User login
CDC issues COVID-19 vaccine guidance for underlying conditions
The Centers for Disease Control and Prevention has issued updated guidance for people with underlying medical conditions who are considering getting the coronavirus vaccine.
“Adults of any age with certain underlying medical conditions are at increased risk for severe illness from the virus that causes COVID-19,” the CDC said in the guidance, posted on Dec. 26. “mRNA COVID-19 vaccines may be administered to people with underlying medical conditions provided they have not had a severe allergic reaction to any of the ingredients in the vaccine.”
Both the Pfizer and Moderna vaccines use mRNA, or messenger RNA.
The CDC guidance had specific information for people with HIV, weakened immune systems, and autoimmune conditions such as Guillain-Barré syndrome (GBS) and Bell’s palsy who are thinking of getting the vaccine.
People with HIV and weakened immune systems “may receive a COVID-19 vaccine. However, they should be aware of the limited safety data,” the CDC said.
There’s no information available yet about the safety of the vaccines for people with weakened immune systems. People with HIV were included in clinical trials, but “safety data specific to this group are not yet available at this time,” the CDC said.
Cases of Bell’s palsy, a temporary facial paralysis, were reported in people receiving the Pfizer and Moderna vaccines in clinical trials, the Food and Drug Administration said Dec. 17.
But the new CDC guidance said that the FDA “does not consider these to be above the rate expected in the general population. They have not concluded these cases were caused by vaccination. Therefore, persons who have previously had Bell’s palsy may receive an mRNA COVID-19 vaccine.”
Researchers have determined the vaccines are safe for people with GBS, a rare autoimmune disorder in which the body’s immune system attacks nerves just as they leave the spinal cord, the CDC said.
“To date, no cases of GBS have been reported following vaccination among participants in the mRNA COVID-19 vaccine clinical trials,” the CDC guidance said. “With few exceptions, the independent Advisory Committee on Immunization Practices general best practice guidelines for immunization do not include a history of GBS as a precaution to vaccination with other vaccines.”
For months, the CDC and other health authorities have said that people with certain medical conditions are at an increased risk of developing severe cases of COVID-19.
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention has issued updated guidance for people with underlying medical conditions who are considering getting the coronavirus vaccine.
“Adults of any age with certain underlying medical conditions are at increased risk for severe illness from the virus that causes COVID-19,” the CDC said in the guidance, posted on Dec. 26. “mRNA COVID-19 vaccines may be administered to people with underlying medical conditions provided they have not had a severe allergic reaction to any of the ingredients in the vaccine.”
Both the Pfizer and Moderna vaccines use mRNA, or messenger RNA.
The CDC guidance had specific information for people with HIV, weakened immune systems, and autoimmune conditions such as Guillain-Barré syndrome (GBS) and Bell’s palsy who are thinking of getting the vaccine.
People with HIV and weakened immune systems “may receive a COVID-19 vaccine. However, they should be aware of the limited safety data,” the CDC said.
There’s no information available yet about the safety of the vaccines for people with weakened immune systems. People with HIV were included in clinical trials, but “safety data specific to this group are not yet available at this time,” the CDC said.
Cases of Bell’s palsy, a temporary facial paralysis, were reported in people receiving the Pfizer and Moderna vaccines in clinical trials, the Food and Drug Administration said Dec. 17.
But the new CDC guidance said that the FDA “does not consider these to be above the rate expected in the general population. They have not concluded these cases were caused by vaccination. Therefore, persons who have previously had Bell’s palsy may receive an mRNA COVID-19 vaccine.”
Researchers have determined the vaccines are safe for people with GBS, a rare autoimmune disorder in which the body’s immune system attacks nerves just as they leave the spinal cord, the CDC said.
“To date, no cases of GBS have been reported following vaccination among participants in the mRNA COVID-19 vaccine clinical trials,” the CDC guidance said. “With few exceptions, the independent Advisory Committee on Immunization Practices general best practice guidelines for immunization do not include a history of GBS as a precaution to vaccination with other vaccines.”
For months, the CDC and other health authorities have said that people with certain medical conditions are at an increased risk of developing severe cases of COVID-19.
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention has issued updated guidance for people with underlying medical conditions who are considering getting the coronavirus vaccine.
“Adults of any age with certain underlying medical conditions are at increased risk for severe illness from the virus that causes COVID-19,” the CDC said in the guidance, posted on Dec. 26. “mRNA COVID-19 vaccines may be administered to people with underlying medical conditions provided they have not had a severe allergic reaction to any of the ingredients in the vaccine.”
Both the Pfizer and Moderna vaccines use mRNA, or messenger RNA.
The CDC guidance had specific information for people with HIV, weakened immune systems, and autoimmune conditions such as Guillain-Barré syndrome (GBS) and Bell’s palsy who are thinking of getting the vaccine.
People with HIV and weakened immune systems “may receive a COVID-19 vaccine. However, they should be aware of the limited safety data,” the CDC said.
There’s no information available yet about the safety of the vaccines for people with weakened immune systems. People with HIV were included in clinical trials, but “safety data specific to this group are not yet available at this time,” the CDC said.
Cases of Bell’s palsy, a temporary facial paralysis, were reported in people receiving the Pfizer and Moderna vaccines in clinical trials, the Food and Drug Administration said Dec. 17.
But the new CDC guidance said that the FDA “does not consider these to be above the rate expected in the general population. They have not concluded these cases were caused by vaccination. Therefore, persons who have previously had Bell’s palsy may receive an mRNA COVID-19 vaccine.”
Researchers have determined the vaccines are safe for people with GBS, a rare autoimmune disorder in which the body’s immune system attacks nerves just as they leave the spinal cord, the CDC said.
“To date, no cases of GBS have been reported following vaccination among participants in the mRNA COVID-19 vaccine clinical trials,” the CDC guidance said. “With few exceptions, the independent Advisory Committee on Immunization Practices general best practice guidelines for immunization do not include a history of GBS as a precaution to vaccination with other vaccines.”
For months, the CDC and other health authorities have said that people with certain medical conditions are at an increased risk of developing severe cases of COVID-19.
A version of this article first appeared on Medscape.com.
Strategies for tracking SARS-CoV-2 could help detect next pandemic
Two recently published studies indicate that COVID-19 infections were already circulating in the United States in December 2019. The question is whether these methodologies that could be applied to track the next pandemic.
One study evaluating blood donations found antibodies on the West coast as early as Dec. 13, 2019, and in blood donated on the East Coast by early January 2020 (Clin Infect Dis. 2020; Nov 30. doi: 10.1093/cid/ciaa1785). Both preceded the first documented COVID-19 infection in the United States, which has been widely reported as occurring on Jan. 19, 2020, in a traveler returning from China.
The other study, utilizing electronic medical record (EMR) analytics, demonstrated a spike in visits or hospitalizations for cough, a trend that persisted from Dec. 22, 2019, onward, exceeding norms for seasonal flu ( J Med Internet Res. 2020;22:e21562). This spike was interpreted as evidence that the SARS-CoV-2 pandemic was already underway before the first case was established.
While the ongoing serologic testing of blood donations for viral antibodies “will advance understanding of the epidemiology” for SARS-CoV-2 and “inform allocation of resources and public health prevention interventions to mitigate morbidity and mortality,” it might also be a strategy for disease surveillance in the next pandemic, according to a team led by investigators at the Centers for Disease Control and Prevention.
Blood donation surveillance is not now used routinely to monitor for population-based health threats, but it is not a new idea, according to the lead author of the study, Sridhar V. Basavaraju, MD, of Emory University and director of the CDC’s Office of Blood, Organ, and Other Tissue Safety, Atlanta, and his coinvestigators. Most recently, blood donation surveillance was used in the United States to track the penetration of the Zika virus.
For early detection of respiratory infections, blood donations might have unique advantages over alternatives, such as surveillance of respiratory specimens from symptomatic patients. Not least, blood donation surveillance captures individuals who are not seeking medical care, according to the investigators.
EMR surveillance might also have unique advantages for population-based monitoring of health threats. For one, aggregate data from large EMR systems have the potential to reveal symptom patterns before they become apparent at level of clinical care, according to a team of collaborating investigators from the University of California, Los Angeles, and the University of Washington, Seattle.
Emphasizing an urgent need for “agile healthcare analytics” to enable “disease surveillance in real time,” the first author of the EMR study, Joann G. Elmore, MD, professor in the department of health policy and management at the University of California, Los Angeles, expressed the hope that the approach will “lead to better preparation and the ability to quickly provide warnings and track the next pandemic.”
In the blood donation surveillance study, the goal was simply to determine whether SARS-CoV-2 reactive antibodies could be found in blood donations before the first case was identified. Of the 7,389 archived blood samples tested between Dec. 13, 2019, and Jan. 17, 2020, 106 (1.4%) were reactive.
These were not true positives, acknowledged the investigators. True positives would require reactive antibodies in the context of a positive molecular diagnostic test or paired acute convalescent sera with rising titers. The investigators also cautioned that false positives could not be completely ruled out, particularly in light of cross-reactivity that has been reported with other human coronaviruses.
Nevertheless, the monitoring of blood donations offers substantial promise for “understanding the dynamics of SARS-CoV-2 pandemic from early introduction,” and the CDC is now collaborating on ongoing surveillance with the goal of contributing information that could be applied “to mitigate morbidity and mortality.”
Lessons learned from this pandemic are potentially relevant to the next.
The EMR study simply looked at whether the word “cough” was included more often in the notes from visits or hospitalizations between December 2019 and February 2020 relative to the preceding 5 years. The investigators drew on data from three hospitals and more than 180 clinics.
From Dec. 22, 2019, onward, cough was noted above the 95% prediction interval for all 10 weeks of the study. The excess was seen in the outpatient setting and among hospitalized patients. There was also significant excess in the number of patients hospitalized with acute respiratory failure during the study period.
“Our approach to analyzing electronic records could be helpful in the future as we included consideration of data from the outpatient clinics in addition to the emergency departments and inpatient settings,” Dr. Elmore reported.
Surveillance of influenza and influenza-like infections has been undertaken in the United States for more than 20 years, but Dr. Elmore contends that EMR data, particularly data from outpatient clinics are “usually a harbinger of what is to come” for emergency department visits and, ultimately, hospitalizations. She thinks that this is a resource not yet fully exploited.
“There are always opportunities to better harness EMR data,” Dr. Elmore said.
These are intriguing studies and “useful” for reconsidering when SARS-CoV-2 was introduced in the United States, according to Janet G. Basemen, PhD, a professor of epidemiology and the associate dean of the University of Washington School of Public Health, Seattle. However, she noted that the task of translating data like these into actionable public health strategies has proven difficult in the past.
Symptom-based surveillance systems “have mostly served as situational awareness rather than early detection tools,” Dr. Baseman said. The problem is timely interpretation of a given signal.
Not that she doubts such tools “would be an incredible resource for humanity” if the current limitations can be resolved or that technological advances will lead to better methods of detecting and monitoring pandemics “at some point.” Rather, “we’re just not there yet,” she said.
SOURCE: Basavaraju SV et al. Clin Infect Dis. 2020 Nov 30. doi: 10.1093/cid/ciaa1785); Elmore JG et al. J Med Internet Res. 2020;22:e21562).
Two recently published studies indicate that COVID-19 infections were already circulating in the United States in December 2019. The question is whether these methodologies that could be applied to track the next pandemic.
One study evaluating blood donations found antibodies on the West coast as early as Dec. 13, 2019, and in blood donated on the East Coast by early January 2020 (Clin Infect Dis. 2020; Nov 30. doi: 10.1093/cid/ciaa1785). Both preceded the first documented COVID-19 infection in the United States, which has been widely reported as occurring on Jan. 19, 2020, in a traveler returning from China.
The other study, utilizing electronic medical record (EMR) analytics, demonstrated a spike in visits or hospitalizations for cough, a trend that persisted from Dec. 22, 2019, onward, exceeding norms for seasonal flu ( J Med Internet Res. 2020;22:e21562). This spike was interpreted as evidence that the SARS-CoV-2 pandemic was already underway before the first case was established.
While the ongoing serologic testing of blood donations for viral antibodies “will advance understanding of the epidemiology” for SARS-CoV-2 and “inform allocation of resources and public health prevention interventions to mitigate morbidity and mortality,” it might also be a strategy for disease surveillance in the next pandemic, according to a team led by investigators at the Centers for Disease Control and Prevention.
Blood donation surveillance is not now used routinely to monitor for population-based health threats, but it is not a new idea, according to the lead author of the study, Sridhar V. Basavaraju, MD, of Emory University and director of the CDC’s Office of Blood, Organ, and Other Tissue Safety, Atlanta, and his coinvestigators. Most recently, blood donation surveillance was used in the United States to track the penetration of the Zika virus.
For early detection of respiratory infections, blood donations might have unique advantages over alternatives, such as surveillance of respiratory specimens from symptomatic patients. Not least, blood donation surveillance captures individuals who are not seeking medical care, according to the investigators.
EMR surveillance might also have unique advantages for population-based monitoring of health threats. For one, aggregate data from large EMR systems have the potential to reveal symptom patterns before they become apparent at level of clinical care, according to a team of collaborating investigators from the University of California, Los Angeles, and the University of Washington, Seattle.
Emphasizing an urgent need for “agile healthcare analytics” to enable “disease surveillance in real time,” the first author of the EMR study, Joann G. Elmore, MD, professor in the department of health policy and management at the University of California, Los Angeles, expressed the hope that the approach will “lead to better preparation and the ability to quickly provide warnings and track the next pandemic.”
In the blood donation surveillance study, the goal was simply to determine whether SARS-CoV-2 reactive antibodies could be found in blood donations before the first case was identified. Of the 7,389 archived blood samples tested between Dec. 13, 2019, and Jan. 17, 2020, 106 (1.4%) were reactive.
These were not true positives, acknowledged the investigators. True positives would require reactive antibodies in the context of a positive molecular diagnostic test or paired acute convalescent sera with rising titers. The investigators also cautioned that false positives could not be completely ruled out, particularly in light of cross-reactivity that has been reported with other human coronaviruses.
Nevertheless, the monitoring of blood donations offers substantial promise for “understanding the dynamics of SARS-CoV-2 pandemic from early introduction,” and the CDC is now collaborating on ongoing surveillance with the goal of contributing information that could be applied “to mitigate morbidity and mortality.”
Lessons learned from this pandemic are potentially relevant to the next.
The EMR study simply looked at whether the word “cough” was included more often in the notes from visits or hospitalizations between December 2019 and February 2020 relative to the preceding 5 years. The investigators drew on data from three hospitals and more than 180 clinics.
From Dec. 22, 2019, onward, cough was noted above the 95% prediction interval for all 10 weeks of the study. The excess was seen in the outpatient setting and among hospitalized patients. There was also significant excess in the number of patients hospitalized with acute respiratory failure during the study period.
“Our approach to analyzing electronic records could be helpful in the future as we included consideration of data from the outpatient clinics in addition to the emergency departments and inpatient settings,” Dr. Elmore reported.
Surveillance of influenza and influenza-like infections has been undertaken in the United States for more than 20 years, but Dr. Elmore contends that EMR data, particularly data from outpatient clinics are “usually a harbinger of what is to come” for emergency department visits and, ultimately, hospitalizations. She thinks that this is a resource not yet fully exploited.
“There are always opportunities to better harness EMR data,” Dr. Elmore said.
These are intriguing studies and “useful” for reconsidering when SARS-CoV-2 was introduced in the United States, according to Janet G. Basemen, PhD, a professor of epidemiology and the associate dean of the University of Washington School of Public Health, Seattle. However, she noted that the task of translating data like these into actionable public health strategies has proven difficult in the past.
Symptom-based surveillance systems “have mostly served as situational awareness rather than early detection tools,” Dr. Baseman said. The problem is timely interpretation of a given signal.
Not that she doubts such tools “would be an incredible resource for humanity” if the current limitations can be resolved or that technological advances will lead to better methods of detecting and monitoring pandemics “at some point.” Rather, “we’re just not there yet,” she said.
SOURCE: Basavaraju SV et al. Clin Infect Dis. 2020 Nov 30. doi: 10.1093/cid/ciaa1785); Elmore JG et al. J Med Internet Res. 2020;22:e21562).
Two recently published studies indicate that COVID-19 infections were already circulating in the United States in December 2019. The question is whether these methodologies that could be applied to track the next pandemic.
One study evaluating blood donations found antibodies on the West coast as early as Dec. 13, 2019, and in blood donated on the East Coast by early January 2020 (Clin Infect Dis. 2020; Nov 30. doi: 10.1093/cid/ciaa1785). Both preceded the first documented COVID-19 infection in the United States, which has been widely reported as occurring on Jan. 19, 2020, in a traveler returning from China.
The other study, utilizing electronic medical record (EMR) analytics, demonstrated a spike in visits or hospitalizations for cough, a trend that persisted from Dec. 22, 2019, onward, exceeding norms for seasonal flu ( J Med Internet Res. 2020;22:e21562). This spike was interpreted as evidence that the SARS-CoV-2 pandemic was already underway before the first case was established.
While the ongoing serologic testing of blood donations for viral antibodies “will advance understanding of the epidemiology” for SARS-CoV-2 and “inform allocation of resources and public health prevention interventions to mitigate morbidity and mortality,” it might also be a strategy for disease surveillance in the next pandemic, according to a team led by investigators at the Centers for Disease Control and Prevention.
Blood donation surveillance is not now used routinely to monitor for population-based health threats, but it is not a new idea, according to the lead author of the study, Sridhar V. Basavaraju, MD, of Emory University and director of the CDC’s Office of Blood, Organ, and Other Tissue Safety, Atlanta, and his coinvestigators. Most recently, blood donation surveillance was used in the United States to track the penetration of the Zika virus.
For early detection of respiratory infections, blood donations might have unique advantages over alternatives, such as surveillance of respiratory specimens from symptomatic patients. Not least, blood donation surveillance captures individuals who are not seeking medical care, according to the investigators.
EMR surveillance might also have unique advantages for population-based monitoring of health threats. For one, aggregate data from large EMR systems have the potential to reveal symptom patterns before they become apparent at level of clinical care, according to a team of collaborating investigators from the University of California, Los Angeles, and the University of Washington, Seattle.
Emphasizing an urgent need for “agile healthcare analytics” to enable “disease surveillance in real time,” the first author of the EMR study, Joann G. Elmore, MD, professor in the department of health policy and management at the University of California, Los Angeles, expressed the hope that the approach will “lead to better preparation and the ability to quickly provide warnings and track the next pandemic.”
In the blood donation surveillance study, the goal was simply to determine whether SARS-CoV-2 reactive antibodies could be found in blood donations before the first case was identified. Of the 7,389 archived blood samples tested between Dec. 13, 2019, and Jan. 17, 2020, 106 (1.4%) were reactive.
These were not true positives, acknowledged the investigators. True positives would require reactive antibodies in the context of a positive molecular diagnostic test or paired acute convalescent sera with rising titers. The investigators also cautioned that false positives could not be completely ruled out, particularly in light of cross-reactivity that has been reported with other human coronaviruses.
Nevertheless, the monitoring of blood donations offers substantial promise for “understanding the dynamics of SARS-CoV-2 pandemic from early introduction,” and the CDC is now collaborating on ongoing surveillance with the goal of contributing information that could be applied “to mitigate morbidity and mortality.”
Lessons learned from this pandemic are potentially relevant to the next.
The EMR study simply looked at whether the word “cough” was included more often in the notes from visits or hospitalizations between December 2019 and February 2020 relative to the preceding 5 years. The investigators drew on data from three hospitals and more than 180 clinics.
From Dec. 22, 2019, onward, cough was noted above the 95% prediction interval for all 10 weeks of the study. The excess was seen in the outpatient setting and among hospitalized patients. There was also significant excess in the number of patients hospitalized with acute respiratory failure during the study period.
“Our approach to analyzing electronic records could be helpful in the future as we included consideration of data from the outpatient clinics in addition to the emergency departments and inpatient settings,” Dr. Elmore reported.
Surveillance of influenza and influenza-like infections has been undertaken in the United States for more than 20 years, but Dr. Elmore contends that EMR data, particularly data from outpatient clinics are “usually a harbinger of what is to come” for emergency department visits and, ultimately, hospitalizations. She thinks that this is a resource not yet fully exploited.
“There are always opportunities to better harness EMR data,” Dr. Elmore said.
These are intriguing studies and “useful” for reconsidering when SARS-CoV-2 was introduced in the United States, according to Janet G. Basemen, PhD, a professor of epidemiology and the associate dean of the University of Washington School of Public Health, Seattle. However, she noted that the task of translating data like these into actionable public health strategies has proven difficult in the past.
Symptom-based surveillance systems “have mostly served as situational awareness rather than early detection tools,” Dr. Baseman said. The problem is timely interpretation of a given signal.
Not that she doubts such tools “would be an incredible resource for humanity” if the current limitations can be resolved or that technological advances will lead to better methods of detecting and monitoring pandemics “at some point.” Rather, “we’re just not there yet,” she said.
SOURCE: Basavaraju SV et al. Clin Infect Dis. 2020 Nov 30. doi: 10.1093/cid/ciaa1785); Elmore JG et al. J Med Internet Res. 2020;22:e21562).
Second COVID-19 vaccine ready for use, CDC panel says
The panel voted 11-0, with three recusals, to recommend use of Moderna’s vaccine for people aged 18 years and older, while seeking more information on risk for anaphylaxis. This vote followed the December 18th decision by the US Food and Drug Administration (FDA) to grant emergency use authorization (EUA) for the vaccine, known as mRNA-1273.
On December 11, the FDA granted the first US emergency clearance for a COVID-19 vaccine to the Pfizer-BioNTech product. ACIP met the following day and voted to endorse the use of that vaccine, with a vote of 11-0 and three recusals. The Pfizer-BioNTech COVID-19 vaccine is recommended for use in people aged 16 years and older.
Moderna’s vaccine is expected to help curb the pandemic, with clinical trial data showing a 94.1% efficacy rate. But there’s also concerns about side effects noted in testing of both vaccines and in the early rollout of the Pfizer vaccine, particularly anaphylaxis.
“There are likely going to be lots of bumps in the road” with the introduction of the COVID-19 vaccines, but these are being disclosed to the public in a way that is “fair and transparent,” said ACIP member Beth P. Bell, MD, MPH.
“Our systems so far appear to be doing what they are supposed to do” in terms of determining risks from the COVID-19 vaccine, added Bell, who is a clinical professor in the department of global health at the University of Washington’s School of Public Health in Seattle. The Moderna EUA “represents progress towards ending this horrific pandemic,” she said.
In a new forecast released this week, the CDC projects that the number of newly reported COVID-19 deaths will likely increase over the next 4 weeks, with 15,800 to 27,700 new deaths likely to be reported in the week ending January 9, 2021. That could bring the total number of COVID-19 deaths in the United States to between 357,000 and 391,000 by this date, according to the agency.
ACIP panelist Lynn Bahta, RN, MPH, CPH, said she had been “eager” to have the panel proceed with its endorsement of the Moderna vaccine, “especially in light of the fact that we are seeing an average 2600 deaths a day.”
Having two COVID-19 vaccines available might help slow down the pandemic, “despite the fact that we still have a lot to learn both about the disease and the vaccine,” said Bahta, who is an immunization consultant with the Minnesota Department of Health in Saint Paul.
ACIP members encouraged Moderna officials who presented at the meeting to continue studies for potential complications associated with the vaccine when given to women who are pregnant or breastfeeding.
Panelists also pressed for more data on the risk for Bell’s palsy, which the FDA staff also had noted in the agency’s review of Moderna’s vaccine. Moderna has reported four cases from a pivotal study, one in the placebo group and three among study participants who received the company’s vaccine. These cases occurred between 15 and 33 days after vaccination, and are all resolved or resolving, according to Moderna.
There was also a question raised about how many doses of vaccine might be squeezed out of a vial. CDC will explore this topic further at its meeting on COVID-19 vaccines December 20, said Nancy Messonnier, MD, director of the agency’s National Center for Immunization and Respiratory Diseases, at the Saturday meeting.
“In this time of public health crisis, none of us would want to squander a single dose of a vaccine that’s potentially lifesaving,” CDC’s Messonnier said. “We’re going to plan to have a short discussion of that issue tomorrow.”
Messonnier also responded to a comment made during the meeting about cases where people who received COVID-19 vaccine were unaware of the CDC’s V-safe tool.
V-safe is a smartphone-based tool that uses text messaging and web surveys to help people keep in touch with the medical community after getting the COVID-19 vaccine and is seen as a way to help spot side effects. Messonnier asked that people listening to the webcast of the ACIP meeting help spread the word about the CDC’s V-safe tool.
“Our perception, based on the number of people who have enrolled in V-safe, is that the message is getting out to many places, but even one site that doesn’t have this information is something that we want to try to correct,” she said.
Anaphylaxis concerns
The chief concern for ACIP members and CDC staff about COVID-19 vaccines appeared to be reports of allergic reactions. Thomas Clark, MD, MPH, a CDC staff member, told the ACIP panel that, as of December 18, the agency had identified six cases of anaphylaxis following administration of the Pfizer-BioNTech vaccine that met a certain standard, known as the Brighton Collaboration criteria.
Additional case reports have been reviewed and determined not to be anaphylaxis, Clark said. All suspect cases were identified through processes such as the federal Vaccine Adverse Event Reporting System (VAERS), he said.
People who experience anaphylaxis following COVID-19 vaccination should not receive additional doses of the shot, Clark said in his presentation to ACIP. Members of the panel asked Clark whether there have been any discernible patterns to these cases, such as geographic clusters.
Clark replied that it was “early” in the process to make reports, with investigations still ongoing. He did note that the people who had anaphylaxis following vaccination had received their doses from more than one production lot, with multiple lots having been distributed.
“You folks may have seen in the news a couple of cases from Alaska, but we’ve had reports from other jurisdictions so there’s no obvious clustering geographically,” Clark said.
Another CDC staff member, Sarah Mbaeyi, MD, MPH, noted in her presentation that there should be an observation period of 30 minutes following COVID-19 vaccination for anyone with a history of anaphylaxis for any reason, and of at least 15 minutes for other recipients.
Disclosure of ingredients used in the COVID-19 vaccines might help people with an allergy assess these products, the representative for the American Medical Association, Sandra Fryhofer, MD, told ACIP. As such, she thanked CDC’s Mbaeyi for including a breakout of ingredients in her presentation to the panel. Fryhofer encouraged Moderna officials to be as transparent as possible in disclosing the ingredients of the company’s COVID-19 vaccine.
“That might be important because I think it’s very essential that we figure out what might be triggering these anaphylactic reactions, because that is definitely going to affect the vaccine implementation,” Fryhofer said.
The three ACIP members who had conflicts that prevented their voting were Robert L. Atmar, MD, who said at the Saturday meeting he had participated in COVID-19 trials, including research on the Moderna vaccine; Sharon E. Frey, MD, who said at the Saturday meeting that she had been involved with research on COVID-19 vaccines, including Moderna’s; and Paul Hunter, MD, who said he has received a grant from Pfizer for pneumococcal vaccines.
The other panel members have reported no relevant financial relationships.
This article first appeared on Medscape.com.
The panel voted 11-0, with three recusals, to recommend use of Moderna’s vaccine for people aged 18 years and older, while seeking more information on risk for anaphylaxis. This vote followed the December 18th decision by the US Food and Drug Administration (FDA) to grant emergency use authorization (EUA) for the vaccine, known as mRNA-1273.
On December 11, the FDA granted the first US emergency clearance for a COVID-19 vaccine to the Pfizer-BioNTech product. ACIP met the following day and voted to endorse the use of that vaccine, with a vote of 11-0 and three recusals. The Pfizer-BioNTech COVID-19 vaccine is recommended for use in people aged 16 years and older.
Moderna’s vaccine is expected to help curb the pandemic, with clinical trial data showing a 94.1% efficacy rate. But there’s also concerns about side effects noted in testing of both vaccines and in the early rollout of the Pfizer vaccine, particularly anaphylaxis.
“There are likely going to be lots of bumps in the road” with the introduction of the COVID-19 vaccines, but these are being disclosed to the public in a way that is “fair and transparent,” said ACIP member Beth P. Bell, MD, MPH.
“Our systems so far appear to be doing what they are supposed to do” in terms of determining risks from the COVID-19 vaccine, added Bell, who is a clinical professor in the department of global health at the University of Washington’s School of Public Health in Seattle. The Moderna EUA “represents progress towards ending this horrific pandemic,” she said.
In a new forecast released this week, the CDC projects that the number of newly reported COVID-19 deaths will likely increase over the next 4 weeks, with 15,800 to 27,700 new deaths likely to be reported in the week ending January 9, 2021. That could bring the total number of COVID-19 deaths in the United States to between 357,000 and 391,000 by this date, according to the agency.
ACIP panelist Lynn Bahta, RN, MPH, CPH, said she had been “eager” to have the panel proceed with its endorsement of the Moderna vaccine, “especially in light of the fact that we are seeing an average 2600 deaths a day.”
Having two COVID-19 vaccines available might help slow down the pandemic, “despite the fact that we still have a lot to learn both about the disease and the vaccine,” said Bahta, who is an immunization consultant with the Minnesota Department of Health in Saint Paul.
ACIP members encouraged Moderna officials who presented at the meeting to continue studies for potential complications associated with the vaccine when given to women who are pregnant or breastfeeding.
Panelists also pressed for more data on the risk for Bell’s palsy, which the FDA staff also had noted in the agency’s review of Moderna’s vaccine. Moderna has reported four cases from a pivotal study, one in the placebo group and three among study participants who received the company’s vaccine. These cases occurred between 15 and 33 days after vaccination, and are all resolved or resolving, according to Moderna.
There was also a question raised about how many doses of vaccine might be squeezed out of a vial. CDC will explore this topic further at its meeting on COVID-19 vaccines December 20, said Nancy Messonnier, MD, director of the agency’s National Center for Immunization and Respiratory Diseases, at the Saturday meeting.
“In this time of public health crisis, none of us would want to squander a single dose of a vaccine that’s potentially lifesaving,” CDC’s Messonnier said. “We’re going to plan to have a short discussion of that issue tomorrow.”
Messonnier also responded to a comment made during the meeting about cases where people who received COVID-19 vaccine were unaware of the CDC’s V-safe tool.
V-safe is a smartphone-based tool that uses text messaging and web surveys to help people keep in touch with the medical community after getting the COVID-19 vaccine and is seen as a way to help spot side effects. Messonnier asked that people listening to the webcast of the ACIP meeting help spread the word about the CDC’s V-safe tool.
“Our perception, based on the number of people who have enrolled in V-safe, is that the message is getting out to many places, but even one site that doesn’t have this information is something that we want to try to correct,” she said.
Anaphylaxis concerns
The chief concern for ACIP members and CDC staff about COVID-19 vaccines appeared to be reports of allergic reactions. Thomas Clark, MD, MPH, a CDC staff member, told the ACIP panel that, as of December 18, the agency had identified six cases of anaphylaxis following administration of the Pfizer-BioNTech vaccine that met a certain standard, known as the Brighton Collaboration criteria.
Additional case reports have been reviewed and determined not to be anaphylaxis, Clark said. All suspect cases were identified through processes such as the federal Vaccine Adverse Event Reporting System (VAERS), he said.
People who experience anaphylaxis following COVID-19 vaccination should not receive additional doses of the shot, Clark said in his presentation to ACIP. Members of the panel asked Clark whether there have been any discernible patterns to these cases, such as geographic clusters.
Clark replied that it was “early” in the process to make reports, with investigations still ongoing. He did note that the people who had anaphylaxis following vaccination had received their doses from more than one production lot, with multiple lots having been distributed.
“You folks may have seen in the news a couple of cases from Alaska, but we’ve had reports from other jurisdictions so there’s no obvious clustering geographically,” Clark said.
Another CDC staff member, Sarah Mbaeyi, MD, MPH, noted in her presentation that there should be an observation period of 30 minutes following COVID-19 vaccination for anyone with a history of anaphylaxis for any reason, and of at least 15 minutes for other recipients.
Disclosure of ingredients used in the COVID-19 vaccines might help people with an allergy assess these products, the representative for the American Medical Association, Sandra Fryhofer, MD, told ACIP. As such, she thanked CDC’s Mbaeyi for including a breakout of ingredients in her presentation to the panel. Fryhofer encouraged Moderna officials to be as transparent as possible in disclosing the ingredients of the company’s COVID-19 vaccine.
“That might be important because I think it’s very essential that we figure out what might be triggering these anaphylactic reactions, because that is definitely going to affect the vaccine implementation,” Fryhofer said.
The three ACIP members who had conflicts that prevented their voting were Robert L. Atmar, MD, who said at the Saturday meeting he had participated in COVID-19 trials, including research on the Moderna vaccine; Sharon E. Frey, MD, who said at the Saturday meeting that she had been involved with research on COVID-19 vaccines, including Moderna’s; and Paul Hunter, MD, who said he has received a grant from Pfizer for pneumococcal vaccines.
The other panel members have reported no relevant financial relationships.
This article first appeared on Medscape.com.
The panel voted 11-0, with three recusals, to recommend use of Moderna’s vaccine for people aged 18 years and older, while seeking more information on risk for anaphylaxis. This vote followed the December 18th decision by the US Food and Drug Administration (FDA) to grant emergency use authorization (EUA) for the vaccine, known as mRNA-1273.
On December 11, the FDA granted the first US emergency clearance for a COVID-19 vaccine to the Pfizer-BioNTech product. ACIP met the following day and voted to endorse the use of that vaccine, with a vote of 11-0 and three recusals. The Pfizer-BioNTech COVID-19 vaccine is recommended for use in people aged 16 years and older.
Moderna’s vaccine is expected to help curb the pandemic, with clinical trial data showing a 94.1% efficacy rate. But there’s also concerns about side effects noted in testing of both vaccines and in the early rollout of the Pfizer vaccine, particularly anaphylaxis.
“There are likely going to be lots of bumps in the road” with the introduction of the COVID-19 vaccines, but these are being disclosed to the public in a way that is “fair and transparent,” said ACIP member Beth P. Bell, MD, MPH.
“Our systems so far appear to be doing what they are supposed to do” in terms of determining risks from the COVID-19 vaccine, added Bell, who is a clinical professor in the department of global health at the University of Washington’s School of Public Health in Seattle. The Moderna EUA “represents progress towards ending this horrific pandemic,” she said.
In a new forecast released this week, the CDC projects that the number of newly reported COVID-19 deaths will likely increase over the next 4 weeks, with 15,800 to 27,700 new deaths likely to be reported in the week ending January 9, 2021. That could bring the total number of COVID-19 deaths in the United States to between 357,000 and 391,000 by this date, according to the agency.
ACIP panelist Lynn Bahta, RN, MPH, CPH, said she had been “eager” to have the panel proceed with its endorsement of the Moderna vaccine, “especially in light of the fact that we are seeing an average 2600 deaths a day.”
Having two COVID-19 vaccines available might help slow down the pandemic, “despite the fact that we still have a lot to learn both about the disease and the vaccine,” said Bahta, who is an immunization consultant with the Minnesota Department of Health in Saint Paul.
ACIP members encouraged Moderna officials who presented at the meeting to continue studies for potential complications associated with the vaccine when given to women who are pregnant or breastfeeding.
Panelists also pressed for more data on the risk for Bell’s palsy, which the FDA staff also had noted in the agency’s review of Moderna’s vaccine. Moderna has reported four cases from a pivotal study, one in the placebo group and three among study participants who received the company’s vaccine. These cases occurred between 15 and 33 days after vaccination, and are all resolved or resolving, according to Moderna.
There was also a question raised about how many doses of vaccine might be squeezed out of a vial. CDC will explore this topic further at its meeting on COVID-19 vaccines December 20, said Nancy Messonnier, MD, director of the agency’s National Center for Immunization and Respiratory Diseases, at the Saturday meeting.
“In this time of public health crisis, none of us would want to squander a single dose of a vaccine that’s potentially lifesaving,” CDC’s Messonnier said. “We’re going to plan to have a short discussion of that issue tomorrow.”
Messonnier also responded to a comment made during the meeting about cases where people who received COVID-19 vaccine were unaware of the CDC’s V-safe tool.
V-safe is a smartphone-based tool that uses text messaging and web surveys to help people keep in touch with the medical community after getting the COVID-19 vaccine and is seen as a way to help spot side effects. Messonnier asked that people listening to the webcast of the ACIP meeting help spread the word about the CDC’s V-safe tool.
“Our perception, based on the number of people who have enrolled in V-safe, is that the message is getting out to many places, but even one site that doesn’t have this information is something that we want to try to correct,” she said.
Anaphylaxis concerns
The chief concern for ACIP members and CDC staff about COVID-19 vaccines appeared to be reports of allergic reactions. Thomas Clark, MD, MPH, a CDC staff member, told the ACIP panel that, as of December 18, the agency had identified six cases of anaphylaxis following administration of the Pfizer-BioNTech vaccine that met a certain standard, known as the Brighton Collaboration criteria.
Additional case reports have been reviewed and determined not to be anaphylaxis, Clark said. All suspect cases were identified through processes such as the federal Vaccine Adverse Event Reporting System (VAERS), he said.
People who experience anaphylaxis following COVID-19 vaccination should not receive additional doses of the shot, Clark said in his presentation to ACIP. Members of the panel asked Clark whether there have been any discernible patterns to these cases, such as geographic clusters.
Clark replied that it was “early” in the process to make reports, with investigations still ongoing. He did note that the people who had anaphylaxis following vaccination had received their doses from more than one production lot, with multiple lots having been distributed.
“You folks may have seen in the news a couple of cases from Alaska, but we’ve had reports from other jurisdictions so there’s no obvious clustering geographically,” Clark said.
Another CDC staff member, Sarah Mbaeyi, MD, MPH, noted in her presentation that there should be an observation period of 30 minutes following COVID-19 vaccination for anyone with a history of anaphylaxis for any reason, and of at least 15 minutes for other recipients.
Disclosure of ingredients used in the COVID-19 vaccines might help people with an allergy assess these products, the representative for the American Medical Association, Sandra Fryhofer, MD, told ACIP. As such, she thanked CDC’s Mbaeyi for including a breakout of ingredients in her presentation to the panel. Fryhofer encouraged Moderna officials to be as transparent as possible in disclosing the ingredients of the company’s COVID-19 vaccine.
“That might be important because I think it’s very essential that we figure out what might be triggering these anaphylactic reactions, because that is definitely going to affect the vaccine implementation,” Fryhofer said.
The three ACIP members who had conflicts that prevented their voting were Robert L. Atmar, MD, who said at the Saturday meeting he had participated in COVID-19 trials, including research on the Moderna vaccine; Sharon E. Frey, MD, who said at the Saturday meeting that she had been involved with research on COVID-19 vaccines, including Moderna’s; and Paul Hunter, MD, who said he has received a grant from Pfizer for pneumococcal vaccines.
The other panel members have reported no relevant financial relationships.
This article first appeared on Medscape.com.
FDA grants emergency use for Moderna COVID-19 vaccine
As expected, the US Food and Drug Administration granted Moderna an emergency use authorization (EUA) for its messenger RNA COVID-19 vaccine December 18.
There is one final step — the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices will need to recommend its use, as it did 2 days after the Pfizer/BioNTech mRNA vaccine received its EUA on December 10.
The EUA for the Moderna vaccine is “a major milestone in trying to contain this pandemic,” Hana Mohammed El Sahly, MD, told Medscape Medical News.
Scaling up distribution of the two vaccine products will come next. She notes that even under less emergent conditions, making sure people who need a vaccine receive it can be hard. “I hope the media attention around this will make more people aware that there are vaccines that might help them,” said El Sahly, chair of the FDA Vaccines and Related Biological Products Advisory Committee (VRBPAC).
The EUA for the Moderna vaccine follows a review by the independent VRBPAC members on December 17, which voted 20-0 with one abstention to recommend the EUA. The vaccine is authorized for use in people 18 and older.
Emergency approval of a second COVID-19 vaccine “is great — we need all the tools we can to fight this pandemic,” Stephen Schrantz, MD, infectious disease specialist and assistant professor of medicine at the University of Chicago, told Medscape Medical News. “The early data coming from Moderna looks good, and I agree with the FDA that an EUA is indicated.
“It’s incumbent upon all us healthcare professionals to put ourselves out there as supporting this vaccine and supporting people getting it,” Schrantz continued. “We want to make sure people who are on the fence understand this is a safe vaccine that has been vetted appropriately through the FDA and through phase 3 clinical trials.”
“I know the critical role physicians play as vaccine influencers,” AMA President Susan Bailey, MD, said during a December 14 webinar for journalists reporting on COVID-19 vaccines. “We have to continue to do what physicians have always done: review the evidence and trust the science. Lives are at stake.” The webinar was cosponsored by the AMA and the Poynter Institute.
Ramping up healthcare provider immunizations
“I am very excited to see the FDA’s positive review of the Moderna vaccine. We have been waiting to have another vaccine we can use for healthcare workers and staff, and now we have it,” Aneesh Mehta, MD, of Emory University School of Medicine in Atlanta, Georgia, told Medscape Medical News.
“We had been hoping for a vaccine with a 70% or 80% efficacy, and to see two vaccines now with greater than 90% efficacy is remarkable,” he added.
The efficacy levels associated with both mRNA vaccines “did exceed expectations for sure — this is not what we built the studies around. It was surprising in the good sense of the word,” said El Sahly, who is also associate professor of molecular virology and microbiology at Baylor College of Medicine in Houston, Texas.
Unanswered questions remain
Schrantz likewise said the high efficacy rate was important but not all that is needed. “[W]hat we know about this vaccine is it is very effective at preventing disease. We don’t have any understanding at this time whether or not these vaccines prevent infection and transmissibility.”
Bailey said, “The jury is still out on whether or not you can still transmit the virus after you’ve had the vaccine. Hopefully not, but we don’t really know that for sure.”
“It’s risky to think that once you get the shot in your arm everything goes back to normal. It doesn’t,” Bailey added.
Another unknown is the duration of protection following immunization. The Pfizer and Moderna products “have similar constructs, seem to have a reasonable safety profile, and excellent short-term efficacy,” El Sahly said. She cautioned, however, that long-term efficacy still needs to be determined.
Whether any rare adverse events will emerge in the long run is another question. Answers could come over time from the ongoing phase 3 trials, as well as from post-EUA surveillance among vaccine recipients.
“Our work is not done after issuing an EUA,” FDA Commissioner Stephen Hahn, MD, said in a JAMA webinar on December 14. The FDA is closely monitoring for any adverse event rates above the normal background incidence. “We are going to be transparent about it if we are seeing anything that is not at base level.”
“The key is to be humble, keep your eyes open and know that once the vaccine is out there, there may be things we learn that we don’t know now. That is true for virtually any medical innovation,” Paul Offit, MD, director of The Vaccine Education Center at Children’s Hospital of Philadelphia and a member of the FDA VRBPAC, said during the AMA/Poynter Institute webinar.
During the same webinar, an attendee asked about prioritizing immunization for spouses and family members of healthcare workers. “My husband wants to know that too,” replied Patricia A. Stinchfield, APRN, CNP, pediatric nurse practitioner in infectious diseases at Children’s Minnesota, St. Paul.
“It is true we should be thinking about our healthcare workers’ family members. But at this point in time we just don’t have the supplies to address it that way,” said Stinchfield, who is also the president-elect of the National Foundation for Infectious Diseases.
Advantages beyond the numbers?
“The major advantage of having two vaccines is sheer volume,” Mehta said. An additional advantage of more than one product is the potential to offer an option when a specific vaccine is contraindicated. “We could offer someone a different vaccine…similar to what we do with the influenza vaccine.”
“The more the merrier in terms of having more vaccine products,” Schrantz said. Despite differences in shipping, storage, minimum age requirements, and dosing intervals, the Pfizer and Moderna vaccines are very similar, he said. “Really the only difference between these two vaccines is the proprietary lipid nanoparticle — the delivery vehicle if you will.”
Both vaccines “appear very similar in their capacity to protect against disease, to protect [people in] various racial and ethnic backgrounds, and in their capacity to protect against severe disease,” Offit said.
In terms of vaccines in the development pipeline, “We don’t know but we might start to see a difference with the Johnson & Johnson vaccine or the Janssen vaccine, which are single dose. They might confer some advantages, but we are waiting on the safety and efficacy data,” Schrantz said.
As a two-dose vaccine, the AstraZeneca product does not offer an advantage on the dosing strategy, “but it is easier to transport than the mRNA vaccines,” he said. Some concerns with the initial data on the AstraZeneca vaccine will likely need to be addressed before the company applies for an EUA, Schrantz added.
“That is an important question,” El Sahly said. The ongoing studies should provide more data from participants of all ages and ethnic backgrounds that “will allow us to make a determination as to whether there is any difference between these two vaccines.
She added that the Pfizer and Moderna vaccines seem comparable from the early data. “We’ll see if this stands in the long run.”
Future outlook
Now that the FDA approved emergency use of two COVID-19 vaccines, “we need each state to quickly implement their plans to get the vaccines into the hands of providers who need to give the vaccines,” Mehta said. “We are seeing very effective rollout in multiple regions of the country. And we hope to see that continue as we get more vaccines from manufacturers over the coming months.”
“Within a year of identifying the sequence of this virus we have two large clinical vaccine trials that show efficacy,” Offit said. “That was an amazing technologic accomplishment, but now comes the hard part. Mass producing this vaccine, getting it out there, making sure everybody who most benefits gets it, is going to be really, really hard.”
“But I’m optimistic,” Offit said. “If we can do this by next Thanksgiving, we’re going to see a dramatic drop in the number of cases, hospitalizations and deaths, and we can get our lives back together again.”
“My greatest hope is that a year from now we look back and realize we did something really amazing together,” Bailey said, “and we have a feeling of accomplishment and appreciation for all the hard work that has been done.”
Mehta shared the important message he shares when walking around the hospital: “While these vaccines are coming and they are very promising, we need to continue to remember the 3 Ws: wearing a mask, washing your hands, and watching your distance,” he said.
“With the combination of those 3Ws and those vaccines, we will hopefully come through this COVID pandemic.”
El Sahly receives funding through the NIH for her research, including her role as co-chair of the Moderna vaccine phase 3 clinical trial. Schrantz is a site investigator for the Moderna and Janssen vaccine trials. Mehta also receives funding through the NIH. None of these experts had any relevant financial disclosures.
This article first appeared on Medscape.com.
As expected, the US Food and Drug Administration granted Moderna an emergency use authorization (EUA) for its messenger RNA COVID-19 vaccine December 18.
There is one final step — the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices will need to recommend its use, as it did 2 days after the Pfizer/BioNTech mRNA vaccine received its EUA on December 10.
The EUA for the Moderna vaccine is “a major milestone in trying to contain this pandemic,” Hana Mohammed El Sahly, MD, told Medscape Medical News.
Scaling up distribution of the two vaccine products will come next. She notes that even under less emergent conditions, making sure people who need a vaccine receive it can be hard. “I hope the media attention around this will make more people aware that there are vaccines that might help them,” said El Sahly, chair of the FDA Vaccines and Related Biological Products Advisory Committee (VRBPAC).
The EUA for the Moderna vaccine follows a review by the independent VRBPAC members on December 17, which voted 20-0 with one abstention to recommend the EUA. The vaccine is authorized for use in people 18 and older.
Emergency approval of a second COVID-19 vaccine “is great — we need all the tools we can to fight this pandemic,” Stephen Schrantz, MD, infectious disease specialist and assistant professor of medicine at the University of Chicago, told Medscape Medical News. “The early data coming from Moderna looks good, and I agree with the FDA that an EUA is indicated.
“It’s incumbent upon all us healthcare professionals to put ourselves out there as supporting this vaccine and supporting people getting it,” Schrantz continued. “We want to make sure people who are on the fence understand this is a safe vaccine that has been vetted appropriately through the FDA and through phase 3 clinical trials.”
“I know the critical role physicians play as vaccine influencers,” AMA President Susan Bailey, MD, said during a December 14 webinar for journalists reporting on COVID-19 vaccines. “We have to continue to do what physicians have always done: review the evidence and trust the science. Lives are at stake.” The webinar was cosponsored by the AMA and the Poynter Institute.
Ramping up healthcare provider immunizations
“I am very excited to see the FDA’s positive review of the Moderna vaccine. We have been waiting to have another vaccine we can use for healthcare workers and staff, and now we have it,” Aneesh Mehta, MD, of Emory University School of Medicine in Atlanta, Georgia, told Medscape Medical News.
“We had been hoping for a vaccine with a 70% or 80% efficacy, and to see two vaccines now with greater than 90% efficacy is remarkable,” he added.
The efficacy levels associated with both mRNA vaccines “did exceed expectations for sure — this is not what we built the studies around. It was surprising in the good sense of the word,” said El Sahly, who is also associate professor of molecular virology and microbiology at Baylor College of Medicine in Houston, Texas.
Unanswered questions remain
Schrantz likewise said the high efficacy rate was important but not all that is needed. “[W]hat we know about this vaccine is it is very effective at preventing disease. We don’t have any understanding at this time whether or not these vaccines prevent infection and transmissibility.”
Bailey said, “The jury is still out on whether or not you can still transmit the virus after you’ve had the vaccine. Hopefully not, but we don’t really know that for sure.”
“It’s risky to think that once you get the shot in your arm everything goes back to normal. It doesn’t,” Bailey added.
Another unknown is the duration of protection following immunization. The Pfizer and Moderna products “have similar constructs, seem to have a reasonable safety profile, and excellent short-term efficacy,” El Sahly said. She cautioned, however, that long-term efficacy still needs to be determined.
Whether any rare adverse events will emerge in the long run is another question. Answers could come over time from the ongoing phase 3 trials, as well as from post-EUA surveillance among vaccine recipients.
“Our work is not done after issuing an EUA,” FDA Commissioner Stephen Hahn, MD, said in a JAMA webinar on December 14. The FDA is closely monitoring for any adverse event rates above the normal background incidence. “We are going to be transparent about it if we are seeing anything that is not at base level.”
“The key is to be humble, keep your eyes open and know that once the vaccine is out there, there may be things we learn that we don’t know now. That is true for virtually any medical innovation,” Paul Offit, MD, director of The Vaccine Education Center at Children’s Hospital of Philadelphia and a member of the FDA VRBPAC, said during the AMA/Poynter Institute webinar.
During the same webinar, an attendee asked about prioritizing immunization for spouses and family members of healthcare workers. “My husband wants to know that too,” replied Patricia A. Stinchfield, APRN, CNP, pediatric nurse practitioner in infectious diseases at Children’s Minnesota, St. Paul.
“It is true we should be thinking about our healthcare workers’ family members. But at this point in time we just don’t have the supplies to address it that way,” said Stinchfield, who is also the president-elect of the National Foundation for Infectious Diseases.
Advantages beyond the numbers?
“The major advantage of having two vaccines is sheer volume,” Mehta said. An additional advantage of more than one product is the potential to offer an option when a specific vaccine is contraindicated. “We could offer someone a different vaccine…similar to what we do with the influenza vaccine.”
“The more the merrier in terms of having more vaccine products,” Schrantz said. Despite differences in shipping, storage, minimum age requirements, and dosing intervals, the Pfizer and Moderna vaccines are very similar, he said. “Really the only difference between these two vaccines is the proprietary lipid nanoparticle — the delivery vehicle if you will.”
Both vaccines “appear very similar in their capacity to protect against disease, to protect [people in] various racial and ethnic backgrounds, and in their capacity to protect against severe disease,” Offit said.
In terms of vaccines in the development pipeline, “We don’t know but we might start to see a difference with the Johnson & Johnson vaccine or the Janssen vaccine, which are single dose. They might confer some advantages, but we are waiting on the safety and efficacy data,” Schrantz said.
As a two-dose vaccine, the AstraZeneca product does not offer an advantage on the dosing strategy, “but it is easier to transport than the mRNA vaccines,” he said. Some concerns with the initial data on the AstraZeneca vaccine will likely need to be addressed before the company applies for an EUA, Schrantz added.
“That is an important question,” El Sahly said. The ongoing studies should provide more data from participants of all ages and ethnic backgrounds that “will allow us to make a determination as to whether there is any difference between these two vaccines.
She added that the Pfizer and Moderna vaccines seem comparable from the early data. “We’ll see if this stands in the long run.”
Future outlook
Now that the FDA approved emergency use of two COVID-19 vaccines, “we need each state to quickly implement their plans to get the vaccines into the hands of providers who need to give the vaccines,” Mehta said. “We are seeing very effective rollout in multiple regions of the country. And we hope to see that continue as we get more vaccines from manufacturers over the coming months.”
“Within a year of identifying the sequence of this virus we have two large clinical vaccine trials that show efficacy,” Offit said. “That was an amazing technologic accomplishment, but now comes the hard part. Mass producing this vaccine, getting it out there, making sure everybody who most benefits gets it, is going to be really, really hard.”
“But I’m optimistic,” Offit said. “If we can do this by next Thanksgiving, we’re going to see a dramatic drop in the number of cases, hospitalizations and deaths, and we can get our lives back together again.”
“My greatest hope is that a year from now we look back and realize we did something really amazing together,” Bailey said, “and we have a feeling of accomplishment and appreciation for all the hard work that has been done.”
Mehta shared the important message he shares when walking around the hospital: “While these vaccines are coming and they are very promising, we need to continue to remember the 3 Ws: wearing a mask, washing your hands, and watching your distance,” he said.
“With the combination of those 3Ws and those vaccines, we will hopefully come through this COVID pandemic.”
El Sahly receives funding through the NIH for her research, including her role as co-chair of the Moderna vaccine phase 3 clinical trial. Schrantz is a site investigator for the Moderna and Janssen vaccine trials. Mehta also receives funding through the NIH. None of these experts had any relevant financial disclosures.
This article first appeared on Medscape.com.
As expected, the US Food and Drug Administration granted Moderna an emergency use authorization (EUA) for its messenger RNA COVID-19 vaccine December 18.
There is one final step — the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices will need to recommend its use, as it did 2 days after the Pfizer/BioNTech mRNA vaccine received its EUA on December 10.
The EUA for the Moderna vaccine is “a major milestone in trying to contain this pandemic,” Hana Mohammed El Sahly, MD, told Medscape Medical News.
Scaling up distribution of the two vaccine products will come next. She notes that even under less emergent conditions, making sure people who need a vaccine receive it can be hard. “I hope the media attention around this will make more people aware that there are vaccines that might help them,” said El Sahly, chair of the FDA Vaccines and Related Biological Products Advisory Committee (VRBPAC).
The EUA for the Moderna vaccine follows a review by the independent VRBPAC members on December 17, which voted 20-0 with one abstention to recommend the EUA. The vaccine is authorized for use in people 18 and older.
Emergency approval of a second COVID-19 vaccine “is great — we need all the tools we can to fight this pandemic,” Stephen Schrantz, MD, infectious disease specialist and assistant professor of medicine at the University of Chicago, told Medscape Medical News. “The early data coming from Moderna looks good, and I agree with the FDA that an EUA is indicated.
“It’s incumbent upon all us healthcare professionals to put ourselves out there as supporting this vaccine and supporting people getting it,” Schrantz continued. “We want to make sure people who are on the fence understand this is a safe vaccine that has been vetted appropriately through the FDA and through phase 3 clinical trials.”
“I know the critical role physicians play as vaccine influencers,” AMA President Susan Bailey, MD, said during a December 14 webinar for journalists reporting on COVID-19 vaccines. “We have to continue to do what physicians have always done: review the evidence and trust the science. Lives are at stake.” The webinar was cosponsored by the AMA and the Poynter Institute.
Ramping up healthcare provider immunizations
“I am very excited to see the FDA’s positive review of the Moderna vaccine. We have been waiting to have another vaccine we can use for healthcare workers and staff, and now we have it,” Aneesh Mehta, MD, of Emory University School of Medicine in Atlanta, Georgia, told Medscape Medical News.
“We had been hoping for a vaccine with a 70% or 80% efficacy, and to see two vaccines now with greater than 90% efficacy is remarkable,” he added.
The efficacy levels associated with both mRNA vaccines “did exceed expectations for sure — this is not what we built the studies around. It was surprising in the good sense of the word,” said El Sahly, who is also associate professor of molecular virology and microbiology at Baylor College of Medicine in Houston, Texas.
Unanswered questions remain
Schrantz likewise said the high efficacy rate was important but not all that is needed. “[W]hat we know about this vaccine is it is very effective at preventing disease. We don’t have any understanding at this time whether or not these vaccines prevent infection and transmissibility.”
Bailey said, “The jury is still out on whether or not you can still transmit the virus after you’ve had the vaccine. Hopefully not, but we don’t really know that for sure.”
“It’s risky to think that once you get the shot in your arm everything goes back to normal. It doesn’t,” Bailey added.
Another unknown is the duration of protection following immunization. The Pfizer and Moderna products “have similar constructs, seem to have a reasonable safety profile, and excellent short-term efficacy,” El Sahly said. She cautioned, however, that long-term efficacy still needs to be determined.
Whether any rare adverse events will emerge in the long run is another question. Answers could come over time from the ongoing phase 3 trials, as well as from post-EUA surveillance among vaccine recipients.
“Our work is not done after issuing an EUA,” FDA Commissioner Stephen Hahn, MD, said in a JAMA webinar on December 14. The FDA is closely monitoring for any adverse event rates above the normal background incidence. “We are going to be transparent about it if we are seeing anything that is not at base level.”
“The key is to be humble, keep your eyes open and know that once the vaccine is out there, there may be things we learn that we don’t know now. That is true for virtually any medical innovation,” Paul Offit, MD, director of The Vaccine Education Center at Children’s Hospital of Philadelphia and a member of the FDA VRBPAC, said during the AMA/Poynter Institute webinar.
During the same webinar, an attendee asked about prioritizing immunization for spouses and family members of healthcare workers. “My husband wants to know that too,” replied Patricia A. Stinchfield, APRN, CNP, pediatric nurse practitioner in infectious diseases at Children’s Minnesota, St. Paul.
“It is true we should be thinking about our healthcare workers’ family members. But at this point in time we just don’t have the supplies to address it that way,” said Stinchfield, who is also the president-elect of the National Foundation for Infectious Diseases.
Advantages beyond the numbers?
“The major advantage of having two vaccines is sheer volume,” Mehta said. An additional advantage of more than one product is the potential to offer an option when a specific vaccine is contraindicated. “We could offer someone a different vaccine…similar to what we do with the influenza vaccine.”
“The more the merrier in terms of having more vaccine products,” Schrantz said. Despite differences in shipping, storage, minimum age requirements, and dosing intervals, the Pfizer and Moderna vaccines are very similar, he said. “Really the only difference between these two vaccines is the proprietary lipid nanoparticle — the delivery vehicle if you will.”
Both vaccines “appear very similar in their capacity to protect against disease, to protect [people in] various racial and ethnic backgrounds, and in their capacity to protect against severe disease,” Offit said.
In terms of vaccines in the development pipeline, “We don’t know but we might start to see a difference with the Johnson & Johnson vaccine or the Janssen vaccine, which are single dose. They might confer some advantages, but we are waiting on the safety and efficacy data,” Schrantz said.
As a two-dose vaccine, the AstraZeneca product does not offer an advantage on the dosing strategy, “but it is easier to transport than the mRNA vaccines,” he said. Some concerns with the initial data on the AstraZeneca vaccine will likely need to be addressed before the company applies for an EUA, Schrantz added.
“That is an important question,” El Sahly said. The ongoing studies should provide more data from participants of all ages and ethnic backgrounds that “will allow us to make a determination as to whether there is any difference between these two vaccines.
She added that the Pfizer and Moderna vaccines seem comparable from the early data. “We’ll see if this stands in the long run.”
Future outlook
Now that the FDA approved emergency use of two COVID-19 vaccines, “we need each state to quickly implement their plans to get the vaccines into the hands of providers who need to give the vaccines,” Mehta said. “We are seeing very effective rollout in multiple regions of the country. And we hope to see that continue as we get more vaccines from manufacturers over the coming months.”
“Within a year of identifying the sequence of this virus we have two large clinical vaccine trials that show efficacy,” Offit said. “That was an amazing technologic accomplishment, but now comes the hard part. Mass producing this vaccine, getting it out there, making sure everybody who most benefits gets it, is going to be really, really hard.”
“But I’m optimistic,” Offit said. “If we can do this by next Thanksgiving, we’re going to see a dramatic drop in the number of cases, hospitalizations and deaths, and we can get our lives back together again.”
“My greatest hope is that a year from now we look back and realize we did something really amazing together,” Bailey said, “and we have a feeling of accomplishment and appreciation for all the hard work that has been done.”
Mehta shared the important message he shares when walking around the hospital: “While these vaccines are coming and they are very promising, we need to continue to remember the 3 Ws: wearing a mask, washing your hands, and watching your distance,” he said.
“With the combination of those 3Ws and those vaccines, we will hopefully come through this COVID pandemic.”
El Sahly receives funding through the NIH for her research, including her role as co-chair of the Moderna vaccine phase 3 clinical trial. Schrantz is a site investigator for the Moderna and Janssen vaccine trials. Mehta also receives funding through the NIH. None of these experts had any relevant financial disclosures.
This article first appeared on Medscape.com.
Moderna COVID-19 vaccine wins decisive recommendation from FDA panel
The US Food and Drug Administration (FDA) put Moderna’s application before its Vaccines and Related Biological Products Advisory Committee. The panel voted 20-0 on this question: “Based on the totality of scientific evidence available, do the benefits of the Moderna COVID-19 Vaccine outweigh its risks for use in individuals 18 years of age and older?” There was one abstention.
The FDA is not bound to act on the recommendations of its advisers, but the agency usually takes the panel’s advice. The FDA cleared the similar Pfizer-BioNTech vaccine on December 11 through an emergency use authorization (EUA), following a positive vote for the product at a December 10 advisory committee meeting. In this case, the FDA staff appeared to be pushing for a broad endorsement of the Moderna vaccine, for which the agency appears likely to soon also grant an EUA.
Marion Gruber, PhD, director of the Office of Vaccines Research and Review at FDA’s Center for Biologics Evaluation and Research, earlier rebuffed attempts by some of the panelists to alter the voting question. Some panelists wanted to make tweaks, including a rephrasing to underscore the limited nature of an EUA, compared with a more complete approval through the biologics license application (BLA) process.
FDA panelist Michael Kurilla, MD, PhD, of the National Institutes of Health was the only panelist to abstain from voting. He said he was uncomfortable with the phrasing of the question.
“In the midst of a pandemic and with limited vaccine supply available, a blanket statement for individuals 18 years and older is just too broad,” he said. “I’m not convinced that for all of those age groups the benefits do actually outweigh the risks.”
In general, though, there was strong support for Moderna’s vaccine. FDA panelist James Hildreth Sr, MD, PhD, of Meharry Medical College in Nashville, Tennessee spoke of the “remarkable achievement” seen in having two vaccines ready for clearance by December for a virus that only emerged as a threat this year.
Study data indicate the primary efficacy endpoint demonstrated vaccine efficacy (VE) of 94.1% (95% CI, 89.3% - 96.8%) for the Moderna vaccine, with 11 COVID-19 cases in the vaccine group and 185 COVID-19 cases in the placebo group, the FDA staff noted during the meeting.
The advisers and FDA staff also honed in on several key issues with COVID-19 vaccines, including the challenge of having people in the placebo groups of studies seek to get cleared vaccines. Also of concern to the panel were early reports of allergic reactions seen with the Pfizer product.
Doran L. Fink, MD, PhD, an FDA official who has been closely involved with the COVID-19 vaccines, told the panel that two healthcare workers in Alaska had allergic reactions minutes after receiving the Pfizer vaccine, one of which was a case of anaphylactic reaction that resulted in hospitalization.
In the United Kingdom, there were two cases reported of notable allergic reactions, leading regulators there to issue a warning that people who have a history of significant allergic reactions should not currently receive the Pfizer-BioNTech vaccine.
The people involved in these incidents have recovered or are recovering, Fink said. But the FDA expects there will be additional reports of allergic reactions to COVID-19 vaccines.
“These cases underscores the need to remain vigilant during the early phase of the vaccination campaign,” Fink said. “To this end, FDA is working with Pfizer to further revise factsheets and prescribing information for their vaccine to draw attention to CDC guidelines for post- vaccination monitoring and management of immediate allergic reactions.”
mRNA vaccines in the lead
An FDA emergency clearance for Moderna’s product would be another vote of confidence in a new approach to making vaccines. Both the Pfizer-BioNTech and Moderna vaccines provide the immune system with a kind of blueprint in the form of genetic material, mRNA. The mRNA sets the stage for the synthesis of the signature spike protein that the SARS-CoV-2 virus uses to attach to and infect human cells.
In a December 15 commentary for this news organization Michael E. Pichichero, MD, wrote that the “revolutionary aspect of mRNA vaccines is the speed at which they can be designed and produced.”
“This is why they lead the pack among the SARS-CoV-2 vaccine candidates and why the National Institute of Allergy and Infectious Diseases provided financial, technical, and/or clinical support. Indeed, once the amino acid sequence of a protein can be determined (a relatively easy task these days) it’s straightforward to synthesize mRNA in the lab — and it can be done incredibly fast,” he wrote.
The FDA allowed one waiver for panelist James K. Hildreth in connection with his personal relationship to a trial participant and his university’s participation in vaccine testing.
This article first appeared on Medscape.com.
The US Food and Drug Administration (FDA) put Moderna’s application before its Vaccines and Related Biological Products Advisory Committee. The panel voted 20-0 on this question: “Based on the totality of scientific evidence available, do the benefits of the Moderna COVID-19 Vaccine outweigh its risks for use in individuals 18 years of age and older?” There was one abstention.
The FDA is not bound to act on the recommendations of its advisers, but the agency usually takes the panel’s advice. The FDA cleared the similar Pfizer-BioNTech vaccine on December 11 through an emergency use authorization (EUA), following a positive vote for the product at a December 10 advisory committee meeting. In this case, the FDA staff appeared to be pushing for a broad endorsement of the Moderna vaccine, for which the agency appears likely to soon also grant an EUA.
Marion Gruber, PhD, director of the Office of Vaccines Research and Review at FDA’s Center for Biologics Evaluation and Research, earlier rebuffed attempts by some of the panelists to alter the voting question. Some panelists wanted to make tweaks, including a rephrasing to underscore the limited nature of an EUA, compared with a more complete approval through the biologics license application (BLA) process.
FDA panelist Michael Kurilla, MD, PhD, of the National Institutes of Health was the only panelist to abstain from voting. He said he was uncomfortable with the phrasing of the question.
“In the midst of a pandemic and with limited vaccine supply available, a blanket statement for individuals 18 years and older is just too broad,” he said. “I’m not convinced that for all of those age groups the benefits do actually outweigh the risks.”
In general, though, there was strong support for Moderna’s vaccine. FDA panelist James Hildreth Sr, MD, PhD, of Meharry Medical College in Nashville, Tennessee spoke of the “remarkable achievement” seen in having two vaccines ready for clearance by December for a virus that only emerged as a threat this year.
Study data indicate the primary efficacy endpoint demonstrated vaccine efficacy (VE) of 94.1% (95% CI, 89.3% - 96.8%) for the Moderna vaccine, with 11 COVID-19 cases in the vaccine group and 185 COVID-19 cases in the placebo group, the FDA staff noted during the meeting.
The advisers and FDA staff also honed in on several key issues with COVID-19 vaccines, including the challenge of having people in the placebo groups of studies seek to get cleared vaccines. Also of concern to the panel were early reports of allergic reactions seen with the Pfizer product.
Doran L. Fink, MD, PhD, an FDA official who has been closely involved with the COVID-19 vaccines, told the panel that two healthcare workers in Alaska had allergic reactions minutes after receiving the Pfizer vaccine, one of which was a case of anaphylactic reaction that resulted in hospitalization.
In the United Kingdom, there were two cases reported of notable allergic reactions, leading regulators there to issue a warning that people who have a history of significant allergic reactions should not currently receive the Pfizer-BioNTech vaccine.
The people involved in these incidents have recovered or are recovering, Fink said. But the FDA expects there will be additional reports of allergic reactions to COVID-19 vaccines.
“These cases underscores the need to remain vigilant during the early phase of the vaccination campaign,” Fink said. “To this end, FDA is working with Pfizer to further revise factsheets and prescribing information for their vaccine to draw attention to CDC guidelines for post- vaccination monitoring and management of immediate allergic reactions.”
mRNA vaccines in the lead
An FDA emergency clearance for Moderna’s product would be another vote of confidence in a new approach to making vaccines. Both the Pfizer-BioNTech and Moderna vaccines provide the immune system with a kind of blueprint in the form of genetic material, mRNA. The mRNA sets the stage for the synthesis of the signature spike protein that the SARS-CoV-2 virus uses to attach to and infect human cells.
In a December 15 commentary for this news organization Michael E. Pichichero, MD, wrote that the “revolutionary aspect of mRNA vaccines is the speed at which they can be designed and produced.”
“This is why they lead the pack among the SARS-CoV-2 vaccine candidates and why the National Institute of Allergy and Infectious Diseases provided financial, technical, and/or clinical support. Indeed, once the amino acid sequence of a protein can be determined (a relatively easy task these days) it’s straightforward to synthesize mRNA in the lab — and it can be done incredibly fast,” he wrote.
The FDA allowed one waiver for panelist James K. Hildreth in connection with his personal relationship to a trial participant and his university’s participation in vaccine testing.
This article first appeared on Medscape.com.
The US Food and Drug Administration (FDA) put Moderna’s application before its Vaccines and Related Biological Products Advisory Committee. The panel voted 20-0 on this question: “Based on the totality of scientific evidence available, do the benefits of the Moderna COVID-19 Vaccine outweigh its risks for use in individuals 18 years of age and older?” There was one abstention.
The FDA is not bound to act on the recommendations of its advisers, but the agency usually takes the panel’s advice. The FDA cleared the similar Pfizer-BioNTech vaccine on December 11 through an emergency use authorization (EUA), following a positive vote for the product at a December 10 advisory committee meeting. In this case, the FDA staff appeared to be pushing for a broad endorsement of the Moderna vaccine, for which the agency appears likely to soon also grant an EUA.
Marion Gruber, PhD, director of the Office of Vaccines Research and Review at FDA’s Center for Biologics Evaluation and Research, earlier rebuffed attempts by some of the panelists to alter the voting question. Some panelists wanted to make tweaks, including a rephrasing to underscore the limited nature of an EUA, compared with a more complete approval through the biologics license application (BLA) process.
FDA panelist Michael Kurilla, MD, PhD, of the National Institutes of Health was the only panelist to abstain from voting. He said he was uncomfortable with the phrasing of the question.
“In the midst of a pandemic and with limited vaccine supply available, a blanket statement for individuals 18 years and older is just too broad,” he said. “I’m not convinced that for all of those age groups the benefits do actually outweigh the risks.”
In general, though, there was strong support for Moderna’s vaccine. FDA panelist James Hildreth Sr, MD, PhD, of Meharry Medical College in Nashville, Tennessee spoke of the “remarkable achievement” seen in having two vaccines ready for clearance by December for a virus that only emerged as a threat this year.
Study data indicate the primary efficacy endpoint demonstrated vaccine efficacy (VE) of 94.1% (95% CI, 89.3% - 96.8%) for the Moderna vaccine, with 11 COVID-19 cases in the vaccine group and 185 COVID-19 cases in the placebo group, the FDA staff noted during the meeting.
The advisers and FDA staff also honed in on several key issues with COVID-19 vaccines, including the challenge of having people in the placebo groups of studies seek to get cleared vaccines. Also of concern to the panel were early reports of allergic reactions seen with the Pfizer product.
Doran L. Fink, MD, PhD, an FDA official who has been closely involved with the COVID-19 vaccines, told the panel that two healthcare workers in Alaska had allergic reactions minutes after receiving the Pfizer vaccine, one of which was a case of anaphylactic reaction that resulted in hospitalization.
In the United Kingdom, there were two cases reported of notable allergic reactions, leading regulators there to issue a warning that people who have a history of significant allergic reactions should not currently receive the Pfizer-BioNTech vaccine.
The people involved in these incidents have recovered or are recovering, Fink said. But the FDA expects there will be additional reports of allergic reactions to COVID-19 vaccines.
“These cases underscores the need to remain vigilant during the early phase of the vaccination campaign,” Fink said. “To this end, FDA is working with Pfizer to further revise factsheets and prescribing information for their vaccine to draw attention to CDC guidelines for post- vaccination monitoring and management of immediate allergic reactions.”
mRNA vaccines in the lead
An FDA emergency clearance for Moderna’s product would be another vote of confidence in a new approach to making vaccines. Both the Pfizer-BioNTech and Moderna vaccines provide the immune system with a kind of blueprint in the form of genetic material, mRNA. The mRNA sets the stage for the synthesis of the signature spike protein that the SARS-CoV-2 virus uses to attach to and infect human cells.
In a December 15 commentary for this news organization Michael E. Pichichero, MD, wrote that the “revolutionary aspect of mRNA vaccines is the speed at which they can be designed and produced.”
“This is why they lead the pack among the SARS-CoV-2 vaccine candidates and why the National Institute of Allergy and Infectious Diseases provided financial, technical, and/or clinical support. Indeed, once the amino acid sequence of a protein can be determined (a relatively easy task these days) it’s straightforward to synthesize mRNA in the lab — and it can be done incredibly fast,” he wrote.
The FDA allowed one waiver for panelist James K. Hildreth in connection with his personal relationship to a trial participant and his university’s participation in vaccine testing.
This article first appeared on Medscape.com.
A new model of care to return holism to family medicine
Here is our problem: Family medicine has allowed itself, and its patients, to be picked apart by the forces of reductionism and a system that profits from the sick and suffering. We have lost sight of our purpose and our vision to care for the whole person. We have lost our way as healers.
The result is not only a decline in the specialty of family medicine as a leader in primary care but declining value and worsening outcomes in health care overall. We need to get our mojo back. We can do this by focusing less on trying to be all things to all people at all times, and more on creating better models for preventing, managing, and reversing chronic disease. This means providing health care that is person centered, relationship based, recovery focused, and paid for comprehensively.
I call this model Advanced Primary Care, or APC (FIGURE). In this article, I describe exemplars of APC from across the United States. I also provide tools to help you recover its central feature, holism—care of the whole person in mind, body, community, and spirit—in your practice, thus returning us to the core purpose of family medicine.
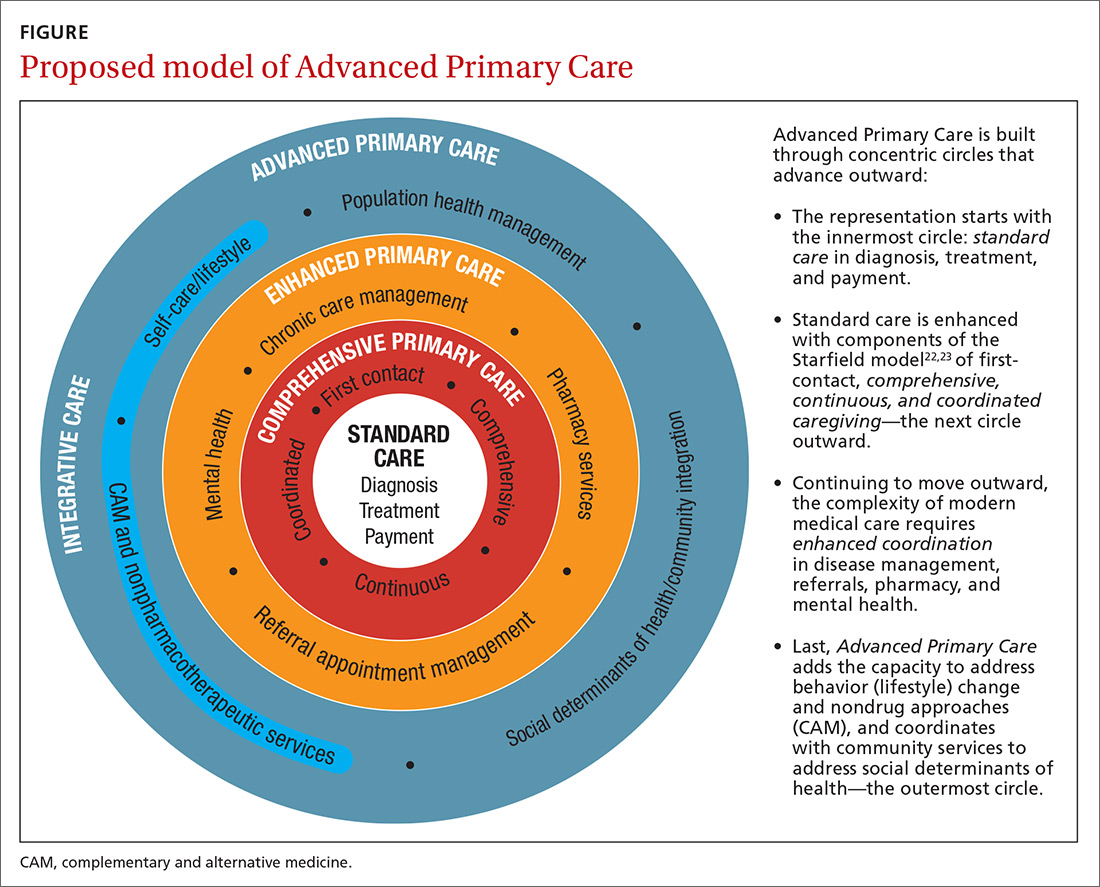
Holism is central to family medicine
More than 40 years ago, psychiatrist George Engel, MD, published a seminal article in Science that inspired a radical vision of how health care should be practiced.1 Called the biopsychosocial model, it stated what, in some ways, is obvious: Human beings are complex organisms embedded in complex environments made up of distinct, yet interacting, dimensions. These dimensions included physical, psychological, and social components. Engel’s radical proposition was that these dimensions are definable and measurable and that good medicine cannot afford to ignore any of them.
Engel’s assertion that good medicine requires holism was a clarion call during a time of rapidly expanding knowledge and subspecialization. That call was the inspiration for a new medical specialty called family medicine, which dared to proclaim that the best way to heal was to care for the whole person within the context of that person’s emotional and social environment. Family medicine reinvigorated primary care and grew rapidly, becoming a preeminent primary care specialty in the United States.
Continue to : Reductionism is relentless
Reductionism is relentless
But the forces of medicine were—and still are—driving relentlessly the other way. The science of the small and particular (reductionism), with dazzling technology and exploding subspecialty knowledge, and backed by powerful economic drivers, rewards health care for pulling the patient and the medical profession apart. We pay more to those who treat small parts of a person over a short period than to those who attend to the whole person over the lifetime.
Today, family medicine—for all of its common sense, scientific soundness, connectedness to patients, and demonstrated value—struggles to survive.2-6 The holistic vision of Engel is declining. The struggle in primary care is that its holistic vision gets co-opted by specialized medical science—and then it desperately attempts to apply those small and specialized tools to the care of patients in their wholeness. Holism is largely dead in health care, and everyone pays the consequences.7
Health care is losing its value
The damage from this decline in holism is not just to primary care but to the value of health care in general. Most medical care being delivered today—comprising diagnosis, treatment, and payment (the innermost circle of the FIGURE)—is not producing good health.8 Only 15% to 20% of the healing of an individual or a population comes from health care.9 The rest—nearly 80%—comes from other factors rarely addressed in the health care system: behavioral and lifestyle choices that people make in their daily life, including those related to food, movement, sleep, stress, and substance use.10 Increasingly, it is the economic and social determinants of health that influence this behavior and have a greater impact on health and lifespan than physiology or genes.11 The same social determinants of health also influence patients’ ability to obtain medical care and pursue a meaningful life.12
The result of this decline in holism and in the value of health care in general has been a relentless rise in the cost of medical care13-15 and the need for social services; declining life expectancy16,17 and quality of life18; growing patient dissatisfaction; and burnout in providers.19,20 Health care has become, as investor and business leader Warren Buffet remarked, the “tapeworm” of the economy and a major contributor to growing disparities in health and well-being between the haves and have-nots.21 Engel’s prediction that good medicine cannot afford to ignore holism has come to pass.
3-step solution:Return to whole-person care
Family medicine needs to return to whole-person care, but it can do so only if it attends to, and effectively delivers on, the prevention, treatment, and reversal of chronic disease and the enhancement of health and well-being. This can happen only if family medicine stops trying to be all things to all people at all times and, instead, focuses on what matters to the patient as a person.
Continue to: This means that the core...
This means that the core interaction in family medicine must be to assess the whole person—mind, body, social, spirit—and help that person make changes that improve his/her/their health and well-being based on his/her/their individualized needs and social context. In other words, family medicine needs to deliver a holistic model of APC that is person centered, relationship based, recovery focused, and paid for comprehensively.
How does one get from “standard” primary care of today (the innermost circle of the FIGURE) to a framework that truly delivers on the promise of healing? I propose 3 steps to return holism to family medicine.
STEP 1: Start with comprehensive, coordinated primary care. We know that this works. Starfield and others demonstrated this 2 decades ago, defining and devising what we know as quality primary care—characterized by first-contact care, comprehensive primary care (CPC), continuous care, and coordinated care.22 This type of primary care improves outcomes, lowers costs, and is satisfying to patients and providers.23 The physician cares for the patient throughout that person’s entire life cycle and provides all evidence-based services needed to prevent and treat common conditions. Comprehensive primary care is positioned in the first circle outward from the innermost circle of the FIGURE.
As medicine has become increasingly complex and subspecialized, however, the ability to coordinate care is often frayed, adding cost and reducing quality.24-26 Today, comprehensive primary care needs enhanced coordination. At a minimum, this means coordinating services for:
- chronic disease management (outpatient and inpatient transitions and emergency department use)
- referral (specialists and tests)
- pharmacy services (including delivery and patient education support).
An example of a primary care system that meets these requirements is the Catalyst Health Network in central Texas, which supplies coordination services to more than 1000 comprehensive primary care practices and 1.5 million patients.27 The Catalyst Network makes money for those practices, saves money in the system, enhances patient and provider satisfaction, and improves population health in the community.27 I call this enhanced primary care (EPC), shown in the second circle out from the innermost circle of the FIGURE.
STEP 2: Add integrative medicine and mental health. EPC improves fragmented care but does not necessarily address a patient’s underlying determinants of healing. We know that health behaviors such as smoking cessation, avoidance of alcohol and drug abuse, improved diet, physical activity, sleep, and stress management contribute 40% to 60% of a person’s and a population’s health.10 In addition, evidence shows that behavioral health services, along with lifestyle change support, can even reverse many chronic diseases seen in primary care, such as obesity, diabetes, hypertension, cardiovascular disease, depression, and substance abuse.28,29
Continue to: Therefore, we need to add...
Therefore, we need to add routine mental health services and nonpharmacotherapeutic approaches (eg, complementary and alternative medicine) to primary care.30 Doing so requires that behavioral change and self-care become a central feature of the doctor–patient dialogue and team skills31 and be added to primary care.30,31 I call this integrative primary care (IPC), shown on the left side in the third circle out from the innermost circle of the FIGURE.
An example of IPC is Whole Health, an initiative of the US Veteran’s Health Administration. Whole Health empowers and informs a person-centered approach and integrates it into the delivery of routine care.32 Evaluation of Whole Health implementation, which involved more than 130,000 veterans followed for 2 years, found a net overall reduction in the total cost of care of 20%—saving nearly $650 million or, on average, more than $4500 per veteran.33
STEP 3: Address social determinants of health. Primary care will not fully be part of the solution for producing health and well-being unless it becomes instrumental in addressing the social determinants of health (SDH), defined as “… conditions in the environments in which people are born, live, learn, work, play, worship, and age that affect a wide range of health, functioning, and quality-of-life outcomes and risks.”34 These determinants include not only basic needs, such as housing, food, safety, and transportation (ie, social needs), but also what are known as structural determinants, such as income, education, language, and racial and ethnic bias. Health care cannot solve all of these social ills,but it is increasingly being called on to be the nexus of coordination for services that address these needs when they affect health outcomes.35,36
Examples of health systems that provide for social needs include the free “food prescription” program of Pennsylvania’s Geisinger Health System, for patients with diabetes who do not have the resources to pay for food.37 This approach improves blood glucose control by patients and saves money on medications and other interventions. Similarly, Kaiser Permanente has experimented with housing vouchers for homeless patients,and most Federally Qualified Health Centers provide bus or other transportation tickets to patients for their appointments and free or discounted tests and specialty care.38
Implementing whole-person care for all
I propose that we make APC the central focus of family medicine. This model would comprise CPC, plus EPC, IPC, and community coordination to address SDH. This is expressed as:
CPC + EPC + IPC + SDH = APC
Continue to: APC would mean...
APC would mean health for the whole person and for all people. Again, the FIGURE shows how this model, encompassing the entire third circle out from the center circle, could be created from current models of care.
How do we pay for this? We already do—and way too much. The problem is not lack of money in the health care system but how it is organized and distributed. The Centers for Medicare and Medicaid Services and other payers are developing value-based payment models to help cover this type of care,39 but payers cannot pay for something if it is unavailable.
Can family physicians deliver APC? I believe they can, and have given a few examples here to show how this is already happening. To help primary care providers start to deliver APC in their system, my team and I have built the HOPE (Healing Oriented Practices & Environments) Note Toolkit to use in daily practice.40 These and other tools are being used by a number of large hospital systems and health care networks around the country. (You can download the HOPE Note Toolkit, at no cost, at https://drwaynejonas.com/resources/hope-note/.)
Whatever we call this new type of primary care, it needs to care for the whole person and to be available to all. It finds expression in these assertions:
- We cannot ignore an essential part of what a human being is and expect them to heal or become whole.
- We cannot ignore essential people in our communities and expect our costs to go down or our compassion to go up.
- We need to stop allowing family medicine to be co-opted by reductionism and its profits.
In sum, we need a new vision of primary care—like Engel’s holistic vision in the 1970s—to motivate us, and we need to return to fundamental concepts of how healing works in medicine.41
CORRESPONDENCE
Wayne B. Jonas, MD, Samueli Integrative Health Programs, 1800 Diagonal Road, Suite 617, Alexandria, VA 22314; [email protected].
1. Engel GL. The need for a new medical model: a challenge for biomedicine. Science. 1977;196:129-136.
2. Schwartz MD, Durning S, Linzer M, et al. Changes in medical students’ views of internal medicine careers from 1990 to 2007. Arch Intern Med. 2011;171:744-749.
3. Bronchetti ET, Christensen GS, Hoynes HW. Local food prices, SNAP purchasing power, and child health. Cambridge, MA: National Bureau of Economic Research. June 2018. www.nber.org/papers/w24762?mc_cid=8c7211d34b&mc_eid=fbbc7df813. Accessed November 24, 2020.
4. Federal Student Aid, US Department of Education. Public Service Loan Forgiveness (PSLF). 2018. https://studentaid.ed.gov/sa/repay-loans/forgiveness-cancellation/public-service. Accessed November 24, 2020.
5. Aten B, Figueroa E, Martin T. Notes on estimating the multi-year regional price parities by 16 expenditure categories: 2005-2009. WP2011-03. Washington, DC: Bureau of Economic Analysis, US Department of Commerce; April 2011. www.bea.gov/system/files/papers/WP2011-3.pdf. Accessed November 24, 2020.
6. Aten BH, Figueroa EB, Martin TM. Regional price parities for states and metropolitan areas, 2006-2010. Washington, DC: Bureau of Economic Analysis, US Department of Commerce; August 2012. https://apps.bea.gov/scb/pdf/2012/08%20August/0812_regional_price_parities.pdf. Accessed November 24, 2020.
7. Stange KC, Ferrer RL. The paradox of primary care. Ann Fam Med. 2009;7:293-299.
8. Panel on Understanding Cross-national Health Differences Among High-income Countries, Committee on Population, Division of Behavioral and Social Sciences and Education, and Board on Population Health and Public Health Practice, National Research Council and Institute of Medicine of the National Academies. US Health in International Perspective: Shorter Lives, Poorer Health. Woolf SH, Aron L, eds. The National Academies Press; 2013.
9. Hood CM, Gennuso KP, Swain GR, et al. County health rankings: relationships between determinant factors and health outcomes. Am J Prev Med. 2016;50:129-135.
10. McGinnis JM, Williams-Russo P, Knickman JR. The case for more active policy attention to health promotion. Health Aff (Millwood). 2002;21:78-93.
11. Roeder A. Zip code better predictor of health than genetic code. Harvard T. H. Chan School of Public Health Web site. News release. August 4, 2014. www.hsph.harvard.edu/news/features/zip-code-better-predictor-of-health-than-genetic-code/. Accessed November 24, 2020.

12. US health map. Seattle, WA: University of Washington Institute for Health Metrics and Evaluation; March 13, 2018. www.healthdata.org/data-visualization/us-health-map. Accessed November 24, 2020.
13. Highfill T. Comparing estimates of U.S. health care expenditures by medical condition, 2000-2012. Survey of Current Business. 2016;1-5. https://apps.bea.gov/scb/pdf/2016/3%20March/0316_comparing_u.s._health_care_expenditures_by_medical_condition.pdf. Accessed November 24, 2020.
14. Waters H, Graf M. The Costs of Chronic Disease in the US. Washington, DC: Milken Institute; August 2018. https://milkeninstitute.org/sites/default/files/reports-pdf/ChronicDiseases-HighRes-FINAL.pdf. Accessed November 24, 2020.
15. Meyer H. Health care spending will hit 19.4% of GDP in the next decade, CMS projects. Modern Health care. February 20, 2019. www.modernhealthcare.com/article/20190220/NEWS/190229989/healthcare-spending-will-hit-19-4-of-gdp-in-the-next-decade-cms-projects. Accessed November 24, 2020.
16. Woolf SH, Schoomaker H. Life expectancy and mortality rates in the United States, 1959-2017. JAMA. 2019;322:1996-2016.
17. Basu S, Berkowitz SA, Phillips RL, et al. Association of primary care physician supply with population mortality in the United States, 2005-2015. JAMA Intern Med. 2019;179:506-514.
18. Zack MM, Moriarty DG, Stroup DF, et al. Worsening trends in adult health-related quality of life and self-rated health—United States, 1993–2001. Public Health Rep. 2004;119:493-505.
19. Windover AK, Martinez K, Mercer, MB, et al. Correlates and outcomes of physician burnout within a large academic medical center. Research letter. JAMA Intern Med. 2018;178:856-858.
20. West CP, Dyrbye LN, Shanafelt TD. Physician burnout: contributors, consequences and solutions. J Intern Med. 2018;283:516-529.
21. Buffett: Health care is a tapeworm on the economic system. CNBC Squawk Box. February 26, 2018. www.cnbc.com/video/2018/02/26/buffett-health-care-is-a-tapeworm-on-the-economic-system.html. Accessed November 24, 2020.
22. Starfield B. Primary Care: Concept, Evaluation, and Policy. Oxford University Press; 1992.
23. Starfield B, Shi L, Macinko J. Contribution of primary care to health systems and health. Milbank Q. 2005;83:457-502.
24. Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. National Academies Press (US); 2001.
25. Burton R. Health policy brief: improving care transitions. Health Affairs. September 13, 2012. www.healthaffairs.org/do/10.1377/hpb20120913.327236/full/healthpolicybrief_76.pdf. Accessed November 24, 2020.
26. Toulany A, Stukel TA, Kurdyak P, et al. Association of primary care continuity with outcomes following transition to adult care for adolescents with severe mental illness. JAMA Netw Open. 2019;2:e198415.
27. Helping communities thrive. Catalyst Health Network Web site. www.catalysthealthnetwork.com/. Accessed November 24, 2020.
28. Diabetes Prevention Program (DPP) Research Group. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25:2165-2171.
29. Scherger JE. Lean and Fit: A Doctor’s Journey to Healthy Nutrition and Greater Wellness. 2nd ed. Scotts Valley, CA: CreateSpace Publishing; 2016.
30. Qaseem A, Wilt TJ, McLean RM, et al; . Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:514-530.
31. Hibbard JH, Greene J. What the evidence shows about patient activation: better health outcomes and care experiences; fewer data on costs. Health Aff (Millwood). 2013;32:207-214.
32. What is whole health? Washington, DC: US Department of Veterans Affairs. October 13, 2020. www.va.gov/patientcenteredcare/explore/about-whole-health.asp. Accessed November 25, 2020.
33. COVER Commission. Creating options for veterans’ expedited recovery. Final report. Washington, DC: US Veterans Administration. January 24, 2020. www.va.gov/COVER/docs/COVER-Commission-Final-Report-2020-01-24.pdf. Accessed November 24, 2020.

34. Social determinants of health. Washington, DC: Office of Disease Prevention and Health Promotion, US Department of Health and Human Services. HealthyPeople.gov Web site. www.healthypeople.gov/2020/topics-objectives/topic/social-determinants-of-health. Accessed November 24, 2020.
35. Breslin E, Lambertino A. Medicaid and social determinants of health: adjusting payment and measuring health outcomes. Princeton University Woodrow Wilson School of Public and International Affairs, State Health and Value Strategies Program Web site. July 2017. www.shvs.org/wp-content/uploads/2017/07/SHVS_SocialDeterminants_HMA_July2017.pdf. Accessed November 24, 2020.
36. James CV. Actively addressing social determinants of health will help us achieve health equity. US Centers for Medicare & Medicaid Services Web site. April 26, 2019. www.cms.gov/blog/actively-addressing-social-determinants-health-will-help-us-achieve-health-equity. Accessed November 24, 2020.
37. Geisinger receives “Innovation in Advancing Health Equity” award. Geisinger Health Web site. April 24, 2018. www.geisinger.org/health-plan/news-releases/2018/04/23/19/28/geisinger-receives-innovation-in-advancing-health-equity-award. Accessed November 24, 2020.
38. Bresnick J. Kaiser Permanente launches full-network social determinants program. HealthITAnalytics Web site. May 6, 2019. https://healthitanalytics.com/news/kaiser-permanente-launches-full-network-social-determinants-program. Accessed November 25, 2020.
39. Medicare Payment Advisory Commission (MEDPAC). Physician and other health Professional services. In: Report to the Congress: Medicare Payment Policy. March 2016: 115-117. http://medpac.gov/docs/default-source/reports/chapter-4-physician-and-other-health-professional-services-march-2016-report-.pdf. Accessed November 24, 2020.
40. Jonas W. Helping patients with chronic diseases and conditions heal with the HOPE Note: integrative primary care case study. https://drwaynejonas.com/wp-content/uploads/2018/09/CS_HOPE-Note_FINAL.pdf. Accessed November 24, 2020.
41. Jonas W. How Healing Works. Berkley, CA: Lorena Jones Books; 2018.
Here is our problem: Family medicine has allowed itself, and its patients, to be picked apart by the forces of reductionism and a system that profits from the sick and suffering. We have lost sight of our purpose and our vision to care for the whole person. We have lost our way as healers.
The result is not only a decline in the specialty of family medicine as a leader in primary care but declining value and worsening outcomes in health care overall. We need to get our mojo back. We can do this by focusing less on trying to be all things to all people at all times, and more on creating better models for preventing, managing, and reversing chronic disease. This means providing health care that is person centered, relationship based, recovery focused, and paid for comprehensively.
I call this model Advanced Primary Care, or APC (FIGURE). In this article, I describe exemplars of APC from across the United States. I also provide tools to help you recover its central feature, holism—care of the whole person in mind, body, community, and spirit—in your practice, thus returning us to the core purpose of family medicine.

Holism is central to family medicine
More than 40 years ago, psychiatrist George Engel, MD, published a seminal article in Science that inspired a radical vision of how health care should be practiced.1 Called the biopsychosocial model, it stated what, in some ways, is obvious: Human beings are complex organisms embedded in complex environments made up of distinct, yet interacting, dimensions. These dimensions included physical, psychological, and social components. Engel’s radical proposition was that these dimensions are definable and measurable and that good medicine cannot afford to ignore any of them.
Engel’s assertion that good medicine requires holism was a clarion call during a time of rapidly expanding knowledge and subspecialization. That call was the inspiration for a new medical specialty called family medicine, which dared to proclaim that the best way to heal was to care for the whole person within the context of that person’s emotional and social environment. Family medicine reinvigorated primary care and grew rapidly, becoming a preeminent primary care specialty in the United States.
Continue to : Reductionism is relentless
Reductionism is relentless
But the forces of medicine were—and still are—driving relentlessly the other way. The science of the small and particular (reductionism), with dazzling technology and exploding subspecialty knowledge, and backed by powerful economic drivers, rewards health care for pulling the patient and the medical profession apart. We pay more to those who treat small parts of a person over a short period than to those who attend to the whole person over the lifetime.
Today, family medicine—for all of its common sense, scientific soundness, connectedness to patients, and demonstrated value—struggles to survive.2-6 The holistic vision of Engel is declining. The struggle in primary care is that its holistic vision gets co-opted by specialized medical science—and then it desperately attempts to apply those small and specialized tools to the care of patients in their wholeness. Holism is largely dead in health care, and everyone pays the consequences.7
Health care is losing its value
The damage from this decline in holism is not just to primary care but to the value of health care in general. Most medical care being delivered today—comprising diagnosis, treatment, and payment (the innermost circle of the FIGURE)—is not producing good health.8 Only 15% to 20% of the healing of an individual or a population comes from health care.9 The rest—nearly 80%—comes from other factors rarely addressed in the health care system: behavioral and lifestyle choices that people make in their daily life, including those related to food, movement, sleep, stress, and substance use.10 Increasingly, it is the economic and social determinants of health that influence this behavior and have a greater impact on health and lifespan than physiology or genes.11 The same social determinants of health also influence patients’ ability to obtain medical care and pursue a meaningful life.12
The result of this decline in holism and in the value of health care in general has been a relentless rise in the cost of medical care13-15 and the need for social services; declining life expectancy16,17 and quality of life18; growing patient dissatisfaction; and burnout in providers.19,20 Health care has become, as investor and business leader Warren Buffet remarked, the “tapeworm” of the economy and a major contributor to growing disparities in health and well-being between the haves and have-nots.21 Engel’s prediction that good medicine cannot afford to ignore holism has come to pass.
3-step solution:Return to whole-person care
Family medicine needs to return to whole-person care, but it can do so only if it attends to, and effectively delivers on, the prevention, treatment, and reversal of chronic disease and the enhancement of health and well-being. This can happen only if family medicine stops trying to be all things to all people at all times and, instead, focuses on what matters to the patient as a person.
Continue to: This means that the core...
This means that the core interaction in family medicine must be to assess the whole person—mind, body, social, spirit—and help that person make changes that improve his/her/their health and well-being based on his/her/their individualized needs and social context. In other words, family medicine needs to deliver a holistic model of APC that is person centered, relationship based, recovery focused, and paid for comprehensively.
How does one get from “standard” primary care of today (the innermost circle of the FIGURE) to a framework that truly delivers on the promise of healing? I propose 3 steps to return holism to family medicine.
STEP 1: Start with comprehensive, coordinated primary care. We know that this works. Starfield and others demonstrated this 2 decades ago, defining and devising what we know as quality primary care—characterized by first-contact care, comprehensive primary care (CPC), continuous care, and coordinated care.22 This type of primary care improves outcomes, lowers costs, and is satisfying to patients and providers.23 The physician cares for the patient throughout that person’s entire life cycle and provides all evidence-based services needed to prevent and treat common conditions. Comprehensive primary care is positioned in the first circle outward from the innermost circle of the FIGURE.
As medicine has become increasingly complex and subspecialized, however, the ability to coordinate care is often frayed, adding cost and reducing quality.24-26 Today, comprehensive primary care needs enhanced coordination. At a minimum, this means coordinating services for:
- chronic disease management (outpatient and inpatient transitions and emergency department use)
- referral (specialists and tests)
- pharmacy services (including delivery and patient education support).
An example of a primary care system that meets these requirements is the Catalyst Health Network in central Texas, which supplies coordination services to more than 1000 comprehensive primary care practices and 1.5 million patients.27 The Catalyst Network makes money for those practices, saves money in the system, enhances patient and provider satisfaction, and improves population health in the community.27 I call this enhanced primary care (EPC), shown in the second circle out from the innermost circle of the FIGURE.
STEP 2: Add integrative medicine and mental health. EPC improves fragmented care but does not necessarily address a patient’s underlying determinants of healing. We know that health behaviors such as smoking cessation, avoidance of alcohol and drug abuse, improved diet, physical activity, sleep, and stress management contribute 40% to 60% of a person’s and a population’s health.10 In addition, evidence shows that behavioral health services, along with lifestyle change support, can even reverse many chronic diseases seen in primary care, such as obesity, diabetes, hypertension, cardiovascular disease, depression, and substance abuse.28,29
Continue to: Therefore, we need to add...
Therefore, we need to add routine mental health services and nonpharmacotherapeutic approaches (eg, complementary and alternative medicine) to primary care.30 Doing so requires that behavioral change and self-care become a central feature of the doctor–patient dialogue and team skills31 and be added to primary care.30,31 I call this integrative primary care (IPC), shown on the left side in the third circle out from the innermost circle of the FIGURE.
An example of IPC is Whole Health, an initiative of the US Veteran’s Health Administration. Whole Health empowers and informs a person-centered approach and integrates it into the delivery of routine care.32 Evaluation of Whole Health implementation, which involved more than 130,000 veterans followed for 2 years, found a net overall reduction in the total cost of care of 20%—saving nearly $650 million or, on average, more than $4500 per veteran.33
STEP 3: Address social determinants of health. Primary care will not fully be part of the solution for producing health and well-being unless it becomes instrumental in addressing the social determinants of health (SDH), defined as “… conditions in the environments in which people are born, live, learn, work, play, worship, and age that affect a wide range of health, functioning, and quality-of-life outcomes and risks.”34 These determinants include not only basic needs, such as housing, food, safety, and transportation (ie, social needs), but also what are known as structural determinants, such as income, education, language, and racial and ethnic bias. Health care cannot solve all of these social ills,but it is increasingly being called on to be the nexus of coordination for services that address these needs when they affect health outcomes.35,36
Examples of health systems that provide for social needs include the free “food prescription” program of Pennsylvania’s Geisinger Health System, for patients with diabetes who do not have the resources to pay for food.37 This approach improves blood glucose control by patients and saves money on medications and other interventions. Similarly, Kaiser Permanente has experimented with housing vouchers for homeless patients,and most Federally Qualified Health Centers provide bus or other transportation tickets to patients for their appointments and free or discounted tests and specialty care.38
Implementing whole-person care for all
I propose that we make APC the central focus of family medicine. This model would comprise CPC, plus EPC, IPC, and community coordination to address SDH. This is expressed as:
CPC + EPC + IPC + SDH = APC
Continue to: APC would mean...
APC would mean health for the whole person and for all people. Again, the FIGURE shows how this model, encompassing the entire third circle out from the center circle, could be created from current models of care.
How do we pay for this? We already do—and way too much. The problem is not lack of money in the health care system but how it is organized and distributed. The Centers for Medicare and Medicaid Services and other payers are developing value-based payment models to help cover this type of care,39 but payers cannot pay for something if it is unavailable.
Can family physicians deliver APC? I believe they can, and have given a few examples here to show how this is already happening. To help primary care providers start to deliver APC in their system, my team and I have built the HOPE (Healing Oriented Practices & Environments) Note Toolkit to use in daily practice.40 These and other tools are being used by a number of large hospital systems and health care networks around the country. (You can download the HOPE Note Toolkit, at no cost, at https://drwaynejonas.com/resources/hope-note/.)
Whatever we call this new type of primary care, it needs to care for the whole person and to be available to all. It finds expression in these assertions:
- We cannot ignore an essential part of what a human being is and expect them to heal or become whole.
- We cannot ignore essential people in our communities and expect our costs to go down or our compassion to go up.
- We need to stop allowing family medicine to be co-opted by reductionism and its profits.
In sum, we need a new vision of primary care—like Engel’s holistic vision in the 1970s—to motivate us, and we need to return to fundamental concepts of how healing works in medicine.41
CORRESPONDENCE
Wayne B. Jonas, MD, Samueli Integrative Health Programs, 1800 Diagonal Road, Suite 617, Alexandria, VA 22314; [email protected].
Here is our problem: Family medicine has allowed itself, and its patients, to be picked apart by the forces of reductionism and a system that profits from the sick and suffering. We have lost sight of our purpose and our vision to care for the whole person. We have lost our way as healers.
The result is not only a decline in the specialty of family medicine as a leader in primary care but declining value and worsening outcomes in health care overall. We need to get our mojo back. We can do this by focusing less on trying to be all things to all people at all times, and more on creating better models for preventing, managing, and reversing chronic disease. This means providing health care that is person centered, relationship based, recovery focused, and paid for comprehensively.
I call this model Advanced Primary Care, or APC (FIGURE). In this article, I describe exemplars of APC from across the United States. I also provide tools to help you recover its central feature, holism—care of the whole person in mind, body, community, and spirit—in your practice, thus returning us to the core purpose of family medicine.

Holism is central to family medicine
More than 40 years ago, psychiatrist George Engel, MD, published a seminal article in Science that inspired a radical vision of how health care should be practiced.1 Called the biopsychosocial model, it stated what, in some ways, is obvious: Human beings are complex organisms embedded in complex environments made up of distinct, yet interacting, dimensions. These dimensions included physical, psychological, and social components. Engel’s radical proposition was that these dimensions are definable and measurable and that good medicine cannot afford to ignore any of them.
Engel’s assertion that good medicine requires holism was a clarion call during a time of rapidly expanding knowledge and subspecialization. That call was the inspiration for a new medical specialty called family medicine, which dared to proclaim that the best way to heal was to care for the whole person within the context of that person’s emotional and social environment. Family medicine reinvigorated primary care and grew rapidly, becoming a preeminent primary care specialty in the United States.
Continue to : Reductionism is relentless
Reductionism is relentless
But the forces of medicine were—and still are—driving relentlessly the other way. The science of the small and particular (reductionism), with dazzling technology and exploding subspecialty knowledge, and backed by powerful economic drivers, rewards health care for pulling the patient and the medical profession apart. We pay more to those who treat small parts of a person over a short period than to those who attend to the whole person over the lifetime.
Today, family medicine—for all of its common sense, scientific soundness, connectedness to patients, and demonstrated value—struggles to survive.2-6 The holistic vision of Engel is declining. The struggle in primary care is that its holistic vision gets co-opted by specialized medical science—and then it desperately attempts to apply those small and specialized tools to the care of patients in their wholeness. Holism is largely dead in health care, and everyone pays the consequences.7
Health care is losing its value
The damage from this decline in holism is not just to primary care but to the value of health care in general. Most medical care being delivered today—comprising diagnosis, treatment, and payment (the innermost circle of the FIGURE)—is not producing good health.8 Only 15% to 20% of the healing of an individual or a population comes from health care.9 The rest—nearly 80%—comes from other factors rarely addressed in the health care system: behavioral and lifestyle choices that people make in their daily life, including those related to food, movement, sleep, stress, and substance use.10 Increasingly, it is the economic and social determinants of health that influence this behavior and have a greater impact on health and lifespan than physiology or genes.11 The same social determinants of health also influence patients’ ability to obtain medical care and pursue a meaningful life.12
The result of this decline in holism and in the value of health care in general has been a relentless rise in the cost of medical care13-15 and the need for social services; declining life expectancy16,17 and quality of life18; growing patient dissatisfaction; and burnout in providers.19,20 Health care has become, as investor and business leader Warren Buffet remarked, the “tapeworm” of the economy and a major contributor to growing disparities in health and well-being between the haves and have-nots.21 Engel’s prediction that good medicine cannot afford to ignore holism has come to pass.
3-step solution:Return to whole-person care
Family medicine needs to return to whole-person care, but it can do so only if it attends to, and effectively delivers on, the prevention, treatment, and reversal of chronic disease and the enhancement of health and well-being. This can happen only if family medicine stops trying to be all things to all people at all times and, instead, focuses on what matters to the patient as a person.
Continue to: This means that the core...
This means that the core interaction in family medicine must be to assess the whole person—mind, body, social, spirit—and help that person make changes that improve his/her/their health and well-being based on his/her/their individualized needs and social context. In other words, family medicine needs to deliver a holistic model of APC that is person centered, relationship based, recovery focused, and paid for comprehensively.
How does one get from “standard” primary care of today (the innermost circle of the FIGURE) to a framework that truly delivers on the promise of healing? I propose 3 steps to return holism to family medicine.
STEP 1: Start with comprehensive, coordinated primary care. We know that this works. Starfield and others demonstrated this 2 decades ago, defining and devising what we know as quality primary care—characterized by first-contact care, comprehensive primary care (CPC), continuous care, and coordinated care.22 This type of primary care improves outcomes, lowers costs, and is satisfying to patients and providers.23 The physician cares for the patient throughout that person’s entire life cycle and provides all evidence-based services needed to prevent and treat common conditions. Comprehensive primary care is positioned in the first circle outward from the innermost circle of the FIGURE.
As medicine has become increasingly complex and subspecialized, however, the ability to coordinate care is often frayed, adding cost and reducing quality.24-26 Today, comprehensive primary care needs enhanced coordination. At a minimum, this means coordinating services for:
- chronic disease management (outpatient and inpatient transitions and emergency department use)
- referral (specialists and tests)
- pharmacy services (including delivery and patient education support).
An example of a primary care system that meets these requirements is the Catalyst Health Network in central Texas, which supplies coordination services to more than 1000 comprehensive primary care practices and 1.5 million patients.27 The Catalyst Network makes money for those practices, saves money in the system, enhances patient and provider satisfaction, and improves population health in the community.27 I call this enhanced primary care (EPC), shown in the second circle out from the innermost circle of the FIGURE.
STEP 2: Add integrative medicine and mental health. EPC improves fragmented care but does not necessarily address a patient’s underlying determinants of healing. We know that health behaviors such as smoking cessation, avoidance of alcohol and drug abuse, improved diet, physical activity, sleep, and stress management contribute 40% to 60% of a person’s and a population’s health.10 In addition, evidence shows that behavioral health services, along with lifestyle change support, can even reverse many chronic diseases seen in primary care, such as obesity, diabetes, hypertension, cardiovascular disease, depression, and substance abuse.28,29
Continue to: Therefore, we need to add...
Therefore, we need to add routine mental health services and nonpharmacotherapeutic approaches (eg, complementary and alternative medicine) to primary care.30 Doing so requires that behavioral change and self-care become a central feature of the doctor–patient dialogue and team skills31 and be added to primary care.30,31 I call this integrative primary care (IPC), shown on the left side in the third circle out from the innermost circle of the FIGURE.
An example of IPC is Whole Health, an initiative of the US Veteran’s Health Administration. Whole Health empowers and informs a person-centered approach and integrates it into the delivery of routine care.32 Evaluation of Whole Health implementation, which involved more than 130,000 veterans followed for 2 years, found a net overall reduction in the total cost of care of 20%—saving nearly $650 million or, on average, more than $4500 per veteran.33
STEP 3: Address social determinants of health. Primary care will not fully be part of the solution for producing health and well-being unless it becomes instrumental in addressing the social determinants of health (SDH), defined as “… conditions in the environments in which people are born, live, learn, work, play, worship, and age that affect a wide range of health, functioning, and quality-of-life outcomes and risks.”34 These determinants include not only basic needs, such as housing, food, safety, and transportation (ie, social needs), but also what are known as structural determinants, such as income, education, language, and racial and ethnic bias. Health care cannot solve all of these social ills,but it is increasingly being called on to be the nexus of coordination for services that address these needs when they affect health outcomes.35,36
Examples of health systems that provide for social needs include the free “food prescription” program of Pennsylvania’s Geisinger Health System, for patients with diabetes who do not have the resources to pay for food.37 This approach improves blood glucose control by patients and saves money on medications and other interventions. Similarly, Kaiser Permanente has experimented with housing vouchers for homeless patients,and most Federally Qualified Health Centers provide bus or other transportation tickets to patients for their appointments and free or discounted tests and specialty care.38
Implementing whole-person care for all
I propose that we make APC the central focus of family medicine. This model would comprise CPC, plus EPC, IPC, and community coordination to address SDH. This is expressed as:
CPC + EPC + IPC + SDH = APC
Continue to: APC would mean...
APC would mean health for the whole person and for all people. Again, the FIGURE shows how this model, encompassing the entire third circle out from the center circle, could be created from current models of care.
How do we pay for this? We already do—and way too much. The problem is not lack of money in the health care system but how it is organized and distributed. The Centers for Medicare and Medicaid Services and other payers are developing value-based payment models to help cover this type of care,39 but payers cannot pay for something if it is unavailable.
Can family physicians deliver APC? I believe they can, and have given a few examples here to show how this is already happening. To help primary care providers start to deliver APC in their system, my team and I have built the HOPE (Healing Oriented Practices & Environments) Note Toolkit to use in daily practice.40 These and other tools are being used by a number of large hospital systems and health care networks around the country. (You can download the HOPE Note Toolkit, at no cost, at https://drwaynejonas.com/resources/hope-note/.)
Whatever we call this new type of primary care, it needs to care for the whole person and to be available to all. It finds expression in these assertions:
- We cannot ignore an essential part of what a human being is and expect them to heal or become whole.
- We cannot ignore essential people in our communities and expect our costs to go down or our compassion to go up.
- We need to stop allowing family medicine to be co-opted by reductionism and its profits.
In sum, we need a new vision of primary care—like Engel’s holistic vision in the 1970s—to motivate us, and we need to return to fundamental concepts of how healing works in medicine.41
CORRESPONDENCE
Wayne B. Jonas, MD, Samueli Integrative Health Programs, 1800 Diagonal Road, Suite 617, Alexandria, VA 22314; [email protected].
1. Engel GL. The need for a new medical model: a challenge for biomedicine. Science. 1977;196:129-136.
2. Schwartz MD, Durning S, Linzer M, et al. Changes in medical students’ views of internal medicine careers from 1990 to 2007. Arch Intern Med. 2011;171:744-749.
3. Bronchetti ET, Christensen GS, Hoynes HW. Local food prices, SNAP purchasing power, and child health. Cambridge, MA: National Bureau of Economic Research. June 2018. www.nber.org/papers/w24762?mc_cid=8c7211d34b&mc_eid=fbbc7df813. Accessed November 24, 2020.
4. Federal Student Aid, US Department of Education. Public Service Loan Forgiveness (PSLF). 2018. https://studentaid.ed.gov/sa/repay-loans/forgiveness-cancellation/public-service. Accessed November 24, 2020.
5. Aten B, Figueroa E, Martin T. Notes on estimating the multi-year regional price parities by 16 expenditure categories: 2005-2009. WP2011-03. Washington, DC: Bureau of Economic Analysis, US Department of Commerce; April 2011. www.bea.gov/system/files/papers/WP2011-3.pdf. Accessed November 24, 2020.
6. Aten BH, Figueroa EB, Martin TM. Regional price parities for states and metropolitan areas, 2006-2010. Washington, DC: Bureau of Economic Analysis, US Department of Commerce; August 2012. https://apps.bea.gov/scb/pdf/2012/08%20August/0812_regional_price_parities.pdf. Accessed November 24, 2020.
7. Stange KC, Ferrer RL. The paradox of primary care. Ann Fam Med. 2009;7:293-299.
8. Panel on Understanding Cross-national Health Differences Among High-income Countries, Committee on Population, Division of Behavioral and Social Sciences and Education, and Board on Population Health and Public Health Practice, National Research Council and Institute of Medicine of the National Academies. US Health in International Perspective: Shorter Lives, Poorer Health. Woolf SH, Aron L, eds. The National Academies Press; 2013.
9. Hood CM, Gennuso KP, Swain GR, et al. County health rankings: relationships between determinant factors and health outcomes. Am J Prev Med. 2016;50:129-135.
10. McGinnis JM, Williams-Russo P, Knickman JR. The case for more active policy attention to health promotion. Health Aff (Millwood). 2002;21:78-93.
11. Roeder A. Zip code better predictor of health than genetic code. Harvard T. H. Chan School of Public Health Web site. News release. August 4, 2014. www.hsph.harvard.edu/news/features/zip-code-better-predictor-of-health-than-genetic-code/. Accessed November 24, 2020.

12. US health map. Seattle, WA: University of Washington Institute for Health Metrics and Evaluation; March 13, 2018. www.healthdata.org/data-visualization/us-health-map. Accessed November 24, 2020.
13. Highfill T. Comparing estimates of U.S. health care expenditures by medical condition, 2000-2012. Survey of Current Business. 2016;1-5. https://apps.bea.gov/scb/pdf/2016/3%20March/0316_comparing_u.s._health_care_expenditures_by_medical_condition.pdf. Accessed November 24, 2020.
14. Waters H, Graf M. The Costs of Chronic Disease in the US. Washington, DC: Milken Institute; August 2018. https://milkeninstitute.org/sites/default/files/reports-pdf/ChronicDiseases-HighRes-FINAL.pdf. Accessed November 24, 2020.
15. Meyer H. Health care spending will hit 19.4% of GDP in the next decade, CMS projects. Modern Health care. February 20, 2019. www.modernhealthcare.com/article/20190220/NEWS/190229989/healthcare-spending-will-hit-19-4-of-gdp-in-the-next-decade-cms-projects. Accessed November 24, 2020.
16. Woolf SH, Schoomaker H. Life expectancy and mortality rates in the United States, 1959-2017. JAMA. 2019;322:1996-2016.
17. Basu S, Berkowitz SA, Phillips RL, et al. Association of primary care physician supply with population mortality in the United States, 2005-2015. JAMA Intern Med. 2019;179:506-514.
18. Zack MM, Moriarty DG, Stroup DF, et al. Worsening trends in adult health-related quality of life and self-rated health—United States, 1993–2001. Public Health Rep. 2004;119:493-505.
19. Windover AK, Martinez K, Mercer, MB, et al. Correlates and outcomes of physician burnout within a large academic medical center. Research letter. JAMA Intern Med. 2018;178:856-858.
20. West CP, Dyrbye LN, Shanafelt TD. Physician burnout: contributors, consequences and solutions. J Intern Med. 2018;283:516-529.
21. Buffett: Health care is a tapeworm on the economic system. CNBC Squawk Box. February 26, 2018. www.cnbc.com/video/2018/02/26/buffett-health-care-is-a-tapeworm-on-the-economic-system.html. Accessed November 24, 2020.
22. Starfield B. Primary Care: Concept, Evaluation, and Policy. Oxford University Press; 1992.
23. Starfield B, Shi L, Macinko J. Contribution of primary care to health systems and health. Milbank Q. 2005;83:457-502.
24. Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. National Academies Press (US); 2001.
25. Burton R. Health policy brief: improving care transitions. Health Affairs. September 13, 2012. www.healthaffairs.org/do/10.1377/hpb20120913.327236/full/healthpolicybrief_76.pdf. Accessed November 24, 2020.
26. Toulany A, Stukel TA, Kurdyak P, et al. Association of primary care continuity with outcomes following transition to adult care for adolescents with severe mental illness. JAMA Netw Open. 2019;2:e198415.
27. Helping communities thrive. Catalyst Health Network Web site. www.catalysthealthnetwork.com/. Accessed November 24, 2020.
28. Diabetes Prevention Program (DPP) Research Group. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25:2165-2171.
29. Scherger JE. Lean and Fit: A Doctor’s Journey to Healthy Nutrition and Greater Wellness. 2nd ed. Scotts Valley, CA: CreateSpace Publishing; 2016.
30. Qaseem A, Wilt TJ, McLean RM, et al; . Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:514-530.
31. Hibbard JH, Greene J. What the evidence shows about patient activation: better health outcomes and care experiences; fewer data on costs. Health Aff (Millwood). 2013;32:207-214.
32. What is whole health? Washington, DC: US Department of Veterans Affairs. October 13, 2020. www.va.gov/patientcenteredcare/explore/about-whole-health.asp. Accessed November 25, 2020.
33. COVER Commission. Creating options for veterans’ expedited recovery. Final report. Washington, DC: US Veterans Administration. January 24, 2020. www.va.gov/COVER/docs/COVER-Commission-Final-Report-2020-01-24.pdf. Accessed November 24, 2020.

34. Social determinants of health. Washington, DC: Office of Disease Prevention and Health Promotion, US Department of Health and Human Services. HealthyPeople.gov Web site. www.healthypeople.gov/2020/topics-objectives/topic/social-determinants-of-health. Accessed November 24, 2020.
35. Breslin E, Lambertino A. Medicaid and social determinants of health: adjusting payment and measuring health outcomes. Princeton University Woodrow Wilson School of Public and International Affairs, State Health and Value Strategies Program Web site. July 2017. www.shvs.org/wp-content/uploads/2017/07/SHVS_SocialDeterminants_HMA_July2017.pdf. Accessed November 24, 2020.
36. James CV. Actively addressing social determinants of health will help us achieve health equity. US Centers for Medicare & Medicaid Services Web site. April 26, 2019. www.cms.gov/blog/actively-addressing-social-determinants-health-will-help-us-achieve-health-equity. Accessed November 24, 2020.
37. Geisinger receives “Innovation in Advancing Health Equity” award. Geisinger Health Web site. April 24, 2018. www.geisinger.org/health-plan/news-releases/2018/04/23/19/28/geisinger-receives-innovation-in-advancing-health-equity-award. Accessed November 24, 2020.
38. Bresnick J. Kaiser Permanente launches full-network social determinants program. HealthITAnalytics Web site. May 6, 2019. https://healthitanalytics.com/news/kaiser-permanente-launches-full-network-social-determinants-program. Accessed November 25, 2020.
39. Medicare Payment Advisory Commission (MEDPAC). Physician and other health Professional services. In: Report to the Congress: Medicare Payment Policy. March 2016: 115-117. http://medpac.gov/docs/default-source/reports/chapter-4-physician-and-other-health-professional-services-march-2016-report-.pdf. Accessed November 24, 2020.
40. Jonas W. Helping patients with chronic diseases and conditions heal with the HOPE Note: integrative primary care case study. https://drwaynejonas.com/wp-content/uploads/2018/09/CS_HOPE-Note_FINAL.pdf. Accessed November 24, 2020.
41. Jonas W. How Healing Works. Berkley, CA: Lorena Jones Books; 2018.
1. Engel GL. The need for a new medical model: a challenge for biomedicine. Science. 1977;196:129-136.
2. Schwartz MD, Durning S, Linzer M, et al. Changes in medical students’ views of internal medicine careers from 1990 to 2007. Arch Intern Med. 2011;171:744-749.
3. Bronchetti ET, Christensen GS, Hoynes HW. Local food prices, SNAP purchasing power, and child health. Cambridge, MA: National Bureau of Economic Research. June 2018. www.nber.org/papers/w24762?mc_cid=8c7211d34b&mc_eid=fbbc7df813. Accessed November 24, 2020.
4. Federal Student Aid, US Department of Education. Public Service Loan Forgiveness (PSLF). 2018. https://studentaid.ed.gov/sa/repay-loans/forgiveness-cancellation/public-service. Accessed November 24, 2020.
5. Aten B, Figueroa E, Martin T. Notes on estimating the multi-year regional price parities by 16 expenditure categories: 2005-2009. WP2011-03. Washington, DC: Bureau of Economic Analysis, US Department of Commerce; April 2011. www.bea.gov/system/files/papers/WP2011-3.pdf. Accessed November 24, 2020.
6. Aten BH, Figueroa EB, Martin TM. Regional price parities for states and metropolitan areas, 2006-2010. Washington, DC: Bureau of Economic Analysis, US Department of Commerce; August 2012. https://apps.bea.gov/scb/pdf/2012/08%20August/0812_regional_price_parities.pdf. Accessed November 24, 2020.
7. Stange KC, Ferrer RL. The paradox of primary care. Ann Fam Med. 2009;7:293-299.
8. Panel on Understanding Cross-national Health Differences Among High-income Countries, Committee on Population, Division of Behavioral and Social Sciences and Education, and Board on Population Health and Public Health Practice, National Research Council and Institute of Medicine of the National Academies. US Health in International Perspective: Shorter Lives, Poorer Health. Woolf SH, Aron L, eds. The National Academies Press; 2013.
9. Hood CM, Gennuso KP, Swain GR, et al. County health rankings: relationships between determinant factors and health outcomes. Am J Prev Med. 2016;50:129-135.
10. McGinnis JM, Williams-Russo P, Knickman JR. The case for more active policy attention to health promotion. Health Aff (Millwood). 2002;21:78-93.
11. Roeder A. Zip code better predictor of health than genetic code. Harvard T. H. Chan School of Public Health Web site. News release. August 4, 2014. www.hsph.harvard.edu/news/features/zip-code-better-predictor-of-health-than-genetic-code/. Accessed November 24, 2020.

12. US health map. Seattle, WA: University of Washington Institute for Health Metrics and Evaluation; March 13, 2018. www.healthdata.org/data-visualization/us-health-map. Accessed November 24, 2020.
13. Highfill T. Comparing estimates of U.S. health care expenditures by medical condition, 2000-2012. Survey of Current Business. 2016;1-5. https://apps.bea.gov/scb/pdf/2016/3%20March/0316_comparing_u.s._health_care_expenditures_by_medical_condition.pdf. Accessed November 24, 2020.
14. Waters H, Graf M. The Costs of Chronic Disease in the US. Washington, DC: Milken Institute; August 2018. https://milkeninstitute.org/sites/default/files/reports-pdf/ChronicDiseases-HighRes-FINAL.pdf. Accessed November 24, 2020.
15. Meyer H. Health care spending will hit 19.4% of GDP in the next decade, CMS projects. Modern Health care. February 20, 2019. www.modernhealthcare.com/article/20190220/NEWS/190229989/healthcare-spending-will-hit-19-4-of-gdp-in-the-next-decade-cms-projects. Accessed November 24, 2020.
16. Woolf SH, Schoomaker H. Life expectancy and mortality rates in the United States, 1959-2017. JAMA. 2019;322:1996-2016.
17. Basu S, Berkowitz SA, Phillips RL, et al. Association of primary care physician supply with population mortality in the United States, 2005-2015. JAMA Intern Med. 2019;179:506-514.
18. Zack MM, Moriarty DG, Stroup DF, et al. Worsening trends in adult health-related quality of life and self-rated health—United States, 1993–2001. Public Health Rep. 2004;119:493-505.
19. Windover AK, Martinez K, Mercer, MB, et al. Correlates and outcomes of physician burnout within a large academic medical center. Research letter. JAMA Intern Med. 2018;178:856-858.
20. West CP, Dyrbye LN, Shanafelt TD. Physician burnout: contributors, consequences and solutions. J Intern Med. 2018;283:516-529.
21. Buffett: Health care is a tapeworm on the economic system. CNBC Squawk Box. February 26, 2018. www.cnbc.com/video/2018/02/26/buffett-health-care-is-a-tapeworm-on-the-economic-system.html. Accessed November 24, 2020.
22. Starfield B. Primary Care: Concept, Evaluation, and Policy. Oxford University Press; 1992.
23. Starfield B, Shi L, Macinko J. Contribution of primary care to health systems and health. Milbank Q. 2005;83:457-502.
24. Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. National Academies Press (US); 2001.
25. Burton R. Health policy brief: improving care transitions. Health Affairs. September 13, 2012. www.healthaffairs.org/do/10.1377/hpb20120913.327236/full/healthpolicybrief_76.pdf. Accessed November 24, 2020.
26. Toulany A, Stukel TA, Kurdyak P, et al. Association of primary care continuity with outcomes following transition to adult care for adolescents with severe mental illness. JAMA Netw Open. 2019;2:e198415.
27. Helping communities thrive. Catalyst Health Network Web site. www.catalysthealthnetwork.com/. Accessed November 24, 2020.
28. Diabetes Prevention Program (DPP) Research Group. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25:2165-2171.
29. Scherger JE. Lean and Fit: A Doctor’s Journey to Healthy Nutrition and Greater Wellness. 2nd ed. Scotts Valley, CA: CreateSpace Publishing; 2016.
30. Qaseem A, Wilt TJ, McLean RM, et al; . Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:514-530.
31. Hibbard JH, Greene J. What the evidence shows about patient activation: better health outcomes and care experiences; fewer data on costs. Health Aff (Millwood). 2013;32:207-214.
32. What is whole health? Washington, DC: US Department of Veterans Affairs. October 13, 2020. www.va.gov/patientcenteredcare/explore/about-whole-health.asp. Accessed November 25, 2020.
33. COVER Commission. Creating options for veterans’ expedited recovery. Final report. Washington, DC: US Veterans Administration. January 24, 2020. www.va.gov/COVER/docs/COVER-Commission-Final-Report-2020-01-24.pdf. Accessed November 24, 2020.

34. Social determinants of health. Washington, DC: Office of Disease Prevention and Health Promotion, US Department of Health and Human Services. HealthyPeople.gov Web site. www.healthypeople.gov/2020/topics-objectives/topic/social-determinants-of-health. Accessed November 24, 2020.
35. Breslin E, Lambertino A. Medicaid and social determinants of health: adjusting payment and measuring health outcomes. Princeton University Woodrow Wilson School of Public and International Affairs, State Health and Value Strategies Program Web site. July 2017. www.shvs.org/wp-content/uploads/2017/07/SHVS_SocialDeterminants_HMA_July2017.pdf. Accessed November 24, 2020.
36. James CV. Actively addressing social determinants of health will help us achieve health equity. US Centers for Medicare & Medicaid Services Web site. April 26, 2019. www.cms.gov/blog/actively-addressing-social-determinants-health-will-help-us-achieve-health-equity. Accessed November 24, 2020.
37. Geisinger receives “Innovation in Advancing Health Equity” award. Geisinger Health Web site. April 24, 2018. www.geisinger.org/health-plan/news-releases/2018/04/23/19/28/geisinger-receives-innovation-in-advancing-health-equity-award. Accessed November 24, 2020.
38. Bresnick J. Kaiser Permanente launches full-network social determinants program. HealthITAnalytics Web site. May 6, 2019. https://healthitanalytics.com/news/kaiser-permanente-launches-full-network-social-determinants-program. Accessed November 25, 2020.
39. Medicare Payment Advisory Commission (MEDPAC). Physician and other health Professional services. In: Report to the Congress: Medicare Payment Policy. March 2016: 115-117. http://medpac.gov/docs/default-source/reports/chapter-4-physician-and-other-health-professional-services-march-2016-report-.pdf. Accessed November 24, 2020.
40. Jonas W. Helping patients with chronic diseases and conditions heal with the HOPE Note: integrative primary care case study. https://drwaynejonas.com/wp-content/uploads/2018/09/CS_HOPE-Note_FINAL.pdf. Accessed November 24, 2020.
41. Jonas W. How Healing Works. Berkley, CA: Lorena Jones Books; 2018.
PRACTICE RECOMMENDATIONS
❯ Build care teams into your practice so that you integrate “what matters” into the center of the clinical encounter. C
❯ Add practice approaches that help patients engage in healthy lifestyles and that remove social and economic barriers for improving health and well-being. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Biden chooses California Attorney General Xavier Becerra to head HHS
If confirmed by the US Senate, Becerra will face the challenge of overseeing the federal agency charged with protecting the health of all Americans in the midst of the COVID-19 pandemic. At the time of the announcement, nearly 15 million Americans had tested positive for COVID-19 and more than 280,000 had died.
Becerra served 12 terms in Congress, representing the Los Angeles area. Although his public health experience is limited, he served on the Congressional Ways and Means Committee overseeing health-related issues. Becerra is known as an advocate for the health and well-being of women in particular.
The American College of Physicians, American Academy of Pediatrics, American College of Obstetricians & Gynecologists, American Academy of Family Physicians, and the American Psychiatric Association wrote a letter to Biden on December 3 urging him to select leaders with medical and healthcare expertise, in particular physicians.
“We believe that your administration and the country would be well-served by the appointment of qualified physicians to serve in key positions critical to advancing the health of our nation,” they wrote. “Therefore, our organizations, which represent more than 400,000 front-line physicians practicing in the United States, write to request that you identify and appoint physicians to healthcare leadership positions within your administration.”
Recent advocacy
Becerra has worked with Republican attorneys general to lobby HHS to increase access to remdesivir to treat people with COVID-19.
As attorney general, Becerra filed more than 100 lawsuits against the Trump administration. In November, he also represented more than 20 states in arguments supporting the Affordable Care Act before the Supreme Court.
On December 4, Becerra joined with attorneys general from 23 states and the District of Columbia opposing a proposed rule from the outgoing Trump administration. The rule would deregulate HHS and “sunset”many agency provisions before Trump leaves office next month.
Becerra will be the first Latino appointed as HHS secretary, which furthers Biden’s goal to create a diverse cabinet. Becerra has been attorney general of California since 2017, replacing Vice President-elect Kamala Harris when she became senator.
Biden’s choice of Becerra was unexpected, according to The New York Times, and he was not the only candidate. Speculation was that Biden initially considered Vivek Murthy, MD, later chosen as the next US surgeon general, as well New Mexico Gov. Michelle Lujan Grisham and Rhode Island Gov. Gina Raimondo.
A huge undertaking
As HHS secretary, Becerra would oversee a wide range of federal agencies, including the US Food and Drug Administration, the Centers for Disease Control and Prevention, the National Institutes of Health, and the Centers for Medicare & Medicaid Services.
The fiscal year 2021 budget proposed for HHS includes $94.5 billion in discretionary budget authority and $1.3 trillion in mandatory funding. Overall, HHS controls nearly one quarter of all federal expenditures and provides more grant money than all other federal agencies combined.
Becerra, 62, grew up in Sacramento, California. He was the first in his family to graduate from college. He received his undergraduate and law degrees from Stanford University.
This article first appeared on Medscape.com.
If confirmed by the US Senate, Becerra will face the challenge of overseeing the federal agency charged with protecting the health of all Americans in the midst of the COVID-19 pandemic. At the time of the announcement, nearly 15 million Americans had tested positive for COVID-19 and more than 280,000 had died.
Becerra served 12 terms in Congress, representing the Los Angeles area. Although his public health experience is limited, he served on the Congressional Ways and Means Committee overseeing health-related issues. Becerra is known as an advocate for the health and well-being of women in particular.
The American College of Physicians, American Academy of Pediatrics, American College of Obstetricians & Gynecologists, American Academy of Family Physicians, and the American Psychiatric Association wrote a letter to Biden on December 3 urging him to select leaders with medical and healthcare expertise, in particular physicians.
“We believe that your administration and the country would be well-served by the appointment of qualified physicians to serve in key positions critical to advancing the health of our nation,” they wrote. “Therefore, our organizations, which represent more than 400,000 front-line physicians practicing in the United States, write to request that you identify and appoint physicians to healthcare leadership positions within your administration.”
Recent advocacy
Becerra has worked with Republican attorneys general to lobby HHS to increase access to remdesivir to treat people with COVID-19.
As attorney general, Becerra filed more than 100 lawsuits against the Trump administration. In November, he also represented more than 20 states in arguments supporting the Affordable Care Act before the Supreme Court.
On December 4, Becerra joined with attorneys general from 23 states and the District of Columbia opposing a proposed rule from the outgoing Trump administration. The rule would deregulate HHS and “sunset”many agency provisions before Trump leaves office next month.
Becerra will be the first Latino appointed as HHS secretary, which furthers Biden’s goal to create a diverse cabinet. Becerra has been attorney general of California since 2017, replacing Vice President-elect Kamala Harris when she became senator.
Biden’s choice of Becerra was unexpected, according to The New York Times, and he was not the only candidate. Speculation was that Biden initially considered Vivek Murthy, MD, later chosen as the next US surgeon general, as well New Mexico Gov. Michelle Lujan Grisham and Rhode Island Gov. Gina Raimondo.
A huge undertaking
As HHS secretary, Becerra would oversee a wide range of federal agencies, including the US Food and Drug Administration, the Centers for Disease Control and Prevention, the National Institutes of Health, and the Centers for Medicare & Medicaid Services.
The fiscal year 2021 budget proposed for HHS includes $94.5 billion in discretionary budget authority and $1.3 trillion in mandatory funding. Overall, HHS controls nearly one quarter of all federal expenditures and provides more grant money than all other federal agencies combined.
Becerra, 62, grew up in Sacramento, California. He was the first in his family to graduate from college. He received his undergraduate and law degrees from Stanford University.
This article first appeared on Medscape.com.
If confirmed by the US Senate, Becerra will face the challenge of overseeing the federal agency charged with protecting the health of all Americans in the midst of the COVID-19 pandemic. At the time of the announcement, nearly 15 million Americans had tested positive for COVID-19 and more than 280,000 had died.
Becerra served 12 terms in Congress, representing the Los Angeles area. Although his public health experience is limited, he served on the Congressional Ways and Means Committee overseeing health-related issues. Becerra is known as an advocate for the health and well-being of women in particular.
The American College of Physicians, American Academy of Pediatrics, American College of Obstetricians & Gynecologists, American Academy of Family Physicians, and the American Psychiatric Association wrote a letter to Biden on December 3 urging him to select leaders with medical and healthcare expertise, in particular physicians.
“We believe that your administration and the country would be well-served by the appointment of qualified physicians to serve in key positions critical to advancing the health of our nation,” they wrote. “Therefore, our organizations, which represent more than 400,000 front-line physicians practicing in the United States, write to request that you identify and appoint physicians to healthcare leadership positions within your administration.”
Recent advocacy
Becerra has worked with Republican attorneys general to lobby HHS to increase access to remdesivir to treat people with COVID-19.
As attorney general, Becerra filed more than 100 lawsuits against the Trump administration. In November, he also represented more than 20 states in arguments supporting the Affordable Care Act before the Supreme Court.
On December 4, Becerra joined with attorneys general from 23 states and the District of Columbia opposing a proposed rule from the outgoing Trump administration. The rule would deregulate HHS and “sunset”many agency provisions before Trump leaves office next month.
Becerra will be the first Latino appointed as HHS secretary, which furthers Biden’s goal to create a diverse cabinet. Becerra has been attorney general of California since 2017, replacing Vice President-elect Kamala Harris when she became senator.
Biden’s choice of Becerra was unexpected, according to The New York Times, and he was not the only candidate. Speculation was that Biden initially considered Vivek Murthy, MD, later chosen as the next US surgeon general, as well New Mexico Gov. Michelle Lujan Grisham and Rhode Island Gov. Gina Raimondo.
A huge undertaking
As HHS secretary, Becerra would oversee a wide range of federal agencies, including the US Food and Drug Administration, the Centers for Disease Control and Prevention, the National Institutes of Health, and the Centers for Medicare & Medicaid Services.
The fiscal year 2021 budget proposed for HHS includes $94.5 billion in discretionary budget authority and $1.3 trillion in mandatory funding. Overall, HHS controls nearly one quarter of all federal expenditures and provides more grant money than all other federal agencies combined.
Becerra, 62, grew up in Sacramento, California. He was the first in his family to graduate from college. He received his undergraduate and law degrees from Stanford University.
This article first appeared on Medscape.com.
HHS, Surgeon General urge action on maternal health
The U.S. Surgeon General and Department of Health & Human Services are calling on health care professionals, hospitals, employers, insurers, women, and the nation to work together to reduce maternal morbidity and mortality – and the disparities that make the risks higher for women of color.
The maternal mortality rate in the United States is the highest among developed countries of the world and continues to rise. In 2018, for every 100,000 live births, approximately 17 women died while pregnant or within 42 days of the end of pregnancy from causes related to pregnancy or delivery – that’s a substantial increase from 7 deaths per 100,000 live births in 1987, according to the surgeon general’s new call to action.
“Our mothers had much lower rates of dying related to pregnancy, compared to women today,” Dorothy Fink, MD, HHS deputy assistant secretary for women’s health, said at a briefing held Dec. 3 to mark the call to action.
Cardiovascular conditions were the most common cause of pregnancy-related deaths between 2011 and 2015, accounting for more than one in three of the deaths. HHS’s related action plan sets a target of achieving blood pressure control in 80% of women of reproductive age with hypertension by 2025.
The plan also seeks to reduce the maternal mortality rate by 50% and decrease low-risk cesarean deliveries by 25% within 5 years.
Surgeon General Jerome Adams, MD, said at the briefing. “This is not just unacceptable, it is just something that we need to understand is not inevitable,” he said, adding that the Centers for Disease Control and Prevention has determined that two thirds of the deaths are preventable.
Dr. Adams also noted that it was important to address maternal health now, especially with COVID-19 raging. “Without attention and action, maternal health could actually worsen because of this pandemic,” he said.
“We cannot discuss maternal health, much less improve it, unless we acknowledge women of color are at a much greater risk of harm related to childbirth,” Dr. Adams said. “Black women are two to three times more likely to die of pregnancy-related causes compared to many other racial and ethnic groups.” The disparity increases with age, according to the CDC.
Studies have shown that education does not eliminate those disparities. Black women with a college degree are twice as likely to die as White or Asian American women who did not finish high school, Dr. Adams said.
He held up a photo of a colleague, Shalone Irving, who he said was a PhD-educated epidemiologist who “died not long ago from pregnancy-related complications.”
Income is also not a factor, said Dr. Adams, noting that pop singer Beyonce had a near-death experience with preeclampsia. He also noted that Serena Williams, a top athlete, also struggled with pregnancy complications.
Recommendations not all funded
The HHS action plan is not explicitly funded, although Dr. Fink and Dr. Adams said that President Donald J. Trump’s fiscal 2021 budget includes some specific requests for improving maternal health. It will be up to Congress to grant the requests.
The budget seeks $80 million for the Health Resources and Services Administration to improve access to and quality of care. It also includes money to expand Medicaid coverage for 1 year after birth for women with substance use disorders. The American Medical Association in 2019 adopted a policy urging Medicaid coverage to be expanded to include all women for a year after childbirth. The American College of Obstetricians and Gynecologists has also encouraged this extension.
“We are encouraged that the HHS action plan includes support for policies to close coverage and care gaps for all postpartum women after pregnancy-related Medicaid coverage expires,” Maureen G. Phipps, MD, MPH, CEO of the American College of Obstetricians and Gynecologists, said in an interview.
The HHS could act immediately by approving Medicaid waivers to extend such coverage, Dr. Phipps said.
The budget also requests $24 million to expand maternal mortality review programs to every state, said Dr. Fink. Currently, 43 states and the District of Columbia, have such committees, which are charged with reviewing deaths of women within a year of pregnancy or birth.
The HHS will also join with the March of Dimes to address the disparities in Black women by implementing evidence-based best practices to improve quality in hospital settings.
It is not the first time the Trump administration has taken aim at reducing maternal morbidity and mortality. In 2018, the president signed the Preventing Maternal Deaths Act, which authorized the CDC to award $50 million over 5 years so that every state could form maternal mortality review committees.
A version of this article originally appeared on Medscape.com.
The U.S. Surgeon General and Department of Health & Human Services are calling on health care professionals, hospitals, employers, insurers, women, and the nation to work together to reduce maternal morbidity and mortality – and the disparities that make the risks higher for women of color.
The maternal mortality rate in the United States is the highest among developed countries of the world and continues to rise. In 2018, for every 100,000 live births, approximately 17 women died while pregnant or within 42 days of the end of pregnancy from causes related to pregnancy or delivery – that’s a substantial increase from 7 deaths per 100,000 live births in 1987, according to the surgeon general’s new call to action.
“Our mothers had much lower rates of dying related to pregnancy, compared to women today,” Dorothy Fink, MD, HHS deputy assistant secretary for women’s health, said at a briefing held Dec. 3 to mark the call to action.
Cardiovascular conditions were the most common cause of pregnancy-related deaths between 2011 and 2015, accounting for more than one in three of the deaths. HHS’s related action plan sets a target of achieving blood pressure control in 80% of women of reproductive age with hypertension by 2025.
The plan also seeks to reduce the maternal mortality rate by 50% and decrease low-risk cesarean deliveries by 25% within 5 years.
Surgeon General Jerome Adams, MD, said at the briefing. “This is not just unacceptable, it is just something that we need to understand is not inevitable,” he said, adding that the Centers for Disease Control and Prevention has determined that two thirds of the deaths are preventable.
Dr. Adams also noted that it was important to address maternal health now, especially with COVID-19 raging. “Without attention and action, maternal health could actually worsen because of this pandemic,” he said.
“We cannot discuss maternal health, much less improve it, unless we acknowledge women of color are at a much greater risk of harm related to childbirth,” Dr. Adams said. “Black women are two to three times more likely to die of pregnancy-related causes compared to many other racial and ethnic groups.” The disparity increases with age, according to the CDC.
Studies have shown that education does not eliminate those disparities. Black women with a college degree are twice as likely to die as White or Asian American women who did not finish high school, Dr. Adams said.
He held up a photo of a colleague, Shalone Irving, who he said was a PhD-educated epidemiologist who “died not long ago from pregnancy-related complications.”
Income is also not a factor, said Dr. Adams, noting that pop singer Beyonce had a near-death experience with preeclampsia. He also noted that Serena Williams, a top athlete, also struggled with pregnancy complications.
Recommendations not all funded
The HHS action plan is not explicitly funded, although Dr. Fink and Dr. Adams said that President Donald J. Trump’s fiscal 2021 budget includes some specific requests for improving maternal health. It will be up to Congress to grant the requests.
The budget seeks $80 million for the Health Resources and Services Administration to improve access to and quality of care. It also includes money to expand Medicaid coverage for 1 year after birth for women with substance use disorders. The American Medical Association in 2019 adopted a policy urging Medicaid coverage to be expanded to include all women for a year after childbirth. The American College of Obstetricians and Gynecologists has also encouraged this extension.
“We are encouraged that the HHS action plan includes support for policies to close coverage and care gaps for all postpartum women after pregnancy-related Medicaid coverage expires,” Maureen G. Phipps, MD, MPH, CEO of the American College of Obstetricians and Gynecologists, said in an interview.
The HHS could act immediately by approving Medicaid waivers to extend such coverage, Dr. Phipps said.
The budget also requests $24 million to expand maternal mortality review programs to every state, said Dr. Fink. Currently, 43 states and the District of Columbia, have such committees, which are charged with reviewing deaths of women within a year of pregnancy or birth.
The HHS will also join with the March of Dimes to address the disparities in Black women by implementing evidence-based best practices to improve quality in hospital settings.
It is not the first time the Trump administration has taken aim at reducing maternal morbidity and mortality. In 2018, the president signed the Preventing Maternal Deaths Act, which authorized the CDC to award $50 million over 5 years so that every state could form maternal mortality review committees.
A version of this article originally appeared on Medscape.com.
The U.S. Surgeon General and Department of Health & Human Services are calling on health care professionals, hospitals, employers, insurers, women, and the nation to work together to reduce maternal morbidity and mortality – and the disparities that make the risks higher for women of color.
The maternal mortality rate in the United States is the highest among developed countries of the world and continues to rise. In 2018, for every 100,000 live births, approximately 17 women died while pregnant or within 42 days of the end of pregnancy from causes related to pregnancy or delivery – that’s a substantial increase from 7 deaths per 100,000 live births in 1987, according to the surgeon general’s new call to action.
“Our mothers had much lower rates of dying related to pregnancy, compared to women today,” Dorothy Fink, MD, HHS deputy assistant secretary for women’s health, said at a briefing held Dec. 3 to mark the call to action.
Cardiovascular conditions were the most common cause of pregnancy-related deaths between 2011 and 2015, accounting for more than one in three of the deaths. HHS’s related action plan sets a target of achieving blood pressure control in 80% of women of reproductive age with hypertension by 2025.
The plan also seeks to reduce the maternal mortality rate by 50% and decrease low-risk cesarean deliveries by 25% within 5 years.
Surgeon General Jerome Adams, MD, said at the briefing. “This is not just unacceptable, it is just something that we need to understand is not inevitable,” he said, adding that the Centers for Disease Control and Prevention has determined that two thirds of the deaths are preventable.
Dr. Adams also noted that it was important to address maternal health now, especially with COVID-19 raging. “Without attention and action, maternal health could actually worsen because of this pandemic,” he said.
“We cannot discuss maternal health, much less improve it, unless we acknowledge women of color are at a much greater risk of harm related to childbirth,” Dr. Adams said. “Black women are two to three times more likely to die of pregnancy-related causes compared to many other racial and ethnic groups.” The disparity increases with age, according to the CDC.
Studies have shown that education does not eliminate those disparities. Black women with a college degree are twice as likely to die as White or Asian American women who did not finish high school, Dr. Adams said.
He held up a photo of a colleague, Shalone Irving, who he said was a PhD-educated epidemiologist who “died not long ago from pregnancy-related complications.”
Income is also not a factor, said Dr. Adams, noting that pop singer Beyonce had a near-death experience with preeclampsia. He also noted that Serena Williams, a top athlete, also struggled with pregnancy complications.
Recommendations not all funded
The HHS action plan is not explicitly funded, although Dr. Fink and Dr. Adams said that President Donald J. Trump’s fiscal 2021 budget includes some specific requests for improving maternal health. It will be up to Congress to grant the requests.
The budget seeks $80 million for the Health Resources and Services Administration to improve access to and quality of care. It also includes money to expand Medicaid coverage for 1 year after birth for women with substance use disorders. The American Medical Association in 2019 adopted a policy urging Medicaid coverage to be expanded to include all women for a year after childbirth. The American College of Obstetricians and Gynecologists has also encouraged this extension.
“We are encouraged that the HHS action plan includes support for policies to close coverage and care gaps for all postpartum women after pregnancy-related Medicaid coverage expires,” Maureen G. Phipps, MD, MPH, CEO of the American College of Obstetricians and Gynecologists, said in an interview.
The HHS could act immediately by approving Medicaid waivers to extend such coverage, Dr. Phipps said.
The budget also requests $24 million to expand maternal mortality review programs to every state, said Dr. Fink. Currently, 43 states and the District of Columbia, have such committees, which are charged with reviewing deaths of women within a year of pregnancy or birth.
The HHS will also join with the March of Dimes to address the disparities in Black women by implementing evidence-based best practices to improve quality in hospital settings.
It is not the first time the Trump administration has taken aim at reducing maternal morbidity and mortality. In 2018, the president signed the Preventing Maternal Deaths Act, which authorized the CDC to award $50 million over 5 years so that every state could form maternal mortality review committees.
A version of this article originally appeared on Medscape.com.
Addressing Maternal Mortality Through Education: The Mommies Methadone Program
From the UT Health Long School of Medicine San Antonio, Texas.
Abstract
Objective: To educate pregnant patients with opioid use disorder (OUD) about the effects of opioids in order to improve understanding and help achieve sustained abstinence.
Methods: The Center for Health Care Services and University Hospital System (UHS) in San Antonio, TX, jointly o
Results: Of 68 women enrolled in the program, 33 completed both the pre-survey and the post-survey (48.5%). Nearly half (48%) were very motivated to quit before pregnancy, but 85% were very motivated to quit once pregnant. All participants said learning more about the effects of opiates would increase motivation for sobriety. Prior to the educational intervention, 39% of participants knew it was safe to breastfeed on methadone, which improved to 97% in the post-survey, and 76% incorrectly thought they would be reported to authorities by their health care providers if they used illegal drugs during pregnancy, while in the post-survey, 100% knew they would not be reported for doing so.
Conclusion: Pregnancy and education about opioids increased patients’ motivation to quit. Patients also advanced their breastfeeding knowledge and learned about patient-provider confidentiality. Our greatest challenge was participant follow-up; however, this improved with the help of a full-time Mommies Program nurse. Our future aim is to increase project awareness and extend the educational research.
Keywords: pregnancy; addiction; opioids; OUD; counseling.
In 2012 more than 259 million prescriptions for opioids were written in the United States, which was a 200% increase since 1998.1 Since the early 2000s, admissions to opioid substance abuse programs and the death rate from opioids have quadrupled.2-4 Specifically, the rate of heroin use increased more than 300% from 2010 to 2014.5 Opioid use in pregnancy has also escalated in recent years, with a 3- to 4-fold increase from 2000 to 2009 and with 4 in 1000 deliveries being complicated by opioid use disorder (OUD) in 2011.6-8
Between 2000 and 2014, the maternal mortality rate in the United States increased 24%, making it the only industrialized nation with a maternal mortality rate that is rising rather than falling.9 The Texas Maternal Mortality and Morbidity Task Force found that between 2012 and 2015 drug overdose was the leading cause of maternal death in the period from delivery to 365 days postpartum, and it has increased dramatically since 2010.10,11
In addition, maternal mortality reviews in several states have identified substance use as a major risk factor for pregnancy-associated deaths.12,13 In Texas between 2012 and 2015, opioids were found in 58% of maternal drug overdoses.10 In 2007, 22.8% of women who were enrolled in Medicaid programs in 46 states filled an opioid prescription during pregnancy.14 Additionally, the rising prevalence of opioid use in pregnancy has led to a sharp increase in neonatal abstinence syndrome (NAS), rising from 1.5 cases per 1000 hospital births in 1999 to 6.0 per 1000 hospital births in 2013.15 Unsurprisingly, states with the highest rates of opioid prescribing also have the highest rates of NAS.16
Methadone combined with counseling and behavioral therapy has been the standard of care for the treatment of OUD in pregnancy since the 1970s. Methadone treatment prevents opioid withdrawal symptoms and increases adherence to prenatal care.17 One of the largest methadone treatment clinics in the San Antonio, TX, area is the Center for Health Care Services (CHCS). University Health System in San Antonio (UHS) has established a clinic called The Mommies Program, where mothers addicted to opioids can receive prenatal care by a dedicated physician, registered nurse, and a certified nurse midwife, who work in collaboration with the CHCS methadone clinic. Pregnant patients with OUD in pregnancy are concurrently enrolled in the Mommies Program and receive prenatal care through UHS and methadone treatment and counseling through CHCS. The continuity effort aims to increase prenatal care rates and adherence to methadone treatment.
Once mothers are off illicit opioids and on methadone, it is essential to discuss breastfeeding with them, as many mothers addicted to illicit opioids may have been told that they should not be breastfeeding. However, breastfeeding should be encouraged in women who are stable on methadone if they are not using illicit drugs and do not have other contraindications, regardless of maternal methadone dose, since the transfer of methadone into breast milk is minimal.18-20 Breastfeeding is beneficial in women taking methadone and has been associated with decreased severity of NAS symptoms, decreased need for pharmacotherapy, and a shorter hospital stay for the baby.21 In addition, breastfeeding contributes to the development of an attachment between mother and infant, while also providing the infant with natural immunity. Women should be counseled about the need to stop breastfeeding in the event of a relapse.22
Finally, the postpartum period represents a time of increased stressors, such as loss of sleep, child protective services involvement, and frustration with constant demands from new baby. For mothers with addiction, this is an especially sensitive time, as the stressors may be exacerbated by their new sobriety and a sudden end to the motivation they experienced from pregnancy.23 Therefore, early and frequent postpartum care with methadone dose evaluation is essential in order to decrease drug relapse and screen for postpartum depression in detail, since patients with a history of drug use are at increased risk of postpartum depression.
In 2017 medical students at UT Health Long School of Medicine in San Antonio created a project to educate women about OUD in pregnancy and provide motivational incentives for sustained abstinence; this project has continued each year since. Students provide education about methadone treatment and the dangers of using illicit opioids during and after pregnancy. Students especially focus on educating patients on the key problem areas in the literature, such as overdose, NAS, breastfeeding, postpartum substance use, and postpartum depression.
Methods
From October 2018 to February 2020, a total of 15 medical students volunteered between 1 and 20 times at the Mommies Program clinic, which was held once or twice per week from 8
The only inclusion criteria for participating in the educational intervention and survey was participants had to be 18 years of age or older and enrolled in the Mommies Program. Patients who met the inclusion criteria and agreed to participate completed a pre-survey administered by the students during the patient’s initial prenatal visit (Figure 2). This survey collected baseline information about the patient’s history with opioid use and their current knowledge of methadone treatment, NAS, legal aspects of drug use disclosure, and drug testing prior to the education portion of the encounter. After the pre-survey was administered, students spent 30 minutes reviewing the correct answers of the survey with the patients by utilizing the standardized handout to help patients understand details of methadone and opioid use in pregnancy (Figure 1). The post-survey was administered by a student once patients entered the third trimester to assess whether the education session increased patients’ knowledge of these topics.

At the time patients completed the post-survey, they received a Baby Bag as well as education regarding each item in the bag. The aim of distributing Baby Bags was to relieve some possible postnatal stressors and educate the patients about infant care. Items included in the bag were diapers, wipes, bottles, clothes, and swaddles. Prenatal vitamins were added in January 2020, as many patients struggle to afford vitamins if they are not currently covered by Medicaid or have other barriers. The Baby Bag items were purchased through a Community Service Learning grant through UT Health San Antonio.
Results
Of 68 women enrolled in the Mommies Program during the intervention period, 33 completed the pre-survey and the post-survey (48.5%). Even though all patients enrolled in the program met the inclusion criteria, patients were not included in the educational program for multiple reasons, including refusal to participate, poor clinic follow-up, or lack of students to collect surveys. However, all patients who completed the pre-survey did complete the post-survey. In the pre-survey, only 39% of participants knew it was safe to breastfeed while on methadone. In the post-survey, 97% knew it safe to breastfeed. Nearly half (48%) reported being very motivated to quit opioids before pregnancy, but 85% were very motivated to quit once pregnant. In the pre-survey, 76% incorrectly thought they would be reported to authorities by their health providers if they used illegal drugs during pregnancy, while in the post-survey, 100% knew they would not be reported for doing so. Also, all participants said learning more about the effects of opiates would increase motivation for sobriety.
Discussion
Questions assessed during the educational surveys revolved around patients’ knowledge of the intricacies, legally and physiologically, of methadone treatment for OUD, as well as beneficial aspects for patients and future child health, such as breastfeeding and motivation to quit and stay sober.
It was clear that there was a lack of knowledge and education about breastfeeding, as only 39% of the participants thought that it was safe to breastfeed while on methadone in the pre-survey; in the post survey, this improved to 97%. Students spent a large portion of the educational time going over the safety of breastfeeding for patients on methadone and the many benefits to mother and baby. Students also reviewed breastfeeding with patients every time patients came in for a visit and debunked any falsehoods about the negatives of breastfeeding while on methadone. This is another testament to the benefits of reinforcement around patient education.
The area of trust between provider and patient is essential in all provider-patient relationships. However, in the area of addiction, a trusting bond is especially important, as patients must feel confident and comfortable to disclose every aspect of their lives so the provider can give the best care. It was clear from our initial data that many patients did not feel this trust or understand the legal aspects regarding the provider-patient relationship in the terms of drug use, as the pre-survey shows 76% of patients originally thought they would be reported to authorities if they told their provider they used illegal drugs during pregnancy. This was an enormous issue in the clinic and something that needed to be addressed because, based on these data, we feared many patients would not be honest about using illegal drugs to supplement their methadone if they believed they would be reported to the authorities or even jailed. The medical student education team continually assured patients that their honesty about illegal drug use during pregnancy would not be revealed to the authorities, and also made it clear to patients that it was essential they were honest about illegal drug use so the optimal care could be provided by the team. These discussions were successful, as the post-survey showed that 100% of patients knew they would not be reported to the authorities if they used illegal drugs during the pregnancy. This showed an increase in knowledge, but also suggested an increase in confidence in the provider-patient relationship by patients, which we speculate allowed for a better patient experience, better patient outcomes, and less emotional stress for the patient and provider.
Last, we wanted to study and address the motivation to quit using drugs and stay sober through learning about the effects of opiates and how this motivation was related to pregnancy. A study by Mitchell et al makes clear that pregnancy is a motivation to seek treatment for drug use and to quit,24 and our survey data support these findings, with 48% of patients motivated to quit before they were pregnant and 85% motivated to quit once they knew they were pregnant. In addition, all patients attested on the pre- and post-survey that learning more about opioids would increase their motivation for sobriety. Therefore, we believe education about the use of opioids and other drugs is a strong motivation towards sobriety and should be further studied in methadone treatment and other drugs as well.
We will continue to focus on sobriety postpartum by using the education in pregnancy as a springboard to further postpartum education, as education seems to be very beneficial to future sobriety. In the future, we believe extending the educational program past pregnancy and discussing opioid use and addiction with patients at multiple follow-up visits will be beneficial to patients’ sobriety.
We faced 2 main challenges in implementing this intervention and survey: patients would often miss multiple appointments during their third trimester or would not attend their postpartum visit if they only had 1 prenatal visit; and many clinic sessions had low student attendance because students often had many other responsibilities in medical school and there were only 15 volunteers over the study time. These challenges decreased our post-survey completion rate. However, there has been improvement in follow-up as the project has continued. The Mommies Program now has a full-time registered nurse, and a larger number of medical student teachers have volunteered to attend the clinic. In the future, we aim to increase awareness of our project and the benefits of participation, expand advertising at our medical school to increase student participation, and increase follow-up education in the postpartum period.
Another future direction is to include local, free doula services, which are offered through Catholic Charities in San Antonio. Doulas provide antepartum, intrapartum, and postpartum services, which we believe will help our patients through advocacy and support for sobriety during this emotional and stressful time.
Conclusion
Counseled participants were receptive to learning about the effects of OUD and methadone on themselves and their newborn. Participants unanimously stated that learning more about OUD increased their motivation for sobriety. It was also clear that the increased motivation to be sober during pregnancy, as compared to before pregnancy, is an opportunity to help these women take steps to get sober. Patients also advanced their breastfeeding knowledge, as we helped debunk falsehoods surrounding breastfeeding while on methadone, and we anticipate this will lead to greater breastfeeding rates for our patients on methadone, although this was not specifically studied. Finally, patients learned about patient-provider confidentiality, which allowed for more open and clear communication with patients so they could be cared for to the greatest degree and trust could remain paramount.
Drug use is a common problem in the health care system, and exposure to patients with addiction is important for medical students in training. We believe that attending the Mommies Program allowed medical students to gain exposure and skills to better help patients with OUD.
Corresponding author: Nicholas Stansbury, MD, [email protected].
Financial disclosures: None.
1. Centers for Disease Control and Prevention. Opioid painkiller prescribing: where you live makes a difference. CDC website. www.cdc.gov/vitalsigns/opioid-prescribing. Accessed October 28, 2020.
2. Substance Abuse and Mental Health Services Administration. Drug Abuse Warning Network, 2011: national estimates of drug-related emergency department visits. HHS Publication No. (SMA) 13-4760, DAWN Series D-39. Rockville (MD): SAMHSA; 2013. www.samhsa.gov/data/sites/default/files/DAWN2k11ED/DAWN2k11ED/DAWN2k11ED.pdf. Accessed October 28, 2020.
3. Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med. 2016;374:154-63.
4. National Center for Health Statistics. NCHS data on drug-poisoning deaths. NCHS Factsheet. https://www.cdc.gov/nchs/data/factsheets/factsheet-drug-poisoning-H.pdf. Accessed October 28, 2020.
5. National Institute on Drug Abuse. America’s addiction to opioids: heroin and prescription drug abuse. Bethesda (MD): NIDA; 2014. www.drugabuse.gov/about-nida/legislative-activities/testimony-to-congress/2016/americas-addiction-to-opioids-heroin-prescription-drug-abuse. Accessed October 28, 2020.
6. Substance Abuse and Mental Health Services Administration (SAMHSA). Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: SAMHSA, 2011 Contract No.: HHS Publication no. (SMA) 11–4658.
7. Maeda A, Bateman BT, Clancy CR, et al. Opioid abuse and dependence during pregnancy: temporal trends and obstetrical outcomes. Anesthesiology. 2014;121:1158-1165.
8. Whiteman VE, Salemi JL, Mogos MF, et al. Maternal opioid drug use during pregnancy and its impact on perinatal morbidity, mortality, and the costs of medical care in the United States. J Pregnancy. 2014;2014:1-8
9. Pregnancy Mortality Surveillance System. www.cdc.gov/reproductivehealth/maternal-mortality/pregnancy-mortality-surveillance-system.htm#trends. Accessed February 4, 2020.
10. Macdorman MF, Declercq E, Cabral H, Morton C. Recent increases in the U.S. maternal mortality rate. Obstet Gynecol. 2016;128:447-455.
11. Texas Health and Human Services. Maternal Mortality and Morbidity Task Force and Department of State Health Services Joint Biennial Report, September 2018. www.dshs.texas.gov/legislative/2018-Reports/MMMTFJointReport2018.pdf
12. Virginia Department of Health. Pregnancy-associated deaths from drug overdose in Virginia, 1999-2007: a report from the Virginia Maternal Mortality Review Team. Richmond, VA: VDH; 2015. www.vdh.virginia.gov/content/uploads/sites/18/2016/04/Final-Pregnancy-Associated-Deaths-Due-to-Drug-Overdose.pdf. Accessed October 28, 2020.
13. Maryland Department of Health and Mental Hygiene. Maryland maternal mortality review 2016 annual report. Baltimore: DHMH; 2016. https://phpa.health.maryland.gov/Documents/Maryland-Maternal-Mortality-Review-2016-Report.pdf. Accessed October 28, 2020.
14. Desai RJ, Hernandez-Diaz S, Bateman BT, Huybrechts KF. Increase in prescription opioid use during pregnancy among Medicaid-enrolled women. Obstet Gynecol. 2014;123:997-1002.
15. Reddy UM, Davis JM, Ren Z, et al. Opioid use in pregnancy, neonatal abstinence syndrome, and childhood outcomes. Obstet Gynecol Survey. 2017;72:703-705.
16. Patrick SW, Davis MM, Lehmann CU, Cooper WO. Increasing incidence and geographic distribution of neonatal abstinence syndrome: United States 2009 to 2012. J Perinatol. 2015;35:650-655.
17. Center for Substance Abuse Treatment. Medication-assisted treatment for opioid addiction during pregnancy. In: Medication-assisted treatment for opioid addiction in opioid treatment programs. Treatment Improvement Protocol (TIP) Series, No. 43. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2005:211-224.
18. Wojnar-Horton RE, Kristensen JH, Yapp P, et al. Methadone distribution and excretion into breast milk of clients in a methadone maintenance programme. Br J Clin Pharmacol. 1997;44:543-547.
19. Reece-Stremtan S, Marinelli KA. ABM clinical protocol #21: guidelines for breastfeeding and substance use or substance use disorder, revised 2015. Breastfeed Med. 2015;10:135-141.
20. Sachs HC. The transfer of drugs and therapeutics into human breast milk: an update on selected topics. Committee on Drugs. Pediatrics. 2013;132:e796-809.
21. Bagley SM, Wachman EM, Holland E, Brogly SB. Review of the assessment and management of neonatal abstinence syndrome. Addict Sci Clin Pract. 2014;9:19.
22. Opioid use and opioid use disorder in pregnancy. Committee Opinion No. 711. Obstet Gynecol. 2017;130:488-489.
23. Gopman S. Prenatal and postpartum care of women with substance use disorders. Obstet Gynecol Clin North Am. 2014;41:213-228.
24. Mitchell M, Severtson S, Latimer W. Pregnancy and race/ethnicity as predictors of motivation for drug treatment. Am J Drug Alcohol Abuse. 2008;34:397-404.
From the UT Health Long School of Medicine San Antonio, Texas.
Abstract
Objective: To educate pregnant patients with opioid use disorder (OUD) about the effects of opioids in order to improve understanding and help achieve sustained abstinence.
Methods: The Center for Health Care Services and University Hospital System (UHS) in San Antonio, TX, jointly o
Results: Of 68 women enrolled in the program, 33 completed both the pre-survey and the post-survey (48.5%). Nearly half (48%) were very motivated to quit before pregnancy, but 85% were very motivated to quit once pregnant. All participants said learning more about the effects of opiates would increase motivation for sobriety. Prior to the educational intervention, 39% of participants knew it was safe to breastfeed on methadone, which improved to 97% in the post-survey, and 76% incorrectly thought they would be reported to authorities by their health care providers if they used illegal drugs during pregnancy, while in the post-survey, 100% knew they would not be reported for doing so.
Conclusion: Pregnancy and education about opioids increased patients’ motivation to quit. Patients also advanced their breastfeeding knowledge and learned about patient-provider confidentiality. Our greatest challenge was participant follow-up; however, this improved with the help of a full-time Mommies Program nurse. Our future aim is to increase project awareness and extend the educational research.
Keywords: pregnancy; addiction; opioids; OUD; counseling.
In 2012 more than 259 million prescriptions for opioids were written in the United States, which was a 200% increase since 1998.1 Since the early 2000s, admissions to opioid substance abuse programs and the death rate from opioids have quadrupled.2-4 Specifically, the rate of heroin use increased more than 300% from 2010 to 2014.5 Opioid use in pregnancy has also escalated in recent years, with a 3- to 4-fold increase from 2000 to 2009 and with 4 in 1000 deliveries being complicated by opioid use disorder (OUD) in 2011.6-8
Between 2000 and 2014, the maternal mortality rate in the United States increased 24%, making it the only industrialized nation with a maternal mortality rate that is rising rather than falling.9 The Texas Maternal Mortality and Morbidity Task Force found that between 2012 and 2015 drug overdose was the leading cause of maternal death in the period from delivery to 365 days postpartum, and it has increased dramatically since 2010.10,11
In addition, maternal mortality reviews in several states have identified substance use as a major risk factor for pregnancy-associated deaths.12,13 In Texas between 2012 and 2015, opioids were found in 58% of maternal drug overdoses.10 In 2007, 22.8% of women who were enrolled in Medicaid programs in 46 states filled an opioid prescription during pregnancy.14 Additionally, the rising prevalence of opioid use in pregnancy has led to a sharp increase in neonatal abstinence syndrome (NAS), rising from 1.5 cases per 1000 hospital births in 1999 to 6.0 per 1000 hospital births in 2013.15 Unsurprisingly, states with the highest rates of opioid prescribing also have the highest rates of NAS.16
Methadone combined with counseling and behavioral therapy has been the standard of care for the treatment of OUD in pregnancy since the 1970s. Methadone treatment prevents opioid withdrawal symptoms and increases adherence to prenatal care.17 One of the largest methadone treatment clinics in the San Antonio, TX, area is the Center for Health Care Services (CHCS). University Health System in San Antonio (UHS) has established a clinic called The Mommies Program, where mothers addicted to opioids can receive prenatal care by a dedicated physician, registered nurse, and a certified nurse midwife, who work in collaboration with the CHCS methadone clinic. Pregnant patients with OUD in pregnancy are concurrently enrolled in the Mommies Program and receive prenatal care through UHS and methadone treatment and counseling through CHCS. The continuity effort aims to increase prenatal care rates and adherence to methadone treatment.
Once mothers are off illicit opioids and on methadone, it is essential to discuss breastfeeding with them, as many mothers addicted to illicit opioids may have been told that they should not be breastfeeding. However, breastfeeding should be encouraged in women who are stable on methadone if they are not using illicit drugs and do not have other contraindications, regardless of maternal methadone dose, since the transfer of methadone into breast milk is minimal.18-20 Breastfeeding is beneficial in women taking methadone and has been associated with decreased severity of NAS symptoms, decreased need for pharmacotherapy, and a shorter hospital stay for the baby.21 In addition, breastfeeding contributes to the development of an attachment between mother and infant, while also providing the infant with natural immunity. Women should be counseled about the need to stop breastfeeding in the event of a relapse.22
Finally, the postpartum period represents a time of increased stressors, such as loss of sleep, child protective services involvement, and frustration with constant demands from new baby. For mothers with addiction, this is an especially sensitive time, as the stressors may be exacerbated by their new sobriety and a sudden end to the motivation they experienced from pregnancy.23 Therefore, early and frequent postpartum care with methadone dose evaluation is essential in order to decrease drug relapse and screen for postpartum depression in detail, since patients with a history of drug use are at increased risk of postpartum depression.
In 2017 medical students at UT Health Long School of Medicine in San Antonio created a project to educate women about OUD in pregnancy and provide motivational incentives for sustained abstinence; this project has continued each year since. Students provide education about methadone treatment and the dangers of using illicit opioids during and after pregnancy. Students especially focus on educating patients on the key problem areas in the literature, such as overdose, NAS, breastfeeding, postpartum substance use, and postpartum depression.
Methods
From October 2018 to February 2020, a total of 15 medical students volunteered between 1 and 20 times at the Mommies Program clinic, which was held once or twice per week from 8

The only inclusion criteria for participating in the educational intervention and survey was participants had to be 18 years of age or older and enrolled in the Mommies Program. Patients who met the inclusion criteria and agreed to participate completed a pre-survey administered by the students during the patient’s initial prenatal visit (Figure 2). This survey collected baseline information about the patient’s history with opioid use and their current knowledge of methadone treatment, NAS, legal aspects of drug use disclosure, and drug testing prior to the education portion of the encounter. After the pre-survey was administered, students spent 30 minutes reviewing the correct answers of the survey with the patients by utilizing the standardized handout to help patients understand details of methadone and opioid use in pregnancy (Figure 1). The post-survey was administered by a student once patients entered the third trimester to assess whether the education session increased patients’ knowledge of these topics.

At the time patients completed the post-survey, they received a Baby Bag as well as education regarding each item in the bag. The aim of distributing Baby Bags was to relieve some possible postnatal stressors and educate the patients about infant care. Items included in the bag were diapers, wipes, bottles, clothes, and swaddles. Prenatal vitamins were added in January 2020, as many patients struggle to afford vitamins if they are not currently covered by Medicaid or have other barriers. The Baby Bag items were purchased through a Community Service Learning grant through UT Health San Antonio.
Results
Of 68 women enrolled in the Mommies Program during the intervention period, 33 completed the pre-survey and the post-survey (48.5%). Even though all patients enrolled in the program met the inclusion criteria, patients were not included in the educational program for multiple reasons, including refusal to participate, poor clinic follow-up, or lack of students to collect surveys. However, all patients who completed the pre-survey did complete the post-survey. In the pre-survey, only 39% of participants knew it was safe to breastfeed while on methadone. In the post-survey, 97% knew it safe to breastfeed. Nearly half (48%) reported being very motivated to quit opioids before pregnancy, but 85% were very motivated to quit once pregnant. In the pre-survey, 76% incorrectly thought they would be reported to authorities by their health providers if they used illegal drugs during pregnancy, while in the post-survey, 100% knew they would not be reported for doing so. Also, all participants said learning more about the effects of opiates would increase motivation for sobriety.
Discussion
Questions assessed during the educational surveys revolved around patients’ knowledge of the intricacies, legally and physiologically, of methadone treatment for OUD, as well as beneficial aspects for patients and future child health, such as breastfeeding and motivation to quit and stay sober.
It was clear that there was a lack of knowledge and education about breastfeeding, as only 39% of the participants thought that it was safe to breastfeed while on methadone in the pre-survey; in the post survey, this improved to 97%. Students spent a large portion of the educational time going over the safety of breastfeeding for patients on methadone and the many benefits to mother and baby. Students also reviewed breastfeeding with patients every time patients came in for a visit and debunked any falsehoods about the negatives of breastfeeding while on methadone. This is another testament to the benefits of reinforcement around patient education.
The area of trust between provider and patient is essential in all provider-patient relationships. However, in the area of addiction, a trusting bond is especially important, as patients must feel confident and comfortable to disclose every aspect of their lives so the provider can give the best care. It was clear from our initial data that many patients did not feel this trust or understand the legal aspects regarding the provider-patient relationship in the terms of drug use, as the pre-survey shows 76% of patients originally thought they would be reported to authorities if they told their provider they used illegal drugs during pregnancy. This was an enormous issue in the clinic and something that needed to be addressed because, based on these data, we feared many patients would not be honest about using illegal drugs to supplement their methadone if they believed they would be reported to the authorities or even jailed. The medical student education team continually assured patients that their honesty about illegal drug use during pregnancy would not be revealed to the authorities, and also made it clear to patients that it was essential they were honest about illegal drug use so the optimal care could be provided by the team. These discussions were successful, as the post-survey showed that 100% of patients knew they would not be reported to the authorities if they used illegal drugs during the pregnancy. This showed an increase in knowledge, but also suggested an increase in confidence in the provider-patient relationship by patients, which we speculate allowed for a better patient experience, better patient outcomes, and less emotional stress for the patient and provider.
Last, we wanted to study and address the motivation to quit using drugs and stay sober through learning about the effects of opiates and how this motivation was related to pregnancy. A study by Mitchell et al makes clear that pregnancy is a motivation to seek treatment for drug use and to quit,24 and our survey data support these findings, with 48% of patients motivated to quit before they were pregnant and 85% motivated to quit once they knew they were pregnant. In addition, all patients attested on the pre- and post-survey that learning more about opioids would increase their motivation for sobriety. Therefore, we believe education about the use of opioids and other drugs is a strong motivation towards sobriety and should be further studied in methadone treatment and other drugs as well.
We will continue to focus on sobriety postpartum by using the education in pregnancy as a springboard to further postpartum education, as education seems to be very beneficial to future sobriety. In the future, we believe extending the educational program past pregnancy and discussing opioid use and addiction with patients at multiple follow-up visits will be beneficial to patients’ sobriety.
We faced 2 main challenges in implementing this intervention and survey: patients would often miss multiple appointments during their third trimester or would not attend their postpartum visit if they only had 1 prenatal visit; and many clinic sessions had low student attendance because students often had many other responsibilities in medical school and there were only 15 volunteers over the study time. These challenges decreased our post-survey completion rate. However, there has been improvement in follow-up as the project has continued. The Mommies Program now has a full-time registered nurse, and a larger number of medical student teachers have volunteered to attend the clinic. In the future, we aim to increase awareness of our project and the benefits of participation, expand advertising at our medical school to increase student participation, and increase follow-up education in the postpartum period.
Another future direction is to include local, free doula services, which are offered through Catholic Charities in San Antonio. Doulas provide antepartum, intrapartum, and postpartum services, which we believe will help our patients through advocacy and support for sobriety during this emotional and stressful time.
Conclusion
Counseled participants were receptive to learning about the effects of OUD and methadone on themselves and their newborn. Participants unanimously stated that learning more about OUD increased their motivation for sobriety. It was also clear that the increased motivation to be sober during pregnancy, as compared to before pregnancy, is an opportunity to help these women take steps to get sober. Patients also advanced their breastfeeding knowledge, as we helped debunk falsehoods surrounding breastfeeding while on methadone, and we anticipate this will lead to greater breastfeeding rates for our patients on methadone, although this was not specifically studied. Finally, patients learned about patient-provider confidentiality, which allowed for more open and clear communication with patients so they could be cared for to the greatest degree and trust could remain paramount.
Drug use is a common problem in the health care system, and exposure to patients with addiction is important for medical students in training. We believe that attending the Mommies Program allowed medical students to gain exposure and skills to better help patients with OUD.
Corresponding author: Nicholas Stansbury, MD, [email protected].
Financial disclosures: None.
From the UT Health Long School of Medicine San Antonio, Texas.
Abstract
Objective: To educate pregnant patients with opioid use disorder (OUD) about the effects of opioids in order to improve understanding and help achieve sustained abstinence.
Methods: The Center for Health Care Services and University Hospital System (UHS) in San Antonio, TX, jointly o
Results: Of 68 women enrolled in the program, 33 completed both the pre-survey and the post-survey (48.5%). Nearly half (48%) were very motivated to quit before pregnancy, but 85% were very motivated to quit once pregnant. All participants said learning more about the effects of opiates would increase motivation for sobriety. Prior to the educational intervention, 39% of participants knew it was safe to breastfeed on methadone, which improved to 97% in the post-survey, and 76% incorrectly thought they would be reported to authorities by their health care providers if they used illegal drugs during pregnancy, while in the post-survey, 100% knew they would not be reported for doing so.
Conclusion: Pregnancy and education about opioids increased patients’ motivation to quit. Patients also advanced their breastfeeding knowledge and learned about patient-provider confidentiality. Our greatest challenge was participant follow-up; however, this improved with the help of a full-time Mommies Program nurse. Our future aim is to increase project awareness and extend the educational research.
Keywords: pregnancy; addiction; opioids; OUD; counseling.
In 2012 more than 259 million prescriptions for opioids were written in the United States, which was a 200% increase since 1998.1 Since the early 2000s, admissions to opioid substance abuse programs and the death rate from opioids have quadrupled.2-4 Specifically, the rate of heroin use increased more than 300% from 2010 to 2014.5 Opioid use in pregnancy has also escalated in recent years, with a 3- to 4-fold increase from 2000 to 2009 and with 4 in 1000 deliveries being complicated by opioid use disorder (OUD) in 2011.6-8
Between 2000 and 2014, the maternal mortality rate in the United States increased 24%, making it the only industrialized nation with a maternal mortality rate that is rising rather than falling.9 The Texas Maternal Mortality and Morbidity Task Force found that between 2012 and 2015 drug overdose was the leading cause of maternal death in the period from delivery to 365 days postpartum, and it has increased dramatically since 2010.10,11
In addition, maternal mortality reviews in several states have identified substance use as a major risk factor for pregnancy-associated deaths.12,13 In Texas between 2012 and 2015, opioids were found in 58% of maternal drug overdoses.10 In 2007, 22.8% of women who were enrolled in Medicaid programs in 46 states filled an opioid prescription during pregnancy.14 Additionally, the rising prevalence of opioid use in pregnancy has led to a sharp increase in neonatal abstinence syndrome (NAS), rising from 1.5 cases per 1000 hospital births in 1999 to 6.0 per 1000 hospital births in 2013.15 Unsurprisingly, states with the highest rates of opioid prescribing also have the highest rates of NAS.16
Methadone combined with counseling and behavioral therapy has been the standard of care for the treatment of OUD in pregnancy since the 1970s. Methadone treatment prevents opioid withdrawal symptoms and increases adherence to prenatal care.17 One of the largest methadone treatment clinics in the San Antonio, TX, area is the Center for Health Care Services (CHCS). University Health System in San Antonio (UHS) has established a clinic called The Mommies Program, where mothers addicted to opioids can receive prenatal care by a dedicated physician, registered nurse, and a certified nurse midwife, who work in collaboration with the CHCS methadone clinic. Pregnant patients with OUD in pregnancy are concurrently enrolled in the Mommies Program and receive prenatal care through UHS and methadone treatment and counseling through CHCS. The continuity effort aims to increase prenatal care rates and adherence to methadone treatment.
Once mothers are off illicit opioids and on methadone, it is essential to discuss breastfeeding with them, as many mothers addicted to illicit opioids may have been told that they should not be breastfeeding. However, breastfeeding should be encouraged in women who are stable on methadone if they are not using illicit drugs and do not have other contraindications, regardless of maternal methadone dose, since the transfer of methadone into breast milk is minimal.18-20 Breastfeeding is beneficial in women taking methadone and has been associated with decreased severity of NAS symptoms, decreased need for pharmacotherapy, and a shorter hospital stay for the baby.21 In addition, breastfeeding contributes to the development of an attachment between mother and infant, while also providing the infant with natural immunity. Women should be counseled about the need to stop breastfeeding in the event of a relapse.22
Finally, the postpartum period represents a time of increased stressors, such as loss of sleep, child protective services involvement, and frustration with constant demands from new baby. For mothers with addiction, this is an especially sensitive time, as the stressors may be exacerbated by their new sobriety and a sudden end to the motivation they experienced from pregnancy.23 Therefore, early and frequent postpartum care with methadone dose evaluation is essential in order to decrease drug relapse and screen for postpartum depression in detail, since patients with a history of drug use are at increased risk of postpartum depression.
In 2017 medical students at UT Health Long School of Medicine in San Antonio created a project to educate women about OUD in pregnancy and provide motivational incentives for sustained abstinence; this project has continued each year since. Students provide education about methadone treatment and the dangers of using illicit opioids during and after pregnancy. Students especially focus on educating patients on the key problem areas in the literature, such as overdose, NAS, breastfeeding, postpartum substance use, and postpartum depression.
Methods
From October 2018 to February 2020, a total of 15 medical students volunteered between 1 and 20 times at the Mommies Program clinic, which was held once or twice per week from 8

The only inclusion criteria for participating in the educational intervention and survey was participants had to be 18 years of age or older and enrolled in the Mommies Program. Patients who met the inclusion criteria and agreed to participate completed a pre-survey administered by the students during the patient’s initial prenatal visit (Figure 2). This survey collected baseline information about the patient’s history with opioid use and their current knowledge of methadone treatment, NAS, legal aspects of drug use disclosure, and drug testing prior to the education portion of the encounter. After the pre-survey was administered, students spent 30 minutes reviewing the correct answers of the survey with the patients by utilizing the standardized handout to help patients understand details of methadone and opioid use in pregnancy (Figure 1). The post-survey was administered by a student once patients entered the third trimester to assess whether the education session increased patients’ knowledge of these topics.

At the time patients completed the post-survey, they received a Baby Bag as well as education regarding each item in the bag. The aim of distributing Baby Bags was to relieve some possible postnatal stressors and educate the patients about infant care. Items included in the bag were diapers, wipes, bottles, clothes, and swaddles. Prenatal vitamins were added in January 2020, as many patients struggle to afford vitamins if they are not currently covered by Medicaid or have other barriers. The Baby Bag items were purchased through a Community Service Learning grant through UT Health San Antonio.
Results
Of 68 women enrolled in the Mommies Program during the intervention period, 33 completed the pre-survey and the post-survey (48.5%). Even though all patients enrolled in the program met the inclusion criteria, patients were not included in the educational program for multiple reasons, including refusal to participate, poor clinic follow-up, or lack of students to collect surveys. However, all patients who completed the pre-survey did complete the post-survey. In the pre-survey, only 39% of participants knew it was safe to breastfeed while on methadone. In the post-survey, 97% knew it safe to breastfeed. Nearly half (48%) reported being very motivated to quit opioids before pregnancy, but 85% were very motivated to quit once pregnant. In the pre-survey, 76% incorrectly thought they would be reported to authorities by their health providers if they used illegal drugs during pregnancy, while in the post-survey, 100% knew they would not be reported for doing so. Also, all participants said learning more about the effects of opiates would increase motivation for sobriety.
Discussion
Questions assessed during the educational surveys revolved around patients’ knowledge of the intricacies, legally and physiologically, of methadone treatment for OUD, as well as beneficial aspects for patients and future child health, such as breastfeeding and motivation to quit and stay sober.
It was clear that there was a lack of knowledge and education about breastfeeding, as only 39% of the participants thought that it was safe to breastfeed while on methadone in the pre-survey; in the post survey, this improved to 97%. Students spent a large portion of the educational time going over the safety of breastfeeding for patients on methadone and the many benefits to mother and baby. Students also reviewed breastfeeding with patients every time patients came in for a visit and debunked any falsehoods about the negatives of breastfeeding while on methadone. This is another testament to the benefits of reinforcement around patient education.
The area of trust between provider and patient is essential in all provider-patient relationships. However, in the area of addiction, a trusting bond is especially important, as patients must feel confident and comfortable to disclose every aspect of their lives so the provider can give the best care. It was clear from our initial data that many patients did not feel this trust or understand the legal aspects regarding the provider-patient relationship in the terms of drug use, as the pre-survey shows 76% of patients originally thought they would be reported to authorities if they told their provider they used illegal drugs during pregnancy. This was an enormous issue in the clinic and something that needed to be addressed because, based on these data, we feared many patients would not be honest about using illegal drugs to supplement their methadone if they believed they would be reported to the authorities or even jailed. The medical student education team continually assured patients that their honesty about illegal drug use during pregnancy would not be revealed to the authorities, and also made it clear to patients that it was essential they were honest about illegal drug use so the optimal care could be provided by the team. These discussions were successful, as the post-survey showed that 100% of patients knew they would not be reported to the authorities if they used illegal drugs during the pregnancy. This showed an increase in knowledge, but also suggested an increase in confidence in the provider-patient relationship by patients, which we speculate allowed for a better patient experience, better patient outcomes, and less emotional stress for the patient and provider.
Last, we wanted to study and address the motivation to quit using drugs and stay sober through learning about the effects of opiates and how this motivation was related to pregnancy. A study by Mitchell et al makes clear that pregnancy is a motivation to seek treatment for drug use and to quit,24 and our survey data support these findings, with 48% of patients motivated to quit before they were pregnant and 85% motivated to quit once they knew they were pregnant. In addition, all patients attested on the pre- and post-survey that learning more about opioids would increase their motivation for sobriety. Therefore, we believe education about the use of opioids and other drugs is a strong motivation towards sobriety and should be further studied in methadone treatment and other drugs as well.
We will continue to focus on sobriety postpartum by using the education in pregnancy as a springboard to further postpartum education, as education seems to be very beneficial to future sobriety. In the future, we believe extending the educational program past pregnancy and discussing opioid use and addiction with patients at multiple follow-up visits will be beneficial to patients’ sobriety.
We faced 2 main challenges in implementing this intervention and survey: patients would often miss multiple appointments during their third trimester or would not attend their postpartum visit if they only had 1 prenatal visit; and many clinic sessions had low student attendance because students often had many other responsibilities in medical school and there were only 15 volunteers over the study time. These challenges decreased our post-survey completion rate. However, there has been improvement in follow-up as the project has continued. The Mommies Program now has a full-time registered nurse, and a larger number of medical student teachers have volunteered to attend the clinic. In the future, we aim to increase awareness of our project and the benefits of participation, expand advertising at our medical school to increase student participation, and increase follow-up education in the postpartum period.
Another future direction is to include local, free doula services, which are offered through Catholic Charities in San Antonio. Doulas provide antepartum, intrapartum, and postpartum services, which we believe will help our patients through advocacy and support for sobriety during this emotional and stressful time.
Conclusion
Counseled participants were receptive to learning about the effects of OUD and methadone on themselves and their newborn. Participants unanimously stated that learning more about OUD increased their motivation for sobriety. It was also clear that the increased motivation to be sober during pregnancy, as compared to before pregnancy, is an opportunity to help these women take steps to get sober. Patients also advanced their breastfeeding knowledge, as we helped debunk falsehoods surrounding breastfeeding while on methadone, and we anticipate this will lead to greater breastfeeding rates for our patients on methadone, although this was not specifically studied. Finally, patients learned about patient-provider confidentiality, which allowed for more open and clear communication with patients so they could be cared for to the greatest degree and trust could remain paramount.
Drug use is a common problem in the health care system, and exposure to patients with addiction is important for medical students in training. We believe that attending the Mommies Program allowed medical students to gain exposure and skills to better help patients with OUD.
Corresponding author: Nicholas Stansbury, MD, [email protected].
Financial disclosures: None.
1. Centers for Disease Control and Prevention. Opioid painkiller prescribing: where you live makes a difference. CDC website. www.cdc.gov/vitalsigns/opioid-prescribing. Accessed October 28, 2020.
2. Substance Abuse and Mental Health Services Administration. Drug Abuse Warning Network, 2011: national estimates of drug-related emergency department visits. HHS Publication No. (SMA) 13-4760, DAWN Series D-39. Rockville (MD): SAMHSA; 2013. www.samhsa.gov/data/sites/default/files/DAWN2k11ED/DAWN2k11ED/DAWN2k11ED.pdf. Accessed October 28, 2020.
3. Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med. 2016;374:154-63.
4. National Center for Health Statistics. NCHS data on drug-poisoning deaths. NCHS Factsheet. https://www.cdc.gov/nchs/data/factsheets/factsheet-drug-poisoning-H.pdf. Accessed October 28, 2020.
5. National Institute on Drug Abuse. America’s addiction to opioids: heroin and prescription drug abuse. Bethesda (MD): NIDA; 2014. www.drugabuse.gov/about-nida/legislative-activities/testimony-to-congress/2016/americas-addiction-to-opioids-heroin-prescription-drug-abuse. Accessed October 28, 2020.
6. Substance Abuse and Mental Health Services Administration (SAMHSA). Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: SAMHSA, 2011 Contract No.: HHS Publication no. (SMA) 11–4658.
7. Maeda A, Bateman BT, Clancy CR, et al. Opioid abuse and dependence during pregnancy: temporal trends and obstetrical outcomes. Anesthesiology. 2014;121:1158-1165.
8. Whiteman VE, Salemi JL, Mogos MF, et al. Maternal opioid drug use during pregnancy and its impact on perinatal morbidity, mortality, and the costs of medical care in the United States. J Pregnancy. 2014;2014:1-8
9. Pregnancy Mortality Surveillance System. www.cdc.gov/reproductivehealth/maternal-mortality/pregnancy-mortality-surveillance-system.htm#trends. Accessed February 4, 2020.
10. Macdorman MF, Declercq E, Cabral H, Morton C. Recent increases in the U.S. maternal mortality rate. Obstet Gynecol. 2016;128:447-455.
11. Texas Health and Human Services. Maternal Mortality and Morbidity Task Force and Department of State Health Services Joint Biennial Report, September 2018. www.dshs.texas.gov/legislative/2018-Reports/MMMTFJointReport2018.pdf
12. Virginia Department of Health. Pregnancy-associated deaths from drug overdose in Virginia, 1999-2007: a report from the Virginia Maternal Mortality Review Team. Richmond, VA: VDH; 2015. www.vdh.virginia.gov/content/uploads/sites/18/2016/04/Final-Pregnancy-Associated-Deaths-Due-to-Drug-Overdose.pdf. Accessed October 28, 2020.
13. Maryland Department of Health and Mental Hygiene. Maryland maternal mortality review 2016 annual report. Baltimore: DHMH; 2016. https://phpa.health.maryland.gov/Documents/Maryland-Maternal-Mortality-Review-2016-Report.pdf. Accessed October 28, 2020.
14. Desai RJ, Hernandez-Diaz S, Bateman BT, Huybrechts KF. Increase in prescription opioid use during pregnancy among Medicaid-enrolled women. Obstet Gynecol. 2014;123:997-1002.
15. Reddy UM, Davis JM, Ren Z, et al. Opioid use in pregnancy, neonatal abstinence syndrome, and childhood outcomes. Obstet Gynecol Survey. 2017;72:703-705.
16. Patrick SW, Davis MM, Lehmann CU, Cooper WO. Increasing incidence and geographic distribution of neonatal abstinence syndrome: United States 2009 to 2012. J Perinatol. 2015;35:650-655.
17. Center for Substance Abuse Treatment. Medication-assisted treatment for opioid addiction during pregnancy. In: Medication-assisted treatment for opioid addiction in opioid treatment programs. Treatment Improvement Protocol (TIP) Series, No. 43. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2005:211-224.
18. Wojnar-Horton RE, Kristensen JH, Yapp P, et al. Methadone distribution and excretion into breast milk of clients in a methadone maintenance programme. Br J Clin Pharmacol. 1997;44:543-547.
19. Reece-Stremtan S, Marinelli KA. ABM clinical protocol #21: guidelines for breastfeeding and substance use or substance use disorder, revised 2015. Breastfeed Med. 2015;10:135-141.
20. Sachs HC. The transfer of drugs and therapeutics into human breast milk: an update on selected topics. Committee on Drugs. Pediatrics. 2013;132:e796-809.
21. Bagley SM, Wachman EM, Holland E, Brogly SB. Review of the assessment and management of neonatal abstinence syndrome. Addict Sci Clin Pract. 2014;9:19.
22. Opioid use and opioid use disorder in pregnancy. Committee Opinion No. 711. Obstet Gynecol. 2017;130:488-489.
23. Gopman S. Prenatal and postpartum care of women with substance use disorders. Obstet Gynecol Clin North Am. 2014;41:213-228.
24. Mitchell M, Severtson S, Latimer W. Pregnancy and race/ethnicity as predictors of motivation for drug treatment. Am J Drug Alcohol Abuse. 2008;34:397-404.
1. Centers for Disease Control and Prevention. Opioid painkiller prescribing: where you live makes a difference. CDC website. www.cdc.gov/vitalsigns/opioid-prescribing. Accessed October 28, 2020.
2. Substance Abuse and Mental Health Services Administration. Drug Abuse Warning Network, 2011: national estimates of drug-related emergency department visits. HHS Publication No. (SMA) 13-4760, DAWN Series D-39. Rockville (MD): SAMHSA; 2013. www.samhsa.gov/data/sites/default/files/DAWN2k11ED/DAWN2k11ED/DAWN2k11ED.pdf. Accessed October 28, 2020.
3. Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med. 2016;374:154-63.
4. National Center for Health Statistics. NCHS data on drug-poisoning deaths. NCHS Factsheet. https://www.cdc.gov/nchs/data/factsheets/factsheet-drug-poisoning-H.pdf. Accessed October 28, 2020.
5. National Institute on Drug Abuse. America’s addiction to opioids: heroin and prescription drug abuse. Bethesda (MD): NIDA; 2014. www.drugabuse.gov/about-nida/legislative-activities/testimony-to-congress/2016/americas-addiction-to-opioids-heroin-prescription-drug-abuse. Accessed October 28, 2020.
6. Substance Abuse and Mental Health Services Administration (SAMHSA). Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: SAMHSA, 2011 Contract No.: HHS Publication no. (SMA) 11–4658.
7. Maeda A, Bateman BT, Clancy CR, et al. Opioid abuse and dependence during pregnancy: temporal trends and obstetrical outcomes. Anesthesiology. 2014;121:1158-1165.
8. Whiteman VE, Salemi JL, Mogos MF, et al. Maternal opioid drug use during pregnancy and its impact on perinatal morbidity, mortality, and the costs of medical care in the United States. J Pregnancy. 2014;2014:1-8
9. Pregnancy Mortality Surveillance System. www.cdc.gov/reproductivehealth/maternal-mortality/pregnancy-mortality-surveillance-system.htm#trends. Accessed February 4, 2020.
10. Macdorman MF, Declercq E, Cabral H, Morton C. Recent increases in the U.S. maternal mortality rate. Obstet Gynecol. 2016;128:447-455.
11. Texas Health and Human Services. Maternal Mortality and Morbidity Task Force and Department of State Health Services Joint Biennial Report, September 2018. www.dshs.texas.gov/legislative/2018-Reports/MMMTFJointReport2018.pdf
12. Virginia Department of Health. Pregnancy-associated deaths from drug overdose in Virginia, 1999-2007: a report from the Virginia Maternal Mortality Review Team. Richmond, VA: VDH; 2015. www.vdh.virginia.gov/content/uploads/sites/18/2016/04/Final-Pregnancy-Associated-Deaths-Due-to-Drug-Overdose.pdf. Accessed October 28, 2020.
13. Maryland Department of Health and Mental Hygiene. Maryland maternal mortality review 2016 annual report. Baltimore: DHMH; 2016. https://phpa.health.maryland.gov/Documents/Maryland-Maternal-Mortality-Review-2016-Report.pdf. Accessed October 28, 2020.
14. Desai RJ, Hernandez-Diaz S, Bateman BT, Huybrechts KF. Increase in prescription opioid use during pregnancy among Medicaid-enrolled women. Obstet Gynecol. 2014;123:997-1002.
15. Reddy UM, Davis JM, Ren Z, et al. Opioid use in pregnancy, neonatal abstinence syndrome, and childhood outcomes. Obstet Gynecol Survey. 2017;72:703-705.
16. Patrick SW, Davis MM, Lehmann CU, Cooper WO. Increasing incidence and geographic distribution of neonatal abstinence syndrome: United States 2009 to 2012. J Perinatol. 2015;35:650-655.
17. Center for Substance Abuse Treatment. Medication-assisted treatment for opioid addiction during pregnancy. In: Medication-assisted treatment for opioid addiction in opioid treatment programs. Treatment Improvement Protocol (TIP) Series, No. 43. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2005:211-224.
18. Wojnar-Horton RE, Kristensen JH, Yapp P, et al. Methadone distribution and excretion into breast milk of clients in a methadone maintenance programme. Br J Clin Pharmacol. 1997;44:543-547.
19. Reece-Stremtan S, Marinelli KA. ABM clinical protocol #21: guidelines for breastfeeding and substance use or substance use disorder, revised 2015. Breastfeed Med. 2015;10:135-141.
20. Sachs HC. The transfer of drugs and therapeutics into human breast milk: an update on selected topics. Committee on Drugs. Pediatrics. 2013;132:e796-809.
21. Bagley SM, Wachman EM, Holland E, Brogly SB. Review of the assessment and management of neonatal abstinence syndrome. Addict Sci Clin Pract. 2014;9:19.
22. Opioid use and opioid use disorder in pregnancy. Committee Opinion No. 711. Obstet Gynecol. 2017;130:488-489.
23. Gopman S. Prenatal and postpartum care of women with substance use disorders. Obstet Gynecol Clin North Am. 2014;41:213-228.
24. Mitchell M, Severtson S, Latimer W. Pregnancy and race/ethnicity as predictors of motivation for drug treatment. Am J Drug Alcohol Abuse. 2008;34:397-404.
Facing systemic racism in health care: Inequities in medical education
Finding inspiration among life’s challenges
Barbara Levy, MD: I am fortunate to have met Pierre serendipitously at a training that we were both attending and was impressed by Dr. Johnson’s life story, his passion and commitment, and his dedication—not only to his personal career but also to raising up other young men of color by trying to break down barriers that face them. His life story highlights those areas of systemic and structural problems that all of us together need to address if we are going to make any progress.
Pierre Johnson, MD: Thank you, Barbara. A little about myself: I am a board-certified ObGyn, and I specialize in minimally invasive surgery. I was born on the South side of Chicago, experiencing gang violence, drugs, and substandard, underserved schools. Long story short, I had a very rough upbringing. I had a single mom and several different issues at home. I am the oldest of 5 siblings, and life was tough.
But I knew that I wanted to do something different with my life. I saw that there was a need in my community as far as health care was concerned, in particular women’s health and childbirth. I knew early on that I wanted to be an ObGyn, and the reason had a lot to do with The Cosby Show. It was the only example of a positive, successful Black man that I saw. No one graduated from college in my family. There weren’t any models of young Black excellence around me. Saying that I wanted to be a doctor planted a seed. I was 9 when my mom became pregnant with my first sibling, and it was fascinating to me. The physiology of pregnancy, and eventually childbirth, was extremely fascinating to me; it set me off on my journey to be an ObGyn.
As I got older, things didn’t get any easier. I went to high school in one of the toughest areas on the South side of Chicago. Gang violence, and violence in and of itself, were all around me, but I was able to stay focused. I went on to Xavier University in Louisiana.
Dr. Levy: There are some important things that I learned from your book and from talking to you at our first meeting. Your mom’s ObGyn, when she was pregnant with your next youngest sibling, was also a Black ObGyn. He took some time to take you under wing?
Dr. Johnson: He did. My mom’s ObGyn was a Black man. Other than The Cosby Show, that’s the only time I saw something like that. When I spoke to him, he really took the time to answer my questions and show me that he was like me; he wasn’t just a far-off mythical person, or something that I could not obtain.
Continue to: Seeing is believing when it comes to success...
Seeing is believing when it comes to success
Dr. Levy: Do you think it was important to have a role model who wasn’t a sports star?
Dr. Johnson: If you can’t see it, you can’t achieve it. He took his time to really talk to me, and it’s the little things for kids that go a long way in their life experience. I still have a relationship with him to this day. How he handled me as a kid made me realize that this is something that I could do. That was extremely important for me.
Dr. Levy: One of the structural things I think we need to point out is that the ability to see yourself as someone successful is critical. When we see 1,000 images a day and they are all White, and they are all so different from where we are that it gets incorporated into our sense of being. I think that’s really difficult for those of us of with privilege to understand what that privilege is.
Dr. Johnson: Absolutely, and I’ll even go further. In residency, 2 White females were my classmates, and both of their parents were doctors. They had grandparents who were doctors. My mom was addicted to drugs; my father was not around. They had been talking medicine since they were 5. You have to make things equitable, but in medicine it’s really not equitable. In medicine, what we don’t realize is that there is an importance for all aspects of someone’s upbringing and environment, and it’s not just what they can regurgitate on a standardized test. If a patient can’t relate to you and tell you what is wrong with them, how can you adequately treat them?
Dr. Levy: Even if they are trying to tell me, but I can’t hear it because I don’t have the language and I don’t have the background. There are really good data to show, in fact, that Black male physicians do a better job at engaging Black men to manage their hypertension.1 When we look at the inequities in birth outcomes for women of color, indigenous women and Black women, there’s evidence that providers who come from a similar background do a better job.
Dr. Johnson: There was the study of Black infants that just came out about them dying at a 3-time higher rate in non-Black physicians’ hands.2 These things need to be recognized. They need to be discussed, and they need to be identified as issues and then, realistically, we need to start talking about solutions, not get offended by what actual statistics are saying.
Foundational inequities in education
Dr. Levy: To address some of the barriers that you faced: I know that you went to a high school that was not geared toward pushing students into professional careers. Your colleagues, however, had educations that prepared them for the standardized tests and other things that they would face academically.
Dr. Johnson: People think I am kidding when I say it, but when I went into college, I didn’t know what a periodic table was. I saw it, but I had no idea what these things meant. I didn’t have any sciences or any AP classes in high school. I did well, but grades are smoke and mirrors. The true test of medicine comes with testing. From the MCATs to the boards, every step of the way there is a standardized test.
Knowledge is something that you can obtain, but test taking is a cultivated skill that happens from a very early age. Trying to teach an adult or someone in their late teens a skill that they should have learned as a kid is difficult. For me, I did not have that, so I had to program myself. I had to learn how to fundamentally take tests as an adult, where most people understand how to do that going into college and professional school.
Dr. Levy: I was impressed with your resilience. I think all of us as human beings, if we fail a test, we take it personally and think it’s about our lack of knowledge. One of the insights that you came to was that failure on those things was not that you didn’t study hard enough. In fact, you probably studied 4 times harder than most other people. You had the knowledge. Being able to get that knowledge into a standardized structured test score was the huge challenge for you.
Dr. Johnson: That’s it. I can remember taking the MCAT, and if you looked at the step 1 book, I could regurgitate to you everything on that page. However, it’s not a test about do you know it or not. It’s an understanding of the English language and how to break things down to make things fit into particular scenarios.
Continue to: A college experience focused on growth and exposure...
A college experience focused on growth and exposure
Dr. Levy: I was impressed by the distinction between your experience at Xavier University where there was a lot of support and guidance and help in your premed program, and what happened to you when you hit medical school.
Dr. Johnson: Xavier University in Louisiana is the number 1 institution in the country for getting minorities into professional school. They understand that they have kids that are brilliant but underprepared, and just have not had the background to actually tackle some of these tough curriculums. I always had good grades in school. But by not being challenged, I didn’t know what I didn’t really know. So now that I was seeing biology, chemistry for the first time, and trying to tackle it; there’s a failure point. I didn’t know how to take tests, and I didn’t know how to study properly. The harder I tried, the worse things got for me.
Xavier has seen that story a multitude of times. If I went to a bigger or predominantly White university, a counselor would have told me, “Well, medicine’s maybe not for you. You can’t handle a premed curriculum.” Instead, I said, “Listen, I’m studying. I’m doing all of these things, and I’m not hacking it.” And they broke it down: “Let’s get you into study groups with kids that have had these type of AP classes before. We’ll have you watch how they study,” and everything started to click. That facilitation of how to adjust to this curriculum was a godsend. It’s the only reason I’m here. I am a prime example of being brilliant enough to be able to do it, but needing the infrastructure and a system set up.
Dr. Levy: There’s a great book by Carol Dweck called Mindset that talks about education of young kids and putting them into silos so early in life; the brilliant kids go into the AP courses and the rest are labeled as inadequate. It’s assumed in a fixed mindset based on their heredity and IQ, and not based on the fact that they have not been exposed to the right things.
Xavier was growing you into the man who could, in fact, do all of those things. I think that is one of the systemic and structural issues that we have—that fixed mindset that frames a kid who is not succeeding as therefore unable to succeed, as opposed to framing that child as not having the correct tools.
New tribulations of medical school
Dr. Johnson: Absolutely. I think what Xavier did for me is to at least let me understand what I needed to do, how to comprehend and retain information, which I never had been exposed to before. Those years were very important to establishing a foundation. When going to medical school, it was like, “There’s no more excuses. What could be the problem now?” Well, now let’s talk about taking tests—a whole different skill. Xavier focused on getting me to understand how to structure my thought process and knowledge base. In medical school I had to apply those skills (because if you can’t apply them, there’s no fit).
My second through fourth year of medical school, I was the only African-American kid in my class. I was spending 20-hour days sometimes just studying, trying to overcompensate by knowing as much as I possibly could and thinking that would propel me from the test-taking standpoint. Even though I didn’t have a lot of classmates in medical school that looked like me, I did have mentors that looked similarly, who really saw potential in me. Dr. Frederick Horvath, a nephrologist in Peoria said, “What are you doing? I want you to get out of these books, and let’s go out to lunch.”
He ended up buying me some instrumental books, really talked to me, listening to my background and understanding how driven I was as a person. He took me under his wing for the rest of medical school and said, “This is how you navigate through these spaces. Yes, you need to have a fund of knowledge to be able to take these tests, but you need to start understanding how to apply it to these questions.” I’m forever grateful to Dr. Horvath for doing that because it was a point in time where I was lost and struggling.
Continue to: Hitting a stride but facing racism head-on...
Hitting a stride but facing racism head-on
Dr. Levy: You talk about the systemic and pervasive racism that was on the wards when you hit them in fourth year. If you don’t mind sharing just a little bit of that, it would help people reading this to have a better understanding of the kinds of barriers that are out there.
Dr. Johnson: Even when I talk about it today, it bothers me.
I went to medical school in Peoria, Illinois, not far from the home of the Ku Klux Klan. At that time, once you got out of Chicago it was a very brutal place, with systemic racism throughout. I was a young Black kid going through a process that not many young Black kids from the South side of Chicago go through, and you had people who had never seen anyone like me. When I was going through my clinical rotations, I knew what I was up against. I was dressed “to the T” every day, arriving early, leaving late, trying to answer questions. I would look at the evaluations, and they would be disparaging. I would look at my counterparts, how their evaluations were, and how people would respond to them, and it would be completely different.
Surgery was the part of ObGyn that I really grew to love more than anything, even more than obstetrics. When general surgery came, I wanted to take it very seriously and learn as much as I possibly could. From the beginning, I knew there was a problem because the chief resident, an older White man, wouldn’t look me in the eye or talk to me. He would make disparaging remarks. The thing that stuck out in my mind the most was when I was in the operating room transporting patients, just like a medical student did, and he came up behind me and said, “You know, Pierre, this is where a small mind and a strong back come into play.” For me, it took me to a place where I had to corral my emotions and thoughts because I just wanted to lash out and just tell him how racist and horrible that was for him to say that to me. I explained this to the powers that be, the director of the department, and they basically blew it off to the side.
When it came down to the end of the evaluation period, I passed with flying colors. But they gave me an incomplete because of that chief resident and his remarks on my evaluations. He had 3 pages of report about me as a person and as a student. He said that he had difficulty in expressing his opinions about me because of possible cultural biases that he may have had. He put “cultural biases” in an evaluation, and they looked at that and said that was enough for me to have to remediate my time. I was required to do an extra month in Pontiac, Illinois, which is even more rural than Peoria, because of a racist person that did not give me a fair opportunity because I was Black.
Like everything else in life, it was a learning experience. It’s why I fight so hard today. It’s why I’m so passionate about equity, not only in medicine but also in all aspects of society. It shows why we have police brutality and Black men dying in the streets. It shows how this happens because there are cultural and implicit biases that play out in every part of life, and we are not honest about it. Until we are honest about it and until we say that this is happening and there is something that needs to be done to address it, it’s going to continue to happen. That is my fight.
Exposing the unspoken power struggle
Dr. Levy: I couldn’t agree more. Attributing things like that to the individual, where you talk about a White man in power and a power structure that didn’t literally physically beat you but did beat you into submission. You talk about how to succeed in medical school, and how you had to suck it up and submit to something that was incredibly unfair. You understood, you were old enough, mature enough, to understand that if you fought back, you were going to lose. The only opportunity you had was to submit to that inequity and push forward.
Dr. Johnson: When I did try to fight, the chair of the department told me that either I accept the consequences or I would not graduate from medical school and be forced to do another year. That struck a chord with me. I think that happens a lot in our society, and it needs to be exposed.
Past experiences reflected in today’s society
Dr. Levy: Can you talk about what you faced in your ObGyn residency in terms of the systemic pushback, people not taking your orders, people questioning you. I know that I have heard that a great deal, and I experienced that myself as a woman.
Dr. Johnson: We look at the things that are happening now, everything from George Floyd’s murder to Colin Kaepernick taking a knee. These things are 10 years past when I first started residency. The year before I started residency, there was a noose hanging on the capitol lawn of Springfield, Illinois’ capital city. There’s systemic racism and hatred there. When I first started on the wards of my first year of ObGyn, again, I was the very first Black resident of my program’s history. Nobody could relate to me.
I went from a year-long general surgery internship at Washington Hospital Center in Washington, DC, to ObGyn residency. In the first 2 months, there were complaints of, “He’s not answering his pages. He’s not being prompt.” I went to my program director and said, “Listen, I have never had one complaint like this. There’s a problem here. And there’s a problem when I’m on the floor: When trying to give orders to nurses, they’re not taking them. I had to tell a couple of nurses, ‘I’m Dr. Johnson. Don’t call me by my first name, especially not in front of patients.’”
My director was just not hearing me, because the entire scenario was something they had never been exposed to. Systemic racism is real, and unless you experience it, it’s very difficult to accept that it is happening. But biases happen when you are not cognizant. People are used to things a certain way. Things play out in the media that make your mind think a certain way, and you don’t even realize it. You may not even want to be that way.
Continue to: Unconscious bias is a barrier to ensuring equity...
Unconscious bias is a barrier to ensuring equity
Dr. Levy: One very important point you just made is that we as the system need to be able to recognize those unconscious things, the language that we use, the disparaging remarks, the things that put people down, as well as the things that keep people out of promotion.
There are some interesting data about both race and gender and the language that we use when we write recommendations for people, that we do things unconsciously. The big message to all of us at the end is to open our minds to where those things can occur. For myself, professionally, I keep a list of words that I use when I write recommendations. I measure myself to ensure that I am using the same language for men and women, for Black and White. I think we need to overcome the system and the structure to create real equity—not equality but equity.
It begins with being real about the issues
Dr. Johnson: It’s a bigger problem than the existence of bias and racism. I think these are systemic issues that have been cultivated over centuries that have never been addressed. The true issue is that we deny that these are problems and refuse to talk about it because it makes us uncomfortable. To truly make things more equitable, we have to push our levels of comfort to be able to talk about things in a healthy manner, be open and transparent, and to start to understand how we are thinking about certain things. When you can see it, you can start to implement changes and start to change mentalities and thought processes.
For me, people say, “You don’t look like a doctor.” I get that all the time—because I have tattoos and earrings. I wear my hair in a mohawk. The image of what success looks like has been manifested through our media and culture, and it has imprinted on our minds as to how things are supposed to be. If someone doesn’t fit those molds, we start to shun them out, or we start to exhibit biases against those things. What I am trying to do is change that thought process of what a successful or a professional person looks like. It doesn’t have a look. It is not a White or Black thing. It’s an intellect, a mindset, a way of living. You have to treat every person as an individual and take all the biases out of it and understand where they are coming from and what they have to offer to the profession.
Dr. Levy: I personally was so impressed by you when I met you. I was impressed by the tattoos and the earrings, and my initial response to them was exactly that biased, “Oh, who is this person?” I checked that at the door, listened to you, and was really impressed at your surgical skill, your knowledge, your background. I am really grateful that you have been willing to spend the time to share that with everyone.
Dr. Johnson: Thank you for this discussion.
To watch the full interview between Drs. Levy and Johnson, visit: https://www.mdedge.com/obgyn/article/228507/facing-systemic-racism-health-care-inequities-medical-education. ●
- The Pulse of Perseverance:
Three Black Doctors on Their Journey to Success Pierre Johnson, MD; Maxime Madhere, MD; and Joseph Semien Jr, MD - Mindset:
The New Psychology of Success
Carol S. Dweck
- Benkert R, Peters R, Tate N, et al. Trust of nurse practitioners and physicians among African Americans with hypertension. J Am Acad Nurse Pract. 2008;20:273-280.
- Greenwood BN, Hardeman RR, Huang L, et al. Physician– patient racial concordance and disparities in birthing mortality for newborns. Proc Natl Acad Sci U S A. 2020; 117:21194-21200.
Finding inspiration among life’s challenges
Barbara Levy, MD: I am fortunate to have met Pierre serendipitously at a training that we were both attending and was impressed by Dr. Johnson’s life story, his passion and commitment, and his dedication—not only to his personal career but also to raising up other young men of color by trying to break down barriers that face them. His life story highlights those areas of systemic and structural problems that all of us together need to address if we are going to make any progress.
Pierre Johnson, MD: Thank you, Barbara. A little about myself: I am a board-certified ObGyn, and I specialize in minimally invasive surgery. I was born on the South side of Chicago, experiencing gang violence, drugs, and substandard, underserved schools. Long story short, I had a very rough upbringing. I had a single mom and several different issues at home. I am the oldest of 5 siblings, and life was tough.
But I knew that I wanted to do something different with my life. I saw that there was a need in my community as far as health care was concerned, in particular women’s health and childbirth. I knew early on that I wanted to be an ObGyn, and the reason had a lot to do with The Cosby Show. It was the only example of a positive, successful Black man that I saw. No one graduated from college in my family. There weren’t any models of young Black excellence around me. Saying that I wanted to be a doctor planted a seed. I was 9 when my mom became pregnant with my first sibling, and it was fascinating to me. The physiology of pregnancy, and eventually childbirth, was extremely fascinating to me; it set me off on my journey to be an ObGyn.
As I got older, things didn’t get any easier. I went to high school in one of the toughest areas on the South side of Chicago. Gang violence, and violence in and of itself, were all around me, but I was able to stay focused. I went on to Xavier University in Louisiana.
Dr. Levy: There are some important things that I learned from your book and from talking to you at our first meeting. Your mom’s ObGyn, when she was pregnant with your next youngest sibling, was also a Black ObGyn. He took some time to take you under wing?
Dr. Johnson: He did. My mom’s ObGyn was a Black man. Other than The Cosby Show, that’s the only time I saw something like that. When I spoke to him, he really took the time to answer my questions and show me that he was like me; he wasn’t just a far-off mythical person, or something that I could not obtain.
Continue to: Seeing is believing when it comes to success...
Seeing is believing when it comes to success
Dr. Levy: Do you think it was important to have a role model who wasn’t a sports star?
Dr. Johnson: If you can’t see it, you can’t achieve it. He took his time to really talk to me, and it’s the little things for kids that go a long way in their life experience. I still have a relationship with him to this day. How he handled me as a kid made me realize that this is something that I could do. That was extremely important for me.
Dr. Levy: One of the structural things I think we need to point out is that the ability to see yourself as someone successful is critical. When we see 1,000 images a day and they are all White, and they are all so different from where we are that it gets incorporated into our sense of being. I think that’s really difficult for those of us of with privilege to understand what that privilege is.
Dr. Johnson: Absolutely, and I’ll even go further. In residency, 2 White females were my classmates, and both of their parents were doctors. They had grandparents who were doctors. My mom was addicted to drugs; my father was not around. They had been talking medicine since they were 5. You have to make things equitable, but in medicine it’s really not equitable. In medicine, what we don’t realize is that there is an importance for all aspects of someone’s upbringing and environment, and it’s not just what they can regurgitate on a standardized test. If a patient can’t relate to you and tell you what is wrong with them, how can you adequately treat them?
Dr. Levy: Even if they are trying to tell me, but I can’t hear it because I don’t have the language and I don’t have the background. There are really good data to show, in fact, that Black male physicians do a better job at engaging Black men to manage their hypertension.1 When we look at the inequities in birth outcomes for women of color, indigenous women and Black women, there’s evidence that providers who come from a similar background do a better job.
Dr. Johnson: There was the study of Black infants that just came out about them dying at a 3-time higher rate in non-Black physicians’ hands.2 These things need to be recognized. They need to be discussed, and they need to be identified as issues and then, realistically, we need to start talking about solutions, not get offended by what actual statistics are saying.
Foundational inequities in education
Dr. Levy: To address some of the barriers that you faced: I know that you went to a high school that was not geared toward pushing students into professional careers. Your colleagues, however, had educations that prepared them for the standardized tests and other things that they would face academically.
Dr. Johnson: People think I am kidding when I say it, but when I went into college, I didn’t know what a periodic table was. I saw it, but I had no idea what these things meant. I didn’t have any sciences or any AP classes in high school. I did well, but grades are smoke and mirrors. The true test of medicine comes with testing. From the MCATs to the boards, every step of the way there is a standardized test.
Knowledge is something that you can obtain, but test taking is a cultivated skill that happens from a very early age. Trying to teach an adult or someone in their late teens a skill that they should have learned as a kid is difficult. For me, I did not have that, so I had to program myself. I had to learn how to fundamentally take tests as an adult, where most people understand how to do that going into college and professional school.
Dr. Levy: I was impressed with your resilience. I think all of us as human beings, if we fail a test, we take it personally and think it’s about our lack of knowledge. One of the insights that you came to was that failure on those things was not that you didn’t study hard enough. In fact, you probably studied 4 times harder than most other people. You had the knowledge. Being able to get that knowledge into a standardized structured test score was the huge challenge for you.
Dr. Johnson: That’s it. I can remember taking the MCAT, and if you looked at the step 1 book, I could regurgitate to you everything on that page. However, it’s not a test about do you know it or not. It’s an understanding of the English language and how to break things down to make things fit into particular scenarios.
Continue to: A college experience focused on growth and exposure...
A college experience focused on growth and exposure
Dr. Levy: I was impressed by the distinction between your experience at Xavier University where there was a lot of support and guidance and help in your premed program, and what happened to you when you hit medical school.
Dr. Johnson: Xavier University in Louisiana is the number 1 institution in the country for getting minorities into professional school. They understand that they have kids that are brilliant but underprepared, and just have not had the background to actually tackle some of these tough curriculums. I always had good grades in school. But by not being challenged, I didn’t know what I didn’t really know. So now that I was seeing biology, chemistry for the first time, and trying to tackle it; there’s a failure point. I didn’t know how to take tests, and I didn’t know how to study properly. The harder I tried, the worse things got for me.
Xavier has seen that story a multitude of times. If I went to a bigger or predominantly White university, a counselor would have told me, “Well, medicine’s maybe not for you. You can’t handle a premed curriculum.” Instead, I said, “Listen, I’m studying. I’m doing all of these things, and I’m not hacking it.” And they broke it down: “Let’s get you into study groups with kids that have had these type of AP classes before. We’ll have you watch how they study,” and everything started to click. That facilitation of how to adjust to this curriculum was a godsend. It’s the only reason I’m here. I am a prime example of being brilliant enough to be able to do it, but needing the infrastructure and a system set up.
Dr. Levy: There’s a great book by Carol Dweck called Mindset that talks about education of young kids and putting them into silos so early in life; the brilliant kids go into the AP courses and the rest are labeled as inadequate. It’s assumed in a fixed mindset based on their heredity and IQ, and not based on the fact that they have not been exposed to the right things.
Xavier was growing you into the man who could, in fact, do all of those things. I think that is one of the systemic and structural issues that we have—that fixed mindset that frames a kid who is not succeeding as therefore unable to succeed, as opposed to framing that child as not having the correct tools.
New tribulations of medical school
Dr. Johnson: Absolutely. I think what Xavier did for me is to at least let me understand what I needed to do, how to comprehend and retain information, which I never had been exposed to before. Those years were very important to establishing a foundation. When going to medical school, it was like, “There’s no more excuses. What could be the problem now?” Well, now let’s talk about taking tests—a whole different skill. Xavier focused on getting me to understand how to structure my thought process and knowledge base. In medical school I had to apply those skills (because if you can’t apply them, there’s no fit).
My second through fourth year of medical school, I was the only African-American kid in my class. I was spending 20-hour days sometimes just studying, trying to overcompensate by knowing as much as I possibly could and thinking that would propel me from the test-taking standpoint. Even though I didn’t have a lot of classmates in medical school that looked like me, I did have mentors that looked similarly, who really saw potential in me. Dr. Frederick Horvath, a nephrologist in Peoria said, “What are you doing? I want you to get out of these books, and let’s go out to lunch.”
He ended up buying me some instrumental books, really talked to me, listening to my background and understanding how driven I was as a person. He took me under his wing for the rest of medical school and said, “This is how you navigate through these spaces. Yes, you need to have a fund of knowledge to be able to take these tests, but you need to start understanding how to apply it to these questions.” I’m forever grateful to Dr. Horvath for doing that because it was a point in time where I was lost and struggling.
Continue to: Hitting a stride but facing racism head-on...
Hitting a stride but facing racism head-on
Dr. Levy: You talk about the systemic and pervasive racism that was on the wards when you hit them in fourth year. If you don’t mind sharing just a little bit of that, it would help people reading this to have a better understanding of the kinds of barriers that are out there.
Dr. Johnson: Even when I talk about it today, it bothers me.
I went to medical school in Peoria, Illinois, not far from the home of the Ku Klux Klan. At that time, once you got out of Chicago it was a very brutal place, with systemic racism throughout. I was a young Black kid going through a process that not many young Black kids from the South side of Chicago go through, and you had people who had never seen anyone like me. When I was going through my clinical rotations, I knew what I was up against. I was dressed “to the T” every day, arriving early, leaving late, trying to answer questions. I would look at the evaluations, and they would be disparaging. I would look at my counterparts, how their evaluations were, and how people would respond to them, and it would be completely different.
Surgery was the part of ObGyn that I really grew to love more than anything, even more than obstetrics. When general surgery came, I wanted to take it very seriously and learn as much as I possibly could. From the beginning, I knew there was a problem because the chief resident, an older White man, wouldn’t look me in the eye or talk to me. He would make disparaging remarks. The thing that stuck out in my mind the most was when I was in the operating room transporting patients, just like a medical student did, and he came up behind me and said, “You know, Pierre, this is where a small mind and a strong back come into play.” For me, it took me to a place where I had to corral my emotions and thoughts because I just wanted to lash out and just tell him how racist and horrible that was for him to say that to me. I explained this to the powers that be, the director of the department, and they basically blew it off to the side.
When it came down to the end of the evaluation period, I passed with flying colors. But they gave me an incomplete because of that chief resident and his remarks on my evaluations. He had 3 pages of report about me as a person and as a student. He said that he had difficulty in expressing his opinions about me because of possible cultural biases that he may have had. He put “cultural biases” in an evaluation, and they looked at that and said that was enough for me to have to remediate my time. I was required to do an extra month in Pontiac, Illinois, which is even more rural than Peoria, because of a racist person that did not give me a fair opportunity because I was Black.
Like everything else in life, it was a learning experience. It’s why I fight so hard today. It’s why I’m so passionate about equity, not only in medicine but also in all aspects of society. It shows why we have police brutality and Black men dying in the streets. It shows how this happens because there are cultural and implicit biases that play out in every part of life, and we are not honest about it. Until we are honest about it and until we say that this is happening and there is something that needs to be done to address it, it’s going to continue to happen. That is my fight.
Exposing the unspoken power struggle
Dr. Levy: I couldn’t agree more. Attributing things like that to the individual, where you talk about a White man in power and a power structure that didn’t literally physically beat you but did beat you into submission. You talk about how to succeed in medical school, and how you had to suck it up and submit to something that was incredibly unfair. You understood, you were old enough, mature enough, to understand that if you fought back, you were going to lose. The only opportunity you had was to submit to that inequity and push forward.
Dr. Johnson: When I did try to fight, the chair of the department told me that either I accept the consequences or I would not graduate from medical school and be forced to do another year. That struck a chord with me. I think that happens a lot in our society, and it needs to be exposed.
Past experiences reflected in today’s society
Dr. Levy: Can you talk about what you faced in your ObGyn residency in terms of the systemic pushback, people not taking your orders, people questioning you. I know that I have heard that a great deal, and I experienced that myself as a woman.
Dr. Johnson: We look at the things that are happening now, everything from George Floyd’s murder to Colin Kaepernick taking a knee. These things are 10 years past when I first started residency. The year before I started residency, there was a noose hanging on the capitol lawn of Springfield, Illinois’ capital city. There’s systemic racism and hatred there. When I first started on the wards of my first year of ObGyn, again, I was the very first Black resident of my program’s history. Nobody could relate to me.
I went from a year-long general surgery internship at Washington Hospital Center in Washington, DC, to ObGyn residency. In the first 2 months, there were complaints of, “He’s not answering his pages. He’s not being prompt.” I went to my program director and said, “Listen, I have never had one complaint like this. There’s a problem here. And there’s a problem when I’m on the floor: When trying to give orders to nurses, they’re not taking them. I had to tell a couple of nurses, ‘I’m Dr. Johnson. Don’t call me by my first name, especially not in front of patients.’”
My director was just not hearing me, because the entire scenario was something they had never been exposed to. Systemic racism is real, and unless you experience it, it’s very difficult to accept that it is happening. But biases happen when you are not cognizant. People are used to things a certain way. Things play out in the media that make your mind think a certain way, and you don’t even realize it. You may not even want to be that way.
Continue to: Unconscious bias is a barrier to ensuring equity...
Unconscious bias is a barrier to ensuring equity
Dr. Levy: One very important point you just made is that we as the system need to be able to recognize those unconscious things, the language that we use, the disparaging remarks, the things that put people down, as well as the things that keep people out of promotion.
There are some interesting data about both race and gender and the language that we use when we write recommendations for people, that we do things unconsciously. The big message to all of us at the end is to open our minds to where those things can occur. For myself, professionally, I keep a list of words that I use when I write recommendations. I measure myself to ensure that I am using the same language for men and women, for Black and White. I think we need to overcome the system and the structure to create real equity—not equality but equity.
It begins with being real about the issues
Dr. Johnson: It’s a bigger problem than the existence of bias and racism. I think these are systemic issues that have been cultivated over centuries that have never been addressed. The true issue is that we deny that these are problems and refuse to talk about it because it makes us uncomfortable. To truly make things more equitable, we have to push our levels of comfort to be able to talk about things in a healthy manner, be open and transparent, and to start to understand how we are thinking about certain things. When you can see it, you can start to implement changes and start to change mentalities and thought processes.
For me, people say, “You don’t look like a doctor.” I get that all the time—because I have tattoos and earrings. I wear my hair in a mohawk. The image of what success looks like has been manifested through our media and culture, and it has imprinted on our minds as to how things are supposed to be. If someone doesn’t fit those molds, we start to shun them out, or we start to exhibit biases against those things. What I am trying to do is change that thought process of what a successful or a professional person looks like. It doesn’t have a look. It is not a White or Black thing. It’s an intellect, a mindset, a way of living. You have to treat every person as an individual and take all the biases out of it and understand where they are coming from and what they have to offer to the profession.
Dr. Levy: I personally was so impressed by you when I met you. I was impressed by the tattoos and the earrings, and my initial response to them was exactly that biased, “Oh, who is this person?” I checked that at the door, listened to you, and was really impressed at your surgical skill, your knowledge, your background. I am really grateful that you have been willing to spend the time to share that with everyone.
Dr. Johnson: Thank you for this discussion.
To watch the full interview between Drs. Levy and Johnson, visit: https://www.mdedge.com/obgyn/article/228507/facing-systemic-racism-health-care-inequities-medical-education. ●
- The Pulse of Perseverance:
Three Black Doctors on Their Journey to Success Pierre Johnson, MD; Maxime Madhere, MD; and Joseph Semien Jr, MD - Mindset:
The New Psychology of Success
Carol S. Dweck
Finding inspiration among life’s challenges
Barbara Levy, MD: I am fortunate to have met Pierre serendipitously at a training that we were both attending and was impressed by Dr. Johnson’s life story, his passion and commitment, and his dedication—not only to his personal career but also to raising up other young men of color by trying to break down barriers that face them. His life story highlights those areas of systemic and structural problems that all of us together need to address if we are going to make any progress.
Pierre Johnson, MD: Thank you, Barbara. A little about myself: I am a board-certified ObGyn, and I specialize in minimally invasive surgery. I was born on the South side of Chicago, experiencing gang violence, drugs, and substandard, underserved schools. Long story short, I had a very rough upbringing. I had a single mom and several different issues at home. I am the oldest of 5 siblings, and life was tough.
But I knew that I wanted to do something different with my life. I saw that there was a need in my community as far as health care was concerned, in particular women’s health and childbirth. I knew early on that I wanted to be an ObGyn, and the reason had a lot to do with The Cosby Show. It was the only example of a positive, successful Black man that I saw. No one graduated from college in my family. There weren’t any models of young Black excellence around me. Saying that I wanted to be a doctor planted a seed. I was 9 when my mom became pregnant with my first sibling, and it was fascinating to me. The physiology of pregnancy, and eventually childbirth, was extremely fascinating to me; it set me off on my journey to be an ObGyn.
As I got older, things didn’t get any easier. I went to high school in one of the toughest areas on the South side of Chicago. Gang violence, and violence in and of itself, were all around me, but I was able to stay focused. I went on to Xavier University in Louisiana.
Dr. Levy: There are some important things that I learned from your book and from talking to you at our first meeting. Your mom’s ObGyn, when she was pregnant with your next youngest sibling, was also a Black ObGyn. He took some time to take you under wing?
Dr. Johnson: He did. My mom’s ObGyn was a Black man. Other than The Cosby Show, that’s the only time I saw something like that. When I spoke to him, he really took the time to answer my questions and show me that he was like me; he wasn’t just a far-off mythical person, or something that I could not obtain.
Continue to: Seeing is believing when it comes to success...
Seeing is believing when it comes to success
Dr. Levy: Do you think it was important to have a role model who wasn’t a sports star?
Dr. Johnson: If you can’t see it, you can’t achieve it. He took his time to really talk to me, and it’s the little things for kids that go a long way in their life experience. I still have a relationship with him to this day. How he handled me as a kid made me realize that this is something that I could do. That was extremely important for me.
Dr. Levy: One of the structural things I think we need to point out is that the ability to see yourself as someone successful is critical. When we see 1,000 images a day and they are all White, and they are all so different from where we are that it gets incorporated into our sense of being. I think that’s really difficult for those of us of with privilege to understand what that privilege is.
Dr. Johnson: Absolutely, and I’ll even go further. In residency, 2 White females were my classmates, and both of their parents were doctors. They had grandparents who were doctors. My mom was addicted to drugs; my father was not around. They had been talking medicine since they were 5. You have to make things equitable, but in medicine it’s really not equitable. In medicine, what we don’t realize is that there is an importance for all aspects of someone’s upbringing and environment, and it’s not just what they can regurgitate on a standardized test. If a patient can’t relate to you and tell you what is wrong with them, how can you adequately treat them?
Dr. Levy: Even if they are trying to tell me, but I can’t hear it because I don’t have the language and I don’t have the background. There are really good data to show, in fact, that Black male physicians do a better job at engaging Black men to manage their hypertension.1 When we look at the inequities in birth outcomes for women of color, indigenous women and Black women, there’s evidence that providers who come from a similar background do a better job.
Dr. Johnson: There was the study of Black infants that just came out about them dying at a 3-time higher rate in non-Black physicians’ hands.2 These things need to be recognized. They need to be discussed, and they need to be identified as issues and then, realistically, we need to start talking about solutions, not get offended by what actual statistics are saying.
Foundational inequities in education
Dr. Levy: To address some of the barriers that you faced: I know that you went to a high school that was not geared toward pushing students into professional careers. Your colleagues, however, had educations that prepared them for the standardized tests and other things that they would face academically.
Dr. Johnson: People think I am kidding when I say it, but when I went into college, I didn’t know what a periodic table was. I saw it, but I had no idea what these things meant. I didn’t have any sciences or any AP classes in high school. I did well, but grades are smoke and mirrors. The true test of medicine comes with testing. From the MCATs to the boards, every step of the way there is a standardized test.
Knowledge is something that you can obtain, but test taking is a cultivated skill that happens from a very early age. Trying to teach an adult or someone in their late teens a skill that they should have learned as a kid is difficult. For me, I did not have that, so I had to program myself. I had to learn how to fundamentally take tests as an adult, where most people understand how to do that going into college and professional school.
Dr. Levy: I was impressed with your resilience. I think all of us as human beings, if we fail a test, we take it personally and think it’s about our lack of knowledge. One of the insights that you came to was that failure on those things was not that you didn’t study hard enough. In fact, you probably studied 4 times harder than most other people. You had the knowledge. Being able to get that knowledge into a standardized structured test score was the huge challenge for you.
Dr. Johnson: That’s it. I can remember taking the MCAT, and if you looked at the step 1 book, I could regurgitate to you everything on that page. However, it’s not a test about do you know it or not. It’s an understanding of the English language and how to break things down to make things fit into particular scenarios.
Continue to: A college experience focused on growth and exposure...
A college experience focused on growth and exposure
Dr. Levy: I was impressed by the distinction between your experience at Xavier University where there was a lot of support and guidance and help in your premed program, and what happened to you when you hit medical school.
Dr. Johnson: Xavier University in Louisiana is the number 1 institution in the country for getting minorities into professional school. They understand that they have kids that are brilliant but underprepared, and just have not had the background to actually tackle some of these tough curriculums. I always had good grades in school. But by not being challenged, I didn’t know what I didn’t really know. So now that I was seeing biology, chemistry for the first time, and trying to tackle it; there’s a failure point. I didn’t know how to take tests, and I didn’t know how to study properly. The harder I tried, the worse things got for me.
Xavier has seen that story a multitude of times. If I went to a bigger or predominantly White university, a counselor would have told me, “Well, medicine’s maybe not for you. You can’t handle a premed curriculum.” Instead, I said, “Listen, I’m studying. I’m doing all of these things, and I’m not hacking it.” And they broke it down: “Let’s get you into study groups with kids that have had these type of AP classes before. We’ll have you watch how they study,” and everything started to click. That facilitation of how to adjust to this curriculum was a godsend. It’s the only reason I’m here. I am a prime example of being brilliant enough to be able to do it, but needing the infrastructure and a system set up.
Dr. Levy: There’s a great book by Carol Dweck called Mindset that talks about education of young kids and putting them into silos so early in life; the brilliant kids go into the AP courses and the rest are labeled as inadequate. It’s assumed in a fixed mindset based on their heredity and IQ, and not based on the fact that they have not been exposed to the right things.
Xavier was growing you into the man who could, in fact, do all of those things. I think that is one of the systemic and structural issues that we have—that fixed mindset that frames a kid who is not succeeding as therefore unable to succeed, as opposed to framing that child as not having the correct tools.
New tribulations of medical school
Dr. Johnson: Absolutely. I think what Xavier did for me is to at least let me understand what I needed to do, how to comprehend and retain information, which I never had been exposed to before. Those years were very important to establishing a foundation. When going to medical school, it was like, “There’s no more excuses. What could be the problem now?” Well, now let’s talk about taking tests—a whole different skill. Xavier focused on getting me to understand how to structure my thought process and knowledge base. In medical school I had to apply those skills (because if you can’t apply them, there’s no fit).
My second through fourth year of medical school, I was the only African-American kid in my class. I was spending 20-hour days sometimes just studying, trying to overcompensate by knowing as much as I possibly could and thinking that would propel me from the test-taking standpoint. Even though I didn’t have a lot of classmates in medical school that looked like me, I did have mentors that looked similarly, who really saw potential in me. Dr. Frederick Horvath, a nephrologist in Peoria said, “What are you doing? I want you to get out of these books, and let’s go out to lunch.”
He ended up buying me some instrumental books, really talked to me, listening to my background and understanding how driven I was as a person. He took me under his wing for the rest of medical school and said, “This is how you navigate through these spaces. Yes, you need to have a fund of knowledge to be able to take these tests, but you need to start understanding how to apply it to these questions.” I’m forever grateful to Dr. Horvath for doing that because it was a point in time where I was lost and struggling.
Continue to: Hitting a stride but facing racism head-on...
Hitting a stride but facing racism head-on
Dr. Levy: You talk about the systemic and pervasive racism that was on the wards when you hit them in fourth year. If you don’t mind sharing just a little bit of that, it would help people reading this to have a better understanding of the kinds of barriers that are out there.
Dr. Johnson: Even when I talk about it today, it bothers me.
I went to medical school in Peoria, Illinois, not far from the home of the Ku Klux Klan. At that time, once you got out of Chicago it was a very brutal place, with systemic racism throughout. I was a young Black kid going through a process that not many young Black kids from the South side of Chicago go through, and you had people who had never seen anyone like me. When I was going through my clinical rotations, I knew what I was up against. I was dressed “to the T” every day, arriving early, leaving late, trying to answer questions. I would look at the evaluations, and they would be disparaging. I would look at my counterparts, how their evaluations were, and how people would respond to them, and it would be completely different.
Surgery was the part of ObGyn that I really grew to love more than anything, even more than obstetrics. When general surgery came, I wanted to take it very seriously and learn as much as I possibly could. From the beginning, I knew there was a problem because the chief resident, an older White man, wouldn’t look me in the eye or talk to me. He would make disparaging remarks. The thing that stuck out in my mind the most was when I was in the operating room transporting patients, just like a medical student did, and he came up behind me and said, “You know, Pierre, this is where a small mind and a strong back come into play.” For me, it took me to a place where I had to corral my emotions and thoughts because I just wanted to lash out and just tell him how racist and horrible that was for him to say that to me. I explained this to the powers that be, the director of the department, and they basically blew it off to the side.
When it came down to the end of the evaluation period, I passed with flying colors. But they gave me an incomplete because of that chief resident and his remarks on my evaluations. He had 3 pages of report about me as a person and as a student. He said that he had difficulty in expressing his opinions about me because of possible cultural biases that he may have had. He put “cultural biases” in an evaluation, and they looked at that and said that was enough for me to have to remediate my time. I was required to do an extra month in Pontiac, Illinois, which is even more rural than Peoria, because of a racist person that did not give me a fair opportunity because I was Black.
Like everything else in life, it was a learning experience. It’s why I fight so hard today. It’s why I’m so passionate about equity, not only in medicine but also in all aspects of society. It shows why we have police brutality and Black men dying in the streets. It shows how this happens because there are cultural and implicit biases that play out in every part of life, and we are not honest about it. Until we are honest about it and until we say that this is happening and there is something that needs to be done to address it, it’s going to continue to happen. That is my fight.
Exposing the unspoken power struggle
Dr. Levy: I couldn’t agree more. Attributing things like that to the individual, where you talk about a White man in power and a power structure that didn’t literally physically beat you but did beat you into submission. You talk about how to succeed in medical school, and how you had to suck it up and submit to something that was incredibly unfair. You understood, you were old enough, mature enough, to understand that if you fought back, you were going to lose. The only opportunity you had was to submit to that inequity and push forward.
Dr. Johnson: When I did try to fight, the chair of the department told me that either I accept the consequences or I would not graduate from medical school and be forced to do another year. That struck a chord with me. I think that happens a lot in our society, and it needs to be exposed.
Past experiences reflected in today’s society
Dr. Levy: Can you talk about what you faced in your ObGyn residency in terms of the systemic pushback, people not taking your orders, people questioning you. I know that I have heard that a great deal, and I experienced that myself as a woman.
Dr. Johnson: We look at the things that are happening now, everything from George Floyd’s murder to Colin Kaepernick taking a knee. These things are 10 years past when I first started residency. The year before I started residency, there was a noose hanging on the capitol lawn of Springfield, Illinois’ capital city. There’s systemic racism and hatred there. When I first started on the wards of my first year of ObGyn, again, I was the very first Black resident of my program’s history. Nobody could relate to me.
I went from a year-long general surgery internship at Washington Hospital Center in Washington, DC, to ObGyn residency. In the first 2 months, there were complaints of, “He’s not answering his pages. He’s not being prompt.” I went to my program director and said, “Listen, I have never had one complaint like this. There’s a problem here. And there’s a problem when I’m on the floor: When trying to give orders to nurses, they’re not taking them. I had to tell a couple of nurses, ‘I’m Dr. Johnson. Don’t call me by my first name, especially not in front of patients.’”
My director was just not hearing me, because the entire scenario was something they had never been exposed to. Systemic racism is real, and unless you experience it, it’s very difficult to accept that it is happening. But biases happen when you are not cognizant. People are used to things a certain way. Things play out in the media that make your mind think a certain way, and you don’t even realize it. You may not even want to be that way.
Continue to: Unconscious bias is a barrier to ensuring equity...
Unconscious bias is a barrier to ensuring equity
Dr. Levy: One very important point you just made is that we as the system need to be able to recognize those unconscious things, the language that we use, the disparaging remarks, the things that put people down, as well as the things that keep people out of promotion.
There are some interesting data about both race and gender and the language that we use when we write recommendations for people, that we do things unconsciously. The big message to all of us at the end is to open our minds to where those things can occur. For myself, professionally, I keep a list of words that I use when I write recommendations. I measure myself to ensure that I am using the same language for men and women, for Black and White. I think we need to overcome the system and the structure to create real equity—not equality but equity.
It begins with being real about the issues
Dr. Johnson: It’s a bigger problem than the existence of bias and racism. I think these are systemic issues that have been cultivated over centuries that have never been addressed. The true issue is that we deny that these are problems and refuse to talk about it because it makes us uncomfortable. To truly make things more equitable, we have to push our levels of comfort to be able to talk about things in a healthy manner, be open and transparent, and to start to understand how we are thinking about certain things. When you can see it, you can start to implement changes and start to change mentalities and thought processes.
For me, people say, “You don’t look like a doctor.” I get that all the time—because I have tattoos and earrings. I wear my hair in a mohawk. The image of what success looks like has been manifested through our media and culture, and it has imprinted on our minds as to how things are supposed to be. If someone doesn’t fit those molds, we start to shun them out, or we start to exhibit biases against those things. What I am trying to do is change that thought process of what a successful or a professional person looks like. It doesn’t have a look. It is not a White or Black thing. It’s an intellect, a mindset, a way of living. You have to treat every person as an individual and take all the biases out of it and understand where they are coming from and what they have to offer to the profession.
Dr. Levy: I personally was so impressed by you when I met you. I was impressed by the tattoos and the earrings, and my initial response to them was exactly that biased, “Oh, who is this person?” I checked that at the door, listened to you, and was really impressed at your surgical skill, your knowledge, your background. I am really grateful that you have been willing to spend the time to share that with everyone.
Dr. Johnson: Thank you for this discussion.
To watch the full interview between Drs. Levy and Johnson, visit: https://www.mdedge.com/obgyn/article/228507/facing-systemic-racism-health-care-inequities-medical-education. ●
- The Pulse of Perseverance:
Three Black Doctors on Their Journey to Success Pierre Johnson, MD; Maxime Madhere, MD; and Joseph Semien Jr, MD - Mindset:
The New Psychology of Success
Carol S. Dweck
- Benkert R, Peters R, Tate N, et al. Trust of nurse practitioners and physicians among African Americans with hypertension. J Am Acad Nurse Pract. 2008;20:273-280.
- Greenwood BN, Hardeman RR, Huang L, et al. Physician– patient racial concordance and disparities in birthing mortality for newborns. Proc Natl Acad Sci U S A. 2020; 117:21194-21200.
- Benkert R, Peters R, Tate N, et al. Trust of nurse practitioners and physicians among African Americans with hypertension. J Am Acad Nurse Pract. 2008;20:273-280.
- Greenwood BN, Hardeman RR, Huang L, et al. Physician– patient racial concordance and disparities in birthing mortality for newborns. Proc Natl Acad Sci U S A. 2020; 117:21194-21200.