User login
CBTI Strategy Reduces Sleeping Pill Use in Canadian Seniors
A strategy developed by Canadian researchers for encouraging older patients with insomnia to wean themselves from sleeping pills and improve their sleep through behavioral techniques is effective, data suggest. If proven helpful for the millions of older Canadians who currently rely on nightly benzodiazepines (BZDs) and non-BZDs (colloquially known as Z drugs) for their sleep, it might yield an additional benefit: Reducing resource utilization.
“We know that cognitive behavioral therapy for insomnia (CBTI) works. It’s recommended as first-line therapy because it works,” study author David Gardner, PharmD, professor of psychiatry at Dalhousie University in Halifax, Nova Scotia, Canada, told this news organization.
“We’re sharing information about sleeping pills, information that has been embedded with behavior-change techniques that lead people to second-guess or rethink their long-term use of sedative hypnotics and then bring that information to their provider or pharmacist to discuss it,” he said.
The results were published in JAMA Psychiatry.
Better Sleep, Fewer Pills
Dr. Gardner and his team created a direct-to-patient, patient-directed, multicomponent knowledge mobilization intervention called Sleepwell. It incorporates best practice– and guideline-based evidence and multiple behavioral change techniques with content and graphics. Dr. Gardner emphasized that it represents a directional shift in care that alleviates providers’ burden without removing it entirely.
To test the intervention’s effectiveness, Dr. Gardner and his team chose New Brunswick as a location for a 6-month, three-arm, open-label, randomized controlled trial; the province has one of the highest rates of sedative use and an older adult population that is vulnerable to the serious side effects of these drugs (eg, cognitive impairment, falls, and frailty). The study was called Your Answers When Needing Sleep in New Brunswick (YAWNS NB).
Eligible participants were aged ≥ 65 years, lived in the community, and had taken benzodiazepine receptor agonists (BZRAs) for ≥ 3 nights per week for 3 or more months. Participants were randomly assigned to a control group or one of the two intervention groups. The YAWNS-1 intervention group (n = 195) received a mailed package containing a cover letter, a booklet outlining how to stop sleeping pills, a booklet on how to “get your sleep back,” and a companion website. The YAWNS-2 group (n = 193) received updated versions of the booklets used in a prior trial. The control group (n = 192) was assigned treatment as usual (TAU).
A greater proportion of YAWNS-1 participants discontinued BZRAs at 6 months (26.2%) and had dose reductions (20.4%), compared with YAWNS-2 participants (20.3% and 14.4%, respectively) and TAU participants (7.5% and 12.8%, respectively). The corresponding numbers needed to mail to achieve an additional discontinuation was 5.3 YAWNS-1 packages and 7.8 YAWNS-2 packages.
At 6 months, BZRA cessation was sustained a mean 13.6 weeks for YAWNS-1, 14.3 weeks for YAWNS-2, and 16.9 weeks for TAU.
Sleep measures also improved with YAWNS-1, compared with YAWNs-2 and TAU. Sleep onset latency was reduced by 26.1 minutes among YAWNS-1 participants, compared with YAWNS-2 (P < .001), and by 27.7 minutes, compared with TAU (P < .001). Wake after sleep onset increased by 4.1 minutes in YAWNS-1, 11.1 minutes in YAWNS-2, and 7.5 minutes in TAU.
Although all participants underwent rigorous assessment before inclusion, less than half of participants receiving either intervention (36% in YAWNS-1 and 43% in YAWNS-2) contacted their provider or pharmacist to discuss BZD dose reductions. This finding may have resulted partly from limited access because of the COVID-19 pandemic, according to the authors.
A Stepped-Care Model
The intervention is intended to help patients “change their approach from sleeping pills to a short-term CBTI course for long-term sleep benefits, and then speak to their provider,” said Dr. Gardner.
He pointed to a post-study follow-up of the study participants’ health providers, most of whom had moderate to extensive experience deprescribing BZRAs, which showed that 87.5%-100% fully or nearly fully agreed with or supported using the Sleepwell strategy and its content with older patients who rely on sedatives.
“Providers said that deprescribing is difficult, time-consuming, and often not a productive use of their time,” said Dr. Gardner. “I see insomnia as a health issue well set up for a stepped-care model. Self-help approaches are at the very bottom of that model and can help shift the initial burden to patients and out of the healthcare system.”
Poor uptake has prevented CBTI from demonstrating its potential, which is a challenge that Charles M. Morin, PhD, professor of psychology at Laval University in Quebec City, Quebec, Canada, attributes to two factors. “Clearly, there aren’t enough providers with this kind of expertise, and it’s not always covered by public health insurance, so people have to pay out of pocket to treat their insomnia,” he said.
“Overall, I think that this was a very nice study, well conducted, with an impressive sample size,” said Dr. Morin, who was not involved in the study. “The results are quite encouraging, telling us that even when older adults have used sleep medications for an average of 10 years, it’s still possible to reduce the medication. But this doesn’t happen alone. People need to be guided in doing that, not only to decrease medication use, but they also need an alternative,” he said.
Dr. Morin questioned how many patients agree to start with a low intensity. “Ideally, it should be a shared decision paradigm, where the physician or whoever sees the patient first presents the available options and explains the pluses and minuses of each. Some patients might choose medication because it’s a quick fix,” he said. “But some might want to do CBTI, even if it takes more work. The results are sustainable over time,” he added.
The study was jointly funded by the Public Health Agency of Canada and the government of New Brunswick as a Healthy Seniors Pilot Project. Dr. Gardner and Dr. Morin reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A strategy developed by Canadian researchers for encouraging older patients with insomnia to wean themselves from sleeping pills and improve their sleep through behavioral techniques is effective, data suggest. If proven helpful for the millions of older Canadians who currently rely on nightly benzodiazepines (BZDs) and non-BZDs (colloquially known as Z drugs) for their sleep, it might yield an additional benefit: Reducing resource utilization.
“We know that cognitive behavioral therapy for insomnia (CBTI) works. It’s recommended as first-line therapy because it works,” study author David Gardner, PharmD, professor of psychiatry at Dalhousie University in Halifax, Nova Scotia, Canada, told this news organization.
“We’re sharing information about sleeping pills, information that has been embedded with behavior-change techniques that lead people to second-guess or rethink their long-term use of sedative hypnotics and then bring that information to their provider or pharmacist to discuss it,” he said.
The results were published in JAMA Psychiatry.
Better Sleep, Fewer Pills
Dr. Gardner and his team created a direct-to-patient, patient-directed, multicomponent knowledge mobilization intervention called Sleepwell. It incorporates best practice– and guideline-based evidence and multiple behavioral change techniques with content and graphics. Dr. Gardner emphasized that it represents a directional shift in care that alleviates providers’ burden without removing it entirely.
To test the intervention’s effectiveness, Dr. Gardner and his team chose New Brunswick as a location for a 6-month, three-arm, open-label, randomized controlled trial; the province has one of the highest rates of sedative use and an older adult population that is vulnerable to the serious side effects of these drugs (eg, cognitive impairment, falls, and frailty). The study was called Your Answers When Needing Sleep in New Brunswick (YAWNS NB).
Eligible participants were aged ≥ 65 years, lived in the community, and had taken benzodiazepine receptor agonists (BZRAs) for ≥ 3 nights per week for 3 or more months. Participants were randomly assigned to a control group or one of the two intervention groups. The YAWNS-1 intervention group (n = 195) received a mailed package containing a cover letter, a booklet outlining how to stop sleeping pills, a booklet on how to “get your sleep back,” and a companion website. The YAWNS-2 group (n = 193) received updated versions of the booklets used in a prior trial. The control group (n = 192) was assigned treatment as usual (TAU).
A greater proportion of YAWNS-1 participants discontinued BZRAs at 6 months (26.2%) and had dose reductions (20.4%), compared with YAWNS-2 participants (20.3% and 14.4%, respectively) and TAU participants (7.5% and 12.8%, respectively). The corresponding numbers needed to mail to achieve an additional discontinuation was 5.3 YAWNS-1 packages and 7.8 YAWNS-2 packages.
At 6 months, BZRA cessation was sustained a mean 13.6 weeks for YAWNS-1, 14.3 weeks for YAWNS-2, and 16.9 weeks for TAU.
Sleep measures also improved with YAWNS-1, compared with YAWNs-2 and TAU. Sleep onset latency was reduced by 26.1 minutes among YAWNS-1 participants, compared with YAWNS-2 (P < .001), and by 27.7 minutes, compared with TAU (P < .001). Wake after sleep onset increased by 4.1 minutes in YAWNS-1, 11.1 minutes in YAWNS-2, and 7.5 minutes in TAU.
Although all participants underwent rigorous assessment before inclusion, less than half of participants receiving either intervention (36% in YAWNS-1 and 43% in YAWNS-2) contacted their provider or pharmacist to discuss BZD dose reductions. This finding may have resulted partly from limited access because of the COVID-19 pandemic, according to the authors.
A Stepped-Care Model
The intervention is intended to help patients “change their approach from sleeping pills to a short-term CBTI course for long-term sleep benefits, and then speak to their provider,” said Dr. Gardner.
He pointed to a post-study follow-up of the study participants’ health providers, most of whom had moderate to extensive experience deprescribing BZRAs, which showed that 87.5%-100% fully or nearly fully agreed with or supported using the Sleepwell strategy and its content with older patients who rely on sedatives.
“Providers said that deprescribing is difficult, time-consuming, and often not a productive use of their time,” said Dr. Gardner. “I see insomnia as a health issue well set up for a stepped-care model. Self-help approaches are at the very bottom of that model and can help shift the initial burden to patients and out of the healthcare system.”
Poor uptake has prevented CBTI from demonstrating its potential, which is a challenge that Charles M. Morin, PhD, professor of psychology at Laval University in Quebec City, Quebec, Canada, attributes to two factors. “Clearly, there aren’t enough providers with this kind of expertise, and it’s not always covered by public health insurance, so people have to pay out of pocket to treat their insomnia,” he said.
“Overall, I think that this was a very nice study, well conducted, with an impressive sample size,” said Dr. Morin, who was not involved in the study. “The results are quite encouraging, telling us that even when older adults have used sleep medications for an average of 10 years, it’s still possible to reduce the medication. But this doesn’t happen alone. People need to be guided in doing that, not only to decrease medication use, but they also need an alternative,” he said.
Dr. Morin questioned how many patients agree to start with a low intensity. “Ideally, it should be a shared decision paradigm, where the physician or whoever sees the patient first presents the available options and explains the pluses and minuses of each. Some patients might choose medication because it’s a quick fix,” he said. “But some might want to do CBTI, even if it takes more work. The results are sustainable over time,” he added.
The study was jointly funded by the Public Health Agency of Canada and the government of New Brunswick as a Healthy Seniors Pilot Project. Dr. Gardner and Dr. Morin reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A strategy developed by Canadian researchers for encouraging older patients with insomnia to wean themselves from sleeping pills and improve their sleep through behavioral techniques is effective, data suggest. If proven helpful for the millions of older Canadians who currently rely on nightly benzodiazepines (BZDs) and non-BZDs (colloquially known as Z drugs) for their sleep, it might yield an additional benefit: Reducing resource utilization.
“We know that cognitive behavioral therapy for insomnia (CBTI) works. It’s recommended as first-line therapy because it works,” study author David Gardner, PharmD, professor of psychiatry at Dalhousie University in Halifax, Nova Scotia, Canada, told this news organization.
“We’re sharing information about sleeping pills, information that has been embedded with behavior-change techniques that lead people to second-guess or rethink their long-term use of sedative hypnotics and then bring that information to their provider or pharmacist to discuss it,” he said.
The results were published in JAMA Psychiatry.
Better Sleep, Fewer Pills
Dr. Gardner and his team created a direct-to-patient, patient-directed, multicomponent knowledge mobilization intervention called Sleepwell. It incorporates best practice– and guideline-based evidence and multiple behavioral change techniques with content and graphics. Dr. Gardner emphasized that it represents a directional shift in care that alleviates providers’ burden without removing it entirely.
To test the intervention’s effectiveness, Dr. Gardner and his team chose New Brunswick as a location for a 6-month, three-arm, open-label, randomized controlled trial; the province has one of the highest rates of sedative use and an older adult population that is vulnerable to the serious side effects of these drugs (eg, cognitive impairment, falls, and frailty). The study was called Your Answers When Needing Sleep in New Brunswick (YAWNS NB).
Eligible participants were aged ≥ 65 years, lived in the community, and had taken benzodiazepine receptor agonists (BZRAs) for ≥ 3 nights per week for 3 or more months. Participants were randomly assigned to a control group or one of the two intervention groups. The YAWNS-1 intervention group (n = 195) received a mailed package containing a cover letter, a booklet outlining how to stop sleeping pills, a booklet on how to “get your sleep back,” and a companion website. The YAWNS-2 group (n = 193) received updated versions of the booklets used in a prior trial. The control group (n = 192) was assigned treatment as usual (TAU).
A greater proportion of YAWNS-1 participants discontinued BZRAs at 6 months (26.2%) and had dose reductions (20.4%), compared with YAWNS-2 participants (20.3% and 14.4%, respectively) and TAU participants (7.5% and 12.8%, respectively). The corresponding numbers needed to mail to achieve an additional discontinuation was 5.3 YAWNS-1 packages and 7.8 YAWNS-2 packages.
At 6 months, BZRA cessation was sustained a mean 13.6 weeks for YAWNS-1, 14.3 weeks for YAWNS-2, and 16.9 weeks for TAU.
Sleep measures also improved with YAWNS-1, compared with YAWNs-2 and TAU. Sleep onset latency was reduced by 26.1 minutes among YAWNS-1 participants, compared with YAWNS-2 (P < .001), and by 27.7 minutes, compared with TAU (P < .001). Wake after sleep onset increased by 4.1 minutes in YAWNS-1, 11.1 minutes in YAWNS-2, and 7.5 minutes in TAU.
Although all participants underwent rigorous assessment before inclusion, less than half of participants receiving either intervention (36% in YAWNS-1 and 43% in YAWNS-2) contacted their provider or pharmacist to discuss BZD dose reductions. This finding may have resulted partly from limited access because of the COVID-19 pandemic, according to the authors.
A Stepped-Care Model
The intervention is intended to help patients “change their approach from sleeping pills to a short-term CBTI course for long-term sleep benefits, and then speak to their provider,” said Dr. Gardner.
He pointed to a post-study follow-up of the study participants’ health providers, most of whom had moderate to extensive experience deprescribing BZRAs, which showed that 87.5%-100% fully or nearly fully agreed with or supported using the Sleepwell strategy and its content with older patients who rely on sedatives.
“Providers said that deprescribing is difficult, time-consuming, and often not a productive use of their time,” said Dr. Gardner. “I see insomnia as a health issue well set up for a stepped-care model. Self-help approaches are at the very bottom of that model and can help shift the initial burden to patients and out of the healthcare system.”
Poor uptake has prevented CBTI from demonstrating its potential, which is a challenge that Charles M. Morin, PhD, professor of psychology at Laval University in Quebec City, Quebec, Canada, attributes to two factors. “Clearly, there aren’t enough providers with this kind of expertise, and it’s not always covered by public health insurance, so people have to pay out of pocket to treat their insomnia,” he said.
“Overall, I think that this was a very nice study, well conducted, with an impressive sample size,” said Dr. Morin, who was not involved in the study. “The results are quite encouraging, telling us that even when older adults have used sleep medications for an average of 10 years, it’s still possible to reduce the medication. But this doesn’t happen alone. People need to be guided in doing that, not only to decrease medication use, but they also need an alternative,” he said.
Dr. Morin questioned how many patients agree to start with a low intensity. “Ideally, it should be a shared decision paradigm, where the physician or whoever sees the patient first presents the available options and explains the pluses and minuses of each. Some patients might choose medication because it’s a quick fix,” he said. “But some might want to do CBTI, even if it takes more work. The results are sustainable over time,” he added.
The study was jointly funded by the Public Health Agency of Canada and the government of New Brunswick as a Healthy Seniors Pilot Project. Dr. Gardner and Dr. Morin reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Age-Friendly Health Systems Transformation: A Whole Person Approach to Support the Well-Being of Older Adults
The COVID-19 pandemic established a new normal for health care delivery, with leaders rethinking core practices to survive and thrive in a changing environment and improve the health and well-being of patients. The Veterans Health Administration (VHA) is embracing a shift in focus from “what is the matter” to “what really matters” to address pre- and postpandemic challenges through a whole health approach.1 Initially conceptualized by the VHA in 2011, whole health “is an approach to health care that empowers and equips people to take charge of their health and well-being so that they can live their life to the fullest.”1 Whole health integrates evidence-based complementary and integrative health (CIH) therapies to manage pain; this includes acupuncture, meditation, tai chi, yoga, massage therapy, guided imagery, biofeedback, and clinical hypnosis.1 The VHA now recognizes well-being as a core value, helping clinicians respond to emerging challenges related to the social determinants of health (eg, access to health care, physical activity, and healthy foods) and guiding health care decision making.1,2
Well-being through empowerment—elements of whole health and Age-Friendly Health Systems (AFHS)—encourages health care institutions to work with employees, patients, and other stakeholders to address global challenges, clinician burnout, and social issues faced by their communities. This approach focuses on life’s purpose and meaning for individuals and inspires leaders to engage with patients, staff, and communities in new, impactful ways by focusing on wellbeing and wholeness rather than illness and disease. Having a higher sense of purpose is associated with lower all-cause mortality, reduced risk of specific diseases, better health behaviors, greater use of preventive services, and fewer hospital days of care.3
This article describes how AFHS supports the well-being of older adults and aligns with the whole health model of care. It also outlines the VHA investment to transform health care to be more person-centered by documenting what matters in the electronic health record (EHR).
AGE-FRIENDLY CARE
Given that nearly half of veterans enrolled in the VHA are aged ≥ 65 years, there is an increased need to identify models of care to support this aging population.4 This is especially critical because older veterans often have multiple chronic conditions and complex care needs that benefit from a whole person approach. The AFHS movement aims to provide evidence-based care aligned with what matters to older adults and provides a mechanism for transforming care to meet the needs of older veterans. This includes addressing age-related health concerns while promoting optimal health outcomes and quality of life. AFHS follows the 4Ms framework: what matters, medication, mentation, and mobility.5 The 4Ms serve as a guide for the health care of older adults in any setting, where each “M” is assessed and acted on to support what matters.5 Since 2020, > 390 teams have developed a plan to implement the 4Ms at 156 VHA facilities, demonstrating the VHA commitment to transforming health care for veterans.6
When VHA teams join the AFHS movement, they may also engage older veterans in a whole health system (WHS) (Figure). While AFHS is designed to improve care for patients aged ≥ 65 years, it also complements whole health, a person-centered approach available to all veterans enrolled in the VHA. Through the WHS and AFHS, veterans are empowered and equipped to take charge of their health and well-being through conversations about their unique goals, preferences, and health priorities.4 Clinicians are challenged to assess what matters by asking questions like, “What brings you joy?” and, “How can we help you meet your health goals?”1,5 These questions shift the conversation from disease-based treatment and enable clinicians to better understand the veteran as a person.1,5
For whole health and AFHS, conversations about what matters are anchored in the veteran’s goals and preferences, especially those facing a significant health change (ie, a new diagnosis or treatment decision).5,7 Together, the veteran’s goals and priorities serve as the foundation for developing person-centered care plans that often go beyond conventional medical treatments to address the physical, mental, emotional, and social aspects of health.
SYSTEM-WIDE DIRECTIVE
The WHS enhances AFHS discussions about what matters to veterans by adding a system-level lens for conceptualizing health care delivery by leveraging the 3 components of WHS: the “pathway,” well-being programs, and whole health clinical care.
The Pathway
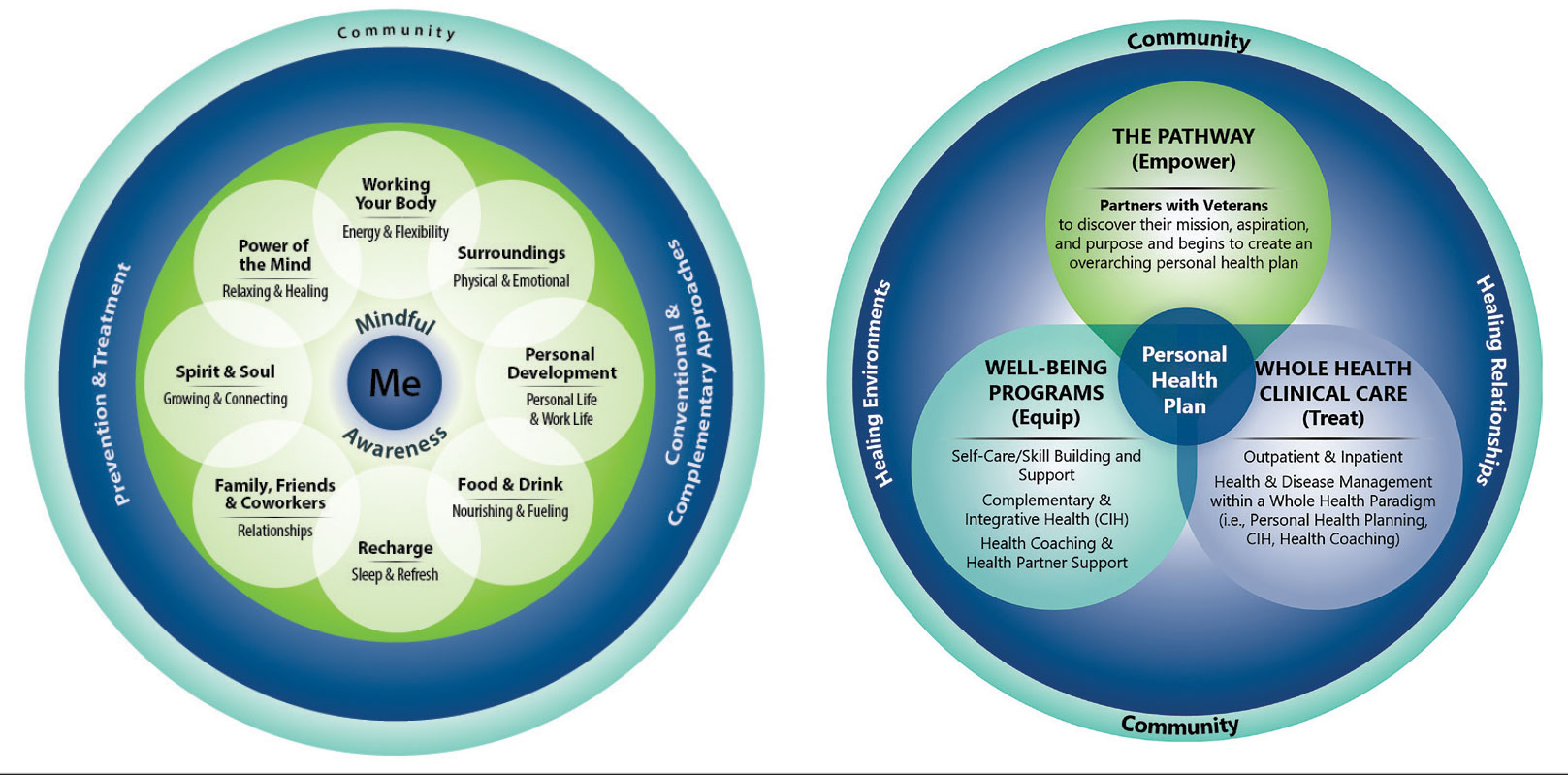
Discovering what matters, or the veteran’s “mission, aspiration, and purpose,” begins with the WHS pathway. When stepping into the pathway, veterans begin completing a personal health inventory, or “walking the circle of health,” which encourages self-reflection that focuses on components of their life that can influence health and well-being.1,8 The circle of health offers a visual representation of the 4 most important aspects of health and well-being: First, “Me” at the center as an individual who is the expert on their life, values, goals, and priorities. Only the individual can know what really matters through mindful awareness and what works for their life. Second, self-care consists of 8 areas that impact health and wellbeing: working your body; surroundings; personal development; food and drink; recharge; family, friends, and coworkers; spirit and soul; and power of the mind. Third, professional care consists of prevention, conventional care, and complementary care. Finally, the community that supports the individual.
Well-Being Programs
VHA provides WHS programs that support veterans in building self-care skills and improving their quality of life, often through integrative care clinics that offer coaching and CIH therapies. For example, a veteran who prioritizes mobility when seeking care at an integrative care clinic will not only receive conventional medical treatment for their physical symptoms but may also be offered CIH therapies depending on their goals. The veteran may set a daily mobility goal with their care team that supports what matters, incorporating CIH approaches, such as yoga and tai chi into the care plan.5 These holistic approaches for moving the body can help alleviate physical symptoms, reduce stress, improve mindful awareness, and provide opportunities for self-discovery and growth, thus promote overall well-being
Whole Health Clinical Care
AFHS and the 4Ms embody the clinical care component of the WHS. Because what matters is the driver of the 4Ms, every action taken by the care team supports wellbeing and quality of life by promoting independence, connection, and support, and addressing external factors, such as social determinants of health. At a minimum, well-being includes “functioning well: the experience of positive emotions such as happiness and contentment as well as the development of one’s potential, having some control over one’s life, having a sense of purpose, and experiencing positive relationships.”9 From a system perspective, the VHA has begun to normalize focusing on what matters to veterans, using an interprofessional approach, one of the first steps to implementing AFHS.
As the programs expand, AFHS teams can learn from whole health well-being programs and increase the capacity for self-care in older veterans. Learning about the key elements included in the circle of health helps clinicians understand each veteran’s perceived strengths and weaknesses to support their self-care. From there, teams can act on the 4Ms and connect older veterans with the most appropriate programs and services at their facility, ensuring continuum of care.
DOCUMENTATION
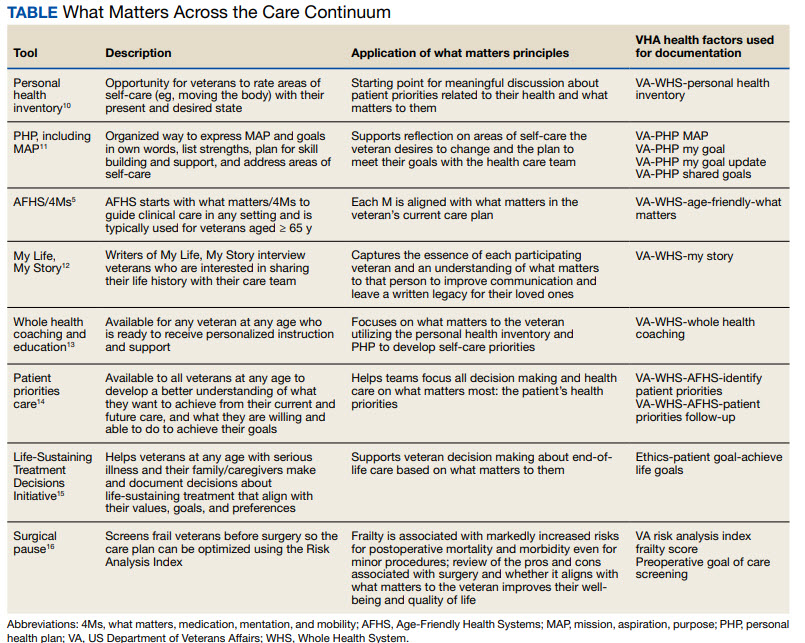
The VHA leverages several tools and evidence-based practices to assess and act on what matters for veterans of all ages (Table).5,10-16 The VHA EHR and associated dashboards contain a wealth of information about whole health and AFHS implementation, scale up, and spread. A national AFHS 4Ms note template contains standardized data elements called health factors, which provide a mechanism for monitoring 4Ms care via its related dashboard. This template was developed by an interprofessional workgroup of VHA staff and underwent a thorough human factors engineering review and testing process prior to its release. Although teams continue to personalize care based on what matters to the veteran, data from the standardized 4Ms note template and dashboard provide a way to establish consistent, equitable care across multiple care settings.17
Between January 2022 and December 2023, > 612,000 participants aged ≥ 65 years identified what matters to them through 1.35 million assessments. During that period, > 36,000 veterans aged ≥ 65 years participated in AFHS and had what matters conversations documented. A personalized health plan was completed by 585,270 veterans for a total of 1.1 million assessments.11 Whole health coaching has been documented for > 57,000 veterans with > 200,000 assessments completed.13 In fiscal year 2023, a total of 1,802,131 veterans participated in whole health.
When teams share information about what matters to the veteran in a clinicianfacing format in the EHR, this helps ensure that the VHA honors veteran preferences throughout transitions of care and across all phases of health care. Although the EHR captures data on what matters, measurement of the overall impact on veteran and health system outcomes is essential. Further evaluation and ongoing education are needed to ensure clinicians are accurately and efficiently capturing the care provided by completing the appropriate EHR. Additional challenges include identifying ways to balance the documentation burden, while ensuring notes include valuable patient-centered information to guide care. EHR tools and templates have helped to unlock important insights on health care delivery in the VHA; however, health systems must consider how these clinical practices support the overall well-being of patients. How leaders empower frontline clinicians in any care setting to use these data to drive meaningful change is also important.
TRANSFORMING VHA CARE DELIVERY
In Achieving Whole Health: A New Approach for Veterans and the Nation, the National Academy of Science proposes a framework for the transformation of health care institutions to provide better whole health to veterans.3 Transformation requires change in entire systems and leaders who mobilize people “for participation in the process of change, encouraging a sense of collective identity and collective efficacy, which in turn brings stronger feelings of self-worth and self-efficacy,” and an enhanced sense of meaningfulness in their work and lives.18
Shifting health care approaches to equipping and empowering veterans and employees with whole health and AFHS resources is transformational and requires radically different assumptions and approaches that cannot be realized through traditional approaches. This change requires robust and multifaceted cultural transformation spanning all levels of the organization. Whole health and AFHS are facilitating this transformation by supporting documentation and data needs, tracking outcomes across settings, and accelerating spread to new facilities and care settings nationwide to support older veterans in improving their health and well-being.
Whole health and AFHS are complementary approaches to care that can work to empower veterans (as well as caregivers and clinicians) to align services with what matters most to veterans. Lessons such as standardizing person-centered assessments of what matters, creating supportive structures to better align care with veterans’ priorities, and identifying meaningful veteran and system-level outcomes to help sustain transformational change can be applied from whole health to AFHS. Together these programs have the potential to enhance overall health outcomes and quality of life for veterans.
- Kligler B, Hyde J, Gantt C, Bokhour B. The Whole Health transformation at the Veterans Health Administration: moving from “what’s the matter with you?” to “what matters to you?” Med Care. 2022;60(5):387-391. doi:10.1097/MLR.0000000000001706
- Centers for Disease Control and Prevention. Social determinants of health (SDOH) at CDC. January 17, 2024. Accessed September 12, 2024. https://www.cdc.gov/public-health-gateway/php/about/social-determinants-of-health.html
- National Academies of Sciences, Engineering, and Medicine. Achieving Whole Health: A New Approach for Veterans and the Nation. The National Academies Press; 2023. Accessed September 9, 2024. doi:10.17226/26854
- Church K, Munro S, Shaughnessy M, Clancy C. Age-friendly health systems: improving care for older adults in the Veterans Health Administration. Health Serv Res. 2023;58 Suppl 1(Suppl 1):5-8. doi:10.1111/1475-6773.14110
- Laderman M, Jackson C, Little K, Duong T, Pelton L. “What Matters” to older adults? A toolkit for health systems to design better care with older adults. Institute for Healthcare Improvement; 2019. Accessed September 9, 2024. https://www.ihi.org/Engage/Initiatives/Age-Friendly-Health-Systems/Documents/IHI_Age_Friendly_What_Matters_to_Older_Adults_Toolkit.pdf
- U.S. Department of Veterans Affairs. Age-Friendly Health Systems. Updated September 4, 2024. Accessed September 9, 2024. https://marketplace.va.gov/innovations/age-friendly-health-systems
- Brown TT, Hurley VB, Rodriguez HP, et al. Shared dec i s i o n - m a k i n g l o w e r s m e d i c a l e x p e n d i t u re s a n d the effect is amplified in racially-ethnically concordant relationships. Med Care. 2023;61(8):528-535. doi:10.1097/MLR.0000000000001881
- Kligler B. Whole Health in the Veterans Health Administration. Glob Adv Health Med. 2022;11:2164957X221077214.
- Ruggeri K, Garcia-Garzon E, Maguire Á, Matz S, Huppert FA. Well-being is more than happiness and life satisfaction: a multidimensional analysis of 21 countries. Health Qual Life Outcomes. 2020;18(1):192. doi:10.1186/s12955-020-01423-y
- U.S. Department of Veterans Affairs. Personal Health Inventory. Updated May 2022. Accessed September 9, 2024. https://www.va.gov/WHOLEHEALTH/docs/PHI-long-May22-fillable-508.pdf doi:10.1177/2164957X221077214
- Veterans Health Administration. Personal Health Plan. Updated March 2019. Accessed September 9, 2024. https:// www.va.gov/WHOLEHEALTH/docs/PersonalHealthPlan_508_03-2019.pdf
- Veterans Health Administration. Whole Health: My Life, My Story. Updated March 20, 2024. Accessed September 9, 2024. https://www.va.gov/WHOLEHEALTH/mylifemystory/index.asp
- U.S. Department of Veterans Affairs. Whole Health Library: Whole Health for Skill Building. Updated April 17, 2024. Accessed September 9, 2024. https://www.va.gov/WHOLEHEALTHLIBRARY/courses/whole-health-skill-building.asp
- U.S. Department of Veterans Affairs. Making Decisions: Current Care Planning. Updated May 21, 2024. Accessed September 9, 2024. https://www.va.gov/geriatrics/pages/making_decisions.asp
- U.S. Department of Veterans Affairs. Life-Sustaining Treatment Decisions Initiative (LSTDI). Updated March 2024. Accessed September 12, 2024. https://marketplace.va.gov/innovations/life-sustaining-treatment-decisions-initiative
- U.S. Department of Veterans Affairs. Center for Health Equity Research and Promotion: Surgical Pause Saving Veterans Lives. Updated September 22, 2021. Accessed September 9, 2024. https://www.cherp.research.va.gov/features/Surgical_Pause_Saving_Veterans_Lives.asp
- Munro S, Church K, Berner C, et al. Implementation of an agefriendly template in the Veterans Health Administration electronic health record. J Inform Nurs. 2023;8(3):6-11.
- Burns JM. Transforming Leadership: A New Pursuit of Happiness. Grove Press; 2003.
- US Department of Veterans Affairs, Veterans Health Administration. Whole Health: Circle of Health Overview. Updated May 20, 2024. Accessed September 12, 2024. https://www.va.gov/WHOLEHEALTH/circle-of-health/index.asp
The COVID-19 pandemic established a new normal for health care delivery, with leaders rethinking core practices to survive and thrive in a changing environment and improve the health and well-being of patients. The Veterans Health Administration (VHA) is embracing a shift in focus from “what is the matter” to “what really matters” to address pre- and postpandemic challenges through a whole health approach.1 Initially conceptualized by the VHA in 2011, whole health “is an approach to health care that empowers and equips people to take charge of their health and well-being so that they can live their life to the fullest.”1 Whole health integrates evidence-based complementary and integrative health (CIH) therapies to manage pain; this includes acupuncture, meditation, tai chi, yoga, massage therapy, guided imagery, biofeedback, and clinical hypnosis.1 The VHA now recognizes well-being as a core value, helping clinicians respond to emerging challenges related to the social determinants of health (eg, access to health care, physical activity, and healthy foods) and guiding health care decision making.1,2
Well-being through empowerment—elements of whole health and Age-Friendly Health Systems (AFHS)—encourages health care institutions to work with employees, patients, and other stakeholders to address global challenges, clinician burnout, and social issues faced by their communities. This approach focuses on life’s purpose and meaning for individuals and inspires leaders to engage with patients, staff, and communities in new, impactful ways by focusing on wellbeing and wholeness rather than illness and disease. Having a higher sense of purpose is associated with lower all-cause mortality, reduced risk of specific diseases, better health behaviors, greater use of preventive services, and fewer hospital days of care.3
This article describes how AFHS supports the well-being of older adults and aligns with the whole health model of care. It also outlines the VHA investment to transform health care to be more person-centered by documenting what matters in the electronic health record (EHR).
AGE-FRIENDLY CARE
Given that nearly half of veterans enrolled in the VHA are aged ≥ 65 years, there is an increased need to identify models of care to support this aging population.4 This is especially critical because older veterans often have multiple chronic conditions and complex care needs that benefit from a whole person approach. The AFHS movement aims to provide evidence-based care aligned with what matters to older adults and provides a mechanism for transforming care to meet the needs of older veterans. This includes addressing age-related health concerns while promoting optimal health outcomes and quality of life. AFHS follows the 4Ms framework: what matters, medication, mentation, and mobility.5 The 4Ms serve as a guide for the health care of older adults in any setting, where each “M” is assessed and acted on to support what matters.5 Since 2020, > 390 teams have developed a plan to implement the 4Ms at 156 VHA facilities, demonstrating the VHA commitment to transforming health care for veterans.6
When VHA teams join the AFHS movement, they may also engage older veterans in a whole health system (WHS) (Figure). While AFHS is designed to improve care for patients aged ≥ 65 years, it also complements whole health, a person-centered approach available to all veterans enrolled in the VHA. Through the WHS and AFHS, veterans are empowered and equipped to take charge of their health and well-being through conversations about their unique goals, preferences, and health priorities.4 Clinicians are challenged to assess what matters by asking questions like, “What brings you joy?” and, “How can we help you meet your health goals?”1,5 These questions shift the conversation from disease-based treatment and enable clinicians to better understand the veteran as a person.1,5

For whole health and AFHS, conversations about what matters are anchored in the veteran’s goals and preferences, especially those facing a significant health change (ie, a new diagnosis or treatment decision).5,7 Together, the veteran’s goals and priorities serve as the foundation for developing person-centered care plans that often go beyond conventional medical treatments to address the physical, mental, emotional, and social aspects of health.
SYSTEM-WIDE DIRECTIVE
The WHS enhances AFHS discussions about what matters to veterans by adding a system-level lens for conceptualizing health care delivery by leveraging the 3 components of WHS: the “pathway,” well-being programs, and whole health clinical care.
The Pathway
Discovering what matters, or the veteran’s “mission, aspiration, and purpose,” begins with the WHS pathway. When stepping into the pathway, veterans begin completing a personal health inventory, or “walking the circle of health,” which encourages self-reflection that focuses on components of their life that can influence health and well-being.1,8 The circle of health offers a visual representation of the 4 most important aspects of health and well-being: First, “Me” at the center as an individual who is the expert on their life, values, goals, and priorities. Only the individual can know what really matters through mindful awareness and what works for their life. Second, self-care consists of 8 areas that impact health and wellbeing: working your body; surroundings; personal development; food and drink; recharge; family, friends, and coworkers; spirit and soul; and power of the mind. Third, professional care consists of prevention, conventional care, and complementary care. Finally, the community that supports the individual.
Well-Being Programs
VHA provides WHS programs that support veterans in building self-care skills and improving their quality of life, often through integrative care clinics that offer coaching and CIH therapies. For example, a veteran who prioritizes mobility when seeking care at an integrative care clinic will not only receive conventional medical treatment for their physical symptoms but may also be offered CIH therapies depending on their goals. The veteran may set a daily mobility goal with their care team that supports what matters, incorporating CIH approaches, such as yoga and tai chi into the care plan.5 These holistic approaches for moving the body can help alleviate physical symptoms, reduce stress, improve mindful awareness, and provide opportunities for self-discovery and growth, thus promote overall well-being
Whole Health Clinical Care
AFHS and the 4Ms embody the clinical care component of the WHS. Because what matters is the driver of the 4Ms, every action taken by the care team supports wellbeing and quality of life by promoting independence, connection, and support, and addressing external factors, such as social determinants of health. At a minimum, well-being includes “functioning well: the experience of positive emotions such as happiness and contentment as well as the development of one’s potential, having some control over one’s life, having a sense of purpose, and experiencing positive relationships.”9 From a system perspective, the VHA has begun to normalize focusing on what matters to veterans, using an interprofessional approach, one of the first steps to implementing AFHS.
As the programs expand, AFHS teams can learn from whole health well-being programs and increase the capacity for self-care in older veterans. Learning about the key elements included in the circle of health helps clinicians understand each veteran’s perceived strengths and weaknesses to support their self-care. From there, teams can act on the 4Ms and connect older veterans with the most appropriate programs and services at their facility, ensuring continuum of care.
DOCUMENTATION
The VHA leverages several tools and evidence-based practices to assess and act on what matters for veterans of all ages (Table).5,10-16 The VHA EHR and associated dashboards contain a wealth of information about whole health and AFHS implementation, scale up, and spread. A national AFHS 4Ms note template contains standardized data elements called health factors, which provide a mechanism for monitoring 4Ms care via its related dashboard. This template was developed by an interprofessional workgroup of VHA staff and underwent a thorough human factors engineering review and testing process prior to its release. Although teams continue to personalize care based on what matters to the veteran, data from the standardized 4Ms note template and dashboard provide a way to establish consistent, equitable care across multiple care settings.17

Between January 2022 and December 2023, > 612,000 participants aged ≥ 65 years identified what matters to them through 1.35 million assessments. During that period, > 36,000 veterans aged ≥ 65 years participated in AFHS and had what matters conversations documented. A personalized health plan was completed by 585,270 veterans for a total of 1.1 million assessments.11 Whole health coaching has been documented for > 57,000 veterans with > 200,000 assessments completed.13 In fiscal year 2023, a total of 1,802,131 veterans participated in whole health.
When teams share information about what matters to the veteran in a clinicianfacing format in the EHR, this helps ensure that the VHA honors veteran preferences throughout transitions of care and across all phases of health care. Although the EHR captures data on what matters, measurement of the overall impact on veteran and health system outcomes is essential. Further evaluation and ongoing education are needed to ensure clinicians are accurately and efficiently capturing the care provided by completing the appropriate EHR. Additional challenges include identifying ways to balance the documentation burden, while ensuring notes include valuable patient-centered information to guide care. EHR tools and templates have helped to unlock important insights on health care delivery in the VHA; however, health systems must consider how these clinical practices support the overall well-being of patients. How leaders empower frontline clinicians in any care setting to use these data to drive meaningful change is also important.
TRANSFORMING VHA CARE DELIVERY
In Achieving Whole Health: A New Approach for Veterans and the Nation, the National Academy of Science proposes a framework for the transformation of health care institutions to provide better whole health to veterans.3 Transformation requires change in entire systems and leaders who mobilize people “for participation in the process of change, encouraging a sense of collective identity and collective efficacy, which in turn brings stronger feelings of self-worth and self-efficacy,” and an enhanced sense of meaningfulness in their work and lives.18
Shifting health care approaches to equipping and empowering veterans and employees with whole health and AFHS resources is transformational and requires radically different assumptions and approaches that cannot be realized through traditional approaches. This change requires robust and multifaceted cultural transformation spanning all levels of the organization. Whole health and AFHS are facilitating this transformation by supporting documentation and data needs, tracking outcomes across settings, and accelerating spread to new facilities and care settings nationwide to support older veterans in improving their health and well-being.
Whole health and AFHS are complementary approaches to care that can work to empower veterans (as well as caregivers and clinicians) to align services with what matters most to veterans. Lessons such as standardizing person-centered assessments of what matters, creating supportive structures to better align care with veterans’ priorities, and identifying meaningful veteran and system-level outcomes to help sustain transformational change can be applied from whole health to AFHS. Together these programs have the potential to enhance overall health outcomes and quality of life for veterans.
The COVID-19 pandemic established a new normal for health care delivery, with leaders rethinking core practices to survive and thrive in a changing environment and improve the health and well-being of patients. The Veterans Health Administration (VHA) is embracing a shift in focus from “what is the matter” to “what really matters” to address pre- and postpandemic challenges through a whole health approach.1 Initially conceptualized by the VHA in 2011, whole health “is an approach to health care that empowers and equips people to take charge of their health and well-being so that they can live their life to the fullest.”1 Whole health integrates evidence-based complementary and integrative health (CIH) therapies to manage pain; this includes acupuncture, meditation, tai chi, yoga, massage therapy, guided imagery, biofeedback, and clinical hypnosis.1 The VHA now recognizes well-being as a core value, helping clinicians respond to emerging challenges related to the social determinants of health (eg, access to health care, physical activity, and healthy foods) and guiding health care decision making.1,2
Well-being through empowerment—elements of whole health and Age-Friendly Health Systems (AFHS)—encourages health care institutions to work with employees, patients, and other stakeholders to address global challenges, clinician burnout, and social issues faced by their communities. This approach focuses on life’s purpose and meaning for individuals and inspires leaders to engage with patients, staff, and communities in new, impactful ways by focusing on wellbeing and wholeness rather than illness and disease. Having a higher sense of purpose is associated with lower all-cause mortality, reduced risk of specific diseases, better health behaviors, greater use of preventive services, and fewer hospital days of care.3
This article describes how AFHS supports the well-being of older adults and aligns with the whole health model of care. It also outlines the VHA investment to transform health care to be more person-centered by documenting what matters in the electronic health record (EHR).
AGE-FRIENDLY CARE
Given that nearly half of veterans enrolled in the VHA are aged ≥ 65 years, there is an increased need to identify models of care to support this aging population.4 This is especially critical because older veterans often have multiple chronic conditions and complex care needs that benefit from a whole person approach. The AFHS movement aims to provide evidence-based care aligned with what matters to older adults and provides a mechanism for transforming care to meet the needs of older veterans. This includes addressing age-related health concerns while promoting optimal health outcomes and quality of life. AFHS follows the 4Ms framework: what matters, medication, mentation, and mobility.5 The 4Ms serve as a guide for the health care of older adults in any setting, where each “M” is assessed and acted on to support what matters.5 Since 2020, > 390 teams have developed a plan to implement the 4Ms at 156 VHA facilities, demonstrating the VHA commitment to transforming health care for veterans.6
When VHA teams join the AFHS movement, they may also engage older veterans in a whole health system (WHS) (Figure). While AFHS is designed to improve care for patients aged ≥ 65 years, it also complements whole health, a person-centered approach available to all veterans enrolled in the VHA. Through the WHS and AFHS, veterans are empowered and equipped to take charge of their health and well-being through conversations about their unique goals, preferences, and health priorities.4 Clinicians are challenged to assess what matters by asking questions like, “What brings you joy?” and, “How can we help you meet your health goals?”1,5 These questions shift the conversation from disease-based treatment and enable clinicians to better understand the veteran as a person.1,5

For whole health and AFHS, conversations about what matters are anchored in the veteran’s goals and preferences, especially those facing a significant health change (ie, a new diagnosis or treatment decision).5,7 Together, the veteran’s goals and priorities serve as the foundation for developing person-centered care plans that often go beyond conventional medical treatments to address the physical, mental, emotional, and social aspects of health.
SYSTEM-WIDE DIRECTIVE
The WHS enhances AFHS discussions about what matters to veterans by adding a system-level lens for conceptualizing health care delivery by leveraging the 3 components of WHS: the “pathway,” well-being programs, and whole health clinical care.
The Pathway
Discovering what matters, or the veteran’s “mission, aspiration, and purpose,” begins with the WHS pathway. When stepping into the pathway, veterans begin completing a personal health inventory, or “walking the circle of health,” which encourages self-reflection that focuses on components of their life that can influence health and well-being.1,8 The circle of health offers a visual representation of the 4 most important aspects of health and well-being: First, “Me” at the center as an individual who is the expert on their life, values, goals, and priorities. Only the individual can know what really matters through mindful awareness and what works for their life. Second, self-care consists of 8 areas that impact health and wellbeing: working your body; surroundings; personal development; food and drink; recharge; family, friends, and coworkers; spirit and soul; and power of the mind. Third, professional care consists of prevention, conventional care, and complementary care. Finally, the community that supports the individual.
Well-Being Programs
VHA provides WHS programs that support veterans in building self-care skills and improving their quality of life, often through integrative care clinics that offer coaching and CIH therapies. For example, a veteran who prioritizes mobility when seeking care at an integrative care clinic will not only receive conventional medical treatment for their physical symptoms but may also be offered CIH therapies depending on their goals. The veteran may set a daily mobility goal with their care team that supports what matters, incorporating CIH approaches, such as yoga and tai chi into the care plan.5 These holistic approaches for moving the body can help alleviate physical symptoms, reduce stress, improve mindful awareness, and provide opportunities for self-discovery and growth, thus promote overall well-being
Whole Health Clinical Care
AFHS and the 4Ms embody the clinical care component of the WHS. Because what matters is the driver of the 4Ms, every action taken by the care team supports wellbeing and quality of life by promoting independence, connection, and support, and addressing external factors, such as social determinants of health. At a minimum, well-being includes “functioning well: the experience of positive emotions such as happiness and contentment as well as the development of one’s potential, having some control over one’s life, having a sense of purpose, and experiencing positive relationships.”9 From a system perspective, the VHA has begun to normalize focusing on what matters to veterans, using an interprofessional approach, one of the first steps to implementing AFHS.
As the programs expand, AFHS teams can learn from whole health well-being programs and increase the capacity for self-care in older veterans. Learning about the key elements included in the circle of health helps clinicians understand each veteran’s perceived strengths and weaknesses to support their self-care. From there, teams can act on the 4Ms and connect older veterans with the most appropriate programs and services at their facility, ensuring continuum of care.
DOCUMENTATION
The VHA leverages several tools and evidence-based practices to assess and act on what matters for veterans of all ages (Table).5,10-16 The VHA EHR and associated dashboards contain a wealth of information about whole health and AFHS implementation, scale up, and spread. A national AFHS 4Ms note template contains standardized data elements called health factors, which provide a mechanism for monitoring 4Ms care via its related dashboard. This template was developed by an interprofessional workgroup of VHA staff and underwent a thorough human factors engineering review and testing process prior to its release. Although teams continue to personalize care based on what matters to the veteran, data from the standardized 4Ms note template and dashboard provide a way to establish consistent, equitable care across multiple care settings.17

Between January 2022 and December 2023, > 612,000 participants aged ≥ 65 years identified what matters to them through 1.35 million assessments. During that period, > 36,000 veterans aged ≥ 65 years participated in AFHS and had what matters conversations documented. A personalized health plan was completed by 585,270 veterans for a total of 1.1 million assessments.11 Whole health coaching has been documented for > 57,000 veterans with > 200,000 assessments completed.13 In fiscal year 2023, a total of 1,802,131 veterans participated in whole health.
When teams share information about what matters to the veteran in a clinicianfacing format in the EHR, this helps ensure that the VHA honors veteran preferences throughout transitions of care and across all phases of health care. Although the EHR captures data on what matters, measurement of the overall impact on veteran and health system outcomes is essential. Further evaluation and ongoing education are needed to ensure clinicians are accurately and efficiently capturing the care provided by completing the appropriate EHR. Additional challenges include identifying ways to balance the documentation burden, while ensuring notes include valuable patient-centered information to guide care. EHR tools and templates have helped to unlock important insights on health care delivery in the VHA; however, health systems must consider how these clinical practices support the overall well-being of patients. How leaders empower frontline clinicians in any care setting to use these data to drive meaningful change is also important.
TRANSFORMING VHA CARE DELIVERY
In Achieving Whole Health: A New Approach for Veterans and the Nation, the National Academy of Science proposes a framework for the transformation of health care institutions to provide better whole health to veterans.3 Transformation requires change in entire systems and leaders who mobilize people “for participation in the process of change, encouraging a sense of collective identity and collective efficacy, which in turn brings stronger feelings of self-worth and self-efficacy,” and an enhanced sense of meaningfulness in their work and lives.18
Shifting health care approaches to equipping and empowering veterans and employees with whole health and AFHS resources is transformational and requires radically different assumptions and approaches that cannot be realized through traditional approaches. This change requires robust and multifaceted cultural transformation spanning all levels of the organization. Whole health and AFHS are facilitating this transformation by supporting documentation and data needs, tracking outcomes across settings, and accelerating spread to new facilities and care settings nationwide to support older veterans in improving their health and well-being.
Whole health and AFHS are complementary approaches to care that can work to empower veterans (as well as caregivers and clinicians) to align services with what matters most to veterans. Lessons such as standardizing person-centered assessments of what matters, creating supportive structures to better align care with veterans’ priorities, and identifying meaningful veteran and system-level outcomes to help sustain transformational change can be applied from whole health to AFHS. Together these programs have the potential to enhance overall health outcomes and quality of life for veterans.
- Kligler B, Hyde J, Gantt C, Bokhour B. The Whole Health transformation at the Veterans Health Administration: moving from “what’s the matter with you?” to “what matters to you?” Med Care. 2022;60(5):387-391. doi:10.1097/MLR.0000000000001706
- Centers for Disease Control and Prevention. Social determinants of health (SDOH) at CDC. January 17, 2024. Accessed September 12, 2024. https://www.cdc.gov/public-health-gateway/php/about/social-determinants-of-health.html
- National Academies of Sciences, Engineering, and Medicine. Achieving Whole Health: A New Approach for Veterans and the Nation. The National Academies Press; 2023. Accessed September 9, 2024. doi:10.17226/26854
- Church K, Munro S, Shaughnessy M, Clancy C. Age-friendly health systems: improving care for older adults in the Veterans Health Administration. Health Serv Res. 2023;58 Suppl 1(Suppl 1):5-8. doi:10.1111/1475-6773.14110
- Laderman M, Jackson C, Little K, Duong T, Pelton L. “What Matters” to older adults? A toolkit for health systems to design better care with older adults. Institute for Healthcare Improvement; 2019. Accessed September 9, 2024. https://www.ihi.org/Engage/Initiatives/Age-Friendly-Health-Systems/Documents/IHI_Age_Friendly_What_Matters_to_Older_Adults_Toolkit.pdf
- U.S. Department of Veterans Affairs. Age-Friendly Health Systems. Updated September 4, 2024. Accessed September 9, 2024. https://marketplace.va.gov/innovations/age-friendly-health-systems
- Brown TT, Hurley VB, Rodriguez HP, et al. Shared dec i s i o n - m a k i n g l o w e r s m e d i c a l e x p e n d i t u re s a n d the effect is amplified in racially-ethnically concordant relationships. Med Care. 2023;61(8):528-535. doi:10.1097/MLR.0000000000001881
- Kligler B. Whole Health in the Veterans Health Administration. Glob Adv Health Med. 2022;11:2164957X221077214.
- Ruggeri K, Garcia-Garzon E, Maguire Á, Matz S, Huppert FA. Well-being is more than happiness and life satisfaction: a multidimensional analysis of 21 countries. Health Qual Life Outcomes. 2020;18(1):192. doi:10.1186/s12955-020-01423-y
- U.S. Department of Veterans Affairs. Personal Health Inventory. Updated May 2022. Accessed September 9, 2024. https://www.va.gov/WHOLEHEALTH/docs/PHI-long-May22-fillable-508.pdf doi:10.1177/2164957X221077214
- Veterans Health Administration. Personal Health Plan. Updated March 2019. Accessed September 9, 2024. https:// www.va.gov/WHOLEHEALTH/docs/PersonalHealthPlan_508_03-2019.pdf
- Veterans Health Administration. Whole Health: My Life, My Story. Updated March 20, 2024. Accessed September 9, 2024. https://www.va.gov/WHOLEHEALTH/mylifemystory/index.asp
- U.S. Department of Veterans Affairs. Whole Health Library: Whole Health for Skill Building. Updated April 17, 2024. Accessed September 9, 2024. https://www.va.gov/WHOLEHEALTHLIBRARY/courses/whole-health-skill-building.asp
- U.S. Department of Veterans Affairs. Making Decisions: Current Care Planning. Updated May 21, 2024. Accessed September 9, 2024. https://www.va.gov/geriatrics/pages/making_decisions.asp
- U.S. Department of Veterans Affairs. Life-Sustaining Treatment Decisions Initiative (LSTDI). Updated March 2024. Accessed September 12, 2024. https://marketplace.va.gov/innovations/life-sustaining-treatment-decisions-initiative
- U.S. Department of Veterans Affairs. Center for Health Equity Research and Promotion: Surgical Pause Saving Veterans Lives. Updated September 22, 2021. Accessed September 9, 2024. https://www.cherp.research.va.gov/features/Surgical_Pause_Saving_Veterans_Lives.asp
- Munro S, Church K, Berner C, et al. Implementation of an agefriendly template in the Veterans Health Administration electronic health record. J Inform Nurs. 2023;8(3):6-11.
- Burns JM. Transforming Leadership: A New Pursuit of Happiness. Grove Press; 2003.
- US Department of Veterans Affairs, Veterans Health Administration. Whole Health: Circle of Health Overview. Updated May 20, 2024. Accessed September 12, 2024. https://www.va.gov/WHOLEHEALTH/circle-of-health/index.asp
- Kligler B, Hyde J, Gantt C, Bokhour B. The Whole Health transformation at the Veterans Health Administration: moving from “what’s the matter with you?” to “what matters to you?” Med Care. 2022;60(5):387-391. doi:10.1097/MLR.0000000000001706
- Centers for Disease Control and Prevention. Social determinants of health (SDOH) at CDC. January 17, 2024. Accessed September 12, 2024. https://www.cdc.gov/public-health-gateway/php/about/social-determinants-of-health.html
- National Academies of Sciences, Engineering, and Medicine. Achieving Whole Health: A New Approach for Veterans and the Nation. The National Academies Press; 2023. Accessed September 9, 2024. doi:10.17226/26854
- Church K, Munro S, Shaughnessy M, Clancy C. Age-friendly health systems: improving care for older adults in the Veterans Health Administration. Health Serv Res. 2023;58 Suppl 1(Suppl 1):5-8. doi:10.1111/1475-6773.14110
- Laderman M, Jackson C, Little K, Duong T, Pelton L. “What Matters” to older adults? A toolkit for health systems to design better care with older adults. Institute for Healthcare Improvement; 2019. Accessed September 9, 2024. https://www.ihi.org/Engage/Initiatives/Age-Friendly-Health-Systems/Documents/IHI_Age_Friendly_What_Matters_to_Older_Adults_Toolkit.pdf
- U.S. Department of Veterans Affairs. Age-Friendly Health Systems. Updated September 4, 2024. Accessed September 9, 2024. https://marketplace.va.gov/innovations/age-friendly-health-systems
- Brown TT, Hurley VB, Rodriguez HP, et al. Shared dec i s i o n - m a k i n g l o w e r s m e d i c a l e x p e n d i t u re s a n d the effect is amplified in racially-ethnically concordant relationships. Med Care. 2023;61(8):528-535. doi:10.1097/MLR.0000000000001881
- Kligler B. Whole Health in the Veterans Health Administration. Glob Adv Health Med. 2022;11:2164957X221077214.
- Ruggeri K, Garcia-Garzon E, Maguire Á, Matz S, Huppert FA. Well-being is more than happiness and life satisfaction: a multidimensional analysis of 21 countries. Health Qual Life Outcomes. 2020;18(1):192. doi:10.1186/s12955-020-01423-y
- U.S. Department of Veterans Affairs. Personal Health Inventory. Updated May 2022. Accessed September 9, 2024. https://www.va.gov/WHOLEHEALTH/docs/PHI-long-May22-fillable-508.pdf doi:10.1177/2164957X221077214
- Veterans Health Administration. Personal Health Plan. Updated March 2019. Accessed September 9, 2024. https:// www.va.gov/WHOLEHEALTH/docs/PersonalHealthPlan_508_03-2019.pdf
- Veterans Health Administration. Whole Health: My Life, My Story. Updated March 20, 2024. Accessed September 9, 2024. https://www.va.gov/WHOLEHEALTH/mylifemystory/index.asp
- U.S. Department of Veterans Affairs. Whole Health Library: Whole Health for Skill Building. Updated April 17, 2024. Accessed September 9, 2024. https://www.va.gov/WHOLEHEALTHLIBRARY/courses/whole-health-skill-building.asp
- U.S. Department of Veterans Affairs. Making Decisions: Current Care Planning. Updated May 21, 2024. Accessed September 9, 2024. https://www.va.gov/geriatrics/pages/making_decisions.asp
- U.S. Department of Veterans Affairs. Life-Sustaining Treatment Decisions Initiative (LSTDI). Updated March 2024. Accessed September 12, 2024. https://marketplace.va.gov/innovations/life-sustaining-treatment-decisions-initiative
- U.S. Department of Veterans Affairs. Center for Health Equity Research and Promotion: Surgical Pause Saving Veterans Lives. Updated September 22, 2021. Accessed September 9, 2024. https://www.cherp.research.va.gov/features/Surgical_Pause_Saving_Veterans_Lives.asp
- Munro S, Church K, Berner C, et al. Implementation of an agefriendly template in the Veterans Health Administration electronic health record. J Inform Nurs. 2023;8(3):6-11.
- Burns JM. Transforming Leadership: A New Pursuit of Happiness. Grove Press; 2003.
- US Department of Veterans Affairs, Veterans Health Administration. Whole Health: Circle of Health Overview. Updated May 20, 2024. Accessed September 12, 2024. https://www.va.gov/WHOLEHEALTH/circle-of-health/index.asp
How Psychedelic Drugs Can Aid Patients at the End of Life
Palliative care has proven to be one of the most promising fields for research on interventions with psychedelic substances. One of the most prominent researchers in this area was the American psychopharmacologist Roland Griffiths, PhD.
In 2016, Dr. Griffiths and his team at Johns Hopkins University in Baltimore, Maryland, published one of the most relevant contributions to the field by demonstrating in a placebo-controlled study that psilocybin can reduce depressive and anxiety symptoms in patients with cancer. The study, conducted with 51 patients diagnosed with advanced-stage cancer, compared the effects of a low dose and a high dose of psilocybin, showing that the high dose resulted in improvements in mood, quality of life, and sense of life, reducing death-related anxiety.
In 2021, after a routine examination, Dr. Griffiths himself was diagnosed with advanced colon cancer. Unexpectedly, the researcher found himself in the position of his research subjects. In an interview with The New York Times in April 2023, he stated that, after some resistance, he agreed to undergo an LSD session.
In the conversation, he revealed that he had a 50% chance of being alive by Halloween. Despite the diagnosis, he showed no discouragement. “As a scientist, I feel like a kid in a candy store, considering all the research and questions that need to be answered about psychedelics and the theme of human flourishing,” he said.
In his last months of life, in the various appearances and interviews he gave, Dr. Griffiths demonstrated a perception of life uncommon in people facing death. “I’m excited to communicate, to shake off the dust and tell people: ‘Come on, wake up!’ ”
He passed away on October 16, 2023, at age 77 years, opening new horizons for clinical research with psychedelics and becoming an example of the therapeutic potential of these substances.
Innovative Treatments
“I believe this will be one of the next conditions, if not the next condition, to be considered for the designation of innovative treatment in future psilocybin regulation in the United States, where the field is more advanced,” said Lucas Maia, PhD, a psychopharmacologist and researcher affiliated with the Advanced Center for Psychedelic Medicine (CAMP) at the Federal University of Rio Grande do Norte (UFRN) and the Interdisciplinary Cooperation for Ayahuasca Research and Outreach (ICARO) at the State University of Campinas in São Paulo, Brazil.
Currently, MDMA (for the treatment of posttraumatic stress disorder), psilocybin (for depressive disorder), and MM120 (an LSD analogue used to treat generalized anxiety disorder) are the only psychedelic substances that have received the designation of innovative treatment by the Food and Drug Administration (FDA).
In 2022, Dr. Maia and a colleague from ICARO, Ana Cláudia Mesquita Garcia, PhD, a professor at the School of Nursing at the Federal University of Alfenas in Brazil and leader of the Interdisciplinary Center for Studies in Palliative Care, published a systematic review in the Journal of Pain and Symptom Management that evaluated the use of psychedelic-assisted treatments for symptom control in patients with serious or terminal illnesses.
Of the 20 articles reviewed, 9 (45%) used LSD, 5 (25%) psilocybin, 2 (10%) dipropyltryptamine (DPT), 1 (5%) used ketamine, and 1 (5%) used MDMA. In 10% of the studies, LSD and DPT were combined. Altogether, 347 participants (54%) received LSD, 116 (18%) psilocybin, 81 (13%) LSD and DPT, 64 (10%) DPT, 18 (3%) MDMA, and 14 (2%) ketamine.
The conclusion of the study is that psychedelics provide therapeutic effects on physical, psychological, social, and existential outcomes. They are associated with a reduction in pain and improvement in sleep. A decrease in depressive and anxiety symptoms is also observed; such symptoms are common in patients with serious diseases. In addition, interpersonal relationships become closer and more empathetic. Finally, there is a reduction in the fear of death and suffering, an increase in acceptance, and a redefinition of the disease.
In 55% of the studies, the adverse effects were mild to moderate and transient. They included nausea, vomiting, dry mouth, and fatigue, as well as anxiety, panic, and hallucinations. The researchers concluded that the scarcity and difficulty of access to professional training in psychedelic-assisted treatments represent a significant challenge for the advancement of these interventions, especially in countries in the Global South.
Another systematic review and meta-analysis published in July by researchers at the University of Michigan in Ann Arbor, Michigan, included seven studies with 132 participants and showed significant improvements in quality of life, pain control, and anxiety relief after psychedelic-assisted psychotherapy with psilocybin. The combined effects indicated statistically significant reductions in anxiety symptoms after 4.0-4.5 months and after 6.0-6.5 months post administration, compared with the initial evaluations.
One of the most advanced research studies currently being conducted is led by Stephen Ross, MD, a psychiatrist affiliated with New York University’s Langone Medical Center, New York City. The phase 2b clinical study is randomized, double blind, and placebo controlled, and involves 300 participants. The study aims to evaluate the effects of psilocybin-assisted psychotherapy on psychiatric and existential distress in patients with advanced cancer. Its expected completion date is in 2027.
“We still lack effective interventions in minimizing psychological, spiritual, and existential suffering,” said Dr. Garcia. “In this sense, respecting the contraindications of a physical nature (including pre-existing illnesses at study initiation, disease staging, patient functionality level, comorbidities, concurrent pharmacological treatments, etc) and of a psychiatric nature for the use of psychedelics, depending on the clinical picture, end-of-life patients facing existential crises and psychological suffering will likely benefit more from psychedelic-assisted psychotherapy, which highlights the need for more research and the integration of this treatment into clinical practice.”
Changing Perceptions
Since 2021, the Cancer Institute of the State of São Paulo (Icesp) has been providing palliative treatment with ketamine — an atypical psychedelic — following a rigorous and carefully monitored clinical protocol. The substance is already used off label to treat refractory depressive disorder. In addition, in 2020, Brazil’s National Health Surveillance Agency approved the use of Spravato, an intranasal antidepressant based on the ketamine derivative esketamine.
Icesp has hospice beds for clinical oncology patients, and a pain management team evaluates which patients meet the inclusion criteria for ketamine use. In addition to difficult-to-control pain, it is important that the patient present emotional, existential, or spiritual symptoms that amplify that pain.
After this evaluation, a psychoeducation process takes place, in which the patient receives clear information about the treatment, its potential benefits and risks, and understands how ketamine can be a viable option for managing their symptoms. Finally, it is essential that the patient accept the referral and demonstrate a willingness to participate in the treatment, agreeing to the proposed terms.
The treatment takes place in a hospital environment, with an ambiance that aims to provide comfort and safety. Clinicians consider not only the substance dose (such as 0.5 mg/kg) but also the emotional state (“set”) and the treatment environment (“setting”). The experience is facilitated through psychological support for the patient during and after treatment.
According to Alessandro Campolina, MD, PhD, a researcher at the Center for Translational Oncology Research at Icesp, it is important to highlight that quality of life is intrinsically linked to the patient’s self-perception, including how they see themselves in terms of health and in the context in which they live.
The doctor explains that psychedelic interventions can provide a “window of opportunity,” allowing a qualified clinician to help the patient explore new perspectives based on their experiences.
“Often, although the intensity of pain remains the same, the way the patient perceives it can change significantly. For example, a patient may report that, despite the pain, they now feel less concerned about it because they were able to contemplate more significant aspects of their life,” said Dr. Campolina.
“This observation shows that treatment is not limited to addressing the pain or primary symptoms, but also addresses the associated suffering. While some patients have profound insights, many others experience more subtle changes that, under the guidance of a competent therapist, can turn into valuable clinical insights, thus improving quality of life and how they deal with their pathologies.”
Dr. Griffiths exemplified this in the interview with the Times when he reflected on his own cancer. He came to believe, as if guided an external observer, that “there is a meaning and a purpose in this [disease] that go beyond your understanding, and the way you are dealing with it is exactly how you should.”
Toshio Chiba, MD, chief physician of the Palliative Care Service at Icesp, emphasized that ketamine is already in use. “It is not feasible to wait years for the approval of psilocybin or for the FDA’s decision on MDMA, especially if the patient needs immediate care,” he said.
Furthermore, recreational and therapeutic uses are distinct. “It is essential to note that responsibilities are shared between the professional and the patient,” said Dr. Chiba. “In the therapeutic setting, there is an ethical and civil responsibility of the medical professional, as well as the patient actively engaging in treatment.”
Early palliative care can also facilitate the establishment of care goals. “I prefer to avoid terms like ‘coping’ or ‘fighting the disease,’” said Dr. Chiba. “Nowadays, dealing with cancer is more about coexisting with the disease properly, as treatments can last for years.
“Of course, there are still highly lethal tumors. However, for neoplasms like breast, colorectal, and prostate cancers, we often talk about 5, 10, or even 15 years of coexistence [with the condition]. The lack of this information [about the disease, treatments, and existential issues] can generate distress in some patients, who end up excessively worrying about the future,” he added.
But palliative treatment with psychedelics as a panacea, he said.
In addition, Marcelo Falchi, MD, medical director of CAMP at UFRN, also emphasized that psychedelics are not a risk-free intervention. Substances like LSD and psilocybin, for example, can cause increases in blood pressure and tachycardia, which, may limit their use for patients at high cardiovascular risk. Crises of anxiety or dissociative symptoms also may occur, and they require mitigation strategies such as psychological support and attention to set and setting.
“But research seems to agree that the risks can be managed effectively through a diligent process, allowing for the responsible exploration of the therapeutic potential of psychedelics,” said Dr. Falchi, who is responsible for CAMP’s postgraduate course in psychedelic therapies. The program provides training in substances used in Brazil, such as ketamine and ibogaine.
The use of psychedelics in palliative care requires a significant shift in how professionals relate to patients.
Unlike in traditional practice, where the prescription is followed by quick consultations, palliative care with psychedelics requires deep and continuous involvement, as Dr. Campolina pointed out. “We joke that it’s not a high-tech specialty, but ‘high touch,’ because it demands the constant presence of the doctor or therapist with the patient. This can involve sessions of several hours, with frequent monitoring and regular contact after sessions. This dynamic emphasizes the importance of human touch and connection during the process, reflecting a new way of practicing medicine.”
In his last months of life, Dr. Griffiths sought to emphasize this point, suggesting that, from a broader perspective, doctors and patients face the same fundamental questions. “We all know we are terminal,” he said. “Essentially, we shouldn’t need a stage 4 cancer diagnosis to awaken to this reality.”
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Palliative care has proven to be one of the most promising fields for research on interventions with psychedelic substances. One of the most prominent researchers in this area was the American psychopharmacologist Roland Griffiths, PhD.
In 2016, Dr. Griffiths and his team at Johns Hopkins University in Baltimore, Maryland, published one of the most relevant contributions to the field by demonstrating in a placebo-controlled study that psilocybin can reduce depressive and anxiety symptoms in patients with cancer. The study, conducted with 51 patients diagnosed with advanced-stage cancer, compared the effects of a low dose and a high dose of psilocybin, showing that the high dose resulted in improvements in mood, quality of life, and sense of life, reducing death-related anxiety.
In 2021, after a routine examination, Dr. Griffiths himself was diagnosed with advanced colon cancer. Unexpectedly, the researcher found himself in the position of his research subjects. In an interview with The New York Times in April 2023, he stated that, after some resistance, he agreed to undergo an LSD session.
In the conversation, he revealed that he had a 50% chance of being alive by Halloween. Despite the diagnosis, he showed no discouragement. “As a scientist, I feel like a kid in a candy store, considering all the research and questions that need to be answered about psychedelics and the theme of human flourishing,” he said.
In his last months of life, in the various appearances and interviews he gave, Dr. Griffiths demonstrated a perception of life uncommon in people facing death. “I’m excited to communicate, to shake off the dust and tell people: ‘Come on, wake up!’ ”
He passed away on October 16, 2023, at age 77 years, opening new horizons for clinical research with psychedelics and becoming an example of the therapeutic potential of these substances.
Innovative Treatments
“I believe this will be one of the next conditions, if not the next condition, to be considered for the designation of innovative treatment in future psilocybin regulation in the United States, where the field is more advanced,” said Lucas Maia, PhD, a psychopharmacologist and researcher affiliated with the Advanced Center for Psychedelic Medicine (CAMP) at the Federal University of Rio Grande do Norte (UFRN) and the Interdisciplinary Cooperation for Ayahuasca Research and Outreach (ICARO) at the State University of Campinas in São Paulo, Brazil.
Currently, MDMA (for the treatment of posttraumatic stress disorder), psilocybin (for depressive disorder), and MM120 (an LSD analogue used to treat generalized anxiety disorder) are the only psychedelic substances that have received the designation of innovative treatment by the Food and Drug Administration (FDA).
In 2022, Dr. Maia and a colleague from ICARO, Ana Cláudia Mesquita Garcia, PhD, a professor at the School of Nursing at the Federal University of Alfenas in Brazil and leader of the Interdisciplinary Center for Studies in Palliative Care, published a systematic review in the Journal of Pain and Symptom Management that evaluated the use of psychedelic-assisted treatments for symptom control in patients with serious or terminal illnesses.
Of the 20 articles reviewed, 9 (45%) used LSD, 5 (25%) psilocybin, 2 (10%) dipropyltryptamine (DPT), 1 (5%) used ketamine, and 1 (5%) used MDMA. In 10% of the studies, LSD and DPT were combined. Altogether, 347 participants (54%) received LSD, 116 (18%) psilocybin, 81 (13%) LSD and DPT, 64 (10%) DPT, 18 (3%) MDMA, and 14 (2%) ketamine.
The conclusion of the study is that psychedelics provide therapeutic effects on physical, psychological, social, and existential outcomes. They are associated with a reduction in pain and improvement in sleep. A decrease in depressive and anxiety symptoms is also observed; such symptoms are common in patients with serious diseases. In addition, interpersonal relationships become closer and more empathetic. Finally, there is a reduction in the fear of death and suffering, an increase in acceptance, and a redefinition of the disease.
In 55% of the studies, the adverse effects were mild to moderate and transient. They included nausea, vomiting, dry mouth, and fatigue, as well as anxiety, panic, and hallucinations. The researchers concluded that the scarcity and difficulty of access to professional training in psychedelic-assisted treatments represent a significant challenge for the advancement of these interventions, especially in countries in the Global South.
Another systematic review and meta-analysis published in July by researchers at the University of Michigan in Ann Arbor, Michigan, included seven studies with 132 participants and showed significant improvements in quality of life, pain control, and anxiety relief after psychedelic-assisted psychotherapy with psilocybin. The combined effects indicated statistically significant reductions in anxiety symptoms after 4.0-4.5 months and after 6.0-6.5 months post administration, compared with the initial evaluations.
One of the most advanced research studies currently being conducted is led by Stephen Ross, MD, a psychiatrist affiliated with New York University’s Langone Medical Center, New York City. The phase 2b clinical study is randomized, double blind, and placebo controlled, and involves 300 participants. The study aims to evaluate the effects of psilocybin-assisted psychotherapy on psychiatric and existential distress in patients with advanced cancer. Its expected completion date is in 2027.
“We still lack effective interventions in minimizing psychological, spiritual, and existential suffering,” said Dr. Garcia. “In this sense, respecting the contraindications of a physical nature (including pre-existing illnesses at study initiation, disease staging, patient functionality level, comorbidities, concurrent pharmacological treatments, etc) and of a psychiatric nature for the use of psychedelics, depending on the clinical picture, end-of-life patients facing existential crises and psychological suffering will likely benefit more from psychedelic-assisted psychotherapy, which highlights the need for more research and the integration of this treatment into clinical practice.”
Changing Perceptions
Since 2021, the Cancer Institute of the State of São Paulo (Icesp) has been providing palliative treatment with ketamine — an atypical psychedelic — following a rigorous and carefully monitored clinical protocol. The substance is already used off label to treat refractory depressive disorder. In addition, in 2020, Brazil’s National Health Surveillance Agency approved the use of Spravato, an intranasal antidepressant based on the ketamine derivative esketamine.
Icesp has hospice beds for clinical oncology patients, and a pain management team evaluates which patients meet the inclusion criteria for ketamine use. In addition to difficult-to-control pain, it is important that the patient present emotional, existential, or spiritual symptoms that amplify that pain.
After this evaluation, a psychoeducation process takes place, in which the patient receives clear information about the treatment, its potential benefits and risks, and understands how ketamine can be a viable option for managing their symptoms. Finally, it is essential that the patient accept the referral and demonstrate a willingness to participate in the treatment, agreeing to the proposed terms.
The treatment takes place in a hospital environment, with an ambiance that aims to provide comfort and safety. Clinicians consider not only the substance dose (such as 0.5 mg/kg) but also the emotional state (“set”) and the treatment environment (“setting”). The experience is facilitated through psychological support for the patient during and after treatment.
According to Alessandro Campolina, MD, PhD, a researcher at the Center for Translational Oncology Research at Icesp, it is important to highlight that quality of life is intrinsically linked to the patient’s self-perception, including how they see themselves in terms of health and in the context in which they live.
The doctor explains that psychedelic interventions can provide a “window of opportunity,” allowing a qualified clinician to help the patient explore new perspectives based on their experiences.
“Often, although the intensity of pain remains the same, the way the patient perceives it can change significantly. For example, a patient may report that, despite the pain, they now feel less concerned about it because they were able to contemplate more significant aspects of their life,” said Dr. Campolina.
“This observation shows that treatment is not limited to addressing the pain or primary symptoms, but also addresses the associated suffering. While some patients have profound insights, many others experience more subtle changes that, under the guidance of a competent therapist, can turn into valuable clinical insights, thus improving quality of life and how they deal with their pathologies.”
Dr. Griffiths exemplified this in the interview with the Times when he reflected on his own cancer. He came to believe, as if guided an external observer, that “there is a meaning and a purpose in this [disease] that go beyond your understanding, and the way you are dealing with it is exactly how you should.”
Toshio Chiba, MD, chief physician of the Palliative Care Service at Icesp, emphasized that ketamine is already in use. “It is not feasible to wait years for the approval of psilocybin or for the FDA’s decision on MDMA, especially if the patient needs immediate care,” he said.
Furthermore, recreational and therapeutic uses are distinct. “It is essential to note that responsibilities are shared between the professional and the patient,” said Dr. Chiba. “In the therapeutic setting, there is an ethical and civil responsibility of the medical professional, as well as the patient actively engaging in treatment.”
Early palliative care can also facilitate the establishment of care goals. “I prefer to avoid terms like ‘coping’ or ‘fighting the disease,’” said Dr. Chiba. “Nowadays, dealing with cancer is more about coexisting with the disease properly, as treatments can last for years.
“Of course, there are still highly lethal tumors. However, for neoplasms like breast, colorectal, and prostate cancers, we often talk about 5, 10, or even 15 years of coexistence [with the condition]. The lack of this information [about the disease, treatments, and existential issues] can generate distress in some patients, who end up excessively worrying about the future,” he added.
But palliative treatment with psychedelics as a panacea, he said.
In addition, Marcelo Falchi, MD, medical director of CAMP at UFRN, also emphasized that psychedelics are not a risk-free intervention. Substances like LSD and psilocybin, for example, can cause increases in blood pressure and tachycardia, which, may limit their use for patients at high cardiovascular risk. Crises of anxiety or dissociative symptoms also may occur, and they require mitigation strategies such as psychological support and attention to set and setting.
“But research seems to agree that the risks can be managed effectively through a diligent process, allowing for the responsible exploration of the therapeutic potential of psychedelics,” said Dr. Falchi, who is responsible for CAMP’s postgraduate course in psychedelic therapies. The program provides training in substances used in Brazil, such as ketamine and ibogaine.
The use of psychedelics in palliative care requires a significant shift in how professionals relate to patients.
Unlike in traditional practice, where the prescription is followed by quick consultations, palliative care with psychedelics requires deep and continuous involvement, as Dr. Campolina pointed out. “We joke that it’s not a high-tech specialty, but ‘high touch,’ because it demands the constant presence of the doctor or therapist with the patient. This can involve sessions of several hours, with frequent monitoring and regular contact after sessions. This dynamic emphasizes the importance of human touch and connection during the process, reflecting a new way of practicing medicine.”
In his last months of life, Dr. Griffiths sought to emphasize this point, suggesting that, from a broader perspective, doctors and patients face the same fundamental questions. “We all know we are terminal,” he said. “Essentially, we shouldn’t need a stage 4 cancer diagnosis to awaken to this reality.”
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Palliative care has proven to be one of the most promising fields for research on interventions with psychedelic substances. One of the most prominent researchers in this area was the American psychopharmacologist Roland Griffiths, PhD.
In 2016, Dr. Griffiths and his team at Johns Hopkins University in Baltimore, Maryland, published one of the most relevant contributions to the field by demonstrating in a placebo-controlled study that psilocybin can reduce depressive and anxiety symptoms in patients with cancer. The study, conducted with 51 patients diagnosed with advanced-stage cancer, compared the effects of a low dose and a high dose of psilocybin, showing that the high dose resulted in improvements in mood, quality of life, and sense of life, reducing death-related anxiety.
In 2021, after a routine examination, Dr. Griffiths himself was diagnosed with advanced colon cancer. Unexpectedly, the researcher found himself in the position of his research subjects. In an interview with The New York Times in April 2023, he stated that, after some resistance, he agreed to undergo an LSD session.
In the conversation, he revealed that he had a 50% chance of being alive by Halloween. Despite the diagnosis, he showed no discouragement. “As a scientist, I feel like a kid in a candy store, considering all the research and questions that need to be answered about psychedelics and the theme of human flourishing,” he said.
In his last months of life, in the various appearances and interviews he gave, Dr. Griffiths demonstrated a perception of life uncommon in people facing death. “I’m excited to communicate, to shake off the dust and tell people: ‘Come on, wake up!’ ”
He passed away on October 16, 2023, at age 77 years, opening new horizons for clinical research with psychedelics and becoming an example of the therapeutic potential of these substances.
Innovative Treatments
“I believe this will be one of the next conditions, if not the next condition, to be considered for the designation of innovative treatment in future psilocybin regulation in the United States, where the field is more advanced,” said Lucas Maia, PhD, a psychopharmacologist and researcher affiliated with the Advanced Center for Psychedelic Medicine (CAMP) at the Federal University of Rio Grande do Norte (UFRN) and the Interdisciplinary Cooperation for Ayahuasca Research and Outreach (ICARO) at the State University of Campinas in São Paulo, Brazil.
Currently, MDMA (for the treatment of posttraumatic stress disorder), psilocybin (for depressive disorder), and MM120 (an LSD analogue used to treat generalized anxiety disorder) are the only psychedelic substances that have received the designation of innovative treatment by the Food and Drug Administration (FDA).
In 2022, Dr. Maia and a colleague from ICARO, Ana Cláudia Mesquita Garcia, PhD, a professor at the School of Nursing at the Federal University of Alfenas in Brazil and leader of the Interdisciplinary Center for Studies in Palliative Care, published a systematic review in the Journal of Pain and Symptom Management that evaluated the use of psychedelic-assisted treatments for symptom control in patients with serious or terminal illnesses.
Of the 20 articles reviewed, 9 (45%) used LSD, 5 (25%) psilocybin, 2 (10%) dipropyltryptamine (DPT), 1 (5%) used ketamine, and 1 (5%) used MDMA. In 10% of the studies, LSD and DPT were combined. Altogether, 347 participants (54%) received LSD, 116 (18%) psilocybin, 81 (13%) LSD and DPT, 64 (10%) DPT, 18 (3%) MDMA, and 14 (2%) ketamine.
The conclusion of the study is that psychedelics provide therapeutic effects on physical, psychological, social, and existential outcomes. They are associated with a reduction in pain and improvement in sleep. A decrease in depressive and anxiety symptoms is also observed; such symptoms are common in patients with serious diseases. In addition, interpersonal relationships become closer and more empathetic. Finally, there is a reduction in the fear of death and suffering, an increase in acceptance, and a redefinition of the disease.
In 55% of the studies, the adverse effects were mild to moderate and transient. They included nausea, vomiting, dry mouth, and fatigue, as well as anxiety, panic, and hallucinations. The researchers concluded that the scarcity and difficulty of access to professional training in psychedelic-assisted treatments represent a significant challenge for the advancement of these interventions, especially in countries in the Global South.
Another systematic review and meta-analysis published in July by researchers at the University of Michigan in Ann Arbor, Michigan, included seven studies with 132 participants and showed significant improvements in quality of life, pain control, and anxiety relief after psychedelic-assisted psychotherapy with psilocybin. The combined effects indicated statistically significant reductions in anxiety symptoms after 4.0-4.5 months and after 6.0-6.5 months post administration, compared with the initial evaluations.
One of the most advanced research studies currently being conducted is led by Stephen Ross, MD, a psychiatrist affiliated with New York University’s Langone Medical Center, New York City. The phase 2b clinical study is randomized, double blind, and placebo controlled, and involves 300 participants. The study aims to evaluate the effects of psilocybin-assisted psychotherapy on psychiatric and existential distress in patients with advanced cancer. Its expected completion date is in 2027.
“We still lack effective interventions in minimizing psychological, spiritual, and existential suffering,” said Dr. Garcia. “In this sense, respecting the contraindications of a physical nature (including pre-existing illnesses at study initiation, disease staging, patient functionality level, comorbidities, concurrent pharmacological treatments, etc) and of a psychiatric nature for the use of psychedelics, depending on the clinical picture, end-of-life patients facing existential crises and psychological suffering will likely benefit more from psychedelic-assisted psychotherapy, which highlights the need for more research and the integration of this treatment into clinical practice.”
Changing Perceptions
Since 2021, the Cancer Institute of the State of São Paulo (Icesp) has been providing palliative treatment with ketamine — an atypical psychedelic — following a rigorous and carefully monitored clinical protocol. The substance is already used off label to treat refractory depressive disorder. In addition, in 2020, Brazil’s National Health Surveillance Agency approved the use of Spravato, an intranasal antidepressant based on the ketamine derivative esketamine.
Icesp has hospice beds for clinical oncology patients, and a pain management team evaluates which patients meet the inclusion criteria for ketamine use. In addition to difficult-to-control pain, it is important that the patient present emotional, existential, or spiritual symptoms that amplify that pain.
After this evaluation, a psychoeducation process takes place, in which the patient receives clear information about the treatment, its potential benefits and risks, and understands how ketamine can be a viable option for managing their symptoms. Finally, it is essential that the patient accept the referral and demonstrate a willingness to participate in the treatment, agreeing to the proposed terms.
The treatment takes place in a hospital environment, with an ambiance that aims to provide comfort and safety. Clinicians consider not only the substance dose (such as 0.5 mg/kg) but also the emotional state (“set”) and the treatment environment (“setting”). The experience is facilitated through psychological support for the patient during and after treatment.
According to Alessandro Campolina, MD, PhD, a researcher at the Center for Translational Oncology Research at Icesp, it is important to highlight that quality of life is intrinsically linked to the patient’s self-perception, including how they see themselves in terms of health and in the context in which they live.
The doctor explains that psychedelic interventions can provide a “window of opportunity,” allowing a qualified clinician to help the patient explore new perspectives based on their experiences.
“Often, although the intensity of pain remains the same, the way the patient perceives it can change significantly. For example, a patient may report that, despite the pain, they now feel less concerned about it because they were able to contemplate more significant aspects of their life,” said Dr. Campolina.
“This observation shows that treatment is not limited to addressing the pain or primary symptoms, but also addresses the associated suffering. While some patients have profound insights, many others experience more subtle changes that, under the guidance of a competent therapist, can turn into valuable clinical insights, thus improving quality of life and how they deal with their pathologies.”
Dr. Griffiths exemplified this in the interview with the Times when he reflected on his own cancer. He came to believe, as if guided an external observer, that “there is a meaning and a purpose in this [disease] that go beyond your understanding, and the way you are dealing with it is exactly how you should.”
Toshio Chiba, MD, chief physician of the Palliative Care Service at Icesp, emphasized that ketamine is already in use. “It is not feasible to wait years for the approval of psilocybin or for the FDA’s decision on MDMA, especially if the patient needs immediate care,” he said.
Furthermore, recreational and therapeutic uses are distinct. “It is essential to note that responsibilities are shared between the professional and the patient,” said Dr. Chiba. “In the therapeutic setting, there is an ethical and civil responsibility of the medical professional, as well as the patient actively engaging in treatment.”
Early palliative care can also facilitate the establishment of care goals. “I prefer to avoid terms like ‘coping’ or ‘fighting the disease,’” said Dr. Chiba. “Nowadays, dealing with cancer is more about coexisting with the disease properly, as treatments can last for years.
“Of course, there are still highly lethal tumors. However, for neoplasms like breast, colorectal, and prostate cancers, we often talk about 5, 10, or even 15 years of coexistence [with the condition]. The lack of this information [about the disease, treatments, and existential issues] can generate distress in some patients, who end up excessively worrying about the future,” he added.
But palliative treatment with psychedelics as a panacea, he said.
In addition, Marcelo Falchi, MD, medical director of CAMP at UFRN, also emphasized that psychedelics are not a risk-free intervention. Substances like LSD and psilocybin, for example, can cause increases in blood pressure and tachycardia, which, may limit their use for patients at high cardiovascular risk. Crises of anxiety or dissociative symptoms also may occur, and they require mitigation strategies such as psychological support and attention to set and setting.
“But research seems to agree that the risks can be managed effectively through a diligent process, allowing for the responsible exploration of the therapeutic potential of psychedelics,” said Dr. Falchi, who is responsible for CAMP’s postgraduate course in psychedelic therapies. The program provides training in substances used in Brazil, such as ketamine and ibogaine.
The use of psychedelics in palliative care requires a significant shift in how professionals relate to patients.
Unlike in traditional practice, where the prescription is followed by quick consultations, palliative care with psychedelics requires deep and continuous involvement, as Dr. Campolina pointed out. “We joke that it’s not a high-tech specialty, but ‘high touch,’ because it demands the constant presence of the doctor or therapist with the patient. This can involve sessions of several hours, with frequent monitoring and regular contact after sessions. This dynamic emphasizes the importance of human touch and connection during the process, reflecting a new way of practicing medicine.”
In his last months of life, Dr. Griffiths sought to emphasize this point, suggesting that, from a broader perspective, doctors and patients face the same fundamental questions. “We all know we are terminal,” he said. “Essentially, we shouldn’t need a stage 4 cancer diagnosis to awaken to this reality.”
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Alzheimer’s and Comorbidities: Implications for Patient Care
Alzheimer’s disease (AD), the most common cause of dementia, is the fifth leading cause of death in the United States. An estimated 6.9 million Americans aged 65 years or older have AD. Comorbid conditions in AD may exacerbate the progression of dementia and negatively affect overall health.
Although the exact mechanisms remain unclear, systemic inflammation is thought to play a significant role in the development of many common comorbidities associated with AD. Among the most frequently observed comorbid conditions are hypertension, diabetes, and depression. The presence of these comorbidities affects the treatment and management of AD, underscoring the need to understand the mechanisms of their interrelationship and develop effective management strategies.
Hypertension
Hypertension is a well-established risk factor for numerous health conditions, including AD. A comprehensive review of five meta-analyses and 52 primary studies revealed that elevated systolic blood pressure (SBP) correlates with an 11 % increased risk of developing AD, raising the question of whether early intervention and control of blood pressure would mitigate the risk for AD later in life.
Findings from the Northern Manhattan Study suggest that although elevated SBP contributes to cognitive decline in older patients, the use of antihypertensive medications can neutralize the effects of high SBP on certain cognitive functions. Furthermore, a systematic review and meta-analysis comprising 12 trials (92,135 participants) demonstrated a significant reduction in the risk for dementia and cognitive impairment with antihypertensive treatment.
Notably, a retrospective cohort study involving 69,081 participants treated with beta-blockers for hypertension found that beta-blockers with high blood-brain barrier permeability were associated with a reduced risk for AD compared with those with low blood-brain barrier permeability. Additionally, a secondary analysis of the SPRINT trial found antihypertensive medications that stimulate vs inhibit type 2 and 4 angiotensin II receptors were associated with a lower incidence of cognitive impairment. Although further clinical trials are necessary to directly assess specific medications, these findings emphasize the potential of antihypertensive treatment as a strategic approach to reduce the risk for AD.
Type 2 Diabetes
The connection between AD and type 2 diabetes is such that AD is sometimes referred to as “type 3 diabetes.” Both diseases share some of the same underlying pathophysiologic mechanisms, particularly the development of insulin resistance and oxidative stress. A prospective cohort study of 10,095 participants showed that diabetes was significantly associated with a higher risk of developing dementia; this risk is even greater in patients who develop diabetes at an earlier age.
In an interview with this news organization, Alvaro Pascual-Leone, MD, PhD, a professor of neurology at Harvard Medical School, Boston, said, “In addition to being a comorbidity factor, diabetes appears to be a predisposing risk factor for AD.” This is supported by a comprehensive literature review showing an increased progression from mild cognitive impairment (MCI) to dementia in patients with diabetes, prediabetes, or metabolic syndrome, with a pooled odds ratio for dementia progression in individuals with diabetes of 1.53.
Owing to the overlapping pathophysiologic mechanisms in AD and diabetes, treating one condition may have beneficial effects on the other. A systematic umbrella review and meta-analysis that included 10 meta-analyses across nine classes of diabetes drugs found a protective effect against dementia with the use of metformin, thiazolidinediones (including pioglitazone), glucagon-like peptide 1 receptor agonists, and sodium-glucose cotransporter 2 inhibitors. Moreover, a cohort study of 12,220 patients who discontinued metformin early (ie, stopped using metformin without a prior history of abnormal kidney function) and 29,126 patients considered routine users found an increased risk for dementia in the early terminator group. Although further research is warranted, the concurrent treatment of AD and diabetes with antidiabetic agents holds considerable promise.
Depression and Anxiety
Anxiety and depression are significant risk factors for AD, and conversely, AD increases the likelihood of developing these psychiatric conditions. A systematic review of 14,760 studies showed dysthymia often emerges during the early stages of AD as an emotional response to cognitive decline.
Data from the Australian Imaging Biomarkers and Lifestyle study showed a markedly elevated risk for AD and MCI among individuals with preexisting anxiety or depression. This study also found that age, sex, and marital status are important determinants, with men and single individuals with depression being particularly susceptible to developing AD. Conversely, a cohort study of 129,410 AD patients with AD, 390,088 patients with all-cause dementia, and 3,900,880 age-matched controls without a history of depression showed a cumulative incidence of depression of 13% in the AD group vs 3% in the control group, suggesting a heightened risk for depression following an AD diagnosis.
These findings underscore the importance of targeted screening and assessment for patients with anxiety and depression who may be at risk for AD or those diagnosed with AD who are at risk for subsequent depression and anxiety. Although antidepressants are effective in treating depression in general, their efficacy in AD-related depression is of variable quality, probably owing to differing pathophysiologic mechanisms of the disease. Further research is necessary to explore both pharmacologic and nonpharmacologic interventions for treating depression in AD patients. Some studies have found that cognitive behavioral-therapy can be effective in improving depression in patients with AD.
Sleep Disorders
Research has shown a strong correlation between AD and sleep disorders, particularly obstructive sleep apnea, insomnia, and circadian rhythm disruptions. Additionally, studies suggest that insomnia and sleep deprivation contribute to increased amyloid beta production and tau pathology, hallmark features of AD. A scoping review of 70 studies proposed that this relationship is mediated by the glymphatic system (glial-dependent waste clearance pathway in the central nervous system), and that sleep deprivation disrupts its function, leading to protein accumulation and subsequent neurologic symptoms of AD. Another study showed that sleep deprivation triggers glial cell activation, initiating an inflammatory cascade that accelerates AD progression.
Given that the gold standard treatment for obstructive sleep apnea is continuous positive airway pressure (CPAP), it has been hypothesized that CPAP could also alleviate AD symptoms owing to shared pathophysiologic mechanisms of these conditions. A large systemic review found that CPAP use improved AD symptoms in patients with mild AD or MCI, though other sleep interventions, such as cognitive-behavioral therapy and melatonin supplementation, have yielded mixed outcomes. However, most studies in this area are small in scale, and there remains a paucity of research on treating sleep disorders in AD patients, indicating a need for further investigation.
Musculoskeletal Disorders
Although no direct causative link has been established, research indicates an association between osteoarthritis (OA) and dementia, likely because of similar pathophysiologic mechanisms, including systemic inflammation. Longitudinal analyses of data from the Alzheimer’s Disease Neuroimaging Initiative study found cognitively normal older individuals with OA experience more rapid declines in hippocampal volumes compared to those without OA, suggesting that OA may elevate the risk of cognitive impairment. Current treatments for OA, such as nonsteroidal anti-inflammatory drugs, glucocorticoids, and disease-modifying OA drugs, might also help alleviate AD symptoms related to inflammation, though the research in this area is limited.
AD has also been linked to osteoporosis. In a longitudinal follow-up study involving 78,994 patients with osteoporosis and 78,994 controls, AD developed in 5856 patients with osteoporosis compared with 3761 patients in the control group. These findings represent a 1.27-fold higher incidence of AD in patients with osteoporosis than in the control group, suggesting that osteoporosis might be a risk factor for AD.
Additionally, research has identified a relationship between AD and increased fracture risk and decreased bone mineral density, with AD patients exhibiting a significantly higher likelihood of bone fractures compared with those without AD. “Falls and fractures, aside from the risk they pose in all geriatric patients, in individuals with cognitive impairment — whether due to AD or another cause — have higher risk to cause delirium and that can result in greater morbidity and mortality and a lasting increase in cognitive disability,” stated Dr. Pascual-Leone. Current recommendations emphasize exercise and fall prevention strategies to reduce fracture risk in patients with AD, but there is a lack of comprehensive research on the safety and efficacy of osteoporosis medications in this population.
Implications for Clinical Practice
The intricate interplay between AD and its comorbidities highlights the need for a comprehensive and integrated approach to patient care. The overlapping pathophysiologic mechanisms suggest that these comorbidities can contribute to the evolution and progression of AD. Likewise, AD can exacerbate comorbid conditions. As such, a holistic assessment strategy that prioritizes early detection and management of comorbid conditions to mitigate their impact on AD progression would be beneficial. Dr. Pascual-Leone added, “The presence of any of these comorbidities suggests a need to screen for MCI earlier than might otherwise be indicated or as part of the treatment for the comorbid condition. In many cases, patients can make lifestyle modifications that improve not only the comorbid condition but also reduce its effect on dementia.” In doing so, healthcare providers can help improve patient outcomes and enhance the overall quality of life for individuals living with AD.
Alissa Hershberger, Professor of Nursing, University of Central Missouri, Lee’s Summit, Missouri, has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Alzheimer’s disease (AD), the most common cause of dementia, is the fifth leading cause of death in the United States. An estimated 6.9 million Americans aged 65 years or older have AD. Comorbid conditions in AD may exacerbate the progression of dementia and negatively affect overall health.
Although the exact mechanisms remain unclear, systemic inflammation is thought to play a significant role in the development of many common comorbidities associated with AD. Among the most frequently observed comorbid conditions are hypertension, diabetes, and depression. The presence of these comorbidities affects the treatment and management of AD, underscoring the need to understand the mechanisms of their interrelationship and develop effective management strategies.
Hypertension
Hypertension is a well-established risk factor for numerous health conditions, including AD. A comprehensive review of five meta-analyses and 52 primary studies revealed that elevated systolic blood pressure (SBP) correlates with an 11 % increased risk of developing AD, raising the question of whether early intervention and control of blood pressure would mitigate the risk for AD later in life.
Findings from the Northern Manhattan Study suggest that although elevated SBP contributes to cognitive decline in older patients, the use of antihypertensive medications can neutralize the effects of high SBP on certain cognitive functions. Furthermore, a systematic review and meta-analysis comprising 12 trials (92,135 participants) demonstrated a significant reduction in the risk for dementia and cognitive impairment with antihypertensive treatment.
Notably, a retrospective cohort study involving 69,081 participants treated with beta-blockers for hypertension found that beta-blockers with high blood-brain barrier permeability were associated with a reduced risk for AD compared with those with low blood-brain barrier permeability. Additionally, a secondary analysis of the SPRINT trial found antihypertensive medications that stimulate vs inhibit type 2 and 4 angiotensin II receptors were associated with a lower incidence of cognitive impairment. Although further clinical trials are necessary to directly assess specific medications, these findings emphasize the potential of antihypertensive treatment as a strategic approach to reduce the risk for AD.
Type 2 Diabetes
The connection between AD and type 2 diabetes is such that AD is sometimes referred to as “type 3 diabetes.” Both diseases share some of the same underlying pathophysiologic mechanisms, particularly the development of insulin resistance and oxidative stress. A prospective cohort study of 10,095 participants showed that diabetes was significantly associated with a higher risk of developing dementia; this risk is even greater in patients who develop diabetes at an earlier age.
In an interview with this news organization, Alvaro Pascual-Leone, MD, PhD, a professor of neurology at Harvard Medical School, Boston, said, “In addition to being a comorbidity factor, diabetes appears to be a predisposing risk factor for AD.” This is supported by a comprehensive literature review showing an increased progression from mild cognitive impairment (MCI) to dementia in patients with diabetes, prediabetes, or metabolic syndrome, with a pooled odds ratio for dementia progression in individuals with diabetes of 1.53.
Owing to the overlapping pathophysiologic mechanisms in AD and diabetes, treating one condition may have beneficial effects on the other. A systematic umbrella review and meta-analysis that included 10 meta-analyses across nine classes of diabetes drugs found a protective effect against dementia with the use of metformin, thiazolidinediones (including pioglitazone), glucagon-like peptide 1 receptor agonists, and sodium-glucose cotransporter 2 inhibitors. Moreover, a cohort study of 12,220 patients who discontinued metformin early (ie, stopped using metformin without a prior history of abnormal kidney function) and 29,126 patients considered routine users found an increased risk for dementia in the early terminator group. Although further research is warranted, the concurrent treatment of AD and diabetes with antidiabetic agents holds considerable promise.
Depression and Anxiety
Anxiety and depression are significant risk factors for AD, and conversely, AD increases the likelihood of developing these psychiatric conditions. A systematic review of 14,760 studies showed dysthymia often emerges during the early stages of AD as an emotional response to cognitive decline.
Data from the Australian Imaging Biomarkers and Lifestyle study showed a markedly elevated risk for AD and MCI among individuals with preexisting anxiety or depression. This study also found that age, sex, and marital status are important determinants, with men and single individuals with depression being particularly susceptible to developing AD. Conversely, a cohort study of 129,410 AD patients with AD, 390,088 patients with all-cause dementia, and 3,900,880 age-matched controls without a history of depression showed a cumulative incidence of depression of 13% in the AD group vs 3% in the control group, suggesting a heightened risk for depression following an AD diagnosis.
These findings underscore the importance of targeted screening and assessment for patients with anxiety and depression who may be at risk for AD or those diagnosed with AD who are at risk for subsequent depression and anxiety. Although antidepressants are effective in treating depression in general, their efficacy in AD-related depression is of variable quality, probably owing to differing pathophysiologic mechanisms of the disease. Further research is necessary to explore both pharmacologic and nonpharmacologic interventions for treating depression in AD patients. Some studies have found that cognitive behavioral-therapy can be effective in improving depression in patients with AD.
Sleep Disorders
Research has shown a strong correlation between AD and sleep disorders, particularly obstructive sleep apnea, insomnia, and circadian rhythm disruptions. Additionally, studies suggest that insomnia and sleep deprivation contribute to increased amyloid beta production and tau pathology, hallmark features of AD. A scoping review of 70 studies proposed that this relationship is mediated by the glymphatic system (glial-dependent waste clearance pathway in the central nervous system), and that sleep deprivation disrupts its function, leading to protein accumulation and subsequent neurologic symptoms of AD. Another study showed that sleep deprivation triggers glial cell activation, initiating an inflammatory cascade that accelerates AD progression.
Given that the gold standard treatment for obstructive sleep apnea is continuous positive airway pressure (CPAP), it has been hypothesized that CPAP could also alleviate AD symptoms owing to shared pathophysiologic mechanisms of these conditions. A large systemic review found that CPAP use improved AD symptoms in patients with mild AD or MCI, though other sleep interventions, such as cognitive-behavioral therapy and melatonin supplementation, have yielded mixed outcomes. However, most studies in this area are small in scale, and there remains a paucity of research on treating sleep disorders in AD patients, indicating a need for further investigation.
Musculoskeletal Disorders
Although no direct causative link has been established, research indicates an association between osteoarthritis (OA) and dementia, likely because of similar pathophysiologic mechanisms, including systemic inflammation. Longitudinal analyses of data from the Alzheimer’s Disease Neuroimaging Initiative study found cognitively normal older individuals with OA experience more rapid declines in hippocampal volumes compared to those without OA, suggesting that OA may elevate the risk of cognitive impairment. Current treatments for OA, such as nonsteroidal anti-inflammatory drugs, glucocorticoids, and disease-modifying OA drugs, might also help alleviate AD symptoms related to inflammation, though the research in this area is limited.
AD has also been linked to osteoporosis. In a longitudinal follow-up study involving 78,994 patients with osteoporosis and 78,994 controls, AD developed in 5856 patients with osteoporosis compared with 3761 patients in the control group. These findings represent a 1.27-fold higher incidence of AD in patients with osteoporosis than in the control group, suggesting that osteoporosis might be a risk factor for AD.
Additionally, research has identified a relationship between AD and increased fracture risk and decreased bone mineral density, with AD patients exhibiting a significantly higher likelihood of bone fractures compared with those without AD. “Falls and fractures, aside from the risk they pose in all geriatric patients, in individuals with cognitive impairment — whether due to AD or another cause — have higher risk to cause delirium and that can result in greater morbidity and mortality and a lasting increase in cognitive disability,” stated Dr. Pascual-Leone. Current recommendations emphasize exercise and fall prevention strategies to reduce fracture risk in patients with AD, but there is a lack of comprehensive research on the safety and efficacy of osteoporosis medications in this population.
Implications for Clinical Practice
The intricate interplay between AD and its comorbidities highlights the need for a comprehensive and integrated approach to patient care. The overlapping pathophysiologic mechanisms suggest that these comorbidities can contribute to the evolution and progression of AD. Likewise, AD can exacerbate comorbid conditions. As such, a holistic assessment strategy that prioritizes early detection and management of comorbid conditions to mitigate their impact on AD progression would be beneficial. Dr. Pascual-Leone added, “The presence of any of these comorbidities suggests a need to screen for MCI earlier than might otherwise be indicated or as part of the treatment for the comorbid condition. In many cases, patients can make lifestyle modifications that improve not only the comorbid condition but also reduce its effect on dementia.” In doing so, healthcare providers can help improve patient outcomes and enhance the overall quality of life for individuals living with AD.
Alissa Hershberger, Professor of Nursing, University of Central Missouri, Lee’s Summit, Missouri, has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Alzheimer’s disease (AD), the most common cause of dementia, is the fifth leading cause of death in the United States. An estimated 6.9 million Americans aged 65 years or older have AD. Comorbid conditions in AD may exacerbate the progression of dementia and negatively affect overall health.
Although the exact mechanisms remain unclear, systemic inflammation is thought to play a significant role in the development of many common comorbidities associated with AD. Among the most frequently observed comorbid conditions are hypertension, diabetes, and depression. The presence of these comorbidities affects the treatment and management of AD, underscoring the need to understand the mechanisms of their interrelationship and develop effective management strategies.
Hypertension
Hypertension is a well-established risk factor for numerous health conditions, including AD. A comprehensive review of five meta-analyses and 52 primary studies revealed that elevated systolic blood pressure (SBP) correlates with an 11 % increased risk of developing AD, raising the question of whether early intervention and control of blood pressure would mitigate the risk for AD later in life.
Findings from the Northern Manhattan Study suggest that although elevated SBP contributes to cognitive decline in older patients, the use of antihypertensive medications can neutralize the effects of high SBP on certain cognitive functions. Furthermore, a systematic review and meta-analysis comprising 12 trials (92,135 participants) demonstrated a significant reduction in the risk for dementia and cognitive impairment with antihypertensive treatment.
Notably, a retrospective cohort study involving 69,081 participants treated with beta-blockers for hypertension found that beta-blockers with high blood-brain barrier permeability were associated with a reduced risk for AD compared with those with low blood-brain barrier permeability. Additionally, a secondary analysis of the SPRINT trial found antihypertensive medications that stimulate vs inhibit type 2 and 4 angiotensin II receptors were associated with a lower incidence of cognitive impairment. Although further clinical trials are necessary to directly assess specific medications, these findings emphasize the potential of antihypertensive treatment as a strategic approach to reduce the risk for AD.
Type 2 Diabetes
The connection between AD and type 2 diabetes is such that AD is sometimes referred to as “type 3 diabetes.” Both diseases share some of the same underlying pathophysiologic mechanisms, particularly the development of insulin resistance and oxidative stress. A prospective cohort study of 10,095 participants showed that diabetes was significantly associated with a higher risk of developing dementia; this risk is even greater in patients who develop diabetes at an earlier age.
In an interview with this news organization, Alvaro Pascual-Leone, MD, PhD, a professor of neurology at Harvard Medical School, Boston, said, “In addition to being a comorbidity factor, diabetes appears to be a predisposing risk factor for AD.” This is supported by a comprehensive literature review showing an increased progression from mild cognitive impairment (MCI) to dementia in patients with diabetes, prediabetes, or metabolic syndrome, with a pooled odds ratio for dementia progression in individuals with diabetes of 1.53.
Owing to the overlapping pathophysiologic mechanisms in AD and diabetes, treating one condition may have beneficial effects on the other. A systematic umbrella review and meta-analysis that included 10 meta-analyses across nine classes of diabetes drugs found a protective effect against dementia with the use of metformin, thiazolidinediones (including pioglitazone), glucagon-like peptide 1 receptor agonists, and sodium-glucose cotransporter 2 inhibitors. Moreover, a cohort study of 12,220 patients who discontinued metformin early (ie, stopped using metformin without a prior history of abnormal kidney function) and 29,126 patients considered routine users found an increased risk for dementia in the early terminator group. Although further research is warranted, the concurrent treatment of AD and diabetes with antidiabetic agents holds considerable promise.
Depression and Anxiety
Anxiety and depression are significant risk factors for AD, and conversely, AD increases the likelihood of developing these psychiatric conditions. A systematic review of 14,760 studies showed dysthymia often emerges during the early stages of AD as an emotional response to cognitive decline.
Data from the Australian Imaging Biomarkers and Lifestyle study showed a markedly elevated risk for AD and MCI among individuals with preexisting anxiety or depression. This study also found that age, sex, and marital status are important determinants, with men and single individuals with depression being particularly susceptible to developing AD. Conversely, a cohort study of 129,410 AD patients with AD, 390,088 patients with all-cause dementia, and 3,900,880 age-matched controls without a history of depression showed a cumulative incidence of depression of 13% in the AD group vs 3% in the control group, suggesting a heightened risk for depression following an AD diagnosis.
These findings underscore the importance of targeted screening and assessment for patients with anxiety and depression who may be at risk for AD or those diagnosed with AD who are at risk for subsequent depression and anxiety. Although antidepressants are effective in treating depression in general, their efficacy in AD-related depression is of variable quality, probably owing to differing pathophysiologic mechanisms of the disease. Further research is necessary to explore both pharmacologic and nonpharmacologic interventions for treating depression in AD patients. Some studies have found that cognitive behavioral-therapy can be effective in improving depression in patients with AD.
Sleep Disorders
Research has shown a strong correlation between AD and sleep disorders, particularly obstructive sleep apnea, insomnia, and circadian rhythm disruptions. Additionally, studies suggest that insomnia and sleep deprivation contribute to increased amyloid beta production and tau pathology, hallmark features of AD. A scoping review of 70 studies proposed that this relationship is mediated by the glymphatic system (glial-dependent waste clearance pathway in the central nervous system), and that sleep deprivation disrupts its function, leading to protein accumulation and subsequent neurologic symptoms of AD. Another study showed that sleep deprivation triggers glial cell activation, initiating an inflammatory cascade that accelerates AD progression.
Given that the gold standard treatment for obstructive sleep apnea is continuous positive airway pressure (CPAP), it has been hypothesized that CPAP could also alleviate AD symptoms owing to shared pathophysiologic mechanisms of these conditions. A large systemic review found that CPAP use improved AD symptoms in patients with mild AD or MCI, though other sleep interventions, such as cognitive-behavioral therapy and melatonin supplementation, have yielded mixed outcomes. However, most studies in this area are small in scale, and there remains a paucity of research on treating sleep disorders in AD patients, indicating a need for further investigation.
Musculoskeletal Disorders
Although no direct causative link has been established, research indicates an association between osteoarthritis (OA) and dementia, likely because of similar pathophysiologic mechanisms, including systemic inflammation. Longitudinal analyses of data from the Alzheimer’s Disease Neuroimaging Initiative study found cognitively normal older individuals with OA experience more rapid declines in hippocampal volumes compared to those without OA, suggesting that OA may elevate the risk of cognitive impairment. Current treatments for OA, such as nonsteroidal anti-inflammatory drugs, glucocorticoids, and disease-modifying OA drugs, might also help alleviate AD symptoms related to inflammation, though the research in this area is limited.
AD has also been linked to osteoporosis. In a longitudinal follow-up study involving 78,994 patients with osteoporosis and 78,994 controls, AD developed in 5856 patients with osteoporosis compared with 3761 patients in the control group. These findings represent a 1.27-fold higher incidence of AD in patients with osteoporosis than in the control group, suggesting that osteoporosis might be a risk factor for AD.
Additionally, research has identified a relationship between AD and increased fracture risk and decreased bone mineral density, with AD patients exhibiting a significantly higher likelihood of bone fractures compared with those without AD. “Falls and fractures, aside from the risk they pose in all geriatric patients, in individuals with cognitive impairment — whether due to AD or another cause — have higher risk to cause delirium and that can result in greater morbidity and mortality and a lasting increase in cognitive disability,” stated Dr. Pascual-Leone. Current recommendations emphasize exercise and fall prevention strategies to reduce fracture risk in patients with AD, but there is a lack of comprehensive research on the safety and efficacy of osteoporosis medications in this population.
Implications for Clinical Practice
The intricate interplay between AD and its comorbidities highlights the need for a comprehensive and integrated approach to patient care. The overlapping pathophysiologic mechanisms suggest that these comorbidities can contribute to the evolution and progression of AD. Likewise, AD can exacerbate comorbid conditions. As such, a holistic assessment strategy that prioritizes early detection and management of comorbid conditions to mitigate their impact on AD progression would be beneficial. Dr. Pascual-Leone added, “The presence of any of these comorbidities suggests a need to screen for MCI earlier than might otherwise be indicated or as part of the treatment for the comorbid condition. In many cases, patients can make lifestyle modifications that improve not only the comorbid condition but also reduce its effect on dementia.” In doing so, healthcare providers can help improve patient outcomes and enhance the overall quality of life for individuals living with AD.
Alissa Hershberger, Professor of Nursing, University of Central Missouri, Lee’s Summit, Missouri, has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Does Screening for CKD Benefit Older Adults?
TOPLINE:
Short-term mortality, hospitalizations, and cardiovascular disease (CVD) events are not significantly different between patients diagnosed with chronic kidney disease (CKD) during routine medical care and those through screening, in a study that found older age, being male, and having a diagnosis of heart failure are associated with an increased risk for mortality in patients with CKD.
METHODOLOGY:
- Researchers conducted a prospective cohort study involving 892 primary care patients aged 60 years or older with CKD from the Oxford Renal Cohort Study in England.
- Participants were categorized into those with existing CKD (n = 257; median age, 75 years), screen-detected CKD (n = 185; median age, roughly 73 years), or temporary reduction in kidney function (n = 450; median age, roughly 73 years).
- The primary outcome was a composite of all-cause mortality, hospitalization, CVD, or end-stage kidney disease.
- The secondary outcomes were the individual components of the composite primary outcome and factors associated with mortality in those with CKD.
TAKEAWAY:
- The composite outcomes were not significantly different between patients with preexisting CKD and kidney disease identified during screening (adjusted hazard ratio [aHR], 0.94; 95% CI, 0.67-1.33).
- Risks for death, hospitalization, CVD, or end-stage kidney disease were not significantly different between the two groups.
- Older age (aHR per year, 1.10; 95% CI, 1.06-1.15), male sex (aHR, 2.31; 95% CI, 1.26-4.24), and heart failure (aHR, 5.18; 95% CI, 2.45-10.97) were associated with higher risks for death.
- No cases of end-stage kidney disease were reported during the study period.
IN PRACTICE:
“Our findings show that the risk of short-term mortality, hospitalization, and CVD is comparable in people diagnosed through screening to those diagnosed routinely in primary care. This suggests that screening older people for CKD may be of value to increase detection and enable disease-modifying treatment to be initiated at an earlier stage,” the study authors wrote.
SOURCE:
The study was led by Anna K. Forbes, MBChB, and José M. Ordóñez-Mena, PhD, of the Nuffield Department of Primary Care Health Sciences at the University of Oxford, England. It was published online in BJGP Open.
LIMITATIONS:
The study had a relatively short follow-up period and a cohort primarily consisting of individuals with early-stage CKD, which may have limited the identification of end-stage cases of the condition. The study population predominantly consisted of White individuals, affecting the generalizability of the results to more diverse populations. Misclassification bias may have occurred due to changes in the kidney function over time.
DISCLOSURES:
The data linkage provided by NHS Digital was supported by funding from the NIHR School of Primary Care Research. Some authors were partly supported by the NIHR Oxford Biomedical Research Centre and NIHR Oxford Thames Valley Applied Research Collaborative. One author reported receiving financial support for attending a conference, while another received consulting fees from various pharmaceutical companies. Another author reported receiving a grant from the Wellcome Trust and payment while working as a presenter for NB Medical and is an unpaid trustee of some charities.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Short-term mortality, hospitalizations, and cardiovascular disease (CVD) events are not significantly different between patients diagnosed with chronic kidney disease (CKD) during routine medical care and those through screening, in a study that found older age, being male, and having a diagnosis of heart failure are associated with an increased risk for mortality in patients with CKD.
METHODOLOGY:
- Researchers conducted a prospective cohort study involving 892 primary care patients aged 60 years or older with CKD from the Oxford Renal Cohort Study in England.
- Participants were categorized into those with existing CKD (n = 257; median age, 75 years), screen-detected CKD (n = 185; median age, roughly 73 years), or temporary reduction in kidney function (n = 450; median age, roughly 73 years).
- The primary outcome was a composite of all-cause mortality, hospitalization, CVD, or end-stage kidney disease.
- The secondary outcomes were the individual components of the composite primary outcome and factors associated with mortality in those with CKD.
TAKEAWAY:
- The composite outcomes were not significantly different between patients with preexisting CKD and kidney disease identified during screening (adjusted hazard ratio [aHR], 0.94; 95% CI, 0.67-1.33).
- Risks for death, hospitalization, CVD, or end-stage kidney disease were not significantly different between the two groups.
- Older age (aHR per year, 1.10; 95% CI, 1.06-1.15), male sex (aHR, 2.31; 95% CI, 1.26-4.24), and heart failure (aHR, 5.18; 95% CI, 2.45-10.97) were associated with higher risks for death.
- No cases of end-stage kidney disease were reported during the study period.
IN PRACTICE:
“Our findings show that the risk of short-term mortality, hospitalization, and CVD is comparable in people diagnosed through screening to those diagnosed routinely in primary care. This suggests that screening older people for CKD may be of value to increase detection and enable disease-modifying treatment to be initiated at an earlier stage,” the study authors wrote.
SOURCE:
The study was led by Anna K. Forbes, MBChB, and José M. Ordóñez-Mena, PhD, of the Nuffield Department of Primary Care Health Sciences at the University of Oxford, England. It was published online in BJGP Open.
LIMITATIONS:
The study had a relatively short follow-up period and a cohort primarily consisting of individuals with early-stage CKD, which may have limited the identification of end-stage cases of the condition. The study population predominantly consisted of White individuals, affecting the generalizability of the results to more diverse populations. Misclassification bias may have occurred due to changes in the kidney function over time.
DISCLOSURES:
The data linkage provided by NHS Digital was supported by funding from the NIHR School of Primary Care Research. Some authors were partly supported by the NIHR Oxford Biomedical Research Centre and NIHR Oxford Thames Valley Applied Research Collaborative. One author reported receiving financial support for attending a conference, while another received consulting fees from various pharmaceutical companies. Another author reported receiving a grant from the Wellcome Trust and payment while working as a presenter for NB Medical and is an unpaid trustee of some charities.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Short-term mortality, hospitalizations, and cardiovascular disease (CVD) events are not significantly different between patients diagnosed with chronic kidney disease (CKD) during routine medical care and those through screening, in a study that found older age, being male, and having a diagnosis of heart failure are associated with an increased risk for mortality in patients with CKD.
METHODOLOGY:
- Researchers conducted a prospective cohort study involving 892 primary care patients aged 60 years or older with CKD from the Oxford Renal Cohort Study in England.
- Participants were categorized into those with existing CKD (n = 257; median age, 75 years), screen-detected CKD (n = 185; median age, roughly 73 years), or temporary reduction in kidney function (n = 450; median age, roughly 73 years).
- The primary outcome was a composite of all-cause mortality, hospitalization, CVD, or end-stage kidney disease.
- The secondary outcomes were the individual components of the composite primary outcome and factors associated with mortality in those with CKD.
TAKEAWAY:
- The composite outcomes were not significantly different between patients with preexisting CKD and kidney disease identified during screening (adjusted hazard ratio [aHR], 0.94; 95% CI, 0.67-1.33).
- Risks for death, hospitalization, CVD, or end-stage kidney disease were not significantly different between the two groups.
- Older age (aHR per year, 1.10; 95% CI, 1.06-1.15), male sex (aHR, 2.31; 95% CI, 1.26-4.24), and heart failure (aHR, 5.18; 95% CI, 2.45-10.97) were associated with higher risks for death.
- No cases of end-stage kidney disease were reported during the study period.
IN PRACTICE:
“Our findings show that the risk of short-term mortality, hospitalization, and CVD is comparable in people diagnosed through screening to those diagnosed routinely in primary care. This suggests that screening older people for CKD may be of value to increase detection and enable disease-modifying treatment to be initiated at an earlier stage,” the study authors wrote.
SOURCE:
The study was led by Anna K. Forbes, MBChB, and José M. Ordóñez-Mena, PhD, of the Nuffield Department of Primary Care Health Sciences at the University of Oxford, England. It was published online in BJGP Open.
LIMITATIONS:
The study had a relatively short follow-up period and a cohort primarily consisting of individuals with early-stage CKD, which may have limited the identification of end-stage cases of the condition. The study population predominantly consisted of White individuals, affecting the generalizability of the results to more diverse populations. Misclassification bias may have occurred due to changes in the kidney function over time.
DISCLOSURES:
The data linkage provided by NHS Digital was supported by funding from the NIHR School of Primary Care Research. Some authors were partly supported by the NIHR Oxford Biomedical Research Centre and NIHR Oxford Thames Valley Applied Research Collaborative. One author reported receiving financial support for attending a conference, while another received consulting fees from various pharmaceutical companies. Another author reported receiving a grant from the Wellcome Trust and payment while working as a presenter for NB Medical and is an unpaid trustee of some charities.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Muscle Relaxants for Chronic Pain: Where Is the Greatest Evidence?
TOPLINE:
The long-term use of muscle relaxants may benefit patients with painful spasms or cramps and neck pain, according to a systematic review of clinical studies, but they do not appear to be beneficial for low back pain, fibromyalgia, or headaches and can have adverse effects such as sedation and dry mouth.
METHODOLOGY:
- Researchers conducted a systematic review to evaluate the effectiveness of long-term use (≥ 4 weeks) of muscle relaxants for chronic pain lasting ≥ 3 months.
- They identified 30 randomized clinical trials involving 1314 patients and 14 cohort studies involving 1168 patients, grouped according to the categories of low back pain, fibromyalgia, painful cramps or spasticity, headaches, and other syndromes.
- Baclofen, tizanidine, cyclobenzaprine, eperisone, quinine, carisoprodol, orphenadrine, chlormezanone, and methocarbamol were the muscle relaxants assessed in comparison with placebo, other treatments, or untreated individuals.
TAKEAWAY:
- The long-term use of muscle relaxants reduced pain intensity in those with painful spasms or cramps and neck pain. Baclofen, orphenadrine, carisoprodol, and methocarbamol improved cramp frequency, while the use of eperisone and chlormezanone improved neck pain and enhanced the quality of sleep, respectively, in those with neck osteoarthritis.
- While some studies suggested that muscle relaxants reduced pain intensity in those with back pain and fibromyalgia, between-group differences were not observed. The benefits seen with some medications diminished after their discontinuation.
- Despite tizanidine improving pain severity in headaches, 25% participants dropped out owing to adverse effects. Although certain muscle relaxants demonstrated pain relief, others did not.
- The most common adverse effects of muscle relaxants were somnolence and dry mouth. Other adverse events included vomiting, diarrhea, nausea, weakness, and constipation.
IN PRACTICE:
“For patients already prescribed long-term SMRs [skeletal muscle relaxants], interventions are needed to assist clinicians to engage in shared decision-making with patients about deprescribing SMRs. This may be particularly true for older patients for whom risks of adverse events may be greater,” the authors wrote. “Clinicians should be vigilant for adverse effects and consider deprescribing if pain-related goals are not met.”
SOURCE:
The study, led by Benjamin J. Oldfield, MD, MHS, Yale School of Medicine, New Haven, Connecticut, was published online on September 19, 2024, in JAMA Network Open
LIMITATIONS:
This systematic review was limited to publications written in English, Spanish, and Italian language, potentially excluding studies from other regions. Variations in clinical sites, definitions of pain syndromes, medications, and durations of therapy prevented the possibility of conducting meta-analyses. Only quantitative studies were included, excluding valuable insights into patient experiences offered by qualitative studies.
DISCLOSURES:
The study was supported by the National Institute on Drug Abuse. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
The long-term use of muscle relaxants may benefit patients with painful spasms or cramps and neck pain, according to a systematic review of clinical studies, but they do not appear to be beneficial for low back pain, fibromyalgia, or headaches and can have adverse effects such as sedation and dry mouth.
METHODOLOGY:
- Researchers conducted a systematic review to evaluate the effectiveness of long-term use (≥ 4 weeks) of muscle relaxants for chronic pain lasting ≥ 3 months.
- They identified 30 randomized clinical trials involving 1314 patients and 14 cohort studies involving 1168 patients, grouped according to the categories of low back pain, fibromyalgia, painful cramps or spasticity, headaches, and other syndromes.
- Baclofen, tizanidine, cyclobenzaprine, eperisone, quinine, carisoprodol, orphenadrine, chlormezanone, and methocarbamol were the muscle relaxants assessed in comparison with placebo, other treatments, or untreated individuals.
TAKEAWAY:
- The long-term use of muscle relaxants reduced pain intensity in those with painful spasms or cramps and neck pain. Baclofen, orphenadrine, carisoprodol, and methocarbamol improved cramp frequency, while the use of eperisone and chlormezanone improved neck pain and enhanced the quality of sleep, respectively, in those with neck osteoarthritis.
- While some studies suggested that muscle relaxants reduced pain intensity in those with back pain and fibromyalgia, between-group differences were not observed. The benefits seen with some medications diminished after their discontinuation.
- Despite tizanidine improving pain severity in headaches, 25% participants dropped out owing to adverse effects. Although certain muscle relaxants demonstrated pain relief, others did not.
- The most common adverse effects of muscle relaxants were somnolence and dry mouth. Other adverse events included vomiting, diarrhea, nausea, weakness, and constipation.
IN PRACTICE:
“For patients already prescribed long-term SMRs [skeletal muscle relaxants], interventions are needed to assist clinicians to engage in shared decision-making with patients about deprescribing SMRs. This may be particularly true for older patients for whom risks of adverse events may be greater,” the authors wrote. “Clinicians should be vigilant for adverse effects and consider deprescribing if pain-related goals are not met.”
SOURCE:
The study, led by Benjamin J. Oldfield, MD, MHS, Yale School of Medicine, New Haven, Connecticut, was published online on September 19, 2024, in JAMA Network Open
LIMITATIONS:
This systematic review was limited to publications written in English, Spanish, and Italian language, potentially excluding studies from other regions. Variations in clinical sites, definitions of pain syndromes, medications, and durations of therapy prevented the possibility of conducting meta-analyses. Only quantitative studies were included, excluding valuable insights into patient experiences offered by qualitative studies.
DISCLOSURES:
The study was supported by the National Institute on Drug Abuse. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
The long-term use of muscle relaxants may benefit patients with painful spasms or cramps and neck pain, according to a systematic review of clinical studies, but they do not appear to be beneficial for low back pain, fibromyalgia, or headaches and can have adverse effects such as sedation and dry mouth.
METHODOLOGY:
- Researchers conducted a systematic review to evaluate the effectiveness of long-term use (≥ 4 weeks) of muscle relaxants for chronic pain lasting ≥ 3 months.
- They identified 30 randomized clinical trials involving 1314 patients and 14 cohort studies involving 1168 patients, grouped according to the categories of low back pain, fibromyalgia, painful cramps or spasticity, headaches, and other syndromes.
- Baclofen, tizanidine, cyclobenzaprine, eperisone, quinine, carisoprodol, orphenadrine, chlormezanone, and methocarbamol were the muscle relaxants assessed in comparison with placebo, other treatments, or untreated individuals.
TAKEAWAY:
- The long-term use of muscle relaxants reduced pain intensity in those with painful spasms or cramps and neck pain. Baclofen, orphenadrine, carisoprodol, and methocarbamol improved cramp frequency, while the use of eperisone and chlormezanone improved neck pain and enhanced the quality of sleep, respectively, in those with neck osteoarthritis.
- While some studies suggested that muscle relaxants reduced pain intensity in those with back pain and fibromyalgia, between-group differences were not observed. The benefits seen with some medications diminished after their discontinuation.
- Despite tizanidine improving pain severity in headaches, 25% participants dropped out owing to adverse effects. Although certain muscle relaxants demonstrated pain relief, others did not.
- The most common adverse effects of muscle relaxants were somnolence and dry mouth. Other adverse events included vomiting, diarrhea, nausea, weakness, and constipation.
IN PRACTICE:
“For patients already prescribed long-term SMRs [skeletal muscle relaxants], interventions are needed to assist clinicians to engage in shared decision-making with patients about deprescribing SMRs. This may be particularly true for older patients for whom risks of adverse events may be greater,” the authors wrote. “Clinicians should be vigilant for adverse effects and consider deprescribing if pain-related goals are not met.”
SOURCE:
The study, led by Benjamin J. Oldfield, MD, MHS, Yale School of Medicine, New Haven, Connecticut, was published online on September 19, 2024, in JAMA Network Open
LIMITATIONS:
This systematic review was limited to publications written in English, Spanish, and Italian language, potentially excluding studies from other regions. Variations in clinical sites, definitions of pain syndromes, medications, and durations of therapy prevented the possibility of conducting meta-analyses. Only quantitative studies were included, excluding valuable insights into patient experiences offered by qualitative studies.
DISCLOSURES:
The study was supported by the National Institute on Drug Abuse. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Elinzanetant Shows Significant Improvement in Menopausal Vasomotor and Sleep Symptoms
CHICAGO — The nonhormonal investigational drug elinzanetant led to significant improvement in hot flashes as well as sleep disturbance and quality of life, according to data from three randomized controlled trials presented at The Menopause Society 2024 Annual Meeting in Chicago. Two phase 3 trials, OASIS 1 and 2, were also published in JAMA, and the longer-term OASIS 3 trial was presented as a poster at the conference.
Elinzanetant is a selective neurokinin (NK) receptor antagonist, similar to fezolinetant, the first drug in this class approved by the US Food and Drug Administration (FDA) for vasomotor symptoms in May 2023. This class of medications targets the estrogen-sensitive kisspeptin/NK B/dynorphin (KNDy) neurons thought to play a role in thermoregulation and hot flashes during menopause. While fezolinetant targets only the NK-3 receptor, elinzanetant is a dual NK receptor antagonist that targets both NK-1 and NK-3. Bayer submitted a New Drug Application for elinzanetant to the FDA on August 1.
For those in whom hormone therapy is contraindicated, “it’s always been difficult for women with really severe symptoms to have a safe and effective therapy,” lead author JoAnn Pinkerton, MD, a professor of ob.gyn. at the University of Virginia in Charlottesville, Virginia, told this news organization. “The nonhormonal therapies we’ve used mostly off-label — the antidepressants, gabapentin, clonidine, oxybutynin — do help the hot flashes, but they don’t work nearly as effectively as these new NK receptor antagonists, and having one that looks like it might have a broader use for hot flashes, night sweats, mood, and sleep is just really exciting.”
Dr. Pinkerton said approximately 80% of the women in the OASIS 1 and 2 studies had at least a 50% reduction in hot flashes. “It was a very strong, dramatic positive finding, but the improvements in sleep and mood have really encouraged us to go further,” she said.
Declining estrogen levels during and after menopause can cause hypertrophy and hyperactivity of the KNDy neurons, which has been linked to thermoregulation disruptions that may trigger hot flashes, James Simon, MD, a clinical professor of ob.gyn. at The George Washington University School of Medicine & Health Sciences and medical director of IntimMedicine in Washington, DC, told attendees. He presented pooled data from OASIS 1 and 2. The NK-1 receptor, targeted by elinzanetant but not fezolinetant, is also thought to play a role in insomnia and possibly in mood.
“Oftentimes the focus on a lot of these drugs is hot flashes, hot flashes, hot flashes, but we know hot flashes do not occur in isolation,” Chrisandra Shufelt, MD, professor and chair of general internal medicine and associate director of the Women’s Health Research Center at Mayo Clinic in Jacksonville, Florida, told this news organization. Elinzanetant is “an interesting compound because it actually works on sleep, and that was critical because sleep disturbance precedes” many other menopausal symptoms, said Dr. Shufelt, who was not involved in the study.
“I think it is an outstanding option for women who don’t have the opportunity to get hormones,” Dr. Shufelt said, and she was particularly pleased to see there were no safety concerns for the liver in the trial data. The FDA issued a warning on September 12 about the risk for rare liver injury with fezolinetant, but the early signals that had been seen in fezolinetant data were not seen in these elinzanetant data.
The OASIS 1 and 2 trials enrolled postmenopausal women, aged 40-65 years, who had at least 50 moderate to severe vasomotor occurrences per week.
“A moderate hot flash is a hot flash that is also associated with sweating, and a severe hot flash is a moderate hot flash that stops a woman in her tracks,” Dr. Simon said. “Namely, it’s severe enough with sweating and central nervous system effects that she is interrupted in whatever it is that she’s doing at the time.”
Exclusion criteria for the trials included a history of arrhythmias, heart block, or QT prolongation; abnormal lab results; history of malignancy within the past 5 years; uncontrolled or treatment-resistant hypertension, hypothyroidism, or hyperthyroidism; unexplained postmenopausal bleeding; clinically relevant abnormal mammogram findings; or disordered proliferative endometrium, endometrial hyperplasia, polyp, or endometrial cancer.
The predominantly White (80%) women were an average 54 years old, with an average body mass index (BMI) of 27.8, and were an average 3.5 years from their last period. For the first 12 weeks of the trials, 399 women were assigned to receive 120 mg once daily of oral elinzanetant and 397 were assigned to once daily placebo. Then the women taking placebo switched to elinzanetant for the final 14 weeks of the study.
The endpoints included mean change in frequency and severity of vasomotor symptoms at weeks 1, 4, and 12 as well as change in sleep disturbance and quality of life at week 12. Sleep was assessed with the Patient-Reported Outcomes Measurement Information System Sleep Disturbance–Short Form score, which ranges from 28.9 to 76.5, with a higher number denoting greater sleep disturbance. The Menopause-Specific Quality-of-Life score ranges from 1 to 8, with a higher score indicating poorer quality of life.
Daily frequency of vasomotor symptoms was 14 per day at baseline in the elinzanetant group, decreasing by 4.8 per day at week 1, 8 per day at week 4, and 9.4 per day at week 12. In the placebo group, women had an average 15.2 occurrences per day at baseline, which decreased by 3.2 at week 1, 5.2 at week 4, and 6.4 at week 12. Comparing the groups at 12 weeks, those receiving elinzanetant had 3.2 fewer daily vasomotor symptoms than those receiving placebo (P < .0001).
The severity of vasomotor symptoms also improved more in the elinzanetant group than in the placebo group over 12 weeks, after which severity improved further in those who switched from placebo to elinzanetant (P < .0001).
Sleep disturbance scores, starting at a mean 61.5 in the elinzanetant group and 60.5 in the placebo group, fell 10.7 points in the elinzanetant group and 5.3 points in the placebo group at 12 weeks, for a difference of 4.9 points (P < .0001). Sleep then further improved in those who switched from placebo to elinzanetant. Quality-of-life scores improved 1.37 points (from 4.52 at baseline) in the elinzanetant group and 0.96 points (from 4.49 at baseline) in the placebo group, for a mean difference at 12 weeks of 0.36 (P < .0001).
Though no head-to-head data exist comparing elinzanetant and fezolinetant, Dr. Simon told this news organization the side effects with fezolinetant “tend to be gastrointestinal, whereas the side effects for elinzanetant tend to be central nervous system,” such as drowsiness and lethargy.
The women who are the best candidates for elinzanetant, Dr. Pinkerton told this news organization, include those who have had an estrogen-sensitive cancer, such as breast or endometrial cancer, or who have fear of it, a family history, or are otherwise high risk. Other ideal candidates include those with a history of venous thromboembolism, people who have migraine with aura (due to concerns about increased risk for stroke), and those who have endometriosis or large fibroids.
“Then the last group might be women who took hormone therapy in their 50s and want to continue, but they’re trying to go off, and they have a recurrence of their hot flashes or night sweats or sleep issues,” Dr. Pinkerton said. “This might be a great group to switch over.”
OASIS 3 assessed the drug for 1 year and “supported the results of OASIS 1 and 2, demonstrating efficacy over a longer study duration and in a population with a vasomotor symptom profile representative of that seen in clinical practice,” Nick Panay, BSc, MBBS, director of the Menopause & PMS Centre at Queen Charlotte’s Hospital & Imperial College London, London, England, and his colleague reported.
Among 628 postmenopausal women aged 40-65, the predominantly White (78.5%) women were an average 54 years old, with an average BMI of 27.6, and were an average 5 years past their last period. Half received 120 mg elinzanetant and half received a placebo for 52 weeks.
At 12 weeks, the women receiving elinzanetant reported an average 1.6 moderate to severe vasomotor symptoms per day, down from 6.7 at baseline. Daily average symptoms in the placebo group fell from 6.8 at baseline to 3.4 at 12 weeks, for a difference of 1.6 fewer occurrences per day in the elinzanetant group (P < .0001).
Sleep disturbances also improved, falling 9.4 points from a baseline 57.4 in the elinzanetant group and 5.7 points from a baseline 58 in the placebo group. Quality-of-life scores improved from 4.1 to 2.8 (−1.3 change) in the elinzanetant group and from 4.4 to 3.3 (−1.1 change) in the placebo group.
In addition to looking at treatment-emergent adverse events, the safety assessments also included endometrial biopsies; bone mineral density in the femoral neck, hip, and lumbar spine; weight; and labs. Adverse events related to the study drug occurred in 30.4% of those in the elinzanetant group and 14.6% of those in the placebo group. The most commonly reported adverse events were headache (9.6% elinzanetant vs 7% placebo), fatigue (7% vs 10.2%), and sleepiness (5.1% vs 1.3%). A higher proportion of women taking elinzanetant (12.5%) than those taking placebo (4.1%) discontinued the study.
No serious adverse events deemed to be treatment-related occurred in either group, and no endometrial hyperplasia or malignant neoplasm occurred in either group. Bone mineral density changes in both groups were within the expected range for the women’s age, and their weight remained stable over the 52 weeks.
Six women taking elinzanetant and four taking placebo met predefined criteria for close liver observation, but none showed hepatotoxicity or evidence of possible drug-induced liver injury.
The research was funded by Bayer. Dr. Pinkerton has run a trial funded by Bayer and is a consultant for Bayer and Pfizer. Dr. Shufelt had no disclosures. Dr. Simon had grant/research support, consulting/advisory board participation, and/or speaking disclosures with AbbVie, Bayer Healthcare, Besins Healthcare, California Institute of Integral Studies, Camargo Pharmaceutical Services, Covance, Daré Bioscience, DEKA M.E.L.A S.r.l., Femasys, Ipsen, KaNDy/NeRRe Therapeutics, Khyria, Madorra, Mayne Pharma, Mitsubishi Tanabe Pharma Development America, Mylan/Viatris Inc, Myovant Sciences, ObsEva SA, Pfizer, Pharmavite, QUE Oncology, Scynexis, Sebela Pharmaceuticals, Sprout Pharmaceuticals, TherapeuticsMD, Vella Bioscience, and Viveve Medical, and he is a stockholder in Sermonix Pharmaceuticals.
A version of this article first appeared on Medscape.com.
CHICAGO — The nonhormonal investigational drug elinzanetant led to significant improvement in hot flashes as well as sleep disturbance and quality of life, according to data from three randomized controlled trials presented at The Menopause Society 2024 Annual Meeting in Chicago. Two phase 3 trials, OASIS 1 and 2, were also published in JAMA, and the longer-term OASIS 3 trial was presented as a poster at the conference.
Elinzanetant is a selective neurokinin (NK) receptor antagonist, similar to fezolinetant, the first drug in this class approved by the US Food and Drug Administration (FDA) for vasomotor symptoms in May 2023. This class of medications targets the estrogen-sensitive kisspeptin/NK B/dynorphin (KNDy) neurons thought to play a role in thermoregulation and hot flashes during menopause. While fezolinetant targets only the NK-3 receptor, elinzanetant is a dual NK receptor antagonist that targets both NK-1 and NK-3. Bayer submitted a New Drug Application for elinzanetant to the FDA on August 1.
For those in whom hormone therapy is contraindicated, “it’s always been difficult for women with really severe symptoms to have a safe and effective therapy,” lead author JoAnn Pinkerton, MD, a professor of ob.gyn. at the University of Virginia in Charlottesville, Virginia, told this news organization. “The nonhormonal therapies we’ve used mostly off-label — the antidepressants, gabapentin, clonidine, oxybutynin — do help the hot flashes, but they don’t work nearly as effectively as these new NK receptor antagonists, and having one that looks like it might have a broader use for hot flashes, night sweats, mood, and sleep is just really exciting.”
Dr. Pinkerton said approximately 80% of the women in the OASIS 1 and 2 studies had at least a 50% reduction in hot flashes. “It was a very strong, dramatic positive finding, but the improvements in sleep and mood have really encouraged us to go further,” she said.
Declining estrogen levels during and after menopause can cause hypertrophy and hyperactivity of the KNDy neurons, which has been linked to thermoregulation disruptions that may trigger hot flashes, James Simon, MD, a clinical professor of ob.gyn. at The George Washington University School of Medicine & Health Sciences and medical director of IntimMedicine in Washington, DC, told attendees. He presented pooled data from OASIS 1 and 2. The NK-1 receptor, targeted by elinzanetant but not fezolinetant, is also thought to play a role in insomnia and possibly in mood.
“Oftentimes the focus on a lot of these drugs is hot flashes, hot flashes, hot flashes, but we know hot flashes do not occur in isolation,” Chrisandra Shufelt, MD, professor and chair of general internal medicine and associate director of the Women’s Health Research Center at Mayo Clinic in Jacksonville, Florida, told this news organization. Elinzanetant is “an interesting compound because it actually works on sleep, and that was critical because sleep disturbance precedes” many other menopausal symptoms, said Dr. Shufelt, who was not involved in the study.
“I think it is an outstanding option for women who don’t have the opportunity to get hormones,” Dr. Shufelt said, and she was particularly pleased to see there were no safety concerns for the liver in the trial data. The FDA issued a warning on September 12 about the risk for rare liver injury with fezolinetant, but the early signals that had been seen in fezolinetant data were not seen in these elinzanetant data.
The OASIS 1 and 2 trials enrolled postmenopausal women, aged 40-65 years, who had at least 50 moderate to severe vasomotor occurrences per week.
“A moderate hot flash is a hot flash that is also associated with sweating, and a severe hot flash is a moderate hot flash that stops a woman in her tracks,” Dr. Simon said. “Namely, it’s severe enough with sweating and central nervous system effects that she is interrupted in whatever it is that she’s doing at the time.”
Exclusion criteria for the trials included a history of arrhythmias, heart block, or QT prolongation; abnormal lab results; history of malignancy within the past 5 years; uncontrolled or treatment-resistant hypertension, hypothyroidism, or hyperthyroidism; unexplained postmenopausal bleeding; clinically relevant abnormal mammogram findings; or disordered proliferative endometrium, endometrial hyperplasia, polyp, or endometrial cancer.
The predominantly White (80%) women were an average 54 years old, with an average body mass index (BMI) of 27.8, and were an average 3.5 years from their last period. For the first 12 weeks of the trials, 399 women were assigned to receive 120 mg once daily of oral elinzanetant and 397 were assigned to once daily placebo. Then the women taking placebo switched to elinzanetant for the final 14 weeks of the study.
The endpoints included mean change in frequency and severity of vasomotor symptoms at weeks 1, 4, and 12 as well as change in sleep disturbance and quality of life at week 12. Sleep was assessed with the Patient-Reported Outcomes Measurement Information System Sleep Disturbance–Short Form score, which ranges from 28.9 to 76.5, with a higher number denoting greater sleep disturbance. The Menopause-Specific Quality-of-Life score ranges from 1 to 8, with a higher score indicating poorer quality of life.
Daily frequency of vasomotor symptoms was 14 per day at baseline in the elinzanetant group, decreasing by 4.8 per day at week 1, 8 per day at week 4, and 9.4 per day at week 12. In the placebo group, women had an average 15.2 occurrences per day at baseline, which decreased by 3.2 at week 1, 5.2 at week 4, and 6.4 at week 12. Comparing the groups at 12 weeks, those receiving elinzanetant had 3.2 fewer daily vasomotor symptoms than those receiving placebo (P < .0001).
The severity of vasomotor symptoms also improved more in the elinzanetant group than in the placebo group over 12 weeks, after which severity improved further in those who switched from placebo to elinzanetant (P < .0001).
Sleep disturbance scores, starting at a mean 61.5 in the elinzanetant group and 60.5 in the placebo group, fell 10.7 points in the elinzanetant group and 5.3 points in the placebo group at 12 weeks, for a difference of 4.9 points (P < .0001). Sleep then further improved in those who switched from placebo to elinzanetant. Quality-of-life scores improved 1.37 points (from 4.52 at baseline) in the elinzanetant group and 0.96 points (from 4.49 at baseline) in the placebo group, for a mean difference at 12 weeks of 0.36 (P < .0001).
Though no head-to-head data exist comparing elinzanetant and fezolinetant, Dr. Simon told this news organization the side effects with fezolinetant “tend to be gastrointestinal, whereas the side effects for elinzanetant tend to be central nervous system,” such as drowsiness and lethargy.
The women who are the best candidates for elinzanetant, Dr. Pinkerton told this news organization, include those who have had an estrogen-sensitive cancer, such as breast or endometrial cancer, or who have fear of it, a family history, or are otherwise high risk. Other ideal candidates include those with a history of venous thromboembolism, people who have migraine with aura (due to concerns about increased risk for stroke), and those who have endometriosis or large fibroids.
“Then the last group might be women who took hormone therapy in their 50s and want to continue, but they’re trying to go off, and they have a recurrence of their hot flashes or night sweats or sleep issues,” Dr. Pinkerton said. “This might be a great group to switch over.”
OASIS 3 assessed the drug for 1 year and “supported the results of OASIS 1 and 2, demonstrating efficacy over a longer study duration and in a population with a vasomotor symptom profile representative of that seen in clinical practice,” Nick Panay, BSc, MBBS, director of the Menopause & PMS Centre at Queen Charlotte’s Hospital & Imperial College London, London, England, and his colleague reported.
Among 628 postmenopausal women aged 40-65, the predominantly White (78.5%) women were an average 54 years old, with an average BMI of 27.6, and were an average 5 years past their last period. Half received 120 mg elinzanetant and half received a placebo for 52 weeks.
At 12 weeks, the women receiving elinzanetant reported an average 1.6 moderate to severe vasomotor symptoms per day, down from 6.7 at baseline. Daily average symptoms in the placebo group fell from 6.8 at baseline to 3.4 at 12 weeks, for a difference of 1.6 fewer occurrences per day in the elinzanetant group (P < .0001).
Sleep disturbances also improved, falling 9.4 points from a baseline 57.4 in the elinzanetant group and 5.7 points from a baseline 58 in the placebo group. Quality-of-life scores improved from 4.1 to 2.8 (−1.3 change) in the elinzanetant group and from 4.4 to 3.3 (−1.1 change) in the placebo group.
In addition to looking at treatment-emergent adverse events, the safety assessments also included endometrial biopsies; bone mineral density in the femoral neck, hip, and lumbar spine; weight; and labs. Adverse events related to the study drug occurred in 30.4% of those in the elinzanetant group and 14.6% of those in the placebo group. The most commonly reported adverse events were headache (9.6% elinzanetant vs 7% placebo), fatigue (7% vs 10.2%), and sleepiness (5.1% vs 1.3%). A higher proportion of women taking elinzanetant (12.5%) than those taking placebo (4.1%) discontinued the study.
No serious adverse events deemed to be treatment-related occurred in either group, and no endometrial hyperplasia or malignant neoplasm occurred in either group. Bone mineral density changes in both groups were within the expected range for the women’s age, and their weight remained stable over the 52 weeks.
Six women taking elinzanetant and four taking placebo met predefined criteria for close liver observation, but none showed hepatotoxicity or evidence of possible drug-induced liver injury.
The research was funded by Bayer. Dr. Pinkerton has run a trial funded by Bayer and is a consultant for Bayer and Pfizer. Dr. Shufelt had no disclosures. Dr. Simon had grant/research support, consulting/advisory board participation, and/or speaking disclosures with AbbVie, Bayer Healthcare, Besins Healthcare, California Institute of Integral Studies, Camargo Pharmaceutical Services, Covance, Daré Bioscience, DEKA M.E.L.A S.r.l., Femasys, Ipsen, KaNDy/NeRRe Therapeutics, Khyria, Madorra, Mayne Pharma, Mitsubishi Tanabe Pharma Development America, Mylan/Viatris Inc, Myovant Sciences, ObsEva SA, Pfizer, Pharmavite, QUE Oncology, Scynexis, Sebela Pharmaceuticals, Sprout Pharmaceuticals, TherapeuticsMD, Vella Bioscience, and Viveve Medical, and he is a stockholder in Sermonix Pharmaceuticals.
A version of this article first appeared on Medscape.com.
CHICAGO — The nonhormonal investigational drug elinzanetant led to significant improvement in hot flashes as well as sleep disturbance and quality of life, according to data from three randomized controlled trials presented at The Menopause Society 2024 Annual Meeting in Chicago. Two phase 3 trials, OASIS 1 and 2, were also published in JAMA, and the longer-term OASIS 3 trial was presented as a poster at the conference.
Elinzanetant is a selective neurokinin (NK) receptor antagonist, similar to fezolinetant, the first drug in this class approved by the US Food and Drug Administration (FDA) for vasomotor symptoms in May 2023. This class of medications targets the estrogen-sensitive kisspeptin/NK B/dynorphin (KNDy) neurons thought to play a role in thermoregulation and hot flashes during menopause. While fezolinetant targets only the NK-3 receptor, elinzanetant is a dual NK receptor antagonist that targets both NK-1 and NK-3. Bayer submitted a New Drug Application for elinzanetant to the FDA on August 1.
For those in whom hormone therapy is contraindicated, “it’s always been difficult for women with really severe symptoms to have a safe and effective therapy,” lead author JoAnn Pinkerton, MD, a professor of ob.gyn. at the University of Virginia in Charlottesville, Virginia, told this news organization. “The nonhormonal therapies we’ve used mostly off-label — the antidepressants, gabapentin, clonidine, oxybutynin — do help the hot flashes, but they don’t work nearly as effectively as these new NK receptor antagonists, and having one that looks like it might have a broader use for hot flashes, night sweats, mood, and sleep is just really exciting.”
Dr. Pinkerton said approximately 80% of the women in the OASIS 1 and 2 studies had at least a 50% reduction in hot flashes. “It was a very strong, dramatic positive finding, but the improvements in sleep and mood have really encouraged us to go further,” she said.
Declining estrogen levels during and after menopause can cause hypertrophy and hyperactivity of the KNDy neurons, which has been linked to thermoregulation disruptions that may trigger hot flashes, James Simon, MD, a clinical professor of ob.gyn. at The George Washington University School of Medicine & Health Sciences and medical director of IntimMedicine in Washington, DC, told attendees. He presented pooled data from OASIS 1 and 2. The NK-1 receptor, targeted by elinzanetant but not fezolinetant, is also thought to play a role in insomnia and possibly in mood.
“Oftentimes the focus on a lot of these drugs is hot flashes, hot flashes, hot flashes, but we know hot flashes do not occur in isolation,” Chrisandra Shufelt, MD, professor and chair of general internal medicine and associate director of the Women’s Health Research Center at Mayo Clinic in Jacksonville, Florida, told this news organization. Elinzanetant is “an interesting compound because it actually works on sleep, and that was critical because sleep disturbance precedes” many other menopausal symptoms, said Dr. Shufelt, who was not involved in the study.
“I think it is an outstanding option for women who don’t have the opportunity to get hormones,” Dr. Shufelt said, and she was particularly pleased to see there were no safety concerns for the liver in the trial data. The FDA issued a warning on September 12 about the risk for rare liver injury with fezolinetant, but the early signals that had been seen in fezolinetant data were not seen in these elinzanetant data.
The OASIS 1 and 2 trials enrolled postmenopausal women, aged 40-65 years, who had at least 50 moderate to severe vasomotor occurrences per week.
“A moderate hot flash is a hot flash that is also associated with sweating, and a severe hot flash is a moderate hot flash that stops a woman in her tracks,” Dr. Simon said. “Namely, it’s severe enough with sweating and central nervous system effects that she is interrupted in whatever it is that she’s doing at the time.”
Exclusion criteria for the trials included a history of arrhythmias, heart block, or QT prolongation; abnormal lab results; history of malignancy within the past 5 years; uncontrolled or treatment-resistant hypertension, hypothyroidism, or hyperthyroidism; unexplained postmenopausal bleeding; clinically relevant abnormal mammogram findings; or disordered proliferative endometrium, endometrial hyperplasia, polyp, or endometrial cancer.
The predominantly White (80%) women were an average 54 years old, with an average body mass index (BMI) of 27.8, and were an average 3.5 years from their last period. For the first 12 weeks of the trials, 399 women were assigned to receive 120 mg once daily of oral elinzanetant and 397 were assigned to once daily placebo. Then the women taking placebo switched to elinzanetant for the final 14 weeks of the study.
The endpoints included mean change in frequency and severity of vasomotor symptoms at weeks 1, 4, and 12 as well as change in sleep disturbance and quality of life at week 12. Sleep was assessed with the Patient-Reported Outcomes Measurement Information System Sleep Disturbance–Short Form score, which ranges from 28.9 to 76.5, with a higher number denoting greater sleep disturbance. The Menopause-Specific Quality-of-Life score ranges from 1 to 8, with a higher score indicating poorer quality of life.
Daily frequency of vasomotor symptoms was 14 per day at baseline in the elinzanetant group, decreasing by 4.8 per day at week 1, 8 per day at week 4, and 9.4 per day at week 12. In the placebo group, women had an average 15.2 occurrences per day at baseline, which decreased by 3.2 at week 1, 5.2 at week 4, and 6.4 at week 12. Comparing the groups at 12 weeks, those receiving elinzanetant had 3.2 fewer daily vasomotor symptoms than those receiving placebo (P < .0001).
The severity of vasomotor symptoms also improved more in the elinzanetant group than in the placebo group over 12 weeks, after which severity improved further in those who switched from placebo to elinzanetant (P < .0001).
Sleep disturbance scores, starting at a mean 61.5 in the elinzanetant group and 60.5 in the placebo group, fell 10.7 points in the elinzanetant group and 5.3 points in the placebo group at 12 weeks, for a difference of 4.9 points (P < .0001). Sleep then further improved in those who switched from placebo to elinzanetant. Quality-of-life scores improved 1.37 points (from 4.52 at baseline) in the elinzanetant group and 0.96 points (from 4.49 at baseline) in the placebo group, for a mean difference at 12 weeks of 0.36 (P < .0001).
Though no head-to-head data exist comparing elinzanetant and fezolinetant, Dr. Simon told this news organization the side effects with fezolinetant “tend to be gastrointestinal, whereas the side effects for elinzanetant tend to be central nervous system,” such as drowsiness and lethargy.
The women who are the best candidates for elinzanetant, Dr. Pinkerton told this news organization, include those who have had an estrogen-sensitive cancer, such as breast or endometrial cancer, or who have fear of it, a family history, or are otherwise high risk. Other ideal candidates include those with a history of venous thromboembolism, people who have migraine with aura (due to concerns about increased risk for stroke), and those who have endometriosis or large fibroids.
“Then the last group might be women who took hormone therapy in their 50s and want to continue, but they’re trying to go off, and they have a recurrence of their hot flashes or night sweats or sleep issues,” Dr. Pinkerton said. “This might be a great group to switch over.”
OASIS 3 assessed the drug for 1 year and “supported the results of OASIS 1 and 2, demonstrating efficacy over a longer study duration and in a population with a vasomotor symptom profile representative of that seen in clinical practice,” Nick Panay, BSc, MBBS, director of the Menopause & PMS Centre at Queen Charlotte’s Hospital & Imperial College London, London, England, and his colleague reported.
Among 628 postmenopausal women aged 40-65, the predominantly White (78.5%) women were an average 54 years old, with an average BMI of 27.6, and were an average 5 years past their last period. Half received 120 mg elinzanetant and half received a placebo for 52 weeks.
At 12 weeks, the women receiving elinzanetant reported an average 1.6 moderate to severe vasomotor symptoms per day, down from 6.7 at baseline. Daily average symptoms in the placebo group fell from 6.8 at baseline to 3.4 at 12 weeks, for a difference of 1.6 fewer occurrences per day in the elinzanetant group (P < .0001).
Sleep disturbances also improved, falling 9.4 points from a baseline 57.4 in the elinzanetant group and 5.7 points from a baseline 58 in the placebo group. Quality-of-life scores improved from 4.1 to 2.8 (−1.3 change) in the elinzanetant group and from 4.4 to 3.3 (−1.1 change) in the placebo group.
In addition to looking at treatment-emergent adverse events, the safety assessments also included endometrial biopsies; bone mineral density in the femoral neck, hip, and lumbar spine; weight; and labs. Adverse events related to the study drug occurred in 30.4% of those in the elinzanetant group and 14.6% of those in the placebo group. The most commonly reported adverse events were headache (9.6% elinzanetant vs 7% placebo), fatigue (7% vs 10.2%), and sleepiness (5.1% vs 1.3%). A higher proportion of women taking elinzanetant (12.5%) than those taking placebo (4.1%) discontinued the study.
No serious adverse events deemed to be treatment-related occurred in either group, and no endometrial hyperplasia or malignant neoplasm occurred in either group. Bone mineral density changes in both groups were within the expected range for the women’s age, and their weight remained stable over the 52 weeks.
Six women taking elinzanetant and four taking placebo met predefined criteria for close liver observation, but none showed hepatotoxicity or evidence of possible drug-induced liver injury.
The research was funded by Bayer. Dr. Pinkerton has run a trial funded by Bayer and is a consultant for Bayer and Pfizer. Dr. Shufelt had no disclosures. Dr. Simon had grant/research support, consulting/advisory board participation, and/or speaking disclosures with AbbVie, Bayer Healthcare, Besins Healthcare, California Institute of Integral Studies, Camargo Pharmaceutical Services, Covance, Daré Bioscience, DEKA M.E.L.A S.r.l., Femasys, Ipsen, KaNDy/NeRRe Therapeutics, Khyria, Madorra, Mayne Pharma, Mitsubishi Tanabe Pharma Development America, Mylan/Viatris Inc, Myovant Sciences, ObsEva SA, Pfizer, Pharmavite, QUE Oncology, Scynexis, Sebela Pharmaceuticals, Sprout Pharmaceuticals, TherapeuticsMD, Vella Bioscience, and Viveve Medical, and he is a stockholder in Sermonix Pharmaceuticals.
A version of this article first appeared on Medscape.com.
FROM THE MENOPAUSE SOCIETY 2024
Most Women With Genitourinary Syndrome of Menopause Do Not Receive Effective Treatment
CHICAGO — The vast majority of women experiencing genitourinary syndrome of menopause (GSM) symptoms did not receive a prescription for hormonal vaginal therapies prior to seeking care at a specialized menopause clinic, according to research presented at the annual meeting of The Menopause Society.
“GSM symptoms are very common and affect women’s health and quality of life, often worsening without effective therapy,” Leticia Hernández Galán, PhD, of the Department of Obstetrics & Gynecology, McMaster University, Hamilton, Ontario, Canada, and colleagues reported. “We have demonstrated that most women seeking specialty care in an urban center with GSM symptoms have not been given a trial of local vaginal therapies by referring providers despite guidelines about safety and lack of contraindications. Given very long wait times for menopause providers in Canada, improved education for both women and their providers is needed to reduce needless suffering and improve care.”
Stephanie Faubion, MD, MBA, director of the Mayo Clinic Women’s Health in Jacksonville, Florida, and medical director of The Menopause Society, was not involved with the study but agreed with the authors’ assessment of the findings.
“This study highlights the treatment gap for women with genitourinary syndrome of menopause,” Dr. Faubion told this news organization. “Clearly, there is underutilization of low-dose vaginal hormonal therapies, which are known to be safe and effective. We still have work to do in terms of educating both women and providers on established treatment options for this common concern in menopausal women.”
The findings match previous ones that found a majority of women with GSM do not receive treatment. A 2017 study, which was cited in the 2020 Menopause Society position statement on the condition, found that half of women with GSM had never used any treatment.
GSM is the current term that replaces previously used “vulvovaginal atrophy” and “atrophic vaginitis” because it encompasses all the menopause symptoms and signs associated with menopause that affect the vagina, vulva, and urinary tract. Anywhere from 50% to 84% of postmenopausal women experience GSM, the authors noted, with symptoms that include “burning, itching, or irritation of the vulva” and “lack of lubrication and discomfort or pain with sexual activity as well as dysuria, increased frequency or urgency of urination, and increased risk for urinary tract infections.”
First-line treatment of mild GSM often includes nonhormonal vaginal lubricants and moisturizers, but vaginal estrogen is considered the most effective treatment for more severe or bothersome cases. Other treatments include systematic hormone therapy and ospemifene or other selective estrogen receptor modulators.
Increased Risk for Urinary Tract Infections (UTIs)
Untreated GSM is not simply a quality of life issue; it increases the risk of developing serious UTIs, explained JoAnn Pinkerton, MD, a professor of obstetrics and gynecology at the University of Virginia, Charlottesville, who was not involved in the study.
“Estrogen depletion alters the vaginal epithelium, with distinct impairments in lubrication, elasticity, pH, and blood flow,” Dr. Pinkerton said. “The vaginal microbiome changes, with increasing pH following menopause and loss of lactobacillus predominance. These alterations allow a more hospitable environment for bacterial growth and increase the risk of UTI.”
Vaginal estrogen, meanwhile, reduces UTI risk because it “increases the presence of lactobacillus in the vagina due to improvements in vaginal pH, rebuilding superficial cells, elasticity, and connectivity,” she said.
The study assessed the incidence of GSM among patients at a single specialized Canadian institution, St. Joseph’s Healthcare Menopause Clinic in Hamilton, Ontario, between January 2021 and August 2024. Patients completed a Menopause Rating Scale that quantified two sets of GSM symptoms relating to “dryness of the vagina” and “bladder problems.” Patients also answered questions about the provider they had seen before coming to the specialized clinic and whether they had been prescribed local vaginal products before their visit.
Among 529 patients, the average age was 51, and the vast majority (88%) had some amount of tertiary education beyond high school. Only 21.5% were still menstruating, whereas the other respondents had stopped menstruating. The patient population was mostly White (85.6%), with Black, Hispanic, Asian, Middle Eastern, and Indigenous patients making up most of the other patient groups.
Among the 521 patients who answered the question on vaginal dryness, answers were similarly split between none (26%), mild (23%), moderate (21%), severe (15%), and very severe (15%). One third of the 526 women (34%) who answered the question on bladder problems said they had none, whereas the remainder reported their problems as mild (24%), moderate (24%), severe (11%), or very severe (7%).
Despite about half the participants reporting moderate to very severe vaginal dryness, 85% of them had not been prescribed local vaginal hormone therapies before their visit to the menopause clinic. Women were more likely to have been prescribed a localized therapy if they were older, were postmenopausal instead of perimenopausal, or had a female healthcare provider prior to this visit.
The survey also asked about the specialty and years in practice for the providers women had seen before visiting the clinic, but neither of these were predictors for receiving a hormone prescription. The patient’s education, partner status, and ethnicity were also not associated with the likelihood of a prescription.
Among 62 women who had been prescribed a vaginal hormone treatment, most were prescribed Vagifem (29%) or Premarin Vaginal cream (26%), followed by Intrarosa (19%), Estragyn cream (16%), Estring (3%), or something else (18%).
Serious Complications of GSM
Dr. Pinkerton described how GSM, particularly in older women, can run the risk of becoming life-threatening if untreated and unrecognized.
“For some women, UTIs can lead to urosepsis, as both the vaginal tissues and bladder tissues are thin with blood vessels close to the surface,” Dr. Pinkerton said. “What may have started as a UTI, can ascend to the kidneys or get into the bloodstream, which, in some, can develop into urosepsis, which can be life-threatening. The bacterial pathogen initiates the disease process, but host immune responses drive whether sepsis develops and its severity.”
The research by Dr. Hernández Galán was funded by the Canadian Institutes of Health Research, the Canadian Menopause Society, and Pfizer. Dr. Faubion had no disclosures, and Dr. Pinkerton has run a trial funded by Bayer and is a consultant for Bayer and Pfizer.
A version of this article first appeared on Medscape.com.
CHICAGO — The vast majority of women experiencing genitourinary syndrome of menopause (GSM) symptoms did not receive a prescription for hormonal vaginal therapies prior to seeking care at a specialized menopause clinic, according to research presented at the annual meeting of The Menopause Society.
“GSM symptoms are very common and affect women’s health and quality of life, often worsening without effective therapy,” Leticia Hernández Galán, PhD, of the Department of Obstetrics & Gynecology, McMaster University, Hamilton, Ontario, Canada, and colleagues reported. “We have demonstrated that most women seeking specialty care in an urban center with GSM symptoms have not been given a trial of local vaginal therapies by referring providers despite guidelines about safety and lack of contraindications. Given very long wait times for menopause providers in Canada, improved education for both women and their providers is needed to reduce needless suffering and improve care.”
Stephanie Faubion, MD, MBA, director of the Mayo Clinic Women’s Health in Jacksonville, Florida, and medical director of The Menopause Society, was not involved with the study but agreed with the authors’ assessment of the findings.
“This study highlights the treatment gap for women with genitourinary syndrome of menopause,” Dr. Faubion told this news organization. “Clearly, there is underutilization of low-dose vaginal hormonal therapies, which are known to be safe and effective. We still have work to do in terms of educating both women and providers on established treatment options for this common concern in menopausal women.”
The findings match previous ones that found a majority of women with GSM do not receive treatment. A 2017 study, which was cited in the 2020 Menopause Society position statement on the condition, found that half of women with GSM had never used any treatment.
GSM is the current term that replaces previously used “vulvovaginal atrophy” and “atrophic vaginitis” because it encompasses all the menopause symptoms and signs associated with menopause that affect the vagina, vulva, and urinary tract. Anywhere from 50% to 84% of postmenopausal women experience GSM, the authors noted, with symptoms that include “burning, itching, or irritation of the vulva” and “lack of lubrication and discomfort or pain with sexual activity as well as dysuria, increased frequency or urgency of urination, and increased risk for urinary tract infections.”
First-line treatment of mild GSM often includes nonhormonal vaginal lubricants and moisturizers, but vaginal estrogen is considered the most effective treatment for more severe or bothersome cases. Other treatments include systematic hormone therapy and ospemifene or other selective estrogen receptor modulators.
Increased Risk for Urinary Tract Infections (UTIs)
Untreated GSM is not simply a quality of life issue; it increases the risk of developing serious UTIs, explained JoAnn Pinkerton, MD, a professor of obstetrics and gynecology at the University of Virginia, Charlottesville, who was not involved in the study.
“Estrogen depletion alters the vaginal epithelium, with distinct impairments in lubrication, elasticity, pH, and blood flow,” Dr. Pinkerton said. “The vaginal microbiome changes, with increasing pH following menopause and loss of lactobacillus predominance. These alterations allow a more hospitable environment for bacterial growth and increase the risk of UTI.”
Vaginal estrogen, meanwhile, reduces UTI risk because it “increases the presence of lactobacillus in the vagina due to improvements in vaginal pH, rebuilding superficial cells, elasticity, and connectivity,” she said.
The study assessed the incidence of GSM among patients at a single specialized Canadian institution, St. Joseph’s Healthcare Menopause Clinic in Hamilton, Ontario, between January 2021 and August 2024. Patients completed a Menopause Rating Scale that quantified two sets of GSM symptoms relating to “dryness of the vagina” and “bladder problems.” Patients also answered questions about the provider they had seen before coming to the specialized clinic and whether they had been prescribed local vaginal products before their visit.
Among 529 patients, the average age was 51, and the vast majority (88%) had some amount of tertiary education beyond high school. Only 21.5% were still menstruating, whereas the other respondents had stopped menstruating. The patient population was mostly White (85.6%), with Black, Hispanic, Asian, Middle Eastern, and Indigenous patients making up most of the other patient groups.
Among the 521 patients who answered the question on vaginal dryness, answers were similarly split between none (26%), mild (23%), moderate (21%), severe (15%), and very severe (15%). One third of the 526 women (34%) who answered the question on bladder problems said they had none, whereas the remainder reported their problems as mild (24%), moderate (24%), severe (11%), or very severe (7%).
Despite about half the participants reporting moderate to very severe vaginal dryness, 85% of them had not been prescribed local vaginal hormone therapies before their visit to the menopause clinic. Women were more likely to have been prescribed a localized therapy if they were older, were postmenopausal instead of perimenopausal, or had a female healthcare provider prior to this visit.
The survey also asked about the specialty and years in practice for the providers women had seen before visiting the clinic, but neither of these were predictors for receiving a hormone prescription. The patient’s education, partner status, and ethnicity were also not associated with the likelihood of a prescription.
Among 62 women who had been prescribed a vaginal hormone treatment, most were prescribed Vagifem (29%) or Premarin Vaginal cream (26%), followed by Intrarosa (19%), Estragyn cream (16%), Estring (3%), or something else (18%).
Serious Complications of GSM
Dr. Pinkerton described how GSM, particularly in older women, can run the risk of becoming life-threatening if untreated and unrecognized.
“For some women, UTIs can lead to urosepsis, as both the vaginal tissues and bladder tissues are thin with blood vessels close to the surface,” Dr. Pinkerton said. “What may have started as a UTI, can ascend to the kidneys or get into the bloodstream, which, in some, can develop into urosepsis, which can be life-threatening. The bacterial pathogen initiates the disease process, but host immune responses drive whether sepsis develops and its severity.”
The research by Dr. Hernández Galán was funded by the Canadian Institutes of Health Research, the Canadian Menopause Society, and Pfizer. Dr. Faubion had no disclosures, and Dr. Pinkerton has run a trial funded by Bayer and is a consultant for Bayer and Pfizer.
A version of this article first appeared on Medscape.com.
CHICAGO — The vast majority of women experiencing genitourinary syndrome of menopause (GSM) symptoms did not receive a prescription for hormonal vaginal therapies prior to seeking care at a specialized menopause clinic, according to research presented at the annual meeting of The Menopause Society.
“GSM symptoms are very common and affect women’s health and quality of life, often worsening without effective therapy,” Leticia Hernández Galán, PhD, of the Department of Obstetrics & Gynecology, McMaster University, Hamilton, Ontario, Canada, and colleagues reported. “We have demonstrated that most women seeking specialty care in an urban center with GSM symptoms have not been given a trial of local vaginal therapies by referring providers despite guidelines about safety and lack of contraindications. Given very long wait times for menopause providers in Canada, improved education for both women and their providers is needed to reduce needless suffering and improve care.”
Stephanie Faubion, MD, MBA, director of the Mayo Clinic Women’s Health in Jacksonville, Florida, and medical director of The Menopause Society, was not involved with the study but agreed with the authors’ assessment of the findings.
“This study highlights the treatment gap for women with genitourinary syndrome of menopause,” Dr. Faubion told this news organization. “Clearly, there is underutilization of low-dose vaginal hormonal therapies, which are known to be safe and effective. We still have work to do in terms of educating both women and providers on established treatment options for this common concern in menopausal women.”
The findings match previous ones that found a majority of women with GSM do not receive treatment. A 2017 study, which was cited in the 2020 Menopause Society position statement on the condition, found that half of women with GSM had never used any treatment.
GSM is the current term that replaces previously used “vulvovaginal atrophy” and “atrophic vaginitis” because it encompasses all the menopause symptoms and signs associated with menopause that affect the vagina, vulva, and urinary tract. Anywhere from 50% to 84% of postmenopausal women experience GSM, the authors noted, with symptoms that include “burning, itching, or irritation of the vulva” and “lack of lubrication and discomfort or pain with sexual activity as well as dysuria, increased frequency or urgency of urination, and increased risk for urinary tract infections.”
First-line treatment of mild GSM often includes nonhormonal vaginal lubricants and moisturizers, but vaginal estrogen is considered the most effective treatment for more severe or bothersome cases. Other treatments include systematic hormone therapy and ospemifene or other selective estrogen receptor modulators.
Increased Risk for Urinary Tract Infections (UTIs)
Untreated GSM is not simply a quality of life issue; it increases the risk of developing serious UTIs, explained JoAnn Pinkerton, MD, a professor of obstetrics and gynecology at the University of Virginia, Charlottesville, who was not involved in the study.
“Estrogen depletion alters the vaginal epithelium, with distinct impairments in lubrication, elasticity, pH, and blood flow,” Dr. Pinkerton said. “The vaginal microbiome changes, with increasing pH following menopause and loss of lactobacillus predominance. These alterations allow a more hospitable environment for bacterial growth and increase the risk of UTI.”
Vaginal estrogen, meanwhile, reduces UTI risk because it “increases the presence of lactobacillus in the vagina due to improvements in vaginal pH, rebuilding superficial cells, elasticity, and connectivity,” she said.
The study assessed the incidence of GSM among patients at a single specialized Canadian institution, St. Joseph’s Healthcare Menopause Clinic in Hamilton, Ontario, between January 2021 and August 2024. Patients completed a Menopause Rating Scale that quantified two sets of GSM symptoms relating to “dryness of the vagina” and “bladder problems.” Patients also answered questions about the provider they had seen before coming to the specialized clinic and whether they had been prescribed local vaginal products before their visit.
Among 529 patients, the average age was 51, and the vast majority (88%) had some amount of tertiary education beyond high school. Only 21.5% were still menstruating, whereas the other respondents had stopped menstruating. The patient population was mostly White (85.6%), with Black, Hispanic, Asian, Middle Eastern, and Indigenous patients making up most of the other patient groups.
Among the 521 patients who answered the question on vaginal dryness, answers were similarly split between none (26%), mild (23%), moderate (21%), severe (15%), and very severe (15%). One third of the 526 women (34%) who answered the question on bladder problems said they had none, whereas the remainder reported their problems as mild (24%), moderate (24%), severe (11%), or very severe (7%).
Despite about half the participants reporting moderate to very severe vaginal dryness, 85% of them had not been prescribed local vaginal hormone therapies before their visit to the menopause clinic. Women were more likely to have been prescribed a localized therapy if they were older, were postmenopausal instead of perimenopausal, or had a female healthcare provider prior to this visit.
The survey also asked about the specialty and years in practice for the providers women had seen before visiting the clinic, but neither of these were predictors for receiving a hormone prescription. The patient’s education, partner status, and ethnicity were also not associated with the likelihood of a prescription.
Among 62 women who had been prescribed a vaginal hormone treatment, most were prescribed Vagifem (29%) or Premarin Vaginal cream (26%), followed by Intrarosa (19%), Estragyn cream (16%), Estring (3%), or something else (18%).
Serious Complications of GSM
Dr. Pinkerton described how GSM, particularly in older women, can run the risk of becoming life-threatening if untreated and unrecognized.
“For some women, UTIs can lead to urosepsis, as both the vaginal tissues and bladder tissues are thin with blood vessels close to the surface,” Dr. Pinkerton said. “What may have started as a UTI, can ascend to the kidneys or get into the bloodstream, which, in some, can develop into urosepsis, which can be life-threatening. The bacterial pathogen initiates the disease process, but host immune responses drive whether sepsis develops and its severity.”
The research by Dr. Hernández Galán was funded by the Canadian Institutes of Health Research, the Canadian Menopause Society, and Pfizer. Dr. Faubion had no disclosures, and Dr. Pinkerton has run a trial funded by Bayer and is a consultant for Bayer and Pfizer.
A version of this article first appeared on Medscape.com.
FROM THE MENOPAUSE SOCIETY 2024
Hormone Therapy for Menopause Remains at Historic Lows Despite Effectiveness and Safety Profile
CHICAGO — before the publication of the 2002 Women’s Health Initiative (WHI) study that misguidedly cast doubt on the safety of HT. Though subsequent research has addressed the flaws of the WHI study and supports the use of HT in most menopausal women younger than 60 years, use of this therapy has never recovered, according to research presented at the annual meeting of The Menopause Society (formerly The North American Menopause Society).
“Despite evidence supporting the efficacy and safety of HT, usage rates of US Food and Drug Administration–approved HT remain low,” Stephanie Faubion, MD, MBA, director of the Mayo Clinic Women’s Health in Jacksonville, Florida, and medical director of The Menopause Society, told attendees. “Improved education of clinicians and patients is critically needed.”
Today, “there is more clarity on the risk/benefit ratio of HT use with the benefits typically outweighing the risks in women who initiate therapy under the age of 60 years and within 10 years of menopause onset.”
Using medical and pharmacy claims data from OptumLabs, Dr. Faubion and her colleagues examined utilization rates from 2007 to 2023 of transdermal vs oral estrogen and of conjugated estrogen vs estradiol in women aged 40 years or older. The data included more than 200 million people throughout the United States covered by commercial insurance or Medicare Advantage. The researchers defined annual rate of HT use as the proportion of women who had at least 180 days of a filled prescription for a systemic HT preparation with estrogen.
The study population increased from an estimated 2 million women in 2007 to 4.5 million women in 2023, and the average age of enrollees increased from 53 in 2007 to 66 in 2023. Starting at 4.6% in 2007, HT use steadily declined to a low of 1.8% in 2023 for the whole cohort of women aged 40 years or older.
Though rates remained highest in women aged 50-64 years, it still declined within each age group: From 6% in 2007 to 3.6% in 2023 among women aged 50-54 years, from 7.3% to 3.8% among women aged 55-59 years, and from 7.5% to 2.9% among women aged 60-64 years. It also declined in younger women, from 3.2% in 2007 to 1.5% in 2023 in those aged 45-50 years. Estradiol was the most common formulation used, and oral administration was the most common route.
The researchers also saw a gradual decline during the study period in the use of high-dose oral HT and an increase in the use of low-dose oral HT, whereas standard dosages remained fairly consistent as the most common dose prescribed. Similarly, the use of high transdermal doses declined, whereas low transdermal doses increased and surpassed the use of standard doses. Conjugated estrogen use plummeted during the study period across all age groups, from 2%-5% in most age groups to < 1% in all age groups by 2023.
One limitation of the study was that it could not examine rates of compounded HT use because those would not be reflected in insurance claims, pointed out JoAnn Pinkerton, MD, a professor of ob.gyn. at the University of Virginia in Charlottesville, Virginia, who was not involved in the study. Dr. Pinkerton found it surprising that the numbers were so low, despite the fact that research estimates suggest less than 15% of menopausal women are receiving adequate treatment, she told this news organization. “You can see there’s a large unmet need to get treatment,” she said. “All major medical societies say the same thing: For healthy, symptomatic menopausal women, you can use hormone therapy safely and effectively.”
The lack of education among providers is likely the biggest reason for the decline, Dr. Pinkerton says. “I think it’s because there’s a whole group of providers that did not receive any training, and that’s OB/GYNs, internal medicine, family practice, endocrinologists,” she said. “Now that people are starting to feel more confident that we can use it safely, we’re trying to get that training out to people about vasomotor symptoms, about hormone therapy, and now about new nonhormone therapies.”
Dr. Pinkerton noted that The Menopause Society has begun a new teaching program, Menopause Step-by-Step, aimed at providing short articles on the basics of menopause, HT, non-HT, and vaginal issues.
A separate poster presented at the conference provides insight into another potential factor contributing to low HT rates. A survey of 1050 American and Canadian women found that 90% discussed their symptoms with their healthcare providers, yet only 25% said their doctor identified the symptoms as likely due to perimenopause or menopause on their first visit — and only 10% of respondents said their doctor was the one to bring up perimenopause/menopause.
The respondents comprised a convenience sample of those who saw the survey on social media, in an email, or on the website of Morphus, a Toronto-based company aimed at providing support, information, and products related to menopause. Though the survey is ongoing, the analyzed responses are from March to May 2024.
Though 40% of the women said their provider attributed their symptoms to perimenopause or menopause on the second or third visit, 18% saw a provider four to five times, and 17% saw a provider more than five times before the provider considered menopause as a cause. About a third of the women (35%) brought it up to their doctor themselves and found their provider receptive, but 40% said the response was dismissive when they brought it up, and 15% said the topic was never broached at all.
Andrea Donsky, RHN, founder of Morphus who conducted the study, found these numbers surprising because she would have hoped that more doctors would have brought up perimenopause/menopause sooner. “We still have a lot of work to do to help educate women and healthcare providers,” Ms. Donsky told this news organization. “A lot of women spend years not knowing they’re in this phase of life, so they visit their doctors/HCPs [healthcare providers] many times because the connection isn’t made on the first visit.”
Danielle Meitiv, MS, a study co-author and health coach based in Silver Spring, Maryland, added, “Everyone wonders why we end up with Dr. Google; that’s the only doctor who’s talking to us about menopause.”
Dr. Pinkerton was less surprised by these survey findings. “As a menopause specialist, my most common new patient is a perimenopausal woman who feels like she hasn’t been listened to,” whether it’s her primary care doctor, her ob.gyn., or another clinician. “If the provider doesn’t ask or if the women doesn’t tell, then you don’t have the conversation,” Dr. Pinkerton said. “So many women in perimenopause are busy with work, families, partnerships, aging parents — all of the issues that they’re dealing with — that when they start to have sleep issues or mood issues or easy crying, they relate it to their life stressors, instead of recognizing that it’s fluctuating hormones.”
When Ms. Donsky examined the 1223 responses they had received through August 2024, the most common treatments advised for symptoms were antidepressants and HT, both recommended by 38% of providers. Other common recommendations were to “lose weight,” “eat less and exercise more,” supplements, or birth control pills.
Dr. Faubion had no disclosures, and her study used no external funding. Dr. Pinkerton has run a trial funded by Bayer and is a consultant for Bayer and Pfizer. Ms. Donsky is the owner of Morphus. Ms. Meitiv had no disclosures. The poster on women’s experiences with providers was funded by Morphus Inc.
A version of this article first appeared on Medscape.com.
CHICAGO — before the publication of the 2002 Women’s Health Initiative (WHI) study that misguidedly cast doubt on the safety of HT. Though subsequent research has addressed the flaws of the WHI study and supports the use of HT in most menopausal women younger than 60 years, use of this therapy has never recovered, according to research presented at the annual meeting of The Menopause Society (formerly The North American Menopause Society).
“Despite evidence supporting the efficacy and safety of HT, usage rates of US Food and Drug Administration–approved HT remain low,” Stephanie Faubion, MD, MBA, director of the Mayo Clinic Women’s Health in Jacksonville, Florida, and medical director of The Menopause Society, told attendees. “Improved education of clinicians and patients is critically needed.”
Today, “there is more clarity on the risk/benefit ratio of HT use with the benefits typically outweighing the risks in women who initiate therapy under the age of 60 years and within 10 years of menopause onset.”
Using medical and pharmacy claims data from OptumLabs, Dr. Faubion and her colleagues examined utilization rates from 2007 to 2023 of transdermal vs oral estrogen and of conjugated estrogen vs estradiol in women aged 40 years or older. The data included more than 200 million people throughout the United States covered by commercial insurance or Medicare Advantage. The researchers defined annual rate of HT use as the proportion of women who had at least 180 days of a filled prescription for a systemic HT preparation with estrogen.
The study population increased from an estimated 2 million women in 2007 to 4.5 million women in 2023, and the average age of enrollees increased from 53 in 2007 to 66 in 2023. Starting at 4.6% in 2007, HT use steadily declined to a low of 1.8% in 2023 for the whole cohort of women aged 40 years or older.
Though rates remained highest in women aged 50-64 years, it still declined within each age group: From 6% in 2007 to 3.6% in 2023 among women aged 50-54 years, from 7.3% to 3.8% among women aged 55-59 years, and from 7.5% to 2.9% among women aged 60-64 years. It also declined in younger women, from 3.2% in 2007 to 1.5% in 2023 in those aged 45-50 years. Estradiol was the most common formulation used, and oral administration was the most common route.
The researchers also saw a gradual decline during the study period in the use of high-dose oral HT and an increase in the use of low-dose oral HT, whereas standard dosages remained fairly consistent as the most common dose prescribed. Similarly, the use of high transdermal doses declined, whereas low transdermal doses increased and surpassed the use of standard doses. Conjugated estrogen use plummeted during the study period across all age groups, from 2%-5% in most age groups to < 1% in all age groups by 2023.
One limitation of the study was that it could not examine rates of compounded HT use because those would not be reflected in insurance claims, pointed out JoAnn Pinkerton, MD, a professor of ob.gyn. at the University of Virginia in Charlottesville, Virginia, who was not involved in the study. Dr. Pinkerton found it surprising that the numbers were so low, despite the fact that research estimates suggest less than 15% of menopausal women are receiving adequate treatment, she told this news organization. “You can see there’s a large unmet need to get treatment,” she said. “All major medical societies say the same thing: For healthy, symptomatic menopausal women, you can use hormone therapy safely and effectively.”
The lack of education among providers is likely the biggest reason for the decline, Dr. Pinkerton says. “I think it’s because there’s a whole group of providers that did not receive any training, and that’s OB/GYNs, internal medicine, family practice, endocrinologists,” she said. “Now that people are starting to feel more confident that we can use it safely, we’re trying to get that training out to people about vasomotor symptoms, about hormone therapy, and now about new nonhormone therapies.”
Dr. Pinkerton noted that The Menopause Society has begun a new teaching program, Menopause Step-by-Step, aimed at providing short articles on the basics of menopause, HT, non-HT, and vaginal issues.
A separate poster presented at the conference provides insight into another potential factor contributing to low HT rates. A survey of 1050 American and Canadian women found that 90% discussed their symptoms with their healthcare providers, yet only 25% said their doctor identified the symptoms as likely due to perimenopause or menopause on their first visit — and only 10% of respondents said their doctor was the one to bring up perimenopause/menopause.
The respondents comprised a convenience sample of those who saw the survey on social media, in an email, or on the website of Morphus, a Toronto-based company aimed at providing support, information, and products related to menopause. Though the survey is ongoing, the analyzed responses are from March to May 2024.
Though 40% of the women said their provider attributed their symptoms to perimenopause or menopause on the second or third visit, 18% saw a provider four to five times, and 17% saw a provider more than five times before the provider considered menopause as a cause. About a third of the women (35%) brought it up to their doctor themselves and found their provider receptive, but 40% said the response was dismissive when they brought it up, and 15% said the topic was never broached at all.
Andrea Donsky, RHN, founder of Morphus who conducted the study, found these numbers surprising because she would have hoped that more doctors would have brought up perimenopause/menopause sooner. “We still have a lot of work to do to help educate women and healthcare providers,” Ms. Donsky told this news organization. “A lot of women spend years not knowing they’re in this phase of life, so they visit their doctors/HCPs [healthcare providers] many times because the connection isn’t made on the first visit.”
Danielle Meitiv, MS, a study co-author and health coach based in Silver Spring, Maryland, added, “Everyone wonders why we end up with Dr. Google; that’s the only doctor who’s talking to us about menopause.”
Dr. Pinkerton was less surprised by these survey findings. “As a menopause specialist, my most common new patient is a perimenopausal woman who feels like she hasn’t been listened to,” whether it’s her primary care doctor, her ob.gyn., or another clinician. “If the provider doesn’t ask or if the women doesn’t tell, then you don’t have the conversation,” Dr. Pinkerton said. “So many women in perimenopause are busy with work, families, partnerships, aging parents — all of the issues that they’re dealing with — that when they start to have sleep issues or mood issues or easy crying, they relate it to their life stressors, instead of recognizing that it’s fluctuating hormones.”
When Ms. Donsky examined the 1223 responses they had received through August 2024, the most common treatments advised for symptoms were antidepressants and HT, both recommended by 38% of providers. Other common recommendations were to “lose weight,” “eat less and exercise more,” supplements, or birth control pills.
Dr. Faubion had no disclosures, and her study used no external funding. Dr. Pinkerton has run a trial funded by Bayer and is a consultant for Bayer and Pfizer. Ms. Donsky is the owner of Morphus. Ms. Meitiv had no disclosures. The poster on women’s experiences with providers was funded by Morphus Inc.
A version of this article first appeared on Medscape.com.
CHICAGO — before the publication of the 2002 Women’s Health Initiative (WHI) study that misguidedly cast doubt on the safety of HT. Though subsequent research has addressed the flaws of the WHI study and supports the use of HT in most menopausal women younger than 60 years, use of this therapy has never recovered, according to research presented at the annual meeting of The Menopause Society (formerly The North American Menopause Society).
“Despite evidence supporting the efficacy and safety of HT, usage rates of US Food and Drug Administration–approved HT remain low,” Stephanie Faubion, MD, MBA, director of the Mayo Clinic Women’s Health in Jacksonville, Florida, and medical director of The Menopause Society, told attendees. “Improved education of clinicians and patients is critically needed.”
Today, “there is more clarity on the risk/benefit ratio of HT use with the benefits typically outweighing the risks in women who initiate therapy under the age of 60 years and within 10 years of menopause onset.”
Using medical and pharmacy claims data from OptumLabs, Dr. Faubion and her colleagues examined utilization rates from 2007 to 2023 of transdermal vs oral estrogen and of conjugated estrogen vs estradiol in women aged 40 years or older. The data included more than 200 million people throughout the United States covered by commercial insurance or Medicare Advantage. The researchers defined annual rate of HT use as the proportion of women who had at least 180 days of a filled prescription for a systemic HT preparation with estrogen.
The study population increased from an estimated 2 million women in 2007 to 4.5 million women in 2023, and the average age of enrollees increased from 53 in 2007 to 66 in 2023. Starting at 4.6% in 2007, HT use steadily declined to a low of 1.8% in 2023 for the whole cohort of women aged 40 years or older.
Though rates remained highest in women aged 50-64 years, it still declined within each age group: From 6% in 2007 to 3.6% in 2023 among women aged 50-54 years, from 7.3% to 3.8% among women aged 55-59 years, and from 7.5% to 2.9% among women aged 60-64 years. It also declined in younger women, from 3.2% in 2007 to 1.5% in 2023 in those aged 45-50 years. Estradiol was the most common formulation used, and oral administration was the most common route.
The researchers also saw a gradual decline during the study period in the use of high-dose oral HT and an increase in the use of low-dose oral HT, whereas standard dosages remained fairly consistent as the most common dose prescribed. Similarly, the use of high transdermal doses declined, whereas low transdermal doses increased and surpassed the use of standard doses. Conjugated estrogen use plummeted during the study period across all age groups, from 2%-5% in most age groups to < 1% in all age groups by 2023.
One limitation of the study was that it could not examine rates of compounded HT use because those would not be reflected in insurance claims, pointed out JoAnn Pinkerton, MD, a professor of ob.gyn. at the University of Virginia in Charlottesville, Virginia, who was not involved in the study. Dr. Pinkerton found it surprising that the numbers were so low, despite the fact that research estimates suggest less than 15% of menopausal women are receiving adequate treatment, she told this news organization. “You can see there’s a large unmet need to get treatment,” she said. “All major medical societies say the same thing: For healthy, symptomatic menopausal women, you can use hormone therapy safely and effectively.”
The lack of education among providers is likely the biggest reason for the decline, Dr. Pinkerton says. “I think it’s because there’s a whole group of providers that did not receive any training, and that’s OB/GYNs, internal medicine, family practice, endocrinologists,” she said. “Now that people are starting to feel more confident that we can use it safely, we’re trying to get that training out to people about vasomotor symptoms, about hormone therapy, and now about new nonhormone therapies.”
Dr. Pinkerton noted that The Menopause Society has begun a new teaching program, Menopause Step-by-Step, aimed at providing short articles on the basics of menopause, HT, non-HT, and vaginal issues.
A separate poster presented at the conference provides insight into another potential factor contributing to low HT rates. A survey of 1050 American and Canadian women found that 90% discussed their symptoms with their healthcare providers, yet only 25% said their doctor identified the symptoms as likely due to perimenopause or menopause on their first visit — and only 10% of respondents said their doctor was the one to bring up perimenopause/menopause.
The respondents comprised a convenience sample of those who saw the survey on social media, in an email, or on the website of Morphus, a Toronto-based company aimed at providing support, information, and products related to menopause. Though the survey is ongoing, the analyzed responses are from March to May 2024.
Though 40% of the women said their provider attributed their symptoms to perimenopause or menopause on the second or third visit, 18% saw a provider four to five times, and 17% saw a provider more than five times before the provider considered menopause as a cause. About a third of the women (35%) brought it up to their doctor themselves and found their provider receptive, but 40% said the response was dismissive when they brought it up, and 15% said the topic was never broached at all.
Andrea Donsky, RHN, founder of Morphus who conducted the study, found these numbers surprising because she would have hoped that more doctors would have brought up perimenopause/menopause sooner. “We still have a lot of work to do to help educate women and healthcare providers,” Ms. Donsky told this news organization. “A lot of women spend years not knowing they’re in this phase of life, so they visit their doctors/HCPs [healthcare providers] many times because the connection isn’t made on the first visit.”
Danielle Meitiv, MS, a study co-author and health coach based in Silver Spring, Maryland, added, “Everyone wonders why we end up with Dr. Google; that’s the only doctor who’s talking to us about menopause.”
Dr. Pinkerton was less surprised by these survey findings. “As a menopause specialist, my most common new patient is a perimenopausal woman who feels like she hasn’t been listened to,” whether it’s her primary care doctor, her ob.gyn., or another clinician. “If the provider doesn’t ask or if the women doesn’t tell, then you don’t have the conversation,” Dr. Pinkerton said. “So many women in perimenopause are busy with work, families, partnerships, aging parents — all of the issues that they’re dealing with — that when they start to have sleep issues or mood issues or easy crying, they relate it to their life stressors, instead of recognizing that it’s fluctuating hormones.”
When Ms. Donsky examined the 1223 responses they had received through August 2024, the most common treatments advised for symptoms were antidepressants and HT, both recommended by 38% of providers. Other common recommendations were to “lose weight,” “eat less and exercise more,” supplements, or birth control pills.
Dr. Faubion had no disclosures, and her study used no external funding. Dr. Pinkerton has run a trial funded by Bayer and is a consultant for Bayer and Pfizer. Ms. Donsky is the owner of Morphus. Ms. Meitiv had no disclosures. The poster on women’s experiences with providers was funded by Morphus Inc.
A version of this article first appeared on Medscape.com.
FROM THE MENOPAUSE SOCIETY 2024
Hot Flashes: Do They Predict CVD and Dementia?
This transcript has been edited for clarity.
I’d like to talk about a recent report in the journal Menopause linking menopausal symptoms to increased risk for cognitive impairment. I’d also like to discuss some of the recent studies that have addressed whether hot flashes are linked to increased risk for heart disease and other forms of cardiovascular disease (CVD).
Given that 75%-80% of perimenopausal and postmenopausal women have hot flashes and vasomotor symptoms, it’s undoubtedly a more complex relationship between hot flashes and these outcomes than a simple one-size-fits-all, yes-or-no question.
Increasing evidence shows that several additional factors are important, including the age at which the symptoms are occurring, the time since menopause, the severity of the symptoms, whether they co-occur with night sweats and sleep disruption, and the cardiovascular status of the woman.
Several studies suggest that women who have more severe hot flashes and vasomotor symptoms are more likely to have prevalent cardiovascular risk factors — hypertension, dyslipidemia, high body mass index, endothelial dysfunction — as measured by flow-mediated vasodilation and other measures.
It is quite plausible that hot flashes could be a marker for increased risk for cognitive impairment. But the question remains, are hot flashes associated with cognitive impairment independent of these other risk factors? It appears that the associations between hot flashes, vasomotor symptoms, and CVD, and other adverse outcomes, may be more likely when hot flashes persist after age 60 or are newly occurring in later menopause. In the Women’s Health Initiative observational study, the presence of hot flashes and vasomotor symptoms in early menopause was not linked to any increased risk for heart attack, stroke, total CVD, or all-cause mortality.
However, the onset of these symptoms, especially new onset of these symptoms after age 60 or in later menopause, was in fact linked to increased risk for CVD and all-cause mortality. With respect to cognitive impairment, if a woman is having hot flashes and night sweats with regular sleep disruption, performance on cognitive testing would not be as favorable as it would be in the absence of these symptoms.
This brings us to the new study in Menopause that included approximately 1300 Latino women in nine Latin American countries, with an average age of 55 years. Looking at the association between severe menopausal symptoms and cognitive impairment, researchers found that women with severe symptoms were more likely to have cognitive impairment.
Conversely, they found that the women who had a favorable CVD risk factor status (physically active, lower BMI, healthier) and were ever users of estrogen were less likely to have cognitive impairment.
Clearly, for estrogen therapy, we need randomized clinical trials of the presence or absence of vasomotor symptoms and cognitive and CVD outcomes. Such analyses are ongoing, and new randomized trials focused specifically on women in early menopause would be very beneficial.
At the present time, it’s important that we not alarm women about the associations seen in some of these studies because often they are not independent associations; they aren’t independent of other risk factors that are commonly linked to hot flashes and night sweats. There are many other complexities in the relationship between hot flashes and cognitive impairment.
We need to appreciate that women who have moderate to severe hot flashes (especially when associated with disrupted sleep) do have impaired quality of life. It’s important to treat these symptoms, especially in early menopause, and very effective hormonal and nonhormonal treatments are available.
For women with symptoms that persist into later menopause or who have new onset of symptoms in later menopause, it’s important to prioritize cardiovascular health. For example, be more vigilant about behavioral lifestyle counseling to lower risk, and be even more aggressive in treating dyslipidemia and diabetes.
JoAnn E. Manson, Professor of Medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School; Chief, Division of Preventive Medicine, Brigham and Women’s Hospital, Boston, Massachusetts; and Past President, North American Menopause Society, 2011-2012, has disclosed the following relevant financial relationships: Received study pill donation and infrastructure support from Mars Symbioscience (for the COSMOS trial).
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’d like to talk about a recent report in the journal Menopause linking menopausal symptoms to increased risk for cognitive impairment. I’d also like to discuss some of the recent studies that have addressed whether hot flashes are linked to increased risk for heart disease and other forms of cardiovascular disease (CVD).
Given that 75%-80% of perimenopausal and postmenopausal women have hot flashes and vasomotor symptoms, it’s undoubtedly a more complex relationship between hot flashes and these outcomes than a simple one-size-fits-all, yes-or-no question.
Increasing evidence shows that several additional factors are important, including the age at which the symptoms are occurring, the time since menopause, the severity of the symptoms, whether they co-occur with night sweats and sleep disruption, and the cardiovascular status of the woman.
Several studies suggest that women who have more severe hot flashes and vasomotor symptoms are more likely to have prevalent cardiovascular risk factors — hypertension, dyslipidemia, high body mass index, endothelial dysfunction — as measured by flow-mediated vasodilation and other measures.
It is quite plausible that hot flashes could be a marker for increased risk for cognitive impairment. But the question remains, are hot flashes associated with cognitive impairment independent of these other risk factors? It appears that the associations between hot flashes, vasomotor symptoms, and CVD, and other adverse outcomes, may be more likely when hot flashes persist after age 60 or are newly occurring in later menopause. In the Women’s Health Initiative observational study, the presence of hot flashes and vasomotor symptoms in early menopause was not linked to any increased risk for heart attack, stroke, total CVD, or all-cause mortality.
However, the onset of these symptoms, especially new onset of these symptoms after age 60 or in later menopause, was in fact linked to increased risk for CVD and all-cause mortality. With respect to cognitive impairment, if a woman is having hot flashes and night sweats with regular sleep disruption, performance on cognitive testing would not be as favorable as it would be in the absence of these symptoms.
This brings us to the new study in Menopause that included approximately 1300 Latino women in nine Latin American countries, with an average age of 55 years. Looking at the association between severe menopausal symptoms and cognitive impairment, researchers found that women with severe symptoms were more likely to have cognitive impairment.
Conversely, they found that the women who had a favorable CVD risk factor status (physically active, lower BMI, healthier) and were ever users of estrogen were less likely to have cognitive impairment.
Clearly, for estrogen therapy, we need randomized clinical trials of the presence or absence of vasomotor symptoms and cognitive and CVD outcomes. Such analyses are ongoing, and new randomized trials focused specifically on women in early menopause would be very beneficial.
At the present time, it’s important that we not alarm women about the associations seen in some of these studies because often they are not independent associations; they aren’t independent of other risk factors that are commonly linked to hot flashes and night sweats. There are many other complexities in the relationship between hot flashes and cognitive impairment.
We need to appreciate that women who have moderate to severe hot flashes (especially when associated with disrupted sleep) do have impaired quality of life. It’s important to treat these symptoms, especially in early menopause, and very effective hormonal and nonhormonal treatments are available.
For women with symptoms that persist into later menopause or who have new onset of symptoms in later menopause, it’s important to prioritize cardiovascular health. For example, be more vigilant about behavioral lifestyle counseling to lower risk, and be even more aggressive in treating dyslipidemia and diabetes.
JoAnn E. Manson, Professor of Medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School; Chief, Division of Preventive Medicine, Brigham and Women’s Hospital, Boston, Massachusetts; and Past President, North American Menopause Society, 2011-2012, has disclosed the following relevant financial relationships: Received study pill donation and infrastructure support from Mars Symbioscience (for the COSMOS trial).
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’d like to talk about a recent report in the journal Menopause linking menopausal symptoms to increased risk for cognitive impairment. I’d also like to discuss some of the recent studies that have addressed whether hot flashes are linked to increased risk for heart disease and other forms of cardiovascular disease (CVD).
Given that 75%-80% of perimenopausal and postmenopausal women have hot flashes and vasomotor symptoms, it’s undoubtedly a more complex relationship between hot flashes and these outcomes than a simple one-size-fits-all, yes-or-no question.
Increasing evidence shows that several additional factors are important, including the age at which the symptoms are occurring, the time since menopause, the severity of the symptoms, whether they co-occur with night sweats and sleep disruption, and the cardiovascular status of the woman.
Several studies suggest that women who have more severe hot flashes and vasomotor symptoms are more likely to have prevalent cardiovascular risk factors — hypertension, dyslipidemia, high body mass index, endothelial dysfunction — as measured by flow-mediated vasodilation and other measures.
It is quite plausible that hot flashes could be a marker for increased risk for cognitive impairment. But the question remains, are hot flashes associated with cognitive impairment independent of these other risk factors? It appears that the associations between hot flashes, vasomotor symptoms, and CVD, and other adverse outcomes, may be more likely when hot flashes persist after age 60 or are newly occurring in later menopause. In the Women’s Health Initiative observational study, the presence of hot flashes and vasomotor symptoms in early menopause was not linked to any increased risk for heart attack, stroke, total CVD, or all-cause mortality.
However, the onset of these symptoms, especially new onset of these symptoms after age 60 or in later menopause, was in fact linked to increased risk for CVD and all-cause mortality. With respect to cognitive impairment, if a woman is having hot flashes and night sweats with regular sleep disruption, performance on cognitive testing would not be as favorable as it would be in the absence of these symptoms.
This brings us to the new study in Menopause that included approximately 1300 Latino women in nine Latin American countries, with an average age of 55 years. Looking at the association between severe menopausal symptoms and cognitive impairment, researchers found that women with severe symptoms were more likely to have cognitive impairment.
Conversely, they found that the women who had a favorable CVD risk factor status (physically active, lower BMI, healthier) and were ever users of estrogen were less likely to have cognitive impairment.
Clearly, for estrogen therapy, we need randomized clinical trials of the presence or absence of vasomotor symptoms and cognitive and CVD outcomes. Such analyses are ongoing, and new randomized trials focused specifically on women in early menopause would be very beneficial.
At the present time, it’s important that we not alarm women about the associations seen in some of these studies because often they are not independent associations; they aren’t independent of other risk factors that are commonly linked to hot flashes and night sweats. There are many other complexities in the relationship between hot flashes and cognitive impairment.
We need to appreciate that women who have moderate to severe hot flashes (especially when associated with disrupted sleep) do have impaired quality of life. It’s important to treat these symptoms, especially in early menopause, and very effective hormonal and nonhormonal treatments are available.
For women with symptoms that persist into later menopause or who have new onset of symptoms in later menopause, it’s important to prioritize cardiovascular health. For example, be more vigilant about behavioral lifestyle counseling to lower risk, and be even more aggressive in treating dyslipidemia and diabetes.
JoAnn E. Manson, Professor of Medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School; Chief, Division of Preventive Medicine, Brigham and Women’s Hospital, Boston, Massachusetts; and Past President, North American Menopause Society, 2011-2012, has disclosed the following relevant financial relationships: Received study pill donation and infrastructure support from Mars Symbioscience (for the COSMOS trial).
A version of this article first appeared on Medscape.com.