User login
Are oncologists ready to confront a second wave of COVID-19?
Canceled appointments, postponed surgeries, and delayed cancer diagnoses – all are a recipe for exhaustion for oncologists around the world, struggling to reach and treat their patients during the pandemic. Physicians and their teams felt the pain as COVID-19 took its initial march around the globe.
“We saw the distress of people with cancer who could no longer get to anyone on the phone. Their medical visit was usually canceled. Their radiotherapy session was postponed or modified, and chemotherapy postponed,” says Axel Kahn, MD, chairman of the board of directors of La Ligue Nationale Contre le Cancer (National League Against Cancer). “In the vast majority of cases, cancer treatment can be postponed or readjusted, without affecting the patient’s chances of survival, but there has been a lot of anxiety because the patients do not know that.”
The stay-at-home factor was one that played out across many months during the first wave.
“I believe that the ‘stay-home’ message that we transmitted was rigorously followed by patients who should have come to the emergency room much earlier and who, therefore, were admitted with a much more deteriorated general condition than in non-COVID-19 times,” says Benjamín Domingo Arrué, MD, from the department of medical oncology at Hospital Universitari i Politècnic La Fe in Valencia, Spain.
And in Brazil, some of the impact from the initial hit of COVID-19 on oncology is only now being felt, according to Laura Testa, MD, head of breast medical oncology, Instituto do Câncer do Estado de São Paulo.
“We are starting to see a lot of cancer cases that didn’t show up at the beginning of the pandemic, but now they are arriving to us already in advanced stages,” she said. “These patients need hospital care. If the situation worsens and goes back to what we saw at the peak of the curve, I fear the public system won’t be able to treat properly the oncology patients that need hospital care and the patients with cancer who also have COVID-19.”
But even as health care worker fatigue and concerns linger, oncologists say that what they have learned in the last 6 months has helped them prepare as COVID-19 cases increase and a second global wave kicks up.
Lessons from the first wave
In the United States, COVID-19 hit different regions at different times and to different degrees. One of the areas hit first was Seattle.
“We jumped on top of this, we were evidence based, we put things in place very, very quickly,” said Julie Gralow, MD, professor at the University of Washington and the Fred Hutchinson Cancer Research Center, both in Seattle.
“We did a really good job keeping COVID out of our cancer centers,” Dr. Gralow said. “We learned how to be super safe, and to keep symptomatic people out of the building, and to limit the extra people they could bring with them. It’s all about the number of contacts you have.”
The story was different, though, for oncologists in several other countries, and sometimes it varied immensely within each nation.
“We treated fewer patients with cancer during the first wave,” says Dirk Arnold, MD, medical director of the Asklepios Tumor Center Hamburg (Germany), in an interview. “In part, this was because staff were quarantined and because we had a completely different infrastructure in all of the hospitals. But also fewer patients with cancer came to the clinic at all. A lot of resources were directed toward COVID-19.”
In Spain, telemedicine helped keep up with visits, but other areas felt the effect of COVID-19 patient loads.
“At least in the oncology department of our center, we have practically maintained 100% of visits, mostly by telephone,” says Dr. Arrué, “but the reality is that our country has not yet been prepared for telemedicine.”
Laura Mezquita, MD, of the department of medical oncology at Hospital Clinic de Barcelona, describes a more dramatic situation: “We have seen how some of our patients, especially with metastatic disease, have been dismissed for intensive care and life-support treatments, as well as specific treatments against COVID-19 (tocilizumab, remdesivir, etc.) due to the general health collapse of the former wave,” she said. She adds that specific oncologic populations, such as those with thoracic tumors, have been more affected.
Distress among oncologists
Many oncologists are still feeling stressed and fatigued after the first wave, just as a second string of outbreaks is on its way.
A survey presented at last month’s ESMO 2020 Congress found that, in July-August, moral distress was reported by one-third of the oncologists who responded, and more than half reported a feeling of exhaustion.
“The tiredness and team exhaustion is noticeable,” said Dr. Arnold. “We recently had a task force discussion about what will happen when we have a second wave and how the department and our services will adapt. It was clear that those who were at the very front in the first wave had only a limited desire to do that again in the second wave.”
Another concern: COVID-19’s effect on staffing levels.
“We have a population of young caregivers who are affected by the COVID-19 disease with an absenteeism rate that is quite unprecedented,” said Sophie Beaupère, general delegate of Unicancer since January.
She said that, in general, the absenteeism rate in the cancer centers averages 5%-6%, depending on the year. But that rate is now skyrocketing.
Stop-start cycle for surgery
As caregivers quarantined around the world, more than 10% of patients with cancer had treatment canceled or delayed during the first wave of the pandemic, according to another survey from ESMO, involving 109 oncologists from 18 countries.
Difficulties were reported for surgeries by 34% of the centers, but also difficulties with delivering chemotherapy (22% of centers), radiotherapy (13.7%), and therapy with checkpoint inhibitors (9.1%), monoclonal antibodies (9%), and oral targeted therapy (3.7%).
Stopping surgery is a real concern in France, noted Dr. Kahn, the National League Against Cancer chair. He says that in regions that were badly hit by COVID-19, “it was not possible to have access to the operating room for people who absolutely needed surgery; for example, patients with lung cancer that was still operable. Most of the recovery rooms were mobilized for resuscitation.”
There may be some solutions, suggested Thierry Breton, director general of the National Institute of Cancer in France. “We are getting prepared, with the health ministry, for a possible increase in hospital tension, which would lead to a situation where we would have to reschedule operations. Nationally, regionally, and locally, we are seeing how we can resume and prioritize surgeries that have not been done.”
Delays in cancer diagnosis
While COVID-19 affected treatment, many oncologists say the major impact of the first wave was a delay in diagnosing cancer. Some of this was a result of the suspension of cancer screening programs, but there was also fear among the general public about visiting clinics and hospitals during a pandemic.
“We didn’t do so well with cancer during the first wave here in the U.K.,” said Karol Sikora, PhD, MBBChir, professor of cancer medicine and founding dean at the University of Buckingham Medical School, London. “Cancer diagnostic pathways virtually stalled partly because patients didn’t seek help, but getting scans and biopsies was also very difficult. Even patients referred urgently under the ‘2-weeks-wait’ rule were turned down.”
In France, “the delay in diagnosis is indisputable,” said Dr. Kahn. “About 50% of the cancer diagnoses one would expect during this period were missed.”
“I am worried that there remains a major traffic jam that has not been caught up with, and, in the meantime, the health crisis is worsening,” he added.
In Seattle, Dr. Gralow said the first COVID-19 wave had little impact on treatment for breast cancer, but it was in screening for breast cancer “where things really got messed up.”
“Even though we’ve been fully ramped up again,” she said, concerns remain. To ensure that screening mammography is maintained, “we have spaced out the visits to keep our waiting rooms less populated, with a longer time between using the machine so we can clean it. To do this, we have extended operating hours and are now opening on Saturday.
“So we’re actually at 100% of our capacity, but I’m really nervous, though, that a lot of people put off their screening mammogram and aren’t going to come in and get it.
“Not only did people get the message to stay home and not do nonessential things, but I think a lot of people lost their health insurance when they lost their jobs,” she said, and without health insurance, they are not covered for cancer screening.
Looking ahead, with a plan
Many oncologists agree that access to care can and must be improved – and there were some positive moves.
“Some regimens changed during the first months of the pandemic, and I don’t see them going back to the way they were anytime soon,” said Dr. Testa. “The changes/adaptations that were made to minimize the chance of SARS-CoV-2 infection are still in place and will go on for a while. In this context, telemedicine helped a lot. The pandemic forced the stakeholders to step up and put it in place in March. And now it’s here to stay.”
The experience gained in the last several months has driven preparation for the next wave.
“We are not going to see the disorganization that we saw during the first wave,” said Florence Joly, MD, PhD, head of medical oncology at the Centre François Baclesse in Caen, France. “The difference between now and earlier this year is that COVID diagnostic tests are available. That was one of the problems in the first wave. We had no way to diagnose.”
On the East Coast of the United States, medical oncologist Charu Aggarwal, MD, MPH, is also optimistic: “I think we’re at a place where we can manage.”
“I believe if there was going to be a new wave of COVID-19 cases we would be: better psychologically prepared and better organized,” said Dr. Aggarwal, assistant professor of medicine in the hematology-oncology division at the University of Pennsylvania, Philadelphia. “We already have experience with all of the tools, we have telemedicine available, we have screening protocols available, we have testing, we are already universally masking, everyone’s hand-washing, so I do think that means we would be okay.”
Dr. Arnold agreed that “we are much better prepared than for the first wave, but … we have immense tasks in the area of patient management, the digitization of patient care, the clear allocation of resources when there is a second or third wave. In many areas of preparation, I believe, unfortunately, we are not as well positioned as we had actually hoped.”
The first wave of COVID hit cancer services in the United Kingdom particularly hard: One modeling study suggested that delays in cancer referrals will lead to thousands of additional deaths and tens of thousands of life-years lost.
“Cancer services are working at near normal levels now, but they are still fragile and could be severely compromised again if the NHS [National Health Service] gets flooded by COVID patients,” said Dr. Sikora.
The second wave may be different. “Although the number of infections has increased, the hospitalizations have only risen a little. Let’s see what happens,” he said in an interview. Since then, however, infections have continued to rise, and there has been an increase in hospitalizations. New social distancing measures in the United Kingdom were put into place on Oct. 12, with the aim of protecting the NHS from overload.
Dr. Arrué describes it this way: “The reality is that the ‘second wave’ has left behind the initial grief and shock that both patients and health professionals experienced when faced with something that, until now, we had only seen in the movies.” The second wave has led to new restrictions – including a partial lockdown since the beginning of October.
Dr. Aggarwal says her department recently had a conference with Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, about the impact of COVID-19 on oncology.
“I asked him what advice he’d give oncologists, and he said to go back to as much screening as you were doing previously as quickly as possible. That’s what must be relayed to our oncologists in the community – and also to primary care physicians – because they are often the ones who are ordering and championing the screening efforts.”
This article was originated by Aude Lecrubier, Medscape French edition, and developed by Zosia Chustecka, Medscape Oncology. With additional reporting by Kate Johnson, freelance medical journalist, Claudia Gottschling for Medscape Germany, Leoleli Schwartz for Medscape em português, Tim Locke for Medscape United Kingdom, and Carla Nieto Martínez, freelance medical journalist for Medscape Spanish edition.
This article first appeared on Medscape.com.
Canceled appointments, postponed surgeries, and delayed cancer diagnoses – all are a recipe for exhaustion for oncologists around the world, struggling to reach and treat their patients during the pandemic. Physicians and their teams felt the pain as COVID-19 took its initial march around the globe.
“We saw the distress of people with cancer who could no longer get to anyone on the phone. Their medical visit was usually canceled. Their radiotherapy session was postponed or modified, and chemotherapy postponed,” says Axel Kahn, MD, chairman of the board of directors of La Ligue Nationale Contre le Cancer (National League Against Cancer). “In the vast majority of cases, cancer treatment can be postponed or readjusted, without affecting the patient’s chances of survival, but there has been a lot of anxiety because the patients do not know that.”
The stay-at-home factor was one that played out across many months during the first wave.
“I believe that the ‘stay-home’ message that we transmitted was rigorously followed by patients who should have come to the emergency room much earlier and who, therefore, were admitted with a much more deteriorated general condition than in non-COVID-19 times,” says Benjamín Domingo Arrué, MD, from the department of medical oncology at Hospital Universitari i Politècnic La Fe in Valencia, Spain.
And in Brazil, some of the impact from the initial hit of COVID-19 on oncology is only now being felt, according to Laura Testa, MD, head of breast medical oncology, Instituto do Câncer do Estado de São Paulo.
“We are starting to see a lot of cancer cases that didn’t show up at the beginning of the pandemic, but now they are arriving to us already in advanced stages,” she said. “These patients need hospital care. If the situation worsens and goes back to what we saw at the peak of the curve, I fear the public system won’t be able to treat properly the oncology patients that need hospital care and the patients with cancer who also have COVID-19.”
But even as health care worker fatigue and concerns linger, oncologists say that what they have learned in the last 6 months has helped them prepare as COVID-19 cases increase and a second global wave kicks up.
Lessons from the first wave
In the United States, COVID-19 hit different regions at different times and to different degrees. One of the areas hit first was Seattle.
“We jumped on top of this, we were evidence based, we put things in place very, very quickly,” said Julie Gralow, MD, professor at the University of Washington and the Fred Hutchinson Cancer Research Center, both in Seattle.
“We did a really good job keeping COVID out of our cancer centers,” Dr. Gralow said. “We learned how to be super safe, and to keep symptomatic people out of the building, and to limit the extra people they could bring with them. It’s all about the number of contacts you have.”
The story was different, though, for oncologists in several other countries, and sometimes it varied immensely within each nation.
“We treated fewer patients with cancer during the first wave,” says Dirk Arnold, MD, medical director of the Asklepios Tumor Center Hamburg (Germany), in an interview. “In part, this was because staff were quarantined and because we had a completely different infrastructure in all of the hospitals. But also fewer patients with cancer came to the clinic at all. A lot of resources were directed toward COVID-19.”
In Spain, telemedicine helped keep up with visits, but other areas felt the effect of COVID-19 patient loads.
“At least in the oncology department of our center, we have practically maintained 100% of visits, mostly by telephone,” says Dr. Arrué, “but the reality is that our country has not yet been prepared for telemedicine.”
Laura Mezquita, MD, of the department of medical oncology at Hospital Clinic de Barcelona, describes a more dramatic situation: “We have seen how some of our patients, especially with metastatic disease, have been dismissed for intensive care and life-support treatments, as well as specific treatments against COVID-19 (tocilizumab, remdesivir, etc.) due to the general health collapse of the former wave,” she said. She adds that specific oncologic populations, such as those with thoracic tumors, have been more affected.
Distress among oncologists
Many oncologists are still feeling stressed and fatigued after the first wave, just as a second string of outbreaks is on its way.
A survey presented at last month’s ESMO 2020 Congress found that, in July-August, moral distress was reported by one-third of the oncologists who responded, and more than half reported a feeling of exhaustion.
“The tiredness and team exhaustion is noticeable,” said Dr. Arnold. “We recently had a task force discussion about what will happen when we have a second wave and how the department and our services will adapt. It was clear that those who were at the very front in the first wave had only a limited desire to do that again in the second wave.”
Another concern: COVID-19’s effect on staffing levels.
“We have a population of young caregivers who are affected by the COVID-19 disease with an absenteeism rate that is quite unprecedented,” said Sophie Beaupère, general delegate of Unicancer since January.
She said that, in general, the absenteeism rate in the cancer centers averages 5%-6%, depending on the year. But that rate is now skyrocketing.
Stop-start cycle for surgery
As caregivers quarantined around the world, more than 10% of patients with cancer had treatment canceled or delayed during the first wave of the pandemic, according to another survey from ESMO, involving 109 oncologists from 18 countries.
Difficulties were reported for surgeries by 34% of the centers, but also difficulties with delivering chemotherapy (22% of centers), radiotherapy (13.7%), and therapy with checkpoint inhibitors (9.1%), monoclonal antibodies (9%), and oral targeted therapy (3.7%).
Stopping surgery is a real concern in France, noted Dr. Kahn, the National League Against Cancer chair. He says that in regions that were badly hit by COVID-19, “it was not possible to have access to the operating room for people who absolutely needed surgery; for example, patients with lung cancer that was still operable. Most of the recovery rooms were mobilized for resuscitation.”
There may be some solutions, suggested Thierry Breton, director general of the National Institute of Cancer in France. “We are getting prepared, with the health ministry, for a possible increase in hospital tension, which would lead to a situation where we would have to reschedule operations. Nationally, regionally, and locally, we are seeing how we can resume and prioritize surgeries that have not been done.”
Delays in cancer diagnosis
While COVID-19 affected treatment, many oncologists say the major impact of the first wave was a delay in diagnosing cancer. Some of this was a result of the suspension of cancer screening programs, but there was also fear among the general public about visiting clinics and hospitals during a pandemic.
“We didn’t do so well with cancer during the first wave here in the U.K.,” said Karol Sikora, PhD, MBBChir, professor of cancer medicine and founding dean at the University of Buckingham Medical School, London. “Cancer diagnostic pathways virtually stalled partly because patients didn’t seek help, but getting scans and biopsies was also very difficult. Even patients referred urgently under the ‘2-weeks-wait’ rule were turned down.”
In France, “the delay in diagnosis is indisputable,” said Dr. Kahn. “About 50% of the cancer diagnoses one would expect during this period were missed.”
“I am worried that there remains a major traffic jam that has not been caught up with, and, in the meantime, the health crisis is worsening,” he added.
In Seattle, Dr. Gralow said the first COVID-19 wave had little impact on treatment for breast cancer, but it was in screening for breast cancer “where things really got messed up.”
“Even though we’ve been fully ramped up again,” she said, concerns remain. To ensure that screening mammography is maintained, “we have spaced out the visits to keep our waiting rooms less populated, with a longer time between using the machine so we can clean it. To do this, we have extended operating hours and are now opening on Saturday.
“So we’re actually at 100% of our capacity, but I’m really nervous, though, that a lot of people put off their screening mammogram and aren’t going to come in and get it.
“Not only did people get the message to stay home and not do nonessential things, but I think a lot of people lost their health insurance when they lost their jobs,” she said, and without health insurance, they are not covered for cancer screening.
Looking ahead, with a plan
Many oncologists agree that access to care can and must be improved – and there were some positive moves.
“Some regimens changed during the first months of the pandemic, and I don’t see them going back to the way they were anytime soon,” said Dr. Testa. “The changes/adaptations that were made to minimize the chance of SARS-CoV-2 infection are still in place and will go on for a while. In this context, telemedicine helped a lot. The pandemic forced the stakeholders to step up and put it in place in March. And now it’s here to stay.”
The experience gained in the last several months has driven preparation for the next wave.
“We are not going to see the disorganization that we saw during the first wave,” said Florence Joly, MD, PhD, head of medical oncology at the Centre François Baclesse in Caen, France. “The difference between now and earlier this year is that COVID diagnostic tests are available. That was one of the problems in the first wave. We had no way to diagnose.”
On the East Coast of the United States, medical oncologist Charu Aggarwal, MD, MPH, is also optimistic: “I think we’re at a place where we can manage.”
“I believe if there was going to be a new wave of COVID-19 cases we would be: better psychologically prepared and better organized,” said Dr. Aggarwal, assistant professor of medicine in the hematology-oncology division at the University of Pennsylvania, Philadelphia. “We already have experience with all of the tools, we have telemedicine available, we have screening protocols available, we have testing, we are already universally masking, everyone’s hand-washing, so I do think that means we would be okay.”
Dr. Arnold agreed that “we are much better prepared than for the first wave, but … we have immense tasks in the area of patient management, the digitization of patient care, the clear allocation of resources when there is a second or third wave. In many areas of preparation, I believe, unfortunately, we are not as well positioned as we had actually hoped.”
The first wave of COVID hit cancer services in the United Kingdom particularly hard: One modeling study suggested that delays in cancer referrals will lead to thousands of additional deaths and tens of thousands of life-years lost.
“Cancer services are working at near normal levels now, but they are still fragile and could be severely compromised again if the NHS [National Health Service] gets flooded by COVID patients,” said Dr. Sikora.
The second wave may be different. “Although the number of infections has increased, the hospitalizations have only risen a little. Let’s see what happens,” he said in an interview. Since then, however, infections have continued to rise, and there has been an increase in hospitalizations. New social distancing measures in the United Kingdom were put into place on Oct. 12, with the aim of protecting the NHS from overload.
Dr. Arrué describes it this way: “The reality is that the ‘second wave’ has left behind the initial grief and shock that both patients and health professionals experienced when faced with something that, until now, we had only seen in the movies.” The second wave has led to new restrictions – including a partial lockdown since the beginning of October.
Dr. Aggarwal says her department recently had a conference with Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, about the impact of COVID-19 on oncology.
“I asked him what advice he’d give oncologists, and he said to go back to as much screening as you were doing previously as quickly as possible. That’s what must be relayed to our oncologists in the community – and also to primary care physicians – because they are often the ones who are ordering and championing the screening efforts.”
This article was originated by Aude Lecrubier, Medscape French edition, and developed by Zosia Chustecka, Medscape Oncology. With additional reporting by Kate Johnson, freelance medical journalist, Claudia Gottschling for Medscape Germany, Leoleli Schwartz for Medscape em português, Tim Locke for Medscape United Kingdom, and Carla Nieto Martínez, freelance medical journalist for Medscape Spanish edition.
This article first appeared on Medscape.com.
Canceled appointments, postponed surgeries, and delayed cancer diagnoses – all are a recipe for exhaustion for oncologists around the world, struggling to reach and treat their patients during the pandemic. Physicians and their teams felt the pain as COVID-19 took its initial march around the globe.
“We saw the distress of people with cancer who could no longer get to anyone on the phone. Their medical visit was usually canceled. Their radiotherapy session was postponed or modified, and chemotherapy postponed,” says Axel Kahn, MD, chairman of the board of directors of La Ligue Nationale Contre le Cancer (National League Against Cancer). “In the vast majority of cases, cancer treatment can be postponed or readjusted, without affecting the patient’s chances of survival, but there has been a lot of anxiety because the patients do not know that.”
The stay-at-home factor was one that played out across many months during the first wave.
“I believe that the ‘stay-home’ message that we transmitted was rigorously followed by patients who should have come to the emergency room much earlier and who, therefore, were admitted with a much more deteriorated general condition than in non-COVID-19 times,” says Benjamín Domingo Arrué, MD, from the department of medical oncology at Hospital Universitari i Politècnic La Fe in Valencia, Spain.
And in Brazil, some of the impact from the initial hit of COVID-19 on oncology is only now being felt, according to Laura Testa, MD, head of breast medical oncology, Instituto do Câncer do Estado de São Paulo.
“We are starting to see a lot of cancer cases that didn’t show up at the beginning of the pandemic, but now they are arriving to us already in advanced stages,” she said. “These patients need hospital care. If the situation worsens and goes back to what we saw at the peak of the curve, I fear the public system won’t be able to treat properly the oncology patients that need hospital care and the patients with cancer who also have COVID-19.”
But even as health care worker fatigue and concerns linger, oncologists say that what they have learned in the last 6 months has helped them prepare as COVID-19 cases increase and a second global wave kicks up.
Lessons from the first wave
In the United States, COVID-19 hit different regions at different times and to different degrees. One of the areas hit first was Seattle.
“We jumped on top of this, we were evidence based, we put things in place very, very quickly,” said Julie Gralow, MD, professor at the University of Washington and the Fred Hutchinson Cancer Research Center, both in Seattle.
“We did a really good job keeping COVID out of our cancer centers,” Dr. Gralow said. “We learned how to be super safe, and to keep symptomatic people out of the building, and to limit the extra people they could bring with them. It’s all about the number of contacts you have.”
The story was different, though, for oncologists in several other countries, and sometimes it varied immensely within each nation.
“We treated fewer patients with cancer during the first wave,” says Dirk Arnold, MD, medical director of the Asklepios Tumor Center Hamburg (Germany), in an interview. “In part, this was because staff were quarantined and because we had a completely different infrastructure in all of the hospitals. But also fewer patients with cancer came to the clinic at all. A lot of resources were directed toward COVID-19.”
In Spain, telemedicine helped keep up with visits, but other areas felt the effect of COVID-19 patient loads.
“At least in the oncology department of our center, we have practically maintained 100% of visits, mostly by telephone,” says Dr. Arrué, “but the reality is that our country has not yet been prepared for telemedicine.”
Laura Mezquita, MD, of the department of medical oncology at Hospital Clinic de Barcelona, describes a more dramatic situation: “We have seen how some of our patients, especially with metastatic disease, have been dismissed for intensive care and life-support treatments, as well as specific treatments against COVID-19 (tocilizumab, remdesivir, etc.) due to the general health collapse of the former wave,” she said. She adds that specific oncologic populations, such as those with thoracic tumors, have been more affected.
Distress among oncologists
Many oncologists are still feeling stressed and fatigued after the first wave, just as a second string of outbreaks is on its way.
A survey presented at last month’s ESMO 2020 Congress found that, in July-August, moral distress was reported by one-third of the oncologists who responded, and more than half reported a feeling of exhaustion.
“The tiredness and team exhaustion is noticeable,” said Dr. Arnold. “We recently had a task force discussion about what will happen when we have a second wave and how the department and our services will adapt. It was clear that those who were at the very front in the first wave had only a limited desire to do that again in the second wave.”
Another concern: COVID-19’s effect on staffing levels.
“We have a population of young caregivers who are affected by the COVID-19 disease with an absenteeism rate that is quite unprecedented,” said Sophie Beaupère, general delegate of Unicancer since January.
She said that, in general, the absenteeism rate in the cancer centers averages 5%-6%, depending on the year. But that rate is now skyrocketing.
Stop-start cycle for surgery
As caregivers quarantined around the world, more than 10% of patients with cancer had treatment canceled or delayed during the first wave of the pandemic, according to another survey from ESMO, involving 109 oncologists from 18 countries.
Difficulties were reported for surgeries by 34% of the centers, but also difficulties with delivering chemotherapy (22% of centers), radiotherapy (13.7%), and therapy with checkpoint inhibitors (9.1%), monoclonal antibodies (9%), and oral targeted therapy (3.7%).
Stopping surgery is a real concern in France, noted Dr. Kahn, the National League Against Cancer chair. He says that in regions that were badly hit by COVID-19, “it was not possible to have access to the operating room for people who absolutely needed surgery; for example, patients with lung cancer that was still operable. Most of the recovery rooms were mobilized for resuscitation.”
There may be some solutions, suggested Thierry Breton, director general of the National Institute of Cancer in France. “We are getting prepared, with the health ministry, for a possible increase in hospital tension, which would lead to a situation where we would have to reschedule operations. Nationally, regionally, and locally, we are seeing how we can resume and prioritize surgeries that have not been done.”
Delays in cancer diagnosis
While COVID-19 affected treatment, many oncologists say the major impact of the first wave was a delay in diagnosing cancer. Some of this was a result of the suspension of cancer screening programs, but there was also fear among the general public about visiting clinics and hospitals during a pandemic.
“We didn’t do so well with cancer during the first wave here in the U.K.,” said Karol Sikora, PhD, MBBChir, professor of cancer medicine and founding dean at the University of Buckingham Medical School, London. “Cancer diagnostic pathways virtually stalled partly because patients didn’t seek help, but getting scans and biopsies was also very difficult. Even patients referred urgently under the ‘2-weeks-wait’ rule were turned down.”
In France, “the delay in diagnosis is indisputable,” said Dr. Kahn. “About 50% of the cancer diagnoses one would expect during this period were missed.”
“I am worried that there remains a major traffic jam that has not been caught up with, and, in the meantime, the health crisis is worsening,” he added.
In Seattle, Dr. Gralow said the first COVID-19 wave had little impact on treatment for breast cancer, but it was in screening for breast cancer “where things really got messed up.”
“Even though we’ve been fully ramped up again,” she said, concerns remain. To ensure that screening mammography is maintained, “we have spaced out the visits to keep our waiting rooms less populated, with a longer time between using the machine so we can clean it. To do this, we have extended operating hours and are now opening on Saturday.
“So we’re actually at 100% of our capacity, but I’m really nervous, though, that a lot of people put off their screening mammogram and aren’t going to come in and get it.
“Not only did people get the message to stay home and not do nonessential things, but I think a lot of people lost their health insurance when they lost their jobs,” she said, and without health insurance, they are not covered for cancer screening.
Looking ahead, with a plan
Many oncologists agree that access to care can and must be improved – and there were some positive moves.
“Some regimens changed during the first months of the pandemic, and I don’t see them going back to the way they were anytime soon,” said Dr. Testa. “The changes/adaptations that were made to minimize the chance of SARS-CoV-2 infection are still in place and will go on for a while. In this context, telemedicine helped a lot. The pandemic forced the stakeholders to step up and put it in place in March. And now it’s here to stay.”
The experience gained in the last several months has driven preparation for the next wave.
“We are not going to see the disorganization that we saw during the first wave,” said Florence Joly, MD, PhD, head of medical oncology at the Centre François Baclesse in Caen, France. “The difference between now and earlier this year is that COVID diagnostic tests are available. That was one of the problems in the first wave. We had no way to diagnose.”
On the East Coast of the United States, medical oncologist Charu Aggarwal, MD, MPH, is also optimistic: “I think we’re at a place where we can manage.”
“I believe if there was going to be a new wave of COVID-19 cases we would be: better psychologically prepared and better organized,” said Dr. Aggarwal, assistant professor of medicine in the hematology-oncology division at the University of Pennsylvania, Philadelphia. “We already have experience with all of the tools, we have telemedicine available, we have screening protocols available, we have testing, we are already universally masking, everyone’s hand-washing, so I do think that means we would be okay.”
Dr. Arnold agreed that “we are much better prepared than for the first wave, but … we have immense tasks in the area of patient management, the digitization of patient care, the clear allocation of resources when there is a second or third wave. In many areas of preparation, I believe, unfortunately, we are not as well positioned as we had actually hoped.”
The first wave of COVID hit cancer services in the United Kingdom particularly hard: One modeling study suggested that delays in cancer referrals will lead to thousands of additional deaths and tens of thousands of life-years lost.
“Cancer services are working at near normal levels now, but they are still fragile and could be severely compromised again if the NHS [National Health Service] gets flooded by COVID patients,” said Dr. Sikora.
The second wave may be different. “Although the number of infections has increased, the hospitalizations have only risen a little. Let’s see what happens,” he said in an interview. Since then, however, infections have continued to rise, and there has been an increase in hospitalizations. New social distancing measures in the United Kingdom were put into place on Oct. 12, with the aim of protecting the NHS from overload.
Dr. Arrué describes it this way: “The reality is that the ‘second wave’ has left behind the initial grief and shock that both patients and health professionals experienced when faced with something that, until now, we had only seen in the movies.” The second wave has led to new restrictions – including a partial lockdown since the beginning of October.
Dr. Aggarwal says her department recently had a conference with Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, about the impact of COVID-19 on oncology.
“I asked him what advice he’d give oncologists, and he said to go back to as much screening as you were doing previously as quickly as possible. That’s what must be relayed to our oncologists in the community – and also to primary care physicians – because they are often the ones who are ordering and championing the screening efforts.”
This article was originated by Aude Lecrubier, Medscape French edition, and developed by Zosia Chustecka, Medscape Oncology. With additional reporting by Kate Johnson, freelance medical journalist, Claudia Gottschling for Medscape Germany, Leoleli Schwartz for Medscape em português, Tim Locke for Medscape United Kingdom, and Carla Nieto Martínez, freelance medical journalist for Medscape Spanish edition.
This article first appeared on Medscape.com.
Two-stage surgery to reduce ovarian cancer risk piques interest
“Many physicians would assume that prevention of cancer, especially cancer as serious as ovarian cancer, trumps all other decision making, but when we really listen to high-risk women, they want to have options,” Karen Lu, MD, chair of gynecologic oncology and reproductive medicine at MD Anderson Cancer Center, Houston, Texas, told Medscape Medical News.
She was commenting on the findings from a UK survey conducted among women at an increased risk for ovarian cancer (OC), some of whom had already undergone salpingo-oophorectomy (RRSO), a standard risk-reducing surgery that involves removal of fallopian tubes and ovaries.
The survey found that these women were just as likely to consider an alternative two-stage surgical approach in which the fallopian tubes are removed but removal of the ovaries is delayed ― risk-reducing early salpingectomy with delayed oophorectomy (RRESDO).
In the survey, women were asked which option they would theorectically prefer. At present, the two-step surgery is recommended only within the context of a research trial (several of which are ongoing).
The UK survey was published online August 16 in the British Journal of Obstetrics and Gynaecology.
It found that premenopausal women concerned about the sexual dysfunction that can occur after RRSO were most likely to embrace the two-step surgery option.
The likelihood of finding this option acceptable was nearly three times higher among this subgroup of patients (odds ratio [RR], 2.9). It was more than five times higher among patients who had already undergone RRSO and had experienced sexual dysfunction after the surgery (OR, 5.3), the authors report.
These findings largely mirror those from a 2014 survey of US women, which set the stage for the Women Choosing Surgical Prevention (WISP) study.
The WISP investigators, led by Lu, are assessing quality-of-life outcomes related to sexual function with RRESDO vs RRSO.
Final results from the WISP study and from a similar Dutch study, TUBA, which is evaluating RRESDO’s effects on menopause-related quality of life, are anticipated in late 2020 or early 2021.
The investigators from both the WISP and the TUBA trials are planning a joint trial to evaluate the safety and efficacy of RRESDO, Lu told Medscape Medical News.
The PROTECTOR study, in the United Kingdom, is currently enrolling patients. Like WISP, its primary endpoint will be quality-of-life measures related to sexual function. The PROTECTOR trial will offer the option of RRESDO to the “large proportion of eligible women” who are interested in this two-stage approach, as evidenced by the UK survey, said Faiza Gaba, MBB, first author on the survey results. Gaba is affiliated with the Wolfson Institute of Preventive Medicine at the Queen Mary University of London and the Department of Gynaecological Oncology at St Bartholomew’s Hospital, London, United Kingdom.
Survey findings
The 39-item survey was offered from October 2017 to June 2019 at multiple clinics in the United Kingdom and to members of a support group for BRCA gene carriers. Of the 683 respondents, 346 had undergone RRSO and 337 had not. Those who had not were significantly younger (38.3 years vs 51.5 years); 262 were premenopausal.
Overall, 88.8% of the premenopausal and 95.2% of the postmenopausal women who had undergone RRSO were satisfied with their decision, but, respectively, 9.4% and 1.2% of these women regretted their decision.
More than half (55.3%) said they would consider participating in a study offering RRESDO, 20.2% said they wouldn’t consider it, and 24% weren’t sure.
Among the premenopausal respondents who had not undergone RRSO, 69.1% said they would consider it, and 30.9% said they would not.
Those wanting to delay hot flashes were five times more likely to find RRESDO acceptable (OR, 5.0).
Willingness to undergo RRESDO in a trial setting was also higher among those who considered it acceptable to undergo two surgeries (OR, 444.1), to undergo interval monitoring between surgeries (OR, 59.0), to have uncertainty about the level of OC risk reduction with RRESDO (OR, 14.6), and to potentially experience interval OC between the two surgeries (OR, 9.6).
Notably, 74.1% of the premenopausal RRSO patients used hormone replacement therapy (HRT), and most said it reduced symptoms of vaginal dryness. HRT use was not significantly associated with satisfaction or regret regarding decisions to undergo RRSO, the authors found.
Rather, the high regret rates among premenopausal women who underwent RRSO were driven largely by certain symptoms. Regret was highest among those who experienced night sweats (OR, 13.8), sleep disturbance (OR, 18.8), sexual dysfunction (OR, 5.3), or urinary incontinence (OR 17.2). More of those women than those who did not experience these symptoms said they regretted their decision (OR, 6.4) and that RRSO did them a lot of harm (OR, 3.9). These women were also significantly more likely to say they would have opted for RRESDO instead of RRSO had they been given the option, whereas those with hot flashes, osteoporosis, or fatigue after RRSO were less likely, retrospectively, to choose RRESDO.
The findings suggest “there is a range of tolerability and acceptability of various symptoms among women which affects surgical decision making,” the authors comment.
RRSO remains the gold standard for OC risk reduction, but about 10% of premenopausal women regret having undergone RRSO, mainly because of the menopausal sequelae, they note.
RRESDO could offer an alternative for relatively young women who wish to delay the onset of menopause, they suggest.
The approach is supported by evidence that most high-grade, serous OC originates in the fallopian tubes, meaning delayed oophorectomy with RRESDO may have a favorable risk-benefit profile for those wishing to avoid surgical menopause.
Preliminary reports from WISP and TUBA were presented at the annual meeting of the Society of Gynecologic Oncology in 2019. These initial results showed, as expected, that menopausal symptoms were worse with RRSO. This was true even among those who used HRT, WISP lead investigator Lu told Medscape Medical News.
She applauded the work by Gaba and colleagues, saying that the survey shows that women appreciate having options.
“It’s quite a daunting dilemma” for a woman at high risk but who is without cancer ― a “previvor” ― to be told that the standard-of-care recommendation is to undergo surgical menopause years earlier than would occur naturally, Lu added.
However, it is most important to know whether a given approach is safe and effective, and that’s where the joint international study planned by her team and the TUBA study investigators comes in.
“Acceptability is important; showing the impact on menopausal symptoms and sexual function is important,” she said. “But ultimately, we really need to know that [RRESDO] protects women from ovarian cancer.”
The UK survey was funded by a grant from Rosetrees Trust. Gaba and Lu have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
“Many physicians would assume that prevention of cancer, especially cancer as serious as ovarian cancer, trumps all other decision making, but when we really listen to high-risk women, they want to have options,” Karen Lu, MD, chair of gynecologic oncology and reproductive medicine at MD Anderson Cancer Center, Houston, Texas, told Medscape Medical News.
She was commenting on the findings from a UK survey conducted among women at an increased risk for ovarian cancer (OC), some of whom had already undergone salpingo-oophorectomy (RRSO), a standard risk-reducing surgery that involves removal of fallopian tubes and ovaries.
The survey found that these women were just as likely to consider an alternative two-stage surgical approach in which the fallopian tubes are removed but removal of the ovaries is delayed ― risk-reducing early salpingectomy with delayed oophorectomy (RRESDO).
In the survey, women were asked which option they would theorectically prefer. At present, the two-step surgery is recommended only within the context of a research trial (several of which are ongoing).
The UK survey was published online August 16 in the British Journal of Obstetrics and Gynaecology.
It found that premenopausal women concerned about the sexual dysfunction that can occur after RRSO were most likely to embrace the two-step surgery option.
The likelihood of finding this option acceptable was nearly three times higher among this subgroup of patients (odds ratio [RR], 2.9). It was more than five times higher among patients who had already undergone RRSO and had experienced sexual dysfunction after the surgery (OR, 5.3), the authors report.
These findings largely mirror those from a 2014 survey of US women, which set the stage for the Women Choosing Surgical Prevention (WISP) study.
The WISP investigators, led by Lu, are assessing quality-of-life outcomes related to sexual function with RRESDO vs RRSO.
Final results from the WISP study and from a similar Dutch study, TUBA, which is evaluating RRESDO’s effects on menopause-related quality of life, are anticipated in late 2020 or early 2021.
The investigators from both the WISP and the TUBA trials are planning a joint trial to evaluate the safety and efficacy of RRESDO, Lu told Medscape Medical News.
The PROTECTOR study, in the United Kingdom, is currently enrolling patients. Like WISP, its primary endpoint will be quality-of-life measures related to sexual function. The PROTECTOR trial will offer the option of RRESDO to the “large proportion of eligible women” who are interested in this two-stage approach, as evidenced by the UK survey, said Faiza Gaba, MBB, first author on the survey results. Gaba is affiliated with the Wolfson Institute of Preventive Medicine at the Queen Mary University of London and the Department of Gynaecological Oncology at St Bartholomew’s Hospital, London, United Kingdom.
Survey findings
The 39-item survey was offered from October 2017 to June 2019 at multiple clinics in the United Kingdom and to members of a support group for BRCA gene carriers. Of the 683 respondents, 346 had undergone RRSO and 337 had not. Those who had not were significantly younger (38.3 years vs 51.5 years); 262 were premenopausal.
Overall, 88.8% of the premenopausal and 95.2% of the postmenopausal women who had undergone RRSO were satisfied with their decision, but, respectively, 9.4% and 1.2% of these women regretted their decision.
More than half (55.3%) said they would consider participating in a study offering RRESDO, 20.2% said they wouldn’t consider it, and 24% weren’t sure.
Among the premenopausal respondents who had not undergone RRSO, 69.1% said they would consider it, and 30.9% said they would not.
Those wanting to delay hot flashes were five times more likely to find RRESDO acceptable (OR, 5.0).
Willingness to undergo RRESDO in a trial setting was also higher among those who considered it acceptable to undergo two surgeries (OR, 444.1), to undergo interval monitoring between surgeries (OR, 59.0), to have uncertainty about the level of OC risk reduction with RRESDO (OR, 14.6), and to potentially experience interval OC between the two surgeries (OR, 9.6).
Notably, 74.1% of the premenopausal RRSO patients used hormone replacement therapy (HRT), and most said it reduced symptoms of vaginal dryness. HRT use was not significantly associated with satisfaction or regret regarding decisions to undergo RRSO, the authors found.
Rather, the high regret rates among premenopausal women who underwent RRSO were driven largely by certain symptoms. Regret was highest among those who experienced night sweats (OR, 13.8), sleep disturbance (OR, 18.8), sexual dysfunction (OR, 5.3), or urinary incontinence (OR 17.2). More of those women than those who did not experience these symptoms said they regretted their decision (OR, 6.4) and that RRSO did them a lot of harm (OR, 3.9). These women were also significantly more likely to say they would have opted for RRESDO instead of RRSO had they been given the option, whereas those with hot flashes, osteoporosis, or fatigue after RRSO were less likely, retrospectively, to choose RRESDO.
The findings suggest “there is a range of tolerability and acceptability of various symptoms among women which affects surgical decision making,” the authors comment.
RRSO remains the gold standard for OC risk reduction, but about 10% of premenopausal women regret having undergone RRSO, mainly because of the menopausal sequelae, they note.
RRESDO could offer an alternative for relatively young women who wish to delay the onset of menopause, they suggest.
The approach is supported by evidence that most high-grade, serous OC originates in the fallopian tubes, meaning delayed oophorectomy with RRESDO may have a favorable risk-benefit profile for those wishing to avoid surgical menopause.
Preliminary reports from WISP and TUBA were presented at the annual meeting of the Society of Gynecologic Oncology in 2019. These initial results showed, as expected, that menopausal symptoms were worse with RRSO. This was true even among those who used HRT, WISP lead investigator Lu told Medscape Medical News.
She applauded the work by Gaba and colleagues, saying that the survey shows that women appreciate having options.
“It’s quite a daunting dilemma” for a woman at high risk but who is without cancer ― a “previvor” ― to be told that the standard-of-care recommendation is to undergo surgical menopause years earlier than would occur naturally, Lu added.
However, it is most important to know whether a given approach is safe and effective, and that’s where the joint international study planned by her team and the TUBA study investigators comes in.
“Acceptability is important; showing the impact on menopausal symptoms and sexual function is important,” she said. “But ultimately, we really need to know that [RRESDO] protects women from ovarian cancer.”
The UK survey was funded by a grant from Rosetrees Trust. Gaba and Lu have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
“Many physicians would assume that prevention of cancer, especially cancer as serious as ovarian cancer, trumps all other decision making, but when we really listen to high-risk women, they want to have options,” Karen Lu, MD, chair of gynecologic oncology and reproductive medicine at MD Anderson Cancer Center, Houston, Texas, told Medscape Medical News.
She was commenting on the findings from a UK survey conducted among women at an increased risk for ovarian cancer (OC), some of whom had already undergone salpingo-oophorectomy (RRSO), a standard risk-reducing surgery that involves removal of fallopian tubes and ovaries.
The survey found that these women were just as likely to consider an alternative two-stage surgical approach in which the fallopian tubes are removed but removal of the ovaries is delayed ― risk-reducing early salpingectomy with delayed oophorectomy (RRESDO).
In the survey, women were asked which option they would theorectically prefer. At present, the two-step surgery is recommended only within the context of a research trial (several of which are ongoing).
The UK survey was published online August 16 in the British Journal of Obstetrics and Gynaecology.
It found that premenopausal women concerned about the sexual dysfunction that can occur after RRSO were most likely to embrace the two-step surgery option.
The likelihood of finding this option acceptable was nearly three times higher among this subgroup of patients (odds ratio [RR], 2.9). It was more than five times higher among patients who had already undergone RRSO and had experienced sexual dysfunction after the surgery (OR, 5.3), the authors report.
These findings largely mirror those from a 2014 survey of US women, which set the stage for the Women Choosing Surgical Prevention (WISP) study.
The WISP investigators, led by Lu, are assessing quality-of-life outcomes related to sexual function with RRESDO vs RRSO.
Final results from the WISP study and from a similar Dutch study, TUBA, which is evaluating RRESDO’s effects on menopause-related quality of life, are anticipated in late 2020 or early 2021.
The investigators from both the WISP and the TUBA trials are planning a joint trial to evaluate the safety and efficacy of RRESDO, Lu told Medscape Medical News.
The PROTECTOR study, in the United Kingdom, is currently enrolling patients. Like WISP, its primary endpoint will be quality-of-life measures related to sexual function. The PROTECTOR trial will offer the option of RRESDO to the “large proportion of eligible women” who are interested in this two-stage approach, as evidenced by the UK survey, said Faiza Gaba, MBB, first author on the survey results. Gaba is affiliated with the Wolfson Institute of Preventive Medicine at the Queen Mary University of London and the Department of Gynaecological Oncology at St Bartholomew’s Hospital, London, United Kingdom.
Survey findings
The 39-item survey was offered from October 2017 to June 2019 at multiple clinics in the United Kingdom and to members of a support group for BRCA gene carriers. Of the 683 respondents, 346 had undergone RRSO and 337 had not. Those who had not were significantly younger (38.3 years vs 51.5 years); 262 were premenopausal.
Overall, 88.8% of the premenopausal and 95.2% of the postmenopausal women who had undergone RRSO were satisfied with their decision, but, respectively, 9.4% and 1.2% of these women regretted their decision.
More than half (55.3%) said they would consider participating in a study offering RRESDO, 20.2% said they wouldn’t consider it, and 24% weren’t sure.
Among the premenopausal respondents who had not undergone RRSO, 69.1% said they would consider it, and 30.9% said they would not.
Those wanting to delay hot flashes were five times more likely to find RRESDO acceptable (OR, 5.0).
Willingness to undergo RRESDO in a trial setting was also higher among those who considered it acceptable to undergo two surgeries (OR, 444.1), to undergo interval monitoring between surgeries (OR, 59.0), to have uncertainty about the level of OC risk reduction with RRESDO (OR, 14.6), and to potentially experience interval OC between the two surgeries (OR, 9.6).
Notably, 74.1% of the premenopausal RRSO patients used hormone replacement therapy (HRT), and most said it reduced symptoms of vaginal dryness. HRT use was not significantly associated with satisfaction or regret regarding decisions to undergo RRSO, the authors found.
Rather, the high regret rates among premenopausal women who underwent RRSO were driven largely by certain symptoms. Regret was highest among those who experienced night sweats (OR, 13.8), sleep disturbance (OR, 18.8), sexual dysfunction (OR, 5.3), or urinary incontinence (OR 17.2). More of those women than those who did not experience these symptoms said they regretted their decision (OR, 6.4) and that RRSO did them a lot of harm (OR, 3.9). These women were also significantly more likely to say they would have opted for RRESDO instead of RRSO had they been given the option, whereas those with hot flashes, osteoporosis, or fatigue after RRSO were less likely, retrospectively, to choose RRESDO.
The findings suggest “there is a range of tolerability and acceptability of various symptoms among women which affects surgical decision making,” the authors comment.
RRSO remains the gold standard for OC risk reduction, but about 10% of premenopausal women regret having undergone RRSO, mainly because of the menopausal sequelae, they note.
RRESDO could offer an alternative for relatively young women who wish to delay the onset of menopause, they suggest.
The approach is supported by evidence that most high-grade, serous OC originates in the fallopian tubes, meaning delayed oophorectomy with RRESDO may have a favorable risk-benefit profile for those wishing to avoid surgical menopause.
Preliminary reports from WISP and TUBA were presented at the annual meeting of the Society of Gynecologic Oncology in 2019. These initial results showed, as expected, that menopausal symptoms were worse with RRSO. This was true even among those who used HRT, WISP lead investigator Lu told Medscape Medical News.
She applauded the work by Gaba and colleagues, saying that the survey shows that women appreciate having options.
“It’s quite a daunting dilemma” for a woman at high risk but who is without cancer ― a “previvor” ― to be told that the standard-of-care recommendation is to undergo surgical menopause years earlier than would occur naturally, Lu added.
However, it is most important to know whether a given approach is safe and effective, and that’s where the joint international study planned by her team and the TUBA study investigators comes in.
“Acceptability is important; showing the impact on menopausal symptoms and sexual function is important,” she said. “But ultimately, we really need to know that [RRESDO] protects women from ovarian cancer.”
The UK survey was funded by a grant from Rosetrees Trust. Gaba and Lu have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Clinical factors and treatment tied to COVID-19 mortality in cancer patients
according to two presentations at the European Society for Medical Oncology Virtual Congress 2020.
Two analyses of data from the COVID-19 and Cancer Consortium (CCC19) were presented at the meeting.
The data suggest that older age, male sex, more comorbidities, poor performance status, progressive cancer or multiple cancers, hematologic malignancy, and recent cancer therapy are all associated with higher mortality among patients with cancer and COVID-19. Anti-CD20 therapy is associated with an especially high mortality rate, according to an investigator.
Among hospitalized patients, increased absolute neutrophil count as well as abnormal D-dimer, high-sensitivity troponin, and C-reactive protein are associated with a higher risk of mortality.
Prior analyses of CCC19 data pointed to several factors associated with higher COVID-19 death rates, according to Petros Grivas, MD, PhD, of University of Washington, Seattle, who presented some CCC19 data at the meeting. However, the prior analyses were limited by weak statistical power and low event rates, Dr. Grivas said.
Clinical and laboratory factors: Abstract LBA72
The aim of Dr. Grivas’s analysis was to validate a priori identified demographic and clinicopathologic factors associated with 30-day all-cause mortality in patients with COVID-19 and cancer. Dr. Grivas and colleagues also explored the potential association between laboratory parameters and 30-day all-cause mortality.
The analysis included 3,899 patients with cancer and COVID-19 from 124 centers. Most centers are in the United States, but 4% are in Canada, and 2% are in Spain. About two-thirds of patients were 60 years of age or younger at baseline, half were men, 79% had solid tumors, and 21% had hematologic malignancies.
Cancer-specific factors associated with an increased risk of 30-day all-cause mortality were having progressive cancer (adjusted odds ratio, 2.9), receiving cancer therapy within 3 months (aOR, 1.2), having a hematologic versus solid tumor (aOR, 1.7), and having multiple malignancies (aOR, 1.5).
Clinical factors associated with an increased risk of 30-day all-cause mortality were Black versus White race (aOR, 1.5), older age (aOR, 1.7 per 10 years), three or more actively treated comorbidities (versus none; aOR, 2.1), and Eastern Cooperative Oncology Group performance status of 2 or more (versus 0; aOR, 4.6).
In hospitalized patients, several laboratory variables were associated with an increased risk of 30-day all-cause mortality. Having an absolute neutrophil count above the upper limit of normal doubled the risk (aOR, 2.0), while abnormal D-dimer, high-sensitivity troponin, and C-reactive protein all more than doubled the risk of mortality (aORs of 2.5, 2.5, and 2.4, respectively).
Further risk modeling with multivariable analysis will be performed after longer follow-up, Dr. Grivas noted.
Treatment-related outcomes: Abstract LBA71
An additional analysis of CCC19 data encompassed 3,654 patients. In this analysis, researchers investigated the correlation between timing of cancer treatment and COVID-19–related complications and 30-day mortality.
Mortality was highest among cancer patients treated 1-3 months prior to COVID-19 diagnosis, with all-cause mortality at 28%, said Trisha M. Wise-Draper, MD, PhD, of University of Cincinnati, when presenting the data at the meeting.
Rates for other complications (hospitalization, oxygen required, ICU admission, and mechanical ventilation) were similar regardless of treatment timing.
The unadjusted 30-day mortality rate was highest for patients treated most recently with chemoimmunotherapy (30%), followed by chemotherapy (18%), chemoradiotherapy (18%), and targeted therapy (17%).
The mortality rate was “particularly high,” at 50%, in patients receiving anti-CD20 therapy 1-3 months prior to COVID-19 diagnosis – the time period for which significant B-cell depletion develops, Dr. Wise-Draper observed.
An analysis of disease status among 1,449 patients treated within 3 months of COVID-19 diagnosis showed mortality risk increasing from 6% among patients in remission or with newly emergent disease, to 22% in patients with any active cancer, to 34% in those with progressing disease, Dr. Wise-Draper said.
Discussant Benjamin Solomon, MD, PhD, of Peter MacCallum Cancer Centre in Melbourne, made note of the high 30-day mortality rate seen in patients receiving anti-CD20 therapy as well as the elevated standardized mortality ratios with recent chemoimmunotherapy and targeted therapy.
“Although there are some limitations of this analysis, it provides the best data we have to date about the effects of treatment on early mortality in patients with COVID-19 and cancer. It points to a modest but heterogeneous effect of treatment on outcome, one which is likely to become clearer with larger cohorts and additional analysis,” Dr. Solomon said.
This research was funded by the American Cancer Society, Hope Foundation for Cancer Research, Jim and Carol O’Hare Fund, National Cancer Institute, National Human Genome Research Institute, Vanderbilt Institute for Clinical and Translational Research, and Fonds de Recherche du Quebec-Sante. Dr. Grivas disclosed relationships with many companies, but none are related to this work. Dr. Wise-Draper disclosed relationships with Merck, Bristol-Myers Squibb, Tesaro, GlaxoSmithKline, AstraZeneca, Shattuck Labs, and Rakuten. Dr. Solomon disclosed relationships with Amgen, AstraZeneca, Merck, Bristol-Myers Squibb, Novartis, Pfizer, and Roche-Genentech.
SOURCES: Grivas P et al. ESMO 2020, Abstract LBA72; Wise-Draper TM et al. ESMO 2020, Abstract LBA71.
according to two presentations at the European Society for Medical Oncology Virtual Congress 2020.
Two analyses of data from the COVID-19 and Cancer Consortium (CCC19) were presented at the meeting.
The data suggest that older age, male sex, more comorbidities, poor performance status, progressive cancer or multiple cancers, hematologic malignancy, and recent cancer therapy are all associated with higher mortality among patients with cancer and COVID-19. Anti-CD20 therapy is associated with an especially high mortality rate, according to an investigator.
Among hospitalized patients, increased absolute neutrophil count as well as abnormal D-dimer, high-sensitivity troponin, and C-reactive protein are associated with a higher risk of mortality.
Prior analyses of CCC19 data pointed to several factors associated with higher COVID-19 death rates, according to Petros Grivas, MD, PhD, of University of Washington, Seattle, who presented some CCC19 data at the meeting. However, the prior analyses were limited by weak statistical power and low event rates, Dr. Grivas said.
Clinical and laboratory factors: Abstract LBA72
The aim of Dr. Grivas’s analysis was to validate a priori identified demographic and clinicopathologic factors associated with 30-day all-cause mortality in patients with COVID-19 and cancer. Dr. Grivas and colleagues also explored the potential association between laboratory parameters and 30-day all-cause mortality.
The analysis included 3,899 patients with cancer and COVID-19 from 124 centers. Most centers are in the United States, but 4% are in Canada, and 2% are in Spain. About two-thirds of patients were 60 years of age or younger at baseline, half were men, 79% had solid tumors, and 21% had hematologic malignancies.
Cancer-specific factors associated with an increased risk of 30-day all-cause mortality were having progressive cancer (adjusted odds ratio, 2.9), receiving cancer therapy within 3 months (aOR, 1.2), having a hematologic versus solid tumor (aOR, 1.7), and having multiple malignancies (aOR, 1.5).
Clinical factors associated with an increased risk of 30-day all-cause mortality were Black versus White race (aOR, 1.5), older age (aOR, 1.7 per 10 years), three or more actively treated comorbidities (versus none; aOR, 2.1), and Eastern Cooperative Oncology Group performance status of 2 or more (versus 0; aOR, 4.6).
In hospitalized patients, several laboratory variables were associated with an increased risk of 30-day all-cause mortality. Having an absolute neutrophil count above the upper limit of normal doubled the risk (aOR, 2.0), while abnormal D-dimer, high-sensitivity troponin, and C-reactive protein all more than doubled the risk of mortality (aORs of 2.5, 2.5, and 2.4, respectively).
Further risk modeling with multivariable analysis will be performed after longer follow-up, Dr. Grivas noted.
Treatment-related outcomes: Abstract LBA71
An additional analysis of CCC19 data encompassed 3,654 patients. In this analysis, researchers investigated the correlation between timing of cancer treatment and COVID-19–related complications and 30-day mortality.
Mortality was highest among cancer patients treated 1-3 months prior to COVID-19 diagnosis, with all-cause mortality at 28%, said Trisha M. Wise-Draper, MD, PhD, of University of Cincinnati, when presenting the data at the meeting.
Rates for other complications (hospitalization, oxygen required, ICU admission, and mechanical ventilation) were similar regardless of treatment timing.
The unadjusted 30-day mortality rate was highest for patients treated most recently with chemoimmunotherapy (30%), followed by chemotherapy (18%), chemoradiotherapy (18%), and targeted therapy (17%).
The mortality rate was “particularly high,” at 50%, in patients receiving anti-CD20 therapy 1-3 months prior to COVID-19 diagnosis – the time period for which significant B-cell depletion develops, Dr. Wise-Draper observed.
An analysis of disease status among 1,449 patients treated within 3 months of COVID-19 diagnosis showed mortality risk increasing from 6% among patients in remission or with newly emergent disease, to 22% in patients with any active cancer, to 34% in those with progressing disease, Dr. Wise-Draper said.
Discussant Benjamin Solomon, MD, PhD, of Peter MacCallum Cancer Centre in Melbourne, made note of the high 30-day mortality rate seen in patients receiving anti-CD20 therapy as well as the elevated standardized mortality ratios with recent chemoimmunotherapy and targeted therapy.
“Although there are some limitations of this analysis, it provides the best data we have to date about the effects of treatment on early mortality in patients with COVID-19 and cancer. It points to a modest but heterogeneous effect of treatment on outcome, one which is likely to become clearer with larger cohorts and additional analysis,” Dr. Solomon said.
This research was funded by the American Cancer Society, Hope Foundation for Cancer Research, Jim and Carol O’Hare Fund, National Cancer Institute, National Human Genome Research Institute, Vanderbilt Institute for Clinical and Translational Research, and Fonds de Recherche du Quebec-Sante. Dr. Grivas disclosed relationships with many companies, but none are related to this work. Dr. Wise-Draper disclosed relationships with Merck, Bristol-Myers Squibb, Tesaro, GlaxoSmithKline, AstraZeneca, Shattuck Labs, and Rakuten. Dr. Solomon disclosed relationships with Amgen, AstraZeneca, Merck, Bristol-Myers Squibb, Novartis, Pfizer, and Roche-Genentech.
SOURCES: Grivas P et al. ESMO 2020, Abstract LBA72; Wise-Draper TM et al. ESMO 2020, Abstract LBA71.
according to two presentations at the European Society for Medical Oncology Virtual Congress 2020.
Two analyses of data from the COVID-19 and Cancer Consortium (CCC19) were presented at the meeting.
The data suggest that older age, male sex, more comorbidities, poor performance status, progressive cancer or multiple cancers, hematologic malignancy, and recent cancer therapy are all associated with higher mortality among patients with cancer and COVID-19. Anti-CD20 therapy is associated with an especially high mortality rate, according to an investigator.
Among hospitalized patients, increased absolute neutrophil count as well as abnormal D-dimer, high-sensitivity troponin, and C-reactive protein are associated with a higher risk of mortality.
Prior analyses of CCC19 data pointed to several factors associated with higher COVID-19 death rates, according to Petros Grivas, MD, PhD, of University of Washington, Seattle, who presented some CCC19 data at the meeting. However, the prior analyses were limited by weak statistical power and low event rates, Dr. Grivas said.
Clinical and laboratory factors: Abstract LBA72
The aim of Dr. Grivas’s analysis was to validate a priori identified demographic and clinicopathologic factors associated with 30-day all-cause mortality in patients with COVID-19 and cancer. Dr. Grivas and colleagues also explored the potential association between laboratory parameters and 30-day all-cause mortality.
The analysis included 3,899 patients with cancer and COVID-19 from 124 centers. Most centers are in the United States, but 4% are in Canada, and 2% are in Spain. About two-thirds of patients were 60 years of age or younger at baseline, half were men, 79% had solid tumors, and 21% had hematologic malignancies.
Cancer-specific factors associated with an increased risk of 30-day all-cause mortality were having progressive cancer (adjusted odds ratio, 2.9), receiving cancer therapy within 3 months (aOR, 1.2), having a hematologic versus solid tumor (aOR, 1.7), and having multiple malignancies (aOR, 1.5).
Clinical factors associated with an increased risk of 30-day all-cause mortality were Black versus White race (aOR, 1.5), older age (aOR, 1.7 per 10 years), three or more actively treated comorbidities (versus none; aOR, 2.1), and Eastern Cooperative Oncology Group performance status of 2 or more (versus 0; aOR, 4.6).
In hospitalized patients, several laboratory variables were associated with an increased risk of 30-day all-cause mortality. Having an absolute neutrophil count above the upper limit of normal doubled the risk (aOR, 2.0), while abnormal D-dimer, high-sensitivity troponin, and C-reactive protein all more than doubled the risk of mortality (aORs of 2.5, 2.5, and 2.4, respectively).
Further risk modeling with multivariable analysis will be performed after longer follow-up, Dr. Grivas noted.
Treatment-related outcomes: Abstract LBA71
An additional analysis of CCC19 data encompassed 3,654 patients. In this analysis, researchers investigated the correlation between timing of cancer treatment and COVID-19–related complications and 30-day mortality.
Mortality was highest among cancer patients treated 1-3 months prior to COVID-19 diagnosis, with all-cause mortality at 28%, said Trisha M. Wise-Draper, MD, PhD, of University of Cincinnati, when presenting the data at the meeting.
Rates for other complications (hospitalization, oxygen required, ICU admission, and mechanical ventilation) were similar regardless of treatment timing.
The unadjusted 30-day mortality rate was highest for patients treated most recently with chemoimmunotherapy (30%), followed by chemotherapy (18%), chemoradiotherapy (18%), and targeted therapy (17%).
The mortality rate was “particularly high,” at 50%, in patients receiving anti-CD20 therapy 1-3 months prior to COVID-19 diagnosis – the time period for which significant B-cell depletion develops, Dr. Wise-Draper observed.
An analysis of disease status among 1,449 patients treated within 3 months of COVID-19 diagnosis showed mortality risk increasing from 6% among patients in remission or with newly emergent disease, to 22% in patients with any active cancer, to 34% in those with progressing disease, Dr. Wise-Draper said.
Discussant Benjamin Solomon, MD, PhD, of Peter MacCallum Cancer Centre in Melbourne, made note of the high 30-day mortality rate seen in patients receiving anti-CD20 therapy as well as the elevated standardized mortality ratios with recent chemoimmunotherapy and targeted therapy.
“Although there are some limitations of this analysis, it provides the best data we have to date about the effects of treatment on early mortality in patients with COVID-19 and cancer. It points to a modest but heterogeneous effect of treatment on outcome, one which is likely to become clearer with larger cohorts and additional analysis,” Dr. Solomon said.
This research was funded by the American Cancer Society, Hope Foundation for Cancer Research, Jim and Carol O’Hare Fund, National Cancer Institute, National Human Genome Research Institute, Vanderbilt Institute for Clinical and Translational Research, and Fonds de Recherche du Quebec-Sante. Dr. Grivas disclosed relationships with many companies, but none are related to this work. Dr. Wise-Draper disclosed relationships with Merck, Bristol-Myers Squibb, Tesaro, GlaxoSmithKline, AstraZeneca, Shattuck Labs, and Rakuten. Dr. Solomon disclosed relationships with Amgen, AstraZeneca, Merck, Bristol-Myers Squibb, Novartis, Pfizer, and Roche-Genentech.
SOURCES: Grivas P et al. ESMO 2020, Abstract LBA72; Wise-Draper TM et al. ESMO 2020, Abstract LBA71.
FROM ESMO 2020
The professional advancement of drug and device innovation

I often say that there are both “guardrail” days and very good days when it comes to the ins and outs of health care builds and product launches. The process is much like starting down the path of a country road in the middle of a blizzard—unless you have dependable wipers and a good defrost system, that path can get murky very quickly. With this article I hope to offer my counsel to inventors, featuring a few of my prior launches as well as case studies of health care launches I was not involved with, and sharing the lessons learned and hurdles that were overcome. I encourage all entrepreneurs to act on their ideas because, in the world of health care startups, the only failure is not acting on an invention.
Case study 1: Cerezyme
Today, Cerezyme is indicated for patients with Gaucher, which is a lysosomal storage disorder. Cerezyme’s first-generation product, called Ceredase, was a human tissue-derived protein that we extracted from human placentas. At the time, the concept of moving this program forward was denied by the Board of Directors because they said that even if you could collect enough placentas to make the enzyme, it would be too expensive to manufacture. In fact, early scale-up modeling for manufacturing the protein demonstrated that Genzyme would need 4 tons of placentas per Gaucher patient per year.
Gaucher is a severe, early-onset disease that has a significant negative outcome for patients. Patients with Gaucher are in dire need of treatment. Genzyme went forward with the Ceredase program by financing it through the families of patients with the disease, by starting an LLC separate from the business and funding the initial clinical trial and the development of the protein through the families of Gaucher patients. That approach was a successful endeavor. A great example of a creative capital structure to advance a program.
This was in the late 1980s/early 1990s, and at the height of the AIDS challenge. Genzyme based the manufacturing in Lille, France, and we cryopreserved placentas in the United States and Europe and shipped them to Lille to be processed into therapy. Genzyme eventually received approval for Ceredase from the US Food and Drug Administration (FDA) and the European Medicines Agency. At the height of the placenta collection, we were gathering about 10% to 15% of the placentas in the United States and 30% to 40% of the placentas in Europe. Resources supply became an issue until we developed a recombinant form of the protein, accomplished by using a manufacturing system called a CHO cell line.
This is a very good success story: If this invention was not pursued, Gaucher patients would not benefit from the treatment today. In addition, there are a plethora of patients with different lysosomal storage disorders treated with additional proteins that have been aided by us going through the entire development, manufacturing, and global commercialization process. We figured out how to manufacture and deliver the treatment, working through multiple countries’ political systems, and today the therapy is paid for by insurance and government systems on a worldwide basis.
Continue to: Case study 2...
Case study 2: ThinPrep
I like to use the approval of ThinPrep as an example of avoiding a false negative—a stoppage in the development of the product or drug for the wrong reasons. False negatives, in my mind, occur when you are developing a technology and you run into issues during the clinical phase and/or with FDA approval, or with a technical failure or you run out of capital prior to knowing whether or not the innovation actually works. In the case of ThinPrep, a poorly run clinical trial almost resulted in a false negative.
The company at the time was Cytyc, and an initial clinical study presented to the FDA yielded a neutral-negative outcome. The FDA said that there were not enough data to show the differentiation from the current Pap smear standard of care.
The founders of the company at that time had inherited the study protocol from a prior leadership team, so they had to finish the trial with the initial protocol. Given the FDA’s advisement, they developed a new trial. It took the persistence of these two founders, who mortgaged their homes and spent their personal dollars to take this through the next wave of clinical development. In the end it was successful. The revised clinical trial yielded an approval for ThinPrep, which is now considered a standard of care.
The use of ThinPrep reduced cervical cancer deaths by 40% from preapproval. The challenging path from clinical development to eventual commercial launch and physician leadership in advancing patient care makes the story of ThinPrep a great example of not allowing an early false negative of a poorly designed and run clinical trial stop important innovation.
Case study 3: Cologuard
The development of Cologuard is a case study demonstrating that, sometimes, when your first attempt does not work, you need to have the persistence to raise additional capital and/or use a slightly different technical approach. The approval story of Cologuard is important to share because it is an important cancer screening diagnostic, using DNA from stool samples to test for colon cancer, giving access to important colon cancer screening to many patients. Currently, caregivers are only scraping the surface with Cologuard’s ability to screen the population. There are many more patients that need access to the test, and I believe they will get it in the years ahead.
Cologuard went through a first- and second-generational technical failure. They could not get the test’s specificity and sensitivity to be at the level of a screening tool; there were too many false-positive results. With the third iteration came the technical breakthrough, and a very large, expensive study was conducted—one the leadership team was criticized for. However, that study yielded the data that achieved a New England Journal of Medicine article, and reimbursement support across the country. The combination of the right technical team and the right leadership team, who planned a proper commercial launch, with a CEO that supported the extensive clinical study, has resulted in the fourth generation of Cologuard—an important breakthrough offering a very useful new standard of care in colon cancer detection and screening.
Continue to: Pearls for moving your innovations forward...
Pearls for moving your innovations forward
Because of my experience in undergoing health care start-ups, and contributing to several of those advancements of innovation, many inventors approach me for advice on their paths from idea to full-concept company. Here are a few of my lessons learned.
Consider purpose, not financial gain, first and foremost. Financial gain is typically the by-product or outcome of a standard-of-care breakthrough for inventors, but it’s a very hard road. Pursue your invention for advancing patient care and moving a new standard of care forward in health care versus financial gain at the end.
Determine whether your invention is a product or a company, or potentially, not capitalizable at all. Figure this out early. Analyze your idea to make sure it is sound and truly novel. Analyze the competition and to make sure it is sound and truly novel. Analyze the competition and the market dynamics to support a new product. Can the development path be defined very clearly to raise capital? Is your innovation a big enough breakthrough in the market with several current products to actually make a difference in patient outcomes (and eventually achieve product reimbursement)? The creation of a company may be the right strategy if the innovation can support a differentiated enough breakthrough where you can actually support all the infrastructure to build the business. If you find that the market is not there to support and develop your idea to eventual success, backing off early is important to preserve invested capital.
Protect early. Is your invention patentable, or has someone else already thought of the idea? What kind of patent(s) are appropriate? Where, geographically, do you want to protect your invention? Find a good patent attorney in your local area, early in the process, to help you answer all of these critical questions. Patents are expensive to file and maintain, but it is not expensive to do a literature search to find out if your idea is novel. A provisional patent, which would be your first step, is an important cost-effective step.
Capital is out there. If your invention or idea deserves capital, it is available. I will address raising capital in more detail in the next section.
Consider regulatory and manufacturing as achievable hurdles. Inventors often get tripped up here, considering the regulatory hurdles and manufacturing too challenging and abandoning their ideas because the risk is too great. Regulatory and manufacturing are very important aspects of health care standard-of-care builds. Cutting corners is not an option. That said, regulatory and manufacturing should not stop you. Challenges often can be worked through as long as the clinical need is there, and the clinical data support bringing that technology forward.
Consider corporate partnerships. I am a fan of corporate partners. But which ones should you target, and when and why? Corporate partnerships can bring significant capital, which is great, but there is enough investor capital out there that you should not pursue a corporate partner just for capital. The main benefit of a corporate partner is enterprise intellect. They typically know more about the field that you are entering than the investors or a small company leadership team.
Establish and listen to advisors. When thinking about who to trust, research their track record. Advisors who have gone through this process before, and specifically in your product area, are important to have access to.
Persistence is key. I have observed a tremendous “compression of innovation” in the health care areas that I have been involved with—human tissue-derived proteins, robotic surgery, stem cell therapy, and digital health (which is still in its infancy). For each of these breakthrough categories, early on, it appeared that it couldn’t be done. However, after the first 2 or 3 major breakthroughs in each one of these areas, a compression of innovation occurred. For instance, after approximately 15 years of protein development, we came out with the recombinant manufacturing systems for proteins. Very quickly, within 10 years, there were more than 70 proteins on the market. The persistence of the inventors to overcome early obstacles in each of these health care areas was critical to future success in each area.
Continue to: Raising capital...
Raising capital
There are different investors who specialize in different types of investment opportunities. The first phase of raising capital is the seed round—where there is typically early data, or even no data and just a concept. From this seed round forward, there is less risk as you develop your technology; thus, there are different investors that support different stages of development and that specialize in different types of investing. It is important to target the right investors and raise enough capital to be able to go achieve multiple operational milestones. Otherwise, when you go through your first round of capital, or the Series A or B financing rounds, there may not be a set of investors out there to fund the company moving forward. Health care investors will make it known that they invest in certain rounds of capital. You can determine who those investors are by doing a search online.
A mistake health care inventors can make is not taking enough capital from investors, because they are concerned about dilution. I advise investors not to focus on dilution but rather on, how big can you make “the pie” (value of the company) worth? The entire process is about bringing a true product through to a new standard-of-care curve.
Trust is the most important thing to earn with investors, and there is zero tolerance for a lack of trust. Share your vision as the inventor with investors, who want to know where this category could be in the next 5 or 10 years. Clinical data will always win, and health care investors and industry leaders should be focused on executing the most robust clinical data to demonstrate the clearest potential clinical outcome. Investors will follow a good plan that has been developed to achieve FDA approval, successful commercialization or “go to market” launch, and eventual reimbursement to support a true standard-of-care change.
Failure is defined by inaction
The 3 case studies that I have shared were success stories because the ideas and inventions were acted upon. When I was at Genzyme, we built the company up to more than $1 billion in revenue. We commercialized proteins in over 50 countries. Most importantly, many patients benefited from the innovation. If you have an invention and an idea, act on it—and surround yourself with great people in every discipline. Having the right people and team is extremely important. ●

I often say that there are both “guardrail” days and very good days when it comes to the ins and outs of health care builds and product launches. The process is much like starting down the path of a country road in the middle of a blizzard—unless you have dependable wipers and a good defrost system, that path can get murky very quickly. With this article I hope to offer my counsel to inventors, featuring a few of my prior launches as well as case studies of health care launches I was not involved with, and sharing the lessons learned and hurdles that were overcome. I encourage all entrepreneurs to act on their ideas because, in the world of health care startups, the only failure is not acting on an invention.
Case study 1: Cerezyme
Today, Cerezyme is indicated for patients with Gaucher, which is a lysosomal storage disorder. Cerezyme’s first-generation product, called Ceredase, was a human tissue-derived protein that we extracted from human placentas. At the time, the concept of moving this program forward was denied by the Board of Directors because they said that even if you could collect enough placentas to make the enzyme, it would be too expensive to manufacture. In fact, early scale-up modeling for manufacturing the protein demonstrated that Genzyme would need 4 tons of placentas per Gaucher patient per year.
Gaucher is a severe, early-onset disease that has a significant negative outcome for patients. Patients with Gaucher are in dire need of treatment. Genzyme went forward with the Ceredase program by financing it through the families of patients with the disease, by starting an LLC separate from the business and funding the initial clinical trial and the development of the protein through the families of Gaucher patients. That approach was a successful endeavor. A great example of a creative capital structure to advance a program.
This was in the late 1980s/early 1990s, and at the height of the AIDS challenge. Genzyme based the manufacturing in Lille, France, and we cryopreserved placentas in the United States and Europe and shipped them to Lille to be processed into therapy. Genzyme eventually received approval for Ceredase from the US Food and Drug Administration (FDA) and the European Medicines Agency. At the height of the placenta collection, we were gathering about 10% to 15% of the placentas in the United States and 30% to 40% of the placentas in Europe. Resources supply became an issue until we developed a recombinant form of the protein, accomplished by using a manufacturing system called a CHO cell line.
This is a very good success story: If this invention was not pursued, Gaucher patients would not benefit from the treatment today. In addition, there are a plethora of patients with different lysosomal storage disorders treated with additional proteins that have been aided by us going through the entire development, manufacturing, and global commercialization process. We figured out how to manufacture and deliver the treatment, working through multiple countries’ political systems, and today the therapy is paid for by insurance and government systems on a worldwide basis.
Continue to: Case study 2...
Case study 2: ThinPrep
I like to use the approval of ThinPrep as an example of avoiding a false negative—a stoppage in the development of the product or drug for the wrong reasons. False negatives, in my mind, occur when you are developing a technology and you run into issues during the clinical phase and/or with FDA approval, or with a technical failure or you run out of capital prior to knowing whether or not the innovation actually works. In the case of ThinPrep, a poorly run clinical trial almost resulted in a false negative.
The company at the time was Cytyc, and an initial clinical study presented to the FDA yielded a neutral-negative outcome. The FDA said that there were not enough data to show the differentiation from the current Pap smear standard of care.
The founders of the company at that time had inherited the study protocol from a prior leadership team, so they had to finish the trial with the initial protocol. Given the FDA’s advisement, they developed a new trial. It took the persistence of these two founders, who mortgaged their homes and spent their personal dollars to take this through the next wave of clinical development. In the end it was successful. The revised clinical trial yielded an approval for ThinPrep, which is now considered a standard of care.
The use of ThinPrep reduced cervical cancer deaths by 40% from preapproval. The challenging path from clinical development to eventual commercial launch and physician leadership in advancing patient care makes the story of ThinPrep a great example of not allowing an early false negative of a poorly designed and run clinical trial stop important innovation.
Case study 3: Cologuard
The development of Cologuard is a case study demonstrating that, sometimes, when your first attempt does not work, you need to have the persistence to raise additional capital and/or use a slightly different technical approach. The approval story of Cologuard is important to share because it is an important cancer screening diagnostic, using DNA from stool samples to test for colon cancer, giving access to important colon cancer screening to many patients. Currently, caregivers are only scraping the surface with Cologuard’s ability to screen the population. There are many more patients that need access to the test, and I believe they will get it in the years ahead.
Cologuard went through a first- and second-generational technical failure. They could not get the test’s specificity and sensitivity to be at the level of a screening tool; there were too many false-positive results. With the third iteration came the technical breakthrough, and a very large, expensive study was conducted—one the leadership team was criticized for. However, that study yielded the data that achieved a New England Journal of Medicine article, and reimbursement support across the country. The combination of the right technical team and the right leadership team, who planned a proper commercial launch, with a CEO that supported the extensive clinical study, has resulted in the fourth generation of Cologuard—an important breakthrough offering a very useful new standard of care in colon cancer detection and screening.
Continue to: Pearls for moving your innovations forward...
Pearls for moving your innovations forward
Because of my experience in undergoing health care start-ups, and contributing to several of those advancements of innovation, many inventors approach me for advice on their paths from idea to full-concept company. Here are a few of my lessons learned.
Consider purpose, not financial gain, first and foremost. Financial gain is typically the by-product or outcome of a standard-of-care breakthrough for inventors, but it’s a very hard road. Pursue your invention for advancing patient care and moving a new standard of care forward in health care versus financial gain at the end.
Determine whether your invention is a product or a company, or potentially, not capitalizable at all. Figure this out early. Analyze your idea to make sure it is sound and truly novel. Analyze the competition and to make sure it is sound and truly novel. Analyze the competition and the market dynamics to support a new product. Can the development path be defined very clearly to raise capital? Is your innovation a big enough breakthrough in the market with several current products to actually make a difference in patient outcomes (and eventually achieve product reimbursement)? The creation of a company may be the right strategy if the innovation can support a differentiated enough breakthrough where you can actually support all the infrastructure to build the business. If you find that the market is not there to support and develop your idea to eventual success, backing off early is important to preserve invested capital.
Protect early. Is your invention patentable, or has someone else already thought of the idea? What kind of patent(s) are appropriate? Where, geographically, do you want to protect your invention? Find a good patent attorney in your local area, early in the process, to help you answer all of these critical questions. Patents are expensive to file and maintain, but it is not expensive to do a literature search to find out if your idea is novel. A provisional patent, which would be your first step, is an important cost-effective step.
Capital is out there. If your invention or idea deserves capital, it is available. I will address raising capital in more detail in the next section.
Consider regulatory and manufacturing as achievable hurdles. Inventors often get tripped up here, considering the regulatory hurdles and manufacturing too challenging and abandoning their ideas because the risk is too great. Regulatory and manufacturing are very important aspects of health care standard-of-care builds. Cutting corners is not an option. That said, regulatory and manufacturing should not stop you. Challenges often can be worked through as long as the clinical need is there, and the clinical data support bringing that technology forward.
Consider corporate partnerships. I am a fan of corporate partners. But which ones should you target, and when and why? Corporate partnerships can bring significant capital, which is great, but there is enough investor capital out there that you should not pursue a corporate partner just for capital. The main benefit of a corporate partner is enterprise intellect. They typically know more about the field that you are entering than the investors or a small company leadership team.
Establish and listen to advisors. When thinking about who to trust, research their track record. Advisors who have gone through this process before, and specifically in your product area, are important to have access to.
Persistence is key. I have observed a tremendous “compression of innovation” in the health care areas that I have been involved with—human tissue-derived proteins, robotic surgery, stem cell therapy, and digital health (which is still in its infancy). For each of these breakthrough categories, early on, it appeared that it couldn’t be done. However, after the first 2 or 3 major breakthroughs in each one of these areas, a compression of innovation occurred. For instance, after approximately 15 years of protein development, we came out with the recombinant manufacturing systems for proteins. Very quickly, within 10 years, there were more than 70 proteins on the market. The persistence of the inventors to overcome early obstacles in each of these health care areas was critical to future success in each area.
Continue to: Raising capital...
Raising capital
There are different investors who specialize in different types of investment opportunities. The first phase of raising capital is the seed round—where there is typically early data, or even no data and just a concept. From this seed round forward, there is less risk as you develop your technology; thus, there are different investors that support different stages of development and that specialize in different types of investing. It is important to target the right investors and raise enough capital to be able to go achieve multiple operational milestones. Otherwise, when you go through your first round of capital, or the Series A or B financing rounds, there may not be a set of investors out there to fund the company moving forward. Health care investors will make it known that they invest in certain rounds of capital. You can determine who those investors are by doing a search online.
A mistake health care inventors can make is not taking enough capital from investors, because they are concerned about dilution. I advise investors not to focus on dilution but rather on, how big can you make “the pie” (value of the company) worth? The entire process is about bringing a true product through to a new standard-of-care curve.
Trust is the most important thing to earn with investors, and there is zero tolerance for a lack of trust. Share your vision as the inventor with investors, who want to know where this category could be in the next 5 or 10 years. Clinical data will always win, and health care investors and industry leaders should be focused on executing the most robust clinical data to demonstrate the clearest potential clinical outcome. Investors will follow a good plan that has been developed to achieve FDA approval, successful commercialization or “go to market” launch, and eventual reimbursement to support a true standard-of-care change.
Failure is defined by inaction
The 3 case studies that I have shared were success stories because the ideas and inventions were acted upon. When I was at Genzyme, we built the company up to more than $1 billion in revenue. We commercialized proteins in over 50 countries. Most importantly, many patients benefited from the innovation. If you have an invention and an idea, act on it—and surround yourself with great people in every discipline. Having the right people and team is extremely important. ●

I often say that there are both “guardrail” days and very good days when it comes to the ins and outs of health care builds and product launches. The process is much like starting down the path of a country road in the middle of a blizzard—unless you have dependable wipers and a good defrost system, that path can get murky very quickly. With this article I hope to offer my counsel to inventors, featuring a few of my prior launches as well as case studies of health care launches I was not involved with, and sharing the lessons learned and hurdles that were overcome. I encourage all entrepreneurs to act on their ideas because, in the world of health care startups, the only failure is not acting on an invention.
Case study 1: Cerezyme
Today, Cerezyme is indicated for patients with Gaucher, which is a lysosomal storage disorder. Cerezyme’s first-generation product, called Ceredase, was a human tissue-derived protein that we extracted from human placentas. At the time, the concept of moving this program forward was denied by the Board of Directors because they said that even if you could collect enough placentas to make the enzyme, it would be too expensive to manufacture. In fact, early scale-up modeling for manufacturing the protein demonstrated that Genzyme would need 4 tons of placentas per Gaucher patient per year.
Gaucher is a severe, early-onset disease that has a significant negative outcome for patients. Patients with Gaucher are in dire need of treatment. Genzyme went forward with the Ceredase program by financing it through the families of patients with the disease, by starting an LLC separate from the business and funding the initial clinical trial and the development of the protein through the families of Gaucher patients. That approach was a successful endeavor. A great example of a creative capital structure to advance a program.
This was in the late 1980s/early 1990s, and at the height of the AIDS challenge. Genzyme based the manufacturing in Lille, France, and we cryopreserved placentas in the United States and Europe and shipped them to Lille to be processed into therapy. Genzyme eventually received approval for Ceredase from the US Food and Drug Administration (FDA) and the European Medicines Agency. At the height of the placenta collection, we were gathering about 10% to 15% of the placentas in the United States and 30% to 40% of the placentas in Europe. Resources supply became an issue until we developed a recombinant form of the protein, accomplished by using a manufacturing system called a CHO cell line.
This is a very good success story: If this invention was not pursued, Gaucher patients would not benefit from the treatment today. In addition, there are a plethora of patients with different lysosomal storage disorders treated with additional proteins that have been aided by us going through the entire development, manufacturing, and global commercialization process. We figured out how to manufacture and deliver the treatment, working through multiple countries’ political systems, and today the therapy is paid for by insurance and government systems on a worldwide basis.
Continue to: Case study 2...
Case study 2: ThinPrep
I like to use the approval of ThinPrep as an example of avoiding a false negative—a stoppage in the development of the product or drug for the wrong reasons. False negatives, in my mind, occur when you are developing a technology and you run into issues during the clinical phase and/or with FDA approval, or with a technical failure or you run out of capital prior to knowing whether or not the innovation actually works. In the case of ThinPrep, a poorly run clinical trial almost resulted in a false negative.
The company at the time was Cytyc, and an initial clinical study presented to the FDA yielded a neutral-negative outcome. The FDA said that there were not enough data to show the differentiation from the current Pap smear standard of care.
The founders of the company at that time had inherited the study protocol from a prior leadership team, so they had to finish the trial with the initial protocol. Given the FDA’s advisement, they developed a new trial. It took the persistence of these two founders, who mortgaged their homes and spent their personal dollars to take this through the next wave of clinical development. In the end it was successful. The revised clinical trial yielded an approval for ThinPrep, which is now considered a standard of care.
The use of ThinPrep reduced cervical cancer deaths by 40% from preapproval. The challenging path from clinical development to eventual commercial launch and physician leadership in advancing patient care makes the story of ThinPrep a great example of not allowing an early false negative of a poorly designed and run clinical trial stop important innovation.
Case study 3: Cologuard
The development of Cologuard is a case study demonstrating that, sometimes, when your first attempt does not work, you need to have the persistence to raise additional capital and/or use a slightly different technical approach. The approval story of Cologuard is important to share because it is an important cancer screening diagnostic, using DNA from stool samples to test for colon cancer, giving access to important colon cancer screening to many patients. Currently, caregivers are only scraping the surface with Cologuard’s ability to screen the population. There are many more patients that need access to the test, and I believe they will get it in the years ahead.
Cologuard went through a first- and second-generational technical failure. They could not get the test’s specificity and sensitivity to be at the level of a screening tool; there were too many false-positive results. With the third iteration came the technical breakthrough, and a very large, expensive study was conducted—one the leadership team was criticized for. However, that study yielded the data that achieved a New England Journal of Medicine article, and reimbursement support across the country. The combination of the right technical team and the right leadership team, who planned a proper commercial launch, with a CEO that supported the extensive clinical study, has resulted in the fourth generation of Cologuard—an important breakthrough offering a very useful new standard of care in colon cancer detection and screening.
Continue to: Pearls for moving your innovations forward...
Pearls for moving your innovations forward
Because of my experience in undergoing health care start-ups, and contributing to several of those advancements of innovation, many inventors approach me for advice on their paths from idea to full-concept company. Here are a few of my lessons learned.
Consider purpose, not financial gain, first and foremost. Financial gain is typically the by-product or outcome of a standard-of-care breakthrough for inventors, but it’s a very hard road. Pursue your invention for advancing patient care and moving a new standard of care forward in health care versus financial gain at the end.
Determine whether your invention is a product or a company, or potentially, not capitalizable at all. Figure this out early. Analyze your idea to make sure it is sound and truly novel. Analyze the competition and to make sure it is sound and truly novel. Analyze the competition and the market dynamics to support a new product. Can the development path be defined very clearly to raise capital? Is your innovation a big enough breakthrough in the market with several current products to actually make a difference in patient outcomes (and eventually achieve product reimbursement)? The creation of a company may be the right strategy if the innovation can support a differentiated enough breakthrough where you can actually support all the infrastructure to build the business. If you find that the market is not there to support and develop your idea to eventual success, backing off early is important to preserve invested capital.
Protect early. Is your invention patentable, or has someone else already thought of the idea? What kind of patent(s) are appropriate? Where, geographically, do you want to protect your invention? Find a good patent attorney in your local area, early in the process, to help you answer all of these critical questions. Patents are expensive to file and maintain, but it is not expensive to do a literature search to find out if your idea is novel. A provisional patent, which would be your first step, is an important cost-effective step.
Capital is out there. If your invention or idea deserves capital, it is available. I will address raising capital in more detail in the next section.
Consider regulatory and manufacturing as achievable hurdles. Inventors often get tripped up here, considering the regulatory hurdles and manufacturing too challenging and abandoning their ideas because the risk is too great. Regulatory and manufacturing are very important aspects of health care standard-of-care builds. Cutting corners is not an option. That said, regulatory and manufacturing should not stop you. Challenges often can be worked through as long as the clinical need is there, and the clinical data support bringing that technology forward.
Consider corporate partnerships. I am a fan of corporate partners. But which ones should you target, and when and why? Corporate partnerships can bring significant capital, which is great, but there is enough investor capital out there that you should not pursue a corporate partner just for capital. The main benefit of a corporate partner is enterprise intellect. They typically know more about the field that you are entering than the investors or a small company leadership team.
Establish and listen to advisors. When thinking about who to trust, research their track record. Advisors who have gone through this process before, and specifically in your product area, are important to have access to.
Persistence is key. I have observed a tremendous “compression of innovation” in the health care areas that I have been involved with—human tissue-derived proteins, robotic surgery, stem cell therapy, and digital health (which is still in its infancy). For each of these breakthrough categories, early on, it appeared that it couldn’t be done. However, after the first 2 or 3 major breakthroughs in each one of these areas, a compression of innovation occurred. For instance, after approximately 15 years of protein development, we came out with the recombinant manufacturing systems for proteins. Very quickly, within 10 years, there were more than 70 proteins on the market. The persistence of the inventors to overcome early obstacles in each of these health care areas was critical to future success in each area.
Continue to: Raising capital...
Raising capital
There are different investors who specialize in different types of investment opportunities. The first phase of raising capital is the seed round—where there is typically early data, or even no data and just a concept. From this seed round forward, there is less risk as you develop your technology; thus, there are different investors that support different stages of development and that specialize in different types of investing. It is important to target the right investors and raise enough capital to be able to go achieve multiple operational milestones. Otherwise, when you go through your first round of capital, or the Series A or B financing rounds, there may not be a set of investors out there to fund the company moving forward. Health care investors will make it known that they invest in certain rounds of capital. You can determine who those investors are by doing a search online.
A mistake health care inventors can make is not taking enough capital from investors, because they are concerned about dilution. I advise investors not to focus on dilution but rather on, how big can you make “the pie” (value of the company) worth? The entire process is about bringing a true product through to a new standard-of-care curve.
Trust is the most important thing to earn with investors, and there is zero tolerance for a lack of trust. Share your vision as the inventor with investors, who want to know where this category could be in the next 5 or 10 years. Clinical data will always win, and health care investors and industry leaders should be focused on executing the most robust clinical data to demonstrate the clearest potential clinical outcome. Investors will follow a good plan that has been developed to achieve FDA approval, successful commercialization or “go to market” launch, and eventual reimbursement to support a true standard-of-care change.
Failure is defined by inaction
The 3 case studies that I have shared were success stories because the ideas and inventions were acted upon. When I was at Genzyme, we built the company up to more than $1 billion in revenue. We commercialized proteins in over 50 countries. Most importantly, many patients benefited from the innovation. If you have an invention and an idea, act on it—and surround yourself with great people in every discipline. Having the right people and team is extremely important. ●
How ObGyns can best work with radiologists to optimize screening for patients with dense breasts
If your ObGyn practices are anything like ours, every time there is news coverage of a study regarding mammography or about efforts to pass a breast density inform law, your phone rings with patient calls. In fact, every density inform law enacted in the United States, except for in Illinois, directs patients to their referring provider—generally their ObGyn—to discuss the screening and risk implications of dense breast tissue.
The steady increased awareness of breast density means that we, as ObGyns and other primary care providers (PCPs), have additional responsibilities in managing the breast health of our patients. This includes guiding discussions with patients about what breast density means and whether supplemental screening beyond mammography might be beneficial.
As members of the Medical Advisory Board for DenseBreast-info.org (an online educational resource dedicated to providing breast density information to patients and health care professionals), we are aware of the growing body of evidence demonstrating improved detection of early breast cancer using supplemental screening in dense breasts. However, we know that there is confusion among clinicians about how and when to facilitate tailored screening for women with dense breasts or other breast cancer risk factors. Here we answer 6 questions focusing on how to navigate patient discussions around the topic and the best way to collaborate with radiologists to improve breast care for patients.
Play an active role
1. What role should ObGyns and PCPs play in women’s breast health?
Elizabeth Etkin-Kramer, MD: I am a firm believer that ObGyns and all women’s health providers should be able to assess their patients’ risk of breast cancer and explain the process for managing this risk with their patients. This explanation includes the clinical implications of breast density and when supplemental screening should be employed. It is also important for providers to know when to offer genetic testing and when a patient’s personal or family history indicates supplemental screening with breast magnetic resonance imaging (MRI).
DaCarla M. Albright, MD: I absolutely agree that PCPs, ObGyns, and family practitioners should spend the time to be educated about breast density and supplemental screening options. While the exact role providers play in managing patients’ breast health may vary depending on the practice type or location, the need for knowledge and comfort when talking with patients to help them make informed decisions is critical. Breast health and screening, including the importance of breast density, happen to be a particular interest of mine. I have participated in educational webinars, invited lectures, and breast cancer awareness media events on this topic in the past.
Continue to: Join forces with imaging centers...
Join forces with imaging centers
2. How can ObGyns and radiologists collaborate most effectively to use screening results to personalize breast care for patients?
Dr. Etkin-Kramer: It is important to have a close relationship with the radiologists that read our patients’ mammograms. We need to be able to easily contact the radiologist and quickly get clarification on a patient’s report or discuss next steps. Imaging centers should consider running outreach programs to educate their referring providers on how to risk assess, with this assessment inclusive of breast density. Dinner lectures or grand round meetings are effective to facilitate communication between the radiology community and the ObGyn community. Finally, as we all know, supplemental screening is often subject to copays and deductibles per insurance coverage. If advocacy groups, who are working to eliminate these types of costs, cannot get insurers to waive these payments, we need a less expensive self-pay option.
Dr. Albright: I definitely have and encourage an open line of communication between my practice and breast radiology, as well as our breast surgeons and cancer center to set up consultations as needed. We also invite our radiologists as guests to monthly practice meetings or grand rounds within our department to further improve access and open communication, as this environment is one in which greater provider education on density and adjunctive screening can be achieved.
Know when to refer a high-risk patient
3. Most ObGyns routinely collect family history and perform formal risk assessment. What do you need to know about referring patients to a high-risk program?
Dr. Etkin-Kramer: It is important as ObGyns to be knowledgeable about breast and ovarian cancer risk assessment and genetic testing for cancer susceptibility genes. Our patients expect that of us. I am comfortable doing risk assessment in my office, but I sometimes refer to other specialists in the community if the patient needs additional counseling. For risk assessment, I look at family and personal history, breast density, and other factors that might lead me to believe the patient might carry a hereditary cancer susceptibility gene, including Ashkenazi Jewish ancestry.1 When indicated, I check lifetime as well as short-term (5- to 10-year) risk, usually using Breast Cancer Surveillance Consortium (BCSC) or Tyrer-Cuzick/International Breast Cancer Intervention Study (IBIS) models, as these include breast density.
I discuss risk-reducing medications. The US Preventive Services Task Force recommends these agents if my patient’s 5-year risk of breast cancer is 1.67% or greater, and I strongly recommend chemoprevention when the patient’s 5-year BCSC risk exceeds 3%, provided likely benefits exceed risks.2,3 I discuss adding screening breast MRI if lifetime risk by Tyrer-Cuzick exceeds 20%. (Note that Gail and BCSC models are not recommended to be used to determine risk for purposes of supplemental screening with MRI as they do not consider paternal family history nor age of relatives at diagnosis.)
Dr. Albright: ObGyns should be able to ascertain a pertinent history and identify patients at risk for breast cancer based on their personal history, family history, and breast imaging/biopsy history, if relevant. We also need to improve our discussions of supplemental screening for patients who have heterogeneously dense or extremely dense breast tissue. I sense that some ObGyns may rely heavily on the radiologist to suggest supplemental screening, but patients actually look to ObGyns as their providers to have this knowledge and give them direction.
Since I practice at a large academic medical center, I have the opportunity to refer patients to our Breast Cancer Genetics Program because I may be limited on time for counseling in the office and do not want to miss salient details. With all of the information I have ascertained about the patient, I am able to determine and encourage appropriate screening and assure insurance coverage for adjunctive breast MRI when appropriate.
Continue to: Consider how you order patients’ screening to reduce barriers and cost...
Consider how you order patients’ screening to reduce barriers and cost
4. How would you suggest reducing barriers when referring patients for supplemental screening, such as MRI for high-risk women or ultrasound for those with dense breasts? Would you prefer it if such screening could be performed without additional script/referral? How does insurance coverage factor in?
Dr. Etkin-Kramer: I would love for a screening mammogram with possible ultrasound, on one script, to be the norm. One of the centers that I work with accepts a script written this way. Further, when a patient receives screening at a freestanding facility as opposed to a hospital, the fee for the supplemental screening may be lower because they do not add on a facility fee.
Dr. Albright: We have an order in our electronic health record that allows for screening mammography but adds on diagnostic mammography/bilateral ultrasonography, if indicated by imaging. I am mostly ordering that option now for all of my screening patients; rarely have I had issues with insurance accepting that script. As for when ordering an MRI, I always try to ensure that I have done the patient’s personal risk assessment and included that lifetime breast cancer risk on the order. If the risk is 20% or higher, I typically do not have any insurance coverage issues. If I am ordering MRI as supplemental screening, I typically order the “Fast MRI” protocol that our center offers. This order incurs a $299 out-of-pocket cost for the patient. Any patient with heterogeneously or extremely dense breasts on mammography should have this option, but it requires patient education, discussion with the provider, and an additional cost. I definitely think that insurers need to consider covering supplemental screening, since breast density is reportable in a majority of the US states and will soon be the national standard.
Pearls for guiding patients
5. How do you discuss breast density and the need for supplemental screening with your patients?
Dr. Etkin-Kramer: I strongly feel that my patients need to know when a screening test has limited ability to do its job. This is the case with dense breasts. Visuals help; when discussing breast density, I like the images supplied by DenseBreast-info.org (FIGURE). I explain the two implications of dense tissue:
- First, dense tissue makes it harder to visualize cancers in the breast—the denser the breasts, the less likely the radiologist can pick up a cancer, so mammographic sensitivity for extremely dense breasts can be as low as 25% to 50%.
- Second, high breast density adds to the risk of developing breast cancer. I explain that supplemental screening will pick up additional cancers in women with dense breasts. For example, breast ultrasound will pick up about 2-3/1000 additional breast cancers per year and MRI or molecular breast imaging (MBI) will pick up much more, perhaps 10/1000.
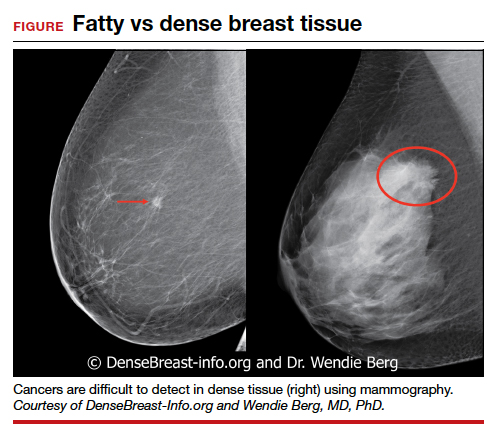
MRI is more invasive than an ultrasound and uses gadolinium, and MBI has more radiation. Supplemental screening is not endorsed by ACOG’s most recent Committee Opinion from 2017; 4 however, patients may choose to have it done. This is where shared-decision making is important.
I strongly recommend that all women’s health care providers complete the CME course on the DenseBreast-info.org website. “ Breast Density: Why It Matters ” is a certified educational program for referring physicians that helps health care professionals learn about breast density, its associated risks, and how best to guide patients regarding breast cancer screening.
Continue to: Dr. Albright...
Dr. Albright: When I discuss breast density, I make sure that patients understand that their mammogram determines the density of their breast tissue. I review that in the higher density categories (heterogeneously dense or extremely dense), there is a higher risk of missing cancer, and that these categories are also associated with a higher risk of breast cancer. I also discuss the potential need for supplemental screening, for which my institution primarily offers Fast MRI. However, we can offer breast ultrasonography instead as an option, especially for those concerned about gadolinium exposure. Our center offers either of these supplemental screenings at a cost of $299. I also review the lack of coverage for supplemental screening by some insurance carriers, as both providers and patients may need to advocate for insurer coverage of adjunct studies.
Educational resources
6. What reference materials, illustrations, or other tools do you use to educate your patients?
Dr. Etkin-Kramer: I frequently use handouts printed from the DenseBreast-info.org website, and there is now a brand new patient fact sheet that I have just started using. I also have an example of breast density categories from fatty replaced to extremely dense on my computer, and I am putting it on a new smart board.
Dr. Albright: The extensive resources available at DenseBreast-info.org can improve both patient and provider knowledge of these important issues, so I suggest patients visit that website, and I use many of the images and visuals to help explain breast density. I even use the materials from the website for educating my resident trainees on breast health and screening. ●
Nearly 16,000 children (up to age 19 years) face cancer-related treatment every year.1 For girls and young women, undergoing chest radiotherapy puts them at higher risk for secondary breast cancer. In fact, they have a 30% chance of developing such cancer by age 50—a risk that is similar to women with a BRCA1 mutation.2 Therefore, current recommendations for breast cancer screening among those who have undergone childhood chest radiation (≥20 Gy) are to begin annual mammography, with adjunct magnetic resonance imaging (MRI), at age 25 years (or 8 years after chest radiotherapy).3
To determine the benefits and risks of these recommendations, as well as of similar strategies, Yeh and colleagues performed simulation modeling using data from the Childhood Cancer Survivor Study and two CISNET (Cancer Intervention and Surveillance Modeling Network) models.4 For their study they targeted a cohort of female childhood cancer survivors having undergone chest radiotherapy and evaluated breast cancer screening with the following strategies:
- mammography plus MRI, starting at ages 25, 30, or 35 years and continuing to age 74
- MRI alone, starting at ages 25, 30, or 35 years and continuing to age 74.
They found that both strategies reduced the risk of breast cancer in the targeted cohort but that screening beginning at the earliest ages prevented most deaths. No screening at all was associated with a 10% to 11% lifetime risk of breast cancer, but mammography plus MRI beginning at age 25 reduced that risk by 56% to 71% depending on the model. Screening with MRI alone reduced mortality risk by 56% to 62%. When considering cost per quality adjusted life-year gained, the researchers found that screening beginning at age 30 to be the most cost-effective.4
Yeh and colleagues addressed concerns with mammography and radiation. Although they said the associated amount of radiation exposure is small, the use of mammography in women younger than age 30 is controversial—and not recommended by the American Cancer Society or the National Comprehensive Cancer Network.5,6
Bottom line. Yeh and colleagues conclude that MRI screening, with or without mammography, beginning between the ages of 25 and 30 should be emphasized in screening guidelines. They note the importance of insurance coverage for MRI in those at risk for breast cancer due to childhood radiation exposure.4
References
- National Cancer Institute. How common is cancer in children? https://www.cancer.gov/types/childhood-cancers/child-adolescentcancers-fact-sheet#how-common-is-cancer-in-children. Accessed September 25, 2020.
- Moskowitz CS, Chou JF, Wolden SL, et al. Breast cancer after chest radiation therapy for childhood cancer. J Clin Oncol. 2014;32:2217- 2223.
- Children’s Oncology Group. Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers. http:// www.survivorshipguidelines.org/pdf/2018/COG_LTFU_Guidelines_v5.pdf. Accessed September 25, 2020.
- Yeh JM, Lowry KP, Schechter CB, et al. Clinical benefits, harms, and cost-effectiveness of breast cancer screening for survivors of childhood cancer treated with chest radiation. Ann Intern Med. 2020;173:331-341.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Breast cancer screening and diagnosis version 1.2019. https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed September 25, 2020.
- Bharucha PP, Chiu KE, Francois FM, et al. Genetic testing and screening recommendations for patients with hereditary breast cancer. RadioGraphics. 2020;40:913-936.
- Freedman AN, Yu B, Gail MH, et al. Benefit/risk assessment for breast cancer chemoprevention with raloxifene or tamoxifen for women age 50 years or older. J Clin Oncol. 2011;29:2327-2333.
- Pruthi S, Heisey RE, Bevers TB. Chemoprevention for breast cancer. Ann Surg Oncol. 2015;22:3230-3235.
- American College of Obstetricians and Gynecologists. Committee opinion no. 625: management of women with dense breasts diagnosed by mammography [published correction appears in Obstet Gynecol. 2016;127:166]. Obstet Gynecol. 2015;125(3):750-751.
If your ObGyn practices are anything like ours, every time there is news coverage of a study regarding mammography or about efforts to pass a breast density inform law, your phone rings with patient calls. In fact, every density inform law enacted in the United States, except for in Illinois, directs patients to their referring provider—generally their ObGyn—to discuss the screening and risk implications of dense breast tissue.
The steady increased awareness of breast density means that we, as ObGyns and other primary care providers (PCPs), have additional responsibilities in managing the breast health of our patients. This includes guiding discussions with patients about what breast density means and whether supplemental screening beyond mammography might be beneficial.
As members of the Medical Advisory Board for DenseBreast-info.org (an online educational resource dedicated to providing breast density information to patients and health care professionals), we are aware of the growing body of evidence demonstrating improved detection of early breast cancer using supplemental screening in dense breasts. However, we know that there is confusion among clinicians about how and when to facilitate tailored screening for women with dense breasts or other breast cancer risk factors. Here we answer 6 questions focusing on how to navigate patient discussions around the topic and the best way to collaborate with radiologists to improve breast care for patients.
Play an active role
1. What role should ObGyns and PCPs play in women’s breast health?
Elizabeth Etkin-Kramer, MD: I am a firm believer that ObGyns and all women’s health providers should be able to assess their patients’ risk of breast cancer and explain the process for managing this risk with their patients. This explanation includes the clinical implications of breast density and when supplemental screening should be employed. It is also important for providers to know when to offer genetic testing and when a patient’s personal or family history indicates supplemental screening with breast magnetic resonance imaging (MRI).
DaCarla M. Albright, MD: I absolutely agree that PCPs, ObGyns, and family practitioners should spend the time to be educated about breast density and supplemental screening options. While the exact role providers play in managing patients’ breast health may vary depending on the practice type or location, the need for knowledge and comfort when talking with patients to help them make informed decisions is critical. Breast health and screening, including the importance of breast density, happen to be a particular interest of mine. I have participated in educational webinars, invited lectures, and breast cancer awareness media events on this topic in the past.
Continue to: Join forces with imaging centers...
Join forces with imaging centers
2. How can ObGyns and radiologists collaborate most effectively to use screening results to personalize breast care for patients?
Dr. Etkin-Kramer: It is important to have a close relationship with the radiologists that read our patients’ mammograms. We need to be able to easily contact the radiologist and quickly get clarification on a patient’s report or discuss next steps. Imaging centers should consider running outreach programs to educate their referring providers on how to risk assess, with this assessment inclusive of breast density. Dinner lectures or grand round meetings are effective to facilitate communication between the radiology community and the ObGyn community. Finally, as we all know, supplemental screening is often subject to copays and deductibles per insurance coverage. If advocacy groups, who are working to eliminate these types of costs, cannot get insurers to waive these payments, we need a less expensive self-pay option.
Dr. Albright: I definitely have and encourage an open line of communication between my practice and breast radiology, as well as our breast surgeons and cancer center to set up consultations as needed. We also invite our radiologists as guests to monthly practice meetings or grand rounds within our department to further improve access and open communication, as this environment is one in which greater provider education on density and adjunctive screening can be achieved.
Know when to refer a high-risk patient
3. Most ObGyns routinely collect family history and perform formal risk assessment. What do you need to know about referring patients to a high-risk program?
Dr. Etkin-Kramer: It is important as ObGyns to be knowledgeable about breast and ovarian cancer risk assessment and genetic testing for cancer susceptibility genes. Our patients expect that of us. I am comfortable doing risk assessment in my office, but I sometimes refer to other specialists in the community if the patient needs additional counseling. For risk assessment, I look at family and personal history, breast density, and other factors that might lead me to believe the patient might carry a hereditary cancer susceptibility gene, including Ashkenazi Jewish ancestry.1 When indicated, I check lifetime as well as short-term (5- to 10-year) risk, usually using Breast Cancer Surveillance Consortium (BCSC) or Tyrer-Cuzick/International Breast Cancer Intervention Study (IBIS) models, as these include breast density.
I discuss risk-reducing medications. The US Preventive Services Task Force recommends these agents if my patient’s 5-year risk of breast cancer is 1.67% or greater, and I strongly recommend chemoprevention when the patient’s 5-year BCSC risk exceeds 3%, provided likely benefits exceed risks.2,3 I discuss adding screening breast MRI if lifetime risk by Tyrer-Cuzick exceeds 20%. (Note that Gail and BCSC models are not recommended to be used to determine risk for purposes of supplemental screening with MRI as they do not consider paternal family history nor age of relatives at diagnosis.)
Dr. Albright: ObGyns should be able to ascertain a pertinent history and identify patients at risk for breast cancer based on their personal history, family history, and breast imaging/biopsy history, if relevant. We also need to improve our discussions of supplemental screening for patients who have heterogeneously dense or extremely dense breast tissue. I sense that some ObGyns may rely heavily on the radiologist to suggest supplemental screening, but patients actually look to ObGyns as their providers to have this knowledge and give them direction.
Since I practice at a large academic medical center, I have the opportunity to refer patients to our Breast Cancer Genetics Program because I may be limited on time for counseling in the office and do not want to miss salient details. With all of the information I have ascertained about the patient, I am able to determine and encourage appropriate screening and assure insurance coverage for adjunctive breast MRI when appropriate.
Continue to: Consider how you order patients’ screening to reduce barriers and cost...
Consider how you order patients’ screening to reduce barriers and cost
4. How would you suggest reducing barriers when referring patients for supplemental screening, such as MRI for high-risk women or ultrasound for those with dense breasts? Would you prefer it if such screening could be performed without additional script/referral? How does insurance coverage factor in?
Dr. Etkin-Kramer: I would love for a screening mammogram with possible ultrasound, on one script, to be the norm. One of the centers that I work with accepts a script written this way. Further, when a patient receives screening at a freestanding facility as opposed to a hospital, the fee for the supplemental screening may be lower because they do not add on a facility fee.
Dr. Albright: We have an order in our electronic health record that allows for screening mammography but adds on diagnostic mammography/bilateral ultrasonography, if indicated by imaging. I am mostly ordering that option now for all of my screening patients; rarely have I had issues with insurance accepting that script. As for when ordering an MRI, I always try to ensure that I have done the patient’s personal risk assessment and included that lifetime breast cancer risk on the order. If the risk is 20% or higher, I typically do not have any insurance coverage issues. If I am ordering MRI as supplemental screening, I typically order the “Fast MRI” protocol that our center offers. This order incurs a $299 out-of-pocket cost for the patient. Any patient with heterogeneously or extremely dense breasts on mammography should have this option, but it requires patient education, discussion with the provider, and an additional cost. I definitely think that insurers need to consider covering supplemental screening, since breast density is reportable in a majority of the US states and will soon be the national standard.
Pearls for guiding patients
5. How do you discuss breast density and the need for supplemental screening with your patients?
Dr. Etkin-Kramer: I strongly feel that my patients need to know when a screening test has limited ability to do its job. This is the case with dense breasts. Visuals help; when discussing breast density, I like the images supplied by DenseBreast-info.org (FIGURE). I explain the two implications of dense tissue:
- First, dense tissue makes it harder to visualize cancers in the breast—the denser the breasts, the less likely the radiologist can pick up a cancer, so mammographic sensitivity for extremely dense breasts can be as low as 25% to 50%.
- Second, high breast density adds to the risk of developing breast cancer. I explain that supplemental screening will pick up additional cancers in women with dense breasts. For example, breast ultrasound will pick up about 2-3/1000 additional breast cancers per year and MRI or molecular breast imaging (MBI) will pick up much more, perhaps 10/1000.

MRI is more invasive than an ultrasound and uses gadolinium, and MBI has more radiation. Supplemental screening is not endorsed by ACOG’s most recent Committee Opinion from 2017; 4 however, patients may choose to have it done. This is where shared-decision making is important.
I strongly recommend that all women’s health care providers complete the CME course on the DenseBreast-info.org website. “ Breast Density: Why It Matters ” is a certified educational program for referring physicians that helps health care professionals learn about breast density, its associated risks, and how best to guide patients regarding breast cancer screening.
Continue to: Dr. Albright...
Dr. Albright: When I discuss breast density, I make sure that patients understand that their mammogram determines the density of their breast tissue. I review that in the higher density categories (heterogeneously dense or extremely dense), there is a higher risk of missing cancer, and that these categories are also associated with a higher risk of breast cancer. I also discuss the potential need for supplemental screening, for which my institution primarily offers Fast MRI. However, we can offer breast ultrasonography instead as an option, especially for those concerned about gadolinium exposure. Our center offers either of these supplemental screenings at a cost of $299. I also review the lack of coverage for supplemental screening by some insurance carriers, as both providers and patients may need to advocate for insurer coverage of adjunct studies.
Educational resources
6. What reference materials, illustrations, or other tools do you use to educate your patients?
Dr. Etkin-Kramer: I frequently use handouts printed from the DenseBreast-info.org website, and there is now a brand new patient fact sheet that I have just started using. I also have an example of breast density categories from fatty replaced to extremely dense on my computer, and I am putting it on a new smart board.
Dr. Albright: The extensive resources available at DenseBreast-info.org can improve both patient and provider knowledge of these important issues, so I suggest patients visit that website, and I use many of the images and visuals to help explain breast density. I even use the materials from the website for educating my resident trainees on breast health and screening. ●
Nearly 16,000 children (up to age 19 years) face cancer-related treatment every year.1 For girls and young women, undergoing chest radiotherapy puts them at higher risk for secondary breast cancer. In fact, they have a 30% chance of developing such cancer by age 50—a risk that is similar to women with a BRCA1 mutation.2 Therefore, current recommendations for breast cancer screening among those who have undergone childhood chest radiation (≥20 Gy) are to begin annual mammography, with adjunct magnetic resonance imaging (MRI), at age 25 years (or 8 years after chest radiotherapy).3
To determine the benefits and risks of these recommendations, as well as of similar strategies, Yeh and colleagues performed simulation modeling using data from the Childhood Cancer Survivor Study and two CISNET (Cancer Intervention and Surveillance Modeling Network) models.4 For their study they targeted a cohort of female childhood cancer survivors having undergone chest radiotherapy and evaluated breast cancer screening with the following strategies:
- mammography plus MRI, starting at ages 25, 30, or 35 years and continuing to age 74
- MRI alone, starting at ages 25, 30, or 35 years and continuing to age 74.
They found that both strategies reduced the risk of breast cancer in the targeted cohort but that screening beginning at the earliest ages prevented most deaths. No screening at all was associated with a 10% to 11% lifetime risk of breast cancer, but mammography plus MRI beginning at age 25 reduced that risk by 56% to 71% depending on the model. Screening with MRI alone reduced mortality risk by 56% to 62%. When considering cost per quality adjusted life-year gained, the researchers found that screening beginning at age 30 to be the most cost-effective.4
Yeh and colleagues addressed concerns with mammography and radiation. Although they said the associated amount of radiation exposure is small, the use of mammography in women younger than age 30 is controversial—and not recommended by the American Cancer Society or the National Comprehensive Cancer Network.5,6
Bottom line. Yeh and colleagues conclude that MRI screening, with or without mammography, beginning between the ages of 25 and 30 should be emphasized in screening guidelines. They note the importance of insurance coverage for MRI in those at risk for breast cancer due to childhood radiation exposure.4
References
- National Cancer Institute. How common is cancer in children? https://www.cancer.gov/types/childhood-cancers/child-adolescentcancers-fact-sheet#how-common-is-cancer-in-children. Accessed September 25, 2020.
- Moskowitz CS, Chou JF, Wolden SL, et al. Breast cancer after chest radiation therapy for childhood cancer. J Clin Oncol. 2014;32:2217- 2223.
- Children’s Oncology Group. Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers. http:// www.survivorshipguidelines.org/pdf/2018/COG_LTFU_Guidelines_v5.pdf. Accessed September 25, 2020.
- Yeh JM, Lowry KP, Schechter CB, et al. Clinical benefits, harms, and cost-effectiveness of breast cancer screening for survivors of childhood cancer treated with chest radiation. Ann Intern Med. 2020;173:331-341.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Breast cancer screening and diagnosis version 1.2019. https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed September 25, 2020.
If your ObGyn practices are anything like ours, every time there is news coverage of a study regarding mammography or about efforts to pass a breast density inform law, your phone rings with patient calls. In fact, every density inform law enacted in the United States, except for in Illinois, directs patients to their referring provider—generally their ObGyn—to discuss the screening and risk implications of dense breast tissue.
The steady increased awareness of breast density means that we, as ObGyns and other primary care providers (PCPs), have additional responsibilities in managing the breast health of our patients. This includes guiding discussions with patients about what breast density means and whether supplemental screening beyond mammography might be beneficial.
As members of the Medical Advisory Board for DenseBreast-info.org (an online educational resource dedicated to providing breast density information to patients and health care professionals), we are aware of the growing body of evidence demonstrating improved detection of early breast cancer using supplemental screening in dense breasts. However, we know that there is confusion among clinicians about how and when to facilitate tailored screening for women with dense breasts or other breast cancer risk factors. Here we answer 6 questions focusing on how to navigate patient discussions around the topic and the best way to collaborate with radiologists to improve breast care for patients.
Play an active role
1. What role should ObGyns and PCPs play in women’s breast health?
Elizabeth Etkin-Kramer, MD: I am a firm believer that ObGyns and all women’s health providers should be able to assess their patients’ risk of breast cancer and explain the process for managing this risk with their patients. This explanation includes the clinical implications of breast density and when supplemental screening should be employed. It is also important for providers to know when to offer genetic testing and when a patient’s personal or family history indicates supplemental screening with breast magnetic resonance imaging (MRI).
DaCarla M. Albright, MD: I absolutely agree that PCPs, ObGyns, and family practitioners should spend the time to be educated about breast density and supplemental screening options. While the exact role providers play in managing patients’ breast health may vary depending on the practice type or location, the need for knowledge and comfort when talking with patients to help them make informed decisions is critical. Breast health and screening, including the importance of breast density, happen to be a particular interest of mine. I have participated in educational webinars, invited lectures, and breast cancer awareness media events on this topic in the past.
Continue to: Join forces with imaging centers...
Join forces with imaging centers
2. How can ObGyns and radiologists collaborate most effectively to use screening results to personalize breast care for patients?
Dr. Etkin-Kramer: It is important to have a close relationship with the radiologists that read our patients’ mammograms. We need to be able to easily contact the radiologist and quickly get clarification on a patient’s report or discuss next steps. Imaging centers should consider running outreach programs to educate their referring providers on how to risk assess, with this assessment inclusive of breast density. Dinner lectures or grand round meetings are effective to facilitate communication between the radiology community and the ObGyn community. Finally, as we all know, supplemental screening is often subject to copays and deductibles per insurance coverage. If advocacy groups, who are working to eliminate these types of costs, cannot get insurers to waive these payments, we need a less expensive self-pay option.
Dr. Albright: I definitely have and encourage an open line of communication between my practice and breast radiology, as well as our breast surgeons and cancer center to set up consultations as needed. We also invite our radiologists as guests to monthly practice meetings or grand rounds within our department to further improve access and open communication, as this environment is one in which greater provider education on density and adjunctive screening can be achieved.
Know when to refer a high-risk patient
3. Most ObGyns routinely collect family history and perform formal risk assessment. What do you need to know about referring patients to a high-risk program?
Dr. Etkin-Kramer: It is important as ObGyns to be knowledgeable about breast and ovarian cancer risk assessment and genetic testing for cancer susceptibility genes. Our patients expect that of us. I am comfortable doing risk assessment in my office, but I sometimes refer to other specialists in the community if the patient needs additional counseling. For risk assessment, I look at family and personal history, breast density, and other factors that might lead me to believe the patient might carry a hereditary cancer susceptibility gene, including Ashkenazi Jewish ancestry.1 When indicated, I check lifetime as well as short-term (5- to 10-year) risk, usually using Breast Cancer Surveillance Consortium (BCSC) or Tyrer-Cuzick/International Breast Cancer Intervention Study (IBIS) models, as these include breast density.
I discuss risk-reducing medications. The US Preventive Services Task Force recommends these agents if my patient’s 5-year risk of breast cancer is 1.67% or greater, and I strongly recommend chemoprevention when the patient’s 5-year BCSC risk exceeds 3%, provided likely benefits exceed risks.2,3 I discuss adding screening breast MRI if lifetime risk by Tyrer-Cuzick exceeds 20%. (Note that Gail and BCSC models are not recommended to be used to determine risk for purposes of supplemental screening with MRI as they do not consider paternal family history nor age of relatives at diagnosis.)
Dr. Albright: ObGyns should be able to ascertain a pertinent history and identify patients at risk for breast cancer based on their personal history, family history, and breast imaging/biopsy history, if relevant. We also need to improve our discussions of supplemental screening for patients who have heterogeneously dense or extremely dense breast tissue. I sense that some ObGyns may rely heavily on the radiologist to suggest supplemental screening, but patients actually look to ObGyns as their providers to have this knowledge and give them direction.
Since I practice at a large academic medical center, I have the opportunity to refer patients to our Breast Cancer Genetics Program because I may be limited on time for counseling in the office and do not want to miss salient details. With all of the information I have ascertained about the patient, I am able to determine and encourage appropriate screening and assure insurance coverage for adjunctive breast MRI when appropriate.
Continue to: Consider how you order patients’ screening to reduce barriers and cost...
Consider how you order patients’ screening to reduce barriers and cost
4. How would you suggest reducing barriers when referring patients for supplemental screening, such as MRI for high-risk women or ultrasound for those with dense breasts? Would you prefer it if such screening could be performed without additional script/referral? How does insurance coverage factor in?
Dr. Etkin-Kramer: I would love for a screening mammogram with possible ultrasound, on one script, to be the norm. One of the centers that I work with accepts a script written this way. Further, when a patient receives screening at a freestanding facility as opposed to a hospital, the fee for the supplemental screening may be lower because they do not add on a facility fee.
Dr. Albright: We have an order in our electronic health record that allows for screening mammography but adds on diagnostic mammography/bilateral ultrasonography, if indicated by imaging. I am mostly ordering that option now for all of my screening patients; rarely have I had issues with insurance accepting that script. As for when ordering an MRI, I always try to ensure that I have done the patient’s personal risk assessment and included that lifetime breast cancer risk on the order. If the risk is 20% or higher, I typically do not have any insurance coverage issues. If I am ordering MRI as supplemental screening, I typically order the “Fast MRI” protocol that our center offers. This order incurs a $299 out-of-pocket cost for the patient. Any patient with heterogeneously or extremely dense breasts on mammography should have this option, but it requires patient education, discussion with the provider, and an additional cost. I definitely think that insurers need to consider covering supplemental screening, since breast density is reportable in a majority of the US states and will soon be the national standard.
Pearls for guiding patients
5. How do you discuss breast density and the need for supplemental screening with your patients?
Dr. Etkin-Kramer: I strongly feel that my patients need to know when a screening test has limited ability to do its job. This is the case with dense breasts. Visuals help; when discussing breast density, I like the images supplied by DenseBreast-info.org (FIGURE). I explain the two implications of dense tissue:
- First, dense tissue makes it harder to visualize cancers in the breast—the denser the breasts, the less likely the radiologist can pick up a cancer, so mammographic sensitivity for extremely dense breasts can be as low as 25% to 50%.
- Second, high breast density adds to the risk of developing breast cancer. I explain that supplemental screening will pick up additional cancers in women with dense breasts. For example, breast ultrasound will pick up about 2-3/1000 additional breast cancers per year and MRI or molecular breast imaging (MBI) will pick up much more, perhaps 10/1000.

MRI is more invasive than an ultrasound and uses gadolinium, and MBI has more radiation. Supplemental screening is not endorsed by ACOG’s most recent Committee Opinion from 2017; 4 however, patients may choose to have it done. This is where shared-decision making is important.
I strongly recommend that all women’s health care providers complete the CME course on the DenseBreast-info.org website. “ Breast Density: Why It Matters ” is a certified educational program for referring physicians that helps health care professionals learn about breast density, its associated risks, and how best to guide patients regarding breast cancer screening.
Continue to: Dr. Albright...
Dr. Albright: When I discuss breast density, I make sure that patients understand that their mammogram determines the density of their breast tissue. I review that in the higher density categories (heterogeneously dense or extremely dense), there is a higher risk of missing cancer, and that these categories are also associated with a higher risk of breast cancer. I also discuss the potential need for supplemental screening, for which my institution primarily offers Fast MRI. However, we can offer breast ultrasonography instead as an option, especially for those concerned about gadolinium exposure. Our center offers either of these supplemental screenings at a cost of $299. I also review the lack of coverage for supplemental screening by some insurance carriers, as both providers and patients may need to advocate for insurer coverage of adjunct studies.
Educational resources
6. What reference materials, illustrations, or other tools do you use to educate your patients?
Dr. Etkin-Kramer: I frequently use handouts printed from the DenseBreast-info.org website, and there is now a brand new patient fact sheet that I have just started using. I also have an example of breast density categories from fatty replaced to extremely dense on my computer, and I am putting it on a new smart board.
Dr. Albright: The extensive resources available at DenseBreast-info.org can improve both patient and provider knowledge of these important issues, so I suggest patients visit that website, and I use many of the images and visuals to help explain breast density. I even use the materials from the website for educating my resident trainees on breast health and screening. ●
Nearly 16,000 children (up to age 19 years) face cancer-related treatment every year.1 For girls and young women, undergoing chest radiotherapy puts them at higher risk for secondary breast cancer. In fact, they have a 30% chance of developing such cancer by age 50—a risk that is similar to women with a BRCA1 mutation.2 Therefore, current recommendations for breast cancer screening among those who have undergone childhood chest radiation (≥20 Gy) are to begin annual mammography, with adjunct magnetic resonance imaging (MRI), at age 25 years (or 8 years after chest radiotherapy).3
To determine the benefits and risks of these recommendations, as well as of similar strategies, Yeh and colleagues performed simulation modeling using data from the Childhood Cancer Survivor Study and two CISNET (Cancer Intervention and Surveillance Modeling Network) models.4 For their study they targeted a cohort of female childhood cancer survivors having undergone chest radiotherapy and evaluated breast cancer screening with the following strategies:
- mammography plus MRI, starting at ages 25, 30, or 35 years and continuing to age 74
- MRI alone, starting at ages 25, 30, or 35 years and continuing to age 74.
They found that both strategies reduced the risk of breast cancer in the targeted cohort but that screening beginning at the earliest ages prevented most deaths. No screening at all was associated with a 10% to 11% lifetime risk of breast cancer, but mammography plus MRI beginning at age 25 reduced that risk by 56% to 71% depending on the model. Screening with MRI alone reduced mortality risk by 56% to 62%. When considering cost per quality adjusted life-year gained, the researchers found that screening beginning at age 30 to be the most cost-effective.4
Yeh and colleagues addressed concerns with mammography and radiation. Although they said the associated amount of radiation exposure is small, the use of mammography in women younger than age 30 is controversial—and not recommended by the American Cancer Society or the National Comprehensive Cancer Network.5,6
Bottom line. Yeh and colleagues conclude that MRI screening, with or without mammography, beginning between the ages of 25 and 30 should be emphasized in screening guidelines. They note the importance of insurance coverage for MRI in those at risk for breast cancer due to childhood radiation exposure.4
References
- National Cancer Institute. How common is cancer in children? https://www.cancer.gov/types/childhood-cancers/child-adolescentcancers-fact-sheet#how-common-is-cancer-in-children. Accessed September 25, 2020.
- Moskowitz CS, Chou JF, Wolden SL, et al. Breast cancer after chest radiation therapy for childhood cancer. J Clin Oncol. 2014;32:2217- 2223.
- Children’s Oncology Group. Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers. http:// www.survivorshipguidelines.org/pdf/2018/COG_LTFU_Guidelines_v5.pdf. Accessed September 25, 2020.
- Yeh JM, Lowry KP, Schechter CB, et al. Clinical benefits, harms, and cost-effectiveness of breast cancer screening for survivors of childhood cancer treated with chest radiation. Ann Intern Med. 2020;173:331-341.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Breast cancer screening and diagnosis version 1.2019. https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed September 25, 2020.
- Bharucha PP, Chiu KE, Francois FM, et al. Genetic testing and screening recommendations for patients with hereditary breast cancer. RadioGraphics. 2020;40:913-936.
- Freedman AN, Yu B, Gail MH, et al. Benefit/risk assessment for breast cancer chemoprevention with raloxifene or tamoxifen for women age 50 years or older. J Clin Oncol. 2011;29:2327-2333.
- Pruthi S, Heisey RE, Bevers TB. Chemoprevention for breast cancer. Ann Surg Oncol. 2015;22:3230-3235.
- American College of Obstetricians and Gynecologists. Committee opinion no. 625: management of women with dense breasts diagnosed by mammography [published correction appears in Obstet Gynecol. 2016;127:166]. Obstet Gynecol. 2015;125(3):750-751.
- Bharucha PP, Chiu KE, Francois FM, et al. Genetic testing and screening recommendations for patients with hereditary breast cancer. RadioGraphics. 2020;40:913-936.
- Freedman AN, Yu B, Gail MH, et al. Benefit/risk assessment for breast cancer chemoprevention with raloxifene or tamoxifen for women age 50 years or older. J Clin Oncol. 2011;29:2327-2333.
- Pruthi S, Heisey RE, Bevers TB. Chemoprevention for breast cancer. Ann Surg Oncol. 2015;22:3230-3235.
- American College of Obstetricians and Gynecologists. Committee opinion no. 625: management of women with dense breasts diagnosed by mammography [published correction appears in Obstet Gynecol. 2016;127:166]. Obstet Gynecol. 2015;125(3):750-751.
Atezolizumab strikes out in ovarian cancer
“Despite notable success with the incorporation of bevacizumab and atezolizumab in the treatment of other solid tumors ... the first such study in ovarian cancer did not meet its first primary endpoint” of extending PFS in the intention-to-treat population or in patients positive for programmed death-ligand 1 (PD-L1), said investigator Kathleen Moore, MD, of the University of Oklahoma in Oklahoma City.
Dr. Moore presented this study, IMagyn050/GOG 3015/ENGOT-OV39, at the European Society for Medical Oncology Virtual Congress 2020.
Explaining the negative results
The rationale for this study was good, said discussant Isabelle Ray-Coquard, MD, PhD, of the University Claude Bernard Lyon I in Villeurbanne, France, who was an investigator on the study but not an author on the meeting report.
Unfortunately, the study’s results were ultimately negative. A prior phase 3 trial of an immune checkpoint inhibitor in ovarian cancer also had negative results. In the JAVELIN OVARIAN 100 trial, adding avelumab to chemotherapy did not improve PFS, and the trial was stopped early for futility.
Dr. Ray-Coquard posed the question of whether checkpoint inhibitors are “dead” for epithelial ovarian cancer but said the answer isn’t clear.
There might be something unique about the tumor microenvironment that shields ovarian cancer from the immune system, or perhaps the PD-L1 pathway is the wrong target for immunotherapy.
It might also be that the first-line setting is the wrong place for checkpoint inhibitors, and they might work better in the second line, Dr. Ray-Coquard said.
Another possibility is that the delayed effect of immunotherapy means that overall survival – which isn’t yet mature for IMagyn050 – might be a better primary outcome than PFS.
Patient and treatment details
IMagyn050 enrolled 1,301 patients with newly diagnosed, stage III/IV epithelial ovarian, primary peritoneal, or fallopian tube cancer.
Patients were randomized to atezolizumab (n = 651) or placebo (n = 650) in combination with bevacizumab, paclitaxel, and carboplatin every 3 weeks for six cycles. For cycles 7-22, patients received bevacizumab with placebo or atezolizumab.
Most patients had ovarian cancer (73% in the placebo arm and 75% in the atezolizumab arm), followed by fallopian tube cancer (17% and 15%, respectively) and primary peritoneal cancer (10% and 9%, respectively).
In both arms, 31% of patients had stage IV disease, and 60% were PD-L1 positive (at least 1% of tumor cells staining positive).
Treatment was given in the neoadjuvant setting for 25% of patients in both arms and after primary cytoreductive surgery for the remaining patients.
Efficacy and safety
In the intention-to-treat population, the median PFS was 19.5 months with atezolizumab and 18.4 months in the placebo arm. In PD-L1–positive patients, the median PFS was 20.8 months and 18.5 months, respectively.
The differences in PFS were not statistically significant or clinically meaningful, Dr. Ray-Coquard said.
She noted that results were not stratified by BRCA status, which has been linked to PFS in ovarian cancer, and a potential imbalance between the groups might have contributed to the negative results.
Overall survival data won’t be mature until 2023, but, in the first interim analysis, “there was no apparent difference in the curves,” Dr. Moore said.
In the intention-to-treat population, the median overall survival was not reached in either treatment arm. In the PD-L1–positive population, the median overall survival was 31.2 months in the placebo arm and was not reached in the atezolizumab arm.
Adverse events were consistent with the known safety profiles of atezolizumab and the other drugs, Dr. Moore said. There were more serious events and more events leading to discontinuation with atezolizumab.
Adverse events more common with atezolizumab included febrile neutropenia, pyrexia, thyroid abnormalities, and rash, including severe cutaneous reactions. Colitis, pancreatitis, and infusion-related reactions were relatively infrequent but more common with atezolizumab, Dr. Moore said.
What the future holds
Dr. Ray-Coquard said there are signals in IMagyn050 that warrant follow-up, including a trend toward improved PFS among women who had high-grade nonserous clear cell histology. In this group, the median PFS was 12.3 months in the placebo arm and 13.6 months in the atezolizumab arm (hazard ratio, 0.64).
Additionally, there was a significant PFS improvement in women with at least 5% of their tumor cells staining positive for PD-L1. The median PFS was 20.2 months in the placebo arm but was not reached in the atezolizumab arm (HR, 0.64; P = .0278).
Dr. Ray-Coquard said we need to know who these PD-L1–high patients are in terms of BRCA status, histology, and residual disease.
She went on to say that companies haven’t given up on checkpoint inhibitors for ovarian cancer. Phase 3 trials are testing the atezolizumab/bevacizumab/chemotherapy combination for late relapsed disease, atezolizumab with niraparib and chemotherapy for recurrent ovarian cancer, and nivolumab with rucaparib following response to chemotherapy.
The IMagyn050 study was funded by F. Hoffmann-La Roche, the company developing atezolizumab. Dr. Moore and Dr. Ray-Coquard disclosed relationships with Roche and many other companies.
SOURCE: Moore K et al. ESMO 2020, Abstract LBA31.
“Despite notable success with the incorporation of bevacizumab and atezolizumab in the treatment of other solid tumors ... the first such study in ovarian cancer did not meet its first primary endpoint” of extending PFS in the intention-to-treat population or in patients positive for programmed death-ligand 1 (PD-L1), said investigator Kathleen Moore, MD, of the University of Oklahoma in Oklahoma City.
Dr. Moore presented this study, IMagyn050/GOG 3015/ENGOT-OV39, at the European Society for Medical Oncology Virtual Congress 2020.
Explaining the negative results
The rationale for this study was good, said discussant Isabelle Ray-Coquard, MD, PhD, of the University Claude Bernard Lyon I in Villeurbanne, France, who was an investigator on the study but not an author on the meeting report.
Unfortunately, the study’s results were ultimately negative. A prior phase 3 trial of an immune checkpoint inhibitor in ovarian cancer also had negative results. In the JAVELIN OVARIAN 100 trial, adding avelumab to chemotherapy did not improve PFS, and the trial was stopped early for futility.
Dr. Ray-Coquard posed the question of whether checkpoint inhibitors are “dead” for epithelial ovarian cancer but said the answer isn’t clear.
There might be something unique about the tumor microenvironment that shields ovarian cancer from the immune system, or perhaps the PD-L1 pathway is the wrong target for immunotherapy.
It might also be that the first-line setting is the wrong place for checkpoint inhibitors, and they might work better in the second line, Dr. Ray-Coquard said.
Another possibility is that the delayed effect of immunotherapy means that overall survival – which isn’t yet mature for IMagyn050 – might be a better primary outcome than PFS.
Patient and treatment details
IMagyn050 enrolled 1,301 patients with newly diagnosed, stage III/IV epithelial ovarian, primary peritoneal, or fallopian tube cancer.
Patients were randomized to atezolizumab (n = 651) or placebo (n = 650) in combination with bevacizumab, paclitaxel, and carboplatin every 3 weeks for six cycles. For cycles 7-22, patients received bevacizumab with placebo or atezolizumab.
Most patients had ovarian cancer (73% in the placebo arm and 75% in the atezolizumab arm), followed by fallopian tube cancer (17% and 15%, respectively) and primary peritoneal cancer (10% and 9%, respectively).
In both arms, 31% of patients had stage IV disease, and 60% were PD-L1 positive (at least 1% of tumor cells staining positive).
Treatment was given in the neoadjuvant setting for 25% of patients in both arms and after primary cytoreductive surgery for the remaining patients.
Efficacy and safety
In the intention-to-treat population, the median PFS was 19.5 months with atezolizumab and 18.4 months in the placebo arm. In PD-L1–positive patients, the median PFS was 20.8 months and 18.5 months, respectively.
The differences in PFS were not statistically significant or clinically meaningful, Dr. Ray-Coquard said.
She noted that results were not stratified by BRCA status, which has been linked to PFS in ovarian cancer, and a potential imbalance between the groups might have contributed to the negative results.
Overall survival data won’t be mature until 2023, but, in the first interim analysis, “there was no apparent difference in the curves,” Dr. Moore said.
In the intention-to-treat population, the median overall survival was not reached in either treatment arm. In the PD-L1–positive population, the median overall survival was 31.2 months in the placebo arm and was not reached in the atezolizumab arm.
Adverse events were consistent with the known safety profiles of atezolizumab and the other drugs, Dr. Moore said. There were more serious events and more events leading to discontinuation with atezolizumab.
Adverse events more common with atezolizumab included febrile neutropenia, pyrexia, thyroid abnormalities, and rash, including severe cutaneous reactions. Colitis, pancreatitis, and infusion-related reactions were relatively infrequent but more common with atezolizumab, Dr. Moore said.
What the future holds
Dr. Ray-Coquard said there are signals in IMagyn050 that warrant follow-up, including a trend toward improved PFS among women who had high-grade nonserous clear cell histology. In this group, the median PFS was 12.3 months in the placebo arm and 13.6 months in the atezolizumab arm (hazard ratio, 0.64).
Additionally, there was a significant PFS improvement in women with at least 5% of their tumor cells staining positive for PD-L1. The median PFS was 20.2 months in the placebo arm but was not reached in the atezolizumab arm (HR, 0.64; P = .0278).
Dr. Ray-Coquard said we need to know who these PD-L1–high patients are in terms of BRCA status, histology, and residual disease.
She went on to say that companies haven’t given up on checkpoint inhibitors for ovarian cancer. Phase 3 trials are testing the atezolizumab/bevacizumab/chemotherapy combination for late relapsed disease, atezolizumab with niraparib and chemotherapy for recurrent ovarian cancer, and nivolumab with rucaparib following response to chemotherapy.
The IMagyn050 study was funded by F. Hoffmann-La Roche, the company developing atezolizumab. Dr. Moore and Dr. Ray-Coquard disclosed relationships with Roche and many other companies.
SOURCE: Moore K et al. ESMO 2020, Abstract LBA31.
“Despite notable success with the incorporation of bevacizumab and atezolizumab in the treatment of other solid tumors ... the first such study in ovarian cancer did not meet its first primary endpoint” of extending PFS in the intention-to-treat population or in patients positive for programmed death-ligand 1 (PD-L1), said investigator Kathleen Moore, MD, of the University of Oklahoma in Oklahoma City.
Dr. Moore presented this study, IMagyn050/GOG 3015/ENGOT-OV39, at the European Society for Medical Oncology Virtual Congress 2020.
Explaining the negative results
The rationale for this study was good, said discussant Isabelle Ray-Coquard, MD, PhD, of the University Claude Bernard Lyon I in Villeurbanne, France, who was an investigator on the study but not an author on the meeting report.
Unfortunately, the study’s results were ultimately negative. A prior phase 3 trial of an immune checkpoint inhibitor in ovarian cancer also had negative results. In the JAVELIN OVARIAN 100 trial, adding avelumab to chemotherapy did not improve PFS, and the trial was stopped early for futility.
Dr. Ray-Coquard posed the question of whether checkpoint inhibitors are “dead” for epithelial ovarian cancer but said the answer isn’t clear.
There might be something unique about the tumor microenvironment that shields ovarian cancer from the immune system, or perhaps the PD-L1 pathway is the wrong target for immunotherapy.
It might also be that the first-line setting is the wrong place for checkpoint inhibitors, and they might work better in the second line, Dr. Ray-Coquard said.
Another possibility is that the delayed effect of immunotherapy means that overall survival – which isn’t yet mature for IMagyn050 – might be a better primary outcome than PFS.
Patient and treatment details
IMagyn050 enrolled 1,301 patients with newly diagnosed, stage III/IV epithelial ovarian, primary peritoneal, or fallopian tube cancer.
Patients were randomized to atezolizumab (n = 651) or placebo (n = 650) in combination with bevacizumab, paclitaxel, and carboplatin every 3 weeks for six cycles. For cycles 7-22, patients received bevacizumab with placebo or atezolizumab.
Most patients had ovarian cancer (73% in the placebo arm and 75% in the atezolizumab arm), followed by fallopian tube cancer (17% and 15%, respectively) and primary peritoneal cancer (10% and 9%, respectively).
In both arms, 31% of patients had stage IV disease, and 60% were PD-L1 positive (at least 1% of tumor cells staining positive).
Treatment was given in the neoadjuvant setting for 25% of patients in both arms and after primary cytoreductive surgery for the remaining patients.
Efficacy and safety
In the intention-to-treat population, the median PFS was 19.5 months with atezolizumab and 18.4 months in the placebo arm. In PD-L1–positive patients, the median PFS was 20.8 months and 18.5 months, respectively.
The differences in PFS were not statistically significant or clinically meaningful, Dr. Ray-Coquard said.
She noted that results were not stratified by BRCA status, which has been linked to PFS in ovarian cancer, and a potential imbalance between the groups might have contributed to the negative results.
Overall survival data won’t be mature until 2023, but, in the first interim analysis, “there was no apparent difference in the curves,” Dr. Moore said.
In the intention-to-treat population, the median overall survival was not reached in either treatment arm. In the PD-L1–positive population, the median overall survival was 31.2 months in the placebo arm and was not reached in the atezolizumab arm.
Adverse events were consistent with the known safety profiles of atezolizumab and the other drugs, Dr. Moore said. There were more serious events and more events leading to discontinuation with atezolizumab.
Adverse events more common with atezolizumab included febrile neutropenia, pyrexia, thyroid abnormalities, and rash, including severe cutaneous reactions. Colitis, pancreatitis, and infusion-related reactions were relatively infrequent but more common with atezolizumab, Dr. Moore said.
What the future holds
Dr. Ray-Coquard said there are signals in IMagyn050 that warrant follow-up, including a trend toward improved PFS among women who had high-grade nonserous clear cell histology. In this group, the median PFS was 12.3 months in the placebo arm and 13.6 months in the atezolizumab arm (hazard ratio, 0.64).
Additionally, there was a significant PFS improvement in women with at least 5% of their tumor cells staining positive for PD-L1. The median PFS was 20.2 months in the placebo arm but was not reached in the atezolizumab arm (HR, 0.64; P = .0278).
Dr. Ray-Coquard said we need to know who these PD-L1–high patients are in terms of BRCA status, histology, and residual disease.
She went on to say that companies haven’t given up on checkpoint inhibitors for ovarian cancer. Phase 3 trials are testing the atezolizumab/bevacizumab/chemotherapy combination for late relapsed disease, atezolizumab with niraparib and chemotherapy for recurrent ovarian cancer, and nivolumab with rucaparib following response to chemotherapy.
The IMagyn050 study was funded by F. Hoffmann-La Roche, the company developing atezolizumab. Dr. Moore and Dr. Ray-Coquard disclosed relationships with Roche and many other companies.
SOURCE: Moore K et al. ESMO 2020, Abstract LBA31.
FROM ESMO 2020
HPV vaccine shown to substantially reduce cervical cancer risk
It’s been shown that the vaccine (Gardasil) helps prevent genital warts and high-grade cervical lesions, but until now, data on the ability of the vaccine to prevent cervical cancer, although widely assumed, had been lacking.
“Our results extend [the] knowledge base by showing that quadrivalent HPV vaccination is also associated with a substantially reduced risk of invasive cervical cancer, which is the ultimate intent of HPV vaccination programs,” said investigators led by Jiayao Lei, PhD, a researcher in the department of medical epidemiology and biostatistics at the Karolinska Institute, Stockholm.
The study was published online Oct. 1 in the New England Journal of Medicine.
“This work provides evidence of actual cancer prevention,” commented Diane Harper, MD, an HPV expert and professor in the departments of family medicine and obstetrics & gynecology at the University of Michigan, Ann Arbor. She was the principal investigator on the original Gardasil trial.
This study “shows that the quadrivalent HPV vaccine provides prevention from the sexually transmitted HPV infection that actually reduces the incidence of cervical cancer in young women up to 30 years of age,” she said when approached for comment.
However, she also added a note of caution. These new results show “that vaccinated women still develop cervical cancer, but at a slower rate. This makes the connection between early-age vaccination and continued adult life screening incredibly important,” Dr. Harper said in an interview
Cervical cancer was diagnosed in 19 of the 527,871 women (0.004%) who had received at least one dose of the vaccine versus 538 among the 1,145,112 women (0.05%) who had not.
The cumulative incidence was 47 cases per 100,000 vaccinated women and 94 cases per 100,000 unvaccinated women. The cervical cancer incidence rate ratio for the comparison of vaccinated versus unvaccinated women was 0.37 (95% confidence interval, 0.21-0.57).
The risk reduction was even greater among women who had been vaccinated before the age of 17, with a cumulative incidence of 4 versus 54 cases per 100,000 for women vaccinated after age 17. The incidence rate ratio was 0.12 (95% CI, 0.00-0.34) for women who had been vaccinated before age 17 versus 0.47 (95% CI, 0.27-0.75) among those vaccinated from age 17 to 30 years.
Overall, “the risk of cervical cancer among participants who had initiated vaccination before the age of 17 years was 88% lower than among those who had never been vaccinated,” the investigators noted.
These results “support the recommendation to administer quadrivalent HPV vaccine before exposure to HPV infection to achieve the most substantial benefit,” the investigators wrote.
Details of the Swedish review
For their review, Dr. Lei and colleagues used several Swedish demographic and health registries to connect vaccination status to incident cervical cancers, using the personal identification numbers Sweden issues to residents.
Participants were followed starting either on their 10th birthday or on Jan. 1, 2006, whichever came later. They were followed until, among other things, diagnosis of invasive cervical cancer; their 31st birthday; or until Dec. 31, 2017, whichever came first.
The quadrivalent HPV vaccine, approved in Sweden in 2006, was used almost exclusively during the study period. Participants were considered vaccinated if they had received only one shot, but the investigators set out to analyze a relationship between the incidence of invasive cervical cancer and the number of shots given.
Among other things, the team controlled for age at follow-up, calendar year, county of residence, maternal disease history, and parental characteristics, including education and household income.
The investigators commented that it’s possible that HPV-vaccinated women could have been generally healthier than unvaccinated women and so would have been at lower risk for cervical cancer.
“Confounding by lifestyle and health factors in the women (such as smoking status, sexual activity, oral contraceptive use, and obesity) cannot be excluded; these factors are known to be associated with a risk of cervical cancer,” the investigators wrote.
HPV is also associated with other types of cancer, including anal and oropharyngeal cancers. But these cancers develop over a longer period than cervical cancer.
Dr. Harper noted that the “probability of HPV 16 cancer by time since infection peaks at 40 years after infection for anal cancers and nearly 50 years after infection for oropharyngeal cancers. This means that registries, such as in Sweden, for the next 40 years will record the evidence to say whether HPV vaccination lasts long enough to prevent [these] other HPV 16–associated cancers occurring at a much later time in life.”
The work was funded by the Swedish Foundation for Strategic Research, the Swedish Cancer Society, and the Swedish Research Council and by the China Scholarship Council. Dr. Lei and two other investigators reported HPV vaccine research funding from Merck, the maker of Gardasil. Harper disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
It’s been shown that the vaccine (Gardasil) helps prevent genital warts and high-grade cervical lesions, but until now, data on the ability of the vaccine to prevent cervical cancer, although widely assumed, had been lacking.
“Our results extend [the] knowledge base by showing that quadrivalent HPV vaccination is also associated with a substantially reduced risk of invasive cervical cancer, which is the ultimate intent of HPV vaccination programs,” said investigators led by Jiayao Lei, PhD, a researcher in the department of medical epidemiology and biostatistics at the Karolinska Institute, Stockholm.
The study was published online Oct. 1 in the New England Journal of Medicine.
“This work provides evidence of actual cancer prevention,” commented Diane Harper, MD, an HPV expert and professor in the departments of family medicine and obstetrics & gynecology at the University of Michigan, Ann Arbor. She was the principal investigator on the original Gardasil trial.
This study “shows that the quadrivalent HPV vaccine provides prevention from the sexually transmitted HPV infection that actually reduces the incidence of cervical cancer in young women up to 30 years of age,” she said when approached for comment.
However, she also added a note of caution. These new results show “that vaccinated women still develop cervical cancer, but at a slower rate. This makes the connection between early-age vaccination and continued adult life screening incredibly important,” Dr. Harper said in an interview
Cervical cancer was diagnosed in 19 of the 527,871 women (0.004%) who had received at least one dose of the vaccine versus 538 among the 1,145,112 women (0.05%) who had not.
The cumulative incidence was 47 cases per 100,000 vaccinated women and 94 cases per 100,000 unvaccinated women. The cervical cancer incidence rate ratio for the comparison of vaccinated versus unvaccinated women was 0.37 (95% confidence interval, 0.21-0.57).
The risk reduction was even greater among women who had been vaccinated before the age of 17, with a cumulative incidence of 4 versus 54 cases per 100,000 for women vaccinated after age 17. The incidence rate ratio was 0.12 (95% CI, 0.00-0.34) for women who had been vaccinated before age 17 versus 0.47 (95% CI, 0.27-0.75) among those vaccinated from age 17 to 30 years.
Overall, “the risk of cervical cancer among participants who had initiated vaccination before the age of 17 years was 88% lower than among those who had never been vaccinated,” the investigators noted.
These results “support the recommendation to administer quadrivalent HPV vaccine before exposure to HPV infection to achieve the most substantial benefit,” the investigators wrote.
Details of the Swedish review
For their review, Dr. Lei and colleagues used several Swedish demographic and health registries to connect vaccination status to incident cervical cancers, using the personal identification numbers Sweden issues to residents.
Participants were followed starting either on their 10th birthday or on Jan. 1, 2006, whichever came later. They were followed until, among other things, diagnosis of invasive cervical cancer; their 31st birthday; or until Dec. 31, 2017, whichever came first.
The quadrivalent HPV vaccine, approved in Sweden in 2006, was used almost exclusively during the study period. Participants were considered vaccinated if they had received only one shot, but the investigators set out to analyze a relationship between the incidence of invasive cervical cancer and the number of shots given.
Among other things, the team controlled for age at follow-up, calendar year, county of residence, maternal disease history, and parental characteristics, including education and household income.
The investigators commented that it’s possible that HPV-vaccinated women could have been generally healthier than unvaccinated women and so would have been at lower risk for cervical cancer.
“Confounding by lifestyle and health factors in the women (such as smoking status, sexual activity, oral contraceptive use, and obesity) cannot be excluded; these factors are known to be associated with a risk of cervical cancer,” the investigators wrote.
HPV is also associated with other types of cancer, including anal and oropharyngeal cancers. But these cancers develop over a longer period than cervical cancer.
Dr. Harper noted that the “probability of HPV 16 cancer by time since infection peaks at 40 years after infection for anal cancers and nearly 50 years after infection for oropharyngeal cancers. This means that registries, such as in Sweden, for the next 40 years will record the evidence to say whether HPV vaccination lasts long enough to prevent [these] other HPV 16–associated cancers occurring at a much later time in life.”
The work was funded by the Swedish Foundation for Strategic Research, the Swedish Cancer Society, and the Swedish Research Council and by the China Scholarship Council. Dr. Lei and two other investigators reported HPV vaccine research funding from Merck, the maker of Gardasil. Harper disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
It’s been shown that the vaccine (Gardasil) helps prevent genital warts and high-grade cervical lesions, but until now, data on the ability of the vaccine to prevent cervical cancer, although widely assumed, had been lacking.
“Our results extend [the] knowledge base by showing that quadrivalent HPV vaccination is also associated with a substantially reduced risk of invasive cervical cancer, which is the ultimate intent of HPV vaccination programs,” said investigators led by Jiayao Lei, PhD, a researcher in the department of medical epidemiology and biostatistics at the Karolinska Institute, Stockholm.
The study was published online Oct. 1 in the New England Journal of Medicine.
“This work provides evidence of actual cancer prevention,” commented Diane Harper, MD, an HPV expert and professor in the departments of family medicine and obstetrics & gynecology at the University of Michigan, Ann Arbor. She was the principal investigator on the original Gardasil trial.
This study “shows that the quadrivalent HPV vaccine provides prevention from the sexually transmitted HPV infection that actually reduces the incidence of cervical cancer in young women up to 30 years of age,” she said when approached for comment.
However, she also added a note of caution. These new results show “that vaccinated women still develop cervical cancer, but at a slower rate. This makes the connection between early-age vaccination and continued adult life screening incredibly important,” Dr. Harper said in an interview
Cervical cancer was diagnosed in 19 of the 527,871 women (0.004%) who had received at least one dose of the vaccine versus 538 among the 1,145,112 women (0.05%) who had not.
The cumulative incidence was 47 cases per 100,000 vaccinated women and 94 cases per 100,000 unvaccinated women. The cervical cancer incidence rate ratio for the comparison of vaccinated versus unvaccinated women was 0.37 (95% confidence interval, 0.21-0.57).
The risk reduction was even greater among women who had been vaccinated before the age of 17, with a cumulative incidence of 4 versus 54 cases per 100,000 for women vaccinated after age 17. The incidence rate ratio was 0.12 (95% CI, 0.00-0.34) for women who had been vaccinated before age 17 versus 0.47 (95% CI, 0.27-0.75) among those vaccinated from age 17 to 30 years.
Overall, “the risk of cervical cancer among participants who had initiated vaccination before the age of 17 years was 88% lower than among those who had never been vaccinated,” the investigators noted.
These results “support the recommendation to administer quadrivalent HPV vaccine before exposure to HPV infection to achieve the most substantial benefit,” the investigators wrote.
Details of the Swedish review
For their review, Dr. Lei and colleagues used several Swedish demographic and health registries to connect vaccination status to incident cervical cancers, using the personal identification numbers Sweden issues to residents.
Participants were followed starting either on their 10th birthday or on Jan. 1, 2006, whichever came later. They were followed until, among other things, diagnosis of invasive cervical cancer; their 31st birthday; or until Dec. 31, 2017, whichever came first.
The quadrivalent HPV vaccine, approved in Sweden in 2006, was used almost exclusively during the study period. Participants were considered vaccinated if they had received only one shot, but the investigators set out to analyze a relationship between the incidence of invasive cervical cancer and the number of shots given.
Among other things, the team controlled for age at follow-up, calendar year, county of residence, maternal disease history, and parental characteristics, including education and household income.
The investigators commented that it’s possible that HPV-vaccinated women could have been generally healthier than unvaccinated women and so would have been at lower risk for cervical cancer.
“Confounding by lifestyle and health factors in the women (such as smoking status, sexual activity, oral contraceptive use, and obesity) cannot be excluded; these factors are known to be associated with a risk of cervical cancer,” the investigators wrote.
HPV is also associated with other types of cancer, including anal and oropharyngeal cancers. But these cancers develop over a longer period than cervical cancer.
Dr. Harper noted that the “probability of HPV 16 cancer by time since infection peaks at 40 years after infection for anal cancers and nearly 50 years after infection for oropharyngeal cancers. This means that registries, such as in Sweden, for the next 40 years will record the evidence to say whether HPV vaccination lasts long enough to prevent [these] other HPV 16–associated cancers occurring at a much later time in life.”
The work was funded by the Swedish Foundation for Strategic Research, the Swedish Cancer Society, and the Swedish Research Council and by the China Scholarship Council. Dr. Lei and two other investigators reported HPV vaccine research funding from Merck, the maker of Gardasil. Harper disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The scope of under- and overtreatment in older adults with cancer
Because of physiological changes with aging and differences in cancer biology, caring for older adults (OAs) with cancer requires careful assessment and planning.
Clark Dumontier, MD, of Brigham and Women’s Hospital in Boston, and colleagues sought to define the meaning of the terms “undertreatment” and “overtreatment” for OAs with cancer in a scoping literature review published in the Journal of Clinical Oncology.
Though OAs are typically defined as adults aged 65 years and older, in this review, the authors defined OAs as patients aged 60 years and older.
The authors theorized that a scoping review of papers about this patient population could provide clues about limitations in the oncology literature and guidance about patient management and future research. Despite comprising the majority of cancer patients, OAs are underrepresented in clinical trials.
About scoping reviews
Scoping reviews are used to identify existing evidence in a field, clarify concepts or definitions in the literature, survey how research on a topic is conducted, and identify knowledge gaps. In addition, scoping reviews summarize available evidence without answering a discrete research question.
Industry standards for scoping reviews have been established by the Johanna Briggs Institute and Preferred Reporting Items for Systematic Reviews and Meta-analyses extension for scoping reviews. According to these standards, scoping reviews should:
- Establish eligibility criteria with a rationale for each criterion clearly explained
- Search multiple databases in multiple languages
- Include “gray literature,” defined as studies that are unpublished or difficult to locate
- Have several independent reviewers screen titles and abstracts
- Ask multiple independent reviewers to review full text articles
- Present results with charts or diagrams that align with the review’s objective
- Graphically depict the decision process for including/excluding sources
- Identify implications for further research.
In their review, Dr. DuMontier and colleagues fulfilled many of the aforementioned criteria. The team searched three English-language databases for titles and abstracts that included the terms undertreatment and/or overtreatment, and were related to OAs with cancer, inclusive of all types of articles, cancer types, and treatments.
Definitions of undertreatment and overtreatment were extracted, and categories underlying these definitions were derived. Within a random subset of articles, two coauthors independently determined final categories of definitions and independently assigned those categories.
Findings and implications
To define OA, Dr. DuMontier and colleagues used a cutoff of 60 years or older. Articles mentioning undertreatment (n = 236), overtreatment (n = 71), or both (n = 51) met criteria for inclusion (n = 256), but only 14 articles (5.5%) explicitly provided formal definitions.
For most of the reviewed articles, the authors judged definitions from the surrounding context. In a random subset of 50 articles, there was a high level of agreement (87.1%; κ = 0.81) between two coauthors in independently assigning categories of definitions.
Undertreatment was applied to therapy that was less than recommended (148 articles; 62.7%) or less than recommended with worse outcomes (88 articles; 37.3%).
Overtreatment most commonly denoted intensive treatment of an OA in whom harms outweighed the benefits of treatment (38 articles; 53.5%) or intensive treatment of a cancer not expected to affect the OA during the patient’s remaining life (33 articles; 46.5%).
Overall, the authors found that undertreatment and overtreatment of OAs with cancer are imprecisely defined concepts. Formal geriatric assessment was recommended in just over half of articles, and only 26.2% recommended formal assessments of age-related vulnerabilities for management. The authors proposed definitions that accounted for both oncologic factors and geriatric domains.
Care of individual patients and clinical research
National Comprehensive Cancer Network (NCCN) guidelines for OAs with cancer recommend initial consideration of overall life expectancy. If a patient is a candidate for cancer treatment on that basis, the next recommended assessment is that of the patient’s capacity to understand the relevant information, appreciate the underlying values and overall medical situation, reason through decisions, and communicate a choice that is consistent with the patient’s articulated goals.
In the pretreatment evaluation of OAs in whom there are no concerns about tolerance to antineoplastic therapy, NCCN guidelines suggest geriatric screening with standardized tools and, if abnormal, comprehensive geriatric screening. The guidelines recommend considering alternative treatment options if nonmodifiable abnormalities are identified.
Referral to a geriatric clinical specialist, use of the Cancer and Aging Research Group’s Chemo Toxicity Calculator, and calculation of Chemotherapy Risk Assessment Scale for High-Age Patients score are specifically suggested if high-risk procedures (such as chemotherapy, radiation, or complex surgery, which most oncologists would consider to be “another day in the office”) are contemplated.
The American Society of Clinical Oncology (ASCO) guidelines for geriatric oncology are similarly detailed and endorse similar evaluations and management.
Employing disease-centric and geriatric domains
Dr. DuMontier and colleagues noted that, for OAs with comorbidity or psychosocial challenges, surrogate survival endpoints are unrelated to quality of life (QOL) outcomes. Nonetheless, QOL is valued by OAs at least as much as survival improvement.
Through no fault of their own, the authors’ conclusion that undertreatment and overtreatment are imperfectly defined concepts has a certain neutrality to it. However, the terms undertreatment and overtreatment are commonly used to signify that inappropriate treatment decisions were made. Therefore, the terms are inherently negative and pejorative.
As with most emotionally charged issues in oncology, it is ideal for professionals in our field to take charge when deficiencies exist. ASCO, NCCN, and the authors of this scoping review have provided a conceptual basis for doing so.
An integrated oncologist-geriatrician approach was shown to be effective in the randomized INTEGERATE trial, showing improved QOL, reduced hospital admissions, and reduced early treatment discontinuation from adverse events (ASCO 2020, Abstract 12011).
Therefore, those clinicians who have not formally, systematically, and routinely supplemented the traditional disease-centric endpoints with patient-centered criteria need to do so.
Similarly, a retrospective study published in JAMA Network Open demonstrated that geriatric and surgical comanagement of OAs with cancer was associated with significantly lower 90-day postoperative mortality and receipt of more supportive care services (physical therapy, occupational therapy, speech and swallow rehabilitation, and nutrition services), in comparison with management from the surgical service only.
These clinical and administrative changes will not only enhance patient management but also facilitate the clinical trials required to clarify optimal treatment intensity. As that occurs, we will be able to apply as much precision to the care of OAs with cancer as we do in other areas of cancer treatment.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
SOURCE: Dumontier C et al. J Clin Oncol. 2020 Aug 1;38(22):2558-2569.
Because of physiological changes with aging and differences in cancer biology, caring for older adults (OAs) with cancer requires careful assessment and planning.
Clark Dumontier, MD, of Brigham and Women’s Hospital in Boston, and colleagues sought to define the meaning of the terms “undertreatment” and “overtreatment” for OAs with cancer in a scoping literature review published in the Journal of Clinical Oncology.
Though OAs are typically defined as adults aged 65 years and older, in this review, the authors defined OAs as patients aged 60 years and older.
The authors theorized that a scoping review of papers about this patient population could provide clues about limitations in the oncology literature and guidance about patient management and future research. Despite comprising the majority of cancer patients, OAs are underrepresented in clinical trials.
About scoping reviews
Scoping reviews are used to identify existing evidence in a field, clarify concepts or definitions in the literature, survey how research on a topic is conducted, and identify knowledge gaps. In addition, scoping reviews summarize available evidence without answering a discrete research question.
Industry standards for scoping reviews have been established by the Johanna Briggs Institute and Preferred Reporting Items for Systematic Reviews and Meta-analyses extension for scoping reviews. According to these standards, scoping reviews should:
- Establish eligibility criteria with a rationale for each criterion clearly explained
- Search multiple databases in multiple languages
- Include “gray literature,” defined as studies that are unpublished or difficult to locate
- Have several independent reviewers screen titles and abstracts
- Ask multiple independent reviewers to review full text articles
- Present results with charts or diagrams that align with the review’s objective
- Graphically depict the decision process for including/excluding sources
- Identify implications for further research.
In their review, Dr. DuMontier and colleagues fulfilled many of the aforementioned criteria. The team searched three English-language databases for titles and abstracts that included the terms undertreatment and/or overtreatment, and were related to OAs with cancer, inclusive of all types of articles, cancer types, and treatments.
Definitions of undertreatment and overtreatment were extracted, and categories underlying these definitions were derived. Within a random subset of articles, two coauthors independently determined final categories of definitions and independently assigned those categories.
Findings and implications
To define OA, Dr. DuMontier and colleagues used a cutoff of 60 years or older. Articles mentioning undertreatment (n = 236), overtreatment (n = 71), or both (n = 51) met criteria for inclusion (n = 256), but only 14 articles (5.5%) explicitly provided formal definitions.
For most of the reviewed articles, the authors judged definitions from the surrounding context. In a random subset of 50 articles, there was a high level of agreement (87.1%; κ = 0.81) between two coauthors in independently assigning categories of definitions.
Undertreatment was applied to therapy that was less than recommended (148 articles; 62.7%) or less than recommended with worse outcomes (88 articles; 37.3%).
Overtreatment most commonly denoted intensive treatment of an OA in whom harms outweighed the benefits of treatment (38 articles; 53.5%) or intensive treatment of a cancer not expected to affect the OA during the patient’s remaining life (33 articles; 46.5%).
Overall, the authors found that undertreatment and overtreatment of OAs with cancer are imprecisely defined concepts. Formal geriatric assessment was recommended in just over half of articles, and only 26.2% recommended formal assessments of age-related vulnerabilities for management. The authors proposed definitions that accounted for both oncologic factors and geriatric domains.
Care of individual patients and clinical research
National Comprehensive Cancer Network (NCCN) guidelines for OAs with cancer recommend initial consideration of overall life expectancy. If a patient is a candidate for cancer treatment on that basis, the next recommended assessment is that of the patient’s capacity to understand the relevant information, appreciate the underlying values and overall medical situation, reason through decisions, and communicate a choice that is consistent with the patient’s articulated goals.
In the pretreatment evaluation of OAs in whom there are no concerns about tolerance to antineoplastic therapy, NCCN guidelines suggest geriatric screening with standardized tools and, if abnormal, comprehensive geriatric screening. The guidelines recommend considering alternative treatment options if nonmodifiable abnormalities are identified.
Referral to a geriatric clinical specialist, use of the Cancer and Aging Research Group’s Chemo Toxicity Calculator, and calculation of Chemotherapy Risk Assessment Scale for High-Age Patients score are specifically suggested if high-risk procedures (such as chemotherapy, radiation, or complex surgery, which most oncologists would consider to be “another day in the office”) are contemplated.
The American Society of Clinical Oncology (ASCO) guidelines for geriatric oncology are similarly detailed and endorse similar evaluations and management.
Employing disease-centric and geriatric domains
Dr. DuMontier and colleagues noted that, for OAs with comorbidity or psychosocial challenges, surrogate survival endpoints are unrelated to quality of life (QOL) outcomes. Nonetheless, QOL is valued by OAs at least as much as survival improvement.
Through no fault of their own, the authors’ conclusion that undertreatment and overtreatment are imperfectly defined concepts has a certain neutrality to it. However, the terms undertreatment and overtreatment are commonly used to signify that inappropriate treatment decisions were made. Therefore, the terms are inherently negative and pejorative.
As with most emotionally charged issues in oncology, it is ideal for professionals in our field to take charge when deficiencies exist. ASCO, NCCN, and the authors of this scoping review have provided a conceptual basis for doing so.
An integrated oncologist-geriatrician approach was shown to be effective in the randomized INTEGERATE trial, showing improved QOL, reduced hospital admissions, and reduced early treatment discontinuation from adverse events (ASCO 2020, Abstract 12011).
Therefore, those clinicians who have not formally, systematically, and routinely supplemented the traditional disease-centric endpoints with patient-centered criteria need to do so.
Similarly, a retrospective study published in JAMA Network Open demonstrated that geriatric and surgical comanagement of OAs with cancer was associated with significantly lower 90-day postoperative mortality and receipt of more supportive care services (physical therapy, occupational therapy, speech and swallow rehabilitation, and nutrition services), in comparison with management from the surgical service only.
These clinical and administrative changes will not only enhance patient management but also facilitate the clinical trials required to clarify optimal treatment intensity. As that occurs, we will be able to apply as much precision to the care of OAs with cancer as we do in other areas of cancer treatment.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
SOURCE: Dumontier C et al. J Clin Oncol. 2020 Aug 1;38(22):2558-2569.
Because of physiological changes with aging and differences in cancer biology, caring for older adults (OAs) with cancer requires careful assessment and planning.
Clark Dumontier, MD, of Brigham and Women’s Hospital in Boston, and colleagues sought to define the meaning of the terms “undertreatment” and “overtreatment” for OAs with cancer in a scoping literature review published in the Journal of Clinical Oncology.
Though OAs are typically defined as adults aged 65 years and older, in this review, the authors defined OAs as patients aged 60 years and older.
The authors theorized that a scoping review of papers about this patient population could provide clues about limitations in the oncology literature and guidance about patient management and future research. Despite comprising the majority of cancer patients, OAs are underrepresented in clinical trials.
About scoping reviews
Scoping reviews are used to identify existing evidence in a field, clarify concepts or definitions in the literature, survey how research on a topic is conducted, and identify knowledge gaps. In addition, scoping reviews summarize available evidence without answering a discrete research question.
Industry standards for scoping reviews have been established by the Johanna Briggs Institute and Preferred Reporting Items for Systematic Reviews and Meta-analyses extension for scoping reviews. According to these standards, scoping reviews should:
- Establish eligibility criteria with a rationale for each criterion clearly explained
- Search multiple databases in multiple languages
- Include “gray literature,” defined as studies that are unpublished or difficult to locate
- Have several independent reviewers screen titles and abstracts
- Ask multiple independent reviewers to review full text articles
- Present results with charts or diagrams that align with the review’s objective
- Graphically depict the decision process for including/excluding sources
- Identify implications for further research.
In their review, Dr. DuMontier and colleagues fulfilled many of the aforementioned criteria. The team searched three English-language databases for titles and abstracts that included the terms undertreatment and/or overtreatment, and were related to OAs with cancer, inclusive of all types of articles, cancer types, and treatments.
Definitions of undertreatment and overtreatment were extracted, and categories underlying these definitions were derived. Within a random subset of articles, two coauthors independently determined final categories of definitions and independently assigned those categories.
Findings and implications
To define OA, Dr. DuMontier and colleagues used a cutoff of 60 years or older. Articles mentioning undertreatment (n = 236), overtreatment (n = 71), or both (n = 51) met criteria for inclusion (n = 256), but only 14 articles (5.5%) explicitly provided formal definitions.
For most of the reviewed articles, the authors judged definitions from the surrounding context. In a random subset of 50 articles, there was a high level of agreement (87.1%; κ = 0.81) between two coauthors in independently assigning categories of definitions.
Undertreatment was applied to therapy that was less than recommended (148 articles; 62.7%) or less than recommended with worse outcomes (88 articles; 37.3%).
Overtreatment most commonly denoted intensive treatment of an OA in whom harms outweighed the benefits of treatment (38 articles; 53.5%) or intensive treatment of a cancer not expected to affect the OA during the patient’s remaining life (33 articles; 46.5%).
Overall, the authors found that undertreatment and overtreatment of OAs with cancer are imprecisely defined concepts. Formal geriatric assessment was recommended in just over half of articles, and only 26.2% recommended formal assessments of age-related vulnerabilities for management. The authors proposed definitions that accounted for both oncologic factors and geriatric domains.
Care of individual patients and clinical research
National Comprehensive Cancer Network (NCCN) guidelines for OAs with cancer recommend initial consideration of overall life expectancy. If a patient is a candidate for cancer treatment on that basis, the next recommended assessment is that of the patient’s capacity to understand the relevant information, appreciate the underlying values and overall medical situation, reason through decisions, and communicate a choice that is consistent with the patient’s articulated goals.
In the pretreatment evaluation of OAs in whom there are no concerns about tolerance to antineoplastic therapy, NCCN guidelines suggest geriatric screening with standardized tools and, if abnormal, comprehensive geriatric screening. The guidelines recommend considering alternative treatment options if nonmodifiable abnormalities are identified.
Referral to a geriatric clinical specialist, use of the Cancer and Aging Research Group’s Chemo Toxicity Calculator, and calculation of Chemotherapy Risk Assessment Scale for High-Age Patients score are specifically suggested if high-risk procedures (such as chemotherapy, radiation, or complex surgery, which most oncologists would consider to be “another day in the office”) are contemplated.
The American Society of Clinical Oncology (ASCO) guidelines for geriatric oncology are similarly detailed and endorse similar evaluations and management.
Employing disease-centric and geriatric domains
Dr. DuMontier and colleagues noted that, for OAs with comorbidity or psychosocial challenges, surrogate survival endpoints are unrelated to quality of life (QOL) outcomes. Nonetheless, QOL is valued by OAs at least as much as survival improvement.
Through no fault of their own, the authors’ conclusion that undertreatment and overtreatment are imperfectly defined concepts has a certain neutrality to it. However, the terms undertreatment and overtreatment are commonly used to signify that inappropriate treatment decisions were made. Therefore, the terms are inherently negative and pejorative.
As with most emotionally charged issues in oncology, it is ideal for professionals in our field to take charge when deficiencies exist. ASCO, NCCN, and the authors of this scoping review have provided a conceptual basis for doing so.
An integrated oncologist-geriatrician approach was shown to be effective in the randomized INTEGERATE trial, showing improved QOL, reduced hospital admissions, and reduced early treatment discontinuation from adverse events (ASCO 2020, Abstract 12011).
Therefore, those clinicians who have not formally, systematically, and routinely supplemented the traditional disease-centric endpoints with patient-centered criteria need to do so.
Similarly, a retrospective study published in JAMA Network Open demonstrated that geriatric and surgical comanagement of OAs with cancer was associated with significantly lower 90-day postoperative mortality and receipt of more supportive care services (physical therapy, occupational therapy, speech and swallow rehabilitation, and nutrition services), in comparison with management from the surgical service only.
These clinical and administrative changes will not only enhance patient management but also facilitate the clinical trials required to clarify optimal treatment intensity. As that occurs, we will be able to apply as much precision to the care of OAs with cancer as we do in other areas of cancer treatment.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
SOURCE: Dumontier C et al. J Clin Oncol. 2020 Aug 1;38(22):2558-2569.
Cancer disparities: One of the most pressing public health issues
“The burden of cancer is not shouldered equally by all segments of the U.S. population,” the AACR adds. “The adverse differences in cancer burden that exist among certain population groups are one of the most pressing public health challenges that we face in the United States.”
AACR president Antoni Ribas, MD, PhD, gave some examples of these disparities at a September 16 Congressional briefing that focused on the inaugural AACR Cancer Disparities Progress Report 2020.
He noted that:
- Black men have more than double the rate of death from prostate cancer compared with men of other racial and ethnic groups.
- Hispanic children are 24% more likely to develop leukemia than non-Hispanic children.
- Non-Hispanic Black children and adolescents with cancer are more than 50% more likely to die from the cancer than non-Hispanic white children and adolescents with cancer.
- Women of low socioeconomic status with early stage ovarian cancer are 50% less likely to receive recommended care than are women of high socioeconomic status.
- In addition to racial and ethnic minority groups, other populations that bear a disproportionate burden when it comes to cancer include individuals lacking adequate health insurance coverage, immigrants, those with disabilities, residents in rural areas, and members of the lesbian, gay, bisexual, and transgender communities.
“It is absolutely unacceptable that advances in cancer care and treatment are not benefiting everyone equally,” Ribas commented.
Making progress against cancer
Progress being made against cancer was highlighted in another publication, the annual AACR Cancer Progress Report 2020.
U.S. cancer deaths declined by 29% between 1991 and 2017, translating to nearly 3 million cancer deaths avoided, the report notes. In addition, 5-year survival rates for all cancers combined increased from 49% in the mid-1970s to 70% for patients diagnosed from 2010-2016.
Between August 2019 and July 31 of this year, the U.S. Food and Drug Administration approved 20 new anticancer drugs for various cancer types and 15 new indications for previously approved cancer drugs, marking the highest number of approvals in one 12-month period since AACR started producing these reports 10 years ago.
A continuing reduction in the cigarette smoking rate among US adults, which is now below 14%, is contributing greatly to declines in lung cancer rates, which have largely driven the improvements in cancer survival, the AACR noted.
This report also notes that progress has been made toward reducing cancer disparities. Overall disparities in cancer death rates among racial and ethnic groups are less pronounced now than they have been in the past two decades. For example, the overall cancer death rate for African American patients was 33% higher than for White patients in 1990 but just 14% higher in 2016.
However, both reports agree that more must be done to reduce cancer disparities even further.
They highlight initiatives that are underway, including:
- The draft guidance issued by the FDA to promote diversification of clinical trial populations.
- The National Institutes of Health’s (NIH’s) Continuing Umbrella of Research Experiences (CURE) program supporting underrepresented students and scientists along their academic and research career pathway.
- The Centers for Disease Control and Prevention’s Racial and Ethnic Approaches to Community Health (REACH) program, a grant-making program focused on encouraging preventive behaviors in underserved communities.
- The NIH’s All of Us program, which is gathering information from the genomes of 1 million healthy individuals with a focus on recruitment from historically underrepresented populations.
Ribas also announced that AACR has established a task force to focus on racial inequalities in cancer research.
Eliminating disparities would save money, argued John D. Carpten, PhD, from the University of Southern California, Los Angeles, who chaired the steering committee that developed the AACR Cancer Disparities Progress Report.
Carpten noted research showing that eliminating disparities for racial and ethnic minorities between 2003 and 2006 would have reduced health care costs by more than $1 trillion in the United States. This underscores the potentially far-reaching impact of efforts to eliminate disparities, he said.
“Without a doubt, socioeconomics and inequities in access to quality care represent major factors influencing cancer health disparities, and these disparities will persist until we address these issues” he said.
Both progress reports culminate in a call to action, largely focused on the need for “unwavering, bipartisan support from Congress, in the form of robust and sustained annual increases in funding for the NIH, NCI [National Cancer Institute], and FDA,” which is vital for accelerating the pace of progress.
The challenge is now compounded by the ongoing COVID-19 pandemic: Both progress reports note that racial and ethnic minorities, including African Americans, are not only affected disproportionately by cancer, but also by COVID-19, further highlighting the “stark inequities in health care.”
Ribas further called for action from national leadership and the scientific community.
“During this unprecedented time in our nation’s history, there is also a need for our nation’s leaders to take on a much bigger role in confronting and combating the structural and systemic racism that contributes to health disparities,” he said. The “pervasive racism and social injustices” that have contributed to disparities in both COVID-19 and cancer underscore the need for “the scientific community to step up and partner with Congress to assess and address this issue within the research community.”
This article first appeared on Medscape.com.
“The burden of cancer is not shouldered equally by all segments of the U.S. population,” the AACR adds. “The adverse differences in cancer burden that exist among certain population groups are one of the most pressing public health challenges that we face in the United States.”
AACR president Antoni Ribas, MD, PhD, gave some examples of these disparities at a September 16 Congressional briefing that focused on the inaugural AACR Cancer Disparities Progress Report 2020.
He noted that:
- Black men have more than double the rate of death from prostate cancer compared with men of other racial and ethnic groups.
- Hispanic children are 24% more likely to develop leukemia than non-Hispanic children.
- Non-Hispanic Black children and adolescents with cancer are more than 50% more likely to die from the cancer than non-Hispanic white children and adolescents with cancer.
- Women of low socioeconomic status with early stage ovarian cancer are 50% less likely to receive recommended care than are women of high socioeconomic status.
- In addition to racial and ethnic minority groups, other populations that bear a disproportionate burden when it comes to cancer include individuals lacking adequate health insurance coverage, immigrants, those with disabilities, residents in rural areas, and members of the lesbian, gay, bisexual, and transgender communities.
“It is absolutely unacceptable that advances in cancer care and treatment are not benefiting everyone equally,” Ribas commented.
Making progress against cancer
Progress being made against cancer was highlighted in another publication, the annual AACR Cancer Progress Report 2020.
U.S. cancer deaths declined by 29% between 1991 and 2017, translating to nearly 3 million cancer deaths avoided, the report notes. In addition, 5-year survival rates for all cancers combined increased from 49% in the mid-1970s to 70% for patients diagnosed from 2010-2016.
Between August 2019 and July 31 of this year, the U.S. Food and Drug Administration approved 20 new anticancer drugs for various cancer types and 15 new indications for previously approved cancer drugs, marking the highest number of approvals in one 12-month period since AACR started producing these reports 10 years ago.
A continuing reduction in the cigarette smoking rate among US adults, which is now below 14%, is contributing greatly to declines in lung cancer rates, which have largely driven the improvements in cancer survival, the AACR noted.
This report also notes that progress has been made toward reducing cancer disparities. Overall disparities in cancer death rates among racial and ethnic groups are less pronounced now than they have been in the past two decades. For example, the overall cancer death rate for African American patients was 33% higher than for White patients in 1990 but just 14% higher in 2016.
However, both reports agree that more must be done to reduce cancer disparities even further.
They highlight initiatives that are underway, including:
- The draft guidance issued by the FDA to promote diversification of clinical trial populations.
- The National Institutes of Health’s (NIH’s) Continuing Umbrella of Research Experiences (CURE) program supporting underrepresented students and scientists along their academic and research career pathway.
- The Centers for Disease Control and Prevention’s Racial and Ethnic Approaches to Community Health (REACH) program, a grant-making program focused on encouraging preventive behaviors in underserved communities.
- The NIH’s All of Us program, which is gathering information from the genomes of 1 million healthy individuals with a focus on recruitment from historically underrepresented populations.
Ribas also announced that AACR has established a task force to focus on racial inequalities in cancer research.
Eliminating disparities would save money, argued John D. Carpten, PhD, from the University of Southern California, Los Angeles, who chaired the steering committee that developed the AACR Cancer Disparities Progress Report.
Carpten noted research showing that eliminating disparities for racial and ethnic minorities between 2003 and 2006 would have reduced health care costs by more than $1 trillion in the United States. This underscores the potentially far-reaching impact of efforts to eliminate disparities, he said.
“Without a doubt, socioeconomics and inequities in access to quality care represent major factors influencing cancer health disparities, and these disparities will persist until we address these issues” he said.
Both progress reports culminate in a call to action, largely focused on the need for “unwavering, bipartisan support from Congress, in the form of robust and sustained annual increases in funding for the NIH, NCI [National Cancer Institute], and FDA,” which is vital for accelerating the pace of progress.
The challenge is now compounded by the ongoing COVID-19 pandemic: Both progress reports note that racial and ethnic minorities, including African Americans, are not only affected disproportionately by cancer, but also by COVID-19, further highlighting the “stark inequities in health care.”
Ribas further called for action from national leadership and the scientific community.
“During this unprecedented time in our nation’s history, there is also a need for our nation’s leaders to take on a much bigger role in confronting and combating the structural and systemic racism that contributes to health disparities,” he said. The “pervasive racism and social injustices” that have contributed to disparities in both COVID-19 and cancer underscore the need for “the scientific community to step up and partner with Congress to assess and address this issue within the research community.”
This article first appeared on Medscape.com.
“The burden of cancer is not shouldered equally by all segments of the U.S. population,” the AACR adds. “The adverse differences in cancer burden that exist among certain population groups are one of the most pressing public health challenges that we face in the United States.”
AACR president Antoni Ribas, MD, PhD, gave some examples of these disparities at a September 16 Congressional briefing that focused on the inaugural AACR Cancer Disparities Progress Report 2020.
He noted that:
- Black men have more than double the rate of death from prostate cancer compared with men of other racial and ethnic groups.
- Hispanic children are 24% more likely to develop leukemia than non-Hispanic children.
- Non-Hispanic Black children and adolescents with cancer are more than 50% more likely to die from the cancer than non-Hispanic white children and adolescents with cancer.
- Women of low socioeconomic status with early stage ovarian cancer are 50% less likely to receive recommended care than are women of high socioeconomic status.
- In addition to racial and ethnic minority groups, other populations that bear a disproportionate burden when it comes to cancer include individuals lacking adequate health insurance coverage, immigrants, those with disabilities, residents in rural areas, and members of the lesbian, gay, bisexual, and transgender communities.
“It is absolutely unacceptable that advances in cancer care and treatment are not benefiting everyone equally,” Ribas commented.
Making progress against cancer
Progress being made against cancer was highlighted in another publication, the annual AACR Cancer Progress Report 2020.
U.S. cancer deaths declined by 29% between 1991 and 2017, translating to nearly 3 million cancer deaths avoided, the report notes. In addition, 5-year survival rates for all cancers combined increased from 49% in the mid-1970s to 70% for patients diagnosed from 2010-2016.
Between August 2019 and July 31 of this year, the U.S. Food and Drug Administration approved 20 new anticancer drugs for various cancer types and 15 new indications for previously approved cancer drugs, marking the highest number of approvals in one 12-month period since AACR started producing these reports 10 years ago.
A continuing reduction in the cigarette smoking rate among US adults, which is now below 14%, is contributing greatly to declines in lung cancer rates, which have largely driven the improvements in cancer survival, the AACR noted.
This report also notes that progress has been made toward reducing cancer disparities. Overall disparities in cancer death rates among racial and ethnic groups are less pronounced now than they have been in the past two decades. For example, the overall cancer death rate for African American patients was 33% higher than for White patients in 1990 but just 14% higher in 2016.
However, both reports agree that more must be done to reduce cancer disparities even further.
They highlight initiatives that are underway, including:
- The draft guidance issued by the FDA to promote diversification of clinical trial populations.
- The National Institutes of Health’s (NIH’s) Continuing Umbrella of Research Experiences (CURE) program supporting underrepresented students and scientists along their academic and research career pathway.
- The Centers for Disease Control and Prevention’s Racial and Ethnic Approaches to Community Health (REACH) program, a grant-making program focused on encouraging preventive behaviors in underserved communities.
- The NIH’s All of Us program, which is gathering information from the genomes of 1 million healthy individuals with a focus on recruitment from historically underrepresented populations.
Ribas also announced that AACR has established a task force to focus on racial inequalities in cancer research.
Eliminating disparities would save money, argued John D. Carpten, PhD, from the University of Southern California, Los Angeles, who chaired the steering committee that developed the AACR Cancer Disparities Progress Report.
Carpten noted research showing that eliminating disparities for racial and ethnic minorities between 2003 and 2006 would have reduced health care costs by more than $1 trillion in the United States. This underscores the potentially far-reaching impact of efforts to eliminate disparities, he said.
“Without a doubt, socioeconomics and inequities in access to quality care represent major factors influencing cancer health disparities, and these disparities will persist until we address these issues” he said.
Both progress reports culminate in a call to action, largely focused on the need for “unwavering, bipartisan support from Congress, in the form of robust and sustained annual increases in funding for the NIH, NCI [National Cancer Institute], and FDA,” which is vital for accelerating the pace of progress.
The challenge is now compounded by the ongoing COVID-19 pandemic: Both progress reports note that racial and ethnic minorities, including African Americans, are not only affected disproportionately by cancer, but also by COVID-19, further highlighting the “stark inequities in health care.”
Ribas further called for action from national leadership and the scientific community.
“During this unprecedented time in our nation’s history, there is also a need for our nation’s leaders to take on a much bigger role in confronting and combating the structural and systemic racism that contributes to health disparities,” he said. The “pervasive racism and social injustices” that have contributed to disparities in both COVID-19 and cancer underscore the need for “the scientific community to step up and partner with Congress to assess and address this issue within the research community.”
This article first appeared on Medscape.com.
Palbociclib plus letrozole improves PFS in advanced endometrial cancer
Adding palbociclib to letrozole significantly prolonged progression-free survival (PFS) in a phase 2 trial of patients with advanced or recurrent estrogen receptor (ER)–positive endometrial cancer.
This was the first randomized trial to evaluate the efficacy of a CDK4/6 inhibitor in combination with an aromatase inhibitor in patients with advanced or recurrent ER-positive endometrial cancer, noted study investigator Mansoor Raza Mirza, MD, PhD, of Rigshospitalet Copenhagen University Hospital.
Dr. Mirza presented results from this study, ENGOT-EN3-NSGO/PALEO, at the European Society for Medical Oncology Virtual Congress 2020.
Palbociclib is a selective inhibitor of CDK4/, both of which are involved in cell-cycle transitions, Dr. Mirza explained. He observed that endometrial endometrioid adenocarcinomas are hormone dependent, and endocrine treatment with an aromatase inhibitor is well established. Palbociclib has been shown to be superior, when combined with letrozole, to letrozole alone in ER-positive breast cancer.
For the ENGOT-EN3-NSGO/PALEO study, investigators enrolled 77 patients with ER-positive advanced/recurrent endometrial cancer. Patients had received at least one prior systemic therapy, no prior endocrine therapy (except medroxyprogesterone acetate and megestrol acetate), and no prior CDK inhibitor.
Patients were randomized 1:1 to receive oral letrozole (2.5 mg on days 1-28) and either palbociclib (125 mg on days 1-21) or placebo (125 mg on days 1-21) in a 28-day cycle until progression. Baseline characteristics were similar between the treatment arms.
Efficacy
Of the 77 patients enrolled, 73 were evaluable. The primary endpoint was PFS.
The median PFS in the intention-to-treat population was 3.0 months in the placebo arm and 8.3 months in the palbociclib arm (hazard ratio, 0.56; 95% confidence interval, 0.32-0.98; P = .0376).
Looking at stratification factors, PFS was higher in the palbociclib arm among the large majority (about 85%) of patients who had received no prior medroxyprogesterone acetate or megestrol acetate (HR, 0.55; 95% CI, 0.29-01.04; P = .0615) and among patients with relapsed disease (HR, 0.61; 95% CI, 0.34-1.09; P = .0916).
The disease control rate at 24 weeks, a secondary endpoint, was 63.6% in the palbociclib arm and 37.8% in the placebo arm.
Safety
Adverse events were more frequent in the palbociclib arm, with neutropenia being the most common.
Rates of adverse event–related dose reduction (to 100 mg or 75 mg) were 36% in the palbociclib arm and 3% in the placebo arm.
Adverse event–related discontinuation rates were 25% and 19% for palbociclib and letrozole, respectively, in the palbociclib arm and 14% and 11% for placebo and letrozole, respectively, in the placebo arm.
“The toxicity of palbociclib plus letrozole combination therapy was manageable, with most patients remaining on treatment until disease progression,” Dr. Mirza said.
He noted that an analysis of patient-reported outcomes revealed no detrimental effect on quality of life with the combination therapy.
Next steps
“Compared with placebo plus letrozole, the combination of palbociclib plus letrozole demonstrated clinically meaningful improvement in PFS,” Dr. Mirza said. “These results merit a phase 3 validation trial.”
“There is a huge rationale for using this drug in endometrial cancer,” commented study discussant Domenica Lorusso, MD, PhD, of Fondazione Policlinico Universitario Agostino Gemelli IRCCS and Catholic University of Sacred Hearth in Rome.
Dr. Lorusso said hormone receptor expression, which has been identified as a strong predictor of CDK4/6 inhibitor activity, is present in up to 90% of patients with type 1 and in about 65% of patients with type 2 endometrial cancer.
Response rates, in experience with aromatase inhibitors, have been “quite disappointing, in the 10%-20% range,” Dr. Lorusso said, with “dismal prognosis” and guidelines stating that “no standard second-line treatment has been identified.”
This research was sponsored by investigators, but Pfizer provided a study grant. Dr. Mirza disclosed relationships with Pfizer, AstraZeneca, Biocad, Clovis Oncology, Genmab, Karyopharm Therapeutics, Merck, Oncology Adventure, Roche, Seattle Genetics, Sera Prognostics, Sotio, GlaxoSmithKline, Zai Lab, and Boehringer Ingelheim. Dr. Lorusso disclosed relationships with AstraZeneca, Biocad, Clovis Oncology, Genmab, Merck, Roche, Tesaro, Amgen, Immunogen, and Pharma Mar.
SOURCE: Mirza MR et al. ESMO 2020, Abstract LBA28.
Adding palbociclib to letrozole significantly prolonged progression-free survival (PFS) in a phase 2 trial of patients with advanced or recurrent estrogen receptor (ER)–positive endometrial cancer.
This was the first randomized trial to evaluate the efficacy of a CDK4/6 inhibitor in combination with an aromatase inhibitor in patients with advanced or recurrent ER-positive endometrial cancer, noted study investigator Mansoor Raza Mirza, MD, PhD, of Rigshospitalet Copenhagen University Hospital.
Dr. Mirza presented results from this study, ENGOT-EN3-NSGO/PALEO, at the European Society for Medical Oncology Virtual Congress 2020.
Palbociclib is a selective inhibitor of CDK4/, both of which are involved in cell-cycle transitions, Dr. Mirza explained. He observed that endometrial endometrioid adenocarcinomas are hormone dependent, and endocrine treatment with an aromatase inhibitor is well established. Palbociclib has been shown to be superior, when combined with letrozole, to letrozole alone in ER-positive breast cancer.
For the ENGOT-EN3-NSGO/PALEO study, investigators enrolled 77 patients with ER-positive advanced/recurrent endometrial cancer. Patients had received at least one prior systemic therapy, no prior endocrine therapy (except medroxyprogesterone acetate and megestrol acetate), and no prior CDK inhibitor.
Patients were randomized 1:1 to receive oral letrozole (2.5 mg on days 1-28) and either palbociclib (125 mg on days 1-21) or placebo (125 mg on days 1-21) in a 28-day cycle until progression. Baseline characteristics were similar between the treatment arms.
Efficacy
Of the 77 patients enrolled, 73 were evaluable. The primary endpoint was PFS.
The median PFS in the intention-to-treat population was 3.0 months in the placebo arm and 8.3 months in the palbociclib arm (hazard ratio, 0.56; 95% confidence interval, 0.32-0.98; P = .0376).
Looking at stratification factors, PFS was higher in the palbociclib arm among the large majority (about 85%) of patients who had received no prior medroxyprogesterone acetate or megestrol acetate (HR, 0.55; 95% CI, 0.29-01.04; P = .0615) and among patients with relapsed disease (HR, 0.61; 95% CI, 0.34-1.09; P = .0916).
The disease control rate at 24 weeks, a secondary endpoint, was 63.6% in the palbociclib arm and 37.8% in the placebo arm.
Safety
Adverse events were more frequent in the palbociclib arm, with neutropenia being the most common.
Rates of adverse event–related dose reduction (to 100 mg or 75 mg) were 36% in the palbociclib arm and 3% in the placebo arm.
Adverse event–related discontinuation rates were 25% and 19% for palbociclib and letrozole, respectively, in the palbociclib arm and 14% and 11% for placebo and letrozole, respectively, in the placebo arm.
“The toxicity of palbociclib plus letrozole combination therapy was manageable, with most patients remaining on treatment until disease progression,” Dr. Mirza said.
He noted that an analysis of patient-reported outcomes revealed no detrimental effect on quality of life with the combination therapy.
Next steps
“Compared with placebo plus letrozole, the combination of palbociclib plus letrozole demonstrated clinically meaningful improvement in PFS,” Dr. Mirza said. “These results merit a phase 3 validation trial.”
“There is a huge rationale for using this drug in endometrial cancer,” commented study discussant Domenica Lorusso, MD, PhD, of Fondazione Policlinico Universitario Agostino Gemelli IRCCS and Catholic University of Sacred Hearth in Rome.
Dr. Lorusso said hormone receptor expression, which has been identified as a strong predictor of CDK4/6 inhibitor activity, is present in up to 90% of patients with type 1 and in about 65% of patients with type 2 endometrial cancer.
Response rates, in experience with aromatase inhibitors, have been “quite disappointing, in the 10%-20% range,” Dr. Lorusso said, with “dismal prognosis” and guidelines stating that “no standard second-line treatment has been identified.”
This research was sponsored by investigators, but Pfizer provided a study grant. Dr. Mirza disclosed relationships with Pfizer, AstraZeneca, Biocad, Clovis Oncology, Genmab, Karyopharm Therapeutics, Merck, Oncology Adventure, Roche, Seattle Genetics, Sera Prognostics, Sotio, GlaxoSmithKline, Zai Lab, and Boehringer Ingelheim. Dr. Lorusso disclosed relationships with AstraZeneca, Biocad, Clovis Oncology, Genmab, Merck, Roche, Tesaro, Amgen, Immunogen, and Pharma Mar.
SOURCE: Mirza MR et al. ESMO 2020, Abstract LBA28.
Adding palbociclib to letrozole significantly prolonged progression-free survival (PFS) in a phase 2 trial of patients with advanced or recurrent estrogen receptor (ER)–positive endometrial cancer.
This was the first randomized trial to evaluate the efficacy of a CDK4/6 inhibitor in combination with an aromatase inhibitor in patients with advanced or recurrent ER-positive endometrial cancer, noted study investigator Mansoor Raza Mirza, MD, PhD, of Rigshospitalet Copenhagen University Hospital.
Dr. Mirza presented results from this study, ENGOT-EN3-NSGO/PALEO, at the European Society for Medical Oncology Virtual Congress 2020.
Palbociclib is a selective inhibitor of CDK4/, both of which are involved in cell-cycle transitions, Dr. Mirza explained. He observed that endometrial endometrioid adenocarcinomas are hormone dependent, and endocrine treatment with an aromatase inhibitor is well established. Palbociclib has been shown to be superior, when combined with letrozole, to letrozole alone in ER-positive breast cancer.
For the ENGOT-EN3-NSGO/PALEO study, investigators enrolled 77 patients with ER-positive advanced/recurrent endometrial cancer. Patients had received at least one prior systemic therapy, no prior endocrine therapy (except medroxyprogesterone acetate and megestrol acetate), and no prior CDK inhibitor.
Patients were randomized 1:1 to receive oral letrozole (2.5 mg on days 1-28) and either palbociclib (125 mg on days 1-21) or placebo (125 mg on days 1-21) in a 28-day cycle until progression. Baseline characteristics were similar between the treatment arms.
Efficacy
Of the 77 patients enrolled, 73 were evaluable. The primary endpoint was PFS.
The median PFS in the intention-to-treat population was 3.0 months in the placebo arm and 8.3 months in the palbociclib arm (hazard ratio, 0.56; 95% confidence interval, 0.32-0.98; P = .0376).
Looking at stratification factors, PFS was higher in the palbociclib arm among the large majority (about 85%) of patients who had received no prior medroxyprogesterone acetate or megestrol acetate (HR, 0.55; 95% CI, 0.29-01.04; P = .0615) and among patients with relapsed disease (HR, 0.61; 95% CI, 0.34-1.09; P = .0916).
The disease control rate at 24 weeks, a secondary endpoint, was 63.6% in the palbociclib arm and 37.8% in the placebo arm.
Safety
Adverse events were more frequent in the palbociclib arm, with neutropenia being the most common.
Rates of adverse event–related dose reduction (to 100 mg or 75 mg) were 36% in the palbociclib arm and 3% in the placebo arm.
Adverse event–related discontinuation rates were 25% and 19% for palbociclib and letrozole, respectively, in the palbociclib arm and 14% and 11% for placebo and letrozole, respectively, in the placebo arm.
“The toxicity of palbociclib plus letrozole combination therapy was manageable, with most patients remaining on treatment until disease progression,” Dr. Mirza said.
He noted that an analysis of patient-reported outcomes revealed no detrimental effect on quality of life with the combination therapy.
Next steps
“Compared with placebo plus letrozole, the combination of palbociclib plus letrozole demonstrated clinically meaningful improvement in PFS,” Dr. Mirza said. “These results merit a phase 3 validation trial.”
“There is a huge rationale for using this drug in endometrial cancer,” commented study discussant Domenica Lorusso, MD, PhD, of Fondazione Policlinico Universitario Agostino Gemelli IRCCS and Catholic University of Sacred Hearth in Rome.
Dr. Lorusso said hormone receptor expression, which has been identified as a strong predictor of CDK4/6 inhibitor activity, is present in up to 90% of patients with type 1 and in about 65% of patients with type 2 endometrial cancer.
Response rates, in experience with aromatase inhibitors, have been “quite disappointing, in the 10%-20% range,” Dr. Lorusso said, with “dismal prognosis” and guidelines stating that “no standard second-line treatment has been identified.”
This research was sponsored by investigators, but Pfizer provided a study grant. Dr. Mirza disclosed relationships with Pfizer, AstraZeneca, Biocad, Clovis Oncology, Genmab, Karyopharm Therapeutics, Merck, Oncology Adventure, Roche, Seattle Genetics, Sera Prognostics, Sotio, GlaxoSmithKline, Zai Lab, and Boehringer Ingelheim. Dr. Lorusso disclosed relationships with AstraZeneca, Biocad, Clovis Oncology, Genmab, Merck, Roche, Tesaro, Amgen, Immunogen, and Pharma Mar.
SOURCE: Mirza MR et al. ESMO 2020, Abstract LBA28.
FROM ESMO 2020