User login
Power-morcellation hysterectomies declined and most performed with no containment bag
The use of laparoscopic power morcellators for minimally invasive hysterectomy has significantly decreased, and while the use of containment bags increased after the U.S. Food and Drug Administration’s 2014 safety warning about power morcellators, most procedures employing them are still performed without bags, according to a large database study in Obstetrics & Gynecology.
Containment bags are thought to limit the dissemination of potentially pathologic tissue, including unsuspected cancerous cells.
Rates of uterine cancer in women having morcellation were similar before and after the 2014 FDA guidance, and containment bags were used in only a small proportion of women with uterine cancer, according to findings from a research group led by Jason D. Wright, MD, of the division of gynecologic oncology at Columbia University, New York.
“Despite warnings from professional societies and regulatory agencies, as well as intense public scrutiny after the FDA warnings, the majority of morcellated uterine cancers occurred with uncontained laparoscopic power morcellation,” Dr. Wright and associates wrote, adding that the findings have important policy implications. First, efforts are needed to ensure morcellation is avoided in women with pathologic abnormalities. Second, despite regulatory approval, the safety and efficacy of containment bags remain uncertain, and the use and outcomes of these devices should be monitored closely.
The authors noted that laparoscopic power morcellation with a containment bag actually carries a small but significant increase in the risk of complications, compared with uncontained morcellation.
The study
Drawing on the Premier Healthcare Database, the researchers looked at deidentified patients aged 18 years or older who underwent laparoscopic supracervical hysterectomy from 2010 to 2018. The largest age group having the procedure consisted of women aged 40-49.
Patients were stratified based on use of laparoscopic power morcellators.
The cohort was further stratified as either pre–FDA guidance (2010 quarter 1 to 2014 quarter 1) or post–FDA guidance (2014 quarter 2 to 2018 quarter 2).
In the final cohort of 67,115 patients, laparoscopic power morcellator use decreased from 66.7% in 2013 quarter 4 to 13.3% by 2018 quarter 2. The likelihood of using this device decreased by 9.5% for each quarter elapsed in the post–FDA warning period (risk ratio, 0.91; 95% confidence interval, 0.90-0.91).
In other findings, containment bag use rose from 5.2% in 2013 quarter 4 to 15.2% by 2018 quarter 2. The likelihood of containment bag use rose by 3% for each quarter elapsed in the post–FDA warning period (RR, 1.03; 95% CI, 1.02-1.05).
Among women who underwent surgery with laparoscopic power morcellator use, uterine cancers or sarcomas were identified in 54 (0.17%) before the FDA guidance, compared with 7 (0.12%) after the guidance (P = .45).
Containment bags were used in 11.1% of women with uterine cancers or sarcomas before the FDA guidance, compared with 14.3% after the guidance (P = .12). The perioperative complication rate was 3.3% among women who had laparoscopic power morcellator use without a containment bag, compared with 4.5% (P = .001) in those with a containment bag (adjusted RR, 1.35; 95% CI, 1.12-1.64).
A related editorial argued that the backlash against power morcellation was unwarranted and an example of “reactionary medicine.”
Ben A. Abdu, MD, and Cameron Lowry, MD, of the department of obstetrics and gynecology at the University of Tennessee Health Science Center in Memphis, noted that with the known advantages of laparoscopy over laparotomy – decreased blood loss, decreased pain, and fewer wound complications and infections – it is of paramount importance to continue to offer minimally invasive surgery whenever possible. After the FDA raised safety concerns, there was a rise in the rate of open abdominal hysterectomy, which was accompanied by an increase in surgical morbidity. “Perhaps for now we should avoid throwing the baby out with the bath water,” they wrote.
The editorialists pointed out that any surgery may entail unintended complications. “It is also important to remember that there is a risk of dissemination of malignant tissue whether or not power morcellation is used, and it has even been observed in laparotomy,” they stated, noting that bag rupture and tissue spillage can occur even when the containment bag remains intact.
The downward trend in the use of power morcellators observed by Dr. Wright’s group is of serious concern, the commentators added, especially because the FDA communication was made in response to a rare occurrence and possibly resting on an overestimation of risk. “Based on their review of the medical literature at the time, the FDA cited prevalence estimates of 1 in 352 for any uterine sarcoma and 1 in 498 for leiomyosarcoma,” they wrote. “Many authors have expressed concern that the FDA data review was overestimated.” For example, they cite a meta-analysis using prospective data in which the prevalence of occult leiomyosarcoma was estimated at 1 in 8,300. Despite this extremely low prevalence, there has been an almost total nationwide hospital moratorium on the use of power morcellation, which will likely continue. Some manufacturers have ceased or limited production, distribution, and sales of these devices, they noted.
According to Dr. Michael L. Nimaroff, MD, however, chief of minimally invasive gynecologic surgery at Northwell Health in New Hyde Park, N.Y., the general post–FDA-guidance backlash did not have much effect on expert practitioners in this surgical field. “Those of us who specialize in minimally invasive gynecologic surgery, which has many benefits for the patients, never pivoted,” he told this news organization. “We continued to perform it but more conscientiously and with more concern for safety.”
As for morcellator use, added Dr. Nimaroff, specialists were so accustomed to doing these surgeries before the containment systems were made available that they don’t miss the power morcellator. “We actually retrieve tissue manually, and most of our morcellations, if they’re not contained manually, are retrieved vaginally or through a slightly bigger incision. So patients still benefit from minimally invasive surgery, and in some cases these techniques actually shorten the operation.”
This study received no external funding. Dr. Wright is editor in chief of Obstetrics & Gynecology. He reported royalties from UpToDate and has received research support from Merck. Coauthor Dr. Hou has served as a consultant for Foundation Medicine and Natera. Dr. Abdu and Dr. Lowry disclosed no competing interests, as did Dr. Nimaroff.
The use of laparoscopic power morcellators for minimally invasive hysterectomy has significantly decreased, and while the use of containment bags increased after the U.S. Food and Drug Administration’s 2014 safety warning about power morcellators, most procedures employing them are still performed without bags, according to a large database study in Obstetrics & Gynecology.
Containment bags are thought to limit the dissemination of potentially pathologic tissue, including unsuspected cancerous cells.
Rates of uterine cancer in women having morcellation were similar before and after the 2014 FDA guidance, and containment bags were used in only a small proportion of women with uterine cancer, according to findings from a research group led by Jason D. Wright, MD, of the division of gynecologic oncology at Columbia University, New York.
“Despite warnings from professional societies and regulatory agencies, as well as intense public scrutiny after the FDA warnings, the majority of morcellated uterine cancers occurred with uncontained laparoscopic power morcellation,” Dr. Wright and associates wrote, adding that the findings have important policy implications. First, efforts are needed to ensure morcellation is avoided in women with pathologic abnormalities. Second, despite regulatory approval, the safety and efficacy of containment bags remain uncertain, and the use and outcomes of these devices should be monitored closely.
The authors noted that laparoscopic power morcellation with a containment bag actually carries a small but significant increase in the risk of complications, compared with uncontained morcellation.
The study
Drawing on the Premier Healthcare Database, the researchers looked at deidentified patients aged 18 years or older who underwent laparoscopic supracervical hysterectomy from 2010 to 2018. The largest age group having the procedure consisted of women aged 40-49.
Patients were stratified based on use of laparoscopic power morcellators.
The cohort was further stratified as either pre–FDA guidance (2010 quarter 1 to 2014 quarter 1) or post–FDA guidance (2014 quarter 2 to 2018 quarter 2).
In the final cohort of 67,115 patients, laparoscopic power morcellator use decreased from 66.7% in 2013 quarter 4 to 13.3% by 2018 quarter 2. The likelihood of using this device decreased by 9.5% for each quarter elapsed in the post–FDA warning period (risk ratio, 0.91; 95% confidence interval, 0.90-0.91).
In other findings, containment bag use rose from 5.2% in 2013 quarter 4 to 15.2% by 2018 quarter 2. The likelihood of containment bag use rose by 3% for each quarter elapsed in the post–FDA warning period (RR, 1.03; 95% CI, 1.02-1.05).
Among women who underwent surgery with laparoscopic power morcellator use, uterine cancers or sarcomas were identified in 54 (0.17%) before the FDA guidance, compared with 7 (0.12%) after the guidance (P = .45).
Containment bags were used in 11.1% of women with uterine cancers or sarcomas before the FDA guidance, compared with 14.3% after the guidance (P = .12). The perioperative complication rate was 3.3% among women who had laparoscopic power morcellator use without a containment bag, compared with 4.5% (P = .001) in those with a containment bag (adjusted RR, 1.35; 95% CI, 1.12-1.64).
A related editorial argued that the backlash against power morcellation was unwarranted and an example of “reactionary medicine.”
Ben A. Abdu, MD, and Cameron Lowry, MD, of the department of obstetrics and gynecology at the University of Tennessee Health Science Center in Memphis, noted that with the known advantages of laparoscopy over laparotomy – decreased blood loss, decreased pain, and fewer wound complications and infections – it is of paramount importance to continue to offer minimally invasive surgery whenever possible. After the FDA raised safety concerns, there was a rise in the rate of open abdominal hysterectomy, which was accompanied by an increase in surgical morbidity. “Perhaps for now we should avoid throwing the baby out with the bath water,” they wrote.
The editorialists pointed out that any surgery may entail unintended complications. “It is also important to remember that there is a risk of dissemination of malignant tissue whether or not power morcellation is used, and it has even been observed in laparotomy,” they stated, noting that bag rupture and tissue spillage can occur even when the containment bag remains intact.
The downward trend in the use of power morcellators observed by Dr. Wright’s group is of serious concern, the commentators added, especially because the FDA communication was made in response to a rare occurrence and possibly resting on an overestimation of risk. “Based on their review of the medical literature at the time, the FDA cited prevalence estimates of 1 in 352 for any uterine sarcoma and 1 in 498 for leiomyosarcoma,” they wrote. “Many authors have expressed concern that the FDA data review was overestimated.” For example, they cite a meta-analysis using prospective data in which the prevalence of occult leiomyosarcoma was estimated at 1 in 8,300. Despite this extremely low prevalence, there has been an almost total nationwide hospital moratorium on the use of power morcellation, which will likely continue. Some manufacturers have ceased or limited production, distribution, and sales of these devices, they noted.
According to Dr. Michael L. Nimaroff, MD, however, chief of minimally invasive gynecologic surgery at Northwell Health in New Hyde Park, N.Y., the general post–FDA-guidance backlash did not have much effect on expert practitioners in this surgical field. “Those of us who specialize in minimally invasive gynecologic surgery, which has many benefits for the patients, never pivoted,” he told this news organization. “We continued to perform it but more conscientiously and with more concern for safety.”
As for morcellator use, added Dr. Nimaroff, specialists were so accustomed to doing these surgeries before the containment systems were made available that they don’t miss the power morcellator. “We actually retrieve tissue manually, and most of our morcellations, if they’re not contained manually, are retrieved vaginally or through a slightly bigger incision. So patients still benefit from minimally invasive surgery, and in some cases these techniques actually shorten the operation.”
This study received no external funding. Dr. Wright is editor in chief of Obstetrics & Gynecology. He reported royalties from UpToDate and has received research support from Merck. Coauthor Dr. Hou has served as a consultant for Foundation Medicine and Natera. Dr. Abdu and Dr. Lowry disclosed no competing interests, as did Dr. Nimaroff.
The use of laparoscopic power morcellators for minimally invasive hysterectomy has significantly decreased, and while the use of containment bags increased after the U.S. Food and Drug Administration’s 2014 safety warning about power morcellators, most procedures employing them are still performed without bags, according to a large database study in Obstetrics & Gynecology.
Containment bags are thought to limit the dissemination of potentially pathologic tissue, including unsuspected cancerous cells.
Rates of uterine cancer in women having morcellation were similar before and after the 2014 FDA guidance, and containment bags were used in only a small proportion of women with uterine cancer, according to findings from a research group led by Jason D. Wright, MD, of the division of gynecologic oncology at Columbia University, New York.
“Despite warnings from professional societies and regulatory agencies, as well as intense public scrutiny after the FDA warnings, the majority of morcellated uterine cancers occurred with uncontained laparoscopic power morcellation,” Dr. Wright and associates wrote, adding that the findings have important policy implications. First, efforts are needed to ensure morcellation is avoided in women with pathologic abnormalities. Second, despite regulatory approval, the safety and efficacy of containment bags remain uncertain, and the use and outcomes of these devices should be monitored closely.
The authors noted that laparoscopic power morcellation with a containment bag actually carries a small but significant increase in the risk of complications, compared with uncontained morcellation.
The study
Drawing on the Premier Healthcare Database, the researchers looked at deidentified patients aged 18 years or older who underwent laparoscopic supracervical hysterectomy from 2010 to 2018. The largest age group having the procedure consisted of women aged 40-49.
Patients were stratified based on use of laparoscopic power morcellators.
The cohort was further stratified as either pre–FDA guidance (2010 quarter 1 to 2014 quarter 1) or post–FDA guidance (2014 quarter 2 to 2018 quarter 2).
In the final cohort of 67,115 patients, laparoscopic power morcellator use decreased from 66.7% in 2013 quarter 4 to 13.3% by 2018 quarter 2. The likelihood of using this device decreased by 9.5% for each quarter elapsed in the post–FDA warning period (risk ratio, 0.91; 95% confidence interval, 0.90-0.91).
In other findings, containment bag use rose from 5.2% in 2013 quarter 4 to 15.2% by 2018 quarter 2. The likelihood of containment bag use rose by 3% for each quarter elapsed in the post–FDA warning period (RR, 1.03; 95% CI, 1.02-1.05).
Among women who underwent surgery with laparoscopic power morcellator use, uterine cancers or sarcomas were identified in 54 (0.17%) before the FDA guidance, compared with 7 (0.12%) after the guidance (P = .45).
Containment bags were used in 11.1% of women with uterine cancers or sarcomas before the FDA guidance, compared with 14.3% after the guidance (P = .12). The perioperative complication rate was 3.3% among women who had laparoscopic power morcellator use without a containment bag, compared with 4.5% (P = .001) in those with a containment bag (adjusted RR, 1.35; 95% CI, 1.12-1.64).
A related editorial argued that the backlash against power morcellation was unwarranted and an example of “reactionary medicine.”
Ben A. Abdu, MD, and Cameron Lowry, MD, of the department of obstetrics and gynecology at the University of Tennessee Health Science Center in Memphis, noted that with the known advantages of laparoscopy over laparotomy – decreased blood loss, decreased pain, and fewer wound complications and infections – it is of paramount importance to continue to offer minimally invasive surgery whenever possible. After the FDA raised safety concerns, there was a rise in the rate of open abdominal hysterectomy, which was accompanied by an increase in surgical morbidity. “Perhaps for now we should avoid throwing the baby out with the bath water,” they wrote.
The editorialists pointed out that any surgery may entail unintended complications. “It is also important to remember that there is a risk of dissemination of malignant tissue whether or not power morcellation is used, and it has even been observed in laparotomy,” they stated, noting that bag rupture and tissue spillage can occur even when the containment bag remains intact.
The downward trend in the use of power morcellators observed by Dr. Wright’s group is of serious concern, the commentators added, especially because the FDA communication was made in response to a rare occurrence and possibly resting on an overestimation of risk. “Based on their review of the medical literature at the time, the FDA cited prevalence estimates of 1 in 352 for any uterine sarcoma and 1 in 498 for leiomyosarcoma,” they wrote. “Many authors have expressed concern that the FDA data review was overestimated.” For example, they cite a meta-analysis using prospective data in which the prevalence of occult leiomyosarcoma was estimated at 1 in 8,300. Despite this extremely low prevalence, there has been an almost total nationwide hospital moratorium on the use of power morcellation, which will likely continue. Some manufacturers have ceased or limited production, distribution, and sales of these devices, they noted.
According to Dr. Michael L. Nimaroff, MD, however, chief of minimally invasive gynecologic surgery at Northwell Health in New Hyde Park, N.Y., the general post–FDA-guidance backlash did not have much effect on expert practitioners in this surgical field. “Those of us who specialize in minimally invasive gynecologic surgery, which has many benefits for the patients, never pivoted,” he told this news organization. “We continued to perform it but more conscientiously and with more concern for safety.”
As for morcellator use, added Dr. Nimaroff, specialists were so accustomed to doing these surgeries before the containment systems were made available that they don’t miss the power morcellator. “We actually retrieve tissue manually, and most of our morcellations, if they’re not contained manually, are retrieved vaginally or through a slightly bigger incision. So patients still benefit from minimally invasive surgery, and in some cases these techniques actually shorten the operation.”
This study received no external funding. Dr. Wright is editor in chief of Obstetrics & Gynecology. He reported royalties from UpToDate and has received research support from Merck. Coauthor Dr. Hou has served as a consultant for Foundation Medicine and Natera. Dr. Abdu and Dr. Lowry disclosed no competing interests, as did Dr. Nimaroff.
FROM OBSTETRICS & GYNECOLOGY
10 new ways docs could face legal troubles post Roe
Doctors in states where abortion is legal are likely to be the next target for antiabortion activists who want to deter residents from seeking abortions across state lines, say legal experts.
Antiabortion legislators in several states are mounting efforts to clamp down on out-of-state abortions, which they view as a legal loophole.
Nineteen states have already banned the use of telemedicine to prescribe medication abortion by requiring the clinician to be physically present when the medication is administered. These states include Arizona, Louisiana, Tennessee, and Texas, which also recently criminalized sending abortion pills through the mail.
Some state legislators plan to introduce legislation based on a Texas abortion ban enacted last year in which private citizens can sue anyone who assists state residents in obtaining an out-of-state abortion.
Meanwhile, legislators in states including New York where abortion is legal have introduced bills to shield doctors involved in reproductive care from possible negative actions by medical malpractice companies and professional misconduct charges.
This news organization asked legal experts for advice on how doctors can protect themselves and still provide appropriate medical care in this rapidly changing legal landscape. Here’s what they had to say.
1. What if patients from states where abortion is banned want to come to my practice in a legal state? What should I be aware of?
“Doctors should do what they think is medically necessary, but they should be aware of potential criminal and/or civil consequences in a patient’s home state, especially if those states have staked out more extreme positions on abortion such as Texas, Oklahoma, and Louisiana,” says Katherine Florey, JD, a professor at the University of California, Davis, School of Law.
The patient’s home state would need to have criminal laws in place that would explicitly ban residents from obtaining out-of-state abortions. “Probably the greater risk on the criminal front is that many states have existing laws that don’t specify their geographical reach but that could be construed to allow for criminal jurisdiction over out-of-state providers who help residents in obtaining an abortion,” she says.
However, criminal laws would be harder to enforce because of constitutional obstacles that would require the U.S. Supreme Court to resolve in a court case. Another barrier is that criminal law typically requires that a significant element of the offense take place in the prosecuting state, says Ms. Florey.
2. Am I likely to be sued by a patient from a state with an abortion ban?
“It’s more likely that states with abortion bans will pursue civil liability cases, particularly in states such as Texas and Oklahoma that allow private individuals to pursue lawsuits against individuals providing or assisting with abortions.”
Such liability is particularly appealing to antiabortion states because it allows them to target abortion care providers rather than the women seeking abortions, an approach that might be both more politically palatable and more effective in achieving abortion-restriction goals, says Ms. Florey.
“It’s not just the threat of jail time that can deter physicians from providing abortions. They can face significant career consequences from civil liability, including being reported to their medical licensing boards and having their malpractice insurance premiums increase,” says Ms. Florey.
3. What if I provide ‘abortion pill’ prescriptions by telemedicine to a patient in another state?
Doctors need to know what the rules are in the patient’s home state because states generally regard where the patient is located as where telemedicine is legally conducted, says Ms. Florey. “It’s more problematic to conduct telemedicine in states where it’s illegal. It could be viewed legally as if the doctor were prescribing a medication abortion in the patient’s state.”
Ms. Florey also advises doctors to find out whether the patient’s home state bans medication abortions. “The courts or states could decide that the physician is practicing in the jurisdiction where the patient is located even if care is provided remotely. In that case, the doctor would have to comply with all the laws of that state.”
She recommends that doctors counsel patients seeking medication abortions to come to the state where abortion is legal and get on their computers there.
“It’s not a perfect solution, but it provides more legal protection than providing medication abortion across state lines,” says Ms. Florey.
4. Can doctors be sued by patients for not informing them of the full range of treatment options, including abortion, when their health is at risk?
If the doctor is in a state that has banned abortion and the procedure is illegal, they can’t recommend something that the law doesn’t allow, says Ms. Florey.
It’s a tough call for doctors in states where abortion is illegal because they could get into legal trouble if they counsel a woman to get an abortion and the court later deems it was not medically necessary, says Ms. Florey.
But doctors could also get into legal trouble if they don’t counsel a woman to get an abortion if her life is in danger and she meets the exception in the abortion ban.
“Ultimately, I think doctors have to follow their conscience and best medical judgment but recognize the legal hazards that exist. If a physician is seeing a lot of out-of-state patients from a single jurisdiction (such as a neighboring state), it would be best to consult with an attorney from that state,” advises Ms. Florey.
5. If a patient from another state comes to me (in a legal state) for abortion care, am I required to provide them with any warnings, information, and so on?
Doctors may be required by some antiabortion states to mention certain risks, especially to the mother’s mental health, that could arise from abortions even if they are not well-supported by evidence, says Ms. Florey.
If a warning is required in a patient’s home state and there were complications from the procedure or the patient became depressed, it could be grounds for a civil lawsuit, says Ms. Florey.
“There is a Montana case, for example, where the plaintiff sued for malpractice after having an abortion. She alleged that she was given medically inaccurate information about the fetus’s HIV status, but she also claimed that she wasn’t informed that she might become depressed as a result of the abortion,” says Ms. Florey. (The case was ultimately decided on a different ground.)
6. What about complications from abortion care that I provided to a patient from another state? What are my responsibilities and risks? Can I be sued for malpractice when the patient returns to her home state?
If physicians can’t monitor the patients after their visit and something goes wrong, the doctors are at greater risk of negligence and being sued for malpractice in the patient’s home state, says Ms. Florey.
She recommends that doctors ask patients to stay for monitoring after the procedure. “I realize that may not be possible for all patients, but it’s a much safer alternative,” says Ms. Florey.
Otherwise, if the doctor communicates with the patient about the complications in her antiabortion state, the state’s courts could view the doctor as having ties to the state and claim they have jurisdiction in the case and apply the state’s laws, says Ms. Florey.
“Criminal jurisdiction would be more of stretch because the central conduct happened out of state, but states could still try to prosecute a case,” she says.
7. If a patient comes to me from another state, are there any residency requirements, or does the person need to find residence in my state for a period of time? Am I responsible for knowing their arrangements?
Generally, as a constitutional principle, a person can go to another state and have the services that a resident is entitled to, says Ms. Florey.
“States can’t normally discriminate against patients from out of state, so it’s not a residency requirement unless a state imposes one. If a state did that, it would probably be unconstitutional,” she says.
It would be less risky legally, though, if a patient remains in the state where she received abortion care for a significant period of time, says Ms. Florey.
8. How can I protect the privacy of patients’ medical records if they received an abortion or other reproductive care in the state?
To some extent, HIPAA accommodates state laws that mandate reporting of patient information, says Lisa C. Ikemoto, JD, also a professor at the UC Davis School of Law.
The Privacy Rule doesn’t require doctors to disclose protected health information about a patient when state laws require reporting. But the rule allows them to disclose private health information when there’s a court order such as a warrant or subpoena, says Ms. Ikemoto.
“Providers should make sure that patient information remains in records that are HIPAA protected. Some states, including California, have enacted privacy laws that are more protective of patient information,” she says.
The Department of Health & Human Services issued new guidance in June for health care professionals to clarify what the Privacy Rule requires them to report in light of the restrictive abortion laws.
9. I practice in a state where abortion is legal. Can I be extradited to another state if I’m prosecuted for crimes relating to reproductive health services?
Yes, generally, if your state allows it, says Ms. Florey. States have a constitutional obligation to extradite citizens of a different state if that person’s home state asks for that, but states do not have to extradite their own citizens.
However, traditionally, states have cooperated with extradition requests and most states have laws in place providing for extradition in those circumstances, which they could change to exempt abortion providers.
A handful of states – Connecticut, New York, Delaware, and New Jersey – have passed laws specifically shielding medical providers from being prosecuted under abortion restrictions passed in other states.
Governors in Massachusetts, Minnesota, New Mexico, and Nevada have issued executive orders saying they will not extradite abortion providers to states that have banned abortion provision, and that state employees will generally not comply with those out-of-state investigations.
10. Should I increase my malpractice insurance in anticipation of more potential legal problems from patients coming to me from antiabortion states?
Yes, I would recommend that doctors increase their malpractice coverage because of the increased legal risks they could face.
“It’s possible that a state might file a lawsuit against out-of-state abortion providers. Criminal prosecution is also a possibility, but the obstacles to prosecuting a case against an out-of-state provider would be considerable, especially if their home state has passed laws shielding abortion providers,” says Ms. Florey.
Individual malpractice claims or some sort of private enforcement action in a state that has established one would be more likely, she adds.
Ms. Florey advises doctors to monitor this rapidly evolving area of law. “Everything I am saying today could change with a single Supreme Court case. There will also be this kind of push/pull as antiabortion states try to crack down on out-of-state residents who provide or assist in abortion and physicians’ home states that try to protect them from legal consequences.”
A version of this article first appeared on Medscape.com.
Doctors in states where abortion is legal are likely to be the next target for antiabortion activists who want to deter residents from seeking abortions across state lines, say legal experts.
Antiabortion legislators in several states are mounting efforts to clamp down on out-of-state abortions, which they view as a legal loophole.
Nineteen states have already banned the use of telemedicine to prescribe medication abortion by requiring the clinician to be physically present when the medication is administered. These states include Arizona, Louisiana, Tennessee, and Texas, which also recently criminalized sending abortion pills through the mail.
Some state legislators plan to introduce legislation based on a Texas abortion ban enacted last year in which private citizens can sue anyone who assists state residents in obtaining an out-of-state abortion.
Meanwhile, legislators in states including New York where abortion is legal have introduced bills to shield doctors involved in reproductive care from possible negative actions by medical malpractice companies and professional misconduct charges.
This news organization asked legal experts for advice on how doctors can protect themselves and still provide appropriate medical care in this rapidly changing legal landscape. Here’s what they had to say.
1. What if patients from states where abortion is banned want to come to my practice in a legal state? What should I be aware of?
“Doctors should do what they think is medically necessary, but they should be aware of potential criminal and/or civil consequences in a patient’s home state, especially if those states have staked out more extreme positions on abortion such as Texas, Oklahoma, and Louisiana,” says Katherine Florey, JD, a professor at the University of California, Davis, School of Law.
The patient’s home state would need to have criminal laws in place that would explicitly ban residents from obtaining out-of-state abortions. “Probably the greater risk on the criminal front is that many states have existing laws that don’t specify their geographical reach but that could be construed to allow for criminal jurisdiction over out-of-state providers who help residents in obtaining an abortion,” she says.
However, criminal laws would be harder to enforce because of constitutional obstacles that would require the U.S. Supreme Court to resolve in a court case. Another barrier is that criminal law typically requires that a significant element of the offense take place in the prosecuting state, says Ms. Florey.
2. Am I likely to be sued by a patient from a state with an abortion ban?
“It’s more likely that states with abortion bans will pursue civil liability cases, particularly in states such as Texas and Oklahoma that allow private individuals to pursue lawsuits against individuals providing or assisting with abortions.”
Such liability is particularly appealing to antiabortion states because it allows them to target abortion care providers rather than the women seeking abortions, an approach that might be both more politically palatable and more effective in achieving abortion-restriction goals, says Ms. Florey.
“It’s not just the threat of jail time that can deter physicians from providing abortions. They can face significant career consequences from civil liability, including being reported to their medical licensing boards and having their malpractice insurance premiums increase,” says Ms. Florey.
3. What if I provide ‘abortion pill’ prescriptions by telemedicine to a patient in another state?
Doctors need to know what the rules are in the patient’s home state because states generally regard where the patient is located as where telemedicine is legally conducted, says Ms. Florey. “It’s more problematic to conduct telemedicine in states where it’s illegal. It could be viewed legally as if the doctor were prescribing a medication abortion in the patient’s state.”
Ms. Florey also advises doctors to find out whether the patient’s home state bans medication abortions. “The courts or states could decide that the physician is practicing in the jurisdiction where the patient is located even if care is provided remotely. In that case, the doctor would have to comply with all the laws of that state.”
She recommends that doctors counsel patients seeking medication abortions to come to the state where abortion is legal and get on their computers there.
“It’s not a perfect solution, but it provides more legal protection than providing medication abortion across state lines,” says Ms. Florey.
4. Can doctors be sued by patients for not informing them of the full range of treatment options, including abortion, when their health is at risk?
If the doctor is in a state that has banned abortion and the procedure is illegal, they can’t recommend something that the law doesn’t allow, says Ms. Florey.
It’s a tough call for doctors in states where abortion is illegal because they could get into legal trouble if they counsel a woman to get an abortion and the court later deems it was not medically necessary, says Ms. Florey.
But doctors could also get into legal trouble if they don’t counsel a woman to get an abortion if her life is in danger and she meets the exception in the abortion ban.
“Ultimately, I think doctors have to follow their conscience and best medical judgment but recognize the legal hazards that exist. If a physician is seeing a lot of out-of-state patients from a single jurisdiction (such as a neighboring state), it would be best to consult with an attorney from that state,” advises Ms. Florey.
5. If a patient from another state comes to me (in a legal state) for abortion care, am I required to provide them with any warnings, information, and so on?
Doctors may be required by some antiabortion states to mention certain risks, especially to the mother’s mental health, that could arise from abortions even if they are not well-supported by evidence, says Ms. Florey.
If a warning is required in a patient’s home state and there were complications from the procedure or the patient became depressed, it could be grounds for a civil lawsuit, says Ms. Florey.
“There is a Montana case, for example, where the plaintiff sued for malpractice after having an abortion. She alleged that she was given medically inaccurate information about the fetus’s HIV status, but she also claimed that she wasn’t informed that she might become depressed as a result of the abortion,” says Ms. Florey. (The case was ultimately decided on a different ground.)
6. What about complications from abortion care that I provided to a patient from another state? What are my responsibilities and risks? Can I be sued for malpractice when the patient returns to her home state?
If physicians can’t monitor the patients after their visit and something goes wrong, the doctors are at greater risk of negligence and being sued for malpractice in the patient’s home state, says Ms. Florey.
She recommends that doctors ask patients to stay for monitoring after the procedure. “I realize that may not be possible for all patients, but it’s a much safer alternative,” says Ms. Florey.
Otherwise, if the doctor communicates with the patient about the complications in her antiabortion state, the state’s courts could view the doctor as having ties to the state and claim they have jurisdiction in the case and apply the state’s laws, says Ms. Florey.
“Criminal jurisdiction would be more of stretch because the central conduct happened out of state, but states could still try to prosecute a case,” she says.
7. If a patient comes to me from another state, are there any residency requirements, or does the person need to find residence in my state for a period of time? Am I responsible for knowing their arrangements?
Generally, as a constitutional principle, a person can go to another state and have the services that a resident is entitled to, says Ms. Florey.
“States can’t normally discriminate against patients from out of state, so it’s not a residency requirement unless a state imposes one. If a state did that, it would probably be unconstitutional,” she says.
It would be less risky legally, though, if a patient remains in the state where she received abortion care for a significant period of time, says Ms. Florey.
8. How can I protect the privacy of patients’ medical records if they received an abortion or other reproductive care in the state?
To some extent, HIPAA accommodates state laws that mandate reporting of patient information, says Lisa C. Ikemoto, JD, also a professor at the UC Davis School of Law.
The Privacy Rule doesn’t require doctors to disclose protected health information about a patient when state laws require reporting. But the rule allows them to disclose private health information when there’s a court order such as a warrant or subpoena, says Ms. Ikemoto.
“Providers should make sure that patient information remains in records that are HIPAA protected. Some states, including California, have enacted privacy laws that are more protective of patient information,” she says.
The Department of Health & Human Services issued new guidance in June for health care professionals to clarify what the Privacy Rule requires them to report in light of the restrictive abortion laws.
9. I practice in a state where abortion is legal. Can I be extradited to another state if I’m prosecuted for crimes relating to reproductive health services?
Yes, generally, if your state allows it, says Ms. Florey. States have a constitutional obligation to extradite citizens of a different state if that person’s home state asks for that, but states do not have to extradite their own citizens.
However, traditionally, states have cooperated with extradition requests and most states have laws in place providing for extradition in those circumstances, which they could change to exempt abortion providers.
A handful of states – Connecticut, New York, Delaware, and New Jersey – have passed laws specifically shielding medical providers from being prosecuted under abortion restrictions passed in other states.
Governors in Massachusetts, Minnesota, New Mexico, and Nevada have issued executive orders saying they will not extradite abortion providers to states that have banned abortion provision, and that state employees will generally not comply with those out-of-state investigations.
10. Should I increase my malpractice insurance in anticipation of more potential legal problems from patients coming to me from antiabortion states?
Yes, I would recommend that doctors increase their malpractice coverage because of the increased legal risks they could face.
“It’s possible that a state might file a lawsuit against out-of-state abortion providers. Criminal prosecution is also a possibility, but the obstacles to prosecuting a case against an out-of-state provider would be considerable, especially if their home state has passed laws shielding abortion providers,” says Ms. Florey.
Individual malpractice claims or some sort of private enforcement action in a state that has established one would be more likely, she adds.
Ms. Florey advises doctors to monitor this rapidly evolving area of law. “Everything I am saying today could change with a single Supreme Court case. There will also be this kind of push/pull as antiabortion states try to crack down on out-of-state residents who provide or assist in abortion and physicians’ home states that try to protect them from legal consequences.”
A version of this article first appeared on Medscape.com.
Doctors in states where abortion is legal are likely to be the next target for antiabortion activists who want to deter residents from seeking abortions across state lines, say legal experts.
Antiabortion legislators in several states are mounting efforts to clamp down on out-of-state abortions, which they view as a legal loophole.
Nineteen states have already banned the use of telemedicine to prescribe medication abortion by requiring the clinician to be physically present when the medication is administered. These states include Arizona, Louisiana, Tennessee, and Texas, which also recently criminalized sending abortion pills through the mail.
Some state legislators plan to introduce legislation based on a Texas abortion ban enacted last year in which private citizens can sue anyone who assists state residents in obtaining an out-of-state abortion.
Meanwhile, legislators in states including New York where abortion is legal have introduced bills to shield doctors involved in reproductive care from possible negative actions by medical malpractice companies and professional misconduct charges.
This news organization asked legal experts for advice on how doctors can protect themselves and still provide appropriate medical care in this rapidly changing legal landscape. Here’s what they had to say.
1. What if patients from states where abortion is banned want to come to my practice in a legal state? What should I be aware of?
“Doctors should do what they think is medically necessary, but they should be aware of potential criminal and/or civil consequences in a patient’s home state, especially if those states have staked out more extreme positions on abortion such as Texas, Oklahoma, and Louisiana,” says Katherine Florey, JD, a professor at the University of California, Davis, School of Law.
The patient’s home state would need to have criminal laws in place that would explicitly ban residents from obtaining out-of-state abortions. “Probably the greater risk on the criminal front is that many states have existing laws that don’t specify their geographical reach but that could be construed to allow for criminal jurisdiction over out-of-state providers who help residents in obtaining an abortion,” she says.
However, criminal laws would be harder to enforce because of constitutional obstacles that would require the U.S. Supreme Court to resolve in a court case. Another barrier is that criminal law typically requires that a significant element of the offense take place in the prosecuting state, says Ms. Florey.
2. Am I likely to be sued by a patient from a state with an abortion ban?
“It’s more likely that states with abortion bans will pursue civil liability cases, particularly in states such as Texas and Oklahoma that allow private individuals to pursue lawsuits against individuals providing or assisting with abortions.”
Such liability is particularly appealing to antiabortion states because it allows them to target abortion care providers rather than the women seeking abortions, an approach that might be both more politically palatable and more effective in achieving abortion-restriction goals, says Ms. Florey.
“It’s not just the threat of jail time that can deter physicians from providing abortions. They can face significant career consequences from civil liability, including being reported to their medical licensing boards and having their malpractice insurance premiums increase,” says Ms. Florey.
3. What if I provide ‘abortion pill’ prescriptions by telemedicine to a patient in another state?
Doctors need to know what the rules are in the patient’s home state because states generally regard where the patient is located as where telemedicine is legally conducted, says Ms. Florey. “It’s more problematic to conduct telemedicine in states where it’s illegal. It could be viewed legally as if the doctor were prescribing a medication abortion in the patient’s state.”
Ms. Florey also advises doctors to find out whether the patient’s home state bans medication abortions. “The courts or states could decide that the physician is practicing in the jurisdiction where the patient is located even if care is provided remotely. In that case, the doctor would have to comply with all the laws of that state.”
She recommends that doctors counsel patients seeking medication abortions to come to the state where abortion is legal and get on their computers there.
“It’s not a perfect solution, but it provides more legal protection than providing medication abortion across state lines,” says Ms. Florey.
4. Can doctors be sued by patients for not informing them of the full range of treatment options, including abortion, when their health is at risk?
If the doctor is in a state that has banned abortion and the procedure is illegal, they can’t recommend something that the law doesn’t allow, says Ms. Florey.
It’s a tough call for doctors in states where abortion is illegal because they could get into legal trouble if they counsel a woman to get an abortion and the court later deems it was not medically necessary, says Ms. Florey.
But doctors could also get into legal trouble if they don’t counsel a woman to get an abortion if her life is in danger and she meets the exception in the abortion ban.
“Ultimately, I think doctors have to follow their conscience and best medical judgment but recognize the legal hazards that exist. If a physician is seeing a lot of out-of-state patients from a single jurisdiction (such as a neighboring state), it would be best to consult with an attorney from that state,” advises Ms. Florey.
5. If a patient from another state comes to me (in a legal state) for abortion care, am I required to provide them with any warnings, information, and so on?
Doctors may be required by some antiabortion states to mention certain risks, especially to the mother’s mental health, that could arise from abortions even if they are not well-supported by evidence, says Ms. Florey.
If a warning is required in a patient’s home state and there were complications from the procedure or the patient became depressed, it could be grounds for a civil lawsuit, says Ms. Florey.
“There is a Montana case, for example, where the plaintiff sued for malpractice after having an abortion. She alleged that she was given medically inaccurate information about the fetus’s HIV status, but she also claimed that she wasn’t informed that she might become depressed as a result of the abortion,” says Ms. Florey. (The case was ultimately decided on a different ground.)
6. What about complications from abortion care that I provided to a patient from another state? What are my responsibilities and risks? Can I be sued for malpractice when the patient returns to her home state?
If physicians can’t monitor the patients after their visit and something goes wrong, the doctors are at greater risk of negligence and being sued for malpractice in the patient’s home state, says Ms. Florey.
She recommends that doctors ask patients to stay for monitoring after the procedure. “I realize that may not be possible for all patients, but it’s a much safer alternative,” says Ms. Florey.
Otherwise, if the doctor communicates with the patient about the complications in her antiabortion state, the state’s courts could view the doctor as having ties to the state and claim they have jurisdiction in the case and apply the state’s laws, says Ms. Florey.
“Criminal jurisdiction would be more of stretch because the central conduct happened out of state, but states could still try to prosecute a case,” she says.
7. If a patient comes to me from another state, are there any residency requirements, or does the person need to find residence in my state for a period of time? Am I responsible for knowing their arrangements?
Generally, as a constitutional principle, a person can go to another state and have the services that a resident is entitled to, says Ms. Florey.
“States can’t normally discriminate against patients from out of state, so it’s not a residency requirement unless a state imposes one. If a state did that, it would probably be unconstitutional,” she says.
It would be less risky legally, though, if a patient remains in the state where she received abortion care for a significant period of time, says Ms. Florey.
8. How can I protect the privacy of patients’ medical records if they received an abortion or other reproductive care in the state?
To some extent, HIPAA accommodates state laws that mandate reporting of patient information, says Lisa C. Ikemoto, JD, also a professor at the UC Davis School of Law.
The Privacy Rule doesn’t require doctors to disclose protected health information about a patient when state laws require reporting. But the rule allows them to disclose private health information when there’s a court order such as a warrant or subpoena, says Ms. Ikemoto.
“Providers should make sure that patient information remains in records that are HIPAA protected. Some states, including California, have enacted privacy laws that are more protective of patient information,” she says.
The Department of Health & Human Services issued new guidance in June for health care professionals to clarify what the Privacy Rule requires them to report in light of the restrictive abortion laws.
9. I practice in a state where abortion is legal. Can I be extradited to another state if I’m prosecuted for crimes relating to reproductive health services?
Yes, generally, if your state allows it, says Ms. Florey. States have a constitutional obligation to extradite citizens of a different state if that person’s home state asks for that, but states do not have to extradite their own citizens.
However, traditionally, states have cooperated with extradition requests and most states have laws in place providing for extradition in those circumstances, which they could change to exempt abortion providers.
A handful of states – Connecticut, New York, Delaware, and New Jersey – have passed laws specifically shielding medical providers from being prosecuted under abortion restrictions passed in other states.
Governors in Massachusetts, Minnesota, New Mexico, and Nevada have issued executive orders saying they will not extradite abortion providers to states that have banned abortion provision, and that state employees will generally not comply with those out-of-state investigations.
10. Should I increase my malpractice insurance in anticipation of more potential legal problems from patients coming to me from antiabortion states?
Yes, I would recommend that doctors increase their malpractice coverage because of the increased legal risks they could face.
“It’s possible that a state might file a lawsuit against out-of-state abortion providers. Criminal prosecution is also a possibility, but the obstacles to prosecuting a case against an out-of-state provider would be considerable, especially if their home state has passed laws shielding abortion providers,” says Ms. Florey.
Individual malpractice claims or some sort of private enforcement action in a state that has established one would be more likely, she adds.
Ms. Florey advises doctors to monitor this rapidly evolving area of law. “Everything I am saying today could change with a single Supreme Court case. There will also be this kind of push/pull as antiabortion states try to crack down on out-of-state residents who provide or assist in abortion and physicians’ home states that try to protect them from legal consequences.”
A version of this article first appeared on Medscape.com.
Should patients undergoing surgical treatment for cervical lesions also receive an HPV vaccination?
Human papillomavirus (HPV) vaccine given around the time women have surgery for precancerous cervical lesions might lead to a reduction in the risk of lesions returning, as well as other HPV-related diseases, but the effects of this remain unclear.
The authors of the new study, published in The BMJ, explained that women who have been treated for high-grade cervical intra-epithelial neoplasia (CIN) have a “lifelong residual high risk of cervical cancer and other malignancies related to HPV infection,” and some research suggests that giving a preventive HPV vaccine alongside treatment for CIN might help to “reduce the risk in these women.”
HPV vaccination is highly effective at preventing the development of precancerous cervical lesions, CIN, and in the U.K., HPV vaccination is offered to girls and boys around the age of 12 or 13.
Eluned Hughes, head of information and engagement at Jo’s Cervical Cancer Trust, said: “Recent evidence has found that cases of cervical cancer have fallen 87% since the introduction of the HPV vaccine program in U.K. schools in 2008.”
“However, women over the age of 27, for whom the vaccine was not available, remain at increased risk of cervical cancer,” she highlighted.
Significant risk of bias and scarcity of data
In the study, researchers set out to explore the efficacy of HPV vaccination on the risk of HPV infection and recurrent diseases related to HPV infection in individuals undergoing local surgical treatment of preinvasive genital disease.
The systematic review and meta-analysis, led by researchers at Imperial College London, screened data from PubMed (Medline), Scopus, Cochrane, Web of Science, and ClinicalTrials.gov from inception to March 31, 2021.
The researchers analyzed the results of 18 studies – two randomized controlled trials (RCTs), 12 observational studies, and four post-hoc analyses of RCTs.
The authors said that the two RCTs were classified as low risk of bias, while in the observational studies and post-hoc analyses, risk of bias was moderate for seven, serious for seven, and critical for two. Average length of follow-up was 36 months.
There was a reduction of 57% in the risk of recurrence of high-grade pre-invasive disease (CIN2+) in individuals who were vaccinated, compared with those who were not vaccinated. “The effect estimate was “even more pronounced” – a relative 74% reduction – when the risk of recurrence of CIN2+ was assessed for disease related to the two high-risk HPV types – HPV16 and HPV18,” explained the authors.
However, the researchers noted that these effects are unclear because of the “scarcity of data” and the “moderate to high overall risk of bias” of the available studies.
Quality of evidence inconclusive – more trials needed
With regards to CIN3, the risk of recurrence of was also reduced in patients who were vaccinated, but there was a high level of uncertainty about the quality of this evidence, cautioned the authors.
Evidence was also lacking on the benefit of HPV vaccination for recurrence of vulvar, vaginal, and anal lesions, as well as genital warts.
Analysis of the post-hoc studies from randomized controlled trial data with historic vaccination at randomization before the development of the disease reported inconsistent results, the authors said.
Several study limitations were acknowledged by the authors, including that most of the studies were observational, of low to moderate quality, and with relatively short follow-up times, which they pointed out prevented assessment of long-term effects. In addition, the average age of participants was not provided in most studies, and factors such as smoking – associated with a higher risk of recurrence – were not controlled for in many studies.
“HPV vaccination might reduce the risk of recurrence of CIN, in particular when related to HPV16 or HPV18, in women treated with local excision,” they concluded. However, they cautioned that “quality of evidence indicated that the data were inconclusive.”
“Large, appropriately powered, randomized controlled trials are required to establish the effectiveness of adjuvant HPV vaccination at the time of local surgical treatment of CIN,” they recommended.
“Given that the incidence of recurrence of high-grade disease is low in quality assured national screening programs, such as in the United Kingdom, absolute risks and a cost effectiveness analysis would be important in determining the implementation strategy of HPV vaccination after treatment,” the authors said.
Ms. Hughes said that the charity was pleased to see emerging research into the value of using the HPV vaccine to prevent the recurrence of cervical cell changes. She said that the charity looks forward to seeing “further large-scale studies into the effectiveness of this method.”
In the meantime, the charity encourages all women and other people with a cervix to attend their cervical screening and for young people to have the HPV vaccination when invited, as “these are the best tools we currently have to prevent cervical cancer,” she said.
A version of this article first appeared on Medscape UK.
Human papillomavirus (HPV) vaccine given around the time women have surgery for precancerous cervical lesions might lead to a reduction in the risk of lesions returning, as well as other HPV-related diseases, but the effects of this remain unclear.
The authors of the new study, published in The BMJ, explained that women who have been treated for high-grade cervical intra-epithelial neoplasia (CIN) have a “lifelong residual high risk of cervical cancer and other malignancies related to HPV infection,” and some research suggests that giving a preventive HPV vaccine alongside treatment for CIN might help to “reduce the risk in these women.”
HPV vaccination is highly effective at preventing the development of precancerous cervical lesions, CIN, and in the U.K., HPV vaccination is offered to girls and boys around the age of 12 or 13.
Eluned Hughes, head of information and engagement at Jo’s Cervical Cancer Trust, said: “Recent evidence has found that cases of cervical cancer have fallen 87% since the introduction of the HPV vaccine program in U.K. schools in 2008.”
“However, women over the age of 27, for whom the vaccine was not available, remain at increased risk of cervical cancer,” she highlighted.
Significant risk of bias and scarcity of data
In the study, researchers set out to explore the efficacy of HPV vaccination on the risk of HPV infection and recurrent diseases related to HPV infection in individuals undergoing local surgical treatment of preinvasive genital disease.
The systematic review and meta-analysis, led by researchers at Imperial College London, screened data from PubMed (Medline), Scopus, Cochrane, Web of Science, and ClinicalTrials.gov from inception to March 31, 2021.
The researchers analyzed the results of 18 studies – two randomized controlled trials (RCTs), 12 observational studies, and four post-hoc analyses of RCTs.
The authors said that the two RCTs were classified as low risk of bias, while in the observational studies and post-hoc analyses, risk of bias was moderate for seven, serious for seven, and critical for two. Average length of follow-up was 36 months.
There was a reduction of 57% in the risk of recurrence of high-grade pre-invasive disease (CIN2+) in individuals who were vaccinated, compared with those who were not vaccinated. “The effect estimate was “even more pronounced” – a relative 74% reduction – when the risk of recurrence of CIN2+ was assessed for disease related to the two high-risk HPV types – HPV16 and HPV18,” explained the authors.
However, the researchers noted that these effects are unclear because of the “scarcity of data” and the “moderate to high overall risk of bias” of the available studies.
Quality of evidence inconclusive – more trials needed
With regards to CIN3, the risk of recurrence of was also reduced in patients who were vaccinated, but there was a high level of uncertainty about the quality of this evidence, cautioned the authors.
Evidence was also lacking on the benefit of HPV vaccination for recurrence of vulvar, vaginal, and anal lesions, as well as genital warts.
Analysis of the post-hoc studies from randomized controlled trial data with historic vaccination at randomization before the development of the disease reported inconsistent results, the authors said.
Several study limitations were acknowledged by the authors, including that most of the studies were observational, of low to moderate quality, and with relatively short follow-up times, which they pointed out prevented assessment of long-term effects. In addition, the average age of participants was not provided in most studies, and factors such as smoking – associated with a higher risk of recurrence – were not controlled for in many studies.
“HPV vaccination might reduce the risk of recurrence of CIN, in particular when related to HPV16 or HPV18, in women treated with local excision,” they concluded. However, they cautioned that “quality of evidence indicated that the data were inconclusive.”
“Large, appropriately powered, randomized controlled trials are required to establish the effectiveness of adjuvant HPV vaccination at the time of local surgical treatment of CIN,” they recommended.
“Given that the incidence of recurrence of high-grade disease is low in quality assured national screening programs, such as in the United Kingdom, absolute risks and a cost effectiveness analysis would be important in determining the implementation strategy of HPV vaccination after treatment,” the authors said.
Ms. Hughes said that the charity was pleased to see emerging research into the value of using the HPV vaccine to prevent the recurrence of cervical cell changes. She said that the charity looks forward to seeing “further large-scale studies into the effectiveness of this method.”
In the meantime, the charity encourages all women and other people with a cervix to attend their cervical screening and for young people to have the HPV vaccination when invited, as “these are the best tools we currently have to prevent cervical cancer,” she said.
A version of this article first appeared on Medscape UK.
Human papillomavirus (HPV) vaccine given around the time women have surgery for precancerous cervical lesions might lead to a reduction in the risk of lesions returning, as well as other HPV-related diseases, but the effects of this remain unclear.
The authors of the new study, published in The BMJ, explained that women who have been treated for high-grade cervical intra-epithelial neoplasia (CIN) have a “lifelong residual high risk of cervical cancer and other malignancies related to HPV infection,” and some research suggests that giving a preventive HPV vaccine alongside treatment for CIN might help to “reduce the risk in these women.”
HPV vaccination is highly effective at preventing the development of precancerous cervical lesions, CIN, and in the U.K., HPV vaccination is offered to girls and boys around the age of 12 or 13.
Eluned Hughes, head of information and engagement at Jo’s Cervical Cancer Trust, said: “Recent evidence has found that cases of cervical cancer have fallen 87% since the introduction of the HPV vaccine program in U.K. schools in 2008.”
“However, women over the age of 27, for whom the vaccine was not available, remain at increased risk of cervical cancer,” she highlighted.
Significant risk of bias and scarcity of data
In the study, researchers set out to explore the efficacy of HPV vaccination on the risk of HPV infection and recurrent diseases related to HPV infection in individuals undergoing local surgical treatment of preinvasive genital disease.
The systematic review and meta-analysis, led by researchers at Imperial College London, screened data from PubMed (Medline), Scopus, Cochrane, Web of Science, and ClinicalTrials.gov from inception to March 31, 2021.
The researchers analyzed the results of 18 studies – two randomized controlled trials (RCTs), 12 observational studies, and four post-hoc analyses of RCTs.
The authors said that the two RCTs were classified as low risk of bias, while in the observational studies and post-hoc analyses, risk of bias was moderate for seven, serious for seven, and critical for two. Average length of follow-up was 36 months.
There was a reduction of 57% in the risk of recurrence of high-grade pre-invasive disease (CIN2+) in individuals who were vaccinated, compared with those who were not vaccinated. “The effect estimate was “even more pronounced” – a relative 74% reduction – when the risk of recurrence of CIN2+ was assessed for disease related to the two high-risk HPV types – HPV16 and HPV18,” explained the authors.
However, the researchers noted that these effects are unclear because of the “scarcity of data” and the “moderate to high overall risk of bias” of the available studies.
Quality of evidence inconclusive – more trials needed
With regards to CIN3, the risk of recurrence of was also reduced in patients who were vaccinated, but there was a high level of uncertainty about the quality of this evidence, cautioned the authors.
Evidence was also lacking on the benefit of HPV vaccination for recurrence of vulvar, vaginal, and anal lesions, as well as genital warts.
Analysis of the post-hoc studies from randomized controlled trial data with historic vaccination at randomization before the development of the disease reported inconsistent results, the authors said.
Several study limitations were acknowledged by the authors, including that most of the studies were observational, of low to moderate quality, and with relatively short follow-up times, which they pointed out prevented assessment of long-term effects. In addition, the average age of participants was not provided in most studies, and factors such as smoking – associated with a higher risk of recurrence – were not controlled for in many studies.
“HPV vaccination might reduce the risk of recurrence of CIN, in particular when related to HPV16 or HPV18, in women treated with local excision,” they concluded. However, they cautioned that “quality of evidence indicated that the data were inconclusive.”
“Large, appropriately powered, randomized controlled trials are required to establish the effectiveness of adjuvant HPV vaccination at the time of local surgical treatment of CIN,” they recommended.
“Given that the incidence of recurrence of high-grade disease is low in quality assured national screening programs, such as in the United Kingdom, absolute risks and a cost effectiveness analysis would be important in determining the implementation strategy of HPV vaccination after treatment,” the authors said.
Ms. Hughes said that the charity was pleased to see emerging research into the value of using the HPV vaccine to prevent the recurrence of cervical cell changes. She said that the charity looks forward to seeing “further large-scale studies into the effectiveness of this method.”
In the meantime, the charity encourages all women and other people with a cervix to attend their cervical screening and for young people to have the HPV vaccination when invited, as “these are the best tools we currently have to prevent cervical cancer,” she said.
A version of this article first appeared on Medscape UK.
A hypogastric nerve-focused approach to nerve-sparing endometriosis surgery
Radical resection of deep infiltrating endometriosis (DIE) or pelvic malignancies can lead to inadvertent damage to the pelvic autonomic nerve bundles, causing urinary dysfunction in up to 41% of cases, as well as anorectal and sexual dysfunction.1 Each of these sequelae can significantly affect the patient’s quality of life.
Nerve-sparing techniques have therefore been a trending topic in gynecologic surgery in the 21st century, starting with papers by Marc Possover, MD, of Switzerland, on the laparoscopic neuronavigation (LANN) technique. In an important 2005 publication, he described how the LANN technique can significantly reduce postoperative functional morbidity in laparoscopic radical pelvic surgery.2
The LANN method utilizes intraoperative neurostimulation to identify and dissect the intrapelvic nerve bundles away from surrounding tissue prior to dissection of the DIE or pelvic malignancies. The nerves are exposed and preserved under direct visualization in a fashion similar to that used to expose and preserve the ureters. Pelvic dissection using the LANN technique is extensive and occurs down to the level of the sacral nerve roots.
Dr. Possover’s 2005 paper and others like it spurred increased awareness of the intrapelvic part of the autonomic nervous system – in particular, the hypogastric nerves, the pelvic splanchnic nerves, and the inferior hypogastric plexus. Across additional published studies, nerve-sparing techniques were shown to be effective in preserving neurologic pelvic functions, with significantly less urinary retention and rectal/sexual dysfunction than seen with traditional laparoscopy techniques.
For example, in a single-center prospective clinical trial reported in 2012, 56 of 65 (86.2%) patients treated with a classical laparoscopic technique for excision of DIE reported neurologic pelvic dysfunctions, compared with 1 of 61 (1.6%) patients treated with a nerve-sparing approach.3
While research has confirmed the importance of nerve-sparing techniques, it also shone light on the reality that the LANN technique is extremely technically challenging and requires a high level of surgical expertise and advanced training. In my teaching of the technique, I also saw that few gynecologic surgeons were able to incorporate the advanced nerve-sparing technique into their practices.
A group consisting of myself and collaborators at the University of Bologna, Italy, and the University of Cambridge, England, recently developed an alternative to the LANN approach that uses the hypogastric nerves as landmarks. The technique requires less dissection and should be technically achievable when the pelvic neuroanatomy and anatomy of the presacral fascia are well understood. The hypogastric nerve is identified and used as a landmark to preserve the deeper autonomic nerve bundles in the pelvis without exposure and without more extensive dissection to the level of the sacral nerve roots.4,5
This hypogastric nerve-based technique will cover the vast majority of radical surgeries for DIE. When more advanced nerve sparing and more extensive dissection is needed for the very deepest levels of disease infiltration, patients can be referred to surgeons with advanced training, comfort, and experience with the LANN technique.
The pelvic neuroanatomy
As described in our video articles published in 2015 in Fertility and Sterility6 and 2019 in the Journal of Minimally Invasive Gynecology,5 the left and right hypogastric nerves are the main sympathetic nerves of the autonomic nervous system in the pelvis. They originate from the superior hypogastric plexus and, at the level of the middle rectal vessels, they join the pelvic sacral splanchnic nerves to form the inferior hypogastric plexus. They are easily identifiable at their origin and are the most superficial and readily identifiable component of the inferior hypogastric plexus.
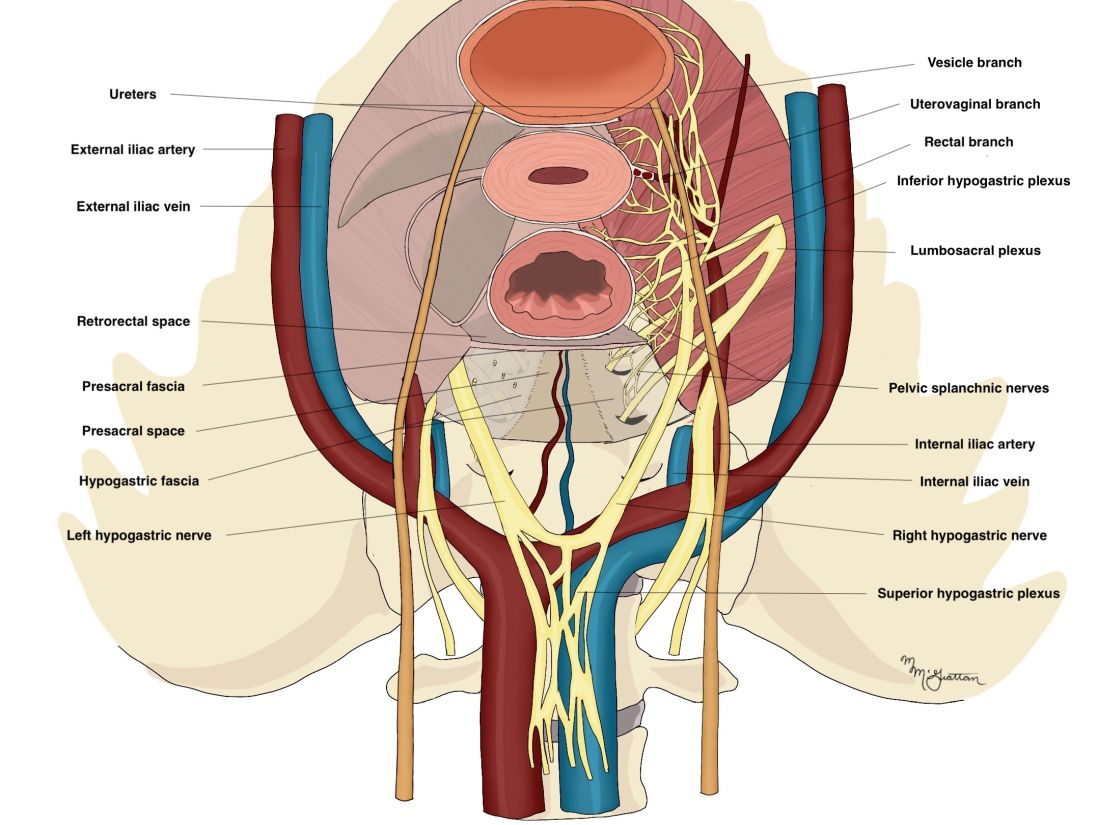
The sympathetic input from the hypogastric nerves causes the internal urethral and anal sphincters to contract, as well as detrusor relaxation and a reduction of peristalsis of the descending colon, sigmoid, and rectum; thus, hypogastric nerve input promotes continence.
The hypogastric nerves also carry afferent signals for pelvic visceral proprioception. Lesion to the hypogastric nerves will usually be subclinical and will put the patient at risk for unnoticeable bladder distension, which usually becomes symptomatic about 7 years after the procedure.7
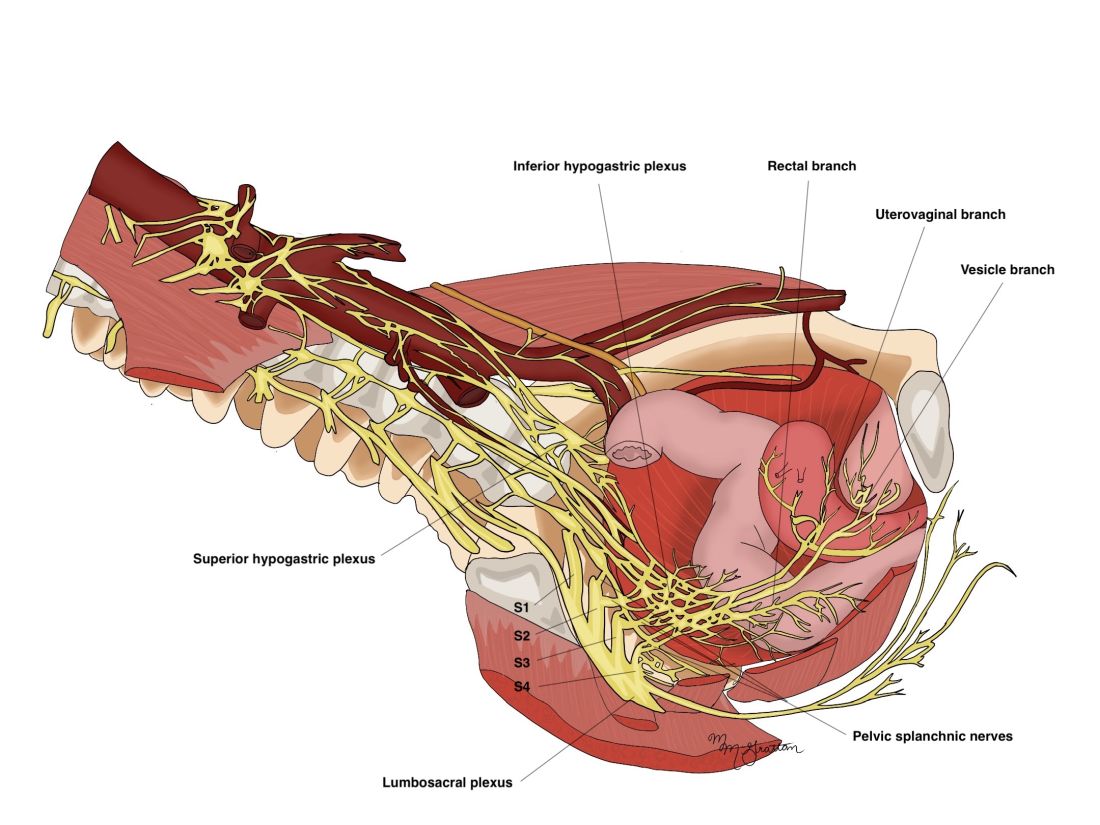
The thin pelvic splanchnic nerves – which merge with the hypogastric nerves into the pararectal fossae to form the inferior hypogastric plexus – arise from nerve roots S2 and S4 and carry all parasympathetic signals to the bladder, rectum, and the sigmoid and left colons. Lesions to these bundles are the main cause of neurogenic urinary retention.
The inferior hypogastric plexi split into the vesical, uterine, and rectal branches, which carry the sympathetic, parasympathetic, and sensory fibers from both the splanchnic and hypogastric nerves. Damage to the inferior hypogastric plexi and/or its branches may induce severe dysfunction to the target organs of the injured fibers.
A focus on the hypogastric nerve
Our approach was developed after we studied the anatomic reliability of the hypogastric nerves through a prospective observational study consisting of measurements during five cadaveric dissections and 10 in-vivo laparoscopic surgeries for rectosigmoid endometriosis.4 We took an interfascial approach to dissection.
Our goal was to clarify the distances between the hypogastric nerves and the ureters, the midsagittal plane, the midcervical plane, and the uterosacral ligaments in each hemipelvis, and in doing so, enable identification of the hypogastric nerves and establish recognizable limits for dissection.
We found quite a bit of variance in the anatomic position and appearance of the hypogastric nerves, but the variances were not very broad. Most notably, the right hypogastric nerve was significantly farther toward the ureter (mean, 14.5 mm; range, 10-25 mm) than the left one (mean, 8.6 mm; range, 7-12 mm).
The ureters were a good landmark for identification of the hypogastric nerves because the nerves were consistently found medially and posteriorly to the ureter at a mean distance of 11.6 mm. Overall, we demonstrated reproducibility in the identification and dissection of the hypogastric nerves using recognizable interfascial planes and anatomic landmarks.4
With good anatomic understanding, a stepwise approach can be taken to identify and preserve the hypogastric nerve and the deeper inferior hypogastric plexus without the need for more extensive dissection.
As shown in our 2019 video, the right hypogastric nerves can be identified transperitoneally in most cases.5 For confirmation, a gentle anterior pulling on the hypogastric nerve causes a caudal movement of the peritoneum overlying the superior hypogastric plexus. (Intermittent pulling on the nerve can also be helpful in localizing the left hypogastric nerve.)
To dissect a hypogastric nerve, the retroperitoneum is opened at the level of the pelvic brim, just inferomedially to the external iliac vessels, and the incision is extended anteriorly, with gentle dissection of the underlying tissue until the ureter is identified.
Once the ureter is identified and lateralized, dissection along the peritoneum is carried deeper and medially into the pelvis until the hypogastric nerve is identified. Lateral to this area are the internal iliac artery, the branching uterine artery, and the obliterated umbilical ligament. In the left hemipelvis, the hypogastric nerve can reliably be found at a mean distance of 8.6 mm from the ureter, while the right one will be found on average 14.5 mm away.
The hypogastric nerves form the posteromedial limit for a safe and simple nerve-sparing dissection. Any dissection posteriorly and laterally to these landmarks should start with the identification of sacral nerve roots and hypogastric nerves.
Dr. Lemos reported that he has no relevant disclosures.
Dr. Lemos is associate professor in the department of obstetrics and gynecology at the University of Toronto.
References
1. Imboden S et al. J Minim Invasive Gynecol. 2021 Aug;28(8):1544-51. doi: 10.1016/j.jmig.2021.01.009.
2. Possover M et al. J Am Coll Surg. 2005;201(6):913-7. doi: 10.1016/j.jamcollsurg.2005.07.006.
3. Ceccaroni M et al. Surg Endosc. 2012;26(7):2029-45. doi: 10.1007/s00464-012-2153-3.
4. Seracchioli R et al. J Minim Invasive Gynecol. 2019;26(7):1340-5. doi: 10.1016/j.jmig.2019.01.010.
5. Zakhari A et al. J Minim Invasive Gynecol. 2020;27(4):813-4. doi: 10.1016/j.jmig.2019.08.001
6. Lemos N et al. Fertil Steril. 2015 Nov;104(5):e11-2. doi: 10.1016/j.fertnstert.2015.07.1138.
7. Possover M. Fertil Steril. 2014 Mar;101(3):754-8. doi: 10.1016/j.fertnstert.2013.12.019.
Radical resection of deep infiltrating endometriosis (DIE) or pelvic malignancies can lead to inadvertent damage to the pelvic autonomic nerve bundles, causing urinary dysfunction in up to 41% of cases, as well as anorectal and sexual dysfunction.1 Each of these sequelae can significantly affect the patient’s quality of life.
Nerve-sparing techniques have therefore been a trending topic in gynecologic surgery in the 21st century, starting with papers by Marc Possover, MD, of Switzerland, on the laparoscopic neuronavigation (LANN) technique. In an important 2005 publication, he described how the LANN technique can significantly reduce postoperative functional morbidity in laparoscopic radical pelvic surgery.2
The LANN method utilizes intraoperative neurostimulation to identify and dissect the intrapelvic nerve bundles away from surrounding tissue prior to dissection of the DIE or pelvic malignancies. The nerves are exposed and preserved under direct visualization in a fashion similar to that used to expose and preserve the ureters. Pelvic dissection using the LANN technique is extensive and occurs down to the level of the sacral nerve roots.
Dr. Possover’s 2005 paper and others like it spurred increased awareness of the intrapelvic part of the autonomic nervous system – in particular, the hypogastric nerves, the pelvic splanchnic nerves, and the inferior hypogastric plexus. Across additional published studies, nerve-sparing techniques were shown to be effective in preserving neurologic pelvic functions, with significantly less urinary retention and rectal/sexual dysfunction than seen with traditional laparoscopy techniques.
For example, in a single-center prospective clinical trial reported in 2012, 56 of 65 (86.2%) patients treated with a classical laparoscopic technique for excision of DIE reported neurologic pelvic dysfunctions, compared with 1 of 61 (1.6%) patients treated with a nerve-sparing approach.3
While research has confirmed the importance of nerve-sparing techniques, it also shone light on the reality that the LANN technique is extremely technically challenging and requires a high level of surgical expertise and advanced training. In my teaching of the technique, I also saw that few gynecologic surgeons were able to incorporate the advanced nerve-sparing technique into their practices.
A group consisting of myself and collaborators at the University of Bologna, Italy, and the University of Cambridge, England, recently developed an alternative to the LANN approach that uses the hypogastric nerves as landmarks. The technique requires less dissection and should be technically achievable when the pelvic neuroanatomy and anatomy of the presacral fascia are well understood. The hypogastric nerve is identified and used as a landmark to preserve the deeper autonomic nerve bundles in the pelvis without exposure and without more extensive dissection to the level of the sacral nerve roots.4,5
This hypogastric nerve-based technique will cover the vast majority of radical surgeries for DIE. When more advanced nerve sparing and more extensive dissection is needed for the very deepest levels of disease infiltration, patients can be referred to surgeons with advanced training, comfort, and experience with the LANN technique.
The pelvic neuroanatomy
As described in our video articles published in 2015 in Fertility and Sterility6 and 2019 in the Journal of Minimally Invasive Gynecology,5 the left and right hypogastric nerves are the main sympathetic nerves of the autonomic nervous system in the pelvis. They originate from the superior hypogastric plexus and, at the level of the middle rectal vessels, they join the pelvic sacral splanchnic nerves to form the inferior hypogastric plexus. They are easily identifiable at their origin and are the most superficial and readily identifiable component of the inferior hypogastric plexus.

The sympathetic input from the hypogastric nerves causes the internal urethral and anal sphincters to contract, as well as detrusor relaxation and a reduction of peristalsis of the descending colon, sigmoid, and rectum; thus, hypogastric nerve input promotes continence.
The hypogastric nerves also carry afferent signals for pelvic visceral proprioception. Lesion to the hypogastric nerves will usually be subclinical and will put the patient at risk for unnoticeable bladder distension, which usually becomes symptomatic about 7 years after the procedure.7

The thin pelvic splanchnic nerves – which merge with the hypogastric nerves into the pararectal fossae to form the inferior hypogastric plexus – arise from nerve roots S2 and S4 and carry all parasympathetic signals to the bladder, rectum, and the sigmoid and left colons. Lesions to these bundles are the main cause of neurogenic urinary retention.
The inferior hypogastric plexi split into the vesical, uterine, and rectal branches, which carry the sympathetic, parasympathetic, and sensory fibers from both the splanchnic and hypogastric nerves. Damage to the inferior hypogastric plexi and/or its branches may induce severe dysfunction to the target organs of the injured fibers.
A focus on the hypogastric nerve
Our approach was developed after we studied the anatomic reliability of the hypogastric nerves through a prospective observational study consisting of measurements during five cadaveric dissections and 10 in-vivo laparoscopic surgeries for rectosigmoid endometriosis.4 We took an interfascial approach to dissection.
Our goal was to clarify the distances between the hypogastric nerves and the ureters, the midsagittal plane, the midcervical plane, and the uterosacral ligaments in each hemipelvis, and in doing so, enable identification of the hypogastric nerves and establish recognizable limits for dissection.
We found quite a bit of variance in the anatomic position and appearance of the hypogastric nerves, but the variances were not very broad. Most notably, the right hypogastric nerve was significantly farther toward the ureter (mean, 14.5 mm; range, 10-25 mm) than the left one (mean, 8.6 mm; range, 7-12 mm).
The ureters were a good landmark for identification of the hypogastric nerves because the nerves were consistently found medially and posteriorly to the ureter at a mean distance of 11.6 mm. Overall, we demonstrated reproducibility in the identification and dissection of the hypogastric nerves using recognizable interfascial planes and anatomic landmarks.4
With good anatomic understanding, a stepwise approach can be taken to identify and preserve the hypogastric nerve and the deeper inferior hypogastric plexus without the need for more extensive dissection.
As shown in our 2019 video, the right hypogastric nerves can be identified transperitoneally in most cases.5 For confirmation, a gentle anterior pulling on the hypogastric nerve causes a caudal movement of the peritoneum overlying the superior hypogastric plexus. (Intermittent pulling on the nerve can also be helpful in localizing the left hypogastric nerve.)
To dissect a hypogastric nerve, the retroperitoneum is opened at the level of the pelvic brim, just inferomedially to the external iliac vessels, and the incision is extended anteriorly, with gentle dissection of the underlying tissue until the ureter is identified.
Once the ureter is identified and lateralized, dissection along the peritoneum is carried deeper and medially into the pelvis until the hypogastric nerve is identified. Lateral to this area are the internal iliac artery, the branching uterine artery, and the obliterated umbilical ligament. In the left hemipelvis, the hypogastric nerve can reliably be found at a mean distance of 8.6 mm from the ureter, while the right one will be found on average 14.5 mm away.
The hypogastric nerves form the posteromedial limit for a safe and simple nerve-sparing dissection. Any dissection posteriorly and laterally to these landmarks should start with the identification of sacral nerve roots and hypogastric nerves.
Dr. Lemos reported that he has no relevant disclosures.
Dr. Lemos is associate professor in the department of obstetrics and gynecology at the University of Toronto.
References
1. Imboden S et al. J Minim Invasive Gynecol. 2021 Aug;28(8):1544-51. doi: 10.1016/j.jmig.2021.01.009.
2. Possover M et al. J Am Coll Surg. 2005;201(6):913-7. doi: 10.1016/j.jamcollsurg.2005.07.006.
3. Ceccaroni M et al. Surg Endosc. 2012;26(7):2029-45. doi: 10.1007/s00464-012-2153-3.
4. Seracchioli R et al. J Minim Invasive Gynecol. 2019;26(7):1340-5. doi: 10.1016/j.jmig.2019.01.010.
5. Zakhari A et al. J Minim Invasive Gynecol. 2020;27(4):813-4. doi: 10.1016/j.jmig.2019.08.001
6. Lemos N et al. Fertil Steril. 2015 Nov;104(5):e11-2. doi: 10.1016/j.fertnstert.2015.07.1138.
7. Possover M. Fertil Steril. 2014 Mar;101(3):754-8. doi: 10.1016/j.fertnstert.2013.12.019.
Radical resection of deep infiltrating endometriosis (DIE) or pelvic malignancies can lead to inadvertent damage to the pelvic autonomic nerve bundles, causing urinary dysfunction in up to 41% of cases, as well as anorectal and sexual dysfunction.1 Each of these sequelae can significantly affect the patient’s quality of life.
Nerve-sparing techniques have therefore been a trending topic in gynecologic surgery in the 21st century, starting with papers by Marc Possover, MD, of Switzerland, on the laparoscopic neuronavigation (LANN) technique. In an important 2005 publication, he described how the LANN technique can significantly reduce postoperative functional morbidity in laparoscopic radical pelvic surgery.2
The LANN method utilizes intraoperative neurostimulation to identify and dissect the intrapelvic nerve bundles away from surrounding tissue prior to dissection of the DIE or pelvic malignancies. The nerves are exposed and preserved under direct visualization in a fashion similar to that used to expose and preserve the ureters. Pelvic dissection using the LANN technique is extensive and occurs down to the level of the sacral nerve roots.
Dr. Possover’s 2005 paper and others like it spurred increased awareness of the intrapelvic part of the autonomic nervous system – in particular, the hypogastric nerves, the pelvic splanchnic nerves, and the inferior hypogastric plexus. Across additional published studies, nerve-sparing techniques were shown to be effective in preserving neurologic pelvic functions, with significantly less urinary retention and rectal/sexual dysfunction than seen with traditional laparoscopy techniques.
For example, in a single-center prospective clinical trial reported in 2012, 56 of 65 (86.2%) patients treated with a classical laparoscopic technique for excision of DIE reported neurologic pelvic dysfunctions, compared with 1 of 61 (1.6%) patients treated with a nerve-sparing approach.3
While research has confirmed the importance of nerve-sparing techniques, it also shone light on the reality that the LANN technique is extremely technically challenging and requires a high level of surgical expertise and advanced training. In my teaching of the technique, I also saw that few gynecologic surgeons were able to incorporate the advanced nerve-sparing technique into their practices.
A group consisting of myself and collaborators at the University of Bologna, Italy, and the University of Cambridge, England, recently developed an alternative to the LANN approach that uses the hypogastric nerves as landmarks. The technique requires less dissection and should be technically achievable when the pelvic neuroanatomy and anatomy of the presacral fascia are well understood. The hypogastric nerve is identified and used as a landmark to preserve the deeper autonomic nerve bundles in the pelvis without exposure and without more extensive dissection to the level of the sacral nerve roots.4,5
This hypogastric nerve-based technique will cover the vast majority of radical surgeries for DIE. When more advanced nerve sparing and more extensive dissection is needed for the very deepest levels of disease infiltration, patients can be referred to surgeons with advanced training, comfort, and experience with the LANN technique.
The pelvic neuroanatomy
As described in our video articles published in 2015 in Fertility and Sterility6 and 2019 in the Journal of Minimally Invasive Gynecology,5 the left and right hypogastric nerves are the main sympathetic nerves of the autonomic nervous system in the pelvis. They originate from the superior hypogastric plexus and, at the level of the middle rectal vessels, they join the pelvic sacral splanchnic nerves to form the inferior hypogastric plexus. They are easily identifiable at their origin and are the most superficial and readily identifiable component of the inferior hypogastric plexus.

The sympathetic input from the hypogastric nerves causes the internal urethral and anal sphincters to contract, as well as detrusor relaxation and a reduction of peristalsis of the descending colon, sigmoid, and rectum; thus, hypogastric nerve input promotes continence.
The hypogastric nerves also carry afferent signals for pelvic visceral proprioception. Lesion to the hypogastric nerves will usually be subclinical and will put the patient at risk for unnoticeable bladder distension, which usually becomes symptomatic about 7 years after the procedure.7

The thin pelvic splanchnic nerves – which merge with the hypogastric nerves into the pararectal fossae to form the inferior hypogastric plexus – arise from nerve roots S2 and S4 and carry all parasympathetic signals to the bladder, rectum, and the sigmoid and left colons. Lesions to these bundles are the main cause of neurogenic urinary retention.
The inferior hypogastric plexi split into the vesical, uterine, and rectal branches, which carry the sympathetic, parasympathetic, and sensory fibers from both the splanchnic and hypogastric nerves. Damage to the inferior hypogastric plexi and/or its branches may induce severe dysfunction to the target organs of the injured fibers.
A focus on the hypogastric nerve
Our approach was developed after we studied the anatomic reliability of the hypogastric nerves through a prospective observational study consisting of measurements during five cadaveric dissections and 10 in-vivo laparoscopic surgeries for rectosigmoid endometriosis.4 We took an interfascial approach to dissection.
Our goal was to clarify the distances between the hypogastric nerves and the ureters, the midsagittal plane, the midcervical plane, and the uterosacral ligaments in each hemipelvis, and in doing so, enable identification of the hypogastric nerves and establish recognizable limits for dissection.
We found quite a bit of variance in the anatomic position and appearance of the hypogastric nerves, but the variances were not very broad. Most notably, the right hypogastric nerve was significantly farther toward the ureter (mean, 14.5 mm; range, 10-25 mm) than the left one (mean, 8.6 mm; range, 7-12 mm).
The ureters were a good landmark for identification of the hypogastric nerves because the nerves were consistently found medially and posteriorly to the ureter at a mean distance of 11.6 mm. Overall, we demonstrated reproducibility in the identification and dissection of the hypogastric nerves using recognizable interfascial planes and anatomic landmarks.4
With good anatomic understanding, a stepwise approach can be taken to identify and preserve the hypogastric nerve and the deeper inferior hypogastric plexus without the need for more extensive dissection.
As shown in our 2019 video, the right hypogastric nerves can be identified transperitoneally in most cases.5 For confirmation, a gentle anterior pulling on the hypogastric nerve causes a caudal movement of the peritoneum overlying the superior hypogastric plexus. (Intermittent pulling on the nerve can also be helpful in localizing the left hypogastric nerve.)
To dissect a hypogastric nerve, the retroperitoneum is opened at the level of the pelvic brim, just inferomedially to the external iliac vessels, and the incision is extended anteriorly, with gentle dissection of the underlying tissue until the ureter is identified.
Once the ureter is identified and lateralized, dissection along the peritoneum is carried deeper and medially into the pelvis until the hypogastric nerve is identified. Lateral to this area are the internal iliac artery, the branching uterine artery, and the obliterated umbilical ligament. In the left hemipelvis, the hypogastric nerve can reliably be found at a mean distance of 8.6 mm from the ureter, while the right one will be found on average 14.5 mm away.
The hypogastric nerves form the posteromedial limit for a safe and simple nerve-sparing dissection. Any dissection posteriorly and laterally to these landmarks should start with the identification of sacral nerve roots and hypogastric nerves.
Dr. Lemos reported that he has no relevant disclosures.
Dr. Lemos is associate professor in the department of obstetrics and gynecology at the University of Toronto.
References
1. Imboden S et al. J Minim Invasive Gynecol. 2021 Aug;28(8):1544-51. doi: 10.1016/j.jmig.2021.01.009.
2. Possover M et al. J Am Coll Surg. 2005;201(6):913-7. doi: 10.1016/j.jamcollsurg.2005.07.006.
3. Ceccaroni M et al. Surg Endosc. 2012;26(7):2029-45. doi: 10.1007/s00464-012-2153-3.
4. Seracchioli R et al. J Minim Invasive Gynecol. 2019;26(7):1340-5. doi: 10.1016/j.jmig.2019.01.010.
5. Zakhari A et al. J Minim Invasive Gynecol. 2020;27(4):813-4. doi: 10.1016/j.jmig.2019.08.001
6. Lemos N et al. Fertil Steril. 2015 Nov;104(5):e11-2. doi: 10.1016/j.fertnstert.2015.07.1138.
7. Possover M. Fertil Steril. 2014 Mar;101(3):754-8. doi: 10.1016/j.fertnstert.2013.12.019.
Spare the nerves in deep infiltrative endometriosis surgery
The pelvic autonomic nerves are responsible for the neurogenic control of the rectum and bladder and for sexual arousal. Over the past 30 years, different nerve-sparing techniques have been recommended and adopted to minimize risk of urinary or rectal dysfunction and incontinence, as well as sexual dysfunction, in radical surgery for rectal and early cervical cancer without compromising surgical outcome.
As the treatment of deep infiltrative endometriosis has become more aggressive and radical, it is certainly feasible to consider nerve-sparing techniques at the time of dissection and endometriosis excision to minimize the known risk of urinary, rectal, and sexual dysfunction. Interestingly, because endometriosis generally follows an asymmetric distribution, effect on bladder function is not as problematic as it is in the case of cancer surgery.
Early innovators include Dr. Marc Possover from Switzerland and Dr. Marcello Ceccaroni from Italy. Both physicians are superior pelvic neuroanatomists. Both describe meticulous and extensive dissection of the nerves of the pelvis at the time of excision of deep infiltrative endometriosis. Unfortunately, their techniques would appear to be beyond the scope of even the most experienced excisional surgeons.
A simplified approach to nerve sparing at the time of excision of deep infiltrative endometriosis has been developed by our guest author, Dr. Nucelio Lemos, in collaboration with physicians at the University of Bologna and the University of Cambridge. By using the hypogastric nerves as the landmark, they have developed a more surgeon friendly and less radical approach to nerve sparing at the time of deep infiltrative endometriosis surgery.
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of both Dr. Lemos and his fellow in advanced gynecologic surgery, Dr. Meghan McGrattan, from Mount Sinai and Women’s College Hospital in Toronto. Dr. McGrattan drew the anatomic illustrations that accompany Dr. Lemos’ description of the new technique.
Dr. Lemos is associate professor in the department of obstetrics and gynecology at the University of Toronto. He specializes in pelvic pain, pelvic floor dysfunction, pelvic organ prolapse, endometriosis, and neuropelveology. Dr. Lemos is a founding member and second vice president of the International Society of Neuropelveology. In addition, Dr. Lemos started the Pelvic Functional Surgery and Neuropelveology Clinic in the department of obstetrics and gynecology of Mount Sinai and Women’s College Hospitals, Toronto.
It is a pleasure and honor to welcome Dr. Lemos and Dr. McGrattan to this addition of the Master Class in Gynecologic Surgery.
Dr. Miller is a professor of obstetrics and gynecology, department of clinical sciences, Rosalind Franklin University of Medicine and Science, North Chicago, Ill. He has no conflicts of interest to report.
The pelvic autonomic nerves are responsible for the neurogenic control of the rectum and bladder and for sexual arousal. Over the past 30 years, different nerve-sparing techniques have been recommended and adopted to minimize risk of urinary or rectal dysfunction and incontinence, as well as sexual dysfunction, in radical surgery for rectal and early cervical cancer without compromising surgical outcome.
As the treatment of deep infiltrative endometriosis has become more aggressive and radical, it is certainly feasible to consider nerve-sparing techniques at the time of dissection and endometriosis excision to minimize the known risk of urinary, rectal, and sexual dysfunction. Interestingly, because endometriosis generally follows an asymmetric distribution, effect on bladder function is not as problematic as it is in the case of cancer surgery.
Early innovators include Dr. Marc Possover from Switzerland and Dr. Marcello Ceccaroni from Italy. Both physicians are superior pelvic neuroanatomists. Both describe meticulous and extensive dissection of the nerves of the pelvis at the time of excision of deep infiltrative endometriosis. Unfortunately, their techniques would appear to be beyond the scope of even the most experienced excisional surgeons.
A simplified approach to nerve sparing at the time of excision of deep infiltrative endometriosis has been developed by our guest author, Dr. Nucelio Lemos, in collaboration with physicians at the University of Bologna and the University of Cambridge. By using the hypogastric nerves as the landmark, they have developed a more surgeon friendly and less radical approach to nerve sparing at the time of deep infiltrative endometriosis surgery.
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of both Dr. Lemos and his fellow in advanced gynecologic surgery, Dr. Meghan McGrattan, from Mount Sinai and Women’s College Hospital in Toronto. Dr. McGrattan drew the anatomic illustrations that accompany Dr. Lemos’ description of the new technique.
Dr. Lemos is associate professor in the department of obstetrics and gynecology at the University of Toronto. He specializes in pelvic pain, pelvic floor dysfunction, pelvic organ prolapse, endometriosis, and neuropelveology. Dr. Lemos is a founding member and second vice president of the International Society of Neuropelveology. In addition, Dr. Lemos started the Pelvic Functional Surgery and Neuropelveology Clinic in the department of obstetrics and gynecology of Mount Sinai and Women’s College Hospitals, Toronto.
It is a pleasure and honor to welcome Dr. Lemos and Dr. McGrattan to this addition of the Master Class in Gynecologic Surgery.
Dr. Miller is a professor of obstetrics and gynecology, department of clinical sciences, Rosalind Franklin University of Medicine and Science, North Chicago, Ill. He has no conflicts of interest to report.
The pelvic autonomic nerves are responsible for the neurogenic control of the rectum and bladder and for sexual arousal. Over the past 30 years, different nerve-sparing techniques have been recommended and adopted to minimize risk of urinary or rectal dysfunction and incontinence, as well as sexual dysfunction, in radical surgery for rectal and early cervical cancer without compromising surgical outcome.
As the treatment of deep infiltrative endometriosis has become more aggressive and radical, it is certainly feasible to consider nerve-sparing techniques at the time of dissection and endometriosis excision to minimize the known risk of urinary, rectal, and sexual dysfunction. Interestingly, because endometriosis generally follows an asymmetric distribution, effect on bladder function is not as problematic as it is in the case of cancer surgery.
Early innovators include Dr. Marc Possover from Switzerland and Dr. Marcello Ceccaroni from Italy. Both physicians are superior pelvic neuroanatomists. Both describe meticulous and extensive dissection of the nerves of the pelvis at the time of excision of deep infiltrative endometriosis. Unfortunately, their techniques would appear to be beyond the scope of even the most experienced excisional surgeons.
A simplified approach to nerve sparing at the time of excision of deep infiltrative endometriosis has been developed by our guest author, Dr. Nucelio Lemos, in collaboration with physicians at the University of Bologna and the University of Cambridge. By using the hypogastric nerves as the landmark, they have developed a more surgeon friendly and less radical approach to nerve sparing at the time of deep infiltrative endometriosis surgery.
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of both Dr. Lemos and his fellow in advanced gynecologic surgery, Dr. Meghan McGrattan, from Mount Sinai and Women’s College Hospital in Toronto. Dr. McGrattan drew the anatomic illustrations that accompany Dr. Lemos’ description of the new technique.
Dr. Lemos is associate professor in the department of obstetrics and gynecology at the University of Toronto. He specializes in pelvic pain, pelvic floor dysfunction, pelvic organ prolapse, endometriosis, and neuropelveology. Dr. Lemos is a founding member and second vice president of the International Society of Neuropelveology. In addition, Dr. Lemos started the Pelvic Functional Surgery and Neuropelveology Clinic in the department of obstetrics and gynecology of Mount Sinai and Women’s College Hospitals, Toronto.
It is a pleasure and honor to welcome Dr. Lemos and Dr. McGrattan to this addition of the Master Class in Gynecologic Surgery.
Dr. Miller is a professor of obstetrics and gynecology, department of clinical sciences, Rosalind Franklin University of Medicine and Science, North Chicago, Ill. He has no conflicts of interest to report.
Add AFib to noncardiac surgery risk evaluation: New support
Practice has gone back and forth on whether atrial fibrillation (AFib) should be considered in the preoperative cardiovascular risk (CV) evaluation of patients slated for noncardiac surgery, and the Revised Cardiac Risk Index (RCRI), currently widely used as an assessment tool, doesn’t include the arrhythmia.
But consideration of preexisting AFib along with the RCRI predicted 30-day mortality more sharply than the RCRI alone in an analysis of data covering several million patients slated for such procedures.
Indeed, AFib emerged as a significant, independent risk factor for a number of bad postoperative outcomes. Mortality within a month of the procedure climbed about 30% for patients with AFib before the noncardiac surgery. Their 30-day risks for stroke and for heart failure hospitalization went up similarly.
The addition of AFib to the RCRI significantly improved its ability to discriminate 30-day postoperative risk levels regardless of age, sex, and type of noncardiac surgery, Amgad Mentias, MD, Cleveland Clinic, told this news organization. And “it was able to correctly up-classify patients to high risk, if AFib was there, and it was able to down-classify some patients to lower risk if it wasn’t there.”
“I think [the findings] are convincing evidence that atrial fib should at least be part of the thought process for the surgical team and the medical team taking care of the patient,” said Dr. Mentias, who is senior author on the study published in the Journal of the American College of Cardiology, with lead author Sameer Prasada, MD, also of the Cleveland Clinic.
The results “call for incorporating AFib as a risk factor in perioperative risk scores for cardiovascular morbidity and mortality,” the published report states.
Supraventricular arrhythmias had been part of the Goldman Risk Index once widely used preoperatively to assess cardiac risk before practice adopted the RCRI in the past decade, observe Anne B. Curtis, MD, and Sai Krishna C. Korada, MD, University at Buffalo, New York, in an accompanying editorial.
The current findings “demonstrate improved prediction of adverse postsurgical outcomes” from supplementing the RCRI with AFib, they write. Given associations between preexisting AFib and serious cardiac events, “it is time to ‘re-revise’ the RCRI and acknowledge the importance of AFib in predicting adverse outcomes” after noncardiac surgery.
The new findings, however, aren’t all straightforward. In one result that remains a bit of a head-scratcher, postoperative risk of myocardial infarction (MI) in patients with preexisting AFib went in the opposite direction of risk for death and other CV outcomes, falling by almost 20%.
That is “hard to explain with the available data,” the report states, but “the use of anticoagulation, whether oral or parenteral (as a bridge therapy in the perioperative period), is a plausible explanation” given the frequent role of thrombosis in triggering MIs.
Consistent with such a mechanism, the group argues, the MI risk reduction was seen primarily among patients with AFib and a CHA2DS2-VASc score of 2 or higher – that is, those at highest risk for stroke and therefore most likely to be on oral anticoagulation. The MI risk reduction wasn’t seen in such patients with a CHA2DS2-VASc score of 0 or 1.
“I think that’s part of the explanation, that anticoagulation can reduce risk of MI. But it’s not the whole explanation,” Dr. Mentias said in an interview. If it were the sole mechanism, he said, then the same oral anticoagulation that protected against MI should have also cut the postoperative stroke risk. Yet that risk climbed 40% among patients with preexisting AFib.
The analysis started with 8.6 million Medicare patients with planned noncardiac surgery, seen from 2015 to 2019, of whom 16.4% had preexisting AFib. Propensity matching for demographics, urgency and type of surgery, CHA2DS2-VASc score, and RCRI index created two cohorts for comparison: 1.13 million patients with and 1.92 million without preexisting AFib.
Preexisting AFib was associated with a higher 30-day risk for death from any cause, the primary endpoint being 8.3% versus 5.8% for those without such AFib (P < .001), for an odds ratio of 1.31 (95% confidence interval, 1.30-1.32).
Corresponding 30-day ORs for other events, all significant at P < .001, were:
- 1.31 (95% CI, 1.30-1.33) for heart failure
- 1.40 (95% CI, 1.37-1.43) for stroke
- 1.59 (95% CI, 1.43-1.75) for systemic embolism
- 1.14 (95% CI, 1.13-1.16) for major bleeding
- 0.81 (95% CI, 0.79-0.82) for MI
Those with preexisting AFib also had longer hospitalizations at a median 5 days, compared with 4 days for those without such AFib (P < .001).
The study has the limitations of most any retrospective cohort analysis. Other limitations, the report notes, include lack of information on any antiarrhythmic meds given during hospitalization or type of AFib.
For example, AFib that is permanent – compared with paroxysmal or persistent – may be associated with more atrial fibrosis, greater atrial dilatation, “and probably higher pressures inside the heart,” Dr. Mentias observed.
“That’s not always the case, but that’s the notion. So presumably people with persistent or permanent atrial fib would have more advanced heart disease, and that could imply more risk. But we did not have that kind of data.”
Dr. Mentias and Dr. Prasada report no relevant financial relationships; disclosures for the other authors are in the report. Dr. Curtis discloses serving on advisory boards for Abbott, Janssen Pharmaceuticals, Sanofi, and Milestone Pharmaceuticals; receiving honoraria for speaking from Medtronic and Zoll; and serving on a data-monitoring board for Medtronic. Dr. Korada reports he has no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Practice has gone back and forth on whether atrial fibrillation (AFib) should be considered in the preoperative cardiovascular risk (CV) evaluation of patients slated for noncardiac surgery, and the Revised Cardiac Risk Index (RCRI), currently widely used as an assessment tool, doesn’t include the arrhythmia.
But consideration of preexisting AFib along with the RCRI predicted 30-day mortality more sharply than the RCRI alone in an analysis of data covering several million patients slated for such procedures.
Indeed, AFib emerged as a significant, independent risk factor for a number of bad postoperative outcomes. Mortality within a month of the procedure climbed about 30% for patients with AFib before the noncardiac surgery. Their 30-day risks for stroke and for heart failure hospitalization went up similarly.
The addition of AFib to the RCRI significantly improved its ability to discriminate 30-day postoperative risk levels regardless of age, sex, and type of noncardiac surgery, Amgad Mentias, MD, Cleveland Clinic, told this news organization. And “it was able to correctly up-classify patients to high risk, if AFib was there, and it was able to down-classify some patients to lower risk if it wasn’t there.”
“I think [the findings] are convincing evidence that atrial fib should at least be part of the thought process for the surgical team and the medical team taking care of the patient,” said Dr. Mentias, who is senior author on the study published in the Journal of the American College of Cardiology, with lead author Sameer Prasada, MD, also of the Cleveland Clinic.
The results “call for incorporating AFib as a risk factor in perioperative risk scores for cardiovascular morbidity and mortality,” the published report states.
Supraventricular arrhythmias had been part of the Goldman Risk Index once widely used preoperatively to assess cardiac risk before practice adopted the RCRI in the past decade, observe Anne B. Curtis, MD, and Sai Krishna C. Korada, MD, University at Buffalo, New York, in an accompanying editorial.
The current findings “demonstrate improved prediction of adverse postsurgical outcomes” from supplementing the RCRI with AFib, they write. Given associations between preexisting AFib and serious cardiac events, “it is time to ‘re-revise’ the RCRI and acknowledge the importance of AFib in predicting adverse outcomes” after noncardiac surgery.
The new findings, however, aren’t all straightforward. In one result that remains a bit of a head-scratcher, postoperative risk of myocardial infarction (MI) in patients with preexisting AFib went in the opposite direction of risk for death and other CV outcomes, falling by almost 20%.
That is “hard to explain with the available data,” the report states, but “the use of anticoagulation, whether oral or parenteral (as a bridge therapy in the perioperative period), is a plausible explanation” given the frequent role of thrombosis in triggering MIs.
Consistent with such a mechanism, the group argues, the MI risk reduction was seen primarily among patients with AFib and a CHA2DS2-VASc score of 2 or higher – that is, those at highest risk for stroke and therefore most likely to be on oral anticoagulation. The MI risk reduction wasn’t seen in such patients with a CHA2DS2-VASc score of 0 or 1.
“I think that’s part of the explanation, that anticoagulation can reduce risk of MI. But it’s not the whole explanation,” Dr. Mentias said in an interview. If it were the sole mechanism, he said, then the same oral anticoagulation that protected against MI should have also cut the postoperative stroke risk. Yet that risk climbed 40% among patients with preexisting AFib.
The analysis started with 8.6 million Medicare patients with planned noncardiac surgery, seen from 2015 to 2019, of whom 16.4% had preexisting AFib. Propensity matching for demographics, urgency and type of surgery, CHA2DS2-VASc score, and RCRI index created two cohorts for comparison: 1.13 million patients with and 1.92 million without preexisting AFib.
Preexisting AFib was associated with a higher 30-day risk for death from any cause, the primary endpoint being 8.3% versus 5.8% for those without such AFib (P < .001), for an odds ratio of 1.31 (95% confidence interval, 1.30-1.32).
Corresponding 30-day ORs for other events, all significant at P < .001, were:
- 1.31 (95% CI, 1.30-1.33) for heart failure
- 1.40 (95% CI, 1.37-1.43) for stroke
- 1.59 (95% CI, 1.43-1.75) for systemic embolism
- 1.14 (95% CI, 1.13-1.16) for major bleeding
- 0.81 (95% CI, 0.79-0.82) for MI
Those with preexisting AFib also had longer hospitalizations at a median 5 days, compared with 4 days for those without such AFib (P < .001).
The study has the limitations of most any retrospective cohort analysis. Other limitations, the report notes, include lack of information on any antiarrhythmic meds given during hospitalization or type of AFib.
For example, AFib that is permanent – compared with paroxysmal or persistent – may be associated with more atrial fibrosis, greater atrial dilatation, “and probably higher pressures inside the heart,” Dr. Mentias observed.
“That’s not always the case, but that’s the notion. So presumably people with persistent or permanent atrial fib would have more advanced heart disease, and that could imply more risk. But we did not have that kind of data.”
Dr. Mentias and Dr. Prasada report no relevant financial relationships; disclosures for the other authors are in the report. Dr. Curtis discloses serving on advisory boards for Abbott, Janssen Pharmaceuticals, Sanofi, and Milestone Pharmaceuticals; receiving honoraria for speaking from Medtronic and Zoll; and serving on a data-monitoring board for Medtronic. Dr. Korada reports he has no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Practice has gone back and forth on whether atrial fibrillation (AFib) should be considered in the preoperative cardiovascular risk (CV) evaluation of patients slated for noncardiac surgery, and the Revised Cardiac Risk Index (RCRI), currently widely used as an assessment tool, doesn’t include the arrhythmia.
But consideration of preexisting AFib along with the RCRI predicted 30-day mortality more sharply than the RCRI alone in an analysis of data covering several million patients slated for such procedures.
Indeed, AFib emerged as a significant, independent risk factor for a number of bad postoperative outcomes. Mortality within a month of the procedure climbed about 30% for patients with AFib before the noncardiac surgery. Their 30-day risks for stroke and for heart failure hospitalization went up similarly.
The addition of AFib to the RCRI significantly improved its ability to discriminate 30-day postoperative risk levels regardless of age, sex, and type of noncardiac surgery, Amgad Mentias, MD, Cleveland Clinic, told this news organization. And “it was able to correctly up-classify patients to high risk, if AFib was there, and it was able to down-classify some patients to lower risk if it wasn’t there.”
“I think [the findings] are convincing evidence that atrial fib should at least be part of the thought process for the surgical team and the medical team taking care of the patient,” said Dr. Mentias, who is senior author on the study published in the Journal of the American College of Cardiology, with lead author Sameer Prasada, MD, also of the Cleveland Clinic.
The results “call for incorporating AFib as a risk factor in perioperative risk scores for cardiovascular morbidity and mortality,” the published report states.
Supraventricular arrhythmias had been part of the Goldman Risk Index once widely used preoperatively to assess cardiac risk before practice adopted the RCRI in the past decade, observe Anne B. Curtis, MD, and Sai Krishna C. Korada, MD, University at Buffalo, New York, in an accompanying editorial.
The current findings “demonstrate improved prediction of adverse postsurgical outcomes” from supplementing the RCRI with AFib, they write. Given associations between preexisting AFib and serious cardiac events, “it is time to ‘re-revise’ the RCRI and acknowledge the importance of AFib in predicting adverse outcomes” after noncardiac surgery.
The new findings, however, aren’t all straightforward. In one result that remains a bit of a head-scratcher, postoperative risk of myocardial infarction (MI) in patients with preexisting AFib went in the opposite direction of risk for death and other CV outcomes, falling by almost 20%.
That is “hard to explain with the available data,” the report states, but “the use of anticoagulation, whether oral or parenteral (as a bridge therapy in the perioperative period), is a plausible explanation” given the frequent role of thrombosis in triggering MIs.
Consistent with such a mechanism, the group argues, the MI risk reduction was seen primarily among patients with AFib and a CHA2DS2-VASc score of 2 or higher – that is, those at highest risk for stroke and therefore most likely to be on oral anticoagulation. The MI risk reduction wasn’t seen in such patients with a CHA2DS2-VASc score of 0 or 1.
“I think that’s part of the explanation, that anticoagulation can reduce risk of MI. But it’s not the whole explanation,” Dr. Mentias said in an interview. If it were the sole mechanism, he said, then the same oral anticoagulation that protected against MI should have also cut the postoperative stroke risk. Yet that risk climbed 40% among patients with preexisting AFib.
The analysis started with 8.6 million Medicare patients with planned noncardiac surgery, seen from 2015 to 2019, of whom 16.4% had preexisting AFib. Propensity matching for demographics, urgency and type of surgery, CHA2DS2-VASc score, and RCRI index created two cohorts for comparison: 1.13 million patients with and 1.92 million without preexisting AFib.
Preexisting AFib was associated with a higher 30-day risk for death from any cause, the primary endpoint being 8.3% versus 5.8% for those without such AFib (P < .001), for an odds ratio of 1.31 (95% confidence interval, 1.30-1.32).
Corresponding 30-day ORs for other events, all significant at P < .001, were:
- 1.31 (95% CI, 1.30-1.33) for heart failure
- 1.40 (95% CI, 1.37-1.43) for stroke
- 1.59 (95% CI, 1.43-1.75) for systemic embolism
- 1.14 (95% CI, 1.13-1.16) for major bleeding
- 0.81 (95% CI, 0.79-0.82) for MI
Those with preexisting AFib also had longer hospitalizations at a median 5 days, compared with 4 days for those without such AFib (P < .001).
The study has the limitations of most any retrospective cohort analysis. Other limitations, the report notes, include lack of information on any antiarrhythmic meds given during hospitalization or type of AFib.
For example, AFib that is permanent – compared with paroxysmal or persistent – may be associated with more atrial fibrosis, greater atrial dilatation, “and probably higher pressures inside the heart,” Dr. Mentias observed.
“That’s not always the case, but that’s the notion. So presumably people with persistent or permanent atrial fib would have more advanced heart disease, and that could imply more risk. But we did not have that kind of data.”
Dr. Mentias and Dr. Prasada report no relevant financial relationships; disclosures for the other authors are in the report. Dr. Curtis discloses serving on advisory boards for Abbott, Janssen Pharmaceuticals, Sanofi, and Milestone Pharmaceuticals; receiving honoraria for speaking from Medtronic and Zoll; and serving on a data-monitoring board for Medtronic. Dr. Korada reports he has no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Surgeons may underestimate recovery from incontinence operation
Surgeons may significantly underestimate how long it will take women to return to normal activities following sling surgery to correct stress urinary incontinence, a new study has found.
The researchers found that just over 40% of women reported returning to work and other normal activities within 2 weeks of having undergone midurethral sling procedures – a much less optimistic forecast than what surgeons typically provide in these cases.
“This is in contrast to a published survey of physicians that showed the majority of surgeons suggested patients return to work within 2 weeks,” Rui Wang, MD, a fellow in female pelvic medicine and reconstructive surgery at Hartford Hospital, Conn., said in an interview.
Dr. Wang referred to a published survey of 135 physicians that was conducted at a 2018 meeting of the Society of Gynecologic Surgeons. In that survey, 88% of respondents indicated that patients could return to sedentary work within 2 weeks after undergoing sling surgery. Most recommended longer waits before returning to manual labor.
The authors of the survey noted a lack of consensus guidelines and wide variations in recommendations for postoperative restrictions after minimally invasive gynecologic and pelvic reconstructive surgery, which the researchers called a “largely unstudied field.”
Dr. Wang said, “The majority of patients may need more than 2 weeks to return to work and normal activities even following minimally invasive outpatient surgeries such as midurethral sling.”
Dr. Wang is scheduled to present the findings June 18 at the annual meeting of the American Urogynecologic Society.
For the new study, Dr. Wang and a colleague examined how patients answered questions about their activity levels during recovery after sling procedures. The patients were enrolled in the Trial of Mid-Urethral Slings (TOMUS), a randomized controlled trial that compared two types of midurethral slings used for the treatment of stress urinary incontinence: the retropubic midurethral mesh sling and the transobturator midurethral sling. Results of the trial were published in 2010.
Of 597 women enrolled in TOMUS, 441 were included in the new analysis. Patients who underwent another surgery at the same time as their sling procedure were excluded from the analysis.
As part of the trial, patients were asked how many paid workdays they took off after surgery; whether they had returned to full normal activities of daily life, including work, if applicable; and how much time it took for them to fully return to normal activities of daily life, including work.
The researchers found that 183 (41.5%) returned to normal activities within 2 weeks of the procedure. Among those patients, the median recovery time was 6 days. Within 6 weeks of surgery, 308 (70%) had returned to normal activities, including work. After 6 months, 407 (98.3%) were back to their normal routines, the study showed.
Multivariate regression analysis yielded no factor that predicted the timing of returning to normal activity and work. Nor did the researchers observe any significant differences in failure rates and adverse outcomes between patients who returned within 2 weeks or after 2 weeks.
Essential information for patient planning
Dr. Wang said she expects that the findings will help physicians in counseling patients and setting postoperative recovery expectations. “For patients planning elective surgery, one of the most important quality-of-life issues is the time they will need to take off from work and recover,” she said.
Although most patients needed more than 2 weeks to recover, the median paid time off after surgery was 4 days. “Many patients would have taken unpaid days off or used vacation time for their postoperative recovery,” Dr. Wang said.
She added that more research is needed to explore whether that discrepancy disproportionately affects women in jobs with fewer employee benefits. “We did not find that age, race/ethnicity, marital status, occupation, symptom severity, and duration of surgery significantly predicted the timing of return to work or normal activities,” she said. “But are there other factors, such as geographic location, insurance status, [or] income, that may affect this timing?”
Sarah Boyd, MD, an assistant professor in the Division of Female Pelvic Medicine and Reconstructive Surgery at Penn State College of Medicine, Hershey, said the new findings add concrete information that can guide patients in planning their recovery.
“Previously, surgeons could only provide general estimates to these patients based on the experience of their patients,” Dr. Boyd, who was not involved in the study, told this news organization.
The analysis has not been published in a peer-reviewed journal, and Dr. Boyd said that the findings may not pertain to all individuals who undergo midurethral sling procedures, such as people who have had prior surgery for incontinence or those who undergo surgery for other pelvic floor disorders at the same time.
Dr. Wang and Dr. Boyd reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Surgeons may significantly underestimate how long it will take women to return to normal activities following sling surgery to correct stress urinary incontinence, a new study has found.
The researchers found that just over 40% of women reported returning to work and other normal activities within 2 weeks of having undergone midurethral sling procedures – a much less optimistic forecast than what surgeons typically provide in these cases.
“This is in contrast to a published survey of physicians that showed the majority of surgeons suggested patients return to work within 2 weeks,” Rui Wang, MD, a fellow in female pelvic medicine and reconstructive surgery at Hartford Hospital, Conn., said in an interview.
Dr. Wang referred to a published survey of 135 physicians that was conducted at a 2018 meeting of the Society of Gynecologic Surgeons. In that survey, 88% of respondents indicated that patients could return to sedentary work within 2 weeks after undergoing sling surgery. Most recommended longer waits before returning to manual labor.
The authors of the survey noted a lack of consensus guidelines and wide variations in recommendations for postoperative restrictions after minimally invasive gynecologic and pelvic reconstructive surgery, which the researchers called a “largely unstudied field.”
Dr. Wang said, “The majority of patients may need more than 2 weeks to return to work and normal activities even following minimally invasive outpatient surgeries such as midurethral sling.”
Dr. Wang is scheduled to present the findings June 18 at the annual meeting of the American Urogynecologic Society.
For the new study, Dr. Wang and a colleague examined how patients answered questions about their activity levels during recovery after sling procedures. The patients were enrolled in the Trial of Mid-Urethral Slings (TOMUS), a randomized controlled trial that compared two types of midurethral slings used for the treatment of stress urinary incontinence: the retropubic midurethral mesh sling and the transobturator midurethral sling. Results of the trial were published in 2010.
Of 597 women enrolled in TOMUS, 441 were included in the new analysis. Patients who underwent another surgery at the same time as their sling procedure were excluded from the analysis.
As part of the trial, patients were asked how many paid workdays they took off after surgery; whether they had returned to full normal activities of daily life, including work, if applicable; and how much time it took for them to fully return to normal activities of daily life, including work.
The researchers found that 183 (41.5%) returned to normal activities within 2 weeks of the procedure. Among those patients, the median recovery time was 6 days. Within 6 weeks of surgery, 308 (70%) had returned to normal activities, including work. After 6 months, 407 (98.3%) were back to their normal routines, the study showed.
Multivariate regression analysis yielded no factor that predicted the timing of returning to normal activity and work. Nor did the researchers observe any significant differences in failure rates and adverse outcomes between patients who returned within 2 weeks or after 2 weeks.
Essential information for patient planning
Dr. Wang said she expects that the findings will help physicians in counseling patients and setting postoperative recovery expectations. “For patients planning elective surgery, one of the most important quality-of-life issues is the time they will need to take off from work and recover,” she said.
Although most patients needed more than 2 weeks to recover, the median paid time off after surgery was 4 days. “Many patients would have taken unpaid days off or used vacation time for their postoperative recovery,” Dr. Wang said.
She added that more research is needed to explore whether that discrepancy disproportionately affects women in jobs with fewer employee benefits. “We did not find that age, race/ethnicity, marital status, occupation, symptom severity, and duration of surgery significantly predicted the timing of return to work or normal activities,” she said. “But are there other factors, such as geographic location, insurance status, [or] income, that may affect this timing?”
Sarah Boyd, MD, an assistant professor in the Division of Female Pelvic Medicine and Reconstructive Surgery at Penn State College of Medicine, Hershey, said the new findings add concrete information that can guide patients in planning their recovery.
“Previously, surgeons could only provide general estimates to these patients based on the experience of their patients,” Dr. Boyd, who was not involved in the study, told this news organization.
The analysis has not been published in a peer-reviewed journal, and Dr. Boyd said that the findings may not pertain to all individuals who undergo midurethral sling procedures, such as people who have had prior surgery for incontinence or those who undergo surgery for other pelvic floor disorders at the same time.
Dr. Wang and Dr. Boyd reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Surgeons may significantly underestimate how long it will take women to return to normal activities following sling surgery to correct stress urinary incontinence, a new study has found.
The researchers found that just over 40% of women reported returning to work and other normal activities within 2 weeks of having undergone midurethral sling procedures – a much less optimistic forecast than what surgeons typically provide in these cases.
“This is in contrast to a published survey of physicians that showed the majority of surgeons suggested patients return to work within 2 weeks,” Rui Wang, MD, a fellow in female pelvic medicine and reconstructive surgery at Hartford Hospital, Conn., said in an interview.
Dr. Wang referred to a published survey of 135 physicians that was conducted at a 2018 meeting of the Society of Gynecologic Surgeons. In that survey, 88% of respondents indicated that patients could return to sedentary work within 2 weeks after undergoing sling surgery. Most recommended longer waits before returning to manual labor.
The authors of the survey noted a lack of consensus guidelines and wide variations in recommendations for postoperative restrictions after minimally invasive gynecologic and pelvic reconstructive surgery, which the researchers called a “largely unstudied field.”
Dr. Wang said, “The majority of patients may need more than 2 weeks to return to work and normal activities even following minimally invasive outpatient surgeries such as midurethral sling.”
Dr. Wang is scheduled to present the findings June 18 at the annual meeting of the American Urogynecologic Society.
For the new study, Dr. Wang and a colleague examined how patients answered questions about their activity levels during recovery after sling procedures. The patients were enrolled in the Trial of Mid-Urethral Slings (TOMUS), a randomized controlled trial that compared two types of midurethral slings used for the treatment of stress urinary incontinence: the retropubic midurethral mesh sling and the transobturator midurethral sling. Results of the trial were published in 2010.
Of 597 women enrolled in TOMUS, 441 were included in the new analysis. Patients who underwent another surgery at the same time as their sling procedure were excluded from the analysis.
As part of the trial, patients were asked how many paid workdays they took off after surgery; whether they had returned to full normal activities of daily life, including work, if applicable; and how much time it took for them to fully return to normal activities of daily life, including work.
The researchers found that 183 (41.5%) returned to normal activities within 2 weeks of the procedure. Among those patients, the median recovery time was 6 days. Within 6 weeks of surgery, 308 (70%) had returned to normal activities, including work. After 6 months, 407 (98.3%) were back to their normal routines, the study showed.
Multivariate regression analysis yielded no factor that predicted the timing of returning to normal activity and work. Nor did the researchers observe any significant differences in failure rates and adverse outcomes between patients who returned within 2 weeks or after 2 weeks.
Essential information for patient planning
Dr. Wang said she expects that the findings will help physicians in counseling patients and setting postoperative recovery expectations. “For patients planning elective surgery, one of the most important quality-of-life issues is the time they will need to take off from work and recover,” she said.
Although most patients needed more than 2 weeks to recover, the median paid time off after surgery was 4 days. “Many patients would have taken unpaid days off or used vacation time for their postoperative recovery,” Dr. Wang said.
She added that more research is needed to explore whether that discrepancy disproportionately affects women in jobs with fewer employee benefits. “We did not find that age, race/ethnicity, marital status, occupation, symptom severity, and duration of surgery significantly predicted the timing of return to work or normal activities,” she said. “But are there other factors, such as geographic location, insurance status, [or] income, that may affect this timing?”
Sarah Boyd, MD, an assistant professor in the Division of Female Pelvic Medicine and Reconstructive Surgery at Penn State College of Medicine, Hershey, said the new findings add concrete information that can guide patients in planning their recovery.
“Previously, surgeons could only provide general estimates to these patients based on the experience of their patients,” Dr. Boyd, who was not involved in the study, told this news organization.
The analysis has not been published in a peer-reviewed journal, and Dr. Boyd said that the findings may not pertain to all individuals who undergo midurethral sling procedures, such as people who have had prior surgery for incontinence or those who undergo surgery for other pelvic floor disorders at the same time.
Dr. Wang and Dr. Boyd reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AUGS 2022
Women are not being warned that anesthetic may reduce birth pill efficacy
The effectiveness of hormonal contraceptives, including the pill and mini-pill, may be compromised by sugammadex, a drug widely used in anesthesia for reversing neuromuscular blockade induced by rocuronium or vecuronium.
Yet women are not routinely informed that the drug may make their contraception less effective, delegates at Euroanaesthesia, the annual meeting of the European Society of Anaesthesiology and Intensive Care in Milan were told.
New research presented at the meeting supports the authors’ experience that “robust methods for identifying at-risk patients and informing them of the associated risk of contraceptive failures is not common practice across anesthetic departments within the United Kingdom, and likely further afield.”
This is according to a survey of almost 150 anesthetic professionals, including consultants, junior doctors, and physician assistants, working at University College London Hospitals NHS Foundation Trust.
Dr. Neha Passi, Dr. Matt Oliver, and colleagues at the trust’s department of anesthesiology sent out a seven-question survey to their 150 colleagues and received 82 responses, 94% of which claimed awareness of the risk of contraceptive failure with sugammadex. However, 70% of the respondents admitted that they do not routinely discuss this with patients who have received the drug.
Risk with all forms of hormonal contraceptive
Yet current guidance is to inform women of child-bearing age that they have received the drug and, because of increased risk of contraceptive failure, advise those taking oral hormonal contraceptives to follow the missed pill advice in the leaflet that comes with their contraceptives. It also counsels that clinicians should advise women using other types of hormonal contraceptive to use an additional nonhormonal means of contraception for 7 days.
The study authors also carried out a retrospective audit of sugammadex use in the trust and reported that during the 6 weeks covered by the audit, 234 patients were administered sugammadex of whom 65 (28%) were women of childbearing age. Of these, 17 had a medical history that meant they weren’t at risk of pregnancy, but the other 48 should have received advice on the risks of contraceptive failure – however there was no record in the medical notes of such advice having been given for any of the at-risk 48 women.
While sugammadex is the only anesthetic drug known to have this effect, it is recognized to interact with progesterone and so may reduce the effectiveness of hormonal contraceptives, including the progesterone-only pill, combined pill, vaginal rings, implants, and intrauterine devices.
Dr. Passi said: “It is concerning that we are so seldom informing patients of the risk of contraceptive failure following sugammadex use.
“Use of sugammadex is expected to rise as it becomes cheaper in the future, and ensuring that women receiving this medicine are aware it may increase their risk of unwanted pregnancy must be a priority.”
She added: “It is important to note, however, that most patients receiving an anesthetic do not need a muscle relaxant and that sugammadex is one of several drugs available to reverse muscle relaxation.”
Dr. Oliver said: “We only studied one hospital trust but we expect the results to be similar in elsewhere in the U.K.”
In response to their findings, the study’s authors have created patient information leaflets and letters and programmed the trust’s electronic patient record system to identify “at-risk” patients and deliver electronic prompts to the anesthetists caring for them in the perioperative period.
A version of this article first appeared on Medscape UK.
The effectiveness of hormonal contraceptives, including the pill and mini-pill, may be compromised by sugammadex, a drug widely used in anesthesia for reversing neuromuscular blockade induced by rocuronium or vecuronium.
Yet women are not routinely informed that the drug may make their contraception less effective, delegates at Euroanaesthesia, the annual meeting of the European Society of Anaesthesiology and Intensive Care in Milan were told.
New research presented at the meeting supports the authors’ experience that “robust methods for identifying at-risk patients and informing them of the associated risk of contraceptive failures is not common practice across anesthetic departments within the United Kingdom, and likely further afield.”
This is according to a survey of almost 150 anesthetic professionals, including consultants, junior doctors, and physician assistants, working at University College London Hospitals NHS Foundation Trust.
Dr. Neha Passi, Dr. Matt Oliver, and colleagues at the trust’s department of anesthesiology sent out a seven-question survey to their 150 colleagues and received 82 responses, 94% of which claimed awareness of the risk of contraceptive failure with sugammadex. However, 70% of the respondents admitted that they do not routinely discuss this with patients who have received the drug.
Risk with all forms of hormonal contraceptive
Yet current guidance is to inform women of child-bearing age that they have received the drug and, because of increased risk of contraceptive failure, advise those taking oral hormonal contraceptives to follow the missed pill advice in the leaflet that comes with their contraceptives. It also counsels that clinicians should advise women using other types of hormonal contraceptive to use an additional nonhormonal means of contraception for 7 days.
The study authors also carried out a retrospective audit of sugammadex use in the trust and reported that during the 6 weeks covered by the audit, 234 patients were administered sugammadex of whom 65 (28%) were women of childbearing age. Of these, 17 had a medical history that meant they weren’t at risk of pregnancy, but the other 48 should have received advice on the risks of contraceptive failure – however there was no record in the medical notes of such advice having been given for any of the at-risk 48 women.
While sugammadex is the only anesthetic drug known to have this effect, it is recognized to interact with progesterone and so may reduce the effectiveness of hormonal contraceptives, including the progesterone-only pill, combined pill, vaginal rings, implants, and intrauterine devices.
Dr. Passi said: “It is concerning that we are so seldom informing patients of the risk of contraceptive failure following sugammadex use.
“Use of sugammadex is expected to rise as it becomes cheaper in the future, and ensuring that women receiving this medicine are aware it may increase their risk of unwanted pregnancy must be a priority.”
She added: “It is important to note, however, that most patients receiving an anesthetic do not need a muscle relaxant and that sugammadex is one of several drugs available to reverse muscle relaxation.”
Dr. Oliver said: “We only studied one hospital trust but we expect the results to be similar in elsewhere in the U.K.”
In response to their findings, the study’s authors have created patient information leaflets and letters and programmed the trust’s electronic patient record system to identify “at-risk” patients and deliver electronic prompts to the anesthetists caring for them in the perioperative period.
A version of this article first appeared on Medscape UK.
The effectiveness of hormonal contraceptives, including the pill and mini-pill, may be compromised by sugammadex, a drug widely used in anesthesia for reversing neuromuscular blockade induced by rocuronium or vecuronium.
Yet women are not routinely informed that the drug may make their contraception less effective, delegates at Euroanaesthesia, the annual meeting of the European Society of Anaesthesiology and Intensive Care in Milan were told.
New research presented at the meeting supports the authors’ experience that “robust methods for identifying at-risk patients and informing them of the associated risk of contraceptive failures is not common practice across anesthetic departments within the United Kingdom, and likely further afield.”
This is according to a survey of almost 150 anesthetic professionals, including consultants, junior doctors, and physician assistants, working at University College London Hospitals NHS Foundation Trust.
Dr. Neha Passi, Dr. Matt Oliver, and colleagues at the trust’s department of anesthesiology sent out a seven-question survey to their 150 colleagues and received 82 responses, 94% of which claimed awareness of the risk of contraceptive failure with sugammadex. However, 70% of the respondents admitted that they do not routinely discuss this with patients who have received the drug.
Risk with all forms of hormonal contraceptive
Yet current guidance is to inform women of child-bearing age that they have received the drug and, because of increased risk of contraceptive failure, advise those taking oral hormonal contraceptives to follow the missed pill advice in the leaflet that comes with their contraceptives. It also counsels that clinicians should advise women using other types of hormonal contraceptive to use an additional nonhormonal means of contraception for 7 days.
The study authors also carried out a retrospective audit of sugammadex use in the trust and reported that during the 6 weeks covered by the audit, 234 patients were administered sugammadex of whom 65 (28%) were women of childbearing age. Of these, 17 had a medical history that meant they weren’t at risk of pregnancy, but the other 48 should have received advice on the risks of contraceptive failure – however there was no record in the medical notes of such advice having been given for any of the at-risk 48 women.
While sugammadex is the only anesthetic drug known to have this effect, it is recognized to interact with progesterone and so may reduce the effectiveness of hormonal contraceptives, including the progesterone-only pill, combined pill, vaginal rings, implants, and intrauterine devices.
Dr. Passi said: “It is concerning that we are so seldom informing patients of the risk of contraceptive failure following sugammadex use.
“Use of sugammadex is expected to rise as it becomes cheaper in the future, and ensuring that women receiving this medicine are aware it may increase their risk of unwanted pregnancy must be a priority.”
She added: “It is important to note, however, that most patients receiving an anesthetic do not need a muscle relaxant and that sugammadex is one of several drugs available to reverse muscle relaxation.”
Dr. Oliver said: “We only studied one hospital trust but we expect the results to be similar in elsewhere in the U.K.”
In response to their findings, the study’s authors have created patient information leaflets and letters and programmed the trust’s electronic patient record system to identify “at-risk” patients and deliver electronic prompts to the anesthetists caring for them in the perioperative period.
A version of this article first appeared on Medscape UK.
FROM EUROANAESTHESIA
Müllerian anomalies – old problem, new approach and classification
The American Society for Reproductive Medicine’s classification system for müllerian anomalies was the standard until the revision in 2021 by ASRM, which updated and expanded the classification presenting nine classes and imaging criteria: müllerian agenesis, cervical agenesis, unicornuate, uterus didelphys, bicornuate, septate, longitudinal vaginal septum, transverse vaginal septum, and complex anomalies. This month’s article addresses müllerian anomalies from embryology to treatment options.
The early embryo has the capability of developing a wolffian (internal male) or müllerian (internal female) system. Unless anti-müllerian hormone (formerly müllerian-inhibiting substance) is produced, the embryo develops a female reproductive system beginning with two lateral uterine anlagen that fuse in the midline and canalize. Müllerian anomalies occur because of accidents during fusion and canalization (see Table).
The incidence of müllerian anomalies is difficult to discern, given the potential for a normal reproductive outcome precluding an evaluation and based on the population studied. Müllerian anomalies are found in approximately 4.3% of fertile women, 3.5%-8% of infertile patients, 12.3%-13% of those with recurrent pregnancy losses, and 24.5% of patients with miscarriage and infertility. Of the müllerian anomalies, the most common is septate (35%), followed by bicornuate (26%), arcuate (18%), unicornuate (10%), didelphys (8%), and agenesis (3%) (Hum Reprod Update. 2001;7[2]:161; Hum Reprod Update. 2011;17[6]:761-71).
In 20%-30% of patients with müllerian anomalies, particularly in women with a unicornuate uterus, renal anomalies exist that are typically ipsilateral to the absent or rudimentary contralateral uterine horn (J Pediatr Adolesc Gynecol. 2021;34[2]:154-60). As there is no definitive evidence to suggest an association between a septate uterus and renal anomalies, the renal system evaluation can be deferred in this population (Fertil Steril. 2021 Nov;116[5]:1238-52).
Diagnosis
2-D ultrasound can be a screen for müllerian anomalies and genitourinary anatomic variants. The diagnostic accuracy of 3-D ultrasound with müllerian anomalies is reported to be 97.6% with sensitivity and specificity of 98.3% and 99.4%, respectively (Hum. Reprod. 2016;31[1]:2-7). As a result, office 3-D has essentially replaced MRI in the diagnosis of müllerian anomalies (Ultrasound Obstet Gynecol. 2015 Nov;46[5]:616-22), with one exception because of the avoidance of a transvaginal probe in the non–sexually active adult and younger adolescent/child. MRI is reserved for diagnosing complex müllerian anomalies or if there is a diagnostic challenge.
Criteria to diagnose müllerian anomalies by radiology begins with the “reference line,” i.e., a line joining both tubal ostia (interostial line). A septate uterus is diagnosed if the distance from the interostial line to the cephalad endometrium is more than 1 cm, otherwise it is considered normal or arcuate based on its appearance. An arcuate uterus has not been associated with impaired reproduction and can be viewed as a normal variant. Alternatively, a bicornuate uterus is diagnosed when the external fundal indentation is more than 1 cm (Fertil Steril. 2021 Nov;116[5]:1238-52).
Clinical course
Women with müllerian anomalies may experience pelvic pain and prolonged and/or abnormal bleeding at the time of menarche. While the ability to conceive may not be impaired from müllerian anomalies with the possible exception of the septate uterus, the pregnancy course can be affected, i.e., recurrent pregnancy loss, preterm birth, perinatal mortality, and malpresentation in labor (Reprod Biomed Online. 2014;29[6]:665). In women with septate, bicornuate, and uterine didelphys, fetal growth restriction appears to be increased. Spontaneous abortion rates of 32% and preterm birth rates of 28% have been reported in patients with uterus didelphys (Obstet Gynecol. 1990;75[6]:906).
Special consideration of the unicornuate is given because of the potential for a rudimentary horn that may communicate with the main uterine cavity and/or have functional endometrium which places the woman at risk of an ectopic pregnancy in the smaller horn. Patients with a unicornuate uterus are at higher risk for preterm labor and breech presentation. An obstructed (noncommunicating) functional rudimentary horn is a risk for endometriosis with cyclic pain because of outflow tract obstruction and an ectopic pregnancy prompting consideration for hemihysterectomy based on symptoms.
The septate uterus – old dogma revisited
The incidence of uterine septa is approximately 1-15 per 1,000. As the most common müllerian anomaly, the septate uterus has traditionally been associated with an increased risk for spontaneous abortion (21%-44%) and preterm birth (12%-33%). The live birth rate ranges from 50% to 72% (Hum Reprod Update. 2001;7[2]:161-74). A uterine septum is believed to develop as a result of failure of resorption of the tissue connecting the two paramesonephric (müllerian) ducts prior to the 20th embryonic week.
Incising the uterine septum (metroplasty) dates back to 1884 when Ruge described a blind transcervical metroplasty in a woman with two previous miscarriages who, postoperatively, delivered a healthy baby. In the early 1900s, Tompkins reported an abdominal metroplasty (Fertil Stertil. 2021;115:1140-2). The decision to proceed with metroplasty is based on only established observational studies (Fertil Steril. 2016;106:530-40). Until recently, the majority of studies suggested that metroplasty is associated with decreased spontaneous abortion rates and improved obstetrical outcomes. A retrospective case series of 361 patients with a septate uterus who had primary infertility of >2 years’ duration, a history of 1-2 spontaneous abortions, or recurrent pregnancy loss suggested a significant improvement in the live birth rate and reduction in miscarriage (Arch Gynecol Obstet. 2003;268:289-92). A meta-analysis found that the overall pregnancy rate after septum incision was 67.8% and the live-birth rate was 53.5% (J Minim Invas Gynecol. 2013;20:22-42).
Recently, two multinational studies question the prevailing dogma (Fertil Steril. 2021 Sep;116[3]:693-4). Both studies could not demonstrate any increase in live birth rate, reduction in preterm birth, or in pregnancy loss after metroplasty. A significant limitation was the lack of a uniform consensus on the definition of the septate uterus and allowing the discretion of the physician to diagnosis a septum (Hum Reprod. 2020;35:1578-88; Hum Reprod. 2021;36:1260-7).
Hysteroscopic metroplasty is not without complications. Uterine rupture during pregnancy or delivery, while rare, may be linked to significant entry into the myometrium and/or overzealous cauterization and perforation, which emphasizes the importance of appropriate techniques.
Conclusion
A diagnosis of müllerian anomalies justifies a comprehensive consultation with the patient given the risk of pregnancy complications. Management of the septate uterus has become controversial. In a patient with infertility, prior pregnancy loss, or poor obstetrical outcome, it is reasonable to consider metroplasty; otherwise, expectant management is an option.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando. Email him at [email protected].
The American Society for Reproductive Medicine’s classification system for müllerian anomalies was the standard until the revision in 2021 by ASRM, which updated and expanded the classification presenting nine classes and imaging criteria: müllerian agenesis, cervical agenesis, unicornuate, uterus didelphys, bicornuate, septate, longitudinal vaginal septum, transverse vaginal septum, and complex anomalies. This month’s article addresses müllerian anomalies from embryology to treatment options.
The early embryo has the capability of developing a wolffian (internal male) or müllerian (internal female) system. Unless anti-müllerian hormone (formerly müllerian-inhibiting substance) is produced, the embryo develops a female reproductive system beginning with two lateral uterine anlagen that fuse in the midline and canalize. Müllerian anomalies occur because of accidents during fusion and canalization (see Table).
The incidence of müllerian anomalies is difficult to discern, given the potential for a normal reproductive outcome precluding an evaluation and based on the population studied. Müllerian anomalies are found in approximately 4.3% of fertile women, 3.5%-8% of infertile patients, 12.3%-13% of those with recurrent pregnancy losses, and 24.5% of patients with miscarriage and infertility. Of the müllerian anomalies, the most common is septate (35%), followed by bicornuate (26%), arcuate (18%), unicornuate (10%), didelphys (8%), and agenesis (3%) (Hum Reprod Update. 2001;7[2]:161; Hum Reprod Update. 2011;17[6]:761-71).
In 20%-30% of patients with müllerian anomalies, particularly in women with a unicornuate uterus, renal anomalies exist that are typically ipsilateral to the absent or rudimentary contralateral uterine horn (J Pediatr Adolesc Gynecol. 2021;34[2]:154-60). As there is no definitive evidence to suggest an association between a septate uterus and renal anomalies, the renal system evaluation can be deferred in this population (Fertil Steril. 2021 Nov;116[5]:1238-52).
Diagnosis
2-D ultrasound can be a screen for müllerian anomalies and genitourinary anatomic variants. The diagnostic accuracy of 3-D ultrasound with müllerian anomalies is reported to be 97.6% with sensitivity and specificity of 98.3% and 99.4%, respectively (Hum. Reprod. 2016;31[1]:2-7). As a result, office 3-D has essentially replaced MRI in the diagnosis of müllerian anomalies (Ultrasound Obstet Gynecol. 2015 Nov;46[5]:616-22), with one exception because of the avoidance of a transvaginal probe in the non–sexually active adult and younger adolescent/child. MRI is reserved for diagnosing complex müllerian anomalies or if there is a diagnostic challenge.
Criteria to diagnose müllerian anomalies by radiology begins with the “reference line,” i.e., a line joining both tubal ostia (interostial line). A septate uterus is diagnosed if the distance from the interostial line to the cephalad endometrium is more than 1 cm, otherwise it is considered normal or arcuate based on its appearance. An arcuate uterus has not been associated with impaired reproduction and can be viewed as a normal variant. Alternatively, a bicornuate uterus is diagnosed when the external fundal indentation is more than 1 cm (Fertil Steril. 2021 Nov;116[5]:1238-52).
Clinical course
Women with müllerian anomalies may experience pelvic pain and prolonged and/or abnormal bleeding at the time of menarche. While the ability to conceive may not be impaired from müllerian anomalies with the possible exception of the septate uterus, the pregnancy course can be affected, i.e., recurrent pregnancy loss, preterm birth, perinatal mortality, and malpresentation in labor (Reprod Biomed Online. 2014;29[6]:665). In women with septate, bicornuate, and uterine didelphys, fetal growth restriction appears to be increased. Spontaneous abortion rates of 32% and preterm birth rates of 28% have been reported in patients with uterus didelphys (Obstet Gynecol. 1990;75[6]:906).
Special consideration of the unicornuate is given because of the potential for a rudimentary horn that may communicate with the main uterine cavity and/or have functional endometrium which places the woman at risk of an ectopic pregnancy in the smaller horn. Patients with a unicornuate uterus are at higher risk for preterm labor and breech presentation. An obstructed (noncommunicating) functional rudimentary horn is a risk for endometriosis with cyclic pain because of outflow tract obstruction and an ectopic pregnancy prompting consideration for hemihysterectomy based on symptoms.
The septate uterus – old dogma revisited
The incidence of uterine septa is approximately 1-15 per 1,000. As the most common müllerian anomaly, the septate uterus has traditionally been associated with an increased risk for spontaneous abortion (21%-44%) and preterm birth (12%-33%). The live birth rate ranges from 50% to 72% (Hum Reprod Update. 2001;7[2]:161-74). A uterine septum is believed to develop as a result of failure of resorption of the tissue connecting the two paramesonephric (müllerian) ducts prior to the 20th embryonic week.
Incising the uterine septum (metroplasty) dates back to 1884 when Ruge described a blind transcervical metroplasty in a woman with two previous miscarriages who, postoperatively, delivered a healthy baby. In the early 1900s, Tompkins reported an abdominal metroplasty (Fertil Stertil. 2021;115:1140-2). The decision to proceed with metroplasty is based on only established observational studies (Fertil Steril. 2016;106:530-40). Until recently, the majority of studies suggested that metroplasty is associated with decreased spontaneous abortion rates and improved obstetrical outcomes. A retrospective case series of 361 patients with a septate uterus who had primary infertility of >2 years’ duration, a history of 1-2 spontaneous abortions, or recurrent pregnancy loss suggested a significant improvement in the live birth rate and reduction in miscarriage (Arch Gynecol Obstet. 2003;268:289-92). A meta-analysis found that the overall pregnancy rate after septum incision was 67.8% and the live-birth rate was 53.5% (J Minim Invas Gynecol. 2013;20:22-42).
Recently, two multinational studies question the prevailing dogma (Fertil Steril. 2021 Sep;116[3]:693-4). Both studies could not demonstrate any increase in live birth rate, reduction in preterm birth, or in pregnancy loss after metroplasty. A significant limitation was the lack of a uniform consensus on the definition of the septate uterus and allowing the discretion of the physician to diagnosis a septum (Hum Reprod. 2020;35:1578-88; Hum Reprod. 2021;36:1260-7).
Hysteroscopic metroplasty is not without complications. Uterine rupture during pregnancy or delivery, while rare, may be linked to significant entry into the myometrium and/or overzealous cauterization and perforation, which emphasizes the importance of appropriate techniques.
Conclusion
A diagnosis of müllerian anomalies justifies a comprehensive consultation with the patient given the risk of pregnancy complications. Management of the septate uterus has become controversial. In a patient with infertility, prior pregnancy loss, or poor obstetrical outcome, it is reasonable to consider metroplasty; otherwise, expectant management is an option.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando. Email him at [email protected].
The American Society for Reproductive Medicine’s classification system for müllerian anomalies was the standard until the revision in 2021 by ASRM, which updated and expanded the classification presenting nine classes and imaging criteria: müllerian agenesis, cervical agenesis, unicornuate, uterus didelphys, bicornuate, septate, longitudinal vaginal septum, transverse vaginal septum, and complex anomalies. This month’s article addresses müllerian anomalies from embryology to treatment options.
The early embryo has the capability of developing a wolffian (internal male) or müllerian (internal female) system. Unless anti-müllerian hormone (formerly müllerian-inhibiting substance) is produced, the embryo develops a female reproductive system beginning with two lateral uterine anlagen that fuse in the midline and canalize. Müllerian anomalies occur because of accidents during fusion and canalization (see Table).
The incidence of müllerian anomalies is difficult to discern, given the potential for a normal reproductive outcome precluding an evaluation and based on the population studied. Müllerian anomalies are found in approximately 4.3% of fertile women, 3.5%-8% of infertile patients, 12.3%-13% of those with recurrent pregnancy losses, and 24.5% of patients with miscarriage and infertility. Of the müllerian anomalies, the most common is septate (35%), followed by bicornuate (26%), arcuate (18%), unicornuate (10%), didelphys (8%), and agenesis (3%) (Hum Reprod Update. 2001;7[2]:161; Hum Reprod Update. 2011;17[6]:761-71).
In 20%-30% of patients with müllerian anomalies, particularly in women with a unicornuate uterus, renal anomalies exist that are typically ipsilateral to the absent or rudimentary contralateral uterine horn (J Pediatr Adolesc Gynecol. 2021;34[2]:154-60). As there is no definitive evidence to suggest an association between a septate uterus and renal anomalies, the renal system evaluation can be deferred in this population (Fertil Steril. 2021 Nov;116[5]:1238-52).
Diagnosis
2-D ultrasound can be a screen for müllerian anomalies and genitourinary anatomic variants. The diagnostic accuracy of 3-D ultrasound with müllerian anomalies is reported to be 97.6% with sensitivity and specificity of 98.3% and 99.4%, respectively (Hum. Reprod. 2016;31[1]:2-7). As a result, office 3-D has essentially replaced MRI in the diagnosis of müllerian anomalies (Ultrasound Obstet Gynecol. 2015 Nov;46[5]:616-22), with one exception because of the avoidance of a transvaginal probe in the non–sexually active adult and younger adolescent/child. MRI is reserved for diagnosing complex müllerian anomalies or if there is a diagnostic challenge.
Criteria to diagnose müllerian anomalies by radiology begins with the “reference line,” i.e., a line joining both tubal ostia (interostial line). A septate uterus is diagnosed if the distance from the interostial line to the cephalad endometrium is more than 1 cm, otherwise it is considered normal or arcuate based on its appearance. An arcuate uterus has not been associated with impaired reproduction and can be viewed as a normal variant. Alternatively, a bicornuate uterus is diagnosed when the external fundal indentation is more than 1 cm (Fertil Steril. 2021 Nov;116[5]:1238-52).
Clinical course
Women with müllerian anomalies may experience pelvic pain and prolonged and/or abnormal bleeding at the time of menarche. While the ability to conceive may not be impaired from müllerian anomalies with the possible exception of the septate uterus, the pregnancy course can be affected, i.e., recurrent pregnancy loss, preterm birth, perinatal mortality, and malpresentation in labor (Reprod Biomed Online. 2014;29[6]:665). In women with septate, bicornuate, and uterine didelphys, fetal growth restriction appears to be increased. Spontaneous abortion rates of 32% and preterm birth rates of 28% have been reported in patients with uterus didelphys (Obstet Gynecol. 1990;75[6]:906).
Special consideration of the unicornuate is given because of the potential for a rudimentary horn that may communicate with the main uterine cavity and/or have functional endometrium which places the woman at risk of an ectopic pregnancy in the smaller horn. Patients with a unicornuate uterus are at higher risk for preterm labor and breech presentation. An obstructed (noncommunicating) functional rudimentary horn is a risk for endometriosis with cyclic pain because of outflow tract obstruction and an ectopic pregnancy prompting consideration for hemihysterectomy based on symptoms.
The septate uterus – old dogma revisited
The incidence of uterine septa is approximately 1-15 per 1,000. As the most common müllerian anomaly, the septate uterus has traditionally been associated with an increased risk for spontaneous abortion (21%-44%) and preterm birth (12%-33%). The live birth rate ranges from 50% to 72% (Hum Reprod Update. 2001;7[2]:161-74). A uterine septum is believed to develop as a result of failure of resorption of the tissue connecting the two paramesonephric (müllerian) ducts prior to the 20th embryonic week.
Incising the uterine septum (metroplasty) dates back to 1884 when Ruge described a blind transcervical metroplasty in a woman with two previous miscarriages who, postoperatively, delivered a healthy baby. In the early 1900s, Tompkins reported an abdominal metroplasty (Fertil Stertil. 2021;115:1140-2). The decision to proceed with metroplasty is based on only established observational studies (Fertil Steril. 2016;106:530-40). Until recently, the majority of studies suggested that metroplasty is associated with decreased spontaneous abortion rates and improved obstetrical outcomes. A retrospective case series of 361 patients with a septate uterus who had primary infertility of >2 years’ duration, a history of 1-2 spontaneous abortions, or recurrent pregnancy loss suggested a significant improvement in the live birth rate and reduction in miscarriage (Arch Gynecol Obstet. 2003;268:289-92). A meta-analysis found that the overall pregnancy rate after septum incision was 67.8% and the live-birth rate was 53.5% (J Minim Invas Gynecol. 2013;20:22-42).
Recently, two multinational studies question the prevailing dogma (Fertil Steril. 2021 Sep;116[3]:693-4). Both studies could not demonstrate any increase in live birth rate, reduction in preterm birth, or in pregnancy loss after metroplasty. A significant limitation was the lack of a uniform consensus on the definition of the septate uterus and allowing the discretion of the physician to diagnosis a septum (Hum Reprod. 2020;35:1578-88; Hum Reprod. 2021;36:1260-7).
Hysteroscopic metroplasty is not without complications. Uterine rupture during pregnancy or delivery, while rare, may be linked to significant entry into the myometrium and/or overzealous cauterization and perforation, which emphasizes the importance of appropriate techniques.
Conclusion
A diagnosis of müllerian anomalies justifies a comprehensive consultation with the patient given the risk of pregnancy complications. Management of the septate uterus has become controversial. In a patient with infertility, prior pregnancy loss, or poor obstetrical outcome, it is reasonable to consider metroplasty; otherwise, expectant management is an option.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando. Email him at [email protected].
Optimizing ‘optimal’ in ovarian cancer cytoreduction
The goal of advanced ovarian cancer surgery is to remove all gross disease, or all visible and palpable disease implants. This became the established standard when improved survival was consistently observed among patients who had undergone complete surgical resection. Traditionally, definitions of no gross residual disease have been left in the hands, and eyes, of the surgeon. However, new technology has emerged which affords surgeons the ability to visualize ovarian cancer deposits that are imperceptible to the naked eye. But will this improve upon the poor cure rates for advanced ovarian cancer?
Many are familiar with the traditional definitions of “optimal” (less than 1 cm–sized deposits at any one location) and “suboptimal” (greater than 1 cm–sized deposits remaining) when referring to surgical cytoreduction of ovarian cancer. This nomenclature was introduced to define, categorize, and prognosticate patient groups after surgery. In recent years we have moved away from these descriptive definitions of ovarian cancer resection, borrowing from surgical oncology measures of surgical outcomes where “R0” defines surgical resection with negative margins, “R1” includes resection with positive microscopic margins (negative for tumor intraoperatively, but positive on microscopic pathology), and “R2” refers to macroscopic residual disease remaining.1
In ovarian cancer, surgeons have adopted the expression R0 to include patients in whom there is no gross visible or palpable residual disease, a special, favorable subgrouping of the previous “optimal” group. R1 is applied to patients with macroscopic, residual disease that fits within the traditional “optimal” cytoreduction classification (<1 cm in any one location). Obviously, these are significant variations to the traditional surgical oncology definitions, but not without supporting data. For example, patients with no gross residual disease (now defined as “R0”) have been observed to have improved survival, compared with patients who are “optimally” debulked but with R1 (<1 cm) residual disease.2 Therefore, this new goal of complete surgical resection has replaced the previous standard of “optimal” cytoreduction in which small macroscopic residual disease was acceptable.
Whether or not a surgery is completed with no gross residual disease is a subjective assessment made by the surgeon, and in practice, highly inaccurate. When a posttrial ad hoc analysis of 1,873 patients with advanced ovarian cancer who had been enrolled in a Gynecologic Oncology Group cooperative trial correlated surgeons’ assessments of “optimal” cytoreduction with objective postoperative radiographic findings (performed, on average, less than 1 month postoperatively) they found that postoperative CT scans identified lesions >1 cm in 40% of cases that had been characterized by surgeons as an “optimal” cytoreduction.3 Most commonly, discrepant lesions were identified in the upper abdominal quadrants and retroperitoneal aortic nodal regions. Therefore, surgeons’ subjective assessment of cytoreduction is prone to error, and given how important the completeness of cytoreduction is for clinical outcomes, there is interest in discovering methods to improve upon surgeons’ ability to discriminate volume of disease.
Pafolacianine (Cytalux, On Target Laboratories) is a novel drug that binds a fluorescent molecule to folic acid targeting the folate alpha receptors which are overexpressed on nonmucinous epithelial ovarian cancer cells compared with adjacent nonmalignant tissues.4 The drug is intravenously infused preoperatively and then visualized with companion near-infrared imaging devices during surgery to visualize its fluorescent signal where it is bound to ovarian cancer implants. In a phase 2 study of 178 patients with confirmed or suspected ovarian cancer, pafolacianine was able to detect implants of ovarian cancer in 26.9% of cases where the surgeon’s visual inspection was negative.5 Of note, the false-positive rate of this drug was not trivial, at 20%. Based on this efficacy data, the drug has been granted FDA approved for use in ovarian cancer surgery to augment the surgeon’s visualization of cancer. However, important questions remain unanswered by these preliminary data.
Will removal of additional microscopic ovarian cancer implants, only seen by pafolacianine, improve the survival of patients with ovarian cancer, and what effect will the addition of this extra surgery have on their surgical morbidity and risk? The use of pafolacianine to augment ovarian cancer debulking surgeries pivots on the premise that ovarian cancer outcomes are determined by surgical “effort” more than the biology of the disease. Otherwise said: The more we surgically remove, the more we cure. But this seems an old-fashioned notion, increasingly challenged by data. It has been shown that, when ovarian cancer debulking surgeries are necessarily more radical because of extensive disease distribution, prognosis is worse, compared with those patients with less extensive disease distribution.6 The effect of surgical effort contributes less than that of predetermined patterns of disease presentation. Additionally, genomic traits are different in tumors that are objectively determined to be not amenable to optimal cytoreduction, compared with resectable tumors.7 These data suggest that it is the disease, more than the surgeon, that most influences outcomes.
Additionally, the question of whether surgical removal of microscopic disease improves ovarian cancer survival has already been addressed with negative findings. The LION trial randomized 647 women with advanced ovarian cancer to primary cytoreductive surgery either with or without routine lymphadenectomy of clinically negative nodes.8 This study found no survival benefit to resecting clinically negative, microscopically positive nodes. In light of these data, it is difficult to imagine that there would be different results with the resection of microscopic peritoneal disease implants identified by pafolacianine.
While pafolacianine promises to move us closer to a true “R0” (negative margins) resection of ovarian cancer, is this even a feasible goal in a disease that is widely metastatic, particularly in the peritoneal cavity? What do “negative margins” mean in the peritoneal cavity? The sensitivity of pafolacianine in detecting microscopic disease is obviously not so high that it can guarantee patients a complete resection of a disseminated disease, and we still do not know what absolute benefit is derived from moving a little bit further on the continuum of surgical resection.
Perhaps augmentation of debulking is not the only, or best, use of pafolacianine for ovarian cancer surgery. Perhaps it might serve a role in diagnostics or staging of the disease rather than for a therapeutic purpose. In the meantime, we await ongoing clinical trials in this space to better inform clinicians what benefits, or harms, they might expect from the addition of this new drug as we continue to define the “optimal” surgical procedure for advanced ovarian cancer.
Dr. Emma Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no conflicts of interest.
References
1. Hermanek P, Wittekind C. Semin Surg Oncol 1994;10:12-20.
2. Elattar A et al. Cochrane Database Syst Rev 2011 Aug 10;2011(8):CD007565.
3. Eskander RN et al. Gynecol Oncol 2018;149:525-30.
4. Randall LM et al. Gynecol Oncol 2019;155:63-8.
5. Food and Drug Administration. FDA approves pafolacianine for identifying malignant ovarian cancer lesions. 2021 Dec 1.
6. Horowitz NS et al. J Clin Oncol 2015;33:937-43.
7. Lee S et al. Cell Rep. 2020;31:107502.
8. Harter P et al. N Engl J Med 2019;380:822-32.
The goal of advanced ovarian cancer surgery is to remove all gross disease, or all visible and palpable disease implants. This became the established standard when improved survival was consistently observed among patients who had undergone complete surgical resection. Traditionally, definitions of no gross residual disease have been left in the hands, and eyes, of the surgeon. However, new technology has emerged which affords surgeons the ability to visualize ovarian cancer deposits that are imperceptible to the naked eye. But will this improve upon the poor cure rates for advanced ovarian cancer?
Many are familiar with the traditional definitions of “optimal” (less than 1 cm–sized deposits at any one location) and “suboptimal” (greater than 1 cm–sized deposits remaining) when referring to surgical cytoreduction of ovarian cancer. This nomenclature was introduced to define, categorize, and prognosticate patient groups after surgery. In recent years we have moved away from these descriptive definitions of ovarian cancer resection, borrowing from surgical oncology measures of surgical outcomes where “R0” defines surgical resection with negative margins, “R1” includes resection with positive microscopic margins (negative for tumor intraoperatively, but positive on microscopic pathology), and “R2” refers to macroscopic residual disease remaining.1
In ovarian cancer, surgeons have adopted the expression R0 to include patients in whom there is no gross visible or palpable residual disease, a special, favorable subgrouping of the previous “optimal” group. R1 is applied to patients with macroscopic, residual disease that fits within the traditional “optimal” cytoreduction classification (<1 cm in any one location). Obviously, these are significant variations to the traditional surgical oncology definitions, but not without supporting data. For example, patients with no gross residual disease (now defined as “R0”) have been observed to have improved survival, compared with patients who are “optimally” debulked but with R1 (<1 cm) residual disease.2 Therefore, this new goal of complete surgical resection has replaced the previous standard of “optimal” cytoreduction in which small macroscopic residual disease was acceptable.
Whether or not a surgery is completed with no gross residual disease is a subjective assessment made by the surgeon, and in practice, highly inaccurate. When a posttrial ad hoc analysis of 1,873 patients with advanced ovarian cancer who had been enrolled in a Gynecologic Oncology Group cooperative trial correlated surgeons’ assessments of “optimal” cytoreduction with objective postoperative radiographic findings (performed, on average, less than 1 month postoperatively) they found that postoperative CT scans identified lesions >1 cm in 40% of cases that had been characterized by surgeons as an “optimal” cytoreduction.3 Most commonly, discrepant lesions were identified in the upper abdominal quadrants and retroperitoneal aortic nodal regions. Therefore, surgeons’ subjective assessment of cytoreduction is prone to error, and given how important the completeness of cytoreduction is for clinical outcomes, there is interest in discovering methods to improve upon surgeons’ ability to discriminate volume of disease.
Pafolacianine (Cytalux, On Target Laboratories) is a novel drug that binds a fluorescent molecule to folic acid targeting the folate alpha receptors which are overexpressed on nonmucinous epithelial ovarian cancer cells compared with adjacent nonmalignant tissues.4 The drug is intravenously infused preoperatively and then visualized with companion near-infrared imaging devices during surgery to visualize its fluorescent signal where it is bound to ovarian cancer implants. In a phase 2 study of 178 patients with confirmed or suspected ovarian cancer, pafolacianine was able to detect implants of ovarian cancer in 26.9% of cases where the surgeon’s visual inspection was negative.5 Of note, the false-positive rate of this drug was not trivial, at 20%. Based on this efficacy data, the drug has been granted FDA approved for use in ovarian cancer surgery to augment the surgeon’s visualization of cancer. However, important questions remain unanswered by these preliminary data.
Will removal of additional microscopic ovarian cancer implants, only seen by pafolacianine, improve the survival of patients with ovarian cancer, and what effect will the addition of this extra surgery have on their surgical morbidity and risk? The use of pafolacianine to augment ovarian cancer debulking surgeries pivots on the premise that ovarian cancer outcomes are determined by surgical “effort” more than the biology of the disease. Otherwise said: The more we surgically remove, the more we cure. But this seems an old-fashioned notion, increasingly challenged by data. It has been shown that, when ovarian cancer debulking surgeries are necessarily more radical because of extensive disease distribution, prognosis is worse, compared with those patients with less extensive disease distribution.6 The effect of surgical effort contributes less than that of predetermined patterns of disease presentation. Additionally, genomic traits are different in tumors that are objectively determined to be not amenable to optimal cytoreduction, compared with resectable tumors.7 These data suggest that it is the disease, more than the surgeon, that most influences outcomes.
Additionally, the question of whether surgical removal of microscopic disease improves ovarian cancer survival has already been addressed with negative findings. The LION trial randomized 647 women with advanced ovarian cancer to primary cytoreductive surgery either with or without routine lymphadenectomy of clinically negative nodes.8 This study found no survival benefit to resecting clinically negative, microscopically positive nodes. In light of these data, it is difficult to imagine that there would be different results with the resection of microscopic peritoneal disease implants identified by pafolacianine.
While pafolacianine promises to move us closer to a true “R0” (negative margins) resection of ovarian cancer, is this even a feasible goal in a disease that is widely metastatic, particularly in the peritoneal cavity? What do “negative margins” mean in the peritoneal cavity? The sensitivity of pafolacianine in detecting microscopic disease is obviously not so high that it can guarantee patients a complete resection of a disseminated disease, and we still do not know what absolute benefit is derived from moving a little bit further on the continuum of surgical resection.
Perhaps augmentation of debulking is not the only, or best, use of pafolacianine for ovarian cancer surgery. Perhaps it might serve a role in diagnostics or staging of the disease rather than for a therapeutic purpose. In the meantime, we await ongoing clinical trials in this space to better inform clinicians what benefits, or harms, they might expect from the addition of this new drug as we continue to define the “optimal” surgical procedure for advanced ovarian cancer.
Dr. Emma Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no conflicts of interest.
References
1. Hermanek P, Wittekind C. Semin Surg Oncol 1994;10:12-20.
2. Elattar A et al. Cochrane Database Syst Rev 2011 Aug 10;2011(8):CD007565.
3. Eskander RN et al. Gynecol Oncol 2018;149:525-30.
4. Randall LM et al. Gynecol Oncol 2019;155:63-8.
5. Food and Drug Administration. FDA approves pafolacianine for identifying malignant ovarian cancer lesions. 2021 Dec 1.
6. Horowitz NS et al. J Clin Oncol 2015;33:937-43.
7. Lee S et al. Cell Rep. 2020;31:107502.
8. Harter P et al. N Engl J Med 2019;380:822-32.
The goal of advanced ovarian cancer surgery is to remove all gross disease, or all visible and palpable disease implants. This became the established standard when improved survival was consistently observed among patients who had undergone complete surgical resection. Traditionally, definitions of no gross residual disease have been left in the hands, and eyes, of the surgeon. However, new technology has emerged which affords surgeons the ability to visualize ovarian cancer deposits that are imperceptible to the naked eye. But will this improve upon the poor cure rates for advanced ovarian cancer?
Many are familiar with the traditional definitions of “optimal” (less than 1 cm–sized deposits at any one location) and “suboptimal” (greater than 1 cm–sized deposits remaining) when referring to surgical cytoreduction of ovarian cancer. This nomenclature was introduced to define, categorize, and prognosticate patient groups after surgery. In recent years we have moved away from these descriptive definitions of ovarian cancer resection, borrowing from surgical oncology measures of surgical outcomes where “R0” defines surgical resection with negative margins, “R1” includes resection with positive microscopic margins (negative for tumor intraoperatively, but positive on microscopic pathology), and “R2” refers to macroscopic residual disease remaining.1
In ovarian cancer, surgeons have adopted the expression R0 to include patients in whom there is no gross visible or palpable residual disease, a special, favorable subgrouping of the previous “optimal” group. R1 is applied to patients with macroscopic, residual disease that fits within the traditional “optimal” cytoreduction classification (<1 cm in any one location). Obviously, these are significant variations to the traditional surgical oncology definitions, but not without supporting data. For example, patients with no gross residual disease (now defined as “R0”) have been observed to have improved survival, compared with patients who are “optimally” debulked but with R1 (<1 cm) residual disease.2 Therefore, this new goal of complete surgical resection has replaced the previous standard of “optimal” cytoreduction in which small macroscopic residual disease was acceptable.
Whether or not a surgery is completed with no gross residual disease is a subjective assessment made by the surgeon, and in practice, highly inaccurate. When a posttrial ad hoc analysis of 1,873 patients with advanced ovarian cancer who had been enrolled in a Gynecologic Oncology Group cooperative trial correlated surgeons’ assessments of “optimal” cytoreduction with objective postoperative radiographic findings (performed, on average, less than 1 month postoperatively) they found that postoperative CT scans identified lesions >1 cm in 40% of cases that had been characterized by surgeons as an “optimal” cytoreduction.3 Most commonly, discrepant lesions were identified in the upper abdominal quadrants and retroperitoneal aortic nodal regions. Therefore, surgeons’ subjective assessment of cytoreduction is prone to error, and given how important the completeness of cytoreduction is for clinical outcomes, there is interest in discovering methods to improve upon surgeons’ ability to discriminate volume of disease.
Pafolacianine (Cytalux, On Target Laboratories) is a novel drug that binds a fluorescent molecule to folic acid targeting the folate alpha receptors which are overexpressed on nonmucinous epithelial ovarian cancer cells compared with adjacent nonmalignant tissues.4 The drug is intravenously infused preoperatively and then visualized with companion near-infrared imaging devices during surgery to visualize its fluorescent signal where it is bound to ovarian cancer implants. In a phase 2 study of 178 patients with confirmed or suspected ovarian cancer, pafolacianine was able to detect implants of ovarian cancer in 26.9% of cases where the surgeon’s visual inspection was negative.5 Of note, the false-positive rate of this drug was not trivial, at 20%. Based on this efficacy data, the drug has been granted FDA approved for use in ovarian cancer surgery to augment the surgeon’s visualization of cancer. However, important questions remain unanswered by these preliminary data.
Will removal of additional microscopic ovarian cancer implants, only seen by pafolacianine, improve the survival of patients with ovarian cancer, and what effect will the addition of this extra surgery have on their surgical morbidity and risk? The use of pafolacianine to augment ovarian cancer debulking surgeries pivots on the premise that ovarian cancer outcomes are determined by surgical “effort” more than the biology of the disease. Otherwise said: The more we surgically remove, the more we cure. But this seems an old-fashioned notion, increasingly challenged by data. It has been shown that, when ovarian cancer debulking surgeries are necessarily more radical because of extensive disease distribution, prognosis is worse, compared with those patients with less extensive disease distribution.6 The effect of surgical effort contributes less than that of predetermined patterns of disease presentation. Additionally, genomic traits are different in tumors that are objectively determined to be not amenable to optimal cytoreduction, compared with resectable tumors.7 These data suggest that it is the disease, more than the surgeon, that most influences outcomes.
Additionally, the question of whether surgical removal of microscopic disease improves ovarian cancer survival has already been addressed with negative findings. The LION trial randomized 647 women with advanced ovarian cancer to primary cytoreductive surgery either with or without routine lymphadenectomy of clinically negative nodes.8 This study found no survival benefit to resecting clinically negative, microscopically positive nodes. In light of these data, it is difficult to imagine that there would be different results with the resection of microscopic peritoneal disease implants identified by pafolacianine.
While pafolacianine promises to move us closer to a true “R0” (negative margins) resection of ovarian cancer, is this even a feasible goal in a disease that is widely metastatic, particularly in the peritoneal cavity? What do “negative margins” mean in the peritoneal cavity? The sensitivity of pafolacianine in detecting microscopic disease is obviously not so high that it can guarantee patients a complete resection of a disseminated disease, and we still do not know what absolute benefit is derived from moving a little bit further on the continuum of surgical resection.
Perhaps augmentation of debulking is not the only, or best, use of pafolacianine for ovarian cancer surgery. Perhaps it might serve a role in diagnostics or staging of the disease rather than for a therapeutic purpose. In the meantime, we await ongoing clinical trials in this space to better inform clinicians what benefits, or harms, they might expect from the addition of this new drug as we continue to define the “optimal” surgical procedure for advanced ovarian cancer.
Dr. Emma Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no conflicts of interest.
References
1. Hermanek P, Wittekind C. Semin Surg Oncol 1994;10:12-20.
2. Elattar A et al. Cochrane Database Syst Rev 2011 Aug 10;2011(8):CD007565.
3. Eskander RN et al. Gynecol Oncol 2018;149:525-30.
4. Randall LM et al. Gynecol Oncol 2019;155:63-8.
5. Food and Drug Administration. FDA approves pafolacianine for identifying malignant ovarian cancer lesions. 2021 Dec 1.
6. Horowitz NS et al. J Clin Oncol 2015;33:937-43.
7. Lee S et al. Cell Rep. 2020;31:107502.
8. Harter P et al. N Engl J Med 2019;380:822-32.












