User login
Rucaparib extends PFS in BRCA-mutated ovarian cancer, with an exception
Investigator-assessed PFS in both an intention-to-treat (ITT) analysis and an efficacy analysis that excluded patients with BRCA reversion mutations was 7.4 months in the rucaparib arm, compared with 5.7 months in patients who received either platinum-based chemotherapy or weekly paclitaxel.
Among the 23 patients with BRCA reversion mutations, however, investigator-assessed PFS was 2.9 months with rucaparib and 5.5 months with chemotherapy.
Overall survival data were not mature at the time of data cutoff in September 2020.
“Although the numbers are very small, the results suggest that presence of a BRCA reversion mutation may predict a reduced benefit from rucaparib,” said Rebecca Kristeleit, MBChB, PhD, of Guy’s and St. Thomas’ NHS Foundation Trust in London.
She presented the findings from ARIEL4 at the Society of Gynecologic Oncology’s Virtual Annual Meeting on Women’s Cancer (Abstract 11479).
Invited discussant Ursula Matulonis, MD, of the Dana-Farber Cancer Institute in Boston, commented that the “BRCA reversion mutation data from ARIEL4 is intriguing. Strategies to overcome and better understand this type of resistance mechanism are needed.”
Study rationale and details
Rucaparib is approved as monotherapy for patients with BRCA-mutated, relapsed ovarian cancer who have received at least two prior lines of platinum-based chemotherapy. The approval was based on results of two phase 1/2 studies. ARIEL4 is a phase 3 confirmatory study, designed in consultation with both the U.S. Food and Drug Administration and the European Medicines Agency.
Women with relapsed, high-grade epithelial ovarian, fallopian tube, or primary peritoneal cancer with deleterious germline or somatic BRCA mutations were eligible for enrollment in ARIEL4. The patients had to have received at least two lines of chemotherapy, including at least one platinum-based regimen, with no prior PARP inhibitor or single-agent paclitaxel treatment.
Overall, 95% of patients had epithelial ovarian cancer, 3% had fallopian tube cancer, and 2% had primary peritoneal cancer. About 90% of cancers were serous in histology. Most patients (84%) had germline BRCA mutations, 16% had somatic mutations, and the status was unknown in the remaining patients.
Patients were randomized on a 2:1 basis to receive rucaparib at 600 mg twice daily (n = 233) or chemotherapy (n = 116), stratified by platinum sensitivity status. Patients assigned to chemotherapy whose disease was considered platinum resistant or partially platinum sensitive were assigned to weekly paclitaxel. Patients with fully platinum-sensitive disease were assigned to platinum-based single-agent or doublet chemotherapy. Treatment cycles were 28 days.
On radiologically confirmed disease progression or unacceptable toxicity, patients assigned to chemotherapy had the option to cross over to the rucaparib arm. The follow-up portion of the study began 28 days after the last treatment dose, with visits every 8 weeks thereafter.
Baseline characteristics in the ITT population were similar between arms. There were 13 patients in the rucaparib arm and 10 in the chemotherapy arm who had BRCA reversion mutations and were excluded from the efficacy population.
Efficacy and safety
Investigator-assessed PFS in the efficacy population was a median of 7.4 months with rucaparib and 5.7 months with chemotherapy, translating to a hazard ratio (HR) of 0.64 (P = .001). In the ITT population, the respective median PFS intervals were identical, although with a slightly less favorable HR of 0.67 (P = .002). In the 23 patients with BRCA reversion mutations, the median PFS was worse with rucaparib, at 2.9 months, compared with 5.5 months for chemotherapy. This translated to a HR of 2.77, although the 95% confidence interval was wide and crossed 1, likely due to the small sample size.
Among patients who had measurable disease at baseline, the overall response rate in the efficacy population was 40.3% with rucaparib and 32.3% with chemotherapy, a difference that was not statistically significant (P = .13). The overall response data were similar in the ITT population (37.9% and 30.2%, respectively).
In the efficacy population, the duration of response was significantly longer in the rucaparib arm, at a median of 9.4 months versus 7.2 months (HR, 0.59; 95% CI, 0.36-0.98). The respective median response durations were identical in the ITT population, but the HR was 0.56 (95% CI, 0.34-0.93).
In both the efficacy and ITT populations, global health status was virtually identical and unchanged from baseline in both treatment arms through cycle 7.
Treatment-emergent adverse events (TEAEs) were more frequent with rucaparib. The most common TEAEs in the rucaparib and chemotherapy arms, respectively, were anemia/decreased hemoglobin (53.9% and 31.9%), nausea (53.4% and 31.9%), asthenia/fatigue (49.6% and 44.2%), ALT/AST increase (34.5% and 11.5%), and vomiting (34.1% and 16.8%).
In all, 8.2% of patients in the rucaparib arm and 12.4% of those in the chemotherapy arm discontinued therapy due to TEAEs.
Four patients in the rucaparib arm developed myelodysplastic syndrome or acute myeloid leukemia – one during treatment and three during follow-up. There were no cases of myelodysplastic syndrome or acute myeloid leukemia in patients who received chemotherapy.
“Data from ARIEL4 fits the paradigm that single-agent activity of PARP inhibitors in BRCA-mutated, recurrent ovarian cancer may be comparable to chemotherapy, and may, at times, be superior, depending on the study population, trial design, and treatment for control patients,” Dr. Matulonis said.
The study was funded by Clovis Oncology. Dr. Kristeleit disclosed relationships with Clovis, Roche, and Tesaro. Dr. Matulonis disclosed relationships with Novartis, Merck, and Immunogen.
Investigator-assessed PFS in both an intention-to-treat (ITT) analysis and an efficacy analysis that excluded patients with BRCA reversion mutations was 7.4 months in the rucaparib arm, compared with 5.7 months in patients who received either platinum-based chemotherapy or weekly paclitaxel.
Among the 23 patients with BRCA reversion mutations, however, investigator-assessed PFS was 2.9 months with rucaparib and 5.5 months with chemotherapy.
Overall survival data were not mature at the time of data cutoff in September 2020.
“Although the numbers are very small, the results suggest that presence of a BRCA reversion mutation may predict a reduced benefit from rucaparib,” said Rebecca Kristeleit, MBChB, PhD, of Guy’s and St. Thomas’ NHS Foundation Trust in London.
She presented the findings from ARIEL4 at the Society of Gynecologic Oncology’s Virtual Annual Meeting on Women’s Cancer (Abstract 11479).
Invited discussant Ursula Matulonis, MD, of the Dana-Farber Cancer Institute in Boston, commented that the “BRCA reversion mutation data from ARIEL4 is intriguing. Strategies to overcome and better understand this type of resistance mechanism are needed.”
Study rationale and details
Rucaparib is approved as monotherapy for patients with BRCA-mutated, relapsed ovarian cancer who have received at least two prior lines of platinum-based chemotherapy. The approval was based on results of two phase 1/2 studies. ARIEL4 is a phase 3 confirmatory study, designed in consultation with both the U.S. Food and Drug Administration and the European Medicines Agency.
Women with relapsed, high-grade epithelial ovarian, fallopian tube, or primary peritoneal cancer with deleterious germline or somatic BRCA mutations were eligible for enrollment in ARIEL4. The patients had to have received at least two lines of chemotherapy, including at least one platinum-based regimen, with no prior PARP inhibitor or single-agent paclitaxel treatment.
Overall, 95% of patients had epithelial ovarian cancer, 3% had fallopian tube cancer, and 2% had primary peritoneal cancer. About 90% of cancers were serous in histology. Most patients (84%) had germline BRCA mutations, 16% had somatic mutations, and the status was unknown in the remaining patients.
Patients were randomized on a 2:1 basis to receive rucaparib at 600 mg twice daily (n = 233) or chemotherapy (n = 116), stratified by platinum sensitivity status. Patients assigned to chemotherapy whose disease was considered platinum resistant or partially platinum sensitive were assigned to weekly paclitaxel. Patients with fully platinum-sensitive disease were assigned to platinum-based single-agent or doublet chemotherapy. Treatment cycles were 28 days.
On radiologically confirmed disease progression or unacceptable toxicity, patients assigned to chemotherapy had the option to cross over to the rucaparib arm. The follow-up portion of the study began 28 days after the last treatment dose, with visits every 8 weeks thereafter.
Baseline characteristics in the ITT population were similar between arms. There were 13 patients in the rucaparib arm and 10 in the chemotherapy arm who had BRCA reversion mutations and were excluded from the efficacy population.
Efficacy and safety
Investigator-assessed PFS in the efficacy population was a median of 7.4 months with rucaparib and 5.7 months with chemotherapy, translating to a hazard ratio (HR) of 0.64 (P = .001). In the ITT population, the respective median PFS intervals were identical, although with a slightly less favorable HR of 0.67 (P = .002). In the 23 patients with BRCA reversion mutations, the median PFS was worse with rucaparib, at 2.9 months, compared with 5.5 months for chemotherapy. This translated to a HR of 2.77, although the 95% confidence interval was wide and crossed 1, likely due to the small sample size.
Among patients who had measurable disease at baseline, the overall response rate in the efficacy population was 40.3% with rucaparib and 32.3% with chemotherapy, a difference that was not statistically significant (P = .13). The overall response data were similar in the ITT population (37.9% and 30.2%, respectively).
In the efficacy population, the duration of response was significantly longer in the rucaparib arm, at a median of 9.4 months versus 7.2 months (HR, 0.59; 95% CI, 0.36-0.98). The respective median response durations were identical in the ITT population, but the HR was 0.56 (95% CI, 0.34-0.93).
In both the efficacy and ITT populations, global health status was virtually identical and unchanged from baseline in both treatment arms through cycle 7.
Treatment-emergent adverse events (TEAEs) were more frequent with rucaparib. The most common TEAEs in the rucaparib and chemotherapy arms, respectively, were anemia/decreased hemoglobin (53.9% and 31.9%), nausea (53.4% and 31.9%), asthenia/fatigue (49.6% and 44.2%), ALT/AST increase (34.5% and 11.5%), and vomiting (34.1% and 16.8%).
In all, 8.2% of patients in the rucaparib arm and 12.4% of those in the chemotherapy arm discontinued therapy due to TEAEs.
Four patients in the rucaparib arm developed myelodysplastic syndrome or acute myeloid leukemia – one during treatment and three during follow-up. There were no cases of myelodysplastic syndrome or acute myeloid leukemia in patients who received chemotherapy.
“Data from ARIEL4 fits the paradigm that single-agent activity of PARP inhibitors in BRCA-mutated, recurrent ovarian cancer may be comparable to chemotherapy, and may, at times, be superior, depending on the study population, trial design, and treatment for control patients,” Dr. Matulonis said.
The study was funded by Clovis Oncology. Dr. Kristeleit disclosed relationships with Clovis, Roche, and Tesaro. Dr. Matulonis disclosed relationships with Novartis, Merck, and Immunogen.
Investigator-assessed PFS in both an intention-to-treat (ITT) analysis and an efficacy analysis that excluded patients with BRCA reversion mutations was 7.4 months in the rucaparib arm, compared with 5.7 months in patients who received either platinum-based chemotherapy or weekly paclitaxel.
Among the 23 patients with BRCA reversion mutations, however, investigator-assessed PFS was 2.9 months with rucaparib and 5.5 months with chemotherapy.
Overall survival data were not mature at the time of data cutoff in September 2020.
“Although the numbers are very small, the results suggest that presence of a BRCA reversion mutation may predict a reduced benefit from rucaparib,” said Rebecca Kristeleit, MBChB, PhD, of Guy’s and St. Thomas’ NHS Foundation Trust in London.
She presented the findings from ARIEL4 at the Society of Gynecologic Oncology’s Virtual Annual Meeting on Women’s Cancer (Abstract 11479).
Invited discussant Ursula Matulonis, MD, of the Dana-Farber Cancer Institute in Boston, commented that the “BRCA reversion mutation data from ARIEL4 is intriguing. Strategies to overcome and better understand this type of resistance mechanism are needed.”
Study rationale and details
Rucaparib is approved as monotherapy for patients with BRCA-mutated, relapsed ovarian cancer who have received at least two prior lines of platinum-based chemotherapy. The approval was based on results of two phase 1/2 studies. ARIEL4 is a phase 3 confirmatory study, designed in consultation with both the U.S. Food and Drug Administration and the European Medicines Agency.
Women with relapsed, high-grade epithelial ovarian, fallopian tube, or primary peritoneal cancer with deleterious germline or somatic BRCA mutations were eligible for enrollment in ARIEL4. The patients had to have received at least two lines of chemotherapy, including at least one platinum-based regimen, with no prior PARP inhibitor or single-agent paclitaxel treatment.
Overall, 95% of patients had epithelial ovarian cancer, 3% had fallopian tube cancer, and 2% had primary peritoneal cancer. About 90% of cancers were serous in histology. Most patients (84%) had germline BRCA mutations, 16% had somatic mutations, and the status was unknown in the remaining patients.
Patients were randomized on a 2:1 basis to receive rucaparib at 600 mg twice daily (n = 233) or chemotherapy (n = 116), stratified by platinum sensitivity status. Patients assigned to chemotherapy whose disease was considered platinum resistant or partially platinum sensitive were assigned to weekly paclitaxel. Patients with fully platinum-sensitive disease were assigned to platinum-based single-agent or doublet chemotherapy. Treatment cycles were 28 days.
On radiologically confirmed disease progression or unacceptable toxicity, patients assigned to chemotherapy had the option to cross over to the rucaparib arm. The follow-up portion of the study began 28 days after the last treatment dose, with visits every 8 weeks thereafter.
Baseline characteristics in the ITT population were similar between arms. There were 13 patients in the rucaparib arm and 10 in the chemotherapy arm who had BRCA reversion mutations and were excluded from the efficacy population.
Efficacy and safety
Investigator-assessed PFS in the efficacy population was a median of 7.4 months with rucaparib and 5.7 months with chemotherapy, translating to a hazard ratio (HR) of 0.64 (P = .001). In the ITT population, the respective median PFS intervals were identical, although with a slightly less favorable HR of 0.67 (P = .002). In the 23 patients with BRCA reversion mutations, the median PFS was worse with rucaparib, at 2.9 months, compared with 5.5 months for chemotherapy. This translated to a HR of 2.77, although the 95% confidence interval was wide and crossed 1, likely due to the small sample size.
Among patients who had measurable disease at baseline, the overall response rate in the efficacy population was 40.3% with rucaparib and 32.3% with chemotherapy, a difference that was not statistically significant (P = .13). The overall response data were similar in the ITT population (37.9% and 30.2%, respectively).
In the efficacy population, the duration of response was significantly longer in the rucaparib arm, at a median of 9.4 months versus 7.2 months (HR, 0.59; 95% CI, 0.36-0.98). The respective median response durations were identical in the ITT population, but the HR was 0.56 (95% CI, 0.34-0.93).
In both the efficacy and ITT populations, global health status was virtually identical and unchanged from baseline in both treatment arms through cycle 7.
Treatment-emergent adverse events (TEAEs) were more frequent with rucaparib. The most common TEAEs in the rucaparib and chemotherapy arms, respectively, were anemia/decreased hemoglobin (53.9% and 31.9%), nausea (53.4% and 31.9%), asthenia/fatigue (49.6% and 44.2%), ALT/AST increase (34.5% and 11.5%), and vomiting (34.1% and 16.8%).
In all, 8.2% of patients in the rucaparib arm and 12.4% of those in the chemotherapy arm discontinued therapy due to TEAEs.
Four patients in the rucaparib arm developed myelodysplastic syndrome or acute myeloid leukemia – one during treatment and three during follow-up. There were no cases of myelodysplastic syndrome or acute myeloid leukemia in patients who received chemotherapy.
“Data from ARIEL4 fits the paradigm that single-agent activity of PARP inhibitors in BRCA-mutated, recurrent ovarian cancer may be comparable to chemotherapy, and may, at times, be superior, depending on the study population, trial design, and treatment for control patients,” Dr. Matulonis said.
The study was funded by Clovis Oncology. Dr. Kristeleit disclosed relationships with Clovis, Roche, and Tesaro. Dr. Matulonis disclosed relationships with Novartis, Merck, and Immunogen.
FROM SGO 2021
Is the WHO’s HPV vaccination target within reach?
The WHO’s goal is to have HPV vaccines delivered to 90% of all adolescent girls by 2030, part of the organization’s larger goal to “eliminate” cervical cancer, or reduce the annual incidence of cervical cancer to below 4 cases per 100,000 people globally.
Laia Bruni, MD, PhD, of Catalan Institute of Oncology in Barcelona, and colleagues outlined the progress made thus far toward reaching the WHO’s goals in an article published in Preventive Medicine.
The authors noted that cervical cancer caused by HPV is a “major public health problem, especially in low- and middle-income countries (LMIC).”
However, vaccines against HPV have been available since 2006 and have been recommended by the WHO since 2009.
HPV vaccines have been introduced into many national immunization schedules. Among the 194 WHO member states, 107 (55%) had introduced HPV vaccination as of June 2020, according to estimates from the WHO and the United Nations International Children’s Emergency Fund (UNICEF).
Still, vaccine introduction and coverages are suboptimal, according to several studies and international agencies.
In their article, Dr. Bruni and colleagues describe the mid-2020 status of HPV vaccine introduction, based on WHO/UNICEF estimates of national HPV immunization coverage from 2010 to 2019.
HPV vaccination by region
The Americas and Europe are by far the WHO regions with the highest rates of HPV vaccination, with 85% and 77% of their countries, respectively, having already introduced HPV vaccination, either partially or nationwide.
In 2019, a record number of introductions, 16, were reported, mostly in LMICs where access has been limited. In prior years, the average had been a relatively steady 7-8 introductions per year.
The percentage of high-income countries (HICs) that have introduced HPV vaccination exceeds 80%. LMICs started introducing HPV vaccination later and at a slower pace, compared with HICs. By the end of 2019, only 41% of LMICs had introduced vaccination. However, of the new introductions in 2019, 87% were in LMICs.
In 2019, the average performance coverage for HPV vaccination programs in 99 countries (both HICs and LMICs) was around 67% for the first vaccine dose and 53% for the final dose.
Median performance coverage was higher in LMICs than in HICs for the first dose (80% and 72%, respectively), but mean dropout rates were higher in LMICs than in HICs (18% and 11%, respectively).
Coverage of more than 90% was achieved for the last dose in only five countries (6%). Twenty-two countries (21%) achieved coverages of 75% or higher, while 35 countries (40%) had final dose coverages of 50% or less.
Global coverage of the final HPV vaccine dose (weighted by population size) was estimated at 15%. According to the authors, that low percentage can be explained by the fact that many of the most populous countries have either not yet introduced HPV vaccination or have low performance.
The countries with highest cervical cancer burden have had limited secondary prevention and have been less likely to provide access to vaccination, the authors noted. However, this trend appears to be reversing, with 14 new LMICs providing HPV vaccination in 2019.
HPV vaccination by sex
By 2019, almost a third of the 107 HPV vaccination programs (n = 33) were “gender neutral,” with girls and boys receiving HPV vaccines. Generally, LMICs targeted younger girls (9-10 years) compared with HICs (11-13 years).
Dr. Bruni and colleagues estimated that 15% of girls and 4% of boys were vaccinated globally with the full course of vaccine. At least one dose was received by 20% of girls and 5% of boys.
From 2010 to 2019, HPV vaccination rates in HICs rose from 42% in girls and 0% in boys to 88% and 44%, respectively. In LMICs, over the same period, rates rose from 4% in girls and 0% in boys to 40% and 5%, respectively.
Obstacles and the path forward
The COVID-19 pandemic has halted HPV vaccine delivery in the majority of countries, Dr. Bruni and colleagues noted. About 70 countries had reported program interruptions by August 2020, and delays to HPV vaccine introductions were anticipated for other countries.
An economic downturn could have further far-reaching effects on plans to introduce HPV vaccines, Dr. Bruni and colleagues observed.
While meeting the 2030 target will be challenging, the authors noted that, in every geographic area, some programs are meeting the 90% target.
“HPV national programs should aim to get 90+% of girls vaccinated before the age of 15,” Dr. Bruni said in an interview. “This is a feasible goal, and some countries have succeeded, such as Norway and Rwanda. Average performance, however, is around 55%, and that shows that it is not an easy task.”
Dr. Bruni underscored the four main actions that should be taken to achieve 90% coverage of HPV vaccination, as outlined in the WHO cervical cancer elimination strategy:
- Secure sufficient and affordable HPV vaccines.
- Increase the quality and coverage of vaccination.
- Improve communication and social mobilization.
- Innovate to improve efficiency of vaccine delivery.
“Addressing vaccine hesitancy adequately is one of the biggest challenges we face, especially for the HPV vaccine,” Dr. Bruni said. “As the WHO document states, understanding social, cultural, societal, and other barriers affecting acceptance and uptake of the vaccine will be critical for overcoming vaccine hesitancy and countering misinformation.”
This research was funded by a grant from Instituto de Salud Carlos III and various other grants. Dr. Bruni and coauthors said they have no relevant disclosures.
The WHO’s goal is to have HPV vaccines delivered to 90% of all adolescent girls by 2030, part of the organization’s larger goal to “eliminate” cervical cancer, or reduce the annual incidence of cervical cancer to below 4 cases per 100,000 people globally.
Laia Bruni, MD, PhD, of Catalan Institute of Oncology in Barcelona, and colleagues outlined the progress made thus far toward reaching the WHO’s goals in an article published in Preventive Medicine.
The authors noted that cervical cancer caused by HPV is a “major public health problem, especially in low- and middle-income countries (LMIC).”
However, vaccines against HPV have been available since 2006 and have been recommended by the WHO since 2009.
HPV vaccines have been introduced into many national immunization schedules. Among the 194 WHO member states, 107 (55%) had introduced HPV vaccination as of June 2020, according to estimates from the WHO and the United Nations International Children’s Emergency Fund (UNICEF).
Still, vaccine introduction and coverages are suboptimal, according to several studies and international agencies.
In their article, Dr. Bruni and colleagues describe the mid-2020 status of HPV vaccine introduction, based on WHO/UNICEF estimates of national HPV immunization coverage from 2010 to 2019.
HPV vaccination by region
The Americas and Europe are by far the WHO regions with the highest rates of HPV vaccination, with 85% and 77% of their countries, respectively, having already introduced HPV vaccination, either partially or nationwide.
In 2019, a record number of introductions, 16, were reported, mostly in LMICs where access has been limited. In prior years, the average had been a relatively steady 7-8 introductions per year.
The percentage of high-income countries (HICs) that have introduced HPV vaccination exceeds 80%. LMICs started introducing HPV vaccination later and at a slower pace, compared with HICs. By the end of 2019, only 41% of LMICs had introduced vaccination. However, of the new introductions in 2019, 87% were in LMICs.
In 2019, the average performance coverage for HPV vaccination programs in 99 countries (both HICs and LMICs) was around 67% for the first vaccine dose and 53% for the final dose.
Median performance coverage was higher in LMICs than in HICs for the first dose (80% and 72%, respectively), but mean dropout rates were higher in LMICs than in HICs (18% and 11%, respectively).
Coverage of more than 90% was achieved for the last dose in only five countries (6%). Twenty-two countries (21%) achieved coverages of 75% or higher, while 35 countries (40%) had final dose coverages of 50% or less.
Global coverage of the final HPV vaccine dose (weighted by population size) was estimated at 15%. According to the authors, that low percentage can be explained by the fact that many of the most populous countries have either not yet introduced HPV vaccination or have low performance.
The countries with highest cervical cancer burden have had limited secondary prevention and have been less likely to provide access to vaccination, the authors noted. However, this trend appears to be reversing, with 14 new LMICs providing HPV vaccination in 2019.
HPV vaccination by sex
By 2019, almost a third of the 107 HPV vaccination programs (n = 33) were “gender neutral,” with girls and boys receiving HPV vaccines. Generally, LMICs targeted younger girls (9-10 years) compared with HICs (11-13 years).
Dr. Bruni and colleagues estimated that 15% of girls and 4% of boys were vaccinated globally with the full course of vaccine. At least one dose was received by 20% of girls and 5% of boys.
From 2010 to 2019, HPV vaccination rates in HICs rose from 42% in girls and 0% in boys to 88% and 44%, respectively. In LMICs, over the same period, rates rose from 4% in girls and 0% in boys to 40% and 5%, respectively.
Obstacles and the path forward
The COVID-19 pandemic has halted HPV vaccine delivery in the majority of countries, Dr. Bruni and colleagues noted. About 70 countries had reported program interruptions by August 2020, and delays to HPV vaccine introductions were anticipated for other countries.
An economic downturn could have further far-reaching effects on plans to introduce HPV vaccines, Dr. Bruni and colleagues observed.
While meeting the 2030 target will be challenging, the authors noted that, in every geographic area, some programs are meeting the 90% target.
“HPV national programs should aim to get 90+% of girls vaccinated before the age of 15,” Dr. Bruni said in an interview. “This is a feasible goal, and some countries have succeeded, such as Norway and Rwanda. Average performance, however, is around 55%, and that shows that it is not an easy task.”
Dr. Bruni underscored the four main actions that should be taken to achieve 90% coverage of HPV vaccination, as outlined in the WHO cervical cancer elimination strategy:
- Secure sufficient and affordable HPV vaccines.
- Increase the quality and coverage of vaccination.
- Improve communication and social mobilization.
- Innovate to improve efficiency of vaccine delivery.
“Addressing vaccine hesitancy adequately is one of the biggest challenges we face, especially for the HPV vaccine,” Dr. Bruni said. “As the WHO document states, understanding social, cultural, societal, and other barriers affecting acceptance and uptake of the vaccine will be critical for overcoming vaccine hesitancy and countering misinformation.”
This research was funded by a grant from Instituto de Salud Carlos III and various other grants. Dr. Bruni and coauthors said they have no relevant disclosures.
The WHO’s goal is to have HPV vaccines delivered to 90% of all adolescent girls by 2030, part of the organization’s larger goal to “eliminate” cervical cancer, or reduce the annual incidence of cervical cancer to below 4 cases per 100,000 people globally.
Laia Bruni, MD, PhD, of Catalan Institute of Oncology in Barcelona, and colleagues outlined the progress made thus far toward reaching the WHO’s goals in an article published in Preventive Medicine.
The authors noted that cervical cancer caused by HPV is a “major public health problem, especially in low- and middle-income countries (LMIC).”
However, vaccines against HPV have been available since 2006 and have been recommended by the WHO since 2009.
HPV vaccines have been introduced into many national immunization schedules. Among the 194 WHO member states, 107 (55%) had introduced HPV vaccination as of June 2020, according to estimates from the WHO and the United Nations International Children’s Emergency Fund (UNICEF).
Still, vaccine introduction and coverages are suboptimal, according to several studies and international agencies.
In their article, Dr. Bruni and colleagues describe the mid-2020 status of HPV vaccine introduction, based on WHO/UNICEF estimates of national HPV immunization coverage from 2010 to 2019.
HPV vaccination by region
The Americas and Europe are by far the WHO regions with the highest rates of HPV vaccination, with 85% and 77% of their countries, respectively, having already introduced HPV vaccination, either partially or nationwide.
In 2019, a record number of introductions, 16, were reported, mostly in LMICs where access has been limited. In prior years, the average had been a relatively steady 7-8 introductions per year.
The percentage of high-income countries (HICs) that have introduced HPV vaccination exceeds 80%. LMICs started introducing HPV vaccination later and at a slower pace, compared with HICs. By the end of 2019, only 41% of LMICs had introduced vaccination. However, of the new introductions in 2019, 87% were in LMICs.
In 2019, the average performance coverage for HPV vaccination programs in 99 countries (both HICs and LMICs) was around 67% for the first vaccine dose and 53% for the final dose.
Median performance coverage was higher in LMICs than in HICs for the first dose (80% and 72%, respectively), but mean dropout rates were higher in LMICs than in HICs (18% and 11%, respectively).
Coverage of more than 90% was achieved for the last dose in only five countries (6%). Twenty-two countries (21%) achieved coverages of 75% or higher, while 35 countries (40%) had final dose coverages of 50% or less.
Global coverage of the final HPV vaccine dose (weighted by population size) was estimated at 15%. According to the authors, that low percentage can be explained by the fact that many of the most populous countries have either not yet introduced HPV vaccination or have low performance.
The countries with highest cervical cancer burden have had limited secondary prevention and have been less likely to provide access to vaccination, the authors noted. However, this trend appears to be reversing, with 14 new LMICs providing HPV vaccination in 2019.
HPV vaccination by sex
By 2019, almost a third of the 107 HPV vaccination programs (n = 33) were “gender neutral,” with girls and boys receiving HPV vaccines. Generally, LMICs targeted younger girls (9-10 years) compared with HICs (11-13 years).
Dr. Bruni and colleagues estimated that 15% of girls and 4% of boys were vaccinated globally with the full course of vaccine. At least one dose was received by 20% of girls and 5% of boys.
From 2010 to 2019, HPV vaccination rates in HICs rose from 42% in girls and 0% in boys to 88% and 44%, respectively. In LMICs, over the same period, rates rose from 4% in girls and 0% in boys to 40% and 5%, respectively.
Obstacles and the path forward
The COVID-19 pandemic has halted HPV vaccine delivery in the majority of countries, Dr. Bruni and colleagues noted. About 70 countries had reported program interruptions by August 2020, and delays to HPV vaccine introductions were anticipated for other countries.
An economic downturn could have further far-reaching effects on plans to introduce HPV vaccines, Dr. Bruni and colleagues observed.
While meeting the 2030 target will be challenging, the authors noted that, in every geographic area, some programs are meeting the 90% target.
“HPV national programs should aim to get 90+% of girls vaccinated before the age of 15,” Dr. Bruni said in an interview. “This is a feasible goal, and some countries have succeeded, such as Norway and Rwanda. Average performance, however, is around 55%, and that shows that it is not an easy task.”
Dr. Bruni underscored the four main actions that should be taken to achieve 90% coverage of HPV vaccination, as outlined in the WHO cervical cancer elimination strategy:
- Secure sufficient and affordable HPV vaccines.
- Increase the quality and coverage of vaccination.
- Improve communication and social mobilization.
- Innovate to improve efficiency of vaccine delivery.
“Addressing vaccine hesitancy adequately is one of the biggest challenges we face, especially for the HPV vaccine,” Dr. Bruni said. “As the WHO document states, understanding social, cultural, societal, and other barriers affecting acceptance and uptake of the vaccine will be critical for overcoming vaccine hesitancy and countering misinformation.”
This research was funded by a grant from Instituto de Salud Carlos III and various other grants. Dr. Bruni and coauthors said they have no relevant disclosures.
FROM PREVENTIVE MEDICINE
SNP chips deemed ‘extremely unreliable’ for identifying rare variants
In fact, SNP chips are “extremely unreliable for genotyping very rare pathogenic variants,” and a positive result for such a variant “is more likely to be wrong than right,” researchers reported in the BMJ.
The authors explained that SNP chips are “DNA microarrays that test genetic variation at many hundreds of thousands of specific locations across the genome.” Although SNP chips have proven accurate in identifying common variants, past reports have suggested that SNP chips perform poorly for genotyping rare variants.
To gain more insight, Caroline Wright, PhD, of the University of Exeter (England) and colleagues conducted a large study.
The researchers analyzed data on 49,908 people from the UK Biobank who had SNP chip and next-generation sequencing results, as well as an additional 21 people who purchased consumer genetic tests and shared their data online via the Personal Genome Project.
The researchers compared the SNP chip and sequencing results. They also selected rare pathogenic variants in BRCA1 and BRCA2 for detailed analysis of clinically actionable variants in the UK Biobank, and they assessed BRCA-related cancers in participants using cancer registry data.
Largest evaluation of SNP chips
SNP chips performed well for common variants, the researchers found. Sensitivity, specificity, positive-predictive value, and negative-predictive value all exceeded 99% for 108,574 common variants.
For rare variants, SNP chips performed poorly, with a positive-predictive value of 16% for variants with a frequency below 0.001% in the UK Biobank.
“The study provides the largest evaluation of the performance of SNP chips for genotyping genetic variants at different frequencies in the population, particularly focusing on very rare variants,” Dr. Wright said. “The biggest surprise was how poorly the SNP chips we evaluated performed for rare variants.”
Dr. Wright noted that there is an inherent problem built into using SNP chip technology to genotype very rare variants.
“The SNP chip technology relies on clustering data from multiple individuals in order to determine what genotype each individual has at a specific position in their genome,” Dr. Wright explained. “Although this method works very well for common variants, the rarer the variant, the harder it is to distinguish from experimental noise.”
False positives and cancer: ‘Don’t trust the results’
The researchers found that, for rare BRCA variants (frequency below 0.01%), SNP chips had a sensitivity of 34.6%, specificity of 98.3%, negative-predictive value of 99.9%, and positive-predictive value of 4.2%.
Rates of BRCA-related cancers in patients with positive SNP chip results were similar to rates in age-matched control subjects because “the vast majority of variants were false positives,” the researchers noted.
“If these variants are incorrectly genotyped – that is, false positives detected – a woman could be offered screening or even prophylactic surgery inappropriately when she is more likely to be at population background risk [for BRCA-related cancers],” Dr. Wright said.
“For very-rare-disease–causing genetic variants, don’t trust the results from SNP chips; for example, those from direct-to-consumer genetic tests. Never use them to guide clinical action without diagnostic validation,” she added.
Heather Hampel, a genetic counselor and researcher at the Ohio State University Comprehensive Cancer Center in Columbus, agreed.
“Positive results on SNP-based tests need to be confirmed by medical-grade genetic testing using a sequencing technology,” she said. “Negative results on an SNP- based test cannot be considered to rule out mutations in BRCA1/2 or other cancer-susceptibility genes, so individuals with strong personal and family histories of cancer should be seen by a genetic counselor to consider medical-grade genetic testing using a sequencing technology.”
Practicing oncologists can trust patients’ prior germline genetic test results if the testing was performed in a cancer genetics clinic, which uses sequencing-based technologies, Ms. Hampel noted.
“If the test was performed before 2013, there are likely new genes that have been discovered for which their patient was not tested, and repeat testing may be warranted,” Ms. Hampel said. “A referral to a cancer genetic counselor would be appropriate.”
Ms. Hampel disclosed relationships with Genome Medical, GI OnDemand, Invitae Genetics, and Promega. Dr. Wright and her coauthors disclosed no conflicts of interest. The group’s research was conducted using the UK Biobank and the University of Exeter High-Performance Computing, with funding from the Wellcome Trust and the National Institute for Health Research.
In fact, SNP chips are “extremely unreliable for genotyping very rare pathogenic variants,” and a positive result for such a variant “is more likely to be wrong than right,” researchers reported in the BMJ.
The authors explained that SNP chips are “DNA microarrays that test genetic variation at many hundreds of thousands of specific locations across the genome.” Although SNP chips have proven accurate in identifying common variants, past reports have suggested that SNP chips perform poorly for genotyping rare variants.
To gain more insight, Caroline Wright, PhD, of the University of Exeter (England) and colleagues conducted a large study.
The researchers analyzed data on 49,908 people from the UK Biobank who had SNP chip and next-generation sequencing results, as well as an additional 21 people who purchased consumer genetic tests and shared their data online via the Personal Genome Project.
The researchers compared the SNP chip and sequencing results. They also selected rare pathogenic variants in BRCA1 and BRCA2 for detailed analysis of clinically actionable variants in the UK Biobank, and they assessed BRCA-related cancers in participants using cancer registry data.
Largest evaluation of SNP chips
SNP chips performed well for common variants, the researchers found. Sensitivity, specificity, positive-predictive value, and negative-predictive value all exceeded 99% for 108,574 common variants.
For rare variants, SNP chips performed poorly, with a positive-predictive value of 16% for variants with a frequency below 0.001% in the UK Biobank.
“The study provides the largest evaluation of the performance of SNP chips for genotyping genetic variants at different frequencies in the population, particularly focusing on very rare variants,” Dr. Wright said. “The biggest surprise was how poorly the SNP chips we evaluated performed for rare variants.”
Dr. Wright noted that there is an inherent problem built into using SNP chip technology to genotype very rare variants.
“The SNP chip technology relies on clustering data from multiple individuals in order to determine what genotype each individual has at a specific position in their genome,” Dr. Wright explained. “Although this method works very well for common variants, the rarer the variant, the harder it is to distinguish from experimental noise.”
False positives and cancer: ‘Don’t trust the results’
The researchers found that, for rare BRCA variants (frequency below 0.01%), SNP chips had a sensitivity of 34.6%, specificity of 98.3%, negative-predictive value of 99.9%, and positive-predictive value of 4.2%.
Rates of BRCA-related cancers in patients with positive SNP chip results were similar to rates in age-matched control subjects because “the vast majority of variants were false positives,” the researchers noted.
“If these variants are incorrectly genotyped – that is, false positives detected – a woman could be offered screening or even prophylactic surgery inappropriately when she is more likely to be at population background risk [for BRCA-related cancers],” Dr. Wright said.
“For very-rare-disease–causing genetic variants, don’t trust the results from SNP chips; for example, those from direct-to-consumer genetic tests. Never use them to guide clinical action without diagnostic validation,” she added.
Heather Hampel, a genetic counselor and researcher at the Ohio State University Comprehensive Cancer Center in Columbus, agreed.
“Positive results on SNP-based tests need to be confirmed by medical-grade genetic testing using a sequencing technology,” she said. “Negative results on an SNP- based test cannot be considered to rule out mutations in BRCA1/2 or other cancer-susceptibility genes, so individuals with strong personal and family histories of cancer should be seen by a genetic counselor to consider medical-grade genetic testing using a sequencing technology.”
Practicing oncologists can trust patients’ prior germline genetic test results if the testing was performed in a cancer genetics clinic, which uses sequencing-based technologies, Ms. Hampel noted.
“If the test was performed before 2013, there are likely new genes that have been discovered for which their patient was not tested, and repeat testing may be warranted,” Ms. Hampel said. “A referral to a cancer genetic counselor would be appropriate.”
Ms. Hampel disclosed relationships with Genome Medical, GI OnDemand, Invitae Genetics, and Promega. Dr. Wright and her coauthors disclosed no conflicts of interest. The group’s research was conducted using the UK Biobank and the University of Exeter High-Performance Computing, with funding from the Wellcome Trust and the National Institute for Health Research.
In fact, SNP chips are “extremely unreliable for genotyping very rare pathogenic variants,” and a positive result for such a variant “is more likely to be wrong than right,” researchers reported in the BMJ.
The authors explained that SNP chips are “DNA microarrays that test genetic variation at many hundreds of thousands of specific locations across the genome.” Although SNP chips have proven accurate in identifying common variants, past reports have suggested that SNP chips perform poorly for genotyping rare variants.
To gain more insight, Caroline Wright, PhD, of the University of Exeter (England) and colleagues conducted a large study.
The researchers analyzed data on 49,908 people from the UK Biobank who had SNP chip and next-generation sequencing results, as well as an additional 21 people who purchased consumer genetic tests and shared their data online via the Personal Genome Project.
The researchers compared the SNP chip and sequencing results. They also selected rare pathogenic variants in BRCA1 and BRCA2 for detailed analysis of clinically actionable variants in the UK Biobank, and they assessed BRCA-related cancers in participants using cancer registry data.
Largest evaluation of SNP chips
SNP chips performed well for common variants, the researchers found. Sensitivity, specificity, positive-predictive value, and negative-predictive value all exceeded 99% for 108,574 common variants.
For rare variants, SNP chips performed poorly, with a positive-predictive value of 16% for variants with a frequency below 0.001% in the UK Biobank.
“The study provides the largest evaluation of the performance of SNP chips for genotyping genetic variants at different frequencies in the population, particularly focusing on very rare variants,” Dr. Wright said. “The biggest surprise was how poorly the SNP chips we evaluated performed for rare variants.”
Dr. Wright noted that there is an inherent problem built into using SNP chip technology to genotype very rare variants.
“The SNP chip technology relies on clustering data from multiple individuals in order to determine what genotype each individual has at a specific position in their genome,” Dr. Wright explained. “Although this method works very well for common variants, the rarer the variant, the harder it is to distinguish from experimental noise.”
False positives and cancer: ‘Don’t trust the results’
The researchers found that, for rare BRCA variants (frequency below 0.01%), SNP chips had a sensitivity of 34.6%, specificity of 98.3%, negative-predictive value of 99.9%, and positive-predictive value of 4.2%.
Rates of BRCA-related cancers in patients with positive SNP chip results were similar to rates in age-matched control subjects because “the vast majority of variants were false positives,” the researchers noted.
“If these variants are incorrectly genotyped – that is, false positives detected – a woman could be offered screening or even prophylactic surgery inappropriately when she is more likely to be at population background risk [for BRCA-related cancers],” Dr. Wright said.
“For very-rare-disease–causing genetic variants, don’t trust the results from SNP chips; for example, those from direct-to-consumer genetic tests. Never use them to guide clinical action without diagnostic validation,” she added.
Heather Hampel, a genetic counselor and researcher at the Ohio State University Comprehensive Cancer Center in Columbus, agreed.
“Positive results on SNP-based tests need to be confirmed by medical-grade genetic testing using a sequencing technology,” she said. “Negative results on an SNP- based test cannot be considered to rule out mutations in BRCA1/2 or other cancer-susceptibility genes, so individuals with strong personal and family histories of cancer should be seen by a genetic counselor to consider medical-grade genetic testing using a sequencing technology.”
Practicing oncologists can trust patients’ prior germline genetic test results if the testing was performed in a cancer genetics clinic, which uses sequencing-based technologies, Ms. Hampel noted.
“If the test was performed before 2013, there are likely new genes that have been discovered for which their patient was not tested, and repeat testing may be warranted,” Ms. Hampel said. “A referral to a cancer genetic counselor would be appropriate.”
Ms. Hampel disclosed relationships with Genome Medical, GI OnDemand, Invitae Genetics, and Promega. Dr. Wright and her coauthors disclosed no conflicts of interest. The group’s research was conducted using the UK Biobank and the University of Exeter High-Performance Computing, with funding from the Wellcome Trust and the National Institute for Health Research.
FROM BMJ
Don’t delay: Cancer patients need both doses of COVID vaccine
The new findings, which are soon to be published as a preprint, cast doubt on the current U.K. policy of delaying the second dose of the vaccine.
Delaying the second dose can leave most patients with cancer wholly or partially unprotected, according to the researchers. Moreover, such a delay has implications for transmission of SARS-CoV-2 in the cancer patient’s environs as well as for the evolution of virus variants that could be of concern, the researchers concluded.
The data come from a British study that included 151 patients with cancer and 54 healthy control persons. All participants received the COVID-19 mRNA BNT162b2 vaccine (Pfizer-BioNTech).
This vaccine requires two doses. The first few participants in this study were given the second dose 21 days after they had received the first dose, but then national guidelines changed, and the remaining participants had to wait 12 weeks to receive their second dose.
The researchers reported that, among health controls, the immune efficacy of the first dose was very high (97% efficacious). By contrast, among patients with solid tumors, the immune efficacy of a single dose was strikingly low (39%), and it was even lower in patients with hematologic malignancies (13%).
The second dose of vaccine greatly and rapidly increased the immune efficacy in patients with solid tumors (95% within 2 weeks of receiving the second dose), the researchers added.
Too few patients with hematologic cancers had received the second dose before the study ended for clear conclusions to be drawn. Nevertheless, the available data suggest that 50% of patients with hematologic cancers who had received the booster at day 21 were seropositive at 5 weeks vs. only 8% of those who had not received the booster.
“Our data provide the first real-world evidence of immune efficacy following one dose of the Pfizer vaccine in immunocompromised patient populations [and] clearly show that the poor one-dose efficacy in cancer patients can be rescued with an early booster at day 21,” commented senior author Sheeba Irshad, MD, senior clinical lecturer, King’s College London.
“Based on our findings, we would recommend an urgent review of the vaccine strategy for clinically extremely vulnerable groups. Until then, it is important that cancer patients continue to observe all public health measures in place, such as social distancing and shielding when attending hospitals, even after vaccination,” Dr. Irshad added.
The paper, with first author Leticia Monin-Aldama, PhD, is scheduled to appear on the preprint server medRxiv. It has not undergone peer review. The paper was distributed to journalists, with comments from experts not involved in the study, by the UK Science Media Centre.
These data are “of immediate importance” to patients with cancer, commented Shoba Amarnath, PhD, Newcastle University research fellow, Laboratory of T-cell Regulation, Newcastle University Center for Cancer, Newcastle upon Tyne, England.
“These findings are consistent with our understanding. … We know that the immune system within cancer patients is compromised as compared to healthy controls,” Dr. Amarnath said. “The data in the study support the notion that, in solid cancer patients, a considerable delay in second dose will extend the period when cancer patients are at risk of SARS-CoV-2 infection.”
Although more data are required, “this study does raise the issue of whether patients with cancer, other diseases, or those undergoing therapies that affect the body’s immune response should be fast-tracked for their second vaccine dose,” commented Lawrence Young, PhD, professor of molecular oncology and director of the Warwick Cancer Research Center, University of Warwick, Coventry, England.
Stephen Evans, MSc, professor of pharmacoepidemiology, London School of Hygiene and Tropical Medicine, underlined that the study is “essentially” observational and “inevitable limitations must be taken into account.
“Nevertheless, these results do suggest that the vaccines may well not protect those patients with cancer as well as those without cancer,” Mr. Evans said. He added that it is “important that this population continues to observe all COVID-19–associated measures, such as social distancing and shielding when attending hospitals, even after vaccination.”
Study details
Previous studies have shown that some patients with cancer have prolonged responses to SARS-CoV-2 infection, with ongoing immune dysregulation, inefficient seroconversion, and prolonged viral shedding.
There are few data, however, on how these patients respond to COVID-19 vaccination. The authors point out that, among the 18,860 individuals who received the Pfizer vaccine during its development trials, “none with an active oncological diagnosis was included.”
To investigate this issue, they launched the SARS-CoV-2 for Cancer Patients (SOAP-02) study.
The 151 patients with cancer who participated in this study were mostly elderly, the authors noted (75% were older than 65 years; the median age was 73 years). The majority (63%) had solid-tumor malignancies. Of those, 8% had late-stage disease and had been living with their cancer for more than 24 months.
The healthy control persons were vaccine-eligible primary health care workers who were not age matched to the cancer patients.
All participants received the first dose of vaccine; 31 (of 151) patients with cancer and 16 (of 54) healthy control persons received the second dose on day 21.
The remaining participants were scheduled to receive their second dose 12 weeks later (after the study ended), in line with the changes in the national guidelines.
The team reported that, approximately 21 days after receiving the first vaccine dose, the immune efficacy of the vaccine was estimated to be 97% among healthy control persons vs. 39% for patients with solid tumors and only 13% for those with hematologic malignancies (P < .0001 for both).
T-cell responses, as assessed via interferon-gamma and/or interleukin-2 production, were observed in 82% of healthy control persons, 71% of patients with solid tumors, and 50% of those with hematologic cancers.
Vaccine boosting at day 21 resulted in immune efficacy of 100% for healthy control persons and 95% for patients with solid tumors. In contrast, only 43% of those who did not receive the second dose were seropositive 2 weeks later.
Further analysis suggested that participants who did not have a serologic response were “spread evenly” across different cancer types, but the reduced responses were more frequent among patients who had received the vaccine within 15 days of cancer treatment, especially chemotherapy, and had undergone intensive treatments.
The SOAP study is sponsored by King’s College London and Guy’s and St. Thomas Trust Foundation NHS Trust. It is funded from grants from the KCL Charity, Cancer Research UK, and program grants from Breast Cancer Now. The investigators have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The new findings, which are soon to be published as a preprint, cast doubt on the current U.K. policy of delaying the second dose of the vaccine.
Delaying the second dose can leave most patients with cancer wholly or partially unprotected, according to the researchers. Moreover, such a delay has implications for transmission of SARS-CoV-2 in the cancer patient’s environs as well as for the evolution of virus variants that could be of concern, the researchers concluded.
The data come from a British study that included 151 patients with cancer and 54 healthy control persons. All participants received the COVID-19 mRNA BNT162b2 vaccine (Pfizer-BioNTech).
This vaccine requires two doses. The first few participants in this study were given the second dose 21 days after they had received the first dose, but then national guidelines changed, and the remaining participants had to wait 12 weeks to receive their second dose.
The researchers reported that, among health controls, the immune efficacy of the first dose was very high (97% efficacious). By contrast, among patients with solid tumors, the immune efficacy of a single dose was strikingly low (39%), and it was even lower in patients with hematologic malignancies (13%).
The second dose of vaccine greatly and rapidly increased the immune efficacy in patients with solid tumors (95% within 2 weeks of receiving the second dose), the researchers added.
Too few patients with hematologic cancers had received the second dose before the study ended for clear conclusions to be drawn. Nevertheless, the available data suggest that 50% of patients with hematologic cancers who had received the booster at day 21 were seropositive at 5 weeks vs. only 8% of those who had not received the booster.
“Our data provide the first real-world evidence of immune efficacy following one dose of the Pfizer vaccine in immunocompromised patient populations [and] clearly show that the poor one-dose efficacy in cancer patients can be rescued with an early booster at day 21,” commented senior author Sheeba Irshad, MD, senior clinical lecturer, King’s College London.
“Based on our findings, we would recommend an urgent review of the vaccine strategy for clinically extremely vulnerable groups. Until then, it is important that cancer patients continue to observe all public health measures in place, such as social distancing and shielding when attending hospitals, even after vaccination,” Dr. Irshad added.
The paper, with first author Leticia Monin-Aldama, PhD, is scheduled to appear on the preprint server medRxiv. It has not undergone peer review. The paper was distributed to journalists, with comments from experts not involved in the study, by the UK Science Media Centre.
These data are “of immediate importance” to patients with cancer, commented Shoba Amarnath, PhD, Newcastle University research fellow, Laboratory of T-cell Regulation, Newcastle University Center for Cancer, Newcastle upon Tyne, England.
“These findings are consistent with our understanding. … We know that the immune system within cancer patients is compromised as compared to healthy controls,” Dr. Amarnath said. “The data in the study support the notion that, in solid cancer patients, a considerable delay in second dose will extend the period when cancer patients are at risk of SARS-CoV-2 infection.”
Although more data are required, “this study does raise the issue of whether patients with cancer, other diseases, or those undergoing therapies that affect the body’s immune response should be fast-tracked for their second vaccine dose,” commented Lawrence Young, PhD, professor of molecular oncology and director of the Warwick Cancer Research Center, University of Warwick, Coventry, England.
Stephen Evans, MSc, professor of pharmacoepidemiology, London School of Hygiene and Tropical Medicine, underlined that the study is “essentially” observational and “inevitable limitations must be taken into account.
“Nevertheless, these results do suggest that the vaccines may well not protect those patients with cancer as well as those without cancer,” Mr. Evans said. He added that it is “important that this population continues to observe all COVID-19–associated measures, such as social distancing and shielding when attending hospitals, even after vaccination.”
Study details
Previous studies have shown that some patients with cancer have prolonged responses to SARS-CoV-2 infection, with ongoing immune dysregulation, inefficient seroconversion, and prolonged viral shedding.
There are few data, however, on how these patients respond to COVID-19 vaccination. The authors point out that, among the 18,860 individuals who received the Pfizer vaccine during its development trials, “none with an active oncological diagnosis was included.”
To investigate this issue, they launched the SARS-CoV-2 for Cancer Patients (SOAP-02) study.
The 151 patients with cancer who participated in this study were mostly elderly, the authors noted (75% were older than 65 years; the median age was 73 years). The majority (63%) had solid-tumor malignancies. Of those, 8% had late-stage disease and had been living with their cancer for more than 24 months.
The healthy control persons were vaccine-eligible primary health care workers who were not age matched to the cancer patients.
All participants received the first dose of vaccine; 31 (of 151) patients with cancer and 16 (of 54) healthy control persons received the second dose on day 21.
The remaining participants were scheduled to receive their second dose 12 weeks later (after the study ended), in line with the changes in the national guidelines.
The team reported that, approximately 21 days after receiving the first vaccine dose, the immune efficacy of the vaccine was estimated to be 97% among healthy control persons vs. 39% for patients with solid tumors and only 13% for those with hematologic malignancies (P < .0001 for both).
T-cell responses, as assessed via interferon-gamma and/or interleukin-2 production, were observed in 82% of healthy control persons, 71% of patients with solid tumors, and 50% of those with hematologic cancers.
Vaccine boosting at day 21 resulted in immune efficacy of 100% for healthy control persons and 95% for patients with solid tumors. In contrast, only 43% of those who did not receive the second dose were seropositive 2 weeks later.
Further analysis suggested that participants who did not have a serologic response were “spread evenly” across different cancer types, but the reduced responses were more frequent among patients who had received the vaccine within 15 days of cancer treatment, especially chemotherapy, and had undergone intensive treatments.
The SOAP study is sponsored by King’s College London and Guy’s and St. Thomas Trust Foundation NHS Trust. It is funded from grants from the KCL Charity, Cancer Research UK, and program grants from Breast Cancer Now. The investigators have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The new findings, which are soon to be published as a preprint, cast doubt on the current U.K. policy of delaying the second dose of the vaccine.
Delaying the second dose can leave most patients with cancer wholly or partially unprotected, according to the researchers. Moreover, such a delay has implications for transmission of SARS-CoV-2 in the cancer patient’s environs as well as for the evolution of virus variants that could be of concern, the researchers concluded.
The data come from a British study that included 151 patients with cancer and 54 healthy control persons. All participants received the COVID-19 mRNA BNT162b2 vaccine (Pfizer-BioNTech).
This vaccine requires two doses. The first few participants in this study were given the second dose 21 days after they had received the first dose, but then national guidelines changed, and the remaining participants had to wait 12 weeks to receive their second dose.
The researchers reported that, among health controls, the immune efficacy of the first dose was very high (97% efficacious). By contrast, among patients with solid tumors, the immune efficacy of a single dose was strikingly low (39%), and it was even lower in patients with hematologic malignancies (13%).
The second dose of vaccine greatly and rapidly increased the immune efficacy in patients with solid tumors (95% within 2 weeks of receiving the second dose), the researchers added.
Too few patients with hematologic cancers had received the second dose before the study ended for clear conclusions to be drawn. Nevertheless, the available data suggest that 50% of patients with hematologic cancers who had received the booster at day 21 were seropositive at 5 weeks vs. only 8% of those who had not received the booster.
“Our data provide the first real-world evidence of immune efficacy following one dose of the Pfizer vaccine in immunocompromised patient populations [and] clearly show that the poor one-dose efficacy in cancer patients can be rescued with an early booster at day 21,” commented senior author Sheeba Irshad, MD, senior clinical lecturer, King’s College London.
“Based on our findings, we would recommend an urgent review of the vaccine strategy for clinically extremely vulnerable groups. Until then, it is important that cancer patients continue to observe all public health measures in place, such as social distancing and shielding when attending hospitals, even after vaccination,” Dr. Irshad added.
The paper, with first author Leticia Monin-Aldama, PhD, is scheduled to appear on the preprint server medRxiv. It has not undergone peer review. The paper was distributed to journalists, with comments from experts not involved in the study, by the UK Science Media Centre.
These data are “of immediate importance” to patients with cancer, commented Shoba Amarnath, PhD, Newcastle University research fellow, Laboratory of T-cell Regulation, Newcastle University Center for Cancer, Newcastle upon Tyne, England.
“These findings are consistent with our understanding. … We know that the immune system within cancer patients is compromised as compared to healthy controls,” Dr. Amarnath said. “The data in the study support the notion that, in solid cancer patients, a considerable delay in second dose will extend the period when cancer patients are at risk of SARS-CoV-2 infection.”
Although more data are required, “this study does raise the issue of whether patients with cancer, other diseases, or those undergoing therapies that affect the body’s immune response should be fast-tracked for their second vaccine dose,” commented Lawrence Young, PhD, professor of molecular oncology and director of the Warwick Cancer Research Center, University of Warwick, Coventry, England.
Stephen Evans, MSc, professor of pharmacoepidemiology, London School of Hygiene and Tropical Medicine, underlined that the study is “essentially” observational and “inevitable limitations must be taken into account.
“Nevertheless, these results do suggest that the vaccines may well not protect those patients with cancer as well as those without cancer,” Mr. Evans said. He added that it is “important that this population continues to observe all COVID-19–associated measures, such as social distancing and shielding when attending hospitals, even after vaccination.”
Study details
Previous studies have shown that some patients with cancer have prolonged responses to SARS-CoV-2 infection, with ongoing immune dysregulation, inefficient seroconversion, and prolonged viral shedding.
There are few data, however, on how these patients respond to COVID-19 vaccination. The authors point out that, among the 18,860 individuals who received the Pfizer vaccine during its development trials, “none with an active oncological diagnosis was included.”
To investigate this issue, they launched the SARS-CoV-2 for Cancer Patients (SOAP-02) study.
The 151 patients with cancer who participated in this study were mostly elderly, the authors noted (75% were older than 65 years; the median age was 73 years). The majority (63%) had solid-tumor malignancies. Of those, 8% had late-stage disease and had been living with their cancer for more than 24 months.
The healthy control persons were vaccine-eligible primary health care workers who were not age matched to the cancer patients.
All participants received the first dose of vaccine; 31 (of 151) patients with cancer and 16 (of 54) healthy control persons received the second dose on day 21.
The remaining participants were scheduled to receive their second dose 12 weeks later (after the study ended), in line with the changes in the national guidelines.
The team reported that, approximately 21 days after receiving the first vaccine dose, the immune efficacy of the vaccine was estimated to be 97% among healthy control persons vs. 39% for patients with solid tumors and only 13% for those with hematologic malignancies (P < .0001 for both).
T-cell responses, as assessed via interferon-gamma and/or interleukin-2 production, were observed in 82% of healthy control persons, 71% of patients with solid tumors, and 50% of those with hematologic cancers.
Vaccine boosting at day 21 resulted in immune efficacy of 100% for healthy control persons and 95% for patients with solid tumors. In contrast, only 43% of those who did not receive the second dose were seropositive 2 weeks later.
Further analysis suggested that participants who did not have a serologic response were “spread evenly” across different cancer types, but the reduced responses were more frequent among patients who had received the vaccine within 15 days of cancer treatment, especially chemotherapy, and had undergone intensive treatments.
The SOAP study is sponsored by King’s College London and Guy’s and St. Thomas Trust Foundation NHS Trust. It is funded from grants from the KCL Charity, Cancer Research UK, and program grants from Breast Cancer Now. The investigators have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Vaginal pH may predict CIN 2 progression in HIV-positive women
Elevated vaginal pH at the time of cervical intraepithelial neoplasia 2 diagnosis may be a useful marker of CIN 2 persistence/progression, as well as the rate of persistence/progression in HIV-positive women, new research suggests.
“We analyzed data from the Women’s Interagency HIV Study [WIHS], an observational, longitudinal cohort of women with and without HIV to determine factors that may influence CIN 2 natural history,” said Kate Michel, PhD, MPH, of Georgetown University, Washington. She presented the results at the Conference on Retroviruses and Opportunistic Infections.
As previous data have shown a high incidence of CIN 2 progression among women with HIV, the researchers evaluated the role of human papillomavirus (HPV) type, local immune response, and markers of the cervicovaginal microbiome on the risk of CIN 2 persistence/progression.
Within the cohort, follow-up visits occur every 6 months, and clinical data is collected via questionnaires, physical and gynecologic exams, and biological samples. As no specific treatment is offered in the WIHS, treatment for cervical abnormalities is abstracted from medical records.
In the present study, Dr. Michel and colleagues selected up to four banked cervicovaginal lavage (CVL) samples per woman, with the first sample selected 6-12 months prior to CIN 2 diagnosis, the second at CIN 2 diagnosis, the third between CIN 2 diagnosis and outcome, and the fourth at the outcome visit.
The investigators performed HPV typing and muiltiplex immune mediator testing on each CVL sample. Lab results from WIHS core testing were also extracted, including plasma CD4+ T-cell count and HIV viral load, as well as vaginal pH and Nugent’s score.
Study outcomes included persistence/progression and regression, defined as a subsequent CIN 2 or CIN 3 diagnosis and subsequent CIN 1 or normal diagnosis, respectively. Logistic regression models were used to determine CIN 2 regression versus persistence/progression.
Results
A total of 337 samples were obtained and 94 women were included in the analysis. Key demographic and behavioral factor were similar at CIN 2 diagnosis.
The majority of participants were African American (53.2%) and on antiretroviral therapy (66.0%). The most prevalent high-risk types were HPV-58 (18.4%) and HPV-16 (17.5%).
After a median 12.5 years of follow-up, 33 participants (35.1%) with incident CIN 2 had a subsequent CIN 2/CIN 3 diagnosis and those who regressed had a higher CD4 T-cell count at CIN 2 diagnosis (P = .02).
Each subsequent high-risk HPV type identified at the pre–CIN 2 visit was associated with higher odds of CIN2 persistence/progression (odds ratio, 2.27; 95% confidence interval, 1.15-4.50).
Bacterial vaginosis (adjusted OR, 5.08; 95% CI, 1.30-19.94) and vaginal pH (aOR, 2.27; 95% CI, 1.15-4.50) at the CIN 2 diagnosis visit were each associated with increased odds of CIN 2 persistence/progression.
Vaginal pH greater than 4.5 at CIN 2 diagnosis was also associated with unadjusted time to CIN 2 persistence/progression (log rank P = .002) and an increased rate of CIN 2 persistence/progression (adjusted hazard ratio, 3.37; 95% CI, 1.26-8.99).
Furthermore, among participants who did not receive CIN 2 treatment, vaginal pH remained associated with greater odds of CIN 2 persistence/progression (OR, 2.46; 95% CI, 1.19-5.13). Cervicovaginal immune mediator levels were not associated with CIN 2 persistence/progression.
“The most striking finding from this work was that vaginal pH was associated with higher odds of, quicker time to, and increased hazard of CIN 2 persistence/progression,” Dr. Michel said. “We postulate this effect is mediated by the cervical microbiome, but more work is needed to establish the exact mechanism.”
“It would be interesting to test whether this association might be explained by different vaginal cleaning techniques, such as douching,” said moderator Ronald T. Mitsuyasu, MD, of the University of California, Los Angeles.
“We’re currently working on an analysis of cervicovaginal bacterial species to explore the microbiome in more detail,” Dr. Michel concluded.
Dr. Michel disclosed no conflicts of interest. The study was supported by multiple sources, including the National Institute of Allergy and Infectious Diseases, the National Cancer Institute, and the Georgetown-Howard Universities Center for Clinical and Translational Science.
Elevated vaginal pH at the time of cervical intraepithelial neoplasia 2 diagnosis may be a useful marker of CIN 2 persistence/progression, as well as the rate of persistence/progression in HIV-positive women, new research suggests.
“We analyzed data from the Women’s Interagency HIV Study [WIHS], an observational, longitudinal cohort of women with and without HIV to determine factors that may influence CIN 2 natural history,” said Kate Michel, PhD, MPH, of Georgetown University, Washington. She presented the results at the Conference on Retroviruses and Opportunistic Infections.
As previous data have shown a high incidence of CIN 2 progression among women with HIV, the researchers evaluated the role of human papillomavirus (HPV) type, local immune response, and markers of the cervicovaginal microbiome on the risk of CIN 2 persistence/progression.
Within the cohort, follow-up visits occur every 6 months, and clinical data is collected via questionnaires, physical and gynecologic exams, and biological samples. As no specific treatment is offered in the WIHS, treatment for cervical abnormalities is abstracted from medical records.
In the present study, Dr. Michel and colleagues selected up to four banked cervicovaginal lavage (CVL) samples per woman, with the first sample selected 6-12 months prior to CIN 2 diagnosis, the second at CIN 2 diagnosis, the third between CIN 2 diagnosis and outcome, and the fourth at the outcome visit.
The investigators performed HPV typing and muiltiplex immune mediator testing on each CVL sample. Lab results from WIHS core testing were also extracted, including plasma CD4+ T-cell count and HIV viral load, as well as vaginal pH and Nugent’s score.
Study outcomes included persistence/progression and regression, defined as a subsequent CIN 2 or CIN 3 diagnosis and subsequent CIN 1 or normal diagnosis, respectively. Logistic regression models were used to determine CIN 2 regression versus persistence/progression.
Results
A total of 337 samples were obtained and 94 women were included in the analysis. Key demographic and behavioral factor were similar at CIN 2 diagnosis.
The majority of participants were African American (53.2%) and on antiretroviral therapy (66.0%). The most prevalent high-risk types were HPV-58 (18.4%) and HPV-16 (17.5%).
After a median 12.5 years of follow-up, 33 participants (35.1%) with incident CIN 2 had a subsequent CIN 2/CIN 3 diagnosis and those who regressed had a higher CD4 T-cell count at CIN 2 diagnosis (P = .02).
Each subsequent high-risk HPV type identified at the pre–CIN 2 visit was associated with higher odds of CIN2 persistence/progression (odds ratio, 2.27; 95% confidence interval, 1.15-4.50).
Bacterial vaginosis (adjusted OR, 5.08; 95% CI, 1.30-19.94) and vaginal pH (aOR, 2.27; 95% CI, 1.15-4.50) at the CIN 2 diagnosis visit were each associated with increased odds of CIN 2 persistence/progression.
Vaginal pH greater than 4.5 at CIN 2 diagnosis was also associated with unadjusted time to CIN 2 persistence/progression (log rank P = .002) and an increased rate of CIN 2 persistence/progression (adjusted hazard ratio, 3.37; 95% CI, 1.26-8.99).
Furthermore, among participants who did not receive CIN 2 treatment, vaginal pH remained associated with greater odds of CIN 2 persistence/progression (OR, 2.46; 95% CI, 1.19-5.13). Cervicovaginal immune mediator levels were not associated with CIN 2 persistence/progression.
“The most striking finding from this work was that vaginal pH was associated with higher odds of, quicker time to, and increased hazard of CIN 2 persistence/progression,” Dr. Michel said. “We postulate this effect is mediated by the cervical microbiome, but more work is needed to establish the exact mechanism.”
“It would be interesting to test whether this association might be explained by different vaginal cleaning techniques, such as douching,” said moderator Ronald T. Mitsuyasu, MD, of the University of California, Los Angeles.
“We’re currently working on an analysis of cervicovaginal bacterial species to explore the microbiome in more detail,” Dr. Michel concluded.
Dr. Michel disclosed no conflicts of interest. The study was supported by multiple sources, including the National Institute of Allergy and Infectious Diseases, the National Cancer Institute, and the Georgetown-Howard Universities Center for Clinical and Translational Science.
Elevated vaginal pH at the time of cervical intraepithelial neoplasia 2 diagnosis may be a useful marker of CIN 2 persistence/progression, as well as the rate of persistence/progression in HIV-positive women, new research suggests.
“We analyzed data from the Women’s Interagency HIV Study [WIHS], an observational, longitudinal cohort of women with and without HIV to determine factors that may influence CIN 2 natural history,” said Kate Michel, PhD, MPH, of Georgetown University, Washington. She presented the results at the Conference on Retroviruses and Opportunistic Infections.
As previous data have shown a high incidence of CIN 2 progression among women with HIV, the researchers evaluated the role of human papillomavirus (HPV) type, local immune response, and markers of the cervicovaginal microbiome on the risk of CIN 2 persistence/progression.
Within the cohort, follow-up visits occur every 6 months, and clinical data is collected via questionnaires, physical and gynecologic exams, and biological samples. As no specific treatment is offered in the WIHS, treatment for cervical abnormalities is abstracted from medical records.
In the present study, Dr. Michel and colleagues selected up to four banked cervicovaginal lavage (CVL) samples per woman, with the first sample selected 6-12 months prior to CIN 2 diagnosis, the second at CIN 2 diagnosis, the third between CIN 2 diagnosis and outcome, and the fourth at the outcome visit.
The investigators performed HPV typing and muiltiplex immune mediator testing on each CVL sample. Lab results from WIHS core testing were also extracted, including plasma CD4+ T-cell count and HIV viral load, as well as vaginal pH and Nugent’s score.
Study outcomes included persistence/progression and regression, defined as a subsequent CIN 2 or CIN 3 diagnosis and subsequent CIN 1 or normal diagnosis, respectively. Logistic regression models were used to determine CIN 2 regression versus persistence/progression.
Results
A total of 337 samples were obtained and 94 women were included in the analysis. Key demographic and behavioral factor were similar at CIN 2 diagnosis.
The majority of participants were African American (53.2%) and on antiretroviral therapy (66.0%). The most prevalent high-risk types were HPV-58 (18.4%) and HPV-16 (17.5%).
After a median 12.5 years of follow-up, 33 participants (35.1%) with incident CIN 2 had a subsequent CIN 2/CIN 3 diagnosis and those who regressed had a higher CD4 T-cell count at CIN 2 diagnosis (P = .02).
Each subsequent high-risk HPV type identified at the pre–CIN 2 visit was associated with higher odds of CIN2 persistence/progression (odds ratio, 2.27; 95% confidence interval, 1.15-4.50).
Bacterial vaginosis (adjusted OR, 5.08; 95% CI, 1.30-19.94) and vaginal pH (aOR, 2.27; 95% CI, 1.15-4.50) at the CIN 2 diagnosis visit were each associated with increased odds of CIN 2 persistence/progression.
Vaginal pH greater than 4.5 at CIN 2 diagnosis was also associated with unadjusted time to CIN 2 persistence/progression (log rank P = .002) and an increased rate of CIN 2 persistence/progression (adjusted hazard ratio, 3.37; 95% CI, 1.26-8.99).
Furthermore, among participants who did not receive CIN 2 treatment, vaginal pH remained associated with greater odds of CIN 2 persistence/progression (OR, 2.46; 95% CI, 1.19-5.13). Cervicovaginal immune mediator levels were not associated with CIN 2 persistence/progression.
“The most striking finding from this work was that vaginal pH was associated with higher odds of, quicker time to, and increased hazard of CIN 2 persistence/progression,” Dr. Michel said. “We postulate this effect is mediated by the cervical microbiome, but more work is needed to establish the exact mechanism.”
“It would be interesting to test whether this association might be explained by different vaginal cleaning techniques, such as douching,” said moderator Ronald T. Mitsuyasu, MD, of the University of California, Los Angeles.
“We’re currently working on an analysis of cervicovaginal bacterial species to explore the microbiome in more detail,” Dr. Michel concluded.
Dr. Michel disclosed no conflicts of interest. The study was supported by multiple sources, including the National Institute of Allergy and Infectious Diseases, the National Cancer Institute, and the Georgetown-Howard Universities Center for Clinical and Translational Science.
FROM CROI 2021
mCODE: Improving data sharing to enhance cancer care
An initiative designed to improve sharing of patient data may provide “tremendous benefits” in cancer care and research, according to authors of a review article.
The goals of the initiative, called Minimal Common Oncology Data Elements (mCODE), were to identify the data elements in electronic health records that are “essential” for making treatment decisions and create “a standardized computable data format” that would improve the exchange of data across EHRs, according to the mCODE website.
Travis J. Osterman, DO, of Vanderbilt University Medical Center in Nashville, Tenn., and colleagues described the mCODE initiative in a review published in JCO Clinical Cancer Informatics.
At present, commercially available EHRs are poorly designed to support modern oncology workflow, requiring laborious data entry and lacking a common library of oncology-specific discrete data elements. As an example, most EHRs poorly support the needs of precision oncology and clinical genetics, since next-generation sequencing and genetic test results are almost universally reported in PDF files.
In addition, basic, operational oncology data (e.g., cancer staging, adverse event documentation, response to treatment, etc.) are captured in EHRs primarily as an unstructured narrative.
Computable, analytical data are found for only the small percentage of patients in clinical trials. Even then, some degree of manual data abstraction is regularly required.
Interoperability of EHRs between practices and health care institutions is often so poor that the transfer of basic cancer-related information as analyzable data is difficult or even impossible.
Making progress: The 21st Century Cures Act
The American Society of Clinical Oncology has a more than 15-year history of developing oncology data standards. Unfortunately, progress in implementing these standards has been glacially slow. Impediments have included:
- A lack of conformance with clinical workflows.
- Failure to test standards on specific-use cases during pilot testing.
- A focus on data exchange, rather than the practical impediments to data entry.
- Poor engagement with EHR vendors in distributing clinical information modules with an oncology-specific focus
- Instability of data interoperability technologies.
The 21st Century Cures Act, which became law in December 2016, mandated improvement in the interoperability of health information through the development of data standards and application programming interfaces.
In early 2020, final rules for implementation required technology vendors to employ application programming interfaces using a single interoperability resource. In addition, payers were required to use the United States Core Data for Interoperability Standard for data exchange. These requirements were intended to provide patients with access to their own health care data “without special effort.”
As a fortunate byproduct, since EHR vendors are required to implement application program interfaces using the Health Level Seven International (HL7) Fast Healthcare Interoperability Resource (FHIR) Specification, the final rules could enable systems like mCODE to be more easily integrated with existing EHRs.
Lessons from CancerLinQ
ASCO created the health technology platform CancerLinQ in 2014, envisioning that it could become an oncology-focused learning health system – a system in which internal data and experience are systematically integrated with external evidence, allowing knowledge to be put into practice.
CancerLinQ extracts data from EHRs and other sources via direct software connections. CancerLinQ then aggregates, harmonizes, and normalizes the data in a cloud-based environment.
The data are available to participating practices for quality improvement in patient care and secondary research. In 2020, records of cancer patients in the CancerLinQ database surpassed 2 million.
CancerLinQ has been successful. However, because of the nature of the EHR ecosystem and the scope and variability of data capture by clinicians, supporting a true learning health system has proven to be a formidable task. Postprocessing manual review using trained human curators is laborious and unsustainable.
The CancerLinQ experience illustrated that basic cancer-pertinent data should be standardized in the EHR and collected prospectively.
The mCODE model
The mCODE initiative seeks to facilitate progress in care quality, clinical research, and health care policy by developing and maintaining a standard, computable, interoperable data format.
Guiding principles that were adopted early in mCODE’s development included:
- A collaborative, noncommercial, use case–driven developmental model.
- Iterative processes.
- User-driven development, refinement, and maintenance.
- Low ongoing maintenance requirements.
A foundational moment in mCODE’s development involved achieving consensus among stakeholders that the project would fail if EHR vendors required additional data entry by users.
After pilot work, a real-world endpoints project, working-group deliberation, public comment, and refinement, the final data standard included six primary domains: patient, disease, laboratory data/vital signs, genomics, treatment, and outcome.
Each domain is further divided into several concepts with specific associated data elements. The data elements are modeled into value sets that specify the possible values for the data element.
To test mCODE, eight organizations representing oncology EHR vendors, standards developers, and research organizations participated in a cancer interoperability track. The comments helped refine mCODE version 1.0, which was released in March 2020 and is accessible via the mCODE website.
Additions will likely be reviewed by a technical review group after external piloting of new use cases.
Innovation, not regulation
Every interaction between a patient and care provider yields information that could lead to improved safety and better outcomes. To be successful, the information must be collected in a computable format so it can be aggregated with data from other patients, analyzed without manual curation, and shared through interoperable systems. Those data should also be secure enough to protect the privacy of individual patients.
mCODE is a consensus data standard for oncology that provides an infrastructure to share patient data between oncology practices and health care systems while promising little to no additional data entry on the part of clinicians. Adoption by sites will be critical, however.
Publishing the standard through the HL7 FHIR technology demonstrated to EHR vendors and regulatory agencies the stability of HL7, an essential requirement for its incorporation into software.
EHR vendors and others are engaged in the CodeX HL7 FHIR Accelerator to design projects to expand and/or modify mCODE. Their creativity and innovativeness via the external advisory mCODE council and/or CodeX will be encouraged to help mCODE reach its full potential.
As part of CodeX, the Community of Practice, an open forum for end users, was established to provide regular updates about mCODE-related initiatives and use cases to solicit in-progress input, according to Robert S. Miller, MD, medical director of CancerLinQ and an author of the mCODE review.
For mCODE to be embraced by all stakeholders, there should be no additional regulations. By engaging stakeholders in an enterprise that supports innovation and collaboration – without additional regulation – mCODE could maximize the potential of EHRs that, until now, have assisted us only marginally in accomplishing those goals.
mCODE is a joint venture of ASCO/CancerLinQ, the Alliance for Clinical Trials in Oncology Foundation, the MITRE Corporation, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
Dr. Osterman disclosed a grant from the National Cancer Institute and relationships with Infostratix, eHealth, AstraZeneca, Outcomes Insights, Biodesix, MD Outlook, GenomOncology, Cota Healthcare, GE Healthcare, and Microsoft. Dr. Miller and the third review author disclosed no conflicts of interest.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
An initiative designed to improve sharing of patient data may provide “tremendous benefits” in cancer care and research, according to authors of a review article.
The goals of the initiative, called Minimal Common Oncology Data Elements (mCODE), were to identify the data elements in electronic health records that are “essential” for making treatment decisions and create “a standardized computable data format” that would improve the exchange of data across EHRs, according to the mCODE website.
Travis J. Osterman, DO, of Vanderbilt University Medical Center in Nashville, Tenn., and colleagues described the mCODE initiative in a review published in JCO Clinical Cancer Informatics.
At present, commercially available EHRs are poorly designed to support modern oncology workflow, requiring laborious data entry and lacking a common library of oncology-specific discrete data elements. As an example, most EHRs poorly support the needs of precision oncology and clinical genetics, since next-generation sequencing and genetic test results are almost universally reported in PDF files.
In addition, basic, operational oncology data (e.g., cancer staging, adverse event documentation, response to treatment, etc.) are captured in EHRs primarily as an unstructured narrative.
Computable, analytical data are found for only the small percentage of patients in clinical trials. Even then, some degree of manual data abstraction is regularly required.
Interoperability of EHRs between practices and health care institutions is often so poor that the transfer of basic cancer-related information as analyzable data is difficult or even impossible.
Making progress: The 21st Century Cures Act
The American Society of Clinical Oncology has a more than 15-year history of developing oncology data standards. Unfortunately, progress in implementing these standards has been glacially slow. Impediments have included:
- A lack of conformance with clinical workflows.
- Failure to test standards on specific-use cases during pilot testing.
- A focus on data exchange, rather than the practical impediments to data entry.
- Poor engagement with EHR vendors in distributing clinical information modules with an oncology-specific focus
- Instability of data interoperability technologies.
The 21st Century Cures Act, which became law in December 2016, mandated improvement in the interoperability of health information through the development of data standards and application programming interfaces.
In early 2020, final rules for implementation required technology vendors to employ application programming interfaces using a single interoperability resource. In addition, payers were required to use the United States Core Data for Interoperability Standard for data exchange. These requirements were intended to provide patients with access to their own health care data “without special effort.”
As a fortunate byproduct, since EHR vendors are required to implement application program interfaces using the Health Level Seven International (HL7) Fast Healthcare Interoperability Resource (FHIR) Specification, the final rules could enable systems like mCODE to be more easily integrated with existing EHRs.
Lessons from CancerLinQ
ASCO created the health technology platform CancerLinQ in 2014, envisioning that it could become an oncology-focused learning health system – a system in which internal data and experience are systematically integrated with external evidence, allowing knowledge to be put into practice.
CancerLinQ extracts data from EHRs and other sources via direct software connections. CancerLinQ then aggregates, harmonizes, and normalizes the data in a cloud-based environment.
The data are available to participating practices for quality improvement in patient care and secondary research. In 2020, records of cancer patients in the CancerLinQ database surpassed 2 million.
CancerLinQ has been successful. However, because of the nature of the EHR ecosystem and the scope and variability of data capture by clinicians, supporting a true learning health system has proven to be a formidable task. Postprocessing manual review using trained human curators is laborious and unsustainable.
The CancerLinQ experience illustrated that basic cancer-pertinent data should be standardized in the EHR and collected prospectively.
The mCODE model
The mCODE initiative seeks to facilitate progress in care quality, clinical research, and health care policy by developing and maintaining a standard, computable, interoperable data format.
Guiding principles that were adopted early in mCODE’s development included:
- A collaborative, noncommercial, use case–driven developmental model.
- Iterative processes.
- User-driven development, refinement, and maintenance.
- Low ongoing maintenance requirements.
A foundational moment in mCODE’s development involved achieving consensus among stakeholders that the project would fail if EHR vendors required additional data entry by users.
After pilot work, a real-world endpoints project, working-group deliberation, public comment, and refinement, the final data standard included six primary domains: patient, disease, laboratory data/vital signs, genomics, treatment, and outcome.
Each domain is further divided into several concepts with specific associated data elements. The data elements are modeled into value sets that specify the possible values for the data element.
To test mCODE, eight organizations representing oncology EHR vendors, standards developers, and research organizations participated in a cancer interoperability track. The comments helped refine mCODE version 1.0, which was released in March 2020 and is accessible via the mCODE website.
Additions will likely be reviewed by a technical review group after external piloting of new use cases.
Innovation, not regulation
Every interaction between a patient and care provider yields information that could lead to improved safety and better outcomes. To be successful, the information must be collected in a computable format so it can be aggregated with data from other patients, analyzed without manual curation, and shared through interoperable systems. Those data should also be secure enough to protect the privacy of individual patients.
mCODE is a consensus data standard for oncology that provides an infrastructure to share patient data between oncology practices and health care systems while promising little to no additional data entry on the part of clinicians. Adoption by sites will be critical, however.
Publishing the standard through the HL7 FHIR technology demonstrated to EHR vendors and regulatory agencies the stability of HL7, an essential requirement for its incorporation into software.
EHR vendors and others are engaged in the CodeX HL7 FHIR Accelerator to design projects to expand and/or modify mCODE. Their creativity and innovativeness via the external advisory mCODE council and/or CodeX will be encouraged to help mCODE reach its full potential.
As part of CodeX, the Community of Practice, an open forum for end users, was established to provide regular updates about mCODE-related initiatives and use cases to solicit in-progress input, according to Robert S. Miller, MD, medical director of CancerLinQ and an author of the mCODE review.
For mCODE to be embraced by all stakeholders, there should be no additional regulations. By engaging stakeholders in an enterprise that supports innovation and collaboration – without additional regulation – mCODE could maximize the potential of EHRs that, until now, have assisted us only marginally in accomplishing those goals.
mCODE is a joint venture of ASCO/CancerLinQ, the Alliance for Clinical Trials in Oncology Foundation, the MITRE Corporation, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
Dr. Osterman disclosed a grant from the National Cancer Institute and relationships with Infostratix, eHealth, AstraZeneca, Outcomes Insights, Biodesix, MD Outlook, GenomOncology, Cota Healthcare, GE Healthcare, and Microsoft. Dr. Miller and the third review author disclosed no conflicts of interest.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
An initiative designed to improve sharing of patient data may provide “tremendous benefits” in cancer care and research, according to authors of a review article.
The goals of the initiative, called Minimal Common Oncology Data Elements (mCODE), were to identify the data elements in electronic health records that are “essential” for making treatment decisions and create “a standardized computable data format” that would improve the exchange of data across EHRs, according to the mCODE website.
Travis J. Osterman, DO, of Vanderbilt University Medical Center in Nashville, Tenn., and colleagues described the mCODE initiative in a review published in JCO Clinical Cancer Informatics.
At present, commercially available EHRs are poorly designed to support modern oncology workflow, requiring laborious data entry and lacking a common library of oncology-specific discrete data elements. As an example, most EHRs poorly support the needs of precision oncology and clinical genetics, since next-generation sequencing and genetic test results are almost universally reported in PDF files.
In addition, basic, operational oncology data (e.g., cancer staging, adverse event documentation, response to treatment, etc.) are captured in EHRs primarily as an unstructured narrative.
Computable, analytical data are found for only the small percentage of patients in clinical trials. Even then, some degree of manual data abstraction is regularly required.
Interoperability of EHRs between practices and health care institutions is often so poor that the transfer of basic cancer-related information as analyzable data is difficult or even impossible.
Making progress: The 21st Century Cures Act
The American Society of Clinical Oncology has a more than 15-year history of developing oncology data standards. Unfortunately, progress in implementing these standards has been glacially slow. Impediments have included:
- A lack of conformance with clinical workflows.
- Failure to test standards on specific-use cases during pilot testing.
- A focus on data exchange, rather than the practical impediments to data entry.
- Poor engagement with EHR vendors in distributing clinical information modules with an oncology-specific focus
- Instability of data interoperability technologies.
The 21st Century Cures Act, which became law in December 2016, mandated improvement in the interoperability of health information through the development of data standards and application programming interfaces.
In early 2020, final rules for implementation required technology vendors to employ application programming interfaces using a single interoperability resource. In addition, payers were required to use the United States Core Data for Interoperability Standard for data exchange. These requirements were intended to provide patients with access to their own health care data “without special effort.”
As a fortunate byproduct, since EHR vendors are required to implement application program interfaces using the Health Level Seven International (HL7) Fast Healthcare Interoperability Resource (FHIR) Specification, the final rules could enable systems like mCODE to be more easily integrated with existing EHRs.
Lessons from CancerLinQ
ASCO created the health technology platform CancerLinQ in 2014, envisioning that it could become an oncology-focused learning health system – a system in which internal data and experience are systematically integrated with external evidence, allowing knowledge to be put into practice.
CancerLinQ extracts data from EHRs and other sources via direct software connections. CancerLinQ then aggregates, harmonizes, and normalizes the data in a cloud-based environment.
The data are available to participating practices for quality improvement in patient care and secondary research. In 2020, records of cancer patients in the CancerLinQ database surpassed 2 million.
CancerLinQ has been successful. However, because of the nature of the EHR ecosystem and the scope and variability of data capture by clinicians, supporting a true learning health system has proven to be a formidable task. Postprocessing manual review using trained human curators is laborious and unsustainable.
The CancerLinQ experience illustrated that basic cancer-pertinent data should be standardized in the EHR and collected prospectively.
The mCODE model
The mCODE initiative seeks to facilitate progress in care quality, clinical research, and health care policy by developing and maintaining a standard, computable, interoperable data format.
Guiding principles that were adopted early in mCODE’s development included:
- A collaborative, noncommercial, use case–driven developmental model.
- Iterative processes.
- User-driven development, refinement, and maintenance.
- Low ongoing maintenance requirements.
A foundational moment in mCODE’s development involved achieving consensus among stakeholders that the project would fail if EHR vendors required additional data entry by users.
After pilot work, a real-world endpoints project, working-group deliberation, public comment, and refinement, the final data standard included six primary domains: patient, disease, laboratory data/vital signs, genomics, treatment, and outcome.
Each domain is further divided into several concepts with specific associated data elements. The data elements are modeled into value sets that specify the possible values for the data element.
To test mCODE, eight organizations representing oncology EHR vendors, standards developers, and research organizations participated in a cancer interoperability track. The comments helped refine mCODE version 1.0, which was released in March 2020 and is accessible via the mCODE website.
Additions will likely be reviewed by a technical review group after external piloting of new use cases.
Innovation, not regulation
Every interaction between a patient and care provider yields information that could lead to improved safety and better outcomes. To be successful, the information must be collected in a computable format so it can be aggregated with data from other patients, analyzed without manual curation, and shared through interoperable systems. Those data should also be secure enough to protect the privacy of individual patients.
mCODE is a consensus data standard for oncology that provides an infrastructure to share patient data between oncology practices and health care systems while promising little to no additional data entry on the part of clinicians. Adoption by sites will be critical, however.
Publishing the standard through the HL7 FHIR technology demonstrated to EHR vendors and regulatory agencies the stability of HL7, an essential requirement for its incorporation into software.
EHR vendors and others are engaged in the CodeX HL7 FHIR Accelerator to design projects to expand and/or modify mCODE. Their creativity and innovativeness via the external advisory mCODE council and/or CodeX will be encouraged to help mCODE reach its full potential.
As part of CodeX, the Community of Practice, an open forum for end users, was established to provide regular updates about mCODE-related initiatives and use cases to solicit in-progress input, according to Robert S. Miller, MD, medical director of CancerLinQ and an author of the mCODE review.
For mCODE to be embraced by all stakeholders, there should be no additional regulations. By engaging stakeholders in an enterprise that supports innovation and collaboration – without additional regulation – mCODE could maximize the potential of EHRs that, until now, have assisted us only marginally in accomplishing those goals.
mCODE is a joint venture of ASCO/CancerLinQ, the Alliance for Clinical Trials in Oncology Foundation, the MITRE Corporation, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
Dr. Osterman disclosed a grant from the National Cancer Institute and relationships with Infostratix, eHealth, AstraZeneca, Outcomes Insights, Biodesix, MD Outlook, GenomOncology, Cota Healthcare, GE Healthcare, and Microsoft. Dr. Miller and the third review author disclosed no conflicts of interest.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
FROM JCO CLINICAL CANCER INFORMATICS
Is there liability if you don’t test for BRCA?
CASE Young woman with family history of breast cancer detects lump
Two weeks after noting a lump on her breast when her cat happened to jump on her in that spot, a 28-year-old woman (G0) went to her primary care provider. She was referred to her gynecologist; breast imaging, ultrasonography, and mammography were obtained, with microcalcifications noted. A fine needle aspiration diagnosed intraductal malignancy. The surgical breast tissue specimen was estrogen receptor (ER)- and progestogen receptor (PR)-positive and HER2-negative. Other tumor markers were obtained, including carcinoembryonic antigen, and tissue polypeptide specific antigen, p53, cathepsin D, cyclin E, and nestin, but results were not available.
With regard to family history, the woman’s mother and maternal grandmother had a history of breast cancer. The patient and her family underwent gene testing. The patient was found to be BRCA1- and BRCA2-positive; her mother was BRCA1-positive, an older sister was BRCA2-positive, and her grandmother was not tested.
The question arose in light of her family history as to why she was not tested for BRCA and appropriately counseled by her gynecologist prior to the cancer diagnosis. Litigation was initiated. While the case did not go forward regarding litigation, it is indeed a case in point. (Please note that this is a hypothetical case. It is based on a composite of several cases.)
Medical considerations
Breast cancer is the most common type of cancer affecting women in the Western world.1 Advances in clinical testing for gene mutations have escalated and allowed for identification of patients at increased risk for breast and ovarian cancer. Along with these advances come professional liability risk. After looking at the medical considerations for BRCA1 and 2 testing, we will consider a number of important legal issues. In the view of some commentators, the failure to diagnose genetic mutations in patients predisposed to cancer is “poised to become the next wave of medical professional liability lawsuits.”2
BRCA1 and BRCA2 genes provide tumor suppressor proteins, and assessment for mutations is recommended for individuals at high risk for breast and/or ovarian cancer; mutations in BRCA genes cause DNA damage, which increases the chance of developing cancer. The other way to look at it is, BRCA1 and 2 are tumor suppressor genes that are integrally involved with DNA damage control. Once there is a mutation, it adversely affects the beneficial effects of the gene. Mutations in these genes account for 5% to 10% of all hereditary breast cancers.3 Of note, men with BRCA2 are at increased risk for prostate cancer.
A patient who presents to her gynecologist stating that there is a family history of breast cancer, without knowledge of genetic components, presents a challenge (and a medicolegal risk) for the provider to assess. Prediction models have been used to determine specific patient risk for carrying a genetic mutation with resultant breast cancer development.4 Risk prediction models do not appear to be a good answer to predicting who is more likely to develop breast or ovarian cancer, however. A Mayo model may assist (FIGURE).5 Clinicians should also be aware of other models of risk assessment, including the Gail Model (TABLE 1).6
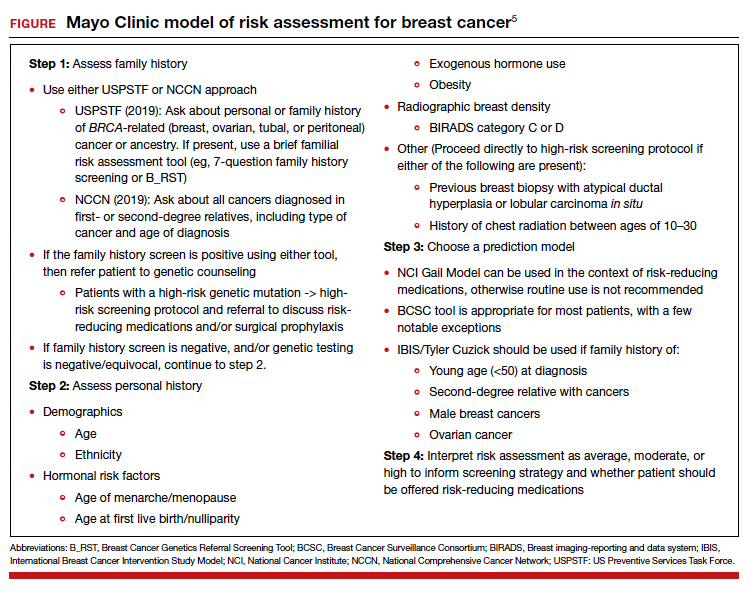
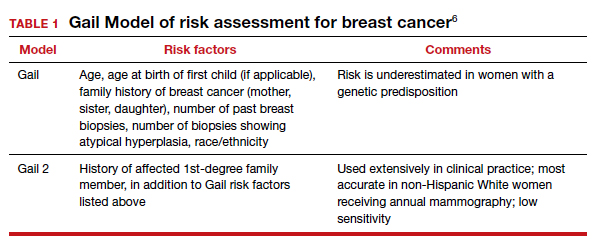
Continue to: Guidelines for genetic testing...
Guidelines for genetic testing
The American College of Obstetricians and Gynecologists states that patient medical history and family history are paramount in obtaining information regarding risk for breast and ovarian cancer. First- and second-degree relatives are allocated to this category. Information regarding age of diagnosis, maternal and paternal lineage, and ethnic background can imply a need for genetic testing (TABLE 2).7,8 A number of genetics national organizations have participated in recommendations and include the American College of Medical Genetics and Genomics, the National Society for Genetic Counselors, and the Society of Gynecologic Oncology.7
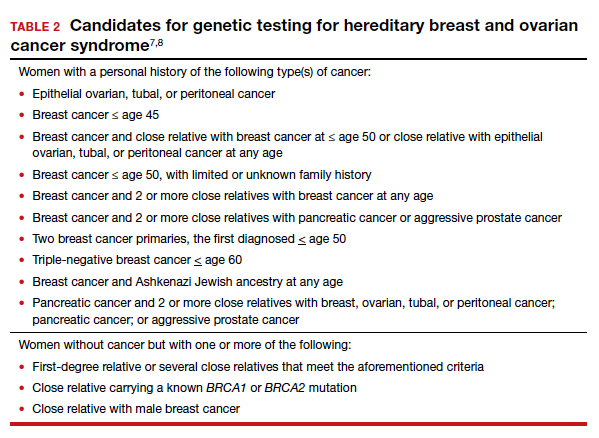
The question always surfaces, could the clinical outcome of the cancer when diagnosed have been changed if screening were undertaken, with earlier diagnosis, or prevented with prophylactic mastectomy, and changed the end result. In addition, it is well known that breast augmentation mammoplasty alters the ability to accurately evaluate mammograms. Patients considering this type of plastic surgery, ideally, should be counselled accordingly.9
Bottom line, we as clinicians must be cognizant of both ACOG and United States Preventive Services Task Force (USPSTF) recommendations regarding screening and gene testing for women considered high risk for breast cancer based on family history.7
Legal considerations
The case presented demonstrates that the discovery of the BRCA1 and BRCA2 genes, and reliable tests for determining the existence of the genes, brought with them legal issues as well as medical advantages. We look at professional liability (malpractice) questions this technology raises, and then consider the outcome of the hypothetical case. (BRCA is used here to apply broadly—not only to BRCA1 and 2 but also to PALB2, CHEK2, and similar genetic abnormalities.)
To date, the most visible BRCA legal issues covered in cases and law reviews have focused more on patent law than malpractice. The most important of these was a decision of the US Supreme Court in Association for Molecular Pathology v Myriad Genetics.10 The US Patent Office was granting patents to companies finding useful, naturally occurring segments of human DNA, and had granted Myriad several patents on BRCA1 and BRCA2 genes. This patent policy had the potential to seriously interfere with broad scientific use of these genes.11 Fortunately, the Supreme Court stepped in and unanimously invalidated such patents. It held that a “naturally occurring DNA segment is a product of nature and not patent eligible merely because it has been isolated.” The Court noted, “Finding the location of the BRCA1 and BRCA2 genes does not render the genes patent eligible ‘new . . . composition[s] of matter.’”8 The Court did allow the patenting of tests for specific gene structures, and artificial changes in naturally occurring genes.
Malpractice and BRCA
While the BRCA patent wars have lingered, the potential for a significant increase in BRCA-related malpractice cases is of increasing concern. Like most malpractice liability, these new claims are based on very old principles of negligence.12 To prevail, the plaintiff (ordinarily, an injured patient) must demonstrate 4 things:
- A duty. That is, the physician owed a duty to the injured party. Usually (but not always) that requires a professional relationship between the physician and the person injured.
- A breach of that duty. Malpractice liability is based on the fact that the physician did something that a reasonably careful physician (generally, of the same specialty) would not have done, or that the physician failed to do something that a reasonable physician would have done. This usually means that the profession itself sees what the physician did (or did not do) as medically inappropriate. In medical malpractice cases, that is ordinarily measured by what the usual or common practice is among prudent physicians. In rare circumstances, courts have found the standard practice of a profession to be negligent. Where, for example, it was custom for a professional not to give an eye pressure test to anyone under age 40, a court found that common standard to be inappropriate.13 In the words of Judge Learned Hand (speaking about a different case), “a whole calling may have unduly lagged in the adoption of new and available devices. It never may set its own tests.”14 Underlying negligence is a cost-benefit analysis (discussed below).
- Damages. There must have been some damage that courts recognize, usually loss of money or opportunity to work, the cost of care, pain and suffering, or loss of enjoyment/quality of life. In malpractice, many states now recognize the “loss of chance” or the “loss of a chance.” That means, if a “physician negligently fails to diagnose a curable disease, and the patient is harmed by the disease, the physician should be liable for causing the ‘loss of a chance of a cure.’”15 (Delay in diagnosis is the most common reason for claims in breast cancer care.)16
- Causation. The breach of duty (negligence) must have caused the damages. The causation must have been reasonably close. If a driver drives through a stop sign, or a physician misreads a test, and someone is injured but there is no connection between the negligence and the injury, there is not tort liability.
The 4 elements of malpractice just described are raised in some way in the possible liability associated with BRCA testing. We next look at the ways in which liability may arise from that testing (or lack of it).
Underlying much of the following discussion is the “cost-benefit” consideration noted above. This concept is that the total cost (financial and health) of testing should be compared with the value of the benefits of testing, taking into account the probabilities that the testing will result in better health outcomes. BRCA testing, for example, is essentially cost-free in terms of physical risk. Its financial cost, while not trivial, is not great, and it is commonly covered by health insurance.17 In terms of benefits, the testing has the potential for providing critical information in making treatment decisions for a meaningful percentage of patients and their families. There are many ways of analyzing the liability risks of genetic malpractice,7,18 and the following is intended to discuss some of the greatest risks related to BRCA testing.
Continue to: Areas of liability...
Areas of liability
The failure to recommend a test. The circumstances in which BRCA testing should be undertaken are set out by professional organizations (noted above). These recommendations are not static, however. They change from time to time. Given the potential harm caused by the failure to test in relevant circumstances, malpractice liability is certainly a possibility when the failure to recommend a test to a patient results in a cancer that might have been prevented had the genetic problem been identified in a timely manner. The circumstances in which testing should be considered continue to change, placing an obligation on clinicians to stay well informed of changing genetic understandings. Another risk is that one specialist may assume that it is the job of another specialist to order the test. Whatever the cause of the failure to test, or unnecessary delay in testing, it appears to be the primary basis for BRCA liability.
The failure to properly interpret a test. Any test that is misinterpreted may lead to harm for the patient. A false negative, of course, may mean that preventive treatment that could have been undertaken will be foregone, as a “loss of a chance.” On the other hand, a false positive can lead to radical, unnecessary surgery or treatment. If a misinterpretation occurred because of carelessness by the testing organization, or confusion by a practitioner, there is a likelihood of negligence.19
A different form of “misinterpretation” could be reasonable—and not negligent. Advances in scientific-medical understanding may result in the outcome of tests being reconsidered and changed. That has been the case with genetic testing and breast cancer. The availability of multiple breast cancer SNPs (single nucleotide polymorphisms), and combining this information with other risk factors for example, results in a polygenic risk score that may be at odds with the level of risk from earlier testing.20,21 This naturally leads to the question of when later, updated testing should be recommended to look for a better current interpretation.22,23
The failure to act on BRCA test results. Testing is of no value, of course, if the results are not used properly. Test results or analyses that are not sent to the proper physicians, or are somehow ignored when properly directed, is a “never” event—it should never happen. It almost always would be considered negligence, and if the patient were injured, could lead to liability. Amazingly, one study found that, in genetic testing liability cases, nearly 20% of the claims arose from failure to return test results to patients.24 In addition, when a patient is found to be BRCA-positive, there is an obligation to discuss the options for dealing with the increased risk associated with the gene mutation(s), as well as to recommend the prudent course of action or to refer the patient to someone who will have that discussion.
Informed consent to the patient. BRCA testing requires informed consent. The physical risks of the testing process are minimal, of course, but it carries a number of other emotional and family risks. The informed consent process is an invitation to an honest discussion between clinicians and patients. It should be an opportunity to discuss what the testing is, and is not, and what the test may mean for treatment. It may also be an opportunity to discuss the implications for other members of the patient’s family (noted below).
One element of informed consent is a discussion of the consequences of failure to consent, or to undertake one of the alternatives. In the case of BRCA testing, this is especially important in cases in which a patient expresses a hesitancy to be tested with an “I’d rather not know philosophy.” Although clinicians should not practice law, some patient concerns about discrimination may be addressed by the protection that the federal Genetic Information Nondiscrimination Act (GINA) and other laws provide (which prohibit insurance and employment discrimination based on genetic information). A good source of information about GINA and related nondiscrimination laws is provided by the National Human Genome Research Institute.25 In addition, the National Institutes of Health has a website that may be helpful to many patients26 (and a much more complex site for health professionals).27 At the same time, courts have resisted plaintiffs/patients who have tried to use informed consent as a way of suing for failure to offer genetic testing.28,29
The failure to refer. In some cases, a patient should be formally referred for genetics consultation. The considerations here are similar to other circumstances in modern, fast developing medical practice that require special sensitivity to those occasions in which a patient will benefit from additional expertise. It is a principle that the AMA Council on Ethical and Judicial Affairs has expressed this way: “In the absence of adequate expertise in pretest and posttest counseling, a physician should refer the patient to an appropriate specialist.”30 The failure to refer, when that deviates from acceptable practice, may result in liability.
Informing others. BRCA testing is an area of medicine in which results may be of great significance not only to the patient but also to the patient’s family.31 Physicians should counsel patients on the importance of informing relatives about relevant results and “should make themselves available to assist patients in communicating with relatives to discuss opportunities for counseling and testing, as appropriate.”30 The question may arise, however, of whether in some circumstances physicians should go a step further in ensuring relatives receive important information regarding their loved one’s health.32 The law has been reluctant to impose liability to “third parties” (someone not a patient). Duties usually arise through the physician-patient relationship. There are exceptions. Perhaps the best known has been the obligation of mental health professionals to take action to protect third parties from patients who have made believable threats against identifiable victims.33 There are indications that some courts could find, in extreme circumstances, a “duty to warn” nonpatients in some instances where it is essential to inform third parties that they should receive a specific form of genetic testing.34,35 Such a duty would, of course, have to protect the privacy rights of the patient to the maximum extent possible. A general duty of this type has not been established widely, but may be part of the future.
Continue to: Was there liability in our example case?...
Was there liability in our example case?
The hypothetical case provided above suggests that there could be liability. Routine medical history by the primary care physician would have produced the fact that the patient’s mother, sister, and maternal grandmother had breast cancer. That would clearly have put her in a category of those who should have received genetic testing. Yet, she was not tested until after her cancer was found. From the limited facts we have, it appears that this timeline of events would have been outside accepted practice—and negligent. The case was not pursued by the patient, however, and this may represent the current state of liability for BRCA issues.
The extent of liability seems to be significant
Our discussion of liability suggests that there is significant potential for BRCA testing negligence within practice, and that the damages in these cases could be substantial. Yet the predicted “tsunami” of malpractice lawsuits related to genetic testing has not appeared.36,37 One study of cases in the United States (through 2016) found a “slowly rising tide” of liability cases instead of a tsunami,24 as the number of claims made was low. On the other hand, the payments where damages were awarded were an order of magnitude larger than other malpractice cases—a mean of $5.3 million and median of $2 million. This is compared with mean values in the range of $275,000 to $600,000 in other areas of malpractice.
The majority of the genetic malpractice cases involve prenatal and newborn testing, and diagnosis/susceptibility/pharmacogenomic accounting for about 25% of cases. In terms of type of errors claimed, approximately 50% were diagnostic-interpretation errors, 30% failure to offer testing, nearly 20% failure to return test results to the patients, and a few remaining cases of failure to properly treat in light of genetic testing.24
Despite a few very large payments, however, the fact remains that there is a surprisingly low number of genetics malpractice cases. Gary Marchant and colleagues suggest that several reasons may account for this:
- the clinical implementation of genetic science has been slower than expected
- the lack of expertise of many physicians in genetic science
- expert witnesses have sometimes been hard to find
- the lack of understanding by plaintiffs’ attorneys of genetic malpractice
- potential plaintiffs’ lack of understanding of the nature of genetic testing and the harms resulting from genetic negligence.17,24,37
The tide is slowly coming in
By all appearances, there is every reason to think that genetic malpractice will be increasing, and that the recent past of much higher damages per claim paid in the genetics area will be part of that tide. The National Human Genome Research LawSeq project has suggested a number of useful ways of dealing with the liability issues.18 In addition to the BRCA issues that we have considered in this article for ObGyns, there are other critical issues of prenatal and newborn genetic testing.38 But those are topics for another day. ●
- Sevilla C, Moatti JP, Reynier CJ, et al. Testing for BRCA1 mutations: a cost-effective analysis. Europ J Human Genetics. 2002;10:599-606.
- Cotton V, Kirkpatrick D. Failure to recommend genetic counseling in breast cancer: is the next wave of medical professional liability lawsuits? Contemp OB/GYN. June 1, 2017.
- Suryavanshi M, Kumar D, Panigrahi M, et al. Detection of false positive mutations in BRCA gene by next generation sequencing. Fam Cancer. 2017;16:311-317.
- Black L, Knoppers B, Avard D, et al. Legal liability and the uncertain nature of risk prediction: the case of breast cancer risk prediction models. Public Health Genomics. 2012;15:335-340.
- McClintock A, Gollab A, Laya M. Breast cancer risk assessment, a step-wise approach for primary care physicians on the front lines of shared decision making. Mayo Clin Proc. 2020;95:1268-1275.
- National Cancer Institute. The Breast Cancer Risk Assessment Tool. https://bcrisktool.cancer.gov/. Accessed February 25, 2021.
- Neff J, Richardson G, Phelps J. Legal liabilities associated with hereditary breast and ovarian cancers. J Reprod Med. 2020;65:227-230.
- American College of Obstetricians and Gynecologists. Practice Bulletin No 182: hereditary breast and ovarian cancer syndrome. Obstet Gynecol. 2017;130:e110-e126.
- Sá dos Reis C, Gremion I, and Meystre NR. Study of breast implants mammography examinations for identification of suitable image quality criteria. Insights Imaging. 2020;11:3.
- Association for Molecular Pathology v Myriad Genetics, 569 U.S. 576 (2013).
- Smith SR. The Supreme Court 2012-2013: dogs, DNA, and DOMA. Register Rep. 2013;39(Fall):26-33.
- Bal BS. An introduction to medical malpractice in the United States. Clin Orthop Relat Res. 2009;467:339-347.
- Helling v Carey, 83 Wn.2d 514, 519 P.2d 981 (1974).
- The T.J. Hooper, 60 F.2d 737, 740 (2d Cir.1932), cert. denied 287 U.S. 662 (1932).
- Fischer DA. Tort recovery for loss of a chance. Wake Forest L Rev. 2001;36:605-655.
- Murphy BL, Ray-Zack MD, Reddy PN, et al. Breast cancer litigation in the 21st century. Ann Surg Oncol. 2018;25:2939- 2947.
- Prince AE. Prevention for those who can pay: insurance reimbursement of genetic-based preventive interventions in the liminal state between health and disease. J Law Biosci. 2015;2:365-395.
- Marchant G, Barnes M, Evans JP, et al; LawSeq Liability Task Force. From genetics to genomics: facing the liability implications in clinical care. J Law Med Ethics. 2020;48:11-43.
- Complaint, Held v Ambry Genetics Corp., No. 15-CV-8683, 2015 WL 6750024 (S.D.N.Y. Nov. 4, 2015); Order of Dismissal, Held v Ambry Genetics Corp., No. 15-CV-8683, (S.D.N.Y. Dec. 6, 2016).
- Pederson HJ. Breast cancer risk assessment and treatment: current concepts in genetics and genomics. Contemp OB/ GYN. 2017; 62:A1-A4.
- Pederson HJ. Who needs breast cancer genetics testing? OBG Manag. 2018;30:34-39.
- Roberts JL, Foulkes A. Genetic duties. William Mary L Rev. 2020;62:143-212.
- Thorogood A, Cook-Deegan R, Knoppers B. Public variant databases: liability? Genet Med. 2017;19:838–841.
- Marchant G, Lindor R. Genomic malpractice: an emerging tide or gentle ripple? Food Drug Law J. 2018;73:1-37.
- National Human Genome Research Institute. Genetic discrimination. https://www.genome.gov/about-genomics /policy-issues/Genetic-Discrimination. Updated September 16, 2020. Accessed February 25, 2021.
- National Cancer Institute. BRCA mutations: cancer risk and genetic testing. https://www.cancer.gov/about-cancer /causes-prevention/genetics/brca-fact-sheet. Reviewed November 19, 2020. Accessed February 25, 2021.
- National Cancer Institute. Genetics of breast and gynecologic cancers (PDQ®)–Health Professional Version. https://www .cancer.gov/types/breast/hp/breast-ovarian-genetics-pdq. Updated February 12, 2021. Accessed February 25, 2021.
- Reed v Campagnolo, 630 A.2d 1145, 1152–54 (Md. 1993).
- Munro v Regents of Univ. of Cal.,263 Cal. Rptr. 878, 885, 988 (1989).
- AMA Council on Ethical and Judicial Affairs. AMA Code of Medical Ethics’ opinions on genetic testing. Opinion 2.131. 2009;11:683-685. https://journalofethics.ama-assn .org/article/ama-code-medical-ethics-opinions-genetictesting/2009-09.
- Gilbar R, Barnoy S. Disclosing genetic test results to the patient’ relatives: how does the law influence clinical practice? J Law Technol Policy. 2019;125-168.
- Song K. Warning third parties of genetic risks in the era of personalized medicine. U.C. Davis L Rev. 2016;49:1987-2018.
- Tarasoff v Regents of the University of California, 551 P.2d 334, 131 Cal. Rptr. 14 (Cal. 1976).
- Safer v Estate of Pack, 677 A.2d 1188 (N.J. App. 1996), cert. denied, 683 A.2d 1163 (N.J. 1996).
- Pate v Threlkel, 661 So.2d 278 (Fla. 1995).
- Rothstein MA. Liability issues in pharmacogenomics. Louisiana L Rev. 2005;66:117-124.
- Marchant G, Lindor R. Personalized medicine and genetic malpractice. Genet Med. 2013;15:921-922.
- Westbrook M. Transforming the physician’s standard of care in the context of whole genome sequencing technologies: finding guidance in best practice standards. Saint Louis U J Health Law Policy. 2015;9:111-148.
CASE Young woman with family history of breast cancer detects lump
Two weeks after noting a lump on her breast when her cat happened to jump on her in that spot, a 28-year-old woman (G0) went to her primary care provider. She was referred to her gynecologist; breast imaging, ultrasonography, and mammography were obtained, with microcalcifications noted. A fine needle aspiration diagnosed intraductal malignancy. The surgical breast tissue specimen was estrogen receptor (ER)- and progestogen receptor (PR)-positive and HER2-negative. Other tumor markers were obtained, including carcinoembryonic antigen, and tissue polypeptide specific antigen, p53, cathepsin D, cyclin E, and nestin, but results were not available.
With regard to family history, the woman’s mother and maternal grandmother had a history of breast cancer. The patient and her family underwent gene testing. The patient was found to be BRCA1- and BRCA2-positive; her mother was BRCA1-positive, an older sister was BRCA2-positive, and her grandmother was not tested.
The question arose in light of her family history as to why she was not tested for BRCA and appropriately counseled by her gynecologist prior to the cancer diagnosis. Litigation was initiated. While the case did not go forward regarding litigation, it is indeed a case in point. (Please note that this is a hypothetical case. It is based on a composite of several cases.)
Medical considerations
Breast cancer is the most common type of cancer affecting women in the Western world.1 Advances in clinical testing for gene mutations have escalated and allowed for identification of patients at increased risk for breast and ovarian cancer. Along with these advances come professional liability risk. After looking at the medical considerations for BRCA1 and 2 testing, we will consider a number of important legal issues. In the view of some commentators, the failure to diagnose genetic mutations in patients predisposed to cancer is “poised to become the next wave of medical professional liability lawsuits.”2
BRCA1 and BRCA2 genes provide tumor suppressor proteins, and assessment for mutations is recommended for individuals at high risk for breast and/or ovarian cancer; mutations in BRCA genes cause DNA damage, which increases the chance of developing cancer. The other way to look at it is, BRCA1 and 2 are tumor suppressor genes that are integrally involved with DNA damage control. Once there is a mutation, it adversely affects the beneficial effects of the gene. Mutations in these genes account for 5% to 10% of all hereditary breast cancers.3 Of note, men with BRCA2 are at increased risk for prostate cancer.
A patient who presents to her gynecologist stating that there is a family history of breast cancer, without knowledge of genetic components, presents a challenge (and a medicolegal risk) for the provider to assess. Prediction models have been used to determine specific patient risk for carrying a genetic mutation with resultant breast cancer development.4 Risk prediction models do not appear to be a good answer to predicting who is more likely to develop breast or ovarian cancer, however. A Mayo model may assist (FIGURE).5 Clinicians should also be aware of other models of risk assessment, including the Gail Model (TABLE 1).6


Continue to: Guidelines for genetic testing...
Guidelines for genetic testing
The American College of Obstetricians and Gynecologists states that patient medical history and family history are paramount in obtaining information regarding risk for breast and ovarian cancer. First- and second-degree relatives are allocated to this category. Information regarding age of diagnosis, maternal and paternal lineage, and ethnic background can imply a need for genetic testing (TABLE 2).7,8 A number of genetics national organizations have participated in recommendations and include the American College of Medical Genetics and Genomics, the National Society for Genetic Counselors, and the Society of Gynecologic Oncology.7

The question always surfaces, could the clinical outcome of the cancer when diagnosed have been changed if screening were undertaken, with earlier diagnosis, or prevented with prophylactic mastectomy, and changed the end result. In addition, it is well known that breast augmentation mammoplasty alters the ability to accurately evaluate mammograms. Patients considering this type of plastic surgery, ideally, should be counselled accordingly.9
Bottom line, we as clinicians must be cognizant of both ACOG and United States Preventive Services Task Force (USPSTF) recommendations regarding screening and gene testing for women considered high risk for breast cancer based on family history.7
Legal considerations
The case presented demonstrates that the discovery of the BRCA1 and BRCA2 genes, and reliable tests for determining the existence of the genes, brought with them legal issues as well as medical advantages. We look at professional liability (malpractice) questions this technology raises, and then consider the outcome of the hypothetical case. (BRCA is used here to apply broadly—not only to BRCA1 and 2 but also to PALB2, CHEK2, and similar genetic abnormalities.)
To date, the most visible BRCA legal issues covered in cases and law reviews have focused more on patent law than malpractice. The most important of these was a decision of the US Supreme Court in Association for Molecular Pathology v Myriad Genetics.10 The US Patent Office was granting patents to companies finding useful, naturally occurring segments of human DNA, and had granted Myriad several patents on BRCA1 and BRCA2 genes. This patent policy had the potential to seriously interfere with broad scientific use of these genes.11 Fortunately, the Supreme Court stepped in and unanimously invalidated such patents. It held that a “naturally occurring DNA segment is a product of nature and not patent eligible merely because it has been isolated.” The Court noted, “Finding the location of the BRCA1 and BRCA2 genes does not render the genes patent eligible ‘new . . . composition[s] of matter.’”8 The Court did allow the patenting of tests for specific gene structures, and artificial changes in naturally occurring genes.
Malpractice and BRCA
While the BRCA patent wars have lingered, the potential for a significant increase in BRCA-related malpractice cases is of increasing concern. Like most malpractice liability, these new claims are based on very old principles of negligence.12 To prevail, the plaintiff (ordinarily, an injured patient) must demonstrate 4 things:
- A duty. That is, the physician owed a duty to the injured party. Usually (but not always) that requires a professional relationship between the physician and the person injured.
- A breach of that duty. Malpractice liability is based on the fact that the physician did something that a reasonably careful physician (generally, of the same specialty) would not have done, or that the physician failed to do something that a reasonable physician would have done. This usually means that the profession itself sees what the physician did (or did not do) as medically inappropriate. In medical malpractice cases, that is ordinarily measured by what the usual or common practice is among prudent physicians. In rare circumstances, courts have found the standard practice of a profession to be negligent. Where, for example, it was custom for a professional not to give an eye pressure test to anyone under age 40, a court found that common standard to be inappropriate.13 In the words of Judge Learned Hand (speaking about a different case), “a whole calling may have unduly lagged in the adoption of new and available devices. It never may set its own tests.”14 Underlying negligence is a cost-benefit analysis (discussed below).
- Damages. There must have been some damage that courts recognize, usually loss of money or opportunity to work, the cost of care, pain and suffering, or loss of enjoyment/quality of life. In malpractice, many states now recognize the “loss of chance” or the “loss of a chance.” That means, if a “physician negligently fails to diagnose a curable disease, and the patient is harmed by the disease, the physician should be liable for causing the ‘loss of a chance of a cure.’”15 (Delay in diagnosis is the most common reason for claims in breast cancer care.)16
- Causation. The breach of duty (negligence) must have caused the damages. The causation must have been reasonably close. If a driver drives through a stop sign, or a physician misreads a test, and someone is injured but there is no connection between the negligence and the injury, there is not tort liability.
The 4 elements of malpractice just described are raised in some way in the possible liability associated with BRCA testing. We next look at the ways in which liability may arise from that testing (or lack of it).
Underlying much of the following discussion is the “cost-benefit” consideration noted above. This concept is that the total cost (financial and health) of testing should be compared with the value of the benefits of testing, taking into account the probabilities that the testing will result in better health outcomes. BRCA testing, for example, is essentially cost-free in terms of physical risk. Its financial cost, while not trivial, is not great, and it is commonly covered by health insurance.17 In terms of benefits, the testing has the potential for providing critical information in making treatment decisions for a meaningful percentage of patients and their families. There are many ways of analyzing the liability risks of genetic malpractice,7,18 and the following is intended to discuss some of the greatest risks related to BRCA testing.
Continue to: Areas of liability...
Areas of liability
The failure to recommend a test. The circumstances in which BRCA testing should be undertaken are set out by professional organizations (noted above). These recommendations are not static, however. They change from time to time. Given the potential harm caused by the failure to test in relevant circumstances, malpractice liability is certainly a possibility when the failure to recommend a test to a patient results in a cancer that might have been prevented had the genetic problem been identified in a timely manner. The circumstances in which testing should be considered continue to change, placing an obligation on clinicians to stay well informed of changing genetic understandings. Another risk is that one specialist may assume that it is the job of another specialist to order the test. Whatever the cause of the failure to test, or unnecessary delay in testing, it appears to be the primary basis for BRCA liability.
The failure to properly interpret a test. Any test that is misinterpreted may lead to harm for the patient. A false negative, of course, may mean that preventive treatment that could have been undertaken will be foregone, as a “loss of a chance.” On the other hand, a false positive can lead to radical, unnecessary surgery or treatment. If a misinterpretation occurred because of carelessness by the testing organization, or confusion by a practitioner, there is a likelihood of negligence.19
A different form of “misinterpretation” could be reasonable—and not negligent. Advances in scientific-medical understanding may result in the outcome of tests being reconsidered and changed. That has been the case with genetic testing and breast cancer. The availability of multiple breast cancer SNPs (single nucleotide polymorphisms), and combining this information with other risk factors for example, results in a polygenic risk score that may be at odds with the level of risk from earlier testing.20,21 This naturally leads to the question of when later, updated testing should be recommended to look for a better current interpretation.22,23
The failure to act on BRCA test results. Testing is of no value, of course, if the results are not used properly. Test results or analyses that are not sent to the proper physicians, or are somehow ignored when properly directed, is a “never” event—it should never happen. It almost always would be considered negligence, and if the patient were injured, could lead to liability. Amazingly, one study found that, in genetic testing liability cases, nearly 20% of the claims arose from failure to return test results to patients.24 In addition, when a patient is found to be BRCA-positive, there is an obligation to discuss the options for dealing with the increased risk associated with the gene mutation(s), as well as to recommend the prudent course of action or to refer the patient to someone who will have that discussion.
Informed consent to the patient. BRCA testing requires informed consent. The physical risks of the testing process are minimal, of course, but it carries a number of other emotional and family risks. The informed consent process is an invitation to an honest discussion between clinicians and patients. It should be an opportunity to discuss what the testing is, and is not, and what the test may mean for treatment. It may also be an opportunity to discuss the implications for other members of the patient’s family (noted below).
One element of informed consent is a discussion of the consequences of failure to consent, or to undertake one of the alternatives. In the case of BRCA testing, this is especially important in cases in which a patient expresses a hesitancy to be tested with an “I’d rather not know philosophy.” Although clinicians should not practice law, some patient concerns about discrimination may be addressed by the protection that the federal Genetic Information Nondiscrimination Act (GINA) and other laws provide (which prohibit insurance and employment discrimination based on genetic information). A good source of information about GINA and related nondiscrimination laws is provided by the National Human Genome Research Institute.25 In addition, the National Institutes of Health has a website that may be helpful to many patients26 (and a much more complex site for health professionals).27 At the same time, courts have resisted plaintiffs/patients who have tried to use informed consent as a way of suing for failure to offer genetic testing.28,29
The failure to refer. In some cases, a patient should be formally referred for genetics consultation. The considerations here are similar to other circumstances in modern, fast developing medical practice that require special sensitivity to those occasions in which a patient will benefit from additional expertise. It is a principle that the AMA Council on Ethical and Judicial Affairs has expressed this way: “In the absence of adequate expertise in pretest and posttest counseling, a physician should refer the patient to an appropriate specialist.”30 The failure to refer, when that deviates from acceptable practice, may result in liability.
Informing others. BRCA testing is an area of medicine in which results may be of great significance not only to the patient but also to the patient’s family.31 Physicians should counsel patients on the importance of informing relatives about relevant results and “should make themselves available to assist patients in communicating with relatives to discuss opportunities for counseling and testing, as appropriate.”30 The question may arise, however, of whether in some circumstances physicians should go a step further in ensuring relatives receive important information regarding their loved one’s health.32 The law has been reluctant to impose liability to “third parties” (someone not a patient). Duties usually arise through the physician-patient relationship. There are exceptions. Perhaps the best known has been the obligation of mental health professionals to take action to protect third parties from patients who have made believable threats against identifiable victims.33 There are indications that some courts could find, in extreme circumstances, a “duty to warn” nonpatients in some instances where it is essential to inform third parties that they should receive a specific form of genetic testing.34,35 Such a duty would, of course, have to protect the privacy rights of the patient to the maximum extent possible. A general duty of this type has not been established widely, but may be part of the future.
Continue to: Was there liability in our example case?...
Was there liability in our example case?
The hypothetical case provided above suggests that there could be liability. Routine medical history by the primary care physician would have produced the fact that the patient’s mother, sister, and maternal grandmother had breast cancer. That would clearly have put her in a category of those who should have received genetic testing. Yet, she was not tested until after her cancer was found. From the limited facts we have, it appears that this timeline of events would have been outside accepted practice—and negligent. The case was not pursued by the patient, however, and this may represent the current state of liability for BRCA issues.
The extent of liability seems to be significant
Our discussion of liability suggests that there is significant potential for BRCA testing negligence within practice, and that the damages in these cases could be substantial. Yet the predicted “tsunami” of malpractice lawsuits related to genetic testing has not appeared.36,37 One study of cases in the United States (through 2016) found a “slowly rising tide” of liability cases instead of a tsunami,24 as the number of claims made was low. On the other hand, the payments where damages were awarded were an order of magnitude larger than other malpractice cases—a mean of $5.3 million and median of $2 million. This is compared with mean values in the range of $275,000 to $600,000 in other areas of malpractice.
The majority of the genetic malpractice cases involve prenatal and newborn testing, and diagnosis/susceptibility/pharmacogenomic accounting for about 25% of cases. In terms of type of errors claimed, approximately 50% were diagnostic-interpretation errors, 30% failure to offer testing, nearly 20% failure to return test results to the patients, and a few remaining cases of failure to properly treat in light of genetic testing.24
Despite a few very large payments, however, the fact remains that there is a surprisingly low number of genetics malpractice cases. Gary Marchant and colleagues suggest that several reasons may account for this:
- the clinical implementation of genetic science has been slower than expected
- the lack of expertise of many physicians in genetic science
- expert witnesses have sometimes been hard to find
- the lack of understanding by plaintiffs’ attorneys of genetic malpractice
- potential plaintiffs’ lack of understanding of the nature of genetic testing and the harms resulting from genetic negligence.17,24,37
The tide is slowly coming in
By all appearances, there is every reason to think that genetic malpractice will be increasing, and that the recent past of much higher damages per claim paid in the genetics area will be part of that tide. The National Human Genome Research LawSeq project has suggested a number of useful ways of dealing with the liability issues.18 In addition to the BRCA issues that we have considered in this article for ObGyns, there are other critical issues of prenatal and newborn genetic testing.38 But those are topics for another day. ●
CASE Young woman with family history of breast cancer detects lump
Two weeks after noting a lump on her breast when her cat happened to jump on her in that spot, a 28-year-old woman (G0) went to her primary care provider. She was referred to her gynecologist; breast imaging, ultrasonography, and mammography were obtained, with microcalcifications noted. A fine needle aspiration diagnosed intraductal malignancy. The surgical breast tissue specimen was estrogen receptor (ER)- and progestogen receptor (PR)-positive and HER2-negative. Other tumor markers were obtained, including carcinoembryonic antigen, and tissue polypeptide specific antigen, p53, cathepsin D, cyclin E, and nestin, but results were not available.
With regard to family history, the woman’s mother and maternal grandmother had a history of breast cancer. The patient and her family underwent gene testing. The patient was found to be BRCA1- and BRCA2-positive; her mother was BRCA1-positive, an older sister was BRCA2-positive, and her grandmother was not tested.
The question arose in light of her family history as to why she was not tested for BRCA and appropriately counseled by her gynecologist prior to the cancer diagnosis. Litigation was initiated. While the case did not go forward regarding litigation, it is indeed a case in point. (Please note that this is a hypothetical case. It is based on a composite of several cases.)
Medical considerations
Breast cancer is the most common type of cancer affecting women in the Western world.1 Advances in clinical testing for gene mutations have escalated and allowed for identification of patients at increased risk for breast and ovarian cancer. Along with these advances come professional liability risk. After looking at the medical considerations for BRCA1 and 2 testing, we will consider a number of important legal issues. In the view of some commentators, the failure to diagnose genetic mutations in patients predisposed to cancer is “poised to become the next wave of medical professional liability lawsuits.”2
BRCA1 and BRCA2 genes provide tumor suppressor proteins, and assessment for mutations is recommended for individuals at high risk for breast and/or ovarian cancer; mutations in BRCA genes cause DNA damage, which increases the chance of developing cancer. The other way to look at it is, BRCA1 and 2 are tumor suppressor genes that are integrally involved with DNA damage control. Once there is a mutation, it adversely affects the beneficial effects of the gene. Mutations in these genes account for 5% to 10% of all hereditary breast cancers.3 Of note, men with BRCA2 are at increased risk for prostate cancer.
A patient who presents to her gynecologist stating that there is a family history of breast cancer, without knowledge of genetic components, presents a challenge (and a medicolegal risk) for the provider to assess. Prediction models have been used to determine specific patient risk for carrying a genetic mutation with resultant breast cancer development.4 Risk prediction models do not appear to be a good answer to predicting who is more likely to develop breast or ovarian cancer, however. A Mayo model may assist (FIGURE).5 Clinicians should also be aware of other models of risk assessment, including the Gail Model (TABLE 1).6


Continue to: Guidelines for genetic testing...
Guidelines for genetic testing
The American College of Obstetricians and Gynecologists states that patient medical history and family history are paramount in obtaining information regarding risk for breast and ovarian cancer. First- and second-degree relatives are allocated to this category. Information regarding age of diagnosis, maternal and paternal lineage, and ethnic background can imply a need for genetic testing (TABLE 2).7,8 A number of genetics national organizations have participated in recommendations and include the American College of Medical Genetics and Genomics, the National Society for Genetic Counselors, and the Society of Gynecologic Oncology.7

The question always surfaces, could the clinical outcome of the cancer when diagnosed have been changed if screening were undertaken, with earlier diagnosis, or prevented with prophylactic mastectomy, and changed the end result. In addition, it is well known that breast augmentation mammoplasty alters the ability to accurately evaluate mammograms. Patients considering this type of plastic surgery, ideally, should be counselled accordingly.9
Bottom line, we as clinicians must be cognizant of both ACOG and United States Preventive Services Task Force (USPSTF) recommendations regarding screening and gene testing for women considered high risk for breast cancer based on family history.7
Legal considerations
The case presented demonstrates that the discovery of the BRCA1 and BRCA2 genes, and reliable tests for determining the existence of the genes, brought with them legal issues as well as medical advantages. We look at professional liability (malpractice) questions this technology raises, and then consider the outcome of the hypothetical case. (BRCA is used here to apply broadly—not only to BRCA1 and 2 but also to PALB2, CHEK2, and similar genetic abnormalities.)
To date, the most visible BRCA legal issues covered in cases and law reviews have focused more on patent law than malpractice. The most important of these was a decision of the US Supreme Court in Association for Molecular Pathology v Myriad Genetics.10 The US Patent Office was granting patents to companies finding useful, naturally occurring segments of human DNA, and had granted Myriad several patents on BRCA1 and BRCA2 genes. This patent policy had the potential to seriously interfere with broad scientific use of these genes.11 Fortunately, the Supreme Court stepped in and unanimously invalidated such patents. It held that a “naturally occurring DNA segment is a product of nature and not patent eligible merely because it has been isolated.” The Court noted, “Finding the location of the BRCA1 and BRCA2 genes does not render the genes patent eligible ‘new . . . composition[s] of matter.’”8 The Court did allow the patenting of tests for specific gene structures, and artificial changes in naturally occurring genes.
Malpractice and BRCA
While the BRCA patent wars have lingered, the potential for a significant increase in BRCA-related malpractice cases is of increasing concern. Like most malpractice liability, these new claims are based on very old principles of negligence.12 To prevail, the plaintiff (ordinarily, an injured patient) must demonstrate 4 things:
- A duty. That is, the physician owed a duty to the injured party. Usually (but not always) that requires a professional relationship between the physician and the person injured.
- A breach of that duty. Malpractice liability is based on the fact that the physician did something that a reasonably careful physician (generally, of the same specialty) would not have done, or that the physician failed to do something that a reasonable physician would have done. This usually means that the profession itself sees what the physician did (or did not do) as medically inappropriate. In medical malpractice cases, that is ordinarily measured by what the usual or common practice is among prudent physicians. In rare circumstances, courts have found the standard practice of a profession to be negligent. Where, for example, it was custom for a professional not to give an eye pressure test to anyone under age 40, a court found that common standard to be inappropriate.13 In the words of Judge Learned Hand (speaking about a different case), “a whole calling may have unduly lagged in the adoption of new and available devices. It never may set its own tests.”14 Underlying negligence is a cost-benefit analysis (discussed below).
- Damages. There must have been some damage that courts recognize, usually loss of money or opportunity to work, the cost of care, pain and suffering, or loss of enjoyment/quality of life. In malpractice, many states now recognize the “loss of chance” or the “loss of a chance.” That means, if a “physician negligently fails to diagnose a curable disease, and the patient is harmed by the disease, the physician should be liable for causing the ‘loss of a chance of a cure.’”15 (Delay in diagnosis is the most common reason for claims in breast cancer care.)16
- Causation. The breach of duty (negligence) must have caused the damages. The causation must have been reasonably close. If a driver drives through a stop sign, or a physician misreads a test, and someone is injured but there is no connection between the negligence and the injury, there is not tort liability.
The 4 elements of malpractice just described are raised in some way in the possible liability associated with BRCA testing. We next look at the ways in which liability may arise from that testing (or lack of it).
Underlying much of the following discussion is the “cost-benefit” consideration noted above. This concept is that the total cost (financial and health) of testing should be compared with the value of the benefits of testing, taking into account the probabilities that the testing will result in better health outcomes. BRCA testing, for example, is essentially cost-free in terms of physical risk. Its financial cost, while not trivial, is not great, and it is commonly covered by health insurance.17 In terms of benefits, the testing has the potential for providing critical information in making treatment decisions for a meaningful percentage of patients and their families. There are many ways of analyzing the liability risks of genetic malpractice,7,18 and the following is intended to discuss some of the greatest risks related to BRCA testing.
Continue to: Areas of liability...
Areas of liability
The failure to recommend a test. The circumstances in which BRCA testing should be undertaken are set out by professional organizations (noted above). These recommendations are not static, however. They change from time to time. Given the potential harm caused by the failure to test in relevant circumstances, malpractice liability is certainly a possibility when the failure to recommend a test to a patient results in a cancer that might have been prevented had the genetic problem been identified in a timely manner. The circumstances in which testing should be considered continue to change, placing an obligation on clinicians to stay well informed of changing genetic understandings. Another risk is that one specialist may assume that it is the job of another specialist to order the test. Whatever the cause of the failure to test, or unnecessary delay in testing, it appears to be the primary basis for BRCA liability.
The failure to properly interpret a test. Any test that is misinterpreted may lead to harm for the patient. A false negative, of course, may mean that preventive treatment that could have been undertaken will be foregone, as a “loss of a chance.” On the other hand, a false positive can lead to radical, unnecessary surgery or treatment. If a misinterpretation occurred because of carelessness by the testing organization, or confusion by a practitioner, there is a likelihood of negligence.19
A different form of “misinterpretation” could be reasonable—and not negligent. Advances in scientific-medical understanding may result in the outcome of tests being reconsidered and changed. That has been the case with genetic testing and breast cancer. The availability of multiple breast cancer SNPs (single nucleotide polymorphisms), and combining this information with other risk factors for example, results in a polygenic risk score that may be at odds with the level of risk from earlier testing.20,21 This naturally leads to the question of when later, updated testing should be recommended to look for a better current interpretation.22,23
The failure to act on BRCA test results. Testing is of no value, of course, if the results are not used properly. Test results or analyses that are not sent to the proper physicians, or are somehow ignored when properly directed, is a “never” event—it should never happen. It almost always would be considered negligence, and if the patient were injured, could lead to liability. Amazingly, one study found that, in genetic testing liability cases, nearly 20% of the claims arose from failure to return test results to patients.24 In addition, when a patient is found to be BRCA-positive, there is an obligation to discuss the options for dealing with the increased risk associated with the gene mutation(s), as well as to recommend the prudent course of action or to refer the patient to someone who will have that discussion.
Informed consent to the patient. BRCA testing requires informed consent. The physical risks of the testing process are minimal, of course, but it carries a number of other emotional and family risks. The informed consent process is an invitation to an honest discussion between clinicians and patients. It should be an opportunity to discuss what the testing is, and is not, and what the test may mean for treatment. It may also be an opportunity to discuss the implications for other members of the patient’s family (noted below).
One element of informed consent is a discussion of the consequences of failure to consent, or to undertake one of the alternatives. In the case of BRCA testing, this is especially important in cases in which a patient expresses a hesitancy to be tested with an “I’d rather not know philosophy.” Although clinicians should not practice law, some patient concerns about discrimination may be addressed by the protection that the federal Genetic Information Nondiscrimination Act (GINA) and other laws provide (which prohibit insurance and employment discrimination based on genetic information). A good source of information about GINA and related nondiscrimination laws is provided by the National Human Genome Research Institute.25 In addition, the National Institutes of Health has a website that may be helpful to many patients26 (and a much more complex site for health professionals).27 At the same time, courts have resisted plaintiffs/patients who have tried to use informed consent as a way of suing for failure to offer genetic testing.28,29
The failure to refer. In some cases, a patient should be formally referred for genetics consultation. The considerations here are similar to other circumstances in modern, fast developing medical practice that require special sensitivity to those occasions in which a patient will benefit from additional expertise. It is a principle that the AMA Council on Ethical and Judicial Affairs has expressed this way: “In the absence of adequate expertise in pretest and posttest counseling, a physician should refer the patient to an appropriate specialist.”30 The failure to refer, when that deviates from acceptable practice, may result in liability.
Informing others. BRCA testing is an area of medicine in which results may be of great significance not only to the patient but also to the patient’s family.31 Physicians should counsel patients on the importance of informing relatives about relevant results and “should make themselves available to assist patients in communicating with relatives to discuss opportunities for counseling and testing, as appropriate.”30 The question may arise, however, of whether in some circumstances physicians should go a step further in ensuring relatives receive important information regarding their loved one’s health.32 The law has been reluctant to impose liability to “third parties” (someone not a patient). Duties usually arise through the physician-patient relationship. There are exceptions. Perhaps the best known has been the obligation of mental health professionals to take action to protect third parties from patients who have made believable threats against identifiable victims.33 There are indications that some courts could find, in extreme circumstances, a “duty to warn” nonpatients in some instances where it is essential to inform third parties that they should receive a specific form of genetic testing.34,35 Such a duty would, of course, have to protect the privacy rights of the patient to the maximum extent possible. A general duty of this type has not been established widely, but may be part of the future.
Continue to: Was there liability in our example case?...
Was there liability in our example case?
The hypothetical case provided above suggests that there could be liability. Routine medical history by the primary care physician would have produced the fact that the patient’s mother, sister, and maternal grandmother had breast cancer. That would clearly have put her in a category of those who should have received genetic testing. Yet, she was not tested until after her cancer was found. From the limited facts we have, it appears that this timeline of events would have been outside accepted practice—and negligent. The case was not pursued by the patient, however, and this may represent the current state of liability for BRCA issues.
The extent of liability seems to be significant
Our discussion of liability suggests that there is significant potential for BRCA testing negligence within practice, and that the damages in these cases could be substantial. Yet the predicted “tsunami” of malpractice lawsuits related to genetic testing has not appeared.36,37 One study of cases in the United States (through 2016) found a “slowly rising tide” of liability cases instead of a tsunami,24 as the number of claims made was low. On the other hand, the payments where damages were awarded were an order of magnitude larger than other malpractice cases—a mean of $5.3 million and median of $2 million. This is compared with mean values in the range of $275,000 to $600,000 in other areas of malpractice.
The majority of the genetic malpractice cases involve prenatal and newborn testing, and diagnosis/susceptibility/pharmacogenomic accounting for about 25% of cases. In terms of type of errors claimed, approximately 50% were diagnostic-interpretation errors, 30% failure to offer testing, nearly 20% failure to return test results to the patients, and a few remaining cases of failure to properly treat in light of genetic testing.24
Despite a few very large payments, however, the fact remains that there is a surprisingly low number of genetics malpractice cases. Gary Marchant and colleagues suggest that several reasons may account for this:
- the clinical implementation of genetic science has been slower than expected
- the lack of expertise of many physicians in genetic science
- expert witnesses have sometimes been hard to find
- the lack of understanding by plaintiffs’ attorneys of genetic malpractice
- potential plaintiffs’ lack of understanding of the nature of genetic testing and the harms resulting from genetic negligence.17,24,37
The tide is slowly coming in
By all appearances, there is every reason to think that genetic malpractice will be increasing, and that the recent past of much higher damages per claim paid in the genetics area will be part of that tide. The National Human Genome Research LawSeq project has suggested a number of useful ways of dealing with the liability issues.18 In addition to the BRCA issues that we have considered in this article for ObGyns, there are other critical issues of prenatal and newborn genetic testing.38 But those are topics for another day. ●
- Sevilla C, Moatti JP, Reynier CJ, et al. Testing for BRCA1 mutations: a cost-effective analysis. Europ J Human Genetics. 2002;10:599-606.
- Cotton V, Kirkpatrick D. Failure to recommend genetic counseling in breast cancer: is the next wave of medical professional liability lawsuits? Contemp OB/GYN. June 1, 2017.
- Suryavanshi M, Kumar D, Panigrahi M, et al. Detection of false positive mutations in BRCA gene by next generation sequencing. Fam Cancer. 2017;16:311-317.
- Black L, Knoppers B, Avard D, et al. Legal liability and the uncertain nature of risk prediction: the case of breast cancer risk prediction models. Public Health Genomics. 2012;15:335-340.
- McClintock A, Gollab A, Laya M. Breast cancer risk assessment, a step-wise approach for primary care physicians on the front lines of shared decision making. Mayo Clin Proc. 2020;95:1268-1275.
- National Cancer Institute. The Breast Cancer Risk Assessment Tool. https://bcrisktool.cancer.gov/. Accessed February 25, 2021.
- Neff J, Richardson G, Phelps J. Legal liabilities associated with hereditary breast and ovarian cancers. J Reprod Med. 2020;65:227-230.
- American College of Obstetricians and Gynecologists. Practice Bulletin No 182: hereditary breast and ovarian cancer syndrome. Obstet Gynecol. 2017;130:e110-e126.
- Sá dos Reis C, Gremion I, and Meystre NR. Study of breast implants mammography examinations for identification of suitable image quality criteria. Insights Imaging. 2020;11:3.
- Association for Molecular Pathology v Myriad Genetics, 569 U.S. 576 (2013).
- Smith SR. The Supreme Court 2012-2013: dogs, DNA, and DOMA. Register Rep. 2013;39(Fall):26-33.
- Bal BS. An introduction to medical malpractice in the United States. Clin Orthop Relat Res. 2009;467:339-347.
- Helling v Carey, 83 Wn.2d 514, 519 P.2d 981 (1974).
- The T.J. Hooper, 60 F.2d 737, 740 (2d Cir.1932), cert. denied 287 U.S. 662 (1932).
- Fischer DA. Tort recovery for loss of a chance. Wake Forest L Rev. 2001;36:605-655.
- Murphy BL, Ray-Zack MD, Reddy PN, et al. Breast cancer litigation in the 21st century. Ann Surg Oncol. 2018;25:2939- 2947.
- Prince AE. Prevention for those who can pay: insurance reimbursement of genetic-based preventive interventions in the liminal state between health and disease. J Law Biosci. 2015;2:365-395.
- Marchant G, Barnes M, Evans JP, et al; LawSeq Liability Task Force. From genetics to genomics: facing the liability implications in clinical care. J Law Med Ethics. 2020;48:11-43.
- Complaint, Held v Ambry Genetics Corp., No. 15-CV-8683, 2015 WL 6750024 (S.D.N.Y. Nov. 4, 2015); Order of Dismissal, Held v Ambry Genetics Corp., No. 15-CV-8683, (S.D.N.Y. Dec. 6, 2016).
- Pederson HJ. Breast cancer risk assessment and treatment: current concepts in genetics and genomics. Contemp OB/ GYN. 2017; 62:A1-A4.
- Pederson HJ. Who needs breast cancer genetics testing? OBG Manag. 2018;30:34-39.
- Roberts JL, Foulkes A. Genetic duties. William Mary L Rev. 2020;62:143-212.
- Thorogood A, Cook-Deegan R, Knoppers B. Public variant databases: liability? Genet Med. 2017;19:838–841.
- Marchant G, Lindor R. Genomic malpractice: an emerging tide or gentle ripple? Food Drug Law J. 2018;73:1-37.
- National Human Genome Research Institute. Genetic discrimination. https://www.genome.gov/about-genomics /policy-issues/Genetic-Discrimination. Updated September 16, 2020. Accessed February 25, 2021.
- National Cancer Institute. BRCA mutations: cancer risk and genetic testing. https://www.cancer.gov/about-cancer /causes-prevention/genetics/brca-fact-sheet. Reviewed November 19, 2020. Accessed February 25, 2021.
- National Cancer Institute. Genetics of breast and gynecologic cancers (PDQ®)–Health Professional Version. https://www .cancer.gov/types/breast/hp/breast-ovarian-genetics-pdq. Updated February 12, 2021. Accessed February 25, 2021.
- Reed v Campagnolo, 630 A.2d 1145, 1152–54 (Md. 1993).
- Munro v Regents of Univ. of Cal.,263 Cal. Rptr. 878, 885, 988 (1989).
- AMA Council on Ethical and Judicial Affairs. AMA Code of Medical Ethics’ opinions on genetic testing. Opinion 2.131. 2009;11:683-685. https://journalofethics.ama-assn .org/article/ama-code-medical-ethics-opinions-genetictesting/2009-09.
- Gilbar R, Barnoy S. Disclosing genetic test results to the patient’ relatives: how does the law influence clinical practice? J Law Technol Policy. 2019;125-168.
- Song K. Warning third parties of genetic risks in the era of personalized medicine. U.C. Davis L Rev. 2016;49:1987-2018.
- Tarasoff v Regents of the University of California, 551 P.2d 334, 131 Cal. Rptr. 14 (Cal. 1976).
- Safer v Estate of Pack, 677 A.2d 1188 (N.J. App. 1996), cert. denied, 683 A.2d 1163 (N.J. 1996).
- Pate v Threlkel, 661 So.2d 278 (Fla. 1995).
- Rothstein MA. Liability issues in pharmacogenomics. Louisiana L Rev. 2005;66:117-124.
- Marchant G, Lindor R. Personalized medicine and genetic malpractice. Genet Med. 2013;15:921-922.
- Westbrook M. Transforming the physician’s standard of care in the context of whole genome sequencing technologies: finding guidance in best practice standards. Saint Louis U J Health Law Policy. 2015;9:111-148.
- Sevilla C, Moatti JP, Reynier CJ, et al. Testing for BRCA1 mutations: a cost-effective analysis. Europ J Human Genetics. 2002;10:599-606.
- Cotton V, Kirkpatrick D. Failure to recommend genetic counseling in breast cancer: is the next wave of medical professional liability lawsuits? Contemp OB/GYN. June 1, 2017.
- Suryavanshi M, Kumar D, Panigrahi M, et al. Detection of false positive mutations in BRCA gene by next generation sequencing. Fam Cancer. 2017;16:311-317.
- Black L, Knoppers B, Avard D, et al. Legal liability and the uncertain nature of risk prediction: the case of breast cancer risk prediction models. Public Health Genomics. 2012;15:335-340.
- McClintock A, Gollab A, Laya M. Breast cancer risk assessment, a step-wise approach for primary care physicians on the front lines of shared decision making. Mayo Clin Proc. 2020;95:1268-1275.
- National Cancer Institute. The Breast Cancer Risk Assessment Tool. https://bcrisktool.cancer.gov/. Accessed February 25, 2021.
- Neff J, Richardson G, Phelps J. Legal liabilities associated with hereditary breast and ovarian cancers. J Reprod Med. 2020;65:227-230.
- American College of Obstetricians and Gynecologists. Practice Bulletin No 182: hereditary breast and ovarian cancer syndrome. Obstet Gynecol. 2017;130:e110-e126.
- Sá dos Reis C, Gremion I, and Meystre NR. Study of breast implants mammography examinations for identification of suitable image quality criteria. Insights Imaging. 2020;11:3.
- Association for Molecular Pathology v Myriad Genetics, 569 U.S. 576 (2013).
- Smith SR. The Supreme Court 2012-2013: dogs, DNA, and DOMA. Register Rep. 2013;39(Fall):26-33.
- Bal BS. An introduction to medical malpractice in the United States. Clin Orthop Relat Res. 2009;467:339-347.
- Helling v Carey, 83 Wn.2d 514, 519 P.2d 981 (1974).
- The T.J. Hooper, 60 F.2d 737, 740 (2d Cir.1932), cert. denied 287 U.S. 662 (1932).
- Fischer DA. Tort recovery for loss of a chance. Wake Forest L Rev. 2001;36:605-655.
- Murphy BL, Ray-Zack MD, Reddy PN, et al. Breast cancer litigation in the 21st century. Ann Surg Oncol. 2018;25:2939- 2947.
- Prince AE. Prevention for those who can pay: insurance reimbursement of genetic-based preventive interventions in the liminal state between health and disease. J Law Biosci. 2015;2:365-395.
- Marchant G, Barnes M, Evans JP, et al; LawSeq Liability Task Force. From genetics to genomics: facing the liability implications in clinical care. J Law Med Ethics. 2020;48:11-43.
- Complaint, Held v Ambry Genetics Corp., No. 15-CV-8683, 2015 WL 6750024 (S.D.N.Y. Nov. 4, 2015); Order of Dismissal, Held v Ambry Genetics Corp., No. 15-CV-8683, (S.D.N.Y. Dec. 6, 2016).
- Pederson HJ. Breast cancer risk assessment and treatment: current concepts in genetics and genomics. Contemp OB/ GYN. 2017; 62:A1-A4.
- Pederson HJ. Who needs breast cancer genetics testing? OBG Manag. 2018;30:34-39.
- Roberts JL, Foulkes A. Genetic duties. William Mary L Rev. 2020;62:143-212.
- Thorogood A, Cook-Deegan R, Knoppers B. Public variant databases: liability? Genet Med. 2017;19:838–841.
- Marchant G, Lindor R. Genomic malpractice: an emerging tide or gentle ripple? Food Drug Law J. 2018;73:1-37.
- National Human Genome Research Institute. Genetic discrimination. https://www.genome.gov/about-genomics /policy-issues/Genetic-Discrimination. Updated September 16, 2020. Accessed February 25, 2021.
- National Cancer Institute. BRCA mutations: cancer risk and genetic testing. https://www.cancer.gov/about-cancer /causes-prevention/genetics/brca-fact-sheet. Reviewed November 19, 2020. Accessed February 25, 2021.
- National Cancer Institute. Genetics of breast and gynecologic cancers (PDQ®)–Health Professional Version. https://www .cancer.gov/types/breast/hp/breast-ovarian-genetics-pdq. Updated February 12, 2021. Accessed February 25, 2021.
- Reed v Campagnolo, 630 A.2d 1145, 1152–54 (Md. 1993).
- Munro v Regents of Univ. of Cal.,263 Cal. Rptr. 878, 885, 988 (1989).
- AMA Council on Ethical and Judicial Affairs. AMA Code of Medical Ethics’ opinions on genetic testing. Opinion 2.131. 2009;11:683-685. https://journalofethics.ama-assn .org/article/ama-code-medical-ethics-opinions-genetictesting/2009-09.
- Gilbar R, Barnoy S. Disclosing genetic test results to the patient’ relatives: how does the law influence clinical practice? J Law Technol Policy. 2019;125-168.
- Song K. Warning third parties of genetic risks in the era of personalized medicine. U.C. Davis L Rev. 2016;49:1987-2018.
- Tarasoff v Regents of the University of California, 551 P.2d 334, 131 Cal. Rptr. 14 (Cal. 1976).
- Safer v Estate of Pack, 677 A.2d 1188 (N.J. App. 1996), cert. denied, 683 A.2d 1163 (N.J. 1996).
- Pate v Threlkel, 661 So.2d 278 (Fla. 1995).
- Rothstein MA. Liability issues in pharmacogenomics. Louisiana L Rev. 2005;66:117-124.
- Marchant G, Lindor R. Personalized medicine and genetic malpractice. Genet Med. 2013;15:921-922.
- Westbrook M. Transforming the physician’s standard of care in the context of whole genome sequencing technologies: finding guidance in best practice standards. Saint Louis U J Health Law Policy. 2015;9:111-148.
2021 Update on gynecologic cancer
Gynecologic malignancies continue to be a major cause of cancer-related mortality in women. In 2020, a number of developments changed practice in gynecologic oncology. In this Update, we highlight 3 important articles. The first showed that human papillomavirus (HPV) vaccination reduced the rate of cervical cancer. The next evaluated a novel targeted therapeutic approach using the combination of pembrolizumab and lenvatinib in women with recurrent endometrial carcinoma that progressed after prior systemic therapy. Finally, the third article showed that talcum powder was not associated with an increased risk of ovarian cancer. We provide here a brief overview of the major findings of these studies and how these results are influencing practice.
Evidence establishes that HPV vaccination cuts risk of invasive cervical cancer
Lei J, Ploner A, Elfström KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:1340-1348.
HPV infection is associated with 99% of cervical cancers, and approximately 65% to 75% of cases involve HPV 16 or 18.1,2 The quadrivalent HPV (6, 11, 16, 18) vaccine was approved by the US Food and Drug Administration in 2006 for the prevention of cervical intraepithelial lesions and genital warts associated with HPV.3-5 Previous studies of the HPV vaccine showed it to be effective in preventing HPV infection, genital warts, and high-grade precancerous cervical lesions, such as cervical intraepithelial neoplasia grade 2 (CIN2) and grade 3 (CIN3).6-8 While the vaccine offers a number of advantages, the long-term goal of the vaccine—to reduce the incidence of invasive cervical cancer—was not shown until recently.
Large study followed HPV vaccinated and unvaccinated women
Lei and colleagues conducted a registry based cohort study from 2006 through 2017 of women aged 10 to 30 years who were living in Sweden.9 They followed the women from their 10th birthday until they were diagnosed with cervical cancer, died, emigrated from Sweden, were lost to follow-up, or turned 31 years of age. In the study, the unique personal identity numbers assigned to all Swedish residents were linked to a number of large national administrative databases. Beginning in 2007 in Sweden, the quadrivalent vaccine was subsidized for use in girls aged 13 to 17, and a subsequent catch-up period that started in 2012 incorporated women who had not been vaccinated.
Continue to: Cervical cancer rates were lowest in women vaccinated before age 17...
Cervical cancer rates were lowest in women vaccinated before age 17
A total of 1,672,983 women were included in the study; 527,871 received at least one dose of the HPV vaccine. During the study period, cervical cancer was diagnosed in 19 women who had received the quadrivalent HPV vaccine and in 538 women who had not received the vaccine. Women who initiated vaccination before age 17 had the lowest rates of cervical cancer (4 cases per 100,000 persons), followed by women vaccinated after age 17 (54 cases per 100,000 persons) and then those who were not vaccinated (94 cases per 100,000 persons).
After adjusting for confounders, the incidence rate ratio (RR) of cervical cancer was significantly lower among vaccinated women compared with unvaccinated women (RR, 0.37; 95% confidence interval [CI], 0.21– 0.57) (FIGURE 1).9 In addition, women who were vaccinated before age 17 demonstrated the greatest benefit. For those vaccinated before age 17 versus those who were unvaccinated, the RR was 0.12 (95% CI, 0.00–0.34). For women vaccinated between age 17 and 30 versus unvaccinated women, the RR was 0.47 (95% CI, 0.27–0.75).
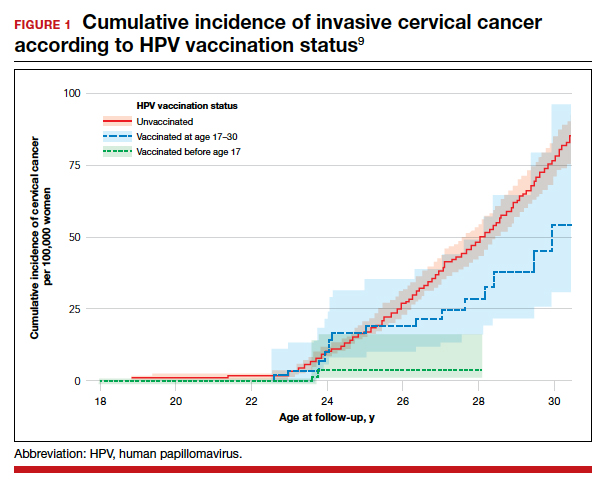
The study by Lei and colleagues showed that HPV vaccination was associated with a substantially lower risk of invasive cervical cancer. While all women who received the vaccine had reduced rates of invasive cervical cancer, those who received the vaccine earlier (before age 17) showed the greatest reduction in invasive cervical cancer. On a population level, this study demonstrates that a program of HPV vaccination can reduce the burden of cervical cancer.
Promising option for patients with advanced endometrial cancer: Lenvatinib plus pembrolizumab
Makker V, Taylor MH, Aghajanian C, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer. J Clin Oncol. 2020;38:2981-2992.
Advanced stage endometrial cancer is associated with a 17% 5-year survival rate.10 Paclitaxel with carboplatin is the standard first-line treatment for advanced, recurrent, and metastatic endometrial cancer; for women who do not respond to this regimen, effective treatment options are limited.11,12
The immunotherapy approach
Immunotherapy is a more recently developed treatment, an approach in which the immune system is activated to target cancer cells. Pembrolizumab is a commonly used agent for many solid tumors.13 This drug binds to the programmed cell death receptor 1 (PD-1) or PD-ligand 1 (PD-L1), a component of the immune checkpoint, which then allows the immune system to target and destroy cancer cells.14
Prembrolizumab is FDA approved for use in the treatment of microsatellite instability-high (MSI-H)/mismatch repair deficient (dMMR) solid tumors that have progressed after prior therapy and for which there are no satisfactory alternative treatment options.15 Endometrial cancers frequently display microsatellite instability and mismatch repair defects.16
Lenvatinib is an oral multikinase inhibitor that targets vascular endothelial growth factor receptors 1, 2, and 3; fibroblast growth factor receptors 1, 2, 3, and 4; and platelet derived growth factor receptor alpha, RET, and KIT.17-19 In a phase 2 study of lenvatinib monotherapy for advanced previously treated endometrial cancer, the response rate was 14.3%.20
While some preclinical studies have examined the combination of immune checkpoint inhibitors with lenvatinib,21-23 a recent study is the first to evaluate this combination in patients with advanced tumors.24
Continue to: Prembrolizumab-lenvatinib combination therapy...
Prembrolizumab-lenvatinib combination therapy
Makker and colleagues conducted an ongoing multinational, open-label, phase 1B/2 study of lenvatinib 20 mg daily orally plus pembrolizumab 200 mg intravenously once every 3 weeks in patients with select solid tumors.24 Women with previously treated endometrial carcinoma (N = 125) were included. Of the study participants, 49% were PD-L1 positive and 10% were MSI-H/dMMR. The primary end point was objective response rate (ORR) at 24 weeks, which was 38.0% (95% CI, 28.8%–47.8%).
The median duration of response was 21.2 months (95% CI, 7.6 months to not estimable). The ORR was similar in patients with PD-L1 expressing tumors (35.8%; 95% CI, 23.1%–50.2%), who are more likely to respond to immunotherapy, compared with those without PD-L1 expression (39.5%; 95% CI, 25.0%–55.6%). For patients with MSI-H/dMMR, there was a higher ORR (63.6%; 95% CI, 30.8%–89.1%, versus 36.2%; 95% CI, 26.5%–46.7%).
Median progression-free survival was 7.4 months (95% CI, 5.3–8.7 months) and median overall survival was 16.7 months (15 months to not estimable). Moderate to severe treatment-related adverse events occurred in 83 patients (66.9%), and 22 patients (17.7%) discontinued 1 or both study drugs because of adverse effects. Two deaths were judged to be treatment related.
This study showed promising results for the combination of pembrolizumab with lenvatinib in women with advanced endometrial carcinoma who have progressed after prior systemic therapy. These data led to an accelerated approval by the FDA for the treatment of women with advanced endometrial carcinoma that is not MSI-H/dMMR, who have disease progression after prior systemic therapy, and who are not candidates for curative surgery or radiation therapy.25 Currently, 2 phase 3 trials of lenvatinib plus pembrolizumab in advanced endometrial carcinoma are underway, which will shed further light on this combination therapy
What is the risk of ovarian cancer in women who use powder in the genital area?
O’Brien KM, Tworoger SS, Harris HR, et al. Association of powder use in the genital area with risk of ovarian cancer. JAMA. 2020;323:49-59.
Women apply talcum powder to their genital area to keep skin dry and to prevent rashes. Powder can be applied by direct application, sanitary napkins, diaphragms, or tampons. Most powder products contain the mineral talc. Because it often is found in nature with asbestos, a known carcinogen, talc’s carcinogenic effects have been investigated.26,27
Talc also might ascend through the genital tract and irritate the epithelial lining of the fallopian tubes or ovaries, possibly triggering an inflammatory response that may promote carcinogenesis.28,29 Case-control studies have reported a possible association between genital powder use and ovarian cancer.30,31 Since these studies, talc-related lawsuits and media coverage have increased.32,33
Large prospective cohorts provide data for analysis
In a pooled analysis of 4 large US-based observational cohorts between 1976 and 2017, O’Brien and colleagues noted that 38% of the 252,745 women included in the study self-reported the use of powder in the genital area.34 With a median of 11.2 years of follow-up, 2,168 women developed ovarian cancer (58 cases/100,000 person-years). Among women who reported using genital powder, the incidence of ovarian cancer was 61 cases/100,000 person-years, while for women who reported never using genital powder, the incidence was 55 cases/100,000 person-years. This corresponded to an estimated hazard ratio (HR) of 1.08 (95% CI, 0.99–1.17).
Frequent powder use, long-term use, and never use. Similar findings were seen for those with frequent use versus never use (HR, 1.09; 95% CI, 0.97–1.23) and long-term use versus never use (HR, 1.01; 95% CI, 0.82– 1.25). When restricting the group to women with a patent reproductive tract at baseline, the HR was 1.13 (95% CI, 1.01–1.26), but the P value for interaction comparing women with versus women without a patent reproductive tract was 0.15 (FIGURE 2).34
Bottom line. In contrast to a prior meta-analysis, in this study there was no statistically significant association between the self-reported use of powder in the genital area and the incidence of ovarian cancer. ●
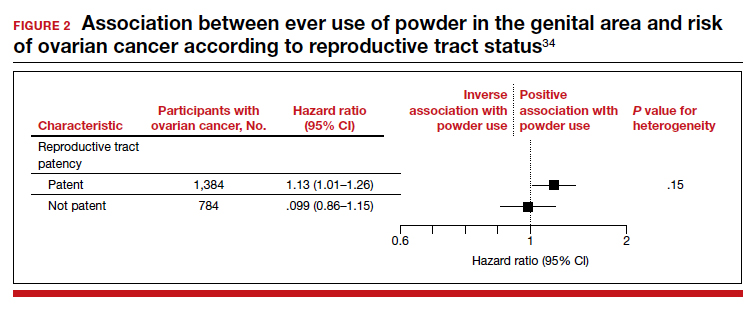
The study by O’Brien and colleagues is the largest study to date with the longest follow-up that examines the possible association between talc-based powder use and ovarian cancer. A strength of this study is the avoidance of recall bias by the selection of administrative data sets that had gathered information on talcum powder use from patients prior to the diagnosis of ovarian cancer. While these findings are reassuring, the study may have been underpowered to identify a small increase in ovarian cancer risk with talc use.
- de Sanjose S, Quint WG, Alemany L, et al; Retrospective International Survey and HPV Time Trends Study Group. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048-1056.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12-19.
- Ault KA; Future II Study Group. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet. 2007;369:1861-1868.
- Garland SM, Hernandez-Avila M, Wheeler CM, et al; Females United to Unilaterally Reduce Endo/Ectocervical disease (FUTURE) I Investigators. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356:1928-1943.
- Joura EA, Leodolter S, Hernandez-Avila M, et al. Efficacy of a quadrivalent prophylactic human papillomavirus (types 6, 11, 16, and 18) L1 virus-like-particle vaccine against highgrade vulval and vaginal lesions: a combined analysis of three randomised clinical trials. Lancet. 2007;369:1693-1702.
- Arbyn M, Xu L, Simoens C, et al. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst Rev. 2018;5(5):CD009069.
- Paavonen J, Naud P, Salmerón J, et al; HPV PATRICIA Study Group. Efficacy of human papillomavirus (HPV)-16/18 AS04- adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301-314.
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915-1927.
- Lei J, Ploner A, Elfström KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:1340-1348.
- American Cancer Society. Survival rates for endometrial cancer. https://www.cancer.org/cancer/endometrial-cancer/ detection-diagnosis-staging/survival-rates.html. Accessed February 9, 2021.
- Miller D, Filiaci V, Fleming G, et al. Late-breaking abstract 1: Randomized phase III noninferiority trial of first line chemotherapy for metastatic or recurrent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2012;125:771.
- National Comprehensive Cancer Network. Clinical practice guidelines in oncology: uterine neoplasms. Version 3.2019. https://www.nccn.org/professionals/physician_gls/pdf /uterine.pdf. Accessed February 9, 2021.
- Marcus L, Lemery SJ, Keegan P, et al. FDA approval summary: pembrolizumab for the treatment of microsatellite instabilityhigh solid tumors. Clin Cancer Res. 2019;25:3753-3758.
- Arora E, Masab M, Mittar P, et al. Role of immune checkpoint inhibitors in advanced or recurrent endometrial cancer. Cureus. 2018;10:e2521.
- Keytruda (pembrolizumab). Package insert. Merck Sharp & Dohme; 2018.
- Cancer Genome Atlas Research Network; Kandoth C, Schultz N, Cherniak AD, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67-73.
- Matsui J, Yamamoto Y, Funahashi Y, et al. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int J Cancer. 2008;122:664-671.
- Okamoto K, Kodama K, Takase K, et al. Antitumor activities of the targeted multi-tyrosine kinase inhibitor lenvatinib (E7080) against RET gene fusion-driven tumor models. Cancer Lett. 2013;340:97-103.
- Tohyama O, Matsui J, Kodama K, et al. Antitumor activity of lenvatinib (E7080): an angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models. J Thyroid Res. 2014;2014: 638747.
- Vergote I, Teneriello M, Powell MA, et al. A phase II trial of lenvatinib in patients with advanced or recurrent endometrial cancer: angiopoietin-2 as a predictive marker for clinical outcomes. J Clin Oncol. 2013;31(15 suppl): abstract 5520.
- Kimura T, Kato Y, Ozawa Y, et al. Immunomodulatory activity of lenvatinib contributes to antitumor activity in the Hepa1-6 hepatocellular carcinoma model. Cancer Sci. 2018;109:3993-4002.
- Kato Y, Tabata K, Hori Y, et al. Effects of lenvatinib on tumorassociated macrophages enhance antitumor activity of PD-1 signal inhibitors. Mol Cancer Ther. 2015;14(12 suppl 2): abstract A92.
- Kato Y, Bao X, Macgrath S, et al. Lenvatinib mesilate (LEN) enhanced antitumor activity of a PD-1 blockade agent by potentiating Th1 immune response. Ann Oncol. 2016;27(suppl 6): abstract 2PD.
- Makker V, Taylor MH, Aghajanian C, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer. J Clin Oncol. 2020;38:2981-2992.
- Lenvima (lenvatinib). Package insert. Woodcliff Lake, NJ: Eisai; 2019.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Carbon black, titanium dioxide, and talc. IARC Monogr Eval Carcinog Risks Hum. 2010;93:1-413.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Arsenic, metals, fibres, and dusts. IARC Monogr Eval Carcinog Risks Hum. 2012;100(pt C):11-465.
- Erickson BK, Conner MG, Landen CN Jr. The role of the fallopian tube in the origin of ovarian cancer. Am J Obstet Gynecol. 2013;209:409-414.
- Ness RB, Cottreau C. Possible role of ovarian epithelial inflammation in ovarian cancer. J Natl Cancer Inst. 1999;91:1459-1467.
- Terry KL, Karageorgi S, Shvetsov YB, et al; Ovarian Cancer Association Consortium. Genital powder use and risk of ovarian cancer: a pooled analysis of 8,525 cases and 9,859 controls. Cancer Prev Res. 2013;6:811-821.
- Penninkilampi R, Eslick GD. Perineal talc use and ovarian cancer: a systematic review and meta-analysis. Epidemiology. 2018;29:41-49.
- Hsu T. Johnson & Johnson told to pay $4.7 billion in baby powder lawsuit. New York Times. July 12, 2018. Accessed February 18, 2021. https://www.nytimes.com/2018/07/12 /business/johnson-johnson-talcum-powder.html.
- McGinley L. Does talcum powder cause ovarian cancer? Washington Post. August 25, 2017. Accessed February 18, 2021. https://www.washingtonpost.com/news/to-your -health/wp/2017/08/23/does-talcum-powder-cause -ovarian-cancer-experts-are-divided/.
- O’Brien KM, Tworoger SS, Harris HR, et al. Association of powder use in the genital area with risk of ovarian cancer. JAMA. 2020;323:49-59.

Gynecologic malignancies continue to be a major cause of cancer-related mortality in women. In 2020, a number of developments changed practice in gynecologic oncology. In this Update, we highlight 3 important articles. The first showed that human papillomavirus (HPV) vaccination reduced the rate of cervical cancer. The next evaluated a novel targeted therapeutic approach using the combination of pembrolizumab and lenvatinib in women with recurrent endometrial carcinoma that progressed after prior systemic therapy. Finally, the third article showed that talcum powder was not associated with an increased risk of ovarian cancer. We provide here a brief overview of the major findings of these studies and how these results are influencing practice.
Evidence establishes that HPV vaccination cuts risk of invasive cervical cancer
Lei J, Ploner A, Elfström KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:1340-1348.
HPV infection is associated with 99% of cervical cancers, and approximately 65% to 75% of cases involve HPV 16 or 18.1,2 The quadrivalent HPV (6, 11, 16, 18) vaccine was approved by the US Food and Drug Administration in 2006 for the prevention of cervical intraepithelial lesions and genital warts associated with HPV.3-5 Previous studies of the HPV vaccine showed it to be effective in preventing HPV infection, genital warts, and high-grade precancerous cervical lesions, such as cervical intraepithelial neoplasia grade 2 (CIN2) and grade 3 (CIN3).6-8 While the vaccine offers a number of advantages, the long-term goal of the vaccine—to reduce the incidence of invasive cervical cancer—was not shown until recently.
Large study followed HPV vaccinated and unvaccinated women
Lei and colleagues conducted a registry based cohort study from 2006 through 2017 of women aged 10 to 30 years who were living in Sweden.9 They followed the women from their 10th birthday until they were diagnosed with cervical cancer, died, emigrated from Sweden, were lost to follow-up, or turned 31 years of age. In the study, the unique personal identity numbers assigned to all Swedish residents were linked to a number of large national administrative databases. Beginning in 2007 in Sweden, the quadrivalent vaccine was subsidized for use in girls aged 13 to 17, and a subsequent catch-up period that started in 2012 incorporated women who had not been vaccinated.
Continue to: Cervical cancer rates were lowest in women vaccinated before age 17...
Cervical cancer rates were lowest in women vaccinated before age 17
A total of 1,672,983 women were included in the study; 527,871 received at least one dose of the HPV vaccine. During the study period, cervical cancer was diagnosed in 19 women who had received the quadrivalent HPV vaccine and in 538 women who had not received the vaccine. Women who initiated vaccination before age 17 had the lowest rates of cervical cancer (4 cases per 100,000 persons), followed by women vaccinated after age 17 (54 cases per 100,000 persons) and then those who were not vaccinated (94 cases per 100,000 persons).
After adjusting for confounders, the incidence rate ratio (RR) of cervical cancer was significantly lower among vaccinated women compared with unvaccinated women (RR, 0.37; 95% confidence interval [CI], 0.21– 0.57) (FIGURE 1).9 In addition, women who were vaccinated before age 17 demonstrated the greatest benefit. For those vaccinated before age 17 versus those who were unvaccinated, the RR was 0.12 (95% CI, 0.00–0.34). For women vaccinated between age 17 and 30 versus unvaccinated women, the RR was 0.47 (95% CI, 0.27–0.75).

The study by Lei and colleagues showed that HPV vaccination was associated with a substantially lower risk of invasive cervical cancer. While all women who received the vaccine had reduced rates of invasive cervical cancer, those who received the vaccine earlier (before age 17) showed the greatest reduction in invasive cervical cancer. On a population level, this study demonstrates that a program of HPV vaccination can reduce the burden of cervical cancer.
Promising option for patients with advanced endometrial cancer: Lenvatinib plus pembrolizumab
Makker V, Taylor MH, Aghajanian C, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer. J Clin Oncol. 2020;38:2981-2992.
Advanced stage endometrial cancer is associated with a 17% 5-year survival rate.10 Paclitaxel with carboplatin is the standard first-line treatment for advanced, recurrent, and metastatic endometrial cancer; for women who do not respond to this regimen, effective treatment options are limited.11,12
The immunotherapy approach
Immunotherapy is a more recently developed treatment, an approach in which the immune system is activated to target cancer cells. Pembrolizumab is a commonly used agent for many solid tumors.13 This drug binds to the programmed cell death receptor 1 (PD-1) or PD-ligand 1 (PD-L1), a component of the immune checkpoint, which then allows the immune system to target and destroy cancer cells.14
Prembrolizumab is FDA approved for use in the treatment of microsatellite instability-high (MSI-H)/mismatch repair deficient (dMMR) solid tumors that have progressed after prior therapy and for which there are no satisfactory alternative treatment options.15 Endometrial cancers frequently display microsatellite instability and mismatch repair defects.16
Lenvatinib is an oral multikinase inhibitor that targets vascular endothelial growth factor receptors 1, 2, and 3; fibroblast growth factor receptors 1, 2, 3, and 4; and platelet derived growth factor receptor alpha, RET, and KIT.17-19 In a phase 2 study of lenvatinib monotherapy for advanced previously treated endometrial cancer, the response rate was 14.3%.20
While some preclinical studies have examined the combination of immune checkpoint inhibitors with lenvatinib,21-23 a recent study is the first to evaluate this combination in patients with advanced tumors.24
Continue to: Prembrolizumab-lenvatinib combination therapy...
Prembrolizumab-lenvatinib combination therapy
Makker and colleagues conducted an ongoing multinational, open-label, phase 1B/2 study of lenvatinib 20 mg daily orally plus pembrolizumab 200 mg intravenously once every 3 weeks in patients with select solid tumors.24 Women with previously treated endometrial carcinoma (N = 125) were included. Of the study participants, 49% were PD-L1 positive and 10% were MSI-H/dMMR. The primary end point was objective response rate (ORR) at 24 weeks, which was 38.0% (95% CI, 28.8%–47.8%).
The median duration of response was 21.2 months (95% CI, 7.6 months to not estimable). The ORR was similar in patients with PD-L1 expressing tumors (35.8%; 95% CI, 23.1%–50.2%), who are more likely to respond to immunotherapy, compared with those without PD-L1 expression (39.5%; 95% CI, 25.0%–55.6%). For patients with MSI-H/dMMR, there was a higher ORR (63.6%; 95% CI, 30.8%–89.1%, versus 36.2%; 95% CI, 26.5%–46.7%).
Median progression-free survival was 7.4 months (95% CI, 5.3–8.7 months) and median overall survival was 16.7 months (15 months to not estimable). Moderate to severe treatment-related adverse events occurred in 83 patients (66.9%), and 22 patients (17.7%) discontinued 1 or both study drugs because of adverse effects. Two deaths were judged to be treatment related.
This study showed promising results for the combination of pembrolizumab with lenvatinib in women with advanced endometrial carcinoma who have progressed after prior systemic therapy. These data led to an accelerated approval by the FDA for the treatment of women with advanced endometrial carcinoma that is not MSI-H/dMMR, who have disease progression after prior systemic therapy, and who are not candidates for curative surgery or radiation therapy.25 Currently, 2 phase 3 trials of lenvatinib plus pembrolizumab in advanced endometrial carcinoma are underway, which will shed further light on this combination therapy
What is the risk of ovarian cancer in women who use powder in the genital area?
O’Brien KM, Tworoger SS, Harris HR, et al. Association of powder use in the genital area with risk of ovarian cancer. JAMA. 2020;323:49-59.
Women apply talcum powder to their genital area to keep skin dry and to prevent rashes. Powder can be applied by direct application, sanitary napkins, diaphragms, or tampons. Most powder products contain the mineral talc. Because it often is found in nature with asbestos, a known carcinogen, talc’s carcinogenic effects have been investigated.26,27
Talc also might ascend through the genital tract and irritate the epithelial lining of the fallopian tubes or ovaries, possibly triggering an inflammatory response that may promote carcinogenesis.28,29 Case-control studies have reported a possible association between genital powder use and ovarian cancer.30,31 Since these studies, talc-related lawsuits and media coverage have increased.32,33
Large prospective cohorts provide data for analysis
In a pooled analysis of 4 large US-based observational cohorts between 1976 and 2017, O’Brien and colleagues noted that 38% of the 252,745 women included in the study self-reported the use of powder in the genital area.34 With a median of 11.2 years of follow-up, 2,168 women developed ovarian cancer (58 cases/100,000 person-years). Among women who reported using genital powder, the incidence of ovarian cancer was 61 cases/100,000 person-years, while for women who reported never using genital powder, the incidence was 55 cases/100,000 person-years. This corresponded to an estimated hazard ratio (HR) of 1.08 (95% CI, 0.99–1.17).
Frequent powder use, long-term use, and never use. Similar findings were seen for those with frequent use versus never use (HR, 1.09; 95% CI, 0.97–1.23) and long-term use versus never use (HR, 1.01; 95% CI, 0.82– 1.25). When restricting the group to women with a patent reproductive tract at baseline, the HR was 1.13 (95% CI, 1.01–1.26), but the P value for interaction comparing women with versus women without a patent reproductive tract was 0.15 (FIGURE 2).34
Bottom line. In contrast to a prior meta-analysis, in this study there was no statistically significant association between the self-reported use of powder in the genital area and the incidence of ovarian cancer. ●

The study by O’Brien and colleagues is the largest study to date with the longest follow-up that examines the possible association between talc-based powder use and ovarian cancer. A strength of this study is the avoidance of recall bias by the selection of administrative data sets that had gathered information on talcum powder use from patients prior to the diagnosis of ovarian cancer. While these findings are reassuring, the study may have been underpowered to identify a small increase in ovarian cancer risk with talc use.

Gynecologic malignancies continue to be a major cause of cancer-related mortality in women. In 2020, a number of developments changed practice in gynecologic oncology. In this Update, we highlight 3 important articles. The first showed that human papillomavirus (HPV) vaccination reduced the rate of cervical cancer. The next evaluated a novel targeted therapeutic approach using the combination of pembrolizumab and lenvatinib in women with recurrent endometrial carcinoma that progressed after prior systemic therapy. Finally, the third article showed that talcum powder was not associated with an increased risk of ovarian cancer. We provide here a brief overview of the major findings of these studies and how these results are influencing practice.
Evidence establishes that HPV vaccination cuts risk of invasive cervical cancer
Lei J, Ploner A, Elfström KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:1340-1348.
HPV infection is associated with 99% of cervical cancers, and approximately 65% to 75% of cases involve HPV 16 or 18.1,2 The quadrivalent HPV (6, 11, 16, 18) vaccine was approved by the US Food and Drug Administration in 2006 for the prevention of cervical intraepithelial lesions and genital warts associated with HPV.3-5 Previous studies of the HPV vaccine showed it to be effective in preventing HPV infection, genital warts, and high-grade precancerous cervical lesions, such as cervical intraepithelial neoplasia grade 2 (CIN2) and grade 3 (CIN3).6-8 While the vaccine offers a number of advantages, the long-term goal of the vaccine—to reduce the incidence of invasive cervical cancer—was not shown until recently.
Large study followed HPV vaccinated and unvaccinated women
Lei and colleagues conducted a registry based cohort study from 2006 through 2017 of women aged 10 to 30 years who were living in Sweden.9 They followed the women from their 10th birthday until they were diagnosed with cervical cancer, died, emigrated from Sweden, were lost to follow-up, or turned 31 years of age. In the study, the unique personal identity numbers assigned to all Swedish residents were linked to a number of large national administrative databases. Beginning in 2007 in Sweden, the quadrivalent vaccine was subsidized for use in girls aged 13 to 17, and a subsequent catch-up period that started in 2012 incorporated women who had not been vaccinated.
Continue to: Cervical cancer rates were lowest in women vaccinated before age 17...
Cervical cancer rates were lowest in women vaccinated before age 17
A total of 1,672,983 women were included in the study; 527,871 received at least one dose of the HPV vaccine. During the study period, cervical cancer was diagnosed in 19 women who had received the quadrivalent HPV vaccine and in 538 women who had not received the vaccine. Women who initiated vaccination before age 17 had the lowest rates of cervical cancer (4 cases per 100,000 persons), followed by women vaccinated after age 17 (54 cases per 100,000 persons) and then those who were not vaccinated (94 cases per 100,000 persons).
After adjusting for confounders, the incidence rate ratio (RR) of cervical cancer was significantly lower among vaccinated women compared with unvaccinated women (RR, 0.37; 95% confidence interval [CI], 0.21– 0.57) (FIGURE 1).9 In addition, women who were vaccinated before age 17 demonstrated the greatest benefit. For those vaccinated before age 17 versus those who were unvaccinated, the RR was 0.12 (95% CI, 0.00–0.34). For women vaccinated between age 17 and 30 versus unvaccinated women, the RR was 0.47 (95% CI, 0.27–0.75).

The study by Lei and colleagues showed that HPV vaccination was associated with a substantially lower risk of invasive cervical cancer. While all women who received the vaccine had reduced rates of invasive cervical cancer, those who received the vaccine earlier (before age 17) showed the greatest reduction in invasive cervical cancer. On a population level, this study demonstrates that a program of HPV vaccination can reduce the burden of cervical cancer.
Promising option for patients with advanced endometrial cancer: Lenvatinib plus pembrolizumab
Makker V, Taylor MH, Aghajanian C, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer. J Clin Oncol. 2020;38:2981-2992.
Advanced stage endometrial cancer is associated with a 17% 5-year survival rate.10 Paclitaxel with carboplatin is the standard first-line treatment for advanced, recurrent, and metastatic endometrial cancer; for women who do not respond to this regimen, effective treatment options are limited.11,12
The immunotherapy approach
Immunotherapy is a more recently developed treatment, an approach in which the immune system is activated to target cancer cells. Pembrolizumab is a commonly used agent for many solid tumors.13 This drug binds to the programmed cell death receptor 1 (PD-1) or PD-ligand 1 (PD-L1), a component of the immune checkpoint, which then allows the immune system to target and destroy cancer cells.14
Prembrolizumab is FDA approved for use in the treatment of microsatellite instability-high (MSI-H)/mismatch repair deficient (dMMR) solid tumors that have progressed after prior therapy and for which there are no satisfactory alternative treatment options.15 Endometrial cancers frequently display microsatellite instability and mismatch repair defects.16
Lenvatinib is an oral multikinase inhibitor that targets vascular endothelial growth factor receptors 1, 2, and 3; fibroblast growth factor receptors 1, 2, 3, and 4; and platelet derived growth factor receptor alpha, RET, and KIT.17-19 In a phase 2 study of lenvatinib monotherapy for advanced previously treated endometrial cancer, the response rate was 14.3%.20
While some preclinical studies have examined the combination of immune checkpoint inhibitors with lenvatinib,21-23 a recent study is the first to evaluate this combination in patients with advanced tumors.24
Continue to: Prembrolizumab-lenvatinib combination therapy...
Prembrolizumab-lenvatinib combination therapy
Makker and colleagues conducted an ongoing multinational, open-label, phase 1B/2 study of lenvatinib 20 mg daily orally plus pembrolizumab 200 mg intravenously once every 3 weeks in patients with select solid tumors.24 Women with previously treated endometrial carcinoma (N = 125) were included. Of the study participants, 49% were PD-L1 positive and 10% were MSI-H/dMMR. The primary end point was objective response rate (ORR) at 24 weeks, which was 38.0% (95% CI, 28.8%–47.8%).
The median duration of response was 21.2 months (95% CI, 7.6 months to not estimable). The ORR was similar in patients with PD-L1 expressing tumors (35.8%; 95% CI, 23.1%–50.2%), who are more likely to respond to immunotherapy, compared with those without PD-L1 expression (39.5%; 95% CI, 25.0%–55.6%). For patients with MSI-H/dMMR, there was a higher ORR (63.6%; 95% CI, 30.8%–89.1%, versus 36.2%; 95% CI, 26.5%–46.7%).
Median progression-free survival was 7.4 months (95% CI, 5.3–8.7 months) and median overall survival was 16.7 months (15 months to not estimable). Moderate to severe treatment-related adverse events occurred in 83 patients (66.9%), and 22 patients (17.7%) discontinued 1 or both study drugs because of adverse effects. Two deaths were judged to be treatment related.
This study showed promising results for the combination of pembrolizumab with lenvatinib in women with advanced endometrial carcinoma who have progressed after prior systemic therapy. These data led to an accelerated approval by the FDA for the treatment of women with advanced endometrial carcinoma that is not MSI-H/dMMR, who have disease progression after prior systemic therapy, and who are not candidates for curative surgery or radiation therapy.25 Currently, 2 phase 3 trials of lenvatinib plus pembrolizumab in advanced endometrial carcinoma are underway, which will shed further light on this combination therapy
What is the risk of ovarian cancer in women who use powder in the genital area?
O’Brien KM, Tworoger SS, Harris HR, et al. Association of powder use in the genital area with risk of ovarian cancer. JAMA. 2020;323:49-59.
Women apply talcum powder to their genital area to keep skin dry and to prevent rashes. Powder can be applied by direct application, sanitary napkins, diaphragms, or tampons. Most powder products contain the mineral talc. Because it often is found in nature with asbestos, a known carcinogen, talc’s carcinogenic effects have been investigated.26,27
Talc also might ascend through the genital tract and irritate the epithelial lining of the fallopian tubes or ovaries, possibly triggering an inflammatory response that may promote carcinogenesis.28,29 Case-control studies have reported a possible association between genital powder use and ovarian cancer.30,31 Since these studies, talc-related lawsuits and media coverage have increased.32,33
Large prospective cohorts provide data for analysis
In a pooled analysis of 4 large US-based observational cohorts between 1976 and 2017, O’Brien and colleagues noted that 38% of the 252,745 women included in the study self-reported the use of powder in the genital area.34 With a median of 11.2 years of follow-up, 2,168 women developed ovarian cancer (58 cases/100,000 person-years). Among women who reported using genital powder, the incidence of ovarian cancer was 61 cases/100,000 person-years, while for women who reported never using genital powder, the incidence was 55 cases/100,000 person-years. This corresponded to an estimated hazard ratio (HR) of 1.08 (95% CI, 0.99–1.17).
Frequent powder use, long-term use, and never use. Similar findings were seen for those with frequent use versus never use (HR, 1.09; 95% CI, 0.97–1.23) and long-term use versus never use (HR, 1.01; 95% CI, 0.82– 1.25). When restricting the group to women with a patent reproductive tract at baseline, the HR was 1.13 (95% CI, 1.01–1.26), but the P value for interaction comparing women with versus women without a patent reproductive tract was 0.15 (FIGURE 2).34
Bottom line. In contrast to a prior meta-analysis, in this study there was no statistically significant association between the self-reported use of powder in the genital area and the incidence of ovarian cancer. ●

The study by O’Brien and colleagues is the largest study to date with the longest follow-up that examines the possible association between talc-based powder use and ovarian cancer. A strength of this study is the avoidance of recall bias by the selection of administrative data sets that had gathered information on talcum powder use from patients prior to the diagnosis of ovarian cancer. While these findings are reassuring, the study may have been underpowered to identify a small increase in ovarian cancer risk with talc use.
- de Sanjose S, Quint WG, Alemany L, et al; Retrospective International Survey and HPV Time Trends Study Group. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048-1056.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12-19.
- Ault KA; Future II Study Group. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet. 2007;369:1861-1868.
- Garland SM, Hernandez-Avila M, Wheeler CM, et al; Females United to Unilaterally Reduce Endo/Ectocervical disease (FUTURE) I Investigators. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356:1928-1943.
- Joura EA, Leodolter S, Hernandez-Avila M, et al. Efficacy of a quadrivalent prophylactic human papillomavirus (types 6, 11, 16, and 18) L1 virus-like-particle vaccine against highgrade vulval and vaginal lesions: a combined analysis of three randomised clinical trials. Lancet. 2007;369:1693-1702.
- Arbyn M, Xu L, Simoens C, et al. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst Rev. 2018;5(5):CD009069.
- Paavonen J, Naud P, Salmerón J, et al; HPV PATRICIA Study Group. Efficacy of human papillomavirus (HPV)-16/18 AS04- adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301-314.
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915-1927.
- Lei J, Ploner A, Elfström KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:1340-1348.
- American Cancer Society. Survival rates for endometrial cancer. https://www.cancer.org/cancer/endometrial-cancer/ detection-diagnosis-staging/survival-rates.html. Accessed February 9, 2021.
- Miller D, Filiaci V, Fleming G, et al. Late-breaking abstract 1: Randomized phase III noninferiority trial of first line chemotherapy for metastatic or recurrent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2012;125:771.
- National Comprehensive Cancer Network. Clinical practice guidelines in oncology: uterine neoplasms. Version 3.2019. https://www.nccn.org/professionals/physician_gls/pdf /uterine.pdf. Accessed February 9, 2021.
- Marcus L, Lemery SJ, Keegan P, et al. FDA approval summary: pembrolizumab for the treatment of microsatellite instabilityhigh solid tumors. Clin Cancer Res. 2019;25:3753-3758.
- Arora E, Masab M, Mittar P, et al. Role of immune checkpoint inhibitors in advanced or recurrent endometrial cancer. Cureus. 2018;10:e2521.
- Keytruda (pembrolizumab). Package insert. Merck Sharp & Dohme; 2018.
- Cancer Genome Atlas Research Network; Kandoth C, Schultz N, Cherniak AD, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67-73.
- Matsui J, Yamamoto Y, Funahashi Y, et al. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int J Cancer. 2008;122:664-671.
- Okamoto K, Kodama K, Takase K, et al. Antitumor activities of the targeted multi-tyrosine kinase inhibitor lenvatinib (E7080) against RET gene fusion-driven tumor models. Cancer Lett. 2013;340:97-103.
- Tohyama O, Matsui J, Kodama K, et al. Antitumor activity of lenvatinib (E7080): an angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models. J Thyroid Res. 2014;2014: 638747.
- Vergote I, Teneriello M, Powell MA, et al. A phase II trial of lenvatinib in patients with advanced or recurrent endometrial cancer: angiopoietin-2 as a predictive marker for clinical outcomes. J Clin Oncol. 2013;31(15 suppl): abstract 5520.
- Kimura T, Kato Y, Ozawa Y, et al. Immunomodulatory activity of lenvatinib contributes to antitumor activity in the Hepa1-6 hepatocellular carcinoma model. Cancer Sci. 2018;109:3993-4002.
- Kato Y, Tabata K, Hori Y, et al. Effects of lenvatinib on tumorassociated macrophages enhance antitumor activity of PD-1 signal inhibitors. Mol Cancer Ther. 2015;14(12 suppl 2): abstract A92.
- Kato Y, Bao X, Macgrath S, et al. Lenvatinib mesilate (LEN) enhanced antitumor activity of a PD-1 blockade agent by potentiating Th1 immune response. Ann Oncol. 2016;27(suppl 6): abstract 2PD.
- Makker V, Taylor MH, Aghajanian C, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer. J Clin Oncol. 2020;38:2981-2992.
- Lenvima (lenvatinib). Package insert. Woodcliff Lake, NJ: Eisai; 2019.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Carbon black, titanium dioxide, and talc. IARC Monogr Eval Carcinog Risks Hum. 2010;93:1-413.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Arsenic, metals, fibres, and dusts. IARC Monogr Eval Carcinog Risks Hum. 2012;100(pt C):11-465.
- Erickson BK, Conner MG, Landen CN Jr. The role of the fallopian tube in the origin of ovarian cancer. Am J Obstet Gynecol. 2013;209:409-414.
- Ness RB, Cottreau C. Possible role of ovarian epithelial inflammation in ovarian cancer. J Natl Cancer Inst. 1999;91:1459-1467.
- Terry KL, Karageorgi S, Shvetsov YB, et al; Ovarian Cancer Association Consortium. Genital powder use and risk of ovarian cancer: a pooled analysis of 8,525 cases and 9,859 controls. Cancer Prev Res. 2013;6:811-821.
- Penninkilampi R, Eslick GD. Perineal talc use and ovarian cancer: a systematic review and meta-analysis. Epidemiology. 2018;29:41-49.
- Hsu T. Johnson & Johnson told to pay $4.7 billion in baby powder lawsuit. New York Times. July 12, 2018. Accessed February 18, 2021. https://www.nytimes.com/2018/07/12 /business/johnson-johnson-talcum-powder.html.
- McGinley L. Does talcum powder cause ovarian cancer? Washington Post. August 25, 2017. Accessed February 18, 2021. https://www.washingtonpost.com/news/to-your -health/wp/2017/08/23/does-talcum-powder-cause -ovarian-cancer-experts-are-divided/.
- O’Brien KM, Tworoger SS, Harris HR, et al. Association of powder use in the genital area with risk of ovarian cancer. JAMA. 2020;323:49-59.
- de Sanjose S, Quint WG, Alemany L, et al; Retrospective International Survey and HPV Time Trends Study Group. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048-1056.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12-19.
- Ault KA; Future II Study Group. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet. 2007;369:1861-1868.
- Garland SM, Hernandez-Avila M, Wheeler CM, et al; Females United to Unilaterally Reduce Endo/Ectocervical disease (FUTURE) I Investigators. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356:1928-1943.
- Joura EA, Leodolter S, Hernandez-Avila M, et al. Efficacy of a quadrivalent prophylactic human papillomavirus (types 6, 11, 16, and 18) L1 virus-like-particle vaccine against highgrade vulval and vaginal lesions: a combined analysis of three randomised clinical trials. Lancet. 2007;369:1693-1702.
- Arbyn M, Xu L, Simoens C, et al. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst Rev. 2018;5(5):CD009069.
- Paavonen J, Naud P, Salmerón J, et al; HPV PATRICIA Study Group. Efficacy of human papillomavirus (HPV)-16/18 AS04- adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301-314.
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915-1927.
- Lei J, Ploner A, Elfström KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:1340-1348.
- American Cancer Society. Survival rates for endometrial cancer. https://www.cancer.org/cancer/endometrial-cancer/ detection-diagnosis-staging/survival-rates.html. Accessed February 9, 2021.
- Miller D, Filiaci V, Fleming G, et al. Late-breaking abstract 1: Randomized phase III noninferiority trial of first line chemotherapy for metastatic or recurrent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2012;125:771.
- National Comprehensive Cancer Network. Clinical practice guidelines in oncology: uterine neoplasms. Version 3.2019. https://www.nccn.org/professionals/physician_gls/pdf /uterine.pdf. Accessed February 9, 2021.
- Marcus L, Lemery SJ, Keegan P, et al. FDA approval summary: pembrolizumab for the treatment of microsatellite instabilityhigh solid tumors. Clin Cancer Res. 2019;25:3753-3758.
- Arora E, Masab M, Mittar P, et al. Role of immune checkpoint inhibitors in advanced or recurrent endometrial cancer. Cureus. 2018;10:e2521.
- Keytruda (pembrolizumab). Package insert. Merck Sharp & Dohme; 2018.
- Cancer Genome Atlas Research Network; Kandoth C, Schultz N, Cherniak AD, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67-73.
- Matsui J, Yamamoto Y, Funahashi Y, et al. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int J Cancer. 2008;122:664-671.
- Okamoto K, Kodama K, Takase K, et al. Antitumor activities of the targeted multi-tyrosine kinase inhibitor lenvatinib (E7080) against RET gene fusion-driven tumor models. Cancer Lett. 2013;340:97-103.
- Tohyama O, Matsui J, Kodama K, et al. Antitumor activity of lenvatinib (E7080): an angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models. J Thyroid Res. 2014;2014: 638747.
- Vergote I, Teneriello M, Powell MA, et al. A phase II trial of lenvatinib in patients with advanced or recurrent endometrial cancer: angiopoietin-2 as a predictive marker for clinical outcomes. J Clin Oncol. 2013;31(15 suppl): abstract 5520.
- Kimura T, Kato Y, Ozawa Y, et al. Immunomodulatory activity of lenvatinib contributes to antitumor activity in the Hepa1-6 hepatocellular carcinoma model. Cancer Sci. 2018;109:3993-4002.
- Kato Y, Tabata K, Hori Y, et al. Effects of lenvatinib on tumorassociated macrophages enhance antitumor activity of PD-1 signal inhibitors. Mol Cancer Ther. 2015;14(12 suppl 2): abstract A92.
- Kato Y, Bao X, Macgrath S, et al. Lenvatinib mesilate (LEN) enhanced antitumor activity of a PD-1 blockade agent by potentiating Th1 immune response. Ann Oncol. 2016;27(suppl 6): abstract 2PD.
- Makker V, Taylor MH, Aghajanian C, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer. J Clin Oncol. 2020;38:2981-2992.
- Lenvima (lenvatinib). Package insert. Woodcliff Lake, NJ: Eisai; 2019.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Carbon black, titanium dioxide, and talc. IARC Monogr Eval Carcinog Risks Hum. 2010;93:1-413.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Arsenic, metals, fibres, and dusts. IARC Monogr Eval Carcinog Risks Hum. 2012;100(pt C):11-465.
- Erickson BK, Conner MG, Landen CN Jr. The role of the fallopian tube in the origin of ovarian cancer. Am J Obstet Gynecol. 2013;209:409-414.
- Ness RB, Cottreau C. Possible role of ovarian epithelial inflammation in ovarian cancer. J Natl Cancer Inst. 1999;91:1459-1467.
- Terry KL, Karageorgi S, Shvetsov YB, et al; Ovarian Cancer Association Consortium. Genital powder use and risk of ovarian cancer: a pooled analysis of 8,525 cases and 9,859 controls. Cancer Prev Res. 2013;6:811-821.
- Penninkilampi R, Eslick GD. Perineal talc use and ovarian cancer: a systematic review and meta-analysis. Epidemiology. 2018;29:41-49.
- Hsu T. Johnson & Johnson told to pay $4.7 billion in baby powder lawsuit. New York Times. July 12, 2018. Accessed February 18, 2021. https://www.nytimes.com/2018/07/12 /business/johnson-johnson-talcum-powder.html.
- McGinley L. Does talcum powder cause ovarian cancer? Washington Post. August 25, 2017. Accessed February 18, 2021. https://www.washingtonpost.com/news/to-your -health/wp/2017/08/23/does-talcum-powder-cause -ovarian-cancer-experts-are-divided/.
- O’Brien KM, Tworoger SS, Harris HR, et al. Association of powder use in the genital area with risk of ovarian cancer. JAMA. 2020;323:49-59.
Genetic testing for breast and ovarian cancer: What has changed and what still needs to change?
Investigators found racial and ethnic disparities in genetic testing as well as “persistent underuse” of testing in patients with ovarian cancer.
The team also discovered that most pathogenic variant (PV) results were in 20 genes associated with breast and/or ovarian cancer, and testing other genes largely revealed variants of uncertain significance (VUS).
Allison W. Kurian, MD, of Stanford (Calif.) University, and colleagues recounted these findings in the Journal of Clinical Oncology.
Because of improvements in sequencing technology, competition among commercial purveyors, and declining cost, genetic testing has been increasingly available to clinicians for patient management and cancer prevention (JAMA. 2015 Sep 8;314[10]:997-8). Although germline testing can guide therapy for several solid tumors, there is little research about how often and how well it is used in practice.
For their study, Dr. Kurian and colleagues used a SEER Genetic Testing Linkage Demonstration Project in a population-based assessment of testing for cancer risk. The investigators analyzed 7-year trends in testing among all women diagnosed with breast or ovarian cancer in Georgia or California from 2013 to 2017, reviewing testing patterns and result interpretation from 2012 to 2019.
Before analyzing the data, the investigators made the following hypotheses:
- Multigene panels (MGP) would entirely replace testing for BRCA1/2 only.
- Testing underutilization in patients with ovarian cancer would improve over time.
- More patients would be tested at lower levels of pretest risk for PVs.
- Sociodemographic differences in testing trends would not be observed.
- Detection of PVs and VUS would increase.
- Racial and ethnic disparities in rates of VUS would diminish.
Study conduct
The investigators examined genetic tests performed from 2012 through the beginning of 2019 at major commercial laboratories and linked that information with data in the SEER registries in Georgia and California on all breast and ovarian cancer patients diagnosed between 2013 and 2017. There were few criteria for exclusion.
Genetic testing results were categorized as identifying a PV or likely PV, VUS, or benign or likely benign mutation by American College of Medical Genetics criteria. When a patient had genetic testing on more than one occasion, the most recent test was used.
If a PV was identified, the types of PVs were grouped according to the level of evidence that supported pathogenicity into the following categories:
- BRCA1 or BRCA2 mutations.
- PVs in other genes designated by the National Comprehensive Cancer Network as associated with breast or ovarian cancer (e.g., ATM, BARD1, BRIP1, CDH1, CHEK2, EPCAM, MLH1, MSH2, MSH6, NBN, NF1, PALB2, MS2, PTEN, RAD51C, RAD51D, STK11, and TP53).
- PVs in other actionable genes (e.g., APC, BMPR1A, MEN1, MUTYH, NF2, RB1, RET, SDHAF2, SDHB, SDHC, SDHD, SMAD4, TSC1, TSC2, and VHL).
- Any other tested genes.
The investigators also tabulated instances in which genetic testing identified a VUS in any gene but no PV. If a VUS was identified originally and was reclassified more recently into the “PV/likely PV” or “benign/likely benign” categories, only the resolved categorization was recorded.
The authors evaluated clinical and sociodemographic correlates of testing trends for breast and ovarian cancer, assessing the relationship between race, age, and geographic site in receipt of any test or type of test.
Among laboratories, the investigators examined trends in the number of genes tested, associations with sociodemographic factors, categories of test results, and whether trends differed by race or ethnicity.
Findings, by hypothesis
Hypothesis #1: MGP will entirely replace testing for BRCA1/2 only.
About 25% of tested patients with breast cancer diagnosed in early 2013 received MGP, compared with more than 80% of those diagnosed in late 2017.
The trend for ovarian cancer was similar. About 40% of patients diagnosed in early 2013 received MGP, compared with more than 90% diagnosed in late 2017. These trends were similar in California and Georgia.
From 2012 to 2019, there was a consistent upward trend in gene number for patients with breast cancer (mean, 19) or ovarian cancer (mean, 21), from approximately 10 genes to 35 genes.
Hypothesis #2: Underutilization of testing in patients with ovarian cancer will improve.
Among the 187,535 patients with breast cancer and the 14,689 patients with ovarian cancer diagnosed in Georgia or California from 2013 through 2017, on average, testing rates increased 2% per year.
In all, 25.2% of breast cancer patients and 34.3% of ovarian cancer patients had genetic testing on one (87.3%) or more (12.7%) occasions.
Prior research suggested that, in 2013 and 2014, 31% of women with ovarian cancer had genetic testing (JAMA Oncol. 2018 Aug 1;4[8]:1066-72/ J Clin Oncol. 2019 May 20;37[15]:1305-15).
The investigators therefore concluded that underutilization of genetic testing in ovarian cancer did not improve substantially during the 7-year interval analyzed.
Hypothesis #3: More patients will be tested at lower levels of pretest risk.
These data were more difficult to abstract from the SEER database, but older patients were more likely to be tested in later years.
In patients older than 60 years of age (who accounted for more than 50% of both cancer cohorts), testing rates increased from 11.1% to 14.9% for breast cancer and 25.3% to 31.4% for ovarian cancer. By contrast, patients younger than 45 years of age were less than 15% of the sample and had lower testing rates over time.
There were no substantial changes in testing rates by other clinical variables. Therefore, in concert with the age-related testing trends, it is likely that women were tested for genetic mutations at increasingly lower levels of pretest risk.
Hypothesis #4: Sociodemographic differences in testing trends will not be observed.
Among patients with breast cancer, approximately 31% of those who had genetic testing were uninsured, 31% had Medicaid, and 26% had private insurance, Medicare, or other insurance.
For patients with ovarian cancer, approximately 28% were uninsured, 27% had Medicaid, and 39% had private insurance, Medicare, or other insurance.
The authors had previously found that less testing was associated with Black race, greater poverty, and less insurance coverage (J Clin Oncol. 2019 May 20;37[15]:1305-15). However, they noted no changes in testing rates by sociodemographic variables over time.
Hypothesis #5: Detection of both PVs and VUS will increase.
The proportion of tested breast cancer patients with PVs in BRCA1/2 decreased from 7.5% to 5.0% (P < .001), whereas PV yield for the two other clinically salient categories (breast or ovarian and other actionable genes) increased.
The proportion of PVs in any breast or ovarian gene increased from 1.3% to 4.6%, and the proportion in any other actionable gene increased from 0.3% to 1.3%.
For breast cancer patients, VUS-only rates increased from 8.5% in early 2013 to 22.4% in late 2017.
For ovarian cancer patients, the yield of PVs in BRCA1/2 decreased from 15.7% to 12.4% (P < .001), whereas the PV yield for breast or ovarian genes increased from 3.9% to 4.3%, and the yield for other actionable genes increased from 0.3% to 2.0%.
In ovarian cancer patients, the PV or VUS-only result rate increased from 30.8% in early 2013 to 43.0% in late 2017, entirely due to the increase in VUS-only rates. VUS were identified in 8.1% of patients diagnosed in early 2013 and increased to 28.3% in patients diagnosed in late 2017.
Hypothesis #6: Racial or ethnic disparities in rates of VUS will diminish.
Among patients with breast cancer, racial or ethnic differences in PV rates were small and did not change over time. For patients with ovarian cancer, PV rates across racial or ethnic groups diminished over time.
However, for both breast and ovarian cancer patients, there were large differences in VUS-only rates by race and ethnicity that persisted during the interval studied.
In 2017, for patients with breast cancer, VUS-only rates were substantially higher in Asian (42.4%), Black (36.6%), and Hispanic (27.7%) patients than in non-Hispanic White patients (24.5%, P < .001).
Similar trends were noted for patients with ovarian cancer. VUS-only rates were substantially higher in Asian (47.8%), Black (46.0%), and Hispanic (36.8%) patients than in non-Hispanic White patients (24.6%, P < .001).
Multivariable logistic regressions were performed separately for tested patients with breast cancer and ovarian cancer, and the results showed no significant interaction between race or ethnicity and date. Therefore, there was no significant change in racial or ethnic differences in VUS-only results across the study period.
Where these findings leave clinicians in 2021
Among the patients studied, there was:
- Marked expansion in the number of genes sequenced.
- A likely modest trend toward testing patients with lower pretest risk of a PV.
- No sociodemographic differences in testing trends.
- A small increase in PV rates and a substantial increase in VUS-only rates.
- Near-complete replacement of selective testing by MGP.
For patients with breast cancer, the proportion of all PVs that were in BRCA1/2 fell substantially. Adoption of MGP testing doubled the probability of detecting a PV in other tested genes. Most of the increase was in genes with an established breast or ovarian cancer association, with fewer PVs found in other actionable genes and very few PVs in other tested genes.
Contrary to their hypothesis, the authors observed a sustained undertesting of patients with ovarian cancer. Only 34.3% performed versus nearly 100% recommended, with little change since 2014.
This finding is surprising – and tremendously disappointing – since the prevalence of BRCA1/2 PVs is higher in ovarian cancer than in other cancers (Gynecol Oncol. 2017 Nov;147[2]:375-380), and germline-targeted therapy with PARP inhibitors has been approved for use since 2014.
Furthermore, insurance carriers provide coverage for genetic testing in most patients with carcinoma of the ovary, fallopian tube, and/or peritoneum.
Action plans: Less could be more
During the period analyzed, the increase in VUS-only results dramatically outpaced the increase in PVs.
Since there is a substantially larger volume of clinical genetic testing in non-Hispanic White patients with breast or ovarian cancer, the spectrum of normal variation is less well-defined in other racial or ethnic groups.
The study showed a widening of the “racial-ethnic VUS gap,” with Black and Asian patients having nearly twofold more VUS, although they were not tested for more genes than non-Hispanic White patients.
This is problematic on several levels. Identification of a VUS is challenging for communicating results to and recommending cascade testing for family members.
There is worrisome information regarding overtreatment or counseling of VUS patients about their results. For example, the PROMPT registry showed that 10%-15% of women with PV/VUS in genes not associated with a high risk of ovarian cancer underwent oophorectomy without a clear indication for the procedure.
Although population-based testing might augment the available data on the spectrum of normal variation in racial and ethnic minorities, it would likely exacerbate the proliferation of VUS over PVs.
It is essential to accelerate ongoing approaches to VUS reclassification.
In addition, the authors suggest that it may be time to reverse the trend in increasing the number of genes tested in MGPs. Their rationale is that, in Georgia and California, most PVs among patients with breast and ovarian cancer were identified in 20 genes (ATM, BARD1, BRCA1, BRCA2, BRIP1, CDH1, CHEK2, EPCAM, MLH1, MSH2, MSH6, NBN, NF1, PMS2, PALB2, PTEN, RAD51C, RAD51D, STK11, and TP53).
If the Georgia and California data are representative of a more generalized pattern, a panel of 20 breast cancer– and/or ovarian cancer–associated genes may be ideal for maximizing the yield of clinically relevant PVs and minimizing VUS results for all patients.
Finally, defining the patient, clinician, and health care system factors that impede widespread genetic testing for ovarian cancer patients must be prioritized. As the authors suggest, quality improvement efforts should focus on getting a lot closer to testing rates of 100% for patients with ovarian cancer and building the database that will help sort VUS in minority patients into their proper context of pathogenicity, rather than adding more genes per test.
This research was supported by the National Cancer Institute, the Centers for Disease Control and Prevention, and the California Department of Public Health. The authors disclosed relationships with Myriad Genetics, Ambry Genetics, Color Genomics, GeneDx/BioReference, InVitae, Genentech, Genomic Health, Roche/Genentech, Oncoquest, Tesaro, and Karyopharm Therapeutics.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Investigators found racial and ethnic disparities in genetic testing as well as “persistent underuse” of testing in patients with ovarian cancer.
The team also discovered that most pathogenic variant (PV) results were in 20 genes associated with breast and/or ovarian cancer, and testing other genes largely revealed variants of uncertain significance (VUS).
Allison W. Kurian, MD, of Stanford (Calif.) University, and colleagues recounted these findings in the Journal of Clinical Oncology.
Because of improvements in sequencing technology, competition among commercial purveyors, and declining cost, genetic testing has been increasingly available to clinicians for patient management and cancer prevention (JAMA. 2015 Sep 8;314[10]:997-8). Although germline testing can guide therapy for several solid tumors, there is little research about how often and how well it is used in practice.
For their study, Dr. Kurian and colleagues used a SEER Genetic Testing Linkage Demonstration Project in a population-based assessment of testing for cancer risk. The investigators analyzed 7-year trends in testing among all women diagnosed with breast or ovarian cancer in Georgia or California from 2013 to 2017, reviewing testing patterns and result interpretation from 2012 to 2019.
Before analyzing the data, the investigators made the following hypotheses:
- Multigene panels (MGP) would entirely replace testing for BRCA1/2 only.
- Testing underutilization in patients with ovarian cancer would improve over time.
- More patients would be tested at lower levels of pretest risk for PVs.
- Sociodemographic differences in testing trends would not be observed.
- Detection of PVs and VUS would increase.
- Racial and ethnic disparities in rates of VUS would diminish.
Study conduct
The investigators examined genetic tests performed from 2012 through the beginning of 2019 at major commercial laboratories and linked that information with data in the SEER registries in Georgia and California on all breast and ovarian cancer patients diagnosed between 2013 and 2017. There were few criteria for exclusion.
Genetic testing results were categorized as identifying a PV or likely PV, VUS, or benign or likely benign mutation by American College of Medical Genetics criteria. When a patient had genetic testing on more than one occasion, the most recent test was used.
If a PV was identified, the types of PVs were grouped according to the level of evidence that supported pathogenicity into the following categories:
- BRCA1 or BRCA2 mutations.
- PVs in other genes designated by the National Comprehensive Cancer Network as associated with breast or ovarian cancer (e.g., ATM, BARD1, BRIP1, CDH1, CHEK2, EPCAM, MLH1, MSH2, MSH6, NBN, NF1, PALB2, MS2, PTEN, RAD51C, RAD51D, STK11, and TP53).
- PVs in other actionable genes (e.g., APC, BMPR1A, MEN1, MUTYH, NF2, RB1, RET, SDHAF2, SDHB, SDHC, SDHD, SMAD4, TSC1, TSC2, and VHL).
- Any other tested genes.
The investigators also tabulated instances in which genetic testing identified a VUS in any gene but no PV. If a VUS was identified originally and was reclassified more recently into the “PV/likely PV” or “benign/likely benign” categories, only the resolved categorization was recorded.
The authors evaluated clinical and sociodemographic correlates of testing trends for breast and ovarian cancer, assessing the relationship between race, age, and geographic site in receipt of any test or type of test.
Among laboratories, the investigators examined trends in the number of genes tested, associations with sociodemographic factors, categories of test results, and whether trends differed by race or ethnicity.
Findings, by hypothesis
Hypothesis #1: MGP will entirely replace testing for BRCA1/2 only.
About 25% of tested patients with breast cancer diagnosed in early 2013 received MGP, compared with more than 80% of those diagnosed in late 2017.
The trend for ovarian cancer was similar. About 40% of patients diagnosed in early 2013 received MGP, compared with more than 90% diagnosed in late 2017. These trends were similar in California and Georgia.
From 2012 to 2019, there was a consistent upward trend in gene number for patients with breast cancer (mean, 19) or ovarian cancer (mean, 21), from approximately 10 genes to 35 genes.
Hypothesis #2: Underutilization of testing in patients with ovarian cancer will improve.
Among the 187,535 patients with breast cancer and the 14,689 patients with ovarian cancer diagnosed in Georgia or California from 2013 through 2017, on average, testing rates increased 2% per year.
In all, 25.2% of breast cancer patients and 34.3% of ovarian cancer patients had genetic testing on one (87.3%) or more (12.7%) occasions.
Prior research suggested that, in 2013 and 2014, 31% of women with ovarian cancer had genetic testing (JAMA Oncol. 2018 Aug 1;4[8]:1066-72/ J Clin Oncol. 2019 May 20;37[15]:1305-15).
The investigators therefore concluded that underutilization of genetic testing in ovarian cancer did not improve substantially during the 7-year interval analyzed.
Hypothesis #3: More patients will be tested at lower levels of pretest risk.
These data were more difficult to abstract from the SEER database, but older patients were more likely to be tested in later years.
In patients older than 60 years of age (who accounted for more than 50% of both cancer cohorts), testing rates increased from 11.1% to 14.9% for breast cancer and 25.3% to 31.4% for ovarian cancer. By contrast, patients younger than 45 years of age were less than 15% of the sample and had lower testing rates over time.
There were no substantial changes in testing rates by other clinical variables. Therefore, in concert with the age-related testing trends, it is likely that women were tested for genetic mutations at increasingly lower levels of pretest risk.
Hypothesis #4: Sociodemographic differences in testing trends will not be observed.
Among patients with breast cancer, approximately 31% of those who had genetic testing were uninsured, 31% had Medicaid, and 26% had private insurance, Medicare, or other insurance.
For patients with ovarian cancer, approximately 28% were uninsured, 27% had Medicaid, and 39% had private insurance, Medicare, or other insurance.
The authors had previously found that less testing was associated with Black race, greater poverty, and less insurance coverage (J Clin Oncol. 2019 May 20;37[15]:1305-15). However, they noted no changes in testing rates by sociodemographic variables over time.
Hypothesis #5: Detection of both PVs and VUS will increase.
The proportion of tested breast cancer patients with PVs in BRCA1/2 decreased from 7.5% to 5.0% (P < .001), whereas PV yield for the two other clinically salient categories (breast or ovarian and other actionable genes) increased.
The proportion of PVs in any breast or ovarian gene increased from 1.3% to 4.6%, and the proportion in any other actionable gene increased from 0.3% to 1.3%.
For breast cancer patients, VUS-only rates increased from 8.5% in early 2013 to 22.4% in late 2017.
For ovarian cancer patients, the yield of PVs in BRCA1/2 decreased from 15.7% to 12.4% (P < .001), whereas the PV yield for breast or ovarian genes increased from 3.9% to 4.3%, and the yield for other actionable genes increased from 0.3% to 2.0%.
In ovarian cancer patients, the PV or VUS-only result rate increased from 30.8% in early 2013 to 43.0% in late 2017, entirely due to the increase in VUS-only rates. VUS were identified in 8.1% of patients diagnosed in early 2013 and increased to 28.3% in patients diagnosed in late 2017.
Hypothesis #6: Racial or ethnic disparities in rates of VUS will diminish.
Among patients with breast cancer, racial or ethnic differences in PV rates were small and did not change over time. For patients with ovarian cancer, PV rates across racial or ethnic groups diminished over time.
However, for both breast and ovarian cancer patients, there were large differences in VUS-only rates by race and ethnicity that persisted during the interval studied.
In 2017, for patients with breast cancer, VUS-only rates were substantially higher in Asian (42.4%), Black (36.6%), and Hispanic (27.7%) patients than in non-Hispanic White patients (24.5%, P < .001).
Similar trends were noted for patients with ovarian cancer. VUS-only rates were substantially higher in Asian (47.8%), Black (46.0%), and Hispanic (36.8%) patients than in non-Hispanic White patients (24.6%, P < .001).
Multivariable logistic regressions were performed separately for tested patients with breast cancer and ovarian cancer, and the results showed no significant interaction between race or ethnicity and date. Therefore, there was no significant change in racial or ethnic differences in VUS-only results across the study period.
Where these findings leave clinicians in 2021
Among the patients studied, there was:
- Marked expansion in the number of genes sequenced.
- A likely modest trend toward testing patients with lower pretest risk of a PV.
- No sociodemographic differences in testing trends.
- A small increase in PV rates and a substantial increase in VUS-only rates.
- Near-complete replacement of selective testing by MGP.
For patients with breast cancer, the proportion of all PVs that were in BRCA1/2 fell substantially. Adoption of MGP testing doubled the probability of detecting a PV in other tested genes. Most of the increase was in genes with an established breast or ovarian cancer association, with fewer PVs found in other actionable genes and very few PVs in other tested genes.
Contrary to their hypothesis, the authors observed a sustained undertesting of patients with ovarian cancer. Only 34.3% performed versus nearly 100% recommended, with little change since 2014.
This finding is surprising – and tremendously disappointing – since the prevalence of BRCA1/2 PVs is higher in ovarian cancer than in other cancers (Gynecol Oncol. 2017 Nov;147[2]:375-380), and germline-targeted therapy with PARP inhibitors has been approved for use since 2014.
Furthermore, insurance carriers provide coverage for genetic testing in most patients with carcinoma of the ovary, fallopian tube, and/or peritoneum.
Action plans: Less could be more
During the period analyzed, the increase in VUS-only results dramatically outpaced the increase in PVs.
Since there is a substantially larger volume of clinical genetic testing in non-Hispanic White patients with breast or ovarian cancer, the spectrum of normal variation is less well-defined in other racial or ethnic groups.
The study showed a widening of the “racial-ethnic VUS gap,” with Black and Asian patients having nearly twofold more VUS, although they were not tested for more genes than non-Hispanic White patients.
This is problematic on several levels. Identification of a VUS is challenging for communicating results to and recommending cascade testing for family members.
There is worrisome information regarding overtreatment or counseling of VUS patients about their results. For example, the PROMPT registry showed that 10%-15% of women with PV/VUS in genes not associated with a high risk of ovarian cancer underwent oophorectomy without a clear indication for the procedure.
Although population-based testing might augment the available data on the spectrum of normal variation in racial and ethnic minorities, it would likely exacerbate the proliferation of VUS over PVs.
It is essential to accelerate ongoing approaches to VUS reclassification.
In addition, the authors suggest that it may be time to reverse the trend in increasing the number of genes tested in MGPs. Their rationale is that, in Georgia and California, most PVs among patients with breast and ovarian cancer were identified in 20 genes (ATM, BARD1, BRCA1, BRCA2, BRIP1, CDH1, CHEK2, EPCAM, MLH1, MSH2, MSH6, NBN, NF1, PMS2, PALB2, PTEN, RAD51C, RAD51D, STK11, and TP53).
If the Georgia and California data are representative of a more generalized pattern, a panel of 20 breast cancer– and/or ovarian cancer–associated genes may be ideal for maximizing the yield of clinically relevant PVs and minimizing VUS results for all patients.
Finally, defining the patient, clinician, and health care system factors that impede widespread genetic testing for ovarian cancer patients must be prioritized. As the authors suggest, quality improvement efforts should focus on getting a lot closer to testing rates of 100% for patients with ovarian cancer and building the database that will help sort VUS in minority patients into their proper context of pathogenicity, rather than adding more genes per test.
This research was supported by the National Cancer Institute, the Centers for Disease Control and Prevention, and the California Department of Public Health. The authors disclosed relationships with Myriad Genetics, Ambry Genetics, Color Genomics, GeneDx/BioReference, InVitae, Genentech, Genomic Health, Roche/Genentech, Oncoquest, Tesaro, and Karyopharm Therapeutics.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Investigators found racial and ethnic disparities in genetic testing as well as “persistent underuse” of testing in patients with ovarian cancer.
The team also discovered that most pathogenic variant (PV) results were in 20 genes associated with breast and/or ovarian cancer, and testing other genes largely revealed variants of uncertain significance (VUS).
Allison W. Kurian, MD, of Stanford (Calif.) University, and colleagues recounted these findings in the Journal of Clinical Oncology.
Because of improvements in sequencing technology, competition among commercial purveyors, and declining cost, genetic testing has been increasingly available to clinicians for patient management and cancer prevention (JAMA. 2015 Sep 8;314[10]:997-8). Although germline testing can guide therapy for several solid tumors, there is little research about how often and how well it is used in practice.
For their study, Dr. Kurian and colleagues used a SEER Genetic Testing Linkage Demonstration Project in a population-based assessment of testing for cancer risk. The investigators analyzed 7-year trends in testing among all women diagnosed with breast or ovarian cancer in Georgia or California from 2013 to 2017, reviewing testing patterns and result interpretation from 2012 to 2019.
Before analyzing the data, the investigators made the following hypotheses:
- Multigene panels (MGP) would entirely replace testing for BRCA1/2 only.
- Testing underutilization in patients with ovarian cancer would improve over time.
- More patients would be tested at lower levels of pretest risk for PVs.
- Sociodemographic differences in testing trends would not be observed.
- Detection of PVs and VUS would increase.
- Racial and ethnic disparities in rates of VUS would diminish.
Study conduct
The investigators examined genetic tests performed from 2012 through the beginning of 2019 at major commercial laboratories and linked that information with data in the SEER registries in Georgia and California on all breast and ovarian cancer patients diagnosed between 2013 and 2017. There were few criteria for exclusion.
Genetic testing results were categorized as identifying a PV or likely PV, VUS, or benign or likely benign mutation by American College of Medical Genetics criteria. When a patient had genetic testing on more than one occasion, the most recent test was used.
If a PV was identified, the types of PVs were grouped according to the level of evidence that supported pathogenicity into the following categories:
- BRCA1 or BRCA2 mutations.
- PVs in other genes designated by the National Comprehensive Cancer Network as associated with breast or ovarian cancer (e.g., ATM, BARD1, BRIP1, CDH1, CHEK2, EPCAM, MLH1, MSH2, MSH6, NBN, NF1, PALB2, MS2, PTEN, RAD51C, RAD51D, STK11, and TP53).
- PVs in other actionable genes (e.g., APC, BMPR1A, MEN1, MUTYH, NF2, RB1, RET, SDHAF2, SDHB, SDHC, SDHD, SMAD4, TSC1, TSC2, and VHL).
- Any other tested genes.
The investigators also tabulated instances in which genetic testing identified a VUS in any gene but no PV. If a VUS was identified originally and was reclassified more recently into the “PV/likely PV” or “benign/likely benign” categories, only the resolved categorization was recorded.
The authors evaluated clinical and sociodemographic correlates of testing trends for breast and ovarian cancer, assessing the relationship between race, age, and geographic site in receipt of any test or type of test.
Among laboratories, the investigators examined trends in the number of genes tested, associations with sociodemographic factors, categories of test results, and whether trends differed by race or ethnicity.
Findings, by hypothesis
Hypothesis #1: MGP will entirely replace testing for BRCA1/2 only.
About 25% of tested patients with breast cancer diagnosed in early 2013 received MGP, compared with more than 80% of those diagnosed in late 2017.
The trend for ovarian cancer was similar. About 40% of patients diagnosed in early 2013 received MGP, compared with more than 90% diagnosed in late 2017. These trends were similar in California and Georgia.
From 2012 to 2019, there was a consistent upward trend in gene number for patients with breast cancer (mean, 19) or ovarian cancer (mean, 21), from approximately 10 genes to 35 genes.
Hypothesis #2: Underutilization of testing in patients with ovarian cancer will improve.
Among the 187,535 patients with breast cancer and the 14,689 patients with ovarian cancer diagnosed in Georgia or California from 2013 through 2017, on average, testing rates increased 2% per year.
In all, 25.2% of breast cancer patients and 34.3% of ovarian cancer patients had genetic testing on one (87.3%) or more (12.7%) occasions.
Prior research suggested that, in 2013 and 2014, 31% of women with ovarian cancer had genetic testing (JAMA Oncol. 2018 Aug 1;4[8]:1066-72/ J Clin Oncol. 2019 May 20;37[15]:1305-15).
The investigators therefore concluded that underutilization of genetic testing in ovarian cancer did not improve substantially during the 7-year interval analyzed.
Hypothesis #3: More patients will be tested at lower levels of pretest risk.
These data were more difficult to abstract from the SEER database, but older patients were more likely to be tested in later years.
In patients older than 60 years of age (who accounted for more than 50% of both cancer cohorts), testing rates increased from 11.1% to 14.9% for breast cancer and 25.3% to 31.4% for ovarian cancer. By contrast, patients younger than 45 years of age were less than 15% of the sample and had lower testing rates over time.
There were no substantial changes in testing rates by other clinical variables. Therefore, in concert with the age-related testing trends, it is likely that women were tested for genetic mutations at increasingly lower levels of pretest risk.
Hypothesis #4: Sociodemographic differences in testing trends will not be observed.
Among patients with breast cancer, approximately 31% of those who had genetic testing were uninsured, 31% had Medicaid, and 26% had private insurance, Medicare, or other insurance.
For patients with ovarian cancer, approximately 28% were uninsured, 27% had Medicaid, and 39% had private insurance, Medicare, or other insurance.
The authors had previously found that less testing was associated with Black race, greater poverty, and less insurance coverage (J Clin Oncol. 2019 May 20;37[15]:1305-15). However, they noted no changes in testing rates by sociodemographic variables over time.
Hypothesis #5: Detection of both PVs and VUS will increase.
The proportion of tested breast cancer patients with PVs in BRCA1/2 decreased from 7.5% to 5.0% (P < .001), whereas PV yield for the two other clinically salient categories (breast or ovarian and other actionable genes) increased.
The proportion of PVs in any breast or ovarian gene increased from 1.3% to 4.6%, and the proportion in any other actionable gene increased from 0.3% to 1.3%.
For breast cancer patients, VUS-only rates increased from 8.5% in early 2013 to 22.4% in late 2017.
For ovarian cancer patients, the yield of PVs in BRCA1/2 decreased from 15.7% to 12.4% (P < .001), whereas the PV yield for breast or ovarian genes increased from 3.9% to 4.3%, and the yield for other actionable genes increased from 0.3% to 2.0%.
In ovarian cancer patients, the PV or VUS-only result rate increased from 30.8% in early 2013 to 43.0% in late 2017, entirely due to the increase in VUS-only rates. VUS were identified in 8.1% of patients diagnosed in early 2013 and increased to 28.3% in patients diagnosed in late 2017.
Hypothesis #6: Racial or ethnic disparities in rates of VUS will diminish.
Among patients with breast cancer, racial or ethnic differences in PV rates were small and did not change over time. For patients with ovarian cancer, PV rates across racial or ethnic groups diminished over time.
However, for both breast and ovarian cancer patients, there were large differences in VUS-only rates by race and ethnicity that persisted during the interval studied.
In 2017, for patients with breast cancer, VUS-only rates were substantially higher in Asian (42.4%), Black (36.6%), and Hispanic (27.7%) patients than in non-Hispanic White patients (24.5%, P < .001).
Similar trends were noted for patients with ovarian cancer. VUS-only rates were substantially higher in Asian (47.8%), Black (46.0%), and Hispanic (36.8%) patients than in non-Hispanic White patients (24.6%, P < .001).
Multivariable logistic regressions were performed separately for tested patients with breast cancer and ovarian cancer, and the results showed no significant interaction between race or ethnicity and date. Therefore, there was no significant change in racial or ethnic differences in VUS-only results across the study period.
Where these findings leave clinicians in 2021
Among the patients studied, there was:
- Marked expansion in the number of genes sequenced.
- A likely modest trend toward testing patients with lower pretest risk of a PV.
- No sociodemographic differences in testing trends.
- A small increase in PV rates and a substantial increase in VUS-only rates.
- Near-complete replacement of selective testing by MGP.
For patients with breast cancer, the proportion of all PVs that were in BRCA1/2 fell substantially. Adoption of MGP testing doubled the probability of detecting a PV in other tested genes. Most of the increase was in genes with an established breast or ovarian cancer association, with fewer PVs found in other actionable genes and very few PVs in other tested genes.
Contrary to their hypothesis, the authors observed a sustained undertesting of patients with ovarian cancer. Only 34.3% performed versus nearly 100% recommended, with little change since 2014.
This finding is surprising – and tremendously disappointing – since the prevalence of BRCA1/2 PVs is higher in ovarian cancer than in other cancers (Gynecol Oncol. 2017 Nov;147[2]:375-380), and germline-targeted therapy with PARP inhibitors has been approved for use since 2014.
Furthermore, insurance carriers provide coverage for genetic testing in most patients with carcinoma of the ovary, fallopian tube, and/or peritoneum.
Action plans: Less could be more
During the period analyzed, the increase in VUS-only results dramatically outpaced the increase in PVs.
Since there is a substantially larger volume of clinical genetic testing in non-Hispanic White patients with breast or ovarian cancer, the spectrum of normal variation is less well-defined in other racial or ethnic groups.
The study showed a widening of the “racial-ethnic VUS gap,” with Black and Asian patients having nearly twofold more VUS, although they were not tested for more genes than non-Hispanic White patients.
This is problematic on several levels. Identification of a VUS is challenging for communicating results to and recommending cascade testing for family members.
There is worrisome information regarding overtreatment or counseling of VUS patients about their results. For example, the PROMPT registry showed that 10%-15% of women with PV/VUS in genes not associated with a high risk of ovarian cancer underwent oophorectomy without a clear indication for the procedure.
Although population-based testing might augment the available data on the spectrum of normal variation in racial and ethnic minorities, it would likely exacerbate the proliferation of VUS over PVs.
It is essential to accelerate ongoing approaches to VUS reclassification.
In addition, the authors suggest that it may be time to reverse the trend in increasing the number of genes tested in MGPs. Their rationale is that, in Georgia and California, most PVs among patients with breast and ovarian cancer were identified in 20 genes (ATM, BARD1, BRCA1, BRCA2, BRIP1, CDH1, CHEK2, EPCAM, MLH1, MSH2, MSH6, NBN, NF1, PMS2, PALB2, PTEN, RAD51C, RAD51D, STK11, and TP53).
If the Georgia and California data are representative of a more generalized pattern, a panel of 20 breast cancer– and/or ovarian cancer–associated genes may be ideal for maximizing the yield of clinically relevant PVs and minimizing VUS results for all patients.
Finally, defining the patient, clinician, and health care system factors that impede widespread genetic testing for ovarian cancer patients must be prioritized. As the authors suggest, quality improvement efforts should focus on getting a lot closer to testing rates of 100% for patients with ovarian cancer and building the database that will help sort VUS in minority patients into their proper context of pathogenicity, rather than adding more genes per test.
This research was supported by the National Cancer Institute, the Centers for Disease Control and Prevention, and the California Department of Public Health. The authors disclosed relationships with Myriad Genetics, Ambry Genetics, Color Genomics, GeneDx/BioReference, InVitae, Genentech, Genomic Health, Roche/Genentech, Oncoquest, Tesaro, and Karyopharm Therapeutics.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Ovarian cancer prevention: How patients decide on surgery
Investigators interviewed 24 premenopausal women with BRCA mutations who were considering prophylactic surgery. Responses showed that women who prioritized cancer risk reduction opted for risk-reducing salpingo-oophorectomy (RRSO), while those who were more concerned about the effects of surgical menopause opted for risk-reducing early salpingectomy with delayed oophorectomy (RRESDO). Factors such as past surgical experience influenced patients’ decisions as well.
The women were participants in the PROTECTOR trial, which was designed to determine the impact of RRESDO on sexual function by comparing RRESDO, RRSO, and no surgery. Study participants made their own decisions regarding surgery, and the interviews with 24 participants provided insight into how patients made their decisions.
Faiza Gaba, MBBS, PhD, of Queen Mary University of London, and colleagues reviewed the results in the Journal of Medical Genetics.
“I think what is most important is that even though this is a tough decision women are being given, they are being given options that allow them to make up their own minds with support from health care providers,” said Barbara A. Goff, MD, of the University of Washington, Seattle, when asked to comment on this research.
Study details and results
The investigators interviewed 24 women aged 34-46 years. Fourteen patients were BRCA1 carriers, and 10 were BRCA2 carriers. Twenty-two women were White, and two were Asian. Nineteen women were married, and four were nulliparous.
The interviews lasted a mean of 55 minutes and were conducted with a predeveloped topic guide.
Eleven of the women interviewed opted for RRESDO, seven opted for RRSO, and six decided against prophylactic surgery. Four women who had previous breast cancer all opted for ovarian cancer prevention surgery.
Among the 18 women who chose surgery, 16 had completed childbearing, and 2 didn’t want children. Among the six women who decided against surgery, four had not completed childbearing, one was unsure of which procedure to choose, and the sixth had strong feelings against removing healthy tissue.
The 11 women who chose RRESDO did so because of concerns about early menopause following oophorectomy, particularly low mood, sexual dysfunction, and poorer quality of life. They were also more likely to have had a previous positive surgical experience, so they were not as concerned about a two-step surgery.
Women who chose RRSO were motivated by a strong family history of ovarian cancer, fear of dying, concurrent benign gynecological issues, lack of screening for ovarian cancer, and physician advice.
Nine women also opted for risk-reducing mastectomy (RRM), which was deemed a more difficult decision than prophylactic ovarian cancer surgery.
Women who decided against RRM did so because they hadn’t completed childbearing, they were concerned about recovery time and the psychological effects of the surgery, or they had confidence in breast cancer screening and treatment.
Among women who chose RRM, two highlighted lack of health care professional support for deciding against reconstruction. One interviewee regretted opting for flap reconstruction due to the resultant cosmetic appearance and chronic pain but did not regret RRM.
Fifteen women said they would have preferred combined RRM and ovarian cancer prevention in one surgery, due to less anxiety, less waiting, fewer appointments, less time off work, and a single surgical recovery.
The women advised fellow BRCA carriers to avoid time pressure with surgical decision-making, talk to other BRCA carriers, do personal research, and ask for second opinions if not satisfied. They also said the opportunity to ask experts questions and meet other BRCA carriers face-to-face was more helpful than online support groups. Women managed in specialist settings said they received better care, better access to hormone replacement therapy, and were more satisfied.
High-risk women need to be supported by a multidisciplinary team of geneticists, gynecologists/oncologists, oncoplastic surgeons, menopause specialists, fertility specialists, psychologists, and specialist nurses, the investigators wrote.
This study was funded by the Rosetrees Trust and Barts Charity. The authors disclosed relationships with AstraZeneca, Merck, Cancer Research UK, The Eve Appeal, Israel National Institute for Health Policy Research, and the UK National Health Service. Dr. Goff has no relevant disclosures.
Investigators interviewed 24 premenopausal women with BRCA mutations who were considering prophylactic surgery. Responses showed that women who prioritized cancer risk reduction opted for risk-reducing salpingo-oophorectomy (RRSO), while those who were more concerned about the effects of surgical menopause opted for risk-reducing early salpingectomy with delayed oophorectomy (RRESDO). Factors such as past surgical experience influenced patients’ decisions as well.
The women were participants in the PROTECTOR trial, which was designed to determine the impact of RRESDO on sexual function by comparing RRESDO, RRSO, and no surgery. Study participants made their own decisions regarding surgery, and the interviews with 24 participants provided insight into how patients made their decisions.
Faiza Gaba, MBBS, PhD, of Queen Mary University of London, and colleagues reviewed the results in the Journal of Medical Genetics.
“I think what is most important is that even though this is a tough decision women are being given, they are being given options that allow them to make up their own minds with support from health care providers,” said Barbara A. Goff, MD, of the University of Washington, Seattle, when asked to comment on this research.
Study details and results
The investigators interviewed 24 women aged 34-46 years. Fourteen patients were BRCA1 carriers, and 10 were BRCA2 carriers. Twenty-two women were White, and two were Asian. Nineteen women were married, and four were nulliparous.
The interviews lasted a mean of 55 minutes and were conducted with a predeveloped topic guide.
Eleven of the women interviewed opted for RRESDO, seven opted for RRSO, and six decided against prophylactic surgery. Four women who had previous breast cancer all opted for ovarian cancer prevention surgery.
Among the 18 women who chose surgery, 16 had completed childbearing, and 2 didn’t want children. Among the six women who decided against surgery, four had not completed childbearing, one was unsure of which procedure to choose, and the sixth had strong feelings against removing healthy tissue.
The 11 women who chose RRESDO did so because of concerns about early menopause following oophorectomy, particularly low mood, sexual dysfunction, and poorer quality of life. They were also more likely to have had a previous positive surgical experience, so they were not as concerned about a two-step surgery.
Women who chose RRSO were motivated by a strong family history of ovarian cancer, fear of dying, concurrent benign gynecological issues, lack of screening for ovarian cancer, and physician advice.
Nine women also opted for risk-reducing mastectomy (RRM), which was deemed a more difficult decision than prophylactic ovarian cancer surgery.
Women who decided against RRM did so because they hadn’t completed childbearing, they were concerned about recovery time and the psychological effects of the surgery, or they had confidence in breast cancer screening and treatment.
Among women who chose RRM, two highlighted lack of health care professional support for deciding against reconstruction. One interviewee regretted opting for flap reconstruction due to the resultant cosmetic appearance and chronic pain but did not regret RRM.
Fifteen women said they would have preferred combined RRM and ovarian cancer prevention in one surgery, due to less anxiety, less waiting, fewer appointments, less time off work, and a single surgical recovery.
The women advised fellow BRCA carriers to avoid time pressure with surgical decision-making, talk to other BRCA carriers, do personal research, and ask for second opinions if not satisfied. They also said the opportunity to ask experts questions and meet other BRCA carriers face-to-face was more helpful than online support groups. Women managed in specialist settings said they received better care, better access to hormone replacement therapy, and were more satisfied.
High-risk women need to be supported by a multidisciplinary team of geneticists, gynecologists/oncologists, oncoplastic surgeons, menopause specialists, fertility specialists, psychologists, and specialist nurses, the investigators wrote.
This study was funded by the Rosetrees Trust and Barts Charity. The authors disclosed relationships with AstraZeneca, Merck, Cancer Research UK, The Eve Appeal, Israel National Institute for Health Policy Research, and the UK National Health Service. Dr. Goff has no relevant disclosures.
Investigators interviewed 24 premenopausal women with BRCA mutations who were considering prophylactic surgery. Responses showed that women who prioritized cancer risk reduction opted for risk-reducing salpingo-oophorectomy (RRSO), while those who were more concerned about the effects of surgical menopause opted for risk-reducing early salpingectomy with delayed oophorectomy (RRESDO). Factors such as past surgical experience influenced patients’ decisions as well.
The women were participants in the PROTECTOR trial, which was designed to determine the impact of RRESDO on sexual function by comparing RRESDO, RRSO, and no surgery. Study participants made their own decisions regarding surgery, and the interviews with 24 participants provided insight into how patients made their decisions.
Faiza Gaba, MBBS, PhD, of Queen Mary University of London, and colleagues reviewed the results in the Journal of Medical Genetics.
“I think what is most important is that even though this is a tough decision women are being given, they are being given options that allow them to make up their own minds with support from health care providers,” said Barbara A. Goff, MD, of the University of Washington, Seattle, when asked to comment on this research.
Study details and results
The investigators interviewed 24 women aged 34-46 years. Fourteen patients were BRCA1 carriers, and 10 were BRCA2 carriers. Twenty-two women were White, and two were Asian. Nineteen women were married, and four were nulliparous.
The interviews lasted a mean of 55 minutes and were conducted with a predeveloped topic guide.
Eleven of the women interviewed opted for RRESDO, seven opted for RRSO, and six decided against prophylactic surgery. Four women who had previous breast cancer all opted for ovarian cancer prevention surgery.
Among the 18 women who chose surgery, 16 had completed childbearing, and 2 didn’t want children. Among the six women who decided against surgery, four had not completed childbearing, one was unsure of which procedure to choose, and the sixth had strong feelings against removing healthy tissue.
The 11 women who chose RRESDO did so because of concerns about early menopause following oophorectomy, particularly low mood, sexual dysfunction, and poorer quality of life. They were also more likely to have had a previous positive surgical experience, so they were not as concerned about a two-step surgery.
Women who chose RRSO were motivated by a strong family history of ovarian cancer, fear of dying, concurrent benign gynecological issues, lack of screening for ovarian cancer, and physician advice.
Nine women also opted for risk-reducing mastectomy (RRM), which was deemed a more difficult decision than prophylactic ovarian cancer surgery.
Women who decided against RRM did so because they hadn’t completed childbearing, they were concerned about recovery time and the psychological effects of the surgery, or they had confidence in breast cancer screening and treatment.
Among women who chose RRM, two highlighted lack of health care professional support for deciding against reconstruction. One interviewee regretted opting for flap reconstruction due to the resultant cosmetic appearance and chronic pain but did not regret RRM.
Fifteen women said they would have preferred combined RRM and ovarian cancer prevention in one surgery, due to less anxiety, less waiting, fewer appointments, less time off work, and a single surgical recovery.
The women advised fellow BRCA carriers to avoid time pressure with surgical decision-making, talk to other BRCA carriers, do personal research, and ask for second opinions if not satisfied. They also said the opportunity to ask experts questions and meet other BRCA carriers face-to-face was more helpful than online support groups. Women managed in specialist settings said they received better care, better access to hormone replacement therapy, and were more satisfied.
High-risk women need to be supported by a multidisciplinary team of geneticists, gynecologists/oncologists, oncoplastic surgeons, menopause specialists, fertility specialists, psychologists, and specialist nurses, the investigators wrote.
This study was funded by the Rosetrees Trust and Barts Charity. The authors disclosed relationships with AstraZeneca, Merck, Cancer Research UK, The Eve Appeal, Israel National Institute for Health Policy Research, and the UK National Health Service. Dr. Goff has no relevant disclosures.
FROM THE JOURNAL OF MEDICAL GENETICS