentially be at risk for liver disease and following the recommendations set out in the joint European Association for the Study of the Liver/EASD/European Association for the Study of Obesity guidelines would result in a huge number of patients being identified and potentially needing referral, he argued.
“At this stage, we need to build friendly, reliable and cost-effective screening process to be applied in the health systems,” Dr. Perseghin suggested. He proposed that liver surveillance would need to be not only personalized on a patient level, but also at the infrastructure level. Measuring liver enzymes, for example, was going to be less accurate in picking up liver disease but blood tests were widely available, whereas imaging methods were not going to be something all diabetes clinics would have immediate access to.
“There are clearly a lot of provocative decisions still to be made,” acknowledged Philip Newsome, PhD, FRCPE, an honorary consultant hepatologist at the University of Birmingham (England) and who cochaired the debate.
“We need to demonstrate that looking for the presence of liver disease in this cohort changes their outcomes in a way that is cost effective,” Dr. Newsome, who is also the secretary general of EASL.
“Tests are evolving, but more importantly, treatments are evolving. So, the decision around cost effectiveness will clearly change,” he added.
NAFLD therapies unclar
“There are still a lot of questions,” Dr. Newsome said during a Novo-Nordisk–sponsored “Meet the Expert” session discussing EASL-EASD-EASO guidelines. “We don’t have any licensed therapies at the moment. But there’s been a huge amount of investment, looking at all sorts of different approaches.”
Dr. Newsome added: “We also don’t know how to monitor these patients. Most of the noninvasive are very useful for staging patients, but we don’t really understand how useful they are for monitoring changes in fibrosis.”
Diabetologist Hannele Yki-Järvinen, MD, PhD, of the University of Helsinki, gave her thoughts on the topic during the same session.
“We should add FIB-4 [Fibrosis-4 index] to the annual exam and ask the lab to calculate FIB-4 automatically,” Dr. Yki-Järvinen said. FIB-4is calculated using the patients age and the results of readily available blood tests that measure the AST/ALT ratio and the platelet count.
Dr. Trauner has received advisory fees and grant support from various companies with an interest in developing liver-directed therapies, and is also a coinventor of 24-norursodeoxycholic acid under development for cholestatic liver disease and potentially NAFLD. Dr. Perseghin has received honoraria and grant support from various pharmaceutical companies with an interest in diabetes care. Dr. Roden did not provide any disclosures. Dr. Newsome has received research grants from Boehringer Ingelheim and Novo Nordisk and acted as a consultant to many pharmaceutical companies. Dr. Yki-Järvinen disclosed receiving consultancy fees from Eli Lilly, MSD, and Novo Nordisk.
SOURCE: Trauner M; Persghin G. EASD 2020, Session S27.
With high rates of fatty liver disease known to occur among people with type 2 diabetes, is it time to introduce routine liver screening into daily diabetes practice? The answer depends on whom you ask, and then there are still some important caveats.
From the hepatologist’s perspective, there is no excuse not to consider liver surveillance now that noninvasive screening methods are available, suggested Michael Trauner, MD, of the Medical University of Vienna.
“From a practical standpoint, I think every type 2 diabetic over 50 years of age is at high risk,” and consequently should be screened at diagnosis, Dr. Trauner said during a debate at the virtual annual meeting of the European Association for the Study of Diabetes. “I would screen at diagnosis and then decide on recall depending on noninvasive fibrosis markers.”
“It’s a rising problem that we are facing these days,” observed Michael Roden, MD, chair and professor of internal medicine, endocrinology and metabolic diseases at Heinrich-Heine University in Düsseldorf, Germany, and who cochaired the session. Not only do people with type 2 diabetes have an increased risk for developing liver diseases, but also there’s a higher risk for those with fatty liver diseases developing type 2 diabetes.
A meta-analysis published in Gut in just last week illustrates just how big a problem this is – nonalcoholic fatty liver disease (NAFLD) “doubled the risk of type 2 diabetes,” said Dr Rosen, who is also the director of the division of endocrinology and diabetology at University Clinics Düsseldorf. That analysis was based on more than 500,000 people, almost 28,000 of whom had incident diabetes over a 5-year period.
Screening tools scarce
This makes liver screening in type 2 diabetes patients “a formidable challenge,” cautioned Gianluca Perseghin, MD, professor of endocrinology at the Monza (Italy) Polyclinic and the University of Milano-Bicocca in Milan.
“Hepatologists generally see only the most severe cases,” Dr. Perseghin said. Diabetologists and endocrinologists would be likely to see huge numbers of patients that could potentially be at risk for liver disease and following the recommendations set out in the joint European Association for the Study of the Liver/EASD/European Association for the Study of Obesity guidelines would result in a huge number of patients being identified and potentially needing referral, he argued.
“At this stage, we need to build friendly, reliable and cost-effective screening process to be applied in the health systems,” Dr. Perseghin suggested. He proposed that liver surveillance would need to be not only personalized on a patient level, but also at the infrastructure level. Measuring liver enzymes, for example, was going to be less accurate in picking up liver disease but blood tests were widely available, whereas imaging methods were not going to be something all diabetes clinics would have immediate access to.
“There are clearly a lot of provocative decisions still to be made,” acknowledged Philip Newsome, PhD, FRCPE, an honorary consultant hepatologist at the University of Birmingham (England) and who cochaired the debate.
“We need to demonstrate that looking for the presence of liver disease in this cohort changes their outcomes in a way that is cost effective,” Dr. Newsome, who is also the secretary general of EASL.
“Tests are evolving, but more importantly, treatments are evolving. So, the decision around cost effectiveness will clearly change,” he added.
NAFLD therapies unclar
“There are still a lot of questions,” Dr. Newsome said during a Novo-Nordisk–sponsored “Meet the Expert” session discussing EASL-EASD-EASO guidelines. “We don’t have any licensed therapies at the moment. But there’s been a huge amount of investment, looking at all sorts of different approaches.”
Dr. Newsome added: “We also don’t know how to monitor these patients. Most of the noninvasive are very useful for staging patients, but we don’t really understand how useful they are for monitoring changes in fibrosis.”
Diabetologist Hannele Yki-Järvinen, MD, PhD, of the University of Helsinki, gave her thoughts on the topic during the same session.
“We should add FIB-4 [Fibrosis-4 index] to the annual exam and ask the lab to calculate FIB-4 automatically,” Dr. Yki-Järvinen said. FIB-4is calculated using the patients age and the results of readily available blood tests that measure the AST/ALT ratio and the platelet count.
Dr. Trauner has received advisory fees and grant support from various companies with an interest in developing liver-directed therapies, and is also a coinventor of 24-norursodeoxycholic acid under development for cholestatic liver disease and potentially NAFLD. Dr. Perseghin has received honoraria and grant support from various pharmaceutical companies with an interest in diabetes care. Dr. Roden did not provide any disclosures. Dr. Newsome has received research grants from Boehringer Ingelheim and Novo Nordisk and acted as a consultant to many pharmaceutical companies. Dr. Yki-Järvinen disclosed receiving consultancy fees from Eli Lilly, MSD, and Novo Nordisk.
SOURCE: Trauner M; Persghin G. EASD 2020, Session S27.
REPORTING FROM EASD 2020
Dapagliflozin’s CKD performance sends heart failure messages
The DAPA-CKD trial results, which proved dapagliflozin’s efficacy for slowing chronic kidney disease progression in patients selected for signs of worsening renal function, also have important messages for cardiologists, especially heart failure physicians.
Those messages include findings that were “consistent” with the results of the earlier DAPA-HF trial, which tested the same sodium-glucose transporter 2 (SGLT2) inhibitor in patients selected for having heart failure with reduced ejection fraction (HFrEF). In addition, a specific action of dapagliflozin (Farxiga) on the patients in DAPA-CKD, which enrolled patients based on markers of chronic kidney disease (CKD), was prevention of first and recurrent heart failure hospitalizations, John J.V. McMurray, MD, said at the virtual annual scientific meeting of the Heart Failure Society of America, further highlighting the role that dapagliflozin has in reducing both heart failure and renal events.
What DAPA-CKD means for heart failure
The main findings from the DAPA-CKD trial, published in September in the New England Journal of Medicine, included as a secondary outcome the combined rate of death from cardiovascular causes or hospitalization for heart failure (HHF). Treatment with dapagliflozin linked with a significant 29% relative reduction in this endpoint, compared with placebo-treated patients. At the HFSA meeting, Dr. McMurray reported for the first time the specific HHF numbers, a prespecified secondary endpoint for the study.
Patients on dapagliflozin had 37 total HHF events (1.7%), including both first-time and subsequent hospitalizations, while patients in the placebo arm had a total of 71 HHF events (3.3%) during the study’s median 2.4 years of follow-up, an absolute reduction of 1.6% that translated into a relative risk reduction of 49%.
The HHF findings from DAPA-CKD importantly showed that SGLT2 inhibition in patients with signs of renal dysfunction “will not only slow progression of kidney disease but will also reduce the risk of developing heart failure, crucially in patients with or without type 2 diabetes,” explained Dr. McMurray in an interview. “Cardiologists often consult in the kidney wards and advise on management of patients with chronic kidney disease, even those without heart failure.”
The DAPA-CKD findings carry another important message for heart failure management regarding the minimum level of renal function a patient can have and still safely receive dapagliflozin or possibly another agent from the same SGLT2 inhibitor class. In DAPA-CKD, patients safely received dapagliflozin with an estimated glomerular filtration rate (eGFR) as low as 25 mL/min per 1.73 m2; 14% of enrolled patients had an eGFR of 25-29 mL/min per 1.73 m2.
“Typically, about 40%-50% of patients with heart failure have chronic kidney disease,” which makes this safety finding important to clinicians who care for heart failure patients, but it’s also important for any patient who might be a candidate for dapagliflozin or another drug from its class. “We had no strong evidence before this trial that SGLT2 inhibition could reduce hard renal endpoints,” specifically need for chronic dialysis, renal transplant, or renal death, “in patients with or without diabetes,” Dr. McMurray said.
DAPA-CKD grows the pool of eligible heart failure patients
A further consequence of the DAPA-CKD findings is that when, as expected, regulatory bodies give dapagliflozin an indication for treating the types of CKD patients enrolled in the trial, it will functionally expand this treatment to an even larger swath of heart failure patients who currently don’t qualify for this treatment, specifically patients with CKD who also have heart failure with preserved ejection fraction (HFpEF). On Oct. 2, 2020, the Food and Drug Administration fast-tracked dapagliflozin for the CKD indication by granting it Breakthrough Therapy Designation based on the DAPA-CKD results.
Results first reported in 2019 from the DAPA-HF trial led to dapagliflozin receiving a labeled indication for treating HFrEF, the types of heart failure patients enrolled in the trial. Direct evidence on the efficacy of SGLT2 inhibitors for patients with HFpEF will not be available until results from a few trials now in progress become available during the next 12 months.
In the meantime, nearly half of patients with HFpEF also have CKD, noted Dr. McMurray, and another large portion of HFpEF patients have type 2 diabetes and hence qualify for SGLT2 inhibitor treatment that way. “Obviously, we would like to know specifically about heart failure outcomes in patients with HFpEF” on SGLT2 inhibitor treatment, he acknowledged. But the recent approval of dapagliflozin for patients with HFrEF and the likely indication coming soon for treating CKD means that the number of patients with heart failure who are not eligible for SGLT2 inhibitor treatment is dwindling down to some extent.
New DAPA-HF results show no drug, device interactions
In a separate session at the HFSA virtual meeting, Dr. McMurray and several collaborators on the DAPA-HF trial presented results from some new analyses. Dr. McMurray looked at the impact of dapagliflozin treatment on the primary endpoint when patients were stratified by the diuretic dosage they received at study entry. The results showed that “the benefits from dapagliflozin were irrespective of the use of background diuretic therapy or the diuretic dose,” he reported. Study findings also showed that roughly three-quarters of patients in the study had no change in their diuretic dosage during the course of the trial, that the fraction of patients who had an increase in their dosage was about the same as those whose diuretic dosage decreased, and that this pattern was similar in both the patients on dapagliflozin and in those randomized to placebo.
Another set of new analyses from DAPA-HF looked at the impact on dapagliflozin efficacy of background medical and device therapies for heart failure, as well as background diabetes therapies. The findings showed no signal of an interaction with background therapies. “The effects of dapagliflozin are incremental and complimentary to conventional therapies for HFrEF,” concluded Lars Kober, MD, a professor and heart failure physician at Copenhagen University Hospital.
DAPA-CKD was funded by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. McMurray’s employer, Glasgow University, has received payments from AstraZeneca and several other companies to compensate for his time overseeing various clinical trials. Dr. Kober has received honoraria for speaking on behalf of several companies including AstraZeneca.
The DAPA-CKD trial results, which proved dapagliflozin’s efficacy for slowing chronic kidney disease progression in patients selected for signs of worsening renal function, also have important messages for cardiologists, especially heart failure physicians.
Those messages include findings that were “consistent” with the results of the earlier DAPA-HF trial, which tested the same sodium-glucose transporter 2 (SGLT2) inhibitor in patients selected for having heart failure with reduced ejection fraction (HFrEF). In addition, a specific action of dapagliflozin (Farxiga) on the patients in DAPA-CKD, which enrolled patients based on markers of chronic kidney disease (CKD), was prevention of first and recurrent heart failure hospitalizations, John J.V. McMurray, MD, said at the virtual annual scientific meeting of the Heart Failure Society of America, further highlighting the role that dapagliflozin has in reducing both heart failure and renal events.
What DAPA-CKD means for heart failure
The main findings from the DAPA-CKD trial, published in September in the New England Journal of Medicine, included as a secondary outcome the combined rate of death from cardiovascular causes or hospitalization for heart failure (HHF). Treatment with dapagliflozin linked with a significant 29% relative reduction in this endpoint, compared with placebo-treated patients. At the HFSA meeting, Dr. McMurray reported for the first time the specific HHF numbers, a prespecified secondary endpoint for the study.
Patients on dapagliflozin had 37 total HHF events (1.7%), including both first-time and subsequent hospitalizations, while patients in the placebo arm had a total of 71 HHF events (3.3%) during the study’s median 2.4 years of follow-up, an absolute reduction of 1.6% that translated into a relative risk reduction of 49%.
The HHF findings from DAPA-CKD importantly showed that SGLT2 inhibition in patients with signs of renal dysfunction “will not only slow progression of kidney disease but will also reduce the risk of developing heart failure, crucially in patients with or without type 2 diabetes,” explained Dr. McMurray in an interview. “Cardiologists often consult in the kidney wards and advise on management of patients with chronic kidney disease, even those without heart failure.”
The DAPA-CKD findings carry another important message for heart failure management regarding the minimum level of renal function a patient can have and still safely receive dapagliflozin or possibly another agent from the same SGLT2 inhibitor class. In DAPA-CKD, patients safely received dapagliflozin with an estimated glomerular filtration rate (eGFR) as low as 25 mL/min per 1.73 m2; 14% of enrolled patients had an eGFR of 25-29 mL/min per 1.73 m2.
“Typically, about 40%-50% of patients with heart failure have chronic kidney disease,” which makes this safety finding important to clinicians who care for heart failure patients, but it’s also important for any patient who might be a candidate for dapagliflozin or another drug from its class. “We had no strong evidence before this trial that SGLT2 inhibition could reduce hard renal endpoints,” specifically need for chronic dialysis, renal transplant, or renal death, “in patients with or without diabetes,” Dr. McMurray said.
DAPA-CKD grows the pool of eligible heart failure patients
A further consequence of the DAPA-CKD findings is that when, as expected, regulatory bodies give dapagliflozin an indication for treating the types of CKD patients enrolled in the trial, it will functionally expand this treatment to an even larger swath of heart failure patients who currently don’t qualify for this treatment, specifically patients with CKD who also have heart failure with preserved ejection fraction (HFpEF). On Oct. 2, 2020, the Food and Drug Administration fast-tracked dapagliflozin for the CKD indication by granting it Breakthrough Therapy Designation based on the DAPA-CKD results.
Results first reported in 2019 from the DAPA-HF trial led to dapagliflozin receiving a labeled indication for treating HFrEF, the types of heart failure patients enrolled in the trial. Direct evidence on the efficacy of SGLT2 inhibitors for patients with HFpEF will not be available until results from a few trials now in progress become available during the next 12 months.
In the meantime, nearly half of patients with HFpEF also have CKD, noted Dr. McMurray, and another large portion of HFpEF patients have type 2 diabetes and hence qualify for SGLT2 inhibitor treatment that way. “Obviously, we would like to know specifically about heart failure outcomes in patients with HFpEF” on SGLT2 inhibitor treatment, he acknowledged. But the recent approval of dapagliflozin for patients with HFrEF and the likely indication coming soon for treating CKD means that the number of patients with heart failure who are not eligible for SGLT2 inhibitor treatment is dwindling down to some extent.
New DAPA-HF results show no drug, device interactions
In a separate session at the HFSA virtual meeting, Dr. McMurray and several collaborators on the DAPA-HF trial presented results from some new analyses. Dr. McMurray looked at the impact of dapagliflozin treatment on the primary endpoint when patients were stratified by the diuretic dosage they received at study entry. The results showed that “the benefits from dapagliflozin were irrespective of the use of background diuretic therapy or the diuretic dose,” he reported. Study findings also showed that roughly three-quarters of patients in the study had no change in their diuretic dosage during the course of the trial, that the fraction of patients who had an increase in their dosage was about the same as those whose diuretic dosage decreased, and that this pattern was similar in both the patients on dapagliflozin and in those randomized to placebo.
Another set of new analyses from DAPA-HF looked at the impact on dapagliflozin efficacy of background medical and device therapies for heart failure, as well as background diabetes therapies. The findings showed no signal of an interaction with background therapies. “The effects of dapagliflozin are incremental and complimentary to conventional therapies for HFrEF,” concluded Lars Kober, MD, a professor and heart failure physician at Copenhagen University Hospital.
DAPA-CKD was funded by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. McMurray’s employer, Glasgow University, has received payments from AstraZeneca and several other companies to compensate for his time overseeing various clinical trials. Dr. Kober has received honoraria for speaking on behalf of several companies including AstraZeneca.
The DAPA-CKD trial results, which proved dapagliflozin’s efficacy for slowing chronic kidney disease progression in patients selected for signs of worsening renal function, also have important messages for cardiologists, especially heart failure physicians.
Those messages include findings that were “consistent” with the results of the earlier DAPA-HF trial, which tested the same sodium-glucose transporter 2 (SGLT2) inhibitor in patients selected for having heart failure with reduced ejection fraction (HFrEF). In addition, a specific action of dapagliflozin (Farxiga) on the patients in DAPA-CKD, which enrolled patients based on markers of chronic kidney disease (CKD), was prevention of first and recurrent heart failure hospitalizations, John J.V. McMurray, MD, said at the virtual annual scientific meeting of the Heart Failure Society of America, further highlighting the role that dapagliflozin has in reducing both heart failure and renal events.
What DAPA-CKD means for heart failure
The main findings from the DAPA-CKD trial, published in September in the New England Journal of Medicine, included as a secondary outcome the combined rate of death from cardiovascular causes or hospitalization for heart failure (HHF). Treatment with dapagliflozin linked with a significant 29% relative reduction in this endpoint, compared with placebo-treated patients. At the HFSA meeting, Dr. McMurray reported for the first time the specific HHF numbers, a prespecified secondary endpoint for the study.
Patients on dapagliflozin had 37 total HHF events (1.7%), including both first-time and subsequent hospitalizations, while patients in the placebo arm had a total of 71 HHF events (3.3%) during the study’s median 2.4 years of follow-up, an absolute reduction of 1.6% that translated into a relative risk reduction of 49%.
The HHF findings from DAPA-CKD importantly showed that SGLT2 inhibition in patients with signs of renal dysfunction “will not only slow progression of kidney disease but will also reduce the risk of developing heart failure, crucially in patients with or without type 2 diabetes,” explained Dr. McMurray in an interview. “Cardiologists often consult in the kidney wards and advise on management of patients with chronic kidney disease, even those without heart failure.”
The DAPA-CKD findings carry another important message for heart failure management regarding the minimum level of renal function a patient can have and still safely receive dapagliflozin or possibly another agent from the same SGLT2 inhibitor class. In DAPA-CKD, patients safely received dapagliflozin with an estimated glomerular filtration rate (eGFR) as low as 25 mL/min per 1.73 m2; 14% of enrolled patients had an eGFR of 25-29 mL/min per 1.73 m2.
“Typically, about 40%-50% of patients with heart failure have chronic kidney disease,” which makes this safety finding important to clinicians who care for heart failure patients, but it’s also important for any patient who might be a candidate for dapagliflozin or another drug from its class. “We had no strong evidence before this trial that SGLT2 inhibition could reduce hard renal endpoints,” specifically need for chronic dialysis, renal transplant, or renal death, “in patients with or without diabetes,” Dr. McMurray said.
DAPA-CKD grows the pool of eligible heart failure patients
A further consequence of the DAPA-CKD findings is that when, as expected, regulatory bodies give dapagliflozin an indication for treating the types of CKD patients enrolled in the trial, it will functionally expand this treatment to an even larger swath of heart failure patients who currently don’t qualify for this treatment, specifically patients with CKD who also have heart failure with preserved ejection fraction (HFpEF). On Oct. 2, 2020, the Food and Drug Administration fast-tracked dapagliflozin for the CKD indication by granting it Breakthrough Therapy Designation based on the DAPA-CKD results.
Results first reported in 2019 from the DAPA-HF trial led to dapagliflozin receiving a labeled indication for treating HFrEF, the types of heart failure patients enrolled in the trial. Direct evidence on the efficacy of SGLT2 inhibitors for patients with HFpEF will not be available until results from a few trials now in progress become available during the next 12 months.
In the meantime, nearly half of patients with HFpEF also have CKD, noted Dr. McMurray, and another large portion of HFpEF patients have type 2 diabetes and hence qualify for SGLT2 inhibitor treatment that way. “Obviously, we would like to know specifically about heart failure outcomes in patients with HFpEF” on SGLT2 inhibitor treatment, he acknowledged. But the recent approval of dapagliflozin for patients with HFrEF and the likely indication coming soon for treating CKD means that the number of patients with heart failure who are not eligible for SGLT2 inhibitor treatment is dwindling down to some extent.
New DAPA-HF results show no drug, device interactions
In a separate session at the HFSA virtual meeting, Dr. McMurray and several collaborators on the DAPA-HF trial presented results from some new analyses. Dr. McMurray looked at the impact of dapagliflozin treatment on the primary endpoint when patients were stratified by the diuretic dosage they received at study entry. The results showed that “the benefits from dapagliflozin were irrespective of the use of background diuretic therapy or the diuretic dose,” he reported. Study findings also showed that roughly three-quarters of patients in the study had no change in their diuretic dosage during the course of the trial, that the fraction of patients who had an increase in their dosage was about the same as those whose diuretic dosage decreased, and that this pattern was similar in both the patients on dapagliflozin and in those randomized to placebo.
Another set of new analyses from DAPA-HF looked at the impact on dapagliflozin efficacy of background medical and device therapies for heart failure, as well as background diabetes therapies. The findings showed no signal of an interaction with background therapies. “The effects of dapagliflozin are incremental and complimentary to conventional therapies for HFrEF,” concluded Lars Kober, MD, a professor and heart failure physician at Copenhagen University Hospital.
DAPA-CKD was funded by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. McMurray’s employer, Glasgow University, has received payments from AstraZeneca and several other companies to compensate for his time overseeing various clinical trials. Dr. Kober has received honoraria for speaking on behalf of several companies including AstraZeneca.
FROM HFSA 2020