User login
Age, other risk factors predict length of MM survival
Younger age of onset and the use of autologous hematopoietic stem cell transplant (ASCT) treatment were key factors improving the length of survival of newly diagnosed, active multiple myeloma (MM) patients, according to the results of a retrospective analysis.
In addition, multivariable analysis showed that a higher level of blood creatinine, the presence of extramedullary disease, a lower level of partial remission, and the use of nonautologous hematopoietic stem cell transplantation were independent risk factors for shorter survival, according to Virginia Bove, MD, of the Asociación Espanola Primera en Socorros Mutuos, Montevideo, Uruguay and colleagues.
Dr. Bove and colleagues retrospectively analyzed clinical characteristics, response to treatment, and survival of 282 patients from multiple institutions who had active newly-diagnosed multiple myeloma. They compared the results between patients age 65 years or younger (53.2%) with those older than 65 years and assessed clinical risk factors, as reported online in Hematology, Transfusion, and Cell Therapy.
The main cause of death in all patients was MM progression and the early mortality rate was not different between the younger and older patients. The main cause of early death in older patients was infection, according to the researchers.
Multiple risk factors
“Although MM patients younger than 66 years of age have an aggressive presentation with an advanced stage, high rate of renal failure and extramedullary disease, this did not translate into an inferior [overall survival] and [progression-free survival],” the researchers reported.
The overall response rate was similar between groups (80.6% vs. 81.4%; P = .866), and the overall survival was significantly longer in young patients (median, 65 months vs. 41 months; P = .001) and higher in those who received autologous hematopoietic stem cell transplantation.
Multivariate analysis was performed on data from the younger patients. The results showed that a creatinine level of less than or equal to 2 mg/dL (P = .048), extramedullary disease (P = .001), a lower VGPR (P = .003) and the use of nonautologous hematopoietic stem cell transplantation (P = .048) were all independent risk factors for shorter survival.
“Older age is an independent adverse prognostic factor. Adequate risk identification, frontline treatment based on novel drugs and ASCT are the best strategies to improve outcomes, both in young and old patients,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Bove V et al. Hematol Transfus Cell Ther. 2020 Aug 20. doi: 10.1016/j.htct.2020.06.014.
Younger age of onset and the use of autologous hematopoietic stem cell transplant (ASCT) treatment were key factors improving the length of survival of newly diagnosed, active multiple myeloma (MM) patients, according to the results of a retrospective analysis.
In addition, multivariable analysis showed that a higher level of blood creatinine, the presence of extramedullary disease, a lower level of partial remission, and the use of nonautologous hematopoietic stem cell transplantation were independent risk factors for shorter survival, according to Virginia Bove, MD, of the Asociación Espanola Primera en Socorros Mutuos, Montevideo, Uruguay and colleagues.
Dr. Bove and colleagues retrospectively analyzed clinical characteristics, response to treatment, and survival of 282 patients from multiple institutions who had active newly-diagnosed multiple myeloma. They compared the results between patients age 65 years or younger (53.2%) with those older than 65 years and assessed clinical risk factors, as reported online in Hematology, Transfusion, and Cell Therapy.
The main cause of death in all patients was MM progression and the early mortality rate was not different between the younger and older patients. The main cause of early death in older patients was infection, according to the researchers.
Multiple risk factors
“Although MM patients younger than 66 years of age have an aggressive presentation with an advanced stage, high rate of renal failure and extramedullary disease, this did not translate into an inferior [overall survival] and [progression-free survival],” the researchers reported.
The overall response rate was similar between groups (80.6% vs. 81.4%; P = .866), and the overall survival was significantly longer in young patients (median, 65 months vs. 41 months; P = .001) and higher in those who received autologous hematopoietic stem cell transplantation.
Multivariate analysis was performed on data from the younger patients. The results showed that a creatinine level of less than or equal to 2 mg/dL (P = .048), extramedullary disease (P = .001), a lower VGPR (P = .003) and the use of nonautologous hematopoietic stem cell transplantation (P = .048) were all independent risk factors for shorter survival.
“Older age is an independent adverse prognostic factor. Adequate risk identification, frontline treatment based on novel drugs and ASCT are the best strategies to improve outcomes, both in young and old patients,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Bove V et al. Hematol Transfus Cell Ther. 2020 Aug 20. doi: 10.1016/j.htct.2020.06.014.
Younger age of onset and the use of autologous hematopoietic stem cell transplant (ASCT) treatment were key factors improving the length of survival of newly diagnosed, active multiple myeloma (MM) patients, according to the results of a retrospective analysis.
In addition, multivariable analysis showed that a higher level of blood creatinine, the presence of extramedullary disease, a lower level of partial remission, and the use of nonautologous hematopoietic stem cell transplantation were independent risk factors for shorter survival, according to Virginia Bove, MD, of the Asociación Espanola Primera en Socorros Mutuos, Montevideo, Uruguay and colleagues.
Dr. Bove and colleagues retrospectively analyzed clinical characteristics, response to treatment, and survival of 282 patients from multiple institutions who had active newly-diagnosed multiple myeloma. They compared the results between patients age 65 years or younger (53.2%) with those older than 65 years and assessed clinical risk factors, as reported online in Hematology, Transfusion, and Cell Therapy.
The main cause of death in all patients was MM progression and the early mortality rate was not different between the younger and older patients. The main cause of early death in older patients was infection, according to the researchers.
Multiple risk factors
“Although MM patients younger than 66 years of age have an aggressive presentation with an advanced stage, high rate of renal failure and extramedullary disease, this did not translate into an inferior [overall survival] and [progression-free survival],” the researchers reported.
The overall response rate was similar between groups (80.6% vs. 81.4%; P = .866), and the overall survival was significantly longer in young patients (median, 65 months vs. 41 months; P = .001) and higher in those who received autologous hematopoietic stem cell transplantation.
Multivariate analysis was performed on data from the younger patients. The results showed that a creatinine level of less than or equal to 2 mg/dL (P = .048), extramedullary disease (P = .001), a lower VGPR (P = .003) and the use of nonautologous hematopoietic stem cell transplantation (P = .048) were all independent risk factors for shorter survival.
“Older age is an independent adverse prognostic factor. Adequate risk identification, frontline treatment based on novel drugs and ASCT are the best strategies to improve outcomes, both in young and old patients,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Bove V et al. Hematol Transfus Cell Ther. 2020 Aug 20. doi: 10.1016/j.htct.2020.06.014.
FROM HEMATOLOGY, TRANSFUSION, AND CELL THERAPY
Age, smoking among leading cancer risk factors for SLE patients
A new study has quantified cancer risk factors in patients with systemic lupus erythematosus, including smoking and the use of certain medications.
“As expected, older age was associated with cancer overall, as well as with the most common cancer subtypes,” wrote Sasha Bernatsky, MD, PhD, of McGill University, Montreal, and coauthors. The study was published in Arthritis Care & Research.
To determine the risk of cancer in people with clinically confirmed incident systemic lupus erythematosus (SLE), the researchers analyzed data from 1,668 newly diagnosed lupus patients with at least one follow-up visit. All patients were enrolled in the Systemic Lupus International Collaborating Clinics inception cohort from across 33 different centers in North America, Europe, and Asia. A total of 89% (n = 1,480) were women, and 49% (n = 824) were white. The average follow-up period was 9 years.
Of the 1,668 SLE patients, 65 developed some type of cancer. The cancers included 15 breast;, 10 nonmelanoma skin; 7 lung; 6 hematologic, 6 prostate; 5 melanoma; 3 cervical; 3 renal; 2 gastric; 2 head and neck; 2 thyroid; and 1 rectal, sarcoma, thymoma, or uterine. No patient had more than one type, and the mean age of the cancer patients at time of SLE diagnosis was 45.6 (standard deviation, 14.5).
Almost half of the 65 cancers occurred in past or current smokers, including all of the lung cancers, while only 33% of patients without cancers smoked prior to baseline. After univariate analysis, characteristics associated with a higher risk of all cancers included older age at SLE diagnosis (adjusted hazard ratio, 1.05; 95% confidence interval, 1.03-1.06), White race/ethnicity (aHR 1.34; 95% CI, 0.76-2.37), and smoking (aHR 1.21; 95% CI, 0.73-2.01).
After multivariate analysis, the two characteristics most associated with increased cancer risk were older age at SLE diagnosis and being male. The analyses also confirmed that older age was a risk factor for breast cancer (aHR 1.06; 95% CI, 1.02-1.10) and nonmelanoma skin cancer (aHR, 1.06; 95% CI, 1.02-1.11), while use of antimalarial drugs was associated with a lower risk of both breast (aHR, 0.28; 95% CI, 0.09-0.90) and nonmelanoma skin (aHR, 0.23; 95% CI, 0.05-0.95) cancers. For lung cancer, the highest risk factor was smoking 15 or more cigarettes a day (aHR, 6.64; 95% CI, 1.43-30.9); for hematologic cancers, it was being in the top quartile of SLE disease activity (aHR, 7.14; 95% CI, 1.13-45.3).
The authors acknowledged their study’s limitations, including the small number of cancers overall and purposefully not comparing cancer risk in SLE patients with risk in the general population. Although their methods – “physicians recording events at annual visits, confirmed by review of charts” – were recognized as very suitable for the current analysis, they noted that a broader comparison would “potentially be problematic due to differential misclassification error” in cancer registry data.
Two of the study’s authors reported potential conflicts of interest, including receiving grants and consulting and personal fees from various pharmaceutical companies. No other potential conflicts were reported.
SOURCE: Bernatsky S et al. Arthritis Care Res. 2020 Aug 19. doi: 10.1002/acr.24425.
A new study has quantified cancer risk factors in patients with systemic lupus erythematosus, including smoking and the use of certain medications.
“As expected, older age was associated with cancer overall, as well as with the most common cancer subtypes,” wrote Sasha Bernatsky, MD, PhD, of McGill University, Montreal, and coauthors. The study was published in Arthritis Care & Research.
To determine the risk of cancer in people with clinically confirmed incident systemic lupus erythematosus (SLE), the researchers analyzed data from 1,668 newly diagnosed lupus patients with at least one follow-up visit. All patients were enrolled in the Systemic Lupus International Collaborating Clinics inception cohort from across 33 different centers in North America, Europe, and Asia. A total of 89% (n = 1,480) were women, and 49% (n = 824) were white. The average follow-up period was 9 years.
Of the 1,668 SLE patients, 65 developed some type of cancer. The cancers included 15 breast;, 10 nonmelanoma skin; 7 lung; 6 hematologic, 6 prostate; 5 melanoma; 3 cervical; 3 renal; 2 gastric; 2 head and neck; 2 thyroid; and 1 rectal, sarcoma, thymoma, or uterine. No patient had more than one type, and the mean age of the cancer patients at time of SLE diagnosis was 45.6 (standard deviation, 14.5).
Almost half of the 65 cancers occurred in past or current smokers, including all of the lung cancers, while only 33% of patients without cancers smoked prior to baseline. After univariate analysis, characteristics associated with a higher risk of all cancers included older age at SLE diagnosis (adjusted hazard ratio, 1.05; 95% confidence interval, 1.03-1.06), White race/ethnicity (aHR 1.34; 95% CI, 0.76-2.37), and smoking (aHR 1.21; 95% CI, 0.73-2.01).
After multivariate analysis, the two characteristics most associated with increased cancer risk were older age at SLE diagnosis and being male. The analyses also confirmed that older age was a risk factor for breast cancer (aHR 1.06; 95% CI, 1.02-1.10) and nonmelanoma skin cancer (aHR, 1.06; 95% CI, 1.02-1.11), while use of antimalarial drugs was associated with a lower risk of both breast (aHR, 0.28; 95% CI, 0.09-0.90) and nonmelanoma skin (aHR, 0.23; 95% CI, 0.05-0.95) cancers. For lung cancer, the highest risk factor was smoking 15 or more cigarettes a day (aHR, 6.64; 95% CI, 1.43-30.9); for hematologic cancers, it was being in the top quartile of SLE disease activity (aHR, 7.14; 95% CI, 1.13-45.3).
The authors acknowledged their study’s limitations, including the small number of cancers overall and purposefully not comparing cancer risk in SLE patients with risk in the general population. Although their methods – “physicians recording events at annual visits, confirmed by review of charts” – were recognized as very suitable for the current analysis, they noted that a broader comparison would “potentially be problematic due to differential misclassification error” in cancer registry data.
Two of the study’s authors reported potential conflicts of interest, including receiving grants and consulting and personal fees from various pharmaceutical companies. No other potential conflicts were reported.
SOURCE: Bernatsky S et al. Arthritis Care Res. 2020 Aug 19. doi: 10.1002/acr.24425.
A new study has quantified cancer risk factors in patients with systemic lupus erythematosus, including smoking and the use of certain medications.
“As expected, older age was associated with cancer overall, as well as with the most common cancer subtypes,” wrote Sasha Bernatsky, MD, PhD, of McGill University, Montreal, and coauthors. The study was published in Arthritis Care & Research.
To determine the risk of cancer in people with clinically confirmed incident systemic lupus erythematosus (SLE), the researchers analyzed data from 1,668 newly diagnosed lupus patients with at least one follow-up visit. All patients were enrolled in the Systemic Lupus International Collaborating Clinics inception cohort from across 33 different centers in North America, Europe, and Asia. A total of 89% (n = 1,480) were women, and 49% (n = 824) were white. The average follow-up period was 9 years.
Of the 1,668 SLE patients, 65 developed some type of cancer. The cancers included 15 breast;, 10 nonmelanoma skin; 7 lung; 6 hematologic, 6 prostate; 5 melanoma; 3 cervical; 3 renal; 2 gastric; 2 head and neck; 2 thyroid; and 1 rectal, sarcoma, thymoma, or uterine. No patient had more than one type, and the mean age of the cancer patients at time of SLE diagnosis was 45.6 (standard deviation, 14.5).
Almost half of the 65 cancers occurred in past or current smokers, including all of the lung cancers, while only 33% of patients without cancers smoked prior to baseline. After univariate analysis, characteristics associated with a higher risk of all cancers included older age at SLE diagnosis (adjusted hazard ratio, 1.05; 95% confidence interval, 1.03-1.06), White race/ethnicity (aHR 1.34; 95% CI, 0.76-2.37), and smoking (aHR 1.21; 95% CI, 0.73-2.01).
After multivariate analysis, the two characteristics most associated with increased cancer risk were older age at SLE diagnosis and being male. The analyses also confirmed that older age was a risk factor for breast cancer (aHR 1.06; 95% CI, 1.02-1.10) and nonmelanoma skin cancer (aHR, 1.06; 95% CI, 1.02-1.11), while use of antimalarial drugs was associated with a lower risk of both breast (aHR, 0.28; 95% CI, 0.09-0.90) and nonmelanoma skin (aHR, 0.23; 95% CI, 0.05-0.95) cancers. For lung cancer, the highest risk factor was smoking 15 or more cigarettes a day (aHR, 6.64; 95% CI, 1.43-30.9); for hematologic cancers, it was being in the top quartile of SLE disease activity (aHR, 7.14; 95% CI, 1.13-45.3).
The authors acknowledged their study’s limitations, including the small number of cancers overall and purposefully not comparing cancer risk in SLE patients with risk in the general population. Although their methods – “physicians recording events at annual visits, confirmed by review of charts” – were recognized as very suitable for the current analysis, they noted that a broader comparison would “potentially be problematic due to differential misclassification error” in cancer registry data.
Two of the study’s authors reported potential conflicts of interest, including receiving grants and consulting and personal fees from various pharmaceutical companies. No other potential conflicts were reported.
SOURCE: Bernatsky S et al. Arthritis Care Res. 2020 Aug 19. doi: 10.1002/acr.24425.
FROM ARTHRITIS CARE & RESEARCH
Polygenic risk score may predict VTE in adolescents, but not adults, with ALL
Although patients with acute lymphoblastic leukemia (ALL) are at known risk of venous thromboembolism (VTE), there was no overall genetic correlation found to be associated with that susceptibility in the overall population. However, a significant genetic predisposition to VTE was found in adolescent ALL patients, according to a report published in Thrombosis Research.
The researchers assessed the prospectively registered VTE events and collected germline DNA in patients aged 1-45.9 years in the Nordic Society of Pediatric Hematology and Oncology (NOPHO) ALL2008 study, which took place from 2008 to 2016. The researchers performed polygenic risk score (PRS) analysis on VTE development in the NOPHO cohort, according to Kirsten Brunsvig Jarvis, MD, of Oslo University Hospital, and colleagues.
The researchers used summary statistics from two large genomewide association studies on VTE in adults (the International Network of Venous Thromboembolism Clinical Research Networks [INVENT] consortium and the UK Biobank).
Of 1,252 patients with ALL in the genetic cohort, 89 developed VTE (2.5-year cumulative incidence, 7.2%; 95% confidence interval,5.7-8.6) at a median 12.7 weeks from diagnosis.
Overall, an analysis of single-nucleotide polymorphisms (SNPs) from INVENT and UK Biobank studies did not reveal evidence of polygenic correlation with VTE in patients with ALL, the researchers reported. However, when separating adolescents aged 10.0-17.9 years (n = 231) from adults aged 18 years or older (n = 127), they saw polygenic overlap between the INVENT study and thromboembolism development in the adolescent population.
The best-fit polygenic risk score, including 16,144 SNPs, was associated with VTE in adolescents with ALL at a hazard ratio of 1.76 (95% CI, 1.23-2.52; P = .02).
Adolescent vs. adult risk
The researchers expressed surprise that they did not find evidence of genetic overlap in adults. But they stated that, in general, VTE occurs more frequently in adults as part of natural aging, while children and adolescents are physiologically protected. This might explain why genetics might play a stronger role in the high-risk situation of cancer and chemotherapy in adolescents who do not have as many additional exogenic risk factors as adults.
“The usefulness of genetic studies on [V]TE in the general adult population is limited when it comes to understanding the etiology of [V]TE in patients with ALL. However, we found evidence of polygenic overlap in subgroup analysis of adolescents aged 10.0-17.9 years with ALL, and we believe the genetics of [V]TE in this group should be further explored in future risk prediction models for identification of those who might benefit from thromboprophylaxis,” the researchers concluded.
The study was supported by research grant from the South-Eastern Norway Regional Health Authority. The authors reported that they had no conflicts of interest.
SOURCE: Jarvis KB et al. Thromb Res. 2020 Aug 11.doi: 10.1016/j.thromres.2020.08.015.
Although patients with acute lymphoblastic leukemia (ALL) are at known risk of venous thromboembolism (VTE), there was no overall genetic correlation found to be associated with that susceptibility in the overall population. However, a significant genetic predisposition to VTE was found in adolescent ALL patients, according to a report published in Thrombosis Research.
The researchers assessed the prospectively registered VTE events and collected germline DNA in patients aged 1-45.9 years in the Nordic Society of Pediatric Hematology and Oncology (NOPHO) ALL2008 study, which took place from 2008 to 2016. The researchers performed polygenic risk score (PRS) analysis on VTE development in the NOPHO cohort, according to Kirsten Brunsvig Jarvis, MD, of Oslo University Hospital, and colleagues.
The researchers used summary statistics from two large genomewide association studies on VTE in adults (the International Network of Venous Thromboembolism Clinical Research Networks [INVENT] consortium and the UK Biobank).
Of 1,252 patients with ALL in the genetic cohort, 89 developed VTE (2.5-year cumulative incidence, 7.2%; 95% confidence interval,5.7-8.6) at a median 12.7 weeks from diagnosis.
Overall, an analysis of single-nucleotide polymorphisms (SNPs) from INVENT and UK Biobank studies did not reveal evidence of polygenic correlation with VTE in patients with ALL, the researchers reported. However, when separating adolescents aged 10.0-17.9 years (n = 231) from adults aged 18 years or older (n = 127), they saw polygenic overlap between the INVENT study and thromboembolism development in the adolescent population.
The best-fit polygenic risk score, including 16,144 SNPs, was associated with VTE in adolescents with ALL at a hazard ratio of 1.76 (95% CI, 1.23-2.52; P = .02).
Adolescent vs. adult risk
The researchers expressed surprise that they did not find evidence of genetic overlap in adults. But they stated that, in general, VTE occurs more frequently in adults as part of natural aging, while children and adolescents are physiologically protected. This might explain why genetics might play a stronger role in the high-risk situation of cancer and chemotherapy in adolescents who do not have as many additional exogenic risk factors as adults.
“The usefulness of genetic studies on [V]TE in the general adult population is limited when it comes to understanding the etiology of [V]TE in patients with ALL. However, we found evidence of polygenic overlap in subgroup analysis of adolescents aged 10.0-17.9 years with ALL, and we believe the genetics of [V]TE in this group should be further explored in future risk prediction models for identification of those who might benefit from thromboprophylaxis,” the researchers concluded.
The study was supported by research grant from the South-Eastern Norway Regional Health Authority. The authors reported that they had no conflicts of interest.
SOURCE: Jarvis KB et al. Thromb Res. 2020 Aug 11.doi: 10.1016/j.thromres.2020.08.015.
Although patients with acute lymphoblastic leukemia (ALL) are at known risk of venous thromboembolism (VTE), there was no overall genetic correlation found to be associated with that susceptibility in the overall population. However, a significant genetic predisposition to VTE was found in adolescent ALL patients, according to a report published in Thrombosis Research.
The researchers assessed the prospectively registered VTE events and collected germline DNA in patients aged 1-45.9 years in the Nordic Society of Pediatric Hematology and Oncology (NOPHO) ALL2008 study, which took place from 2008 to 2016. The researchers performed polygenic risk score (PRS) analysis on VTE development in the NOPHO cohort, according to Kirsten Brunsvig Jarvis, MD, of Oslo University Hospital, and colleagues.
The researchers used summary statistics from two large genomewide association studies on VTE in adults (the International Network of Venous Thromboembolism Clinical Research Networks [INVENT] consortium and the UK Biobank).
Of 1,252 patients with ALL in the genetic cohort, 89 developed VTE (2.5-year cumulative incidence, 7.2%; 95% confidence interval,5.7-8.6) at a median 12.7 weeks from diagnosis.
Overall, an analysis of single-nucleotide polymorphisms (SNPs) from INVENT and UK Biobank studies did not reveal evidence of polygenic correlation with VTE in patients with ALL, the researchers reported. However, when separating adolescents aged 10.0-17.9 years (n = 231) from adults aged 18 years or older (n = 127), they saw polygenic overlap between the INVENT study and thromboembolism development in the adolescent population.
The best-fit polygenic risk score, including 16,144 SNPs, was associated with VTE in adolescents with ALL at a hazard ratio of 1.76 (95% CI, 1.23-2.52; P = .02).
Adolescent vs. adult risk
The researchers expressed surprise that they did not find evidence of genetic overlap in adults. But they stated that, in general, VTE occurs more frequently in adults as part of natural aging, while children and adolescents are physiologically protected. This might explain why genetics might play a stronger role in the high-risk situation of cancer and chemotherapy in adolescents who do not have as many additional exogenic risk factors as adults.
“The usefulness of genetic studies on [V]TE in the general adult population is limited when it comes to understanding the etiology of [V]TE in patients with ALL. However, we found evidence of polygenic overlap in subgroup analysis of adolescents aged 10.0-17.9 years with ALL, and we believe the genetics of [V]TE in this group should be further explored in future risk prediction models for identification of those who might benefit from thromboprophylaxis,” the researchers concluded.
The study was supported by research grant from the South-Eastern Norway Regional Health Authority. The authors reported that they had no conflicts of interest.
SOURCE: Jarvis KB et al. Thromb Res. 2020 Aug 11.doi: 10.1016/j.thromres.2020.08.015.
FROM THROMBOSIS RESEARCH
BALL score predicts benefit from ibrutinib therapy in relapsed/refractory CLL patients
The BALL score was able to identify a subset of patients with chronic lymphocytic leukemia (CLL) who particularly benefit from single-agent ibrutinib therapy, according to the results of a study of 111 patients followed from two different institutions.
The BALL model consists of four factors: serum beta₂-microglobulin at 5 mg/dL or greater, hemoglobin < 110 g/L for women or < 120 g/L for men, lactate dehydrogenase [LDH] > upper limit of normal [UNL], and time elapsed from last therapy less than 24 months. Each parameter was alloted 1 point, leading to a stratification of patients into three different prognostic groups: low risk (score 0-1), intermediate risk (2-3), and high risk (score 4), according to a report published online in Leukemia Research.
According to Stefano Molica, MD, of the Azienda Ospedaliera Pugliese-Ciaccio, Catanzaro, Italy, and his colleagues, the majority of patients (82%) were clinical Rai stage II-IV. The median patient age was 63 years and nearly 68% were men.
The researchers assessed four models for predicting overall survival. The modified version of CLL-International Prognostic Index (CLL-IPI) failed to provide prognostic information in relapsed/refractory (R/R) CLL (P = .77) as did the Ahn et al. model (P = .95) and a simplified BALL model (P = .09). In contrast, the full BALL score captured two groups of patients with significant differences in survival (hazard ratio, 0.240; 95 % confidence interval, 0.10-0.54; P = .0005); however, because of the low number of patients in the high-risk category, these cases were combined with the intermediate-risk group.
The BALL score identified a subset of patients, accounting for about 50% of the whole population, who particularly benefit from single-agent ibrutinib, according to Dr. Molica and his colleagues. These patients had a survival rate of 85% at 3 years.
“In contrast, the outcome of subjects with intermediate-high risk is disappointing. These patients should be considered for a combination of targeted drugs or cellular-based therapies,” the researchers concluded.
The authors reported that they had no conflicts.
SOURCE: Molica S et al. Leuk Res. 2020 Jun 10. https://doi.org/10.1016/j.leukres.2020.
The BALL score was able to identify a subset of patients with chronic lymphocytic leukemia (CLL) who particularly benefit from single-agent ibrutinib therapy, according to the results of a study of 111 patients followed from two different institutions.
The BALL model consists of four factors: serum beta₂-microglobulin at 5 mg/dL or greater, hemoglobin < 110 g/L for women or < 120 g/L for men, lactate dehydrogenase [LDH] > upper limit of normal [UNL], and time elapsed from last therapy less than 24 months. Each parameter was alloted 1 point, leading to a stratification of patients into three different prognostic groups: low risk (score 0-1), intermediate risk (2-3), and high risk (score 4), according to a report published online in Leukemia Research.
According to Stefano Molica, MD, of the Azienda Ospedaliera Pugliese-Ciaccio, Catanzaro, Italy, and his colleagues, the majority of patients (82%) were clinical Rai stage II-IV. The median patient age was 63 years and nearly 68% were men.
The researchers assessed four models for predicting overall survival. The modified version of CLL-International Prognostic Index (CLL-IPI) failed to provide prognostic information in relapsed/refractory (R/R) CLL (P = .77) as did the Ahn et al. model (P = .95) and a simplified BALL model (P = .09). In contrast, the full BALL score captured two groups of patients with significant differences in survival (hazard ratio, 0.240; 95 % confidence interval, 0.10-0.54; P = .0005); however, because of the low number of patients in the high-risk category, these cases were combined with the intermediate-risk group.
The BALL score identified a subset of patients, accounting for about 50% of the whole population, who particularly benefit from single-agent ibrutinib, according to Dr. Molica and his colleagues. These patients had a survival rate of 85% at 3 years.
“In contrast, the outcome of subjects with intermediate-high risk is disappointing. These patients should be considered for a combination of targeted drugs or cellular-based therapies,” the researchers concluded.
The authors reported that they had no conflicts.
SOURCE: Molica S et al. Leuk Res. 2020 Jun 10. https://doi.org/10.1016/j.leukres.2020.
The BALL score was able to identify a subset of patients with chronic lymphocytic leukemia (CLL) who particularly benefit from single-agent ibrutinib therapy, according to the results of a study of 111 patients followed from two different institutions.
The BALL model consists of four factors: serum beta₂-microglobulin at 5 mg/dL or greater, hemoglobin < 110 g/L for women or < 120 g/L for men, lactate dehydrogenase [LDH] > upper limit of normal [UNL], and time elapsed from last therapy less than 24 months. Each parameter was alloted 1 point, leading to a stratification of patients into three different prognostic groups: low risk (score 0-1), intermediate risk (2-3), and high risk (score 4), according to a report published online in Leukemia Research.
According to Stefano Molica, MD, of the Azienda Ospedaliera Pugliese-Ciaccio, Catanzaro, Italy, and his colleagues, the majority of patients (82%) were clinical Rai stage II-IV. The median patient age was 63 years and nearly 68% were men.
The researchers assessed four models for predicting overall survival. The modified version of CLL-International Prognostic Index (CLL-IPI) failed to provide prognostic information in relapsed/refractory (R/R) CLL (P = .77) as did the Ahn et al. model (P = .95) and a simplified BALL model (P = .09). In contrast, the full BALL score captured two groups of patients with significant differences in survival (hazard ratio, 0.240; 95 % confidence interval, 0.10-0.54; P = .0005); however, because of the low number of patients in the high-risk category, these cases were combined with the intermediate-risk group.
The BALL score identified a subset of patients, accounting for about 50% of the whole population, who particularly benefit from single-agent ibrutinib, according to Dr. Molica and his colleagues. These patients had a survival rate of 85% at 3 years.
“In contrast, the outcome of subjects with intermediate-high risk is disappointing. These patients should be considered for a combination of targeted drugs or cellular-based therapies,” the researchers concluded.
The authors reported that they had no conflicts.
SOURCE: Molica S et al. Leuk Res. 2020 Jun 10. https://doi.org/10.1016/j.leukres.2020.
FROM LEUKEMIA RESEARCH
FDA approves new drug for diffuse large B-cell lymphoma
A novel drug, tafasitamab-cxix (Monjuvi, MorphoSys US), has been approved by the Food and Drug Administration for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL).
The product is a humanized Fc-modified cytolytic CD19 targeting monoclonal antibody. It mediates B-cell lysis through apoptosis and immune effector mechanism, including antibody-dependent cell-mediated cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP).
It is indicated for use in combination with lenalidomide for adult patients with relapsed/refractory DLBCL that is not otherwise specified, including DLBCL arising from low-grade lymphoma, and in patients who are not eligible for autologous stem cell transplant (ASCT).
Tafasitamab-cxix in combination with lenalidomide is the first treatment that approved by the FDA for second-line use for patients with relapsed or refractory DLBCL, notes the manufacturer.
The approval “brings a new treatment option to patients in dire need across the United States,” said Gilles Salles, MD, chair of the clinical hematology department at the University of Lyon (France), and lead investigator of the L-MIND study.
The FDA granted an accelerated approval on the basis of overall response rate from an open-label, single-arm, phase 2 trial in 81 patients (known as L-MIND). Further trials are underway to confirm clinical benefit.
The L-MIND trial was conducted in patients with relapsed or refractory DLBCL who had received at least one, but no more than three, prior lines of therapy, including an anti-CD20 targeting therapy (e.g., rituximab), who were not eligible for high-dose chemotherapy or who refused subsequent ASCT.
All patients received tafasitamab-cxix 12 mg/kg intravenously with lenalidomide (25 mg orally on days 1-21 of each 28-day cycle) for a maximum of 12 cycles, followed by tafasitamab-cxix as monotherapy.
The best ORR (defined as complete and partial responders) in 71 patients with a diagnosis of DLBCL confirmed by central pathology was 55%, with complete responses in 37% and partial responses in 18% of patients. The median response duration was 21.7 months (range, 0-24).
The most common adverse reactions (≥20%) were neutropenia, fatigue, anemia, diarrhea, thrombocytopenia, cough, fever, peripheral edema, respiratory tract infection, and decreased appetite.
Precautions and warnings include infusion-related reactions (6%), serious or severe myelosuppression (including neutropenia [50%], thrombocytopenia [18%], and anemia [7%]), infections (73%), and embryo-fetal toxicity.
DLBCL is the most common type of non-Hodgkin lymphoma in adults worldwide, characterized by rapidly growing masses of malignant B-cells in the lymph nodes, spleen, liver, bone marrow or other organs. It is an aggressive disease with about one in three patients not responding to initial therapy or relapsing thereafter, notes the manufacturer. In the United States each year approximately 10,000 patients who are not eligible for ASCT are diagnosed with relapsed or refractory DLBCL.
This article first appeared on Medscape.com.
A novel drug, tafasitamab-cxix (Monjuvi, MorphoSys US), has been approved by the Food and Drug Administration for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL).
The product is a humanized Fc-modified cytolytic CD19 targeting monoclonal antibody. It mediates B-cell lysis through apoptosis and immune effector mechanism, including antibody-dependent cell-mediated cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP).
It is indicated for use in combination with lenalidomide for adult patients with relapsed/refractory DLBCL that is not otherwise specified, including DLBCL arising from low-grade lymphoma, and in patients who are not eligible for autologous stem cell transplant (ASCT).
Tafasitamab-cxix in combination with lenalidomide is the first treatment that approved by the FDA for second-line use for patients with relapsed or refractory DLBCL, notes the manufacturer.
The approval “brings a new treatment option to patients in dire need across the United States,” said Gilles Salles, MD, chair of the clinical hematology department at the University of Lyon (France), and lead investigator of the L-MIND study.
The FDA granted an accelerated approval on the basis of overall response rate from an open-label, single-arm, phase 2 trial in 81 patients (known as L-MIND). Further trials are underway to confirm clinical benefit.
The L-MIND trial was conducted in patients with relapsed or refractory DLBCL who had received at least one, but no more than three, prior lines of therapy, including an anti-CD20 targeting therapy (e.g., rituximab), who were not eligible for high-dose chemotherapy or who refused subsequent ASCT.
All patients received tafasitamab-cxix 12 mg/kg intravenously with lenalidomide (25 mg orally on days 1-21 of each 28-day cycle) for a maximum of 12 cycles, followed by tafasitamab-cxix as monotherapy.
The best ORR (defined as complete and partial responders) in 71 patients with a diagnosis of DLBCL confirmed by central pathology was 55%, with complete responses in 37% and partial responses in 18% of patients. The median response duration was 21.7 months (range, 0-24).
The most common adverse reactions (≥20%) were neutropenia, fatigue, anemia, diarrhea, thrombocytopenia, cough, fever, peripheral edema, respiratory tract infection, and decreased appetite.
Precautions and warnings include infusion-related reactions (6%), serious or severe myelosuppression (including neutropenia [50%], thrombocytopenia [18%], and anemia [7%]), infections (73%), and embryo-fetal toxicity.
DLBCL is the most common type of non-Hodgkin lymphoma in adults worldwide, characterized by rapidly growing masses of malignant B-cells in the lymph nodes, spleen, liver, bone marrow or other organs. It is an aggressive disease with about one in three patients not responding to initial therapy or relapsing thereafter, notes the manufacturer. In the United States each year approximately 10,000 patients who are not eligible for ASCT are diagnosed with relapsed or refractory DLBCL.
This article first appeared on Medscape.com.
A novel drug, tafasitamab-cxix (Monjuvi, MorphoSys US), has been approved by the Food and Drug Administration for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL).
The product is a humanized Fc-modified cytolytic CD19 targeting monoclonal antibody. It mediates B-cell lysis through apoptosis and immune effector mechanism, including antibody-dependent cell-mediated cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP).
It is indicated for use in combination with lenalidomide for adult patients with relapsed/refractory DLBCL that is not otherwise specified, including DLBCL arising from low-grade lymphoma, and in patients who are not eligible for autologous stem cell transplant (ASCT).
Tafasitamab-cxix in combination with lenalidomide is the first treatment that approved by the FDA for second-line use for patients with relapsed or refractory DLBCL, notes the manufacturer.
The approval “brings a new treatment option to patients in dire need across the United States,” said Gilles Salles, MD, chair of the clinical hematology department at the University of Lyon (France), and lead investigator of the L-MIND study.
The FDA granted an accelerated approval on the basis of overall response rate from an open-label, single-arm, phase 2 trial in 81 patients (known as L-MIND). Further trials are underway to confirm clinical benefit.
The L-MIND trial was conducted in patients with relapsed or refractory DLBCL who had received at least one, but no more than three, prior lines of therapy, including an anti-CD20 targeting therapy (e.g., rituximab), who were not eligible for high-dose chemotherapy or who refused subsequent ASCT.
All patients received tafasitamab-cxix 12 mg/kg intravenously with lenalidomide (25 mg orally on days 1-21 of each 28-day cycle) for a maximum of 12 cycles, followed by tafasitamab-cxix as monotherapy.
The best ORR (defined as complete and partial responders) in 71 patients with a diagnosis of DLBCL confirmed by central pathology was 55%, with complete responses in 37% and partial responses in 18% of patients. The median response duration was 21.7 months (range, 0-24).
The most common adverse reactions (≥20%) were neutropenia, fatigue, anemia, diarrhea, thrombocytopenia, cough, fever, peripheral edema, respiratory tract infection, and decreased appetite.
Precautions and warnings include infusion-related reactions (6%), serious or severe myelosuppression (including neutropenia [50%], thrombocytopenia [18%], and anemia [7%]), infections (73%), and embryo-fetal toxicity.
DLBCL is the most common type of non-Hodgkin lymphoma in adults worldwide, characterized by rapidly growing masses of malignant B-cells in the lymph nodes, spleen, liver, bone marrow or other organs. It is an aggressive disease with about one in three patients not responding to initial therapy or relapsing thereafter, notes the manufacturer. In the United States each year approximately 10,000 patients who are not eligible for ASCT are diagnosed with relapsed or refractory DLBCL.
This article first appeared on Medscape.com.
No rise in major hemorrhagic events with antiplatelet therapy after ICH
Background: Antiplatelet agents reduce the risk of major vascular events in patient with established vaso-occlusive disease, but they may increase the risk of ICH. Patients with prior ICH are at risk for both vaso-occlusive and hemorrhagic events. Clarification of the relative risk and benefit of antiplatelet agent use in this clinical scenario would serve to guide therapy.
Study design: Prospective, open-label, randomized parallel group trial.
Setting: 122 hospitals located in the United Kingdom.
Synopsis: The study included 537 adult patients with imaging-confirmed, nontraumatic intracerebral hemorrhage who were previously prescribed antithrombotic medications were randomized in 1:1 fashion to either start or avoid antiplatelet therapy. Participants were followed up on an annual basis with postal questionnaires both to the participants and their primary care providers. No significant difference was identified in rates of recurrent ICH (adjusted hazard ratio, 0.51; 95% confidence interval, 0.25-1.03), major hemorrhagic events (aHR, 0.71; 95% CI, 0.39-1.30), or major occlusive vascular events (aHR, 1.02; 95% CI, 0.65-1.60) between groups.
Hospitalists should be aware that these data suggest that the risk assessment for resumption of antiplatelet agents should not be affected by a history of nontraumatic intracerebral hemorrhage when weighed against the benefit of these medications in patients with occlusive vascular disease.
Bottom line: Resumption of antiplatelet agents following intracerebral hemorrhage showed no evidence of increased risk of recurrent intracerebral hemorrhage or major hemorrhagic events.
Citation: RESTART Collaboration. Effects of antiplatelet therapy after stroke due to intracerebral haemorrhage (RESTART): A randomized, open-label trial. Lancet. 2019. doi: 10.1016/S0140-6736(19)30840-2.
Dr. Deitelzweig is system department chair of hospital medicine at Ochsner Health System, New Orleans.
Background: Antiplatelet agents reduce the risk of major vascular events in patient with established vaso-occlusive disease, but they may increase the risk of ICH. Patients with prior ICH are at risk for both vaso-occlusive and hemorrhagic events. Clarification of the relative risk and benefit of antiplatelet agent use in this clinical scenario would serve to guide therapy.
Study design: Prospective, open-label, randomized parallel group trial.
Setting: 122 hospitals located in the United Kingdom.
Synopsis: The study included 537 adult patients with imaging-confirmed, nontraumatic intracerebral hemorrhage who were previously prescribed antithrombotic medications were randomized in 1:1 fashion to either start or avoid antiplatelet therapy. Participants were followed up on an annual basis with postal questionnaires both to the participants and their primary care providers. No significant difference was identified in rates of recurrent ICH (adjusted hazard ratio, 0.51; 95% confidence interval, 0.25-1.03), major hemorrhagic events (aHR, 0.71; 95% CI, 0.39-1.30), or major occlusive vascular events (aHR, 1.02; 95% CI, 0.65-1.60) between groups.
Hospitalists should be aware that these data suggest that the risk assessment for resumption of antiplatelet agents should not be affected by a history of nontraumatic intracerebral hemorrhage when weighed against the benefit of these medications in patients with occlusive vascular disease.
Bottom line: Resumption of antiplatelet agents following intracerebral hemorrhage showed no evidence of increased risk of recurrent intracerebral hemorrhage or major hemorrhagic events.
Citation: RESTART Collaboration. Effects of antiplatelet therapy after stroke due to intracerebral haemorrhage (RESTART): A randomized, open-label trial. Lancet. 2019. doi: 10.1016/S0140-6736(19)30840-2.
Dr. Deitelzweig is system department chair of hospital medicine at Ochsner Health System, New Orleans.
Background: Antiplatelet agents reduce the risk of major vascular events in patient with established vaso-occlusive disease, but they may increase the risk of ICH. Patients with prior ICH are at risk for both vaso-occlusive and hemorrhagic events. Clarification of the relative risk and benefit of antiplatelet agent use in this clinical scenario would serve to guide therapy.
Study design: Prospective, open-label, randomized parallel group trial.
Setting: 122 hospitals located in the United Kingdom.
Synopsis: The study included 537 adult patients with imaging-confirmed, nontraumatic intracerebral hemorrhage who were previously prescribed antithrombotic medications were randomized in 1:1 fashion to either start or avoid antiplatelet therapy. Participants were followed up on an annual basis with postal questionnaires both to the participants and their primary care providers. No significant difference was identified in rates of recurrent ICH (adjusted hazard ratio, 0.51; 95% confidence interval, 0.25-1.03), major hemorrhagic events (aHR, 0.71; 95% CI, 0.39-1.30), or major occlusive vascular events (aHR, 1.02; 95% CI, 0.65-1.60) between groups.
Hospitalists should be aware that these data suggest that the risk assessment for resumption of antiplatelet agents should not be affected by a history of nontraumatic intracerebral hemorrhage when weighed against the benefit of these medications in patients with occlusive vascular disease.
Bottom line: Resumption of antiplatelet agents following intracerebral hemorrhage showed no evidence of increased risk of recurrent intracerebral hemorrhage or major hemorrhagic events.
Citation: RESTART Collaboration. Effects of antiplatelet therapy after stroke due to intracerebral haemorrhage (RESTART): A randomized, open-label trial. Lancet. 2019. doi: 10.1016/S0140-6736(19)30840-2.
Dr. Deitelzweig is system department chair of hospital medicine at Ochsner Health System, New Orleans.
Large cohort study: Bevacizumab safe, effective for severe HHT bleeds
Systemic bevacizumab is safe and highly effective for the management of chronic bleeding and anemia in patients with hereditary hemorrhagic telangiectasia (HHT), according to findings from an international observational study.
In 238 patients from 12 international centers who were treated with bevacizumab for a median of 12 months, mean hemoglobin levels increased by 3.2 g/dL (mean pre- and posttreatment levels, 8.6 vs. 11.8 g/dL), and epistaxis severity scores (ESS) decreased by a mean of 3.4 points (pre- and posttreatment scores, 6.8 vs. 3.4 points), Hanny Al-Samkari, MD, reported at the International Society on Thrombosis and Haemostasis virtual congress.
As established in prior studies, the minimal clinically important difference in the ESS, a well-validated 10-point bleeding score in HHT, is 0.71 points; the mean reduction seen in this study was 4.75 times that, noted Dr. Al-Samkari, a clinical investigator and hematologist at Massachusetts General Hospital, Boston.
Further, the median number of red blood cell units transfused during the first year of treatment decreased by 82%, compared with the 6 months prior to treatment (9.0 vs. 0 units), and median iron infusions decreased by 70% during the same period (8.0 vs. 2.0 infusions), he said, adding that these improvements occurred within the first 6 months of treatment and were maintained through 12 months.
Study subjects were adults with a mean age of 63 years who were treated with systemic bevacizumab, a vascular endothelial growth factor receptor (VEGF) monoclonal antibody, between 2011 and 2019 for the primary indication of moderate to severe chronic HHT-related bleeding and anemia. Treatment involved four to eight induction infusions – typically at a dose of 5 mg/kg and given 4 weeks apart – followed by bevacizumab maintenance, either as continuous or scheduled maintenance at 4-, 8-, or 12-week intervals regardless of symptoms, or on an as-needed basis, with 2-6 infusions for signs or symptoms of rebleeding.
Patients received a median of 11 infusions, and 181 received maintenance treatment, including continuous or scheduled maintenance in 136 patients and as-needed maintenance in 45 patients, Dr. Al-Samkari said.
The treatment was generally well tolerated; the most common adverse events during 344 patient-years of treatment were hypertension, fatigue, and proteinuria. No fatal treatment-related adverse events occurred, and no increase in adverse events occurred with longer treatment, he noted
Venous thromboembolism occurred in five patients, including two patients who had “provoked events immediately following joint replacement surgery,” he said.
Thirteen patients (5%) discontinued treatment – 11 for inadequate effect and 2 for side effects, he noted.
Subgroup analyses showed that outcomes were similar regardless of underlying pathogenic mutation, but among those receiving bevacizumab maintenance, the continuous approach, compared with as-needed maintenance, was associated with greater improvement in hemoglobin (10.8 vs. 12.3 g/dL) and ESS (mean, 4.96 vs. 2.88) during months 7-12 of treatment, “which is the time most reflective of the effect of maintenance,” he said.
HHT, also known as Osler-Weber-Rendu disease, is a “rare, genetic, progressive, multisystem bleeding disorder resulting from disorder of angiogenesis,” Dr. Al-Samkari explained, adding that the condition is characterized by severe, recurrent epistaxis and chronic gastrointestinal bleeding.
“Bleeding frequently leads to iron deficiency anemia, which may be severe and dependent on regular iron infusions and/or blood transfusions,” he said.
Though rare, it is the second most common bleeding disorder worldwide, with a prevalence about twice that of hemophilia A and six times that of hemophilia B.
“Despite this, it has no FDA-approved therapies,” he said. “The current mainstay of care is surgical or procedural local hemostatic intervention – which is usually temporizing – and hematological support in the form of blood and iron.”
However, given that the underlying genetic defects that cause HHT result in elevations in VEGF, targeting VEGF with existing antiangiogenic agents is a promising approach.
In fact, several centers have been using bevacizumab for several years as an off-label treatment for bleeding in this setting, and while scattered case reports and small case series suggest efficacy, no “large or definitive studies” have been conducted, and safety hasn’t been carefully evaluated, he said.
To that end, the International Hereditary Hemorrhagic Telangiectasia Intravenous Bevacizumab Investigative Team (InHIBIT) was formed. The current report, “the largest study of antiangiogenic therapy to date in HHT,” represents the results of the InHIBIT-Bleed study, the first completed by the team. The next study will address bevacizumab for the treatment of high-output cardiac failure in HHT (the InHIBIT-HF study), Dr. Al-Samkari said.
Though limited by its retrospective nature and lack of a unified treatment protocol, the InHIBIT-Bleed study provides important information and is strengthened by the large cohort size, especially given that HHT is a rare disease, and by other factors, such as the substantial number of patient-years of treatment.
“Bevacizumab was effective in the management of severe HHT-related epistaxis and GI bleeds,” he said, noting the “significant and striking improvements” on a variety of measures.
Questioned about the durability of treatment effects after treatment discontinuation, Dr. Al-Samkari said outcomes are variable, “highly patient dependent,” and “something that we really need to investigate thoroughly.”
As for the potential for anti-VEGF therapy for bleeding in certain non-HHT settings, session moderator Michael Makris, MD, professor of haemostasis and thrombosis at the University of Sheffield, England, said the possibilities are intriguing.
“I work with lots of patients with von Willebrand disease and angiodysplasia,” he said, adding that angiodysplasia-related bleeding in type 2A von Willebrand disease is a major issue.
Dr. Al-Samkari agreed that the possibility is worth exploring.
“We have looked at this in a small number of patients, and the jury is still out,” he said. “But there is a publication in Gastroenterology – Albitar et al. – that evaluated bevacizumab in patients without HHT [who had] angiodysplasias from other causes – not specifically in type 2 von Willebrand syndrome ... and did find that it was effective at causing the angiodysplasias to regress, hemoglobin to improve.
“So the non-HHT use of this agent is certainly an important one [and] we do have retrospective evidence in a small group of patients who don’t have HHT, who do have angiodysplasias and bleeding, that it may be effective as well.”
Dr. Al-Samkari reported receiving research support and/or consulting fees from Agios, Amgen, and Dova.
SOURCE: Al-Samkari H et al. ISTH 2020, Abstract OC 09.2.
Systemic bevacizumab is safe and highly effective for the management of chronic bleeding and anemia in patients with hereditary hemorrhagic telangiectasia (HHT), according to findings from an international observational study.
In 238 patients from 12 international centers who were treated with bevacizumab for a median of 12 months, mean hemoglobin levels increased by 3.2 g/dL (mean pre- and posttreatment levels, 8.6 vs. 11.8 g/dL), and epistaxis severity scores (ESS) decreased by a mean of 3.4 points (pre- and posttreatment scores, 6.8 vs. 3.4 points), Hanny Al-Samkari, MD, reported at the International Society on Thrombosis and Haemostasis virtual congress.
As established in prior studies, the minimal clinically important difference in the ESS, a well-validated 10-point bleeding score in HHT, is 0.71 points; the mean reduction seen in this study was 4.75 times that, noted Dr. Al-Samkari, a clinical investigator and hematologist at Massachusetts General Hospital, Boston.
Further, the median number of red blood cell units transfused during the first year of treatment decreased by 82%, compared with the 6 months prior to treatment (9.0 vs. 0 units), and median iron infusions decreased by 70% during the same period (8.0 vs. 2.0 infusions), he said, adding that these improvements occurred within the first 6 months of treatment and were maintained through 12 months.
Study subjects were adults with a mean age of 63 years who were treated with systemic bevacizumab, a vascular endothelial growth factor receptor (VEGF) monoclonal antibody, between 2011 and 2019 for the primary indication of moderate to severe chronic HHT-related bleeding and anemia. Treatment involved four to eight induction infusions – typically at a dose of 5 mg/kg and given 4 weeks apart – followed by bevacizumab maintenance, either as continuous or scheduled maintenance at 4-, 8-, or 12-week intervals regardless of symptoms, or on an as-needed basis, with 2-6 infusions for signs or symptoms of rebleeding.
Patients received a median of 11 infusions, and 181 received maintenance treatment, including continuous or scheduled maintenance in 136 patients and as-needed maintenance in 45 patients, Dr. Al-Samkari said.
The treatment was generally well tolerated; the most common adverse events during 344 patient-years of treatment were hypertension, fatigue, and proteinuria. No fatal treatment-related adverse events occurred, and no increase in adverse events occurred with longer treatment, he noted
Venous thromboembolism occurred in five patients, including two patients who had “provoked events immediately following joint replacement surgery,” he said.
Thirteen patients (5%) discontinued treatment – 11 for inadequate effect and 2 for side effects, he noted.
Subgroup analyses showed that outcomes were similar regardless of underlying pathogenic mutation, but among those receiving bevacizumab maintenance, the continuous approach, compared with as-needed maintenance, was associated with greater improvement in hemoglobin (10.8 vs. 12.3 g/dL) and ESS (mean, 4.96 vs. 2.88) during months 7-12 of treatment, “which is the time most reflective of the effect of maintenance,” he said.
HHT, also known as Osler-Weber-Rendu disease, is a “rare, genetic, progressive, multisystem bleeding disorder resulting from disorder of angiogenesis,” Dr. Al-Samkari explained, adding that the condition is characterized by severe, recurrent epistaxis and chronic gastrointestinal bleeding.
“Bleeding frequently leads to iron deficiency anemia, which may be severe and dependent on regular iron infusions and/or blood transfusions,” he said.
Though rare, it is the second most common bleeding disorder worldwide, with a prevalence about twice that of hemophilia A and six times that of hemophilia B.
“Despite this, it has no FDA-approved therapies,” he said. “The current mainstay of care is surgical or procedural local hemostatic intervention – which is usually temporizing – and hematological support in the form of blood and iron.”
However, given that the underlying genetic defects that cause HHT result in elevations in VEGF, targeting VEGF with existing antiangiogenic agents is a promising approach.
In fact, several centers have been using bevacizumab for several years as an off-label treatment for bleeding in this setting, and while scattered case reports and small case series suggest efficacy, no “large or definitive studies” have been conducted, and safety hasn’t been carefully evaluated, he said.
To that end, the International Hereditary Hemorrhagic Telangiectasia Intravenous Bevacizumab Investigative Team (InHIBIT) was formed. The current report, “the largest study of antiangiogenic therapy to date in HHT,” represents the results of the InHIBIT-Bleed study, the first completed by the team. The next study will address bevacizumab for the treatment of high-output cardiac failure in HHT (the InHIBIT-HF study), Dr. Al-Samkari said.
Though limited by its retrospective nature and lack of a unified treatment protocol, the InHIBIT-Bleed study provides important information and is strengthened by the large cohort size, especially given that HHT is a rare disease, and by other factors, such as the substantial number of patient-years of treatment.
“Bevacizumab was effective in the management of severe HHT-related epistaxis and GI bleeds,” he said, noting the “significant and striking improvements” on a variety of measures.
Questioned about the durability of treatment effects after treatment discontinuation, Dr. Al-Samkari said outcomes are variable, “highly patient dependent,” and “something that we really need to investigate thoroughly.”
As for the potential for anti-VEGF therapy for bleeding in certain non-HHT settings, session moderator Michael Makris, MD, professor of haemostasis and thrombosis at the University of Sheffield, England, said the possibilities are intriguing.
“I work with lots of patients with von Willebrand disease and angiodysplasia,” he said, adding that angiodysplasia-related bleeding in type 2A von Willebrand disease is a major issue.
Dr. Al-Samkari agreed that the possibility is worth exploring.
“We have looked at this in a small number of patients, and the jury is still out,” he said. “But there is a publication in Gastroenterology – Albitar et al. – that evaluated bevacizumab in patients without HHT [who had] angiodysplasias from other causes – not specifically in type 2 von Willebrand syndrome ... and did find that it was effective at causing the angiodysplasias to regress, hemoglobin to improve.
“So the non-HHT use of this agent is certainly an important one [and] we do have retrospective evidence in a small group of patients who don’t have HHT, who do have angiodysplasias and bleeding, that it may be effective as well.”
Dr. Al-Samkari reported receiving research support and/or consulting fees from Agios, Amgen, and Dova.
SOURCE: Al-Samkari H et al. ISTH 2020, Abstract OC 09.2.
Systemic bevacizumab is safe and highly effective for the management of chronic bleeding and anemia in patients with hereditary hemorrhagic telangiectasia (HHT), according to findings from an international observational study.
In 238 patients from 12 international centers who were treated with bevacizumab for a median of 12 months, mean hemoglobin levels increased by 3.2 g/dL (mean pre- and posttreatment levels, 8.6 vs. 11.8 g/dL), and epistaxis severity scores (ESS) decreased by a mean of 3.4 points (pre- and posttreatment scores, 6.8 vs. 3.4 points), Hanny Al-Samkari, MD, reported at the International Society on Thrombosis and Haemostasis virtual congress.
As established in prior studies, the minimal clinically important difference in the ESS, a well-validated 10-point bleeding score in HHT, is 0.71 points; the mean reduction seen in this study was 4.75 times that, noted Dr. Al-Samkari, a clinical investigator and hematologist at Massachusetts General Hospital, Boston.
Further, the median number of red blood cell units transfused during the first year of treatment decreased by 82%, compared with the 6 months prior to treatment (9.0 vs. 0 units), and median iron infusions decreased by 70% during the same period (8.0 vs. 2.0 infusions), he said, adding that these improvements occurred within the first 6 months of treatment and were maintained through 12 months.
Study subjects were adults with a mean age of 63 years who were treated with systemic bevacizumab, a vascular endothelial growth factor receptor (VEGF) monoclonal antibody, between 2011 and 2019 for the primary indication of moderate to severe chronic HHT-related bleeding and anemia. Treatment involved four to eight induction infusions – typically at a dose of 5 mg/kg and given 4 weeks apart – followed by bevacizumab maintenance, either as continuous or scheduled maintenance at 4-, 8-, or 12-week intervals regardless of symptoms, or on an as-needed basis, with 2-6 infusions for signs or symptoms of rebleeding.
Patients received a median of 11 infusions, and 181 received maintenance treatment, including continuous or scheduled maintenance in 136 patients and as-needed maintenance in 45 patients, Dr. Al-Samkari said.
The treatment was generally well tolerated; the most common adverse events during 344 patient-years of treatment were hypertension, fatigue, and proteinuria. No fatal treatment-related adverse events occurred, and no increase in adverse events occurred with longer treatment, he noted
Venous thromboembolism occurred in five patients, including two patients who had “provoked events immediately following joint replacement surgery,” he said.
Thirteen patients (5%) discontinued treatment – 11 for inadequate effect and 2 for side effects, he noted.
Subgroup analyses showed that outcomes were similar regardless of underlying pathogenic mutation, but among those receiving bevacizumab maintenance, the continuous approach, compared with as-needed maintenance, was associated with greater improvement in hemoglobin (10.8 vs. 12.3 g/dL) and ESS (mean, 4.96 vs. 2.88) during months 7-12 of treatment, “which is the time most reflective of the effect of maintenance,” he said.
HHT, also known as Osler-Weber-Rendu disease, is a “rare, genetic, progressive, multisystem bleeding disorder resulting from disorder of angiogenesis,” Dr. Al-Samkari explained, adding that the condition is characterized by severe, recurrent epistaxis and chronic gastrointestinal bleeding.
“Bleeding frequently leads to iron deficiency anemia, which may be severe and dependent on regular iron infusions and/or blood transfusions,” he said.
Though rare, it is the second most common bleeding disorder worldwide, with a prevalence about twice that of hemophilia A and six times that of hemophilia B.
“Despite this, it has no FDA-approved therapies,” he said. “The current mainstay of care is surgical or procedural local hemostatic intervention – which is usually temporizing – and hematological support in the form of blood and iron.”
However, given that the underlying genetic defects that cause HHT result in elevations in VEGF, targeting VEGF with existing antiangiogenic agents is a promising approach.
In fact, several centers have been using bevacizumab for several years as an off-label treatment for bleeding in this setting, and while scattered case reports and small case series suggest efficacy, no “large or definitive studies” have been conducted, and safety hasn’t been carefully evaluated, he said.
To that end, the International Hereditary Hemorrhagic Telangiectasia Intravenous Bevacizumab Investigative Team (InHIBIT) was formed. The current report, “the largest study of antiangiogenic therapy to date in HHT,” represents the results of the InHIBIT-Bleed study, the first completed by the team. The next study will address bevacizumab for the treatment of high-output cardiac failure in HHT (the InHIBIT-HF study), Dr. Al-Samkari said.
Though limited by its retrospective nature and lack of a unified treatment protocol, the InHIBIT-Bleed study provides important information and is strengthened by the large cohort size, especially given that HHT is a rare disease, and by other factors, such as the substantial number of patient-years of treatment.
“Bevacizumab was effective in the management of severe HHT-related epistaxis and GI bleeds,” he said, noting the “significant and striking improvements” on a variety of measures.
Questioned about the durability of treatment effects after treatment discontinuation, Dr. Al-Samkari said outcomes are variable, “highly patient dependent,” and “something that we really need to investigate thoroughly.”
As for the potential for anti-VEGF therapy for bleeding in certain non-HHT settings, session moderator Michael Makris, MD, professor of haemostasis and thrombosis at the University of Sheffield, England, said the possibilities are intriguing.
“I work with lots of patients with von Willebrand disease and angiodysplasia,” he said, adding that angiodysplasia-related bleeding in type 2A von Willebrand disease is a major issue.
Dr. Al-Samkari agreed that the possibility is worth exploring.
“We have looked at this in a small number of patients, and the jury is still out,” he said. “But there is a publication in Gastroenterology – Albitar et al. – that evaluated bevacizumab in patients without HHT [who had] angiodysplasias from other causes – not specifically in type 2 von Willebrand syndrome ... and did find that it was effective at causing the angiodysplasias to regress, hemoglobin to improve.
“So the non-HHT use of this agent is certainly an important one [and] we do have retrospective evidence in a small group of patients who don’t have HHT, who do have angiodysplasias and bleeding, that it may be effective as well.”
Dr. Al-Samkari reported receiving research support and/or consulting fees from Agios, Amgen, and Dova.
SOURCE: Al-Samkari H et al. ISTH 2020, Abstract OC 09.2.
FROM THE 2020 ISTH CONGRESS
Hematologic manifestations of COVID-19
While SARS-CoV-2 causes frequent and potentially severe pulmonary disease, extrapulmonary manifestations may be a prominent part of the clinical spectrum, according to a review published in Nature Medicine.
In this comprehensive literature review, Aakriti Gupta, MD, of New York-Presbyterian/Columbia University Irving Medical Center and colleagues detailed the epidemiologic and clinical multisystem effects of COVID-19. The authors explained what is known and/or suspected about the pathophysiology of those effects and outlined the resultant management considerations.
Key mechanisms for multiorgan injury include direct viral toxicity, endothelial cell damage with inflammatory mediation of thrombosis, aberrant immune response, and dysregulation of the renin-angiotensin-aldosterone system.
The relative importance of each pathway in the clinical presentation of COVID-19 and the mechanism for extrapulmonary spread of SARS-CoV-2 infection are imperfectly understood, Dr. Gupta and colleagues noted.
As for the hematologic effects of COVID-19, patients may present with several laboratory abnormalities, but the most clinically relevant complications are thromboembolic.
COVID-19-associated coagulopathy
Dr. Gupta and colleagues noted that COVID-19–associated coagulopathy (CAC) is accompanied by elevated levels of D-dimer and fibrinogen, with minor abnormalities in prothrombin time, activated partial thromboplastin time, and platelet counts in the initial stage of infection.
Elevated D-dimer levels have been reported in up to 46% of hospitalized patients, and a longitudinal increase while hospitalized is associated with higher mortality.
In initial reports from China and the Netherlands, thrombotic complications were seen in up to 30% of COVID-19 patients in ICUs. Thromboembolic events have been reported in 17%-22% of critically ill COVID-19 patients in studies from Italy and France.
Globally, in severely affected COVID-19 patients, there have been reports of thromboses in intravenous catheters and extracorporeal circuits as well as arterial vascular occlusive events, including myocardial infarction, acute limb ischemia, and stroke.
There have been multiple small studies in which critically ill COVID-19 patients were routinely screened for thrombotic disease. In these studies, rates of thrombotic complications ranged from 69% to 85%, despite thromboprophylaxis. Variability in prophylactic and screening protocols explain discrepancies in event rates.
Pathophysiology
The abnormally high blood levels of D-dimer and fibrinogen during the early stages of SARS-CoV-2 infection are reflective of excessive inflammation rather than overt disseminated intravascular coagulation (DIC), which may develop in later stages of illness, according to Dr. Gupta and colleagues. The authors theorized that uninhibited inflammation, along with hypoxia and direct viral-mediated cellular injury, contribute to thrombotic complications in COVID-19 patients.
“The increased expression of ACE2 in endothelial cells after infection with SARS-CoV-2 may perpetuate a vicious cycle of endothelialitis that promotes thromboinflammation,” the authors wrote. “Collectively, hemostatic and inflammatory changes, which reflect endothelial damage and activation as well as critical illness, constitute a prothrombotic milieu.”
The authors noted that small autopsy series have shown high rates of microvascular and macrovascular thromboses, particularly in the pulmonary circulation, in COVID-19 patients.
Management considerations
Dr. Gupta and colleagues referenced interim guidelines from the International Society of Thrombosis and Haemostasis that recommend serial complete blood counts, with white blood cell differential and assessment of D-dimer, prothrombin time, and fibrinogen for hospitalized patients with COVID-19. The authors also cited guidelines published in the Journal of the American College of Cardiology that recommend routine risk assessment for venous thromboembolism in all hospitalized patients with COVID-19 and the consideration of standard-dose pharmaco-prophylaxis in patients who lack absolute contraindications.
Empiric use of higher-than-routine prophylactic-dose or therapeutic-dose anticoagulation in ICU patients in the absence of proven thromboses has been implemented in some institutions, Dr. Gupta and colleagues noted. Parenteral anticoagulants (such as low-molecular-weight or unfractionated heparin) are preferred to oral anticoagulants because of short half-life, available reversal agents, and the potential for drug interactions between oral agents and antiviral and/or antibacterial treatment, according to the authors.
They wrote that randomized clinical trials “will be crucial to establishing effective and safe strategies” for anticoagulation in COVID-19 patients. To this point, few randomized trials have been published to guide management of COVID-19–associated extrapulmonary manifestations, including CAC.
Research priorities
A more complete understanding of the organ-specific pathophysiology of this multisystem disease is vital, according to Dr. Gupta and colleagues.
“Regional, national, and international collaborations of clinicians and scientists focused on high-quality, transparent, ethical, and evidence-based research practices would help propel the global community toward achieving success against this pandemic,” the authors wrote.
They noted that common definitions and data standards for research are key for cross-institutional and international collaborations.
Initial attention to high-quality prospective scientific documentation standards would have been valuable and will be required for dedicated trials to address the multisystem effects of COVID-19.
Community of learners
As much as at any prior time in their careers, during the COVID-19 pandemic, health care providers have been enveloped in a community of learners – a group of people who share values and beliefs and who actively engage in learning from one another.
Through a patchwork of sources – news media, social media, traditional medical journals, general and COVID-focused meetings, and, most importantly, patients – we have been living in a learning-centered environment. Academicians, clinicians, practicing physicians, researchers, patients, family members, and caregivers have been actively and intentionally building a knowledge base together.
Through their published review, Dr. Gupta and colleagues have contributed meaningfully to the understanding our learning community has of the various extrapulmonary manifestations of COVID-19. The authors have provided a nice template for further research and clinical advances.
Dr. Gupta and colleagues disclosed financial relationships with a range of pharmaceutical companies and other organizations.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Source: Gupta A et al. Nat Med. 2020 Jul;26(7):1017-32.
While SARS-CoV-2 causes frequent and potentially severe pulmonary disease, extrapulmonary manifestations may be a prominent part of the clinical spectrum, according to a review published in Nature Medicine.
In this comprehensive literature review, Aakriti Gupta, MD, of New York-Presbyterian/Columbia University Irving Medical Center and colleagues detailed the epidemiologic and clinical multisystem effects of COVID-19. The authors explained what is known and/or suspected about the pathophysiology of those effects and outlined the resultant management considerations.
Key mechanisms for multiorgan injury include direct viral toxicity, endothelial cell damage with inflammatory mediation of thrombosis, aberrant immune response, and dysregulation of the renin-angiotensin-aldosterone system.
The relative importance of each pathway in the clinical presentation of COVID-19 and the mechanism for extrapulmonary spread of SARS-CoV-2 infection are imperfectly understood, Dr. Gupta and colleagues noted.
As for the hematologic effects of COVID-19, patients may present with several laboratory abnormalities, but the most clinically relevant complications are thromboembolic.
COVID-19-associated coagulopathy
Dr. Gupta and colleagues noted that COVID-19–associated coagulopathy (CAC) is accompanied by elevated levels of D-dimer and fibrinogen, with minor abnormalities in prothrombin time, activated partial thromboplastin time, and platelet counts in the initial stage of infection.
Elevated D-dimer levels have been reported in up to 46% of hospitalized patients, and a longitudinal increase while hospitalized is associated with higher mortality.
In initial reports from China and the Netherlands, thrombotic complications were seen in up to 30% of COVID-19 patients in ICUs. Thromboembolic events have been reported in 17%-22% of critically ill COVID-19 patients in studies from Italy and France.
Globally, in severely affected COVID-19 patients, there have been reports of thromboses in intravenous catheters and extracorporeal circuits as well as arterial vascular occlusive events, including myocardial infarction, acute limb ischemia, and stroke.
There have been multiple small studies in which critically ill COVID-19 patients were routinely screened for thrombotic disease. In these studies, rates of thrombotic complications ranged from 69% to 85%, despite thromboprophylaxis. Variability in prophylactic and screening protocols explain discrepancies in event rates.
Pathophysiology
The abnormally high blood levels of D-dimer and fibrinogen during the early stages of SARS-CoV-2 infection are reflective of excessive inflammation rather than overt disseminated intravascular coagulation (DIC), which may develop in later stages of illness, according to Dr. Gupta and colleagues. The authors theorized that uninhibited inflammation, along with hypoxia and direct viral-mediated cellular injury, contribute to thrombotic complications in COVID-19 patients.
“The increased expression of ACE2 in endothelial cells after infection with SARS-CoV-2 may perpetuate a vicious cycle of endothelialitis that promotes thromboinflammation,” the authors wrote. “Collectively, hemostatic and inflammatory changes, which reflect endothelial damage and activation as well as critical illness, constitute a prothrombotic milieu.”
The authors noted that small autopsy series have shown high rates of microvascular and macrovascular thromboses, particularly in the pulmonary circulation, in COVID-19 patients.
Management considerations
Dr. Gupta and colleagues referenced interim guidelines from the International Society of Thrombosis and Haemostasis that recommend serial complete blood counts, with white blood cell differential and assessment of D-dimer, prothrombin time, and fibrinogen for hospitalized patients with COVID-19. The authors also cited guidelines published in the Journal of the American College of Cardiology that recommend routine risk assessment for venous thromboembolism in all hospitalized patients with COVID-19 and the consideration of standard-dose pharmaco-prophylaxis in patients who lack absolute contraindications.
Empiric use of higher-than-routine prophylactic-dose or therapeutic-dose anticoagulation in ICU patients in the absence of proven thromboses has been implemented in some institutions, Dr. Gupta and colleagues noted. Parenteral anticoagulants (such as low-molecular-weight or unfractionated heparin) are preferred to oral anticoagulants because of short half-life, available reversal agents, and the potential for drug interactions between oral agents and antiviral and/or antibacterial treatment, according to the authors.
They wrote that randomized clinical trials “will be crucial to establishing effective and safe strategies” for anticoagulation in COVID-19 patients. To this point, few randomized trials have been published to guide management of COVID-19–associated extrapulmonary manifestations, including CAC.
Research priorities
A more complete understanding of the organ-specific pathophysiology of this multisystem disease is vital, according to Dr. Gupta and colleagues.
“Regional, national, and international collaborations of clinicians and scientists focused on high-quality, transparent, ethical, and evidence-based research practices would help propel the global community toward achieving success against this pandemic,” the authors wrote.
They noted that common definitions and data standards for research are key for cross-institutional and international collaborations.
Initial attention to high-quality prospective scientific documentation standards would have been valuable and will be required for dedicated trials to address the multisystem effects of COVID-19.
Community of learners
As much as at any prior time in their careers, during the COVID-19 pandemic, health care providers have been enveloped in a community of learners – a group of people who share values and beliefs and who actively engage in learning from one another.
Through a patchwork of sources – news media, social media, traditional medical journals, general and COVID-focused meetings, and, most importantly, patients – we have been living in a learning-centered environment. Academicians, clinicians, practicing physicians, researchers, patients, family members, and caregivers have been actively and intentionally building a knowledge base together.
Through their published review, Dr. Gupta and colleagues have contributed meaningfully to the understanding our learning community has of the various extrapulmonary manifestations of COVID-19. The authors have provided a nice template for further research and clinical advances.
Dr. Gupta and colleagues disclosed financial relationships with a range of pharmaceutical companies and other organizations.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Source: Gupta A et al. Nat Med. 2020 Jul;26(7):1017-32.
While SARS-CoV-2 causes frequent and potentially severe pulmonary disease, extrapulmonary manifestations may be a prominent part of the clinical spectrum, according to a review published in Nature Medicine.
In this comprehensive literature review, Aakriti Gupta, MD, of New York-Presbyterian/Columbia University Irving Medical Center and colleagues detailed the epidemiologic and clinical multisystem effects of COVID-19. The authors explained what is known and/or suspected about the pathophysiology of those effects and outlined the resultant management considerations.
Key mechanisms for multiorgan injury include direct viral toxicity, endothelial cell damage with inflammatory mediation of thrombosis, aberrant immune response, and dysregulation of the renin-angiotensin-aldosterone system.
The relative importance of each pathway in the clinical presentation of COVID-19 and the mechanism for extrapulmonary spread of SARS-CoV-2 infection are imperfectly understood, Dr. Gupta and colleagues noted.
As for the hematologic effects of COVID-19, patients may present with several laboratory abnormalities, but the most clinically relevant complications are thromboembolic.
COVID-19-associated coagulopathy
Dr. Gupta and colleagues noted that COVID-19–associated coagulopathy (CAC) is accompanied by elevated levels of D-dimer and fibrinogen, with minor abnormalities in prothrombin time, activated partial thromboplastin time, and platelet counts in the initial stage of infection.
Elevated D-dimer levels have been reported in up to 46% of hospitalized patients, and a longitudinal increase while hospitalized is associated with higher mortality.
In initial reports from China and the Netherlands, thrombotic complications were seen in up to 30% of COVID-19 patients in ICUs. Thromboembolic events have been reported in 17%-22% of critically ill COVID-19 patients in studies from Italy and France.
Globally, in severely affected COVID-19 patients, there have been reports of thromboses in intravenous catheters and extracorporeal circuits as well as arterial vascular occlusive events, including myocardial infarction, acute limb ischemia, and stroke.
There have been multiple small studies in which critically ill COVID-19 patients were routinely screened for thrombotic disease. In these studies, rates of thrombotic complications ranged from 69% to 85%, despite thromboprophylaxis. Variability in prophylactic and screening protocols explain discrepancies in event rates.
Pathophysiology
The abnormally high blood levels of D-dimer and fibrinogen during the early stages of SARS-CoV-2 infection are reflective of excessive inflammation rather than overt disseminated intravascular coagulation (DIC), which may develop in later stages of illness, according to Dr. Gupta and colleagues. The authors theorized that uninhibited inflammation, along with hypoxia and direct viral-mediated cellular injury, contribute to thrombotic complications in COVID-19 patients.
“The increased expression of ACE2 in endothelial cells after infection with SARS-CoV-2 may perpetuate a vicious cycle of endothelialitis that promotes thromboinflammation,” the authors wrote. “Collectively, hemostatic and inflammatory changes, which reflect endothelial damage and activation as well as critical illness, constitute a prothrombotic milieu.”
The authors noted that small autopsy series have shown high rates of microvascular and macrovascular thromboses, particularly in the pulmonary circulation, in COVID-19 patients.
Management considerations
Dr. Gupta and colleagues referenced interim guidelines from the International Society of Thrombosis and Haemostasis that recommend serial complete blood counts, with white blood cell differential and assessment of D-dimer, prothrombin time, and fibrinogen for hospitalized patients with COVID-19. The authors also cited guidelines published in the Journal of the American College of Cardiology that recommend routine risk assessment for venous thromboembolism in all hospitalized patients with COVID-19 and the consideration of standard-dose pharmaco-prophylaxis in patients who lack absolute contraindications.
Empiric use of higher-than-routine prophylactic-dose or therapeutic-dose anticoagulation in ICU patients in the absence of proven thromboses has been implemented in some institutions, Dr. Gupta and colleagues noted. Parenteral anticoagulants (such as low-molecular-weight or unfractionated heparin) are preferred to oral anticoagulants because of short half-life, available reversal agents, and the potential for drug interactions between oral agents and antiviral and/or antibacterial treatment, according to the authors.
They wrote that randomized clinical trials “will be crucial to establishing effective and safe strategies” for anticoagulation in COVID-19 patients. To this point, few randomized trials have been published to guide management of COVID-19–associated extrapulmonary manifestations, including CAC.
Research priorities
A more complete understanding of the organ-specific pathophysiology of this multisystem disease is vital, according to Dr. Gupta and colleagues.
“Regional, national, and international collaborations of clinicians and scientists focused on high-quality, transparent, ethical, and evidence-based research practices would help propel the global community toward achieving success against this pandemic,” the authors wrote.
They noted that common definitions and data standards for research are key for cross-institutional and international collaborations.
Initial attention to high-quality prospective scientific documentation standards would have been valuable and will be required for dedicated trials to address the multisystem effects of COVID-19.
Community of learners
As much as at any prior time in their careers, during the COVID-19 pandemic, health care providers have been enveloped in a community of learners – a group of people who share values and beliefs and who actively engage in learning from one another.
Through a patchwork of sources – news media, social media, traditional medical journals, general and COVID-focused meetings, and, most importantly, patients – we have been living in a learning-centered environment. Academicians, clinicians, practicing physicians, researchers, patients, family members, and caregivers have been actively and intentionally building a knowledge base together.
Through their published review, Dr. Gupta and colleagues have contributed meaningfully to the understanding our learning community has of the various extrapulmonary manifestations of COVID-19. The authors have provided a nice template for further research and clinical advances.
Dr. Gupta and colleagues disclosed financial relationships with a range of pharmaceutical companies and other organizations.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Source: Gupta A et al. Nat Med. 2020 Jul;26(7):1017-32.
FROM NATURE MEDICINE
A Multidisciplinary Ambulation Protocol to Reduce Postoperative Venous Thromboembolism After Colorectal Surgery
From the Department of Surgery, Washington University School of Medicine, St. Louis, MO.
Abstract
Background: Patients undergoing colorectal surgery are at high risk for postoperative venous thromboembolism (VTE). Early ambulation has been encouraged to lower rates of VTE, but evidence demonstrating its effectiveness outside of a bundle is limited.
Objective: To create a multidisciplinary ambulation protocol in an effort to reduce postoperative VTE.
Methods: A single-center, retrospective, comparative study of patients who underwent colectomy or proctectomy was conducted. Outcomes of patients operated on prior to protocol implementation were compared with a cohort after implementation. The intervention studied was the implementation of a multidisciplinary ambulation protocol. The primary endpoint was postoperative VTE.
Results: There was no difference between the pre-intervention group (n = 1762) and the postintervention group (n = 253) in terms of sex, race, origin, emergency status, operative time, and the majority of medical comorbidities (with the exception of smoking status and congestive heart failure). After the protocol was implemented, ambulation rates on postoperative days 0, 1, and 2 improved from 36.4%, 47.3%, and 50.2% to 36.8%, 74.7%, and 82.6%, respectively The VTE rate in the pre-intervention group was 2.7% versus a rate of 0.4% in the postintervention group (P = 0.02).
Conclusion: Creation of an ambulation protocol is associated with a significant reduction in VTE. Commitment from patients, families, nurses, physician extenders, and physicians is critical to the success of the program.
Keywords: VTE; pulmonary embolism; deep vein thrombosis; postoperative; quality improvement.
Postoperative venous thromboembolism (VTE) is a significant source of morbidity, mortality, and cost.1,2 Colorectal surgery patients are at particularly high risk for VTE due to positioning during surgery, pelvic dissection, and other conditions often found in these patients, such as cancer and inflammatory bowel disease.3 A National Surgical Quality Improvement Program (NSQIP) analysis demonstrated an overall rate of VTE in colorectal surgery patients of 2.4%, although other studies have demonstrated rates up to 9%, even in those receiving appropriate chemoprophylaxis.4-6 Many of these VTEs occur in the postdischarge setting. In a NSQIP study of colorectal surgery patients, the rate of VTE between discharge and 30 days was 0.47%.7 The cost burdenfor a postoperative VTE has been estimated to be more than $18,000.8
Studies from NSQIP have identified multiple factors associated with VTE in colorectal surgery patients, but NSQIP does not record ambulation as a standard variable.9 Multiple strategies have been implemented to reduce postoperative VTE. Often, these studies focus on increasing compliance with appropriate chemoprophylaxis, risk stratification, or bundling multiple strategies.10,11 However, despite the fact that postsurgical ambulation is widely encouraged and recommended by the American Society of Colon and Rectal Surgeons clinical practice guidelines, there is little evidence demonstrating the role of ambulation alone in the reduction of VTE.4,12 The purpose of this study was to create a multidisciplinary protocol to increase postoperative ambulation and evaluate its effect on VTE.
Methods
Setting
This study was conducted at a single academic tertiary care center.
Patients and Outcome Measures
All patients undergoing colectomy or proctectomy by surgeons in the section of colon and rectal surgery at a single institution between January 2011 and March 2017 were included. Colectomy and proctectomy were defined by CPT codes 44140, 44141, 44143, 44144, 44145, 44146, 44147, 44150, 44151, 44155, 44156, 44157, 44158, 44160, 44204, 44205, 44206, 44207, 44208, 44210, 44211, 44212, 44213, 45110, 45111, 45112, 45113, 45114, 45116, 45119, 45120, 45121, 45123, 45126, 45160, 45395, and 45397. The primary outcome of VTE within 30 days, including deep venous thrombosis (DVT) and pulmonary embolism (PE), was measured using institution-specific data from NSQIP in both the pre-intervention and postintervention setting. The occurrence of both DVT and PE in 1 patient was counted as a single event of VTE. Ambulation rate on postoperative day (POD) 0, 1, and 2 was calculated by NSQIP in the pre-intervention setting (our institution-specific NSQIP recorded ambulation data for an unrelated project) and by review of the electronic health record in the postintervention setting, as this institution-specific variable was no longer being collected. Ambulation was defined as getting out of bed and taking at least 1 step. The threshold for ambulating each day was once on POD 0 and twice on PODs 1 and 2. Patients with missing ambulation data were excluded from the analysis. Both prior to and throughout the intervention, all patients were given VTE chemoprophylaxis with either low-dose unfractionated heparin or low-molecular-weight heparin prior to induction of anesthesia, with chemoprophylaxis extending an additional 21 days after discharge (unless specifically contraindicated); sequential compression devices; and standard orders to ambulate 3 times daily from POD 0 as part of the standard Enhanced Recovery After Surgery protocol.
Analysis
Statistical analysis was performed using univariate analysis. Chi-square test and univariate logistic regression were used to determine the association between ambulation rates and VTE in the pre-intervention group. Chi-square test was also used to compare ambulation and VTE rates between the pre-intervention and postintervention groups. Plan-Do-Study-Act (PDSA) cycle fidelity (the degree to which a PDSA cycle is carried out in accordance with the guiding principles of its use) was measured by recording the ambulation rates both before and after the intervention.13 Statistical analysis was performed using SAS Version 9.4 (SAS Institute, Cary, NC). This study was reviewed by the Washington University School of Medicine Institutional Review Board and deemed to be quality improvement, not human subjects research, and therefore did not require formal approval.
Baseline Outcome Rates
A total of 1762 patients were identified during the pre-intervention period. The overall VTE rate in the pre-intervention group was 2.7% (n = 48), with 39 DVTs (2.2%) and 13 PEs (0.7%). Pre-intervention ambulation data were available on 590 patients. Baseline ambulation rates on PODs 0, 1, and 2 were 36.4% (213/590), 47.3% (279/590), and 50.2% (296/590), respectively. Patients who did not ambulate on POD 0 had a VTE rate of 4.3%, as compared to 0.9% in those who did ambulate (Table 1). Patients who did not ambulate twice on POD 1 had a VTE rate of 4.8%, compared to 1.1% in those who did ambulate (odds ratio [OR], 4.66; 95% confidence interval [CI], 1.34 to 16.28). Patients who did not ambulate twice on POD 2 had a VTE rate of 5.4%, compared to 0.7% in those who did. Finally, those who ambulated twice on both PODs 1 and 2 had a 0% rate of VTE, compared to 4.9% in those who did not ambulate on both PODs.
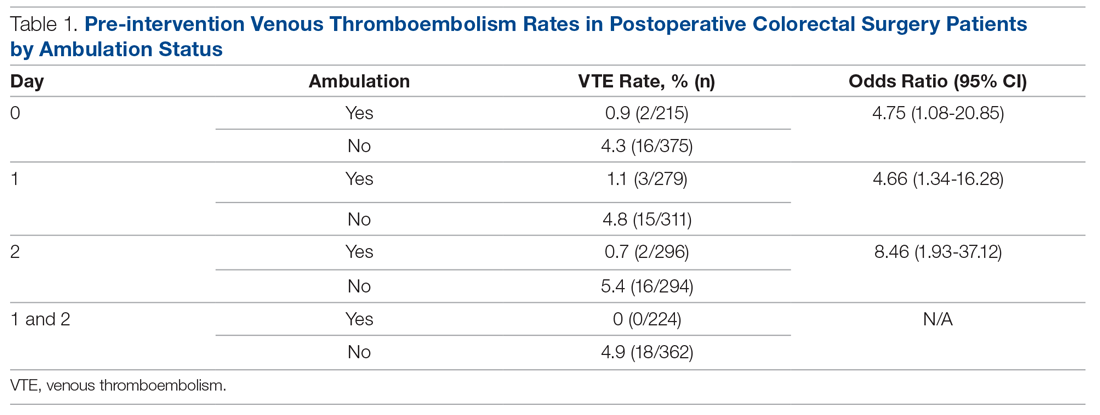
Ambulation Protocol
After baseline outcome rates had been established, a multidisciplinary team of medical assistants, nurses, nurse practitioners, and physicians worked together to identify all processes that involved postoperative ambulation. Given the significant differences in VTE rates between patients who ambulated and those that did not, we created a multidisciplinary ambulation protocol using the PDSA method.14 Multiple points of patient contact were chosen for intervention, and the ambulation protocol was implemented in June 2018 and continued for 7 months.
Patients were observed from their initial office visit with a surgeon, during the preoperative education encounter, and in the operating room and on the surgical ward until discharge. Representatives from multiple disciplines who encountered patients at various times in the process, including medical assistants, patient care technicians, nurses, nurse practitioners, physical therapists, and physicians, participated in a kick-off meeting to identify difficulties they encounter when encouraging patient ambulation. The following 4 areas were identified.
Barriers to Patient Ambulation
Patient Expectations. Patients did not appear to have a clear expectation of what their ambulation goals were postoperatively, despite the fact that each patient is given an operative pathway booklet that includes their goals for each day, including ambulation. The consensus was that patients were overwhelmed with the amount of information and, oftentimes, the severity of their diagnosis, so the information regarding ambulation was not retained. Nurses commented that patients frequently stated that they did not think their surgeon wanted them to get out of bed postoperatively.
Electronic Orders. There was confusion within the nursing staff regarding orders in the electronic health record compared to physician expectations. Orders stated patients should ambulate 3 times daily, but did not specify on which postoperative day this should start. Often, nursing verbal sign-out from the post-anesthesia care unit (PACU) would be an order for bedrest, despite no clear origin of this order. This created confusion among the nursing staff as to what the appropriate ambulation orders should be.
Nursing Workflow. The initial state of the nursing workflow was not conducive to evaluating for, or assisting with, ambulation. With no set time to assist and evaluate patients for ambulation, it turned into a task nurses needed to accomplish when they had extra time. With increasing demands of charting in the electronic health record, nurses often had to skip ambulation in order to accomplish other tasks.
Family Expectations. In addition to patient expectations, family members often had expectations that were not congruent with the planned postoperative course. Nurses stated family members would often tell them that they did not feel that their family member should be ambulating so soon after surgery. Often these family members had not attended preoperative education sessions with the patient. This was compounded by the uncertainty among the nursing staff regarding what exactly the ambulation orders were.
Interventions
Targeted interventions were created to address these 4 barriers to ambulation identified by staff.
Preoperative Education. Although all elective patients received a printed operative pathway booklet describing daily goals, including ambulation, patients still did not have a sufficient understanding of what was expected of them. The education session was modified to increase the time spent on both the expectation for and the rationale behind ambulation. That section of the education session ended with a verbal commitment and read-back of the expectations for ambulation by the patient.
Clarification of Electronic Orders. Postoperative orders within the colorectal standard pathway were changed, including specific time frames and frequency, to match the information provided in the patient education booklet. These orders were for ambulation within 4 hours of arrival to the floor, and the orders also noted that no patient should be on bedrest unless explicitly stated. From POD 1, all patients were to ambulate at least twice daily for the remainder of the hospital stay (patients were encouraged to walk 4 times daily, but we set a minimum expectation of twice daily for the order set). These orders were clarified with in-person meetings with the nursing staff and leadership from the PACU and the colorectal surgical ward.
Adjusted Nursing Workflow. Nurses were interviewed and asked to create a plan regarding how they could better incorporate ambulation into their daily workflow. Ambulation assessment was incorporated into the twice-per-shift recording of vital signs and patient safety assessment. This was recorded into the electronic health record at the same time as the patients’ vital signs. This allowed nurses to keep track of which patients would need extra assistance in ambulation and which patients were doing well on their own with the assistance of family. It also helped focus the resources of physical therapy and the single ambulation technician on the floor and to assist patients who needed more assistance.
Creation of Ambulation Encouragement Signs. The authors discovered that despite patients being told preoperatively about ambulation expectations, friends and family are not always included in these conversations. As nurses frequently cited both patients and family as reasons patients thought they should not walk, multiple signs inviting patients to take an active role in their recovery by ambulating were created and placed around the unit. The signs outlined the expectations of being out of bed and taking at least 1 step on the day of surgery and walking at least 4 times per day thereafter. In addition, we addressed frequently asked questions around issues such as walking with intravenous poles and urinary catheters. The posters were signed by all staff colorectal surgeons.
Results
Over the course of 7 months (June 2018 to December 2018), 253 postintervention patients were identified (Table 2). There was no difference between the pre-intervention group (n = 1762) and the postintervention group in terms of sex, race, origin, emergency status, operative time, and the majority of medical comorbidities (with the exception of smoking status and congestive heart failure). The postintervention group was slightly older (60 versus 57 years) and had a higher percentage of patients with an American Society of Anesthesiologists physical status score greater than 2 (66.8% versus 51.2%). The postintervention group also had higher rates of both malignancy (53.4% versus 33.3%) and inflammatory bowel disease (18.2% versus 14.4%).
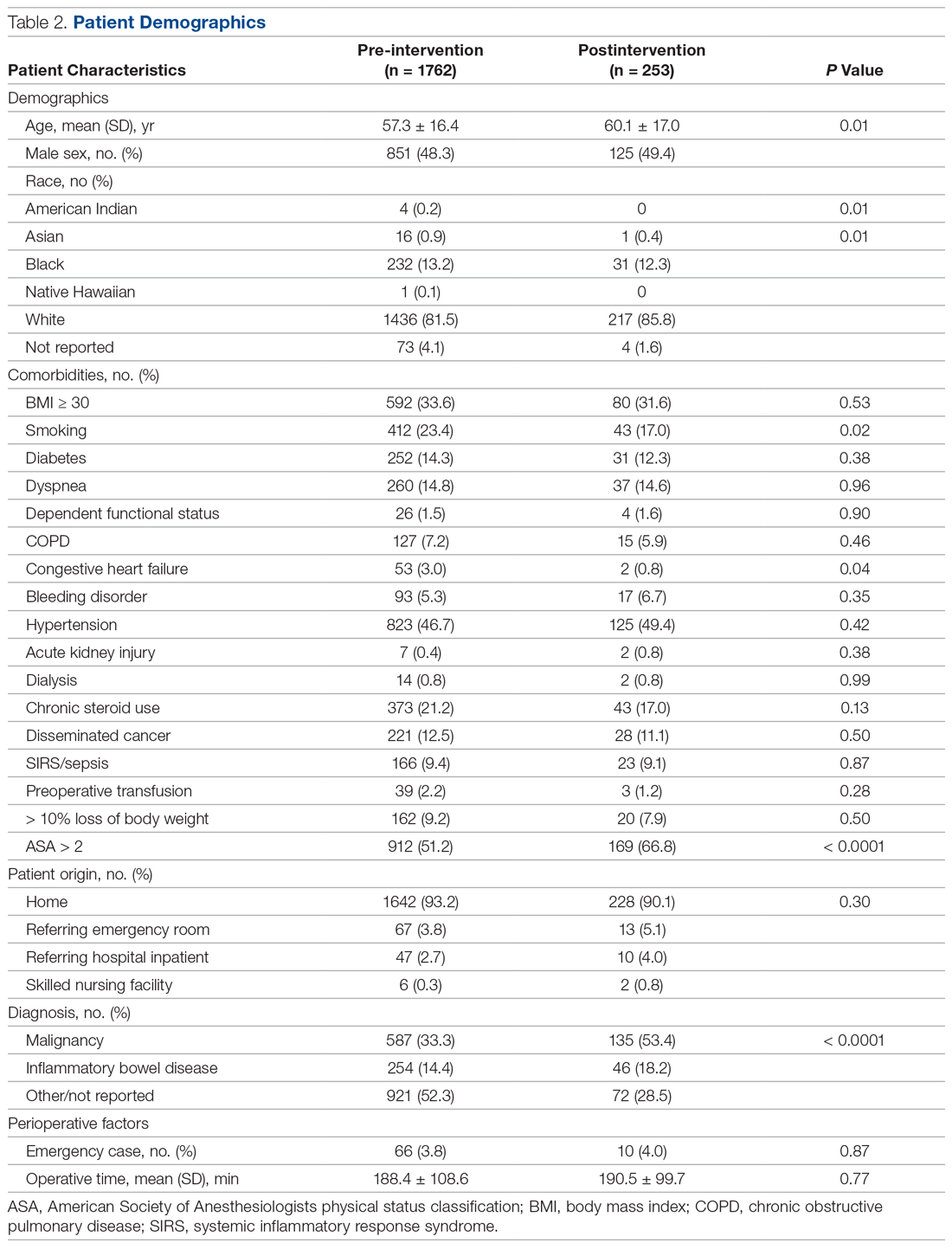
The fidelity of the PDSA cycle was measured by pre-intervention and postintervention ambulation rates. Ambulation rates on POD 0, 1, and 2 improved from 36.4%, 47.3%, and 50.2% to 36.8%, 74.7%, and 82.6%, respectively (Table 3). The VTE rate decreased from 2.7% to 0.4% (P = 0.02), with 1 DVT and 0 PEs. It should be noted that the only patient who developed a VTE postintervention did not ambulate on PODs 0, 1, or 2.
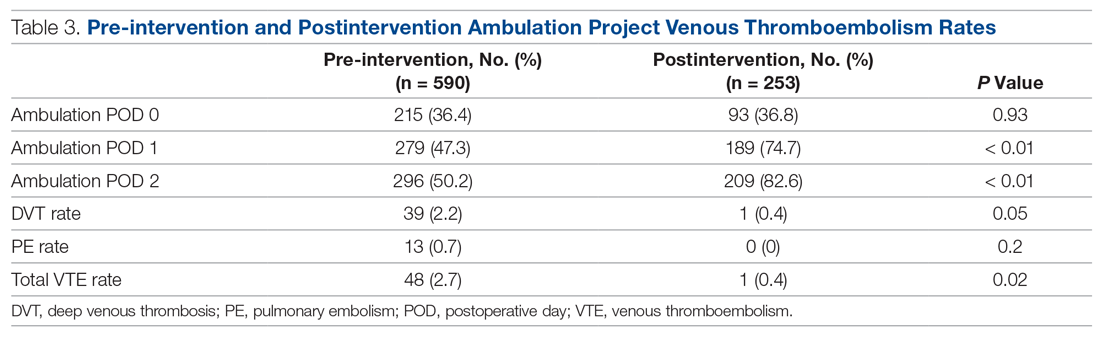
Discussion
Postoperative VTE is a severe complication for postoperative colorectal surgery patients. Previous studies have demonstrated that increasing ambulation is associated with a lower rate of overall complications, and, when incorporated into a bundle, is associated with decreased rates of VTE.11,15 However, this is the first study to our knowledge demonstrating that creation of an ambulation protocol alone is associated with a decrease in VTE.
Analysis of pre-intervention data demonstrated a strong association between ambulation and an absence of VTE. No patient who ambulated on PODs 0, 1, and 2 developed a VTE. Based on those results, we moved forward with creating the ambulation protocol. While ambulation stayed stable on POD 0, there were 60% and 65% increases on PODs 1 and 2, respectively. Nurses cited late arrival to the floor for second and third start cases as the primary difficulty in getting patients to ambulate more on POD 0.
We believe the key to the success of the ambulation protocol was its multidisciplinary nature. Certainly, the easiest way to create an ambulation protocol is to change the postoperative orders to state patients must walk 4 times per day. However, if the nursing staff is unable or unwilling to carry out these orders, the orders serve little purpose. In order to make lasting changes, all stakeholders in the process must be identified. In our case, stakeholders included surgery and nursing leadership, surgeons, nurse practitioners, nurses, medical assistants, physical therapists, patient care technicians, and patients. This is where we utilized kaizen, a core principle of Lean methodology that empowers employees at the level of the work being carried out to propose ideas for improvement.16 From the beginning of the patient experience, the health care practitioners who were carrying out each step of the process were best able to identify the problems and create solutions. In addition, stakeholders were given regular updates regarding how their efforts were increasing ambulation rates and the results at the end of the study period.
This study also demonstrates that, in a health care system increasingly focused on both quality and cost, significant improvements in quality can be made without increasing cost or resource utilization. Early in the process, it was proposed that the only way to increase the ambulation rate would be to increase the number of physical therapists, nurses, and nursing assistants. However, after identifying the root causes of the problem, the solutions had more to do with improving workflow and fixing problem areas identified by the staff.
In addition to having a positive effect on the outcome studied, collaborative projects such as this between physicians and nurses may lead to increased nursing job satisfaction. A meta-analysis of 31 studies identified nurse-physician collaboration and autonomy as 2 factors that correlate most strongly with nursing satisfaction.17 A Cochrane review also suggests that practice-based interprofessional collaboration may lead to improved health care processes and outcomes.18
This study has several limitations. Pre-intervention ambulation rates were abstracted from institution-specific NSQIP data, and missing data were excluded from analysis. Also, due to the retrospective collection of the pre-intervention data, the distance of ambulation could not be quantified. The bar for ambulation is low, as patients were only required to get out of bed and walk 1 step. However, we feel that getting out of bed and taking even 1 step is substantially better than complete bedrest. It is likely that once patients cross the threshold of taking 1 step, they are more likely to ambulate. An area of future study may be to more precisely define the relationship between the quantity of ambulation in steps and its effect on VTE. Finally, we acknowledge that while there is no direct increase in costs, implementing an ambulation protocol does take time from all who participate in the project.
Conclusion
Creation of an ambulation protocol is associated with a decrease in postoperative VTE rates in colorectal surgery patients. A multidisciplinary approach is critical to identify the underlying problems and propose effective solutions. Further studies are required to better correlate the distance of ambulation and its effect on VTE. However, this study shows that even a minimum of 1 step is associated with decreased VTE rates.
Corresponding author: Aneel Damle, MD, MBA, Colon & Rectal Surgery Associates, 3433 Broadway St. NE, Suite 115, Minneapolis, MN 55413; [email protected].
Financial disclosures: None.
1. Gangireddy C, Rectenwald JR, Upchurch GR, et al. Risk factors and clinical impact of postoperative symptomatic venous thromboembolism. J Vasc Surg. 2007;45:341-342.
2. Newhook TE, LaPar DJ, Walters DM, et al. Impact of postoperative venous thromboembolism on postoperative morbidity, mortality, and resource utilization after hepatectomy. Am Surg. 2015;81:1216-1223.
3. Bergqvist D. Venous thromboembolism: a review of risk and prevention in colorectal surgery patients. Dis Colon Rectum. 2006;49:1620-1628.
4. Fleming F, Gaertner W, Ternent CA, et al. The American society of colon and rectal surgeons clinical practice guideline for the prevention of venous thromboembolic disease in colorectal surgery. Dis Colon Rectum. 2018;61:14-20.
5. McLeod RS, Geerts WH, Sniderman KW, et al. Canadian Colorectal Surgery DVT Prophylaxis Trial investigators. Subcutaneous heparin versus low-molecular-weight heparin as thromboprophylaxis in patients undergoing colorectal surgery: results of the Canadian colorectal DV prophylaxis trial: a randomized, double-blind trial. Ann Surg. 2001;233:438-444.
6. Shapiro R, Vogel JD, Kiran RP. Risk of postoperative venous thromboembolism after laparoscopic and open colorectal surgery: an additional benefit of the minimally invasive approach? Dis Colon Rectum. 2011;54:1496-1502.
7. Dimick JB, Chen SL, Taheri PA, et al. Hospital costs associated with surgical complications: a report from the private-sector National Surgical Quality Improvement Program. J Am Coll Surg. 2004;199:531-537.
8. Fleming FJ, Kim MJ, Salloum RM, et al. How much do we need to worry about venous thromboembolism after hospital discharge? A study of colorectal surgery patients using the National Surgical Quality Improvement Program database. Dis Colon Rectum. 2010;53:1355-1360.
9. ACS NSQIP. User guide for the 2016 ACS NSQIP participant use data file (PUF). 2017. www.facs.org/~/media/files/quality%20programs/nsqip/nsqip_puf_userguide_2016.ashx Accessed July 10, 2020.
10. Caprini JA. Risk assessment as a guide for the prevention of the many faces of venous thromboembolism. Am J Surg. 2010;199(1 Suppl):S3-S10.
11. Cassidy MR, Rosenkranz P, McAney D. Reducing postoperative venous thromboembolism complications with a standardized risk-stratified prophylaxis protocol and mobilization protocol. J Am Coll Surg. 2014;218:1095-1104.
12. Lau BD, Streiff MB, Kraus PS, et al. No evidence to support ambulation for reducing postoperative venous thromboembolism. J Am Coll Surg. 2014;219:1101-1103.
13. McNicholas C, Lennox L, Woodcock T, et al. Evolving quality improvement support strategies to improve Plan–Do–Study–Act cycle fidelity: a retrospective mixed-methods study. BMJ Qual Saf. 2019;28:356-365.
14. Taylor MJ, McNicholas C, Nicolay C, et al. Systematic review of the application of the plan–do–study–act method to improve quality in healthcare. BMC Qual Saf. 2014;23:290-298.
15. Nevo Y, Shaltiel T, Constantini N, et al. Effect of ambulation and physical activity on postoperative complications. J Am Coll Surg. 2016;223(Suppl 1):S61.
16. Mazzocato P, Stenfors-Hayes T, von Thiele Schwarz U, et al. Kaizen practice in healthcare: a qualitative analysis of hospital employees’ suggestions for improvement. BMJ Open. 2016;6:e012256.
17. Zangaro GA, Soeken KL. A meta-analysis of studies of nurses’ job satisfaction. Res Nursing Health. 2007;30:445-458.
18. Reeves S, Pelone F, Harrison R, et al. Interprofessional collaboration to improve professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2017;6(6):CD000072.
From the Department of Surgery, Washington University School of Medicine, St. Louis, MO.
Abstract
Background: Patients undergoing colorectal surgery are at high risk for postoperative venous thromboembolism (VTE). Early ambulation has been encouraged to lower rates of VTE, but evidence demonstrating its effectiveness outside of a bundle is limited.
Objective: To create a multidisciplinary ambulation protocol in an effort to reduce postoperative VTE.
Methods: A single-center, retrospective, comparative study of patients who underwent colectomy or proctectomy was conducted. Outcomes of patients operated on prior to protocol implementation were compared with a cohort after implementation. The intervention studied was the implementation of a multidisciplinary ambulation protocol. The primary endpoint was postoperative VTE.
Results: There was no difference between the pre-intervention group (n = 1762) and the postintervention group (n = 253) in terms of sex, race, origin, emergency status, operative time, and the majority of medical comorbidities (with the exception of smoking status and congestive heart failure). After the protocol was implemented, ambulation rates on postoperative days 0, 1, and 2 improved from 36.4%, 47.3%, and 50.2% to 36.8%, 74.7%, and 82.6%, respectively The VTE rate in the pre-intervention group was 2.7% versus a rate of 0.4% in the postintervention group (P = 0.02).
Conclusion: Creation of an ambulation protocol is associated with a significant reduction in VTE. Commitment from patients, families, nurses, physician extenders, and physicians is critical to the success of the program.
Keywords: VTE; pulmonary embolism; deep vein thrombosis; postoperative; quality improvement.
Postoperative venous thromboembolism (VTE) is a significant source of morbidity, mortality, and cost.1,2 Colorectal surgery patients are at particularly high risk for VTE due to positioning during surgery, pelvic dissection, and other conditions often found in these patients, such as cancer and inflammatory bowel disease.3 A National Surgical Quality Improvement Program (NSQIP) analysis demonstrated an overall rate of VTE in colorectal surgery patients of 2.4%, although other studies have demonstrated rates up to 9%, even in those receiving appropriate chemoprophylaxis.4-6 Many of these VTEs occur in the postdischarge setting. In a NSQIP study of colorectal surgery patients, the rate of VTE between discharge and 30 days was 0.47%.7 The cost burdenfor a postoperative VTE has been estimated to be more than $18,000.8
Studies from NSQIP have identified multiple factors associated with VTE in colorectal surgery patients, but NSQIP does not record ambulation as a standard variable.9 Multiple strategies have been implemented to reduce postoperative VTE. Often, these studies focus on increasing compliance with appropriate chemoprophylaxis, risk stratification, or bundling multiple strategies.10,11 However, despite the fact that postsurgical ambulation is widely encouraged and recommended by the American Society of Colon and Rectal Surgeons clinical practice guidelines, there is little evidence demonstrating the role of ambulation alone in the reduction of VTE.4,12 The purpose of this study was to create a multidisciplinary protocol to increase postoperative ambulation and evaluate its effect on VTE.
Methods
Setting
This study was conducted at a single academic tertiary care center.
Patients and Outcome Measures
All patients undergoing colectomy or proctectomy by surgeons in the section of colon and rectal surgery at a single institution between January 2011 and March 2017 were included. Colectomy and proctectomy were defined by CPT codes 44140, 44141, 44143, 44144, 44145, 44146, 44147, 44150, 44151, 44155, 44156, 44157, 44158, 44160, 44204, 44205, 44206, 44207, 44208, 44210, 44211, 44212, 44213, 45110, 45111, 45112, 45113, 45114, 45116, 45119, 45120, 45121, 45123, 45126, 45160, 45395, and 45397. The primary outcome of VTE within 30 days, including deep venous thrombosis (DVT) and pulmonary embolism (PE), was measured using institution-specific data from NSQIP in both the pre-intervention and postintervention setting. The occurrence of both DVT and PE in 1 patient was counted as a single event of VTE. Ambulation rate on postoperative day (POD) 0, 1, and 2 was calculated by NSQIP in the pre-intervention setting (our institution-specific NSQIP recorded ambulation data for an unrelated project) and by review of the electronic health record in the postintervention setting, as this institution-specific variable was no longer being collected. Ambulation was defined as getting out of bed and taking at least 1 step. The threshold for ambulating each day was once on POD 0 and twice on PODs 1 and 2. Patients with missing ambulation data were excluded from the analysis. Both prior to and throughout the intervention, all patients were given VTE chemoprophylaxis with either low-dose unfractionated heparin or low-molecular-weight heparin prior to induction of anesthesia, with chemoprophylaxis extending an additional 21 days after discharge (unless specifically contraindicated); sequential compression devices; and standard orders to ambulate 3 times daily from POD 0 as part of the standard Enhanced Recovery After Surgery protocol.
Analysis
Statistical analysis was performed using univariate analysis. Chi-square test and univariate logistic regression were used to determine the association between ambulation rates and VTE in the pre-intervention group. Chi-square test was also used to compare ambulation and VTE rates between the pre-intervention and postintervention groups. Plan-Do-Study-Act (PDSA) cycle fidelity (the degree to which a PDSA cycle is carried out in accordance with the guiding principles of its use) was measured by recording the ambulation rates both before and after the intervention.13 Statistical analysis was performed using SAS Version 9.4 (SAS Institute, Cary, NC). This study was reviewed by the Washington University School of Medicine Institutional Review Board and deemed to be quality improvement, not human subjects research, and therefore did not require formal approval.
Baseline Outcome Rates
A total of 1762 patients were identified during the pre-intervention period. The overall VTE rate in the pre-intervention group was 2.7% (n = 48), with 39 DVTs (2.2%) and 13 PEs (0.7%). Pre-intervention ambulation data were available on 590 patients. Baseline ambulation rates on PODs 0, 1, and 2 were 36.4% (213/590), 47.3% (279/590), and 50.2% (296/590), respectively. Patients who did not ambulate on POD 0 had a VTE rate of 4.3%, as compared to 0.9% in those who did ambulate (Table 1). Patients who did not ambulate twice on POD 1 had a VTE rate of 4.8%, compared to 1.1% in those who did ambulate (odds ratio [OR], 4.66; 95% confidence interval [CI], 1.34 to 16.28). Patients who did not ambulate twice on POD 2 had a VTE rate of 5.4%, compared to 0.7% in those who did. Finally, those who ambulated twice on both PODs 1 and 2 had a 0% rate of VTE, compared to 4.9% in those who did not ambulate on both PODs.

Ambulation Protocol
After baseline outcome rates had been established, a multidisciplinary team of medical assistants, nurses, nurse practitioners, and physicians worked together to identify all processes that involved postoperative ambulation. Given the significant differences in VTE rates between patients who ambulated and those that did not, we created a multidisciplinary ambulation protocol using the PDSA method.14 Multiple points of patient contact were chosen for intervention, and the ambulation protocol was implemented in June 2018 and continued for 7 months.
Patients were observed from their initial office visit with a surgeon, during the preoperative education encounter, and in the operating room and on the surgical ward until discharge. Representatives from multiple disciplines who encountered patients at various times in the process, including medical assistants, patient care technicians, nurses, nurse practitioners, physical therapists, and physicians, participated in a kick-off meeting to identify difficulties they encounter when encouraging patient ambulation. The following 4 areas were identified.
Barriers to Patient Ambulation
Patient Expectations. Patients did not appear to have a clear expectation of what their ambulation goals were postoperatively, despite the fact that each patient is given an operative pathway booklet that includes their goals for each day, including ambulation. The consensus was that patients were overwhelmed with the amount of information and, oftentimes, the severity of their diagnosis, so the information regarding ambulation was not retained. Nurses commented that patients frequently stated that they did not think their surgeon wanted them to get out of bed postoperatively.
Electronic Orders. There was confusion within the nursing staff regarding orders in the electronic health record compared to physician expectations. Orders stated patients should ambulate 3 times daily, but did not specify on which postoperative day this should start. Often, nursing verbal sign-out from the post-anesthesia care unit (PACU) would be an order for bedrest, despite no clear origin of this order. This created confusion among the nursing staff as to what the appropriate ambulation orders should be.
Nursing Workflow. The initial state of the nursing workflow was not conducive to evaluating for, or assisting with, ambulation. With no set time to assist and evaluate patients for ambulation, it turned into a task nurses needed to accomplish when they had extra time. With increasing demands of charting in the electronic health record, nurses often had to skip ambulation in order to accomplish other tasks.
Family Expectations. In addition to patient expectations, family members often had expectations that were not congruent with the planned postoperative course. Nurses stated family members would often tell them that they did not feel that their family member should be ambulating so soon after surgery. Often these family members had not attended preoperative education sessions with the patient. This was compounded by the uncertainty among the nursing staff regarding what exactly the ambulation orders were.
Interventions
Targeted interventions were created to address these 4 barriers to ambulation identified by staff.
Preoperative Education. Although all elective patients received a printed operative pathway booklet describing daily goals, including ambulation, patients still did not have a sufficient understanding of what was expected of them. The education session was modified to increase the time spent on both the expectation for and the rationale behind ambulation. That section of the education session ended with a verbal commitment and read-back of the expectations for ambulation by the patient.
Clarification of Electronic Orders. Postoperative orders within the colorectal standard pathway were changed, including specific time frames and frequency, to match the information provided in the patient education booklet. These orders were for ambulation within 4 hours of arrival to the floor, and the orders also noted that no patient should be on bedrest unless explicitly stated. From POD 1, all patients were to ambulate at least twice daily for the remainder of the hospital stay (patients were encouraged to walk 4 times daily, but we set a minimum expectation of twice daily for the order set). These orders were clarified with in-person meetings with the nursing staff and leadership from the PACU and the colorectal surgical ward.
Adjusted Nursing Workflow. Nurses were interviewed and asked to create a plan regarding how they could better incorporate ambulation into their daily workflow. Ambulation assessment was incorporated into the twice-per-shift recording of vital signs and patient safety assessment. This was recorded into the electronic health record at the same time as the patients’ vital signs. This allowed nurses to keep track of which patients would need extra assistance in ambulation and which patients were doing well on their own with the assistance of family. It also helped focus the resources of physical therapy and the single ambulation technician on the floor and to assist patients who needed more assistance.
Creation of Ambulation Encouragement Signs. The authors discovered that despite patients being told preoperatively about ambulation expectations, friends and family are not always included in these conversations. As nurses frequently cited both patients and family as reasons patients thought they should not walk, multiple signs inviting patients to take an active role in their recovery by ambulating were created and placed around the unit. The signs outlined the expectations of being out of bed and taking at least 1 step on the day of surgery and walking at least 4 times per day thereafter. In addition, we addressed frequently asked questions around issues such as walking with intravenous poles and urinary catheters. The posters were signed by all staff colorectal surgeons.
Results
Over the course of 7 months (June 2018 to December 2018), 253 postintervention patients were identified (Table 2). There was no difference between the pre-intervention group (n = 1762) and the postintervention group in terms of sex, race, origin, emergency status, operative time, and the majority of medical comorbidities (with the exception of smoking status and congestive heart failure). The postintervention group was slightly older (60 versus 57 years) and had a higher percentage of patients with an American Society of Anesthesiologists physical status score greater than 2 (66.8% versus 51.2%). The postintervention group also had higher rates of both malignancy (53.4% versus 33.3%) and inflammatory bowel disease (18.2% versus 14.4%).

The fidelity of the PDSA cycle was measured by pre-intervention and postintervention ambulation rates. Ambulation rates on POD 0, 1, and 2 improved from 36.4%, 47.3%, and 50.2% to 36.8%, 74.7%, and 82.6%, respectively (Table 3). The VTE rate decreased from 2.7% to 0.4% (P = 0.02), with 1 DVT and 0 PEs. It should be noted that the only patient who developed a VTE postintervention did not ambulate on PODs 0, 1, or 2.

Discussion
Postoperative VTE is a severe complication for postoperative colorectal surgery patients. Previous studies have demonstrated that increasing ambulation is associated with a lower rate of overall complications, and, when incorporated into a bundle, is associated with decreased rates of VTE.11,15 However, this is the first study to our knowledge demonstrating that creation of an ambulation protocol alone is associated with a decrease in VTE.
Analysis of pre-intervention data demonstrated a strong association between ambulation and an absence of VTE. No patient who ambulated on PODs 0, 1, and 2 developed a VTE. Based on those results, we moved forward with creating the ambulation protocol. While ambulation stayed stable on POD 0, there were 60% and 65% increases on PODs 1 and 2, respectively. Nurses cited late arrival to the floor for second and third start cases as the primary difficulty in getting patients to ambulate more on POD 0.
We believe the key to the success of the ambulation protocol was its multidisciplinary nature. Certainly, the easiest way to create an ambulation protocol is to change the postoperative orders to state patients must walk 4 times per day. However, if the nursing staff is unable or unwilling to carry out these orders, the orders serve little purpose. In order to make lasting changes, all stakeholders in the process must be identified. In our case, stakeholders included surgery and nursing leadership, surgeons, nurse practitioners, nurses, medical assistants, physical therapists, patient care technicians, and patients. This is where we utilized kaizen, a core principle of Lean methodology that empowers employees at the level of the work being carried out to propose ideas for improvement.16 From the beginning of the patient experience, the health care practitioners who were carrying out each step of the process were best able to identify the problems and create solutions. In addition, stakeholders were given regular updates regarding how their efforts were increasing ambulation rates and the results at the end of the study period.
This study also demonstrates that, in a health care system increasingly focused on both quality and cost, significant improvements in quality can be made without increasing cost or resource utilization. Early in the process, it was proposed that the only way to increase the ambulation rate would be to increase the number of physical therapists, nurses, and nursing assistants. However, after identifying the root causes of the problem, the solutions had more to do with improving workflow and fixing problem areas identified by the staff.
In addition to having a positive effect on the outcome studied, collaborative projects such as this between physicians and nurses may lead to increased nursing job satisfaction. A meta-analysis of 31 studies identified nurse-physician collaboration and autonomy as 2 factors that correlate most strongly with nursing satisfaction.17 A Cochrane review also suggests that practice-based interprofessional collaboration may lead to improved health care processes and outcomes.18
This study has several limitations. Pre-intervention ambulation rates were abstracted from institution-specific NSQIP data, and missing data were excluded from analysis. Also, due to the retrospective collection of the pre-intervention data, the distance of ambulation could not be quantified. The bar for ambulation is low, as patients were only required to get out of bed and walk 1 step. However, we feel that getting out of bed and taking even 1 step is substantially better than complete bedrest. It is likely that once patients cross the threshold of taking 1 step, they are more likely to ambulate. An area of future study may be to more precisely define the relationship between the quantity of ambulation in steps and its effect on VTE. Finally, we acknowledge that while there is no direct increase in costs, implementing an ambulation protocol does take time from all who participate in the project.
Conclusion
Creation of an ambulation protocol is associated with a decrease in postoperative VTE rates in colorectal surgery patients. A multidisciplinary approach is critical to identify the underlying problems and propose effective solutions. Further studies are required to better correlate the distance of ambulation and its effect on VTE. However, this study shows that even a minimum of 1 step is associated with decreased VTE rates.
Corresponding author: Aneel Damle, MD, MBA, Colon & Rectal Surgery Associates, 3433 Broadway St. NE, Suite 115, Minneapolis, MN 55413; [email protected].
Financial disclosures: None.
From the Department of Surgery, Washington University School of Medicine, St. Louis, MO.
Abstract
Background: Patients undergoing colorectal surgery are at high risk for postoperative venous thromboembolism (VTE). Early ambulation has been encouraged to lower rates of VTE, but evidence demonstrating its effectiveness outside of a bundle is limited.
Objective: To create a multidisciplinary ambulation protocol in an effort to reduce postoperative VTE.
Methods: A single-center, retrospective, comparative study of patients who underwent colectomy or proctectomy was conducted. Outcomes of patients operated on prior to protocol implementation were compared with a cohort after implementation. The intervention studied was the implementation of a multidisciplinary ambulation protocol. The primary endpoint was postoperative VTE.
Results: There was no difference between the pre-intervention group (n = 1762) and the postintervention group (n = 253) in terms of sex, race, origin, emergency status, operative time, and the majority of medical comorbidities (with the exception of smoking status and congestive heart failure). After the protocol was implemented, ambulation rates on postoperative days 0, 1, and 2 improved from 36.4%, 47.3%, and 50.2% to 36.8%, 74.7%, and 82.6%, respectively The VTE rate in the pre-intervention group was 2.7% versus a rate of 0.4% in the postintervention group (P = 0.02).
Conclusion: Creation of an ambulation protocol is associated with a significant reduction in VTE. Commitment from patients, families, nurses, physician extenders, and physicians is critical to the success of the program.
Keywords: VTE; pulmonary embolism; deep vein thrombosis; postoperative; quality improvement.
Postoperative venous thromboembolism (VTE) is a significant source of morbidity, mortality, and cost.1,2 Colorectal surgery patients are at particularly high risk for VTE due to positioning during surgery, pelvic dissection, and other conditions often found in these patients, such as cancer and inflammatory bowel disease.3 A National Surgical Quality Improvement Program (NSQIP) analysis demonstrated an overall rate of VTE in colorectal surgery patients of 2.4%, although other studies have demonstrated rates up to 9%, even in those receiving appropriate chemoprophylaxis.4-6 Many of these VTEs occur in the postdischarge setting. In a NSQIP study of colorectal surgery patients, the rate of VTE between discharge and 30 days was 0.47%.7 The cost burdenfor a postoperative VTE has been estimated to be more than $18,000.8
Studies from NSQIP have identified multiple factors associated with VTE in colorectal surgery patients, but NSQIP does not record ambulation as a standard variable.9 Multiple strategies have been implemented to reduce postoperative VTE. Often, these studies focus on increasing compliance with appropriate chemoprophylaxis, risk stratification, or bundling multiple strategies.10,11 However, despite the fact that postsurgical ambulation is widely encouraged and recommended by the American Society of Colon and Rectal Surgeons clinical practice guidelines, there is little evidence demonstrating the role of ambulation alone in the reduction of VTE.4,12 The purpose of this study was to create a multidisciplinary protocol to increase postoperative ambulation and evaluate its effect on VTE.
Methods
Setting
This study was conducted at a single academic tertiary care center.
Patients and Outcome Measures
All patients undergoing colectomy or proctectomy by surgeons in the section of colon and rectal surgery at a single institution between January 2011 and March 2017 were included. Colectomy and proctectomy were defined by CPT codes 44140, 44141, 44143, 44144, 44145, 44146, 44147, 44150, 44151, 44155, 44156, 44157, 44158, 44160, 44204, 44205, 44206, 44207, 44208, 44210, 44211, 44212, 44213, 45110, 45111, 45112, 45113, 45114, 45116, 45119, 45120, 45121, 45123, 45126, 45160, 45395, and 45397. The primary outcome of VTE within 30 days, including deep venous thrombosis (DVT) and pulmonary embolism (PE), was measured using institution-specific data from NSQIP in both the pre-intervention and postintervention setting. The occurrence of both DVT and PE in 1 patient was counted as a single event of VTE. Ambulation rate on postoperative day (POD) 0, 1, and 2 was calculated by NSQIP in the pre-intervention setting (our institution-specific NSQIP recorded ambulation data for an unrelated project) and by review of the electronic health record in the postintervention setting, as this institution-specific variable was no longer being collected. Ambulation was defined as getting out of bed and taking at least 1 step. The threshold for ambulating each day was once on POD 0 and twice on PODs 1 and 2. Patients with missing ambulation data were excluded from the analysis. Both prior to and throughout the intervention, all patients were given VTE chemoprophylaxis with either low-dose unfractionated heparin or low-molecular-weight heparin prior to induction of anesthesia, with chemoprophylaxis extending an additional 21 days after discharge (unless specifically contraindicated); sequential compression devices; and standard orders to ambulate 3 times daily from POD 0 as part of the standard Enhanced Recovery After Surgery protocol.
Analysis
Statistical analysis was performed using univariate analysis. Chi-square test and univariate logistic regression were used to determine the association between ambulation rates and VTE in the pre-intervention group. Chi-square test was also used to compare ambulation and VTE rates between the pre-intervention and postintervention groups. Plan-Do-Study-Act (PDSA) cycle fidelity (the degree to which a PDSA cycle is carried out in accordance with the guiding principles of its use) was measured by recording the ambulation rates both before and after the intervention.13 Statistical analysis was performed using SAS Version 9.4 (SAS Institute, Cary, NC). This study was reviewed by the Washington University School of Medicine Institutional Review Board and deemed to be quality improvement, not human subjects research, and therefore did not require formal approval.
Baseline Outcome Rates
A total of 1762 patients were identified during the pre-intervention period. The overall VTE rate in the pre-intervention group was 2.7% (n = 48), with 39 DVTs (2.2%) and 13 PEs (0.7%). Pre-intervention ambulation data were available on 590 patients. Baseline ambulation rates on PODs 0, 1, and 2 were 36.4% (213/590), 47.3% (279/590), and 50.2% (296/590), respectively. Patients who did not ambulate on POD 0 had a VTE rate of 4.3%, as compared to 0.9% in those who did ambulate (Table 1). Patients who did not ambulate twice on POD 1 had a VTE rate of 4.8%, compared to 1.1% in those who did ambulate (odds ratio [OR], 4.66; 95% confidence interval [CI], 1.34 to 16.28). Patients who did not ambulate twice on POD 2 had a VTE rate of 5.4%, compared to 0.7% in those who did. Finally, those who ambulated twice on both PODs 1 and 2 had a 0% rate of VTE, compared to 4.9% in those who did not ambulate on both PODs.

Ambulation Protocol
After baseline outcome rates had been established, a multidisciplinary team of medical assistants, nurses, nurse practitioners, and physicians worked together to identify all processes that involved postoperative ambulation. Given the significant differences in VTE rates between patients who ambulated and those that did not, we created a multidisciplinary ambulation protocol using the PDSA method.14 Multiple points of patient contact were chosen for intervention, and the ambulation protocol was implemented in June 2018 and continued for 7 months.
Patients were observed from their initial office visit with a surgeon, during the preoperative education encounter, and in the operating room and on the surgical ward until discharge. Representatives from multiple disciplines who encountered patients at various times in the process, including medical assistants, patient care technicians, nurses, nurse practitioners, physical therapists, and physicians, participated in a kick-off meeting to identify difficulties they encounter when encouraging patient ambulation. The following 4 areas were identified.
Barriers to Patient Ambulation
Patient Expectations. Patients did not appear to have a clear expectation of what their ambulation goals were postoperatively, despite the fact that each patient is given an operative pathway booklet that includes their goals for each day, including ambulation. The consensus was that patients were overwhelmed with the amount of information and, oftentimes, the severity of their diagnosis, so the information regarding ambulation was not retained. Nurses commented that patients frequently stated that they did not think their surgeon wanted them to get out of bed postoperatively.
Electronic Orders. There was confusion within the nursing staff regarding orders in the electronic health record compared to physician expectations. Orders stated patients should ambulate 3 times daily, but did not specify on which postoperative day this should start. Often, nursing verbal sign-out from the post-anesthesia care unit (PACU) would be an order for bedrest, despite no clear origin of this order. This created confusion among the nursing staff as to what the appropriate ambulation orders should be.
Nursing Workflow. The initial state of the nursing workflow was not conducive to evaluating for, or assisting with, ambulation. With no set time to assist and evaluate patients for ambulation, it turned into a task nurses needed to accomplish when they had extra time. With increasing demands of charting in the electronic health record, nurses often had to skip ambulation in order to accomplish other tasks.
Family Expectations. In addition to patient expectations, family members often had expectations that were not congruent with the planned postoperative course. Nurses stated family members would often tell them that they did not feel that their family member should be ambulating so soon after surgery. Often these family members had not attended preoperative education sessions with the patient. This was compounded by the uncertainty among the nursing staff regarding what exactly the ambulation orders were.
Interventions
Targeted interventions were created to address these 4 barriers to ambulation identified by staff.
Preoperative Education. Although all elective patients received a printed operative pathway booklet describing daily goals, including ambulation, patients still did not have a sufficient understanding of what was expected of them. The education session was modified to increase the time spent on both the expectation for and the rationale behind ambulation. That section of the education session ended with a verbal commitment and read-back of the expectations for ambulation by the patient.
Clarification of Electronic Orders. Postoperative orders within the colorectal standard pathway were changed, including specific time frames and frequency, to match the information provided in the patient education booklet. These orders were for ambulation within 4 hours of arrival to the floor, and the orders also noted that no patient should be on bedrest unless explicitly stated. From POD 1, all patients were to ambulate at least twice daily for the remainder of the hospital stay (patients were encouraged to walk 4 times daily, but we set a minimum expectation of twice daily for the order set). These orders were clarified with in-person meetings with the nursing staff and leadership from the PACU and the colorectal surgical ward.
Adjusted Nursing Workflow. Nurses were interviewed and asked to create a plan regarding how they could better incorporate ambulation into their daily workflow. Ambulation assessment was incorporated into the twice-per-shift recording of vital signs and patient safety assessment. This was recorded into the electronic health record at the same time as the patients’ vital signs. This allowed nurses to keep track of which patients would need extra assistance in ambulation and which patients were doing well on their own with the assistance of family. It also helped focus the resources of physical therapy and the single ambulation technician on the floor and to assist patients who needed more assistance.
Creation of Ambulation Encouragement Signs. The authors discovered that despite patients being told preoperatively about ambulation expectations, friends and family are not always included in these conversations. As nurses frequently cited both patients and family as reasons patients thought they should not walk, multiple signs inviting patients to take an active role in their recovery by ambulating were created and placed around the unit. The signs outlined the expectations of being out of bed and taking at least 1 step on the day of surgery and walking at least 4 times per day thereafter. In addition, we addressed frequently asked questions around issues such as walking with intravenous poles and urinary catheters. The posters were signed by all staff colorectal surgeons.
Results
Over the course of 7 months (June 2018 to December 2018), 253 postintervention patients were identified (Table 2). There was no difference between the pre-intervention group (n = 1762) and the postintervention group in terms of sex, race, origin, emergency status, operative time, and the majority of medical comorbidities (with the exception of smoking status and congestive heart failure). The postintervention group was slightly older (60 versus 57 years) and had a higher percentage of patients with an American Society of Anesthesiologists physical status score greater than 2 (66.8% versus 51.2%). The postintervention group also had higher rates of both malignancy (53.4% versus 33.3%) and inflammatory bowel disease (18.2% versus 14.4%).

The fidelity of the PDSA cycle was measured by pre-intervention and postintervention ambulation rates. Ambulation rates on POD 0, 1, and 2 improved from 36.4%, 47.3%, and 50.2% to 36.8%, 74.7%, and 82.6%, respectively (Table 3). The VTE rate decreased from 2.7% to 0.4% (P = 0.02), with 1 DVT and 0 PEs. It should be noted that the only patient who developed a VTE postintervention did not ambulate on PODs 0, 1, or 2.

Discussion
Postoperative VTE is a severe complication for postoperative colorectal surgery patients. Previous studies have demonstrated that increasing ambulation is associated with a lower rate of overall complications, and, when incorporated into a bundle, is associated with decreased rates of VTE.11,15 However, this is the first study to our knowledge demonstrating that creation of an ambulation protocol alone is associated with a decrease in VTE.
Analysis of pre-intervention data demonstrated a strong association between ambulation and an absence of VTE. No patient who ambulated on PODs 0, 1, and 2 developed a VTE. Based on those results, we moved forward with creating the ambulation protocol. While ambulation stayed stable on POD 0, there were 60% and 65% increases on PODs 1 and 2, respectively. Nurses cited late arrival to the floor for second and third start cases as the primary difficulty in getting patients to ambulate more on POD 0.
We believe the key to the success of the ambulation protocol was its multidisciplinary nature. Certainly, the easiest way to create an ambulation protocol is to change the postoperative orders to state patients must walk 4 times per day. However, if the nursing staff is unable or unwilling to carry out these orders, the orders serve little purpose. In order to make lasting changes, all stakeholders in the process must be identified. In our case, stakeholders included surgery and nursing leadership, surgeons, nurse practitioners, nurses, medical assistants, physical therapists, patient care technicians, and patients. This is where we utilized kaizen, a core principle of Lean methodology that empowers employees at the level of the work being carried out to propose ideas for improvement.16 From the beginning of the patient experience, the health care practitioners who were carrying out each step of the process were best able to identify the problems and create solutions. In addition, stakeholders were given regular updates regarding how their efforts were increasing ambulation rates and the results at the end of the study period.
This study also demonstrates that, in a health care system increasingly focused on both quality and cost, significant improvements in quality can be made without increasing cost or resource utilization. Early in the process, it was proposed that the only way to increase the ambulation rate would be to increase the number of physical therapists, nurses, and nursing assistants. However, after identifying the root causes of the problem, the solutions had more to do with improving workflow and fixing problem areas identified by the staff.
In addition to having a positive effect on the outcome studied, collaborative projects such as this between physicians and nurses may lead to increased nursing job satisfaction. A meta-analysis of 31 studies identified nurse-physician collaboration and autonomy as 2 factors that correlate most strongly with nursing satisfaction.17 A Cochrane review also suggests that practice-based interprofessional collaboration may lead to improved health care processes and outcomes.18
This study has several limitations. Pre-intervention ambulation rates were abstracted from institution-specific NSQIP data, and missing data were excluded from analysis. Also, due to the retrospective collection of the pre-intervention data, the distance of ambulation could not be quantified. The bar for ambulation is low, as patients were only required to get out of bed and walk 1 step. However, we feel that getting out of bed and taking even 1 step is substantially better than complete bedrest. It is likely that once patients cross the threshold of taking 1 step, they are more likely to ambulate. An area of future study may be to more precisely define the relationship between the quantity of ambulation in steps and its effect on VTE. Finally, we acknowledge that while there is no direct increase in costs, implementing an ambulation protocol does take time from all who participate in the project.
Conclusion
Creation of an ambulation protocol is associated with a decrease in postoperative VTE rates in colorectal surgery patients. A multidisciplinary approach is critical to identify the underlying problems and propose effective solutions. Further studies are required to better correlate the distance of ambulation and its effect on VTE. However, this study shows that even a minimum of 1 step is associated with decreased VTE rates.
Corresponding author: Aneel Damle, MD, MBA, Colon & Rectal Surgery Associates, 3433 Broadway St. NE, Suite 115, Minneapolis, MN 55413; [email protected].
Financial disclosures: None.
1. Gangireddy C, Rectenwald JR, Upchurch GR, et al. Risk factors and clinical impact of postoperative symptomatic venous thromboembolism. J Vasc Surg. 2007;45:341-342.
2. Newhook TE, LaPar DJ, Walters DM, et al. Impact of postoperative venous thromboembolism on postoperative morbidity, mortality, and resource utilization after hepatectomy. Am Surg. 2015;81:1216-1223.
3. Bergqvist D. Venous thromboembolism: a review of risk and prevention in colorectal surgery patients. Dis Colon Rectum. 2006;49:1620-1628.
4. Fleming F, Gaertner W, Ternent CA, et al. The American society of colon and rectal surgeons clinical practice guideline for the prevention of venous thromboembolic disease in colorectal surgery. Dis Colon Rectum. 2018;61:14-20.
5. McLeod RS, Geerts WH, Sniderman KW, et al. Canadian Colorectal Surgery DVT Prophylaxis Trial investigators. Subcutaneous heparin versus low-molecular-weight heparin as thromboprophylaxis in patients undergoing colorectal surgery: results of the Canadian colorectal DV prophylaxis trial: a randomized, double-blind trial. Ann Surg. 2001;233:438-444.
6. Shapiro R, Vogel JD, Kiran RP. Risk of postoperative venous thromboembolism after laparoscopic and open colorectal surgery: an additional benefit of the minimally invasive approach? Dis Colon Rectum. 2011;54:1496-1502.
7. Dimick JB, Chen SL, Taheri PA, et al. Hospital costs associated with surgical complications: a report from the private-sector National Surgical Quality Improvement Program. J Am Coll Surg. 2004;199:531-537.
8. Fleming FJ, Kim MJ, Salloum RM, et al. How much do we need to worry about venous thromboembolism after hospital discharge? A study of colorectal surgery patients using the National Surgical Quality Improvement Program database. Dis Colon Rectum. 2010;53:1355-1360.
9. ACS NSQIP. User guide for the 2016 ACS NSQIP participant use data file (PUF). 2017. www.facs.org/~/media/files/quality%20programs/nsqip/nsqip_puf_userguide_2016.ashx Accessed July 10, 2020.
10. Caprini JA. Risk assessment as a guide for the prevention of the many faces of venous thromboembolism. Am J Surg. 2010;199(1 Suppl):S3-S10.
11. Cassidy MR, Rosenkranz P, McAney D. Reducing postoperative venous thromboembolism complications with a standardized risk-stratified prophylaxis protocol and mobilization protocol. J Am Coll Surg. 2014;218:1095-1104.
12. Lau BD, Streiff MB, Kraus PS, et al. No evidence to support ambulation for reducing postoperative venous thromboembolism. J Am Coll Surg. 2014;219:1101-1103.
13. McNicholas C, Lennox L, Woodcock T, et al. Evolving quality improvement support strategies to improve Plan–Do–Study–Act cycle fidelity: a retrospective mixed-methods study. BMJ Qual Saf. 2019;28:356-365.
14. Taylor MJ, McNicholas C, Nicolay C, et al. Systematic review of the application of the plan–do–study–act method to improve quality in healthcare. BMC Qual Saf. 2014;23:290-298.
15. Nevo Y, Shaltiel T, Constantini N, et al. Effect of ambulation and physical activity on postoperative complications. J Am Coll Surg. 2016;223(Suppl 1):S61.
16. Mazzocato P, Stenfors-Hayes T, von Thiele Schwarz U, et al. Kaizen practice in healthcare: a qualitative analysis of hospital employees’ suggestions for improvement. BMJ Open. 2016;6:e012256.
17. Zangaro GA, Soeken KL. A meta-analysis of studies of nurses’ job satisfaction. Res Nursing Health. 2007;30:445-458.
18. Reeves S, Pelone F, Harrison R, et al. Interprofessional collaboration to improve professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2017;6(6):CD000072.
1. Gangireddy C, Rectenwald JR, Upchurch GR, et al. Risk factors and clinical impact of postoperative symptomatic venous thromboembolism. J Vasc Surg. 2007;45:341-342.
2. Newhook TE, LaPar DJ, Walters DM, et al. Impact of postoperative venous thromboembolism on postoperative morbidity, mortality, and resource utilization after hepatectomy. Am Surg. 2015;81:1216-1223.
3. Bergqvist D. Venous thromboembolism: a review of risk and prevention in colorectal surgery patients. Dis Colon Rectum. 2006;49:1620-1628.
4. Fleming F, Gaertner W, Ternent CA, et al. The American society of colon and rectal surgeons clinical practice guideline for the prevention of venous thromboembolic disease in colorectal surgery. Dis Colon Rectum. 2018;61:14-20.
5. McLeod RS, Geerts WH, Sniderman KW, et al. Canadian Colorectal Surgery DVT Prophylaxis Trial investigators. Subcutaneous heparin versus low-molecular-weight heparin as thromboprophylaxis in patients undergoing colorectal surgery: results of the Canadian colorectal DV prophylaxis trial: a randomized, double-blind trial. Ann Surg. 2001;233:438-444.
6. Shapiro R, Vogel JD, Kiran RP. Risk of postoperative venous thromboembolism after laparoscopic and open colorectal surgery: an additional benefit of the minimally invasive approach? Dis Colon Rectum. 2011;54:1496-1502.
7. Dimick JB, Chen SL, Taheri PA, et al. Hospital costs associated with surgical complications: a report from the private-sector National Surgical Quality Improvement Program. J Am Coll Surg. 2004;199:531-537.
8. Fleming FJ, Kim MJ, Salloum RM, et al. How much do we need to worry about venous thromboembolism after hospital discharge? A study of colorectal surgery patients using the National Surgical Quality Improvement Program database. Dis Colon Rectum. 2010;53:1355-1360.
9. ACS NSQIP. User guide for the 2016 ACS NSQIP participant use data file (PUF). 2017. www.facs.org/~/media/files/quality%20programs/nsqip/nsqip_puf_userguide_2016.ashx Accessed July 10, 2020.
10. Caprini JA. Risk assessment as a guide for the prevention of the many faces of venous thromboembolism. Am J Surg. 2010;199(1 Suppl):S3-S10.
11. Cassidy MR, Rosenkranz P, McAney D. Reducing postoperative venous thromboembolism complications with a standardized risk-stratified prophylaxis protocol and mobilization protocol. J Am Coll Surg. 2014;218:1095-1104.
12. Lau BD, Streiff MB, Kraus PS, et al. No evidence to support ambulation for reducing postoperative venous thromboembolism. J Am Coll Surg. 2014;219:1101-1103.
13. McNicholas C, Lennox L, Woodcock T, et al. Evolving quality improvement support strategies to improve Plan–Do–Study–Act cycle fidelity: a retrospective mixed-methods study. BMJ Qual Saf. 2019;28:356-365.
14. Taylor MJ, McNicholas C, Nicolay C, et al. Systematic review of the application of the plan–do–study–act method to improve quality in healthcare. BMC Qual Saf. 2014;23:290-298.
15. Nevo Y, Shaltiel T, Constantini N, et al. Effect of ambulation and physical activity on postoperative complications. J Am Coll Surg. 2016;223(Suppl 1):S61.
16. Mazzocato P, Stenfors-Hayes T, von Thiele Schwarz U, et al. Kaizen practice in healthcare: a qualitative analysis of hospital employees’ suggestions for improvement. BMJ Open. 2016;6:e012256.
17. Zangaro GA, Soeken KL. A meta-analysis of studies of nurses’ job satisfaction. Res Nursing Health. 2007;30:445-458.
18. Reeves S, Pelone F, Harrison R, et al. Interprofessional collaboration to improve professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2017;6(6):CD000072.
FDA okays new CAR T therapy, first for mantle cell lymphoma
The Food and Drug Administration granted accelerated approval to brexucabtagene autoleucel (Tecartus, Kite Pharma), the first approved chimeric antigen receptor (CAR) T cell therapy for the treatment of adult patients with relapsed or refractory mantle cell lymphoma (MCL).
The new agent is the second approved CAR T cell product developed by Kite and follows the 2017 approval of axicabtagene ciloleucel (Yescarta) for diffuse large B-cell lymphoma.
“Despite promising advances, there are still major gaps in treatment for patients with MCL who progress following initial therapy,” investigator Michael Wang, MD, of the University of Texas MD Anderson Cancer Center in Houston, said in a company statement. “Many patients have high-risk disease and are more likely to keep progressing, even after subsequent treatments.”
In the same press statement, Meghan Gutierrez, chief executive officer, Lymphoma Research Foundation, said: “This approval marks the first CAR T cell therapy approved for mantle cell lymphoma patients and represents a new frontier in the treatment of this disease.”
The approval of the single-infusion therapy is based on efficacy and safety data from the ongoing, single-arm ZUMA-2 pivotal trial, which enrolled 74 adult patients. All patients had previously received anthracycline- or bendamustine-containing chemotherapy, an anti-CD20 antibody therapy and a Bruton tyrosine kinase inhibitor (ibrutinib or acalabrutinib).
In the trial, there was an objective response rate, which was the primary outcome measure, of 87% among 60 patients who were evaluable for efficacy analysis; 62% had a complete response.
Among all patients, follow-up was at least 6 months after their first objective disease response. Median duration of response has not yet been reached.
In terms of adverse events, 18% of the 82 patients evaluable for safety experienced > grade 3 cytokine release syndrome and 37% experienced neurologic events, per the company statement. The most common (≥ 10%) grade 3 or higher adverse reactions were anemia, neutropenia, thrombocytopenia, hypotension, hypophosphatemia, encephalopathy, leukopenia, hypoxia, pyrexia, hyponatremia, hypertension, infection-pathogen unspecified, pneumonia, hypocalcemia, and lymphopenia.
Brexucabtagene autoleucel will be manufactured in Kite’s facility in California. In the pivotal trial, there was a 96% manufacturing success rate and a median manufacturing turnaround time of 15 days from leukapheresis to product delivery.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration granted accelerated approval to brexucabtagene autoleucel (Tecartus, Kite Pharma), the first approved chimeric antigen receptor (CAR) T cell therapy for the treatment of adult patients with relapsed or refractory mantle cell lymphoma (MCL).
The new agent is the second approved CAR T cell product developed by Kite and follows the 2017 approval of axicabtagene ciloleucel (Yescarta) for diffuse large B-cell lymphoma.
“Despite promising advances, there are still major gaps in treatment for patients with MCL who progress following initial therapy,” investigator Michael Wang, MD, of the University of Texas MD Anderson Cancer Center in Houston, said in a company statement. “Many patients have high-risk disease and are more likely to keep progressing, even after subsequent treatments.”
In the same press statement, Meghan Gutierrez, chief executive officer, Lymphoma Research Foundation, said: “This approval marks the first CAR T cell therapy approved for mantle cell lymphoma patients and represents a new frontier in the treatment of this disease.”
The approval of the single-infusion therapy is based on efficacy and safety data from the ongoing, single-arm ZUMA-2 pivotal trial, which enrolled 74 adult patients. All patients had previously received anthracycline- or bendamustine-containing chemotherapy, an anti-CD20 antibody therapy and a Bruton tyrosine kinase inhibitor (ibrutinib or acalabrutinib).
In the trial, there was an objective response rate, which was the primary outcome measure, of 87% among 60 patients who were evaluable for efficacy analysis; 62% had a complete response.
Among all patients, follow-up was at least 6 months after their first objective disease response. Median duration of response has not yet been reached.
In terms of adverse events, 18% of the 82 patients evaluable for safety experienced > grade 3 cytokine release syndrome and 37% experienced neurologic events, per the company statement. The most common (≥ 10%) grade 3 or higher adverse reactions were anemia, neutropenia, thrombocytopenia, hypotension, hypophosphatemia, encephalopathy, leukopenia, hypoxia, pyrexia, hyponatremia, hypertension, infection-pathogen unspecified, pneumonia, hypocalcemia, and lymphopenia.
Brexucabtagene autoleucel will be manufactured in Kite’s facility in California. In the pivotal trial, there was a 96% manufacturing success rate and a median manufacturing turnaround time of 15 days from leukapheresis to product delivery.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration granted accelerated approval to brexucabtagene autoleucel (Tecartus, Kite Pharma), the first approved chimeric antigen receptor (CAR) T cell therapy for the treatment of adult patients with relapsed or refractory mantle cell lymphoma (MCL).
The new agent is the second approved CAR T cell product developed by Kite and follows the 2017 approval of axicabtagene ciloleucel (Yescarta) for diffuse large B-cell lymphoma.
“Despite promising advances, there are still major gaps in treatment for patients with MCL who progress following initial therapy,” investigator Michael Wang, MD, of the University of Texas MD Anderson Cancer Center in Houston, said in a company statement. “Many patients have high-risk disease and are more likely to keep progressing, even after subsequent treatments.”
In the same press statement, Meghan Gutierrez, chief executive officer, Lymphoma Research Foundation, said: “This approval marks the first CAR T cell therapy approved for mantle cell lymphoma patients and represents a new frontier in the treatment of this disease.”
The approval of the single-infusion therapy is based on efficacy and safety data from the ongoing, single-arm ZUMA-2 pivotal trial, which enrolled 74 adult patients. All patients had previously received anthracycline- or bendamustine-containing chemotherapy, an anti-CD20 antibody therapy and a Bruton tyrosine kinase inhibitor (ibrutinib or acalabrutinib).
In the trial, there was an objective response rate, which was the primary outcome measure, of 87% among 60 patients who were evaluable for efficacy analysis; 62% had a complete response.
Among all patients, follow-up was at least 6 months after their first objective disease response. Median duration of response has not yet been reached.
In terms of adverse events, 18% of the 82 patients evaluable for safety experienced > grade 3 cytokine release syndrome and 37% experienced neurologic events, per the company statement. The most common (≥ 10%) grade 3 or higher adverse reactions were anemia, neutropenia, thrombocytopenia, hypotension, hypophosphatemia, encephalopathy, leukopenia, hypoxia, pyrexia, hyponatremia, hypertension, infection-pathogen unspecified, pneumonia, hypocalcemia, and lymphopenia.
Brexucabtagene autoleucel will be manufactured in Kite’s facility in California. In the pivotal trial, there was a 96% manufacturing success rate and a median manufacturing turnaround time of 15 days from leukapheresis to product delivery.
A version of this article originally appeared on Medscape.com.



