User login
FDA approves generic naloxone spray for opioid overdose treatment
The Food and Drug Administration on April 19 approved the first generic naloxone hydrochloride nasal spray (Narcan) as treatment for stopping or reversing an opioid overdose.
“In the wake of the opioid crisis, a number of efforts are underway to make this emergency overdose reversal treatment more readily available and more accessible,” said Douglas Throckmorton, MD, deputy center director for regulatory programs in the FDA’s Center for Drug Evaluation and Research, in a press release. “In addition to this approval of the first generic naloxone nasal spray, moving forward, we will prioritize our review of generic drug applications for naloxone.”
The agency said the naloxone nasal spray does not need assembly and can be used by anyone, regardless of medical training. If the spray is administered quickly after the overdose begins, the effect of the opioid will be countered, often within minutes. However, patients should still seek immediate medical attention.
The FDA cautioned that, when used on a patient with an opioid dependence, naloxone can cause severe opioid withdrawal, characterized by symptoms such as body aches, diarrhea, tachycardia, fever, runny nose, sneezing, goose bumps, sweating, yawning, nausea or vomiting, nervousness, restlessness or irritability, shivering or trembling, abdominal cramps, weakness, and increased blood pressure.
Find the full press release on the FDA website.
The Food and Drug Administration on April 19 approved the first generic naloxone hydrochloride nasal spray (Narcan) as treatment for stopping or reversing an opioid overdose.
“In the wake of the opioid crisis, a number of efforts are underway to make this emergency overdose reversal treatment more readily available and more accessible,” said Douglas Throckmorton, MD, deputy center director for regulatory programs in the FDA’s Center for Drug Evaluation and Research, in a press release. “In addition to this approval of the first generic naloxone nasal spray, moving forward, we will prioritize our review of generic drug applications for naloxone.”
The agency said the naloxone nasal spray does not need assembly and can be used by anyone, regardless of medical training. If the spray is administered quickly after the overdose begins, the effect of the opioid will be countered, often within minutes. However, patients should still seek immediate medical attention.
The FDA cautioned that, when used on a patient with an opioid dependence, naloxone can cause severe opioid withdrawal, characterized by symptoms such as body aches, diarrhea, tachycardia, fever, runny nose, sneezing, goose bumps, sweating, yawning, nausea or vomiting, nervousness, restlessness or irritability, shivering or trembling, abdominal cramps, weakness, and increased blood pressure.
Find the full press release on the FDA website.
The Food and Drug Administration on April 19 approved the first generic naloxone hydrochloride nasal spray (Narcan) as treatment for stopping or reversing an opioid overdose.
“In the wake of the opioid crisis, a number of efforts are underway to make this emergency overdose reversal treatment more readily available and more accessible,” said Douglas Throckmorton, MD, deputy center director for regulatory programs in the FDA’s Center for Drug Evaluation and Research, in a press release. “In addition to this approval of the first generic naloxone nasal spray, moving forward, we will prioritize our review of generic drug applications for naloxone.”
The agency said the naloxone nasal spray does not need assembly and can be used by anyone, regardless of medical training. If the spray is administered quickly after the overdose begins, the effect of the opioid will be countered, often within minutes. However, patients should still seek immediate medical attention.
The FDA cautioned that, when used on a patient with an opioid dependence, naloxone can cause severe opioid withdrawal, characterized by symptoms such as body aches, diarrhea, tachycardia, fever, runny nose, sneezing, goose bumps, sweating, yawning, nausea or vomiting, nervousness, restlessness or irritability, shivering or trembling, abdominal cramps, weakness, and increased blood pressure.
Find the full press release on the FDA website.
Poor response to statins hikes risk of cardiovascular events
About half of patients taking statins for hyperlipidemia don’t adequately respond, leaving them at a 22% increased risk of cardiovascular disease, compared with optimal responders.
Over 6 years, there were about 2,000 more cardiovascular events among those who failed to experience the national treatment target of at least a 40% reduction in LDL cholesterol, according to Stephen F. Weng, MD, and his colleagues. The report is in Heart.
Physicians’ choice of initial statin weighed heavily in the outcomes. Patients who ended up with an optimal response were more likely to get a more potent statin right off, while those with a poorer response were more likely to get a less-potent statin.
“This study provides ‘real world evidence’ that 50% of patients started on statins do not derive the intended therapeutic benefit from them, significantly increasing their risk of future cardiovascular disease,” wrote Dr. Weng of the University of Nottingham, England, and his colleagues. “These findings contribute to the debate on the effectiveness of statin therapy and highlight the need for personalized medicine in lipid management for patients.”
The study comprised 165,411 primary care patients who had hypercholesterolemia but were free of cardiovascular disease at baseline. Statins were prescribed with the goal of at least a 40% reduction in baseline LDL within 24 months of the start of therapy.
Patients had a mean age of 62 years, with a mean baseline LDL of 4.1 mmol/L (158 mg/dL). About 49% were women.
The primary endpoints were the number of patients who did not achieve the 40% or higher reduction in baseline LDL and the between-group risk differences in cardiovascular events (coronary heart disease, stroke or transient ischemic attack, peripheral vascular disease, cardiovascular death).
After 24 months, 51.2% of patients experienced a suboptimal LDL response, with a mean reduction of 2.1 mmol/L (81 mg/dL) compared with 3.1 mmol/L (120 mg/dL). Compared with optimal responders, these patients were significantly more likely to have received a low-potency statin (29% vs. 18%).
Incident cardiovascular events occurred in 14% of the overall group (coronary artery disease, 8%; stroke/TIA, 3%; peripheral vascular disease 1.9%; cardiovascular death, 1%). All of these outcomes were significantly more common among suboptimal responders than optimal responders.
During a mean of 6 years of follow-up, there were 22,798 cardiovascular disease events overall, with significantly more occurring in suboptimal than optimal responders (12,142 vs. 10,656). This translated to a cardiovascular disease rate of 22.6 and 19.7 per 1,000 person-years, respectively.
In a multivariate analysis controlling for age and baseline LDL level, suboptimal responders were 22% more likely to have a cardiovascular disease incident than were optimal responders.
Among suboptimal responders, every unit decrease of 1 mmol/L (39 mg/dL) conferred a significant 6% risk reduction in cardiovascular disease (odds ratio, 0.94).
The benefit was not universal, the authors pointed out. “In this group, the decreased risk remained significant for only stroke/TIA and was not significant for other constituent cardiovascular disease outcomes. However, in patients with an optimal response, an even greater protective effect of LDL reduction and future cardiovascular disease was seen [13%; OR, 0.87],” and this reduction was significant for all of the individual outcomes.
“The study also highlights the benefit of reducing LDL to optimal values, which would lead to better cardiovascular disease outcomes for patients currently on statins,” the authors concluded.
None of the authors had any relevant financial disclosures.
SOURCE: Weng S. et al. Heart 2019 Apr. doi: 10.1136/heartjnl-2018-314253.
Guidelines always look good on paper, but they’re only as good as their implementation, Márcio S. Bittencourt, MD, wrote in an accompanying editorial.
In the United Kingdom, the National Institute for Health and Care Excellence (NICE) guideline pinned effective statin therapy as a lowering of LDL cholesterol by at least 40%. This target aligns well with data accumulated in randomized controlled studies, but it doesn’t benefit patients unless it can be put into practice.
“An important step after a guideline publication is the assessment of its uptake among health practitioners and patients in the real world, as well as of the impact of its adherence on clinical outcomes. These analyses may not only verify its appropriateness, providing feedback for continuous improvement of recommendations, but also identify targets to optimize delivery of health to the society.”
To understand suboptimal statin response, we must understand the many possible reasons behind it – on the part of both physicians and patients.
Physicians may prefer to prescribe low-potency statins for several reasons, including unawareness of guideline recommendations, doubtfulness of better outcomes with higher potent statins or when a lower LDL is attained, and fear of adverse reactions or drug interactions, Dr. Bittencourt noted. “Moreover, doctors may be reluctant to up-titrate drugs when the treatment goals are not achieved, the so-called therapeutic inertia.”
In this study, for example, optimal responders were more likely to initially receive moderately potent statins. Suboptimal responders, on the other hand, were more likely to receive low-potency statins.
“This probably explains why baseline LDL was higher in optimal responders, indicating that higher LDL motivates the physician to be more aggressive upfront.”
Patients bring their own issues to the treatment table.
“Although an inter-individual response to statins may occur according to the genetic background, most cases where LDL response is less than expected are probably due to lack of adherence or persistence to the treatment. ... Of note, poor adherence to lipid-lowering therapy, together with low-intensity therapy, as opposed to high-intensity treatment, is associated with higher cardiovascular risk.”
Effective implementation of guidelines “has been a challenge for a long time. Both physicians and patients should be targets for approaches aiming at improving adherence to guidelines.”
For clinicians, these could include continuing medical education and simplified treatment algorithms. Patients, too, would benefit from some teaching.
“Patients and society should be educated on the scientific evidence documenting the benefits of lipid-lowering therapy, and antistatin propaganda based on pseudoscience should be strongly disavowed and demystified by health authorities.”
Dr. Bittencourt is an internist at the University Hospital San Paolo, Brazil.
Guidelines always look good on paper, but they’re only as good as their implementation, Márcio S. Bittencourt, MD, wrote in an accompanying editorial.
In the United Kingdom, the National Institute for Health and Care Excellence (NICE) guideline pinned effective statin therapy as a lowering of LDL cholesterol by at least 40%. This target aligns well with data accumulated in randomized controlled studies, but it doesn’t benefit patients unless it can be put into practice.
“An important step after a guideline publication is the assessment of its uptake among health practitioners and patients in the real world, as well as of the impact of its adherence on clinical outcomes. These analyses may not only verify its appropriateness, providing feedback for continuous improvement of recommendations, but also identify targets to optimize delivery of health to the society.”
To understand suboptimal statin response, we must understand the many possible reasons behind it – on the part of both physicians and patients.
Physicians may prefer to prescribe low-potency statins for several reasons, including unawareness of guideline recommendations, doubtfulness of better outcomes with higher potent statins or when a lower LDL is attained, and fear of adverse reactions or drug interactions, Dr. Bittencourt noted. “Moreover, doctors may be reluctant to up-titrate drugs when the treatment goals are not achieved, the so-called therapeutic inertia.”
In this study, for example, optimal responders were more likely to initially receive moderately potent statins. Suboptimal responders, on the other hand, were more likely to receive low-potency statins.
“This probably explains why baseline LDL was higher in optimal responders, indicating that higher LDL motivates the physician to be more aggressive upfront.”
Patients bring their own issues to the treatment table.
“Although an inter-individual response to statins may occur according to the genetic background, most cases where LDL response is less than expected are probably due to lack of adherence or persistence to the treatment. ... Of note, poor adherence to lipid-lowering therapy, together with low-intensity therapy, as opposed to high-intensity treatment, is associated with higher cardiovascular risk.”
Effective implementation of guidelines “has been a challenge for a long time. Both physicians and patients should be targets for approaches aiming at improving adherence to guidelines.”
For clinicians, these could include continuing medical education and simplified treatment algorithms. Patients, too, would benefit from some teaching.
“Patients and society should be educated on the scientific evidence documenting the benefits of lipid-lowering therapy, and antistatin propaganda based on pseudoscience should be strongly disavowed and demystified by health authorities.”
Dr. Bittencourt is an internist at the University Hospital San Paolo, Brazil.
Guidelines always look good on paper, but they’re only as good as their implementation, Márcio S. Bittencourt, MD, wrote in an accompanying editorial.
In the United Kingdom, the National Institute for Health and Care Excellence (NICE) guideline pinned effective statin therapy as a lowering of LDL cholesterol by at least 40%. This target aligns well with data accumulated in randomized controlled studies, but it doesn’t benefit patients unless it can be put into practice.
“An important step after a guideline publication is the assessment of its uptake among health practitioners and patients in the real world, as well as of the impact of its adherence on clinical outcomes. These analyses may not only verify its appropriateness, providing feedback for continuous improvement of recommendations, but also identify targets to optimize delivery of health to the society.”
To understand suboptimal statin response, we must understand the many possible reasons behind it – on the part of both physicians and patients.
Physicians may prefer to prescribe low-potency statins for several reasons, including unawareness of guideline recommendations, doubtfulness of better outcomes with higher potent statins or when a lower LDL is attained, and fear of adverse reactions or drug interactions, Dr. Bittencourt noted. “Moreover, doctors may be reluctant to up-titrate drugs when the treatment goals are not achieved, the so-called therapeutic inertia.”
In this study, for example, optimal responders were more likely to initially receive moderately potent statins. Suboptimal responders, on the other hand, were more likely to receive low-potency statins.
“This probably explains why baseline LDL was higher in optimal responders, indicating that higher LDL motivates the physician to be more aggressive upfront.”
Patients bring their own issues to the treatment table.
“Although an inter-individual response to statins may occur according to the genetic background, most cases where LDL response is less than expected are probably due to lack of adherence or persistence to the treatment. ... Of note, poor adherence to lipid-lowering therapy, together with low-intensity therapy, as opposed to high-intensity treatment, is associated with higher cardiovascular risk.”
Effective implementation of guidelines “has been a challenge for a long time. Both physicians and patients should be targets for approaches aiming at improving adherence to guidelines.”
For clinicians, these could include continuing medical education and simplified treatment algorithms. Patients, too, would benefit from some teaching.
“Patients and society should be educated on the scientific evidence documenting the benefits of lipid-lowering therapy, and antistatin propaganda based on pseudoscience should be strongly disavowed and demystified by health authorities.”
Dr. Bittencourt is an internist at the University Hospital San Paolo, Brazil.
About half of patients taking statins for hyperlipidemia don’t adequately respond, leaving them at a 22% increased risk of cardiovascular disease, compared with optimal responders.
Over 6 years, there were about 2,000 more cardiovascular events among those who failed to experience the national treatment target of at least a 40% reduction in LDL cholesterol, according to Stephen F. Weng, MD, and his colleagues. The report is in Heart.
Physicians’ choice of initial statin weighed heavily in the outcomes. Patients who ended up with an optimal response were more likely to get a more potent statin right off, while those with a poorer response were more likely to get a less-potent statin.
“This study provides ‘real world evidence’ that 50% of patients started on statins do not derive the intended therapeutic benefit from them, significantly increasing their risk of future cardiovascular disease,” wrote Dr. Weng of the University of Nottingham, England, and his colleagues. “These findings contribute to the debate on the effectiveness of statin therapy and highlight the need for personalized medicine in lipid management for patients.”
The study comprised 165,411 primary care patients who had hypercholesterolemia but were free of cardiovascular disease at baseline. Statins were prescribed with the goal of at least a 40% reduction in baseline LDL within 24 months of the start of therapy.
Patients had a mean age of 62 years, with a mean baseline LDL of 4.1 mmol/L (158 mg/dL). About 49% were women.
The primary endpoints were the number of patients who did not achieve the 40% or higher reduction in baseline LDL and the between-group risk differences in cardiovascular events (coronary heart disease, stroke or transient ischemic attack, peripheral vascular disease, cardiovascular death).
After 24 months, 51.2% of patients experienced a suboptimal LDL response, with a mean reduction of 2.1 mmol/L (81 mg/dL) compared with 3.1 mmol/L (120 mg/dL). Compared with optimal responders, these patients were significantly more likely to have received a low-potency statin (29% vs. 18%).
Incident cardiovascular events occurred in 14% of the overall group (coronary artery disease, 8%; stroke/TIA, 3%; peripheral vascular disease 1.9%; cardiovascular death, 1%). All of these outcomes were significantly more common among suboptimal responders than optimal responders.
During a mean of 6 years of follow-up, there were 22,798 cardiovascular disease events overall, with significantly more occurring in suboptimal than optimal responders (12,142 vs. 10,656). This translated to a cardiovascular disease rate of 22.6 and 19.7 per 1,000 person-years, respectively.
In a multivariate analysis controlling for age and baseline LDL level, suboptimal responders were 22% more likely to have a cardiovascular disease incident than were optimal responders.
Among suboptimal responders, every unit decrease of 1 mmol/L (39 mg/dL) conferred a significant 6% risk reduction in cardiovascular disease (odds ratio, 0.94).
The benefit was not universal, the authors pointed out. “In this group, the decreased risk remained significant for only stroke/TIA and was not significant for other constituent cardiovascular disease outcomes. However, in patients with an optimal response, an even greater protective effect of LDL reduction and future cardiovascular disease was seen [13%; OR, 0.87],” and this reduction was significant for all of the individual outcomes.
“The study also highlights the benefit of reducing LDL to optimal values, which would lead to better cardiovascular disease outcomes for patients currently on statins,” the authors concluded.
None of the authors had any relevant financial disclosures.
SOURCE: Weng S. et al. Heart 2019 Apr. doi: 10.1136/heartjnl-2018-314253.
About half of patients taking statins for hyperlipidemia don’t adequately respond, leaving them at a 22% increased risk of cardiovascular disease, compared with optimal responders.
Over 6 years, there were about 2,000 more cardiovascular events among those who failed to experience the national treatment target of at least a 40% reduction in LDL cholesterol, according to Stephen F. Weng, MD, and his colleagues. The report is in Heart.
Physicians’ choice of initial statin weighed heavily in the outcomes. Patients who ended up with an optimal response were more likely to get a more potent statin right off, while those with a poorer response were more likely to get a less-potent statin.
“This study provides ‘real world evidence’ that 50% of patients started on statins do not derive the intended therapeutic benefit from them, significantly increasing their risk of future cardiovascular disease,” wrote Dr. Weng of the University of Nottingham, England, and his colleagues. “These findings contribute to the debate on the effectiveness of statin therapy and highlight the need for personalized medicine in lipid management for patients.”
The study comprised 165,411 primary care patients who had hypercholesterolemia but were free of cardiovascular disease at baseline. Statins were prescribed with the goal of at least a 40% reduction in baseline LDL within 24 months of the start of therapy.
Patients had a mean age of 62 years, with a mean baseline LDL of 4.1 mmol/L (158 mg/dL). About 49% were women.
The primary endpoints were the number of patients who did not achieve the 40% or higher reduction in baseline LDL and the between-group risk differences in cardiovascular events (coronary heart disease, stroke or transient ischemic attack, peripheral vascular disease, cardiovascular death).
After 24 months, 51.2% of patients experienced a suboptimal LDL response, with a mean reduction of 2.1 mmol/L (81 mg/dL) compared with 3.1 mmol/L (120 mg/dL). Compared with optimal responders, these patients were significantly more likely to have received a low-potency statin (29% vs. 18%).
Incident cardiovascular events occurred in 14% of the overall group (coronary artery disease, 8%; stroke/TIA, 3%; peripheral vascular disease 1.9%; cardiovascular death, 1%). All of these outcomes were significantly more common among suboptimal responders than optimal responders.
During a mean of 6 years of follow-up, there were 22,798 cardiovascular disease events overall, with significantly more occurring in suboptimal than optimal responders (12,142 vs. 10,656). This translated to a cardiovascular disease rate of 22.6 and 19.7 per 1,000 person-years, respectively.
In a multivariate analysis controlling for age and baseline LDL level, suboptimal responders were 22% more likely to have a cardiovascular disease incident than were optimal responders.
Among suboptimal responders, every unit decrease of 1 mmol/L (39 mg/dL) conferred a significant 6% risk reduction in cardiovascular disease (odds ratio, 0.94).
The benefit was not universal, the authors pointed out. “In this group, the decreased risk remained significant for only stroke/TIA and was not significant for other constituent cardiovascular disease outcomes. However, in patients with an optimal response, an even greater protective effect of LDL reduction and future cardiovascular disease was seen [13%; OR, 0.87],” and this reduction was significant for all of the individual outcomes.
“The study also highlights the benefit of reducing LDL to optimal values, which would lead to better cardiovascular disease outcomes for patients currently on statins,” the authors concluded.
None of the authors had any relevant financial disclosures.
SOURCE: Weng S. et al. Heart 2019 Apr. doi: 10.1136/heartjnl-2018-314253.
FROM HEART
2018 at a glance: Recently approved therapies in oncology
Advances in genomics and technology perpetually change and improve therapies in oncology. Enhanced comprehension of cellular signaling, division, and replication has created a platform to selectively restrict neoplastic growth while preserving the integrity of benign cells.
This article reviews therapies that were newly approved in 2018, as well as those previously approved whose indications were expanded this past year. The list highlights the most clinically important approvals, as well as adverse events that are unique or especially severe.
Click on the PDF above to download the full article and charts in an easy-to-print format.
Apalutamide (Erleada)
Class: Androgen receptor inhibitor.
Disease: Nonmetastatic castration-resistant prostate cancer.
Dose: 240 mg orally, once daily.
Adverse Events (AEs): Hyperkalemia and increased risks of seizures, falls, and fractures.
Phase 3 SPARTAN trial (NCT01946204): 40.5-month metastasis-free survival rate, compared with 16.2 months in the placebo group.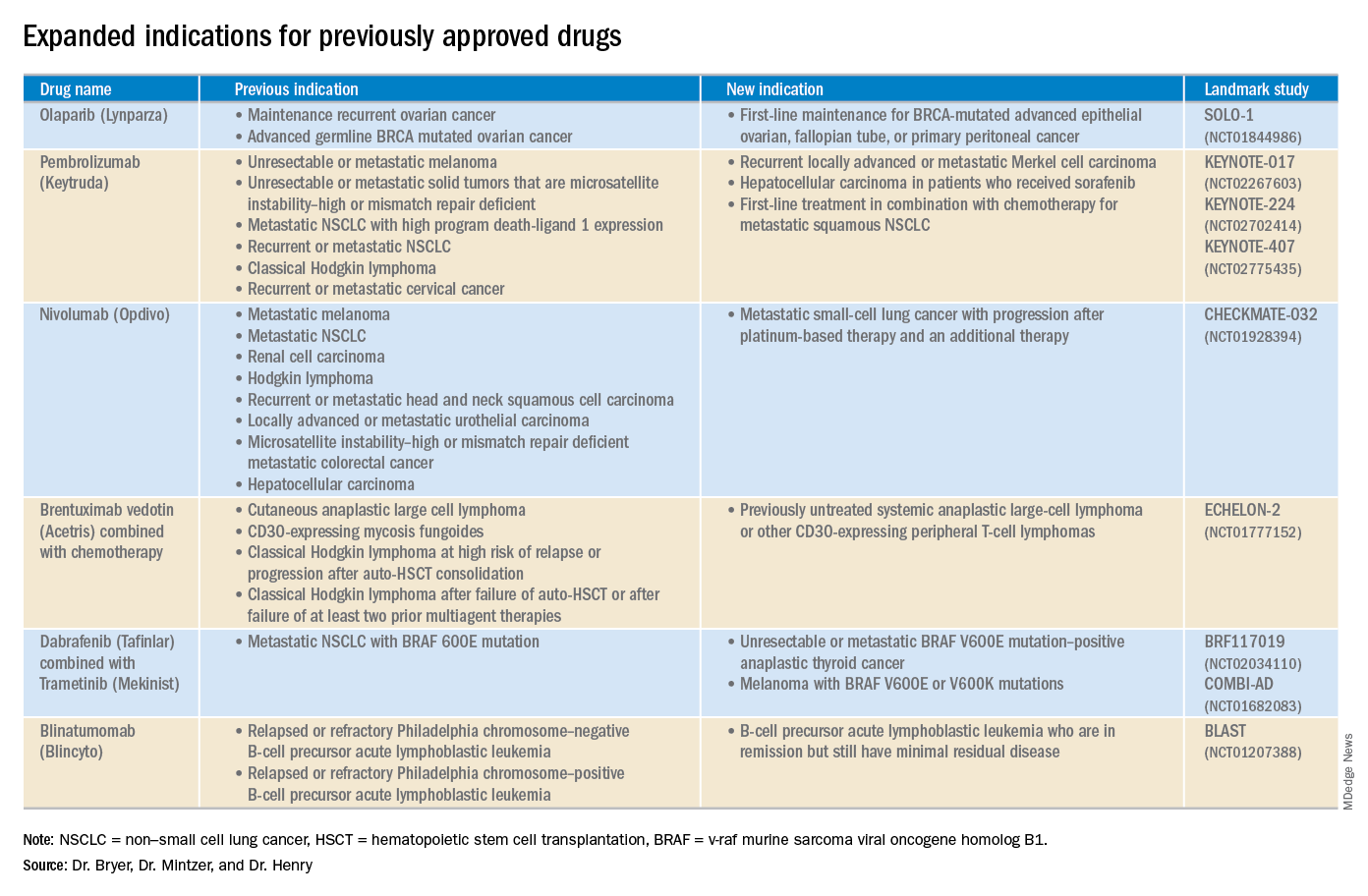
Cemiplimab (Libtayo)
Class: Antibody against programmed cell death protein-1 (PD-1).
Disease: Metastatic cutaneous squamous cell carcinoma (CSCC) or locally advanced CSCC that is ineligible for curative surgery/radiation.
Dose: 350 mg intravenous infusion every 3 weeks.
AEs: Pneumonitis, autoimmune myocarditis, hepatitis, and aseptic meningitis.
1423 and 1540 trials (NCT02383212 and NCT02760498): 47.2% of patients who received cemiplimab had complete disappearance of the tumor or a decrease in tumor size.
Dacomitinib (Vizimpro)
Class: Second-generation tyrosine kinase inhibitor.
Disease: Metastatic non–small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) exon 19 deletion or exon 21 L858R substitution mutation.
Dose: 45 mg orally once daily.
AEs: Dermatotoxicity and diarrhea.
ARCHER1050 trial (NCT01774721): Patients who received dacomitinib demonstrated an improved overall survival, with a median of 34.1 months, compared with 26.8 months with gefitinib.
Duvelisib (Copiktra)
Class: Dual inhibitor of phosphatidylinositol 3-kinase delta and gamma.
Disease: Relapsed or refractory chronic lymphocytic leukemia (CLL), small lymphocytic lymphoma, or relapsed or refractory follicular lymphoma after at least two prior systemic therapies.
Dose: 25 mg orally twice daily.
AEs: Infection, diarrhea or colitis, and pneumonia.
Phase 3 DUO trial (NCT02004522): Progression-free survival in the duvelisib arm was 7.3 months longer than that in the ofatumumab arm. The overall response rate for patients receiving duvelisib was 78%, compared with 39% for those receiving ofatumumab.
Gilteritinib (Xospata)
Class: Inhibits the FLT3 internal tandem duplication (ITD) and FLT3 tyrosine kinase domain (TKD).
Disease: Relapsed or refractory acute myeloid leukemia (AML) with an FLT3 mutation.
Dose: 120 mg orally daily.
ADMIRAL trial (NCT02421939): 21% of the patients who received gilteritinib exhibited complete remission or complete remission with partial hematologic recovery.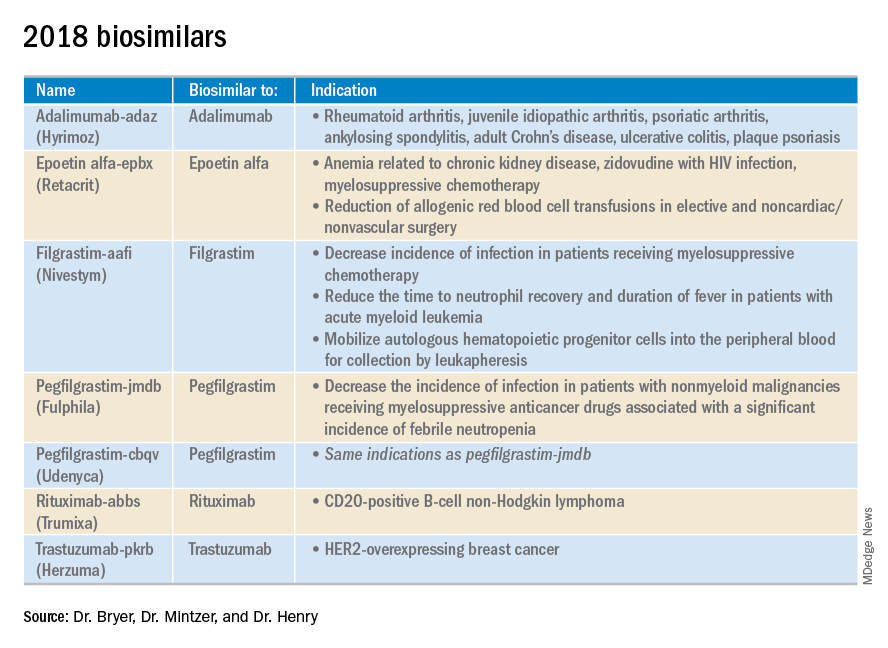
Glasdegib (Daurismo)
Class: Hedgehog pathway inhibitor.
Disease: Adults over age 75 years with newly diagnosed AML and other medical comorbidities that preclude them from intensive chemotherapy.
Dose: The recommended dose is 100 mg orally continuously in 28-day cycles.
AE: QT prolongation and embryo-fetal toxicity
Phase 2 BRIGHT 1003 trial (NCT01546038): 3.9-month overall survival advantage for glasdegib plus cytarabine, compared with cytarabine alone. Overall, 15% of the glasdegib plus low dose cytarabine arm achieved complete remission, compared with the 1% complete remission rate in patients who received cytarabine alone.
Iobenguane I 131 (Azedra)
Class: Radiopharmaceutical agent; induces cell death within the noradrenaline transporter.
Disease: Iobenguane scan–positive, unresectable, locally advanced or metastatic pheochromocytoma or paraganglioma
Dose: Initial intravenous dosimetric dose, followed by two therapeutic doses.
AE: Pancytopenia and elevated international normalized ratio (INR).
IB12B trial (NCT00874614): One-quarter of patients receiving this therapy had at least a 50% reduction in the dose and number of antihypertensives for at least 6 months; almost all patients had a tumor response.
Ivosidenib (Tibsovo)
Class: Small-molecule inhibitor of mutant isocitrate dehydrogenase (IDH1).
Disease: Refractory AML and an IDH1 mutation
Dose: 500 mg orally daily.
AG120-C-001 trial (NCT02074839): Overall response rate of 41.6% in patients who received ivosidenib, with a 30.4% rate of complete remission or complete remission with partial hematologic recovery.
Larotrectinib (Vitrakvi)
Class: Oral tyrosine kinase inhibitor.
Disease: Advanced solid tumors harboring a neurotrophic tyrosine receptor kinase (NTRK) gene fusion.
Dose: 100 mg orally twice daily.
LOXO-TRK-14001, SCOUT, and NAVIGATE trials (NCT02122913, NCT02637687, and NCT02576431): Patients who received larotrectinib had durable responses regardless of patient age, tumor type, and fusion status.
Lutetium Lu 177 dotatate (Lutathera)
Class: Radiolabeled somatostatin analogue.
Disease: Somatostatin receptor–positive gastroenteropancreatic neuroendocrine tumors (GEP-NETs).
Dose: Intravenous infusion 7.4 GBq (200 mCi) every 8 weeks for a total of four doses.
NETTER-1 trial (NCT01578239): 65% of adults who received lutetium Lu 177 showed improved progression-free survival at 20 months, compared with just 10.8% in the control group.
Mogamulizumab (Poteligeo)
Class: Monoclonal antibody that binds to a protein (CC chemokine receptor type 4).
Disease: Relapsed or refractory mycosis fungoides or Sézary syndrome.
Dose: Intravenous infusion 1 mg/kg.
AE: Dermatologic toxicity.
MAVORIC trial (NCT01728805): Patients who received mogamulizumab had improved progression-free survival (median 7.7 months), compared with those taking vorinostat (median 3.1 months).
Moxetumomab pasudotox-tdfk (Lumoxiti)
Class: CD22-directed cytotoxin fused with a fragment of Pseudomonas exotoxin A.
Disease: Relapsed or refractory hairy cell leukemia previously treated with at least two prior systemic therapies, including a purine nucleoside analogue.
Dose: Intravenously as 0.04 mg/kg.
AE: Hemolytic uremic syndrome.
1053 trial (NCT01829711): 30% of the patients who received moxetumomab pasudotox-tdfk had a durable complete response confirmed by maintenance hematologic remission.
Talazoparib (Talzenna)
Class: Poly (ADP-ribose) polymerase (PARP) inhibitor.
Disease: gBRCAm HER2-negative locally advanced or metastatic breast cancer.
Dose: 1 mg orally per day.
EMBRACA trial (NCT01945775): Patients who received talazoparib demonstrated significantly longer progression-free survival, with a median of 8.6 months versis 5.6 months in the control arm.
Dr. Bryer is a resident in the department of internal medicine at the University of Pennsylvania, Philadelphia. Dr. Mentzer is chief of hematology-oncology at Pennsylvania Hospital and professor of medicine at the University of Pennsylvania. Dr. Henry is a hematologist-oncologist at Pennsylvania Hospital and a professor of medicine at the University of Pennsylvania.
Advances in genomics and technology perpetually change and improve therapies in oncology. Enhanced comprehension of cellular signaling, division, and replication has created a platform to selectively restrict neoplastic growth while preserving the integrity of benign cells.
This article reviews therapies that were newly approved in 2018, as well as those previously approved whose indications were expanded this past year. The list highlights the most clinically important approvals, as well as adverse events that are unique or especially severe.
Click on the PDF above to download the full article and charts in an easy-to-print format.
Apalutamide (Erleada)
Class: Androgen receptor inhibitor.
Disease: Nonmetastatic castration-resistant prostate cancer.
Dose: 240 mg orally, once daily.
Adverse Events (AEs): Hyperkalemia and increased risks of seizures, falls, and fractures.
Phase 3 SPARTAN trial (NCT01946204): 40.5-month metastasis-free survival rate, compared with 16.2 months in the placebo group.
Cemiplimab (Libtayo)
Class: Antibody against programmed cell death protein-1 (PD-1).
Disease: Metastatic cutaneous squamous cell carcinoma (CSCC) or locally advanced CSCC that is ineligible for curative surgery/radiation.
Dose: 350 mg intravenous infusion every 3 weeks.
AEs: Pneumonitis, autoimmune myocarditis, hepatitis, and aseptic meningitis.
1423 and 1540 trials (NCT02383212 and NCT02760498): 47.2% of patients who received cemiplimab had complete disappearance of the tumor or a decrease in tumor size.
Dacomitinib (Vizimpro)
Class: Second-generation tyrosine kinase inhibitor.
Disease: Metastatic non–small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) exon 19 deletion or exon 21 L858R substitution mutation.
Dose: 45 mg orally once daily.
AEs: Dermatotoxicity and diarrhea.
ARCHER1050 trial (NCT01774721): Patients who received dacomitinib demonstrated an improved overall survival, with a median of 34.1 months, compared with 26.8 months with gefitinib.
Duvelisib (Copiktra)
Class: Dual inhibitor of phosphatidylinositol 3-kinase delta and gamma.
Disease: Relapsed or refractory chronic lymphocytic leukemia (CLL), small lymphocytic lymphoma, or relapsed or refractory follicular lymphoma after at least two prior systemic therapies.
Dose: 25 mg orally twice daily.
AEs: Infection, diarrhea or colitis, and pneumonia.
Phase 3 DUO trial (NCT02004522): Progression-free survival in the duvelisib arm was 7.3 months longer than that in the ofatumumab arm. The overall response rate for patients receiving duvelisib was 78%, compared with 39% for those receiving ofatumumab.
Gilteritinib (Xospata)
Class: Inhibits the FLT3 internal tandem duplication (ITD) and FLT3 tyrosine kinase domain (TKD).
Disease: Relapsed or refractory acute myeloid leukemia (AML) with an FLT3 mutation.
Dose: 120 mg orally daily.
ADMIRAL trial (NCT02421939): 21% of the patients who received gilteritinib exhibited complete remission or complete remission with partial hematologic recovery.
Glasdegib (Daurismo)
Class: Hedgehog pathway inhibitor.
Disease: Adults over age 75 years with newly diagnosed AML and other medical comorbidities that preclude them from intensive chemotherapy.
Dose: The recommended dose is 100 mg orally continuously in 28-day cycles.
AE: QT prolongation and embryo-fetal toxicity
Phase 2 BRIGHT 1003 trial (NCT01546038): 3.9-month overall survival advantage for glasdegib plus cytarabine, compared with cytarabine alone. Overall, 15% of the glasdegib plus low dose cytarabine arm achieved complete remission, compared with the 1% complete remission rate in patients who received cytarabine alone.
Iobenguane I 131 (Azedra)
Class: Radiopharmaceutical agent; induces cell death within the noradrenaline transporter.
Disease: Iobenguane scan–positive, unresectable, locally advanced or metastatic pheochromocytoma or paraganglioma
Dose: Initial intravenous dosimetric dose, followed by two therapeutic doses.
AE: Pancytopenia and elevated international normalized ratio (INR).
IB12B trial (NCT00874614): One-quarter of patients receiving this therapy had at least a 50% reduction in the dose and number of antihypertensives for at least 6 months; almost all patients had a tumor response.
Ivosidenib (Tibsovo)
Class: Small-molecule inhibitor of mutant isocitrate dehydrogenase (IDH1).
Disease: Refractory AML and an IDH1 mutation
Dose: 500 mg orally daily.
AG120-C-001 trial (NCT02074839): Overall response rate of 41.6% in patients who received ivosidenib, with a 30.4% rate of complete remission or complete remission with partial hematologic recovery.
Larotrectinib (Vitrakvi)
Class: Oral tyrosine kinase inhibitor.
Disease: Advanced solid tumors harboring a neurotrophic tyrosine receptor kinase (NTRK) gene fusion.
Dose: 100 mg orally twice daily.
LOXO-TRK-14001, SCOUT, and NAVIGATE trials (NCT02122913, NCT02637687, and NCT02576431): Patients who received larotrectinib had durable responses regardless of patient age, tumor type, and fusion status.
Lutetium Lu 177 dotatate (Lutathera)
Class: Radiolabeled somatostatin analogue.
Disease: Somatostatin receptor–positive gastroenteropancreatic neuroendocrine tumors (GEP-NETs).
Dose: Intravenous infusion 7.4 GBq (200 mCi) every 8 weeks for a total of four doses.
NETTER-1 trial (NCT01578239): 65% of adults who received lutetium Lu 177 showed improved progression-free survival at 20 months, compared with just 10.8% in the control group.
Mogamulizumab (Poteligeo)
Class: Monoclonal antibody that binds to a protein (CC chemokine receptor type 4).
Disease: Relapsed or refractory mycosis fungoides or Sézary syndrome.
Dose: Intravenous infusion 1 mg/kg.
AE: Dermatologic toxicity.
MAVORIC trial (NCT01728805): Patients who received mogamulizumab had improved progression-free survival (median 7.7 months), compared with those taking vorinostat (median 3.1 months).
Moxetumomab pasudotox-tdfk (Lumoxiti)
Class: CD22-directed cytotoxin fused with a fragment of Pseudomonas exotoxin A.
Disease: Relapsed or refractory hairy cell leukemia previously treated with at least two prior systemic therapies, including a purine nucleoside analogue.
Dose: Intravenously as 0.04 mg/kg.
AE: Hemolytic uremic syndrome.
1053 trial (NCT01829711): 30% of the patients who received moxetumomab pasudotox-tdfk had a durable complete response confirmed by maintenance hematologic remission.
Talazoparib (Talzenna)
Class: Poly (ADP-ribose) polymerase (PARP) inhibitor.
Disease: gBRCAm HER2-negative locally advanced or metastatic breast cancer.
Dose: 1 mg orally per day.
EMBRACA trial (NCT01945775): Patients who received talazoparib demonstrated significantly longer progression-free survival, with a median of 8.6 months versis 5.6 months in the control arm.
Dr. Bryer is a resident in the department of internal medicine at the University of Pennsylvania, Philadelphia. Dr. Mentzer is chief of hematology-oncology at Pennsylvania Hospital and professor of medicine at the University of Pennsylvania. Dr. Henry is a hematologist-oncologist at Pennsylvania Hospital and a professor of medicine at the University of Pennsylvania.
Advances in genomics and technology perpetually change and improve therapies in oncology. Enhanced comprehension of cellular signaling, division, and replication has created a platform to selectively restrict neoplastic growth while preserving the integrity of benign cells.
This article reviews therapies that were newly approved in 2018, as well as those previously approved whose indications were expanded this past year. The list highlights the most clinically important approvals, as well as adverse events that are unique or especially severe.
Click on the PDF above to download the full article and charts in an easy-to-print format.
Apalutamide (Erleada)
Class: Androgen receptor inhibitor.
Disease: Nonmetastatic castration-resistant prostate cancer.
Dose: 240 mg orally, once daily.
Adverse Events (AEs): Hyperkalemia and increased risks of seizures, falls, and fractures.
Phase 3 SPARTAN trial (NCT01946204): 40.5-month metastasis-free survival rate, compared with 16.2 months in the placebo group.
Cemiplimab (Libtayo)
Class: Antibody against programmed cell death protein-1 (PD-1).
Disease: Metastatic cutaneous squamous cell carcinoma (CSCC) or locally advanced CSCC that is ineligible for curative surgery/radiation.
Dose: 350 mg intravenous infusion every 3 weeks.
AEs: Pneumonitis, autoimmune myocarditis, hepatitis, and aseptic meningitis.
1423 and 1540 trials (NCT02383212 and NCT02760498): 47.2% of patients who received cemiplimab had complete disappearance of the tumor or a decrease in tumor size.
Dacomitinib (Vizimpro)
Class: Second-generation tyrosine kinase inhibitor.
Disease: Metastatic non–small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) exon 19 deletion or exon 21 L858R substitution mutation.
Dose: 45 mg orally once daily.
AEs: Dermatotoxicity and diarrhea.
ARCHER1050 trial (NCT01774721): Patients who received dacomitinib demonstrated an improved overall survival, with a median of 34.1 months, compared with 26.8 months with gefitinib.
Duvelisib (Copiktra)
Class: Dual inhibitor of phosphatidylinositol 3-kinase delta and gamma.
Disease: Relapsed or refractory chronic lymphocytic leukemia (CLL), small lymphocytic lymphoma, or relapsed or refractory follicular lymphoma after at least two prior systemic therapies.
Dose: 25 mg orally twice daily.
AEs: Infection, diarrhea or colitis, and pneumonia.
Phase 3 DUO trial (NCT02004522): Progression-free survival in the duvelisib arm was 7.3 months longer than that in the ofatumumab arm. The overall response rate for patients receiving duvelisib was 78%, compared with 39% for those receiving ofatumumab.
Gilteritinib (Xospata)
Class: Inhibits the FLT3 internal tandem duplication (ITD) and FLT3 tyrosine kinase domain (TKD).
Disease: Relapsed or refractory acute myeloid leukemia (AML) with an FLT3 mutation.
Dose: 120 mg orally daily.
ADMIRAL trial (NCT02421939): 21% of the patients who received gilteritinib exhibited complete remission or complete remission with partial hematologic recovery.
Glasdegib (Daurismo)
Class: Hedgehog pathway inhibitor.
Disease: Adults over age 75 years with newly diagnosed AML and other medical comorbidities that preclude them from intensive chemotherapy.
Dose: The recommended dose is 100 mg orally continuously in 28-day cycles.
AE: QT prolongation and embryo-fetal toxicity
Phase 2 BRIGHT 1003 trial (NCT01546038): 3.9-month overall survival advantage for glasdegib plus cytarabine, compared with cytarabine alone. Overall, 15% of the glasdegib plus low dose cytarabine arm achieved complete remission, compared with the 1% complete remission rate in patients who received cytarabine alone.
Iobenguane I 131 (Azedra)
Class: Radiopharmaceutical agent; induces cell death within the noradrenaline transporter.
Disease: Iobenguane scan–positive, unresectable, locally advanced or metastatic pheochromocytoma or paraganglioma
Dose: Initial intravenous dosimetric dose, followed by two therapeutic doses.
AE: Pancytopenia and elevated international normalized ratio (INR).
IB12B trial (NCT00874614): One-quarter of patients receiving this therapy had at least a 50% reduction in the dose and number of antihypertensives for at least 6 months; almost all patients had a tumor response.
Ivosidenib (Tibsovo)
Class: Small-molecule inhibitor of mutant isocitrate dehydrogenase (IDH1).
Disease: Refractory AML and an IDH1 mutation
Dose: 500 mg orally daily.
AG120-C-001 trial (NCT02074839): Overall response rate of 41.6% in patients who received ivosidenib, with a 30.4% rate of complete remission or complete remission with partial hematologic recovery.
Larotrectinib (Vitrakvi)
Class: Oral tyrosine kinase inhibitor.
Disease: Advanced solid tumors harboring a neurotrophic tyrosine receptor kinase (NTRK) gene fusion.
Dose: 100 mg orally twice daily.
LOXO-TRK-14001, SCOUT, and NAVIGATE trials (NCT02122913, NCT02637687, and NCT02576431): Patients who received larotrectinib had durable responses regardless of patient age, tumor type, and fusion status.
Lutetium Lu 177 dotatate (Lutathera)
Class: Radiolabeled somatostatin analogue.
Disease: Somatostatin receptor–positive gastroenteropancreatic neuroendocrine tumors (GEP-NETs).
Dose: Intravenous infusion 7.4 GBq (200 mCi) every 8 weeks for a total of four doses.
NETTER-1 trial (NCT01578239): 65% of adults who received lutetium Lu 177 showed improved progression-free survival at 20 months, compared with just 10.8% in the control group.
Mogamulizumab (Poteligeo)
Class: Monoclonal antibody that binds to a protein (CC chemokine receptor type 4).
Disease: Relapsed or refractory mycosis fungoides or Sézary syndrome.
Dose: Intravenous infusion 1 mg/kg.
AE: Dermatologic toxicity.
MAVORIC trial (NCT01728805): Patients who received mogamulizumab had improved progression-free survival (median 7.7 months), compared with those taking vorinostat (median 3.1 months).
Moxetumomab pasudotox-tdfk (Lumoxiti)
Class: CD22-directed cytotoxin fused with a fragment of Pseudomonas exotoxin A.
Disease: Relapsed or refractory hairy cell leukemia previously treated with at least two prior systemic therapies, including a purine nucleoside analogue.
Dose: Intravenously as 0.04 mg/kg.
AE: Hemolytic uremic syndrome.
1053 trial (NCT01829711): 30% of the patients who received moxetumomab pasudotox-tdfk had a durable complete response confirmed by maintenance hematologic remission.
Talazoparib (Talzenna)
Class: Poly (ADP-ribose) polymerase (PARP) inhibitor.
Disease: gBRCAm HER2-negative locally advanced or metastatic breast cancer.
Dose: 1 mg orally per day.
EMBRACA trial (NCT01945775): Patients who received talazoparib demonstrated significantly longer progression-free survival, with a median of 8.6 months versis 5.6 months in the control arm.
Dr. Bryer is a resident in the department of internal medicine at the University of Pennsylvania, Philadelphia. Dr. Mentzer is chief of hematology-oncology at Pennsylvania Hospital and professor of medicine at the University of Pennsylvania. Dr. Henry is a hematologist-oncologist at Pennsylvania Hospital and a professor of medicine at the University of Pennsylvania.
ACE inhibitors may improve neuropsychiatric lupus
SAN FRANCISCO – The announcement of a first-ever multicenter randomized trial of ACE inhibitors for the treatment of cognitive impairment in systemic lupus erythematosus patients generated some of the biggest buzz at LUPUS 2019.
“The time has come to implement clinical trials in lupus to see if we can address what is one of the most distressing aspects of the disease to patients,” Betty Diamond, MD, said in announcing the planned trial at an international congress on systemic lupus erythematosus (SLE).
Neuropsychiatric lupus, characterized most often by cognitive impairment, affects 40%-90% of SLE patients, according to various epidemiologic studies. This confusion and memory loss has been shown to be independent of systemic disease activity.
“Cognitive impairment is a very common problem and a very insidious problem in lupus patients,” emphasized Dr. Diamond, professor and head of the Center for Autoimmune Musculoskeletal and Hematopoietic Diseases at the Feinstein Institute for Medical Research in Manhasset, New York.
Dr. Diamond was the recipient of the 2018 Lupus Insight Prize awarded by the Lupus Research Alliance, which is funding the multicenter randomized trial. For the study, roughly 70 SLE patients with cognitive impairment not associated with a focal brain lesion will be randomized to receive a centrally acting ACE inhibitor – captopril was the one she and her coinvestigators have used in their mouse model and preliminary clinical studies – or to a non–centrally acting ACE inhibitor, such as enalapril.
“The clinical trial compares an ACE inhibitor that crosses the blood-brain barrier with one that doesn’t. They both have the same renal protection and systemic anti-inflammatory effects,” explained Dr. Diamond, who is also professor of molecular medicine and of medicine at the Zucker School of Medicine at Hofstra/Northwell, East Garden City, N.Y.
In a recent publication (J Exp Med. 2018 Oct 1;215[10]:2554-66), Dr. Diamond and her coinvestigators presented much of the background work that underpins the upcoming randomized trial. Sixteen years ago, they discovered two anti-DNA antibodies that cross-react with the N-methyl-D-aspartate receptor (NMDAR) and enhance NMDAR signaling. They showed that while the NMDARs are critical in learning and memory, their prolonged stimulation results in a high degree of calcium influx, causing neuronal death. These anti-DNA/anti-NMDAR antibodies, known as DNRAbs, are present at elevated titers in 30%-40% of SLE patients, and in a higher proportion of those with neuropsychiatric lupus.
“Most importantly, DNRAbs are present in the cerebrospinal fluid of patients who have nonfocal CNS manifestations of lupus,” Dr. Diamond said.
She and her coworkers developed a mouse model of cognitive impairment in lupus and utilized it to identify a two-stage model of the pathogenesis of DNRAb-mediated neuropsychiatric lupus. First, a traumatic event such as an infection causes a temporary opening in the blood-brain barrier, allowing the DNRAbs to reach the brain. This results in acute excitotoxic neuronal death. This is followed by a second stage, which entails activation of microglia – known as the macrophages of the CNS – with resultant loss of neuronal dendritic arborization and complexity. In the mouse model, this causes a selective impairment in spatial memory that corresponds well to the spatial memory deficit the investigators documented in DNRAb-positive SLE patients, compared with healthy controls and DNRAb-negative lupus patients.
A key finding in this project, Dr. Diamond continued, was that activated microglia turn out to be critical for dendritic loss. If the microglia are deactivated, regrowth of the dendritic processes occurs. This raises a possibility that attendees at LUPUS 2019 found thrilling: Perhaps the cognitive impairment of neuropsychiatric SLE can be prevented and even reversed by suppressing microglial activation.
Promising work in the field of Alzheimer’s disease suggests that centrally acting ACE inhibitors can indeed suppress microglial activation and actually improve cognition. Dr. Diamond and her colleagues showed this also was the case in their mouse model. Moreover, in a small clinical study they used PET brain imaging to show that captopril reduced the increased glucose uptake and hippocampal hypermetabolism associated with DNRAb-positive neuropsychiatric lupus, an effect maintained through 18 months of follow-up.
“We think DNRAbs contribute to cognitive impairment in SLE patients, but we certainly wouldn’t say that antibodies are the only mechanism. Other investigators have shown that interferon can also do this, and that, like the antibodies, interferon acts through microglial activation. We think that this microglial activation is going to be a general paradigm in cognitive impairment in lupus and in other diseases, so microglia are a very good therapeutic target,” Dr. Diamond said.
The primary outcomes in the forthcoming randomized trial involve PET neuronal imaging. They investigators are hoping to see reduced hippocampal hypermetabolism and suppression of activated microglial cells.
“We’ll see if we’re actually hitting our target. We’re doing neuropsychologic testing, too, but we’re really concentrating on imaging outcomes, because those are objective and have many fewer variables to confound them,” according to Dr. Diamond.
Microglial activation is a current topic of intense research interest within the pharmaceutical industry, she added. If the imaging study is positive, she anticipates drug companies will quickly ramp up and conduct large clinical trials powered to show significant results in terms of neuropsychologic test scores and clinical outcomes.
Dr. Diamond reported having no financial conflicts regarding her work, which has been supported largely by the National Institutes of Health.
SAN FRANCISCO – The announcement of a first-ever multicenter randomized trial of ACE inhibitors for the treatment of cognitive impairment in systemic lupus erythematosus patients generated some of the biggest buzz at LUPUS 2019.
“The time has come to implement clinical trials in lupus to see if we can address what is one of the most distressing aspects of the disease to patients,” Betty Diamond, MD, said in announcing the planned trial at an international congress on systemic lupus erythematosus (SLE).
Neuropsychiatric lupus, characterized most often by cognitive impairment, affects 40%-90% of SLE patients, according to various epidemiologic studies. This confusion and memory loss has been shown to be independent of systemic disease activity.
“Cognitive impairment is a very common problem and a very insidious problem in lupus patients,” emphasized Dr. Diamond, professor and head of the Center for Autoimmune Musculoskeletal and Hematopoietic Diseases at the Feinstein Institute for Medical Research in Manhasset, New York.
Dr. Diamond was the recipient of the 2018 Lupus Insight Prize awarded by the Lupus Research Alliance, which is funding the multicenter randomized trial. For the study, roughly 70 SLE patients with cognitive impairment not associated with a focal brain lesion will be randomized to receive a centrally acting ACE inhibitor – captopril was the one she and her coinvestigators have used in their mouse model and preliminary clinical studies – or to a non–centrally acting ACE inhibitor, such as enalapril.
“The clinical trial compares an ACE inhibitor that crosses the blood-brain barrier with one that doesn’t. They both have the same renal protection and systemic anti-inflammatory effects,” explained Dr. Diamond, who is also professor of molecular medicine and of medicine at the Zucker School of Medicine at Hofstra/Northwell, East Garden City, N.Y.
In a recent publication (J Exp Med. 2018 Oct 1;215[10]:2554-66), Dr. Diamond and her coinvestigators presented much of the background work that underpins the upcoming randomized trial. Sixteen years ago, they discovered two anti-DNA antibodies that cross-react with the N-methyl-D-aspartate receptor (NMDAR) and enhance NMDAR signaling. They showed that while the NMDARs are critical in learning and memory, their prolonged stimulation results in a high degree of calcium influx, causing neuronal death. These anti-DNA/anti-NMDAR antibodies, known as DNRAbs, are present at elevated titers in 30%-40% of SLE patients, and in a higher proportion of those with neuropsychiatric lupus.
“Most importantly, DNRAbs are present in the cerebrospinal fluid of patients who have nonfocal CNS manifestations of lupus,” Dr. Diamond said.
She and her coworkers developed a mouse model of cognitive impairment in lupus and utilized it to identify a two-stage model of the pathogenesis of DNRAb-mediated neuropsychiatric lupus. First, a traumatic event such as an infection causes a temporary opening in the blood-brain barrier, allowing the DNRAbs to reach the brain. This results in acute excitotoxic neuronal death. This is followed by a second stage, which entails activation of microglia – known as the macrophages of the CNS – with resultant loss of neuronal dendritic arborization and complexity. In the mouse model, this causes a selective impairment in spatial memory that corresponds well to the spatial memory deficit the investigators documented in DNRAb-positive SLE patients, compared with healthy controls and DNRAb-negative lupus patients.
A key finding in this project, Dr. Diamond continued, was that activated microglia turn out to be critical for dendritic loss. If the microglia are deactivated, regrowth of the dendritic processes occurs. This raises a possibility that attendees at LUPUS 2019 found thrilling: Perhaps the cognitive impairment of neuropsychiatric SLE can be prevented and even reversed by suppressing microglial activation.
Promising work in the field of Alzheimer’s disease suggests that centrally acting ACE inhibitors can indeed suppress microglial activation and actually improve cognition. Dr. Diamond and her colleagues showed this also was the case in their mouse model. Moreover, in a small clinical study they used PET brain imaging to show that captopril reduced the increased glucose uptake and hippocampal hypermetabolism associated with DNRAb-positive neuropsychiatric lupus, an effect maintained through 18 months of follow-up.
“We think DNRAbs contribute to cognitive impairment in SLE patients, but we certainly wouldn’t say that antibodies are the only mechanism. Other investigators have shown that interferon can also do this, and that, like the antibodies, interferon acts through microglial activation. We think that this microglial activation is going to be a general paradigm in cognitive impairment in lupus and in other diseases, so microglia are a very good therapeutic target,” Dr. Diamond said.
The primary outcomes in the forthcoming randomized trial involve PET neuronal imaging. They investigators are hoping to see reduced hippocampal hypermetabolism and suppression of activated microglial cells.
“We’ll see if we’re actually hitting our target. We’re doing neuropsychologic testing, too, but we’re really concentrating on imaging outcomes, because those are objective and have many fewer variables to confound them,” according to Dr. Diamond.
Microglial activation is a current topic of intense research interest within the pharmaceutical industry, she added. If the imaging study is positive, she anticipates drug companies will quickly ramp up and conduct large clinical trials powered to show significant results in terms of neuropsychologic test scores and clinical outcomes.
Dr. Diamond reported having no financial conflicts regarding her work, which has been supported largely by the National Institutes of Health.
SAN FRANCISCO – The announcement of a first-ever multicenter randomized trial of ACE inhibitors for the treatment of cognitive impairment in systemic lupus erythematosus patients generated some of the biggest buzz at LUPUS 2019.
“The time has come to implement clinical trials in lupus to see if we can address what is one of the most distressing aspects of the disease to patients,” Betty Diamond, MD, said in announcing the planned trial at an international congress on systemic lupus erythematosus (SLE).
Neuropsychiatric lupus, characterized most often by cognitive impairment, affects 40%-90% of SLE patients, according to various epidemiologic studies. This confusion and memory loss has been shown to be independent of systemic disease activity.
“Cognitive impairment is a very common problem and a very insidious problem in lupus patients,” emphasized Dr. Diamond, professor and head of the Center for Autoimmune Musculoskeletal and Hematopoietic Diseases at the Feinstein Institute for Medical Research in Manhasset, New York.
Dr. Diamond was the recipient of the 2018 Lupus Insight Prize awarded by the Lupus Research Alliance, which is funding the multicenter randomized trial. For the study, roughly 70 SLE patients with cognitive impairment not associated with a focal brain lesion will be randomized to receive a centrally acting ACE inhibitor – captopril was the one she and her coinvestigators have used in their mouse model and preliminary clinical studies – or to a non–centrally acting ACE inhibitor, such as enalapril.
“The clinical trial compares an ACE inhibitor that crosses the blood-brain barrier with one that doesn’t. They both have the same renal protection and systemic anti-inflammatory effects,” explained Dr. Diamond, who is also professor of molecular medicine and of medicine at the Zucker School of Medicine at Hofstra/Northwell, East Garden City, N.Y.
In a recent publication (J Exp Med. 2018 Oct 1;215[10]:2554-66), Dr. Diamond and her coinvestigators presented much of the background work that underpins the upcoming randomized trial. Sixteen years ago, they discovered two anti-DNA antibodies that cross-react with the N-methyl-D-aspartate receptor (NMDAR) and enhance NMDAR signaling. They showed that while the NMDARs are critical in learning and memory, their prolonged stimulation results in a high degree of calcium influx, causing neuronal death. These anti-DNA/anti-NMDAR antibodies, known as DNRAbs, are present at elevated titers in 30%-40% of SLE patients, and in a higher proportion of those with neuropsychiatric lupus.
“Most importantly, DNRAbs are present in the cerebrospinal fluid of patients who have nonfocal CNS manifestations of lupus,” Dr. Diamond said.
She and her coworkers developed a mouse model of cognitive impairment in lupus and utilized it to identify a two-stage model of the pathogenesis of DNRAb-mediated neuropsychiatric lupus. First, a traumatic event such as an infection causes a temporary opening in the blood-brain barrier, allowing the DNRAbs to reach the brain. This results in acute excitotoxic neuronal death. This is followed by a second stage, which entails activation of microglia – known as the macrophages of the CNS – with resultant loss of neuronal dendritic arborization and complexity. In the mouse model, this causes a selective impairment in spatial memory that corresponds well to the spatial memory deficit the investigators documented in DNRAb-positive SLE patients, compared with healthy controls and DNRAb-negative lupus patients.
A key finding in this project, Dr. Diamond continued, was that activated microglia turn out to be critical for dendritic loss. If the microglia are deactivated, regrowth of the dendritic processes occurs. This raises a possibility that attendees at LUPUS 2019 found thrilling: Perhaps the cognitive impairment of neuropsychiatric SLE can be prevented and even reversed by suppressing microglial activation.
Promising work in the field of Alzheimer’s disease suggests that centrally acting ACE inhibitors can indeed suppress microglial activation and actually improve cognition. Dr. Diamond and her colleagues showed this also was the case in their mouse model. Moreover, in a small clinical study they used PET brain imaging to show that captopril reduced the increased glucose uptake and hippocampal hypermetabolism associated with DNRAb-positive neuropsychiatric lupus, an effect maintained through 18 months of follow-up.
“We think DNRAbs contribute to cognitive impairment in SLE patients, but we certainly wouldn’t say that antibodies are the only mechanism. Other investigators have shown that interferon can also do this, and that, like the antibodies, interferon acts through microglial activation. We think that this microglial activation is going to be a general paradigm in cognitive impairment in lupus and in other diseases, so microglia are a very good therapeutic target,” Dr. Diamond said.
The primary outcomes in the forthcoming randomized trial involve PET neuronal imaging. They investigators are hoping to see reduced hippocampal hypermetabolism and suppression of activated microglial cells.
“We’ll see if we’re actually hitting our target. We’re doing neuropsychologic testing, too, but we’re really concentrating on imaging outcomes, because those are objective and have many fewer variables to confound them,” according to Dr. Diamond.
Microglial activation is a current topic of intense research interest within the pharmaceutical industry, she added. If the imaging study is positive, she anticipates drug companies will quickly ramp up and conduct large clinical trials powered to show significant results in terms of neuropsychologic test scores and clinical outcomes.
Dr. Diamond reported having no financial conflicts regarding her work, which has been supported largely by the National Institutes of Health.
REPORTING FROM LUPUS 2019
Key clinical point: ACE inhibitor therapy may prevent and/or improve neuropsychiatric lupus.
Major finding: The first-ever multicenter randomized trial of ACE inhibitor therapy for neuropsychiatric SLE will soon get underway.
Study details: The planned – and funded – trial will include roughly 70 patients with neuropsychiatric lupus.
Disclosures: The presenter reported having no financial conflicts regarding her work, which has been supported largely by the National Institutes of Health.
Dr. Louis Weiner: AACR presentations highlight new “transformative strategies”
ATLANTA – Several studies featured during a press briefing at the annual meeting of the American Association for Cancer Research highlight the types of “transformative strategies” currently being developed and implemented, according to Louis Weiner, MD.
“Had this been the AACR [meeting] 20 years ago ... each one of them would have been a main plenary presentation and would have been the talk of the meeting,” Dr. Weiner, director of the Georgetown Lombardi Cancer Center at Georgetown University, Washington, and press briefing moderator, said of the findings.
While they are well accepted as being high quality presentations of great value, they don’t cause the same amount of stir, he said, adding: “I wouldn’t say we’re jaded, but we’ve come to the point where we almost expect great results at these meetings, and isn’t that wonderful?”
In this video interview he discussed the findings of two of the studies, including the phase 2 UNITY-NHL study and a preclinical Lynch syndrome mouse model used to develop a potential cancer preventive vaccine.
The Lynch syndrome data “suggest the strong possibility that we might be able to immunize people and combine that treatment with standard nonsteroidal anti-inflammatory agents such as naproxen to delay or reduce the impact of Lynch syndrome.”
“This set of findings ... opens the door to investigators in many different areas of cancer research to explore whether or not there are common frameshift mutations that might create novel neoantigens that we can go after with vaccines – be they for therapeutic benefit or for prevention,” he said.
The UNITY-NHL study, which showed that umbralisib is active and well tolerated as single-agent therapy in patients with relapsed or refractory marginal zone lymphoma, suggests “it’s quite possible that [the phosphoinositide 3-kinase delta inhibitor] is going to become a very important element in the treatment of patients with marginal zone lymphomas, and obviously it can be then used in earlier stages of diseases since it’s well tolerated, and it may well have useful activity in other B-cell malignancies,” he said.
Dr. Weiner reported having no relevant disclosures.
ATLANTA – Several studies featured during a press briefing at the annual meeting of the American Association for Cancer Research highlight the types of “transformative strategies” currently being developed and implemented, according to Louis Weiner, MD.
“Had this been the AACR [meeting] 20 years ago ... each one of them would have been a main plenary presentation and would have been the talk of the meeting,” Dr. Weiner, director of the Georgetown Lombardi Cancer Center at Georgetown University, Washington, and press briefing moderator, said of the findings.
While they are well accepted as being high quality presentations of great value, they don’t cause the same amount of stir, he said, adding: “I wouldn’t say we’re jaded, but we’ve come to the point where we almost expect great results at these meetings, and isn’t that wonderful?”
In this video interview he discussed the findings of two of the studies, including the phase 2 UNITY-NHL study and a preclinical Lynch syndrome mouse model used to develop a potential cancer preventive vaccine.
The Lynch syndrome data “suggest the strong possibility that we might be able to immunize people and combine that treatment with standard nonsteroidal anti-inflammatory agents such as naproxen to delay or reduce the impact of Lynch syndrome.”
“This set of findings ... opens the door to investigators in many different areas of cancer research to explore whether or not there are common frameshift mutations that might create novel neoantigens that we can go after with vaccines – be they for therapeutic benefit or for prevention,” he said.
The UNITY-NHL study, which showed that umbralisib is active and well tolerated as single-agent therapy in patients with relapsed or refractory marginal zone lymphoma, suggests “it’s quite possible that [the phosphoinositide 3-kinase delta inhibitor] is going to become a very important element in the treatment of patients with marginal zone lymphomas, and obviously it can be then used in earlier stages of diseases since it’s well tolerated, and it may well have useful activity in other B-cell malignancies,” he said.
Dr. Weiner reported having no relevant disclosures.
ATLANTA – Several studies featured during a press briefing at the annual meeting of the American Association for Cancer Research highlight the types of “transformative strategies” currently being developed and implemented, according to Louis Weiner, MD.
“Had this been the AACR [meeting] 20 years ago ... each one of them would have been a main plenary presentation and would have been the talk of the meeting,” Dr. Weiner, director of the Georgetown Lombardi Cancer Center at Georgetown University, Washington, and press briefing moderator, said of the findings.
While they are well accepted as being high quality presentations of great value, they don’t cause the same amount of stir, he said, adding: “I wouldn’t say we’re jaded, but we’ve come to the point where we almost expect great results at these meetings, and isn’t that wonderful?”
In this video interview he discussed the findings of two of the studies, including the phase 2 UNITY-NHL study and a preclinical Lynch syndrome mouse model used to develop a potential cancer preventive vaccine.
The Lynch syndrome data “suggest the strong possibility that we might be able to immunize people and combine that treatment with standard nonsteroidal anti-inflammatory agents such as naproxen to delay or reduce the impact of Lynch syndrome.”
“This set of findings ... opens the door to investigators in many different areas of cancer research to explore whether or not there are common frameshift mutations that might create novel neoantigens that we can go after with vaccines – be they for therapeutic benefit or for prevention,” he said.
The UNITY-NHL study, which showed that umbralisib is active and well tolerated as single-agent therapy in patients with relapsed or refractory marginal zone lymphoma, suggests “it’s quite possible that [the phosphoinositide 3-kinase delta inhibitor] is going to become a very important element in the treatment of patients with marginal zone lymphomas, and obviously it can be then used in earlier stages of diseases since it’s well tolerated, and it may well have useful activity in other B-cell malignancies,” he said.
Dr. Weiner reported having no relevant disclosures.
REPORTING FROM AACR
Research coalition issues plan for curing hepatitis B virus
VIENNA – They hope either to have a cure or to have made substantial progress toward this goal over the next 10 years.
Treatments already are on the market that effectively inhibit hepatitis B replication in infected patients (and an effective preventive vaccine also exists). Still, these treatments are not curative, and for the vast majority of patients treatment must continue indefinitely, while their risk for liver cancer and their virally induced immune system abnormalities remain, Peter A. Revill, PhD, said during a press briefing that introduced a strategy for hepatitis B virus (HBV) cure development from the International Coalition to Eliminate HBV. Concurrently with the briefing session, the strategy appeared in an article published online (Lancet Gastroenterol Hepatol. 2019 Apr 10. doi: 10.1016/s2468-1253(19)30119-0).
The way forward will likely be a “two-pronged approach or restoring immune responses and targeting the virus,” Dr. Revill, head of molecular virology at the Doherty Institute in Melbourne, said in a video interview.
The new strategy recognizes the huge challenge of devising a treatment that produces a total cure that includes elimination of all traces of viral DNA from patients and for the immediate future focuses on the goal of functional cure. The term functional cure means a sustained period without detectable HBV surface antigen or HBV DNA in a patient’s serum, as well as suppressed virus release. Another feature of a functional cure would be a halt to progression of liver disease, replaced by liver regeneration, said Anna S. Lok, MD, professor of medicine and director of clinical hepatology at the University of Michigan, Ann Arbor, and a member of the strategy-writing group. She and her colleagues who wrote the strategy foresee the need for drug combinations with agents that can hit multiple viral targets as well as agents that restore normal immune function.
Several novel drug classes aimed at new viral targets, such as capsid inhibitors, are in various stages of clinical development, said Fabien Zoulim, MD, head of the gastroenterology and hepatology service at the Red Cross Hospital in Lyon, France, and another member of the writing panel. “We have many drug candidates” that use novel approaches to further restrict viral growth, roughly 50 agents in phase 1 and 2 studies, he said during the press briefing, held during the meeting sponsored by the European Association for the Study of the Liver. The other, immunologic aspect of the two-part cure strategy – restoring the “exhausted” HBV-specific T-cell population and stimulating production of neutralizing antibody to HBV – remains hypothetical right now, however. “It’s a concept that needs development,” Dr. Zoulim said.
A reason members of the coalition are optimistic about eventual prospects for a cure is that currently about 1% of patients on HBV antiviral treatments have a functional cure after relatively brief treatment, and the percentage of cured patients plateaus at about 10% among those who remain on current HBV antiviral drugs for several years. In addition, a substantial fraction of patients spontaneously resolve their HBV infection without any treatment. Experts estimate that more than 1 billion people worldwide have been infected by HBV and then later had their infection clear “naturally,” said Dr. Revill. But the mechanism by which this happens is currently a mystery. “We don’t know how or why” so many infected people are “cured” naturally, Dr. Revill admitted, but it gives him and his colleagues hope that the numbers can expand once more and better treatments for HBV infection are available.
VIENNA – They hope either to have a cure or to have made substantial progress toward this goal over the next 10 years.
Treatments already are on the market that effectively inhibit hepatitis B replication in infected patients (and an effective preventive vaccine also exists). Still, these treatments are not curative, and for the vast majority of patients treatment must continue indefinitely, while their risk for liver cancer and their virally induced immune system abnormalities remain, Peter A. Revill, PhD, said during a press briefing that introduced a strategy for hepatitis B virus (HBV) cure development from the International Coalition to Eliminate HBV. Concurrently with the briefing session, the strategy appeared in an article published online (Lancet Gastroenterol Hepatol. 2019 Apr 10. doi: 10.1016/s2468-1253(19)30119-0).
The way forward will likely be a “two-pronged approach or restoring immune responses and targeting the virus,” Dr. Revill, head of molecular virology at the Doherty Institute in Melbourne, said in a video interview.
The new strategy recognizes the huge challenge of devising a treatment that produces a total cure that includes elimination of all traces of viral DNA from patients and for the immediate future focuses on the goal of functional cure. The term functional cure means a sustained period without detectable HBV surface antigen or HBV DNA in a patient’s serum, as well as suppressed virus release. Another feature of a functional cure would be a halt to progression of liver disease, replaced by liver regeneration, said Anna S. Lok, MD, professor of medicine and director of clinical hepatology at the University of Michigan, Ann Arbor, and a member of the strategy-writing group. She and her colleagues who wrote the strategy foresee the need for drug combinations with agents that can hit multiple viral targets as well as agents that restore normal immune function.
Several novel drug classes aimed at new viral targets, such as capsid inhibitors, are in various stages of clinical development, said Fabien Zoulim, MD, head of the gastroenterology and hepatology service at the Red Cross Hospital in Lyon, France, and another member of the writing panel. “We have many drug candidates” that use novel approaches to further restrict viral growth, roughly 50 agents in phase 1 and 2 studies, he said during the press briefing, held during the meeting sponsored by the European Association for the Study of the Liver. The other, immunologic aspect of the two-part cure strategy – restoring the “exhausted” HBV-specific T-cell population and stimulating production of neutralizing antibody to HBV – remains hypothetical right now, however. “It’s a concept that needs development,” Dr. Zoulim said.
A reason members of the coalition are optimistic about eventual prospects for a cure is that currently about 1% of patients on HBV antiviral treatments have a functional cure after relatively brief treatment, and the percentage of cured patients plateaus at about 10% among those who remain on current HBV antiviral drugs for several years. In addition, a substantial fraction of patients spontaneously resolve their HBV infection without any treatment. Experts estimate that more than 1 billion people worldwide have been infected by HBV and then later had their infection clear “naturally,” said Dr. Revill. But the mechanism by which this happens is currently a mystery. “We don’t know how or why” so many infected people are “cured” naturally, Dr. Revill admitted, but it gives him and his colleagues hope that the numbers can expand once more and better treatments for HBV infection are available.
VIENNA – They hope either to have a cure or to have made substantial progress toward this goal over the next 10 years.
Treatments already are on the market that effectively inhibit hepatitis B replication in infected patients (and an effective preventive vaccine also exists). Still, these treatments are not curative, and for the vast majority of patients treatment must continue indefinitely, while their risk for liver cancer and their virally induced immune system abnormalities remain, Peter A. Revill, PhD, said during a press briefing that introduced a strategy for hepatitis B virus (HBV) cure development from the International Coalition to Eliminate HBV. Concurrently with the briefing session, the strategy appeared in an article published online (Lancet Gastroenterol Hepatol. 2019 Apr 10. doi: 10.1016/s2468-1253(19)30119-0).
The way forward will likely be a “two-pronged approach or restoring immune responses and targeting the virus,” Dr. Revill, head of molecular virology at the Doherty Institute in Melbourne, said in a video interview.
The new strategy recognizes the huge challenge of devising a treatment that produces a total cure that includes elimination of all traces of viral DNA from patients and for the immediate future focuses on the goal of functional cure. The term functional cure means a sustained period without detectable HBV surface antigen or HBV DNA in a patient’s serum, as well as suppressed virus release. Another feature of a functional cure would be a halt to progression of liver disease, replaced by liver regeneration, said Anna S. Lok, MD, professor of medicine and director of clinical hepatology at the University of Michigan, Ann Arbor, and a member of the strategy-writing group. She and her colleagues who wrote the strategy foresee the need for drug combinations with agents that can hit multiple viral targets as well as agents that restore normal immune function.
Several novel drug classes aimed at new viral targets, such as capsid inhibitors, are in various stages of clinical development, said Fabien Zoulim, MD, head of the gastroenterology and hepatology service at the Red Cross Hospital in Lyon, France, and another member of the writing panel. “We have many drug candidates” that use novel approaches to further restrict viral growth, roughly 50 agents in phase 1 and 2 studies, he said during the press briefing, held during the meeting sponsored by the European Association for the Study of the Liver. The other, immunologic aspect of the two-part cure strategy – restoring the “exhausted” HBV-specific T-cell population and stimulating production of neutralizing antibody to HBV – remains hypothetical right now, however. “It’s a concept that needs development,” Dr. Zoulim said.
A reason members of the coalition are optimistic about eventual prospects for a cure is that currently about 1% of patients on HBV antiviral treatments have a functional cure after relatively brief treatment, and the percentage of cured patients plateaus at about 10% among those who remain on current HBV antiviral drugs for several years. In addition, a substantial fraction of patients spontaneously resolve their HBV infection without any treatment. Experts estimate that more than 1 billion people worldwide have been infected by HBV and then later had their infection clear “naturally,” said Dr. Revill. But the mechanism by which this happens is currently a mystery. “We don’t know how or why” so many infected people are “cured” naturally, Dr. Revill admitted, but it gives him and his colleagues hope that the numbers can expand once more and better treatments for HBV infection are available.
REPORTING FROM ILC 2019
Survey finds psoriasis patients seek relief with alternative therapies
Treatment (CAMs), despite limited documentation supporting their efficacy, reported Emily C. Murphy and her associates, in the department of dermatology, George Washington University, Washington.
They performed a survey-based statistical analysis to identify specific types of commonly used CAMs, and to explore reasons patients increasingly turn to alternative therapies. The survey was distributed in the National Psoriasis Foundation’s (NPF) October 2018 newsletter to its 100,927 members. Their results were published in a letter to the editor of the Journal of the American Academy of Dermatology.
Of the 6,101 NPF members who opened the newsletter, 324 clicked on the survey link. Of the 219 who completed the survey, almost 70% were women. The majority were white (84.1%), compared with Hispanic (6.2%), Asian (3.1%), and black (2.6%) participants. Most of the survey respondents had a dermatologist diagnosis of psoriasis, as well as access to health insurance to cover any prescribed medicines needed.
Of the 41% of respondents who reported using alternative therapies, usage was especially high among those who considered their psoriasis as severe (50% vs. 33.6% of those with nonsevere disease). Among the respondents, women were more likely than were men to use CAMs (45.6% vs. 26.5%, P = .002).
Only 4% cited access to care as a reason for choosing alternative therapies; the majority said they used CAMs because “traditional medications did not help or had side effects.”
While men were more likely than were women to use vitamins (24% vs. 18.9%, respectively), Dead Sea bath salts (17% vs. 7.8%), and cupping (3% vs. 0.8%), women were more likely to use herbals/botanicals (17% vs. 14%) and yoga (9.6% vs. 2%).
Patients with moderate psoriasis were significantly more likely than were those with mild or severe cases of the disease to recommend CAMs, regardless of insurance status (52.4% vs. 35% among those with mild disease and 40.4% for those with severe disease).
For some of the commonly used treatments, such as vitamins D and B12, there is insufficient evidence documenting their efficacy, although Dead Sea treatments have been shown to have therapeutic effects. And while there is efficacy evidence for indigo naturalis and meditation, these were not mentioned or were not commonly reported by respondents, the authors pointed out.
Although just 43% of patients said they would recommend a CAM to other people with psoriasis, its use remains widespread. For this reason, “educational initiatives that enable physicians to discuss evidence-based CAMs may improve patient satisfaction and outcomes,” observed Ms. Murphy, a research fellow, and her associates.
Previous studies have cited rates of CAM usage among patients with psoriasis as high as 62%, but researchers have failed to examine the reasons motivating usage. Not surprisingly, patients often use but misunderstand the benefits of alternative therapies.
“The onus is on us as physicians to not only ask our patients if they are using nonallopathic therapies for their psoriasis, but also to create an accepting environment that enables further discussion regarding said treatments to ensure patient safety and ultimately good outcomes,” senior author Adam Friedman, MD, professor and interim chair of dermatology at George Washington University, said in an interview.
The authors had no financial sources or conflicts of interest to disclose; there was no funding source.
SOURCE: Murphy E et al. J Am Acad Dermatol. 2019 Mar 29. pii: S0190-9622(19)30503-1. doi: 10.1016/j.jaad.2019.03.059.
Treatment (CAMs), despite limited documentation supporting their efficacy, reported Emily C. Murphy and her associates, in the department of dermatology, George Washington University, Washington.
They performed a survey-based statistical analysis to identify specific types of commonly used CAMs, and to explore reasons patients increasingly turn to alternative therapies. The survey was distributed in the National Psoriasis Foundation’s (NPF) October 2018 newsletter to its 100,927 members. Their results were published in a letter to the editor of the Journal of the American Academy of Dermatology.
Of the 6,101 NPF members who opened the newsletter, 324 clicked on the survey link. Of the 219 who completed the survey, almost 70% were women. The majority were white (84.1%), compared with Hispanic (6.2%), Asian (3.1%), and black (2.6%) participants. Most of the survey respondents had a dermatologist diagnosis of psoriasis, as well as access to health insurance to cover any prescribed medicines needed.
Of the 41% of respondents who reported using alternative therapies, usage was especially high among those who considered their psoriasis as severe (50% vs. 33.6% of those with nonsevere disease). Among the respondents, women were more likely than were men to use CAMs (45.6% vs. 26.5%, P = .002).
Only 4% cited access to care as a reason for choosing alternative therapies; the majority said they used CAMs because “traditional medications did not help or had side effects.”
While men were more likely than were women to use vitamins (24% vs. 18.9%, respectively), Dead Sea bath salts (17% vs. 7.8%), and cupping (3% vs. 0.8%), women were more likely to use herbals/botanicals (17% vs. 14%) and yoga (9.6% vs. 2%).
Patients with moderate psoriasis were significantly more likely than were those with mild or severe cases of the disease to recommend CAMs, regardless of insurance status (52.4% vs. 35% among those with mild disease and 40.4% for those with severe disease).
For some of the commonly used treatments, such as vitamins D and B12, there is insufficient evidence documenting their efficacy, although Dead Sea treatments have been shown to have therapeutic effects. And while there is efficacy evidence for indigo naturalis and meditation, these were not mentioned or were not commonly reported by respondents, the authors pointed out.
Although just 43% of patients said they would recommend a CAM to other people with psoriasis, its use remains widespread. For this reason, “educational initiatives that enable physicians to discuss evidence-based CAMs may improve patient satisfaction and outcomes,” observed Ms. Murphy, a research fellow, and her associates.
Previous studies have cited rates of CAM usage among patients with psoriasis as high as 62%, but researchers have failed to examine the reasons motivating usage. Not surprisingly, patients often use but misunderstand the benefits of alternative therapies.
“The onus is on us as physicians to not only ask our patients if they are using nonallopathic therapies for their psoriasis, but also to create an accepting environment that enables further discussion regarding said treatments to ensure patient safety and ultimately good outcomes,” senior author Adam Friedman, MD, professor and interim chair of dermatology at George Washington University, said in an interview.
The authors had no financial sources or conflicts of interest to disclose; there was no funding source.
SOURCE: Murphy E et al. J Am Acad Dermatol. 2019 Mar 29. pii: S0190-9622(19)30503-1. doi: 10.1016/j.jaad.2019.03.059.
Treatment (CAMs), despite limited documentation supporting their efficacy, reported Emily C. Murphy and her associates, in the department of dermatology, George Washington University, Washington.
They performed a survey-based statistical analysis to identify specific types of commonly used CAMs, and to explore reasons patients increasingly turn to alternative therapies. The survey was distributed in the National Psoriasis Foundation’s (NPF) October 2018 newsletter to its 100,927 members. Their results were published in a letter to the editor of the Journal of the American Academy of Dermatology.
Of the 6,101 NPF members who opened the newsletter, 324 clicked on the survey link. Of the 219 who completed the survey, almost 70% were women. The majority were white (84.1%), compared with Hispanic (6.2%), Asian (3.1%), and black (2.6%) participants. Most of the survey respondents had a dermatologist diagnosis of psoriasis, as well as access to health insurance to cover any prescribed medicines needed.
Of the 41% of respondents who reported using alternative therapies, usage was especially high among those who considered their psoriasis as severe (50% vs. 33.6% of those with nonsevere disease). Among the respondents, women were more likely than were men to use CAMs (45.6% vs. 26.5%, P = .002).
Only 4% cited access to care as a reason for choosing alternative therapies; the majority said they used CAMs because “traditional medications did not help or had side effects.”
While men were more likely than were women to use vitamins (24% vs. 18.9%, respectively), Dead Sea bath salts (17% vs. 7.8%), and cupping (3% vs. 0.8%), women were more likely to use herbals/botanicals (17% vs. 14%) and yoga (9.6% vs. 2%).
Patients with moderate psoriasis were significantly more likely than were those with mild or severe cases of the disease to recommend CAMs, regardless of insurance status (52.4% vs. 35% among those with mild disease and 40.4% for those with severe disease).
For some of the commonly used treatments, such as vitamins D and B12, there is insufficient evidence documenting their efficacy, although Dead Sea treatments have been shown to have therapeutic effects. And while there is efficacy evidence for indigo naturalis and meditation, these were not mentioned or were not commonly reported by respondents, the authors pointed out.
Although just 43% of patients said they would recommend a CAM to other people with psoriasis, its use remains widespread. For this reason, “educational initiatives that enable physicians to discuss evidence-based CAMs may improve patient satisfaction and outcomes,” observed Ms. Murphy, a research fellow, and her associates.
Previous studies have cited rates of CAM usage among patients with psoriasis as high as 62%, but researchers have failed to examine the reasons motivating usage. Not surprisingly, patients often use but misunderstand the benefits of alternative therapies.
“The onus is on us as physicians to not only ask our patients if they are using nonallopathic therapies for their psoriasis, but also to create an accepting environment that enables further discussion regarding said treatments to ensure patient safety and ultimately good outcomes,” senior author Adam Friedman, MD, professor and interim chair of dermatology at George Washington University, said in an interview.
The authors had no financial sources or conflicts of interest to disclose; there was no funding source.
SOURCE: Murphy E et al. J Am Acad Dermatol. 2019 Mar 29. pii: S0190-9622(19)30503-1. doi: 10.1016/j.jaad.2019.03.059.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
FDA to expand opioid labeling with instructions on proper tapering
The Food and Drug Administration is making changes to opioid analgesic labeling to give better information to clinicians on how to properly taper patients dependent on opioid use, according to Douglas Throckmorton, MD, deputy director for regulatory programs in the FDA’s Center for Drug Evaluation and Research.
, such as withdrawal symptoms, uncontrolled pain, and suicide. Both the FDA and the Centers for Disease Control and Prevention offer guidelines on how to properly taper opioids, Dr. Throckmorton said, but more needs to be done to ensure that patients are being provided with the correct advice and care.
The changes to the labels will include expanded information to health care clinicians and are intended to be used when both the clinician and patient have agreed to reduce the opioid dosage. When this is discussed, factors that should be considered include the dose of the drug, the duration of treatment, the type of pain being treated, and the physical and psychological attributes of the patient.
Other actions the FDA is pursuing to combat opioid use disorder include working with the National Academies of Sciences, Engineering, and Medicine on guidelines for the proper opioid analgesic prescribing for acute pain resulting from specific conditions or procedures, and advancing policies that make immediate-release opioid formulations available in fixed-quantity packaging for 1 or 2 days.
“The FDA remains committed to addressing the opioid crisis on all fronts, with a significant focus on decreasing unnecessary exposure to opioids and preventing new addiction; supporting the treatment of those with opioid use disorder; fostering the development of novel pain treatment therapies and opioids more resistant to abuse and misuse; and taking action against those involved in the illegal importation and sale of opioids,” Dr. Throckmorton said.
Find the full statement by Dr. Throckmorton on the FDA website.
The Food and Drug Administration is making changes to opioid analgesic labeling to give better information to clinicians on how to properly taper patients dependent on opioid use, according to Douglas Throckmorton, MD, deputy director for regulatory programs in the FDA’s Center for Drug Evaluation and Research.
, such as withdrawal symptoms, uncontrolled pain, and suicide. Both the FDA and the Centers for Disease Control and Prevention offer guidelines on how to properly taper opioids, Dr. Throckmorton said, but more needs to be done to ensure that patients are being provided with the correct advice and care.
The changes to the labels will include expanded information to health care clinicians and are intended to be used when both the clinician and patient have agreed to reduce the opioid dosage. When this is discussed, factors that should be considered include the dose of the drug, the duration of treatment, the type of pain being treated, and the physical and psychological attributes of the patient.
Other actions the FDA is pursuing to combat opioid use disorder include working with the National Academies of Sciences, Engineering, and Medicine on guidelines for the proper opioid analgesic prescribing for acute pain resulting from specific conditions or procedures, and advancing policies that make immediate-release opioid formulations available in fixed-quantity packaging for 1 or 2 days.
“The FDA remains committed to addressing the opioid crisis on all fronts, with a significant focus on decreasing unnecessary exposure to opioids and preventing new addiction; supporting the treatment of those with opioid use disorder; fostering the development of novel pain treatment therapies and opioids more resistant to abuse and misuse; and taking action against those involved in the illegal importation and sale of opioids,” Dr. Throckmorton said.
Find the full statement by Dr. Throckmorton on the FDA website.
The Food and Drug Administration is making changes to opioid analgesic labeling to give better information to clinicians on how to properly taper patients dependent on opioid use, according to Douglas Throckmorton, MD, deputy director for regulatory programs in the FDA’s Center for Drug Evaluation and Research.
, such as withdrawal symptoms, uncontrolled pain, and suicide. Both the FDA and the Centers for Disease Control and Prevention offer guidelines on how to properly taper opioids, Dr. Throckmorton said, but more needs to be done to ensure that patients are being provided with the correct advice and care.
The changes to the labels will include expanded information to health care clinicians and are intended to be used when both the clinician and patient have agreed to reduce the opioid dosage. When this is discussed, factors that should be considered include the dose of the drug, the duration of treatment, the type of pain being treated, and the physical and psychological attributes of the patient.
Other actions the FDA is pursuing to combat opioid use disorder include working with the National Academies of Sciences, Engineering, and Medicine on guidelines for the proper opioid analgesic prescribing for acute pain resulting from specific conditions or procedures, and advancing policies that make immediate-release opioid formulations available in fixed-quantity packaging for 1 or 2 days.
“The FDA remains committed to addressing the opioid crisis on all fronts, with a significant focus on decreasing unnecessary exposure to opioids and preventing new addiction; supporting the treatment of those with opioid use disorder; fostering the development of novel pain treatment therapies and opioids more resistant to abuse and misuse; and taking action against those involved in the illegal importation and sale of opioids,” Dr. Throckmorton said.
Find the full statement by Dr. Throckmorton on the FDA website.
Romosozumab gets FDA approval for treating osteoporosis
“These are women who have a history of osteoporotic fracture or multiple risk factors or have failed other treatments for osteoporosis,” according to a news release from the agency.
The monthly treatment of two injections (given one after the other at one visit) mainly works by increasing new bone formation, but these effects wane after 12 doses. If patients still need osteoporosis therapy after that maximum of 12 doses, it’s recommended they are put on treatments that reduce bone breakdown. Romosozumab-aqqg is “a monoclonal antibody that blocks the effects of the protein sclerostin,” according to the news release.
The treatment’s efficacy and safety was evaluated in two clinical trials of more than 11,000 women with postmenopausal osteoporosis. In one trial, women received 12 months of either romosozumab-aqqg or placebo. The treatment arm had a 73% lower risk of vertebral fracture than did the placebo arm, and this benefit was maintained over a second year when both groups were switched to denosumab, another osteoporosis therapy. In the second trial, one group received romosozumab-aqqg for 1 year and then a year of alendronate, and the other group received 2 years of alendronate, another osteoporosis therapy, according to the news release. In this trial, the romosozumab-aqqg arm had 50% less risk of vertebral fractures than did the alendronate-only arm, as well as reduced risk of nonvertebral fractures.
Romosozumab-aqqg was associated with higher risks of cardiovascular death, heart attack, and stroke in the alendronate trial, so the treatment comes with a boxed warning regarding those risks and recommends that the drug not be used in patients who have had a heart attack or stroke within the previous year, according to the news release. Common side effects include joint pain and headache, as well as injection-site reactions.
“These are women who have a history of osteoporotic fracture or multiple risk factors or have failed other treatments for osteoporosis,” according to a news release from the agency.
The monthly treatment of two injections (given one after the other at one visit) mainly works by increasing new bone formation, but these effects wane after 12 doses. If patients still need osteoporosis therapy after that maximum of 12 doses, it’s recommended they are put on treatments that reduce bone breakdown. Romosozumab-aqqg is “a monoclonal antibody that blocks the effects of the protein sclerostin,” according to the news release.
The treatment’s efficacy and safety was evaluated in two clinical trials of more than 11,000 women with postmenopausal osteoporosis. In one trial, women received 12 months of either romosozumab-aqqg or placebo. The treatment arm had a 73% lower risk of vertebral fracture than did the placebo arm, and this benefit was maintained over a second year when both groups were switched to denosumab, another osteoporosis therapy. In the second trial, one group received romosozumab-aqqg for 1 year and then a year of alendronate, and the other group received 2 years of alendronate, another osteoporosis therapy, according to the news release. In this trial, the romosozumab-aqqg arm had 50% less risk of vertebral fractures than did the alendronate-only arm, as well as reduced risk of nonvertebral fractures.
Romosozumab-aqqg was associated with higher risks of cardiovascular death, heart attack, and stroke in the alendronate trial, so the treatment comes with a boxed warning regarding those risks and recommends that the drug not be used in patients who have had a heart attack or stroke within the previous year, according to the news release. Common side effects include joint pain and headache, as well as injection-site reactions.
“These are women who have a history of osteoporotic fracture or multiple risk factors or have failed other treatments for osteoporosis,” according to a news release from the agency.
The monthly treatment of two injections (given one after the other at one visit) mainly works by increasing new bone formation, but these effects wane after 12 doses. If patients still need osteoporosis therapy after that maximum of 12 doses, it’s recommended they are put on treatments that reduce bone breakdown. Romosozumab-aqqg is “a monoclonal antibody that blocks the effects of the protein sclerostin,” according to the news release.
The treatment’s efficacy and safety was evaluated in two clinical trials of more than 11,000 women with postmenopausal osteoporosis. In one trial, women received 12 months of either romosozumab-aqqg or placebo. The treatment arm had a 73% lower risk of vertebral fracture than did the placebo arm, and this benefit was maintained over a second year when both groups were switched to denosumab, another osteoporosis therapy. In the second trial, one group received romosozumab-aqqg for 1 year and then a year of alendronate, and the other group received 2 years of alendronate, another osteoporosis therapy, according to the news release. In this trial, the romosozumab-aqqg arm had 50% less risk of vertebral fractures than did the alendronate-only arm, as well as reduced risk of nonvertebral fractures.
Romosozumab-aqqg was associated with higher risks of cardiovascular death, heart attack, and stroke in the alendronate trial, so the treatment comes with a boxed warning regarding those risks and recommends that the drug not be used in patients who have had a heart attack or stroke within the previous year, according to the news release. Common side effects include joint pain and headache, as well as injection-site reactions.
NIH’s HEAL initiative seeks coordinated effort to tackle pain, addiction
MILWAUKEE – Congress has allocated a half billion dollars annually to the National Institutes of Health for a program that seeks to end America’s opioid crisis. The agency is putting in place over two-dozen projects spanning basic and translational research, clinical trials, and implementation of new strategies to address pain and fight addiction.
The , said Walter Koroshetz, MD, speaking at the scientific meeting of the American Pain Society. This represents carryover from 2018, a planning year for the initiative, along with the 2019 $500 million annual supplement to the NIH’s base appropriation.
In 2018, NIH and other federal agencies successfully convinced Congress that funding a coordinated use of resources was necessary to overcome the country’s dual opioid and chronic pain crises. “Luck happens to the prepared,” said Dr. Koroshetz, director of the National Institute of Neurological Disorders and Stroke (NINDS), Bethesda, Md., adding that many hours went into putting together a national pain strategy that is multidisciplinary and multi-layered, and involves multiple players.
The two aims of research under the initiative are to improve treatments for misuse and addiction, and to enhance pain management. Focusing on this latter aim, Dr. Koroshetz said that the initiative has several research priorities to enhance pain management.
First, the biological basis for chronic pain needs to be understood in order to formulate effective therapies and interventions. “We need to understand the transition from acute to chronic pain,” he commented. “We need to see if we can learn about the risk factors for developing chronic pain; if we get really lucky, we might identify some biological markers” that identify who is at risk for this transition “in a high-risk acute pain situation.”
Next, a key request of industry and academia will be development of more drugs that avoid the dual-target program of opioids, which affect reward circuitry along with pain circuitry. “Drugs affecting the pain circuit and the reward circuit will always result in addiction” potential, said Dr. Koroshetz. “We’re still using drugs for pain from the poppy plant that were discovered 8,000 years ago.”
The hope with the HEAL initiative is to bring together academic centers with patient populations and research capabilities with industry, to accelerate moving nonaddictive treatments through to phase 3 trials.
The initiative also aims to promote discovery of new biologic targets for safe and effective pain treatment. New understanding of the physiology of pain has led to a multitude of candidate targets, said Dr. Koroshetz: “The good news is that there are so many potential targets. When I started in neurology in the ‘90s, I wouldn’t have said there were many, but now I’d say the list is long.”
Support for this work will require the development of human cell and tissue models, such as induced pluripotent stem cells, 3D printed organoids, and tissue chips. Several HEAL-funded grant mechanisms also seek research-industry collaboration to move investigational drugs for new targets through the pipeline quickly. The agency is hoping to see grantees apply new technologies, such as artificial intelligence, which can help identify new chemical structures and pinpoint new therapeutic targets for drug repurposing.
In addition to rapid drug discovery and accelerated clinical trials, Dr. Koroshetz said that HEAL leaders are hoping to see cross-pollination from two other NIH initiatives to boost pain-targeted medical device development. Both the Brain Research through Advancing Innovative Neurotechnologies (BRAIN) and the Stimulating Peripheral Activity to Relieve Conditions (SPARC) initiatives have already shown promise in identifying targets for effective, noninvasive pain relief devices, he said. Technologies being developed from these programs are “truly amazing,” he added.
A new focus on data and asset sharing among industry, academia, and NIH will “improve the quality, consistency, and efficiency of early-phase pain clinical trials,” Dr. Koroshetz continued. The Early Phase Pain Investigation Clinical Network (EPPIC-Net) will coordinate data and biosample hosting.
Through a competitive submission process, EPPIC-net will review dossiers from institutions or consortia that can serve as assets around which clinical trials can be designed and executed. These early-phase trials will focus on well-defined pain conditions with unmet need, such as chronic regional pain syndrome and tic douloureux, he said.
“We want to find patients who have well-defined conditions. We know the phenotypes, we know the natural history. We’re looking for clinical sites to work on these projects as part of one large team to bring new therapies to patients,” noted Dr. Koroshetz.
Further along the spectrum of research, comparative effectiveness research networks will provide a reality check to compare both pharmacologic and nonpharmacologic interventions all along the spectrum from acute to chronic pain. Here, data elements and storage will also be coordinated through EPPIC-Net.
Implementation science research will fine-tune the practicalities of bringing research to practice as the final piece of the puzzle, said Dr. Koroshetz.
Under NIH director Francis Collins, MD, PhD, Dr. Koroshetz is co-leading the HEAL initiative, along with Nora Volkow, MD, director of the National Institute on Drug Abuse. They wrote about the initiative in JAMA last year (JAMA. 2018 Jul 10;320[2]:129-30).
Dr. Koroshetz reported no conflicts of interest.
MILWAUKEE – Congress has allocated a half billion dollars annually to the National Institutes of Health for a program that seeks to end America’s opioid crisis. The agency is putting in place over two-dozen projects spanning basic and translational research, clinical trials, and implementation of new strategies to address pain and fight addiction.
The , said Walter Koroshetz, MD, speaking at the scientific meeting of the American Pain Society. This represents carryover from 2018, a planning year for the initiative, along with the 2019 $500 million annual supplement to the NIH’s base appropriation.
In 2018, NIH and other federal agencies successfully convinced Congress that funding a coordinated use of resources was necessary to overcome the country’s dual opioid and chronic pain crises. “Luck happens to the prepared,” said Dr. Koroshetz, director of the National Institute of Neurological Disorders and Stroke (NINDS), Bethesda, Md., adding that many hours went into putting together a national pain strategy that is multidisciplinary and multi-layered, and involves multiple players.
The two aims of research under the initiative are to improve treatments for misuse and addiction, and to enhance pain management. Focusing on this latter aim, Dr. Koroshetz said that the initiative has several research priorities to enhance pain management.
First, the biological basis for chronic pain needs to be understood in order to formulate effective therapies and interventions. “We need to understand the transition from acute to chronic pain,” he commented. “We need to see if we can learn about the risk factors for developing chronic pain; if we get really lucky, we might identify some biological markers” that identify who is at risk for this transition “in a high-risk acute pain situation.”
Next, a key request of industry and academia will be development of more drugs that avoid the dual-target program of opioids, which affect reward circuitry along with pain circuitry. “Drugs affecting the pain circuit and the reward circuit will always result in addiction” potential, said Dr. Koroshetz. “We’re still using drugs for pain from the poppy plant that were discovered 8,000 years ago.”
The hope with the HEAL initiative is to bring together academic centers with patient populations and research capabilities with industry, to accelerate moving nonaddictive treatments through to phase 3 trials.
The initiative also aims to promote discovery of new biologic targets for safe and effective pain treatment. New understanding of the physiology of pain has led to a multitude of candidate targets, said Dr. Koroshetz: “The good news is that there are so many potential targets. When I started in neurology in the ‘90s, I wouldn’t have said there were many, but now I’d say the list is long.”
Support for this work will require the development of human cell and tissue models, such as induced pluripotent stem cells, 3D printed organoids, and tissue chips. Several HEAL-funded grant mechanisms also seek research-industry collaboration to move investigational drugs for new targets through the pipeline quickly. The agency is hoping to see grantees apply new technologies, such as artificial intelligence, which can help identify new chemical structures and pinpoint new therapeutic targets for drug repurposing.
In addition to rapid drug discovery and accelerated clinical trials, Dr. Koroshetz said that HEAL leaders are hoping to see cross-pollination from two other NIH initiatives to boost pain-targeted medical device development. Both the Brain Research through Advancing Innovative Neurotechnologies (BRAIN) and the Stimulating Peripheral Activity to Relieve Conditions (SPARC) initiatives have already shown promise in identifying targets for effective, noninvasive pain relief devices, he said. Technologies being developed from these programs are “truly amazing,” he added.
A new focus on data and asset sharing among industry, academia, and NIH will “improve the quality, consistency, and efficiency of early-phase pain clinical trials,” Dr. Koroshetz continued. The Early Phase Pain Investigation Clinical Network (EPPIC-Net) will coordinate data and biosample hosting.
Through a competitive submission process, EPPIC-net will review dossiers from institutions or consortia that can serve as assets around which clinical trials can be designed and executed. These early-phase trials will focus on well-defined pain conditions with unmet need, such as chronic regional pain syndrome and tic douloureux, he said.
“We want to find patients who have well-defined conditions. We know the phenotypes, we know the natural history. We’re looking for clinical sites to work on these projects as part of one large team to bring new therapies to patients,” noted Dr. Koroshetz.
Further along the spectrum of research, comparative effectiveness research networks will provide a reality check to compare both pharmacologic and nonpharmacologic interventions all along the spectrum from acute to chronic pain. Here, data elements and storage will also be coordinated through EPPIC-Net.
Implementation science research will fine-tune the practicalities of bringing research to practice as the final piece of the puzzle, said Dr. Koroshetz.
Under NIH director Francis Collins, MD, PhD, Dr. Koroshetz is co-leading the HEAL initiative, along with Nora Volkow, MD, director of the National Institute on Drug Abuse. They wrote about the initiative in JAMA last year (JAMA. 2018 Jul 10;320[2]:129-30).
Dr. Koroshetz reported no conflicts of interest.
MILWAUKEE – Congress has allocated a half billion dollars annually to the National Institutes of Health for a program that seeks to end America’s opioid crisis. The agency is putting in place over two-dozen projects spanning basic and translational research, clinical trials, and implementation of new strategies to address pain and fight addiction.
The , said Walter Koroshetz, MD, speaking at the scientific meeting of the American Pain Society. This represents carryover from 2018, a planning year for the initiative, along with the 2019 $500 million annual supplement to the NIH’s base appropriation.
In 2018, NIH and other federal agencies successfully convinced Congress that funding a coordinated use of resources was necessary to overcome the country’s dual opioid and chronic pain crises. “Luck happens to the prepared,” said Dr. Koroshetz, director of the National Institute of Neurological Disorders and Stroke (NINDS), Bethesda, Md., adding that many hours went into putting together a national pain strategy that is multidisciplinary and multi-layered, and involves multiple players.
The two aims of research under the initiative are to improve treatments for misuse and addiction, and to enhance pain management. Focusing on this latter aim, Dr. Koroshetz said that the initiative has several research priorities to enhance pain management.
First, the biological basis for chronic pain needs to be understood in order to formulate effective therapies and interventions. “We need to understand the transition from acute to chronic pain,” he commented. “We need to see if we can learn about the risk factors for developing chronic pain; if we get really lucky, we might identify some biological markers” that identify who is at risk for this transition “in a high-risk acute pain situation.”
Next, a key request of industry and academia will be development of more drugs that avoid the dual-target program of opioids, which affect reward circuitry along with pain circuitry. “Drugs affecting the pain circuit and the reward circuit will always result in addiction” potential, said Dr. Koroshetz. “We’re still using drugs for pain from the poppy plant that were discovered 8,000 years ago.”
The hope with the HEAL initiative is to bring together academic centers with patient populations and research capabilities with industry, to accelerate moving nonaddictive treatments through to phase 3 trials.
The initiative also aims to promote discovery of new biologic targets for safe and effective pain treatment. New understanding of the physiology of pain has led to a multitude of candidate targets, said Dr. Koroshetz: “The good news is that there are so many potential targets. When I started in neurology in the ‘90s, I wouldn’t have said there were many, but now I’d say the list is long.”
Support for this work will require the development of human cell and tissue models, such as induced pluripotent stem cells, 3D printed organoids, and tissue chips. Several HEAL-funded grant mechanisms also seek research-industry collaboration to move investigational drugs for new targets through the pipeline quickly. The agency is hoping to see grantees apply new technologies, such as artificial intelligence, which can help identify new chemical structures and pinpoint new therapeutic targets for drug repurposing.
In addition to rapid drug discovery and accelerated clinical trials, Dr. Koroshetz said that HEAL leaders are hoping to see cross-pollination from two other NIH initiatives to boost pain-targeted medical device development. Both the Brain Research through Advancing Innovative Neurotechnologies (BRAIN) and the Stimulating Peripheral Activity to Relieve Conditions (SPARC) initiatives have already shown promise in identifying targets for effective, noninvasive pain relief devices, he said. Technologies being developed from these programs are “truly amazing,” he added.
A new focus on data and asset sharing among industry, academia, and NIH will “improve the quality, consistency, and efficiency of early-phase pain clinical trials,” Dr. Koroshetz continued. The Early Phase Pain Investigation Clinical Network (EPPIC-Net) will coordinate data and biosample hosting.
Through a competitive submission process, EPPIC-net will review dossiers from institutions or consortia that can serve as assets around which clinical trials can be designed and executed. These early-phase trials will focus on well-defined pain conditions with unmet need, such as chronic regional pain syndrome and tic douloureux, he said.
“We want to find patients who have well-defined conditions. We know the phenotypes, we know the natural history. We’re looking for clinical sites to work on these projects as part of one large team to bring new therapies to patients,” noted Dr. Koroshetz.
Further along the spectrum of research, comparative effectiveness research networks will provide a reality check to compare both pharmacologic and nonpharmacologic interventions all along the spectrum from acute to chronic pain. Here, data elements and storage will also be coordinated through EPPIC-Net.
Implementation science research will fine-tune the practicalities of bringing research to practice as the final piece of the puzzle, said Dr. Koroshetz.
Under NIH director Francis Collins, MD, PhD, Dr. Koroshetz is co-leading the HEAL initiative, along with Nora Volkow, MD, director of the National Institute on Drug Abuse. They wrote about the initiative in JAMA last year (JAMA. 2018 Jul 10;320[2]:129-30).
Dr. Koroshetz reported no conflicts of interest.
REPORTING FROM APS 2019











