User login
Vitamin D deficiency: Can we improve diagnosis?
CHICAGO – , new research suggests.
The study supports previous data suggesting that a ratio cut-off of greater than 100 is associated with the development of secondary hyperparathyroidism and the need for correction with supplementation, while a level greater than 50 suggests mild to moderate deficiency, Zhinous Shahidzadeh Yazdi, MD, noted in a poster presented at the annual meeting of the Endocrine Society
Current Endocrine Society guidelines published in 2011 advise measurement of plasma circulating 25(OH)D levels to evaluate vitamin D status in patients at risk for deficiency, defined as < 20 ng/mL (50 nmol/L). Revised guidelines are due out in early 2024.
“We don’t think measuring 25 hydroxy D is optimal because of the impact of vitamin D binding protein,” Dr. Yazdi said in an interview.
“Over 99% of all metabolites are bound to vitamin D binding protein, but only the free fraction is biologically active. By measuring total plasma 25(OH)D – as we do right now in clinic – we cannot account for the impact of vitamin D binding proteins, which vary by threefold across the population,” she added.
Thus, the total 25(OH)D deficiency cut-off of < 20 mg/mL currently recommended by the Endocrine Society may signal clinically significant vitamin D deficiency in one person but not another, noted Dr. Yazdi, a postdoctoral fellow at the University of Maryland, Baltimore.
Directly measuring binding protein or the free fraction would be ideal, but “there aren’t good commercial assays for those, and it’s more difficult to do. So, as an alternative, the vitamin D metabolite ratios implicitly adjust for individual differences in vitamin D binding protein,” she explained.
The ratio that Dr. Yazdi and colleagues propose to measure is that of the vitamin D metabolites 1,25(OH)2D/24,25 (OH)2D (shortened to 1,25D/24,25D), which they say reflect the body’s homeostatic response to vitamin D levels, and which rises in the setting of deficiency. It is a measurement > 100 in this ratio that they believe means the patient should receive vitamin D supplementation.
Controversial topic, ratio proposal is “very early in the game”
The issue of vitamin D deficiency has long generated controversy, particularly since publication of findings from the VITAL study in 2022, which showed vitamin D supplements did not significantly reduce the risk of fracture among adults in midlife and older compared with placebo.
According to the senior author of the new study, Simeon I. Taylor, MD, professor of medicine at the University of Maryland, what still remains controversial after VITAL is the question: “How can you identify people who have sufficiently bad vitamin D deficiency that it’s adversely impacting their bones?”
He added that there is a suggestion that small subpopulations in VITAL really did benefit from vitamin D supplementation, but the study “wasn’t designed to look at that.”
Indeed, the authors of an editorial accompanying the publication of the VITAL study said the findings mean there is no justification for measuring 25(OH)D in the general population or for treating to a target level.
Asked to comment on Dr. Yazdi and colleagues’ ratio proposal for diagnosing vitamin D deficiency, the coauthor of the VITAL study editorial, Clifford J. Rosen, MD, said in an interview: “I do think it’s important to point out that changes in the vitamin D binding protein can have a significant impact on the level of 25 [OH] D ... People should recognize that.”
And, Dr. Rosen noted, “I like the idea that the ... [ratio] is a measure of what’s happening in the body in response to vitamin D stores. So, when you supplement, it comes back up ... In certain individuals at high risk for fractures, for example, you might want to consider a more extensive workup like they’re suggesting.”
However, Dr. Rosen, of the Rosen Musculoskeletal Laboratory at Maine Medical Center Research Institute, Scarborough, added: “If the 25[OH]D level is below 20 [ng/mL] you’re going to treat regardless. When we think about sensitivity, a 25[OH]D level less than 20 [ng/mL] is a good screen ... Those individuals need to be treated, especially if they have low bone mass or fractures.”
To validate the ratio for clinical use, Dr. Rosen said, larger numbers of individuals would need to be evaluated. Moreover, “you’d need to run a standard of vitamin D binding protein by mass spectrometry versus their assumed method using ratios. Ratios are always a little tricky to interpret. So, I think this is very early in the game.”
And measuring the ratio of 1,25D/24,25D “is quite expensive,” he added.
He also pointed out that “calcium intake is really critical. You can have a [25(OH)D] level of 18 ng/mL and not have any of those secondary changes because [you’re] taking adequate calcium ... So, that always is a consideration that has to be worked into the evaluation.”
Same 25(OH)D, different risk level
In their poster, Dr. Yazdi and colleagues explain that to assess vitamin D status “one needs to understand regulation of vitamin D metabolism.” 25(OH)D undergoes two alternative fates: 1α-hydroxylation in the kidney, generating 1,25D (the biologically active form) or 24-hydroxylation leading to 24,25D (a biologically inactive metabolite).
For their study, they analyzed pilot data from 11 otherwise healthy individuals who had total baseline plasma 25(OH)D levels < 20 ng/mL, and compared 25(OH)D, 1,25D, 24,25D, and parathyroid hormone before versus after treating them with vitamin D3 supplementation of 50,000 IU per week for 4-6 weeks, aiming for a total 25D level above 30 ng/mL.
They then modeled how the body maintains 1,25D in a normal range and calculated/compared two vitamin D metabolite ratios in vitamin D deficient versus sufficient states: 25(OH)D/1,25D and 1,25D/24,25D. They then evaluated the applicability of these ratios for assessment of vitamin D status.
They explained that suppression of 24-hydroxylase is the first line of defense to maintain 1,25D levels. Secondary hyperparathyroidism is the second line of defense and occurs in severe vitamin D deficiency when the first line is maximally deployed.
Overall, there was poor correlation between 25[OH]D and 1,25D, “consistent with previous evidence that in mild to moderate vitamin D deficiency, 1,25D is maintained in the normal range, and therefore not a useful index for assessing vitamin D status,” the researchers said in their poster.
Hence, they said, the need to add the ratio of 1,25D/24,25D.
They presented a comparison of two study participants: one with a baseline 25[OH]D of 12.3 ng/mL, the other of 11.7 ng/mL. Although both would therefore be classified as deficient according to current guidelines, their 1,25D/24,25D ratios were 20 and 110, respectively.
In the first participant, the parathyroid hormone response to vitamin D supplementation was negligible, at +5%, compared with a dramatic 34% drop in the second participant.
“We think only the one with very high 1,25D/24,25D [ratio of 110] and a significant drop in parathyroid hormone after vitamin D supplementation [-34%] was vitamin D deficient,” the researchers said.
However, Dr. Taylor noted: “The diagnostic cut-offs we describe should be viewed as tentative for the time being. Additional research will be required to fully validate the optimal diagnostic criteria.”
Dr. Yazdi and Dr. Rosen have reported no relevant financial relationships. Dr. Taylor has reported being a consultant for Ionis Pharmaceuticals.
A version of this article originally appeared on Medscape.com.
CHICAGO – , new research suggests.
The study supports previous data suggesting that a ratio cut-off of greater than 100 is associated with the development of secondary hyperparathyroidism and the need for correction with supplementation, while a level greater than 50 suggests mild to moderate deficiency, Zhinous Shahidzadeh Yazdi, MD, noted in a poster presented at the annual meeting of the Endocrine Society
Current Endocrine Society guidelines published in 2011 advise measurement of plasma circulating 25(OH)D levels to evaluate vitamin D status in patients at risk for deficiency, defined as < 20 ng/mL (50 nmol/L). Revised guidelines are due out in early 2024.
“We don’t think measuring 25 hydroxy D is optimal because of the impact of vitamin D binding protein,” Dr. Yazdi said in an interview.
“Over 99% of all metabolites are bound to vitamin D binding protein, but only the free fraction is biologically active. By measuring total plasma 25(OH)D – as we do right now in clinic – we cannot account for the impact of vitamin D binding proteins, which vary by threefold across the population,” she added.
Thus, the total 25(OH)D deficiency cut-off of < 20 mg/mL currently recommended by the Endocrine Society may signal clinically significant vitamin D deficiency in one person but not another, noted Dr. Yazdi, a postdoctoral fellow at the University of Maryland, Baltimore.
Directly measuring binding protein or the free fraction would be ideal, but “there aren’t good commercial assays for those, and it’s more difficult to do. So, as an alternative, the vitamin D metabolite ratios implicitly adjust for individual differences in vitamin D binding protein,” she explained.
The ratio that Dr. Yazdi and colleagues propose to measure is that of the vitamin D metabolites 1,25(OH)2D/24,25 (OH)2D (shortened to 1,25D/24,25D), which they say reflect the body’s homeostatic response to vitamin D levels, and which rises in the setting of deficiency. It is a measurement > 100 in this ratio that they believe means the patient should receive vitamin D supplementation.
Controversial topic, ratio proposal is “very early in the game”
The issue of vitamin D deficiency has long generated controversy, particularly since publication of findings from the VITAL study in 2022, which showed vitamin D supplements did not significantly reduce the risk of fracture among adults in midlife and older compared with placebo.
According to the senior author of the new study, Simeon I. Taylor, MD, professor of medicine at the University of Maryland, what still remains controversial after VITAL is the question: “How can you identify people who have sufficiently bad vitamin D deficiency that it’s adversely impacting their bones?”
He added that there is a suggestion that small subpopulations in VITAL really did benefit from vitamin D supplementation, but the study “wasn’t designed to look at that.”
Indeed, the authors of an editorial accompanying the publication of the VITAL study said the findings mean there is no justification for measuring 25(OH)D in the general population or for treating to a target level.
Asked to comment on Dr. Yazdi and colleagues’ ratio proposal for diagnosing vitamin D deficiency, the coauthor of the VITAL study editorial, Clifford J. Rosen, MD, said in an interview: “I do think it’s important to point out that changes in the vitamin D binding protein can have a significant impact on the level of 25 [OH] D ... People should recognize that.”
And, Dr. Rosen noted, “I like the idea that the ... [ratio] is a measure of what’s happening in the body in response to vitamin D stores. So, when you supplement, it comes back up ... In certain individuals at high risk for fractures, for example, you might want to consider a more extensive workup like they’re suggesting.”
However, Dr. Rosen, of the Rosen Musculoskeletal Laboratory at Maine Medical Center Research Institute, Scarborough, added: “If the 25[OH]D level is below 20 [ng/mL] you’re going to treat regardless. When we think about sensitivity, a 25[OH]D level less than 20 [ng/mL] is a good screen ... Those individuals need to be treated, especially if they have low bone mass or fractures.”
To validate the ratio for clinical use, Dr. Rosen said, larger numbers of individuals would need to be evaluated. Moreover, “you’d need to run a standard of vitamin D binding protein by mass spectrometry versus their assumed method using ratios. Ratios are always a little tricky to interpret. So, I think this is very early in the game.”
And measuring the ratio of 1,25D/24,25D “is quite expensive,” he added.
He also pointed out that “calcium intake is really critical. You can have a [25(OH)D] level of 18 ng/mL and not have any of those secondary changes because [you’re] taking adequate calcium ... So, that always is a consideration that has to be worked into the evaluation.”
Same 25(OH)D, different risk level
In their poster, Dr. Yazdi and colleagues explain that to assess vitamin D status “one needs to understand regulation of vitamin D metabolism.” 25(OH)D undergoes two alternative fates: 1α-hydroxylation in the kidney, generating 1,25D (the biologically active form) or 24-hydroxylation leading to 24,25D (a biologically inactive metabolite).
For their study, they analyzed pilot data from 11 otherwise healthy individuals who had total baseline plasma 25(OH)D levels < 20 ng/mL, and compared 25(OH)D, 1,25D, 24,25D, and parathyroid hormone before versus after treating them with vitamin D3 supplementation of 50,000 IU per week for 4-6 weeks, aiming for a total 25D level above 30 ng/mL.
They then modeled how the body maintains 1,25D in a normal range and calculated/compared two vitamin D metabolite ratios in vitamin D deficient versus sufficient states: 25(OH)D/1,25D and 1,25D/24,25D. They then evaluated the applicability of these ratios for assessment of vitamin D status.
They explained that suppression of 24-hydroxylase is the first line of defense to maintain 1,25D levels. Secondary hyperparathyroidism is the second line of defense and occurs in severe vitamin D deficiency when the first line is maximally deployed.
Overall, there was poor correlation between 25[OH]D and 1,25D, “consistent with previous evidence that in mild to moderate vitamin D deficiency, 1,25D is maintained in the normal range, and therefore not a useful index for assessing vitamin D status,” the researchers said in their poster.
Hence, they said, the need to add the ratio of 1,25D/24,25D.
They presented a comparison of two study participants: one with a baseline 25[OH]D of 12.3 ng/mL, the other of 11.7 ng/mL. Although both would therefore be classified as deficient according to current guidelines, their 1,25D/24,25D ratios were 20 and 110, respectively.
In the first participant, the parathyroid hormone response to vitamin D supplementation was negligible, at +5%, compared with a dramatic 34% drop in the second participant.
“We think only the one with very high 1,25D/24,25D [ratio of 110] and a significant drop in parathyroid hormone after vitamin D supplementation [-34%] was vitamin D deficient,” the researchers said.
However, Dr. Taylor noted: “The diagnostic cut-offs we describe should be viewed as tentative for the time being. Additional research will be required to fully validate the optimal diagnostic criteria.”
Dr. Yazdi and Dr. Rosen have reported no relevant financial relationships. Dr. Taylor has reported being a consultant for Ionis Pharmaceuticals.
A version of this article originally appeared on Medscape.com.
CHICAGO – , new research suggests.
The study supports previous data suggesting that a ratio cut-off of greater than 100 is associated with the development of secondary hyperparathyroidism and the need for correction with supplementation, while a level greater than 50 suggests mild to moderate deficiency, Zhinous Shahidzadeh Yazdi, MD, noted in a poster presented at the annual meeting of the Endocrine Society
Current Endocrine Society guidelines published in 2011 advise measurement of plasma circulating 25(OH)D levels to evaluate vitamin D status in patients at risk for deficiency, defined as < 20 ng/mL (50 nmol/L). Revised guidelines are due out in early 2024.
“We don’t think measuring 25 hydroxy D is optimal because of the impact of vitamin D binding protein,” Dr. Yazdi said in an interview.
“Over 99% of all metabolites are bound to vitamin D binding protein, but only the free fraction is biologically active. By measuring total plasma 25(OH)D – as we do right now in clinic – we cannot account for the impact of vitamin D binding proteins, which vary by threefold across the population,” she added.
Thus, the total 25(OH)D deficiency cut-off of < 20 mg/mL currently recommended by the Endocrine Society may signal clinically significant vitamin D deficiency in one person but not another, noted Dr. Yazdi, a postdoctoral fellow at the University of Maryland, Baltimore.
Directly measuring binding protein or the free fraction would be ideal, but “there aren’t good commercial assays for those, and it’s more difficult to do. So, as an alternative, the vitamin D metabolite ratios implicitly adjust for individual differences in vitamin D binding protein,” she explained.
The ratio that Dr. Yazdi and colleagues propose to measure is that of the vitamin D metabolites 1,25(OH)2D/24,25 (OH)2D (shortened to 1,25D/24,25D), which they say reflect the body’s homeostatic response to vitamin D levels, and which rises in the setting of deficiency. It is a measurement > 100 in this ratio that they believe means the patient should receive vitamin D supplementation.
Controversial topic, ratio proposal is “very early in the game”
The issue of vitamin D deficiency has long generated controversy, particularly since publication of findings from the VITAL study in 2022, which showed vitamin D supplements did not significantly reduce the risk of fracture among adults in midlife and older compared with placebo.
According to the senior author of the new study, Simeon I. Taylor, MD, professor of medicine at the University of Maryland, what still remains controversial after VITAL is the question: “How can you identify people who have sufficiently bad vitamin D deficiency that it’s adversely impacting their bones?”
He added that there is a suggestion that small subpopulations in VITAL really did benefit from vitamin D supplementation, but the study “wasn’t designed to look at that.”
Indeed, the authors of an editorial accompanying the publication of the VITAL study said the findings mean there is no justification for measuring 25(OH)D in the general population or for treating to a target level.
Asked to comment on Dr. Yazdi and colleagues’ ratio proposal for diagnosing vitamin D deficiency, the coauthor of the VITAL study editorial, Clifford J. Rosen, MD, said in an interview: “I do think it’s important to point out that changes in the vitamin D binding protein can have a significant impact on the level of 25 [OH] D ... People should recognize that.”
And, Dr. Rosen noted, “I like the idea that the ... [ratio] is a measure of what’s happening in the body in response to vitamin D stores. So, when you supplement, it comes back up ... In certain individuals at high risk for fractures, for example, you might want to consider a more extensive workup like they’re suggesting.”
However, Dr. Rosen, of the Rosen Musculoskeletal Laboratory at Maine Medical Center Research Institute, Scarborough, added: “If the 25[OH]D level is below 20 [ng/mL] you’re going to treat regardless. When we think about sensitivity, a 25[OH]D level less than 20 [ng/mL] is a good screen ... Those individuals need to be treated, especially if they have low bone mass or fractures.”
To validate the ratio for clinical use, Dr. Rosen said, larger numbers of individuals would need to be evaluated. Moreover, “you’d need to run a standard of vitamin D binding protein by mass spectrometry versus their assumed method using ratios. Ratios are always a little tricky to interpret. So, I think this is very early in the game.”
And measuring the ratio of 1,25D/24,25D “is quite expensive,” he added.
He also pointed out that “calcium intake is really critical. You can have a [25(OH)D] level of 18 ng/mL and not have any of those secondary changes because [you’re] taking adequate calcium ... So, that always is a consideration that has to be worked into the evaluation.”
Same 25(OH)D, different risk level
In their poster, Dr. Yazdi and colleagues explain that to assess vitamin D status “one needs to understand regulation of vitamin D metabolism.” 25(OH)D undergoes two alternative fates: 1α-hydroxylation in the kidney, generating 1,25D (the biologically active form) or 24-hydroxylation leading to 24,25D (a biologically inactive metabolite).
For their study, they analyzed pilot data from 11 otherwise healthy individuals who had total baseline plasma 25(OH)D levels < 20 ng/mL, and compared 25(OH)D, 1,25D, 24,25D, and parathyroid hormone before versus after treating them with vitamin D3 supplementation of 50,000 IU per week for 4-6 weeks, aiming for a total 25D level above 30 ng/mL.
They then modeled how the body maintains 1,25D in a normal range and calculated/compared two vitamin D metabolite ratios in vitamin D deficient versus sufficient states: 25(OH)D/1,25D and 1,25D/24,25D. They then evaluated the applicability of these ratios for assessment of vitamin D status.
They explained that suppression of 24-hydroxylase is the first line of defense to maintain 1,25D levels. Secondary hyperparathyroidism is the second line of defense and occurs in severe vitamin D deficiency when the first line is maximally deployed.
Overall, there was poor correlation between 25[OH]D and 1,25D, “consistent with previous evidence that in mild to moderate vitamin D deficiency, 1,25D is maintained in the normal range, and therefore not a useful index for assessing vitamin D status,” the researchers said in their poster.
Hence, they said, the need to add the ratio of 1,25D/24,25D.
They presented a comparison of two study participants: one with a baseline 25[OH]D of 12.3 ng/mL, the other of 11.7 ng/mL. Although both would therefore be classified as deficient according to current guidelines, their 1,25D/24,25D ratios were 20 and 110, respectively.
In the first participant, the parathyroid hormone response to vitamin D supplementation was negligible, at +5%, compared with a dramatic 34% drop in the second participant.
“We think only the one with very high 1,25D/24,25D [ratio of 110] and a significant drop in parathyroid hormone after vitamin D supplementation [-34%] was vitamin D deficient,” the researchers said.
However, Dr. Taylor noted: “The diagnostic cut-offs we describe should be viewed as tentative for the time being. Additional research will be required to fully validate the optimal diagnostic criteria.”
Dr. Yazdi and Dr. Rosen have reported no relevant financial relationships. Dr. Taylor has reported being a consultant for Ionis Pharmaceuticals.
A version of this article originally appeared on Medscape.com.
AT ENDO 2023
64-year-old woman • hot flashes, facial flushing, excessive sweating, and palpitations • daily headaches • history of hypertension • Dx?
THE CASE
A 64-year-old woman sought care after having hot flashes, facial flushing, excessive sweating, palpitations, and daily headaches for 1 month. She had a history of hypertension that was well controlled with hydrochlorothiazide 25 mg/d but over the previous month, it had become more difficult to control. Her blood pressure remained elevated to 150/100 mm Hg despite the addition of lisinopril 40 mg/d and amlodipine 10 mg/d, indicating resistant hypertension. She had no family history of hypertension, diabetes, or obesity or any other pertinent medical or surgical history. Physical examination was negative for weight gain, stretch marks, or muscle weakness.
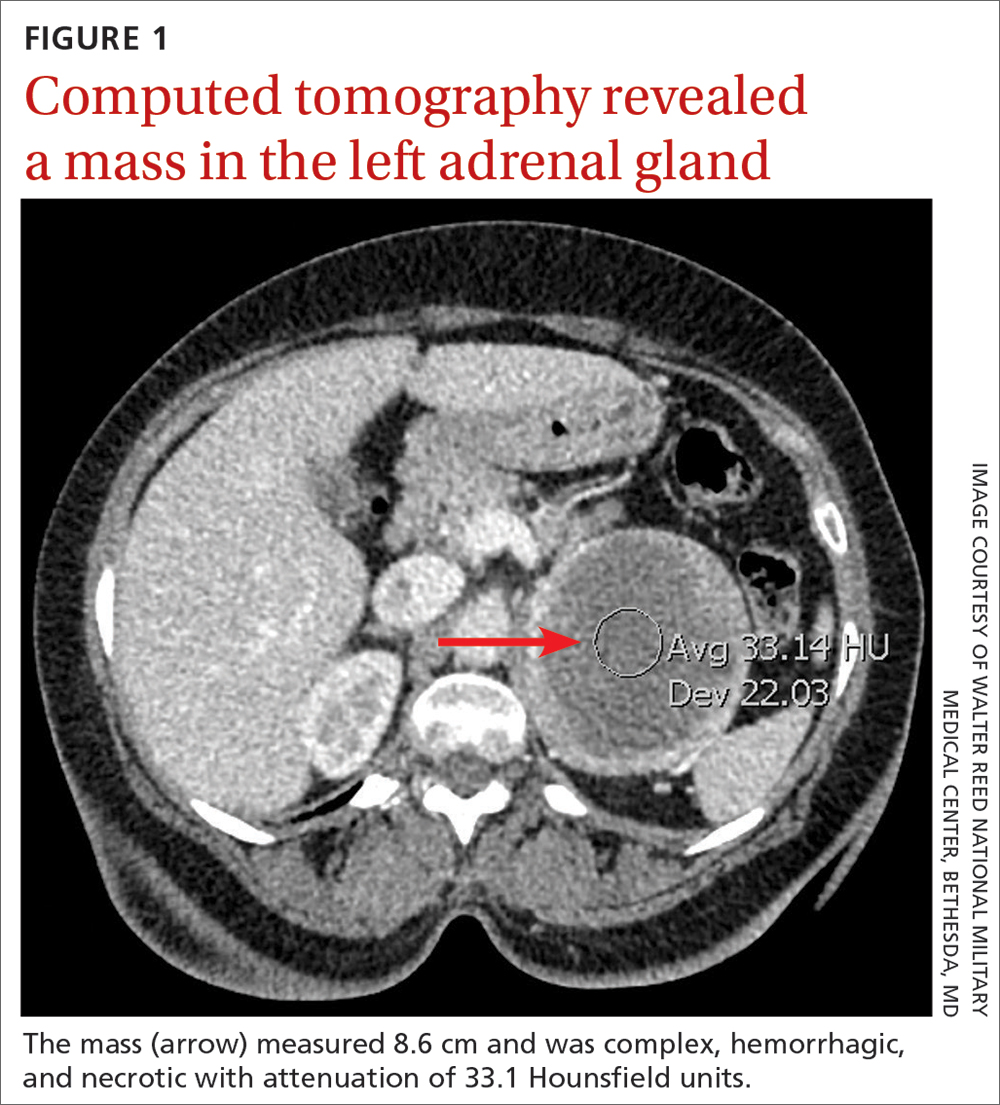
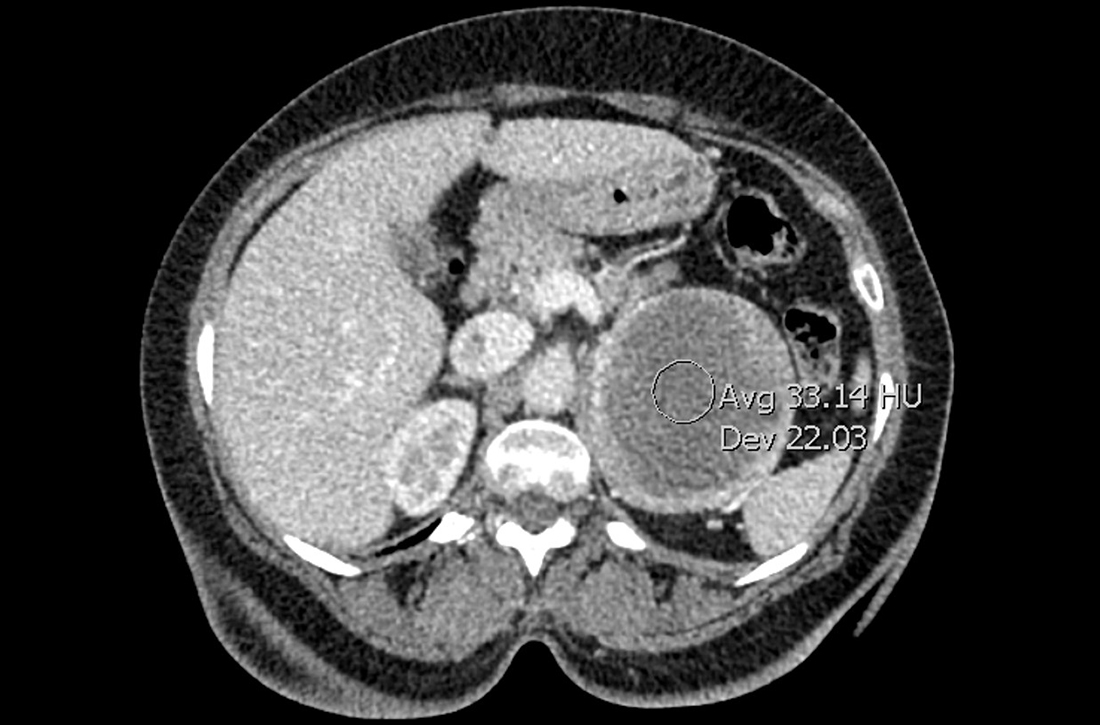
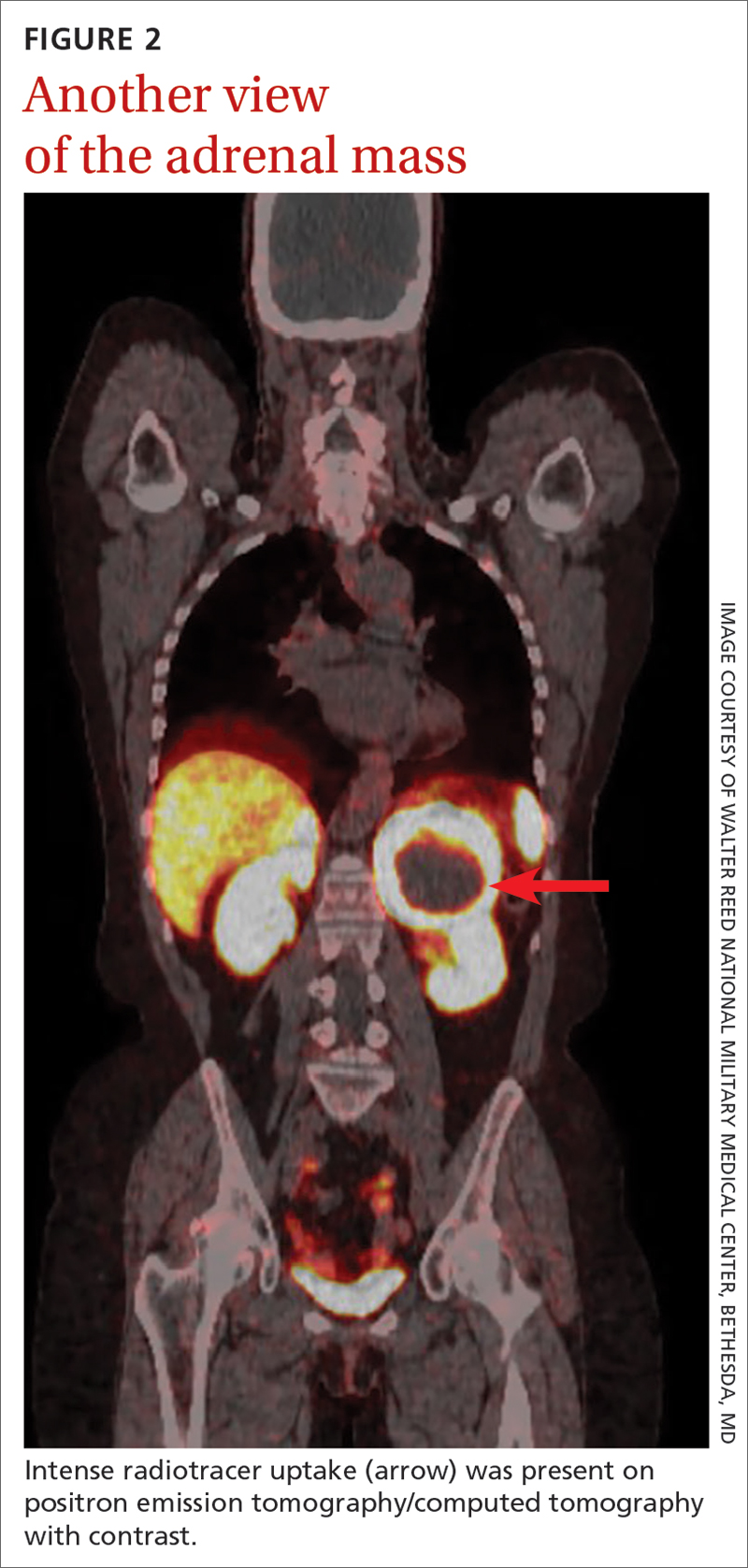
Laboratory tests revealed a normal serum aldosterone-renin ratio, renal function, and thyroid function; however, she had elevated levels of normetanephrine (2429 pg/mL; normal range, 0-145 pg/mL) and metanephrine (143 pg/mL; normal range, 0-62 pg/mL). Computed tomography (CT) revealed an 8.6-cm complex, hemorrhagic, necrotic left adrenal mass with attenuation of 33.1 Hounsfield units (HU) (FIGURE 1). Magnetic resonance imaging (MRI) demonstrated a T2 hyperintense left adrenal mass. An evaluation for Cushing syndrome was negative, and positron emission tomography (PET)/CT with gallium-68 dotatate was ordered. It showed intense radiotracer uptake in the left adrenal gland, with a maximum standardized uptake value of 70.1 (FIGURE 2).
THE DIAGNOSIS
After appropriate preparation with alpha blockade (phenoxybenzamine 20 mg twice daily for 7 days) and fluid resuscitation (normal saline run over 12 hours preoperatively), the patient underwent successful open surgical resection of the adrenal mass, during which her blood pressure was controlled with a nitroprusside infusion and boluses of esmolol and labetalol. Pathology results showed cells in a nested pattern with round to oval nuclei in a vascular background. There was no necrosis, increased mitotic figures, capsular invasion, or increased cellularity. Chromogranin immunohistochemical staining was positive. Given her resistant hypertension, clinical symptoms, and pathology results, the patient was given a diagnosis of pheochromocytoma.
DISCUSSION
Resistant hypertension is defined as blood pressure that is elevated above goal despite the use of 3 maximally titrated antihypertensive agents from different classes or that is well controlled with at least 4 antihypertensive medications.1 The prevalence of resistant hypertension is 12% to 18% in adults being treated for hypertension.1 Patients with resistant hypertension have a higher risk for cardiovascular events and death, are more likely to have a secondary cause of hypertension, and may benefit from special diagnostic testing or treatment approaches to control their blood pressure.1
There are many causes of resistant hypertension; primary aldosteronism is the most common cause (prevalence as high as 20%).2 Given the increased risk for cardiovascular/cerebrovascular disease, all patients with resistant hypertension should be screened for this condition.2 Other causes of resistant hypertension include renal parenchymal disease, renal artery stenosis, coarctation of the aorta, thyroid dysfunction, Cushing syndrome, paraganglioma, and as seen in our case, pheochromocytoma. Although pheochromocytoma is a rare cause of resistant hypertension (0.01%-4%),1 it is associated with high rates of morbidity and mortality if left untreated and may be inherited, making it an essential diagnosis to consider in all patients with resistant hypertension.1,3
Common symptoms of pheochromocytoma are hypertension (paroxysmal or sustained), headaches, palpitations, pallor, and piloerection (or cold sweats).1 Patients with pheochromocytoma typically exhibit metanephrine levels that are more than 4 times the upper limit of normal.4 Therefore, measurement of plasma free metanephrines or urinary fractionated metanephrines is recommended.5 Elevated metanephrine levels also are caused by obesity, obstructive sleep apnea, and certain medications and should be ruled out.5
All pheochromocytomas are potentially malignant. Despite the existence of pathologic scoring systems6,7 and radiographic features that suggest malignancy,8,9 no single risk-stratification tool is recommended in the current literature.10 Ultimately, the only way to confirm malignancy is to see metastases where chromaffin tissue is not normally found on imaging.10
Continue to: Pathologic features to look for...
Pathologic features to look for include capsular/periadrenal adipose invasion, increased cellularity, necrosis, tumor cell spindling, increased/atypical mitotic figures, and nuclear pleomorphism. Radiographic features include larger size (≥ 4-6 cm),11 an irregular shape, necrosis, calcifications, attenuation of 10 HU or higher on noncontrast CT, absolute washout of 60% or lower, and relative washout of 40% or lower.8,12 On MRI, malignant lesions appear hypointense on T1-weighted imaging and hyperintense on T2-weighted imaging.9 Fluorodeoxyglucose avidity on PET scan also is indicative of malignancy.8,9
Treatment for pheochromocytoma is surgical resection. An experienced surgical team and proper preoperative preparation are necessary because the induction of anesthesia, endotracheal intubation, and tumor manipulation can lead to a release of catecholamines, potentially resulting in an intraoperative hypertensive crisis, cardiac arrhythmias, and multiorgan failure.
Proper preoperative preparation includes taking an alpha-adrenergic blocker, such as phenoxybenzamine, prazosin, terazosin, or doxazosin, for at least 7 days to normalize the patient’s blood pressure. Patients should be counseled that they may experience nasal congestion, orthostasis, and fatigue while taking these medications. Volume expansion with intravenous fluids also should be performed and a high-salt diet considered. Beta-adrenergic blockade can be initiated once appropriate alpha-adrenergic blockade is achieved to control the patient’s heart rate; beta-blockers should never be started first because of the risk for severe hypertension. Careful hemodynamic monitoring is vital intraoperatively and postoperatively.5,13 Because metastatic lesions can occur decades after resection, long-term follow-up is critical.5,10
Following tumor resection, our patient’s blood pressure was supported with intravenous fluids and phenylephrine. She was able to discontinue all her antihypertensive medications postoperatively, and her plasma free and urinary fractionated metanephrine levels returned to within normal limits 8 weeks after surgery. Five years after surgery, she continues to have no signs of recurrence, as evidenced by annual negative plasma free metanephrines testing and abdominal/pelvic CT.
THE TAKEAWAY
This case highlights the importance of recognizing resistant hypertension and a potential secondary cause of this disease—pheochromocytoma. Although rare, pheochromocytomas confer increased risk for cardiovascular disease and death. Thus, swift recognition and proper preparation for surgical resection are necessary. Malignant lesions can be diagnosed only upon discovery of metastatic disease and can recur for decades after surgical resection, making diligent long-term follow-up imperative.
CORRESPONDENCE
Nicole O. Vietor, MD, Division of Endocrinology, Walter Reed National Military Medical Center, 8901 Wisconsin Avenue, Bethesda, MD 20889; [email protected]
1. Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:e53-e90. doi: 10.1161/HYP.0000000000000084
2. Young WF Jr. Diagnosis and treatment of primary aldosteronism: practical clinical perspectives. J Intern Med. 2019;285:126-148. doi: 10.1111/joim.12831
3. Young WF Jr, Calhoun DA, Lenders JWM, et al. Screening for endocrine hypertension: an Endocrine Society Scientific Statement. Endocr Rev. 2017;38:103-122. doi: 10.1210/er.2017-00054
4. Lenders JWM, Pacak K, Walther MM, et al. Biochemical diagnosis of pheochromocytoma: which test is best? JAMA. 2002;287:1427-1434. doi: 10.1001/jama.287.11.1427
5. Lenders JW, Duh Q-Y, Eisenhofer G, et al. Pheochromocytoma and paraganglioma: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2014;99:1915-1942. doi: 10.1210/jc.2014-1498
6. Kimura N, Takayanagi R, Takizawa N, et al. Pathological grading for predicting metastasis in phaeochromocytoma and paraganglioma. Endocr Relat Cancer. 2014;21:405-414. doi: 10.1530/ERC-13-0494
7. Thompson LDR. Pheochromocytoma of the Adrenal gland Scaled Score (PASS) to separate benign from malignant neoplasms: a clinicopathologic and immunophenotypic study of 100 cases. Am J Surg Pathol. 2002;26:551-566. doi: 10.1097/00000478-200205000-00002
8. Vaidya A, Hamrahian A, Bancos I, et al. The evaluation of incidentally discovered adrenal masses. Endocr Pract. 2019;25:178-192. doi: 10.4158/DSCR-2018-0565
9. Young WF Jr. Conventional imaging in adrenocortical carcinoma: update and perspectives. Horm Cancer. 2011;2:341-347. doi: 10.1007/s12672-011-0089-z
10. Neumann HPH, Young WF Jr, Eng C. Pheochromocytoma and paraganglioma. N Engl J Med. 2019;381:552-565. doi: 10.1056/NEJMra1806651
11. Iñiguez-Ariza NM, Kohlenberg JD, Delivanis DA, et al. Clinical, biochemical, and radiological characteristics of a single-center retrospective cohort of 705 large adrenal tumors. Mayo Clin Proc Innov Qual Outcomes. 2017;2:30-39. doi: 10.1016/j.mayocpiqo.2017.11.002
12. Marty M, Gaye D, Perez P, et al. Diagnostic accuracy of computed tomography to identify adenomas among adrenal incidentalomas in an endocrinological population. Eur J Endocrinol. 2018;178:439-446. doi: 10.1530/EJE-17-1056
13. Pacak K. Preoperative management of the pheochromocytoma patient. J Clin Endocrinol Metab. 2007;92:4069-4079. doi: 10.1210/jc.2007-1720
THE CASE
A 64-year-old woman sought care after having hot flashes, facial flushing, excessive sweating, palpitations, and daily headaches for 1 month. She had a history of hypertension that was well controlled with hydrochlorothiazide 25 mg/d but over the previous month, it had become more difficult to control. Her blood pressure remained elevated to 150/100 mm Hg despite the addition of lisinopril 40 mg/d and amlodipine 10 mg/d, indicating resistant hypertension. She had no family history of hypertension, diabetes, or obesity or any other pertinent medical or surgical history. Physical examination was negative for weight gain, stretch marks, or muscle weakness.

Laboratory tests revealed a normal serum aldosterone-renin ratio, renal function, and thyroid function; however, she had elevated levels of normetanephrine (2429 pg/mL; normal range, 0-145 pg/mL) and metanephrine (143 pg/mL; normal range, 0-62 pg/mL). Computed tomography (CT) revealed an 8.6-cm complex, hemorrhagic, necrotic left adrenal mass with attenuation of 33.1 Hounsfield units (HU) (FIGURE 1). Magnetic resonance imaging (MRI) demonstrated a T2 hyperintense left adrenal mass. An evaluation for Cushing syndrome was negative, and positron emission tomography (PET)/CT with gallium-68 dotatate was ordered. It showed intense radiotracer uptake in the left adrenal gland, with a maximum standardized uptake value of 70.1 (FIGURE 2).

THE DIAGNOSIS
After appropriate preparation with alpha blockade (phenoxybenzamine 20 mg twice daily for 7 days) and fluid resuscitation (normal saline run over 12 hours preoperatively), the patient underwent successful open surgical resection of the adrenal mass, during which her blood pressure was controlled with a nitroprusside infusion and boluses of esmolol and labetalol. Pathology results showed cells in a nested pattern with round to oval nuclei in a vascular background. There was no necrosis, increased mitotic figures, capsular invasion, or increased cellularity. Chromogranin immunohistochemical staining was positive. Given her resistant hypertension, clinical symptoms, and pathology results, the patient was given a diagnosis of pheochromocytoma.
DISCUSSION
Resistant hypertension is defined as blood pressure that is elevated above goal despite the use of 3 maximally titrated antihypertensive agents from different classes or that is well controlled with at least 4 antihypertensive medications.1 The prevalence of resistant hypertension is 12% to 18% in adults being treated for hypertension.1 Patients with resistant hypertension have a higher risk for cardiovascular events and death, are more likely to have a secondary cause of hypertension, and may benefit from special diagnostic testing or treatment approaches to control their blood pressure.1
There are many causes of resistant hypertension; primary aldosteronism is the most common cause (prevalence as high as 20%).2 Given the increased risk for cardiovascular/cerebrovascular disease, all patients with resistant hypertension should be screened for this condition.2 Other causes of resistant hypertension include renal parenchymal disease, renal artery stenosis, coarctation of the aorta, thyroid dysfunction, Cushing syndrome, paraganglioma, and as seen in our case, pheochromocytoma. Although pheochromocytoma is a rare cause of resistant hypertension (0.01%-4%),1 it is associated with high rates of morbidity and mortality if left untreated and may be inherited, making it an essential diagnosis to consider in all patients with resistant hypertension.1,3
Common symptoms of pheochromocytoma are hypertension (paroxysmal or sustained), headaches, palpitations, pallor, and piloerection (or cold sweats).1 Patients with pheochromocytoma typically exhibit metanephrine levels that are more than 4 times the upper limit of normal.4 Therefore, measurement of plasma free metanephrines or urinary fractionated metanephrines is recommended.5 Elevated metanephrine levels also are caused by obesity, obstructive sleep apnea, and certain medications and should be ruled out.5
All pheochromocytomas are potentially malignant. Despite the existence of pathologic scoring systems6,7 and radiographic features that suggest malignancy,8,9 no single risk-stratification tool is recommended in the current literature.10 Ultimately, the only way to confirm malignancy is to see metastases where chromaffin tissue is not normally found on imaging.10
Continue to: Pathologic features to look for...
Pathologic features to look for include capsular/periadrenal adipose invasion, increased cellularity, necrosis, tumor cell spindling, increased/atypical mitotic figures, and nuclear pleomorphism. Radiographic features include larger size (≥ 4-6 cm),11 an irregular shape, necrosis, calcifications, attenuation of 10 HU or higher on noncontrast CT, absolute washout of 60% or lower, and relative washout of 40% or lower.8,12 On MRI, malignant lesions appear hypointense on T1-weighted imaging and hyperintense on T2-weighted imaging.9 Fluorodeoxyglucose avidity on PET scan also is indicative of malignancy.8,9
Treatment for pheochromocytoma is surgical resection. An experienced surgical team and proper preoperative preparation are necessary because the induction of anesthesia, endotracheal intubation, and tumor manipulation can lead to a release of catecholamines, potentially resulting in an intraoperative hypertensive crisis, cardiac arrhythmias, and multiorgan failure.
Proper preoperative preparation includes taking an alpha-adrenergic blocker, such as phenoxybenzamine, prazosin, terazosin, or doxazosin, for at least 7 days to normalize the patient’s blood pressure. Patients should be counseled that they may experience nasal congestion, orthostasis, and fatigue while taking these medications. Volume expansion with intravenous fluids also should be performed and a high-salt diet considered. Beta-adrenergic blockade can be initiated once appropriate alpha-adrenergic blockade is achieved to control the patient’s heart rate; beta-blockers should never be started first because of the risk for severe hypertension. Careful hemodynamic monitoring is vital intraoperatively and postoperatively.5,13 Because metastatic lesions can occur decades after resection, long-term follow-up is critical.5,10
Following tumor resection, our patient’s blood pressure was supported with intravenous fluids and phenylephrine. She was able to discontinue all her antihypertensive medications postoperatively, and her plasma free and urinary fractionated metanephrine levels returned to within normal limits 8 weeks after surgery. Five years after surgery, she continues to have no signs of recurrence, as evidenced by annual negative plasma free metanephrines testing and abdominal/pelvic CT.
THE TAKEAWAY
This case highlights the importance of recognizing resistant hypertension and a potential secondary cause of this disease—pheochromocytoma. Although rare, pheochromocytomas confer increased risk for cardiovascular disease and death. Thus, swift recognition and proper preparation for surgical resection are necessary. Malignant lesions can be diagnosed only upon discovery of metastatic disease and can recur for decades after surgical resection, making diligent long-term follow-up imperative.
CORRESPONDENCE
Nicole O. Vietor, MD, Division of Endocrinology, Walter Reed National Military Medical Center, 8901 Wisconsin Avenue, Bethesda, MD 20889; [email protected]
THE CASE
A 64-year-old woman sought care after having hot flashes, facial flushing, excessive sweating, palpitations, and daily headaches for 1 month. She had a history of hypertension that was well controlled with hydrochlorothiazide 25 mg/d but over the previous month, it had become more difficult to control. Her blood pressure remained elevated to 150/100 mm Hg despite the addition of lisinopril 40 mg/d and amlodipine 10 mg/d, indicating resistant hypertension. She had no family history of hypertension, diabetes, or obesity or any other pertinent medical or surgical history. Physical examination was negative for weight gain, stretch marks, or muscle weakness.

Laboratory tests revealed a normal serum aldosterone-renin ratio, renal function, and thyroid function; however, she had elevated levels of normetanephrine (2429 pg/mL; normal range, 0-145 pg/mL) and metanephrine (143 pg/mL; normal range, 0-62 pg/mL). Computed tomography (CT) revealed an 8.6-cm complex, hemorrhagic, necrotic left adrenal mass with attenuation of 33.1 Hounsfield units (HU) (FIGURE 1). Magnetic resonance imaging (MRI) demonstrated a T2 hyperintense left adrenal mass. An evaluation for Cushing syndrome was negative, and positron emission tomography (PET)/CT with gallium-68 dotatate was ordered. It showed intense radiotracer uptake in the left adrenal gland, with a maximum standardized uptake value of 70.1 (FIGURE 2).

THE DIAGNOSIS
After appropriate preparation with alpha blockade (phenoxybenzamine 20 mg twice daily for 7 days) and fluid resuscitation (normal saline run over 12 hours preoperatively), the patient underwent successful open surgical resection of the adrenal mass, during which her blood pressure was controlled with a nitroprusside infusion and boluses of esmolol and labetalol. Pathology results showed cells in a nested pattern with round to oval nuclei in a vascular background. There was no necrosis, increased mitotic figures, capsular invasion, or increased cellularity. Chromogranin immunohistochemical staining was positive. Given her resistant hypertension, clinical symptoms, and pathology results, the patient was given a diagnosis of pheochromocytoma.
DISCUSSION
Resistant hypertension is defined as blood pressure that is elevated above goal despite the use of 3 maximally titrated antihypertensive agents from different classes or that is well controlled with at least 4 antihypertensive medications.1 The prevalence of resistant hypertension is 12% to 18% in adults being treated for hypertension.1 Patients with resistant hypertension have a higher risk for cardiovascular events and death, are more likely to have a secondary cause of hypertension, and may benefit from special diagnostic testing or treatment approaches to control their blood pressure.1
There are many causes of resistant hypertension; primary aldosteronism is the most common cause (prevalence as high as 20%).2 Given the increased risk for cardiovascular/cerebrovascular disease, all patients with resistant hypertension should be screened for this condition.2 Other causes of resistant hypertension include renal parenchymal disease, renal artery stenosis, coarctation of the aorta, thyroid dysfunction, Cushing syndrome, paraganglioma, and as seen in our case, pheochromocytoma. Although pheochromocytoma is a rare cause of resistant hypertension (0.01%-4%),1 it is associated with high rates of morbidity and mortality if left untreated and may be inherited, making it an essential diagnosis to consider in all patients with resistant hypertension.1,3
Common symptoms of pheochromocytoma are hypertension (paroxysmal or sustained), headaches, palpitations, pallor, and piloerection (or cold sweats).1 Patients with pheochromocytoma typically exhibit metanephrine levels that are more than 4 times the upper limit of normal.4 Therefore, measurement of plasma free metanephrines or urinary fractionated metanephrines is recommended.5 Elevated metanephrine levels also are caused by obesity, obstructive sleep apnea, and certain medications and should be ruled out.5
All pheochromocytomas are potentially malignant. Despite the existence of pathologic scoring systems6,7 and radiographic features that suggest malignancy,8,9 no single risk-stratification tool is recommended in the current literature.10 Ultimately, the only way to confirm malignancy is to see metastases where chromaffin tissue is not normally found on imaging.10
Continue to: Pathologic features to look for...
Pathologic features to look for include capsular/periadrenal adipose invasion, increased cellularity, necrosis, tumor cell spindling, increased/atypical mitotic figures, and nuclear pleomorphism. Radiographic features include larger size (≥ 4-6 cm),11 an irregular shape, necrosis, calcifications, attenuation of 10 HU or higher on noncontrast CT, absolute washout of 60% or lower, and relative washout of 40% or lower.8,12 On MRI, malignant lesions appear hypointense on T1-weighted imaging and hyperintense on T2-weighted imaging.9 Fluorodeoxyglucose avidity on PET scan also is indicative of malignancy.8,9
Treatment for pheochromocytoma is surgical resection. An experienced surgical team and proper preoperative preparation are necessary because the induction of anesthesia, endotracheal intubation, and tumor manipulation can lead to a release of catecholamines, potentially resulting in an intraoperative hypertensive crisis, cardiac arrhythmias, and multiorgan failure.
Proper preoperative preparation includes taking an alpha-adrenergic blocker, such as phenoxybenzamine, prazosin, terazosin, or doxazosin, for at least 7 days to normalize the patient’s blood pressure. Patients should be counseled that they may experience nasal congestion, orthostasis, and fatigue while taking these medications. Volume expansion with intravenous fluids also should be performed and a high-salt diet considered. Beta-adrenergic blockade can be initiated once appropriate alpha-adrenergic blockade is achieved to control the patient’s heart rate; beta-blockers should never be started first because of the risk for severe hypertension. Careful hemodynamic monitoring is vital intraoperatively and postoperatively.5,13 Because metastatic lesions can occur decades after resection, long-term follow-up is critical.5,10
Following tumor resection, our patient’s blood pressure was supported with intravenous fluids and phenylephrine. She was able to discontinue all her antihypertensive medications postoperatively, and her plasma free and urinary fractionated metanephrine levels returned to within normal limits 8 weeks after surgery. Five years after surgery, she continues to have no signs of recurrence, as evidenced by annual negative plasma free metanephrines testing and abdominal/pelvic CT.
THE TAKEAWAY
This case highlights the importance of recognizing resistant hypertension and a potential secondary cause of this disease—pheochromocytoma. Although rare, pheochromocytomas confer increased risk for cardiovascular disease and death. Thus, swift recognition and proper preparation for surgical resection are necessary. Malignant lesions can be diagnosed only upon discovery of metastatic disease and can recur for decades after surgical resection, making diligent long-term follow-up imperative.
CORRESPONDENCE
Nicole O. Vietor, MD, Division of Endocrinology, Walter Reed National Military Medical Center, 8901 Wisconsin Avenue, Bethesda, MD 20889; [email protected]
1. Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:e53-e90. doi: 10.1161/HYP.0000000000000084
2. Young WF Jr. Diagnosis and treatment of primary aldosteronism: practical clinical perspectives. J Intern Med. 2019;285:126-148. doi: 10.1111/joim.12831
3. Young WF Jr, Calhoun DA, Lenders JWM, et al. Screening for endocrine hypertension: an Endocrine Society Scientific Statement. Endocr Rev. 2017;38:103-122. doi: 10.1210/er.2017-00054
4. Lenders JWM, Pacak K, Walther MM, et al. Biochemical diagnosis of pheochromocytoma: which test is best? JAMA. 2002;287:1427-1434. doi: 10.1001/jama.287.11.1427
5. Lenders JW, Duh Q-Y, Eisenhofer G, et al. Pheochromocytoma and paraganglioma: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2014;99:1915-1942. doi: 10.1210/jc.2014-1498
6. Kimura N, Takayanagi R, Takizawa N, et al. Pathological grading for predicting metastasis in phaeochromocytoma and paraganglioma. Endocr Relat Cancer. 2014;21:405-414. doi: 10.1530/ERC-13-0494
7. Thompson LDR. Pheochromocytoma of the Adrenal gland Scaled Score (PASS) to separate benign from malignant neoplasms: a clinicopathologic and immunophenotypic study of 100 cases. Am J Surg Pathol. 2002;26:551-566. doi: 10.1097/00000478-200205000-00002
8. Vaidya A, Hamrahian A, Bancos I, et al. The evaluation of incidentally discovered adrenal masses. Endocr Pract. 2019;25:178-192. doi: 10.4158/DSCR-2018-0565
9. Young WF Jr. Conventional imaging in adrenocortical carcinoma: update and perspectives. Horm Cancer. 2011;2:341-347. doi: 10.1007/s12672-011-0089-z
10. Neumann HPH, Young WF Jr, Eng C. Pheochromocytoma and paraganglioma. N Engl J Med. 2019;381:552-565. doi: 10.1056/NEJMra1806651
11. Iñiguez-Ariza NM, Kohlenberg JD, Delivanis DA, et al. Clinical, biochemical, and radiological characteristics of a single-center retrospective cohort of 705 large adrenal tumors. Mayo Clin Proc Innov Qual Outcomes. 2017;2:30-39. doi: 10.1016/j.mayocpiqo.2017.11.002
12. Marty M, Gaye D, Perez P, et al. Diagnostic accuracy of computed tomography to identify adenomas among adrenal incidentalomas in an endocrinological population. Eur J Endocrinol. 2018;178:439-446. doi: 10.1530/EJE-17-1056
13. Pacak K. Preoperative management of the pheochromocytoma patient. J Clin Endocrinol Metab. 2007;92:4069-4079. doi: 10.1210/jc.2007-1720
1. Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:e53-e90. doi: 10.1161/HYP.0000000000000084
2. Young WF Jr. Diagnosis and treatment of primary aldosteronism: practical clinical perspectives. J Intern Med. 2019;285:126-148. doi: 10.1111/joim.12831
3. Young WF Jr, Calhoun DA, Lenders JWM, et al. Screening for endocrine hypertension: an Endocrine Society Scientific Statement. Endocr Rev. 2017;38:103-122. doi: 10.1210/er.2017-00054
4. Lenders JWM, Pacak K, Walther MM, et al. Biochemical diagnosis of pheochromocytoma: which test is best? JAMA. 2002;287:1427-1434. doi: 10.1001/jama.287.11.1427
5. Lenders JW, Duh Q-Y, Eisenhofer G, et al. Pheochromocytoma and paraganglioma: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2014;99:1915-1942. doi: 10.1210/jc.2014-1498
6. Kimura N, Takayanagi R, Takizawa N, et al. Pathological grading for predicting metastasis in phaeochromocytoma and paraganglioma. Endocr Relat Cancer. 2014;21:405-414. doi: 10.1530/ERC-13-0494
7. Thompson LDR. Pheochromocytoma of the Adrenal gland Scaled Score (PASS) to separate benign from malignant neoplasms: a clinicopathologic and immunophenotypic study of 100 cases. Am J Surg Pathol. 2002;26:551-566. doi: 10.1097/00000478-200205000-00002
8. Vaidya A, Hamrahian A, Bancos I, et al. The evaluation of incidentally discovered adrenal masses. Endocr Pract. 2019;25:178-192. doi: 10.4158/DSCR-2018-0565
9. Young WF Jr. Conventional imaging in adrenocortical carcinoma: update and perspectives. Horm Cancer. 2011;2:341-347. doi: 10.1007/s12672-011-0089-z
10. Neumann HPH, Young WF Jr, Eng C. Pheochromocytoma and paraganglioma. N Engl J Med. 2019;381:552-565. doi: 10.1056/NEJMra1806651
11. Iñiguez-Ariza NM, Kohlenberg JD, Delivanis DA, et al. Clinical, biochemical, and radiological characteristics of a single-center retrospective cohort of 705 large adrenal tumors. Mayo Clin Proc Innov Qual Outcomes. 2017;2:30-39. doi: 10.1016/j.mayocpiqo.2017.11.002
12. Marty M, Gaye D, Perez P, et al. Diagnostic accuracy of computed tomography to identify adenomas among adrenal incidentalomas in an endocrinological population. Eur J Endocrinol. 2018;178:439-446. doi: 10.1530/EJE-17-1056
13. Pacak K. Preoperative management of the pheochromocytoma patient. J Clin Endocrinol Metab. 2007;92:4069-4079. doi: 10.1210/jc.2007-1720
► Hot flashes, facial flushing, excessive sweating, and palpitations
► Daily headaches
► History of hypertension
Hormone therapies still ‘most effective’ in treating menopausal vasomotor symptoms
Despite new options in non–hormone-based treatments,
This recommendation emerged from an updated position statement from the North American Menopause Society in its first review of the scientific literature since 2015. The statement specifically targets nonhormonal management of symptoms such as hot flashes and night sweats, which occur in as many as 80% of menopausal women but are undertreated. The statement appears in the June issue of the Journal of The North American Menopause Society.
“Women with contraindications or objections to hormone treatment should be informed by professionals of evidence-based effective nonhormone treatment options,” stated a NAMS advisory panel led by Chrisandra L. Shufelt, MD, MS, professor and chair of the division of general internal medicine and associate director of the Women’s Health Research Center at the Mayo Clinic in Jacksonville, Fla. The statement is one of multiple NAMS updates performed at regular intervals, said Dr. Shufelt, also past president of NAMS, in an interview. “But the research has changed, and we wanted to make clinicians aware of new medications. One of our interesting findings was more evidence that off-label use of the nonhormonal overactive bladder drug oxybutynin can lower the rate of hot flashes.”
Dr. Shufelt noted that many of the current update’s findings align with previous research, and stressed that the therapeutic recommendations apply specifically to VMS. “Not all menopause-related symptoms are vasomotor, however,” she said. “While a lot of the lifestyle options such as cooling techniques and exercise are not recommended for controlling hot flashes, diet and exercise changes can be beneficial for other health reasons.”
Although it’s the most effective option for VMS, hormone therapy is not suitable for women with contraindications such as a previous blood clot, an estrogen-dependent cancer, a family history of such cancers, or a personal preference against hormone use, Dr. Shufelt added, so nonhormonal alternatives are important to prevent women from wasting time and money on ineffective remedies. “Women need to know what works and what doesn’t,” she said.
Recommended nonhormonal therapies
Based on a rigorous review of the scientific evidence to date, NAMS found the following therapies to be effective: cognitive-behavioral therapy; clinical hypnosis; SSRIs and serotonin-norepinephrine reuptake inhibitors – which yield mild to moderate improvements; gabapentin – which lessens the frequency and severity of hot flashes; fezolinetant (Veozah), a novel first-in-class neurokinin B antagonist that was Food and Drug Administration–approved in May for VSM; and oxybutynin, an antimuscarinic, anticholinergic drug, that reduces moderate to severe VMS, although long-term use in older adults may be linked to cognitive decline, weight loss, and stellate ganglion block.
Therapies that were ineffective, associated with adverse effects (AEs), or lacking adequate evidence of efficacy and thus not recommended for VMS included: paced respiration; supplemental and herbal remedies such as black cohosh, milk thistle, and evening primrose; cooling techniques; trigger avoidance; exercise and yoga; mindfulness-based intervention and relaxation; suvorexant, a dual orexin-receptor antagonist used for insomnia; soy foods, extracts, and the soy metabolite equol; cannabinoids; acupuncture; calibration of neural oscillations; chiropractics; clonidine, an alpha-2 adrenergic agonist that is associated with significant AEs with no recent evidence of benefit over placebo; dietary modification; and pregabalin – which is associated with significant AEs and has controlled-substance prescribing restrictions.
Ultimately, clinicians should individualize menopause care to each patient. For example, “if a patient says that avoiding caffeine in the morning stops her from having hot flashes in the afternoon, that’s fine,” Dr. Shufelt said.
HT still most effective
“This statement is excellent, comprehensive, and evidence-based,” commented Jill M. Rabin MD, vice chair of education and development, obstetrics and gynecology, at Northshore University Hospital/LIJ Medical Center in Manhasset, N.Y., and professor of obstetrics and gynecology at the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell Health in Hempstead, N.Y.
Dr. Rabin, coauthor of Mind Over Bladder was not involved in compiling the statement.
She agreed that hormone therapy is the most effective option for VMS and regularly prescribes it for suitable candidates in different forms depending on the type and severity of menopausal symptoms. As for nonhormonal options, Dr. Rabin added in an interview, some of those not recommended in the current NAMS statement could yet prove to be effective as more data accumulate. Suvorexant may be one to watch, for instance, but currently there are not enough data on its effectiveness.
“It’s really important to keep up on this nonhormonal research,” Dr. Rabin said. “As the population ages, more and more women will be in the peri- and postmenopausal periods and some have medical reasons for not taking hormone therapy.” It’s important to recommend nonhormonal therapies of proven benefit according to current high-level evidence, she said, “but also to keep your ear to the ground about those still under investigation.”
As for the lifestyle and alternative remedies of unproven benefit, Dr. Rabin added, there’s little harm in trying them. “As far as I know, no one’s ever died of relaxation and paced breathing.” In addition, a patient’s interaction with and sense of control over her own physiology provided by these techniques may be beneficial in themselves.
Dr. Shufelt reported grant support from the National Institutes of Health. Numerous authors reported consulting fees from and other financial ties to private-sector companies. Dr. Rabin had no relevant competing interests to disclose with regard to her comments.
Despite new options in non–hormone-based treatments,
This recommendation emerged from an updated position statement from the North American Menopause Society in its first review of the scientific literature since 2015. The statement specifically targets nonhormonal management of symptoms such as hot flashes and night sweats, which occur in as many as 80% of menopausal women but are undertreated. The statement appears in the June issue of the Journal of The North American Menopause Society.
“Women with contraindications or objections to hormone treatment should be informed by professionals of evidence-based effective nonhormone treatment options,” stated a NAMS advisory panel led by Chrisandra L. Shufelt, MD, MS, professor and chair of the division of general internal medicine and associate director of the Women’s Health Research Center at the Mayo Clinic in Jacksonville, Fla. The statement is one of multiple NAMS updates performed at regular intervals, said Dr. Shufelt, also past president of NAMS, in an interview. “But the research has changed, and we wanted to make clinicians aware of new medications. One of our interesting findings was more evidence that off-label use of the nonhormonal overactive bladder drug oxybutynin can lower the rate of hot flashes.”
Dr. Shufelt noted that many of the current update’s findings align with previous research, and stressed that the therapeutic recommendations apply specifically to VMS. “Not all menopause-related symptoms are vasomotor, however,” she said. “While a lot of the lifestyle options such as cooling techniques and exercise are not recommended for controlling hot flashes, diet and exercise changes can be beneficial for other health reasons.”
Although it’s the most effective option for VMS, hormone therapy is not suitable for women with contraindications such as a previous blood clot, an estrogen-dependent cancer, a family history of such cancers, or a personal preference against hormone use, Dr. Shufelt added, so nonhormonal alternatives are important to prevent women from wasting time and money on ineffective remedies. “Women need to know what works and what doesn’t,” she said.
Recommended nonhormonal therapies
Based on a rigorous review of the scientific evidence to date, NAMS found the following therapies to be effective: cognitive-behavioral therapy; clinical hypnosis; SSRIs and serotonin-norepinephrine reuptake inhibitors – which yield mild to moderate improvements; gabapentin – which lessens the frequency and severity of hot flashes; fezolinetant (Veozah), a novel first-in-class neurokinin B antagonist that was Food and Drug Administration–approved in May for VSM; and oxybutynin, an antimuscarinic, anticholinergic drug, that reduces moderate to severe VMS, although long-term use in older adults may be linked to cognitive decline, weight loss, and stellate ganglion block.
Therapies that were ineffective, associated with adverse effects (AEs), or lacking adequate evidence of efficacy and thus not recommended for VMS included: paced respiration; supplemental and herbal remedies such as black cohosh, milk thistle, and evening primrose; cooling techniques; trigger avoidance; exercise and yoga; mindfulness-based intervention and relaxation; suvorexant, a dual orexin-receptor antagonist used for insomnia; soy foods, extracts, and the soy metabolite equol; cannabinoids; acupuncture; calibration of neural oscillations; chiropractics; clonidine, an alpha-2 adrenergic agonist that is associated with significant AEs with no recent evidence of benefit over placebo; dietary modification; and pregabalin – which is associated with significant AEs and has controlled-substance prescribing restrictions.
Ultimately, clinicians should individualize menopause care to each patient. For example, “if a patient says that avoiding caffeine in the morning stops her from having hot flashes in the afternoon, that’s fine,” Dr. Shufelt said.
HT still most effective
“This statement is excellent, comprehensive, and evidence-based,” commented Jill M. Rabin MD, vice chair of education and development, obstetrics and gynecology, at Northshore University Hospital/LIJ Medical Center in Manhasset, N.Y., and professor of obstetrics and gynecology at the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell Health in Hempstead, N.Y.
Dr. Rabin, coauthor of Mind Over Bladder was not involved in compiling the statement.
She agreed that hormone therapy is the most effective option for VMS and regularly prescribes it for suitable candidates in different forms depending on the type and severity of menopausal symptoms. As for nonhormonal options, Dr. Rabin added in an interview, some of those not recommended in the current NAMS statement could yet prove to be effective as more data accumulate. Suvorexant may be one to watch, for instance, but currently there are not enough data on its effectiveness.
“It’s really important to keep up on this nonhormonal research,” Dr. Rabin said. “As the population ages, more and more women will be in the peri- and postmenopausal periods and some have medical reasons for not taking hormone therapy.” It’s important to recommend nonhormonal therapies of proven benefit according to current high-level evidence, she said, “but also to keep your ear to the ground about those still under investigation.”
As for the lifestyle and alternative remedies of unproven benefit, Dr. Rabin added, there’s little harm in trying them. “As far as I know, no one’s ever died of relaxation and paced breathing.” In addition, a patient’s interaction with and sense of control over her own physiology provided by these techniques may be beneficial in themselves.
Dr. Shufelt reported grant support from the National Institutes of Health. Numerous authors reported consulting fees from and other financial ties to private-sector companies. Dr. Rabin had no relevant competing interests to disclose with regard to her comments.
Despite new options in non–hormone-based treatments,
This recommendation emerged from an updated position statement from the North American Menopause Society in its first review of the scientific literature since 2015. The statement specifically targets nonhormonal management of symptoms such as hot flashes and night sweats, which occur in as many as 80% of menopausal women but are undertreated. The statement appears in the June issue of the Journal of The North American Menopause Society.
“Women with contraindications or objections to hormone treatment should be informed by professionals of evidence-based effective nonhormone treatment options,” stated a NAMS advisory panel led by Chrisandra L. Shufelt, MD, MS, professor and chair of the division of general internal medicine and associate director of the Women’s Health Research Center at the Mayo Clinic in Jacksonville, Fla. The statement is one of multiple NAMS updates performed at regular intervals, said Dr. Shufelt, also past president of NAMS, in an interview. “But the research has changed, and we wanted to make clinicians aware of new medications. One of our interesting findings was more evidence that off-label use of the nonhormonal overactive bladder drug oxybutynin can lower the rate of hot flashes.”
Dr. Shufelt noted that many of the current update’s findings align with previous research, and stressed that the therapeutic recommendations apply specifically to VMS. “Not all menopause-related symptoms are vasomotor, however,” she said. “While a lot of the lifestyle options such as cooling techniques and exercise are not recommended for controlling hot flashes, diet and exercise changes can be beneficial for other health reasons.”
Although it’s the most effective option for VMS, hormone therapy is not suitable for women with contraindications such as a previous blood clot, an estrogen-dependent cancer, a family history of such cancers, or a personal preference against hormone use, Dr. Shufelt added, so nonhormonal alternatives are important to prevent women from wasting time and money on ineffective remedies. “Women need to know what works and what doesn’t,” she said.
Recommended nonhormonal therapies
Based on a rigorous review of the scientific evidence to date, NAMS found the following therapies to be effective: cognitive-behavioral therapy; clinical hypnosis; SSRIs and serotonin-norepinephrine reuptake inhibitors – which yield mild to moderate improvements; gabapentin – which lessens the frequency and severity of hot flashes; fezolinetant (Veozah), a novel first-in-class neurokinin B antagonist that was Food and Drug Administration–approved in May for VSM; and oxybutynin, an antimuscarinic, anticholinergic drug, that reduces moderate to severe VMS, although long-term use in older adults may be linked to cognitive decline, weight loss, and stellate ganglion block.
Therapies that were ineffective, associated with adverse effects (AEs), or lacking adequate evidence of efficacy and thus not recommended for VMS included: paced respiration; supplemental and herbal remedies such as black cohosh, milk thistle, and evening primrose; cooling techniques; trigger avoidance; exercise and yoga; mindfulness-based intervention and relaxation; suvorexant, a dual orexin-receptor antagonist used for insomnia; soy foods, extracts, and the soy metabolite equol; cannabinoids; acupuncture; calibration of neural oscillations; chiropractics; clonidine, an alpha-2 adrenergic agonist that is associated with significant AEs with no recent evidence of benefit over placebo; dietary modification; and pregabalin – which is associated with significant AEs and has controlled-substance prescribing restrictions.
Ultimately, clinicians should individualize menopause care to each patient. For example, “if a patient says that avoiding caffeine in the morning stops her from having hot flashes in the afternoon, that’s fine,” Dr. Shufelt said.
HT still most effective
“This statement is excellent, comprehensive, and evidence-based,” commented Jill M. Rabin MD, vice chair of education and development, obstetrics and gynecology, at Northshore University Hospital/LIJ Medical Center in Manhasset, N.Y., and professor of obstetrics and gynecology at the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell Health in Hempstead, N.Y.
Dr. Rabin, coauthor of Mind Over Bladder was not involved in compiling the statement.
She agreed that hormone therapy is the most effective option for VMS and regularly prescribes it for suitable candidates in different forms depending on the type and severity of menopausal symptoms. As for nonhormonal options, Dr. Rabin added in an interview, some of those not recommended in the current NAMS statement could yet prove to be effective as more data accumulate. Suvorexant may be one to watch, for instance, but currently there are not enough data on its effectiveness.
“It’s really important to keep up on this nonhormonal research,” Dr. Rabin said. “As the population ages, more and more women will be in the peri- and postmenopausal periods and some have medical reasons for not taking hormone therapy.” It’s important to recommend nonhormonal therapies of proven benefit according to current high-level evidence, she said, “but also to keep your ear to the ground about those still under investigation.”
As for the lifestyle and alternative remedies of unproven benefit, Dr. Rabin added, there’s little harm in trying them. “As far as I know, no one’s ever died of relaxation and paced breathing.” In addition, a patient’s interaction with and sense of control over her own physiology provided by these techniques may be beneficial in themselves.
Dr. Shufelt reported grant support from the National Institutes of Health. Numerous authors reported consulting fees from and other financial ties to private-sector companies. Dr. Rabin had no relevant competing interests to disclose with regard to her comments.
FROM THE JOURNAL OF THE NORTH AMERICAN MENOPAUSE SOCIETY
Does weight loss surgery up the risk for bone fractures?
Currently, the two most common types of weight loss surgery performed include sleeve gastrectomy and Roux-en-Y gastric bypass (RYGB). Sleeve gastrectomy involves removing a large portion of the stomach so that its capacity is significantly decreased (to about 20%), reducing the ability to consume large quantities of food. Also, the procedure leads to marked reductions in ghrelin (an appetite-stimulating hormone), and some studies have reported increases in glucagon-like peptide 1 (GLP-1) and peptide YY (PYY), hormones that induce satiety. Gastric bypass involves creating a small stomach pouch and rerouting the small intestine so that it bypasses much of the stomach and also the upper portion of the small intestine. This reduces the amount of food that can be consumed at any time, increases levels of GLP-1 and PYY, and reduces absorption of nutrients with resultant weight loss. Less common bariatric surgeries include gastric banding and biliopancreatic diversion with duodenal switch (BPD-DS). Gastric banding involves placing a ring in the upper portion of the stomach, and the size of the pouch created can be altered by injecting more or less saline through a port inserted under the skin. BPD-DS includes sleeve gastrectomy, resection of a large section of the small intestine, and diversion of the pancreatic and biliary duct to a point below the junction of the ends of the resected gut.
Weight loss surgery is currently recommended for people who have a body mass index greater than or equal to 35 regardless of obesity-related complication and may be considered for those with a BMI greater than or equal to 30. BMI is calculated by dividing the weight (in kilograms) by the height (in meters). In children and adolescents, weight loss surgery should be considered in those with a BMI greater than 120% of the 95th percentile and with a major comorbidity or in those with a BMI greater than 140% of the 95th percentile.
What impact does weight loss surgery have on bone?
Multiple studies in both adults and teenagers have demonstrated that sleeve gastrectomy, RYGB, and BPD-DS (but not gastric banding) are associated with a decrease in bone density, impaired bone structure, and reduced strength estimates over time (Beavers et al; Gagnon, Schafer; Misra, Bredella). The relative risk for fracture after RYGB and BPD-DS is reported to be 1.2-2.3 (that is, 20%-130% more than normal), whereas fracture risk after sleeve gastrectomy is still under study with some conflicting results. Fracture risk starts to increase 2-3 years after surgery and peaks at 5-plus years after surgery. Most of the data for fractures come from studies in adults. With the rising use of weight loss surgery, particularly sleeve gastrectomy, in teenagers, studies are needed to determine fracture risk in this younger age group, who also seem to experience marked reductions in bone density, altered bone structure, and reduced bone strength after bariatric surgery.
What contributes to impaired bone health after weight loss surgery?
The deleterious effect of weight loss surgery on bone appears to be caused by various factors, including the massive and rapid weight loss that occurs after surgery, because body weight has a mechanical loading effect on bone and otherwise promotes bone formation. Weight loss results in mechanical unloading and thus a decrease in bone density. Further, when weight loss occurs, there is loss of both muscle and fat mass, and the reduction in muscle mass is deleterious to bone.
Other possible causes of bone density reduction include reduced absorption of certain nutrients, such as calcium and vitamin D critical for bone mineralization, and alterations in certain hormones that impact bone health. These include increases in parathyroid hormone, which increases bone loss when secreted in excess; increases in PYY (a hormone that reduces bone formation); decreases in ghrelin (a hormone that typically increases bone formation), particularly after sleeve gastrectomy; and decreases in estrone (a kind of estrogen that like other estrogens prevents bone loss). Further, age and gender may modify the bone consequences of surgery as outcomes in postmenopausal women appear to be worse than in younger women and men.
Preventing bone density loss
Given the many benefits of weight loss surgery, what can we do to prevent this decrease in bone density after surgery? It’s important for people undergoing weight loss surgery to be cognizant of this potentially negative outcome and to take appropriate precautions to mitigate this concern.
We should monitor bone density after surgery with the help of dual energy x-ray absorptiometry, starting a few years after surgery, particularly in those who are at greatest risk for fracture, so that we can be proactive about addressing any severe bone loss that warrants pharmacologic intervention.
More general recommendations include optimizing intake of calcium (1,200-1,500 mg/d), vitamin D (2,000-3,000 IUs/d), and protein (60-75 g/d) via diet and/or as supplements and engaging in weight-bearing physical activity because this exerts mechanical loading effects on the skeleton leading to increased bone formation and also increases muscle mass over time, which is beneficial to bone. A progressive resistance training program has been demonstrated to have beneficial effects on bone, and measures should be taken to reduce the risk for falls, which increases after certain kinds of weight loss surgery, such as gastric bypass.
Meeting with a dietitian can help determine any other nutrients that need to be optimized.
Though many hormonal changes after surgery have been linked to reductions in bone density, there are still no recommended hormonal therapies at this time, and more work is required to determine whether specific pharmacologic therapies might help improve bone outcomes after surgery.
Dr. Misra is chief of the division of pediatric endocrinology, Mass General for Children; associate director, Harvard Catalyst Translation and Clinical Research Center; director, Pediatric Endocrine-Sports Endocrine-Neuroendocrine Lab, Mass General Hospital; and professor, department of pediatrics, Harvard Medical School, Boston.
A version of this article originally appeared on Medscape.com.
Currently, the two most common types of weight loss surgery performed include sleeve gastrectomy and Roux-en-Y gastric bypass (RYGB). Sleeve gastrectomy involves removing a large portion of the stomach so that its capacity is significantly decreased (to about 20%), reducing the ability to consume large quantities of food. Also, the procedure leads to marked reductions in ghrelin (an appetite-stimulating hormone), and some studies have reported increases in glucagon-like peptide 1 (GLP-1) and peptide YY (PYY), hormones that induce satiety. Gastric bypass involves creating a small stomach pouch and rerouting the small intestine so that it bypasses much of the stomach and also the upper portion of the small intestine. This reduces the amount of food that can be consumed at any time, increases levels of GLP-1 and PYY, and reduces absorption of nutrients with resultant weight loss. Less common bariatric surgeries include gastric banding and biliopancreatic diversion with duodenal switch (BPD-DS). Gastric banding involves placing a ring in the upper portion of the stomach, and the size of the pouch created can be altered by injecting more or less saline through a port inserted under the skin. BPD-DS includes sleeve gastrectomy, resection of a large section of the small intestine, and diversion of the pancreatic and biliary duct to a point below the junction of the ends of the resected gut.
Weight loss surgery is currently recommended for people who have a body mass index greater than or equal to 35 regardless of obesity-related complication and may be considered for those with a BMI greater than or equal to 30. BMI is calculated by dividing the weight (in kilograms) by the height (in meters). In children and adolescents, weight loss surgery should be considered in those with a BMI greater than 120% of the 95th percentile and with a major comorbidity or in those with a BMI greater than 140% of the 95th percentile.
What impact does weight loss surgery have on bone?
Multiple studies in both adults and teenagers have demonstrated that sleeve gastrectomy, RYGB, and BPD-DS (but not gastric banding) are associated with a decrease in bone density, impaired bone structure, and reduced strength estimates over time (Beavers et al; Gagnon, Schafer; Misra, Bredella). The relative risk for fracture after RYGB and BPD-DS is reported to be 1.2-2.3 (that is, 20%-130% more than normal), whereas fracture risk after sleeve gastrectomy is still under study with some conflicting results. Fracture risk starts to increase 2-3 years after surgery and peaks at 5-plus years after surgery. Most of the data for fractures come from studies in adults. With the rising use of weight loss surgery, particularly sleeve gastrectomy, in teenagers, studies are needed to determine fracture risk in this younger age group, who also seem to experience marked reductions in bone density, altered bone structure, and reduced bone strength after bariatric surgery.
What contributes to impaired bone health after weight loss surgery?
The deleterious effect of weight loss surgery on bone appears to be caused by various factors, including the massive and rapid weight loss that occurs after surgery, because body weight has a mechanical loading effect on bone and otherwise promotes bone formation. Weight loss results in mechanical unloading and thus a decrease in bone density. Further, when weight loss occurs, there is loss of both muscle and fat mass, and the reduction in muscle mass is deleterious to bone.
Other possible causes of bone density reduction include reduced absorption of certain nutrients, such as calcium and vitamin D critical for bone mineralization, and alterations in certain hormones that impact bone health. These include increases in parathyroid hormone, which increases bone loss when secreted in excess; increases in PYY (a hormone that reduces bone formation); decreases in ghrelin (a hormone that typically increases bone formation), particularly after sleeve gastrectomy; and decreases in estrone (a kind of estrogen that like other estrogens prevents bone loss). Further, age and gender may modify the bone consequences of surgery as outcomes in postmenopausal women appear to be worse than in younger women and men.
Preventing bone density loss
Given the many benefits of weight loss surgery, what can we do to prevent this decrease in bone density after surgery? It’s important for people undergoing weight loss surgery to be cognizant of this potentially negative outcome and to take appropriate precautions to mitigate this concern.
We should monitor bone density after surgery with the help of dual energy x-ray absorptiometry, starting a few years after surgery, particularly in those who are at greatest risk for fracture, so that we can be proactive about addressing any severe bone loss that warrants pharmacologic intervention.
More general recommendations include optimizing intake of calcium (1,200-1,500 mg/d), vitamin D (2,000-3,000 IUs/d), and protein (60-75 g/d) via diet and/or as supplements and engaging in weight-bearing physical activity because this exerts mechanical loading effects on the skeleton leading to increased bone formation and also increases muscle mass over time, which is beneficial to bone. A progressive resistance training program has been demonstrated to have beneficial effects on bone, and measures should be taken to reduce the risk for falls, which increases after certain kinds of weight loss surgery, such as gastric bypass.
Meeting with a dietitian can help determine any other nutrients that need to be optimized.
Though many hormonal changes after surgery have been linked to reductions in bone density, there are still no recommended hormonal therapies at this time, and more work is required to determine whether specific pharmacologic therapies might help improve bone outcomes after surgery.
Dr. Misra is chief of the division of pediatric endocrinology, Mass General for Children; associate director, Harvard Catalyst Translation and Clinical Research Center; director, Pediatric Endocrine-Sports Endocrine-Neuroendocrine Lab, Mass General Hospital; and professor, department of pediatrics, Harvard Medical School, Boston.
A version of this article originally appeared on Medscape.com.
Currently, the two most common types of weight loss surgery performed include sleeve gastrectomy and Roux-en-Y gastric bypass (RYGB). Sleeve gastrectomy involves removing a large portion of the stomach so that its capacity is significantly decreased (to about 20%), reducing the ability to consume large quantities of food. Also, the procedure leads to marked reductions in ghrelin (an appetite-stimulating hormone), and some studies have reported increases in glucagon-like peptide 1 (GLP-1) and peptide YY (PYY), hormones that induce satiety. Gastric bypass involves creating a small stomach pouch and rerouting the small intestine so that it bypasses much of the stomach and also the upper portion of the small intestine. This reduces the amount of food that can be consumed at any time, increases levels of GLP-1 and PYY, and reduces absorption of nutrients with resultant weight loss. Less common bariatric surgeries include gastric banding and biliopancreatic diversion with duodenal switch (BPD-DS). Gastric banding involves placing a ring in the upper portion of the stomach, and the size of the pouch created can be altered by injecting more or less saline through a port inserted under the skin. BPD-DS includes sleeve gastrectomy, resection of a large section of the small intestine, and diversion of the pancreatic and biliary duct to a point below the junction of the ends of the resected gut.
Weight loss surgery is currently recommended for people who have a body mass index greater than or equal to 35 regardless of obesity-related complication and may be considered for those with a BMI greater than or equal to 30. BMI is calculated by dividing the weight (in kilograms) by the height (in meters). In children and adolescents, weight loss surgery should be considered in those with a BMI greater than 120% of the 95th percentile and with a major comorbidity or in those with a BMI greater than 140% of the 95th percentile.
What impact does weight loss surgery have on bone?
Multiple studies in both adults and teenagers have demonstrated that sleeve gastrectomy, RYGB, and BPD-DS (but not gastric banding) are associated with a decrease in bone density, impaired bone structure, and reduced strength estimates over time (Beavers et al; Gagnon, Schafer; Misra, Bredella). The relative risk for fracture after RYGB and BPD-DS is reported to be 1.2-2.3 (that is, 20%-130% more than normal), whereas fracture risk after sleeve gastrectomy is still under study with some conflicting results. Fracture risk starts to increase 2-3 years after surgery and peaks at 5-plus years after surgery. Most of the data for fractures come from studies in adults. With the rising use of weight loss surgery, particularly sleeve gastrectomy, in teenagers, studies are needed to determine fracture risk in this younger age group, who also seem to experience marked reductions in bone density, altered bone structure, and reduced bone strength after bariatric surgery.
What contributes to impaired bone health after weight loss surgery?
The deleterious effect of weight loss surgery on bone appears to be caused by various factors, including the massive and rapid weight loss that occurs after surgery, because body weight has a mechanical loading effect on bone and otherwise promotes bone formation. Weight loss results in mechanical unloading and thus a decrease in bone density. Further, when weight loss occurs, there is loss of both muscle and fat mass, and the reduction in muscle mass is deleterious to bone.
Other possible causes of bone density reduction include reduced absorption of certain nutrients, such as calcium and vitamin D critical for bone mineralization, and alterations in certain hormones that impact bone health. These include increases in parathyroid hormone, which increases bone loss when secreted in excess; increases in PYY (a hormone that reduces bone formation); decreases in ghrelin (a hormone that typically increases bone formation), particularly after sleeve gastrectomy; and decreases in estrone (a kind of estrogen that like other estrogens prevents bone loss). Further, age and gender may modify the bone consequences of surgery as outcomes in postmenopausal women appear to be worse than in younger women and men.
Preventing bone density loss
Given the many benefits of weight loss surgery, what can we do to prevent this decrease in bone density after surgery? It’s important for people undergoing weight loss surgery to be cognizant of this potentially negative outcome and to take appropriate precautions to mitigate this concern.
We should monitor bone density after surgery with the help of dual energy x-ray absorptiometry, starting a few years after surgery, particularly in those who are at greatest risk for fracture, so that we can be proactive about addressing any severe bone loss that warrants pharmacologic intervention.
More general recommendations include optimizing intake of calcium (1,200-1,500 mg/d), vitamin D (2,000-3,000 IUs/d), and protein (60-75 g/d) via diet and/or as supplements and engaging in weight-bearing physical activity because this exerts mechanical loading effects on the skeleton leading to increased bone formation and also increases muscle mass over time, which is beneficial to bone. A progressive resistance training program has been demonstrated to have beneficial effects on bone, and measures should be taken to reduce the risk for falls, which increases after certain kinds of weight loss surgery, such as gastric bypass.
Meeting with a dietitian can help determine any other nutrients that need to be optimized.
Though many hormonal changes after surgery have been linked to reductions in bone density, there are still no recommended hormonal therapies at this time, and more work is required to determine whether specific pharmacologic therapies might help improve bone outcomes after surgery.
Dr. Misra is chief of the division of pediatric endocrinology, Mass General for Children; associate director, Harvard Catalyst Translation and Clinical Research Center; director, Pediatric Endocrine-Sports Endocrine-Neuroendocrine Lab, Mass General Hospital; and professor, department of pediatrics, Harvard Medical School, Boston.
A version of this article originally appeared on Medscape.com.
Menopause and long COVID: What women should know
British researchers have noted that women at midlife who have long COVID seem to get specific, and severe, symptoms, including brain fog, fatigue, new-onset dizziness, and difficulty sleeping through the night.
Doctors also think it’s possible that long COVID worsens the symptoms of perimenopause and menopause. Lower levels of estrogen and testosterone appear to be the reason.
“A long COVID theory is that there is a temporary disruption to physiological ovarian steroid hormone production, which could [worsen] symptoms of perimenopause and menopause,” said JoAnn V. Pinkerton, MD, professor of obstetrics at the University of Virginia, Charlottesville, and executive director of the North American Menopause Society.
Long COVID symptoms and menopause symptoms can also be very hard to tell apart.
Another U.K. study cautions that because of this kind of symptom overlap, women at midlife may be misdiagnosed. Research from the North American Menopause Society shows that many women may have trouble recovering from long COVID unless their hormone deficiency is treated.
What are the symptoms of long COVID?
There are over 200 symptoms that have been associated with long COVID, according to the American Medical Association. Some common symptoms are currently defined as the following: feeling extremely tired, feeling depleted after exertion, cognitive issues such as brain fog, heart beating over 100 times a minute, and a loss of sense of smell and taste.
Long COVID symptoms begin a few weeks to a few months after a COVID infection. They can last an indefinite amount of time, but “the hope is that long COVID will not be lifelong,” said Clare Flannery, MD, an endocrinologist and associate professor in the departments of obstetrics, gynecology and reproductive sciences and internal medicine at Yale University, New Haven, Conn.
What are the symptoms of menopause?
Some symptoms of menopause include vaginal infections, irregular bleeding, urinary problems, and sexual problems.
Women in their middle years have other symptoms that can be the same as perimenopause/menopause symptoms.
“Common symptoms of perimenopause and menopause which may also be symptoms ascribed to long COVID include hot flashes, night sweats, disrupted sleep, low mood, depression or anxiety, decreased concentration, memory problems, joint and muscle pains, and headaches,” Dr. Pinkerton said.
Can long COVID actually bring on menopause?
In short: Possibly.
A new study from the Massachusetts Institute of Technology/Patient-Led Research Collaborative/University of California, San Francisco, found that long COVID can cause disruptions to a woman’s menstrual cycle, ovaries, fertility, and menopause itself.
This could be caused by chronic inflammation caused by long COVID on hormones as well. This kind of inflammatory response could explain irregularities in a woman’s menstrual cycle, according to the Newson Health Research and Education study. For instance, “when the body has inflammation, ovulation can happen,” Dr. Flannery said.
The mechanism for how long COVID could spur menopause can also involve a woman’s ovaries.
“Since the theory is that COVID affects the ovary with declines in ovarian reserve and ovarian function, it makes sense that long COVID could bring on symptoms of perimenopause or menopause more acutely or more severely and lengthen the symptoms of the perimenopause and menopausal transition,” Dr. Pinkerton said.
How can hormone replacement therapy benefit women dealing with long COVID during menopause?
Estradiol, the strongest estrogen hormone in a woman’s body, has already been shown to have a positive effect against COVID.
“Estradiol therapy treats symptoms more aggressively in the setting of long COVID,” said Dr. Flannery.
Estradiol is also a form of hormone therapy for menopause symptoms.
“Estradiol has been shown to help hot flashes, night sweats, and sleep and improve mood during perimenopause,” said Dr. Pinkerton. “So it’s likely that perimenopausal or menopausal women with long COVID would see improvements both due to the action of estradiol on the ovary seen during COVID and the improvements in symptoms.”
Estrogen-based hormone therapy has been linked to an increased risk for endometrial, breast, and ovarian cancer, according to the American Cancer Society. This means you should carefully consider how comfortable you are with those additional risks before starting this kind of therapy.
“Which of your symptoms are the most difficult to manage? You may see if you can navigate one to three of them. What are you willing to do for your symptoms? If a woman is willing to favor her sleep for the next 6 months to a year, she may be willing to change how she perceives her risk for cancer,” Dr. Flannery said. “What risk is a woman willing to take? I think if someone has a very low concern about a risk of cancer, and she’s suffering a disrupted life, then taking estradiol in a 1- to 2-year trial period could be critical to help.”
What else can help ease long COVID during menopause?
Getting the COVID vaccine, as well as getting a booster, could help. Not only will this help prevent people from being reinfected with COVID, which can worsen symptoms, but a new Swedish study says there is no evidence that it will cause postmenopausal problems like irregular bleeding.
“Weak and inconsistent associations were observed between SARS-CoV-2 vaccination and healthcare contacts for bleeding in women who are postmenopausal, and even less evidence was recorded of an association for menstrual disturbance or bleeding in women who were premenopausal,” said study coauthor Rickard Ljung, MD, PhD, MPH, professor and acting head of the pharmacoepidemiology and analysis department in the division of use and information of the Swedish Medical Products Agency in Uppsala.
A version of this article first appeared on WebMD.com.
British researchers have noted that women at midlife who have long COVID seem to get specific, and severe, symptoms, including brain fog, fatigue, new-onset dizziness, and difficulty sleeping through the night.
Doctors also think it’s possible that long COVID worsens the symptoms of perimenopause and menopause. Lower levels of estrogen and testosterone appear to be the reason.
“A long COVID theory is that there is a temporary disruption to physiological ovarian steroid hormone production, which could [worsen] symptoms of perimenopause and menopause,” said JoAnn V. Pinkerton, MD, professor of obstetrics at the University of Virginia, Charlottesville, and executive director of the North American Menopause Society.
Long COVID symptoms and menopause symptoms can also be very hard to tell apart.
Another U.K. study cautions that because of this kind of symptom overlap, women at midlife may be misdiagnosed. Research from the North American Menopause Society shows that many women may have trouble recovering from long COVID unless their hormone deficiency is treated.
What are the symptoms of long COVID?
There are over 200 symptoms that have been associated with long COVID, according to the American Medical Association. Some common symptoms are currently defined as the following: feeling extremely tired, feeling depleted after exertion, cognitive issues such as brain fog, heart beating over 100 times a minute, and a loss of sense of smell and taste.
Long COVID symptoms begin a few weeks to a few months after a COVID infection. They can last an indefinite amount of time, but “the hope is that long COVID will not be lifelong,” said Clare Flannery, MD, an endocrinologist and associate professor in the departments of obstetrics, gynecology and reproductive sciences and internal medicine at Yale University, New Haven, Conn.
What are the symptoms of menopause?
Some symptoms of menopause include vaginal infections, irregular bleeding, urinary problems, and sexual problems.
Women in their middle years have other symptoms that can be the same as perimenopause/menopause symptoms.
“Common symptoms of perimenopause and menopause which may also be symptoms ascribed to long COVID include hot flashes, night sweats, disrupted sleep, low mood, depression or anxiety, decreased concentration, memory problems, joint and muscle pains, and headaches,” Dr. Pinkerton said.
Can long COVID actually bring on menopause?
In short: Possibly.
A new study from the Massachusetts Institute of Technology/Patient-Led Research Collaborative/University of California, San Francisco, found that long COVID can cause disruptions to a woman’s menstrual cycle, ovaries, fertility, and menopause itself.
This could be caused by chronic inflammation caused by long COVID on hormones as well. This kind of inflammatory response could explain irregularities in a woman’s menstrual cycle, according to the Newson Health Research and Education study. For instance, “when the body has inflammation, ovulation can happen,” Dr. Flannery said.
The mechanism for how long COVID could spur menopause can also involve a woman’s ovaries.
“Since the theory is that COVID affects the ovary with declines in ovarian reserve and ovarian function, it makes sense that long COVID could bring on symptoms of perimenopause or menopause more acutely or more severely and lengthen the symptoms of the perimenopause and menopausal transition,” Dr. Pinkerton said.
How can hormone replacement therapy benefit women dealing with long COVID during menopause?
Estradiol, the strongest estrogen hormone in a woman’s body, has already been shown to have a positive effect against COVID.
“Estradiol therapy treats symptoms more aggressively in the setting of long COVID,” said Dr. Flannery.
Estradiol is also a form of hormone therapy for menopause symptoms.
“Estradiol has been shown to help hot flashes, night sweats, and sleep and improve mood during perimenopause,” said Dr. Pinkerton. “So it’s likely that perimenopausal or menopausal women with long COVID would see improvements both due to the action of estradiol on the ovary seen during COVID and the improvements in symptoms.”
Estrogen-based hormone therapy has been linked to an increased risk for endometrial, breast, and ovarian cancer, according to the American Cancer Society. This means you should carefully consider how comfortable you are with those additional risks before starting this kind of therapy.
“Which of your symptoms are the most difficult to manage? You may see if you can navigate one to three of them. What are you willing to do for your symptoms? If a woman is willing to favor her sleep for the next 6 months to a year, she may be willing to change how she perceives her risk for cancer,” Dr. Flannery said. “What risk is a woman willing to take? I think if someone has a very low concern about a risk of cancer, and she’s suffering a disrupted life, then taking estradiol in a 1- to 2-year trial period could be critical to help.”
What else can help ease long COVID during menopause?
Getting the COVID vaccine, as well as getting a booster, could help. Not only will this help prevent people from being reinfected with COVID, which can worsen symptoms, but a new Swedish study says there is no evidence that it will cause postmenopausal problems like irregular bleeding.
“Weak and inconsistent associations were observed between SARS-CoV-2 vaccination and healthcare contacts for bleeding in women who are postmenopausal, and even less evidence was recorded of an association for menstrual disturbance or bleeding in women who were premenopausal,” said study coauthor Rickard Ljung, MD, PhD, MPH, professor and acting head of the pharmacoepidemiology and analysis department in the division of use and information of the Swedish Medical Products Agency in Uppsala.
A version of this article first appeared on WebMD.com.
British researchers have noted that women at midlife who have long COVID seem to get specific, and severe, symptoms, including brain fog, fatigue, new-onset dizziness, and difficulty sleeping through the night.
Doctors also think it’s possible that long COVID worsens the symptoms of perimenopause and menopause. Lower levels of estrogen and testosterone appear to be the reason.
“A long COVID theory is that there is a temporary disruption to physiological ovarian steroid hormone production, which could [worsen] symptoms of perimenopause and menopause,” said JoAnn V. Pinkerton, MD, professor of obstetrics at the University of Virginia, Charlottesville, and executive director of the North American Menopause Society.
Long COVID symptoms and menopause symptoms can also be very hard to tell apart.
Another U.K. study cautions that because of this kind of symptom overlap, women at midlife may be misdiagnosed. Research from the North American Menopause Society shows that many women may have trouble recovering from long COVID unless their hormone deficiency is treated.
What are the symptoms of long COVID?
There are over 200 symptoms that have been associated with long COVID, according to the American Medical Association. Some common symptoms are currently defined as the following: feeling extremely tired, feeling depleted after exertion, cognitive issues such as brain fog, heart beating over 100 times a minute, and a loss of sense of smell and taste.
Long COVID symptoms begin a few weeks to a few months after a COVID infection. They can last an indefinite amount of time, but “the hope is that long COVID will not be lifelong,” said Clare Flannery, MD, an endocrinologist and associate professor in the departments of obstetrics, gynecology and reproductive sciences and internal medicine at Yale University, New Haven, Conn.
What are the symptoms of menopause?
Some symptoms of menopause include vaginal infections, irregular bleeding, urinary problems, and sexual problems.
Women in their middle years have other symptoms that can be the same as perimenopause/menopause symptoms.
“Common symptoms of perimenopause and menopause which may also be symptoms ascribed to long COVID include hot flashes, night sweats, disrupted sleep, low mood, depression or anxiety, decreased concentration, memory problems, joint and muscle pains, and headaches,” Dr. Pinkerton said.
Can long COVID actually bring on menopause?
In short: Possibly.
A new study from the Massachusetts Institute of Technology/Patient-Led Research Collaborative/University of California, San Francisco, found that long COVID can cause disruptions to a woman’s menstrual cycle, ovaries, fertility, and menopause itself.
This could be caused by chronic inflammation caused by long COVID on hormones as well. This kind of inflammatory response could explain irregularities in a woman’s menstrual cycle, according to the Newson Health Research and Education study. For instance, “when the body has inflammation, ovulation can happen,” Dr. Flannery said.
The mechanism for how long COVID could spur menopause can also involve a woman’s ovaries.
“Since the theory is that COVID affects the ovary with declines in ovarian reserve and ovarian function, it makes sense that long COVID could bring on symptoms of perimenopause or menopause more acutely or more severely and lengthen the symptoms of the perimenopause and menopausal transition,” Dr. Pinkerton said.
How can hormone replacement therapy benefit women dealing with long COVID during menopause?
Estradiol, the strongest estrogen hormone in a woman’s body, has already been shown to have a positive effect against COVID.
“Estradiol therapy treats symptoms more aggressively in the setting of long COVID,” said Dr. Flannery.
Estradiol is also a form of hormone therapy for menopause symptoms.
“Estradiol has been shown to help hot flashes, night sweats, and sleep and improve mood during perimenopause,” said Dr. Pinkerton. “So it’s likely that perimenopausal or menopausal women with long COVID would see improvements both due to the action of estradiol on the ovary seen during COVID and the improvements in symptoms.”
Estrogen-based hormone therapy has been linked to an increased risk for endometrial, breast, and ovarian cancer, according to the American Cancer Society. This means you should carefully consider how comfortable you are with those additional risks before starting this kind of therapy.
“Which of your symptoms are the most difficult to manage? You may see if you can navigate one to three of them. What are you willing to do for your symptoms? If a woman is willing to favor her sleep for the next 6 months to a year, she may be willing to change how she perceives her risk for cancer,” Dr. Flannery said. “What risk is a woman willing to take? I think if someone has a very low concern about a risk of cancer, and she’s suffering a disrupted life, then taking estradiol in a 1- to 2-year trial period could be critical to help.”
What else can help ease long COVID during menopause?
Getting the COVID vaccine, as well as getting a booster, could help. Not only will this help prevent people from being reinfected with COVID, which can worsen symptoms, but a new Swedish study says there is no evidence that it will cause postmenopausal problems like irregular bleeding.
“Weak and inconsistent associations were observed between SARS-CoV-2 vaccination and healthcare contacts for bleeding in women who are postmenopausal, and even less evidence was recorded of an association for menstrual disturbance or bleeding in women who were premenopausal,” said study coauthor Rickard Ljung, MD, PhD, MPH, professor and acting head of the pharmacoepidemiology and analysis department in the division of use and information of the Swedish Medical Products Agency in Uppsala.
A version of this article first appeared on WebMD.com.
Significant increase in vitamin D deficiency in kids with major depressive disorder
SAN FRANCISCO – , according to new findings that suggest spending more time indoors may have fueled this uptick.
“We suspect that this may be due to the COVID lockdowns and kids schooling from home and having less time outside,” study investigator Oluwatomiwa Babade, MD, MPH, with Virginia Tech Carilion School of Medicine, Roanoke, Va., said in an interview.
The study was presented at the annual meeting of the American Psychiatric Association.
Anecdotal observation confirmed
During the pandemic, investigators noticed an uptick in the number of children and adolescents attending their clinic for psychiatric hospitalization who had low vitamin D levels.
To investigate, they analyzed the records of all patients aged 6-17 years with psychiatric diagnoses and vitamin D level assessment who were admitted into the inpatient psychiatry unit from March 18, 2020, to June 30, 2021.
Among 599 unique patients, 275 (83% female) had a diagnosis of MDD and 226 of these patients were vitamin D deficient (< 30 ng/mL) – a prevalence rate of roughly 82%. Among 246 patients with psychiatric disorders other than MDD, the prevalence of vitamin D deficiency was 76%.
“This was very surprising and much higher than prior to the pandemic. Prior to COVID, the prevalence of vitamin D deficiency was around 14% in similar patients,” Dr. Babade said.
“Now that we are post-lockdown, it would be good to repeat the study. I think the prevalence should drop. That’s my guess,” he added.
Important research, no surprises
In a comment, Cemre Robinson, MD, director of the Mount Sinai Pediatric Bone Health and Calcium Metabolism Clinic, New York, said that although the study’s findings aren’t surprising, “it’s important to present such data in adolescents with major depression.”
“These findings reiterate the importance of screening for vitamin D deficiency in children and adolescents, with or without depression, particularly during winter, which is associated with less sun exposure,” Dr. Robinson, assistant professor of pediatrics, endocrinology, and diabetes at Icahn School of Medicine at Mount Sinai, said.
She noted that vitamin D deficiency is prevalent in the general population, and it can be easily corrected with supplementation.
“Vitamin D is important for bone growth, mineralization, and accretion as well as calcium absorption. Adolescence, in particular, is a period of rapid physical, cognitive, and psychosocial growth,” Dr. Robinson said.
“The requirement of all minerals and vitamins changes in this phase of life. Therefore, it is important to have sufficient vitamin D levels during adolescence for several health benefits,” she noted.
Dr. Robinson said that “more research is needed to validate the present findings in adolescents with major depression, and larger studies, including randomized control trials, are required to establish a causal association between MDD and vitamin D deficiency.”
The study had no specific funding. Dr. Babade and Dr. Robinson report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO – , according to new findings that suggest spending more time indoors may have fueled this uptick.
“We suspect that this may be due to the COVID lockdowns and kids schooling from home and having less time outside,” study investigator Oluwatomiwa Babade, MD, MPH, with Virginia Tech Carilion School of Medicine, Roanoke, Va., said in an interview.
The study was presented at the annual meeting of the American Psychiatric Association.
Anecdotal observation confirmed
During the pandemic, investigators noticed an uptick in the number of children and adolescents attending their clinic for psychiatric hospitalization who had low vitamin D levels.
To investigate, they analyzed the records of all patients aged 6-17 years with psychiatric diagnoses and vitamin D level assessment who were admitted into the inpatient psychiatry unit from March 18, 2020, to June 30, 2021.
Among 599 unique patients, 275 (83% female) had a diagnosis of MDD and 226 of these patients were vitamin D deficient (< 30 ng/mL) – a prevalence rate of roughly 82%. Among 246 patients with psychiatric disorders other than MDD, the prevalence of vitamin D deficiency was 76%.
“This was very surprising and much higher than prior to the pandemic. Prior to COVID, the prevalence of vitamin D deficiency was around 14% in similar patients,” Dr. Babade said.
“Now that we are post-lockdown, it would be good to repeat the study. I think the prevalence should drop. That’s my guess,” he added.
Important research, no surprises
In a comment, Cemre Robinson, MD, director of the Mount Sinai Pediatric Bone Health and Calcium Metabolism Clinic, New York, said that although the study’s findings aren’t surprising, “it’s important to present such data in adolescents with major depression.”
“These findings reiterate the importance of screening for vitamin D deficiency in children and adolescents, with or without depression, particularly during winter, which is associated with less sun exposure,” Dr. Robinson, assistant professor of pediatrics, endocrinology, and diabetes at Icahn School of Medicine at Mount Sinai, said.
She noted that vitamin D deficiency is prevalent in the general population, and it can be easily corrected with supplementation.
“Vitamin D is important for bone growth, mineralization, and accretion as well as calcium absorption. Adolescence, in particular, is a period of rapid physical, cognitive, and psychosocial growth,” Dr. Robinson said.
“The requirement of all minerals and vitamins changes in this phase of life. Therefore, it is important to have sufficient vitamin D levels during adolescence for several health benefits,” she noted.
Dr. Robinson said that “more research is needed to validate the present findings in adolescents with major depression, and larger studies, including randomized control trials, are required to establish a causal association between MDD and vitamin D deficiency.”
The study had no specific funding. Dr. Babade and Dr. Robinson report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO – , according to new findings that suggest spending more time indoors may have fueled this uptick.
“We suspect that this may be due to the COVID lockdowns and kids schooling from home and having less time outside,” study investigator Oluwatomiwa Babade, MD, MPH, with Virginia Tech Carilion School of Medicine, Roanoke, Va., said in an interview.
The study was presented at the annual meeting of the American Psychiatric Association.
Anecdotal observation confirmed
During the pandemic, investigators noticed an uptick in the number of children and adolescents attending their clinic for psychiatric hospitalization who had low vitamin D levels.
To investigate, they analyzed the records of all patients aged 6-17 years with psychiatric diagnoses and vitamin D level assessment who were admitted into the inpatient psychiatry unit from March 18, 2020, to June 30, 2021.
Among 599 unique patients, 275 (83% female) had a diagnosis of MDD and 226 of these patients were vitamin D deficient (< 30 ng/mL) – a prevalence rate of roughly 82%. Among 246 patients with psychiatric disorders other than MDD, the prevalence of vitamin D deficiency was 76%.
“This was very surprising and much higher than prior to the pandemic. Prior to COVID, the prevalence of vitamin D deficiency was around 14% in similar patients,” Dr. Babade said.
“Now that we are post-lockdown, it would be good to repeat the study. I think the prevalence should drop. That’s my guess,” he added.
Important research, no surprises
In a comment, Cemre Robinson, MD, director of the Mount Sinai Pediatric Bone Health and Calcium Metabolism Clinic, New York, said that although the study’s findings aren’t surprising, “it’s important to present such data in adolescents with major depression.”
“These findings reiterate the importance of screening for vitamin D deficiency in children and adolescents, with or without depression, particularly during winter, which is associated with less sun exposure,” Dr. Robinson, assistant professor of pediatrics, endocrinology, and diabetes at Icahn School of Medicine at Mount Sinai, said.
She noted that vitamin D deficiency is prevalent in the general population, and it can be easily corrected with supplementation.
“Vitamin D is important for bone growth, mineralization, and accretion as well as calcium absorption. Adolescence, in particular, is a period of rapid physical, cognitive, and psychosocial growth,” Dr. Robinson said.
“The requirement of all minerals and vitamins changes in this phase of life. Therefore, it is important to have sufficient vitamin D levels during adolescence for several health benefits,” she noted.
Dr. Robinson said that “more research is needed to validate the present findings in adolescents with major depression, and larger studies, including randomized control trials, are required to establish a causal association between MDD and vitamin D deficiency.”
The study had no specific funding. Dr. Babade and Dr. Robinson report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT APA 2023
Common fracture risk predictors often fail for women of any race
according to a study published in JAMA Internal Medicine.
One of the screenings, the U.S. Fracture Risk Assessment Tool (FRAX), proved relatively ineffective at identifying women who developed osteoporosis. The other screening, the Osteoporosis Self-Assessment Tool (OST), excelled at identifying osteoporosis for women in every racial and ethnic group, but also failed at identifying who was most likely to experience a fracture. Osteoporosis experts say that primary care physicians should test for the condition in anyone with any risk factor for it, even if a screening tool suggests doing so is unnecessary.
The United States Preventive Services Task Force (USPSTF) recommends routine testing of bone mineral density in women age 65 years and older to detect risk of developing osteoporosis, which in turn leads to an increased risk for fractures of the hip, spine, shoulder, or forearm. For women aged 50-64, whether bone mineral density accurately reflects who will develop osteoporosis is less clear. In this age range, the USPSTF recommends using either FRAX or OST rather than routine bone mineral density tests.
“I have the utmost respect for the United States Preventive Services Task Force, which lists both of these as valid screening tools for younger postmenopausal women. What I hope this study does is to inform the next iteration of the screening guidelines,” by maintaining the recommendation to use the OST while not keeping FRAX, said Carolyn J. Crandall, MD, MS, an internal medicine physician and health services researcher at University of California, Los Angeles, who helped conduct the research.
The U.S. version of FRAX requires identifying someone’s race, height, and weight, then answering whether they have different risk factors for a fracture such as a previous fracture, rheumatoid arthritis, or smoking. The result was thought to indicate a cumulative risk for major fracture over the next 10 years. Patients at significant risk should then undergo a bone density test.
The tool can also incorporate information about bone mineral density, if available, but the FRAX analyses in Dr. Crandall’s study did not include those data because the study aimed to test the measure’s predictive ability in the absence of a bone scan.
The OST includes only two variables – weight and age – to calculate risk for osteoporosis, and generally takes seconds to complete. It does not include race. As with FRAX, anyone deemed at significant risk for developing osteoporosis should undergo a bone density test.
“OST is really simple; that makes it very appealing,” Dr. Crandall said. “OST could probably be automatically calculated in the electronic medical record.”
Using data from the Women’s Health Initiative, Dr. Crandall and colleagues tracked more than 67,000 women aged 50-64 years for 10 years following enrollment in the study to see who experienced a fracture or developed osteoporosis over that decade. The investigators found that neither FRAX nor OST was particularly good at predicting who went on to experience a fracture.
The accuracy of FRAX at fracture prediction peaked at 65% for Asian women (area under the receiver operating curve, 0.65; 95% confidence interval, 0.58-0.71), and was lowest for Black women (AUC 0.55; 95% CI, 0.52-0.59). OST also was most accurate for Asian women, but only up to 62% (AUC 0.62; 95% CI, 0.56-0.69), and was again lowest for Black women (AUC 0.53; 95% CI, 0.50 - 0.57)
“It is just very hard to predict fractures in this age group,” Dr. Crandall said, noting that more evidence exists about risk for fracture in people older than 65.
The story diverges with predicting risk of osteoporosis in the neck. The OST did this roughly 80% of the time, for all racial groups. That figure proved better than FRAX, without including race.
Treatment gap
“This evidence supports using OST instead of FRAX” for selecting younger postmenopausal women who should undergo a bone mineral density exam, said E. Michael Lewiecki, MD, director of the New Mexico Clinical Research & Osteoporosis Center in Albuquerque.

Dr. Lewiecki, who was not involved in the new study, noted that the U.S. version of FRAX specifies race because of some clinical evidence that different races have different rates of fracture. But he and Dr. Crandall said the validity of race-based algorithms to guide clinical care is a controversial and evolving topic in medicine. Dr. Lewiecki said the Canadian version of FRAX, which is similarly applied to a diverse population as in the United States, omits race and works as well as the U.S. version. Future iterations of the instrument in the United States may not include race, Dr. Lewiecki said.
“The study is perfectly valid as far as it goes. But the big gorilla in the room is that most patients who need a bone density test are not getting it,” Dr. Lewiecki added. Sometimes a patient might break a bone in their wrist, for example, and tell their primary care provider that anyone would have broken that bone because the fall was so hard. Even if that’s true, Dr. Lewiecki said, any woman older than 45 who has broken a bone should undergo a bone density test to determine if they have osteoporosis, even if it seems like there are other possible reasons for why the break occurred.
“Most of the clinical practice guidelines that are used by physicians recommend getting a bone density test in postmenopausal women under the age of 65 who have a risk factor for fracture,” Dr. Lewiecki said, with a primary risk factor being a prior fracture. Dr. Lewiecki said he would rather that anyone who could benefit from a bone density test receive it, rather than someone foregoing a scan based on a screening tool that may be flawed.
“Most patients – men and women – who have osteoporosis are currently not being identified. Even when they are being identified, they are commonly not being treated. And when they are started on treatment, many patients discontinue treatment before they’ve taken it long enough to benefit,” Dr. Lewiecki said.
Dr. Crandall and Dr. Lewiecki report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a study published in JAMA Internal Medicine.
One of the screenings, the U.S. Fracture Risk Assessment Tool (FRAX), proved relatively ineffective at identifying women who developed osteoporosis. The other screening, the Osteoporosis Self-Assessment Tool (OST), excelled at identifying osteoporosis for women in every racial and ethnic group, but also failed at identifying who was most likely to experience a fracture. Osteoporosis experts say that primary care physicians should test for the condition in anyone with any risk factor for it, even if a screening tool suggests doing so is unnecessary.
The United States Preventive Services Task Force (USPSTF) recommends routine testing of bone mineral density in women age 65 years and older to detect risk of developing osteoporosis, which in turn leads to an increased risk for fractures of the hip, spine, shoulder, or forearm. For women aged 50-64, whether bone mineral density accurately reflects who will develop osteoporosis is less clear. In this age range, the USPSTF recommends using either FRAX or OST rather than routine bone mineral density tests.
“I have the utmost respect for the United States Preventive Services Task Force, which lists both of these as valid screening tools for younger postmenopausal women. What I hope this study does is to inform the next iteration of the screening guidelines,” by maintaining the recommendation to use the OST while not keeping FRAX, said Carolyn J. Crandall, MD, MS, an internal medicine physician and health services researcher at University of California, Los Angeles, who helped conduct the research.
The U.S. version of FRAX requires identifying someone’s race, height, and weight, then answering whether they have different risk factors for a fracture such as a previous fracture, rheumatoid arthritis, or smoking. The result was thought to indicate a cumulative risk for major fracture over the next 10 years. Patients at significant risk should then undergo a bone density test.
The tool can also incorporate information about bone mineral density, if available, but the FRAX analyses in Dr. Crandall’s study did not include those data because the study aimed to test the measure’s predictive ability in the absence of a bone scan.
The OST includes only two variables – weight and age – to calculate risk for osteoporosis, and generally takes seconds to complete. It does not include race. As with FRAX, anyone deemed at significant risk for developing osteoporosis should undergo a bone density test.
“OST is really simple; that makes it very appealing,” Dr. Crandall said. “OST could probably be automatically calculated in the electronic medical record.”
Using data from the Women’s Health Initiative, Dr. Crandall and colleagues tracked more than 67,000 women aged 50-64 years for 10 years following enrollment in the study to see who experienced a fracture or developed osteoporosis over that decade. The investigators found that neither FRAX nor OST was particularly good at predicting who went on to experience a fracture.
The accuracy of FRAX at fracture prediction peaked at 65% for Asian women (area under the receiver operating curve, 0.65; 95% confidence interval, 0.58-0.71), and was lowest for Black women (AUC 0.55; 95% CI, 0.52-0.59). OST also was most accurate for Asian women, but only up to 62% (AUC 0.62; 95% CI, 0.56-0.69), and was again lowest for Black women (AUC 0.53; 95% CI, 0.50 - 0.57)
“It is just very hard to predict fractures in this age group,” Dr. Crandall said, noting that more evidence exists about risk for fracture in people older than 65.
The story diverges with predicting risk of osteoporosis in the neck. The OST did this roughly 80% of the time, for all racial groups. That figure proved better than FRAX, without including race.
Treatment gap
“This evidence supports using OST instead of FRAX” for selecting younger postmenopausal women who should undergo a bone mineral density exam, said E. Michael Lewiecki, MD, director of the New Mexico Clinical Research & Osteoporosis Center in Albuquerque.

Dr. Lewiecki, who was not involved in the new study, noted that the U.S. version of FRAX specifies race because of some clinical evidence that different races have different rates of fracture. But he and Dr. Crandall said the validity of race-based algorithms to guide clinical care is a controversial and evolving topic in medicine. Dr. Lewiecki said the Canadian version of FRAX, which is similarly applied to a diverse population as in the United States, omits race and works as well as the U.S. version. Future iterations of the instrument in the United States may not include race, Dr. Lewiecki said.
“The study is perfectly valid as far as it goes. But the big gorilla in the room is that most patients who need a bone density test are not getting it,” Dr. Lewiecki added. Sometimes a patient might break a bone in their wrist, for example, and tell their primary care provider that anyone would have broken that bone because the fall was so hard. Even if that’s true, Dr. Lewiecki said, any woman older than 45 who has broken a bone should undergo a bone density test to determine if they have osteoporosis, even if it seems like there are other possible reasons for why the break occurred.
“Most of the clinical practice guidelines that are used by physicians recommend getting a bone density test in postmenopausal women under the age of 65 who have a risk factor for fracture,” Dr. Lewiecki said, with a primary risk factor being a prior fracture. Dr. Lewiecki said he would rather that anyone who could benefit from a bone density test receive it, rather than someone foregoing a scan based on a screening tool that may be flawed.
“Most patients – men and women – who have osteoporosis are currently not being identified. Even when they are being identified, they are commonly not being treated. And when they are started on treatment, many patients discontinue treatment before they’ve taken it long enough to benefit,” Dr. Lewiecki said.
Dr. Crandall and Dr. Lewiecki report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a study published in JAMA Internal Medicine.
One of the screenings, the U.S. Fracture Risk Assessment Tool (FRAX), proved relatively ineffective at identifying women who developed osteoporosis. The other screening, the Osteoporosis Self-Assessment Tool (OST), excelled at identifying osteoporosis for women in every racial and ethnic group, but also failed at identifying who was most likely to experience a fracture. Osteoporosis experts say that primary care physicians should test for the condition in anyone with any risk factor for it, even if a screening tool suggests doing so is unnecessary.
The United States Preventive Services Task Force (USPSTF) recommends routine testing of bone mineral density in women age 65 years and older to detect risk of developing osteoporosis, which in turn leads to an increased risk for fractures of the hip, spine, shoulder, or forearm. For women aged 50-64, whether bone mineral density accurately reflects who will develop osteoporosis is less clear. In this age range, the USPSTF recommends using either FRAX or OST rather than routine bone mineral density tests.
“I have the utmost respect for the United States Preventive Services Task Force, which lists both of these as valid screening tools for younger postmenopausal women. What I hope this study does is to inform the next iteration of the screening guidelines,” by maintaining the recommendation to use the OST while not keeping FRAX, said Carolyn J. Crandall, MD, MS, an internal medicine physician and health services researcher at University of California, Los Angeles, who helped conduct the research.
The U.S. version of FRAX requires identifying someone’s race, height, and weight, then answering whether they have different risk factors for a fracture such as a previous fracture, rheumatoid arthritis, or smoking. The result was thought to indicate a cumulative risk for major fracture over the next 10 years. Patients at significant risk should then undergo a bone density test.
The tool can also incorporate information about bone mineral density, if available, but the FRAX analyses in Dr. Crandall’s study did not include those data because the study aimed to test the measure’s predictive ability in the absence of a bone scan.
The OST includes only two variables – weight and age – to calculate risk for osteoporosis, and generally takes seconds to complete. It does not include race. As with FRAX, anyone deemed at significant risk for developing osteoporosis should undergo a bone density test.
“OST is really simple; that makes it very appealing,” Dr. Crandall said. “OST could probably be automatically calculated in the electronic medical record.”
Using data from the Women’s Health Initiative, Dr. Crandall and colleagues tracked more than 67,000 women aged 50-64 years for 10 years following enrollment in the study to see who experienced a fracture or developed osteoporosis over that decade. The investigators found that neither FRAX nor OST was particularly good at predicting who went on to experience a fracture.
The accuracy of FRAX at fracture prediction peaked at 65% for Asian women (area under the receiver operating curve, 0.65; 95% confidence interval, 0.58-0.71), and was lowest for Black women (AUC 0.55; 95% CI, 0.52-0.59). OST also was most accurate for Asian women, but only up to 62% (AUC 0.62; 95% CI, 0.56-0.69), and was again lowest for Black women (AUC 0.53; 95% CI, 0.50 - 0.57)
“It is just very hard to predict fractures in this age group,” Dr. Crandall said, noting that more evidence exists about risk for fracture in people older than 65.
The story diverges with predicting risk of osteoporosis in the neck. The OST did this roughly 80% of the time, for all racial groups. That figure proved better than FRAX, without including race.
Treatment gap
“This evidence supports using OST instead of FRAX” for selecting younger postmenopausal women who should undergo a bone mineral density exam, said E. Michael Lewiecki, MD, director of the New Mexico Clinical Research & Osteoporosis Center in Albuquerque.

Dr. Lewiecki, who was not involved in the new study, noted that the U.S. version of FRAX specifies race because of some clinical evidence that different races have different rates of fracture. But he and Dr. Crandall said the validity of race-based algorithms to guide clinical care is a controversial and evolving topic in medicine. Dr. Lewiecki said the Canadian version of FRAX, which is similarly applied to a diverse population as in the United States, omits race and works as well as the U.S. version. Future iterations of the instrument in the United States may not include race, Dr. Lewiecki said.
“The study is perfectly valid as far as it goes. But the big gorilla in the room is that most patients who need a bone density test are not getting it,” Dr. Lewiecki added. Sometimes a patient might break a bone in their wrist, for example, and tell their primary care provider that anyone would have broken that bone because the fall was so hard. Even if that’s true, Dr. Lewiecki said, any woman older than 45 who has broken a bone should undergo a bone density test to determine if they have osteoporosis, even if it seems like there are other possible reasons for why the break occurred.
“Most of the clinical practice guidelines that are used by physicians recommend getting a bone density test in postmenopausal women under the age of 65 who have a risk factor for fracture,” Dr. Lewiecki said, with a primary risk factor being a prior fracture. Dr. Lewiecki said he would rather that anyone who could benefit from a bone density test receive it, rather than someone foregoing a scan based on a screening tool that may be flawed.
“Most patients – men and women – who have osteoporosis are currently not being identified. Even when they are being identified, they are commonly not being treated. And when they are started on treatment, many patients discontinue treatment before they’ve taken it long enough to benefit,” Dr. Lewiecki said.
Dr. Crandall and Dr. Lewiecki report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA INTERNAL MEDICINE
Should you prescribe bioidentical hormones for menopause?
BALTIMORE – according to an expert at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists (ACOG).
Clinicians write an estimated 26 to 33 million prescriptions for compounded bioidentical hormone therapy (cBHT) every year, and almost 41% of menopausal women who need treatment try cBHT during their lives. But these drugs lack the approval for this indication from the Food and Drug Administration.
“There is a public perception that this is natural, safer, and anti-aging,” said Robert Kauffman, MD, a professor of obstetrics and gynecology and assistant dean for research at Texas Tech University Health Sciences Center in Amarillo.
Following the 2002 Women’s Health Initiative report showing a link between hormone therapy (HT) and an increase in the incidence of breast cancer, medical schools have slowed or paused instructing trainees on the traditional treatment, Dr. Kauffman said. The association was later determined to be spurious: HT is not associated with a risk for all-cause mortality or deaths from cardiovascular disease or cancer. However, HT still is largely ignored by younger physicians, Dr. Kauffman said, because of unsubstantiated “dangers” such as heart attack, stroke, and deep vein thrombosis.
The lack of education on HT for medical school students and residents has “opened the door to unsubstantiated marketing claims and practices” for cBHT, Dr. Kauffman said. “Hence, the use of compounded bioidentical hormone therapy has increased” as clinicians look for alternatives.
Groups including ACOG, the North American Menopause Society (NAMS), and the U.S. Preventive Services Task Force recommend against the use of Non–FDA-approved therapies such as cBHT, except for narrow indications. Dr. Kauffman said that drug manufacturers have not conducted randomized controlled trials or observational studies on cBHT in treating menopause.
He cited studies showing quality problems with the compounding process of these drugs, and wide variations in the amount of actual ingredients from product labels. One 2021 study published in Menopause comparing patients taking cBHT or FDA-approved HT found that side effects were significantly higher in the cBHT group (57.6% vs. 14.8%; P < .0001).
But manufacturers of cBHT claim that their products prevent cardiovascular disease and Alzheimer’s disease and decrease the risk for breast cancer and stroke – assertions that are at best unproven, according to Dr. Kauffman.
The National Academies of Sciences, Engineering, and Medicine in 2020 said that clinicians have a duty to inform patients of the insufficient evidence to support clinical use of cBHT and should prescribe the products only to patients with documented allergies to an active ingredient in an FDA-approved agent or who require an alternative dosage.
Patients may also have to pay much more out of pocket for cBHT products because they often are not covered by insurance. Generic HT products, meanwhile, are relatively inexpensive and typically are covered, he noted.
“We have to be careful to avoid financial harm to patients by prescribing things, which are much more expensive than those which are usually available,” Dr. Kauffman said.
Prescribing any non–FDA-approved product, especially when biosimilars are available, places physicians at legal risk, Dr. Kauffman said. Physicians who recommend cBHT should inform patients that the products are not FDA approved and carefully document this discussion in the patient’s electronic health record. State boards of medicine can sanction physicians for “coercion” for prescribing cBHT products without mentioning alternatives, he added.
JoAnn Pinkerton, MD, professor of obstetrics and gynecology at the University of Virginia, Charlottesville, and executive director emeritus of NAMS, who attended the session, praised Dr. Kauffman for providing a balanced and evidence-based overview of the subject.
“There are issues concerning safety, contaminants, and not knowing exactly what dose you’re getting,” with compounded hormones, Dr. Pinkerton said. “They’re being hyped as safer and more effective when in reality, we don’t have any studies that show that information.”
Dr. Pinkerton noted that while a compounded form of physiological testosterone might be relatively reliable, “if you’re using something like a pellet that is super physiologic with incredibly high doses, that you really don’t have any information to stand on that it’s safe or effective ... it might be putting your license at risk.”
A version of this article first appeared on Medscape.com.
BALTIMORE – according to an expert at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists (ACOG).
Clinicians write an estimated 26 to 33 million prescriptions for compounded bioidentical hormone therapy (cBHT) every year, and almost 41% of menopausal women who need treatment try cBHT during their lives. But these drugs lack the approval for this indication from the Food and Drug Administration.
“There is a public perception that this is natural, safer, and anti-aging,” said Robert Kauffman, MD, a professor of obstetrics and gynecology and assistant dean for research at Texas Tech University Health Sciences Center in Amarillo.
Following the 2002 Women’s Health Initiative report showing a link between hormone therapy (HT) and an increase in the incidence of breast cancer, medical schools have slowed or paused instructing trainees on the traditional treatment, Dr. Kauffman said. The association was later determined to be spurious: HT is not associated with a risk for all-cause mortality or deaths from cardiovascular disease or cancer. However, HT still is largely ignored by younger physicians, Dr. Kauffman said, because of unsubstantiated “dangers” such as heart attack, stroke, and deep vein thrombosis.
The lack of education on HT for medical school students and residents has “opened the door to unsubstantiated marketing claims and practices” for cBHT, Dr. Kauffman said. “Hence, the use of compounded bioidentical hormone therapy has increased” as clinicians look for alternatives.
Groups including ACOG, the North American Menopause Society (NAMS), and the U.S. Preventive Services Task Force recommend against the use of Non–FDA-approved therapies such as cBHT, except for narrow indications. Dr. Kauffman said that drug manufacturers have not conducted randomized controlled trials or observational studies on cBHT in treating menopause.
He cited studies showing quality problems with the compounding process of these drugs, and wide variations in the amount of actual ingredients from product labels. One 2021 study published in Menopause comparing patients taking cBHT or FDA-approved HT found that side effects were significantly higher in the cBHT group (57.6% vs. 14.8%; P < .0001).
But manufacturers of cBHT claim that their products prevent cardiovascular disease and Alzheimer’s disease and decrease the risk for breast cancer and stroke – assertions that are at best unproven, according to Dr. Kauffman.
The National Academies of Sciences, Engineering, and Medicine in 2020 said that clinicians have a duty to inform patients of the insufficient evidence to support clinical use of cBHT and should prescribe the products only to patients with documented allergies to an active ingredient in an FDA-approved agent or who require an alternative dosage.
Patients may also have to pay much more out of pocket for cBHT products because they often are not covered by insurance. Generic HT products, meanwhile, are relatively inexpensive and typically are covered, he noted.
“We have to be careful to avoid financial harm to patients by prescribing things, which are much more expensive than those which are usually available,” Dr. Kauffman said.
Prescribing any non–FDA-approved product, especially when biosimilars are available, places physicians at legal risk, Dr. Kauffman said. Physicians who recommend cBHT should inform patients that the products are not FDA approved and carefully document this discussion in the patient’s electronic health record. State boards of medicine can sanction physicians for “coercion” for prescribing cBHT products without mentioning alternatives, he added.
JoAnn Pinkerton, MD, professor of obstetrics and gynecology at the University of Virginia, Charlottesville, and executive director emeritus of NAMS, who attended the session, praised Dr. Kauffman for providing a balanced and evidence-based overview of the subject.
“There are issues concerning safety, contaminants, and not knowing exactly what dose you’re getting,” with compounded hormones, Dr. Pinkerton said. “They’re being hyped as safer and more effective when in reality, we don’t have any studies that show that information.”
Dr. Pinkerton noted that while a compounded form of physiological testosterone might be relatively reliable, “if you’re using something like a pellet that is super physiologic with incredibly high doses, that you really don’t have any information to stand on that it’s safe or effective ... it might be putting your license at risk.”
A version of this article first appeared on Medscape.com.
BALTIMORE – according to an expert at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists (ACOG).
Clinicians write an estimated 26 to 33 million prescriptions for compounded bioidentical hormone therapy (cBHT) every year, and almost 41% of menopausal women who need treatment try cBHT during their lives. But these drugs lack the approval for this indication from the Food and Drug Administration.
“There is a public perception that this is natural, safer, and anti-aging,” said Robert Kauffman, MD, a professor of obstetrics and gynecology and assistant dean for research at Texas Tech University Health Sciences Center in Amarillo.
Following the 2002 Women’s Health Initiative report showing a link between hormone therapy (HT) and an increase in the incidence of breast cancer, medical schools have slowed or paused instructing trainees on the traditional treatment, Dr. Kauffman said. The association was later determined to be spurious: HT is not associated with a risk for all-cause mortality or deaths from cardiovascular disease or cancer. However, HT still is largely ignored by younger physicians, Dr. Kauffman said, because of unsubstantiated “dangers” such as heart attack, stroke, and deep vein thrombosis.
The lack of education on HT for medical school students and residents has “opened the door to unsubstantiated marketing claims and practices” for cBHT, Dr. Kauffman said. “Hence, the use of compounded bioidentical hormone therapy has increased” as clinicians look for alternatives.
Groups including ACOG, the North American Menopause Society (NAMS), and the U.S. Preventive Services Task Force recommend against the use of Non–FDA-approved therapies such as cBHT, except for narrow indications. Dr. Kauffman said that drug manufacturers have not conducted randomized controlled trials or observational studies on cBHT in treating menopause.
He cited studies showing quality problems with the compounding process of these drugs, and wide variations in the amount of actual ingredients from product labels. One 2021 study published in Menopause comparing patients taking cBHT or FDA-approved HT found that side effects were significantly higher in the cBHT group (57.6% vs. 14.8%; P < .0001).
But manufacturers of cBHT claim that their products prevent cardiovascular disease and Alzheimer’s disease and decrease the risk for breast cancer and stroke – assertions that are at best unproven, according to Dr. Kauffman.
The National Academies of Sciences, Engineering, and Medicine in 2020 said that clinicians have a duty to inform patients of the insufficient evidence to support clinical use of cBHT and should prescribe the products only to patients with documented allergies to an active ingredient in an FDA-approved agent or who require an alternative dosage.
Patients may also have to pay much more out of pocket for cBHT products because they often are not covered by insurance. Generic HT products, meanwhile, are relatively inexpensive and typically are covered, he noted.
“We have to be careful to avoid financial harm to patients by prescribing things, which are much more expensive than those which are usually available,” Dr. Kauffman said.
Prescribing any non–FDA-approved product, especially when biosimilars are available, places physicians at legal risk, Dr. Kauffman said. Physicians who recommend cBHT should inform patients that the products are not FDA approved and carefully document this discussion in the patient’s electronic health record. State boards of medicine can sanction physicians for “coercion” for prescribing cBHT products without mentioning alternatives, he added.
JoAnn Pinkerton, MD, professor of obstetrics and gynecology at the University of Virginia, Charlottesville, and executive director emeritus of NAMS, who attended the session, praised Dr. Kauffman for providing a balanced and evidence-based overview of the subject.
“There are issues concerning safety, contaminants, and not knowing exactly what dose you’re getting,” with compounded hormones, Dr. Pinkerton said. “They’re being hyped as safer and more effective when in reality, we don’t have any studies that show that information.”
Dr. Pinkerton noted that while a compounded form of physiological testosterone might be relatively reliable, “if you’re using something like a pellet that is super physiologic with incredibly high doses, that you really don’t have any information to stand on that it’s safe or effective ... it might be putting your license at risk.”
A version of this article first appeared on Medscape.com.
AT ACOG 2023
Half of deaths from homozygous FH occur before age 32 years
MANNHEIM, GERMANY –
The researchers looked at almost 40 patients from the HoFH International Clinical Collaborators (HICC) registry who had died before data entry, finding that they had a mean age of diagnosis of 12 years.
Even those who received treatment had high LDL cholesterol levels, and 70% developed atherosclerotic cardiovascular disease (ASCVD) at a median age of 28 years.
Worryingly, the results showed that the median age at death was 32 years. Results were presented at the annual congress of the European Atherosclerosis Society.
Patients with HoFH “have severe atherosclerotic cardiovascular disease risk,” said study presenter Janneke Mulder, a PhD candidate at the department of internal medicine, Erasmus University Medical Center, Rotterdam, the Netherlands.
“Therefore, early diagnosis and initiation of treatments, and also a combination of treatments, is really crucial,” she added.
Call to action
Approached for comment, Maciej Banach, MD, PhD, full professor of cardiology, Polish Mother’s Memorial Hospital Research Institute, Lodz, and Secretary of the EAS, described the results as “terrifying.”
He said in an interview that they are a “call to action,” especially given that so few patients in the study received intensive combination lipid-lowering therapy despite having a baseline LDL cholesterol level that was “very, very high.”
Banach underlined that patients who receive triple lipid-lowering therapy with a high-intensity statin, ezetimibe (Nustendi), and a proprotein convertase subtilisin/kexin type 9 inhibitor, could expect, based on current evidence, to see their LDL cholesterol levels reduced by 85% and be on target.
“Obviously, this is kind of academic,” because in the real-world “this 85% is not observed very often,” but it offers a target for steep reductions in cholesterol levels.
“This is something that we should focus on for these patients from the beginning,” said Dr. Banach, either with a stepwise approach “or for experts in pediatric HoFH, “maybe immediately.”
He emphasized that clinicians have everything at hand to “be both effective in the early diagnosis of HoFH, the earlier the better, and obviously to be effective with its treatment.”
“We should do something to prolong the lives of those people,” because the current results are “terrifying,” Dr. Banach added.
Rare genetic condition
Presenting her findings, Ms. Mulder began by highlighting that HoFH is a “rare genetic condition that occurs due to mutations in cholesterol metabolism.”
This, she continued, leads to “severely increased LDL cholesterol levels, and consequently to very premature cardiovascular disease,” with patients potentially experiencing their first cardiovascular event before age 20 years.
Ms. Mulder pointed out that, although there have been case series in the literature on HoFH, they have had “limited numbers” and patients have typically spent decades being treated at the same lipid management clinic.
To broaden the understanding of the clinical characteristics and management of patients dying with HoFH, the team examined data from the HICC registry, which is “the largest contemporary database of homozygous FH patients,” Ms. Mulder said.
It includes 751 patients with HoFH from 88 centers in 38 countries who were alive in 2010 or later. Data entry was between 2016 and 2020. The current analysis focused on 37 patients who had already died by the time they were included on the registry.
Of those, 49% were women, 38% were of White ethnicity, and 43% were from high-income countries.
The median age at diagnosis was 12 years, Ms. Mulder said, explaining that this is similar to that seen in other studies. The majority (86%) underwent genetic testing, and 92% presented with xanthomas.
Ms. Mulder also noted that, at their final clinical evaluation, which was conducted a median age of 18 years after their initial diagnosis, 43% of patients were recorded as current or former smokers.
In terms of their lipid-lowering therapy, 94% were taking a statin, whereas 68% were on ezetimibe, and 23% were undergoing apheresis.
Ms. Mulder said that the median number of lipid-lowering therapies per patient was two, and that “sadly ... 26% of the deceased patients had only one or no treatment.”
Therefore, perhaps unsurprisingly even those patients who were receiving treatment had LDL cholesterol levels that were “too high,” at 9.4 mmol/L versus 15.6 mmol/L among those who were untreated.
There was a high prevalence of ASCVD, at 70% overall, or 41% for aortic stenosis, 30% for myocardial infarction, 30% for angina pectoris, and 22% each for aortic valve replacement and coronary artery bypass grafting. In addition, 19% underwent percutaneous coronary intervention.
The median age of onset for ASCVD was 28 years. Ms. Mulder pointed out, however, that, as data were not available for all patients, “this might be an underestimation.” About 70% of patients experienced recurrent ASCVD.
There was a wide range in the age at which patients with HoFH died, although the median was, “strikingly,” 32 years, Ms. Mulder said. Death was confirmed as stemming from cardiovascular causes in 76% of cases.
During the postpresentation discussion, session chair Antonio J. Vallejo-Vaz, PhD, from the Research Group of Clinical Epidemiology and Vascular Risk, Institute of Biomedicine of Seville (Spain), highlighted that, if 38% of the patients were of White ethnicity, then the remainder must therefore be from other ethnic groups.
“There could be potential issues with accessibility to lipid centers” for these patients, which could affect the findings, noted Dr. Vallejo-Vaz, who is also chief scientist of the EAS Familial Hypercholesterolaemia Studies Collaboration.
Ms. Mulder agreed, replying that their results, though already striking, may be an underestimation because the patients were all from either high or middle-income countries, “so it would be good to have some data on low-income countries.”
She was also asked about two patients who died at a much older age than did the others, at ages 70 years and 86 years, respectively, and whether they had, for example, a protective genetic mutation.
Ms. Mulder said that they do not yet know, but they are planning an extended case series on these and other long-lived patients so that they can be investigated further.
No funding or relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
MANNHEIM, GERMANY –
The researchers looked at almost 40 patients from the HoFH International Clinical Collaborators (HICC) registry who had died before data entry, finding that they had a mean age of diagnosis of 12 years.
Even those who received treatment had high LDL cholesterol levels, and 70% developed atherosclerotic cardiovascular disease (ASCVD) at a median age of 28 years.
Worryingly, the results showed that the median age at death was 32 years. Results were presented at the annual congress of the European Atherosclerosis Society.
Patients with HoFH “have severe atherosclerotic cardiovascular disease risk,” said study presenter Janneke Mulder, a PhD candidate at the department of internal medicine, Erasmus University Medical Center, Rotterdam, the Netherlands.
“Therefore, early diagnosis and initiation of treatments, and also a combination of treatments, is really crucial,” she added.
Call to action
Approached for comment, Maciej Banach, MD, PhD, full professor of cardiology, Polish Mother’s Memorial Hospital Research Institute, Lodz, and Secretary of the EAS, described the results as “terrifying.”
He said in an interview that they are a “call to action,” especially given that so few patients in the study received intensive combination lipid-lowering therapy despite having a baseline LDL cholesterol level that was “very, very high.”
Banach underlined that patients who receive triple lipid-lowering therapy with a high-intensity statin, ezetimibe (Nustendi), and a proprotein convertase subtilisin/kexin type 9 inhibitor, could expect, based on current evidence, to see their LDL cholesterol levels reduced by 85% and be on target.
“Obviously, this is kind of academic,” because in the real-world “this 85% is not observed very often,” but it offers a target for steep reductions in cholesterol levels.
“This is something that we should focus on for these patients from the beginning,” said Dr. Banach, either with a stepwise approach “or for experts in pediatric HoFH, “maybe immediately.”
He emphasized that clinicians have everything at hand to “be both effective in the early diagnosis of HoFH, the earlier the better, and obviously to be effective with its treatment.”
“We should do something to prolong the lives of those people,” because the current results are “terrifying,” Dr. Banach added.
Rare genetic condition
Presenting her findings, Ms. Mulder began by highlighting that HoFH is a “rare genetic condition that occurs due to mutations in cholesterol metabolism.”
This, she continued, leads to “severely increased LDL cholesterol levels, and consequently to very premature cardiovascular disease,” with patients potentially experiencing their first cardiovascular event before age 20 years.
Ms. Mulder pointed out that, although there have been case series in the literature on HoFH, they have had “limited numbers” and patients have typically spent decades being treated at the same lipid management clinic.
To broaden the understanding of the clinical characteristics and management of patients dying with HoFH, the team examined data from the HICC registry, which is “the largest contemporary database of homozygous FH patients,” Ms. Mulder said.
It includes 751 patients with HoFH from 88 centers in 38 countries who were alive in 2010 or later. Data entry was between 2016 and 2020. The current analysis focused on 37 patients who had already died by the time they were included on the registry.
Of those, 49% were women, 38% were of White ethnicity, and 43% were from high-income countries.
The median age at diagnosis was 12 years, Ms. Mulder said, explaining that this is similar to that seen in other studies. The majority (86%) underwent genetic testing, and 92% presented with xanthomas.
Ms. Mulder also noted that, at their final clinical evaluation, which was conducted a median age of 18 years after their initial diagnosis, 43% of patients were recorded as current or former smokers.
In terms of their lipid-lowering therapy, 94% were taking a statin, whereas 68% were on ezetimibe, and 23% were undergoing apheresis.
Ms. Mulder said that the median number of lipid-lowering therapies per patient was two, and that “sadly ... 26% of the deceased patients had only one or no treatment.”
Therefore, perhaps unsurprisingly even those patients who were receiving treatment had LDL cholesterol levels that were “too high,” at 9.4 mmol/L versus 15.6 mmol/L among those who were untreated.
There was a high prevalence of ASCVD, at 70% overall, or 41% for aortic stenosis, 30% for myocardial infarction, 30% for angina pectoris, and 22% each for aortic valve replacement and coronary artery bypass grafting. In addition, 19% underwent percutaneous coronary intervention.
The median age of onset for ASCVD was 28 years. Ms. Mulder pointed out, however, that, as data were not available for all patients, “this might be an underestimation.” About 70% of patients experienced recurrent ASCVD.
There was a wide range in the age at which patients with HoFH died, although the median was, “strikingly,” 32 years, Ms. Mulder said. Death was confirmed as stemming from cardiovascular causes in 76% of cases.
During the postpresentation discussion, session chair Antonio J. Vallejo-Vaz, PhD, from the Research Group of Clinical Epidemiology and Vascular Risk, Institute of Biomedicine of Seville (Spain), highlighted that, if 38% of the patients were of White ethnicity, then the remainder must therefore be from other ethnic groups.
“There could be potential issues with accessibility to lipid centers” for these patients, which could affect the findings, noted Dr. Vallejo-Vaz, who is also chief scientist of the EAS Familial Hypercholesterolaemia Studies Collaboration.
Ms. Mulder agreed, replying that their results, though already striking, may be an underestimation because the patients were all from either high or middle-income countries, “so it would be good to have some data on low-income countries.”
She was also asked about two patients who died at a much older age than did the others, at ages 70 years and 86 years, respectively, and whether they had, for example, a protective genetic mutation.
Ms. Mulder said that they do not yet know, but they are planning an extended case series on these and other long-lived patients so that they can be investigated further.
No funding or relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
MANNHEIM, GERMANY –
The researchers looked at almost 40 patients from the HoFH International Clinical Collaborators (HICC) registry who had died before data entry, finding that they had a mean age of diagnosis of 12 years.
Even those who received treatment had high LDL cholesterol levels, and 70% developed atherosclerotic cardiovascular disease (ASCVD) at a median age of 28 years.
Worryingly, the results showed that the median age at death was 32 years. Results were presented at the annual congress of the European Atherosclerosis Society.
Patients with HoFH “have severe atherosclerotic cardiovascular disease risk,” said study presenter Janneke Mulder, a PhD candidate at the department of internal medicine, Erasmus University Medical Center, Rotterdam, the Netherlands.
“Therefore, early diagnosis and initiation of treatments, and also a combination of treatments, is really crucial,” she added.
Call to action
Approached for comment, Maciej Banach, MD, PhD, full professor of cardiology, Polish Mother’s Memorial Hospital Research Institute, Lodz, and Secretary of the EAS, described the results as “terrifying.”
He said in an interview that they are a “call to action,” especially given that so few patients in the study received intensive combination lipid-lowering therapy despite having a baseline LDL cholesterol level that was “very, very high.”
Banach underlined that patients who receive triple lipid-lowering therapy with a high-intensity statin, ezetimibe (Nustendi), and a proprotein convertase subtilisin/kexin type 9 inhibitor, could expect, based on current evidence, to see their LDL cholesterol levels reduced by 85% and be on target.
“Obviously, this is kind of academic,” because in the real-world “this 85% is not observed very often,” but it offers a target for steep reductions in cholesterol levels.
“This is something that we should focus on for these patients from the beginning,” said Dr. Banach, either with a stepwise approach “or for experts in pediatric HoFH, “maybe immediately.”
He emphasized that clinicians have everything at hand to “be both effective in the early diagnosis of HoFH, the earlier the better, and obviously to be effective with its treatment.”
“We should do something to prolong the lives of those people,” because the current results are “terrifying,” Dr. Banach added.
Rare genetic condition
Presenting her findings, Ms. Mulder began by highlighting that HoFH is a “rare genetic condition that occurs due to mutations in cholesterol metabolism.”
This, she continued, leads to “severely increased LDL cholesterol levels, and consequently to very premature cardiovascular disease,” with patients potentially experiencing their first cardiovascular event before age 20 years.
Ms. Mulder pointed out that, although there have been case series in the literature on HoFH, they have had “limited numbers” and patients have typically spent decades being treated at the same lipid management clinic.
To broaden the understanding of the clinical characteristics and management of patients dying with HoFH, the team examined data from the HICC registry, which is “the largest contemporary database of homozygous FH patients,” Ms. Mulder said.
It includes 751 patients with HoFH from 88 centers in 38 countries who were alive in 2010 or later. Data entry was between 2016 and 2020. The current analysis focused on 37 patients who had already died by the time they were included on the registry.
Of those, 49% were women, 38% were of White ethnicity, and 43% were from high-income countries.
The median age at diagnosis was 12 years, Ms. Mulder said, explaining that this is similar to that seen in other studies. The majority (86%) underwent genetic testing, and 92% presented with xanthomas.
Ms. Mulder also noted that, at their final clinical evaluation, which was conducted a median age of 18 years after their initial diagnosis, 43% of patients were recorded as current or former smokers.
In terms of their lipid-lowering therapy, 94% were taking a statin, whereas 68% were on ezetimibe, and 23% were undergoing apheresis.
Ms. Mulder said that the median number of lipid-lowering therapies per patient was two, and that “sadly ... 26% of the deceased patients had only one or no treatment.”
Therefore, perhaps unsurprisingly even those patients who were receiving treatment had LDL cholesterol levels that were “too high,” at 9.4 mmol/L versus 15.6 mmol/L among those who were untreated.
There was a high prevalence of ASCVD, at 70% overall, or 41% for aortic stenosis, 30% for myocardial infarction, 30% for angina pectoris, and 22% each for aortic valve replacement and coronary artery bypass grafting. In addition, 19% underwent percutaneous coronary intervention.
The median age of onset for ASCVD was 28 years. Ms. Mulder pointed out, however, that, as data were not available for all patients, “this might be an underestimation.” About 70% of patients experienced recurrent ASCVD.
There was a wide range in the age at which patients with HoFH died, although the median was, “strikingly,” 32 years, Ms. Mulder said. Death was confirmed as stemming from cardiovascular causes in 76% of cases.
During the postpresentation discussion, session chair Antonio J. Vallejo-Vaz, PhD, from the Research Group of Clinical Epidemiology and Vascular Risk, Institute of Biomedicine of Seville (Spain), highlighted that, if 38% of the patients were of White ethnicity, then the remainder must therefore be from other ethnic groups.
“There could be potential issues with accessibility to lipid centers” for these patients, which could affect the findings, noted Dr. Vallejo-Vaz, who is also chief scientist of the EAS Familial Hypercholesterolaemia Studies Collaboration.
Ms. Mulder agreed, replying that their results, though already striking, may be an underestimation because the patients were all from either high or middle-income countries, “so it would be good to have some data on low-income countries.”
She was also asked about two patients who died at a much older age than did the others, at ages 70 years and 86 years, respectively, and whether they had, for example, a protective genetic mutation.
Ms. Mulder said that they do not yet know, but they are planning an extended case series on these and other long-lived patients so that they can be investigated further.
No funding or relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.