User login
Women’s Cancers: Clinicians Research, Advise on Sexual Dysfunction
Decreased sexual function is a side effect of many types of cancer, notably uterine, cervical, ovarian, and breast cancer, that often goes unaddressed, according to the authors of several studies presented at the Society of Gynecologic Oncology (SGO)’s Annual Meeting on Women’s Cancer.
Patients want to talk about sex, but not necessarily at the start of their diagnosis or treatment, suggest the findings of a study presented at the meeting. Jesse T. Brewer of Weill Cornell Medicine in New York City and colleagues enrolled 63 patients who underwent surgery with documented hereditary breast cancer, ovarian cancer, or Lynch syndrome in a cross-sectional survey.
Overall, 86% said that sexuality and intimacy were very or somewhat important, and 78% said that the healthcare team addressing the issue was very or somewhat important, the researchers found. However, only 40% of the respondents said that they wanted to discuss sexuality at the time of diagnosis because the idea was too overwhelming.
Oncologists are more aware of sexual side effects and the potential for sexual issues that persist long after treatment, but many patients may not have opportunities to talk about sexual concerns, said Don S. Dizon, MD, an oncologist specializing in women’s cancers at Brown University, Providence, Rhode Island, in an interview.
“It is important that we [oncologists] be the ones to open the door to these conversations; people with cancer will not bring it up spontaneously, for fear of making their provider uncomfortable, especially if they’ve never been asked about it before,” Dr. Dizon said in an interview.
He advised clinicians to find a network within their health systems so they can refer patients to specialized services, such as sex therapy, couples counseling, pelvic rehabilitation, or menopausal experts as needed.
In another study presented at the meeting, Naaman Mehta, MD, of NYU Langone Health, and colleagues reviewed data from 166 healthcare providers who completed a 23-item survey about evaluating and managing sexual health concerns of their patients. Most of the respondents were gynecologic oncologists (93.4%), but one radiation oncologist and 10 other healthcare providers also completed the survey.
Overall, approximately 60% of the respondents routinely asked about the sexual health concerns of their patients, and 98% of these said they believed that sexual health discussions should be held with a gynecologic oncologist. Just over half (54%) also said that the patient should be the one to initiate a discussion of sexual health concerns.
Female providers were significantly more likely to discuss sexual health with patients, compared with male providers, after controlling for the hospital setting and training level, the researchers noted (odds ratio, 1.4;P < .01).
The results suggest a need for more ways to integrate sexual health screening into gynecologic oncologic clinics, the researchers concluded.
The provider survey findings are similar to the results of a survey conducted by Dr. Dizon and colleagues in 2007. In that study, less than half of respondents took a sexual history, but 80% felt there was insufficient time to explore sexual issues.
“It is critical to understand that people with cancer do not expect their oncologists to be sexual health experts, but as with all other side effects caused by treatment and the diagnosis, we can be the ones who recognize it,” Dr. Dizon noted, in an interview.
Common Complaints and Causes
In Dr. Dizon’s experience, local symptoms including vaginal dryness, pain with penetration, and vaginal thinning, are common sexual complaints in women with cancer, as are systemic issues such as lack of interest and menopause-type symptoms.
“For those undergoing radiation, the vaginal tunnel can actually develop adhesions, and if not treated proactively this can lead to vaginal stenosis,” said Dr. Dizon, who was not involved in the studies presented at the meeting.
Comorbidities such as diabetes, cardiovascular disease, and musculoskeletal conditions can contribute to sexual issues in women with cancer, according to Nora Lersch, DNP, FNP-BC, AOCNP, and Nicole Dreibelbis, CRNP, the authors of other research presented at the meeting.
Culture, religion, fitness level, history of sexual violence, and gender spectrum health also play a role, as do anxiety and depression, dementia, and substance abuse disorders, the authors wrote in their presentation, “Prioritizing Sexual Health in Gynecological Oncology Care.”
Low libido is a frequent complaint across all cancer types, Ms. Dreibelbis, a nurse practitioner specializing in gynecologic oncology at the UPMC Hillman Cancer Center, Pittsburgh, Pennsylvania, said in an interview.
“Breast cancer patients, especially those on [aromatase inhibitor] therapy, often experience vaginal dryness and therefore dyspareunia,” she added.
The pelvic floor muscles, with their important role in sexual response, can be weakened by cancer treatment or surgery, and the pudendal nerves, which are the primary nerves responsible for sexual response in women, can be affected as well, Dr. Lersch and Ms. Dreibelbis wrote.
Taking Sex Seriously
Researchers are exploring the impact of different cancer prevention treatments for women to mitigate sexual side effects, as illustrated by another study presented at the meeting.
Dr. Barbara Norquist, MD, a gynecologic oncologist at the University of Washington, Seattle, and colleagues compared the sexual function and menopausal symptoms of patients at high risk of ovarian carcinoma who underwent either interval salpingectomy/delayed oophorectomy (ISDO) or risk-reducing salpingo-oophorectomy (RRSO).
“For patients at high risk for ovarian cancer, surgical removal of the tubes and ovaries is the mainstay of prevention, as screening is not effective at reducing death from ovarian cancer. As a result of surgery, many patients become suddenly postmenopausal from losing their ovaries,” Dr. Norquist said in an interview.
Some patients delay surgery out of concern for health and quality of life, including sexual function, she said.
In the study (known as the WISP trial) the researchers compared data from 166 patients who underwent immediate removal of the fallopian tubes and ovaries and 171 who underwent fallopian tube removal and delayed oophorectomy. All patients completed questionnaires about sexual function. The primary outcome was change in sexual function based on the sexual function index (FSFI) from baseline to 6 months after surgery.
Overall, changes in sexual function were significantly greater in the immediate oophorectomy group, compared with the delayed oophorectomy group at 6 months (33% vs 17%) and also at 12 months (43% vs 20%).
A further review of patients using hormone therapy showed that those in the immediate oophorectomy group still had greater decreases in sexual function, compared with the delayed group, though the difference between groups of patients using hormone therapy was less dramatic.
“I was surprised that, even with hormone replacement therapy, patients undergoing removal of the ovaries still had significant detrimental changes to sexual function when compared to those having the tubes removed, although this was even worse in those who could not take HRT,” Dr. Norquist said, in an interview. “I was reassured that menopausal symptoms in general were well managed with HRT, as these patients did not score differently on menopause symptoms, compared with those having their tubes removed,” she said.
Patients deserve accurate information about predicted changes in menopausal symptoms and sexual function as a result of ovary removal, and HRT should be provided when there is no contraindication, Dr. Norquist told this news organization.
Dr. Norquist and colleagues are awaiting the results of clinical trials investigating the safety of salpingectomy with delayed oophorectomy in terms of ovarian cancer prevention, but more research is needed to identify optimal management of the menopausal and sexual side effects associated with surgical menopause, she noted.
“Findings from the WISP study show the importance of hormones in women undergoing prophylactic surgery,” Dr. Dizon said. The findings indicate that salpingectomy has less of a negative influence on sexual function compared to removal of the ovaries, and the impact of hormone therapy and the relatively young age of the patients who took hormones reinforces current knowledge about hormones and sex, he added.
Barriers and Solutions
Barriers to asking women with cancer about sexual issues reported by providers include limited time, lack of training in sexual health, a desire to avoid offending the patient or making them uncomfortable, and uncertainty about how to answer the questions, Dr. Lersch and Ms. Dreibelbis wrote in their presentation.
Barriers to asking healthcare providers about their sexual issues reported by patients include the beliefs that the clinician should initiate the discussion, that sexual function will not be taken seriously, and that they might make the provider uncomfortable.
“Fortunately, more information and research has been done on sexual health and gynecological cancer in recent years, so oncologists are becoming more aware of the issues women may have,” said Dr. Lersch who is an oncology nurse practitioner at Providence Franz Cancer Institute in Portland, Oregon, in an interview.
Telling patients early in their cancer treatment about potential sexual side effects and opportunities for help is essential, she added.
Although oncologists have become more aware of the importance of sexual health and well-being for their patients, “I think there has historically been a disconnect in including sexual health education in medical training,” Ms. Dreibelbis said in an interview.
Dr. Lersch and Ms. Dreibelbis advised a multidimensional approach to managing sexual problems in cancer patients that includes consideration of biological and psychological symptoms, but also social, cultural, and interpersonal factors, in their presentation.
Their suggestions include discussing dyspareunia with their patients, asking for details such as whether the pain is internal or external, whether it occurs with activities outside of sex including masturbation, and whether bleeding is present.
Oncology therapies and surgeries can decrease or eliminate an individual’s ability to produce their own lubricant; for example, removal of the cervix eliminates cervical mucous, which helps with internal lubrication, they wrote in their presentation.
For patients with dyspareunia, Dr. Lersch and Ms. Dreibelbis recommend a vaginal moisturizer especially formulated for vaginal tissue that can be absorbed by the mucosal tissue of the vagina. Use of this type of product can increase the effectiveness of lubricants and help restore integrity of the vaginal tissue. Such moisturizers are available as gels, creams, or suppositories over the counter, and do not contain hormones.
Vaginal estrogen can be helpful for burning, itching, irritation, tissue fragility, and pain with sex, according to Dr. Lersch and Ms. Dreibelbis. Adequate estrogen therapy can promote normalization of vaginal pH and microflora, as well increase vaginal secretion and reduce pain and dryness with intercourse, the presenters stated in their presentation. In addition, dilator therapy can be used to help prevent vaginal stenosis, and penetration bumpers can help relieve discomfort during intercourse, they wrote.
Looking ahead, more research is needed to serve a wider patient population, Ms. Dreibelbis said, in an interview.
“LGBTQIA [individuals] have not been included in sexual health research and there are more people than ever who identify within this group of people. I know there has also been some very early work on shielding the clitoris from the impacts of radiation, and I believe this is extremely important up-and-coming research,” she said.
Dr. Lersch, Ms. Dreibelbi, Dr. Dizon, Dr. Norquist, Ms. Brewer, and Dr. Mehta had no financial conflicts to disclose.
Decreased sexual function is a side effect of many types of cancer, notably uterine, cervical, ovarian, and breast cancer, that often goes unaddressed, according to the authors of several studies presented at the Society of Gynecologic Oncology (SGO)’s Annual Meeting on Women’s Cancer.
Patients want to talk about sex, but not necessarily at the start of their diagnosis or treatment, suggest the findings of a study presented at the meeting. Jesse T. Brewer of Weill Cornell Medicine in New York City and colleagues enrolled 63 patients who underwent surgery with documented hereditary breast cancer, ovarian cancer, or Lynch syndrome in a cross-sectional survey.
Overall, 86% said that sexuality and intimacy were very or somewhat important, and 78% said that the healthcare team addressing the issue was very or somewhat important, the researchers found. However, only 40% of the respondents said that they wanted to discuss sexuality at the time of diagnosis because the idea was too overwhelming.
Oncologists are more aware of sexual side effects and the potential for sexual issues that persist long after treatment, but many patients may not have opportunities to talk about sexual concerns, said Don S. Dizon, MD, an oncologist specializing in women’s cancers at Brown University, Providence, Rhode Island, in an interview.
“It is important that we [oncologists] be the ones to open the door to these conversations; people with cancer will not bring it up spontaneously, for fear of making their provider uncomfortable, especially if they’ve never been asked about it before,” Dr. Dizon said in an interview.
He advised clinicians to find a network within their health systems so they can refer patients to specialized services, such as sex therapy, couples counseling, pelvic rehabilitation, or menopausal experts as needed.
In another study presented at the meeting, Naaman Mehta, MD, of NYU Langone Health, and colleagues reviewed data from 166 healthcare providers who completed a 23-item survey about evaluating and managing sexual health concerns of their patients. Most of the respondents were gynecologic oncologists (93.4%), but one radiation oncologist and 10 other healthcare providers also completed the survey.
Overall, approximately 60% of the respondents routinely asked about the sexual health concerns of their patients, and 98% of these said they believed that sexual health discussions should be held with a gynecologic oncologist. Just over half (54%) also said that the patient should be the one to initiate a discussion of sexual health concerns.
Female providers were significantly more likely to discuss sexual health with patients, compared with male providers, after controlling for the hospital setting and training level, the researchers noted (odds ratio, 1.4;P < .01).
The results suggest a need for more ways to integrate sexual health screening into gynecologic oncologic clinics, the researchers concluded.
The provider survey findings are similar to the results of a survey conducted by Dr. Dizon and colleagues in 2007. In that study, less than half of respondents took a sexual history, but 80% felt there was insufficient time to explore sexual issues.
“It is critical to understand that people with cancer do not expect their oncologists to be sexual health experts, but as with all other side effects caused by treatment and the diagnosis, we can be the ones who recognize it,” Dr. Dizon noted, in an interview.
Common Complaints and Causes
In Dr. Dizon’s experience, local symptoms including vaginal dryness, pain with penetration, and vaginal thinning, are common sexual complaints in women with cancer, as are systemic issues such as lack of interest and menopause-type symptoms.
“For those undergoing radiation, the vaginal tunnel can actually develop adhesions, and if not treated proactively this can lead to vaginal stenosis,” said Dr. Dizon, who was not involved in the studies presented at the meeting.
Comorbidities such as diabetes, cardiovascular disease, and musculoskeletal conditions can contribute to sexual issues in women with cancer, according to Nora Lersch, DNP, FNP-BC, AOCNP, and Nicole Dreibelbis, CRNP, the authors of other research presented at the meeting.
Culture, religion, fitness level, history of sexual violence, and gender spectrum health also play a role, as do anxiety and depression, dementia, and substance abuse disorders, the authors wrote in their presentation, “Prioritizing Sexual Health in Gynecological Oncology Care.”
Low libido is a frequent complaint across all cancer types, Ms. Dreibelbis, a nurse practitioner specializing in gynecologic oncology at the UPMC Hillman Cancer Center, Pittsburgh, Pennsylvania, said in an interview.
“Breast cancer patients, especially those on [aromatase inhibitor] therapy, often experience vaginal dryness and therefore dyspareunia,” she added.
The pelvic floor muscles, with their important role in sexual response, can be weakened by cancer treatment or surgery, and the pudendal nerves, which are the primary nerves responsible for sexual response in women, can be affected as well, Dr. Lersch and Ms. Dreibelbis wrote.
Taking Sex Seriously
Researchers are exploring the impact of different cancer prevention treatments for women to mitigate sexual side effects, as illustrated by another study presented at the meeting.
Dr. Barbara Norquist, MD, a gynecologic oncologist at the University of Washington, Seattle, and colleagues compared the sexual function and menopausal symptoms of patients at high risk of ovarian carcinoma who underwent either interval salpingectomy/delayed oophorectomy (ISDO) or risk-reducing salpingo-oophorectomy (RRSO).
“For patients at high risk for ovarian cancer, surgical removal of the tubes and ovaries is the mainstay of prevention, as screening is not effective at reducing death from ovarian cancer. As a result of surgery, many patients become suddenly postmenopausal from losing their ovaries,” Dr. Norquist said in an interview.
Some patients delay surgery out of concern for health and quality of life, including sexual function, she said.
In the study (known as the WISP trial) the researchers compared data from 166 patients who underwent immediate removal of the fallopian tubes and ovaries and 171 who underwent fallopian tube removal and delayed oophorectomy. All patients completed questionnaires about sexual function. The primary outcome was change in sexual function based on the sexual function index (FSFI) from baseline to 6 months after surgery.
Overall, changes in sexual function were significantly greater in the immediate oophorectomy group, compared with the delayed oophorectomy group at 6 months (33% vs 17%) and also at 12 months (43% vs 20%).
A further review of patients using hormone therapy showed that those in the immediate oophorectomy group still had greater decreases in sexual function, compared with the delayed group, though the difference between groups of patients using hormone therapy was less dramatic.
“I was surprised that, even with hormone replacement therapy, patients undergoing removal of the ovaries still had significant detrimental changes to sexual function when compared to those having the tubes removed, although this was even worse in those who could not take HRT,” Dr. Norquist said, in an interview. “I was reassured that menopausal symptoms in general were well managed with HRT, as these patients did not score differently on menopause symptoms, compared with those having their tubes removed,” she said.
Patients deserve accurate information about predicted changes in menopausal symptoms and sexual function as a result of ovary removal, and HRT should be provided when there is no contraindication, Dr. Norquist told this news organization.
Dr. Norquist and colleagues are awaiting the results of clinical trials investigating the safety of salpingectomy with delayed oophorectomy in terms of ovarian cancer prevention, but more research is needed to identify optimal management of the menopausal and sexual side effects associated with surgical menopause, she noted.
“Findings from the WISP study show the importance of hormones in women undergoing prophylactic surgery,” Dr. Dizon said. The findings indicate that salpingectomy has less of a negative influence on sexual function compared to removal of the ovaries, and the impact of hormone therapy and the relatively young age of the patients who took hormones reinforces current knowledge about hormones and sex, he added.
Barriers and Solutions
Barriers to asking women with cancer about sexual issues reported by providers include limited time, lack of training in sexual health, a desire to avoid offending the patient or making them uncomfortable, and uncertainty about how to answer the questions, Dr. Lersch and Ms. Dreibelbis wrote in their presentation.
Barriers to asking healthcare providers about their sexual issues reported by patients include the beliefs that the clinician should initiate the discussion, that sexual function will not be taken seriously, and that they might make the provider uncomfortable.
“Fortunately, more information and research has been done on sexual health and gynecological cancer in recent years, so oncologists are becoming more aware of the issues women may have,” said Dr. Lersch who is an oncology nurse practitioner at Providence Franz Cancer Institute in Portland, Oregon, in an interview.
Telling patients early in their cancer treatment about potential sexual side effects and opportunities for help is essential, she added.
Although oncologists have become more aware of the importance of sexual health and well-being for their patients, “I think there has historically been a disconnect in including sexual health education in medical training,” Ms. Dreibelbis said in an interview.
Dr. Lersch and Ms. Dreibelbis advised a multidimensional approach to managing sexual problems in cancer patients that includes consideration of biological and psychological symptoms, but also social, cultural, and interpersonal factors, in their presentation.
Their suggestions include discussing dyspareunia with their patients, asking for details such as whether the pain is internal or external, whether it occurs with activities outside of sex including masturbation, and whether bleeding is present.
Oncology therapies and surgeries can decrease or eliminate an individual’s ability to produce their own lubricant; for example, removal of the cervix eliminates cervical mucous, which helps with internal lubrication, they wrote in their presentation.
For patients with dyspareunia, Dr. Lersch and Ms. Dreibelbis recommend a vaginal moisturizer especially formulated for vaginal tissue that can be absorbed by the mucosal tissue of the vagina. Use of this type of product can increase the effectiveness of lubricants and help restore integrity of the vaginal tissue. Such moisturizers are available as gels, creams, or suppositories over the counter, and do not contain hormones.
Vaginal estrogen can be helpful for burning, itching, irritation, tissue fragility, and pain with sex, according to Dr. Lersch and Ms. Dreibelbis. Adequate estrogen therapy can promote normalization of vaginal pH and microflora, as well increase vaginal secretion and reduce pain and dryness with intercourse, the presenters stated in their presentation. In addition, dilator therapy can be used to help prevent vaginal stenosis, and penetration bumpers can help relieve discomfort during intercourse, they wrote.
Looking ahead, more research is needed to serve a wider patient population, Ms. Dreibelbis said, in an interview.
“LGBTQIA [individuals] have not been included in sexual health research and there are more people than ever who identify within this group of people. I know there has also been some very early work on shielding the clitoris from the impacts of radiation, and I believe this is extremely important up-and-coming research,” she said.
Dr. Lersch, Ms. Dreibelbi, Dr. Dizon, Dr. Norquist, Ms. Brewer, and Dr. Mehta had no financial conflicts to disclose.
Decreased sexual function is a side effect of many types of cancer, notably uterine, cervical, ovarian, and breast cancer, that often goes unaddressed, according to the authors of several studies presented at the Society of Gynecologic Oncology (SGO)’s Annual Meeting on Women’s Cancer.
Patients want to talk about sex, but not necessarily at the start of their diagnosis or treatment, suggest the findings of a study presented at the meeting. Jesse T. Brewer of Weill Cornell Medicine in New York City and colleagues enrolled 63 patients who underwent surgery with documented hereditary breast cancer, ovarian cancer, or Lynch syndrome in a cross-sectional survey.
Overall, 86% said that sexuality and intimacy were very or somewhat important, and 78% said that the healthcare team addressing the issue was very or somewhat important, the researchers found. However, only 40% of the respondents said that they wanted to discuss sexuality at the time of diagnosis because the idea was too overwhelming.
Oncologists are more aware of sexual side effects and the potential for sexual issues that persist long after treatment, but many patients may not have opportunities to talk about sexual concerns, said Don S. Dizon, MD, an oncologist specializing in women’s cancers at Brown University, Providence, Rhode Island, in an interview.
“It is important that we [oncologists] be the ones to open the door to these conversations; people with cancer will not bring it up spontaneously, for fear of making their provider uncomfortable, especially if they’ve never been asked about it before,” Dr. Dizon said in an interview.
He advised clinicians to find a network within their health systems so they can refer patients to specialized services, such as sex therapy, couples counseling, pelvic rehabilitation, or menopausal experts as needed.
In another study presented at the meeting, Naaman Mehta, MD, of NYU Langone Health, and colleagues reviewed data from 166 healthcare providers who completed a 23-item survey about evaluating and managing sexual health concerns of their patients. Most of the respondents were gynecologic oncologists (93.4%), but one radiation oncologist and 10 other healthcare providers also completed the survey.
Overall, approximately 60% of the respondents routinely asked about the sexual health concerns of their patients, and 98% of these said they believed that sexual health discussions should be held with a gynecologic oncologist. Just over half (54%) also said that the patient should be the one to initiate a discussion of sexual health concerns.
Female providers were significantly more likely to discuss sexual health with patients, compared with male providers, after controlling for the hospital setting and training level, the researchers noted (odds ratio, 1.4;P < .01).
The results suggest a need for more ways to integrate sexual health screening into gynecologic oncologic clinics, the researchers concluded.
The provider survey findings are similar to the results of a survey conducted by Dr. Dizon and colleagues in 2007. In that study, less than half of respondents took a sexual history, but 80% felt there was insufficient time to explore sexual issues.
“It is critical to understand that people with cancer do not expect their oncologists to be sexual health experts, but as with all other side effects caused by treatment and the diagnosis, we can be the ones who recognize it,” Dr. Dizon noted, in an interview.
Common Complaints and Causes
In Dr. Dizon’s experience, local symptoms including vaginal dryness, pain with penetration, and vaginal thinning, are common sexual complaints in women with cancer, as are systemic issues such as lack of interest and menopause-type symptoms.
“For those undergoing radiation, the vaginal tunnel can actually develop adhesions, and if not treated proactively this can lead to vaginal stenosis,” said Dr. Dizon, who was not involved in the studies presented at the meeting.
Comorbidities such as diabetes, cardiovascular disease, and musculoskeletal conditions can contribute to sexual issues in women with cancer, according to Nora Lersch, DNP, FNP-BC, AOCNP, and Nicole Dreibelbis, CRNP, the authors of other research presented at the meeting.
Culture, religion, fitness level, history of sexual violence, and gender spectrum health also play a role, as do anxiety and depression, dementia, and substance abuse disorders, the authors wrote in their presentation, “Prioritizing Sexual Health in Gynecological Oncology Care.”
Low libido is a frequent complaint across all cancer types, Ms. Dreibelbis, a nurse practitioner specializing in gynecologic oncology at the UPMC Hillman Cancer Center, Pittsburgh, Pennsylvania, said in an interview.
“Breast cancer patients, especially those on [aromatase inhibitor] therapy, often experience vaginal dryness and therefore dyspareunia,” she added.
The pelvic floor muscles, with their important role in sexual response, can be weakened by cancer treatment or surgery, and the pudendal nerves, which are the primary nerves responsible for sexual response in women, can be affected as well, Dr. Lersch and Ms. Dreibelbis wrote.
Taking Sex Seriously
Researchers are exploring the impact of different cancer prevention treatments for women to mitigate sexual side effects, as illustrated by another study presented at the meeting.
Dr. Barbara Norquist, MD, a gynecologic oncologist at the University of Washington, Seattle, and colleagues compared the sexual function and menopausal symptoms of patients at high risk of ovarian carcinoma who underwent either interval salpingectomy/delayed oophorectomy (ISDO) or risk-reducing salpingo-oophorectomy (RRSO).
“For patients at high risk for ovarian cancer, surgical removal of the tubes and ovaries is the mainstay of prevention, as screening is not effective at reducing death from ovarian cancer. As a result of surgery, many patients become suddenly postmenopausal from losing their ovaries,” Dr. Norquist said in an interview.
Some patients delay surgery out of concern for health and quality of life, including sexual function, she said.
In the study (known as the WISP trial) the researchers compared data from 166 patients who underwent immediate removal of the fallopian tubes and ovaries and 171 who underwent fallopian tube removal and delayed oophorectomy. All patients completed questionnaires about sexual function. The primary outcome was change in sexual function based on the sexual function index (FSFI) from baseline to 6 months after surgery.
Overall, changes in sexual function were significantly greater in the immediate oophorectomy group, compared with the delayed oophorectomy group at 6 months (33% vs 17%) and also at 12 months (43% vs 20%).
A further review of patients using hormone therapy showed that those in the immediate oophorectomy group still had greater decreases in sexual function, compared with the delayed group, though the difference between groups of patients using hormone therapy was less dramatic.
“I was surprised that, even with hormone replacement therapy, patients undergoing removal of the ovaries still had significant detrimental changes to sexual function when compared to those having the tubes removed, although this was even worse in those who could not take HRT,” Dr. Norquist said, in an interview. “I was reassured that menopausal symptoms in general were well managed with HRT, as these patients did not score differently on menopause symptoms, compared with those having their tubes removed,” she said.
Patients deserve accurate information about predicted changes in menopausal symptoms and sexual function as a result of ovary removal, and HRT should be provided when there is no contraindication, Dr. Norquist told this news organization.
Dr. Norquist and colleagues are awaiting the results of clinical trials investigating the safety of salpingectomy with delayed oophorectomy in terms of ovarian cancer prevention, but more research is needed to identify optimal management of the menopausal and sexual side effects associated with surgical menopause, she noted.
“Findings from the WISP study show the importance of hormones in women undergoing prophylactic surgery,” Dr. Dizon said. The findings indicate that salpingectomy has less of a negative influence on sexual function compared to removal of the ovaries, and the impact of hormone therapy and the relatively young age of the patients who took hormones reinforces current knowledge about hormones and sex, he added.
Barriers and Solutions
Barriers to asking women with cancer about sexual issues reported by providers include limited time, lack of training in sexual health, a desire to avoid offending the patient or making them uncomfortable, and uncertainty about how to answer the questions, Dr. Lersch and Ms. Dreibelbis wrote in their presentation.
Barriers to asking healthcare providers about their sexual issues reported by patients include the beliefs that the clinician should initiate the discussion, that sexual function will not be taken seriously, and that they might make the provider uncomfortable.
“Fortunately, more information and research has been done on sexual health and gynecological cancer in recent years, so oncologists are becoming more aware of the issues women may have,” said Dr. Lersch who is an oncology nurse practitioner at Providence Franz Cancer Institute in Portland, Oregon, in an interview.
Telling patients early in their cancer treatment about potential sexual side effects and opportunities for help is essential, she added.
Although oncologists have become more aware of the importance of sexual health and well-being for their patients, “I think there has historically been a disconnect in including sexual health education in medical training,” Ms. Dreibelbis said in an interview.
Dr. Lersch and Ms. Dreibelbis advised a multidimensional approach to managing sexual problems in cancer patients that includes consideration of biological and psychological symptoms, but also social, cultural, and interpersonal factors, in their presentation.
Their suggestions include discussing dyspareunia with their patients, asking for details such as whether the pain is internal or external, whether it occurs with activities outside of sex including masturbation, and whether bleeding is present.
Oncology therapies and surgeries can decrease or eliminate an individual’s ability to produce their own lubricant; for example, removal of the cervix eliminates cervical mucous, which helps with internal lubrication, they wrote in their presentation.
For patients with dyspareunia, Dr. Lersch and Ms. Dreibelbis recommend a vaginal moisturizer especially formulated for vaginal tissue that can be absorbed by the mucosal tissue of the vagina. Use of this type of product can increase the effectiveness of lubricants and help restore integrity of the vaginal tissue. Such moisturizers are available as gels, creams, or suppositories over the counter, and do not contain hormones.
Vaginal estrogen can be helpful for burning, itching, irritation, tissue fragility, and pain with sex, according to Dr. Lersch and Ms. Dreibelbis. Adequate estrogen therapy can promote normalization of vaginal pH and microflora, as well increase vaginal secretion and reduce pain and dryness with intercourse, the presenters stated in their presentation. In addition, dilator therapy can be used to help prevent vaginal stenosis, and penetration bumpers can help relieve discomfort during intercourse, they wrote.
Looking ahead, more research is needed to serve a wider patient population, Ms. Dreibelbis said, in an interview.
“LGBTQIA [individuals] have not been included in sexual health research and there are more people than ever who identify within this group of people. I know there has also been some very early work on shielding the clitoris from the impacts of radiation, and I believe this is extremely important up-and-coming research,” she said.
Dr. Lersch, Ms. Dreibelbi, Dr. Dizon, Dr. Norquist, Ms. Brewer, and Dr. Mehta had no financial conflicts to disclose.
FROM SGO 2024
Few Childhood Cancer Survivors Get Recommended Screenings
Among childhood cancer survivors in Ontario, Canada, who faced an elevated risk due to chemotherapy or radiation treatments, 53% followed screening recommendations for cardiomyopathy, 13% met colorectal cancer screening guidelines, and 6% adhered to breast cancer screening guidelines.
“Although over 80% of children newly diagnosed with cancer will become long-term survivors, as many as four out of five of these survivors will develop a serious or life-threatening late effect of their cancer therapy by age 45,” lead author Jennifer Shuldiner, PhD, MPH, a scientist at Women’s College Hospital Institute for Health Systems Solutions and Virtual Care in Toronto, told this news organization.
For instance, the risk for colorectal cancer in childhood cancer survivors is two to three times higher than it is among the general population, and the risk for breast cancer is similar between those who underwent chest radiation and those with a BRCA mutation. As many as 50% of those who received anthracycline chemotherapy or radiation involving the heart later develop cardiotoxicity.
The North American Children’s Oncology Group has published long-term follow-up guidelines for survivors of childhood cancer, yet many survivors don’t follow them because of lack of awareness or other barriers, said Dr. Shuldiner.
“Prior research has shown that many survivors do not complete these recommended tests,” she said. “With better knowledge of this at-risk population, we can design, test, and implement appropriate interventions and supports to tackle the issues.”
The study was published online on March 11 in CMAJ.
Changes in Adherence
The researchers conducted a retrospective population-based cohort study analyzing Ontario healthcare administrative data for adult survivors of childhood cancer diagnosed between 1986 and 2014 who faced an elevated risk for therapy-related colorectal cancer, breast cancer, or cardiomyopathy. The research team then assessed long-term adherence to the North American Children’s Oncology Group guidelines and predictors of adherence.
Among 3241 survivors, 3205 (99%) were at elevated risk for cardiomyopathy, 327 (10%) were at elevated risk for colorectal cancer, and 234 (7%) were at elevated risk for breast cancer. In addition, 2806 (87%) were at risk for one late effect, 345 (11%) were at risk for two late effects, and 90 (3%) were at risk for three late effects.
Overall, 53%, 13%, and 6% were adherent to their recommended surveillance for cardiomyopathy, colorectal cancer, and breast cancer, respectively. Over time, adherence increased for colorectal cancer and cardiomyopathy but decreased for breast cancer.
In addition, patients who were older at diagnosis were more likely to follow screening guidelines for colorectal and breast cancers, whereas those who were younger at diagnosis were more likely to follow screening guidelines for cardiomyopathy.
During a median follow-up of 7.8 years, the proportion of time spent adherent was 43% for cardiomyopathy, 14% for colorectal cancer, and 10% for breast cancer.
Survivors who attended a long-term follow-up clinic in the previous year had low adherence rates as well, though they were higher than in the rest of the cohort. In this group, the proportion of time that was spent adherent was 71% for cardiomyopathy, 27% for colorectal cancer, and 15% for breast cancer.
Shuldiner and colleagues are launching a research trial to determine whether a provincial support system can help childhood cancer survivors receive the recommended surveillance. The support system provides information about screening recommendations to survivors as well as reminders and sends key information to their family doctors.
“We now understand that childhood cancer survivors need help to complete the recommended tests,” said Dr. Shuldiner. “If the trial is successful, we hope it will be implemented in Ontario.”
Survivorship Care Plans
Low screening rates may result from a lack of awareness about screening recommendations and the negative long-term effects of cancer treatments, the study authors wrote. Cancer survivors, caregivers, family physicians, specialists, and survivor support groups can share the responsibility of spreading awareness and adhering to guidelines, they noted. In some cases, a survivorship care plan (SCP) may help.
“SCPs are intended to improve adherence by providing follow-up information and facilitating the transition from cancer treatment to survivorship and from pediatric to adult care,” Adam Yan, MD, a staff oncologist and oncology informatics lead at the Hospital for Sick Children in Toronto, told this news organization.
Dr. Yan, who wasn’t involved with this study, has researched surveillance adherence for secondary cancers and cardiac dysfunction among childhood cancer survivors. He and his colleagues found that screening rates were typically low among survivors who faced high risks for cardiac dysfunction and breast, colorectal, or skin cancers.
However, having a survivorship care plan seemed to help, and survivors treated after 1990 were more likely to have an SCP.
“SCP possession by high-risk survivors was associated with increased breast, skin, and cardiac surveillance,” he said. “It is uncertain whether SCP possession leads to adherence or whether SCP possession is a marker of survivors who are focused on their health and thus likely to adhere to preventive health practices, including surveillance.”
The study was funded by the Canadian Institutes of Health Research and ICES, which receives support from the Ontario Ministry of Health and the Ministry of Long-Term Care. Dr. Shuldiner received a Canadian Institutes of Health Research Health System Impact Postdoctoral Fellowship in support of the work. Dr. Yan disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Among childhood cancer survivors in Ontario, Canada, who faced an elevated risk due to chemotherapy or radiation treatments, 53% followed screening recommendations for cardiomyopathy, 13% met colorectal cancer screening guidelines, and 6% adhered to breast cancer screening guidelines.
“Although over 80% of children newly diagnosed with cancer will become long-term survivors, as many as four out of five of these survivors will develop a serious or life-threatening late effect of their cancer therapy by age 45,” lead author Jennifer Shuldiner, PhD, MPH, a scientist at Women’s College Hospital Institute for Health Systems Solutions and Virtual Care in Toronto, told this news organization.
For instance, the risk for colorectal cancer in childhood cancer survivors is two to three times higher than it is among the general population, and the risk for breast cancer is similar between those who underwent chest radiation and those with a BRCA mutation. As many as 50% of those who received anthracycline chemotherapy or radiation involving the heart later develop cardiotoxicity.
The North American Children’s Oncology Group has published long-term follow-up guidelines for survivors of childhood cancer, yet many survivors don’t follow them because of lack of awareness or other barriers, said Dr. Shuldiner.
“Prior research has shown that many survivors do not complete these recommended tests,” she said. “With better knowledge of this at-risk population, we can design, test, and implement appropriate interventions and supports to tackle the issues.”
The study was published online on March 11 in CMAJ.
Changes in Adherence
The researchers conducted a retrospective population-based cohort study analyzing Ontario healthcare administrative data for adult survivors of childhood cancer diagnosed between 1986 and 2014 who faced an elevated risk for therapy-related colorectal cancer, breast cancer, or cardiomyopathy. The research team then assessed long-term adherence to the North American Children’s Oncology Group guidelines and predictors of adherence.
Among 3241 survivors, 3205 (99%) were at elevated risk for cardiomyopathy, 327 (10%) were at elevated risk for colorectal cancer, and 234 (7%) were at elevated risk for breast cancer. In addition, 2806 (87%) were at risk for one late effect, 345 (11%) were at risk for two late effects, and 90 (3%) were at risk for three late effects.
Overall, 53%, 13%, and 6% were adherent to their recommended surveillance for cardiomyopathy, colorectal cancer, and breast cancer, respectively. Over time, adherence increased for colorectal cancer and cardiomyopathy but decreased for breast cancer.
In addition, patients who were older at diagnosis were more likely to follow screening guidelines for colorectal and breast cancers, whereas those who were younger at diagnosis were more likely to follow screening guidelines for cardiomyopathy.
During a median follow-up of 7.8 years, the proportion of time spent adherent was 43% for cardiomyopathy, 14% for colorectal cancer, and 10% for breast cancer.
Survivors who attended a long-term follow-up clinic in the previous year had low adherence rates as well, though they were higher than in the rest of the cohort. In this group, the proportion of time that was spent adherent was 71% for cardiomyopathy, 27% for colorectal cancer, and 15% for breast cancer.
Shuldiner and colleagues are launching a research trial to determine whether a provincial support system can help childhood cancer survivors receive the recommended surveillance. The support system provides information about screening recommendations to survivors as well as reminders and sends key information to their family doctors.
“We now understand that childhood cancer survivors need help to complete the recommended tests,” said Dr. Shuldiner. “If the trial is successful, we hope it will be implemented in Ontario.”
Survivorship Care Plans
Low screening rates may result from a lack of awareness about screening recommendations and the negative long-term effects of cancer treatments, the study authors wrote. Cancer survivors, caregivers, family physicians, specialists, and survivor support groups can share the responsibility of spreading awareness and adhering to guidelines, they noted. In some cases, a survivorship care plan (SCP) may help.
“SCPs are intended to improve adherence by providing follow-up information and facilitating the transition from cancer treatment to survivorship and from pediatric to adult care,” Adam Yan, MD, a staff oncologist and oncology informatics lead at the Hospital for Sick Children in Toronto, told this news organization.
Dr. Yan, who wasn’t involved with this study, has researched surveillance adherence for secondary cancers and cardiac dysfunction among childhood cancer survivors. He and his colleagues found that screening rates were typically low among survivors who faced high risks for cardiac dysfunction and breast, colorectal, or skin cancers.
However, having a survivorship care plan seemed to help, and survivors treated after 1990 were more likely to have an SCP.
“SCP possession by high-risk survivors was associated with increased breast, skin, and cardiac surveillance,” he said. “It is uncertain whether SCP possession leads to adherence or whether SCP possession is a marker of survivors who are focused on their health and thus likely to adhere to preventive health practices, including surveillance.”
The study was funded by the Canadian Institutes of Health Research and ICES, which receives support from the Ontario Ministry of Health and the Ministry of Long-Term Care. Dr. Shuldiner received a Canadian Institutes of Health Research Health System Impact Postdoctoral Fellowship in support of the work. Dr. Yan disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Among childhood cancer survivors in Ontario, Canada, who faced an elevated risk due to chemotherapy or radiation treatments, 53% followed screening recommendations for cardiomyopathy, 13% met colorectal cancer screening guidelines, and 6% adhered to breast cancer screening guidelines.
“Although over 80% of children newly diagnosed with cancer will become long-term survivors, as many as four out of five of these survivors will develop a serious or life-threatening late effect of their cancer therapy by age 45,” lead author Jennifer Shuldiner, PhD, MPH, a scientist at Women’s College Hospital Institute for Health Systems Solutions and Virtual Care in Toronto, told this news organization.
For instance, the risk for colorectal cancer in childhood cancer survivors is two to three times higher than it is among the general population, and the risk for breast cancer is similar between those who underwent chest radiation and those with a BRCA mutation. As many as 50% of those who received anthracycline chemotherapy or radiation involving the heart later develop cardiotoxicity.
The North American Children’s Oncology Group has published long-term follow-up guidelines for survivors of childhood cancer, yet many survivors don’t follow them because of lack of awareness or other barriers, said Dr. Shuldiner.
“Prior research has shown that many survivors do not complete these recommended tests,” she said. “With better knowledge of this at-risk population, we can design, test, and implement appropriate interventions and supports to tackle the issues.”
The study was published online on March 11 in CMAJ.
Changes in Adherence
The researchers conducted a retrospective population-based cohort study analyzing Ontario healthcare administrative data for adult survivors of childhood cancer diagnosed between 1986 and 2014 who faced an elevated risk for therapy-related colorectal cancer, breast cancer, or cardiomyopathy. The research team then assessed long-term adherence to the North American Children’s Oncology Group guidelines and predictors of adherence.
Among 3241 survivors, 3205 (99%) were at elevated risk for cardiomyopathy, 327 (10%) were at elevated risk for colorectal cancer, and 234 (7%) were at elevated risk for breast cancer. In addition, 2806 (87%) were at risk for one late effect, 345 (11%) were at risk for two late effects, and 90 (3%) were at risk for three late effects.
Overall, 53%, 13%, and 6% were adherent to their recommended surveillance for cardiomyopathy, colorectal cancer, and breast cancer, respectively. Over time, adherence increased for colorectal cancer and cardiomyopathy but decreased for breast cancer.
In addition, patients who were older at diagnosis were more likely to follow screening guidelines for colorectal and breast cancers, whereas those who were younger at diagnosis were more likely to follow screening guidelines for cardiomyopathy.
During a median follow-up of 7.8 years, the proportion of time spent adherent was 43% for cardiomyopathy, 14% for colorectal cancer, and 10% for breast cancer.
Survivors who attended a long-term follow-up clinic in the previous year had low adherence rates as well, though they were higher than in the rest of the cohort. In this group, the proportion of time that was spent adherent was 71% for cardiomyopathy, 27% for colorectal cancer, and 15% for breast cancer.
Shuldiner and colleagues are launching a research trial to determine whether a provincial support system can help childhood cancer survivors receive the recommended surveillance. The support system provides information about screening recommendations to survivors as well as reminders and sends key information to their family doctors.
“We now understand that childhood cancer survivors need help to complete the recommended tests,” said Dr. Shuldiner. “If the trial is successful, we hope it will be implemented in Ontario.”
Survivorship Care Plans
Low screening rates may result from a lack of awareness about screening recommendations and the negative long-term effects of cancer treatments, the study authors wrote. Cancer survivors, caregivers, family physicians, specialists, and survivor support groups can share the responsibility of spreading awareness and adhering to guidelines, they noted. In some cases, a survivorship care plan (SCP) may help.
“SCPs are intended to improve adherence by providing follow-up information and facilitating the transition from cancer treatment to survivorship and from pediatric to adult care,” Adam Yan, MD, a staff oncologist and oncology informatics lead at the Hospital for Sick Children in Toronto, told this news organization.
Dr. Yan, who wasn’t involved with this study, has researched surveillance adherence for secondary cancers and cardiac dysfunction among childhood cancer survivors. He and his colleagues found that screening rates were typically low among survivors who faced high risks for cardiac dysfunction and breast, colorectal, or skin cancers.
However, having a survivorship care plan seemed to help, and survivors treated after 1990 were more likely to have an SCP.
“SCP possession by high-risk survivors was associated with increased breast, skin, and cardiac surveillance,” he said. “It is uncertain whether SCP possession leads to adherence or whether SCP possession is a marker of survivors who are focused on their health and thus likely to adhere to preventive health practices, including surveillance.”
The study was funded by the Canadian Institutes of Health Research and ICES, which receives support from the Ontario Ministry of Health and the Ministry of Long-Term Care. Dr. Shuldiner received a Canadian Institutes of Health Research Health System Impact Postdoctoral Fellowship in support of the work. Dr. Yan disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
VA to Expand Cancer Prevention Services
The US Department of Veterans Affairs (VA) announced plans to expand preventive services, health care, and benefits for veterans with cancer.
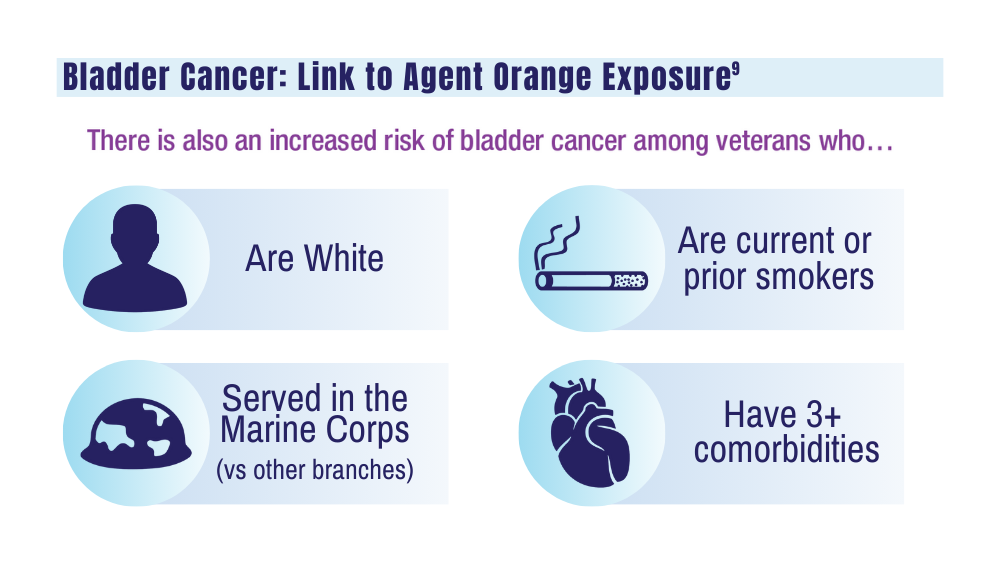
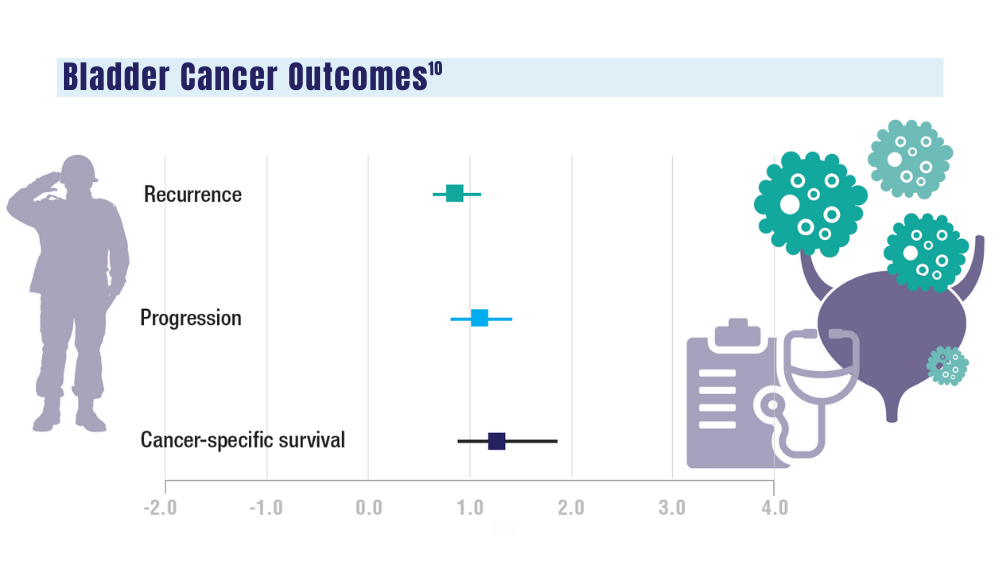
Urethral cancers are set to be added to the list of > 300 conditions considered presumptive under the Sergeant First Class Heath Robinson Honoring our Promise to Address Comprehensive Toxics (PACT) Act of 2022. Veterans deployed to Iraq, Afghanistan, Somalia, Djibouti, Egypt, Jordan, Lebanon, Syria, Yemen, Uzbekistan, and the entire Southwest Asia theater will not need to prove their service caused their urethral cancer in order to receive treatment for it. Additionally, the VA plans to evaluate whether there is a relationship between urinary bladder and ureteral cancers and toxic exposures for these veterans, and determine whether these conditions are presumptive. The VA has already screened > 5 million veterans for toxic exposures under the PACT Act, as part of an ongoing mission to expand cancer care services.
The VA is also set to expand access to screening programs in 2024 by providing:
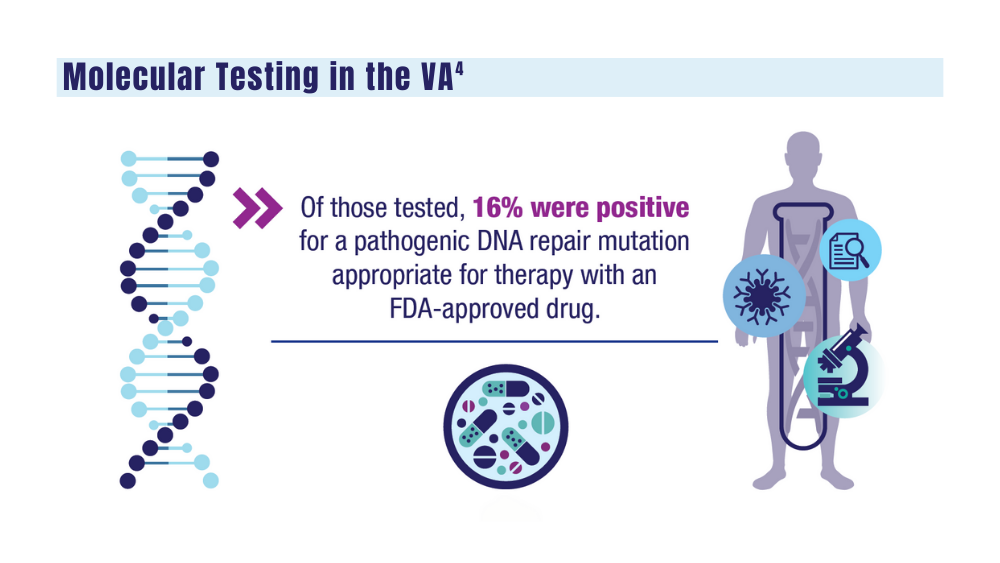
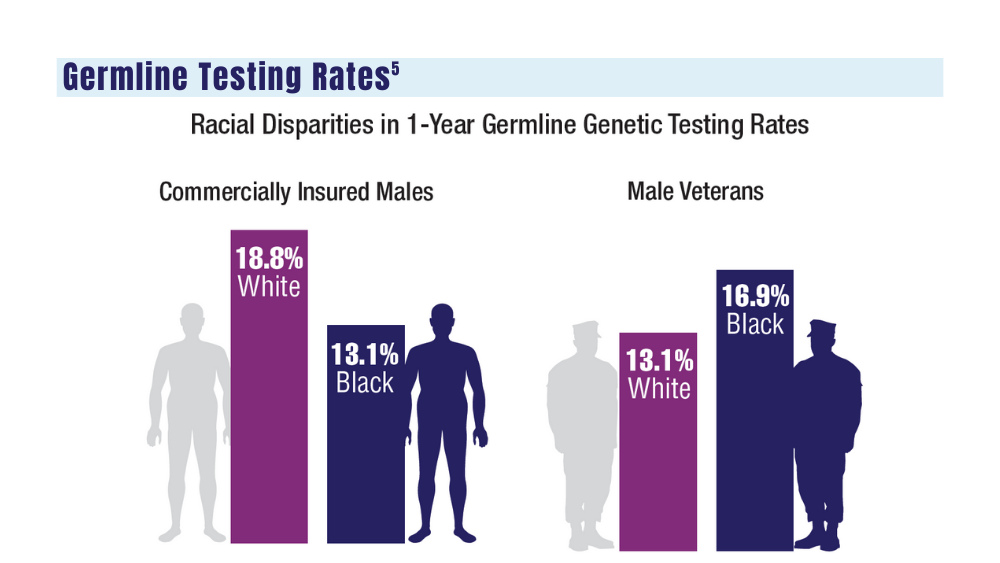
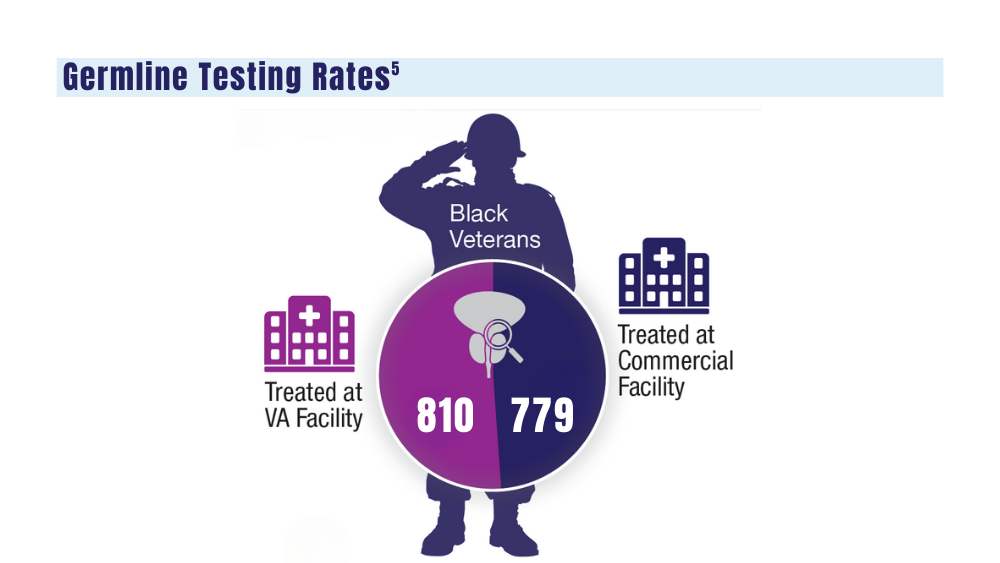
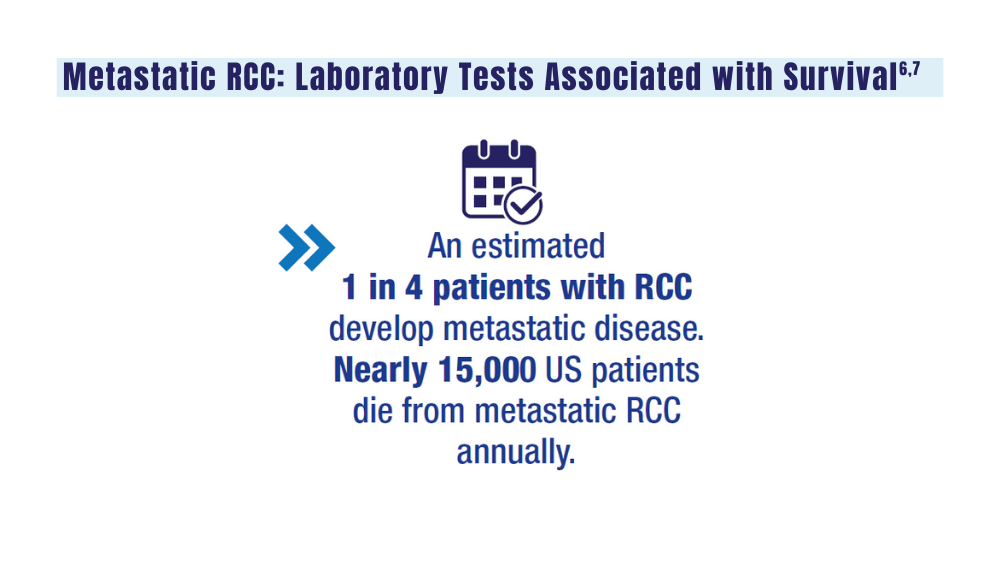
- genetic testing to every veteran who may need it;
- lung cancer screening programs to every VA medical center; and
- home tests for colorectal cancer to > 1 million veterans nationwide.
The VA continues to expand the reach of smoking cessation services, with ≥ 6 additional sites added to the Quit VET eReferral program by the end of 2024, and a new pilot program to integrate smoking cessation services into lung cancer screening.
The VA has already taken steps to build on the Biden-Harris Administration Cancer Moonshot program, which has the goals of preventing ≥ 4 million cancer deaths by 2047 and to improve the experience of individuals with cancer. For instance, it has prioritized claims processing for veterans with cancer and expanded cancer risk assessments and mammograms to veterans aged < 40 years, regardless of age, symptoms, family history, or whether they are enrolled in VA health care. In September, the VA and the National Cancer Institute announced a data-sharing collaboration to better understand and treat cancer among veterans.
“VA is planting the seeds for the future of cancer care,” said VHA Under Secretary for Health Shereef Elnahal, MD. “By investing in screenings, expanding access, and embracing cutting-edge technologies, VA is revolutionizing cancer care delivery, providing the best care possible to our nation’s heroes.”
The US Department of Veterans Affairs (VA) announced plans to expand preventive services, health care, and benefits for veterans with cancer.
Urethral cancers are set to be added to the list of > 300 conditions considered presumptive under the Sergeant First Class Heath Robinson Honoring our Promise to Address Comprehensive Toxics (PACT) Act of 2022. Veterans deployed to Iraq, Afghanistan, Somalia, Djibouti, Egypt, Jordan, Lebanon, Syria, Yemen, Uzbekistan, and the entire Southwest Asia theater will not need to prove their service caused their urethral cancer in order to receive treatment for it. Additionally, the VA plans to evaluate whether there is a relationship between urinary bladder and ureteral cancers and toxic exposures for these veterans, and determine whether these conditions are presumptive. The VA has already screened > 5 million veterans for toxic exposures under the PACT Act, as part of an ongoing mission to expand cancer care services.
The VA is also set to expand access to screening programs in 2024 by providing:
- genetic testing to every veteran who may need it;
- lung cancer screening programs to every VA medical center; and
- home tests for colorectal cancer to > 1 million veterans nationwide.
The VA continues to expand the reach of smoking cessation services, with ≥ 6 additional sites added to the Quit VET eReferral program by the end of 2024, and a new pilot program to integrate smoking cessation services into lung cancer screening.
The VA has already taken steps to build on the Biden-Harris Administration Cancer Moonshot program, which has the goals of preventing ≥ 4 million cancer deaths by 2047 and to improve the experience of individuals with cancer. For instance, it has prioritized claims processing for veterans with cancer and expanded cancer risk assessments and mammograms to veterans aged < 40 years, regardless of age, symptoms, family history, or whether they are enrolled in VA health care. In September, the VA and the National Cancer Institute announced a data-sharing collaboration to better understand and treat cancer among veterans.
“VA is planting the seeds for the future of cancer care,” said VHA Under Secretary for Health Shereef Elnahal, MD. “By investing in screenings, expanding access, and embracing cutting-edge technologies, VA is revolutionizing cancer care delivery, providing the best care possible to our nation’s heroes.”
The US Department of Veterans Affairs (VA) announced plans to expand preventive services, health care, and benefits for veterans with cancer.
Urethral cancers are set to be added to the list of > 300 conditions considered presumptive under the Sergeant First Class Heath Robinson Honoring our Promise to Address Comprehensive Toxics (PACT) Act of 2022. Veterans deployed to Iraq, Afghanistan, Somalia, Djibouti, Egypt, Jordan, Lebanon, Syria, Yemen, Uzbekistan, and the entire Southwest Asia theater will not need to prove their service caused their urethral cancer in order to receive treatment for it. Additionally, the VA plans to evaluate whether there is a relationship between urinary bladder and ureteral cancers and toxic exposures for these veterans, and determine whether these conditions are presumptive. The VA has already screened > 5 million veterans for toxic exposures under the PACT Act, as part of an ongoing mission to expand cancer care services.
The VA is also set to expand access to screening programs in 2024 by providing:
- genetic testing to every veteran who may need it;
- lung cancer screening programs to every VA medical center; and
- home tests for colorectal cancer to > 1 million veterans nationwide.
The VA continues to expand the reach of smoking cessation services, with ≥ 6 additional sites added to the Quit VET eReferral program by the end of 2024, and a new pilot program to integrate smoking cessation services into lung cancer screening.
The VA has already taken steps to build on the Biden-Harris Administration Cancer Moonshot program, which has the goals of preventing ≥ 4 million cancer deaths by 2047 and to improve the experience of individuals with cancer. For instance, it has prioritized claims processing for veterans with cancer and expanded cancer risk assessments and mammograms to veterans aged < 40 years, regardless of age, symptoms, family history, or whether they are enrolled in VA health care. In September, the VA and the National Cancer Institute announced a data-sharing collaboration to better understand and treat cancer among veterans.
“VA is planting the seeds for the future of cancer care,” said VHA Under Secretary for Health Shereef Elnahal, MD. “By investing in screenings, expanding access, and embracing cutting-edge technologies, VA is revolutionizing cancer care delivery, providing the best care possible to our nation’s heroes.”
New Guidelines: Start PSA Screening Earlier in Black Men
Lowering the recommended age for baseline prostate-specific antigen (PSA) would reduce prostate cancer deaths by about 30% in Black men without significantly increasing the rate of overdiagnosis, according to new screening guidelines from the Prostate Cancer Foundation.
Specifically, , a multidisciplinary panel of experts and patient advocates determined based on a comprehensive literature review.
The panel’s findings were presented in a poster at the ASCO Genitourinary Cancers Symposium.
“Black men in the United States are considered a high-risk population for being diagnosed with and dying from prostate cancer,” wrote lead author Isla Garraway, MD, PhD, of the University of California, Los Angeles, and colleagues. Specifically, Black men are about two times more likely to be diagnosed with and die from prostate cancer than White men. But, the authors continued, “few guidelines have outlined specific recommendations for PSA-based prostate cancer screening among Black men.”
The US Preventive Services Task Force recommendations, which are currently being updated, set the PSA screening start age at 55. The task force recommendations, which dictate insurance coverage in the United States, acknowledged “a potential mortality benefit for African American men when beginning screening before age 55 years” but did not explicitly recommend screening earlier.
Current guidelines from the American Cancer Society call for discussions about screening in average-risk men to begin at age 50-55. The recommendations do specify lowering the age to 45 for those at a high risk for prostate cancer, which includes Black men as well as those with a first-degree relative diagnosed with prostate cancer before age 65. In some cases, screening can begin at age 40 in the highest risk men — those with more than one first-degree relative who had prostate cancer at a young age.
The Prostate Cancer Foundation “wanted to address the confusion around different guideline statements and the lack of clarity around screening recommendations for Black men,” said William K. Oh, MD, of The Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, New York City, who chaired the panel for the new guidelines. “We thus convened a distinguished panel of experts from diverse backgrounds and expertise to create six guidelines statements to help Black men, their families, and their healthcare providers to consider options for prostate cancer screening based on the best available evidence.”
After reviewing 287, the expert panel developed six new guideline statements, reaching at least 80% consensus among panel members, addressing screening for Black men:
- Because Black men are at a high risk for prostate cancer, the benefits of screening generally outweigh the risks.
- PSA testing should be considered first line for prostate cancer screening, although some providers may recommend an optional digital rectal exam in addition to the PSA test.
- Black men should engage in shared decision-making with their healthcare providers and other trusted sources of information to learn about the pros and cons of screening.
- For Black men who elect screening, a baseline PSA test should be done between ages 40 and 45, and annual PSA screening should be strongly considered based on the PSA value and the individual’s health status.
- Black men over age 70 who have been undergoing prostate cancer screening should talk with their healthcare provider about whether to continue PSA testing and make an informed decision based on their age, life expectancy, health status, family history, and prior PSA levels.
- Black men who are at even higher risk due to a strong family history and/or known carriers of high-risk genetic variants should consider initiating annual PSA screening as early as age 40.
These statements are based on “the best available evidence, which overwhelmingly supports the conclusion that Black men in the US could benefit from a risk-adapted PSA screening,” the investigators concluded, noting that the latest evidence “warrants revisiting current recommendations for early [prostate cancer] detection in Black men from other national guideline groups.”
“We believe that the outcome of these more directed guidelines will be to give clarity to these men,” added Oh, who is also chief medical officer for the Prostate Cancer Foundation.
This research was funded by the Prostate Cancer Foundation, National Cancer Institute, Veterans Affairs, Jean Perkins Foundation, and Department of Defense. Garraway reported having no disclosures.
A version of this article appeared on Medscape.com.
Lowering the recommended age for baseline prostate-specific antigen (PSA) would reduce prostate cancer deaths by about 30% in Black men without significantly increasing the rate of overdiagnosis, according to new screening guidelines from the Prostate Cancer Foundation.
Specifically, , a multidisciplinary panel of experts and patient advocates determined based on a comprehensive literature review.
The panel’s findings were presented in a poster at the ASCO Genitourinary Cancers Symposium.
“Black men in the United States are considered a high-risk population for being diagnosed with and dying from prostate cancer,” wrote lead author Isla Garraway, MD, PhD, of the University of California, Los Angeles, and colleagues. Specifically, Black men are about two times more likely to be diagnosed with and die from prostate cancer than White men. But, the authors continued, “few guidelines have outlined specific recommendations for PSA-based prostate cancer screening among Black men.”
The US Preventive Services Task Force recommendations, which are currently being updated, set the PSA screening start age at 55. The task force recommendations, which dictate insurance coverage in the United States, acknowledged “a potential mortality benefit for African American men when beginning screening before age 55 years” but did not explicitly recommend screening earlier.
Current guidelines from the American Cancer Society call for discussions about screening in average-risk men to begin at age 50-55. The recommendations do specify lowering the age to 45 for those at a high risk for prostate cancer, which includes Black men as well as those with a first-degree relative diagnosed with prostate cancer before age 65. In some cases, screening can begin at age 40 in the highest risk men — those with more than one first-degree relative who had prostate cancer at a young age.
The Prostate Cancer Foundation “wanted to address the confusion around different guideline statements and the lack of clarity around screening recommendations for Black men,” said William K. Oh, MD, of The Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, New York City, who chaired the panel for the new guidelines. “We thus convened a distinguished panel of experts from diverse backgrounds and expertise to create six guidelines statements to help Black men, their families, and their healthcare providers to consider options for prostate cancer screening based on the best available evidence.”
After reviewing 287, the expert panel developed six new guideline statements, reaching at least 80% consensus among panel members, addressing screening for Black men:
- Because Black men are at a high risk for prostate cancer, the benefits of screening generally outweigh the risks.
- PSA testing should be considered first line for prostate cancer screening, although some providers may recommend an optional digital rectal exam in addition to the PSA test.
- Black men should engage in shared decision-making with their healthcare providers and other trusted sources of information to learn about the pros and cons of screening.
- For Black men who elect screening, a baseline PSA test should be done between ages 40 and 45, and annual PSA screening should be strongly considered based on the PSA value and the individual’s health status.
- Black men over age 70 who have been undergoing prostate cancer screening should talk with their healthcare provider about whether to continue PSA testing and make an informed decision based on their age, life expectancy, health status, family history, and prior PSA levels.
- Black men who are at even higher risk due to a strong family history and/or known carriers of high-risk genetic variants should consider initiating annual PSA screening as early as age 40.
These statements are based on “the best available evidence, which overwhelmingly supports the conclusion that Black men in the US could benefit from a risk-adapted PSA screening,” the investigators concluded, noting that the latest evidence “warrants revisiting current recommendations for early [prostate cancer] detection in Black men from other national guideline groups.”
“We believe that the outcome of these more directed guidelines will be to give clarity to these men,” added Oh, who is also chief medical officer for the Prostate Cancer Foundation.
This research was funded by the Prostate Cancer Foundation, National Cancer Institute, Veterans Affairs, Jean Perkins Foundation, and Department of Defense. Garraway reported having no disclosures.
A version of this article appeared on Medscape.com.
Lowering the recommended age for baseline prostate-specific antigen (PSA) would reduce prostate cancer deaths by about 30% in Black men without significantly increasing the rate of overdiagnosis, according to new screening guidelines from the Prostate Cancer Foundation.
Specifically, , a multidisciplinary panel of experts and patient advocates determined based on a comprehensive literature review.
The panel’s findings were presented in a poster at the ASCO Genitourinary Cancers Symposium.
“Black men in the United States are considered a high-risk population for being diagnosed with and dying from prostate cancer,” wrote lead author Isla Garraway, MD, PhD, of the University of California, Los Angeles, and colleagues. Specifically, Black men are about two times more likely to be diagnosed with and die from prostate cancer than White men. But, the authors continued, “few guidelines have outlined specific recommendations for PSA-based prostate cancer screening among Black men.”
The US Preventive Services Task Force recommendations, which are currently being updated, set the PSA screening start age at 55. The task force recommendations, which dictate insurance coverage in the United States, acknowledged “a potential mortality benefit for African American men when beginning screening before age 55 years” but did not explicitly recommend screening earlier.
Current guidelines from the American Cancer Society call for discussions about screening in average-risk men to begin at age 50-55. The recommendations do specify lowering the age to 45 for those at a high risk for prostate cancer, which includes Black men as well as those with a first-degree relative diagnosed with prostate cancer before age 65. In some cases, screening can begin at age 40 in the highest risk men — those with more than one first-degree relative who had prostate cancer at a young age.
The Prostate Cancer Foundation “wanted to address the confusion around different guideline statements and the lack of clarity around screening recommendations for Black men,” said William K. Oh, MD, of The Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, New York City, who chaired the panel for the new guidelines. “We thus convened a distinguished panel of experts from diverse backgrounds and expertise to create six guidelines statements to help Black men, their families, and their healthcare providers to consider options for prostate cancer screening based on the best available evidence.”
After reviewing 287, the expert panel developed six new guideline statements, reaching at least 80% consensus among panel members, addressing screening for Black men:
- Because Black men are at a high risk for prostate cancer, the benefits of screening generally outweigh the risks.
- PSA testing should be considered first line for prostate cancer screening, although some providers may recommend an optional digital rectal exam in addition to the PSA test.
- Black men should engage in shared decision-making with their healthcare providers and other trusted sources of information to learn about the pros and cons of screening.
- For Black men who elect screening, a baseline PSA test should be done between ages 40 and 45, and annual PSA screening should be strongly considered based on the PSA value and the individual’s health status.
- Black men over age 70 who have been undergoing prostate cancer screening should talk with their healthcare provider about whether to continue PSA testing and make an informed decision based on their age, life expectancy, health status, family history, and prior PSA levels.
- Black men who are at even higher risk due to a strong family history and/or known carriers of high-risk genetic variants should consider initiating annual PSA screening as early as age 40.
These statements are based on “the best available evidence, which overwhelmingly supports the conclusion that Black men in the US could benefit from a risk-adapted PSA screening,” the investigators concluded, noting that the latest evidence “warrants revisiting current recommendations for early [prostate cancer] detection in Black men from other national guideline groups.”
“We believe that the outcome of these more directed guidelines will be to give clarity to these men,” added Oh, who is also chief medical officer for the Prostate Cancer Foundation.
This research was funded by the Prostate Cancer Foundation, National Cancer Institute, Veterans Affairs, Jean Perkins Foundation, and Department of Defense. Garraway reported having no disclosures.
A version of this article appeared on Medscape.com.
FROM ASCO GU 2024
Most Cancer Trial Centers Located Closer to White, Affluent Populations
This inequity may be potentiating the underrepresentation of racially minoritized and socioeconomically disadvantaged populations in clinical trials, suggesting that employment of satellite hospitals is needed to expand access to investigational therapies, reported lead author Hassal Lee, MD, PhD, of Cold Spring Harbor Laboratory, Cold Spring Harbor, New York, and colleagues.
“Minoritized and socioeconomically disadvantaged populations are underrepresented in clinical trials,” the investigators wrote in JAMA Oncology. “This may reduce the generalizability of trial results and propagate health disparities. Contributors to inequitable trial participation include individual-level factors and structural factors.”
Specifically, travel time to trial centers, as well as socioeconomic deprivation, can reduce likelihood of trial participation.
“Data on these parameters and population data on self-identified race exist, but their interrelation with clinical research facilities has not been systematically analyzed,” they wrote.
To try to draw comparisons between the distribution of patients of different races and socioeconomic statuses and the locations of clinical research facilities, Dr. Lee and colleagues aggregated data from the US Census, National Trial registry, Nature Index of Cancer Research Health Institutions, OpenStreetMap, National Cancer Institute–designated Cancer Centers list, and National Homeland Infrastructure Foundation. They then characterized catchment population demographics within 30-, 60-, and 120-minute driving commute times of all US hospitals, along with a more focused look at centers capable of conducting phase 1, phase 2, and phase 3 trials.
These efforts revealed broad geographic inequity.The 78 major centers that conduct 94% of all US cancer trials are located within 30 minutes of populations that have a 10.1% higher proportion of self-identified White individuals than the average US county, and a median income $18,900 higher than average (unpaired mean differences).
The publication also includes several maps characterizing racial and socioeconomic demographics within various catchment areas. For example, centers in New York City, Houston, and Chicago have the most diverse catchment populations within a 30-minute commute. Maps of all cities in the United States with populations greater than 500,000 are available in a supplementary index.
“This study indicates that geographical population distributions may present barriers to equitable clinical trial access and that data are available to proactively strategize about reduction of such barriers,” Dr. Lee and colleagues wrote.
The findings call attention to modifiable socioeconomic factors associated with trial participation, they added, like financial toxicity and affordable transportation, noting that ethnic and racial groups consent to trials at similar rates after controlling for income.
In addition, Dr. Lee and colleagues advised clinical trial designers to enlist satellite hospitals to increase participant diversity, since long commutes exacerbate “socioeconomic burdens associated with clinical trial participation,” with trial participation decreasing as commute time increases.
“Existing clinical trial centers may build collaborative efforts with nearby hospitals closer to underrepresented populations or set up community centers to support new collaborative networks to improve geographical access equity,” they wrote. “Methodologically, our approach is transferable to any country, region, or global effort with sufficient source data and can inform decision-making along the continuum of cancer care, from screening to implementing specialist care.”
A coauthor disclosed relationships with Flagship Therapeutics, Leidos Holding Ltd, Pershing Square Foundation, and others.
This inequity may be potentiating the underrepresentation of racially minoritized and socioeconomically disadvantaged populations in clinical trials, suggesting that employment of satellite hospitals is needed to expand access to investigational therapies, reported lead author Hassal Lee, MD, PhD, of Cold Spring Harbor Laboratory, Cold Spring Harbor, New York, and colleagues.
“Minoritized and socioeconomically disadvantaged populations are underrepresented in clinical trials,” the investigators wrote in JAMA Oncology. “This may reduce the generalizability of trial results and propagate health disparities. Contributors to inequitable trial participation include individual-level factors and structural factors.”
Specifically, travel time to trial centers, as well as socioeconomic deprivation, can reduce likelihood of trial participation.
“Data on these parameters and population data on self-identified race exist, but their interrelation with clinical research facilities has not been systematically analyzed,” they wrote.
To try to draw comparisons between the distribution of patients of different races and socioeconomic statuses and the locations of clinical research facilities, Dr. Lee and colleagues aggregated data from the US Census, National Trial registry, Nature Index of Cancer Research Health Institutions, OpenStreetMap, National Cancer Institute–designated Cancer Centers list, and National Homeland Infrastructure Foundation. They then characterized catchment population demographics within 30-, 60-, and 120-minute driving commute times of all US hospitals, along with a more focused look at centers capable of conducting phase 1, phase 2, and phase 3 trials.
These efforts revealed broad geographic inequity.The 78 major centers that conduct 94% of all US cancer trials are located within 30 minutes of populations that have a 10.1% higher proportion of self-identified White individuals than the average US county, and a median income $18,900 higher than average (unpaired mean differences).
The publication also includes several maps characterizing racial and socioeconomic demographics within various catchment areas. For example, centers in New York City, Houston, and Chicago have the most diverse catchment populations within a 30-minute commute. Maps of all cities in the United States with populations greater than 500,000 are available in a supplementary index.
“This study indicates that geographical population distributions may present barriers to equitable clinical trial access and that data are available to proactively strategize about reduction of such barriers,” Dr. Lee and colleagues wrote.
The findings call attention to modifiable socioeconomic factors associated with trial participation, they added, like financial toxicity and affordable transportation, noting that ethnic and racial groups consent to trials at similar rates after controlling for income.
In addition, Dr. Lee and colleagues advised clinical trial designers to enlist satellite hospitals to increase participant diversity, since long commutes exacerbate “socioeconomic burdens associated with clinical trial participation,” with trial participation decreasing as commute time increases.
“Existing clinical trial centers may build collaborative efforts with nearby hospitals closer to underrepresented populations or set up community centers to support new collaborative networks to improve geographical access equity,” they wrote. “Methodologically, our approach is transferable to any country, region, or global effort with sufficient source data and can inform decision-making along the continuum of cancer care, from screening to implementing specialist care.”
A coauthor disclosed relationships with Flagship Therapeutics, Leidos Holding Ltd, Pershing Square Foundation, and others.
This inequity may be potentiating the underrepresentation of racially minoritized and socioeconomically disadvantaged populations in clinical trials, suggesting that employment of satellite hospitals is needed to expand access to investigational therapies, reported lead author Hassal Lee, MD, PhD, of Cold Spring Harbor Laboratory, Cold Spring Harbor, New York, and colleagues.
“Minoritized and socioeconomically disadvantaged populations are underrepresented in clinical trials,” the investigators wrote in JAMA Oncology. “This may reduce the generalizability of trial results and propagate health disparities. Contributors to inequitable trial participation include individual-level factors and structural factors.”
Specifically, travel time to trial centers, as well as socioeconomic deprivation, can reduce likelihood of trial participation.
“Data on these parameters and population data on self-identified race exist, but their interrelation with clinical research facilities has not been systematically analyzed,” they wrote.
To try to draw comparisons between the distribution of patients of different races and socioeconomic statuses and the locations of clinical research facilities, Dr. Lee and colleagues aggregated data from the US Census, National Trial registry, Nature Index of Cancer Research Health Institutions, OpenStreetMap, National Cancer Institute–designated Cancer Centers list, and National Homeland Infrastructure Foundation. They then characterized catchment population demographics within 30-, 60-, and 120-minute driving commute times of all US hospitals, along with a more focused look at centers capable of conducting phase 1, phase 2, and phase 3 trials.
These efforts revealed broad geographic inequity.The 78 major centers that conduct 94% of all US cancer trials are located within 30 minutes of populations that have a 10.1% higher proportion of self-identified White individuals than the average US county, and a median income $18,900 higher than average (unpaired mean differences).
The publication also includes several maps characterizing racial and socioeconomic demographics within various catchment areas. For example, centers in New York City, Houston, and Chicago have the most diverse catchment populations within a 30-minute commute. Maps of all cities in the United States with populations greater than 500,000 are available in a supplementary index.
“This study indicates that geographical population distributions may present barriers to equitable clinical trial access and that data are available to proactively strategize about reduction of such barriers,” Dr. Lee and colleagues wrote.
The findings call attention to modifiable socioeconomic factors associated with trial participation, they added, like financial toxicity and affordable transportation, noting that ethnic and racial groups consent to trials at similar rates after controlling for income.
In addition, Dr. Lee and colleagues advised clinical trial designers to enlist satellite hospitals to increase participant diversity, since long commutes exacerbate “socioeconomic burdens associated with clinical trial participation,” with trial participation decreasing as commute time increases.
“Existing clinical trial centers may build collaborative efforts with nearby hospitals closer to underrepresented populations or set up community centers to support new collaborative networks to improve geographical access equity,” they wrote. “Methodologically, our approach is transferable to any country, region, or global effort with sufficient source data and can inform decision-making along the continuum of cancer care, from screening to implementing specialist care.”
A coauthor disclosed relationships with Flagship Therapeutics, Leidos Holding Ltd, Pershing Square Foundation, and others.
FROM JAMA ONCOLOGY
New Drug Approvals Are the Wrong Metric for Cancer Policy
How should we define success in cancer policy — what should the endpoint be?
It’s debatable. Is it fewer cancer deaths? Perhaps improved access to therapies or a reduction in disparities?
One thing I know with certainty: The number of new cancer drugs approved by the US Food and Drug Administration (FDA) is not and should not be our primary endpoint in and of itself.
I’ll go a step further: It is not even a surrogate marker for success.
Unfortunately, a new drug approval does not necessarily mean improved patient outcomes. In fact, the majority of cancer drugs approved these days improve neither survival nor quality of life. Our previous work has shown better mortality outcomes in other high-income countries that have not approved or do not fund several cancer drugs that the FDA has approved.
Even if a drug has a meaningful benefit, at an average cost of more than $250,000 per year, if a new drug cannot reach patients because of access or cost issues, it’s meaningless.
However, regulators and media celebrate the number (and speed) of drug approvals every year as if it were a marker of success in and of itself. But approving more drugs should not be the goal; improving outcomes should. The FDA’s current approach is akin to a university celebrating its graduation rate by lowering the requirements to pass.
When US patients lack access to cisplatin and carboplatin, any talk of a Moonshot or precision medicine ‘ending cancer as we know it’ is premature and even embarrassing.
This is exactly what the FDA has been doing with our regulatory standards for drug approval. They have gradually lowered the requirements for approval from two randomized trials to one randomized trial, then further to one randomized trial with a surrogate endpoint. In many instances, they have gone even further, demanding merely single-arm trials. They’ve also gone from requiring overall survival benefits to celebrating nondetrimental effects on overall survival. It’s no wonder that we approve more drugs today than we did in the past — the bar for approval is pretty low nowadays.
In 2019, our lab found an interesting phenomenon: The number of approvals based on surrogate endpoints has been increasing while the number of accelerated approvals has been decreasing. This made no sense at first, because you’d think surrogate-based approvals and accelerated approvals would be collinear. However, we realized that the recent approvals based on surrogate endpoints were regular approvals instead of accelerated approvals, which explained the phenomenon. Not only is the FDA approving more drugs on the basis of lower levels of evidence, but the agency is also offering regular instead of accelerated approval, thereby removing the safety net of a confirmatory trial.
Nearly everybody sees this as a cause for celebration. Pharma celebrates record profits, regulators celebrate record numbers of drug approvals, insurance companies celebrate because they can pass these costs on as insurance premiums and make even more money, and physicians and patients celebrate access to the shiniest, sexiest new cancer drug.
Everybody is happy in this system. The only problem is that patient outcomes don’t improve, resources are taken away from other priorities, and society suffers a net harm.
When you contrast this celebration with the reality on the ground, the difference is stark and sobering. In our clinics, patients lack access to even old chemotherapeutic drugs that are already generic and cheap but make a meaningful difference in patient outcomes. Citing a current lack of incentives, several generic cancer drug manufacturers have stopped making these drugs; the US supply now relies heavily on importing them from emerging economies such as India. When US patients lack access to cisplatin and carboplatin, any talk of a Moonshot or precision medicine “ending cancer as we know it” is premature and even embarrassing.
5-Fluorouracil, methotrexate, and the platinums are backbones of cancer treatment. Cisplatin and carboplatin are not drugs we use with the hope of improving survival by a couple of months; these drugs are the difference between life and death for patients with testicular and ovarian cancers. In a survey of 948 global oncologists, these were considered among the most essential cancer drugs by oncologists in high-income and low- and middle-income countries alike. Although oncologists in low- and middle-income countries sometimes argue that even these cheap generic drugs may be unaffordable to their patients, they usually remain available; access is a function of both availability and affordability. However, the shortage situation in the US is unique in that availability — rather than affordability — is impacting access.
Our profit-over-patients policy has landed us in a terrible paradox.
Generic drugs are cheap, and any industrialized country can manufacture them. This is why so few companies actually do so; the profit margins are low and companies have little incentive to produce them, despite their benefit. Meanwhile, the FDA is approving and offering access to new shiny molecules that cost more than $15,000 per month yet offer less than a month of progression-free survival benefit and no overall survival benefit (see margetuximab in breast cancer). We have a literal fatal attraction to everything new and shiny.
This is a clear misalignment of priorities in US cancer drug policy. Our profit-over-patients policy has landed us in a terrible paradox: If a drug is cheap and meaningful, it won’t be available, but if it is marginal and expensive, we will do everything to ensure patients can get it. It’s no wonder that patients on Medicaid are disproportionately affected by these drug shortages. Unless all patients have easy access to cisplatin, carboplatin, and 5-fluorouracil, it is frankly embarrassing to celebrate the number of new cancer drugs approved each year.
We all have a responsibility in this — policymakers and lawmakers, regulators and payers, manufacturers and distributors, the American Society of Clinical Oncology and other oncology societies, and physicians and patients. This is where our advocacy work should focus. The primary endpoint of our cancer policy should not be how many new treatments we can approve or how many expensive drugs a rich person with the best insurance can get at a leading cancer center. The true measure of our civilization is how it treats its most vulnerable members.
Dr. Gyawali has disclosed the following relevant financial relationship: Received consulting fees from Vivio Health.
Dr. Gyawali is an associate professor in the Departments of Oncology and Public Health Sciences and a scientist in the Division of Cancer Care and Epidemiology at Queen’s University in Kingston, Ontario, Canada, and is also affiliated faculty at the Program on Regulation, Therapeutics, and Law in the Department of Medicine at Brigham and Women’s Hospital in Boston. His clinical and research interests revolve around cancer policy, global oncology, evidence-based oncology, financial toxicities of cancer treatment, clinical trial methods, and supportive care. He tweets at @oncology_bg.
A version of this article appeared on Medscape.com.
How should we define success in cancer policy — what should the endpoint be?
It’s debatable. Is it fewer cancer deaths? Perhaps improved access to therapies or a reduction in disparities?
One thing I know with certainty: The number of new cancer drugs approved by the US Food and Drug Administration (FDA) is not and should not be our primary endpoint in and of itself.
I’ll go a step further: It is not even a surrogate marker for success.
Unfortunately, a new drug approval does not necessarily mean improved patient outcomes. In fact, the majority of cancer drugs approved these days improve neither survival nor quality of life. Our previous work has shown better mortality outcomes in other high-income countries that have not approved or do not fund several cancer drugs that the FDA has approved.
Even if a drug has a meaningful benefit, at an average cost of more than $250,000 per year, if a new drug cannot reach patients because of access or cost issues, it’s meaningless.
However, regulators and media celebrate the number (and speed) of drug approvals every year as if it were a marker of success in and of itself. But approving more drugs should not be the goal; improving outcomes should. The FDA’s current approach is akin to a university celebrating its graduation rate by lowering the requirements to pass.
When US patients lack access to cisplatin and carboplatin, any talk of a Moonshot or precision medicine ‘ending cancer as we know it’ is premature and even embarrassing.
This is exactly what the FDA has been doing with our regulatory standards for drug approval. They have gradually lowered the requirements for approval from two randomized trials to one randomized trial, then further to one randomized trial with a surrogate endpoint. In many instances, they have gone even further, demanding merely single-arm trials. They’ve also gone from requiring overall survival benefits to celebrating nondetrimental effects on overall survival. It’s no wonder that we approve more drugs today than we did in the past — the bar for approval is pretty low nowadays.
In 2019, our lab found an interesting phenomenon: The number of approvals based on surrogate endpoints has been increasing while the number of accelerated approvals has been decreasing. This made no sense at first, because you’d think surrogate-based approvals and accelerated approvals would be collinear. However, we realized that the recent approvals based on surrogate endpoints were regular approvals instead of accelerated approvals, which explained the phenomenon. Not only is the FDA approving more drugs on the basis of lower levels of evidence, but the agency is also offering regular instead of accelerated approval, thereby removing the safety net of a confirmatory trial.
Nearly everybody sees this as a cause for celebration. Pharma celebrates record profits, regulators celebrate record numbers of drug approvals, insurance companies celebrate because they can pass these costs on as insurance premiums and make even more money, and physicians and patients celebrate access to the shiniest, sexiest new cancer drug.
Everybody is happy in this system. The only problem is that patient outcomes don’t improve, resources are taken away from other priorities, and society suffers a net harm.
When you contrast this celebration with the reality on the ground, the difference is stark and sobering. In our clinics, patients lack access to even old chemotherapeutic drugs that are already generic and cheap but make a meaningful difference in patient outcomes. Citing a current lack of incentives, several generic cancer drug manufacturers have stopped making these drugs; the US supply now relies heavily on importing them from emerging economies such as India. When US patients lack access to cisplatin and carboplatin, any talk of a Moonshot or precision medicine “ending cancer as we know it” is premature and even embarrassing.
5-Fluorouracil, methotrexate, and the platinums are backbones of cancer treatment. Cisplatin and carboplatin are not drugs we use with the hope of improving survival by a couple of months; these drugs are the difference between life and death for patients with testicular and ovarian cancers. In a survey of 948 global oncologists, these were considered among the most essential cancer drugs by oncologists in high-income and low- and middle-income countries alike. Although oncologists in low- and middle-income countries sometimes argue that even these cheap generic drugs may be unaffordable to their patients, they usually remain available; access is a function of both availability and affordability. However, the shortage situation in the US is unique in that availability — rather than affordability — is impacting access.
Our profit-over-patients policy has landed us in a terrible paradox.
Generic drugs are cheap, and any industrialized country can manufacture them. This is why so few companies actually do so; the profit margins are low and companies have little incentive to produce them, despite their benefit. Meanwhile, the FDA is approving and offering access to new shiny molecules that cost more than $15,000 per month yet offer less than a month of progression-free survival benefit and no overall survival benefit (see margetuximab in breast cancer). We have a literal fatal attraction to everything new and shiny.
This is a clear misalignment of priorities in US cancer drug policy. Our profit-over-patients policy has landed us in a terrible paradox: If a drug is cheap and meaningful, it won’t be available, but if it is marginal and expensive, we will do everything to ensure patients can get it. It’s no wonder that patients on Medicaid are disproportionately affected by these drug shortages. Unless all patients have easy access to cisplatin, carboplatin, and 5-fluorouracil, it is frankly embarrassing to celebrate the number of new cancer drugs approved each year.
We all have a responsibility in this — policymakers and lawmakers, regulators and payers, manufacturers and distributors, the American Society of Clinical Oncology and other oncology societies, and physicians and patients. This is where our advocacy work should focus. The primary endpoint of our cancer policy should not be how many new treatments we can approve or how many expensive drugs a rich person with the best insurance can get at a leading cancer center. The true measure of our civilization is how it treats its most vulnerable members.
Dr. Gyawali has disclosed the following relevant financial relationship: Received consulting fees from Vivio Health.
Dr. Gyawali is an associate professor in the Departments of Oncology and Public Health Sciences and a scientist in the Division of Cancer Care and Epidemiology at Queen’s University in Kingston, Ontario, Canada, and is also affiliated faculty at the Program on Regulation, Therapeutics, and Law in the Department of Medicine at Brigham and Women’s Hospital in Boston. His clinical and research interests revolve around cancer policy, global oncology, evidence-based oncology, financial toxicities of cancer treatment, clinical trial methods, and supportive care. He tweets at @oncology_bg.
A version of this article appeared on Medscape.com.
How should we define success in cancer policy — what should the endpoint be?
It’s debatable. Is it fewer cancer deaths? Perhaps improved access to therapies or a reduction in disparities?
One thing I know with certainty: The number of new cancer drugs approved by the US Food and Drug Administration (FDA) is not and should not be our primary endpoint in and of itself.
I’ll go a step further: It is not even a surrogate marker for success.
Unfortunately, a new drug approval does not necessarily mean improved patient outcomes. In fact, the majority of cancer drugs approved these days improve neither survival nor quality of life. Our previous work has shown better mortality outcomes in other high-income countries that have not approved or do not fund several cancer drugs that the FDA has approved.
Even if a drug has a meaningful benefit, at an average cost of more than $250,000 per year, if a new drug cannot reach patients because of access or cost issues, it’s meaningless.
However, regulators and media celebrate the number (and speed) of drug approvals every year as if it were a marker of success in and of itself. But approving more drugs should not be the goal; improving outcomes should. The FDA’s current approach is akin to a university celebrating its graduation rate by lowering the requirements to pass.
When US patients lack access to cisplatin and carboplatin, any talk of a Moonshot or precision medicine ‘ending cancer as we know it’ is premature and even embarrassing.
This is exactly what the FDA has been doing with our regulatory standards for drug approval. They have gradually lowered the requirements for approval from two randomized trials to one randomized trial, then further to one randomized trial with a surrogate endpoint. In many instances, they have gone even further, demanding merely single-arm trials. They’ve also gone from requiring overall survival benefits to celebrating nondetrimental effects on overall survival. It’s no wonder that we approve more drugs today than we did in the past — the bar for approval is pretty low nowadays.
In 2019, our lab found an interesting phenomenon: The number of approvals based on surrogate endpoints has been increasing while the number of accelerated approvals has been decreasing. This made no sense at first, because you’d think surrogate-based approvals and accelerated approvals would be collinear. However, we realized that the recent approvals based on surrogate endpoints were regular approvals instead of accelerated approvals, which explained the phenomenon. Not only is the FDA approving more drugs on the basis of lower levels of evidence, but the agency is also offering regular instead of accelerated approval, thereby removing the safety net of a confirmatory trial.
Nearly everybody sees this as a cause for celebration. Pharma celebrates record profits, regulators celebrate record numbers of drug approvals, insurance companies celebrate because they can pass these costs on as insurance premiums and make even more money, and physicians and patients celebrate access to the shiniest, sexiest new cancer drug.
Everybody is happy in this system. The only problem is that patient outcomes don’t improve, resources are taken away from other priorities, and society suffers a net harm.
When you contrast this celebration with the reality on the ground, the difference is stark and sobering. In our clinics, patients lack access to even old chemotherapeutic drugs that are already generic and cheap but make a meaningful difference in patient outcomes. Citing a current lack of incentives, several generic cancer drug manufacturers have stopped making these drugs; the US supply now relies heavily on importing them from emerging economies such as India. When US patients lack access to cisplatin and carboplatin, any talk of a Moonshot or precision medicine “ending cancer as we know it” is premature and even embarrassing.
5-Fluorouracil, methotrexate, and the platinums are backbones of cancer treatment. Cisplatin and carboplatin are not drugs we use with the hope of improving survival by a couple of months; these drugs are the difference between life and death for patients with testicular and ovarian cancers. In a survey of 948 global oncologists, these were considered among the most essential cancer drugs by oncologists in high-income and low- and middle-income countries alike. Although oncologists in low- and middle-income countries sometimes argue that even these cheap generic drugs may be unaffordable to their patients, they usually remain available; access is a function of both availability and affordability. However, the shortage situation in the US is unique in that availability — rather than affordability — is impacting access.
Our profit-over-patients policy has landed us in a terrible paradox.
Generic drugs are cheap, and any industrialized country can manufacture them. This is why so few companies actually do so; the profit margins are low and companies have little incentive to produce them, despite their benefit. Meanwhile, the FDA is approving and offering access to new shiny molecules that cost more than $15,000 per month yet offer less than a month of progression-free survival benefit and no overall survival benefit (see margetuximab in breast cancer). We have a literal fatal attraction to everything new and shiny.
This is a clear misalignment of priorities in US cancer drug policy. Our profit-over-patients policy has landed us in a terrible paradox: If a drug is cheap and meaningful, it won’t be available, but if it is marginal and expensive, we will do everything to ensure patients can get it. It’s no wonder that patients on Medicaid are disproportionately affected by these drug shortages. Unless all patients have easy access to cisplatin, carboplatin, and 5-fluorouracil, it is frankly embarrassing to celebrate the number of new cancer drugs approved each year.
We all have a responsibility in this — policymakers and lawmakers, regulators and payers, manufacturers and distributors, the American Society of Clinical Oncology and other oncology societies, and physicians and patients. This is where our advocacy work should focus. The primary endpoint of our cancer policy should not be how many new treatments we can approve or how many expensive drugs a rich person with the best insurance can get at a leading cancer center. The true measure of our civilization is how it treats its most vulnerable members.
Dr. Gyawali has disclosed the following relevant financial relationship: Received consulting fees from Vivio Health.
Dr. Gyawali is an associate professor in the Departments of Oncology and Public Health Sciences and a scientist in the Division of Cancer Care and Epidemiology at Queen’s University in Kingston, Ontario, Canada, and is also affiliated faculty at the Program on Regulation, Therapeutics, and Law in the Department of Medicine at Brigham and Women’s Hospital in Boston. His clinical and research interests revolve around cancer policy, global oncology, evidence-based oncology, financial toxicities of cancer treatment, clinical trial methods, and supportive care. He tweets at @oncology_bg.
A version of this article appeared on Medscape.com.
Non-Radical Surgery a Win-Win for Early Cervical Cancer
according to results of the GOG-278 trial.
In fact, patients’ quality of life was improved after surgery in both groups, and their concerns about cancer recurrence decreased, especially for those undergoing simple hysterectomy, said Allan Covens, MD, in his late-breaking abstract presentation at the Society of Gynecologic Oncology (SGO)’s Annual Meeting on Women’s Cancer.
“Cone biopsy patients reported less concerns about reproductive fertility after surgery and over time compared to preop assessments,” he added.
Due to screening in developed countries, a large proportion of cervical cancers are discovered at an early stage. Treatment of these cancers with radical surgery is associated with high cure rates but significant adverse effects on quality of life, said Dr. Covens, who is with the University of Toronto, Toronto, Ontario, Canada.
He and his colleagues wanted to see if non-radical surgery could be safely used instead. “Multiple case series have indicated that non-radical surgery is associated with less morbidity and improved quality of life,” he explained. “If this can be proven in a prospective evaluation, it will change future practice.”
GOG-278 was a prospective cohort study of women with stage IA1 (lymph-vascular space invasion+) and IA2-IB1 (≤ 2 cm) carcinoma of the cervix who underwent non-radical surgery (simple hysterectomy or fertility-preserving cone biopsy) and pelvic lymphadenectomy. Criteria included ≤ 10 mm stromal invasion and negative margins on the final cone biopsy.
The primary objectives were to assess changes in functional outcomes of quality of life (bladder/bowel function, sexual function, cancer worry, and reproductive concerns), using validated instruments. Findings were based on 55 patients who underwent cone biopsy and 113 who underwent simple hysterectomy.
Both simple hysterectomy and cone biopsy were associated with “small” declines in sexual function and bladder/bowel function at 4-6 weeks after surgery, but function “quickly” recovered to baseline by 6 months, Dr. Covens reported.
Twelve patients reported a diagnosis of lymphedema, with a Gynecologic Cancer Lymphedema Questionnaire score change of 4 or higher on at least two consecutive evaluations from baseline. This occurred in six cone biopsy and six simple hysterectomy patients.
In a separate presentation, Dr. Covens reported secondary oncologic outcomes from GOG-278, which suggest that non-radical surgery for early-stage cervical cancer is safe, with low perioperative morbidity, although longer follow-up is needed.
He also reported 16 pregnancies in 15 patients who had undergone cone biopsies; 12 of these were successful, and there were four early pregnancy losses.
‘Impressive’ Data
Study discussant Kristin Bixel, MD, with The Ohio State University, Columbus, Ohio, said the data are “impressive” and clearly show that non-radical surgery has “minimal impact on bladder/bowel function, with no long-term differences from baseline.”
She added that the incidence of lymphedema was “honestly significantly lower than what I typically counsel patients about” and wondered if the percentage of patients with lymphedema would increase over time.
Dr. Bixel particularly noted the decrease in cancer worry scores after surgery, as sometimes patients who have less radical procedures fear that this comes with an increased risk for recurrence.
The “growing body of data suggests that less radical surgery is safe and effective for early-stage low-risk cervical cancer and highlights the potential reproductive success,” she concluded.
Funding for the study was provided by grants from NRG Oncology. Dr. Covens had no disclosures. Dr. Bixel has received research funding from the Intuitive Foundation.
A version of this article appeared on Medscape.com.
according to results of the GOG-278 trial.
In fact, patients’ quality of life was improved after surgery in both groups, and their concerns about cancer recurrence decreased, especially for those undergoing simple hysterectomy, said Allan Covens, MD, in his late-breaking abstract presentation at the Society of Gynecologic Oncology (SGO)’s Annual Meeting on Women’s Cancer.
“Cone biopsy patients reported less concerns about reproductive fertility after surgery and over time compared to preop assessments,” he added.
Due to screening in developed countries, a large proportion of cervical cancers are discovered at an early stage. Treatment of these cancers with radical surgery is associated with high cure rates but significant adverse effects on quality of life, said Dr. Covens, who is with the University of Toronto, Toronto, Ontario, Canada.
He and his colleagues wanted to see if non-radical surgery could be safely used instead. “Multiple case series have indicated that non-radical surgery is associated with less morbidity and improved quality of life,” he explained. “If this can be proven in a prospective evaluation, it will change future practice.”
GOG-278 was a prospective cohort study of women with stage IA1 (lymph-vascular space invasion+) and IA2-IB1 (≤ 2 cm) carcinoma of the cervix who underwent non-radical surgery (simple hysterectomy or fertility-preserving cone biopsy) and pelvic lymphadenectomy. Criteria included ≤ 10 mm stromal invasion and negative margins on the final cone biopsy.
The primary objectives were to assess changes in functional outcomes of quality of life (bladder/bowel function, sexual function, cancer worry, and reproductive concerns), using validated instruments. Findings were based on 55 patients who underwent cone biopsy and 113 who underwent simple hysterectomy.
Both simple hysterectomy and cone biopsy were associated with “small” declines in sexual function and bladder/bowel function at 4-6 weeks after surgery, but function “quickly” recovered to baseline by 6 months, Dr. Covens reported.
Twelve patients reported a diagnosis of lymphedema, with a Gynecologic Cancer Lymphedema Questionnaire score change of 4 or higher on at least two consecutive evaluations from baseline. This occurred in six cone biopsy and six simple hysterectomy patients.
In a separate presentation, Dr. Covens reported secondary oncologic outcomes from GOG-278, which suggest that non-radical surgery for early-stage cervical cancer is safe, with low perioperative morbidity, although longer follow-up is needed.
He also reported 16 pregnancies in 15 patients who had undergone cone biopsies; 12 of these were successful, and there were four early pregnancy losses.
‘Impressive’ Data
Study discussant Kristin Bixel, MD, with The Ohio State University, Columbus, Ohio, said the data are “impressive” and clearly show that non-radical surgery has “minimal impact on bladder/bowel function, with no long-term differences from baseline.”
She added that the incidence of lymphedema was “honestly significantly lower than what I typically counsel patients about” and wondered if the percentage of patients with lymphedema would increase over time.
Dr. Bixel particularly noted the decrease in cancer worry scores after surgery, as sometimes patients who have less radical procedures fear that this comes with an increased risk for recurrence.
The “growing body of data suggests that less radical surgery is safe and effective for early-stage low-risk cervical cancer and highlights the potential reproductive success,” she concluded.
Funding for the study was provided by grants from NRG Oncology. Dr. Covens had no disclosures. Dr. Bixel has received research funding from the Intuitive Foundation.
A version of this article appeared on Medscape.com.
according to results of the GOG-278 trial.
In fact, patients’ quality of life was improved after surgery in both groups, and their concerns about cancer recurrence decreased, especially for those undergoing simple hysterectomy, said Allan Covens, MD, in his late-breaking abstract presentation at the Society of Gynecologic Oncology (SGO)’s Annual Meeting on Women’s Cancer.
“Cone biopsy patients reported less concerns about reproductive fertility after surgery and over time compared to preop assessments,” he added.
Due to screening in developed countries, a large proportion of cervical cancers are discovered at an early stage. Treatment of these cancers with radical surgery is associated with high cure rates but significant adverse effects on quality of life, said Dr. Covens, who is with the University of Toronto, Toronto, Ontario, Canada.
He and his colleagues wanted to see if non-radical surgery could be safely used instead. “Multiple case series have indicated that non-radical surgery is associated with less morbidity and improved quality of life,” he explained. “If this can be proven in a prospective evaluation, it will change future practice.”
GOG-278 was a prospective cohort study of women with stage IA1 (lymph-vascular space invasion+) and IA2-IB1 (≤ 2 cm) carcinoma of the cervix who underwent non-radical surgery (simple hysterectomy or fertility-preserving cone biopsy) and pelvic lymphadenectomy. Criteria included ≤ 10 mm stromal invasion and negative margins on the final cone biopsy.
The primary objectives were to assess changes in functional outcomes of quality of life (bladder/bowel function, sexual function, cancer worry, and reproductive concerns), using validated instruments. Findings were based on 55 patients who underwent cone biopsy and 113 who underwent simple hysterectomy.
Both simple hysterectomy and cone biopsy were associated with “small” declines in sexual function and bladder/bowel function at 4-6 weeks after surgery, but function “quickly” recovered to baseline by 6 months, Dr. Covens reported.
Twelve patients reported a diagnosis of lymphedema, with a Gynecologic Cancer Lymphedema Questionnaire score change of 4 or higher on at least two consecutive evaluations from baseline. This occurred in six cone biopsy and six simple hysterectomy patients.
In a separate presentation, Dr. Covens reported secondary oncologic outcomes from GOG-278, which suggest that non-radical surgery for early-stage cervical cancer is safe, with low perioperative morbidity, although longer follow-up is needed.
He also reported 16 pregnancies in 15 patients who had undergone cone biopsies; 12 of these were successful, and there were four early pregnancy losses.
‘Impressive’ Data
Study discussant Kristin Bixel, MD, with The Ohio State University, Columbus, Ohio, said the data are “impressive” and clearly show that non-radical surgery has “minimal impact on bladder/bowel function, with no long-term differences from baseline.”
She added that the incidence of lymphedema was “honestly significantly lower than what I typically counsel patients about” and wondered if the percentage of patients with lymphedema would increase over time.
Dr. Bixel particularly noted the decrease in cancer worry scores after surgery, as sometimes patients who have less radical procedures fear that this comes with an increased risk for recurrence.
The “growing body of data suggests that less radical surgery is safe and effective for early-stage low-risk cervical cancer and highlights the potential reproductive success,” she concluded.
Funding for the study was provided by grants from NRG Oncology. Dr. Covens had no disclosures. Dr. Bixel has received research funding from the Intuitive Foundation.
A version of this article appeared on Medscape.com.
FROM SGO 2024
Extraordinary Patients Inspired Father of Cancer Immunotherapy
His pioneering research established interleukin-2 (IL-2) as the first U.S. Food and Drug Administration–approved cancer immunotherapy in 1992.
To recognize his trailblazing work and other achievements, the American Association for Cancer Research (AACR) will award Dr. Rosenberg with the 2024 AACR Award for Lifetime Achievement in Cancer Research at its annual meeting in April.
Dr. Rosenberg, a senior investigator for the Center for Cancer Research at the National Cancer Institute (NCI), and chief of the NCI Surgery Branch, shared the history behind his novel research and the patient stories that inspired his discoveries, during an interview.
Tell us a little about yourself and where you grew up.
Dr. Rosenberg: I grew up in the Bronx. My parents both immigrated to the United States from Poland as teenagers.
As a young boy, did you always want to become a doctor?
Dr. Rosenberg: I think some defining moments on why I decided to go into medicine occurred when I was 6 or 7 years old. The second world war was over, and many of the horrors of the Holocaust became apparent to me. I was brought up as an Orthodox Jew. My parents were quite religious, and I remember postcards coming in one after another about relatives that had died in the death camps. That had a profound influence on me.
How did that experience impact your aspirations?
Dr. Rosenberg: It was an example to me of how evil certain people and groups can be toward one another. I decided at that point, that I wanted to do something good for people, and medicine seemed the most likely way to do that. But also, I was developing a broad scientific interest. I ended up at the Bronx High School of Science and knew that I not only wanted to practice the medicine of today, but I wanted to play a role in helping develop the medicine.
What led to your interest in cancer treatment?
Dr. Rosenberg: Well, as a medical student and resident, it became clear that the field of cancer needed major improvement. We had three major ways to treat cancer: surgery, radiation therapy, and chemotherapy. That could cure about half of the people [who] had cancer. But despite the best application of those three specialties, there were over 600,000 deaths from cancer each year in the United States alone. It was clear to me that new approaches were needed, and I became very interested in taking advantage of the body’s immune system as a source of information to try to make progress.
Were there patients who inspired your research?
Dr. Rosenberg: There were two patients that I saw early in my career that impressed me a great deal. One was a patient that I saw when working in the emergency ward as a resident. A patient came in with right upper quadrant pain that looked like a gallbladder attack. That’s what it was. But when I went through his chart, I saw that he had been at that hospital 12 years earlier with a metastatic gastric cancer. The surgeons had operated. They saw tumor had spread to the liver and could not be removed. They closed the belly, not expecting him to survive. Yet he kept showing up for follow-up visits.
Here he was 12 years later. When I helped operate to take out his gallbladder, there was no evidence of any cancer. The cancer had disappeared in the absence of any external treatment. One of the rarest events in medicine, the spontaneous regression of a cancer. Somehow his body had learned how to destroy the tumor.
Was the second patient’s case as impressive?
Dr. Rosenberg: This patient had received a kidney transplant from a gentleman who died in an auto accident. [The donor’s] kidney contained a cancer deposit, a kidney cancer, unbeknownst to the transplant surgeons. [When the kidney was transplanted], the recipient developed widespread metastatic kidney cancer.
[The recipient] was on immunosuppressive drugs, and so the drugs had to be stopped. [When the immunosuppressive drugs were stopped], the patient’s body rejected the kidney and his cancer disappeared.
That showed me that, in fact, if you could stimulate a strong enough immune reaction, in this case, an [allogeneic] reaction, against foreign tissues from a different individual, that you could make large vascularized, invasive cancers disappear based on immune reactivities. Those were clues that led me toward studying the immune system’s impact on cancer.
From there, how did your work evolve?
Dr. Rosenberg: As chief of the surgery branch at NIH, I began doing research. It was very difficult to manipulate immune cells in the laboratory. They wouldn’t stay alive. But I tried to study immune reactions in patients with cancer to see if there was such a thing as an immune reaction against the cancer. There was no such thing known at the time. There were no cancer antigens and no known immune reactions against the disease in the human.
Around this time, investigators were publishing studies about interleukin-2 (IL-2), or white blood cells known as leukocytes. How did interleukin-2 further your research?
Dr. Rosenberg: The advent of interleukin-2 enabled scientists to grow lymphocytes outside the body. [This] enabled us to grow t-lymphocytes, which are some of the major warriors of the immune system against foreign tissue. After [studying] 66 patients in which we studied interleukin-2 and cells that would develop from it, we finally saw a disappearance of melanoma in a patient that received interleukin-2. And we went on to treat hundreds of patients with that hormone, interleukin-2. In fact, interleukin-2 became the first immunotherapy ever approved by the Food and Drug Administration for the treatment of cancer in humans.
How did this finding impact your future discoveries?
Dr. Rosenberg: [It] led to studies of the mechanism of action of interleukin-2 and to do that, we identified a kind of cell called a tumor infiltrating lymphocyte. What better place, intuitively to look for cells doing battle against the cancer than within the cancer itself?
In 1988, we demonstrated for the first time that transfer of lymphocytes with antitumor activity could cause the regression of melanoma. This was a living drug obtained from melanoma deposits that could be grown outside the body and then readministered to the patient under suitable conditions. Interestingly, [in February the FDA approved that drug as treatment for patients with melanoma]. A company developed it to the point where in multi-institutional studies, they reproduced our results.
And we’ve now emphasized the value of using T cell therapy, t cell transfer, for the treatment of patients with the common solid cancers, the cancers that start anywhere from the colon up through the intestine, the stomach, the pancreas, and the esophagus. Solid tumors such as ovarian cancer, uterine cancer and so on, are also potentially susceptible to this T cell therapy.
We’ve published several papers showing in isolated patients that you could cause major regressions, if not complete regressions, of these solid cancers in the liver, in the breast, the cervix, the colon. That’s a major aspect of what we’re doing now.
I think immunotherapy has come to be recognized as a major fourth arm that can be used to attack cancers, adding to surgery, radiation, and chemotherapy.
What guidance would you have for other physician-investigators or young doctors who want to follow in your path?
Dr. Rosenberg: You have to have a broad base of knowledge. You have to be willing to immerse yourself in a problem so that your mind is working on it when you’re doing things where you can only think. [When] you’re taking a shower, [or] waiting at a red light, your mind is working on this problem because you’re immersed in trying to understand it.
You need to have a laser focus on the goals that you have and not get sidetracked by issues that may be interesting but not directly related to the goals that you’re attempting to achieve.
His pioneering research established interleukin-2 (IL-2) as the first U.S. Food and Drug Administration–approved cancer immunotherapy in 1992.
To recognize his trailblazing work and other achievements, the American Association for Cancer Research (AACR) will award Dr. Rosenberg with the 2024 AACR Award for Lifetime Achievement in Cancer Research at its annual meeting in April.
Dr. Rosenberg, a senior investigator for the Center for Cancer Research at the National Cancer Institute (NCI), and chief of the NCI Surgery Branch, shared the history behind his novel research and the patient stories that inspired his discoveries, during an interview.
Tell us a little about yourself and where you grew up.
Dr. Rosenberg: I grew up in the Bronx. My parents both immigrated to the United States from Poland as teenagers.
As a young boy, did you always want to become a doctor?
Dr. Rosenberg: I think some defining moments on why I decided to go into medicine occurred when I was 6 or 7 years old. The second world war was over, and many of the horrors of the Holocaust became apparent to me. I was brought up as an Orthodox Jew. My parents were quite religious, and I remember postcards coming in one after another about relatives that had died in the death camps. That had a profound influence on me.
How did that experience impact your aspirations?
Dr. Rosenberg: It was an example to me of how evil certain people and groups can be toward one another. I decided at that point, that I wanted to do something good for people, and medicine seemed the most likely way to do that. But also, I was developing a broad scientific interest. I ended up at the Bronx High School of Science and knew that I not only wanted to practice the medicine of today, but I wanted to play a role in helping develop the medicine.
What led to your interest in cancer treatment?
Dr. Rosenberg: Well, as a medical student and resident, it became clear that the field of cancer needed major improvement. We had three major ways to treat cancer: surgery, radiation therapy, and chemotherapy. That could cure about half of the people [who] had cancer. But despite the best application of those three specialties, there were over 600,000 deaths from cancer each year in the United States alone. It was clear to me that new approaches were needed, and I became very interested in taking advantage of the body’s immune system as a source of information to try to make progress.
Were there patients who inspired your research?
Dr. Rosenberg: There were two patients that I saw early in my career that impressed me a great deal. One was a patient that I saw when working in the emergency ward as a resident. A patient came in with right upper quadrant pain that looked like a gallbladder attack. That’s what it was. But when I went through his chart, I saw that he had been at that hospital 12 years earlier with a metastatic gastric cancer. The surgeons had operated. They saw tumor had spread to the liver and could not be removed. They closed the belly, not expecting him to survive. Yet he kept showing up for follow-up visits.
Here he was 12 years later. When I helped operate to take out his gallbladder, there was no evidence of any cancer. The cancer had disappeared in the absence of any external treatment. One of the rarest events in medicine, the spontaneous regression of a cancer. Somehow his body had learned how to destroy the tumor.
Was the second patient’s case as impressive?
Dr. Rosenberg: This patient had received a kidney transplant from a gentleman who died in an auto accident. [The donor’s] kidney contained a cancer deposit, a kidney cancer, unbeknownst to the transplant surgeons. [When the kidney was transplanted], the recipient developed widespread metastatic kidney cancer.
[The recipient] was on immunosuppressive drugs, and so the drugs had to be stopped. [When the immunosuppressive drugs were stopped], the patient’s body rejected the kidney and his cancer disappeared.
That showed me that, in fact, if you could stimulate a strong enough immune reaction, in this case, an [allogeneic] reaction, against foreign tissues from a different individual, that you could make large vascularized, invasive cancers disappear based on immune reactivities. Those were clues that led me toward studying the immune system’s impact on cancer.
From there, how did your work evolve?
Dr. Rosenberg: As chief of the surgery branch at NIH, I began doing research. It was very difficult to manipulate immune cells in the laboratory. They wouldn’t stay alive. But I tried to study immune reactions in patients with cancer to see if there was such a thing as an immune reaction against the cancer. There was no such thing known at the time. There were no cancer antigens and no known immune reactions against the disease in the human.
Around this time, investigators were publishing studies about interleukin-2 (IL-2), or white blood cells known as leukocytes. How did interleukin-2 further your research?
Dr. Rosenberg: The advent of interleukin-2 enabled scientists to grow lymphocytes outside the body. [This] enabled us to grow t-lymphocytes, which are some of the major warriors of the immune system against foreign tissue. After [studying] 66 patients in which we studied interleukin-2 and cells that would develop from it, we finally saw a disappearance of melanoma in a patient that received interleukin-2. And we went on to treat hundreds of patients with that hormone, interleukin-2. In fact, interleukin-2 became the first immunotherapy ever approved by the Food and Drug Administration for the treatment of cancer in humans.
How did this finding impact your future discoveries?
Dr. Rosenberg: [It] led to studies of the mechanism of action of interleukin-2 and to do that, we identified a kind of cell called a tumor infiltrating lymphocyte. What better place, intuitively to look for cells doing battle against the cancer than within the cancer itself?
In 1988, we demonstrated for the first time that transfer of lymphocytes with antitumor activity could cause the regression of melanoma. This was a living drug obtained from melanoma deposits that could be grown outside the body and then readministered to the patient under suitable conditions. Interestingly, [in February the FDA approved that drug as treatment for patients with melanoma]. A company developed it to the point where in multi-institutional studies, they reproduced our results.
And we’ve now emphasized the value of using T cell therapy, t cell transfer, for the treatment of patients with the common solid cancers, the cancers that start anywhere from the colon up through the intestine, the stomach, the pancreas, and the esophagus. Solid tumors such as ovarian cancer, uterine cancer and so on, are also potentially susceptible to this T cell therapy.
We’ve published several papers showing in isolated patients that you could cause major regressions, if not complete regressions, of these solid cancers in the liver, in the breast, the cervix, the colon. That’s a major aspect of what we’re doing now.
I think immunotherapy has come to be recognized as a major fourth arm that can be used to attack cancers, adding to surgery, radiation, and chemotherapy.
What guidance would you have for other physician-investigators or young doctors who want to follow in your path?
Dr. Rosenberg: You have to have a broad base of knowledge. You have to be willing to immerse yourself in a problem so that your mind is working on it when you’re doing things where you can only think. [When] you’re taking a shower, [or] waiting at a red light, your mind is working on this problem because you’re immersed in trying to understand it.
You need to have a laser focus on the goals that you have and not get sidetracked by issues that may be interesting but not directly related to the goals that you’re attempting to achieve.
His pioneering research established interleukin-2 (IL-2) as the first U.S. Food and Drug Administration–approved cancer immunotherapy in 1992.
To recognize his trailblazing work and other achievements, the American Association for Cancer Research (AACR) will award Dr. Rosenberg with the 2024 AACR Award for Lifetime Achievement in Cancer Research at its annual meeting in April.
Dr. Rosenberg, a senior investigator for the Center for Cancer Research at the National Cancer Institute (NCI), and chief of the NCI Surgery Branch, shared the history behind his novel research and the patient stories that inspired his discoveries, during an interview.
Tell us a little about yourself and where you grew up.
Dr. Rosenberg: I grew up in the Bronx. My parents both immigrated to the United States from Poland as teenagers.
As a young boy, did you always want to become a doctor?
Dr. Rosenberg: I think some defining moments on why I decided to go into medicine occurred when I was 6 or 7 years old. The second world war was over, and many of the horrors of the Holocaust became apparent to me. I was brought up as an Orthodox Jew. My parents were quite religious, and I remember postcards coming in one after another about relatives that had died in the death camps. That had a profound influence on me.
How did that experience impact your aspirations?
Dr. Rosenberg: It was an example to me of how evil certain people and groups can be toward one another. I decided at that point, that I wanted to do something good for people, and medicine seemed the most likely way to do that. But also, I was developing a broad scientific interest. I ended up at the Bronx High School of Science and knew that I not only wanted to practice the medicine of today, but I wanted to play a role in helping develop the medicine.
What led to your interest in cancer treatment?
Dr. Rosenberg: Well, as a medical student and resident, it became clear that the field of cancer needed major improvement. We had three major ways to treat cancer: surgery, radiation therapy, and chemotherapy. That could cure about half of the people [who] had cancer. But despite the best application of those three specialties, there were over 600,000 deaths from cancer each year in the United States alone. It was clear to me that new approaches were needed, and I became very interested in taking advantage of the body’s immune system as a source of information to try to make progress.
Were there patients who inspired your research?
Dr. Rosenberg: There were two patients that I saw early in my career that impressed me a great deal. One was a patient that I saw when working in the emergency ward as a resident. A patient came in with right upper quadrant pain that looked like a gallbladder attack. That’s what it was. But when I went through his chart, I saw that he had been at that hospital 12 years earlier with a metastatic gastric cancer. The surgeons had operated. They saw tumor had spread to the liver and could not be removed. They closed the belly, not expecting him to survive. Yet he kept showing up for follow-up visits.
Here he was 12 years later. When I helped operate to take out his gallbladder, there was no evidence of any cancer. The cancer had disappeared in the absence of any external treatment. One of the rarest events in medicine, the spontaneous regression of a cancer. Somehow his body had learned how to destroy the tumor.
Was the second patient’s case as impressive?
Dr. Rosenberg: This patient had received a kidney transplant from a gentleman who died in an auto accident. [The donor’s] kidney contained a cancer deposit, a kidney cancer, unbeknownst to the transplant surgeons. [When the kidney was transplanted], the recipient developed widespread metastatic kidney cancer.
[The recipient] was on immunosuppressive drugs, and so the drugs had to be stopped. [When the immunosuppressive drugs were stopped], the patient’s body rejected the kidney and his cancer disappeared.
That showed me that, in fact, if you could stimulate a strong enough immune reaction, in this case, an [allogeneic] reaction, against foreign tissues from a different individual, that you could make large vascularized, invasive cancers disappear based on immune reactivities. Those were clues that led me toward studying the immune system’s impact on cancer.
From there, how did your work evolve?
Dr. Rosenberg: As chief of the surgery branch at NIH, I began doing research. It was very difficult to manipulate immune cells in the laboratory. They wouldn’t stay alive. But I tried to study immune reactions in patients with cancer to see if there was such a thing as an immune reaction against the cancer. There was no such thing known at the time. There were no cancer antigens and no known immune reactions against the disease in the human.
Around this time, investigators were publishing studies about interleukin-2 (IL-2), or white blood cells known as leukocytes. How did interleukin-2 further your research?
Dr. Rosenberg: The advent of interleukin-2 enabled scientists to grow lymphocytes outside the body. [This] enabled us to grow t-lymphocytes, which are some of the major warriors of the immune system against foreign tissue. After [studying] 66 patients in which we studied interleukin-2 and cells that would develop from it, we finally saw a disappearance of melanoma in a patient that received interleukin-2. And we went on to treat hundreds of patients with that hormone, interleukin-2. In fact, interleukin-2 became the first immunotherapy ever approved by the Food and Drug Administration for the treatment of cancer in humans.
How did this finding impact your future discoveries?
Dr. Rosenberg: [It] led to studies of the mechanism of action of interleukin-2 and to do that, we identified a kind of cell called a tumor infiltrating lymphocyte. What better place, intuitively to look for cells doing battle against the cancer than within the cancer itself?
In 1988, we demonstrated for the first time that transfer of lymphocytes with antitumor activity could cause the regression of melanoma. This was a living drug obtained from melanoma deposits that could be grown outside the body and then readministered to the patient under suitable conditions. Interestingly, [in February the FDA approved that drug as treatment for patients with melanoma]. A company developed it to the point where in multi-institutional studies, they reproduced our results.
And we’ve now emphasized the value of using T cell therapy, t cell transfer, for the treatment of patients with the common solid cancers, the cancers that start anywhere from the colon up through the intestine, the stomach, the pancreas, and the esophagus. Solid tumors such as ovarian cancer, uterine cancer and so on, are also potentially susceptible to this T cell therapy.
We’ve published several papers showing in isolated patients that you could cause major regressions, if not complete regressions, of these solid cancers in the liver, in the breast, the cervix, the colon. That’s a major aspect of what we’re doing now.
I think immunotherapy has come to be recognized as a major fourth arm that can be used to attack cancers, adding to surgery, radiation, and chemotherapy.
What guidance would you have for other physician-investigators or young doctors who want to follow in your path?
Dr. Rosenberg: You have to have a broad base of knowledge. You have to be willing to immerse yourself in a problem so that your mind is working on it when you’re doing things where you can only think. [When] you’re taking a shower, [or] waiting at a red light, your mind is working on this problem because you’re immersed in trying to understand it.
You need to have a laser focus on the goals that you have and not get sidetracked by issues that may be interesting but not directly related to the goals that you’re attempting to achieve.
Cancer Data Trends 2024
The annual issue of Cancer Data Trends, produced in collaboration with the Association of VA Hematology/Oncology (AVAHO), highlights the latest research in some of the top cancers impacting US veterans.
Click to view the Digital Edition.
In this issue:
Hepatocellular Carcinoma
Special care for veterans, changes in staging, and biomarkers for early diagnosis
Lung Cancer
Guideline updates and racial disparities in veterans
Multiple Myeloma
Improving survival in the VA
Colorectal Cancer
Barriers to follow-up colonoscopies after FIT testing
B-Cell Lymphomas
Findings from the VA's National TeleOncology Program and recent therapy updates
Breast Cancer
A look at the VA's Risk Assessment Pipeline and incidence among veterans vs the general population
Genitourinary Cancers
Molecular testing in prostate cancer, improving survival for metastatic RCC, and links between bladder cancer and Agent Orange exposure
The annual issue of Cancer Data Trends, produced in collaboration with the Association of VA Hematology/Oncology (AVAHO), highlights the latest research in some of the top cancers impacting US veterans.
Click to view the Digital Edition.
In this issue:
Hepatocellular Carcinoma
Special care for veterans, changes in staging, and biomarkers for early diagnosis
Lung Cancer
Guideline updates and racial disparities in veterans
Multiple Myeloma
Improving survival in the VA
Colorectal Cancer
Barriers to follow-up colonoscopies after FIT testing
B-Cell Lymphomas
Findings from the VA's National TeleOncology Program and recent therapy updates
Breast Cancer
A look at the VA's Risk Assessment Pipeline and incidence among veterans vs the general population
Genitourinary Cancers
Molecular testing in prostate cancer, improving survival for metastatic RCC, and links between bladder cancer and Agent Orange exposure
The annual issue of Cancer Data Trends, produced in collaboration with the Association of VA Hematology/Oncology (AVAHO), highlights the latest research in some of the top cancers impacting US veterans.
Click to view the Digital Edition.
In this issue:
Hepatocellular Carcinoma
Special care for veterans, changes in staging, and biomarkers for early diagnosis
Lung Cancer
Guideline updates and racial disparities in veterans
Multiple Myeloma
Improving survival in the VA
Colorectal Cancer
Barriers to follow-up colonoscopies after FIT testing
B-Cell Lymphomas
Findings from the VA's National TeleOncology Program and recent therapy updates
Breast Cancer
A look at the VA's Risk Assessment Pipeline and incidence among veterans vs the general population
Genitourinary Cancers
Molecular testing in prostate cancer, improving survival for metastatic RCC, and links between bladder cancer and Agent Orange exposure
Cancer Data Trends 2024: Genitourinary Cancers
1. Sokolova A, Cheng H. Germline testing in prostate cancer: when and who to test. Oncology (Williston Park). 2021;35(10):645-653. doi:10.46883/ONC.2021.3510.0645
2. Tuffaha H, Edmunds K, Fairbairn D, et al. Guidelines for genetic testing in prostate cancer: a scoping review. Prostate Cancer Prostatic Dis. 2023 May 18. doi:10.1038/s41391-023-00676-0
3. National Comprehensive Cancer Network. NCCN clinical practice guidelines for prostate cancer. Version 4.2023. September 7, 2023. Accessed December 20, 2023. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf
4. National Precision Oncology Program. PMID 26149669 (e-mail, December 13, 2023).
5. Shevach J, Lynch J, Candelieri-Surette D, et al. Racial disparities in germline testing among men with pancreas, breast and metastatic prostate cancers in two health systems. J Clin Oncol. 2023;41(16 suppl):abstract 10549. https://ascopubs.org/doi/abs/10.1200/JCO.2023.41.16_suppl.10549
6. Velaer K, Thomas IC, Yang J, et al. Clinical laboratory tests associated with survival in patients with metastatic renal cell carcinoma: a laboratory wide association study (LWAS). Urol Oncol. 2022;40(1):12.e23-12.e30. doi:10.1016/j.urolonc.2021.08.011
7. Heng DYC, Xie W, Regan MM, et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: a population-based study. Lancet Oncol. 2013;14(2):141-148. doi:10.1016/S1470-2045(12)70559-4
8. Aguilar Palacios D, Wilson B, Michael P, et al. A novel metric for hospital quality in kidney cancer surgery: a Veterans Affairs National Health System validation of concept. Urol Pract. 2022;9(3):237-245. doi:10.1097/UPJ.0000000000000294
9. Williams SB, Janes JL, Howard LE, et al. Exposure to Agent Orange and risk of bladder cancer among US veterans. JAMA Netw Open. 2023;6(6):e2320593. doi:10.1001/jamanetworkopen.2023.20593
10. Penn T, Borza T, Liou JI, et al. Impact of Agent Orange exposure on non-muscle invasive bladder cancer outcomes. Urology. 2023;182:175-180. doi:10.1016/j.urology.2023.08.037
1. Sokolova A, Cheng H. Germline testing in prostate cancer: when and who to test. Oncology (Williston Park). 2021;35(10):645-653. doi:10.46883/ONC.2021.3510.0645
2. Tuffaha H, Edmunds K, Fairbairn D, et al. Guidelines for genetic testing in prostate cancer: a scoping review. Prostate Cancer Prostatic Dis. 2023 May 18. doi:10.1038/s41391-023-00676-0
3. National Comprehensive Cancer Network. NCCN clinical practice guidelines for prostate cancer. Version 4.2023. September 7, 2023. Accessed December 20, 2023. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf
4. National Precision Oncology Program. PMID 26149669 (e-mail, December 13, 2023).
5. Shevach J, Lynch J, Candelieri-Surette D, et al. Racial disparities in germline testing among men with pancreas, breast and metastatic prostate cancers in two health systems. J Clin Oncol. 2023;41(16 suppl):abstract 10549. https://ascopubs.org/doi/abs/10.1200/JCO.2023.41.16_suppl.10549
6. Velaer K, Thomas IC, Yang J, et al. Clinical laboratory tests associated with survival in patients with metastatic renal cell carcinoma: a laboratory wide association study (LWAS). Urol Oncol. 2022;40(1):12.e23-12.e30. doi:10.1016/j.urolonc.2021.08.011
7. Heng DYC, Xie W, Regan MM, et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: a population-based study. Lancet Oncol. 2013;14(2):141-148. doi:10.1016/S1470-2045(12)70559-4
8. Aguilar Palacios D, Wilson B, Michael P, et al. A novel metric for hospital quality in kidney cancer surgery: a Veterans Affairs National Health System validation of concept. Urol Pract. 2022;9(3):237-245. doi:10.1097/UPJ.0000000000000294
9. Williams SB, Janes JL, Howard LE, et al. Exposure to Agent Orange and risk of bladder cancer among US veterans. JAMA Netw Open. 2023;6(6):e2320593. doi:10.1001/jamanetworkopen.2023.20593
10. Penn T, Borza T, Liou JI, et al. Impact of Agent Orange exposure on non-muscle invasive bladder cancer outcomes. Urology. 2023;182:175-180. doi:10.1016/j.urology.2023.08.037
1. Sokolova A, Cheng H. Germline testing in prostate cancer: when and who to test. Oncology (Williston Park). 2021;35(10):645-653. doi:10.46883/ONC.2021.3510.0645
2. Tuffaha H, Edmunds K, Fairbairn D, et al. Guidelines for genetic testing in prostate cancer: a scoping review. Prostate Cancer Prostatic Dis. 2023 May 18. doi:10.1038/s41391-023-00676-0
3. National Comprehensive Cancer Network. NCCN clinical practice guidelines for prostate cancer. Version 4.2023. September 7, 2023. Accessed December 20, 2023. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf
4. National Precision Oncology Program. PMID 26149669 (e-mail, December 13, 2023).
5. Shevach J, Lynch J, Candelieri-Surette D, et al. Racial disparities in germline testing among men with pancreas, breast and metastatic prostate cancers in two health systems. J Clin Oncol. 2023;41(16 suppl):abstract 10549. https://ascopubs.org/doi/abs/10.1200/JCO.2023.41.16_suppl.10549
6. Velaer K, Thomas IC, Yang J, et al. Clinical laboratory tests associated with survival in patients with metastatic renal cell carcinoma: a laboratory wide association study (LWAS). Urol Oncol. 2022;40(1):12.e23-12.e30. doi:10.1016/j.urolonc.2021.08.011
7. Heng DYC, Xie W, Regan MM, et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: a population-based study. Lancet Oncol. 2013;14(2):141-148. doi:10.1016/S1470-2045(12)70559-4
8. Aguilar Palacios D, Wilson B, Michael P, et al. A novel metric for hospital quality in kidney cancer surgery: a Veterans Affairs National Health System validation of concept. Urol Pract. 2022;9(3):237-245. doi:10.1097/UPJ.0000000000000294
9. Williams SB, Janes JL, Howard LE, et al. Exposure to Agent Orange and risk of bladder cancer among US veterans. JAMA Netw Open. 2023;6(6):e2320593. doi:10.1001/jamanetworkopen.2023.20593
10. Penn T, Borza T, Liou JI, et al. Impact of Agent Orange exposure on non-muscle invasive bladder cancer outcomes. Urology. 2023;182:175-180. doi:10.1016/j.urology.2023.08.037