User login
An Interdisciplinary Clinic for Former Prisoners of War
Since the beginning of the American Republic, servicemen have been captured and held as prisoners of war (POWs), including > 130,000 in World War II , > 7,100 in the Korean War, > 700 in the Vietnam War, and 37 in Operation Desert Storm and recent conflicts.1,2 Also, > 80 servicewomen have been held during these conflicts.1-3 Of those living former POWs (FPOWs), almost all are geriatric (aged > 65 years) with a significant portion aged ≥ 85 years.
The physical hardships and psychological stress endured by FPOWs have lifelong deleterious sequelae on health and social functioning.3-5 The experiences of FPOWs are associated with higher prevalence of chronic diseases and diminished functional performance in later life as demonstrated by a survey of FPOWs from World War II.4 The survey assessed health and functional status in a random sample of 101 FPOWs and a group of 107 non-POW combatants from the same military operations. FPOWs reported a higher mean number of somatic symptoms than did non-POWs (7.2 vs 5.4, respectively; P = .002), a higher mean number of diagnosed health conditions (9.4 vs 7.7, respectively; P < .001), and used a greater mean number of medications (4.5 vs 3.4, respectively; P = .001). Among 15 broad categories of diagnoses, differences were found in gastrointestinal disorders (FPOWs 63% vs non-POWs 49%, P = .032), musculoskeletal disorders (FPOWs 76% vs non-POWs 60%, P = .001), and cognitive disorders (FPOWs 31% vs non-POWs 15%, P = .006). FPOWs had a significantly higher proportion of 7 extrapyramidal signs and 6 signs relating to ataxia. On the Instrumental Activities of Daily Living scale, FPOWs were more likely to be impaired than were non-POWs (33% vs 17%, respectively; P = .01). In addition, FPOWs have an increased risk of developing dementia, and this risk is doubled in FPOWs with posttraumatic stress disorder (PTSD) compared with non-FPOWs without PTSD.5
These data indicate that FPOW status is associated with increased risk of disability and loss of independence. Federal statutes established the presumption of a relationship between FPOW status and many comorbidities for VA disability determinations in recognition of such data and to overcome lack of medical records during POW confinement and to accord benefit of the doubt where medical science cannot conclusively link disease etiology to FPOW status, to FPOWs.
Service-Connected Conditions
The historical development of conditions with a presumption of service connection for adjudication of VA compensation/disability claims began in 1921 with the Act to Establish a Veterans’ Bureau and to Improve the Facilities.1 The act simplified and streamlined the claims adjudication process by eliminating the need to obtain evidence on the part of the veteran. The presumption of service connection also facilitated increased accuracy and consistency in adjudications by requiring similar treatment for similar claims. This “presumptive” process relieved claimants and VA of the necessity of producing direct evidence when it was impractical to do so.
In 1970, the first presumptives specific to FPOWs were legislatively established and covered 17 diseases for a FPOW who had been confined for ≥ 30 days (Pub. L. 91-376). The 30-day confinement requirement was later relaxed, and additional presumptives were established that related to diseases that were more common among FPOWs than they were among non-FPOWs. These disorders included traumatic arthritis, stroke, heart disease, osteoporosis, peripheral neuropathy, cold injuries, as well as a variety of digestive and neuropsychiatric disorders. If a FPOW is diagnosed as having ≥ 1 of these conditions and it is judged to be ≥ 10% disabling, the condition is presumed to be a sequelae of the POW experience, and it is classified as a service-connected disability (Table).
FPOW Care And Benefits Teams
Several Veterans Health Administration (VHA) directives have been issued, including the recent VHA directive 1650, which requires that each VHA medical facility have a special Care and Benefits Team (CBT) that is charged with the evaluation and treatment of FPOWs to ensure that “FPOWs receive the highest quality care and benefit services.”6 CBTs must be composed of a clinician trained in internal medicine or family practice; a clinician who is certified through the VA Office of Disability and Medical Assessment to conduct General Medical Compensation and Pension evaluations; a FPOW advocate who typically is a VHA clinical social worker; and a Veterans Benefits Administration (VBA) FPOW coordinator appointed by the local VBA regional office. CBTs can be expanded to include other members as needed. The CBTs are tasked with facilitating interactions between FPOWs, the VHA, and the VBA.
CBTs face several challenges in meeting their responsibilities. For example, the POW experience often results in psychological trauma that foments denial and distrust; hence, thoughtful sensitivity to the sequelae of captivity when approaching FPOWs about personal issues, such as health care, is required. Establishing trusting relationships with FPOWs is necessary if their needs are to be effectively addressed.
While the VHA is mandated to provide priority treatment for FPOWs, including hospital, nursing home, dental, and outpatient treatment, a significant number of FPOWs do not avail themselves of benefits to which they are entitled. Often these FPOWs have not used VA programs and facilities because they are uninformed or confused about VA benefits for FPOWs. As a result, referrals of eligible FPOWs to appropriate programs can be overlooked. Maximizing the service-connected disability rating of FPOWs not only impacts the disability pensions received by these veterans, but also impacts their eligibility for VHA programs, including long-term care and Dependency and Indemnity Compensation, a monthly benefit paid to spouses, children, and/or surviving parents.
In 2013, the FPOW Committee of the South Texas Veterans Health Care System (STVHCS) noted that 40% of FPOWs in our region had no VA primary care or clinic assignment. In consideration of the commitment of the VA to care for FPOWs, the unique POW-related medical and psychological issues, the geriatric age of many FPOWs, and the surprising number of FPOWs currently not receiving VA care, we expanded the concept of the CBT team to create a specialized interdisciplinary FPOW Clinic to address the unique needs of this predominantly elderly population and to involve more FPOWs in the VA system.
The main purpose of this clinic was to advise FPOWs of all VA benefits and services to which they may be entitled by identifying overlooked FPOW presumptives. As the number of FPOWs continues to decrease, outreach to FPOWs and family members has become critical, especially as increased benefits and special services might be available to this increasingly dependent older population. An informal survey of FPOW advocates across the nation found that 21% of FPOWs had disability ratings from the VA of ≤ 60%, including some who had no VA disability rating at all. Thus, an additional goal of the project was to develop a clinic model that could be disseminated throughout the VHA.
Design
The design of the FPOW Clinic team is based on an interdisciplinary model that has proven successful in geriatric medicine.7 The team comprises a physician, a social worker, and a registered nurse.8 All members have expertise in geriatric medicine and specific training in FPOW-related issues by completing a VA employee education training session on FPOW case management. Completion of this training ensured that team members were:
- Familiar with the experiences of FPOWs as well as about the medical, psychosocial, and mental health conditions that affect FPOWs;
- Knowledgeable about FPOW presumptive conditions;
- Familiar with the VBA process for rating FPOW disability claims; and
- Capable of FPOW case coordination, workflow, and communications between the FPOW Clinic team and the VBA to avail FPOWs and their families of all eligible benefits.
In-person FPOW clinic visits and chart reviews helped identify overlooked FPOW benefits. To facilitate case management, a representative of the VBA attended the initial evaluation of each FPOW in the clinic to confirm any overlooked presumptive benefits and to familiarize FPOWs with the claims process. FPOWs were also given the choice to officially enroll in the FPOW clinic for primary care or to remain with their current health care provider. Special efforts were made to enroll those FPOWs who had no STVHCS assigned primary care clinic.
The clinic was scheduled for 4 hours every week. Initial patient visits were 2 hours each and consisted of separate evaluations by each of the 3 FPOW Clinic team members who then met as a team with the addition of the VBA representative. The purpose of this meeting was to discuss overlooked benefits, address any other specific issues noted, and to devise an appropriate interdisciplinary plan. Findings of overlooked benefits and other relevant outcomes then were conveyed to the FPOW. For FPOWs who opted to continue in the clinic for their primary care, subsequent appointments were 1 hour.
Implementation
STVHCS FPOW advocates identified and sent letters to FPOWs announcing the opening of the clinic and its goals. Phone calls were made to each FPOW to address questions and to ascertain their interest. The FPOW advocates then worked directly with schedulers to make clinic appointments. Forty-one FPOWs responded to this initial invitation and attended the new clinic. Subsequently, this number increased through FPOW consults placed by STVHCS primary care providers.
The service-connected disability rating of clinic patients ranged from none (6% of attendees) to 100% (28% of attendees). For 34% of patients, clinic attendance resulted in identification application for overlooked presumptives. VBA evaluation resulted in increased service-connected disability ratings for nearly one-third of clinic patients. All clinic patients without a service-connected disability prior to FPOW clinic evaluation received an increased service-connected disability rating. Overall, 60% of the FPOWs who attended the clinic opted to receive their primary care at the FPOW clinic.
The FPOW Clinic successfully identified overlooked presumptives and facilitated the determination of appropriate service-connected disabilities. Interestingly, the FPOW Clinic encountered an unanticipated challenge to identifying overlooked FPOW benefits—veterans’ medical conditions that are listed by the VHA as being service-connected in the Computerized Patient Record System did not always reflect those listed officially in VBA records. This led to occasional identification of apparently overlooked FPOW presumptives that were already recognized by the VBA but not reflected in VHA records. This issue was addressed by ensuring that VBA representatives attended postclinic meetings with clinic staff and avoided the need to pursue supposedly unrecognized benefits that were recognized.
Telehealth
At present, FPOWs from World War II outnumber those of all other conflicts; however, this group is rapidly dwindling in numbers. World War II FPOWs are aged > 85 years, and therefore among the most frail and dependent of veterans. Often they are homebound and unable to physically travel to clinics for assessment. To serve these veterans, we are modifying the FPOW Clinic to utilize telehealth. The Telehealth FPOW Clinic will obtain relevant data from review of the electronic health record and telehealth-based clinic visits. Telehealth also may be used for assessments of Vietnam War veterans (eg, Agent Orange exposure), atomic veterans, and Gulf War veterans. Once fully designed and implemented, we believe that telehealth will prove to be a cost-effective way to provide clinic benefits to rural and older veterans.
Conclusions
The VHA provides priority medical treatment to FPOWs as well as timely and appropriate assessment of their eligibility for veterans’ benefits. The complexities benefit programs established for FPOWs is often beyond the ken of VHA physicians, social workers, and nurses. Because of this unfamiliarity, referrals of eligible FPOWs to appropriate programs can be overlooked. We established a clinic-based interdisciplinary team (FPOW Clinic) that was fully trained in FPOW benefit programs to identify overlooked benefits for FPOWs and were able to increase the disability rating on approximately one-third of the FPOWs seen in the FPOW Clinic. A telehealth-based version of the FPOW clinic is now being developed.
1. Henning CA; Congressional Research Service. POWs and MIAs: status and accounting issues. https://fas.org/sgp/crs/natsec/RL33452.pdf. Published June 1, 2006. Accessed March 16, 2020.
2. Klein RE, Wells MR, Somers JM. American Prisoners of War (POWs) and Missing in Action (MIAs). Washington, DC: US Department of Veterans Affairs, Office of Policy, Planning, and Preparedness; 2006.
3. Skelton WP 3rd. American ex-prisoners of war. https://m.vfwilserviceoffice.com/upload/VA%20Report%20on%20Former%20POWs.pdf. Updated April 2002. Accessed March 16, 2020.
4. Creasey H, Sulway MR, Dent O, Broe GA, Jorm A, Tennant C. Is experience as a prisoner of war a risk factor for accelerated age-related illness and disability? J Am Geriatr Soc. 1999;47(1):60-64.
5. Meziab O, Kirby KA, Williams B, Yaffe K, Byers AL, Barnes DE. Prisoner of war status, posttraumatic stress disorder, and dementia in older veterans. Alzheimers Dement. 2014;10(3)(suppl):S236-S241.
6. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1650. Special Care and Benefits Teams Evaluating or Treating Former Prisoners of War. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=7481. Published July 31, 2018. Accessed March 16, 2020.
7. Boult C, Boult LB, Morishita L, Dowd B, Kane RL, Urdangarin CF. A randomized clinical trial of outpatient geriatric evaluation and management. J Am Geriatr Soc. 2001;49(4):351-359.
8. Kellogg, DL Jr. Geriatric Research, Education and Clinical Center (GRECC): former prisoners of war (FPOW) clinic, methods, procedures & training manual. https://www.southtexas.va.gov/grecc/docs/FPOW_toolkit.pdf. Updated January 28, 2015. Accessed March 16, 2020.
Since the beginning of the American Republic, servicemen have been captured and held as prisoners of war (POWs), including > 130,000 in World War II , > 7,100 in the Korean War, > 700 in the Vietnam War, and 37 in Operation Desert Storm and recent conflicts.1,2 Also, > 80 servicewomen have been held during these conflicts.1-3 Of those living former POWs (FPOWs), almost all are geriatric (aged > 65 years) with a significant portion aged ≥ 85 years.
The physical hardships and psychological stress endured by FPOWs have lifelong deleterious sequelae on health and social functioning.3-5 The experiences of FPOWs are associated with higher prevalence of chronic diseases and diminished functional performance in later life as demonstrated by a survey of FPOWs from World War II.4 The survey assessed health and functional status in a random sample of 101 FPOWs and a group of 107 non-POW combatants from the same military operations. FPOWs reported a higher mean number of somatic symptoms than did non-POWs (7.2 vs 5.4, respectively; P = .002), a higher mean number of diagnosed health conditions (9.4 vs 7.7, respectively; P < .001), and used a greater mean number of medications (4.5 vs 3.4, respectively; P = .001). Among 15 broad categories of diagnoses, differences were found in gastrointestinal disorders (FPOWs 63% vs non-POWs 49%, P = .032), musculoskeletal disorders (FPOWs 76% vs non-POWs 60%, P = .001), and cognitive disorders (FPOWs 31% vs non-POWs 15%, P = .006). FPOWs had a significantly higher proportion of 7 extrapyramidal signs and 6 signs relating to ataxia. On the Instrumental Activities of Daily Living scale, FPOWs were more likely to be impaired than were non-POWs (33% vs 17%, respectively; P = .01). In addition, FPOWs have an increased risk of developing dementia, and this risk is doubled in FPOWs with posttraumatic stress disorder (PTSD) compared with non-FPOWs without PTSD.5
These data indicate that FPOW status is associated with increased risk of disability and loss of independence. Federal statutes established the presumption of a relationship between FPOW status and many comorbidities for VA disability determinations in recognition of such data and to overcome lack of medical records during POW confinement and to accord benefit of the doubt where medical science cannot conclusively link disease etiology to FPOW status, to FPOWs.
Service-Connected Conditions
The historical development of conditions with a presumption of service connection for adjudication of VA compensation/disability claims began in 1921 with the Act to Establish a Veterans’ Bureau and to Improve the Facilities.1 The act simplified and streamlined the claims adjudication process by eliminating the need to obtain evidence on the part of the veteran. The presumption of service connection also facilitated increased accuracy and consistency in adjudications by requiring similar treatment for similar claims. This “presumptive” process relieved claimants and VA of the necessity of producing direct evidence when it was impractical to do so.
In 1970, the first presumptives specific to FPOWs were legislatively established and covered 17 diseases for a FPOW who had been confined for ≥ 30 days (Pub. L. 91-376). The 30-day confinement requirement was later relaxed, and additional presumptives were established that related to diseases that were more common among FPOWs than they were among non-FPOWs. These disorders included traumatic arthritis, stroke, heart disease, osteoporosis, peripheral neuropathy, cold injuries, as well as a variety of digestive and neuropsychiatric disorders. If a FPOW is diagnosed as having ≥ 1 of these conditions and it is judged to be ≥ 10% disabling, the condition is presumed to be a sequelae of the POW experience, and it is classified as a service-connected disability (Table).
FPOW Care And Benefits Teams
Several Veterans Health Administration (VHA) directives have been issued, including the recent VHA directive 1650, which requires that each VHA medical facility have a special Care and Benefits Team (CBT) that is charged with the evaluation and treatment of FPOWs to ensure that “FPOWs receive the highest quality care and benefit services.”6 CBTs must be composed of a clinician trained in internal medicine or family practice; a clinician who is certified through the VA Office of Disability and Medical Assessment to conduct General Medical Compensation and Pension evaluations; a FPOW advocate who typically is a VHA clinical social worker; and a Veterans Benefits Administration (VBA) FPOW coordinator appointed by the local VBA regional office. CBTs can be expanded to include other members as needed. The CBTs are tasked with facilitating interactions between FPOWs, the VHA, and the VBA.
CBTs face several challenges in meeting their responsibilities. For example, the POW experience often results in psychological trauma that foments denial and distrust; hence, thoughtful sensitivity to the sequelae of captivity when approaching FPOWs about personal issues, such as health care, is required. Establishing trusting relationships with FPOWs is necessary if their needs are to be effectively addressed.
While the VHA is mandated to provide priority treatment for FPOWs, including hospital, nursing home, dental, and outpatient treatment, a significant number of FPOWs do not avail themselves of benefits to which they are entitled. Often these FPOWs have not used VA programs and facilities because they are uninformed or confused about VA benefits for FPOWs. As a result, referrals of eligible FPOWs to appropriate programs can be overlooked. Maximizing the service-connected disability rating of FPOWs not only impacts the disability pensions received by these veterans, but also impacts their eligibility for VHA programs, including long-term care and Dependency and Indemnity Compensation, a monthly benefit paid to spouses, children, and/or surviving parents.
In 2013, the FPOW Committee of the South Texas Veterans Health Care System (STVHCS) noted that 40% of FPOWs in our region had no VA primary care or clinic assignment. In consideration of the commitment of the VA to care for FPOWs, the unique POW-related medical and psychological issues, the geriatric age of many FPOWs, and the surprising number of FPOWs currently not receiving VA care, we expanded the concept of the CBT team to create a specialized interdisciplinary FPOW Clinic to address the unique needs of this predominantly elderly population and to involve more FPOWs in the VA system.
The main purpose of this clinic was to advise FPOWs of all VA benefits and services to which they may be entitled by identifying overlooked FPOW presumptives. As the number of FPOWs continues to decrease, outreach to FPOWs and family members has become critical, especially as increased benefits and special services might be available to this increasingly dependent older population. An informal survey of FPOW advocates across the nation found that 21% of FPOWs had disability ratings from the VA of ≤ 60%, including some who had no VA disability rating at all. Thus, an additional goal of the project was to develop a clinic model that could be disseminated throughout the VHA.
Design
The design of the FPOW Clinic team is based on an interdisciplinary model that has proven successful in geriatric medicine.7 The team comprises a physician, a social worker, and a registered nurse.8 All members have expertise in geriatric medicine and specific training in FPOW-related issues by completing a VA employee education training session on FPOW case management. Completion of this training ensured that team members were:
- Familiar with the experiences of FPOWs as well as about the medical, psychosocial, and mental health conditions that affect FPOWs;
- Knowledgeable about FPOW presumptive conditions;
- Familiar with the VBA process for rating FPOW disability claims; and
- Capable of FPOW case coordination, workflow, and communications between the FPOW Clinic team and the VBA to avail FPOWs and their families of all eligible benefits.
In-person FPOW clinic visits and chart reviews helped identify overlooked FPOW benefits. To facilitate case management, a representative of the VBA attended the initial evaluation of each FPOW in the clinic to confirm any overlooked presumptive benefits and to familiarize FPOWs with the claims process. FPOWs were also given the choice to officially enroll in the FPOW clinic for primary care or to remain with their current health care provider. Special efforts were made to enroll those FPOWs who had no STVHCS assigned primary care clinic.
The clinic was scheduled for 4 hours every week. Initial patient visits were 2 hours each and consisted of separate evaluations by each of the 3 FPOW Clinic team members who then met as a team with the addition of the VBA representative. The purpose of this meeting was to discuss overlooked benefits, address any other specific issues noted, and to devise an appropriate interdisciplinary plan. Findings of overlooked benefits and other relevant outcomes then were conveyed to the FPOW. For FPOWs who opted to continue in the clinic for their primary care, subsequent appointments were 1 hour.
Implementation
STVHCS FPOW advocates identified and sent letters to FPOWs announcing the opening of the clinic and its goals. Phone calls were made to each FPOW to address questions and to ascertain their interest. The FPOW advocates then worked directly with schedulers to make clinic appointments. Forty-one FPOWs responded to this initial invitation and attended the new clinic. Subsequently, this number increased through FPOW consults placed by STVHCS primary care providers.
The service-connected disability rating of clinic patients ranged from none (6% of attendees) to 100% (28% of attendees). For 34% of patients, clinic attendance resulted in identification application for overlooked presumptives. VBA evaluation resulted in increased service-connected disability ratings for nearly one-third of clinic patients. All clinic patients without a service-connected disability prior to FPOW clinic evaluation received an increased service-connected disability rating. Overall, 60% of the FPOWs who attended the clinic opted to receive their primary care at the FPOW clinic.
The FPOW Clinic successfully identified overlooked presumptives and facilitated the determination of appropriate service-connected disabilities. Interestingly, the FPOW Clinic encountered an unanticipated challenge to identifying overlooked FPOW benefits—veterans’ medical conditions that are listed by the VHA as being service-connected in the Computerized Patient Record System did not always reflect those listed officially in VBA records. This led to occasional identification of apparently overlooked FPOW presumptives that were already recognized by the VBA but not reflected in VHA records. This issue was addressed by ensuring that VBA representatives attended postclinic meetings with clinic staff and avoided the need to pursue supposedly unrecognized benefits that were recognized.
Telehealth
At present, FPOWs from World War II outnumber those of all other conflicts; however, this group is rapidly dwindling in numbers. World War II FPOWs are aged > 85 years, and therefore among the most frail and dependent of veterans. Often they are homebound and unable to physically travel to clinics for assessment. To serve these veterans, we are modifying the FPOW Clinic to utilize telehealth. The Telehealth FPOW Clinic will obtain relevant data from review of the electronic health record and telehealth-based clinic visits. Telehealth also may be used for assessments of Vietnam War veterans (eg, Agent Orange exposure), atomic veterans, and Gulf War veterans. Once fully designed and implemented, we believe that telehealth will prove to be a cost-effective way to provide clinic benefits to rural and older veterans.
Conclusions
The VHA provides priority medical treatment to FPOWs as well as timely and appropriate assessment of their eligibility for veterans’ benefits. The complexities benefit programs established for FPOWs is often beyond the ken of VHA physicians, social workers, and nurses. Because of this unfamiliarity, referrals of eligible FPOWs to appropriate programs can be overlooked. We established a clinic-based interdisciplinary team (FPOW Clinic) that was fully trained in FPOW benefit programs to identify overlooked benefits for FPOWs and were able to increase the disability rating on approximately one-third of the FPOWs seen in the FPOW Clinic. A telehealth-based version of the FPOW clinic is now being developed.
Since the beginning of the American Republic, servicemen have been captured and held as prisoners of war (POWs), including > 130,000 in World War II , > 7,100 in the Korean War, > 700 in the Vietnam War, and 37 in Operation Desert Storm and recent conflicts.1,2 Also, > 80 servicewomen have been held during these conflicts.1-3 Of those living former POWs (FPOWs), almost all are geriatric (aged > 65 years) with a significant portion aged ≥ 85 years.
The physical hardships and psychological stress endured by FPOWs have lifelong deleterious sequelae on health and social functioning.3-5 The experiences of FPOWs are associated with higher prevalence of chronic diseases and diminished functional performance in later life as demonstrated by a survey of FPOWs from World War II.4 The survey assessed health and functional status in a random sample of 101 FPOWs and a group of 107 non-POW combatants from the same military operations. FPOWs reported a higher mean number of somatic symptoms than did non-POWs (7.2 vs 5.4, respectively; P = .002), a higher mean number of diagnosed health conditions (9.4 vs 7.7, respectively; P < .001), and used a greater mean number of medications (4.5 vs 3.4, respectively; P = .001). Among 15 broad categories of diagnoses, differences were found in gastrointestinal disorders (FPOWs 63% vs non-POWs 49%, P = .032), musculoskeletal disorders (FPOWs 76% vs non-POWs 60%, P = .001), and cognitive disorders (FPOWs 31% vs non-POWs 15%, P = .006). FPOWs had a significantly higher proportion of 7 extrapyramidal signs and 6 signs relating to ataxia. On the Instrumental Activities of Daily Living scale, FPOWs were more likely to be impaired than were non-POWs (33% vs 17%, respectively; P = .01). In addition, FPOWs have an increased risk of developing dementia, and this risk is doubled in FPOWs with posttraumatic stress disorder (PTSD) compared with non-FPOWs without PTSD.5
These data indicate that FPOW status is associated with increased risk of disability and loss of independence. Federal statutes established the presumption of a relationship between FPOW status and many comorbidities for VA disability determinations in recognition of such data and to overcome lack of medical records during POW confinement and to accord benefit of the doubt where medical science cannot conclusively link disease etiology to FPOW status, to FPOWs.
Service-Connected Conditions
The historical development of conditions with a presumption of service connection for adjudication of VA compensation/disability claims began in 1921 with the Act to Establish a Veterans’ Bureau and to Improve the Facilities.1 The act simplified and streamlined the claims adjudication process by eliminating the need to obtain evidence on the part of the veteran. The presumption of service connection also facilitated increased accuracy and consistency in adjudications by requiring similar treatment for similar claims. This “presumptive” process relieved claimants and VA of the necessity of producing direct evidence when it was impractical to do so.
In 1970, the first presumptives specific to FPOWs were legislatively established and covered 17 diseases for a FPOW who had been confined for ≥ 30 days (Pub. L. 91-376). The 30-day confinement requirement was later relaxed, and additional presumptives were established that related to diseases that were more common among FPOWs than they were among non-FPOWs. These disorders included traumatic arthritis, stroke, heart disease, osteoporosis, peripheral neuropathy, cold injuries, as well as a variety of digestive and neuropsychiatric disorders. If a FPOW is diagnosed as having ≥ 1 of these conditions and it is judged to be ≥ 10% disabling, the condition is presumed to be a sequelae of the POW experience, and it is classified as a service-connected disability (Table).
FPOW Care And Benefits Teams
Several Veterans Health Administration (VHA) directives have been issued, including the recent VHA directive 1650, which requires that each VHA medical facility have a special Care and Benefits Team (CBT) that is charged with the evaluation and treatment of FPOWs to ensure that “FPOWs receive the highest quality care and benefit services.”6 CBTs must be composed of a clinician trained in internal medicine or family practice; a clinician who is certified through the VA Office of Disability and Medical Assessment to conduct General Medical Compensation and Pension evaluations; a FPOW advocate who typically is a VHA clinical social worker; and a Veterans Benefits Administration (VBA) FPOW coordinator appointed by the local VBA regional office. CBTs can be expanded to include other members as needed. The CBTs are tasked with facilitating interactions between FPOWs, the VHA, and the VBA.
CBTs face several challenges in meeting their responsibilities. For example, the POW experience often results in psychological trauma that foments denial and distrust; hence, thoughtful sensitivity to the sequelae of captivity when approaching FPOWs about personal issues, such as health care, is required. Establishing trusting relationships with FPOWs is necessary if their needs are to be effectively addressed.
While the VHA is mandated to provide priority treatment for FPOWs, including hospital, nursing home, dental, and outpatient treatment, a significant number of FPOWs do not avail themselves of benefits to which they are entitled. Often these FPOWs have not used VA programs and facilities because they are uninformed or confused about VA benefits for FPOWs. As a result, referrals of eligible FPOWs to appropriate programs can be overlooked. Maximizing the service-connected disability rating of FPOWs not only impacts the disability pensions received by these veterans, but also impacts their eligibility for VHA programs, including long-term care and Dependency and Indemnity Compensation, a monthly benefit paid to spouses, children, and/or surviving parents.
In 2013, the FPOW Committee of the South Texas Veterans Health Care System (STVHCS) noted that 40% of FPOWs in our region had no VA primary care or clinic assignment. In consideration of the commitment of the VA to care for FPOWs, the unique POW-related medical and psychological issues, the geriatric age of many FPOWs, and the surprising number of FPOWs currently not receiving VA care, we expanded the concept of the CBT team to create a specialized interdisciplinary FPOW Clinic to address the unique needs of this predominantly elderly population and to involve more FPOWs in the VA system.
The main purpose of this clinic was to advise FPOWs of all VA benefits and services to which they may be entitled by identifying overlooked FPOW presumptives. As the number of FPOWs continues to decrease, outreach to FPOWs and family members has become critical, especially as increased benefits and special services might be available to this increasingly dependent older population. An informal survey of FPOW advocates across the nation found that 21% of FPOWs had disability ratings from the VA of ≤ 60%, including some who had no VA disability rating at all. Thus, an additional goal of the project was to develop a clinic model that could be disseminated throughout the VHA.
Design
The design of the FPOW Clinic team is based on an interdisciplinary model that has proven successful in geriatric medicine.7 The team comprises a physician, a social worker, and a registered nurse.8 All members have expertise in geriatric medicine and specific training in FPOW-related issues by completing a VA employee education training session on FPOW case management. Completion of this training ensured that team members were:
- Familiar with the experiences of FPOWs as well as about the medical, psychosocial, and mental health conditions that affect FPOWs;
- Knowledgeable about FPOW presumptive conditions;
- Familiar with the VBA process for rating FPOW disability claims; and
- Capable of FPOW case coordination, workflow, and communications between the FPOW Clinic team and the VBA to avail FPOWs and their families of all eligible benefits.
In-person FPOW clinic visits and chart reviews helped identify overlooked FPOW benefits. To facilitate case management, a representative of the VBA attended the initial evaluation of each FPOW in the clinic to confirm any overlooked presumptive benefits and to familiarize FPOWs with the claims process. FPOWs were also given the choice to officially enroll in the FPOW clinic for primary care or to remain with their current health care provider. Special efforts were made to enroll those FPOWs who had no STVHCS assigned primary care clinic.
The clinic was scheduled for 4 hours every week. Initial patient visits were 2 hours each and consisted of separate evaluations by each of the 3 FPOW Clinic team members who then met as a team with the addition of the VBA representative. The purpose of this meeting was to discuss overlooked benefits, address any other specific issues noted, and to devise an appropriate interdisciplinary plan. Findings of overlooked benefits and other relevant outcomes then were conveyed to the FPOW. For FPOWs who opted to continue in the clinic for their primary care, subsequent appointments were 1 hour.
Implementation
STVHCS FPOW advocates identified and sent letters to FPOWs announcing the opening of the clinic and its goals. Phone calls were made to each FPOW to address questions and to ascertain their interest. The FPOW advocates then worked directly with schedulers to make clinic appointments. Forty-one FPOWs responded to this initial invitation and attended the new clinic. Subsequently, this number increased through FPOW consults placed by STVHCS primary care providers.
The service-connected disability rating of clinic patients ranged from none (6% of attendees) to 100% (28% of attendees). For 34% of patients, clinic attendance resulted in identification application for overlooked presumptives. VBA evaluation resulted in increased service-connected disability ratings for nearly one-third of clinic patients. All clinic patients without a service-connected disability prior to FPOW clinic evaluation received an increased service-connected disability rating. Overall, 60% of the FPOWs who attended the clinic opted to receive their primary care at the FPOW clinic.
The FPOW Clinic successfully identified overlooked presumptives and facilitated the determination of appropriate service-connected disabilities. Interestingly, the FPOW Clinic encountered an unanticipated challenge to identifying overlooked FPOW benefits—veterans’ medical conditions that are listed by the VHA as being service-connected in the Computerized Patient Record System did not always reflect those listed officially in VBA records. This led to occasional identification of apparently overlooked FPOW presumptives that were already recognized by the VBA but not reflected in VHA records. This issue was addressed by ensuring that VBA representatives attended postclinic meetings with clinic staff and avoided the need to pursue supposedly unrecognized benefits that were recognized.
Telehealth
At present, FPOWs from World War II outnumber those of all other conflicts; however, this group is rapidly dwindling in numbers. World War II FPOWs are aged > 85 years, and therefore among the most frail and dependent of veterans. Often they are homebound and unable to physically travel to clinics for assessment. To serve these veterans, we are modifying the FPOW Clinic to utilize telehealth. The Telehealth FPOW Clinic will obtain relevant data from review of the electronic health record and telehealth-based clinic visits. Telehealth also may be used for assessments of Vietnam War veterans (eg, Agent Orange exposure), atomic veterans, and Gulf War veterans. Once fully designed and implemented, we believe that telehealth will prove to be a cost-effective way to provide clinic benefits to rural and older veterans.
Conclusions
The VHA provides priority medical treatment to FPOWs as well as timely and appropriate assessment of their eligibility for veterans’ benefits. The complexities benefit programs established for FPOWs is often beyond the ken of VHA physicians, social workers, and nurses. Because of this unfamiliarity, referrals of eligible FPOWs to appropriate programs can be overlooked. We established a clinic-based interdisciplinary team (FPOW Clinic) that was fully trained in FPOW benefit programs to identify overlooked benefits for FPOWs and were able to increase the disability rating on approximately one-third of the FPOWs seen in the FPOW Clinic. A telehealth-based version of the FPOW clinic is now being developed.
1. Henning CA; Congressional Research Service. POWs and MIAs: status and accounting issues. https://fas.org/sgp/crs/natsec/RL33452.pdf. Published June 1, 2006. Accessed March 16, 2020.
2. Klein RE, Wells MR, Somers JM. American Prisoners of War (POWs) and Missing in Action (MIAs). Washington, DC: US Department of Veterans Affairs, Office of Policy, Planning, and Preparedness; 2006.
3. Skelton WP 3rd. American ex-prisoners of war. https://m.vfwilserviceoffice.com/upload/VA%20Report%20on%20Former%20POWs.pdf. Updated April 2002. Accessed March 16, 2020.
4. Creasey H, Sulway MR, Dent O, Broe GA, Jorm A, Tennant C. Is experience as a prisoner of war a risk factor for accelerated age-related illness and disability? J Am Geriatr Soc. 1999;47(1):60-64.
5. Meziab O, Kirby KA, Williams B, Yaffe K, Byers AL, Barnes DE. Prisoner of war status, posttraumatic stress disorder, and dementia in older veterans. Alzheimers Dement. 2014;10(3)(suppl):S236-S241.
6. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1650. Special Care and Benefits Teams Evaluating or Treating Former Prisoners of War. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=7481. Published July 31, 2018. Accessed March 16, 2020.
7. Boult C, Boult LB, Morishita L, Dowd B, Kane RL, Urdangarin CF. A randomized clinical trial of outpatient geriatric evaluation and management. J Am Geriatr Soc. 2001;49(4):351-359.
8. Kellogg, DL Jr. Geriatric Research, Education and Clinical Center (GRECC): former prisoners of war (FPOW) clinic, methods, procedures & training manual. https://www.southtexas.va.gov/grecc/docs/FPOW_toolkit.pdf. Updated January 28, 2015. Accessed March 16, 2020.
1. Henning CA; Congressional Research Service. POWs and MIAs: status and accounting issues. https://fas.org/sgp/crs/natsec/RL33452.pdf. Published June 1, 2006. Accessed March 16, 2020.
2. Klein RE, Wells MR, Somers JM. American Prisoners of War (POWs) and Missing in Action (MIAs). Washington, DC: US Department of Veterans Affairs, Office of Policy, Planning, and Preparedness; 2006.
3. Skelton WP 3rd. American ex-prisoners of war. https://m.vfwilserviceoffice.com/upload/VA%20Report%20on%20Former%20POWs.pdf. Updated April 2002. Accessed March 16, 2020.
4. Creasey H, Sulway MR, Dent O, Broe GA, Jorm A, Tennant C. Is experience as a prisoner of war a risk factor for accelerated age-related illness and disability? J Am Geriatr Soc. 1999;47(1):60-64.
5. Meziab O, Kirby KA, Williams B, Yaffe K, Byers AL, Barnes DE. Prisoner of war status, posttraumatic stress disorder, and dementia in older veterans. Alzheimers Dement. 2014;10(3)(suppl):S236-S241.
6. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1650. Special Care and Benefits Teams Evaluating or Treating Former Prisoners of War. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=7481. Published July 31, 2018. Accessed March 16, 2020.
7. Boult C, Boult LB, Morishita L, Dowd B, Kane RL, Urdangarin CF. A randomized clinical trial of outpatient geriatric evaluation and management. J Am Geriatr Soc. 2001;49(4):351-359.
8. Kellogg, DL Jr. Geriatric Research, Education and Clinical Center (GRECC): former prisoners of war (FPOW) clinic, methods, procedures & training manual. https://www.southtexas.va.gov/grecc/docs/FPOW_toolkit.pdf. Updated January 28, 2015. Accessed March 16, 2020.
Aspirin, Yes, for at-risk elderly—but what about the healthy elderly?
ILLUSTRATIVE CASE
A healthy 72-year-old man with well-controlled hypertension on amlodipine 10 mg/d presents to you for an annual exam. He has no history of coronary artery disease or stroke. Should you recommend that he start aspirin for primary prevention of cardiovascular disease?
Cardiovascular disease (CVD) remains the leading cause of death in the United States.2 Aspirin therapy remains the standard of care for secondary prevention of CVD in patients with known coronary artery disease (CAD).3 Aspirin reduces the risk of atherothrombosis by irreversibly inhibiting platelet function. At the same time, it increases the risk of major bleeding, including gastrointestinal bleeds and hemorrhagic strokes. Even though the benefit of aspirin in patients with known CAD is well established, the benefit of aspirin as primary prevention is less certain.
Two recent large randomized controlled trials (RCTs) examined the benefits and risks of aspirin in a variety of patient populations. The ARRIVE trial looked at more than 12,000 patients with a mean age of 63 years with moderate risk of CVD (approximately 15% risk of a cardiovascular event in 10 years) and randomly assigned them to receive aspirin or placebo.4 After an average follow-up period of 5 years, researchers observed that actual cardiovascular event risk was < 10% in both groups, and there was no significant difference in the primary outcome of first cardiovascular event or all-cause mortality. There was, however, a significant increase in bleeding events in the group receiving aspirin.4
The ASCEND trial evaluated aspirin vs placebo in more than 15,000 adult patients with type 2 diabetes mellitus and a low risk of CVD (< 10% risk of cardiovascular event in 5 years). 5 The primary endpoint of the study was first cardiovascular event. The authors found a significantly lower rate of cardiovascular events in the aspirin group, as well as more major bleeding events. Additionally, there was no difference between the aspirin and placebo groups in all-cause mortality after 7 years. The authors concluded that the benefits of aspirin in this group were counterbalanced by the harms.5
Currently, several organizations offer recommendations on aspirin use in people 40 to 70 years of age based on a patient’s risk of bleeding and risk of CVD.6-8 Recommendations regarding aspirin use as primary prevention have been less clear for patients < 40 and > 70 years of age.6
Elderly patients are at higher risk of CVD and bleeding, but until recently, few studies had evaluated elderly populations to assess the benefits vs the risks of aspirin for primary CVD prevention. As of 2016, the US Preventive Services Task Force (USPSTF) stated the evidence was insufficient to assess the balance of the benefits and harms of initiating aspirin use for primary prevention of CVD in patients older than 70 years of age.6 This trial focuses on aspirin use for primary prevention of CVD in healthy elderly adults.
STUDY SUMMARY
Don’t use aspirin as primary prevention of CVD in the elderly
This secondary analysis of a prior double-blind RCT, which found low-dose aspirin did not prolong survival in elderly patients, examined the effect of aspirin on CVD and hemorrhage in 19,114 elderly patients without known CVD.1 The patients were ≥ 70 years of age (≥ 65 years for blacks and Hispanics) with a mean age of 74 years and were from Australia (87%) and the United States (13%). Approximately one-third of the patients were taking a statin, and 14% were taking a nonsteroidal anti-inflammatory drug (NSAID) regularly. Patients were randomized to either aspirin 100 mg/d or matching placebo and were followed for an average of 4.7 years.
Continue to: Outcomes
Outcomes. The outcome of CVD was a composite of fatal coronary heart disease, nonfatal myocardial infarction (MI), fatal or nonfatal ischemic stroke, or hospitalization for heart failure, and the outcome of major adverse cardiovascular event was a composite of fatal cardiovascular disease (excluding death from heart failure), nonfatal MI, or fatal and nonfatal ischemic stroke.
Results. No difference was seen between the aspirin and placebo groups in CVD outcomes (10.7 events per 1000 person-years vs 11.3 events per 1000 person-years, respectively; hazard ratio [HR] = 0.95; 95% confidence interval [CI], 0.83-1.08) or major cardiovascular events (7.8 events per 1000 person-years vs 8.8 events per 1000 person-years, respectively; HR = 0.89; 95% CI, 0.77-1.03). The composite and individual endpoints of fatal cardiovascular disease, heart failure hospitalizations, fatal and nonfatal MI, and ischemic stroke also did not differ significantly between the groups.
The rate of major hemorrhagic events (composite of hemorrhagic stroke, intracranial bleed, or extracranial bleed), however, was higher in the aspirin vs the placebo group (8.6 events per 1000 person-years vs 6.2 events per 1000 person-years, respectively; HR = 1.4; 95% CI, 1.2-1.6; number needed to harm = 98).
WHAT’S NEW
Finding of more harm than good leads to change in ACC/AHA guidelines
Although the most recent USPSTF guidelines state the evidence is insufficient to assess the risks and benefits of aspirin for the primary prevention of cardiovascular disease in this age group, this trial reveals there is a greater risk of hemorrhagic events than there is prevention of cardiovascular outcomes with aspirin use in healthy elderly patients > 70 years of age.6 Because of this trial, the American College of Cardiology (ACC) and the American Heart Association (AHA) have updated their guidelines on the primary prevention of cardiovascular disease to recommend that aspirin not be used routinely in patients > 70 years of age.7
CAVEATS
Potential benefit to people at higher risk?
The rate of cardiovascular disease was lower than expected in this overall healthy population, so it is not known if cardiovascular benefits may outweigh the risk of bleeding in a higher-risk population. The trial also didn’t address the potential harms of deprescribing aspirin. Additionally, although aspirin may not be protective for cardiovascular events and may lead to more bleeding, there may be other benefits to aspirin in this patient population that were not addressed by this study.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
Popular beliefs and wide availability may make tide difficult to change
Patients have been told for years to take a daily aspirin to “protect their heart”; this behavior may be difficult to change. And because aspirin is widely available over the counter, patients may take it without their physician’s knowledge.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. McNeil JJ, Wolfe R, Woods RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379:1509-1518.
2. Murphy SL, Xu JQ, Kochanek KD, et al. Mortality in the United States, 2017. NCHS Data Brief, no. 328. Hyattsville, MD: National Center for Health Statistics. 2018.
3. Smith SC Jr, Benjamin EJ, Bonow RO, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124:2458-2473.
4. Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet. 2018;392:1036-1046.
5. Bowman L, Mafham M, Wallendszus K, et al; ASCEND Study Collaborative Group. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379:1529-1539.
6. Bibbins-Domingo K; U.S. Preventive Services Task Force. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2016;164:836-845.
7. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Amer Coll Cardiol. 2019;74:1376-1414.
8. American Diabetes Association. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S103-S123.
ILLUSTRATIVE CASE
A healthy 72-year-old man with well-controlled hypertension on amlodipine 10 mg/d presents to you for an annual exam. He has no history of coronary artery disease or stroke. Should you recommend that he start aspirin for primary prevention of cardiovascular disease?
Cardiovascular disease (CVD) remains the leading cause of death in the United States.2 Aspirin therapy remains the standard of care for secondary prevention of CVD in patients with known coronary artery disease (CAD).3 Aspirin reduces the risk of atherothrombosis by irreversibly inhibiting platelet function. At the same time, it increases the risk of major bleeding, including gastrointestinal bleeds and hemorrhagic strokes. Even though the benefit of aspirin in patients with known CAD is well established, the benefit of aspirin as primary prevention is less certain.
Two recent large randomized controlled trials (RCTs) examined the benefits and risks of aspirin in a variety of patient populations. The ARRIVE trial looked at more than 12,000 patients with a mean age of 63 years with moderate risk of CVD (approximately 15% risk of a cardiovascular event in 10 years) and randomly assigned them to receive aspirin or placebo.4 After an average follow-up period of 5 years, researchers observed that actual cardiovascular event risk was < 10% in both groups, and there was no significant difference in the primary outcome of first cardiovascular event or all-cause mortality. There was, however, a significant increase in bleeding events in the group receiving aspirin.4
The ASCEND trial evaluated aspirin vs placebo in more than 15,000 adult patients with type 2 diabetes mellitus and a low risk of CVD (< 10% risk of cardiovascular event in 5 years). 5 The primary endpoint of the study was first cardiovascular event. The authors found a significantly lower rate of cardiovascular events in the aspirin group, as well as more major bleeding events. Additionally, there was no difference between the aspirin and placebo groups in all-cause mortality after 7 years. The authors concluded that the benefits of aspirin in this group were counterbalanced by the harms.5
Currently, several organizations offer recommendations on aspirin use in people 40 to 70 years of age based on a patient’s risk of bleeding and risk of CVD.6-8 Recommendations regarding aspirin use as primary prevention have been less clear for patients < 40 and > 70 years of age.6
Elderly patients are at higher risk of CVD and bleeding, but until recently, few studies had evaluated elderly populations to assess the benefits vs the risks of aspirin for primary CVD prevention. As of 2016, the US Preventive Services Task Force (USPSTF) stated the evidence was insufficient to assess the balance of the benefits and harms of initiating aspirin use for primary prevention of CVD in patients older than 70 years of age.6 This trial focuses on aspirin use for primary prevention of CVD in healthy elderly adults.
STUDY SUMMARY
Don’t use aspirin as primary prevention of CVD in the elderly
This secondary analysis of a prior double-blind RCT, which found low-dose aspirin did not prolong survival in elderly patients, examined the effect of aspirin on CVD and hemorrhage in 19,114 elderly patients without known CVD.1 The patients were ≥ 70 years of age (≥ 65 years for blacks and Hispanics) with a mean age of 74 years and were from Australia (87%) and the United States (13%). Approximately one-third of the patients were taking a statin, and 14% were taking a nonsteroidal anti-inflammatory drug (NSAID) regularly. Patients were randomized to either aspirin 100 mg/d or matching placebo and were followed for an average of 4.7 years.
Continue to: Outcomes
Outcomes. The outcome of CVD was a composite of fatal coronary heart disease, nonfatal myocardial infarction (MI), fatal or nonfatal ischemic stroke, or hospitalization for heart failure, and the outcome of major adverse cardiovascular event was a composite of fatal cardiovascular disease (excluding death from heart failure), nonfatal MI, or fatal and nonfatal ischemic stroke.
Results. No difference was seen between the aspirin and placebo groups in CVD outcomes (10.7 events per 1000 person-years vs 11.3 events per 1000 person-years, respectively; hazard ratio [HR] = 0.95; 95% confidence interval [CI], 0.83-1.08) or major cardiovascular events (7.8 events per 1000 person-years vs 8.8 events per 1000 person-years, respectively; HR = 0.89; 95% CI, 0.77-1.03). The composite and individual endpoints of fatal cardiovascular disease, heart failure hospitalizations, fatal and nonfatal MI, and ischemic stroke also did not differ significantly between the groups.
The rate of major hemorrhagic events (composite of hemorrhagic stroke, intracranial bleed, or extracranial bleed), however, was higher in the aspirin vs the placebo group (8.6 events per 1000 person-years vs 6.2 events per 1000 person-years, respectively; HR = 1.4; 95% CI, 1.2-1.6; number needed to harm = 98).
WHAT’S NEW
Finding of more harm than good leads to change in ACC/AHA guidelines
Although the most recent USPSTF guidelines state the evidence is insufficient to assess the risks and benefits of aspirin for the primary prevention of cardiovascular disease in this age group, this trial reveals there is a greater risk of hemorrhagic events than there is prevention of cardiovascular outcomes with aspirin use in healthy elderly patients > 70 years of age.6 Because of this trial, the American College of Cardiology (ACC) and the American Heart Association (AHA) have updated their guidelines on the primary prevention of cardiovascular disease to recommend that aspirin not be used routinely in patients > 70 years of age.7
CAVEATS
Potential benefit to people at higher risk?
The rate of cardiovascular disease was lower than expected in this overall healthy population, so it is not known if cardiovascular benefits may outweigh the risk of bleeding in a higher-risk population. The trial also didn’t address the potential harms of deprescribing aspirin. Additionally, although aspirin may not be protective for cardiovascular events and may lead to more bleeding, there may be other benefits to aspirin in this patient population that were not addressed by this study.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
Popular beliefs and wide availability may make tide difficult to change
Patients have been told for years to take a daily aspirin to “protect their heart”; this behavior may be difficult to change. And because aspirin is widely available over the counter, patients may take it without their physician’s knowledge.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A healthy 72-year-old man with well-controlled hypertension on amlodipine 10 mg/d presents to you for an annual exam. He has no history of coronary artery disease or stroke. Should you recommend that he start aspirin for primary prevention of cardiovascular disease?
Cardiovascular disease (CVD) remains the leading cause of death in the United States.2 Aspirin therapy remains the standard of care for secondary prevention of CVD in patients with known coronary artery disease (CAD).3 Aspirin reduces the risk of atherothrombosis by irreversibly inhibiting platelet function. At the same time, it increases the risk of major bleeding, including gastrointestinal bleeds and hemorrhagic strokes. Even though the benefit of aspirin in patients with known CAD is well established, the benefit of aspirin as primary prevention is less certain.
Two recent large randomized controlled trials (RCTs) examined the benefits and risks of aspirin in a variety of patient populations. The ARRIVE trial looked at more than 12,000 patients with a mean age of 63 years with moderate risk of CVD (approximately 15% risk of a cardiovascular event in 10 years) and randomly assigned them to receive aspirin or placebo.4 After an average follow-up period of 5 years, researchers observed that actual cardiovascular event risk was < 10% in both groups, and there was no significant difference in the primary outcome of first cardiovascular event or all-cause mortality. There was, however, a significant increase in bleeding events in the group receiving aspirin.4
The ASCEND trial evaluated aspirin vs placebo in more than 15,000 adult patients with type 2 diabetes mellitus and a low risk of CVD (< 10% risk of cardiovascular event in 5 years). 5 The primary endpoint of the study was first cardiovascular event. The authors found a significantly lower rate of cardiovascular events in the aspirin group, as well as more major bleeding events. Additionally, there was no difference between the aspirin and placebo groups in all-cause mortality after 7 years. The authors concluded that the benefits of aspirin in this group were counterbalanced by the harms.5
Currently, several organizations offer recommendations on aspirin use in people 40 to 70 years of age based on a patient’s risk of bleeding and risk of CVD.6-8 Recommendations regarding aspirin use as primary prevention have been less clear for patients < 40 and > 70 years of age.6
Elderly patients are at higher risk of CVD and bleeding, but until recently, few studies had evaluated elderly populations to assess the benefits vs the risks of aspirin for primary CVD prevention. As of 2016, the US Preventive Services Task Force (USPSTF) stated the evidence was insufficient to assess the balance of the benefits and harms of initiating aspirin use for primary prevention of CVD in patients older than 70 years of age.6 This trial focuses on aspirin use for primary prevention of CVD in healthy elderly adults.
STUDY SUMMARY
Don’t use aspirin as primary prevention of CVD in the elderly
This secondary analysis of a prior double-blind RCT, which found low-dose aspirin did not prolong survival in elderly patients, examined the effect of aspirin on CVD and hemorrhage in 19,114 elderly patients without known CVD.1 The patients were ≥ 70 years of age (≥ 65 years for blacks and Hispanics) with a mean age of 74 years and were from Australia (87%) and the United States (13%). Approximately one-third of the patients were taking a statin, and 14% were taking a nonsteroidal anti-inflammatory drug (NSAID) regularly. Patients were randomized to either aspirin 100 mg/d or matching placebo and were followed for an average of 4.7 years.
Continue to: Outcomes
Outcomes. The outcome of CVD was a composite of fatal coronary heart disease, nonfatal myocardial infarction (MI), fatal or nonfatal ischemic stroke, or hospitalization for heart failure, and the outcome of major adverse cardiovascular event was a composite of fatal cardiovascular disease (excluding death from heart failure), nonfatal MI, or fatal and nonfatal ischemic stroke.
Results. No difference was seen between the aspirin and placebo groups in CVD outcomes (10.7 events per 1000 person-years vs 11.3 events per 1000 person-years, respectively; hazard ratio [HR] = 0.95; 95% confidence interval [CI], 0.83-1.08) or major cardiovascular events (7.8 events per 1000 person-years vs 8.8 events per 1000 person-years, respectively; HR = 0.89; 95% CI, 0.77-1.03). The composite and individual endpoints of fatal cardiovascular disease, heart failure hospitalizations, fatal and nonfatal MI, and ischemic stroke also did not differ significantly between the groups.
The rate of major hemorrhagic events (composite of hemorrhagic stroke, intracranial bleed, or extracranial bleed), however, was higher in the aspirin vs the placebo group (8.6 events per 1000 person-years vs 6.2 events per 1000 person-years, respectively; HR = 1.4; 95% CI, 1.2-1.6; number needed to harm = 98).
WHAT’S NEW
Finding of more harm than good leads to change in ACC/AHA guidelines
Although the most recent USPSTF guidelines state the evidence is insufficient to assess the risks and benefits of aspirin for the primary prevention of cardiovascular disease in this age group, this trial reveals there is a greater risk of hemorrhagic events than there is prevention of cardiovascular outcomes with aspirin use in healthy elderly patients > 70 years of age.6 Because of this trial, the American College of Cardiology (ACC) and the American Heart Association (AHA) have updated their guidelines on the primary prevention of cardiovascular disease to recommend that aspirin not be used routinely in patients > 70 years of age.7
CAVEATS
Potential benefit to people at higher risk?
The rate of cardiovascular disease was lower than expected in this overall healthy population, so it is not known if cardiovascular benefits may outweigh the risk of bleeding in a higher-risk population. The trial also didn’t address the potential harms of deprescribing aspirin. Additionally, although aspirin may not be protective for cardiovascular events and may lead to more bleeding, there may be other benefits to aspirin in this patient population that were not addressed by this study.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
Popular beliefs and wide availability may make tide difficult to change
Patients have been told for years to take a daily aspirin to “protect their heart”; this behavior may be difficult to change. And because aspirin is widely available over the counter, patients may take it without their physician’s knowledge.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. McNeil JJ, Wolfe R, Woods RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379:1509-1518.
2. Murphy SL, Xu JQ, Kochanek KD, et al. Mortality in the United States, 2017. NCHS Data Brief, no. 328. Hyattsville, MD: National Center for Health Statistics. 2018.
3. Smith SC Jr, Benjamin EJ, Bonow RO, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124:2458-2473.
4. Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet. 2018;392:1036-1046.
5. Bowman L, Mafham M, Wallendszus K, et al; ASCEND Study Collaborative Group. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379:1529-1539.
6. Bibbins-Domingo K; U.S. Preventive Services Task Force. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2016;164:836-845.
7. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Amer Coll Cardiol. 2019;74:1376-1414.
8. American Diabetes Association. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S103-S123.
1. McNeil JJ, Wolfe R, Woods RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379:1509-1518.
2. Murphy SL, Xu JQ, Kochanek KD, et al. Mortality in the United States, 2017. NCHS Data Brief, no. 328. Hyattsville, MD: National Center for Health Statistics. 2018.
3. Smith SC Jr, Benjamin EJ, Bonow RO, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124:2458-2473.
4. Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet. 2018;392:1036-1046.
5. Bowman L, Mafham M, Wallendszus K, et al; ASCEND Study Collaborative Group. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379:1529-1539.
6. Bibbins-Domingo K; U.S. Preventive Services Task Force. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2016;164:836-845.
7. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Amer Coll Cardiol. 2019;74:1376-1414.
8. American Diabetes Association. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S103-S123.
PRACTICE CHANGER
Do not prescribe aspirin for primary prevention of cardiovascular disease in your elderly patients. Aspirin does not improve cardiovascular outcomes and it significantly increases the risk of bleeding events.
STRENGTH OF RECOMMENDATION
B: Based on a single randomized controlled trial.
McNeil JJ, Wolfe R, Woods RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379:1509-1518.1
Polypharmacy in older adults
Mrs. B, age 66, presents to the emergency department with altered mental status, impaired gait, and tremors. Her son says she has had these symptoms for 3 days. He adds that she has been experiencing more knee pain than usual, and began taking naproxen, 220 mg twice daily, approximately 1 week ago.
Mrs. B’s medical history includes coronary artery disease (CAD), gastroesophageal reflux disease (GERD), hip fracture, osteoarthritis, and osteoporosis. She also has a history of insomnia and bipolar disorder.
Further, Mrs. B reports that 2 months ago, after watching a television program about mental health, she began taking ginkgo biloba, 60 mg/d by mouth for “memory,” and kava kava, 100 mg by mouth 3 times a day for “anxiety.” She did not tell her physician or pharmacist that she began using these supplements because she believes that “natural supplements wouldn’t affect her prescription medications.”
In addition to naproxen, gingko biloba, and kava kava, Mrs. B takes the following medications orally:
Mrs. B’s blood pressure is 132/74 mm Hg (at goal for her age) and her laboratory workup is unremarkable, except for the following results: serum creatinine level of 1.1 mg/dL, blood urea nitrogen/serum creatinine ratio of 40, and creatinine clearance rate of approximately 85 mL/min. An electrocardiogram shows normal sinus rhythm with a QTc of 489 ms. A lithium serum concentration level, drawn randomly, is 1.6 mEq/mL, suggesting lithium toxicity.
Although there is no consensus definition of polypharmacy, the most commonly referenced is concurrent use of ≥5 medications.1 During the last 2 decades, the percentage of adults who report receiving polypharmacy has markedly increased, from 8.2% to 15%.2 Geriatric patients, defined as those age >65, typically receive ≥5 prescription medications.2 Polypharmacy is associated with increased1:
- mortality
- adverse drug reactions
- falls
- length of hospital stay
- readmission rates.
Older adults are particularly vulnerable to the negative outcomes associated with polypharmacy because both increasing age and number of medications received are positively correlated with the risk of adverse events.3 However, the use of multiple medications may be clinically appropriate and necessary in patients with multiple chronic conditions. Recent research suggests that in addition to prescription medications, over-the-counter (OTC) medications and dietary supplements also pose polypharmacy concerns for geriatric patients.3 Here we discuss the risks of OTC medications and dietary supplements for older patients who may be receiving polypharmacy, and highlight specific agents and interactions to watch for in these individuals based on Mrs. B’s case.
Continue to: Factors that increase the risks of OTC medications
Factors that increase the risks of OTC medications
Although older adults account for only 15% of the present population, they purchase 40% of all OTC medications.4 These patients may inadvertently use OTC medications containing unnecessary or potentially harmful active ingredients because of unfamiliarity with the specific product, variability among products, or decreased health literacy. According to research presented at a 2010 Institute of Medicine Workshop on Safe Use Initiative and Health Literacy, many patients have a limited understanding of OTC medication indications and therapeutic duplication.5 For example, researchers found that almost 70% of patients thought they could take 2 products containing the same ingredient.5 Most patients were not able to determine the active ingredients or maximum daily dose of an OTC medication. Patients who were older, had lower literacy, or were African American were more likely to misunderstand medication labeling.5 Additional literature suggests that up to 20% of medical admissions can be attributed to adverse effects of OTC medications.6
Misconceptions regarding dietary supplements
The use of alternative and complementary medicine also is on the rise among geriatric patients.7-9 A recent study found that 70% of older adults in the United States consumed at least 1 dietary supplement in the past 30 days, with 29% consuming ≥4 natural products. Women consumed twice as many supplements as men.10
The perceived safety of natural medicines and dietary supplements is a common and potentially dangerous misconception.11 Because patients typically assume dietary supplements are safe, they often do not report their use to their clinicians, especially if clinicians do not explicitly ask them about supplement use.12 This is especially concerning because the FDA does not have the authority to review or regulate natural medicines or dietary supplements.13,14
With no requirements or regulations regarding quality control of these products, the obvious question is: “How do patients know what they’re ingesting?” The uncertainty regarding the true composition of dietary supplements is a cause for concern because federal regulations do not provide a standard way to verify the purity, quality, and safety. As a result, there is a dearth of information regarding drug–dietary supplement interactions and drug–dietary supplement–disease state interactions.8,15

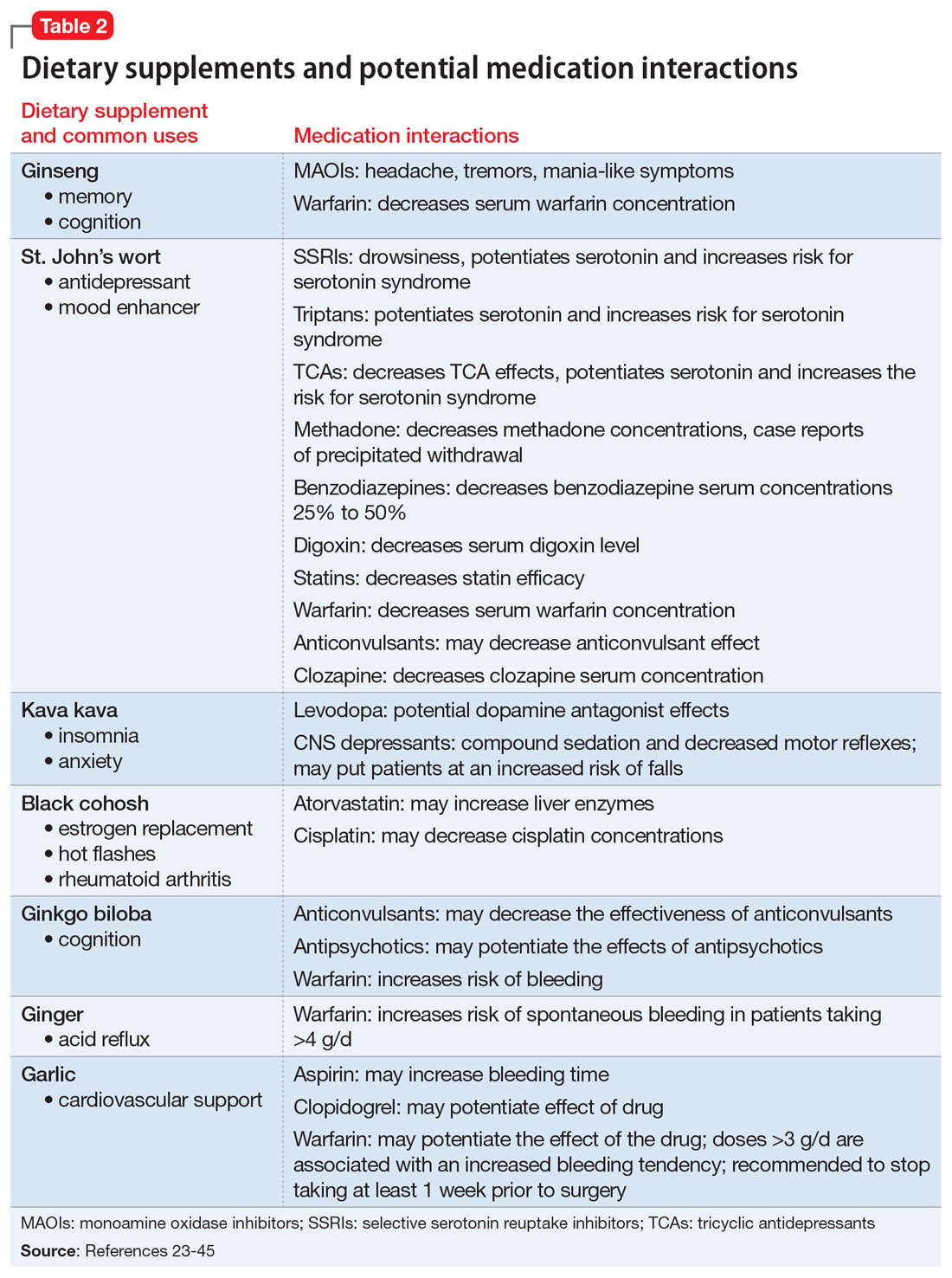
What to watch for
Table 116-22 outlines OTC medication classes and potential medication and/or disease state interactions. Table 223-45 outlines potential interactions between select dietary supplements, medications, and disease states. Here we discuss several of these potential interactions based on the medications that Mrs. B was taking.
Continue to: Nonsteroidal anti-inflammatory drugs (NSAIDs)
Nonsteroidal anti-inflammatory drugs (NSAIDs). All OTC NSAIDs, except aspirin and salicylates, increase the risk for lithium toxicity by decreasing glomerular filtration rate and promoting lithium reabsorption in the kidneys.16 Additionally, NSAIDs increase the risk of developing gastric ulcers and may initiate or exacerbate GERD by suppressing gastric prostaglandin synthesis. Gastric prostaglandins facilitate the formation of a protective lipid-layer in the gastrointestinal (GI) tract.18,46-48 For Mrs. B, the naproxen she was taking resulted in lithium toxicity.
Ginkgo biloba is a plant used most commonly for its reported effect on memory. However, many drug–dietary supplement interactions have been associated with ginkgo biloba that may pose a problem for geriatric patients who receive polypharmacy.49 Mrs. B may have experienced decreased effectiveness of omeprazole and increased sedation or orthostatic hypotension with trazodone.
Kava kava is a natural sedative that can worsen cognition, increase the risk of falls, and potentially cause hepatotoxicity.50 The sedative effects of kava kava are thought to be a direct result of gamma-aminobutyric acid (GABA) modulation via the blockage of voltage-gated sodium ion channels.51 In Mrs. B’s case, when used in combination with diphenhydramine and trazodone, kava kava had the potential to further increase her risk of sedation and falls.
Gastroesophageal reflux disease medications. Older adults may be at an increased risk of GERD due to diseases that affect the esophagus and GI tract, such as diabetes, Parkinson’s disease, and Alzheimer’s disease. Medications may also contribute to gastric reflux by loosening the esophageal tone. Nitrates, benzodiazepines, anticholinergics, antidepressants, and lidocaine have been implicated in precipitating or exacerbating GERD.52
Numerous OTC products can be used to treat heartburn. Calcium carbonate supplements are typically recommended as first-line agents to treat occasional heartburn; histamine-2 receptor antagonists (H2RAs) and proton pump inhibitors (PPIs) generally are reserved for patients who experience heartburn more frequently.47 Per the American Geriatrics Society Beers Criteria for Potentially Inappropriate Medication Use in Older Adults, H2RAs were removed from the “avoid” list for patients with dementia or cognitive impairment due to a lack of strong evidence; however, H2RAs remain on the “avoid” list for patients with delirium.17 Low-dose H2RAs can be used safely in geriatric patients who have renal impairment. Although PPIs are not listedon the Beers Criteria, they have been associated with an increased risk of dementia, osteoporosis, and infections.53,54 There is robust evidence to support bone loss and fractures associated with chronic use of PPIs. However, the data linking PPI use and dementia is controversial due to multiple confounders identified in the studies, such as concomitant use of benzodiazepines.48 PPIs should be prescribed sparingly and judiciously in geriatric patients, and the need for continued PPI therapy should frequently be reassessed.48 Mrs. B’s use of omeprazole, a PPI, may put her at an increased risk for hip fracture compounded by an elevated fall risk associated with other medications she was taking.
Continue to: Trazodone
Trazodone causes sedative effects via anti-alpha 1 activity, which is thought to be responsible for orthostasis and may further increase the risk of falls.51 Mrs. B’s use of trazodone may have increased her risk of sedation and falls.
Antihistaminergic medications are associated with sedation, confusion, cognitive dysfunction, falls, and delirium in geriatric patients. Medications that act on histamine receptors can be particularly detrimental in the geriatric population because of their decreased clearance, smaller volume of distribution, and decreased tolerance.17,18
Anticholinergic medications. Although atropine and benztropine are widely recognized as anticholinergic agents, other medications, such as digoxin, paroxetine, and colchicine, also demonstrate anticholinergic activity that can cause problematic central and peripheral effects in geriatric patients.55 Central anticholinergic inhibition can lead to reduced cognitive function and impairments in attention and short-term memory. The peripheral effects of anticholinergic medications are similar to those of antihistamines and may include, but are not limited to, dry eyes and mouth via increased inhibition of acetylcholine-mediated muscle contraction of salivary glands.55 These effects can be compounded by the use of OTC medications that exhibit anticholinergic activity.
Diphenhydramine causes sedation through its activity on cholinergic and histaminergic receptors. Patients may not be aware that many OTC cough-and-cold combination products (such as NyQuil, Theraflu, etc.) and OTC nighttime analgesic products (such as Tylenol PM, Aleve PM, Motrin PM, etc.) contain diphenhydramine. For a geriatric patient, such as Mrs. B, diphenhydramine may increase the risk of falls and worsen cognition.
Teach patients to disclose everything they take
Polypharmacy can be detrimental to older patients’ health due to the increased risk of toxicity caused by therapeutic duplication, drug–drug interactions, and drug-disease interactions. Most patients are unable to navigate the nuances of medication indications, maximum dosages, and therapeutic duplications. Older adults frequently take OTC medications and have the greatest risk of developing adverse effects from these medications due to decreased renal and hepatic clearance, increased drug sensitivity, and decreased volume of distribution. Dietary supplements pose a unique risk because they are not FDA-regulated and their purity, quality, and content cannot be verified. Educating patients and family members about the importance of reporting all their prescription medications, OTC medications, and dietary supplements to their pharmacists and clinicians is critical in order to identify and mitigate the risks associated with polypharmacy in geriatric patients.
Continue to: CASE
CASE CONTINUED
Mrs. B is diagnosed with lithium toxicity due to a drug–drug interaction with naproxen. Her lithium is held, and IV fluids are administered. Her symptoms resolve over the next few days. Mrs. B and her son are taught about the interaction between lithium and NSAIDs, and she is counseled to avoid all OTC NSAIDs other than aspirin. Her clinician recommends taking acetaminophen because it will not interact with her medications and is the recommended OTC treatment for mild or moderate pain in geriatric patients.17,56
Next, the clinician addresses Mrs. B’s GERD. Although Mrs. B had been taking PPIs twice daily, her physician recommends decreasing the omeprazole frequency to once daily to minimize adverse effects and pill burden. She also decreases Mrs. B’s aspirin from 325 to 81 mg/d because evidence suggests that when used to prevent CAD, lower-dose aspirin is effective as high-dose aspirin and has fewer adverse effects.57 Finally, she advises Mrs. B to stop taking ginkgo biloba and kava kava and to always check with her primary care physician or pharmacist before beginning any new medication, dietary supplement, or vitamin.
Mrs. B agrees to first check with her clinicians before following advice from mass media. A follow-up appointment is scheduled for 2 weeks to assess renal function, a lithium serum concentration, and adherence to her simplified medication regimen.
Related Resources
- US Department of Health and Human Services. National Institutes of Health. MedlinePlus. Herbs and supplements. https://medlineplus.gov/druginfo/herb_All.html.
- US Department of Health and Human Services. National Center for Complementary and Integrative Health. https://nccih.nih.gov/.
Drug Brand Names
Atorvastatin • Lipitor
Atropine • Atropen
Benztropine • Cogentin
Clozapine • Clozaril
Clopidogrel • Plavix
Colchicine • Colcrys, Gloperba
Digoxin • Cardoxin, Digitek
Lidocaine • Lidoderm, Xylocaine Viscous
Lithium • Eskalith, Lithobid
Methadone • Methadose
Morphine • Kadian, Morphabond
Paroxetine • Paxil
Trazodone • Desyrel
Warfarin • Coumadin, Jantoven
1. Masnoon N, Shakib S, Kalisch-Ellett, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17:230.
2. Kantor ED, Rehm CD, Haas JS, et al. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314(17):1818-1831.
3. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13(1):57-65.
4. Maiese DR. Healthy People 2010-leading health indicators for women. Womens Health Issues. 2002;12(4):155-164.
5. National Academy of Sciences. Institute of Medicine (US) Roundtable on Health Literacy. The Safe Use Initiative and Health Literacy: workshop summary. https://www.ncbi.nlm.nih.gov/books/NBK209756/. Published 2010. Accessed January 22, 2020.
6. Caranasos GJ, Stewart RB, Cluff LE. Drug-induced illness leading to hospitalisation. JAMA. 1974;228(6):713-717.
7. Agbabiaka T. Prevalence of drug–herb and drug-supplement interactions in older adults: a cross-sectional survey. Br J Gen Pract. 2018;68(675):e711-e717. doi: 10.3399/bjgp18X699101.
8. Agbabiaka T, Wider B, Watson L, et al. Concurrent use of prescription drugs and herbal medicinal products in older adults: a systematic review. Drugs Aging. 2017;34(12):891-905.
9. de Souza Silva JE, Santos Souza CA, da Silva TB, et al. Use of herbal medicines by elderly patients: a systematic review. Arch Gerontol Geriatr. 2014;59(2):227-233.
10. Gahche J, Bailey RL, Potischman N, et al. Dietary supplement use was very high among older adults in the United States in 2011-2014. J Nutr. 2017;147(10):1968-1976.
11. Nisly NL, Gryzlak BM, Zimmerman MB et al. Dietary supplement polypharmacy: an unrecognized public health problem? Evid Based Complement Alternat Med. 2010;7(1):107-113.
12. Kennedy J, Wang CC, Wu CH. Patient disclosure about herb and supplement use among adults in the US. Evid Based Complement Alternat Med. 2008;5(4):451-456.
13. Dickinson A. History and overview of DSHEA. Fitoterapia. 2011;82(1):5-10.
14. Dietary Supplement Health and Education Act of 1994. Public Law 103-417,103rd Congress. https://www.congress.gov/bill/103rd-congress/senate-bill/784. Accessed February 20, 2020.
15. US Department of Health & Human Services. National Institute on Aging. Dietary supplements. https://www.nia.nih.gov/health/dietary-supplements. Reviewed November 30, 2017. Accessed January 22, 2020.
16. Ragheb M. The clinical significance of lithium-nonsteroidal. J Clin Psychopharmacol. 1990;10(5):350-354.
17. 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 Updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694.
18. Cho H, Myung J, Suh HS, et al. Antihistamine use and the risk of injurious falls or fracture in elderly patients: a systematic review and meta-analysis. Osteoporos Int. 2018;29(10):2163-2170.
19. Manlucu J, Tonelli M, Ray JG, et al. Dose-reducing H2 receptor antagonists in the presence of low glomerular filtration rate: a systematic review of the evidence. Nephrol Dial Transplant. 2005;20(11):2376-2384.
20. Sudafed [package insert]. Fort Washington, PA: McNeil Consumer Healthcare Division; 2018.
21. US National Library of Medicine. National Center for Biotechnology Information. PubChem Compound Summary: Dextromethorphan; CID=5360696. https://pubchem.ncbi.nlm.nih.gov/compound/5360696. Accessed January 22, 2020.
22. Hedya SA, Swoboda HD. Lithium toxicity. https://www.ncbi.nlm.nih.gov/books/NBK499992/. Updated August 14, 2019. Accessed January 22, 2020.
23. US Department of Health & Human Services. National Center for Complementary and Integrative Health. Herb-drug interactions: what the science says. https://www.nccih.nih.gov/health/providers/digest/herb-drug-interactions-science. Published September 2015. Accessed January 22, 2020.
24. Shader RI, Greenblatt DJ. Bees, ginseng and MAOIs revisited. J Clin Psychopharmacol. 1988;8(4):235.
25. Chua YT. Interaction between warfarin and Chinese herbal medicines. Singapore Med J. 2015;56(1):11-18.
26. Bonetto N, Santelli L, Battistin L, et al. Serotonin syndrome and rhabdomyolysis induced by concomitant use of triptans, fluoxetine and hypericum. Cephalalgia. 2007;27(12):1421-1423.
27. Henderson L, Yue QY, Bergquist C, et al. St John’s wort (Hypericum perforatum): drug interactions and clinical outcomes. Br J Clin Pharmacol. 2002;54(4):349-356.
28. Johne A, Schmider J, Brockmöller J, et al. Decreased plasma levels of amitriptyline and its metabolites on comedication with an extract from St John’s wort (Hypericum perforatum). J Clin Psychopharmacol. 2002;22(1):46-54.
29. Eich-Höchli D, Oppliger R, Golay KP, et al. Methadone maintenance treatment and St John’s wort: a case report. Pharmacopsychiatry. 2003;36(1):35-37.
30. Johne A, Brockmöller J, Bauer S, et al. Pharmacokinetic interaction of digoxin with an herbal extract from St John’s wort (Hypericum perforatum). Clin Pharmacol Ther. 1999;66(4):338-345.
31. Andrén L, Andreasson A, Eggertsen R. Interaction between a commercially available St John’s wort product (Movina) and atorvastatin in patients with hypercholesterolemia. Eur J Clin Pharmacol. 2007;63(10):913-916.
32. Van Strater AC. Interaction of St John’s wort (Hypericum perforatum) with clozapine. Int Clin Psychopharmacol. 2012;27(2):121-124.
33. Nöldner M, Chatterjee SS. Inhibition of haloperidol-induced catalepsy in rats by root extracts from Piper methysticum F. Phytomedicine. 1999;6(4):285-286.
34. Boerner RJ, Klement S. Attenuation of neuroleptic-induced extrapyramidal side effects by kava special extract WS 1490. Wien Med Wochenschr. 2004;154(21-22):508-510.
35. Schelosky L, Raffauf C, Jendroska K, et al. Kava and dopamine antagonism. J Neurol Neurosurg Psychiatry. 1995;58(5):639-640.
36. Singh YN. Potential for interaction of kava and St. John’s wort with drugs. J Ethnopharmacol. 2005;100(1-2):108-113.
37. Patel NM, Derkits R. Possible increase in liver enzymes secondary to atorvastatin and black cohosh administration. J Pharm Prac. 2007;20(4):341-346.
38. Rockwell S, Liu Y, Higgins SA. Alteration of the effects of cancer therapy agents on breast cancer cells by the herbal medicine black cohosh. Breast Cancer Res Treat. 2005;90(3):233-239.
39. Granger AS. Ginkgo biloba precipitating epileptic seizures. Age Ageing. 2001;30(6):523-525.
40. Mohutsky MA, Anderson GD, Miller JW, et al. Ginkgo biloba: evaluation of CYP2C9 drug interactions in vitro and in vivo. Am J Ther. 2006;13(1):24-31.
41. Zhang XY, Zhou DF, Zhang PY, et al. A double-blind, placebo controlled trial of extract of Ginkgo biloba added to haloperidol in treatment-resistant patients with schizophrenia. J Clin Psychiatry. 2001;62(11):878-883.
42. Atmaca M, Tezcan E, Kuloglu M, et al. The effect of extract of ginkgo biloba addition to olanzapine on therapeutic effect and antioxidant enzyme levels in patients with schizophrenia. Psychiatry Clin Neurosci. 2005;59(6):652-656.
43. Doruk A, Uzun O, Ozsahin A. A placebo-controlled study of extract of ginkgo biloba added to clozapine in patients with treatment-resistant schizophrenia. Int Clin Psychopharmacol. 2008;23(4):223-237.
44. Vaes LP. Interactions of warfarin with garlic, ginger, ginkgo, or ginseng: nature of the evidence. Ann Pharmacother. 2000;34(12):1478-1482.
45. Kanji S, Seely D, Yazdi F, et al. Interactions of commonly used dietary supplements with cardiovascular drugs: a systematic review. Syst Rev. 2012;1:26.
46. Wallace JL. Pathogenesis of NSAID-induced gastroduodenal mucosal injury. Best Pract Res Clin Gastroenterol. 2001;15(5):691-703.
47. Triadafilopoulos G, Sharma R. Features of symptomatic gastroesophageal reflux disease in elderly patients. Am J Gastroenterol. 1997;92(11):2007-2011.
48. Haastrup PF, Thompson W, Søndergaard J, et al. Side effects of long-term proton pump inhibitor use: a review. Basic Clin Pharmacol Toxicol. 2018;123(2):114-121.
49. Diamond BJ, Bailey MR. Ginkgo biloba: indications, mechanisms and safety. Psychiatr Clin N Am. 2013;36:73-83.
50. White CM. The pharmacology, pharmacokinetics, efficacy, and adverse events associated with kava. J Clin Pharmacol. 2018;58(11):1396-1405.
51. Gleitz J, Beile A, Peters T. (+/-)-Kavain inhibits veratridine-activated voltage-dependent Na(+)-channels in synaptosomes prepared from rat cerebral cortex. Neuropharmacology. 1995;34(9):1133-1138.
52. Kahrilas PJ. Gastroesophageal reflux disease and its complications. In: Feldman M, ed. Sleisenger & Fordtran’s Gastrointestinal and Liver Disease. 6th ed. Philadelphia, PA: WB Saunders Company; 1998:498-516.
53. Haenisch B, von Holt K, Wiese B, et al. Risk of dementia in elderly patients with the use of proton pump inhibitors. Eur Arch Psychiatry Clin Neurosci. 2015;265(5):419-428.
54. Sheen E, Triadafilopoulos G. Adverse effects of long-term proton pump inhibitor therapy. Dig Dis Sci. 2011;56(4):931-950.
55. Pitkälä KH, Suominen MH, Bell JS, et al. Herbal medications and other dietary supplements. A clinical review for physicians caring for older people. Ann Medicine. 2016;48(8):586-602.
56. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. Arthritis Rheum. 2000;43(9):1905-1915.
57. Vandvik PO, Lincoff AM, Core JM, et al. Primary and secondary prevention of cardiovascular disease: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e637S-e668S. doi: 10.1378/chest.11-2306.
Mrs. B, age 66, presents to the emergency department with altered mental status, impaired gait, and tremors. Her son says she has had these symptoms for 3 days. He adds that she has been experiencing more knee pain than usual, and began taking naproxen, 220 mg twice daily, approximately 1 week ago.
Mrs. B’s medical history includes coronary artery disease (CAD), gastroesophageal reflux disease (GERD), hip fracture, osteoarthritis, and osteoporosis. She also has a history of insomnia and bipolar disorder.
Further, Mrs. B reports that 2 months ago, after watching a television program about mental health, she began taking ginkgo biloba, 60 mg/d by mouth for “memory,” and kava kava, 100 mg by mouth 3 times a day for “anxiety.” She did not tell her physician or pharmacist that she began using these supplements because she believes that “natural supplements wouldn’t affect her prescription medications.”
In addition to naproxen, gingko biloba, and kava kava, Mrs. B takes the following medications orally:
Mrs. B’s blood pressure is 132/74 mm Hg (at goal for her age) and her laboratory workup is unremarkable, except for the following results: serum creatinine level of 1.1 mg/dL, blood urea nitrogen/serum creatinine ratio of 40, and creatinine clearance rate of approximately 85 mL/min. An electrocardiogram shows normal sinus rhythm with a QTc of 489 ms. A lithium serum concentration level, drawn randomly, is 1.6 mEq/mL, suggesting lithium toxicity.
Although there is no consensus definition of polypharmacy, the most commonly referenced is concurrent use of ≥5 medications.1 During the last 2 decades, the percentage of adults who report receiving polypharmacy has markedly increased, from 8.2% to 15%.2 Geriatric patients, defined as those age >65, typically receive ≥5 prescription medications.2 Polypharmacy is associated with increased1:
- mortality
- adverse drug reactions
- falls
- length of hospital stay
- readmission rates.
Older adults are particularly vulnerable to the negative outcomes associated with polypharmacy because both increasing age and number of medications received are positively correlated with the risk of adverse events.3 However, the use of multiple medications may be clinically appropriate and necessary in patients with multiple chronic conditions. Recent research suggests that in addition to prescription medications, over-the-counter (OTC) medications and dietary supplements also pose polypharmacy concerns for geriatric patients.3 Here we discuss the risks of OTC medications and dietary supplements for older patients who may be receiving polypharmacy, and highlight specific agents and interactions to watch for in these individuals based on Mrs. B’s case.
Continue to: Factors that increase the risks of OTC medications
Factors that increase the risks of OTC medications
Although older adults account for only 15% of the present population, they purchase 40% of all OTC medications.4 These patients may inadvertently use OTC medications containing unnecessary or potentially harmful active ingredients because of unfamiliarity with the specific product, variability among products, or decreased health literacy. According to research presented at a 2010 Institute of Medicine Workshop on Safe Use Initiative and Health Literacy, many patients have a limited understanding of OTC medication indications and therapeutic duplication.5 For example, researchers found that almost 70% of patients thought they could take 2 products containing the same ingredient.5 Most patients were not able to determine the active ingredients or maximum daily dose of an OTC medication. Patients who were older, had lower literacy, or were African American were more likely to misunderstand medication labeling.5 Additional literature suggests that up to 20% of medical admissions can be attributed to adverse effects of OTC medications.6
Misconceptions regarding dietary supplements
The use of alternative and complementary medicine also is on the rise among geriatric patients.7-9 A recent study found that 70% of older adults in the United States consumed at least 1 dietary supplement in the past 30 days, with 29% consuming ≥4 natural products. Women consumed twice as many supplements as men.10
The perceived safety of natural medicines and dietary supplements is a common and potentially dangerous misconception.11 Because patients typically assume dietary supplements are safe, they often do not report their use to their clinicians, especially if clinicians do not explicitly ask them about supplement use.12 This is especially concerning because the FDA does not have the authority to review or regulate natural medicines or dietary supplements.13,14
With no requirements or regulations regarding quality control of these products, the obvious question is: “How do patients know what they’re ingesting?” The uncertainty regarding the true composition of dietary supplements is a cause for concern because federal regulations do not provide a standard way to verify the purity, quality, and safety. As a result, there is a dearth of information regarding drug–dietary supplement interactions and drug–dietary supplement–disease state interactions.8,15

What to watch for
Table 116-22 outlines OTC medication classes and potential medication and/or disease state interactions. Table 223-45 outlines potential interactions between select dietary supplements, medications, and disease states. Here we discuss several of these potential interactions based on the medications that Mrs. B was taking.

Continue to: Nonsteroidal anti-inflammatory drugs (NSAIDs)
Nonsteroidal anti-inflammatory drugs (NSAIDs). All OTC NSAIDs, except aspirin and salicylates, increase the risk for lithium toxicity by decreasing glomerular filtration rate and promoting lithium reabsorption in the kidneys.16 Additionally, NSAIDs increase the risk of developing gastric ulcers and may initiate or exacerbate GERD by suppressing gastric prostaglandin synthesis. Gastric prostaglandins facilitate the formation of a protective lipid-layer in the gastrointestinal (GI) tract.18,46-48 For Mrs. B, the naproxen she was taking resulted in lithium toxicity.
Ginkgo biloba is a plant used most commonly for its reported effect on memory. However, many drug–dietary supplement interactions have been associated with ginkgo biloba that may pose a problem for geriatric patients who receive polypharmacy.49 Mrs. B may have experienced decreased effectiveness of omeprazole and increased sedation or orthostatic hypotension with trazodone.
Kava kava is a natural sedative that can worsen cognition, increase the risk of falls, and potentially cause hepatotoxicity.50 The sedative effects of kava kava are thought to be a direct result of gamma-aminobutyric acid (GABA) modulation via the blockage of voltage-gated sodium ion channels.51 In Mrs. B’s case, when used in combination with diphenhydramine and trazodone, kava kava had the potential to further increase her risk of sedation and falls.
Gastroesophageal reflux disease medications. Older adults may be at an increased risk of GERD due to diseases that affect the esophagus and GI tract, such as diabetes, Parkinson’s disease, and Alzheimer’s disease. Medications may also contribute to gastric reflux by loosening the esophageal tone. Nitrates, benzodiazepines, anticholinergics, antidepressants, and lidocaine have been implicated in precipitating or exacerbating GERD.52
Numerous OTC products can be used to treat heartburn. Calcium carbonate supplements are typically recommended as first-line agents to treat occasional heartburn; histamine-2 receptor antagonists (H2RAs) and proton pump inhibitors (PPIs) generally are reserved for patients who experience heartburn more frequently.47 Per the American Geriatrics Society Beers Criteria for Potentially Inappropriate Medication Use in Older Adults, H2RAs were removed from the “avoid” list for patients with dementia or cognitive impairment due to a lack of strong evidence; however, H2RAs remain on the “avoid” list for patients with delirium.17 Low-dose H2RAs can be used safely in geriatric patients who have renal impairment. Although PPIs are not listedon the Beers Criteria, they have been associated with an increased risk of dementia, osteoporosis, and infections.53,54 There is robust evidence to support bone loss and fractures associated with chronic use of PPIs. However, the data linking PPI use and dementia is controversial due to multiple confounders identified in the studies, such as concomitant use of benzodiazepines.48 PPIs should be prescribed sparingly and judiciously in geriatric patients, and the need for continued PPI therapy should frequently be reassessed.48 Mrs. B’s use of omeprazole, a PPI, may put her at an increased risk for hip fracture compounded by an elevated fall risk associated with other medications she was taking.
Continue to: Trazodone
Trazodone causes sedative effects via anti-alpha 1 activity, which is thought to be responsible for orthostasis and may further increase the risk of falls.51 Mrs. B’s use of trazodone may have increased her risk of sedation and falls.
Antihistaminergic medications are associated with sedation, confusion, cognitive dysfunction, falls, and delirium in geriatric patients. Medications that act on histamine receptors can be particularly detrimental in the geriatric population because of their decreased clearance, smaller volume of distribution, and decreased tolerance.17,18
Anticholinergic medications. Although atropine and benztropine are widely recognized as anticholinergic agents, other medications, such as digoxin, paroxetine, and colchicine, also demonstrate anticholinergic activity that can cause problematic central and peripheral effects in geriatric patients.55 Central anticholinergic inhibition can lead to reduced cognitive function and impairments in attention and short-term memory. The peripheral effects of anticholinergic medications are similar to those of antihistamines and may include, but are not limited to, dry eyes and mouth via increased inhibition of acetylcholine-mediated muscle contraction of salivary glands.55 These effects can be compounded by the use of OTC medications that exhibit anticholinergic activity.
Diphenhydramine causes sedation through its activity on cholinergic and histaminergic receptors. Patients may not be aware that many OTC cough-and-cold combination products (such as NyQuil, Theraflu, etc.) and OTC nighttime analgesic products (such as Tylenol PM, Aleve PM, Motrin PM, etc.) contain diphenhydramine. For a geriatric patient, such as Mrs. B, diphenhydramine may increase the risk of falls and worsen cognition.
Teach patients to disclose everything they take
Polypharmacy can be detrimental to older patients’ health due to the increased risk of toxicity caused by therapeutic duplication, drug–drug interactions, and drug-disease interactions. Most patients are unable to navigate the nuances of medication indications, maximum dosages, and therapeutic duplications. Older adults frequently take OTC medications and have the greatest risk of developing adverse effects from these medications due to decreased renal and hepatic clearance, increased drug sensitivity, and decreased volume of distribution. Dietary supplements pose a unique risk because they are not FDA-regulated and their purity, quality, and content cannot be verified. Educating patients and family members about the importance of reporting all their prescription medications, OTC medications, and dietary supplements to their pharmacists and clinicians is critical in order to identify and mitigate the risks associated with polypharmacy in geriatric patients.
Continue to: CASE
CASE CONTINUED
Mrs. B is diagnosed with lithium toxicity due to a drug–drug interaction with naproxen. Her lithium is held, and IV fluids are administered. Her symptoms resolve over the next few days. Mrs. B and her son are taught about the interaction between lithium and NSAIDs, and she is counseled to avoid all OTC NSAIDs other than aspirin. Her clinician recommends taking acetaminophen because it will not interact with her medications and is the recommended OTC treatment for mild or moderate pain in geriatric patients.17,56
Next, the clinician addresses Mrs. B’s GERD. Although Mrs. B had been taking PPIs twice daily, her physician recommends decreasing the omeprazole frequency to once daily to minimize adverse effects and pill burden. She also decreases Mrs. B’s aspirin from 325 to 81 mg/d because evidence suggests that when used to prevent CAD, lower-dose aspirin is effective as high-dose aspirin and has fewer adverse effects.57 Finally, she advises Mrs. B to stop taking ginkgo biloba and kava kava and to always check with her primary care physician or pharmacist before beginning any new medication, dietary supplement, or vitamin.
Mrs. B agrees to first check with her clinicians before following advice from mass media. A follow-up appointment is scheduled for 2 weeks to assess renal function, a lithium serum concentration, and adherence to her simplified medication regimen.
Related Resources
- US Department of Health and Human Services. National Institutes of Health. MedlinePlus. Herbs and supplements. https://medlineplus.gov/druginfo/herb_All.html.
- US Department of Health and Human Services. National Center for Complementary and Integrative Health. https://nccih.nih.gov/.
Drug Brand Names
Atorvastatin • Lipitor
Atropine • Atropen
Benztropine • Cogentin
Clozapine • Clozaril
Clopidogrel • Plavix
Colchicine • Colcrys, Gloperba
Digoxin • Cardoxin, Digitek
Lidocaine • Lidoderm, Xylocaine Viscous
Lithium • Eskalith, Lithobid
Methadone • Methadose
Morphine • Kadian, Morphabond
Paroxetine • Paxil
Trazodone • Desyrel
Warfarin • Coumadin, Jantoven
Mrs. B, age 66, presents to the emergency department with altered mental status, impaired gait, and tremors. Her son says she has had these symptoms for 3 days. He adds that she has been experiencing more knee pain than usual, and began taking naproxen, 220 mg twice daily, approximately 1 week ago.
Mrs. B’s medical history includes coronary artery disease (CAD), gastroesophageal reflux disease (GERD), hip fracture, osteoarthritis, and osteoporosis. She also has a history of insomnia and bipolar disorder.
Further, Mrs. B reports that 2 months ago, after watching a television program about mental health, she began taking ginkgo biloba, 60 mg/d by mouth for “memory,” and kava kava, 100 mg by mouth 3 times a day for “anxiety.” She did not tell her physician or pharmacist that she began using these supplements because she believes that “natural supplements wouldn’t affect her prescription medications.”
In addition to naproxen, gingko biloba, and kava kava, Mrs. B takes the following medications orally:
Mrs. B’s blood pressure is 132/74 mm Hg (at goal for her age) and her laboratory workup is unremarkable, except for the following results: serum creatinine level of 1.1 mg/dL, blood urea nitrogen/serum creatinine ratio of 40, and creatinine clearance rate of approximately 85 mL/min. An electrocardiogram shows normal sinus rhythm with a QTc of 489 ms. A lithium serum concentration level, drawn randomly, is 1.6 mEq/mL, suggesting lithium toxicity.
Although there is no consensus definition of polypharmacy, the most commonly referenced is concurrent use of ≥5 medications.1 During the last 2 decades, the percentage of adults who report receiving polypharmacy has markedly increased, from 8.2% to 15%.2 Geriatric patients, defined as those age >65, typically receive ≥5 prescription medications.2 Polypharmacy is associated with increased1:
- mortality
- adverse drug reactions
- falls
- length of hospital stay
- readmission rates.
Older adults are particularly vulnerable to the negative outcomes associated with polypharmacy because both increasing age and number of medications received are positively correlated with the risk of adverse events.3 However, the use of multiple medications may be clinically appropriate and necessary in patients with multiple chronic conditions. Recent research suggests that in addition to prescription medications, over-the-counter (OTC) medications and dietary supplements also pose polypharmacy concerns for geriatric patients.3 Here we discuss the risks of OTC medications and dietary supplements for older patients who may be receiving polypharmacy, and highlight specific agents and interactions to watch for in these individuals based on Mrs. B’s case.
Continue to: Factors that increase the risks of OTC medications
Factors that increase the risks of OTC medications
Although older adults account for only 15% of the present population, they purchase 40% of all OTC medications.4 These patients may inadvertently use OTC medications containing unnecessary or potentially harmful active ingredients because of unfamiliarity with the specific product, variability among products, or decreased health literacy. According to research presented at a 2010 Institute of Medicine Workshop on Safe Use Initiative and Health Literacy, many patients have a limited understanding of OTC medication indications and therapeutic duplication.5 For example, researchers found that almost 70% of patients thought they could take 2 products containing the same ingredient.5 Most patients were not able to determine the active ingredients or maximum daily dose of an OTC medication. Patients who were older, had lower literacy, or were African American were more likely to misunderstand medication labeling.5 Additional literature suggests that up to 20% of medical admissions can be attributed to adverse effects of OTC medications.6
Misconceptions regarding dietary supplements
The use of alternative and complementary medicine also is on the rise among geriatric patients.7-9 A recent study found that 70% of older adults in the United States consumed at least 1 dietary supplement in the past 30 days, with 29% consuming ≥4 natural products. Women consumed twice as many supplements as men.10
The perceived safety of natural medicines and dietary supplements is a common and potentially dangerous misconception.11 Because patients typically assume dietary supplements are safe, they often do not report their use to their clinicians, especially if clinicians do not explicitly ask them about supplement use.12 This is especially concerning because the FDA does not have the authority to review or regulate natural medicines or dietary supplements.13,14
With no requirements or regulations regarding quality control of these products, the obvious question is: “How do patients know what they’re ingesting?” The uncertainty regarding the true composition of dietary supplements is a cause for concern because federal regulations do not provide a standard way to verify the purity, quality, and safety. As a result, there is a dearth of information regarding drug–dietary supplement interactions and drug–dietary supplement–disease state interactions.8,15

What to watch for
Table 116-22 outlines OTC medication classes and potential medication and/or disease state interactions. Table 223-45 outlines potential interactions between select dietary supplements, medications, and disease states. Here we discuss several of these potential interactions based on the medications that Mrs. B was taking.

Continue to: Nonsteroidal anti-inflammatory drugs (NSAIDs)
Nonsteroidal anti-inflammatory drugs (NSAIDs). All OTC NSAIDs, except aspirin and salicylates, increase the risk for lithium toxicity by decreasing glomerular filtration rate and promoting lithium reabsorption in the kidneys.16 Additionally, NSAIDs increase the risk of developing gastric ulcers and may initiate or exacerbate GERD by suppressing gastric prostaglandin synthesis. Gastric prostaglandins facilitate the formation of a protective lipid-layer in the gastrointestinal (GI) tract.18,46-48 For Mrs. B, the naproxen she was taking resulted in lithium toxicity.
Ginkgo biloba is a plant used most commonly for its reported effect on memory. However, many drug–dietary supplement interactions have been associated with ginkgo biloba that may pose a problem for geriatric patients who receive polypharmacy.49 Mrs. B may have experienced decreased effectiveness of omeprazole and increased sedation or orthostatic hypotension with trazodone.
Kava kava is a natural sedative that can worsen cognition, increase the risk of falls, and potentially cause hepatotoxicity.50 The sedative effects of kava kava are thought to be a direct result of gamma-aminobutyric acid (GABA) modulation via the blockage of voltage-gated sodium ion channels.51 In Mrs. B’s case, when used in combination with diphenhydramine and trazodone, kava kava had the potential to further increase her risk of sedation and falls.
Gastroesophageal reflux disease medications. Older adults may be at an increased risk of GERD due to diseases that affect the esophagus and GI tract, such as diabetes, Parkinson’s disease, and Alzheimer’s disease. Medications may also contribute to gastric reflux by loosening the esophageal tone. Nitrates, benzodiazepines, anticholinergics, antidepressants, and lidocaine have been implicated in precipitating or exacerbating GERD.52
Numerous OTC products can be used to treat heartburn. Calcium carbonate supplements are typically recommended as first-line agents to treat occasional heartburn; histamine-2 receptor antagonists (H2RAs) and proton pump inhibitors (PPIs) generally are reserved for patients who experience heartburn more frequently.47 Per the American Geriatrics Society Beers Criteria for Potentially Inappropriate Medication Use in Older Adults, H2RAs were removed from the “avoid” list for patients with dementia or cognitive impairment due to a lack of strong evidence; however, H2RAs remain on the “avoid” list for patients with delirium.17 Low-dose H2RAs can be used safely in geriatric patients who have renal impairment. Although PPIs are not listedon the Beers Criteria, they have been associated with an increased risk of dementia, osteoporosis, and infections.53,54 There is robust evidence to support bone loss and fractures associated with chronic use of PPIs. However, the data linking PPI use and dementia is controversial due to multiple confounders identified in the studies, such as concomitant use of benzodiazepines.48 PPIs should be prescribed sparingly and judiciously in geriatric patients, and the need for continued PPI therapy should frequently be reassessed.48 Mrs. B’s use of omeprazole, a PPI, may put her at an increased risk for hip fracture compounded by an elevated fall risk associated with other medications she was taking.
Continue to: Trazodone
Trazodone causes sedative effects via anti-alpha 1 activity, which is thought to be responsible for orthostasis and may further increase the risk of falls.51 Mrs. B’s use of trazodone may have increased her risk of sedation and falls.
Antihistaminergic medications are associated with sedation, confusion, cognitive dysfunction, falls, and delirium in geriatric patients. Medications that act on histamine receptors can be particularly detrimental in the geriatric population because of their decreased clearance, smaller volume of distribution, and decreased tolerance.17,18
Anticholinergic medications. Although atropine and benztropine are widely recognized as anticholinergic agents, other medications, such as digoxin, paroxetine, and colchicine, also demonstrate anticholinergic activity that can cause problematic central and peripheral effects in geriatric patients.55 Central anticholinergic inhibition can lead to reduced cognitive function and impairments in attention and short-term memory. The peripheral effects of anticholinergic medications are similar to those of antihistamines and may include, but are not limited to, dry eyes and mouth via increased inhibition of acetylcholine-mediated muscle contraction of salivary glands.55 These effects can be compounded by the use of OTC medications that exhibit anticholinergic activity.
Diphenhydramine causes sedation through its activity on cholinergic and histaminergic receptors. Patients may not be aware that many OTC cough-and-cold combination products (such as NyQuil, Theraflu, etc.) and OTC nighttime analgesic products (such as Tylenol PM, Aleve PM, Motrin PM, etc.) contain diphenhydramine. For a geriatric patient, such as Mrs. B, diphenhydramine may increase the risk of falls and worsen cognition.
Teach patients to disclose everything they take
Polypharmacy can be detrimental to older patients’ health due to the increased risk of toxicity caused by therapeutic duplication, drug–drug interactions, and drug-disease interactions. Most patients are unable to navigate the nuances of medication indications, maximum dosages, and therapeutic duplications. Older adults frequently take OTC medications and have the greatest risk of developing adverse effects from these medications due to decreased renal and hepatic clearance, increased drug sensitivity, and decreased volume of distribution. Dietary supplements pose a unique risk because they are not FDA-regulated and their purity, quality, and content cannot be verified. Educating patients and family members about the importance of reporting all their prescription medications, OTC medications, and dietary supplements to their pharmacists and clinicians is critical in order to identify and mitigate the risks associated with polypharmacy in geriatric patients.
Continue to: CASE
CASE CONTINUED
Mrs. B is diagnosed with lithium toxicity due to a drug–drug interaction with naproxen. Her lithium is held, and IV fluids are administered. Her symptoms resolve over the next few days. Mrs. B and her son are taught about the interaction between lithium and NSAIDs, and she is counseled to avoid all OTC NSAIDs other than aspirin. Her clinician recommends taking acetaminophen because it will not interact with her medications and is the recommended OTC treatment for mild or moderate pain in geriatric patients.17,56
Next, the clinician addresses Mrs. B’s GERD. Although Mrs. B had been taking PPIs twice daily, her physician recommends decreasing the omeprazole frequency to once daily to minimize adverse effects and pill burden. She also decreases Mrs. B’s aspirin from 325 to 81 mg/d because evidence suggests that when used to prevent CAD, lower-dose aspirin is effective as high-dose aspirin and has fewer adverse effects.57 Finally, she advises Mrs. B to stop taking ginkgo biloba and kava kava and to always check with her primary care physician or pharmacist before beginning any new medication, dietary supplement, or vitamin.
Mrs. B agrees to first check with her clinicians before following advice from mass media. A follow-up appointment is scheduled for 2 weeks to assess renal function, a lithium serum concentration, and adherence to her simplified medication regimen.
Related Resources
- US Department of Health and Human Services. National Institutes of Health. MedlinePlus. Herbs and supplements. https://medlineplus.gov/druginfo/herb_All.html.
- US Department of Health and Human Services. National Center for Complementary and Integrative Health. https://nccih.nih.gov/.
Drug Brand Names
Atorvastatin • Lipitor
Atropine • Atropen
Benztropine • Cogentin
Clozapine • Clozaril
Clopidogrel • Plavix
Colchicine • Colcrys, Gloperba
Digoxin • Cardoxin, Digitek
Lidocaine • Lidoderm, Xylocaine Viscous
Lithium • Eskalith, Lithobid
Methadone • Methadose
Morphine • Kadian, Morphabond
Paroxetine • Paxil
Trazodone • Desyrel
Warfarin • Coumadin, Jantoven
1. Masnoon N, Shakib S, Kalisch-Ellett, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17:230.
2. Kantor ED, Rehm CD, Haas JS, et al. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314(17):1818-1831.
3. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13(1):57-65.
4. Maiese DR. Healthy People 2010-leading health indicators for women. Womens Health Issues. 2002;12(4):155-164.
5. National Academy of Sciences. Institute of Medicine (US) Roundtable on Health Literacy. The Safe Use Initiative and Health Literacy: workshop summary. https://www.ncbi.nlm.nih.gov/books/NBK209756/. Published 2010. Accessed January 22, 2020.
6. Caranasos GJ, Stewart RB, Cluff LE. Drug-induced illness leading to hospitalisation. JAMA. 1974;228(6):713-717.
7. Agbabiaka T. Prevalence of drug–herb and drug-supplement interactions in older adults: a cross-sectional survey. Br J Gen Pract. 2018;68(675):e711-e717. doi: 10.3399/bjgp18X699101.
8. Agbabiaka T, Wider B, Watson L, et al. Concurrent use of prescription drugs and herbal medicinal products in older adults: a systematic review. Drugs Aging. 2017;34(12):891-905.
9. de Souza Silva JE, Santos Souza CA, da Silva TB, et al. Use of herbal medicines by elderly patients: a systematic review. Arch Gerontol Geriatr. 2014;59(2):227-233.
10. Gahche J, Bailey RL, Potischman N, et al. Dietary supplement use was very high among older adults in the United States in 2011-2014. J Nutr. 2017;147(10):1968-1976.
11. Nisly NL, Gryzlak BM, Zimmerman MB et al. Dietary supplement polypharmacy: an unrecognized public health problem? Evid Based Complement Alternat Med. 2010;7(1):107-113.
12. Kennedy J, Wang CC, Wu CH. Patient disclosure about herb and supplement use among adults in the US. Evid Based Complement Alternat Med. 2008;5(4):451-456.
13. Dickinson A. History and overview of DSHEA. Fitoterapia. 2011;82(1):5-10.
14. Dietary Supplement Health and Education Act of 1994. Public Law 103-417,103rd Congress. https://www.congress.gov/bill/103rd-congress/senate-bill/784. Accessed February 20, 2020.
15. US Department of Health & Human Services. National Institute on Aging. Dietary supplements. https://www.nia.nih.gov/health/dietary-supplements. Reviewed November 30, 2017. Accessed January 22, 2020.
16. Ragheb M. The clinical significance of lithium-nonsteroidal. J Clin Psychopharmacol. 1990;10(5):350-354.
17. 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 Updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694.
18. Cho H, Myung J, Suh HS, et al. Antihistamine use and the risk of injurious falls or fracture in elderly patients: a systematic review and meta-analysis. Osteoporos Int. 2018;29(10):2163-2170.
19. Manlucu J, Tonelli M, Ray JG, et al. Dose-reducing H2 receptor antagonists in the presence of low glomerular filtration rate: a systematic review of the evidence. Nephrol Dial Transplant. 2005;20(11):2376-2384.
20. Sudafed [package insert]. Fort Washington, PA: McNeil Consumer Healthcare Division; 2018.
21. US National Library of Medicine. National Center for Biotechnology Information. PubChem Compound Summary: Dextromethorphan; CID=5360696. https://pubchem.ncbi.nlm.nih.gov/compound/5360696. Accessed January 22, 2020.
22. Hedya SA, Swoboda HD. Lithium toxicity. https://www.ncbi.nlm.nih.gov/books/NBK499992/. Updated August 14, 2019. Accessed January 22, 2020.
23. US Department of Health & Human Services. National Center for Complementary and Integrative Health. Herb-drug interactions: what the science says. https://www.nccih.nih.gov/health/providers/digest/herb-drug-interactions-science. Published September 2015. Accessed January 22, 2020.
24. Shader RI, Greenblatt DJ. Bees, ginseng and MAOIs revisited. J Clin Psychopharmacol. 1988;8(4):235.
25. Chua YT. Interaction between warfarin and Chinese herbal medicines. Singapore Med J. 2015;56(1):11-18.
26. Bonetto N, Santelli L, Battistin L, et al. Serotonin syndrome and rhabdomyolysis induced by concomitant use of triptans, fluoxetine and hypericum. Cephalalgia. 2007;27(12):1421-1423.
27. Henderson L, Yue QY, Bergquist C, et al. St John’s wort (Hypericum perforatum): drug interactions and clinical outcomes. Br J Clin Pharmacol. 2002;54(4):349-356.
28. Johne A, Schmider J, Brockmöller J, et al. Decreased plasma levels of amitriptyline and its metabolites on comedication with an extract from St John’s wort (Hypericum perforatum). J Clin Psychopharmacol. 2002;22(1):46-54.
29. Eich-Höchli D, Oppliger R, Golay KP, et al. Methadone maintenance treatment and St John’s wort: a case report. Pharmacopsychiatry. 2003;36(1):35-37.
30. Johne A, Brockmöller J, Bauer S, et al. Pharmacokinetic interaction of digoxin with an herbal extract from St John’s wort (Hypericum perforatum). Clin Pharmacol Ther. 1999;66(4):338-345.
31. Andrén L, Andreasson A, Eggertsen R. Interaction between a commercially available St John’s wort product (Movina) and atorvastatin in patients with hypercholesterolemia. Eur J Clin Pharmacol. 2007;63(10):913-916.
32. Van Strater AC. Interaction of St John’s wort (Hypericum perforatum) with clozapine. Int Clin Psychopharmacol. 2012;27(2):121-124.
33. Nöldner M, Chatterjee SS. Inhibition of haloperidol-induced catalepsy in rats by root extracts from Piper methysticum F. Phytomedicine. 1999;6(4):285-286.
34. Boerner RJ, Klement S. Attenuation of neuroleptic-induced extrapyramidal side effects by kava special extract WS 1490. Wien Med Wochenschr. 2004;154(21-22):508-510.
35. Schelosky L, Raffauf C, Jendroska K, et al. Kava and dopamine antagonism. J Neurol Neurosurg Psychiatry. 1995;58(5):639-640.
36. Singh YN. Potential for interaction of kava and St. John’s wort with drugs. J Ethnopharmacol. 2005;100(1-2):108-113.
37. Patel NM, Derkits R. Possible increase in liver enzymes secondary to atorvastatin and black cohosh administration. J Pharm Prac. 2007;20(4):341-346.
38. Rockwell S, Liu Y, Higgins SA. Alteration of the effects of cancer therapy agents on breast cancer cells by the herbal medicine black cohosh. Breast Cancer Res Treat. 2005;90(3):233-239.
39. Granger AS. Ginkgo biloba precipitating epileptic seizures. Age Ageing. 2001;30(6):523-525.
40. Mohutsky MA, Anderson GD, Miller JW, et al. Ginkgo biloba: evaluation of CYP2C9 drug interactions in vitro and in vivo. Am J Ther. 2006;13(1):24-31.
41. Zhang XY, Zhou DF, Zhang PY, et al. A double-blind, placebo controlled trial of extract of Ginkgo biloba added to haloperidol in treatment-resistant patients with schizophrenia. J Clin Psychiatry. 2001;62(11):878-883.
42. Atmaca M, Tezcan E, Kuloglu M, et al. The effect of extract of ginkgo biloba addition to olanzapine on therapeutic effect and antioxidant enzyme levels in patients with schizophrenia. Psychiatry Clin Neurosci. 2005;59(6):652-656.
43. Doruk A, Uzun O, Ozsahin A. A placebo-controlled study of extract of ginkgo biloba added to clozapine in patients with treatment-resistant schizophrenia. Int Clin Psychopharmacol. 2008;23(4):223-237.
44. Vaes LP. Interactions of warfarin with garlic, ginger, ginkgo, or ginseng: nature of the evidence. Ann Pharmacother. 2000;34(12):1478-1482.
45. Kanji S, Seely D, Yazdi F, et al. Interactions of commonly used dietary supplements with cardiovascular drugs: a systematic review. Syst Rev. 2012;1:26.
46. Wallace JL. Pathogenesis of NSAID-induced gastroduodenal mucosal injury. Best Pract Res Clin Gastroenterol. 2001;15(5):691-703.
47. Triadafilopoulos G, Sharma R. Features of symptomatic gastroesophageal reflux disease in elderly patients. Am J Gastroenterol. 1997;92(11):2007-2011.
48. Haastrup PF, Thompson W, Søndergaard J, et al. Side effects of long-term proton pump inhibitor use: a review. Basic Clin Pharmacol Toxicol. 2018;123(2):114-121.
49. Diamond BJ, Bailey MR. Ginkgo biloba: indications, mechanisms and safety. Psychiatr Clin N Am. 2013;36:73-83.
50. White CM. The pharmacology, pharmacokinetics, efficacy, and adverse events associated with kava. J Clin Pharmacol. 2018;58(11):1396-1405.
51. Gleitz J, Beile A, Peters T. (+/-)-Kavain inhibits veratridine-activated voltage-dependent Na(+)-channels in synaptosomes prepared from rat cerebral cortex. Neuropharmacology. 1995;34(9):1133-1138.
52. Kahrilas PJ. Gastroesophageal reflux disease and its complications. In: Feldman M, ed. Sleisenger & Fordtran’s Gastrointestinal and Liver Disease. 6th ed. Philadelphia, PA: WB Saunders Company; 1998:498-516.
53. Haenisch B, von Holt K, Wiese B, et al. Risk of dementia in elderly patients with the use of proton pump inhibitors. Eur Arch Psychiatry Clin Neurosci. 2015;265(5):419-428.
54. Sheen E, Triadafilopoulos G. Adverse effects of long-term proton pump inhibitor therapy. Dig Dis Sci. 2011;56(4):931-950.
55. Pitkälä KH, Suominen MH, Bell JS, et al. Herbal medications and other dietary supplements. A clinical review for physicians caring for older people. Ann Medicine. 2016;48(8):586-602.
56. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. Arthritis Rheum. 2000;43(9):1905-1915.
57. Vandvik PO, Lincoff AM, Core JM, et al. Primary and secondary prevention of cardiovascular disease: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e637S-e668S. doi: 10.1378/chest.11-2306.
1. Masnoon N, Shakib S, Kalisch-Ellett, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17:230.
2. Kantor ED, Rehm CD, Haas JS, et al. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314(17):1818-1831.
3. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13(1):57-65.
4. Maiese DR. Healthy People 2010-leading health indicators for women. Womens Health Issues. 2002;12(4):155-164.
5. National Academy of Sciences. Institute of Medicine (US) Roundtable on Health Literacy. The Safe Use Initiative and Health Literacy: workshop summary. https://www.ncbi.nlm.nih.gov/books/NBK209756/. Published 2010. Accessed January 22, 2020.
6. Caranasos GJ, Stewart RB, Cluff LE. Drug-induced illness leading to hospitalisation. JAMA. 1974;228(6):713-717.
7. Agbabiaka T. Prevalence of drug–herb and drug-supplement interactions in older adults: a cross-sectional survey. Br J Gen Pract. 2018;68(675):e711-e717. doi: 10.3399/bjgp18X699101.
8. Agbabiaka T, Wider B, Watson L, et al. Concurrent use of prescription drugs and herbal medicinal products in older adults: a systematic review. Drugs Aging. 2017;34(12):891-905.
9. de Souza Silva JE, Santos Souza CA, da Silva TB, et al. Use of herbal medicines by elderly patients: a systematic review. Arch Gerontol Geriatr. 2014;59(2):227-233.
10. Gahche J, Bailey RL, Potischman N, et al. Dietary supplement use was very high among older adults in the United States in 2011-2014. J Nutr. 2017;147(10):1968-1976.
11. Nisly NL, Gryzlak BM, Zimmerman MB et al. Dietary supplement polypharmacy: an unrecognized public health problem? Evid Based Complement Alternat Med. 2010;7(1):107-113.
12. Kennedy J, Wang CC, Wu CH. Patient disclosure about herb and supplement use among adults in the US. Evid Based Complement Alternat Med. 2008;5(4):451-456.
13. Dickinson A. History and overview of DSHEA. Fitoterapia. 2011;82(1):5-10.
14. Dietary Supplement Health and Education Act of 1994. Public Law 103-417,103rd Congress. https://www.congress.gov/bill/103rd-congress/senate-bill/784. Accessed February 20, 2020.
15. US Department of Health & Human Services. National Institute on Aging. Dietary supplements. https://www.nia.nih.gov/health/dietary-supplements. Reviewed November 30, 2017. Accessed January 22, 2020.
16. Ragheb M. The clinical significance of lithium-nonsteroidal. J Clin Psychopharmacol. 1990;10(5):350-354.
17. 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 Updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694.
18. Cho H, Myung J, Suh HS, et al. Antihistamine use and the risk of injurious falls or fracture in elderly patients: a systematic review and meta-analysis. Osteoporos Int. 2018;29(10):2163-2170.
19. Manlucu J, Tonelli M, Ray JG, et al. Dose-reducing H2 receptor antagonists in the presence of low glomerular filtration rate: a systematic review of the evidence. Nephrol Dial Transplant. 2005;20(11):2376-2384.
20. Sudafed [package insert]. Fort Washington, PA: McNeil Consumer Healthcare Division; 2018.
21. US National Library of Medicine. National Center for Biotechnology Information. PubChem Compound Summary: Dextromethorphan; CID=5360696. https://pubchem.ncbi.nlm.nih.gov/compound/5360696. Accessed January 22, 2020.
22. Hedya SA, Swoboda HD. Lithium toxicity. https://www.ncbi.nlm.nih.gov/books/NBK499992/. Updated August 14, 2019. Accessed January 22, 2020.
23. US Department of Health & Human Services. National Center for Complementary and Integrative Health. Herb-drug interactions: what the science says. https://www.nccih.nih.gov/health/providers/digest/herb-drug-interactions-science. Published September 2015. Accessed January 22, 2020.
24. Shader RI, Greenblatt DJ. Bees, ginseng and MAOIs revisited. J Clin Psychopharmacol. 1988;8(4):235.
25. Chua YT. Interaction between warfarin and Chinese herbal medicines. Singapore Med J. 2015;56(1):11-18.
26. Bonetto N, Santelli L, Battistin L, et al. Serotonin syndrome and rhabdomyolysis induced by concomitant use of triptans, fluoxetine and hypericum. Cephalalgia. 2007;27(12):1421-1423.
27. Henderson L, Yue QY, Bergquist C, et al. St John’s wort (Hypericum perforatum): drug interactions and clinical outcomes. Br J Clin Pharmacol. 2002;54(4):349-356.
28. Johne A, Schmider J, Brockmöller J, et al. Decreased plasma levels of amitriptyline and its metabolites on comedication with an extract from St John’s wort (Hypericum perforatum). J Clin Psychopharmacol. 2002;22(1):46-54.
29. Eich-Höchli D, Oppliger R, Golay KP, et al. Methadone maintenance treatment and St John’s wort: a case report. Pharmacopsychiatry. 2003;36(1):35-37.
30. Johne A, Brockmöller J, Bauer S, et al. Pharmacokinetic interaction of digoxin with an herbal extract from St John’s wort (Hypericum perforatum). Clin Pharmacol Ther. 1999;66(4):338-345.
31. Andrén L, Andreasson A, Eggertsen R. Interaction between a commercially available St John’s wort product (Movina) and atorvastatin in patients with hypercholesterolemia. Eur J Clin Pharmacol. 2007;63(10):913-916.
32. Van Strater AC. Interaction of St John’s wort (Hypericum perforatum) with clozapine. Int Clin Psychopharmacol. 2012;27(2):121-124.
33. Nöldner M, Chatterjee SS. Inhibition of haloperidol-induced catalepsy in rats by root extracts from Piper methysticum F. Phytomedicine. 1999;6(4):285-286.
34. Boerner RJ, Klement S. Attenuation of neuroleptic-induced extrapyramidal side effects by kava special extract WS 1490. Wien Med Wochenschr. 2004;154(21-22):508-510.
35. Schelosky L, Raffauf C, Jendroska K, et al. Kava and dopamine antagonism. J Neurol Neurosurg Psychiatry. 1995;58(5):639-640.
36. Singh YN. Potential for interaction of kava and St. John’s wort with drugs. J Ethnopharmacol. 2005;100(1-2):108-113.
37. Patel NM, Derkits R. Possible increase in liver enzymes secondary to atorvastatin and black cohosh administration. J Pharm Prac. 2007;20(4):341-346.
38. Rockwell S, Liu Y, Higgins SA. Alteration of the effects of cancer therapy agents on breast cancer cells by the herbal medicine black cohosh. Breast Cancer Res Treat. 2005;90(3):233-239.
39. Granger AS. Ginkgo biloba precipitating epileptic seizures. Age Ageing. 2001;30(6):523-525.
40. Mohutsky MA, Anderson GD, Miller JW, et al. Ginkgo biloba: evaluation of CYP2C9 drug interactions in vitro and in vivo. Am J Ther. 2006;13(1):24-31.
41. Zhang XY, Zhou DF, Zhang PY, et al. A double-blind, placebo controlled trial of extract of Ginkgo biloba added to haloperidol in treatment-resistant patients with schizophrenia. J Clin Psychiatry. 2001;62(11):878-883.
42. Atmaca M, Tezcan E, Kuloglu M, et al. The effect of extract of ginkgo biloba addition to olanzapine on therapeutic effect and antioxidant enzyme levels in patients with schizophrenia. Psychiatry Clin Neurosci. 2005;59(6):652-656.
43. Doruk A, Uzun O, Ozsahin A. A placebo-controlled study of extract of ginkgo biloba added to clozapine in patients with treatment-resistant schizophrenia. Int Clin Psychopharmacol. 2008;23(4):223-237.
44. Vaes LP. Interactions of warfarin with garlic, ginger, ginkgo, or ginseng: nature of the evidence. Ann Pharmacother. 2000;34(12):1478-1482.
45. Kanji S, Seely D, Yazdi F, et al. Interactions of commonly used dietary supplements with cardiovascular drugs: a systematic review. Syst Rev. 2012;1:26.
46. Wallace JL. Pathogenesis of NSAID-induced gastroduodenal mucosal injury. Best Pract Res Clin Gastroenterol. 2001;15(5):691-703.
47. Triadafilopoulos G, Sharma R. Features of symptomatic gastroesophageal reflux disease in elderly patients. Am J Gastroenterol. 1997;92(11):2007-2011.
48. Haastrup PF, Thompson W, Søndergaard J, et al. Side effects of long-term proton pump inhibitor use: a review. Basic Clin Pharmacol Toxicol. 2018;123(2):114-121.
49. Diamond BJ, Bailey MR. Ginkgo biloba: indications, mechanisms and safety. Psychiatr Clin N Am. 2013;36:73-83.
50. White CM. The pharmacology, pharmacokinetics, efficacy, and adverse events associated with kava. J Clin Pharmacol. 2018;58(11):1396-1405.
51. Gleitz J, Beile A, Peters T. (+/-)-Kavain inhibits veratridine-activated voltage-dependent Na(+)-channels in synaptosomes prepared from rat cerebral cortex. Neuropharmacology. 1995;34(9):1133-1138.
52. Kahrilas PJ. Gastroesophageal reflux disease and its complications. In: Feldman M, ed. Sleisenger & Fordtran’s Gastrointestinal and Liver Disease. 6th ed. Philadelphia, PA: WB Saunders Company; 1998:498-516.
53. Haenisch B, von Holt K, Wiese B, et al. Risk of dementia in elderly patients with the use of proton pump inhibitors. Eur Arch Psychiatry Clin Neurosci. 2015;265(5):419-428.
54. Sheen E, Triadafilopoulos G. Adverse effects of long-term proton pump inhibitor therapy. Dig Dis Sci. 2011;56(4):931-950.
55. Pitkälä KH, Suominen MH, Bell JS, et al. Herbal medications and other dietary supplements. A clinical review for physicians caring for older people. Ann Medicine. 2016;48(8):586-602.
56. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. Arthritis Rheum. 2000;43(9):1905-1915.
57. Vandvik PO, Lincoff AM, Core JM, et al. Primary and secondary prevention of cardiovascular disease: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e637S-e668S. doi: 10.1378/chest.11-2306.
Geriatric Assessment and Collaborative Medication Review for Older Adults With Polypharmacy
Study Overview
Objective. To examine the effect of clinical geriatric assessments and collaborative medication review by geriatricians and family physicians on quality of life and other patient outcomes in home-dwelling older adults with polypharmacy.
Design. The study was a single-blind, cluster randomized clinical trial enrolling home-dwelling adults aged 70 years and older who were taking 7 or more medications. Family physicians in Norway were recruited to participate in the trial with their patients. Randomization was at the family physician level to avoid contamination between intervention and control groups.
Setting and participants. The study was conducted in Akershus and Oslo, Norway. Family physicians were recruited to participate in the trial with their patients. A total of 84 family physicians were recruited, of which 70 were included in the trial and randomized to intervention versus control; 14 were excluded because they had no eligible patients. The cluster size of each family physician was limited to 5 patients per physician to avoid large variation in cluster sizes. Patients were eligible for enrollment if they were home-dwelling, aged 70 years or older, and were taking 7 or more systemic medications regularly and had medications administered by the home nursing service. Patients were excluded if they were expected to die or be institutionalized within 6 months, or if they were discouraged from participation by their family physician. A total of 174 patients were recruited, with 87 patients in each group (34 family physicians were in the control group and 36 in the intervention group).
Intervention. The intervention included a geriatric assessment performed by a physician trained in geriatric medicine and supervised by a senior consultant. The geriatric assessment consisted of review of medical history; systematic screening for current problems; clinical examination; supplementary tests, if indicated; and review of each medication being used. The review of medication included the indication for each medication, dosage, adverse effects, and interactions. The geriatric assessment consultation took 1 hour to complete, on average. After the geriatric assessment, the family physician and the geriatrician met to discuss each medication and to establish a collaborative plan for adjustments and follow-up; this meeting was approximately 15 minutes in duration. Lastly, clinical follow-up with the older adult was conducted by the geriatrician or the family physician, as agreed upon in the plan, with most follow-up conducted by the family physician. Participants randomized to the control group received usual care without any intervention.
Main outcome measures. Outcomes were assessed at 16-week and 24-week follow-up. The main study outcome measure was health-related quality of life (HRQoL), as measured by the 15D instrument, at 16 weeks. The quality-of-life measure included the following aspects, each rated on an ordinal scale of 5 levels: mobility, vision, hearing, breathing, sleeping, eating, speech, elimination, usual activities, mental function, discomfort or symptoms, depression, distress, vitality, and sexual activity. The index scale including all aspects is in the range of 0 to 1, with a higher score indicating better quality of life. A predetermined change of 0.015 or more is considered clinically important, and a positive change of 0.035 indicates much better HRQoL. Other outcomes included: appropriateness of medications measured by the Medication Appropriateness Index and the Assessment of Underutilization; physical function (short Physical Performance battery); gait speed; grip strength; cognitive functioning; physical and cognitive disability (Functional Independence Measure); caregiver burden (Relative Stress Scale); physical measures, including orthostatic blood pressure, falls, and weight; hospital admissions; use of home nursing service; incidence of institutionalization; and mortality.
Main results. The study included 174 patients with an average age of 83.3 years (SD, 7.3); 67.8% were women. Of those who were randomized to the intervention and control groups, 158 (90.8%) completed the trial. The average number of regularly used medications was 10.1 (SD, 2.7) in the intervention group and 9.5 (SD, 2.6) in the control group. At week 16 of follow-up, patients in the intervention group had an improved HRQoL score measured by the 15D instrument; the difference between the intervention group and control groups was 0.045 (95% confidence interval [CI], 0.004 -0.086; P = 0.03). Medication appropriateness was better in the intervention group, as compared with the control group at both 16 weeks and 24 weeks. Nearly all (99%) patients in the intervention group experienced medication changes, which included withdrawal of medications, dosage adjustment, or new drug regimens. There was a trend towards a higher rate of hospitalization during follow-up in the intervention group (adjusted risk ratio, 2.03; 95% CI, 0.98-4.24; P = 0.06). Other secondary outcomes were not substantially different between the intervention and control groups.
Conclusion. The study demonstrated that a clinical geriatric assessment and collaborative medication review by geriatrician and family physician led to improved HRQoL and improved medication use.
Commentary
The use of multiple medications in older adults is common, with almost 20% of older adults over age 65 taking 10 or more medications.1 Polypharmacy in older adults is associated with lower adherence rates and increases the potential for interactions between medications.2 Age-related changes, such as changes in absorption, metabolism, and excretion, affect pharmacokinetics of medications and potentiate adverse drug reactions, requiring adjustments in use and dosing to optimize safety and outcomes. Recognizing the potential effects of medications in older adults, evidence-based guidelines, such as the Beers criteria3 and START/STOPP criteria,4 have been developed to identify potentially inappropriate medications in older adults and to improve prescribing. Randomized trials using the START/STOPP criteria have demonstrated improved medication appropriateness, reduced polypharmacy, and reduced adverse drug reactions.5 Although this study did not use a criteria-based approach for improving medication use, it demonstrated that in a population of older adults with polypharmacy, medication review with geriatricians can lead to improved HRQoL while improving medication appropriateness. The collaborative approach between the family physician and geriatrician, rather than a consultative approach with recommendations from a geriatrician, may have contributed to increased uptake of medication changes. Such an approach may be a reasonable strategy to improve medication use in older adults.
A limitation of the study is that the improvement in HRQoL could have been the result of medication changes, but could also have been due to other changes in the plan of care that resulted from the geriatric assessment. As noted by the authors, the increase in hospital admissions, though not statistically significant, could have resulted from the medication modifications; however, it was also noted that the geriatric assessments could have identified severe illnesses that required hospitalization, as the timeline from geriatric assessment to hospitalization suggested was the case. Thus, the increase in hospitalization resulting from timely identification of severe illness was more likely a benefit than an adverse effect; however, further studies should be done to elucidate this.
Applications for Clinical Practice
Older adults with multiple chronic conditions and complex medication regimens are at risk for poor health outcomes, and a purposeful medication review to improve medication use, leading to the removal of unnecessary and potentially harmful medications, adjustment of dosages, and initiation of appropriate medications, may yield health benefits, such as improved HRQoL. The present study utilized an approach that could be scalable, which is important given the limited number of clinicians with geriatrics expertise. For health systems with geriatrics clinical expertise, it may be reasonable to consider adopting a similar collaborative approach in order to improve care for older adults most at risk. Further reports on how patients and family physicians perceive this intervention will enhance our understanding of whether it could be implemented widely.
–William W. Hung, MD, MPH
1. Steinman MA, Hanlon JT. Managing medications in clinically complex elders: “There’s got to be a happy medium”. JAMA. 2010;304:1592-1601.
2. Vik SA, Maxwell CJ, Hogan DB. Measurement, correlates, and health outcomes of medication adherence among seniors. Ann Pharmacother. 2004;38:303-312.
3. American Geriatrics Society 2015 Updated Beers criteria for potentially inappropriate medication use in older Adults. J Am Geriatr Soc. 2015;63:2227-2246.
4. Hill-Taylor B, Sketris I, Hayden J, et al. Application of the STOPP/START criteria: a systematic review of the prevalence of potentially inappropriate prescribing in older adults, and evidence of clinical, humanistic and economic impact. J Clin Pharm Ther. 2013;38:360-372.
5. O’Mahony D. STOPP/START criteria for potentially inappropriate medications/ potential prescribing omissions in older people: origin and progress. Expert Rev Clin Pharmacol. 2020;13:15-22.
Study Overview
Objective. To examine the effect of clinical geriatric assessments and collaborative medication review by geriatricians and family physicians on quality of life and other patient outcomes in home-dwelling older adults with polypharmacy.
Design. The study was a single-blind, cluster randomized clinical trial enrolling home-dwelling adults aged 70 years and older who were taking 7 or more medications. Family physicians in Norway were recruited to participate in the trial with their patients. Randomization was at the family physician level to avoid contamination between intervention and control groups.
Setting and participants. The study was conducted in Akershus and Oslo, Norway. Family physicians were recruited to participate in the trial with their patients. A total of 84 family physicians were recruited, of which 70 were included in the trial and randomized to intervention versus control; 14 were excluded because they had no eligible patients. The cluster size of each family physician was limited to 5 patients per physician to avoid large variation in cluster sizes. Patients were eligible for enrollment if they were home-dwelling, aged 70 years or older, and were taking 7 or more systemic medications regularly and had medications administered by the home nursing service. Patients were excluded if they were expected to die or be institutionalized within 6 months, or if they were discouraged from participation by their family physician. A total of 174 patients were recruited, with 87 patients in each group (34 family physicians were in the control group and 36 in the intervention group).
Intervention. The intervention included a geriatric assessment performed by a physician trained in geriatric medicine and supervised by a senior consultant. The geriatric assessment consisted of review of medical history; systematic screening for current problems; clinical examination; supplementary tests, if indicated; and review of each medication being used. The review of medication included the indication for each medication, dosage, adverse effects, and interactions. The geriatric assessment consultation took 1 hour to complete, on average. After the geriatric assessment, the family physician and the geriatrician met to discuss each medication and to establish a collaborative plan for adjustments and follow-up; this meeting was approximately 15 minutes in duration. Lastly, clinical follow-up with the older adult was conducted by the geriatrician or the family physician, as agreed upon in the plan, with most follow-up conducted by the family physician. Participants randomized to the control group received usual care without any intervention.
Main outcome measures. Outcomes were assessed at 16-week and 24-week follow-up. The main study outcome measure was health-related quality of life (HRQoL), as measured by the 15D instrument, at 16 weeks. The quality-of-life measure included the following aspects, each rated on an ordinal scale of 5 levels: mobility, vision, hearing, breathing, sleeping, eating, speech, elimination, usual activities, mental function, discomfort or symptoms, depression, distress, vitality, and sexual activity. The index scale including all aspects is in the range of 0 to 1, with a higher score indicating better quality of life. A predetermined change of 0.015 or more is considered clinically important, and a positive change of 0.035 indicates much better HRQoL. Other outcomes included: appropriateness of medications measured by the Medication Appropriateness Index and the Assessment of Underutilization; physical function (short Physical Performance battery); gait speed; grip strength; cognitive functioning; physical and cognitive disability (Functional Independence Measure); caregiver burden (Relative Stress Scale); physical measures, including orthostatic blood pressure, falls, and weight; hospital admissions; use of home nursing service; incidence of institutionalization; and mortality.
Main results. The study included 174 patients with an average age of 83.3 years (SD, 7.3); 67.8% were women. Of those who were randomized to the intervention and control groups, 158 (90.8%) completed the trial. The average number of regularly used medications was 10.1 (SD, 2.7) in the intervention group and 9.5 (SD, 2.6) in the control group. At week 16 of follow-up, patients in the intervention group had an improved HRQoL score measured by the 15D instrument; the difference between the intervention group and control groups was 0.045 (95% confidence interval [CI], 0.004 -0.086; P = 0.03). Medication appropriateness was better in the intervention group, as compared with the control group at both 16 weeks and 24 weeks. Nearly all (99%) patients in the intervention group experienced medication changes, which included withdrawal of medications, dosage adjustment, or new drug regimens. There was a trend towards a higher rate of hospitalization during follow-up in the intervention group (adjusted risk ratio, 2.03; 95% CI, 0.98-4.24; P = 0.06). Other secondary outcomes were not substantially different between the intervention and control groups.
Conclusion. The study demonstrated that a clinical geriatric assessment and collaborative medication review by geriatrician and family physician led to improved HRQoL and improved medication use.
Commentary
The use of multiple medications in older adults is common, with almost 20% of older adults over age 65 taking 10 or more medications.1 Polypharmacy in older adults is associated with lower adherence rates and increases the potential for interactions between medications.2 Age-related changes, such as changes in absorption, metabolism, and excretion, affect pharmacokinetics of medications and potentiate adverse drug reactions, requiring adjustments in use and dosing to optimize safety and outcomes. Recognizing the potential effects of medications in older adults, evidence-based guidelines, such as the Beers criteria3 and START/STOPP criteria,4 have been developed to identify potentially inappropriate medications in older adults and to improve prescribing. Randomized trials using the START/STOPP criteria have demonstrated improved medication appropriateness, reduced polypharmacy, and reduced adverse drug reactions.5 Although this study did not use a criteria-based approach for improving medication use, it demonstrated that in a population of older adults with polypharmacy, medication review with geriatricians can lead to improved HRQoL while improving medication appropriateness. The collaborative approach between the family physician and geriatrician, rather than a consultative approach with recommendations from a geriatrician, may have contributed to increased uptake of medication changes. Such an approach may be a reasonable strategy to improve medication use in older adults.
A limitation of the study is that the improvement in HRQoL could have been the result of medication changes, but could also have been due to other changes in the plan of care that resulted from the geriatric assessment. As noted by the authors, the increase in hospital admissions, though not statistically significant, could have resulted from the medication modifications; however, it was also noted that the geriatric assessments could have identified severe illnesses that required hospitalization, as the timeline from geriatric assessment to hospitalization suggested was the case. Thus, the increase in hospitalization resulting from timely identification of severe illness was more likely a benefit than an adverse effect; however, further studies should be done to elucidate this.
Applications for Clinical Practice
Older adults with multiple chronic conditions and complex medication regimens are at risk for poor health outcomes, and a purposeful medication review to improve medication use, leading to the removal of unnecessary and potentially harmful medications, adjustment of dosages, and initiation of appropriate medications, may yield health benefits, such as improved HRQoL. The present study utilized an approach that could be scalable, which is important given the limited number of clinicians with geriatrics expertise. For health systems with geriatrics clinical expertise, it may be reasonable to consider adopting a similar collaborative approach in order to improve care for older adults most at risk. Further reports on how patients and family physicians perceive this intervention will enhance our understanding of whether it could be implemented widely.
–William W. Hung, MD, MPH
Study Overview
Objective. To examine the effect of clinical geriatric assessments and collaborative medication review by geriatricians and family physicians on quality of life and other patient outcomes in home-dwelling older adults with polypharmacy.
Design. The study was a single-blind, cluster randomized clinical trial enrolling home-dwelling adults aged 70 years and older who were taking 7 or more medications. Family physicians in Norway were recruited to participate in the trial with their patients. Randomization was at the family physician level to avoid contamination between intervention and control groups.
Setting and participants. The study was conducted in Akershus and Oslo, Norway. Family physicians were recruited to participate in the trial with their patients. A total of 84 family physicians were recruited, of which 70 were included in the trial and randomized to intervention versus control; 14 were excluded because they had no eligible patients. The cluster size of each family physician was limited to 5 patients per physician to avoid large variation in cluster sizes. Patients were eligible for enrollment if they were home-dwelling, aged 70 years or older, and were taking 7 or more systemic medications regularly and had medications administered by the home nursing service. Patients were excluded if they were expected to die or be institutionalized within 6 months, or if they were discouraged from participation by their family physician. A total of 174 patients were recruited, with 87 patients in each group (34 family physicians were in the control group and 36 in the intervention group).
Intervention. The intervention included a geriatric assessment performed by a physician trained in geriatric medicine and supervised by a senior consultant. The geriatric assessment consisted of review of medical history; systematic screening for current problems; clinical examination; supplementary tests, if indicated; and review of each medication being used. The review of medication included the indication for each medication, dosage, adverse effects, and interactions. The geriatric assessment consultation took 1 hour to complete, on average. After the geriatric assessment, the family physician and the geriatrician met to discuss each medication and to establish a collaborative plan for adjustments and follow-up; this meeting was approximately 15 minutes in duration. Lastly, clinical follow-up with the older adult was conducted by the geriatrician or the family physician, as agreed upon in the plan, with most follow-up conducted by the family physician. Participants randomized to the control group received usual care without any intervention.
Main outcome measures. Outcomes were assessed at 16-week and 24-week follow-up. The main study outcome measure was health-related quality of life (HRQoL), as measured by the 15D instrument, at 16 weeks. The quality-of-life measure included the following aspects, each rated on an ordinal scale of 5 levels: mobility, vision, hearing, breathing, sleeping, eating, speech, elimination, usual activities, mental function, discomfort or symptoms, depression, distress, vitality, and sexual activity. The index scale including all aspects is in the range of 0 to 1, with a higher score indicating better quality of life. A predetermined change of 0.015 or more is considered clinically important, and a positive change of 0.035 indicates much better HRQoL. Other outcomes included: appropriateness of medications measured by the Medication Appropriateness Index and the Assessment of Underutilization; physical function (short Physical Performance battery); gait speed; grip strength; cognitive functioning; physical and cognitive disability (Functional Independence Measure); caregiver burden (Relative Stress Scale); physical measures, including orthostatic blood pressure, falls, and weight; hospital admissions; use of home nursing service; incidence of institutionalization; and mortality.
Main results. The study included 174 patients with an average age of 83.3 years (SD, 7.3); 67.8% were women. Of those who were randomized to the intervention and control groups, 158 (90.8%) completed the trial. The average number of regularly used medications was 10.1 (SD, 2.7) in the intervention group and 9.5 (SD, 2.6) in the control group. At week 16 of follow-up, patients in the intervention group had an improved HRQoL score measured by the 15D instrument; the difference between the intervention group and control groups was 0.045 (95% confidence interval [CI], 0.004 -0.086; P = 0.03). Medication appropriateness was better in the intervention group, as compared with the control group at both 16 weeks and 24 weeks. Nearly all (99%) patients in the intervention group experienced medication changes, which included withdrawal of medications, dosage adjustment, or new drug regimens. There was a trend towards a higher rate of hospitalization during follow-up in the intervention group (adjusted risk ratio, 2.03; 95% CI, 0.98-4.24; P = 0.06). Other secondary outcomes were not substantially different between the intervention and control groups.
Conclusion. The study demonstrated that a clinical geriatric assessment and collaborative medication review by geriatrician and family physician led to improved HRQoL and improved medication use.
Commentary
The use of multiple medications in older adults is common, with almost 20% of older adults over age 65 taking 10 or more medications.1 Polypharmacy in older adults is associated with lower adherence rates and increases the potential for interactions between medications.2 Age-related changes, such as changes in absorption, metabolism, and excretion, affect pharmacokinetics of medications and potentiate adverse drug reactions, requiring adjustments in use and dosing to optimize safety and outcomes. Recognizing the potential effects of medications in older adults, evidence-based guidelines, such as the Beers criteria3 and START/STOPP criteria,4 have been developed to identify potentially inappropriate medications in older adults and to improve prescribing. Randomized trials using the START/STOPP criteria have demonstrated improved medication appropriateness, reduced polypharmacy, and reduced adverse drug reactions.5 Although this study did not use a criteria-based approach for improving medication use, it demonstrated that in a population of older adults with polypharmacy, medication review with geriatricians can lead to improved HRQoL while improving medication appropriateness. The collaborative approach between the family physician and geriatrician, rather than a consultative approach with recommendations from a geriatrician, may have contributed to increased uptake of medication changes. Such an approach may be a reasonable strategy to improve medication use in older adults.
A limitation of the study is that the improvement in HRQoL could have been the result of medication changes, but could also have been due to other changes in the plan of care that resulted from the geriatric assessment. As noted by the authors, the increase in hospital admissions, though not statistically significant, could have resulted from the medication modifications; however, it was also noted that the geriatric assessments could have identified severe illnesses that required hospitalization, as the timeline from geriatric assessment to hospitalization suggested was the case. Thus, the increase in hospitalization resulting from timely identification of severe illness was more likely a benefit than an adverse effect; however, further studies should be done to elucidate this.
Applications for Clinical Practice
Older adults with multiple chronic conditions and complex medication regimens are at risk for poor health outcomes, and a purposeful medication review to improve medication use, leading to the removal of unnecessary and potentially harmful medications, adjustment of dosages, and initiation of appropriate medications, may yield health benefits, such as improved HRQoL. The present study utilized an approach that could be scalable, which is important given the limited number of clinicians with geriatrics expertise. For health systems with geriatrics clinical expertise, it may be reasonable to consider adopting a similar collaborative approach in order to improve care for older adults most at risk. Further reports on how patients and family physicians perceive this intervention will enhance our understanding of whether it could be implemented widely.
–William W. Hung, MD, MPH
1. Steinman MA, Hanlon JT. Managing medications in clinically complex elders: “There’s got to be a happy medium”. JAMA. 2010;304:1592-1601.
2. Vik SA, Maxwell CJ, Hogan DB. Measurement, correlates, and health outcomes of medication adherence among seniors. Ann Pharmacother. 2004;38:303-312.
3. American Geriatrics Society 2015 Updated Beers criteria for potentially inappropriate medication use in older Adults. J Am Geriatr Soc. 2015;63:2227-2246.
4. Hill-Taylor B, Sketris I, Hayden J, et al. Application of the STOPP/START criteria: a systematic review of the prevalence of potentially inappropriate prescribing in older adults, and evidence of clinical, humanistic and economic impact. J Clin Pharm Ther. 2013;38:360-372.
5. O’Mahony D. STOPP/START criteria for potentially inappropriate medications/ potential prescribing omissions in older people: origin and progress. Expert Rev Clin Pharmacol. 2020;13:15-22.
1. Steinman MA, Hanlon JT. Managing medications in clinically complex elders: “There’s got to be a happy medium”. JAMA. 2010;304:1592-1601.
2. Vik SA, Maxwell CJ, Hogan DB. Measurement, correlates, and health outcomes of medication adherence among seniors. Ann Pharmacother. 2004;38:303-312.
3. American Geriatrics Society 2015 Updated Beers criteria for potentially inappropriate medication use in older Adults. J Am Geriatr Soc. 2015;63:2227-2246.
4. Hill-Taylor B, Sketris I, Hayden J, et al. Application of the STOPP/START criteria: a systematic review of the prevalence of potentially inappropriate prescribing in older adults, and evidence of clinical, humanistic and economic impact. J Clin Pharm Ther. 2013;38:360-372.
5. O’Mahony D. STOPP/START criteria for potentially inappropriate medications/ potential prescribing omissions in older people: origin and progress. Expert Rev Clin Pharmacol. 2020;13:15-22.
Targeting gut bacteria may improve levodopa uptake
Differences in metabolism of levodopa between patients with Parkinson’s disease may be caused by variations in gut bacteria, according to investigators.
Specifically, patients with a higher abundance of Enterococcus faecalis may be converting levodopa into dopamine via decarboxylation before it can cross the blood-brain barrier, reported Emily P. Balskus, PhD, of Harvard University in Cambridge, Mass.
Although existing decarboxylase inhibitors, such as carbidopa, can reduce metabolism of levodopa by host enzymes, these drugs are unable to inhibit the enzymatic activity of E. faecalis in the gut, Dr. Balskus said at the annual Gut Microbiota for Health World Summit, sponsored by the American Gastroenterological Association and the European Society for Neurogastroenterology and Motility.
“[Carbidopa] is actually completely ineffective at inhibiting decarboxylation in human fecal suspension,” Dr. Balskus said, referring to research led by PhD student Vayu Maini Rekdal. “We think that this could indicate that patients who are taking carbidopa are not inhibiting any bacterial metabolism that they may have.”
While previous research showed that E. faecalis could decarboxylate levodopa, Dr. Balskus and colleagues linked this process with the tyrosine decarboxylase gene (TyrDC), and showed that the of abundance E. faecalis and TyrDC correlate with levodopa metabolism.
Unlike the human enzyme responsible for decarboxylation of levodopa, the E. faecalis enzyme preferentially binds with L-tyrosine. This could explain why existing decarboxylase inhibitors have little impact on decarboxylation in the gut, Dr. Balskus said.
She also noted that this unique characteristic may open doors to new therapeutics. In the lab, Dr. Balskus and colleagues tested a decarboxylase inhibitor that resembled L-tyrosine, (S)-alpha-fluoromethyltyrosine (AFMT). Indeed, AFMT completely inhibited of decarboxylation of levodopa in both E. faecalis cells and complex human microbiome samples.
“We think this is pretty exciting,” Dr. Balskus said.
Early animal studies support this enthusiasm, as germ-free mice colonized with E. faecalis maintain higher serum levels of levodopa with concurrent administration of AFMT.
“We think that this could indicate that a promising way to improve levodopa therapy for Parkinson’s patients would be to develop compounds that inhibit bacterial drug metabolism activity,” Dr. Balskus said.
Concluding her presentation, Dr. Balskus emphasized the importance of a biochemical approach to microbiome research. “Studying enzymes opens up new, exciting opportunities for microbiome manipulation. We can design or develop inhibitors of enzymes, use those inhibitors as tools to study the roles of individual metabolic activities, and potentially use them as therapeutics. We are very excited about the possibility of treating or preventing human disease not just by manipulating processes in our own cells, but by targeting activities in the microbiota.”
Dr. Balskus reported funding from HHMI, the Bill and Melinda Gates Foundation, the David and Lucile Packard Foundation, and Merck.
Differences in metabolism of levodopa between patients with Parkinson’s disease may be caused by variations in gut bacteria, according to investigators.
Specifically, patients with a higher abundance of Enterococcus faecalis may be converting levodopa into dopamine via decarboxylation before it can cross the blood-brain barrier, reported Emily P. Balskus, PhD, of Harvard University in Cambridge, Mass.
Although existing decarboxylase inhibitors, such as carbidopa, can reduce metabolism of levodopa by host enzymes, these drugs are unable to inhibit the enzymatic activity of E. faecalis in the gut, Dr. Balskus said at the annual Gut Microbiota for Health World Summit, sponsored by the American Gastroenterological Association and the European Society for Neurogastroenterology and Motility.
“[Carbidopa] is actually completely ineffective at inhibiting decarboxylation in human fecal suspension,” Dr. Balskus said, referring to research led by PhD student Vayu Maini Rekdal. “We think that this could indicate that patients who are taking carbidopa are not inhibiting any bacterial metabolism that they may have.”
While previous research showed that E. faecalis could decarboxylate levodopa, Dr. Balskus and colleagues linked this process with the tyrosine decarboxylase gene (TyrDC), and showed that the of abundance E. faecalis and TyrDC correlate with levodopa metabolism.
Unlike the human enzyme responsible for decarboxylation of levodopa, the E. faecalis enzyme preferentially binds with L-tyrosine. This could explain why existing decarboxylase inhibitors have little impact on decarboxylation in the gut, Dr. Balskus said.
She also noted that this unique characteristic may open doors to new therapeutics. In the lab, Dr. Balskus and colleagues tested a decarboxylase inhibitor that resembled L-tyrosine, (S)-alpha-fluoromethyltyrosine (AFMT). Indeed, AFMT completely inhibited of decarboxylation of levodopa in both E. faecalis cells and complex human microbiome samples.
“We think this is pretty exciting,” Dr. Balskus said.
Early animal studies support this enthusiasm, as germ-free mice colonized with E. faecalis maintain higher serum levels of levodopa with concurrent administration of AFMT.
“We think that this could indicate that a promising way to improve levodopa therapy for Parkinson’s patients would be to develop compounds that inhibit bacterial drug metabolism activity,” Dr. Balskus said.
Concluding her presentation, Dr. Balskus emphasized the importance of a biochemical approach to microbiome research. “Studying enzymes opens up new, exciting opportunities for microbiome manipulation. We can design or develop inhibitors of enzymes, use those inhibitors as tools to study the roles of individual metabolic activities, and potentially use them as therapeutics. We are very excited about the possibility of treating or preventing human disease not just by manipulating processes in our own cells, but by targeting activities in the microbiota.”
Dr. Balskus reported funding from HHMI, the Bill and Melinda Gates Foundation, the David and Lucile Packard Foundation, and Merck.
Differences in metabolism of levodopa between patients with Parkinson’s disease may be caused by variations in gut bacteria, according to investigators.
Specifically, patients with a higher abundance of Enterococcus faecalis may be converting levodopa into dopamine via decarboxylation before it can cross the blood-brain barrier, reported Emily P. Balskus, PhD, of Harvard University in Cambridge, Mass.
Although existing decarboxylase inhibitors, such as carbidopa, can reduce metabolism of levodopa by host enzymes, these drugs are unable to inhibit the enzymatic activity of E. faecalis in the gut, Dr. Balskus said at the annual Gut Microbiota for Health World Summit, sponsored by the American Gastroenterological Association and the European Society for Neurogastroenterology and Motility.
“[Carbidopa] is actually completely ineffective at inhibiting decarboxylation in human fecal suspension,” Dr. Balskus said, referring to research led by PhD student Vayu Maini Rekdal. “We think that this could indicate that patients who are taking carbidopa are not inhibiting any bacterial metabolism that they may have.”
While previous research showed that E. faecalis could decarboxylate levodopa, Dr. Balskus and colleagues linked this process with the tyrosine decarboxylase gene (TyrDC), and showed that the of abundance E. faecalis and TyrDC correlate with levodopa metabolism.
Unlike the human enzyme responsible for decarboxylation of levodopa, the E. faecalis enzyme preferentially binds with L-tyrosine. This could explain why existing decarboxylase inhibitors have little impact on decarboxylation in the gut, Dr. Balskus said.
She also noted that this unique characteristic may open doors to new therapeutics. In the lab, Dr. Balskus and colleagues tested a decarboxylase inhibitor that resembled L-tyrosine, (S)-alpha-fluoromethyltyrosine (AFMT). Indeed, AFMT completely inhibited of decarboxylation of levodopa in both E. faecalis cells and complex human microbiome samples.
“We think this is pretty exciting,” Dr. Balskus said.
Early animal studies support this enthusiasm, as germ-free mice colonized with E. faecalis maintain higher serum levels of levodopa with concurrent administration of AFMT.
“We think that this could indicate that a promising way to improve levodopa therapy for Parkinson’s patients would be to develop compounds that inhibit bacterial drug metabolism activity,” Dr. Balskus said.
Concluding her presentation, Dr. Balskus emphasized the importance of a biochemical approach to microbiome research. “Studying enzymes opens up new, exciting opportunities for microbiome manipulation. We can design or develop inhibitors of enzymes, use those inhibitors as tools to study the roles of individual metabolic activities, and potentially use them as therapeutics. We are very excited about the possibility of treating or preventing human disease not just by manipulating processes in our own cells, but by targeting activities in the microbiota.”
Dr. Balskus reported funding from HHMI, the Bill and Melinda Gates Foundation, the David and Lucile Packard Foundation, and Merck.
FROM GMFH 2020
Elderly Americans carry heavier opioid burden
according to the Agency for Healthcare Quality and Research.
Elderly adults with chronic and acute pain obtained an average of 774 morphine milligram equivalents (MMEs) of prescription opioids annually during 2015-2016 from outpatient clinicians, compared with 376 MMEs a year for nonelderly adults, said Asako S. Moriya, PhD, and G. Edward Miller, PhD, of the AHRQ.
Narrowing the age groups shows that opioid MMEs increased with age, starting at 49 MMEs for 18- to 26-year-olds and rising to a high of 856 MMEs in the 65- to 74-year-old group, before dropping off in the oldest adults, the investigators said in a Medical Expenditure Panel Survey (MEPS) research findings report.
The analysis included “all opioid medications that are commonly used to treat pain” and excluded respiratory agents, antitussives, and drugs used for medication-assisted treatment, they noted. The MEPS data cover prescriptions purchased or obtained in outpatient settings but not those administered in inpatient settings or in clinics or physician offices.
according to the Agency for Healthcare Quality and Research.

Elderly adults with chronic and acute pain obtained an average of 774 morphine milligram equivalents (MMEs) of prescription opioids annually during 2015-2016 from outpatient clinicians, compared with 376 MMEs a year for nonelderly adults, said Asako S. Moriya, PhD, and G. Edward Miller, PhD, of the AHRQ.
Narrowing the age groups shows that opioid MMEs increased with age, starting at 49 MMEs for 18- to 26-year-olds and rising to a high of 856 MMEs in the 65- to 74-year-old group, before dropping off in the oldest adults, the investigators said in a Medical Expenditure Panel Survey (MEPS) research findings report.
The analysis included “all opioid medications that are commonly used to treat pain” and excluded respiratory agents, antitussives, and drugs used for medication-assisted treatment, they noted. The MEPS data cover prescriptions purchased or obtained in outpatient settings but not those administered in inpatient settings or in clinics or physician offices.
according to the Agency for Healthcare Quality and Research.

Elderly adults with chronic and acute pain obtained an average of 774 morphine milligram equivalents (MMEs) of prescription opioids annually during 2015-2016 from outpatient clinicians, compared with 376 MMEs a year for nonelderly adults, said Asako S. Moriya, PhD, and G. Edward Miller, PhD, of the AHRQ.
Narrowing the age groups shows that opioid MMEs increased with age, starting at 49 MMEs for 18- to 26-year-olds and rising to a high of 856 MMEs in the 65- to 74-year-old group, before dropping off in the oldest adults, the investigators said in a Medical Expenditure Panel Survey (MEPS) research findings report.
The analysis included “all opioid medications that are commonly used to treat pain” and excluded respiratory agents, antitussives, and drugs used for medication-assisted treatment, they noted. The MEPS data cover prescriptions purchased or obtained in outpatient settings but not those administered in inpatient settings or in clinics or physician offices.
Screen asymptomatic older adults for cognitive impairment? Not so fast
Reference
1. US Preventive Services Task Force. Final recommendation statement: cognitive impairment in older adults: screening. www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cognitive-impairment-in-older-adults-screening. Published February 2020. Accessed March 19, 2020.
Reference
1. US Preventive Services Task Force. Final recommendation statement: cognitive impairment in older adults: screening. www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cognitive-impairment-in-older-adults-screening. Published February 2020. Accessed March 19, 2020.
Reference
1. US Preventive Services Task Force. Final recommendation statement: cognitive impairment in older adults: screening. www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cognitive-impairment-in-older-adults-screening. Published February 2020. Accessed March 19, 2020.
Review highlights shortage of data on elderly cancer patients
Phase 3 clinical trials for cancer are underreporting safety and efficacy data for elderly patients, according to a systematic review of 159 articles.
Roughly 40% of articles reporting efficacy data and 9% of articles reporting safety data had results stratified by age, Karlynn BrintzenhofeSzoc, PhD, of the University of Cincinnati, and colleagues noted in the Journal of Geriatric Oncology.
“Results of our systematic review suggest that there is inadequate reporting of treatment efficacy and adverse events as well as discrepancies as to how older age is defined, considered, and reported,” the investigators wrote. “This sparse and varied reporting critically limits the evidence base for treating older patients with cancer.”
This study was inspired by the American Society of Clinical Oncology, which turned a spotlight on the age-specific data shortage in 2015, when it published a statement that called for inclusion of more elderly patients in cancer trials (J Clin Oncol. 2015 Nov 10;33[32]:3826-33).
According to Tammy Hshieh, MD, a geriatrician at Dana-Farber Cancer Institute in Boston, data for elderly patients with cancer are needed more than ever.
“Cancer care has become, increasingly, a field where precision medicine is at its strongest,” Dr. Hshieh said in an interview. “[Oncologists] have a lot of data on patients that allow them to tailor their care to each individual patient’s profile, and so the fact that there is not a lot of evidence looking at toxicities and side effects for older patients makes it basically harder for oncologists to practice evidence-based medicine for this vulnerable but growing population.” This leads to poorer and more variable outcomes, Dr. Hshieh said. When data aren’t available, clinicians must rely on experience and recognize that patient age isn’t as simple as date of birth.
“Oncologists looking at older patients really have to trust their gestalt and their experience in determining how to provide the best care for their older patients,” she said. “They have to look at the chronological age of the patient and try to determine whether that actually matches more of what we’re saying is the physiological age of the patient and use that to guide their treatment.”
Study details
The study included phase 3 clinical trials of adult cancer patients that were conducted from mid-2016 through mid-2017. After identifying 929 manuscripts, the investigators removed duplicates and those that did not meet criteria. This left 159 articles published in 36 journals and covering 25 cancer types.
Of the 159 articles, 73.6% included age-specific medians (in addition to age means), and 47.2% had data stratified by age.
Efficacy was often reported (96.2%), but only 39.9% of articles specified age when describing effectiveness. Although most articles (84.9%) included safety data, only 8.9% had safety findings stratified by age.
In article discussion sections, age was mentioned infrequently in relation to treatment efficacy (13.8%) and rarely in relation to complications and adverse events (5.7%).Beyond underreporting of age-specific data, the investigators found that age categories themselves may be an area in need of improvement.“When outcomes pertaining to older adults were reported, the results were inconsistent as evidenced by the array of age distributions and varying categorization of ‘older adults,’” the investigators wrote. “There is a significant and timely need to design all clinical trials to include older adults and utilize a broad array of geriatric-specific outcomes.” Dr. Hshieh said these findings are concerning, but the study itself suggests the medical community is making efforts to correct the data shortage.“It was actually an important study, even though the results are a little discouraging,” Dr. Hshieh said. “What I’m hoping is that [these findings], combined with all the other literature that’s starting to come out about the need for more research in older patients with cancer, is going to be an impetus for us to do more research, and to be more open to treating older patients, and not to be afraid to confront this head on.”When asked about strategies for managing elderly patients, Dr. Hshieh first recommended the 2018 ASCO Guideline for Geriatric Oncology (J Clin Oncol. 2018 Aug 1;36[22]:2326-47).
“It’s very well written,” she said. “It is clear and user-friendly.”
Dr. Hshieh also offered some simple principles that may help guide clinical decision making.“I’m thinking of three things that an oncologist in the community would want to look at when they see an older patient and they’re trying to determine their treatment plan,” she said. “I would say [the oncologist] should look at [the patient’s] function; their psychosocial status, which includes mood and the support that they have in the community; and cognition.”
Dr. Hshieh and the study authors reported no conflicts of interest.
SOURCE: BrintzenhofeSzoc K et al. J Geriatr Oncol. 2020 Jan 10. pii: S1879-4068(19)30501-6.
Phase 3 clinical trials for cancer are underreporting safety and efficacy data for elderly patients, according to a systematic review of 159 articles.
Roughly 40% of articles reporting efficacy data and 9% of articles reporting safety data had results stratified by age, Karlynn BrintzenhofeSzoc, PhD, of the University of Cincinnati, and colleagues noted in the Journal of Geriatric Oncology.
“Results of our systematic review suggest that there is inadequate reporting of treatment efficacy and adverse events as well as discrepancies as to how older age is defined, considered, and reported,” the investigators wrote. “This sparse and varied reporting critically limits the evidence base for treating older patients with cancer.”
This study was inspired by the American Society of Clinical Oncology, which turned a spotlight on the age-specific data shortage in 2015, when it published a statement that called for inclusion of more elderly patients in cancer trials (J Clin Oncol. 2015 Nov 10;33[32]:3826-33).
According to Tammy Hshieh, MD, a geriatrician at Dana-Farber Cancer Institute in Boston, data for elderly patients with cancer are needed more than ever.
“Cancer care has become, increasingly, a field where precision medicine is at its strongest,” Dr. Hshieh said in an interview. “[Oncologists] have a lot of data on patients that allow them to tailor their care to each individual patient’s profile, and so the fact that there is not a lot of evidence looking at toxicities and side effects for older patients makes it basically harder for oncologists to practice evidence-based medicine for this vulnerable but growing population.” This leads to poorer and more variable outcomes, Dr. Hshieh said. When data aren’t available, clinicians must rely on experience and recognize that patient age isn’t as simple as date of birth.
“Oncologists looking at older patients really have to trust their gestalt and their experience in determining how to provide the best care for their older patients,” she said. “They have to look at the chronological age of the patient and try to determine whether that actually matches more of what we’re saying is the physiological age of the patient and use that to guide their treatment.”
Study details
The study included phase 3 clinical trials of adult cancer patients that were conducted from mid-2016 through mid-2017. After identifying 929 manuscripts, the investigators removed duplicates and those that did not meet criteria. This left 159 articles published in 36 journals and covering 25 cancer types.
Of the 159 articles, 73.6% included age-specific medians (in addition to age means), and 47.2% had data stratified by age.
Efficacy was often reported (96.2%), but only 39.9% of articles specified age when describing effectiveness. Although most articles (84.9%) included safety data, only 8.9% had safety findings stratified by age.
In article discussion sections, age was mentioned infrequently in relation to treatment efficacy (13.8%) and rarely in relation to complications and adverse events (5.7%).Beyond underreporting of age-specific data, the investigators found that age categories themselves may be an area in need of improvement.“When outcomes pertaining to older adults were reported, the results were inconsistent as evidenced by the array of age distributions and varying categorization of ‘older adults,’” the investigators wrote. “There is a significant and timely need to design all clinical trials to include older adults and utilize a broad array of geriatric-specific outcomes.” Dr. Hshieh said these findings are concerning, but the study itself suggests the medical community is making efforts to correct the data shortage.“It was actually an important study, even though the results are a little discouraging,” Dr. Hshieh said. “What I’m hoping is that [these findings], combined with all the other literature that’s starting to come out about the need for more research in older patients with cancer, is going to be an impetus for us to do more research, and to be more open to treating older patients, and not to be afraid to confront this head on.”When asked about strategies for managing elderly patients, Dr. Hshieh first recommended the 2018 ASCO Guideline for Geriatric Oncology (J Clin Oncol. 2018 Aug 1;36[22]:2326-47).
“It’s very well written,” she said. “It is clear and user-friendly.”
Dr. Hshieh also offered some simple principles that may help guide clinical decision making.“I’m thinking of three things that an oncologist in the community would want to look at when they see an older patient and they’re trying to determine their treatment plan,” she said. “I would say [the oncologist] should look at [the patient’s] function; their psychosocial status, which includes mood and the support that they have in the community; and cognition.”
Dr. Hshieh and the study authors reported no conflicts of interest.
SOURCE: BrintzenhofeSzoc K et al. J Geriatr Oncol. 2020 Jan 10. pii: S1879-4068(19)30501-6.
Phase 3 clinical trials for cancer are underreporting safety and efficacy data for elderly patients, according to a systematic review of 159 articles.
Roughly 40% of articles reporting efficacy data and 9% of articles reporting safety data had results stratified by age, Karlynn BrintzenhofeSzoc, PhD, of the University of Cincinnati, and colleagues noted in the Journal of Geriatric Oncology.
“Results of our systematic review suggest that there is inadequate reporting of treatment efficacy and adverse events as well as discrepancies as to how older age is defined, considered, and reported,” the investigators wrote. “This sparse and varied reporting critically limits the evidence base for treating older patients with cancer.”
This study was inspired by the American Society of Clinical Oncology, which turned a spotlight on the age-specific data shortage in 2015, when it published a statement that called for inclusion of more elderly patients in cancer trials (J Clin Oncol. 2015 Nov 10;33[32]:3826-33).
According to Tammy Hshieh, MD, a geriatrician at Dana-Farber Cancer Institute in Boston, data for elderly patients with cancer are needed more than ever.
“Cancer care has become, increasingly, a field where precision medicine is at its strongest,” Dr. Hshieh said in an interview. “[Oncologists] have a lot of data on patients that allow them to tailor their care to each individual patient’s profile, and so the fact that there is not a lot of evidence looking at toxicities and side effects for older patients makes it basically harder for oncologists to practice evidence-based medicine for this vulnerable but growing population.” This leads to poorer and more variable outcomes, Dr. Hshieh said. When data aren’t available, clinicians must rely on experience and recognize that patient age isn’t as simple as date of birth.
“Oncologists looking at older patients really have to trust their gestalt and their experience in determining how to provide the best care for their older patients,” she said. “They have to look at the chronological age of the patient and try to determine whether that actually matches more of what we’re saying is the physiological age of the patient and use that to guide their treatment.”
Study details
The study included phase 3 clinical trials of adult cancer patients that were conducted from mid-2016 through mid-2017. After identifying 929 manuscripts, the investigators removed duplicates and those that did not meet criteria. This left 159 articles published in 36 journals and covering 25 cancer types.
Of the 159 articles, 73.6% included age-specific medians (in addition to age means), and 47.2% had data stratified by age.
Efficacy was often reported (96.2%), but only 39.9% of articles specified age when describing effectiveness. Although most articles (84.9%) included safety data, only 8.9% had safety findings stratified by age.
In article discussion sections, age was mentioned infrequently in relation to treatment efficacy (13.8%) and rarely in relation to complications and adverse events (5.7%).Beyond underreporting of age-specific data, the investigators found that age categories themselves may be an area in need of improvement.“When outcomes pertaining to older adults were reported, the results were inconsistent as evidenced by the array of age distributions and varying categorization of ‘older adults,’” the investigators wrote. “There is a significant and timely need to design all clinical trials to include older adults and utilize a broad array of geriatric-specific outcomes.” Dr. Hshieh said these findings are concerning, but the study itself suggests the medical community is making efforts to correct the data shortage.“It was actually an important study, even though the results are a little discouraging,” Dr. Hshieh said. “What I’m hoping is that [these findings], combined with all the other literature that’s starting to come out about the need for more research in older patients with cancer, is going to be an impetus for us to do more research, and to be more open to treating older patients, and not to be afraid to confront this head on.”When asked about strategies for managing elderly patients, Dr. Hshieh first recommended the 2018 ASCO Guideline for Geriatric Oncology (J Clin Oncol. 2018 Aug 1;36[22]:2326-47).
“It’s very well written,” she said. “It is clear and user-friendly.”
Dr. Hshieh also offered some simple principles that may help guide clinical decision making.“I’m thinking of three things that an oncologist in the community would want to look at when they see an older patient and they’re trying to determine their treatment plan,” she said. “I would say [the oncologist] should look at [the patient’s] function; their psychosocial status, which includes mood and the support that they have in the community; and cognition.”
Dr. Hshieh and the study authors reported no conflicts of interest.
SOURCE: BrintzenhofeSzoc K et al. J Geriatr Oncol. 2020 Jan 10. pii: S1879-4068(19)30501-6.
FROM JOURNAL OF GERIATRIC ONCOLOGY
DMT use is common in older patients with MS
WEST PALM BEACH, FLA. –
MS disease activity typically declines with age. At the same time, evidence to support the efficacy of MS drugs in older patients is limited, said Yinan Zhang, MD, a researcher at Icahn School of Medicine at Mount Sinai, New York. Clinical trials have tended to enroll younger patients and to include only patients with active disease, which is not representative of most older patients in the real world, Dr. Zhang said.
“DMTs for MS may be less efficacious in the elderly, especially in the absence of active disease, yet real-world prescribing patterns still show widespread use of DMTs in older patients,” Dr. Zhang and colleagues said. Physicians may be able to use the presence of disease activity to identify older patients who should receive therapy. “Continuing DMTs in elderly patients who have no evidence of disease activity should be questioned rather than accepted,” they said at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
To investigate whether age affects the efficacy of DMTs in patients with relapsing-remitting MS and how often DMTs are used in different age groups, Dr. Zhang and coinvestigators conducted a meta-analysis of group-level data from clinical trial, analyzed individual-level data from one of the trials, and reviewed survey data from two registries.
The meta-analysis included 26 clinical trials of 13 DMTs with more than 12,400 patients. Participants had an average age of about 37 years. “An age-dependent relationship of DMTs on relapse rate in RRMS [relapsing-remitting MS] cannot be established with currently published aggregate summary data,” the researchers said. “The meta-analysis was limited by the use of group-level data resulting in a narrow range of mean age.”
In an effort to overcome the limitations of group-level data, they analyzed individual-level data from approximately 1,000 patients in the CombiRx trial, which compared interferon beta-1a plus glatiramer acetate versus the agents alone. Thirty-seven of the patients were aged 55 years or older. The results suggest that each “1-year increase in baseline age was associated with a 3.2% reduction in the odds of having a relapse” during the trial, the investigators said. Change in annualized relapse rate was not significantly associated with age group, which may have resulted from “enrollment criteria selecting for patients with active disease, where DMTs are expected to show the greatest efficacy,” the researchers said.
Finally, Dr. Zhang and colleagues reviewed data on DMT use by age group from the North American Research Committee on Multiple Sclerosis (NARCOMS) and the Multiple Sclerosis Surveillance Registry (MSSR) from Veterans Affairs. In a 2018 survey of nearly 7,000 patients in the NARCOMS registry, 39.2% of patients older than 60 years were taking a DMT, including 44.5% of patients aged 61-70, 28.6% of patients aged 71-80, and 11% of patients aged 81 years and older. In comparison, about 62% of patients aged 41-50 years were taking DMT.
A 2019 survey of about 1,700 veterans in the MSSR found that 36.3% of patients older than 60 years were taking a DMT, including 41.1% of patients aged 61-70, 27.2% of patients aged 71-80, and 7.1% of patients aged 81 years and older. Among patients aged 41-50 years, more than 72% were taking a DMT. “The continued use of DMTs in the elderly may be the result of the perceived notion that disease inactivity is due to the effect of DMTs rather than the natural disease course with aging,” they said.
Dr. Zhang had no relevant disclosures. Coauthors disclosed consulting for and grant support from various pharmaceutical companies.
SOURCE: Zhang Y et al. ACTRIMS Forum 2020. Abstract P263.
WEST PALM BEACH, FLA. –
MS disease activity typically declines with age. At the same time, evidence to support the efficacy of MS drugs in older patients is limited, said Yinan Zhang, MD, a researcher at Icahn School of Medicine at Mount Sinai, New York. Clinical trials have tended to enroll younger patients and to include only patients with active disease, which is not representative of most older patients in the real world, Dr. Zhang said.
“DMTs for MS may be less efficacious in the elderly, especially in the absence of active disease, yet real-world prescribing patterns still show widespread use of DMTs in older patients,” Dr. Zhang and colleagues said. Physicians may be able to use the presence of disease activity to identify older patients who should receive therapy. “Continuing DMTs in elderly patients who have no evidence of disease activity should be questioned rather than accepted,” they said at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
To investigate whether age affects the efficacy of DMTs in patients with relapsing-remitting MS and how often DMTs are used in different age groups, Dr. Zhang and coinvestigators conducted a meta-analysis of group-level data from clinical trial, analyzed individual-level data from one of the trials, and reviewed survey data from two registries.
The meta-analysis included 26 clinical trials of 13 DMTs with more than 12,400 patients. Participants had an average age of about 37 years. “An age-dependent relationship of DMTs on relapse rate in RRMS [relapsing-remitting MS] cannot be established with currently published aggregate summary data,” the researchers said. “The meta-analysis was limited by the use of group-level data resulting in a narrow range of mean age.”
In an effort to overcome the limitations of group-level data, they analyzed individual-level data from approximately 1,000 patients in the CombiRx trial, which compared interferon beta-1a plus glatiramer acetate versus the agents alone. Thirty-seven of the patients were aged 55 years or older. The results suggest that each “1-year increase in baseline age was associated with a 3.2% reduction in the odds of having a relapse” during the trial, the investigators said. Change in annualized relapse rate was not significantly associated with age group, which may have resulted from “enrollment criteria selecting for patients with active disease, where DMTs are expected to show the greatest efficacy,” the researchers said.
Finally, Dr. Zhang and colleagues reviewed data on DMT use by age group from the North American Research Committee on Multiple Sclerosis (NARCOMS) and the Multiple Sclerosis Surveillance Registry (MSSR) from Veterans Affairs. In a 2018 survey of nearly 7,000 patients in the NARCOMS registry, 39.2% of patients older than 60 years were taking a DMT, including 44.5% of patients aged 61-70, 28.6% of patients aged 71-80, and 11% of patients aged 81 years and older. In comparison, about 62% of patients aged 41-50 years were taking DMT.
A 2019 survey of about 1,700 veterans in the MSSR found that 36.3% of patients older than 60 years were taking a DMT, including 41.1% of patients aged 61-70, 27.2% of patients aged 71-80, and 7.1% of patients aged 81 years and older. Among patients aged 41-50 years, more than 72% were taking a DMT. “The continued use of DMTs in the elderly may be the result of the perceived notion that disease inactivity is due to the effect of DMTs rather than the natural disease course with aging,” they said.
Dr. Zhang had no relevant disclosures. Coauthors disclosed consulting for and grant support from various pharmaceutical companies.
SOURCE: Zhang Y et al. ACTRIMS Forum 2020. Abstract P263.
WEST PALM BEACH, FLA. –
MS disease activity typically declines with age. At the same time, evidence to support the efficacy of MS drugs in older patients is limited, said Yinan Zhang, MD, a researcher at Icahn School of Medicine at Mount Sinai, New York. Clinical trials have tended to enroll younger patients and to include only patients with active disease, which is not representative of most older patients in the real world, Dr. Zhang said.
“DMTs for MS may be less efficacious in the elderly, especially in the absence of active disease, yet real-world prescribing patterns still show widespread use of DMTs in older patients,” Dr. Zhang and colleagues said. Physicians may be able to use the presence of disease activity to identify older patients who should receive therapy. “Continuing DMTs in elderly patients who have no evidence of disease activity should be questioned rather than accepted,” they said at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
To investigate whether age affects the efficacy of DMTs in patients with relapsing-remitting MS and how often DMTs are used in different age groups, Dr. Zhang and coinvestigators conducted a meta-analysis of group-level data from clinical trial, analyzed individual-level data from one of the trials, and reviewed survey data from two registries.
The meta-analysis included 26 clinical trials of 13 DMTs with more than 12,400 patients. Participants had an average age of about 37 years. “An age-dependent relationship of DMTs on relapse rate in RRMS [relapsing-remitting MS] cannot be established with currently published aggregate summary data,” the researchers said. “The meta-analysis was limited by the use of group-level data resulting in a narrow range of mean age.”
In an effort to overcome the limitations of group-level data, they analyzed individual-level data from approximately 1,000 patients in the CombiRx trial, which compared interferon beta-1a plus glatiramer acetate versus the agents alone. Thirty-seven of the patients were aged 55 years or older. The results suggest that each “1-year increase in baseline age was associated with a 3.2% reduction in the odds of having a relapse” during the trial, the investigators said. Change in annualized relapse rate was not significantly associated with age group, which may have resulted from “enrollment criteria selecting for patients with active disease, where DMTs are expected to show the greatest efficacy,” the researchers said.
Finally, Dr. Zhang and colleagues reviewed data on DMT use by age group from the North American Research Committee on Multiple Sclerosis (NARCOMS) and the Multiple Sclerosis Surveillance Registry (MSSR) from Veterans Affairs. In a 2018 survey of nearly 7,000 patients in the NARCOMS registry, 39.2% of patients older than 60 years were taking a DMT, including 44.5% of patients aged 61-70, 28.6% of patients aged 71-80, and 11% of patients aged 81 years and older. In comparison, about 62% of patients aged 41-50 years were taking DMT.
A 2019 survey of about 1,700 veterans in the MSSR found that 36.3% of patients older than 60 years were taking a DMT, including 41.1% of patients aged 61-70, 27.2% of patients aged 71-80, and 7.1% of patients aged 81 years and older. Among patients aged 41-50 years, more than 72% were taking a DMT. “The continued use of DMTs in the elderly may be the result of the perceived notion that disease inactivity is due to the effect of DMTs rather than the natural disease course with aging,” they said.
Dr. Zhang had no relevant disclosures. Coauthors disclosed consulting for and grant support from various pharmaceutical companies.
SOURCE: Zhang Y et al. ACTRIMS Forum 2020. Abstract P263.
REPORTING FROM ACTRIMS FORUM 2020
Stress-related disorders linked to later neurodegenerative diseases
Individuals with posttraumatic stress disorder (PTSD), acute stress reaction, adjustment disorder, or other stress reactions had an 80% increased risk of vascular neurodegenerative diseases, according to results of the study, which was based on Swedish population registry data.
Risk of primary neurodegenerative diseases was increased as well in people with those conditions, but only by 31%, according to lead author Huan Song, MD, PhD, of Sichuan University in Chengdu, China.
“The stronger association observed for neurodegenerative diseases with a vascular component, compared with primary neurodegenerative diseases, suggested a considerable role of a possible cerebrovascular pathway,” Dr. Song and coauthors said in a report on the study appearing in JAMA Neurology.
While some previous studies have linked stress-related disorders to neurodegenerative diseases – particularly PTSD and dementia – this is believed to be the first, according to the investigators, to comprehensively evaluate all stress-related disorders in relation to the most common neurodegenerative conditions.
When considering neurodegenerative conditions separately, they found a statistically significant association between stress-related disorders and Alzheimer’s disease, while linkages with Parkinson’s disease and amyotrophic lateral sclerosis (ALS) were “comparable” but associations did not reach statistical significance, according to investigators.
Based on these findings, stress reduction should be recommended in addition to daily physical activity, mental activity, and a heart-healthy diet to potentially reduce risk of onset or worsening of cognitive decline, according to Chun Lim, MD, PhD, medical director of the cognitive neurology unit at Beth Israel Deaconess Medical Center in Boston.
“We don’t really have great evidence that anything slows down the progression of Alzheimer’s disease, but there are some suggestions that for people who lead heart-healthy lifestyles or adhere to a Mediterranean diet, fewer develop cognitive issues over 5-10 years,” Dr. Lim said in an interview. “Because of this paper, stress reduction may be one additional way to hopefully help these patients these patients that have or are concerned about cognitive issues.”
The population-matched cohort of the study included 61,748 individuals with stress-related disorders and 595,335 matched individuals without those disorders, while the sibling-matched cohort included 44,839 individuals with those disorders and 78,482 without. The median age at the start of follow-up was 47 years and 39.4% of those with stress-related disorders were male.
During follow-up, the incidence of neurodegenerative diseases per 1,000 person-years was 1.50 for individuals with stress-related disorders, versus 0.82 for those without stress-related disorders, according to the report. Risk of primary neurodegenerative diseases was increased among those with stress-related disorders, compared with those without, with a hazard ratio of 1.31 (95% confidence interval, 1.15-1.48). However, the risk of vascular neurodegenerative diseases was significantly higher, with an HR of 1.80 (95% CI, 1.40-2.31; P = .03 for the difference between hazard ratios).
Results of the matched sibling cohort supported results of the population-matched cohort, though the elevated risk of vascular neurodegenerative diseases among those with stress-related disorders was “slightly lower” than in the population-based cohort, Dr. Song and coauthors wrote in their report.
Beyond causing a host of hormonal and medical issues, stress can lead to sleep issues that may have long-term consequences, Dr. Lim noted in the interview.
“There’s some thought that quality sleep is important for memory formation, and if people are under a fair amount of stress and they have really poor sleep, that can also lead to cognitive issues including memory impairment,” he said.
“There are these multiple avenues that may be contributing to the accelerated development of these kinds of issues,” he added, “so I think this paper suggests more ways to counsel the patients about using lifestyle modifications to slow down the development of these cognitive impairments.”
Funding for the study came from the Swedish Research Council, Icelandic Research Fund; ,European Research Council the Karolinska Institutet, Swedish Research Council, and West China Hospital. Authors of the study provided disclosures related to those organizations as well as Shire/Takeda and Evolan.
SOURCE: Song H et al. JAMA Neurol. 2020 Mar 9. doi: 10.1001/jamaneurol.2020.0117.
Individuals with posttraumatic stress disorder (PTSD), acute stress reaction, adjustment disorder, or other stress reactions had an 80% increased risk of vascular neurodegenerative diseases, according to results of the study, which was based on Swedish population registry data.
Risk of primary neurodegenerative diseases was increased as well in people with those conditions, but only by 31%, according to lead author Huan Song, MD, PhD, of Sichuan University in Chengdu, China.
“The stronger association observed for neurodegenerative diseases with a vascular component, compared with primary neurodegenerative diseases, suggested a considerable role of a possible cerebrovascular pathway,” Dr. Song and coauthors said in a report on the study appearing in JAMA Neurology.
While some previous studies have linked stress-related disorders to neurodegenerative diseases – particularly PTSD and dementia – this is believed to be the first, according to the investigators, to comprehensively evaluate all stress-related disorders in relation to the most common neurodegenerative conditions.
When considering neurodegenerative conditions separately, they found a statistically significant association between stress-related disorders and Alzheimer’s disease, while linkages with Parkinson’s disease and amyotrophic lateral sclerosis (ALS) were “comparable” but associations did not reach statistical significance, according to investigators.
Based on these findings, stress reduction should be recommended in addition to daily physical activity, mental activity, and a heart-healthy diet to potentially reduce risk of onset or worsening of cognitive decline, according to Chun Lim, MD, PhD, medical director of the cognitive neurology unit at Beth Israel Deaconess Medical Center in Boston.
“We don’t really have great evidence that anything slows down the progression of Alzheimer’s disease, but there are some suggestions that for people who lead heart-healthy lifestyles or adhere to a Mediterranean diet, fewer develop cognitive issues over 5-10 years,” Dr. Lim said in an interview. “Because of this paper, stress reduction may be one additional way to hopefully help these patients these patients that have or are concerned about cognitive issues.”
The population-matched cohort of the study included 61,748 individuals with stress-related disorders and 595,335 matched individuals without those disorders, while the sibling-matched cohort included 44,839 individuals with those disorders and 78,482 without. The median age at the start of follow-up was 47 years and 39.4% of those with stress-related disorders were male.
During follow-up, the incidence of neurodegenerative diseases per 1,000 person-years was 1.50 for individuals with stress-related disorders, versus 0.82 for those without stress-related disorders, according to the report. Risk of primary neurodegenerative diseases was increased among those with stress-related disorders, compared with those without, with a hazard ratio of 1.31 (95% confidence interval, 1.15-1.48). However, the risk of vascular neurodegenerative diseases was significantly higher, with an HR of 1.80 (95% CI, 1.40-2.31; P = .03 for the difference between hazard ratios).
Results of the matched sibling cohort supported results of the population-matched cohort, though the elevated risk of vascular neurodegenerative diseases among those with stress-related disorders was “slightly lower” than in the population-based cohort, Dr. Song and coauthors wrote in their report.
Beyond causing a host of hormonal and medical issues, stress can lead to sleep issues that may have long-term consequences, Dr. Lim noted in the interview.
“There’s some thought that quality sleep is important for memory formation, and if people are under a fair amount of stress and they have really poor sleep, that can also lead to cognitive issues including memory impairment,” he said.
“There are these multiple avenues that may be contributing to the accelerated development of these kinds of issues,” he added, “so I think this paper suggests more ways to counsel the patients about using lifestyle modifications to slow down the development of these cognitive impairments.”
Funding for the study came from the Swedish Research Council, Icelandic Research Fund; ,European Research Council the Karolinska Institutet, Swedish Research Council, and West China Hospital. Authors of the study provided disclosures related to those organizations as well as Shire/Takeda and Evolan.
SOURCE: Song H et al. JAMA Neurol. 2020 Mar 9. doi: 10.1001/jamaneurol.2020.0117.
Individuals with posttraumatic stress disorder (PTSD), acute stress reaction, adjustment disorder, or other stress reactions had an 80% increased risk of vascular neurodegenerative diseases, according to results of the study, which was based on Swedish population registry data.
Risk of primary neurodegenerative diseases was increased as well in people with those conditions, but only by 31%, according to lead author Huan Song, MD, PhD, of Sichuan University in Chengdu, China.
“The stronger association observed for neurodegenerative diseases with a vascular component, compared with primary neurodegenerative diseases, suggested a considerable role of a possible cerebrovascular pathway,” Dr. Song and coauthors said in a report on the study appearing in JAMA Neurology.
While some previous studies have linked stress-related disorders to neurodegenerative diseases – particularly PTSD and dementia – this is believed to be the first, according to the investigators, to comprehensively evaluate all stress-related disorders in relation to the most common neurodegenerative conditions.
When considering neurodegenerative conditions separately, they found a statistically significant association between stress-related disorders and Alzheimer’s disease, while linkages with Parkinson’s disease and amyotrophic lateral sclerosis (ALS) were “comparable” but associations did not reach statistical significance, according to investigators.
Based on these findings, stress reduction should be recommended in addition to daily physical activity, mental activity, and a heart-healthy diet to potentially reduce risk of onset or worsening of cognitive decline, according to Chun Lim, MD, PhD, medical director of the cognitive neurology unit at Beth Israel Deaconess Medical Center in Boston.
“We don’t really have great evidence that anything slows down the progression of Alzheimer’s disease, but there are some suggestions that for people who lead heart-healthy lifestyles or adhere to a Mediterranean diet, fewer develop cognitive issues over 5-10 years,” Dr. Lim said in an interview. “Because of this paper, stress reduction may be one additional way to hopefully help these patients these patients that have or are concerned about cognitive issues.”
The population-matched cohort of the study included 61,748 individuals with stress-related disorders and 595,335 matched individuals without those disorders, while the sibling-matched cohort included 44,839 individuals with those disorders and 78,482 without. The median age at the start of follow-up was 47 years and 39.4% of those with stress-related disorders were male.
During follow-up, the incidence of neurodegenerative diseases per 1,000 person-years was 1.50 for individuals with stress-related disorders, versus 0.82 for those without stress-related disorders, according to the report. Risk of primary neurodegenerative diseases was increased among those with stress-related disorders, compared with those without, with a hazard ratio of 1.31 (95% confidence interval, 1.15-1.48). However, the risk of vascular neurodegenerative diseases was significantly higher, with an HR of 1.80 (95% CI, 1.40-2.31; P = .03 for the difference between hazard ratios).
Results of the matched sibling cohort supported results of the population-matched cohort, though the elevated risk of vascular neurodegenerative diseases among those with stress-related disorders was “slightly lower” than in the population-based cohort, Dr. Song and coauthors wrote in their report.
Beyond causing a host of hormonal and medical issues, stress can lead to sleep issues that may have long-term consequences, Dr. Lim noted in the interview.
“There’s some thought that quality sleep is important for memory formation, and if people are under a fair amount of stress and they have really poor sleep, that can also lead to cognitive issues including memory impairment,” he said.
“There are these multiple avenues that may be contributing to the accelerated development of these kinds of issues,” he added, “so I think this paper suggests more ways to counsel the patients about using lifestyle modifications to slow down the development of these cognitive impairments.”
Funding for the study came from the Swedish Research Council, Icelandic Research Fund; ,European Research Council the Karolinska Institutet, Swedish Research Council, and West China Hospital. Authors of the study provided disclosures related to those organizations as well as Shire/Takeda and Evolan.
SOURCE: Song H et al. JAMA Neurol. 2020 Mar 9. doi: 10.1001/jamaneurol.2020.0117.
FROM JAMA NEUROLOGY