User login
COVID-19 death rate was twice as high in cancer patients in NYC study
COVID-19 patients with cancer had double the fatality rate of COVID-19 patients without cancer treated in an urban New York hospital system, according to data from a retrospective study.
with COVID-19 treated during the same time period in the same hospital system.
Vikas Mehta, MD, of Montefiore Medical Center, New York, and colleagues reported these results in Cancer Discovery.
“As New York has emerged as the current epicenter of the pandemic, we sought to investigate the risk posed by COVID-19 to our cancer population,” the authors wrote.
They identified 218 cancer patients treated for COVID-19 in the Montefiore Health System between March 18 and April 8, 2020. Three-quarters of patients had solid tumors, and 25% had hematologic malignancies. Most patients were adults (98.6%), their median age was 69 years (range, 10-92 years), and 58% were men.
In all, 28% of the cancer patients (61/218) died from COVID-19, including 25% (41/164) of those with solid tumors and 37% (20/54) of those with hematologic malignancies.
Deaths by cancer type
Among the 164 patients with solid tumors, case fatality rates were as follows:
- Pancreatic – 67% (2/3)
- Lung – 55% (6/11)
- Colorectal – 38% (8/21)
- Upper gastrointestinal – 38% (3/8)
- Gynecologic – 38% (5/13)
- Skin – 33% (1/3)
- Hepatobiliary – 29% (2/7)
- Bone/soft tissue – 20% (1/5)
- Genitourinary – 15% (7/46)
- Breast – 14% (4/28)
- Neurologic – 13% (1/8)
- Head and neck – 13% (1/8).
None of the three patients with neuroendocrine tumors died.
Among the 54 patients with hematologic malignancies, case fatality rates were as follows:
- Chronic myeloid leukemia – 100% (1/1)
- Hodgkin lymphoma – 60% (3/5)
- Myelodysplastic syndromes – 60% (3/5)
- Multiple myeloma – 38% (5/13)
- Non-Hodgkin lymphoma – 33% (5/15)
- Chronic lymphocytic leukemia – 33% (1/3)
- Myeloproliferative neoplasms – 29% (2/7).
None of the four patients with acute lymphoblastic leukemia died, and there was one patient with acute myeloid leukemia who did not die.
Factors associated with increased mortality
The researchers compared the 218 cancer patients with COVID-19 with 1,090 age- and sex-matched noncancer patients with COVID-19 treated in the Montefiore Health System between March 18 and April 8, 2020.
Case fatality rates in cancer patients with COVID-19 were significantly increased in all age groups, but older age was associated with higher mortality.
“We observed case fatality rates were elevated in all age cohorts in cancer patients and achieved statistical significance in the age groups 45-64 and in patients older than 75 years of age,” the authors reported.
Other factors significantly associated with higher mortality in a multivariable analysis included the presence of multiple comorbidities; the need for ICU support; and increased levels of d-dimer, lactate, and lactate dehydrogenase.
Additional factors, such as socioeconomic and health disparities, may also be significant predictors of mortality, according to the authors. They noted that this cohort largely consisted of patients from a socioeconomically underprivileged community where mortality because of COVID-19 is reportedly higher.
Proactive strategies moving forward
“We have been addressing the significant burden of the COVID-19 pandemic on our vulnerable cancer patients through a variety of ways,” said study author Balazs Halmos, MD, of Montefiore Medical Center.
The center set up a separate infusion unit exclusively for COVID-positive patients and established separate inpatient areas. Dr. Halmos and colleagues are also providing telemedicine, virtual supportive care services, telephonic counseling, and bilingual peer-support programs.
“Many questions remain as we continue to establish new practices for our cancer patients,” Dr. Halmos said. “We will find answers to these questions as we continue to focus on adaptation and not acceptance in response to the COVID crisis. Our patients deserve nothing less.”
The Albert Einstein Cancer Center supported this study. The authors reported having no conflicts of interest.
SOURCE: Mehta V et al. Cancer Discov. 2020 May 1. doi: 10.1158/2159-8290.CD-20-0516.
COVID-19 patients with cancer had double the fatality rate of COVID-19 patients without cancer treated in an urban New York hospital system, according to data from a retrospective study.
with COVID-19 treated during the same time period in the same hospital system.
Vikas Mehta, MD, of Montefiore Medical Center, New York, and colleagues reported these results in Cancer Discovery.
“As New York has emerged as the current epicenter of the pandemic, we sought to investigate the risk posed by COVID-19 to our cancer population,” the authors wrote.
They identified 218 cancer patients treated for COVID-19 in the Montefiore Health System between March 18 and April 8, 2020. Three-quarters of patients had solid tumors, and 25% had hematologic malignancies. Most patients were adults (98.6%), their median age was 69 years (range, 10-92 years), and 58% were men.
In all, 28% of the cancer patients (61/218) died from COVID-19, including 25% (41/164) of those with solid tumors and 37% (20/54) of those with hematologic malignancies.
Deaths by cancer type
Among the 164 patients with solid tumors, case fatality rates were as follows:
- Pancreatic – 67% (2/3)
- Lung – 55% (6/11)
- Colorectal – 38% (8/21)
- Upper gastrointestinal – 38% (3/8)
- Gynecologic – 38% (5/13)
- Skin – 33% (1/3)
- Hepatobiliary – 29% (2/7)
- Bone/soft tissue – 20% (1/5)
- Genitourinary – 15% (7/46)
- Breast – 14% (4/28)
- Neurologic – 13% (1/8)
- Head and neck – 13% (1/8).
None of the three patients with neuroendocrine tumors died.
Among the 54 patients with hematologic malignancies, case fatality rates were as follows:
- Chronic myeloid leukemia – 100% (1/1)
- Hodgkin lymphoma – 60% (3/5)
- Myelodysplastic syndromes – 60% (3/5)
- Multiple myeloma – 38% (5/13)
- Non-Hodgkin lymphoma – 33% (5/15)
- Chronic lymphocytic leukemia – 33% (1/3)
- Myeloproliferative neoplasms – 29% (2/7).
None of the four patients with acute lymphoblastic leukemia died, and there was one patient with acute myeloid leukemia who did not die.
Factors associated with increased mortality
The researchers compared the 218 cancer patients with COVID-19 with 1,090 age- and sex-matched noncancer patients with COVID-19 treated in the Montefiore Health System between March 18 and April 8, 2020.
Case fatality rates in cancer patients with COVID-19 were significantly increased in all age groups, but older age was associated with higher mortality.
“We observed case fatality rates were elevated in all age cohorts in cancer patients and achieved statistical significance in the age groups 45-64 and in patients older than 75 years of age,” the authors reported.
Other factors significantly associated with higher mortality in a multivariable analysis included the presence of multiple comorbidities; the need for ICU support; and increased levels of d-dimer, lactate, and lactate dehydrogenase.
Additional factors, such as socioeconomic and health disparities, may also be significant predictors of mortality, according to the authors. They noted that this cohort largely consisted of patients from a socioeconomically underprivileged community where mortality because of COVID-19 is reportedly higher.
Proactive strategies moving forward
“We have been addressing the significant burden of the COVID-19 pandemic on our vulnerable cancer patients through a variety of ways,” said study author Balazs Halmos, MD, of Montefiore Medical Center.
The center set up a separate infusion unit exclusively for COVID-positive patients and established separate inpatient areas. Dr. Halmos and colleagues are also providing telemedicine, virtual supportive care services, telephonic counseling, and bilingual peer-support programs.
“Many questions remain as we continue to establish new practices for our cancer patients,” Dr. Halmos said. “We will find answers to these questions as we continue to focus on adaptation and not acceptance in response to the COVID crisis. Our patients deserve nothing less.”
The Albert Einstein Cancer Center supported this study. The authors reported having no conflicts of interest.
SOURCE: Mehta V et al. Cancer Discov. 2020 May 1. doi: 10.1158/2159-8290.CD-20-0516.
COVID-19 patients with cancer had double the fatality rate of COVID-19 patients without cancer treated in an urban New York hospital system, according to data from a retrospective study.
with COVID-19 treated during the same time period in the same hospital system.
Vikas Mehta, MD, of Montefiore Medical Center, New York, and colleagues reported these results in Cancer Discovery.
“As New York has emerged as the current epicenter of the pandemic, we sought to investigate the risk posed by COVID-19 to our cancer population,” the authors wrote.
They identified 218 cancer patients treated for COVID-19 in the Montefiore Health System between March 18 and April 8, 2020. Three-quarters of patients had solid tumors, and 25% had hematologic malignancies. Most patients were adults (98.6%), their median age was 69 years (range, 10-92 years), and 58% were men.
In all, 28% of the cancer patients (61/218) died from COVID-19, including 25% (41/164) of those with solid tumors and 37% (20/54) of those with hematologic malignancies.
Deaths by cancer type
Among the 164 patients with solid tumors, case fatality rates were as follows:
- Pancreatic – 67% (2/3)
- Lung – 55% (6/11)
- Colorectal – 38% (8/21)
- Upper gastrointestinal – 38% (3/8)
- Gynecologic – 38% (5/13)
- Skin – 33% (1/3)
- Hepatobiliary – 29% (2/7)
- Bone/soft tissue – 20% (1/5)
- Genitourinary – 15% (7/46)
- Breast – 14% (4/28)
- Neurologic – 13% (1/8)
- Head and neck – 13% (1/8).
None of the three patients with neuroendocrine tumors died.
Among the 54 patients with hematologic malignancies, case fatality rates were as follows:
- Chronic myeloid leukemia – 100% (1/1)
- Hodgkin lymphoma – 60% (3/5)
- Myelodysplastic syndromes – 60% (3/5)
- Multiple myeloma – 38% (5/13)
- Non-Hodgkin lymphoma – 33% (5/15)
- Chronic lymphocytic leukemia – 33% (1/3)
- Myeloproliferative neoplasms – 29% (2/7).
None of the four patients with acute lymphoblastic leukemia died, and there was one patient with acute myeloid leukemia who did not die.
Factors associated with increased mortality
The researchers compared the 218 cancer patients with COVID-19 with 1,090 age- and sex-matched noncancer patients with COVID-19 treated in the Montefiore Health System between March 18 and April 8, 2020.
Case fatality rates in cancer patients with COVID-19 were significantly increased in all age groups, but older age was associated with higher mortality.
“We observed case fatality rates were elevated in all age cohorts in cancer patients and achieved statistical significance in the age groups 45-64 and in patients older than 75 years of age,” the authors reported.
Other factors significantly associated with higher mortality in a multivariable analysis included the presence of multiple comorbidities; the need for ICU support; and increased levels of d-dimer, lactate, and lactate dehydrogenase.
Additional factors, such as socioeconomic and health disparities, may also be significant predictors of mortality, according to the authors. They noted that this cohort largely consisted of patients from a socioeconomically underprivileged community where mortality because of COVID-19 is reportedly higher.
Proactive strategies moving forward
“We have been addressing the significant burden of the COVID-19 pandemic on our vulnerable cancer patients through a variety of ways,” said study author Balazs Halmos, MD, of Montefiore Medical Center.
The center set up a separate infusion unit exclusively for COVID-positive patients and established separate inpatient areas. Dr. Halmos and colleagues are also providing telemedicine, virtual supportive care services, telephonic counseling, and bilingual peer-support programs.
“Many questions remain as we continue to establish new practices for our cancer patients,” Dr. Halmos said. “We will find answers to these questions as we continue to focus on adaptation and not acceptance in response to the COVID crisis. Our patients deserve nothing less.”
The Albert Einstein Cancer Center supported this study. The authors reported having no conflicts of interest.
SOURCE: Mehta V et al. Cancer Discov. 2020 May 1. doi: 10.1158/2159-8290.CD-20-0516.
FROM CANCER DISCOVERY
Three months of COVID-19 may mean 80,000 missed cancer diagnoses
, according to a report by the IQVIA Institute for Human Data Science looking at trends in the United States.
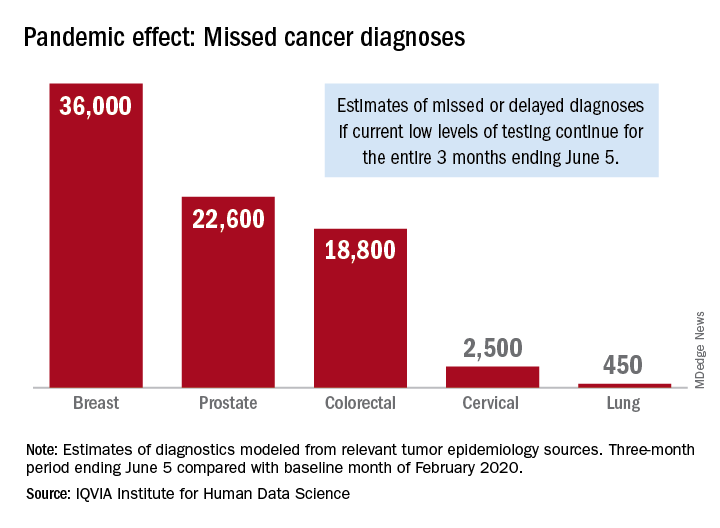
Screening and monitoring tests for breast, prostate, colorectal, cervical, and lung cancer were down 39%-90% in early April, compared with the baseline month of February, according to report authors Murray Aitken and Michael Kleinrock, both of IQVIA.
These findings are based on data from IQVIA’s medical claims database, which includes more than 205 million patients, over 1.7 billion claims, and 3 billion service records obtained annually.
The data suggest that, at current positivity rates, there could be 36,000 missed or delayed diagnoses of breast cancer during the 3-month period from early March through early June. Estimates for missed diagnoses of the four other cancers analyzed include 450 for lung cancer, 2,500 for cervical cancer, 18,800 for colorectal cancer, and 22,600 for prostate cancer.
The authors project a total of 22 million canceled or delayed tests for the five cancers over the 3-month period ending June 5, based on a comparison of claims data for early April with the February baseline. Catching up on this backlog will be problematic, according to the authors.
“Current excess health care capacity ... would require providers to shift priorities to make time and space in schedules and facilities as well as the cooperation of patients to return to health care providers,” the authors wrote. “Both of these could be further disrupted by economic factors or reintroduction of social distancing in a reemergence of the outbreak.”
The report was produced by the IQVIA Institute for Human Data Science without industry or government funding.
SOURCE: Murray A and Kleinrock M. Shifts in healthcare demand, delivery and care during the COVID-19 era. IQVIA Institute for Human Data Science. April 2020.
, according to a report by the IQVIA Institute for Human Data Science looking at trends in the United States.

Screening and monitoring tests for breast, prostate, colorectal, cervical, and lung cancer were down 39%-90% in early April, compared with the baseline month of February, according to report authors Murray Aitken and Michael Kleinrock, both of IQVIA.
These findings are based on data from IQVIA’s medical claims database, which includes more than 205 million patients, over 1.7 billion claims, and 3 billion service records obtained annually.
The data suggest that, at current positivity rates, there could be 36,000 missed or delayed diagnoses of breast cancer during the 3-month period from early March through early June. Estimates for missed diagnoses of the four other cancers analyzed include 450 for lung cancer, 2,500 for cervical cancer, 18,800 for colorectal cancer, and 22,600 for prostate cancer.
The authors project a total of 22 million canceled or delayed tests for the five cancers over the 3-month period ending June 5, based on a comparison of claims data for early April with the February baseline. Catching up on this backlog will be problematic, according to the authors.
“Current excess health care capacity ... would require providers to shift priorities to make time and space in schedules and facilities as well as the cooperation of patients to return to health care providers,” the authors wrote. “Both of these could be further disrupted by economic factors or reintroduction of social distancing in a reemergence of the outbreak.”
The report was produced by the IQVIA Institute for Human Data Science without industry or government funding.
SOURCE: Murray A and Kleinrock M. Shifts in healthcare demand, delivery and care during the COVID-19 era. IQVIA Institute for Human Data Science. April 2020.
, according to a report by the IQVIA Institute for Human Data Science looking at trends in the United States.

Screening and monitoring tests for breast, prostate, colorectal, cervical, and lung cancer were down 39%-90% in early April, compared with the baseline month of February, according to report authors Murray Aitken and Michael Kleinrock, both of IQVIA.
These findings are based on data from IQVIA’s medical claims database, which includes more than 205 million patients, over 1.7 billion claims, and 3 billion service records obtained annually.
The data suggest that, at current positivity rates, there could be 36,000 missed or delayed diagnoses of breast cancer during the 3-month period from early March through early June. Estimates for missed diagnoses of the four other cancers analyzed include 450 for lung cancer, 2,500 for cervical cancer, 18,800 for colorectal cancer, and 22,600 for prostate cancer.
The authors project a total of 22 million canceled or delayed tests for the five cancers over the 3-month period ending June 5, based on a comparison of claims data for early April with the February baseline. Catching up on this backlog will be problematic, according to the authors.
“Current excess health care capacity ... would require providers to shift priorities to make time and space in schedules and facilities as well as the cooperation of patients to return to health care providers,” the authors wrote. “Both of these could be further disrupted by economic factors or reintroduction of social distancing in a reemergence of the outbreak.”
The report was produced by the IQVIA Institute for Human Data Science without industry or government funding.
SOURCE: Murray A and Kleinrock M. Shifts in healthcare demand, delivery and care during the COVID-19 era. IQVIA Institute for Human Data Science. April 2020.
Cancer screening, monitoring down during pandemic
according to a report by the IQVIA Institute for Human Data Science.
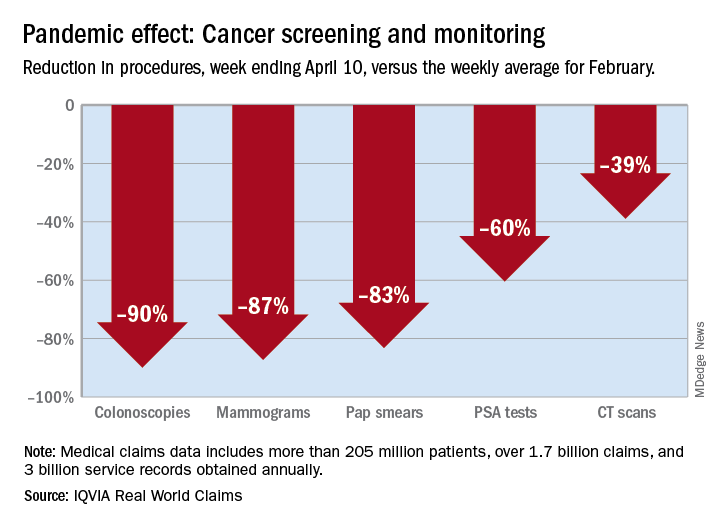
There were 90% fewer colonoscopies ordered during the week ending April 10, compared with the weekly average for Feb. 1-28, based on claims data analyzed by IQVIA.
IQVIA’s medical claims database includes more than 205 million patients, over 1.7 billion claims, and 3 billion service records obtained annually.
The data also showed an 87% reduction in mammograms and an 83% reduction in Pap smears during the week ending April 10. Prostate-specific antigen tests for prostate cancer decreased by 60%, and CT scans for lung cancer decreased by 39%.
The smaller decrease in CT scans for lung cancer “may reflect the generally more serious nature of those tumors or be due to concerns about ruling out COVID-related issues in some patients,” according to report authors Murray Aitken and Michael Kleinrock, both of IQVIA.
The report also showed that overall patient interactions with oncologists were down by 20% through April 3, based on medical and pharmacy claims processed since February, but there was variation by tumor type.
The authors noted “little or no disruption” in oncologist visits in March for patients with aggressive tumors or those diagnosed at advanced stages, compared with February. However, for patients with skin cancer or prostate cancer, visit rates were down by 20%-50% in March.
“This may reflect that oncologists who are providing care across multiple tumor types are prioritizing their time and efforts to those patients with more advanced or aggressive tumors,” the authors wrote.
This report was produced by the IQVIA Institute for Human Data Science without industry or government funding.
SOURCE: Murray A and Kleinrock M. Shifts in healthcare demand, delivery and care during the COVID-19 era. IQVIA Institute for Human Data Science. April 2020.
according to a report by the IQVIA Institute for Human Data Science.

There were 90% fewer colonoscopies ordered during the week ending April 10, compared with the weekly average for Feb. 1-28, based on claims data analyzed by IQVIA.
IQVIA’s medical claims database includes more than 205 million patients, over 1.7 billion claims, and 3 billion service records obtained annually.
The data also showed an 87% reduction in mammograms and an 83% reduction in Pap smears during the week ending April 10. Prostate-specific antigen tests for prostate cancer decreased by 60%, and CT scans for lung cancer decreased by 39%.
The smaller decrease in CT scans for lung cancer “may reflect the generally more serious nature of those tumors or be due to concerns about ruling out COVID-related issues in some patients,” according to report authors Murray Aitken and Michael Kleinrock, both of IQVIA.
The report also showed that overall patient interactions with oncologists were down by 20% through April 3, based on medical and pharmacy claims processed since February, but there was variation by tumor type.
The authors noted “little or no disruption” in oncologist visits in March for patients with aggressive tumors or those diagnosed at advanced stages, compared with February. However, for patients with skin cancer or prostate cancer, visit rates were down by 20%-50% in March.
“This may reflect that oncologists who are providing care across multiple tumor types are prioritizing their time and efforts to those patients with more advanced or aggressive tumors,” the authors wrote.
This report was produced by the IQVIA Institute for Human Data Science without industry or government funding.
SOURCE: Murray A and Kleinrock M. Shifts in healthcare demand, delivery and care during the COVID-19 era. IQVIA Institute for Human Data Science. April 2020.
according to a report by the IQVIA Institute for Human Data Science.

There were 90% fewer colonoscopies ordered during the week ending April 10, compared with the weekly average for Feb. 1-28, based on claims data analyzed by IQVIA.
IQVIA’s medical claims database includes more than 205 million patients, over 1.7 billion claims, and 3 billion service records obtained annually.
The data also showed an 87% reduction in mammograms and an 83% reduction in Pap smears during the week ending April 10. Prostate-specific antigen tests for prostate cancer decreased by 60%, and CT scans for lung cancer decreased by 39%.
The smaller decrease in CT scans for lung cancer “may reflect the generally more serious nature of those tumors or be due to concerns about ruling out COVID-related issues in some patients,” according to report authors Murray Aitken and Michael Kleinrock, both of IQVIA.
The report also showed that overall patient interactions with oncologists were down by 20% through April 3, based on medical and pharmacy claims processed since February, but there was variation by tumor type.
The authors noted “little or no disruption” in oncologist visits in March for patients with aggressive tumors or those diagnosed at advanced stages, compared with February. However, for patients with skin cancer or prostate cancer, visit rates were down by 20%-50% in March.
“This may reflect that oncologists who are providing care across multiple tumor types are prioritizing their time and efforts to those patients with more advanced or aggressive tumors,” the authors wrote.
This report was produced by the IQVIA Institute for Human Data Science without industry or government funding.
SOURCE: Murray A and Kleinrock M. Shifts in healthcare demand, delivery and care during the COVID-19 era. IQVIA Institute for Human Data Science. April 2020.
Excess cancer deaths predicted as care is disrupted by COVID-19
The majority of patients who have cancer or are suspected of having cancer are not accessing healthcare services in the United Kingdom or the United States because of the COVID-19 pandemic, the first report of its kind estimates.
As a result, there will be an excess of deaths among patients who have cancer and multiple comorbidities in both countries during the current coronavirus emergency, the report warns.
The authors calculate that there will be 6,270 excess deaths among cancer patients 1 year from now in England and 33,890 excess deaths among cancer patients in the United States. (In the United States, the estimated excess number of deaths applies only to patients older than 40 years, they note.)
“The recorded underlying cause of these excess deaths may be cancer, COVID-19, or comorbidity (such as myocardial infarction),” Alvina Lai, PhD, University College London, United Kingdom, and colleagues observe.
“Our data have highlighted how cancer patients with multimorbidity are a particularly at-risk group during the current pandemic,” they emphasize.
The study was published on ResearchGate as a preprint and has not undergone peer review.
Commenting on the study on the UK Science Media Center, several experts emphasized the lack of peer review, noting that interpretation of these data needs to be further refined on the basis of that input. One expert suggested that there are “substantial uncertainties that this paper does not adequately communicate.” But others argued that this topic was important enough to warrant early release of the data.
Chris Bunce, PhD, University of Birmingham, United Kingdom, said this study represents “a highly valuable contribution.”
“It is universally accepted that early diagnosis and treatment and adherence to treatment regimens saves lives,” he pointed out.
“Therefore, these COVID-19-related impacts will cost lives,” Bunce said.
“And if this information is to influence cancer care and guide policy during the COVID-19 crisis, then it is important that the findings are disseminated and discussed immediately, warranting their release ahead of peer view,” he added.
In a Medscape UK commentary, oncologist Karol Sikora, MD, PhD, argues that “restarting cancer services can’t come soon enough.”
“Resonably Argued Numerical Estimate”
“It’s well known that there have been considerable changes in the provision of health care for many conditions, including cancers, as a result of all the measures to deal with the COVID-19 crisis,” said Kevin McConway, PhD, professor emeritus of applied statistics, the Open University, Milton Keynes, United Kingdom.
“It seems inevitable that there will be increased deaths in cancer patients if they are infected with the virus or because of changes in the health services available to them, and quite possibly also from socio-economic effects of the responses to the crisis,” he continued.
“This study is the first that I have seen that produces a reasonably argued numerical estimate of the number of excess deaths of people with cancer arising from these factors in the UK and the USA,” he added.
Declines in Urgent Referrals and Chemo Attendance
For the study, the team used DATA-CAN, the UK National Health Data Research Hub for Cancer, to assess weekly returns for urgent cancer referrals for early diagnosis and also chemotherapy attendances for hospitals in Leeds, London, and Northern Ireland going back to 2018.
The data revealed that there have been major declines in chemotherapy attendances. There has been, on average, a 60% decrease from prepandemic levels in eight hospitals in the three regions that were assessed.
Urgent cancer referrals have dropped by an average of 76% compared to prepandemic levels in the three regions.
On the conservative assumption that the COVID-19 pandemic will only affect patients with newly diagnosed cancer (incident cases), the researchers estimate that the proportion of the population affected by the emergency (PAE) is 40% and that the relative impact of the emergency (RIE) is 1.5.
PAE is a summary measure of exposure to the adverse health consequences of the emergency; RIE is a summary measure of the combined impact on mortality of infection, health service change, physical distancing, and economic downturn, the authors explain.
Comorbidities Common
“Comorbidities were common in people with cancer,” the study authors note. For example, more than one quarter of the study population had at least one comorbidity; more than 14% had two.
For incident cancers, the number of excess deaths steadily increased in conjunction with an increase in the number of comorbidities, such that more than 80% of deaths occurred in patients with one or more comorbidities.
“When considering both prevalent and incident cancers together with a COVID-19 PAE of 40%, we estimated 17,991 excess deaths at a RIE of 1.5; 78.1% of these deaths occur in patients with ≥1 comorbidities,” the authors report.
“The excess risk of death in people living with cancer during the COVID-19 emergency may be due not only to COVID-19 infection, but also to the unintended health consequences of changes in health service provision, the physical or psychological effects of social distancing, and economic upheaval,” they state.
“This is the first study demonstrating profound recent changes in cancer care delivery in multiple centers,” the authors observe.
Lai has disclosed no relevant financial relationships. Several coauthors have various relationships with industry, as listed in their article. The commentators have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The majority of patients who have cancer or are suspected of having cancer are not accessing healthcare services in the United Kingdom or the United States because of the COVID-19 pandemic, the first report of its kind estimates.
As a result, there will be an excess of deaths among patients who have cancer and multiple comorbidities in both countries during the current coronavirus emergency, the report warns.
The authors calculate that there will be 6,270 excess deaths among cancer patients 1 year from now in England and 33,890 excess deaths among cancer patients in the United States. (In the United States, the estimated excess number of deaths applies only to patients older than 40 years, they note.)
“The recorded underlying cause of these excess deaths may be cancer, COVID-19, or comorbidity (such as myocardial infarction),” Alvina Lai, PhD, University College London, United Kingdom, and colleagues observe.
“Our data have highlighted how cancer patients with multimorbidity are a particularly at-risk group during the current pandemic,” they emphasize.
The study was published on ResearchGate as a preprint and has not undergone peer review.
Commenting on the study on the UK Science Media Center, several experts emphasized the lack of peer review, noting that interpretation of these data needs to be further refined on the basis of that input. One expert suggested that there are “substantial uncertainties that this paper does not adequately communicate.” But others argued that this topic was important enough to warrant early release of the data.
Chris Bunce, PhD, University of Birmingham, United Kingdom, said this study represents “a highly valuable contribution.”
“It is universally accepted that early diagnosis and treatment and adherence to treatment regimens saves lives,” he pointed out.
“Therefore, these COVID-19-related impacts will cost lives,” Bunce said.
“And if this information is to influence cancer care and guide policy during the COVID-19 crisis, then it is important that the findings are disseminated and discussed immediately, warranting their release ahead of peer view,” he added.
In a Medscape UK commentary, oncologist Karol Sikora, MD, PhD, argues that “restarting cancer services can’t come soon enough.”
“Resonably Argued Numerical Estimate”
“It’s well known that there have been considerable changes in the provision of health care for many conditions, including cancers, as a result of all the measures to deal with the COVID-19 crisis,” said Kevin McConway, PhD, professor emeritus of applied statistics, the Open University, Milton Keynes, United Kingdom.
“It seems inevitable that there will be increased deaths in cancer patients if they are infected with the virus or because of changes in the health services available to them, and quite possibly also from socio-economic effects of the responses to the crisis,” he continued.
“This study is the first that I have seen that produces a reasonably argued numerical estimate of the number of excess deaths of people with cancer arising from these factors in the UK and the USA,” he added.
Declines in Urgent Referrals and Chemo Attendance
For the study, the team used DATA-CAN, the UK National Health Data Research Hub for Cancer, to assess weekly returns for urgent cancer referrals for early diagnosis and also chemotherapy attendances for hospitals in Leeds, London, and Northern Ireland going back to 2018.
The data revealed that there have been major declines in chemotherapy attendances. There has been, on average, a 60% decrease from prepandemic levels in eight hospitals in the three regions that were assessed.
Urgent cancer referrals have dropped by an average of 76% compared to prepandemic levels in the three regions.
On the conservative assumption that the COVID-19 pandemic will only affect patients with newly diagnosed cancer (incident cases), the researchers estimate that the proportion of the population affected by the emergency (PAE) is 40% and that the relative impact of the emergency (RIE) is 1.5.
PAE is a summary measure of exposure to the adverse health consequences of the emergency; RIE is a summary measure of the combined impact on mortality of infection, health service change, physical distancing, and economic downturn, the authors explain.
Comorbidities Common
“Comorbidities were common in people with cancer,” the study authors note. For example, more than one quarter of the study population had at least one comorbidity; more than 14% had two.
For incident cancers, the number of excess deaths steadily increased in conjunction with an increase in the number of comorbidities, such that more than 80% of deaths occurred in patients with one or more comorbidities.
“When considering both prevalent and incident cancers together with a COVID-19 PAE of 40%, we estimated 17,991 excess deaths at a RIE of 1.5; 78.1% of these deaths occur in patients with ≥1 comorbidities,” the authors report.
“The excess risk of death in people living with cancer during the COVID-19 emergency may be due not only to COVID-19 infection, but also to the unintended health consequences of changes in health service provision, the physical or psychological effects of social distancing, and economic upheaval,” they state.
“This is the first study demonstrating profound recent changes in cancer care delivery in multiple centers,” the authors observe.
Lai has disclosed no relevant financial relationships. Several coauthors have various relationships with industry, as listed in their article. The commentators have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The majority of patients who have cancer or are suspected of having cancer are not accessing healthcare services in the United Kingdom or the United States because of the COVID-19 pandemic, the first report of its kind estimates.
As a result, there will be an excess of deaths among patients who have cancer and multiple comorbidities in both countries during the current coronavirus emergency, the report warns.
The authors calculate that there will be 6,270 excess deaths among cancer patients 1 year from now in England and 33,890 excess deaths among cancer patients in the United States. (In the United States, the estimated excess number of deaths applies only to patients older than 40 years, they note.)
“The recorded underlying cause of these excess deaths may be cancer, COVID-19, or comorbidity (such as myocardial infarction),” Alvina Lai, PhD, University College London, United Kingdom, and colleagues observe.
“Our data have highlighted how cancer patients with multimorbidity are a particularly at-risk group during the current pandemic,” they emphasize.
The study was published on ResearchGate as a preprint and has not undergone peer review.
Commenting on the study on the UK Science Media Center, several experts emphasized the lack of peer review, noting that interpretation of these data needs to be further refined on the basis of that input. One expert suggested that there are “substantial uncertainties that this paper does not adequately communicate.” But others argued that this topic was important enough to warrant early release of the data.
Chris Bunce, PhD, University of Birmingham, United Kingdom, said this study represents “a highly valuable contribution.”
“It is universally accepted that early diagnosis and treatment and adherence to treatment regimens saves lives,” he pointed out.
“Therefore, these COVID-19-related impacts will cost lives,” Bunce said.
“And if this information is to influence cancer care and guide policy during the COVID-19 crisis, then it is important that the findings are disseminated and discussed immediately, warranting their release ahead of peer view,” he added.
In a Medscape UK commentary, oncologist Karol Sikora, MD, PhD, argues that “restarting cancer services can’t come soon enough.”
“Resonably Argued Numerical Estimate”
“It’s well known that there have been considerable changes in the provision of health care for many conditions, including cancers, as a result of all the measures to deal with the COVID-19 crisis,” said Kevin McConway, PhD, professor emeritus of applied statistics, the Open University, Milton Keynes, United Kingdom.
“It seems inevitable that there will be increased deaths in cancer patients if they are infected with the virus or because of changes in the health services available to them, and quite possibly also from socio-economic effects of the responses to the crisis,” he continued.
“This study is the first that I have seen that produces a reasonably argued numerical estimate of the number of excess deaths of people with cancer arising from these factors in the UK and the USA,” he added.
Declines in Urgent Referrals and Chemo Attendance
For the study, the team used DATA-CAN, the UK National Health Data Research Hub for Cancer, to assess weekly returns for urgent cancer referrals for early diagnosis and also chemotherapy attendances for hospitals in Leeds, London, and Northern Ireland going back to 2018.
The data revealed that there have been major declines in chemotherapy attendances. There has been, on average, a 60% decrease from prepandemic levels in eight hospitals in the three regions that were assessed.
Urgent cancer referrals have dropped by an average of 76% compared to prepandemic levels in the three regions.
On the conservative assumption that the COVID-19 pandemic will only affect patients with newly diagnosed cancer (incident cases), the researchers estimate that the proportion of the population affected by the emergency (PAE) is 40% and that the relative impact of the emergency (RIE) is 1.5.
PAE is a summary measure of exposure to the adverse health consequences of the emergency; RIE is a summary measure of the combined impact on mortality of infection, health service change, physical distancing, and economic downturn, the authors explain.
Comorbidities Common
“Comorbidities were common in people with cancer,” the study authors note. For example, more than one quarter of the study population had at least one comorbidity; more than 14% had two.
For incident cancers, the number of excess deaths steadily increased in conjunction with an increase in the number of comorbidities, such that more than 80% of deaths occurred in patients with one or more comorbidities.
“When considering both prevalent and incident cancers together with a COVID-19 PAE of 40%, we estimated 17,991 excess deaths at a RIE of 1.5; 78.1% of these deaths occur in patients with ≥1 comorbidities,” the authors report.
“The excess risk of death in people living with cancer during the COVID-19 emergency may be due not only to COVID-19 infection, but also to the unintended health consequences of changes in health service provision, the physical or psychological effects of social distancing, and economic upheaval,” they state.
“This is the first study demonstrating profound recent changes in cancer care delivery in multiple centers,” the authors observe.
Lai has disclosed no relevant financial relationships. Several coauthors have various relationships with industry, as listed in their article. The commentators have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Antitumor treatment may increase risk of severe events in COVID-19 patients
Cancer patients who received antitumor treatment within 14 days of COVID-19 diagnosis had an increased risk of severe events, according to data from three hospitals in Wuhan.
Patients with patchy consolidation at hospital admission also had an increased risk of severe events, defined as ICU admission, mechanical ventilation, or death.
However, these findings are limited by the small number of patients studied and the retrospective nature of the analysis, according to researchers.
Li Zhang, MD, PhD, of Tongji Hospital in Wuhan, China, presented this research at the AACR virtual meeting I. Some of the data were previously published in Annals of Oncology.
The researchers studied 28 patients with cancer among 1,276 patients with COVID-19 treated at three hospitals in Wuhan. The most common cancer types were lung (n = 7), esophageal (n = 4), and breast (n = 3). Patients had other gastrointestinal, gynecologic, genitourinary, and head and neck cancers as well.
The patients’ median age was 65 years (range, 56-70 years), 60.9% were men, 35.7% had stage IV cancer, and 28.6% had hospital-acquired COVID-19. Antitumor treatments included chemotherapy (n = 22), surgery (n = 21), radiotherapy (n = 21), targeted therapy (n = 5), and immune checkpoint inhibitors (n = 2).
COVID-19 treatment
Most patients (n = 22) received oxygen as their only respiratory intervention, although 10 received mechanical ventilation.
For systemic therapy, patients received antibiotic treatment (n = 23), corticosteroids (n = 15), intravenous immunoglobulin (n = 10), and tocilizumab (n = 1).
Antiviral treatments included umifenovir (n = 14), lopinavir/ritonavir (n = 10), ganciclovir (n = 9), ribavirin (n = 1), or a combination of antiviral drugs (n = 9).
“No cancer patients were enrolled in clinical trials, so no one received hydroxychloroquine or remdesivir,” Dr. Zhang noted.
Outcomes
In all, 15 patients (53.6%) had severe events. The median time from COVID-19 diagnosis to severe events was 7 days (range, 5-15 days).
A total of eight patients (28.6%) died – three with lung cancer, two with prostate cancer, one with liver cancer, one with rectal cancer, and one with testicular cancer.
Causes of death were acute respiratory distress syndrome (n = 5), septic shock (n = 1), suspected pulmonary embolism (n = 1), and acute myocardial infarction (n = 1).
By April 4, 14 patients had been discharged from the hospital, and 6 were still hospitalized. The median duration of hospitalization was 18.4 days for discharged patients and 29.4 days for patients still in hospital.
Follow-up CT scans showed improvement in 13 patients, no changes in 5 patients, and deterioration in 6 patients.
Factors associated with severe events
In a multivariable analysis, receiving antitumor treatment within 14 days of COVID-19 diagnosis was associated with severe events (hazard ratio, 4.079; P = .037).
However, only seven patients received antitumor treatments within 14 days of COVID-19 diagnosis – three chemotherapy, two targeted therapy, one radiotherapy, and one immune checkpoint inhibitor. Five of these seven patients had severe events.
Another factor associated with severe events in multivariable analysis was patchy consolidation on CT scan at admission (HR, 5.438; P = .01). Age and gender were not significantly associated with severe events.
Immune checkpoint inhibitors
Dr. Zhang and colleagues also analyzed a second group of cancer patients and their family members to determine if patients on immune checkpoint inhibitors have an increased risk of COVID-19.
This group included 124 cancer patients treated with immune checkpoint inhibitors for at least 2 months. The patients had a median age of 59 years (range, 54-65 years), and 61.8% were men. Most patients (95.2%) had stage IV cancer, and the most common cancers were lung (54.0%), esophageal (18.6%), and head and neck (10.7%).
In this group, only one cancer patient developed COVID-19 (via nosocomial infection). In another case, a patient’s spouse developed COVID-19, but the patient did not.
Dr. Zhang said this “limited information did not suggest cancer patients treated with immune checkpoint inhibitors were more vulnerable to COVID infection.”
Dr. Zhang and colleagues reported no conflicts of interest. This research was funded by the National Natural Science Foundation of China and Huazhong University of Science and Technology COVID-19 Rapid Response Call China.
SOURCE: Zhang L et al. Ann Oncol. 2020 Mar 26. doi: 10.1016/j.annonc.2020.03.296.
Cancer patients who received antitumor treatment within 14 days of COVID-19 diagnosis had an increased risk of severe events, according to data from three hospitals in Wuhan.
Patients with patchy consolidation at hospital admission also had an increased risk of severe events, defined as ICU admission, mechanical ventilation, or death.
However, these findings are limited by the small number of patients studied and the retrospective nature of the analysis, according to researchers.
Li Zhang, MD, PhD, of Tongji Hospital in Wuhan, China, presented this research at the AACR virtual meeting I. Some of the data were previously published in Annals of Oncology.
The researchers studied 28 patients with cancer among 1,276 patients with COVID-19 treated at three hospitals in Wuhan. The most common cancer types were lung (n = 7), esophageal (n = 4), and breast (n = 3). Patients had other gastrointestinal, gynecologic, genitourinary, and head and neck cancers as well.
The patients’ median age was 65 years (range, 56-70 years), 60.9% were men, 35.7% had stage IV cancer, and 28.6% had hospital-acquired COVID-19. Antitumor treatments included chemotherapy (n = 22), surgery (n = 21), radiotherapy (n = 21), targeted therapy (n = 5), and immune checkpoint inhibitors (n = 2).
COVID-19 treatment
Most patients (n = 22) received oxygen as their only respiratory intervention, although 10 received mechanical ventilation.
For systemic therapy, patients received antibiotic treatment (n = 23), corticosteroids (n = 15), intravenous immunoglobulin (n = 10), and tocilizumab (n = 1).
Antiviral treatments included umifenovir (n = 14), lopinavir/ritonavir (n = 10), ganciclovir (n = 9), ribavirin (n = 1), or a combination of antiviral drugs (n = 9).
“No cancer patients were enrolled in clinical trials, so no one received hydroxychloroquine or remdesivir,” Dr. Zhang noted.
Outcomes
In all, 15 patients (53.6%) had severe events. The median time from COVID-19 diagnosis to severe events was 7 days (range, 5-15 days).
A total of eight patients (28.6%) died – three with lung cancer, two with prostate cancer, one with liver cancer, one with rectal cancer, and one with testicular cancer.
Causes of death were acute respiratory distress syndrome (n = 5), septic shock (n = 1), suspected pulmonary embolism (n = 1), and acute myocardial infarction (n = 1).
By April 4, 14 patients had been discharged from the hospital, and 6 were still hospitalized. The median duration of hospitalization was 18.4 days for discharged patients and 29.4 days for patients still in hospital.
Follow-up CT scans showed improvement in 13 patients, no changes in 5 patients, and deterioration in 6 patients.
Factors associated with severe events
In a multivariable analysis, receiving antitumor treatment within 14 days of COVID-19 diagnosis was associated with severe events (hazard ratio, 4.079; P = .037).
However, only seven patients received antitumor treatments within 14 days of COVID-19 diagnosis – three chemotherapy, two targeted therapy, one radiotherapy, and one immune checkpoint inhibitor. Five of these seven patients had severe events.
Another factor associated with severe events in multivariable analysis was patchy consolidation on CT scan at admission (HR, 5.438; P = .01). Age and gender were not significantly associated with severe events.
Immune checkpoint inhibitors
Dr. Zhang and colleagues also analyzed a second group of cancer patients and their family members to determine if patients on immune checkpoint inhibitors have an increased risk of COVID-19.
This group included 124 cancer patients treated with immune checkpoint inhibitors for at least 2 months. The patients had a median age of 59 years (range, 54-65 years), and 61.8% were men. Most patients (95.2%) had stage IV cancer, and the most common cancers were lung (54.0%), esophageal (18.6%), and head and neck (10.7%).
In this group, only one cancer patient developed COVID-19 (via nosocomial infection). In another case, a patient’s spouse developed COVID-19, but the patient did not.
Dr. Zhang said this “limited information did not suggest cancer patients treated with immune checkpoint inhibitors were more vulnerable to COVID infection.”
Dr. Zhang and colleagues reported no conflicts of interest. This research was funded by the National Natural Science Foundation of China and Huazhong University of Science and Technology COVID-19 Rapid Response Call China.
SOURCE: Zhang L et al. Ann Oncol. 2020 Mar 26. doi: 10.1016/j.annonc.2020.03.296.
Cancer patients who received antitumor treatment within 14 days of COVID-19 diagnosis had an increased risk of severe events, according to data from three hospitals in Wuhan.
Patients with patchy consolidation at hospital admission also had an increased risk of severe events, defined as ICU admission, mechanical ventilation, or death.
However, these findings are limited by the small number of patients studied and the retrospective nature of the analysis, according to researchers.
Li Zhang, MD, PhD, of Tongji Hospital in Wuhan, China, presented this research at the AACR virtual meeting I. Some of the data were previously published in Annals of Oncology.
The researchers studied 28 patients with cancer among 1,276 patients with COVID-19 treated at three hospitals in Wuhan. The most common cancer types were lung (n = 7), esophageal (n = 4), and breast (n = 3). Patients had other gastrointestinal, gynecologic, genitourinary, and head and neck cancers as well.
The patients’ median age was 65 years (range, 56-70 years), 60.9% were men, 35.7% had stage IV cancer, and 28.6% had hospital-acquired COVID-19. Antitumor treatments included chemotherapy (n = 22), surgery (n = 21), radiotherapy (n = 21), targeted therapy (n = 5), and immune checkpoint inhibitors (n = 2).
COVID-19 treatment
Most patients (n = 22) received oxygen as their only respiratory intervention, although 10 received mechanical ventilation.
For systemic therapy, patients received antibiotic treatment (n = 23), corticosteroids (n = 15), intravenous immunoglobulin (n = 10), and tocilizumab (n = 1).
Antiviral treatments included umifenovir (n = 14), lopinavir/ritonavir (n = 10), ganciclovir (n = 9), ribavirin (n = 1), or a combination of antiviral drugs (n = 9).
“No cancer patients were enrolled in clinical trials, so no one received hydroxychloroquine or remdesivir,” Dr. Zhang noted.
Outcomes
In all, 15 patients (53.6%) had severe events. The median time from COVID-19 diagnosis to severe events was 7 days (range, 5-15 days).
A total of eight patients (28.6%) died – three with lung cancer, two with prostate cancer, one with liver cancer, one with rectal cancer, and one with testicular cancer.
Causes of death were acute respiratory distress syndrome (n = 5), septic shock (n = 1), suspected pulmonary embolism (n = 1), and acute myocardial infarction (n = 1).
By April 4, 14 patients had been discharged from the hospital, and 6 were still hospitalized. The median duration of hospitalization was 18.4 days for discharged patients and 29.4 days for patients still in hospital.
Follow-up CT scans showed improvement in 13 patients, no changes in 5 patients, and deterioration in 6 patients.
Factors associated with severe events
In a multivariable analysis, receiving antitumor treatment within 14 days of COVID-19 diagnosis was associated with severe events (hazard ratio, 4.079; P = .037).
However, only seven patients received antitumor treatments within 14 days of COVID-19 diagnosis – three chemotherapy, two targeted therapy, one radiotherapy, and one immune checkpoint inhibitor. Five of these seven patients had severe events.
Another factor associated with severe events in multivariable analysis was patchy consolidation on CT scan at admission (HR, 5.438; P = .01). Age and gender were not significantly associated with severe events.
Immune checkpoint inhibitors
Dr. Zhang and colleagues also analyzed a second group of cancer patients and their family members to determine if patients on immune checkpoint inhibitors have an increased risk of COVID-19.
This group included 124 cancer patients treated with immune checkpoint inhibitors for at least 2 months. The patients had a median age of 59 years (range, 54-65 years), and 61.8% were men. Most patients (95.2%) had stage IV cancer, and the most common cancers were lung (54.0%), esophageal (18.6%), and head and neck (10.7%).
In this group, only one cancer patient developed COVID-19 (via nosocomial infection). In another case, a patient’s spouse developed COVID-19, but the patient did not.
Dr. Zhang said this “limited information did not suggest cancer patients treated with immune checkpoint inhibitors were more vulnerable to COVID infection.”
Dr. Zhang and colleagues reported no conflicts of interest. This research was funded by the National Natural Science Foundation of China and Huazhong University of Science and Technology COVID-19 Rapid Response Call China.
SOURCE: Zhang L et al. Ann Oncol. 2020 Mar 26. doi: 10.1016/j.annonc.2020.03.296.
FROM AACR 2020
It’s a bust: Adding immunotherapy confers no benefit in mCRPC
No clinical benefit was seen from adding the immune checkpoint inhibitor atezolizumab (Tecentriq, Genentech) to standard treatment with the androgen receptor inhibitor enzalutamide (Xtandi; Astellas Pharma) for men with metastatic castration-resistant prostate cancer (mCRPC).
Median overall survival, the trial’s primary endpoint, was numerically but not statistically longer among patients with mCRPC who were randomly assigned to receive treatment with enzalutamide alone, reported lead investigator Christopher J. Sweeney, MBBS, from the Dana-Farber Cancer Institute in Boston.
The finding comes from the phase 3 IMbassador250 trial.
This was “the first phase 3 trial to investigate a checkpoint inhibitor therapy combination in metastatic CRPC. It revealed no evidence of a difference in cancer control between arms, whether it be measured by radiographic progression-free survival or time to PSA [prostate-specific antigen] progression,” he said.
Adding insult to injury, the investigators could not identify, on the basis of expression of programmed death–ligand 1 (PD-L1), a subpopulation of patients who might have benefited from the combination therapy, and adverse events were more frequent than with enzalutamide alone, Sweeney said.
Sweeney presented the results online during the American Association for Cancer Research (AACR) 2020 virtual meeting.
What happened?
It sounded good on paper: the clinical rationale for combining an immune checkpoint inhibitor with enzalutamide was that there was evidence that immunotherapy with sipuleucel-T (Provenge, Dendreon Pharmaceuticals) had efficacy in CRPC, and initial investigations with ipilimumab (Yervoy, Bristol-Myers Squibb) that showed an increase in antigen-specific T cells following treatment with sipuleucel-T, Sweeney said.
In addition, there has been evidence that programmed death–1 (PD-1) blockade may have activity following the development of resistance to enzalutamide and that monotherapy with atezolizumab, an inhibitor of PD-1 and PD-L1, was associated with long-term disease control in mCRPC, he added.
However, these new results “indicate that neither anti-PD-1/PD-L1 monotherapy or the anti-PD-1/PD-L1 combination therapy with enzalutamide are likely to provide improved clinical benefit over standard-of-care agents for patients with mCRPC whose disease progressed on prior hormonal therapy and prior chemotherapy,” commented Padmanee Sharma, MD, PhD, from the University of Texas MD Anderson Cancer Center in Houston, who was the invited discussant.
Discussing why the IMbassador250 trial may have failed, she noted that prostate cancer has few T cells, and therefore targeting PD-1 or PD-L1 would not have much of an effect.
“There are multiple immunosuppressive pathways within the prostate tumor microenvironment, and the PD-1/PD-L1-targeting agents may not sufficiently target all these immunosuppressive pathways,” she said.
In addition, “there are few mutations in prostate cancer, and as a result, the effector T cells may not be able to recognize an adequate number of antigens to lead to an antitumor response,” Sharma said.
Study details
The IMbassador250 study was a phase 3, multicenter, randomized, open-label study involving 759 men with metastatic, locally advanced, or incurable CRPC who had experienced disease progression with abiraterone (Zytiga, Janssen), were ineligible or refused a taxane-based regimen, or for whom a taxane regimen had failed.
After stratification on the basis of prior taxane therapy, presence of liver metastases, lactate dehydrogenase levels, and pain severity in the past 24 hours, the patients were randomly assigned to receive either enzalutamide 160 mg daily or the same regimen plus atezolizumab 1200 mg intravenously every 3 weeks. Treatment continued until loss of clinical benefit or unacceptable toxicity.
Median overall survival (OS) was 15.2 months with the combination, compared with 16.6 months for enzalutamide alone, which translated into a hazard ratio for death with the combination of 1.12 (P = .28).
The 12-month OS rates were 60.6% for enzalutamide alone versus 64.7% for the combination.
An analysis of OS by clinical subgroup found no advantage from the combination over enzalutamide alone for prior docetaxel exposure, prior local therapy, measurable disease status, the presence of visceral or nonvisceral metastases, or PD-L1 expression.
Radiographic progression-free survival was 4.2 months with atezolizumab/enzalutamide versus 4.1 months with enzalutamide alone (P = ns). Time to PSA progression was 2.8 months in each arm.
Among patients with measurable disease at baseline, the overall response rates were 14% in the combination group and 7% in the group that received enzalutamide alone. Two patients in the combination group and one in the enzalutamide-only group had complete responses.
Grade 3 or 4 treatment-related adverse events (AEs) occurred in 28% of patients who received the combination, versus 10% who received enzalutamide alone. Seven patients in the combination arm and one in the enzalutamide arm died from treatment-related causes. Treatment-related serious AEs were also more frequent with the combination (14% vs. 3%), and AEs that led to discontinuation of any treatment component occurred in 14% of patients in the combination arm, versus 6% in the enzalutamide-alone arm.
The study was sponsored by F. Hoffman–La Roche. Enzalutamide was provided by Astellas and Pfizer. Sweeny has advisory or consulting roles and/or has received research funding from the companies. Sharma has consulting roles, has engaged in advisory board activities, or owns stock in for various companies, not including the sponsors.
This article first appeared on Medscape.com.
No clinical benefit was seen from adding the immune checkpoint inhibitor atezolizumab (Tecentriq, Genentech) to standard treatment with the androgen receptor inhibitor enzalutamide (Xtandi; Astellas Pharma) for men with metastatic castration-resistant prostate cancer (mCRPC).
Median overall survival, the trial’s primary endpoint, was numerically but not statistically longer among patients with mCRPC who were randomly assigned to receive treatment with enzalutamide alone, reported lead investigator Christopher J. Sweeney, MBBS, from the Dana-Farber Cancer Institute in Boston.
The finding comes from the phase 3 IMbassador250 trial.
This was “the first phase 3 trial to investigate a checkpoint inhibitor therapy combination in metastatic CRPC. It revealed no evidence of a difference in cancer control between arms, whether it be measured by radiographic progression-free survival or time to PSA [prostate-specific antigen] progression,” he said.
Adding insult to injury, the investigators could not identify, on the basis of expression of programmed death–ligand 1 (PD-L1), a subpopulation of patients who might have benefited from the combination therapy, and adverse events were more frequent than with enzalutamide alone, Sweeney said.
Sweeney presented the results online during the American Association for Cancer Research (AACR) 2020 virtual meeting.
What happened?
It sounded good on paper: the clinical rationale for combining an immune checkpoint inhibitor with enzalutamide was that there was evidence that immunotherapy with sipuleucel-T (Provenge, Dendreon Pharmaceuticals) had efficacy in CRPC, and initial investigations with ipilimumab (Yervoy, Bristol-Myers Squibb) that showed an increase in antigen-specific T cells following treatment with sipuleucel-T, Sweeney said.
In addition, there has been evidence that programmed death–1 (PD-1) blockade may have activity following the development of resistance to enzalutamide and that monotherapy with atezolizumab, an inhibitor of PD-1 and PD-L1, was associated with long-term disease control in mCRPC, he added.
However, these new results “indicate that neither anti-PD-1/PD-L1 monotherapy or the anti-PD-1/PD-L1 combination therapy with enzalutamide are likely to provide improved clinical benefit over standard-of-care agents for patients with mCRPC whose disease progressed on prior hormonal therapy and prior chemotherapy,” commented Padmanee Sharma, MD, PhD, from the University of Texas MD Anderson Cancer Center in Houston, who was the invited discussant.
Discussing why the IMbassador250 trial may have failed, she noted that prostate cancer has few T cells, and therefore targeting PD-1 or PD-L1 would not have much of an effect.
“There are multiple immunosuppressive pathways within the prostate tumor microenvironment, and the PD-1/PD-L1-targeting agents may not sufficiently target all these immunosuppressive pathways,” she said.
In addition, “there are few mutations in prostate cancer, and as a result, the effector T cells may not be able to recognize an adequate number of antigens to lead to an antitumor response,” Sharma said.
Study details
The IMbassador250 study was a phase 3, multicenter, randomized, open-label study involving 759 men with metastatic, locally advanced, or incurable CRPC who had experienced disease progression with abiraterone (Zytiga, Janssen), were ineligible or refused a taxane-based regimen, or for whom a taxane regimen had failed.
After stratification on the basis of prior taxane therapy, presence of liver metastases, lactate dehydrogenase levels, and pain severity in the past 24 hours, the patients were randomly assigned to receive either enzalutamide 160 mg daily or the same regimen plus atezolizumab 1200 mg intravenously every 3 weeks. Treatment continued until loss of clinical benefit or unacceptable toxicity.
Median overall survival (OS) was 15.2 months with the combination, compared with 16.6 months for enzalutamide alone, which translated into a hazard ratio for death with the combination of 1.12 (P = .28).
The 12-month OS rates were 60.6% for enzalutamide alone versus 64.7% for the combination.
An analysis of OS by clinical subgroup found no advantage from the combination over enzalutamide alone for prior docetaxel exposure, prior local therapy, measurable disease status, the presence of visceral or nonvisceral metastases, or PD-L1 expression.
Radiographic progression-free survival was 4.2 months with atezolizumab/enzalutamide versus 4.1 months with enzalutamide alone (P = ns). Time to PSA progression was 2.8 months in each arm.
Among patients with measurable disease at baseline, the overall response rates were 14% in the combination group and 7% in the group that received enzalutamide alone. Two patients in the combination group and one in the enzalutamide-only group had complete responses.
Grade 3 or 4 treatment-related adverse events (AEs) occurred in 28% of patients who received the combination, versus 10% who received enzalutamide alone. Seven patients in the combination arm and one in the enzalutamide arm died from treatment-related causes. Treatment-related serious AEs were also more frequent with the combination (14% vs. 3%), and AEs that led to discontinuation of any treatment component occurred in 14% of patients in the combination arm, versus 6% in the enzalutamide-alone arm.
The study was sponsored by F. Hoffman–La Roche. Enzalutamide was provided by Astellas and Pfizer. Sweeny has advisory or consulting roles and/or has received research funding from the companies. Sharma has consulting roles, has engaged in advisory board activities, or owns stock in for various companies, not including the sponsors.
This article first appeared on Medscape.com.
No clinical benefit was seen from adding the immune checkpoint inhibitor atezolizumab (Tecentriq, Genentech) to standard treatment with the androgen receptor inhibitor enzalutamide (Xtandi; Astellas Pharma) for men with metastatic castration-resistant prostate cancer (mCRPC).
Median overall survival, the trial’s primary endpoint, was numerically but not statistically longer among patients with mCRPC who were randomly assigned to receive treatment with enzalutamide alone, reported lead investigator Christopher J. Sweeney, MBBS, from the Dana-Farber Cancer Institute in Boston.
The finding comes from the phase 3 IMbassador250 trial.
This was “the first phase 3 trial to investigate a checkpoint inhibitor therapy combination in metastatic CRPC. It revealed no evidence of a difference in cancer control between arms, whether it be measured by radiographic progression-free survival or time to PSA [prostate-specific antigen] progression,” he said.
Adding insult to injury, the investigators could not identify, on the basis of expression of programmed death–ligand 1 (PD-L1), a subpopulation of patients who might have benefited from the combination therapy, and adverse events were more frequent than with enzalutamide alone, Sweeney said.
Sweeney presented the results online during the American Association for Cancer Research (AACR) 2020 virtual meeting.
What happened?
It sounded good on paper: the clinical rationale for combining an immune checkpoint inhibitor with enzalutamide was that there was evidence that immunotherapy with sipuleucel-T (Provenge, Dendreon Pharmaceuticals) had efficacy in CRPC, and initial investigations with ipilimumab (Yervoy, Bristol-Myers Squibb) that showed an increase in antigen-specific T cells following treatment with sipuleucel-T, Sweeney said.
In addition, there has been evidence that programmed death–1 (PD-1) blockade may have activity following the development of resistance to enzalutamide and that monotherapy with atezolizumab, an inhibitor of PD-1 and PD-L1, was associated with long-term disease control in mCRPC, he added.
However, these new results “indicate that neither anti-PD-1/PD-L1 monotherapy or the anti-PD-1/PD-L1 combination therapy with enzalutamide are likely to provide improved clinical benefit over standard-of-care agents for patients with mCRPC whose disease progressed on prior hormonal therapy and prior chemotherapy,” commented Padmanee Sharma, MD, PhD, from the University of Texas MD Anderson Cancer Center in Houston, who was the invited discussant.
Discussing why the IMbassador250 trial may have failed, she noted that prostate cancer has few T cells, and therefore targeting PD-1 or PD-L1 would not have much of an effect.
“There are multiple immunosuppressive pathways within the prostate tumor microenvironment, and the PD-1/PD-L1-targeting agents may not sufficiently target all these immunosuppressive pathways,” she said.
In addition, “there are few mutations in prostate cancer, and as a result, the effector T cells may not be able to recognize an adequate number of antigens to lead to an antitumor response,” Sharma said.
Study details
The IMbassador250 study was a phase 3, multicenter, randomized, open-label study involving 759 men with metastatic, locally advanced, or incurable CRPC who had experienced disease progression with abiraterone (Zytiga, Janssen), were ineligible or refused a taxane-based regimen, or for whom a taxane regimen had failed.
After stratification on the basis of prior taxane therapy, presence of liver metastases, lactate dehydrogenase levels, and pain severity in the past 24 hours, the patients were randomly assigned to receive either enzalutamide 160 mg daily or the same regimen plus atezolizumab 1200 mg intravenously every 3 weeks. Treatment continued until loss of clinical benefit or unacceptable toxicity.
Median overall survival (OS) was 15.2 months with the combination, compared with 16.6 months for enzalutamide alone, which translated into a hazard ratio for death with the combination of 1.12 (P = .28).
The 12-month OS rates were 60.6% for enzalutamide alone versus 64.7% for the combination.
An analysis of OS by clinical subgroup found no advantage from the combination over enzalutamide alone for prior docetaxel exposure, prior local therapy, measurable disease status, the presence of visceral or nonvisceral metastases, or PD-L1 expression.
Radiographic progression-free survival was 4.2 months with atezolizumab/enzalutamide versus 4.1 months with enzalutamide alone (P = ns). Time to PSA progression was 2.8 months in each arm.
Among patients with measurable disease at baseline, the overall response rates were 14% in the combination group and 7% in the group that received enzalutamide alone. Two patients in the combination group and one in the enzalutamide-only group had complete responses.
Grade 3 or 4 treatment-related adverse events (AEs) occurred in 28% of patients who received the combination, versus 10% who received enzalutamide alone. Seven patients in the combination arm and one in the enzalutamide arm died from treatment-related causes. Treatment-related serious AEs were also more frequent with the combination (14% vs. 3%), and AEs that led to discontinuation of any treatment component occurred in 14% of patients in the combination arm, versus 6% in the enzalutamide-alone arm.
The study was sponsored by F. Hoffman–La Roche. Enzalutamide was provided by Astellas and Pfizer. Sweeny has advisory or consulting roles and/or has received research funding from the companies. Sharma has consulting roles, has engaged in advisory board activities, or owns stock in for various companies, not including the sponsors.
This article first appeared on Medscape.com.
ASCO panel outlines cancer care challenges during COVID-19 pandemic
The COVID-19 pandemic continues to exact a heavy price on cancer patients, cancer care, and clinical trials, an expert panel reported during a presscast.
“Limited data available thus far are sobering: In Italy, about 20% of COVID-related deaths occurred in people with cancer, and, in China, COVID-19 patients who had cancer were about five times more likely than others to die or be placed on a ventilator in an intensive care unit,” said Howard A “Skip” Burris, MD, president of the American Society of Clinical Oncology and president and CEO of the Sarah Cannon Cancer Institute in Nashville, Tenn.
“We also have little evidence on returning COVID-19 patients with cancer. Physicians have to rely on limited data, anecdotal reports, and their own professional expertise” regarding the extent of increased risk to cancer patients with COVID-19, whether to interrupt or modify treatment, and the effects of cancer on recovery from COVID-19 infection, Dr. Burris said during the ASCO-sponsored online presscast.
Care of COVID-free patients
For cancer patients without COVID-19, the picture is equally dim, with the prospect of delayed surgery, chemotherapy, or screening; shortages of medications and equipment needed for critical care; the shift to telemedicine that may increase patient anxiety; and the potential loss of access to innovative therapies through clinical trials, Dr. Burris said.
“We’re concerned that some hospitals have effectively deemed all cancer surgeries to be elective, requiring them to be postponed. For patients with fast-moving or hard-to-treat cancer, this delay may be devastating,” he said.
Dr. Burris also cited concerns about delayed cancer diagnosis. “In a typical month, roughly 150,000 Americans are diagnosed with cancer. But right now, routine screening visits are postponed, and patients with pain or other warning signs may put off a doctor’s visit because of social distancing,” he said.
The pandemic has also exacerbated shortages of sedatives and opioid analgesics required for intubation and mechanical ventilation of patients.
Trials halted or slowed
Dr. Burris also briefly discussed results of a new survey, which were posted online ahead of publication in JCO Oncology Practice. The survey showed that, of 14 academic and 18 community-based cancer programs, 59.4% reported halting screening and/or enrollment for at least some clinical trials and suspending research-based clinical visits except for those where cancer treatment was delivered.
“Half of respondents reported ceasing research-only blood and/or tissue collections,” the authors of the article reported.
“Trial interruptions are devastating news for thousands of patients; in many cases, clinical trials are the best or only appropriate option for care,” Dr. Burris said.
The article authors, led by David Waterhouse, MD, of Oncology Hematology Care in Cincinnati, pointed to a silver lining in the pandemic cloud in the form of opportunities to improve clinical trials going forward.
“Nearly all respondents (90.3%) identified telehealth visits for participants as a potential improvement to clinical trial conduct, and more than three-quarters (77.4%) indicated that remote patient review of symptoms held similar potential,” the authors wrote.
Other potential improvements included remote site visits from trial sponsors and/or contract research organizations, more efficient study enrollment through secure electronic platforms, direct shipment of oral drugs to patients, remote assessments of adverse events, and streamlined data collection.
Lessons from the front lines
Another member of the presscast panel, Melissa Dillmon, MD, of the Harbin Clinic Cancer Center in Rome, Georgia, described the experience of community oncologists during the pandemic.
Her community, located in northeastern Georgia, experienced a COVID-19 outbreak in early March linked to services at two large churches. Community public health authorities issued a shelter-in-place order before the state government issued stay-at-home guidelines and shuttered all but essential business, some of which were allowed by state order to reopen as of April 24.
Dr. Dillmon’s center began screening patients for COVID-19 symptoms at the door, limited visitors or companions, instituted virtual visits and tumor boards, and set up a cancer treatment triage system that would allow essential surgeries to proceed and most infusions to continue, while delaying the start of chemotherapy when possible.
“We have encouraged patients to continue on treatment, especially if treatment is being given with curative intent, or if the cancer is responding well already to treatment,” she said.
The center, located in a community with a high prevalence of comorbidities and high incidence of lung cancer, has seen a sharp decline in colonoscopies, mammograms, and lung scans as patient shelter in place.
“We have great concerns about patients missing their screening lung scans, as this program has already proven to be finding earlier lung cancers that are curable,” Dr. Dillmon said.
A view from Washington state
Another panel member, Gary Lyman, MD, of the Fred Hutchinson Cancer Research Center in Seattle, described the response by the state of Washington, the initial epicenter of the COVID-19 outbreak in the United States.
Following identification of infections in hospitalized patients and at a nursing home in Kirkland, Washington, “our response, which began in early March and progressed through the second and third week in March at the state level, was to restrict large gatherings; progressively, schools were closed; larger businesses closed; and, by March 23, a stay-at-home policy was implemented, and all nonessential businesses were closed,” Dr. Lyman said.
“We believe, based on what has happened since that time, that this has considerably flattened the curve,” he continued.
Lessons from the Washington experience include the need to plan for a long-term disruption or alteration of cancer care, expand COVID-19 testing to all patients coming into hospitals or major clinics, institute aggressive supportive care measures, prepare for subsequent waves of infection, collect and share data, and, for remote or rural areas, identify lifelines to needed resources, Dr. Lyman said.
ASCO resources
Also speaking at the presscast, Jonathan Marron, MD, of Boston Children’s Hospital and Harvard Medical School, Boston, outlined ASCO’s guidance on allocation of scarce resources during the COVID-19 pandemic.
Richard L. Schilsky, MD, ASCO chief medical officer and executive vice president, outlined community-wide collaborations, data initiatives, and online resources for both clinicians and patients.
The COVID-19 pandemic continues to exact a heavy price on cancer patients, cancer care, and clinical trials, an expert panel reported during a presscast.
“Limited data available thus far are sobering: In Italy, about 20% of COVID-related deaths occurred in people with cancer, and, in China, COVID-19 patients who had cancer were about five times more likely than others to die or be placed on a ventilator in an intensive care unit,” said Howard A “Skip” Burris, MD, president of the American Society of Clinical Oncology and president and CEO of the Sarah Cannon Cancer Institute in Nashville, Tenn.
“We also have little evidence on returning COVID-19 patients with cancer. Physicians have to rely on limited data, anecdotal reports, and their own professional expertise” regarding the extent of increased risk to cancer patients with COVID-19, whether to interrupt or modify treatment, and the effects of cancer on recovery from COVID-19 infection, Dr. Burris said during the ASCO-sponsored online presscast.
Care of COVID-free patients
For cancer patients without COVID-19, the picture is equally dim, with the prospect of delayed surgery, chemotherapy, or screening; shortages of medications and equipment needed for critical care; the shift to telemedicine that may increase patient anxiety; and the potential loss of access to innovative therapies through clinical trials, Dr. Burris said.
“We’re concerned that some hospitals have effectively deemed all cancer surgeries to be elective, requiring them to be postponed. For patients with fast-moving or hard-to-treat cancer, this delay may be devastating,” he said.
Dr. Burris also cited concerns about delayed cancer diagnosis. “In a typical month, roughly 150,000 Americans are diagnosed with cancer. But right now, routine screening visits are postponed, and patients with pain or other warning signs may put off a doctor’s visit because of social distancing,” he said.
The pandemic has also exacerbated shortages of sedatives and opioid analgesics required for intubation and mechanical ventilation of patients.
Trials halted or slowed
Dr. Burris also briefly discussed results of a new survey, which were posted online ahead of publication in JCO Oncology Practice. The survey showed that, of 14 academic and 18 community-based cancer programs, 59.4% reported halting screening and/or enrollment for at least some clinical trials and suspending research-based clinical visits except for those where cancer treatment was delivered.
“Half of respondents reported ceasing research-only blood and/or tissue collections,” the authors of the article reported.
“Trial interruptions are devastating news for thousands of patients; in many cases, clinical trials are the best or only appropriate option for care,” Dr. Burris said.
The article authors, led by David Waterhouse, MD, of Oncology Hematology Care in Cincinnati, pointed to a silver lining in the pandemic cloud in the form of opportunities to improve clinical trials going forward.
“Nearly all respondents (90.3%) identified telehealth visits for participants as a potential improvement to clinical trial conduct, and more than three-quarters (77.4%) indicated that remote patient review of symptoms held similar potential,” the authors wrote.
Other potential improvements included remote site visits from trial sponsors and/or contract research organizations, more efficient study enrollment through secure electronic platforms, direct shipment of oral drugs to patients, remote assessments of adverse events, and streamlined data collection.
Lessons from the front lines
Another member of the presscast panel, Melissa Dillmon, MD, of the Harbin Clinic Cancer Center in Rome, Georgia, described the experience of community oncologists during the pandemic.
Her community, located in northeastern Georgia, experienced a COVID-19 outbreak in early March linked to services at two large churches. Community public health authorities issued a shelter-in-place order before the state government issued stay-at-home guidelines and shuttered all but essential business, some of which were allowed by state order to reopen as of April 24.
Dr. Dillmon’s center began screening patients for COVID-19 symptoms at the door, limited visitors or companions, instituted virtual visits and tumor boards, and set up a cancer treatment triage system that would allow essential surgeries to proceed and most infusions to continue, while delaying the start of chemotherapy when possible.
“We have encouraged patients to continue on treatment, especially if treatment is being given with curative intent, or if the cancer is responding well already to treatment,” she said.
The center, located in a community with a high prevalence of comorbidities and high incidence of lung cancer, has seen a sharp decline in colonoscopies, mammograms, and lung scans as patient shelter in place.
“We have great concerns about patients missing their screening lung scans, as this program has already proven to be finding earlier lung cancers that are curable,” Dr. Dillmon said.
A view from Washington state
Another panel member, Gary Lyman, MD, of the Fred Hutchinson Cancer Research Center in Seattle, described the response by the state of Washington, the initial epicenter of the COVID-19 outbreak in the United States.
Following identification of infections in hospitalized patients and at a nursing home in Kirkland, Washington, “our response, which began in early March and progressed through the second and third week in March at the state level, was to restrict large gatherings; progressively, schools were closed; larger businesses closed; and, by March 23, a stay-at-home policy was implemented, and all nonessential businesses were closed,” Dr. Lyman said.
“We believe, based on what has happened since that time, that this has considerably flattened the curve,” he continued.
Lessons from the Washington experience include the need to plan for a long-term disruption or alteration of cancer care, expand COVID-19 testing to all patients coming into hospitals or major clinics, institute aggressive supportive care measures, prepare for subsequent waves of infection, collect and share data, and, for remote or rural areas, identify lifelines to needed resources, Dr. Lyman said.
ASCO resources
Also speaking at the presscast, Jonathan Marron, MD, of Boston Children’s Hospital and Harvard Medical School, Boston, outlined ASCO’s guidance on allocation of scarce resources during the COVID-19 pandemic.
Richard L. Schilsky, MD, ASCO chief medical officer and executive vice president, outlined community-wide collaborations, data initiatives, and online resources for both clinicians and patients.
The COVID-19 pandemic continues to exact a heavy price on cancer patients, cancer care, and clinical trials, an expert panel reported during a presscast.
“Limited data available thus far are sobering: In Italy, about 20% of COVID-related deaths occurred in people with cancer, and, in China, COVID-19 patients who had cancer were about five times more likely than others to die or be placed on a ventilator in an intensive care unit,” said Howard A “Skip” Burris, MD, president of the American Society of Clinical Oncology and president and CEO of the Sarah Cannon Cancer Institute in Nashville, Tenn.
“We also have little evidence on returning COVID-19 patients with cancer. Physicians have to rely on limited data, anecdotal reports, and their own professional expertise” regarding the extent of increased risk to cancer patients with COVID-19, whether to interrupt or modify treatment, and the effects of cancer on recovery from COVID-19 infection, Dr. Burris said during the ASCO-sponsored online presscast.
Care of COVID-free patients
For cancer patients without COVID-19, the picture is equally dim, with the prospect of delayed surgery, chemotherapy, or screening; shortages of medications and equipment needed for critical care; the shift to telemedicine that may increase patient anxiety; and the potential loss of access to innovative therapies through clinical trials, Dr. Burris said.
“We’re concerned that some hospitals have effectively deemed all cancer surgeries to be elective, requiring them to be postponed. For patients with fast-moving or hard-to-treat cancer, this delay may be devastating,” he said.
Dr. Burris also cited concerns about delayed cancer diagnosis. “In a typical month, roughly 150,000 Americans are diagnosed with cancer. But right now, routine screening visits are postponed, and patients with pain or other warning signs may put off a doctor’s visit because of social distancing,” he said.
The pandemic has also exacerbated shortages of sedatives and opioid analgesics required for intubation and mechanical ventilation of patients.
Trials halted or slowed
Dr. Burris also briefly discussed results of a new survey, which were posted online ahead of publication in JCO Oncology Practice. The survey showed that, of 14 academic and 18 community-based cancer programs, 59.4% reported halting screening and/or enrollment for at least some clinical trials and suspending research-based clinical visits except for those where cancer treatment was delivered.
“Half of respondents reported ceasing research-only blood and/or tissue collections,” the authors of the article reported.
“Trial interruptions are devastating news for thousands of patients; in many cases, clinical trials are the best or only appropriate option for care,” Dr. Burris said.
The article authors, led by David Waterhouse, MD, of Oncology Hematology Care in Cincinnati, pointed to a silver lining in the pandemic cloud in the form of opportunities to improve clinical trials going forward.
“Nearly all respondents (90.3%) identified telehealth visits for participants as a potential improvement to clinical trial conduct, and more than three-quarters (77.4%) indicated that remote patient review of symptoms held similar potential,” the authors wrote.
Other potential improvements included remote site visits from trial sponsors and/or contract research organizations, more efficient study enrollment through secure electronic platforms, direct shipment of oral drugs to patients, remote assessments of adverse events, and streamlined data collection.
Lessons from the front lines
Another member of the presscast panel, Melissa Dillmon, MD, of the Harbin Clinic Cancer Center in Rome, Georgia, described the experience of community oncologists during the pandemic.
Her community, located in northeastern Georgia, experienced a COVID-19 outbreak in early March linked to services at two large churches. Community public health authorities issued a shelter-in-place order before the state government issued stay-at-home guidelines and shuttered all but essential business, some of which were allowed by state order to reopen as of April 24.
Dr. Dillmon’s center began screening patients for COVID-19 symptoms at the door, limited visitors or companions, instituted virtual visits and tumor boards, and set up a cancer treatment triage system that would allow essential surgeries to proceed and most infusions to continue, while delaying the start of chemotherapy when possible.
“We have encouraged patients to continue on treatment, especially if treatment is being given with curative intent, or if the cancer is responding well already to treatment,” she said.
The center, located in a community with a high prevalence of comorbidities and high incidence of lung cancer, has seen a sharp decline in colonoscopies, mammograms, and lung scans as patient shelter in place.
“We have great concerns about patients missing their screening lung scans, as this program has already proven to be finding earlier lung cancers that are curable,” Dr. Dillmon said.
A view from Washington state
Another panel member, Gary Lyman, MD, of the Fred Hutchinson Cancer Research Center in Seattle, described the response by the state of Washington, the initial epicenter of the COVID-19 outbreak in the United States.
Following identification of infections in hospitalized patients and at a nursing home in Kirkland, Washington, “our response, which began in early March and progressed through the second and third week in March at the state level, was to restrict large gatherings; progressively, schools were closed; larger businesses closed; and, by March 23, a stay-at-home policy was implemented, and all nonessential businesses were closed,” Dr. Lyman said.
“We believe, based on what has happened since that time, that this has considerably flattened the curve,” he continued.
Lessons from the Washington experience include the need to plan for a long-term disruption or alteration of cancer care, expand COVID-19 testing to all patients coming into hospitals or major clinics, institute aggressive supportive care measures, prepare for subsequent waves of infection, collect and share data, and, for remote or rural areas, identify lifelines to needed resources, Dr. Lyman said.
ASCO resources
Also speaking at the presscast, Jonathan Marron, MD, of Boston Children’s Hospital and Harvard Medical School, Boston, outlined ASCO’s guidance on allocation of scarce resources during the COVID-19 pandemic.
Richard L. Schilsky, MD, ASCO chief medical officer and executive vice president, outlined community-wide collaborations, data initiatives, and online resources for both clinicians and patients.
Will coronavirus restrictions lead to more advanced cancers?
My pathology lab once faced a daily flood of colon polyps, pap smears, and prostate biopsies. Suddenly, our work has dried up. The coronavirus pandemic has cleared out operating rooms and clinics across the country. Endoscopy and radiology suites have gone dark.
Pathology is largely driven by mass screening programs, and the machinery of screening has grinded to a halt during the COVID-19 pandemic. The American Cancer Society currently recommends that “no one should go to a health care facility for routine cancer screening at this time.”
But malignancies are still growing and spreading even though a great deal of medical care is on hold. The most urgent cancer care is still taking place; the risks of delaying treatment for patients with advanced or symptomatic cancer are obvious—these tumors can cause severe pain and life-threatening complications.
But that leaves us with a more complex and uncomfortable question: Will the pause in screening ultimately leave patients with tiny, asymptomatic cancers or precursor lesions worse off? What will a delay mean for those with ductal carcinoma in situ or small breast cancers? What’s the long-term effect of all those dysplastic nevi and early melanoma left unexcised by dermatologists? Perhaps more troubling, what about the spreading kidney cancer that may have turned up as an incidental finding on a CT scan?
COVID-19: A natural experiment
For many years, we’ve been dealing with the other side of the screening question: overdiagnosing and treating cancers that would probably never harm the patient. Overdiagnosis has been on a decades-long rise due to organized screening like PSA testing and mammography, as well as through ad hoc detection from heavier use of medical imaging. All of these have been disrupted by the pandemic.
Because the correlation between medical interventions and cancer overdiagnosis is clear, we can safely assume that overdiagnosis will decline during the pandemic. But what will be the net effect? Early detection of cancer undoubtedly saves some lives, but how many and at what cost has been a seemingly intractable debate.
Until now.
The coronavirus outbreak will be a natural experiment like no other. Economists and epidemiologists love to study “natural experiments” – systemic shocks that shed light on a complex phenomenon.
The unexpected nationwide delay in screening will undoubtedly inform the debate on overdiagnosis. For one, we can learn whether less intensive screening leads to more advanced cancers. Because screening will probably return to normal at different times across the country, we can almost simulate a randomized trial. Will this transformative data be a silver lining to this awful time?
The pressure to ‘fight’
The pandemic has also raised a question about cancer screening that goes beyond data: Why has the loud epidemic of coronavirus so thoroughly trumped cancer’s silent one? To me, the necessary urgency of our coronavirus response stands in stark contrast to the overly aggressive public health messaging used for cancer screening.
The tools used to fight the coronavirus epidemic have been forceful. We’re all diligently washing our hands and staying inside. We’re making sacrifices in our jobs and personal lives to stop the virus’ spread.
Cancer screening has similarly been touted as dogma – an urgent public health intervention that only a fool would turn down. The American Cancer Society once ran an infamous advertisement suggesting that if you decline mammography, you “need more than your breasts examined.” Even today, well-intentioned organizations run cancer screening drives pushing people to pledge to “get screened now.” It is no surprise, then, that I have had patients and family members confide in me that they feel guilty about not pursuing all of their recommended screening tests. The thought of anyone feeling like they caused their own cancer appalls me.
This pressure extends into the clinic. In many practices, primary care doctors are evaluated based on how many patients “comply” with screening recommendations. There seems to be a relentless drive to reach 100% screening penetration. These oversimplified tactics run counter to the shared decision making and informed consent we profess to value in medicine.
The tricky thing about cancer screening is that because most people will never develop the cancer being screened for, we know that most people can also never be helped by it. This doesn’t make screening useless, just as washing your hands can help even if it doesn’t guarantee that you won’t catch coronavirus. We know that some individuals benefit, which we detect at the population level. Overdiagnosis arises in the same way, as a phenomenon detected within populations and not individuals. These aspects of screening are what has led to cancer being viewed as a “societal disease” requiring a uniform response – 100% screening compliance.
Metaphors of war
These assumptions fall apart now that we are facing a real societal disease, an infectious disease outbreak. Coronavirus has made us reflect on what actions individuals should take in order to protect others. But cancer is not a contagion. When we decide whether and how to screen, we make intimate decisions affecting primarily ourselves and our family – not society at large.
Countless articles have been written about the use of metaphor in cancer, perhaps most famously by essayist and breast cancer patient Susan Sontag. Sontag and others have been critical of the rampant use of war metaphors in the cancer community. Wars invoke sacrifice, duty, and suffering. The “battle” against coronavirus really puts the “war on cancer” in perspective. These pandemic weeks have terrified me. I have been willing to do anything to protect myself and others. They’ve also exhausted me. We can’t be at war forever.
When this current war ends, will the “war on cancer” resume unchanged? Screening will no doubt begin again, hopefully improved by data from the coronavirus natural experiment. But I wonder whether we will tolerate the same kinds of public health messages – and whether we should – having now experienced an infectious disease outbreak where our actions as individuals really do have an impact on the health of others.
After feeling helpless, besieged, and even guilt-ridden during the pandemic, I think many people would appreciate regaining a sense of control over other aspects of their health. Cancer screening can save lives, but it’s a choice we should make for ourselves based on an understanding of the trade-offs and our own preferences. When screening restarts, I hope its paternalistic dogma can be replaced by nuanced, empowering tactics more appropriate for peacetime.
Benjamin Mazer, MD, MBA, is an anatomic and clinical pathology resident at Yale with interests in diagnostic surgical pathology, laboratory management, and evidence-based medicine.
This article first appeared on Medscape.com.
My pathology lab once faced a daily flood of colon polyps, pap smears, and prostate biopsies. Suddenly, our work has dried up. The coronavirus pandemic has cleared out operating rooms and clinics across the country. Endoscopy and radiology suites have gone dark.
Pathology is largely driven by mass screening programs, and the machinery of screening has grinded to a halt during the COVID-19 pandemic. The American Cancer Society currently recommends that “no one should go to a health care facility for routine cancer screening at this time.”
But malignancies are still growing and spreading even though a great deal of medical care is on hold. The most urgent cancer care is still taking place; the risks of delaying treatment for patients with advanced or symptomatic cancer are obvious—these tumors can cause severe pain and life-threatening complications.
But that leaves us with a more complex and uncomfortable question: Will the pause in screening ultimately leave patients with tiny, asymptomatic cancers or precursor lesions worse off? What will a delay mean for those with ductal carcinoma in situ or small breast cancers? What’s the long-term effect of all those dysplastic nevi and early melanoma left unexcised by dermatologists? Perhaps more troubling, what about the spreading kidney cancer that may have turned up as an incidental finding on a CT scan?
COVID-19: A natural experiment
For many years, we’ve been dealing with the other side of the screening question: overdiagnosing and treating cancers that would probably never harm the patient. Overdiagnosis has been on a decades-long rise due to organized screening like PSA testing and mammography, as well as through ad hoc detection from heavier use of medical imaging. All of these have been disrupted by the pandemic.
Because the correlation between medical interventions and cancer overdiagnosis is clear, we can safely assume that overdiagnosis will decline during the pandemic. But what will be the net effect? Early detection of cancer undoubtedly saves some lives, but how many and at what cost has been a seemingly intractable debate.
Until now.
The coronavirus outbreak will be a natural experiment like no other. Economists and epidemiologists love to study “natural experiments” – systemic shocks that shed light on a complex phenomenon.
The unexpected nationwide delay in screening will undoubtedly inform the debate on overdiagnosis. For one, we can learn whether less intensive screening leads to more advanced cancers. Because screening will probably return to normal at different times across the country, we can almost simulate a randomized trial. Will this transformative data be a silver lining to this awful time?
The pressure to ‘fight’
The pandemic has also raised a question about cancer screening that goes beyond data: Why has the loud epidemic of coronavirus so thoroughly trumped cancer’s silent one? To me, the necessary urgency of our coronavirus response stands in stark contrast to the overly aggressive public health messaging used for cancer screening.
The tools used to fight the coronavirus epidemic have been forceful. We’re all diligently washing our hands and staying inside. We’re making sacrifices in our jobs and personal lives to stop the virus’ spread.
Cancer screening has similarly been touted as dogma – an urgent public health intervention that only a fool would turn down. The American Cancer Society once ran an infamous advertisement suggesting that if you decline mammography, you “need more than your breasts examined.” Even today, well-intentioned organizations run cancer screening drives pushing people to pledge to “get screened now.” It is no surprise, then, that I have had patients and family members confide in me that they feel guilty about not pursuing all of their recommended screening tests. The thought of anyone feeling like they caused their own cancer appalls me.
This pressure extends into the clinic. In many practices, primary care doctors are evaluated based on how many patients “comply” with screening recommendations. There seems to be a relentless drive to reach 100% screening penetration. These oversimplified tactics run counter to the shared decision making and informed consent we profess to value in medicine.
The tricky thing about cancer screening is that because most people will never develop the cancer being screened for, we know that most people can also never be helped by it. This doesn’t make screening useless, just as washing your hands can help even if it doesn’t guarantee that you won’t catch coronavirus. We know that some individuals benefit, which we detect at the population level. Overdiagnosis arises in the same way, as a phenomenon detected within populations and not individuals. These aspects of screening are what has led to cancer being viewed as a “societal disease” requiring a uniform response – 100% screening compliance.
Metaphors of war
These assumptions fall apart now that we are facing a real societal disease, an infectious disease outbreak. Coronavirus has made us reflect on what actions individuals should take in order to protect others. But cancer is not a contagion. When we decide whether and how to screen, we make intimate decisions affecting primarily ourselves and our family – not society at large.
Countless articles have been written about the use of metaphor in cancer, perhaps most famously by essayist and breast cancer patient Susan Sontag. Sontag and others have been critical of the rampant use of war metaphors in the cancer community. Wars invoke sacrifice, duty, and suffering. The “battle” against coronavirus really puts the “war on cancer” in perspective. These pandemic weeks have terrified me. I have been willing to do anything to protect myself and others. They’ve also exhausted me. We can’t be at war forever.
When this current war ends, will the “war on cancer” resume unchanged? Screening will no doubt begin again, hopefully improved by data from the coronavirus natural experiment. But I wonder whether we will tolerate the same kinds of public health messages – and whether we should – having now experienced an infectious disease outbreak where our actions as individuals really do have an impact on the health of others.
After feeling helpless, besieged, and even guilt-ridden during the pandemic, I think many people would appreciate regaining a sense of control over other aspects of their health. Cancer screening can save lives, but it’s a choice we should make for ourselves based on an understanding of the trade-offs and our own preferences. When screening restarts, I hope its paternalistic dogma can be replaced by nuanced, empowering tactics more appropriate for peacetime.
Benjamin Mazer, MD, MBA, is an anatomic and clinical pathology resident at Yale with interests in diagnostic surgical pathology, laboratory management, and evidence-based medicine.
This article first appeared on Medscape.com.
My pathology lab once faced a daily flood of colon polyps, pap smears, and prostate biopsies. Suddenly, our work has dried up. The coronavirus pandemic has cleared out operating rooms and clinics across the country. Endoscopy and radiology suites have gone dark.
Pathology is largely driven by mass screening programs, and the machinery of screening has grinded to a halt during the COVID-19 pandemic. The American Cancer Society currently recommends that “no one should go to a health care facility for routine cancer screening at this time.”
But malignancies are still growing and spreading even though a great deal of medical care is on hold. The most urgent cancer care is still taking place; the risks of delaying treatment for patients with advanced or symptomatic cancer are obvious—these tumors can cause severe pain and life-threatening complications.
But that leaves us with a more complex and uncomfortable question: Will the pause in screening ultimately leave patients with tiny, asymptomatic cancers or precursor lesions worse off? What will a delay mean for those with ductal carcinoma in situ or small breast cancers? What’s the long-term effect of all those dysplastic nevi and early melanoma left unexcised by dermatologists? Perhaps more troubling, what about the spreading kidney cancer that may have turned up as an incidental finding on a CT scan?
COVID-19: A natural experiment
For many years, we’ve been dealing with the other side of the screening question: overdiagnosing and treating cancers that would probably never harm the patient. Overdiagnosis has been on a decades-long rise due to organized screening like PSA testing and mammography, as well as through ad hoc detection from heavier use of medical imaging. All of these have been disrupted by the pandemic.
Because the correlation between medical interventions and cancer overdiagnosis is clear, we can safely assume that overdiagnosis will decline during the pandemic. But what will be the net effect? Early detection of cancer undoubtedly saves some lives, but how many and at what cost has been a seemingly intractable debate.
Until now.
The coronavirus outbreak will be a natural experiment like no other. Economists and epidemiologists love to study “natural experiments” – systemic shocks that shed light on a complex phenomenon.
The unexpected nationwide delay in screening will undoubtedly inform the debate on overdiagnosis. For one, we can learn whether less intensive screening leads to more advanced cancers. Because screening will probably return to normal at different times across the country, we can almost simulate a randomized trial. Will this transformative data be a silver lining to this awful time?
The pressure to ‘fight’
The pandemic has also raised a question about cancer screening that goes beyond data: Why has the loud epidemic of coronavirus so thoroughly trumped cancer’s silent one? To me, the necessary urgency of our coronavirus response stands in stark contrast to the overly aggressive public health messaging used for cancer screening.
The tools used to fight the coronavirus epidemic have been forceful. We’re all diligently washing our hands and staying inside. We’re making sacrifices in our jobs and personal lives to stop the virus’ spread.
Cancer screening has similarly been touted as dogma – an urgent public health intervention that only a fool would turn down. The American Cancer Society once ran an infamous advertisement suggesting that if you decline mammography, you “need more than your breasts examined.” Even today, well-intentioned organizations run cancer screening drives pushing people to pledge to “get screened now.” It is no surprise, then, that I have had patients and family members confide in me that they feel guilty about not pursuing all of their recommended screening tests. The thought of anyone feeling like they caused their own cancer appalls me.
This pressure extends into the clinic. In many practices, primary care doctors are evaluated based on how many patients “comply” with screening recommendations. There seems to be a relentless drive to reach 100% screening penetration. These oversimplified tactics run counter to the shared decision making and informed consent we profess to value in medicine.
The tricky thing about cancer screening is that because most people will never develop the cancer being screened for, we know that most people can also never be helped by it. This doesn’t make screening useless, just as washing your hands can help even if it doesn’t guarantee that you won’t catch coronavirus. We know that some individuals benefit, which we detect at the population level. Overdiagnosis arises in the same way, as a phenomenon detected within populations and not individuals. These aspects of screening are what has led to cancer being viewed as a “societal disease” requiring a uniform response – 100% screening compliance.
Metaphors of war
These assumptions fall apart now that we are facing a real societal disease, an infectious disease outbreak. Coronavirus has made us reflect on what actions individuals should take in order to protect others. But cancer is not a contagion. When we decide whether and how to screen, we make intimate decisions affecting primarily ourselves and our family – not society at large.
Countless articles have been written about the use of metaphor in cancer, perhaps most famously by essayist and breast cancer patient Susan Sontag. Sontag and others have been critical of the rampant use of war metaphors in the cancer community. Wars invoke sacrifice, duty, and suffering. The “battle” against coronavirus really puts the “war on cancer” in perspective. These pandemic weeks have terrified me. I have been willing to do anything to protect myself and others. They’ve also exhausted me. We can’t be at war forever.
When this current war ends, will the “war on cancer” resume unchanged? Screening will no doubt begin again, hopefully improved by data from the coronavirus natural experiment. But I wonder whether we will tolerate the same kinds of public health messages – and whether we should – having now experienced an infectious disease outbreak where our actions as individuals really do have an impact on the health of others.
After feeling helpless, besieged, and even guilt-ridden during the pandemic, I think many people would appreciate regaining a sense of control over other aspects of their health. Cancer screening can save lives, but it’s a choice we should make for ourselves based on an understanding of the trade-offs and our own preferences. When screening restarts, I hope its paternalistic dogma can be replaced by nuanced, empowering tactics more appropriate for peacetime.
Benjamin Mazer, MD, MBA, is an anatomic and clinical pathology resident at Yale with interests in diagnostic surgical pathology, laboratory management, and evidence-based medicine.
This article first appeared on Medscape.com.
European cancer centers restructure care in the era of COVID-19
Delivering cancer care during the COVID-19 pandemic has proved particularly challenging, as minimizing the risk of infection must be balanced with maintaining optimal outcomes.
Healthcare systems and oncologists have had to reorganize standard oncologic care in order to protect vulnerable patients from exposure to COVID-19 as well as deal with pandemic-related issues of equipment and staffing shortages.
A new article now describes how seven cancer centers in Europe rapidly reorganized their oncologic services and are tackling this crisis, as well as offering guidance to other institutions.
This was a major undertaking, to work out a system where patients can still get care but in a safer manner, explained coauthor Emile Voest, MD, medical director of the Netherlands Cancer Institute in Amsterdam.
“Decisions needed to be taken based on availability of personnel, protective materials, and urgencies,” he told Medscape Medical News. “Because every country had its own speed of development of the COVID pandemic, there were different scenarios in all institutions, but all with a common factor of key expertise on how to de-escalate in a safe manner.”
The article was published April 16 in Nature Medicine.
The Netherlands Cancer Institute (the Netherlands), Karolinska Institute (Sweden), Institute Gustave Roussy (France), Cambridge Cancer Center (United Kingdom), Istituto Nazionale dei Tumori di Milano (Italy), German Cancer Research Center (Germany), and Vall d’Hebron Institute of Oncology (Spain) have been working closely together in a legal entity since 2014, and have created ‘Cancer Core Europe’ (CCE). The goal is to “maximize coherence and critical mass in cancer research,” the authors note.
The consortium represents roughly 60,000 patients with newly diagnosed cancer, delivers approximately 300,000 treatment courses, and conducts about 1.2 million consultations annually, with more than 1,500 ongoing clinical trials. In a joint effort, the centers collected, translated, and compared the guidelines that had been put in place to treat patients with cancer during the COVID-19 pandemic.
Cancer treatment is multidisciplinary and involves many specialties including surgery, radiology, pathology, radiation oncology, and medical oncology. Coordinating care among disciplines is a very complex process, Voest noted.
“Changing treatment also means that you need to reconsider capacities and requirements,” he said. “Hospitals have installed crisis teams that were very good at coordinating these efforts.”
Restructuring care
Cancer care had to be reorganized on multiple levels, and the CCE centers looked at several aspects that needed to be accounted for, to ensure continuity in cancer care.
“The biggest challenge for the NHS and other healthcare systems is the surge of patients requiring oxygen and/or intensive care, and the nature and infectiousness of the virus,” said coauthor Carlos Caldas, MD, FMedSci, professor of cancer medicine at the University of Cambridge, United Kingdom. “In hospitals that are mostly run close to capacity, and where all kinds of patients are treated, this has created major resource and logistical problems.”
For regular clinical activities, the institutions with dedicated cancer centers (German Cancer Research Center, Institute Gustave Roussy, Istituto Nazionale dei Tumori di Milano, and Netherlands Cancer Institute) have attempted to stay COVID-19 free. This policy would in turn help ensure that sufficient clinical and intensive-care capacity could be reserved for critical cancer surgeries or management of treatment-related side effects, and allow hospitals outside of the CCE to transfer patients with cancer to these centers. The general hospitals can then focus on caring for patients with COVID-19, as well as other illnesses/injuries that require inpatient care.
As the CCE centers located within general hospitals (Cambridge Cancer Center, Vall d’Hebron Institute of Oncology and Karolinska Institute) have to admit patients with suspected and positive cases of COVID-19, being “COVID-19 free” was never a realistic or pursued goal.
The authors note that it is the responsibility of all healthcare professionals to ensure patients are not exposed to COVID-19, and this has meant minimizing hospital visits and person-to-person contact. For example, whenever possible, consultations take place via telephone calls or over the Internet, and nonurgent appointments that would require a patient’s physical presence at the clinic have been postponed. Visitors are also not permitted to accompany patients when admitted to the hospital or during procedures.
Standard-of-care treatment regimens have been adapted across all centers to minimize the number of hospital visits and hospitalizations and prevent “anticancer treatment-induced” complications of COVID-19.
To minimize visits and hospitalizations, strategies include converting intravenous treatments to oral or subcutaneous regimens when possible; switching from cytotoxic chemotherapy to a less-toxic approach to minimize the risk of complications requiring hospitalization; or to pause therapies when possible (stable disease reached or better). In addition, nonemergency surgeries have been postponed or replaced by radiotherapy.
To prevent anticancer treatment-induced complications of COVID-19, most centers use the paradigm that the added benefit for tumor control should be weighed against the potential risk for COVID-19–related morbidity and mortality. To prevent or reduce the risk of neutropenia and lymphopenia, for example, all centers have suggested a de-escalation of cytotoxic chemotherapy or targeted treatment strategies, or to forgo second or subsequent lines of palliative treatments if response rates from up-front therapy are low.
Some of these changes may be here to stay, noted Caldas. “One of the positive messages that comes out of this is that, clearly, care can be delivered in a safe and compassionate manner without requiring as many hospital visits as in the pre-COVID-19 era,” he said. “In the future, we will take heed of the COVID-19 experience to improve delivery of cancer care.”
Capacity of facilities
Many healthcare systems have become overwhelmed as the pandemic has intensified, thus making it necessary to prioritize. To prepare for this possibility, CCE centers have established protocols to categorize and prioritize patients for systemic treatment or surgery. While the protocols vary by center, they are comparable with one another as they prioritize on the basis of anticipated treatment outcome, the authors note.
The guidelines in CCE centers unanimously recommend that neoadjuvant therapies and curative surgeries be the top priority, for the times when operating room and/or ICU capacity is limited. As an alternative, neoadjuvant systemic treatments may be initiated or extended to postpone surgery, and other nonsurgical interventions can be considered.
In addition, some centers agree that certain elective surgeries can be safely delayed if backed by scientific evidence. As an example, an 11-week deferment of surgery may be acceptable for patients with rectal cancer after downstaging.
Cancer centers may also need to upscale and downscale quickly, depending on how the pandemic evolves, and many have already outlined scenarios to prepare for increasing or decreasing their capacity using phased approaches.
The Netherlands Cancer Institute, for example, has defined four phases of increasing severity; in Germany, capacity planning has been coordinated among 18 hospitals and the federal ministry of health, in order to prevent shortages of cancer services.
“We note that the optimal downscaling strategies depend on country- and center-specific capacities and preferences,” they write. “Therefore, it is difficult to propose a common schedule, and it will be most effective if hospitals outline their own phase-specific downscaling strategies based on the prioritization schemes and practical handles discussed above.”
Future research
Better strategies will be needed to reduce the impact of COVID-19 in cancer care, and four research priorities were identified to allow for evidence-based adjustments of cancer care protocols while the pandemic continues:
- Collect real-world data about the effects of adjustment and de-escalation of treatment regimens on outcomes
- Determine the incidence of COVID-19 in both the general population and among patients with cancer who have received systemic therapies, with large-scale serological testing
- Develop an epidemiological model that will allow estimates of the cumulative incidence of COVID-19 for a patient with cancer, within a specific time frame
- Determine COVID-19 related morbidity and mortality in patients with cancer who have been treated with systemic therapies and/or granulocyte colony-stimulating factor (G-CSF). Several projects are currently underway, such as the UK Coronavirus Cancer Monitoring Project.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Delivering cancer care during the COVID-19 pandemic has proved particularly challenging, as minimizing the risk of infection must be balanced with maintaining optimal outcomes.
Healthcare systems and oncologists have had to reorganize standard oncologic care in order to protect vulnerable patients from exposure to COVID-19 as well as deal with pandemic-related issues of equipment and staffing shortages.
A new article now describes how seven cancer centers in Europe rapidly reorganized their oncologic services and are tackling this crisis, as well as offering guidance to other institutions.
This was a major undertaking, to work out a system where patients can still get care but in a safer manner, explained coauthor Emile Voest, MD, medical director of the Netherlands Cancer Institute in Amsterdam.
“Decisions needed to be taken based on availability of personnel, protective materials, and urgencies,” he told Medscape Medical News. “Because every country had its own speed of development of the COVID pandemic, there were different scenarios in all institutions, but all with a common factor of key expertise on how to de-escalate in a safe manner.”
The article was published April 16 in Nature Medicine.
The Netherlands Cancer Institute (the Netherlands), Karolinska Institute (Sweden), Institute Gustave Roussy (France), Cambridge Cancer Center (United Kingdom), Istituto Nazionale dei Tumori di Milano (Italy), German Cancer Research Center (Germany), and Vall d’Hebron Institute of Oncology (Spain) have been working closely together in a legal entity since 2014, and have created ‘Cancer Core Europe’ (CCE). The goal is to “maximize coherence and critical mass in cancer research,” the authors note.
The consortium represents roughly 60,000 patients with newly diagnosed cancer, delivers approximately 300,000 treatment courses, and conducts about 1.2 million consultations annually, with more than 1,500 ongoing clinical trials. In a joint effort, the centers collected, translated, and compared the guidelines that had been put in place to treat patients with cancer during the COVID-19 pandemic.
Cancer treatment is multidisciplinary and involves many specialties including surgery, radiology, pathology, radiation oncology, and medical oncology. Coordinating care among disciplines is a very complex process, Voest noted.
“Changing treatment also means that you need to reconsider capacities and requirements,” he said. “Hospitals have installed crisis teams that were very good at coordinating these efforts.”
Restructuring care
Cancer care had to be reorganized on multiple levels, and the CCE centers looked at several aspects that needed to be accounted for, to ensure continuity in cancer care.
“The biggest challenge for the NHS and other healthcare systems is the surge of patients requiring oxygen and/or intensive care, and the nature and infectiousness of the virus,” said coauthor Carlos Caldas, MD, FMedSci, professor of cancer medicine at the University of Cambridge, United Kingdom. “In hospitals that are mostly run close to capacity, and where all kinds of patients are treated, this has created major resource and logistical problems.”
For regular clinical activities, the institutions with dedicated cancer centers (German Cancer Research Center, Institute Gustave Roussy, Istituto Nazionale dei Tumori di Milano, and Netherlands Cancer Institute) have attempted to stay COVID-19 free. This policy would in turn help ensure that sufficient clinical and intensive-care capacity could be reserved for critical cancer surgeries or management of treatment-related side effects, and allow hospitals outside of the CCE to transfer patients with cancer to these centers. The general hospitals can then focus on caring for patients with COVID-19, as well as other illnesses/injuries that require inpatient care.
As the CCE centers located within general hospitals (Cambridge Cancer Center, Vall d’Hebron Institute of Oncology and Karolinska Institute) have to admit patients with suspected and positive cases of COVID-19, being “COVID-19 free” was never a realistic or pursued goal.
The authors note that it is the responsibility of all healthcare professionals to ensure patients are not exposed to COVID-19, and this has meant minimizing hospital visits and person-to-person contact. For example, whenever possible, consultations take place via telephone calls or over the Internet, and nonurgent appointments that would require a patient’s physical presence at the clinic have been postponed. Visitors are also not permitted to accompany patients when admitted to the hospital or during procedures.
Standard-of-care treatment regimens have been adapted across all centers to minimize the number of hospital visits and hospitalizations and prevent “anticancer treatment-induced” complications of COVID-19.
To minimize visits and hospitalizations, strategies include converting intravenous treatments to oral or subcutaneous regimens when possible; switching from cytotoxic chemotherapy to a less-toxic approach to minimize the risk of complications requiring hospitalization; or to pause therapies when possible (stable disease reached or better). In addition, nonemergency surgeries have been postponed or replaced by radiotherapy.
To prevent anticancer treatment-induced complications of COVID-19, most centers use the paradigm that the added benefit for tumor control should be weighed against the potential risk for COVID-19–related morbidity and mortality. To prevent or reduce the risk of neutropenia and lymphopenia, for example, all centers have suggested a de-escalation of cytotoxic chemotherapy or targeted treatment strategies, or to forgo second or subsequent lines of palliative treatments if response rates from up-front therapy are low.
Some of these changes may be here to stay, noted Caldas. “One of the positive messages that comes out of this is that, clearly, care can be delivered in a safe and compassionate manner without requiring as many hospital visits as in the pre-COVID-19 era,” he said. “In the future, we will take heed of the COVID-19 experience to improve delivery of cancer care.”
Capacity of facilities
Many healthcare systems have become overwhelmed as the pandemic has intensified, thus making it necessary to prioritize. To prepare for this possibility, CCE centers have established protocols to categorize and prioritize patients for systemic treatment or surgery. While the protocols vary by center, they are comparable with one another as they prioritize on the basis of anticipated treatment outcome, the authors note.
The guidelines in CCE centers unanimously recommend that neoadjuvant therapies and curative surgeries be the top priority, for the times when operating room and/or ICU capacity is limited. As an alternative, neoadjuvant systemic treatments may be initiated or extended to postpone surgery, and other nonsurgical interventions can be considered.
In addition, some centers agree that certain elective surgeries can be safely delayed if backed by scientific evidence. As an example, an 11-week deferment of surgery may be acceptable for patients with rectal cancer after downstaging.
Cancer centers may also need to upscale and downscale quickly, depending on how the pandemic evolves, and many have already outlined scenarios to prepare for increasing or decreasing their capacity using phased approaches.
The Netherlands Cancer Institute, for example, has defined four phases of increasing severity; in Germany, capacity planning has been coordinated among 18 hospitals and the federal ministry of health, in order to prevent shortages of cancer services.
“We note that the optimal downscaling strategies depend on country- and center-specific capacities and preferences,” they write. “Therefore, it is difficult to propose a common schedule, and it will be most effective if hospitals outline their own phase-specific downscaling strategies based on the prioritization schemes and practical handles discussed above.”
Future research
Better strategies will be needed to reduce the impact of COVID-19 in cancer care, and four research priorities were identified to allow for evidence-based adjustments of cancer care protocols while the pandemic continues:
- Collect real-world data about the effects of adjustment and de-escalation of treatment regimens on outcomes
- Determine the incidence of COVID-19 in both the general population and among patients with cancer who have received systemic therapies, with large-scale serological testing
- Develop an epidemiological model that will allow estimates of the cumulative incidence of COVID-19 for a patient with cancer, within a specific time frame
- Determine COVID-19 related morbidity and mortality in patients with cancer who have been treated with systemic therapies and/or granulocyte colony-stimulating factor (G-CSF). Several projects are currently underway, such as the UK Coronavirus Cancer Monitoring Project.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Delivering cancer care during the COVID-19 pandemic has proved particularly challenging, as minimizing the risk of infection must be balanced with maintaining optimal outcomes.
Healthcare systems and oncologists have had to reorganize standard oncologic care in order to protect vulnerable patients from exposure to COVID-19 as well as deal with pandemic-related issues of equipment and staffing shortages.
A new article now describes how seven cancer centers in Europe rapidly reorganized their oncologic services and are tackling this crisis, as well as offering guidance to other institutions.
This was a major undertaking, to work out a system where patients can still get care but in a safer manner, explained coauthor Emile Voest, MD, medical director of the Netherlands Cancer Institute in Amsterdam.
“Decisions needed to be taken based on availability of personnel, protective materials, and urgencies,” he told Medscape Medical News. “Because every country had its own speed of development of the COVID pandemic, there were different scenarios in all institutions, but all with a common factor of key expertise on how to de-escalate in a safe manner.”
The article was published April 16 in Nature Medicine.
The Netherlands Cancer Institute (the Netherlands), Karolinska Institute (Sweden), Institute Gustave Roussy (France), Cambridge Cancer Center (United Kingdom), Istituto Nazionale dei Tumori di Milano (Italy), German Cancer Research Center (Germany), and Vall d’Hebron Institute of Oncology (Spain) have been working closely together in a legal entity since 2014, and have created ‘Cancer Core Europe’ (CCE). The goal is to “maximize coherence and critical mass in cancer research,” the authors note.
The consortium represents roughly 60,000 patients with newly diagnosed cancer, delivers approximately 300,000 treatment courses, and conducts about 1.2 million consultations annually, with more than 1,500 ongoing clinical trials. In a joint effort, the centers collected, translated, and compared the guidelines that had been put in place to treat patients with cancer during the COVID-19 pandemic.
Cancer treatment is multidisciplinary and involves many specialties including surgery, radiology, pathology, radiation oncology, and medical oncology. Coordinating care among disciplines is a very complex process, Voest noted.
“Changing treatment also means that you need to reconsider capacities and requirements,” he said. “Hospitals have installed crisis teams that were very good at coordinating these efforts.”
Restructuring care
Cancer care had to be reorganized on multiple levels, and the CCE centers looked at several aspects that needed to be accounted for, to ensure continuity in cancer care.
“The biggest challenge for the NHS and other healthcare systems is the surge of patients requiring oxygen and/or intensive care, and the nature and infectiousness of the virus,” said coauthor Carlos Caldas, MD, FMedSci, professor of cancer medicine at the University of Cambridge, United Kingdom. “In hospitals that are mostly run close to capacity, and where all kinds of patients are treated, this has created major resource and logistical problems.”
For regular clinical activities, the institutions with dedicated cancer centers (German Cancer Research Center, Institute Gustave Roussy, Istituto Nazionale dei Tumori di Milano, and Netherlands Cancer Institute) have attempted to stay COVID-19 free. This policy would in turn help ensure that sufficient clinical and intensive-care capacity could be reserved for critical cancer surgeries or management of treatment-related side effects, and allow hospitals outside of the CCE to transfer patients with cancer to these centers. The general hospitals can then focus on caring for patients with COVID-19, as well as other illnesses/injuries that require inpatient care.
As the CCE centers located within general hospitals (Cambridge Cancer Center, Vall d’Hebron Institute of Oncology and Karolinska Institute) have to admit patients with suspected and positive cases of COVID-19, being “COVID-19 free” was never a realistic or pursued goal.
The authors note that it is the responsibility of all healthcare professionals to ensure patients are not exposed to COVID-19, and this has meant minimizing hospital visits and person-to-person contact. For example, whenever possible, consultations take place via telephone calls or over the Internet, and nonurgent appointments that would require a patient’s physical presence at the clinic have been postponed. Visitors are also not permitted to accompany patients when admitted to the hospital or during procedures.
Standard-of-care treatment regimens have been adapted across all centers to minimize the number of hospital visits and hospitalizations and prevent “anticancer treatment-induced” complications of COVID-19.
To minimize visits and hospitalizations, strategies include converting intravenous treatments to oral or subcutaneous regimens when possible; switching from cytotoxic chemotherapy to a less-toxic approach to minimize the risk of complications requiring hospitalization; or to pause therapies when possible (stable disease reached or better). In addition, nonemergency surgeries have been postponed or replaced by radiotherapy.
To prevent anticancer treatment-induced complications of COVID-19, most centers use the paradigm that the added benefit for tumor control should be weighed against the potential risk for COVID-19–related morbidity and mortality. To prevent or reduce the risk of neutropenia and lymphopenia, for example, all centers have suggested a de-escalation of cytotoxic chemotherapy or targeted treatment strategies, or to forgo second or subsequent lines of palliative treatments if response rates from up-front therapy are low.
Some of these changes may be here to stay, noted Caldas. “One of the positive messages that comes out of this is that, clearly, care can be delivered in a safe and compassionate manner without requiring as many hospital visits as in the pre-COVID-19 era,” he said. “In the future, we will take heed of the COVID-19 experience to improve delivery of cancer care.”
Capacity of facilities
Many healthcare systems have become overwhelmed as the pandemic has intensified, thus making it necessary to prioritize. To prepare for this possibility, CCE centers have established protocols to categorize and prioritize patients for systemic treatment or surgery. While the protocols vary by center, they are comparable with one another as they prioritize on the basis of anticipated treatment outcome, the authors note.
The guidelines in CCE centers unanimously recommend that neoadjuvant therapies and curative surgeries be the top priority, for the times when operating room and/or ICU capacity is limited. As an alternative, neoadjuvant systemic treatments may be initiated or extended to postpone surgery, and other nonsurgical interventions can be considered.
In addition, some centers agree that certain elective surgeries can be safely delayed if backed by scientific evidence. As an example, an 11-week deferment of surgery may be acceptable for patients with rectal cancer after downstaging.
Cancer centers may also need to upscale and downscale quickly, depending on how the pandemic evolves, and many have already outlined scenarios to prepare for increasing or decreasing their capacity using phased approaches.
The Netherlands Cancer Institute, for example, has defined four phases of increasing severity; in Germany, capacity planning has been coordinated among 18 hospitals and the federal ministry of health, in order to prevent shortages of cancer services.
“We note that the optimal downscaling strategies depend on country- and center-specific capacities and preferences,” they write. “Therefore, it is difficult to propose a common schedule, and it will be most effective if hospitals outline their own phase-specific downscaling strategies based on the prioritization schemes and practical handles discussed above.”
Future research
Better strategies will be needed to reduce the impact of COVID-19 in cancer care, and four research priorities were identified to allow for evidence-based adjustments of cancer care protocols while the pandemic continues:
- Collect real-world data about the effects of adjustment and de-escalation of treatment regimens on outcomes
- Determine the incidence of COVID-19 in both the general population and among patients with cancer who have received systemic therapies, with large-scale serological testing
- Develop an epidemiological model that will allow estimates of the cumulative incidence of COVID-19 for a patient with cancer, within a specific time frame
- Determine COVID-19 related morbidity and mortality in patients with cancer who have been treated with systemic therapies and/or granulocyte colony-stimulating factor (G-CSF). Several projects are currently underway, such as the UK Coronavirus Cancer Monitoring Project.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Want to keep cancer patients and providers safe during the pandemic? Here’s how
according to the authors of a special feature article in the Journal of the National Comprehensive Cancer Network.
Prescreening, telemedicine, and limiting procedures top the authors’ list of 10 recommendations for ensuring patient safety in U.S. oncology practices. Assuring appropriate personal proctective equipment (PPE), encouraging telecommuting, and providing wellness/stress management are a few of the ways to look out for health care worker safety during the crisis.
These recommendations were drafted to provide guidance during the rapidly evolving global pandemic that, in some cases, has deluged health care delivery systems and strained the ability of providers to assure safe and effective care, said lead author Pelin Cinar, MD, of the Hellen Diller Family Comprehensive Cancer Center at the University of California, San Francisco.
“I think we have been so overwhelmed that sometimes it’s difficult to get organized in our thought processes,” Dr. Cinar said in an interview. “So this [article] was really trying to provide some structure to each of the different steps that we should be addressing at minimum.”
Screening patients
Prescreening systems are a critical first step to ensure cancer centers are helping control community spread of the virus, according to the article. Whether done by phone or online, prescreening 1-2 days before a patient’s visit can help identify COVID-19 symptoms and exposure history, guiding whether patients need to be evaluated, monitored, or referred to an ED.
Next, screening clinics can help ensure cancer patients with COVID-19 symptoms are evaluated and tested in a unit with dedicated staff, according to the article.
“If symptomatic patients present to the cancer center for treatment after a negative prescreening assessment, they must be provided with a mask and directed to a screening clinic for evaluation and potential testing before moving forward with any cancer-directed therapy,” the article states.
Telemedicine and treatment
Telemedicine visits should be done whenever possible to avoid in-person visits, according to the article. Dr. Cinar said that her center, like other cancer centers, has seen a major uptick in these visits, which are typically done over video. In February, there were a total of 232 video visits at her center, which jumped to 1,702 in March, or an approximate 600% increase.
“Even though we had a relatively robust presence [before the pandemic], we still weren’t at a level where we are now,” Dr. Cinar said.
When it comes to cancer treatment, surgeries and procedures should be limited to essential or urgent cases, and, if possible, chemotherapy and systemic therapy regimens can be modified to allow for fewer visits to the cancer center or infusion center, according to the article.
Transitions to outpatient care can help further reduce the need for in-person visits, while intervals between scans can be increased, or biochemical markers can be used instead of scans.
Protecting providers
Health care workers providing cancer care should be assured appropriate PPE, and websites or other centralized resources should be in place to make sure workers are aware of current PPE guidelines and changes in workflow, according to the article.
The authors note that daily screening tools or temperature checks of symptomatic workers can help decrease the risk of exposure to others. The authors also recommend establishing clear rules for when health care workers with suspected or confirmed COVID-19 should be staying at home and returning to the job.
Telecommuting should be encouraged, with limited staff participating in onsite rotations to further reduce exposure risks, the article states.
Anxiety, insomnia, and distress have been reported among frontline health care workers managing patients with COVID-19, according to the article, which recommends wellness and stress management resources be available as an “invaluable resource” in cancer centers.
“We have to take care of ourselves to be able to take care of others,” Dr. Cinar said. “With PPE, you’re physically protecting yourself, while self-care, stress management, and wellness are also a big component of protecting ourselves.”
The report by Dr. Cinar and colleagues was an invited article from the NCCN Best Practices Committee. One coauthor reported relationships with Abbvie, Adaptive Biotechnologies, Aduro, and several other companies. Dr. Cinar and the remaining authors said they had no relevant conflicts of interest.
SOURCE: Cinar P et al. J Natl Compr Canc Netw. 2020 Apr 15. doi: 10.6004/jnccn.2020.7572.
according to the authors of a special feature article in the Journal of the National Comprehensive Cancer Network.
Prescreening, telemedicine, and limiting procedures top the authors’ list of 10 recommendations for ensuring patient safety in U.S. oncology practices. Assuring appropriate personal proctective equipment (PPE), encouraging telecommuting, and providing wellness/stress management are a few of the ways to look out for health care worker safety during the crisis.
These recommendations were drafted to provide guidance during the rapidly evolving global pandemic that, in some cases, has deluged health care delivery systems and strained the ability of providers to assure safe and effective care, said lead author Pelin Cinar, MD, of the Hellen Diller Family Comprehensive Cancer Center at the University of California, San Francisco.
“I think we have been so overwhelmed that sometimes it’s difficult to get organized in our thought processes,” Dr. Cinar said in an interview. “So this [article] was really trying to provide some structure to each of the different steps that we should be addressing at minimum.”
Screening patients
Prescreening systems are a critical first step to ensure cancer centers are helping control community spread of the virus, according to the article. Whether done by phone or online, prescreening 1-2 days before a patient’s visit can help identify COVID-19 symptoms and exposure history, guiding whether patients need to be evaluated, monitored, or referred to an ED.
Next, screening clinics can help ensure cancer patients with COVID-19 symptoms are evaluated and tested in a unit with dedicated staff, according to the article.
“If symptomatic patients present to the cancer center for treatment after a negative prescreening assessment, they must be provided with a mask and directed to a screening clinic for evaluation and potential testing before moving forward with any cancer-directed therapy,” the article states.
Telemedicine and treatment
Telemedicine visits should be done whenever possible to avoid in-person visits, according to the article. Dr. Cinar said that her center, like other cancer centers, has seen a major uptick in these visits, which are typically done over video. In February, there were a total of 232 video visits at her center, which jumped to 1,702 in March, or an approximate 600% increase.
“Even though we had a relatively robust presence [before the pandemic], we still weren’t at a level where we are now,” Dr. Cinar said.
When it comes to cancer treatment, surgeries and procedures should be limited to essential or urgent cases, and, if possible, chemotherapy and systemic therapy regimens can be modified to allow for fewer visits to the cancer center or infusion center, according to the article.
Transitions to outpatient care can help further reduce the need for in-person visits, while intervals between scans can be increased, or biochemical markers can be used instead of scans.
Protecting providers
Health care workers providing cancer care should be assured appropriate PPE, and websites or other centralized resources should be in place to make sure workers are aware of current PPE guidelines and changes in workflow, according to the article.
The authors note that daily screening tools or temperature checks of symptomatic workers can help decrease the risk of exposure to others. The authors also recommend establishing clear rules for when health care workers with suspected or confirmed COVID-19 should be staying at home and returning to the job.
Telecommuting should be encouraged, with limited staff participating in onsite rotations to further reduce exposure risks, the article states.
Anxiety, insomnia, and distress have been reported among frontline health care workers managing patients with COVID-19, according to the article, which recommends wellness and stress management resources be available as an “invaluable resource” in cancer centers.
“We have to take care of ourselves to be able to take care of others,” Dr. Cinar said. “With PPE, you’re physically protecting yourself, while self-care, stress management, and wellness are also a big component of protecting ourselves.”
The report by Dr. Cinar and colleagues was an invited article from the NCCN Best Practices Committee. One coauthor reported relationships with Abbvie, Adaptive Biotechnologies, Aduro, and several other companies. Dr. Cinar and the remaining authors said they had no relevant conflicts of interest.
SOURCE: Cinar P et al. J Natl Compr Canc Netw. 2020 Apr 15. doi: 10.6004/jnccn.2020.7572.
according to the authors of a special feature article in the Journal of the National Comprehensive Cancer Network.
Prescreening, telemedicine, and limiting procedures top the authors’ list of 10 recommendations for ensuring patient safety in U.S. oncology practices. Assuring appropriate personal proctective equipment (PPE), encouraging telecommuting, and providing wellness/stress management are a few of the ways to look out for health care worker safety during the crisis.
These recommendations were drafted to provide guidance during the rapidly evolving global pandemic that, in some cases, has deluged health care delivery systems and strained the ability of providers to assure safe and effective care, said lead author Pelin Cinar, MD, of the Hellen Diller Family Comprehensive Cancer Center at the University of California, San Francisco.
“I think we have been so overwhelmed that sometimes it’s difficult to get organized in our thought processes,” Dr. Cinar said in an interview. “So this [article] was really trying to provide some structure to each of the different steps that we should be addressing at minimum.”
Screening patients
Prescreening systems are a critical first step to ensure cancer centers are helping control community spread of the virus, according to the article. Whether done by phone or online, prescreening 1-2 days before a patient’s visit can help identify COVID-19 symptoms and exposure history, guiding whether patients need to be evaluated, monitored, or referred to an ED.
Next, screening clinics can help ensure cancer patients with COVID-19 symptoms are evaluated and tested in a unit with dedicated staff, according to the article.
“If symptomatic patients present to the cancer center for treatment after a negative prescreening assessment, they must be provided with a mask and directed to a screening clinic for evaluation and potential testing before moving forward with any cancer-directed therapy,” the article states.
Telemedicine and treatment
Telemedicine visits should be done whenever possible to avoid in-person visits, according to the article. Dr. Cinar said that her center, like other cancer centers, has seen a major uptick in these visits, which are typically done over video. In February, there were a total of 232 video visits at her center, which jumped to 1,702 in March, or an approximate 600% increase.
“Even though we had a relatively robust presence [before the pandemic], we still weren’t at a level where we are now,” Dr. Cinar said.
When it comes to cancer treatment, surgeries and procedures should be limited to essential or urgent cases, and, if possible, chemotherapy and systemic therapy regimens can be modified to allow for fewer visits to the cancer center or infusion center, according to the article.
Transitions to outpatient care can help further reduce the need for in-person visits, while intervals between scans can be increased, or biochemical markers can be used instead of scans.
Protecting providers
Health care workers providing cancer care should be assured appropriate PPE, and websites or other centralized resources should be in place to make sure workers are aware of current PPE guidelines and changes in workflow, according to the article.
The authors note that daily screening tools or temperature checks of symptomatic workers can help decrease the risk of exposure to others. The authors also recommend establishing clear rules for when health care workers with suspected or confirmed COVID-19 should be staying at home and returning to the job.
Telecommuting should be encouraged, with limited staff participating in onsite rotations to further reduce exposure risks, the article states.
Anxiety, insomnia, and distress have been reported among frontline health care workers managing patients with COVID-19, according to the article, which recommends wellness and stress management resources be available as an “invaluable resource” in cancer centers.
“We have to take care of ourselves to be able to take care of others,” Dr. Cinar said. “With PPE, you’re physically protecting yourself, while self-care, stress management, and wellness are also a big component of protecting ourselves.”
The report by Dr. Cinar and colleagues was an invited article from the NCCN Best Practices Committee. One coauthor reported relationships with Abbvie, Adaptive Biotechnologies, Aduro, and several other companies. Dr. Cinar and the remaining authors said they had no relevant conflicts of interest.
SOURCE: Cinar P et al. J Natl Compr Canc Netw. 2020 Apr 15. doi: 10.6004/jnccn.2020.7572.
FROM THE JOURNAL OF THE NATIONAL COMPREHENSIVE CANCER NETWORK

