User login
Over half of pregnant patients not properly screened for thyroid disease
BALTIMORE – Less than half of the pregnant patients who met the criteria for thyroid screening were actually screened by their clinician, according to a retrospective cohort study presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists in Baltimore. Those who met criteria and did receive screening had higher live birth rates and lower miscarriage rates than those who met the criteria but did not undergo screening, the study found.
“These results suggest that improving thyroid screening adherence may lead to improved pregnancy outcomes,” lead author Allan Dong, MD, of Advocate Lutheran General Hospital in Des Plaines, Ill., told attendees. “However, following targeted screening guidelines can be difficult for clinicians. In practice, universal screening for diabetes and pregnancy may provide more comprehensive screening coverage and potentially lead to improved outcomes.”
Instead of universal screening for thyroid disease, ACOG and the American Thyroid Association recommend targeted screening of high-risk patients, though ATA’s criteria are substantially broader than ACOG’s. But, Dr. Dong told attendees, “guidelines are only beneficial if they are followed appropriately,” and Ob.Gyns. have limited time to screen for risk factors in the midst of other clinical priorities. So he aimed to learn whether Ob.Gyns. were following the guidelines of either organization in screening people at higher risk for thyroid disease.
Dr. Dong and his coauthor, Melisa Lott, DO, reviewed the charts of all 1,025 patients who presented at their institution for new obstetrical visits in 2020 to determine which ones had risk factors that would qualify them for screening under ATA or ACOG guidelines. ACOG’s screening criteria included having a personal or family history of thyroid disease or type 1 diabetes, or there being clinical suspicion for thyroid disease. ATA’s screening criteria included the following:
- Personal or family history of thyroid disease.
- History of head or neck radiation.
- History of a prior thyroid surgery.
- Over age 30.
- Any autoimmune disease.
- A body mass index greater than 40 kg/m2.
- History of pregnancy loss, preterm delivery, or infertility.
- Recently used amiodarone lithium or iodine-based contrast.
- Lived in an area of known iodine deficiency.
- Clinical suspicion of thyroid disease.
ATA screening criteria identified four times as many patients requiring screening than did ACOG criteria, Dr. Dong noted. Of the 198 patients who met ACOG’s criteria, 43.9% were screened with thyroid function testing. Meanwhile, 826 patients – including all those who met ACOG’s criteria – met ATA’s criteria for screening, but only 13.1% of them underwent thyroid function testing.
Live birth rates were significantly higher among patients who met ATA criteria and were screened (92.6%) than among patients who met ATA criteria but were not screened (83.3%, P = .006). Similarly, the miscarriage rate was 4.6% in patients who met ATA criteria and were screened, compared to 12.4% in patients who met the criteria but did not undergo thyroid function testing (P = .009).
“A similar difference, although not statistically significant, was noted when comparing patients who were screened appropriately per ACOG criteria with those who met criteria for screening but were not screened,” Dr. Dong told attendees. “However, our study was underpowered to detect this difference due to the lower number of patients who meet criteria for screening under ACOG guidelines.”
The researchers did not find any significant difference in preterm delivery rates.
Anna Whelan, MD, of Women & Infants Hospital of Brown University, Providence, R.I., was not involved in the study but viewed the poster and pointed out that many of the patients, if seen by a primary care provider prior to pregnancy, would likely have been screened by their PCP. The rate of underscreening therefore suggests that patients “are not getting good, consistent primary care because there’s a lack of primary care physicians,” Dr. Whelan said in an interview.
In addition, she added, “maybe not all obstetricians and those providing care, such as midwives and other providers, are aware of the [ATA] guidelines on who should be screened.” She added that additional education about thyroid screening guidelines might be helpful for providers.
Dr. Dong reported being a stock shareholder in 3M, AbbVie, General Electric, Johnson & Johnson, Medtronic, Pfizer, and Viking Therapeutics. Dr. Whelan had no disclosures.
BALTIMORE – Less than half of the pregnant patients who met the criteria for thyroid screening were actually screened by their clinician, according to a retrospective cohort study presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists in Baltimore. Those who met criteria and did receive screening had higher live birth rates and lower miscarriage rates than those who met the criteria but did not undergo screening, the study found.
“These results suggest that improving thyroid screening adherence may lead to improved pregnancy outcomes,” lead author Allan Dong, MD, of Advocate Lutheran General Hospital in Des Plaines, Ill., told attendees. “However, following targeted screening guidelines can be difficult for clinicians. In practice, universal screening for diabetes and pregnancy may provide more comprehensive screening coverage and potentially lead to improved outcomes.”
Instead of universal screening for thyroid disease, ACOG and the American Thyroid Association recommend targeted screening of high-risk patients, though ATA’s criteria are substantially broader than ACOG’s. But, Dr. Dong told attendees, “guidelines are only beneficial if they are followed appropriately,” and Ob.Gyns. have limited time to screen for risk factors in the midst of other clinical priorities. So he aimed to learn whether Ob.Gyns. were following the guidelines of either organization in screening people at higher risk for thyroid disease.
Dr. Dong and his coauthor, Melisa Lott, DO, reviewed the charts of all 1,025 patients who presented at their institution for new obstetrical visits in 2020 to determine which ones had risk factors that would qualify them for screening under ATA or ACOG guidelines. ACOG’s screening criteria included having a personal or family history of thyroid disease or type 1 diabetes, or there being clinical suspicion for thyroid disease. ATA’s screening criteria included the following:
- Personal or family history of thyroid disease.
- History of head or neck radiation.
- History of a prior thyroid surgery.
- Over age 30.
- Any autoimmune disease.
- A body mass index greater than 40 kg/m2.
- History of pregnancy loss, preterm delivery, or infertility.
- Recently used amiodarone lithium or iodine-based contrast.
- Lived in an area of known iodine deficiency.
- Clinical suspicion of thyroid disease.
ATA screening criteria identified four times as many patients requiring screening than did ACOG criteria, Dr. Dong noted. Of the 198 patients who met ACOG’s criteria, 43.9% were screened with thyroid function testing. Meanwhile, 826 patients – including all those who met ACOG’s criteria – met ATA’s criteria for screening, but only 13.1% of them underwent thyroid function testing.
Live birth rates were significantly higher among patients who met ATA criteria and were screened (92.6%) than among patients who met ATA criteria but were not screened (83.3%, P = .006). Similarly, the miscarriage rate was 4.6% in patients who met ATA criteria and were screened, compared to 12.4% in patients who met the criteria but did not undergo thyroid function testing (P = .009).
“A similar difference, although not statistically significant, was noted when comparing patients who were screened appropriately per ACOG criteria with those who met criteria for screening but were not screened,” Dr. Dong told attendees. “However, our study was underpowered to detect this difference due to the lower number of patients who meet criteria for screening under ACOG guidelines.”
The researchers did not find any significant difference in preterm delivery rates.
Anna Whelan, MD, of Women & Infants Hospital of Brown University, Providence, R.I., was not involved in the study but viewed the poster and pointed out that many of the patients, if seen by a primary care provider prior to pregnancy, would likely have been screened by their PCP. The rate of underscreening therefore suggests that patients “are not getting good, consistent primary care because there’s a lack of primary care physicians,” Dr. Whelan said in an interview.
In addition, she added, “maybe not all obstetricians and those providing care, such as midwives and other providers, are aware of the [ATA] guidelines on who should be screened.” She added that additional education about thyroid screening guidelines might be helpful for providers.
Dr. Dong reported being a stock shareholder in 3M, AbbVie, General Electric, Johnson & Johnson, Medtronic, Pfizer, and Viking Therapeutics. Dr. Whelan had no disclosures.
BALTIMORE – Less than half of the pregnant patients who met the criteria for thyroid screening were actually screened by their clinician, according to a retrospective cohort study presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists in Baltimore. Those who met criteria and did receive screening had higher live birth rates and lower miscarriage rates than those who met the criteria but did not undergo screening, the study found.
“These results suggest that improving thyroid screening adherence may lead to improved pregnancy outcomes,” lead author Allan Dong, MD, of Advocate Lutheran General Hospital in Des Plaines, Ill., told attendees. “However, following targeted screening guidelines can be difficult for clinicians. In practice, universal screening for diabetes and pregnancy may provide more comprehensive screening coverage and potentially lead to improved outcomes.”
Instead of universal screening for thyroid disease, ACOG and the American Thyroid Association recommend targeted screening of high-risk patients, though ATA’s criteria are substantially broader than ACOG’s. But, Dr. Dong told attendees, “guidelines are only beneficial if they are followed appropriately,” and Ob.Gyns. have limited time to screen for risk factors in the midst of other clinical priorities. So he aimed to learn whether Ob.Gyns. were following the guidelines of either organization in screening people at higher risk for thyroid disease.
Dr. Dong and his coauthor, Melisa Lott, DO, reviewed the charts of all 1,025 patients who presented at their institution for new obstetrical visits in 2020 to determine which ones had risk factors that would qualify them for screening under ATA or ACOG guidelines. ACOG’s screening criteria included having a personal or family history of thyroid disease or type 1 diabetes, or there being clinical suspicion for thyroid disease. ATA’s screening criteria included the following:
- Personal or family history of thyroid disease.
- History of head or neck radiation.
- History of a prior thyroid surgery.
- Over age 30.
- Any autoimmune disease.
- A body mass index greater than 40 kg/m2.
- History of pregnancy loss, preterm delivery, or infertility.
- Recently used amiodarone lithium or iodine-based contrast.
- Lived in an area of known iodine deficiency.
- Clinical suspicion of thyroid disease.
ATA screening criteria identified four times as many patients requiring screening than did ACOG criteria, Dr. Dong noted. Of the 198 patients who met ACOG’s criteria, 43.9% were screened with thyroid function testing. Meanwhile, 826 patients – including all those who met ACOG’s criteria – met ATA’s criteria for screening, but only 13.1% of them underwent thyroid function testing.
Live birth rates were significantly higher among patients who met ATA criteria and were screened (92.6%) than among patients who met ATA criteria but were not screened (83.3%, P = .006). Similarly, the miscarriage rate was 4.6% in patients who met ATA criteria and were screened, compared to 12.4% in patients who met the criteria but did not undergo thyroid function testing (P = .009).
“A similar difference, although not statistically significant, was noted when comparing patients who were screened appropriately per ACOG criteria with those who met criteria for screening but were not screened,” Dr. Dong told attendees. “However, our study was underpowered to detect this difference due to the lower number of patients who meet criteria for screening under ACOG guidelines.”
The researchers did not find any significant difference in preterm delivery rates.
Anna Whelan, MD, of Women & Infants Hospital of Brown University, Providence, R.I., was not involved in the study but viewed the poster and pointed out that many of the patients, if seen by a primary care provider prior to pregnancy, would likely have been screened by their PCP. The rate of underscreening therefore suggests that patients “are not getting good, consistent primary care because there’s a lack of primary care physicians,” Dr. Whelan said in an interview.
In addition, she added, “maybe not all obstetricians and those providing care, such as midwives and other providers, are aware of the [ATA] guidelines on who should be screened.” She added that additional education about thyroid screening guidelines might be helpful for providers.
Dr. Dong reported being a stock shareholder in 3M, AbbVie, General Electric, Johnson & Johnson, Medtronic, Pfizer, and Viking Therapeutics. Dr. Whelan had no disclosures.
FROM ACOG 2023
Ear acupuncture with diet aids weight loss
DUBLIN – with high levels of visceral fat and overweight/obesity.
Three months of auricular acupuncture stimulation and dietary restriction led to a mean weight loss of nearly 9 kg plus a drop in waist circumference of more than 10 cm.
According to the researchers, acupuncture beads, used in Japan to augment weight loss for more than 30 years, are thought to stimulate nerves and organs that regulate appetite, satiety, hunger, and food cravings.
Findings of the observational study were presented by Takahiro Fujimoto, MD, PhD, Clinic F, Tokyo, at this year’s European Congress on Obesity.
Together with a prior study using the same intervention in women, Dr. Fujimoto and colleagues have now gathered data in more than 1,000 individuals, he said. “We wanted a method that was simple and noninvasive that would serve as a support to exercise and dietary therapy,” Dr. Fujimoto said in an interview.
“We believe there is an effect,” he asserted. “Acupuncture’s effect lies in stimulating the satiety center with benefits in helping individuals to control their food cravings and intake when reducing meals,” he said, pointing out that similar techniques have been used in patients undergoing withdrawal from drug addiction and in smoking cessation. He explained that acupuncture beads are believed to help individuals change their lifestyle habits, and added that “the relapse rate after 6 months is addressed in another paper, and it is very low.”
Professor Jason C.G. Halford, PhD, head of school at the University of Leeds, England, and president of the European Association for the Study of Obesity, commented on the findings. “There is no control group here receiving everything but the acupuncture,” he noted. “As such, it could be other elements of the intervention driving this [effect] including the act of keeping a food diary increasing awareness of one’s diet. A randomized controlled trial would be the next step.”
In women, the technique led to significantly more weight loss than in those who were untreated, and weight loss was maintained for 6 months after the end of treatment.
The researchers added that acupuncture stimulation with beads was a simpler method than traditional use of intradermal needles requiring expert acupuncturists. The stimulation is applied with 1.5-mm metal ear beads on 6 points of the outer ear (shen men, food pipe, upper stomach opening, stomach, lungs, and endocrine system) that correspond to meridian lines, and as such, restores the flow of qi by resolving any blockages or disruption. This may help with a variety of health conditions, according to the researchers. Placed on both ears, surgical tape was used to keep the beads in place to ensure participants continuously received uniform pressure on each of the six acupuncture points.
Dietary guidance was provided to participants to help reduce food intake by half, and nutritional supplements were given to compensate for any deficiencies. Participants attended twice-weekly clinic visits for bead sticking and diet progress monitoring. Body weight, body fat percentage, fat mass, lean mass, muscle mass, body mass index (BMI), and abdominal fat were assessed at the start and end of the study period.
“Since these tiny metal beads are attached to six points on the outer ear that stimulate nerves and organs which regulate appetite, satiety, and hunger, this type of acupuncture does not require complex knowledge or skill,” explained Dr. Fujimoto.
The results of the latest study, in men only, build on a prior study of more than 1,300 women who also received auricular acupuncture stimulation with beads as well as a halving of their food intake. In women, the weight loss program led to total body weight loss of 11.2% over 3 months.
At baseline, the 81 male participants, ages 21-78 years, had a mean BMI of 28.4 kg/m2 and mean waist circumference of 98.4 cm. Body fat percentage was 28.2%.
After 3 months, participants lost a mean of 8.6 kg (P < .001), decreased waist circumference by a mean of 10.4 cm (P < .001), and lost a mean of 4.0% of total body fat (P < .001). Visceral fat levels also fell by 2.2 points (P < .001), from 15.2 points at baseline to 13.0 points after 3 months. (A healthy visceral fat rating is between 1 and 12 points.) BMI decreased by almost 3 kg/m2 (from 28.4 at baseline to 25.5 at 3 months; P < .001).
Improvement in muscle-to-fat ratio was greater in men than women, whereas women had a greater decrease in percentage body fat than men.
“Whilst receiving ear acupuncture, the investigators asked participants to cut their food intake by half. It’s not unreasonable to expect that this major dietary change was the main reason participants lost weight,” remarked Graham Wheeler, PhD, statistical ambassador at the Royal Statistical Society, United Kingdom.
He also commented on the lack of a control group: “This study does not show us the impact of ear acupuncture on weight loss.”
Dr. Fujimoto and Dr. Halford have reported no relevant financial relationships. Dr. Wheeler is a statistical ambassador for the Royal Statistical Society, is employed by GSK, and holds an honorary senior lecturer post at Imperial College London.
A version of this article first appeared on Medscape.com.
DUBLIN – with high levels of visceral fat and overweight/obesity.
Three months of auricular acupuncture stimulation and dietary restriction led to a mean weight loss of nearly 9 kg plus a drop in waist circumference of more than 10 cm.
According to the researchers, acupuncture beads, used in Japan to augment weight loss for more than 30 years, are thought to stimulate nerves and organs that regulate appetite, satiety, hunger, and food cravings.
Findings of the observational study were presented by Takahiro Fujimoto, MD, PhD, Clinic F, Tokyo, at this year’s European Congress on Obesity.
Together with a prior study using the same intervention in women, Dr. Fujimoto and colleagues have now gathered data in more than 1,000 individuals, he said. “We wanted a method that was simple and noninvasive that would serve as a support to exercise and dietary therapy,” Dr. Fujimoto said in an interview.
“We believe there is an effect,” he asserted. “Acupuncture’s effect lies in stimulating the satiety center with benefits in helping individuals to control their food cravings and intake when reducing meals,” he said, pointing out that similar techniques have been used in patients undergoing withdrawal from drug addiction and in smoking cessation. He explained that acupuncture beads are believed to help individuals change their lifestyle habits, and added that “the relapse rate after 6 months is addressed in another paper, and it is very low.”
Professor Jason C.G. Halford, PhD, head of school at the University of Leeds, England, and president of the European Association for the Study of Obesity, commented on the findings. “There is no control group here receiving everything but the acupuncture,” he noted. “As such, it could be other elements of the intervention driving this [effect] including the act of keeping a food diary increasing awareness of one’s diet. A randomized controlled trial would be the next step.”
In women, the technique led to significantly more weight loss than in those who were untreated, and weight loss was maintained for 6 months after the end of treatment.
The researchers added that acupuncture stimulation with beads was a simpler method than traditional use of intradermal needles requiring expert acupuncturists. The stimulation is applied with 1.5-mm metal ear beads on 6 points of the outer ear (shen men, food pipe, upper stomach opening, stomach, lungs, and endocrine system) that correspond to meridian lines, and as such, restores the flow of qi by resolving any blockages or disruption. This may help with a variety of health conditions, according to the researchers. Placed on both ears, surgical tape was used to keep the beads in place to ensure participants continuously received uniform pressure on each of the six acupuncture points.
Dietary guidance was provided to participants to help reduce food intake by half, and nutritional supplements were given to compensate for any deficiencies. Participants attended twice-weekly clinic visits for bead sticking and diet progress monitoring. Body weight, body fat percentage, fat mass, lean mass, muscle mass, body mass index (BMI), and abdominal fat were assessed at the start and end of the study period.
“Since these tiny metal beads are attached to six points on the outer ear that stimulate nerves and organs which regulate appetite, satiety, and hunger, this type of acupuncture does not require complex knowledge or skill,” explained Dr. Fujimoto.
The results of the latest study, in men only, build on a prior study of more than 1,300 women who also received auricular acupuncture stimulation with beads as well as a halving of their food intake. In women, the weight loss program led to total body weight loss of 11.2% over 3 months.
At baseline, the 81 male participants, ages 21-78 years, had a mean BMI of 28.4 kg/m2 and mean waist circumference of 98.4 cm. Body fat percentage was 28.2%.
After 3 months, participants lost a mean of 8.6 kg (P < .001), decreased waist circumference by a mean of 10.4 cm (P < .001), and lost a mean of 4.0% of total body fat (P < .001). Visceral fat levels also fell by 2.2 points (P < .001), from 15.2 points at baseline to 13.0 points after 3 months. (A healthy visceral fat rating is between 1 and 12 points.) BMI decreased by almost 3 kg/m2 (from 28.4 at baseline to 25.5 at 3 months; P < .001).
Improvement in muscle-to-fat ratio was greater in men than women, whereas women had a greater decrease in percentage body fat than men.
“Whilst receiving ear acupuncture, the investigators asked participants to cut their food intake by half. It’s not unreasonable to expect that this major dietary change was the main reason participants lost weight,” remarked Graham Wheeler, PhD, statistical ambassador at the Royal Statistical Society, United Kingdom.
He also commented on the lack of a control group: “This study does not show us the impact of ear acupuncture on weight loss.”
Dr. Fujimoto and Dr. Halford have reported no relevant financial relationships. Dr. Wheeler is a statistical ambassador for the Royal Statistical Society, is employed by GSK, and holds an honorary senior lecturer post at Imperial College London.
A version of this article first appeared on Medscape.com.
DUBLIN – with high levels of visceral fat and overweight/obesity.
Three months of auricular acupuncture stimulation and dietary restriction led to a mean weight loss of nearly 9 kg plus a drop in waist circumference of more than 10 cm.
According to the researchers, acupuncture beads, used in Japan to augment weight loss for more than 30 years, are thought to stimulate nerves and organs that regulate appetite, satiety, hunger, and food cravings.
Findings of the observational study were presented by Takahiro Fujimoto, MD, PhD, Clinic F, Tokyo, at this year’s European Congress on Obesity.
Together with a prior study using the same intervention in women, Dr. Fujimoto and colleagues have now gathered data in more than 1,000 individuals, he said. “We wanted a method that was simple and noninvasive that would serve as a support to exercise and dietary therapy,” Dr. Fujimoto said in an interview.
“We believe there is an effect,” he asserted. “Acupuncture’s effect lies in stimulating the satiety center with benefits in helping individuals to control their food cravings and intake when reducing meals,” he said, pointing out that similar techniques have been used in patients undergoing withdrawal from drug addiction and in smoking cessation. He explained that acupuncture beads are believed to help individuals change their lifestyle habits, and added that “the relapse rate after 6 months is addressed in another paper, and it is very low.”
Professor Jason C.G. Halford, PhD, head of school at the University of Leeds, England, and president of the European Association for the Study of Obesity, commented on the findings. “There is no control group here receiving everything but the acupuncture,” he noted. “As such, it could be other elements of the intervention driving this [effect] including the act of keeping a food diary increasing awareness of one’s diet. A randomized controlled trial would be the next step.”
In women, the technique led to significantly more weight loss than in those who were untreated, and weight loss was maintained for 6 months after the end of treatment.
The researchers added that acupuncture stimulation with beads was a simpler method than traditional use of intradermal needles requiring expert acupuncturists. The stimulation is applied with 1.5-mm metal ear beads on 6 points of the outer ear (shen men, food pipe, upper stomach opening, stomach, lungs, and endocrine system) that correspond to meridian lines, and as such, restores the flow of qi by resolving any blockages or disruption. This may help with a variety of health conditions, according to the researchers. Placed on both ears, surgical tape was used to keep the beads in place to ensure participants continuously received uniform pressure on each of the six acupuncture points.
Dietary guidance was provided to participants to help reduce food intake by half, and nutritional supplements were given to compensate for any deficiencies. Participants attended twice-weekly clinic visits for bead sticking and diet progress monitoring. Body weight, body fat percentage, fat mass, lean mass, muscle mass, body mass index (BMI), and abdominal fat were assessed at the start and end of the study period.
“Since these tiny metal beads are attached to six points on the outer ear that stimulate nerves and organs which regulate appetite, satiety, and hunger, this type of acupuncture does not require complex knowledge or skill,” explained Dr. Fujimoto.
The results of the latest study, in men only, build on a prior study of more than 1,300 women who also received auricular acupuncture stimulation with beads as well as a halving of their food intake. In women, the weight loss program led to total body weight loss of 11.2% over 3 months.
At baseline, the 81 male participants, ages 21-78 years, had a mean BMI of 28.4 kg/m2 and mean waist circumference of 98.4 cm. Body fat percentage was 28.2%.
After 3 months, participants lost a mean of 8.6 kg (P < .001), decreased waist circumference by a mean of 10.4 cm (P < .001), and lost a mean of 4.0% of total body fat (P < .001). Visceral fat levels also fell by 2.2 points (P < .001), from 15.2 points at baseline to 13.0 points after 3 months. (A healthy visceral fat rating is between 1 and 12 points.) BMI decreased by almost 3 kg/m2 (from 28.4 at baseline to 25.5 at 3 months; P < .001).
Improvement in muscle-to-fat ratio was greater in men than women, whereas women had a greater decrease in percentage body fat than men.
“Whilst receiving ear acupuncture, the investigators asked participants to cut their food intake by half. It’s not unreasonable to expect that this major dietary change was the main reason participants lost weight,” remarked Graham Wheeler, PhD, statistical ambassador at the Royal Statistical Society, United Kingdom.
He also commented on the lack of a control group: “This study does not show us the impact of ear acupuncture on weight loss.”
Dr. Fujimoto and Dr. Halford have reported no relevant financial relationships. Dr. Wheeler is a statistical ambassador for the Royal Statistical Society, is employed by GSK, and holds an honorary senior lecturer post at Imperial College London.
A version of this article first appeared on Medscape.com.
AT ECO 2023
Glucagon Prescription Rates for Individuals With Type 1 Diabetes Mellitus Following Implementation of an Electronic Health Records Intervention
From Vanderbilt University School of Medicine, and Vanderbilt University Medical Center, Nashville, TN.
ABSTRACT
Objective: Severe hypoglycemia can alter consciousness and inhibit oral intake, requiring nonoral rescue glucagon administration to raise blood glucose to safe levels. Thus, current guidelines recommend glucagon kit prescriptions for all patients at risk for hypoglycemia, especially patients with type 1 diabetes mellitus (T1DM). At the diabetes outpatient clinic at a tertiary medical center, glucagon prescription rates for T1DM patients remained suboptimal.
Methods: A quality improvement team analyzed patient flow through the endocrinology clinic and identified the lack of a systematic approach to assessing patients for home glucagon prescriptions as a major barrier. The team implemented 2 successive interventions. First, intake staff indicated whether patients lacked an active glucagon prescription on patients’ face sheets. Second, clinical pharmacists reviewed patient prescriptions prior to scheduled visits and pended glucagon orders for patients without active prescriptions. Of note, when a pharmacy pends an order, the pharmacist enters an order into the electronic health record (EHR) but does not sign it. The order is saved for a provider to later access and sign. A statistical process control p-chart tracked monthly prescription rates.
Results: After 7 months, glucagon prescription rates increased from a baseline of 59% to 72% as the new steady state.
Conclusion: This project demonstrates that a series of interventions can improve glucagon prescription rates for patients at risk for hypoglycemia. The project’s success stemmed from combining an EHR-generated report and interdisciplinary staff members’ involvement. Other endocrinology clinics may incorporate this approach to implement similar processes and improve glucagon prescription rates.
Keywords: diabetes, hypoglycemia, glucagon, quality improvement, prescription rates, medical student.
Hypoglycemia limits the management of blood glucose in patients with type 1 diabetes mellitus (T1DM). Severe hypoglycemia, characterized by altered mental status (AMS) or physical status requiring assistance for recovery, can lead to seizure, coma, or death.1 Hypoglycemia in diabetes often occurs iatrogenically, primarily from insulin therapy: 30% to 40% of patients with T1DM and 10% to 30% of patients with insulin-treated type 2 diabetes mellitus experience severe hypoglycemia in a given year.2 One study estimated that nearly 100,000 emergency department visits for hypoglycemia occur in the United States per year, with almost one-third resulting in hospitalization.3
Most patients self-treat mild hypoglycemia with oral intake of carbohydrates. However, since hypoglycemia-induced nausea and AMS can make oral intake more difficult or prevent it entirely, patients require a treatment that family, friends, or coworkers can administer. Rescue glucagon, prescribed as intramuscular injections or intranasal sprays, raises blood glucose to safe levels in 10 to 15 minutes.4 Therefore, the American Diabetes Association (ADA) recommends glucagon for all patients at risk for hypoglycemia, especially patients with T1DM.5 Despite the ADA’s recommendation, current evidence suggests suboptimal glucagon prescription rates, particularly in patients with T1DM. One study reported that, although 85% of US adults with T1DM had formerly been prescribed glucagon, only 68% of these patients (57.8% overall) had a current prescription.4 Few quality improvement efforts have tackled increasing prescription rates. Prior successful studies have attempted to do so via pharmacist-led educational interventions for providers6 and via electronic health record (EHR) notifications for patient risk.7 The project described here aimed to expand upon prior studies with a quality improvement project to increase glucagon prescription rates among patients at risk for severe hypoglycemia.
This study was conducted at a tertiary medical center’s outpatient diabetes clinic; the clinic treats more than 9500 patients with DM annually, more than 2700 of whom have T1DM. In the clinic’s multidisciplinary care model, patients typically follow up every 3 to 6 months, alternating between appointments with fellowship-trained endocrinologists and advanced practice providers (APPs). In addition to having certified diabetes educators, the clinic employs 2 dedicated clinical pharmacists whose duties include assisting providers in prescription management, helping patients identify the most affordable way to obtain their medications, and educating patients regarding their medications.
Patient flow through the clinic involves close coordination with multiple health professionals. Medical assistants (MAs) and licensed practical nurses (LPNs) perform patient intake, document vital signs, and ask screening questions, including dates of patients’ last hemoglobin A1c tests and diabetic eye examination. After intake, the provider (endocrinologist or APP) sees the patient. Once the appointment concludes, patients proceed to the in-house phlebotomy laboratory as indicated and check out with administrative staff to schedule future appointments.
From August 2021 through June 2022, teams of medical students at the tertiary center completed this project as part of a 4-week integrated science course on diabetes. Longitudinal supervision by an endocrinology faculty member ensured project continuity. The project employed the Standards for QUality Improvement Reporting Excellence (SQUIRE 2.0) method for reporting.8
Stakeholder analysis took place in August 2021. Surveyed clinic providers identified patients with T1DM as the most appropriate population and the outpatient setting as the most appropriate site for intervention. A fishbone diagram illustrated stakeholders to interview, impacts of the clinical flow, information technology to leverage, and potential holes contributing to glucagon prescription conversations falling through.
Interviews with T1DM patients, clinical pharmacists, APPs, MAs/LPNs, and endocrinologists identified barriers to glucagon prescription. The interviews and a process map analysis revealed several themes. While patients and providers understood the importance of glucagon prescription, barriers included glucagon cost, prescription fill burden, and, most pervasively, providers forgetting to ask patients whether they have a glucagon prescription and failing to consider glucagon prescriptions.For this study, each team of medical students worked on the project for 1 month. The revolving teams of medical students met approximately once per week for the duration of the project to review data and implementation phases. At the end of each month, the current team recorded the steps they had taken and information they had analyzed in a shared document, prepared short videos summarizing the work completed, and proposed next steps for the incoming team to support knowledge generation and continuity. Students from outgoing teams were available to contact if incoming teams had any questions.
Interventions
In the first implementation phase, which was carried out over 4 months (December 2021 to March 2022), the patient care manager trained MAs/LPNs to write a glucagon reminder on patients’ face sheets. At check-in, MAs/LPNs screened for a current glucagon prescription. If the patient lacked an up-to-date prescription, the MAs/LPNs hand-wrote a reminder on the patient’s face sheet, which was given to the provider immediately prior to seeing the patient. The clinical staff received an email explaining the intervention beforehand; the daily intake staff email included project reminders.
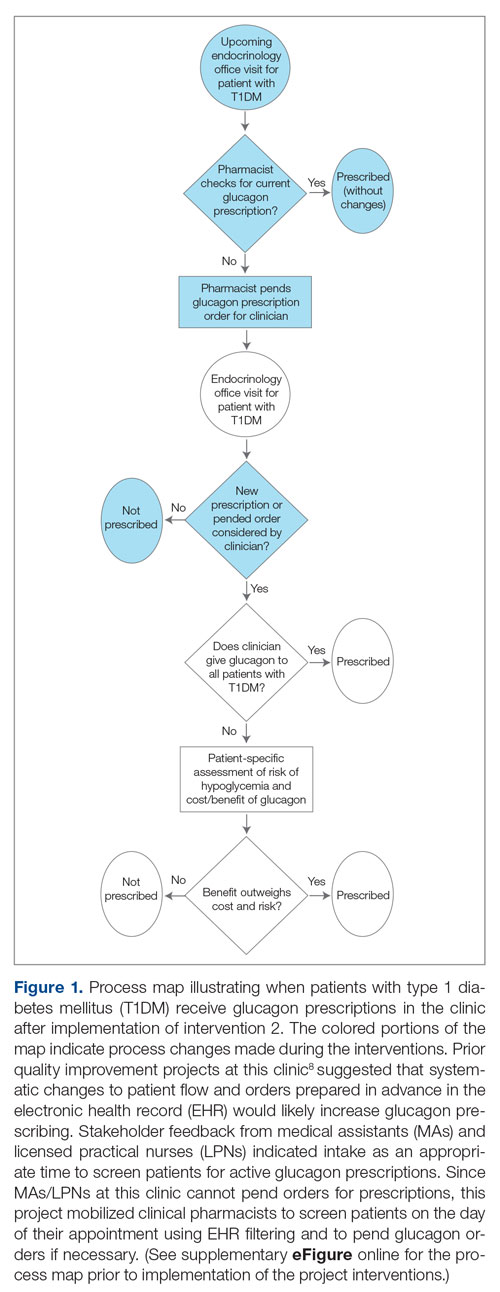
In the second implementation phase, which started in April 2022, had been carried out for 3 months at the time of this report, and is ongoing, clinical pharmacists have been pending glucagon prescriptions ahead of patients’ appointments. Each week, the pharmacists generate an EHR report that includes all patients with T1DM who have attended at least 1 appointment at the clinic within the past year (regardless of whether each patient possessed an active and up-to-date glucagon prescription) and the date of each patient’s next appointment. For patients who have an appointment in the upcoming week and lack an active glucagon prescription, the pharmacists run a benefits investigation to determine the insurance-preferred glucagon formulation and then pend the appropriate order in the EHR. During the patient’s next appointment, the EHR prompts the provider to review and sign the pharmacist’s pended order (Figure 1).
This project used a process measure in its analysis: the percentage of patients with T1DM with an active glucagon prescription at the time of their visit to the clinic. The patient population included all patients with a visit diagnosis of T1DM seen by an APP at the clinic during the time scope of the project. The project’s scope was limited to patients seen by APPs to help standardize appointment comparisons, with the intent to expand to the endocrinologist staff if the interventions proved successful with APPs. Patients seen by APPs were also under the care of endocrinologists and seen by them during this time period. The project excluded no patients.
Each individual patient appointment represented a data point: a time at which an APP could prescribe glucagon for a patient with T1DM. Thus, a single patient who had multiple appointments during the study period would generate multiple data points in this study.
For all T1DM patients at the clinic seen by an APP during the study period, the project aimed to increase the percentage with an active and up-to-date glucagon prescription from 58.8% to 70% over a 6-month period, a relatively modest goal appropriate for the time constraints and that would be similar to the changes seen in previous work in the same clinic.9
This project analyzed de-identified data using a statistical process control chart (specifically, a p-chart) and standard rules for assessing special-cause signals and thus statistical significance.
Results
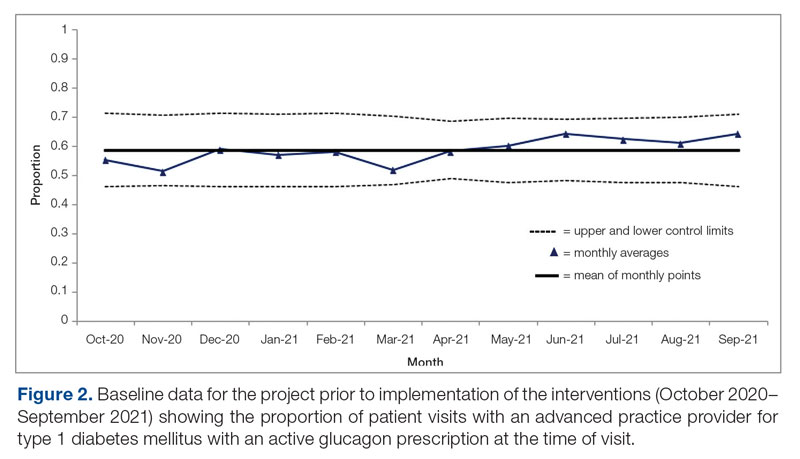
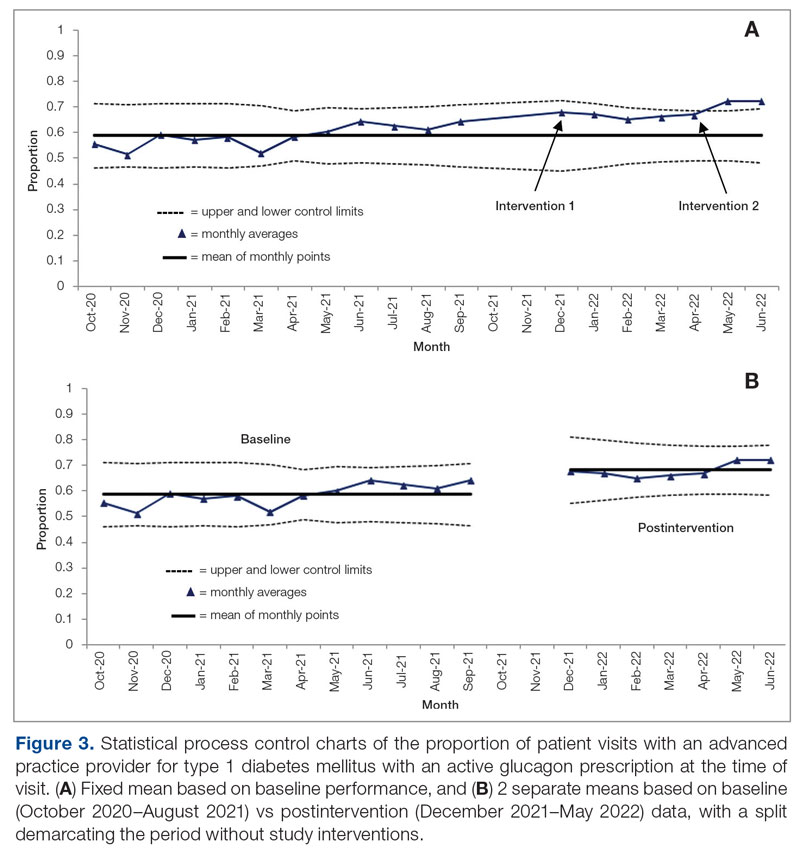
Baseline data were collected from October 2020 to September 2021. During this time, APPs saw 1959 T1DM patients, of whom 1152 (58.8%) had an active glucagon prescription at the time of visit and 41.2% lacked a glucagon prescription (Figure 2). During the 4 months of implementation phase 1, analysis of the statistical process control chart identified no special cause signal. Therefore, the project moved to a second intervention with implementation phase 2 in April 2022 (3 months of postintervention data are reported). During the entire intervention, 731 of 1080 (67.7%) patients had a glucagon prescription. The average for the last 2 months, with phase 2 fully implemented, was 72.3%, surpassing the 70% threshold identified as the study target (Figure 3).
Interviews with clinical pharmacists during implementation phase 2 revealed that generating the EHR report and reviewing patients with glucagon prescription indications resulted in variable daily workload increases ranging from approximately 15 to 45 minutes, depending on the number of patients requiring intervention that day. During the first month of implementation phase 2, the EHR report required repeated modification to fulfill the intervention needs. Staffing changes over the intervention period potentially impacted the pattern of glucagon prescribing. This project excluded the 2 months immediately prior to implementation phase 1, from October 2021 to November 2021, because the staff had begun having discussions about this initiative, which may have influenced glucagon prescription rates.
Discussion
This project evaluated 2 interventions over the course of 7 months to determine their efficacy in increasing the frequency of glucagon prescribing for individuals with T1DM in an endocrinology clinic. These interventions were associated with increased prescribing from a baseline of 58.8% to 72.3% over the last 2 months of the project. In the first intervention, performed over 4 months, MAs/LPNs wrote reminders on the appropriate patients’ face sheets, which were given to providers prior to appointments. This project adapted the approach from a successful previous quality improvement study on increasing microalbuminuria screening rates.9 However, glucagon prescription rates did not increase significantly, likely because, unlike with microalbuminuria screenings, MAs/LPNs could not pend glucagon prescriptions.
In the second intervention, performed over 3 months, clinical pharmacists pended glucagon prescriptions for identified eligible patients. Glucagon prescribing rates increased considerably, with rates of 72.3% and 72.4% over May and June 2021, respectively, indicating that the intervention successfully established a new higher steady state of proportion of patient visits with active glucagon prescriptions compared with the baseline rate of 58.8%. Given that the baseline data for this clinic were higher than the baseline glucagon prescription rates reported in other studies (49.3%),10 this intervention could have a major impact in clinics with a baseline more comparable to conditions in that study.
This project demonstrated how a combination of an EHR-generated report and interdisciplinary involvement provides an actionable process to increase glucagon prescription rates for patients with T1DM. Compared to prior studies that implemented passive interventions, such as a note template that relies on provider adherence,7 this project emphasizes the benefit of implementing an active systems-level intervention with a pre-pended order.
Regarding prior studies, 1 large, 2-arm study of clinical pharmacists proactively pending orders for appropriate patients showed a 56% glucagon prescription rate in the intervention group, compared with 0.9% in the control group with no pharmacist intervention.11 Our project had a much higher baseline rate: 58.8% prior to intervention vs 0.9% in the nonintervention group for the previous study—likely due to its chosen location’s status as an endocrinology clinic rather than a general health care setting.
A different study that focused on patient education rather than glucagon prescription rates used similar EHR-generated reports to identify appropriate patients and assessed glucagon prescription needs during check-in. Following the educational interventions in that study, patients reporting self-comfort and education with glucagon administration significantly increased from 66.2% to 83.2%, and household member comfort and education with glucagon administration increased from 50.8% to 79.7%. This suggests the possibility of expanding the use of the EHR-generated report to assist not only with increasing glucagon prescription rates, but also with patient education on glucagon use rates and possibly fill rates.7 While novel glucagon products may change uptake rates, no new glucagon products arose or were prescribed at this clinic during the course of data collection.
Of note, our project increased the workload on clinical pharmacists. The pharmacists agreed to participate, despite the increased work, after a collaborative discussion about how to best address the need to increase glucagon prescriptions or patient safety; the pharmacy department had initially agreed to collaborate specifically to identify and attend to unmet needs such as this one. Although this project greatly benefited from the expertise and enthusiasm of the clinical pharmacists involved, this tradeoff requires further study to determine sustainability.
This project had several limitations. Because of the structure in which this intervention occurred (a year-long course with rotating groups of medical students), there was a necessary component of time constraint, and this project had just 2 implementation phases, for a total of 7 months of postintervention data. The clinic has permanently implemented these changes into its workflow, but subsequent assessments are needed to monitor the effects and assess sustainability.
The specific clinical site chosen for this study benefited from dedicated onsite clinical pharmacists, who are not available at all comparable clinical sites. Due to feasibility, this project only assessed whether the providers prescribed the glucagon, not whether the patients filled the prescriptions and used the glucagon when necessary. Although prescribing rates increased in our study, it cannot be assumed that fill rates increased identically.
Finally, interventions relying on EHR-generated reports carry inherent limitations, such as the risk of misidentification or omission of patients who had indications for a glucagon prescription. The project attempted to mitigate this limitation through random sampling of the EHR report to ensure accuracy. Additionally, EHR-generated reports encourage sustainability and expansion to all clinic patients, with far less required overhead work compared to manually derived data.
Future investigations may focus on expanding this intervention to all patients at risk for hypoglycemia, as well as to study further interventions into prescription fill rates and glucagon use rates.
Conclusion
This project indicates that a proactive, interdisciplinary quality improvement project can increase glucagon prescription rates for patients with T1DM in the outpatient setting. The most effective intervention mobilized clinical pharmacists to identify patients with indications for a glucagon prescription using an integrated EHR-generated report and subsequently pend a glucagon order for the endocrinology provider to sign during the visit. The strengths of the approach included using a multidisciplinary team, minimizing costs to patients by leveraging the pharmacists’ expertise to ensure insurance coverage of specific formulations, and utilizing automatic EHR reporting to streamline patient identification. Ideally, improvements in glucagon prescription rates should ultimately decrease hospitalizations and improve treatment of severe hypoglycemia for at-risk patients.
Corresponding author: Chase D. Hendrickson, MD, MPH; [email protected]
Disclosures: None reported.
1. Weinstock RS, Aleppo G, Bailey TS, et al. The Role of Blood Glucose Monitoring in Diabetes Management. American Diabetes Association; 2020.
2. Lamounier RN, Geloneze B, Leite SO, et al. Hypoglycemia incidence and awareness among insulin-treated patients with diabetes: the HAT study in Brazil. Diabetol Metab Syndr. 2018;10:83. doi:10.1186/s13098-018-0379-5
3. Li P, Geng Z, Ladage VP, et al. Early hypoglycaemia and adherence after basal insulin initiation in a nationally representative sample of Medicare beneficiaries with type 2 diabetes. Diabetes Obes Metab. 2019;21(11):2486-2495. doi:10.1111/dom.13832
4. Haymond MW, Liu J, Bispham J, et al. Use of glucagon in patients with type 1 diabetes. Clin Diabetes. 2019;37(2):162-166. doi:10.2337/cd18-0028
5. American Diabetes Association Professional Practice Committee. 6. Glycemic targets: standards of medical care in diabetes-2022. Diabetes Care. 2022; 45(Suppl 1):S83-S96. doi:10.2337/dc22-S006
6. O’Reilly EA, Cross LV, Hayes JS, et al. Impact of pharmacist intervention on glucagon prescribing patterns in an outpatient internal medicine teaching clinic. J Am Pharm Assoc (2003). 2020;60(2):384-390. doi:10.1016/j.japh.2019.04.0097.
7. Cobb EC, Watson NA, Wardian J, et al. Diabetes Center of Excellence Hypoglycemia Emergency Preparedness Project. Clin Diabetes. 2018;36(2):184-186. doi:10.2337/cd17-0040
8. Ogrinc G, Davies L, Goodman D, et al. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. doi:10.1136/bmjqs-2015-004411
9. Kam S, Angaramo S, Antoun J, et al. Improving annual albuminuria testing for individuals with diabetes. BMJ Open Qual. 2022;11(1):e001591. doi:10.1136/bmjoq-2021-001591
10. Mitchell BD, He X, Sturdy IM, et al. Glucagon prescription patterns in patients with either type 1 or 2 diabetes with newly prescribed insulin. Endocr Pract. 2016;22(2):123-135. doi:10.4158/EP15831.OR
11. Whitfield N, Gregory P, Liu B, et al. Impact of pharmacist outreach on glucagon prescribing. J Am Pharm Assoc. 2022;62(4):1384-1388.e.1. doi:10.1016/j.japh.2022.01.017
From Vanderbilt University School of Medicine, and Vanderbilt University Medical Center, Nashville, TN.
ABSTRACT
Objective: Severe hypoglycemia can alter consciousness and inhibit oral intake, requiring nonoral rescue glucagon administration to raise blood glucose to safe levels. Thus, current guidelines recommend glucagon kit prescriptions for all patients at risk for hypoglycemia, especially patients with type 1 diabetes mellitus (T1DM). At the diabetes outpatient clinic at a tertiary medical center, glucagon prescription rates for T1DM patients remained suboptimal.
Methods: A quality improvement team analyzed patient flow through the endocrinology clinic and identified the lack of a systematic approach to assessing patients for home glucagon prescriptions as a major barrier. The team implemented 2 successive interventions. First, intake staff indicated whether patients lacked an active glucagon prescription on patients’ face sheets. Second, clinical pharmacists reviewed patient prescriptions prior to scheduled visits and pended glucagon orders for patients without active prescriptions. Of note, when a pharmacy pends an order, the pharmacist enters an order into the electronic health record (EHR) but does not sign it. The order is saved for a provider to later access and sign. A statistical process control p-chart tracked monthly prescription rates.
Results: After 7 months, glucagon prescription rates increased from a baseline of 59% to 72% as the new steady state.
Conclusion: This project demonstrates that a series of interventions can improve glucagon prescription rates for patients at risk for hypoglycemia. The project’s success stemmed from combining an EHR-generated report and interdisciplinary staff members’ involvement. Other endocrinology clinics may incorporate this approach to implement similar processes and improve glucagon prescription rates.
Keywords: diabetes, hypoglycemia, glucagon, quality improvement, prescription rates, medical student.
Hypoglycemia limits the management of blood glucose in patients with type 1 diabetes mellitus (T1DM). Severe hypoglycemia, characterized by altered mental status (AMS) or physical status requiring assistance for recovery, can lead to seizure, coma, or death.1 Hypoglycemia in diabetes often occurs iatrogenically, primarily from insulin therapy: 30% to 40% of patients with T1DM and 10% to 30% of patients with insulin-treated type 2 diabetes mellitus experience severe hypoglycemia in a given year.2 One study estimated that nearly 100,000 emergency department visits for hypoglycemia occur in the United States per year, with almost one-third resulting in hospitalization.3
Most patients self-treat mild hypoglycemia with oral intake of carbohydrates. However, since hypoglycemia-induced nausea and AMS can make oral intake more difficult or prevent it entirely, patients require a treatment that family, friends, or coworkers can administer. Rescue glucagon, prescribed as intramuscular injections or intranasal sprays, raises blood glucose to safe levels in 10 to 15 minutes.4 Therefore, the American Diabetes Association (ADA) recommends glucagon for all patients at risk for hypoglycemia, especially patients with T1DM.5 Despite the ADA’s recommendation, current evidence suggests suboptimal glucagon prescription rates, particularly in patients with T1DM. One study reported that, although 85% of US adults with T1DM had formerly been prescribed glucagon, only 68% of these patients (57.8% overall) had a current prescription.4 Few quality improvement efforts have tackled increasing prescription rates. Prior successful studies have attempted to do so via pharmacist-led educational interventions for providers6 and via electronic health record (EHR) notifications for patient risk.7 The project described here aimed to expand upon prior studies with a quality improvement project to increase glucagon prescription rates among patients at risk for severe hypoglycemia.
This study was conducted at a tertiary medical center’s outpatient diabetes clinic; the clinic treats more than 9500 patients with DM annually, more than 2700 of whom have T1DM. In the clinic’s multidisciplinary care model, patients typically follow up every 3 to 6 months, alternating between appointments with fellowship-trained endocrinologists and advanced practice providers (APPs). In addition to having certified diabetes educators, the clinic employs 2 dedicated clinical pharmacists whose duties include assisting providers in prescription management, helping patients identify the most affordable way to obtain their medications, and educating patients regarding their medications.
Patient flow through the clinic involves close coordination with multiple health professionals. Medical assistants (MAs) and licensed practical nurses (LPNs) perform patient intake, document vital signs, and ask screening questions, including dates of patients’ last hemoglobin A1c tests and diabetic eye examination. After intake, the provider (endocrinologist or APP) sees the patient. Once the appointment concludes, patients proceed to the in-house phlebotomy laboratory as indicated and check out with administrative staff to schedule future appointments.
From August 2021 through June 2022, teams of medical students at the tertiary center completed this project as part of a 4-week integrated science course on diabetes. Longitudinal supervision by an endocrinology faculty member ensured project continuity. The project employed the Standards for QUality Improvement Reporting Excellence (SQUIRE 2.0) method for reporting.8
Stakeholder analysis took place in August 2021. Surveyed clinic providers identified patients with T1DM as the most appropriate population and the outpatient setting as the most appropriate site for intervention. A fishbone diagram illustrated stakeholders to interview, impacts of the clinical flow, information technology to leverage, and potential holes contributing to glucagon prescription conversations falling through.
Interviews with T1DM patients, clinical pharmacists, APPs, MAs/LPNs, and endocrinologists identified barriers to glucagon prescription. The interviews and a process map analysis revealed several themes. While patients and providers understood the importance of glucagon prescription, barriers included glucagon cost, prescription fill burden, and, most pervasively, providers forgetting to ask patients whether they have a glucagon prescription and failing to consider glucagon prescriptions.For this study, each team of medical students worked on the project for 1 month. The revolving teams of medical students met approximately once per week for the duration of the project to review data and implementation phases. At the end of each month, the current team recorded the steps they had taken and information they had analyzed in a shared document, prepared short videos summarizing the work completed, and proposed next steps for the incoming team to support knowledge generation and continuity. Students from outgoing teams were available to contact if incoming teams had any questions.
Interventions
In the first implementation phase, which was carried out over 4 months (December 2021 to March 2022), the patient care manager trained MAs/LPNs to write a glucagon reminder on patients’ face sheets. At check-in, MAs/LPNs screened for a current glucagon prescription. If the patient lacked an up-to-date prescription, the MAs/LPNs hand-wrote a reminder on the patient’s face sheet, which was given to the provider immediately prior to seeing the patient. The clinical staff received an email explaining the intervention beforehand; the daily intake staff email included project reminders.

In the second implementation phase, which started in April 2022, had been carried out for 3 months at the time of this report, and is ongoing, clinical pharmacists have been pending glucagon prescriptions ahead of patients’ appointments. Each week, the pharmacists generate an EHR report that includes all patients with T1DM who have attended at least 1 appointment at the clinic within the past year (regardless of whether each patient possessed an active and up-to-date glucagon prescription) and the date of each patient’s next appointment. For patients who have an appointment in the upcoming week and lack an active glucagon prescription, the pharmacists run a benefits investigation to determine the insurance-preferred glucagon formulation and then pend the appropriate order in the EHR. During the patient’s next appointment, the EHR prompts the provider to review and sign the pharmacist’s pended order (Figure 1).

This project used a process measure in its analysis: the percentage of patients with T1DM with an active glucagon prescription at the time of their visit to the clinic. The patient population included all patients with a visit diagnosis of T1DM seen by an APP at the clinic during the time scope of the project. The project’s scope was limited to patients seen by APPs to help standardize appointment comparisons, with the intent to expand to the endocrinologist staff if the interventions proved successful with APPs. Patients seen by APPs were also under the care of endocrinologists and seen by them during this time period. The project excluded no patients.
Each individual patient appointment represented a data point: a time at which an APP could prescribe glucagon for a patient with T1DM. Thus, a single patient who had multiple appointments during the study period would generate multiple data points in this study.
For all T1DM patients at the clinic seen by an APP during the study period, the project aimed to increase the percentage with an active and up-to-date glucagon prescription from 58.8% to 70% over a 6-month period, a relatively modest goal appropriate for the time constraints and that would be similar to the changes seen in previous work in the same clinic.9
This project analyzed de-identified data using a statistical process control chart (specifically, a p-chart) and standard rules for assessing special-cause signals and thus statistical significance.
Results
Baseline data were collected from October 2020 to September 2021. During this time, APPs saw 1959 T1DM patients, of whom 1152 (58.8%) had an active glucagon prescription at the time of visit and 41.2% lacked a glucagon prescription (Figure 2). During the 4 months of implementation phase 1, analysis of the statistical process control chart identified no special cause signal. Therefore, the project moved to a second intervention with implementation phase 2 in April 2022 (3 months of postintervention data are reported). During the entire intervention, 731 of 1080 (67.7%) patients had a glucagon prescription. The average for the last 2 months, with phase 2 fully implemented, was 72.3%, surpassing the 70% threshold identified as the study target (Figure 3).

Interviews with clinical pharmacists during implementation phase 2 revealed that generating the EHR report and reviewing patients with glucagon prescription indications resulted in variable daily workload increases ranging from approximately 15 to 45 minutes, depending on the number of patients requiring intervention that day. During the first month of implementation phase 2, the EHR report required repeated modification to fulfill the intervention needs. Staffing changes over the intervention period potentially impacted the pattern of glucagon prescribing. This project excluded the 2 months immediately prior to implementation phase 1, from October 2021 to November 2021, because the staff had begun having discussions about this initiative, which may have influenced glucagon prescription rates.

Discussion
This project evaluated 2 interventions over the course of 7 months to determine their efficacy in increasing the frequency of glucagon prescribing for individuals with T1DM in an endocrinology clinic. These interventions were associated with increased prescribing from a baseline of 58.8% to 72.3% over the last 2 months of the project. In the first intervention, performed over 4 months, MAs/LPNs wrote reminders on the appropriate patients’ face sheets, which were given to providers prior to appointments. This project adapted the approach from a successful previous quality improvement study on increasing microalbuminuria screening rates.9 However, glucagon prescription rates did not increase significantly, likely because, unlike with microalbuminuria screenings, MAs/LPNs could not pend glucagon prescriptions.
In the second intervention, performed over 3 months, clinical pharmacists pended glucagon prescriptions for identified eligible patients. Glucagon prescribing rates increased considerably, with rates of 72.3% and 72.4% over May and June 2021, respectively, indicating that the intervention successfully established a new higher steady state of proportion of patient visits with active glucagon prescriptions compared with the baseline rate of 58.8%. Given that the baseline data for this clinic were higher than the baseline glucagon prescription rates reported in other studies (49.3%),10 this intervention could have a major impact in clinics with a baseline more comparable to conditions in that study.
This project demonstrated how a combination of an EHR-generated report and interdisciplinary involvement provides an actionable process to increase glucagon prescription rates for patients with T1DM. Compared to prior studies that implemented passive interventions, such as a note template that relies on provider adherence,7 this project emphasizes the benefit of implementing an active systems-level intervention with a pre-pended order.
Regarding prior studies, 1 large, 2-arm study of clinical pharmacists proactively pending orders for appropriate patients showed a 56% glucagon prescription rate in the intervention group, compared with 0.9% in the control group with no pharmacist intervention.11 Our project had a much higher baseline rate: 58.8% prior to intervention vs 0.9% in the nonintervention group for the previous study—likely due to its chosen location’s status as an endocrinology clinic rather than a general health care setting.
A different study that focused on patient education rather than glucagon prescription rates used similar EHR-generated reports to identify appropriate patients and assessed glucagon prescription needs during check-in. Following the educational interventions in that study, patients reporting self-comfort and education with glucagon administration significantly increased from 66.2% to 83.2%, and household member comfort and education with glucagon administration increased from 50.8% to 79.7%. This suggests the possibility of expanding the use of the EHR-generated report to assist not only with increasing glucagon prescription rates, but also with patient education on glucagon use rates and possibly fill rates.7 While novel glucagon products may change uptake rates, no new glucagon products arose or were prescribed at this clinic during the course of data collection.
Of note, our project increased the workload on clinical pharmacists. The pharmacists agreed to participate, despite the increased work, after a collaborative discussion about how to best address the need to increase glucagon prescriptions or patient safety; the pharmacy department had initially agreed to collaborate specifically to identify and attend to unmet needs such as this one. Although this project greatly benefited from the expertise and enthusiasm of the clinical pharmacists involved, this tradeoff requires further study to determine sustainability.
This project had several limitations. Because of the structure in which this intervention occurred (a year-long course with rotating groups of medical students), there was a necessary component of time constraint, and this project had just 2 implementation phases, for a total of 7 months of postintervention data. The clinic has permanently implemented these changes into its workflow, but subsequent assessments are needed to monitor the effects and assess sustainability.
The specific clinical site chosen for this study benefited from dedicated onsite clinical pharmacists, who are not available at all comparable clinical sites. Due to feasibility, this project only assessed whether the providers prescribed the glucagon, not whether the patients filled the prescriptions and used the glucagon when necessary. Although prescribing rates increased in our study, it cannot be assumed that fill rates increased identically.
Finally, interventions relying on EHR-generated reports carry inherent limitations, such as the risk of misidentification or omission of patients who had indications for a glucagon prescription. The project attempted to mitigate this limitation through random sampling of the EHR report to ensure accuracy. Additionally, EHR-generated reports encourage sustainability and expansion to all clinic patients, with far less required overhead work compared to manually derived data.
Future investigations may focus on expanding this intervention to all patients at risk for hypoglycemia, as well as to study further interventions into prescription fill rates and glucagon use rates.
Conclusion
This project indicates that a proactive, interdisciplinary quality improvement project can increase glucagon prescription rates for patients with T1DM in the outpatient setting. The most effective intervention mobilized clinical pharmacists to identify patients with indications for a glucagon prescription using an integrated EHR-generated report and subsequently pend a glucagon order for the endocrinology provider to sign during the visit. The strengths of the approach included using a multidisciplinary team, minimizing costs to patients by leveraging the pharmacists’ expertise to ensure insurance coverage of specific formulations, and utilizing automatic EHR reporting to streamline patient identification. Ideally, improvements in glucagon prescription rates should ultimately decrease hospitalizations and improve treatment of severe hypoglycemia for at-risk patients.
Corresponding author: Chase D. Hendrickson, MD, MPH; [email protected]
Disclosures: None reported.
From Vanderbilt University School of Medicine, and Vanderbilt University Medical Center, Nashville, TN.
ABSTRACT
Objective: Severe hypoglycemia can alter consciousness and inhibit oral intake, requiring nonoral rescue glucagon administration to raise blood glucose to safe levels. Thus, current guidelines recommend glucagon kit prescriptions for all patients at risk for hypoglycemia, especially patients with type 1 diabetes mellitus (T1DM). At the diabetes outpatient clinic at a tertiary medical center, glucagon prescription rates for T1DM patients remained suboptimal.
Methods: A quality improvement team analyzed patient flow through the endocrinology clinic and identified the lack of a systematic approach to assessing patients for home glucagon prescriptions as a major barrier. The team implemented 2 successive interventions. First, intake staff indicated whether patients lacked an active glucagon prescription on patients’ face sheets. Second, clinical pharmacists reviewed patient prescriptions prior to scheduled visits and pended glucagon orders for patients without active prescriptions. Of note, when a pharmacy pends an order, the pharmacist enters an order into the electronic health record (EHR) but does not sign it. The order is saved for a provider to later access and sign. A statistical process control p-chart tracked monthly prescription rates.
Results: After 7 months, glucagon prescription rates increased from a baseline of 59% to 72% as the new steady state.
Conclusion: This project demonstrates that a series of interventions can improve glucagon prescription rates for patients at risk for hypoglycemia. The project’s success stemmed from combining an EHR-generated report and interdisciplinary staff members’ involvement. Other endocrinology clinics may incorporate this approach to implement similar processes and improve glucagon prescription rates.
Keywords: diabetes, hypoglycemia, glucagon, quality improvement, prescription rates, medical student.
Hypoglycemia limits the management of blood glucose in patients with type 1 diabetes mellitus (T1DM). Severe hypoglycemia, characterized by altered mental status (AMS) or physical status requiring assistance for recovery, can lead to seizure, coma, or death.1 Hypoglycemia in diabetes often occurs iatrogenically, primarily from insulin therapy: 30% to 40% of patients with T1DM and 10% to 30% of patients with insulin-treated type 2 diabetes mellitus experience severe hypoglycemia in a given year.2 One study estimated that nearly 100,000 emergency department visits for hypoglycemia occur in the United States per year, with almost one-third resulting in hospitalization.3
Most patients self-treat mild hypoglycemia with oral intake of carbohydrates. However, since hypoglycemia-induced nausea and AMS can make oral intake more difficult or prevent it entirely, patients require a treatment that family, friends, or coworkers can administer. Rescue glucagon, prescribed as intramuscular injections or intranasal sprays, raises blood glucose to safe levels in 10 to 15 minutes.4 Therefore, the American Diabetes Association (ADA) recommends glucagon for all patients at risk for hypoglycemia, especially patients with T1DM.5 Despite the ADA’s recommendation, current evidence suggests suboptimal glucagon prescription rates, particularly in patients with T1DM. One study reported that, although 85% of US adults with T1DM had formerly been prescribed glucagon, only 68% of these patients (57.8% overall) had a current prescription.4 Few quality improvement efforts have tackled increasing prescription rates. Prior successful studies have attempted to do so via pharmacist-led educational interventions for providers6 and via electronic health record (EHR) notifications for patient risk.7 The project described here aimed to expand upon prior studies with a quality improvement project to increase glucagon prescription rates among patients at risk for severe hypoglycemia.
This study was conducted at a tertiary medical center’s outpatient diabetes clinic; the clinic treats more than 9500 patients with DM annually, more than 2700 of whom have T1DM. In the clinic’s multidisciplinary care model, patients typically follow up every 3 to 6 months, alternating between appointments with fellowship-trained endocrinologists and advanced practice providers (APPs). In addition to having certified diabetes educators, the clinic employs 2 dedicated clinical pharmacists whose duties include assisting providers in prescription management, helping patients identify the most affordable way to obtain their medications, and educating patients regarding their medications.
Patient flow through the clinic involves close coordination with multiple health professionals. Medical assistants (MAs) and licensed practical nurses (LPNs) perform patient intake, document vital signs, and ask screening questions, including dates of patients’ last hemoglobin A1c tests and diabetic eye examination. After intake, the provider (endocrinologist or APP) sees the patient. Once the appointment concludes, patients proceed to the in-house phlebotomy laboratory as indicated and check out with administrative staff to schedule future appointments.
From August 2021 through June 2022, teams of medical students at the tertiary center completed this project as part of a 4-week integrated science course on diabetes. Longitudinal supervision by an endocrinology faculty member ensured project continuity. The project employed the Standards for QUality Improvement Reporting Excellence (SQUIRE 2.0) method for reporting.8
Stakeholder analysis took place in August 2021. Surveyed clinic providers identified patients with T1DM as the most appropriate population and the outpatient setting as the most appropriate site for intervention. A fishbone diagram illustrated stakeholders to interview, impacts of the clinical flow, information technology to leverage, and potential holes contributing to glucagon prescription conversations falling through.
Interviews with T1DM patients, clinical pharmacists, APPs, MAs/LPNs, and endocrinologists identified barriers to glucagon prescription. The interviews and a process map analysis revealed several themes. While patients and providers understood the importance of glucagon prescription, barriers included glucagon cost, prescription fill burden, and, most pervasively, providers forgetting to ask patients whether they have a glucagon prescription and failing to consider glucagon prescriptions.For this study, each team of medical students worked on the project for 1 month. The revolving teams of medical students met approximately once per week for the duration of the project to review data and implementation phases. At the end of each month, the current team recorded the steps they had taken and information they had analyzed in a shared document, prepared short videos summarizing the work completed, and proposed next steps for the incoming team to support knowledge generation and continuity. Students from outgoing teams were available to contact if incoming teams had any questions.
Interventions
In the first implementation phase, which was carried out over 4 months (December 2021 to March 2022), the patient care manager trained MAs/LPNs to write a glucagon reminder on patients’ face sheets. At check-in, MAs/LPNs screened for a current glucagon prescription. If the patient lacked an up-to-date prescription, the MAs/LPNs hand-wrote a reminder on the patient’s face sheet, which was given to the provider immediately prior to seeing the patient. The clinical staff received an email explaining the intervention beforehand; the daily intake staff email included project reminders.

In the second implementation phase, which started in April 2022, had been carried out for 3 months at the time of this report, and is ongoing, clinical pharmacists have been pending glucagon prescriptions ahead of patients’ appointments. Each week, the pharmacists generate an EHR report that includes all patients with T1DM who have attended at least 1 appointment at the clinic within the past year (regardless of whether each patient possessed an active and up-to-date glucagon prescription) and the date of each patient’s next appointment. For patients who have an appointment in the upcoming week and lack an active glucagon prescription, the pharmacists run a benefits investigation to determine the insurance-preferred glucagon formulation and then pend the appropriate order in the EHR. During the patient’s next appointment, the EHR prompts the provider to review and sign the pharmacist’s pended order (Figure 1).

This project used a process measure in its analysis: the percentage of patients with T1DM with an active glucagon prescription at the time of their visit to the clinic. The patient population included all patients with a visit diagnosis of T1DM seen by an APP at the clinic during the time scope of the project. The project’s scope was limited to patients seen by APPs to help standardize appointment comparisons, with the intent to expand to the endocrinologist staff if the interventions proved successful with APPs. Patients seen by APPs were also under the care of endocrinologists and seen by them during this time period. The project excluded no patients.
Each individual patient appointment represented a data point: a time at which an APP could prescribe glucagon for a patient with T1DM. Thus, a single patient who had multiple appointments during the study period would generate multiple data points in this study.
For all T1DM patients at the clinic seen by an APP during the study period, the project aimed to increase the percentage with an active and up-to-date glucagon prescription from 58.8% to 70% over a 6-month period, a relatively modest goal appropriate for the time constraints and that would be similar to the changes seen in previous work in the same clinic.9
This project analyzed de-identified data using a statistical process control chart (specifically, a p-chart) and standard rules for assessing special-cause signals and thus statistical significance.
Results
Baseline data were collected from October 2020 to September 2021. During this time, APPs saw 1959 T1DM patients, of whom 1152 (58.8%) had an active glucagon prescription at the time of visit and 41.2% lacked a glucagon prescription (Figure 2). During the 4 months of implementation phase 1, analysis of the statistical process control chart identified no special cause signal. Therefore, the project moved to a second intervention with implementation phase 2 in April 2022 (3 months of postintervention data are reported). During the entire intervention, 731 of 1080 (67.7%) patients had a glucagon prescription. The average for the last 2 months, with phase 2 fully implemented, was 72.3%, surpassing the 70% threshold identified as the study target (Figure 3).

Interviews with clinical pharmacists during implementation phase 2 revealed that generating the EHR report and reviewing patients with glucagon prescription indications resulted in variable daily workload increases ranging from approximately 15 to 45 minutes, depending on the number of patients requiring intervention that day. During the first month of implementation phase 2, the EHR report required repeated modification to fulfill the intervention needs. Staffing changes over the intervention period potentially impacted the pattern of glucagon prescribing. This project excluded the 2 months immediately prior to implementation phase 1, from October 2021 to November 2021, because the staff had begun having discussions about this initiative, which may have influenced glucagon prescription rates.

Discussion
This project evaluated 2 interventions over the course of 7 months to determine their efficacy in increasing the frequency of glucagon prescribing for individuals with T1DM in an endocrinology clinic. These interventions were associated with increased prescribing from a baseline of 58.8% to 72.3% over the last 2 months of the project. In the first intervention, performed over 4 months, MAs/LPNs wrote reminders on the appropriate patients’ face sheets, which were given to providers prior to appointments. This project adapted the approach from a successful previous quality improvement study on increasing microalbuminuria screening rates.9 However, glucagon prescription rates did not increase significantly, likely because, unlike with microalbuminuria screenings, MAs/LPNs could not pend glucagon prescriptions.
In the second intervention, performed over 3 months, clinical pharmacists pended glucagon prescriptions for identified eligible patients. Glucagon prescribing rates increased considerably, with rates of 72.3% and 72.4% over May and June 2021, respectively, indicating that the intervention successfully established a new higher steady state of proportion of patient visits with active glucagon prescriptions compared with the baseline rate of 58.8%. Given that the baseline data for this clinic were higher than the baseline glucagon prescription rates reported in other studies (49.3%),10 this intervention could have a major impact in clinics with a baseline more comparable to conditions in that study.
This project demonstrated how a combination of an EHR-generated report and interdisciplinary involvement provides an actionable process to increase glucagon prescription rates for patients with T1DM. Compared to prior studies that implemented passive interventions, such as a note template that relies on provider adherence,7 this project emphasizes the benefit of implementing an active systems-level intervention with a pre-pended order.
Regarding prior studies, 1 large, 2-arm study of clinical pharmacists proactively pending orders for appropriate patients showed a 56% glucagon prescription rate in the intervention group, compared with 0.9% in the control group with no pharmacist intervention.11 Our project had a much higher baseline rate: 58.8% prior to intervention vs 0.9% in the nonintervention group for the previous study—likely due to its chosen location’s status as an endocrinology clinic rather than a general health care setting.
A different study that focused on patient education rather than glucagon prescription rates used similar EHR-generated reports to identify appropriate patients and assessed glucagon prescription needs during check-in. Following the educational interventions in that study, patients reporting self-comfort and education with glucagon administration significantly increased from 66.2% to 83.2%, and household member comfort and education with glucagon administration increased from 50.8% to 79.7%. This suggests the possibility of expanding the use of the EHR-generated report to assist not only with increasing glucagon prescription rates, but also with patient education on glucagon use rates and possibly fill rates.7 While novel glucagon products may change uptake rates, no new glucagon products arose or were prescribed at this clinic during the course of data collection.
Of note, our project increased the workload on clinical pharmacists. The pharmacists agreed to participate, despite the increased work, after a collaborative discussion about how to best address the need to increase glucagon prescriptions or patient safety; the pharmacy department had initially agreed to collaborate specifically to identify and attend to unmet needs such as this one. Although this project greatly benefited from the expertise and enthusiasm of the clinical pharmacists involved, this tradeoff requires further study to determine sustainability.
This project had several limitations. Because of the structure in which this intervention occurred (a year-long course with rotating groups of medical students), there was a necessary component of time constraint, and this project had just 2 implementation phases, for a total of 7 months of postintervention data. The clinic has permanently implemented these changes into its workflow, but subsequent assessments are needed to monitor the effects and assess sustainability.
The specific clinical site chosen for this study benefited from dedicated onsite clinical pharmacists, who are not available at all comparable clinical sites. Due to feasibility, this project only assessed whether the providers prescribed the glucagon, not whether the patients filled the prescriptions and used the glucagon when necessary. Although prescribing rates increased in our study, it cannot be assumed that fill rates increased identically.
Finally, interventions relying on EHR-generated reports carry inherent limitations, such as the risk of misidentification or omission of patients who had indications for a glucagon prescription. The project attempted to mitigate this limitation through random sampling of the EHR report to ensure accuracy. Additionally, EHR-generated reports encourage sustainability and expansion to all clinic patients, with far less required overhead work compared to manually derived data.
Future investigations may focus on expanding this intervention to all patients at risk for hypoglycemia, as well as to study further interventions into prescription fill rates and glucagon use rates.
Conclusion
This project indicates that a proactive, interdisciplinary quality improvement project can increase glucagon prescription rates for patients with T1DM in the outpatient setting. The most effective intervention mobilized clinical pharmacists to identify patients with indications for a glucagon prescription using an integrated EHR-generated report and subsequently pend a glucagon order for the endocrinology provider to sign during the visit. The strengths of the approach included using a multidisciplinary team, minimizing costs to patients by leveraging the pharmacists’ expertise to ensure insurance coverage of specific formulations, and utilizing automatic EHR reporting to streamline patient identification. Ideally, improvements in glucagon prescription rates should ultimately decrease hospitalizations and improve treatment of severe hypoglycemia for at-risk patients.
Corresponding author: Chase D. Hendrickson, MD, MPH; [email protected]
Disclosures: None reported.
1. Weinstock RS, Aleppo G, Bailey TS, et al. The Role of Blood Glucose Monitoring in Diabetes Management. American Diabetes Association; 2020.
2. Lamounier RN, Geloneze B, Leite SO, et al. Hypoglycemia incidence and awareness among insulin-treated patients with diabetes: the HAT study in Brazil. Diabetol Metab Syndr. 2018;10:83. doi:10.1186/s13098-018-0379-5
3. Li P, Geng Z, Ladage VP, et al. Early hypoglycaemia and adherence after basal insulin initiation in a nationally representative sample of Medicare beneficiaries with type 2 diabetes. Diabetes Obes Metab. 2019;21(11):2486-2495. doi:10.1111/dom.13832
4. Haymond MW, Liu J, Bispham J, et al. Use of glucagon in patients with type 1 diabetes. Clin Diabetes. 2019;37(2):162-166. doi:10.2337/cd18-0028
5. American Diabetes Association Professional Practice Committee. 6. Glycemic targets: standards of medical care in diabetes-2022. Diabetes Care. 2022; 45(Suppl 1):S83-S96. doi:10.2337/dc22-S006
6. O’Reilly EA, Cross LV, Hayes JS, et al. Impact of pharmacist intervention on glucagon prescribing patterns in an outpatient internal medicine teaching clinic. J Am Pharm Assoc (2003). 2020;60(2):384-390. doi:10.1016/j.japh.2019.04.0097.
7. Cobb EC, Watson NA, Wardian J, et al. Diabetes Center of Excellence Hypoglycemia Emergency Preparedness Project. Clin Diabetes. 2018;36(2):184-186. doi:10.2337/cd17-0040
8. Ogrinc G, Davies L, Goodman D, et al. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. doi:10.1136/bmjqs-2015-004411
9. Kam S, Angaramo S, Antoun J, et al. Improving annual albuminuria testing for individuals with diabetes. BMJ Open Qual. 2022;11(1):e001591. doi:10.1136/bmjoq-2021-001591
10. Mitchell BD, He X, Sturdy IM, et al. Glucagon prescription patterns in patients with either type 1 or 2 diabetes with newly prescribed insulin. Endocr Pract. 2016;22(2):123-135. doi:10.4158/EP15831.OR
11. Whitfield N, Gregory P, Liu B, et al. Impact of pharmacist outreach on glucagon prescribing. J Am Pharm Assoc. 2022;62(4):1384-1388.e.1. doi:10.1016/j.japh.2022.01.017
1. Weinstock RS, Aleppo G, Bailey TS, et al. The Role of Blood Glucose Monitoring in Diabetes Management. American Diabetes Association; 2020.
2. Lamounier RN, Geloneze B, Leite SO, et al. Hypoglycemia incidence and awareness among insulin-treated patients with diabetes: the HAT study in Brazil. Diabetol Metab Syndr. 2018;10:83. doi:10.1186/s13098-018-0379-5
3. Li P, Geng Z, Ladage VP, et al. Early hypoglycaemia and adherence after basal insulin initiation in a nationally representative sample of Medicare beneficiaries with type 2 diabetes. Diabetes Obes Metab. 2019;21(11):2486-2495. doi:10.1111/dom.13832
4. Haymond MW, Liu J, Bispham J, et al. Use of glucagon in patients with type 1 diabetes. Clin Diabetes. 2019;37(2):162-166. doi:10.2337/cd18-0028
5. American Diabetes Association Professional Practice Committee. 6. Glycemic targets: standards of medical care in diabetes-2022. Diabetes Care. 2022; 45(Suppl 1):S83-S96. doi:10.2337/dc22-S006
6. O’Reilly EA, Cross LV, Hayes JS, et al. Impact of pharmacist intervention on glucagon prescribing patterns in an outpatient internal medicine teaching clinic. J Am Pharm Assoc (2003). 2020;60(2):384-390. doi:10.1016/j.japh.2019.04.0097.
7. Cobb EC, Watson NA, Wardian J, et al. Diabetes Center of Excellence Hypoglycemia Emergency Preparedness Project. Clin Diabetes. 2018;36(2):184-186. doi:10.2337/cd17-0040
8. Ogrinc G, Davies L, Goodman D, et al. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. doi:10.1136/bmjqs-2015-004411
9. Kam S, Angaramo S, Antoun J, et al. Improving annual albuminuria testing for individuals with diabetes. BMJ Open Qual. 2022;11(1):e001591. doi:10.1136/bmjoq-2021-001591
10. Mitchell BD, He X, Sturdy IM, et al. Glucagon prescription patterns in patients with either type 1 or 2 diabetes with newly prescribed insulin. Endocr Pract. 2016;22(2):123-135. doi:10.4158/EP15831.OR
11. Whitfield N, Gregory P, Liu B, et al. Impact of pharmacist outreach on glucagon prescribing. J Am Pharm Assoc. 2022;62(4):1384-1388.e.1. doi:10.1016/j.japh.2022.01.017
TransCon PTH nears U.S. approval for hypoparathyroidism?
SEATTLE –
Findings from 110-week phase 2 data for the once-daily investigational parathyroid hormone (PTH) replacement drug were recently presented at the annual scientific & clinical congress of the American Association of Clinical Endocrinology.
Overall, the drug was associated with independence from conventional calcium and active vitamin D therapy in most patients at 110 weeks, with no discontinuations due to adverse effects.
“Patients with hypoparathyroidism have low serum calcium levels and struggle with quality of life and biochemical abnormalities. The data from the TransCon PTH studies seem to show that a lot of these abnormalities can be reversed,” presenter Mishaela R. Rubin, MD, said in an interview.
Other PTH replacement therapies such as Nupara (now discontinued) and teriparatide (off-label) have been used in some patients with hypoparathyroidism.
However, “[TransCon PTH] is delivered in such a way as to have a prolonged half-life, so that’s kind of a special benefit that it has,” added Dr. Rubin of the division of endocrinology and metabolic bone disease, department of medicine, Columbia University, New York.
Asked to comment, session moderator Thanh Hoang, DO, of Walter Reed National Military Medical Center, Silver Spring, Md., said: “I think it’s a very promising medication because right now we don’t have a lot of options ... I think it would help a lot of patients.”
Approval denied, company addressing concerns
On May 1, the Food and Drug Administration issued a complete response letter, signaling denial of approval for the TransCon PTH, citing concerns related to manufacturing control of the product’s drug/device combination product, but not about the product’s safety and efficacy, according to an Ascendis statement.
The company is now working with the FDA to address these issues and is awaiting a European Union decision later this year.
The FDA did not request that the company conduct further clinical trials of TransCon PTH, which now include published 26-week phase 2 and phase 3 data along with the current longer-term phase 2 data presented at AACE.
“The company has said that they’re hopeful the issues will be addressable and that the FDA did not have any concerns about safety,” Dr. Rubin said in an interview.
Calcium normalized, bone turnover improved
Dr. Rubin presented long-term efficacy and safety data from the Phase 2 PaTH Forward trial, which involved 57 of the initial 59 participants who completed week 110 of an open-label extension of the trial.
During the first 4 weeks, patients had been randomized to TransCon PTH at fixed doses of 15 µg/day, 18 µg/day, 21 µg/day, or placebo. After week 4, all patients switched to TransCon PTH titrated to doses of 6-60 µg/day along with conventional therapy, with the goal of maintaining normocalcemia.
Participants were a mean age of 50 years, 81% were women, and 92% were White. Causes of hypoparathyroidism were neck surgery in 80%, autoimmune disease in 2%, and idiopathic disease in 19%. Disease duration was 12 years (range 1-39), and all were taking conventional therapy including calcium and active vitamin D (calcitriol or alfacaldiol).
At 110 weeks, all 57 patients were able to stop taking active vitamin D, and 53 of the 57 (93%) patients achieved independence from conventional therapy, defined as taking 0 µg/day of active vitamin D and no more than 600 mg/day of calcium (the dietary supplement dose). A total of 44 (77%) patients were not taking any calcium or active vitamin D.
“This really establishes the durability up to 2 years of keeping people off conventional therapy,” Dr. Rubin said during her presentation.
There was an initial uptick to 9.4 mg/dL in mean serum calcium, as some participants were still taking active vitamin D, but that dropped to 8.9 mg/dL by week 26. Mean 24-hour urine calcium dropped from 428 mg/day at baseline to 173 mg/day by week 26. Both serum calcium and urine calcium remained in the normal range through week 110 in all patients, at 8.6 mg/dL and 167 mg/day, respectively.
“This is a really important outcome because we know that high urine calcium in these patients sets them at risk for going on to develop nephrocalcinosis, nephrolithiasis, and ultimately, chronic kidney disease,” Dr. Rubin said.
Serum levels of two bone formation markers peaked at 12 weeks after initiation of TransCon PTH. Both trended downward thereafter through week 110 to levels approximating those of age- and sex-matched controls.
“Both markers started off low, consistent with hypoparathyroidism, but with initiation of TransCon PTH we see a robust increase in bone turnover markers, almost as if the bone is ‘waking up,’ if you will. And this is consistent with calcium being mobilized from the skeleton and going into the circulation,” Dr. Rubin explained.
Bone mineral density assessed by dual-energy x-ray absorptiometry normalized, primarily in the first 26 weeks. For lumbar spine L1-L4, mean Z-scores dropped from 1.6 to 1.0 at 26 weeks and down to 0.7 by week 100. For total hip, those values were 1.0, 0.6, and 0.4, respectively. The values approached age- and sex-matched norms, Dr. Rubin noted, to “perhaps where their skeleton would be if they hadn’t had hypoparathyroidism.”
Overall 56 of the 57 (94.9%) patients reported treatment-emergent adverse events, of which 25 (42.4%) were treatment related and none were deemed serious. There were no treatment-emergent adverse events related to hypercalcemia or hypocalcemia leading to health care visits or hospitalization, none leading to discontinuation of study drug, and none to death.
“So overall, a reassuring safety profile,” Dr. Rubin said. “We look forward to presenting the next 2 years’ worth of data to the end of the open-label extension study.”
Dr. Rubin is a paid researcher for Ascendis, which funded the study. Dr. Hoang has reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
SEATTLE –
Findings from 110-week phase 2 data for the once-daily investigational parathyroid hormone (PTH) replacement drug were recently presented at the annual scientific & clinical congress of the American Association of Clinical Endocrinology.
Overall, the drug was associated with independence from conventional calcium and active vitamin D therapy in most patients at 110 weeks, with no discontinuations due to adverse effects.
“Patients with hypoparathyroidism have low serum calcium levels and struggle with quality of life and biochemical abnormalities. The data from the TransCon PTH studies seem to show that a lot of these abnormalities can be reversed,” presenter Mishaela R. Rubin, MD, said in an interview.
Other PTH replacement therapies such as Nupara (now discontinued) and teriparatide (off-label) have been used in some patients with hypoparathyroidism.
However, “[TransCon PTH] is delivered in such a way as to have a prolonged half-life, so that’s kind of a special benefit that it has,” added Dr. Rubin of the division of endocrinology and metabolic bone disease, department of medicine, Columbia University, New York.
Asked to comment, session moderator Thanh Hoang, DO, of Walter Reed National Military Medical Center, Silver Spring, Md., said: “I think it’s a very promising medication because right now we don’t have a lot of options ... I think it would help a lot of patients.”
Approval denied, company addressing concerns
On May 1, the Food and Drug Administration issued a complete response letter, signaling denial of approval for the TransCon PTH, citing concerns related to manufacturing control of the product’s drug/device combination product, but not about the product’s safety and efficacy, according to an Ascendis statement.
The company is now working with the FDA to address these issues and is awaiting a European Union decision later this year.
The FDA did not request that the company conduct further clinical trials of TransCon PTH, which now include published 26-week phase 2 and phase 3 data along with the current longer-term phase 2 data presented at AACE.
“The company has said that they’re hopeful the issues will be addressable and that the FDA did not have any concerns about safety,” Dr. Rubin said in an interview.
Calcium normalized, bone turnover improved
Dr. Rubin presented long-term efficacy and safety data from the Phase 2 PaTH Forward trial, which involved 57 of the initial 59 participants who completed week 110 of an open-label extension of the trial.
During the first 4 weeks, patients had been randomized to TransCon PTH at fixed doses of 15 µg/day, 18 µg/day, 21 µg/day, or placebo. After week 4, all patients switched to TransCon PTH titrated to doses of 6-60 µg/day along with conventional therapy, with the goal of maintaining normocalcemia.
Participants were a mean age of 50 years, 81% were women, and 92% were White. Causes of hypoparathyroidism were neck surgery in 80%, autoimmune disease in 2%, and idiopathic disease in 19%. Disease duration was 12 years (range 1-39), and all were taking conventional therapy including calcium and active vitamin D (calcitriol or alfacaldiol).
At 110 weeks, all 57 patients were able to stop taking active vitamin D, and 53 of the 57 (93%) patients achieved independence from conventional therapy, defined as taking 0 µg/day of active vitamin D and no more than 600 mg/day of calcium (the dietary supplement dose). A total of 44 (77%) patients were not taking any calcium or active vitamin D.
“This really establishes the durability up to 2 years of keeping people off conventional therapy,” Dr. Rubin said during her presentation.
There was an initial uptick to 9.4 mg/dL in mean serum calcium, as some participants were still taking active vitamin D, but that dropped to 8.9 mg/dL by week 26. Mean 24-hour urine calcium dropped from 428 mg/day at baseline to 173 mg/day by week 26. Both serum calcium and urine calcium remained in the normal range through week 110 in all patients, at 8.6 mg/dL and 167 mg/day, respectively.
“This is a really important outcome because we know that high urine calcium in these patients sets them at risk for going on to develop nephrocalcinosis, nephrolithiasis, and ultimately, chronic kidney disease,” Dr. Rubin said.
Serum levels of two bone formation markers peaked at 12 weeks after initiation of TransCon PTH. Both trended downward thereafter through week 110 to levels approximating those of age- and sex-matched controls.
“Both markers started off low, consistent with hypoparathyroidism, but with initiation of TransCon PTH we see a robust increase in bone turnover markers, almost as if the bone is ‘waking up,’ if you will. And this is consistent with calcium being mobilized from the skeleton and going into the circulation,” Dr. Rubin explained.
Bone mineral density assessed by dual-energy x-ray absorptiometry normalized, primarily in the first 26 weeks. For lumbar spine L1-L4, mean Z-scores dropped from 1.6 to 1.0 at 26 weeks and down to 0.7 by week 100. For total hip, those values were 1.0, 0.6, and 0.4, respectively. The values approached age- and sex-matched norms, Dr. Rubin noted, to “perhaps where their skeleton would be if they hadn’t had hypoparathyroidism.”
Overall 56 of the 57 (94.9%) patients reported treatment-emergent adverse events, of which 25 (42.4%) were treatment related and none were deemed serious. There were no treatment-emergent adverse events related to hypercalcemia or hypocalcemia leading to health care visits or hospitalization, none leading to discontinuation of study drug, and none to death.
“So overall, a reassuring safety profile,” Dr. Rubin said. “We look forward to presenting the next 2 years’ worth of data to the end of the open-label extension study.”
Dr. Rubin is a paid researcher for Ascendis, which funded the study. Dr. Hoang has reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
SEATTLE –
Findings from 110-week phase 2 data for the once-daily investigational parathyroid hormone (PTH) replacement drug were recently presented at the annual scientific & clinical congress of the American Association of Clinical Endocrinology.
Overall, the drug was associated with independence from conventional calcium and active vitamin D therapy in most patients at 110 weeks, with no discontinuations due to adverse effects.
“Patients with hypoparathyroidism have low serum calcium levels and struggle with quality of life and biochemical abnormalities. The data from the TransCon PTH studies seem to show that a lot of these abnormalities can be reversed,” presenter Mishaela R. Rubin, MD, said in an interview.
Other PTH replacement therapies such as Nupara (now discontinued) and teriparatide (off-label) have been used in some patients with hypoparathyroidism.
However, “[TransCon PTH] is delivered in such a way as to have a prolonged half-life, so that’s kind of a special benefit that it has,” added Dr. Rubin of the division of endocrinology and metabolic bone disease, department of medicine, Columbia University, New York.
Asked to comment, session moderator Thanh Hoang, DO, of Walter Reed National Military Medical Center, Silver Spring, Md., said: “I think it’s a very promising medication because right now we don’t have a lot of options ... I think it would help a lot of patients.”
Approval denied, company addressing concerns
On May 1, the Food and Drug Administration issued a complete response letter, signaling denial of approval for the TransCon PTH, citing concerns related to manufacturing control of the product’s drug/device combination product, but not about the product’s safety and efficacy, according to an Ascendis statement.
The company is now working with the FDA to address these issues and is awaiting a European Union decision later this year.
The FDA did not request that the company conduct further clinical trials of TransCon PTH, which now include published 26-week phase 2 and phase 3 data along with the current longer-term phase 2 data presented at AACE.
“The company has said that they’re hopeful the issues will be addressable and that the FDA did not have any concerns about safety,” Dr. Rubin said in an interview.
Calcium normalized, bone turnover improved
Dr. Rubin presented long-term efficacy and safety data from the Phase 2 PaTH Forward trial, which involved 57 of the initial 59 participants who completed week 110 of an open-label extension of the trial.
During the first 4 weeks, patients had been randomized to TransCon PTH at fixed doses of 15 µg/day, 18 µg/day, 21 µg/day, or placebo. After week 4, all patients switched to TransCon PTH titrated to doses of 6-60 µg/day along with conventional therapy, with the goal of maintaining normocalcemia.
Participants were a mean age of 50 years, 81% were women, and 92% were White. Causes of hypoparathyroidism were neck surgery in 80%, autoimmune disease in 2%, and idiopathic disease in 19%. Disease duration was 12 years (range 1-39), and all were taking conventional therapy including calcium and active vitamin D (calcitriol or alfacaldiol).
At 110 weeks, all 57 patients were able to stop taking active vitamin D, and 53 of the 57 (93%) patients achieved independence from conventional therapy, defined as taking 0 µg/day of active vitamin D and no more than 600 mg/day of calcium (the dietary supplement dose). A total of 44 (77%) patients were not taking any calcium or active vitamin D.
“This really establishes the durability up to 2 years of keeping people off conventional therapy,” Dr. Rubin said during her presentation.
There was an initial uptick to 9.4 mg/dL in mean serum calcium, as some participants were still taking active vitamin D, but that dropped to 8.9 mg/dL by week 26. Mean 24-hour urine calcium dropped from 428 mg/day at baseline to 173 mg/day by week 26. Both serum calcium and urine calcium remained in the normal range through week 110 in all patients, at 8.6 mg/dL and 167 mg/day, respectively.
“This is a really important outcome because we know that high urine calcium in these patients sets them at risk for going on to develop nephrocalcinosis, nephrolithiasis, and ultimately, chronic kidney disease,” Dr. Rubin said.
Serum levels of two bone formation markers peaked at 12 weeks after initiation of TransCon PTH. Both trended downward thereafter through week 110 to levels approximating those of age- and sex-matched controls.
“Both markers started off low, consistent with hypoparathyroidism, but with initiation of TransCon PTH we see a robust increase in bone turnover markers, almost as if the bone is ‘waking up,’ if you will. And this is consistent with calcium being mobilized from the skeleton and going into the circulation,” Dr. Rubin explained.
Bone mineral density assessed by dual-energy x-ray absorptiometry normalized, primarily in the first 26 weeks. For lumbar spine L1-L4, mean Z-scores dropped from 1.6 to 1.0 at 26 weeks and down to 0.7 by week 100. For total hip, those values were 1.0, 0.6, and 0.4, respectively. The values approached age- and sex-matched norms, Dr. Rubin noted, to “perhaps where their skeleton would be if they hadn’t had hypoparathyroidism.”
Overall 56 of the 57 (94.9%) patients reported treatment-emergent adverse events, of which 25 (42.4%) were treatment related and none were deemed serious. There were no treatment-emergent adverse events related to hypercalcemia or hypocalcemia leading to health care visits or hospitalization, none leading to discontinuation of study drug, and none to death.
“So overall, a reassuring safety profile,” Dr. Rubin said. “We look forward to presenting the next 2 years’ worth of data to the end of the open-label extension study.”
Dr. Rubin is a paid researcher for Ascendis, which funded the study. Dr. Hoang has reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
AT AACE 2023
Could vitamin D supplementation help in long COVID?
, in a retrospective, case-matched study.
The lower levels of vitamin D in patients with long COVID were most notable in those with brain fog.
These findings, by Luigi di Filippo, MD, and colleagues, were recently presented at the European Congress of Endocrinology and published in the Journal of Clinical Endocrinology & Metabolism.
“Our data suggest that vitamin D levels should be evaluated in COVID-19 patients after hospital discharge,” wrote the researchers, from San Raffaele Hospital, Milan.
“The role of vitamin D supplementation as a preventive strategy of COVID-19 sequelae should be tested in randomized controlled trials,” they urged.
The researchers also stressed that this was a controlled study in a homogeneous population, it included multiple signs and symptoms of long COVID, and it had a longer follow-up than most previous studies (6 vs. 3 months).
“The highly controlled nature of our study helps us better understand the role of vitamin D deficiency in long COVID and establish that there is likely a link between vitamin D deficiency and long COVID,” senior author Andrea Giustina, MD, said in a press release from the ECE.
“Our study shows that COVID-19 patients with low vitamin D levels are more likely to develop long COVID, but it is not yet known whether vitamin D supplements could improve the symptoms or reduce this risk altogether,” he cautioned.
“If confirmed in large, interventional, randomized controlled trials, [our data suggest] that vitamin D supplementation could represent a possible preventive strategy in reducing the burden of COVID-19 sequelae,” Dr. Giustina and colleagues wrote.
Reasonable to test vitamin D levels, consider supplementation
Invited to comment, Amiel Dror, MD, PhD, who led a related study that showed that people with a vitamin D deficiency were more likely to have severe COVID-19, agreed.
“The novelty and significance of this [new] study lie in the fact that it expands on our current understanding of the interplay between vitamin D and COVID-19, taking it beyond the acute phase of the disease,” said Dr. Dror, from Bar-Ilan University, Safed, Israel.
“It’s striking to see how vitamin D levels continue to influence patients’ health even after recovery from the initial infection,” he noted.
“The findings certainly add weight to the argument for conducting a randomized control trial [RCT],” he continued, which “would enable us to conclusively determine whether vitamin D supplementation can effectively reduce the risk or severity of long COVID.”
“In the interim,” Dr. Dror said, “given the safety profile of vitamin D and its broad health benefits, it could be reasonable to test for vitamin D levels in patients admitted with COVID-19. If levels are found to be low, supplementation could be considered.”
“However, it’s important to note that this should be done under medical supervision,” he cautioned, “and further studies are needed to establish the optimal timing and dosage of supplementation.”
“I anticipate that we’ll see more RCTs [of this] in the future,” he speculated.
Low vitamin D and risk of long COVID
Long COVID is an emerging syndrome that affects 50%-70% of COVID-19 survivors.
Low levels of vitamin D have been associated with increased likelihood of needing mechanical ventilation and worse survival in patients hospitalized with COVID-19, but the risk of long COVID associated with vitamin D has not been known.
Researchers analyzed data from adults aged 18 and older hospitalized at San Raffaele Hospital with a confirmed diagnosis of COVID-19 and discharged during the first pandemic wave, from March to May 2020, and then seen 6-months later for follow-up.
Patients were excluded if they had been admitted to the intensive care unit during hospitalization or had missing medical data or blood samples available to determine (OH) vitamin D levels, at admission and the 6-month follow-up.
Long COVID-19 was defined based on the U.K. National Institute for Health and Care Excellence guidelines as the concomitant presence of at least two or more of 17 signs and symptoms that were absent prior to the COVID-19 infection and could only be attributed to that acute disease.
Researchers identified 50 patients with long COVID at the 6-month follow-up and matched them with 50 patients without long COVID at that time point, based on age, sex, concomitant comorbidities, need for noninvasive mechanical ventilation, and week of evaluation.
Patients were a mean age of 61 years (range, 51-73) and 56% were men; 28% had been on a ventilator during hospitalization for COVID-19.
The most frequent signs and symptoms at 6 months in the patients with long COVID were asthenia (weakness, 38% of patients), dysgeusia (bad taste in the mouth, 34%), dyspnea (shortness of breath, 34%), and anosmia (loss of sense of smell, 24%).
Most symptoms were related to the cardiorespiratory system (42%), the feeling of well-being (42%), or the senses (36%), and fewer patients had symptoms related to neurocognitive impairment (headache or brain fog, 14%), or ear, nose, and throat (12%), or gastrointestinal system (4%).
Patients with long COVID had lower mean 25(OH) vitamin D levels than patients without long COVID (20.1 vs 23.2 ng/mL; P = .03). However, actual vitamin D deficiency levels were similar in both groups.
Two-thirds of patients with low vitamin D levels at hospital admission still presented with low levels at the 6-month follow-up.
Vitamin D levels were significantly lower in patients with neurocognitive symptoms at follow-up (n = 7) than in those without such symptoms (n = 93) (14.6 vs. 20.6 ng/mL; P = .042).
In patients with vitamin D deficiency (< 20 ng/mL) at admission and at follow-up (n = 42), those with long COVID (n = 22) had lower vitamin D levels at follow-up than those without long COVID (n = 20) (12.7 vs. 15.2 ng/mL; P = .041).
And in multiple regression analyses, a lower 25(OH) vitamin D level at follow-up was the only variable that was significantly associated with long COVID (odds ratio, 1.09; 95% confidence interval, 1.01-1.16; P = .008).
The findings “strongly reinforce the clinical usefulness of 25(OH) vitamin D evaluation as a possible modifiable pathophysiological factor underlying this emerging worldwide critical health issue,” the researchers concluded.
The study was supported by Abiogen Pharma. One study author is an employee at Abiogen. Dr. Giustina has reported being a consultant for Abiogen and Takeda and receiving a research grant to his institution from Takeda. Dr. Di Filippo and the other authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, in a retrospective, case-matched study.
The lower levels of vitamin D in patients with long COVID were most notable in those with brain fog.
These findings, by Luigi di Filippo, MD, and colleagues, were recently presented at the European Congress of Endocrinology and published in the Journal of Clinical Endocrinology & Metabolism.
“Our data suggest that vitamin D levels should be evaluated in COVID-19 patients after hospital discharge,” wrote the researchers, from San Raffaele Hospital, Milan.
“The role of vitamin D supplementation as a preventive strategy of COVID-19 sequelae should be tested in randomized controlled trials,” they urged.
The researchers also stressed that this was a controlled study in a homogeneous population, it included multiple signs and symptoms of long COVID, and it had a longer follow-up than most previous studies (6 vs. 3 months).
“The highly controlled nature of our study helps us better understand the role of vitamin D deficiency in long COVID and establish that there is likely a link between vitamin D deficiency and long COVID,” senior author Andrea Giustina, MD, said in a press release from the ECE.
“Our study shows that COVID-19 patients with low vitamin D levels are more likely to develop long COVID, but it is not yet known whether vitamin D supplements could improve the symptoms or reduce this risk altogether,” he cautioned.
“If confirmed in large, interventional, randomized controlled trials, [our data suggest] that vitamin D supplementation could represent a possible preventive strategy in reducing the burden of COVID-19 sequelae,” Dr. Giustina and colleagues wrote.
Reasonable to test vitamin D levels, consider supplementation
Invited to comment, Amiel Dror, MD, PhD, who led a related study that showed that people with a vitamin D deficiency were more likely to have severe COVID-19, agreed.
“The novelty and significance of this [new] study lie in the fact that it expands on our current understanding of the interplay between vitamin D and COVID-19, taking it beyond the acute phase of the disease,” said Dr. Dror, from Bar-Ilan University, Safed, Israel.
“It’s striking to see how vitamin D levels continue to influence patients’ health even after recovery from the initial infection,” he noted.
“The findings certainly add weight to the argument for conducting a randomized control trial [RCT],” he continued, which “would enable us to conclusively determine whether vitamin D supplementation can effectively reduce the risk or severity of long COVID.”
“In the interim,” Dr. Dror said, “given the safety profile of vitamin D and its broad health benefits, it could be reasonable to test for vitamin D levels in patients admitted with COVID-19. If levels are found to be low, supplementation could be considered.”
“However, it’s important to note that this should be done under medical supervision,” he cautioned, “and further studies are needed to establish the optimal timing and dosage of supplementation.”
“I anticipate that we’ll see more RCTs [of this] in the future,” he speculated.
Low vitamin D and risk of long COVID
Long COVID is an emerging syndrome that affects 50%-70% of COVID-19 survivors.
Low levels of vitamin D have been associated with increased likelihood of needing mechanical ventilation and worse survival in patients hospitalized with COVID-19, but the risk of long COVID associated with vitamin D has not been known.
Researchers analyzed data from adults aged 18 and older hospitalized at San Raffaele Hospital with a confirmed diagnosis of COVID-19 and discharged during the first pandemic wave, from March to May 2020, and then seen 6-months later for follow-up.
Patients were excluded if they had been admitted to the intensive care unit during hospitalization or had missing medical data or blood samples available to determine (OH) vitamin D levels, at admission and the 6-month follow-up.
Long COVID-19 was defined based on the U.K. National Institute for Health and Care Excellence guidelines as the concomitant presence of at least two or more of 17 signs and symptoms that were absent prior to the COVID-19 infection and could only be attributed to that acute disease.
Researchers identified 50 patients with long COVID at the 6-month follow-up and matched them with 50 patients without long COVID at that time point, based on age, sex, concomitant comorbidities, need for noninvasive mechanical ventilation, and week of evaluation.
Patients were a mean age of 61 years (range, 51-73) and 56% were men; 28% had been on a ventilator during hospitalization for COVID-19.
The most frequent signs and symptoms at 6 months in the patients with long COVID were asthenia (weakness, 38% of patients), dysgeusia (bad taste in the mouth, 34%), dyspnea (shortness of breath, 34%), and anosmia (loss of sense of smell, 24%).
Most symptoms were related to the cardiorespiratory system (42%), the feeling of well-being (42%), or the senses (36%), and fewer patients had symptoms related to neurocognitive impairment (headache or brain fog, 14%), or ear, nose, and throat (12%), or gastrointestinal system (4%).
Patients with long COVID had lower mean 25(OH) vitamin D levels than patients without long COVID (20.1 vs 23.2 ng/mL; P = .03). However, actual vitamin D deficiency levels were similar in both groups.
Two-thirds of patients with low vitamin D levels at hospital admission still presented with low levels at the 6-month follow-up.
Vitamin D levels were significantly lower in patients with neurocognitive symptoms at follow-up (n = 7) than in those without such symptoms (n = 93) (14.6 vs. 20.6 ng/mL; P = .042).
In patients with vitamin D deficiency (< 20 ng/mL) at admission and at follow-up (n = 42), those with long COVID (n = 22) had lower vitamin D levels at follow-up than those without long COVID (n = 20) (12.7 vs. 15.2 ng/mL; P = .041).
And in multiple regression analyses, a lower 25(OH) vitamin D level at follow-up was the only variable that was significantly associated with long COVID (odds ratio, 1.09; 95% confidence interval, 1.01-1.16; P = .008).
The findings “strongly reinforce the clinical usefulness of 25(OH) vitamin D evaluation as a possible modifiable pathophysiological factor underlying this emerging worldwide critical health issue,” the researchers concluded.
The study was supported by Abiogen Pharma. One study author is an employee at Abiogen. Dr. Giustina has reported being a consultant for Abiogen and Takeda and receiving a research grant to his institution from Takeda. Dr. Di Filippo and the other authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, in a retrospective, case-matched study.
The lower levels of vitamin D in patients with long COVID were most notable in those with brain fog.
These findings, by Luigi di Filippo, MD, and colleagues, were recently presented at the European Congress of Endocrinology and published in the Journal of Clinical Endocrinology & Metabolism.
“Our data suggest that vitamin D levels should be evaluated in COVID-19 patients after hospital discharge,” wrote the researchers, from San Raffaele Hospital, Milan.
“The role of vitamin D supplementation as a preventive strategy of COVID-19 sequelae should be tested in randomized controlled trials,” they urged.
The researchers also stressed that this was a controlled study in a homogeneous population, it included multiple signs and symptoms of long COVID, and it had a longer follow-up than most previous studies (6 vs. 3 months).
“The highly controlled nature of our study helps us better understand the role of vitamin D deficiency in long COVID and establish that there is likely a link between vitamin D deficiency and long COVID,” senior author Andrea Giustina, MD, said in a press release from the ECE.
“Our study shows that COVID-19 patients with low vitamin D levels are more likely to develop long COVID, but it is not yet known whether vitamin D supplements could improve the symptoms or reduce this risk altogether,” he cautioned.
“If confirmed in large, interventional, randomized controlled trials, [our data suggest] that vitamin D supplementation could represent a possible preventive strategy in reducing the burden of COVID-19 sequelae,” Dr. Giustina and colleagues wrote.
Reasonable to test vitamin D levels, consider supplementation
Invited to comment, Amiel Dror, MD, PhD, who led a related study that showed that people with a vitamin D deficiency were more likely to have severe COVID-19, agreed.
“The novelty and significance of this [new] study lie in the fact that it expands on our current understanding of the interplay between vitamin D and COVID-19, taking it beyond the acute phase of the disease,” said Dr. Dror, from Bar-Ilan University, Safed, Israel.
“It’s striking to see how vitamin D levels continue to influence patients’ health even after recovery from the initial infection,” he noted.
“The findings certainly add weight to the argument for conducting a randomized control trial [RCT],” he continued, which “would enable us to conclusively determine whether vitamin D supplementation can effectively reduce the risk or severity of long COVID.”
“In the interim,” Dr. Dror said, “given the safety profile of vitamin D and its broad health benefits, it could be reasonable to test for vitamin D levels in patients admitted with COVID-19. If levels are found to be low, supplementation could be considered.”
“However, it’s important to note that this should be done under medical supervision,” he cautioned, “and further studies are needed to establish the optimal timing and dosage of supplementation.”
“I anticipate that we’ll see more RCTs [of this] in the future,” he speculated.
Low vitamin D and risk of long COVID
Long COVID is an emerging syndrome that affects 50%-70% of COVID-19 survivors.
Low levels of vitamin D have been associated with increased likelihood of needing mechanical ventilation and worse survival in patients hospitalized with COVID-19, but the risk of long COVID associated with vitamin D has not been known.
Researchers analyzed data from adults aged 18 and older hospitalized at San Raffaele Hospital with a confirmed diagnosis of COVID-19 and discharged during the first pandemic wave, from March to May 2020, and then seen 6-months later for follow-up.
Patients were excluded if they had been admitted to the intensive care unit during hospitalization or had missing medical data or blood samples available to determine (OH) vitamin D levels, at admission and the 6-month follow-up.
Long COVID-19 was defined based on the U.K. National Institute for Health and Care Excellence guidelines as the concomitant presence of at least two or more of 17 signs and symptoms that were absent prior to the COVID-19 infection and could only be attributed to that acute disease.
Researchers identified 50 patients with long COVID at the 6-month follow-up and matched them with 50 patients without long COVID at that time point, based on age, sex, concomitant comorbidities, need for noninvasive mechanical ventilation, and week of evaluation.
Patients were a mean age of 61 years (range, 51-73) and 56% were men; 28% had been on a ventilator during hospitalization for COVID-19.
The most frequent signs and symptoms at 6 months in the patients with long COVID were asthenia (weakness, 38% of patients), dysgeusia (bad taste in the mouth, 34%), dyspnea (shortness of breath, 34%), and anosmia (loss of sense of smell, 24%).
Most symptoms were related to the cardiorespiratory system (42%), the feeling of well-being (42%), or the senses (36%), and fewer patients had symptoms related to neurocognitive impairment (headache or brain fog, 14%), or ear, nose, and throat (12%), or gastrointestinal system (4%).
Patients with long COVID had lower mean 25(OH) vitamin D levels than patients without long COVID (20.1 vs 23.2 ng/mL; P = .03). However, actual vitamin D deficiency levels were similar in both groups.
Two-thirds of patients with low vitamin D levels at hospital admission still presented with low levels at the 6-month follow-up.
Vitamin D levels were significantly lower in patients with neurocognitive symptoms at follow-up (n = 7) than in those without such symptoms (n = 93) (14.6 vs. 20.6 ng/mL; P = .042).
In patients with vitamin D deficiency (< 20 ng/mL) at admission and at follow-up (n = 42), those with long COVID (n = 22) had lower vitamin D levels at follow-up than those without long COVID (n = 20) (12.7 vs. 15.2 ng/mL; P = .041).
And in multiple regression analyses, a lower 25(OH) vitamin D level at follow-up was the only variable that was significantly associated with long COVID (odds ratio, 1.09; 95% confidence interval, 1.01-1.16; P = .008).
The findings “strongly reinforce the clinical usefulness of 25(OH) vitamin D evaluation as a possible modifiable pathophysiological factor underlying this emerging worldwide critical health issue,” the researchers concluded.
The study was supported by Abiogen Pharma. One study author is an employee at Abiogen. Dr. Giustina has reported being a consultant for Abiogen and Takeda and receiving a research grant to his institution from Takeda. Dr. Di Filippo and the other authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ECE 2023
Metabolic abnormalities boost obesity-related cancer risk
, and an even higher risk, two- to threefold higher, for specific cancers, such as endometrial, liver, and renal cell cancers, compared with metabolically healthy normal weight.
Even in people with so-called “metabolically healthy” obesity, the risk for overall obesity-related cancer is increased, compared with normal-weight, metabolically healthy individuals; however, the associations here are weaker than in people with metabolically unhealthy obesity.
“The type of metabolic obesity phenotype is important when assessing obesity-related cancer risk,” lead researcher Ming Sun, PhD, from Lund University, Malmö, Sweden, said in an interview. “In general, metabolic aberrations further increased the obesity-induced cancer risk, suggesting that obesity and metabolic aberrations are useful targets for prevention.”
“This synergy means that when obesity and metabolic unhealth occur together, that’s particularly bad,” added Tanja Stocks, PhD, senior author, also of Lund University.
“But the data also highlight that even obesity and overweight alone comprise an increased risk of cancer,” Dr. Stocks noted.
Dr. Sun said the findings have important public health implications, suggesting that “a significant number of cancer cases could potentially be prevented by targeting the coexistence of metabolic problems and obesity, in particular for obesity-related cancers among men.”
The results will be presented as a poster by Dr. Sun at the European Congress on Obesity 2023, being held in Dublin, and have been published in the Journal of the National Cancer Institute.
Metabolically unhealthy obesity worst for cancer risks
Andrew G. Renehan, PhD, FRCS, professor of cancer studies and surgery, University of Manchester, England, welcomed the new work, saying it addresses the issue with very large study numbers. “[It] nicely demonstrates that there are clear examples where metabolically unhealthy overweight and obese phenotypes have increased cancer risk relative to [metabolically] healthy overweight and obese phenotypes,” he said.
“There is a clear need for clinically based research addressing these hypotheses ... but these studies will additionally need to factor in other dimensions such as the selection of treatment for metabolic aberrations, both medical and surgical, and the consequent metabolic control resulting from these interventions,” Dr. Renehan observed.
Vibhu Chittajallu, MD, a gastroenterologist based at University Hospitals Cleveland Medical Center, said it was beneficial to see another study further validating the association of obesity with the development of obesity-associated cancers.
“This is an interesting study [because it focuses] on the role of metabolic syndrome in obesity and how it affects the risk of development of obesity-associated cancers,” he said in an interview.
“I believe that the results of this study further strengthen the need for improved management of obesity and metabolic syndrome to reduce the risk of obesity-associated cancer formation that plays a role in preventable and premature deaths in adult patients with obesity.”
Synergy between metabolic aberrations and obesity, and cancer risk
Dr. Sun and colleagues note that obesity is an established risk factor for several cancers. It is often accompanied by metabolic aberrations, which have been a commonly proposed mechanism to link obesity with cancer. During the last decade, obesity with or without metabolic aberrations – commonly termed “metabolically unhealthy” or “healthy obesity” – has been extensively investigated in the cardiovascular field; however, studies regarding cancer are limited.
According to Dr. Sun, this new study is the first to look at the synergistic effect of unhealthy metabolism and body mass index – the latter was further categorized as normal weight (BMI < 25 kg/m2), overweight (BMI < 30) and obesity (BMI ≤ 30) – and the association with cancer risk, both overall and in relation to site-specific cancers.
Data were drawn from 797,193 European individuals (in Norway, Sweden, and Austria), of whom 23,630 developed an obesity-related cancer during the follow-up period. A metabolic score comprising mid-blood pressure, plasma glucose, and triglycerides was used to provide a measure of healthy or unhealthy metabolic status. Relative risks (hazard ratios) for overall and site-specific cancers were determined. Comparisons were made with metabolically healthy people of normal weight (effectively controls).
When different metabolic scores and BMIs were combined, participants fell into six categories: metabolically unhealthy obesity (6.8% of participants); metabolically healthy obesity (3.4%), metabolically unhealthy overweight (15.4%), metabolically healthy overweight (19.8%), metabolically unhealthy normal weight (12.5%), and metabolically healthy normal weight (42.0%).
Metabolically unhealthy women with obesity had a hazard ratio of 1.43 for overall obesity-related cancers, compared with metabolically healthy women of normal weight. Of particular note were risks of two cancer types in women with metabolically unhealthy obesity: renal cancer, with an HR of 2.43, and endometrial cancer, with an HR of 3.0, compared with controls.
Even in metabolically healthy women with obesity, compared with metabolically healthy women of normal weight, there was an increased risk of endometrial cancer, with an HR of 2.36.
“If you look at individual cancers, in particular endometrial cancer, this seems to be very much driven by obesity and not so much by the metabolic factor,” remarked Dr. Stocks.
In males, compared with metabolically healthy men of normal weight, metabolically unhealthy men with obesity had an overall obesity-related cancer risk HR of 1.91. Specifically, the risk of renal cell cancer was more than doubled, with an HR of 2.59. The HR for colon cancer was 1.85, and that for rectal cancer and pancreatic cancer was similar, both having HRs of 1.32.
Again, risk was lower in metabolically healthy men with obesity, although still higher than for metabolically healthy normal-weight men.
A version of this article first appeared on Medscape.com.
, and an even higher risk, two- to threefold higher, for specific cancers, such as endometrial, liver, and renal cell cancers, compared with metabolically healthy normal weight.
Even in people with so-called “metabolically healthy” obesity, the risk for overall obesity-related cancer is increased, compared with normal-weight, metabolically healthy individuals; however, the associations here are weaker than in people with metabolically unhealthy obesity.
“The type of metabolic obesity phenotype is important when assessing obesity-related cancer risk,” lead researcher Ming Sun, PhD, from Lund University, Malmö, Sweden, said in an interview. “In general, metabolic aberrations further increased the obesity-induced cancer risk, suggesting that obesity and metabolic aberrations are useful targets for prevention.”
“This synergy means that when obesity and metabolic unhealth occur together, that’s particularly bad,” added Tanja Stocks, PhD, senior author, also of Lund University.
“But the data also highlight that even obesity and overweight alone comprise an increased risk of cancer,” Dr. Stocks noted.
Dr. Sun said the findings have important public health implications, suggesting that “a significant number of cancer cases could potentially be prevented by targeting the coexistence of metabolic problems and obesity, in particular for obesity-related cancers among men.”
The results will be presented as a poster by Dr. Sun at the European Congress on Obesity 2023, being held in Dublin, and have been published in the Journal of the National Cancer Institute.
Metabolically unhealthy obesity worst for cancer risks
Andrew G. Renehan, PhD, FRCS, professor of cancer studies and surgery, University of Manchester, England, welcomed the new work, saying it addresses the issue with very large study numbers. “[It] nicely demonstrates that there are clear examples where metabolically unhealthy overweight and obese phenotypes have increased cancer risk relative to [metabolically] healthy overweight and obese phenotypes,” he said.
“There is a clear need for clinically based research addressing these hypotheses ... but these studies will additionally need to factor in other dimensions such as the selection of treatment for metabolic aberrations, both medical and surgical, and the consequent metabolic control resulting from these interventions,” Dr. Renehan observed.
Vibhu Chittajallu, MD, a gastroenterologist based at University Hospitals Cleveland Medical Center, said it was beneficial to see another study further validating the association of obesity with the development of obesity-associated cancers.
“This is an interesting study [because it focuses] on the role of metabolic syndrome in obesity and how it affects the risk of development of obesity-associated cancers,” he said in an interview.
“I believe that the results of this study further strengthen the need for improved management of obesity and metabolic syndrome to reduce the risk of obesity-associated cancer formation that plays a role in preventable and premature deaths in adult patients with obesity.”
Synergy between metabolic aberrations and obesity, and cancer risk
Dr. Sun and colleagues note that obesity is an established risk factor for several cancers. It is often accompanied by metabolic aberrations, which have been a commonly proposed mechanism to link obesity with cancer. During the last decade, obesity with or without metabolic aberrations – commonly termed “metabolically unhealthy” or “healthy obesity” – has been extensively investigated in the cardiovascular field; however, studies regarding cancer are limited.
According to Dr. Sun, this new study is the first to look at the synergistic effect of unhealthy metabolism and body mass index – the latter was further categorized as normal weight (BMI < 25 kg/m2), overweight (BMI < 30) and obesity (BMI ≤ 30) – and the association with cancer risk, both overall and in relation to site-specific cancers.
Data were drawn from 797,193 European individuals (in Norway, Sweden, and Austria), of whom 23,630 developed an obesity-related cancer during the follow-up period. A metabolic score comprising mid-blood pressure, plasma glucose, and triglycerides was used to provide a measure of healthy or unhealthy metabolic status. Relative risks (hazard ratios) for overall and site-specific cancers were determined. Comparisons were made with metabolically healthy people of normal weight (effectively controls).
When different metabolic scores and BMIs were combined, participants fell into six categories: metabolically unhealthy obesity (6.8% of participants); metabolically healthy obesity (3.4%), metabolically unhealthy overweight (15.4%), metabolically healthy overweight (19.8%), metabolically unhealthy normal weight (12.5%), and metabolically healthy normal weight (42.0%).
Metabolically unhealthy women with obesity had a hazard ratio of 1.43 for overall obesity-related cancers, compared with metabolically healthy women of normal weight. Of particular note were risks of two cancer types in women with metabolically unhealthy obesity: renal cancer, with an HR of 2.43, and endometrial cancer, with an HR of 3.0, compared with controls.
Even in metabolically healthy women with obesity, compared with metabolically healthy women of normal weight, there was an increased risk of endometrial cancer, with an HR of 2.36.
“If you look at individual cancers, in particular endometrial cancer, this seems to be very much driven by obesity and not so much by the metabolic factor,” remarked Dr. Stocks.
In males, compared with metabolically healthy men of normal weight, metabolically unhealthy men with obesity had an overall obesity-related cancer risk HR of 1.91. Specifically, the risk of renal cell cancer was more than doubled, with an HR of 2.59. The HR for colon cancer was 1.85, and that for rectal cancer and pancreatic cancer was similar, both having HRs of 1.32.
Again, risk was lower in metabolically healthy men with obesity, although still higher than for metabolically healthy normal-weight men.
A version of this article first appeared on Medscape.com.
, and an even higher risk, two- to threefold higher, for specific cancers, such as endometrial, liver, and renal cell cancers, compared with metabolically healthy normal weight.
Even in people with so-called “metabolically healthy” obesity, the risk for overall obesity-related cancer is increased, compared with normal-weight, metabolically healthy individuals; however, the associations here are weaker than in people with metabolically unhealthy obesity.
“The type of metabolic obesity phenotype is important when assessing obesity-related cancer risk,” lead researcher Ming Sun, PhD, from Lund University, Malmö, Sweden, said in an interview. “In general, metabolic aberrations further increased the obesity-induced cancer risk, suggesting that obesity and metabolic aberrations are useful targets for prevention.”
“This synergy means that when obesity and metabolic unhealth occur together, that’s particularly bad,” added Tanja Stocks, PhD, senior author, also of Lund University.
“But the data also highlight that even obesity and overweight alone comprise an increased risk of cancer,” Dr. Stocks noted.
Dr. Sun said the findings have important public health implications, suggesting that “a significant number of cancer cases could potentially be prevented by targeting the coexistence of metabolic problems and obesity, in particular for obesity-related cancers among men.”
The results will be presented as a poster by Dr. Sun at the European Congress on Obesity 2023, being held in Dublin, and have been published in the Journal of the National Cancer Institute.
Metabolically unhealthy obesity worst for cancer risks
Andrew G. Renehan, PhD, FRCS, professor of cancer studies and surgery, University of Manchester, England, welcomed the new work, saying it addresses the issue with very large study numbers. “[It] nicely demonstrates that there are clear examples where metabolically unhealthy overweight and obese phenotypes have increased cancer risk relative to [metabolically] healthy overweight and obese phenotypes,” he said.
“There is a clear need for clinically based research addressing these hypotheses ... but these studies will additionally need to factor in other dimensions such as the selection of treatment for metabolic aberrations, both medical and surgical, and the consequent metabolic control resulting from these interventions,” Dr. Renehan observed.
Vibhu Chittajallu, MD, a gastroenterologist based at University Hospitals Cleveland Medical Center, said it was beneficial to see another study further validating the association of obesity with the development of obesity-associated cancers.
“This is an interesting study [because it focuses] on the role of metabolic syndrome in obesity and how it affects the risk of development of obesity-associated cancers,” he said in an interview.
“I believe that the results of this study further strengthen the need for improved management of obesity and metabolic syndrome to reduce the risk of obesity-associated cancer formation that plays a role in preventable and premature deaths in adult patients with obesity.”
Synergy between metabolic aberrations and obesity, and cancer risk
Dr. Sun and colleagues note that obesity is an established risk factor for several cancers. It is often accompanied by metabolic aberrations, which have been a commonly proposed mechanism to link obesity with cancer. During the last decade, obesity with or without metabolic aberrations – commonly termed “metabolically unhealthy” or “healthy obesity” – has been extensively investigated in the cardiovascular field; however, studies regarding cancer are limited.
According to Dr. Sun, this new study is the first to look at the synergistic effect of unhealthy metabolism and body mass index – the latter was further categorized as normal weight (BMI < 25 kg/m2), overweight (BMI < 30) and obesity (BMI ≤ 30) – and the association with cancer risk, both overall and in relation to site-specific cancers.
Data were drawn from 797,193 European individuals (in Norway, Sweden, and Austria), of whom 23,630 developed an obesity-related cancer during the follow-up period. A metabolic score comprising mid-blood pressure, plasma glucose, and triglycerides was used to provide a measure of healthy or unhealthy metabolic status. Relative risks (hazard ratios) for overall and site-specific cancers were determined. Comparisons were made with metabolically healthy people of normal weight (effectively controls).
When different metabolic scores and BMIs were combined, participants fell into six categories: metabolically unhealthy obesity (6.8% of participants); metabolically healthy obesity (3.4%), metabolically unhealthy overweight (15.4%), metabolically healthy overweight (19.8%), metabolically unhealthy normal weight (12.5%), and metabolically healthy normal weight (42.0%).
Metabolically unhealthy women with obesity had a hazard ratio of 1.43 for overall obesity-related cancers, compared with metabolically healthy women of normal weight. Of particular note were risks of two cancer types in women with metabolically unhealthy obesity: renal cancer, with an HR of 2.43, and endometrial cancer, with an HR of 3.0, compared with controls.
Even in metabolically healthy women with obesity, compared with metabolically healthy women of normal weight, there was an increased risk of endometrial cancer, with an HR of 2.36.
“If you look at individual cancers, in particular endometrial cancer, this seems to be very much driven by obesity and not so much by the metabolic factor,” remarked Dr. Stocks.
In males, compared with metabolically healthy men of normal weight, metabolically unhealthy men with obesity had an overall obesity-related cancer risk HR of 1.91. Specifically, the risk of renal cell cancer was more than doubled, with an HR of 2.59. The HR for colon cancer was 1.85, and that for rectal cancer and pancreatic cancer was similar, both having HRs of 1.32.
Again, risk was lower in metabolically healthy men with obesity, although still higher than for metabolically healthy normal-weight men.
A version of this article first appeared on Medscape.com.
FROM ECO 2023
Subclinical hypothyroidism: Let the evidence be your guide
Subclinical hypothyroidism (SCH) is a biochemical state in which the thyroid-stimulating hormone (TSH) is elevated while the free thyroxine (T4) level is normal. Overt hypothyroidism is not diagnosed until the free T4 level is decreased, regardless of the degree of TSH elevation.
The overall prevalence of SCH in iodine-rich areas is 4% to 10%, with a risk for progression to overt hypothyroidism of between 2% and 6% annually.1 The prevalence of SCH varies depending on the TSH reference range used.1 The normal reference range for TSH varies depending on the laboratory and/or the reference population surveyed, with the range likely widening with increasing age.
SCH is most common among women, the elderly, and White individuals.2 The discovery of SCH is often incidental, given that usually it is detected by laboratory findings alone without associated symptoms of overt hypothyroidism.3
The not-so-significant role of symptoms in subclinical hypothyroidism
Symptoms associated with overt hypothyroidism include constipation, dry skin, fatigue, slow thinking, poor memory, muscle cramps, weakness, and cold intolerance. In SCH, these symptoms are inconsistent, with around 1 in 3 patients having no symptoms
One study reported that roughly 18% of euthyroid individuals, 22% of SCH patients, and 26% of those with overt hypothyroidism reported 4 or more symptoms classically thought to be related to hypothyroidism.4 A large Danish cohort study found that hypothyroid symptoms were no more common in patients with SCH than in euthyroid individuals in the general population.5 These studies question the validity of attributing symptoms to SCH.
Adverse health associations
Observational data suggest that SCH is associated with an increased risk for dyslipidemia, coronary heart disease, heart failure, and cardiovascular mortality, particularly in those with TSH levels ≥ 10 mIU/L.6,7 Such associations were not found for most adults with TSH levels between 5 and 10 mIU/L.8 There are also potential associations of SCH with obesity, nonalcoholic fatty liver disease, and nonalcoholic steatohepatitis.9,10 Despite thyroid studies being commonly ordered as part of a mental health evaluation, SCH has not been statistically associated with depressive symptoms.11,12
Caveats with laboratory testing
There are several issues to consider when performing a laboratory assessment of thyroid function. TSH levels fluctuate considerably during the day, as TSH secretion has a circadian rhythm. TSH values are 50% higher at night and in the early morning than during the rest of the day.13 TSH values also may rise in response to current illness or stress. Due to this biologic variability, repeat testing to confirm TSH levels is recommended if an initial test result is abnormal.14
Continue to: An exact reference range...
An exact reference range for TSH is not widely agreed upon—although most laboratories regard 4.0 to 5.0 mIU/L as the high-end cutoff for normal. Also, “normal” TSH levels appear to differ by age. Accordingly, some experts have recommended an age-based reference range for TSH levels,15 although this is not implemented widely by laboratories. A TSH level of 6.0 mIU/L (or even higher) may be more appropriate for adults older than 65 years.1
Biotin supplementation has been shown to cause spurious thyroid testing results (TSH, T3, T4) depending on the type of assay used. Therefore, supplements containing biotin should be withheld for several days before assessing thyroid function.16Patients with SCH are often categorized as having TSH levels between 4.5 and 10 mIU/L (around 90% of patients) or levels ≥ 10 mIU/L.8,17 If followed for 5 years, approximately 60% of patients with SCH and TSH levels between 4 and 10 mIU/L will normalize without intervention.18 Normalization is less common in patients with a TSH level greater than 10 mIU/L.18
The risk for progression to overt hypothyroidism also appears to be higher for those with certain risk factors. These include higher baseline TSH levels, presence of thyroid peroxidase antibodies (TPOAbs), or history of neck irradiation or radioactive iodine uptake.1 Other risk factors for eventual thyroid dysfunction include female sex, older age, goiter, and high iodine intake.13
Evidence for treatment varies
Guidelines for the treatment of SCH (TABLE 18,14,19,20) are founded on the condition’s risk for progression to overt hypothyroidism and its association with health consequences such as cardiovascular disease. Guidelines of the American Thyroid Association (ATA) and European Thyroid Association (ETA), and those of the United Kingdom–based National Institute for Health and Care Excellence (NICE), prioritize treatment for individuals with a TSH level > 10 mIU/La and for those with
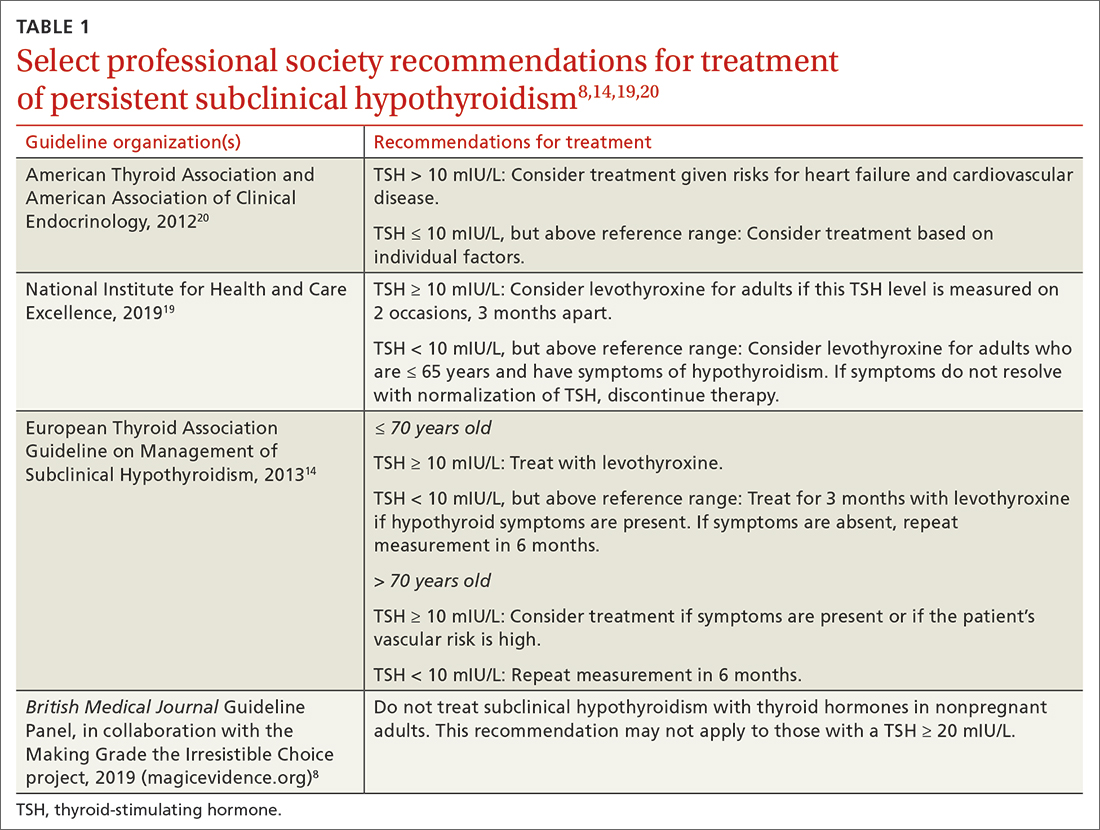
There are few large RCTs of treatment outcomes for SCH. A 2017 RCT (the Thyroid Hormone Replacement for Untreated Older Adults with Subclinical Hypothyroidism, or TRUST, trial) of 737 adults older than 65 years with SCH evaluated the ability of levothyroxine to normalize TSH values compared with placebo. At 1 year, there was no difference in hypothyroid symptoms or tiredness scale scores with levothyroxine treatment compared with placebo.21 This finding was consistent even in the subgroup with a higher baseline symptom burden.22
Continue to: Two small RCTs evaluated...
Two small RCTs evaluated treatment of SCH with depressive symptoms and cognitive function, neither finding benefit compared with placebo.12,23 A 2018 systematic review and meta-analysis of 21 studies and 2192 adults did not show a benefit to quality of life or thyroid-specific symptoms in those treated for SCH compared with controls.24
RCT support also is lacking for a reduction in cardiovascular mortality following treatment for SCH. A large population-level retrospective cohort from Denmark showed no difference in cardiovascular mortality or myocardial infarction in those treated for SCH compared with controls.25 Pooled results from 2 RCTs (for patients older than 65 years, and those older than 80 years) showed no change in risk for cardiovascular outcomes in older adults treated for SCH.26 Older adults treated for SCH in the TRUST trial showed no improvements in systolic or diastolic function on echocardiography.27 Two trials showed no difference in carotid intima-media thickness with treatment of SCH compared with placebo.28,29
While most of the RCT data come from older adults, a retrospective cohort study in the United Kingdom of younger (ages 40-70 years; n = 3093) and older (age > 70 years; n = 1642) patients showed a reduction in cardiovascular mortality among treated patients who were younger (hazard ratio [HR] = 0.61; 4.2% vs. 6.6%) but not those who were older (HR = 0.99; 12.7% vs. 10.7%).30 There is also evidence that thyroid size in those with goiter can be reduced with treatment of SCH.31
A measured approach to treating subclinical hypothyroidism
Consider several factors when deciding whether to treat SCH. For instance, RCT data suggest a lack of treatment benefit in relieving depression, improving cognition, or reducing general hypothyroid symptoms. Treatment of SCH in older adults does not appear to improve cardiovascular outcomes. The question of whether long-term treatment of SCH in younger patients reduces cardiovascular morbidity or mortality lacks answers from RCTs. Before diagnosing SCH or starting treatment, always confirm SCH with repeat testing in 2 to 3 months, as a high percentage of those with untreated SCH will have normal thyroid function on repeat testing.
In the event you and your patient elect to treat SCH, guidelines and trials generally support a low initial daily dose of 25 to 50 mcg of levothyroxine (T4), followed with dose changes every 4 to 8 weeks and a goal of normalizing TSH to within the lower half of the reference range (0.4-2.5 mIU/L).14 This is generally similar to published treatment goals for primary hypothyroidism and is based on studies suggesting the lower half of the reference range is normal for young, healthy, euthyroid individuals.32 Though full replacement doses (1.6-1.8 mcg/kg of ideal body weight) can be started for those who are elderly or who have ischemic heart disease or angina, this approach should be avoided in favor of low-dose initial therapy.33 Thyroid supplements are best absorbed when taken apart from food, calcium, or iron supplements. The ATA suggests taking thyroid medication 60 minutes before breakfast or at bedtime (3 or more hours after the evening meal).33
Continue to: Screening guidelines differ
Screening guidelines differ
Lacking population-level screening data from RCTs, most organizations do not recommend screening for thyroid dysfunction or they note insufficient evidence to make a screening recommendation (TABLE 217,19,20,34). In their most recent recommendation statement on the subject in 2015, the US Preventive Services Task Force (USPSTF) concluded the current evidence was insufficient to recommend for or against thyroid dysfunction screening in nonpregnant, asymptomatic adults.17 This differs from the ATA and the American Association of Clinical Endocrinology (AACE; formerly known as the American Association of Clinical Endocrinologists), which both recommend targeted screening for thyroid dysfunction based on symptoms or risk factors.20
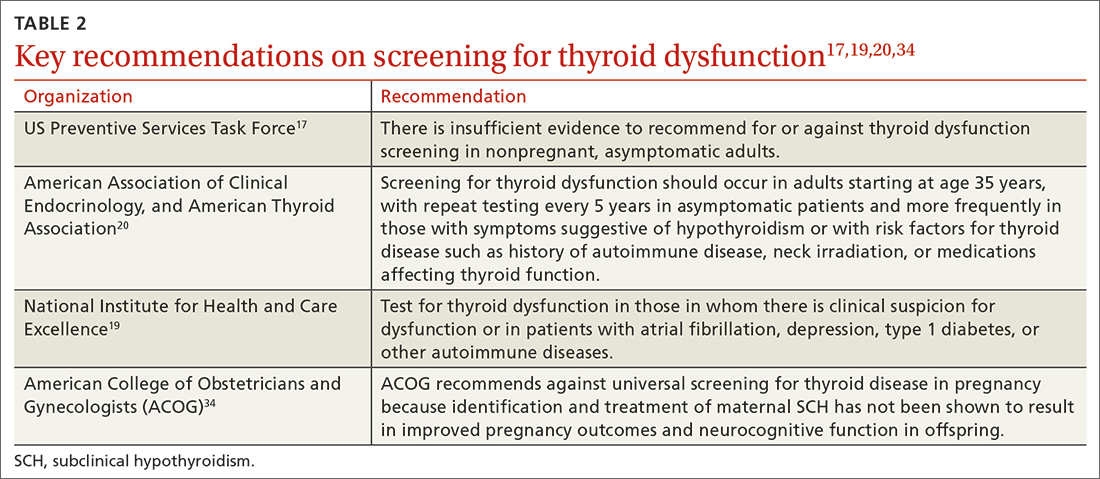
What about subclinical hypothyroidism in pregnancy?
Overt hypothyroidism is associated with adverse events during pregnancy and with subsequent neurodevelopmental complications in children, although the effects of SCH during pregnancy remain less certain. Concerns have been raised over the potential association of SCH with pregnancy loss, placental abruption, premature rupture of membranes, and neonatal death.35 Historically, the prevalence of SCH during pregnancy has ranged from 2% to 2.5%, but using lower trimester-based TSH reference ranges, the prevalence of SCH in pregnancy may be as high as 15%.35
Guided by a large RCT that failed to find benefit (pregnancy outcomes, neurodevelopmental outcomes in children) following treatment of SCH in pregnancy,36 the American College of Obstetricians and Gynecologists (ACOG) recommends against routine screening for thyroid disease in pregnancy.34 The ATA notes insufficient evidence to rec-ommend universal screening for thyroid dysfunction in pregnancy but recommends targeted screening of those with risk factors.37 Data are conflicting on the benefit of treating known or recently detected SCH on pregnancy outcomes including pregnancy loss.35,38 As such, the American Society of Reproductive Medicine and the ATA both generally recommend treatment of SCH in pregnant patients, particularly when the TSH is ≥ 4.0 mIU/L and TPOAbs are present.37,39
a The ATA, ETA, and NICE have slightly different recommendations when a TSH level = 10 mIU/L. ETA and NICE recommend prioritizing treatment for individuals with this level, while ATA recommends treatment when individual factors are also considered.
ACKNOWLEDGEMENT
The authors thank Family Medicine Medical Librarian Gwen Wilson, MLS, AHIP, for her assistance with literature searches.
CORRESPONDENCE
Nicholas LeFevre, MD, Family and Community Medicine, University of Missouri–Columbia School of Medicine, One Hospital Drive, M224 Medical Science Building, Columbia, MO 65212; [email protected]
1. Reyes Domingo F, Avey MT, Doull M. Screening for thyroid dysfunction and treatment of screen-detected thyroid dysfunction in asymptomatic, community-dwelling adults: a systematic review. Syst Rev. 2019;8:260. doi: 10.1186/s13643-019-1181-7
2. Cooper DS, Biondi B. Subclinical thyroid disease. Lancet. 2012;379:1142-1154. doi: 10.1016/S0140-6736(11)60276-6
3. Bauer BS, Azcoaga-Lorenzo A, Agrawal U, et al. Management strategies for patients with subclinical hypothyroidism: a protocol for an umbrella review. Syst Rev. 2021;10:290. doi: 10.1186/s13643-021-01842-y
4. Canaris GJ, Manowitz NR, Mayor G, et al. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160:526-534. doi: 10.1001/archinte.160.4.526
5. Carlé A, Karmisholt JS, Knudsen N, et al. Does subclinical hypothyroidism add any symptoms? Evidence from a Danish population-based study. Am J Med. 2021;134:1115-1126.e1. doi: 10.1016/j.amjmed.2021.03.009
6. Gencer B, Collet TH, Virgini V, et al. Subclinical thyroid dysfunction and the risk of heart failure events: an individual participant data analysis from 6 prospective cohorts. Circulation. 2012;126:1040-1049. doi: 10.1161/CIRCULATIONAHA.112.096024
7. Rodondi N, den Elzen WP, Bauer DC, et al. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA. 2010;304:1365-1374. doi: 10.1001/jama.2010.1361
8. Bekkering GE, Agoritsas T, Lytvyn L, et al. Thyroid hormones treatment for subclinical hypothyroidism: a clinical practice guideline. BMJ. 2019;365:l2006. doi: 10.1136/bmj.l2006
9. Chung GE, Kim D, Kim W, et al. Non-alcoholic fatty liver disease across the spectrum of hypothyroidism. J Hepatol. 2012;57:150-156. doi: 10.1016/j.jhep.2012.02.027
10. Kim D, Kim W, Joo SK, et al. Subclinical hypothyroidism and low-normal thyroid function are associated with nonalcoholic steatohepatitis and fibrosis. Clin Gastroenterol Hepatol. 2018;16:123-131.e1. doi: 10.1016/j.cgh.2017.08.014
11. Kim JS, Zhang Y, Chang Y, et al. Subclinical hypothyroidism and incident depression in young and middle-age adults. J Clin Endocrinol Metab. 2018;103:1827-1833. doi: 10.1210/jc.2017-01247
12. Jorde R, Waterloo K, Storhaug H, et al. Neuropsychological function and symptoms in subjects with subclinical hypothyroidism and the effect of thyroxine treatment. J Clin Endocrinol Metab. 2006;91:145-53. doi: 10.1210/jc.2005-1775
13. Azim S, Nasr C. Subclinical hypothyroidism: when to treat. Cleve Clin J Med. 2019;86:101-110. doi: 10.3949/ccjm.86a.17053
14. Pearce SH, Brabant G, Duntas LH, et al. 2013 ETA Guideline: Management of subclinical hypothyroidism. Eur Thyroid J. 2013;2:215-228. doi: 10.1159/000356507
15. Cappola AR. The thyrotropin reference range should be changed in older patients. JAMA. 2019;322:1961-1962. doi: 10.1001/jama.2019.14728
16. Li D, Radulescu A, Shrestha RT, et al. Association of biotin ingestion with performance of hormone and nonhormone assays in healthy adults. JAMA. 2017;318:1150-1160.
17. LeFevre ML, USPSTF. Screening for thyroid dysfunction: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;162:641-650. doi: 10.7326/M15-0483
18. Meyerovitch J, Rotman-Pikielni P, Sherf M, et al. Serum thyrotropin measurements in the community: five-year follow-up in a large network of primary care physicians. Arch Intern Med. 2007;167:1533-1538. doi: 10.1001/archinte.167.14.1533
19. NICE. Thyroid Disease: assessment and management (NICE guideline NG145). 2019. Accessed March 14, 2023. www.nice.org.uk/guidance/ng145/resources/thyroid-disease-assessment-and-management-pdf-66141781496773
20. Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid. 2012;22:1200-1235. doi: 10.1089/thy.2012.0205
21. Stott DJ, Rodondi N, Kearney PM, et al. Thyroid hormone therapy for older adults with subclinical hypothyroidism. N Engl J Med. 2017;376:2534-2544. doi: 10.1056/NEJMoa1603825
22. de Montmollin M, Feller M, Beglinger S, et al. L-thyroxine therapy for older adults with subclinical hypothyroidism and hypothyroid symptoms: secondary analysis of a randomized trial. Ann Intern Med. 2020;172:709-716. doi: 10.7326/M19-3193
23. Parle J, Roberts L, Wilson S, et al. A randomized controlled trial of the effect of thyroxine replacement on cognitive function in community-living elderly subjects with subclinical hypothyroidism: the Birmingham Elderly Thyroid study. J Clin Endocrinol Metab. 2010;95:3623-3632. doi: 10.1210/jc.2009-2571
24. Feller M, Snel M, Moutzouri E, et al. Association of thyroid hormone therapy with quality of life and thyroid-related symptoms in patients with subclinical hypothyroidism: a systematic review and meta-analysis. JAMA. 2018;320:1349-1359. doi: 10.1001/jama.2018.13770
25. Andersen MN, Schjerning Olsen A-M, Madsen JC, et al. Levothyroxine substitution in patients with subclinical hypothyroidism and the risk of myocardial infarction and mortality. PLoS One. 2015;10:e0129793. doi: 10.1371/journal.pone.0129793
26. Zijlstra LE, Jukema JW, Westendorp RG, et al. Levothyroxine treatment and cardiovascular outcomes in older people with subclinical hypothyroidism: pooled individual results of two randomised controlled trials. Front Endocrinol (Lausanne). 2021;12:674841. doi: 10.3389/fendo.2021.674841
27. Gencer B, Moutzouri E, Blum MR, et al. The impact of levothyroxine on cardiac function in older adults with mild subclinical hypothyroidism: a randomized clinical trial. Am J Med. 2020;133:848-856.e5. doi: 10.1016/j.amjmed.2020.01.018
28. Blum MR, Gencer B, Adam L, et al. Impact of thyroid hormone therapy on atherosclerosis in the elderly with subclinical hypothyroidism: a randomized trial. J Clin Endocrinol Metab. 2018;103:2988-2997. doi: 10.1210/jc.2018-00279
29. Aziz M, Kandimalla Y, Machavarapu A, et al. Effect of thyroxin treatment on carotid intima-media thickness (CIMT) reduction in patients with subclinical hypothyroidism (SCH): a meta-analysis of clinical trials. J Atheroscler Thromb. 2017;24:643-659. doi: 10.5551/jat.39917
30. Razvi S, Weaver JU, Butler TJ, et al. Levothyroxine treatment of subclinical hypothyroidism, fatal and nonfatal cardiovascular events, and mortality. Arch Intern Med. 2012;172:811-817. doi: 10.1001/archinternmed.2012.1159
31. Romaldini JH, Biancalana MM, Figueiredo DI, et al. Effect of L-thyroxine administration on antithyroid antibody levels, lipid profile, and thyroid volume in patients with Hashimoto’s thyroiditis. Thyroid. 1996;6:183-188. doi: 10.1089/thy.1996.6.183
32. Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev. 2008;29:76-131. doi: 10.1210/er.2006-0043
33. Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism: prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid. 2014;24:1670-1751. doi: 10.1089/thy.2014.0028
34. ACOG. Thyroid disease in pregnancy: ACOG practice bulletin, Number 223. Obstet Gynecol. 2020;135:e261-e274. doi: 10.1097/AOG.0000000000003893
35. Maraka S, Ospina NM, O’Keeffe ET, et al. Subclinical hypothyroidism in pregnancy: a systematic review and meta-analysis. Thyroid. 2016;26:580-590. doi: 10.1089/thy.2015.0418
36. Casey BM, Thom EA, Peaceman AM, et al. Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. N Engl J Med. 2017;376:815-825. doi: 10.1056/NEJMoa1606205
37. Alexander EK, Pearce EN, Brent FA, et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid. 2017;27:315-389. doi: 10.1089/thy.2016.0457
38. Dong AC, Morgan J, Kane M, et al. Subclinical hypothyroidism and thyroid autoimmunity in recurrent pregnancy loss: a systematic review and meta-analysis. Fertil Steril. 2020;113:587-600.e1. doi: 10.1016/j.fertnstert.2019.11.003
39. Practice Committee of the American Society for Reproductive Medicine. Subclinical hypothyroidism in the infertile female population: a guideline. Fertil Steril. 2015;104:545-553. doi: 10.1016/j.fertnstert.2015.05.028
Subclinical hypothyroidism (SCH) is a biochemical state in which the thyroid-stimulating hormone (TSH) is elevated while the free thyroxine (T4) level is normal. Overt hypothyroidism is not diagnosed until the free T4 level is decreased, regardless of the degree of TSH elevation.
The overall prevalence of SCH in iodine-rich areas is 4% to 10%, with a risk for progression to overt hypothyroidism of between 2% and 6% annually.1 The prevalence of SCH varies depending on the TSH reference range used.1 The normal reference range for TSH varies depending on the laboratory and/or the reference population surveyed, with the range likely widening with increasing age.
SCH is most common among women, the elderly, and White individuals.2 The discovery of SCH is often incidental, given that usually it is detected by laboratory findings alone without associated symptoms of overt hypothyroidism.3
The not-so-significant role of symptoms in subclinical hypothyroidism
Symptoms associated with overt hypothyroidism include constipation, dry skin, fatigue, slow thinking, poor memory, muscle cramps, weakness, and cold intolerance. In SCH, these symptoms are inconsistent, with around 1 in 3 patients having no symptoms
One study reported that roughly 18% of euthyroid individuals, 22% of SCH patients, and 26% of those with overt hypothyroidism reported 4 or more symptoms classically thought to be related to hypothyroidism.4 A large Danish cohort study found that hypothyroid symptoms were no more common in patients with SCH than in euthyroid individuals in the general population.5 These studies question the validity of attributing symptoms to SCH.
Adverse health associations
Observational data suggest that SCH is associated with an increased risk for dyslipidemia, coronary heart disease, heart failure, and cardiovascular mortality, particularly in those with TSH levels ≥ 10 mIU/L.6,7 Such associations were not found for most adults with TSH levels between 5 and 10 mIU/L.8 There are also potential associations of SCH with obesity, nonalcoholic fatty liver disease, and nonalcoholic steatohepatitis.9,10 Despite thyroid studies being commonly ordered as part of a mental health evaluation, SCH has not been statistically associated with depressive symptoms.11,12
Caveats with laboratory testing
There are several issues to consider when performing a laboratory assessment of thyroid function. TSH levels fluctuate considerably during the day, as TSH secretion has a circadian rhythm. TSH values are 50% higher at night and in the early morning than during the rest of the day.13 TSH values also may rise in response to current illness or stress. Due to this biologic variability, repeat testing to confirm TSH levels is recommended if an initial test result is abnormal.14
Continue to: An exact reference range...
An exact reference range for TSH is not widely agreed upon—although most laboratories regard 4.0 to 5.0 mIU/L as the high-end cutoff for normal. Also, “normal” TSH levels appear to differ by age. Accordingly, some experts have recommended an age-based reference range for TSH levels,15 although this is not implemented widely by laboratories. A TSH level of 6.0 mIU/L (or even higher) may be more appropriate for adults older than 65 years.1
Biotin supplementation has been shown to cause spurious thyroid testing results (TSH, T3, T4) depending on the type of assay used. Therefore, supplements containing biotin should be withheld for several days before assessing thyroid function.16Patients with SCH are often categorized as having TSH levels between 4.5 and 10 mIU/L (around 90% of patients) or levels ≥ 10 mIU/L.8,17 If followed for 5 years, approximately 60% of patients with SCH and TSH levels between 4 and 10 mIU/L will normalize without intervention.18 Normalization is less common in patients with a TSH level greater than 10 mIU/L.18
The risk for progression to overt hypothyroidism also appears to be higher for those with certain risk factors. These include higher baseline TSH levels, presence of thyroid peroxidase antibodies (TPOAbs), or history of neck irradiation or radioactive iodine uptake.1 Other risk factors for eventual thyroid dysfunction include female sex, older age, goiter, and high iodine intake.13
Evidence for treatment varies
Guidelines for the treatment of SCH (TABLE 18,14,19,20) are founded on the condition’s risk for progression to overt hypothyroidism and its association with health consequences such as cardiovascular disease. Guidelines of the American Thyroid Association (ATA) and European Thyroid Association (ETA), and those of the United Kingdom–based National Institute for Health and Care Excellence (NICE), prioritize treatment for individuals with a TSH level > 10 mIU/La and for those with

There are few large RCTs of treatment outcomes for SCH. A 2017 RCT (the Thyroid Hormone Replacement for Untreated Older Adults with Subclinical Hypothyroidism, or TRUST, trial) of 737 adults older than 65 years with SCH evaluated the ability of levothyroxine to normalize TSH values compared with placebo. At 1 year, there was no difference in hypothyroid symptoms or tiredness scale scores with levothyroxine treatment compared with placebo.21 This finding was consistent even in the subgroup with a higher baseline symptom burden.22
Continue to: Two small RCTs evaluated...
Two small RCTs evaluated treatment of SCH with depressive symptoms and cognitive function, neither finding benefit compared with placebo.12,23 A 2018 systematic review and meta-analysis of 21 studies and 2192 adults did not show a benefit to quality of life or thyroid-specific symptoms in those treated for SCH compared with controls.24
RCT support also is lacking for a reduction in cardiovascular mortality following treatment for SCH. A large population-level retrospective cohort from Denmark showed no difference in cardiovascular mortality or myocardial infarction in those treated for SCH compared with controls.25 Pooled results from 2 RCTs (for patients older than 65 years, and those older than 80 years) showed no change in risk for cardiovascular outcomes in older adults treated for SCH.26 Older adults treated for SCH in the TRUST trial showed no improvements in systolic or diastolic function on echocardiography.27 Two trials showed no difference in carotid intima-media thickness with treatment of SCH compared with placebo.28,29
While most of the RCT data come from older adults, a retrospective cohort study in the United Kingdom of younger (ages 40-70 years; n = 3093) and older (age > 70 years; n = 1642) patients showed a reduction in cardiovascular mortality among treated patients who were younger (hazard ratio [HR] = 0.61; 4.2% vs. 6.6%) but not those who were older (HR = 0.99; 12.7% vs. 10.7%).30 There is also evidence that thyroid size in those with goiter can be reduced with treatment of SCH.31
A measured approach to treating subclinical hypothyroidism
Consider several factors when deciding whether to treat SCH. For instance, RCT data suggest a lack of treatment benefit in relieving depression, improving cognition, or reducing general hypothyroid symptoms. Treatment of SCH in older adults does not appear to improve cardiovascular outcomes. The question of whether long-term treatment of SCH in younger patients reduces cardiovascular morbidity or mortality lacks answers from RCTs. Before diagnosing SCH or starting treatment, always confirm SCH with repeat testing in 2 to 3 months, as a high percentage of those with untreated SCH will have normal thyroid function on repeat testing.
In the event you and your patient elect to treat SCH, guidelines and trials generally support a low initial daily dose of 25 to 50 mcg of levothyroxine (T4), followed with dose changes every 4 to 8 weeks and a goal of normalizing TSH to within the lower half of the reference range (0.4-2.5 mIU/L).14 This is generally similar to published treatment goals for primary hypothyroidism and is based on studies suggesting the lower half of the reference range is normal for young, healthy, euthyroid individuals.32 Though full replacement doses (1.6-1.8 mcg/kg of ideal body weight) can be started for those who are elderly or who have ischemic heart disease or angina, this approach should be avoided in favor of low-dose initial therapy.33 Thyroid supplements are best absorbed when taken apart from food, calcium, or iron supplements. The ATA suggests taking thyroid medication 60 minutes before breakfast or at bedtime (3 or more hours after the evening meal).33
Continue to: Screening guidelines differ
Screening guidelines differ
Lacking population-level screening data from RCTs, most organizations do not recommend screening for thyroid dysfunction or they note insufficient evidence to make a screening recommendation (TABLE 217,19,20,34). In their most recent recommendation statement on the subject in 2015, the US Preventive Services Task Force (USPSTF) concluded the current evidence was insufficient to recommend for or against thyroid dysfunction screening in nonpregnant, asymptomatic adults.17 This differs from the ATA and the American Association of Clinical Endocrinology (AACE; formerly known as the American Association of Clinical Endocrinologists), which both recommend targeted screening for thyroid dysfunction based on symptoms or risk factors.20

What about subclinical hypothyroidism in pregnancy?
Overt hypothyroidism is associated with adverse events during pregnancy and with subsequent neurodevelopmental complications in children, although the effects of SCH during pregnancy remain less certain. Concerns have been raised over the potential association of SCH with pregnancy loss, placental abruption, premature rupture of membranes, and neonatal death.35 Historically, the prevalence of SCH during pregnancy has ranged from 2% to 2.5%, but using lower trimester-based TSH reference ranges, the prevalence of SCH in pregnancy may be as high as 15%.35
Guided by a large RCT that failed to find benefit (pregnancy outcomes, neurodevelopmental outcomes in children) following treatment of SCH in pregnancy,36 the American College of Obstetricians and Gynecologists (ACOG) recommends against routine screening for thyroid disease in pregnancy.34 The ATA notes insufficient evidence to rec-ommend universal screening for thyroid dysfunction in pregnancy but recommends targeted screening of those with risk factors.37 Data are conflicting on the benefit of treating known or recently detected SCH on pregnancy outcomes including pregnancy loss.35,38 As such, the American Society of Reproductive Medicine and the ATA both generally recommend treatment of SCH in pregnant patients, particularly when the TSH is ≥ 4.0 mIU/L and TPOAbs are present.37,39
a The ATA, ETA, and NICE have slightly different recommendations when a TSH level = 10 mIU/L. ETA and NICE recommend prioritizing treatment for individuals with this level, while ATA recommends treatment when individual factors are also considered.
ACKNOWLEDGEMENT
The authors thank Family Medicine Medical Librarian Gwen Wilson, MLS, AHIP, for her assistance with literature searches.
CORRESPONDENCE
Nicholas LeFevre, MD, Family and Community Medicine, University of Missouri–Columbia School of Medicine, One Hospital Drive, M224 Medical Science Building, Columbia, MO 65212; [email protected]
Subclinical hypothyroidism (SCH) is a biochemical state in which the thyroid-stimulating hormone (TSH) is elevated while the free thyroxine (T4) level is normal. Overt hypothyroidism is not diagnosed until the free T4 level is decreased, regardless of the degree of TSH elevation.
The overall prevalence of SCH in iodine-rich areas is 4% to 10%, with a risk for progression to overt hypothyroidism of between 2% and 6% annually.1 The prevalence of SCH varies depending on the TSH reference range used.1 The normal reference range for TSH varies depending on the laboratory and/or the reference population surveyed, with the range likely widening with increasing age.
SCH is most common among women, the elderly, and White individuals.2 The discovery of SCH is often incidental, given that usually it is detected by laboratory findings alone without associated symptoms of overt hypothyroidism.3
The not-so-significant role of symptoms in subclinical hypothyroidism
Symptoms associated with overt hypothyroidism include constipation, dry skin, fatigue, slow thinking, poor memory, muscle cramps, weakness, and cold intolerance. In SCH, these symptoms are inconsistent, with around 1 in 3 patients having no symptoms
One study reported that roughly 18% of euthyroid individuals, 22% of SCH patients, and 26% of those with overt hypothyroidism reported 4 or more symptoms classically thought to be related to hypothyroidism.4 A large Danish cohort study found that hypothyroid symptoms were no more common in patients with SCH than in euthyroid individuals in the general population.5 These studies question the validity of attributing symptoms to SCH.
Adverse health associations
Observational data suggest that SCH is associated with an increased risk for dyslipidemia, coronary heart disease, heart failure, and cardiovascular mortality, particularly in those with TSH levels ≥ 10 mIU/L.6,7 Such associations were not found for most adults with TSH levels between 5 and 10 mIU/L.8 There are also potential associations of SCH with obesity, nonalcoholic fatty liver disease, and nonalcoholic steatohepatitis.9,10 Despite thyroid studies being commonly ordered as part of a mental health evaluation, SCH has not been statistically associated with depressive symptoms.11,12
Caveats with laboratory testing
There are several issues to consider when performing a laboratory assessment of thyroid function. TSH levels fluctuate considerably during the day, as TSH secretion has a circadian rhythm. TSH values are 50% higher at night and in the early morning than during the rest of the day.13 TSH values also may rise in response to current illness or stress. Due to this biologic variability, repeat testing to confirm TSH levels is recommended if an initial test result is abnormal.14
Continue to: An exact reference range...
An exact reference range for TSH is not widely agreed upon—although most laboratories regard 4.0 to 5.0 mIU/L as the high-end cutoff for normal. Also, “normal” TSH levels appear to differ by age. Accordingly, some experts have recommended an age-based reference range for TSH levels,15 although this is not implemented widely by laboratories. A TSH level of 6.0 mIU/L (or even higher) may be more appropriate for adults older than 65 years.1
Biotin supplementation has been shown to cause spurious thyroid testing results (TSH, T3, T4) depending on the type of assay used. Therefore, supplements containing biotin should be withheld for several days before assessing thyroid function.16Patients with SCH are often categorized as having TSH levels between 4.5 and 10 mIU/L (around 90% of patients) or levels ≥ 10 mIU/L.8,17 If followed for 5 years, approximately 60% of patients with SCH and TSH levels between 4 and 10 mIU/L will normalize without intervention.18 Normalization is less common in patients with a TSH level greater than 10 mIU/L.18
The risk for progression to overt hypothyroidism also appears to be higher for those with certain risk factors. These include higher baseline TSH levels, presence of thyroid peroxidase antibodies (TPOAbs), or history of neck irradiation or radioactive iodine uptake.1 Other risk factors for eventual thyroid dysfunction include female sex, older age, goiter, and high iodine intake.13
Evidence for treatment varies
Guidelines for the treatment of SCH (TABLE 18,14,19,20) are founded on the condition’s risk for progression to overt hypothyroidism and its association with health consequences such as cardiovascular disease. Guidelines of the American Thyroid Association (ATA) and European Thyroid Association (ETA), and those of the United Kingdom–based National Institute for Health and Care Excellence (NICE), prioritize treatment for individuals with a TSH level > 10 mIU/La and for those with

There are few large RCTs of treatment outcomes for SCH. A 2017 RCT (the Thyroid Hormone Replacement for Untreated Older Adults with Subclinical Hypothyroidism, or TRUST, trial) of 737 adults older than 65 years with SCH evaluated the ability of levothyroxine to normalize TSH values compared with placebo. At 1 year, there was no difference in hypothyroid symptoms or tiredness scale scores with levothyroxine treatment compared with placebo.21 This finding was consistent even in the subgroup with a higher baseline symptom burden.22
Continue to: Two small RCTs evaluated...
Two small RCTs evaluated treatment of SCH with depressive symptoms and cognitive function, neither finding benefit compared with placebo.12,23 A 2018 systematic review and meta-analysis of 21 studies and 2192 adults did not show a benefit to quality of life or thyroid-specific symptoms in those treated for SCH compared with controls.24
RCT support also is lacking for a reduction in cardiovascular mortality following treatment for SCH. A large population-level retrospective cohort from Denmark showed no difference in cardiovascular mortality or myocardial infarction in those treated for SCH compared with controls.25 Pooled results from 2 RCTs (for patients older than 65 years, and those older than 80 years) showed no change in risk for cardiovascular outcomes in older adults treated for SCH.26 Older adults treated for SCH in the TRUST trial showed no improvements in systolic or diastolic function on echocardiography.27 Two trials showed no difference in carotid intima-media thickness with treatment of SCH compared with placebo.28,29
While most of the RCT data come from older adults, a retrospective cohort study in the United Kingdom of younger (ages 40-70 years; n = 3093) and older (age > 70 years; n = 1642) patients showed a reduction in cardiovascular mortality among treated patients who were younger (hazard ratio [HR] = 0.61; 4.2% vs. 6.6%) but not those who were older (HR = 0.99; 12.7% vs. 10.7%).30 There is also evidence that thyroid size in those with goiter can be reduced with treatment of SCH.31
A measured approach to treating subclinical hypothyroidism
Consider several factors when deciding whether to treat SCH. For instance, RCT data suggest a lack of treatment benefit in relieving depression, improving cognition, or reducing general hypothyroid symptoms. Treatment of SCH in older adults does not appear to improve cardiovascular outcomes. The question of whether long-term treatment of SCH in younger patients reduces cardiovascular morbidity or mortality lacks answers from RCTs. Before diagnosing SCH or starting treatment, always confirm SCH with repeat testing in 2 to 3 months, as a high percentage of those with untreated SCH will have normal thyroid function on repeat testing.
In the event you and your patient elect to treat SCH, guidelines and trials generally support a low initial daily dose of 25 to 50 mcg of levothyroxine (T4), followed with dose changes every 4 to 8 weeks and a goal of normalizing TSH to within the lower half of the reference range (0.4-2.5 mIU/L).14 This is generally similar to published treatment goals for primary hypothyroidism and is based on studies suggesting the lower half of the reference range is normal for young, healthy, euthyroid individuals.32 Though full replacement doses (1.6-1.8 mcg/kg of ideal body weight) can be started for those who are elderly or who have ischemic heart disease or angina, this approach should be avoided in favor of low-dose initial therapy.33 Thyroid supplements are best absorbed when taken apart from food, calcium, or iron supplements. The ATA suggests taking thyroid medication 60 minutes before breakfast or at bedtime (3 or more hours after the evening meal).33
Continue to: Screening guidelines differ
Screening guidelines differ
Lacking population-level screening data from RCTs, most organizations do not recommend screening for thyroid dysfunction or they note insufficient evidence to make a screening recommendation (TABLE 217,19,20,34). In their most recent recommendation statement on the subject in 2015, the US Preventive Services Task Force (USPSTF) concluded the current evidence was insufficient to recommend for or against thyroid dysfunction screening in nonpregnant, asymptomatic adults.17 This differs from the ATA and the American Association of Clinical Endocrinology (AACE; formerly known as the American Association of Clinical Endocrinologists), which both recommend targeted screening for thyroid dysfunction based on symptoms or risk factors.20

What about subclinical hypothyroidism in pregnancy?
Overt hypothyroidism is associated with adverse events during pregnancy and with subsequent neurodevelopmental complications in children, although the effects of SCH during pregnancy remain less certain. Concerns have been raised over the potential association of SCH with pregnancy loss, placental abruption, premature rupture of membranes, and neonatal death.35 Historically, the prevalence of SCH during pregnancy has ranged from 2% to 2.5%, but using lower trimester-based TSH reference ranges, the prevalence of SCH in pregnancy may be as high as 15%.35
Guided by a large RCT that failed to find benefit (pregnancy outcomes, neurodevelopmental outcomes in children) following treatment of SCH in pregnancy,36 the American College of Obstetricians and Gynecologists (ACOG) recommends against routine screening for thyroid disease in pregnancy.34 The ATA notes insufficient evidence to rec-ommend universal screening for thyroid dysfunction in pregnancy but recommends targeted screening of those with risk factors.37 Data are conflicting on the benefit of treating known or recently detected SCH on pregnancy outcomes including pregnancy loss.35,38 As such, the American Society of Reproductive Medicine and the ATA both generally recommend treatment of SCH in pregnant patients, particularly when the TSH is ≥ 4.0 mIU/L and TPOAbs are present.37,39
a The ATA, ETA, and NICE have slightly different recommendations when a TSH level = 10 mIU/L. ETA and NICE recommend prioritizing treatment for individuals with this level, while ATA recommends treatment when individual factors are also considered.
ACKNOWLEDGEMENT
The authors thank Family Medicine Medical Librarian Gwen Wilson, MLS, AHIP, for her assistance with literature searches.
CORRESPONDENCE
Nicholas LeFevre, MD, Family and Community Medicine, University of Missouri–Columbia School of Medicine, One Hospital Drive, M224 Medical Science Building, Columbia, MO 65212; [email protected]
1. Reyes Domingo F, Avey MT, Doull M. Screening for thyroid dysfunction and treatment of screen-detected thyroid dysfunction in asymptomatic, community-dwelling adults: a systematic review. Syst Rev. 2019;8:260. doi: 10.1186/s13643-019-1181-7
2. Cooper DS, Biondi B. Subclinical thyroid disease. Lancet. 2012;379:1142-1154. doi: 10.1016/S0140-6736(11)60276-6
3. Bauer BS, Azcoaga-Lorenzo A, Agrawal U, et al. Management strategies for patients with subclinical hypothyroidism: a protocol for an umbrella review. Syst Rev. 2021;10:290. doi: 10.1186/s13643-021-01842-y
4. Canaris GJ, Manowitz NR, Mayor G, et al. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160:526-534. doi: 10.1001/archinte.160.4.526
5. Carlé A, Karmisholt JS, Knudsen N, et al. Does subclinical hypothyroidism add any symptoms? Evidence from a Danish population-based study. Am J Med. 2021;134:1115-1126.e1. doi: 10.1016/j.amjmed.2021.03.009
6. Gencer B, Collet TH, Virgini V, et al. Subclinical thyroid dysfunction and the risk of heart failure events: an individual participant data analysis from 6 prospective cohorts. Circulation. 2012;126:1040-1049. doi: 10.1161/CIRCULATIONAHA.112.096024
7. Rodondi N, den Elzen WP, Bauer DC, et al. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA. 2010;304:1365-1374. doi: 10.1001/jama.2010.1361
8. Bekkering GE, Agoritsas T, Lytvyn L, et al. Thyroid hormones treatment for subclinical hypothyroidism: a clinical practice guideline. BMJ. 2019;365:l2006. doi: 10.1136/bmj.l2006
9. Chung GE, Kim D, Kim W, et al. Non-alcoholic fatty liver disease across the spectrum of hypothyroidism. J Hepatol. 2012;57:150-156. doi: 10.1016/j.jhep.2012.02.027
10. Kim D, Kim W, Joo SK, et al. Subclinical hypothyroidism and low-normal thyroid function are associated with nonalcoholic steatohepatitis and fibrosis. Clin Gastroenterol Hepatol. 2018;16:123-131.e1. doi: 10.1016/j.cgh.2017.08.014
11. Kim JS, Zhang Y, Chang Y, et al. Subclinical hypothyroidism and incident depression in young and middle-age adults. J Clin Endocrinol Metab. 2018;103:1827-1833. doi: 10.1210/jc.2017-01247
12. Jorde R, Waterloo K, Storhaug H, et al. Neuropsychological function and symptoms in subjects with subclinical hypothyroidism and the effect of thyroxine treatment. J Clin Endocrinol Metab. 2006;91:145-53. doi: 10.1210/jc.2005-1775
13. Azim S, Nasr C. Subclinical hypothyroidism: when to treat. Cleve Clin J Med. 2019;86:101-110. doi: 10.3949/ccjm.86a.17053
14. Pearce SH, Brabant G, Duntas LH, et al. 2013 ETA Guideline: Management of subclinical hypothyroidism. Eur Thyroid J. 2013;2:215-228. doi: 10.1159/000356507
15. Cappola AR. The thyrotropin reference range should be changed in older patients. JAMA. 2019;322:1961-1962. doi: 10.1001/jama.2019.14728
16. Li D, Radulescu A, Shrestha RT, et al. Association of biotin ingestion with performance of hormone and nonhormone assays in healthy adults. JAMA. 2017;318:1150-1160.
17. LeFevre ML, USPSTF. Screening for thyroid dysfunction: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;162:641-650. doi: 10.7326/M15-0483
18. Meyerovitch J, Rotman-Pikielni P, Sherf M, et al. Serum thyrotropin measurements in the community: five-year follow-up in a large network of primary care physicians. Arch Intern Med. 2007;167:1533-1538. doi: 10.1001/archinte.167.14.1533
19. NICE. Thyroid Disease: assessment and management (NICE guideline NG145). 2019. Accessed March 14, 2023. www.nice.org.uk/guidance/ng145/resources/thyroid-disease-assessment-and-management-pdf-66141781496773
20. Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid. 2012;22:1200-1235. doi: 10.1089/thy.2012.0205
21. Stott DJ, Rodondi N, Kearney PM, et al. Thyroid hormone therapy for older adults with subclinical hypothyroidism. N Engl J Med. 2017;376:2534-2544. doi: 10.1056/NEJMoa1603825
22. de Montmollin M, Feller M, Beglinger S, et al. L-thyroxine therapy for older adults with subclinical hypothyroidism and hypothyroid symptoms: secondary analysis of a randomized trial. Ann Intern Med. 2020;172:709-716. doi: 10.7326/M19-3193
23. Parle J, Roberts L, Wilson S, et al. A randomized controlled trial of the effect of thyroxine replacement on cognitive function in community-living elderly subjects with subclinical hypothyroidism: the Birmingham Elderly Thyroid study. J Clin Endocrinol Metab. 2010;95:3623-3632. doi: 10.1210/jc.2009-2571
24. Feller M, Snel M, Moutzouri E, et al. Association of thyroid hormone therapy with quality of life and thyroid-related symptoms in patients with subclinical hypothyroidism: a systematic review and meta-analysis. JAMA. 2018;320:1349-1359. doi: 10.1001/jama.2018.13770
25. Andersen MN, Schjerning Olsen A-M, Madsen JC, et al. Levothyroxine substitution in patients with subclinical hypothyroidism and the risk of myocardial infarction and mortality. PLoS One. 2015;10:e0129793. doi: 10.1371/journal.pone.0129793
26. Zijlstra LE, Jukema JW, Westendorp RG, et al. Levothyroxine treatment and cardiovascular outcomes in older people with subclinical hypothyroidism: pooled individual results of two randomised controlled trials. Front Endocrinol (Lausanne). 2021;12:674841. doi: 10.3389/fendo.2021.674841
27. Gencer B, Moutzouri E, Blum MR, et al. The impact of levothyroxine on cardiac function in older adults with mild subclinical hypothyroidism: a randomized clinical trial. Am J Med. 2020;133:848-856.e5. doi: 10.1016/j.amjmed.2020.01.018
28. Blum MR, Gencer B, Adam L, et al. Impact of thyroid hormone therapy on atherosclerosis in the elderly with subclinical hypothyroidism: a randomized trial. J Clin Endocrinol Metab. 2018;103:2988-2997. doi: 10.1210/jc.2018-00279
29. Aziz M, Kandimalla Y, Machavarapu A, et al. Effect of thyroxin treatment on carotid intima-media thickness (CIMT) reduction in patients with subclinical hypothyroidism (SCH): a meta-analysis of clinical trials. J Atheroscler Thromb. 2017;24:643-659. doi: 10.5551/jat.39917
30. Razvi S, Weaver JU, Butler TJ, et al. Levothyroxine treatment of subclinical hypothyroidism, fatal and nonfatal cardiovascular events, and mortality. Arch Intern Med. 2012;172:811-817. doi: 10.1001/archinternmed.2012.1159
31. Romaldini JH, Biancalana MM, Figueiredo DI, et al. Effect of L-thyroxine administration on antithyroid antibody levels, lipid profile, and thyroid volume in patients with Hashimoto’s thyroiditis. Thyroid. 1996;6:183-188. doi: 10.1089/thy.1996.6.183
32. Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev. 2008;29:76-131. doi: 10.1210/er.2006-0043
33. Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism: prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid. 2014;24:1670-1751. doi: 10.1089/thy.2014.0028
34. ACOG. Thyroid disease in pregnancy: ACOG practice bulletin, Number 223. Obstet Gynecol. 2020;135:e261-e274. doi: 10.1097/AOG.0000000000003893
35. Maraka S, Ospina NM, O’Keeffe ET, et al. Subclinical hypothyroidism in pregnancy: a systematic review and meta-analysis. Thyroid. 2016;26:580-590. doi: 10.1089/thy.2015.0418
36. Casey BM, Thom EA, Peaceman AM, et al. Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. N Engl J Med. 2017;376:815-825. doi: 10.1056/NEJMoa1606205
37. Alexander EK, Pearce EN, Brent FA, et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid. 2017;27:315-389. doi: 10.1089/thy.2016.0457
38. Dong AC, Morgan J, Kane M, et al. Subclinical hypothyroidism and thyroid autoimmunity in recurrent pregnancy loss: a systematic review and meta-analysis. Fertil Steril. 2020;113:587-600.e1. doi: 10.1016/j.fertnstert.2019.11.003
39. Practice Committee of the American Society for Reproductive Medicine. Subclinical hypothyroidism in the infertile female population: a guideline. Fertil Steril. 2015;104:545-553. doi: 10.1016/j.fertnstert.2015.05.028
1. Reyes Domingo F, Avey MT, Doull M. Screening for thyroid dysfunction and treatment of screen-detected thyroid dysfunction in asymptomatic, community-dwelling adults: a systematic review. Syst Rev. 2019;8:260. doi: 10.1186/s13643-019-1181-7
2. Cooper DS, Biondi B. Subclinical thyroid disease. Lancet. 2012;379:1142-1154. doi: 10.1016/S0140-6736(11)60276-6
3. Bauer BS, Azcoaga-Lorenzo A, Agrawal U, et al. Management strategies for patients with subclinical hypothyroidism: a protocol for an umbrella review. Syst Rev. 2021;10:290. doi: 10.1186/s13643-021-01842-y
4. Canaris GJ, Manowitz NR, Mayor G, et al. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160:526-534. doi: 10.1001/archinte.160.4.526
5. Carlé A, Karmisholt JS, Knudsen N, et al. Does subclinical hypothyroidism add any symptoms? Evidence from a Danish population-based study. Am J Med. 2021;134:1115-1126.e1. doi: 10.1016/j.amjmed.2021.03.009
6. Gencer B, Collet TH, Virgini V, et al. Subclinical thyroid dysfunction and the risk of heart failure events: an individual participant data analysis from 6 prospective cohorts. Circulation. 2012;126:1040-1049. doi: 10.1161/CIRCULATIONAHA.112.096024
7. Rodondi N, den Elzen WP, Bauer DC, et al. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA. 2010;304:1365-1374. doi: 10.1001/jama.2010.1361
8. Bekkering GE, Agoritsas T, Lytvyn L, et al. Thyroid hormones treatment for subclinical hypothyroidism: a clinical practice guideline. BMJ. 2019;365:l2006. doi: 10.1136/bmj.l2006
9. Chung GE, Kim D, Kim W, et al. Non-alcoholic fatty liver disease across the spectrum of hypothyroidism. J Hepatol. 2012;57:150-156. doi: 10.1016/j.jhep.2012.02.027
10. Kim D, Kim W, Joo SK, et al. Subclinical hypothyroidism and low-normal thyroid function are associated with nonalcoholic steatohepatitis and fibrosis. Clin Gastroenterol Hepatol. 2018;16:123-131.e1. doi: 10.1016/j.cgh.2017.08.014
11. Kim JS, Zhang Y, Chang Y, et al. Subclinical hypothyroidism and incident depression in young and middle-age adults. J Clin Endocrinol Metab. 2018;103:1827-1833. doi: 10.1210/jc.2017-01247
12. Jorde R, Waterloo K, Storhaug H, et al. Neuropsychological function and symptoms in subjects with subclinical hypothyroidism and the effect of thyroxine treatment. J Clin Endocrinol Metab. 2006;91:145-53. doi: 10.1210/jc.2005-1775
13. Azim S, Nasr C. Subclinical hypothyroidism: when to treat. Cleve Clin J Med. 2019;86:101-110. doi: 10.3949/ccjm.86a.17053
14. Pearce SH, Brabant G, Duntas LH, et al. 2013 ETA Guideline: Management of subclinical hypothyroidism. Eur Thyroid J. 2013;2:215-228. doi: 10.1159/000356507
15. Cappola AR. The thyrotropin reference range should be changed in older patients. JAMA. 2019;322:1961-1962. doi: 10.1001/jama.2019.14728
16. Li D, Radulescu A, Shrestha RT, et al. Association of biotin ingestion with performance of hormone and nonhormone assays in healthy adults. JAMA. 2017;318:1150-1160.
17. LeFevre ML, USPSTF. Screening for thyroid dysfunction: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;162:641-650. doi: 10.7326/M15-0483
18. Meyerovitch J, Rotman-Pikielni P, Sherf M, et al. Serum thyrotropin measurements in the community: five-year follow-up in a large network of primary care physicians. Arch Intern Med. 2007;167:1533-1538. doi: 10.1001/archinte.167.14.1533
19. NICE. Thyroid Disease: assessment and management (NICE guideline NG145). 2019. Accessed March 14, 2023. www.nice.org.uk/guidance/ng145/resources/thyroid-disease-assessment-and-management-pdf-66141781496773
20. Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid. 2012;22:1200-1235. doi: 10.1089/thy.2012.0205
21. Stott DJ, Rodondi N, Kearney PM, et al. Thyroid hormone therapy for older adults with subclinical hypothyroidism. N Engl J Med. 2017;376:2534-2544. doi: 10.1056/NEJMoa1603825
22. de Montmollin M, Feller M, Beglinger S, et al. L-thyroxine therapy for older adults with subclinical hypothyroidism and hypothyroid symptoms: secondary analysis of a randomized trial. Ann Intern Med. 2020;172:709-716. doi: 10.7326/M19-3193
23. Parle J, Roberts L, Wilson S, et al. A randomized controlled trial of the effect of thyroxine replacement on cognitive function in community-living elderly subjects with subclinical hypothyroidism: the Birmingham Elderly Thyroid study. J Clin Endocrinol Metab. 2010;95:3623-3632. doi: 10.1210/jc.2009-2571
24. Feller M, Snel M, Moutzouri E, et al. Association of thyroid hormone therapy with quality of life and thyroid-related symptoms in patients with subclinical hypothyroidism: a systematic review and meta-analysis. JAMA. 2018;320:1349-1359. doi: 10.1001/jama.2018.13770
25. Andersen MN, Schjerning Olsen A-M, Madsen JC, et al. Levothyroxine substitution in patients with subclinical hypothyroidism and the risk of myocardial infarction and mortality. PLoS One. 2015;10:e0129793. doi: 10.1371/journal.pone.0129793
26. Zijlstra LE, Jukema JW, Westendorp RG, et al. Levothyroxine treatment and cardiovascular outcomes in older people with subclinical hypothyroidism: pooled individual results of two randomised controlled trials. Front Endocrinol (Lausanne). 2021;12:674841. doi: 10.3389/fendo.2021.674841
27. Gencer B, Moutzouri E, Blum MR, et al. The impact of levothyroxine on cardiac function in older adults with mild subclinical hypothyroidism: a randomized clinical trial. Am J Med. 2020;133:848-856.e5. doi: 10.1016/j.amjmed.2020.01.018
28. Blum MR, Gencer B, Adam L, et al. Impact of thyroid hormone therapy on atherosclerosis in the elderly with subclinical hypothyroidism: a randomized trial. J Clin Endocrinol Metab. 2018;103:2988-2997. doi: 10.1210/jc.2018-00279
29. Aziz M, Kandimalla Y, Machavarapu A, et al. Effect of thyroxin treatment on carotid intima-media thickness (CIMT) reduction in patients with subclinical hypothyroidism (SCH): a meta-analysis of clinical trials. J Atheroscler Thromb. 2017;24:643-659. doi: 10.5551/jat.39917
30. Razvi S, Weaver JU, Butler TJ, et al. Levothyroxine treatment of subclinical hypothyroidism, fatal and nonfatal cardiovascular events, and mortality. Arch Intern Med. 2012;172:811-817. doi: 10.1001/archinternmed.2012.1159
31. Romaldini JH, Biancalana MM, Figueiredo DI, et al. Effect of L-thyroxine administration on antithyroid antibody levels, lipid profile, and thyroid volume in patients with Hashimoto’s thyroiditis. Thyroid. 1996;6:183-188. doi: 10.1089/thy.1996.6.183
32. Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev. 2008;29:76-131. doi: 10.1210/er.2006-0043
33. Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism: prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid. 2014;24:1670-1751. doi: 10.1089/thy.2014.0028
34. ACOG. Thyroid disease in pregnancy: ACOG practice bulletin, Number 223. Obstet Gynecol. 2020;135:e261-e274. doi: 10.1097/AOG.0000000000003893
35. Maraka S, Ospina NM, O’Keeffe ET, et al. Subclinical hypothyroidism in pregnancy: a systematic review and meta-analysis. Thyroid. 2016;26:580-590. doi: 10.1089/thy.2015.0418
36. Casey BM, Thom EA, Peaceman AM, et al. Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. N Engl J Med. 2017;376:815-825. doi: 10.1056/NEJMoa1606205
37. Alexander EK, Pearce EN, Brent FA, et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid. 2017;27:315-389. doi: 10.1089/thy.2016.0457
38. Dong AC, Morgan J, Kane M, et al. Subclinical hypothyroidism and thyroid autoimmunity in recurrent pregnancy loss: a systematic review and meta-analysis. Fertil Steril. 2020;113:587-600.e1. doi: 10.1016/j.fertnstert.2019.11.003
39. Practice Committee of the American Society for Reproductive Medicine. Subclinical hypothyroidism in the infertile female population: a guideline. Fertil Steril. 2015;104:545-553. doi: 10.1016/j.fertnstert.2015.05.028
PRACTICE RECOMMENDATIONS
› Do not routinely screen for subclinical or overt hypothyroidism in asymptomatic nonpregnant adults. B
› Consider treatment of known or screening-detected subclinical hypothyroidism (SCH) in patients who are pregnant or trying to conceive. C
› Consider treating SCH in younger adults whose thyroidstimulating hormone level is ≥ 10 mIU/L. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Noninvasive skin test may aid in Cushing diagnosis
SEATTLE – new research suggests.
Tissue accumulation of AGEs – harmful compounds formed by glycation of macromolecules – has been implicated in aging, diabetes, and cardiovascular disease. Now, in a new single-center prospective study, a group of 208 patients with endogenous hypercortisolism was found to have significantly higher median tissue AGE levels than 103 reference subjects without hypercortisolism.
The findings were presented at the annual meeting of the American Association of Clinical Endocrinology by Rashi Sandooja, MD, an endocrinology fellow at the Mayo Clinic, Rochester, Minn.
“Diagnosis of endogenous hypercortisolism can be quite challenging. Often patients can have nonspecific symptoms with biochemical testing being equivocal. In these situations, new biomarkers of hypercortisolism such as AGE measurement could potentially be useful,” Dr. Sandooja said in an interview.
“After proper validation, it could help clinicians in cases which may not be straightforward and could serve as an additional” instrument in the toolkit to reach a conclusive diagnosis, she added.
Asked to comment, session moderator Anupam Kotwal, MD, said in an interview: “I think it’s very exciting data. ... I envision its use in mild autonomous cortisol secretion, where there are not a lot of overt Cushing features but they may have a small adrenal mass. ... It might be used to guide care when there’s not a clear-cut answer.”
However, he cautioned that more validation is needed to determine the correlates of AGEs by different etiologies and magnitudes of cortisol excess.
Moreover, “skin can become thin in hypercortisolism, so is [the reader device] just detecting it more with skin testing? I think a blood test for validation would be a very good next step,” added Dr. Kotwal, who is an assistant professor in the division of diabetes, endocrinology and metabolism at the University of Nebraska, Omaha.
More work will be needed
Future directions for research should include adding a longitudinal arm and looking at the impact on AGE after patients undergo curative surgery and achieve remission, Dr. Sandooja explained.
“It will be interesting to see if AGE levels continue to be persistently high or decrease after patients achieve sustained remission of hypercortisolism. We are also interested in whether AGE measurement at baseline, prior to surgery may be associated with glucocorticoid withdrawal, myopathy, and metabolic outcomes following the surgery.”
Dr. Kotwal observed: “If the answer is clear for Cushing disease, I don’t know what extra information this would give. Maybe they would monitor people more closely afterward. It would be useful to see, but I think the first low-hanging fruit is use it in a way to guide the care of patients where we’re unclear as to whether initial treatment of this [mild autonomous cortisol secretion] is going to improve their outcomes.”
But, he added, “keeping in mind issues of skin ... we don’t want to distract clinicians and patients from using the tried and tested methods of characterizing Cushing syndrome. I’m always hesitant to bring something into practice before there is a little more information on how it can be used.”
Dr. Sandooja and Dr. Kotwal reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SEATTLE – new research suggests.
Tissue accumulation of AGEs – harmful compounds formed by glycation of macromolecules – has been implicated in aging, diabetes, and cardiovascular disease. Now, in a new single-center prospective study, a group of 208 patients with endogenous hypercortisolism was found to have significantly higher median tissue AGE levels than 103 reference subjects without hypercortisolism.
The findings were presented at the annual meeting of the American Association of Clinical Endocrinology by Rashi Sandooja, MD, an endocrinology fellow at the Mayo Clinic, Rochester, Minn.
“Diagnosis of endogenous hypercortisolism can be quite challenging. Often patients can have nonspecific symptoms with biochemical testing being equivocal. In these situations, new biomarkers of hypercortisolism such as AGE measurement could potentially be useful,” Dr. Sandooja said in an interview.
“After proper validation, it could help clinicians in cases which may not be straightforward and could serve as an additional” instrument in the toolkit to reach a conclusive diagnosis, she added.
Asked to comment, session moderator Anupam Kotwal, MD, said in an interview: “I think it’s very exciting data. ... I envision its use in mild autonomous cortisol secretion, where there are not a lot of overt Cushing features but they may have a small adrenal mass. ... It might be used to guide care when there’s not a clear-cut answer.”
However, he cautioned that more validation is needed to determine the correlates of AGEs by different etiologies and magnitudes of cortisol excess.
Moreover, “skin can become thin in hypercortisolism, so is [the reader device] just detecting it more with skin testing? I think a blood test for validation would be a very good next step,” added Dr. Kotwal, who is an assistant professor in the division of diabetes, endocrinology and metabolism at the University of Nebraska, Omaha.
More work will be needed
Future directions for research should include adding a longitudinal arm and looking at the impact on AGE after patients undergo curative surgery and achieve remission, Dr. Sandooja explained.
“It will be interesting to see if AGE levels continue to be persistently high or decrease after patients achieve sustained remission of hypercortisolism. We are also interested in whether AGE measurement at baseline, prior to surgery may be associated with glucocorticoid withdrawal, myopathy, and metabolic outcomes following the surgery.”
Dr. Kotwal observed: “If the answer is clear for Cushing disease, I don’t know what extra information this would give. Maybe they would monitor people more closely afterward. It would be useful to see, but I think the first low-hanging fruit is use it in a way to guide the care of patients where we’re unclear as to whether initial treatment of this [mild autonomous cortisol secretion] is going to improve their outcomes.”
But, he added, “keeping in mind issues of skin ... we don’t want to distract clinicians and patients from using the tried and tested methods of characterizing Cushing syndrome. I’m always hesitant to bring something into practice before there is a little more information on how it can be used.”
Dr. Sandooja and Dr. Kotwal reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SEATTLE – new research suggests.
Tissue accumulation of AGEs – harmful compounds formed by glycation of macromolecules – has been implicated in aging, diabetes, and cardiovascular disease. Now, in a new single-center prospective study, a group of 208 patients with endogenous hypercortisolism was found to have significantly higher median tissue AGE levels than 103 reference subjects without hypercortisolism.
The findings were presented at the annual meeting of the American Association of Clinical Endocrinology by Rashi Sandooja, MD, an endocrinology fellow at the Mayo Clinic, Rochester, Minn.
“Diagnosis of endogenous hypercortisolism can be quite challenging. Often patients can have nonspecific symptoms with biochemical testing being equivocal. In these situations, new biomarkers of hypercortisolism such as AGE measurement could potentially be useful,” Dr. Sandooja said in an interview.
“After proper validation, it could help clinicians in cases which may not be straightforward and could serve as an additional” instrument in the toolkit to reach a conclusive diagnosis, she added.
Asked to comment, session moderator Anupam Kotwal, MD, said in an interview: “I think it’s very exciting data. ... I envision its use in mild autonomous cortisol secretion, where there are not a lot of overt Cushing features but they may have a small adrenal mass. ... It might be used to guide care when there’s not a clear-cut answer.”
However, he cautioned that more validation is needed to determine the correlates of AGEs by different etiologies and magnitudes of cortisol excess.
Moreover, “skin can become thin in hypercortisolism, so is [the reader device] just detecting it more with skin testing? I think a blood test for validation would be a very good next step,” added Dr. Kotwal, who is an assistant professor in the division of diabetes, endocrinology and metabolism at the University of Nebraska, Omaha.
More work will be needed
Future directions for research should include adding a longitudinal arm and looking at the impact on AGE after patients undergo curative surgery and achieve remission, Dr. Sandooja explained.
“It will be interesting to see if AGE levels continue to be persistently high or decrease after patients achieve sustained remission of hypercortisolism. We are also interested in whether AGE measurement at baseline, prior to surgery may be associated with glucocorticoid withdrawal, myopathy, and metabolic outcomes following the surgery.”
Dr. Kotwal observed: “If the answer is clear for Cushing disease, I don’t know what extra information this would give. Maybe they would monitor people more closely afterward. It would be useful to see, but I think the first low-hanging fruit is use it in a way to guide the care of patients where we’re unclear as to whether initial treatment of this [mild autonomous cortisol secretion] is going to improve their outcomes.”
But, he added, “keeping in mind issues of skin ... we don’t want to distract clinicians and patients from using the tried and tested methods of characterizing Cushing syndrome. I’m always hesitant to bring something into practice before there is a little more information on how it can be used.”
Dr. Sandooja and Dr. Kotwal reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT AACE 2023
Statins appear to guard against liver disease progression
The Swedish population-based study found that adults with noncirrhotic CLD who were on statin therapy had a statistically significant 40% lower risk of developing severe liver disease, compared with matched patients who were not on statin therapy.
The statin users were also less apt to progress to cirrhosis or hepatocellular carcinoma (HCC) and to die of liver disease, Rajani Sharma, MD, MSc, division of digestive and liver diseases, Columbia University Irving Medical Center, New York, and colleagues reported.
Their study was published online in Clinical Gastroenterology and Hepatology.
More than just cholesterol lowering
The study “continues the theme that cholesterol-lowering statins are good for a lot more things than just lowering cholesterol,” William Carey, MD, who wasn’t involved with the study, said in an interview.
The results are “very consistent with other trials that show that people with liver disease on statins do better in many respects than those who are not on statins,” said Dr. Carey, acting head of the hepatology section, department of gastroenterology, hepatology, and nutrition, Cleveland Clinic.
“The effects are not trivial,” Dr. Carey added. “It’s a very significant advantage in terms of fibrosis progression and survival.”
Statins have been shown to inhibit inflammatory pathways, promote endothelial cell function, and reduce hepatic stellate cell activity, suggesting that statins could lessen the progression of liver fibrosis, Dr. Sharma and coauthors wrote.
A few prior studies have looked at the effects of statins in noncirrhotic CLD specifically, but most only included patients with viral hepatitis, and the identification of precirrhotic liver disease was largely based on fibrosis scores or ICD coding, leading to a risk for misclassification and heterogeneity in results, they wrote.
Using histopathology data in a nationwide Swedish cohort, Dr. Sharma and colleagues identified 3862 adults with noncirrhotic CLD who were statin users and a like number of propensity score–matched nonstatin users with noncirrhotic CLD. The adults with CLD included in the study were required to have a liver biopsy showing fibrosis or inflammation between the years 1969 and 2017 and at least one ICD code for CLD.
In both groups, 45% of patients had nonalcoholic fatty liver disease (NAFLD), 22% had alcohol-related liver disease (ALD), 18% had viral hepatitis, and 15% had autoimmune hepatitis (AIH).
The analysis found 234 (6.1%) statin users developed severe liver disease versus 276 (7.1%) nonusers, with incidence rates of 10.5 versus 18.1 per 1,000 person-years, respectively.
Statin use was associated with a statistically significant 40% lower rate of severe liver disease (hazard ratio, 0.60; 95% confidence interval, 0.48-0.74).
This was the case in ALD (HR, 0.30; 95% CI, 0.19-0.49) and NAFLD (HR, 0.68; 95% CI 0.45-1.00), but the results were not statistically significant for individuals with viral hepatitis (HR, 0.76; 95% CI, 0.51-1.14) or AIH (HR, 0.88; 0.48-1.58).
Statin use had a protective association in both prefibrosis and fibrosis stages at diagnosis, the researchers reported.
Statin use was also associated with lower rates of progression to cirrhosis (HR, 0.62; 95% CI, 0.49-0.78), HCC (HR, 0.44; 95% CI, 0.27-0.71) and liver-related death or liver transplant (HR, 0.55; 95% CI, 0.36-0.82).
The authors noted that their “study provides the most robust estimates available thus far.” However, they cautioned that “prospective randomized controlled trials are necessary in order to recommend statin use in clinical practice.”
‘Reassuring and pleasantly surprising’
The study is “very interesting, reassuring, and pleasantly surprising,” Scott L. Friedman, MD, chief of the division of liver diseases and dean for therapeutic discovery at the Icahn School of Medicine at Mount Sinai. New York, said in an interview.
“Statins have been around for a long time, and in earlier days, there was fear of using them because they might induce liver injury. But ample and consistent data exclude the possibility that they are more toxic in patients with liver disease,” said Dr. Friedman, who was not associated with this research.
“What’s interesting and new about this paper is that those studies that have looked at the effects of statins on liver disease have primarily focused on patients who have cirrhosis because there’s some scientific evidence [that] statins can lead to vasodilation and reduce the elevated liver blood flow that occurs in cirrhosis,” he explained.
“Instead, this study, which is quite sizable, includes patients who do not have evidence of cirrhosis based on biopsies. The results suggest that statins have a significant protective effect in these patients,” Dr. Friedman said.
The study was supported by the Karolinska Institute in Stockholm, the Columbia University Irving Medical Center, the Swedish Research Council, the Swedish Cancer Society, and the U.S. National Institutes of Health. Dr. Sharma is a consultant for Takeda and Volv. Other coauthors reported current or past relationships with Bristol-Myers Squibb, Gilead, Salix, and GlaxoSmithKline. Dr. Carey and Dr. Friedman reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The Swedish population-based study found that adults with noncirrhotic CLD who were on statin therapy had a statistically significant 40% lower risk of developing severe liver disease, compared with matched patients who were not on statin therapy.
The statin users were also less apt to progress to cirrhosis or hepatocellular carcinoma (HCC) and to die of liver disease, Rajani Sharma, MD, MSc, division of digestive and liver diseases, Columbia University Irving Medical Center, New York, and colleagues reported.
Their study was published online in Clinical Gastroenterology and Hepatology.
More than just cholesterol lowering
The study “continues the theme that cholesterol-lowering statins are good for a lot more things than just lowering cholesterol,” William Carey, MD, who wasn’t involved with the study, said in an interview.
The results are “very consistent with other trials that show that people with liver disease on statins do better in many respects than those who are not on statins,” said Dr. Carey, acting head of the hepatology section, department of gastroenterology, hepatology, and nutrition, Cleveland Clinic.
“The effects are not trivial,” Dr. Carey added. “It’s a very significant advantage in terms of fibrosis progression and survival.”
Statins have been shown to inhibit inflammatory pathways, promote endothelial cell function, and reduce hepatic stellate cell activity, suggesting that statins could lessen the progression of liver fibrosis, Dr. Sharma and coauthors wrote.
A few prior studies have looked at the effects of statins in noncirrhotic CLD specifically, but most only included patients with viral hepatitis, and the identification of precirrhotic liver disease was largely based on fibrosis scores or ICD coding, leading to a risk for misclassification and heterogeneity in results, they wrote.
Using histopathology data in a nationwide Swedish cohort, Dr. Sharma and colleagues identified 3862 adults with noncirrhotic CLD who were statin users and a like number of propensity score–matched nonstatin users with noncirrhotic CLD. The adults with CLD included in the study were required to have a liver biopsy showing fibrosis or inflammation between the years 1969 and 2017 and at least one ICD code for CLD.
In both groups, 45% of patients had nonalcoholic fatty liver disease (NAFLD), 22% had alcohol-related liver disease (ALD), 18% had viral hepatitis, and 15% had autoimmune hepatitis (AIH).
The analysis found 234 (6.1%) statin users developed severe liver disease versus 276 (7.1%) nonusers, with incidence rates of 10.5 versus 18.1 per 1,000 person-years, respectively.
Statin use was associated with a statistically significant 40% lower rate of severe liver disease (hazard ratio, 0.60; 95% confidence interval, 0.48-0.74).
This was the case in ALD (HR, 0.30; 95% CI, 0.19-0.49) and NAFLD (HR, 0.68; 95% CI 0.45-1.00), but the results were not statistically significant for individuals with viral hepatitis (HR, 0.76; 95% CI, 0.51-1.14) or AIH (HR, 0.88; 0.48-1.58).
Statin use had a protective association in both prefibrosis and fibrosis stages at diagnosis, the researchers reported.
Statin use was also associated with lower rates of progression to cirrhosis (HR, 0.62; 95% CI, 0.49-0.78), HCC (HR, 0.44; 95% CI, 0.27-0.71) and liver-related death or liver transplant (HR, 0.55; 95% CI, 0.36-0.82).
The authors noted that their “study provides the most robust estimates available thus far.” However, they cautioned that “prospective randomized controlled trials are necessary in order to recommend statin use in clinical practice.”
‘Reassuring and pleasantly surprising’
The study is “very interesting, reassuring, and pleasantly surprising,” Scott L. Friedman, MD, chief of the division of liver diseases and dean for therapeutic discovery at the Icahn School of Medicine at Mount Sinai. New York, said in an interview.
“Statins have been around for a long time, and in earlier days, there was fear of using them because they might induce liver injury. But ample and consistent data exclude the possibility that they are more toxic in patients with liver disease,” said Dr. Friedman, who was not associated with this research.
“What’s interesting and new about this paper is that those studies that have looked at the effects of statins on liver disease have primarily focused on patients who have cirrhosis because there’s some scientific evidence [that] statins can lead to vasodilation and reduce the elevated liver blood flow that occurs in cirrhosis,” he explained.
“Instead, this study, which is quite sizable, includes patients who do not have evidence of cirrhosis based on biopsies. The results suggest that statins have a significant protective effect in these patients,” Dr. Friedman said.
The study was supported by the Karolinska Institute in Stockholm, the Columbia University Irving Medical Center, the Swedish Research Council, the Swedish Cancer Society, and the U.S. National Institutes of Health. Dr. Sharma is a consultant for Takeda and Volv. Other coauthors reported current or past relationships with Bristol-Myers Squibb, Gilead, Salix, and GlaxoSmithKline. Dr. Carey and Dr. Friedman reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The Swedish population-based study found that adults with noncirrhotic CLD who were on statin therapy had a statistically significant 40% lower risk of developing severe liver disease, compared with matched patients who were not on statin therapy.
The statin users were also less apt to progress to cirrhosis or hepatocellular carcinoma (HCC) and to die of liver disease, Rajani Sharma, MD, MSc, division of digestive and liver diseases, Columbia University Irving Medical Center, New York, and colleagues reported.
Their study was published online in Clinical Gastroenterology and Hepatology.
More than just cholesterol lowering
The study “continues the theme that cholesterol-lowering statins are good for a lot more things than just lowering cholesterol,” William Carey, MD, who wasn’t involved with the study, said in an interview.
The results are “very consistent with other trials that show that people with liver disease on statins do better in many respects than those who are not on statins,” said Dr. Carey, acting head of the hepatology section, department of gastroenterology, hepatology, and nutrition, Cleveland Clinic.
“The effects are not trivial,” Dr. Carey added. “It’s a very significant advantage in terms of fibrosis progression and survival.”
Statins have been shown to inhibit inflammatory pathways, promote endothelial cell function, and reduce hepatic stellate cell activity, suggesting that statins could lessen the progression of liver fibrosis, Dr. Sharma and coauthors wrote.
A few prior studies have looked at the effects of statins in noncirrhotic CLD specifically, but most only included patients with viral hepatitis, and the identification of precirrhotic liver disease was largely based on fibrosis scores or ICD coding, leading to a risk for misclassification and heterogeneity in results, they wrote.
Using histopathology data in a nationwide Swedish cohort, Dr. Sharma and colleagues identified 3862 adults with noncirrhotic CLD who were statin users and a like number of propensity score–matched nonstatin users with noncirrhotic CLD. The adults with CLD included in the study were required to have a liver biopsy showing fibrosis or inflammation between the years 1969 and 2017 and at least one ICD code for CLD.
In both groups, 45% of patients had nonalcoholic fatty liver disease (NAFLD), 22% had alcohol-related liver disease (ALD), 18% had viral hepatitis, and 15% had autoimmune hepatitis (AIH).
The analysis found 234 (6.1%) statin users developed severe liver disease versus 276 (7.1%) nonusers, with incidence rates of 10.5 versus 18.1 per 1,000 person-years, respectively.
Statin use was associated with a statistically significant 40% lower rate of severe liver disease (hazard ratio, 0.60; 95% confidence interval, 0.48-0.74).
This was the case in ALD (HR, 0.30; 95% CI, 0.19-0.49) and NAFLD (HR, 0.68; 95% CI 0.45-1.00), but the results were not statistically significant for individuals with viral hepatitis (HR, 0.76; 95% CI, 0.51-1.14) or AIH (HR, 0.88; 0.48-1.58).
Statin use had a protective association in both prefibrosis and fibrosis stages at diagnosis, the researchers reported.
Statin use was also associated with lower rates of progression to cirrhosis (HR, 0.62; 95% CI, 0.49-0.78), HCC (HR, 0.44; 95% CI, 0.27-0.71) and liver-related death or liver transplant (HR, 0.55; 95% CI, 0.36-0.82).
The authors noted that their “study provides the most robust estimates available thus far.” However, they cautioned that “prospective randomized controlled trials are necessary in order to recommend statin use in clinical practice.”
‘Reassuring and pleasantly surprising’
The study is “very interesting, reassuring, and pleasantly surprising,” Scott L. Friedman, MD, chief of the division of liver diseases and dean for therapeutic discovery at the Icahn School of Medicine at Mount Sinai. New York, said in an interview.
“Statins have been around for a long time, and in earlier days, there was fear of using them because they might induce liver injury. But ample and consistent data exclude the possibility that they are more toxic in patients with liver disease,” said Dr. Friedman, who was not associated with this research.
“What’s interesting and new about this paper is that those studies that have looked at the effects of statins on liver disease have primarily focused on patients who have cirrhosis because there’s some scientific evidence [that] statins can lead to vasodilation and reduce the elevated liver blood flow that occurs in cirrhosis,” he explained.
“Instead, this study, which is quite sizable, includes patients who do not have evidence of cirrhosis based on biopsies. The results suggest that statins have a significant protective effect in these patients,” Dr. Friedman said.
The study was supported by the Karolinska Institute in Stockholm, the Columbia University Irving Medical Center, the Swedish Research Council, the Swedish Cancer Society, and the U.S. National Institutes of Health. Dr. Sharma is a consultant for Takeda and Volv. Other coauthors reported current or past relationships with Bristol-Myers Squibb, Gilead, Salix, and GlaxoSmithKline. Dr. Carey and Dr. Friedman reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
BMI has greater impact on survival in younger breast cancer patients
new data suggest.
Obesity is a well-known risk factor for breast cancer in postmenopausal women and has been associated with adverse prognosis, said Senna W.M. Lammers, MD, of Maastricht (the Netherlands) University during a presentation at the European Society for Medical Oncology (ESMO) Breast Cancer annual congress. In addition, some studies suggest that patients with higher body mass index (BMI) experience reduced benefits from endocrine therapy, she said.
Dr. Lammers and colleagues conducted a study to determine the prognostic and predictive effect of BMI on disease-free survival in postmenopausal women with hormone receptor–positive (HR+) breast cancer who were treated with extended endocrine therapy.
The study population included participants in the randomized, phase III DATA trial, which evaluated the use of 6 years vs. 3 years of anastrozole in postmenopausal women with HR+ breast cancer who were disease-free after 2-3 years of adjuvant tamoxifen therapy.
Patients were categorized based on BMI as having normal weight (18.5-24.9 kg/m2), overweight (25-29.9 kg/m2), or obese (30 kg/m2 or higher). The primary outcome was disease-free survival (DFS); the median follow-up period was 13.1 years.
DFS for patients with normal weight, overweight, and obesity was 66.2%, 59.5%, and 52.4%, with a P value of less than .001 for the trend, Dr. Lammers said. “These results were confirmed in multivariable analysis,” she said. Overall, patients with overweight and obesity had a worse DFS when compared with patients with normal weight (hazard ratio, 1.16; P = .10, for patients with overweight and HR, 1.26; P = .03 for patients with obesity).
“Next, we aimed to determine whether the prognostic effect of BMI differed by age,” Dr. Lammers said.
In women younger than 60 years, overweight and obesity were significantly associated with worse DFS (HR, 1.29; P = .05 and HR 1.83, P less than .001, respectively). However, this effect was not observed in women aged 60 years and older.
The researchers also examined the treatment effect of extended anastrozole on adapted DFS by weight, and found no significant differences among patients with normal weight, overweight, and obesity (HR, 1.00; HR, 0.74; and HR, 0.97, respectively), said Dr. Lammers.
In the question and answer session, Dr. Lammers was asked about possible explanations for the difference in DFS by age. Potential explanations include possible survival bias “as only the healthier [patients with obesity] survive to old age,” she said. Other potential explanations are biological, such as the potentially higher levels of bone density in older [patients with obesity], she said.
When asked about additional clinical implications, Dr. Lammers emphasized the importance of maintaining a healthy BMI for breast cancer patients of all ages. Other research areas might involve the use of lifestyle interventions, although these are challenging to implement, she noted.
Data draw attention to quality of life and lifestyle factors
The need to “look at drug development with new eyes” is particularly important when reviewing patient-reported outcomes, said Otto Metzger, MD, of the Dana Farber Cancer Institute, Boston, who served as the discussant for the session.
Dr. Metzger brought up the association between age and the effect of BMI on DFS, specifically.
Based on data from multiple studies and meta-analyses, “I do believe that obesity does play a role in prognosis,” he said, but the question is how long will researchers continue to simply record data without acting to add lifestyle interventions while also trying to develop new drugs, he said. Although convincing patients to make lifestyle changes remains a challenge, patients are often more motivated to make such changes after a cancer diagnosis, Dr. Metzger noted.
“I am a firm believer in the use of digital therapeutics in the context of clinical trials,” said Dr. Metzger. Digital technology offers great potential to educate patients on [adverse effects] and also to improve treatment adherence and quality of life, he concluded.
The study was supported by AstraZeneca, and Dr. Lammers disclosed financial relationships with AstraZeneca and Eli Lilly. Dr. Metzger disclosed receiving research funding to his institution from Pfizer, Genentech/Roche, and Sanofi, and serving as an adviser/consultant to AstraZeneca, Merck, Oncoclinicas, Resilience, and Roche.
new data suggest.
Obesity is a well-known risk factor for breast cancer in postmenopausal women and has been associated with adverse prognosis, said Senna W.M. Lammers, MD, of Maastricht (the Netherlands) University during a presentation at the European Society for Medical Oncology (ESMO) Breast Cancer annual congress. In addition, some studies suggest that patients with higher body mass index (BMI) experience reduced benefits from endocrine therapy, she said.
Dr. Lammers and colleagues conducted a study to determine the prognostic and predictive effect of BMI on disease-free survival in postmenopausal women with hormone receptor–positive (HR+) breast cancer who were treated with extended endocrine therapy.
The study population included participants in the randomized, phase III DATA trial, which evaluated the use of 6 years vs. 3 years of anastrozole in postmenopausal women with HR+ breast cancer who were disease-free after 2-3 years of adjuvant tamoxifen therapy.
Patients were categorized based on BMI as having normal weight (18.5-24.9 kg/m2), overweight (25-29.9 kg/m2), or obese (30 kg/m2 or higher). The primary outcome was disease-free survival (DFS); the median follow-up period was 13.1 years.
DFS for patients with normal weight, overweight, and obesity was 66.2%, 59.5%, and 52.4%, with a P value of less than .001 for the trend, Dr. Lammers said. “These results were confirmed in multivariable analysis,” she said. Overall, patients with overweight and obesity had a worse DFS when compared with patients with normal weight (hazard ratio, 1.16; P = .10, for patients with overweight and HR, 1.26; P = .03 for patients with obesity).
“Next, we aimed to determine whether the prognostic effect of BMI differed by age,” Dr. Lammers said.
In women younger than 60 years, overweight and obesity were significantly associated with worse DFS (HR, 1.29; P = .05 and HR 1.83, P less than .001, respectively). However, this effect was not observed in women aged 60 years and older.
The researchers also examined the treatment effect of extended anastrozole on adapted DFS by weight, and found no significant differences among patients with normal weight, overweight, and obesity (HR, 1.00; HR, 0.74; and HR, 0.97, respectively), said Dr. Lammers.
In the question and answer session, Dr. Lammers was asked about possible explanations for the difference in DFS by age. Potential explanations include possible survival bias “as only the healthier [patients with obesity] survive to old age,” she said. Other potential explanations are biological, such as the potentially higher levels of bone density in older [patients with obesity], she said.
When asked about additional clinical implications, Dr. Lammers emphasized the importance of maintaining a healthy BMI for breast cancer patients of all ages. Other research areas might involve the use of lifestyle interventions, although these are challenging to implement, she noted.
Data draw attention to quality of life and lifestyle factors
The need to “look at drug development with new eyes” is particularly important when reviewing patient-reported outcomes, said Otto Metzger, MD, of the Dana Farber Cancer Institute, Boston, who served as the discussant for the session.
Dr. Metzger brought up the association between age and the effect of BMI on DFS, specifically.
Based on data from multiple studies and meta-analyses, “I do believe that obesity does play a role in prognosis,” he said, but the question is how long will researchers continue to simply record data without acting to add lifestyle interventions while also trying to develop new drugs, he said. Although convincing patients to make lifestyle changes remains a challenge, patients are often more motivated to make such changes after a cancer diagnosis, Dr. Metzger noted.
“I am a firm believer in the use of digital therapeutics in the context of clinical trials,” said Dr. Metzger. Digital technology offers great potential to educate patients on [adverse effects] and also to improve treatment adherence and quality of life, he concluded.
The study was supported by AstraZeneca, and Dr. Lammers disclosed financial relationships with AstraZeneca and Eli Lilly. Dr. Metzger disclosed receiving research funding to his institution from Pfizer, Genentech/Roche, and Sanofi, and serving as an adviser/consultant to AstraZeneca, Merck, Oncoclinicas, Resilience, and Roche.
new data suggest.
Obesity is a well-known risk factor for breast cancer in postmenopausal women and has been associated with adverse prognosis, said Senna W.M. Lammers, MD, of Maastricht (the Netherlands) University during a presentation at the European Society for Medical Oncology (ESMO) Breast Cancer annual congress. In addition, some studies suggest that patients with higher body mass index (BMI) experience reduced benefits from endocrine therapy, she said.
Dr. Lammers and colleagues conducted a study to determine the prognostic and predictive effect of BMI on disease-free survival in postmenopausal women with hormone receptor–positive (HR+) breast cancer who were treated with extended endocrine therapy.
The study population included participants in the randomized, phase III DATA trial, which evaluated the use of 6 years vs. 3 years of anastrozole in postmenopausal women with HR+ breast cancer who were disease-free after 2-3 years of adjuvant tamoxifen therapy.
Patients were categorized based on BMI as having normal weight (18.5-24.9 kg/m2), overweight (25-29.9 kg/m2), or obese (30 kg/m2 or higher). The primary outcome was disease-free survival (DFS); the median follow-up period was 13.1 years.
DFS for patients with normal weight, overweight, and obesity was 66.2%, 59.5%, and 52.4%, with a P value of less than .001 for the trend, Dr. Lammers said. “These results were confirmed in multivariable analysis,” she said. Overall, patients with overweight and obesity had a worse DFS when compared with patients with normal weight (hazard ratio, 1.16; P = .10, for patients with overweight and HR, 1.26; P = .03 for patients with obesity).
“Next, we aimed to determine whether the prognostic effect of BMI differed by age,” Dr. Lammers said.
In women younger than 60 years, overweight and obesity were significantly associated with worse DFS (HR, 1.29; P = .05 and HR 1.83, P less than .001, respectively). However, this effect was not observed in women aged 60 years and older.
The researchers also examined the treatment effect of extended anastrozole on adapted DFS by weight, and found no significant differences among patients with normal weight, overweight, and obesity (HR, 1.00; HR, 0.74; and HR, 0.97, respectively), said Dr. Lammers.
In the question and answer session, Dr. Lammers was asked about possible explanations for the difference in DFS by age. Potential explanations include possible survival bias “as only the healthier [patients with obesity] survive to old age,” she said. Other potential explanations are biological, such as the potentially higher levels of bone density in older [patients with obesity], she said.
When asked about additional clinical implications, Dr. Lammers emphasized the importance of maintaining a healthy BMI for breast cancer patients of all ages. Other research areas might involve the use of lifestyle interventions, although these are challenging to implement, she noted.
Data draw attention to quality of life and lifestyle factors
The need to “look at drug development with new eyes” is particularly important when reviewing patient-reported outcomes, said Otto Metzger, MD, of the Dana Farber Cancer Institute, Boston, who served as the discussant for the session.
Dr. Metzger brought up the association between age and the effect of BMI on DFS, specifically.
Based on data from multiple studies and meta-analyses, “I do believe that obesity does play a role in prognosis,” he said, but the question is how long will researchers continue to simply record data without acting to add lifestyle interventions while also trying to develop new drugs, he said. Although convincing patients to make lifestyle changes remains a challenge, patients are often more motivated to make such changes after a cancer diagnosis, Dr. Metzger noted.
“I am a firm believer in the use of digital therapeutics in the context of clinical trials,” said Dr. Metzger. Digital technology offers great potential to educate patients on [adverse effects] and also to improve treatment adherence and quality of life, he concluded.
The study was supported by AstraZeneca, and Dr. Lammers disclosed financial relationships with AstraZeneca and Eli Lilly. Dr. Metzger disclosed receiving research funding to his institution from Pfizer, Genentech/Roche, and Sanofi, and serving as an adviser/consultant to AstraZeneca, Merck, Oncoclinicas, Resilience, and Roche.
FROM ESMO BREAST CANCER 2023