User login
Refractive Outcomes for Cataract Surgery With Toric Intraocular Lenses at a Veterans Affairs Medical Center
Cataract surgery is one of the most common ambulatory procedures performed in the US.1-3 With the aging of the US population, the number of Americans with cataracts is projected to increase from 24.4 million in 2010 to 38.7 million in 2030.4
Approximately 20% of all cataract patients have preoperative astigmatism of > 1.5 diopters (D), underscoring the importance of training residents in the placement of toric intraocular lenses (IOLs).5 However, the implantation of toric IOLs is more challenging than monofocal IOLs, requiring precise surgical alignment of the IOL.6 Successful toric IOL implantation also requires accurate calculation of the IOL cylinder power and target axis of alignment. It is unclear which toric IOL calculation formula offers the most accurate refractive predictions, and practitioners have designed strategies to apply different formulae depending on the biometric dimensions of the target eye.7-9
Previous studies of resident-performed cataract surgery using toric IOLs6,10-13 and studies that compare the performance of the Barrett and Holladay toric formulae have been limited by their small sample sizes (< 107 eyes).7,14-16 Moreover, none of the studies that evaluate the comparative effectiveness of these biometric formulae were conducted at a teaching hospital.7,14-16
Given the added complexity of toric IOL placement and variable surgical experience of residents as ophthalmologists-in-training, it is important to assess outcomes in teaching hospitals.13 The primary aims of this study were to assess the visual and refractive outcomes of cataract surgery using toric IOLs in a US Department of Veterans Affairs (VA) teaching hospital and to compare the relative accuracy of the Holladay 2 or Barrett toric biometric formulae in predicting postoperative refraction outcomes.
Methods
The Providence VA Medical Center (PVAMC) Institutional Review Board approved this study. This retrospective chart review included patients with cataract and corneal astigmatism who underwent cataract surgery using Acrysof toric IOLs, model SN6AT (Alcon) at the PVAMC teaching hospital between November 2013 and May 2018.
Only 1 eye was included from each study subject to avoid compounding of data with the use of bilateral eyes.17 In addition, bilateral cataract surgery was only performed on some patients at the PVAMC, so including both eyes from eligible patients would disproportionately weigh those patients’ outcomes. If both eyes had cataract surgery and their postoperative visual acuities were unequal, we chose the eye with the better postoperative visual acuity since refraction accuracy decreases with worsening best-corrected visual acuity (BCVA). If both eyes had cataract surgery and the postoperative visual acuity was the same, the first operated eye was chosen.17,18
Exclusion criteria included worse than 20/40 BCVA, posterior capsular rupture, sulcus IOL, history of corneal disease, history of refractive surgery (laser-assisted in situ keratomileusis [LASIK]/photorefractive keratectomy [PRK]), axial length not measurable by the Lenstar optical biometer (Haag-Streit USA), or no postoperative refraction within 3 weeks to 4 months.19,20
Patient age, race/ethnicity, gender, preoperative refraction, preoperative BCVA, postoperative refraction, postoperative BCVA, and IOL power were recorded from patient charts (Table 1). Preoperative and postoperative refractive values were converted to spherical equivalents. The preoperative biometry and most of the postoperative refractions were performed by experienced technicians certified by the Joint Commission on Allied Health Personnel in Ophthalmology. The main outcomes for the assessment of surgeries included the postoperative BCVA, postoperative spherical equivalent refraction, and postoperative residual refractive astigmatism.
Axial length (AL), preoperative anterior chamber depth (ACD), preoperative flat corneal front power (K1), preoperative steep corneal front power (K2), lens thickness, horizontal white-to-white (WTW) corneal diameter, and central corneal thickness (CCT) were recorded from the Lenstar biometric device. Predicted postoperative refractions for the Holladay 2 formula were calculated using Holladay IOL Consultant software (Holladay Consulting). Predicted postoperative refractions for the Barrett toric IOL formula were calculated using the online Barrett toric formula calculator.21 Since previous studies have shown that both the Holladay and Barrett formulae account for posterior corneal astigmatism, a comparison of refractive outcomes in eyes with against-the-rule astigmatism vs with-the-rule astigmatism was not performed.14 An estimated standardized value for surgically-induced astigmatism was entered into both formulae; 0.3 diopter (D) was chosen based on previously published averages.22-24
A formula’s prediction error is defined as the predicted postoperative refraction minus the actual postoperative refraction. The mean absolute prediction error (MAE), defined as the mean of the absolute values of the prediction errors, and the median absolute prediction error (MedAE), defined as the median of the absolute values of the prediction errors, were used to assess the overall accuracy of each formula. Also, the percentages of eyes with postoperative refraction within ≥ 0.25 D, ≥ 0.50 D, and ≥ 1.0 D were calculated for both formulae. Two-tailed t tests were performed to compare the MAE between the formulae. Subgroup analyses were performed for short eyes (AL < 22 mm), medium length eyes (AL = 22-25 mm), and long eyes (AL > 25 mm). Statistical analysis was performed using STATA 11 (STATA Corp). The preoperative corneal astigmatism and postoperative refractive astigmatism of all the cases were compared in double-angle plots to assess how well the toric IOL neutralized the corneal astigmatism.
Results
Of 325 charts reviewed during the study period, 34 patients were excluded due to lack of postoperative refraction within the designated follow-up period, 5 for worse than 20/40 postoperative BCVA (4 had preexisting ocular disease), 2 for complications, and 1 for missing data. We included 283 eyes from 283 patients in the final study. Resident ophthalmologists were the primary surgeons in 87.6% (248/283) of the cases.
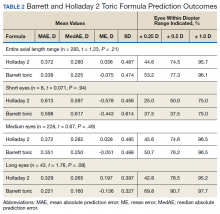
The median postoperative BCVA was 20/20, and 92% of patients had a postoperative BCVA of 20/25 or better. The prediction outcomes of the toric SN6AT IOLs are shown in Table 2. The Barrett toric formula had a lower MAE than the Holladay 2 formula, but this difference was not statistically significant. The Barrett toric formula also predicted a higher percentage of eyes with postoperative refraction within ≥ 0.25 D (53.2%), ≥ 0.5 D (77.3%), and ≥ 1.0 D (96.1%). For both formulae, > 95% of eyes had prediction errors that fell within 1.0 D.
While the Barrett formula demonstrated a lower MAE in all 3 AL groups, no statistically significant differences were found between the Barrett and Holladay formulae (P = .94, P = .49, and P = .08 for short, medium, and long eyes, respectively). Both formulae produced the lowest MAE in the long AL group: Barrett had a MAE of 0.221 D and Holladay 2 had one of 0.329 D. The Barrett formula produced its highest percentage of eyes with prediction errors falling within 0.25 D and 0.5 D in the long AL group. In comparison, both formulae had the highest MAEs in the short AL group (Barrett toric, 0.598 D; Holladay 2, 0.613 D) and produced the lowest percentage of eyes with prediction errors falling within ≥ 0.25 D and ≥ 0.5 D in the short AL group.
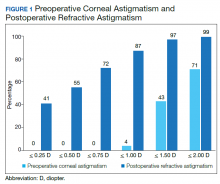
A cumulative histogram of the preoperative corneal and postoperative refractive astigmatism magnitude is shown in Figure 1. The same data are presented as double-angle plots in the Appendix, which shows that the centroid values for preoperative corneal astigmatism were greatlyreduced when compared with the postoperative refractive astigmatism (mean absolute value of 1.77 D ≥ 0.73 D to 0.5 D ≥ 0.50 D).
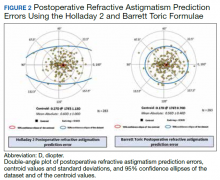
Preoperative corneal astigmatism and postoperative refractive astigmatism were compared since preoperative refractive astigmatism has noncorneal contributions, including lenticular astigmatism, and there is minimal expected change between preoperative and postoperative corneal astigmatism.14 For comparison, double-angle plots of postoperative refractive astigmatism prediction errors for the Holladay and Barrett formulae are shown in Figure 2.
Discussion
To our knowledge, this is the largest study of resident-performed cataract surgery using toric IOLs, the largest study that compared the performance of the Barrett toric and Holladay 2 formulae, and the first that compared these formulae in a teaching hospital setting. This study found no significant difference in the predictive accuracy of the Barrett and Holladay 2 biometric formulae for cataract surgery using toric IOLs. In addition, our refractive outcomes were consistent with the results of previous toric IOL outcome studies conducted in teaching and nonteaching hospital settings.6,10-13
In 4 previous studies that compared the MAE of the Barrett and Holladay formulae for toric IOLs, the Barrett formula produced a lower MAE than the Holladay 2 formula.7,14-16 However, this difference was significant in only 2 of the studies, which had sample sizes of only 68 and 107 eyes.14,16 Furthermore, the Barrett toric formula produced the lower MAE for the entire AL range, though this was not statistically significant at our sample size. In addition, both formulae produced the lowest MAE in the long AL group and the highest MAE in the short AL group. The unique anatomy and high IOL power needed in short eyes may explain the challenges in attaining accurate IOL power predictions in this AL group.19,25
Limitations
The sample size of this study may have prevented us from detecting statistically significant differences in the performance of the Barrett and Holladay formulae. However, our findings are consistent with studies that compare the accuracy of these formulae in teaching and nonteaching hospital settings. Second, the study was conducted at a VA hospital, and a high proportion of patients were male; thus, our findings may not be generalizable to patients who receive cataract surgery with toric IOLs in other settings.
Conclusions
In a single VA teaching hospital, the Barrett and Holladay 2 biometric formulae provide similar refractive predictions for cataract surgery using toric IOLs. Larger studies would be necessary to detect smaller differences in the relative performance of the biometric formulae.
1. Schein OD, Cassard SD, Tielsch JM, Gower EW. Cataract surgery among Medicare beneficiaries. Ophthalmic Epidemiol. 2012;19(5):257-264.
2. Congdon N, O’Colmain B, Klaver CC, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122(4):477-485.
3. Congdon N, Vingerling JR, Klein BE, et al. Prevalence of cataract and pseudophakia/aphakia among adults in the United States. Arch Ophthalmol. 2004;122(4):487-494.
4. National Eye Institute. Cataract tables: cataract defined. https://www.nei.nih.gov/learn-about-eye-health/resources-for-health-educators/eye-health-data-and-statistics/cataract-data-and-statistics/cataract-tables. Updated February 7, 2020. Accessed February 10, 2020.
5. Ostri C, Falck L, Boberg-Ans G, Kessel L. The need for toric intra-ocular lens implantation in public ophthalmology departments. Acta Ophthalmol. 2015;93(5):e396-e397.
6. Sundy M, McKnight D, Eck C, Rieger F 3rd. Visual acuity outcomes of toric lens implantation in patients undergoing cataract surgery at a residency training program. Mo Med. 2016;113(1):40-43.
7. Ferreira TB, Ribeiro P, Ribeiro FJ, O’Neill JG. Comparison of methodologies using estimated or measured values of total corneal astigmatism for toric intraocular lens power calculation. J Refract Surg. 2017;33(12):794-800.
8. Reitblat O, Levy A, Kleinmann G, Abulafia A, Assia EI. Effect of posterior corneal astigmatism on power calculation and alignment of toric intraocular lenses: comparison of methodologies. J Cataract Refract Surg. 2016;42(2):217-225.
9. Aristodemou P, Knox Cartwright NE, Sparrow JM, Johnston RL. Formula choice: Hoffer Q, Holladay 1, or SRK/T and refractive outcomes in 8108 eyes after cataract surgery with biometry by partial coherence interferometry. J Cataract Refract Surg. 2011;37(1):63-71.
10. Moreira HR, Hatch KM, Greenberg PB. Benchmarking outcomes in resident-performed cataract surgery with toric intraocular lenses [published correction appears in: Clin Experiment Ophthalmol. 2013;41(8):819]. Clin Exp Ophthalmol. 2013;41(6):624-626.
11. Retzlaff JA, Sanders DR, Kraff MC. Development of the SRK/T intraocular lens implant power calculation formula [published correction appears in: J Cataract Refract Surg. 1990;16(4):528]. J Cataract Refract Surg. 1990;16(3):333-340.
12. Roensch MA, Charton JW, Blomquist PH, Aggarwal NK, McCulley JP. Resident experience with toric and multifocal intraocular lenses in a public county hospital system. J Cataract Refract Surg. 2012;38(5):793-798.
13. Pouyeh B, Galor A, Junk AK, et al. Surgical and refractive outcomes of cataract surgery with toric intraocular lens implantation at a resident-teaching institution. J Cataract Refract Surg. 2011;37(9):1623-1628.
14. Ferreira TB, Ribeiro P, Ribeiro FJ, O’Neill JG. Comparison of astigmatic prediction errors associated with new calculation methods for toric intraocular lenses. J Cataract Refract Surg. 2017;43(3):340-347.
15. Abulafia A, Hill WE, Franchina M, Barrett GD. Comparison of methods to predict residual astigmatism after intraocular lens implantation. J Refract Surg. 2015;31(10):699-707.
16. Abulafia A, Barrett GD, Kleinmann G, et al. Prediction of refractive outcomes with toric intraocular lens implantation. J Cataract Refract Surg. 2015;41(5):936-944.
17. Wang Q, Jiang W, Lin T, Wu X, Lin H, Chen W. Meta-analysis of accuracy of intraocular lens power calculation formulas in short eyes. Clin Exp Ophthalmol. 2018;46(4):356-363.
18. Melles RB, Holladay JT, Chang WJ. Accuracy of intraocular lens calculation formulas. Ophthalmology. 2018;125(2):169-178.
19. Hoffer KJ. The Hoffer Q formula: a comparison of theoretic and regression formulas. J Cataract Refract Surg. 1993;19(6):700-712.
20. Cooke DL, Cooke TL. Comparison of 9 intraocular lens power calculation formulas. J Cataract Refract Surg. 2016;42(8):1157-1164.
21. American Society of Cataract and Refractive Surgery. Barrett toric calculator. www.ascrs.org/barrett-toric-calculator. Accessed February 5, 2020.
22. Holladay JT, Pettit G. Improving toric intraocular lens calculations using total surgically induced astigmatism for a 2.5 mm temporal incision. J Cataract Refract Surg. 2019;45(3):272-283.
23. Canovas C, Alarcon A, Rosén R, et al. New algorithm for toric intraocular lens power calculation considering the posterior corneal astigmatism. J Cataract Refract Surg. 2018;44(2):168-174.
24. Visser N, Berendschot TT, Bauer NJ, Nuijts RM. Vector analysis of corneal and refractive astigmatism changes following toric pseudophakic and toric phakic IOL implantation. Invest Ophthalmol Vis Sci. 2012;53(4):1865-1873.
25. Narváez J, Zimmerman G, Stulting RD, Chang DH. Accuracy of intraocular lens power prediction using the Hoffer Q, Holladay 1, Holladay 2, and SRK/T formulas. J Cataract Refract Surg. 2006;32(12):2050-2053.
Cataract surgery is one of the most common ambulatory procedures performed in the US.1-3 With the aging of the US population, the number of Americans with cataracts is projected to increase from 24.4 million in 2010 to 38.7 million in 2030.4
Approximately 20% of all cataract patients have preoperative astigmatism of > 1.5 diopters (D), underscoring the importance of training residents in the placement of toric intraocular lenses (IOLs).5 However, the implantation of toric IOLs is more challenging than monofocal IOLs, requiring precise surgical alignment of the IOL.6 Successful toric IOL implantation also requires accurate calculation of the IOL cylinder power and target axis of alignment. It is unclear which toric IOL calculation formula offers the most accurate refractive predictions, and practitioners have designed strategies to apply different formulae depending on the biometric dimensions of the target eye.7-9
Previous studies of resident-performed cataract surgery using toric IOLs6,10-13 and studies that compare the performance of the Barrett and Holladay toric formulae have been limited by their small sample sizes (< 107 eyes).7,14-16 Moreover, none of the studies that evaluate the comparative effectiveness of these biometric formulae were conducted at a teaching hospital.7,14-16
Given the added complexity of toric IOL placement and variable surgical experience of residents as ophthalmologists-in-training, it is important to assess outcomes in teaching hospitals.13 The primary aims of this study were to assess the visual and refractive outcomes of cataract surgery using toric IOLs in a US Department of Veterans Affairs (VA) teaching hospital and to compare the relative accuracy of the Holladay 2 or Barrett toric biometric formulae in predicting postoperative refraction outcomes.
Methods
The Providence VA Medical Center (PVAMC) Institutional Review Board approved this study. This retrospective chart review included patients with cataract and corneal astigmatism who underwent cataract surgery using Acrysof toric IOLs, model SN6AT (Alcon) at the PVAMC teaching hospital between November 2013 and May 2018.
Only 1 eye was included from each study subject to avoid compounding of data with the use of bilateral eyes.17 In addition, bilateral cataract surgery was only performed on some patients at the PVAMC, so including both eyes from eligible patients would disproportionately weigh those patients’ outcomes. If both eyes had cataract surgery and their postoperative visual acuities were unequal, we chose the eye with the better postoperative visual acuity since refraction accuracy decreases with worsening best-corrected visual acuity (BCVA). If both eyes had cataract surgery and the postoperative visual acuity was the same, the first operated eye was chosen.17,18
Exclusion criteria included worse than 20/40 BCVA, posterior capsular rupture, sulcus IOL, history of corneal disease, history of refractive surgery (laser-assisted in situ keratomileusis [LASIK]/photorefractive keratectomy [PRK]), axial length not measurable by the Lenstar optical biometer (Haag-Streit USA), or no postoperative refraction within 3 weeks to 4 months.19,20
Patient age, race/ethnicity, gender, preoperative refraction, preoperative BCVA, postoperative refraction, postoperative BCVA, and IOL power were recorded from patient charts (Table 1). Preoperative and postoperative refractive values were converted to spherical equivalents. The preoperative biometry and most of the postoperative refractions were performed by experienced technicians certified by the Joint Commission on Allied Health Personnel in Ophthalmology. The main outcomes for the assessment of surgeries included the postoperative BCVA, postoperative spherical equivalent refraction, and postoperative residual refractive astigmatism.
Axial length (AL), preoperative anterior chamber depth (ACD), preoperative flat corneal front power (K1), preoperative steep corneal front power (K2), lens thickness, horizontal white-to-white (WTW) corneal diameter, and central corneal thickness (CCT) were recorded from the Lenstar biometric device. Predicted postoperative refractions for the Holladay 2 formula were calculated using Holladay IOL Consultant software (Holladay Consulting). Predicted postoperative refractions for the Barrett toric IOL formula were calculated using the online Barrett toric formula calculator.21 Since previous studies have shown that both the Holladay and Barrett formulae account for posterior corneal astigmatism, a comparison of refractive outcomes in eyes with against-the-rule astigmatism vs with-the-rule astigmatism was not performed.14 An estimated standardized value for surgically-induced astigmatism was entered into both formulae; 0.3 diopter (D) was chosen based on previously published averages.22-24
A formula’s prediction error is defined as the predicted postoperative refraction minus the actual postoperative refraction. The mean absolute prediction error (MAE), defined as the mean of the absolute values of the prediction errors, and the median absolute prediction error (MedAE), defined as the median of the absolute values of the prediction errors, were used to assess the overall accuracy of each formula. Also, the percentages of eyes with postoperative refraction within ≥ 0.25 D, ≥ 0.50 D, and ≥ 1.0 D were calculated for both formulae. Two-tailed t tests were performed to compare the MAE between the formulae. Subgroup analyses were performed for short eyes (AL < 22 mm), medium length eyes (AL = 22-25 mm), and long eyes (AL > 25 mm). Statistical analysis was performed using STATA 11 (STATA Corp). The preoperative corneal astigmatism and postoperative refractive astigmatism of all the cases were compared in double-angle plots to assess how well the toric IOL neutralized the corneal astigmatism.
Results
Of 325 charts reviewed during the study period, 34 patients were excluded due to lack of postoperative refraction within the designated follow-up period, 5 for worse than 20/40 postoperative BCVA (4 had preexisting ocular disease), 2 for complications, and 1 for missing data. We included 283 eyes from 283 patients in the final study. Resident ophthalmologists were the primary surgeons in 87.6% (248/283) of the cases.
The median postoperative BCVA was 20/20, and 92% of patients had a postoperative BCVA of 20/25 or better. The prediction outcomes of the toric SN6AT IOLs are shown in Table 2. The Barrett toric formula had a lower MAE than the Holladay 2 formula, but this difference was not statistically significant. The Barrett toric formula also predicted a higher percentage of eyes with postoperative refraction within ≥ 0.25 D (53.2%), ≥ 0.5 D (77.3%), and ≥ 1.0 D (96.1%). For both formulae, > 95% of eyes had prediction errors that fell within 1.0 D.
While the Barrett formula demonstrated a lower MAE in all 3 AL groups, no statistically significant differences were found between the Barrett and Holladay formulae (P = .94, P = .49, and P = .08 for short, medium, and long eyes, respectively). Both formulae produced the lowest MAE in the long AL group: Barrett had a MAE of 0.221 D and Holladay 2 had one of 0.329 D. The Barrett formula produced its highest percentage of eyes with prediction errors falling within 0.25 D and 0.5 D in the long AL group. In comparison, both formulae had the highest MAEs in the short AL group (Barrett toric, 0.598 D; Holladay 2, 0.613 D) and produced the lowest percentage of eyes with prediction errors falling within ≥ 0.25 D and ≥ 0.5 D in the short AL group.
A cumulative histogram of the preoperative corneal and postoperative refractive astigmatism magnitude is shown in Figure 1. The same data are presented as double-angle plots in the Appendix, which shows that the centroid values for preoperative corneal astigmatism were greatlyreduced when compared with the postoperative refractive astigmatism (mean absolute value of 1.77 D ≥ 0.73 D to 0.5 D ≥ 0.50 D).
Preoperative corneal astigmatism and postoperative refractive astigmatism were compared since preoperative refractive astigmatism has noncorneal contributions, including lenticular astigmatism, and there is minimal expected change between preoperative and postoperative corneal astigmatism.14 For comparison, double-angle plots of postoperative refractive astigmatism prediction errors for the Holladay and Barrett formulae are shown in Figure 2.
Discussion
To our knowledge, this is the largest study of resident-performed cataract surgery using toric IOLs, the largest study that compared the performance of the Barrett toric and Holladay 2 formulae, and the first that compared these formulae in a teaching hospital setting. This study found no significant difference in the predictive accuracy of the Barrett and Holladay 2 biometric formulae for cataract surgery using toric IOLs. In addition, our refractive outcomes were consistent with the results of previous toric IOL outcome studies conducted in teaching and nonteaching hospital settings.6,10-13
In 4 previous studies that compared the MAE of the Barrett and Holladay formulae for toric IOLs, the Barrett formula produced a lower MAE than the Holladay 2 formula.7,14-16 However, this difference was significant in only 2 of the studies, which had sample sizes of only 68 and 107 eyes.14,16 Furthermore, the Barrett toric formula produced the lower MAE for the entire AL range, though this was not statistically significant at our sample size. In addition, both formulae produced the lowest MAE in the long AL group and the highest MAE in the short AL group. The unique anatomy and high IOL power needed in short eyes may explain the challenges in attaining accurate IOL power predictions in this AL group.19,25
Limitations
The sample size of this study may have prevented us from detecting statistically significant differences in the performance of the Barrett and Holladay formulae. However, our findings are consistent with studies that compare the accuracy of these formulae in teaching and nonteaching hospital settings. Second, the study was conducted at a VA hospital, and a high proportion of patients were male; thus, our findings may not be generalizable to patients who receive cataract surgery with toric IOLs in other settings.
Conclusions
In a single VA teaching hospital, the Barrett and Holladay 2 biometric formulae provide similar refractive predictions for cataract surgery using toric IOLs. Larger studies would be necessary to detect smaller differences in the relative performance of the biometric formulae.
Cataract surgery is one of the most common ambulatory procedures performed in the US.1-3 With the aging of the US population, the number of Americans with cataracts is projected to increase from 24.4 million in 2010 to 38.7 million in 2030.4
Approximately 20% of all cataract patients have preoperative astigmatism of > 1.5 diopters (D), underscoring the importance of training residents in the placement of toric intraocular lenses (IOLs).5 However, the implantation of toric IOLs is more challenging than monofocal IOLs, requiring precise surgical alignment of the IOL.6 Successful toric IOL implantation also requires accurate calculation of the IOL cylinder power and target axis of alignment. It is unclear which toric IOL calculation formula offers the most accurate refractive predictions, and practitioners have designed strategies to apply different formulae depending on the biometric dimensions of the target eye.7-9
Previous studies of resident-performed cataract surgery using toric IOLs6,10-13 and studies that compare the performance of the Barrett and Holladay toric formulae have been limited by their small sample sizes (< 107 eyes).7,14-16 Moreover, none of the studies that evaluate the comparative effectiveness of these biometric formulae were conducted at a teaching hospital.7,14-16
Given the added complexity of toric IOL placement and variable surgical experience of residents as ophthalmologists-in-training, it is important to assess outcomes in teaching hospitals.13 The primary aims of this study were to assess the visual and refractive outcomes of cataract surgery using toric IOLs in a US Department of Veterans Affairs (VA) teaching hospital and to compare the relative accuracy of the Holladay 2 or Barrett toric biometric formulae in predicting postoperative refraction outcomes.
Methods
The Providence VA Medical Center (PVAMC) Institutional Review Board approved this study. This retrospective chart review included patients with cataract and corneal astigmatism who underwent cataract surgery using Acrysof toric IOLs, model SN6AT (Alcon) at the PVAMC teaching hospital between November 2013 and May 2018.
Only 1 eye was included from each study subject to avoid compounding of data with the use of bilateral eyes.17 In addition, bilateral cataract surgery was only performed on some patients at the PVAMC, so including both eyes from eligible patients would disproportionately weigh those patients’ outcomes. If both eyes had cataract surgery and their postoperative visual acuities were unequal, we chose the eye with the better postoperative visual acuity since refraction accuracy decreases with worsening best-corrected visual acuity (BCVA). If both eyes had cataract surgery and the postoperative visual acuity was the same, the first operated eye was chosen.17,18
Exclusion criteria included worse than 20/40 BCVA, posterior capsular rupture, sulcus IOL, history of corneal disease, history of refractive surgery (laser-assisted in situ keratomileusis [LASIK]/photorefractive keratectomy [PRK]), axial length not measurable by the Lenstar optical biometer (Haag-Streit USA), or no postoperative refraction within 3 weeks to 4 months.19,20
Patient age, race/ethnicity, gender, preoperative refraction, preoperative BCVA, postoperative refraction, postoperative BCVA, and IOL power were recorded from patient charts (Table 1). Preoperative and postoperative refractive values were converted to spherical equivalents. The preoperative biometry and most of the postoperative refractions were performed by experienced technicians certified by the Joint Commission on Allied Health Personnel in Ophthalmology. The main outcomes for the assessment of surgeries included the postoperative BCVA, postoperative spherical equivalent refraction, and postoperative residual refractive astigmatism.
Axial length (AL), preoperative anterior chamber depth (ACD), preoperative flat corneal front power (K1), preoperative steep corneal front power (K2), lens thickness, horizontal white-to-white (WTW) corneal diameter, and central corneal thickness (CCT) were recorded from the Lenstar biometric device. Predicted postoperative refractions for the Holladay 2 formula were calculated using Holladay IOL Consultant software (Holladay Consulting). Predicted postoperative refractions for the Barrett toric IOL formula were calculated using the online Barrett toric formula calculator.21 Since previous studies have shown that both the Holladay and Barrett formulae account for posterior corneal astigmatism, a comparison of refractive outcomes in eyes with against-the-rule astigmatism vs with-the-rule astigmatism was not performed.14 An estimated standardized value for surgically-induced astigmatism was entered into both formulae; 0.3 diopter (D) was chosen based on previously published averages.22-24
A formula’s prediction error is defined as the predicted postoperative refraction minus the actual postoperative refraction. The mean absolute prediction error (MAE), defined as the mean of the absolute values of the prediction errors, and the median absolute prediction error (MedAE), defined as the median of the absolute values of the prediction errors, were used to assess the overall accuracy of each formula. Also, the percentages of eyes with postoperative refraction within ≥ 0.25 D, ≥ 0.50 D, and ≥ 1.0 D were calculated for both formulae. Two-tailed t tests were performed to compare the MAE between the formulae. Subgroup analyses were performed for short eyes (AL < 22 mm), medium length eyes (AL = 22-25 mm), and long eyes (AL > 25 mm). Statistical analysis was performed using STATA 11 (STATA Corp). The preoperative corneal astigmatism and postoperative refractive astigmatism of all the cases were compared in double-angle plots to assess how well the toric IOL neutralized the corneal astigmatism.
Results
Of 325 charts reviewed during the study period, 34 patients were excluded due to lack of postoperative refraction within the designated follow-up period, 5 for worse than 20/40 postoperative BCVA (4 had preexisting ocular disease), 2 for complications, and 1 for missing data. We included 283 eyes from 283 patients in the final study. Resident ophthalmologists were the primary surgeons in 87.6% (248/283) of the cases.
The median postoperative BCVA was 20/20, and 92% of patients had a postoperative BCVA of 20/25 or better. The prediction outcomes of the toric SN6AT IOLs are shown in Table 2. The Barrett toric formula had a lower MAE than the Holladay 2 formula, but this difference was not statistically significant. The Barrett toric formula also predicted a higher percentage of eyes with postoperative refraction within ≥ 0.25 D (53.2%), ≥ 0.5 D (77.3%), and ≥ 1.0 D (96.1%). For both formulae, > 95% of eyes had prediction errors that fell within 1.0 D.
While the Barrett formula demonstrated a lower MAE in all 3 AL groups, no statistically significant differences were found between the Barrett and Holladay formulae (P = .94, P = .49, and P = .08 for short, medium, and long eyes, respectively). Both formulae produced the lowest MAE in the long AL group: Barrett had a MAE of 0.221 D and Holladay 2 had one of 0.329 D. The Barrett formula produced its highest percentage of eyes with prediction errors falling within 0.25 D and 0.5 D in the long AL group. In comparison, both formulae had the highest MAEs in the short AL group (Barrett toric, 0.598 D; Holladay 2, 0.613 D) and produced the lowest percentage of eyes with prediction errors falling within ≥ 0.25 D and ≥ 0.5 D in the short AL group.
A cumulative histogram of the preoperative corneal and postoperative refractive astigmatism magnitude is shown in Figure 1. The same data are presented as double-angle plots in the Appendix, which shows that the centroid values for preoperative corneal astigmatism were greatlyreduced when compared with the postoperative refractive astigmatism (mean absolute value of 1.77 D ≥ 0.73 D to 0.5 D ≥ 0.50 D).
Preoperative corneal astigmatism and postoperative refractive astigmatism were compared since preoperative refractive astigmatism has noncorneal contributions, including lenticular astigmatism, and there is minimal expected change between preoperative and postoperative corneal astigmatism.14 For comparison, double-angle plots of postoperative refractive astigmatism prediction errors for the Holladay and Barrett formulae are shown in Figure 2.
Discussion
To our knowledge, this is the largest study of resident-performed cataract surgery using toric IOLs, the largest study that compared the performance of the Barrett toric and Holladay 2 formulae, and the first that compared these formulae in a teaching hospital setting. This study found no significant difference in the predictive accuracy of the Barrett and Holladay 2 biometric formulae for cataract surgery using toric IOLs. In addition, our refractive outcomes were consistent with the results of previous toric IOL outcome studies conducted in teaching and nonteaching hospital settings.6,10-13
In 4 previous studies that compared the MAE of the Barrett and Holladay formulae for toric IOLs, the Barrett formula produced a lower MAE than the Holladay 2 formula.7,14-16 However, this difference was significant in only 2 of the studies, which had sample sizes of only 68 and 107 eyes.14,16 Furthermore, the Barrett toric formula produced the lower MAE for the entire AL range, though this was not statistically significant at our sample size. In addition, both formulae produced the lowest MAE in the long AL group and the highest MAE in the short AL group. The unique anatomy and high IOL power needed in short eyes may explain the challenges in attaining accurate IOL power predictions in this AL group.19,25
Limitations
The sample size of this study may have prevented us from detecting statistically significant differences in the performance of the Barrett and Holladay formulae. However, our findings are consistent with studies that compare the accuracy of these formulae in teaching and nonteaching hospital settings. Second, the study was conducted at a VA hospital, and a high proportion of patients were male; thus, our findings may not be generalizable to patients who receive cataract surgery with toric IOLs in other settings.
Conclusions
In a single VA teaching hospital, the Barrett and Holladay 2 biometric formulae provide similar refractive predictions for cataract surgery using toric IOLs. Larger studies would be necessary to detect smaller differences in the relative performance of the biometric formulae.
1. Schein OD, Cassard SD, Tielsch JM, Gower EW. Cataract surgery among Medicare beneficiaries. Ophthalmic Epidemiol. 2012;19(5):257-264.
2. Congdon N, O’Colmain B, Klaver CC, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122(4):477-485.
3. Congdon N, Vingerling JR, Klein BE, et al. Prevalence of cataract and pseudophakia/aphakia among adults in the United States. Arch Ophthalmol. 2004;122(4):487-494.
4. National Eye Institute. Cataract tables: cataract defined. https://www.nei.nih.gov/learn-about-eye-health/resources-for-health-educators/eye-health-data-and-statistics/cataract-data-and-statistics/cataract-tables. Updated February 7, 2020. Accessed February 10, 2020.
5. Ostri C, Falck L, Boberg-Ans G, Kessel L. The need for toric intra-ocular lens implantation in public ophthalmology departments. Acta Ophthalmol. 2015;93(5):e396-e397.
6. Sundy M, McKnight D, Eck C, Rieger F 3rd. Visual acuity outcomes of toric lens implantation in patients undergoing cataract surgery at a residency training program. Mo Med. 2016;113(1):40-43.
7. Ferreira TB, Ribeiro P, Ribeiro FJ, O’Neill JG. Comparison of methodologies using estimated or measured values of total corneal astigmatism for toric intraocular lens power calculation. J Refract Surg. 2017;33(12):794-800.
8. Reitblat O, Levy A, Kleinmann G, Abulafia A, Assia EI. Effect of posterior corneal astigmatism on power calculation and alignment of toric intraocular lenses: comparison of methodologies. J Cataract Refract Surg. 2016;42(2):217-225.
9. Aristodemou P, Knox Cartwright NE, Sparrow JM, Johnston RL. Formula choice: Hoffer Q, Holladay 1, or SRK/T and refractive outcomes in 8108 eyes after cataract surgery with biometry by partial coherence interferometry. J Cataract Refract Surg. 2011;37(1):63-71.
10. Moreira HR, Hatch KM, Greenberg PB. Benchmarking outcomes in resident-performed cataract surgery with toric intraocular lenses [published correction appears in: Clin Experiment Ophthalmol. 2013;41(8):819]. Clin Exp Ophthalmol. 2013;41(6):624-626.
11. Retzlaff JA, Sanders DR, Kraff MC. Development of the SRK/T intraocular lens implant power calculation formula [published correction appears in: J Cataract Refract Surg. 1990;16(4):528]. J Cataract Refract Surg. 1990;16(3):333-340.
12. Roensch MA, Charton JW, Blomquist PH, Aggarwal NK, McCulley JP. Resident experience with toric and multifocal intraocular lenses in a public county hospital system. J Cataract Refract Surg. 2012;38(5):793-798.
13. Pouyeh B, Galor A, Junk AK, et al. Surgical and refractive outcomes of cataract surgery with toric intraocular lens implantation at a resident-teaching institution. J Cataract Refract Surg. 2011;37(9):1623-1628.
14. Ferreira TB, Ribeiro P, Ribeiro FJ, O’Neill JG. Comparison of astigmatic prediction errors associated with new calculation methods for toric intraocular lenses. J Cataract Refract Surg. 2017;43(3):340-347.
15. Abulafia A, Hill WE, Franchina M, Barrett GD. Comparison of methods to predict residual astigmatism after intraocular lens implantation. J Refract Surg. 2015;31(10):699-707.
16. Abulafia A, Barrett GD, Kleinmann G, et al. Prediction of refractive outcomes with toric intraocular lens implantation. J Cataract Refract Surg. 2015;41(5):936-944.
17. Wang Q, Jiang W, Lin T, Wu X, Lin H, Chen W. Meta-analysis of accuracy of intraocular lens power calculation formulas in short eyes. Clin Exp Ophthalmol. 2018;46(4):356-363.
18. Melles RB, Holladay JT, Chang WJ. Accuracy of intraocular lens calculation formulas. Ophthalmology. 2018;125(2):169-178.
19. Hoffer KJ. The Hoffer Q formula: a comparison of theoretic and regression formulas. J Cataract Refract Surg. 1993;19(6):700-712.
20. Cooke DL, Cooke TL. Comparison of 9 intraocular lens power calculation formulas. J Cataract Refract Surg. 2016;42(8):1157-1164.
21. American Society of Cataract and Refractive Surgery. Barrett toric calculator. www.ascrs.org/barrett-toric-calculator. Accessed February 5, 2020.
22. Holladay JT, Pettit G. Improving toric intraocular lens calculations using total surgically induced astigmatism for a 2.5 mm temporal incision. J Cataract Refract Surg. 2019;45(3):272-283.
23. Canovas C, Alarcon A, Rosén R, et al. New algorithm for toric intraocular lens power calculation considering the posterior corneal astigmatism. J Cataract Refract Surg. 2018;44(2):168-174.
24. Visser N, Berendschot TT, Bauer NJ, Nuijts RM. Vector analysis of corneal and refractive astigmatism changes following toric pseudophakic and toric phakic IOL implantation. Invest Ophthalmol Vis Sci. 2012;53(4):1865-1873.
25. Narváez J, Zimmerman G, Stulting RD, Chang DH. Accuracy of intraocular lens power prediction using the Hoffer Q, Holladay 1, Holladay 2, and SRK/T formulas. J Cataract Refract Surg. 2006;32(12):2050-2053.
1. Schein OD, Cassard SD, Tielsch JM, Gower EW. Cataract surgery among Medicare beneficiaries. Ophthalmic Epidemiol. 2012;19(5):257-264.
2. Congdon N, O’Colmain B, Klaver CC, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122(4):477-485.
3. Congdon N, Vingerling JR, Klein BE, et al. Prevalence of cataract and pseudophakia/aphakia among adults in the United States. Arch Ophthalmol. 2004;122(4):487-494.
4. National Eye Institute. Cataract tables: cataract defined. https://www.nei.nih.gov/learn-about-eye-health/resources-for-health-educators/eye-health-data-and-statistics/cataract-data-and-statistics/cataract-tables. Updated February 7, 2020. Accessed February 10, 2020.
5. Ostri C, Falck L, Boberg-Ans G, Kessel L. The need for toric intra-ocular lens implantation in public ophthalmology departments. Acta Ophthalmol. 2015;93(5):e396-e397.
6. Sundy M, McKnight D, Eck C, Rieger F 3rd. Visual acuity outcomes of toric lens implantation in patients undergoing cataract surgery at a residency training program. Mo Med. 2016;113(1):40-43.
7. Ferreira TB, Ribeiro P, Ribeiro FJ, O’Neill JG. Comparison of methodologies using estimated or measured values of total corneal astigmatism for toric intraocular lens power calculation. J Refract Surg. 2017;33(12):794-800.
8. Reitblat O, Levy A, Kleinmann G, Abulafia A, Assia EI. Effect of posterior corneal astigmatism on power calculation and alignment of toric intraocular lenses: comparison of methodologies. J Cataract Refract Surg. 2016;42(2):217-225.
9. Aristodemou P, Knox Cartwright NE, Sparrow JM, Johnston RL. Formula choice: Hoffer Q, Holladay 1, or SRK/T and refractive outcomes in 8108 eyes after cataract surgery with biometry by partial coherence interferometry. J Cataract Refract Surg. 2011;37(1):63-71.
10. Moreira HR, Hatch KM, Greenberg PB. Benchmarking outcomes in resident-performed cataract surgery with toric intraocular lenses [published correction appears in: Clin Experiment Ophthalmol. 2013;41(8):819]. Clin Exp Ophthalmol. 2013;41(6):624-626.
11. Retzlaff JA, Sanders DR, Kraff MC. Development of the SRK/T intraocular lens implant power calculation formula [published correction appears in: J Cataract Refract Surg. 1990;16(4):528]. J Cataract Refract Surg. 1990;16(3):333-340.
12. Roensch MA, Charton JW, Blomquist PH, Aggarwal NK, McCulley JP. Resident experience with toric and multifocal intraocular lenses in a public county hospital system. J Cataract Refract Surg. 2012;38(5):793-798.
13. Pouyeh B, Galor A, Junk AK, et al. Surgical and refractive outcomes of cataract surgery with toric intraocular lens implantation at a resident-teaching institution. J Cataract Refract Surg. 2011;37(9):1623-1628.
14. Ferreira TB, Ribeiro P, Ribeiro FJ, O’Neill JG. Comparison of astigmatic prediction errors associated with new calculation methods for toric intraocular lenses. J Cataract Refract Surg. 2017;43(3):340-347.
15. Abulafia A, Hill WE, Franchina M, Barrett GD. Comparison of methods to predict residual astigmatism after intraocular lens implantation. J Refract Surg. 2015;31(10):699-707.
16. Abulafia A, Barrett GD, Kleinmann G, et al. Prediction of refractive outcomes with toric intraocular lens implantation. J Cataract Refract Surg. 2015;41(5):936-944.
17. Wang Q, Jiang W, Lin T, Wu X, Lin H, Chen W. Meta-analysis of accuracy of intraocular lens power calculation formulas in short eyes. Clin Exp Ophthalmol. 2018;46(4):356-363.
18. Melles RB, Holladay JT, Chang WJ. Accuracy of intraocular lens calculation formulas. Ophthalmology. 2018;125(2):169-178.
19. Hoffer KJ. The Hoffer Q formula: a comparison of theoretic and regression formulas. J Cataract Refract Surg. 1993;19(6):700-712.
20. Cooke DL, Cooke TL. Comparison of 9 intraocular lens power calculation formulas. J Cataract Refract Surg. 2016;42(8):1157-1164.
21. American Society of Cataract and Refractive Surgery. Barrett toric calculator. www.ascrs.org/barrett-toric-calculator. Accessed February 5, 2020.
22. Holladay JT, Pettit G. Improving toric intraocular lens calculations using total surgically induced astigmatism for a 2.5 mm temporal incision. J Cataract Refract Surg. 2019;45(3):272-283.
23. Canovas C, Alarcon A, Rosén R, et al. New algorithm for toric intraocular lens power calculation considering the posterior corneal astigmatism. J Cataract Refract Surg. 2018;44(2):168-174.
24. Visser N, Berendschot TT, Bauer NJ, Nuijts RM. Vector analysis of corneal and refractive astigmatism changes following toric pseudophakic and toric phakic IOL implantation. Invest Ophthalmol Vis Sci. 2012;53(4):1865-1873.
25. Narváez J, Zimmerman G, Stulting RD, Chang DH. Accuracy of intraocular lens power prediction using the Hoffer Q, Holladay 1, Holladay 2, and SRK/T formulas. J Cataract Refract Surg. 2006;32(12):2050-2053.
Loneliness, social isolation in seniors need urgent attention
Health care systems need to take urgent action to address social isolation and loneliness among U.S. seniors, experts say.
A new report from the National Academies of Sciences, Engineering, and Medicine (NAS) points out that social isolation in this population is a major public health concern that contributes to heart disease, depression, and premature death.
The report authors note that the health care system remains an underused partner in preventing, identifying, and intervening in social isolation and loneliness among adults over age 50.
For seniors who are homebound, have no family, or do not belong to community or faith groups, a medical appointment or home health visit may be one of the few social interactions they have, the report notes.
Health care providers and systems may be “first responders” in recognizing lonely or socially isolated patients, committee chair Dan Blazer, MD, from Duke University School of Medicine, Durham, N.C., said during a press briefing.
As deadly as obesity, smoking
Committee member Julianne Holt-Lunstad, PhD, from Brigham Young University, Provo, Utah, noted that social isolation and loneliness are “distinctly different.”
Social isolation is defined as an objective lack of (or limited) social connections, while loneliness is a subjective perception of social isolation or the subjective feeling of being lonely.
Not all older adults are isolated or lonely, but they are more likely to face predisposing factors such as living alone and the loss of loved ones, she explained.
The issue may be compounded for LGBT, minority, and immigrant older adults, who may already face barriers to care, stigma, and discrimination. Social isolation and loneliness may also directly stem from chronic illness, hearing or vision loss, or mobility issues. In these cases, health care providers might be able to help prevent or reduce social isolation and loneliness by directly addressing the underlying health-related causes.
Holt-Lunstad told the briefing. The report offers a vision for how the health care system can identify people at risk of social isolation and loneliness, intervene, and engage other community partners.
It recommends that providers use validated tools to periodically assess patients who may be at risk for social isolation and loneliness and connect them to community resources for help.
The report also calls for greater education and training among health providers. Schools of health professions and training programs for direct care workers (eg, home health aides, nurse aides, and personal care aides) should incorporate social isolation and loneliness in their curricula, the report says.
It also offers recommendations for leveraging digital health and health technology, improving community partnerships, increasing funding for research, and creation of a national resource center under the Department of Health and Human Services.
Blazer said there remains “much to be learned” about what approaches to mitigating social isolation and loneliness work best in which populations.
The report, from the Committee on the Health and Medical Dimensions of Social Isolation and Loneliness in Older Adults, was sponsored by the AARP Foundation.
This article first appeared on Medscape.com.
Health care systems need to take urgent action to address social isolation and loneliness among U.S. seniors, experts say.
A new report from the National Academies of Sciences, Engineering, and Medicine (NAS) points out that social isolation in this population is a major public health concern that contributes to heart disease, depression, and premature death.
The report authors note that the health care system remains an underused partner in preventing, identifying, and intervening in social isolation and loneliness among adults over age 50.
For seniors who are homebound, have no family, or do not belong to community or faith groups, a medical appointment or home health visit may be one of the few social interactions they have, the report notes.
Health care providers and systems may be “first responders” in recognizing lonely or socially isolated patients, committee chair Dan Blazer, MD, from Duke University School of Medicine, Durham, N.C., said during a press briefing.
As deadly as obesity, smoking
Committee member Julianne Holt-Lunstad, PhD, from Brigham Young University, Provo, Utah, noted that social isolation and loneliness are “distinctly different.”
Social isolation is defined as an objective lack of (or limited) social connections, while loneliness is a subjective perception of social isolation or the subjective feeling of being lonely.
Not all older adults are isolated or lonely, but they are more likely to face predisposing factors such as living alone and the loss of loved ones, she explained.
The issue may be compounded for LGBT, minority, and immigrant older adults, who may already face barriers to care, stigma, and discrimination. Social isolation and loneliness may also directly stem from chronic illness, hearing or vision loss, or mobility issues. In these cases, health care providers might be able to help prevent or reduce social isolation and loneliness by directly addressing the underlying health-related causes.
Holt-Lunstad told the briefing. The report offers a vision for how the health care system can identify people at risk of social isolation and loneliness, intervene, and engage other community partners.
It recommends that providers use validated tools to periodically assess patients who may be at risk for social isolation and loneliness and connect them to community resources for help.
The report also calls for greater education and training among health providers. Schools of health professions and training programs for direct care workers (eg, home health aides, nurse aides, and personal care aides) should incorporate social isolation and loneliness in their curricula, the report says.
It also offers recommendations for leveraging digital health and health technology, improving community partnerships, increasing funding for research, and creation of a national resource center under the Department of Health and Human Services.
Blazer said there remains “much to be learned” about what approaches to mitigating social isolation and loneliness work best in which populations.
The report, from the Committee on the Health and Medical Dimensions of Social Isolation and Loneliness in Older Adults, was sponsored by the AARP Foundation.
This article first appeared on Medscape.com.
Health care systems need to take urgent action to address social isolation and loneliness among U.S. seniors, experts say.
A new report from the National Academies of Sciences, Engineering, and Medicine (NAS) points out that social isolation in this population is a major public health concern that contributes to heart disease, depression, and premature death.
The report authors note that the health care system remains an underused partner in preventing, identifying, and intervening in social isolation and loneliness among adults over age 50.
For seniors who are homebound, have no family, or do not belong to community or faith groups, a medical appointment or home health visit may be one of the few social interactions they have, the report notes.
Health care providers and systems may be “first responders” in recognizing lonely or socially isolated patients, committee chair Dan Blazer, MD, from Duke University School of Medicine, Durham, N.C., said during a press briefing.
As deadly as obesity, smoking
Committee member Julianne Holt-Lunstad, PhD, from Brigham Young University, Provo, Utah, noted that social isolation and loneliness are “distinctly different.”
Social isolation is defined as an objective lack of (or limited) social connections, while loneliness is a subjective perception of social isolation or the subjective feeling of being lonely.
Not all older adults are isolated or lonely, but they are more likely to face predisposing factors such as living alone and the loss of loved ones, she explained.
The issue may be compounded for LGBT, minority, and immigrant older adults, who may already face barriers to care, stigma, and discrimination. Social isolation and loneliness may also directly stem from chronic illness, hearing or vision loss, or mobility issues. In these cases, health care providers might be able to help prevent or reduce social isolation and loneliness by directly addressing the underlying health-related causes.
Holt-Lunstad told the briefing. The report offers a vision for how the health care system can identify people at risk of social isolation and loneliness, intervene, and engage other community partners.
It recommends that providers use validated tools to periodically assess patients who may be at risk for social isolation and loneliness and connect them to community resources for help.
The report also calls for greater education and training among health providers. Schools of health professions and training programs for direct care workers (eg, home health aides, nurse aides, and personal care aides) should incorporate social isolation and loneliness in their curricula, the report says.
It also offers recommendations for leveraging digital health and health technology, improving community partnerships, increasing funding for research, and creation of a national resource center under the Department of Health and Human Services.
Blazer said there remains “much to be learned” about what approaches to mitigating social isolation and loneliness work best in which populations.
The report, from the Committee on the Health and Medical Dimensions of Social Isolation and Loneliness in Older Adults, was sponsored by the AARP Foundation.
This article first appeared on Medscape.com.
RA magnifies fragility fracture risk in ESRD
MAUI, HAWAII – Comorbid rheumatoid arthritis is a force multiplier for fragility fracture risk in patients with end-stage renal disease, Renée Peterkin-McCalman, MD, reported at the 2020 Rheumatology Winter Clinical Symposium.
“Patients with RA and ESRD are at substantially increased risk of osteoporotic fragility fractures compared to the overall population of ESRD patients. So fracture prevention prior to initiation of dialysis should be a focus of care in patients with RA,” said Dr. Peterkin-McCalman, a rheumatology fellow at the Medical College of Georgia, Augusta.
She presented a retrospective cohort study of 10,706 adults who initiated hemodialysis or peritoneal dialysis for ESRD during 2005-2008, including 1,040 who also had RA. All subjects were drawn from the United States Renal Data System. The impetus for the study, Dr. Peterkin-McCalman explained in an interview, was that although prior studies have established that RA and ESRD are independent risk factors for osteoporotic fractures, the interplay between the two was previously unknown.
The risk of incident osteoporotic fractures during the first 3 years after going on renal dialysis was 14.7% in patients with ESRD only, vaulting to 25.6% in those with comorbid RA. Individuals with both RA and ESRD were at an adjusted 1.83-fold increased overall risk for new fragility fractures and at 1.85-fold increased risk for hip fracture, compared to those without RA.
Far and away the strongest risk factor for incident osteoporotic fractures in the group with RA plus ESRD was a history of a fracture sustained within 5 years prior to initiation of dialysis, with an associated 11.5-fold increased fracture risk overall and an 8.2-fold increased risk of hip fracture.
“The reason that’s important is we don’t really have any medications to reduce fracture risk once you get to ESRD. Of course, we have bisphosphonates and Prolia (denosumab) and things like that, but that’s in patients with milder CKD [chronic kidney disease] or no renal disease at all. So the goal is to identify the patients early who are at higher risk so that we can protect those bones before they get to ESRD and we have nothing left to treat them with,” she said.
In addition to a history of prevalent fracture prior to starting ESRD, the other risk factors for fracture in patients with ESRD and comorbid RA Dr. Peterkin-McCalman identified in her study included age greater than 50 years at the start of dialysis and female gender, which was associated with a twofold greater fracture risk than in men. Black patients with ESRD and RA were 64% less likely than whites to experience an incident fragility fracture. And the fracture risk was higher in patients on hemodialysis than with peritoneal dialysis.
Her study was supported by the Medical College of Georgia and a research grant from Dialysis Clinic Inc.
SOURCE: Peterkin-McCalman R et al. RWCS 2020.
MAUI, HAWAII – Comorbid rheumatoid arthritis is a force multiplier for fragility fracture risk in patients with end-stage renal disease, Renée Peterkin-McCalman, MD, reported at the 2020 Rheumatology Winter Clinical Symposium.
“Patients with RA and ESRD are at substantially increased risk of osteoporotic fragility fractures compared to the overall population of ESRD patients. So fracture prevention prior to initiation of dialysis should be a focus of care in patients with RA,” said Dr. Peterkin-McCalman, a rheumatology fellow at the Medical College of Georgia, Augusta.
She presented a retrospective cohort study of 10,706 adults who initiated hemodialysis or peritoneal dialysis for ESRD during 2005-2008, including 1,040 who also had RA. All subjects were drawn from the United States Renal Data System. The impetus for the study, Dr. Peterkin-McCalman explained in an interview, was that although prior studies have established that RA and ESRD are independent risk factors for osteoporotic fractures, the interplay between the two was previously unknown.
The risk of incident osteoporotic fractures during the first 3 years after going on renal dialysis was 14.7% in patients with ESRD only, vaulting to 25.6% in those with comorbid RA. Individuals with both RA and ESRD were at an adjusted 1.83-fold increased overall risk for new fragility fractures and at 1.85-fold increased risk for hip fracture, compared to those without RA.
Far and away the strongest risk factor for incident osteoporotic fractures in the group with RA plus ESRD was a history of a fracture sustained within 5 years prior to initiation of dialysis, with an associated 11.5-fold increased fracture risk overall and an 8.2-fold increased risk of hip fracture.
“The reason that’s important is we don’t really have any medications to reduce fracture risk once you get to ESRD. Of course, we have bisphosphonates and Prolia (denosumab) and things like that, but that’s in patients with milder CKD [chronic kidney disease] or no renal disease at all. So the goal is to identify the patients early who are at higher risk so that we can protect those bones before they get to ESRD and we have nothing left to treat them with,” she said.
In addition to a history of prevalent fracture prior to starting ESRD, the other risk factors for fracture in patients with ESRD and comorbid RA Dr. Peterkin-McCalman identified in her study included age greater than 50 years at the start of dialysis and female gender, which was associated with a twofold greater fracture risk than in men. Black patients with ESRD and RA were 64% less likely than whites to experience an incident fragility fracture. And the fracture risk was higher in patients on hemodialysis than with peritoneal dialysis.
Her study was supported by the Medical College of Georgia and a research grant from Dialysis Clinic Inc.
SOURCE: Peterkin-McCalman R et al. RWCS 2020.
MAUI, HAWAII – Comorbid rheumatoid arthritis is a force multiplier for fragility fracture risk in patients with end-stage renal disease, Renée Peterkin-McCalman, MD, reported at the 2020 Rheumatology Winter Clinical Symposium.
“Patients with RA and ESRD are at substantially increased risk of osteoporotic fragility fractures compared to the overall population of ESRD patients. So fracture prevention prior to initiation of dialysis should be a focus of care in patients with RA,” said Dr. Peterkin-McCalman, a rheumatology fellow at the Medical College of Georgia, Augusta.
She presented a retrospective cohort study of 10,706 adults who initiated hemodialysis or peritoneal dialysis for ESRD during 2005-2008, including 1,040 who also had RA. All subjects were drawn from the United States Renal Data System. The impetus for the study, Dr. Peterkin-McCalman explained in an interview, was that although prior studies have established that RA and ESRD are independent risk factors for osteoporotic fractures, the interplay between the two was previously unknown.
The risk of incident osteoporotic fractures during the first 3 years after going on renal dialysis was 14.7% in patients with ESRD only, vaulting to 25.6% in those with comorbid RA. Individuals with both RA and ESRD were at an adjusted 1.83-fold increased overall risk for new fragility fractures and at 1.85-fold increased risk for hip fracture, compared to those without RA.
Far and away the strongest risk factor for incident osteoporotic fractures in the group with RA plus ESRD was a history of a fracture sustained within 5 years prior to initiation of dialysis, with an associated 11.5-fold increased fracture risk overall and an 8.2-fold increased risk of hip fracture.
“The reason that’s important is we don’t really have any medications to reduce fracture risk once you get to ESRD. Of course, we have bisphosphonates and Prolia (denosumab) and things like that, but that’s in patients with milder CKD [chronic kidney disease] or no renal disease at all. So the goal is to identify the patients early who are at higher risk so that we can protect those bones before they get to ESRD and we have nothing left to treat them with,” she said.
In addition to a history of prevalent fracture prior to starting ESRD, the other risk factors for fracture in patients with ESRD and comorbid RA Dr. Peterkin-McCalman identified in her study included age greater than 50 years at the start of dialysis and female gender, which was associated with a twofold greater fracture risk than in men. Black patients with ESRD and RA were 64% less likely than whites to experience an incident fragility fracture. And the fracture risk was higher in patients on hemodialysis than with peritoneal dialysis.
Her study was supported by the Medical College of Georgia and a research grant from Dialysis Clinic Inc.
SOURCE: Peterkin-McCalman R et al. RWCS 2020.
REPORTING FROM RWCS 2020
Refining your approach to hypothyroidism treatment
CASE
A 38-year-old woman presents for a routine physical. Other than urgent care visits for 1 episode of influenza and 2 upper respiratory illnesses, she has not seen a physician for a physical in 5 years. She denies any significant medical history. She takes naproxen occasionally for chronic right knee pain. She does not use tobacco or alcohol. Recently, she has started using a meal replacement shake at lunchtime for weight management. She performs aerobic exercise 30 to 40 minutes per day, 5 days per week. Her family history is significant for type 2 diabetes mellitus, arthritis, heart disease, and hyperlipidemia on her mother’s side. She is single, is not currently sexually active, works as a pharmacy technician, and has no children. A high-risk human papillomavirus test was normal 4 years ago.
A review of systems is notable for a 20-pound weight gain over the past year, worsening heartburn over the past 2 weeks, and chronic knee pain, which is greater in the right knee than the left. She denies weakness, fatigue, nausea, diarrhea, constipation, or abdominal pain. Vital signs reveal a blood pressure of 146/88 mm Hg, a heart rate of 63 bpm, a temperature of 98°F (36.7°C), a respiratory rate of 16, a height of 5’7’’ (1.7 m), a weight of 217 lbs (98.4 kg), and a peripheral capillary oxygen saturation (SpO2) of 99% on room air. The physical exam reveals a body mass index (BMI) of 34, warm dry skin, and coarse brittle hair.
Lab results reveal a thyroid-stimulating hormone (TSH) level of 11.17 mIU/L (reference range, 0.45-4.5 mIU/L) and a free thyroxine (T4) of 0.58 ng/dL (reference range, 0.8-2.8 ng/dL). A basic metabolic panel and hemoglobin A1C level are normal.
What would you recommend?
In the United States, the prevalence of overt hypothyroidism (defined as a TSH level > 4.5 mIU/L and a low free T4) among people ≥ 12 years of age was estimated at 0.3% based on National Health and Nutrition Examination Survey (NHANES) data from 1999-2002.1 Subclinical hypothyroidism (TSH level > 4.5 mIU/L but < 10 mIU/L and a normal T4 level) is even more common, with an estimated prevalence of 3.4%.1 Hypothyroidism is more common in females and occurs more frequently in Caucasian Americans and Mexican Americans than in African Americans.1
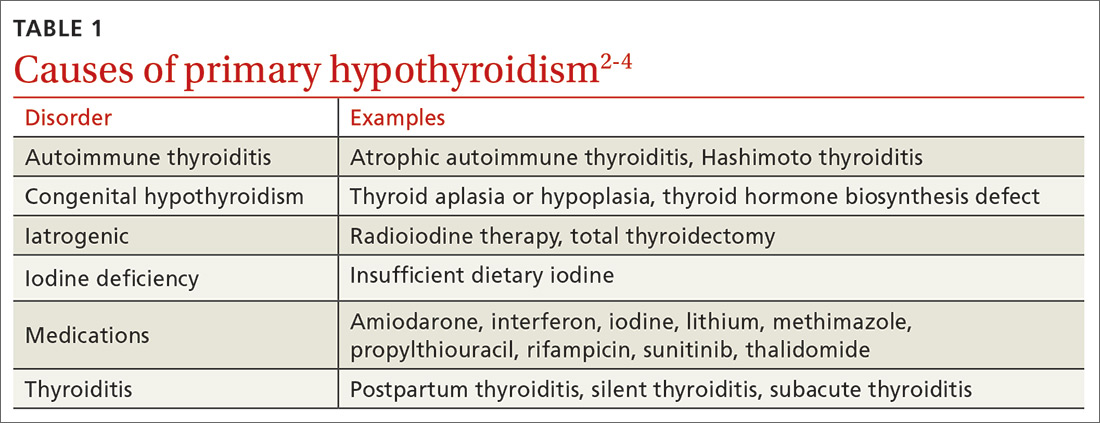
The most common etiologies of hypothyroidism include autoimmune thyroiditis (eg, Hashimoto thyroiditis, atrophic autoimmune thyroiditis) and iatrogenic causes (eg, after radioactive iodine ablation or thyroidectomy) (TABLE 1).2-4
Initiating thyroid hormone replacement
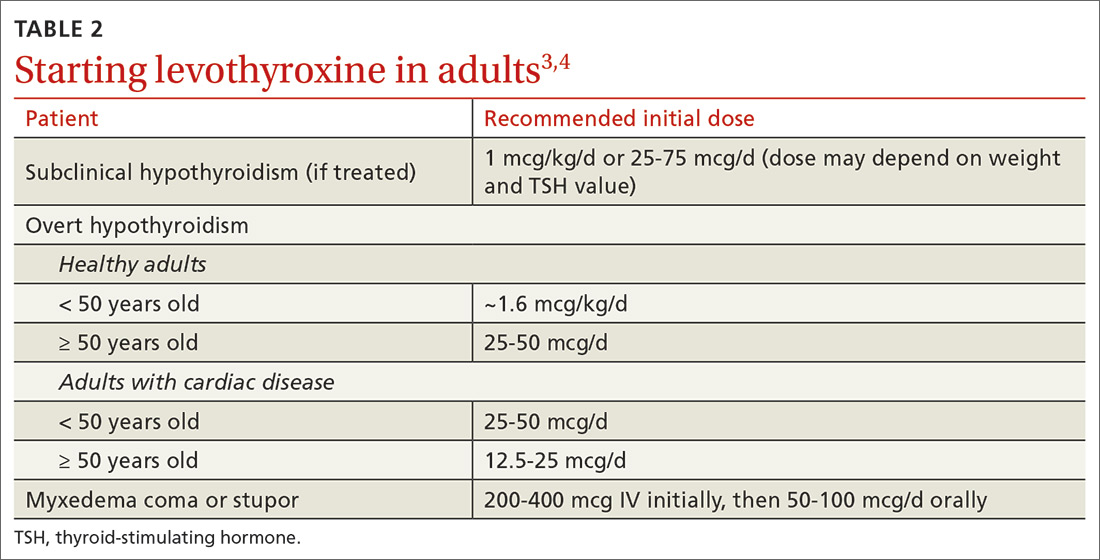
Factors to consider when starting a patient on thyroid hormone replacement include age, weight, symptom severity, TSH level, goal TSH value, adverse effects from thyroid supplements, history of cardiac disease, and, for women of child-bearing age, the desire for pregnancy vs the use of contraceptives. Most adult patients < 50 years with overt hypothyroidism can begin a weight-based dose of levothyroxine: ~1.6 mcg/kg/d (based on ideal body weight).3
Continue to: For adults with cardiac disease...
For adults with cardiac disease, the risk of over-replacement limits initial dosing to 25 to 50 mcg/d for patients < 50 years (12.5-25 mcg/d; ≥ 50 years).3 For adults with subclinical hypothyroidism, it is reasonable to begin therapy at a lower daily dose (eg, 25-75 mcg/d) depending on baseline TSH level, symptoms (the patient may be asymptomatic), and the presence of cardiac disease (TABLE 23,4). Consider treatment in patients with subclinical hypothyroidism particularly when patients have a goiter or dyslipidemia and in women contemplating pregnancy in the near future. Elderly patients may require a dose 20% to 25% lower than younger adults because of decreased body mass.3
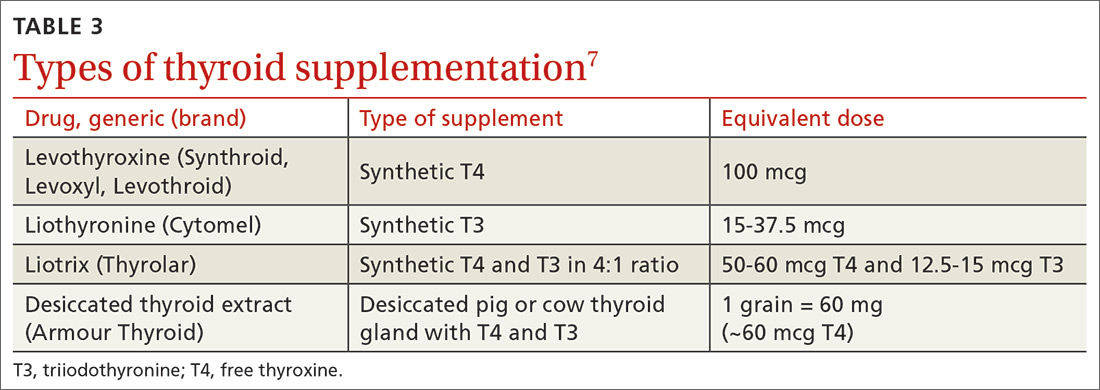
Levothyroxine is considered first-line therapy for hypothyroidism because of its low cost, dose consistency, low risk of allergic reactions, and potential to cause fewer cardiac adverse effects than triiodothyronine (T3) products such as desiccated thyroid extract.5 Although data have not shown an absolute increase in cardiovascular adverse effects, T3 products have a higher T3 vs T4 ratio, giving them a theoretically increased risk.5,6 Desiccated thyroid extract also has been associated with allergic reactions.5
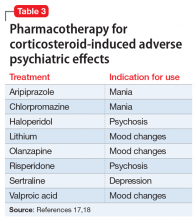
Use of liothyronine alone or in combination with levothyroxine lacks evidence and guideline support.4 Furthermore, it is dosed twice daily, which makes it less convenient, and concerns still exist that there may be an increase in cardiovascular adverse effects.4,6 See TABLE 37 for a summary of available products and their equivalent doses.
Maintaining patients on therapy
The maintenance phase begins once hypothyroidism is diagnosed and treatment is initiated. This phase includes regular monitoring with laboratory studies, office visits, and as-needed adjustments in hormone replacement dosing. The frequency at which all of these occur is variable and based on a number of factors including the patient’s other medical conditions, use of other medications including over-the-counter agents, the patient’s age, weight changes, and pregnancy status.3,4,8 In general, dosage adjustments of 12.5 to 25 mcg can be made at 6- to 8-week intervals based on repeat TSH measurements, patient symptoms, and comorbidities.3
Once a patient is symptomatically stable and laboratory values have normalized, the recommended frequency of laboratory evaluation and office visits is every 12 months, barring significant changes in any of the factors mentioned above. At each visit, physicians should perform medication (including supplements) reconciliation and discuss any health condition updates. Changes to the therapy plan, including frequency or timing of laboratory tests, may be necessary if patients begin taking medications that alter the absorption or function of levothyroxine (eg, steroids).
Continue to: To maximize absorption...
To maximize absorption, providers should review with patients the optimal way to take thyroid hormones. Levothyroxine is approximately 70% to 80% absorbed under ideal conditions, which means taking it in the morning at least 30 to 60 minutes before eating or 3 to 4 hours after the last meal of the day.3,9-13 Of note, TSH levels may increase slightly in patients taking proton pump inhibitors, but this does not usually require a dose increase of thyroid hormone.11 Given that some supplements, particularly iron and calcium, can interfere with absorption, it is recommended to maintain a 3- to 4-hour gap between taking those supplements and taking levothyroxine.12-14 For those patients unable or unwilling to adhere to these recommendations, an increase in levothyroxine dose may be required in order to compensate for the decreased absorption.
Don’t adjust hormone therapy based on clinical presentation alone. While clinical symptoms are important, it is not recommended to adjust hormone therapy based solely on clinical presentation. Common hypothyroid symptoms of dry skin, edema, weight gain, and fatigue may be caused by other medical conditions. While indices including Achilles reflex time and basal metabolic rate have shown some correlation to thyroid dysfunction, there has been limited evidence to show that longitudinal index changes reflect subtle changes in thyroid hormone levels.3
The most recent guidelines from the American Thyroid Association recommend that, “Symptoms should be followed, but considered in the context of serum thyrotropin values, relevant comorbidities, and other potential causes.”3
Special populations/circumstances to keep in mind
Malabsorption conditions. When a higher than expected weight-based dose of levothyroxine is required, physicians should review administration timing, adherence, and comorbid medical conditions that can affect absorption.
Several studies, for example, have demonstrated the impact of Helicobacter pylori gastritis on levothyroxine absorption and subsequent TSH levels.15-17 In one nonrandomized prospective study, patients with H pylori and hypothyroidism who were previously thought to be unresponsive to levothyroxine therapy had a decrease in average TSH level from 30.5 mIU/L to 4.2 mIU/L after H pylori was eradicated.15 Autoimmune atrophic gastritis and celiac disease, both of which are more common in those with other autoimmune diseases, are also associated with the need for higher than expected levothyroxine doses.17,18
Continue to: A history of gastric bypass surgery...
A history of gastric bypass surgery alone is not considered a risk factor for poor absorption of thyroid hormone, given that the majority of levothyroxine absorption occurs in the ileum.19,20 However, advancing age (> 70 years) and extreme obesity (BMI > 40) are independent risk factors for decreased levothyroxine absorption.20,21
Women of reproductive age and pregnant women. Overt untreated or undertreated hypothyroidism can be associated with increased risk of maternal and fetal complications including decreased fertility, miscarriage, preterm delivery, lower birth rates, and infant cognitive deficits.3,22 Therefore, the main focus should be optimization of thyroid hormone levels prior to and during pregnancy.3,4,8,22 Thyroid hormone replacement needs to be increased during pregnancy in approximately 50% to 85% of women using thyroid replacement prior to pregnancy, but the dose requirements vary based on the underlying etiology of thyroid dysfunction.
One initial option for patients on a stable dose before pregnancy is to increase their daily dose by a half tablet (1.5 × daily dose) immediately after home confirmation of pregnancy, until finer dose adjustments (usually increases of 25%-60% ) can be made by a physician. Experts recommend that a TSH level be obtained every 4 weeks until mid-gestation and then at least once around 30 weeks’ gestation to ensure specific targets are being met with dose adjustments.22 Optimal thyrotropin reference ranges during conception and pregnancy can be found in the literature.23
Patients who have positive antibodies and normal thyroid function tests. Patients who are screened for thyroid disorders may demonstrate normal thyroid function (ie, euthyroid) with TSH, free T4, and, if checked, free T3, all within normal ranges. Despite these normal lab results, patients may have additional test results that demonstrate positive thyroid autoantibodies including thyroglobulin antibodies and/or thyroid peroxidase antibodies. Thyroid autoimmunity itself has been associated with a range of other autoimmune conditions as well as an increased risk of thyroid cancer in those with Hashimoto thyroiditis.24 Two studies showed that prophylactic treatment of euthyroid patients with levothyroxine led to a reduction in antibody levels and a lower TSH level.25,26 However, no studies have focused on patient-oriented outcomes such as hospitalizations, quality of life, or symptoms. If the patient remains asymptomatic, we recommend no treatment, but that the patient’s TSH levels be monitored every 12 months.27
Elderly patients. Population data have shown that TSH increases normally with age, with a TSH level of 7.5 mIU/L being the upper limit of normal for a population of healthy adults > 80 years of age.28,29 Overall, studies have failed to show any benefit in treating elderly patients with subclinical hypothyroidism unless their TSH level exceeds 10 mIU/L.6,21 The one exception is elderly patients with heart failure in whom untreated subclinical hypothyroidism has been shown to be associated with higher mortality.30
Continue to: Elderly patients are at higher risk...
Elderly patients are at higher risk for adverse effects of thyroid over-replacement, including atrial fibrillation and osteoporosis. While there have been no randomized trials examining target TSH levels in this population, a reasonable recommendation is a goal TSH level of 4 to 6 mIU/L for elderly patients ≥ 70 years.4
CASE
As a result of the patient’s elevated TSH level and symptoms of hypothyroidism, you start levothyroxine 150 mcg/d by mouth, counsel her on potential adverse effects, and schedule a follow-up visit with another TSH check in 6 weeks.
Follow-up laboratory studies 6 weeks later reveal a TSH level of 5.86 mIU/L (reference range, 0.45-4.5 mIU/L) and a free T4 level of 0.74 ng/dL (reference range, 0.8-2.8 ng/dL). Based on those results, you increase the dose of levothyroxine to 175 mcg/d.
At her follow-up visit 12 weeks after initial presentation, her TSH level is 3.85 mIU/L. She reports feeling better overall with less fatigue, and she has lost 5 pounds since her last visit. You recommend she continue levothyroxine 175 mcg/d after reviewing medication compliance with the patient and ensuring she is indeed taking it in the morning, at least 30 minutes prior to eating. With improved but not resolved symptoms, she agrees to follow-up with repeat TSH laboratory studies in 6 weeks to determine whether further dose adjustments are necessary. Given that she is of reproductive age and her TSH level is suboptimal for pregnancy, you caution her about heightened pregnancy/fetal risks with a suboptimal TSH and recommend that she use reliable contraception.
CORRESPONDENCE
Christopher Bunt, MD, FAAFP, 5 Charleston Center Drive, Suite 263, MSC 192,Charleston, SC 29425; [email protected]
1. Aoki Y, Belin RM, Clickner R, et al. Serum TSH and total T4 in the United States population and their association with participant characteristics: National Health and Nutrition Examination Survey (NHANES 1999-2002). Thyroid. 2007;17:1211-1223.
2. Vaidya B, Pearce SH. Management of hypothyroidism in adults. BMJ. 2008;337:a801.
3. Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012;18:988-1028.
4. Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association task force on thyroid hormone replacement. Thyroid. 2014;24:1670-1751.
5. Toft AD. Thyroxine therapy. N Engl J Med. 1994;331:174-180.
6. Floriani C, Gencer B, Collet TH, et al. Subclinical thyroid dysfunction and cardiovascular diseases: 2016 update. Eur Heart J. 2018;39:503-507.
7. Lexi-Comp, Inc. (Lexi-Drugs®). https://online.lexi.com/lco/action/login. Accessed July 7, 2017.
8. Okosieme O, Gilbert J, Abraham P, et al. Management of primary hypothyroidism: statement by the British Thyroid Association Executive Committee. Clin Endocrinol (Oxf). 2016;84:799-808.
9. Fish LH, Schwartz HL, Cavanaugh J, et al. Replacement dose, metabolism, and bioavailability of levothyroxine in the treatment of hypothyroidism. Role of triiodothyronine in pituitary feedback in humans. N Engl J Med. 1987;316:764-770.
10. John-Kalarickal J, Pearlman G, Carlson HE. New medications which decrease levothyroxine absorption. Thyroid. 2007;17:763-765.
11. Sachmechi I, Reich DM, Aninyei M, et al. Effect of proton pump inhibitors on serum thyroid-stimulating hormone level in euthyroid patients treated with levothyroxine for hypothyroidism. Endocr Pract. 2007;13:345-349.
12. Sperber AD, Liel Y. Evidence for interference with the intestinal absorption of levothyroxine sodium by aluminum hydroxide. Arch Intern Med. 1992;152:183-184.
13. Zamfirescu I, Carlson HE. Absorption of levothyroxine when coadministered with various calcium formulations. Thyroid. 2011;21:483-486.
14. Campbell NR, Hasinoff BB, Stalts H, et al. Ferrous sulfate reduces thyroxine efficacy in patients with hypothyroidism. Ann Intern Med. 1992;117:1010-1013.
15. Bugdaci MS, Zuhur SS, Sokmen M, et al. The role of Helicobacter pylori in patients with hypothyroidism in whom could not be achieved normal thyrotropin levels despite treatment with high doses of thyroxine. Helicobacter. 2011;16:124-130.
16. Centanni M, Gargano L, Canettieri G, et al. Thyroxine in goiter, Helicobacter pylori infection, and chronic gastritis. N Engl J Med. 2006;354:1787-1795.
17. Centanni M, Marignani M, Gargano L, et al. Atrophic body gastritis in patients with autoimmune thyroid disease: an underdiagnosed association. Arch Intern Med. 1999;159:1726-1730.
18. Collins D, Wilcox R, Nathan M, et al. Celiac disease and hypothyroidism. Am J Med. 2012;125:278-282.
19. Azizi F, Belur R, Albano J. Malabsorption of thyroid hormones after jejunoileal bypass for obesity. Ann Intern Med. 1979;90:941-942.
20. Gkotsina M, Michalaki M, Mamali I, et al. Improved levothyroxine pharmacokinetics after bariatric surgery. Thyroid. 2013;23:414-419.
21. Hennessey JV, Espaillat R. Diagnosis and management of subclinical hypothyroidism in elderly adults: a review of the literature. J Am Geriatr Soc. 2015;63:1663-1673.
22. Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017;27:315-389.
23. Carney LA, Quinlan JD, West JM. Thyroid disease in pregnancy. Am Fam Physician. 2014;89:273-278.
24. Fröhlich E, Wahl R. Thyroid autoimmunity: role of anti-thyroid antibodies in thyroid and extra-thyroidal diseases. Front Immunol. 2017;8:521.
25. Aksoy DY, Kerimoglu U, Okur H, et al. Effects of prophylactic thyroid hormone replacement in euthyroid Hashimoto’s thyroiditis. Endocr J. 2005;52:337-343.
26. Padberg S, Heller K, Usadel KH, et al. One-year prophylactic treatment of euthyroid Hashimoto’s thyroiditis patients with levothyroxine: is there a benefit? Thyroid. 2001;11:249-255.
27. Rugge B, Balshem H, Sehgal R, et al. Screening and Treatment of Subclinical Hypothyroidism or Hyperthyroidism [Internet]. Comparative Effectiveness Reviews, No. 24. Rockville, MD: Agency for Healthcare Research and Quality; October 2011. www.ncbi.nlm.nih.gov/books/NBK83492/. Accessed February 21, 2020.
28. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489-499.
29. Surks MI, Hollowell JG. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab. 2007;92:4575-4582.
30. Pasqualetti G, Tognini S, Polini A, et al. Is subclinical hypothyroidism a cardiovascular risk factor in the elderly? J Clin Endocrinol Metab. 2013;98:2256-2266.
CASE
A 38-year-old woman presents for a routine physical. Other than urgent care visits for 1 episode of influenza and 2 upper respiratory illnesses, she has not seen a physician for a physical in 5 years. She denies any significant medical history. She takes naproxen occasionally for chronic right knee pain. She does not use tobacco or alcohol. Recently, she has started using a meal replacement shake at lunchtime for weight management. She performs aerobic exercise 30 to 40 minutes per day, 5 days per week. Her family history is significant for type 2 diabetes mellitus, arthritis, heart disease, and hyperlipidemia on her mother’s side. She is single, is not currently sexually active, works as a pharmacy technician, and has no children. A high-risk human papillomavirus test was normal 4 years ago.
A review of systems is notable for a 20-pound weight gain over the past year, worsening heartburn over the past 2 weeks, and chronic knee pain, which is greater in the right knee than the left. She denies weakness, fatigue, nausea, diarrhea, constipation, or abdominal pain. Vital signs reveal a blood pressure of 146/88 mm Hg, a heart rate of 63 bpm, a temperature of 98°F (36.7°C), a respiratory rate of 16, a height of 5’7’’ (1.7 m), a weight of 217 lbs (98.4 kg), and a peripheral capillary oxygen saturation (SpO2) of 99% on room air. The physical exam reveals a body mass index (BMI) of 34, warm dry skin, and coarse brittle hair.
Lab results reveal a thyroid-stimulating hormone (TSH) level of 11.17 mIU/L (reference range, 0.45-4.5 mIU/L) and a free thyroxine (T4) of 0.58 ng/dL (reference range, 0.8-2.8 ng/dL). A basic metabolic panel and hemoglobin A1C level are normal.
What would you recommend?
In the United States, the prevalence of overt hypothyroidism (defined as a TSH level > 4.5 mIU/L and a low free T4) among people ≥ 12 years of age was estimated at 0.3% based on National Health and Nutrition Examination Survey (NHANES) data from 1999-2002.1 Subclinical hypothyroidism (TSH level > 4.5 mIU/L but < 10 mIU/L and a normal T4 level) is even more common, with an estimated prevalence of 3.4%.1 Hypothyroidism is more common in females and occurs more frequently in Caucasian Americans and Mexican Americans than in African Americans.1
The most common etiologies of hypothyroidism include autoimmune thyroiditis (eg, Hashimoto thyroiditis, atrophic autoimmune thyroiditis) and iatrogenic causes (eg, after radioactive iodine ablation or thyroidectomy) (TABLE 1).2-4

Initiating thyroid hormone replacement
Factors to consider when starting a patient on thyroid hormone replacement include age, weight, symptom severity, TSH level, goal TSH value, adverse effects from thyroid supplements, history of cardiac disease, and, for women of child-bearing age, the desire for pregnancy vs the use of contraceptives. Most adult patients < 50 years with overt hypothyroidism can begin a weight-based dose of levothyroxine: ~1.6 mcg/kg/d (based on ideal body weight).3
Continue to: For adults with cardiac disease...
For adults with cardiac disease, the risk of over-replacement limits initial dosing to 25 to 50 mcg/d for patients < 50 years (12.5-25 mcg/d; ≥ 50 years).3 For adults with subclinical hypothyroidism, it is reasonable to begin therapy at a lower daily dose (eg, 25-75 mcg/d) depending on baseline TSH level, symptoms (the patient may be asymptomatic), and the presence of cardiac disease (TABLE 23,4). Consider treatment in patients with subclinical hypothyroidism particularly when patients have a goiter or dyslipidemia and in women contemplating pregnancy in the near future. Elderly patients may require a dose 20% to 25% lower than younger adults because of decreased body mass.3

Levothyroxine is considered first-line therapy for hypothyroidism because of its low cost, dose consistency, low risk of allergic reactions, and potential to cause fewer cardiac adverse effects than triiodothyronine (T3) products such as desiccated thyroid extract.5 Although data have not shown an absolute increase in cardiovascular adverse effects, T3 products have a higher T3 vs T4 ratio, giving them a theoretically increased risk.5,6 Desiccated thyroid extract also has been associated with allergic reactions.5
Use of liothyronine alone or in combination with levothyroxine lacks evidence and guideline support.4 Furthermore, it is dosed twice daily, which makes it less convenient, and concerns still exist that there may be an increase in cardiovascular adverse effects.4,6 See TABLE 37 for a summary of available products and their equivalent doses.

Maintaining patients on therapy
The maintenance phase begins once hypothyroidism is diagnosed and treatment is initiated. This phase includes regular monitoring with laboratory studies, office visits, and as-needed adjustments in hormone replacement dosing. The frequency at which all of these occur is variable and based on a number of factors including the patient’s other medical conditions, use of other medications including over-the-counter agents, the patient’s age, weight changes, and pregnancy status.3,4,8 In general, dosage adjustments of 12.5 to 25 mcg can be made at 6- to 8-week intervals based on repeat TSH measurements, patient symptoms, and comorbidities.3
Once a patient is symptomatically stable and laboratory values have normalized, the recommended frequency of laboratory evaluation and office visits is every 12 months, barring significant changes in any of the factors mentioned above. At each visit, physicians should perform medication (including supplements) reconciliation and discuss any health condition updates. Changes to the therapy plan, including frequency or timing of laboratory tests, may be necessary if patients begin taking medications that alter the absorption or function of levothyroxine (eg, steroids).
Continue to: To maximize absorption...
To maximize absorption, providers should review with patients the optimal way to take thyroid hormones. Levothyroxine is approximately 70% to 80% absorbed under ideal conditions, which means taking it in the morning at least 30 to 60 minutes before eating or 3 to 4 hours after the last meal of the day.3,9-13 Of note, TSH levels may increase slightly in patients taking proton pump inhibitors, but this does not usually require a dose increase of thyroid hormone.11 Given that some supplements, particularly iron and calcium, can interfere with absorption, it is recommended to maintain a 3- to 4-hour gap between taking those supplements and taking levothyroxine.12-14 For those patients unable or unwilling to adhere to these recommendations, an increase in levothyroxine dose may be required in order to compensate for the decreased absorption.
Don’t adjust hormone therapy based on clinical presentation alone. While clinical symptoms are important, it is not recommended to adjust hormone therapy based solely on clinical presentation. Common hypothyroid symptoms of dry skin, edema, weight gain, and fatigue may be caused by other medical conditions. While indices including Achilles reflex time and basal metabolic rate have shown some correlation to thyroid dysfunction, there has been limited evidence to show that longitudinal index changes reflect subtle changes in thyroid hormone levels.3
The most recent guidelines from the American Thyroid Association recommend that, “Symptoms should be followed, but considered in the context of serum thyrotropin values, relevant comorbidities, and other potential causes.”3
Special populations/circumstances to keep in mind
Malabsorption conditions. When a higher than expected weight-based dose of levothyroxine is required, physicians should review administration timing, adherence, and comorbid medical conditions that can affect absorption.
Several studies, for example, have demonstrated the impact of Helicobacter pylori gastritis on levothyroxine absorption and subsequent TSH levels.15-17 In one nonrandomized prospective study, patients with H pylori and hypothyroidism who were previously thought to be unresponsive to levothyroxine therapy had a decrease in average TSH level from 30.5 mIU/L to 4.2 mIU/L after H pylori was eradicated.15 Autoimmune atrophic gastritis and celiac disease, both of which are more common in those with other autoimmune diseases, are also associated with the need for higher than expected levothyroxine doses.17,18
Continue to: A history of gastric bypass surgery...
A history of gastric bypass surgery alone is not considered a risk factor for poor absorption of thyroid hormone, given that the majority of levothyroxine absorption occurs in the ileum.19,20 However, advancing age (> 70 years) and extreme obesity (BMI > 40) are independent risk factors for decreased levothyroxine absorption.20,21
Women of reproductive age and pregnant women. Overt untreated or undertreated hypothyroidism can be associated with increased risk of maternal and fetal complications including decreased fertility, miscarriage, preterm delivery, lower birth rates, and infant cognitive deficits.3,22 Therefore, the main focus should be optimization of thyroid hormone levels prior to and during pregnancy.3,4,8,22 Thyroid hormone replacement needs to be increased during pregnancy in approximately 50% to 85% of women using thyroid replacement prior to pregnancy, but the dose requirements vary based on the underlying etiology of thyroid dysfunction.
One initial option for patients on a stable dose before pregnancy is to increase their daily dose by a half tablet (1.5 × daily dose) immediately after home confirmation of pregnancy, until finer dose adjustments (usually increases of 25%-60% ) can be made by a physician. Experts recommend that a TSH level be obtained every 4 weeks until mid-gestation and then at least once around 30 weeks’ gestation to ensure specific targets are being met with dose adjustments.22 Optimal thyrotropin reference ranges during conception and pregnancy can be found in the literature.23
Patients who have positive antibodies and normal thyroid function tests. Patients who are screened for thyroid disorders may demonstrate normal thyroid function (ie, euthyroid) with TSH, free T4, and, if checked, free T3, all within normal ranges. Despite these normal lab results, patients may have additional test results that demonstrate positive thyroid autoantibodies including thyroglobulin antibodies and/or thyroid peroxidase antibodies. Thyroid autoimmunity itself has been associated with a range of other autoimmune conditions as well as an increased risk of thyroid cancer in those with Hashimoto thyroiditis.24 Two studies showed that prophylactic treatment of euthyroid patients with levothyroxine led to a reduction in antibody levels and a lower TSH level.25,26 However, no studies have focused on patient-oriented outcomes such as hospitalizations, quality of life, or symptoms. If the patient remains asymptomatic, we recommend no treatment, but that the patient’s TSH levels be monitored every 12 months.27
Elderly patients. Population data have shown that TSH increases normally with age, with a TSH level of 7.5 mIU/L being the upper limit of normal for a population of healthy adults > 80 years of age.28,29 Overall, studies have failed to show any benefit in treating elderly patients with subclinical hypothyroidism unless their TSH level exceeds 10 mIU/L.6,21 The one exception is elderly patients with heart failure in whom untreated subclinical hypothyroidism has been shown to be associated with higher mortality.30
Continue to: Elderly patients are at higher risk...
Elderly patients are at higher risk for adverse effects of thyroid over-replacement, including atrial fibrillation and osteoporosis. While there have been no randomized trials examining target TSH levels in this population, a reasonable recommendation is a goal TSH level of 4 to 6 mIU/L for elderly patients ≥ 70 years.4
CASE
As a result of the patient’s elevated TSH level and symptoms of hypothyroidism, you start levothyroxine 150 mcg/d by mouth, counsel her on potential adverse effects, and schedule a follow-up visit with another TSH check in 6 weeks.
Follow-up laboratory studies 6 weeks later reveal a TSH level of 5.86 mIU/L (reference range, 0.45-4.5 mIU/L) and a free T4 level of 0.74 ng/dL (reference range, 0.8-2.8 ng/dL). Based on those results, you increase the dose of levothyroxine to 175 mcg/d.
At her follow-up visit 12 weeks after initial presentation, her TSH level is 3.85 mIU/L. She reports feeling better overall with less fatigue, and she has lost 5 pounds since her last visit. You recommend she continue levothyroxine 175 mcg/d after reviewing medication compliance with the patient and ensuring she is indeed taking it in the morning, at least 30 minutes prior to eating. With improved but not resolved symptoms, she agrees to follow-up with repeat TSH laboratory studies in 6 weeks to determine whether further dose adjustments are necessary. Given that she is of reproductive age and her TSH level is suboptimal for pregnancy, you caution her about heightened pregnancy/fetal risks with a suboptimal TSH and recommend that she use reliable contraception.
CORRESPONDENCE
Christopher Bunt, MD, FAAFP, 5 Charleston Center Drive, Suite 263, MSC 192,Charleston, SC 29425; [email protected]
CASE
A 38-year-old woman presents for a routine physical. Other than urgent care visits for 1 episode of influenza and 2 upper respiratory illnesses, she has not seen a physician for a physical in 5 years. She denies any significant medical history. She takes naproxen occasionally for chronic right knee pain. She does not use tobacco or alcohol. Recently, she has started using a meal replacement shake at lunchtime for weight management. She performs aerobic exercise 30 to 40 minutes per day, 5 days per week. Her family history is significant for type 2 diabetes mellitus, arthritis, heart disease, and hyperlipidemia on her mother’s side. She is single, is not currently sexually active, works as a pharmacy technician, and has no children. A high-risk human papillomavirus test was normal 4 years ago.
A review of systems is notable for a 20-pound weight gain over the past year, worsening heartburn over the past 2 weeks, and chronic knee pain, which is greater in the right knee than the left. She denies weakness, fatigue, nausea, diarrhea, constipation, or abdominal pain. Vital signs reveal a blood pressure of 146/88 mm Hg, a heart rate of 63 bpm, a temperature of 98°F (36.7°C), a respiratory rate of 16, a height of 5’7’’ (1.7 m), a weight of 217 lbs (98.4 kg), and a peripheral capillary oxygen saturation (SpO2) of 99% on room air. The physical exam reveals a body mass index (BMI) of 34, warm dry skin, and coarse brittle hair.
Lab results reveal a thyroid-stimulating hormone (TSH) level of 11.17 mIU/L (reference range, 0.45-4.5 mIU/L) and a free thyroxine (T4) of 0.58 ng/dL (reference range, 0.8-2.8 ng/dL). A basic metabolic panel and hemoglobin A1C level are normal.
What would you recommend?
In the United States, the prevalence of overt hypothyroidism (defined as a TSH level > 4.5 mIU/L and a low free T4) among people ≥ 12 years of age was estimated at 0.3% based on National Health and Nutrition Examination Survey (NHANES) data from 1999-2002.1 Subclinical hypothyroidism (TSH level > 4.5 mIU/L but < 10 mIU/L and a normal T4 level) is even more common, with an estimated prevalence of 3.4%.1 Hypothyroidism is more common in females and occurs more frequently in Caucasian Americans and Mexican Americans than in African Americans.1
The most common etiologies of hypothyroidism include autoimmune thyroiditis (eg, Hashimoto thyroiditis, atrophic autoimmune thyroiditis) and iatrogenic causes (eg, after radioactive iodine ablation or thyroidectomy) (TABLE 1).2-4

Initiating thyroid hormone replacement
Factors to consider when starting a patient on thyroid hormone replacement include age, weight, symptom severity, TSH level, goal TSH value, adverse effects from thyroid supplements, history of cardiac disease, and, for women of child-bearing age, the desire for pregnancy vs the use of contraceptives. Most adult patients < 50 years with overt hypothyroidism can begin a weight-based dose of levothyroxine: ~1.6 mcg/kg/d (based on ideal body weight).3
Continue to: For adults with cardiac disease...
For adults with cardiac disease, the risk of over-replacement limits initial dosing to 25 to 50 mcg/d for patients < 50 years (12.5-25 mcg/d; ≥ 50 years).3 For adults with subclinical hypothyroidism, it is reasonable to begin therapy at a lower daily dose (eg, 25-75 mcg/d) depending on baseline TSH level, symptoms (the patient may be asymptomatic), and the presence of cardiac disease (TABLE 23,4). Consider treatment in patients with subclinical hypothyroidism particularly when patients have a goiter or dyslipidemia and in women contemplating pregnancy in the near future. Elderly patients may require a dose 20% to 25% lower than younger adults because of decreased body mass.3

Levothyroxine is considered first-line therapy for hypothyroidism because of its low cost, dose consistency, low risk of allergic reactions, and potential to cause fewer cardiac adverse effects than triiodothyronine (T3) products such as desiccated thyroid extract.5 Although data have not shown an absolute increase in cardiovascular adverse effects, T3 products have a higher T3 vs T4 ratio, giving them a theoretically increased risk.5,6 Desiccated thyroid extract also has been associated with allergic reactions.5
Use of liothyronine alone or in combination with levothyroxine lacks evidence and guideline support.4 Furthermore, it is dosed twice daily, which makes it less convenient, and concerns still exist that there may be an increase in cardiovascular adverse effects.4,6 See TABLE 37 for a summary of available products and their equivalent doses.

Maintaining patients on therapy
The maintenance phase begins once hypothyroidism is diagnosed and treatment is initiated. This phase includes regular monitoring with laboratory studies, office visits, and as-needed adjustments in hormone replacement dosing. The frequency at which all of these occur is variable and based on a number of factors including the patient’s other medical conditions, use of other medications including over-the-counter agents, the patient’s age, weight changes, and pregnancy status.3,4,8 In general, dosage adjustments of 12.5 to 25 mcg can be made at 6- to 8-week intervals based on repeat TSH measurements, patient symptoms, and comorbidities.3
Once a patient is symptomatically stable and laboratory values have normalized, the recommended frequency of laboratory evaluation and office visits is every 12 months, barring significant changes in any of the factors mentioned above. At each visit, physicians should perform medication (including supplements) reconciliation and discuss any health condition updates. Changes to the therapy plan, including frequency or timing of laboratory tests, may be necessary if patients begin taking medications that alter the absorption or function of levothyroxine (eg, steroids).
Continue to: To maximize absorption...
To maximize absorption, providers should review with patients the optimal way to take thyroid hormones. Levothyroxine is approximately 70% to 80% absorbed under ideal conditions, which means taking it in the morning at least 30 to 60 minutes before eating or 3 to 4 hours after the last meal of the day.3,9-13 Of note, TSH levels may increase slightly in patients taking proton pump inhibitors, but this does not usually require a dose increase of thyroid hormone.11 Given that some supplements, particularly iron and calcium, can interfere with absorption, it is recommended to maintain a 3- to 4-hour gap between taking those supplements and taking levothyroxine.12-14 For those patients unable or unwilling to adhere to these recommendations, an increase in levothyroxine dose may be required in order to compensate for the decreased absorption.
Don’t adjust hormone therapy based on clinical presentation alone. While clinical symptoms are important, it is not recommended to adjust hormone therapy based solely on clinical presentation. Common hypothyroid symptoms of dry skin, edema, weight gain, and fatigue may be caused by other medical conditions. While indices including Achilles reflex time and basal metabolic rate have shown some correlation to thyroid dysfunction, there has been limited evidence to show that longitudinal index changes reflect subtle changes in thyroid hormone levels.3
The most recent guidelines from the American Thyroid Association recommend that, “Symptoms should be followed, but considered in the context of serum thyrotropin values, relevant comorbidities, and other potential causes.”3
Special populations/circumstances to keep in mind
Malabsorption conditions. When a higher than expected weight-based dose of levothyroxine is required, physicians should review administration timing, adherence, and comorbid medical conditions that can affect absorption.
Several studies, for example, have demonstrated the impact of Helicobacter pylori gastritis on levothyroxine absorption and subsequent TSH levels.15-17 In one nonrandomized prospective study, patients with H pylori and hypothyroidism who were previously thought to be unresponsive to levothyroxine therapy had a decrease in average TSH level from 30.5 mIU/L to 4.2 mIU/L after H pylori was eradicated.15 Autoimmune atrophic gastritis and celiac disease, both of which are more common in those with other autoimmune diseases, are also associated with the need for higher than expected levothyroxine doses.17,18
Continue to: A history of gastric bypass surgery...
A history of gastric bypass surgery alone is not considered a risk factor for poor absorption of thyroid hormone, given that the majority of levothyroxine absorption occurs in the ileum.19,20 However, advancing age (> 70 years) and extreme obesity (BMI > 40) are independent risk factors for decreased levothyroxine absorption.20,21
Women of reproductive age and pregnant women. Overt untreated or undertreated hypothyroidism can be associated with increased risk of maternal and fetal complications including decreased fertility, miscarriage, preterm delivery, lower birth rates, and infant cognitive deficits.3,22 Therefore, the main focus should be optimization of thyroid hormone levels prior to and during pregnancy.3,4,8,22 Thyroid hormone replacement needs to be increased during pregnancy in approximately 50% to 85% of women using thyroid replacement prior to pregnancy, but the dose requirements vary based on the underlying etiology of thyroid dysfunction.
One initial option for patients on a stable dose before pregnancy is to increase their daily dose by a half tablet (1.5 × daily dose) immediately after home confirmation of pregnancy, until finer dose adjustments (usually increases of 25%-60% ) can be made by a physician. Experts recommend that a TSH level be obtained every 4 weeks until mid-gestation and then at least once around 30 weeks’ gestation to ensure specific targets are being met with dose adjustments.22 Optimal thyrotropin reference ranges during conception and pregnancy can be found in the literature.23
Patients who have positive antibodies and normal thyroid function tests. Patients who are screened for thyroid disorders may demonstrate normal thyroid function (ie, euthyroid) with TSH, free T4, and, if checked, free T3, all within normal ranges. Despite these normal lab results, patients may have additional test results that demonstrate positive thyroid autoantibodies including thyroglobulin antibodies and/or thyroid peroxidase antibodies. Thyroid autoimmunity itself has been associated with a range of other autoimmune conditions as well as an increased risk of thyroid cancer in those with Hashimoto thyroiditis.24 Two studies showed that prophylactic treatment of euthyroid patients with levothyroxine led to a reduction in antibody levels and a lower TSH level.25,26 However, no studies have focused on patient-oriented outcomes such as hospitalizations, quality of life, or symptoms. If the patient remains asymptomatic, we recommend no treatment, but that the patient’s TSH levels be monitored every 12 months.27
Elderly patients. Population data have shown that TSH increases normally with age, with a TSH level of 7.5 mIU/L being the upper limit of normal for a population of healthy adults > 80 years of age.28,29 Overall, studies have failed to show any benefit in treating elderly patients with subclinical hypothyroidism unless their TSH level exceeds 10 mIU/L.6,21 The one exception is elderly patients with heart failure in whom untreated subclinical hypothyroidism has been shown to be associated with higher mortality.30
Continue to: Elderly patients are at higher risk...
Elderly patients are at higher risk for adverse effects of thyroid over-replacement, including atrial fibrillation and osteoporosis. While there have been no randomized trials examining target TSH levels in this population, a reasonable recommendation is a goal TSH level of 4 to 6 mIU/L for elderly patients ≥ 70 years.4
CASE
As a result of the patient’s elevated TSH level and symptoms of hypothyroidism, you start levothyroxine 150 mcg/d by mouth, counsel her on potential adverse effects, and schedule a follow-up visit with another TSH check in 6 weeks.
Follow-up laboratory studies 6 weeks later reveal a TSH level of 5.86 mIU/L (reference range, 0.45-4.5 mIU/L) and a free T4 level of 0.74 ng/dL (reference range, 0.8-2.8 ng/dL). Based on those results, you increase the dose of levothyroxine to 175 mcg/d.
At her follow-up visit 12 weeks after initial presentation, her TSH level is 3.85 mIU/L. She reports feeling better overall with less fatigue, and she has lost 5 pounds since her last visit. You recommend she continue levothyroxine 175 mcg/d after reviewing medication compliance with the patient and ensuring she is indeed taking it in the morning, at least 30 minutes prior to eating. With improved but not resolved symptoms, she agrees to follow-up with repeat TSH laboratory studies in 6 weeks to determine whether further dose adjustments are necessary. Given that she is of reproductive age and her TSH level is suboptimal for pregnancy, you caution her about heightened pregnancy/fetal risks with a suboptimal TSH and recommend that she use reliable contraception.
CORRESPONDENCE
Christopher Bunt, MD, FAAFP, 5 Charleston Center Drive, Suite 263, MSC 192,Charleston, SC 29425; [email protected]
1. Aoki Y, Belin RM, Clickner R, et al. Serum TSH and total T4 in the United States population and their association with participant characteristics: National Health and Nutrition Examination Survey (NHANES 1999-2002). Thyroid. 2007;17:1211-1223.
2. Vaidya B, Pearce SH. Management of hypothyroidism in adults. BMJ. 2008;337:a801.
3. Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012;18:988-1028.
4. Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association task force on thyroid hormone replacement. Thyroid. 2014;24:1670-1751.
5. Toft AD. Thyroxine therapy. N Engl J Med. 1994;331:174-180.
6. Floriani C, Gencer B, Collet TH, et al. Subclinical thyroid dysfunction and cardiovascular diseases: 2016 update. Eur Heart J. 2018;39:503-507.
7. Lexi-Comp, Inc. (Lexi-Drugs®). https://online.lexi.com/lco/action/login. Accessed July 7, 2017.
8. Okosieme O, Gilbert J, Abraham P, et al. Management of primary hypothyroidism: statement by the British Thyroid Association Executive Committee. Clin Endocrinol (Oxf). 2016;84:799-808.
9. Fish LH, Schwartz HL, Cavanaugh J, et al. Replacement dose, metabolism, and bioavailability of levothyroxine in the treatment of hypothyroidism. Role of triiodothyronine in pituitary feedback in humans. N Engl J Med. 1987;316:764-770.
10. John-Kalarickal J, Pearlman G, Carlson HE. New medications which decrease levothyroxine absorption. Thyroid. 2007;17:763-765.
11. Sachmechi I, Reich DM, Aninyei M, et al. Effect of proton pump inhibitors on serum thyroid-stimulating hormone level in euthyroid patients treated with levothyroxine for hypothyroidism. Endocr Pract. 2007;13:345-349.
12. Sperber AD, Liel Y. Evidence for interference with the intestinal absorption of levothyroxine sodium by aluminum hydroxide. Arch Intern Med. 1992;152:183-184.
13. Zamfirescu I, Carlson HE. Absorption of levothyroxine when coadministered with various calcium formulations. Thyroid. 2011;21:483-486.
14. Campbell NR, Hasinoff BB, Stalts H, et al. Ferrous sulfate reduces thyroxine efficacy in patients with hypothyroidism. Ann Intern Med. 1992;117:1010-1013.
15. Bugdaci MS, Zuhur SS, Sokmen M, et al. The role of Helicobacter pylori in patients with hypothyroidism in whom could not be achieved normal thyrotropin levels despite treatment with high doses of thyroxine. Helicobacter. 2011;16:124-130.
16. Centanni M, Gargano L, Canettieri G, et al. Thyroxine in goiter, Helicobacter pylori infection, and chronic gastritis. N Engl J Med. 2006;354:1787-1795.
17. Centanni M, Marignani M, Gargano L, et al. Atrophic body gastritis in patients with autoimmune thyroid disease: an underdiagnosed association. Arch Intern Med. 1999;159:1726-1730.
18. Collins D, Wilcox R, Nathan M, et al. Celiac disease and hypothyroidism. Am J Med. 2012;125:278-282.
19. Azizi F, Belur R, Albano J. Malabsorption of thyroid hormones after jejunoileal bypass for obesity. Ann Intern Med. 1979;90:941-942.
20. Gkotsina M, Michalaki M, Mamali I, et al. Improved levothyroxine pharmacokinetics after bariatric surgery. Thyroid. 2013;23:414-419.
21. Hennessey JV, Espaillat R. Diagnosis and management of subclinical hypothyroidism in elderly adults: a review of the literature. J Am Geriatr Soc. 2015;63:1663-1673.
22. Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017;27:315-389.
23. Carney LA, Quinlan JD, West JM. Thyroid disease in pregnancy. Am Fam Physician. 2014;89:273-278.
24. Fröhlich E, Wahl R. Thyroid autoimmunity: role of anti-thyroid antibodies in thyroid and extra-thyroidal diseases. Front Immunol. 2017;8:521.
25. Aksoy DY, Kerimoglu U, Okur H, et al. Effects of prophylactic thyroid hormone replacement in euthyroid Hashimoto’s thyroiditis. Endocr J. 2005;52:337-343.
26. Padberg S, Heller K, Usadel KH, et al. One-year prophylactic treatment of euthyroid Hashimoto’s thyroiditis patients with levothyroxine: is there a benefit? Thyroid. 2001;11:249-255.
27. Rugge B, Balshem H, Sehgal R, et al. Screening and Treatment of Subclinical Hypothyroidism or Hyperthyroidism [Internet]. Comparative Effectiveness Reviews, No. 24. Rockville, MD: Agency for Healthcare Research and Quality; October 2011. www.ncbi.nlm.nih.gov/books/NBK83492/. Accessed February 21, 2020.
28. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489-499.
29. Surks MI, Hollowell JG. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab. 2007;92:4575-4582.
30. Pasqualetti G, Tognini S, Polini A, et al. Is subclinical hypothyroidism a cardiovascular risk factor in the elderly? J Clin Endocrinol Metab. 2013;98:2256-2266.
1. Aoki Y, Belin RM, Clickner R, et al. Serum TSH and total T4 in the United States population and their association with participant characteristics: National Health and Nutrition Examination Survey (NHANES 1999-2002). Thyroid. 2007;17:1211-1223.
2. Vaidya B, Pearce SH. Management of hypothyroidism in adults. BMJ. 2008;337:a801.
3. Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012;18:988-1028.
4. Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association task force on thyroid hormone replacement. Thyroid. 2014;24:1670-1751.
5. Toft AD. Thyroxine therapy. N Engl J Med. 1994;331:174-180.
6. Floriani C, Gencer B, Collet TH, et al. Subclinical thyroid dysfunction and cardiovascular diseases: 2016 update. Eur Heart J. 2018;39:503-507.
7. Lexi-Comp, Inc. (Lexi-Drugs®). https://online.lexi.com/lco/action/login. Accessed July 7, 2017.
8. Okosieme O, Gilbert J, Abraham P, et al. Management of primary hypothyroidism: statement by the British Thyroid Association Executive Committee. Clin Endocrinol (Oxf). 2016;84:799-808.
9. Fish LH, Schwartz HL, Cavanaugh J, et al. Replacement dose, metabolism, and bioavailability of levothyroxine in the treatment of hypothyroidism. Role of triiodothyronine in pituitary feedback in humans. N Engl J Med. 1987;316:764-770.
10. John-Kalarickal J, Pearlman G, Carlson HE. New medications which decrease levothyroxine absorption. Thyroid. 2007;17:763-765.
11. Sachmechi I, Reich DM, Aninyei M, et al. Effect of proton pump inhibitors on serum thyroid-stimulating hormone level in euthyroid patients treated with levothyroxine for hypothyroidism. Endocr Pract. 2007;13:345-349.
12. Sperber AD, Liel Y. Evidence for interference with the intestinal absorption of levothyroxine sodium by aluminum hydroxide. Arch Intern Med. 1992;152:183-184.
13. Zamfirescu I, Carlson HE. Absorption of levothyroxine when coadministered with various calcium formulations. Thyroid. 2011;21:483-486.
14. Campbell NR, Hasinoff BB, Stalts H, et al. Ferrous sulfate reduces thyroxine efficacy in patients with hypothyroidism. Ann Intern Med. 1992;117:1010-1013.
15. Bugdaci MS, Zuhur SS, Sokmen M, et al. The role of Helicobacter pylori in patients with hypothyroidism in whom could not be achieved normal thyrotropin levels despite treatment with high doses of thyroxine. Helicobacter. 2011;16:124-130.
16. Centanni M, Gargano L, Canettieri G, et al. Thyroxine in goiter, Helicobacter pylori infection, and chronic gastritis. N Engl J Med. 2006;354:1787-1795.
17. Centanni M, Marignani M, Gargano L, et al. Atrophic body gastritis in patients with autoimmune thyroid disease: an underdiagnosed association. Arch Intern Med. 1999;159:1726-1730.
18. Collins D, Wilcox R, Nathan M, et al. Celiac disease and hypothyroidism. Am J Med. 2012;125:278-282.
19. Azizi F, Belur R, Albano J. Malabsorption of thyroid hormones after jejunoileal bypass for obesity. Ann Intern Med. 1979;90:941-942.
20. Gkotsina M, Michalaki M, Mamali I, et al. Improved levothyroxine pharmacokinetics after bariatric surgery. Thyroid. 2013;23:414-419.
21. Hennessey JV, Espaillat R. Diagnosis and management of subclinical hypothyroidism in elderly adults: a review of the literature. J Am Geriatr Soc. 2015;63:1663-1673.
22. Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017;27:315-389.
23. Carney LA, Quinlan JD, West JM. Thyroid disease in pregnancy. Am Fam Physician. 2014;89:273-278.
24. Fröhlich E, Wahl R. Thyroid autoimmunity: role of anti-thyroid antibodies in thyroid and extra-thyroidal diseases. Front Immunol. 2017;8:521.
25. Aksoy DY, Kerimoglu U, Okur H, et al. Effects of prophylactic thyroid hormone replacement in euthyroid Hashimoto’s thyroiditis. Endocr J. 2005;52:337-343.
26. Padberg S, Heller K, Usadel KH, et al. One-year prophylactic treatment of euthyroid Hashimoto’s thyroiditis patients with levothyroxine: is there a benefit? Thyroid. 2001;11:249-255.
27. Rugge B, Balshem H, Sehgal R, et al. Screening and Treatment of Subclinical Hypothyroidism or Hyperthyroidism [Internet]. Comparative Effectiveness Reviews, No. 24. Rockville, MD: Agency for Healthcare Research and Quality; October 2011. www.ncbi.nlm.nih.gov/books/NBK83492/. Accessed February 21, 2020.
28. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489-499.
29. Surks MI, Hollowell JG. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab. 2007;92:4575-4582.
30. Pasqualetti G, Tognini S, Polini A, et al. Is subclinical hypothyroidism a cardiovascular risk factor in the elderly? J Clin Endocrinol Metab. 2013;98:2256-2266.
PRACTICE RECOMMENDATIONS
› Prescribe levothyroxine 1.6 mcg/kg/d for healthy adult patients < 50 years of age with overt hypothyroidism. B
› Consider lower initial doses of levothyroxine in patients with cardiac disease (12.5-50 mcg/d) or subclinical hypothyroidism (25-75 mcg/d). B
› Titrate levothyroxine by 12.5 to 25 mcg/d at 6- to 8-week intervals based on thyroid-stimulating hormone measurements, comorbidities, and symptoms. C
› Closely monitor and provide thyroid supplementation to female patients who are pregnant or of reproductive age with concomitant hypothyroidism. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Depression, or something else?
CASE Suicidal behavior, severe headaches
Ms. A, age 60, presents to the emergency department (ED) with depression, suicidal behavior, and 3 days of severe headaches. Neurology is consulted and an MRI is ordered, which shows a 3.0-cm mass lesion in the left temporal lobe with associated vasogenic edema that is suspicious for metastatic disease (Figure).
Ms. A is admitted to the hospital for further workup of her brain lesion. She is started on IV dexamethasone, 10 mg every 6 hours, a glucocorticosteroid, for brain edema, and levetiracetam, 500 mg twice a day, for seizure prophylaxis.
Upon admission, in addition to oncology and neurosurgery, psychiatry is also consulted to evaluate Ms. A for depression and suicidality.
EVALUATION Mood changes and poor judgment
Ms. A has a psychiatric history of depression and alcohol use disorder but says she has not consumed any alcohol in years. Her medical history includes hypertension, diabetes, and stage 4 non-small–cell lung cancer, for which she received surgery and adjuvant chemoradiotherapy 1 year ago.
On initial intake, Ms. A reports that in addition to the headaches, she has also been experiencing worsening depression and suicidal behavior. For the past 2 months, she has had a severely depressed mood, with notable anhedonia, poor appetite, insomnia, low energy, and decreased concentration. The changes in her mental health were triggered by her mother’s death. Three days prior to admission, the patient planned to overdose on antihypertensive pills, but her suicide attempt was interrupted when her family called. She denies any current suicidal ideation, intent, or plan.
According to her family, Ms. A has been increasingly irritable and her personality has changed in the past month. She also has been repeatedly sorting through her neighbors’ garbage.
Ms. A’s current psychiatric medications are duloxetine, 30 mg/d; quetiapine, 50 mg every night at bedtime; and buspirone, 10 mg/d. However, it is unclear if she is consistently taking these medications.
Continue to: On mental status examination...
On mental status examination, Ms. A is calm and she has no abnormal movements. She says she is depressed. Her affect is reactive and labile. She is alert and oriented to person, place, and time. Her attention, registration, and recall are intact. Her executive function is not tested. However, Ms. A’s insight and judgment seem poor.
To address Ms. A’s worsening depression, the psychiatry team increases her duloxetine from 30 to 60 mg/d, and she continues quetiapine, 50 mg every night at bedtime, for mood lability. Buspirone is not continued because she was not taking a therapeutic dosage in the community.
Within 4 days, Ms. A shows improvement in sleep, appetite, and mood. She has no further suicidal ideation.
[polldaddy:10511743]
The authors’ observations
Ms. A had a recurrence of what was presumed to be major depressive disorder (MDD) in the context of her mother’s death. However, she also exhibited irritability, mood lability, and impulsivity, all of which could be part of her depression, or a separate problem related to her brain tumor. Because Ms. A had never displayed bizarre behavior before the past few weeks, it is likely that her CNS lesion was directly affecting her personality and possibly underlying her planned suicide attempt.
Fifty to 80% of patients with CNS tumors, either primary or metastatic, present with psychiatric symptoms.1 Table 11-3 lists common psychiatric symptoms of brain tumors. Unfortunately, there is little reliable evidence that directly correlates tumor location with specific psychiatric symptoms. A 2010 meta-analysis found a statistically significant link between anorexia nervosa and hypothalamic tumors.1 However, for other brain regions, there is only an increased likelihood that any given tumor location will produce psychiatric symptoms.1,4 For instance, compared to patients with tumors in other locations, those with temporal lobe tumors are more likely to present with mood disorders, personality changes, and memory problems.1 In contrast, patients with frontal lobe tumors have an increased likelihood of psychosis, mood disorders, and personality changes.1 Patients with tumors in the pituitary region often present with anxiety.1
Continue to: When considering treatment options...
When considering treatment options for Ms. A, alcohol withdrawal was unlikely given the remote history of alcohol use, low alcohol blood level, and lack of evidence of unstable vital signs or tremor. Although she might have benefited from inpatient psychiatric treatment, this needed to wait until there was a definitive treatment plan for her brain tumor. Finally, although a paraneoplastic syndrome, such as limbic encephalitis, could be causing her psychiatric symptoms, this scenario is less likely with non-small–cell lung cancer.
Although uncommon, CNS tumors can present with psychiatric symptoms as the only manifestation. This is more likely when a patient exhibits new-onset or atypical symptoms, or fails to respond to standard psychiatric treatment.4 Case reports have described patients with brain tumors being misdiagnosed as having a primary psychiatric condition, which delays treatment of their CNS cancer.2 Additionally, frontal and limbic tumors are more likely to present with psychiatric manifestations; up to 90% of patients exhibit altered mental status or personality changes, as did Ms. A.1,4 Clearly, it is easier to identify patients with psychiatric symptoms resulting from a brain tumor when they also present with focal neurologic deficits or systemic symptoms, such as headache or nausea and vomiting. Ms. A presented with severe headaches, which is what led to her early imaging and prompt diagnosis.
Numerous proposed mechanisms might account for the psychiatric symptoms that occur during the course of a brain tumor, including direct injury to neuronal cells, secretion of hormones or other tumor-derived substances, and peri-ictal phenomena.3
TREATMENT Tumor is removed, but memory is impaired
Ms. A is scheduled for craniotomy and surgical resection of the frontal mass. Prior to surgery, Ms. A shows interest in improving her health, cooperates with staff, and seeks her daughter’s input on treatment. One week after admission, Ms. A has her mass resected, which is confirmed on biopsy to be a lung metastasis. Post-surgery, Ms. A receives codeine, 30 mg every 6 hours as needed, for pain; she continues dexamethasone, 4 mg IV every 6 hours, for brain edema and levetiracetam, 500 mg twice a day, for seizure prophylaxis.
On Day 2 after surgery, Ms. A attempts to elope. When she is approached by a psychiatrist on the treatment team, she does not recognize him. Although her long-term memory seems intact, she is unable to remember the details of recent events, including her medical and surgical treatments.
[polldaddy:10511745]
Continue to: The authors' observations
The authors’ observations
Ms. A’s memory impairment may be secondary to a surgically acquired neurocognitive deficit. In the United States, brain metastases represent a significant public health issue, affecting >100,000 patients per year.5 Metastatic lesions are the most common brain tumors. Lung cancer, breast cancer, and melanoma are the leading solid tumors to spread to the CNS.5 In cases of single brain metastasis, similar to Ms. A’s solitary left temporal lobe lesion, surgical resection plays a critical role in treatment. It provides histological confirmation of metastatic disease and can relieve mass effect if present. Studies have shown that combined surgical resection with radiation improves survival relative to patients who undergo radiation therapy alone.6,7
However, the benefits of surgical resection need to be balanced with preservation of neurologic function. Emerging evidence suggests that a majority of patients have surgically-acquired cognitive deficits due to damage of normal surrounding tissues, and these deficits are associated with reduced quality of life.8,9 Further, a study examining glioma surgical resections found that patients with left temporal lobe tumors exhibit more frequent and severe neurocognitive decline than patients with right temporal lobe tumors, especially in domains such as verbal memory.8 Ms. A’s memory impairment was persistent during her postoperative course, which suggests that it was not just an immediate post-surgical phenomenon, but a longer-lasting cognitive change directly related to the resection.
It is also possible that Ms. A had a prior neurocognitive disorder that manifested to a greater degree as a result of the CNS tumor. Ms. A might have had early-onset Alzheimer’s disease, although her intact memory before surgery makes this less likely. Alternatively, she could have had vascular dementia, especially given her long-standing hypertension and diabetes. This might have been missed in the initial evaluation because executive function was not tested. However, the relatively abrupt onset of memory problems after surgery suggests that she had no underlying neurocognitive disorder.
Ms. A’s presumed episode of MDD might also explain her memory changes. Major depressive disorder is increasingly common among geriatric patients, affecting approximately 5% of community-dwelling older adults.10 Its incidence increases with medical comorbidities, as suggested by depression rates of 5% to 10% in the primary care setting vs 37% in patients after critical-care hospitalizations.10 Late-life depression (LLD) occurs in adults age ≥60. Unlike depression in younger patients, LLD is more likely to be associated with cognitive impairment, specifically impairment of executive function and memory.11 The incidence of cognitive impairment in LLD is higher in patients with a history of depression, such as Ms. A.11,12 However, in general, patients who are depressed have memory complaints out of proportion to the clinical findings, and they show poor effort on cognitive testing. Ms. A exhibited neither of these, which makes it less likely that LLD was the exclusive cause of her memory loss.13 Table 214 outlines the management of cognitive deficits in a patient with a brain tumor.
EVALUATION Increasingly agitated and paranoid
After the tumor resection, Ms. A becomes increasingly irritable, uncooperative, and agitated. She repeatedly demands to be discharged. She insists she is fine and refuses medications and further laboratory workup. She becomes paranoid about the nursing staff and believes they are trying to kill her.
Continue to: On psychiatric re-evaluation...
On psychiatric re-evaluation, Ms. A demonstrates pressured speech, perseveration about going home, paranoid delusions, and anger at her family and physicians.
[polldaddy:10511747]
The authors’ observations
Ms. A’s refusal of medications and agitation may be explained by postoperative delirium, a surgical complication that is increasingly common among geriatric patients and is associated with poor clinical outcomes. Delirium is characterized by an acute onset and fluctuating course of symptoms that include inattention, motoric hypo- or hyperactivity, inappropriate behavior, emotional lability, cognitive dysfunction, and psychotic symptoms.15 Risk factors that contribute to postoperative delirium include older age, alcohol use, and poor baseline functional and cognitive status.16 The pathophysiology of delirium is not fully understood, but accumulating evidence suggests that different sets of interacting biologic factors (ie, neurotransmitters and inflammation) contribute to a disruption of large-scale neuronal networks in the brain, resulting in cognitive dysfunction.15 Patients who develop postoperative delirium are more likely to develop long-term cognitive dysfunction and have an increased risk of dementia.16
Another potential source of Ms. A’s agitation is steroid use. Ms. A received IV dexamethasone, 8 to 16 mg/d, around the time of her surgery. Steroids are commonly used to treat brain tumors, particularly when there is vasogenic edema. Steroid psychosis is a term loosely used to describe a wide range of psychiatric symptoms induced by corticosteroids that includes, but is not limited to, depression, mania, psychosis, delirium, and cognitive impairment.17 Steroid-induced psychiatric adverse effects occur in 5% to 18% of patients receiving corticosteroids and often happen early in treatment, although they can occur at any point.18 Corticosteroids influence brain activity via glucocorticoid and mineralocorticoid receptors. These receptors are widely distributed throughout the brain and affect neurotransmitter systems, such as the serotonergic system, that are associated with changes in mood, behavior, and cognition.17 While the adverse psychiatric manifestations of steroid use vary, higher dosages are associated with an increased risk of psychiatric complications; mania is more prevalent early in the course of treatment, and depression is more common with long-term use.17,19 Table 317,18 outlines the evidence-based treatment of corticosteroid-induced adverse psychiatric effects.
Although there are no clinical guidelines or FDA-approved medications for treating steroid-induced psychiatric adverse events, these are best managed by tapering and discontinuing steroids when possible and simultaneously using psychotropic medications to treat psychiatric symptoms. Case reports and limited evidence-based literature have demonstrated that steroid-induced mania responds to mood stabilizers or antipsychotics, while depression can be managed with antidepressants or lithium.17
Additionally, patients with CNS tumors are at risk for seizures and often are prescribed antiepileptics. Because it is easy to administer and does not need to be titrated, levetiracetam is a commonly used agent. However, levetiracetam can cause psychiatric adverse effects, including behavior changes and frank psychosis.20
Continue to: Finally, Ms. A's altered mental status...
Finally, Ms. A’s altered mental status could have been related to opioid intoxication. Opioids are used to manage postsurgical pain, and studies have shown these medications can be a precipitating factor for delirium in geriatric patients.21
TREATMENT Medication adjustments
At the request of the psychiatry team, levetiracetam is discontinued due to its potential for psychiatric adverse effects. The neurosurgery team replaces it with valproic acid, 500 mg every 12 hours. Ms. A is also tapered off steroids fairly rapidly because of the potential for steroid-induced psychiatric adverse effects. Her quetiapine is titrated from 50 to 150 mg every night at bedtime, and duloxetine is discontinued.
OUTCOME Agitation improves dramatically
Ms. A’s new medication regimen dramatically improves her agitation, which allows Ms. A, her family, and the medical team to work together to establish treatment goals. Ms. A ultimately returns home with the assistance of her family. She continues to have memory issues, but with improved emotion regulation. Several months later, Ms. A is readmitted to the hospital because her cancer has progressed despite treatment.
Bottom Line
Brain tumors may present with various psychiatric manifestations that can change during the course of the patient’s treatment. A comprehensive psychiatric evaluation should parse out the interplay between direct effects of the tumor and any adverse effects that are the result of medical and/or surgical interventions to determine the cause of psychiatric symptoms and their appropriate management.
Related Resource
Madhusoodanan S, Ting MB, Farah T, et al. Psychiatric aspects of brain tumors: a review. World J Psychiatry. 2015;5(3):273-285.
Drug Brand Names
Aripiprazole • Abilify
Buspirone • Buspar
Chlorpromazine • Thorazine
Codeine • Codeine systemic
Dexamethasone • Decadron
Duloxetine • Cymbalta
Haloperidol • Haldol
Levetiracetam • Keppra
Lorazepam • Ativan
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Valproic acid • Depakene
1. Madhusoodanan S, Opler MG, Moise D, et al. Brain tumor location and psychiatric symptoms: is there any association? A meta-analysis of published case studies. Expert Rev Neurother. 2010;10(10):1529-1536.
2. Bunevicius A, Deltuva VP, Deltuviene D, et al. Brain lesions manifesting as psychiatric disorders: eight cases. CNS Spectr. 2008;13(11):950-958.
3. Pearl ML, Talgat G, Valea FA, et al. Psychiatric symptoms due to brain metastases. Med Update Psychiatr. 1998;3(4):91-94.
4. Madhusoodanan S, Danan D, Moise D. Psychiatric manifestations of brain tumors: diagnostic implications. Expert Rev Neurother. 2007;7(4):343-349.
5. Ferguson SD, Wagner KM, Prabhu SS, et al. Neurosurgical management of brain metastases. Clin Exp Metastasis. 2017;34(6-7):377-389.
6. Husain ZA, Regine WF, Kwok Y, et al. Brain metastases: contemporary management and future directions. Eur J Clin Med Oncol. 2011;3(3):38-45.
7. Vecht CJ, Haaxmareiche H, Noordijk EM, et al. Treatment of single brain metastasis - radiotherapy alone or combined with neurosurgery. Ann Neurol. 1993;33(6):583-590.
8. Barry RL, Byun NE, Tantawy MN, et al. In vivo neuroimaging and behavioral correlates in a rat model of chemotherapy-induced cognitive dysfunction. Brain Imaging Behav. 2018;12(1):87-95.
9. Wu AS, Witgert ME, Lang FF, et al. Neurocognitive function before and after surgery for insular gliomas. J Neurosurg. 2011;115(6):1115-1125.
10. Taylor WD. Depression in the elderly. N Engl J Med. 2014;371(13):1228-1236.
11. Liguori C, Pierantozzi M, Chiaravalloti A, et al. When cognitive decline and depression coexist in the elderly: CSF biomarkers analysis can differentiate Alzheimer’s disease from late-life depression. Front Aging Neurosci. 2018;10:38.
12. Luijendijk HJ, van den Berg JF, Dekker MJHJ, et al. Incidence and recurrence of late-life depression. Arch Gen Psychiatry. 2008;65(12):1394-1401.
13. Potter GG, Steffens DC. Contribution of depression to cognitive impairment and dementia in older adults. Neurologist. 2007;13(3):105-117.
14. Taphoorn MJB, Klein M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 2004;3(3):159-168.
15. Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922.
16. Sprung J, Roberts RO, Weingarten TN, et al. Postoperative delirium in elderly patients is associated with subsequent cognitive impairment. Br J Anaesth. 2017;119(2):316-323.
17. Kusljic S, Manias E, Gogos A. Corticosteroid-induced psychiatric disturbances: it is time for pharmacists to take notice. Res Soc Adm Pharm. 2016;12(2):355-360.
18. Cerullo MA. Corticosteroid-induced mania: prepare for the unpredictable. Current Psychiatry. 2006;5(6):43-50.
19. Dubovsky AN, Arvikar S, Stern TA, et al. Steroid psychosis revisited. Psychosomatics. 2012;53(2):103-115.
20. Habets JGV, Leentjens AFG, Schijns OEMG. Serious and reversible levetiracetam-induced psychiatric symptoms after resection of frontal low-grade glioma: two case histories. Br J Neurosurg. 2017;31(4):471-473.
21
CASE Suicidal behavior, severe headaches
Ms. A, age 60, presents to the emergency department (ED) with depression, suicidal behavior, and 3 days of severe headaches. Neurology is consulted and an MRI is ordered, which shows a 3.0-cm mass lesion in the left temporal lobe with associated vasogenic edema that is suspicious for metastatic disease (Figure).
Ms. A is admitted to the hospital for further workup of her brain lesion. She is started on IV dexamethasone, 10 mg every 6 hours, a glucocorticosteroid, for brain edema, and levetiracetam, 500 mg twice a day, for seizure prophylaxis.
Upon admission, in addition to oncology and neurosurgery, psychiatry is also consulted to evaluate Ms. A for depression and suicidality.
EVALUATION Mood changes and poor judgment
Ms. A has a psychiatric history of depression and alcohol use disorder but says she has not consumed any alcohol in years. Her medical history includes hypertension, diabetes, and stage 4 non-small–cell lung cancer, for which she received surgery and adjuvant chemoradiotherapy 1 year ago.
On initial intake, Ms. A reports that in addition to the headaches, she has also been experiencing worsening depression and suicidal behavior. For the past 2 months, she has had a severely depressed mood, with notable anhedonia, poor appetite, insomnia, low energy, and decreased concentration. The changes in her mental health were triggered by her mother’s death. Three days prior to admission, the patient planned to overdose on antihypertensive pills, but her suicide attempt was interrupted when her family called. She denies any current suicidal ideation, intent, or plan.
According to her family, Ms. A has been increasingly irritable and her personality has changed in the past month. She also has been repeatedly sorting through her neighbors’ garbage.
Ms. A’s current psychiatric medications are duloxetine, 30 mg/d; quetiapine, 50 mg every night at bedtime; and buspirone, 10 mg/d. However, it is unclear if she is consistently taking these medications.
Continue to: On mental status examination...
On mental status examination, Ms. A is calm and she has no abnormal movements. She says she is depressed. Her affect is reactive and labile. She is alert and oriented to person, place, and time. Her attention, registration, and recall are intact. Her executive function is not tested. However, Ms. A’s insight and judgment seem poor.
To address Ms. A’s worsening depression, the psychiatry team increases her duloxetine from 30 to 60 mg/d, and she continues quetiapine, 50 mg every night at bedtime, for mood lability. Buspirone is not continued because she was not taking a therapeutic dosage in the community.
Within 4 days, Ms. A shows improvement in sleep, appetite, and mood. She has no further suicidal ideation.
[polldaddy:10511743]
The authors’ observations
Ms. A had a recurrence of what was presumed to be major depressive disorder (MDD) in the context of her mother’s death. However, she also exhibited irritability, mood lability, and impulsivity, all of which could be part of her depression, or a separate problem related to her brain tumor. Because Ms. A had never displayed bizarre behavior before the past few weeks, it is likely that her CNS lesion was directly affecting her personality and possibly underlying her planned suicide attempt.
Fifty to 80% of patients with CNS tumors, either primary or metastatic, present with psychiatric symptoms.1 Table 11-3 lists common psychiatric symptoms of brain tumors. Unfortunately, there is little reliable evidence that directly correlates tumor location with specific psychiatric symptoms. A 2010 meta-analysis found a statistically significant link between anorexia nervosa and hypothalamic tumors.1 However, for other brain regions, there is only an increased likelihood that any given tumor location will produce psychiatric symptoms.1,4 For instance, compared to patients with tumors in other locations, those with temporal lobe tumors are more likely to present with mood disorders, personality changes, and memory problems.1 In contrast, patients with frontal lobe tumors have an increased likelihood of psychosis, mood disorders, and personality changes.1 Patients with tumors in the pituitary region often present with anxiety.1
Continue to: When considering treatment options...
When considering treatment options for Ms. A, alcohol withdrawal was unlikely given the remote history of alcohol use, low alcohol blood level, and lack of evidence of unstable vital signs or tremor. Although she might have benefited from inpatient psychiatric treatment, this needed to wait until there was a definitive treatment plan for her brain tumor. Finally, although a paraneoplastic syndrome, such as limbic encephalitis, could be causing her psychiatric symptoms, this scenario is less likely with non-small–cell lung cancer.
Although uncommon, CNS tumors can present with psychiatric symptoms as the only manifestation. This is more likely when a patient exhibits new-onset or atypical symptoms, or fails to respond to standard psychiatric treatment.4 Case reports have described patients with brain tumors being misdiagnosed as having a primary psychiatric condition, which delays treatment of their CNS cancer.2 Additionally, frontal and limbic tumors are more likely to present with psychiatric manifestations; up to 90% of patients exhibit altered mental status or personality changes, as did Ms. A.1,4 Clearly, it is easier to identify patients with psychiatric symptoms resulting from a brain tumor when they also present with focal neurologic deficits or systemic symptoms, such as headache or nausea and vomiting. Ms. A presented with severe headaches, which is what led to her early imaging and prompt diagnosis.
Numerous proposed mechanisms might account for the psychiatric symptoms that occur during the course of a brain tumor, including direct injury to neuronal cells, secretion of hormones or other tumor-derived substances, and peri-ictal phenomena.3
TREATMENT Tumor is removed, but memory is impaired
Ms. A is scheduled for craniotomy and surgical resection of the frontal mass. Prior to surgery, Ms. A shows interest in improving her health, cooperates with staff, and seeks her daughter’s input on treatment. One week after admission, Ms. A has her mass resected, which is confirmed on biopsy to be a lung metastasis. Post-surgery, Ms. A receives codeine, 30 mg every 6 hours as needed, for pain; she continues dexamethasone, 4 mg IV every 6 hours, for brain edema and levetiracetam, 500 mg twice a day, for seizure prophylaxis.
On Day 2 after surgery, Ms. A attempts to elope. When she is approached by a psychiatrist on the treatment team, she does not recognize him. Although her long-term memory seems intact, she is unable to remember the details of recent events, including her medical and surgical treatments.
[polldaddy:10511745]
Continue to: The authors' observations
The authors’ observations
Ms. A’s memory impairment may be secondary to a surgically acquired neurocognitive deficit. In the United States, brain metastases represent a significant public health issue, affecting >100,000 patients per year.5 Metastatic lesions are the most common brain tumors. Lung cancer, breast cancer, and melanoma are the leading solid tumors to spread to the CNS.5 In cases of single brain metastasis, similar to Ms. A’s solitary left temporal lobe lesion, surgical resection plays a critical role in treatment. It provides histological confirmation of metastatic disease and can relieve mass effect if present. Studies have shown that combined surgical resection with radiation improves survival relative to patients who undergo radiation therapy alone.6,7
However, the benefits of surgical resection need to be balanced with preservation of neurologic function. Emerging evidence suggests that a majority of patients have surgically-acquired cognitive deficits due to damage of normal surrounding tissues, and these deficits are associated with reduced quality of life.8,9 Further, a study examining glioma surgical resections found that patients with left temporal lobe tumors exhibit more frequent and severe neurocognitive decline than patients with right temporal lobe tumors, especially in domains such as verbal memory.8 Ms. A’s memory impairment was persistent during her postoperative course, which suggests that it was not just an immediate post-surgical phenomenon, but a longer-lasting cognitive change directly related to the resection.
It is also possible that Ms. A had a prior neurocognitive disorder that manifested to a greater degree as a result of the CNS tumor. Ms. A might have had early-onset Alzheimer’s disease, although her intact memory before surgery makes this less likely. Alternatively, she could have had vascular dementia, especially given her long-standing hypertension and diabetes. This might have been missed in the initial evaluation because executive function was not tested. However, the relatively abrupt onset of memory problems after surgery suggests that she had no underlying neurocognitive disorder.
Ms. A’s presumed episode of MDD might also explain her memory changes. Major depressive disorder is increasingly common among geriatric patients, affecting approximately 5% of community-dwelling older adults.10 Its incidence increases with medical comorbidities, as suggested by depression rates of 5% to 10% in the primary care setting vs 37% in patients after critical-care hospitalizations.10 Late-life depression (LLD) occurs in adults age ≥60. Unlike depression in younger patients, LLD is more likely to be associated with cognitive impairment, specifically impairment of executive function and memory.11 The incidence of cognitive impairment in LLD is higher in patients with a history of depression, such as Ms. A.11,12 However, in general, patients who are depressed have memory complaints out of proportion to the clinical findings, and they show poor effort on cognitive testing. Ms. A exhibited neither of these, which makes it less likely that LLD was the exclusive cause of her memory loss.13 Table 214 outlines the management of cognitive deficits in a patient with a brain tumor.
EVALUATION Increasingly agitated and paranoid
After the tumor resection, Ms. A becomes increasingly irritable, uncooperative, and agitated. She repeatedly demands to be discharged. She insists she is fine and refuses medications and further laboratory workup. She becomes paranoid about the nursing staff and believes they are trying to kill her.
Continue to: On psychiatric re-evaluation...
On psychiatric re-evaluation, Ms. A demonstrates pressured speech, perseveration about going home, paranoid delusions, and anger at her family and physicians.
[polldaddy:10511747]
The authors’ observations
Ms. A’s refusal of medications and agitation may be explained by postoperative delirium, a surgical complication that is increasingly common among geriatric patients and is associated with poor clinical outcomes. Delirium is characterized by an acute onset and fluctuating course of symptoms that include inattention, motoric hypo- or hyperactivity, inappropriate behavior, emotional lability, cognitive dysfunction, and psychotic symptoms.15 Risk factors that contribute to postoperative delirium include older age, alcohol use, and poor baseline functional and cognitive status.16 The pathophysiology of delirium is not fully understood, but accumulating evidence suggests that different sets of interacting biologic factors (ie, neurotransmitters and inflammation) contribute to a disruption of large-scale neuronal networks in the brain, resulting in cognitive dysfunction.15 Patients who develop postoperative delirium are more likely to develop long-term cognitive dysfunction and have an increased risk of dementia.16
Another potential source of Ms. A’s agitation is steroid use. Ms. A received IV dexamethasone, 8 to 16 mg/d, around the time of her surgery. Steroids are commonly used to treat brain tumors, particularly when there is vasogenic edema. Steroid psychosis is a term loosely used to describe a wide range of psychiatric symptoms induced by corticosteroids that includes, but is not limited to, depression, mania, psychosis, delirium, and cognitive impairment.17 Steroid-induced psychiatric adverse effects occur in 5% to 18% of patients receiving corticosteroids and often happen early in treatment, although they can occur at any point.18 Corticosteroids influence brain activity via glucocorticoid and mineralocorticoid receptors. These receptors are widely distributed throughout the brain and affect neurotransmitter systems, such as the serotonergic system, that are associated with changes in mood, behavior, and cognition.17 While the adverse psychiatric manifestations of steroid use vary, higher dosages are associated with an increased risk of psychiatric complications; mania is more prevalent early in the course of treatment, and depression is more common with long-term use.17,19 Table 317,18 outlines the evidence-based treatment of corticosteroid-induced adverse psychiatric effects.
Although there are no clinical guidelines or FDA-approved medications for treating steroid-induced psychiatric adverse events, these are best managed by tapering and discontinuing steroids when possible and simultaneously using psychotropic medications to treat psychiatric symptoms. Case reports and limited evidence-based literature have demonstrated that steroid-induced mania responds to mood stabilizers or antipsychotics, while depression can be managed with antidepressants or lithium.17
Additionally, patients with CNS tumors are at risk for seizures and often are prescribed antiepileptics. Because it is easy to administer and does not need to be titrated, levetiracetam is a commonly used agent. However, levetiracetam can cause psychiatric adverse effects, including behavior changes and frank psychosis.20
Continue to: Finally, Ms. A's altered mental status...
Finally, Ms. A’s altered mental status could have been related to opioid intoxication. Opioids are used to manage postsurgical pain, and studies have shown these medications can be a precipitating factor for delirium in geriatric patients.21
TREATMENT Medication adjustments
At the request of the psychiatry team, levetiracetam is discontinued due to its potential for psychiatric adverse effects. The neurosurgery team replaces it with valproic acid, 500 mg every 12 hours. Ms. A is also tapered off steroids fairly rapidly because of the potential for steroid-induced psychiatric adverse effects. Her quetiapine is titrated from 50 to 150 mg every night at bedtime, and duloxetine is discontinued.
OUTCOME Agitation improves dramatically
Ms. A’s new medication regimen dramatically improves her agitation, which allows Ms. A, her family, and the medical team to work together to establish treatment goals. Ms. A ultimately returns home with the assistance of her family. She continues to have memory issues, but with improved emotion regulation. Several months later, Ms. A is readmitted to the hospital because her cancer has progressed despite treatment.
Bottom Line
Brain tumors may present with various psychiatric manifestations that can change during the course of the patient’s treatment. A comprehensive psychiatric evaluation should parse out the interplay between direct effects of the tumor and any adverse effects that are the result of medical and/or surgical interventions to determine the cause of psychiatric symptoms and their appropriate management.
Related Resource
Madhusoodanan S, Ting MB, Farah T, et al. Psychiatric aspects of brain tumors: a review. World J Psychiatry. 2015;5(3):273-285.
Drug Brand Names
Aripiprazole • Abilify
Buspirone • Buspar
Chlorpromazine • Thorazine
Codeine • Codeine systemic
Dexamethasone • Decadron
Duloxetine • Cymbalta
Haloperidol • Haldol
Levetiracetam • Keppra
Lorazepam • Ativan
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Valproic acid • Depakene
CASE Suicidal behavior, severe headaches
Ms. A, age 60, presents to the emergency department (ED) with depression, suicidal behavior, and 3 days of severe headaches. Neurology is consulted and an MRI is ordered, which shows a 3.0-cm mass lesion in the left temporal lobe with associated vasogenic edema that is suspicious for metastatic disease (Figure).
Ms. A is admitted to the hospital for further workup of her brain lesion. She is started on IV dexamethasone, 10 mg every 6 hours, a glucocorticosteroid, for brain edema, and levetiracetam, 500 mg twice a day, for seizure prophylaxis.
Upon admission, in addition to oncology and neurosurgery, psychiatry is also consulted to evaluate Ms. A for depression and suicidality.
EVALUATION Mood changes and poor judgment
Ms. A has a psychiatric history of depression and alcohol use disorder but says she has not consumed any alcohol in years. Her medical history includes hypertension, diabetes, and stage 4 non-small–cell lung cancer, for which she received surgery and adjuvant chemoradiotherapy 1 year ago.
On initial intake, Ms. A reports that in addition to the headaches, she has also been experiencing worsening depression and suicidal behavior. For the past 2 months, she has had a severely depressed mood, with notable anhedonia, poor appetite, insomnia, low energy, and decreased concentration. The changes in her mental health were triggered by her mother’s death. Three days prior to admission, the patient planned to overdose on antihypertensive pills, but her suicide attempt was interrupted when her family called. She denies any current suicidal ideation, intent, or plan.
According to her family, Ms. A has been increasingly irritable and her personality has changed in the past month. She also has been repeatedly sorting through her neighbors’ garbage.
Ms. A’s current psychiatric medications are duloxetine, 30 mg/d; quetiapine, 50 mg every night at bedtime; and buspirone, 10 mg/d. However, it is unclear if she is consistently taking these medications.
Continue to: On mental status examination...
On mental status examination, Ms. A is calm and she has no abnormal movements. She says she is depressed. Her affect is reactive and labile. She is alert and oriented to person, place, and time. Her attention, registration, and recall are intact. Her executive function is not tested. However, Ms. A’s insight and judgment seem poor.
To address Ms. A’s worsening depression, the psychiatry team increases her duloxetine from 30 to 60 mg/d, and she continues quetiapine, 50 mg every night at bedtime, for mood lability. Buspirone is not continued because she was not taking a therapeutic dosage in the community.
Within 4 days, Ms. A shows improvement in sleep, appetite, and mood. She has no further suicidal ideation.
[polldaddy:10511743]
The authors’ observations
Ms. A had a recurrence of what was presumed to be major depressive disorder (MDD) in the context of her mother’s death. However, she also exhibited irritability, mood lability, and impulsivity, all of which could be part of her depression, or a separate problem related to her brain tumor. Because Ms. A had never displayed bizarre behavior before the past few weeks, it is likely that her CNS lesion was directly affecting her personality and possibly underlying her planned suicide attempt.
Fifty to 80% of patients with CNS tumors, either primary or metastatic, present with psychiatric symptoms.1 Table 11-3 lists common psychiatric symptoms of brain tumors. Unfortunately, there is little reliable evidence that directly correlates tumor location with specific psychiatric symptoms. A 2010 meta-analysis found a statistically significant link between anorexia nervosa and hypothalamic tumors.1 However, for other brain regions, there is only an increased likelihood that any given tumor location will produce psychiatric symptoms.1,4 For instance, compared to patients with tumors in other locations, those with temporal lobe tumors are more likely to present with mood disorders, personality changes, and memory problems.1 In contrast, patients with frontal lobe tumors have an increased likelihood of psychosis, mood disorders, and personality changes.1 Patients with tumors in the pituitary region often present with anxiety.1
Continue to: When considering treatment options...
When considering treatment options for Ms. A, alcohol withdrawal was unlikely given the remote history of alcohol use, low alcohol blood level, and lack of evidence of unstable vital signs or tremor. Although she might have benefited from inpatient psychiatric treatment, this needed to wait until there was a definitive treatment plan for her brain tumor. Finally, although a paraneoplastic syndrome, such as limbic encephalitis, could be causing her psychiatric symptoms, this scenario is less likely with non-small–cell lung cancer.
Although uncommon, CNS tumors can present with psychiatric symptoms as the only manifestation. This is more likely when a patient exhibits new-onset or atypical symptoms, or fails to respond to standard psychiatric treatment.4 Case reports have described patients with brain tumors being misdiagnosed as having a primary psychiatric condition, which delays treatment of their CNS cancer.2 Additionally, frontal and limbic tumors are more likely to present with psychiatric manifestations; up to 90% of patients exhibit altered mental status or personality changes, as did Ms. A.1,4 Clearly, it is easier to identify patients with psychiatric symptoms resulting from a brain tumor when they also present with focal neurologic deficits or systemic symptoms, such as headache or nausea and vomiting. Ms. A presented with severe headaches, which is what led to her early imaging and prompt diagnosis.
Numerous proposed mechanisms might account for the psychiatric symptoms that occur during the course of a brain tumor, including direct injury to neuronal cells, secretion of hormones or other tumor-derived substances, and peri-ictal phenomena.3
TREATMENT Tumor is removed, but memory is impaired
Ms. A is scheduled for craniotomy and surgical resection of the frontal mass. Prior to surgery, Ms. A shows interest in improving her health, cooperates with staff, and seeks her daughter’s input on treatment. One week after admission, Ms. A has her mass resected, which is confirmed on biopsy to be a lung metastasis. Post-surgery, Ms. A receives codeine, 30 mg every 6 hours as needed, for pain; she continues dexamethasone, 4 mg IV every 6 hours, for brain edema and levetiracetam, 500 mg twice a day, for seizure prophylaxis.
On Day 2 after surgery, Ms. A attempts to elope. When she is approached by a psychiatrist on the treatment team, she does not recognize him. Although her long-term memory seems intact, she is unable to remember the details of recent events, including her medical and surgical treatments.
[polldaddy:10511745]
Continue to: The authors' observations
The authors’ observations
Ms. A’s memory impairment may be secondary to a surgically acquired neurocognitive deficit. In the United States, brain metastases represent a significant public health issue, affecting >100,000 patients per year.5 Metastatic lesions are the most common brain tumors. Lung cancer, breast cancer, and melanoma are the leading solid tumors to spread to the CNS.5 In cases of single brain metastasis, similar to Ms. A’s solitary left temporal lobe lesion, surgical resection plays a critical role in treatment. It provides histological confirmation of metastatic disease and can relieve mass effect if present. Studies have shown that combined surgical resection with radiation improves survival relative to patients who undergo radiation therapy alone.6,7
However, the benefits of surgical resection need to be balanced with preservation of neurologic function. Emerging evidence suggests that a majority of patients have surgically-acquired cognitive deficits due to damage of normal surrounding tissues, and these deficits are associated with reduced quality of life.8,9 Further, a study examining glioma surgical resections found that patients with left temporal lobe tumors exhibit more frequent and severe neurocognitive decline than patients with right temporal lobe tumors, especially in domains such as verbal memory.8 Ms. A’s memory impairment was persistent during her postoperative course, which suggests that it was not just an immediate post-surgical phenomenon, but a longer-lasting cognitive change directly related to the resection.
It is also possible that Ms. A had a prior neurocognitive disorder that manifested to a greater degree as a result of the CNS tumor. Ms. A might have had early-onset Alzheimer’s disease, although her intact memory before surgery makes this less likely. Alternatively, she could have had vascular dementia, especially given her long-standing hypertension and diabetes. This might have been missed in the initial evaluation because executive function was not tested. However, the relatively abrupt onset of memory problems after surgery suggests that she had no underlying neurocognitive disorder.
Ms. A’s presumed episode of MDD might also explain her memory changes. Major depressive disorder is increasingly common among geriatric patients, affecting approximately 5% of community-dwelling older adults.10 Its incidence increases with medical comorbidities, as suggested by depression rates of 5% to 10% in the primary care setting vs 37% in patients after critical-care hospitalizations.10 Late-life depression (LLD) occurs in adults age ≥60. Unlike depression in younger patients, LLD is more likely to be associated with cognitive impairment, specifically impairment of executive function and memory.11 The incidence of cognitive impairment in LLD is higher in patients with a history of depression, such as Ms. A.11,12 However, in general, patients who are depressed have memory complaints out of proportion to the clinical findings, and they show poor effort on cognitive testing. Ms. A exhibited neither of these, which makes it less likely that LLD was the exclusive cause of her memory loss.13 Table 214 outlines the management of cognitive deficits in a patient with a brain tumor.
EVALUATION Increasingly agitated and paranoid
After the tumor resection, Ms. A becomes increasingly irritable, uncooperative, and agitated. She repeatedly demands to be discharged. She insists she is fine and refuses medications and further laboratory workup. She becomes paranoid about the nursing staff and believes they are trying to kill her.
Continue to: On psychiatric re-evaluation...
On psychiatric re-evaluation, Ms. A demonstrates pressured speech, perseveration about going home, paranoid delusions, and anger at her family and physicians.
[polldaddy:10511747]
The authors’ observations
Ms. A’s refusal of medications and agitation may be explained by postoperative delirium, a surgical complication that is increasingly common among geriatric patients and is associated with poor clinical outcomes. Delirium is characterized by an acute onset and fluctuating course of symptoms that include inattention, motoric hypo- or hyperactivity, inappropriate behavior, emotional lability, cognitive dysfunction, and psychotic symptoms.15 Risk factors that contribute to postoperative delirium include older age, alcohol use, and poor baseline functional and cognitive status.16 The pathophysiology of delirium is not fully understood, but accumulating evidence suggests that different sets of interacting biologic factors (ie, neurotransmitters and inflammation) contribute to a disruption of large-scale neuronal networks in the brain, resulting in cognitive dysfunction.15 Patients who develop postoperative delirium are more likely to develop long-term cognitive dysfunction and have an increased risk of dementia.16
Another potential source of Ms. A’s agitation is steroid use. Ms. A received IV dexamethasone, 8 to 16 mg/d, around the time of her surgery. Steroids are commonly used to treat brain tumors, particularly when there is vasogenic edema. Steroid psychosis is a term loosely used to describe a wide range of psychiatric symptoms induced by corticosteroids that includes, but is not limited to, depression, mania, psychosis, delirium, and cognitive impairment.17 Steroid-induced psychiatric adverse effects occur in 5% to 18% of patients receiving corticosteroids and often happen early in treatment, although they can occur at any point.18 Corticosteroids influence brain activity via glucocorticoid and mineralocorticoid receptors. These receptors are widely distributed throughout the brain and affect neurotransmitter systems, such as the serotonergic system, that are associated with changes in mood, behavior, and cognition.17 While the adverse psychiatric manifestations of steroid use vary, higher dosages are associated with an increased risk of psychiatric complications; mania is more prevalent early in the course of treatment, and depression is more common with long-term use.17,19 Table 317,18 outlines the evidence-based treatment of corticosteroid-induced adverse psychiatric effects.
Although there are no clinical guidelines or FDA-approved medications for treating steroid-induced psychiatric adverse events, these are best managed by tapering and discontinuing steroids when possible and simultaneously using psychotropic medications to treat psychiatric symptoms. Case reports and limited evidence-based literature have demonstrated that steroid-induced mania responds to mood stabilizers or antipsychotics, while depression can be managed with antidepressants or lithium.17
Additionally, patients with CNS tumors are at risk for seizures and often are prescribed antiepileptics. Because it is easy to administer and does not need to be titrated, levetiracetam is a commonly used agent. However, levetiracetam can cause psychiatric adverse effects, including behavior changes and frank psychosis.20
Continue to: Finally, Ms. A's altered mental status...
Finally, Ms. A’s altered mental status could have been related to opioid intoxication. Opioids are used to manage postsurgical pain, and studies have shown these medications can be a precipitating factor for delirium in geriatric patients.21
TREATMENT Medication adjustments
At the request of the psychiatry team, levetiracetam is discontinued due to its potential for psychiatric adverse effects. The neurosurgery team replaces it with valproic acid, 500 mg every 12 hours. Ms. A is also tapered off steroids fairly rapidly because of the potential for steroid-induced psychiatric adverse effects. Her quetiapine is titrated from 50 to 150 mg every night at bedtime, and duloxetine is discontinued.
OUTCOME Agitation improves dramatically
Ms. A’s new medication regimen dramatically improves her agitation, which allows Ms. A, her family, and the medical team to work together to establish treatment goals. Ms. A ultimately returns home with the assistance of her family. She continues to have memory issues, but with improved emotion regulation. Several months later, Ms. A is readmitted to the hospital because her cancer has progressed despite treatment.
Bottom Line
Brain tumors may present with various psychiatric manifestations that can change during the course of the patient’s treatment. A comprehensive psychiatric evaluation should parse out the interplay between direct effects of the tumor and any adverse effects that are the result of medical and/or surgical interventions to determine the cause of psychiatric symptoms and their appropriate management.
Related Resource
Madhusoodanan S, Ting MB, Farah T, et al. Psychiatric aspects of brain tumors: a review. World J Psychiatry. 2015;5(3):273-285.
Drug Brand Names
Aripiprazole • Abilify
Buspirone • Buspar
Chlorpromazine • Thorazine
Codeine • Codeine systemic
Dexamethasone • Decadron
Duloxetine • Cymbalta
Haloperidol • Haldol
Levetiracetam • Keppra
Lorazepam • Ativan
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Valproic acid • Depakene
1. Madhusoodanan S, Opler MG, Moise D, et al. Brain tumor location and psychiatric symptoms: is there any association? A meta-analysis of published case studies. Expert Rev Neurother. 2010;10(10):1529-1536.
2. Bunevicius A, Deltuva VP, Deltuviene D, et al. Brain lesions manifesting as psychiatric disorders: eight cases. CNS Spectr. 2008;13(11):950-958.
3. Pearl ML, Talgat G, Valea FA, et al. Psychiatric symptoms due to brain metastases. Med Update Psychiatr. 1998;3(4):91-94.
4. Madhusoodanan S, Danan D, Moise D. Psychiatric manifestations of brain tumors: diagnostic implications. Expert Rev Neurother. 2007;7(4):343-349.
5. Ferguson SD, Wagner KM, Prabhu SS, et al. Neurosurgical management of brain metastases. Clin Exp Metastasis. 2017;34(6-7):377-389.
6. Husain ZA, Regine WF, Kwok Y, et al. Brain metastases: contemporary management and future directions. Eur J Clin Med Oncol. 2011;3(3):38-45.
7. Vecht CJ, Haaxmareiche H, Noordijk EM, et al. Treatment of single brain metastasis - radiotherapy alone or combined with neurosurgery. Ann Neurol. 1993;33(6):583-590.
8. Barry RL, Byun NE, Tantawy MN, et al. In vivo neuroimaging and behavioral correlates in a rat model of chemotherapy-induced cognitive dysfunction. Brain Imaging Behav. 2018;12(1):87-95.
9. Wu AS, Witgert ME, Lang FF, et al. Neurocognitive function before and after surgery for insular gliomas. J Neurosurg. 2011;115(6):1115-1125.
10. Taylor WD. Depression in the elderly. N Engl J Med. 2014;371(13):1228-1236.
11. Liguori C, Pierantozzi M, Chiaravalloti A, et al. When cognitive decline and depression coexist in the elderly: CSF biomarkers analysis can differentiate Alzheimer’s disease from late-life depression. Front Aging Neurosci. 2018;10:38.
12. Luijendijk HJ, van den Berg JF, Dekker MJHJ, et al. Incidence and recurrence of late-life depression. Arch Gen Psychiatry. 2008;65(12):1394-1401.
13. Potter GG, Steffens DC. Contribution of depression to cognitive impairment and dementia in older adults. Neurologist. 2007;13(3):105-117.
14. Taphoorn MJB, Klein M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 2004;3(3):159-168.
15. Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922.
16. Sprung J, Roberts RO, Weingarten TN, et al. Postoperative delirium in elderly patients is associated with subsequent cognitive impairment. Br J Anaesth. 2017;119(2):316-323.
17. Kusljic S, Manias E, Gogos A. Corticosteroid-induced psychiatric disturbances: it is time for pharmacists to take notice. Res Soc Adm Pharm. 2016;12(2):355-360.
18. Cerullo MA. Corticosteroid-induced mania: prepare for the unpredictable. Current Psychiatry. 2006;5(6):43-50.
19. Dubovsky AN, Arvikar S, Stern TA, et al. Steroid psychosis revisited. Psychosomatics. 2012;53(2):103-115.
20. Habets JGV, Leentjens AFG, Schijns OEMG. Serious and reversible levetiracetam-induced psychiatric symptoms after resection of frontal low-grade glioma: two case histories. Br J Neurosurg. 2017;31(4):471-473.
21
1. Madhusoodanan S, Opler MG, Moise D, et al. Brain tumor location and psychiatric symptoms: is there any association? A meta-analysis of published case studies. Expert Rev Neurother. 2010;10(10):1529-1536.
2. Bunevicius A, Deltuva VP, Deltuviene D, et al. Brain lesions manifesting as psychiatric disorders: eight cases. CNS Spectr. 2008;13(11):950-958.
3. Pearl ML, Talgat G, Valea FA, et al. Psychiatric symptoms due to brain metastases. Med Update Psychiatr. 1998;3(4):91-94.
4. Madhusoodanan S, Danan D, Moise D. Psychiatric manifestations of brain tumors: diagnostic implications. Expert Rev Neurother. 2007;7(4):343-349.
5. Ferguson SD, Wagner KM, Prabhu SS, et al. Neurosurgical management of brain metastases. Clin Exp Metastasis. 2017;34(6-7):377-389.
6. Husain ZA, Regine WF, Kwok Y, et al. Brain metastases: contemporary management and future directions. Eur J Clin Med Oncol. 2011;3(3):38-45.
7. Vecht CJ, Haaxmareiche H, Noordijk EM, et al. Treatment of single brain metastasis - radiotherapy alone or combined with neurosurgery. Ann Neurol. 1993;33(6):583-590.
8. Barry RL, Byun NE, Tantawy MN, et al. In vivo neuroimaging and behavioral correlates in a rat model of chemotherapy-induced cognitive dysfunction. Brain Imaging Behav. 2018;12(1):87-95.
9. Wu AS, Witgert ME, Lang FF, et al. Neurocognitive function before and after surgery for insular gliomas. J Neurosurg. 2011;115(6):1115-1125.
10. Taylor WD. Depression in the elderly. N Engl J Med. 2014;371(13):1228-1236.
11. Liguori C, Pierantozzi M, Chiaravalloti A, et al. When cognitive decline and depression coexist in the elderly: CSF biomarkers analysis can differentiate Alzheimer’s disease from late-life depression. Front Aging Neurosci. 2018;10:38.
12. Luijendijk HJ, van den Berg JF, Dekker MJHJ, et al. Incidence and recurrence of late-life depression. Arch Gen Psychiatry. 2008;65(12):1394-1401.
13. Potter GG, Steffens DC. Contribution of depression to cognitive impairment and dementia in older adults. Neurologist. 2007;13(3):105-117.
14. Taphoorn MJB, Klein M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 2004;3(3):159-168.
15. Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922.
16. Sprung J, Roberts RO, Weingarten TN, et al. Postoperative delirium in elderly patients is associated with subsequent cognitive impairment. Br J Anaesth. 2017;119(2):316-323.
17. Kusljic S, Manias E, Gogos A. Corticosteroid-induced psychiatric disturbances: it is time for pharmacists to take notice. Res Soc Adm Pharm. 2016;12(2):355-360.
18. Cerullo MA. Corticosteroid-induced mania: prepare for the unpredictable. Current Psychiatry. 2006;5(6):43-50.
19. Dubovsky AN, Arvikar S, Stern TA, et al. Steroid psychosis revisited. Psychosomatics. 2012;53(2):103-115.
20. Habets JGV, Leentjens AFG, Schijns OEMG. Serious and reversible levetiracetam-induced psychiatric symptoms after resection of frontal low-grade glioma: two case histories. Br J Neurosurg. 2017;31(4):471-473.
21
ACIP: Flu vaccines for older adults show similar safety profiles
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) recommends that age-appropriate vaccines be used when possible, said Kenneth E. Schmader, MD, professor of medicine at Duke University, Durham, N.C. However, no study to date had directly compared the safety of the trivalent high dose (HD-IIV3) and adjuvanted (aIIV3) vaccines or their impact on health-related quality of life. Dr. Schmader presented findings from a randomized trial at the February ACIP meeting.
To compare the safety of the vaccines, the researchers recruited community-dwelling volunteers aged 65 years and older who were cognitively intact, not immunosuppressed, and had no contraindications for influenza vaccination. A total of 378 individuals were randomized to aIIV3 and 379 to HD-IIV3. The average age was 72 years; 80 individuals in the aIIV3 group and 83 in the HDIIV3 group were 80 years and older. The primary outcome was moderate or severe injection site pain.
Overall, the proportion of participants with moderate or severe injection site pain was not significantly different after aIIV3 vs. HD-IIV3 (3.2% vs. 5.8%).
Nine participants in the aIIV3 group and three participants in the HD-IIV3 group experienced at least one serious adverse event, but no serious adverse events were deemed vaccine related, and the occurrence of serious adverse events was not significantly different between groups.
In addition, measures of short-term, postvaccination health-related quality of life were not significantly different between the groups. Changes in scores from day 1 prevaccination to day 3 postvaccination on the EuroQOL-5 dimensions-5 levels (EQ-5D-5L) were –0.05 for both groups.
The findings were limited in part by the lack of inclusion of older adults in nursing homes or similar settings, Dr. Schmader noted. However, the results suggest that “from the standpoint of safety, either vaccine is an acceptable option for the prevention of influenza in older adults.”
Studies comparing the immunogenicity of the vaccines are ongoing, and the data should be available within the next few months, he noted.
Dr. Schmader had no financial conflicts to disclose.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) recommends that age-appropriate vaccines be used when possible, said Kenneth E. Schmader, MD, professor of medicine at Duke University, Durham, N.C. However, no study to date had directly compared the safety of the trivalent high dose (HD-IIV3) and adjuvanted (aIIV3) vaccines or their impact on health-related quality of life. Dr. Schmader presented findings from a randomized trial at the February ACIP meeting.
To compare the safety of the vaccines, the researchers recruited community-dwelling volunteers aged 65 years and older who were cognitively intact, not immunosuppressed, and had no contraindications for influenza vaccination. A total of 378 individuals were randomized to aIIV3 and 379 to HD-IIV3. The average age was 72 years; 80 individuals in the aIIV3 group and 83 in the HDIIV3 group were 80 years and older. The primary outcome was moderate or severe injection site pain.
Overall, the proportion of participants with moderate or severe injection site pain was not significantly different after aIIV3 vs. HD-IIV3 (3.2% vs. 5.8%).
Nine participants in the aIIV3 group and three participants in the HD-IIV3 group experienced at least one serious adverse event, but no serious adverse events were deemed vaccine related, and the occurrence of serious adverse events was not significantly different between groups.
In addition, measures of short-term, postvaccination health-related quality of life were not significantly different between the groups. Changes in scores from day 1 prevaccination to day 3 postvaccination on the EuroQOL-5 dimensions-5 levels (EQ-5D-5L) were –0.05 for both groups.
The findings were limited in part by the lack of inclusion of older adults in nursing homes or similar settings, Dr. Schmader noted. However, the results suggest that “from the standpoint of safety, either vaccine is an acceptable option for the prevention of influenza in older adults.”
Studies comparing the immunogenicity of the vaccines are ongoing, and the data should be available within the next few months, he noted.
Dr. Schmader had no financial conflicts to disclose.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) recommends that age-appropriate vaccines be used when possible, said Kenneth E. Schmader, MD, professor of medicine at Duke University, Durham, N.C. However, no study to date had directly compared the safety of the trivalent high dose (HD-IIV3) and adjuvanted (aIIV3) vaccines or their impact on health-related quality of life. Dr. Schmader presented findings from a randomized trial at the February ACIP meeting.
To compare the safety of the vaccines, the researchers recruited community-dwelling volunteers aged 65 years and older who were cognitively intact, not immunosuppressed, and had no contraindications for influenza vaccination. A total of 378 individuals were randomized to aIIV3 and 379 to HD-IIV3. The average age was 72 years; 80 individuals in the aIIV3 group and 83 in the HDIIV3 group were 80 years and older. The primary outcome was moderate or severe injection site pain.
Overall, the proportion of participants with moderate or severe injection site pain was not significantly different after aIIV3 vs. HD-IIV3 (3.2% vs. 5.8%).
Nine participants in the aIIV3 group and three participants in the HD-IIV3 group experienced at least one serious adverse event, but no serious adverse events were deemed vaccine related, and the occurrence of serious adverse events was not significantly different between groups.
In addition, measures of short-term, postvaccination health-related quality of life were not significantly different between the groups. Changes in scores from day 1 prevaccination to day 3 postvaccination on the EuroQOL-5 dimensions-5 levels (EQ-5D-5L) were –0.05 for both groups.
The findings were limited in part by the lack of inclusion of older adults in nursing homes or similar settings, Dr. Schmader noted. However, the results suggest that “from the standpoint of safety, either vaccine is an acceptable option for the prevention of influenza in older adults.”
Studies comparing the immunogenicity of the vaccines are ongoing, and the data should be available within the next few months, he noted.
Dr. Schmader had no financial conflicts to disclose.
FROM AN ACIP MEETING
Joint replacement: What’s new in 2020
MAUI, HAWAII – Outpatient total hip and knee replacement is “the latest craze” in orthopedic surgery, and it’s being driven by the might of Medicare, William Bugbee, MD, said at the 2020 Rheumatology Winter Clinical Symposium.
“In 2019, Medicare took total knee replacement off the inpatient-only list, meaning you could do it as an outpatient. And just in January 2020, they took total hips off that list. So I have to designate most of my hip and knee replacements as outpatients, even if I do it in the hospital and keep them for 1 night. And some of the private insurers have already gone to that, so they’ll deny coverage if I say I want a 1-day hospital stay, believe it or not,” according to Dr. Bugbee, chief of joint reconstruction in the department of orthopedics at the Scripps Clinic in La Jolla, Calif.
He provided a behind-the-scenes look at contemporary trends in joint replacement as well as tips on how rheumatologists can best help their patients get through the experience with excellent outcomes.
Joint replacement remains the best treatment for advanced arthritis of the hips and knees, he said. There is a high degree of confidence about the predictability and durability of the results. But joint replacement has become highly commoditized.
“We’re getting pummeled by Medicare to make this as cheap as possible,” the orthopedic surgeon explained. “An implant costs the hospital $3,000-$6,000. A care episode for a primary total joint replacement should cost a hospital $8,000-$15,000, which is about what Medicare pays for the [Diagnosis Related Group], so the margins are small. That’s why we’re being drilled on about how much we spend on every little thing. We hardly do any labs, x-rays, anything.”
As a result of recent advances in pre-, peri-, and postoperative management, outpatient joint replacement has become a safe and comparatively economical option for generally healthy patients.
“We’ve engineered a much better patient experience, so the assault and battery of 5, 10, 15 years ago isn’t so bad anymore,” Dr. Bugbee said.
Rheumatologists can expect to see a growing number of their patients undergoing total knee or hip replacement at outpatient surgery centers. That’s not a bad thing so long as the procedure is being done there because the outpatient center employs best practices in order to provide a highly efficient episode of care supported by excellent outcome data, he continued.
State-of-the-art perioperative management in 2020 includes accelerated-care pathways that allow ambulation within an hour or 2 after surgery along with same-day discharge, regional anesthesia with motor-sparing nerve blocks, and multimodal pain management with avoidance of intravenous narcotics except in opioid-tolerant patients. Tranexamic acid is now widely used in order to reduce operative blood loss.
“When I started practice 25 years ago, 50% of patients got a blood transfusion. I haven’t given a blood transfusion to a patient in probably 2 years. Tranexamic acid reduces blood loss by 500-700 cc with no discernible adverse effects. It’s truly remarkable,” he said.
Another important technical advance has been the routine use of oral dexamethasone. “Decadron is an antiemetic, it has anti-inflammatory effects, and it makes people happy. It’s a simple, cheap drug that has revolutionized care,” the surgeon continued.
Postoperative management has been streamlined. Dr. Bugbee is among many orthopedic surgeons who no longer routinely prescribe therapist-directed formal physical therapy for total hip arthroplasty patients, relying instead upon online tools and apps for self-administered physical therapy. Pedal exercise devices available online for $30 or so have been shown to be as effective as supervised physical therapy for knee rehabilitation.
What patients want to know about joint replacement
The question patients most often ask both their referring physician and the orthopedic surgeon is, “How long will my joint replacement last?” The best available data come from a couple of recent paired meta-analyses. The investigators reported 82% implant survivorship 25 years after primary total knee arthroplasty and 70% after unicondylar knee arthroplasty as well as a 25-year implant survivorship rate of 77% for total hip arthroplasty.
“I expected that hip arthroplasty survivorship rate to be much higher than 77%. The reason it’s not is probably because of the metal-on-metal bearing surface debacle of about 10 years ago. There’ve been lots of revisions because of that. We thought metal-on-metal implants were going to be all that, with microscopically low wear, but they turned out to be a nightmare because of metal ion release,” Dr. Bugbee observed.
The long-term joint survivorship data are based upon older implants. Encouraging albeit still preliminary data suggest contemporary implants may last significantly longer. The “clear winner,” he said, is a 36-mm ceramic head and a highly crosslinked polyethylene liner.
“That’s been a game changer, with a 10- to 20-fold decrease in wear compared to plastics for weight-bearing surfaces,” Dr. Bugbee said.
In terms of functional improvement, by various measures 85%-97% of patients are satisfied with the results of their total hip replacement, and 60% report returning to high-level recreational activities. Patient satisfaction scores are lower – 75%-90% – after total knee arthroplasty.
“The total knee replacement just doesn’t work like a regular joint,” the surgeon observed. “When I think of hip and knee replacements, I think of a hip as a Ferrari – it’s a high-performance joint replacement – and I think of the knee as a Ford – it’s serviceable, it does the job, and it’s okay but not fantastic.”
How referring physicians can optimize preoperative management and long-term follow-up
Orthopedic surgeons would appreciate help from rheumatologists and primary care physicians in preoperatively addressing the known modifiable risk factors for poor outcomes of joint replacement. These include obesity, smoking, depression, a hemoglobin A1c of 7% or more, and being on opioids. These risk factors are incompatible with outpatient hip or knee replacement.
“Let the surgeon know if you think outpatient joint replacement is a bad idea in your patient for medical reasons,” Dr. Bugbee urged.
Also, orthopedic surgeons can generally benefit from rheumatologist input regarding perioperative management of patients on standard disease-modifying antirheumatic drugs, biologics, or Janus kinase inhibitors as recommended in the guidelines published jointly by the American College of Rheumatology and the American Association of Hip and Knee Surgeons.
“I can guarantee you that most orthopedic surgeons don’t know about these guidelines. The evidence base for these recommendations is not great, but these are the best guidelines we have,” Dr. Bugbee said.
After joint replacement surgery a patient should get an x-ray of the replacement every 5 years. And if a patient develops a painful hip after arthroplasty, it’s worthwhile to order blood chromium and cobalt levels.
“The implant weight-bearing surface matters. You can’t necessarily tell on x-ray what’s a metal-on-metal hip and what’s metal-on-plastic or ceramic. You already send people for a lot of labs. If you see a patient with a painful total hip replacement, just add a cobalt and chromium. If they’re elevated, talk to the orthopedist,” he advised.
The road ahead
Hip and knee replacement is an $18 billion market today. And it’s a major growth industry: According to a recent projection, there will be 1 million total hip replacements and 4 million total knee replacements annually 10 years from now, figures four times greater than projected for 2030 in an earlier 2005 estimate. The rapid growth is coming from the expanding elderly population combined with a virtual epidemic of posttraumatic arthritis in young people – but decidedly not from patients with joint failure attributable to rheumatoid arthritis.
“Congratulations! You’ve eradicated rheumatoid arthritis from my practice,” Dr. Bugbee declared. “Most of the rheumatoid arthritis patients who come to me come because they have osteoarthritis in their joint, not because of their rheumatoid arthritis.”
He reported serving as a consultant to Orthalign, Insight Medical, and Arthrex, and receiving royalties from Smith and Nephew and Depuy.
MAUI, HAWAII – Outpatient total hip and knee replacement is “the latest craze” in orthopedic surgery, and it’s being driven by the might of Medicare, William Bugbee, MD, said at the 2020 Rheumatology Winter Clinical Symposium.
“In 2019, Medicare took total knee replacement off the inpatient-only list, meaning you could do it as an outpatient. And just in January 2020, they took total hips off that list. So I have to designate most of my hip and knee replacements as outpatients, even if I do it in the hospital and keep them for 1 night. And some of the private insurers have already gone to that, so they’ll deny coverage if I say I want a 1-day hospital stay, believe it or not,” according to Dr. Bugbee, chief of joint reconstruction in the department of orthopedics at the Scripps Clinic in La Jolla, Calif.
He provided a behind-the-scenes look at contemporary trends in joint replacement as well as tips on how rheumatologists can best help their patients get through the experience with excellent outcomes.
Joint replacement remains the best treatment for advanced arthritis of the hips and knees, he said. There is a high degree of confidence about the predictability and durability of the results. But joint replacement has become highly commoditized.
“We’re getting pummeled by Medicare to make this as cheap as possible,” the orthopedic surgeon explained. “An implant costs the hospital $3,000-$6,000. A care episode for a primary total joint replacement should cost a hospital $8,000-$15,000, which is about what Medicare pays for the [Diagnosis Related Group], so the margins are small. That’s why we’re being drilled on about how much we spend on every little thing. We hardly do any labs, x-rays, anything.”
As a result of recent advances in pre-, peri-, and postoperative management, outpatient joint replacement has become a safe and comparatively economical option for generally healthy patients.
“We’ve engineered a much better patient experience, so the assault and battery of 5, 10, 15 years ago isn’t so bad anymore,” Dr. Bugbee said.
Rheumatologists can expect to see a growing number of their patients undergoing total knee or hip replacement at outpatient surgery centers. That’s not a bad thing so long as the procedure is being done there because the outpatient center employs best practices in order to provide a highly efficient episode of care supported by excellent outcome data, he continued.
State-of-the-art perioperative management in 2020 includes accelerated-care pathways that allow ambulation within an hour or 2 after surgery along with same-day discharge, regional anesthesia with motor-sparing nerve blocks, and multimodal pain management with avoidance of intravenous narcotics except in opioid-tolerant patients. Tranexamic acid is now widely used in order to reduce operative blood loss.
“When I started practice 25 years ago, 50% of patients got a blood transfusion. I haven’t given a blood transfusion to a patient in probably 2 years. Tranexamic acid reduces blood loss by 500-700 cc with no discernible adverse effects. It’s truly remarkable,” he said.
Another important technical advance has been the routine use of oral dexamethasone. “Decadron is an antiemetic, it has anti-inflammatory effects, and it makes people happy. It’s a simple, cheap drug that has revolutionized care,” the surgeon continued.
Postoperative management has been streamlined. Dr. Bugbee is among many orthopedic surgeons who no longer routinely prescribe therapist-directed formal physical therapy for total hip arthroplasty patients, relying instead upon online tools and apps for self-administered physical therapy. Pedal exercise devices available online for $30 or so have been shown to be as effective as supervised physical therapy for knee rehabilitation.
What patients want to know about joint replacement
The question patients most often ask both their referring physician and the orthopedic surgeon is, “How long will my joint replacement last?” The best available data come from a couple of recent paired meta-analyses. The investigators reported 82% implant survivorship 25 years after primary total knee arthroplasty and 70% after unicondylar knee arthroplasty as well as a 25-year implant survivorship rate of 77% for total hip arthroplasty.
“I expected that hip arthroplasty survivorship rate to be much higher than 77%. The reason it’s not is probably because of the metal-on-metal bearing surface debacle of about 10 years ago. There’ve been lots of revisions because of that. We thought metal-on-metal implants were going to be all that, with microscopically low wear, but they turned out to be a nightmare because of metal ion release,” Dr. Bugbee observed.
The long-term joint survivorship data are based upon older implants. Encouraging albeit still preliminary data suggest contemporary implants may last significantly longer. The “clear winner,” he said, is a 36-mm ceramic head and a highly crosslinked polyethylene liner.
“That’s been a game changer, with a 10- to 20-fold decrease in wear compared to plastics for weight-bearing surfaces,” Dr. Bugbee said.
In terms of functional improvement, by various measures 85%-97% of patients are satisfied with the results of their total hip replacement, and 60% report returning to high-level recreational activities. Patient satisfaction scores are lower – 75%-90% – after total knee arthroplasty.
“The total knee replacement just doesn’t work like a regular joint,” the surgeon observed. “When I think of hip and knee replacements, I think of a hip as a Ferrari – it’s a high-performance joint replacement – and I think of the knee as a Ford – it’s serviceable, it does the job, and it’s okay but not fantastic.”
How referring physicians can optimize preoperative management and long-term follow-up
Orthopedic surgeons would appreciate help from rheumatologists and primary care physicians in preoperatively addressing the known modifiable risk factors for poor outcomes of joint replacement. These include obesity, smoking, depression, a hemoglobin A1c of 7% or more, and being on opioids. These risk factors are incompatible with outpatient hip or knee replacement.
“Let the surgeon know if you think outpatient joint replacement is a bad idea in your patient for medical reasons,” Dr. Bugbee urged.
Also, orthopedic surgeons can generally benefit from rheumatologist input regarding perioperative management of patients on standard disease-modifying antirheumatic drugs, biologics, or Janus kinase inhibitors as recommended in the guidelines published jointly by the American College of Rheumatology and the American Association of Hip and Knee Surgeons.
“I can guarantee you that most orthopedic surgeons don’t know about these guidelines. The evidence base for these recommendations is not great, but these are the best guidelines we have,” Dr. Bugbee said.
After joint replacement surgery a patient should get an x-ray of the replacement every 5 years. And if a patient develops a painful hip after arthroplasty, it’s worthwhile to order blood chromium and cobalt levels.
“The implant weight-bearing surface matters. You can’t necessarily tell on x-ray what’s a metal-on-metal hip and what’s metal-on-plastic or ceramic. You already send people for a lot of labs. If you see a patient with a painful total hip replacement, just add a cobalt and chromium. If they’re elevated, talk to the orthopedist,” he advised.
The road ahead
Hip and knee replacement is an $18 billion market today. And it’s a major growth industry: According to a recent projection, there will be 1 million total hip replacements and 4 million total knee replacements annually 10 years from now, figures four times greater than projected for 2030 in an earlier 2005 estimate. The rapid growth is coming from the expanding elderly population combined with a virtual epidemic of posttraumatic arthritis in young people – but decidedly not from patients with joint failure attributable to rheumatoid arthritis.
“Congratulations! You’ve eradicated rheumatoid arthritis from my practice,” Dr. Bugbee declared. “Most of the rheumatoid arthritis patients who come to me come because they have osteoarthritis in their joint, not because of their rheumatoid arthritis.”
He reported serving as a consultant to Orthalign, Insight Medical, and Arthrex, and receiving royalties from Smith and Nephew and Depuy.
MAUI, HAWAII – Outpatient total hip and knee replacement is “the latest craze” in orthopedic surgery, and it’s being driven by the might of Medicare, William Bugbee, MD, said at the 2020 Rheumatology Winter Clinical Symposium.
“In 2019, Medicare took total knee replacement off the inpatient-only list, meaning you could do it as an outpatient. And just in January 2020, they took total hips off that list. So I have to designate most of my hip and knee replacements as outpatients, even if I do it in the hospital and keep them for 1 night. And some of the private insurers have already gone to that, so they’ll deny coverage if I say I want a 1-day hospital stay, believe it or not,” according to Dr. Bugbee, chief of joint reconstruction in the department of orthopedics at the Scripps Clinic in La Jolla, Calif.
He provided a behind-the-scenes look at contemporary trends in joint replacement as well as tips on how rheumatologists can best help their patients get through the experience with excellent outcomes.
Joint replacement remains the best treatment for advanced arthritis of the hips and knees, he said. There is a high degree of confidence about the predictability and durability of the results. But joint replacement has become highly commoditized.
“We’re getting pummeled by Medicare to make this as cheap as possible,” the orthopedic surgeon explained. “An implant costs the hospital $3,000-$6,000. A care episode for a primary total joint replacement should cost a hospital $8,000-$15,000, which is about what Medicare pays for the [Diagnosis Related Group], so the margins are small. That’s why we’re being drilled on about how much we spend on every little thing. We hardly do any labs, x-rays, anything.”
As a result of recent advances in pre-, peri-, and postoperative management, outpatient joint replacement has become a safe and comparatively economical option for generally healthy patients.
“We’ve engineered a much better patient experience, so the assault and battery of 5, 10, 15 years ago isn’t so bad anymore,” Dr. Bugbee said.
Rheumatologists can expect to see a growing number of their patients undergoing total knee or hip replacement at outpatient surgery centers. That’s not a bad thing so long as the procedure is being done there because the outpatient center employs best practices in order to provide a highly efficient episode of care supported by excellent outcome data, he continued.
State-of-the-art perioperative management in 2020 includes accelerated-care pathways that allow ambulation within an hour or 2 after surgery along with same-day discharge, regional anesthesia with motor-sparing nerve blocks, and multimodal pain management with avoidance of intravenous narcotics except in opioid-tolerant patients. Tranexamic acid is now widely used in order to reduce operative blood loss.
“When I started practice 25 years ago, 50% of patients got a blood transfusion. I haven’t given a blood transfusion to a patient in probably 2 years. Tranexamic acid reduces blood loss by 500-700 cc with no discernible adverse effects. It’s truly remarkable,” he said.
Another important technical advance has been the routine use of oral dexamethasone. “Decadron is an antiemetic, it has anti-inflammatory effects, and it makes people happy. It’s a simple, cheap drug that has revolutionized care,” the surgeon continued.
Postoperative management has been streamlined. Dr. Bugbee is among many orthopedic surgeons who no longer routinely prescribe therapist-directed formal physical therapy for total hip arthroplasty patients, relying instead upon online tools and apps for self-administered physical therapy. Pedal exercise devices available online for $30 or so have been shown to be as effective as supervised physical therapy for knee rehabilitation.
What patients want to know about joint replacement
The question patients most often ask both their referring physician and the orthopedic surgeon is, “How long will my joint replacement last?” The best available data come from a couple of recent paired meta-analyses. The investigators reported 82% implant survivorship 25 years after primary total knee arthroplasty and 70% after unicondylar knee arthroplasty as well as a 25-year implant survivorship rate of 77% for total hip arthroplasty.
“I expected that hip arthroplasty survivorship rate to be much higher than 77%. The reason it’s not is probably because of the metal-on-metal bearing surface debacle of about 10 years ago. There’ve been lots of revisions because of that. We thought metal-on-metal implants were going to be all that, with microscopically low wear, but they turned out to be a nightmare because of metal ion release,” Dr. Bugbee observed.
The long-term joint survivorship data are based upon older implants. Encouraging albeit still preliminary data suggest contemporary implants may last significantly longer. The “clear winner,” he said, is a 36-mm ceramic head and a highly crosslinked polyethylene liner.
“That’s been a game changer, with a 10- to 20-fold decrease in wear compared to plastics for weight-bearing surfaces,” Dr. Bugbee said.
In terms of functional improvement, by various measures 85%-97% of patients are satisfied with the results of their total hip replacement, and 60% report returning to high-level recreational activities. Patient satisfaction scores are lower – 75%-90% – after total knee arthroplasty.
“The total knee replacement just doesn’t work like a regular joint,” the surgeon observed. “When I think of hip and knee replacements, I think of a hip as a Ferrari – it’s a high-performance joint replacement – and I think of the knee as a Ford – it’s serviceable, it does the job, and it’s okay but not fantastic.”
How referring physicians can optimize preoperative management and long-term follow-up
Orthopedic surgeons would appreciate help from rheumatologists and primary care physicians in preoperatively addressing the known modifiable risk factors for poor outcomes of joint replacement. These include obesity, smoking, depression, a hemoglobin A1c of 7% or more, and being on opioids. These risk factors are incompatible with outpatient hip or knee replacement.
“Let the surgeon know if you think outpatient joint replacement is a bad idea in your patient for medical reasons,” Dr. Bugbee urged.
Also, orthopedic surgeons can generally benefit from rheumatologist input regarding perioperative management of patients on standard disease-modifying antirheumatic drugs, biologics, or Janus kinase inhibitors as recommended in the guidelines published jointly by the American College of Rheumatology and the American Association of Hip and Knee Surgeons.
“I can guarantee you that most orthopedic surgeons don’t know about these guidelines. The evidence base for these recommendations is not great, but these are the best guidelines we have,” Dr. Bugbee said.
After joint replacement surgery a patient should get an x-ray of the replacement every 5 years. And if a patient develops a painful hip after arthroplasty, it’s worthwhile to order blood chromium and cobalt levels.
“The implant weight-bearing surface matters. You can’t necessarily tell on x-ray what’s a metal-on-metal hip and what’s metal-on-plastic or ceramic. You already send people for a lot of labs. If you see a patient with a painful total hip replacement, just add a cobalt and chromium. If they’re elevated, talk to the orthopedist,” he advised.
The road ahead
Hip and knee replacement is an $18 billion market today. And it’s a major growth industry: According to a recent projection, there will be 1 million total hip replacements and 4 million total knee replacements annually 10 years from now, figures four times greater than projected for 2030 in an earlier 2005 estimate. The rapid growth is coming from the expanding elderly population combined with a virtual epidemic of posttraumatic arthritis in young people – but decidedly not from patients with joint failure attributable to rheumatoid arthritis.
“Congratulations! You’ve eradicated rheumatoid arthritis from my practice,” Dr. Bugbee declared. “Most of the rheumatoid arthritis patients who come to me come because they have osteoarthritis in their joint, not because of their rheumatoid arthritis.”
He reported serving as a consultant to Orthalign, Insight Medical, and Arthrex, and receiving royalties from Smith and Nephew and Depuy.
REPORTING FROM RWCS 2020
USPSTF again deems evidence insufficient to recommend cognitive impairment screening in older adults
The U.S. Preventive Services Task Force has deemed the current evidence “insufficient” to make a recommendation in regard to screening for cognitive impairment in adults aged 65 years or older.
“More research is needed on the effect of screening and early detection of cognitive impairment on important patient, caregiver, and societal outcomes, including decision making, advance planning, and caregiver outcomes,” wrote lead author Douglas K. Owens, MD, of Stanford (Calif.) University and fellow members of the task force. The statement was published in JAMA.
To update a 2014 recommendation from the USPSTF, which also found insufficient evidence to properly assess cognitive screening’s benefits and harms, the task force commissioned a systematic review of studies applicable to community-dwelling older adults who are not exhibiting signs or symptoms of cognitive impairment. For their statement, “cognitive impairment” is defined as mild cognitive impairment and mild to moderate dementia.
Ultimately, they determined several factors that limited the overall evidence, including the short duration of most trials and the heterogenous nature of interventions and inconsistencies in outcomes reported. Any evidence that suggested improvements was mostly applicable to patients with moderate dementia, meaning “its applicability to a screen-detected population is uncertain.”
Updating 2014 recommendations
Their statement was based on an evidence report, also published in JAMA, in which a team of researchers reviewed 287 studies that included more than 285,000 older adults; 92 of the studies were newly identified, while the other 195 were carried forward from the 2014 recommendation’s review. The researchers sought the answers to five key questions, carrying over the framework from the previous review.
“Despite the accumulation of new data, the conclusions for these key questions are essentially unchanged from the prior review,” wrote lead author Carrie D. Patnode, PhD, of the Kaiser Permanente Center for Health Research in Portland, Ore., and coauthors.
Of the questions – which concerned the accuracy of screening instruments; the harms of screening; the harms of interventions; and if screening or interventions improved decision making or outcomes for the patient, family/caregiver, or society – moderate evidence was found to support the accuracy of the instruments, treatment with acetylcholinesterase inhibitors and memantine for patients with moderate dementia, and psychoeducation interventions for caregivers of patients with moderate dementia. At the same time, there was moderate evidence of adverse effects from acetylcholinesterase inhibitors and memantine in patients with moderate dementia.
“I think, eventually, there will be sufficient evidence to justify screening, once we have what I call a tiered approach,” Marwan Sabbagh, MD, of the Cleveland Clinic Lou Ruvo Center for Brain Health in Las Vegas, said in an interview. “The very near future will include blood tests for Alzheimer’s, or PET scans, or genetics, or something else. Right now, the cognitive screens lack the specificity and sensitivity, and the secondary screening infrastructure that would improve the accuracy doesn’t exist yet.
“I think this is a ‘not now,’ ” he added, “but I wouldn’t say ‘not ever.’ ”
Dr. Patnode and coauthors noted specific limitations in the evidence, including a lack of studies on how screening for and treating cognitive impairment affects decision making. In addition, details like quality of life and institutionalization were inconsistently reported, and “consistent and standardized reporting of results according to meaningful thresholds of clinical significance” would have been valuable across all measures.
Clinical implications
The implications of this report’s conclusions are substantial, especially as the rising prevalence of mild cognitive impairment and dementia becomes a worldwide concern, wrote Ronald C. Petersen, PhD, MD, of the Mayo Clinic in Rochester, Minn., and Kristine Yaffe, MD, of the University of California, San Francisco, in an accompanying editorial.
Though the data does not explicitly support screening, Dr. Petersen and Dr. Yaffe noted that it still may have benefits. An estimated 10% of cognitive impairment is caused by at least somewhat reversible causes, and screening could also be used to improve care in medical problems that are worsened by cognitive impairment. To find the true value of these efforts, they wrote, researchers need to design and execute additional clinical trials that “answer many of the important questions surrounding screening and treatment of cognitive impairment.”
“The absence of evidence for benefit may lead to inaction,” they added, noting that clinicians screening should still consider the value of screening on a case-by-case basis in order to keep up with the impact of new disease-modifying therapies for certain neurodegenerative diseases.
All members of the USPSTF received travel reimbursement and an honorarium for participating in meetings. One member reported receiving grants and personal fees from Healthwise. The study was funded by the Department of Health & Human Services. One of the authors reported receiving grants from the National Institutes of Health and the Food and Drug Administration. Dr. Petersen and Dr. Yaffe reported consulting for, and receiving funding from, various pharmaceutical companies, foundations, and government organizations.
SOURCES: Owens DK et al. JAMA. 2020 Feb 25. doi: 10.1001/jama.2020.0435; Patnode CD et al. JAMA. 2020 Feb 25. doi: 10.1001/jama.2019.22258.
The U.S. Preventive Services Task Force has deemed the current evidence “insufficient” to make a recommendation in regard to screening for cognitive impairment in adults aged 65 years or older.
“More research is needed on the effect of screening and early detection of cognitive impairment on important patient, caregiver, and societal outcomes, including decision making, advance planning, and caregiver outcomes,” wrote lead author Douglas K. Owens, MD, of Stanford (Calif.) University and fellow members of the task force. The statement was published in JAMA.
To update a 2014 recommendation from the USPSTF, which also found insufficient evidence to properly assess cognitive screening’s benefits and harms, the task force commissioned a systematic review of studies applicable to community-dwelling older adults who are not exhibiting signs or symptoms of cognitive impairment. For their statement, “cognitive impairment” is defined as mild cognitive impairment and mild to moderate dementia.
Ultimately, they determined several factors that limited the overall evidence, including the short duration of most trials and the heterogenous nature of interventions and inconsistencies in outcomes reported. Any evidence that suggested improvements was mostly applicable to patients with moderate dementia, meaning “its applicability to a screen-detected population is uncertain.”
Updating 2014 recommendations
Their statement was based on an evidence report, also published in JAMA, in which a team of researchers reviewed 287 studies that included more than 285,000 older adults; 92 of the studies were newly identified, while the other 195 were carried forward from the 2014 recommendation’s review. The researchers sought the answers to five key questions, carrying over the framework from the previous review.
“Despite the accumulation of new data, the conclusions for these key questions are essentially unchanged from the prior review,” wrote lead author Carrie D. Patnode, PhD, of the Kaiser Permanente Center for Health Research in Portland, Ore., and coauthors.
Of the questions – which concerned the accuracy of screening instruments; the harms of screening; the harms of interventions; and if screening or interventions improved decision making or outcomes for the patient, family/caregiver, or society – moderate evidence was found to support the accuracy of the instruments, treatment with acetylcholinesterase inhibitors and memantine for patients with moderate dementia, and psychoeducation interventions for caregivers of patients with moderate dementia. At the same time, there was moderate evidence of adverse effects from acetylcholinesterase inhibitors and memantine in patients with moderate dementia.
“I think, eventually, there will be sufficient evidence to justify screening, once we have what I call a tiered approach,” Marwan Sabbagh, MD, of the Cleveland Clinic Lou Ruvo Center for Brain Health in Las Vegas, said in an interview. “The very near future will include blood tests for Alzheimer’s, or PET scans, or genetics, or something else. Right now, the cognitive screens lack the specificity and sensitivity, and the secondary screening infrastructure that would improve the accuracy doesn’t exist yet.
“I think this is a ‘not now,’ ” he added, “but I wouldn’t say ‘not ever.’ ”
Dr. Patnode and coauthors noted specific limitations in the evidence, including a lack of studies on how screening for and treating cognitive impairment affects decision making. In addition, details like quality of life and institutionalization were inconsistently reported, and “consistent and standardized reporting of results according to meaningful thresholds of clinical significance” would have been valuable across all measures.
Clinical implications
The implications of this report’s conclusions are substantial, especially as the rising prevalence of mild cognitive impairment and dementia becomes a worldwide concern, wrote Ronald C. Petersen, PhD, MD, of the Mayo Clinic in Rochester, Minn., and Kristine Yaffe, MD, of the University of California, San Francisco, in an accompanying editorial.
Though the data does not explicitly support screening, Dr. Petersen and Dr. Yaffe noted that it still may have benefits. An estimated 10% of cognitive impairment is caused by at least somewhat reversible causes, and screening could also be used to improve care in medical problems that are worsened by cognitive impairment. To find the true value of these efforts, they wrote, researchers need to design and execute additional clinical trials that “answer many of the important questions surrounding screening and treatment of cognitive impairment.”
“The absence of evidence for benefit may lead to inaction,” they added, noting that clinicians screening should still consider the value of screening on a case-by-case basis in order to keep up with the impact of new disease-modifying therapies for certain neurodegenerative diseases.
All members of the USPSTF received travel reimbursement and an honorarium for participating in meetings. One member reported receiving grants and personal fees from Healthwise. The study was funded by the Department of Health & Human Services. One of the authors reported receiving grants from the National Institutes of Health and the Food and Drug Administration. Dr. Petersen and Dr. Yaffe reported consulting for, and receiving funding from, various pharmaceutical companies, foundations, and government organizations.
SOURCES: Owens DK et al. JAMA. 2020 Feb 25. doi: 10.1001/jama.2020.0435; Patnode CD et al. JAMA. 2020 Feb 25. doi: 10.1001/jama.2019.22258.
The U.S. Preventive Services Task Force has deemed the current evidence “insufficient” to make a recommendation in regard to screening for cognitive impairment in adults aged 65 years or older.
“More research is needed on the effect of screening and early detection of cognitive impairment on important patient, caregiver, and societal outcomes, including decision making, advance planning, and caregiver outcomes,” wrote lead author Douglas K. Owens, MD, of Stanford (Calif.) University and fellow members of the task force. The statement was published in JAMA.
To update a 2014 recommendation from the USPSTF, which also found insufficient evidence to properly assess cognitive screening’s benefits and harms, the task force commissioned a systematic review of studies applicable to community-dwelling older adults who are not exhibiting signs or symptoms of cognitive impairment. For their statement, “cognitive impairment” is defined as mild cognitive impairment and mild to moderate dementia.
Ultimately, they determined several factors that limited the overall evidence, including the short duration of most trials and the heterogenous nature of interventions and inconsistencies in outcomes reported. Any evidence that suggested improvements was mostly applicable to patients with moderate dementia, meaning “its applicability to a screen-detected population is uncertain.”
Updating 2014 recommendations
Their statement was based on an evidence report, also published in JAMA, in which a team of researchers reviewed 287 studies that included more than 285,000 older adults; 92 of the studies were newly identified, while the other 195 were carried forward from the 2014 recommendation’s review. The researchers sought the answers to five key questions, carrying over the framework from the previous review.
“Despite the accumulation of new data, the conclusions for these key questions are essentially unchanged from the prior review,” wrote lead author Carrie D. Patnode, PhD, of the Kaiser Permanente Center for Health Research in Portland, Ore., and coauthors.
Of the questions – which concerned the accuracy of screening instruments; the harms of screening; the harms of interventions; and if screening or interventions improved decision making or outcomes for the patient, family/caregiver, or society – moderate evidence was found to support the accuracy of the instruments, treatment with acetylcholinesterase inhibitors and memantine for patients with moderate dementia, and psychoeducation interventions for caregivers of patients with moderate dementia. At the same time, there was moderate evidence of adverse effects from acetylcholinesterase inhibitors and memantine in patients with moderate dementia.
“I think, eventually, there will be sufficient evidence to justify screening, once we have what I call a tiered approach,” Marwan Sabbagh, MD, of the Cleveland Clinic Lou Ruvo Center for Brain Health in Las Vegas, said in an interview. “The very near future will include blood tests for Alzheimer’s, or PET scans, or genetics, or something else. Right now, the cognitive screens lack the specificity and sensitivity, and the secondary screening infrastructure that would improve the accuracy doesn’t exist yet.
“I think this is a ‘not now,’ ” he added, “but I wouldn’t say ‘not ever.’ ”
Dr. Patnode and coauthors noted specific limitations in the evidence, including a lack of studies on how screening for and treating cognitive impairment affects decision making. In addition, details like quality of life and institutionalization were inconsistently reported, and “consistent and standardized reporting of results according to meaningful thresholds of clinical significance” would have been valuable across all measures.
Clinical implications
The implications of this report’s conclusions are substantial, especially as the rising prevalence of mild cognitive impairment and dementia becomes a worldwide concern, wrote Ronald C. Petersen, PhD, MD, of the Mayo Clinic in Rochester, Minn., and Kristine Yaffe, MD, of the University of California, San Francisco, in an accompanying editorial.
Though the data does not explicitly support screening, Dr. Petersen and Dr. Yaffe noted that it still may have benefits. An estimated 10% of cognitive impairment is caused by at least somewhat reversible causes, and screening could also be used to improve care in medical problems that are worsened by cognitive impairment. To find the true value of these efforts, they wrote, researchers need to design and execute additional clinical trials that “answer many of the important questions surrounding screening and treatment of cognitive impairment.”
“The absence of evidence for benefit may lead to inaction,” they added, noting that clinicians screening should still consider the value of screening on a case-by-case basis in order to keep up with the impact of new disease-modifying therapies for certain neurodegenerative diseases.
All members of the USPSTF received travel reimbursement and an honorarium for participating in meetings. One member reported receiving grants and personal fees from Healthwise. The study was funded by the Department of Health & Human Services. One of the authors reported receiving grants from the National Institutes of Health and the Food and Drug Administration. Dr. Petersen and Dr. Yaffe reported consulting for, and receiving funding from, various pharmaceutical companies, foundations, and government organizations.
SOURCES: Owens DK et al. JAMA. 2020 Feb 25. doi: 10.1001/jama.2020.0435; Patnode CD et al. JAMA. 2020 Feb 25. doi: 10.1001/jama.2019.22258.
FROM JAMA
Medicare beneficiaries get few home health visits after ICU stay
ORLANDO – , an analysis of hospital and home health claims data suggests.
The beneficiaries, all discharged directly to home health after an intensive care unit stay, received an average of less than one visit per week in the ensuing month, while a full third received no visits at all, according to authors of the analysis, presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
Living alone and living in a rural area were associated with significantly fewer home health rehabilitation visits, according to investigator Jason Raymond Falvey, PT, DPT, PhD, of Yale University, New Haven, Conn.
“We identified that these patients are receiving low doses of rehabilitation in home health care settings, and perhaps doses low enough to not be physiologically adequate to overcome the deconditioning and aerobic capacity concerns that these patients have,” Dr. Falvey said.
These findings reflect an “underrecognition” of the importance of rehabilitation both outside and inside the hospital setting, according to Patricia J. Posa, RN, of Saint Joseph Mercy Hospital, Northville, Mich.
“We even struggle to provide sufficient rehabilitation while they’re in the hospital,” Ms. Posa said in an interview. “So I think that we still have a major gap in providing rehab services across the continuum, and part of that is recognizing the deficits that patients, especially our elderly patients, might be leaving the hospital with.”
Medicare beneficiaries who survive a critical illness are often discharged with referrals for physical, occupational, or speech therapy, yet there are not much data on the delivery of that care or how many visits actually take place, according to Dr. Falvey.
He and coinvestigators analyzed data on 3,176 Medicare beneficiaries discharged to home health right after an acute hospitalization with an ICU stay of at least 24 hours. To do this, they linked 2012 Medicare hospital and home health claims data with Medicare demographic and patient assessment data.
They found that the beneficiaries received just 3.5 home rehabilitation visits in 30 days, while 33% had no visits on record.
The factors most strongly associated with receiving fewer rehabilitation visits, in adjusted models, included living in a rural setting, with a rate ratio (RR) of 0.87 and living alone, with an RR of 0.88.
Higher comorbidity count also was associated with fewer visits (RR, 0.98), according to the investigators.
On the other hand, Medicare beneficiaries who received more visits were more likely to be older (RR, 1.03; 1.01-1.04; for every 5 years), more likely to have higher disability scores (RR, 1.03; 1.02-1.04; per point on the Elixhauser Comorbidity Index), and more likely to have reported severe dyspnea (RR, 1.12; 1.04-1.21), according to the report.
More research will be needed to determine the appropriate number of home health rehabilitation visits for older hospitalized patients, according to Ms. Pena, a member of the Society of Critical Care Medicine’s ICU Liberation initiative, which aims to free patients from the harmful effects of pain, agitation/sedation, delirium, immobility, and sleep disruption in the ICU, as well as improve patient outcomes after an ICU stay.
The literature is already fairly robust, she said, on how frequently visits are warranted following specific scenarios such as postsurgical hip or knee replacement or stroke.
“For the general hospitalized patients that are just losing function because they were sick and didn’t get out of bed enough, we don’t really have good data to say, ‘you know, they need three visits a week, or they need two visits a week for an hour in order to improve,’ ” she said, “so the science is still not caught up with the frequency.”
In the absence of data, the number of visits may be left up to an individual clinician’s knowledge and past experience as well as what insurance will pay for, Ms. Pena said.
Dr. Falvey reported royalties related to an online continuing education course on hospital readmissions. No other disclosures were reported.
SOURCE: Falvey J et al. Crit Care Med. 2020 Jan;48(1):28.
ORLANDO – , an analysis of hospital and home health claims data suggests.
The beneficiaries, all discharged directly to home health after an intensive care unit stay, received an average of less than one visit per week in the ensuing month, while a full third received no visits at all, according to authors of the analysis, presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
Living alone and living in a rural area were associated with significantly fewer home health rehabilitation visits, according to investigator Jason Raymond Falvey, PT, DPT, PhD, of Yale University, New Haven, Conn.
“We identified that these patients are receiving low doses of rehabilitation in home health care settings, and perhaps doses low enough to not be physiologically adequate to overcome the deconditioning and aerobic capacity concerns that these patients have,” Dr. Falvey said.
These findings reflect an “underrecognition” of the importance of rehabilitation both outside and inside the hospital setting, according to Patricia J. Posa, RN, of Saint Joseph Mercy Hospital, Northville, Mich.
“We even struggle to provide sufficient rehabilitation while they’re in the hospital,” Ms. Posa said in an interview. “So I think that we still have a major gap in providing rehab services across the continuum, and part of that is recognizing the deficits that patients, especially our elderly patients, might be leaving the hospital with.”
Medicare beneficiaries who survive a critical illness are often discharged with referrals for physical, occupational, or speech therapy, yet there are not much data on the delivery of that care or how many visits actually take place, according to Dr. Falvey.
He and coinvestigators analyzed data on 3,176 Medicare beneficiaries discharged to home health right after an acute hospitalization with an ICU stay of at least 24 hours. To do this, they linked 2012 Medicare hospital and home health claims data with Medicare demographic and patient assessment data.
They found that the beneficiaries received just 3.5 home rehabilitation visits in 30 days, while 33% had no visits on record.
The factors most strongly associated with receiving fewer rehabilitation visits, in adjusted models, included living in a rural setting, with a rate ratio (RR) of 0.87 and living alone, with an RR of 0.88.
Higher comorbidity count also was associated with fewer visits (RR, 0.98), according to the investigators.
On the other hand, Medicare beneficiaries who received more visits were more likely to be older (RR, 1.03; 1.01-1.04; for every 5 years), more likely to have higher disability scores (RR, 1.03; 1.02-1.04; per point on the Elixhauser Comorbidity Index), and more likely to have reported severe dyspnea (RR, 1.12; 1.04-1.21), according to the report.
More research will be needed to determine the appropriate number of home health rehabilitation visits for older hospitalized patients, according to Ms. Pena, a member of the Society of Critical Care Medicine’s ICU Liberation initiative, which aims to free patients from the harmful effects of pain, agitation/sedation, delirium, immobility, and sleep disruption in the ICU, as well as improve patient outcomes after an ICU stay.
The literature is already fairly robust, she said, on how frequently visits are warranted following specific scenarios such as postsurgical hip or knee replacement or stroke.
“For the general hospitalized patients that are just losing function because they were sick and didn’t get out of bed enough, we don’t really have good data to say, ‘you know, they need three visits a week, or they need two visits a week for an hour in order to improve,’ ” she said, “so the science is still not caught up with the frequency.”
In the absence of data, the number of visits may be left up to an individual clinician’s knowledge and past experience as well as what insurance will pay for, Ms. Pena said.
Dr. Falvey reported royalties related to an online continuing education course on hospital readmissions. No other disclosures were reported.
SOURCE: Falvey J et al. Crit Care Med. 2020 Jan;48(1):28.
ORLANDO – , an analysis of hospital and home health claims data suggests.
The beneficiaries, all discharged directly to home health after an intensive care unit stay, received an average of less than one visit per week in the ensuing month, while a full third received no visits at all, according to authors of the analysis, presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
Living alone and living in a rural area were associated with significantly fewer home health rehabilitation visits, according to investigator Jason Raymond Falvey, PT, DPT, PhD, of Yale University, New Haven, Conn.
“We identified that these patients are receiving low doses of rehabilitation in home health care settings, and perhaps doses low enough to not be physiologically adequate to overcome the deconditioning and aerobic capacity concerns that these patients have,” Dr. Falvey said.
These findings reflect an “underrecognition” of the importance of rehabilitation both outside and inside the hospital setting, according to Patricia J. Posa, RN, of Saint Joseph Mercy Hospital, Northville, Mich.
“We even struggle to provide sufficient rehabilitation while they’re in the hospital,” Ms. Posa said in an interview. “So I think that we still have a major gap in providing rehab services across the continuum, and part of that is recognizing the deficits that patients, especially our elderly patients, might be leaving the hospital with.”
Medicare beneficiaries who survive a critical illness are often discharged with referrals for physical, occupational, or speech therapy, yet there are not much data on the delivery of that care or how many visits actually take place, according to Dr. Falvey.
He and coinvestigators analyzed data on 3,176 Medicare beneficiaries discharged to home health right after an acute hospitalization with an ICU stay of at least 24 hours. To do this, they linked 2012 Medicare hospital and home health claims data with Medicare demographic and patient assessment data.
They found that the beneficiaries received just 3.5 home rehabilitation visits in 30 days, while 33% had no visits on record.
The factors most strongly associated with receiving fewer rehabilitation visits, in adjusted models, included living in a rural setting, with a rate ratio (RR) of 0.87 and living alone, with an RR of 0.88.
Higher comorbidity count also was associated with fewer visits (RR, 0.98), according to the investigators.
On the other hand, Medicare beneficiaries who received more visits were more likely to be older (RR, 1.03; 1.01-1.04; for every 5 years), more likely to have higher disability scores (RR, 1.03; 1.02-1.04; per point on the Elixhauser Comorbidity Index), and more likely to have reported severe dyspnea (RR, 1.12; 1.04-1.21), according to the report.
More research will be needed to determine the appropriate number of home health rehabilitation visits for older hospitalized patients, according to Ms. Pena, a member of the Society of Critical Care Medicine’s ICU Liberation initiative, which aims to free patients from the harmful effects of pain, agitation/sedation, delirium, immobility, and sleep disruption in the ICU, as well as improve patient outcomes after an ICU stay.
The literature is already fairly robust, she said, on how frequently visits are warranted following specific scenarios such as postsurgical hip or knee replacement or stroke.
“For the general hospitalized patients that are just losing function because they were sick and didn’t get out of bed enough, we don’t really have good data to say, ‘you know, they need three visits a week, or they need two visits a week for an hour in order to improve,’ ” she said, “so the science is still not caught up with the frequency.”
In the absence of data, the number of visits may be left up to an individual clinician’s knowledge and past experience as well as what insurance will pay for, Ms. Pena said.
Dr. Falvey reported royalties related to an online continuing education course on hospital readmissions. No other disclosures were reported.
SOURCE: Falvey J et al. Crit Care Med. 2020 Jan;48(1):28.
REPORTING FROM CCC49
Carotid endarterectomy surpasses stenting in elderly, asymptomatic patients
LOS ANGELES – Carotid artery stenting in older, asymptomatic patients with severe carotid artery stenosis is, in general, as bad an idea as it has already proven to be in symptomatic patients, with a multifold increase in adverse short- and mid-term outcomes, compared with similar older, asymptomatic patients who underwent endarterectomy, according to a combined-study analysis with more than 2,500 patients.
The risk for poor outcomes in patients with severe but asymptomatic carotid artery disease who underwent carotid artery stenting (CAS), compared with patients who instead underwent carotid endarterectomy (CEA) “abruptly increased around age 75,” in an analysis that combined data from the two major, published, randomized trials that compared these two interventions in this patient population, Jenifer H. Voeks, PhD said at the International Stroke Conference sponsored by the American Heart Association.
These results “largely mirror” the findings from a similar combined analysis of data from four major, randomized trials that compared CEA and CAS in patients with symptomatic carotid disease, she noted (Lancet. 2016 Mar 26;387[10025]:1305-11). The new findings in an expanded population of asymptomatic patients derived from two separate studies showed that, in patients aged 70 years or less, “CAS appears to be a reasonable alternative to CEA, but above age 70, and certainly above age 75, age-related risk factors such as cerebrovascular anatomy and underlying cerebral pathology should be carefully considered before selecting patients for CAS,” said Dr. Voeks, a neurology researcher at the Medical University of South Carolina, Charleston. Many experts also believe that, for asymptomatic patients, intensive medical management may have returned as an alternative to either of these invasive approaches for treating severe carotid stenosis and has achieved a level of equipoise that led to the launch of CREST 2 (Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis Trial). CREST 2 is comparing CEA and CAS with medical management, and is scheduled to report results in 2021.
The data for this analysis in asymptomatic patients came from the first CREST (Carotid Revascularization Endarterectomy Versus Stenting Trial; N Engl J Med. 2010 Jul 1;363[1]:11-23), which included 1,181 asymptomatic patients (nearly half the total enrollment, with symptomatic patients making up the balance) and had no age ceiling, as well as all 1,453 patients from the ACT 1 trial, which enrolled exclusively asymptomatic patients and limited enrollment to patients aged 79 years or less (N Engl J Med. 2016 Mar 17;374[11]: 1011-20). Because the maximum age of patients in ACT 1 was 79 years, for this analysis Dr. Voeks and associates only included the 1,091 asymptomatic CREST patients who also were within the same age ceiling. The resulting cohort of 2,544 included 1,637 patients who underwent CAS and 907 who underwent CEA (because of a 3:1 randomization ratio in ACT 1), creating the largest data set to compare CAS and CEA by age in asymptomatic patients, Dr. Voeks noted. When subdivided by age, 30% of the cohort was younger that 65 years, 54% were 65-74, and 16% were 75-79.
The primary outcome the researchers used for their analysis was the combined incidence of periprocedural stroke, MI, or death, plus the incidence of ipsilateral stroke during 4 years of follow-up post procedure. Among patients who underwent CAS, this outcome occurred in roughly 9% of patients aged 75-79 years and in about 3% of those younger than 65 years, a hazard ratio of 2.9 that was statistically significant. In contrast, the incidence of the primary outcome among patients aged 65-74 years was just 30% higher, compared with patients aged less than 65 years, a difference that was not statistically significant.
Patients who underwent CEA showed no similar relationship between age and outcome. The incidence of the primary outcome among the CEA patients was roughly the same, about 3.5%, regardless of their age.
A second analysis that considered age as a continuous variable showed a sharply spiked increase in the risk for CAS patients, compared with CEA patients once they reached about age 73-75 years. Until about age 72, the rate of the primary outcome was nearly the same regardless of whether patients underwent CAS or CEA, but the risk for adverse outcomes rose “steeply” starting at about age 75 so that by age 79 the rate of the primary outcome approached 300% higher among the CAS patients compared with CEA patients, Dr. Voeks said.
She cautioned that the analysis included just 115 total primary-outcome events, which makes the incidence rate estimates somewhat imprecise, and that the data reflect outcomes in patients who were treated more than a decade ago, but these data remain the only reported results from large randomized trials that compared CAS and CEA in asymptomatic patients.
Dr. Voeks reported no disclosures.
SOURCE: Voeks JH al. Stroke. 2020 Feb 12;51[suppl 1], Abstract 70.
The role for carotid intervention in asymptomatic patients with severe carotid stenosis, usually defined as a stenosis that obstructs at least 70% of the carotid lumen, is controversial right now because intensive medical management has not been compared with invasive treatments, such as carotid endarterectomy and carotid stenting, for well over a decade. New drugs and new regimens have become treatment options for patients with advanced atherosclerotic carotid artery disease, and this has returned us to a state of equipoise for medical versus interventional management. That’s the premise behind CREST 2 (Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis Trial), which is comparing medical treatment against endarterectomy and against carotid stenting in a randomized study. The results may be available in 2021.
The new findings are very important for helping patients and their families make informed decisions. CAS is often perceived as the safer option for older patients because it is less traumatic and invasive than CEA. The data that Dr. Voeks reported show once again that this intuitive impression about CAS in the elderly is belied by the evidence. But the findings also require cautious interpretation because they came from a post hoc, subgroup analysis.
Mai N. Nguyen-Huynh, MD , is a vascular neurologist with Kaiser Permanente Northern California in Oakland. She had no relevant disclosures. She made these comments in an interview.
The role for carotid intervention in asymptomatic patients with severe carotid stenosis, usually defined as a stenosis that obstructs at least 70% of the carotid lumen, is controversial right now because intensive medical management has not been compared with invasive treatments, such as carotid endarterectomy and carotid stenting, for well over a decade. New drugs and new regimens have become treatment options for patients with advanced atherosclerotic carotid artery disease, and this has returned us to a state of equipoise for medical versus interventional management. That’s the premise behind CREST 2 (Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis Trial), which is comparing medical treatment against endarterectomy and against carotid stenting in a randomized study. The results may be available in 2021.
The new findings are very important for helping patients and their families make informed decisions. CAS is often perceived as the safer option for older patients because it is less traumatic and invasive than CEA. The data that Dr. Voeks reported show once again that this intuitive impression about CAS in the elderly is belied by the evidence. But the findings also require cautious interpretation because they came from a post hoc, subgroup analysis.
Mai N. Nguyen-Huynh, MD , is a vascular neurologist with Kaiser Permanente Northern California in Oakland. She had no relevant disclosures. She made these comments in an interview.
The role for carotid intervention in asymptomatic patients with severe carotid stenosis, usually defined as a stenosis that obstructs at least 70% of the carotid lumen, is controversial right now because intensive medical management has not been compared with invasive treatments, such as carotid endarterectomy and carotid stenting, for well over a decade. New drugs and new regimens have become treatment options for patients with advanced atherosclerotic carotid artery disease, and this has returned us to a state of equipoise for medical versus interventional management. That’s the premise behind CREST 2 (Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis Trial), which is comparing medical treatment against endarterectomy and against carotid stenting in a randomized study. The results may be available in 2021.
The new findings are very important for helping patients and their families make informed decisions. CAS is often perceived as the safer option for older patients because it is less traumatic and invasive than CEA. The data that Dr. Voeks reported show once again that this intuitive impression about CAS in the elderly is belied by the evidence. But the findings also require cautious interpretation because they came from a post hoc, subgroup analysis.
Mai N. Nguyen-Huynh, MD , is a vascular neurologist with Kaiser Permanente Northern California in Oakland. She had no relevant disclosures. She made these comments in an interview.
LOS ANGELES – Carotid artery stenting in older, asymptomatic patients with severe carotid artery stenosis is, in general, as bad an idea as it has already proven to be in symptomatic patients, with a multifold increase in adverse short- and mid-term outcomes, compared with similar older, asymptomatic patients who underwent endarterectomy, according to a combined-study analysis with more than 2,500 patients.
The risk for poor outcomes in patients with severe but asymptomatic carotid artery disease who underwent carotid artery stenting (CAS), compared with patients who instead underwent carotid endarterectomy (CEA) “abruptly increased around age 75,” in an analysis that combined data from the two major, published, randomized trials that compared these two interventions in this patient population, Jenifer H. Voeks, PhD said at the International Stroke Conference sponsored by the American Heart Association.
These results “largely mirror” the findings from a similar combined analysis of data from four major, randomized trials that compared CEA and CAS in patients with symptomatic carotid disease, she noted (Lancet. 2016 Mar 26;387[10025]:1305-11). The new findings in an expanded population of asymptomatic patients derived from two separate studies showed that, in patients aged 70 years or less, “CAS appears to be a reasonable alternative to CEA, but above age 70, and certainly above age 75, age-related risk factors such as cerebrovascular anatomy and underlying cerebral pathology should be carefully considered before selecting patients for CAS,” said Dr. Voeks, a neurology researcher at the Medical University of South Carolina, Charleston. Many experts also believe that, for asymptomatic patients, intensive medical management may have returned as an alternative to either of these invasive approaches for treating severe carotid stenosis and has achieved a level of equipoise that led to the launch of CREST 2 (Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis Trial). CREST 2 is comparing CEA and CAS with medical management, and is scheduled to report results in 2021.
The data for this analysis in asymptomatic patients came from the first CREST (Carotid Revascularization Endarterectomy Versus Stenting Trial; N Engl J Med. 2010 Jul 1;363[1]:11-23), which included 1,181 asymptomatic patients (nearly half the total enrollment, with symptomatic patients making up the balance) and had no age ceiling, as well as all 1,453 patients from the ACT 1 trial, which enrolled exclusively asymptomatic patients and limited enrollment to patients aged 79 years or less (N Engl J Med. 2016 Mar 17;374[11]: 1011-20). Because the maximum age of patients in ACT 1 was 79 years, for this analysis Dr. Voeks and associates only included the 1,091 asymptomatic CREST patients who also were within the same age ceiling. The resulting cohort of 2,544 included 1,637 patients who underwent CAS and 907 who underwent CEA (because of a 3:1 randomization ratio in ACT 1), creating the largest data set to compare CAS and CEA by age in asymptomatic patients, Dr. Voeks noted. When subdivided by age, 30% of the cohort was younger that 65 years, 54% were 65-74, and 16% were 75-79.
The primary outcome the researchers used for their analysis was the combined incidence of periprocedural stroke, MI, or death, plus the incidence of ipsilateral stroke during 4 years of follow-up post procedure. Among patients who underwent CAS, this outcome occurred in roughly 9% of patients aged 75-79 years and in about 3% of those younger than 65 years, a hazard ratio of 2.9 that was statistically significant. In contrast, the incidence of the primary outcome among patients aged 65-74 years was just 30% higher, compared with patients aged less than 65 years, a difference that was not statistically significant.
Patients who underwent CEA showed no similar relationship between age and outcome. The incidence of the primary outcome among the CEA patients was roughly the same, about 3.5%, regardless of their age.
A second analysis that considered age as a continuous variable showed a sharply spiked increase in the risk for CAS patients, compared with CEA patients once they reached about age 73-75 years. Until about age 72, the rate of the primary outcome was nearly the same regardless of whether patients underwent CAS or CEA, but the risk for adverse outcomes rose “steeply” starting at about age 75 so that by age 79 the rate of the primary outcome approached 300% higher among the CAS patients compared with CEA patients, Dr. Voeks said.
She cautioned that the analysis included just 115 total primary-outcome events, which makes the incidence rate estimates somewhat imprecise, and that the data reflect outcomes in patients who were treated more than a decade ago, but these data remain the only reported results from large randomized trials that compared CAS and CEA in asymptomatic patients.
Dr. Voeks reported no disclosures.
SOURCE: Voeks JH al. Stroke. 2020 Feb 12;51[suppl 1], Abstract 70.
LOS ANGELES – Carotid artery stenting in older, asymptomatic patients with severe carotid artery stenosis is, in general, as bad an idea as it has already proven to be in symptomatic patients, with a multifold increase in adverse short- and mid-term outcomes, compared with similar older, asymptomatic patients who underwent endarterectomy, according to a combined-study analysis with more than 2,500 patients.
The risk for poor outcomes in patients with severe but asymptomatic carotid artery disease who underwent carotid artery stenting (CAS), compared with patients who instead underwent carotid endarterectomy (CEA) “abruptly increased around age 75,” in an analysis that combined data from the two major, published, randomized trials that compared these two interventions in this patient population, Jenifer H. Voeks, PhD said at the International Stroke Conference sponsored by the American Heart Association.
These results “largely mirror” the findings from a similar combined analysis of data from four major, randomized trials that compared CEA and CAS in patients with symptomatic carotid disease, she noted (Lancet. 2016 Mar 26;387[10025]:1305-11). The new findings in an expanded population of asymptomatic patients derived from two separate studies showed that, in patients aged 70 years or less, “CAS appears to be a reasonable alternative to CEA, but above age 70, and certainly above age 75, age-related risk factors such as cerebrovascular anatomy and underlying cerebral pathology should be carefully considered before selecting patients for CAS,” said Dr. Voeks, a neurology researcher at the Medical University of South Carolina, Charleston. Many experts also believe that, for asymptomatic patients, intensive medical management may have returned as an alternative to either of these invasive approaches for treating severe carotid stenosis and has achieved a level of equipoise that led to the launch of CREST 2 (Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis Trial). CREST 2 is comparing CEA and CAS with medical management, and is scheduled to report results in 2021.
The data for this analysis in asymptomatic patients came from the first CREST (Carotid Revascularization Endarterectomy Versus Stenting Trial; N Engl J Med. 2010 Jul 1;363[1]:11-23), which included 1,181 asymptomatic patients (nearly half the total enrollment, with symptomatic patients making up the balance) and had no age ceiling, as well as all 1,453 patients from the ACT 1 trial, which enrolled exclusively asymptomatic patients and limited enrollment to patients aged 79 years or less (N Engl J Med. 2016 Mar 17;374[11]: 1011-20). Because the maximum age of patients in ACT 1 was 79 years, for this analysis Dr. Voeks and associates only included the 1,091 asymptomatic CREST patients who also were within the same age ceiling. The resulting cohort of 2,544 included 1,637 patients who underwent CAS and 907 who underwent CEA (because of a 3:1 randomization ratio in ACT 1), creating the largest data set to compare CAS and CEA by age in asymptomatic patients, Dr. Voeks noted. When subdivided by age, 30% of the cohort was younger that 65 years, 54% were 65-74, and 16% were 75-79.
The primary outcome the researchers used for their analysis was the combined incidence of periprocedural stroke, MI, or death, plus the incidence of ipsilateral stroke during 4 years of follow-up post procedure. Among patients who underwent CAS, this outcome occurred in roughly 9% of patients aged 75-79 years and in about 3% of those younger than 65 years, a hazard ratio of 2.9 that was statistically significant. In contrast, the incidence of the primary outcome among patients aged 65-74 years was just 30% higher, compared with patients aged less than 65 years, a difference that was not statistically significant.
Patients who underwent CEA showed no similar relationship between age and outcome. The incidence of the primary outcome among the CEA patients was roughly the same, about 3.5%, regardless of their age.
A second analysis that considered age as a continuous variable showed a sharply spiked increase in the risk for CAS patients, compared with CEA patients once they reached about age 73-75 years. Until about age 72, the rate of the primary outcome was nearly the same regardless of whether patients underwent CAS or CEA, but the risk for adverse outcomes rose “steeply” starting at about age 75 so that by age 79 the rate of the primary outcome approached 300% higher among the CAS patients compared with CEA patients, Dr. Voeks said.
She cautioned that the analysis included just 115 total primary-outcome events, which makes the incidence rate estimates somewhat imprecise, and that the data reflect outcomes in patients who were treated more than a decade ago, but these data remain the only reported results from large randomized trials that compared CAS and CEA in asymptomatic patients.
Dr. Voeks reported no disclosures.
SOURCE: Voeks JH al. Stroke. 2020 Feb 12;51[suppl 1], Abstract 70.
REPORTING FROM ISC 2020