User login
Reducing Inappropriate Laboratory Testing in the Hospital Setting: How Low Can We Go?
From the University of Toronto (Dr. Basuita, Corey L. Kamen, and Dr. Soong) and Sinai Health System (Corey L. Kamen, Cheryl Ethier, and Dr. Soong), Toronto, Ontario, Canada. Co-first authors are Manpreet Basuita, MD, and Corey L. Kamen, BSc.
Abstract
- Objective: Routine laboratory testing is common among medical inpatients; however, when ordered inappropriately testing can represent low-value care. We examined the impact of an evidence-based intervention bundle on utilization.
- Participants/setting: This prospective cohort study took place at a tertiary academic medical center and included 6424 patients admitted to the general internal medicine service between April 2016 and March 2018.
- Intervention: An intervention bundle, whose first components were implemented in July 2016, included computer order entry restrictions on repetitive laboratory testing, education, and audit-feedback.
- Measures: Data were extracted from the hospital electronic health record. The primary outcome was the number of routine blood tests (complete blood count, creatinine, and electrolytes) ordered per inpatient day.
- Analysis: Descriptive statistics were calculated for demographic variables. We used statistical process control charts to compare the baseline period (April 2016-June 2017) and the intervention period (July 2017-March 2018) for the primary outcome.
- Results: The mean number of combined routine laboratory tests ordered per inpatient day decreased from 1.19 (SD, 0.21) tests to 1.11 (SD, 0.05), a relative reduction of 6.7% (P < 0.0001). Mean cost per case related to laboratory tests decreased from $17.24 in the pre-intervention period to $16.17 in the post-intervention period (relative reduction of 6.2%). This resulted in savings of $26,851 in the intervention year.
- Conclusion: A laboratory intervention bundle was associated with small reductions in testing and costs. A routine test performed less than once per inpatient day may not be clinically appropriate or possible.
Keywords: utilization; clinical costs; quality improvement; QI intervention; internal medicine; inpatient.
Routine laboratory blood testing is a commonly used diagnostic tool that physicians rely on to provide patient care. Although routine blood testing represents less than 5% of most hospital budgets, routine use and over-reliance on testing among physicians makes it a target of cost-reduction efforts.1-3 A variety of interventions have been proposed to reduce inappropriate laboratory tests, with varying results.1,4-6 Successful interventions include providing physicians with fee data associated with ordered laboratory tests, unbundling panels of tests, and multicomponent interventions.6 We conducted a multifaceted quality improvement study to promote and develop interventions to adopt appropriate blood test ordering practices.
Methods
Setting
This prospective cohort study took place at Mount Sinai Hospital, a 443-bed academic hospital affiliated with the University of Toronto, where more than 2400 learners rotate through annually. The study was approved by the Mount Sinai Hospital Research Ethics Board.
Participants
We included all inpatient admissions to the general internal medicine service between April 2016 and March 2018. Exclusion criteria included a length of stay (LOS) longer than 365 days and admission to a critical care unit. Patients with more than 1 admission were counted as separate hospital inpatient visits.
Intervention
Based on internal data, we targeted the top 3 most frequently ordered routine blood tests: complete blood count (CBC), creatinine, and electrolytes. Trainee interviews revealed that habit, bundled order sets, and fear of “missing something” contributed to inappropriate routine blood test ordering. Based on these root causes, we used the Model for Improvement to iteratively develop a multimodal intervention that began in July 2016.7,8 This included a change to the computerized provider order entry (CPOE) to nudge clinicians to a restrictive ordering strategy by substituting the “Daily x3” frequency of blood test ordering with a “Daily x1” option on a pick list of order options. Clinicians could still order daily routine blood tests for any specified duration, but would have to do so by manually changing the default setting within the CPOE.
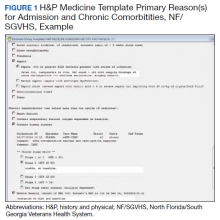
From July 2017 to March 2018, the research team educated residents on appropriate laboratory test ordering and provided audit and feedback data to the clinicians. Diagnostic uncertainty was addressed in teaching sessions. Attending physicians were surveyed on appropriate indications for daily laboratory testing for each of CBC, electrolytes, and creatinine. Appropriate indications (Figure 1) were displayed in visible clinical areas and incorporated into teaching sessions.9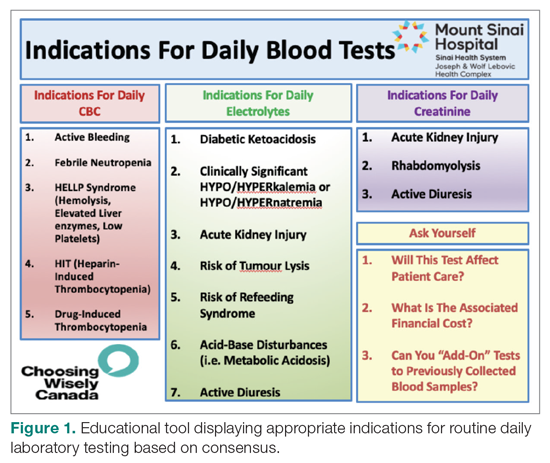
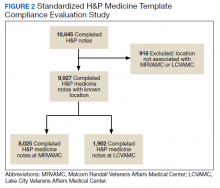
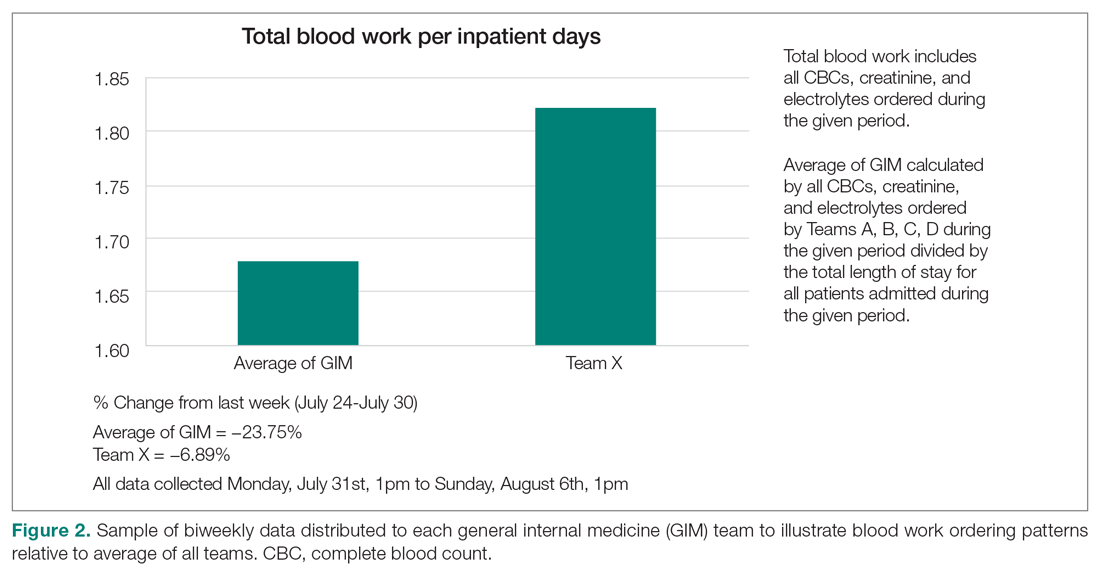
Clinician teams received real-time performance data on their routine blood test ordering patterns compared with an institutional benchmark. Bar graphs of blood work ordering rates (sum of CBCs, creatinine, and electrolytes ordered for all patients on a given team divided by the total LOS for all patients) were distributed to each internal medicine team via email every 2 weeks (Figure 2).1,10-12
Data Collection and Analysis
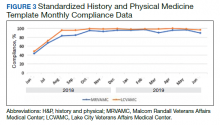
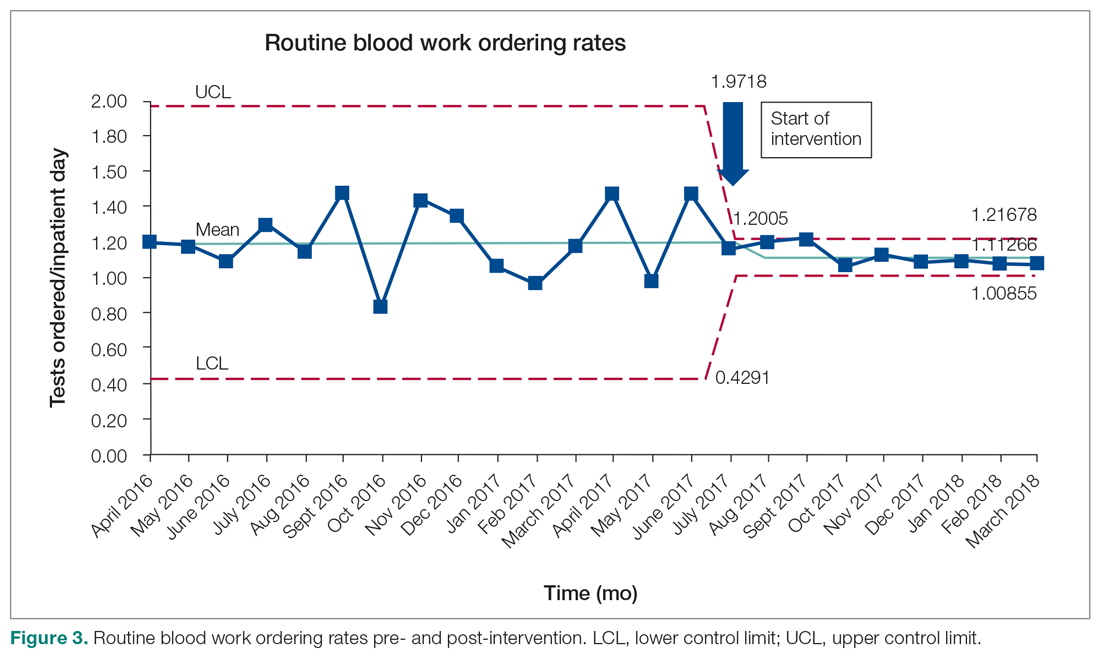
Data were extracted from the hospital electronic health record (EHR). The primary outcome was the number of routine blood tests (CBC, creatinine, and electrolytes) ordered per inpatient day. Descriptive statistics were calculated for demographic variables. We used statistical process control (SPC) charts to compare the baseline period (April 2016-June 2017) and the intervention period (July 2017-March 2018) for the primary outcome. SPC charts display process changes over time. Data are plotted in chronological order, with the central line representing the outcome mean, an upper line representing the upper control limit, and a lower line representing the lower control limit. The upper and lower limits were set at 3δ, which correspond to 3 standard deviations above and below the mean. Six successive points above or beyond the mean suggests “special cause variation,” indicating that observed results are unlikely due to secular trends. SPC charts are commonly used quality tools for process improvement as well as research.13-16 These charts were created using QI Macros SPC software for Excel V. 2012.07 (KnowWare International, Denver, CO).
The direct cost of each laboratory test was acquired from the hospital laboratory department. The cost of each laboratory test (CBC = $7.54/test, electrolytes = $2.04/test, creatinine = $1.28/test, in Canadian dollars) was subsequently added together and multiplied by the pre- and post-intervention difference of total blood tests saved per inpatient day and then multiplied by 365 to arrive at an estimated cost savings per year.
Results
Over the study period, there were 6424 unique patient admissions on the general internal medicine service, with a median LOS of 3.5 days (Table). 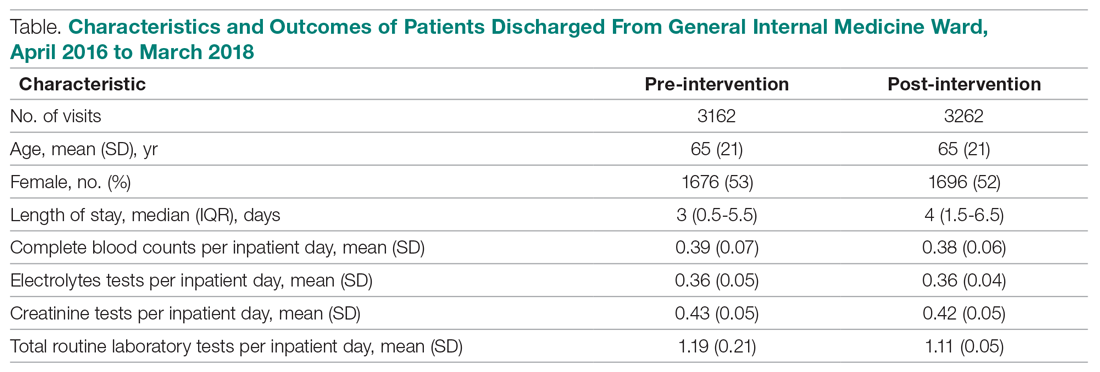
The majority of inpatient visits had at least 1 test of CBC (80%; mean, 3.6 tests/visit), creatinine (79.3%; mean, 3.5 tests/visit), or electrolytes (81.6%; mean, 3.9 tests/visit) completed. In total, 56,767 laboratory tests were ordered.
Following the intervention, there was a reduction in both rates of routine blood test orders and their associated costs, with a shift below the mean. The mean number of tests ordered (combined CBC, creatinine, and electrolytes) per inpatient day decreased from 1.19 (SD, 0.21) in the pre-intervention period to 1.11 (SD, 0.05) in the post-intervention period (P < 0.0001), representing a 6.7% relative reduction (Figure 3). We observed a 6.2% relative reduction in costs per inpatient day, translating to a total savings of $26,851 over 1 year for the intervention period.
Discussion
Our study suggests that a multimodal intervention, including CPOE restrictions, resident education with posters, and audit and feedback strategies, can reduce lab test ordering on general internal medicine wards. This finding is similar to those of previous studies using a similar intervention, although different laboratory tests were targeted.1,2,5,6,10,17
Our study found lower test result reductions than those reported by a previous study, which reported a relative reduction of 17% to 30%,18 and by another investigation that was conducted recently in a similar setting.17 In the latter study, reductions in laboratory testing were mostly found in nonroutine tests, and no significant improvements were noted in CBC, electrolytes, and creatine, the 3 tests we studied over the same duration.17 This may represent a ceiling effect to reducing laboratory testing, and efforts to reduce CBC, electrolytes, and creatinine testing beyond 0.3 to 0.4 tests per inpatient day (or combined 1.16 tests per inpatient day) may not be clinically appropriate or possible. This information can guide institutions to include other areas of overuse based on rates of utilization in order to maximize the benefits from a resource intensive intervention.
There are a number of limitations that merit discussion. First, observational studies do not demonstrate causation; however, to our knowledge, there were no other co-interventions that were being conducted during the study duration. One important note is that our project’s intervention began in July, at which point there are new internal medicine residents beginning their training. As the concept of resource allocation becomes more important, medical schools are spending more time educating students about Choosing Wisely, and, therefore, newer cohorts of residents may be more cognizant of appropriate blood testing. Second, this is a single-center study, limiting generalizability; however, we note that many other centers have reported similar findings. Another limitation is that we do not know whether there were any adverse clinical events associated with blood work ordering that was too restrictive, although informal tracking of STAT laboratory testing remained stable throughout the study period. It is important to ensure that blood work is ordered in moderation and tailored to patients using one’s clinical judgment.
Future Directions
We observed modest reductions in the quantity and costs associated with a quality improvement intervention aimed at reducing routine blood testing. A baseline rate of laboratory testing of less than 1 test per inpatient day may require including other target tests to drive down absolute utilization.
Corresponding author: Christine Soong, MD, MSc, 433-600 University Avenue, Toronto, Ontario, Canada M5G 1X5; [email protected].
Financial disclosures: None.
1. Eaton KP, Levy K, Soong C, et al. Evidence-based guidelines to eliminate repetitive laboratory testing. JAMA Intern Med. 2017;178:431.
2. May TA, Clancy M, Critchfield J, et al. Reducing unnecessary inpatient laboratory testing in a teaching hospital. Am J Clin Pathol. 2006;126:200-206.
3. Thavendiranathan P, Bagai A, Ebidia A, et al. Do blood tests cause anemia in hospitalized patients? The effect of diagnostic phlebotomy on hemoglobin and hematocrit levels. J Gen Intern Med. 2005;20:520-524.
4. Feldman LS, Shihab HM, Thiemann D, et al. Impact of providing fee data on laboratory test ordering: a controlled clinical trial. JAMA Intern Med. 2013;173:903-908.
5. Attali, M, Barel Y, Somin M, et al. A cost-effective method for reducing the volume of laboratory tests in a university-associated teaching hospital. Mt Sinai J Med. 2006;73:787-794.
6. Faisal A, Andres K, Rind JAK, et al. Reducing the number of unnecessary routine laboratory tests through education of internal medicine residents. Postgrad Med J. 2018;94:716-719.
7. How to Improve. Institute for Healthcare Improvement. 2009. http://www.ihi.org/resources/Pages/HowtoImprove/default.aspx. Accessed June 5, 2019.
8. Langley GL, Moen R, Nolan KM, et al. The Improvement Guide: A Practical Approach to Enhancing Organizational Performance. 2nd ed. San Francisco: Jossey-Bass Publishers; 2009.
9. Hicks L. Blood Draws Toolkit. Choosing Wisely Canada. 2017. https://choosingwiselycanada.org/wpcontent/uploads/2017/10/CWC_BloodDraws_Toolkit.pdf. Accessed March 5, 2019.
10. Sadowski BW, Lane AB, Wood SM, et al. High-value, cost-conscious care: iterative systems-based interventions to reduce unnecessary laboratory testing. Am J Med. 2017;130:1112e1-1112e7.
11. Minerowicz C, Abel N, Hunter K, et al. Impact of weekly feedback on test ordering patterns. Am J Manag Care. 2015;21:763-768.
12. Calderon-Margalit R, Mor-Yosef S, et al. An administrative intervention to improve the utilization of laboratory tests within a university hospital. Int J Qual Health Care. 2005;17:243-248.
13. Benneyan JC, Lloyd RC, Plsek PE. Statistical process control as a tool for research and healthcare improvement. Qual Saf Health Care. 2003;12:458-64.
14. American Society for Quality. Control chart. ASM website. https://asq.org/quality-resources/control-chart. Accessed November 5, 2020.
15. American Society for Quality. The 7 Basic Quality Tools For Process Improvement. ASM website. https://asq.org/quality-resources/seven-basic-quality-tools. Accessed November 5, 2020.
16. Benneyan JC, Lloyd RC, Plsek PE. Statistical process control as a tool for research and healthcare improvement. Qual Saf Health Care. 2003;12:458-464.
17. Ambasta A, Ma IWY, Woo S, et al. Impact of an education and multilevel social comparison-based intervention bundle on use of routine blood tests in hospitalised patients at an academic tertiary care hospital: a controlled pre-intervention post-intervention study. BMJ Qual Saf. 2020;29:1-2.
18. Lee VS, Kawamoto K, Hess R, et al. Implementation of a value-driven outcomes program to identify high variability in clinical costs and outcomes and association with reduced cost and improved quality. JAMA. 2016;316:1061-1072.
From the University of Toronto (Dr. Basuita, Corey L. Kamen, and Dr. Soong) and Sinai Health System (Corey L. Kamen, Cheryl Ethier, and Dr. Soong), Toronto, Ontario, Canada. Co-first authors are Manpreet Basuita, MD, and Corey L. Kamen, BSc.
Abstract
- Objective: Routine laboratory testing is common among medical inpatients; however, when ordered inappropriately testing can represent low-value care. We examined the impact of an evidence-based intervention bundle on utilization.
- Participants/setting: This prospective cohort study took place at a tertiary academic medical center and included 6424 patients admitted to the general internal medicine service between April 2016 and March 2018.
- Intervention: An intervention bundle, whose first components were implemented in July 2016, included computer order entry restrictions on repetitive laboratory testing, education, and audit-feedback.
- Measures: Data were extracted from the hospital electronic health record. The primary outcome was the number of routine blood tests (complete blood count, creatinine, and electrolytes) ordered per inpatient day.
- Analysis: Descriptive statistics were calculated for demographic variables. We used statistical process control charts to compare the baseline period (April 2016-June 2017) and the intervention period (July 2017-March 2018) for the primary outcome.
- Results: The mean number of combined routine laboratory tests ordered per inpatient day decreased from 1.19 (SD, 0.21) tests to 1.11 (SD, 0.05), a relative reduction of 6.7% (P < 0.0001). Mean cost per case related to laboratory tests decreased from $17.24 in the pre-intervention period to $16.17 in the post-intervention period (relative reduction of 6.2%). This resulted in savings of $26,851 in the intervention year.
- Conclusion: A laboratory intervention bundle was associated with small reductions in testing and costs. A routine test performed less than once per inpatient day may not be clinically appropriate or possible.
Keywords: utilization; clinical costs; quality improvement; QI intervention; internal medicine; inpatient.
Routine laboratory blood testing is a commonly used diagnostic tool that physicians rely on to provide patient care. Although routine blood testing represents less than 5% of most hospital budgets, routine use and over-reliance on testing among physicians makes it a target of cost-reduction efforts.1-3 A variety of interventions have been proposed to reduce inappropriate laboratory tests, with varying results.1,4-6 Successful interventions include providing physicians with fee data associated with ordered laboratory tests, unbundling panels of tests, and multicomponent interventions.6 We conducted a multifaceted quality improvement study to promote and develop interventions to adopt appropriate blood test ordering practices.
Methods
Setting
This prospective cohort study took place at Mount Sinai Hospital, a 443-bed academic hospital affiliated with the University of Toronto, where more than 2400 learners rotate through annually. The study was approved by the Mount Sinai Hospital Research Ethics Board.
Participants
We included all inpatient admissions to the general internal medicine service between April 2016 and March 2018. Exclusion criteria included a length of stay (LOS) longer than 365 days and admission to a critical care unit. Patients with more than 1 admission were counted as separate hospital inpatient visits.
Intervention
Based on internal data, we targeted the top 3 most frequently ordered routine blood tests: complete blood count (CBC), creatinine, and electrolytes. Trainee interviews revealed that habit, bundled order sets, and fear of “missing something” contributed to inappropriate routine blood test ordering. Based on these root causes, we used the Model for Improvement to iteratively develop a multimodal intervention that began in July 2016.7,8 This included a change to the computerized provider order entry (CPOE) to nudge clinicians to a restrictive ordering strategy by substituting the “Daily x3” frequency of blood test ordering with a “Daily x1” option on a pick list of order options. Clinicians could still order daily routine blood tests for any specified duration, but would have to do so by manually changing the default setting within the CPOE.
From July 2017 to March 2018, the research team educated residents on appropriate laboratory test ordering and provided audit and feedback data to the clinicians. Diagnostic uncertainty was addressed in teaching sessions. Attending physicians were surveyed on appropriate indications for daily laboratory testing for each of CBC, electrolytes, and creatinine. Appropriate indications (Figure 1) were displayed in visible clinical areas and incorporated into teaching sessions.9
Clinician teams received real-time performance data on their routine blood test ordering patterns compared with an institutional benchmark. Bar graphs of blood work ordering rates (sum of CBCs, creatinine, and electrolytes ordered for all patients on a given team divided by the total LOS for all patients) were distributed to each internal medicine team via email every 2 weeks (Figure 2).1,10-12

Data Collection and Analysis
Data were extracted from the hospital electronic health record (EHR). The primary outcome was the number of routine blood tests (CBC, creatinine, and electrolytes) ordered per inpatient day. Descriptive statistics were calculated for demographic variables. We used statistical process control (SPC) charts to compare the baseline period (April 2016-June 2017) and the intervention period (July 2017-March 2018) for the primary outcome. SPC charts display process changes over time. Data are plotted in chronological order, with the central line representing the outcome mean, an upper line representing the upper control limit, and a lower line representing the lower control limit. The upper and lower limits were set at 3δ, which correspond to 3 standard deviations above and below the mean. Six successive points above or beyond the mean suggests “special cause variation,” indicating that observed results are unlikely due to secular trends. SPC charts are commonly used quality tools for process improvement as well as research.13-16 These charts were created using QI Macros SPC software for Excel V. 2012.07 (KnowWare International, Denver, CO).
The direct cost of each laboratory test was acquired from the hospital laboratory department. The cost of each laboratory test (CBC = $7.54/test, electrolytes = $2.04/test, creatinine = $1.28/test, in Canadian dollars) was subsequently added together and multiplied by the pre- and post-intervention difference of total blood tests saved per inpatient day and then multiplied by 365 to arrive at an estimated cost savings per year.
Results
Over the study period, there were 6424 unique patient admissions on the general internal medicine service, with a median LOS of 3.5 days (Table). 
The majority of inpatient visits had at least 1 test of CBC (80%; mean, 3.6 tests/visit), creatinine (79.3%; mean, 3.5 tests/visit), or electrolytes (81.6%; mean, 3.9 tests/visit) completed. In total, 56,767 laboratory tests were ordered.
Following the intervention, there was a reduction in both rates of routine blood test orders and their associated costs, with a shift below the mean. The mean number of tests ordered (combined CBC, creatinine, and electrolytes) per inpatient day decreased from 1.19 (SD, 0.21) in the pre-intervention period to 1.11 (SD, 0.05) in the post-intervention period (P < 0.0001), representing a 6.7% relative reduction (Figure 3). We observed a 6.2% relative reduction in costs per inpatient day, translating to a total savings of $26,851 over 1 year for the intervention period.

Discussion
Our study suggests that a multimodal intervention, including CPOE restrictions, resident education with posters, and audit and feedback strategies, can reduce lab test ordering on general internal medicine wards. This finding is similar to those of previous studies using a similar intervention, although different laboratory tests were targeted.1,2,5,6,10,17
Our study found lower test result reductions than those reported by a previous study, which reported a relative reduction of 17% to 30%,18 and by another investigation that was conducted recently in a similar setting.17 In the latter study, reductions in laboratory testing were mostly found in nonroutine tests, and no significant improvements were noted in CBC, electrolytes, and creatine, the 3 tests we studied over the same duration.17 This may represent a ceiling effect to reducing laboratory testing, and efforts to reduce CBC, electrolytes, and creatinine testing beyond 0.3 to 0.4 tests per inpatient day (or combined 1.16 tests per inpatient day) may not be clinically appropriate or possible. This information can guide institutions to include other areas of overuse based on rates of utilization in order to maximize the benefits from a resource intensive intervention.
There are a number of limitations that merit discussion. First, observational studies do not demonstrate causation; however, to our knowledge, there were no other co-interventions that were being conducted during the study duration. One important note is that our project’s intervention began in July, at which point there are new internal medicine residents beginning their training. As the concept of resource allocation becomes more important, medical schools are spending more time educating students about Choosing Wisely, and, therefore, newer cohorts of residents may be more cognizant of appropriate blood testing. Second, this is a single-center study, limiting generalizability; however, we note that many other centers have reported similar findings. Another limitation is that we do not know whether there were any adverse clinical events associated with blood work ordering that was too restrictive, although informal tracking of STAT laboratory testing remained stable throughout the study period. It is important to ensure that blood work is ordered in moderation and tailored to patients using one’s clinical judgment.
Future Directions
We observed modest reductions in the quantity and costs associated with a quality improvement intervention aimed at reducing routine blood testing. A baseline rate of laboratory testing of less than 1 test per inpatient day may require including other target tests to drive down absolute utilization.
Corresponding author: Christine Soong, MD, MSc, 433-600 University Avenue, Toronto, Ontario, Canada M5G 1X5; [email protected].
Financial disclosures: None.
From the University of Toronto (Dr. Basuita, Corey L. Kamen, and Dr. Soong) and Sinai Health System (Corey L. Kamen, Cheryl Ethier, and Dr. Soong), Toronto, Ontario, Canada. Co-first authors are Manpreet Basuita, MD, and Corey L. Kamen, BSc.
Abstract
- Objective: Routine laboratory testing is common among medical inpatients; however, when ordered inappropriately testing can represent low-value care. We examined the impact of an evidence-based intervention bundle on utilization.
- Participants/setting: This prospective cohort study took place at a tertiary academic medical center and included 6424 patients admitted to the general internal medicine service between April 2016 and March 2018.
- Intervention: An intervention bundle, whose first components were implemented in July 2016, included computer order entry restrictions on repetitive laboratory testing, education, and audit-feedback.
- Measures: Data were extracted from the hospital electronic health record. The primary outcome was the number of routine blood tests (complete blood count, creatinine, and electrolytes) ordered per inpatient day.
- Analysis: Descriptive statistics were calculated for demographic variables. We used statistical process control charts to compare the baseline period (April 2016-June 2017) and the intervention period (July 2017-March 2018) for the primary outcome.
- Results: The mean number of combined routine laboratory tests ordered per inpatient day decreased from 1.19 (SD, 0.21) tests to 1.11 (SD, 0.05), a relative reduction of 6.7% (P < 0.0001). Mean cost per case related to laboratory tests decreased from $17.24 in the pre-intervention period to $16.17 in the post-intervention period (relative reduction of 6.2%). This resulted in savings of $26,851 in the intervention year.
- Conclusion: A laboratory intervention bundle was associated with small reductions in testing and costs. A routine test performed less than once per inpatient day may not be clinically appropriate or possible.
Keywords: utilization; clinical costs; quality improvement; QI intervention; internal medicine; inpatient.
Routine laboratory blood testing is a commonly used diagnostic tool that physicians rely on to provide patient care. Although routine blood testing represents less than 5% of most hospital budgets, routine use and over-reliance on testing among physicians makes it a target of cost-reduction efforts.1-3 A variety of interventions have been proposed to reduce inappropriate laboratory tests, with varying results.1,4-6 Successful interventions include providing physicians with fee data associated with ordered laboratory tests, unbundling panels of tests, and multicomponent interventions.6 We conducted a multifaceted quality improvement study to promote and develop interventions to adopt appropriate blood test ordering practices.
Methods
Setting
This prospective cohort study took place at Mount Sinai Hospital, a 443-bed academic hospital affiliated with the University of Toronto, where more than 2400 learners rotate through annually. The study was approved by the Mount Sinai Hospital Research Ethics Board.
Participants
We included all inpatient admissions to the general internal medicine service between April 2016 and March 2018. Exclusion criteria included a length of stay (LOS) longer than 365 days and admission to a critical care unit. Patients with more than 1 admission were counted as separate hospital inpatient visits.
Intervention
Based on internal data, we targeted the top 3 most frequently ordered routine blood tests: complete blood count (CBC), creatinine, and electrolytes. Trainee interviews revealed that habit, bundled order sets, and fear of “missing something” contributed to inappropriate routine blood test ordering. Based on these root causes, we used the Model for Improvement to iteratively develop a multimodal intervention that began in July 2016.7,8 This included a change to the computerized provider order entry (CPOE) to nudge clinicians to a restrictive ordering strategy by substituting the “Daily x3” frequency of blood test ordering with a “Daily x1” option on a pick list of order options. Clinicians could still order daily routine blood tests for any specified duration, but would have to do so by manually changing the default setting within the CPOE.
From July 2017 to March 2018, the research team educated residents on appropriate laboratory test ordering and provided audit and feedback data to the clinicians. Diagnostic uncertainty was addressed in teaching sessions. Attending physicians were surveyed on appropriate indications for daily laboratory testing for each of CBC, electrolytes, and creatinine. Appropriate indications (Figure 1) were displayed in visible clinical areas and incorporated into teaching sessions.9
Clinician teams received real-time performance data on their routine blood test ordering patterns compared with an institutional benchmark. Bar graphs of blood work ordering rates (sum of CBCs, creatinine, and electrolytes ordered for all patients on a given team divided by the total LOS for all patients) were distributed to each internal medicine team via email every 2 weeks (Figure 2).1,10-12

Data Collection and Analysis
Data were extracted from the hospital electronic health record (EHR). The primary outcome was the number of routine blood tests (CBC, creatinine, and electrolytes) ordered per inpatient day. Descriptive statistics were calculated for demographic variables. We used statistical process control (SPC) charts to compare the baseline period (April 2016-June 2017) and the intervention period (July 2017-March 2018) for the primary outcome. SPC charts display process changes over time. Data are plotted in chronological order, with the central line representing the outcome mean, an upper line representing the upper control limit, and a lower line representing the lower control limit. The upper and lower limits were set at 3δ, which correspond to 3 standard deviations above and below the mean. Six successive points above or beyond the mean suggests “special cause variation,” indicating that observed results are unlikely due to secular trends. SPC charts are commonly used quality tools for process improvement as well as research.13-16 These charts were created using QI Macros SPC software for Excel V. 2012.07 (KnowWare International, Denver, CO).
The direct cost of each laboratory test was acquired from the hospital laboratory department. The cost of each laboratory test (CBC = $7.54/test, electrolytes = $2.04/test, creatinine = $1.28/test, in Canadian dollars) was subsequently added together and multiplied by the pre- and post-intervention difference of total blood tests saved per inpatient day and then multiplied by 365 to arrive at an estimated cost savings per year.
Results
Over the study period, there were 6424 unique patient admissions on the general internal medicine service, with a median LOS of 3.5 days (Table). 
The majority of inpatient visits had at least 1 test of CBC (80%; mean, 3.6 tests/visit), creatinine (79.3%; mean, 3.5 tests/visit), or electrolytes (81.6%; mean, 3.9 tests/visit) completed. In total, 56,767 laboratory tests were ordered.
Following the intervention, there was a reduction in both rates of routine blood test orders and their associated costs, with a shift below the mean. The mean number of tests ordered (combined CBC, creatinine, and electrolytes) per inpatient day decreased from 1.19 (SD, 0.21) in the pre-intervention period to 1.11 (SD, 0.05) in the post-intervention period (P < 0.0001), representing a 6.7% relative reduction (Figure 3). We observed a 6.2% relative reduction in costs per inpatient day, translating to a total savings of $26,851 over 1 year for the intervention period.

Discussion
Our study suggests that a multimodal intervention, including CPOE restrictions, resident education with posters, and audit and feedback strategies, can reduce lab test ordering on general internal medicine wards. This finding is similar to those of previous studies using a similar intervention, although different laboratory tests were targeted.1,2,5,6,10,17
Our study found lower test result reductions than those reported by a previous study, which reported a relative reduction of 17% to 30%,18 and by another investigation that was conducted recently in a similar setting.17 In the latter study, reductions in laboratory testing were mostly found in nonroutine tests, and no significant improvements were noted in CBC, electrolytes, and creatine, the 3 tests we studied over the same duration.17 This may represent a ceiling effect to reducing laboratory testing, and efforts to reduce CBC, electrolytes, and creatinine testing beyond 0.3 to 0.4 tests per inpatient day (or combined 1.16 tests per inpatient day) may not be clinically appropriate or possible. This information can guide institutions to include other areas of overuse based on rates of utilization in order to maximize the benefits from a resource intensive intervention.
There are a number of limitations that merit discussion. First, observational studies do not demonstrate causation; however, to our knowledge, there were no other co-interventions that were being conducted during the study duration. One important note is that our project’s intervention began in July, at which point there are new internal medicine residents beginning their training. As the concept of resource allocation becomes more important, medical schools are spending more time educating students about Choosing Wisely, and, therefore, newer cohorts of residents may be more cognizant of appropriate blood testing. Second, this is a single-center study, limiting generalizability; however, we note that many other centers have reported similar findings. Another limitation is that we do not know whether there were any adverse clinical events associated with blood work ordering that was too restrictive, although informal tracking of STAT laboratory testing remained stable throughout the study period. It is important to ensure that blood work is ordered in moderation and tailored to patients using one’s clinical judgment.
Future Directions
We observed modest reductions in the quantity and costs associated with a quality improvement intervention aimed at reducing routine blood testing. A baseline rate of laboratory testing of less than 1 test per inpatient day may require including other target tests to drive down absolute utilization.
Corresponding author: Christine Soong, MD, MSc, 433-600 University Avenue, Toronto, Ontario, Canada M5G 1X5; [email protected].
Financial disclosures: None.
1. Eaton KP, Levy K, Soong C, et al. Evidence-based guidelines to eliminate repetitive laboratory testing. JAMA Intern Med. 2017;178:431.
2. May TA, Clancy M, Critchfield J, et al. Reducing unnecessary inpatient laboratory testing in a teaching hospital. Am J Clin Pathol. 2006;126:200-206.
3. Thavendiranathan P, Bagai A, Ebidia A, et al. Do blood tests cause anemia in hospitalized patients? The effect of diagnostic phlebotomy on hemoglobin and hematocrit levels. J Gen Intern Med. 2005;20:520-524.
4. Feldman LS, Shihab HM, Thiemann D, et al. Impact of providing fee data on laboratory test ordering: a controlled clinical trial. JAMA Intern Med. 2013;173:903-908.
5. Attali, M, Barel Y, Somin M, et al. A cost-effective method for reducing the volume of laboratory tests in a university-associated teaching hospital. Mt Sinai J Med. 2006;73:787-794.
6. Faisal A, Andres K, Rind JAK, et al. Reducing the number of unnecessary routine laboratory tests through education of internal medicine residents. Postgrad Med J. 2018;94:716-719.
7. How to Improve. Institute for Healthcare Improvement. 2009. http://www.ihi.org/resources/Pages/HowtoImprove/default.aspx. Accessed June 5, 2019.
8. Langley GL, Moen R, Nolan KM, et al. The Improvement Guide: A Practical Approach to Enhancing Organizational Performance. 2nd ed. San Francisco: Jossey-Bass Publishers; 2009.
9. Hicks L. Blood Draws Toolkit. Choosing Wisely Canada. 2017. https://choosingwiselycanada.org/wpcontent/uploads/2017/10/CWC_BloodDraws_Toolkit.pdf. Accessed March 5, 2019.
10. Sadowski BW, Lane AB, Wood SM, et al. High-value, cost-conscious care: iterative systems-based interventions to reduce unnecessary laboratory testing. Am J Med. 2017;130:1112e1-1112e7.
11. Minerowicz C, Abel N, Hunter K, et al. Impact of weekly feedback on test ordering patterns. Am J Manag Care. 2015;21:763-768.
12. Calderon-Margalit R, Mor-Yosef S, et al. An administrative intervention to improve the utilization of laboratory tests within a university hospital. Int J Qual Health Care. 2005;17:243-248.
13. Benneyan JC, Lloyd RC, Plsek PE. Statistical process control as a tool for research and healthcare improvement. Qual Saf Health Care. 2003;12:458-64.
14. American Society for Quality. Control chart. ASM website. https://asq.org/quality-resources/control-chart. Accessed November 5, 2020.
15. American Society for Quality. The 7 Basic Quality Tools For Process Improvement. ASM website. https://asq.org/quality-resources/seven-basic-quality-tools. Accessed November 5, 2020.
16. Benneyan JC, Lloyd RC, Plsek PE. Statistical process control as a tool for research and healthcare improvement. Qual Saf Health Care. 2003;12:458-464.
17. Ambasta A, Ma IWY, Woo S, et al. Impact of an education and multilevel social comparison-based intervention bundle on use of routine blood tests in hospitalised patients at an academic tertiary care hospital: a controlled pre-intervention post-intervention study. BMJ Qual Saf. 2020;29:1-2.
18. Lee VS, Kawamoto K, Hess R, et al. Implementation of a value-driven outcomes program to identify high variability in clinical costs and outcomes and association with reduced cost and improved quality. JAMA. 2016;316:1061-1072.
1. Eaton KP, Levy K, Soong C, et al. Evidence-based guidelines to eliminate repetitive laboratory testing. JAMA Intern Med. 2017;178:431.
2. May TA, Clancy M, Critchfield J, et al. Reducing unnecessary inpatient laboratory testing in a teaching hospital. Am J Clin Pathol. 2006;126:200-206.
3. Thavendiranathan P, Bagai A, Ebidia A, et al. Do blood tests cause anemia in hospitalized patients? The effect of diagnostic phlebotomy on hemoglobin and hematocrit levels. J Gen Intern Med. 2005;20:520-524.
4. Feldman LS, Shihab HM, Thiemann D, et al. Impact of providing fee data on laboratory test ordering: a controlled clinical trial. JAMA Intern Med. 2013;173:903-908.
5. Attali, M, Barel Y, Somin M, et al. A cost-effective method for reducing the volume of laboratory tests in a university-associated teaching hospital. Mt Sinai J Med. 2006;73:787-794.
6. Faisal A, Andres K, Rind JAK, et al. Reducing the number of unnecessary routine laboratory tests through education of internal medicine residents. Postgrad Med J. 2018;94:716-719.
7. How to Improve. Institute for Healthcare Improvement. 2009. http://www.ihi.org/resources/Pages/HowtoImprove/default.aspx. Accessed June 5, 2019.
8. Langley GL, Moen R, Nolan KM, et al. The Improvement Guide: A Practical Approach to Enhancing Organizational Performance. 2nd ed. San Francisco: Jossey-Bass Publishers; 2009.
9. Hicks L. Blood Draws Toolkit. Choosing Wisely Canada. 2017. https://choosingwiselycanada.org/wpcontent/uploads/2017/10/CWC_BloodDraws_Toolkit.pdf. Accessed March 5, 2019.
10. Sadowski BW, Lane AB, Wood SM, et al. High-value, cost-conscious care: iterative systems-based interventions to reduce unnecessary laboratory testing. Am J Med. 2017;130:1112e1-1112e7.
11. Minerowicz C, Abel N, Hunter K, et al. Impact of weekly feedback on test ordering patterns. Am J Manag Care. 2015;21:763-768.
12. Calderon-Margalit R, Mor-Yosef S, et al. An administrative intervention to improve the utilization of laboratory tests within a university hospital. Int J Qual Health Care. 2005;17:243-248.
13. Benneyan JC, Lloyd RC, Plsek PE. Statistical process control as a tool for research and healthcare improvement. Qual Saf Health Care. 2003;12:458-64.
14. American Society for Quality. Control chart. ASM website. https://asq.org/quality-resources/control-chart. Accessed November 5, 2020.
15. American Society for Quality. The 7 Basic Quality Tools For Process Improvement. ASM website. https://asq.org/quality-resources/seven-basic-quality-tools. Accessed November 5, 2020.
16. Benneyan JC, Lloyd RC, Plsek PE. Statistical process control as a tool for research and healthcare improvement. Qual Saf Health Care. 2003;12:458-464.
17. Ambasta A, Ma IWY, Woo S, et al. Impact of an education and multilevel social comparison-based intervention bundle on use of routine blood tests in hospitalised patients at an academic tertiary care hospital: a controlled pre-intervention post-intervention study. BMJ Qual Saf. 2020;29:1-2.
18. Lee VS, Kawamoto K, Hess R, et al. Implementation of a value-driven outcomes program to identify high variability in clinical costs and outcomes and association with reduced cost and improved quality. JAMA. 2016;316:1061-1072.
CDC panel delves into priorities for COVID vaccine distribution
On Monday, members of an influential federal panel delved into the challenges ahead in deciding who will get the first doses of COVID-19 vaccines, including questions about which healthcare workers need those initial vaccinations the most.
The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) did not take any votes or seek to establish formal positions. Instead, the meeting served as a forum for experts to discuss the thorny issues ahead. The US Food and Drug Administration (FDA) could make a decision next month regarding clearance for the first COVID-19 vaccine.
An FDA advisory committee will meet December 10 to review the request for emergency use authorization (EUA) of a COVID-19 vaccine from Pfizer, in partnership with BioNTech. Moderna Inc said on November 16 that it expects to soon ask the FDA for an EUA of its rival COVID vaccine.
ACIP will face a two-part task after the FDA clears COVID-19 vaccines, said Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases. ACIP will need to first decide whether to recommend use of the vaccine and then address the “complicated and difficult” question of which groups should get the initial limited quantities.
“There aren’t any perfect decisions,” she told the ACIP members. “I know this is something that most of you didn’t anticipate doing, making these kinds of huge decisions in the midst of a pandemic.”
There has been considerable public discussion of prioritization of COVID-19 vaccines, including a set of recommendations offered by a special committee created by the National Academies of Sciences, Engineering and Medicine. In addition, CDC staff and members of ACIP outlined what they termed the “four ethical principles” meant to guide these decisions in a November 23 report in the agency’s Morbidity and Mortality Weekly Report. These four principles are to maximize benefits and minimize harms; promote justice; mitigate health inequities; and promote transparency.
But as the issuing of the first EUA nears, it falls to ACIP to move beyond endorsing broad goals. The panel will need to make decisions as to which groups will have to wait for COVID-19 vaccines.
ACIP members on Monday delved into these kinds of more detailed questions, using a proposed three-stage model as a discussion point.
In phase 1a of this model, healthcare workers and residents of long-term care facilities would be the first people to be vaccinated. Phase 1b would include those deemed essential workers, including police officers, firefighters, and those in education, transportation, food, and agriculture sectors. Phase 1c would include adults with high-risk medical conditions and those aged 65 years and older.
ACIP member Grace M. Lee, MD, MPH, of Stanford University, Stanford, California, questioned whether healthcare workers who are not seeing patients in person should wait to get the vaccines. There has been a marked rise in the use of telehealth during the pandemic, which has spared some clinicians from in-person COVID-19 patient visits in their practices.
“Close partnership with our public health colleagues will be critically important to make sure that we are not trying to vaccinate 100% of our healthcare workforce, if some proportion of our workforce can work from home,” Lee said.
ACIP member Pablo Sánchez, MD, of the Research Institute at Nationwide Children’s Hospital in Columbus, Ohio, concurred. Some clinicians, he noted, may have better access to personal protective equipment than others, he said.
“Unfortunately, not all healthcare workers are equal in terms of risk,” Sánchez said. “Within institutions, we’re going to have to prioritize which ones will get” the vaccine.
Clinicians may also make judgments about their own risk and need for early access to COVID-19 vaccinations, Sánchez said.
“I’m 66, and I’d rather give it to somebody much older and sicker than me,” he said.
Broader access
Fairly large populations will essentially be competing for limited doses of the first vaccines to reach the market.
The overlap is significant in the four priority groups put forward by CDC. The CDC staff estimated that about 21 million people would fall into the healthcare personnel category, which includes hospital staff, pharmacists, and those working in long-term care facilities. There are about 87 million people in the essential workers groups. More than 100 million adults in the United States, such as those with diabetes and cancers, fall into the high-risk medical conditions group. Another 53 million people are aged 65 and older.
Department of Health and Human Services Secretary Alex Azar on November 18 said the federal government expects to have about 40 million doses of these two vaccines by the end of December, which is enough to provide the two-dose regimen for about 20 million. If all goes as expected, Pfizer and Moderna will ramp up production.
Moderna has said that it expects by the end of this year to have approximately 20 million doses of its vaccine ready to ship in the United States and that it is on track to manufacture 500 million to 1 billion doses globally in 2021. Pfizer and BioNTech have said they expect to produce globally up to 50 million doses in 2020 and up to 1.3 billion doses by the end of 2021.
At the Monday meeting, several ACIP panelists stressed the need to ensure that essential workers get early doses of vaccines.
In many cases, these workers serve in jobs with significant public interaction and live in poor communities. They put themselves and their families at risk. Many of them lack the resources to take precautions available to those better able to isolate, said ACIP member Beth Bell, MD, MPH, of the University of Washington, Seattle, Washington.
“These essential workers are out there putting themselves at risk to allow the rest of us to socially distance,” she said. “Recognizing that not all of them may want to be vaccinated at this stage, we need to provide them with the opportunity early on in the process.”
In Bell’s view, the initial rollout of COVID-19 vaccines will send an important message about sharing this resource.
“If we’re serious about valuing equity, we need to have that baked in early on in the vaccination program,” she said.
Bell also said she was in favor of including people living in nursing homes in the initial wave of vaccinations. Concerns were raised about the frailty of this population.
“Given the mortality impact on the healthcare system from the number of nursing home residents that have been dying, I think on balance it makes sense to include them in phase 1a,” Bell said.
Other ACIP panelists said missteps with early vaccination of people in nursing homes could undermine faith in the treatments. Because of the ages and medical conditions of people in nursing homes, many of them may die after receiving the COVID-19 vaccine. Such deaths would not be associated with vaccine, but the medical community would not yet have evidence to disprove a connection.
There could be a backlash, with people falsely linking the death of a grandparent to the vaccine.
Fellow ACIP member Robert L. Atmar, MD, Baylor College of Medicine, Houston, Texas, was among those who had raised concerns about including people living in long-term care facilities in phase 1a. He said there are not yet enough data to judge the balance of benefits and harms of vaccination for this population.
The Pfizer and Moderna vaccines are “reactagenic,” meaning people may not feel well in the days after receiving the shots. The symptoms could lead to additional health evaluations of older people in nursing homes as clinicians try to figure out whether the patient’s reactions to the vaccine are caused by some condition or infection, Atmar said.
“Those of us who see these patients in the hospital recognize that there are often medical interventions that are done in the pursuit of a diagnosis, of a change in clinical status, that in and of themselves can lead to harm,” Atmar said.
Clinicians likely will have to encourage their patients of all ages to receive second doses of COVID-19 vaccines, despite the malaise they may provoke.
“We really need to make patients aware that this is not going to be a walk in the park. I mean, they’re going to know they had a vaccine, they’re probably not going to feel wonderful, but they’ve got to come back for that second dose,” said Sandra Adamson Fryhofer, MD, who represented the American Medical Association.
ACIP is expected to meet again to offer specific recommendations on the Pfizer and Moderna vaccines. ACIP’s recommendations trigger reimbursement processes, Azar said at a Tuesday press conference. ACIP’s work will inform decisions made by the federal government and governors about deploying shipments of COVID-19 vaccines, he said.
“At the end of the day, that is a decision, though, of the US government to make, which is where to recommend the prioritization,” Azar said. “It will be our nation’s governors in implementing the distribution plans to tell us” where to ship the vaccine.
This article first appeared on Medscape.com.
On Monday, members of an influential federal panel delved into the challenges ahead in deciding who will get the first doses of COVID-19 vaccines, including questions about which healthcare workers need those initial vaccinations the most.
The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) did not take any votes or seek to establish formal positions. Instead, the meeting served as a forum for experts to discuss the thorny issues ahead. The US Food and Drug Administration (FDA) could make a decision next month regarding clearance for the first COVID-19 vaccine.
An FDA advisory committee will meet December 10 to review the request for emergency use authorization (EUA) of a COVID-19 vaccine from Pfizer, in partnership with BioNTech. Moderna Inc said on November 16 that it expects to soon ask the FDA for an EUA of its rival COVID vaccine.
ACIP will face a two-part task after the FDA clears COVID-19 vaccines, said Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases. ACIP will need to first decide whether to recommend use of the vaccine and then address the “complicated and difficult” question of which groups should get the initial limited quantities.
“There aren’t any perfect decisions,” she told the ACIP members. “I know this is something that most of you didn’t anticipate doing, making these kinds of huge decisions in the midst of a pandemic.”
There has been considerable public discussion of prioritization of COVID-19 vaccines, including a set of recommendations offered by a special committee created by the National Academies of Sciences, Engineering and Medicine. In addition, CDC staff and members of ACIP outlined what they termed the “four ethical principles” meant to guide these decisions in a November 23 report in the agency’s Morbidity and Mortality Weekly Report. These four principles are to maximize benefits and minimize harms; promote justice; mitigate health inequities; and promote transparency.
But as the issuing of the first EUA nears, it falls to ACIP to move beyond endorsing broad goals. The panel will need to make decisions as to which groups will have to wait for COVID-19 vaccines.
ACIP members on Monday delved into these kinds of more detailed questions, using a proposed three-stage model as a discussion point.
In phase 1a of this model, healthcare workers and residents of long-term care facilities would be the first people to be vaccinated. Phase 1b would include those deemed essential workers, including police officers, firefighters, and those in education, transportation, food, and agriculture sectors. Phase 1c would include adults with high-risk medical conditions and those aged 65 years and older.
ACIP member Grace M. Lee, MD, MPH, of Stanford University, Stanford, California, questioned whether healthcare workers who are not seeing patients in person should wait to get the vaccines. There has been a marked rise in the use of telehealth during the pandemic, which has spared some clinicians from in-person COVID-19 patient visits in their practices.
“Close partnership with our public health colleagues will be critically important to make sure that we are not trying to vaccinate 100% of our healthcare workforce, if some proportion of our workforce can work from home,” Lee said.
ACIP member Pablo Sánchez, MD, of the Research Institute at Nationwide Children’s Hospital in Columbus, Ohio, concurred. Some clinicians, he noted, may have better access to personal protective equipment than others, he said.
“Unfortunately, not all healthcare workers are equal in terms of risk,” Sánchez said. “Within institutions, we’re going to have to prioritize which ones will get” the vaccine.
Clinicians may also make judgments about their own risk and need for early access to COVID-19 vaccinations, Sánchez said.
“I’m 66, and I’d rather give it to somebody much older and sicker than me,” he said.
Broader access
Fairly large populations will essentially be competing for limited doses of the first vaccines to reach the market.
The overlap is significant in the four priority groups put forward by CDC. The CDC staff estimated that about 21 million people would fall into the healthcare personnel category, which includes hospital staff, pharmacists, and those working in long-term care facilities. There are about 87 million people in the essential workers groups. More than 100 million adults in the United States, such as those with diabetes and cancers, fall into the high-risk medical conditions group. Another 53 million people are aged 65 and older.
Department of Health and Human Services Secretary Alex Azar on November 18 said the federal government expects to have about 40 million doses of these two vaccines by the end of December, which is enough to provide the two-dose regimen for about 20 million. If all goes as expected, Pfizer and Moderna will ramp up production.
Moderna has said that it expects by the end of this year to have approximately 20 million doses of its vaccine ready to ship in the United States and that it is on track to manufacture 500 million to 1 billion doses globally in 2021. Pfizer and BioNTech have said they expect to produce globally up to 50 million doses in 2020 and up to 1.3 billion doses by the end of 2021.
At the Monday meeting, several ACIP panelists stressed the need to ensure that essential workers get early doses of vaccines.
In many cases, these workers serve in jobs with significant public interaction and live in poor communities. They put themselves and their families at risk. Many of them lack the resources to take precautions available to those better able to isolate, said ACIP member Beth Bell, MD, MPH, of the University of Washington, Seattle, Washington.
“These essential workers are out there putting themselves at risk to allow the rest of us to socially distance,” she said. “Recognizing that not all of them may want to be vaccinated at this stage, we need to provide them with the opportunity early on in the process.”
In Bell’s view, the initial rollout of COVID-19 vaccines will send an important message about sharing this resource.
“If we’re serious about valuing equity, we need to have that baked in early on in the vaccination program,” she said.
Bell also said she was in favor of including people living in nursing homes in the initial wave of vaccinations. Concerns were raised about the frailty of this population.
“Given the mortality impact on the healthcare system from the number of nursing home residents that have been dying, I think on balance it makes sense to include them in phase 1a,” Bell said.
Other ACIP panelists said missteps with early vaccination of people in nursing homes could undermine faith in the treatments. Because of the ages and medical conditions of people in nursing homes, many of them may die after receiving the COVID-19 vaccine. Such deaths would not be associated with vaccine, but the medical community would not yet have evidence to disprove a connection.
There could be a backlash, with people falsely linking the death of a grandparent to the vaccine.
Fellow ACIP member Robert L. Atmar, MD, Baylor College of Medicine, Houston, Texas, was among those who had raised concerns about including people living in long-term care facilities in phase 1a. He said there are not yet enough data to judge the balance of benefits and harms of vaccination for this population.
The Pfizer and Moderna vaccines are “reactagenic,” meaning people may not feel well in the days after receiving the shots. The symptoms could lead to additional health evaluations of older people in nursing homes as clinicians try to figure out whether the patient’s reactions to the vaccine are caused by some condition or infection, Atmar said.
“Those of us who see these patients in the hospital recognize that there are often medical interventions that are done in the pursuit of a diagnosis, of a change in clinical status, that in and of themselves can lead to harm,” Atmar said.
Clinicians likely will have to encourage their patients of all ages to receive second doses of COVID-19 vaccines, despite the malaise they may provoke.
“We really need to make patients aware that this is not going to be a walk in the park. I mean, they’re going to know they had a vaccine, they’re probably not going to feel wonderful, but they’ve got to come back for that second dose,” said Sandra Adamson Fryhofer, MD, who represented the American Medical Association.
ACIP is expected to meet again to offer specific recommendations on the Pfizer and Moderna vaccines. ACIP’s recommendations trigger reimbursement processes, Azar said at a Tuesday press conference. ACIP’s work will inform decisions made by the federal government and governors about deploying shipments of COVID-19 vaccines, he said.
“At the end of the day, that is a decision, though, of the US government to make, which is where to recommend the prioritization,” Azar said. “It will be our nation’s governors in implementing the distribution plans to tell us” where to ship the vaccine.
This article first appeared on Medscape.com.
On Monday, members of an influential federal panel delved into the challenges ahead in deciding who will get the first doses of COVID-19 vaccines, including questions about which healthcare workers need those initial vaccinations the most.
The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) did not take any votes or seek to establish formal positions. Instead, the meeting served as a forum for experts to discuss the thorny issues ahead. The US Food and Drug Administration (FDA) could make a decision next month regarding clearance for the first COVID-19 vaccine.
An FDA advisory committee will meet December 10 to review the request for emergency use authorization (EUA) of a COVID-19 vaccine from Pfizer, in partnership with BioNTech. Moderna Inc said on November 16 that it expects to soon ask the FDA for an EUA of its rival COVID vaccine.
ACIP will face a two-part task after the FDA clears COVID-19 vaccines, said Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases. ACIP will need to first decide whether to recommend use of the vaccine and then address the “complicated and difficult” question of which groups should get the initial limited quantities.
“There aren’t any perfect decisions,” she told the ACIP members. “I know this is something that most of you didn’t anticipate doing, making these kinds of huge decisions in the midst of a pandemic.”
There has been considerable public discussion of prioritization of COVID-19 vaccines, including a set of recommendations offered by a special committee created by the National Academies of Sciences, Engineering and Medicine. In addition, CDC staff and members of ACIP outlined what they termed the “four ethical principles” meant to guide these decisions in a November 23 report in the agency’s Morbidity and Mortality Weekly Report. These four principles are to maximize benefits and minimize harms; promote justice; mitigate health inequities; and promote transparency.
But as the issuing of the first EUA nears, it falls to ACIP to move beyond endorsing broad goals. The panel will need to make decisions as to which groups will have to wait for COVID-19 vaccines.
ACIP members on Monday delved into these kinds of more detailed questions, using a proposed three-stage model as a discussion point.
In phase 1a of this model, healthcare workers and residents of long-term care facilities would be the first people to be vaccinated. Phase 1b would include those deemed essential workers, including police officers, firefighters, and those in education, transportation, food, and agriculture sectors. Phase 1c would include adults with high-risk medical conditions and those aged 65 years and older.
ACIP member Grace M. Lee, MD, MPH, of Stanford University, Stanford, California, questioned whether healthcare workers who are not seeing patients in person should wait to get the vaccines. There has been a marked rise in the use of telehealth during the pandemic, which has spared some clinicians from in-person COVID-19 patient visits in their practices.
“Close partnership with our public health colleagues will be critically important to make sure that we are not trying to vaccinate 100% of our healthcare workforce, if some proportion of our workforce can work from home,” Lee said.
ACIP member Pablo Sánchez, MD, of the Research Institute at Nationwide Children’s Hospital in Columbus, Ohio, concurred. Some clinicians, he noted, may have better access to personal protective equipment than others, he said.
“Unfortunately, not all healthcare workers are equal in terms of risk,” Sánchez said. “Within institutions, we’re going to have to prioritize which ones will get” the vaccine.
Clinicians may also make judgments about their own risk and need for early access to COVID-19 vaccinations, Sánchez said.
“I’m 66, and I’d rather give it to somebody much older and sicker than me,” he said.
Broader access
Fairly large populations will essentially be competing for limited doses of the first vaccines to reach the market.
The overlap is significant in the four priority groups put forward by CDC. The CDC staff estimated that about 21 million people would fall into the healthcare personnel category, which includes hospital staff, pharmacists, and those working in long-term care facilities. There are about 87 million people in the essential workers groups. More than 100 million adults in the United States, such as those with diabetes and cancers, fall into the high-risk medical conditions group. Another 53 million people are aged 65 and older.
Department of Health and Human Services Secretary Alex Azar on November 18 said the federal government expects to have about 40 million doses of these two vaccines by the end of December, which is enough to provide the two-dose regimen for about 20 million. If all goes as expected, Pfizer and Moderna will ramp up production.
Moderna has said that it expects by the end of this year to have approximately 20 million doses of its vaccine ready to ship in the United States and that it is on track to manufacture 500 million to 1 billion doses globally in 2021. Pfizer and BioNTech have said they expect to produce globally up to 50 million doses in 2020 and up to 1.3 billion doses by the end of 2021.
At the Monday meeting, several ACIP panelists stressed the need to ensure that essential workers get early doses of vaccines.
In many cases, these workers serve in jobs with significant public interaction and live in poor communities. They put themselves and their families at risk. Many of them lack the resources to take precautions available to those better able to isolate, said ACIP member Beth Bell, MD, MPH, of the University of Washington, Seattle, Washington.
“These essential workers are out there putting themselves at risk to allow the rest of us to socially distance,” she said. “Recognizing that not all of them may want to be vaccinated at this stage, we need to provide them with the opportunity early on in the process.”
In Bell’s view, the initial rollout of COVID-19 vaccines will send an important message about sharing this resource.
“If we’re serious about valuing equity, we need to have that baked in early on in the vaccination program,” she said.
Bell also said she was in favor of including people living in nursing homes in the initial wave of vaccinations. Concerns were raised about the frailty of this population.
“Given the mortality impact on the healthcare system from the number of nursing home residents that have been dying, I think on balance it makes sense to include them in phase 1a,” Bell said.
Other ACIP panelists said missteps with early vaccination of people in nursing homes could undermine faith in the treatments. Because of the ages and medical conditions of people in nursing homes, many of them may die after receiving the COVID-19 vaccine. Such deaths would not be associated with vaccine, but the medical community would not yet have evidence to disprove a connection.
There could be a backlash, with people falsely linking the death of a grandparent to the vaccine.
Fellow ACIP member Robert L. Atmar, MD, Baylor College of Medicine, Houston, Texas, was among those who had raised concerns about including people living in long-term care facilities in phase 1a. He said there are not yet enough data to judge the balance of benefits and harms of vaccination for this population.
The Pfizer and Moderna vaccines are “reactagenic,” meaning people may not feel well in the days after receiving the shots. The symptoms could lead to additional health evaluations of older people in nursing homes as clinicians try to figure out whether the patient’s reactions to the vaccine are caused by some condition or infection, Atmar said.
“Those of us who see these patients in the hospital recognize that there are often medical interventions that are done in the pursuit of a diagnosis, of a change in clinical status, that in and of themselves can lead to harm,” Atmar said.
Clinicians likely will have to encourage their patients of all ages to receive second doses of COVID-19 vaccines, despite the malaise they may provoke.
“We really need to make patients aware that this is not going to be a walk in the park. I mean, they’re going to know they had a vaccine, they’re probably not going to feel wonderful, but they’ve got to come back for that second dose,” said Sandra Adamson Fryhofer, MD, who represented the American Medical Association.
ACIP is expected to meet again to offer specific recommendations on the Pfizer and Moderna vaccines. ACIP’s recommendations trigger reimbursement processes, Azar said at a Tuesday press conference. ACIP’s work will inform decisions made by the federal government and governors about deploying shipments of COVID-19 vaccines, he said.
“At the end of the day, that is a decision, though, of the US government to make, which is where to recommend the prioritization,” Azar said. “It will be our nation’s governors in implementing the distribution plans to tell us” where to ship the vaccine.
This article first appeared on Medscape.com.
FDA expands Xofluza indication to include postexposure flu prophylaxis
The US Food and Drug Administration (FDA) has expanded the indication for the antiviral baloxavir marboxil (Xofluza) to include postexposure prophylaxis of uncomplicated influenza in people aged 12 years and older.
“This expanded indication for Xofluza will provide an important option to help prevent influenza just in time for a flu season that is anticipated to be unlike any other because it will coincide with the coronavirus pandemic,” Debra Birnkrant, MD, director, Division of Antiviral Products, FDA Center for Drug Evaluation and Research, said in a press release.
In addition, Xofluza, which was previously available only in tablet form, is also now available as granules for mixing in water, the FDA said.
The agency first approved baloxavir marboxil in 2018 for the treatment of acute uncomplicated influenza in people aged 12 years or older who have been symptomatic for no more than 48 hours.
A year later, the FDA expanded the indication to include people at high risk of developing influenza-related complications, such as those with asthma, chronic lung disease, diabetes, heart disease, or morbid obesity, as well as adults aged 65 years or older.
The safety and efficacy of Xofluza for influenza postexposure prophylaxis is supported by a randomized, double-blind, controlled trial involving 607 people aged 12 years and older. After exposure to a person with influenza in their household, they received a single dose of Xofluza or placebo.
The primary endpoint was the proportion of individuals who became infected with influenza and presented with fever and at least one respiratory symptom from day 1 to day 10.
Of the 303 people who received Xofluza, 1% of individuals met these criteria, compared with 13% of those who received placebo.
The most common adverse effects of Xofluza include diarrhea, bronchitis, nausea, sinusitis, and headache.
Hypersensitivity, including anaphylaxis, can occur in patients taking Xofluza. The antiviral is contraindicated in people with a known hypersensitivity reaction to Xofluza.
Xofluza should not be coadministered with dairy products, calcium-fortified beverages, laxatives, antacids, or oral supplements containing calcium, iron, magnesium, selenium, aluminium, or zinc.
Full prescribing information is available online.
This article first appeared on Medscape.com.
The US Food and Drug Administration (FDA) has expanded the indication for the antiviral baloxavir marboxil (Xofluza) to include postexposure prophylaxis of uncomplicated influenza in people aged 12 years and older.
“This expanded indication for Xofluza will provide an important option to help prevent influenza just in time for a flu season that is anticipated to be unlike any other because it will coincide with the coronavirus pandemic,” Debra Birnkrant, MD, director, Division of Antiviral Products, FDA Center for Drug Evaluation and Research, said in a press release.
In addition, Xofluza, which was previously available only in tablet form, is also now available as granules for mixing in water, the FDA said.
The agency first approved baloxavir marboxil in 2018 for the treatment of acute uncomplicated influenza in people aged 12 years or older who have been symptomatic for no more than 48 hours.
A year later, the FDA expanded the indication to include people at high risk of developing influenza-related complications, such as those with asthma, chronic lung disease, diabetes, heart disease, or morbid obesity, as well as adults aged 65 years or older.
The safety and efficacy of Xofluza for influenza postexposure prophylaxis is supported by a randomized, double-blind, controlled trial involving 607 people aged 12 years and older. After exposure to a person with influenza in their household, they received a single dose of Xofluza or placebo.
The primary endpoint was the proportion of individuals who became infected with influenza and presented with fever and at least one respiratory symptom from day 1 to day 10.
Of the 303 people who received Xofluza, 1% of individuals met these criteria, compared with 13% of those who received placebo.
The most common adverse effects of Xofluza include diarrhea, bronchitis, nausea, sinusitis, and headache.
Hypersensitivity, including anaphylaxis, can occur in patients taking Xofluza. The antiviral is contraindicated in people with a known hypersensitivity reaction to Xofluza.
Xofluza should not be coadministered with dairy products, calcium-fortified beverages, laxatives, antacids, or oral supplements containing calcium, iron, magnesium, selenium, aluminium, or zinc.
Full prescribing information is available online.
This article first appeared on Medscape.com.
The US Food and Drug Administration (FDA) has expanded the indication for the antiviral baloxavir marboxil (Xofluza) to include postexposure prophylaxis of uncomplicated influenza in people aged 12 years and older.
“This expanded indication for Xofluza will provide an important option to help prevent influenza just in time for a flu season that is anticipated to be unlike any other because it will coincide with the coronavirus pandemic,” Debra Birnkrant, MD, director, Division of Antiviral Products, FDA Center for Drug Evaluation and Research, said in a press release.
In addition, Xofluza, which was previously available only in tablet form, is also now available as granules for mixing in water, the FDA said.
The agency first approved baloxavir marboxil in 2018 for the treatment of acute uncomplicated influenza in people aged 12 years or older who have been symptomatic for no more than 48 hours.
A year later, the FDA expanded the indication to include people at high risk of developing influenza-related complications, such as those with asthma, chronic lung disease, diabetes, heart disease, or morbid obesity, as well as adults aged 65 years or older.
The safety and efficacy of Xofluza for influenza postexposure prophylaxis is supported by a randomized, double-blind, controlled trial involving 607 people aged 12 years and older. After exposure to a person with influenza in their household, they received a single dose of Xofluza or placebo.
The primary endpoint was the proportion of individuals who became infected with influenza and presented with fever and at least one respiratory symptom from day 1 to day 10.
Of the 303 people who received Xofluza, 1% of individuals met these criteria, compared with 13% of those who received placebo.
The most common adverse effects of Xofluza include diarrhea, bronchitis, nausea, sinusitis, and headache.
Hypersensitivity, including anaphylaxis, can occur in patients taking Xofluza. The antiviral is contraindicated in people with a known hypersensitivity reaction to Xofluza.
Xofluza should not be coadministered with dairy products, calcium-fortified beverages, laxatives, antacids, or oral supplements containing calcium, iron, magnesium, selenium, aluminium, or zinc.
Full prescribing information is available online.
This article first appeared on Medscape.com.
Equitable Post-COVID-19 Care: A Practical Framework to Integrate Health Equity in Diabetes Management
From T1D Exchange, Boston, MA (Dr. Ebekozien, Dr. Odugbesan, and Nicole Rioles); Barbara Davis Center, University of Colorado, Boulder, CO (Dr. Majidi); Cincinnati Children’s Hospital Medical Center, Cincinnati, OH (Dr. Jones); and Nationwide Children’s Hospital, Columbus, OH (Dr. Kamboj)
Health equity has been described as the opportunity for all persons to obtain their highest level of health possible.1 Unfortunately, even with advances in technology and care practices, disparities persist in health care outcomes. Disparities in prevalence, prognosis, and outcomes still exist in diabetes management.2 Non-Hispanic Black and/or Hispanic populations are more likely to have worse glycemic control,3,4 to encounter more barriers in access to care,5 and to have higher levels of acute complications,4 and to use advanced technologies less frequently.4 Diabetes is one of the preexisting conditions that increase morbidity and mortality in COVID-19.6,7 Unfortunately, adverse outcomes from COVID-19 also disproportionately impact a specific vulnerable population.8,9 The urgent transition to managing diabetes remotely during the COVID-19 pandemic may exacerbate long-term inequities because some vulnerable patients might not have access to technology devices necessary for effective remote management.
Here, we describe how quality improvement (QI) tools and principles can be adapted into a framework for advancing health equity. Specifically, we describe a 10-step framework that may be applied in diabetes care management to achieve improvement, using a hypothetical example of increasing use of continuous glucose monitors (CGMs) among patients with type 1 diabetes mellitus.10 This framework was developed to address the literature gap on practical ways health care providers can address inequities using QI principles, and was implemented by 1 of the authors at a local public health department.11 The framework’s iterative and comprehensive design makes it ideal for addressing inequities in chronic diseases like diabetes, which have multiple root causes with no easy solutions. The improvement program pilot received a national model practice award.11,12
10-Step Framework
Step 1: Review program/project baseline data for existing disparities. Diabetes programs and routine QI processes encourage existing data review to determine how effective the current system is working and if the existing process has a predictable pattern.13,14 Our equity-revised framework proposes a more in-depth review to stratify baseline data based on factors that might contribute to inequities, including race, age, income levels, ethnicity, language, sexual orientation, insurance type, and zip code. This process will identify patients not served or unfairly impacted due to socioeconomic factors. For example, using the hypothetical example of improving CGM use, a team completes a preliminary data review and determines that baseline CGM use is 30% in the clinic population. However, in a review to assess for disparities, they also identify that patients on public insurance have a significantly lower CGM uptake of only 15%.
Step 2: Build an equitable project team, including patients with lived experiences. Routine projects typically have clinicians, administrative staff, and analytic staff as members of their team. In a post-COVID-19 world, every team needs to learn directly from people impacted and share decision-making power. The traditional approach to receiving feedback has generally been to collect responses using surveys or focus groups. We propose that individuals/families who are disproportionately impacted be included as active members on QI teams. For example, in the hypothetical example of the CGM project, team members would include patients with type 1 diabetes who are on public insurance and their families.
Step 3: Develop equity-focused goals. The traditional program involves the development of aims that are SMART (specific, measurable, achievable, realistic, time-bound).15 The proposed framework encourages the inclusion of equity-revised goals (SMARTer) using insights from Steps 1 and 2. For example, your typical smart goal might be to increase the percentage of patients using CGM by 20% in 6 months, while a SMARTer goal would be to increase the proportion of patients using CGM by 20% and reduce the disparities among public and private insurance patients by 30% in 6 months.
Step 4: Identify inequitable processes/pathways. Traditional QI programs might use a process map or flow diagram to depict the current state of a process visually.16 For example, in Figure 1, the process map diagram depicts some differences in the process for patients with public insurance as opposed to those with private insurance. The framework also advocates for using visual tools like process maps to depict how there might be inequitable pathways in a system. Visually identifying inequitable processes/pathways can help a team see barriers, address challenges, and pilot innovative solutions.
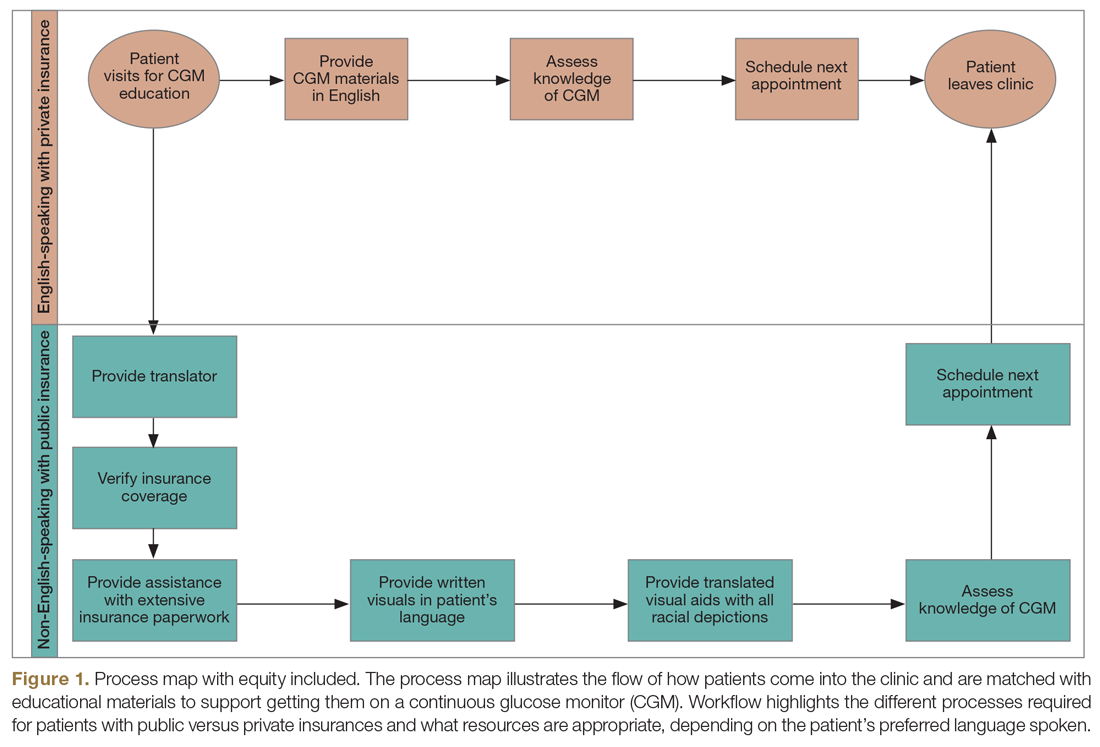
Step 5: Identify how socioeconomic factors are contributing to the current outcome. A good understanding of factors that contribute to the problem is an essential part of finding fundamental solutions. The fishbone diagram16 is a visualization tool used to identify contributing factors. When investigating contributing factors, it is commonplace to identify factors that fit into 1 of 5 categories: people, process, place, product, and policies (5 Ps). An equity-focused process will include equity as a new major factor category, and the socioeconomic impacts that contribute to inequities will be brainstormed and visually represented. For example, in the hypothetical CGM improvement example, an equity contributing factor is extensive CGM application paperwork for patients on public insurance as compared to those on private insurance. Figure 2 shows equity integrated into a fishbone diagram.
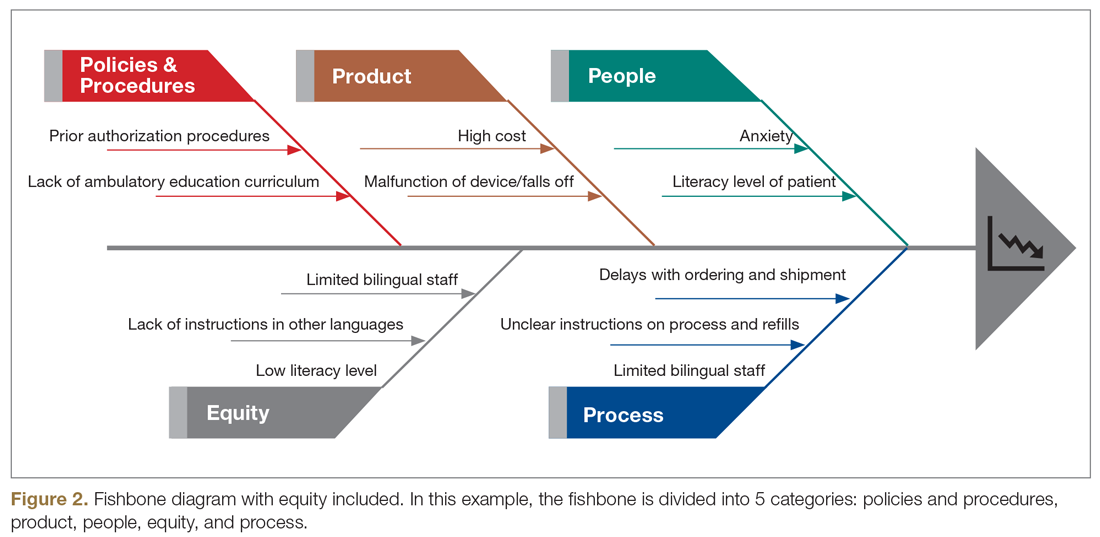
Step 6: Brainstorm possible improvements. Potential improvement ideas for the hypothetical CGM example might include redesigning the existing workflow, piloting CGM educational classes, and using a CGM barrier assessment tool to identify and address barriers to adoption.
Step 7: Use the decision matrix with equity as a criterion to prioritize improvement ideas. Decision matrix15 is a great tool that is frequently used to help teams prioritize potential ideas. Project team members must decide what criteria are important in prioritizing ideas to implement. Common criteria include implementation cost, time, and resources, but in addition to the common criteria, the team can specify ”impact on equity” as one of their criteria, alongside other standard criteria like impact.
Step 8: Test one small change at a time. This step is consistent with other traditional improvement models using the Plan, Do, Study, Act (PDSA) model for improvement.17 During this phase, the team should make predictions on the expected impact of the intervention on outcomes. For example, in the hypothetical example, the team predicts that testing and expanding CGM classes will reduce disparities among public versus private health insurance users by 5% and increase overall CGM uptake by 10%.
Step 9: Measure and compare results with predictions to identify inequitable practices or consequences. After each test of change, the team should review the results, including implementation cost considerations, and compare them to the predictions in the earlier step. The team should also document the potential reasons why their predictions were correct or inaccurate, and whether there were any unforeseen outcomes from the intervention.
Step 10: Celebrate small wins and repeat the process. Making fundamental and equitable changes takes time. This framework aimed at undoing inequities, particularly those inequities that have been amplified by the COVID-19 pandemic, is iterative and ongoing.18,19 Not every test of change will impact the outcome or reduce inequity, but over time, each change will impact the next, generating sustainable effects.
Conclusion
There are ongoing studies examining the adverse outcomes and potential health inequities for patients with diabetes impacted by COVID-19.20 Health care providers need to plan for post-COVID-19 care, keeping in mind that the pandemic might worsen already existing health disparities in diabetes management.3,4,21 This work will involve an intentional approach to address structural and systemic racism.22 Therefore, the work of building health equity solutions must be rooted in racial justice, economic equity, and equitable access to health care and education.
Initiatives like this are currently being funded through foundation grants as well as state and federal research or program grants. Regional and national payors, including the Centers for Medicare & Medicaid Services, are currently piloting long-term sustainable funding models through programs like accountable care organizations and the Accountable Health Communities Model.23
Health systems can successfully address health equity and racial justice, using a framework as described above, to identify determinants of health, develop policies to expand access to care for the most vulnerable patients, distribute decision-making power, and train staff by naming structural racism as a driver of health inequities.
Acknowledgment: The authors acknowledge the contributions of patients, families, diabetes care teams, and collaborators within the T1D Exchange Quality Improvement Collaborative, who continually seek to improve care and outcomes for people living with diabetes.
Corresponding author: Osagie Ebekozien, MD, 11 Avenue De La Fayette, Boston, MA 02115; [email protected].
Financial disclosures: None.
Funding: T1D Exchange QI Collaborative is funded by The Leona M. and Harry B. Helmsley Charitable Trust. No specific funding was received for this manuscript or the development of this framework.
Keywords: type 1 diabetes; quality improvement; QI framework; racial justice; health disparities.
1. American Public Health Association Health Equity web site. https://www.apha.org/topics-and-issues/health-equity. Accessed June 4, 2020.
2. Lado J, Lipman T. Racial and ethnic disparities in the incidence, treatment, and outcomes of youth with type 1 diabetes. Endocrinol Metab Clin North Am. 2016;45:453-461.
3. Kahkoska AR, Shay CM, Crandell J, et al. Association of race and ethnicity with glycemic control and hemoglobin A1c levels in youth with type 1 diabetes. JAMA Netw Open. 2018;1:e181851.
4. Willi SM, Miller KM, DiMeglio LA, et al; T1D Exchange Clinic Network. Racial-ethnic disparities in management and outcomes among children with type 1 diabetes. Pediatrics. 2015;135:424-434.
5. Valenzuela JM, Seid M, Waitzfelder B, et al. Prevalence of and disparities in barriers to care experienced by youth with type 1 diabetes. J Pediatr. 2014;164:1369-1375.
6. Hussain A, Bhowmik B, do Vale Moreira NC. COVID-19 and diabetes: Knowledge in progress. Diabetes Res Clin Pract. 2020;162:108142.
7. Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle Region - case series. N Engl J Med. 2020;382:2012-2022.
8. Laurencin CT, McClinton A. The COVID-19 pandemic: a call to action to identify and address racial and ethnic disparities. J Racial Ethn Health Disparities. 2020;7:398-402.
9. Shah M, Sachdeva M, Dodiuk-Gad RP. COVID-19 and racial disparities. J Am Acad Dermatol. 2020;83:e35.
10. Ebekozien O, Rioles N, DeSalvo D, et al. Improving continuous glucose monitoring (CGM) use across national centers: results from the T1D Exchange Quality Improvement Collaborative (T1DX-QI). Diabetes. 2020;69(Supplement 1):145-LB.
11. Ebekozien O. QI methodology to address health equity. Presented at American Society of Quality BOSCON 2018; Boston, MA; March 19 and 20, 2018.
12. 2019 Model Practice Award, Building A Culture of Improvement. National Association of County and City Health Officials web site. www.naccho.org/membership/awards/model-practices. Accessed June 4, 2020.
13. Nuckols TK, Keeler E, Anderson LJ, et al. Economic evaluation of quality improvement interventions designed to improve glycemic control in diabetes: a systematic review and weighted regression analysis. Diabetes Care. 2018;41:985‐993.
14. Rossi MC, Nicolucci A, Arcangeli A, et al. Baseline quality-of-care data from a quality-improvement program implemented by a network of diabetes outpatient clinics. Diabetes Care. 2008;31:2166‐2168.
15. McQuillan RF, Silver SA, Harel Z, et al. How to measure and interpret quality improvement data. Clin J Am Soc Nephrol. 2016;11:908-914.
16. Siddiqi FS. Quality improvement in diabetes care: time for us to step up? Can J Diabetes. 2019;43:233.
17. Taylor MJ, McNicholas C, Nicolay C, et al. Systematic review of the application of the plan-do-study-act method to improve quality in healthcare. BMJ Qual Saf. 2014;23:290‐298.
18. Ferdinand KC, Nasser SA. African American COVID-19 mortality: a sentinel event. J Am Coll Cardiol. 2020;75:2746-2748..
19. Muniyappa R, Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020;318:E736-E741.
20. Ebekozien OA, Noor N, Gallagher MP, Alonso GT. Type 1 diabetes and COVID-19: preliminary findings from a multicenter surveillance study in the U.S. Diabetes Care. 2020;43:e83-e85.
21. Majidi S, Ebekozien O, Noor N, et al. Inequities in health outcomes among patients in the T1D Exchange-QI Collaborative. Diabetes. 2020;69(Supplement 1):1220-P. https://doi.org/10.2337/ db20-1220.-P.
22. Williams DR, Mohammed SA. Discrimination and racial disparities in health: evidence and needed research. J Behav Med. 2009;32:20-47.
23. Centers for Medicare & Medicaid Services. Accountable Health Communities Model. CMS.gov web site. https://innovation.cms.gov/innovation-models/ahcm. Accessed October 10, 2020.
From T1D Exchange, Boston, MA (Dr. Ebekozien, Dr. Odugbesan, and Nicole Rioles); Barbara Davis Center, University of Colorado, Boulder, CO (Dr. Majidi); Cincinnati Children’s Hospital Medical Center, Cincinnati, OH (Dr. Jones); and Nationwide Children’s Hospital, Columbus, OH (Dr. Kamboj)
Health equity has been described as the opportunity for all persons to obtain their highest level of health possible.1 Unfortunately, even with advances in technology and care practices, disparities persist in health care outcomes. Disparities in prevalence, prognosis, and outcomes still exist in diabetes management.2 Non-Hispanic Black and/or Hispanic populations are more likely to have worse glycemic control,3,4 to encounter more barriers in access to care,5 and to have higher levels of acute complications,4 and to use advanced technologies less frequently.4 Diabetes is one of the preexisting conditions that increase morbidity and mortality in COVID-19.6,7 Unfortunately, adverse outcomes from COVID-19 also disproportionately impact a specific vulnerable population.8,9 The urgent transition to managing diabetes remotely during the COVID-19 pandemic may exacerbate long-term inequities because some vulnerable patients might not have access to technology devices necessary for effective remote management.
Here, we describe how quality improvement (QI) tools and principles can be adapted into a framework for advancing health equity. Specifically, we describe a 10-step framework that may be applied in diabetes care management to achieve improvement, using a hypothetical example of increasing use of continuous glucose monitors (CGMs) among patients with type 1 diabetes mellitus.10 This framework was developed to address the literature gap on practical ways health care providers can address inequities using QI principles, and was implemented by 1 of the authors at a local public health department.11 The framework’s iterative and comprehensive design makes it ideal for addressing inequities in chronic diseases like diabetes, which have multiple root causes with no easy solutions. The improvement program pilot received a national model practice award.11,12
10-Step Framework
Step 1: Review program/project baseline data for existing disparities. Diabetes programs and routine QI processes encourage existing data review to determine how effective the current system is working and if the existing process has a predictable pattern.13,14 Our equity-revised framework proposes a more in-depth review to stratify baseline data based on factors that might contribute to inequities, including race, age, income levels, ethnicity, language, sexual orientation, insurance type, and zip code. This process will identify patients not served or unfairly impacted due to socioeconomic factors. For example, using the hypothetical example of improving CGM use, a team completes a preliminary data review and determines that baseline CGM use is 30% in the clinic population. However, in a review to assess for disparities, they also identify that patients on public insurance have a significantly lower CGM uptake of only 15%.
Step 2: Build an equitable project team, including patients with lived experiences. Routine projects typically have clinicians, administrative staff, and analytic staff as members of their team. In a post-COVID-19 world, every team needs to learn directly from people impacted and share decision-making power. The traditional approach to receiving feedback has generally been to collect responses using surveys or focus groups. We propose that individuals/families who are disproportionately impacted be included as active members on QI teams. For example, in the hypothetical example of the CGM project, team members would include patients with type 1 diabetes who are on public insurance and their families.
Step 3: Develop equity-focused goals. The traditional program involves the development of aims that are SMART (specific, measurable, achievable, realistic, time-bound).15 The proposed framework encourages the inclusion of equity-revised goals (SMARTer) using insights from Steps 1 and 2. For example, your typical smart goal might be to increase the percentage of patients using CGM by 20% in 6 months, while a SMARTer goal would be to increase the proportion of patients using CGM by 20% and reduce the disparities among public and private insurance patients by 30% in 6 months.
Step 4: Identify inequitable processes/pathways. Traditional QI programs might use a process map or flow diagram to depict the current state of a process visually.16 For example, in Figure 1, the process map diagram depicts some differences in the process for patients with public insurance as opposed to those with private insurance. The framework also advocates for using visual tools like process maps to depict how there might be inequitable pathways in a system. Visually identifying inequitable processes/pathways can help a team see barriers, address challenges, and pilot innovative solutions.

Step 5: Identify how socioeconomic factors are contributing to the current outcome. A good understanding of factors that contribute to the problem is an essential part of finding fundamental solutions. The fishbone diagram16 is a visualization tool used to identify contributing factors. When investigating contributing factors, it is commonplace to identify factors that fit into 1 of 5 categories: people, process, place, product, and policies (5 Ps). An equity-focused process will include equity as a new major factor category, and the socioeconomic impacts that contribute to inequities will be brainstormed and visually represented. For example, in the hypothetical CGM improvement example, an equity contributing factor is extensive CGM application paperwork for patients on public insurance as compared to those on private insurance. Figure 2 shows equity integrated into a fishbone diagram.

Step 6: Brainstorm possible improvements. Potential improvement ideas for the hypothetical CGM example might include redesigning the existing workflow, piloting CGM educational classes, and using a CGM barrier assessment tool to identify and address barriers to adoption.
Step 7: Use the decision matrix with equity as a criterion to prioritize improvement ideas. Decision matrix15 is a great tool that is frequently used to help teams prioritize potential ideas. Project team members must decide what criteria are important in prioritizing ideas to implement. Common criteria include implementation cost, time, and resources, but in addition to the common criteria, the team can specify ”impact on equity” as one of their criteria, alongside other standard criteria like impact.
Step 8: Test one small change at a time. This step is consistent with other traditional improvement models using the Plan, Do, Study, Act (PDSA) model for improvement.17 During this phase, the team should make predictions on the expected impact of the intervention on outcomes. For example, in the hypothetical example, the team predicts that testing and expanding CGM classes will reduce disparities among public versus private health insurance users by 5% and increase overall CGM uptake by 10%.
Step 9: Measure and compare results with predictions to identify inequitable practices or consequences. After each test of change, the team should review the results, including implementation cost considerations, and compare them to the predictions in the earlier step. The team should also document the potential reasons why their predictions were correct or inaccurate, and whether there were any unforeseen outcomes from the intervention.
Step 10: Celebrate small wins and repeat the process. Making fundamental and equitable changes takes time. This framework aimed at undoing inequities, particularly those inequities that have been amplified by the COVID-19 pandemic, is iterative and ongoing.18,19 Not every test of change will impact the outcome or reduce inequity, but over time, each change will impact the next, generating sustainable effects.
Conclusion
There are ongoing studies examining the adverse outcomes and potential health inequities for patients with diabetes impacted by COVID-19.20 Health care providers need to plan for post-COVID-19 care, keeping in mind that the pandemic might worsen already existing health disparities in diabetes management.3,4,21 This work will involve an intentional approach to address structural and systemic racism.22 Therefore, the work of building health equity solutions must be rooted in racial justice, economic equity, and equitable access to health care and education.
Initiatives like this are currently being funded through foundation grants as well as state and federal research or program grants. Regional and national payors, including the Centers for Medicare & Medicaid Services, are currently piloting long-term sustainable funding models through programs like accountable care organizations and the Accountable Health Communities Model.23
Health systems can successfully address health equity and racial justice, using a framework as described above, to identify determinants of health, develop policies to expand access to care for the most vulnerable patients, distribute decision-making power, and train staff by naming structural racism as a driver of health inequities.
Acknowledgment: The authors acknowledge the contributions of patients, families, diabetes care teams, and collaborators within the T1D Exchange Quality Improvement Collaborative, who continually seek to improve care and outcomes for people living with diabetes.
Corresponding author: Osagie Ebekozien, MD, 11 Avenue De La Fayette, Boston, MA 02115; [email protected].
Financial disclosures: None.
Funding: T1D Exchange QI Collaborative is funded by The Leona M. and Harry B. Helmsley Charitable Trust. No specific funding was received for this manuscript or the development of this framework.
Keywords: type 1 diabetes; quality improvement; QI framework; racial justice; health disparities.
From T1D Exchange, Boston, MA (Dr. Ebekozien, Dr. Odugbesan, and Nicole Rioles); Barbara Davis Center, University of Colorado, Boulder, CO (Dr. Majidi); Cincinnati Children’s Hospital Medical Center, Cincinnati, OH (Dr. Jones); and Nationwide Children’s Hospital, Columbus, OH (Dr. Kamboj)
Health equity has been described as the opportunity for all persons to obtain their highest level of health possible.1 Unfortunately, even with advances in technology and care practices, disparities persist in health care outcomes. Disparities in prevalence, prognosis, and outcomes still exist in diabetes management.2 Non-Hispanic Black and/or Hispanic populations are more likely to have worse glycemic control,3,4 to encounter more barriers in access to care,5 and to have higher levels of acute complications,4 and to use advanced technologies less frequently.4 Diabetes is one of the preexisting conditions that increase morbidity and mortality in COVID-19.6,7 Unfortunately, adverse outcomes from COVID-19 also disproportionately impact a specific vulnerable population.8,9 The urgent transition to managing diabetes remotely during the COVID-19 pandemic may exacerbate long-term inequities because some vulnerable patients might not have access to technology devices necessary for effective remote management.
Here, we describe how quality improvement (QI) tools and principles can be adapted into a framework for advancing health equity. Specifically, we describe a 10-step framework that may be applied in diabetes care management to achieve improvement, using a hypothetical example of increasing use of continuous glucose monitors (CGMs) among patients with type 1 diabetes mellitus.10 This framework was developed to address the literature gap on practical ways health care providers can address inequities using QI principles, and was implemented by 1 of the authors at a local public health department.11 The framework’s iterative and comprehensive design makes it ideal for addressing inequities in chronic diseases like diabetes, which have multiple root causes with no easy solutions. The improvement program pilot received a national model practice award.11,12
10-Step Framework
Step 1: Review program/project baseline data for existing disparities. Diabetes programs and routine QI processes encourage existing data review to determine how effective the current system is working and if the existing process has a predictable pattern.13,14 Our equity-revised framework proposes a more in-depth review to stratify baseline data based on factors that might contribute to inequities, including race, age, income levels, ethnicity, language, sexual orientation, insurance type, and zip code. This process will identify patients not served or unfairly impacted due to socioeconomic factors. For example, using the hypothetical example of improving CGM use, a team completes a preliminary data review and determines that baseline CGM use is 30% in the clinic population. However, in a review to assess for disparities, they also identify that patients on public insurance have a significantly lower CGM uptake of only 15%.
Step 2: Build an equitable project team, including patients with lived experiences. Routine projects typically have clinicians, administrative staff, and analytic staff as members of their team. In a post-COVID-19 world, every team needs to learn directly from people impacted and share decision-making power. The traditional approach to receiving feedback has generally been to collect responses using surveys or focus groups. We propose that individuals/families who are disproportionately impacted be included as active members on QI teams. For example, in the hypothetical example of the CGM project, team members would include patients with type 1 diabetes who are on public insurance and their families.
Step 3: Develop equity-focused goals. The traditional program involves the development of aims that are SMART (specific, measurable, achievable, realistic, time-bound).15 The proposed framework encourages the inclusion of equity-revised goals (SMARTer) using insights from Steps 1 and 2. For example, your typical smart goal might be to increase the percentage of patients using CGM by 20% in 6 months, while a SMARTer goal would be to increase the proportion of patients using CGM by 20% and reduce the disparities among public and private insurance patients by 30% in 6 months.
Step 4: Identify inequitable processes/pathways. Traditional QI programs might use a process map or flow diagram to depict the current state of a process visually.16 For example, in Figure 1, the process map diagram depicts some differences in the process for patients with public insurance as opposed to those with private insurance. The framework also advocates for using visual tools like process maps to depict how there might be inequitable pathways in a system. Visually identifying inequitable processes/pathways can help a team see barriers, address challenges, and pilot innovative solutions.

Step 5: Identify how socioeconomic factors are contributing to the current outcome. A good understanding of factors that contribute to the problem is an essential part of finding fundamental solutions. The fishbone diagram16 is a visualization tool used to identify contributing factors. When investigating contributing factors, it is commonplace to identify factors that fit into 1 of 5 categories: people, process, place, product, and policies (5 Ps). An equity-focused process will include equity as a new major factor category, and the socioeconomic impacts that contribute to inequities will be brainstormed and visually represented. For example, in the hypothetical CGM improvement example, an equity contributing factor is extensive CGM application paperwork for patients on public insurance as compared to those on private insurance. Figure 2 shows equity integrated into a fishbone diagram.

Step 6: Brainstorm possible improvements. Potential improvement ideas for the hypothetical CGM example might include redesigning the existing workflow, piloting CGM educational classes, and using a CGM barrier assessment tool to identify and address barriers to adoption.
Step 7: Use the decision matrix with equity as a criterion to prioritize improvement ideas. Decision matrix15 is a great tool that is frequently used to help teams prioritize potential ideas. Project team members must decide what criteria are important in prioritizing ideas to implement. Common criteria include implementation cost, time, and resources, but in addition to the common criteria, the team can specify ”impact on equity” as one of their criteria, alongside other standard criteria like impact.
Step 8: Test one small change at a time. This step is consistent with other traditional improvement models using the Plan, Do, Study, Act (PDSA) model for improvement.17 During this phase, the team should make predictions on the expected impact of the intervention on outcomes. For example, in the hypothetical example, the team predicts that testing and expanding CGM classes will reduce disparities among public versus private health insurance users by 5% and increase overall CGM uptake by 10%.
Step 9: Measure and compare results with predictions to identify inequitable practices or consequences. After each test of change, the team should review the results, including implementation cost considerations, and compare them to the predictions in the earlier step. The team should also document the potential reasons why their predictions were correct or inaccurate, and whether there were any unforeseen outcomes from the intervention.
Step 10: Celebrate small wins and repeat the process. Making fundamental and equitable changes takes time. This framework aimed at undoing inequities, particularly those inequities that have been amplified by the COVID-19 pandemic, is iterative and ongoing.18,19 Not every test of change will impact the outcome or reduce inequity, but over time, each change will impact the next, generating sustainable effects.
Conclusion
There are ongoing studies examining the adverse outcomes and potential health inequities for patients with diabetes impacted by COVID-19.20 Health care providers need to plan for post-COVID-19 care, keeping in mind that the pandemic might worsen already existing health disparities in diabetes management.3,4,21 This work will involve an intentional approach to address structural and systemic racism.22 Therefore, the work of building health equity solutions must be rooted in racial justice, economic equity, and equitable access to health care and education.
Initiatives like this are currently being funded through foundation grants as well as state and federal research or program grants. Regional and national payors, including the Centers for Medicare & Medicaid Services, are currently piloting long-term sustainable funding models through programs like accountable care organizations and the Accountable Health Communities Model.23
Health systems can successfully address health equity and racial justice, using a framework as described above, to identify determinants of health, develop policies to expand access to care for the most vulnerable patients, distribute decision-making power, and train staff by naming structural racism as a driver of health inequities.
Acknowledgment: The authors acknowledge the contributions of patients, families, diabetes care teams, and collaborators within the T1D Exchange Quality Improvement Collaborative, who continually seek to improve care and outcomes for people living with diabetes.
Corresponding author: Osagie Ebekozien, MD, 11 Avenue De La Fayette, Boston, MA 02115; [email protected].
Financial disclosures: None.
Funding: T1D Exchange QI Collaborative is funded by The Leona M. and Harry B. Helmsley Charitable Trust. No specific funding was received for this manuscript or the development of this framework.
Keywords: type 1 diabetes; quality improvement; QI framework; racial justice; health disparities.
1. American Public Health Association Health Equity web site. https://www.apha.org/topics-and-issues/health-equity. Accessed June 4, 2020.
2. Lado J, Lipman T. Racial and ethnic disparities in the incidence, treatment, and outcomes of youth with type 1 diabetes. Endocrinol Metab Clin North Am. 2016;45:453-461.
3. Kahkoska AR, Shay CM, Crandell J, et al. Association of race and ethnicity with glycemic control and hemoglobin A1c levels in youth with type 1 diabetes. JAMA Netw Open. 2018;1:e181851.
4. Willi SM, Miller KM, DiMeglio LA, et al; T1D Exchange Clinic Network. Racial-ethnic disparities in management and outcomes among children with type 1 diabetes. Pediatrics. 2015;135:424-434.
5. Valenzuela JM, Seid M, Waitzfelder B, et al. Prevalence of and disparities in barriers to care experienced by youth with type 1 diabetes. J Pediatr. 2014;164:1369-1375.
6. Hussain A, Bhowmik B, do Vale Moreira NC. COVID-19 and diabetes: Knowledge in progress. Diabetes Res Clin Pract. 2020;162:108142.
7. Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle Region - case series. N Engl J Med. 2020;382:2012-2022.
8. Laurencin CT, McClinton A. The COVID-19 pandemic: a call to action to identify and address racial and ethnic disparities. J Racial Ethn Health Disparities. 2020;7:398-402.
9. Shah M, Sachdeva M, Dodiuk-Gad RP. COVID-19 and racial disparities. J Am Acad Dermatol. 2020;83:e35.
10. Ebekozien O, Rioles N, DeSalvo D, et al. Improving continuous glucose monitoring (CGM) use across national centers: results from the T1D Exchange Quality Improvement Collaborative (T1DX-QI). Diabetes. 2020;69(Supplement 1):145-LB.
11. Ebekozien O. QI methodology to address health equity. Presented at American Society of Quality BOSCON 2018; Boston, MA; March 19 and 20, 2018.
12. 2019 Model Practice Award, Building A Culture of Improvement. National Association of County and City Health Officials web site. www.naccho.org/membership/awards/model-practices. Accessed June 4, 2020.
13. Nuckols TK, Keeler E, Anderson LJ, et al. Economic evaluation of quality improvement interventions designed to improve glycemic control in diabetes: a systematic review and weighted regression analysis. Diabetes Care. 2018;41:985‐993.
14. Rossi MC, Nicolucci A, Arcangeli A, et al. Baseline quality-of-care data from a quality-improvement program implemented by a network of diabetes outpatient clinics. Diabetes Care. 2008;31:2166‐2168.
15. McQuillan RF, Silver SA, Harel Z, et al. How to measure and interpret quality improvement data. Clin J Am Soc Nephrol. 2016;11:908-914.
16. Siddiqi FS. Quality improvement in diabetes care: time for us to step up? Can J Diabetes. 2019;43:233.
17. Taylor MJ, McNicholas C, Nicolay C, et al. Systematic review of the application of the plan-do-study-act method to improve quality in healthcare. BMJ Qual Saf. 2014;23:290‐298.
18. Ferdinand KC, Nasser SA. African American COVID-19 mortality: a sentinel event. J Am Coll Cardiol. 2020;75:2746-2748..
19. Muniyappa R, Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020;318:E736-E741.
20. Ebekozien OA, Noor N, Gallagher MP, Alonso GT. Type 1 diabetes and COVID-19: preliminary findings from a multicenter surveillance study in the U.S. Diabetes Care. 2020;43:e83-e85.
21. Majidi S, Ebekozien O, Noor N, et al. Inequities in health outcomes among patients in the T1D Exchange-QI Collaborative. Diabetes. 2020;69(Supplement 1):1220-P. https://doi.org/10.2337/ db20-1220.-P.
22. Williams DR, Mohammed SA. Discrimination and racial disparities in health: evidence and needed research. J Behav Med. 2009;32:20-47.
23. Centers for Medicare & Medicaid Services. Accountable Health Communities Model. CMS.gov web site. https://innovation.cms.gov/innovation-models/ahcm. Accessed October 10, 2020.
1. American Public Health Association Health Equity web site. https://www.apha.org/topics-and-issues/health-equity. Accessed June 4, 2020.
2. Lado J, Lipman T. Racial and ethnic disparities in the incidence, treatment, and outcomes of youth with type 1 diabetes. Endocrinol Metab Clin North Am. 2016;45:453-461.
3. Kahkoska AR, Shay CM, Crandell J, et al. Association of race and ethnicity with glycemic control and hemoglobin A1c levels in youth with type 1 diabetes. JAMA Netw Open. 2018;1:e181851.
4. Willi SM, Miller KM, DiMeglio LA, et al; T1D Exchange Clinic Network. Racial-ethnic disparities in management and outcomes among children with type 1 diabetes. Pediatrics. 2015;135:424-434.
5. Valenzuela JM, Seid M, Waitzfelder B, et al. Prevalence of and disparities in barriers to care experienced by youth with type 1 diabetes. J Pediatr. 2014;164:1369-1375.
6. Hussain A, Bhowmik B, do Vale Moreira NC. COVID-19 and diabetes: Knowledge in progress. Diabetes Res Clin Pract. 2020;162:108142.
7. Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle Region - case series. N Engl J Med. 2020;382:2012-2022.
8. Laurencin CT, McClinton A. The COVID-19 pandemic: a call to action to identify and address racial and ethnic disparities. J Racial Ethn Health Disparities. 2020;7:398-402.
9. Shah M, Sachdeva M, Dodiuk-Gad RP. COVID-19 and racial disparities. J Am Acad Dermatol. 2020;83:e35.
10. Ebekozien O, Rioles N, DeSalvo D, et al. Improving continuous glucose monitoring (CGM) use across national centers: results from the T1D Exchange Quality Improvement Collaborative (T1DX-QI). Diabetes. 2020;69(Supplement 1):145-LB.
11. Ebekozien O. QI methodology to address health equity. Presented at American Society of Quality BOSCON 2018; Boston, MA; March 19 and 20, 2018.
12. 2019 Model Practice Award, Building A Culture of Improvement. National Association of County and City Health Officials web site. www.naccho.org/membership/awards/model-practices. Accessed June 4, 2020.
13. Nuckols TK, Keeler E, Anderson LJ, et al. Economic evaluation of quality improvement interventions designed to improve glycemic control in diabetes: a systematic review and weighted regression analysis. Diabetes Care. 2018;41:985‐993.
14. Rossi MC, Nicolucci A, Arcangeli A, et al. Baseline quality-of-care data from a quality-improvement program implemented by a network of diabetes outpatient clinics. Diabetes Care. 2008;31:2166‐2168.
15. McQuillan RF, Silver SA, Harel Z, et al. How to measure and interpret quality improvement data. Clin J Am Soc Nephrol. 2016;11:908-914.
16. Siddiqi FS. Quality improvement in diabetes care: time for us to step up? Can J Diabetes. 2019;43:233.
17. Taylor MJ, McNicholas C, Nicolay C, et al. Systematic review of the application of the plan-do-study-act method to improve quality in healthcare. BMJ Qual Saf. 2014;23:290‐298.
18. Ferdinand KC, Nasser SA. African American COVID-19 mortality: a sentinel event. J Am Coll Cardiol. 2020;75:2746-2748..
19. Muniyappa R, Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020;318:E736-E741.
20. Ebekozien OA, Noor N, Gallagher MP, Alonso GT. Type 1 diabetes and COVID-19: preliminary findings from a multicenter surveillance study in the U.S. Diabetes Care. 2020;43:e83-e85.
21. Majidi S, Ebekozien O, Noor N, et al. Inequities in health outcomes among patients in the T1D Exchange-QI Collaborative. Diabetes. 2020;69(Supplement 1):1220-P. https://doi.org/10.2337/ db20-1220.-P.
22. Williams DR, Mohammed SA. Discrimination and racial disparities in health: evidence and needed research. J Behav Med. 2009;32:20-47.
23. Centers for Medicare & Medicaid Services. Accountable Health Communities Model. CMS.gov web site. https://innovation.cms.gov/innovation-models/ahcm. Accessed October 10, 2020.
AMA takes on vaccine misinformation, physician vaccines, racism
The American Medical Association House of Delegates has adopted a policy to educate physicians on how to speak with patients about COVID-19 vaccination to counteract widespread misinformation about the vaccine development process.
Other highlights of the AMA’s recent special meeting include a new policy on the ethics of physicians getting immunized against COVID-19 and a far-reaching statement about racism.
Under the organization’s new vaccination education policy, the AMA will provide physicians with “culturally appropriate patient education materials,” according to a news release.
This campaign will be conducted “bearing in mind the historical context of ‘experimentation’ with vaccines and other medication in communities of color,” the AMA said, apparently alluding to the infamous Tuskegee study of syphilis in Black men.
Educating the public about the safety and efficacy of the COVID-19 vaccine programs is an “urgent priority,” the AMA said. This is especially true among populations that have been disproportionately affected by the disease. Black and Latino people are being hospitalized for COVID-19 at far higher rates than White Americans.
“Under the new policy, the AMA will help address patient concerns, dispel misinformation, and build confidence in COVID-19 vaccination,” the release states. The AMA also plans to build a coalition of health care and public health organizations to develop and implement a joint public education program.
Polls have indicated that many people will not get vaccinated when supplies of the new COVID-19 vaccines are available, although public support is rising. A recent Gallup poll found that 58% of surveyed adults were willing to be inoculated, up from 50% in September.
A Kaiser Family Foundation survey in September found that a majority of Americans were skeptical of a rushed vaccine, because they were concerned that the Trump administration was pressuring the Food and Drug Administration to approve a vaccine before the election.
“Given the unprecedented situation with COVID-19 and with vaccine development moving at a rapid pace, many of our patients and the public have questions and concerns,” said AMA President Susan R. Bailey, MD, in the release. “It is essential that we speak together as a strong, unified voice across health care and public health, inclusive of organizations respected in communities of color; to use scientific, fact-based evidence to help allay public concerns; and build confidence in COVID-19 vaccine candidates that are determined to be safe and effective.”
Physician, immunize thyself
The AMA also adopted a new ethics policy about physician immunization. On Monday, the AMA House of Delegates stated that physicians who are not immunized from a vaccine-preventable disease have an ethical responsibility to take appropriate actions to protect patients and colleagues.
The AMA code of ethics has long maintained that physicians have a strong ethical duty to accept immunizations when a safe, effective vaccine is available. However, the organization said in a news release, “it is not ethically problematic to exempt individuals when a specific vaccine poses a risk due to underlying medical conditions.”
Ethical concerns arise when physicians are allowed to decline vaccinations for nonmedical reasons, according to a report presented to the House of Delegates by the AMA Council on Ethical and Judicial Affairs.
According to the newly amended AMA ethical guidance, “physicians who are not or cannot be immunized have a responsibility to voluntarily take appropriate actions to protect patients, fellow health care workers and others.” This includes refraining from direct patient contact.
The delegates also approved a guidance asserting that physician practices and health care institutions are responsible for developing policies and procedures for responding to pandemics and epidemics. These policies and procedures should outline appropriate protective equipment allocation, staff immunization programs, and infection control practices.
Combating systemic racism
In an effort to reduce racial disparities in healthcare, the AMA House of Delegates adopted new policies recognizing race as a social construct, rather than a biological construct.
“The policies aim to advance data-driven, antiracist concepts challenging the current clinical application of race and its effects on vulnerable patient populations,” an AMA statement said.
The new AMA policies “reflect an understanding of race as a socially constructed category different from ethnicity, genetic ancestry, or biology, and aim to end the misinterpretation of race as a biological category defined by genetic traits or biological differences,” the AMA said.
According to the AMA, the practice of accepting race as a biological construct “exacerbates health disparities and results in detrimental health outcomes for marginalized and minoritized communities.”
Specifically, the AMA said it supports ending the practice of using race as a proxy for biology in medical education, research, and clinical practice. It also encourages medical education programs to recognize the harmful effects of this approach. It recommends that clinicians and researchers focus on genetics and biology, the experience of racism, and social determinants of health when describing risk factors for disease.
“The AMA is dedicated to dismantling racist and discriminatory policies and practices across all of health care, and that includes the way we define race in medicine,” said AMA board member Michael Suk, MD, in its statement. “We believe it is not sufficient for medicine to be nonracist, which is why the AMA is committed to pushing for a shift in thinking from race as a biological risk factor to a deeper understanding of racism as a determinant of health.”
The AMA also plans to partner with physician organizations and other stakeholders “to identify any problematic aspects of medical education that may perpetuate institutional and structural racism.” For example, the AMA will work with other organizations to improve clinical algorithms that incorrectly adjust for race and lead to less-than-optimal care for minority patients.
A version of this article originally appeared on Medscape.com.
The American Medical Association House of Delegates has adopted a policy to educate physicians on how to speak with patients about COVID-19 vaccination to counteract widespread misinformation about the vaccine development process.
Other highlights of the AMA’s recent special meeting include a new policy on the ethics of physicians getting immunized against COVID-19 and a far-reaching statement about racism.
Under the organization’s new vaccination education policy, the AMA will provide physicians with “culturally appropriate patient education materials,” according to a news release.
This campaign will be conducted “bearing in mind the historical context of ‘experimentation’ with vaccines and other medication in communities of color,” the AMA said, apparently alluding to the infamous Tuskegee study of syphilis in Black men.
Educating the public about the safety and efficacy of the COVID-19 vaccine programs is an “urgent priority,” the AMA said. This is especially true among populations that have been disproportionately affected by the disease. Black and Latino people are being hospitalized for COVID-19 at far higher rates than White Americans.
“Under the new policy, the AMA will help address patient concerns, dispel misinformation, and build confidence in COVID-19 vaccination,” the release states. The AMA also plans to build a coalition of health care and public health organizations to develop and implement a joint public education program.
Polls have indicated that many people will not get vaccinated when supplies of the new COVID-19 vaccines are available, although public support is rising. A recent Gallup poll found that 58% of surveyed adults were willing to be inoculated, up from 50% in September.
A Kaiser Family Foundation survey in September found that a majority of Americans were skeptical of a rushed vaccine, because they were concerned that the Trump administration was pressuring the Food and Drug Administration to approve a vaccine before the election.
“Given the unprecedented situation with COVID-19 and with vaccine development moving at a rapid pace, many of our patients and the public have questions and concerns,” said AMA President Susan R. Bailey, MD, in the release. “It is essential that we speak together as a strong, unified voice across health care and public health, inclusive of organizations respected in communities of color; to use scientific, fact-based evidence to help allay public concerns; and build confidence in COVID-19 vaccine candidates that are determined to be safe and effective.”
Physician, immunize thyself
The AMA also adopted a new ethics policy about physician immunization. On Monday, the AMA House of Delegates stated that physicians who are not immunized from a vaccine-preventable disease have an ethical responsibility to take appropriate actions to protect patients and colleagues.
The AMA code of ethics has long maintained that physicians have a strong ethical duty to accept immunizations when a safe, effective vaccine is available. However, the organization said in a news release, “it is not ethically problematic to exempt individuals when a specific vaccine poses a risk due to underlying medical conditions.”
Ethical concerns arise when physicians are allowed to decline vaccinations for nonmedical reasons, according to a report presented to the House of Delegates by the AMA Council on Ethical and Judicial Affairs.
According to the newly amended AMA ethical guidance, “physicians who are not or cannot be immunized have a responsibility to voluntarily take appropriate actions to protect patients, fellow health care workers and others.” This includes refraining from direct patient contact.
The delegates also approved a guidance asserting that physician practices and health care institutions are responsible for developing policies and procedures for responding to pandemics and epidemics. These policies and procedures should outline appropriate protective equipment allocation, staff immunization programs, and infection control practices.
Combating systemic racism
In an effort to reduce racial disparities in healthcare, the AMA House of Delegates adopted new policies recognizing race as a social construct, rather than a biological construct.
“The policies aim to advance data-driven, antiracist concepts challenging the current clinical application of race and its effects on vulnerable patient populations,” an AMA statement said.
The new AMA policies “reflect an understanding of race as a socially constructed category different from ethnicity, genetic ancestry, or biology, and aim to end the misinterpretation of race as a biological category defined by genetic traits or biological differences,” the AMA said.
According to the AMA, the practice of accepting race as a biological construct “exacerbates health disparities and results in detrimental health outcomes for marginalized and minoritized communities.”
Specifically, the AMA said it supports ending the practice of using race as a proxy for biology in medical education, research, and clinical practice. It also encourages medical education programs to recognize the harmful effects of this approach. It recommends that clinicians and researchers focus on genetics and biology, the experience of racism, and social determinants of health when describing risk factors for disease.
“The AMA is dedicated to dismantling racist and discriminatory policies and practices across all of health care, and that includes the way we define race in medicine,” said AMA board member Michael Suk, MD, in its statement. “We believe it is not sufficient for medicine to be nonracist, which is why the AMA is committed to pushing for a shift in thinking from race as a biological risk factor to a deeper understanding of racism as a determinant of health.”
The AMA also plans to partner with physician organizations and other stakeholders “to identify any problematic aspects of medical education that may perpetuate institutional and structural racism.” For example, the AMA will work with other organizations to improve clinical algorithms that incorrectly adjust for race and lead to less-than-optimal care for minority patients.
A version of this article originally appeared on Medscape.com.
The American Medical Association House of Delegates has adopted a policy to educate physicians on how to speak with patients about COVID-19 vaccination to counteract widespread misinformation about the vaccine development process.
Other highlights of the AMA’s recent special meeting include a new policy on the ethics of physicians getting immunized against COVID-19 and a far-reaching statement about racism.
Under the organization’s new vaccination education policy, the AMA will provide physicians with “culturally appropriate patient education materials,” according to a news release.
This campaign will be conducted “bearing in mind the historical context of ‘experimentation’ with vaccines and other medication in communities of color,” the AMA said, apparently alluding to the infamous Tuskegee study of syphilis in Black men.
Educating the public about the safety and efficacy of the COVID-19 vaccine programs is an “urgent priority,” the AMA said. This is especially true among populations that have been disproportionately affected by the disease. Black and Latino people are being hospitalized for COVID-19 at far higher rates than White Americans.
“Under the new policy, the AMA will help address patient concerns, dispel misinformation, and build confidence in COVID-19 vaccination,” the release states. The AMA also plans to build a coalition of health care and public health organizations to develop and implement a joint public education program.
Polls have indicated that many people will not get vaccinated when supplies of the new COVID-19 vaccines are available, although public support is rising. A recent Gallup poll found that 58% of surveyed adults were willing to be inoculated, up from 50% in September.
A Kaiser Family Foundation survey in September found that a majority of Americans were skeptical of a rushed vaccine, because they were concerned that the Trump administration was pressuring the Food and Drug Administration to approve a vaccine before the election.
“Given the unprecedented situation with COVID-19 and with vaccine development moving at a rapid pace, many of our patients and the public have questions and concerns,” said AMA President Susan R. Bailey, MD, in the release. “It is essential that we speak together as a strong, unified voice across health care and public health, inclusive of organizations respected in communities of color; to use scientific, fact-based evidence to help allay public concerns; and build confidence in COVID-19 vaccine candidates that are determined to be safe and effective.”
Physician, immunize thyself
The AMA also adopted a new ethics policy about physician immunization. On Monday, the AMA House of Delegates stated that physicians who are not immunized from a vaccine-preventable disease have an ethical responsibility to take appropriate actions to protect patients and colleagues.
The AMA code of ethics has long maintained that physicians have a strong ethical duty to accept immunizations when a safe, effective vaccine is available. However, the organization said in a news release, “it is not ethically problematic to exempt individuals when a specific vaccine poses a risk due to underlying medical conditions.”
Ethical concerns arise when physicians are allowed to decline vaccinations for nonmedical reasons, according to a report presented to the House of Delegates by the AMA Council on Ethical and Judicial Affairs.
According to the newly amended AMA ethical guidance, “physicians who are not or cannot be immunized have a responsibility to voluntarily take appropriate actions to protect patients, fellow health care workers and others.” This includes refraining from direct patient contact.
The delegates also approved a guidance asserting that physician practices and health care institutions are responsible for developing policies and procedures for responding to pandemics and epidemics. These policies and procedures should outline appropriate protective equipment allocation, staff immunization programs, and infection control practices.
Combating systemic racism
In an effort to reduce racial disparities in healthcare, the AMA House of Delegates adopted new policies recognizing race as a social construct, rather than a biological construct.
“The policies aim to advance data-driven, antiracist concepts challenging the current clinical application of race and its effects on vulnerable patient populations,” an AMA statement said.
The new AMA policies “reflect an understanding of race as a socially constructed category different from ethnicity, genetic ancestry, or biology, and aim to end the misinterpretation of race as a biological category defined by genetic traits or biological differences,” the AMA said.
According to the AMA, the practice of accepting race as a biological construct “exacerbates health disparities and results in detrimental health outcomes for marginalized and minoritized communities.”
Specifically, the AMA said it supports ending the practice of using race as a proxy for biology in medical education, research, and clinical practice. It also encourages medical education programs to recognize the harmful effects of this approach. It recommends that clinicians and researchers focus on genetics and biology, the experience of racism, and social determinants of health when describing risk factors for disease.
“The AMA is dedicated to dismantling racist and discriminatory policies and practices across all of health care, and that includes the way we define race in medicine,” said AMA board member Michael Suk, MD, in its statement. “We believe it is not sufficient for medicine to be nonracist, which is why the AMA is committed to pushing for a shift in thinking from race as a biological risk factor to a deeper understanding of racism as a determinant of health.”
The AMA also plans to partner with physician organizations and other stakeholders “to identify any problematic aspects of medical education that may perpetuate institutional and structural racism.” For example, the AMA will work with other organizations to improve clinical algorithms that incorrectly adjust for race and lead to less-than-optimal care for minority patients.
A version of this article originally appeared on Medscape.com.
Trump could clean house at health agencies
Others may soon depart voluntarily. Politico reported in late October that more than two dozen political appointees had already left the U.S. Department Health and Human Services (HHS) since the start of the COVID-19 pandemic in February and that potentially dozens of the more than 100 in the department would leave if Trump was not reelected.
Trump hasn’t conceded, he is challenging the election results, and he has already fired his Defense Secretary, Mark Esper.
Among those possibly in Trump’s sights: HHS Secretary Alex Azar, US Food and Drug Administration (FDA) Commissioner Stephen Hahn, MD, Centers for Disease Control and Prevention (CDC) Director Robert Redfield, MD, and White House Coronavirus Task Force member Anthony Fauci, MD, who is also the director of the National Institutes of Allergy and Infectious Diseases.
Seema Verma, the administrator of the Centers for Medicare & Medicaid Services (CMS), is likely safe. According to Politico, Verma is expected to leave on her own terms.
Azar has had a long run as a Trump appointee. He took office in January 2018 and has been a staunch loyalist. But he’s frequently been the butt of grousing by Trump for not doing enough to help lower drug prices and for his handling of the coronavirus pandemic. Azar was initially in charge of the Trump virus effort but was quickly replaced by Vice President Mike Pence.
It was widely reported in late April that Trump was considering firing Azar, but the president called that “fake news” in a tweet.
Azar has complained about Hahn, who was confirmed in December 2019. According to Politico, Azar was looking into how to remove Hahn as commissioner because of the FDA’s battle with the White House over standards for emergency use authorization of a coronavirus vaccine.
In addition, Trump was infuriated by the agency’s insistence that it stick to the highest bar for an emergency approval. “The deep state, or whoever, over at the FDA is making it very difficult for drug companies to get people in order to test the vaccines and therapeutics. Obviously, they are hoping to delay the answer until after November 3rd,” Trump tweeted at Hahn.
Fauci on the firing line?
Most of the president’s ire has been directed at Fauci. As far back as April, Trump retweeted a call for Fauci’s firing. Twitter removed the original tweet but kept Trump’s comments on the original tweet.
The president has frequently questioned Fauci’s advice, sidelined him from task force meetings, and infrequently met with him. Trump called Fauci a “disaster” during a call with supporters in October, and then, at a campaign rally in November, intimated that he would fire the scientist after the election, according to The Washington Post.
But such a firing cannot be easily done. Some have speculated that Trump could pressure Fauci’s boss, Francis Collins, MD, PhD — the director of the National Institutes of Health (NIH), who is a political appointee — to get rid of him. But Collins would have to come up with a reason to fire Fauci. Because he is not a political appointee, Fauci is afforded a raft of protections given to civil service employees of the federal government.
To demote or fire Fauci, Collins would have to give him 30 days’ notice unless there’s a belief that he committed a crime. Fauci would have at least a week to offer evidence and affidavits in support of his service.
He’d also be entitled to legal representation, a written decision, and the specific reasons for the action being taken quickly. He could also request a hearing, and he’d be able to appeal any action to the Merit Systems Protection Board. The process could take months, if not years.
In late October, Trump issued an executive order that would reclassify certain federal employees so that they wouldn’t have such protections. But agencies have until mid-January to come up with lists of such workers, according to Government Executive.
Collins has been with NIH since 1993, when he headed the Human Genome Project and the National Human Genome Research Institute. Politico has speculated that Collins, 70, might retire if Trump was reelected. It’s unclear what he’ll do now.
Redfield, who has taken heat for his leadership from many in public health — and was asked in October to stand up to Trump by former CDC Director William H. Foege, MD — has been openly contradicted by the president on more than one occasion, according to The New York Times.
In September, The Hill reported that Trump told reporters that he’d chastised Redfield by phone soon after Redfield had told a Senate committee that a coronavirus vaccine would not be available until mid-2021.
This article first appeared on Medscape.com.
Others may soon depart voluntarily. Politico reported in late October that more than two dozen political appointees had already left the U.S. Department Health and Human Services (HHS) since the start of the COVID-19 pandemic in February and that potentially dozens of the more than 100 in the department would leave if Trump was not reelected.
Trump hasn’t conceded, he is challenging the election results, and he has already fired his Defense Secretary, Mark Esper.
Among those possibly in Trump’s sights: HHS Secretary Alex Azar, US Food and Drug Administration (FDA) Commissioner Stephen Hahn, MD, Centers for Disease Control and Prevention (CDC) Director Robert Redfield, MD, and White House Coronavirus Task Force member Anthony Fauci, MD, who is also the director of the National Institutes of Allergy and Infectious Diseases.
Seema Verma, the administrator of the Centers for Medicare & Medicaid Services (CMS), is likely safe. According to Politico, Verma is expected to leave on her own terms.
Azar has had a long run as a Trump appointee. He took office in January 2018 and has been a staunch loyalist. But he’s frequently been the butt of grousing by Trump for not doing enough to help lower drug prices and for his handling of the coronavirus pandemic. Azar was initially in charge of the Trump virus effort but was quickly replaced by Vice President Mike Pence.
It was widely reported in late April that Trump was considering firing Azar, but the president called that “fake news” in a tweet.
Azar has complained about Hahn, who was confirmed in December 2019. According to Politico, Azar was looking into how to remove Hahn as commissioner because of the FDA’s battle with the White House over standards for emergency use authorization of a coronavirus vaccine.
In addition, Trump was infuriated by the agency’s insistence that it stick to the highest bar for an emergency approval. “The deep state, or whoever, over at the FDA is making it very difficult for drug companies to get people in order to test the vaccines and therapeutics. Obviously, they are hoping to delay the answer until after November 3rd,” Trump tweeted at Hahn.
Fauci on the firing line?
Most of the president’s ire has been directed at Fauci. As far back as April, Trump retweeted a call for Fauci’s firing. Twitter removed the original tweet but kept Trump’s comments on the original tweet.
The president has frequently questioned Fauci’s advice, sidelined him from task force meetings, and infrequently met with him. Trump called Fauci a “disaster” during a call with supporters in October, and then, at a campaign rally in November, intimated that he would fire the scientist after the election, according to The Washington Post.
But such a firing cannot be easily done. Some have speculated that Trump could pressure Fauci’s boss, Francis Collins, MD, PhD — the director of the National Institutes of Health (NIH), who is a political appointee — to get rid of him. But Collins would have to come up with a reason to fire Fauci. Because he is not a political appointee, Fauci is afforded a raft of protections given to civil service employees of the federal government.
To demote or fire Fauci, Collins would have to give him 30 days’ notice unless there’s a belief that he committed a crime. Fauci would have at least a week to offer evidence and affidavits in support of his service.
He’d also be entitled to legal representation, a written decision, and the specific reasons for the action being taken quickly. He could also request a hearing, and he’d be able to appeal any action to the Merit Systems Protection Board. The process could take months, if not years.
In late October, Trump issued an executive order that would reclassify certain federal employees so that they wouldn’t have such protections. But agencies have until mid-January to come up with lists of such workers, according to Government Executive.
Collins has been with NIH since 1993, when he headed the Human Genome Project and the National Human Genome Research Institute. Politico has speculated that Collins, 70, might retire if Trump was reelected. It’s unclear what he’ll do now.
Redfield, who has taken heat for his leadership from many in public health — and was asked in October to stand up to Trump by former CDC Director William H. Foege, MD — has been openly contradicted by the president on more than one occasion, according to The New York Times.
In September, The Hill reported that Trump told reporters that he’d chastised Redfield by phone soon after Redfield had told a Senate committee that a coronavirus vaccine would not be available until mid-2021.
This article first appeared on Medscape.com.
Others may soon depart voluntarily. Politico reported in late October that more than two dozen political appointees had already left the U.S. Department Health and Human Services (HHS) since the start of the COVID-19 pandemic in February and that potentially dozens of the more than 100 in the department would leave if Trump was not reelected.
Trump hasn’t conceded, he is challenging the election results, and he has already fired his Defense Secretary, Mark Esper.
Among those possibly in Trump’s sights: HHS Secretary Alex Azar, US Food and Drug Administration (FDA) Commissioner Stephen Hahn, MD, Centers for Disease Control and Prevention (CDC) Director Robert Redfield, MD, and White House Coronavirus Task Force member Anthony Fauci, MD, who is also the director of the National Institutes of Allergy and Infectious Diseases.
Seema Verma, the administrator of the Centers for Medicare & Medicaid Services (CMS), is likely safe. According to Politico, Verma is expected to leave on her own terms.
Azar has had a long run as a Trump appointee. He took office in January 2018 and has been a staunch loyalist. But he’s frequently been the butt of grousing by Trump for not doing enough to help lower drug prices and for his handling of the coronavirus pandemic. Azar was initially in charge of the Trump virus effort but was quickly replaced by Vice President Mike Pence.
It was widely reported in late April that Trump was considering firing Azar, but the president called that “fake news” in a tweet.
Azar has complained about Hahn, who was confirmed in December 2019. According to Politico, Azar was looking into how to remove Hahn as commissioner because of the FDA’s battle with the White House over standards for emergency use authorization of a coronavirus vaccine.
In addition, Trump was infuriated by the agency’s insistence that it stick to the highest bar for an emergency approval. “The deep state, or whoever, over at the FDA is making it very difficult for drug companies to get people in order to test the vaccines and therapeutics. Obviously, they are hoping to delay the answer until after November 3rd,” Trump tweeted at Hahn.
Fauci on the firing line?
Most of the president’s ire has been directed at Fauci. As far back as April, Trump retweeted a call for Fauci’s firing. Twitter removed the original tweet but kept Trump’s comments on the original tweet.
The president has frequently questioned Fauci’s advice, sidelined him from task force meetings, and infrequently met with him. Trump called Fauci a “disaster” during a call with supporters in October, and then, at a campaign rally in November, intimated that he would fire the scientist after the election, according to The Washington Post.
But such a firing cannot be easily done. Some have speculated that Trump could pressure Fauci’s boss, Francis Collins, MD, PhD — the director of the National Institutes of Health (NIH), who is a political appointee — to get rid of him. But Collins would have to come up with a reason to fire Fauci. Because he is not a political appointee, Fauci is afforded a raft of protections given to civil service employees of the federal government.
To demote or fire Fauci, Collins would have to give him 30 days’ notice unless there’s a belief that he committed a crime. Fauci would have at least a week to offer evidence and affidavits in support of his service.
He’d also be entitled to legal representation, a written decision, and the specific reasons for the action being taken quickly. He could also request a hearing, and he’d be able to appeal any action to the Merit Systems Protection Board. The process could take months, if not years.
In late October, Trump issued an executive order that would reclassify certain federal employees so that they wouldn’t have such protections. But agencies have until mid-January to come up with lists of such workers, according to Government Executive.
Collins has been with NIH since 1993, when he headed the Human Genome Project and the National Human Genome Research Institute. Politico has speculated that Collins, 70, might retire if Trump was reelected. It’s unclear what he’ll do now.
Redfield, who has taken heat for his leadership from many in public health — and was asked in October to stand up to Trump by former CDC Director William H. Foege, MD — has been openly contradicted by the president on more than one occasion, according to The New York Times.
In September, The Hill reported that Trump told reporters that he’d chastised Redfield by phone soon after Redfield had told a Senate committee that a coronavirus vaccine would not be available until mid-2021.
This article first appeared on Medscape.com.
FDA grants emergency use authorization to Lilly’s antibody COVID-19 therapy
The monoclonal antibody therapy has emergency authorization for treating patients who have tested positive for SARS-CoV-2 infection and who are considered to be at high risk for progression to severe COVID-19 or hospitalization. To be eligible for treatment with bamlanivimab, patients must be at least 12 years of age and weigh at least 40 kg (approximately 88 lb). The agency notes that this includes patients aged 65 years and older or people with certain chronic conditions.
Bamlanivimab is not authorized for use in patients who are hospitalized or who require oxygen therapy because of COVID-19. The FDA’s action comes less than 2 weeks after Eli Lilly halted the ACTIV-3 study of the therapy for severe, hospitalized COVID-19 patients after evidence showed that adding the antibody therapy to standard care did not improve outcomes over standard care alone for patients with advanced COVID-19.
The government contract with Eli Lilly involves the purchase of 300,000 doses through December, with the option to procure another 650,000 doses through June 2021.
Because of Operation Warp Speed, “we have supplies to distribute now. Product distribution will begin this week,” US Health & Human Services (HHS) Secretary Alex Azar said at a news conference today.
“We talked about building the bridge to safe and effective vaccines” for COVID-19, Azar added. “With this therapeutic, the bridge is taking shape.”
Bamlanivimab 700 mg will be administered as a 1-hour infusion followed by a 1-hour observation period for detecting any infusion-related side effects. The authorized dose is 700 mg, which was on the lower end of the dose range evaluated in studies.
During the press conference, a reporter asked whether the lower dose was chosen in order that more doses of the antibody could be made available. “The lower dose is a rational choice in this situation because we don’t want to give more of a drug than you need,” said Janet Woodcock, MD, the therapeutics lead for Operation Warp Speed. “I think we could probably go lower.”
Bamlanivimab works by attaching to the virus and blocking its entry into the cells and possibly by helping the patients’ immune system clear the virus, said Woodcock, who is also director of the FDA’s Center for Drug Evaluation and Research.
“The goal is to treat high-risk people as soon as possible after they show symptoms and are diagnosed,” she added.
Infusions an initial challenge?
There could be some logistic challenges at first because the antibody is administered via infusion. “We expect there will initially be a challenge in administering ... these infusions and setting up infusion centers,” Woodcock said.
Outpatient intravenous infusions are normally performed at infusion centers for patients with cancer and immune disorders, she noted. “You really don’t want them mixing with people who have COVID-19 disease, so we will need to set up separate sites.”
Bamlanivimab will be provided free of cost to patients, Azar said. Patients should be aware that coinsurance may be required for the infusion.
“Fair and equitable” distribution planned
During phase 1 of distribution, the agent will first be allocated to hospitals and hospital-affiliated locations only, John Redd, MD, MPH, chief medical officer, Office of the Assistant Secretary for Preparedness and Response at HHS, said at the press conference.
During phase 2, “there will be expanded distribution to outpatient sites,” he said. In an effort to keep the process transparent, a new website features the latest updates on the distribution of bamlanivimab.
Allocation will be based on two factors: the number of new cases reported in a state or territory in the prior 7 days, and rates of COVID-19 hospitalization during the same period.
Asked why the government would determine distribution of the antibody on the basis of the number of hospitalized patients when the indication includes prevention of admission, Woodcock replied that hospitalization is a surrogate measure that can reflect risk factors in a particular state population, such as obesity, diabetes, or the proportion of older people.
Furthermore, the confirmed cases are a “leading indicator,” she said, that can help identify a steep rise in COVID-19 cases that could indicate more hospitalizations are likely soon. “We don’t want to miss that.”
Data underlying the EUA decision
A decrease in hospitalizations or emergency department visits within 28 days of treatment in preclinical studies was “the most important evidence that bamlanivimab may be effective,” the agency noted in the press release announcing the EUA. Among patients at high risk for progression, 3% required such interventions, compared with 10% of placebo-treated patients.
Potential side effects of bamlanivimab include anaphylaxis, infusion-related reactions, nausea, diarrhea, dizziness, headache, itching, and vomiting.
“As illustrated by today’s action, the FDA remains committed to expediting the development and availability of potential COVID-19 treatments and providing sick patients timely access to new therapies where appropriate,” FDA Commissioner Stephen M. Hahn, MD, said in the news release.
Healthcare providers can download a detailed FDA fact sheet on the EUA for bamlanivimab, which includes dosing instructions.
This article first appeared on Medscape.com.
The monoclonal antibody therapy has emergency authorization for treating patients who have tested positive for SARS-CoV-2 infection and who are considered to be at high risk for progression to severe COVID-19 or hospitalization. To be eligible for treatment with bamlanivimab, patients must be at least 12 years of age and weigh at least 40 kg (approximately 88 lb). The agency notes that this includes patients aged 65 years and older or people with certain chronic conditions.
Bamlanivimab is not authorized for use in patients who are hospitalized or who require oxygen therapy because of COVID-19. The FDA’s action comes less than 2 weeks after Eli Lilly halted the ACTIV-3 study of the therapy for severe, hospitalized COVID-19 patients after evidence showed that adding the antibody therapy to standard care did not improve outcomes over standard care alone for patients with advanced COVID-19.
The government contract with Eli Lilly involves the purchase of 300,000 doses through December, with the option to procure another 650,000 doses through June 2021.
Because of Operation Warp Speed, “we have supplies to distribute now. Product distribution will begin this week,” US Health & Human Services (HHS) Secretary Alex Azar said at a news conference today.
“We talked about building the bridge to safe and effective vaccines” for COVID-19, Azar added. “With this therapeutic, the bridge is taking shape.”
Bamlanivimab 700 mg will be administered as a 1-hour infusion followed by a 1-hour observation period for detecting any infusion-related side effects. The authorized dose is 700 mg, which was on the lower end of the dose range evaluated in studies.
During the press conference, a reporter asked whether the lower dose was chosen in order that more doses of the antibody could be made available. “The lower dose is a rational choice in this situation because we don’t want to give more of a drug than you need,” said Janet Woodcock, MD, the therapeutics lead for Operation Warp Speed. “I think we could probably go lower.”
Bamlanivimab works by attaching to the virus and blocking its entry into the cells and possibly by helping the patients’ immune system clear the virus, said Woodcock, who is also director of the FDA’s Center for Drug Evaluation and Research.
“The goal is to treat high-risk people as soon as possible after they show symptoms and are diagnosed,” she added.
Infusions an initial challenge?
There could be some logistic challenges at first because the antibody is administered via infusion. “We expect there will initially be a challenge in administering ... these infusions and setting up infusion centers,” Woodcock said.
Outpatient intravenous infusions are normally performed at infusion centers for patients with cancer and immune disorders, she noted. “You really don’t want them mixing with people who have COVID-19 disease, so we will need to set up separate sites.”
Bamlanivimab will be provided free of cost to patients, Azar said. Patients should be aware that coinsurance may be required for the infusion.
“Fair and equitable” distribution planned
During phase 1 of distribution, the agent will first be allocated to hospitals and hospital-affiliated locations only, John Redd, MD, MPH, chief medical officer, Office of the Assistant Secretary for Preparedness and Response at HHS, said at the press conference.
During phase 2, “there will be expanded distribution to outpatient sites,” he said. In an effort to keep the process transparent, a new website features the latest updates on the distribution of bamlanivimab.
Allocation will be based on two factors: the number of new cases reported in a state or territory in the prior 7 days, and rates of COVID-19 hospitalization during the same period.
Asked why the government would determine distribution of the antibody on the basis of the number of hospitalized patients when the indication includes prevention of admission, Woodcock replied that hospitalization is a surrogate measure that can reflect risk factors in a particular state population, such as obesity, diabetes, or the proportion of older people.
Furthermore, the confirmed cases are a “leading indicator,” she said, that can help identify a steep rise in COVID-19 cases that could indicate more hospitalizations are likely soon. “We don’t want to miss that.”
Data underlying the EUA decision
A decrease in hospitalizations or emergency department visits within 28 days of treatment in preclinical studies was “the most important evidence that bamlanivimab may be effective,” the agency noted in the press release announcing the EUA. Among patients at high risk for progression, 3% required such interventions, compared with 10% of placebo-treated patients.
Potential side effects of bamlanivimab include anaphylaxis, infusion-related reactions, nausea, diarrhea, dizziness, headache, itching, and vomiting.
“As illustrated by today’s action, the FDA remains committed to expediting the development and availability of potential COVID-19 treatments and providing sick patients timely access to new therapies where appropriate,” FDA Commissioner Stephen M. Hahn, MD, said in the news release.
Healthcare providers can download a detailed FDA fact sheet on the EUA for bamlanivimab, which includes dosing instructions.
This article first appeared on Medscape.com.
The monoclonal antibody therapy has emergency authorization for treating patients who have tested positive for SARS-CoV-2 infection and who are considered to be at high risk for progression to severe COVID-19 or hospitalization. To be eligible for treatment with bamlanivimab, patients must be at least 12 years of age and weigh at least 40 kg (approximately 88 lb). The agency notes that this includes patients aged 65 years and older or people with certain chronic conditions.
Bamlanivimab is not authorized for use in patients who are hospitalized or who require oxygen therapy because of COVID-19. The FDA’s action comes less than 2 weeks after Eli Lilly halted the ACTIV-3 study of the therapy for severe, hospitalized COVID-19 patients after evidence showed that adding the antibody therapy to standard care did not improve outcomes over standard care alone for patients with advanced COVID-19.
The government contract with Eli Lilly involves the purchase of 300,000 doses through December, with the option to procure another 650,000 doses through June 2021.
Because of Operation Warp Speed, “we have supplies to distribute now. Product distribution will begin this week,” US Health & Human Services (HHS) Secretary Alex Azar said at a news conference today.
“We talked about building the bridge to safe and effective vaccines” for COVID-19, Azar added. “With this therapeutic, the bridge is taking shape.”
Bamlanivimab 700 mg will be administered as a 1-hour infusion followed by a 1-hour observation period for detecting any infusion-related side effects. The authorized dose is 700 mg, which was on the lower end of the dose range evaluated in studies.
During the press conference, a reporter asked whether the lower dose was chosen in order that more doses of the antibody could be made available. “The lower dose is a rational choice in this situation because we don’t want to give more of a drug than you need,” said Janet Woodcock, MD, the therapeutics lead for Operation Warp Speed. “I think we could probably go lower.”
Bamlanivimab works by attaching to the virus and blocking its entry into the cells and possibly by helping the patients’ immune system clear the virus, said Woodcock, who is also director of the FDA’s Center for Drug Evaluation and Research.
“The goal is to treat high-risk people as soon as possible after they show symptoms and are diagnosed,” she added.
Infusions an initial challenge?
There could be some logistic challenges at first because the antibody is administered via infusion. “We expect there will initially be a challenge in administering ... these infusions and setting up infusion centers,” Woodcock said.
Outpatient intravenous infusions are normally performed at infusion centers for patients with cancer and immune disorders, she noted. “You really don’t want them mixing with people who have COVID-19 disease, so we will need to set up separate sites.”
Bamlanivimab will be provided free of cost to patients, Azar said. Patients should be aware that coinsurance may be required for the infusion.
“Fair and equitable” distribution planned
During phase 1 of distribution, the agent will first be allocated to hospitals and hospital-affiliated locations only, John Redd, MD, MPH, chief medical officer, Office of the Assistant Secretary for Preparedness and Response at HHS, said at the press conference.
During phase 2, “there will be expanded distribution to outpatient sites,” he said. In an effort to keep the process transparent, a new website features the latest updates on the distribution of bamlanivimab.
Allocation will be based on two factors: the number of new cases reported in a state or territory in the prior 7 days, and rates of COVID-19 hospitalization during the same period.
Asked why the government would determine distribution of the antibody on the basis of the number of hospitalized patients when the indication includes prevention of admission, Woodcock replied that hospitalization is a surrogate measure that can reflect risk factors in a particular state population, such as obesity, diabetes, or the proportion of older people.
Furthermore, the confirmed cases are a “leading indicator,” she said, that can help identify a steep rise in COVID-19 cases that could indicate more hospitalizations are likely soon. “We don’t want to miss that.”
Data underlying the EUA decision
A decrease in hospitalizations or emergency department visits within 28 days of treatment in preclinical studies was “the most important evidence that bamlanivimab may be effective,” the agency noted in the press release announcing the EUA. Among patients at high risk for progression, 3% required such interventions, compared with 10% of placebo-treated patients.
Potential side effects of bamlanivimab include anaphylaxis, infusion-related reactions, nausea, diarrhea, dizziness, headache, itching, and vomiting.
“As illustrated by today’s action, the FDA remains committed to expediting the development and availability of potential COVID-19 treatments and providing sick patients timely access to new therapies where appropriate,” FDA Commissioner Stephen M. Hahn, MD, said in the news release.
Healthcare providers can download a detailed FDA fact sheet on the EUA for bamlanivimab, which includes dosing instructions.
This article first appeared on Medscape.com.
Template Design and Analysis: Integrating Informatics Solutions to Improve Clinical Documentation
Standardized template design is a useful tool to improve clinical documentation and reliable reporting of health care outcomes when constructed with clear objectives and with collaboration of key stakeholders. A standardized template should not only capture accurate diagnostic information, but also inform quality improvement (QI) measures and best practices.
Kang and colleagues showed that a correlation exists between organizational satisfaction and improved quality outcomes.1 A new initiative should have a well-defined purpose reinforced by collaborative workgroups and engaged employees who understand their clinical care role with electronic health record (EHR) modifications.
Several studies have shown how the usefulness of templates achieve multipurpose goals, such as accurate documentation and improved care. Valluru and colleagues showed a significant increase in vaccination rates for patients with inflammatory bowel disease after implementing a standardized template.2 By using a standardized template, Thaker and colleagues showed improved documentation regarding obesity and increased nutritional and physical activity counseling.3 Furthermore, Grogan and colleagues showed that templates are useful for house staff education on International Classification of Diseases (ICD) terminology and demonstrated improved documentation in the postintervention group.4,5
This article discusses the US Department of Veterans Affairs (VA) North Florida/South Georgia Veterans Health System (NF/SGVHS) integrated informatics solutions within template design in the Veterans Health Administration (VHA) EHR system that was associated with an increase in its case severity index (CSI) through improved clinical documentation capture.
Methods
According to policy activities that constitute research at NF/SGVHS, institutional review board approval was not required as this work met the criteria for operational improvement activities exempt from ethics review.
NF/SGVHS includes 2 hospitals: Malcom Randall VA Medical Center (MRVAMC) in Gainesville, Florida, and Lake City VA Medical Center (LCVAMC) in Lake City, Florida. MRVAMC is a large, 1a, academic VA facility composed of rotating residents and fellows and includes multiple specialty care services. LCVAMC is a smaller, nonteaching facility.
Template Design Impact
CSI is a risk-adjusted formula developed by the Inpatient Evaluation Center within VHA. CSI is incorporated into the VHA quality metrics reporting system, Strategic Analytics for Improvement and Learning (SAIL). CSI risk-adjusts metrics such as length of stay and mortality before releasing SAIL reports. CSI is calculated separately for acute level of care (LOC) and for the intensive care unit (ICU). In fiscal year (FY) 2017, acute LOC preimplementation data for CSI at NF/SGVHS were 0.76 for MRVAMC and 0.81 for LCVAMC, which was significantly below the national VHA average of 0.96 (Table).
A below-average CSI conveys a less complicated case mix compared with most other VA facilities. Although smaller VA facilities may have a less complicated case mix, it is unusual for large, tertiary care 1a VA facilities to have a low CSI. This low CSI is usually due to inadequate documentation, which affects not only risk-adjusted quality metrics outcomes, but also potential reimbursement.6
An interdisciplinary team composed of attendings, residents, and a clinical document improvement specialist identified the below-average acute LOC CSI for MRVAMC and LCVAMC compared with that of the national VHA average. Further analysis by chart reviews showed inconsistencies with standardized documentation despite prior health care provider education on ICD terminology and specific groups of common comorbidities analyzed in administrative data reviews for risk-adjustment purposes, known as Elixhauser comorbidities.5,7
A chart review showed lack of clarity regarding primary reason(s) for admission and chronic comorbidities within NF/SGVHS. Using Pareto chart analysis, the template team designed a standardized history and physical (H&P) medicine template based on NF/SGVHS common medicine admissions (Figure 1). A Pareto chart is a valuable QI tool that assists with identifying majority contributors to a problem(s) being analyzed when evaluating a large set of data points. Subsequently, this tool helps focus direction on QI efforts.8
The template had the usual H&P elements not shown (eg, chief complaint, history of present illness, etc), and highlights the assessment/plan section containing primary reason(s) for admission and chronic comorbidities (Figure 1). The complete assessment and plan section on the template can be found in the Appendix.
To simplify the template interface, only single clicks were required to expand diagnostic and chronic comorbidity checkboxes. Subcategories then appeared to select diagnosis and chronic comorbidities along with free text for additional documentation.
In addition, data objects were created within the template that permitted the ability to retrieve information from the VHA EHR and insert specific data points of interest in the template; for example, body mass index to assess degree of obesity and estimated glomerular filtration rate to determine the stage of chronic kidney disease. This allowed users to easily reference data in one template in lieu of searching for data in multiple places in the EHR.9
Results
The standardized H&P medicine template was implemented at MRVAMC and LCVAMC in June 2018 (the final month of the third quarter of FY 2018). As clinical providers throughout NF/SGVHS used the standardized template, acute LOC postimplementation data for CSI significantly improved. Although the national VHA average slightly decreased from 0.96 in the first quarter of FY 2017 to 0.89, in the first quarter of FY 2019, MRVAMC acute LOC CSI improved from 0.76 to 0.97, and LCVAMC acute LOC CSI improved from 0.81 to 1.07 during the same period.
In addition, compliance also was monitored within MRVAMC and LCVAMC for about 1 year after standardized H&P medicine template implementation. Compliance was determined by how often the standardized H&P medicine template was used for inpatient medicine admissions to the acute care wards vs other H&P notes used (such as personalized templates).
Methodology for compliance analysis included acquisition of completed H&P medicine notes from June 18, 2018 to June 30, 2019, within the VHA Veterans Information Systems and Technology Architecture (VistA) clinical and business information system using the search strings: “H&P admission history and physical” and “history of present illness.”10
A review identified 10,845 completed medicine H&P notes. Nine hundred eighteen notes were excluded as their search function yielded a location not corresponding to MRVAMC or LCVAMC. Of the 9,927 notes remaining, 8,025 of these were completed medicine H&P notes at MRVAMC and 1,902 were completed medicine H&P notes at LCVAMC (Figure 2).
From June 18, 2018 to June 30, 2019 at MRVAMC, compliance was reviewed monthly for the 8,025 completed H&P medicine notes. Of the completed H&P medicine notes, the standardized H&P medicine template was used 43.2% in June 2018. By June 2019, MRVAMC clinical providers demonstrated significant improvement for standardized H&P medicine template use at 89.9% (Figure 3). Total average compliance from June 18, 2018 to June 30, 2019, was 88.4%, which doubled compliance from the initial introduction of the standardized H&P medicine template.
Compliance was reviewed monthly for the 1,902 completed H&P medicine notes from June 18, 2018 to June 30, 2019, at LCVAMC. Of the completed H&P medicine notes, the standardized template was used 48.2% of the time in June 2018. By June 2019, LCVAMC clinical providers demonstrated significant improvement for standardized H&P medicine template use, which increased to 96.9%. Total average compliance from June 18, 2018 to June 30, 2019, was 93.8%, which was almost double the baseline compliance rate.
Discussion
Template design with clear objectives, strategic collaboration, and integrated informatics solutions has the potential to increase accuracy of documentation. As shown, the NF/SGVHS template design was associated with significant improvement in acute LOC CSI for both MRVAMC and LCVAMC due to more accurate documentation using the standardized H&P medicine template.
Numerous factors contributed to the success of this template design. First, a clear vision for application of the template was communicated with key stakeholders. In addition, the template design team was focused on specific goals rather than a one size fits all approach, which was crucial for sustainable execution. Although interdisciplinary teamwork has the potential to result in innovative practices, large multidisciplinary teams also may have difficulty establishing a shared vision that can result in barriers to achieving project goals.
Balancing standardization and customization was essential for user buy-in. As noted by Gardner and Pearce, inviting clinical providers to participate in template design and allowing for customization has the potential to increase acceptance and use of templates.11 Although the original design for the standardized H&P medicine template started with the medicine service at NF/SGVHS, the design framework is applicable to numerous services where various clinical care elements can be customized.
Explaining the informatics tools built into the template allowed clinicians to see opportunities to improve clinical documentation and the impact it has on reporting health care outcomes. When improvement work involves integrating clinical care delivery and administrative expectations, it is essential that health care systems understand and strategically execute project initiatives at this critical juncture.
Finally, incorporation of a sustainability plan when process improvement strategies are implemented is vital. In addition to collaboration with the clinical providers during design and implementation of the standardized template, leadership buy-in was key. Compliance with standardized H&P medicine template use was monitored monthly and reviewed by the NF/SGVHS Chief of Staff.
As noted, LCVAMC postimplementation acute LOC CSI was higher than that of MRVAMC despite being a smaller facility. This might be due to the MRVAMC designation as a teaching institution. Medicine is the only inpatient service at LCVAMC staffed by hospitalists with limited specialists available for consultation, whereas MRVAMC is a tertiary care teaching facility with numerous inpatient services and subspecialties. As LCVAMC has more continuity, house staff rotating at MRVAMC require continued training/education on new templates and process changes.
Limitations
Although standardized template design was successful at NF/SGVHS, limitations should be noted. Our clinical documentation improvement (CDI) program also was expanded about the same time as the new templates were released. The expansion of the CDI program in addition to new template design likely had a synergistic effect on acute LOC CSI.
CSI is a complex, risk-adjusted model that includes numerous factors, including but not limited to diagnosis and comorbid conditions. Other factors include age, marital status, procedures, source of admission, specific laboratory values, medical or surgical diagnosis-related group, intensive care unit stays, and immunosuppressive status. CSI also includes operative and nonoperative components that average into an overall CSI. As the majority of CSI is composed of nonoperative constituents within NF/SGVHS, we do not believe this had any substantial impact on reporting of CSI improvements.
In addition, template entry into VHA EHR requires a location selection (such as a clinic name or ward name following an inpatient admission). Of the 10,845 completed H&P medicine notes identified in VistA, 918 notes were excluded from analysis as their search function yielded a location not corresponding to MRVAMC or LCVAMC. For the 918 notes excluded, we believe this was likely due to user error where locations not related to MRVAMC or LCVAMC were selected during standardized H&P medicine template entry.
Conclusions
After the NF/SGVHS implementation of a uniquely designed template embedded with informatics solutions within the VHA EHR, the CSI increased due to more accurate documentation.
Next steps include determining the impact of the NF/SGVHS template design on potential reimbursement and expanding template design into the outpatient setting where there are additional opportunities to improve clinical documentation and reliable reporting of health care outcomes.
Acknowledgments
The authors thank the following individuals for their experience and contribution: Beverley White is the Clinical Documentation Improvement Coordinator at North Florida/South Georgia Veterans Health System and provided expertise on documentation requirements. Russell Jacobitz and Susan Rozelle provided technical expertise on electronic health record system enhancements and implemented the template design. Jess Delaune, MD, and Robert Carroll, MD, provided additional physician input during template design. We also acknowledge the Inpatient Evaluation Center (IPEC) within the Veterans Health Administration (VHA). IPEC developed the case severity index, a risk-adjusted formula incorporated into the VHA quality metric reporting system, Strategic Analytics for Improvement and Learning (SAIL).
1. Kang R, Kunkel S, Columbo J, et al. Association of Hospital Employee satisfaction with patient safety and satisfaction within Veterans Affairs Medical Centers. Am J Med. 2019;132(4):530-534.e1. doi: 10.1016/j.amjmed.2018.11.031
2. Valluru, N, Kang L, Gaidos JK. Health maintenance documentation improves for veterans with IBD using a template in the Computerized Patient Record System. Dig Dis Sci. 2018;63(7):1782-1786. doi:10.1007%2Fs10620-018-5093-5
3. Thaker VV, Lee F, Bottino CJ, et al. Impact of an electronic template on documentation of obesity in a primary care clinic. Clin Pediatr. 2016;55(12):1152-1159. doi:10.1177/0009922815621331
4. Grogan EL, Speroff T, Deppen S, et al. Improving documentation of patient acuity level using a progress note template. J Am Coll Surg. 2004;199(3):468-475. doi:10.1016/j.jamcollsurg.2004.05.254
5. Centers for Disease Control and Prevention. Classification of diseases, functioning, and disability. https://www .cdc.gov/nchs/icd/index.htm. Updated June 30, 2020. Accessed October 12, 2020.
6. Marill K A, Gauharou ES, Nelson BK, et al. Prospective, randomized trial of template-assisted versus undirected written recording of physician records in the emergency department. Ann Emerg Med. 1999;33(5):500- 509. doi:10.1016/S0196-0644(99)70336-7
7. Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. doi:10.1097/00005650-199801000-00004
8. Hart KA, Steinfeldt BA, Braun RD. Formulation and applications of a probalistic Pareto chart. AIAA. 2015;0804. doi:10.2514/6.2015-0804
9. IBM. IBM knowledge center: overview of data objects. https://www.ibm.com/support/knowledgecenter /en/SSLTBW_2.3.0/com.ibm.zos.v2r3.cbclx01/data _objects.htm. Accessed October 12, 2020.
10. US Department of Veterans Affairs. History of IT at VA. https://www.oit.va.gov/about/history.cfm. Accessed October 18, 2020.
11. Gardner CL, Pearce PF. Customization of electronic medical record templates to improve end-user satisfaction. Comput Inform Nurs. 2013;31(3):115-121. doi:10.1097/NXN.0b013e3182771814
Standardized template design is a useful tool to improve clinical documentation and reliable reporting of health care outcomes when constructed with clear objectives and with collaboration of key stakeholders. A standardized template should not only capture accurate diagnostic information, but also inform quality improvement (QI) measures and best practices.
Kang and colleagues showed that a correlation exists between organizational satisfaction and improved quality outcomes.1 A new initiative should have a well-defined purpose reinforced by collaborative workgroups and engaged employees who understand their clinical care role with electronic health record (EHR) modifications.
Several studies have shown how the usefulness of templates achieve multipurpose goals, such as accurate documentation and improved care. Valluru and colleagues showed a significant increase in vaccination rates for patients with inflammatory bowel disease after implementing a standardized template.2 By using a standardized template, Thaker and colleagues showed improved documentation regarding obesity and increased nutritional and physical activity counseling.3 Furthermore, Grogan and colleagues showed that templates are useful for house staff education on International Classification of Diseases (ICD) terminology and demonstrated improved documentation in the postintervention group.4,5
This article discusses the US Department of Veterans Affairs (VA) North Florida/South Georgia Veterans Health System (NF/SGVHS) integrated informatics solutions within template design in the Veterans Health Administration (VHA) EHR system that was associated with an increase in its case severity index (CSI) through improved clinical documentation capture.
Methods
According to policy activities that constitute research at NF/SGVHS, institutional review board approval was not required as this work met the criteria for operational improvement activities exempt from ethics review.
NF/SGVHS includes 2 hospitals: Malcom Randall VA Medical Center (MRVAMC) in Gainesville, Florida, and Lake City VA Medical Center (LCVAMC) in Lake City, Florida. MRVAMC is a large, 1a, academic VA facility composed of rotating residents and fellows and includes multiple specialty care services. LCVAMC is a smaller, nonteaching facility.
Template Design Impact
CSI is a risk-adjusted formula developed by the Inpatient Evaluation Center within VHA. CSI is incorporated into the VHA quality metrics reporting system, Strategic Analytics for Improvement and Learning (SAIL). CSI risk-adjusts metrics such as length of stay and mortality before releasing SAIL reports. CSI is calculated separately for acute level of care (LOC) and for the intensive care unit (ICU). In fiscal year (FY) 2017, acute LOC preimplementation data for CSI at NF/SGVHS were 0.76 for MRVAMC and 0.81 for LCVAMC, which was significantly below the national VHA average of 0.96 (Table).
A below-average CSI conveys a less complicated case mix compared with most other VA facilities. Although smaller VA facilities may have a less complicated case mix, it is unusual for large, tertiary care 1a VA facilities to have a low CSI. This low CSI is usually due to inadequate documentation, which affects not only risk-adjusted quality metrics outcomes, but also potential reimbursement.6
An interdisciplinary team composed of attendings, residents, and a clinical document improvement specialist identified the below-average acute LOC CSI for MRVAMC and LCVAMC compared with that of the national VHA average. Further analysis by chart reviews showed inconsistencies with standardized documentation despite prior health care provider education on ICD terminology and specific groups of common comorbidities analyzed in administrative data reviews for risk-adjustment purposes, known as Elixhauser comorbidities.5,7
A chart review showed lack of clarity regarding primary reason(s) for admission and chronic comorbidities within NF/SGVHS. Using Pareto chart analysis, the template team designed a standardized history and physical (H&P) medicine template based on NF/SGVHS common medicine admissions (Figure 1). A Pareto chart is a valuable QI tool that assists with identifying majority contributors to a problem(s) being analyzed when evaluating a large set of data points. Subsequently, this tool helps focus direction on QI efforts.8
The template had the usual H&P elements not shown (eg, chief complaint, history of present illness, etc), and highlights the assessment/plan section containing primary reason(s) for admission and chronic comorbidities (Figure 1). The complete assessment and plan section on the template can be found in the Appendix.
To simplify the template interface, only single clicks were required to expand diagnostic and chronic comorbidity checkboxes. Subcategories then appeared to select diagnosis and chronic comorbidities along with free text for additional documentation.
In addition, data objects were created within the template that permitted the ability to retrieve information from the VHA EHR and insert specific data points of interest in the template; for example, body mass index to assess degree of obesity and estimated glomerular filtration rate to determine the stage of chronic kidney disease. This allowed users to easily reference data in one template in lieu of searching for data in multiple places in the EHR.9
Results
The standardized H&P medicine template was implemented at MRVAMC and LCVAMC in June 2018 (the final month of the third quarter of FY 2018). As clinical providers throughout NF/SGVHS used the standardized template, acute LOC postimplementation data for CSI significantly improved. Although the national VHA average slightly decreased from 0.96 in the first quarter of FY 2017 to 0.89, in the first quarter of FY 2019, MRVAMC acute LOC CSI improved from 0.76 to 0.97, and LCVAMC acute LOC CSI improved from 0.81 to 1.07 during the same period.
In addition, compliance also was monitored within MRVAMC and LCVAMC for about 1 year after standardized H&P medicine template implementation. Compliance was determined by how often the standardized H&P medicine template was used for inpatient medicine admissions to the acute care wards vs other H&P notes used (such as personalized templates).
Methodology for compliance analysis included acquisition of completed H&P medicine notes from June 18, 2018 to June 30, 2019, within the VHA Veterans Information Systems and Technology Architecture (VistA) clinical and business information system using the search strings: “H&P admission history and physical” and “history of present illness.”10
A review identified 10,845 completed medicine H&P notes. Nine hundred eighteen notes were excluded as their search function yielded a location not corresponding to MRVAMC or LCVAMC. Of the 9,927 notes remaining, 8,025 of these were completed medicine H&P notes at MRVAMC and 1,902 were completed medicine H&P notes at LCVAMC (Figure 2).
From June 18, 2018 to June 30, 2019 at MRVAMC, compliance was reviewed monthly for the 8,025 completed H&P medicine notes. Of the completed H&P medicine notes, the standardized H&P medicine template was used 43.2% in June 2018. By June 2019, MRVAMC clinical providers demonstrated significant improvement for standardized H&P medicine template use at 89.9% (Figure 3). Total average compliance from June 18, 2018 to June 30, 2019, was 88.4%, which doubled compliance from the initial introduction of the standardized H&P medicine template.
Compliance was reviewed monthly for the 1,902 completed H&P medicine notes from June 18, 2018 to June 30, 2019, at LCVAMC. Of the completed H&P medicine notes, the standardized template was used 48.2% of the time in June 2018. By June 2019, LCVAMC clinical providers demonstrated significant improvement for standardized H&P medicine template use, which increased to 96.9%. Total average compliance from June 18, 2018 to June 30, 2019, was 93.8%, which was almost double the baseline compliance rate.
Discussion
Template design with clear objectives, strategic collaboration, and integrated informatics solutions has the potential to increase accuracy of documentation. As shown, the NF/SGVHS template design was associated with significant improvement in acute LOC CSI for both MRVAMC and LCVAMC due to more accurate documentation using the standardized H&P medicine template.
Numerous factors contributed to the success of this template design. First, a clear vision for application of the template was communicated with key stakeholders. In addition, the template design team was focused on specific goals rather than a one size fits all approach, which was crucial for sustainable execution. Although interdisciplinary teamwork has the potential to result in innovative practices, large multidisciplinary teams also may have difficulty establishing a shared vision that can result in barriers to achieving project goals.
Balancing standardization and customization was essential for user buy-in. As noted by Gardner and Pearce, inviting clinical providers to participate in template design and allowing for customization has the potential to increase acceptance and use of templates.11 Although the original design for the standardized H&P medicine template started with the medicine service at NF/SGVHS, the design framework is applicable to numerous services where various clinical care elements can be customized.
Explaining the informatics tools built into the template allowed clinicians to see opportunities to improve clinical documentation and the impact it has on reporting health care outcomes. When improvement work involves integrating clinical care delivery and administrative expectations, it is essential that health care systems understand and strategically execute project initiatives at this critical juncture.
Finally, incorporation of a sustainability plan when process improvement strategies are implemented is vital. In addition to collaboration with the clinical providers during design and implementation of the standardized template, leadership buy-in was key. Compliance with standardized H&P medicine template use was monitored monthly and reviewed by the NF/SGVHS Chief of Staff.
As noted, LCVAMC postimplementation acute LOC CSI was higher than that of MRVAMC despite being a smaller facility. This might be due to the MRVAMC designation as a teaching institution. Medicine is the only inpatient service at LCVAMC staffed by hospitalists with limited specialists available for consultation, whereas MRVAMC is a tertiary care teaching facility with numerous inpatient services and subspecialties. As LCVAMC has more continuity, house staff rotating at MRVAMC require continued training/education on new templates and process changes.
Limitations
Although standardized template design was successful at NF/SGVHS, limitations should be noted. Our clinical documentation improvement (CDI) program also was expanded about the same time as the new templates were released. The expansion of the CDI program in addition to new template design likely had a synergistic effect on acute LOC CSI.
CSI is a complex, risk-adjusted model that includes numerous factors, including but not limited to diagnosis and comorbid conditions. Other factors include age, marital status, procedures, source of admission, specific laboratory values, medical or surgical diagnosis-related group, intensive care unit stays, and immunosuppressive status. CSI also includes operative and nonoperative components that average into an overall CSI. As the majority of CSI is composed of nonoperative constituents within NF/SGVHS, we do not believe this had any substantial impact on reporting of CSI improvements.
In addition, template entry into VHA EHR requires a location selection (such as a clinic name or ward name following an inpatient admission). Of the 10,845 completed H&P medicine notes identified in VistA, 918 notes were excluded from analysis as their search function yielded a location not corresponding to MRVAMC or LCVAMC. For the 918 notes excluded, we believe this was likely due to user error where locations not related to MRVAMC or LCVAMC were selected during standardized H&P medicine template entry.
Conclusions
After the NF/SGVHS implementation of a uniquely designed template embedded with informatics solutions within the VHA EHR, the CSI increased due to more accurate documentation.
Next steps include determining the impact of the NF/SGVHS template design on potential reimbursement and expanding template design into the outpatient setting where there are additional opportunities to improve clinical documentation and reliable reporting of health care outcomes.
Acknowledgments
The authors thank the following individuals for their experience and contribution: Beverley White is the Clinical Documentation Improvement Coordinator at North Florida/South Georgia Veterans Health System and provided expertise on documentation requirements. Russell Jacobitz and Susan Rozelle provided technical expertise on electronic health record system enhancements and implemented the template design. Jess Delaune, MD, and Robert Carroll, MD, provided additional physician input during template design. We also acknowledge the Inpatient Evaluation Center (IPEC) within the Veterans Health Administration (VHA). IPEC developed the case severity index, a risk-adjusted formula incorporated into the VHA quality metric reporting system, Strategic Analytics for Improvement and Learning (SAIL).
Standardized template design is a useful tool to improve clinical documentation and reliable reporting of health care outcomes when constructed with clear objectives and with collaboration of key stakeholders. A standardized template should not only capture accurate diagnostic information, but also inform quality improvement (QI) measures and best practices.
Kang and colleagues showed that a correlation exists between organizational satisfaction and improved quality outcomes.1 A new initiative should have a well-defined purpose reinforced by collaborative workgroups and engaged employees who understand their clinical care role with electronic health record (EHR) modifications.
Several studies have shown how the usefulness of templates achieve multipurpose goals, such as accurate documentation and improved care. Valluru and colleagues showed a significant increase in vaccination rates for patients with inflammatory bowel disease after implementing a standardized template.2 By using a standardized template, Thaker and colleagues showed improved documentation regarding obesity and increased nutritional and physical activity counseling.3 Furthermore, Grogan and colleagues showed that templates are useful for house staff education on International Classification of Diseases (ICD) terminology and demonstrated improved documentation in the postintervention group.4,5
This article discusses the US Department of Veterans Affairs (VA) North Florida/South Georgia Veterans Health System (NF/SGVHS) integrated informatics solutions within template design in the Veterans Health Administration (VHA) EHR system that was associated with an increase in its case severity index (CSI) through improved clinical documentation capture.
Methods
According to policy activities that constitute research at NF/SGVHS, institutional review board approval was not required as this work met the criteria for operational improvement activities exempt from ethics review.
NF/SGVHS includes 2 hospitals: Malcom Randall VA Medical Center (MRVAMC) in Gainesville, Florida, and Lake City VA Medical Center (LCVAMC) in Lake City, Florida. MRVAMC is a large, 1a, academic VA facility composed of rotating residents and fellows and includes multiple specialty care services. LCVAMC is a smaller, nonteaching facility.
Template Design Impact
CSI is a risk-adjusted formula developed by the Inpatient Evaluation Center within VHA. CSI is incorporated into the VHA quality metrics reporting system, Strategic Analytics for Improvement and Learning (SAIL). CSI risk-adjusts metrics such as length of stay and mortality before releasing SAIL reports. CSI is calculated separately for acute level of care (LOC) and for the intensive care unit (ICU). In fiscal year (FY) 2017, acute LOC preimplementation data for CSI at NF/SGVHS were 0.76 for MRVAMC and 0.81 for LCVAMC, which was significantly below the national VHA average of 0.96 (Table).
A below-average CSI conveys a less complicated case mix compared with most other VA facilities. Although smaller VA facilities may have a less complicated case mix, it is unusual for large, tertiary care 1a VA facilities to have a low CSI. This low CSI is usually due to inadequate documentation, which affects not only risk-adjusted quality metrics outcomes, but also potential reimbursement.6
An interdisciplinary team composed of attendings, residents, and a clinical document improvement specialist identified the below-average acute LOC CSI for MRVAMC and LCVAMC compared with that of the national VHA average. Further analysis by chart reviews showed inconsistencies with standardized documentation despite prior health care provider education on ICD terminology and specific groups of common comorbidities analyzed in administrative data reviews for risk-adjustment purposes, known as Elixhauser comorbidities.5,7
A chart review showed lack of clarity regarding primary reason(s) for admission and chronic comorbidities within NF/SGVHS. Using Pareto chart analysis, the template team designed a standardized history and physical (H&P) medicine template based on NF/SGVHS common medicine admissions (Figure 1). A Pareto chart is a valuable QI tool that assists with identifying majority contributors to a problem(s) being analyzed when evaluating a large set of data points. Subsequently, this tool helps focus direction on QI efforts.8
The template had the usual H&P elements not shown (eg, chief complaint, history of present illness, etc), and highlights the assessment/plan section containing primary reason(s) for admission and chronic comorbidities (Figure 1). The complete assessment and plan section on the template can be found in the Appendix.
To simplify the template interface, only single clicks were required to expand diagnostic and chronic comorbidity checkboxes. Subcategories then appeared to select diagnosis and chronic comorbidities along with free text for additional documentation.
In addition, data objects were created within the template that permitted the ability to retrieve information from the VHA EHR and insert specific data points of interest in the template; for example, body mass index to assess degree of obesity and estimated glomerular filtration rate to determine the stage of chronic kidney disease. This allowed users to easily reference data in one template in lieu of searching for data in multiple places in the EHR.9
Results
The standardized H&P medicine template was implemented at MRVAMC and LCVAMC in June 2018 (the final month of the third quarter of FY 2018). As clinical providers throughout NF/SGVHS used the standardized template, acute LOC postimplementation data for CSI significantly improved. Although the national VHA average slightly decreased from 0.96 in the first quarter of FY 2017 to 0.89, in the first quarter of FY 2019, MRVAMC acute LOC CSI improved from 0.76 to 0.97, and LCVAMC acute LOC CSI improved from 0.81 to 1.07 during the same period.
In addition, compliance also was monitored within MRVAMC and LCVAMC for about 1 year after standardized H&P medicine template implementation. Compliance was determined by how often the standardized H&P medicine template was used for inpatient medicine admissions to the acute care wards vs other H&P notes used (such as personalized templates).
Methodology for compliance analysis included acquisition of completed H&P medicine notes from June 18, 2018 to June 30, 2019, within the VHA Veterans Information Systems and Technology Architecture (VistA) clinical and business information system using the search strings: “H&P admission history and physical” and “history of present illness.”10
A review identified 10,845 completed medicine H&P notes. Nine hundred eighteen notes were excluded as their search function yielded a location not corresponding to MRVAMC or LCVAMC. Of the 9,927 notes remaining, 8,025 of these were completed medicine H&P notes at MRVAMC and 1,902 were completed medicine H&P notes at LCVAMC (Figure 2).
From June 18, 2018 to June 30, 2019 at MRVAMC, compliance was reviewed monthly for the 8,025 completed H&P medicine notes. Of the completed H&P medicine notes, the standardized H&P medicine template was used 43.2% in June 2018. By June 2019, MRVAMC clinical providers demonstrated significant improvement for standardized H&P medicine template use at 89.9% (Figure 3). Total average compliance from June 18, 2018 to June 30, 2019, was 88.4%, which doubled compliance from the initial introduction of the standardized H&P medicine template.
Compliance was reviewed monthly for the 1,902 completed H&P medicine notes from June 18, 2018 to June 30, 2019, at LCVAMC. Of the completed H&P medicine notes, the standardized template was used 48.2% of the time in June 2018. By June 2019, LCVAMC clinical providers demonstrated significant improvement for standardized H&P medicine template use, which increased to 96.9%. Total average compliance from June 18, 2018 to June 30, 2019, was 93.8%, which was almost double the baseline compliance rate.
Discussion
Template design with clear objectives, strategic collaboration, and integrated informatics solutions has the potential to increase accuracy of documentation. As shown, the NF/SGVHS template design was associated with significant improvement in acute LOC CSI for both MRVAMC and LCVAMC due to more accurate documentation using the standardized H&P medicine template.
Numerous factors contributed to the success of this template design. First, a clear vision for application of the template was communicated with key stakeholders. In addition, the template design team was focused on specific goals rather than a one size fits all approach, which was crucial for sustainable execution. Although interdisciplinary teamwork has the potential to result in innovative practices, large multidisciplinary teams also may have difficulty establishing a shared vision that can result in barriers to achieving project goals.
Balancing standardization and customization was essential for user buy-in. As noted by Gardner and Pearce, inviting clinical providers to participate in template design and allowing for customization has the potential to increase acceptance and use of templates.11 Although the original design for the standardized H&P medicine template started with the medicine service at NF/SGVHS, the design framework is applicable to numerous services where various clinical care elements can be customized.
Explaining the informatics tools built into the template allowed clinicians to see opportunities to improve clinical documentation and the impact it has on reporting health care outcomes. When improvement work involves integrating clinical care delivery and administrative expectations, it is essential that health care systems understand and strategically execute project initiatives at this critical juncture.
Finally, incorporation of a sustainability plan when process improvement strategies are implemented is vital. In addition to collaboration with the clinical providers during design and implementation of the standardized template, leadership buy-in was key. Compliance with standardized H&P medicine template use was monitored monthly and reviewed by the NF/SGVHS Chief of Staff.
As noted, LCVAMC postimplementation acute LOC CSI was higher than that of MRVAMC despite being a smaller facility. This might be due to the MRVAMC designation as a teaching institution. Medicine is the only inpatient service at LCVAMC staffed by hospitalists with limited specialists available for consultation, whereas MRVAMC is a tertiary care teaching facility with numerous inpatient services and subspecialties. As LCVAMC has more continuity, house staff rotating at MRVAMC require continued training/education on new templates and process changes.
Limitations
Although standardized template design was successful at NF/SGVHS, limitations should be noted. Our clinical documentation improvement (CDI) program also was expanded about the same time as the new templates were released. The expansion of the CDI program in addition to new template design likely had a synergistic effect on acute LOC CSI.
CSI is a complex, risk-adjusted model that includes numerous factors, including but not limited to diagnosis and comorbid conditions. Other factors include age, marital status, procedures, source of admission, specific laboratory values, medical or surgical diagnosis-related group, intensive care unit stays, and immunosuppressive status. CSI also includes operative and nonoperative components that average into an overall CSI. As the majority of CSI is composed of nonoperative constituents within NF/SGVHS, we do not believe this had any substantial impact on reporting of CSI improvements.
In addition, template entry into VHA EHR requires a location selection (such as a clinic name or ward name following an inpatient admission). Of the 10,845 completed H&P medicine notes identified in VistA, 918 notes were excluded from analysis as their search function yielded a location not corresponding to MRVAMC or LCVAMC. For the 918 notes excluded, we believe this was likely due to user error where locations not related to MRVAMC or LCVAMC were selected during standardized H&P medicine template entry.
Conclusions
After the NF/SGVHS implementation of a uniquely designed template embedded with informatics solutions within the VHA EHR, the CSI increased due to more accurate documentation.
Next steps include determining the impact of the NF/SGVHS template design on potential reimbursement and expanding template design into the outpatient setting where there are additional opportunities to improve clinical documentation and reliable reporting of health care outcomes.
Acknowledgments
The authors thank the following individuals for their experience and contribution: Beverley White is the Clinical Documentation Improvement Coordinator at North Florida/South Georgia Veterans Health System and provided expertise on documentation requirements. Russell Jacobitz and Susan Rozelle provided technical expertise on electronic health record system enhancements and implemented the template design. Jess Delaune, MD, and Robert Carroll, MD, provided additional physician input during template design. We also acknowledge the Inpatient Evaluation Center (IPEC) within the Veterans Health Administration (VHA). IPEC developed the case severity index, a risk-adjusted formula incorporated into the VHA quality metric reporting system, Strategic Analytics for Improvement and Learning (SAIL).
1. Kang R, Kunkel S, Columbo J, et al. Association of Hospital Employee satisfaction with patient safety and satisfaction within Veterans Affairs Medical Centers. Am J Med. 2019;132(4):530-534.e1. doi: 10.1016/j.amjmed.2018.11.031
2. Valluru, N, Kang L, Gaidos JK. Health maintenance documentation improves for veterans with IBD using a template in the Computerized Patient Record System. Dig Dis Sci. 2018;63(7):1782-1786. doi:10.1007%2Fs10620-018-5093-5
3. Thaker VV, Lee F, Bottino CJ, et al. Impact of an electronic template on documentation of obesity in a primary care clinic. Clin Pediatr. 2016;55(12):1152-1159. doi:10.1177/0009922815621331
4. Grogan EL, Speroff T, Deppen S, et al. Improving documentation of patient acuity level using a progress note template. J Am Coll Surg. 2004;199(3):468-475. doi:10.1016/j.jamcollsurg.2004.05.254
5. Centers for Disease Control and Prevention. Classification of diseases, functioning, and disability. https://www .cdc.gov/nchs/icd/index.htm. Updated June 30, 2020. Accessed October 12, 2020.
6. Marill K A, Gauharou ES, Nelson BK, et al. Prospective, randomized trial of template-assisted versus undirected written recording of physician records in the emergency department. Ann Emerg Med. 1999;33(5):500- 509. doi:10.1016/S0196-0644(99)70336-7
7. Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. doi:10.1097/00005650-199801000-00004
8. Hart KA, Steinfeldt BA, Braun RD. Formulation and applications of a probalistic Pareto chart. AIAA. 2015;0804. doi:10.2514/6.2015-0804
9. IBM. IBM knowledge center: overview of data objects. https://www.ibm.com/support/knowledgecenter /en/SSLTBW_2.3.0/com.ibm.zos.v2r3.cbclx01/data _objects.htm. Accessed October 12, 2020.
10. US Department of Veterans Affairs. History of IT at VA. https://www.oit.va.gov/about/history.cfm. Accessed October 18, 2020.
11. Gardner CL, Pearce PF. Customization of electronic medical record templates to improve end-user satisfaction. Comput Inform Nurs. 2013;31(3):115-121. doi:10.1097/NXN.0b013e3182771814
1. Kang R, Kunkel S, Columbo J, et al. Association of Hospital Employee satisfaction with patient safety and satisfaction within Veterans Affairs Medical Centers. Am J Med. 2019;132(4):530-534.e1. doi: 10.1016/j.amjmed.2018.11.031
2. Valluru, N, Kang L, Gaidos JK. Health maintenance documentation improves for veterans with IBD using a template in the Computerized Patient Record System. Dig Dis Sci. 2018;63(7):1782-1786. doi:10.1007%2Fs10620-018-5093-5
3. Thaker VV, Lee F, Bottino CJ, et al. Impact of an electronic template on documentation of obesity in a primary care clinic. Clin Pediatr. 2016;55(12):1152-1159. doi:10.1177/0009922815621331
4. Grogan EL, Speroff T, Deppen S, et al. Improving documentation of patient acuity level using a progress note template. J Am Coll Surg. 2004;199(3):468-475. doi:10.1016/j.jamcollsurg.2004.05.254
5. Centers for Disease Control and Prevention. Classification of diseases, functioning, and disability. https://www .cdc.gov/nchs/icd/index.htm. Updated June 30, 2020. Accessed October 12, 2020.
6. Marill K A, Gauharou ES, Nelson BK, et al. Prospective, randomized trial of template-assisted versus undirected written recording of physician records in the emergency department. Ann Emerg Med. 1999;33(5):500- 509. doi:10.1016/S0196-0644(99)70336-7
7. Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. doi:10.1097/00005650-199801000-00004
8. Hart KA, Steinfeldt BA, Braun RD. Formulation and applications of a probalistic Pareto chart. AIAA. 2015;0804. doi:10.2514/6.2015-0804
9. IBM. IBM knowledge center: overview of data objects. https://www.ibm.com/support/knowledgecenter /en/SSLTBW_2.3.0/com.ibm.zos.v2r3.cbclx01/data _objects.htm. Accessed October 12, 2020.
10. US Department of Veterans Affairs. History of IT at VA. https://www.oit.va.gov/about/history.cfm. Accessed October 18, 2020.
11. Gardner CL, Pearce PF. Customization of electronic medical record templates to improve end-user satisfaction. Comput Inform Nurs. 2013;31(3):115-121. doi:10.1097/NXN.0b013e3182771814
Biden victory: What it means for COVID, health care
The former vice president has sketched out a big health agenda: ramping up the federal response to COVID-19, boosting the Affordable Care Act, creating a new “public option” to cover uninsured Americans, and expanding Medicare and Medicaid.
But the president-elect’s long to-do list on health is likely to face significant roadblocks in Congress and the courts, experts say.
For instance, Biden’s ambitious proposals on COVID-19 -- including his recent call for a national mask mandate -- could be waylaid by legal challenges and run into political hurdles on Capitol Hill, where he may face a divided Congress.
Joseph Antos, PhD, a health policy expert with the conservative American Enterprise Institute, predicts Biden will encounter the same type of congressional “gridlock situation” that President Barack Obama ran into during his second term.
“We have a situation that has been like this for a very, very long time -- lack of cooperation, lack of recognition that either party is capable of rising above their own electoral views to deal with problems that the country actually has.”
Antos also suggests that Biden may also face enormous political pressure to address the economic fallout from the coronavirus, including record unemployment and business closures, before anything else.
“I think it’s really going to be efforts that are intended to promote economic development and promote the economy,” he says.
In addition, Biden’s plans to expand Obamacare might face a new challenge from the Supreme Court in the year ahead. This month, the high court will take up a new case seeking to overturn the law.
Even so, experts say Biden’s plans on COVID-19 and expanding health care are likely to define his tenure in the White House as a central focus of his presidency.
“Health care will be at the very top of the list of the president’s priorities,” says Sabrina Corlette, JD, co-director of the Center on Health Insurance Reforms at Georgetown University’s McCourt School of Public Policy. “I do think, however, that the administration is going to be very preoccupied with the response to COVID-19 and the economic fallout … particularly in the first year.”
Here’s a closer look at what we can expect from a Biden presidency.
COVID-19: Federalizing response efforts
Biden will move to federalize the response to COVID-19. He has said he will take back major responsibilities from the states -- such as setting national policies on mask wearing, social distancing, and the reopening of schools and businesses, based on CDC guidance. In the days leading up to the election, Biden called for a national mask mandate, after waffling on the issue throughout the summer.
He has said he will let public health science drive political policy. Biden is also planning to create his own task force to advise officials during the transition on managing the new surge in COVID-19 cases, vaccine safety and protecting at-risk populations, Politico reported this week. He received a virtual briefing on the pandemic from a panel of experts as he awaited the election’s outcome.
“I think we will no longer have this confused and contradictory public messaging,” Corlette says, “but I also think there will be humility and the recognition that the evidence is evolving -- that we don’t have all the answers, but we’re learning as we go.”
But national mandates on masks and social distancing will be challenging to enforce, experts say. They are also likely to face pushback from business interests, opposition from public officials in GOP-led states, and even legal challenges.
Biden’s ability to work with Congress -- or not -- may determine whether he is able to implement some of the key components of his coronavirus action plan, which includes:
- Providing free COVID-19 testing for all Americans
- Hiring 100,000 contact tracers
- Eliminating out-of-pocket expenses for coronavirus treatment
- Delivering “sufficient” PPE for essential workers
- Supporting science-backed vaccines and medical treatments being developed
- Requiring the reopening of businesses, workplaces, and schools only after “sufficient” reductions in community transmission -- under evidence-based protocols put forward by the CDC
- Giving emergency paid leave for workers dislocated by the pandemic and more financial aid for workers, families, and small businesses
- Shoring up safeguards to protect at-risk Americans, including older people
- Boosting pay for health care workers on the front lines
Biden has not detailed how he would pay for many of these, beyond promising to force wealthy Americans to “pay their fair share” of taxes to help. He has proposed a tax increase on Americans making more than $400,000 a year, which would require congressional approval.
Antos says he expects Biden’s proposed COVID-19 action plan to be virtually the same as Trump’s in two areas: efforts to develop a vaccine and antiviral treatments.
The administration has spent some $225 million on COVID-19 testing efforts, with a particular focus on rural areas.
Trump launched Operation Warp Speed to fast-track a vaccine. As part of that, the federal government has contracted with six drug companies, spending nearly $11 billion. The operation aims to provide at least 300 million doses of a coronavirus vaccine by January 2021.
Antos would like to see “a more sophisticated approach to social distancing” from the president-elect that takes into account the different challenges facing Americans depending on their income, work situation, and other factors during the pandemic.
“There are a lot of people in this country where working from home is fine and their jobs are secure,” he notes. “It’s the person who used to work at a restaurant that closed, it’s the line worker at a factory that has severely cut back its hours. It’s basically lower-middle-class people, low-income people, middle-class people, and it’s not the elite.
“And the policies have not given enough consideration to the fact that their circumstances and their tradeoffs would differ from the tradeoffs of somebody who doesn’t have anything to worry about economically.
“So, what we need is a more supple policy [that] will give people the information they need and give them the financial support that they also need … so they can make good decisions for themselves and their families. And we basically haven’t done that.”
Obamacare on the blocks?
The Supreme Court’s decision to take up another case seeking to overturn the Affordable Care Act could hand Biden’s health agenda a major setback -- and put the medical care for millions of Americans in jeopardy.
On Nov. 10, the high court will hear oral arguments on a lawsuit that would strike down all of Obamacare. A decision is not expected until next year.
The court has previously upheld the 2010 law, which Biden helped usher through Congress as vice president. But the addition of right-leaning Supreme Court Justice Amy Coney Barrett to the bench last month gives the court a clear conservative majority that could mean the end of Obamacare, legal experts say.
Republicans have opposed the law since its passage, but they have been unable to muster the votes to repeal it, or to pass an alternative
Antos, from the American Enterprise Institute, notes conservatives believe the law has increased costs for health care and insurance over the past decade, in part because of its protections for Americans with preexisting conditions and requiring insurers to provide comprehensive “gold-plated” policies.
“It’s driven up costs, offers plans that are not very strong, put high-risk folks into the same [insurance pool], which has increased costs for everyone, the employer mandate … these are all the reasons,” he says.
The Supreme Court isn’t expected to deliver a decision on the Affordable Care Act before the middle of next year. But the uncertainty will likely push back Biden’s proposals to expand on the law.
Overturning Obamacare would have huge impacts on millions of Americans:
- As many as 133 million Americans -- roughly half the U.S. population -- with preexisting conditions could find it harder, if not impossible, to find affordable health insurance. That figure does not include Americans infected with COVID-19.
- About 165 million who require expensive treatments -- for cancer and other conditions -- would no longer be protected from huge costs for care by federal caps on out-of-pocket expenditures the Affordable Care Act requires.
- An estimated 21 million who now buy insurance through the Obamacare Marketplaces could lose their coverage.
- Another 12 million on Medicaid could find themselves without insurance.
- At least 2 million young adults ages 26 and under, now on their parents’ health policies, could be kicked off.
- Millions of people who use Medicare could face higher costs.
- Federal subsidies for lower-income Americans to buy policies would disappear.
Throughout the campaign, Biden repeatedly stressed the need to preserve the law’s provision barring insurance companies from refusing coverage for Americans with preexisting conditions, such as diabetes, cancer, and heart disease. It also outlaws charging higher premiums on the basis of health status, age, or gender.
Biden has also pledged to bolster the law as president.
He has proposed a variety of add-ons to the Affordable Care Act he says will “insure more than an estimated 97% of Americans,” according to the Biden campaign site.
Biden’s proposals include offering larger federal subsidies to help low- and middle-income Americans pay for policies purchased through Obamacare insurance Marketplaces.
The boldest of Biden’s proposals is the creation of a “public option” for insurance -- a Medicare-like program that small businesses and individuals could choose if they do not have coverage, cannot afford it, or don’t like their employer-based coverage.
It would also automatically enroll millions of uninsured Americans living in the 14 states that have not expanded Medicaid, which covers low-income people.
But such a plan would require congressional approval -- including a “super majority” of 60 Senate votes to block a likely GOP filibuster. That will be a significant challenge Biden will have to overcome, with Congress so evenly divided.
The White House would also have to defeat heavy lobbying from some of the most influential industry interest groups in Washington, Corlette says.
“I’m not even confident they would get all the Democrat votes,” she says.
“So, it’s a going to be an uphill battle to get a public option passed.”
Taken together, Biden’s plans for expanding Obamacare are projected to cost $750 billion over 10 years. He has said much of that financing would come from increasing taxes on the wealthy.
That means it would likely require congressional approval, which Antos suggests is unlikely given the polarization on Capitol Hill.
Medicare, Medicaid, and drug costs
Biden has called for a host of reforms targeting Medicare, Medicaid, and rising drug costs.
On Medicare, which primarily covers seniors 65 and older, Biden has proposed lowering the eligibility age from 65 to 60. That could extend Medicare to up to 20 million more Americans.
On Medicaid, the health care safety net for low-income and disabled Americans, the president-elect supports increased federal funding to states during the current economic crisis, and potentially beyond.
Medicare is likely to become a key focus of the new administration, in light of the pressures the pandemic is placing on Medicare funding.
In April, Medicare’s trustees said that the Part A trust fund for the program, which pays for hospital and inpatient care, could start to run dry in 2026.
But those projections did not include the impact of COVID-19. Some economists have since projected that Medicare Part A could become insolvent as early as 2022.
Medicare Part B, which pays for doctor and outpatient costs, is funded by general tax funding and beneficiary insurance premiums, so it is not in danger of drying up.
Adding to those pressures is an executive order Trump signed in August temporarily deferring payroll taxes, a primary funding vehicle for Medicare and Social Security.
Under these taxes, employees pay 6.2% of their earnings (on annual income up to $137,700) toward Social Security and 1.45% for Medicare taxes each pay period. Employers pay the same rate per paycheck, adding up to a combined 12.4% Social Security tax and 2.9% Medicare tax.
Biden has said he would reverse the tax cut when he takes office.
But to get a handle on Medicare and Medicaid funding issues, he is likely to need congressional support. Corlette and other experts say that could be a challenge while the nation remains in the grip of the coronavirus pandemic.
In addition to his Medicare and Medicaid reforms, Biden has proposed several plans to lower drug prices, a subset of rising health care and insurance costs.
U.S. spending on prescription drugs has increased nearly 42% over the past decade -- from $253.1 billion in 2010 to $358.7 billion in 2020 (projected) -- according to the Centers for Medicare & Medicaid Services.
In 2020, retail prices for 460 commonly prescribed drugs have spiked an average of 5.2%, according to new analysis by 3 Axis Advisors, a health research firm.
That’s more than double the projected rate of inflation.
To control drug costs, Biden supports legislation approved by the Democratic-led House of Representatives last year that would empower Medicare to negotiate drug prices with drug companies, as private insurers do.
Federal law now bars Medicare from negotiating prices on behalf of the 67.7 million Americans who use it. Drug companies and many GOP leaders argue that the current law is necessary to allow them to spend more on research and development of new medications.
In addition, Biden supports the idea of lifting bans on importing drugs from foreign countries with lower costs.
He also backs creating an independent review board to set price caps for new medications with no competitors; making high-quality generics more available; ending tax breaks for drug company advertising; and limiting their leeway in raising prices.
All of these proposals would likely require congressional approval and could face legal challenges in the courts.
This article first appeared on WebMD.com.
The former vice president has sketched out a big health agenda: ramping up the federal response to COVID-19, boosting the Affordable Care Act, creating a new “public option” to cover uninsured Americans, and expanding Medicare and Medicaid.
But the president-elect’s long to-do list on health is likely to face significant roadblocks in Congress and the courts, experts say.
For instance, Biden’s ambitious proposals on COVID-19 -- including his recent call for a national mask mandate -- could be waylaid by legal challenges and run into political hurdles on Capitol Hill, where he may face a divided Congress.
Joseph Antos, PhD, a health policy expert with the conservative American Enterprise Institute, predicts Biden will encounter the same type of congressional “gridlock situation” that President Barack Obama ran into during his second term.
“We have a situation that has been like this for a very, very long time -- lack of cooperation, lack of recognition that either party is capable of rising above their own electoral views to deal with problems that the country actually has.”
Antos also suggests that Biden may also face enormous political pressure to address the economic fallout from the coronavirus, including record unemployment and business closures, before anything else.
“I think it’s really going to be efforts that are intended to promote economic development and promote the economy,” he says.
In addition, Biden’s plans to expand Obamacare might face a new challenge from the Supreme Court in the year ahead. This month, the high court will take up a new case seeking to overturn the law.
Even so, experts say Biden’s plans on COVID-19 and expanding health care are likely to define his tenure in the White House as a central focus of his presidency.
“Health care will be at the very top of the list of the president’s priorities,” says Sabrina Corlette, JD, co-director of the Center on Health Insurance Reforms at Georgetown University’s McCourt School of Public Policy. “I do think, however, that the administration is going to be very preoccupied with the response to COVID-19 and the economic fallout … particularly in the first year.”
Here’s a closer look at what we can expect from a Biden presidency.
COVID-19: Federalizing response efforts
Biden will move to federalize the response to COVID-19. He has said he will take back major responsibilities from the states -- such as setting national policies on mask wearing, social distancing, and the reopening of schools and businesses, based on CDC guidance. In the days leading up to the election, Biden called for a national mask mandate, after waffling on the issue throughout the summer.
He has said he will let public health science drive political policy. Biden is also planning to create his own task force to advise officials during the transition on managing the new surge in COVID-19 cases, vaccine safety and protecting at-risk populations, Politico reported this week. He received a virtual briefing on the pandemic from a panel of experts as he awaited the election’s outcome.
“I think we will no longer have this confused and contradictory public messaging,” Corlette says, “but I also think there will be humility and the recognition that the evidence is evolving -- that we don’t have all the answers, but we’re learning as we go.”
But national mandates on masks and social distancing will be challenging to enforce, experts say. They are also likely to face pushback from business interests, opposition from public officials in GOP-led states, and even legal challenges.
Biden’s ability to work with Congress -- or not -- may determine whether he is able to implement some of the key components of his coronavirus action plan, which includes:
- Providing free COVID-19 testing for all Americans
- Hiring 100,000 contact tracers
- Eliminating out-of-pocket expenses for coronavirus treatment
- Delivering “sufficient” PPE for essential workers
- Supporting science-backed vaccines and medical treatments being developed
- Requiring the reopening of businesses, workplaces, and schools only after “sufficient” reductions in community transmission -- under evidence-based protocols put forward by the CDC
- Giving emergency paid leave for workers dislocated by the pandemic and more financial aid for workers, families, and small businesses
- Shoring up safeguards to protect at-risk Americans, including older people
- Boosting pay for health care workers on the front lines
Biden has not detailed how he would pay for many of these, beyond promising to force wealthy Americans to “pay their fair share” of taxes to help. He has proposed a tax increase on Americans making more than $400,000 a year, which would require congressional approval.
Antos says he expects Biden’s proposed COVID-19 action plan to be virtually the same as Trump’s in two areas: efforts to develop a vaccine and antiviral treatments.
The administration has spent some $225 million on COVID-19 testing efforts, with a particular focus on rural areas.
Trump launched Operation Warp Speed to fast-track a vaccine. As part of that, the federal government has contracted with six drug companies, spending nearly $11 billion. The operation aims to provide at least 300 million doses of a coronavirus vaccine by January 2021.
Antos would like to see “a more sophisticated approach to social distancing” from the president-elect that takes into account the different challenges facing Americans depending on their income, work situation, and other factors during the pandemic.
“There are a lot of people in this country where working from home is fine and their jobs are secure,” he notes. “It’s the person who used to work at a restaurant that closed, it’s the line worker at a factory that has severely cut back its hours. It’s basically lower-middle-class people, low-income people, middle-class people, and it’s not the elite.
“And the policies have not given enough consideration to the fact that their circumstances and their tradeoffs would differ from the tradeoffs of somebody who doesn’t have anything to worry about economically.
“So, what we need is a more supple policy [that] will give people the information they need and give them the financial support that they also need … so they can make good decisions for themselves and their families. And we basically haven’t done that.”
Obamacare on the blocks?
The Supreme Court’s decision to take up another case seeking to overturn the Affordable Care Act could hand Biden’s health agenda a major setback -- and put the medical care for millions of Americans in jeopardy.
On Nov. 10, the high court will hear oral arguments on a lawsuit that would strike down all of Obamacare. A decision is not expected until next year.
The court has previously upheld the 2010 law, which Biden helped usher through Congress as vice president. But the addition of right-leaning Supreme Court Justice Amy Coney Barrett to the bench last month gives the court a clear conservative majority that could mean the end of Obamacare, legal experts say.
Republicans have opposed the law since its passage, but they have been unable to muster the votes to repeal it, or to pass an alternative
Antos, from the American Enterprise Institute, notes conservatives believe the law has increased costs for health care and insurance over the past decade, in part because of its protections for Americans with preexisting conditions and requiring insurers to provide comprehensive “gold-plated” policies.
“It’s driven up costs, offers plans that are not very strong, put high-risk folks into the same [insurance pool], which has increased costs for everyone, the employer mandate … these are all the reasons,” he says.
The Supreme Court isn’t expected to deliver a decision on the Affordable Care Act before the middle of next year. But the uncertainty will likely push back Biden’s proposals to expand on the law.
Overturning Obamacare would have huge impacts on millions of Americans:
- As many as 133 million Americans -- roughly half the U.S. population -- with preexisting conditions could find it harder, if not impossible, to find affordable health insurance. That figure does not include Americans infected with COVID-19.
- About 165 million who require expensive treatments -- for cancer and other conditions -- would no longer be protected from huge costs for care by federal caps on out-of-pocket expenditures the Affordable Care Act requires.
- An estimated 21 million who now buy insurance through the Obamacare Marketplaces could lose their coverage.
- Another 12 million on Medicaid could find themselves without insurance.
- At least 2 million young adults ages 26 and under, now on their parents’ health policies, could be kicked off.
- Millions of people who use Medicare could face higher costs.
- Federal subsidies for lower-income Americans to buy policies would disappear.
Throughout the campaign, Biden repeatedly stressed the need to preserve the law’s provision barring insurance companies from refusing coverage for Americans with preexisting conditions, such as diabetes, cancer, and heart disease. It also outlaws charging higher premiums on the basis of health status, age, or gender.
Biden has also pledged to bolster the law as president.
He has proposed a variety of add-ons to the Affordable Care Act he says will “insure more than an estimated 97% of Americans,” according to the Biden campaign site.
Biden’s proposals include offering larger federal subsidies to help low- and middle-income Americans pay for policies purchased through Obamacare insurance Marketplaces.
The boldest of Biden’s proposals is the creation of a “public option” for insurance -- a Medicare-like program that small businesses and individuals could choose if they do not have coverage, cannot afford it, or don’t like their employer-based coverage.
It would also automatically enroll millions of uninsured Americans living in the 14 states that have not expanded Medicaid, which covers low-income people.
But such a plan would require congressional approval -- including a “super majority” of 60 Senate votes to block a likely GOP filibuster. That will be a significant challenge Biden will have to overcome, with Congress so evenly divided.
The White House would also have to defeat heavy lobbying from some of the most influential industry interest groups in Washington, Corlette says.
“I’m not even confident they would get all the Democrat votes,” she says.
“So, it’s a going to be an uphill battle to get a public option passed.”
Taken together, Biden’s plans for expanding Obamacare are projected to cost $750 billion over 10 years. He has said much of that financing would come from increasing taxes on the wealthy.
That means it would likely require congressional approval, which Antos suggests is unlikely given the polarization on Capitol Hill.
Medicare, Medicaid, and drug costs
Biden has called for a host of reforms targeting Medicare, Medicaid, and rising drug costs.
On Medicare, which primarily covers seniors 65 and older, Biden has proposed lowering the eligibility age from 65 to 60. That could extend Medicare to up to 20 million more Americans.
On Medicaid, the health care safety net for low-income and disabled Americans, the president-elect supports increased federal funding to states during the current economic crisis, and potentially beyond.
Medicare is likely to become a key focus of the new administration, in light of the pressures the pandemic is placing on Medicare funding.
In April, Medicare’s trustees said that the Part A trust fund for the program, which pays for hospital and inpatient care, could start to run dry in 2026.
But those projections did not include the impact of COVID-19. Some economists have since projected that Medicare Part A could become insolvent as early as 2022.
Medicare Part B, which pays for doctor and outpatient costs, is funded by general tax funding and beneficiary insurance premiums, so it is not in danger of drying up.
Adding to those pressures is an executive order Trump signed in August temporarily deferring payroll taxes, a primary funding vehicle for Medicare and Social Security.
Under these taxes, employees pay 6.2% of their earnings (on annual income up to $137,700) toward Social Security and 1.45% for Medicare taxes each pay period. Employers pay the same rate per paycheck, adding up to a combined 12.4% Social Security tax and 2.9% Medicare tax.
Biden has said he would reverse the tax cut when he takes office.
But to get a handle on Medicare and Medicaid funding issues, he is likely to need congressional support. Corlette and other experts say that could be a challenge while the nation remains in the grip of the coronavirus pandemic.
In addition to his Medicare and Medicaid reforms, Biden has proposed several plans to lower drug prices, a subset of rising health care and insurance costs.
U.S. spending on prescription drugs has increased nearly 42% over the past decade -- from $253.1 billion in 2010 to $358.7 billion in 2020 (projected) -- according to the Centers for Medicare & Medicaid Services.
In 2020, retail prices for 460 commonly prescribed drugs have spiked an average of 5.2%, according to new analysis by 3 Axis Advisors, a health research firm.
That’s more than double the projected rate of inflation.
To control drug costs, Biden supports legislation approved by the Democratic-led House of Representatives last year that would empower Medicare to negotiate drug prices with drug companies, as private insurers do.
Federal law now bars Medicare from negotiating prices on behalf of the 67.7 million Americans who use it. Drug companies and many GOP leaders argue that the current law is necessary to allow them to spend more on research and development of new medications.
In addition, Biden supports the idea of lifting bans on importing drugs from foreign countries with lower costs.
He also backs creating an independent review board to set price caps for new medications with no competitors; making high-quality generics more available; ending tax breaks for drug company advertising; and limiting their leeway in raising prices.
All of these proposals would likely require congressional approval and could face legal challenges in the courts.
This article first appeared on WebMD.com.
The former vice president has sketched out a big health agenda: ramping up the federal response to COVID-19, boosting the Affordable Care Act, creating a new “public option” to cover uninsured Americans, and expanding Medicare and Medicaid.
But the president-elect’s long to-do list on health is likely to face significant roadblocks in Congress and the courts, experts say.
For instance, Biden’s ambitious proposals on COVID-19 -- including his recent call for a national mask mandate -- could be waylaid by legal challenges and run into political hurdles on Capitol Hill, where he may face a divided Congress.
Joseph Antos, PhD, a health policy expert with the conservative American Enterprise Institute, predicts Biden will encounter the same type of congressional “gridlock situation” that President Barack Obama ran into during his second term.
“We have a situation that has been like this for a very, very long time -- lack of cooperation, lack of recognition that either party is capable of rising above their own electoral views to deal with problems that the country actually has.”
Antos also suggests that Biden may also face enormous political pressure to address the economic fallout from the coronavirus, including record unemployment and business closures, before anything else.
“I think it’s really going to be efforts that are intended to promote economic development and promote the economy,” he says.
In addition, Biden’s plans to expand Obamacare might face a new challenge from the Supreme Court in the year ahead. This month, the high court will take up a new case seeking to overturn the law.
Even so, experts say Biden’s plans on COVID-19 and expanding health care are likely to define his tenure in the White House as a central focus of his presidency.
“Health care will be at the very top of the list of the president’s priorities,” says Sabrina Corlette, JD, co-director of the Center on Health Insurance Reforms at Georgetown University’s McCourt School of Public Policy. “I do think, however, that the administration is going to be very preoccupied with the response to COVID-19 and the economic fallout … particularly in the first year.”
Here’s a closer look at what we can expect from a Biden presidency.
COVID-19: Federalizing response efforts
Biden will move to federalize the response to COVID-19. He has said he will take back major responsibilities from the states -- such as setting national policies on mask wearing, social distancing, and the reopening of schools and businesses, based on CDC guidance. In the days leading up to the election, Biden called for a national mask mandate, after waffling on the issue throughout the summer.
He has said he will let public health science drive political policy. Biden is also planning to create his own task force to advise officials during the transition on managing the new surge in COVID-19 cases, vaccine safety and protecting at-risk populations, Politico reported this week. He received a virtual briefing on the pandemic from a panel of experts as he awaited the election’s outcome.
“I think we will no longer have this confused and contradictory public messaging,” Corlette says, “but I also think there will be humility and the recognition that the evidence is evolving -- that we don’t have all the answers, but we’re learning as we go.”
But national mandates on masks and social distancing will be challenging to enforce, experts say. They are also likely to face pushback from business interests, opposition from public officials in GOP-led states, and even legal challenges.
Biden’s ability to work with Congress -- or not -- may determine whether he is able to implement some of the key components of his coronavirus action plan, which includes:
- Providing free COVID-19 testing for all Americans
- Hiring 100,000 contact tracers
- Eliminating out-of-pocket expenses for coronavirus treatment
- Delivering “sufficient” PPE for essential workers
- Supporting science-backed vaccines and medical treatments being developed
- Requiring the reopening of businesses, workplaces, and schools only after “sufficient” reductions in community transmission -- under evidence-based protocols put forward by the CDC
- Giving emergency paid leave for workers dislocated by the pandemic and more financial aid for workers, families, and small businesses
- Shoring up safeguards to protect at-risk Americans, including older people
- Boosting pay for health care workers on the front lines
Biden has not detailed how he would pay for many of these, beyond promising to force wealthy Americans to “pay their fair share” of taxes to help. He has proposed a tax increase on Americans making more than $400,000 a year, which would require congressional approval.
Antos says he expects Biden’s proposed COVID-19 action plan to be virtually the same as Trump’s in two areas: efforts to develop a vaccine and antiviral treatments.
The administration has spent some $225 million on COVID-19 testing efforts, with a particular focus on rural areas.
Trump launched Operation Warp Speed to fast-track a vaccine. As part of that, the federal government has contracted with six drug companies, spending nearly $11 billion. The operation aims to provide at least 300 million doses of a coronavirus vaccine by January 2021.
Antos would like to see “a more sophisticated approach to social distancing” from the president-elect that takes into account the different challenges facing Americans depending on their income, work situation, and other factors during the pandemic.
“There are a lot of people in this country where working from home is fine and their jobs are secure,” he notes. “It’s the person who used to work at a restaurant that closed, it’s the line worker at a factory that has severely cut back its hours. It’s basically lower-middle-class people, low-income people, middle-class people, and it’s not the elite.
“And the policies have not given enough consideration to the fact that their circumstances and their tradeoffs would differ from the tradeoffs of somebody who doesn’t have anything to worry about economically.
“So, what we need is a more supple policy [that] will give people the information they need and give them the financial support that they also need … so they can make good decisions for themselves and their families. And we basically haven’t done that.”
Obamacare on the blocks?
The Supreme Court’s decision to take up another case seeking to overturn the Affordable Care Act could hand Biden’s health agenda a major setback -- and put the medical care for millions of Americans in jeopardy.
On Nov. 10, the high court will hear oral arguments on a lawsuit that would strike down all of Obamacare. A decision is not expected until next year.
The court has previously upheld the 2010 law, which Biden helped usher through Congress as vice president. But the addition of right-leaning Supreme Court Justice Amy Coney Barrett to the bench last month gives the court a clear conservative majority that could mean the end of Obamacare, legal experts say.
Republicans have opposed the law since its passage, but they have been unable to muster the votes to repeal it, or to pass an alternative
Antos, from the American Enterprise Institute, notes conservatives believe the law has increased costs for health care and insurance over the past decade, in part because of its protections for Americans with preexisting conditions and requiring insurers to provide comprehensive “gold-plated” policies.
“It’s driven up costs, offers plans that are not very strong, put high-risk folks into the same [insurance pool], which has increased costs for everyone, the employer mandate … these are all the reasons,” he says.
The Supreme Court isn’t expected to deliver a decision on the Affordable Care Act before the middle of next year. But the uncertainty will likely push back Biden’s proposals to expand on the law.
Overturning Obamacare would have huge impacts on millions of Americans:
- As many as 133 million Americans -- roughly half the U.S. population -- with preexisting conditions could find it harder, if not impossible, to find affordable health insurance. That figure does not include Americans infected with COVID-19.
- About 165 million who require expensive treatments -- for cancer and other conditions -- would no longer be protected from huge costs for care by federal caps on out-of-pocket expenditures the Affordable Care Act requires.
- An estimated 21 million who now buy insurance through the Obamacare Marketplaces could lose their coverage.
- Another 12 million on Medicaid could find themselves without insurance.
- At least 2 million young adults ages 26 and under, now on their parents’ health policies, could be kicked off.
- Millions of people who use Medicare could face higher costs.
- Federal subsidies for lower-income Americans to buy policies would disappear.
Throughout the campaign, Biden repeatedly stressed the need to preserve the law’s provision barring insurance companies from refusing coverage for Americans with preexisting conditions, such as diabetes, cancer, and heart disease. It also outlaws charging higher premiums on the basis of health status, age, or gender.
Biden has also pledged to bolster the law as president.
He has proposed a variety of add-ons to the Affordable Care Act he says will “insure more than an estimated 97% of Americans,” according to the Biden campaign site.
Biden’s proposals include offering larger federal subsidies to help low- and middle-income Americans pay for policies purchased through Obamacare insurance Marketplaces.
The boldest of Biden’s proposals is the creation of a “public option” for insurance -- a Medicare-like program that small businesses and individuals could choose if they do not have coverage, cannot afford it, or don’t like their employer-based coverage.
It would also automatically enroll millions of uninsured Americans living in the 14 states that have not expanded Medicaid, which covers low-income people.
But such a plan would require congressional approval -- including a “super majority” of 60 Senate votes to block a likely GOP filibuster. That will be a significant challenge Biden will have to overcome, with Congress so evenly divided.
The White House would also have to defeat heavy lobbying from some of the most influential industry interest groups in Washington, Corlette says.
“I’m not even confident they would get all the Democrat votes,” she says.
“So, it’s a going to be an uphill battle to get a public option passed.”
Taken together, Biden’s plans for expanding Obamacare are projected to cost $750 billion over 10 years. He has said much of that financing would come from increasing taxes on the wealthy.
That means it would likely require congressional approval, which Antos suggests is unlikely given the polarization on Capitol Hill.
Medicare, Medicaid, and drug costs
Biden has called for a host of reforms targeting Medicare, Medicaid, and rising drug costs.
On Medicare, which primarily covers seniors 65 and older, Biden has proposed lowering the eligibility age from 65 to 60. That could extend Medicare to up to 20 million more Americans.
On Medicaid, the health care safety net for low-income and disabled Americans, the president-elect supports increased federal funding to states during the current economic crisis, and potentially beyond.
Medicare is likely to become a key focus of the new administration, in light of the pressures the pandemic is placing on Medicare funding.
In April, Medicare’s trustees said that the Part A trust fund for the program, which pays for hospital and inpatient care, could start to run dry in 2026.
But those projections did not include the impact of COVID-19. Some economists have since projected that Medicare Part A could become insolvent as early as 2022.
Medicare Part B, which pays for doctor and outpatient costs, is funded by general tax funding and beneficiary insurance premiums, so it is not in danger of drying up.
Adding to those pressures is an executive order Trump signed in August temporarily deferring payroll taxes, a primary funding vehicle for Medicare and Social Security.
Under these taxes, employees pay 6.2% of their earnings (on annual income up to $137,700) toward Social Security and 1.45% for Medicare taxes each pay period. Employers pay the same rate per paycheck, adding up to a combined 12.4% Social Security tax and 2.9% Medicare tax.
Biden has said he would reverse the tax cut when he takes office.
But to get a handle on Medicare and Medicaid funding issues, he is likely to need congressional support. Corlette and other experts say that could be a challenge while the nation remains in the grip of the coronavirus pandemic.
In addition to his Medicare and Medicaid reforms, Biden has proposed several plans to lower drug prices, a subset of rising health care and insurance costs.
U.S. spending on prescription drugs has increased nearly 42% over the past decade -- from $253.1 billion in 2010 to $358.7 billion in 2020 (projected) -- according to the Centers for Medicare & Medicaid Services.
In 2020, retail prices for 460 commonly prescribed drugs have spiked an average of 5.2%, according to new analysis by 3 Axis Advisors, a health research firm.
That’s more than double the projected rate of inflation.
To control drug costs, Biden supports legislation approved by the Democratic-led House of Representatives last year that would empower Medicare to negotiate drug prices with drug companies, as private insurers do.
Federal law now bars Medicare from negotiating prices on behalf of the 67.7 million Americans who use it. Drug companies and many GOP leaders argue that the current law is necessary to allow them to spend more on research and development of new medications.
In addition, Biden supports the idea of lifting bans on importing drugs from foreign countries with lower costs.
He also backs creating an independent review board to set price caps for new medications with no competitors; making high-quality generics more available; ending tax breaks for drug company advertising; and limiting their leeway in raising prices.
All of these proposals would likely require congressional approval and could face legal challenges in the courts.
This article first appeared on WebMD.com.
Why Accept a VA Detail or Short-Term Assignment? Benefits to Employees and the Service
In the Veterans Health Administration (VHA), there are frequent e-mails and requests for employees to accept a detail or short-term assignment across a wide range of positions from administrative to executive leadership. These opportunities afford an employee and the service line valuable benefits and growth opportunities; however, there are reasons why some may be reluctant to pursue these opportunities. In this article, we discuss the barriers to applying for and accepting detail positions and the benefits for the employee and the service lines during periods of standard operations as well as during emergencies requiring alternative staffing strategies.
Details are short-term assignments used to fill a vacant position while hiring for the permanent position or to fill a short-term need (eg, during a pandemic). Details usually last 30 to 120 days, though they may be extended, depending on the position, the number of people willing to serve in the detailed role, and the time to select a candidate for the permanent position. Details can be created for any skill level or type of position to meet an identified need, but they are most often needed for supervisory or leadership roles.
The COVID-19 pandemic has shed light on the importance of individuals’ flexibility and adaptability both within and between roles. Many US Department of Veterans Affairs (VA) facilities stood up Incident Command structures to support the changes required to adapt to the needs created by the pandemic. Establishing an Incident Command means that people within the organization must take on new responsibilities, and in many cases, they are detailed to new positions that were not needed or prioritized before the pandemic.
Barriers
An employee may be reluctant to apply for or accept a detail because he or she has little to no experience; feels uncomfortable stepping into an unfamiliar role; is concerned about making a leap from a clinical to administrative role; has uncertainty whether the job is a good professional fit; dislikes the lack of a pay increase during the detail period even if the new role has more responsibility; and has concern that serving in the detail may make them ineligible to apply for the permanent position due to a perception of being preselected. Additionally, the employee may recognize the added stress on colleagues because the same amount of work must be completed.
Benefits
Although leaving a position for a period of months can be stressful, serving in a detail position provides significant opportunities for professional growth. An employee can gain knowledge and experience in an unfamiliar role before applying for or committing to a permanent position. Those serving in temporary details are often given more support as colleagues and supervisors understand that the role was accepted on short notice with little time to prepare. Other benefits include expanding professional contacts, gaining perspective on a different part of the VHA, and working on skills, such as flexibility, time management, and perseverance. By succeeding in a detail, employees build professional acumen. After taking on additional challenges they become more competitive for future jobs. The VHA Executive Candidate Development Program requires a 120-day detail, serving as either assistant or associate director, chief of staff, or associate director for patient care services/nurse executive as part of the program.1
Temporarily leaving a service line to detail in a different service line has an impact on the home service because of the restrictions imposed. These restrictions guarantee that the employee can return to the original position at the end of a detail, thus providing a sense of job security; however, the home service line is down an employee.
Given these considerations, the following are key points to establish before undertaking the detail: (1) length of assignment; (2) once started, potential for the assignment to be extended; (3) will the employee be doing any of their prior job or just the new job or a blend of both; (4) possible changes in hours and site of work of the employee; (5) who will supervise the employee; (6) who will write the employee’s review; (7) training or skills needed prior to starting; (8) necessary paperwork; (9) how will the new assignment be communicated to others; (10) what happens if the detail ends sooner than planned; and (11) approval and support of all involved parties.
The employee’s home service may need a temporary plan to cover the employee’s workload, especially if the employee will be detailed to a different service line. The temporary plan may require creativity and flexibility and can be a way to trial the contingency plans for staffing the home service. One benefit to the home service is that the employee will have additional skills on returning that may benefit the home service, and the service will gain a potential leader.
When an employee goes to a different service, that service gains an employee who may bring a new perspective to help solve existing conflicts or problems. This can serve as a time to reset expectations or set new goals prior to the arrival of new leadership. If the detail is a good fit, then there is the chance that the employee may return in the future or refer others to it as a professional opportunity.
Conclusions
A detail can benefit the employee and the home and host services if planned in advance, and all parties support the process. A short-term leadership or administrative assignment can help an employee gain valuable experience for the future.
1. US Department of Veterans Affairs. Improve VA’s employee experience.obamaadministration.archives.performance.gov/node/65741.html. Published 2017. Accessed October 19, 2020.
In the Veterans Health Administration (VHA), there are frequent e-mails and requests for employees to accept a detail or short-term assignment across a wide range of positions from administrative to executive leadership. These opportunities afford an employee and the service line valuable benefits and growth opportunities; however, there are reasons why some may be reluctant to pursue these opportunities. In this article, we discuss the barriers to applying for and accepting detail positions and the benefits for the employee and the service lines during periods of standard operations as well as during emergencies requiring alternative staffing strategies.
Details are short-term assignments used to fill a vacant position while hiring for the permanent position or to fill a short-term need (eg, during a pandemic). Details usually last 30 to 120 days, though they may be extended, depending on the position, the number of people willing to serve in the detailed role, and the time to select a candidate for the permanent position. Details can be created for any skill level or type of position to meet an identified need, but they are most often needed for supervisory or leadership roles.
The COVID-19 pandemic has shed light on the importance of individuals’ flexibility and adaptability both within and between roles. Many US Department of Veterans Affairs (VA) facilities stood up Incident Command structures to support the changes required to adapt to the needs created by the pandemic. Establishing an Incident Command means that people within the organization must take on new responsibilities, and in many cases, they are detailed to new positions that were not needed or prioritized before the pandemic.
Barriers
An employee may be reluctant to apply for or accept a detail because he or she has little to no experience; feels uncomfortable stepping into an unfamiliar role; is concerned about making a leap from a clinical to administrative role; has uncertainty whether the job is a good professional fit; dislikes the lack of a pay increase during the detail period even if the new role has more responsibility; and has concern that serving in the detail may make them ineligible to apply for the permanent position due to a perception of being preselected. Additionally, the employee may recognize the added stress on colleagues because the same amount of work must be completed.
Benefits
Although leaving a position for a period of months can be stressful, serving in a detail position provides significant opportunities for professional growth. An employee can gain knowledge and experience in an unfamiliar role before applying for or committing to a permanent position. Those serving in temporary details are often given more support as colleagues and supervisors understand that the role was accepted on short notice with little time to prepare. Other benefits include expanding professional contacts, gaining perspective on a different part of the VHA, and working on skills, such as flexibility, time management, and perseverance. By succeeding in a detail, employees build professional acumen. After taking on additional challenges they become more competitive for future jobs. The VHA Executive Candidate Development Program requires a 120-day detail, serving as either assistant or associate director, chief of staff, or associate director for patient care services/nurse executive as part of the program.1
Temporarily leaving a service line to detail in a different service line has an impact on the home service because of the restrictions imposed. These restrictions guarantee that the employee can return to the original position at the end of a detail, thus providing a sense of job security; however, the home service line is down an employee.
Given these considerations, the following are key points to establish before undertaking the detail: (1) length of assignment; (2) once started, potential for the assignment to be extended; (3) will the employee be doing any of their prior job or just the new job or a blend of both; (4) possible changes in hours and site of work of the employee; (5) who will supervise the employee; (6) who will write the employee’s review; (7) training or skills needed prior to starting; (8) necessary paperwork; (9) how will the new assignment be communicated to others; (10) what happens if the detail ends sooner than planned; and (11) approval and support of all involved parties.
The employee’s home service may need a temporary plan to cover the employee’s workload, especially if the employee will be detailed to a different service line. The temporary plan may require creativity and flexibility and can be a way to trial the contingency plans for staffing the home service. One benefit to the home service is that the employee will have additional skills on returning that may benefit the home service, and the service will gain a potential leader.
When an employee goes to a different service, that service gains an employee who may bring a new perspective to help solve existing conflicts or problems. This can serve as a time to reset expectations or set new goals prior to the arrival of new leadership. If the detail is a good fit, then there is the chance that the employee may return in the future or refer others to it as a professional opportunity.
Conclusions
A detail can benefit the employee and the home and host services if planned in advance, and all parties support the process. A short-term leadership or administrative assignment can help an employee gain valuable experience for the future.
In the Veterans Health Administration (VHA), there are frequent e-mails and requests for employees to accept a detail or short-term assignment across a wide range of positions from administrative to executive leadership. These opportunities afford an employee and the service line valuable benefits and growth opportunities; however, there are reasons why some may be reluctant to pursue these opportunities. In this article, we discuss the barriers to applying for and accepting detail positions and the benefits for the employee and the service lines during periods of standard operations as well as during emergencies requiring alternative staffing strategies.
Details are short-term assignments used to fill a vacant position while hiring for the permanent position or to fill a short-term need (eg, during a pandemic). Details usually last 30 to 120 days, though they may be extended, depending on the position, the number of people willing to serve in the detailed role, and the time to select a candidate for the permanent position. Details can be created for any skill level or type of position to meet an identified need, but they are most often needed for supervisory or leadership roles.
The COVID-19 pandemic has shed light on the importance of individuals’ flexibility and adaptability both within and between roles. Many US Department of Veterans Affairs (VA) facilities stood up Incident Command structures to support the changes required to adapt to the needs created by the pandemic. Establishing an Incident Command means that people within the organization must take on new responsibilities, and in many cases, they are detailed to new positions that were not needed or prioritized before the pandemic.
Barriers
An employee may be reluctant to apply for or accept a detail because he or she has little to no experience; feels uncomfortable stepping into an unfamiliar role; is concerned about making a leap from a clinical to administrative role; has uncertainty whether the job is a good professional fit; dislikes the lack of a pay increase during the detail period even if the new role has more responsibility; and has concern that serving in the detail may make them ineligible to apply for the permanent position due to a perception of being preselected. Additionally, the employee may recognize the added stress on colleagues because the same amount of work must be completed.
Benefits
Although leaving a position for a period of months can be stressful, serving in a detail position provides significant opportunities for professional growth. An employee can gain knowledge and experience in an unfamiliar role before applying for or committing to a permanent position. Those serving in temporary details are often given more support as colleagues and supervisors understand that the role was accepted on short notice with little time to prepare. Other benefits include expanding professional contacts, gaining perspective on a different part of the VHA, and working on skills, such as flexibility, time management, and perseverance. By succeeding in a detail, employees build professional acumen. After taking on additional challenges they become more competitive for future jobs. The VHA Executive Candidate Development Program requires a 120-day detail, serving as either assistant or associate director, chief of staff, or associate director for patient care services/nurse executive as part of the program.1
Temporarily leaving a service line to detail in a different service line has an impact on the home service because of the restrictions imposed. These restrictions guarantee that the employee can return to the original position at the end of a detail, thus providing a sense of job security; however, the home service line is down an employee.
Given these considerations, the following are key points to establish before undertaking the detail: (1) length of assignment; (2) once started, potential for the assignment to be extended; (3) will the employee be doing any of their prior job or just the new job or a blend of both; (4) possible changes in hours and site of work of the employee; (5) who will supervise the employee; (6) who will write the employee’s review; (7) training or skills needed prior to starting; (8) necessary paperwork; (9) how will the new assignment be communicated to others; (10) what happens if the detail ends sooner than planned; and (11) approval and support of all involved parties.
The employee’s home service may need a temporary plan to cover the employee’s workload, especially if the employee will be detailed to a different service line. The temporary plan may require creativity and flexibility and can be a way to trial the contingency plans for staffing the home service. One benefit to the home service is that the employee will have additional skills on returning that may benefit the home service, and the service will gain a potential leader.
When an employee goes to a different service, that service gains an employee who may bring a new perspective to help solve existing conflicts or problems. This can serve as a time to reset expectations or set new goals prior to the arrival of new leadership. If the detail is a good fit, then there is the chance that the employee may return in the future or refer others to it as a professional opportunity.
Conclusions
A detail can benefit the employee and the home and host services if planned in advance, and all parties support the process. A short-term leadership or administrative assignment can help an employee gain valuable experience for the future.
1. US Department of Veterans Affairs. Improve VA’s employee experience.obamaadministration.archives.performance.gov/node/65741.html. Published 2017. Accessed October 19, 2020.
1. US Department of Veterans Affairs. Improve VA’s employee experience.obamaadministration.archives.performance.gov/node/65741.html. Published 2017. Accessed October 19, 2020.