User login
JAK inhibitors look good for severe alopecia areata treatment
said Lucy Yichu Liu, MD, and Brett Andrew King, MD, of Yale University, New Haven, Conn.
Standard medical therapies for alopecia areata – usually topical or injected corticosteroids and allergic contact sensitization – are not very effective for severe disease, particularly alopecia totalis and alopecia universalis. The Janus kinase (JAK) pathway recently has been suggested as a target for treatment.
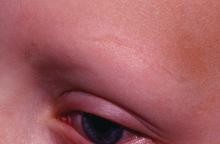
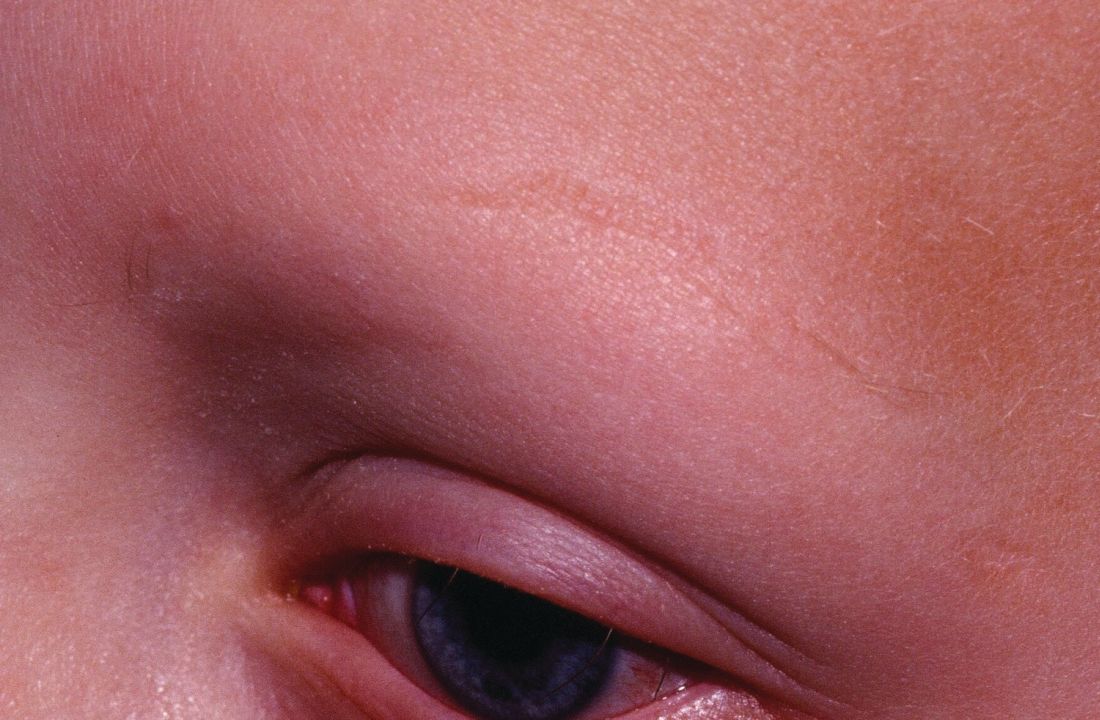
Dr. Liu and Dr. King reviewed several studies, including a retrospective cohort study of 13 patients aged 12-17 years, in which 7 patients had 100% hair loss and 6 had 20%-70% scalp hair loss. The adolescents were treated with the JAK1/3 inhibitor tofacitinib citrate 5 mg twice daily for 2-16 months (median, 5 months). That led to 93% median improvement in Severity of Alopecia Tool (SALT) score (range, 1%-100%) from baseline. Nine patients experienced hair regrowth. There were mild adverse effects, such as upper respiratory infections and headaches.
In a retrospective cohort study of 90 adults taking tofacitinib at a dosage of 5-10 mg twice daily for 4 months or longer with or without prednisone (300 mg once monthly for three doses), patients were divided into those who were more or less likely to respond based on duration of disease. Of 65 patients with alopecia totalis, or alopecia universalis that had lasted 10 years or less, or alopecia areata, 77% had some hair regrowth; 58% had more than 50% improvement from baseline, and 20% achieved full regrowth of hair, Dr. Liu and Dr. King reported in the Journal of Investigative Dermatology Symposium Proceedings.
“Given the finding in adults that complete scalp hair loss for more than 10 years is less likely to respond to treatment, there may be merit to pursuing treatment, even if only intermittently, in adolescents or even younger patients with stable, severe alopecia areata, to prevent irreversible hair loss in the future,” they wrote.
A patient with alopecia universalis achieved partial scalp hair regrowth and complete eyebrow regrowth with compounded ruxolitinib, a topical JAK inhibitor, according to a 2016 case report. Dr. Liu and Dr. King reported that clinical trials with topical JAK inhibitors, including topical tofacitinib and topical ruxolitinib, currently are ongoing.
SOURCE: Liu LY et al. J Investig Dermatol Symp Proc. 2018 Jan. doi: 10.1016/j.jisp.2017.10.003.
said Lucy Yichu Liu, MD, and Brett Andrew King, MD, of Yale University, New Haven, Conn.
Standard medical therapies for alopecia areata – usually topical or injected corticosteroids and allergic contact sensitization – are not very effective for severe disease, particularly alopecia totalis and alopecia universalis. The Janus kinase (JAK) pathway recently has been suggested as a target for treatment.
Dr. Liu and Dr. King reviewed several studies, including a retrospective cohort study of 13 patients aged 12-17 years, in which 7 patients had 100% hair loss and 6 had 20%-70% scalp hair loss. The adolescents were treated with the JAK1/3 inhibitor tofacitinib citrate 5 mg twice daily for 2-16 months (median, 5 months). That led to 93% median improvement in Severity of Alopecia Tool (SALT) score (range, 1%-100%) from baseline. Nine patients experienced hair regrowth. There were mild adverse effects, such as upper respiratory infections and headaches.
In a retrospective cohort study of 90 adults taking tofacitinib at a dosage of 5-10 mg twice daily for 4 months or longer with or without prednisone (300 mg once monthly for three doses), patients were divided into those who were more or less likely to respond based on duration of disease. Of 65 patients with alopecia totalis, or alopecia universalis that had lasted 10 years or less, or alopecia areata, 77% had some hair regrowth; 58% had more than 50% improvement from baseline, and 20% achieved full regrowth of hair, Dr. Liu and Dr. King reported in the Journal of Investigative Dermatology Symposium Proceedings.
“Given the finding in adults that complete scalp hair loss for more than 10 years is less likely to respond to treatment, there may be merit to pursuing treatment, even if only intermittently, in adolescents or even younger patients with stable, severe alopecia areata, to prevent irreversible hair loss in the future,” they wrote.
A patient with alopecia universalis achieved partial scalp hair regrowth and complete eyebrow regrowth with compounded ruxolitinib, a topical JAK inhibitor, according to a 2016 case report. Dr. Liu and Dr. King reported that clinical trials with topical JAK inhibitors, including topical tofacitinib and topical ruxolitinib, currently are ongoing.
SOURCE: Liu LY et al. J Investig Dermatol Symp Proc. 2018 Jan. doi: 10.1016/j.jisp.2017.10.003.
said Lucy Yichu Liu, MD, and Brett Andrew King, MD, of Yale University, New Haven, Conn.
Standard medical therapies for alopecia areata – usually topical or injected corticosteroids and allergic contact sensitization – are not very effective for severe disease, particularly alopecia totalis and alopecia universalis. The Janus kinase (JAK) pathway recently has been suggested as a target for treatment.
Dr. Liu and Dr. King reviewed several studies, including a retrospective cohort study of 13 patients aged 12-17 years, in which 7 patients had 100% hair loss and 6 had 20%-70% scalp hair loss. The adolescents were treated with the JAK1/3 inhibitor tofacitinib citrate 5 mg twice daily for 2-16 months (median, 5 months). That led to 93% median improvement in Severity of Alopecia Tool (SALT) score (range, 1%-100%) from baseline. Nine patients experienced hair regrowth. There were mild adverse effects, such as upper respiratory infections and headaches.
In a retrospective cohort study of 90 adults taking tofacitinib at a dosage of 5-10 mg twice daily for 4 months or longer with or without prednisone (300 mg once monthly for three doses), patients were divided into those who were more or less likely to respond based on duration of disease. Of 65 patients with alopecia totalis, or alopecia universalis that had lasted 10 years or less, or alopecia areata, 77% had some hair regrowth; 58% had more than 50% improvement from baseline, and 20% achieved full regrowth of hair, Dr. Liu and Dr. King reported in the Journal of Investigative Dermatology Symposium Proceedings.
“Given the finding in adults that complete scalp hair loss for more than 10 years is less likely to respond to treatment, there may be merit to pursuing treatment, even if only intermittently, in adolescents or even younger patients with stable, severe alopecia areata, to prevent irreversible hair loss in the future,” they wrote.
A patient with alopecia universalis achieved partial scalp hair regrowth and complete eyebrow regrowth with compounded ruxolitinib, a topical JAK inhibitor, according to a 2016 case report. Dr. Liu and Dr. King reported that clinical trials with topical JAK inhibitors, including topical tofacitinib and topical ruxolitinib, currently are ongoing.
SOURCE: Liu LY et al. J Investig Dermatol Symp Proc. 2018 Jan. doi: 10.1016/j.jisp.2017.10.003.
FROM JOURNAL OF INVESTIGATIVE DERMATOLOGY SYMPOSIUM PROCEEDINGS
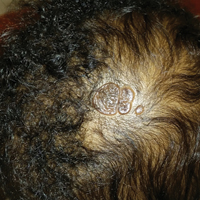
Yellow-Orange Hairless Plaque on the Scalp
The Diagnosis: Nevus Sebaceous
The patient presented with a typical solitary scalp lesion characteristic of nevus sebaceous (NS). The lesion was present at birth as a flat and smooth hairless plaque; however, over time it became more thickened and noticeable, which prompted the parents to seek medical advice.
Nevus sebaceous, also known as NS of Jadassohn, is a benign congenital hamartoma of the sebaceous gland that usually is present at birth and frequently involves the scalp and/or the face. The classic NS lesion is solitary and appears as a well-circumscribed, waxy, yellow-orange or tan, hairless plaque. Despite the presence of these lesions at birth, they may not be noted until early childhood or rarely until adulthood. Generally, the lesion tends to thicken and become more verrucous and velvety over time, particularly around the time of reaching puberty.1 Clinically, NS lesions vary in size from 1 cm to several centimeters. Lesions initially tend to grow proportionately with the child until puberty when they become notably thicker, greasier, and verrucous or nodular under hormonal influences. The yellow discoloration of the lesion is due to sebaceous gland secretion, and the characteristic color usually becomes less evident with age.
Nevus sebaceous occurs in approximately 0.3% of newborns and tends to be sporadic in nature; however, rare familial forms have been reported.2,3 Nevus sebaceous can present as multiple nevi that tend to be extensive and distributed along the Blaschko lines, and they usually are associated with neurologic, ocular, or skeletal defects. Involvement of the central nervous system frequently is associated with large sebaceous nevi located on the face or scalp. This association has been termed NS syndrome.4 Neurologic abnormalities associated with NS syndrome include seizures, mental retardation, and hemimegalencephaly.5 Ocular findings most communally associated with the syndrome are choristomas and colobomas.6-8
There are several benign and malignant epithelial neoplasms that may develop within sebaceous nevi. Benign tumors include trichoblastoma, syringocystadenoma papilliferum, trichilemmoma, sebaceoma, nodular hidradenoma, and hidrocystoma.1,8,9 Malignant neoplasms include basal cell carcinoma (BCC), apocrine carcinoma, sebaceous carcinoma, and squamous cell carcinoma. The lifetime risk of malignancy in NS is unknown. In an extensive literature review by Moody et al10 of 4923 cases of NS for the development of secondary benign and malignant neoplasms, 16% developed benign tumors while 8% developed malignant tumors such as BCC. However, subsequent studies suggested that the incidence of BCC may have been overestimated due to misinterpretation of trichoblastoma and may be less than 1%.11-13
Usually the diagnosis of NS is made clinically and rarely a biopsy for histopathologic confirmation may be needed when the diagnosis is uncertain. Typically, these histopathologic findings include immature hair follicles, hyperplastic immature sebaceous glands, dilated apocrine glands, and epidermal hyperplasia.9 For patients with suspected NS syndrome, additional neurologic and ophthalmologic evaluations should be performed including neuroimaging studies, skeletal radiography, and analysis of liver and renal function.14
The current standard of care in treating NS is full-thickness excision. However, the decision should be individualized based on patient age, extension and location of the lesion, concerns about the cosmetic appearance, and the risk for malignancy.
The 2 main reasons to excise NS include concern about malignancy and undesirable cosmetic appearance. Once a malignant lesion develops within NS, it generally is agreed that the tumor and the entire nevus should be removed; however, recommendations vary for excising NS prophylactically to decrease the risk for malignant growths. Because the risk for malignant transformation seems to be lower than previously thought, observation can be a reasonable choice for lesions that are not associated with cosmetic concern.12,13
Photodynamic therapy, CO2 laser resurfacing, and dermabrasion have been reported as alternative therapeutic approaches. However, there is a growing concern on how effective these treatment modalities are in completely removing the lesion and whether the risk for recurrence and potential for neoplasm development remains.1,9
This patient was healthy with normal development and growth and no signs of neurologic or ocular involvement. The parents were counseled about the risk for malignancy and the long-term cosmetic appearance of the lesion. They opted for surgical excision of the lesion at 18 months of age.
- Eisen DB, Michael DJ. Sebaceous lesions and their associated syndromes: part I. J Am Acad Dermatol. 2009;61:549-560; quiz 561-562.
- Happle R, König A. Familial naevus sebaceus may be explained by paradominant transmission. Br J Dermatol. 1999;141:377.
- Hughes SM, Wilkerson AE, Winfield HL, et al. Familial nevus sebaceus in dizygotic male twins. J Am Acad Dermatol. 2006;54(2 suppl):S47-S48.
- Sugarman JL. Epidermal nevus syndromes. Semin Cutan Med Surg. 2007;26:221-230.
- Davies D, Rogers M. Review of neurological manifestations in 196 patients with sebaceous naevi. Australas J Dermatol. 2002;43:20-23.
- Trivedi N, Nehete G. Complex limbal choristoma in linear nevus sebaceous syndrome managed with scleral grafting. Indian J Ophthalmol. 2016;64:692-694.
- Nema N, Singh K, Verma A. Complex limbal choristoma in nevus sebaceous syndrome [published online February 14, 2012]. Pediatr Dermatol. 2012;29:227-229.
- Park JM, Kim DS, Kim J, et al. Epibulbar complex choristoma and hemimegalencephaly in linear sebaceous naevus syndrome [published online July 2, 2009]. Clin Exp Dermatol. 2009;34:E686-E689.
- Simi CM, Rajalakshmi T, Correa M. Clinicopathologic analysis of 21 cases of nevus sebaceus: a retrospective study. Indian J Dermatol Venereol Leprol. 2008;74:625-627.
- Moody MN, Landau JM, Goldberg LH. Nevus sebaceous revisited. Pediatr Dermatol. 2012;29:15-23.
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000;42(2 pt 1):263-268.
- Santibanez-Gallerani A, Marshall D, Duarte AM, et al. Should nevus sebaceus of Jadassohn in children be excised? a study of 757 cases, and literature review. J Craniofac Surg. 2003;14:658-660.
- Rosen H, Schmidt B, Lam HP, et al. Management of nevus sebaceous and the risk of basal cell carcinoma: an 18-year review. Pediatr Dermatol. 2009;26:676-681.
- Brandling-Bennett HA, Morel KD. Epidermal nevi. Pediatr Clin North Am. 2010;57:1177-1198.
The Diagnosis: Nevus Sebaceous
The patient presented with a typical solitary scalp lesion characteristic of nevus sebaceous (NS). The lesion was present at birth as a flat and smooth hairless plaque; however, over time it became more thickened and noticeable, which prompted the parents to seek medical advice.
Nevus sebaceous, also known as NS of Jadassohn, is a benign congenital hamartoma of the sebaceous gland that usually is present at birth and frequently involves the scalp and/or the face. The classic NS lesion is solitary and appears as a well-circumscribed, waxy, yellow-orange or tan, hairless plaque. Despite the presence of these lesions at birth, they may not be noted until early childhood or rarely until adulthood. Generally, the lesion tends to thicken and become more verrucous and velvety over time, particularly around the time of reaching puberty.1 Clinically, NS lesions vary in size from 1 cm to several centimeters. Lesions initially tend to grow proportionately with the child until puberty when they become notably thicker, greasier, and verrucous or nodular under hormonal influences. The yellow discoloration of the lesion is due to sebaceous gland secretion, and the characteristic color usually becomes less evident with age.
Nevus sebaceous occurs in approximately 0.3% of newborns and tends to be sporadic in nature; however, rare familial forms have been reported.2,3 Nevus sebaceous can present as multiple nevi that tend to be extensive and distributed along the Blaschko lines, and they usually are associated with neurologic, ocular, or skeletal defects. Involvement of the central nervous system frequently is associated with large sebaceous nevi located on the face or scalp. This association has been termed NS syndrome.4 Neurologic abnormalities associated with NS syndrome include seizures, mental retardation, and hemimegalencephaly.5 Ocular findings most communally associated with the syndrome are choristomas and colobomas.6-8
There are several benign and malignant epithelial neoplasms that may develop within sebaceous nevi. Benign tumors include trichoblastoma, syringocystadenoma papilliferum, trichilemmoma, sebaceoma, nodular hidradenoma, and hidrocystoma.1,8,9 Malignant neoplasms include basal cell carcinoma (BCC), apocrine carcinoma, sebaceous carcinoma, and squamous cell carcinoma. The lifetime risk of malignancy in NS is unknown. In an extensive literature review by Moody et al10 of 4923 cases of NS for the development of secondary benign and malignant neoplasms, 16% developed benign tumors while 8% developed malignant tumors such as BCC. However, subsequent studies suggested that the incidence of BCC may have been overestimated due to misinterpretation of trichoblastoma and may be less than 1%.11-13
Usually the diagnosis of NS is made clinically and rarely a biopsy for histopathologic confirmation may be needed when the diagnosis is uncertain. Typically, these histopathologic findings include immature hair follicles, hyperplastic immature sebaceous glands, dilated apocrine glands, and epidermal hyperplasia.9 For patients with suspected NS syndrome, additional neurologic and ophthalmologic evaluations should be performed including neuroimaging studies, skeletal radiography, and analysis of liver and renal function.14
The current standard of care in treating NS is full-thickness excision. However, the decision should be individualized based on patient age, extension and location of the lesion, concerns about the cosmetic appearance, and the risk for malignancy.
The 2 main reasons to excise NS include concern about malignancy and undesirable cosmetic appearance. Once a malignant lesion develops within NS, it generally is agreed that the tumor and the entire nevus should be removed; however, recommendations vary for excising NS prophylactically to decrease the risk for malignant growths. Because the risk for malignant transformation seems to be lower than previously thought, observation can be a reasonable choice for lesions that are not associated with cosmetic concern.12,13
Photodynamic therapy, CO2 laser resurfacing, and dermabrasion have been reported as alternative therapeutic approaches. However, there is a growing concern on how effective these treatment modalities are in completely removing the lesion and whether the risk for recurrence and potential for neoplasm development remains.1,9
This patient was healthy with normal development and growth and no signs of neurologic or ocular involvement. The parents were counseled about the risk for malignancy and the long-term cosmetic appearance of the lesion. They opted for surgical excision of the lesion at 18 months of age.
The Diagnosis: Nevus Sebaceous
The patient presented with a typical solitary scalp lesion characteristic of nevus sebaceous (NS). The lesion was present at birth as a flat and smooth hairless plaque; however, over time it became more thickened and noticeable, which prompted the parents to seek medical advice.
Nevus sebaceous, also known as NS of Jadassohn, is a benign congenital hamartoma of the sebaceous gland that usually is present at birth and frequently involves the scalp and/or the face. The classic NS lesion is solitary and appears as a well-circumscribed, waxy, yellow-orange or tan, hairless plaque. Despite the presence of these lesions at birth, they may not be noted until early childhood or rarely until adulthood. Generally, the lesion tends to thicken and become more verrucous and velvety over time, particularly around the time of reaching puberty.1 Clinically, NS lesions vary in size from 1 cm to several centimeters. Lesions initially tend to grow proportionately with the child until puberty when they become notably thicker, greasier, and verrucous or nodular under hormonal influences. The yellow discoloration of the lesion is due to sebaceous gland secretion, and the characteristic color usually becomes less evident with age.
Nevus sebaceous occurs in approximately 0.3% of newborns and tends to be sporadic in nature; however, rare familial forms have been reported.2,3 Nevus sebaceous can present as multiple nevi that tend to be extensive and distributed along the Blaschko lines, and they usually are associated with neurologic, ocular, or skeletal defects. Involvement of the central nervous system frequently is associated with large sebaceous nevi located on the face or scalp. This association has been termed NS syndrome.4 Neurologic abnormalities associated with NS syndrome include seizures, mental retardation, and hemimegalencephaly.5 Ocular findings most communally associated with the syndrome are choristomas and colobomas.6-8
There are several benign and malignant epithelial neoplasms that may develop within sebaceous nevi. Benign tumors include trichoblastoma, syringocystadenoma papilliferum, trichilemmoma, sebaceoma, nodular hidradenoma, and hidrocystoma.1,8,9 Malignant neoplasms include basal cell carcinoma (BCC), apocrine carcinoma, sebaceous carcinoma, and squamous cell carcinoma. The lifetime risk of malignancy in NS is unknown. In an extensive literature review by Moody et al10 of 4923 cases of NS for the development of secondary benign and malignant neoplasms, 16% developed benign tumors while 8% developed malignant tumors such as BCC. However, subsequent studies suggested that the incidence of BCC may have been overestimated due to misinterpretation of trichoblastoma and may be less than 1%.11-13
Usually the diagnosis of NS is made clinically and rarely a biopsy for histopathologic confirmation may be needed when the diagnosis is uncertain. Typically, these histopathologic findings include immature hair follicles, hyperplastic immature sebaceous glands, dilated apocrine glands, and epidermal hyperplasia.9 For patients with suspected NS syndrome, additional neurologic and ophthalmologic evaluations should be performed including neuroimaging studies, skeletal radiography, and analysis of liver and renal function.14
The current standard of care in treating NS is full-thickness excision. However, the decision should be individualized based on patient age, extension and location of the lesion, concerns about the cosmetic appearance, and the risk for malignancy.
The 2 main reasons to excise NS include concern about malignancy and undesirable cosmetic appearance. Once a malignant lesion develops within NS, it generally is agreed that the tumor and the entire nevus should be removed; however, recommendations vary for excising NS prophylactically to decrease the risk for malignant growths. Because the risk for malignant transformation seems to be lower than previously thought, observation can be a reasonable choice for lesions that are not associated with cosmetic concern.12,13
Photodynamic therapy, CO2 laser resurfacing, and dermabrasion have been reported as alternative therapeutic approaches. However, there is a growing concern on how effective these treatment modalities are in completely removing the lesion and whether the risk for recurrence and potential for neoplasm development remains.1,9
This patient was healthy with normal development and growth and no signs of neurologic or ocular involvement. The parents were counseled about the risk for malignancy and the long-term cosmetic appearance of the lesion. They opted for surgical excision of the lesion at 18 months of age.
- Eisen DB, Michael DJ. Sebaceous lesions and their associated syndromes: part I. J Am Acad Dermatol. 2009;61:549-560; quiz 561-562.
- Happle R, König A. Familial naevus sebaceus may be explained by paradominant transmission. Br J Dermatol. 1999;141:377.
- Hughes SM, Wilkerson AE, Winfield HL, et al. Familial nevus sebaceus in dizygotic male twins. J Am Acad Dermatol. 2006;54(2 suppl):S47-S48.
- Sugarman JL. Epidermal nevus syndromes. Semin Cutan Med Surg. 2007;26:221-230.
- Davies D, Rogers M. Review of neurological manifestations in 196 patients with sebaceous naevi. Australas J Dermatol. 2002;43:20-23.
- Trivedi N, Nehete G. Complex limbal choristoma in linear nevus sebaceous syndrome managed with scleral grafting. Indian J Ophthalmol. 2016;64:692-694.
- Nema N, Singh K, Verma A. Complex limbal choristoma in nevus sebaceous syndrome [published online February 14, 2012]. Pediatr Dermatol. 2012;29:227-229.
- Park JM, Kim DS, Kim J, et al. Epibulbar complex choristoma and hemimegalencephaly in linear sebaceous naevus syndrome [published online July 2, 2009]. Clin Exp Dermatol. 2009;34:E686-E689.
- Simi CM, Rajalakshmi T, Correa M. Clinicopathologic analysis of 21 cases of nevus sebaceus: a retrospective study. Indian J Dermatol Venereol Leprol. 2008;74:625-627.
- Moody MN, Landau JM, Goldberg LH. Nevus sebaceous revisited. Pediatr Dermatol. 2012;29:15-23.
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000;42(2 pt 1):263-268.
- Santibanez-Gallerani A, Marshall D, Duarte AM, et al. Should nevus sebaceus of Jadassohn in children be excised? a study of 757 cases, and literature review. J Craniofac Surg. 2003;14:658-660.
- Rosen H, Schmidt B, Lam HP, et al. Management of nevus sebaceous and the risk of basal cell carcinoma: an 18-year review. Pediatr Dermatol. 2009;26:676-681.
- Brandling-Bennett HA, Morel KD. Epidermal nevi. Pediatr Clin North Am. 2010;57:1177-1198.
- Eisen DB, Michael DJ. Sebaceous lesions and their associated syndromes: part I. J Am Acad Dermatol. 2009;61:549-560; quiz 561-562.
- Happle R, König A. Familial naevus sebaceus may be explained by paradominant transmission. Br J Dermatol. 1999;141:377.
- Hughes SM, Wilkerson AE, Winfield HL, et al. Familial nevus sebaceus in dizygotic male twins. J Am Acad Dermatol. 2006;54(2 suppl):S47-S48.
- Sugarman JL. Epidermal nevus syndromes. Semin Cutan Med Surg. 2007;26:221-230.
- Davies D, Rogers M. Review of neurological manifestations in 196 patients with sebaceous naevi. Australas J Dermatol. 2002;43:20-23.
- Trivedi N, Nehete G. Complex limbal choristoma in linear nevus sebaceous syndrome managed with scleral grafting. Indian J Ophthalmol. 2016;64:692-694.
- Nema N, Singh K, Verma A. Complex limbal choristoma in nevus sebaceous syndrome [published online February 14, 2012]. Pediatr Dermatol. 2012;29:227-229.
- Park JM, Kim DS, Kim J, et al. Epibulbar complex choristoma and hemimegalencephaly in linear sebaceous naevus syndrome [published online July 2, 2009]. Clin Exp Dermatol. 2009;34:E686-E689.
- Simi CM, Rajalakshmi T, Correa M. Clinicopathologic analysis of 21 cases of nevus sebaceus: a retrospective study. Indian J Dermatol Venereol Leprol. 2008;74:625-627.
- Moody MN, Landau JM, Goldberg LH. Nevus sebaceous revisited. Pediatr Dermatol. 2012;29:15-23.
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000;42(2 pt 1):263-268.
- Santibanez-Gallerani A, Marshall D, Duarte AM, et al. Should nevus sebaceus of Jadassohn in children be excised? a study of 757 cases, and literature review. J Craniofac Surg. 2003;14:658-660.
- Rosen H, Schmidt B, Lam HP, et al. Management of nevus sebaceous and the risk of basal cell carcinoma: an 18-year review. Pediatr Dermatol. 2009;26:676-681.
- Brandling-Bennett HA, Morel KD. Epidermal nevi. Pediatr Clin North Am. 2010;57:1177-1198.
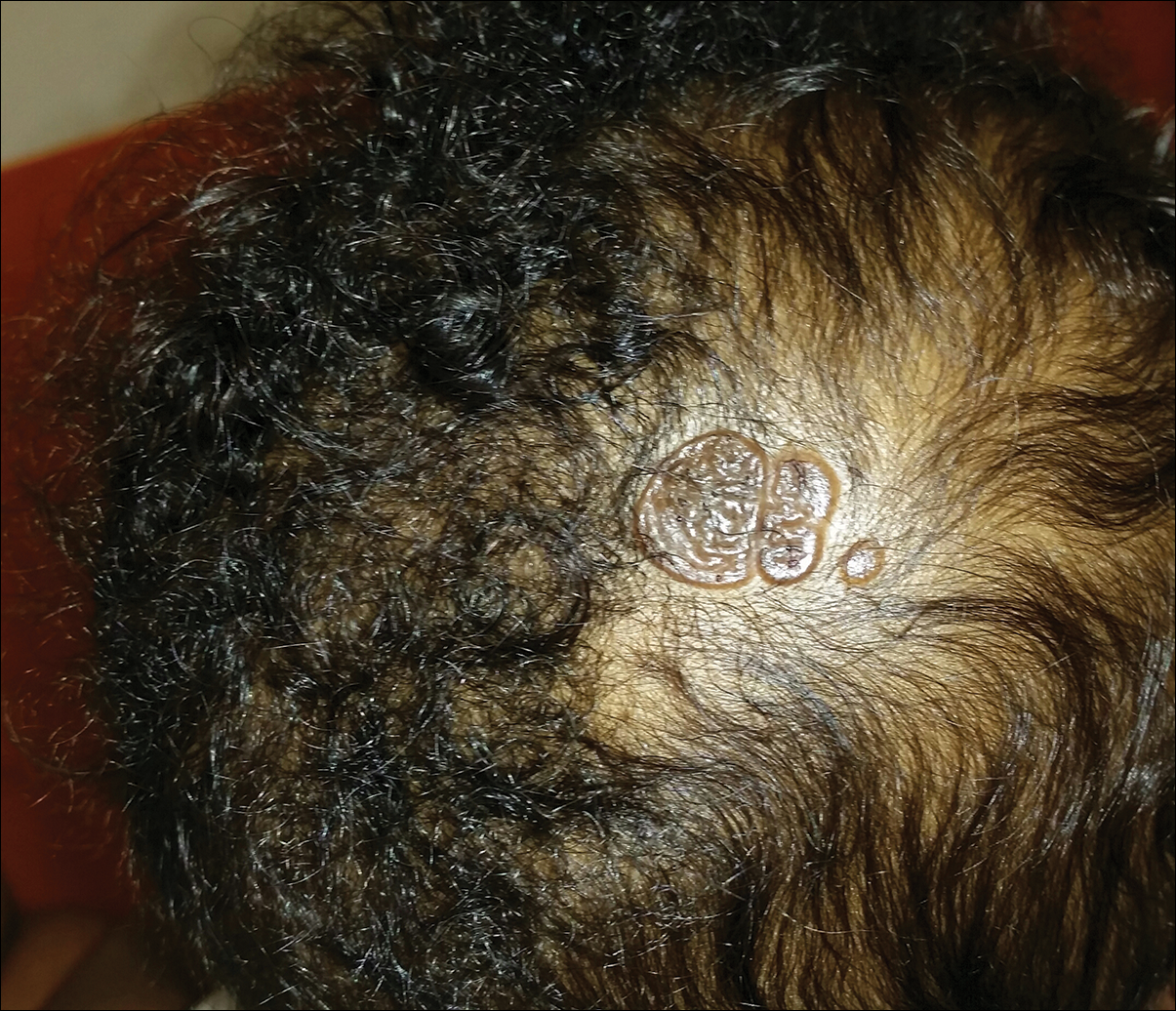
An otherwise healthy 13-month-old boy presented with a well-circumscribed, 3×4-cm, yellow-orange plaque with a verrucous velvety surface on the right side of the posterior scalp. The patient was born at 33 weeks' gestation and had an uneventful perinatal course with a normal head ultrasound at 4 days of age. The lesion had been present since birth and initially was comprised of waxy, yellow-orange, hairless plaques that became more thickened and noticeable over time. The mother recalled that the surface of the plaque initially was flat and smooth but gradually became bumpier and greasier in consistency in the months prior to presentation. The patient was otherwise asymptomatic.
Alopecia tied to nearly fivefold increase in fibroids in African American women
based on data from more than 400,000 women.
In a study published in JAMA Dermatology, researchers reviewed data from 487,104 black women seen at a single center between Aug. 1, 2013, and Aug. 1, 2017. Overall, 14% of women with central centrifugal cicatricial alopecia (CCCA) also had a history of uterine fibroids, compared with 3% percent of black women without CCCA.
“Alopecia is more than just a cosmetic problem. … It could signal an increased risk of developing other conditions,” corresponding author Crystal Aguh, MD, of Johns Hopkins University in Baltimore said in an interview. “To our knowledge, this is the first time that an association has been noted between these two conditions. We believe that the fact that both are related to excess scarring and fibrous tissue deposition may reflect similarities in how both [conditions] develop, but this is still unknown.”
Overall, 62 of 447 women who met criteria for CCCA also had fibroids, representing a nearly fivefold increase in fibroid risk for women with CCCA.
“I was definitely surprised by the findings,” said Dr. Aguh. “I thought it would be interesting to look at any possible correlation between the two diseases, but did not expect to see such a large difference between black women with and without this form of hair loss,” she noted.
As fibroids are often asymptomatic, “physicians should screen their patients with CCCA for symptoms of fibroids such as painful menstrual cycles, heavy bleeding, unexplained anemia, or difficulty conceiving,” said Dr. Aguh. “In those patients who may not know they have fibroids, early recognition that allows for treatment will be especially beneficial.”
The findings were limited by the retrospective nature of the study. “I believe that larger studies are warranted to help us fully understand how these two conditions are connected,” Dr. Aguh said.
Lead author Yemisi Dina of Meharry Medical College, Nashville, Tenn., is supported in part by a grant from the National Institutes of Health. The other researchers had no financial conflicts to disclose.
SOURCE: Dina Y et al. JAMA Dermatol. 2017 Dec 27. doi: 10.1001/jamadermatol.2017.5163
based on data from more than 400,000 women.
In a study published in JAMA Dermatology, researchers reviewed data from 487,104 black women seen at a single center between Aug. 1, 2013, and Aug. 1, 2017. Overall, 14% of women with central centrifugal cicatricial alopecia (CCCA) also had a history of uterine fibroids, compared with 3% percent of black women without CCCA.
“Alopecia is more than just a cosmetic problem. … It could signal an increased risk of developing other conditions,” corresponding author Crystal Aguh, MD, of Johns Hopkins University in Baltimore said in an interview. “To our knowledge, this is the first time that an association has been noted between these two conditions. We believe that the fact that both are related to excess scarring and fibrous tissue deposition may reflect similarities in how both [conditions] develop, but this is still unknown.”
Overall, 62 of 447 women who met criteria for CCCA also had fibroids, representing a nearly fivefold increase in fibroid risk for women with CCCA.
“I was definitely surprised by the findings,” said Dr. Aguh. “I thought it would be interesting to look at any possible correlation between the two diseases, but did not expect to see such a large difference between black women with and without this form of hair loss,” she noted.
As fibroids are often asymptomatic, “physicians should screen their patients with CCCA for symptoms of fibroids such as painful menstrual cycles, heavy bleeding, unexplained anemia, or difficulty conceiving,” said Dr. Aguh. “In those patients who may not know they have fibroids, early recognition that allows for treatment will be especially beneficial.”
The findings were limited by the retrospective nature of the study. “I believe that larger studies are warranted to help us fully understand how these two conditions are connected,” Dr. Aguh said.
Lead author Yemisi Dina of Meharry Medical College, Nashville, Tenn., is supported in part by a grant from the National Institutes of Health. The other researchers had no financial conflicts to disclose.
SOURCE: Dina Y et al. JAMA Dermatol. 2017 Dec 27. doi: 10.1001/jamadermatol.2017.5163
based on data from more than 400,000 women.
In a study published in JAMA Dermatology, researchers reviewed data from 487,104 black women seen at a single center between Aug. 1, 2013, and Aug. 1, 2017. Overall, 14% of women with central centrifugal cicatricial alopecia (CCCA) also had a history of uterine fibroids, compared with 3% percent of black women without CCCA.
“Alopecia is more than just a cosmetic problem. … It could signal an increased risk of developing other conditions,” corresponding author Crystal Aguh, MD, of Johns Hopkins University in Baltimore said in an interview. “To our knowledge, this is the first time that an association has been noted between these two conditions. We believe that the fact that both are related to excess scarring and fibrous tissue deposition may reflect similarities in how both [conditions] develop, but this is still unknown.”
Overall, 62 of 447 women who met criteria for CCCA also had fibroids, representing a nearly fivefold increase in fibroid risk for women with CCCA.
“I was definitely surprised by the findings,” said Dr. Aguh. “I thought it would be interesting to look at any possible correlation between the two diseases, but did not expect to see such a large difference between black women with and without this form of hair loss,” she noted.
As fibroids are often asymptomatic, “physicians should screen their patients with CCCA for symptoms of fibroids such as painful menstrual cycles, heavy bleeding, unexplained anemia, or difficulty conceiving,” said Dr. Aguh. “In those patients who may not know they have fibroids, early recognition that allows for treatment will be especially beneficial.”
The findings were limited by the retrospective nature of the study. “I believe that larger studies are warranted to help us fully understand how these two conditions are connected,” Dr. Aguh said.
Lead author Yemisi Dina of Meharry Medical College, Nashville, Tenn., is supported in part by a grant from the National Institutes of Health. The other researchers had no financial conflicts to disclose.
SOURCE: Dina Y et al. JAMA Dermatol. 2017 Dec 27. doi: 10.1001/jamadermatol.2017.5163
FROM JAMA DERMATOLOGY
Key clinical point: Dermatologists should screen patients with central centrifugal cicatricial alopecia for potential fibroids.
Major finding: Women with CCCA were nearly five times more likely to have fibroids, compared with controls.
Data source: The data come from a review of 487,104 black women seen at a single center between Aug. 1, 2013, and Aug. 1, 2017.
Disclosures: Lead author Yemisi Dina of Meharry Medical College, Nashville, Tenn., is supported in part by a grant from the National Institutes of Health. The other researchers had no financial conflicts to disclose.
Source: Dina Y et al. JAMA Dermatol. 2017 Dec 27. doi: 10.1001/jamadermatol.2017.5163.
No Sulfates, No Parabens, and the “No-Poo” Method: A New Patient Perspective on Common Shampoo Ingredients
Shampoo is a staple in hair grooming that is ever-evolving along with cultural trends. The global shampoo market is expected to reach an estimated value of $25.73 billion by 2019. A major driver of this upward trend in market growth is the increasing demand for natural and organic hair shampoos.1 Society today has a growing fixation on healthy living practices, and as of late, the ingredients in shampoos and other cosmetic products have become one of the latest targets in the health-consciousness craze. In the age of the Internet where information—and misinformation—is widely accessible and dispersed, the general public often strives to self-educate on specialized matters that are out of their expertise. As a result, individuals have developed an aversion to using certain shampoos out of fear that the ingredients, often referred to as “chemicals” by patients due to their complex names, are unnatural and therefore unhealthy.1,2 Product developers are working to meet the demand by reformulating shampoos with labels that indicate sulfate free or paraben free, despite the lack of proof that these formulations are an improvement over traditional approaches to hair health. Additionally, alternative methods of cleansing the hair and scalp, also known as the no-shampoo or “no-poo” method, have begun to gain popularity.2,3
It is essential that dermatologists acknowledge the concerns that their patients have about common shampoo ingredients to dispel the myths that may misinform patient decision-making. This article reviews the controversy surrounding the use of sulfates and parabens in shampoos as well as commonly used shampoo alternatives. Due to the increased prevalence of dry hair shafts in the skin of color population, especially black women, this group is particularly interested in products that will minimize breakage and dryness of the hair. To that end, this population has great interest in the removal of chemical ingredients that may cause damage to the hair shafts, despite the lack of data to support sulfates and paraben damage to hair shafts or scalp skin. Blogs and uninformed hairstylists may propagate these beliefs in a group of consumers who are desperate for new approaches to hair fragility and breakage.
Surfactants and Sulfates
The cleansing ability of a shampoo depends on the surface activity of its detergents. Surface-active ingredients, or surfactants, reduce the surface tension between water and dirt, thus facilitating the removal of environmental dirt from the hair and scalp,4 which is achieved by a molecular structure containing both a hydrophilic and a lipophilic group. Sebum and dirt are bound by the lipophilic ends of the surfactant, becoming the center of a micelle structure with the hydrophilic molecule ends pointing outward. Dirt particles become water soluble and are removed from the scalp and hair shaft upon rinsing with water.4
Surfactants are classified according to the electric charge of the hydrophilic polar group as either anionic, cationic, amphoteric (zwitterionic), or nonionic.5 Each possesses different hair conditioning and cleansing qualities, and multiple surfactants are used in shampoos in differing ratios to accommodate different hair types. In most shampoos, the base consists of anionic and amphoteric surfactants. Depending on individual product requirements, nonionic and cationic surfactants are used to either modify the effects of the surfactants or as conditioning agents.4,5
One subcategory of surfactants that receives much attention is the group of anionic surfactants known as sulfates. Sulfates, particularly sodium lauryl sulfate (SLS), recently have developed a negative reputation as cosmetic ingredients, as reports from various unscientific sources have labeled them as hazardous to one’s health; SLS has been described as a skin and scalp irritant, has been linked to cataract formation, and has even been wrongly labeled as carcinogenic.6 The origins of some of these claims are not clear, though they likely arose from the misinterpretation of complex scientific studies that are easily accessible to laypeople. The link between SLS and ocular irritation or cataract formation is a good illustration of this unsubstantiated fear. A study by Green et al7 showed that corneal exposure to extremely high concentrations of SLS following physical or chemical damage to the eye can result in a slowed healing process. The results of this study have since been wrongly quoted to state that SLS-containing products lead to blindness or severe corneal damage.8 A different study tested for possible ocular irritation in vivo by submerging the lens of an eye into a 20% SLS solution, which accurately approximates the concentration of SLS in rinse-off consumer products.9 However, to achieve ocular irritation, the eyes of laboratory animals were exposed to SLS constantly for 14 days, which would not occur in practical use.9 Similarly, a third study achieved cataract formation in a laboratory only by immersing the lens of an eye into a highly concentrated solution of SLS.10 Such studies are not appropriate representations of how SLS-containing products are used by consumers and have unfortunately been vulnerable to misinterpretation by the general public.
There is no known study that has shown SLS to be carcinogenic. One possible origin of this idea may be from the wrongful interpretation of studies that used SLS as a vehicle substance to test agents that were deemed to be carcinogenic.11 Another possible source of the idea that SLS is carcinogenic comes from its association with 1,4-dioxane, a by-product of the synthesis of certain sulfates such as sodium laureth sulfate due to a process known as ethoxylation.6,12 Although SLS does not undergo this process in its formation and is not linked to 1,4-dioxane, there is potential for cross-contamination of SLS with 1,4-dioxane, which cannot be overlooked. 1,4-Dioxane is classified as “possibly carcinogenic to humans (Group 2B)” by the International Agency for Research on Cancer,13 but screening of SLS for this substance prior to its use in commercial products is standard.
Sulfates are inexpensive detergents that are responsible for lather formation in shampoos as well as in many household cleaning agents.5 Sulfates, similar to all anionic surfactants, are characterized by a negatively charged hydrophilic polar group. The best-known and most commonly used anionic surfactants are sulfated fatty alcohols, alkyl sulfates, and their polyethoxylated analogues alkyl ether sulfates.5,6 Sodium lauryl sulfate (also known as sodium laurilsulfate or sodium dodecyl sulfate) is the most common of them all, found in shampoo and conditioner formulations. Ammonium lauryl sulfate and sodium laureth sulfate are other sulfates commonly used in shampoos and household cleansing products. Sodium lauryl sulfate is a nonvolatile, water-soluble compound. Its partition coefficient (P0), a measure of a substance’s hydrophilic or lipophilic nature, is low at 1.6, making it a rather hydrophilic substance.6 Hydrophilic substances tend to have low bioaccumulation profiles in the body. Additionally, SLS is readily biodegradable. It can be derived from both synthetic and naturally occurring sources; for example, palm kernel oil, petrolatum, and coconut oil are all sources of lauric acid, the starting ingredient used to synthesize SLS. Sodium lauryl sulfate is created by reacting lauryl alcohol with sulfur trioxide gas, followed by neutralization with sodium carbonate (also a naturally occurring compound).6 Sodium lauryl sulfate and other sulfate-containing shampoos widely replaced the usage of traditional soaps formulated from animal or vegetable fats, as these latter formations created a film of insoluble calcium salts on the hair strands upon contact with water, resulting in tangled, dull-appearing hair.5 Additionally, sulfates were preferred to the alkaline pH of traditional soap, which can be harsh on hair strands and cause irritation of the skin and mucous membranes.14 Because they are highly water soluble, sulfates enable the formulation of clear shampoos. They exhibit remarkable cleaning properties and lather formation.5,14
Because sulfates are potent surfactants, they can remove dirt and debris as well as naturally produced healthy oils from the hair and scalp. As a result, sulfates can leave the hair feeling dry and stripped of moisture.4,5 Sulfates are used as the primary detergents in the formulation of deep-cleaning shampoos, which are designed for people who accumulate a heavy buildup of dirt, sebum, and debris from frequent use of styling products. Due to their potent detergency, these shampoos typically are not used on a daily basis but rather at longer intervals.15 A downside to sulfates is that they can have cosmetically unpleasant properties, which can be compensated for by including appropriate softening additives in shampoo formulations.4 A number of anionic surfactants such as olefin sulfonate, alkyl sulfosuccinate, acyl peptides, and alkyl ether carboxylates are well tolerated by the skin and are used together with other anionic and amphoteric surfactants to optimize shampoo properties. Alternatively, sulfate-free shampoos are cleansers compounded by the removal of the anionic group and switched for surfactants with less detergency.4,5
Preservatives and Parabens
Parabens refer to a group of esters of 4-hydroxybenzoic acid commonly used as preservatives in foods, pharmaceuticals, and cosmetics whose widespread use dates back to 1923.16 Concerns over the presence of parabens in shampoos and other cosmetics have been raised by patients for their reputed estrogenic and antiandrogenic effects and suspected involvement in carcinogenesis via endocrine modulation.16,17 In in vitro studies done on yeast assays, parabens have shown weak estrogenic activity that increases in proportion to both the length and increased branching of the alkyl side chains in the paraben’s molecular structure.18 They are 10,000-fold less potent than 17β-estradiol. In in vivo animal studies, parabens show weak estrogenic activity and are 100,000-fold less potent than 17β-estradiol.18 4-Hydroxybenzoic acid, a common metabolite, showed no estrogenic activity when tested both in vitro and in vivo.19 Some concerning research has implicated a link between parabens used in underarm cosmetics, such as deodorants and antiperspirants, and breast cancer16; however, the studies have been conflicting, and there is simply not enough data to assert that parabens cause breast cancer.
The Cosmetic Ingredient Review expert panel first reviewed parabens in 1984 and concluded that “methylparaben, ethylparaben, propylparaben, and butylparaben are safe as cosmetic ingredients in the present practices of use.”20 They extended this statement to include isopropylparaben and isobutylparaben in a later review.21 In 2005, the Scientific Committee on Consumer Products (now known as the Scientific Committee for Consumer Safety) in Europe stated that methylparaben and ethylparaben can be used at levels up to 0.4% in products.22 This decision was reached due to reports of decreased sperm counts and testosterone levels in male juvenile rats exposed to these parabens; however, these reults were not successfully replicated in larger studies.16,22 In 2010, the Scientific Committee for Consumer Safety revisited its stance on parabens, and they then revised their recommendations to say that concentrations of propylparaben and butylparaben should not exceed concentrations of 0.19%, based on “the conservative choice for the calculation of the [Margin-of-Safety] of butyl- and propylparaben.”23 However, in 2011 the use of propylparaben and butylparaben was banned in Denmark for cosmetic products used in children 3 years or younger,16 and the European Commission subsequently amended their directive in 2014, banning isopropylparaben, isobutylparaben, phenylparaben, benzylparaben, and pentylparaben due to lack of data available to evaluate the human risk of these products.24
Contrary to the trends in Europe, there currently are no regulations against the use of parabens in shampoos or other cosmetics in the United States. The American Cancer Society found that there is no evidence to suggest that the current levels of parabens in cosmetic products (eg, antiperspirants) increase one’s risk of breast cancer.25 Parabens are readily absorbed into the body both transdermally and through ingestion but also are believed to be rapidly transformed into harmless and nonspecific metabolites; they are readily metabolized by the liver and excreted in urine, and there is no measured accumulation in tissues.17
Parabens continue to be the most widely used preservatives in personal care products, usually in conjunction with other preservatives. Parabens are good biocides; short-chain esters (eg, methylparabens, ethylparabens) are effective against gram-positive bacteria and are weakly effective against gram-negative bacteria. Long-chain paraben esters (eg, propylparabens, butylparabens) are effective against mold and yeast. The addition of other preservatives creates a broad spectrum of antimicrobial defense in consumer products. Other preservatives include formaldehyde releasers or phenoxyethanol, as well as chelating agents such as EDTA, which improve the stability of these cosmetic products when exposed to air.16 Parabens are naturally occurring substances found in foods such as blueberries, barley, strawberries, yeast, olives, and grapes. As a colorless, odorless, and inexpensive substance, their use has been heavily favored in cosmetic and food products.16
Shampoo Alternatives and the No-Poo Method
Although research has not demonstrated any long-term danger to using shampoo, certain chemicals found in shampoos have the potential to irritate the scalp. Commonly cited allergens in shampoos include cocamidopropyl betaine, propylene glycol, vitamin E (tocopherol), parabens, and benzophenones.5 Additionally, the rising use of formaldehyde-releasing preservatives and isothiazolinones due to mounting pressures to move away from parabens has led to an increase in cases of allergic contact dermatitis (ACD).16 However, the irritability (rather than allergenicity) of these substances often is established during patch testing, a method of detecting delayed-type allergic reactions, which is important to note because patch testing requires a substance to be exposed to the skin for 24 to 48 hours, whereas exposure to shampoo ingredients may last a matter of minutes at most and occur in lesser concentrations because the ingredients are diluted by water in the rinsing process. Given these differences, it is unlikely that a patient would develop a true allergic response from regular shampoo use. Nevertheless, in patients who are already sensitized, exposure could conceivably trigger ACD, and patients must be cognizant of the composition of their shampoos.16
The no-poo method refers to the avoidance of commercial shampoo products when cleansing the hair and scalp and encompasses different methods of cleansing the hair, such as the use of household items (eg, baking soda, apple cider vinegar [ACV]), the use of conditioners to wash the hair (also known as conditioner-only washing or co-washing), treating the scalp with tea tree oil, or simply rinsing the hair with water. Proponents of the no-poo method believe that abstaining from shampoo use leads to healthier hair, retained natural oils, and less exposure to supposedly dangerous chemicals such as parabens or sulfates.2,3,26-28 However, there are no known studies in the literature that assess or support the hypotheses of the no-poo method.
Baking Soda and ACV
Baking soda (sodium bicarbonate) is a substance commonly found in the average household. It has been used in toothpaste formulas and cosmetic products and is known for its acid-neutralizing properties. Baking soda has been shown to have some antifungal and viricidal properties through an unknown mechanism of action.28 It has gained popularity for its use as a means of reducing the appearance of excessive greasiness of the hair shafts. Users also have reported that when washing their hair with baking soda, they are able to achieve a clean scalp and hair that feels soft to the touch.2,3,26,27,29 Despite these reports, users must beware of using baking soda without adequately diluting it with water. Baking soda is a known alkaline irritant.26,30 With a pH of 9, baking soda causes the cuticle layer of the hair fiber to open, increasing the capacity for water absorption. Water penetrates the scales that open, breaking the hydrogen bonds of the keratin molecule.31 Keratin is a spiral helical molecule that keeps its shape due to hydrogen, disulfide, and ionic bonds, as well as Van der Waals force.30 Hydrolysis of these bonds due to exposure to baking soda lowers the elasticity of the hair and increases the negative electrical net charge of the hair fiber surface, which leads to increased friction between fibers, cuticle damage, hair fragility, and fiber breakage.32,33
Apple cider vinegar is an apple-derived acetic acid solution with a pH ranging from 3.1 to 5.28 The pH range of ACV is considered to be ideal for hair by no-poo proponents, as it is similar to the natural pH of the scalp. Its acidic properties are responsible for its antimicrobial abilities, particularly its effectiveness against gram-negative bacteria.30 The acetic acid of ACV can partially interrupt oil interfaces, which contributes to its mild ability to remove product residue and scalp buildup from the hair shaft; the acetic acid also tightens the cuticles on hair fibers.33 Apple cider vinegar is used as a means of cleansing the hair and scalp by no-poo proponents2,3,26; other uses for ACV include using it as a rinse following washing and/or conditioning of the hair or as a means of preserving color in color-treated hair. There also is evidence that ACV may have antifungal properties.28 However, consumers must be aware that if it is not diluted in water, ACV may be too caustic for direct application to the hair and may lead to damage; it can be irritating to eyes, mucus membranes, and acutely inflamed skin. Also, vinegar rinses used on processed or chemically damaged hair may lead to increased hair fragility.2,3
Hair fibers have a pH of 3.67, while the scalp has a pH between 4.5 and 6.2. This slightly acidic film acts as a barrier to viruses, bacteria, and other potential contaminants.33 Studies have shown that the pH of skin increases in proportion to the pH of the cleanser used.34 Therefore, due to the naturally acidic pH of the scalp, acid-balanced shampoos generally are recommended. Shampoos should not have a pH higher than 5.5, as hair shafts can swell due to alkalinization, which can be prevented by pH balancing the shampoo through the addition of an acidic substance (eg, glycolic acid, citric acid) to lower the pH down to approximately 5.5. Apple cider vinegar often is used for this purpose. However, one study revealed that 82% of shampoos already have an acidic pH.34
Conditioner-Only Washing (Co-washing)
Conditioner-only washing, or co-washing, is a widely practiced method of hair grooming. It is popular among individuals who find that commercial shampoos strip too much of the natural hair oils away, leaving the hair rough or unmanageable. Co-washing is not harmful to the hair; however, the molecular structure and function of a conditioner and that of a shampoo are very different.5,35,36 Conditioners are not formulated to remove dirt and buildup in the hair but rather to add substances to the hair, and thus cannot provide extensive cleansing of the hair and scalp; therefore, it is inappropriate to use co-washing as a replacement for shampooing. Quaternary conditioning agents are an exception because they contain amphoteric detergents comprised of both anionic and cationic groups, which allow them both the ability to remove dirt and sebum with its anionic group, typically found in shampoos, as well as the ability to coat and condition the hair due to the high affinity of the cationic group for the negatively charged hair fibers.36,37 Amphoteric detergents are commonly found in 2-in-1 conditioning cleansers, among other ingredients, such as hydrolyzed animal proteins that temporarily plug surface defects on the hair fiber, and dimethicone, a synthetic oil that creates a thin film over the hair shaft, increasing shine and manageability. Of note, these conditioning shampoos are ideal for individuals with minimal product buildup on the hair and scalp and are not adequate scalp cleansers for individuals who either wash their hair infrequently or who regularly use hairstyling products.36,37
Tea Tree Oil
Tea tree oil is an essential oil extracted from the Melaleuca alternifolia plant of the Myrtaceae family. It is native to the coast of northeastern Australia. A holy grail of natural cosmetics, tea tree oil is widely known for its antiviral, antifungal, and antiseptic properties.38 Although not used as a stand-alone cleanser, it is often added to a number of cosmetic products, including shampoos and co-washes. Although deemed safe for topical use, it has been shown to be quite toxic when ingested. Symptoms of ingestion include nausea, vomiting, hallucinations, and coma. The common concern with tea tree oil is its ability to cause ACD. In particular, it is believed that the oxidation products of tea tree oil are allergenic rather than the tea tree oil itself. The evaluation of tea tree oil as a potential contact allergen has been quite difficult; it consists of more than 100 distinct compounds and is often mislabeled, or does not meet the guidelines of the International Organization for Standardization. Nonetheless, the prevalence of ACD due to tea tree oil is low (approximately 1.4%). Despite its low prevalence, tea tree oil should remain in the differential as an ACD-inducing agent. Patch testing with the patient’s supply of tea tree oil is advised when possible.38
Conclusion
It is customary that the ingredients used in shampoos undergo periodic testing and monitoring to assure the safety of their use. Although it is encouraging that patients are proactive in their efforts to stay abreast of the literature, it is still important that cosmetic scientists, dermatologists, and other experts remain at the forefront of educating the public about these substances. Not doing so can result in the propagation of misinformation and unnecessary fears, which can lead to the adaptation of unhygienic or even unsafe hair care practices. As dermatologists, we must ensure that patients are educated about the benefits and hazards of off-label use of household ingredients to the extent that evidence-based medicine permits. Patients must be informed that not all synthetic substances are harmful, and likewise not all naturally occurring substances are safe.
- The global shampoo market 2014-2019 trends, forecast, and opportunity analysis [press release]. New York, NY: Reportlinker; May 21, 2015.
- Is the ‘no shampoo’ trend healthy or harmful? Mercola website. Published January 16, 2016. Accessed December 8, 2017.
- Feltman R. The science (or lack thereof) behind the ‘no-poo’ hair trend. Washington Post. March 10, 2016. https://www.washingtonpost.com/news/speaking-of-science/wp/2016/03/10/the-science-or-lack-thereof-behind-the-no-poo-hair-trend/?utm_term=.9a61edf3fd5a. Accessed December 11, 2017.
- Bouillon C. Shampoos. Clin Dermatol. 1996;14:113-121.
- Trueb RM. Shampoos: ingredients, efficacy, and adverse effects. J Dtsch Dermatol Ges. 2007;5:356-365.
- Bondi CA, Marks JL, Wroblewski LB, et al. Human and environmental toxicity of sodium lauryl sulfate (SLS): evidence for safe use in household cleaning products. Environ Health Insights. 2015;9:27-32.
- Green K, Johnson RE, Chapman JM, et al. Preservative effects on the healing rate of rabbit corneal epithelium. Lens Eye Toxic Res. 1989;6:37-41.
- Sodium lauryl sulphate. Healthy Choices website. http://www.healthychoices.co.uk/sls.html. Accessed December 8, 2017.
- Tekbas¸ ÖF, Uysal Y, Og˘ur R, et al. Non-irritant baby shampoos may cause cataract development. TSK Koruyucu Hekimlik Bülteni. 2008;1:1-6.
- Cater KC, Harbell JW. Prediction of eye irritation potential of surfactant-based rinse-off personal care formulations by the bovine corneal opacity and permeability (BCOP) assay. Cutan Ocul Toxicol. 2006;25:217-233.
- Birt DF, Lawson TA, Julius AD, et al. Inhibition by dietary selenium of colon cancer induced in the rat by bis(2-oxopropyl) nitrosamine. Cancer Res. 1982;42:4455-4459.
- Rastogi SC. Headspace analysis of 1,4-dioxane in products containing polyethoxylated surfactants by GC-MS. Chromatographia. 1990;29:441-445.
- 1,4-Dioxane. IARC Monogr Eval Carcinog Risks Hum. 1999;71, pt 2:589-602.
- Trueb RM. Dermocosmetic aspects of hair and scalp. J Investig Dermatol Symp Proc. 2005;10:289-292.
- D’Souza P, Rathi SK. Shampoo and conditioners: what a dermatologist should know? Indian J Dermatol. 2015;60:248-254.
- Sasseville D, Alfalah M, Lacroix JP. “Parabenoia” debunked, or “who’s afraid of parabens?” Dermatitis. 2015;26:254-259.
- Krowka JF, Loretz L, Geis PA, et al. Preserving the facts on parabens: an overview of these important tools of the trade. Cosmetics & Toiletries. http://www.cosmeticsandtoiletries.com/research/chemistry/Preserving-the-Facts-on-Parabens-An-Overview-of-These-Important-Tools-of-the Trade-425784294.html. Published June 1, 2017. Accessed December 20, 2017.
- Routledge EJ, Parker J, Odum J, et al. Some alkyl hydroxy benzoate preservatives (parabens) are estrogenic. Toxicol Appl Pharmacol. 1998;153:12Y19.
- Hossaini A, Larsen JJ, Larsen JC. Lack of oestrogenic effects of food preservatives (parabens) in uterotrophic assays. Food Chem Toxicol. 2000;38:319-323.
- Cosmetic Ingredient Review. Final report on the safety assessment of methylparaben, ethylparaben, propylparaben and butylparaben. J Am Coll Toxicol. 1984;3:147-209.
- Cosmetic Ingredient Review. Final report on the safety assessment of isobutylparaben and isopropylparaben. J Am Coll Toxicol. 1995;14:364-372.
- Scientific Committee on Consumer Products. Extended Opinion on the Safety Evaluation of Parabens. European Commission website. https://ec.europa.eu/health/ph_risk/committees/04_sccp/docs/sccp_o_019.pdf. Published January 28, 2005. Accessed December 20, 2017.
- Scientific Committee on Consumer Products. Opinion on Parabens. European Commission website. http://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_041.pdf. Revised March 22, 2011. Accessed December 20, 2017.
- European Commission. Commission Regulation (EU) No 258/2014 of 9 April 2014 amending Annexes II and V to Regulation (EC) No 1223/2009 of the European Parliament and of the Council on cosmetic products. EUR-Lex website. http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv:OJ.L_.2014.107.01.0005.01.ENG. Accessed December 20, 2017.
- American Cancer Society. Antiperspirants and breast cancer risk. https://www.cancer.org/cancer/cancer-causes/antiperspirants-and-breast-cancer-risk.html#references. Revised October 14, 2014. Accessed January 2, 2018.
- MacMillan A. Cutting back on shampoo? 15 things you should know. Health. February 25, 2014. http://www.health.com/health/gallery/0,,20788089,00.html#should-you-go-no-poo--1. Accessed December 10, 2017.
- The ‘no poo’ method. https://www.nopoomethod.com/. Accessed December 10, 2017.
- Fong, D, Gaulin C, Le M, et al. Effectiveness of alternative antimicrobial agents for disinfection of hard surfaces. National Collaborating Centre for Environmental Health website. http://www.ncceh.ca/sites/default/files/Alternative_Antimicrobial_Agents_Aug_2014.pdf. Published August 2014. Accessed December 10, 2017.
- Is baking soda too harsh for natural hair? Black Girl With Long Hair website. http://blackgirllonghair.com/2012/02/is-baking-soda-too-harsh-for-hair/2/. Published February 5, 2012. Accessed December 12, 2017.
- O’Lenick T. Anionic/cationic complexes in hair care. J Cosmet Sci. 2011;62:209-228.
- Gavazzoni Dias MF, de Almeida AM, Cecato PM, et al. The shampoo pH can affect the hair: myth or reality? Int J Trichology. 2014;6:95-99.
- Goodman H. The acid mantle of the skin surface. Ind Med Surg. 1958;27:105-108.
- Korting HC, Kober M, Mueller M, et al. Influence of repeated washings with soap and synthetic detergents on pH and resident flora of the skin of forehead and forearm. results of a cross-over trial in health probationers. Acta Derm Venereol. 1987;67:41-47.
- Tarun J, Susan J, Suria J, et al. Evaluation of pH of bathing soaps and shampoos for skin and hair care. Indian J Dermatol. 2014;59:442-444.
- Corbett JF. The chemistry of hair-care products. J Soc Dyers Colour. 1976;92:285-303.
- McMichael AJ, Hordinsky M. Hair Diseases: Medical, Surgical, and Cosmetic Treatments. New York, NY: Taylor & Francis; 2008:59-72.
- Allardice A, Gummo G. Hair conditioning: quaternary ammonium compounds on various hair types. Cosmet Toiletries. 1993;108:107-109.
- Larson D, Jacob SE. Tea tree oil. Dermatitis. 2012;23:48-49.
Shampoo is a staple in hair grooming that is ever-evolving along with cultural trends. The global shampoo market is expected to reach an estimated value of $25.73 billion by 2019. A major driver of this upward trend in market growth is the increasing demand for natural and organic hair shampoos.1 Society today has a growing fixation on healthy living practices, and as of late, the ingredients in shampoos and other cosmetic products have become one of the latest targets in the health-consciousness craze. In the age of the Internet where information—and misinformation—is widely accessible and dispersed, the general public often strives to self-educate on specialized matters that are out of their expertise. As a result, individuals have developed an aversion to using certain shampoos out of fear that the ingredients, often referred to as “chemicals” by patients due to their complex names, are unnatural and therefore unhealthy.1,2 Product developers are working to meet the demand by reformulating shampoos with labels that indicate sulfate free or paraben free, despite the lack of proof that these formulations are an improvement over traditional approaches to hair health. Additionally, alternative methods of cleansing the hair and scalp, also known as the no-shampoo or “no-poo” method, have begun to gain popularity.2,3
It is essential that dermatologists acknowledge the concerns that their patients have about common shampoo ingredients to dispel the myths that may misinform patient decision-making. This article reviews the controversy surrounding the use of sulfates and parabens in shampoos as well as commonly used shampoo alternatives. Due to the increased prevalence of dry hair shafts in the skin of color population, especially black women, this group is particularly interested in products that will minimize breakage and dryness of the hair. To that end, this population has great interest in the removal of chemical ingredients that may cause damage to the hair shafts, despite the lack of data to support sulfates and paraben damage to hair shafts or scalp skin. Blogs and uninformed hairstylists may propagate these beliefs in a group of consumers who are desperate for new approaches to hair fragility and breakage.
Surfactants and Sulfates
The cleansing ability of a shampoo depends on the surface activity of its detergents. Surface-active ingredients, or surfactants, reduce the surface tension between water and dirt, thus facilitating the removal of environmental dirt from the hair and scalp,4 which is achieved by a molecular structure containing both a hydrophilic and a lipophilic group. Sebum and dirt are bound by the lipophilic ends of the surfactant, becoming the center of a micelle structure with the hydrophilic molecule ends pointing outward. Dirt particles become water soluble and are removed from the scalp and hair shaft upon rinsing with water.4
Surfactants are classified according to the electric charge of the hydrophilic polar group as either anionic, cationic, amphoteric (zwitterionic), or nonionic.5 Each possesses different hair conditioning and cleansing qualities, and multiple surfactants are used in shampoos in differing ratios to accommodate different hair types. In most shampoos, the base consists of anionic and amphoteric surfactants. Depending on individual product requirements, nonionic and cationic surfactants are used to either modify the effects of the surfactants or as conditioning agents.4,5
One subcategory of surfactants that receives much attention is the group of anionic surfactants known as sulfates. Sulfates, particularly sodium lauryl sulfate (SLS), recently have developed a negative reputation as cosmetic ingredients, as reports from various unscientific sources have labeled them as hazardous to one’s health; SLS has been described as a skin and scalp irritant, has been linked to cataract formation, and has even been wrongly labeled as carcinogenic.6 The origins of some of these claims are not clear, though they likely arose from the misinterpretation of complex scientific studies that are easily accessible to laypeople. The link between SLS and ocular irritation or cataract formation is a good illustration of this unsubstantiated fear. A study by Green et al7 showed that corneal exposure to extremely high concentrations of SLS following physical or chemical damage to the eye can result in a slowed healing process. The results of this study have since been wrongly quoted to state that SLS-containing products lead to blindness or severe corneal damage.8 A different study tested for possible ocular irritation in vivo by submerging the lens of an eye into a 20% SLS solution, which accurately approximates the concentration of SLS in rinse-off consumer products.9 However, to achieve ocular irritation, the eyes of laboratory animals were exposed to SLS constantly for 14 days, which would not occur in practical use.9 Similarly, a third study achieved cataract formation in a laboratory only by immersing the lens of an eye into a highly concentrated solution of SLS.10 Such studies are not appropriate representations of how SLS-containing products are used by consumers and have unfortunately been vulnerable to misinterpretation by the general public.
There is no known study that has shown SLS to be carcinogenic. One possible origin of this idea may be from the wrongful interpretation of studies that used SLS as a vehicle substance to test agents that were deemed to be carcinogenic.11 Another possible source of the idea that SLS is carcinogenic comes from its association with 1,4-dioxane, a by-product of the synthesis of certain sulfates such as sodium laureth sulfate due to a process known as ethoxylation.6,12 Although SLS does not undergo this process in its formation and is not linked to 1,4-dioxane, there is potential for cross-contamination of SLS with 1,4-dioxane, which cannot be overlooked. 1,4-Dioxane is classified as “possibly carcinogenic to humans (Group 2B)” by the International Agency for Research on Cancer,13 but screening of SLS for this substance prior to its use in commercial products is standard.
Sulfates are inexpensive detergents that are responsible for lather formation in shampoos as well as in many household cleaning agents.5 Sulfates, similar to all anionic surfactants, are characterized by a negatively charged hydrophilic polar group. The best-known and most commonly used anionic surfactants are sulfated fatty alcohols, alkyl sulfates, and their polyethoxylated analogues alkyl ether sulfates.5,6 Sodium lauryl sulfate (also known as sodium laurilsulfate or sodium dodecyl sulfate) is the most common of them all, found in shampoo and conditioner formulations. Ammonium lauryl sulfate and sodium laureth sulfate are other sulfates commonly used in shampoos and household cleansing products. Sodium lauryl sulfate is a nonvolatile, water-soluble compound. Its partition coefficient (P0), a measure of a substance’s hydrophilic or lipophilic nature, is low at 1.6, making it a rather hydrophilic substance.6 Hydrophilic substances tend to have low bioaccumulation profiles in the body. Additionally, SLS is readily biodegradable. It can be derived from both synthetic and naturally occurring sources; for example, palm kernel oil, petrolatum, and coconut oil are all sources of lauric acid, the starting ingredient used to synthesize SLS. Sodium lauryl sulfate is created by reacting lauryl alcohol with sulfur trioxide gas, followed by neutralization with sodium carbonate (also a naturally occurring compound).6 Sodium lauryl sulfate and other sulfate-containing shampoos widely replaced the usage of traditional soaps formulated from animal or vegetable fats, as these latter formations created a film of insoluble calcium salts on the hair strands upon contact with water, resulting in tangled, dull-appearing hair.5 Additionally, sulfates were preferred to the alkaline pH of traditional soap, which can be harsh on hair strands and cause irritation of the skin and mucous membranes.14 Because they are highly water soluble, sulfates enable the formulation of clear shampoos. They exhibit remarkable cleaning properties and lather formation.5,14
Because sulfates are potent surfactants, they can remove dirt and debris as well as naturally produced healthy oils from the hair and scalp. As a result, sulfates can leave the hair feeling dry and stripped of moisture.4,5 Sulfates are used as the primary detergents in the formulation of deep-cleaning shampoos, which are designed for people who accumulate a heavy buildup of dirt, sebum, and debris from frequent use of styling products. Due to their potent detergency, these shampoos typically are not used on a daily basis but rather at longer intervals.15 A downside to sulfates is that they can have cosmetically unpleasant properties, which can be compensated for by including appropriate softening additives in shampoo formulations.4 A number of anionic surfactants such as olefin sulfonate, alkyl sulfosuccinate, acyl peptides, and alkyl ether carboxylates are well tolerated by the skin and are used together with other anionic and amphoteric surfactants to optimize shampoo properties. Alternatively, sulfate-free shampoos are cleansers compounded by the removal of the anionic group and switched for surfactants with less detergency.4,5
Preservatives and Parabens
Parabens refer to a group of esters of 4-hydroxybenzoic acid commonly used as preservatives in foods, pharmaceuticals, and cosmetics whose widespread use dates back to 1923.16 Concerns over the presence of parabens in shampoos and other cosmetics have been raised by patients for their reputed estrogenic and antiandrogenic effects and suspected involvement in carcinogenesis via endocrine modulation.16,17 In in vitro studies done on yeast assays, parabens have shown weak estrogenic activity that increases in proportion to both the length and increased branching of the alkyl side chains in the paraben’s molecular structure.18 They are 10,000-fold less potent than 17β-estradiol. In in vivo animal studies, parabens show weak estrogenic activity and are 100,000-fold less potent than 17β-estradiol.18 4-Hydroxybenzoic acid, a common metabolite, showed no estrogenic activity when tested both in vitro and in vivo.19 Some concerning research has implicated a link between parabens used in underarm cosmetics, such as deodorants and antiperspirants, and breast cancer16; however, the studies have been conflicting, and there is simply not enough data to assert that parabens cause breast cancer.
The Cosmetic Ingredient Review expert panel first reviewed parabens in 1984 and concluded that “methylparaben, ethylparaben, propylparaben, and butylparaben are safe as cosmetic ingredients in the present practices of use.”20 They extended this statement to include isopropylparaben and isobutylparaben in a later review.21 In 2005, the Scientific Committee on Consumer Products (now known as the Scientific Committee for Consumer Safety) in Europe stated that methylparaben and ethylparaben can be used at levels up to 0.4% in products.22 This decision was reached due to reports of decreased sperm counts and testosterone levels in male juvenile rats exposed to these parabens; however, these reults were not successfully replicated in larger studies.16,22 In 2010, the Scientific Committee for Consumer Safety revisited its stance on parabens, and they then revised their recommendations to say that concentrations of propylparaben and butylparaben should not exceed concentrations of 0.19%, based on “the conservative choice for the calculation of the [Margin-of-Safety] of butyl- and propylparaben.”23 However, in 2011 the use of propylparaben and butylparaben was banned in Denmark for cosmetic products used in children 3 years or younger,16 and the European Commission subsequently amended their directive in 2014, banning isopropylparaben, isobutylparaben, phenylparaben, benzylparaben, and pentylparaben due to lack of data available to evaluate the human risk of these products.24
Contrary to the trends in Europe, there currently are no regulations against the use of parabens in shampoos or other cosmetics in the United States. The American Cancer Society found that there is no evidence to suggest that the current levels of parabens in cosmetic products (eg, antiperspirants) increase one’s risk of breast cancer.25 Parabens are readily absorbed into the body both transdermally and through ingestion but also are believed to be rapidly transformed into harmless and nonspecific metabolites; they are readily metabolized by the liver and excreted in urine, and there is no measured accumulation in tissues.17
Parabens continue to be the most widely used preservatives in personal care products, usually in conjunction with other preservatives. Parabens are good biocides; short-chain esters (eg, methylparabens, ethylparabens) are effective against gram-positive bacteria and are weakly effective against gram-negative bacteria. Long-chain paraben esters (eg, propylparabens, butylparabens) are effective against mold and yeast. The addition of other preservatives creates a broad spectrum of antimicrobial defense in consumer products. Other preservatives include formaldehyde releasers or phenoxyethanol, as well as chelating agents such as EDTA, which improve the stability of these cosmetic products when exposed to air.16 Parabens are naturally occurring substances found in foods such as blueberries, barley, strawberries, yeast, olives, and grapes. As a colorless, odorless, and inexpensive substance, their use has been heavily favored in cosmetic and food products.16
Shampoo Alternatives and the No-Poo Method
Although research has not demonstrated any long-term danger to using shampoo, certain chemicals found in shampoos have the potential to irritate the scalp. Commonly cited allergens in shampoos include cocamidopropyl betaine, propylene glycol, vitamin E (tocopherol), parabens, and benzophenones.5 Additionally, the rising use of formaldehyde-releasing preservatives and isothiazolinones due to mounting pressures to move away from parabens has led to an increase in cases of allergic contact dermatitis (ACD).16 However, the irritability (rather than allergenicity) of these substances often is established during patch testing, a method of detecting delayed-type allergic reactions, which is important to note because patch testing requires a substance to be exposed to the skin for 24 to 48 hours, whereas exposure to shampoo ingredients may last a matter of minutes at most and occur in lesser concentrations because the ingredients are diluted by water in the rinsing process. Given these differences, it is unlikely that a patient would develop a true allergic response from regular shampoo use. Nevertheless, in patients who are already sensitized, exposure could conceivably trigger ACD, and patients must be cognizant of the composition of their shampoos.16
The no-poo method refers to the avoidance of commercial shampoo products when cleansing the hair and scalp and encompasses different methods of cleansing the hair, such as the use of household items (eg, baking soda, apple cider vinegar [ACV]), the use of conditioners to wash the hair (also known as conditioner-only washing or co-washing), treating the scalp with tea tree oil, or simply rinsing the hair with water. Proponents of the no-poo method believe that abstaining from shampoo use leads to healthier hair, retained natural oils, and less exposure to supposedly dangerous chemicals such as parabens or sulfates.2,3,26-28 However, there are no known studies in the literature that assess or support the hypotheses of the no-poo method.
Baking Soda and ACV
Baking soda (sodium bicarbonate) is a substance commonly found in the average household. It has been used in toothpaste formulas and cosmetic products and is known for its acid-neutralizing properties. Baking soda has been shown to have some antifungal and viricidal properties through an unknown mechanism of action.28 It has gained popularity for its use as a means of reducing the appearance of excessive greasiness of the hair shafts. Users also have reported that when washing their hair with baking soda, they are able to achieve a clean scalp and hair that feels soft to the touch.2,3,26,27,29 Despite these reports, users must beware of using baking soda without adequately diluting it with water. Baking soda is a known alkaline irritant.26,30 With a pH of 9, baking soda causes the cuticle layer of the hair fiber to open, increasing the capacity for water absorption. Water penetrates the scales that open, breaking the hydrogen bonds of the keratin molecule.31 Keratin is a spiral helical molecule that keeps its shape due to hydrogen, disulfide, and ionic bonds, as well as Van der Waals force.30 Hydrolysis of these bonds due to exposure to baking soda lowers the elasticity of the hair and increases the negative electrical net charge of the hair fiber surface, which leads to increased friction between fibers, cuticle damage, hair fragility, and fiber breakage.32,33
Apple cider vinegar is an apple-derived acetic acid solution with a pH ranging from 3.1 to 5.28 The pH range of ACV is considered to be ideal for hair by no-poo proponents, as it is similar to the natural pH of the scalp. Its acidic properties are responsible for its antimicrobial abilities, particularly its effectiveness against gram-negative bacteria.30 The acetic acid of ACV can partially interrupt oil interfaces, which contributes to its mild ability to remove product residue and scalp buildup from the hair shaft; the acetic acid also tightens the cuticles on hair fibers.33 Apple cider vinegar is used as a means of cleansing the hair and scalp by no-poo proponents2,3,26; other uses for ACV include using it as a rinse following washing and/or conditioning of the hair or as a means of preserving color in color-treated hair. There also is evidence that ACV may have antifungal properties.28 However, consumers must be aware that if it is not diluted in water, ACV may be too caustic for direct application to the hair and may lead to damage; it can be irritating to eyes, mucus membranes, and acutely inflamed skin. Also, vinegar rinses used on processed or chemically damaged hair may lead to increased hair fragility.2,3
Hair fibers have a pH of 3.67, while the scalp has a pH between 4.5 and 6.2. This slightly acidic film acts as a barrier to viruses, bacteria, and other potential contaminants.33 Studies have shown that the pH of skin increases in proportion to the pH of the cleanser used.34 Therefore, due to the naturally acidic pH of the scalp, acid-balanced shampoos generally are recommended. Shampoos should not have a pH higher than 5.5, as hair shafts can swell due to alkalinization, which can be prevented by pH balancing the shampoo through the addition of an acidic substance (eg, glycolic acid, citric acid) to lower the pH down to approximately 5.5. Apple cider vinegar often is used for this purpose. However, one study revealed that 82% of shampoos already have an acidic pH.34
Conditioner-Only Washing (Co-washing)
Conditioner-only washing, or co-washing, is a widely practiced method of hair grooming. It is popular among individuals who find that commercial shampoos strip too much of the natural hair oils away, leaving the hair rough or unmanageable. Co-washing is not harmful to the hair; however, the molecular structure and function of a conditioner and that of a shampoo are very different.5,35,36 Conditioners are not formulated to remove dirt and buildup in the hair but rather to add substances to the hair, and thus cannot provide extensive cleansing of the hair and scalp; therefore, it is inappropriate to use co-washing as a replacement for shampooing. Quaternary conditioning agents are an exception because they contain amphoteric detergents comprised of both anionic and cationic groups, which allow them both the ability to remove dirt and sebum with its anionic group, typically found in shampoos, as well as the ability to coat and condition the hair due to the high affinity of the cationic group for the negatively charged hair fibers.36,37 Amphoteric detergents are commonly found in 2-in-1 conditioning cleansers, among other ingredients, such as hydrolyzed animal proteins that temporarily plug surface defects on the hair fiber, and dimethicone, a synthetic oil that creates a thin film over the hair shaft, increasing shine and manageability. Of note, these conditioning shampoos are ideal for individuals with minimal product buildup on the hair and scalp and are not adequate scalp cleansers for individuals who either wash their hair infrequently or who regularly use hairstyling products.36,37
Tea Tree Oil
Tea tree oil is an essential oil extracted from the Melaleuca alternifolia plant of the Myrtaceae family. It is native to the coast of northeastern Australia. A holy grail of natural cosmetics, tea tree oil is widely known for its antiviral, antifungal, and antiseptic properties.38 Although not used as a stand-alone cleanser, it is often added to a number of cosmetic products, including shampoos and co-washes. Although deemed safe for topical use, it has been shown to be quite toxic when ingested. Symptoms of ingestion include nausea, vomiting, hallucinations, and coma. The common concern with tea tree oil is its ability to cause ACD. In particular, it is believed that the oxidation products of tea tree oil are allergenic rather than the tea tree oil itself. The evaluation of tea tree oil as a potential contact allergen has been quite difficult; it consists of more than 100 distinct compounds and is often mislabeled, or does not meet the guidelines of the International Organization for Standardization. Nonetheless, the prevalence of ACD due to tea tree oil is low (approximately 1.4%). Despite its low prevalence, tea tree oil should remain in the differential as an ACD-inducing agent. Patch testing with the patient’s supply of tea tree oil is advised when possible.38
Conclusion
It is customary that the ingredients used in shampoos undergo periodic testing and monitoring to assure the safety of their use. Although it is encouraging that patients are proactive in their efforts to stay abreast of the literature, it is still important that cosmetic scientists, dermatologists, and other experts remain at the forefront of educating the public about these substances. Not doing so can result in the propagation of misinformation and unnecessary fears, which can lead to the adaptation of unhygienic or even unsafe hair care practices. As dermatologists, we must ensure that patients are educated about the benefits and hazards of off-label use of household ingredients to the extent that evidence-based medicine permits. Patients must be informed that not all synthetic substances are harmful, and likewise not all naturally occurring substances are safe.
Shampoo is a staple in hair grooming that is ever-evolving along with cultural trends. The global shampoo market is expected to reach an estimated value of $25.73 billion by 2019. A major driver of this upward trend in market growth is the increasing demand for natural and organic hair shampoos.1 Society today has a growing fixation on healthy living practices, and as of late, the ingredients in shampoos and other cosmetic products have become one of the latest targets in the health-consciousness craze. In the age of the Internet where information—and misinformation—is widely accessible and dispersed, the general public often strives to self-educate on specialized matters that are out of their expertise. As a result, individuals have developed an aversion to using certain shampoos out of fear that the ingredients, often referred to as “chemicals” by patients due to their complex names, are unnatural and therefore unhealthy.1,2 Product developers are working to meet the demand by reformulating shampoos with labels that indicate sulfate free or paraben free, despite the lack of proof that these formulations are an improvement over traditional approaches to hair health. Additionally, alternative methods of cleansing the hair and scalp, also known as the no-shampoo or “no-poo” method, have begun to gain popularity.2,3
It is essential that dermatologists acknowledge the concerns that their patients have about common shampoo ingredients to dispel the myths that may misinform patient decision-making. This article reviews the controversy surrounding the use of sulfates and parabens in shampoos as well as commonly used shampoo alternatives. Due to the increased prevalence of dry hair shafts in the skin of color population, especially black women, this group is particularly interested in products that will minimize breakage and dryness of the hair. To that end, this population has great interest in the removal of chemical ingredients that may cause damage to the hair shafts, despite the lack of data to support sulfates and paraben damage to hair shafts or scalp skin. Blogs and uninformed hairstylists may propagate these beliefs in a group of consumers who are desperate for new approaches to hair fragility and breakage.
Surfactants and Sulfates
The cleansing ability of a shampoo depends on the surface activity of its detergents. Surface-active ingredients, or surfactants, reduce the surface tension between water and dirt, thus facilitating the removal of environmental dirt from the hair and scalp,4 which is achieved by a molecular structure containing both a hydrophilic and a lipophilic group. Sebum and dirt are bound by the lipophilic ends of the surfactant, becoming the center of a micelle structure with the hydrophilic molecule ends pointing outward. Dirt particles become water soluble and are removed from the scalp and hair shaft upon rinsing with water.4
Surfactants are classified according to the electric charge of the hydrophilic polar group as either anionic, cationic, amphoteric (zwitterionic), or nonionic.5 Each possesses different hair conditioning and cleansing qualities, and multiple surfactants are used in shampoos in differing ratios to accommodate different hair types. In most shampoos, the base consists of anionic and amphoteric surfactants. Depending on individual product requirements, nonionic and cationic surfactants are used to either modify the effects of the surfactants or as conditioning agents.4,5
One subcategory of surfactants that receives much attention is the group of anionic surfactants known as sulfates. Sulfates, particularly sodium lauryl sulfate (SLS), recently have developed a negative reputation as cosmetic ingredients, as reports from various unscientific sources have labeled them as hazardous to one’s health; SLS has been described as a skin and scalp irritant, has been linked to cataract formation, and has even been wrongly labeled as carcinogenic.6 The origins of some of these claims are not clear, though they likely arose from the misinterpretation of complex scientific studies that are easily accessible to laypeople. The link between SLS and ocular irritation or cataract formation is a good illustration of this unsubstantiated fear. A study by Green et al7 showed that corneal exposure to extremely high concentrations of SLS following physical or chemical damage to the eye can result in a slowed healing process. The results of this study have since been wrongly quoted to state that SLS-containing products lead to blindness or severe corneal damage.8 A different study tested for possible ocular irritation in vivo by submerging the lens of an eye into a 20% SLS solution, which accurately approximates the concentration of SLS in rinse-off consumer products.9 However, to achieve ocular irritation, the eyes of laboratory animals were exposed to SLS constantly for 14 days, which would not occur in practical use.9 Similarly, a third study achieved cataract formation in a laboratory only by immersing the lens of an eye into a highly concentrated solution of SLS.10 Such studies are not appropriate representations of how SLS-containing products are used by consumers and have unfortunately been vulnerable to misinterpretation by the general public.
There is no known study that has shown SLS to be carcinogenic. One possible origin of this idea may be from the wrongful interpretation of studies that used SLS as a vehicle substance to test agents that were deemed to be carcinogenic.11 Another possible source of the idea that SLS is carcinogenic comes from its association with 1,4-dioxane, a by-product of the synthesis of certain sulfates such as sodium laureth sulfate due to a process known as ethoxylation.6,12 Although SLS does not undergo this process in its formation and is not linked to 1,4-dioxane, there is potential for cross-contamination of SLS with 1,4-dioxane, which cannot be overlooked. 1,4-Dioxane is classified as “possibly carcinogenic to humans (Group 2B)” by the International Agency for Research on Cancer,13 but screening of SLS for this substance prior to its use in commercial products is standard.
Sulfates are inexpensive detergents that are responsible for lather formation in shampoos as well as in many household cleaning agents.5 Sulfates, similar to all anionic surfactants, are characterized by a negatively charged hydrophilic polar group. The best-known and most commonly used anionic surfactants are sulfated fatty alcohols, alkyl sulfates, and their polyethoxylated analogues alkyl ether sulfates.5,6 Sodium lauryl sulfate (also known as sodium laurilsulfate or sodium dodecyl sulfate) is the most common of them all, found in shampoo and conditioner formulations. Ammonium lauryl sulfate and sodium laureth sulfate are other sulfates commonly used in shampoos and household cleansing products. Sodium lauryl sulfate is a nonvolatile, water-soluble compound. Its partition coefficient (P0), a measure of a substance’s hydrophilic or lipophilic nature, is low at 1.6, making it a rather hydrophilic substance.6 Hydrophilic substances tend to have low bioaccumulation profiles in the body. Additionally, SLS is readily biodegradable. It can be derived from both synthetic and naturally occurring sources; for example, palm kernel oil, petrolatum, and coconut oil are all sources of lauric acid, the starting ingredient used to synthesize SLS. Sodium lauryl sulfate is created by reacting lauryl alcohol with sulfur trioxide gas, followed by neutralization with sodium carbonate (also a naturally occurring compound).6 Sodium lauryl sulfate and other sulfate-containing shampoos widely replaced the usage of traditional soaps formulated from animal or vegetable fats, as these latter formations created a film of insoluble calcium salts on the hair strands upon contact with water, resulting in tangled, dull-appearing hair.5 Additionally, sulfates were preferred to the alkaline pH of traditional soap, which can be harsh on hair strands and cause irritation of the skin and mucous membranes.14 Because they are highly water soluble, sulfates enable the formulation of clear shampoos. They exhibit remarkable cleaning properties and lather formation.5,14
Because sulfates are potent surfactants, they can remove dirt and debris as well as naturally produced healthy oils from the hair and scalp. As a result, sulfates can leave the hair feeling dry and stripped of moisture.4,5 Sulfates are used as the primary detergents in the formulation of deep-cleaning shampoos, which are designed for people who accumulate a heavy buildup of dirt, sebum, and debris from frequent use of styling products. Due to their potent detergency, these shampoos typically are not used on a daily basis but rather at longer intervals.15 A downside to sulfates is that they can have cosmetically unpleasant properties, which can be compensated for by including appropriate softening additives in shampoo formulations.4 A number of anionic surfactants such as olefin sulfonate, alkyl sulfosuccinate, acyl peptides, and alkyl ether carboxylates are well tolerated by the skin and are used together with other anionic and amphoteric surfactants to optimize shampoo properties. Alternatively, sulfate-free shampoos are cleansers compounded by the removal of the anionic group and switched for surfactants with less detergency.4,5
Preservatives and Parabens
Parabens refer to a group of esters of 4-hydroxybenzoic acid commonly used as preservatives in foods, pharmaceuticals, and cosmetics whose widespread use dates back to 1923.16 Concerns over the presence of parabens in shampoos and other cosmetics have been raised by patients for their reputed estrogenic and antiandrogenic effects and suspected involvement in carcinogenesis via endocrine modulation.16,17 In in vitro studies done on yeast assays, parabens have shown weak estrogenic activity that increases in proportion to both the length and increased branching of the alkyl side chains in the paraben’s molecular structure.18 They are 10,000-fold less potent than 17β-estradiol. In in vivo animal studies, parabens show weak estrogenic activity and are 100,000-fold less potent than 17β-estradiol.18 4-Hydroxybenzoic acid, a common metabolite, showed no estrogenic activity when tested both in vitro and in vivo.19 Some concerning research has implicated a link between parabens used in underarm cosmetics, such as deodorants and antiperspirants, and breast cancer16; however, the studies have been conflicting, and there is simply not enough data to assert that parabens cause breast cancer.
The Cosmetic Ingredient Review expert panel first reviewed parabens in 1984 and concluded that “methylparaben, ethylparaben, propylparaben, and butylparaben are safe as cosmetic ingredients in the present practices of use.”20 They extended this statement to include isopropylparaben and isobutylparaben in a later review.21 In 2005, the Scientific Committee on Consumer Products (now known as the Scientific Committee for Consumer Safety) in Europe stated that methylparaben and ethylparaben can be used at levels up to 0.4% in products.22 This decision was reached due to reports of decreased sperm counts and testosterone levels in male juvenile rats exposed to these parabens; however, these reults were not successfully replicated in larger studies.16,22 In 2010, the Scientific Committee for Consumer Safety revisited its stance on parabens, and they then revised their recommendations to say that concentrations of propylparaben and butylparaben should not exceed concentrations of 0.19%, based on “the conservative choice for the calculation of the [Margin-of-Safety] of butyl- and propylparaben.”23 However, in 2011 the use of propylparaben and butylparaben was banned in Denmark for cosmetic products used in children 3 years or younger,16 and the European Commission subsequently amended their directive in 2014, banning isopropylparaben, isobutylparaben, phenylparaben, benzylparaben, and pentylparaben due to lack of data available to evaluate the human risk of these products.24
Contrary to the trends in Europe, there currently are no regulations against the use of parabens in shampoos or other cosmetics in the United States. The American Cancer Society found that there is no evidence to suggest that the current levels of parabens in cosmetic products (eg, antiperspirants) increase one’s risk of breast cancer.25 Parabens are readily absorbed into the body both transdermally and through ingestion but also are believed to be rapidly transformed into harmless and nonspecific metabolites; they are readily metabolized by the liver and excreted in urine, and there is no measured accumulation in tissues.17
Parabens continue to be the most widely used preservatives in personal care products, usually in conjunction with other preservatives. Parabens are good biocides; short-chain esters (eg, methylparabens, ethylparabens) are effective against gram-positive bacteria and are weakly effective against gram-negative bacteria. Long-chain paraben esters (eg, propylparabens, butylparabens) are effective against mold and yeast. The addition of other preservatives creates a broad spectrum of antimicrobial defense in consumer products. Other preservatives include formaldehyde releasers or phenoxyethanol, as well as chelating agents such as EDTA, which improve the stability of these cosmetic products when exposed to air.16 Parabens are naturally occurring substances found in foods such as blueberries, barley, strawberries, yeast, olives, and grapes. As a colorless, odorless, and inexpensive substance, their use has been heavily favored in cosmetic and food products.16
Shampoo Alternatives and the No-Poo Method
Although research has not demonstrated any long-term danger to using shampoo, certain chemicals found in shampoos have the potential to irritate the scalp. Commonly cited allergens in shampoos include cocamidopropyl betaine, propylene glycol, vitamin E (tocopherol), parabens, and benzophenones.5 Additionally, the rising use of formaldehyde-releasing preservatives and isothiazolinones due to mounting pressures to move away from parabens has led to an increase in cases of allergic contact dermatitis (ACD).16 However, the irritability (rather than allergenicity) of these substances often is established during patch testing, a method of detecting delayed-type allergic reactions, which is important to note because patch testing requires a substance to be exposed to the skin for 24 to 48 hours, whereas exposure to shampoo ingredients may last a matter of minutes at most and occur in lesser concentrations because the ingredients are diluted by water in the rinsing process. Given these differences, it is unlikely that a patient would develop a true allergic response from regular shampoo use. Nevertheless, in patients who are already sensitized, exposure could conceivably trigger ACD, and patients must be cognizant of the composition of their shampoos.16
The no-poo method refers to the avoidance of commercial shampoo products when cleansing the hair and scalp and encompasses different methods of cleansing the hair, such as the use of household items (eg, baking soda, apple cider vinegar [ACV]), the use of conditioners to wash the hair (also known as conditioner-only washing or co-washing), treating the scalp with tea tree oil, or simply rinsing the hair with water. Proponents of the no-poo method believe that abstaining from shampoo use leads to healthier hair, retained natural oils, and less exposure to supposedly dangerous chemicals such as parabens or sulfates.2,3,26-28 However, there are no known studies in the literature that assess or support the hypotheses of the no-poo method.
Baking Soda and ACV
Baking soda (sodium bicarbonate) is a substance commonly found in the average household. It has been used in toothpaste formulas and cosmetic products and is known for its acid-neutralizing properties. Baking soda has been shown to have some antifungal and viricidal properties through an unknown mechanism of action.28 It has gained popularity for its use as a means of reducing the appearance of excessive greasiness of the hair shafts. Users also have reported that when washing their hair with baking soda, they are able to achieve a clean scalp and hair that feels soft to the touch.2,3,26,27,29 Despite these reports, users must beware of using baking soda without adequately diluting it with water. Baking soda is a known alkaline irritant.26,30 With a pH of 9, baking soda causes the cuticle layer of the hair fiber to open, increasing the capacity for water absorption. Water penetrates the scales that open, breaking the hydrogen bonds of the keratin molecule.31 Keratin is a spiral helical molecule that keeps its shape due to hydrogen, disulfide, and ionic bonds, as well as Van der Waals force.30 Hydrolysis of these bonds due to exposure to baking soda lowers the elasticity of the hair and increases the negative electrical net charge of the hair fiber surface, which leads to increased friction between fibers, cuticle damage, hair fragility, and fiber breakage.32,33
Apple cider vinegar is an apple-derived acetic acid solution with a pH ranging from 3.1 to 5.28 The pH range of ACV is considered to be ideal for hair by no-poo proponents, as it is similar to the natural pH of the scalp. Its acidic properties are responsible for its antimicrobial abilities, particularly its effectiveness against gram-negative bacteria.30 The acetic acid of ACV can partially interrupt oil interfaces, which contributes to its mild ability to remove product residue and scalp buildup from the hair shaft; the acetic acid also tightens the cuticles on hair fibers.33 Apple cider vinegar is used as a means of cleansing the hair and scalp by no-poo proponents2,3,26; other uses for ACV include using it as a rinse following washing and/or conditioning of the hair or as a means of preserving color in color-treated hair. There also is evidence that ACV may have antifungal properties.28 However, consumers must be aware that if it is not diluted in water, ACV may be too caustic for direct application to the hair and may lead to damage; it can be irritating to eyes, mucus membranes, and acutely inflamed skin. Also, vinegar rinses used on processed or chemically damaged hair may lead to increased hair fragility.2,3
Hair fibers have a pH of 3.67, while the scalp has a pH between 4.5 and 6.2. This slightly acidic film acts as a barrier to viruses, bacteria, and other potential contaminants.33 Studies have shown that the pH of skin increases in proportion to the pH of the cleanser used.34 Therefore, due to the naturally acidic pH of the scalp, acid-balanced shampoos generally are recommended. Shampoos should not have a pH higher than 5.5, as hair shafts can swell due to alkalinization, which can be prevented by pH balancing the shampoo through the addition of an acidic substance (eg, glycolic acid, citric acid) to lower the pH down to approximately 5.5. Apple cider vinegar often is used for this purpose. However, one study revealed that 82% of shampoos already have an acidic pH.34
Conditioner-Only Washing (Co-washing)
Conditioner-only washing, or co-washing, is a widely practiced method of hair grooming. It is popular among individuals who find that commercial shampoos strip too much of the natural hair oils away, leaving the hair rough or unmanageable. Co-washing is not harmful to the hair; however, the molecular structure and function of a conditioner and that of a shampoo are very different.5,35,36 Conditioners are not formulated to remove dirt and buildup in the hair but rather to add substances to the hair, and thus cannot provide extensive cleansing of the hair and scalp; therefore, it is inappropriate to use co-washing as a replacement for shampooing. Quaternary conditioning agents are an exception because they contain amphoteric detergents comprised of both anionic and cationic groups, which allow them both the ability to remove dirt and sebum with its anionic group, typically found in shampoos, as well as the ability to coat and condition the hair due to the high affinity of the cationic group for the negatively charged hair fibers.36,37 Amphoteric detergents are commonly found in 2-in-1 conditioning cleansers, among other ingredients, such as hydrolyzed animal proteins that temporarily plug surface defects on the hair fiber, and dimethicone, a synthetic oil that creates a thin film over the hair shaft, increasing shine and manageability. Of note, these conditioning shampoos are ideal for individuals with minimal product buildup on the hair and scalp and are not adequate scalp cleansers for individuals who either wash their hair infrequently or who regularly use hairstyling products.36,37
Tea Tree Oil
Tea tree oil is an essential oil extracted from the Melaleuca alternifolia plant of the Myrtaceae family. It is native to the coast of northeastern Australia. A holy grail of natural cosmetics, tea tree oil is widely known for its antiviral, antifungal, and antiseptic properties.38 Although not used as a stand-alone cleanser, it is often added to a number of cosmetic products, including shampoos and co-washes. Although deemed safe for topical use, it has been shown to be quite toxic when ingested. Symptoms of ingestion include nausea, vomiting, hallucinations, and coma. The common concern with tea tree oil is its ability to cause ACD. In particular, it is believed that the oxidation products of tea tree oil are allergenic rather than the tea tree oil itself. The evaluation of tea tree oil as a potential contact allergen has been quite difficult; it consists of more than 100 distinct compounds and is often mislabeled, or does not meet the guidelines of the International Organization for Standardization. Nonetheless, the prevalence of ACD due to tea tree oil is low (approximately 1.4%). Despite its low prevalence, tea tree oil should remain in the differential as an ACD-inducing agent. Patch testing with the patient’s supply of tea tree oil is advised when possible.38
Conclusion
It is customary that the ingredients used in shampoos undergo periodic testing and monitoring to assure the safety of their use. Although it is encouraging that patients are proactive in their efforts to stay abreast of the literature, it is still important that cosmetic scientists, dermatologists, and other experts remain at the forefront of educating the public about these substances. Not doing so can result in the propagation of misinformation and unnecessary fears, which can lead to the adaptation of unhygienic or even unsafe hair care practices. As dermatologists, we must ensure that patients are educated about the benefits and hazards of off-label use of household ingredients to the extent that evidence-based medicine permits. Patients must be informed that not all synthetic substances are harmful, and likewise not all naturally occurring substances are safe.
- The global shampoo market 2014-2019 trends, forecast, and opportunity analysis [press release]. New York, NY: Reportlinker; May 21, 2015.
- Is the ‘no shampoo’ trend healthy or harmful? Mercola website. Published January 16, 2016. Accessed December 8, 2017.
- Feltman R. The science (or lack thereof) behind the ‘no-poo’ hair trend. Washington Post. March 10, 2016. https://www.washingtonpost.com/news/speaking-of-science/wp/2016/03/10/the-science-or-lack-thereof-behind-the-no-poo-hair-trend/?utm_term=.9a61edf3fd5a. Accessed December 11, 2017.
- Bouillon C. Shampoos. Clin Dermatol. 1996;14:113-121.
- Trueb RM. Shampoos: ingredients, efficacy, and adverse effects. J Dtsch Dermatol Ges. 2007;5:356-365.
- Bondi CA, Marks JL, Wroblewski LB, et al. Human and environmental toxicity of sodium lauryl sulfate (SLS): evidence for safe use in household cleaning products. Environ Health Insights. 2015;9:27-32.
- Green K, Johnson RE, Chapman JM, et al. Preservative effects on the healing rate of rabbit corneal epithelium. Lens Eye Toxic Res. 1989;6:37-41.
- Sodium lauryl sulphate. Healthy Choices website. http://www.healthychoices.co.uk/sls.html. Accessed December 8, 2017.
- Tekbas¸ ÖF, Uysal Y, Og˘ur R, et al. Non-irritant baby shampoos may cause cataract development. TSK Koruyucu Hekimlik Bülteni. 2008;1:1-6.
- Cater KC, Harbell JW. Prediction of eye irritation potential of surfactant-based rinse-off personal care formulations by the bovine corneal opacity and permeability (BCOP) assay. Cutan Ocul Toxicol. 2006;25:217-233.
- Birt DF, Lawson TA, Julius AD, et al. Inhibition by dietary selenium of colon cancer induced in the rat by bis(2-oxopropyl) nitrosamine. Cancer Res. 1982;42:4455-4459.
- Rastogi SC. Headspace analysis of 1,4-dioxane in products containing polyethoxylated surfactants by GC-MS. Chromatographia. 1990;29:441-445.
- 1,4-Dioxane. IARC Monogr Eval Carcinog Risks Hum. 1999;71, pt 2:589-602.
- Trueb RM. Dermocosmetic aspects of hair and scalp. J Investig Dermatol Symp Proc. 2005;10:289-292.
- D’Souza P, Rathi SK. Shampoo and conditioners: what a dermatologist should know? Indian J Dermatol. 2015;60:248-254.
- Sasseville D, Alfalah M, Lacroix JP. “Parabenoia” debunked, or “who’s afraid of parabens?” Dermatitis. 2015;26:254-259.
- Krowka JF, Loretz L, Geis PA, et al. Preserving the facts on parabens: an overview of these important tools of the trade. Cosmetics & Toiletries. http://www.cosmeticsandtoiletries.com/research/chemistry/Preserving-the-Facts-on-Parabens-An-Overview-of-These-Important-Tools-of-the Trade-425784294.html. Published June 1, 2017. Accessed December 20, 2017.
- Routledge EJ, Parker J, Odum J, et al. Some alkyl hydroxy benzoate preservatives (parabens) are estrogenic. Toxicol Appl Pharmacol. 1998;153:12Y19.
- Hossaini A, Larsen JJ, Larsen JC. Lack of oestrogenic effects of food preservatives (parabens) in uterotrophic assays. Food Chem Toxicol. 2000;38:319-323.
- Cosmetic Ingredient Review. Final report on the safety assessment of methylparaben, ethylparaben, propylparaben and butylparaben. J Am Coll Toxicol. 1984;3:147-209.
- Cosmetic Ingredient Review. Final report on the safety assessment of isobutylparaben and isopropylparaben. J Am Coll Toxicol. 1995;14:364-372.
- Scientific Committee on Consumer Products. Extended Opinion on the Safety Evaluation of Parabens. European Commission website. https://ec.europa.eu/health/ph_risk/committees/04_sccp/docs/sccp_o_019.pdf. Published January 28, 2005. Accessed December 20, 2017.
- Scientific Committee on Consumer Products. Opinion on Parabens. European Commission website. http://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_041.pdf. Revised March 22, 2011. Accessed December 20, 2017.
- European Commission. Commission Regulation (EU) No 258/2014 of 9 April 2014 amending Annexes II and V to Regulation (EC) No 1223/2009 of the European Parliament and of the Council on cosmetic products. EUR-Lex website. http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv:OJ.L_.2014.107.01.0005.01.ENG. Accessed December 20, 2017.
- American Cancer Society. Antiperspirants and breast cancer risk. https://www.cancer.org/cancer/cancer-causes/antiperspirants-and-breast-cancer-risk.html#references. Revised October 14, 2014. Accessed January 2, 2018.
- MacMillan A. Cutting back on shampoo? 15 things you should know. Health. February 25, 2014. http://www.health.com/health/gallery/0,,20788089,00.html#should-you-go-no-poo--1. Accessed December 10, 2017.
- The ‘no poo’ method. https://www.nopoomethod.com/. Accessed December 10, 2017.
- Fong, D, Gaulin C, Le M, et al. Effectiveness of alternative antimicrobial agents for disinfection of hard surfaces. National Collaborating Centre for Environmental Health website. http://www.ncceh.ca/sites/default/files/Alternative_Antimicrobial_Agents_Aug_2014.pdf. Published August 2014. Accessed December 10, 2017.
- Is baking soda too harsh for natural hair? Black Girl With Long Hair website. http://blackgirllonghair.com/2012/02/is-baking-soda-too-harsh-for-hair/2/. Published February 5, 2012. Accessed December 12, 2017.
- O’Lenick T. Anionic/cationic complexes in hair care. J Cosmet Sci. 2011;62:209-228.
- Gavazzoni Dias MF, de Almeida AM, Cecato PM, et al. The shampoo pH can affect the hair: myth or reality? Int J Trichology. 2014;6:95-99.
- Goodman H. The acid mantle of the skin surface. Ind Med Surg. 1958;27:105-108.
- Korting HC, Kober M, Mueller M, et al. Influence of repeated washings with soap and synthetic detergents on pH and resident flora of the skin of forehead and forearm. results of a cross-over trial in health probationers. Acta Derm Venereol. 1987;67:41-47.
- Tarun J, Susan J, Suria J, et al. Evaluation of pH of bathing soaps and shampoos for skin and hair care. Indian J Dermatol. 2014;59:442-444.
- Corbett JF. The chemistry of hair-care products. J Soc Dyers Colour. 1976;92:285-303.
- McMichael AJ, Hordinsky M. Hair Diseases: Medical, Surgical, and Cosmetic Treatments. New York, NY: Taylor & Francis; 2008:59-72.
- Allardice A, Gummo G. Hair conditioning: quaternary ammonium compounds on various hair types. Cosmet Toiletries. 1993;108:107-109.
- Larson D, Jacob SE. Tea tree oil. Dermatitis. 2012;23:48-49.
- The global shampoo market 2014-2019 trends, forecast, and opportunity analysis [press release]. New York, NY: Reportlinker; May 21, 2015.
- Is the ‘no shampoo’ trend healthy or harmful? Mercola website. Published January 16, 2016. Accessed December 8, 2017.
- Feltman R. The science (or lack thereof) behind the ‘no-poo’ hair trend. Washington Post. March 10, 2016. https://www.washingtonpost.com/news/speaking-of-science/wp/2016/03/10/the-science-or-lack-thereof-behind-the-no-poo-hair-trend/?utm_term=.9a61edf3fd5a. Accessed December 11, 2017.
- Bouillon C. Shampoos. Clin Dermatol. 1996;14:113-121.
- Trueb RM. Shampoos: ingredients, efficacy, and adverse effects. J Dtsch Dermatol Ges. 2007;5:356-365.
- Bondi CA, Marks JL, Wroblewski LB, et al. Human and environmental toxicity of sodium lauryl sulfate (SLS): evidence for safe use in household cleaning products. Environ Health Insights. 2015;9:27-32.
- Green K, Johnson RE, Chapman JM, et al. Preservative effects on the healing rate of rabbit corneal epithelium. Lens Eye Toxic Res. 1989;6:37-41.
- Sodium lauryl sulphate. Healthy Choices website. http://www.healthychoices.co.uk/sls.html. Accessed December 8, 2017.
- Tekbas¸ ÖF, Uysal Y, Og˘ur R, et al. Non-irritant baby shampoos may cause cataract development. TSK Koruyucu Hekimlik Bülteni. 2008;1:1-6.
- Cater KC, Harbell JW. Prediction of eye irritation potential of surfactant-based rinse-off personal care formulations by the bovine corneal opacity and permeability (BCOP) assay. Cutan Ocul Toxicol. 2006;25:217-233.
- Birt DF, Lawson TA, Julius AD, et al. Inhibition by dietary selenium of colon cancer induced in the rat by bis(2-oxopropyl) nitrosamine. Cancer Res. 1982;42:4455-4459.
- Rastogi SC. Headspace analysis of 1,4-dioxane in products containing polyethoxylated surfactants by GC-MS. Chromatographia. 1990;29:441-445.
- 1,4-Dioxane. IARC Monogr Eval Carcinog Risks Hum. 1999;71, pt 2:589-602.
- Trueb RM. Dermocosmetic aspects of hair and scalp. J Investig Dermatol Symp Proc. 2005;10:289-292.
- D’Souza P, Rathi SK. Shampoo and conditioners: what a dermatologist should know? Indian J Dermatol. 2015;60:248-254.
- Sasseville D, Alfalah M, Lacroix JP. “Parabenoia” debunked, or “who’s afraid of parabens?” Dermatitis. 2015;26:254-259.
- Krowka JF, Loretz L, Geis PA, et al. Preserving the facts on parabens: an overview of these important tools of the trade. Cosmetics & Toiletries. http://www.cosmeticsandtoiletries.com/research/chemistry/Preserving-the-Facts-on-Parabens-An-Overview-of-These-Important-Tools-of-the Trade-425784294.html. Published June 1, 2017. Accessed December 20, 2017.
- Routledge EJ, Parker J, Odum J, et al. Some alkyl hydroxy benzoate preservatives (parabens) are estrogenic. Toxicol Appl Pharmacol. 1998;153:12Y19.
- Hossaini A, Larsen JJ, Larsen JC. Lack of oestrogenic effects of food preservatives (parabens) in uterotrophic assays. Food Chem Toxicol. 2000;38:319-323.
- Cosmetic Ingredient Review. Final report on the safety assessment of methylparaben, ethylparaben, propylparaben and butylparaben. J Am Coll Toxicol. 1984;3:147-209.
- Cosmetic Ingredient Review. Final report on the safety assessment of isobutylparaben and isopropylparaben. J Am Coll Toxicol. 1995;14:364-372.
- Scientific Committee on Consumer Products. Extended Opinion on the Safety Evaluation of Parabens. European Commission website. https://ec.europa.eu/health/ph_risk/committees/04_sccp/docs/sccp_o_019.pdf. Published January 28, 2005. Accessed December 20, 2017.
- Scientific Committee on Consumer Products. Opinion on Parabens. European Commission website. http://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_041.pdf. Revised March 22, 2011. Accessed December 20, 2017.
- European Commission. Commission Regulation (EU) No 258/2014 of 9 April 2014 amending Annexes II and V to Regulation (EC) No 1223/2009 of the European Parliament and of the Council on cosmetic products. EUR-Lex website. http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv:OJ.L_.2014.107.01.0005.01.ENG. Accessed December 20, 2017.
- American Cancer Society. Antiperspirants and breast cancer risk. https://www.cancer.org/cancer/cancer-causes/antiperspirants-and-breast-cancer-risk.html#references. Revised October 14, 2014. Accessed January 2, 2018.
- MacMillan A. Cutting back on shampoo? 15 things you should know. Health. February 25, 2014. http://www.health.com/health/gallery/0,,20788089,00.html#should-you-go-no-poo--1. Accessed December 10, 2017.
- The ‘no poo’ method. https://www.nopoomethod.com/. Accessed December 10, 2017.
- Fong, D, Gaulin C, Le M, et al. Effectiveness of alternative antimicrobial agents for disinfection of hard surfaces. National Collaborating Centre for Environmental Health website. http://www.ncceh.ca/sites/default/files/Alternative_Antimicrobial_Agents_Aug_2014.pdf. Published August 2014. Accessed December 10, 2017.
- Is baking soda too harsh for natural hair? Black Girl With Long Hair website. http://blackgirllonghair.com/2012/02/is-baking-soda-too-harsh-for-hair/2/. Published February 5, 2012. Accessed December 12, 2017.
- O’Lenick T. Anionic/cationic complexes in hair care. J Cosmet Sci. 2011;62:209-228.
- Gavazzoni Dias MF, de Almeida AM, Cecato PM, et al. The shampoo pH can affect the hair: myth or reality? Int J Trichology. 2014;6:95-99.
- Goodman H. The acid mantle of the skin surface. Ind Med Surg. 1958;27:105-108.
- Korting HC, Kober M, Mueller M, et al. Influence of repeated washings with soap and synthetic detergents on pH and resident flora of the skin of forehead and forearm. results of a cross-over trial in health probationers. Acta Derm Venereol. 1987;67:41-47.
- Tarun J, Susan J, Suria J, et al. Evaluation of pH of bathing soaps and shampoos for skin and hair care. Indian J Dermatol. 2014;59:442-444.
- Corbett JF. The chemistry of hair-care products. J Soc Dyers Colour. 1976;92:285-303.
- McMichael AJ, Hordinsky M. Hair Diseases: Medical, Surgical, and Cosmetic Treatments. New York, NY: Taylor & Francis; 2008:59-72.
- Allardice A, Gummo G. Hair conditioning: quaternary ammonium compounds on various hair types. Cosmet Toiletries. 1993;108:107-109.
- Larson D, Jacob SE. Tea tree oil. Dermatitis. 2012;23:48-49.
Practice Points
- The ingredients in shampoos and other cosmetic products have become scrutinized by the general public and the Internet has contributed to misinformation about certain shampoos.
- Dermatologists must be prepared to acknowledge the concerns that their patients have about common shampoo ingredients to dispel the myths that may misinform patient decision-making.
- This article reviews the controversy surrounding the use of sulfates and parabens in shampoos, as well as commonly used shampoo alternatives, often called the “no-poo” method.
Long-term Pubic Dermatitis Diagnosed as White Piedra
Case Report
A 58-year-old man presented for evaluation of a pruritic rash involving the pubic area of 30 years’ duration. Multiple primary care physicians and dermatologists had evaluated the patient during this period, but he noted a specific diagnosis had not been rendered and multiple treatments had been unsuccessful. The patient described a rash, which was absent at the time of evaluation, as a self-remitting and exacerbating irritation typically induced by sweating and physical activity. The patient also stated that the irritation was associated with a strong, distinct, musty odor that severely interrupted his sex life and decreased his quality of life. Prior treatments included various topical corticosteroids, topical and oral antibiotics, and various homeopathic treatments that were minimally efficacious or nonefficacious. He was unsure if antifungals had previously been prescribed.
The patient’s medical history was notable for pulmonary interstitial fibrosis, anxiety, posttraumatic stress disorder, and mild glucose intolerance. The patient had no pertinent surgical history and no known drug allergies. Current medications included a bronchodilating inhaler, escitalopram, trazodone, buspirone, clonazepam, prazosin, gabapentin, and azithromycin for current upper respiratory tract infection. The patient was a former smoker and a social drinker.
On physical evaluation the pubic area displayed slight patchy erythema without a papular component and was otherwise unremarkable to the unaided eye. Upon palpation of the skin, there were no remarkable findings. Under dermoscopic evaluation, small white-yellow concretions along the hair shaft were noticed. Evaluation with a Wood lamp is shown in Figure 1.
The patient was treated empirically with ketoconazole cream 2% applied to the affected area once daily until follow-up 3 weeks later. The patient also was advised to shave the pubic area to remove potentially infected hairs, as white piedra (WP) was suspected. A diagnosis of WP was confirmed on histologic evaluation of pubic hair samples approximately 1 to 2 weeks later (Figure 2).
At 3-week follow-up, Wood lamp evaluation did not identify concretions along the pubic hair shafts. The patient was symptom free and extremely pleased. Of note, the patient did not shave the pubic area and was counseled on recurrence.
Comment
Piedra (meaning stone in Spanish) describes a group of fungal infections that present with gritty concretions on the hair shaft.1,2 In 1911, Horta3 classified piedra into 2 subtypes: black piedra, caused by Piedraia hortae, and WP, caused by Trichosporon species. Black piedra occurs more frequently in tropical countries and commonly affects hair shafts on the scalp.4 White piedra most commonly affects the pubic area, with rare cases in scalp and facial hair.1,5-7
Epidemiology
White piedra is seen worldwide, including Europe, South America, India, Southeast Asia, Africa, South America, and southern parts of the United States. The majority of cases occur in tropical and temperate regions.1 White piedra likely is underdiagnosed; for example, in a study of 166 young men with genital concerns in Houston, Texas, Trichosporon was isolated in 40% of cultured scrotal hairs.8
Species Identification
There are several species of WP; special techniques must be used to differentiate them, which is beyond the scope of this case. The known species include Trichosporon asahii, Trichosporon asteroides, Trichosporon cutaneum, Trichosporon inkin, Trichosporon mucoides, and Trichosporon ovoides.1Trichosporon asahii and T mucoides have been known to cause systemic infections in immunocompromised hosts known as trichosporonosis.1,9 As an example of a special technique used for species recognition, Sugita et al10 used sequence analysis of the ribosomal DNA intergenic spacer 1 regions to distinguish T asahii isolates. Identification of species may be warranted in the proper clinical scenario; however, histologic evaluation by an experienced dermatopathologist frequently is sufficient to identify the Trichosporon genus.
Transmission
The 2 most common causative organisms of WP are T inkin and T ovoides. Furthermore, T inkin causes the vast majority of WP in the pubic region.8
Diagnosis and Differential
White piedra is characterized by the presence of adherent tan to white nodules along the hair shafts. The concretions tend to be softer than black piedra and, unlike trichomycosis, normally do not fluoresce.1 They do not encircle the hair shaft as hair casts do and can be readily distinguished from Trichomycosis axillaris, black piedra, pediculosis, and trichorrhexis nodosa on microscopic examination.14 The hair shaft concretions of WP are difficult to visualize with the unaided eye. As a result, it is easily misdiagnosed.15 Upon palpation of the infected hair shafts, a grainy sensation is evident. Dermoscopy improves visualization, and fluorescence was useful in our case. Microscopic evaluation will identify the adherent organism and is readily cultured on Sabouraud agar.16 Although Trichosporon species typically do not fluoresce,1 Wood lamp examination occasionally may reveal the organism, such as in our case. A possible explanation for this finding is the synergistic relationship with Corynebacterium,17 some producing fluorescent chemicals. Growth of Trichosporon species may be enhanced by or even dependent on Corynebacterium; therefore, WP is likely a coinfection of fungus and bacteria.17,18 Studies also name a novel species of Brevibacterium in relationship with genital WP.19 This species was described as producing a foul odor, as in our patient.
Treatment
The American Academy of Dermatology’s Guidelines Committee recommends complete removal of the infected hairs.1 The recommendation traditionally is hair removal in conjunction with topical or oral medications,1 such as topical imidazoles, ciclopirox olamine, selenium sulfide 2%, chlorhexidine solution, zinc pyrithione, amphotericin B lotion, and oral itraconazole. Recurrence rates are high and spontaneous remission sometimes occurs.9,20 Triazole antifungals currently are preferred for treatment of Trichosporon infections.5 Patients should be counseled to dispose of undergarments, as the organism has been recovered from cotton fibers and are a source of reinfection.1,21
Conclusion
White piedra, though relatively uncommon, is likely underdiagnosed in the United States and should be suspected in any patient presenting with irritation and a foul odor in the genital area or multiple failed therapies for a nonspecific genital dermatitis. This clinical scenario warrants dermoscopic and Wood lamp examination of the affected skin and hair shafts in addition to microscopic examination of pubic hair shafts by a dermatopathologist. Fluorescence under Wood lamp may aid in diagnosis, and conflicting findings may be attributed to its synergistic relationship with Corynebacterium and Brevibacterium coinfection. Proper treatment includes shaving of the affected hair, oral or topical antifungal treatment, and disposal of affected clothing.
- Kiken DA, Sekaran A, Antaya RJ, et al. White piedra in children [published online September 18, 2006]. J Am Acad Dermatol. 2006;55:956-961.
- Walzam M, Leeming JG. White piedra and Trichosporon beigelii: the incidence in patients attending a clinic in genitourinary medicine. Genitourin Med. 1989;16:331-334.
- Horta P. Sobre una nova forma de piedra. Mem Inst Oswaldo Cruz. 1911;3:86-107.
- Fischman O. Black piedra in Brazil: a contribution to its study in Manaus (State of Amazonas). Mycopathol Mycol Appl. 1965;25:201-204.
- Benson PM, Lapins NA, Odom RB. White piedra. Arch Dermatol. 1983;119:602-604.
- Fischman O, Pires de Camargo Z, Meireles MCA. Genital white piedra: an emerging fungal disease? Fifth International Conference on Mycoses. PAHO Sci Publ. 1989;396:70-76.
- Tambe SA, Dhurat SR, Kumar CA, et al. Two cases of scalp piedra caused by Trichosporon ovoides. Indian J Dermatol Venereol Leprol. 2009;75:293-295.
- Kalter DC, Tschen JA, Cernoch PL, et al. Genital white piedra: epidemiology, microbiology, and therapy. J Am Acad Dermatol. 1986;14:982-993.
- James WD, Berger TG, Elston DM. Andrews’ Diseases of the Skin: Clinical Dermatology. 11th ed. United Kingdom: Saunders Elsevier; 2011.
- Sugita T, Nakajima M, Ikeda R, et al. Sequence analysis of the ribosomal DNA intergenic spacer 1 regions of Trichosporon species. J Clin Microbiol. 2002;40:1826-1830.
- Kaplan W. Piedra in lower animals: a case report of white piedra in a monkey and a review of the literature. J Am Vet Med Assoc. 1959;134:113-117.
- Carneiro JA, Assis FA, Filho JT. Piedra branca genital. An Bras Dermatol. 1971;46:265-269.
- Avram A, Buot G, Binet A, et al. Étude clinique et mycologique concernant 11 cas de trichosporie noueuse (piedra blanche) génito-pubienne. Ann Dermatol Venereol. 1987;114:819-827.
- So
bera JO, Elewski BE. Fungal diseases. In: Bolognia JL, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. New York, NY: Mosby; 2003:1135-1138. - Gold I, Sommer B, Urson S, et al. White piedra: a frequently misdiagnosed infection of hair. Int J Dermatol. 1984;23:621-623.
- Smith JD, Murtishaw WA, McBride ME. White piedra (Trichosporosis). Arch Dermatol. 1973;107:439-442.
- Youker SR, Andreozzi RJ, Appelbaum PC, et al. White piedra: further evidence of a synergistic infection. J Am Acad Dermatol. 2003;49:746-749.
- Ellner KM, McBride ME, Kalter DC, et al. White piedra: evidence for a synergistic infection. Br J Dermatol. 1990;123:355-363.
- McBride ME, Ellner KM, Black HS, et al. A new Brevibacterium sp. isolated from infected genital hair of patients with white piedra. J Med Microbiol. 1993;39:255-261.
- Drake LA, Dinehart SM, Farmer ER, et al. Guidelines of care for superficial mycotic infections of the skin: piedra. J Am Acad Dermatol. 1996;34:122-124.
- de Almeida HL Jr, Rivitti EA, Jaeger RG. White piedra: ultrastructure and a new microecological aspect. Mycoses. 1990;33:491-497.
Case Report
A 58-year-old man presented for evaluation of a pruritic rash involving the pubic area of 30 years’ duration. Multiple primary care physicians and dermatologists had evaluated the patient during this period, but he noted a specific diagnosis had not been rendered and multiple treatments had been unsuccessful. The patient described a rash, which was absent at the time of evaluation, as a self-remitting and exacerbating irritation typically induced by sweating and physical activity. The patient also stated that the irritation was associated with a strong, distinct, musty odor that severely interrupted his sex life and decreased his quality of life. Prior treatments included various topical corticosteroids, topical and oral antibiotics, and various homeopathic treatments that were minimally efficacious or nonefficacious. He was unsure if antifungals had previously been prescribed.
The patient’s medical history was notable for pulmonary interstitial fibrosis, anxiety, posttraumatic stress disorder, and mild glucose intolerance. The patient had no pertinent surgical history and no known drug allergies. Current medications included a bronchodilating inhaler, escitalopram, trazodone, buspirone, clonazepam, prazosin, gabapentin, and azithromycin for current upper respiratory tract infection. The patient was a former smoker and a social drinker.
On physical evaluation the pubic area displayed slight patchy erythema without a papular component and was otherwise unremarkable to the unaided eye. Upon palpation of the skin, there were no remarkable findings. Under dermoscopic evaluation, small white-yellow concretions along the hair shaft were noticed. Evaluation with a Wood lamp is shown in Figure 1.

The patient was treated empirically with ketoconazole cream 2% applied to the affected area once daily until follow-up 3 weeks later. The patient also was advised to shave the pubic area to remove potentially infected hairs, as white piedra (WP) was suspected. A diagnosis of WP was confirmed on histologic evaluation of pubic hair samples approximately 1 to 2 weeks later (Figure 2).

At 3-week follow-up, Wood lamp evaluation did not identify concretions along the pubic hair shafts. The patient was symptom free and extremely pleased. Of note, the patient did not shave the pubic area and was counseled on recurrence.
Comment
Piedra (meaning stone in Spanish) describes a group of fungal infections that present with gritty concretions on the hair shaft.1,2 In 1911, Horta3 classified piedra into 2 subtypes: black piedra, caused by Piedraia hortae, and WP, caused by Trichosporon species. Black piedra occurs more frequently in tropical countries and commonly affects hair shafts on the scalp.4 White piedra most commonly affects the pubic area, with rare cases in scalp and facial hair.1,5-7
Epidemiology
White piedra is seen worldwide, including Europe, South America, India, Southeast Asia, Africa, South America, and southern parts of the United States. The majority of cases occur in tropical and temperate regions.1 White piedra likely is underdiagnosed; for example, in a study of 166 young men with genital concerns in Houston, Texas, Trichosporon was isolated in 40% of cultured scrotal hairs.8
Species Identification
There are several species of WP; special techniques must be used to differentiate them, which is beyond the scope of this case. The known species include Trichosporon asahii, Trichosporon asteroides, Trichosporon cutaneum, Trichosporon inkin, Trichosporon mucoides, and Trichosporon ovoides.1Trichosporon asahii and T mucoides have been known to cause systemic infections in immunocompromised hosts known as trichosporonosis.1,9 As an example of a special technique used for species recognition, Sugita et al10 used sequence analysis of the ribosomal DNA intergenic spacer 1 regions to distinguish T asahii isolates. Identification of species may be warranted in the proper clinical scenario; however, histologic evaluation by an experienced dermatopathologist frequently is sufficient to identify the Trichosporon genus.
Transmission
The 2 most common causative organisms of WP are T inkin and T ovoides. Furthermore, T inkin causes the vast majority of WP in the pubic region.8
Diagnosis and Differential
White piedra is characterized by the presence of adherent tan to white nodules along the hair shafts. The concretions tend to be softer than black piedra and, unlike trichomycosis, normally do not fluoresce.1 They do not encircle the hair shaft as hair casts do and can be readily distinguished from Trichomycosis axillaris, black piedra, pediculosis, and trichorrhexis nodosa on microscopic examination.14 The hair shaft concretions of WP are difficult to visualize with the unaided eye. As a result, it is easily misdiagnosed.15 Upon palpation of the infected hair shafts, a grainy sensation is evident. Dermoscopy improves visualization, and fluorescence was useful in our case. Microscopic evaluation will identify the adherent organism and is readily cultured on Sabouraud agar.16 Although Trichosporon species typically do not fluoresce,1 Wood lamp examination occasionally may reveal the organism, such as in our case. A possible explanation for this finding is the synergistic relationship with Corynebacterium,17 some producing fluorescent chemicals. Growth of Trichosporon species may be enhanced by or even dependent on Corynebacterium; therefore, WP is likely a coinfection of fungus and bacteria.17,18 Studies also name a novel species of Brevibacterium in relationship with genital WP.19 This species was described as producing a foul odor, as in our patient.
Treatment
The American Academy of Dermatology’s Guidelines Committee recommends complete removal of the infected hairs.1 The recommendation traditionally is hair removal in conjunction with topical or oral medications,1 such as topical imidazoles, ciclopirox olamine, selenium sulfide 2%, chlorhexidine solution, zinc pyrithione, amphotericin B lotion, and oral itraconazole. Recurrence rates are high and spontaneous remission sometimes occurs.9,20 Triazole antifungals currently are preferred for treatment of Trichosporon infections.5 Patients should be counseled to dispose of undergarments, as the organism has been recovered from cotton fibers and are a source of reinfection.1,21
Conclusion
White piedra, though relatively uncommon, is likely underdiagnosed in the United States and should be suspected in any patient presenting with irritation and a foul odor in the genital area or multiple failed therapies for a nonspecific genital dermatitis. This clinical scenario warrants dermoscopic and Wood lamp examination of the affected skin and hair shafts in addition to microscopic examination of pubic hair shafts by a dermatopathologist. Fluorescence under Wood lamp may aid in diagnosis, and conflicting findings may be attributed to its synergistic relationship with Corynebacterium and Brevibacterium coinfection. Proper treatment includes shaving of the affected hair, oral or topical antifungal treatment, and disposal of affected clothing.
Case Report
A 58-year-old man presented for evaluation of a pruritic rash involving the pubic area of 30 years’ duration. Multiple primary care physicians and dermatologists had evaluated the patient during this period, but he noted a specific diagnosis had not been rendered and multiple treatments had been unsuccessful. The patient described a rash, which was absent at the time of evaluation, as a self-remitting and exacerbating irritation typically induced by sweating and physical activity. The patient also stated that the irritation was associated with a strong, distinct, musty odor that severely interrupted his sex life and decreased his quality of life. Prior treatments included various topical corticosteroids, topical and oral antibiotics, and various homeopathic treatments that were minimally efficacious or nonefficacious. He was unsure if antifungals had previously been prescribed.
The patient’s medical history was notable for pulmonary interstitial fibrosis, anxiety, posttraumatic stress disorder, and mild glucose intolerance. The patient had no pertinent surgical history and no known drug allergies. Current medications included a bronchodilating inhaler, escitalopram, trazodone, buspirone, clonazepam, prazosin, gabapentin, and azithromycin for current upper respiratory tract infection. The patient was a former smoker and a social drinker.
On physical evaluation the pubic area displayed slight patchy erythema without a papular component and was otherwise unremarkable to the unaided eye. Upon palpation of the skin, there were no remarkable findings. Under dermoscopic evaluation, small white-yellow concretions along the hair shaft were noticed. Evaluation with a Wood lamp is shown in Figure 1.

The patient was treated empirically with ketoconazole cream 2% applied to the affected area once daily until follow-up 3 weeks later. The patient also was advised to shave the pubic area to remove potentially infected hairs, as white piedra (WP) was suspected. A diagnosis of WP was confirmed on histologic evaluation of pubic hair samples approximately 1 to 2 weeks later (Figure 2).

At 3-week follow-up, Wood lamp evaluation did not identify concretions along the pubic hair shafts. The patient was symptom free and extremely pleased. Of note, the patient did not shave the pubic area and was counseled on recurrence.
Comment
Piedra (meaning stone in Spanish) describes a group of fungal infections that present with gritty concretions on the hair shaft.1,2 In 1911, Horta3 classified piedra into 2 subtypes: black piedra, caused by Piedraia hortae, and WP, caused by Trichosporon species. Black piedra occurs more frequently in tropical countries and commonly affects hair shafts on the scalp.4 White piedra most commonly affects the pubic area, with rare cases in scalp and facial hair.1,5-7
Epidemiology
White piedra is seen worldwide, including Europe, South America, India, Southeast Asia, Africa, South America, and southern parts of the United States. The majority of cases occur in tropical and temperate regions.1 White piedra likely is underdiagnosed; for example, in a study of 166 young men with genital concerns in Houston, Texas, Trichosporon was isolated in 40% of cultured scrotal hairs.8
Species Identification
There are several species of WP; special techniques must be used to differentiate them, which is beyond the scope of this case. The known species include Trichosporon asahii, Trichosporon asteroides, Trichosporon cutaneum, Trichosporon inkin, Trichosporon mucoides, and Trichosporon ovoides.1Trichosporon asahii and T mucoides have been known to cause systemic infections in immunocompromised hosts known as trichosporonosis.1,9 As an example of a special technique used for species recognition, Sugita et al10 used sequence analysis of the ribosomal DNA intergenic spacer 1 regions to distinguish T asahii isolates. Identification of species may be warranted in the proper clinical scenario; however, histologic evaluation by an experienced dermatopathologist frequently is sufficient to identify the Trichosporon genus.
Transmission
The 2 most common causative organisms of WP are T inkin and T ovoides. Furthermore, T inkin causes the vast majority of WP in the pubic region.8
Diagnosis and Differential
White piedra is characterized by the presence of adherent tan to white nodules along the hair shafts. The concretions tend to be softer than black piedra and, unlike trichomycosis, normally do not fluoresce.1 They do not encircle the hair shaft as hair casts do and can be readily distinguished from Trichomycosis axillaris, black piedra, pediculosis, and trichorrhexis nodosa on microscopic examination.14 The hair shaft concretions of WP are difficult to visualize with the unaided eye. As a result, it is easily misdiagnosed.15 Upon palpation of the infected hair shafts, a grainy sensation is evident. Dermoscopy improves visualization, and fluorescence was useful in our case. Microscopic evaluation will identify the adherent organism and is readily cultured on Sabouraud agar.16 Although Trichosporon species typically do not fluoresce,1 Wood lamp examination occasionally may reveal the organism, such as in our case. A possible explanation for this finding is the synergistic relationship with Corynebacterium,17 some producing fluorescent chemicals. Growth of Trichosporon species may be enhanced by or even dependent on Corynebacterium; therefore, WP is likely a coinfection of fungus and bacteria.17,18 Studies also name a novel species of Brevibacterium in relationship with genital WP.19 This species was described as producing a foul odor, as in our patient.
Treatment
The American Academy of Dermatology’s Guidelines Committee recommends complete removal of the infected hairs.1 The recommendation traditionally is hair removal in conjunction with topical or oral medications,1 such as topical imidazoles, ciclopirox olamine, selenium sulfide 2%, chlorhexidine solution, zinc pyrithione, amphotericin B lotion, and oral itraconazole. Recurrence rates are high and spontaneous remission sometimes occurs.9,20 Triazole antifungals currently are preferred for treatment of Trichosporon infections.5 Patients should be counseled to dispose of undergarments, as the organism has been recovered from cotton fibers and are a source of reinfection.1,21
Conclusion
White piedra, though relatively uncommon, is likely underdiagnosed in the United States and should be suspected in any patient presenting with irritation and a foul odor in the genital area or multiple failed therapies for a nonspecific genital dermatitis. This clinical scenario warrants dermoscopic and Wood lamp examination of the affected skin and hair shafts in addition to microscopic examination of pubic hair shafts by a dermatopathologist. Fluorescence under Wood lamp may aid in diagnosis, and conflicting findings may be attributed to its synergistic relationship with Corynebacterium and Brevibacterium coinfection. Proper treatment includes shaving of the affected hair, oral or topical antifungal treatment, and disposal of affected clothing.
- Kiken DA, Sekaran A, Antaya RJ, et al. White piedra in children [published online September 18, 2006]. J Am Acad Dermatol. 2006;55:956-961.
- Walzam M, Leeming JG. White piedra and Trichosporon beigelii: the incidence in patients attending a clinic in genitourinary medicine. Genitourin Med. 1989;16:331-334.
- Horta P. Sobre una nova forma de piedra. Mem Inst Oswaldo Cruz. 1911;3:86-107.
- Fischman O. Black piedra in Brazil: a contribution to its study in Manaus (State of Amazonas). Mycopathol Mycol Appl. 1965;25:201-204.
- Benson PM, Lapins NA, Odom RB. White piedra. Arch Dermatol. 1983;119:602-604.
- Fischman O, Pires de Camargo Z, Meireles MCA. Genital white piedra: an emerging fungal disease? Fifth International Conference on Mycoses. PAHO Sci Publ. 1989;396:70-76.
- Tambe SA, Dhurat SR, Kumar CA, et al. Two cases of scalp piedra caused by Trichosporon ovoides. Indian J Dermatol Venereol Leprol. 2009;75:293-295.
- Kalter DC, Tschen JA, Cernoch PL, et al. Genital white piedra: epidemiology, microbiology, and therapy. J Am Acad Dermatol. 1986;14:982-993.
- James WD, Berger TG, Elston DM. Andrews’ Diseases of the Skin: Clinical Dermatology. 11th ed. United Kingdom: Saunders Elsevier; 2011.
- Sugita T, Nakajima M, Ikeda R, et al. Sequence analysis of the ribosomal DNA intergenic spacer 1 regions of Trichosporon species. J Clin Microbiol. 2002;40:1826-1830.
- Kaplan W. Piedra in lower animals: a case report of white piedra in a monkey and a review of the literature. J Am Vet Med Assoc. 1959;134:113-117.
- Carneiro JA, Assis FA, Filho JT. Piedra branca genital. An Bras Dermatol. 1971;46:265-269.
- Avram A, Buot G, Binet A, et al. Étude clinique et mycologique concernant 11 cas de trichosporie noueuse (piedra blanche) génito-pubienne. Ann Dermatol Venereol. 1987;114:819-827.
- So
bera JO, Elewski BE. Fungal diseases. In: Bolognia JL, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. New York, NY: Mosby; 2003:1135-1138. - Gold I, Sommer B, Urson S, et al. White piedra: a frequently misdiagnosed infection of hair. Int J Dermatol. 1984;23:621-623.
- Smith JD, Murtishaw WA, McBride ME. White piedra (Trichosporosis). Arch Dermatol. 1973;107:439-442.
- Youker SR, Andreozzi RJ, Appelbaum PC, et al. White piedra: further evidence of a synergistic infection. J Am Acad Dermatol. 2003;49:746-749.
- Ellner KM, McBride ME, Kalter DC, et al. White piedra: evidence for a synergistic infection. Br J Dermatol. 1990;123:355-363.
- McBride ME, Ellner KM, Black HS, et al. A new Brevibacterium sp. isolated from infected genital hair of patients with white piedra. J Med Microbiol. 1993;39:255-261.
- Drake LA, Dinehart SM, Farmer ER, et al. Guidelines of care for superficial mycotic infections of the skin: piedra. J Am Acad Dermatol. 1996;34:122-124.
- de Almeida HL Jr, Rivitti EA, Jaeger RG. White piedra: ultrastructure and a new microecological aspect. Mycoses. 1990;33:491-497.
- Kiken DA, Sekaran A, Antaya RJ, et al. White piedra in children [published online September 18, 2006]. J Am Acad Dermatol. 2006;55:956-961.
- Walzam M, Leeming JG. White piedra and Trichosporon beigelii: the incidence in patients attending a clinic in genitourinary medicine. Genitourin Med. 1989;16:331-334.
- Horta P. Sobre una nova forma de piedra. Mem Inst Oswaldo Cruz. 1911;3:86-107.
- Fischman O. Black piedra in Brazil: a contribution to its study in Manaus (State of Amazonas). Mycopathol Mycol Appl. 1965;25:201-204.
- Benson PM, Lapins NA, Odom RB. White piedra. Arch Dermatol. 1983;119:602-604.
- Fischman O, Pires de Camargo Z, Meireles MCA. Genital white piedra: an emerging fungal disease? Fifth International Conference on Mycoses. PAHO Sci Publ. 1989;396:70-76.
- Tambe SA, Dhurat SR, Kumar CA, et al. Two cases of scalp piedra caused by Trichosporon ovoides. Indian J Dermatol Venereol Leprol. 2009;75:293-295.
- Kalter DC, Tschen JA, Cernoch PL, et al. Genital white piedra: epidemiology, microbiology, and therapy. J Am Acad Dermatol. 1986;14:982-993.
- James WD, Berger TG, Elston DM. Andrews’ Diseases of the Skin: Clinical Dermatology. 11th ed. United Kingdom: Saunders Elsevier; 2011.
- Sugita T, Nakajima M, Ikeda R, et al. Sequence analysis of the ribosomal DNA intergenic spacer 1 regions of Trichosporon species. J Clin Microbiol. 2002;40:1826-1830.
- Kaplan W. Piedra in lower animals: a case report of white piedra in a monkey and a review of the literature. J Am Vet Med Assoc. 1959;134:113-117.
- Carneiro JA, Assis FA, Filho JT. Piedra branca genital. An Bras Dermatol. 1971;46:265-269.
- Avram A, Buot G, Binet A, et al. Étude clinique et mycologique concernant 11 cas de trichosporie noueuse (piedra blanche) génito-pubienne. Ann Dermatol Venereol. 1987;114:819-827.
- So
bera JO, Elewski BE. Fungal diseases. In: Bolognia JL, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. New York, NY: Mosby; 2003:1135-1138. - Gold I, Sommer B, Urson S, et al. White piedra: a frequently misdiagnosed infection of hair. Int J Dermatol. 1984;23:621-623.
- Smith JD, Murtishaw WA, McBride ME. White piedra (Trichosporosis). Arch Dermatol. 1973;107:439-442.
- Youker SR, Andreozzi RJ, Appelbaum PC, et al. White piedra: further evidence of a synergistic infection. J Am Acad Dermatol. 2003;49:746-749.
- Ellner KM, McBride ME, Kalter DC, et al. White piedra: evidence for a synergistic infection. Br J Dermatol. 1990;123:355-363.
- McBride ME, Ellner KM, Black HS, et al. A new Brevibacterium sp. isolated from infected genital hair of patients with white piedra. J Med Microbiol. 1993;39:255-261.
- Drake LA, Dinehart SM, Farmer ER, et al. Guidelines of care for superficial mycotic infections of the skin: piedra. J Am Acad Dermatol. 1996;34:122-124.
- de Almeida HL Jr, Rivitti EA, Jaeger RG. White piedra: ultrastructure and a new microecological aspect. Mycoses. 1990;33:491-497.
- Although relatively uncommon, white piedra should be suspected in any patient presenting with irritation and foul odor in the genital area or multiple failed therapies for a nonspecific genital dermatitis.
- Wood lamp and dermoscopy should be used to evaluate for parasitic infections of the pubic hair shafts when nonspecific dermatitis presents in this area.
What’s Eating You? Head Lice (Pediculus humanus capitis)
The head louse (Pediculus humanus capitis) is a blood-sucking arthropod of the suborder Anoplura. Lice are obligate human parasites that have infested humans since antiquity. Pediculosis capitis is an infestation of the scalp by head lice. It is estimated that 6 to 12 million individuals in the United States are affected with head lice per year.1 Resistance to topical chemical pediculicides is widespread, and new agents have been developed to address this gap in care.
Characteristics of Head Lice
The head louse is a tan-gray–colored, wingless insect measuring approximately 2- to 3-mm long with 3 body segments. It has 6 legs with claws used to grasp individual hairs, and it moves by crawling; it does not fly or jump.2,3 The head louse has an elongated abdomen and a small head with short antennae and anterior piercing mouthparts (Figure 1).4 Nits are transparent, flask-shaped, 0.5- to 0.8-mm egg cases found firmly cemented to the hair shafts approximately 1 to 4 mm above the level of the scalp (Figure 2).5 The head louse resides on scalp hair and feeds off the scalp itself. Both lice and nits can be present throughout the scalp but are most commonly found in the postauricular and occipital scalp.3,4
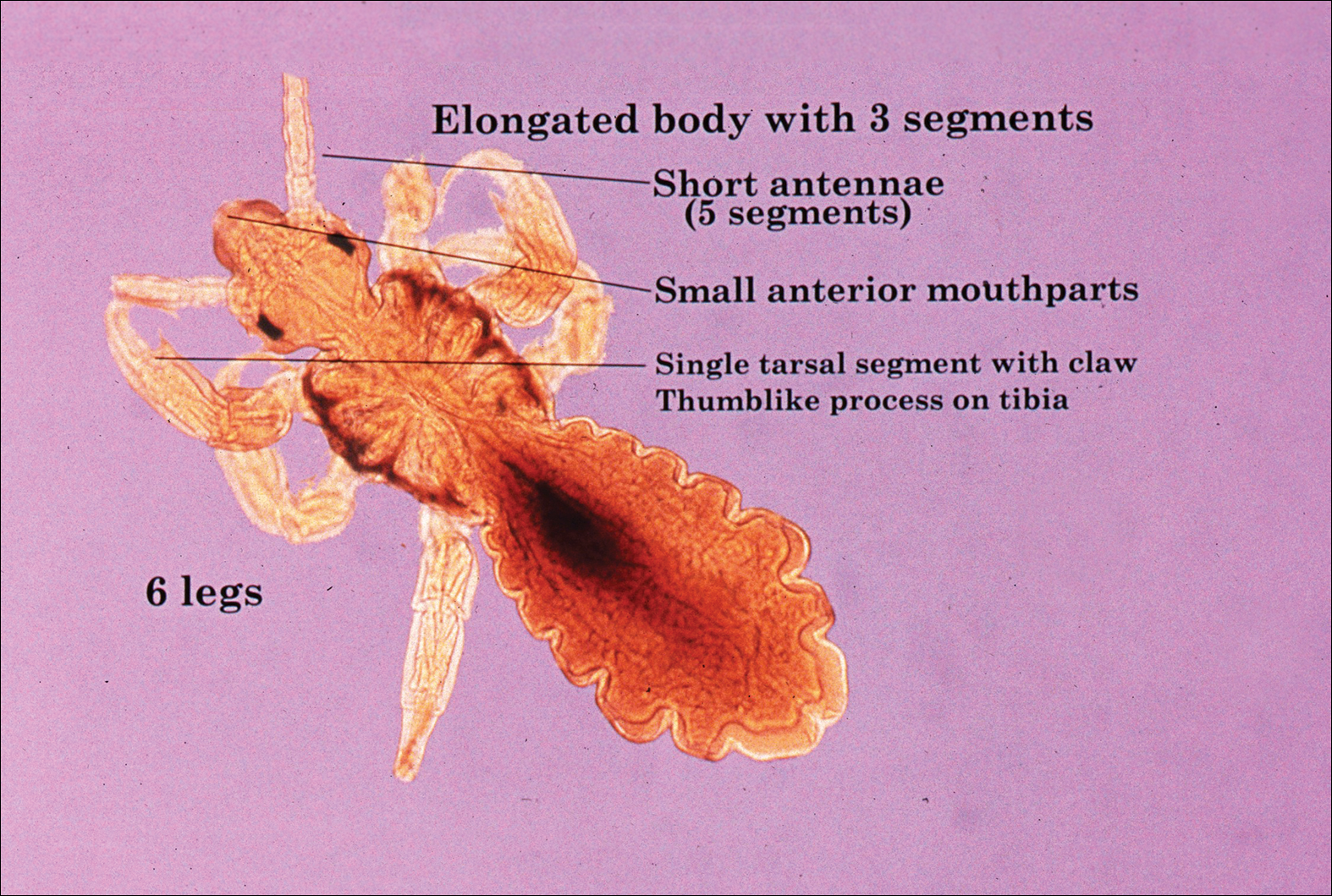
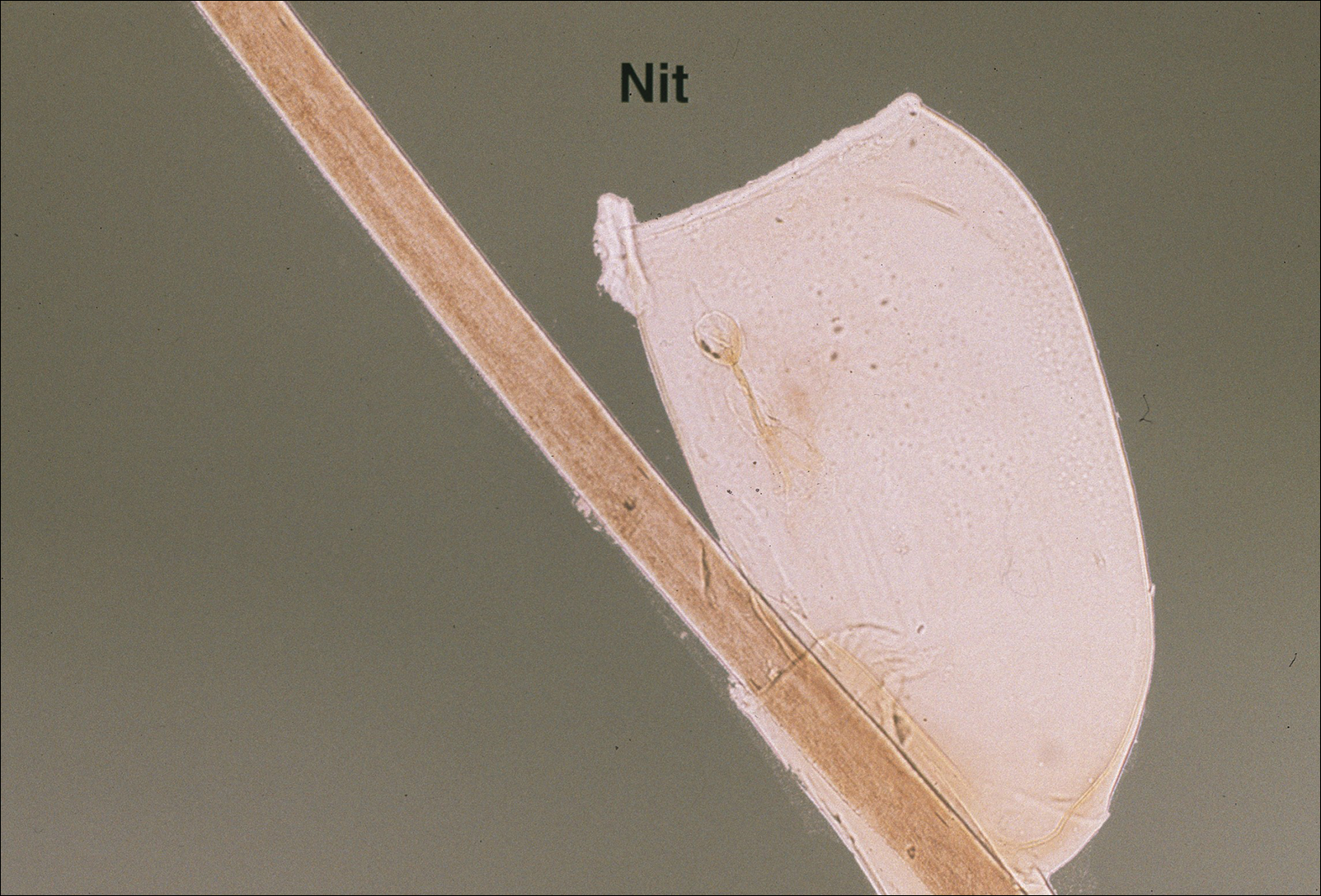
Female lice live approximately 30 days and lay 5 to 10 eggs per day. Eggs incubate individually in nits laid close to the scalp for 8 to 10 days before hatching.1,6 The newly hatched nymphs (also called instars) have multiple exoskeletons that are shed as they grow.7 Nymphs mature into adults in approximately 2 weeks, and the life cycle begins again.8 Head lice are obligate human parasites, feeding approximately every 4 to 6 hours on the blood of the host; however, they can survive up to 4 days without a blood meal on fomites if the climate and conditions are favorable.5,9
Epidemiology and Transmission
Head lice infestations commonly occur in children aged 3 to 11 years and are more prevalent in girls and women.1,10 Infestation rates are not reliably recorded, and few population-based studies have been performed; however, it is estimated that 6 to 12 million individuals are infested annually in the United States.1 Prevalence in some European populations has been estimated to range from 1% to 20%.11 A 2008 literature review found that worldwide prevalence varied across populations from 0.7% to 59%.10
Transmission occurs most frequently from direct head-to-head contact. One study found that transmission is most likely to occur when hairs are arranged in a parallel alignment and move slowly in relation to one another.12 Although controversial and probably less notable, transmission also may occur indirectly via fomites or the sharing of hairbrushes, hats, or other headgear.13,14 Classrooms are a common place for transmission.1 A 2009 study in Germany found an increase in health department consultations for head lice when schools reopened after vacations. The investigators also found that pediculicide sales peaked from mid-September through October, subsequent to schools reopening after the summer holiday.15 There is some evidence that overcrowded housing also can lead to increased incidence and transmission.16,17 There is no consistent correlation of infestation with socioeconomic status.1,17,18
Clinical Manifestations and Diagnosis
Clinically, patients with head lice present with scalp pruritus and sometimes posterior cervical or occipital lymphadenopathy. Pediculosis also can be asymptomatic. With the first exposure, symptoms may not develop for up to 4 to 6 weeks as the immune system develops sensitivity to the louse saliva.6 Bite reactions consisting of papules or wheals are related to immune sensitization.5 Louse feces and excoriations from scratching to relieve itch also may be present on examination. Secondary infection of excoriations also is possible.1
Diagnosis of an active infestation is made by identifying living lice. Because lice move quickly and can be difficult to detect, tightly attached nits on the hair shaft within 4 mm of the scalp are at least indicative of a historic infestation and can be suggestive of active infestation.1,19 Dermoscopy is a helpful tool in differentiating eggs containing nymphs from the empty cases of hatched lice and also from amorphous pseudonits (hair casts)(Figure 3).19,20 Wet combing improves the accuracy of diagnosing an active infection.21

Treatment
Effective treatment of head lice requires eradication of all living lice as well as louse eggs. Topically applied pyrethroids, including pyrethrin shampoos and mousses and permethrin lotion 1%, are considered the first-line therapy.8 Pyrethroids are over-the-counter treatments that act by interfering with sodium transport in the louse, causing depolarization of the neuromembranes and respiratory paralysis.22 Pyrethrins are natural compounds derived from the chrysanthemum plant; permethrin is a synthetic compound. Pyrethrins often are combined with piperonyl butoxide, an insecticide synergist that improves efficacy by inhibiting pyrethrin catabolism.23 Resistance to pyrethroids has become an increasingly important problem in the United States and worldwide.
Malathion lotion 0.5% is another therapeutic option for head lice. Malathion is a prescription organophosphate cholinesterase inhibitor that also causes respiratory paralysis of the louse and is one of the few treatments that is ovicidal.22 It was withdrawn from the market in 1995 due to its flammability and a theoretical risk of respiratory depression if ingested; however, it was reintroduced in 1999 and remains an effective treatment option with little resistance in the United States.24
Lindane 1% (shampoo and lotion), an organochloride compound that acts by causing neuronal hyperstimulation and eventual paralysis of lice, is no longer recommended due to its serious side effects, including central nervous system toxicity and increased risk of seizure.8,24
New US Food and Drug Administration–Approved Therapies
Newer topical treatments include benzyl alcohol lotion 5%, spinosad topical suspension 0.9%, ivermectin lotion 0.5%, and dimethicone-based products. Benzyl alcohol was approved by the US Food and Drug Administration (FDA) in 2009 and is available in the United States by prescription.25 Benzyl alcohol kills lice by asphyxiation. Phase 2 and 3 clinical trials showed significant treatment success 1 day posttreatment (fewer live lice than the vehicle alone; P=.004) and 2 weeks posttreatment (absence of live lice compared to the vehicle alone; P=.001).26
Spinosad was approved by the FDA in 2011 and is available in the United States by prescription.25 It contains the compounds spinosyn A and spinosyn D, which are naturally derived through fermentation by the soil bacterium Saccharopolyspora spinosa. It also contains benzyl alcohol. Spinosad paralyzes lice by disrupting neuronal activity and is at least partially ovicidal.27 Phase 3 clinical trials published in 2009 showed that spinosad was significantly more effective than permethrin in eradicating head lice (P<.001).28
Topical ivermectin was approved by the FDA in 2012 for prescription use.25 It acts on chloride ion channels, causing hyperpolarization of the muscle cells of lice and resulting in paralysis and death. Oral ivermectin (200 μg/kg) given once and repeated in 10 days is not FDA approved for the treatment of head lice but has shown some effectiveness and is sometimes used.8 A comparison study of topical versus oral ivermectin published in 2014 found that eradication was achieved in 88% (n=27) of topical ivermectin users after 1 treatment and 100% (n=31) after 2 treatments. Oral ivermectin produced cure rates of 45% (n=14) after 1 treatment and 97% (n=30) after 2 treatments. Both topical and oral ivermectin treatments are well tolerated.29
Physically Acting Preparations
Products with a physical mode of action are a new attractive option for treatment of pediculosis because the development of resistance is less likely. Studies of silicone-based fluids that physically occlude the respiratory system of the louse, such as dimethicone liquid gel 4%, have shown superiority over treatment with pyrethroids.30,31 Although the safety of dimethicone has been demonstrated, silicone-based treatments have not yet been widely adopted in the United States and are not currently used as a first-line treatment.32 However, use of such physically acting pediculicides may in time surpass traditional neurotoxic treatments due to their low susceptibility to resistance and good safety profile.33,34
Alternative Therapies
Nonchemical treatments for head lice that have shown variable success include wet combing, hot air treatments, and varying occlusive treatments. Physical removal via wet combing requires persistent repeated treatments over several weeks; for example, wet combing may be performed every 3 days for at least 2 weeks or until no head lice are detected on 4 consecutive occasions.35 Cure rates range from 38% to 75% with wet combing as a sole treatment of head lice.36 Because this treatment has minimal risks and no adverse side effects, it can be considered as an alternative treatment for some patients.
Hot air treatments also have been studied. A 2006 study showed that a hot air treatment device had the potential to eradicate head lice, most likely by desiccation. Specifically, 30 minutes of exposure to hot air (at 58.9°F, slightly cooler than a standard hair dryer) using the custom-built device resulted in 98% mortality of eggs and 80% mortality of hatched lice.37 Large randomized controlled trials of hot air treatments have not been performed.
Other alternative treatments include plant-derived oils. A laboratory study of essential oils found that spearmint, cassia, and clove showed pediculicidal activity similar to malathion with improved ovicidal activity.38 However, there is a potential for development of contact dermatitis from essential oils.
Complete Eradication of Head Lice
Removal of nits is an important component of effective lice eradication. Biochemical analysis has revealed that the nit sheath of the head louse is similar in composition to amyloid, rendering it difficult to design products that will unravel the nit sheath while leaving human hair undamaged.39 Because pediculicides are not necessarily ovicidal and complete physical nit removal is difficult to achieve, re-treatment in 7 to 10 days often is advisable to ensure that lice in all stages of the life cycle have been killed.4 Treatment of any secondary bacterial infection also is important. Although transmission of lice via fomites is less likely than from head-to-head contact, the cleaning of hats, hairbrushes, and linens is prudent. Diagnosing and treating infested close contacts also is essential to achieving eradication.4 Coordinated surveillance, education, and treatment efforts in high-risk communities can help detect asymptomatic cases and control local epidemics in a cost-effective manner.40 However, “no nit” policies at schools likely cause a net harm, as nit removal is difficult and children with nonviable nits are then excluded from the classroom.5
Treatment Resistance
Resistance to topical neurotoxic treatments is becoming increasingly common.41-43 Therefore, it is important to identify local patterns of resistance, if possible, when selecting a therapy for head lice. Improper usage, changes in pediculicide formulations and packaging, decreased product efficacy, and natural selection have all contributed to this rise in resistance.7 Additionally, due to protection from multiple exoskeletons and the natural molting process as they mature into adults, nymphs may only receive a sublethal dose when exposed to pediculicides, contributing further to resistance.7 Resistance to synthetic pyrethroids is most predominant, likely due to selection pressure because permethrin historically has been the most widely used insecticide for pediculosis. A 2014 study found that the frequency of sodium-channel insensitivity to pyrethroids, also known as knockdown resistance (or kdr), in US head louse populations collected over a 10-year period was 84.4% and approached 100% in some communities in recent years.44 This evidence strongly supports the use of alternative therapeutic categories to effectively eradicate head lice infestations.
Conclusion
Head lice infestation is common in children, and although it is not harmful to the host, it can be an irritating and symptomatic problem and can lead to notable distress, missed days of school, and secondary infections. Identifying active adult lice is the gold standard for diagnosis. Current recommended treatments include pyrethroids as the first-line therapy; however, resistance to these neurotoxic agents is becoming increasingly common. Alternative therapies such as newer neurotoxic agents or pediculicides with physical mechanisms of action (eg, dimethicone-based products) should be considered, particularly in regions where resistance is known to be high. Education about head lice, proper use of treatment, and coordinated diagnosis are necessary for effective management of this problem.
- Chosidow O. Scabies and pediculosis. Lancet. 2000;355:819-826.
- Centers for Disease Control and Prevention. Head lice. http://www.cdc.gov/parasites/lice/head/index.html. Updated September 24, 2013. Accessed November 9, 2017.
- Hurwitz S. Lice (pediculosis). In: Hurwitz S. Hurwitz Clinical Pediatric Dermatology: A Textbook of Skin Disorders of Childhood and Adolescence. 2nd ed. Philadelphia, PA: WB Saunders Company; 1993:416-419.
- Elston DM. What’s eating you? Pediculus humanus (head louse and body louse). Cutis. 1999;63:259-264.
- Ko CJ, Elston DM. Pediculosis. J Am Acad Dermatol. 2004;50:1-12.
- Frankowski BL, Weiner LB. Head lice. Pediatrics. 2002;110:638-643.
- Meinking TL. Clinical update on resistance and treatment of pediculosis capitis. Am J Manag Care. 2004;10(9 suppl):S264-S268.
- Devore CD, Schutze GE. Head lice. Pediatrics. 2015;135:E1355-E1365.
- Burkhart CN. Fomite transmission with head lice: a continuing controversy. Lancet. 2003;361:99-100.
- Falagas ME, Matthaiou DK, Rafailidis PI, et al. Worldwide prevalence of head lice. Emerg Infect Dis. 2008;14:1493-1494.
- Feldmeier H. Pediculosis capitis: new insights into epidemiology, diagnosis and treatment. Eur J Clin Microbiol Infect Dis. 2012;31:2105-2110.
- Canyon DV, Speare R, Muller R. Spatial and kinetic factors for the transfer of head lice (Pediculus capitis) between hairs. J Invest Dermatol. 2002;119:629-631.
- Burkhart CN, Burkhart CG. Fomite transmission in head lice. J Am Acad Dermatol. 2007;56:1044-1047.
- Canyon DV, Speare R. Indirect transmission of head lice via inanimate objects. Open Dermatol J. 2010;4:72-76.
- Bauer E, Jahnke C, Feldmeier H. Seasonal fluctuations of head lice infestation in Germany. Parasitol Res. 2009;104:677-681.
- Balcioglu IC, Kurt O, Limoncu ME, et al. Rural life, lower socioeconomic status and parasitic infections. Parasitol Int. 2007;56:129-133.
- Lesshafft H, Baier A, Guerra H, et al. Prevalence and risk factors associated with pediculosis capitis in an impoverished urban community in Lima, Peru. J Glob Infect Dis. 2013;5:138-143.
- Tagka A, Lambrou GI, Braoudaki M, et al. Socioeconomical factors associated with pediculosis (Phthiraptera: Pediculidae) in Athens, Greece. J Med Entomol. 2016;53:919-922.
- Di Stefani A, Hofmann-Wellenhof R, Zalaudek I. Dermoscopy for diagnosis and treatment monitoring of pediculosis capitis. J Am Acad Dermatol. 2006;54:909-911.
- Bakos RM, Bakos L. Dermoscopy for diagnosis of pediculosis capitis. J Am Acad Dermatol. 2007;57:727-728.
- Jahnke C, Bauer E, Hengge UR, et al. Accuracy of diagnosis of pediculosis capitis: visual inspection vs wet combing. Arch Dermatol. 2009;145:309-313.
- Elston DM. Drugs used in the treatment of pediculosis. J Drugs Dermatol. 2005;4:207-211.
- National Pesticide Information Center. Piperonyl butoxide (general fact sheet). http://npic.orst.edu/factsheets/pbogen.pdf/. Accessed November 13, 2017.
- Diamantis SA, Morrell DS, Burkhart CN. Treatment of head lice. Dermatol Ther. 2009;22:273-278.
- United States Food and Drug Administration. Treating and preventing head lice. http://www.fda.gov/forconsumers/consumerupdates/ucm171730.htm. Published July 13, 2010. Updated November 8, 2017. Accessed November 13, 2017.
- Meinking TL, Villar ME, Vicaria M, et al. The clinical trials supporting benzyl alcohol lotion 5% (UlesfiaTM): a safe and effective topical treatment for head lice (Pediculosis Humanus Capitis). Pediatr Dermatol. 2010;27:19-24.
- McCormack PL. Spinosad in pediculosis capitis. Am J Clin Dermatol. 2011;12:349-353.
- Stough D, Shellabarger S, Quiring J, et al. Efficacy and safety of spinosad and permethrin creme rinses for pediculosis capitis (head lice). Pediatrics. 2009;124:E389-E395.
- Ahmad HM, Abdel-Azim ES, Abdel-Aziz RT. Assessment of topical versus oral ivermectin as a treatment for head lice. Dermatol Ther. 2014;27:307-310.
- Heukelbach J, Pilger D, Oliveira FA, et al. A highly efficacious pediculicide based on dimethicone: randomized observer blinded comparative trial. BMC Infect Dis. 2008;8:115.
- Burgess IF, Brunton ER, Burgess NA. Single application of 4% dimethicone liquid gel versus two applications of 1% permethrin creme rinse for treatment of head louse infestation: a randomised controlled trial. BMC Dermatol. 2013;13:5.
- Ihde ES, Boscamp JR, Loh JM, et al. Safety and efficacy of a 100% dimethicone pediculocide in school-age children. BMC Pediatr. 2015;15:70.
- Heukelbach J, Oliveira FA, Richter J, et al. Dimethicone-based pediculicides: a physical approach to eradicate head lice. Open Dermatol J. 2010;4:77-81.
- Feldmeier H. Treatment of pediculosis capitis: a critical appraisal of the current literature. Am J Clin Dermatol. 2014;15:401-412.
- Glasziou P, Bennett J, Greenberg P, et al; Handbook Of Non Drug Intervention (HANDI) Project Team. Wet combing for the eradication of head lice. Aust Fam Physician. 2013;42:129-130.
- Tebruegge M, Runnacles J. Is wet combing effective in children with pediculosis capitis infestation? Arch Dis Child. 2007;92:818-820.
- Goates BM, Atkin JS, Wilding KG, et al. An effective nonchemical treatment for head lice: a lot of hot air. Pediatrics. 2006;118:1962-1970.
- Yones DA, Bakir HY, Bayoumi SA. Chemical composition and efficacy of some selected plant oils against Pediculus humanus capitis in vitro. Parasitol Res. 2016;115:3209-3218.
- Burkhart CN, Burkhart CG. Head lice: scientific assessment of the nit sheath with clinical ramifications and therapeutic options. J Am Acad Dermatol. 2005;53:129-133.
- Ibarra J, Fry F, Wickenden C, et al. The impact of well-developed preventative strategies on the eradication of head lice. Perspect Public Health. 2009;129:165-173.
- Mumcuoglu KY, Hemingway J, Miller J, et al. Permethrin resistance in the head louse pediculus humanus capitis from Israel. Med Vet Entomol. 1995;9:427-432.
- Meinking TL, Serrano L, Hard B, et al. Comparative in vitro pediculicidal efficacy of treatments in a resistant head lice population in the United States. Arch Dermatol. 2002;138:220-224.
- Hemingway J, Miller J, Mumcuoglu KY. Pyrethroid resistance mechanisms in the head louse Pediculus capitis from Israel: implications for control. Med Vet Entomol. 1999;13:89-96.
- Yoon KS, Previte DJ, Hodgdon HE, et al. Knockdown resistance allele frequencies in North American head louse (Anoplura: Pediculidae) populations. J Med Entomol. 2014;51:450-457.
The head louse (Pediculus humanus capitis) is a blood-sucking arthropod of the suborder Anoplura. Lice are obligate human parasites that have infested humans since antiquity. Pediculosis capitis is an infestation of the scalp by head lice. It is estimated that 6 to 12 million individuals in the United States are affected with head lice per year.1 Resistance to topical chemical pediculicides is widespread, and new agents have been developed to address this gap in care.
Characteristics of Head Lice
The head louse is a tan-gray–colored, wingless insect measuring approximately 2- to 3-mm long with 3 body segments. It has 6 legs with claws used to grasp individual hairs, and it moves by crawling; it does not fly or jump.2,3 The head louse has an elongated abdomen and a small head with short antennae and anterior piercing mouthparts (Figure 1).4 Nits are transparent, flask-shaped, 0.5- to 0.8-mm egg cases found firmly cemented to the hair shafts approximately 1 to 4 mm above the level of the scalp (Figure 2).5 The head louse resides on scalp hair and feeds off the scalp itself. Both lice and nits can be present throughout the scalp but are most commonly found in the postauricular and occipital scalp.3,4


Female lice live approximately 30 days and lay 5 to 10 eggs per day. Eggs incubate individually in nits laid close to the scalp for 8 to 10 days before hatching.1,6 The newly hatched nymphs (also called instars) have multiple exoskeletons that are shed as they grow.7 Nymphs mature into adults in approximately 2 weeks, and the life cycle begins again.8 Head lice are obligate human parasites, feeding approximately every 4 to 6 hours on the blood of the host; however, they can survive up to 4 days without a blood meal on fomites if the climate and conditions are favorable.5,9
Epidemiology and Transmission
Head lice infestations commonly occur in children aged 3 to 11 years and are more prevalent in girls and women.1,10 Infestation rates are not reliably recorded, and few population-based studies have been performed; however, it is estimated that 6 to 12 million individuals are infested annually in the United States.1 Prevalence in some European populations has been estimated to range from 1% to 20%.11 A 2008 literature review found that worldwide prevalence varied across populations from 0.7% to 59%.10
Transmission occurs most frequently from direct head-to-head contact. One study found that transmission is most likely to occur when hairs are arranged in a parallel alignment and move slowly in relation to one another.12 Although controversial and probably less notable, transmission also may occur indirectly via fomites or the sharing of hairbrushes, hats, or other headgear.13,14 Classrooms are a common place for transmission.1 A 2009 study in Germany found an increase in health department consultations for head lice when schools reopened after vacations. The investigators also found that pediculicide sales peaked from mid-September through October, subsequent to schools reopening after the summer holiday.15 There is some evidence that overcrowded housing also can lead to increased incidence and transmission.16,17 There is no consistent correlation of infestation with socioeconomic status.1,17,18
Clinical Manifestations and Diagnosis
Clinically, patients with head lice present with scalp pruritus and sometimes posterior cervical or occipital lymphadenopathy. Pediculosis also can be asymptomatic. With the first exposure, symptoms may not develop for up to 4 to 6 weeks as the immune system develops sensitivity to the louse saliva.6 Bite reactions consisting of papules or wheals are related to immune sensitization.5 Louse feces and excoriations from scratching to relieve itch also may be present on examination. Secondary infection of excoriations also is possible.1
Diagnosis of an active infestation is made by identifying living lice. Because lice move quickly and can be difficult to detect, tightly attached nits on the hair shaft within 4 mm of the scalp are at least indicative of a historic infestation and can be suggestive of active infestation.1,19 Dermoscopy is a helpful tool in differentiating eggs containing nymphs from the empty cases of hatched lice and also from amorphous pseudonits (hair casts)(Figure 3).19,20 Wet combing improves the accuracy of diagnosing an active infection.21

Treatment
Effective treatment of head lice requires eradication of all living lice as well as louse eggs. Topically applied pyrethroids, including pyrethrin shampoos and mousses and permethrin lotion 1%, are considered the first-line therapy.8 Pyrethroids are over-the-counter treatments that act by interfering with sodium transport in the louse, causing depolarization of the neuromembranes and respiratory paralysis.22 Pyrethrins are natural compounds derived from the chrysanthemum plant; permethrin is a synthetic compound. Pyrethrins often are combined with piperonyl butoxide, an insecticide synergist that improves efficacy by inhibiting pyrethrin catabolism.23 Resistance to pyrethroids has become an increasingly important problem in the United States and worldwide.
Malathion lotion 0.5% is another therapeutic option for head lice. Malathion is a prescription organophosphate cholinesterase inhibitor that also causes respiratory paralysis of the louse and is one of the few treatments that is ovicidal.22 It was withdrawn from the market in 1995 due to its flammability and a theoretical risk of respiratory depression if ingested; however, it was reintroduced in 1999 and remains an effective treatment option with little resistance in the United States.24
Lindane 1% (shampoo and lotion), an organochloride compound that acts by causing neuronal hyperstimulation and eventual paralysis of lice, is no longer recommended due to its serious side effects, including central nervous system toxicity and increased risk of seizure.8,24
New US Food and Drug Administration–Approved Therapies
Newer topical treatments include benzyl alcohol lotion 5%, spinosad topical suspension 0.9%, ivermectin lotion 0.5%, and dimethicone-based products. Benzyl alcohol was approved by the US Food and Drug Administration (FDA) in 2009 and is available in the United States by prescription.25 Benzyl alcohol kills lice by asphyxiation. Phase 2 and 3 clinical trials showed significant treatment success 1 day posttreatment (fewer live lice than the vehicle alone; P=.004) and 2 weeks posttreatment (absence of live lice compared to the vehicle alone; P=.001).26
Spinosad was approved by the FDA in 2011 and is available in the United States by prescription.25 It contains the compounds spinosyn A and spinosyn D, which are naturally derived through fermentation by the soil bacterium Saccharopolyspora spinosa. It also contains benzyl alcohol. Spinosad paralyzes lice by disrupting neuronal activity and is at least partially ovicidal.27 Phase 3 clinical trials published in 2009 showed that spinosad was significantly more effective than permethrin in eradicating head lice (P<.001).28
Topical ivermectin was approved by the FDA in 2012 for prescription use.25 It acts on chloride ion channels, causing hyperpolarization of the muscle cells of lice and resulting in paralysis and death. Oral ivermectin (200 μg/kg) given once and repeated in 10 days is not FDA approved for the treatment of head lice but has shown some effectiveness and is sometimes used.8 A comparison study of topical versus oral ivermectin published in 2014 found that eradication was achieved in 88% (n=27) of topical ivermectin users after 1 treatment and 100% (n=31) after 2 treatments. Oral ivermectin produced cure rates of 45% (n=14) after 1 treatment and 97% (n=30) after 2 treatments. Both topical and oral ivermectin treatments are well tolerated.29
Physically Acting Preparations
Products with a physical mode of action are a new attractive option for treatment of pediculosis because the development of resistance is less likely. Studies of silicone-based fluids that physically occlude the respiratory system of the louse, such as dimethicone liquid gel 4%, have shown superiority over treatment with pyrethroids.30,31 Although the safety of dimethicone has been demonstrated, silicone-based treatments have not yet been widely adopted in the United States and are not currently used as a first-line treatment.32 However, use of such physically acting pediculicides may in time surpass traditional neurotoxic treatments due to their low susceptibility to resistance and good safety profile.33,34
Alternative Therapies
Nonchemical treatments for head lice that have shown variable success include wet combing, hot air treatments, and varying occlusive treatments. Physical removal via wet combing requires persistent repeated treatments over several weeks; for example, wet combing may be performed every 3 days for at least 2 weeks or until no head lice are detected on 4 consecutive occasions.35 Cure rates range from 38% to 75% with wet combing as a sole treatment of head lice.36 Because this treatment has minimal risks and no adverse side effects, it can be considered as an alternative treatment for some patients.
Hot air treatments also have been studied. A 2006 study showed that a hot air treatment device had the potential to eradicate head lice, most likely by desiccation. Specifically, 30 minutes of exposure to hot air (at 58.9°F, slightly cooler than a standard hair dryer) using the custom-built device resulted in 98% mortality of eggs and 80% mortality of hatched lice.37 Large randomized controlled trials of hot air treatments have not been performed.
Other alternative treatments include plant-derived oils. A laboratory study of essential oils found that spearmint, cassia, and clove showed pediculicidal activity similar to malathion with improved ovicidal activity.38 However, there is a potential for development of contact dermatitis from essential oils.
Complete Eradication of Head Lice
Removal of nits is an important component of effective lice eradication. Biochemical analysis has revealed that the nit sheath of the head louse is similar in composition to amyloid, rendering it difficult to design products that will unravel the nit sheath while leaving human hair undamaged.39 Because pediculicides are not necessarily ovicidal and complete physical nit removal is difficult to achieve, re-treatment in 7 to 10 days often is advisable to ensure that lice in all stages of the life cycle have been killed.4 Treatment of any secondary bacterial infection also is important. Although transmission of lice via fomites is less likely than from head-to-head contact, the cleaning of hats, hairbrushes, and linens is prudent. Diagnosing and treating infested close contacts also is essential to achieving eradication.4 Coordinated surveillance, education, and treatment efforts in high-risk communities can help detect asymptomatic cases and control local epidemics in a cost-effective manner.40 However, “no nit” policies at schools likely cause a net harm, as nit removal is difficult and children with nonviable nits are then excluded from the classroom.5
Treatment Resistance
Resistance to topical neurotoxic treatments is becoming increasingly common.41-43 Therefore, it is important to identify local patterns of resistance, if possible, when selecting a therapy for head lice. Improper usage, changes in pediculicide formulations and packaging, decreased product efficacy, and natural selection have all contributed to this rise in resistance.7 Additionally, due to protection from multiple exoskeletons and the natural molting process as they mature into adults, nymphs may only receive a sublethal dose when exposed to pediculicides, contributing further to resistance.7 Resistance to synthetic pyrethroids is most predominant, likely due to selection pressure because permethrin historically has been the most widely used insecticide for pediculosis. A 2014 study found that the frequency of sodium-channel insensitivity to pyrethroids, also known as knockdown resistance (or kdr), in US head louse populations collected over a 10-year period was 84.4% and approached 100% in some communities in recent years.44 This evidence strongly supports the use of alternative therapeutic categories to effectively eradicate head lice infestations.
Conclusion
Head lice infestation is common in children, and although it is not harmful to the host, it can be an irritating and symptomatic problem and can lead to notable distress, missed days of school, and secondary infections. Identifying active adult lice is the gold standard for diagnosis. Current recommended treatments include pyrethroids as the first-line therapy; however, resistance to these neurotoxic agents is becoming increasingly common. Alternative therapies such as newer neurotoxic agents or pediculicides with physical mechanisms of action (eg, dimethicone-based products) should be considered, particularly in regions where resistance is known to be high. Education about head lice, proper use of treatment, and coordinated diagnosis are necessary for effective management of this problem.
The head louse (Pediculus humanus capitis) is a blood-sucking arthropod of the suborder Anoplura. Lice are obligate human parasites that have infested humans since antiquity. Pediculosis capitis is an infestation of the scalp by head lice. It is estimated that 6 to 12 million individuals in the United States are affected with head lice per year.1 Resistance to topical chemical pediculicides is widespread, and new agents have been developed to address this gap in care.
Characteristics of Head Lice
The head louse is a tan-gray–colored, wingless insect measuring approximately 2- to 3-mm long with 3 body segments. It has 6 legs with claws used to grasp individual hairs, and it moves by crawling; it does not fly or jump.2,3 The head louse has an elongated abdomen and a small head with short antennae and anterior piercing mouthparts (Figure 1).4 Nits are transparent, flask-shaped, 0.5- to 0.8-mm egg cases found firmly cemented to the hair shafts approximately 1 to 4 mm above the level of the scalp (Figure 2).5 The head louse resides on scalp hair and feeds off the scalp itself. Both lice and nits can be present throughout the scalp but are most commonly found in the postauricular and occipital scalp.3,4


Female lice live approximately 30 days and lay 5 to 10 eggs per day. Eggs incubate individually in nits laid close to the scalp for 8 to 10 days before hatching.1,6 The newly hatched nymphs (also called instars) have multiple exoskeletons that are shed as they grow.7 Nymphs mature into adults in approximately 2 weeks, and the life cycle begins again.8 Head lice are obligate human parasites, feeding approximately every 4 to 6 hours on the blood of the host; however, they can survive up to 4 days without a blood meal on fomites if the climate and conditions are favorable.5,9
Epidemiology and Transmission
Head lice infestations commonly occur in children aged 3 to 11 years and are more prevalent in girls and women.1,10 Infestation rates are not reliably recorded, and few population-based studies have been performed; however, it is estimated that 6 to 12 million individuals are infested annually in the United States.1 Prevalence in some European populations has been estimated to range from 1% to 20%.11 A 2008 literature review found that worldwide prevalence varied across populations from 0.7% to 59%.10
Transmission occurs most frequently from direct head-to-head contact. One study found that transmission is most likely to occur when hairs are arranged in a parallel alignment and move slowly in relation to one another.12 Although controversial and probably less notable, transmission also may occur indirectly via fomites or the sharing of hairbrushes, hats, or other headgear.13,14 Classrooms are a common place for transmission.1 A 2009 study in Germany found an increase in health department consultations for head lice when schools reopened after vacations. The investigators also found that pediculicide sales peaked from mid-September through October, subsequent to schools reopening after the summer holiday.15 There is some evidence that overcrowded housing also can lead to increased incidence and transmission.16,17 There is no consistent correlation of infestation with socioeconomic status.1,17,18
Clinical Manifestations and Diagnosis
Clinically, patients with head lice present with scalp pruritus and sometimes posterior cervical or occipital lymphadenopathy. Pediculosis also can be asymptomatic. With the first exposure, symptoms may not develop for up to 4 to 6 weeks as the immune system develops sensitivity to the louse saliva.6 Bite reactions consisting of papules or wheals are related to immune sensitization.5 Louse feces and excoriations from scratching to relieve itch also may be present on examination. Secondary infection of excoriations also is possible.1
Diagnosis of an active infestation is made by identifying living lice. Because lice move quickly and can be difficult to detect, tightly attached nits on the hair shaft within 4 mm of the scalp are at least indicative of a historic infestation and can be suggestive of active infestation.1,19 Dermoscopy is a helpful tool in differentiating eggs containing nymphs from the empty cases of hatched lice and also from amorphous pseudonits (hair casts)(Figure 3).19,20 Wet combing improves the accuracy of diagnosing an active infection.21

Treatment
Effective treatment of head lice requires eradication of all living lice as well as louse eggs. Topically applied pyrethroids, including pyrethrin shampoos and mousses and permethrin lotion 1%, are considered the first-line therapy.8 Pyrethroids are over-the-counter treatments that act by interfering with sodium transport in the louse, causing depolarization of the neuromembranes and respiratory paralysis.22 Pyrethrins are natural compounds derived from the chrysanthemum plant; permethrin is a synthetic compound. Pyrethrins often are combined with piperonyl butoxide, an insecticide synergist that improves efficacy by inhibiting pyrethrin catabolism.23 Resistance to pyrethroids has become an increasingly important problem in the United States and worldwide.
Malathion lotion 0.5% is another therapeutic option for head lice. Malathion is a prescription organophosphate cholinesterase inhibitor that also causes respiratory paralysis of the louse and is one of the few treatments that is ovicidal.22 It was withdrawn from the market in 1995 due to its flammability and a theoretical risk of respiratory depression if ingested; however, it was reintroduced in 1999 and remains an effective treatment option with little resistance in the United States.24
Lindane 1% (shampoo and lotion), an organochloride compound that acts by causing neuronal hyperstimulation and eventual paralysis of lice, is no longer recommended due to its serious side effects, including central nervous system toxicity and increased risk of seizure.8,24
New US Food and Drug Administration–Approved Therapies
Newer topical treatments include benzyl alcohol lotion 5%, spinosad topical suspension 0.9%, ivermectin lotion 0.5%, and dimethicone-based products. Benzyl alcohol was approved by the US Food and Drug Administration (FDA) in 2009 and is available in the United States by prescription.25 Benzyl alcohol kills lice by asphyxiation. Phase 2 and 3 clinical trials showed significant treatment success 1 day posttreatment (fewer live lice than the vehicle alone; P=.004) and 2 weeks posttreatment (absence of live lice compared to the vehicle alone; P=.001).26
Spinosad was approved by the FDA in 2011 and is available in the United States by prescription.25 It contains the compounds spinosyn A and spinosyn D, which are naturally derived through fermentation by the soil bacterium Saccharopolyspora spinosa. It also contains benzyl alcohol. Spinosad paralyzes lice by disrupting neuronal activity and is at least partially ovicidal.27 Phase 3 clinical trials published in 2009 showed that spinosad was significantly more effective than permethrin in eradicating head lice (P<.001).28
Topical ivermectin was approved by the FDA in 2012 for prescription use.25 It acts on chloride ion channels, causing hyperpolarization of the muscle cells of lice and resulting in paralysis and death. Oral ivermectin (200 μg/kg) given once and repeated in 10 days is not FDA approved for the treatment of head lice but has shown some effectiveness and is sometimes used.8 A comparison study of topical versus oral ivermectin published in 2014 found that eradication was achieved in 88% (n=27) of topical ivermectin users after 1 treatment and 100% (n=31) after 2 treatments. Oral ivermectin produced cure rates of 45% (n=14) after 1 treatment and 97% (n=30) after 2 treatments. Both topical and oral ivermectin treatments are well tolerated.29
Physically Acting Preparations
Products with a physical mode of action are a new attractive option for treatment of pediculosis because the development of resistance is less likely. Studies of silicone-based fluids that physically occlude the respiratory system of the louse, such as dimethicone liquid gel 4%, have shown superiority over treatment with pyrethroids.30,31 Although the safety of dimethicone has been demonstrated, silicone-based treatments have not yet been widely adopted in the United States and are not currently used as a first-line treatment.32 However, use of such physically acting pediculicides may in time surpass traditional neurotoxic treatments due to their low susceptibility to resistance and good safety profile.33,34
Alternative Therapies
Nonchemical treatments for head lice that have shown variable success include wet combing, hot air treatments, and varying occlusive treatments. Physical removal via wet combing requires persistent repeated treatments over several weeks; for example, wet combing may be performed every 3 days for at least 2 weeks or until no head lice are detected on 4 consecutive occasions.35 Cure rates range from 38% to 75% with wet combing as a sole treatment of head lice.36 Because this treatment has minimal risks and no adverse side effects, it can be considered as an alternative treatment for some patients.
Hot air treatments also have been studied. A 2006 study showed that a hot air treatment device had the potential to eradicate head lice, most likely by desiccation. Specifically, 30 minutes of exposure to hot air (at 58.9°F, slightly cooler than a standard hair dryer) using the custom-built device resulted in 98% mortality of eggs and 80% mortality of hatched lice.37 Large randomized controlled trials of hot air treatments have not been performed.
Other alternative treatments include plant-derived oils. A laboratory study of essential oils found that spearmint, cassia, and clove showed pediculicidal activity similar to malathion with improved ovicidal activity.38 However, there is a potential for development of contact dermatitis from essential oils.
Complete Eradication of Head Lice
Removal of nits is an important component of effective lice eradication. Biochemical analysis has revealed that the nit sheath of the head louse is similar in composition to amyloid, rendering it difficult to design products that will unravel the nit sheath while leaving human hair undamaged.39 Because pediculicides are not necessarily ovicidal and complete physical nit removal is difficult to achieve, re-treatment in 7 to 10 days often is advisable to ensure that lice in all stages of the life cycle have been killed.4 Treatment of any secondary bacterial infection also is important. Although transmission of lice via fomites is less likely than from head-to-head contact, the cleaning of hats, hairbrushes, and linens is prudent. Diagnosing and treating infested close contacts also is essential to achieving eradication.4 Coordinated surveillance, education, and treatment efforts in high-risk communities can help detect asymptomatic cases and control local epidemics in a cost-effective manner.40 However, “no nit” policies at schools likely cause a net harm, as nit removal is difficult and children with nonviable nits are then excluded from the classroom.5
Treatment Resistance
Resistance to topical neurotoxic treatments is becoming increasingly common.41-43 Therefore, it is important to identify local patterns of resistance, if possible, when selecting a therapy for head lice. Improper usage, changes in pediculicide formulations and packaging, decreased product efficacy, and natural selection have all contributed to this rise in resistance.7 Additionally, due to protection from multiple exoskeletons and the natural molting process as they mature into adults, nymphs may only receive a sublethal dose when exposed to pediculicides, contributing further to resistance.7 Resistance to synthetic pyrethroids is most predominant, likely due to selection pressure because permethrin historically has been the most widely used insecticide for pediculosis. A 2014 study found that the frequency of sodium-channel insensitivity to pyrethroids, also known as knockdown resistance (or kdr), in US head louse populations collected over a 10-year period was 84.4% and approached 100% in some communities in recent years.44 This evidence strongly supports the use of alternative therapeutic categories to effectively eradicate head lice infestations.
Conclusion
Head lice infestation is common in children, and although it is not harmful to the host, it can be an irritating and symptomatic problem and can lead to notable distress, missed days of school, and secondary infections. Identifying active adult lice is the gold standard for diagnosis. Current recommended treatments include pyrethroids as the first-line therapy; however, resistance to these neurotoxic agents is becoming increasingly common. Alternative therapies such as newer neurotoxic agents or pediculicides with physical mechanisms of action (eg, dimethicone-based products) should be considered, particularly in regions where resistance is known to be high. Education about head lice, proper use of treatment, and coordinated diagnosis are necessary for effective management of this problem.
- Chosidow O. Scabies and pediculosis. Lancet. 2000;355:819-826.
- Centers for Disease Control and Prevention. Head lice. http://www.cdc.gov/parasites/lice/head/index.html. Updated September 24, 2013. Accessed November 9, 2017.
- Hurwitz S. Lice (pediculosis). In: Hurwitz S. Hurwitz Clinical Pediatric Dermatology: A Textbook of Skin Disorders of Childhood and Adolescence. 2nd ed. Philadelphia, PA: WB Saunders Company; 1993:416-419.
- Elston DM. What’s eating you? Pediculus humanus (head louse and body louse). Cutis. 1999;63:259-264.
- Ko CJ, Elston DM. Pediculosis. J Am Acad Dermatol. 2004;50:1-12.
- Frankowski BL, Weiner LB. Head lice. Pediatrics. 2002;110:638-643.
- Meinking TL. Clinical update on resistance and treatment of pediculosis capitis. Am J Manag Care. 2004;10(9 suppl):S264-S268.
- Devore CD, Schutze GE. Head lice. Pediatrics. 2015;135:E1355-E1365.
- Burkhart CN. Fomite transmission with head lice: a continuing controversy. Lancet. 2003;361:99-100.
- Falagas ME, Matthaiou DK, Rafailidis PI, et al. Worldwide prevalence of head lice. Emerg Infect Dis. 2008;14:1493-1494.
- Feldmeier H. Pediculosis capitis: new insights into epidemiology, diagnosis and treatment. Eur J Clin Microbiol Infect Dis. 2012;31:2105-2110.
- Canyon DV, Speare R, Muller R. Spatial and kinetic factors for the transfer of head lice (Pediculus capitis) between hairs. J Invest Dermatol. 2002;119:629-631.
- Burkhart CN, Burkhart CG. Fomite transmission in head lice. J Am Acad Dermatol. 2007;56:1044-1047.
- Canyon DV, Speare R. Indirect transmission of head lice via inanimate objects. Open Dermatol J. 2010;4:72-76.
- Bauer E, Jahnke C, Feldmeier H. Seasonal fluctuations of head lice infestation in Germany. Parasitol Res. 2009;104:677-681.
- Balcioglu IC, Kurt O, Limoncu ME, et al. Rural life, lower socioeconomic status and parasitic infections. Parasitol Int. 2007;56:129-133.
- Lesshafft H, Baier A, Guerra H, et al. Prevalence and risk factors associated with pediculosis capitis in an impoverished urban community in Lima, Peru. J Glob Infect Dis. 2013;5:138-143.
- Tagka A, Lambrou GI, Braoudaki M, et al. Socioeconomical factors associated with pediculosis (Phthiraptera: Pediculidae) in Athens, Greece. J Med Entomol. 2016;53:919-922.
- Di Stefani A, Hofmann-Wellenhof R, Zalaudek I. Dermoscopy for diagnosis and treatment monitoring of pediculosis capitis. J Am Acad Dermatol. 2006;54:909-911.
- Bakos RM, Bakos L. Dermoscopy for diagnosis of pediculosis capitis. J Am Acad Dermatol. 2007;57:727-728.
- Jahnke C, Bauer E, Hengge UR, et al. Accuracy of diagnosis of pediculosis capitis: visual inspection vs wet combing. Arch Dermatol. 2009;145:309-313.
- Elston DM. Drugs used in the treatment of pediculosis. J Drugs Dermatol. 2005;4:207-211.
- National Pesticide Information Center. Piperonyl butoxide (general fact sheet). http://npic.orst.edu/factsheets/pbogen.pdf/. Accessed November 13, 2017.
- Diamantis SA, Morrell DS, Burkhart CN. Treatment of head lice. Dermatol Ther. 2009;22:273-278.
- United States Food and Drug Administration. Treating and preventing head lice. http://www.fda.gov/forconsumers/consumerupdates/ucm171730.htm. Published July 13, 2010. Updated November 8, 2017. Accessed November 13, 2017.
- Meinking TL, Villar ME, Vicaria M, et al. The clinical trials supporting benzyl alcohol lotion 5% (UlesfiaTM): a safe and effective topical treatment for head lice (Pediculosis Humanus Capitis). Pediatr Dermatol. 2010;27:19-24.
- McCormack PL. Spinosad in pediculosis capitis. Am J Clin Dermatol. 2011;12:349-353.
- Stough D, Shellabarger S, Quiring J, et al. Efficacy and safety of spinosad and permethrin creme rinses for pediculosis capitis (head lice). Pediatrics. 2009;124:E389-E395.
- Ahmad HM, Abdel-Azim ES, Abdel-Aziz RT. Assessment of topical versus oral ivermectin as a treatment for head lice. Dermatol Ther. 2014;27:307-310.
- Heukelbach J, Pilger D, Oliveira FA, et al. A highly efficacious pediculicide based on dimethicone: randomized observer blinded comparative trial. BMC Infect Dis. 2008;8:115.
- Burgess IF, Brunton ER, Burgess NA. Single application of 4% dimethicone liquid gel versus two applications of 1% permethrin creme rinse for treatment of head louse infestation: a randomised controlled trial. BMC Dermatol. 2013;13:5.
- Ihde ES, Boscamp JR, Loh JM, et al. Safety and efficacy of a 100% dimethicone pediculocide in school-age children. BMC Pediatr. 2015;15:70.
- Heukelbach J, Oliveira FA, Richter J, et al. Dimethicone-based pediculicides: a physical approach to eradicate head lice. Open Dermatol J. 2010;4:77-81.
- Feldmeier H. Treatment of pediculosis capitis: a critical appraisal of the current literature. Am J Clin Dermatol. 2014;15:401-412.
- Glasziou P, Bennett J, Greenberg P, et al; Handbook Of Non Drug Intervention (HANDI) Project Team. Wet combing for the eradication of head lice. Aust Fam Physician. 2013;42:129-130.
- Tebruegge M, Runnacles J. Is wet combing effective in children with pediculosis capitis infestation? Arch Dis Child. 2007;92:818-820.
- Goates BM, Atkin JS, Wilding KG, et al. An effective nonchemical treatment for head lice: a lot of hot air. Pediatrics. 2006;118:1962-1970.
- Yones DA, Bakir HY, Bayoumi SA. Chemical composition and efficacy of some selected plant oils against Pediculus humanus capitis in vitro. Parasitol Res. 2016;115:3209-3218.
- Burkhart CN, Burkhart CG. Head lice: scientific assessment of the nit sheath with clinical ramifications and therapeutic options. J Am Acad Dermatol. 2005;53:129-133.
- Ibarra J, Fry F, Wickenden C, et al. The impact of well-developed preventative strategies on the eradication of head lice. Perspect Public Health. 2009;129:165-173.
- Mumcuoglu KY, Hemingway J, Miller J, et al. Permethrin resistance in the head louse pediculus humanus capitis from Israel. Med Vet Entomol. 1995;9:427-432.
- Meinking TL, Serrano L, Hard B, et al. Comparative in vitro pediculicidal efficacy of treatments in a resistant head lice population in the United States. Arch Dermatol. 2002;138:220-224.
- Hemingway J, Miller J, Mumcuoglu KY. Pyrethroid resistance mechanisms in the head louse Pediculus capitis from Israel: implications for control. Med Vet Entomol. 1999;13:89-96.
- Yoon KS, Previte DJ, Hodgdon HE, et al. Knockdown resistance allele frequencies in North American head louse (Anoplura: Pediculidae) populations. J Med Entomol. 2014;51:450-457.
- Chosidow O. Scabies and pediculosis. Lancet. 2000;355:819-826.
- Centers for Disease Control and Prevention. Head lice. http://www.cdc.gov/parasites/lice/head/index.html. Updated September 24, 2013. Accessed November 9, 2017.
- Hurwitz S. Lice (pediculosis). In: Hurwitz S. Hurwitz Clinical Pediatric Dermatology: A Textbook of Skin Disorders of Childhood and Adolescence. 2nd ed. Philadelphia, PA: WB Saunders Company; 1993:416-419.
- Elston DM. What’s eating you? Pediculus humanus (head louse and body louse). Cutis. 1999;63:259-264.
- Ko CJ, Elston DM. Pediculosis. J Am Acad Dermatol. 2004;50:1-12.
- Frankowski BL, Weiner LB. Head lice. Pediatrics. 2002;110:638-643.
- Meinking TL. Clinical update on resistance and treatment of pediculosis capitis. Am J Manag Care. 2004;10(9 suppl):S264-S268.
- Devore CD, Schutze GE. Head lice. Pediatrics. 2015;135:E1355-E1365.
- Burkhart CN. Fomite transmission with head lice: a continuing controversy. Lancet. 2003;361:99-100.
- Falagas ME, Matthaiou DK, Rafailidis PI, et al. Worldwide prevalence of head lice. Emerg Infect Dis. 2008;14:1493-1494.
- Feldmeier H. Pediculosis capitis: new insights into epidemiology, diagnosis and treatment. Eur J Clin Microbiol Infect Dis. 2012;31:2105-2110.
- Canyon DV, Speare R, Muller R. Spatial and kinetic factors for the transfer of head lice (Pediculus capitis) between hairs. J Invest Dermatol. 2002;119:629-631.
- Burkhart CN, Burkhart CG. Fomite transmission in head lice. J Am Acad Dermatol. 2007;56:1044-1047.
- Canyon DV, Speare R. Indirect transmission of head lice via inanimate objects. Open Dermatol J. 2010;4:72-76.
- Bauer E, Jahnke C, Feldmeier H. Seasonal fluctuations of head lice infestation in Germany. Parasitol Res. 2009;104:677-681.
- Balcioglu IC, Kurt O, Limoncu ME, et al. Rural life, lower socioeconomic status and parasitic infections. Parasitol Int. 2007;56:129-133.
- Lesshafft H, Baier A, Guerra H, et al. Prevalence and risk factors associated with pediculosis capitis in an impoverished urban community in Lima, Peru. J Glob Infect Dis. 2013;5:138-143.
- Tagka A, Lambrou GI, Braoudaki M, et al. Socioeconomical factors associated with pediculosis (Phthiraptera: Pediculidae) in Athens, Greece. J Med Entomol. 2016;53:919-922.
- Di Stefani A, Hofmann-Wellenhof R, Zalaudek I. Dermoscopy for diagnosis and treatment monitoring of pediculosis capitis. J Am Acad Dermatol. 2006;54:909-911.
- Bakos RM, Bakos L. Dermoscopy for diagnosis of pediculosis capitis. J Am Acad Dermatol. 2007;57:727-728.
- Jahnke C, Bauer E, Hengge UR, et al. Accuracy of diagnosis of pediculosis capitis: visual inspection vs wet combing. Arch Dermatol. 2009;145:309-313.
- Elston DM. Drugs used in the treatment of pediculosis. J Drugs Dermatol. 2005;4:207-211.
- National Pesticide Information Center. Piperonyl butoxide (general fact sheet). http://npic.orst.edu/factsheets/pbogen.pdf/. Accessed November 13, 2017.
- Diamantis SA, Morrell DS, Burkhart CN. Treatment of head lice. Dermatol Ther. 2009;22:273-278.
- United States Food and Drug Administration. Treating and preventing head lice. http://www.fda.gov/forconsumers/consumerupdates/ucm171730.htm. Published July 13, 2010. Updated November 8, 2017. Accessed November 13, 2017.
- Meinking TL, Villar ME, Vicaria M, et al. The clinical trials supporting benzyl alcohol lotion 5% (UlesfiaTM): a safe and effective topical treatment for head lice (Pediculosis Humanus Capitis). Pediatr Dermatol. 2010;27:19-24.
- McCormack PL. Spinosad in pediculosis capitis. Am J Clin Dermatol. 2011;12:349-353.
- Stough D, Shellabarger S, Quiring J, et al. Efficacy and safety of spinosad and permethrin creme rinses for pediculosis capitis (head lice). Pediatrics. 2009;124:E389-E395.
- Ahmad HM, Abdel-Azim ES, Abdel-Aziz RT. Assessment of topical versus oral ivermectin as a treatment for head lice. Dermatol Ther. 2014;27:307-310.
- Heukelbach J, Pilger D, Oliveira FA, et al. A highly efficacious pediculicide based on dimethicone: randomized observer blinded comparative trial. BMC Infect Dis. 2008;8:115.
- Burgess IF, Brunton ER, Burgess NA. Single application of 4% dimethicone liquid gel versus two applications of 1% permethrin creme rinse for treatment of head louse infestation: a randomised controlled trial. BMC Dermatol. 2013;13:5.
- Ihde ES, Boscamp JR, Loh JM, et al. Safety and efficacy of a 100% dimethicone pediculocide in school-age children. BMC Pediatr. 2015;15:70.
- Heukelbach J, Oliveira FA, Richter J, et al. Dimethicone-based pediculicides: a physical approach to eradicate head lice. Open Dermatol J. 2010;4:77-81.
- Feldmeier H. Treatment of pediculosis capitis: a critical appraisal of the current literature. Am J Clin Dermatol. 2014;15:401-412.
- Glasziou P, Bennett J, Greenberg P, et al; Handbook Of Non Drug Intervention (HANDI) Project Team. Wet combing for the eradication of head lice. Aust Fam Physician. 2013;42:129-130.
- Tebruegge M, Runnacles J. Is wet combing effective in children with pediculosis capitis infestation? Arch Dis Child. 2007;92:818-820.
- Goates BM, Atkin JS, Wilding KG, et al. An effective nonchemical treatment for head lice: a lot of hot air. Pediatrics. 2006;118:1962-1970.
- Yones DA, Bakir HY, Bayoumi SA. Chemical composition and efficacy of some selected plant oils against Pediculus humanus capitis in vitro. Parasitol Res. 2016;115:3209-3218.
- Burkhart CN, Burkhart CG. Head lice: scientific assessment of the nit sheath with clinical ramifications and therapeutic options. J Am Acad Dermatol. 2005;53:129-133.
- Ibarra J, Fry F, Wickenden C, et al. The impact of well-developed preventative strategies on the eradication of head lice. Perspect Public Health. 2009;129:165-173.
- Mumcuoglu KY, Hemingway J, Miller J, et al. Permethrin resistance in the head louse pediculus humanus capitis from Israel. Med Vet Entomol. 1995;9:427-432.
- Meinking TL, Serrano L, Hard B, et al. Comparative in vitro pediculicidal efficacy of treatments in a resistant head lice population in the United States. Arch Dermatol. 2002;138:220-224.
- Hemingway J, Miller J, Mumcuoglu KY. Pyrethroid resistance mechanisms in the head louse Pediculus capitis from Israel: implications for control. Med Vet Entomol. 1999;13:89-96.
- Yoon KS, Previte DJ, Hodgdon HE, et al. Knockdown resistance allele frequencies in North American head louse (Anoplura: Pediculidae) populations. J Med Entomol. 2014;51:450-457.
Practice Points
- Transmission of head lice occurs most frequently from direct head-to-head contact; however, head lice can survive up to 4 days on fomites.
- Patients present with scalp pruritus and bite reactions (papules or wheals), but pediculosis can be asymptomatic, particularly with the first exposure before the immune system has developed sensitivity to the louse saliva.
- Topical pyrethroids are available over-the-counter and are considered first-line therapy; however, resistance to pyrethroids has become an important problem in the United States and worldwide.
- Newer topical treatments such as benzyl alcohol lotion 5%, spinosad topical suspension 0.9%, and ivermectin lotion 0.5% can be prescribed as alternative therapies, particularly if resistance to pyrethroids is a concern.
Emerging treatments tackling hair loss challenges include light therapies
according to Maria Hordinsky, MD.
“Although the precise mechanism remains unclear, it has been postulated that photobiomodulation acts through oxidative metabolism and transcription factor stimulation,” Dr. Hordinsky said in a presentation at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
She referred to one trial, which found that men with androgenetic alopecia who used the HairMax Lasercomb showed an increase in mean hair density at 26 weeks of daily use, compared with a group that used a sham device.
Photobiomodulation devices use either laser light or light-emitting diodes. Comparing the two types is a challenge, and the question of which is more effective remains unanswered, Dr. Hordinsky said.
Other issues to be addressed in future research include finding the optimal wavelength to use for different indications for light-based treatments, determining whether pulse or continuous wave is more effective, and evaluating the potential for systemic side effects of these therapies, she noted.
No treatment for alopecia areata is currently approved by the Food and Drug Administration, but factors to consider when choosing a treatment include the patient’s age, location and extent of hair loss, and the presence of other medical problems, as well as a scalp biopsy report with information on the hair cycle and inflammation. Patients and/or their parents should understand the risks and benefits associated with various treatments to make an informed decision, Dr. Hordinsky said.
Patients and their families “have heard the ‘buzz’ about potential new treatments for alopecia areata, and the discussion needs to include a conversation about ongoing and future clinical research opportunities, as well as off-label use of Janus kinase inhibitors,” particularly oral tofacitinib, she said.
Approximately two-thirds of patients in recent studies of oral tofacitinib have had clinically acceptable hair regrowth after 6 months, Dr. Hordinsky said. Ruxolitinib is also being studied. However, “until clinical research studies are completed, there will be ongoing debate regarding the risks and benefits, cost, and sustainability” of JAK inhibitors or other new treatments, she said.
Dr. Hordinsky disclosed that she is a consultant for companies including Procter & Gamble and Concert, and has received grant/research support from Incyte, Allergan, and the National Alopecia Areata Foundation.
SDEF and this news organization are owned by Frontline Medical Communications.
according to Maria Hordinsky, MD.
“Although the precise mechanism remains unclear, it has been postulated that photobiomodulation acts through oxidative metabolism and transcription factor stimulation,” Dr. Hordinsky said in a presentation at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
She referred to one trial, which found that men with androgenetic alopecia who used the HairMax Lasercomb showed an increase in mean hair density at 26 weeks of daily use, compared with a group that used a sham device.
Photobiomodulation devices use either laser light or light-emitting diodes. Comparing the two types is a challenge, and the question of which is more effective remains unanswered, Dr. Hordinsky said.
Other issues to be addressed in future research include finding the optimal wavelength to use for different indications for light-based treatments, determining whether pulse or continuous wave is more effective, and evaluating the potential for systemic side effects of these therapies, she noted.
No treatment for alopecia areata is currently approved by the Food and Drug Administration, but factors to consider when choosing a treatment include the patient’s age, location and extent of hair loss, and the presence of other medical problems, as well as a scalp biopsy report with information on the hair cycle and inflammation. Patients and/or their parents should understand the risks and benefits associated with various treatments to make an informed decision, Dr. Hordinsky said.
Patients and their families “have heard the ‘buzz’ about potential new treatments for alopecia areata, and the discussion needs to include a conversation about ongoing and future clinical research opportunities, as well as off-label use of Janus kinase inhibitors,” particularly oral tofacitinib, she said.
Approximately two-thirds of patients in recent studies of oral tofacitinib have had clinically acceptable hair regrowth after 6 months, Dr. Hordinsky said. Ruxolitinib is also being studied. However, “until clinical research studies are completed, there will be ongoing debate regarding the risks and benefits, cost, and sustainability” of JAK inhibitors or other new treatments, she said.
Dr. Hordinsky disclosed that she is a consultant for companies including Procter & Gamble and Concert, and has received grant/research support from Incyte, Allergan, and the National Alopecia Areata Foundation.
SDEF and this news organization are owned by Frontline Medical Communications.
according to Maria Hordinsky, MD.
“Although the precise mechanism remains unclear, it has been postulated that photobiomodulation acts through oxidative metabolism and transcription factor stimulation,” Dr. Hordinsky said in a presentation at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
She referred to one trial, which found that men with androgenetic alopecia who used the HairMax Lasercomb showed an increase in mean hair density at 26 weeks of daily use, compared with a group that used a sham device.
Photobiomodulation devices use either laser light or light-emitting diodes. Comparing the two types is a challenge, and the question of which is more effective remains unanswered, Dr. Hordinsky said.
Other issues to be addressed in future research include finding the optimal wavelength to use for different indications for light-based treatments, determining whether pulse or continuous wave is more effective, and evaluating the potential for systemic side effects of these therapies, she noted.
No treatment for alopecia areata is currently approved by the Food and Drug Administration, but factors to consider when choosing a treatment include the patient’s age, location and extent of hair loss, and the presence of other medical problems, as well as a scalp biopsy report with information on the hair cycle and inflammation. Patients and/or their parents should understand the risks and benefits associated with various treatments to make an informed decision, Dr. Hordinsky said.
Patients and their families “have heard the ‘buzz’ about potential new treatments for alopecia areata, and the discussion needs to include a conversation about ongoing and future clinical research opportunities, as well as off-label use of Janus kinase inhibitors,” particularly oral tofacitinib, she said.
Approximately two-thirds of patients in recent studies of oral tofacitinib have had clinically acceptable hair regrowth after 6 months, Dr. Hordinsky said. Ruxolitinib is also being studied. However, “until clinical research studies are completed, there will be ongoing debate regarding the risks and benefits, cost, and sustainability” of JAK inhibitors or other new treatments, she said.
Dr. Hordinsky disclosed that she is a consultant for companies including Procter & Gamble and Concert, and has received grant/research support from Incyte, Allergan, and the National Alopecia Areata Foundation.
SDEF and this news organization are owned by Frontline Medical Communications.
FROM SDEF WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR
Consider different T. capitis presentations in children with hair loss
Categorizing hair loss in children depends on many factors, but it is important to rule out an infectious etiology as early as possible, according to Sheila Fallon Friedlander, MD.
“What can Tinea capitis look like? Anything,” she said in a presentation at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
Although T. capitis most often presents in children aged 3-7 years as a pattern of localized hair loss, often with scaling, sometimes with nodules, other possibilities include pustules, boggy masses, and diffuse hair loss, said Dr. Friedlander, professor of pediatrics and dermatology at the University of California, San Diego.
Sometimes the hair loss may be so subtle that families come in complaining of “dandruff” rather than hair loss, she noted. Evaluating the patient for the presence of cervical or occipital lymph nodes is crucial; big nodes are usually a tip-off that infection is present.
Clinicians treating T. capitis should ask about family pets, advised Dr. Friedlander, adding that city dwellers’ conditions may be more likely caused by Trichophyton tonsurans, T. violaceum, or Trichophyton soudanense. Also consider immigration status and family history when evaluating T. capitis, and use a Wood’s lamp for diagnosis if one is available, she advised. M. canis will fluoresce and T. tonsurans will not, she pointed out.
Other strategies to evaluate the condition include KOH, culture, polymerase chain reaction, and trichoscopy.
The optimal treatment plan for T. capitis depends on the source, Dr. Friedlander explained. If M. canis is the cause, “griseofulvin is the drug of choice,” along with a twice-weekly sporicidal shampoo, she said.
Other treatment options include terbinafine, itraconazole, and fluconazole, and each have their pros and cons, she said. Terbinafine – which persists for months in the skin, nails, and hair – is the least expensive, and is her first choice for infections caused by T. tonsurans.
Itraconazole is available as a liquid, but costs more, causes diarrhea, and comes with a boxed warning about the potential for cardiac complications; fluconazole is the most expensive, but may be used in infants, she added.
Other high-risk groups for T. capitis include female caretakers of high-risk individuals, such as “grandma”; wrestlers; and Buddhist monks, she said. “Short hair, sharing combs, and unclean barbers” contributed to a documented increased risk of T. capitis according to a recently published study of 60 Buddhist monks whose average age was 11.6 years, she added. (Pediatr Dermatol. 2017 May;34[3]:371-3).
Dr. Friedlander had no relevant financial conflicts to disclose.
SDEF and this news organization are owned by Frontline Medical Communications.
Categorizing hair loss in children depends on many factors, but it is important to rule out an infectious etiology as early as possible, according to Sheila Fallon Friedlander, MD.
“What can Tinea capitis look like? Anything,” she said in a presentation at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
Although T. capitis most often presents in children aged 3-7 years as a pattern of localized hair loss, often with scaling, sometimes with nodules, other possibilities include pustules, boggy masses, and diffuse hair loss, said Dr. Friedlander, professor of pediatrics and dermatology at the University of California, San Diego.
Sometimes the hair loss may be so subtle that families come in complaining of “dandruff” rather than hair loss, she noted. Evaluating the patient for the presence of cervical or occipital lymph nodes is crucial; big nodes are usually a tip-off that infection is present.
Clinicians treating T. capitis should ask about family pets, advised Dr. Friedlander, adding that city dwellers’ conditions may be more likely caused by Trichophyton tonsurans, T. violaceum, or Trichophyton soudanense. Also consider immigration status and family history when evaluating T. capitis, and use a Wood’s lamp for diagnosis if one is available, she advised. M. canis will fluoresce and T. tonsurans will not, she pointed out.
Other strategies to evaluate the condition include KOH, culture, polymerase chain reaction, and trichoscopy.
The optimal treatment plan for T. capitis depends on the source, Dr. Friedlander explained. If M. canis is the cause, “griseofulvin is the drug of choice,” along with a twice-weekly sporicidal shampoo, she said.
Other treatment options include terbinafine, itraconazole, and fluconazole, and each have their pros and cons, she said. Terbinafine – which persists for months in the skin, nails, and hair – is the least expensive, and is her first choice for infections caused by T. tonsurans.
Itraconazole is available as a liquid, but costs more, causes diarrhea, and comes with a boxed warning about the potential for cardiac complications; fluconazole is the most expensive, but may be used in infants, she added.
Other high-risk groups for T. capitis include female caretakers of high-risk individuals, such as “grandma”; wrestlers; and Buddhist monks, she said. “Short hair, sharing combs, and unclean barbers” contributed to a documented increased risk of T. capitis according to a recently published study of 60 Buddhist monks whose average age was 11.6 years, she added. (Pediatr Dermatol. 2017 May;34[3]:371-3).
Dr. Friedlander had no relevant financial conflicts to disclose.
SDEF and this news organization are owned by Frontline Medical Communications.
Categorizing hair loss in children depends on many factors, but it is important to rule out an infectious etiology as early as possible, according to Sheila Fallon Friedlander, MD.
“What can Tinea capitis look like? Anything,” she said in a presentation at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
Although T. capitis most often presents in children aged 3-7 years as a pattern of localized hair loss, often with scaling, sometimes with nodules, other possibilities include pustules, boggy masses, and diffuse hair loss, said Dr. Friedlander, professor of pediatrics and dermatology at the University of California, San Diego.
Sometimes the hair loss may be so subtle that families come in complaining of “dandruff” rather than hair loss, she noted. Evaluating the patient for the presence of cervical or occipital lymph nodes is crucial; big nodes are usually a tip-off that infection is present.
Clinicians treating T. capitis should ask about family pets, advised Dr. Friedlander, adding that city dwellers’ conditions may be more likely caused by Trichophyton tonsurans, T. violaceum, or Trichophyton soudanense. Also consider immigration status and family history when evaluating T. capitis, and use a Wood’s lamp for diagnosis if one is available, she advised. M. canis will fluoresce and T. tonsurans will not, she pointed out.
Other strategies to evaluate the condition include KOH, culture, polymerase chain reaction, and trichoscopy.
The optimal treatment plan for T. capitis depends on the source, Dr. Friedlander explained. If M. canis is the cause, “griseofulvin is the drug of choice,” along with a twice-weekly sporicidal shampoo, she said.
Other treatment options include terbinafine, itraconazole, and fluconazole, and each have their pros and cons, she said. Terbinafine – which persists for months in the skin, nails, and hair – is the least expensive, and is her first choice for infections caused by T. tonsurans.
Itraconazole is available as a liquid, but costs more, causes diarrhea, and comes with a boxed warning about the potential for cardiac complications; fluconazole is the most expensive, but may be used in infants, she added.
Other high-risk groups for T. capitis include female caretakers of high-risk individuals, such as “grandma”; wrestlers; and Buddhist monks, she said. “Short hair, sharing combs, and unclean barbers” contributed to a documented increased risk of T. capitis according to a recently published study of 60 Buddhist monks whose average age was 11.6 years, she added. (Pediatr Dermatol. 2017 May;34[3]:371-3).
Dr. Friedlander had no relevant financial conflicts to disclose.
SDEF and this news organization are owned by Frontline Medical Communications.
FROM SDEF WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR
Nail biopsies made simple
CHICAGO – Maral Skelsey, MD, doesn’t get flowers from her patients very often. But, she said, a big bouquet recently landed on her desk after she had performed a nail biopsy on a patient. The note from the patient read, “That wasn’t as bad as I thought it would be!”
The patient’s relief after the procedure highlights the apprehension that both patients and dermatologists can feel when a nail biopsy becomes necessary, said Dr. Skelsey, director of dermatologic surgery at Georgetown University, Washington, D.C.
Speaking at the summer meeting of the American Academy of Dermatology, Dr. Skelsey said that the most important advice she can give about the nail biopsy is, “Do it early and often.”
Dr. Skelsey reminded the audience that the musician Bob Marley died of malignant melanoma; the first sign of his cancer was a longitudinal melanonychia that went unbiopsied. “The biggest mistake we make is not doing it,” she said.
In performing a nail biopsy, said Dr. Skelsey, the goals are, first and foremost, to optimize the pathologic diagnosis. Correct technique can help avoid complications such as bleeding, infection, and nail dystrophy; the right approach can minimize pain and anxiety, she added.
In preparing for a biopsy for melanonychia, “dermoscopy can be very helpful” in assessing the location of the pigment and fine-tuning planning for the biopsy, said Dr. Skelsey. Also, if the streak of melanonychia has reached the distal nail, sending the clipping for pathology can be useful as well.
For dorsal pigmentation, the proximal nail matrix should be biopsied.
“Do not use a punch biopsy on the nail fold to diagnose melanoma – you will get a false negative,” Dr. Skelsey said. It’s not possible to get an accurate diagnosis going through the nail plate to the nail bed, she said.
The preoperative assessment is usually straightforward. Pertinent items in the patient’s history include any medication allergies, current anticoagulation, and any history of prior trauma to the digit to be biopsied. Occasionally, imaging may be helpful, and patients should always be assessed for vascular insufficiency, she noted.
Preoperatively, she asks her patients to remove nail polish and pretreat the area with povidone iodine for 2 days prior to the procedure. Patients need to have a ride home after the procedure, and should be prepared to elevate the affected extremity for 48 hours post procedure. If a toenail is biopsied, they’re advised to come with a postop shoe.
Her patients receive a 5-minute isopropyl alcohol wash of the area to be biopsied just before the procedure, followed by air drying and a 5-minute scrub with 7.5% povidone iodine, which then is wiped off preprocedure.
For hemostasis, a tourniquet can be improvised with a sterile glove finger and a hemostat; there are also dedicated finger cots available that work well for this purpose, she said. In addition to nail nippers and a nail elevator, an English nail splitter can be helpful, said Dr. Skelsey.
For anesthesia, she said she ordinarily uses a 30 gauge needle with buffered lidocaine and epinephrine at room temperature to deliver a wing block. Beginning about 1 cm proximal and lateral to the junction of the proximal and lateral nail fold, the dermatologist can slowly inject about 1.5 cc per side. As the block takes effect, the lateral nail fold will blanch distally in a wing-shaped pattern. This technique, she said, also has the benefit of acting as a volumetric tourniquet.
“To avulse or not to avulse?” asked Dr. Skelsey. “I used to avulse almost everything,” she said, but noted that a complete avulsion is a “pretty traumatic” procedure. Now, unless a full avulsion is required for complete and accurate pathology, she will usually perform a partial nail plate avulsion.
A partial avulsion can reduce pain and morbidity, and can be done by two different methods: the partial proximal avulsion, and the “trap door” avulsion. In a trap door avulsion, she said, the distal matrix is primarily visualized, so this may be a good option for a longitudinal melanonychia arising from the distal matrix. A Freer elevator is used to detach the nail plate from the bed and the matrix, after which the nail plate can be lifted with a hemostat.
In a partial proximal avulsion, the proximal nail fold is reflected, so it’s a better option when the proximal nail matrix needs evaluation, she said.
After the avulsion has been done, “the matrix has been exposed. Now what? Punch or shave?” asked Dr. Skelsey. She noted that she used to perform punch biopsies on “everything,” and that it’s a good option if the pigmented area spans 3 mm or less. One issue, though, is that the specimen can get stuck in the puncher, and extraction can make it difficult to deliver an intact specimen.
Shave biopsies, Dr. Skelsey said, are effective in dealing with nail matrix lesions. They can yield an accurate pathologic diagnosis, and the biopsied digits healed without nail dystrophy in about three quarters of the cases in one study, she said. Potential recurrence of pigmentation is one drawback of the shave technique, she said.
With a shave biopsy, she performs tangential incisions of the proximal and lateral nail folds, and scores and reflects the nail. Then, the band of pigment is shaved tangentially. She cauterizes the area, and sometimes will use a bit of an absorbable gelatin sponge (Gelfoam) as well. Then the proximal nail fold and nail plate are sutured.
Replacing the nail plate results in better cosmesis and is much more comfortable for the patient, she said. An 18-gauge needle can be used to bore a hole through the avulsed nail plate, which may be held in an antiseptic solution soak during the biopsy. The sutures should then be placed from skin to nail plate, so nail fragments aren’t driven into the skin during the suturing process. Finally, specimen margins should be inked, and separate labeled formalin jars are needed for the nail plate, nail bed, and the matrix.
Dr. Skelsey reported that she had no conflicts of interest.
[email protected]
On Twitter @karioakes
CHICAGO – Maral Skelsey, MD, doesn’t get flowers from her patients very often. But, she said, a big bouquet recently landed on her desk after she had performed a nail biopsy on a patient. The note from the patient read, “That wasn’t as bad as I thought it would be!”
The patient’s relief after the procedure highlights the apprehension that both patients and dermatologists can feel when a nail biopsy becomes necessary, said Dr. Skelsey, director of dermatologic surgery at Georgetown University, Washington, D.C.
Speaking at the summer meeting of the American Academy of Dermatology, Dr. Skelsey said that the most important advice she can give about the nail biopsy is, “Do it early and often.”
Dr. Skelsey reminded the audience that the musician Bob Marley died of malignant melanoma; the first sign of his cancer was a longitudinal melanonychia that went unbiopsied. “The biggest mistake we make is not doing it,” she said.
In performing a nail biopsy, said Dr. Skelsey, the goals are, first and foremost, to optimize the pathologic diagnosis. Correct technique can help avoid complications such as bleeding, infection, and nail dystrophy; the right approach can minimize pain and anxiety, she added.
In preparing for a biopsy for melanonychia, “dermoscopy can be very helpful” in assessing the location of the pigment and fine-tuning planning for the biopsy, said Dr. Skelsey. Also, if the streak of melanonychia has reached the distal nail, sending the clipping for pathology can be useful as well.
For dorsal pigmentation, the proximal nail matrix should be biopsied.
“Do not use a punch biopsy on the nail fold to diagnose melanoma – you will get a false negative,” Dr. Skelsey said. It’s not possible to get an accurate diagnosis going through the nail plate to the nail bed, she said.
The preoperative assessment is usually straightforward. Pertinent items in the patient’s history include any medication allergies, current anticoagulation, and any history of prior trauma to the digit to be biopsied. Occasionally, imaging may be helpful, and patients should always be assessed for vascular insufficiency, she noted.
Preoperatively, she asks her patients to remove nail polish and pretreat the area with povidone iodine for 2 days prior to the procedure. Patients need to have a ride home after the procedure, and should be prepared to elevate the affected extremity for 48 hours post procedure. If a toenail is biopsied, they’re advised to come with a postop shoe.
Her patients receive a 5-minute isopropyl alcohol wash of the area to be biopsied just before the procedure, followed by air drying and a 5-minute scrub with 7.5% povidone iodine, which then is wiped off preprocedure.
For hemostasis, a tourniquet can be improvised with a sterile glove finger and a hemostat; there are also dedicated finger cots available that work well for this purpose, she said. In addition to nail nippers and a nail elevator, an English nail splitter can be helpful, said Dr. Skelsey.
For anesthesia, she said she ordinarily uses a 30 gauge needle with buffered lidocaine and epinephrine at room temperature to deliver a wing block. Beginning about 1 cm proximal and lateral to the junction of the proximal and lateral nail fold, the dermatologist can slowly inject about 1.5 cc per side. As the block takes effect, the lateral nail fold will blanch distally in a wing-shaped pattern. This technique, she said, also has the benefit of acting as a volumetric tourniquet.
“To avulse or not to avulse?” asked Dr. Skelsey. “I used to avulse almost everything,” she said, but noted that a complete avulsion is a “pretty traumatic” procedure. Now, unless a full avulsion is required for complete and accurate pathology, she will usually perform a partial nail plate avulsion.
A partial avulsion can reduce pain and morbidity, and can be done by two different methods: the partial proximal avulsion, and the “trap door” avulsion. In a trap door avulsion, she said, the distal matrix is primarily visualized, so this may be a good option for a longitudinal melanonychia arising from the distal matrix. A Freer elevator is used to detach the nail plate from the bed and the matrix, after which the nail plate can be lifted with a hemostat.
In a partial proximal avulsion, the proximal nail fold is reflected, so it’s a better option when the proximal nail matrix needs evaluation, she said.
After the avulsion has been done, “the matrix has been exposed. Now what? Punch or shave?” asked Dr. Skelsey. She noted that she used to perform punch biopsies on “everything,” and that it’s a good option if the pigmented area spans 3 mm or less. One issue, though, is that the specimen can get stuck in the puncher, and extraction can make it difficult to deliver an intact specimen.
Shave biopsies, Dr. Skelsey said, are effective in dealing with nail matrix lesions. They can yield an accurate pathologic diagnosis, and the biopsied digits healed without nail dystrophy in about three quarters of the cases in one study, she said. Potential recurrence of pigmentation is one drawback of the shave technique, she said.
With a shave biopsy, she performs tangential incisions of the proximal and lateral nail folds, and scores and reflects the nail. Then, the band of pigment is shaved tangentially. She cauterizes the area, and sometimes will use a bit of an absorbable gelatin sponge (Gelfoam) as well. Then the proximal nail fold and nail plate are sutured.
Replacing the nail plate results in better cosmesis and is much more comfortable for the patient, she said. An 18-gauge needle can be used to bore a hole through the avulsed nail plate, which may be held in an antiseptic solution soak during the biopsy. The sutures should then be placed from skin to nail plate, so nail fragments aren’t driven into the skin during the suturing process. Finally, specimen margins should be inked, and separate labeled formalin jars are needed for the nail plate, nail bed, and the matrix.
Dr. Skelsey reported that she had no conflicts of interest.
[email protected]
On Twitter @karioakes
CHICAGO – Maral Skelsey, MD, doesn’t get flowers from her patients very often. But, she said, a big bouquet recently landed on her desk after she had performed a nail biopsy on a patient. The note from the patient read, “That wasn’t as bad as I thought it would be!”
The patient’s relief after the procedure highlights the apprehension that both patients and dermatologists can feel when a nail biopsy becomes necessary, said Dr. Skelsey, director of dermatologic surgery at Georgetown University, Washington, D.C.
Speaking at the summer meeting of the American Academy of Dermatology, Dr. Skelsey said that the most important advice she can give about the nail biopsy is, “Do it early and often.”
Dr. Skelsey reminded the audience that the musician Bob Marley died of malignant melanoma; the first sign of his cancer was a longitudinal melanonychia that went unbiopsied. “The biggest mistake we make is not doing it,” she said.
In performing a nail biopsy, said Dr. Skelsey, the goals are, first and foremost, to optimize the pathologic diagnosis. Correct technique can help avoid complications such as bleeding, infection, and nail dystrophy; the right approach can minimize pain and anxiety, she added.
In preparing for a biopsy for melanonychia, “dermoscopy can be very helpful” in assessing the location of the pigment and fine-tuning planning for the biopsy, said Dr. Skelsey. Also, if the streak of melanonychia has reached the distal nail, sending the clipping for pathology can be useful as well.
For dorsal pigmentation, the proximal nail matrix should be biopsied.
“Do not use a punch biopsy on the nail fold to diagnose melanoma – you will get a false negative,” Dr. Skelsey said. It’s not possible to get an accurate diagnosis going through the nail plate to the nail bed, she said.
The preoperative assessment is usually straightforward. Pertinent items in the patient’s history include any medication allergies, current anticoagulation, and any history of prior trauma to the digit to be biopsied. Occasionally, imaging may be helpful, and patients should always be assessed for vascular insufficiency, she noted.
Preoperatively, she asks her patients to remove nail polish and pretreat the area with povidone iodine for 2 days prior to the procedure. Patients need to have a ride home after the procedure, and should be prepared to elevate the affected extremity for 48 hours post procedure. If a toenail is biopsied, they’re advised to come with a postop shoe.
Her patients receive a 5-minute isopropyl alcohol wash of the area to be biopsied just before the procedure, followed by air drying and a 5-minute scrub with 7.5% povidone iodine, which then is wiped off preprocedure.
For hemostasis, a tourniquet can be improvised with a sterile glove finger and a hemostat; there are also dedicated finger cots available that work well for this purpose, she said. In addition to nail nippers and a nail elevator, an English nail splitter can be helpful, said Dr. Skelsey.
For anesthesia, she said she ordinarily uses a 30 gauge needle with buffered lidocaine and epinephrine at room temperature to deliver a wing block. Beginning about 1 cm proximal and lateral to the junction of the proximal and lateral nail fold, the dermatologist can slowly inject about 1.5 cc per side. As the block takes effect, the lateral nail fold will blanch distally in a wing-shaped pattern. This technique, she said, also has the benefit of acting as a volumetric tourniquet.
“To avulse or not to avulse?” asked Dr. Skelsey. “I used to avulse almost everything,” she said, but noted that a complete avulsion is a “pretty traumatic” procedure. Now, unless a full avulsion is required for complete and accurate pathology, she will usually perform a partial nail plate avulsion.
A partial avulsion can reduce pain and morbidity, and can be done by two different methods: the partial proximal avulsion, and the “trap door” avulsion. In a trap door avulsion, she said, the distal matrix is primarily visualized, so this may be a good option for a longitudinal melanonychia arising from the distal matrix. A Freer elevator is used to detach the nail plate from the bed and the matrix, after which the nail plate can be lifted with a hemostat.
In a partial proximal avulsion, the proximal nail fold is reflected, so it’s a better option when the proximal nail matrix needs evaluation, she said.
After the avulsion has been done, “the matrix has been exposed. Now what? Punch or shave?” asked Dr. Skelsey. She noted that she used to perform punch biopsies on “everything,” and that it’s a good option if the pigmented area spans 3 mm or less. One issue, though, is that the specimen can get stuck in the puncher, and extraction can make it difficult to deliver an intact specimen.
Shave biopsies, Dr. Skelsey said, are effective in dealing with nail matrix lesions. They can yield an accurate pathologic diagnosis, and the biopsied digits healed without nail dystrophy in about three quarters of the cases in one study, she said. Potential recurrence of pigmentation is one drawback of the shave technique, she said.
With a shave biopsy, she performs tangential incisions of the proximal and lateral nail folds, and scores and reflects the nail. Then, the band of pigment is shaved tangentially. She cauterizes the area, and sometimes will use a bit of an absorbable gelatin sponge (Gelfoam) as well. Then the proximal nail fold and nail plate are sutured.
Replacing the nail plate results in better cosmesis and is much more comfortable for the patient, she said. An 18-gauge needle can be used to bore a hole through the avulsed nail plate, which may be held in an antiseptic solution soak during the biopsy. The sutures should then be placed from skin to nail plate, so nail fragments aren’t driven into the skin during the suturing process. Finally, specimen margins should be inked, and separate labeled formalin jars are needed for the nail plate, nail bed, and the matrix.
Dr. Skelsey reported that she had no conflicts of interest.
[email protected]
On Twitter @karioakes
AT THE 2017 AAD SUMMER MEETING
Clinical Trial Designs for Topical Antifungal Treatments of Onychomycosis and Implications on Clinical Practice
Onychomycosis is a fungal nail infection primarily caused by dermatophytes.1 If left untreated, the infection can cause nail destruction and deformities,1 resulting in pain and discomfort,2 impaired foot mobility,3 and an overall reduced quality of life.1 Onychomycosis is a chronic condition that requires long treatment periods due to the slow growth rates of toenails.1 To successfully cure the condition, fungal eradication must be achieved.
Prior to the US Food and Drug Administration (FDA) approval of tavaborole and efinaconazole, ciclopirox was the only approved topical treatment for onychomycosis.4 The recent approval of tavaborole and efinaconazole has increased treatment options available to patients and has started to pave the way for future topical treatments. This article discusses the 3 approved topical treatments for onychomycosis and focuses on the design of the phase 3 clinical trials that led to their approval.
Topical Agents Used to Treat Onychomycosis
Tavaborole, efinaconazole, and ciclopirox have undergone extensive clinical investigation to receive FDA approval. Results from pivotal phase 3 studies establishing the efficacy and safety of each agent formed the basis for regulatory submission. Although it may seem intuitive to compare the relative performance of these agents based on their respective phase 3 clinical trial data, there are important differences in study methodology, conduct, and populations that prevent direct comparisons. The FDA provides limited guidance to the pharmaceutical industry on how to conduct clinical trials for potential onychomycosis treatments. Comparative efficacy and safety claims are limited based on cross-study comparisons. The details of the phase 3 trial designs are summarized in the Table.
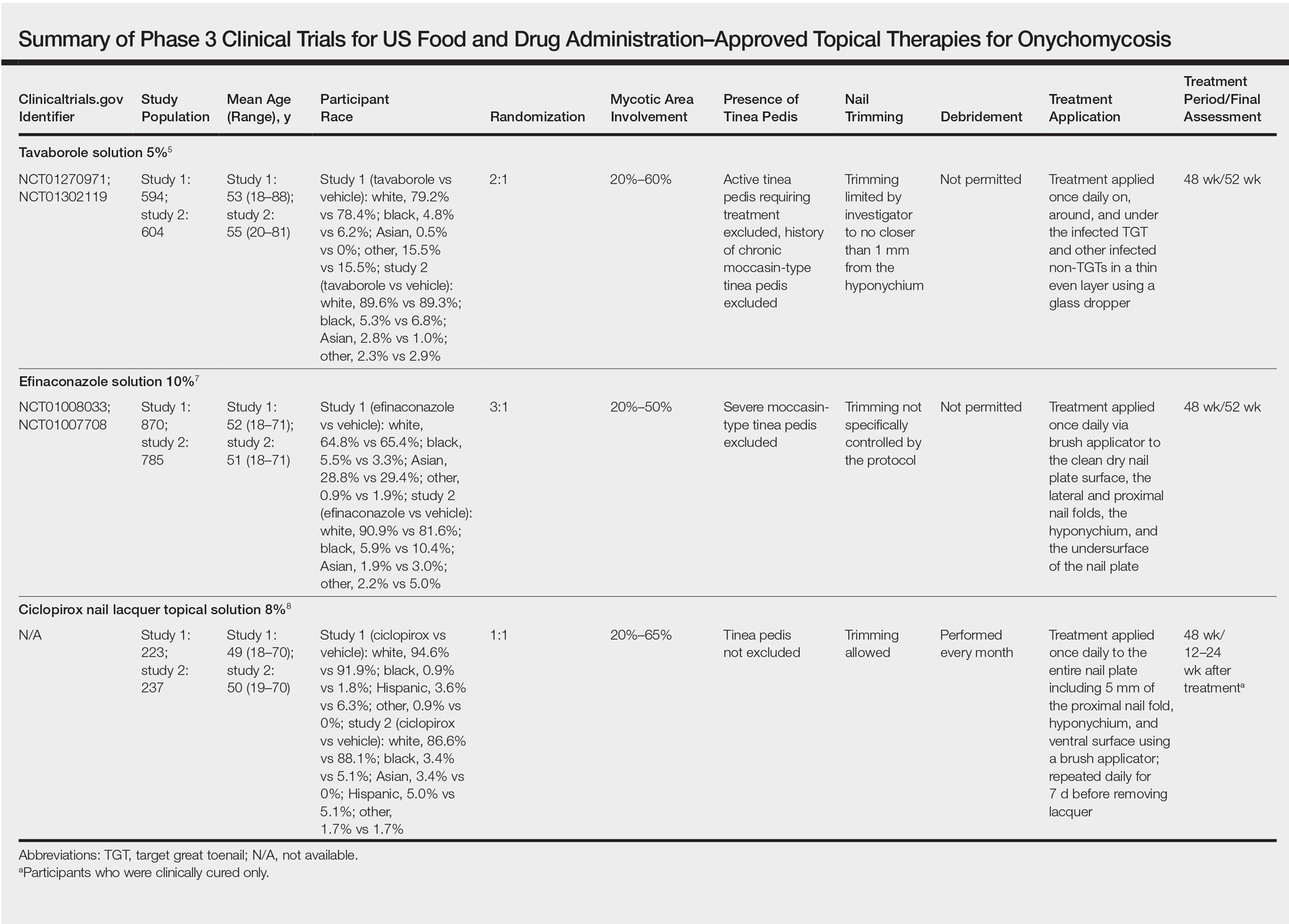
Tavaborole
Tavaborole is a boron-based treatment with a novel mechanism of action.5 Tavaborole binds to the editing domain of leucyl–transfer ribonucleic acid synthetase via an integrated boron atom and inhibits fungal protein synthesis.6 Two identical randomized, double-blind, vehicle-controlled, parallel-group, phase 3 clinical trials evaluating tavaborole were performed.5 The first study (registered at www.clinicaltrials.gov with the identifier NCT01270971) included 594 participants from27 sites in the United States and Mexico and was conducted between December 2010 and November 2012. The second study (NCT01302119) included 604 participants from 32 sites in the United States and Canada and was conducted between February 2011 and January 2013.
Eligible participants 18 years and older had distal subungual onychomycosis (DSO) of the toenails affecting 20% to 60% of 1 or more target great toenails (TGTs), tested positive for fungus using potassium hydroxide (KOH) wet mounts and positive for Trichophyton rubrum and Trichophyton mentagrophytes on fungal culture diagnostic tests, had distal TGT thickness of 3 mm or less, and had 3 mm or more of clear nail between the proximal nail fold and the most proximal visible mycotic border.5 Those with active tinea pedis requiring treatment or with a history of chronic moccasin-type tinea pedis were excluded. Participants were randomized to receive either tavaborole or vehicle (2:1). Treatments were applied once daily to all infected toenails for a total of 48 weeks, and nail debridement (defined as partial or complete removal of the toenail) was not permitted. Notably, controlled trimming of the nail was allowed to 1 mm of the leading nail edge. Regular assessments of each toenail for disease involvement, onycholysis, and subungual hyperkeratosis were made at screening, baseline, week 2, week 6, and every 6 weeks thereafter until week 52. Subungual TGT samples were taken at screening and every 12 weeks during the study for examination at a mycology laboratory, which performed KOH and fungal culture tests. A follow-up assessment was made at week 52.5
The primary end point was complete cure of the TGT at week 52, with secondary end points of completely or almost clear TGT nail (≤10% dystrophic nail), completely or almost clear TGT nail (≤10% dystrophic nail) plus negative mycology, and negative mycology of TGT.5 Examples of TGTs in participants who achieved complete cure and almost clear nails with negative mycology before and after treatment with tavaborole are shown in Figure 1. An example of a patient considered to have treatment failure is shown in Figure 2. This patient showed marked improvement in nail appearance and had a negative culture result but had a positive KOH test, which demonstrates the stringency in which topical agents are judged in onychomycosis trials.5
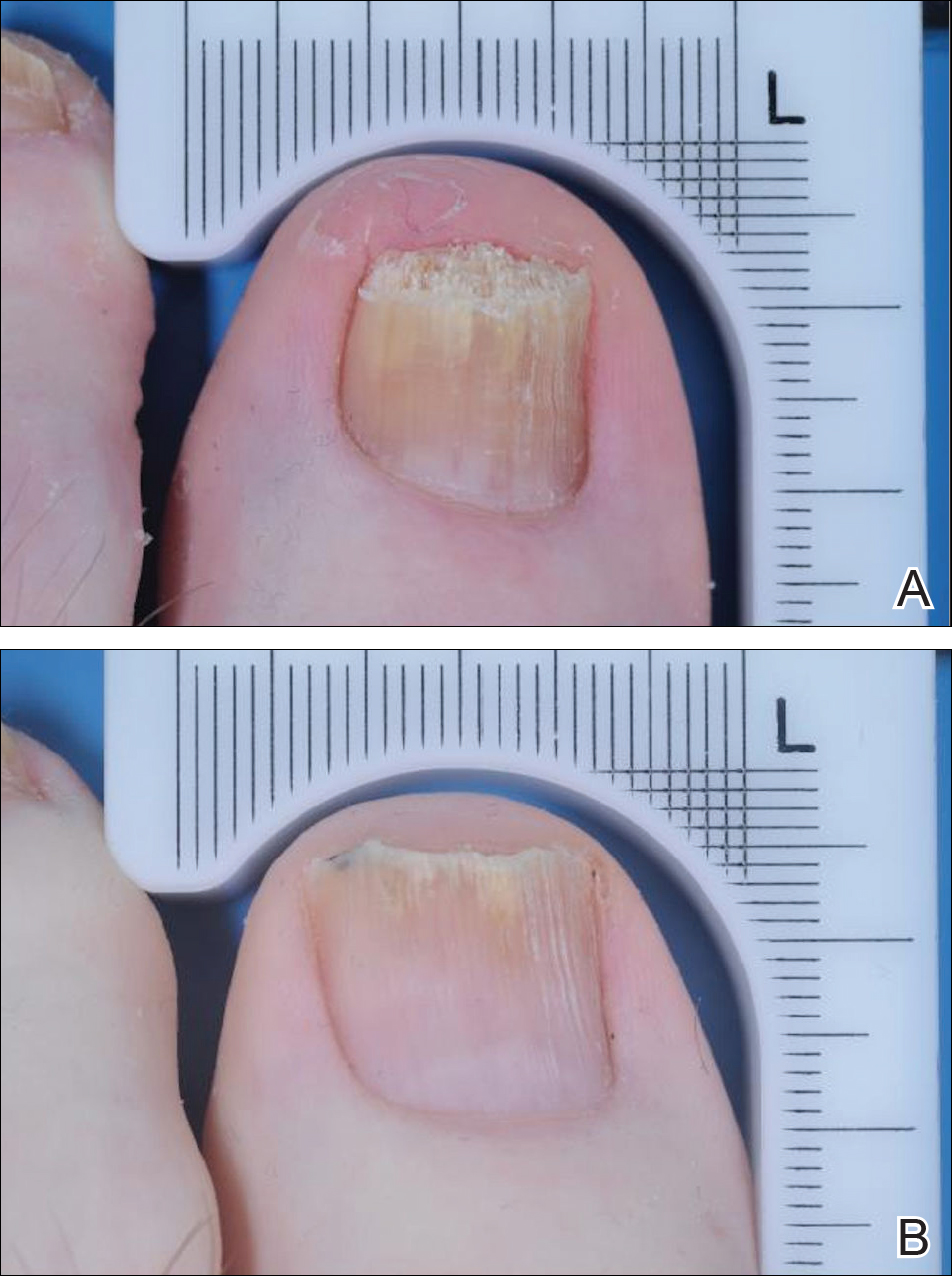
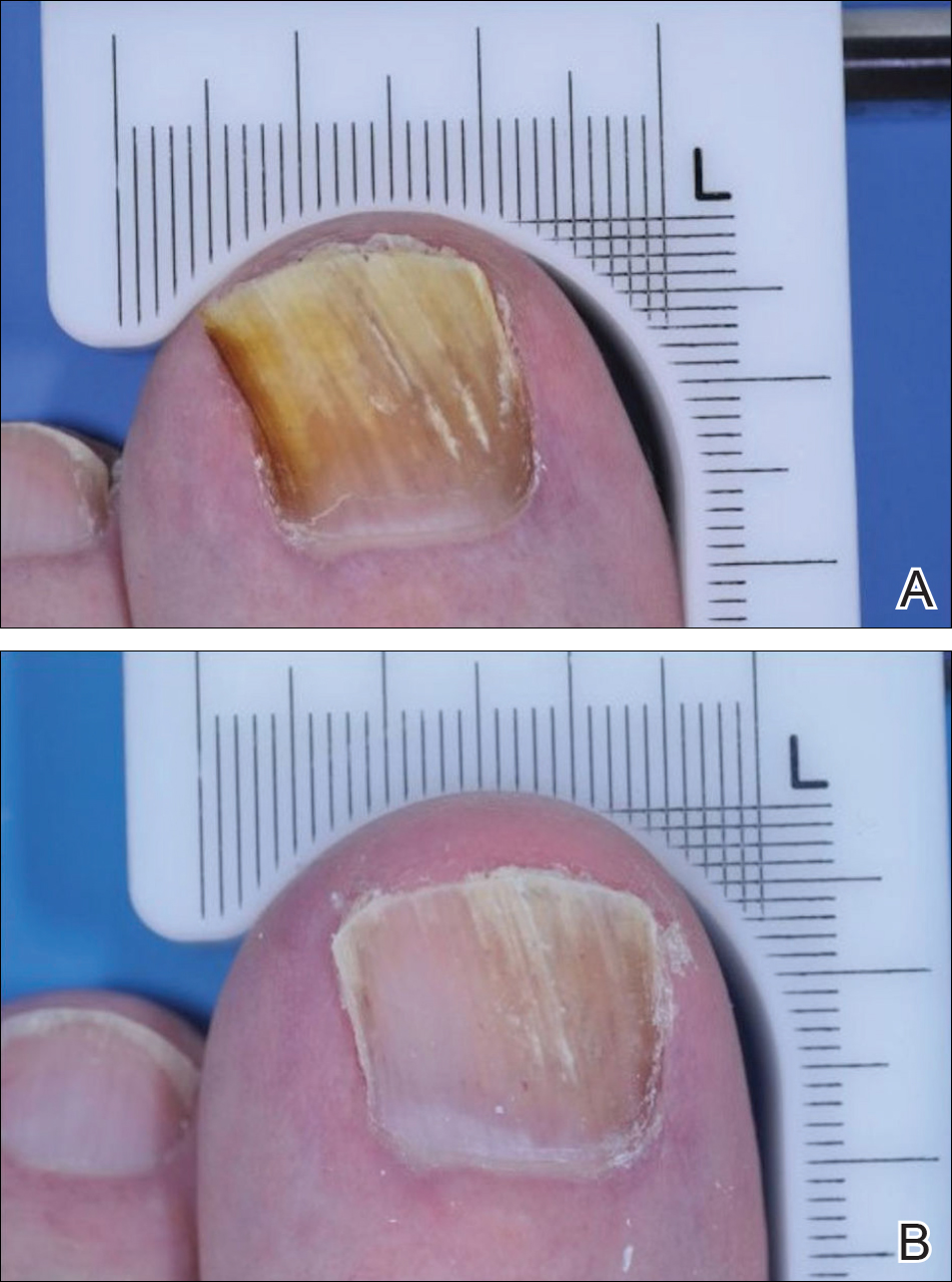
Efinaconazole
Efinaconazole is a topical triazole antifungal specifically indicated to treat onychomycosis. Two identical randomized, vehicle-controlled, double-blind, multicenter trials were performed to assess the safety and efficacy of efinaconazole solution 10%.7 The first study (NCT01008033) involved 870 participants and was conducted at a total of 74 sites in Japan (33 sites), Canada (7 sites), and the United States (34 sites) between December 2009 and September 2011. The second study (NCT01007708) had 785 participants and was conducted at 44 sites in Canada (8 sites) and the United States (36 sites) between December 2009 and October 2011.
Participants aged 18 to 70 years with a clinical diagnosis of DSO affecting 1 or more TGT were eligible to participate.7 Other eligibility criteria included an uninfected toenail length 3 mm or more from the proximal nail fold, a maximum toenail thickness of 3 mm, positive KOH wet mounts, and positive dermatophyte or mixed dermatophyte/candida cultures. Dermatophytes included T rubrum and T mentagrophytes. Those with severe moccasin-type tinea pedis were excluded. Participants were randomized to receive efinaconazole or vehicle (3:1). Once-daily treatments were self-applied to nails for 48 weeks. Clinical assessments were made at baseline and every 12 weeks until week 48, with a follow-up assessment at week 52. No nail trimming protocol was provided.7
The primary end point of the efinaconazole phase 3 trials was complete cure at week 52, with secondary end points including mycologic cure, treatment success (≤5% mycotic nail), and complete or almost complete cure (negative culture and KOH, ≤5% mycotic nail). An example of a complete cure from baseline to week 52 is shown in Figure 3.7
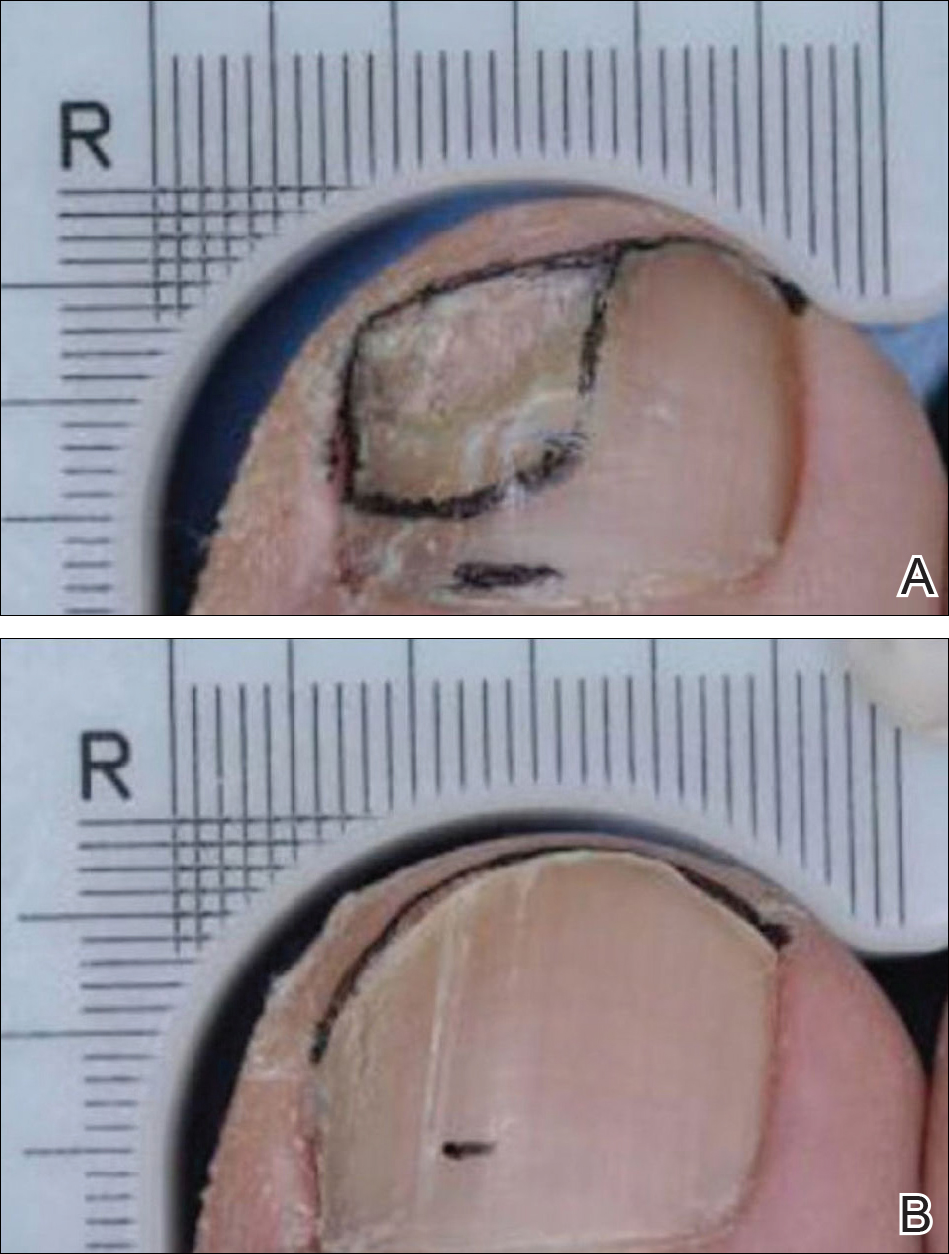
Ciclopirox
Ciclopirox was the first topical therapy to be approved for the treatment of onychomycosis. Ciclopirox is a broad-spectrum antifungal agent that inhibits metal-dependent enzymes, which are responsible for the degradation of toxic peroxides in fungal cells. The safety and efficacy of ciclopirox nail lacquer topical solution 8% also was investigated in 2 identical phase 3 clinical trials.8 The first study was conducted at 9 sites in the United States between June 1994 and June 1996 and included 223 participants. The second study was conducted at 9 sites in the United States between July 1994 and April 1996 and included 237 participants.
Eligible participants were required to have DSO in at least one TGT, positive KOH wet mount with positive dermatophyte culture, and 20% to 65% nail involvement.8 Those with tinea pedis were not excluded. Participants were randomized to receive once-daily treatment with ciclopirox or vehicle (1:1)(applied to all toenails and affected fingernails) for 48 weeks. The product was to be removed by the patient with alcohol on a weekly basis. Trimming was allowed as necessary, and mechanical debridement by the physician could be performed monthly. Assessments were made every 4 weeks, and mycologic examinations were performed every 12 weeks. Participants who were clinically cured were assessed further in a 12- to 24-week posttreatment follow-up period.8
The primary end point of complete cure and secondary end points of treatment success (negative culture and KOH, ≤10% mycotic nail), mycologic cure, and negative mycologic culture were assessed at week 48.8
Phase 3 Clinical Trial Similarities and Differences
The phase 3 clinical trials used to investigate the safety and efficacy of tavaborole,5 efinaconazole,7 and ciclopirox8 were similar in their overall design. All trials were randomized, double-blind, vehicle-controlled studies in patients with DSO. Each agent was assessed using a once-daily application for a treatment period of 48 weeks.
Primary differences among study designs included the age range of participants, the range of mycotic nail involvement, the presence/absence of tinea pedis, and the nail trimming/debridement protocols used. Differences were observed in the patient eligibility criteria of these trials. Both mycotic area and participant age range were inconsistent for each agent (eTable). Participants with larger mycotic areas usually have a poorer prognosis, as they tend to have a greater fungal load.9 A baseline mycotic area of 20% to 60%,5 20% to 50%,7 and 20% to 65%8 at baseline was required for the tavaborole, efinaconazole, and ciclopirox trials, respectively. Variations in mycotic area between trials can affect treatment efficacy, as clinical cures can be reached quicker by patients with smaller areas of infection. Of note, the average mycotic area of involvement was not reported in the tavaborole studies but was 36% and 40% for the efinaconazole and ciclopirox studies, respectively.5,8 It also is more difficult to achieve complete cure in older patients, as they have poor circulation and reduced nail growth rates.1,10 The participant age range was 18 to 88 years in the tavaborole trials, with 8% of the participants older than 70 years,5 compared to 18 to 71 years in both the efinaconazole and ciclopirox trials.7,8 The average age of participants in each study was approximately 54, 51, and 50 years for tavaborole, efinaconazole, and ciclopirox, respectively. Because factors impacting treatment failure can increase with age, efficacy results can be confounded by differing age distributions across different studies.
Another important feature that differed between the clinical trials was the approach to nail trimming—defined as shortening of the free edge of the nail distal to the hyponychium—which varies from debridement in that the nail plate is removed or reduced in thickness proximal to the hyponychium. In the tavaborole trials, trimming was controlled to within 1 mm of the free edge of the nail,5 whereas the protocol used for the ciclopirox trials allowed nail trimming as necessary as well as moderate debridement before treatment application and on a monthly basis.8 Debridement is an important component in all ciclopirox trials, as it is used to reduce fungal load.11 No trimming control was provided during the efinaconazole trials; however, debridement was prohibited.7 These differences can dramatically affect the study results, as residual fungal elements and portions of infected nails are removed during the trimming process in an uncontrolled manner, which can affect mycologic testing results as well as the clinical efficacy results determined through investigator evaluation. Discrepancies regarding nail trimming approach inevitably makes the trial results difficult to compare, as mycologic cure is not translatable between studies.
Furthermore, somewhat unusually, complete cure rate variations were observed between different study centers in the efinaconazole trials. Japanese centers in the first efinaconazole study (NCT01008033) had higher complete cure rates in both the efinaconazole and vehicle treatment arms, which is notable because approximately 29% of participants in this study were Asian, mostly hailing from 33 Japanese centers. The reason for these confounding results is unknown and requires further analysis.
Lastly, the presence or absence of tinea pedis can affect the response to onychomycosis treatment. In the tavaborole trials, patients with active interdigital tinea pedis or exclusively plantar tinea pedis or chronic moccasin-type tinea pedis requiring treatment were excluded from the studies.5 In contrast, only patients with severe moccasin-type tinea pedis were excluded in efinaconazole trials.7 The ciclopirox studies had no exclusions based on presence of tinea pedis.8 These differences are noteworthy, as tinea pedis can serve as a reservoir for fungal infection if not treated and can lead to recurrence of onychomycosis.12
Conclusion
In recent years, disappointing efficacy has resulted in the failure of several topical agents for onychomycosis during their development; however, there are several aspects to consider when examining efficacy data in onychomycosis studies. Obtaining a complete cure in onychomycosis is difficult. Because patients applying treatments at home are unlikely to undergo mycologic testing to confirm complete cure, visual inspections are helpful to determine treatment efficacy.
Despite similar overall designs, notable differences in the study designs of the phase 3 clinical trials investigating tavaborole, efinaconazole, and ciclopirox are likely to have had an effect on the reported results, making the efficacy of the agents difficult to compare. It is particularly tempting to compare the primary end point results of each trial, especially considering tavaborole and efinaconazole had primary end points with the same parameters; however, there are several other factors (eg, age range of study population, extent of infection, nail trimming, patient demographics) that may have affected the outcomes of the studies and precluded a direct comparison of any end points. Without head-to-head investigations, there is room for prescribing clinicians to interpret results differently.
Acknowledgment
Writing and editorial assistance was provided by ApotheCom Associates, LLC, Yardley, Pennsylvania, and was supported by Sandoz, a Novartis division.
- Elewski BE. Onychomycosis: pathogenesis, diagnosis, and management. Clin Microbiol Rev. 1998;11:415-429.
- Thomas J, Jacobson GA, Narkowicz CK, et al. Toenail onychomycosis: an important global disease burden. J Clin Pharm Ther. 2010;35:497-519.
- Scher RK. Onychomycosis: a significant medical disorder. J Am Acad Dermatol. 1996;35(3, pt 2):S2-S5.
- Del Rosso JQ. The role of topical antifungal therapy for onychomycosis and the emergence of newer agents. J Clin Aesthet Dermatol. 2014;7:10-18.
- Elewski BE, Aly R, Baldwin SL, et al. Efficacy and safety of tavaborole topical solution, 5%, a novel boron-based antifungal agent, for the treatment of toenail onychomycosis: results from 2 randomized phase-III studies. J Am Acad Dermatol. 2015;73:62-69.
- Rock FL, Mao W, Yaremchuk A, et al. An antifungal agent inhibits an aminoacyl-tRNA synthetase by trapping tRNA in the editing site. Science. 2007;316:1759-1761.
- Elewski BE, Rich P, Pollak R, et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: two phase III multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013;68:600-608.
- Gupta AK, Joseph WS. Ciclopirox 8% nail lacquer in the treatment of onychomycosis of the toenails in the United States. J Am Pod Med Assoc. 2000;90:495-501.
- Carney C, Tosti A, Daniel R, et al. A new classification system for grading the severity of onychomycosis: Onychomycosis Severity Index. Arch Dermatol. 2011;147:1277-1282.
- Gupta AK. Onychomycosis in the elderly. Drugs Aging. 2000;16:397-407.
- Gupta AK, Malkin KF. Ciclopirox nail lacquer and podiatric practice. J Am Podiatr Med Assoc. 2000;90:502-507.
- Scher RK, Baran R. Onychomycosis in clinical practice: factors contributing to recurrence. Br J Dermatol. 2003;149(suppl 65):5-9.
Onychomycosis is a fungal nail infection primarily caused by dermatophytes.1 If left untreated, the infection can cause nail destruction and deformities,1 resulting in pain and discomfort,2 impaired foot mobility,3 and an overall reduced quality of life.1 Onychomycosis is a chronic condition that requires long treatment periods due to the slow growth rates of toenails.1 To successfully cure the condition, fungal eradication must be achieved.
Prior to the US Food and Drug Administration (FDA) approval of tavaborole and efinaconazole, ciclopirox was the only approved topical treatment for onychomycosis.4 The recent approval of tavaborole and efinaconazole has increased treatment options available to patients and has started to pave the way for future topical treatments. This article discusses the 3 approved topical treatments for onychomycosis and focuses on the design of the phase 3 clinical trials that led to their approval.
Topical Agents Used to Treat Onychomycosis
Tavaborole, efinaconazole, and ciclopirox have undergone extensive clinical investigation to receive FDA approval. Results from pivotal phase 3 studies establishing the efficacy and safety of each agent formed the basis for regulatory submission. Although it may seem intuitive to compare the relative performance of these agents based on their respective phase 3 clinical trial data, there are important differences in study methodology, conduct, and populations that prevent direct comparisons. The FDA provides limited guidance to the pharmaceutical industry on how to conduct clinical trials for potential onychomycosis treatments. Comparative efficacy and safety claims are limited based on cross-study comparisons. The details of the phase 3 trial designs are summarized in the Table.

Tavaborole
Tavaborole is a boron-based treatment with a novel mechanism of action.5 Tavaborole binds to the editing domain of leucyl–transfer ribonucleic acid synthetase via an integrated boron atom and inhibits fungal protein synthesis.6 Two identical randomized, double-blind, vehicle-controlled, parallel-group, phase 3 clinical trials evaluating tavaborole were performed.5 The first study (registered at www.clinicaltrials.gov with the identifier NCT01270971) included 594 participants from27 sites in the United States and Mexico and was conducted between December 2010 and November 2012. The second study (NCT01302119) included 604 participants from 32 sites in the United States and Canada and was conducted between February 2011 and January 2013.
Eligible participants 18 years and older had distal subungual onychomycosis (DSO) of the toenails affecting 20% to 60% of 1 or more target great toenails (TGTs), tested positive for fungus using potassium hydroxide (KOH) wet mounts and positive for Trichophyton rubrum and Trichophyton mentagrophytes on fungal culture diagnostic tests, had distal TGT thickness of 3 mm or less, and had 3 mm or more of clear nail between the proximal nail fold and the most proximal visible mycotic border.5 Those with active tinea pedis requiring treatment or with a history of chronic moccasin-type tinea pedis were excluded. Participants were randomized to receive either tavaborole or vehicle (2:1). Treatments were applied once daily to all infected toenails for a total of 48 weeks, and nail debridement (defined as partial or complete removal of the toenail) was not permitted. Notably, controlled trimming of the nail was allowed to 1 mm of the leading nail edge. Regular assessments of each toenail for disease involvement, onycholysis, and subungual hyperkeratosis were made at screening, baseline, week 2, week 6, and every 6 weeks thereafter until week 52. Subungual TGT samples were taken at screening and every 12 weeks during the study for examination at a mycology laboratory, which performed KOH and fungal culture tests. A follow-up assessment was made at week 52.5
The primary end point was complete cure of the TGT at week 52, with secondary end points of completely or almost clear TGT nail (≤10% dystrophic nail), completely or almost clear TGT nail (≤10% dystrophic nail) plus negative mycology, and negative mycology of TGT.5 Examples of TGTs in participants who achieved complete cure and almost clear nails with negative mycology before and after treatment with tavaborole are shown in Figure 1. An example of a patient considered to have treatment failure is shown in Figure 2. This patient showed marked improvement in nail appearance and had a negative culture result but had a positive KOH test, which demonstrates the stringency in which topical agents are judged in onychomycosis trials.5


Efinaconazole
Efinaconazole is a topical triazole antifungal specifically indicated to treat onychomycosis. Two identical randomized, vehicle-controlled, double-blind, multicenter trials were performed to assess the safety and efficacy of efinaconazole solution 10%.7 The first study (NCT01008033) involved 870 participants and was conducted at a total of 74 sites in Japan (33 sites), Canada (7 sites), and the United States (34 sites) between December 2009 and September 2011. The second study (NCT01007708) had 785 participants and was conducted at 44 sites in Canada (8 sites) and the United States (36 sites) between December 2009 and October 2011.
Participants aged 18 to 70 years with a clinical diagnosis of DSO affecting 1 or more TGT were eligible to participate.7 Other eligibility criteria included an uninfected toenail length 3 mm or more from the proximal nail fold, a maximum toenail thickness of 3 mm, positive KOH wet mounts, and positive dermatophyte or mixed dermatophyte/candida cultures. Dermatophytes included T rubrum and T mentagrophytes. Those with severe moccasin-type tinea pedis were excluded. Participants were randomized to receive efinaconazole or vehicle (3:1). Once-daily treatments were self-applied to nails for 48 weeks. Clinical assessments were made at baseline and every 12 weeks until week 48, with a follow-up assessment at week 52. No nail trimming protocol was provided.7
The primary end point of the efinaconazole phase 3 trials was complete cure at week 52, with secondary end points including mycologic cure, treatment success (≤5% mycotic nail), and complete or almost complete cure (negative culture and KOH, ≤5% mycotic nail). An example of a complete cure from baseline to week 52 is shown in Figure 3.7

Ciclopirox
Ciclopirox was the first topical therapy to be approved for the treatment of onychomycosis. Ciclopirox is a broad-spectrum antifungal agent that inhibits metal-dependent enzymes, which are responsible for the degradation of toxic peroxides in fungal cells. The safety and efficacy of ciclopirox nail lacquer topical solution 8% also was investigated in 2 identical phase 3 clinical trials.8 The first study was conducted at 9 sites in the United States between June 1994 and June 1996 and included 223 participants. The second study was conducted at 9 sites in the United States between July 1994 and April 1996 and included 237 participants.
Eligible participants were required to have DSO in at least one TGT, positive KOH wet mount with positive dermatophyte culture, and 20% to 65% nail involvement.8 Those with tinea pedis were not excluded. Participants were randomized to receive once-daily treatment with ciclopirox or vehicle (1:1)(applied to all toenails and affected fingernails) for 48 weeks. The product was to be removed by the patient with alcohol on a weekly basis. Trimming was allowed as necessary, and mechanical debridement by the physician could be performed monthly. Assessments were made every 4 weeks, and mycologic examinations were performed every 12 weeks. Participants who were clinically cured were assessed further in a 12- to 24-week posttreatment follow-up period.8
The primary end point of complete cure and secondary end points of treatment success (negative culture and KOH, ≤10% mycotic nail), mycologic cure, and negative mycologic culture were assessed at week 48.8
Phase 3 Clinical Trial Similarities and Differences
The phase 3 clinical trials used to investigate the safety and efficacy of tavaborole,5 efinaconazole,7 and ciclopirox8 were similar in their overall design. All trials were randomized, double-blind, vehicle-controlled studies in patients with DSO. Each agent was assessed using a once-daily application for a treatment period of 48 weeks.
Primary differences among study designs included the age range of participants, the range of mycotic nail involvement, the presence/absence of tinea pedis, and the nail trimming/debridement protocols used. Differences were observed in the patient eligibility criteria of these trials. Both mycotic area and participant age range were inconsistent for each agent (eTable). Participants with larger mycotic areas usually have a poorer prognosis, as they tend to have a greater fungal load.9 A baseline mycotic area of 20% to 60%,5 20% to 50%,7 and 20% to 65%8 at baseline was required for the tavaborole, efinaconazole, and ciclopirox trials, respectively. Variations in mycotic area between trials can affect treatment efficacy, as clinical cures can be reached quicker by patients with smaller areas of infection. Of note, the average mycotic area of involvement was not reported in the tavaborole studies but was 36% and 40% for the efinaconazole and ciclopirox studies, respectively.5,8 It also is more difficult to achieve complete cure in older patients, as they have poor circulation and reduced nail growth rates.1,10 The participant age range was 18 to 88 years in the tavaborole trials, with 8% of the participants older than 70 years,5 compared to 18 to 71 years in both the efinaconazole and ciclopirox trials.7,8 The average age of participants in each study was approximately 54, 51, and 50 years for tavaborole, efinaconazole, and ciclopirox, respectively. Because factors impacting treatment failure can increase with age, efficacy results can be confounded by differing age distributions across different studies.
Another important feature that differed between the clinical trials was the approach to nail trimming—defined as shortening of the free edge of the nail distal to the hyponychium—which varies from debridement in that the nail plate is removed or reduced in thickness proximal to the hyponychium. In the tavaborole trials, trimming was controlled to within 1 mm of the free edge of the nail,5 whereas the protocol used for the ciclopirox trials allowed nail trimming as necessary as well as moderate debridement before treatment application and on a monthly basis.8 Debridement is an important component in all ciclopirox trials, as it is used to reduce fungal load.11 No trimming control was provided during the efinaconazole trials; however, debridement was prohibited.7 These differences can dramatically affect the study results, as residual fungal elements and portions of infected nails are removed during the trimming process in an uncontrolled manner, which can affect mycologic testing results as well as the clinical efficacy results determined through investigator evaluation. Discrepancies regarding nail trimming approach inevitably makes the trial results difficult to compare, as mycologic cure is not translatable between studies.
Furthermore, somewhat unusually, complete cure rate variations were observed between different study centers in the efinaconazole trials. Japanese centers in the first efinaconazole study (NCT01008033) had higher complete cure rates in both the efinaconazole and vehicle treatment arms, which is notable because approximately 29% of participants in this study were Asian, mostly hailing from 33 Japanese centers. The reason for these confounding results is unknown and requires further analysis.
Lastly, the presence or absence of tinea pedis can affect the response to onychomycosis treatment. In the tavaborole trials, patients with active interdigital tinea pedis or exclusively plantar tinea pedis or chronic moccasin-type tinea pedis requiring treatment were excluded from the studies.5 In contrast, only patients with severe moccasin-type tinea pedis were excluded in efinaconazole trials.7 The ciclopirox studies had no exclusions based on presence of tinea pedis.8 These differences are noteworthy, as tinea pedis can serve as a reservoir for fungal infection if not treated and can lead to recurrence of onychomycosis.12
Conclusion
In recent years, disappointing efficacy has resulted in the failure of several topical agents for onychomycosis during their development; however, there are several aspects to consider when examining efficacy data in onychomycosis studies. Obtaining a complete cure in onychomycosis is difficult. Because patients applying treatments at home are unlikely to undergo mycologic testing to confirm complete cure, visual inspections are helpful to determine treatment efficacy.
Despite similar overall designs, notable differences in the study designs of the phase 3 clinical trials investigating tavaborole, efinaconazole, and ciclopirox are likely to have had an effect on the reported results, making the efficacy of the agents difficult to compare. It is particularly tempting to compare the primary end point results of each trial, especially considering tavaborole and efinaconazole had primary end points with the same parameters; however, there are several other factors (eg, age range of study population, extent of infection, nail trimming, patient demographics) that may have affected the outcomes of the studies and precluded a direct comparison of any end points. Without head-to-head investigations, there is room for prescribing clinicians to interpret results differently.
Acknowledgment
Writing and editorial assistance was provided by ApotheCom Associates, LLC, Yardley, Pennsylvania, and was supported by Sandoz, a Novartis division.
Onychomycosis is a fungal nail infection primarily caused by dermatophytes.1 If left untreated, the infection can cause nail destruction and deformities,1 resulting in pain and discomfort,2 impaired foot mobility,3 and an overall reduced quality of life.1 Onychomycosis is a chronic condition that requires long treatment periods due to the slow growth rates of toenails.1 To successfully cure the condition, fungal eradication must be achieved.
Prior to the US Food and Drug Administration (FDA) approval of tavaborole and efinaconazole, ciclopirox was the only approved topical treatment for onychomycosis.4 The recent approval of tavaborole and efinaconazole has increased treatment options available to patients and has started to pave the way for future topical treatments. This article discusses the 3 approved topical treatments for onychomycosis and focuses on the design of the phase 3 clinical trials that led to their approval.
Topical Agents Used to Treat Onychomycosis
Tavaborole, efinaconazole, and ciclopirox have undergone extensive clinical investigation to receive FDA approval. Results from pivotal phase 3 studies establishing the efficacy and safety of each agent formed the basis for regulatory submission. Although it may seem intuitive to compare the relative performance of these agents based on their respective phase 3 clinical trial data, there are important differences in study methodology, conduct, and populations that prevent direct comparisons. The FDA provides limited guidance to the pharmaceutical industry on how to conduct clinical trials for potential onychomycosis treatments. Comparative efficacy and safety claims are limited based on cross-study comparisons. The details of the phase 3 trial designs are summarized in the Table.

Tavaborole
Tavaborole is a boron-based treatment with a novel mechanism of action.5 Tavaborole binds to the editing domain of leucyl–transfer ribonucleic acid synthetase via an integrated boron atom and inhibits fungal protein synthesis.6 Two identical randomized, double-blind, vehicle-controlled, parallel-group, phase 3 clinical trials evaluating tavaborole were performed.5 The first study (registered at www.clinicaltrials.gov with the identifier NCT01270971) included 594 participants from27 sites in the United States and Mexico and was conducted between December 2010 and November 2012. The second study (NCT01302119) included 604 participants from 32 sites in the United States and Canada and was conducted between February 2011 and January 2013.
Eligible participants 18 years and older had distal subungual onychomycosis (DSO) of the toenails affecting 20% to 60% of 1 or more target great toenails (TGTs), tested positive for fungus using potassium hydroxide (KOH) wet mounts and positive for Trichophyton rubrum and Trichophyton mentagrophytes on fungal culture diagnostic tests, had distal TGT thickness of 3 mm or less, and had 3 mm or more of clear nail between the proximal nail fold and the most proximal visible mycotic border.5 Those with active tinea pedis requiring treatment or with a history of chronic moccasin-type tinea pedis were excluded. Participants were randomized to receive either tavaborole or vehicle (2:1). Treatments were applied once daily to all infected toenails for a total of 48 weeks, and nail debridement (defined as partial or complete removal of the toenail) was not permitted. Notably, controlled trimming of the nail was allowed to 1 mm of the leading nail edge. Regular assessments of each toenail for disease involvement, onycholysis, and subungual hyperkeratosis were made at screening, baseline, week 2, week 6, and every 6 weeks thereafter until week 52. Subungual TGT samples were taken at screening and every 12 weeks during the study for examination at a mycology laboratory, which performed KOH and fungal culture tests. A follow-up assessment was made at week 52.5
The primary end point was complete cure of the TGT at week 52, with secondary end points of completely or almost clear TGT nail (≤10% dystrophic nail), completely or almost clear TGT nail (≤10% dystrophic nail) plus negative mycology, and negative mycology of TGT.5 Examples of TGTs in participants who achieved complete cure and almost clear nails with negative mycology before and after treatment with tavaborole are shown in Figure 1. An example of a patient considered to have treatment failure is shown in Figure 2. This patient showed marked improvement in nail appearance and had a negative culture result but had a positive KOH test, which demonstrates the stringency in which topical agents are judged in onychomycosis trials.5


Efinaconazole
Efinaconazole is a topical triazole antifungal specifically indicated to treat onychomycosis. Two identical randomized, vehicle-controlled, double-blind, multicenter trials were performed to assess the safety and efficacy of efinaconazole solution 10%.7 The first study (NCT01008033) involved 870 participants and was conducted at a total of 74 sites in Japan (33 sites), Canada (7 sites), and the United States (34 sites) between December 2009 and September 2011. The second study (NCT01007708) had 785 participants and was conducted at 44 sites in Canada (8 sites) and the United States (36 sites) between December 2009 and October 2011.
Participants aged 18 to 70 years with a clinical diagnosis of DSO affecting 1 or more TGT were eligible to participate.7 Other eligibility criteria included an uninfected toenail length 3 mm or more from the proximal nail fold, a maximum toenail thickness of 3 mm, positive KOH wet mounts, and positive dermatophyte or mixed dermatophyte/candida cultures. Dermatophytes included T rubrum and T mentagrophytes. Those with severe moccasin-type tinea pedis were excluded. Participants were randomized to receive efinaconazole or vehicle (3:1). Once-daily treatments were self-applied to nails for 48 weeks. Clinical assessments were made at baseline and every 12 weeks until week 48, with a follow-up assessment at week 52. No nail trimming protocol was provided.7
The primary end point of the efinaconazole phase 3 trials was complete cure at week 52, with secondary end points including mycologic cure, treatment success (≤5% mycotic nail), and complete or almost complete cure (negative culture and KOH, ≤5% mycotic nail). An example of a complete cure from baseline to week 52 is shown in Figure 3.7

Ciclopirox
Ciclopirox was the first topical therapy to be approved for the treatment of onychomycosis. Ciclopirox is a broad-spectrum antifungal agent that inhibits metal-dependent enzymes, which are responsible for the degradation of toxic peroxides in fungal cells. The safety and efficacy of ciclopirox nail lacquer topical solution 8% also was investigated in 2 identical phase 3 clinical trials.8 The first study was conducted at 9 sites in the United States between June 1994 and June 1996 and included 223 participants. The second study was conducted at 9 sites in the United States between July 1994 and April 1996 and included 237 participants.
Eligible participants were required to have DSO in at least one TGT, positive KOH wet mount with positive dermatophyte culture, and 20% to 65% nail involvement.8 Those with tinea pedis were not excluded. Participants were randomized to receive once-daily treatment with ciclopirox or vehicle (1:1)(applied to all toenails and affected fingernails) for 48 weeks. The product was to be removed by the patient with alcohol on a weekly basis. Trimming was allowed as necessary, and mechanical debridement by the physician could be performed monthly. Assessments were made every 4 weeks, and mycologic examinations were performed every 12 weeks. Participants who were clinically cured were assessed further in a 12- to 24-week posttreatment follow-up period.8
The primary end point of complete cure and secondary end points of treatment success (negative culture and KOH, ≤10% mycotic nail), mycologic cure, and negative mycologic culture were assessed at week 48.8
Phase 3 Clinical Trial Similarities and Differences
The phase 3 clinical trials used to investigate the safety and efficacy of tavaborole,5 efinaconazole,7 and ciclopirox8 were similar in their overall design. All trials were randomized, double-blind, vehicle-controlled studies in patients with DSO. Each agent was assessed using a once-daily application for a treatment period of 48 weeks.
Primary differences among study designs included the age range of participants, the range of mycotic nail involvement, the presence/absence of tinea pedis, and the nail trimming/debridement protocols used. Differences were observed in the patient eligibility criteria of these trials. Both mycotic area and participant age range were inconsistent for each agent (eTable). Participants with larger mycotic areas usually have a poorer prognosis, as they tend to have a greater fungal load.9 A baseline mycotic area of 20% to 60%,5 20% to 50%,7 and 20% to 65%8 at baseline was required for the tavaborole, efinaconazole, and ciclopirox trials, respectively. Variations in mycotic area between trials can affect treatment efficacy, as clinical cures can be reached quicker by patients with smaller areas of infection. Of note, the average mycotic area of involvement was not reported in the tavaborole studies but was 36% and 40% for the efinaconazole and ciclopirox studies, respectively.5,8 It also is more difficult to achieve complete cure in older patients, as they have poor circulation and reduced nail growth rates.1,10 The participant age range was 18 to 88 years in the tavaborole trials, with 8% of the participants older than 70 years,5 compared to 18 to 71 years in both the efinaconazole and ciclopirox trials.7,8 The average age of participants in each study was approximately 54, 51, and 50 years for tavaborole, efinaconazole, and ciclopirox, respectively. Because factors impacting treatment failure can increase with age, efficacy results can be confounded by differing age distributions across different studies.
Another important feature that differed between the clinical trials was the approach to nail trimming—defined as shortening of the free edge of the nail distal to the hyponychium—which varies from debridement in that the nail plate is removed or reduced in thickness proximal to the hyponychium. In the tavaborole trials, trimming was controlled to within 1 mm of the free edge of the nail,5 whereas the protocol used for the ciclopirox trials allowed nail trimming as necessary as well as moderate debridement before treatment application and on a monthly basis.8 Debridement is an important component in all ciclopirox trials, as it is used to reduce fungal load.11 No trimming control was provided during the efinaconazole trials; however, debridement was prohibited.7 These differences can dramatically affect the study results, as residual fungal elements and portions of infected nails are removed during the trimming process in an uncontrolled manner, which can affect mycologic testing results as well as the clinical efficacy results determined through investigator evaluation. Discrepancies regarding nail trimming approach inevitably makes the trial results difficult to compare, as mycologic cure is not translatable between studies.
Furthermore, somewhat unusually, complete cure rate variations were observed between different study centers in the efinaconazole trials. Japanese centers in the first efinaconazole study (NCT01008033) had higher complete cure rates in both the efinaconazole and vehicle treatment arms, which is notable because approximately 29% of participants in this study were Asian, mostly hailing from 33 Japanese centers. The reason for these confounding results is unknown and requires further analysis.
Lastly, the presence or absence of tinea pedis can affect the response to onychomycosis treatment. In the tavaborole trials, patients with active interdigital tinea pedis or exclusively plantar tinea pedis or chronic moccasin-type tinea pedis requiring treatment were excluded from the studies.5 In contrast, only patients with severe moccasin-type tinea pedis were excluded in efinaconazole trials.7 The ciclopirox studies had no exclusions based on presence of tinea pedis.8 These differences are noteworthy, as tinea pedis can serve as a reservoir for fungal infection if not treated and can lead to recurrence of onychomycosis.12
Conclusion
In recent years, disappointing efficacy has resulted in the failure of several topical agents for onychomycosis during their development; however, there are several aspects to consider when examining efficacy data in onychomycosis studies. Obtaining a complete cure in onychomycosis is difficult. Because patients applying treatments at home are unlikely to undergo mycologic testing to confirm complete cure, visual inspections are helpful to determine treatment efficacy.
Despite similar overall designs, notable differences in the study designs of the phase 3 clinical trials investigating tavaborole, efinaconazole, and ciclopirox are likely to have had an effect on the reported results, making the efficacy of the agents difficult to compare. It is particularly tempting to compare the primary end point results of each trial, especially considering tavaborole and efinaconazole had primary end points with the same parameters; however, there are several other factors (eg, age range of study population, extent of infection, nail trimming, patient demographics) that may have affected the outcomes of the studies and precluded a direct comparison of any end points. Without head-to-head investigations, there is room for prescribing clinicians to interpret results differently.
Acknowledgment
Writing and editorial assistance was provided by ApotheCom Associates, LLC, Yardley, Pennsylvania, and was supported by Sandoz, a Novartis division.
- Elewski BE. Onychomycosis: pathogenesis, diagnosis, and management. Clin Microbiol Rev. 1998;11:415-429.
- Thomas J, Jacobson GA, Narkowicz CK, et al. Toenail onychomycosis: an important global disease burden. J Clin Pharm Ther. 2010;35:497-519.
- Scher RK. Onychomycosis: a significant medical disorder. J Am Acad Dermatol. 1996;35(3, pt 2):S2-S5.
- Del Rosso JQ. The role of topical antifungal therapy for onychomycosis and the emergence of newer agents. J Clin Aesthet Dermatol. 2014;7:10-18.
- Elewski BE, Aly R, Baldwin SL, et al. Efficacy and safety of tavaborole topical solution, 5%, a novel boron-based antifungal agent, for the treatment of toenail onychomycosis: results from 2 randomized phase-III studies. J Am Acad Dermatol. 2015;73:62-69.
- Rock FL, Mao W, Yaremchuk A, et al. An antifungal agent inhibits an aminoacyl-tRNA synthetase by trapping tRNA in the editing site. Science. 2007;316:1759-1761.
- Elewski BE, Rich P, Pollak R, et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: two phase III multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013;68:600-608.
- Gupta AK, Joseph WS. Ciclopirox 8% nail lacquer in the treatment of onychomycosis of the toenails in the United States. J Am Pod Med Assoc. 2000;90:495-501.
- Carney C, Tosti A, Daniel R, et al. A new classification system for grading the severity of onychomycosis: Onychomycosis Severity Index. Arch Dermatol. 2011;147:1277-1282.
- Gupta AK. Onychomycosis in the elderly. Drugs Aging. 2000;16:397-407.
- Gupta AK, Malkin KF. Ciclopirox nail lacquer and podiatric practice. J Am Podiatr Med Assoc. 2000;90:502-507.
- Scher RK, Baran R. Onychomycosis in clinical practice: factors contributing to recurrence. Br J Dermatol. 2003;149(suppl 65):5-9.
- Elewski BE. Onychomycosis: pathogenesis, diagnosis, and management. Clin Microbiol Rev. 1998;11:415-429.
- Thomas J, Jacobson GA, Narkowicz CK, et al. Toenail onychomycosis: an important global disease burden. J Clin Pharm Ther. 2010;35:497-519.
- Scher RK. Onychomycosis: a significant medical disorder. J Am Acad Dermatol. 1996;35(3, pt 2):S2-S5.
- Del Rosso JQ. The role of topical antifungal therapy for onychomycosis and the emergence of newer agents. J Clin Aesthet Dermatol. 2014;7:10-18.
- Elewski BE, Aly R, Baldwin SL, et al. Efficacy and safety of tavaborole topical solution, 5%, a novel boron-based antifungal agent, for the treatment of toenail onychomycosis: results from 2 randomized phase-III studies. J Am Acad Dermatol. 2015;73:62-69.
- Rock FL, Mao W, Yaremchuk A, et al. An antifungal agent inhibits an aminoacyl-tRNA synthetase by trapping tRNA in the editing site. Science. 2007;316:1759-1761.
- Elewski BE, Rich P, Pollak R, et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: two phase III multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013;68:600-608.
- Gupta AK, Joseph WS. Ciclopirox 8% nail lacquer in the treatment of onychomycosis of the toenails in the United States. J Am Pod Med Assoc. 2000;90:495-501.
- Carney C, Tosti A, Daniel R, et al. A new classification system for grading the severity of onychomycosis: Onychomycosis Severity Index. Arch Dermatol. 2011;147:1277-1282.
- Gupta AK. Onychomycosis in the elderly. Drugs Aging. 2000;16:397-407.
- Gupta AK, Malkin KF. Ciclopirox nail lacquer and podiatric practice. J Am Podiatr Med Assoc. 2000;90:502-507.
- Scher RK, Baran R. Onychomycosis in clinical practice: factors contributing to recurrence. Br J Dermatol. 2003;149(suppl 65):5-9.
Practice Points
- Despite similar overall designs, notable differences in the study designs of phase 3 clinical trials investigating tavaborole, efinaconazole, and ciclopirox for the treatment of onychomycosis are likely to have had an effect on the reported results, making the efficacy of these agents difficult to compare.
- The primary difference between studies for tavaborole, efinaconazole, and ciclopirox include the age range of participants, the range of mycotic nail involvement, the presence/absence of tinea pedis, and the nail trimming/debridement protocols used.
- Without head-to-head investigations, there is room for prescribing clinicians to interpret study results for these agents differently.