User login
FDA approves first two-drug tablet for HIV
The U.S. Food and Drug Administration has approved the first two-drug, fixed-dose, complete regimen for HIV-infected adults, according to an FDA press announcement.
Dovato (dolutegravir and lamivudine), a product of ViiV Healthcare, is intended to serve “as a complete regimen” for the treatment of HIV-1 infection in adults who have had no previous antiretroviral treatment and who have an infection with no known or suspected genetic substitutions associated with resistance to the individual components of Dovato.
“With this approval, patients who have never been treated have the option of taking a two-drug regimen in a single tablet while eliminating additional toxicity and potential drug interactions from a third drug,” said Debra Birnkrant, MD, director of the FDA’s Division of Antiviral Products.
The Dovato labeling includes a Boxed Warning that patients infected with both HIV and hepatitis B should add additional treatment for their HBV or consider a different drug regimen. The most common adverse reactions with Dovato were headache, diarrhea, nausea, insomnia, and fatigue. In addition, the FDA warned that, as there is a known risk for neural tube defects with dolutegravir, patients are advised to avoid use of Dovato at the time of conception through the first trimester of pregnancy.
[email protected]
The U.S. Food and Drug Administration has approved the first two-drug, fixed-dose, complete regimen for HIV-infected adults, according to an FDA press announcement.
Dovato (dolutegravir and lamivudine), a product of ViiV Healthcare, is intended to serve “as a complete regimen” for the treatment of HIV-1 infection in adults who have had no previous antiretroviral treatment and who have an infection with no known or suspected genetic substitutions associated with resistance to the individual components of Dovato.
“With this approval, patients who have never been treated have the option of taking a two-drug regimen in a single tablet while eliminating additional toxicity and potential drug interactions from a third drug,” said Debra Birnkrant, MD, director of the FDA’s Division of Antiviral Products.
The Dovato labeling includes a Boxed Warning that patients infected with both HIV and hepatitis B should add additional treatment for their HBV or consider a different drug regimen. The most common adverse reactions with Dovato were headache, diarrhea, nausea, insomnia, and fatigue. In addition, the FDA warned that, as there is a known risk for neural tube defects with dolutegravir, patients are advised to avoid use of Dovato at the time of conception through the first trimester of pregnancy.
[email protected]
The U.S. Food and Drug Administration has approved the first two-drug, fixed-dose, complete regimen for HIV-infected adults, according to an FDA press announcement.
Dovato (dolutegravir and lamivudine), a product of ViiV Healthcare, is intended to serve “as a complete regimen” for the treatment of HIV-1 infection in adults who have had no previous antiretroviral treatment and who have an infection with no known or suspected genetic substitutions associated with resistance to the individual components of Dovato.
“With this approval, patients who have never been treated have the option of taking a two-drug regimen in a single tablet while eliminating additional toxicity and potential drug interactions from a third drug,” said Debra Birnkrant, MD, director of the FDA’s Division of Antiviral Products.
The Dovato labeling includes a Boxed Warning that patients infected with both HIV and hepatitis B should add additional treatment for their HBV or consider a different drug regimen. The most common adverse reactions with Dovato were headache, diarrhea, nausea, insomnia, and fatigue. In addition, the FDA warned that, as there is a known risk for neural tube defects with dolutegravir, patients are advised to avoid use of Dovato at the time of conception through the first trimester of pregnancy.
[email protected]
Cigna, Express Scripts to offer $25 cap on 30-day insulin supply
Cigna and Express Scripts have announced
The new program, open to Cigna members who are covered in commercial plans, would cap out-of-pocket costs for a 30-day supply of insulin at $25. For plan members, the only eligibility requirement is having an out-of-pocket cost higher than $25, according to a press release.
For a member to participate in the program, the plan administrator at the member’s place of employment has to opt in to it. There are no eligibility requirements imposed on the employer, other than a willingness to opt in.
A spokeswoman for Express Scripts said that there is no charge to sign up for the program, and most plans will not see an additional cost to get the copayment to $25 for the patient.
The announcement comes in the wake of the first of two hearings by the House Committee on Energy & Commerce aimed at understanding why insulin prices have spiked in recent years. The first hearing, held on April 2, examined the impact that the high list price of insulin is having on patients, and how out-of-pocket expenses are limiting access to this life-saving drug. The second hearing, expected to occur during the week of April 8 (the date had not been scheduled as of press time), will bring together various players in the supply chain, including the three major manufacturers of insulin.
“We are confident that our new program will remove cost as a barrier for people in participating plans who need insulin,” Steve Miller, MD, executive vice president and chief clinical officer at Cigna, said in a statement.
The Express Scripts spokeswoman noted that there were more than 700,000 people in a commercially insured plan across Cigna and Express Scripts who had a claim for insulin in 2018. The average out-of-pocket cost of a 30-day supply of insulin in 2018 across this population was $41.50.
Cigna and Express Scripts have announced
The new program, open to Cigna members who are covered in commercial plans, would cap out-of-pocket costs for a 30-day supply of insulin at $25. For plan members, the only eligibility requirement is having an out-of-pocket cost higher than $25, according to a press release.
For a member to participate in the program, the plan administrator at the member’s place of employment has to opt in to it. There are no eligibility requirements imposed on the employer, other than a willingness to opt in.
A spokeswoman for Express Scripts said that there is no charge to sign up for the program, and most plans will not see an additional cost to get the copayment to $25 for the patient.
The announcement comes in the wake of the first of two hearings by the House Committee on Energy & Commerce aimed at understanding why insulin prices have spiked in recent years. The first hearing, held on April 2, examined the impact that the high list price of insulin is having on patients, and how out-of-pocket expenses are limiting access to this life-saving drug. The second hearing, expected to occur during the week of April 8 (the date had not been scheduled as of press time), will bring together various players in the supply chain, including the three major manufacturers of insulin.
“We are confident that our new program will remove cost as a barrier for people in participating plans who need insulin,” Steve Miller, MD, executive vice president and chief clinical officer at Cigna, said in a statement.
The Express Scripts spokeswoman noted that there were more than 700,000 people in a commercially insured plan across Cigna and Express Scripts who had a claim for insulin in 2018. The average out-of-pocket cost of a 30-day supply of insulin in 2018 across this population was $41.50.
Cigna and Express Scripts have announced
The new program, open to Cigna members who are covered in commercial plans, would cap out-of-pocket costs for a 30-day supply of insulin at $25. For plan members, the only eligibility requirement is having an out-of-pocket cost higher than $25, according to a press release.
For a member to participate in the program, the plan administrator at the member’s place of employment has to opt in to it. There are no eligibility requirements imposed on the employer, other than a willingness to opt in.
A spokeswoman for Express Scripts said that there is no charge to sign up for the program, and most plans will not see an additional cost to get the copayment to $25 for the patient.
The announcement comes in the wake of the first of two hearings by the House Committee on Energy & Commerce aimed at understanding why insulin prices have spiked in recent years. The first hearing, held on April 2, examined the impact that the high list price of insulin is having on patients, and how out-of-pocket expenses are limiting access to this life-saving drug. The second hearing, expected to occur during the week of April 8 (the date had not been scheduled as of press time), will bring together various players in the supply chain, including the three major manufacturers of insulin.
“We are confident that our new program will remove cost as a barrier for people in participating plans who need insulin,” Steve Miller, MD, executive vice president and chief clinical officer at Cigna, said in a statement.
The Express Scripts spokeswoman noted that there were more than 700,000 people in a commercially insured plan across Cigna and Express Scripts who had a claim for insulin in 2018. The average out-of-pocket cost of a 30-day supply of insulin in 2018 across this population was $41.50.
Aspirin for primary prevention: USPSTF recommendations for CVD and colorectal cancer
Which patients are likely to benefit from using aspirin for primary prevention? In this article, we review the evidence to date, summarized for primary care settings in guidelines issued by the US Preventive Services Task Force (USPSTF). We supplement this summary with a rundown of the risks associated with aspirin use. And then we wrap up by identifying a clinical decision tool that is available to help make personalized decisions in a busy clinic setting, where determining an individual’s potential cardiovascular benefits and bleeding risk can be challenging.
The “roadmap” from the guidelines. In 2014, after performing a review of the literature, the US Food and Drug Administration recommended against the routine use of aspirin for primary prevention of cardiovascular disease (CVD).1 In 2016, the USPSTF published 4 separate systematic reviews along with a decision analysis using a microsimulation model, which informed their position statement on aspirin for primary prevention.2-6 These USPSTF reviews and recommendations incorporated both CVD and colorectal cancer (CRC) benefits with the bleeding risks from aspirin. Generally, for individuals 50 to 59 years old, the benefits are deemed to outweigh the harms; shared decision making is advised with those 60 to 69 years of age. For patients younger than 50 or 70 and older, evidence is inconclusive.
The benefits of primary prevention with aspirin
Cardiovascular disease
The Antithrombotic Trialists’ (ATT) Collaboration was one of the first meta-analyses that addressed the benefit-to-harm balance and called into question the routine use of aspirin for primary prevention.7 The USPSTF systematic review included the studies from the ATT Collaboration as well as trials performed after its publication, bringing the total number of eligible randomized controlled trials reviewed to 11.2
The benefit of aspirin for primary prevention of nonfatal myocardial infarction (MI) has been shown in multiple randomized controlled trials. The USPSTF systematic review showed a statistically significant relative risk reduction of 17% in patients taking low-dose aspirin (≤ 100 mg; relative risk [RR] = 0.83; confidence interval [95% CI], 0.74-0.94), although the heterogeneity of the studies was high. The same low dose of aspirin showed a statistically significant reduction in nonfatal stroke (RR = 0.86; 95% CI, 0.76-0.98), although the same benefit was not observed when all doses of aspirin were included. Cardiovascular disease mortality and all-cause mortality were not statistically different for patients taking low-dose aspirin when compared with placebo (RR = 0.97; 95% CI, 0.85-1.10 for CVD mortality; RR = 0.95; 95% CI, 0.89-1.01 for all-cause mortality).2
One study of more than 14,000 older (≥ 60 years) Japanese patients showed a statistically significant reduction in nonfatal MI (hazard ratio [HR] = 0.53; 95% CI, 0.31-0.91, P = .02) and nonfatal strokes (HR = 0.57; 95% CI, 0.32-0.99; P = .04). The study was stopped early because at 5 years of follow-up there was no statistically significant difference in a composite primary outcome, which included death from cardiovascular causes, nonfatal MI, and nonfatal stroke (HR = 0.94; 95% CI, 0.77-1.15; P = .54).8
Several recent landmark studies have called into question the benefit of aspirin for cardiovascular primary prevention, especially in obese individuals, patients with diabetes, and the elderly. A meta-analysis of 10 trials showed that the effectiveness of aspirin doses between 75 mg and 100 mg for primary prevention decreased as weight increased; patients weighing 70 kg or more received no benefit.9 The ASCEND (A Study of Cardiovascular Events in Diabetes) trial included more than 15,000 patients with diabetes but no cardiovascular disease. Patients randomized to receive the low-dose aspirin did have fewer serious vascular events (incidence rate ratio [IRR] = 0.88; 95% CI, 0.79-0.97; P = .01), but they also had high risk of major bleeding events (IRR = 1.29; 95% CI, 1.09-1.52; P = .003).10 The ASPREE (Aspirin in Reducing Events in the Elderly) trial included more than 19,000 patients ages 70 years and older with no cardiovascular disease and compared low-dose aspirin to placebo. There was no statistically significant cardiovascular benefit, although there was an increase of major hemorrhage (HR = 1.38; 95% CI, 1.18-1.62; P < .001).11 The ARRIVE (A Randomized Trial of Induction Versus Expectant Management) trial included 12,546 moderate atherosclerotic CVD (ASCVD) risk patients. Although a per-protocol analysis showed a decrease in rates of fatal and nonfatal MI (HR = 0.53; 95% CI, 0.36-0.79; P = .0014), the more reliable intention-to-treat analysis showed no improvement for any outcomes.12
[polldaddy:10286821]
Colorectal cancer
The literature base on prevention of cancer has been growing rapidly. However, the deluge of findings over the past 2 decades of trials and analyses has also introduced ambiguity and, often, conflicting results. The first journal article suggesting aspirin for primary prevention of cancer, published in 1988, was a case-control study wherein a population with CRC was matched to controls to look for potential protective factors.13 The most notable finding was the CRC risk reduction for those taking aspirin or aspirin-containing medications. Since then numerous studies and analyses have explored aspirin’s potential in primary prevention of many types of cancer, with overall unclear findings as denoted in the 2016 USPSTF systemic reviews and recommendations.
Continue to: One major limiting factor...
One major limiting factor is that most data come from CVD prevention trials, and only a limited number of trials have focused specifically on cancer prevention. For the USPSTF, these data showed no statistically significant risk reduction in overall cancer mortality (RR = 0.96; 95% CI, 0.87-1.06) or in total cancer incidence (RR = 0.98; 95% CI, 0.93-1.04).4 Other ongoing trials may yield more definitive data.14
The particular interest in CRC was due to it being the first cancer found to be preventable with aspirin therapy. The USPSTF, while acknowledging the homogeneous nature of supporting studies, noted that their significant number and resulting evidence made CRC the only cancer warranting evaluation. Population studies have now shown more benefit than the few randomized control trials. The Women’s Health Study and the Physicians’ Health Study were both limited by their duration. But such studies conducted over a longer period revealed notable benefits in the second decade of use, with a statistically significant lower CRC incidence (RR = 0.60; 95% CI, 0.47-0.76). Additionally, CRC mortality at 20 years was decreased in patients taking aspirin regularly (RR = 0.67; 95% CI, 0.52-0.86).4 Multiple studies are in progress to better establish aspirin’s CRC benefit.
While not directly applicable to the general population, use of aspirin for patients with Lynch syndrome to prevent CRC has strong supporting evidence.15 Beyond CRC, there is nascent evidence from limited observational studies that aspirin may have a preventive effect on melanoma and ovarian and pancreatic cancers.16-18 Further studies or compilations of data would be needed to draw more significant conclusions on other types of cancers. Larger studies would prove more difficult to do, given the smaller incidences of these cancers.
Interestingly, a recent study showed that for individuals 70 years and older, aspirin might increase the risk for all-cause mortality, primarily due to increased cancer mortality across all types.19 Although this result was unexpected, caution should be used when prescribing aspirin particularly for patients 70 or older with active cancer.
A look at the harms associated with aspirin use
Aspirin has long been known to cause clinically significant bleeding. Aspirin inhibits platelet-derived cyclooxygenase-1 (COX-1), a potent vasoconstrictor, and thereby decreases platelet aggregation, reducing thromboembolic potential and prolonging bleeding time. These effects can confer health benefits but also carry the potential for risks. A decision to initiate aspirin therapy for primary prevention relies on an understanding of the benefit-to-harm balance.
Continue to: Initial aspirin studies...
Initial aspirin studies did not show a statistically significant increase in bleeding, likely due to too few events and inadequate powering. Subsequent meta-analyses from multiple evaluations have consistently shown bleeding to be a risk.3,7 The risk for bleeding with aspirin has also been examined in multiple cohort studies, which has helped elucidate the risk in greater detail.
Gastrointestinal bleeding
Epidemiologic data show that among patients who do not use nonsteroidal anti-inflammatory drugs (NSAIDs), the rate of upper gastrointestinal (GI) complications is 1 case per 1000 patient-years.20 Multiple studies have consistently shown that aspirin use increases the rate of significant upper GI bleeding over baseline risk (odds ratio [OR] = 1.54-1.58).3,21,22 Interestingly, these increases seem not to be influenced by other factors, such as comorbidities that increase the risk for ASCVD. Analysis of cancer prevention studies showed similar epidemiologic trends, with aspirin use exceeding a baseline bleeding risk of 0.7 cases of upper GI complications per 1000 patient-years (
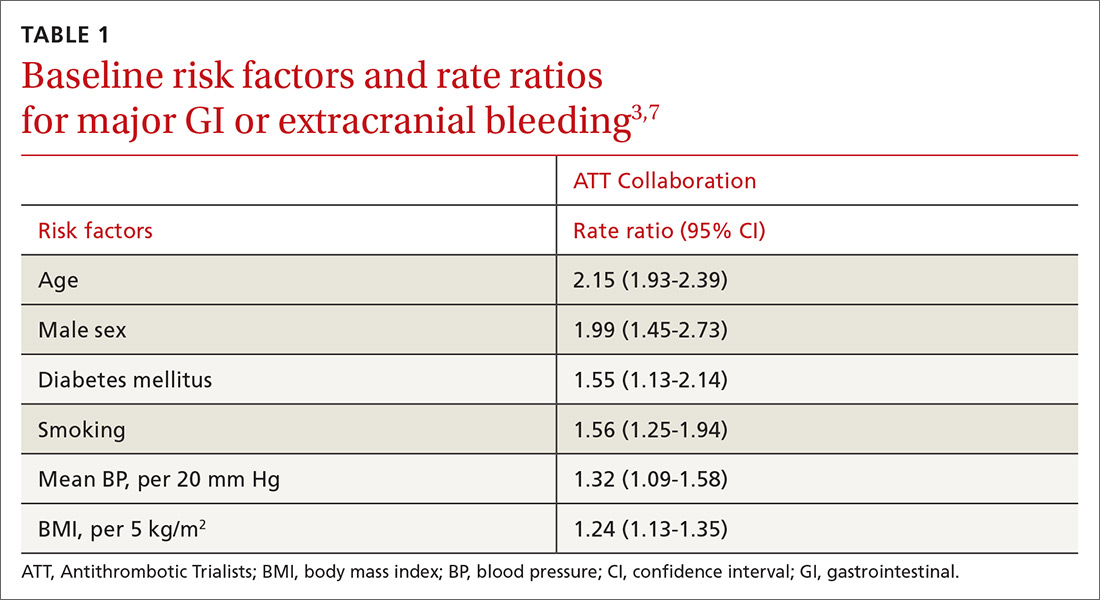
Other risk factors. Evaluation of risk factors for bleeding primarily comes from 2 studies.3,7 Most data concern the impact of individual factors on significant GI bleeding, with fewer data available for evaluating risk for intracerebral hemorrhage (ICH). Initial analysis of individual prospective studies showed little or no correlation between risk for bleeding and such factors as gender, age, or history of hypertension or ASCVD.21 Subsequent analysis of meta-data and large cohorts did show statistically significant impact on rates of bleeding across several factors (TABLE 13,7).
Of note is a large heterogeneous cohort study conducted in Spain. Data showed significant increases in baseline risk for GI bleeding in older men with a history of GI bleeding and NSAID use. The absolute risk for GI bleed in this group was potentially as high as 150 cases per 1000 patient-years, well above the risk level assumed for the average patient.24 A seemingly small OR of 1.5 could dramatically increase the absolute risk for bleeding in such patients, and it suggests that a generalized risk for bleeding probably shouldn’t be applied to all patients. Individuals may be better served by a baseline risk calculation reflecting multiple factors.
Intracerebral hemorrhage
Due to the comparatively uncommon nature of ICH, fewer data are available to support definitive conclusions about its increased risk with aspirin use. Aspirin use appeared to increase the risk for ICH with ratios between 1.27 and 1.32 in meta-analyses (measured as an OR or as an RR),3,7,21 with an IRR of 1.54 in a cohort study.22 The only statistically significant factors suspected to increase the risk of ICH at baseline were smoking (RR = 2.18) and mean BP > 20 mm Hg over normal (OR = 2.18). Age, gender, and diabetes all showed a nonsignificant trend toward risk increase.7
Continue to: Risk based on dose and formulation
Risk based on dose and formulation
The effect of aspirin dose and formulation on bleeding risk is uncertain. Some studies have shown an increased risk for bleeding with daily doses of aspirin ≥ 300 mg, while others have shown no significant increase in rates for bleeding with differing doses.21,25 Enteric coating does appear to lower the rates of gastric mucosal injury, although there are few data on the effect toward reducing clinically significant bleeding.26 Currently, several prospective studies are underway to help clarify the evidence.27
Putting it all together
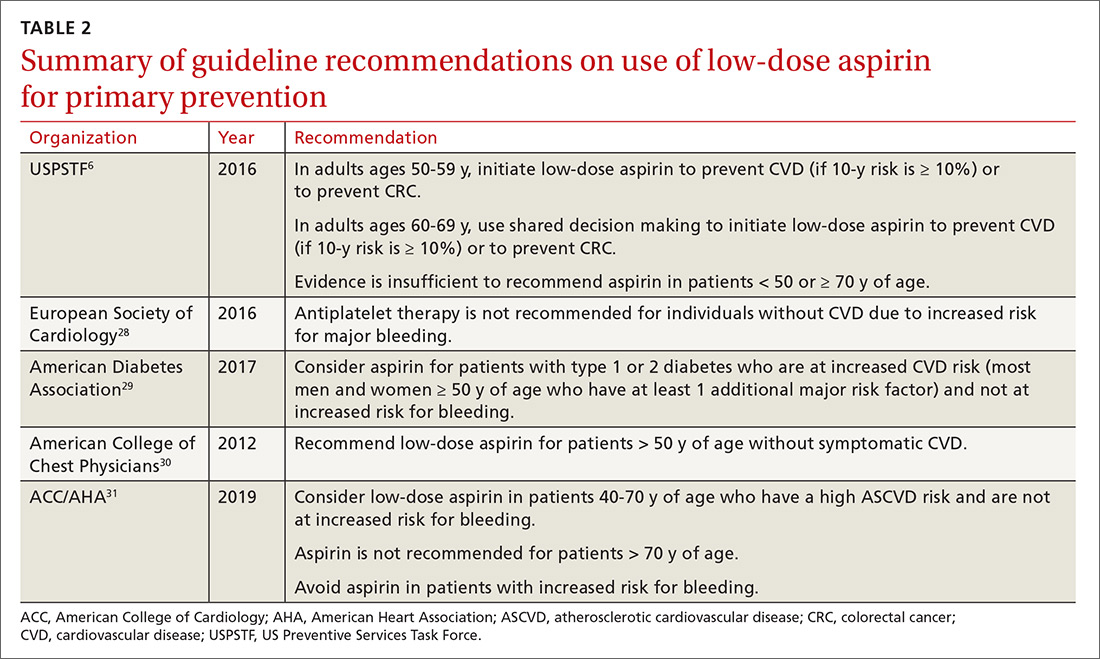
For the general population, the evidence shows that the benefits and harms of aspirin for primary prevention are relatively even. The USPSTF guidelines are the first to recommend aspirin for both CVD and cancer prevention while taking into account the bleeding risk. According to the findings of the USPSTF, the balance of benefits and harms of aspirin use is contingent on 4 factors: age, baseline CVD risk, risk for bleeding, and preferences about taking aspirin.6 The complete recommendations from the USPSTF, along with other leading organizations, are outlined in TABLE 2.6,28-31
Applying the evidence and varying guidelines in practice can feel daunting. Some practical tools have been developed to help clinicians understand patients’ bleeding risk and potential benefits with aspirin use. One such tool is highlighted below. Others are also available, and each has its own strengths and weaknesses.
Aspirin-Guide (www.aspiringuide.com) is a Web-based clinical decision support tool with an associated mobile application. It uses internal calculators (including the pooled cohort calculator prepared jointly by the American College of Cardiology and the American Heart Association) to assess CVD risk as well as bleeding risk. This tool gives clinicians patient-specific numbers-needed-to-treat and numbers-needed-to-harm when considering starting aspirin for primary prevention. It gives specific recommendations for aspirin use based on the data entered, and it also gives providers information to help guide shared decision-making with patients.32 Unfortunately, this decision support tool and others do not take into account the data from the most recent trials, so they should be used with caution.
CORRESPONDENCE
LCDR Dustin K. Smith, DO, Naval Branch Clinic Diego Garcia, PSC 466, Box 301, FPO, AP 96595; [email protected].
1. FDA. Use of aspirin for primary prevention of heart attack and stroke. https://www.fda.gov/Drugs/ResourcesForYou/Consumers/ucm390574.htm. Accessed March 22, 2019.
2. Guirguis-Blake JM, Evans CV, Senger CA, et al. Aspirin for the primary prevention of cardiovascular events: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:804-813.
3. Whitlock EP, Burda BU, Williams SB, et al. Bleeding risks with aspirin use for primary prevention in adults: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:826-835.
4. Chubak J, Whitlock EP, Williams SB, et al. Aspirin for the prevention of cancer incidence and mortality: systematic evidence reviews for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:814-825.
5. Dehmer SP, Maciosek MV, Flottemesch TJ, et al. Aspirin for the primary prevention of cardiovascular disease and colorectal cancer: a decision analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:777-786.
6. Bibbins-Domingo K. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2016;164:836-845.
7. Baigent C, Blackwell L, Colins R, et al; Antithrombotic Trialists (ATT) Collaboration. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participation data from randomised trials. Lancet. 2009:373:1849-1860.
8. Ikeda Y, Shimada K, Teramoto T, et al. Low-dose aspirin for primary prevention of cardiovascular events in Japanese patients 60 years or older with atherosclerotic risk factors: a randomized clinical trial. JAMA. 2014;312:2510-2520.
9. Rothwell PM, Cook NR, Gaziano JM, et al. Effects of aspirin on risks of vascular events and cancer according to bodyweight and dose: analysis of individual patient data from randomised trials. Lancet. 2018;392:387-399.
10. Bowman L, Mafham M, Wallendszus K, et al; ASCEND Study Collaborative Group. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379:1529-1539.
11. McNeil JJ, Wolfe R, Woods RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379:1509-1518.
12. Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet. 2018;392:1036-1046.
13. Kune GA, Kune S, Watson LF. Colorectal cancer risk, chronic illness, operations, and medications: case control results from Melbourne Colorectal Cancer Study. Cancer Res. 1988;48:4399-4404.
14. Sutcliffe P, Connock M, Gurung T, et al. Aspirin for prophylactic use in the primary prevention of cardiovascular disease and cancer: a systematic review and overview of reviews. Health Technol Assess. 2013;17:1-253.
15. Burn J, Gerdes AM, Macrae F, et al. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet. 2011;378:2081-2087.
16. Gamba CA, Swetter SM, Stefanick ML, et al. Aspirin is associated with lower melanoma risk among postmenopausal Caucasian women: the Women’s Health Initiative. Cancer. 2013;119:1562-1569.
17. Trabert B, Ness RB, Lo-Ciganic WH, et al. Aspirin, nonaspirin nonsteroidal anti-inflammatory drug, and acetaminophen use and risk of invasive epithelial ovarian cancer: a pooled analysis in the Ovarian Cancer Association Consortium. J Natl Cancer Inst. 2014;106:djt431.
18. Risch H, Lu L, Streicher SA, et al. Aspirin use and reduced risk of pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2016;26:68-74.
19. McNeil JJ, Nelson MR, Woods RL, et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med. 2018;379:1519-1528.
20. Hernández-Díaz S, Rodríguez LA. Incidence of serious upper gastrointestinal bleeding/perforation in the general population: review of epidemiologic studies. J Clin Epidemiol. 2002;55:157-163.
21. Guirguis-Blake JM, Evans CV, Senger CA, et al. Aspirin for the primary prevention of cardiovascular events: a systematic evidence review for the U.S. Preventive Services Task Force. Evidence Synthesis no 131. Rockville, MD: Agency for Healthcare Research and Quality; 2015. https://www.ncbi.nlm.nih.gov/books/NBK321623/. Accessed March 22, 2019.
22. De Berardis G, Lucisano G, D’Ettorre A, et al. Association of aspirin use with major bleeding in patients with and without diabetes. JAMA. 2012;307:2286-2294.
23. Thorat MA, Cuzick J. Prophylactic use of aspirin: systematic review of harms and approaches to mitigation in the general population. Eur J Epidemiol. 2015;30:5-18.
24. Hernández-Díaz S, García Rodríguez LA. Cardioprotective aspirin users and their excess risk of upper gastrointestinal complications. BMC Med. 2006;4:22.
25. Huang ES, Strate LL, Ho WW, et al. Long term use of aspirin and the risk of gastrointestinal bleeding. Am J Med. 2011:124;426-433.
26. Walker J, Robinson J, Stewart J, et al. Does enteric-coated aspirin result in a lower incidence of gastrointestinal complications compared to normal aspirin? Interact Cardiovasc Thorac Surg. 2007:6;519-522.
27. NIH. Aspirin dosing: a patient-centric trial assessing benefits and long-term effectiveness (ADAPTABLE). https://clinicaltrials.gov/ct2/show/NCT02697916. Accessed March 22, 2019.
28. Piepoli MF, Hoes AW, Agewall S, et al. 2016 European guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. Eur Heart J. 2016;37:2315-2381.
29. ADA. Standards of medical care in diabetes – 2017. Diabetes Care. 2017;40(suppl 1). http://care.diabetesjournals.org/content/diacare/suppl/2016/12/15/40.Supplement_1.DC1/DC_40_S1_final.pdf. Accessed March 22, 2019.
30. Vandvik PO, Lincoff AM, Gore JM, et al. Primary and secondary prevention of cardiovascular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl):e637S-e668S.
31. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease. J Am Col Cardiol. 2019. doi: https://doi.org/10.1016/j.jacc.2019.03.010. Accessed March 22, 2019.
32. Mora S, Manson JE. Aspirin for primary prevention of atherosclerotic cardiovascular disease: advances in diagnosis and treatment. JAMA Intern Med. 2016;176:1195-1204.
Which patients are likely to benefit from using aspirin for primary prevention? In this article, we review the evidence to date, summarized for primary care settings in guidelines issued by the US Preventive Services Task Force (USPSTF). We supplement this summary with a rundown of the risks associated with aspirin use. And then we wrap up by identifying a clinical decision tool that is available to help make personalized decisions in a busy clinic setting, where determining an individual’s potential cardiovascular benefits and bleeding risk can be challenging.
The “roadmap” from the guidelines. In 2014, after performing a review of the literature, the US Food and Drug Administration recommended against the routine use of aspirin for primary prevention of cardiovascular disease (CVD).1 In 2016, the USPSTF published 4 separate systematic reviews along with a decision analysis using a microsimulation model, which informed their position statement on aspirin for primary prevention.2-6 These USPSTF reviews and recommendations incorporated both CVD and colorectal cancer (CRC) benefits with the bleeding risks from aspirin. Generally, for individuals 50 to 59 years old, the benefits are deemed to outweigh the harms; shared decision making is advised with those 60 to 69 years of age. For patients younger than 50 or 70 and older, evidence is inconclusive.
The benefits of primary prevention with aspirin
Cardiovascular disease
The Antithrombotic Trialists’ (ATT) Collaboration was one of the first meta-analyses that addressed the benefit-to-harm balance and called into question the routine use of aspirin for primary prevention.7 The USPSTF systematic review included the studies from the ATT Collaboration as well as trials performed after its publication, bringing the total number of eligible randomized controlled trials reviewed to 11.2
The benefit of aspirin for primary prevention of nonfatal myocardial infarction (MI) has been shown in multiple randomized controlled trials. The USPSTF systematic review showed a statistically significant relative risk reduction of 17% in patients taking low-dose aspirin (≤ 100 mg; relative risk [RR] = 0.83; confidence interval [95% CI], 0.74-0.94), although the heterogeneity of the studies was high. The same low dose of aspirin showed a statistically significant reduction in nonfatal stroke (RR = 0.86; 95% CI, 0.76-0.98), although the same benefit was not observed when all doses of aspirin were included. Cardiovascular disease mortality and all-cause mortality were not statistically different for patients taking low-dose aspirin when compared with placebo (RR = 0.97; 95% CI, 0.85-1.10 for CVD mortality; RR = 0.95; 95% CI, 0.89-1.01 for all-cause mortality).2
One study of more than 14,000 older (≥ 60 years) Japanese patients showed a statistically significant reduction in nonfatal MI (hazard ratio [HR] = 0.53; 95% CI, 0.31-0.91, P = .02) and nonfatal strokes (HR = 0.57; 95% CI, 0.32-0.99; P = .04). The study was stopped early because at 5 years of follow-up there was no statistically significant difference in a composite primary outcome, which included death from cardiovascular causes, nonfatal MI, and nonfatal stroke (HR = 0.94; 95% CI, 0.77-1.15; P = .54).8
Several recent landmark studies have called into question the benefit of aspirin for cardiovascular primary prevention, especially in obese individuals, patients with diabetes, and the elderly. A meta-analysis of 10 trials showed that the effectiveness of aspirin doses between 75 mg and 100 mg for primary prevention decreased as weight increased; patients weighing 70 kg or more received no benefit.9 The ASCEND (A Study of Cardiovascular Events in Diabetes) trial included more than 15,000 patients with diabetes but no cardiovascular disease. Patients randomized to receive the low-dose aspirin did have fewer serious vascular events (incidence rate ratio [IRR] = 0.88; 95% CI, 0.79-0.97; P = .01), but they also had high risk of major bleeding events (IRR = 1.29; 95% CI, 1.09-1.52; P = .003).10 The ASPREE (Aspirin in Reducing Events in the Elderly) trial included more than 19,000 patients ages 70 years and older with no cardiovascular disease and compared low-dose aspirin to placebo. There was no statistically significant cardiovascular benefit, although there was an increase of major hemorrhage (HR = 1.38; 95% CI, 1.18-1.62; P < .001).11 The ARRIVE (A Randomized Trial of Induction Versus Expectant Management) trial included 12,546 moderate atherosclerotic CVD (ASCVD) risk patients. Although a per-protocol analysis showed a decrease in rates of fatal and nonfatal MI (HR = 0.53; 95% CI, 0.36-0.79; P = .0014), the more reliable intention-to-treat analysis showed no improvement for any outcomes.12
[polldaddy:10286821]
Colorectal cancer
The literature base on prevention of cancer has been growing rapidly. However, the deluge of findings over the past 2 decades of trials and analyses has also introduced ambiguity and, often, conflicting results. The first journal article suggesting aspirin for primary prevention of cancer, published in 1988, was a case-control study wherein a population with CRC was matched to controls to look for potential protective factors.13 The most notable finding was the CRC risk reduction for those taking aspirin or aspirin-containing medications. Since then numerous studies and analyses have explored aspirin’s potential in primary prevention of many types of cancer, with overall unclear findings as denoted in the 2016 USPSTF systemic reviews and recommendations.
Continue to: One major limiting factor...
One major limiting factor is that most data come from CVD prevention trials, and only a limited number of trials have focused specifically on cancer prevention. For the USPSTF, these data showed no statistically significant risk reduction in overall cancer mortality (RR = 0.96; 95% CI, 0.87-1.06) or in total cancer incidence (RR = 0.98; 95% CI, 0.93-1.04).4 Other ongoing trials may yield more definitive data.14
The particular interest in CRC was due to it being the first cancer found to be preventable with aspirin therapy. The USPSTF, while acknowledging the homogeneous nature of supporting studies, noted that their significant number and resulting evidence made CRC the only cancer warranting evaluation. Population studies have now shown more benefit than the few randomized control trials. The Women’s Health Study and the Physicians’ Health Study were both limited by their duration. But such studies conducted over a longer period revealed notable benefits in the second decade of use, with a statistically significant lower CRC incidence (RR = 0.60; 95% CI, 0.47-0.76). Additionally, CRC mortality at 20 years was decreased in patients taking aspirin regularly (RR = 0.67; 95% CI, 0.52-0.86).4 Multiple studies are in progress to better establish aspirin’s CRC benefit.
While not directly applicable to the general population, use of aspirin for patients with Lynch syndrome to prevent CRC has strong supporting evidence.15 Beyond CRC, there is nascent evidence from limited observational studies that aspirin may have a preventive effect on melanoma and ovarian and pancreatic cancers.16-18 Further studies or compilations of data would be needed to draw more significant conclusions on other types of cancers. Larger studies would prove more difficult to do, given the smaller incidences of these cancers.
Interestingly, a recent study showed that for individuals 70 years and older, aspirin might increase the risk for all-cause mortality, primarily due to increased cancer mortality across all types.19 Although this result was unexpected, caution should be used when prescribing aspirin particularly for patients 70 or older with active cancer.
A look at the harms associated with aspirin use
Aspirin has long been known to cause clinically significant bleeding. Aspirin inhibits platelet-derived cyclooxygenase-1 (COX-1), a potent vasoconstrictor, and thereby decreases platelet aggregation, reducing thromboembolic potential and prolonging bleeding time. These effects can confer health benefits but also carry the potential for risks. A decision to initiate aspirin therapy for primary prevention relies on an understanding of the benefit-to-harm balance.
Continue to: Initial aspirin studies...
Initial aspirin studies did not show a statistically significant increase in bleeding, likely due to too few events and inadequate powering. Subsequent meta-analyses from multiple evaluations have consistently shown bleeding to be a risk.3,7 The risk for bleeding with aspirin has also been examined in multiple cohort studies, which has helped elucidate the risk in greater detail.
Gastrointestinal bleeding
Epidemiologic data show that among patients who do not use nonsteroidal anti-inflammatory drugs (NSAIDs), the rate of upper gastrointestinal (GI) complications is 1 case per 1000 patient-years.20 Multiple studies have consistently shown that aspirin use increases the rate of significant upper GI bleeding over baseline risk (odds ratio [OR] = 1.54-1.58).3,21,22 Interestingly, these increases seem not to be influenced by other factors, such as comorbidities that increase the risk for ASCVD. Analysis of cancer prevention studies showed similar epidemiologic trends, with aspirin use exceeding a baseline bleeding risk of 0.7 cases of upper GI complications per 1000 patient-years (

Other risk factors. Evaluation of risk factors for bleeding primarily comes from 2 studies.3,7 Most data concern the impact of individual factors on significant GI bleeding, with fewer data available for evaluating risk for intracerebral hemorrhage (ICH). Initial analysis of individual prospective studies showed little or no correlation between risk for bleeding and such factors as gender, age, or history of hypertension or ASCVD.21 Subsequent analysis of meta-data and large cohorts did show statistically significant impact on rates of bleeding across several factors (TABLE 13,7).
Of note is a large heterogeneous cohort study conducted in Spain. Data showed significant increases in baseline risk for GI bleeding in older men with a history of GI bleeding and NSAID use. The absolute risk for GI bleed in this group was potentially as high as 150 cases per 1000 patient-years, well above the risk level assumed for the average patient.24 A seemingly small OR of 1.5 could dramatically increase the absolute risk for bleeding in such patients, and it suggests that a generalized risk for bleeding probably shouldn’t be applied to all patients. Individuals may be better served by a baseline risk calculation reflecting multiple factors.
Intracerebral hemorrhage
Due to the comparatively uncommon nature of ICH, fewer data are available to support definitive conclusions about its increased risk with aspirin use. Aspirin use appeared to increase the risk for ICH with ratios between 1.27 and 1.32 in meta-analyses (measured as an OR or as an RR),3,7,21 with an IRR of 1.54 in a cohort study.22 The only statistically significant factors suspected to increase the risk of ICH at baseline were smoking (RR = 2.18) and mean BP > 20 mm Hg over normal (OR = 2.18). Age, gender, and diabetes all showed a nonsignificant trend toward risk increase.7
Continue to: Risk based on dose and formulation
Risk based on dose and formulation
The effect of aspirin dose and formulation on bleeding risk is uncertain. Some studies have shown an increased risk for bleeding with daily doses of aspirin ≥ 300 mg, while others have shown no significant increase in rates for bleeding with differing doses.21,25 Enteric coating does appear to lower the rates of gastric mucosal injury, although there are few data on the effect toward reducing clinically significant bleeding.26 Currently, several prospective studies are underway to help clarify the evidence.27
Putting it all together
For the general population, the evidence shows that the benefits and harms of aspirin for primary prevention are relatively even. The USPSTF guidelines are the first to recommend aspirin for both CVD and cancer prevention while taking into account the bleeding risk. According to the findings of the USPSTF, the balance of benefits and harms of aspirin use is contingent on 4 factors: age, baseline CVD risk, risk for bleeding, and preferences about taking aspirin.6 The complete recommendations from the USPSTF, along with other leading organizations, are outlined in TABLE 2.6,28-31

Applying the evidence and varying guidelines in practice can feel daunting. Some practical tools have been developed to help clinicians understand patients’ bleeding risk and potential benefits with aspirin use. One such tool is highlighted below. Others are also available, and each has its own strengths and weaknesses.
Aspirin-Guide (www.aspiringuide.com) is a Web-based clinical decision support tool with an associated mobile application. It uses internal calculators (including the pooled cohort calculator prepared jointly by the American College of Cardiology and the American Heart Association) to assess CVD risk as well as bleeding risk. This tool gives clinicians patient-specific numbers-needed-to-treat and numbers-needed-to-harm when considering starting aspirin for primary prevention. It gives specific recommendations for aspirin use based on the data entered, and it also gives providers information to help guide shared decision-making with patients.32 Unfortunately, this decision support tool and others do not take into account the data from the most recent trials, so they should be used with caution.
CORRESPONDENCE
LCDR Dustin K. Smith, DO, Naval Branch Clinic Diego Garcia, PSC 466, Box 301, FPO, AP 96595; [email protected].
Which patients are likely to benefit from using aspirin for primary prevention? In this article, we review the evidence to date, summarized for primary care settings in guidelines issued by the US Preventive Services Task Force (USPSTF). We supplement this summary with a rundown of the risks associated with aspirin use. And then we wrap up by identifying a clinical decision tool that is available to help make personalized decisions in a busy clinic setting, where determining an individual’s potential cardiovascular benefits and bleeding risk can be challenging.
The “roadmap” from the guidelines. In 2014, after performing a review of the literature, the US Food and Drug Administration recommended against the routine use of aspirin for primary prevention of cardiovascular disease (CVD).1 In 2016, the USPSTF published 4 separate systematic reviews along with a decision analysis using a microsimulation model, which informed their position statement on aspirin for primary prevention.2-6 These USPSTF reviews and recommendations incorporated both CVD and colorectal cancer (CRC) benefits with the bleeding risks from aspirin. Generally, for individuals 50 to 59 years old, the benefits are deemed to outweigh the harms; shared decision making is advised with those 60 to 69 years of age. For patients younger than 50 or 70 and older, evidence is inconclusive.
The benefits of primary prevention with aspirin
Cardiovascular disease
The Antithrombotic Trialists’ (ATT) Collaboration was one of the first meta-analyses that addressed the benefit-to-harm balance and called into question the routine use of aspirin for primary prevention.7 The USPSTF systematic review included the studies from the ATT Collaboration as well as trials performed after its publication, bringing the total number of eligible randomized controlled trials reviewed to 11.2
The benefit of aspirin for primary prevention of nonfatal myocardial infarction (MI) has been shown in multiple randomized controlled trials. The USPSTF systematic review showed a statistically significant relative risk reduction of 17% in patients taking low-dose aspirin (≤ 100 mg; relative risk [RR] = 0.83; confidence interval [95% CI], 0.74-0.94), although the heterogeneity of the studies was high. The same low dose of aspirin showed a statistically significant reduction in nonfatal stroke (RR = 0.86; 95% CI, 0.76-0.98), although the same benefit was not observed when all doses of aspirin were included. Cardiovascular disease mortality and all-cause mortality were not statistically different for patients taking low-dose aspirin when compared with placebo (RR = 0.97; 95% CI, 0.85-1.10 for CVD mortality; RR = 0.95; 95% CI, 0.89-1.01 for all-cause mortality).2
One study of more than 14,000 older (≥ 60 years) Japanese patients showed a statistically significant reduction in nonfatal MI (hazard ratio [HR] = 0.53; 95% CI, 0.31-0.91, P = .02) and nonfatal strokes (HR = 0.57; 95% CI, 0.32-0.99; P = .04). The study was stopped early because at 5 years of follow-up there was no statistically significant difference in a composite primary outcome, which included death from cardiovascular causes, nonfatal MI, and nonfatal stroke (HR = 0.94; 95% CI, 0.77-1.15; P = .54).8
Several recent landmark studies have called into question the benefit of aspirin for cardiovascular primary prevention, especially in obese individuals, patients with diabetes, and the elderly. A meta-analysis of 10 trials showed that the effectiveness of aspirin doses between 75 mg and 100 mg for primary prevention decreased as weight increased; patients weighing 70 kg or more received no benefit.9 The ASCEND (A Study of Cardiovascular Events in Diabetes) trial included more than 15,000 patients with diabetes but no cardiovascular disease. Patients randomized to receive the low-dose aspirin did have fewer serious vascular events (incidence rate ratio [IRR] = 0.88; 95% CI, 0.79-0.97; P = .01), but they also had high risk of major bleeding events (IRR = 1.29; 95% CI, 1.09-1.52; P = .003).10 The ASPREE (Aspirin in Reducing Events in the Elderly) trial included more than 19,000 patients ages 70 years and older with no cardiovascular disease and compared low-dose aspirin to placebo. There was no statistically significant cardiovascular benefit, although there was an increase of major hemorrhage (HR = 1.38; 95% CI, 1.18-1.62; P < .001).11 The ARRIVE (A Randomized Trial of Induction Versus Expectant Management) trial included 12,546 moderate atherosclerotic CVD (ASCVD) risk patients. Although a per-protocol analysis showed a decrease in rates of fatal and nonfatal MI (HR = 0.53; 95% CI, 0.36-0.79; P = .0014), the more reliable intention-to-treat analysis showed no improvement for any outcomes.12
[polldaddy:10286821]
Colorectal cancer
The literature base on prevention of cancer has been growing rapidly. However, the deluge of findings over the past 2 decades of trials and analyses has also introduced ambiguity and, often, conflicting results. The first journal article suggesting aspirin for primary prevention of cancer, published in 1988, was a case-control study wherein a population with CRC was matched to controls to look for potential protective factors.13 The most notable finding was the CRC risk reduction for those taking aspirin or aspirin-containing medications. Since then numerous studies and analyses have explored aspirin’s potential in primary prevention of many types of cancer, with overall unclear findings as denoted in the 2016 USPSTF systemic reviews and recommendations.
Continue to: One major limiting factor...
One major limiting factor is that most data come from CVD prevention trials, and only a limited number of trials have focused specifically on cancer prevention. For the USPSTF, these data showed no statistically significant risk reduction in overall cancer mortality (RR = 0.96; 95% CI, 0.87-1.06) or in total cancer incidence (RR = 0.98; 95% CI, 0.93-1.04).4 Other ongoing trials may yield more definitive data.14
The particular interest in CRC was due to it being the first cancer found to be preventable with aspirin therapy. The USPSTF, while acknowledging the homogeneous nature of supporting studies, noted that their significant number and resulting evidence made CRC the only cancer warranting evaluation. Population studies have now shown more benefit than the few randomized control trials. The Women’s Health Study and the Physicians’ Health Study were both limited by their duration. But such studies conducted over a longer period revealed notable benefits in the second decade of use, with a statistically significant lower CRC incidence (RR = 0.60; 95% CI, 0.47-0.76). Additionally, CRC mortality at 20 years was decreased in patients taking aspirin regularly (RR = 0.67; 95% CI, 0.52-0.86).4 Multiple studies are in progress to better establish aspirin’s CRC benefit.
While not directly applicable to the general population, use of aspirin for patients with Lynch syndrome to prevent CRC has strong supporting evidence.15 Beyond CRC, there is nascent evidence from limited observational studies that aspirin may have a preventive effect on melanoma and ovarian and pancreatic cancers.16-18 Further studies or compilations of data would be needed to draw more significant conclusions on other types of cancers. Larger studies would prove more difficult to do, given the smaller incidences of these cancers.
Interestingly, a recent study showed that for individuals 70 years and older, aspirin might increase the risk for all-cause mortality, primarily due to increased cancer mortality across all types.19 Although this result was unexpected, caution should be used when prescribing aspirin particularly for patients 70 or older with active cancer.
A look at the harms associated with aspirin use
Aspirin has long been known to cause clinically significant bleeding. Aspirin inhibits platelet-derived cyclooxygenase-1 (COX-1), a potent vasoconstrictor, and thereby decreases platelet aggregation, reducing thromboembolic potential and prolonging bleeding time. These effects can confer health benefits but also carry the potential for risks. A decision to initiate aspirin therapy for primary prevention relies on an understanding of the benefit-to-harm balance.
Continue to: Initial aspirin studies...
Initial aspirin studies did not show a statistically significant increase in bleeding, likely due to too few events and inadequate powering. Subsequent meta-analyses from multiple evaluations have consistently shown bleeding to be a risk.3,7 The risk for bleeding with aspirin has also been examined in multiple cohort studies, which has helped elucidate the risk in greater detail.
Gastrointestinal bleeding
Epidemiologic data show that among patients who do not use nonsteroidal anti-inflammatory drugs (NSAIDs), the rate of upper gastrointestinal (GI) complications is 1 case per 1000 patient-years.20 Multiple studies have consistently shown that aspirin use increases the rate of significant upper GI bleeding over baseline risk (odds ratio [OR] = 1.54-1.58).3,21,22 Interestingly, these increases seem not to be influenced by other factors, such as comorbidities that increase the risk for ASCVD. Analysis of cancer prevention studies showed similar epidemiologic trends, with aspirin use exceeding a baseline bleeding risk of 0.7 cases of upper GI complications per 1000 patient-years (

Other risk factors. Evaluation of risk factors for bleeding primarily comes from 2 studies.3,7 Most data concern the impact of individual factors on significant GI bleeding, with fewer data available for evaluating risk for intracerebral hemorrhage (ICH). Initial analysis of individual prospective studies showed little or no correlation between risk for bleeding and such factors as gender, age, or history of hypertension or ASCVD.21 Subsequent analysis of meta-data and large cohorts did show statistically significant impact on rates of bleeding across several factors (TABLE 13,7).
Of note is a large heterogeneous cohort study conducted in Spain. Data showed significant increases in baseline risk for GI bleeding in older men with a history of GI bleeding and NSAID use. The absolute risk for GI bleed in this group was potentially as high as 150 cases per 1000 patient-years, well above the risk level assumed for the average patient.24 A seemingly small OR of 1.5 could dramatically increase the absolute risk for bleeding in such patients, and it suggests that a generalized risk for bleeding probably shouldn’t be applied to all patients. Individuals may be better served by a baseline risk calculation reflecting multiple factors.
Intracerebral hemorrhage
Due to the comparatively uncommon nature of ICH, fewer data are available to support definitive conclusions about its increased risk with aspirin use. Aspirin use appeared to increase the risk for ICH with ratios between 1.27 and 1.32 in meta-analyses (measured as an OR or as an RR),3,7,21 with an IRR of 1.54 in a cohort study.22 The only statistically significant factors suspected to increase the risk of ICH at baseline were smoking (RR = 2.18) and mean BP > 20 mm Hg over normal (OR = 2.18). Age, gender, and diabetes all showed a nonsignificant trend toward risk increase.7
Continue to: Risk based on dose and formulation
Risk based on dose and formulation
The effect of aspirin dose and formulation on bleeding risk is uncertain. Some studies have shown an increased risk for bleeding with daily doses of aspirin ≥ 300 mg, while others have shown no significant increase in rates for bleeding with differing doses.21,25 Enteric coating does appear to lower the rates of gastric mucosal injury, although there are few data on the effect toward reducing clinically significant bleeding.26 Currently, several prospective studies are underway to help clarify the evidence.27
Putting it all together
For the general population, the evidence shows that the benefits and harms of aspirin for primary prevention are relatively even. The USPSTF guidelines are the first to recommend aspirin for both CVD and cancer prevention while taking into account the bleeding risk. According to the findings of the USPSTF, the balance of benefits and harms of aspirin use is contingent on 4 factors: age, baseline CVD risk, risk for bleeding, and preferences about taking aspirin.6 The complete recommendations from the USPSTF, along with other leading organizations, are outlined in TABLE 2.6,28-31

Applying the evidence and varying guidelines in practice can feel daunting. Some practical tools have been developed to help clinicians understand patients’ bleeding risk and potential benefits with aspirin use. One such tool is highlighted below. Others are also available, and each has its own strengths and weaknesses.
Aspirin-Guide (www.aspiringuide.com) is a Web-based clinical decision support tool with an associated mobile application. It uses internal calculators (including the pooled cohort calculator prepared jointly by the American College of Cardiology and the American Heart Association) to assess CVD risk as well as bleeding risk. This tool gives clinicians patient-specific numbers-needed-to-treat and numbers-needed-to-harm when considering starting aspirin for primary prevention. It gives specific recommendations for aspirin use based on the data entered, and it also gives providers information to help guide shared decision-making with patients.32 Unfortunately, this decision support tool and others do not take into account the data from the most recent trials, so they should be used with caution.
CORRESPONDENCE
LCDR Dustin K. Smith, DO, Naval Branch Clinic Diego Garcia, PSC 466, Box 301, FPO, AP 96595; [email protected].
1. FDA. Use of aspirin for primary prevention of heart attack and stroke. https://www.fda.gov/Drugs/ResourcesForYou/Consumers/ucm390574.htm. Accessed March 22, 2019.
2. Guirguis-Blake JM, Evans CV, Senger CA, et al. Aspirin for the primary prevention of cardiovascular events: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:804-813.
3. Whitlock EP, Burda BU, Williams SB, et al. Bleeding risks with aspirin use for primary prevention in adults: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:826-835.
4. Chubak J, Whitlock EP, Williams SB, et al. Aspirin for the prevention of cancer incidence and mortality: systematic evidence reviews for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:814-825.
5. Dehmer SP, Maciosek MV, Flottemesch TJ, et al. Aspirin for the primary prevention of cardiovascular disease and colorectal cancer: a decision analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:777-786.
6. Bibbins-Domingo K. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2016;164:836-845.
7. Baigent C, Blackwell L, Colins R, et al; Antithrombotic Trialists (ATT) Collaboration. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participation data from randomised trials. Lancet. 2009:373:1849-1860.
8. Ikeda Y, Shimada K, Teramoto T, et al. Low-dose aspirin for primary prevention of cardiovascular events in Japanese patients 60 years or older with atherosclerotic risk factors: a randomized clinical trial. JAMA. 2014;312:2510-2520.
9. Rothwell PM, Cook NR, Gaziano JM, et al. Effects of aspirin on risks of vascular events and cancer according to bodyweight and dose: analysis of individual patient data from randomised trials. Lancet. 2018;392:387-399.
10. Bowman L, Mafham M, Wallendszus K, et al; ASCEND Study Collaborative Group. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379:1529-1539.
11. McNeil JJ, Wolfe R, Woods RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379:1509-1518.
12. Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet. 2018;392:1036-1046.
13. Kune GA, Kune S, Watson LF. Colorectal cancer risk, chronic illness, operations, and medications: case control results from Melbourne Colorectal Cancer Study. Cancer Res. 1988;48:4399-4404.
14. Sutcliffe P, Connock M, Gurung T, et al. Aspirin for prophylactic use in the primary prevention of cardiovascular disease and cancer: a systematic review and overview of reviews. Health Technol Assess. 2013;17:1-253.
15. Burn J, Gerdes AM, Macrae F, et al. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet. 2011;378:2081-2087.
16. Gamba CA, Swetter SM, Stefanick ML, et al. Aspirin is associated with lower melanoma risk among postmenopausal Caucasian women: the Women’s Health Initiative. Cancer. 2013;119:1562-1569.
17. Trabert B, Ness RB, Lo-Ciganic WH, et al. Aspirin, nonaspirin nonsteroidal anti-inflammatory drug, and acetaminophen use and risk of invasive epithelial ovarian cancer: a pooled analysis in the Ovarian Cancer Association Consortium. J Natl Cancer Inst. 2014;106:djt431.
18. Risch H, Lu L, Streicher SA, et al. Aspirin use and reduced risk of pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2016;26:68-74.
19. McNeil JJ, Nelson MR, Woods RL, et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med. 2018;379:1519-1528.
20. Hernández-Díaz S, Rodríguez LA. Incidence of serious upper gastrointestinal bleeding/perforation in the general population: review of epidemiologic studies. J Clin Epidemiol. 2002;55:157-163.
21. Guirguis-Blake JM, Evans CV, Senger CA, et al. Aspirin for the primary prevention of cardiovascular events: a systematic evidence review for the U.S. Preventive Services Task Force. Evidence Synthesis no 131. Rockville, MD: Agency for Healthcare Research and Quality; 2015. https://www.ncbi.nlm.nih.gov/books/NBK321623/. Accessed March 22, 2019.
22. De Berardis G, Lucisano G, D’Ettorre A, et al. Association of aspirin use with major bleeding in patients with and without diabetes. JAMA. 2012;307:2286-2294.
23. Thorat MA, Cuzick J. Prophylactic use of aspirin: systematic review of harms and approaches to mitigation in the general population. Eur J Epidemiol. 2015;30:5-18.
24. Hernández-Díaz S, García Rodríguez LA. Cardioprotective aspirin users and their excess risk of upper gastrointestinal complications. BMC Med. 2006;4:22.
25. Huang ES, Strate LL, Ho WW, et al. Long term use of aspirin and the risk of gastrointestinal bleeding. Am J Med. 2011:124;426-433.
26. Walker J, Robinson J, Stewart J, et al. Does enteric-coated aspirin result in a lower incidence of gastrointestinal complications compared to normal aspirin? Interact Cardiovasc Thorac Surg. 2007:6;519-522.
27. NIH. Aspirin dosing: a patient-centric trial assessing benefits and long-term effectiveness (ADAPTABLE). https://clinicaltrials.gov/ct2/show/NCT02697916. Accessed March 22, 2019.
28. Piepoli MF, Hoes AW, Agewall S, et al. 2016 European guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. Eur Heart J. 2016;37:2315-2381.
29. ADA. Standards of medical care in diabetes – 2017. Diabetes Care. 2017;40(suppl 1). http://care.diabetesjournals.org/content/diacare/suppl/2016/12/15/40.Supplement_1.DC1/DC_40_S1_final.pdf. Accessed March 22, 2019.
30. Vandvik PO, Lincoff AM, Gore JM, et al. Primary and secondary prevention of cardiovascular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl):e637S-e668S.
31. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease. J Am Col Cardiol. 2019. doi: https://doi.org/10.1016/j.jacc.2019.03.010. Accessed March 22, 2019.
32. Mora S, Manson JE. Aspirin for primary prevention of atherosclerotic cardiovascular disease: advances in diagnosis and treatment. JAMA Intern Med. 2016;176:1195-1204.
1. FDA. Use of aspirin for primary prevention of heart attack and stroke. https://www.fda.gov/Drugs/ResourcesForYou/Consumers/ucm390574.htm. Accessed March 22, 2019.
2. Guirguis-Blake JM, Evans CV, Senger CA, et al. Aspirin for the primary prevention of cardiovascular events: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:804-813.
3. Whitlock EP, Burda BU, Williams SB, et al. Bleeding risks with aspirin use for primary prevention in adults: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:826-835.
4. Chubak J, Whitlock EP, Williams SB, et al. Aspirin for the prevention of cancer incidence and mortality: systematic evidence reviews for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:814-825.
5. Dehmer SP, Maciosek MV, Flottemesch TJ, et al. Aspirin for the primary prevention of cardiovascular disease and colorectal cancer: a decision analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:777-786.
6. Bibbins-Domingo K. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2016;164:836-845.
7. Baigent C, Blackwell L, Colins R, et al; Antithrombotic Trialists (ATT) Collaboration. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participation data from randomised trials. Lancet. 2009:373:1849-1860.
8. Ikeda Y, Shimada K, Teramoto T, et al. Low-dose aspirin for primary prevention of cardiovascular events in Japanese patients 60 years or older with atherosclerotic risk factors: a randomized clinical trial. JAMA. 2014;312:2510-2520.
9. Rothwell PM, Cook NR, Gaziano JM, et al. Effects of aspirin on risks of vascular events and cancer according to bodyweight and dose: analysis of individual patient data from randomised trials. Lancet. 2018;392:387-399.
10. Bowman L, Mafham M, Wallendszus K, et al; ASCEND Study Collaborative Group. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379:1529-1539.
11. McNeil JJ, Wolfe R, Woods RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379:1509-1518.
12. Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet. 2018;392:1036-1046.
13. Kune GA, Kune S, Watson LF. Colorectal cancer risk, chronic illness, operations, and medications: case control results from Melbourne Colorectal Cancer Study. Cancer Res. 1988;48:4399-4404.
14. Sutcliffe P, Connock M, Gurung T, et al. Aspirin for prophylactic use in the primary prevention of cardiovascular disease and cancer: a systematic review and overview of reviews. Health Technol Assess. 2013;17:1-253.
15. Burn J, Gerdes AM, Macrae F, et al. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet. 2011;378:2081-2087.
16. Gamba CA, Swetter SM, Stefanick ML, et al. Aspirin is associated with lower melanoma risk among postmenopausal Caucasian women: the Women’s Health Initiative. Cancer. 2013;119:1562-1569.
17. Trabert B, Ness RB, Lo-Ciganic WH, et al. Aspirin, nonaspirin nonsteroidal anti-inflammatory drug, and acetaminophen use and risk of invasive epithelial ovarian cancer: a pooled analysis in the Ovarian Cancer Association Consortium. J Natl Cancer Inst. 2014;106:djt431.
18. Risch H, Lu L, Streicher SA, et al. Aspirin use and reduced risk of pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2016;26:68-74.
19. McNeil JJ, Nelson MR, Woods RL, et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med. 2018;379:1519-1528.
20. Hernández-Díaz S, Rodríguez LA. Incidence of serious upper gastrointestinal bleeding/perforation in the general population: review of epidemiologic studies. J Clin Epidemiol. 2002;55:157-163.
21. Guirguis-Blake JM, Evans CV, Senger CA, et al. Aspirin for the primary prevention of cardiovascular events: a systematic evidence review for the U.S. Preventive Services Task Force. Evidence Synthesis no 131. Rockville, MD: Agency for Healthcare Research and Quality; 2015. https://www.ncbi.nlm.nih.gov/books/NBK321623/. Accessed March 22, 2019.
22. De Berardis G, Lucisano G, D’Ettorre A, et al. Association of aspirin use with major bleeding in patients with and without diabetes. JAMA. 2012;307:2286-2294.
23. Thorat MA, Cuzick J. Prophylactic use of aspirin: systematic review of harms and approaches to mitigation in the general population. Eur J Epidemiol. 2015;30:5-18.
24. Hernández-Díaz S, García Rodríguez LA. Cardioprotective aspirin users and their excess risk of upper gastrointestinal complications. BMC Med. 2006;4:22.
25. Huang ES, Strate LL, Ho WW, et al. Long term use of aspirin and the risk of gastrointestinal bleeding. Am J Med. 2011:124;426-433.
26. Walker J, Robinson J, Stewart J, et al. Does enteric-coated aspirin result in a lower incidence of gastrointestinal complications compared to normal aspirin? Interact Cardiovasc Thorac Surg. 2007:6;519-522.
27. NIH. Aspirin dosing: a patient-centric trial assessing benefits and long-term effectiveness (ADAPTABLE). https://clinicaltrials.gov/ct2/show/NCT02697916. Accessed March 22, 2019.
28. Piepoli MF, Hoes AW, Agewall S, et al. 2016 European guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. Eur Heart J. 2016;37:2315-2381.
29. ADA. Standards of medical care in diabetes – 2017. Diabetes Care. 2017;40(suppl 1). http://care.diabetesjournals.org/content/diacare/suppl/2016/12/15/40.Supplement_1.DC1/DC_40_S1_final.pdf. Accessed March 22, 2019.
30. Vandvik PO, Lincoff AM, Gore JM, et al. Primary and secondary prevention of cardiovascular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl):e637S-e668S.
31. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease. J Am Col Cardiol. 2019. doi: https://doi.org/10.1016/j.jacc.2019.03.010. Accessed March 22, 2019.
32. Mora S, Manson JE. Aspirin for primary prevention of atherosclerotic cardiovascular disease: advances in diagnosis and treatment. JAMA Intern Med. 2016;176:1195-1204.
PRACTICE RECOMMENDATIONS
› Consider aspirin for patients 50 to 59 years of age who have a 10-year cardiovascular disease (CVD) risk of ≥ 10% and low bleeding risk. C
› Discuss prophylactic aspirin (using a shared decision-making model) with patients 60 to 69 years of age who have a 10-year CVD risk of ≥ 10% and low bleeding risk. C
› Avoid using aspirin for primary prevention in patients ≥ 70 years of age. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Addressing insulin price spikes will require supply chain reform
WASHINGTON – panelists said at a House Committee on Energy & Commerce hearing on insulin affordability.
“Each member of the supply chain has a responsibility to help solve this problem,” said Alvin C. Powers, MD, director of the Vanderbilt Diabetes Center at Vanderbilt University, who was speaking on behalf of the Endocrine Society during the April 2 hearing of the committee’s oversight & investigations subcommittee.
Dr. Powers identified all members – manufacturers, payers, pharmacy benefit managers, patients, providers, and Congress – as having a role in developing a solution that will encourage more access to the treatment.
The hearing was the first of two in a series specifically examining the price of insulin. This one focused on the role pricing issues play in terms of access to insulin and patient outcomes.
To highlight the pricing issues, it was noted that a vial of Humalog (insulin lispro) cost $21 when it was launched by Eli Lilly in 1996. It now costs $275 even though it has gone through no changes in formulation or innovation during that time.
Kasia Lipska, MD, of Yale University School of Medicine noted that a summer 2017 survey conducted by the Yale Diabetes Center found that one in four patients took less than the prescribed dose of insulin specifically because of the cost of insulin.
William Cefalu, MD, chief scientific, medical, and mission officer at the American Diabetes Association, echoed comments from Dr. Powers about pricing and suggested that simply going after list price is not a complete solution.
“There is also no guarantee that if the list price drops there [will] be substantive changes throughout the supply chain,” Dr. Cefalu said, adding that there needs to be a move away from a system based on high list prices and rebates and toward a system that ensures that any negotiated rebate or discount will find its way to the patient at the pharmacy counter.
“That’s what is not happening now,” Dr. Cefalu added. “Unless you can control what happens downstream in the intermediaries and what happens to the patient, there is no guarantee that just dropping list prices ... is going to get the job done.”
Aaron Kowalski, PhD, chief mission officer of JDRF, an organization that funds research into type 1 diabetes, also called out insurers as a part of the problem.
“What we are seeing in the community is people being switched [from their prescribed insulin for nonmedical reasons] by their insurance companies, not by the choice of their physician or the patient, which is just not the right way to practice medicine.”
He relayed an anecdote about a woman who went from having her blood sugar well controlled to dealing with severe cases of hyperglycemia because of changes in the medical coverage of her insulin. It took 8 hours on the phone with the insurance company, not to mention countless hours spent by the physician, to get the situation corrected and to get the proper insulin covered.
“This is a broken part of the system,” Dr. Kowalski said.
Dr. Cefalu noted that data are needed on the medical impact of switching for nonmedical reasons, such as changes to insurance coverage.
Christel Marchand Aprigliano, chief executive officer of the Diabetes Patient Advocacy Coalition, also relayed an anecdote of a friend who had suffered medical consequences of nonmedical switching of his insulin and then having to deal with his insurer’s fail-first policy before they would cover his original, medically effective insulin.
“Insurance has been denied twice because they believe that insulins are interchangeable, which they aren’t,” she said.
Michael Burgess, MD, (R-Texas) asked rhetorically during the hearing whether it would make sense for payers to simply provide insulin at no cost to patients, given the cost of medical complications resulting from lack of proper use as a result of pricing likely is much higher than covering insulin completely.
While specific legislative proposals were not discussed during the hearing, one thing that the panelists agreed would help to clarify all the factors that are contributing to the pricing increases is clear, transparent information about the finances surrounding the insulin as the product moves through the supply chain.
The Food and Drug Administration is also doing its part. Although the agency was not a participant in the hearing, the agency’s commissioner, Scott Gottlieb, MD, released a statement on the same day as the hearing in which he touted efforts in the biosimilar space that could spur competition.
“Once an interchangeable insulin product is approved and available on the market, it can be substituted for the reference product at the pharmacy, potentially leading to increased access [to insulin] and lower costs for patients,” he said in the statement. “The FDA anticipates that biosimilar and interchangeable insulin products will bring the competition that’s needed to help [deliver] affordable treatment options to patients.”
Dr Gottlieb did not say when a biosimilar insulin might be available on the market.
The second hearing in this series has not been scheduled, but is expected to take place the week of April 8 and will feature representatives from three insulin manufacturers and other participants in the supply chain.
WASHINGTON – panelists said at a House Committee on Energy & Commerce hearing on insulin affordability.
“Each member of the supply chain has a responsibility to help solve this problem,” said Alvin C. Powers, MD, director of the Vanderbilt Diabetes Center at Vanderbilt University, who was speaking on behalf of the Endocrine Society during the April 2 hearing of the committee’s oversight & investigations subcommittee.
Dr. Powers identified all members – manufacturers, payers, pharmacy benefit managers, patients, providers, and Congress – as having a role in developing a solution that will encourage more access to the treatment.
The hearing was the first of two in a series specifically examining the price of insulin. This one focused on the role pricing issues play in terms of access to insulin and patient outcomes.
To highlight the pricing issues, it was noted that a vial of Humalog (insulin lispro) cost $21 when it was launched by Eli Lilly in 1996. It now costs $275 even though it has gone through no changes in formulation or innovation during that time.
Kasia Lipska, MD, of Yale University School of Medicine noted that a summer 2017 survey conducted by the Yale Diabetes Center found that one in four patients took less than the prescribed dose of insulin specifically because of the cost of insulin.
William Cefalu, MD, chief scientific, medical, and mission officer at the American Diabetes Association, echoed comments from Dr. Powers about pricing and suggested that simply going after list price is not a complete solution.
“There is also no guarantee that if the list price drops there [will] be substantive changes throughout the supply chain,” Dr. Cefalu said, adding that there needs to be a move away from a system based on high list prices and rebates and toward a system that ensures that any negotiated rebate or discount will find its way to the patient at the pharmacy counter.
“That’s what is not happening now,” Dr. Cefalu added. “Unless you can control what happens downstream in the intermediaries and what happens to the patient, there is no guarantee that just dropping list prices ... is going to get the job done.”
Aaron Kowalski, PhD, chief mission officer of JDRF, an organization that funds research into type 1 diabetes, also called out insurers as a part of the problem.
“What we are seeing in the community is people being switched [from their prescribed insulin for nonmedical reasons] by their insurance companies, not by the choice of their physician or the patient, which is just not the right way to practice medicine.”
He relayed an anecdote about a woman who went from having her blood sugar well controlled to dealing with severe cases of hyperglycemia because of changes in the medical coverage of her insulin. It took 8 hours on the phone with the insurance company, not to mention countless hours spent by the physician, to get the situation corrected and to get the proper insulin covered.
“This is a broken part of the system,” Dr. Kowalski said.
Dr. Cefalu noted that data are needed on the medical impact of switching for nonmedical reasons, such as changes to insurance coverage.
Christel Marchand Aprigliano, chief executive officer of the Diabetes Patient Advocacy Coalition, also relayed an anecdote of a friend who had suffered medical consequences of nonmedical switching of his insulin and then having to deal with his insurer’s fail-first policy before they would cover his original, medically effective insulin.
“Insurance has been denied twice because they believe that insulins are interchangeable, which they aren’t,” she said.
Michael Burgess, MD, (R-Texas) asked rhetorically during the hearing whether it would make sense for payers to simply provide insulin at no cost to patients, given the cost of medical complications resulting from lack of proper use as a result of pricing likely is much higher than covering insulin completely.
While specific legislative proposals were not discussed during the hearing, one thing that the panelists agreed would help to clarify all the factors that are contributing to the pricing increases is clear, transparent information about the finances surrounding the insulin as the product moves through the supply chain.
The Food and Drug Administration is also doing its part. Although the agency was not a participant in the hearing, the agency’s commissioner, Scott Gottlieb, MD, released a statement on the same day as the hearing in which he touted efforts in the biosimilar space that could spur competition.
“Once an interchangeable insulin product is approved and available on the market, it can be substituted for the reference product at the pharmacy, potentially leading to increased access [to insulin] and lower costs for patients,” he said in the statement. “The FDA anticipates that biosimilar and interchangeable insulin products will bring the competition that’s needed to help [deliver] affordable treatment options to patients.”
Dr Gottlieb did not say when a biosimilar insulin might be available on the market.
The second hearing in this series has not been scheduled, but is expected to take place the week of April 8 and will feature representatives from three insulin manufacturers and other participants in the supply chain.
WASHINGTON – panelists said at a House Committee on Energy & Commerce hearing on insulin affordability.
“Each member of the supply chain has a responsibility to help solve this problem,” said Alvin C. Powers, MD, director of the Vanderbilt Diabetes Center at Vanderbilt University, who was speaking on behalf of the Endocrine Society during the April 2 hearing of the committee’s oversight & investigations subcommittee.
Dr. Powers identified all members – manufacturers, payers, pharmacy benefit managers, patients, providers, and Congress – as having a role in developing a solution that will encourage more access to the treatment.
The hearing was the first of two in a series specifically examining the price of insulin. This one focused on the role pricing issues play in terms of access to insulin and patient outcomes.
To highlight the pricing issues, it was noted that a vial of Humalog (insulin lispro) cost $21 when it was launched by Eli Lilly in 1996. It now costs $275 even though it has gone through no changes in formulation or innovation during that time.
Kasia Lipska, MD, of Yale University School of Medicine noted that a summer 2017 survey conducted by the Yale Diabetes Center found that one in four patients took less than the prescribed dose of insulin specifically because of the cost of insulin.
William Cefalu, MD, chief scientific, medical, and mission officer at the American Diabetes Association, echoed comments from Dr. Powers about pricing and suggested that simply going after list price is not a complete solution.
“There is also no guarantee that if the list price drops there [will] be substantive changes throughout the supply chain,” Dr. Cefalu said, adding that there needs to be a move away from a system based on high list prices and rebates and toward a system that ensures that any negotiated rebate or discount will find its way to the patient at the pharmacy counter.
“That’s what is not happening now,” Dr. Cefalu added. “Unless you can control what happens downstream in the intermediaries and what happens to the patient, there is no guarantee that just dropping list prices ... is going to get the job done.”
Aaron Kowalski, PhD, chief mission officer of JDRF, an organization that funds research into type 1 diabetes, also called out insurers as a part of the problem.
“What we are seeing in the community is people being switched [from their prescribed insulin for nonmedical reasons] by their insurance companies, not by the choice of their physician or the patient, which is just not the right way to practice medicine.”
He relayed an anecdote about a woman who went from having her blood sugar well controlled to dealing with severe cases of hyperglycemia because of changes in the medical coverage of her insulin. It took 8 hours on the phone with the insurance company, not to mention countless hours spent by the physician, to get the situation corrected and to get the proper insulin covered.
“This is a broken part of the system,” Dr. Kowalski said.
Dr. Cefalu noted that data are needed on the medical impact of switching for nonmedical reasons, such as changes to insurance coverage.
Christel Marchand Aprigliano, chief executive officer of the Diabetes Patient Advocacy Coalition, also relayed an anecdote of a friend who had suffered medical consequences of nonmedical switching of his insulin and then having to deal with his insurer’s fail-first policy before they would cover his original, medically effective insulin.
“Insurance has been denied twice because they believe that insulins are interchangeable, which they aren’t,” she said.
Michael Burgess, MD, (R-Texas) asked rhetorically during the hearing whether it would make sense for payers to simply provide insulin at no cost to patients, given the cost of medical complications resulting from lack of proper use as a result of pricing likely is much higher than covering insulin completely.
While specific legislative proposals were not discussed during the hearing, one thing that the panelists agreed would help to clarify all the factors that are contributing to the pricing increases is clear, transparent information about the finances surrounding the insulin as the product moves through the supply chain.
The Food and Drug Administration is also doing its part. Although the agency was not a participant in the hearing, the agency’s commissioner, Scott Gottlieb, MD, released a statement on the same day as the hearing in which he touted efforts in the biosimilar space that could spur competition.
“Once an interchangeable insulin product is approved and available on the market, it can be substituted for the reference product at the pharmacy, potentially leading to increased access [to insulin] and lower costs for patients,” he said in the statement. “The FDA anticipates that biosimilar and interchangeable insulin products will bring the competition that’s needed to help [deliver] affordable treatment options to patients.”
Dr Gottlieb did not say when a biosimilar insulin might be available on the market.
The second hearing in this series has not been scheduled, but is expected to take place the week of April 8 and will feature representatives from three insulin manufacturers and other participants in the supply chain.
REPORTING FROM A HOUSE ENERGY & COMMERCE SUBCOMMITTEE HEARING
Unusual effects of common antibiotics
A 60-year-old man is admitted for respiratory failure following a massive myocardial infarction. He develops ventilator-associated pneumonia and is treated with cefepime and vancomycin. Three days later, he develops prolonged atypical absence seizures.
What caused these seizures? The neurologist thinks it might be the cefepime. Do you agree?
Antibiotics are widely used in the United States, with 269 million courses of oral therapy prescribed in 2011.1 Adverse effects such as rash are well known, but rare effects such as seizure, hypoglycemia, and hypoxemia may not be immediately attributed to these drugs.
In this article, we review less-recognized but potentially serious adverse effects of antibiotics commonly prescribed in the United States. We have structured our discussion by organ system for ease of reference.
NERVOUS SYSTEM
The potential adverse effects of antibiotics on the nervous system range from encephalopathy and seizure to nonconvulsive status epilepticus.
Encephalopathy and seizure
Encephalopathy has been reported with penicillins, cephalosporins, sulfamethoxazole-trimethoprim, quinolones, and oxazolidinones such as linezolid.2,3
Seizures are known to occur with penicillins, cephalosporins, carbapenems, and quinolones.2–4 For cephalosporins, these effects are more common at higher doses, in elderly patients, and in patients with renal impairment. Carbapenems are associated with seizure activity in elderly patients.2–4
Encephalopathy and seizure can also occur on a continuum, as is the case with piperacillin-induced encephalopathy, with progressive dysarthria, tremor, and progressive confusion culminating in tonic-clonic seizures.2
Nonconvulsive status epilepticus
Nonconvulsive status epilepticus, marked by prolonged atypical absence seizures, has complicated the use of penicillins, quinolones, clarithromycin, and cephalosporins, specifically cefepime.2,3,5 Diagnosis can be difficult and requires clinical awareness and confirmation with electroencephalography.
Class-specific neurologic effects
Certain antibiotics have class-specific effects:
Tetracyclines: cranial nerve toxicity, neuromuscular blockade, and intracranial hypertension.2
Sulfamethoxazole-trimethoprim: tremors and psychosis, with visual and auditory hallucinations.6
Macrolides: dysequilibrium and potentially irreversible hearing loss.2
Quinolones: orofacial dyskinesia and a Tourette-like syndrome, with a higher incidence reported with newer quinolones.7
Linezolid: optic and peripheral neuropathy2; neuropathy can be persistent and can lead to loss of vision. The package insert recommends monitoring visual function in patients taking linezolid for more than 3 months and in any patient reporting visual symptoms.8
Linezolid is also associated with serotonin syndrome when combined with a drug that potentiates serotonergic activity, most commonly selective serotonin reuptake inhibitors. The syndrome is characterized by a triad of cognitive or behavioral changes, autonomic instability, and neuromuscular excitability such as spontaneous clonus.9
Metronidazole: optic and peripheral neuropathy, in addition to cerebellar toxicity and central nervous system lesions on magnetic resonance imaging of the brain. In a series of 11 cases of cerebellar toxicity, most patients presented with ataxia and dysarthria associated with high total doses of metronidazole, and in most cases, magnetic resonance imaging showed resolution of the lesions upon discontinuation of metronidazole.10
HEMATOLOGIC AND RHEUMATOLOGIC EFFECTS
Agranulocytosis has been associated with beta-lactams, in most cases with prolonged exposure. In one report, the average exposure before onset of agranulocytosis was 22 days for nafcillin and 25 days for penicillin. For penicillins, more than 50% of cases involved high daily doses.11
Likewise, most episodes of vancomycin-induced neutropenia were reported to occur after 20 days of therapy.12
In another study, most cases of drug-induced anemia were due to ceftriaxone and piperacillin.13
Drug-induced thrombocytopenia has been described with penicillins, cephalosporins, sulfonamides, and vancomycin14 and is a well-recognized effect of linezolid. The syndrome of drug reaction with eosinophilia and systemic symptoms, a severe and rare adverse reaction, has been reported with minocycline, sulfamethoxazole, and vancomycin.15
The tetracycline minocycline has been reported to cause drug-induced lupus and polyarteritis nodosa-like vasculitis.16 Drug-induced lupus presents as myalgias and arthralgias, serositis, constitutional symptoms, and positive antinuclear antibody titers. The effect is not dose-dependent. Penicillin, cefuroxime, and nitrofurantoin have also been implicated.16
Kermani et al17 described 9 cases of polyarteritis nodosa, in which 5 patients (56%) had systemic involvement including renal artery microaneurysm, mononeuritis multiplex, and mesenteric vasculitis, and some of these patients also had cutaneous involvement. All patients had positive antineutrophil cytoplasmic antibody in a perinuclear pattern. The median time from start of the minocycline to symptom onset was 9 months, and the median duration of use was 2 years.
Quinolones have also been reported to cause fatal hypersensitivity vasculitis.18,19
CARDIOVASCULAR SYSTEM
Macrolides and quinolones have been reported to cause QT-interval prolongation and torsades de pointes. The risk is greatest when a macrolide is co-administered with a CYP3A4 inhibitor.
Of the macrolides, azithromycin is the safest, as clarithromycin and erythromycin are more likely to cause QT prolongation.
While QT prolongation is a class effect of quinolones, there is variability within the class. Ciprofloxacin is thought to be the safest in terms of cardiovascular adverse effects.20 In addition, Owens and Nolin20 reported that quinolone-associated QT prolongation was more likely to occur in patients with pre-existing QT prolongation, electrolyte abnormalities, organic heart disease, and bradycardia, and especially in women. Other risk factors for QT prolongation with quinolone use include underlying cardiac disease and advanced age.21
Quinolones have also been associated with an increased risk of aortic dissection. The US Food and Drug Administration has issued a warning advising clinicians to avoid quinolones in patients who have aneurysms or are at risk for aneurysms, such as patients with advanced age, peripheral atherosclerotic vascular disease, hypertension and conditions such as Marfan and Ehlers-Danlos syndrome.22
DIGESTIVE SYSTEM
Tetracyclines are known to cause esophagitis from direct contact with and disruption of the mucosal lining. Doxycycline is the most frequent offender.23
Amoxicillin-clavulanate is the antibiotic most commonly associated with drug-induced liver injury, mainly attributable to the clavulanate component.24 It is more common in men over age 50 and with prolonged and repeated dosing and is sometimes fatal. Other adverse effects include Stevens-Johnson syndrome, interstitial nephritis, and thrombotic thrombocytopenic purpura.25
Cholestatic hepatitis has been reported with penicillins, particularly dicloxacillin, oxacillin, and amoxicillin-clavulanate; cephalosporins; doxycycline; sulfamethoxazole-trimethoprim; macrolides; and ciprofloxacin.24–26 Hepatocellular injury is linked to amoxicillin-clavulanate and doxycycline. Drug-induced mixed liver injury has been observed with amoxicillin-clavulanate, sulfamethoxazole-trimethoprim and, rarely, cephalosporins.
Liver injury is classified as cholestatic if the alkaline phosphatase level is more than 2 times higher than normal, or if the ratio of alanine aminotransferase to alkaline phosphatase is less than 2; if the ratio is greater than 5, the injury is considered hepatocellular.24 Mixed liver injury, the most common, is defined as a ratio from 2 to 5.
Nitrofurantoin has also been linked to hepatotoxicity, cirrhosis, and end-stage liver disease, and to death if the drug is continued after the onset of jaundice.26 Death from liver injury has been reported with amoxicillin-clavulanate, sulfamethoxazole-trimethoprim, and erythromycin, and jaundice indicates a poor prognosis, associated with a 10% mortality rate or need for liver transplant in all patients.24
ENDOCRINE SYSTEM
Clarithromycin, sulfonamides, and quinolones are known to precipitate hypoglycemia by interacting with sulfonylureas. A study of Medicare patients age 66 or older who were taking glipizide or glyburide reported that female sex, older age, and a history of hypoglycemic episodes were associated with antibiotic-related hypoglycemia.27 The odds ratio for hypoglycemia was highest for clarithromycin (3.96), sulfamethoxazole-trimethoprim (2.56), metronidazole (2.11), and ciprofloxacin (1.62) when compared with antibiotics that do not cause hypoglycemia. There was no signal for levofloxacin-mediated hypoglycemia in this series.27
RESPIRATORY SYSTEM
Hypersensitivity lung disease has been reported with penicillin, ampicillin, cephalosporins, ciprofloxacin, and sulfonamides including sulfamethoxazole-trimethoprim.28 The lipopeptide daptomycin has been reported to cause acute eosinophilic pneumonia defined as fever for less than 5 days, pulmonary infiltrates, hypoxemia, and a bronchoalveolar lavage or biopsy study with eosinophils. Daptomycin should be stopped early in these cases, and the patient should not be rechallenged, as the reaction can be deadly.29
Nitrofurantoin has a long history of hypersensitivity pneumonitis in its acute form and a chronic allergic response. While more widely recognized, nitrofurantoin pulmonary toxicity is rare, occurring in 1 in 5,000 patients.30
RENAL SYSTEM
Acute interstitial nephritis has been reported with penicillins, cephalosporins, macrolides, quinolones, sulfonamides, and vancomycin.31–33 Acute tubular necrosis has been linked to cephalosporins and tetracyclines. Crystal nephropathy has been seen with quinolones and sulfonamides.
Advanced age is an important risk factor for renal dysfunction from quinolones,18 and penicillin G has been reported to cause glomerulonephritis.31
MUSCULOSKELETAL SYSTEM
Quinolones have been associated with arthropathy or tendinitis at a rate of 1%, including cases of Achilles tendon rupture.18 The US Food and Drug Administration announced in 2016 that the serious adverse events with fluoroquinolones outweigh the benefits in patients with acute sinusitis, acute bronchitis, and uncomplicated urinary tract infection, and that they should be used only if there are no other options.34
Daptomycin is known to cause elevations of creatine kinase.34 Weekly monitoring is recommended based on postmarketing data reports of elevations in 2.5% of patients; myopathy is a rarer effect, occurring in 0.2% of patients.35
REPRODUCTIVE SYSTEM
Antibiotics have long been reported to interact with oral contraceptives, but the data are not compelling for commonly used antibiotics. The strongest association is with rifampicin, which reduces oral contraceptive efficacy and warrants an alternative mode of contraception.36
BACK TO OUR PATIENT
Antibiotics can have serious adverse effects, and it is important for clinicians to be cognizant of this. Our 60-year-old patient who was taking cefepime and vancomycin for pneumonia developed prolonged atypical absence seizures. When the cefepime was discontinued, his mental status improved, and no other seizures were observed.
- Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010–2011. JAMA 2016; 315(17):1864–1873. doi:10.1001/jama.2016.4151
- Grill MF, Maganti RK. Neurotoxic effects associated with antibiotic use: management considerations. Br J Clin Pharmacol 2011; 72(3):381–393. doi:10.1111/j.1365-2125.2011.03991.x
- Dakdouki GK, Al-Awar GN. Cefepime-induced encephalopathy. Int J Infect Dis 2004; 8(1):59–61. pmid:14690782
- Bazan JA, Martin SI, Kaye KM. Newer beta-lactam antiobiotics: doripenem, ceftobiprole, and cefepime. Infect Dis Clin North Am 2009; 23(4):983–999. doi:10.1016/j.idc.2009.06.007
- Bandettini di Poggio M, Anfosso S, Audenino D, Primavera A. Clarithromycin-induced neurotoxicity in adults. J Clin Neurosci 2011; 18(3):313–318. doi:10.1016/j.jocn.2010.08.014
- Saidinejad M, Ewald MB, Shannon MW. Transient psychosis in an immune-competent patient after oral trimethoprim-sulfamethoxazole administration. Pediatrics 2005; 115(6):e739–e741. doi:10.1542/peds.2004-1352
- Thomas RJ, Reagan DR. Association of a Tourette-like syndrome with ofloxacin. Ann Pharmacother 1996; 30(2):138–141. doi:10.1177/106002809603000205
- Pharmacia and Upjohn Company LLC. Zyvox® Package Insert. http://labeling.pfizer.com/showlabeling.aspx?id=649. Accessed March 5, 2019.
- Lawrence KR, Adra M, Gillman PK. Serotonin toxicity associated with the use of linezolid: a review of postmarketing data. Clin Infect Dis 2006; 42(11):1578–1583. doi:10.1086/503839
- Patel K, Green-Hopkins I, Lu S, Tunkel AR. Cerebellar ataxia following prolonged use of metronidazole: case report and literature review. Int J Infect Dis 2008; 12(6):e111–e114. doi:10.1016/j.ijid.2008.03.006
- Andersohn F, Konzen C, Garbe E. Systematic review: agranulocytosis induced by nonchemotherapy drugs. Ann Intern Med 2007; 146(9):657–665. pmid:17470834
- Black E, Lau TT, Ensom MH. Vancomycin-induced neutropenia: is it dose- or duration-related? Ann Pharmacother 2011; 45(5):629–638. doi:10.1345/aph.1P583
- Garratty G. Drug-induced immune hemolytic anemia. Hematology Am Soc Hematol Educ Program 2009: 73–79. doi:10.1182/asheducation-2009.1.73
- Chong Bh, Choi PY, Khachigian L, Perdomo J. Drug-induced immune thrombocytopenia. Hematol Oncol Clin North Am 2013; 27(3):521–540. doi:10.1016/j.hoc.2013.02.003
- Cacoub P, Musette P, Descamps V, et al. The DRESS syndrome: a literature review. Am J Med 2011; 124(7):588–597. doi:10.1016/j.amjmed.2011.01.017
- Chang C, Gershwin ME. Drugs and autoimmunity—a contemporary review and mechanistic approach. J Autoimmun 2010; 34(3):J266–J275. doi:10.1016/j.jaut.2009.11.012
- Kermani TA, Ham EK, Camilleri MJ, Warrington KJ. Polyarteritis nodosa-like vasculitis in association with minocycline use: a single-center case series. Semin Arthritis Rheum 2012; 42(2):213–221. doi:10.1016/j.semarthrit.2012.03.006
- Mandell LA, Ball P, Tillotson G. Antimicrobial safety and tolerability: differences and dilemmas. Clin Infect Dis 2001; 32(suppl 1):S72–S79. doi:10.1086/319379
- Christ W, Esch B. Session III: safety. Adverse reactions to fluoroquinolones in adults and children. Infect Dis Clin Pract 1994; 3(3 suppl 3):S168–S176.
- Owens RC, Nolin TD. Antimicrobial-associated QT interval prolongation: pointes of interest. Clin Infect Dis 2006; 43(12):1603–1611. doi:10.1086/508873
- Rubinstein E, Camm J. Cardiotoxicity of fluoroquinolones. J Antimicrob Chemother 2002; 49(4):593–596. pmid:11909831
- US Food and Drug Administration (FDA). FDA drug safety communication: FDA warns about increased risk of ruptures or tears in the aorta blood vessel with fluoroquinolones antibiotics in certain patients. https://www.fda.gov/Drugs/DrugSafety/ucm628753.htm. Accessed March 15, 2019.
- Seminerio J, McGrath K, Arnold CA, Voltaggio L, Singhi AD. Medication-associated lesions of the GI tract. Gastrointest Endosc 2014; 79(1):140–150. doi:10.1016/j.gie.2013.08.027
- Bjornsson ES, Jonasson JG. Drug-induced cholestasis. Clin Liver Dis 2013; 17(2):191–209. doi:10.1016/j.cld.2012.11.002
- Fontana RJ, Shakil AO, Greenson JK, Boyd I, Lee WM. Acute liver failure due to amoxicillin and amoxicillin/clavulanate. Dig Dis Sci 2005; 50(10):1785–1790. doi:10.1007/s10620-005-2938-5
- Sakaan SA, Twilla JD, Usery JB, Winton JC, Self TH. Nitrofurantoin-induced hepatotoxicity: a rare yet serious complication. South Med J 2014; 107(2):107–113. doi:10.1097/SMJ.0000000000000059
- Parekh TM, Raji M, Lin YL, Tan A, Kuo YF, Goodwin JS. Hypoglycemia after antimicrobial drug prescription for older patients using sulfonylureas. JAMA Intern Med 2014; 174(10):1605–1612. doi:10.1001/jamainternmed.2014.3293
- Prasad R, Gupta P, Singh A, Goel N. Drug induced pulmonary parenchymal disease. Drug Discov Ther 2014; 8(6):232–237. doi:10.5582/ddt.2014.01046
- Miller BA, Gray A, Leblanc TW, Sexton DJ, Martin AR, Slama TG. Acute eosinophilic pneumonia secondary to daptomycin: a report of three cases. Clin Infect Dis 2010; 50(11):e63–e68. doi:10.1086/652656
- Kabbara WK, Kordahi MC. Nitrofurantoin-induced pulmonary toxicity: a case report and review of the literature. J Infect Public Health 2015; 8(4):309–313. doi:10.1016/j.jiph.2015.01.007
- Ghane Shahrbaf F, Assadi F. Drug-induced renal disorders. J Renal Inj Prev 2015; 4(3):57–60. doi:10.12861/jrip.2015.12
- Mac K, Chavada R, Paull S, Howlin K, Wong J. Cefepime induced acute interstitial nephritis—a case report. BMC Nephrol 2015; 16:15. doi:10.1186/s12882-015-0004-x
- Woodruff AE, Meaney CJ, Hansen EA, Prescott GM. Azithromycin-induced, biopsy-proven cute interstitial nephritis in an adult successfully treated with low-dose corticosteroids. Pharmacotherapy 2015; 35(11):e169–e174. doi:10.1002/phar.1660
- US Food and Drug Administration (FDA). FDA drug safety communication: FDA advises restricting fluoroquinolone antibiotic use for certain uncomplicated infections; warns about disabling side effects that can occur together. https://www.fda.gov/Drugs/DrugSafety/ucm500143.htm. Accessed March 7, 2019.
- Hawkey PM. Pre-clinical experience with daptomycin. J Antimicrob Chemother 2008; 62(suppl 3):iii7–iii14. doi:10.1093/jac/dkn367
- ACOG Committee on Practice Bulletins–Gynecology. ACOG practice bulletin. No. 73: Use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol 2006; 107(6):1453–1472. pmid:16738183
A 60-year-old man is admitted for respiratory failure following a massive myocardial infarction. He develops ventilator-associated pneumonia and is treated with cefepime and vancomycin. Three days later, he develops prolonged atypical absence seizures.
What caused these seizures? The neurologist thinks it might be the cefepime. Do you agree?
Antibiotics are widely used in the United States, with 269 million courses of oral therapy prescribed in 2011.1 Adverse effects such as rash are well known, but rare effects such as seizure, hypoglycemia, and hypoxemia may not be immediately attributed to these drugs.
In this article, we review less-recognized but potentially serious adverse effects of antibiotics commonly prescribed in the United States. We have structured our discussion by organ system for ease of reference.
NERVOUS SYSTEM
The potential adverse effects of antibiotics on the nervous system range from encephalopathy and seizure to nonconvulsive status epilepticus.
Encephalopathy and seizure
Encephalopathy has been reported with penicillins, cephalosporins, sulfamethoxazole-trimethoprim, quinolones, and oxazolidinones such as linezolid.2,3
Seizures are known to occur with penicillins, cephalosporins, carbapenems, and quinolones.2–4 For cephalosporins, these effects are more common at higher doses, in elderly patients, and in patients with renal impairment. Carbapenems are associated with seizure activity in elderly patients.2–4
Encephalopathy and seizure can also occur on a continuum, as is the case with piperacillin-induced encephalopathy, with progressive dysarthria, tremor, and progressive confusion culminating in tonic-clonic seizures.2
Nonconvulsive status epilepticus
Nonconvulsive status epilepticus, marked by prolonged atypical absence seizures, has complicated the use of penicillins, quinolones, clarithromycin, and cephalosporins, specifically cefepime.2,3,5 Diagnosis can be difficult and requires clinical awareness and confirmation with electroencephalography.
Class-specific neurologic effects
Certain antibiotics have class-specific effects:
Tetracyclines: cranial nerve toxicity, neuromuscular blockade, and intracranial hypertension.2
Sulfamethoxazole-trimethoprim: tremors and psychosis, with visual and auditory hallucinations.6
Macrolides: dysequilibrium and potentially irreversible hearing loss.2
Quinolones: orofacial dyskinesia and a Tourette-like syndrome, with a higher incidence reported with newer quinolones.7
Linezolid: optic and peripheral neuropathy2; neuropathy can be persistent and can lead to loss of vision. The package insert recommends monitoring visual function in patients taking linezolid for more than 3 months and in any patient reporting visual symptoms.8
Linezolid is also associated with serotonin syndrome when combined with a drug that potentiates serotonergic activity, most commonly selective serotonin reuptake inhibitors. The syndrome is characterized by a triad of cognitive or behavioral changes, autonomic instability, and neuromuscular excitability such as spontaneous clonus.9
Metronidazole: optic and peripheral neuropathy, in addition to cerebellar toxicity and central nervous system lesions on magnetic resonance imaging of the brain. In a series of 11 cases of cerebellar toxicity, most patients presented with ataxia and dysarthria associated with high total doses of metronidazole, and in most cases, magnetic resonance imaging showed resolution of the lesions upon discontinuation of metronidazole.10
HEMATOLOGIC AND RHEUMATOLOGIC EFFECTS
Agranulocytosis has been associated with beta-lactams, in most cases with prolonged exposure. In one report, the average exposure before onset of agranulocytosis was 22 days for nafcillin and 25 days for penicillin. For penicillins, more than 50% of cases involved high daily doses.11
Likewise, most episodes of vancomycin-induced neutropenia were reported to occur after 20 days of therapy.12
In another study, most cases of drug-induced anemia were due to ceftriaxone and piperacillin.13
Drug-induced thrombocytopenia has been described with penicillins, cephalosporins, sulfonamides, and vancomycin14 and is a well-recognized effect of linezolid. The syndrome of drug reaction with eosinophilia and systemic symptoms, a severe and rare adverse reaction, has been reported with minocycline, sulfamethoxazole, and vancomycin.15
The tetracycline minocycline has been reported to cause drug-induced lupus and polyarteritis nodosa-like vasculitis.16 Drug-induced lupus presents as myalgias and arthralgias, serositis, constitutional symptoms, and positive antinuclear antibody titers. The effect is not dose-dependent. Penicillin, cefuroxime, and nitrofurantoin have also been implicated.16
Kermani et al17 described 9 cases of polyarteritis nodosa, in which 5 patients (56%) had systemic involvement including renal artery microaneurysm, mononeuritis multiplex, and mesenteric vasculitis, and some of these patients also had cutaneous involvement. All patients had positive antineutrophil cytoplasmic antibody in a perinuclear pattern. The median time from start of the minocycline to symptom onset was 9 months, and the median duration of use was 2 years.
Quinolones have also been reported to cause fatal hypersensitivity vasculitis.18,19
CARDIOVASCULAR SYSTEM
Macrolides and quinolones have been reported to cause QT-interval prolongation and torsades de pointes. The risk is greatest when a macrolide is co-administered with a CYP3A4 inhibitor.
Of the macrolides, azithromycin is the safest, as clarithromycin and erythromycin are more likely to cause QT prolongation.
While QT prolongation is a class effect of quinolones, there is variability within the class. Ciprofloxacin is thought to be the safest in terms of cardiovascular adverse effects.20 In addition, Owens and Nolin20 reported that quinolone-associated QT prolongation was more likely to occur in patients with pre-existing QT prolongation, electrolyte abnormalities, organic heart disease, and bradycardia, and especially in women. Other risk factors for QT prolongation with quinolone use include underlying cardiac disease and advanced age.21
Quinolones have also been associated with an increased risk of aortic dissection. The US Food and Drug Administration has issued a warning advising clinicians to avoid quinolones in patients who have aneurysms or are at risk for aneurysms, such as patients with advanced age, peripheral atherosclerotic vascular disease, hypertension and conditions such as Marfan and Ehlers-Danlos syndrome.22
DIGESTIVE SYSTEM
Tetracyclines are known to cause esophagitis from direct contact with and disruption of the mucosal lining. Doxycycline is the most frequent offender.23
Amoxicillin-clavulanate is the antibiotic most commonly associated with drug-induced liver injury, mainly attributable to the clavulanate component.24 It is more common in men over age 50 and with prolonged and repeated dosing and is sometimes fatal. Other adverse effects include Stevens-Johnson syndrome, interstitial nephritis, and thrombotic thrombocytopenic purpura.25
Cholestatic hepatitis has been reported with penicillins, particularly dicloxacillin, oxacillin, and amoxicillin-clavulanate; cephalosporins; doxycycline; sulfamethoxazole-trimethoprim; macrolides; and ciprofloxacin.24–26 Hepatocellular injury is linked to amoxicillin-clavulanate and doxycycline. Drug-induced mixed liver injury has been observed with amoxicillin-clavulanate, sulfamethoxazole-trimethoprim and, rarely, cephalosporins.
Liver injury is classified as cholestatic if the alkaline phosphatase level is more than 2 times higher than normal, or if the ratio of alanine aminotransferase to alkaline phosphatase is less than 2; if the ratio is greater than 5, the injury is considered hepatocellular.24 Mixed liver injury, the most common, is defined as a ratio from 2 to 5.
Nitrofurantoin has also been linked to hepatotoxicity, cirrhosis, and end-stage liver disease, and to death if the drug is continued after the onset of jaundice.26 Death from liver injury has been reported with amoxicillin-clavulanate, sulfamethoxazole-trimethoprim, and erythromycin, and jaundice indicates a poor prognosis, associated with a 10% mortality rate or need for liver transplant in all patients.24
ENDOCRINE SYSTEM
Clarithromycin, sulfonamides, and quinolones are known to precipitate hypoglycemia by interacting with sulfonylureas. A study of Medicare patients age 66 or older who were taking glipizide or glyburide reported that female sex, older age, and a history of hypoglycemic episodes were associated with antibiotic-related hypoglycemia.27 The odds ratio for hypoglycemia was highest for clarithromycin (3.96), sulfamethoxazole-trimethoprim (2.56), metronidazole (2.11), and ciprofloxacin (1.62) when compared with antibiotics that do not cause hypoglycemia. There was no signal for levofloxacin-mediated hypoglycemia in this series.27
RESPIRATORY SYSTEM
Hypersensitivity lung disease has been reported with penicillin, ampicillin, cephalosporins, ciprofloxacin, and sulfonamides including sulfamethoxazole-trimethoprim.28 The lipopeptide daptomycin has been reported to cause acute eosinophilic pneumonia defined as fever for less than 5 days, pulmonary infiltrates, hypoxemia, and a bronchoalveolar lavage or biopsy study with eosinophils. Daptomycin should be stopped early in these cases, and the patient should not be rechallenged, as the reaction can be deadly.29
Nitrofurantoin has a long history of hypersensitivity pneumonitis in its acute form and a chronic allergic response. While more widely recognized, nitrofurantoin pulmonary toxicity is rare, occurring in 1 in 5,000 patients.30
RENAL SYSTEM
Acute interstitial nephritis has been reported with penicillins, cephalosporins, macrolides, quinolones, sulfonamides, and vancomycin.31–33 Acute tubular necrosis has been linked to cephalosporins and tetracyclines. Crystal nephropathy has been seen with quinolones and sulfonamides.
Advanced age is an important risk factor for renal dysfunction from quinolones,18 and penicillin G has been reported to cause glomerulonephritis.31
MUSCULOSKELETAL SYSTEM
Quinolones have been associated with arthropathy or tendinitis at a rate of 1%, including cases of Achilles tendon rupture.18 The US Food and Drug Administration announced in 2016 that the serious adverse events with fluoroquinolones outweigh the benefits in patients with acute sinusitis, acute bronchitis, and uncomplicated urinary tract infection, and that they should be used only if there are no other options.34
Daptomycin is known to cause elevations of creatine kinase.34 Weekly monitoring is recommended based on postmarketing data reports of elevations in 2.5% of patients; myopathy is a rarer effect, occurring in 0.2% of patients.35
REPRODUCTIVE SYSTEM
Antibiotics have long been reported to interact with oral contraceptives, but the data are not compelling for commonly used antibiotics. The strongest association is with rifampicin, which reduces oral contraceptive efficacy and warrants an alternative mode of contraception.36
BACK TO OUR PATIENT
Antibiotics can have serious adverse effects, and it is important for clinicians to be cognizant of this. Our 60-year-old patient who was taking cefepime and vancomycin for pneumonia developed prolonged atypical absence seizures. When the cefepime was discontinued, his mental status improved, and no other seizures were observed.
A 60-year-old man is admitted for respiratory failure following a massive myocardial infarction. He develops ventilator-associated pneumonia and is treated with cefepime and vancomycin. Three days later, he develops prolonged atypical absence seizures.
What caused these seizures? The neurologist thinks it might be the cefepime. Do you agree?
Antibiotics are widely used in the United States, with 269 million courses of oral therapy prescribed in 2011.1 Adverse effects such as rash are well known, but rare effects such as seizure, hypoglycemia, and hypoxemia may not be immediately attributed to these drugs.
In this article, we review less-recognized but potentially serious adverse effects of antibiotics commonly prescribed in the United States. We have structured our discussion by organ system for ease of reference.
NERVOUS SYSTEM
The potential adverse effects of antibiotics on the nervous system range from encephalopathy and seizure to nonconvulsive status epilepticus.
Encephalopathy and seizure
Encephalopathy has been reported with penicillins, cephalosporins, sulfamethoxazole-trimethoprim, quinolones, and oxazolidinones such as linezolid.2,3
Seizures are known to occur with penicillins, cephalosporins, carbapenems, and quinolones.2–4 For cephalosporins, these effects are more common at higher doses, in elderly patients, and in patients with renal impairment. Carbapenems are associated with seizure activity in elderly patients.2–4
Encephalopathy and seizure can also occur on a continuum, as is the case with piperacillin-induced encephalopathy, with progressive dysarthria, tremor, and progressive confusion culminating in tonic-clonic seizures.2
Nonconvulsive status epilepticus
Nonconvulsive status epilepticus, marked by prolonged atypical absence seizures, has complicated the use of penicillins, quinolones, clarithromycin, and cephalosporins, specifically cefepime.2,3,5 Diagnosis can be difficult and requires clinical awareness and confirmation with electroencephalography.
Class-specific neurologic effects
Certain antibiotics have class-specific effects:
Tetracyclines: cranial nerve toxicity, neuromuscular blockade, and intracranial hypertension.2
Sulfamethoxazole-trimethoprim: tremors and psychosis, with visual and auditory hallucinations.6
Macrolides: dysequilibrium and potentially irreversible hearing loss.2
Quinolones: orofacial dyskinesia and a Tourette-like syndrome, with a higher incidence reported with newer quinolones.7
Linezolid: optic and peripheral neuropathy2; neuropathy can be persistent and can lead to loss of vision. The package insert recommends monitoring visual function in patients taking linezolid for more than 3 months and in any patient reporting visual symptoms.8
Linezolid is also associated with serotonin syndrome when combined with a drug that potentiates serotonergic activity, most commonly selective serotonin reuptake inhibitors. The syndrome is characterized by a triad of cognitive or behavioral changes, autonomic instability, and neuromuscular excitability such as spontaneous clonus.9
Metronidazole: optic and peripheral neuropathy, in addition to cerebellar toxicity and central nervous system lesions on magnetic resonance imaging of the brain. In a series of 11 cases of cerebellar toxicity, most patients presented with ataxia and dysarthria associated with high total doses of metronidazole, and in most cases, magnetic resonance imaging showed resolution of the lesions upon discontinuation of metronidazole.10
HEMATOLOGIC AND RHEUMATOLOGIC EFFECTS
Agranulocytosis has been associated with beta-lactams, in most cases with prolonged exposure. In one report, the average exposure before onset of agranulocytosis was 22 days for nafcillin and 25 days for penicillin. For penicillins, more than 50% of cases involved high daily doses.11
Likewise, most episodes of vancomycin-induced neutropenia were reported to occur after 20 days of therapy.12
In another study, most cases of drug-induced anemia were due to ceftriaxone and piperacillin.13
Drug-induced thrombocytopenia has been described with penicillins, cephalosporins, sulfonamides, and vancomycin14 and is a well-recognized effect of linezolid. The syndrome of drug reaction with eosinophilia and systemic symptoms, a severe and rare adverse reaction, has been reported with minocycline, sulfamethoxazole, and vancomycin.15
The tetracycline minocycline has been reported to cause drug-induced lupus and polyarteritis nodosa-like vasculitis.16 Drug-induced lupus presents as myalgias and arthralgias, serositis, constitutional symptoms, and positive antinuclear antibody titers. The effect is not dose-dependent. Penicillin, cefuroxime, and nitrofurantoin have also been implicated.16
Kermani et al17 described 9 cases of polyarteritis nodosa, in which 5 patients (56%) had systemic involvement including renal artery microaneurysm, mononeuritis multiplex, and mesenteric vasculitis, and some of these patients also had cutaneous involvement. All patients had positive antineutrophil cytoplasmic antibody in a perinuclear pattern. The median time from start of the minocycline to symptom onset was 9 months, and the median duration of use was 2 years.
Quinolones have also been reported to cause fatal hypersensitivity vasculitis.18,19
CARDIOVASCULAR SYSTEM
Macrolides and quinolones have been reported to cause QT-interval prolongation and torsades de pointes. The risk is greatest when a macrolide is co-administered with a CYP3A4 inhibitor.
Of the macrolides, azithromycin is the safest, as clarithromycin and erythromycin are more likely to cause QT prolongation.
While QT prolongation is a class effect of quinolones, there is variability within the class. Ciprofloxacin is thought to be the safest in terms of cardiovascular adverse effects.20 In addition, Owens and Nolin20 reported that quinolone-associated QT prolongation was more likely to occur in patients with pre-existing QT prolongation, electrolyte abnormalities, organic heart disease, and bradycardia, and especially in women. Other risk factors for QT prolongation with quinolone use include underlying cardiac disease and advanced age.21
Quinolones have also been associated with an increased risk of aortic dissection. The US Food and Drug Administration has issued a warning advising clinicians to avoid quinolones in patients who have aneurysms or are at risk for aneurysms, such as patients with advanced age, peripheral atherosclerotic vascular disease, hypertension and conditions such as Marfan and Ehlers-Danlos syndrome.22
DIGESTIVE SYSTEM
Tetracyclines are known to cause esophagitis from direct contact with and disruption of the mucosal lining. Doxycycline is the most frequent offender.23
Amoxicillin-clavulanate is the antibiotic most commonly associated with drug-induced liver injury, mainly attributable to the clavulanate component.24 It is more common in men over age 50 and with prolonged and repeated dosing and is sometimes fatal. Other adverse effects include Stevens-Johnson syndrome, interstitial nephritis, and thrombotic thrombocytopenic purpura.25
Cholestatic hepatitis has been reported with penicillins, particularly dicloxacillin, oxacillin, and amoxicillin-clavulanate; cephalosporins; doxycycline; sulfamethoxazole-trimethoprim; macrolides; and ciprofloxacin.24–26 Hepatocellular injury is linked to amoxicillin-clavulanate and doxycycline. Drug-induced mixed liver injury has been observed with amoxicillin-clavulanate, sulfamethoxazole-trimethoprim and, rarely, cephalosporins.
Liver injury is classified as cholestatic if the alkaline phosphatase level is more than 2 times higher than normal, or if the ratio of alanine aminotransferase to alkaline phosphatase is less than 2; if the ratio is greater than 5, the injury is considered hepatocellular.24 Mixed liver injury, the most common, is defined as a ratio from 2 to 5.
Nitrofurantoin has also been linked to hepatotoxicity, cirrhosis, and end-stage liver disease, and to death if the drug is continued after the onset of jaundice.26 Death from liver injury has been reported with amoxicillin-clavulanate, sulfamethoxazole-trimethoprim, and erythromycin, and jaundice indicates a poor prognosis, associated with a 10% mortality rate or need for liver transplant in all patients.24
ENDOCRINE SYSTEM
Clarithromycin, sulfonamides, and quinolones are known to precipitate hypoglycemia by interacting with sulfonylureas. A study of Medicare patients age 66 or older who were taking glipizide or glyburide reported that female sex, older age, and a history of hypoglycemic episodes were associated with antibiotic-related hypoglycemia.27 The odds ratio for hypoglycemia was highest for clarithromycin (3.96), sulfamethoxazole-trimethoprim (2.56), metronidazole (2.11), and ciprofloxacin (1.62) when compared with antibiotics that do not cause hypoglycemia. There was no signal for levofloxacin-mediated hypoglycemia in this series.27
RESPIRATORY SYSTEM
Hypersensitivity lung disease has been reported with penicillin, ampicillin, cephalosporins, ciprofloxacin, and sulfonamides including sulfamethoxazole-trimethoprim.28 The lipopeptide daptomycin has been reported to cause acute eosinophilic pneumonia defined as fever for less than 5 days, pulmonary infiltrates, hypoxemia, and a bronchoalveolar lavage or biopsy study with eosinophils. Daptomycin should be stopped early in these cases, and the patient should not be rechallenged, as the reaction can be deadly.29
Nitrofurantoin has a long history of hypersensitivity pneumonitis in its acute form and a chronic allergic response. While more widely recognized, nitrofurantoin pulmonary toxicity is rare, occurring in 1 in 5,000 patients.30
RENAL SYSTEM
Acute interstitial nephritis has been reported with penicillins, cephalosporins, macrolides, quinolones, sulfonamides, and vancomycin.31–33 Acute tubular necrosis has been linked to cephalosporins and tetracyclines. Crystal nephropathy has been seen with quinolones and sulfonamides.
Advanced age is an important risk factor for renal dysfunction from quinolones,18 and penicillin G has been reported to cause glomerulonephritis.31
MUSCULOSKELETAL SYSTEM
Quinolones have been associated with arthropathy or tendinitis at a rate of 1%, including cases of Achilles tendon rupture.18 The US Food and Drug Administration announced in 2016 that the serious adverse events with fluoroquinolones outweigh the benefits in patients with acute sinusitis, acute bronchitis, and uncomplicated urinary tract infection, and that they should be used only if there are no other options.34
Daptomycin is known to cause elevations of creatine kinase.34 Weekly monitoring is recommended based on postmarketing data reports of elevations in 2.5% of patients; myopathy is a rarer effect, occurring in 0.2% of patients.35
REPRODUCTIVE SYSTEM
Antibiotics have long been reported to interact with oral contraceptives, but the data are not compelling for commonly used antibiotics. The strongest association is with rifampicin, which reduces oral contraceptive efficacy and warrants an alternative mode of contraception.36
BACK TO OUR PATIENT
Antibiotics can have serious adverse effects, and it is important for clinicians to be cognizant of this. Our 60-year-old patient who was taking cefepime and vancomycin for pneumonia developed prolonged atypical absence seizures. When the cefepime was discontinued, his mental status improved, and no other seizures were observed.
- Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010–2011. JAMA 2016; 315(17):1864–1873. doi:10.1001/jama.2016.4151
- Grill MF, Maganti RK. Neurotoxic effects associated with antibiotic use: management considerations. Br J Clin Pharmacol 2011; 72(3):381–393. doi:10.1111/j.1365-2125.2011.03991.x
- Dakdouki GK, Al-Awar GN. Cefepime-induced encephalopathy. Int J Infect Dis 2004; 8(1):59–61. pmid:14690782
- Bazan JA, Martin SI, Kaye KM. Newer beta-lactam antiobiotics: doripenem, ceftobiprole, and cefepime. Infect Dis Clin North Am 2009; 23(4):983–999. doi:10.1016/j.idc.2009.06.007
- Bandettini di Poggio M, Anfosso S, Audenino D, Primavera A. Clarithromycin-induced neurotoxicity in adults. J Clin Neurosci 2011; 18(3):313–318. doi:10.1016/j.jocn.2010.08.014
- Saidinejad M, Ewald MB, Shannon MW. Transient psychosis in an immune-competent patient after oral trimethoprim-sulfamethoxazole administration. Pediatrics 2005; 115(6):e739–e741. doi:10.1542/peds.2004-1352
- Thomas RJ, Reagan DR. Association of a Tourette-like syndrome with ofloxacin. Ann Pharmacother 1996; 30(2):138–141. doi:10.1177/106002809603000205
- Pharmacia and Upjohn Company LLC. Zyvox® Package Insert. http://labeling.pfizer.com/showlabeling.aspx?id=649. Accessed March 5, 2019.
- Lawrence KR, Adra M, Gillman PK. Serotonin toxicity associated with the use of linezolid: a review of postmarketing data. Clin Infect Dis 2006; 42(11):1578–1583. doi:10.1086/503839
- Patel K, Green-Hopkins I, Lu S, Tunkel AR. Cerebellar ataxia following prolonged use of metronidazole: case report and literature review. Int J Infect Dis 2008; 12(6):e111–e114. doi:10.1016/j.ijid.2008.03.006
- Andersohn F, Konzen C, Garbe E. Systematic review: agranulocytosis induced by nonchemotherapy drugs. Ann Intern Med 2007; 146(9):657–665. pmid:17470834
- Black E, Lau TT, Ensom MH. Vancomycin-induced neutropenia: is it dose- or duration-related? Ann Pharmacother 2011; 45(5):629–638. doi:10.1345/aph.1P583
- Garratty G. Drug-induced immune hemolytic anemia. Hematology Am Soc Hematol Educ Program 2009: 73–79. doi:10.1182/asheducation-2009.1.73
- Chong Bh, Choi PY, Khachigian L, Perdomo J. Drug-induced immune thrombocytopenia. Hematol Oncol Clin North Am 2013; 27(3):521–540. doi:10.1016/j.hoc.2013.02.003
- Cacoub P, Musette P, Descamps V, et al. The DRESS syndrome: a literature review. Am J Med 2011; 124(7):588–597. doi:10.1016/j.amjmed.2011.01.017
- Chang C, Gershwin ME. Drugs and autoimmunity—a contemporary review and mechanistic approach. J Autoimmun 2010; 34(3):J266–J275. doi:10.1016/j.jaut.2009.11.012
- Kermani TA, Ham EK, Camilleri MJ, Warrington KJ. Polyarteritis nodosa-like vasculitis in association with minocycline use: a single-center case series. Semin Arthritis Rheum 2012; 42(2):213–221. doi:10.1016/j.semarthrit.2012.03.006
- Mandell LA, Ball P, Tillotson G. Antimicrobial safety and tolerability: differences and dilemmas. Clin Infect Dis 2001; 32(suppl 1):S72–S79. doi:10.1086/319379
- Christ W, Esch B. Session III: safety. Adverse reactions to fluoroquinolones in adults and children. Infect Dis Clin Pract 1994; 3(3 suppl 3):S168–S176.
- Owens RC, Nolin TD. Antimicrobial-associated QT interval prolongation: pointes of interest. Clin Infect Dis 2006; 43(12):1603–1611. doi:10.1086/508873
- Rubinstein E, Camm J. Cardiotoxicity of fluoroquinolones. J Antimicrob Chemother 2002; 49(4):593–596. pmid:11909831
- US Food and Drug Administration (FDA). FDA drug safety communication: FDA warns about increased risk of ruptures or tears in the aorta blood vessel with fluoroquinolones antibiotics in certain patients. https://www.fda.gov/Drugs/DrugSafety/ucm628753.htm. Accessed March 15, 2019.
- Seminerio J, McGrath K, Arnold CA, Voltaggio L, Singhi AD. Medication-associated lesions of the GI tract. Gastrointest Endosc 2014; 79(1):140–150. doi:10.1016/j.gie.2013.08.027
- Bjornsson ES, Jonasson JG. Drug-induced cholestasis. Clin Liver Dis 2013; 17(2):191–209. doi:10.1016/j.cld.2012.11.002
- Fontana RJ, Shakil AO, Greenson JK, Boyd I, Lee WM. Acute liver failure due to amoxicillin and amoxicillin/clavulanate. Dig Dis Sci 2005; 50(10):1785–1790. doi:10.1007/s10620-005-2938-5
- Sakaan SA, Twilla JD, Usery JB, Winton JC, Self TH. Nitrofurantoin-induced hepatotoxicity: a rare yet serious complication. South Med J 2014; 107(2):107–113. doi:10.1097/SMJ.0000000000000059
- Parekh TM, Raji M, Lin YL, Tan A, Kuo YF, Goodwin JS. Hypoglycemia after antimicrobial drug prescription for older patients using sulfonylureas. JAMA Intern Med 2014; 174(10):1605–1612. doi:10.1001/jamainternmed.2014.3293
- Prasad R, Gupta P, Singh A, Goel N. Drug induced pulmonary parenchymal disease. Drug Discov Ther 2014; 8(6):232–237. doi:10.5582/ddt.2014.01046
- Miller BA, Gray A, Leblanc TW, Sexton DJ, Martin AR, Slama TG. Acute eosinophilic pneumonia secondary to daptomycin: a report of three cases. Clin Infect Dis 2010; 50(11):e63–e68. doi:10.1086/652656
- Kabbara WK, Kordahi MC. Nitrofurantoin-induced pulmonary toxicity: a case report and review of the literature. J Infect Public Health 2015; 8(4):309–313. doi:10.1016/j.jiph.2015.01.007
- Ghane Shahrbaf F, Assadi F. Drug-induced renal disorders. J Renal Inj Prev 2015; 4(3):57–60. doi:10.12861/jrip.2015.12
- Mac K, Chavada R, Paull S, Howlin K, Wong J. Cefepime induced acute interstitial nephritis—a case report. BMC Nephrol 2015; 16:15. doi:10.1186/s12882-015-0004-x
- Woodruff AE, Meaney CJ, Hansen EA, Prescott GM. Azithromycin-induced, biopsy-proven cute interstitial nephritis in an adult successfully treated with low-dose corticosteroids. Pharmacotherapy 2015; 35(11):e169–e174. doi:10.1002/phar.1660
- US Food and Drug Administration (FDA). FDA drug safety communication: FDA advises restricting fluoroquinolone antibiotic use for certain uncomplicated infections; warns about disabling side effects that can occur together. https://www.fda.gov/Drugs/DrugSafety/ucm500143.htm. Accessed March 7, 2019.
- Hawkey PM. Pre-clinical experience with daptomycin. J Antimicrob Chemother 2008; 62(suppl 3):iii7–iii14. doi:10.1093/jac/dkn367
- ACOG Committee on Practice Bulletins–Gynecology. ACOG practice bulletin. No. 73: Use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol 2006; 107(6):1453–1472. pmid:16738183
- Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010–2011. JAMA 2016; 315(17):1864–1873. doi:10.1001/jama.2016.4151
- Grill MF, Maganti RK. Neurotoxic effects associated with antibiotic use: management considerations. Br J Clin Pharmacol 2011; 72(3):381–393. doi:10.1111/j.1365-2125.2011.03991.x
- Dakdouki GK, Al-Awar GN. Cefepime-induced encephalopathy. Int J Infect Dis 2004; 8(1):59–61. pmid:14690782
- Bazan JA, Martin SI, Kaye KM. Newer beta-lactam antiobiotics: doripenem, ceftobiprole, and cefepime. Infect Dis Clin North Am 2009; 23(4):983–999. doi:10.1016/j.idc.2009.06.007
- Bandettini di Poggio M, Anfosso S, Audenino D, Primavera A. Clarithromycin-induced neurotoxicity in adults. J Clin Neurosci 2011; 18(3):313–318. doi:10.1016/j.jocn.2010.08.014
- Saidinejad M, Ewald MB, Shannon MW. Transient psychosis in an immune-competent patient after oral trimethoprim-sulfamethoxazole administration. Pediatrics 2005; 115(6):e739–e741. doi:10.1542/peds.2004-1352
- Thomas RJ, Reagan DR. Association of a Tourette-like syndrome with ofloxacin. Ann Pharmacother 1996; 30(2):138–141. doi:10.1177/106002809603000205
- Pharmacia and Upjohn Company LLC. Zyvox® Package Insert. http://labeling.pfizer.com/showlabeling.aspx?id=649. Accessed March 5, 2019.
- Lawrence KR, Adra M, Gillman PK. Serotonin toxicity associated with the use of linezolid: a review of postmarketing data. Clin Infect Dis 2006; 42(11):1578–1583. doi:10.1086/503839
- Patel K, Green-Hopkins I, Lu S, Tunkel AR. Cerebellar ataxia following prolonged use of metronidazole: case report and literature review. Int J Infect Dis 2008; 12(6):e111–e114. doi:10.1016/j.ijid.2008.03.006
- Andersohn F, Konzen C, Garbe E. Systematic review: agranulocytosis induced by nonchemotherapy drugs. Ann Intern Med 2007; 146(9):657–665. pmid:17470834
- Black E, Lau TT, Ensom MH. Vancomycin-induced neutropenia: is it dose- or duration-related? Ann Pharmacother 2011; 45(5):629–638. doi:10.1345/aph.1P583
- Garratty G. Drug-induced immune hemolytic anemia. Hematology Am Soc Hematol Educ Program 2009: 73–79. doi:10.1182/asheducation-2009.1.73
- Chong Bh, Choi PY, Khachigian L, Perdomo J. Drug-induced immune thrombocytopenia. Hematol Oncol Clin North Am 2013; 27(3):521–540. doi:10.1016/j.hoc.2013.02.003
- Cacoub P, Musette P, Descamps V, et al. The DRESS syndrome: a literature review. Am J Med 2011; 124(7):588–597. doi:10.1016/j.amjmed.2011.01.017
- Chang C, Gershwin ME. Drugs and autoimmunity—a contemporary review and mechanistic approach. J Autoimmun 2010; 34(3):J266–J275. doi:10.1016/j.jaut.2009.11.012
- Kermani TA, Ham EK, Camilleri MJ, Warrington KJ. Polyarteritis nodosa-like vasculitis in association with minocycline use: a single-center case series. Semin Arthritis Rheum 2012; 42(2):213–221. doi:10.1016/j.semarthrit.2012.03.006
- Mandell LA, Ball P, Tillotson G. Antimicrobial safety and tolerability: differences and dilemmas. Clin Infect Dis 2001; 32(suppl 1):S72–S79. doi:10.1086/319379
- Christ W, Esch B. Session III: safety. Adverse reactions to fluoroquinolones in adults and children. Infect Dis Clin Pract 1994; 3(3 suppl 3):S168–S176.
- Owens RC, Nolin TD. Antimicrobial-associated QT interval prolongation: pointes of interest. Clin Infect Dis 2006; 43(12):1603–1611. doi:10.1086/508873
- Rubinstein E, Camm J. Cardiotoxicity of fluoroquinolones. J Antimicrob Chemother 2002; 49(4):593–596. pmid:11909831
- US Food and Drug Administration (FDA). FDA drug safety communication: FDA warns about increased risk of ruptures or tears in the aorta blood vessel with fluoroquinolones antibiotics in certain patients. https://www.fda.gov/Drugs/DrugSafety/ucm628753.htm. Accessed March 15, 2019.
- Seminerio J, McGrath K, Arnold CA, Voltaggio L, Singhi AD. Medication-associated lesions of the GI tract. Gastrointest Endosc 2014; 79(1):140–150. doi:10.1016/j.gie.2013.08.027
- Bjornsson ES, Jonasson JG. Drug-induced cholestasis. Clin Liver Dis 2013; 17(2):191–209. doi:10.1016/j.cld.2012.11.002
- Fontana RJ, Shakil AO, Greenson JK, Boyd I, Lee WM. Acute liver failure due to amoxicillin and amoxicillin/clavulanate. Dig Dis Sci 2005; 50(10):1785–1790. doi:10.1007/s10620-005-2938-5
- Sakaan SA, Twilla JD, Usery JB, Winton JC, Self TH. Nitrofurantoin-induced hepatotoxicity: a rare yet serious complication. South Med J 2014; 107(2):107–113. doi:10.1097/SMJ.0000000000000059
- Parekh TM, Raji M, Lin YL, Tan A, Kuo YF, Goodwin JS. Hypoglycemia after antimicrobial drug prescription for older patients using sulfonylureas. JAMA Intern Med 2014; 174(10):1605–1612. doi:10.1001/jamainternmed.2014.3293
- Prasad R, Gupta P, Singh A, Goel N. Drug induced pulmonary parenchymal disease. Drug Discov Ther 2014; 8(6):232–237. doi:10.5582/ddt.2014.01046
- Miller BA, Gray A, Leblanc TW, Sexton DJ, Martin AR, Slama TG. Acute eosinophilic pneumonia secondary to daptomycin: a report of three cases. Clin Infect Dis 2010; 50(11):e63–e68. doi:10.1086/652656
- Kabbara WK, Kordahi MC. Nitrofurantoin-induced pulmonary toxicity: a case report and review of the literature. J Infect Public Health 2015; 8(4):309–313. doi:10.1016/j.jiph.2015.01.007
- Ghane Shahrbaf F, Assadi F. Drug-induced renal disorders. J Renal Inj Prev 2015; 4(3):57–60. doi:10.12861/jrip.2015.12
- Mac K, Chavada R, Paull S, Howlin K, Wong J. Cefepime induced acute interstitial nephritis—a case report. BMC Nephrol 2015; 16:15. doi:10.1186/s12882-015-0004-x
- Woodruff AE, Meaney CJ, Hansen EA, Prescott GM. Azithromycin-induced, biopsy-proven cute interstitial nephritis in an adult successfully treated with low-dose corticosteroids. Pharmacotherapy 2015; 35(11):e169–e174. doi:10.1002/phar.1660
- US Food and Drug Administration (FDA). FDA drug safety communication: FDA advises restricting fluoroquinolone antibiotic use for certain uncomplicated infections; warns about disabling side effects that can occur together. https://www.fda.gov/Drugs/DrugSafety/ucm500143.htm. Accessed March 7, 2019.
- Hawkey PM. Pre-clinical experience with daptomycin. J Antimicrob Chemother 2008; 62(suppl 3):iii7–iii14. doi:10.1093/jac/dkn367
- ACOG Committee on Practice Bulletins–Gynecology. ACOG practice bulletin. No. 73: Use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol 2006; 107(6):1453–1472. pmid:16738183
KEY POINTS
- Piperacillin-induced encephalopathy and seizure can occur on a continuum, with progressive dysarthria, tremor, and confusion culminating in tonic-clonic seizures.
- Monocycline-induced lupus can present as myalgia, arthralgia, serositis, constitutional symptoms, and a positive antinuclear antibody titer. The effect is not dose-dependent.
- Acute tubular necrosis has been linked to cephalosporins and tetracyclines. Crystal nephropathy has been reported with quinolones and sulfonamides.
- QT-interval prolongation is a class effect of quinolones and is more likely to occur in patients with pre-existing QT prolongation, electrolyte abnormalities, organic heart disease, or bradycardia, or in women.
How should I treat acute agitation in pregnancy?
Acute agitation in the pregnant patient should be treated as an obstetric emergency, as it jeopardizes the safety of the patient and fetus, as well as others in the emergency room. Uncontrolled agitation is associated with obstetric complications such as preterm delivery, placental abnormalities, postnatal death, and spontaneous abortion.1
Current data on the reproductive safety of drugs commonly used to treat acute agitation—benzodiazepines, typical (first-generation) antipsychotics, atypical (second-generation) antipsychotics, and diphenhydramine—suggest no increase in risk beyond the 2% to 3% risk of congenital malformations in the general population when used in the first trimester.2,3
FOCUS OF THE EMERGENCY EVALUATION
Agitation is defined as the physical manifestation of internal distress, due to an underlying medical condition such as delirium or to a psychiatric condition such as acute intoxication or withdrawal, psychosis, mania, or personality disorder.4
For the agitated pregnant woman who is not belligerent at presentation, triage should start with a basic assessment of airways, breathing, and circulation, as well as vital signs and glucose level.5 A thorough medical history and a description of events leading to the presentation, obtained from the patient or the patient’s family or friends, are vital for narrowing the diagnosis and deciding treatment.
The initial evaluation should include consideration of delirium, trauma, intracranial hemorrhage, coagulopathy, thrombocytopenia, amniotic and venous thromboembolism, hypoxia and hypercapnia, and signs and symptoms of intoxication or withdrawal from substances such as alcohol, cocaine, phencyclidine, methamphetamine, and substituted cathinones (“bath salts”). From 20 weeks of gestation to 6 weeks postpartum, eclampsia should also be considered in the differential diagnosis.1 Ruling out these conditions is important since the management of each differs vastly from the protocol for agitation secondary to psychosis, mania, or delirium.
NEW SYSTEM TO DETERMINE RISK DURING PREGNANCY, LACTATION
The US Food and Drug Administration (FDA) has discontinued its pregnancy category labeling system that used the letters A, B, C, D, and X to convey reproductive and lactation safety. The new system, established under the FDA Pregnancy and Lactation Labeling Rule,6 provides descriptive, up-to-date explanations of risk, as well as previously absent context regarding baseline risk for major malformations in the general population to help with informed decision-making.7 This allows the healthcare provider to interpret the risk for an individual patient.
FIRST-GENERATION ANTIPSYCHOTICS SAFE, EFFECTIVE IN PREGNANCY
Reproductive safety of first-generation (ie, typical) neuroleptics such as haloperidol is supported by extensive data accumulated over the past 50 years.2,3,8 No significant teratogenic effect has been documented with this drug class,7 although a 1996 meta-analysis found a small increase in the relative risk of congenital malformations in offspring exposed to low-potency antipsychotics compared with those exposed to high-potency antipsychotics.2
In general, mid- and high-potency antipsychotics (eg, haloperidol, perphenazine) are often recommended because they are less likely to have associated sedative or hypotensive effects than low-potency antipsychotics (eg, chlorpromazine, perphenazine), which may be a significant consideration for a pregnant patient.2,8
There is a theoretical risk of neonatal extrapyramidal symptoms with exposure to first-generation antipsychotics in the third trimester, but the data to support this are from sparse case reports and small observational cohorts.9
NEWER ANTIPSYCHOTICS ALSO SAFE IN PREGNANCY
Newer antipsychotics such as the second-generation antipsychotics, available since the mid-1990s, are increasingly used as primary or adjunctive therapy across a wide range of psychiatric disorders.10 Recent data from large, prospective cohort studies investigating reproductive safety of these agents are reassuring, with no specific patterns of organ malformation.11,12
DIPHENHYDRAMINE
Recent studies of antihistamines such as diphenhydramine have not reported any risk of major malformations with first-trimester exposure to antihistamines.13,14 Dose-dependent anticholinergic adverse effects of antihistamines can induce or exacerbate delirium and agitation, although these effects are classically seen in elderly, nonpregnant patients.15 Thus, given the paucity of adverse effects and the low risk, diphenhydramine is considered safe to use in pregnancy.13
BENZODIAZEPINES
Benzodiazepines are not contraindicated for the treatment of acute agitation in pregnancy.16 Reproductive safety data from meta-analyses and large population-based cohort studies have found no evidence of increased risk of major malformations in neonates born to mothers on prescription benzodiazepines in the first trimester.17,18 While third-trimester exposure to benzodiazepines has been associated with “floppy-baby” syndrome and neonatal withdrawal syndrome,16 these are more likely to occur in women on long-term prescription benzodiazepine therapy. No study has yet assessed the risk of these outcomes with a 1-time acute exposure in the emergency department; however, the risk is likely minimal given the aforementioned data observed in women on long-term prescription benzodiazepine therapy.
STEPWISE MANAGEMENT OF AGITATION IN PREGNANCY
If untreated, agitation in pregnancy is independently associated with outcomes that include premature delivery, low birth weight, growth retardation, postnatal death, and spontaneous abortion.1 The risk of these outcomes greatly outweighs any potential risk from psychotropic medications during pregnancy.
Nevertheless, intervention should progress in a stepwise manner, starting with the least restrictive and progressing toward more restrictive interventions, including pharmacotherapy, use of a seclusion room, and physical restraints (Figure 1).4,19
Before medications are considered, attempts should be made to engage with and “de-escalate” the patient in a safe, nonstimulating environment.19 If this approach is not effective, the patient should be offered oral medications to help with her agitation. However, if the patient’s behavior continues to escalate, presenting a danger to herself or staff, the use of emergency medications is clearly indicated. Providers should succinctly inform the patient of the need for immediate intervention.
If the patient has had a good response in the past to one of these medications or is currently taking one as needed, the same medication should be offered. If the patient has never been treated for agitation, it is important to consider the presenting symptoms, differential diagnosis, and the route and rapidity of administration of medication. If the patient has experienced a fall or other trauma, confirming a viable fetal heart rate between 10 to 22 weeks of gestation with Doppler ultrasonography and obstetric consultation should be considered.
DRUG THERAPY RECOMMENDATIONS
Mild to moderate agitation in pregnancy should be managed conservatively with diphenhydramine. Other options include a benzodiazepine, particularly lorazepam, if alcohol withdrawal is suspected. A second-generation antipsychotic such as olanzapine in a rapidly dissolving form or ziprasidone is another option if a rapid response is required.20 Table 1 provides a summary of pharmacotherapy recommendations.
Severe agitation may require a combination of agents. A commonly used, safe regimen—colloquially called the “B52 bomb”—is haloperidol 5 mg, lorazepam 2 mg, and diphenhydramine 25 to 50 mg for prophylaxis of dystonia.20
The patient’s response should be monitored closely, as dosing may require modification as a result of pregnancy-related changes in drug distribution, metabolism, and clearance.21
Although no study to our knowledge has assessed risk associated with 1-time exposure to any of these classes of medications in pregnant women, the aforementioned data on long-term exposure provide reassurance that single exposure in emergency departments likely has little or no effect for the developing fetus.
PHYSICAL RESTRAINTS FOR AGITATION IN PREGNANCY
Physical restraints along with emergency medications (ie, chemical restraint) may be indicated when the patient poses a danger to herself or others. In some cases, both types of restraint may be required, whether in the emergency room or an inpatient setting.
However, during the second and third trimesters, physical restraints such as 4-point restraints may predispose the patient to inferior vena cava compression syndrome and compromise placental blood flow.4 Therefore, pregnant patients after 20 weeks of gestation should be positioned in the left lateral decubitus position, with the right hip positioned 10 to 12 cm off the bed with pillows or blankets. And when restraints are used in pregnant patients, frequent checking of vital signs and physical assessment is needed to mitigate risks.4
- Aftab A, Shah AA. Behavioral emergencies: special considerations in the pregnant patient. Psychiatr Clin North Am 2017; 40(3):435–448. doi:10.1016/j.psc.2017.05.017
- Altshuler LL, Cohen L, Szuba MP, Burt VK, Gitlin M, Mintz J. Pharmacologic management of psychiatric illness during pregnancy: dilemmas and guidelines. Am J Psychiatry 1996; 153(5):592–606. doi:10.1176/ajp.153.5.592
- Einarson A. Safety of psychotropic drug use during pregnancy: a review. MedGenMed 2005; 7(4):3. pmid:16614625
- Wilson MP, Nordstrom K, Shah AA, Vilke GM. Psychiatric emergencies in pregnant women. Emerg Med Clin North Am 2015; 33(4):841–851. doi:10.1016/j.emc.2015.07.010
- Brown HE, Stoklosa J, Freundenreich O. How to stabilize an acutely psychotic patient. Curr Psychiatry 2012; 11(12):10–16.
- US Food and Drug Administration. Pregnancy and lactation labeling (drugs) final rule. www.fda.gov/drugs/developmentapprovalprocess/developmentresources/labeling/ucm093307.htm. Accessed January 8, 2019.
- Brucker MC, King TL. The 2015 US Food and Drug Administration pregnancy and lactation labeling rule. J Midwifery Womens Health 2017; 62(3):308–316. doi:10.1111/jmwh.12611
- Diav-Citrin O, Shechtman S, Ornoy S, et al. Safety of haloperidol and penfluridol in pregnancy: a multicenter, prospective, controlled study. J Clin Psychiatry 2005; 66(3):317–322. pmid:15766297
- Galbally M, Snellen M, Power J. Antipsychotic drugs in pregnancy: a review of their maternal and fetal effects. Ther Adv Drug Saf 2014; 5(2):100–109. doi:10.1177/2042098614522682
- Kulkarni J, Storch A, Baraniuk A, Gilbert H, Gavrilidis E, Worsley R. Antipsychotic use in pregnancy. Expert Opin Pharmacother 2015; 16(9):1335–1345. doi:10.1517/14656566.2015.1041501
- Huybrechts KF, Hernández-Díaz S, Patorno E, et al. Antipsychotic use in pregnancy and the risk for congenital malformations. JAMA Psychiatry 2016; 73(9):938–946. doi:10.1001/jamapsychiatry.2016.1520
- Cohen LS, Viguera AC, McInerney KA, et al. Reproductive safety of second-generation antipsychotics: current data from the Massachusetts General Hospital national pregnancy registry for atypical antipsychotics. Am J Psychiatry 2016; 173(3):263–270. doi:10.1176/appi.ajp.2015.15040506
- Li Q, Mitchell AA, Werler MM, Yau WP, Hernández-Díaz S. Assessment of antihistamine use in early pregnancy and birth defects. J Allergy Clin Immunol Pract 2013; 1(6):666–674.e1. doi:10.1016/j.jaip.2013.07.008
- Gilboa SM, Strickland MJ, Olshan AF, Werler MM, Correa A; National Birth Defects Prevention Study. Use of antihistamine medications during early pregnancy and isolated major malformations. Birth Defects Res A Clin Mol Teratol 2009; 85(2):137–150. doi:10.1002/bdra.20513
- Meuleman JR. Association of diphenhydramine use with adverse effects in hospitalized older patients: possible confounders. Arch Intern Med 2002; 162(6):720–721. pmid:11911733
- Enato E, Moretti M, Koren G. The fetal safety of benzodiazepines: an updated meta-analysis. J Obstet Gynaecol Can 2011; 33(1):46–48. doi:10.1016/S1701-2163(16)34772-7
- Dolovich LR, Addis A, Vaillancourt JM, Power JD, Koren G, Einarson TR. Benzodiazepine use in pregnancy and major malformations or oral cleft: meta-analysis of cohort and case-control studies. BMJ 1998; 317(7162):839–843. pmid:9748174
- Bellantuono C, Tofani S, Di Sciascio G, Santone G. Benzodiazepine exposure in pregnancy and risk of major malformations: a critical overview. Gen Hosp Psychiatry 2013; 35(1):3–8. doi:10.1016/j.genhosppsych.2012.09.003
- Richmond JS, Berlin JS, Fishkind AB, et al. Verbal de-escalation of the agitated patient: consensus statement of the American Association for Emergency Psychiatry project BETA De-escalation Workgroup. West J Emerg Med 2012; 13(1):17–25. doi:10.5811/westjem.2011.9.6864
- Prager LM, Ivkovic A. Emergency psychiatry. In: Stern TA, Fava M, Wilens TE, Rosenbaum JF, eds. The Massachusetts General Hospital Comprehensive Clinical Psychiatry. 2nd ed. London: Elsevier; 2016:937–949.
- Feghali M, Venkataramanan R, Caritis S. Pharmacokinetics of drugs in pregnancy. Semin Perinatol 2015; 39(7):512–519. doi:10.1053/j.semperi.2015.08.003
Acute agitation in the pregnant patient should be treated as an obstetric emergency, as it jeopardizes the safety of the patient and fetus, as well as others in the emergency room. Uncontrolled agitation is associated with obstetric complications such as preterm delivery, placental abnormalities, postnatal death, and spontaneous abortion.1
Current data on the reproductive safety of drugs commonly used to treat acute agitation—benzodiazepines, typical (first-generation) antipsychotics, atypical (second-generation) antipsychotics, and diphenhydramine—suggest no increase in risk beyond the 2% to 3% risk of congenital malformations in the general population when used in the first trimester.2,3
FOCUS OF THE EMERGENCY EVALUATION
Agitation is defined as the physical manifestation of internal distress, due to an underlying medical condition such as delirium or to a psychiatric condition such as acute intoxication or withdrawal, psychosis, mania, or personality disorder.4
For the agitated pregnant woman who is not belligerent at presentation, triage should start with a basic assessment of airways, breathing, and circulation, as well as vital signs and glucose level.5 A thorough medical history and a description of events leading to the presentation, obtained from the patient or the patient’s family or friends, are vital for narrowing the diagnosis and deciding treatment.
The initial evaluation should include consideration of delirium, trauma, intracranial hemorrhage, coagulopathy, thrombocytopenia, amniotic and venous thromboembolism, hypoxia and hypercapnia, and signs and symptoms of intoxication or withdrawal from substances such as alcohol, cocaine, phencyclidine, methamphetamine, and substituted cathinones (“bath salts”). From 20 weeks of gestation to 6 weeks postpartum, eclampsia should also be considered in the differential diagnosis.1 Ruling out these conditions is important since the management of each differs vastly from the protocol for agitation secondary to psychosis, mania, or delirium.
NEW SYSTEM TO DETERMINE RISK DURING PREGNANCY, LACTATION
The US Food and Drug Administration (FDA) has discontinued its pregnancy category labeling system that used the letters A, B, C, D, and X to convey reproductive and lactation safety. The new system, established under the FDA Pregnancy and Lactation Labeling Rule,6 provides descriptive, up-to-date explanations of risk, as well as previously absent context regarding baseline risk for major malformations in the general population to help with informed decision-making.7 This allows the healthcare provider to interpret the risk for an individual patient.
FIRST-GENERATION ANTIPSYCHOTICS SAFE, EFFECTIVE IN PREGNANCY
Reproductive safety of first-generation (ie, typical) neuroleptics such as haloperidol is supported by extensive data accumulated over the past 50 years.2,3,8 No significant teratogenic effect has been documented with this drug class,7 although a 1996 meta-analysis found a small increase in the relative risk of congenital malformations in offspring exposed to low-potency antipsychotics compared with those exposed to high-potency antipsychotics.2
In general, mid- and high-potency antipsychotics (eg, haloperidol, perphenazine) are often recommended because they are less likely to have associated sedative or hypotensive effects than low-potency antipsychotics (eg, chlorpromazine, perphenazine), which may be a significant consideration for a pregnant patient.2,8
There is a theoretical risk of neonatal extrapyramidal symptoms with exposure to first-generation antipsychotics in the third trimester, but the data to support this are from sparse case reports and small observational cohorts.9
NEWER ANTIPSYCHOTICS ALSO SAFE IN PREGNANCY
Newer antipsychotics such as the second-generation antipsychotics, available since the mid-1990s, are increasingly used as primary or adjunctive therapy across a wide range of psychiatric disorders.10 Recent data from large, prospective cohort studies investigating reproductive safety of these agents are reassuring, with no specific patterns of organ malformation.11,12
DIPHENHYDRAMINE
Recent studies of antihistamines such as diphenhydramine have not reported any risk of major malformations with first-trimester exposure to antihistamines.13,14 Dose-dependent anticholinergic adverse effects of antihistamines can induce or exacerbate delirium and agitation, although these effects are classically seen in elderly, nonpregnant patients.15 Thus, given the paucity of adverse effects and the low risk, diphenhydramine is considered safe to use in pregnancy.13
BENZODIAZEPINES
Benzodiazepines are not contraindicated for the treatment of acute agitation in pregnancy.16 Reproductive safety data from meta-analyses and large population-based cohort studies have found no evidence of increased risk of major malformations in neonates born to mothers on prescription benzodiazepines in the first trimester.17,18 While third-trimester exposure to benzodiazepines has been associated with “floppy-baby” syndrome and neonatal withdrawal syndrome,16 these are more likely to occur in women on long-term prescription benzodiazepine therapy. No study has yet assessed the risk of these outcomes with a 1-time acute exposure in the emergency department; however, the risk is likely minimal given the aforementioned data observed in women on long-term prescription benzodiazepine therapy.
STEPWISE MANAGEMENT OF AGITATION IN PREGNANCY
If untreated, agitation in pregnancy is independently associated with outcomes that include premature delivery, low birth weight, growth retardation, postnatal death, and spontaneous abortion.1 The risk of these outcomes greatly outweighs any potential risk from psychotropic medications during pregnancy.
Nevertheless, intervention should progress in a stepwise manner, starting with the least restrictive and progressing toward more restrictive interventions, including pharmacotherapy, use of a seclusion room, and physical restraints (Figure 1).4,19
Before medications are considered, attempts should be made to engage with and “de-escalate” the patient in a safe, nonstimulating environment.19 If this approach is not effective, the patient should be offered oral medications to help with her agitation. However, if the patient’s behavior continues to escalate, presenting a danger to herself or staff, the use of emergency medications is clearly indicated. Providers should succinctly inform the patient of the need for immediate intervention.
If the patient has had a good response in the past to one of these medications or is currently taking one as needed, the same medication should be offered. If the patient has never been treated for agitation, it is important to consider the presenting symptoms, differential diagnosis, and the route and rapidity of administration of medication. If the patient has experienced a fall or other trauma, confirming a viable fetal heart rate between 10 to 22 weeks of gestation with Doppler ultrasonography and obstetric consultation should be considered.
DRUG THERAPY RECOMMENDATIONS
Mild to moderate agitation in pregnancy should be managed conservatively with diphenhydramine. Other options include a benzodiazepine, particularly lorazepam, if alcohol withdrawal is suspected. A second-generation antipsychotic such as olanzapine in a rapidly dissolving form or ziprasidone is another option if a rapid response is required.20 Table 1 provides a summary of pharmacotherapy recommendations.
Severe agitation may require a combination of agents. A commonly used, safe regimen—colloquially called the “B52 bomb”—is haloperidol 5 mg, lorazepam 2 mg, and diphenhydramine 25 to 50 mg for prophylaxis of dystonia.20
The patient’s response should be monitored closely, as dosing may require modification as a result of pregnancy-related changes in drug distribution, metabolism, and clearance.21
Although no study to our knowledge has assessed risk associated with 1-time exposure to any of these classes of medications in pregnant women, the aforementioned data on long-term exposure provide reassurance that single exposure in emergency departments likely has little or no effect for the developing fetus.
PHYSICAL RESTRAINTS FOR AGITATION IN PREGNANCY
Physical restraints along with emergency medications (ie, chemical restraint) may be indicated when the patient poses a danger to herself or others. In some cases, both types of restraint may be required, whether in the emergency room or an inpatient setting.
However, during the second and third trimesters, physical restraints such as 4-point restraints may predispose the patient to inferior vena cava compression syndrome and compromise placental blood flow.4 Therefore, pregnant patients after 20 weeks of gestation should be positioned in the left lateral decubitus position, with the right hip positioned 10 to 12 cm off the bed with pillows or blankets. And when restraints are used in pregnant patients, frequent checking of vital signs and physical assessment is needed to mitigate risks.4
Acute agitation in the pregnant patient should be treated as an obstetric emergency, as it jeopardizes the safety of the patient and fetus, as well as others in the emergency room. Uncontrolled agitation is associated with obstetric complications such as preterm delivery, placental abnormalities, postnatal death, and spontaneous abortion.1
Current data on the reproductive safety of drugs commonly used to treat acute agitation—benzodiazepines, typical (first-generation) antipsychotics, atypical (second-generation) antipsychotics, and diphenhydramine—suggest no increase in risk beyond the 2% to 3% risk of congenital malformations in the general population when used in the first trimester.2,3
FOCUS OF THE EMERGENCY EVALUATION
Agitation is defined as the physical manifestation of internal distress, due to an underlying medical condition such as delirium or to a psychiatric condition such as acute intoxication or withdrawal, psychosis, mania, or personality disorder.4
For the agitated pregnant woman who is not belligerent at presentation, triage should start with a basic assessment of airways, breathing, and circulation, as well as vital signs and glucose level.5 A thorough medical history and a description of events leading to the presentation, obtained from the patient or the patient’s family or friends, are vital for narrowing the diagnosis and deciding treatment.
The initial evaluation should include consideration of delirium, trauma, intracranial hemorrhage, coagulopathy, thrombocytopenia, amniotic and venous thromboembolism, hypoxia and hypercapnia, and signs and symptoms of intoxication or withdrawal from substances such as alcohol, cocaine, phencyclidine, methamphetamine, and substituted cathinones (“bath salts”). From 20 weeks of gestation to 6 weeks postpartum, eclampsia should also be considered in the differential diagnosis.1 Ruling out these conditions is important since the management of each differs vastly from the protocol for agitation secondary to psychosis, mania, or delirium.
NEW SYSTEM TO DETERMINE RISK DURING PREGNANCY, LACTATION
The US Food and Drug Administration (FDA) has discontinued its pregnancy category labeling system that used the letters A, B, C, D, and X to convey reproductive and lactation safety. The new system, established under the FDA Pregnancy and Lactation Labeling Rule,6 provides descriptive, up-to-date explanations of risk, as well as previously absent context regarding baseline risk for major malformations in the general population to help with informed decision-making.7 This allows the healthcare provider to interpret the risk for an individual patient.
FIRST-GENERATION ANTIPSYCHOTICS SAFE, EFFECTIVE IN PREGNANCY
Reproductive safety of first-generation (ie, typical) neuroleptics such as haloperidol is supported by extensive data accumulated over the past 50 years.2,3,8 No significant teratogenic effect has been documented with this drug class,7 although a 1996 meta-analysis found a small increase in the relative risk of congenital malformations in offspring exposed to low-potency antipsychotics compared with those exposed to high-potency antipsychotics.2
In general, mid- and high-potency antipsychotics (eg, haloperidol, perphenazine) are often recommended because they are less likely to have associated sedative or hypotensive effects than low-potency antipsychotics (eg, chlorpromazine, perphenazine), which may be a significant consideration for a pregnant patient.2,8
There is a theoretical risk of neonatal extrapyramidal symptoms with exposure to first-generation antipsychotics in the third trimester, but the data to support this are from sparse case reports and small observational cohorts.9
NEWER ANTIPSYCHOTICS ALSO SAFE IN PREGNANCY
Newer antipsychotics such as the second-generation antipsychotics, available since the mid-1990s, are increasingly used as primary or adjunctive therapy across a wide range of psychiatric disorders.10 Recent data from large, prospective cohort studies investigating reproductive safety of these agents are reassuring, with no specific patterns of organ malformation.11,12
DIPHENHYDRAMINE
Recent studies of antihistamines such as diphenhydramine have not reported any risk of major malformations with first-trimester exposure to antihistamines.13,14 Dose-dependent anticholinergic adverse effects of antihistamines can induce or exacerbate delirium and agitation, although these effects are classically seen in elderly, nonpregnant patients.15 Thus, given the paucity of adverse effects and the low risk, diphenhydramine is considered safe to use in pregnancy.13
BENZODIAZEPINES
Benzodiazepines are not contraindicated for the treatment of acute agitation in pregnancy.16 Reproductive safety data from meta-analyses and large population-based cohort studies have found no evidence of increased risk of major malformations in neonates born to mothers on prescription benzodiazepines in the first trimester.17,18 While third-trimester exposure to benzodiazepines has been associated with “floppy-baby” syndrome and neonatal withdrawal syndrome,16 these are more likely to occur in women on long-term prescription benzodiazepine therapy. No study has yet assessed the risk of these outcomes with a 1-time acute exposure in the emergency department; however, the risk is likely minimal given the aforementioned data observed in women on long-term prescription benzodiazepine therapy.
STEPWISE MANAGEMENT OF AGITATION IN PREGNANCY
If untreated, agitation in pregnancy is independently associated with outcomes that include premature delivery, low birth weight, growth retardation, postnatal death, and spontaneous abortion.1 The risk of these outcomes greatly outweighs any potential risk from psychotropic medications during pregnancy.
Nevertheless, intervention should progress in a stepwise manner, starting with the least restrictive and progressing toward more restrictive interventions, including pharmacotherapy, use of a seclusion room, and physical restraints (Figure 1).4,19
Before medications are considered, attempts should be made to engage with and “de-escalate” the patient in a safe, nonstimulating environment.19 If this approach is not effective, the patient should be offered oral medications to help with her agitation. However, if the patient’s behavior continues to escalate, presenting a danger to herself or staff, the use of emergency medications is clearly indicated. Providers should succinctly inform the patient of the need for immediate intervention.
If the patient has had a good response in the past to one of these medications or is currently taking one as needed, the same medication should be offered. If the patient has never been treated for agitation, it is important to consider the presenting symptoms, differential diagnosis, and the route and rapidity of administration of medication. If the patient has experienced a fall or other trauma, confirming a viable fetal heart rate between 10 to 22 weeks of gestation with Doppler ultrasonography and obstetric consultation should be considered.
DRUG THERAPY RECOMMENDATIONS
Mild to moderate agitation in pregnancy should be managed conservatively with diphenhydramine. Other options include a benzodiazepine, particularly lorazepam, if alcohol withdrawal is suspected. A second-generation antipsychotic such as olanzapine in a rapidly dissolving form or ziprasidone is another option if a rapid response is required.20 Table 1 provides a summary of pharmacotherapy recommendations.
Severe agitation may require a combination of agents. A commonly used, safe regimen—colloquially called the “B52 bomb”—is haloperidol 5 mg, lorazepam 2 mg, and diphenhydramine 25 to 50 mg for prophylaxis of dystonia.20
The patient’s response should be monitored closely, as dosing may require modification as a result of pregnancy-related changes in drug distribution, metabolism, and clearance.21
Although no study to our knowledge has assessed risk associated with 1-time exposure to any of these classes of medications in pregnant women, the aforementioned data on long-term exposure provide reassurance that single exposure in emergency departments likely has little or no effect for the developing fetus.
PHYSICAL RESTRAINTS FOR AGITATION IN PREGNANCY
Physical restraints along with emergency medications (ie, chemical restraint) may be indicated when the patient poses a danger to herself or others. In some cases, both types of restraint may be required, whether in the emergency room or an inpatient setting.
However, during the second and third trimesters, physical restraints such as 4-point restraints may predispose the patient to inferior vena cava compression syndrome and compromise placental blood flow.4 Therefore, pregnant patients after 20 weeks of gestation should be positioned in the left lateral decubitus position, with the right hip positioned 10 to 12 cm off the bed with pillows or blankets. And when restraints are used in pregnant patients, frequent checking of vital signs and physical assessment is needed to mitigate risks.4
- Aftab A, Shah AA. Behavioral emergencies: special considerations in the pregnant patient. Psychiatr Clin North Am 2017; 40(3):435–448. doi:10.1016/j.psc.2017.05.017
- Altshuler LL, Cohen L, Szuba MP, Burt VK, Gitlin M, Mintz J. Pharmacologic management of psychiatric illness during pregnancy: dilemmas and guidelines. Am J Psychiatry 1996; 153(5):592–606. doi:10.1176/ajp.153.5.592
- Einarson A. Safety of psychotropic drug use during pregnancy: a review. MedGenMed 2005; 7(4):3. pmid:16614625
- Wilson MP, Nordstrom K, Shah AA, Vilke GM. Psychiatric emergencies in pregnant women. Emerg Med Clin North Am 2015; 33(4):841–851. doi:10.1016/j.emc.2015.07.010
- Brown HE, Stoklosa J, Freundenreich O. How to stabilize an acutely psychotic patient. Curr Psychiatry 2012; 11(12):10–16.
- US Food and Drug Administration. Pregnancy and lactation labeling (drugs) final rule. www.fda.gov/drugs/developmentapprovalprocess/developmentresources/labeling/ucm093307.htm. Accessed January 8, 2019.
- Brucker MC, King TL. The 2015 US Food and Drug Administration pregnancy and lactation labeling rule. J Midwifery Womens Health 2017; 62(3):308–316. doi:10.1111/jmwh.12611
- Diav-Citrin O, Shechtman S, Ornoy S, et al. Safety of haloperidol and penfluridol in pregnancy: a multicenter, prospective, controlled study. J Clin Psychiatry 2005; 66(3):317–322. pmid:15766297
- Galbally M, Snellen M, Power J. Antipsychotic drugs in pregnancy: a review of their maternal and fetal effects. Ther Adv Drug Saf 2014; 5(2):100–109. doi:10.1177/2042098614522682
- Kulkarni J, Storch A, Baraniuk A, Gilbert H, Gavrilidis E, Worsley R. Antipsychotic use in pregnancy. Expert Opin Pharmacother 2015; 16(9):1335–1345. doi:10.1517/14656566.2015.1041501
- Huybrechts KF, Hernández-Díaz S, Patorno E, et al. Antipsychotic use in pregnancy and the risk for congenital malformations. JAMA Psychiatry 2016; 73(9):938–946. doi:10.1001/jamapsychiatry.2016.1520
- Cohen LS, Viguera AC, McInerney KA, et al. Reproductive safety of second-generation antipsychotics: current data from the Massachusetts General Hospital national pregnancy registry for atypical antipsychotics. Am J Psychiatry 2016; 173(3):263–270. doi:10.1176/appi.ajp.2015.15040506
- Li Q, Mitchell AA, Werler MM, Yau WP, Hernández-Díaz S. Assessment of antihistamine use in early pregnancy and birth defects. J Allergy Clin Immunol Pract 2013; 1(6):666–674.e1. doi:10.1016/j.jaip.2013.07.008
- Gilboa SM, Strickland MJ, Olshan AF, Werler MM, Correa A; National Birth Defects Prevention Study. Use of antihistamine medications during early pregnancy and isolated major malformations. Birth Defects Res A Clin Mol Teratol 2009; 85(2):137–150. doi:10.1002/bdra.20513
- Meuleman JR. Association of diphenhydramine use with adverse effects in hospitalized older patients: possible confounders. Arch Intern Med 2002; 162(6):720–721. pmid:11911733
- Enato E, Moretti M, Koren G. The fetal safety of benzodiazepines: an updated meta-analysis. J Obstet Gynaecol Can 2011; 33(1):46–48. doi:10.1016/S1701-2163(16)34772-7
- Dolovich LR, Addis A, Vaillancourt JM, Power JD, Koren G, Einarson TR. Benzodiazepine use in pregnancy and major malformations or oral cleft: meta-analysis of cohort and case-control studies. BMJ 1998; 317(7162):839–843. pmid:9748174
- Bellantuono C, Tofani S, Di Sciascio G, Santone G. Benzodiazepine exposure in pregnancy and risk of major malformations: a critical overview. Gen Hosp Psychiatry 2013; 35(1):3–8. doi:10.1016/j.genhosppsych.2012.09.003
- Richmond JS, Berlin JS, Fishkind AB, et al. Verbal de-escalation of the agitated patient: consensus statement of the American Association for Emergency Psychiatry project BETA De-escalation Workgroup. West J Emerg Med 2012; 13(1):17–25. doi:10.5811/westjem.2011.9.6864
- Prager LM, Ivkovic A. Emergency psychiatry. In: Stern TA, Fava M, Wilens TE, Rosenbaum JF, eds. The Massachusetts General Hospital Comprehensive Clinical Psychiatry. 2nd ed. London: Elsevier; 2016:937–949.
- Feghali M, Venkataramanan R, Caritis S. Pharmacokinetics of drugs in pregnancy. Semin Perinatol 2015; 39(7):512–519. doi:10.1053/j.semperi.2015.08.003
- Aftab A, Shah AA. Behavioral emergencies: special considerations in the pregnant patient. Psychiatr Clin North Am 2017; 40(3):435–448. doi:10.1016/j.psc.2017.05.017
- Altshuler LL, Cohen L, Szuba MP, Burt VK, Gitlin M, Mintz J. Pharmacologic management of psychiatric illness during pregnancy: dilemmas and guidelines. Am J Psychiatry 1996; 153(5):592–606. doi:10.1176/ajp.153.5.592
- Einarson A. Safety of psychotropic drug use during pregnancy: a review. MedGenMed 2005; 7(4):3. pmid:16614625
- Wilson MP, Nordstrom K, Shah AA, Vilke GM. Psychiatric emergencies in pregnant women. Emerg Med Clin North Am 2015; 33(4):841–851. doi:10.1016/j.emc.2015.07.010
- Brown HE, Stoklosa J, Freundenreich O. How to stabilize an acutely psychotic patient. Curr Psychiatry 2012; 11(12):10–16.
- US Food and Drug Administration. Pregnancy and lactation labeling (drugs) final rule. www.fda.gov/drugs/developmentapprovalprocess/developmentresources/labeling/ucm093307.htm. Accessed January 8, 2019.
- Brucker MC, King TL. The 2015 US Food and Drug Administration pregnancy and lactation labeling rule. J Midwifery Womens Health 2017; 62(3):308–316. doi:10.1111/jmwh.12611
- Diav-Citrin O, Shechtman S, Ornoy S, et al. Safety of haloperidol and penfluridol in pregnancy: a multicenter, prospective, controlled study. J Clin Psychiatry 2005; 66(3):317–322. pmid:15766297
- Galbally M, Snellen M, Power J. Antipsychotic drugs in pregnancy: a review of their maternal and fetal effects. Ther Adv Drug Saf 2014; 5(2):100–109. doi:10.1177/2042098614522682
- Kulkarni J, Storch A, Baraniuk A, Gilbert H, Gavrilidis E, Worsley R. Antipsychotic use in pregnancy. Expert Opin Pharmacother 2015; 16(9):1335–1345. doi:10.1517/14656566.2015.1041501
- Huybrechts KF, Hernández-Díaz S, Patorno E, et al. Antipsychotic use in pregnancy and the risk for congenital malformations. JAMA Psychiatry 2016; 73(9):938–946. doi:10.1001/jamapsychiatry.2016.1520
- Cohen LS, Viguera AC, McInerney KA, et al. Reproductive safety of second-generation antipsychotics: current data from the Massachusetts General Hospital national pregnancy registry for atypical antipsychotics. Am J Psychiatry 2016; 173(3):263–270. doi:10.1176/appi.ajp.2015.15040506
- Li Q, Mitchell AA, Werler MM, Yau WP, Hernández-Díaz S. Assessment of antihistamine use in early pregnancy and birth defects. J Allergy Clin Immunol Pract 2013; 1(6):666–674.e1. doi:10.1016/j.jaip.2013.07.008
- Gilboa SM, Strickland MJ, Olshan AF, Werler MM, Correa A; National Birth Defects Prevention Study. Use of antihistamine medications during early pregnancy and isolated major malformations. Birth Defects Res A Clin Mol Teratol 2009; 85(2):137–150. doi:10.1002/bdra.20513
- Meuleman JR. Association of diphenhydramine use with adverse effects in hospitalized older patients: possible confounders. Arch Intern Med 2002; 162(6):720–721. pmid:11911733
- Enato E, Moretti M, Koren G. The fetal safety of benzodiazepines: an updated meta-analysis. J Obstet Gynaecol Can 2011; 33(1):46–48. doi:10.1016/S1701-2163(16)34772-7
- Dolovich LR, Addis A, Vaillancourt JM, Power JD, Koren G, Einarson TR. Benzodiazepine use in pregnancy and major malformations or oral cleft: meta-analysis of cohort and case-control studies. BMJ 1998; 317(7162):839–843. pmid:9748174
- Bellantuono C, Tofani S, Di Sciascio G, Santone G. Benzodiazepine exposure in pregnancy and risk of major malformations: a critical overview. Gen Hosp Psychiatry 2013; 35(1):3–8. doi:10.1016/j.genhosppsych.2012.09.003
- Richmond JS, Berlin JS, Fishkind AB, et al. Verbal de-escalation of the agitated patient: consensus statement of the American Association for Emergency Psychiatry project BETA De-escalation Workgroup. West J Emerg Med 2012; 13(1):17–25. doi:10.5811/westjem.2011.9.6864
- Prager LM, Ivkovic A. Emergency psychiatry. In: Stern TA, Fava M, Wilens TE, Rosenbaum JF, eds. The Massachusetts General Hospital Comprehensive Clinical Psychiatry. 2nd ed. London: Elsevier; 2016:937–949.
- Feghali M, Venkataramanan R, Caritis S. Pharmacokinetics of drugs in pregnancy. Semin Perinatol 2015; 39(7):512–519. doi:10.1053/j.semperi.2015.08.003
Cost-Effective Treatment Option for von Willebrand Disease
Click here to read the supplement.
What can be done to provide a cost-effective treatment option for von Willebrand Disease?
Topics include:
- Review of Key Clinical Studies and Surgical Procedures
- Pharmacokinetics Comparison
- Pharmacoeconomic Comparisons
Click here to read the supplement.
Click here to read the supplement.
What can be done to provide a cost-effective treatment option for von Willebrand Disease?
Topics include:
- Review of Key Clinical Studies and Surgical Procedures
- Pharmacokinetics Comparison
- Pharmacoeconomic Comparisons
Click here to read the supplement.
Click here to read the supplement.
What can be done to provide a cost-effective treatment option for von Willebrand Disease?
Topics include:
- Review of Key Clinical Studies and Surgical Procedures
- Pharmacokinetics Comparison
- Pharmacoeconomic Comparisons
Click here to read the supplement.
Management of Cardiovascular Disease Risk in Rheumatoid Arthritis
From the Division of Rh
Abstract
- Objective: To review the management of traditional and nontraditional CVD cardiovascular disease risk factors in rheumatoid arthritis (RA).
- Methods: Literature review of the management of CVD risk in RA.
- Results: Because of the increased risk of CVD events and CVD mortality among RA patients, aggressive management of CVD risk is essential. Providers should follow national guidelines for the management of traditional CVD risk factors, including dyslipidemia, hypertension, and diabetes mellitus. Similar efforts are needed in counseling on lifestyle modifications, including smoking cessation, regular exercise, and maintaining a healthy body weight. Because higher RA disease activity is also linked with CVD risk, aggressive treatment of RA to a target of low disease activity or remission is critical. Furthermore, the selection of potentially “cardioprotective” agents such as methotrexate and tumor necrosis factor inhibitors, while limiting use of nonsteroidal anti-inflammatory drugs and glucocorticoids, are strategies that could be employed by rheumatologists to help mitigate CVD risk in their patients with RA.
- Conclusion: Routine assessment of CVD risk, management of traditional CVD risk factors, counseling on healthy lifestyle habits, and aggressive treatment of RA are essential to minimize CVD risk in this population.
Keywords: rheumatoid arthritis; cardiovascular disease; cardiovascular risk assessment; cardiovascular risk management.
Editor’s note: This article is part 2 of a 2-part article. “Assessment of Cardiovascular Disease Risk in Rheumatoid Arthritis” was published in the January/February 2019 issue.
Rheumatoid arthritis (RA) is a systemic autoimmune condition that contributes to an increased risk for cardiovascular disease (CVD) among affected patients. In persons with RA, the risk of incident CVD and CVD mortality are increased by approximately 50% compared with the general population.1,2 To minimize CVD risk in this population, providers must routinely assess for CVD risk factors3 and aggressively manage both traditional and nontraditional CVD risk factors.
Managing Traditional Risk Factors
As in the general population, identification and management of traditional CVD risk factors are crucial to minimize CVD risk in the RA population. A prospective study of 201 RA patients demonstrated that traditional CVD risk factors were in fact more predictive of endothelial dysfunction and carotid atherosclerosis than were disease-related inflammatory markers in RA.4 Management of traditional risk factors is detailed in the following sections, and recommendations for managing all traditional CVD risk factors are summarized in the Table.
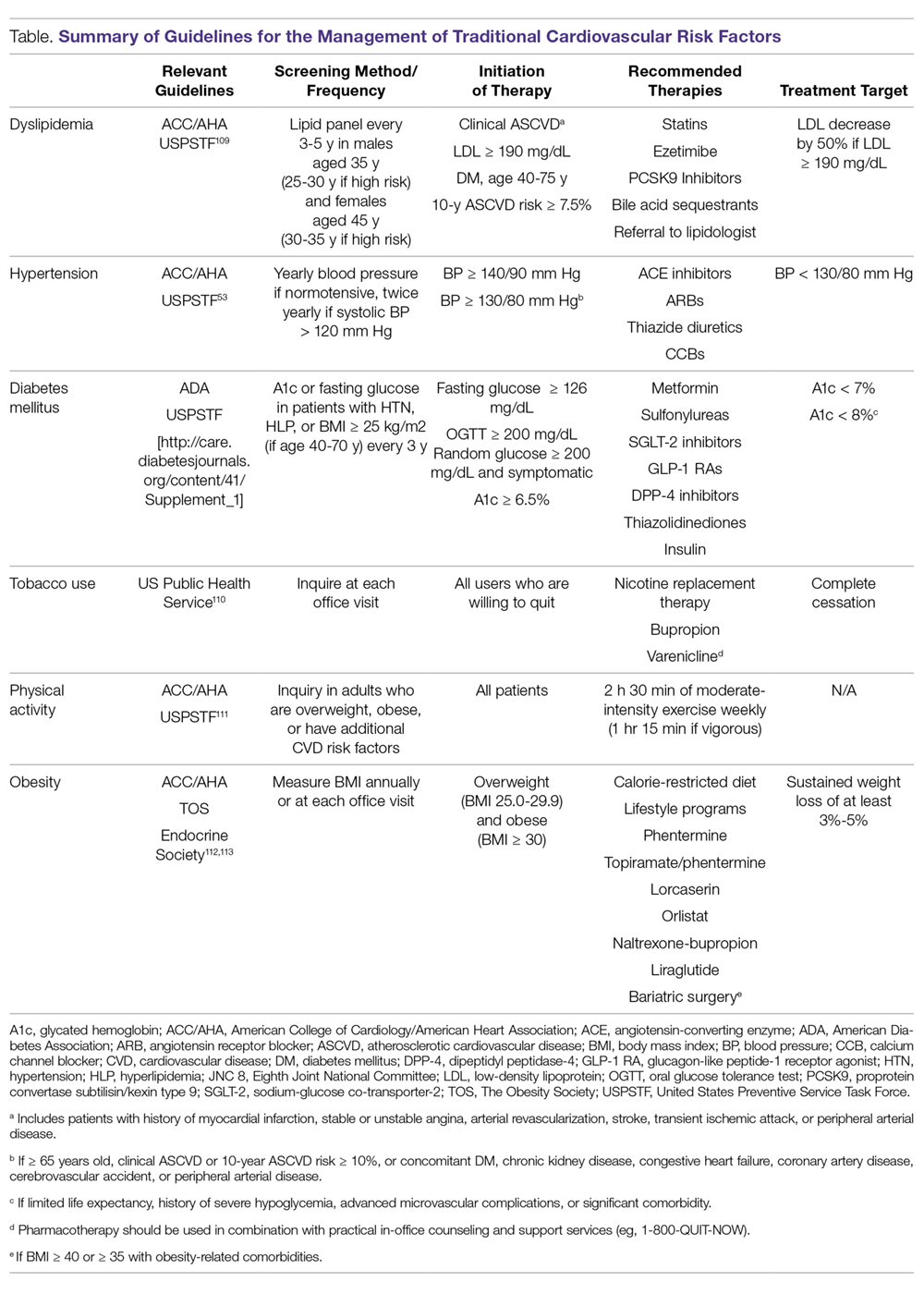
Dyslipidemia
The role of dyslipidemia in atherogenesis is well established, and as a result, lipid levels are nearly universally included in CVD risk stratification tools. However, the interpretation of lipid levels in the context of RA is challenging because of the effects of systemic inflammation on their absolute values. Compared to the general population, patients with RA have lower total cholesterol (TC) and low-density lipoprotein (LDL) levels independent of lipid-lowering therapy.5,6 Despite this, RA patients are at increased risk for CVD. There is even some evidence to suggest a “lipid paradox” in RA, whereby lower TC (< 4 mmol/L) and LDL levels suggest an increased risk of CVD.7,8 In contrast to LDL, higher levels of high-density lipoprotein (HDL) are typically associated with reduced CVD risk, as in the general population.8,9 Interestingly, in a cohort of 16,085 RA patients and 48,499 age- and sex-matched controls, there was no significant difference in the relationship between LDL and CVD risk, suggesting that quantitative lipid levels alone may not entirely explain the CVD mortality gap in RA.9 As such, there is substantial interest in lipoprotein function within the context of CVD risk in RA. Recent investigations have identified impaired HDL function, with reduced cholesterol efflux capacity and antioxidant properties, as well as increased scavenger receptor expression and foam cell formation, in patients with RA.10,11 More research is needed to elucidate how these alterations affect CVD morbidity and mortality and how their measurement could be integrated into improved CVD risk assessment.
Meta-analyses of randomized controlled trials have estimated that lipid-lowering therapy with HMG-CoA reductase inhibitors (statins) reduces the risk of CVD by 25% to 30%; as such, statin therapy has become the standard of care for reduction of CVD risk in the general population.12 Benefits for primary prevention of CVD in RA have also been observed; statin therapy was associated with a reduced risk of CVD events (hazard ratio [HR], 0.45; 95% confidence interval [CI], 0.20-0.98) and all-cause mortality (HR, 0.43; 95% CI, 0.20-0.92) in a population-based cohort study.13 Statins appear to have similar lipid-lowering effects and result in similar CVD risk reduction when used for primary or secondary prevention in RA patients compared to non-RA controls.14-16 Additionally, anti-inflammatory properties of statins may act in synergy with disease-modifying antirheumatic drugs (DMARDs) to improve RA disease activity. In a small study of RA patients, statin therapy improved subjective and objective markers of RA disease activity in conjunction with methotrexate.17
While statins provide robust reduction in CVD risk, some individuals cannot tolerate statin therapy or do not achieve goal LDL levels with statin therapy. Select non-statin LDL-cholesterol-lowering agents have shown promise for reducing CVD events in the general population.18 Ezetimibe, which inhibits cholesterol absorption in the small intestine, very modestly reduced CVD events when added to atorvastatin (relative risk [RR], 0.94; 95% CI, 0.89-0.99) in a double-blind randomized controlled trial.19 Novel monoclonal antibodies to proprotein convertase subtilisin/kexin type 9 (PCSK-9) inhibit the internalization of surface LDL receptors, promoting LDL clearance. Two PCSK-9 inhibitors, alirocumab and evolocumab, were approved by the US Food and Drug Administration (FDA) after randomized controlled trials demonstrated their efficacy in lowering LDL by approximately 60% and reducing CVD events by approximately 15% in patients on maximum-tolerated statin therapy.20-22 To date, non-statin LDL-cholesterol-lowering agents have been subject to limited study in RA.23
Identification and management of dyslipidemia offers an opportunity for substantial CVD risk reduction at the RA population level. Unfortunately, current rates of lipid screening are inadequate in this high-risk group. In a study of 3298 Medicare patients with RA, less than half of RA patients with an indication underwent appropriate lipid screening.24 Additionally, statins are often underutilized for both primary and secondary prevention in RA patients. Only 27% of RA patients meeting National Cholesterol Education Program Adult Treatment Panel III criteria were initiated on statin therapy in a population-based cohort study.25 Among patients discharged after a first myocardial infarction (MI), the odds of receiving lipid-lowering therapy were 31% lower for RA patients (odds ratio [OR], 0.69; 95% CI, 0.58-0.82).26 Similar to the general population, adherence to statins in RA patients appears to be poor.27-30 This raises particular concern considering that a population-based cohort study of RA patients demonstrated a 67% increased risk of MI associated with statin discontinuation, regardless of prior MI status.27 Providers—rheumatologists, primary care providers, and cardiologists alike—need to remain vigilant in efforts to assess CVD risk to identify patients who will benefit from lipid-lowering therapy and to emphasize the importance to patients of statin adherence. Novel models of health-care delivery, health technologies, and patient engagement in care may prove useful for improving lipid screening and management in RA.
Tobacco Use
Cigarette smoking is a shared risk factor for both CVD and RA. Large cohort studies have identified a dose-dependent increased risk of incident RA, particularly seropositive RA, among smokers.31-34 Tobacco smoking has also been associated with increased levels of inflammation and RA disease activity.35 The consequences of tobacco use in the general population are staggering. Among individuals over the age of 30 years, tobacco use is responsible for 12% of all deaths and 10% of all CVD deaths.36 Similar findings are observed in RA; a recent meta-analysis estimated there is a 50% increased risk of CVD events in RA related to smoking tobacco.37 In the general population, smoking cessation markedly lowers CVD risk, and over time CVD risk may approach that of nonsmokers.38,39 Thus, regular counseling and interventions to facilitate smoking cessation are critical to reducing CVD risk in RA patients. RA-specific smoking cessation programs have been proposed, but have yet to outperform standard smoking cessation programs.40
Diabetes Mellitus
It is estimated that almost 10% of the US population has diabetes mellitus (DM), which in isolation portends substantial CVD risk.41 There is an increased prevalence of DM in RA, perhaps owing to factors such as physical inactivity and chronic glucocorticoid use, though a higher level of RA disease activity itself has been associated with increased insulin resistance.42-45 In a cohort of 100 RA patients who were neither obese nor diabetic, RA patients had significantly higher fasting blood glucose and insulin levels than age- and sex-matched controls. These findings were even more pronounced in RA patients with higher levels of disease activity.44 Similar to the general population, DM is associated with poor CVD outcomes in RA.37 Therefore, both appropriate management of diabetes and control of RA disease activity are vitally important to minimize CVD risk related to DM.
Hypertension
Though not a universal finding, there may be an increased prevalence of hypertension in RA patients.31,46 Nonsteroidal anti-inflammatory drug (NSAID) and glucocorticoid use may play a role in the development of hypertension, while DMARDs appear to exert a less substantial effect on blood pressure.47,48 At least one study found that DMARD initiation (particularly for methotrexate and hydroxychloroquine) was associated with significant, albeit small, declines in both systolic and diastolic blood pressure over the first 6 months of treatment.49
Despite its potentially higher prevalence in this population, hypertension is both underdiagnosed and undertreated in RA patients.24,50-52 This is an important deficiency to target because, as in the general population, hypertension is associated with an increased risk of MI (RR, 1.84; 95% CI, 1.38-2.46) and composite CVD outcomes (RR, 2.24; 95% CI, 1.42-3.06) in RA.37 Thresholds for initiation and escalation of antihypertensive therapy are not specific to the RA population; thus, diagnosis and management of hypertension should be informed by the American College of Cardiology/American Heart Association guidelines, treating those with in-office blood pressures exceeding 140/90 mm Hg (> 130/80 mm Hg if aged > 65 years or with concomitant CVD, DM, chronic kidney disease, or 10-year atherosclerotic cardiovascular disease risk > 10%), typically with angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, calcium channel blockers, or thiazide diuretics as comorbidities may dictate or allow.53 Also, the use of NSAIDs and glucocorticoids should be minimized, particularly in those with concomitant hypertension.
Physical Activity
Likely due to factors such as articular pain and stiffness, as well as physical limitations, RA patients are more sedentary than the general population.54,55 In a study of objectively assessed sedentary behavior in RA patients, greater average sedentary time per day and greater number of sedentary bouts (> 20 min) were associated with increased 10-year risk of CVD as assessed by the QRISK2.56 Conversely, the beneficial effects of exercise are well documented. Light to moderate physical activity has been associated with improved cardiovascular outcomes, greater physical function, higher levels of HDL, as well as reduced systemic inflammation and disease activity, and improved endothelial function in RA patients.57-61 While there has been concern that physical activity may result in accelerated joint damage, even high-intensity exercise was shown to be safe without causing significant progression of joint damage.58
Obesity, Weight Loss, and Diet
While obesity is clearly associated with CVD risk in the general population, this relationship is much more complex in RA, as underweight RA patients are also at higher risk for CVD and CVD-related mortality.62-64 One potential explanation for this finding is that pathological weight loss resulting in an underweight body mass index (BMI) is an independent predictor of CVD. In a study of US Veterans with RA, higher rates of weight loss (> 3 kg/m2/year) were associated with increased CVD mortality (HR, 2.27; 95% CI, 1.61-3.19) independent of BMI.65 Systemic inflammation in RA can lead to “rheumatoid cachexia,” characterized by decreased muscle mass, increased adiposity, and increased CVD risk despite a normal or potentially decreased BMI.66 Practitioners should be mindful of not only current body weight, but also patients’ weight trajectories when counseling on lifestyle practices such as healthy diet and regular exercise in RA patients. For obese individuals with RA, healthy weight loss should be encouraged. Interestingly, bariatric surgery in RA patients may improve RA disease activity in addition to its known effects on body weight and DM.67
Counseling on healthy diet with a focus on limiting foods high in saturated- and trans-fatty acids and high glycemic index foods, and increasing consumption of fruits, vegetables, and mono-unsaturated fatty acids is a well-accepted and common practice to help minimize CVD risk in the general population.68 No studies to date have investigated the effect of specific diets on CVD risk in RA patients, and thus we recommend adherence to general population recommendations.
Managing RA-related CVD Risk Factors
Disease Activity
In addition to traditional risk factors, several studies have identified associations between the level of RA disease activity and risk of CVD. In a cohort of US Veterans with RA, CVD-related mortality increased in a dose-dependent manner with higher disease activity categories. In stark contrast, the CVD mortality rates of those in remission paralleled the rates from the general population (standardized mortality ratio [SMR], 0.68; 95% CI, 0.37-1.27).69 In a separate cohort of 1157 RA patients without prior CVD, achieving low disease activity was associated with a lower risk of incident CVD events (HR, 0.65; 95% CI, 0.43-0.99).70 Additionally, high disease activity has been associated with surrogate markers of CVD and other CVD risk factors including NT-proBNP and systolic blood pressure.71,72 While no randomized controlled trial data is available to inform this recommendation, observational data suggest RA should be aggressively treated (ideally to achieve and maintain remission or low disease activity) to minimize CVD risk. While keeping this treatment goal in mind, the differential effects of specific RA therapies on CVD must also be considered.
Glucocorticoids and NSAIDs
With the expanding repertoire of DMARDs available and more aggressive treatment approaches, the role of glucocorticoids and NSAIDs in RA treatment is decreasing over time. While their efficacy for improving pain and stiffness is well established, concern regarding their contribution to CVD risk in RA patients is warranted.
Glucocorticoids are known to have detrimental effects on traditional CVD risk factors such as hypertension, insulin resistance, and dyslipidemia in the general population, as well as in RA patients.73,74 In a meta-analysis of predominantly observational studies of RA patients, glucocorticoid use was associated with an increased risk of CVD events (RR, 1.47; 95% CI, 1.34-1.60), including MI, congestive heart failure (CHF), and cerebrovascular accident (CVA).75 Evidence is conflicting in regards to a clear dose threshold that leads to increased CVD risk with glucocorticoids, though higher doses are associated with greater risk.76-81 As RA patients requiring glucocorticoids typically have higher disease activity, confounding by indication remains a complicating factor in assessing the relative contributions of glucocorticoid use and RA disease activity to elevated CVD risk in many analyses.
The increased CVD risk with NSAID use is not specific to RA and has been well established in the general population.82-84 In the previously mentioned meta-analysis, an increased overall risk of CVD events was observed with NSAID use in RA (RR, 1.18; 95% CI, 1.01-1.38). It should be noted that cyclo-oxygenase 2 (COX-2) inhibitors, in particular rofecoxib (now removed from the market), appeared to drive the majority of this risk (RR, 1.36; 95% CI, 1.10-1.67 in COX-2 inhibitors and RR 1.08, 95% CI, 0.94-1.24 in nonselective NSAIDs), suggesting a potential differential risk among NSAIDs.75 While naproxen has been thought to carry the lowest risk of CVD based on initial studies, this has not been universally observed, including in a recent randomized controlled trial of more than 24,000 RA and osteoarthritis patients.82,85,86
Providers should use the lowest possible dose and duration of glucocorticoids and NSAIDs to achieve symptom relief, with continual efforts to taper or discontinue. Candidates for glucocorticoid and NSAID therapy should be selected carefully, and use of these therapies should be avoided in those with prior CVD or at high risk for CVD based on traditional CVD risk factors. Most importantly, providers should focus on utilizing DMARDs for the management of RA, which more effectively treat RA as well as reduce CVD risk.
Methotrexate
Methotrexate (MTX), a mainstay in the treatment of RA, is a conventional DMARD observed to improve overall survival and mitigate CVD risk in multiple RA cohorts.75,87,88 In a recent meta-analysis comprised of 236,525 RA patients and 5410 CVD events, MTX use was associated with a 28% reduction in overall CVD events across 8 studies (RR, 0.72; 95% CI, 0.57-0.91), substantiating similar findings in a prior meta-analysis.75,88 MTX use was specifically associated with a decreased risk of MI (RR, 0.81; 95% CI, 0.68-0.96). Case-control and cohort studies have cited a 20% to 50% reduced risk of CHF with MTX use.89,90 The potential cardioprotective effect of MTX appears to be both multifactorial and complex, likely mediated through both direct and indirect mechanisms. MTX directly promotes anti-atherogenic lipoprotein function, improves endothelial function, and scavenges free radicals.91,92 Indirectly, MTX likely reduces CVD risk by effectively reducing RA disease activity. Based on these and other data, MTX remains the cornerstone of DMARD therapy in RA patients when targeting CVD risk reduction.
Hydroxychloroquine
Emerging evidence suggests that hydroxychloroquine (HCQ), an antimalarial most often utilized in combination with alternative DMARDs in RA, prevents DM and has beneficial effects on lipid profiles. A recent meta-analysis compiled 3 homogenous observational studies that investigated the effect of HCQ on incident DM. RA patients ever exposed to HCQ had a 40% lower incidence of DM (HR, 0.59; 95% CI, 0.49-0.70).93 Increased duration of HCQ use was shown to further reduce risk of incident DM.94 The aforementioned meta-analysis also pooled 5 studies investigating the effect of HCQ on lipid profiles, with favorable mean differences in TC (–9.82 mg/dL), LDL (–10.61 mg/dL), HDL (4.13 mg/dL), and triglycerides (–19.15 mg/dL) in HCQ users compared to non-users.93 Given these favorable changes to traditional CVD risk factors, it is not surprising that in a retrospective study of 1266 RA patients without prior CVD, HCQ was associated with significantly lower risk of incident CVD. While external validation of these findings is needed, HCQ is an attractive conventional DMARD to be used in RA for CVD risk reduction. Moreover, its combination with MTX and sulfasalazine also shows promise for CVD risk reduction.95,96
TNF Inhibitors
Tumor necrosis factor (TNF) inhibitors are often the initial biologic DMARD therapy used in RA patients not responding to conventional DMARDs. In the previously described meta-analysis, TNF inhibitors were associated with similar reductions in CVD events as MTX (RR, 0.70; 95% CI, 0.54-0.90).75 Of note, there was a trend toward reduced risk of CHF (RR, 0.75; 95% CI, 0.49-1.15) in this same meta-analysis, an area of concern with TNF inhibitor use due to a prior randomized controlled trial demonstrating worsening clinical status in patients with existing moderate-to-severe CHF treated with high-dose infliximab.97 Current RA treatment guidelines recommend avoiding TNF inhibitor use in individuals with CHF.98
Aside from the risk of CHF exacerbation, TNF inhibitors appear to be cardioprotective. Similar to MTX, the mechanism by which TNF inhibition reduces cardiovascular risk is complex and likely due to both direct and indirect mechanisms. Substantial research has been conducted on the effect of TNF inhibition on lipids, with a recent meta-analysis demonstrating increases in HDL and TC, with stable LDL and atherogenic index over treatment follow-up.99 A subsequent meta-analysis not limited to RA patients yielded similar results.100 In addition to quantitative lipid changes, alteration of lipoprotein function, improvement in myocardial function, reduced aortic stiffness, improved blood pressure, and reduced RA disease activity may also be responsible for cardioprotective benefits of these agents.101,102
Non-TNF Biologic and Traditional Synthetic DMARDs
Tocilizumab, an IL-6 inhibitor, can potently increase LDL levels, but it does not appear to increase the risk of CVD events and may actually promote more favorable anti-atherogenic lipoprotein function.103-106 Although these quantitative lipid changes received significant attention in the wake of early reports detailing this effect, similar lipid changes appear to accompany other DMARDs including TNF inhibitors and tofacitinib.107 There have been few studies evaluating the risk of CVD with other non-TNF inhibitor biologic DMARDs and traditional synthetic DMARDs, warranting future study.
Conclusion
To mitigate the increased risk of CVD in RA, primary care and subspecialty providers alike must be aware of this heightened risk in RA, perform frequent assessments of CVD risk,3 and aggressively manage both traditional and nontraditional CVD risk factors. The differential roles in this effort may not be clear; thus, we have proposed a co-management strategy detailed in the Figure. Clear communication between providers is of the utmost importance to ensure effective management of CVD risk.
Given limited evidence for RA-specific CVD risk assessments and traditional risk factor treatment targets, management should follow pertinent national guidelines. The importance of lifestyle counseling should not be overlooked, with a focus on smoking cessation, healthy diet and body weight, and regular aerobic exercise. Finally, rheumatologists should aggressively manage RA using a treat-to-target approach, minimize the use of glucocorticoids and NSAIDs, and preferentially select DMARDs that have been associated with lower CVD risk. Through this comprehensive approach, recent trends of improved CVD outcomes in RA will hopefully become more widespread.108
Corresponding author: Bryant R. England, MD; 986270 Nebraska Medical Center, Omaha, NE 68198-6270; [email protected].
Financial disclosures: Dr. England is supported by UNMC Internal Medicine Scientist Development Award, UNMC Physician-Scientist Training Program, the UNMC Mentored Scholars Program, and the Rheumatology Research Foundation Scientist Development Award. Dr. Mikuls is supported by a VA Merit Award (CX000896) and grants from the National Institutes of Health: National Institute of General Medical Sciences (U54GM115458), National Institute on Alcohol Abuse and Alcoholism (R25AA020818), and National Institute of Arthritis and Musculoskeletal and Skin Diseases (2P50AR60772).
1. Avina-Zubieta JA, Choi HK, Sadatsafavi M, et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690-1697.
2. Avina-Zubieta JA, Thomas J, Sadatsafavi M, et al. Risk of incident cardiovascular events in patients with rheumatoid arthritis: A meta-analysis of observational studies. Ann Rheum Dis. 2012;71:1524-1529.
3. Johnson TM, Mikuls TR, England BR. Assessment of cardiovascular risk in rheumatoid arthritis. J Clin Outcomes Manage. 2019;26:41-47.
4. Sandoo A, Chanchlani N, Hodson J, et al. Classical cardiovascular disease risk factors associate with vascular function and morphology in rheumatoid arthritis: A six-year prospective study. Arthritis Res Ther. 2013;15:R203.
5. Myasoedova E, Crowson CS, Kremers HM, et al. Total cholesterol and LDL levels decrease before rheumatoid arthritis. Ann Rheum Dis. 2010;69:1310-1314.
6. Liao KP, Cai T, Gainer VS, et al. Lipid and lipoprotein levels and trend in rheumatoid arthritis compared to the general population. Arthritis Care Res (Hoboken). 2013;65:2046-2050.
7. Myasoedova E, Crowson CS, Kremers HM, et al. Lipid paradox in rheumatoid arthritis: The impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann Rheum Dis. 2011;70:482-487.
8. Zhang J, Chen L, Delzell E, et al. Republished: The association between inflammatory markers, serum lipids and the risk of cardiovascular events in patients with rheumatoid arthritis. Postgrad Med J. 2014;90:722-729.
9. Liao KP, Liu J, Lu B, et al. Association between lipid levels and major adverse cardiovascular events in rheumatoid arthritis compared to non-rheumatoid arthritis patients. Arthritis Rheumatol. 2015;67:2004-2010.
10. Charles-Schoeman C, Lee YY, Grijalva V, et al. Cholesterol efflux by high density lipoproteins is impaired in patients with active rheumatoid arthritis. Ann Rheum Dis. 2012;71:1157-1162.
11. Voloshyna I, Modayil S, Littlefield MJ, et al. Plasma from rheumatoid arthritis patients promotes pro-atherogenic cholesterol transport gene expression in THP-1 human macrophages. Exp Biol Med (Maywood). 2013 238:1192-1197.
12. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;(1):CD004816.
13. Sheng X, Murphy MJ, Macdonald TM, Wei L. Effectiveness of statins on total cholesterol and cardiovascular disease and all-cause mortality in osteoarthritis and rheumatoid arthritis. J Rheumatol. 2012;39:32-40.
14. An J, Alemao E, Reynolds K, et al. Cardiovascular outcomes associated with lowering low-density lipoprotein cholesterol in rheumatoid arthritis and matched nonrheumatoid arthritis. J Rheumatol. 2016;43:1989-1996.
15. Semb AG, Holme I, Kvien TK, Pedersen TR. Intensive lipid lowering in patients with rheumatoid arthritis and previous myocardial infarction: An explorative analysis from the incremental decrease in endpoints through aggressive lipid lowering (IDEAL) trial. Rheumatology (Oxford). 2011;50:324-329.
16. Semb AG, Kvien TK, DeMicco DA, et al. Effect of intensive lipid-lowering therapy on cardiovascular outcome in patients with and those without inflammatory joint disease. Arthritis Rheum. 2012;64:2836-2846.
17. El-Barbary AM, Hussein MS, Rageh EM, et al. Effect of atorvastatin on inflammation and modification of vascular risk factors in rheumatoid arthritis. J Rheumatol. 2011;38:229-235.
18. Writing Committee, Lloyd-Jones DM, Morris PB, et al. 2016 ACC expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: A report of the American college of cardiology task force on clinical expert consensus documents. J Am Coll Cardiol. 2016;68:92-125.
19. Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
20. Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500-1509.
21. Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489-1499.
22. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
23. Maki-Petaja KM, Booth AD, Hall FC, et al. Ezetimibe and simvastatin reduce inflammation, disease activity, and aortic stiffness and improve endothelial function in rheumatoid arthritis. J Am Coll Cardiol. 2007;50:852-858.
24. Bartels CM, Kind AJ, Everett C, et al. Low frequency of primary lipid screening among medicare patients with rheumatoid arthritis. Arthritis Rheum. 2011;63:1221-1230.
25. Akkara Veetil BM, Myasoedova E, Matteson EL, et al. Use of lipid-lowering agents in rheumatoid arthritis: A population-based cohort study. J Rheumatol. 2013;40:1082-1088.
26. Lindhardsen J, Ahlehoff O, Gislason GH, et al. Initiation and adherence to secondary prevention pharmacotherapy after myocardial infarction in patients with rheumatoid arthritis: A nationwide cohort study. Ann Rheum Dis. 2012;71:1496-1501.
27. De Vera MA, Choi H, Abrahamowicz M, et al. Statin discontinuation and risk of acute myocardial infarction in patients with rheumatoid arthritis: A population-based cohort study. Ann Rheum Dis. 2011;70:1020-1024.
28. Zhang H, Plutzky J, Skentzos S, et al. Discontinuation of statins in routine care settings: A cohort study. Ann Intern Med. 2013;158:526-534.
29. Zhang H, Plutzky J, Shubina M, Turchin A. Continued statin prescriptions after adverse reactions and patient outcomes: A cohort study. Ann Intern Med. 2017;167:221-227.
30. Lemstra M, Blackburn D, Crawley A, Fung R. Proportion and risk indicators of nonadherence to statin therapy: A meta-analysis. Can J Cardiol. 2012;28:574-580.
31. Boyer JF, Gourraud PA, Cantagrel A, et al. Traditional cardiovascular risk factors in rheumatoid arthritis: A meta-analysis. Joint Bone Spine. 2011;78:179-183.
32. Bergstrom U, Jacobsson LT, Nilsson JA, et al. Pulmonary dysfunction, smoking, socioeconomic status and the risk of developing rheumatoid arthritis. Rheumatology (Oxford). 2011;50:2005-2013.
33. Costenbader KH, Feskanich D, Mandl LA, Karlson EW. Smoking intensity, duration, and cessation, and the risk of rheumatoid arthritis in women. Am J Med. 2006;119:503.e1,503.e9.
34. Klareskog L, Stolt P, Lundberg K, et al. A new model for an etiology of rheumatoid arthritis: Smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006;54:38-46.
35. Sokolove J, Wagner CA, Lahey LJ, et al. Increased inflammation and disease activity among current cigarette smokers with rheumatoid arthritis: A cross-sectional analysis of US veterans. Rheumatology (Oxford). 2016;55:1969-1977.
36. World Health Organization. WHO Global Report: Mortality Attributable to Tobacco. Geneva, World Health Organization, 2012.
37. Baghdadi LR, Woodman RJ, Shanahan EM, Mangoni AA. The impact of traditional cardiovascular risk factors on cardiovascular outcomes in patients with rheumatoid arthritis: A systematic review and meta-analysis. PLoS One. 2015;10:e0117952.
38. Centers for Disease Control and Prevention; National Center for Chronic Disease Prevention and Health Promotion. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention; 2010. 6, Cardiovascular Diseases. Available from: https://ncbi.nlm.nih.gov/books/NBK53012/
39. Mons U, Muezzinler A, Gellert C, et al. Impact of smoking and smoking cessation on cardiovascular events and mortality among older adults: Meta-analysis of individual participant data from prospective cohort studies of the CHANCES consortium. BMJ. 2015;350:h1551.
40. Aimer P, Treharne GJ, Stebbings S, Frampton C, Cameron V, Kirby S, et al. Efficacy of a rheumatoid arthritis-specific smoking cessation program: A randomized controlled pilot trial. Arthritis Care Res (Hoboken). 2017;69:28-37.
41. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services; 2017.
42. Jiang P, Li H, Li X. Diabetes mellitus risk factors in rheumatoid arthritis: A systematic review and meta-analysis. Clin Exp Rheumatol. 2015;33:115-121.
43. Shahin D, Eltoraby E, Mesbah A, Houssen M. Insulin resistance in early untreated rheumatoid arthritis patients. Clin Biochem. 2010;43:661-335.
44. Arias de la Rosa I, Escudero-Contreras A, Rodriguez-Cuenca S, et al. Defective glucose and lipid metabolism in rheumatoid arthritis is determined by chronic inflammation in metabolic tissues. J Intern Med. 2018;84(1):61-77.
45. Wilson JC, Sarsour K, Gale S, et al. Incidence and risk of glucocorticoid-associated adverse effects in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2018 Jun 1. doi: 10.1002/acr.23611.
46. Chung CP, Giles JT, Petri M, et al. Prevalence of traditional modifiable cardiovascular risk factors in patients with rheumatoid arthritis: Comparison with control subjects from the multi-ethnic study of atherosclerosis. Semin Arthritis Rheum. 2012;41:535-544.
47. Goodwin JE, Geller DS. Glucocorticoid-induced hypertension. Pediatr Nephrol. 2012;27:1059-1066.
48. Snowden S, Nelson R. The effects of nonsteroidal anti-inflammatory drugs on blood pressure in hypertensive patients. Cardiol Rev. 2011;19:184-191.
49. Baker JF, Sauer B, Teng CC, et al. Initiation of disease-modifying therapies in rheumatoid arthritis is associated with changes in blood pressure. J Clin Rheumatol. 2018;24:203-209.
50. Panoulas VF, Douglas KM, Milionis HJ, et al. Prevalence and associations of hypertension and its control in patients with rheumatoid arthritis. Rheumatology (Oxford). 2007;46:1477-1482.
51. Protogerou AD, Panagiotakos DB, Zampeli E, et al. Arterial hypertension assessed “out-of-office” in a contemporary cohort of rheumatoid arthritis patients free of cardiovascular disease is characterized by high prevalence, low awareness, poor control and increased vascular damage-associated “white coat” phenomenon. Arthritis Res Ther. 2013;15:R142.
52. van Breukelen-van der Stoep DF, van Zeben D, Klop B, et al. Marked underdiagnosis and undertreatment of hypertension and hypercholesterolaemia in rheumatoid arthritis. Rheumatology (Oxford). 2016;55:1210-1216.
53. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2018;71:e127-248.
54. Lee J, Dunlop D, Ehrlich-Jones L, et al. Public health impact of risk factors for physical inactivity in adults with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64:488-493.
55. Sokka T, Hakkinen A, Kautiainen H, et al. Physical inactivity in patients with rheumatoid arthritis: Data from twenty-one countries in a cross-sectional, international study. Arthritis Rheum. 2008;59:42-50.
56. Fenton SAM, Veldhuijzen van Zanten JJCS, Kitas GD, et al. Sedentary behaviour is associated with increased long-term cardiovascular risk in patients with rheumatoid arthritis independently of moderate-to-vigorous physical activity. BMC Musculoskelet Disord. 2017;18:131,017-1473-9.
57. Byram KW, Oeser AM, Linton MF, et al. Exercise is associated with increased small HDL particle concentration and decreased vascular stiffness in rheumatoid arthritis. J Clin Rheumatol. 2018 May 25. 9.
58. de Jong Z, Munneke M, Zwinderman AH, et al. Is a long-term high-intensity exercise program effective and safe in patients with rheumatoid arthritis? results of a randomized controlled trial. Arthritis Rheum. 2003;48:2415-2424.
59. Stavropoulos-Kalinoglou A, Metsios GS, Veldhuijzen van Zanten JJ, et al. Individualised aerobic and resistance exercise training improves cardiorespiratory fitness and reduces cardiovascular risk in patients with rheumatoid arthritis. Ann Rheum Dis. 2013;72:1819-1825.
60. Khoja SS, Almeida GJ, Chester Wasko M, et al. Association of light-intensity physical activity with lower cardiovascular disease risk burden in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2016;68:424-431.
61. Metsios GS, Koutedakis Y, Veldhuijzen van Zanten JJ, et al. Cardiorespiratory fitness levels and their association with cardiovascular profile in patients with rheumatoid arthritis: A cross-sectional study. Rheumatology (Oxford). 2015;54:2215-2220.
62. Escalante A, Haas RW, del Rincon I. Paradoxical effect of body mass index on survival in rheumatoid arthritis: Role of comorbidity and systemic inflammation. Arch Intern Med. 2005;165:1624-1629.
63. Kremers HM, Nicola PJ, Crowson CS, et al. Prognostic importance of low body mass index in relation to cardiovascular mortality in rheumatoid arthritis. Arthritis Rheum. 2004;50:3450-3457.
64. Wolfe F, Michaud K. Effect of body mass index on mortality and clinical status in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64:1471-1479.
65. England BR, Baker JF, Sayles H, et al. Body mass index, weight loss, and cause-specific mortality in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2018;70:11-18.
66. Dessein PH, Solomon A, Hollan I. Metabolic abnormalities in patients with inflammatory rheumatic diseases. Best Pract Res Clin Rheumatol. 2016;30:901-915.
67. Sparks JA, Halperin F, Karlson JC, et al. Impact of bariatric surgery on patients with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2015;67:1619-1626.
68. Mente A, de Koning L, Shannon HS, Anand SS. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Intern Med. 2009;169:659-669.
69. England BR, Sayles H, Michaud K, et al. Cause-specific mortality in male US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2016;68:36-45.
70. Arts EE, Fransen J, Den Broeder AA, et al. Low disease activity (DAS28≤3.2) reduces the risk of first cardiovascular event in rheumatoid arthritis: a time-dependent Cox regression analysis in a large cohort study. Ann Rheum Dis. 2017;76(10):1693-1699.
71. Provan SA, Semb AG, Hisdal J, et al. Remission is the goal for cardiovascular risk management in patients with rheumatoid arthritis: A cross-sectional comparative study. Ann Rheum Dis. 2011;70:812-817.
72. Klarenbeek NB, van der Kooij SM, Huizinga TJ, et al. Blood pressure changes in patients with recent-onset rheumatoid arthritis treated with four different treatment strategies: A post hoc analysis from the BeSt trial. Ann Rheum Dis. 2010;69:1342-1345.
73. Hafstrom I, Rohani M, Deneberg S, et al. Effects of low-dose prednisolone on endothelial function, atherosclerosis, and traditional risk factors for atherosclerosis in patients with rheumatoid arthritis—a randomized study. J Rheumatol. 2007;34:1810-1816.
74. Hoes JN, van der Goes MC, van Raalte DH, et al. Glucose tolerance, insulin sensitivity and beta-cell function in patients with rheumatoid arthritis treated with or without low-to-medium dose glucocorticoids. Ann Rheum Dis. 2011;70:1887-1894.
75. Roubille C. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: A systematic review and meta-analysis. Ann Rheum Dis. 2003;74:480-489.
76. Ajeganova S, Svensson B, Hafstrom I, BARFOT Study Group. Low-dose prednisolone treatment of early rheumatoid arthritis and late cardiovascular outcome and survival: 10-year follow-up of a 2-year randomised trial. BMJ Open. 2014;4:e004259,2013-004259.
77. Avina-Zubieta JA, Choi HK, Sadatsafavi M, et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690-1697.
78. del Rincon I, Battafarano DF, Restrepo JF, et al. Glucocorticoid dose thresholds associated with all-cause and cardiovascular mortality in rheumatoid arthritis. Arthritis Rheumatol. 2014;66:264-272.
79. Davis JM,3rd, Maradit Kremers H, Crowson CS, et al. Glucocorticoids and cardiovascular events in rheumatoid arthritis: A population-based cohort study. Arthritis Rheum. 2007;56:820-830.
80. Zhang J, Xie F, Yun H, et al. Comparative effects of biologics on cardiovascular risk among older patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75:1813-1818.
81. Greenberg JD, Kremer JM, Curtis JR, et al. Tumour necrosis factor antagonist use and associated risk reduction of cardiovascular events among patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70:576-582.
82. Lindhardsen J, Gislason GH, Jacobsen S, et al. Non-steroidal anti-inflammatory drugs and risk of cardiovascular disease in patients with rheumatoid arthritis: A nationwide cohort study. Ann Rheum Dis. 2014;73:1515-1521.
83. Schjerning Olsen AM, Fosbol EL, Lindhardsen J, et al. Duration of treatment with nonsteroidal anti-inflammatory drugs and impact on risk of death and recurrent myocardial infarction in patients with prior myocardial infarction: A nationwide cohort study. Circulation. 2011;123:2226-2235.
84. Gislason GH, Rasmussen JN, Abildstrom SZ, et al. Increased mortality and cardiovascular morbidity associated with use of nonsteroidal anti-inflammatory drugs in chronic heart failure. Arch Intern Med. 2009;169:141-149.
85. Trelle S, Reichenbach S, Wandel S, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: Network meta-analysis. BMJ. 2011;342:c7086.
86. Nissen SE, Yeomans ND, Solomon DH, et al. Cardiovascular safety of celecoxib, naproxen, or ibuprofen for arthritis. N Engl J Med. 2016;375:2519-2529.
87. Wasko MC, Dasgupta A, Hubert Het al. Propensity-adjusted association of methotrexate with overall survival in rheumatoid arthritis. Arthritis Rheum. 2013;65:334-342.
88. Micha R, Imamura F, Wyler von Ballmoos M, et al. Systematic review and meta-analysis of methotrexate use and risk of cardiovascular disease. Am J Cardiol. 2011;108:1362-1370.
89. Bernatsky S, Hudson M, Suissa S. Anti-rheumatic drug use and risk of hospitalization for congestive heart failure in rheumatoid arthritis. Rheumatology (Oxford). 2005;44:677-680.
90. Myasoedova E, Crowson CS, Nicola PJ, et al. The influence of rheumatoid arthritis disease characteristics on heart failure. J Rheumatol. 2011;38:1601-1606.
91. Ronda N, Greco D, Adorni MP, et al. Newly identified antiatherosclerotic activity of methotrexate and adalimumab: Complementary effects on lipoprotein function and macrophage cholesterol metabolism. Arthritis Rheumatol. 2015;67:1155-1164.
92. Zimmerman MC, Clemens DL, Duryee MJ, et al. Direct antioxidant properties of methotrexate: Inhibition of malondialdehyde-acetaldehyde-protein adduct formation and superoxide scavenging. Redox Biol. 2017;13:588-593.
93. Rempenault C, Combe B, Barnetche T, et al. Metabolic and cardiovascular benefits of hydroxychloroquine in patients with rheumatoid arthritis: A systematic review and meta-analysis. Ann Rheum Dis. 2018;77:98-103.
94. Wasko MC, Hubert HB, Lingala VB, et al. Hydroxychloroquine and risk of diabetes in patients with rheumatoid arthritis. JAMA. 2007;298:187-193.
95. Charles-Schoeman C, Wang X, Lee YY, et al. Association of triple therapy with improvement in cholesterol profiles over two-year followup in the treatment of early aggressive rheumatoid arthritis trial. Arthritis Rheumatol. 2016;68:577-586.
96. Charles-Schoeman C, Yin Lee Y, Shahbazian A, et al. Improvement of high-density lipoprotein function in patients with early rheumatoid arthritis treated with methotrexate monotherapy or combination therapies in a randomized controlled trial. Arthritis Rheumatol. 2017;69:46-57.
97. Chung ES, Packer M, Lo KH, , Anti-TNF Therapy Against Congestive Heart Failure Investigators. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: Results of the anti-TNF therapy against congestive heart failure (ATTACH) trial. Circulation. 2003;107:3133-3140.
98. Singh JA, Saag KG, Bridges SL, Jr, et al. 2015 American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68:1-26.
99. Daien CI, Duny Y, Barnetche Tet al. Effect of TNF inhibitors on lipid profile in rheumatoid arthritis: A systematic review with meta-analysis. Ann Rheum Dis. 2012;71:862-868.
100. Di Minno MN, Ambrosino P, Peluso R, et al. Lipid profile changes in patients with rheumatic diseases receiving a treatment with TNF-alpha blockers: A meta-analysis of prospective studies. Ann Med. 2014;46:73-83.
101. Popa C, van Tits LJ, Barrera P, et al. Anti-inflammatory therapy with tumour necrosis factor alpha inhibitors improves high-density lipoprotein cholesterol antioxidative capacity in rheumatoid arthritis patients. Ann Rheum Dis. 2009;68:868-872.
102. O’Neill F, Charakida M, Topham E, et al. Anti-inflammatory treatment improves high-density lipoprotein function in rheumatoid arthritis. Heart. 2017;103:766-773.
103. Nishimoto N, Ito K, Takagi N. Safety and efficacy profiles of tocilizumab monotherapy in Japanese patients with rheumatoid arthritis: Meta-analysis of six initial trials and five long-term extensions. Mod Rheumatol. 2010;20:222-232.
104. Rao VU, Pavlov A, Klearman M, et al. An evaluation of risk factors for major adverse cardiovascular events during tocilizumab therapy. Arthritis Rheumatol. 2015;67:372-380.
105. Gabay C, McInnes IB, Kavanaugh A, et al. Comparison of lipid and lipid-associated cardiovascular risk marker changes after treatment with tocilizumab or adalimumab in patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75:1806-1812.
106. McInnes IB, Thompson L, Giles JT, et al. Effect of interleukin-6 receptor blockade on surrogates of vascular risk in rheumatoid arthritis: MEASURE, a randomised, placebo-controlled study. Ann Rheum Dis. 2015;74:694-702.
107. Souto A, Salgado E, Maneiro JR, et al. Lipid profile changes in patients with chronic inflammatory arthritis treated with biologic agents and tofacitinib in randomized clinical trials: A systematic review and meta-analysis. Arthritis Rheumatol. 2015;67:117-127.
108. Myasoedova E, Gabriel SE, Matteson EL, et al. Decreased cardiovascular mortality in patients with incident rheumatoid arthritis (RA) in recent years: Dawn of a new era in cardiovascular disease in RA? J Rheumatol. 2017;44:732-739.
109. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2889-2934.
110. Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U.S. public health service report. Am J Prev Med. 2008;35:158-176.
111. Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: A report of the American college of cardiology/American heart association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2960-2984.
112. Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:342-362.
113. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American college of cardiology/American heart association task force on practice guidelines and the obesity society. J Am Coll Cardiol. 2014;63:2985-3023.
From the Division of Rh
Abstract
- Objective: To review the management of traditional and nontraditional CVD cardiovascular disease risk factors in rheumatoid arthritis (RA).
- Methods: Literature review of the management of CVD risk in RA.
- Results: Because of the increased risk of CVD events and CVD mortality among RA patients, aggressive management of CVD risk is essential. Providers should follow national guidelines for the management of traditional CVD risk factors, including dyslipidemia, hypertension, and diabetes mellitus. Similar efforts are needed in counseling on lifestyle modifications, including smoking cessation, regular exercise, and maintaining a healthy body weight. Because higher RA disease activity is also linked with CVD risk, aggressive treatment of RA to a target of low disease activity or remission is critical. Furthermore, the selection of potentially “cardioprotective” agents such as methotrexate and tumor necrosis factor inhibitors, while limiting use of nonsteroidal anti-inflammatory drugs and glucocorticoids, are strategies that could be employed by rheumatologists to help mitigate CVD risk in their patients with RA.
- Conclusion: Routine assessment of CVD risk, management of traditional CVD risk factors, counseling on healthy lifestyle habits, and aggressive treatment of RA are essential to minimize CVD risk in this population.
Keywords: rheumatoid arthritis; cardiovascular disease; cardiovascular risk assessment; cardiovascular risk management.
Editor’s note: This article is part 2 of a 2-part article. “Assessment of Cardiovascular Disease Risk in Rheumatoid Arthritis” was published in the January/February 2019 issue.
Rheumatoid arthritis (RA) is a systemic autoimmune condition that contributes to an increased risk for cardiovascular disease (CVD) among affected patients. In persons with RA, the risk of incident CVD and CVD mortality are increased by approximately 50% compared with the general population.1,2 To minimize CVD risk in this population, providers must routinely assess for CVD risk factors3 and aggressively manage both traditional and nontraditional CVD risk factors.
Managing Traditional Risk Factors
As in the general population, identification and management of traditional CVD risk factors are crucial to minimize CVD risk in the RA population. A prospective study of 201 RA patients demonstrated that traditional CVD risk factors were in fact more predictive of endothelial dysfunction and carotid atherosclerosis than were disease-related inflammatory markers in RA.4 Management of traditional risk factors is detailed in the following sections, and recommendations for managing all traditional CVD risk factors are summarized in the Table.

Dyslipidemia
The role of dyslipidemia in atherogenesis is well established, and as a result, lipid levels are nearly universally included in CVD risk stratification tools. However, the interpretation of lipid levels in the context of RA is challenging because of the effects of systemic inflammation on their absolute values. Compared to the general population, patients with RA have lower total cholesterol (TC) and low-density lipoprotein (LDL) levels independent of lipid-lowering therapy.5,6 Despite this, RA patients are at increased risk for CVD. There is even some evidence to suggest a “lipid paradox” in RA, whereby lower TC (< 4 mmol/L) and LDL levels suggest an increased risk of CVD.7,8 In contrast to LDL, higher levels of high-density lipoprotein (HDL) are typically associated with reduced CVD risk, as in the general population.8,9 Interestingly, in a cohort of 16,085 RA patients and 48,499 age- and sex-matched controls, there was no significant difference in the relationship between LDL and CVD risk, suggesting that quantitative lipid levels alone may not entirely explain the CVD mortality gap in RA.9 As such, there is substantial interest in lipoprotein function within the context of CVD risk in RA. Recent investigations have identified impaired HDL function, with reduced cholesterol efflux capacity and antioxidant properties, as well as increased scavenger receptor expression and foam cell formation, in patients with RA.10,11 More research is needed to elucidate how these alterations affect CVD morbidity and mortality and how their measurement could be integrated into improved CVD risk assessment.
Meta-analyses of randomized controlled trials have estimated that lipid-lowering therapy with HMG-CoA reductase inhibitors (statins) reduces the risk of CVD by 25% to 30%; as such, statin therapy has become the standard of care for reduction of CVD risk in the general population.12 Benefits for primary prevention of CVD in RA have also been observed; statin therapy was associated with a reduced risk of CVD events (hazard ratio [HR], 0.45; 95% confidence interval [CI], 0.20-0.98) and all-cause mortality (HR, 0.43; 95% CI, 0.20-0.92) in a population-based cohort study.13 Statins appear to have similar lipid-lowering effects and result in similar CVD risk reduction when used for primary or secondary prevention in RA patients compared to non-RA controls.14-16 Additionally, anti-inflammatory properties of statins may act in synergy with disease-modifying antirheumatic drugs (DMARDs) to improve RA disease activity. In a small study of RA patients, statin therapy improved subjective and objective markers of RA disease activity in conjunction with methotrexate.17
While statins provide robust reduction in CVD risk, some individuals cannot tolerate statin therapy or do not achieve goal LDL levels with statin therapy. Select non-statin LDL-cholesterol-lowering agents have shown promise for reducing CVD events in the general population.18 Ezetimibe, which inhibits cholesterol absorption in the small intestine, very modestly reduced CVD events when added to atorvastatin (relative risk [RR], 0.94; 95% CI, 0.89-0.99) in a double-blind randomized controlled trial.19 Novel monoclonal antibodies to proprotein convertase subtilisin/kexin type 9 (PCSK-9) inhibit the internalization of surface LDL receptors, promoting LDL clearance. Two PCSK-9 inhibitors, alirocumab and evolocumab, were approved by the US Food and Drug Administration (FDA) after randomized controlled trials demonstrated their efficacy in lowering LDL by approximately 60% and reducing CVD events by approximately 15% in patients on maximum-tolerated statin therapy.20-22 To date, non-statin LDL-cholesterol-lowering agents have been subject to limited study in RA.23
Identification and management of dyslipidemia offers an opportunity for substantial CVD risk reduction at the RA population level. Unfortunately, current rates of lipid screening are inadequate in this high-risk group. In a study of 3298 Medicare patients with RA, less than half of RA patients with an indication underwent appropriate lipid screening.24 Additionally, statins are often underutilized for both primary and secondary prevention in RA patients. Only 27% of RA patients meeting National Cholesterol Education Program Adult Treatment Panel III criteria were initiated on statin therapy in a population-based cohort study.25 Among patients discharged after a first myocardial infarction (MI), the odds of receiving lipid-lowering therapy were 31% lower for RA patients (odds ratio [OR], 0.69; 95% CI, 0.58-0.82).26 Similar to the general population, adherence to statins in RA patients appears to be poor.27-30 This raises particular concern considering that a population-based cohort study of RA patients demonstrated a 67% increased risk of MI associated with statin discontinuation, regardless of prior MI status.27 Providers—rheumatologists, primary care providers, and cardiologists alike—need to remain vigilant in efforts to assess CVD risk to identify patients who will benefit from lipid-lowering therapy and to emphasize the importance to patients of statin adherence. Novel models of health-care delivery, health technologies, and patient engagement in care may prove useful for improving lipid screening and management in RA.
Tobacco Use
Cigarette smoking is a shared risk factor for both CVD and RA. Large cohort studies have identified a dose-dependent increased risk of incident RA, particularly seropositive RA, among smokers.31-34 Tobacco smoking has also been associated with increased levels of inflammation and RA disease activity.35 The consequences of tobacco use in the general population are staggering. Among individuals over the age of 30 years, tobacco use is responsible for 12% of all deaths and 10% of all CVD deaths.36 Similar findings are observed in RA; a recent meta-analysis estimated there is a 50% increased risk of CVD events in RA related to smoking tobacco.37 In the general population, smoking cessation markedly lowers CVD risk, and over time CVD risk may approach that of nonsmokers.38,39 Thus, regular counseling and interventions to facilitate smoking cessation are critical to reducing CVD risk in RA patients. RA-specific smoking cessation programs have been proposed, but have yet to outperform standard smoking cessation programs.40
Diabetes Mellitus
It is estimated that almost 10% of the US population has diabetes mellitus (DM), which in isolation portends substantial CVD risk.41 There is an increased prevalence of DM in RA, perhaps owing to factors such as physical inactivity and chronic glucocorticoid use, though a higher level of RA disease activity itself has been associated with increased insulin resistance.42-45 In a cohort of 100 RA patients who were neither obese nor diabetic, RA patients had significantly higher fasting blood glucose and insulin levels than age- and sex-matched controls. These findings were even more pronounced in RA patients with higher levels of disease activity.44 Similar to the general population, DM is associated with poor CVD outcomes in RA.37 Therefore, both appropriate management of diabetes and control of RA disease activity are vitally important to minimize CVD risk related to DM.
Hypertension
Though not a universal finding, there may be an increased prevalence of hypertension in RA patients.31,46 Nonsteroidal anti-inflammatory drug (NSAID) and glucocorticoid use may play a role in the development of hypertension, while DMARDs appear to exert a less substantial effect on blood pressure.47,48 At least one study found that DMARD initiation (particularly for methotrexate and hydroxychloroquine) was associated with significant, albeit small, declines in both systolic and diastolic blood pressure over the first 6 months of treatment.49
Despite its potentially higher prevalence in this population, hypertension is both underdiagnosed and undertreated in RA patients.24,50-52 This is an important deficiency to target because, as in the general population, hypertension is associated with an increased risk of MI (RR, 1.84; 95% CI, 1.38-2.46) and composite CVD outcomes (RR, 2.24; 95% CI, 1.42-3.06) in RA.37 Thresholds for initiation and escalation of antihypertensive therapy are not specific to the RA population; thus, diagnosis and management of hypertension should be informed by the American College of Cardiology/American Heart Association guidelines, treating those with in-office blood pressures exceeding 140/90 mm Hg (> 130/80 mm Hg if aged > 65 years or with concomitant CVD, DM, chronic kidney disease, or 10-year atherosclerotic cardiovascular disease risk > 10%), typically with angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, calcium channel blockers, or thiazide diuretics as comorbidities may dictate or allow.53 Also, the use of NSAIDs and glucocorticoids should be minimized, particularly in those with concomitant hypertension.
Physical Activity
Likely due to factors such as articular pain and stiffness, as well as physical limitations, RA patients are more sedentary than the general population.54,55 In a study of objectively assessed sedentary behavior in RA patients, greater average sedentary time per day and greater number of sedentary bouts (> 20 min) were associated with increased 10-year risk of CVD as assessed by the QRISK2.56 Conversely, the beneficial effects of exercise are well documented. Light to moderate physical activity has been associated with improved cardiovascular outcomes, greater physical function, higher levels of HDL, as well as reduced systemic inflammation and disease activity, and improved endothelial function in RA patients.57-61 While there has been concern that physical activity may result in accelerated joint damage, even high-intensity exercise was shown to be safe without causing significant progression of joint damage.58
Obesity, Weight Loss, and Diet
While obesity is clearly associated with CVD risk in the general population, this relationship is much more complex in RA, as underweight RA patients are also at higher risk for CVD and CVD-related mortality.62-64 One potential explanation for this finding is that pathological weight loss resulting in an underweight body mass index (BMI) is an independent predictor of CVD. In a study of US Veterans with RA, higher rates of weight loss (> 3 kg/m2/year) were associated with increased CVD mortality (HR, 2.27; 95% CI, 1.61-3.19) independent of BMI.65 Systemic inflammation in RA can lead to “rheumatoid cachexia,” characterized by decreased muscle mass, increased adiposity, and increased CVD risk despite a normal or potentially decreased BMI.66 Practitioners should be mindful of not only current body weight, but also patients’ weight trajectories when counseling on lifestyle practices such as healthy diet and regular exercise in RA patients. For obese individuals with RA, healthy weight loss should be encouraged. Interestingly, bariatric surgery in RA patients may improve RA disease activity in addition to its known effects on body weight and DM.67
Counseling on healthy diet with a focus on limiting foods high in saturated- and trans-fatty acids and high glycemic index foods, and increasing consumption of fruits, vegetables, and mono-unsaturated fatty acids is a well-accepted and common practice to help minimize CVD risk in the general population.68 No studies to date have investigated the effect of specific diets on CVD risk in RA patients, and thus we recommend adherence to general population recommendations.
Managing RA-related CVD Risk Factors
Disease Activity
In addition to traditional risk factors, several studies have identified associations between the level of RA disease activity and risk of CVD. In a cohort of US Veterans with RA, CVD-related mortality increased in a dose-dependent manner with higher disease activity categories. In stark contrast, the CVD mortality rates of those in remission paralleled the rates from the general population (standardized mortality ratio [SMR], 0.68; 95% CI, 0.37-1.27).69 In a separate cohort of 1157 RA patients without prior CVD, achieving low disease activity was associated with a lower risk of incident CVD events (HR, 0.65; 95% CI, 0.43-0.99).70 Additionally, high disease activity has been associated with surrogate markers of CVD and other CVD risk factors including NT-proBNP and systolic blood pressure.71,72 While no randomized controlled trial data is available to inform this recommendation, observational data suggest RA should be aggressively treated (ideally to achieve and maintain remission or low disease activity) to minimize CVD risk. While keeping this treatment goal in mind, the differential effects of specific RA therapies on CVD must also be considered.
Glucocorticoids and NSAIDs
With the expanding repertoire of DMARDs available and more aggressive treatment approaches, the role of glucocorticoids and NSAIDs in RA treatment is decreasing over time. While their efficacy for improving pain and stiffness is well established, concern regarding their contribution to CVD risk in RA patients is warranted.
Glucocorticoids are known to have detrimental effects on traditional CVD risk factors such as hypertension, insulin resistance, and dyslipidemia in the general population, as well as in RA patients.73,74 In a meta-analysis of predominantly observational studies of RA patients, glucocorticoid use was associated with an increased risk of CVD events (RR, 1.47; 95% CI, 1.34-1.60), including MI, congestive heart failure (CHF), and cerebrovascular accident (CVA).75 Evidence is conflicting in regards to a clear dose threshold that leads to increased CVD risk with glucocorticoids, though higher doses are associated with greater risk.76-81 As RA patients requiring glucocorticoids typically have higher disease activity, confounding by indication remains a complicating factor in assessing the relative contributions of glucocorticoid use and RA disease activity to elevated CVD risk in many analyses.
The increased CVD risk with NSAID use is not specific to RA and has been well established in the general population.82-84 In the previously mentioned meta-analysis, an increased overall risk of CVD events was observed with NSAID use in RA (RR, 1.18; 95% CI, 1.01-1.38). It should be noted that cyclo-oxygenase 2 (COX-2) inhibitors, in particular rofecoxib (now removed from the market), appeared to drive the majority of this risk (RR, 1.36; 95% CI, 1.10-1.67 in COX-2 inhibitors and RR 1.08, 95% CI, 0.94-1.24 in nonselective NSAIDs), suggesting a potential differential risk among NSAIDs.75 While naproxen has been thought to carry the lowest risk of CVD based on initial studies, this has not been universally observed, including in a recent randomized controlled trial of more than 24,000 RA and osteoarthritis patients.82,85,86
Providers should use the lowest possible dose and duration of glucocorticoids and NSAIDs to achieve symptom relief, with continual efforts to taper or discontinue. Candidates for glucocorticoid and NSAID therapy should be selected carefully, and use of these therapies should be avoided in those with prior CVD or at high risk for CVD based on traditional CVD risk factors. Most importantly, providers should focus on utilizing DMARDs for the management of RA, which more effectively treat RA as well as reduce CVD risk.
Methotrexate
Methotrexate (MTX), a mainstay in the treatment of RA, is a conventional DMARD observed to improve overall survival and mitigate CVD risk in multiple RA cohorts.75,87,88 In a recent meta-analysis comprised of 236,525 RA patients and 5410 CVD events, MTX use was associated with a 28% reduction in overall CVD events across 8 studies (RR, 0.72; 95% CI, 0.57-0.91), substantiating similar findings in a prior meta-analysis.75,88 MTX use was specifically associated with a decreased risk of MI (RR, 0.81; 95% CI, 0.68-0.96). Case-control and cohort studies have cited a 20% to 50% reduced risk of CHF with MTX use.89,90 The potential cardioprotective effect of MTX appears to be both multifactorial and complex, likely mediated through both direct and indirect mechanisms. MTX directly promotes anti-atherogenic lipoprotein function, improves endothelial function, and scavenges free radicals.91,92 Indirectly, MTX likely reduces CVD risk by effectively reducing RA disease activity. Based on these and other data, MTX remains the cornerstone of DMARD therapy in RA patients when targeting CVD risk reduction.
Hydroxychloroquine
Emerging evidence suggests that hydroxychloroquine (HCQ), an antimalarial most often utilized in combination with alternative DMARDs in RA, prevents DM and has beneficial effects on lipid profiles. A recent meta-analysis compiled 3 homogenous observational studies that investigated the effect of HCQ on incident DM. RA patients ever exposed to HCQ had a 40% lower incidence of DM (HR, 0.59; 95% CI, 0.49-0.70).93 Increased duration of HCQ use was shown to further reduce risk of incident DM.94 The aforementioned meta-analysis also pooled 5 studies investigating the effect of HCQ on lipid profiles, with favorable mean differences in TC (–9.82 mg/dL), LDL (–10.61 mg/dL), HDL (4.13 mg/dL), and triglycerides (–19.15 mg/dL) in HCQ users compared to non-users.93 Given these favorable changes to traditional CVD risk factors, it is not surprising that in a retrospective study of 1266 RA patients without prior CVD, HCQ was associated with significantly lower risk of incident CVD. While external validation of these findings is needed, HCQ is an attractive conventional DMARD to be used in RA for CVD risk reduction. Moreover, its combination with MTX and sulfasalazine also shows promise for CVD risk reduction.95,96
TNF Inhibitors
Tumor necrosis factor (TNF) inhibitors are often the initial biologic DMARD therapy used in RA patients not responding to conventional DMARDs. In the previously described meta-analysis, TNF inhibitors were associated with similar reductions in CVD events as MTX (RR, 0.70; 95% CI, 0.54-0.90).75 Of note, there was a trend toward reduced risk of CHF (RR, 0.75; 95% CI, 0.49-1.15) in this same meta-analysis, an area of concern with TNF inhibitor use due to a prior randomized controlled trial demonstrating worsening clinical status in patients with existing moderate-to-severe CHF treated with high-dose infliximab.97 Current RA treatment guidelines recommend avoiding TNF inhibitor use in individuals with CHF.98
Aside from the risk of CHF exacerbation, TNF inhibitors appear to be cardioprotective. Similar to MTX, the mechanism by which TNF inhibition reduces cardiovascular risk is complex and likely due to both direct and indirect mechanisms. Substantial research has been conducted on the effect of TNF inhibition on lipids, with a recent meta-analysis demonstrating increases in HDL and TC, with stable LDL and atherogenic index over treatment follow-up.99 A subsequent meta-analysis not limited to RA patients yielded similar results.100 In addition to quantitative lipid changes, alteration of lipoprotein function, improvement in myocardial function, reduced aortic stiffness, improved blood pressure, and reduced RA disease activity may also be responsible for cardioprotective benefits of these agents.101,102
Non-TNF Biologic and Traditional Synthetic DMARDs
Tocilizumab, an IL-6 inhibitor, can potently increase LDL levels, but it does not appear to increase the risk of CVD events and may actually promote more favorable anti-atherogenic lipoprotein function.103-106 Although these quantitative lipid changes received significant attention in the wake of early reports detailing this effect, similar lipid changes appear to accompany other DMARDs including TNF inhibitors and tofacitinib.107 There have been few studies evaluating the risk of CVD with other non-TNF inhibitor biologic DMARDs and traditional synthetic DMARDs, warranting future study.
Conclusion
To mitigate the increased risk of CVD in RA, primary care and subspecialty providers alike must be aware of this heightened risk in RA, perform frequent assessments of CVD risk,3 and aggressively manage both traditional and nontraditional CVD risk factors. The differential roles in this effort may not be clear; thus, we have proposed a co-management strategy detailed in the Figure. Clear communication between providers is of the utmost importance to ensure effective management of CVD risk.

Given limited evidence for RA-specific CVD risk assessments and traditional risk factor treatment targets, management should follow pertinent national guidelines. The importance of lifestyle counseling should not be overlooked, with a focus on smoking cessation, healthy diet and body weight, and regular aerobic exercise. Finally, rheumatologists should aggressively manage RA using a treat-to-target approach, minimize the use of glucocorticoids and NSAIDs, and preferentially select DMARDs that have been associated with lower CVD risk. Through this comprehensive approach, recent trends of improved CVD outcomes in RA will hopefully become more widespread.108
Corresponding author: Bryant R. England, MD; 986270 Nebraska Medical Center, Omaha, NE 68198-6270; [email protected].
Financial disclosures: Dr. England is supported by UNMC Internal Medicine Scientist Development Award, UNMC Physician-Scientist Training Program, the UNMC Mentored Scholars Program, and the Rheumatology Research Foundation Scientist Development Award. Dr. Mikuls is supported by a VA Merit Award (CX000896) and grants from the National Institutes of Health: National Institute of General Medical Sciences (U54GM115458), National Institute on Alcohol Abuse and Alcoholism (R25AA020818), and National Institute of Arthritis and Musculoskeletal and Skin Diseases (2P50AR60772).
From the Division of Rh
Abstract
- Objective: To review the management of traditional and nontraditional CVD cardiovascular disease risk factors in rheumatoid arthritis (RA).
- Methods: Literature review of the management of CVD risk in RA.
- Results: Because of the increased risk of CVD events and CVD mortality among RA patients, aggressive management of CVD risk is essential. Providers should follow national guidelines for the management of traditional CVD risk factors, including dyslipidemia, hypertension, and diabetes mellitus. Similar efforts are needed in counseling on lifestyle modifications, including smoking cessation, regular exercise, and maintaining a healthy body weight. Because higher RA disease activity is also linked with CVD risk, aggressive treatment of RA to a target of low disease activity or remission is critical. Furthermore, the selection of potentially “cardioprotective” agents such as methotrexate and tumor necrosis factor inhibitors, while limiting use of nonsteroidal anti-inflammatory drugs and glucocorticoids, are strategies that could be employed by rheumatologists to help mitigate CVD risk in their patients with RA.
- Conclusion: Routine assessment of CVD risk, management of traditional CVD risk factors, counseling on healthy lifestyle habits, and aggressive treatment of RA are essential to minimize CVD risk in this population.
Keywords: rheumatoid arthritis; cardiovascular disease; cardiovascular risk assessment; cardiovascular risk management.
Editor’s note: This article is part 2 of a 2-part article. “Assessment of Cardiovascular Disease Risk in Rheumatoid Arthritis” was published in the January/February 2019 issue.
Rheumatoid arthritis (RA) is a systemic autoimmune condition that contributes to an increased risk for cardiovascular disease (CVD) among affected patients. In persons with RA, the risk of incident CVD and CVD mortality are increased by approximately 50% compared with the general population.1,2 To minimize CVD risk in this population, providers must routinely assess for CVD risk factors3 and aggressively manage both traditional and nontraditional CVD risk factors.
Managing Traditional Risk Factors
As in the general population, identification and management of traditional CVD risk factors are crucial to minimize CVD risk in the RA population. A prospective study of 201 RA patients demonstrated that traditional CVD risk factors were in fact more predictive of endothelial dysfunction and carotid atherosclerosis than were disease-related inflammatory markers in RA.4 Management of traditional risk factors is detailed in the following sections, and recommendations for managing all traditional CVD risk factors are summarized in the Table.

Dyslipidemia
The role of dyslipidemia in atherogenesis is well established, and as a result, lipid levels are nearly universally included in CVD risk stratification tools. However, the interpretation of lipid levels in the context of RA is challenging because of the effects of systemic inflammation on their absolute values. Compared to the general population, patients with RA have lower total cholesterol (TC) and low-density lipoprotein (LDL) levels independent of lipid-lowering therapy.5,6 Despite this, RA patients are at increased risk for CVD. There is even some evidence to suggest a “lipid paradox” in RA, whereby lower TC (< 4 mmol/L) and LDL levels suggest an increased risk of CVD.7,8 In contrast to LDL, higher levels of high-density lipoprotein (HDL) are typically associated with reduced CVD risk, as in the general population.8,9 Interestingly, in a cohort of 16,085 RA patients and 48,499 age- and sex-matched controls, there was no significant difference in the relationship between LDL and CVD risk, suggesting that quantitative lipid levels alone may not entirely explain the CVD mortality gap in RA.9 As such, there is substantial interest in lipoprotein function within the context of CVD risk in RA. Recent investigations have identified impaired HDL function, with reduced cholesterol efflux capacity and antioxidant properties, as well as increased scavenger receptor expression and foam cell formation, in patients with RA.10,11 More research is needed to elucidate how these alterations affect CVD morbidity and mortality and how their measurement could be integrated into improved CVD risk assessment.
Meta-analyses of randomized controlled trials have estimated that lipid-lowering therapy with HMG-CoA reductase inhibitors (statins) reduces the risk of CVD by 25% to 30%; as such, statin therapy has become the standard of care for reduction of CVD risk in the general population.12 Benefits for primary prevention of CVD in RA have also been observed; statin therapy was associated with a reduced risk of CVD events (hazard ratio [HR], 0.45; 95% confidence interval [CI], 0.20-0.98) and all-cause mortality (HR, 0.43; 95% CI, 0.20-0.92) in a population-based cohort study.13 Statins appear to have similar lipid-lowering effects and result in similar CVD risk reduction when used for primary or secondary prevention in RA patients compared to non-RA controls.14-16 Additionally, anti-inflammatory properties of statins may act in synergy with disease-modifying antirheumatic drugs (DMARDs) to improve RA disease activity. In a small study of RA patients, statin therapy improved subjective and objective markers of RA disease activity in conjunction with methotrexate.17
While statins provide robust reduction in CVD risk, some individuals cannot tolerate statin therapy or do not achieve goal LDL levels with statin therapy. Select non-statin LDL-cholesterol-lowering agents have shown promise for reducing CVD events in the general population.18 Ezetimibe, which inhibits cholesterol absorption in the small intestine, very modestly reduced CVD events when added to atorvastatin (relative risk [RR], 0.94; 95% CI, 0.89-0.99) in a double-blind randomized controlled trial.19 Novel monoclonal antibodies to proprotein convertase subtilisin/kexin type 9 (PCSK-9) inhibit the internalization of surface LDL receptors, promoting LDL clearance. Two PCSK-9 inhibitors, alirocumab and evolocumab, were approved by the US Food and Drug Administration (FDA) after randomized controlled trials demonstrated their efficacy in lowering LDL by approximately 60% and reducing CVD events by approximately 15% in patients on maximum-tolerated statin therapy.20-22 To date, non-statin LDL-cholesterol-lowering agents have been subject to limited study in RA.23
Identification and management of dyslipidemia offers an opportunity for substantial CVD risk reduction at the RA population level. Unfortunately, current rates of lipid screening are inadequate in this high-risk group. In a study of 3298 Medicare patients with RA, less than half of RA patients with an indication underwent appropriate lipid screening.24 Additionally, statins are often underutilized for both primary and secondary prevention in RA patients. Only 27% of RA patients meeting National Cholesterol Education Program Adult Treatment Panel III criteria were initiated on statin therapy in a population-based cohort study.25 Among patients discharged after a first myocardial infarction (MI), the odds of receiving lipid-lowering therapy were 31% lower for RA patients (odds ratio [OR], 0.69; 95% CI, 0.58-0.82).26 Similar to the general population, adherence to statins in RA patients appears to be poor.27-30 This raises particular concern considering that a population-based cohort study of RA patients demonstrated a 67% increased risk of MI associated with statin discontinuation, regardless of prior MI status.27 Providers—rheumatologists, primary care providers, and cardiologists alike—need to remain vigilant in efforts to assess CVD risk to identify patients who will benefit from lipid-lowering therapy and to emphasize the importance to patients of statin adherence. Novel models of health-care delivery, health technologies, and patient engagement in care may prove useful for improving lipid screening and management in RA.
Tobacco Use
Cigarette smoking is a shared risk factor for both CVD and RA. Large cohort studies have identified a dose-dependent increased risk of incident RA, particularly seropositive RA, among smokers.31-34 Tobacco smoking has also been associated with increased levels of inflammation and RA disease activity.35 The consequences of tobacco use in the general population are staggering. Among individuals over the age of 30 years, tobacco use is responsible for 12% of all deaths and 10% of all CVD deaths.36 Similar findings are observed in RA; a recent meta-analysis estimated there is a 50% increased risk of CVD events in RA related to smoking tobacco.37 In the general population, smoking cessation markedly lowers CVD risk, and over time CVD risk may approach that of nonsmokers.38,39 Thus, regular counseling and interventions to facilitate smoking cessation are critical to reducing CVD risk in RA patients. RA-specific smoking cessation programs have been proposed, but have yet to outperform standard smoking cessation programs.40
Diabetes Mellitus
It is estimated that almost 10% of the US population has diabetes mellitus (DM), which in isolation portends substantial CVD risk.41 There is an increased prevalence of DM in RA, perhaps owing to factors such as physical inactivity and chronic glucocorticoid use, though a higher level of RA disease activity itself has been associated with increased insulin resistance.42-45 In a cohort of 100 RA patients who were neither obese nor diabetic, RA patients had significantly higher fasting blood glucose and insulin levels than age- and sex-matched controls. These findings were even more pronounced in RA patients with higher levels of disease activity.44 Similar to the general population, DM is associated with poor CVD outcomes in RA.37 Therefore, both appropriate management of diabetes and control of RA disease activity are vitally important to minimize CVD risk related to DM.
Hypertension
Though not a universal finding, there may be an increased prevalence of hypertension in RA patients.31,46 Nonsteroidal anti-inflammatory drug (NSAID) and glucocorticoid use may play a role in the development of hypertension, while DMARDs appear to exert a less substantial effect on blood pressure.47,48 At least one study found that DMARD initiation (particularly for methotrexate and hydroxychloroquine) was associated with significant, albeit small, declines in both systolic and diastolic blood pressure over the first 6 months of treatment.49
Despite its potentially higher prevalence in this population, hypertension is both underdiagnosed and undertreated in RA patients.24,50-52 This is an important deficiency to target because, as in the general population, hypertension is associated with an increased risk of MI (RR, 1.84; 95% CI, 1.38-2.46) and composite CVD outcomes (RR, 2.24; 95% CI, 1.42-3.06) in RA.37 Thresholds for initiation and escalation of antihypertensive therapy are not specific to the RA population; thus, diagnosis and management of hypertension should be informed by the American College of Cardiology/American Heart Association guidelines, treating those with in-office blood pressures exceeding 140/90 mm Hg (> 130/80 mm Hg if aged > 65 years or with concomitant CVD, DM, chronic kidney disease, or 10-year atherosclerotic cardiovascular disease risk > 10%), typically with angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, calcium channel blockers, or thiazide diuretics as comorbidities may dictate or allow.53 Also, the use of NSAIDs and glucocorticoids should be minimized, particularly in those with concomitant hypertension.
Physical Activity
Likely due to factors such as articular pain and stiffness, as well as physical limitations, RA patients are more sedentary than the general population.54,55 In a study of objectively assessed sedentary behavior in RA patients, greater average sedentary time per day and greater number of sedentary bouts (> 20 min) were associated with increased 10-year risk of CVD as assessed by the QRISK2.56 Conversely, the beneficial effects of exercise are well documented. Light to moderate physical activity has been associated with improved cardiovascular outcomes, greater physical function, higher levels of HDL, as well as reduced systemic inflammation and disease activity, and improved endothelial function in RA patients.57-61 While there has been concern that physical activity may result in accelerated joint damage, even high-intensity exercise was shown to be safe without causing significant progression of joint damage.58
Obesity, Weight Loss, and Diet
While obesity is clearly associated with CVD risk in the general population, this relationship is much more complex in RA, as underweight RA patients are also at higher risk for CVD and CVD-related mortality.62-64 One potential explanation for this finding is that pathological weight loss resulting in an underweight body mass index (BMI) is an independent predictor of CVD. In a study of US Veterans with RA, higher rates of weight loss (> 3 kg/m2/year) were associated with increased CVD mortality (HR, 2.27; 95% CI, 1.61-3.19) independent of BMI.65 Systemic inflammation in RA can lead to “rheumatoid cachexia,” characterized by decreased muscle mass, increased adiposity, and increased CVD risk despite a normal or potentially decreased BMI.66 Practitioners should be mindful of not only current body weight, but also patients’ weight trajectories when counseling on lifestyle practices such as healthy diet and regular exercise in RA patients. For obese individuals with RA, healthy weight loss should be encouraged. Interestingly, bariatric surgery in RA patients may improve RA disease activity in addition to its known effects on body weight and DM.67
Counseling on healthy diet with a focus on limiting foods high in saturated- and trans-fatty acids and high glycemic index foods, and increasing consumption of fruits, vegetables, and mono-unsaturated fatty acids is a well-accepted and common practice to help minimize CVD risk in the general population.68 No studies to date have investigated the effect of specific diets on CVD risk in RA patients, and thus we recommend adherence to general population recommendations.
Managing RA-related CVD Risk Factors
Disease Activity
In addition to traditional risk factors, several studies have identified associations between the level of RA disease activity and risk of CVD. In a cohort of US Veterans with RA, CVD-related mortality increased in a dose-dependent manner with higher disease activity categories. In stark contrast, the CVD mortality rates of those in remission paralleled the rates from the general population (standardized mortality ratio [SMR], 0.68; 95% CI, 0.37-1.27).69 In a separate cohort of 1157 RA patients without prior CVD, achieving low disease activity was associated with a lower risk of incident CVD events (HR, 0.65; 95% CI, 0.43-0.99).70 Additionally, high disease activity has been associated with surrogate markers of CVD and other CVD risk factors including NT-proBNP and systolic blood pressure.71,72 While no randomized controlled trial data is available to inform this recommendation, observational data suggest RA should be aggressively treated (ideally to achieve and maintain remission or low disease activity) to minimize CVD risk. While keeping this treatment goal in mind, the differential effects of specific RA therapies on CVD must also be considered.
Glucocorticoids and NSAIDs
With the expanding repertoire of DMARDs available and more aggressive treatment approaches, the role of glucocorticoids and NSAIDs in RA treatment is decreasing over time. While their efficacy for improving pain and stiffness is well established, concern regarding their contribution to CVD risk in RA patients is warranted.
Glucocorticoids are known to have detrimental effects on traditional CVD risk factors such as hypertension, insulin resistance, and dyslipidemia in the general population, as well as in RA patients.73,74 In a meta-analysis of predominantly observational studies of RA patients, glucocorticoid use was associated with an increased risk of CVD events (RR, 1.47; 95% CI, 1.34-1.60), including MI, congestive heart failure (CHF), and cerebrovascular accident (CVA).75 Evidence is conflicting in regards to a clear dose threshold that leads to increased CVD risk with glucocorticoids, though higher doses are associated with greater risk.76-81 As RA patients requiring glucocorticoids typically have higher disease activity, confounding by indication remains a complicating factor in assessing the relative contributions of glucocorticoid use and RA disease activity to elevated CVD risk in many analyses.
The increased CVD risk with NSAID use is not specific to RA and has been well established in the general population.82-84 In the previously mentioned meta-analysis, an increased overall risk of CVD events was observed with NSAID use in RA (RR, 1.18; 95% CI, 1.01-1.38). It should be noted that cyclo-oxygenase 2 (COX-2) inhibitors, in particular rofecoxib (now removed from the market), appeared to drive the majority of this risk (RR, 1.36; 95% CI, 1.10-1.67 in COX-2 inhibitors and RR 1.08, 95% CI, 0.94-1.24 in nonselective NSAIDs), suggesting a potential differential risk among NSAIDs.75 While naproxen has been thought to carry the lowest risk of CVD based on initial studies, this has not been universally observed, including in a recent randomized controlled trial of more than 24,000 RA and osteoarthritis patients.82,85,86
Providers should use the lowest possible dose and duration of glucocorticoids and NSAIDs to achieve symptom relief, with continual efforts to taper or discontinue. Candidates for glucocorticoid and NSAID therapy should be selected carefully, and use of these therapies should be avoided in those with prior CVD or at high risk for CVD based on traditional CVD risk factors. Most importantly, providers should focus on utilizing DMARDs for the management of RA, which more effectively treat RA as well as reduce CVD risk.
Methotrexate
Methotrexate (MTX), a mainstay in the treatment of RA, is a conventional DMARD observed to improve overall survival and mitigate CVD risk in multiple RA cohorts.75,87,88 In a recent meta-analysis comprised of 236,525 RA patients and 5410 CVD events, MTX use was associated with a 28% reduction in overall CVD events across 8 studies (RR, 0.72; 95% CI, 0.57-0.91), substantiating similar findings in a prior meta-analysis.75,88 MTX use was specifically associated with a decreased risk of MI (RR, 0.81; 95% CI, 0.68-0.96). Case-control and cohort studies have cited a 20% to 50% reduced risk of CHF with MTX use.89,90 The potential cardioprotective effect of MTX appears to be both multifactorial and complex, likely mediated through both direct and indirect mechanisms. MTX directly promotes anti-atherogenic lipoprotein function, improves endothelial function, and scavenges free radicals.91,92 Indirectly, MTX likely reduces CVD risk by effectively reducing RA disease activity. Based on these and other data, MTX remains the cornerstone of DMARD therapy in RA patients when targeting CVD risk reduction.
Hydroxychloroquine
Emerging evidence suggests that hydroxychloroquine (HCQ), an antimalarial most often utilized in combination with alternative DMARDs in RA, prevents DM and has beneficial effects on lipid profiles. A recent meta-analysis compiled 3 homogenous observational studies that investigated the effect of HCQ on incident DM. RA patients ever exposed to HCQ had a 40% lower incidence of DM (HR, 0.59; 95% CI, 0.49-0.70).93 Increased duration of HCQ use was shown to further reduce risk of incident DM.94 The aforementioned meta-analysis also pooled 5 studies investigating the effect of HCQ on lipid profiles, with favorable mean differences in TC (–9.82 mg/dL), LDL (–10.61 mg/dL), HDL (4.13 mg/dL), and triglycerides (–19.15 mg/dL) in HCQ users compared to non-users.93 Given these favorable changes to traditional CVD risk factors, it is not surprising that in a retrospective study of 1266 RA patients without prior CVD, HCQ was associated with significantly lower risk of incident CVD. While external validation of these findings is needed, HCQ is an attractive conventional DMARD to be used in RA for CVD risk reduction. Moreover, its combination with MTX and sulfasalazine also shows promise for CVD risk reduction.95,96
TNF Inhibitors
Tumor necrosis factor (TNF) inhibitors are often the initial biologic DMARD therapy used in RA patients not responding to conventional DMARDs. In the previously described meta-analysis, TNF inhibitors were associated with similar reductions in CVD events as MTX (RR, 0.70; 95% CI, 0.54-0.90).75 Of note, there was a trend toward reduced risk of CHF (RR, 0.75; 95% CI, 0.49-1.15) in this same meta-analysis, an area of concern with TNF inhibitor use due to a prior randomized controlled trial demonstrating worsening clinical status in patients with existing moderate-to-severe CHF treated with high-dose infliximab.97 Current RA treatment guidelines recommend avoiding TNF inhibitor use in individuals with CHF.98
Aside from the risk of CHF exacerbation, TNF inhibitors appear to be cardioprotective. Similar to MTX, the mechanism by which TNF inhibition reduces cardiovascular risk is complex and likely due to both direct and indirect mechanisms. Substantial research has been conducted on the effect of TNF inhibition on lipids, with a recent meta-analysis demonstrating increases in HDL and TC, with stable LDL and atherogenic index over treatment follow-up.99 A subsequent meta-analysis not limited to RA patients yielded similar results.100 In addition to quantitative lipid changes, alteration of lipoprotein function, improvement in myocardial function, reduced aortic stiffness, improved blood pressure, and reduced RA disease activity may also be responsible for cardioprotective benefits of these agents.101,102
Non-TNF Biologic and Traditional Synthetic DMARDs
Tocilizumab, an IL-6 inhibitor, can potently increase LDL levels, but it does not appear to increase the risk of CVD events and may actually promote more favorable anti-atherogenic lipoprotein function.103-106 Although these quantitative lipid changes received significant attention in the wake of early reports detailing this effect, similar lipid changes appear to accompany other DMARDs including TNF inhibitors and tofacitinib.107 There have been few studies evaluating the risk of CVD with other non-TNF inhibitor biologic DMARDs and traditional synthetic DMARDs, warranting future study.
Conclusion
To mitigate the increased risk of CVD in RA, primary care and subspecialty providers alike must be aware of this heightened risk in RA, perform frequent assessments of CVD risk,3 and aggressively manage both traditional and nontraditional CVD risk factors. The differential roles in this effort may not be clear; thus, we have proposed a co-management strategy detailed in the Figure. Clear communication between providers is of the utmost importance to ensure effective management of CVD risk.

Given limited evidence for RA-specific CVD risk assessments and traditional risk factor treatment targets, management should follow pertinent national guidelines. The importance of lifestyle counseling should not be overlooked, with a focus on smoking cessation, healthy diet and body weight, and regular aerobic exercise. Finally, rheumatologists should aggressively manage RA using a treat-to-target approach, minimize the use of glucocorticoids and NSAIDs, and preferentially select DMARDs that have been associated with lower CVD risk. Through this comprehensive approach, recent trends of improved CVD outcomes in RA will hopefully become more widespread.108
Corresponding author: Bryant R. England, MD; 986270 Nebraska Medical Center, Omaha, NE 68198-6270; [email protected].
Financial disclosures: Dr. England is supported by UNMC Internal Medicine Scientist Development Award, UNMC Physician-Scientist Training Program, the UNMC Mentored Scholars Program, and the Rheumatology Research Foundation Scientist Development Award. Dr. Mikuls is supported by a VA Merit Award (CX000896) and grants from the National Institutes of Health: National Institute of General Medical Sciences (U54GM115458), National Institute on Alcohol Abuse and Alcoholism (R25AA020818), and National Institute of Arthritis and Musculoskeletal and Skin Diseases (2P50AR60772).
1. Avina-Zubieta JA, Choi HK, Sadatsafavi M, et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690-1697.
2. Avina-Zubieta JA, Thomas J, Sadatsafavi M, et al. Risk of incident cardiovascular events in patients with rheumatoid arthritis: A meta-analysis of observational studies. Ann Rheum Dis. 2012;71:1524-1529.
3. Johnson TM, Mikuls TR, England BR. Assessment of cardiovascular risk in rheumatoid arthritis. J Clin Outcomes Manage. 2019;26:41-47.
4. Sandoo A, Chanchlani N, Hodson J, et al. Classical cardiovascular disease risk factors associate with vascular function and morphology in rheumatoid arthritis: A six-year prospective study. Arthritis Res Ther. 2013;15:R203.
5. Myasoedova E, Crowson CS, Kremers HM, et al. Total cholesterol and LDL levels decrease before rheumatoid arthritis. Ann Rheum Dis. 2010;69:1310-1314.
6. Liao KP, Cai T, Gainer VS, et al. Lipid and lipoprotein levels and trend in rheumatoid arthritis compared to the general population. Arthritis Care Res (Hoboken). 2013;65:2046-2050.
7. Myasoedova E, Crowson CS, Kremers HM, et al. Lipid paradox in rheumatoid arthritis: The impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann Rheum Dis. 2011;70:482-487.
8. Zhang J, Chen L, Delzell E, et al. Republished: The association between inflammatory markers, serum lipids and the risk of cardiovascular events in patients with rheumatoid arthritis. Postgrad Med J. 2014;90:722-729.
9. Liao KP, Liu J, Lu B, et al. Association between lipid levels and major adverse cardiovascular events in rheumatoid arthritis compared to non-rheumatoid arthritis patients. Arthritis Rheumatol. 2015;67:2004-2010.
10. Charles-Schoeman C, Lee YY, Grijalva V, et al. Cholesterol efflux by high density lipoproteins is impaired in patients with active rheumatoid arthritis. Ann Rheum Dis. 2012;71:1157-1162.
11. Voloshyna I, Modayil S, Littlefield MJ, et al. Plasma from rheumatoid arthritis patients promotes pro-atherogenic cholesterol transport gene expression in THP-1 human macrophages. Exp Biol Med (Maywood). 2013 238:1192-1197.
12. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;(1):CD004816.
13. Sheng X, Murphy MJ, Macdonald TM, Wei L. Effectiveness of statins on total cholesterol and cardiovascular disease and all-cause mortality in osteoarthritis and rheumatoid arthritis. J Rheumatol. 2012;39:32-40.
14. An J, Alemao E, Reynolds K, et al. Cardiovascular outcomes associated with lowering low-density lipoprotein cholesterol in rheumatoid arthritis and matched nonrheumatoid arthritis. J Rheumatol. 2016;43:1989-1996.
15. Semb AG, Holme I, Kvien TK, Pedersen TR. Intensive lipid lowering in patients with rheumatoid arthritis and previous myocardial infarction: An explorative analysis from the incremental decrease in endpoints through aggressive lipid lowering (IDEAL) trial. Rheumatology (Oxford). 2011;50:324-329.
16. Semb AG, Kvien TK, DeMicco DA, et al. Effect of intensive lipid-lowering therapy on cardiovascular outcome in patients with and those without inflammatory joint disease. Arthritis Rheum. 2012;64:2836-2846.
17. El-Barbary AM, Hussein MS, Rageh EM, et al. Effect of atorvastatin on inflammation and modification of vascular risk factors in rheumatoid arthritis. J Rheumatol. 2011;38:229-235.
18. Writing Committee, Lloyd-Jones DM, Morris PB, et al. 2016 ACC expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: A report of the American college of cardiology task force on clinical expert consensus documents. J Am Coll Cardiol. 2016;68:92-125.
19. Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
20. Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500-1509.
21. Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489-1499.
22. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
23. Maki-Petaja KM, Booth AD, Hall FC, et al. Ezetimibe and simvastatin reduce inflammation, disease activity, and aortic stiffness and improve endothelial function in rheumatoid arthritis. J Am Coll Cardiol. 2007;50:852-858.
24. Bartels CM, Kind AJ, Everett C, et al. Low frequency of primary lipid screening among medicare patients with rheumatoid arthritis. Arthritis Rheum. 2011;63:1221-1230.
25. Akkara Veetil BM, Myasoedova E, Matteson EL, et al. Use of lipid-lowering agents in rheumatoid arthritis: A population-based cohort study. J Rheumatol. 2013;40:1082-1088.
26. Lindhardsen J, Ahlehoff O, Gislason GH, et al. Initiation and adherence to secondary prevention pharmacotherapy after myocardial infarction in patients with rheumatoid arthritis: A nationwide cohort study. Ann Rheum Dis. 2012;71:1496-1501.
27. De Vera MA, Choi H, Abrahamowicz M, et al. Statin discontinuation and risk of acute myocardial infarction in patients with rheumatoid arthritis: A population-based cohort study. Ann Rheum Dis. 2011;70:1020-1024.
28. Zhang H, Plutzky J, Skentzos S, et al. Discontinuation of statins in routine care settings: A cohort study. Ann Intern Med. 2013;158:526-534.
29. Zhang H, Plutzky J, Shubina M, Turchin A. Continued statin prescriptions after adverse reactions and patient outcomes: A cohort study. Ann Intern Med. 2017;167:221-227.
30. Lemstra M, Blackburn D, Crawley A, Fung R. Proportion and risk indicators of nonadherence to statin therapy: A meta-analysis. Can J Cardiol. 2012;28:574-580.
31. Boyer JF, Gourraud PA, Cantagrel A, et al. Traditional cardiovascular risk factors in rheumatoid arthritis: A meta-analysis. Joint Bone Spine. 2011;78:179-183.
32. Bergstrom U, Jacobsson LT, Nilsson JA, et al. Pulmonary dysfunction, smoking, socioeconomic status and the risk of developing rheumatoid arthritis. Rheumatology (Oxford). 2011;50:2005-2013.
33. Costenbader KH, Feskanich D, Mandl LA, Karlson EW. Smoking intensity, duration, and cessation, and the risk of rheumatoid arthritis in women. Am J Med. 2006;119:503.e1,503.e9.
34. Klareskog L, Stolt P, Lundberg K, et al. A new model for an etiology of rheumatoid arthritis: Smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006;54:38-46.
35. Sokolove J, Wagner CA, Lahey LJ, et al. Increased inflammation and disease activity among current cigarette smokers with rheumatoid arthritis: A cross-sectional analysis of US veterans. Rheumatology (Oxford). 2016;55:1969-1977.
36. World Health Organization. WHO Global Report: Mortality Attributable to Tobacco. Geneva, World Health Organization, 2012.
37. Baghdadi LR, Woodman RJ, Shanahan EM, Mangoni AA. The impact of traditional cardiovascular risk factors on cardiovascular outcomes in patients with rheumatoid arthritis: A systematic review and meta-analysis. PLoS One. 2015;10:e0117952.
38. Centers for Disease Control and Prevention; National Center for Chronic Disease Prevention and Health Promotion. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention; 2010. 6, Cardiovascular Diseases. Available from: https://ncbi.nlm.nih.gov/books/NBK53012/
39. Mons U, Muezzinler A, Gellert C, et al. Impact of smoking and smoking cessation on cardiovascular events and mortality among older adults: Meta-analysis of individual participant data from prospective cohort studies of the CHANCES consortium. BMJ. 2015;350:h1551.
40. Aimer P, Treharne GJ, Stebbings S, Frampton C, Cameron V, Kirby S, et al. Efficacy of a rheumatoid arthritis-specific smoking cessation program: A randomized controlled pilot trial. Arthritis Care Res (Hoboken). 2017;69:28-37.
41. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services; 2017.
42. Jiang P, Li H, Li X. Diabetes mellitus risk factors in rheumatoid arthritis: A systematic review and meta-analysis. Clin Exp Rheumatol. 2015;33:115-121.
43. Shahin D, Eltoraby E, Mesbah A, Houssen M. Insulin resistance in early untreated rheumatoid arthritis patients. Clin Biochem. 2010;43:661-335.
44. Arias de la Rosa I, Escudero-Contreras A, Rodriguez-Cuenca S, et al. Defective glucose and lipid metabolism in rheumatoid arthritis is determined by chronic inflammation in metabolic tissues. J Intern Med. 2018;84(1):61-77.
45. Wilson JC, Sarsour K, Gale S, et al. Incidence and risk of glucocorticoid-associated adverse effects in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2018 Jun 1. doi: 10.1002/acr.23611.
46. Chung CP, Giles JT, Petri M, et al. Prevalence of traditional modifiable cardiovascular risk factors in patients with rheumatoid arthritis: Comparison with control subjects from the multi-ethnic study of atherosclerosis. Semin Arthritis Rheum. 2012;41:535-544.
47. Goodwin JE, Geller DS. Glucocorticoid-induced hypertension. Pediatr Nephrol. 2012;27:1059-1066.
48. Snowden S, Nelson R. The effects of nonsteroidal anti-inflammatory drugs on blood pressure in hypertensive patients. Cardiol Rev. 2011;19:184-191.
49. Baker JF, Sauer B, Teng CC, et al. Initiation of disease-modifying therapies in rheumatoid arthritis is associated with changes in blood pressure. J Clin Rheumatol. 2018;24:203-209.
50. Panoulas VF, Douglas KM, Milionis HJ, et al. Prevalence and associations of hypertension and its control in patients with rheumatoid arthritis. Rheumatology (Oxford). 2007;46:1477-1482.
51. Protogerou AD, Panagiotakos DB, Zampeli E, et al. Arterial hypertension assessed “out-of-office” in a contemporary cohort of rheumatoid arthritis patients free of cardiovascular disease is characterized by high prevalence, low awareness, poor control and increased vascular damage-associated “white coat” phenomenon. Arthritis Res Ther. 2013;15:R142.
52. van Breukelen-van der Stoep DF, van Zeben D, Klop B, et al. Marked underdiagnosis and undertreatment of hypertension and hypercholesterolaemia in rheumatoid arthritis. Rheumatology (Oxford). 2016;55:1210-1216.
53. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2018;71:e127-248.
54. Lee J, Dunlop D, Ehrlich-Jones L, et al. Public health impact of risk factors for physical inactivity in adults with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64:488-493.
55. Sokka T, Hakkinen A, Kautiainen H, et al. Physical inactivity in patients with rheumatoid arthritis: Data from twenty-one countries in a cross-sectional, international study. Arthritis Rheum. 2008;59:42-50.
56. Fenton SAM, Veldhuijzen van Zanten JJCS, Kitas GD, et al. Sedentary behaviour is associated with increased long-term cardiovascular risk in patients with rheumatoid arthritis independently of moderate-to-vigorous physical activity. BMC Musculoskelet Disord. 2017;18:131,017-1473-9.
57. Byram KW, Oeser AM, Linton MF, et al. Exercise is associated with increased small HDL particle concentration and decreased vascular stiffness in rheumatoid arthritis. J Clin Rheumatol. 2018 May 25. 9.
58. de Jong Z, Munneke M, Zwinderman AH, et al. Is a long-term high-intensity exercise program effective and safe in patients with rheumatoid arthritis? results of a randomized controlled trial. Arthritis Rheum. 2003;48:2415-2424.
59. Stavropoulos-Kalinoglou A, Metsios GS, Veldhuijzen van Zanten JJ, et al. Individualised aerobic and resistance exercise training improves cardiorespiratory fitness and reduces cardiovascular risk in patients with rheumatoid arthritis. Ann Rheum Dis. 2013;72:1819-1825.
60. Khoja SS, Almeida GJ, Chester Wasko M, et al. Association of light-intensity physical activity with lower cardiovascular disease risk burden in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2016;68:424-431.
61. Metsios GS, Koutedakis Y, Veldhuijzen van Zanten JJ, et al. Cardiorespiratory fitness levels and their association with cardiovascular profile in patients with rheumatoid arthritis: A cross-sectional study. Rheumatology (Oxford). 2015;54:2215-2220.
62. Escalante A, Haas RW, del Rincon I. Paradoxical effect of body mass index on survival in rheumatoid arthritis: Role of comorbidity and systemic inflammation. Arch Intern Med. 2005;165:1624-1629.
63. Kremers HM, Nicola PJ, Crowson CS, et al. Prognostic importance of low body mass index in relation to cardiovascular mortality in rheumatoid arthritis. Arthritis Rheum. 2004;50:3450-3457.
64. Wolfe F, Michaud K. Effect of body mass index on mortality and clinical status in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64:1471-1479.
65. England BR, Baker JF, Sayles H, et al. Body mass index, weight loss, and cause-specific mortality in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2018;70:11-18.
66. Dessein PH, Solomon A, Hollan I. Metabolic abnormalities in patients with inflammatory rheumatic diseases. Best Pract Res Clin Rheumatol. 2016;30:901-915.
67. Sparks JA, Halperin F, Karlson JC, et al. Impact of bariatric surgery on patients with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2015;67:1619-1626.
68. Mente A, de Koning L, Shannon HS, Anand SS. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Intern Med. 2009;169:659-669.
69. England BR, Sayles H, Michaud K, et al. Cause-specific mortality in male US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2016;68:36-45.
70. Arts EE, Fransen J, Den Broeder AA, et al. Low disease activity (DAS28≤3.2) reduces the risk of first cardiovascular event in rheumatoid arthritis: a time-dependent Cox regression analysis in a large cohort study. Ann Rheum Dis. 2017;76(10):1693-1699.
71. Provan SA, Semb AG, Hisdal J, et al. Remission is the goal for cardiovascular risk management in patients with rheumatoid arthritis: A cross-sectional comparative study. Ann Rheum Dis. 2011;70:812-817.
72. Klarenbeek NB, van der Kooij SM, Huizinga TJ, et al. Blood pressure changes in patients with recent-onset rheumatoid arthritis treated with four different treatment strategies: A post hoc analysis from the BeSt trial. Ann Rheum Dis. 2010;69:1342-1345.
73. Hafstrom I, Rohani M, Deneberg S, et al. Effects of low-dose prednisolone on endothelial function, atherosclerosis, and traditional risk factors for atherosclerosis in patients with rheumatoid arthritis—a randomized study. J Rheumatol. 2007;34:1810-1816.
74. Hoes JN, van der Goes MC, van Raalte DH, et al. Glucose tolerance, insulin sensitivity and beta-cell function in patients with rheumatoid arthritis treated with or without low-to-medium dose glucocorticoids. Ann Rheum Dis. 2011;70:1887-1894.
75. Roubille C. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: A systematic review and meta-analysis. Ann Rheum Dis. 2003;74:480-489.
76. Ajeganova S, Svensson B, Hafstrom I, BARFOT Study Group. Low-dose prednisolone treatment of early rheumatoid arthritis and late cardiovascular outcome and survival: 10-year follow-up of a 2-year randomised trial. BMJ Open. 2014;4:e004259,2013-004259.
77. Avina-Zubieta JA, Choi HK, Sadatsafavi M, et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690-1697.
78. del Rincon I, Battafarano DF, Restrepo JF, et al. Glucocorticoid dose thresholds associated with all-cause and cardiovascular mortality in rheumatoid arthritis. Arthritis Rheumatol. 2014;66:264-272.
79. Davis JM,3rd, Maradit Kremers H, Crowson CS, et al. Glucocorticoids and cardiovascular events in rheumatoid arthritis: A population-based cohort study. Arthritis Rheum. 2007;56:820-830.
80. Zhang J, Xie F, Yun H, et al. Comparative effects of biologics on cardiovascular risk among older patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75:1813-1818.
81. Greenberg JD, Kremer JM, Curtis JR, et al. Tumour necrosis factor antagonist use and associated risk reduction of cardiovascular events among patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70:576-582.
82. Lindhardsen J, Gislason GH, Jacobsen S, et al. Non-steroidal anti-inflammatory drugs and risk of cardiovascular disease in patients with rheumatoid arthritis: A nationwide cohort study. Ann Rheum Dis. 2014;73:1515-1521.
83. Schjerning Olsen AM, Fosbol EL, Lindhardsen J, et al. Duration of treatment with nonsteroidal anti-inflammatory drugs and impact on risk of death and recurrent myocardial infarction in patients with prior myocardial infarction: A nationwide cohort study. Circulation. 2011;123:2226-2235.
84. Gislason GH, Rasmussen JN, Abildstrom SZ, et al. Increased mortality and cardiovascular morbidity associated with use of nonsteroidal anti-inflammatory drugs in chronic heart failure. Arch Intern Med. 2009;169:141-149.
85. Trelle S, Reichenbach S, Wandel S, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: Network meta-analysis. BMJ. 2011;342:c7086.
86. Nissen SE, Yeomans ND, Solomon DH, et al. Cardiovascular safety of celecoxib, naproxen, or ibuprofen for arthritis. N Engl J Med. 2016;375:2519-2529.
87. Wasko MC, Dasgupta A, Hubert Het al. Propensity-adjusted association of methotrexate with overall survival in rheumatoid arthritis. Arthritis Rheum. 2013;65:334-342.
88. Micha R, Imamura F, Wyler von Ballmoos M, et al. Systematic review and meta-analysis of methotrexate use and risk of cardiovascular disease. Am J Cardiol. 2011;108:1362-1370.
89. Bernatsky S, Hudson M, Suissa S. Anti-rheumatic drug use and risk of hospitalization for congestive heart failure in rheumatoid arthritis. Rheumatology (Oxford). 2005;44:677-680.
90. Myasoedova E, Crowson CS, Nicola PJ, et al. The influence of rheumatoid arthritis disease characteristics on heart failure. J Rheumatol. 2011;38:1601-1606.
91. Ronda N, Greco D, Adorni MP, et al. Newly identified antiatherosclerotic activity of methotrexate and adalimumab: Complementary effects on lipoprotein function and macrophage cholesterol metabolism. Arthritis Rheumatol. 2015;67:1155-1164.
92. Zimmerman MC, Clemens DL, Duryee MJ, et al. Direct antioxidant properties of methotrexate: Inhibition of malondialdehyde-acetaldehyde-protein adduct formation and superoxide scavenging. Redox Biol. 2017;13:588-593.
93. Rempenault C, Combe B, Barnetche T, et al. Metabolic and cardiovascular benefits of hydroxychloroquine in patients with rheumatoid arthritis: A systematic review and meta-analysis. Ann Rheum Dis. 2018;77:98-103.
94. Wasko MC, Hubert HB, Lingala VB, et al. Hydroxychloroquine and risk of diabetes in patients with rheumatoid arthritis. JAMA. 2007;298:187-193.
95. Charles-Schoeman C, Wang X, Lee YY, et al. Association of triple therapy with improvement in cholesterol profiles over two-year followup in the treatment of early aggressive rheumatoid arthritis trial. Arthritis Rheumatol. 2016;68:577-586.
96. Charles-Schoeman C, Yin Lee Y, Shahbazian A, et al. Improvement of high-density lipoprotein function in patients with early rheumatoid arthritis treated with methotrexate monotherapy or combination therapies in a randomized controlled trial. Arthritis Rheumatol. 2017;69:46-57.
97. Chung ES, Packer M, Lo KH, , Anti-TNF Therapy Against Congestive Heart Failure Investigators. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: Results of the anti-TNF therapy against congestive heart failure (ATTACH) trial. Circulation. 2003;107:3133-3140.
98. Singh JA, Saag KG, Bridges SL, Jr, et al. 2015 American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68:1-26.
99. Daien CI, Duny Y, Barnetche Tet al. Effect of TNF inhibitors on lipid profile in rheumatoid arthritis: A systematic review with meta-analysis. Ann Rheum Dis. 2012;71:862-868.
100. Di Minno MN, Ambrosino P, Peluso R, et al. Lipid profile changes in patients with rheumatic diseases receiving a treatment with TNF-alpha blockers: A meta-analysis of prospective studies. Ann Med. 2014;46:73-83.
101. Popa C, van Tits LJ, Barrera P, et al. Anti-inflammatory therapy with tumour necrosis factor alpha inhibitors improves high-density lipoprotein cholesterol antioxidative capacity in rheumatoid arthritis patients. Ann Rheum Dis. 2009;68:868-872.
102. O’Neill F, Charakida M, Topham E, et al. Anti-inflammatory treatment improves high-density lipoprotein function in rheumatoid arthritis. Heart. 2017;103:766-773.
103. Nishimoto N, Ito K, Takagi N. Safety and efficacy profiles of tocilizumab monotherapy in Japanese patients with rheumatoid arthritis: Meta-analysis of six initial trials and five long-term extensions. Mod Rheumatol. 2010;20:222-232.
104. Rao VU, Pavlov A, Klearman M, et al. An evaluation of risk factors for major adverse cardiovascular events during tocilizumab therapy. Arthritis Rheumatol. 2015;67:372-380.
105. Gabay C, McInnes IB, Kavanaugh A, et al. Comparison of lipid and lipid-associated cardiovascular risk marker changes after treatment with tocilizumab or adalimumab in patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75:1806-1812.
106. McInnes IB, Thompson L, Giles JT, et al. Effect of interleukin-6 receptor blockade on surrogates of vascular risk in rheumatoid arthritis: MEASURE, a randomised, placebo-controlled study. Ann Rheum Dis. 2015;74:694-702.
107. Souto A, Salgado E, Maneiro JR, et al. Lipid profile changes in patients with chronic inflammatory arthritis treated with biologic agents and tofacitinib in randomized clinical trials: A systematic review and meta-analysis. Arthritis Rheumatol. 2015;67:117-127.
108. Myasoedova E, Gabriel SE, Matteson EL, et al. Decreased cardiovascular mortality in patients with incident rheumatoid arthritis (RA) in recent years: Dawn of a new era in cardiovascular disease in RA? J Rheumatol. 2017;44:732-739.
109. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2889-2934.
110. Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U.S. public health service report. Am J Prev Med. 2008;35:158-176.
111. Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: A report of the American college of cardiology/American heart association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2960-2984.
112. Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:342-362.
113. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American college of cardiology/American heart association task force on practice guidelines and the obesity society. J Am Coll Cardiol. 2014;63:2985-3023.
1. Avina-Zubieta JA, Choi HK, Sadatsafavi M, et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690-1697.
2. Avina-Zubieta JA, Thomas J, Sadatsafavi M, et al. Risk of incident cardiovascular events in patients with rheumatoid arthritis: A meta-analysis of observational studies. Ann Rheum Dis. 2012;71:1524-1529.
3. Johnson TM, Mikuls TR, England BR. Assessment of cardiovascular risk in rheumatoid arthritis. J Clin Outcomes Manage. 2019;26:41-47.
4. Sandoo A, Chanchlani N, Hodson J, et al. Classical cardiovascular disease risk factors associate with vascular function and morphology in rheumatoid arthritis: A six-year prospective study. Arthritis Res Ther. 2013;15:R203.
5. Myasoedova E, Crowson CS, Kremers HM, et al. Total cholesterol and LDL levels decrease before rheumatoid arthritis. Ann Rheum Dis. 2010;69:1310-1314.
6. Liao KP, Cai T, Gainer VS, et al. Lipid and lipoprotein levels and trend in rheumatoid arthritis compared to the general population. Arthritis Care Res (Hoboken). 2013;65:2046-2050.
7. Myasoedova E, Crowson CS, Kremers HM, et al. Lipid paradox in rheumatoid arthritis: The impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann Rheum Dis. 2011;70:482-487.
8. Zhang J, Chen L, Delzell E, et al. Republished: The association between inflammatory markers, serum lipids and the risk of cardiovascular events in patients with rheumatoid arthritis. Postgrad Med J. 2014;90:722-729.
9. Liao KP, Liu J, Lu B, et al. Association between lipid levels and major adverse cardiovascular events in rheumatoid arthritis compared to non-rheumatoid arthritis patients. Arthritis Rheumatol. 2015;67:2004-2010.
10. Charles-Schoeman C, Lee YY, Grijalva V, et al. Cholesterol efflux by high density lipoproteins is impaired in patients with active rheumatoid arthritis. Ann Rheum Dis. 2012;71:1157-1162.
11. Voloshyna I, Modayil S, Littlefield MJ, et al. Plasma from rheumatoid arthritis patients promotes pro-atherogenic cholesterol transport gene expression in THP-1 human macrophages. Exp Biol Med (Maywood). 2013 238:1192-1197.
12. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;(1):CD004816.
13. Sheng X, Murphy MJ, Macdonald TM, Wei L. Effectiveness of statins on total cholesterol and cardiovascular disease and all-cause mortality in osteoarthritis and rheumatoid arthritis. J Rheumatol. 2012;39:32-40.
14. An J, Alemao E, Reynolds K, et al. Cardiovascular outcomes associated with lowering low-density lipoprotein cholesterol in rheumatoid arthritis and matched nonrheumatoid arthritis. J Rheumatol. 2016;43:1989-1996.
15. Semb AG, Holme I, Kvien TK, Pedersen TR. Intensive lipid lowering in patients with rheumatoid arthritis and previous myocardial infarction: An explorative analysis from the incremental decrease in endpoints through aggressive lipid lowering (IDEAL) trial. Rheumatology (Oxford). 2011;50:324-329.
16. Semb AG, Kvien TK, DeMicco DA, et al. Effect of intensive lipid-lowering therapy on cardiovascular outcome in patients with and those without inflammatory joint disease. Arthritis Rheum. 2012;64:2836-2846.
17. El-Barbary AM, Hussein MS, Rageh EM, et al. Effect of atorvastatin on inflammation and modification of vascular risk factors in rheumatoid arthritis. J Rheumatol. 2011;38:229-235.
18. Writing Committee, Lloyd-Jones DM, Morris PB, et al. 2016 ACC expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: A report of the American college of cardiology task force on clinical expert consensus documents. J Am Coll Cardiol. 2016;68:92-125.
19. Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
20. Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500-1509.
21. Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489-1499.
22. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
23. Maki-Petaja KM, Booth AD, Hall FC, et al. Ezetimibe and simvastatin reduce inflammation, disease activity, and aortic stiffness and improve endothelial function in rheumatoid arthritis. J Am Coll Cardiol. 2007;50:852-858.
24. Bartels CM, Kind AJ, Everett C, et al. Low frequency of primary lipid screening among medicare patients with rheumatoid arthritis. Arthritis Rheum. 2011;63:1221-1230.
25. Akkara Veetil BM, Myasoedova E, Matteson EL, et al. Use of lipid-lowering agents in rheumatoid arthritis: A population-based cohort study. J Rheumatol. 2013;40:1082-1088.
26. Lindhardsen J, Ahlehoff O, Gislason GH, et al. Initiation and adherence to secondary prevention pharmacotherapy after myocardial infarction in patients with rheumatoid arthritis: A nationwide cohort study. Ann Rheum Dis. 2012;71:1496-1501.
27. De Vera MA, Choi H, Abrahamowicz M, et al. Statin discontinuation and risk of acute myocardial infarction in patients with rheumatoid arthritis: A population-based cohort study. Ann Rheum Dis. 2011;70:1020-1024.
28. Zhang H, Plutzky J, Skentzos S, et al. Discontinuation of statins in routine care settings: A cohort study. Ann Intern Med. 2013;158:526-534.
29. Zhang H, Plutzky J, Shubina M, Turchin A. Continued statin prescriptions after adverse reactions and patient outcomes: A cohort study. Ann Intern Med. 2017;167:221-227.
30. Lemstra M, Blackburn D, Crawley A, Fung R. Proportion and risk indicators of nonadherence to statin therapy: A meta-analysis. Can J Cardiol. 2012;28:574-580.
31. Boyer JF, Gourraud PA, Cantagrel A, et al. Traditional cardiovascular risk factors in rheumatoid arthritis: A meta-analysis. Joint Bone Spine. 2011;78:179-183.
32. Bergstrom U, Jacobsson LT, Nilsson JA, et al. Pulmonary dysfunction, smoking, socioeconomic status and the risk of developing rheumatoid arthritis. Rheumatology (Oxford). 2011;50:2005-2013.
33. Costenbader KH, Feskanich D, Mandl LA, Karlson EW. Smoking intensity, duration, and cessation, and the risk of rheumatoid arthritis in women. Am J Med. 2006;119:503.e1,503.e9.
34. Klareskog L, Stolt P, Lundberg K, et al. A new model for an etiology of rheumatoid arthritis: Smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006;54:38-46.
35. Sokolove J, Wagner CA, Lahey LJ, et al. Increased inflammation and disease activity among current cigarette smokers with rheumatoid arthritis: A cross-sectional analysis of US veterans. Rheumatology (Oxford). 2016;55:1969-1977.
36. World Health Organization. WHO Global Report: Mortality Attributable to Tobacco. Geneva, World Health Organization, 2012.
37. Baghdadi LR, Woodman RJ, Shanahan EM, Mangoni AA. The impact of traditional cardiovascular risk factors on cardiovascular outcomes in patients with rheumatoid arthritis: A systematic review and meta-analysis. PLoS One. 2015;10:e0117952.
38. Centers for Disease Control and Prevention; National Center for Chronic Disease Prevention and Health Promotion. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention; 2010. 6, Cardiovascular Diseases. Available from: https://ncbi.nlm.nih.gov/books/NBK53012/
39. Mons U, Muezzinler A, Gellert C, et al. Impact of smoking and smoking cessation on cardiovascular events and mortality among older adults: Meta-analysis of individual participant data from prospective cohort studies of the CHANCES consortium. BMJ. 2015;350:h1551.
40. Aimer P, Treharne GJ, Stebbings S, Frampton C, Cameron V, Kirby S, et al. Efficacy of a rheumatoid arthritis-specific smoking cessation program: A randomized controlled pilot trial. Arthritis Care Res (Hoboken). 2017;69:28-37.
41. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services; 2017.
42. Jiang P, Li H, Li X. Diabetes mellitus risk factors in rheumatoid arthritis: A systematic review and meta-analysis. Clin Exp Rheumatol. 2015;33:115-121.
43. Shahin D, Eltoraby E, Mesbah A, Houssen M. Insulin resistance in early untreated rheumatoid arthritis patients. Clin Biochem. 2010;43:661-335.
44. Arias de la Rosa I, Escudero-Contreras A, Rodriguez-Cuenca S, et al. Defective glucose and lipid metabolism in rheumatoid arthritis is determined by chronic inflammation in metabolic tissues. J Intern Med. 2018;84(1):61-77.
45. Wilson JC, Sarsour K, Gale S, et al. Incidence and risk of glucocorticoid-associated adverse effects in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2018 Jun 1. doi: 10.1002/acr.23611.
46. Chung CP, Giles JT, Petri M, et al. Prevalence of traditional modifiable cardiovascular risk factors in patients with rheumatoid arthritis: Comparison with control subjects from the multi-ethnic study of atherosclerosis. Semin Arthritis Rheum. 2012;41:535-544.
47. Goodwin JE, Geller DS. Glucocorticoid-induced hypertension. Pediatr Nephrol. 2012;27:1059-1066.
48. Snowden S, Nelson R. The effects of nonsteroidal anti-inflammatory drugs on blood pressure in hypertensive patients. Cardiol Rev. 2011;19:184-191.
49. Baker JF, Sauer B, Teng CC, et al. Initiation of disease-modifying therapies in rheumatoid arthritis is associated with changes in blood pressure. J Clin Rheumatol. 2018;24:203-209.
50. Panoulas VF, Douglas KM, Milionis HJ, et al. Prevalence and associations of hypertension and its control in patients with rheumatoid arthritis. Rheumatology (Oxford). 2007;46:1477-1482.
51. Protogerou AD, Panagiotakos DB, Zampeli E, et al. Arterial hypertension assessed “out-of-office” in a contemporary cohort of rheumatoid arthritis patients free of cardiovascular disease is characterized by high prevalence, low awareness, poor control and increased vascular damage-associated “white coat” phenomenon. Arthritis Res Ther. 2013;15:R142.
52. van Breukelen-van der Stoep DF, van Zeben D, Klop B, et al. Marked underdiagnosis and undertreatment of hypertension and hypercholesterolaemia in rheumatoid arthritis. Rheumatology (Oxford). 2016;55:1210-1216.
53. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2018;71:e127-248.
54. Lee J, Dunlop D, Ehrlich-Jones L, et al. Public health impact of risk factors for physical inactivity in adults with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64:488-493.
55. Sokka T, Hakkinen A, Kautiainen H, et al. Physical inactivity in patients with rheumatoid arthritis: Data from twenty-one countries in a cross-sectional, international study. Arthritis Rheum. 2008;59:42-50.
56. Fenton SAM, Veldhuijzen van Zanten JJCS, Kitas GD, et al. Sedentary behaviour is associated with increased long-term cardiovascular risk in patients with rheumatoid arthritis independently of moderate-to-vigorous physical activity. BMC Musculoskelet Disord. 2017;18:131,017-1473-9.
57. Byram KW, Oeser AM, Linton MF, et al. Exercise is associated with increased small HDL particle concentration and decreased vascular stiffness in rheumatoid arthritis. J Clin Rheumatol. 2018 May 25. 9.
58. de Jong Z, Munneke M, Zwinderman AH, et al. Is a long-term high-intensity exercise program effective and safe in patients with rheumatoid arthritis? results of a randomized controlled trial. Arthritis Rheum. 2003;48:2415-2424.
59. Stavropoulos-Kalinoglou A, Metsios GS, Veldhuijzen van Zanten JJ, et al. Individualised aerobic and resistance exercise training improves cardiorespiratory fitness and reduces cardiovascular risk in patients with rheumatoid arthritis. Ann Rheum Dis. 2013;72:1819-1825.
60. Khoja SS, Almeida GJ, Chester Wasko M, et al. Association of light-intensity physical activity with lower cardiovascular disease risk burden in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2016;68:424-431.
61. Metsios GS, Koutedakis Y, Veldhuijzen van Zanten JJ, et al. Cardiorespiratory fitness levels and their association with cardiovascular profile in patients with rheumatoid arthritis: A cross-sectional study. Rheumatology (Oxford). 2015;54:2215-2220.
62. Escalante A, Haas RW, del Rincon I. Paradoxical effect of body mass index on survival in rheumatoid arthritis: Role of comorbidity and systemic inflammation. Arch Intern Med. 2005;165:1624-1629.
63. Kremers HM, Nicola PJ, Crowson CS, et al. Prognostic importance of low body mass index in relation to cardiovascular mortality in rheumatoid arthritis. Arthritis Rheum. 2004;50:3450-3457.
64. Wolfe F, Michaud K. Effect of body mass index on mortality and clinical status in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64:1471-1479.
65. England BR, Baker JF, Sayles H, et al. Body mass index, weight loss, and cause-specific mortality in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2018;70:11-18.
66. Dessein PH, Solomon A, Hollan I. Metabolic abnormalities in patients with inflammatory rheumatic diseases. Best Pract Res Clin Rheumatol. 2016;30:901-915.
67. Sparks JA, Halperin F, Karlson JC, et al. Impact of bariatric surgery on patients with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2015;67:1619-1626.
68. Mente A, de Koning L, Shannon HS, Anand SS. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Intern Med. 2009;169:659-669.
69. England BR, Sayles H, Michaud K, et al. Cause-specific mortality in male US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2016;68:36-45.
70. Arts EE, Fransen J, Den Broeder AA, et al. Low disease activity (DAS28≤3.2) reduces the risk of first cardiovascular event in rheumatoid arthritis: a time-dependent Cox regression analysis in a large cohort study. Ann Rheum Dis. 2017;76(10):1693-1699.
71. Provan SA, Semb AG, Hisdal J, et al. Remission is the goal for cardiovascular risk management in patients with rheumatoid arthritis: A cross-sectional comparative study. Ann Rheum Dis. 2011;70:812-817.
72. Klarenbeek NB, van der Kooij SM, Huizinga TJ, et al. Blood pressure changes in patients with recent-onset rheumatoid arthritis treated with four different treatment strategies: A post hoc analysis from the BeSt trial. Ann Rheum Dis. 2010;69:1342-1345.
73. Hafstrom I, Rohani M, Deneberg S, et al. Effects of low-dose prednisolone on endothelial function, atherosclerosis, and traditional risk factors for atherosclerosis in patients with rheumatoid arthritis—a randomized study. J Rheumatol. 2007;34:1810-1816.
74. Hoes JN, van der Goes MC, van Raalte DH, et al. Glucose tolerance, insulin sensitivity and beta-cell function in patients with rheumatoid arthritis treated with or without low-to-medium dose glucocorticoids. Ann Rheum Dis. 2011;70:1887-1894.
75. Roubille C. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: A systematic review and meta-analysis. Ann Rheum Dis. 2003;74:480-489.
76. Ajeganova S, Svensson B, Hafstrom I, BARFOT Study Group. Low-dose prednisolone treatment of early rheumatoid arthritis and late cardiovascular outcome and survival: 10-year follow-up of a 2-year randomised trial. BMJ Open. 2014;4:e004259,2013-004259.
77. Avina-Zubieta JA, Choi HK, Sadatsafavi M, et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690-1697.
78. del Rincon I, Battafarano DF, Restrepo JF, et al. Glucocorticoid dose thresholds associated with all-cause and cardiovascular mortality in rheumatoid arthritis. Arthritis Rheumatol. 2014;66:264-272.
79. Davis JM,3rd, Maradit Kremers H, Crowson CS, et al. Glucocorticoids and cardiovascular events in rheumatoid arthritis: A population-based cohort study. Arthritis Rheum. 2007;56:820-830.
80. Zhang J, Xie F, Yun H, et al. Comparative effects of biologics on cardiovascular risk among older patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75:1813-1818.
81. Greenberg JD, Kremer JM, Curtis JR, et al. Tumour necrosis factor antagonist use and associated risk reduction of cardiovascular events among patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70:576-582.
82. Lindhardsen J, Gislason GH, Jacobsen S, et al. Non-steroidal anti-inflammatory drugs and risk of cardiovascular disease in patients with rheumatoid arthritis: A nationwide cohort study. Ann Rheum Dis. 2014;73:1515-1521.
83. Schjerning Olsen AM, Fosbol EL, Lindhardsen J, et al. Duration of treatment with nonsteroidal anti-inflammatory drugs and impact on risk of death and recurrent myocardial infarction in patients with prior myocardial infarction: A nationwide cohort study. Circulation. 2011;123:2226-2235.
84. Gislason GH, Rasmussen JN, Abildstrom SZ, et al. Increased mortality and cardiovascular morbidity associated with use of nonsteroidal anti-inflammatory drugs in chronic heart failure. Arch Intern Med. 2009;169:141-149.
85. Trelle S, Reichenbach S, Wandel S, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: Network meta-analysis. BMJ. 2011;342:c7086.
86. Nissen SE, Yeomans ND, Solomon DH, et al. Cardiovascular safety of celecoxib, naproxen, or ibuprofen for arthritis. N Engl J Med. 2016;375:2519-2529.
87. Wasko MC, Dasgupta A, Hubert Het al. Propensity-adjusted association of methotrexate with overall survival in rheumatoid arthritis. Arthritis Rheum. 2013;65:334-342.
88. Micha R, Imamura F, Wyler von Ballmoos M, et al. Systematic review and meta-analysis of methotrexate use and risk of cardiovascular disease. Am J Cardiol. 2011;108:1362-1370.
89. Bernatsky S, Hudson M, Suissa S. Anti-rheumatic drug use and risk of hospitalization for congestive heart failure in rheumatoid arthritis. Rheumatology (Oxford). 2005;44:677-680.
90. Myasoedova E, Crowson CS, Nicola PJ, et al. The influence of rheumatoid arthritis disease characteristics on heart failure. J Rheumatol. 2011;38:1601-1606.
91. Ronda N, Greco D, Adorni MP, et al. Newly identified antiatherosclerotic activity of methotrexate and adalimumab: Complementary effects on lipoprotein function and macrophage cholesterol metabolism. Arthritis Rheumatol. 2015;67:1155-1164.
92. Zimmerman MC, Clemens DL, Duryee MJ, et al. Direct antioxidant properties of methotrexate: Inhibition of malondialdehyde-acetaldehyde-protein adduct formation and superoxide scavenging. Redox Biol. 2017;13:588-593.
93. Rempenault C, Combe B, Barnetche T, et al. Metabolic and cardiovascular benefits of hydroxychloroquine in patients with rheumatoid arthritis: A systematic review and meta-analysis. Ann Rheum Dis. 2018;77:98-103.
94. Wasko MC, Hubert HB, Lingala VB, et al. Hydroxychloroquine and risk of diabetes in patients with rheumatoid arthritis. JAMA. 2007;298:187-193.
95. Charles-Schoeman C, Wang X, Lee YY, et al. Association of triple therapy with improvement in cholesterol profiles over two-year followup in the treatment of early aggressive rheumatoid arthritis trial. Arthritis Rheumatol. 2016;68:577-586.
96. Charles-Schoeman C, Yin Lee Y, Shahbazian A, et al. Improvement of high-density lipoprotein function in patients with early rheumatoid arthritis treated with methotrexate monotherapy or combination therapies in a randomized controlled trial. Arthritis Rheumatol. 2017;69:46-57.
97. Chung ES, Packer M, Lo KH, , Anti-TNF Therapy Against Congestive Heart Failure Investigators. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: Results of the anti-TNF therapy against congestive heart failure (ATTACH) trial. Circulation. 2003;107:3133-3140.
98. Singh JA, Saag KG, Bridges SL, Jr, et al. 2015 American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68:1-26.
99. Daien CI, Duny Y, Barnetche Tet al. Effect of TNF inhibitors on lipid profile in rheumatoid arthritis: A systematic review with meta-analysis. Ann Rheum Dis. 2012;71:862-868.
100. Di Minno MN, Ambrosino P, Peluso R, et al. Lipid profile changes in patients with rheumatic diseases receiving a treatment with TNF-alpha blockers: A meta-analysis of prospective studies. Ann Med. 2014;46:73-83.
101. Popa C, van Tits LJ, Barrera P, et al. Anti-inflammatory therapy with tumour necrosis factor alpha inhibitors improves high-density lipoprotein cholesterol antioxidative capacity in rheumatoid arthritis patients. Ann Rheum Dis. 2009;68:868-872.
102. O’Neill F, Charakida M, Topham E, et al. Anti-inflammatory treatment improves high-density lipoprotein function in rheumatoid arthritis. Heart. 2017;103:766-773.
103. Nishimoto N, Ito K, Takagi N. Safety and efficacy profiles of tocilizumab monotherapy in Japanese patients with rheumatoid arthritis: Meta-analysis of six initial trials and five long-term extensions. Mod Rheumatol. 2010;20:222-232.
104. Rao VU, Pavlov A, Klearman M, et al. An evaluation of risk factors for major adverse cardiovascular events during tocilizumab therapy. Arthritis Rheumatol. 2015;67:372-380.
105. Gabay C, McInnes IB, Kavanaugh A, et al. Comparison of lipid and lipid-associated cardiovascular risk marker changes after treatment with tocilizumab or adalimumab in patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75:1806-1812.
106. McInnes IB, Thompson L, Giles JT, et al. Effect of interleukin-6 receptor blockade on surrogates of vascular risk in rheumatoid arthritis: MEASURE, a randomised, placebo-controlled study. Ann Rheum Dis. 2015;74:694-702.
107. Souto A, Salgado E, Maneiro JR, et al. Lipid profile changes in patients with chronic inflammatory arthritis treated with biologic agents and tofacitinib in randomized clinical trials: A systematic review and meta-analysis. Arthritis Rheumatol. 2015;67:117-127.
108. Myasoedova E, Gabriel SE, Matteson EL, et al. Decreased cardiovascular mortality in patients with incident rheumatoid arthritis (RA) in recent years: Dawn of a new era in cardiovascular disease in RA? J Rheumatol. 2017;44:732-739.
109. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2889-2934.
110. Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U.S. public health service report. Am J Prev Med. 2008;35:158-176.
111. Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: A report of the American college of cardiology/American heart association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2960-2984.
112. Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:342-362.
113. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American college of cardiology/American heart association task force on practice guidelines and the obesity society. J Am Coll Cardiol. 2014;63:2985-3023.
How much difference will Eli Lilly’s half-price insulin make?
When Erin Gilmer filled her insulin prescription at a Denver-area Walgreens in January, she paid $8.50. U.S. taxpayers paid another $280.51.
“It eats at me to know that taxpayer money is being wasted,” said Gilmer, who has Medicare and was diagnosed with type 1 diabetes while a sophomore at the University of Colorado in 2002.
The diagnosis meant that for the rest of her life she’d require daily insulin shots to stay alive. But the price of that insulin is skyrocketing.
Between 2009 and 2017 the wholesale price of a single vial of Humalog, the Eli Lilly and Co.–manufactured insulin Gilmer uses, nearly tripled – rising from $92.70 to $274.70, according to data from IBM Watson Health.
Six years ago, Gilmer qualified for Social Security Disability Insurance – and thus, Medicare – because of a range of health issues. At the time, the insulin she needed cost $167.70 per vial, according to IBM Watson Health.
“When it’s taxpayer money paying for medication for someone like me, it makes it a national issue, not just a diabetic issue,” Gilmer said.
Stories about people with type 1 diabetes dying when they couldn’t afford insulin have made headlines. Patient activists like Gilmer have protested high prices outside Lilly’s headquarters in Indianapolis.
Last October in Minnesota, State Attorney General Lori Swanson sued insulin manufacturers, alleging price gouging. Pharmaceutical executives were grilled about high drug prices by the Senate Finance Committee on Feb. 26.
This is the backdrop for Lilly’s announcement March 4 that it is rolling out a half-priced, generic version of Humalog called “insulin lispro.” The list price: $137.35 per vial.
“Patients, doctors and policymakers are demanding lower list prices for medicines and lower patient costs at the pharmacy counter,” Eli Lilly CEO David Ricks wrote in a blog post about the move. “You might be surprised to hear that we agree – it’s time for change in our system and for consumer prices to come down.”
No panacea
When Lilly’s Humalog, the first short-acting insulin, came to market in 1996, the list price was about $21 per vial. The price didn’t reach $275 overnight, but yearly price increases added up.
In February 2009, for example, the wholesale price was $92.70, according to IBM Watson Health. It rose to $99.65 in December 2009, then to $107.60 in September 2010, $115.70 in May 2011, and so on.
“There’s no justification for why prices should keep increasing at an average rate of 10% every year,” said Inmaculada Hernandez of the University of Pittsburgh School of Pharmacy, who was lead author of a January report in Health Affairs attributing the skyrocketing cost of prescription drugs to accumulated yearly price hikes.
“The public perception that we have in general is that drugs are so expensive because we need to pay for research and development, and that’s true,” Hernandez said. “However, usually research and development is paid for in the first years of life of a drug.”
At $137.35 per vial, Lilly’s generic insulin is priced at about the same level as Humalog was in 2012, 16 years after it came to market.
“We want to recognize that this is not a panacea,” said company spokesman Greg Kueterman. “This is an option that we hope can help people in the current system that we work with.”
It’s worth noting that Humalog is a rapid-acting insulin, but that’s only one of the two types of insulin most people with type 1 diabetes use every day. The second kind is long-lasting. Lilly makes one called Basaglar. The most popular long-lasting insulin is Lantus, produced by Sanofi. Neither has a lower-cost alternative.
Still, Lilly’s move on Humalog could put pressure on the other two big makers of insulin to act.
Novo Nordisk called Lilly’s lower-priced generic insulin “an important development,” in an emailed statement.
“Bringing affordable insulin to the market requires ideas from all stakeholders,” Novo Nordisk’s Ken Inchausti said in an email, which also listed steps the company has taken, such as a patient assistance program. The statement didn’t say whether Novo Nordisk is considering offering a lower-priced version of its popular insulin Novolog, a rival of Humalog.
A statement from Sanofi, the third major insulin maker, also didn’t say whether the company would offer lower-priced versions of its insulins.
“Sanofi supports any actions that increase access to insulins for patients living with diabetes at an affordable price,” spokeswoman Ashleigh Koss said in the email, which also touted the company’s patient assistance program.
A different kind of generic
One twist in this story is that Lilly’s new insulin is just a repackaged version of Humalog, minus the brand name. It’s called an “authorized generic.”
“Whoever came up with the term ‘authorized generic’?” Dr. Vincent Rajkumar said, laughing. Rajkumar is a hematologist at the Mayo Clinic in Rochester, Minn.
“It’s the same exact drug” as the brand name, he continued.
Typically, Rajkumar said, authorized generics are introduced by brand-name drugmakers to compete with generic versions of their drugs made by rival companies.
But in the case of Humalog and other insulins, there are no generics made by competitors, as there are for, say, the cholesterol medicine Lipitor or even other diabetes drugs, such as metformin.
So when Lilly’s authorized generic comes to market, the company will have both Humalog insulin and the authorized generic version of that medicine on the market.
Rajkumar said it’s a public relations move.
“There’s outrage over the price of insulin that is being discussed in Congress and elsewhere. And so the company basically says, ‘Hey, we will make the identical product available at half price.’ On the surface that sounds great,” Rajkumar said.
“But you look at the problems and you think, ‘OK, how crazy is this that someone is actually going to be buying the brand-name drug?’ ”
In fact, it’s possible that Lilly could break even or profit off its authorized generic compared to the name-brand Humalog, according to University of Pittsburgh’s Hernandez.
The profit margin would depend on the rebates paid by the company to insurers and pharmacy benefit managers. Rebates are getting a lot of attention these days as one factor that pushes drug prices higher. They’re usually not disclosed and increase as a drug’s price increases, providing an incentive to some companies to raise prices.
“Doing an authorized generic is nothing else than giving insurers two options,” Hernandez said: Pay the full list price for a brand-name drug and receive a higher rebate, or pay the lower price for the authorized generic and receive a presumably smaller rebate.
“What we really need to get insulin prices down is to get generics into the market, and we need more than one,” Hernandez said, adding that previous research has shown that prices begin to go down when two or three generics are competing in the marketplace.
Even so, Lillly’s Kueterman said the authorized generic insulin “is going to help hopefully move the system toward a more sustainable model.”
“I can guarantee you the reason that we’re doing this is to help people,” Kueterman said, noting the company’s Diabetes Solution Center has also helped “10,000 people each month pay significantly less for their insulin” since it opened in August.
For Erin Gilmer, the news about an authorized generic insulin from Lilly has left her mildly encouraged.
“It sounds really good, and it will help some people, which is great,” Gilmer said. “It’s Eli Lilly and pharma starting to understand that grassroots activism has to be taken seriously, and we are at a tipping point.”
This story is part of a partnership that includes NPR and Kaiser Health News. Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
When Erin Gilmer filled her insulin prescription at a Denver-area Walgreens in January, she paid $8.50. U.S. taxpayers paid another $280.51.
“It eats at me to know that taxpayer money is being wasted,” said Gilmer, who has Medicare and was diagnosed with type 1 diabetes while a sophomore at the University of Colorado in 2002.
The diagnosis meant that for the rest of her life she’d require daily insulin shots to stay alive. But the price of that insulin is skyrocketing.
Between 2009 and 2017 the wholesale price of a single vial of Humalog, the Eli Lilly and Co.–manufactured insulin Gilmer uses, nearly tripled – rising from $92.70 to $274.70, according to data from IBM Watson Health.
Six years ago, Gilmer qualified for Social Security Disability Insurance – and thus, Medicare – because of a range of health issues. At the time, the insulin she needed cost $167.70 per vial, according to IBM Watson Health.
“When it’s taxpayer money paying for medication for someone like me, it makes it a national issue, not just a diabetic issue,” Gilmer said.
Stories about people with type 1 diabetes dying when they couldn’t afford insulin have made headlines. Patient activists like Gilmer have protested high prices outside Lilly’s headquarters in Indianapolis.
Last October in Minnesota, State Attorney General Lori Swanson sued insulin manufacturers, alleging price gouging. Pharmaceutical executives were grilled about high drug prices by the Senate Finance Committee on Feb. 26.
This is the backdrop for Lilly’s announcement March 4 that it is rolling out a half-priced, generic version of Humalog called “insulin lispro.” The list price: $137.35 per vial.
“Patients, doctors and policymakers are demanding lower list prices for medicines and lower patient costs at the pharmacy counter,” Eli Lilly CEO David Ricks wrote in a blog post about the move. “You might be surprised to hear that we agree – it’s time for change in our system and for consumer prices to come down.”
No panacea
When Lilly’s Humalog, the first short-acting insulin, came to market in 1996, the list price was about $21 per vial. The price didn’t reach $275 overnight, but yearly price increases added up.
In February 2009, for example, the wholesale price was $92.70, according to IBM Watson Health. It rose to $99.65 in December 2009, then to $107.60 in September 2010, $115.70 in May 2011, and so on.
“There’s no justification for why prices should keep increasing at an average rate of 10% every year,” said Inmaculada Hernandez of the University of Pittsburgh School of Pharmacy, who was lead author of a January report in Health Affairs attributing the skyrocketing cost of prescription drugs to accumulated yearly price hikes.
“The public perception that we have in general is that drugs are so expensive because we need to pay for research and development, and that’s true,” Hernandez said. “However, usually research and development is paid for in the first years of life of a drug.”
At $137.35 per vial, Lilly’s generic insulin is priced at about the same level as Humalog was in 2012, 16 years after it came to market.
“We want to recognize that this is not a panacea,” said company spokesman Greg Kueterman. “This is an option that we hope can help people in the current system that we work with.”
It’s worth noting that Humalog is a rapid-acting insulin, but that’s only one of the two types of insulin most people with type 1 diabetes use every day. The second kind is long-lasting. Lilly makes one called Basaglar. The most popular long-lasting insulin is Lantus, produced by Sanofi. Neither has a lower-cost alternative.
Still, Lilly’s move on Humalog could put pressure on the other two big makers of insulin to act.
Novo Nordisk called Lilly’s lower-priced generic insulin “an important development,” in an emailed statement.
“Bringing affordable insulin to the market requires ideas from all stakeholders,” Novo Nordisk’s Ken Inchausti said in an email, which also listed steps the company has taken, such as a patient assistance program. The statement didn’t say whether Novo Nordisk is considering offering a lower-priced version of its popular insulin Novolog, a rival of Humalog.
A statement from Sanofi, the third major insulin maker, also didn’t say whether the company would offer lower-priced versions of its insulins.
“Sanofi supports any actions that increase access to insulins for patients living with diabetes at an affordable price,” spokeswoman Ashleigh Koss said in the email, which also touted the company’s patient assistance program.
A different kind of generic
One twist in this story is that Lilly’s new insulin is just a repackaged version of Humalog, minus the brand name. It’s called an “authorized generic.”
“Whoever came up with the term ‘authorized generic’?” Dr. Vincent Rajkumar said, laughing. Rajkumar is a hematologist at the Mayo Clinic in Rochester, Minn.
“It’s the same exact drug” as the brand name, he continued.
Typically, Rajkumar said, authorized generics are introduced by brand-name drugmakers to compete with generic versions of their drugs made by rival companies.
But in the case of Humalog and other insulins, there are no generics made by competitors, as there are for, say, the cholesterol medicine Lipitor or even other diabetes drugs, such as metformin.
So when Lilly’s authorized generic comes to market, the company will have both Humalog insulin and the authorized generic version of that medicine on the market.
Rajkumar said it’s a public relations move.
“There’s outrage over the price of insulin that is being discussed in Congress and elsewhere. And so the company basically says, ‘Hey, we will make the identical product available at half price.’ On the surface that sounds great,” Rajkumar said.
“But you look at the problems and you think, ‘OK, how crazy is this that someone is actually going to be buying the brand-name drug?’ ”
In fact, it’s possible that Lilly could break even or profit off its authorized generic compared to the name-brand Humalog, according to University of Pittsburgh’s Hernandez.
The profit margin would depend on the rebates paid by the company to insurers and pharmacy benefit managers. Rebates are getting a lot of attention these days as one factor that pushes drug prices higher. They’re usually not disclosed and increase as a drug’s price increases, providing an incentive to some companies to raise prices.
“Doing an authorized generic is nothing else than giving insurers two options,” Hernandez said: Pay the full list price for a brand-name drug and receive a higher rebate, or pay the lower price for the authorized generic and receive a presumably smaller rebate.
“What we really need to get insulin prices down is to get generics into the market, and we need more than one,” Hernandez said, adding that previous research has shown that prices begin to go down when two or three generics are competing in the marketplace.
Even so, Lillly’s Kueterman said the authorized generic insulin “is going to help hopefully move the system toward a more sustainable model.”
“I can guarantee you the reason that we’re doing this is to help people,” Kueterman said, noting the company’s Diabetes Solution Center has also helped “10,000 people each month pay significantly less for their insulin” since it opened in August.
For Erin Gilmer, the news about an authorized generic insulin from Lilly has left her mildly encouraged.
“It sounds really good, and it will help some people, which is great,” Gilmer said. “It’s Eli Lilly and pharma starting to understand that grassroots activism has to be taken seriously, and we are at a tipping point.”
This story is part of a partnership that includes NPR and Kaiser Health News. Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
When Erin Gilmer filled her insulin prescription at a Denver-area Walgreens in January, she paid $8.50. U.S. taxpayers paid another $280.51.
“It eats at me to know that taxpayer money is being wasted,” said Gilmer, who has Medicare and was diagnosed with type 1 diabetes while a sophomore at the University of Colorado in 2002.
The diagnosis meant that for the rest of her life she’d require daily insulin shots to stay alive. But the price of that insulin is skyrocketing.
Between 2009 and 2017 the wholesale price of a single vial of Humalog, the Eli Lilly and Co.–manufactured insulin Gilmer uses, nearly tripled – rising from $92.70 to $274.70, according to data from IBM Watson Health.
Six years ago, Gilmer qualified for Social Security Disability Insurance – and thus, Medicare – because of a range of health issues. At the time, the insulin she needed cost $167.70 per vial, according to IBM Watson Health.
“When it’s taxpayer money paying for medication for someone like me, it makes it a national issue, not just a diabetic issue,” Gilmer said.
Stories about people with type 1 diabetes dying when they couldn’t afford insulin have made headlines. Patient activists like Gilmer have protested high prices outside Lilly’s headquarters in Indianapolis.
Last October in Minnesota, State Attorney General Lori Swanson sued insulin manufacturers, alleging price gouging. Pharmaceutical executives were grilled about high drug prices by the Senate Finance Committee on Feb. 26.
This is the backdrop for Lilly’s announcement March 4 that it is rolling out a half-priced, generic version of Humalog called “insulin lispro.” The list price: $137.35 per vial.
“Patients, doctors and policymakers are demanding lower list prices for medicines and lower patient costs at the pharmacy counter,” Eli Lilly CEO David Ricks wrote in a blog post about the move. “You might be surprised to hear that we agree – it’s time for change in our system and for consumer prices to come down.”
No panacea
When Lilly’s Humalog, the first short-acting insulin, came to market in 1996, the list price was about $21 per vial. The price didn’t reach $275 overnight, but yearly price increases added up.
In February 2009, for example, the wholesale price was $92.70, according to IBM Watson Health. It rose to $99.65 in December 2009, then to $107.60 in September 2010, $115.70 in May 2011, and so on.
“There’s no justification for why prices should keep increasing at an average rate of 10% every year,” said Inmaculada Hernandez of the University of Pittsburgh School of Pharmacy, who was lead author of a January report in Health Affairs attributing the skyrocketing cost of prescription drugs to accumulated yearly price hikes.
“The public perception that we have in general is that drugs are so expensive because we need to pay for research and development, and that’s true,” Hernandez said. “However, usually research and development is paid for in the first years of life of a drug.”
At $137.35 per vial, Lilly’s generic insulin is priced at about the same level as Humalog was in 2012, 16 years after it came to market.
“We want to recognize that this is not a panacea,” said company spokesman Greg Kueterman. “This is an option that we hope can help people in the current system that we work with.”
It’s worth noting that Humalog is a rapid-acting insulin, but that’s only one of the two types of insulin most people with type 1 diabetes use every day. The second kind is long-lasting. Lilly makes one called Basaglar. The most popular long-lasting insulin is Lantus, produced by Sanofi. Neither has a lower-cost alternative.
Still, Lilly’s move on Humalog could put pressure on the other two big makers of insulin to act.
Novo Nordisk called Lilly’s lower-priced generic insulin “an important development,” in an emailed statement.
“Bringing affordable insulin to the market requires ideas from all stakeholders,” Novo Nordisk’s Ken Inchausti said in an email, which also listed steps the company has taken, such as a patient assistance program. The statement didn’t say whether Novo Nordisk is considering offering a lower-priced version of its popular insulin Novolog, a rival of Humalog.
A statement from Sanofi, the third major insulin maker, also didn’t say whether the company would offer lower-priced versions of its insulins.
“Sanofi supports any actions that increase access to insulins for patients living with diabetes at an affordable price,” spokeswoman Ashleigh Koss said in the email, which also touted the company’s patient assistance program.
A different kind of generic
One twist in this story is that Lilly’s new insulin is just a repackaged version of Humalog, minus the brand name. It’s called an “authorized generic.”
“Whoever came up with the term ‘authorized generic’?” Dr. Vincent Rajkumar said, laughing. Rajkumar is a hematologist at the Mayo Clinic in Rochester, Minn.
“It’s the same exact drug” as the brand name, he continued.
Typically, Rajkumar said, authorized generics are introduced by brand-name drugmakers to compete with generic versions of their drugs made by rival companies.
But in the case of Humalog and other insulins, there are no generics made by competitors, as there are for, say, the cholesterol medicine Lipitor or even other diabetes drugs, such as metformin.
So when Lilly’s authorized generic comes to market, the company will have both Humalog insulin and the authorized generic version of that medicine on the market.
Rajkumar said it’s a public relations move.
“There’s outrage over the price of insulin that is being discussed in Congress and elsewhere. And so the company basically says, ‘Hey, we will make the identical product available at half price.’ On the surface that sounds great,” Rajkumar said.
“But you look at the problems and you think, ‘OK, how crazy is this that someone is actually going to be buying the brand-name drug?’ ”
In fact, it’s possible that Lilly could break even or profit off its authorized generic compared to the name-brand Humalog, according to University of Pittsburgh’s Hernandez.
The profit margin would depend on the rebates paid by the company to insurers and pharmacy benefit managers. Rebates are getting a lot of attention these days as one factor that pushes drug prices higher. They’re usually not disclosed and increase as a drug’s price increases, providing an incentive to some companies to raise prices.
“Doing an authorized generic is nothing else than giving insurers two options,” Hernandez said: Pay the full list price for a brand-name drug and receive a higher rebate, or pay the lower price for the authorized generic and receive a presumably smaller rebate.
“What we really need to get insulin prices down is to get generics into the market, and we need more than one,” Hernandez said, adding that previous research has shown that prices begin to go down when two or three generics are competing in the marketplace.
Even so, Lillly’s Kueterman said the authorized generic insulin “is going to help hopefully move the system toward a more sustainable model.”
“I can guarantee you the reason that we’re doing this is to help people,” Kueterman said, noting the company’s Diabetes Solution Center has also helped “10,000 people each month pay significantly less for their insulin” since it opened in August.
For Erin Gilmer, the news about an authorized generic insulin from Lilly has left her mildly encouraged.
“It sounds really good, and it will help some people, which is great,” Gilmer said. “It’s Eli Lilly and pharma starting to understand that grassroots activism has to be taken seriously, and we are at a tipping point.”
This story is part of a partnership that includes NPR and Kaiser Health News. Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Flu or strep? Rapid tests can mislead
A 62-year-old woman presented to our emergency department with fever, chills, hoarseness, pain on swallowing, and a painful neck. Her symptoms had begun 1 day earlier. Because acetaminophen brought no improvement, she went to an urgent care facility, where a nasal swab polymerase chain reaction test was positive for influenza A, and a throat swab rapid test was positive for group A streptococci. She was then referred to our emergency department.
She reported no pre-existing conditions predisposing her to infection. Her temperature was 99.9°F (37.7°C), pulse 112 beats per minute, and respiratory rate 24 breaths per minute. The physical examination was unremarkable except for bilateral anterior cervical adenopathy and bilateral anterior neck tenderness. Her pharynx was not injected, and no exudate, palatal edema, or petechiae were noted.
Results of initial laboratory testing were as follows:
- White blood cell count 20.5 × 109/L (reference range 3.9–11)
- Neutrophils 76% (42%–75%)
- Bands 15% (0%–5%)
- Lymphocytes 3% (21%–51%)
- Erythrocyte sedimentation rate 75 mm/h (< 20 mm/h)
- C-reactive protein 247.14 mg/L (≤ 3 mg/L)
- Serum aminotransferase levels were normal.
- Polymerase chain reaction testing of a nasal swab was negative for viral infection.
Throat swabs and blood samples were sent for culture.
She was started on ceftriaxone 1 g intravenously every 24 hours, with close observation in the medical intensive care unit, where she was admitted because of epiglottitis. On hospital day 3, the throat culture was reported as negative, but the blood culture was reported as positive for Haemophilus influenzae. Thus, the clinical diagnosis was acute epiglottitis due to H influenzae, not group A streptococci.
The patient completed 10 days of ceftriaxone therapy; her recovery was uneventful, and she was discharged on hospital day 10.
INFLUENZA: CHALLENGES TO PROMPT, ACCURATE DIAGNOSIS
During influenza season, emergency departments are inundated with adults with influenza A and other viral respiratory infections. This makes prompt, accurate diagnosis a challenge,1 given the broad differential diagnosis.2,3 Adults with influenza and its complications as well as unrelated conditions can present a special challenge.4
Our patient presented with acute-onset influenza A and was then found to have acute epiglottitis, an unexpected complication of influenza A.5 A positive rapid test for group A streptococci done at an urgent care facility led emergency department physicians to assume that the acute epiglottitis was due to group A streptococci. Unless correlated with clinical findings, results of rapid diagnostic tests may mislead the unwary practitioner. Accurate diagnosis should be based mainly on the history and physical findings. Results of rapid diagnostic tests can be helpful if interpreted in the clinical context.6–8
The rapid test for streptococci is appropriate for the diagnosis of pharyngitis due to group A streptococci in people under age 30 with acute-onset sore throat, fever, and bilateral acute cervical adenopathy, without fatigue or myalgias. However, the rapid test does not differentiate colonization from infection. Group A streptococci are common colonizers with viral pharyngitis. In 30% of cases of Epstein-Barr virus pharyngitis, there is colonization with group A streptococci. A positive rapid test in such cases can result in the wrong diagnosis, ie, pharyngitis due to group A streptococci rather than Epstein-Barr virus.
- Cunha BA. The clinical diagnosis of severe viral influenza A. Infection 2008; 36(1):92–93. doi:10.1007/s15010-007-7255-9
- Cunha BA, Klein NC, Strollo S, Syed U, Mickail N, Laguerre M. Legionnaires’ disease mimicking swine influenza (H1N1) pneumonia during the “herald wave” of the pandemic. Heart Lung 2010; 39(3):242–248. doi:10.1016/j.hrtlng.2009.10.009
- Cunha BA, Raza M. During influenza season: all influenza-like illnesses are not due to influenza: dengue mimicking influenza. J Emerg Med 2015; 48(5):e117–e120. doi:10.1016/j.jemermed.2014.12.051
- Cunha CB. Infectious disease differential diagnosis. In: Cunha CB, Cunha BA, eds. Antibiotic Essentials. Jaypee Brothers Medical Pub: New Delhi, India; 2017:493–526.
- Cunha BA. Pharyngitis. In: Cunha CB, Cunha BA, eds. Antibiotic Essentials. Jaypee Brothers Medical Pub: New Delhi, India; 2017:42–47.
- Cohen JF, Chalumeau M, Levy C, et al. Effect of clinical spectrum, inoculum size and physician characteristics on sensitivity of rapid antigen detection test for group A streptococcal pharyngitis. Eur J Clin Microbiol Infect Dis 2013; 32(6):787–793. doi:10.1007/s10096-012-1809-1
- Dimatteo LA, Lowenstein SR, Brimhall B, Reiquam W, Gonzales R. The relationship between the clinical features of pharyngitis and the sensitivity of a rapid antigen test: evidence of spectrum bias. Ann Emerg Med 2001; 38(6):648–652. doi:10.1067/mem.2001.119850
- Cunha BA. A positive rapid strep test in a young adult with acute pharyngitis: be careful what you wish for! IDCases 2017; 10:58–59. doi:10.1016/j.idcr.2017.08.012
A 62-year-old woman presented to our emergency department with fever, chills, hoarseness, pain on swallowing, and a painful neck. Her symptoms had begun 1 day earlier. Because acetaminophen brought no improvement, she went to an urgent care facility, where a nasal swab polymerase chain reaction test was positive for influenza A, and a throat swab rapid test was positive for group A streptococci. She was then referred to our emergency department.
She reported no pre-existing conditions predisposing her to infection. Her temperature was 99.9°F (37.7°C), pulse 112 beats per minute, and respiratory rate 24 breaths per minute. The physical examination was unremarkable except for bilateral anterior cervical adenopathy and bilateral anterior neck tenderness. Her pharynx was not injected, and no exudate, palatal edema, or petechiae were noted.
Results of initial laboratory testing were as follows:
- White blood cell count 20.5 × 109/L (reference range 3.9–11)
- Neutrophils 76% (42%–75%)
- Bands 15% (0%–5%)
- Lymphocytes 3% (21%–51%)
- Erythrocyte sedimentation rate 75 mm/h (< 20 mm/h)
- C-reactive protein 247.14 mg/L (≤ 3 mg/L)
- Serum aminotransferase levels were normal.
- Polymerase chain reaction testing of a nasal swab was negative for viral infection.
Throat swabs and blood samples were sent for culture.
She was started on ceftriaxone 1 g intravenously every 24 hours, with close observation in the medical intensive care unit, where she was admitted because of epiglottitis. On hospital day 3, the throat culture was reported as negative, but the blood culture was reported as positive for Haemophilus influenzae. Thus, the clinical diagnosis was acute epiglottitis due to H influenzae, not group A streptococci.
The patient completed 10 days of ceftriaxone therapy; her recovery was uneventful, and she was discharged on hospital day 10.
INFLUENZA: CHALLENGES TO PROMPT, ACCURATE DIAGNOSIS
During influenza season, emergency departments are inundated with adults with influenza A and other viral respiratory infections. This makes prompt, accurate diagnosis a challenge,1 given the broad differential diagnosis.2,3 Adults with influenza and its complications as well as unrelated conditions can present a special challenge.4
Our patient presented with acute-onset influenza A and was then found to have acute epiglottitis, an unexpected complication of influenza A.5 A positive rapid test for group A streptococci done at an urgent care facility led emergency department physicians to assume that the acute epiglottitis was due to group A streptococci. Unless correlated with clinical findings, results of rapid diagnostic tests may mislead the unwary practitioner. Accurate diagnosis should be based mainly on the history and physical findings. Results of rapid diagnostic tests can be helpful if interpreted in the clinical context.6–8
The rapid test for streptococci is appropriate for the diagnosis of pharyngitis due to group A streptococci in people under age 30 with acute-onset sore throat, fever, and bilateral acute cervical adenopathy, without fatigue or myalgias. However, the rapid test does not differentiate colonization from infection. Group A streptococci are common colonizers with viral pharyngitis. In 30% of cases of Epstein-Barr virus pharyngitis, there is colonization with group A streptococci. A positive rapid test in such cases can result in the wrong diagnosis, ie, pharyngitis due to group A streptococci rather than Epstein-Barr virus.
A 62-year-old woman presented to our emergency department with fever, chills, hoarseness, pain on swallowing, and a painful neck. Her symptoms had begun 1 day earlier. Because acetaminophen brought no improvement, she went to an urgent care facility, where a nasal swab polymerase chain reaction test was positive for influenza A, and a throat swab rapid test was positive for group A streptococci. She was then referred to our emergency department.
She reported no pre-existing conditions predisposing her to infection. Her temperature was 99.9°F (37.7°C), pulse 112 beats per minute, and respiratory rate 24 breaths per minute. The physical examination was unremarkable except for bilateral anterior cervical adenopathy and bilateral anterior neck tenderness. Her pharynx was not injected, and no exudate, palatal edema, or petechiae were noted.
Results of initial laboratory testing were as follows:
- White blood cell count 20.5 × 109/L (reference range 3.9–11)
- Neutrophils 76% (42%–75%)
- Bands 15% (0%–5%)
- Lymphocytes 3% (21%–51%)
- Erythrocyte sedimentation rate 75 mm/h (< 20 mm/h)
- C-reactive protein 247.14 mg/L (≤ 3 mg/L)
- Serum aminotransferase levels were normal.
- Polymerase chain reaction testing of a nasal swab was negative for viral infection.
Throat swabs and blood samples were sent for culture.
She was started on ceftriaxone 1 g intravenously every 24 hours, with close observation in the medical intensive care unit, where she was admitted because of epiglottitis. On hospital day 3, the throat culture was reported as negative, but the blood culture was reported as positive for Haemophilus influenzae. Thus, the clinical diagnosis was acute epiglottitis due to H influenzae, not group A streptococci.
The patient completed 10 days of ceftriaxone therapy; her recovery was uneventful, and she was discharged on hospital day 10.
INFLUENZA: CHALLENGES TO PROMPT, ACCURATE DIAGNOSIS
During influenza season, emergency departments are inundated with adults with influenza A and other viral respiratory infections. This makes prompt, accurate diagnosis a challenge,1 given the broad differential diagnosis.2,3 Adults with influenza and its complications as well as unrelated conditions can present a special challenge.4
Our patient presented with acute-onset influenza A and was then found to have acute epiglottitis, an unexpected complication of influenza A.5 A positive rapid test for group A streptococci done at an urgent care facility led emergency department physicians to assume that the acute epiglottitis was due to group A streptococci. Unless correlated with clinical findings, results of rapid diagnostic tests may mislead the unwary practitioner. Accurate diagnosis should be based mainly on the history and physical findings. Results of rapid diagnostic tests can be helpful if interpreted in the clinical context.6–8
The rapid test for streptococci is appropriate for the diagnosis of pharyngitis due to group A streptococci in people under age 30 with acute-onset sore throat, fever, and bilateral acute cervical adenopathy, without fatigue or myalgias. However, the rapid test does not differentiate colonization from infection. Group A streptococci are common colonizers with viral pharyngitis. In 30% of cases of Epstein-Barr virus pharyngitis, there is colonization with group A streptococci. A positive rapid test in such cases can result in the wrong diagnosis, ie, pharyngitis due to group A streptococci rather than Epstein-Barr virus.
- Cunha BA. The clinical diagnosis of severe viral influenza A. Infection 2008; 36(1):92–93. doi:10.1007/s15010-007-7255-9
- Cunha BA, Klein NC, Strollo S, Syed U, Mickail N, Laguerre M. Legionnaires’ disease mimicking swine influenza (H1N1) pneumonia during the “herald wave” of the pandemic. Heart Lung 2010; 39(3):242–248. doi:10.1016/j.hrtlng.2009.10.009
- Cunha BA, Raza M. During influenza season: all influenza-like illnesses are not due to influenza: dengue mimicking influenza. J Emerg Med 2015; 48(5):e117–e120. doi:10.1016/j.jemermed.2014.12.051
- Cunha CB. Infectious disease differential diagnosis. In: Cunha CB, Cunha BA, eds. Antibiotic Essentials. Jaypee Brothers Medical Pub: New Delhi, India; 2017:493–526.
- Cunha BA. Pharyngitis. In: Cunha CB, Cunha BA, eds. Antibiotic Essentials. Jaypee Brothers Medical Pub: New Delhi, India; 2017:42–47.
- Cohen JF, Chalumeau M, Levy C, et al. Effect of clinical spectrum, inoculum size and physician characteristics on sensitivity of rapid antigen detection test for group A streptococcal pharyngitis. Eur J Clin Microbiol Infect Dis 2013; 32(6):787–793. doi:10.1007/s10096-012-1809-1
- Dimatteo LA, Lowenstein SR, Brimhall B, Reiquam W, Gonzales R. The relationship between the clinical features of pharyngitis and the sensitivity of a rapid antigen test: evidence of spectrum bias. Ann Emerg Med 2001; 38(6):648–652. doi:10.1067/mem.2001.119850
- Cunha BA. A positive rapid strep test in a young adult with acute pharyngitis: be careful what you wish for! IDCases 2017; 10:58–59. doi:10.1016/j.idcr.2017.08.012
- Cunha BA. The clinical diagnosis of severe viral influenza A. Infection 2008; 36(1):92–93. doi:10.1007/s15010-007-7255-9
- Cunha BA, Klein NC, Strollo S, Syed U, Mickail N, Laguerre M. Legionnaires’ disease mimicking swine influenza (H1N1) pneumonia during the “herald wave” of the pandemic. Heart Lung 2010; 39(3):242–248. doi:10.1016/j.hrtlng.2009.10.009
- Cunha BA, Raza M. During influenza season: all influenza-like illnesses are not due to influenza: dengue mimicking influenza. J Emerg Med 2015; 48(5):e117–e120. doi:10.1016/j.jemermed.2014.12.051
- Cunha CB. Infectious disease differential diagnosis. In: Cunha CB, Cunha BA, eds. Antibiotic Essentials. Jaypee Brothers Medical Pub: New Delhi, India; 2017:493–526.
- Cunha BA. Pharyngitis. In: Cunha CB, Cunha BA, eds. Antibiotic Essentials. Jaypee Brothers Medical Pub: New Delhi, India; 2017:42–47.
- Cohen JF, Chalumeau M, Levy C, et al. Effect of clinical spectrum, inoculum size and physician characteristics on sensitivity of rapid antigen detection test for group A streptococcal pharyngitis. Eur J Clin Microbiol Infect Dis 2013; 32(6):787–793. doi:10.1007/s10096-012-1809-1
- Dimatteo LA, Lowenstein SR, Brimhall B, Reiquam W, Gonzales R. The relationship between the clinical features of pharyngitis and the sensitivity of a rapid antigen test: evidence of spectrum bias. Ann Emerg Med 2001; 38(6):648–652. doi:10.1067/mem.2001.119850
- Cunha BA. A positive rapid strep test in a young adult with acute pharyngitis: be careful what you wish for! IDCases 2017; 10:58–59. doi:10.1016/j.idcr.2017.08.012