User login
A complication of enoxaparin injection
A 78-year-old woman presented to the emergency department with shortness of breath and palpitations and was found to have atrial fibrillation with rapid ventricular response. Medical therapy with drug therapy and cardioversion proved ineffective. She then underwent atrioventricular node ablation and placement of a pacemaker.
At the time of admission, anticoagulation was started with full-dose enoxaparin, injected subcutaneously on the left side of the abdominal wall, as her CHA2DS2-VASc score (http://chadvasc.org) was 5, due to age, female sex, and history of heart failure and hypertension.
Four days after admission, she reported lower abdominal pain, and her urine output was minimal. A bladder scan showed more than 500 mL of residual urine. She was hemodynamically stable, but physical examination revealed mild abdominal distention and tenderness in the suprapubic region. Laboratory testing showed a sharp rise in serum creatinine and a drop in hematocrit.
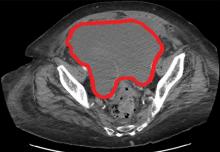
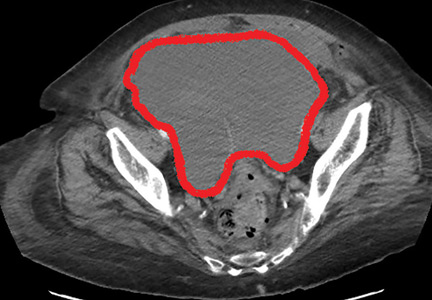
The patient was initially managed conservatively with serial physical examinations, monitoring of the hematocrit, serial imaging studies, and discontinuation of anticoagulation, but the pain and anuria persisted. Repeat computed tomography 15 days after admission showed that the hematoma had expanded, and she now had hydronephrosis on the right side as well, requiring urologic intervention with bilateral nephrostomy tube placement.
The size of the hematoma was evaluated with serial abdominal and pelvic examinations. After several days, her urine output had improved, the nephrostomy tubes were removed, and she was discharged.
RECTUS SHEATH HEMATOMA
Our patient had a giant pelvic hematoma, probably arising from the rectus sheath. This uncommon problem can arise from trauma, anticoagulation, or increased intra-abdominal pressure, but it can also occur spontaneously.1
In rectus sheath hematoma, a branch of the inferior epigastric artery is injured at its insertion into the rectus abdominis muscle. Symptoms arise if bleeding does not stop spontaneously from a tamponade effect.2
We speculate that in our patient, deep injection of enoxaparin into the abdominal wall injured the inferior epigastric artery, which started the hematoma, and the bleeding was exacerbated by the anticoagulation effect of the enoxaparin.
Another form of pelvic hematoma is retroperitoneal. It is most commonly caused by trauma but can occur due to rupture of the aorta, compression from tumors, or, infrequently, anticoagulation therapy.3
The role of anticoagulation
Spontaneous pelvic hematoma is usually missed as a cause of abdominal pain in patients on anticoagulation therapy and is mistaken for common acute conditions such as ulcer, diverticulitis, appendicitis, ovarian cyst torsion, and tumor.4 It usually develops within 5 days of starting anticoagulation therapy. Symptoms vary depending on the location of the hematoma and are best diagnosed with abdominal computed tomography, with sensitivity as high as 100%.
MANAGEMENT
Conservative management, reserved for patients in stable condition, includes temporarily stopping and reevaluating the risks and benefits of anticoagulation and antiplatelet agents, giving blood transfusions, and controlling pain. If conservative measures fail, options are arterial embolization, stent grafting, and blood vessel ligation.5 If these measures fail, patients should undergo surgical evacuation of the hematoma and ligation of bleeding vessels.6
TAKE-HOME MESSAGE
Subcutaneous injections, especially of anticoagulants, into the abdominal wall can increase the risk of hematoma. Other risk factors are older age, female sex, and thin body habitus with less abdominal fat.7 Healthcare professionals should avoid deep injections into the abdomen and should counsel patients and their caregivers about this, as well. The deltoid region could be a safer alternative.
- Cherry WB, Mueller PS. Rectus sheath hematoma: review of 126 cases at a single institution. Medicine (Baltimore) 2006; 85(2):105–110. doi:10.1097/01.md.0000216818.13067.5a
- Hatjipetrou A, Anyfantakis D, Kastanakis M. Rectus sheath hematoma: a review of the literature. Int J Surg 2015; 13:267–271. doi:10.1016/j.ijsu.2014.12.015
- Haq MM, Taimur SDM, Khan SR, Rahman MA. Retroperitoneal hematoma following enoxaparin treatment in an elderly woman—a case report. Cardiovasc J 2010; 3(1):94–97. doi:10.3329/cardio.v3i1.6434
- Luhmann A, Williams EV. Rectus sheath hematoma: a series of unfortunate events. World J Surg 2006; 30(11):2050–2055. doi:10.1007/s00268-005-0702-9
- Pace F, Colombo GM, Del Vecchio LR, et al. Low molecular weight heparin and fatal spontaneous extraperitoneal hematoma in the elderly. Geriatr Gerontol Int 2012; 12(1):172–174. doi:10.1111/j.1447-0594.2011.00742.x
- Velicki L, Cemerlic-Adic N, Bogdanovic D, Mrdanin T. Rectus sheath haematoma: enoxaparin-related complication. Acta Clin Belg 2013; 68(2):147–149. doi:10.2143/ACB.68.2.3213
- Sheth HS, Kumar R, DiNella J, Janov C, Kaldas H, Smith RE. Evaluation of risk factors for rectus sheath hematoma. Clin Appl Thromb Hemost 2016; 22(3):292–296. doi:10.1177/1076029614553024
A 78-year-old woman presented to the emergency department with shortness of breath and palpitations and was found to have atrial fibrillation with rapid ventricular response. Medical therapy with drug therapy and cardioversion proved ineffective. She then underwent atrioventricular node ablation and placement of a pacemaker.
At the time of admission, anticoagulation was started with full-dose enoxaparin, injected subcutaneously on the left side of the abdominal wall, as her CHA2DS2-VASc score (http://chadvasc.org) was 5, due to age, female sex, and history of heart failure and hypertension.
Four days after admission, she reported lower abdominal pain, and her urine output was minimal. A bladder scan showed more than 500 mL of residual urine. She was hemodynamically stable, but physical examination revealed mild abdominal distention and tenderness in the suprapubic region. Laboratory testing showed a sharp rise in serum creatinine and a drop in hematocrit.
The patient was initially managed conservatively with serial physical examinations, monitoring of the hematocrit, serial imaging studies, and discontinuation of anticoagulation, but the pain and anuria persisted. Repeat computed tomography 15 days after admission showed that the hematoma had expanded, and she now had hydronephrosis on the right side as well, requiring urologic intervention with bilateral nephrostomy tube placement.
The size of the hematoma was evaluated with serial abdominal and pelvic examinations. After several days, her urine output had improved, the nephrostomy tubes were removed, and she was discharged.
RECTUS SHEATH HEMATOMA
Our patient had a giant pelvic hematoma, probably arising from the rectus sheath. This uncommon problem can arise from trauma, anticoagulation, or increased intra-abdominal pressure, but it can also occur spontaneously.1
In rectus sheath hematoma, a branch of the inferior epigastric artery is injured at its insertion into the rectus abdominis muscle. Symptoms arise if bleeding does not stop spontaneously from a tamponade effect.2
We speculate that in our patient, deep injection of enoxaparin into the abdominal wall injured the inferior epigastric artery, which started the hematoma, and the bleeding was exacerbated by the anticoagulation effect of the enoxaparin.
Another form of pelvic hematoma is retroperitoneal. It is most commonly caused by trauma but can occur due to rupture of the aorta, compression from tumors, or, infrequently, anticoagulation therapy.3
The role of anticoagulation
Spontaneous pelvic hematoma is usually missed as a cause of abdominal pain in patients on anticoagulation therapy and is mistaken for common acute conditions such as ulcer, diverticulitis, appendicitis, ovarian cyst torsion, and tumor.4 It usually develops within 5 days of starting anticoagulation therapy. Symptoms vary depending on the location of the hematoma and are best diagnosed with abdominal computed tomography, with sensitivity as high as 100%.
MANAGEMENT
Conservative management, reserved for patients in stable condition, includes temporarily stopping and reevaluating the risks and benefits of anticoagulation and antiplatelet agents, giving blood transfusions, and controlling pain. If conservative measures fail, options are arterial embolization, stent grafting, and blood vessel ligation.5 If these measures fail, patients should undergo surgical evacuation of the hematoma and ligation of bleeding vessels.6
TAKE-HOME MESSAGE
Subcutaneous injections, especially of anticoagulants, into the abdominal wall can increase the risk of hematoma. Other risk factors are older age, female sex, and thin body habitus with less abdominal fat.7 Healthcare professionals should avoid deep injections into the abdomen and should counsel patients and their caregivers about this, as well. The deltoid region could be a safer alternative.
A 78-year-old woman presented to the emergency department with shortness of breath and palpitations and was found to have atrial fibrillation with rapid ventricular response. Medical therapy with drug therapy and cardioversion proved ineffective. She then underwent atrioventricular node ablation and placement of a pacemaker.
At the time of admission, anticoagulation was started with full-dose enoxaparin, injected subcutaneously on the left side of the abdominal wall, as her CHA2DS2-VASc score (http://chadvasc.org) was 5, due to age, female sex, and history of heart failure and hypertension.
Four days after admission, she reported lower abdominal pain, and her urine output was minimal. A bladder scan showed more than 500 mL of residual urine. She was hemodynamically stable, but physical examination revealed mild abdominal distention and tenderness in the suprapubic region. Laboratory testing showed a sharp rise in serum creatinine and a drop in hematocrit.
The patient was initially managed conservatively with serial physical examinations, monitoring of the hematocrit, serial imaging studies, and discontinuation of anticoagulation, but the pain and anuria persisted. Repeat computed tomography 15 days after admission showed that the hematoma had expanded, and she now had hydronephrosis on the right side as well, requiring urologic intervention with bilateral nephrostomy tube placement.
The size of the hematoma was evaluated with serial abdominal and pelvic examinations. After several days, her urine output had improved, the nephrostomy tubes were removed, and she was discharged.
RECTUS SHEATH HEMATOMA
Our patient had a giant pelvic hematoma, probably arising from the rectus sheath. This uncommon problem can arise from trauma, anticoagulation, or increased intra-abdominal pressure, but it can also occur spontaneously.1
In rectus sheath hematoma, a branch of the inferior epigastric artery is injured at its insertion into the rectus abdominis muscle. Symptoms arise if bleeding does not stop spontaneously from a tamponade effect.2
We speculate that in our patient, deep injection of enoxaparin into the abdominal wall injured the inferior epigastric artery, which started the hematoma, and the bleeding was exacerbated by the anticoagulation effect of the enoxaparin.
Another form of pelvic hematoma is retroperitoneal. It is most commonly caused by trauma but can occur due to rupture of the aorta, compression from tumors, or, infrequently, anticoagulation therapy.3
The role of anticoagulation
Spontaneous pelvic hematoma is usually missed as a cause of abdominal pain in patients on anticoagulation therapy and is mistaken for common acute conditions such as ulcer, diverticulitis, appendicitis, ovarian cyst torsion, and tumor.4 It usually develops within 5 days of starting anticoagulation therapy. Symptoms vary depending on the location of the hematoma and are best diagnosed with abdominal computed tomography, with sensitivity as high as 100%.
MANAGEMENT
Conservative management, reserved for patients in stable condition, includes temporarily stopping and reevaluating the risks and benefits of anticoagulation and antiplatelet agents, giving blood transfusions, and controlling pain. If conservative measures fail, options are arterial embolization, stent grafting, and blood vessel ligation.5 If these measures fail, patients should undergo surgical evacuation of the hematoma and ligation of bleeding vessels.6
TAKE-HOME MESSAGE
Subcutaneous injections, especially of anticoagulants, into the abdominal wall can increase the risk of hematoma. Other risk factors are older age, female sex, and thin body habitus with less abdominal fat.7 Healthcare professionals should avoid deep injections into the abdomen and should counsel patients and their caregivers about this, as well. The deltoid region could be a safer alternative.
- Cherry WB, Mueller PS. Rectus sheath hematoma: review of 126 cases at a single institution. Medicine (Baltimore) 2006; 85(2):105–110. doi:10.1097/01.md.0000216818.13067.5a
- Hatjipetrou A, Anyfantakis D, Kastanakis M. Rectus sheath hematoma: a review of the literature. Int J Surg 2015; 13:267–271. doi:10.1016/j.ijsu.2014.12.015
- Haq MM, Taimur SDM, Khan SR, Rahman MA. Retroperitoneal hematoma following enoxaparin treatment in an elderly woman—a case report. Cardiovasc J 2010; 3(1):94–97. doi:10.3329/cardio.v3i1.6434
- Luhmann A, Williams EV. Rectus sheath hematoma: a series of unfortunate events. World J Surg 2006; 30(11):2050–2055. doi:10.1007/s00268-005-0702-9
- Pace F, Colombo GM, Del Vecchio LR, et al. Low molecular weight heparin and fatal spontaneous extraperitoneal hematoma in the elderly. Geriatr Gerontol Int 2012; 12(1):172–174. doi:10.1111/j.1447-0594.2011.00742.x
- Velicki L, Cemerlic-Adic N, Bogdanovic D, Mrdanin T. Rectus sheath haematoma: enoxaparin-related complication. Acta Clin Belg 2013; 68(2):147–149. doi:10.2143/ACB.68.2.3213
- Sheth HS, Kumar R, DiNella J, Janov C, Kaldas H, Smith RE. Evaluation of risk factors for rectus sheath hematoma. Clin Appl Thromb Hemost 2016; 22(3):292–296. doi:10.1177/1076029614553024
- Cherry WB, Mueller PS. Rectus sheath hematoma: review of 126 cases at a single institution. Medicine (Baltimore) 2006; 85(2):105–110. doi:10.1097/01.md.0000216818.13067.5a
- Hatjipetrou A, Anyfantakis D, Kastanakis M. Rectus sheath hematoma: a review of the literature. Int J Surg 2015; 13:267–271. doi:10.1016/j.ijsu.2014.12.015
- Haq MM, Taimur SDM, Khan SR, Rahman MA. Retroperitoneal hematoma following enoxaparin treatment in an elderly woman—a case report. Cardiovasc J 2010; 3(1):94–97. doi:10.3329/cardio.v3i1.6434
- Luhmann A, Williams EV. Rectus sheath hematoma: a series of unfortunate events. World J Surg 2006; 30(11):2050–2055. doi:10.1007/s00268-005-0702-9
- Pace F, Colombo GM, Del Vecchio LR, et al. Low molecular weight heparin and fatal spontaneous extraperitoneal hematoma in the elderly. Geriatr Gerontol Int 2012; 12(1):172–174. doi:10.1111/j.1447-0594.2011.00742.x
- Velicki L, Cemerlic-Adic N, Bogdanovic D, Mrdanin T. Rectus sheath haematoma: enoxaparin-related complication. Acta Clin Belg 2013; 68(2):147–149. doi:10.2143/ACB.68.2.3213
- Sheth HS, Kumar R, DiNella J, Janov C, Kaldas H, Smith RE. Evaluation of risk factors for rectus sheath hematoma. Clin Appl Thromb Hemost 2016; 22(3):292–296. doi:10.1177/1076029614553024
Q&A: Drug costs and value in cancer
Skyrocketing drug costs are a key issue facing physicians, patients, and policymakers, but an even thornier problem may be determining a drug’s value.
In this Q&A, Richard L. Schilsky, MD, senior vice president and chief medical officer at the American Society of Clinical Oncology (ASCO), weighs in on the value proposition for cancer drugs and the implications for physicians.
Q: What tools exist for determining a drug’s value?
A: A number of organizations have developed tools to try to determine the value of cancer drug treatments. ASCO, the European Society for Medical Oncology (ESMO), the Institute for Clinical and Economic Review, Memorial Sloan Kettering Cancer Center, and the National Comprehensive Cancer Network have all developed tools for this purpose.
Our tool, the ASCO Value Framework, assesses the value of new cancer drug treatments based on clinical benefit, side effects, and improvements in patient symptoms or quality of life in the context of cost. While it’s hard to directly compare frameworks – given differences in methodology and the many nuances of evaluating clinical trial results – in 2018, ASCO and ESMO published a joint analysis of our value frameworks in the Journal of Clinical Oncology (2018; 37[4]:336-49).
The analysis found that the frameworks produce comparable measures of the clinical benefits of new therapies in approximately two-thirds of the more than 100 treatment comparisons that were examined. It also identified a number of factors that may contribute to the discordant scores, revealing potential ways for both of our organizations to refine our frameworks in the future.
That said, ASCO’s Value Framework is just one part of our broader, multifaceted effort to achieve high-quality, high-value care for all patients with cancer. Other efforts include ASCO’s proposed Patient-Centered Oncology Payment model, the Choosing Wisely campaign to identify low-value clinical strategies, and CancerLinQ and the Quality Oncology Practice Initiative to implement quality measurement and improvement.
Q: How can the issues around drug price and value be addressed earlier in the context of clinical trials?
A: The definition of value ultimately comes down to the price that must be paid to achieve meaningfully improved health outcomes for individual patients or the broader population of affected individuals. Optimizing the value of a new cancer drug treatment begins with an innovation to address an unmet medical need, followed by defining and achieving clinically meaningful improvements in health outcomes through well-designed and efficiently conducted clinical trials. Effectiveness research is also essential to determining how well new treatments perform compared with available alternatives and how they perform in more diverse populations than those typically included in the clinical trials used to establish efficacy.
Patient goals, preferences, and choices shape the real-world experience of a new product, and the direct and indirect costs of a treatment to patients and their families significantly affect whether it is adopted widely. Until their value is clearly established, new and costly products should be deployed judiciously and after careful consideration of the goals of treatment, available options, and the unique needs, preferences, and goals of individual patients.
More research is needed to improve how we assess the value of new cancer drug treatments. New clinical efficacy endpoints – both provider- and patient-reported ones – that accurately describe how a patient feels and functions must be developed and should reflect outcomes of value to patients other than survival, particularly in noncurative settings.
Better predictive biomarkers can transform a drug of modest efficacy in an unselected population to one of high efficacy in a biomarker-defined subgroup and thereby contribute to improving the value of a treatment.
Regulatory and policy initiatives such as adaptive licensing, value-based insurance, and indication-specific pricing that affect marketing approval, reimbursement, or price, respectively, based on treatment effectiveness, also deserve careful consideration and further research to determine their effects on aligning cost with benefit while ensuring patient access to potentially life-extending therapies and continued innovation in drug development.
Q: Aside from the policy options, what’s the role of the oncologist in discussing the value of drugs with patients when determining a treatment plan?
A: Since oncologists don’t control drug prices, our role in improving the value of cancer care involves appropriately managing how resources are used and guiding patients during discussions around the right treatment plan for their particular diagnosis, prognosis, and treatment goals.
Adopting and adhering to high-quality oncology clinical pathways is an important way to improve the quality, efficiency, and value of cancer care. High-quality oncology pathways are detailed, evidence-based treatment protocols for delivering cancer care to patients with specific disease types and stages. When properly designed and implemented, oncology pathways serve as an important tool in appropriately managing cancer care resources and improving the quality of care that patients with cancer receive, while also reducing costs.
Dr. Schilsky is the senior vice president and chief medical officer of ASCO. Formerly the chief of hematology/oncology in the department of medicine and deputy director of the University of Chicago Comprehensive Cancer Center, he is a leader in the field of clinical oncology, specializing in new drug development and the treatment of gastrointestinal cancers. Dr. Schilsky reported research funding from several pharmaceutical companies to ASCO for the Targeted Agent and Profiling Utilization Registry (TAPUR) clinical trial. He also reported travel/accommodation/expense support from Varian.
Skyrocketing drug costs are a key issue facing physicians, patients, and policymakers, but an even thornier problem may be determining a drug’s value.
In this Q&A, Richard L. Schilsky, MD, senior vice president and chief medical officer at the American Society of Clinical Oncology (ASCO), weighs in on the value proposition for cancer drugs and the implications for physicians.
Q: What tools exist for determining a drug’s value?
A: A number of organizations have developed tools to try to determine the value of cancer drug treatments. ASCO, the European Society for Medical Oncology (ESMO), the Institute for Clinical and Economic Review, Memorial Sloan Kettering Cancer Center, and the National Comprehensive Cancer Network have all developed tools for this purpose.
Our tool, the ASCO Value Framework, assesses the value of new cancer drug treatments based on clinical benefit, side effects, and improvements in patient symptoms or quality of life in the context of cost. While it’s hard to directly compare frameworks – given differences in methodology and the many nuances of evaluating clinical trial results – in 2018, ASCO and ESMO published a joint analysis of our value frameworks in the Journal of Clinical Oncology (2018; 37[4]:336-49).
The analysis found that the frameworks produce comparable measures of the clinical benefits of new therapies in approximately two-thirds of the more than 100 treatment comparisons that were examined. It also identified a number of factors that may contribute to the discordant scores, revealing potential ways for both of our organizations to refine our frameworks in the future.
That said, ASCO’s Value Framework is just one part of our broader, multifaceted effort to achieve high-quality, high-value care for all patients with cancer. Other efforts include ASCO’s proposed Patient-Centered Oncology Payment model, the Choosing Wisely campaign to identify low-value clinical strategies, and CancerLinQ and the Quality Oncology Practice Initiative to implement quality measurement and improvement.
Q: How can the issues around drug price and value be addressed earlier in the context of clinical trials?
A: The definition of value ultimately comes down to the price that must be paid to achieve meaningfully improved health outcomes for individual patients or the broader population of affected individuals. Optimizing the value of a new cancer drug treatment begins with an innovation to address an unmet medical need, followed by defining and achieving clinically meaningful improvements in health outcomes through well-designed and efficiently conducted clinical trials. Effectiveness research is also essential to determining how well new treatments perform compared with available alternatives and how they perform in more diverse populations than those typically included in the clinical trials used to establish efficacy.
Patient goals, preferences, and choices shape the real-world experience of a new product, and the direct and indirect costs of a treatment to patients and their families significantly affect whether it is adopted widely. Until their value is clearly established, new and costly products should be deployed judiciously and after careful consideration of the goals of treatment, available options, and the unique needs, preferences, and goals of individual patients.
More research is needed to improve how we assess the value of new cancer drug treatments. New clinical efficacy endpoints – both provider- and patient-reported ones – that accurately describe how a patient feels and functions must be developed and should reflect outcomes of value to patients other than survival, particularly in noncurative settings.
Better predictive biomarkers can transform a drug of modest efficacy in an unselected population to one of high efficacy in a biomarker-defined subgroup and thereby contribute to improving the value of a treatment.
Regulatory and policy initiatives such as adaptive licensing, value-based insurance, and indication-specific pricing that affect marketing approval, reimbursement, or price, respectively, based on treatment effectiveness, also deserve careful consideration and further research to determine their effects on aligning cost with benefit while ensuring patient access to potentially life-extending therapies and continued innovation in drug development.
Q: Aside from the policy options, what’s the role of the oncologist in discussing the value of drugs with patients when determining a treatment plan?
A: Since oncologists don’t control drug prices, our role in improving the value of cancer care involves appropriately managing how resources are used and guiding patients during discussions around the right treatment plan for their particular diagnosis, prognosis, and treatment goals.
Adopting and adhering to high-quality oncology clinical pathways is an important way to improve the quality, efficiency, and value of cancer care. High-quality oncology pathways are detailed, evidence-based treatment protocols for delivering cancer care to patients with specific disease types and stages. When properly designed and implemented, oncology pathways serve as an important tool in appropriately managing cancer care resources and improving the quality of care that patients with cancer receive, while also reducing costs.
Dr. Schilsky is the senior vice president and chief medical officer of ASCO. Formerly the chief of hematology/oncology in the department of medicine and deputy director of the University of Chicago Comprehensive Cancer Center, he is a leader in the field of clinical oncology, specializing in new drug development and the treatment of gastrointestinal cancers. Dr. Schilsky reported research funding from several pharmaceutical companies to ASCO for the Targeted Agent and Profiling Utilization Registry (TAPUR) clinical trial. He also reported travel/accommodation/expense support from Varian.
Skyrocketing drug costs are a key issue facing physicians, patients, and policymakers, but an even thornier problem may be determining a drug’s value.
In this Q&A, Richard L. Schilsky, MD, senior vice president and chief medical officer at the American Society of Clinical Oncology (ASCO), weighs in on the value proposition for cancer drugs and the implications for physicians.
Q: What tools exist for determining a drug’s value?
A: A number of organizations have developed tools to try to determine the value of cancer drug treatments. ASCO, the European Society for Medical Oncology (ESMO), the Institute for Clinical and Economic Review, Memorial Sloan Kettering Cancer Center, and the National Comprehensive Cancer Network have all developed tools for this purpose.
Our tool, the ASCO Value Framework, assesses the value of new cancer drug treatments based on clinical benefit, side effects, and improvements in patient symptoms or quality of life in the context of cost. While it’s hard to directly compare frameworks – given differences in methodology and the many nuances of evaluating clinical trial results – in 2018, ASCO and ESMO published a joint analysis of our value frameworks in the Journal of Clinical Oncology (2018; 37[4]:336-49).
The analysis found that the frameworks produce comparable measures of the clinical benefits of new therapies in approximately two-thirds of the more than 100 treatment comparisons that were examined. It also identified a number of factors that may contribute to the discordant scores, revealing potential ways for both of our organizations to refine our frameworks in the future.
That said, ASCO’s Value Framework is just one part of our broader, multifaceted effort to achieve high-quality, high-value care for all patients with cancer. Other efforts include ASCO’s proposed Patient-Centered Oncology Payment model, the Choosing Wisely campaign to identify low-value clinical strategies, and CancerLinQ and the Quality Oncology Practice Initiative to implement quality measurement and improvement.
Q: How can the issues around drug price and value be addressed earlier in the context of clinical trials?
A: The definition of value ultimately comes down to the price that must be paid to achieve meaningfully improved health outcomes for individual patients or the broader population of affected individuals. Optimizing the value of a new cancer drug treatment begins with an innovation to address an unmet medical need, followed by defining and achieving clinically meaningful improvements in health outcomes through well-designed and efficiently conducted clinical trials. Effectiveness research is also essential to determining how well new treatments perform compared with available alternatives and how they perform in more diverse populations than those typically included in the clinical trials used to establish efficacy.
Patient goals, preferences, and choices shape the real-world experience of a new product, and the direct and indirect costs of a treatment to patients and their families significantly affect whether it is adopted widely. Until their value is clearly established, new and costly products should be deployed judiciously and after careful consideration of the goals of treatment, available options, and the unique needs, preferences, and goals of individual patients.
More research is needed to improve how we assess the value of new cancer drug treatments. New clinical efficacy endpoints – both provider- and patient-reported ones – that accurately describe how a patient feels and functions must be developed and should reflect outcomes of value to patients other than survival, particularly in noncurative settings.
Better predictive biomarkers can transform a drug of modest efficacy in an unselected population to one of high efficacy in a biomarker-defined subgroup and thereby contribute to improving the value of a treatment.
Regulatory and policy initiatives such as adaptive licensing, value-based insurance, and indication-specific pricing that affect marketing approval, reimbursement, or price, respectively, based on treatment effectiveness, also deserve careful consideration and further research to determine their effects on aligning cost with benefit while ensuring patient access to potentially life-extending therapies and continued innovation in drug development.
Q: Aside from the policy options, what’s the role of the oncologist in discussing the value of drugs with patients when determining a treatment plan?
A: Since oncologists don’t control drug prices, our role in improving the value of cancer care involves appropriately managing how resources are used and guiding patients during discussions around the right treatment plan for their particular diagnosis, prognosis, and treatment goals.
Adopting and adhering to high-quality oncology clinical pathways is an important way to improve the quality, efficiency, and value of cancer care. High-quality oncology pathways are detailed, evidence-based treatment protocols for delivering cancer care to patients with specific disease types and stages. When properly designed and implemented, oncology pathways serve as an important tool in appropriately managing cancer care resources and improving the quality of care that patients with cancer receive, while also reducing costs.
Dr. Schilsky is the senior vice president and chief medical officer of ASCO. Formerly the chief of hematology/oncology in the department of medicine and deputy director of the University of Chicago Comprehensive Cancer Center, he is a leader in the field of clinical oncology, specializing in new drug development and the treatment of gastrointestinal cancers. Dr. Schilsky reported research funding from several pharmaceutical companies to ASCO for the Targeted Agent and Profiling Utilization Registry (TAPUR) clinical trial. He also reported travel/accommodation/expense support from Varian.
Ongoing research aims to improve transplant outcomes in sickle cell
Researchers are leading several studies designed to improve hematopoietic stem cell transplantation (HSCT) for patients with sickle cell disease (SCD), experts at the National Heart, Lung, and Blood Institute reported during a recent webinar.
“HSCT offers a potential cure [for SCD], which may improve quantity and quality of life [for patients],” said Courtney D. Fitzhugh, MD, a Lasker Clinical Research Scholar in the Laboratory of Early Sickle Mortality Prevention at NHLBI.
Currently, HLA-matched sibling and matched unrelated donor sources provide the best outcomes for sickle cell patients undergoing allogeneic HSCT, she explained. Alternative stem cell sources include umbilical cord blood and haploidentical donors.
Over the past 2 years, the majority of novel transplant techniques have been primarily aimed at improving conditioning regimens and lowering rates of graft-versus-host disease (GVHD).
Recent evidence
A recent international survey found high survival rates in patients with SCD who underwent HLA-matched sibling HSCT during 1986-2013. At 5-years, overall- and event-free survival rates were 92.9% and 91.4%, respectively, with even higher rates (95% and 93%) seen in children aged younger than 16 years.
With respect to safety, the cumulative incidence rates of acute and chronic GVHD were 14.8% and 14.3%, Dr. Fitzhugh reported.
Much of the success seen with HLA-matched sibling donors is attributable to recent data demonstrating that complete transformation of patient’s bone marrow is unnecessary to illicit a curative effect.
With donor myeloid chimerism levels of at least 20%, the sickle disease phenotype can be reversed, and there’s a reduced risk of GVHD, she said.
In mouse models, researchers have found that inclusion of sirolimus in HLA-matched pretransplant conditioning regimens leads to higher levels of donor cell engraftment. As a result, some conditioning regimens now administer sirolimus (target 10-15 ng/dL) one-day prior to transplantation.
In 55 patients transplanted using this technique, overall- and event-free survival rates of 93% and 87% have been reported, with no transplant-related mortality or evidence of GVHD. Other institutions have also begun to adopt this technique, and have reported similar findings, Dr. Fitzhugh reported.
“When you [administer high-dose] chemotherapy, you don’t expect that patients are able to have children, but we are excited to report that 8 of our patients have had 13 healthy babies post transplant,” Dr. Fitzhugh said.
As a whole, several recent studies have emphasized the importance of the conditioning regimen in successful transplantation for patients with SCD.
With HLA-matched sibling donors, myeloablative regimens that include antithymocyte globulin have demonstrated greater efficacy, she said.
In patients receiving a transplant from a matched unrelated donor, early use of alemtuzumab is linked to higher rates of GVHD, while ongoing studies are exploring whether abatacept reduces the risk of GVHD, she further explained.
With respect to haploidentical and unrelated umbilical cord donors, T-cell depletion and higher-intensity conditioning have been shown to reduce graft rejection rates, she said.
Dr. Fitzhugh acknowledged that long-term efficacy and safety of these novel conditioning regimens is largely unknown. Thus, ongoing follow-up is essential to monitor for potential late effects.
NHLBI-funded trials
Nancy L. DiFronzo, PhD, program director at NHLBI, explained that the agency has funded specific clinical studies evaluating allogeneic HSCT in patients with severe SCD.
“[Surprisingly], this treatment modality is [actually] quite rare, with [only] approximately 9,000 allogeneic transplants occurring in the United States each year,” she said.
One of the primary barriers to HSCT for SCD is a lack of compatible donors. Currently, fewer than 20% of sickle cell patients have a matched unrelated donor or HLA-matched sibling donor, she reported.
Another common barrier are the risks associated with the procedure, including treatment-related toxicities and death. Active participation in a clinical trial is one strategy that can mitigate these risks, she said.
The Blood and Marrow Transplant Clinical Trials Network (BMT CTN) is a group of transplant centers that are recognized experts in HSCT. Dr. DiFronzo explained that the consortium is cosponsored by the National Cancer Institute and NHLBI, with the goal of improving outcomes for both pediatric and adult patients with SCD undergoing HSCT.
At present, the BMT CTN has directly funded three multicenter clinical studies for SCD, including the SCURT study, which has now been completed, as well as the STRIDE2 and Haploidentical HCT trials, both of which are currently enrolling patients.
“The goal of these new approaches [being studied in these 3 trials] is cure, where individuals can live longer with a better quality of life,” Dr. DiFronzo said. “We’ve [specifically] adjusted regimens with [this goal] in mind.”
Dr. Fitzhugh and Dr. DiFronzo did not provide information on financial disclosures.
Researchers are leading several studies designed to improve hematopoietic stem cell transplantation (HSCT) for patients with sickle cell disease (SCD), experts at the National Heart, Lung, and Blood Institute reported during a recent webinar.
“HSCT offers a potential cure [for SCD], which may improve quantity and quality of life [for patients],” said Courtney D. Fitzhugh, MD, a Lasker Clinical Research Scholar in the Laboratory of Early Sickle Mortality Prevention at NHLBI.
Currently, HLA-matched sibling and matched unrelated donor sources provide the best outcomes for sickle cell patients undergoing allogeneic HSCT, she explained. Alternative stem cell sources include umbilical cord blood and haploidentical donors.
Over the past 2 years, the majority of novel transplant techniques have been primarily aimed at improving conditioning regimens and lowering rates of graft-versus-host disease (GVHD).
Recent evidence
A recent international survey found high survival rates in patients with SCD who underwent HLA-matched sibling HSCT during 1986-2013. At 5-years, overall- and event-free survival rates were 92.9% and 91.4%, respectively, with even higher rates (95% and 93%) seen in children aged younger than 16 years.
With respect to safety, the cumulative incidence rates of acute and chronic GVHD were 14.8% and 14.3%, Dr. Fitzhugh reported.
Much of the success seen with HLA-matched sibling donors is attributable to recent data demonstrating that complete transformation of patient’s bone marrow is unnecessary to illicit a curative effect.
With donor myeloid chimerism levels of at least 20%, the sickle disease phenotype can be reversed, and there’s a reduced risk of GVHD, she said.
In mouse models, researchers have found that inclusion of sirolimus in HLA-matched pretransplant conditioning regimens leads to higher levels of donor cell engraftment. As a result, some conditioning regimens now administer sirolimus (target 10-15 ng/dL) one-day prior to transplantation.
In 55 patients transplanted using this technique, overall- and event-free survival rates of 93% and 87% have been reported, with no transplant-related mortality or evidence of GVHD. Other institutions have also begun to adopt this technique, and have reported similar findings, Dr. Fitzhugh reported.
“When you [administer high-dose] chemotherapy, you don’t expect that patients are able to have children, but we are excited to report that 8 of our patients have had 13 healthy babies post transplant,” Dr. Fitzhugh said.
As a whole, several recent studies have emphasized the importance of the conditioning regimen in successful transplantation for patients with SCD.
With HLA-matched sibling donors, myeloablative regimens that include antithymocyte globulin have demonstrated greater efficacy, she said.
In patients receiving a transplant from a matched unrelated donor, early use of alemtuzumab is linked to higher rates of GVHD, while ongoing studies are exploring whether abatacept reduces the risk of GVHD, she further explained.
With respect to haploidentical and unrelated umbilical cord donors, T-cell depletion and higher-intensity conditioning have been shown to reduce graft rejection rates, she said.
Dr. Fitzhugh acknowledged that long-term efficacy and safety of these novel conditioning regimens is largely unknown. Thus, ongoing follow-up is essential to monitor for potential late effects.
NHLBI-funded trials
Nancy L. DiFronzo, PhD, program director at NHLBI, explained that the agency has funded specific clinical studies evaluating allogeneic HSCT in patients with severe SCD.
“[Surprisingly], this treatment modality is [actually] quite rare, with [only] approximately 9,000 allogeneic transplants occurring in the United States each year,” she said.
One of the primary barriers to HSCT for SCD is a lack of compatible donors. Currently, fewer than 20% of sickle cell patients have a matched unrelated donor or HLA-matched sibling donor, she reported.
Another common barrier are the risks associated with the procedure, including treatment-related toxicities and death. Active participation in a clinical trial is one strategy that can mitigate these risks, she said.
The Blood and Marrow Transplant Clinical Trials Network (BMT CTN) is a group of transplant centers that are recognized experts in HSCT. Dr. DiFronzo explained that the consortium is cosponsored by the National Cancer Institute and NHLBI, with the goal of improving outcomes for both pediatric and adult patients with SCD undergoing HSCT.
At present, the BMT CTN has directly funded three multicenter clinical studies for SCD, including the SCURT study, which has now been completed, as well as the STRIDE2 and Haploidentical HCT trials, both of which are currently enrolling patients.
“The goal of these new approaches [being studied in these 3 trials] is cure, where individuals can live longer with a better quality of life,” Dr. DiFronzo said. “We’ve [specifically] adjusted regimens with [this goal] in mind.”
Dr. Fitzhugh and Dr. DiFronzo did not provide information on financial disclosures.
Researchers are leading several studies designed to improve hematopoietic stem cell transplantation (HSCT) for patients with sickle cell disease (SCD), experts at the National Heart, Lung, and Blood Institute reported during a recent webinar.
“HSCT offers a potential cure [for SCD], which may improve quantity and quality of life [for patients],” said Courtney D. Fitzhugh, MD, a Lasker Clinical Research Scholar in the Laboratory of Early Sickle Mortality Prevention at NHLBI.
Currently, HLA-matched sibling and matched unrelated donor sources provide the best outcomes for sickle cell patients undergoing allogeneic HSCT, she explained. Alternative stem cell sources include umbilical cord blood and haploidentical donors.
Over the past 2 years, the majority of novel transplant techniques have been primarily aimed at improving conditioning regimens and lowering rates of graft-versus-host disease (GVHD).
Recent evidence
A recent international survey found high survival rates in patients with SCD who underwent HLA-matched sibling HSCT during 1986-2013. At 5-years, overall- and event-free survival rates were 92.9% and 91.4%, respectively, with even higher rates (95% and 93%) seen in children aged younger than 16 years.
With respect to safety, the cumulative incidence rates of acute and chronic GVHD were 14.8% and 14.3%, Dr. Fitzhugh reported.
Much of the success seen with HLA-matched sibling donors is attributable to recent data demonstrating that complete transformation of patient’s bone marrow is unnecessary to illicit a curative effect.
With donor myeloid chimerism levels of at least 20%, the sickle disease phenotype can be reversed, and there’s a reduced risk of GVHD, she said.
In mouse models, researchers have found that inclusion of sirolimus in HLA-matched pretransplant conditioning regimens leads to higher levels of donor cell engraftment. As a result, some conditioning regimens now administer sirolimus (target 10-15 ng/dL) one-day prior to transplantation.
In 55 patients transplanted using this technique, overall- and event-free survival rates of 93% and 87% have been reported, with no transplant-related mortality or evidence of GVHD. Other institutions have also begun to adopt this technique, and have reported similar findings, Dr. Fitzhugh reported.
“When you [administer high-dose] chemotherapy, you don’t expect that patients are able to have children, but we are excited to report that 8 of our patients have had 13 healthy babies post transplant,” Dr. Fitzhugh said.
As a whole, several recent studies have emphasized the importance of the conditioning regimen in successful transplantation for patients with SCD.
With HLA-matched sibling donors, myeloablative regimens that include antithymocyte globulin have demonstrated greater efficacy, she said.
In patients receiving a transplant from a matched unrelated donor, early use of alemtuzumab is linked to higher rates of GVHD, while ongoing studies are exploring whether abatacept reduces the risk of GVHD, she further explained.
With respect to haploidentical and unrelated umbilical cord donors, T-cell depletion and higher-intensity conditioning have been shown to reduce graft rejection rates, she said.
Dr. Fitzhugh acknowledged that long-term efficacy and safety of these novel conditioning regimens is largely unknown. Thus, ongoing follow-up is essential to monitor for potential late effects.
NHLBI-funded trials
Nancy L. DiFronzo, PhD, program director at NHLBI, explained that the agency has funded specific clinical studies evaluating allogeneic HSCT in patients with severe SCD.
“[Surprisingly], this treatment modality is [actually] quite rare, with [only] approximately 9,000 allogeneic transplants occurring in the United States each year,” she said.
One of the primary barriers to HSCT for SCD is a lack of compatible donors. Currently, fewer than 20% of sickle cell patients have a matched unrelated donor or HLA-matched sibling donor, she reported.
Another common barrier are the risks associated with the procedure, including treatment-related toxicities and death. Active participation in a clinical trial is one strategy that can mitigate these risks, she said.
The Blood and Marrow Transplant Clinical Trials Network (BMT CTN) is a group of transplant centers that are recognized experts in HSCT. Dr. DiFronzo explained that the consortium is cosponsored by the National Cancer Institute and NHLBI, with the goal of improving outcomes for both pediatric and adult patients with SCD undergoing HSCT.
At present, the BMT CTN has directly funded three multicenter clinical studies for SCD, including the SCURT study, which has now been completed, as well as the STRIDE2 and Haploidentical HCT trials, both of which are currently enrolling patients.
“The goal of these new approaches [being studied in these 3 trials] is cure, where individuals can live longer with a better quality of life,” Dr. DiFronzo said. “We’ve [specifically] adjusted regimens with [this goal] in mind.”
Dr. Fitzhugh and Dr. DiFronzo did not provide information on financial disclosures.
Is it safe to discharge patients with anemia?
Background: Anemia is common in hospitalized patients and is associated with short- and long-term morbidity and mortality. Current evidence shows that reduced red blood cell (RBC) use and more restrictive transfusion practices do not increase short-term mortality; however, few data exist on the long-term outcomes of anemia.
Study design: Retrospective cohort study.
Setting: Integrated health care system (Kaiser Permanente) with 21 hospitals located in Northern California.
Synopsis: From 2010 to 2014, there were 801,261 hospitalizations among 445,371 patients who survived to discharge. The prevalence of moderate anemia (hemoglobin between 7 and 10 g/dL) at hospital discharge increased from 20% to 25% (P less than .001) while RBC transfusions decreased by 28% (P less than .001). Resolution of moderate anemia within 6 months of hospital discharge decreased from 42% to 34% (P less than .001). RBC transfusion and rehospitalization rates at 6 months decreased as well. During the study period, adjusted 6-month mortality decreased from 16.1% to 15.6% (P = .04) in patients with moderate anemia.
Given the retrospective design of this study, data must be interpreted with caution in determining a causal relationship. The authors also acknowledge that there may be unmeasured confounding variables not accounted for in the study results.
Bottom line: Despite higher rates of moderate anemia at discharge, there was not an associated rise in subsequent RBC transfusions, readmissions, or mortality in the 6 months after hospital discharge.
Citation: Roubinian NH et al. Long-term outcomes among patients discharged from the hospital with moderate anemia: A retrospective cohort study. Ann Intern Med. 2019 Jan 14. doi: 10.7326/M17-3253.
Dr. Schmit is an associate professor of medicine in the division of general and hospital medicine at UT Health San Antonio and a hospitalist at South Texas Veterans Health Care System, also in San Antonio.
Background: Anemia is common in hospitalized patients and is associated with short- and long-term morbidity and mortality. Current evidence shows that reduced red blood cell (RBC) use and more restrictive transfusion practices do not increase short-term mortality; however, few data exist on the long-term outcomes of anemia.
Study design: Retrospective cohort study.
Setting: Integrated health care system (Kaiser Permanente) with 21 hospitals located in Northern California.
Synopsis: From 2010 to 2014, there were 801,261 hospitalizations among 445,371 patients who survived to discharge. The prevalence of moderate anemia (hemoglobin between 7 and 10 g/dL) at hospital discharge increased from 20% to 25% (P less than .001) while RBC transfusions decreased by 28% (P less than .001). Resolution of moderate anemia within 6 months of hospital discharge decreased from 42% to 34% (P less than .001). RBC transfusion and rehospitalization rates at 6 months decreased as well. During the study period, adjusted 6-month mortality decreased from 16.1% to 15.6% (P = .04) in patients with moderate anemia.
Given the retrospective design of this study, data must be interpreted with caution in determining a causal relationship. The authors also acknowledge that there may be unmeasured confounding variables not accounted for in the study results.
Bottom line: Despite higher rates of moderate anemia at discharge, there was not an associated rise in subsequent RBC transfusions, readmissions, or mortality in the 6 months after hospital discharge.
Citation: Roubinian NH et al. Long-term outcomes among patients discharged from the hospital with moderate anemia: A retrospective cohort study. Ann Intern Med. 2019 Jan 14. doi: 10.7326/M17-3253.
Dr. Schmit is an associate professor of medicine in the division of general and hospital medicine at UT Health San Antonio and a hospitalist at South Texas Veterans Health Care System, also in San Antonio.
Background: Anemia is common in hospitalized patients and is associated with short- and long-term morbidity and mortality. Current evidence shows that reduced red blood cell (RBC) use and more restrictive transfusion practices do not increase short-term mortality; however, few data exist on the long-term outcomes of anemia.
Study design: Retrospective cohort study.
Setting: Integrated health care system (Kaiser Permanente) with 21 hospitals located in Northern California.
Synopsis: From 2010 to 2014, there were 801,261 hospitalizations among 445,371 patients who survived to discharge. The prevalence of moderate anemia (hemoglobin between 7 and 10 g/dL) at hospital discharge increased from 20% to 25% (P less than .001) while RBC transfusions decreased by 28% (P less than .001). Resolution of moderate anemia within 6 months of hospital discharge decreased from 42% to 34% (P less than .001). RBC transfusion and rehospitalization rates at 6 months decreased as well. During the study period, adjusted 6-month mortality decreased from 16.1% to 15.6% (P = .04) in patients with moderate anemia.
Given the retrospective design of this study, data must be interpreted with caution in determining a causal relationship. The authors also acknowledge that there may be unmeasured confounding variables not accounted for in the study results.
Bottom line: Despite higher rates of moderate anemia at discharge, there was not an associated rise in subsequent RBC transfusions, readmissions, or mortality in the 6 months after hospital discharge.
Citation: Roubinian NH et al. Long-term outcomes among patients discharged from the hospital with moderate anemia: A retrospective cohort study. Ann Intern Med. 2019 Jan 14. doi: 10.7326/M17-3253.
Dr. Schmit is an associate professor of medicine in the division of general and hospital medicine at UT Health San Antonio and a hospitalist at South Texas Veterans Health Care System, also in San Antonio.
CAR T-cell therapy found safe, effective for HIV-associated lymphoma
HIV positivity does not preclude chimeric antigen receptor (CAR) T-cell therapy for patients with aggressive lymphoma, a report of two cases suggests. Both of the HIV-positive patients, one of whom had long-term psychiatric comorbidity, achieved durable remission on axicabtagene ciloleucel (Yescarta) without undue toxicity.
“To our knowledge, these are the first reported cases of CAR T-cell therapy administered to HIV-infected patients with lymphoma,” Jeremy S. Abramson, MD, of Massachusetts General Hospital, Boston and his colleagues wrote in Cancer. “Patients with HIV and AIDS, as well as those with preexisting mental illness, should not be considered disqualified from CAR T-cell therapy and deserve ongoing studies to optimize efficacy and safety in this population.”
The Food and Drug Administration has approved two CAR T-cell products that target the B-cell antigen CD19 for the treatment of refractory lymphoma. But their efficacy and safety in HIV-positive patients are unknown because this group has been excluded from pivotal clinical trials.
Dr. Abramson and coauthors detail the two cases of successful anti-CD19 CAR T-cell therapy with axicabtagene ciloleucel in patients with HIV-associated, refractory, high-grade B-cell lymphoma.
The first patient was an HIV-positive man with diffuse large B-cell lymphoma (DLBCL) of germinal center B-cell subtype who was intermittently adherent to antiretroviral therapy. His comorbidities included posttraumatic stress disorder and schizoaffective disorder.
Previous treatments for DLBCL included dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (EPOCH-R), and rituximab, ifosfamide, carboplatin, and etoposide (RICE). A recurrence precluded high-dose chemotherapy with autologous stem cell support.
With close multidisciplinary management, including psychiatric consultation, the patient became a candidate for CAR T-cell therapy and received axicabtagene ciloleucel. He experienced grade 2 cytokine release syndrome and grade 3 neurologic toxicity, both of which resolved with treatment. Imaging showed complete remission at approximately 3 months that was sustained at 1 year. Additionally, he had an undetectable HIV viral load and was psychiatrically stable.
The second patient was a man with AIDS-associated, non–germinal center B-cell, Epstein-Barr virus–positive DLBCL who was adherent to antiretroviral therapy. His lymphoma had recurred rapidly after initially responding to dose-adjusted EPOCH-R and then was refractory to combination rituximab and lenalidomide. He previously had hepatitis B virus, cytomegalovirus, and Mycobacterium avium complex infections.
Because of prolonged cytopenias and infectious complications after the previous lymphoma treatments, the patient was considered a poor candidate for high-dose chemotherapy. He underwent CAR T-cell therapy with axicabtagene ciloleucel and had a complete remission on day 28. Additionally, his HIV infection remained well controlled.
“Although much remains to be learned regarding CAR T-cell therapy in patients with refractory hematologic malignancies, with or without HIV infection, the cases presented herein demonstrate that patients with chemotherapy-refractory, high-grade B-cell lymphoma can successfully undergo autologous CAR T-cell manufacturing, and subsequently can safely tolerate CAR T-cell therapy and achieve a durable complete remission,” the researchers wrote. “These cases have further demonstrated the proactive, multidisciplinary care required to navigate a patient with high-risk lymphoma through CAR T-cell therapy with attention to significant medical and psychiatric comorbidities.”
Dr. Abramson reported that he has acted as a paid member of the scientific advisory board and as a paid consultant for Kite Pharma, which markets Yescarta, and several other companies.
SOURCE: Abramson JS et al. Cancer. 2019 Sep 10. doi: 10.1002/cncr.32411.
HIV positivity does not preclude chimeric antigen receptor (CAR) T-cell therapy for patients with aggressive lymphoma, a report of two cases suggests. Both of the HIV-positive patients, one of whom had long-term psychiatric comorbidity, achieved durable remission on axicabtagene ciloleucel (Yescarta) without undue toxicity.
“To our knowledge, these are the first reported cases of CAR T-cell therapy administered to HIV-infected patients with lymphoma,” Jeremy S. Abramson, MD, of Massachusetts General Hospital, Boston and his colleagues wrote in Cancer. “Patients with HIV and AIDS, as well as those with preexisting mental illness, should not be considered disqualified from CAR T-cell therapy and deserve ongoing studies to optimize efficacy and safety in this population.”
The Food and Drug Administration has approved two CAR T-cell products that target the B-cell antigen CD19 for the treatment of refractory lymphoma. But their efficacy and safety in HIV-positive patients are unknown because this group has been excluded from pivotal clinical trials.
Dr. Abramson and coauthors detail the two cases of successful anti-CD19 CAR T-cell therapy with axicabtagene ciloleucel in patients with HIV-associated, refractory, high-grade B-cell lymphoma.
The first patient was an HIV-positive man with diffuse large B-cell lymphoma (DLBCL) of germinal center B-cell subtype who was intermittently adherent to antiretroviral therapy. His comorbidities included posttraumatic stress disorder and schizoaffective disorder.
Previous treatments for DLBCL included dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (EPOCH-R), and rituximab, ifosfamide, carboplatin, and etoposide (RICE). A recurrence precluded high-dose chemotherapy with autologous stem cell support.
With close multidisciplinary management, including psychiatric consultation, the patient became a candidate for CAR T-cell therapy and received axicabtagene ciloleucel. He experienced grade 2 cytokine release syndrome and grade 3 neurologic toxicity, both of which resolved with treatment. Imaging showed complete remission at approximately 3 months that was sustained at 1 year. Additionally, he had an undetectable HIV viral load and was psychiatrically stable.
The second patient was a man with AIDS-associated, non–germinal center B-cell, Epstein-Barr virus–positive DLBCL who was adherent to antiretroviral therapy. His lymphoma had recurred rapidly after initially responding to dose-adjusted EPOCH-R and then was refractory to combination rituximab and lenalidomide. He previously had hepatitis B virus, cytomegalovirus, and Mycobacterium avium complex infections.
Because of prolonged cytopenias and infectious complications after the previous lymphoma treatments, the patient was considered a poor candidate for high-dose chemotherapy. He underwent CAR T-cell therapy with axicabtagene ciloleucel and had a complete remission on day 28. Additionally, his HIV infection remained well controlled.
“Although much remains to be learned regarding CAR T-cell therapy in patients with refractory hematologic malignancies, with or without HIV infection, the cases presented herein demonstrate that patients with chemotherapy-refractory, high-grade B-cell lymphoma can successfully undergo autologous CAR T-cell manufacturing, and subsequently can safely tolerate CAR T-cell therapy and achieve a durable complete remission,” the researchers wrote. “These cases have further demonstrated the proactive, multidisciplinary care required to navigate a patient with high-risk lymphoma through CAR T-cell therapy with attention to significant medical and psychiatric comorbidities.”
Dr. Abramson reported that he has acted as a paid member of the scientific advisory board and as a paid consultant for Kite Pharma, which markets Yescarta, and several other companies.
SOURCE: Abramson JS et al. Cancer. 2019 Sep 10. doi: 10.1002/cncr.32411.
HIV positivity does not preclude chimeric antigen receptor (CAR) T-cell therapy for patients with aggressive lymphoma, a report of two cases suggests. Both of the HIV-positive patients, one of whom had long-term psychiatric comorbidity, achieved durable remission on axicabtagene ciloleucel (Yescarta) without undue toxicity.
“To our knowledge, these are the first reported cases of CAR T-cell therapy administered to HIV-infected patients with lymphoma,” Jeremy S. Abramson, MD, of Massachusetts General Hospital, Boston and his colleagues wrote in Cancer. “Patients with HIV and AIDS, as well as those with preexisting mental illness, should not be considered disqualified from CAR T-cell therapy and deserve ongoing studies to optimize efficacy and safety in this population.”
The Food and Drug Administration has approved two CAR T-cell products that target the B-cell antigen CD19 for the treatment of refractory lymphoma. But their efficacy and safety in HIV-positive patients are unknown because this group has been excluded from pivotal clinical trials.
Dr. Abramson and coauthors detail the two cases of successful anti-CD19 CAR T-cell therapy with axicabtagene ciloleucel in patients with HIV-associated, refractory, high-grade B-cell lymphoma.
The first patient was an HIV-positive man with diffuse large B-cell lymphoma (DLBCL) of germinal center B-cell subtype who was intermittently adherent to antiretroviral therapy. His comorbidities included posttraumatic stress disorder and schizoaffective disorder.
Previous treatments for DLBCL included dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (EPOCH-R), and rituximab, ifosfamide, carboplatin, and etoposide (RICE). A recurrence precluded high-dose chemotherapy with autologous stem cell support.
With close multidisciplinary management, including psychiatric consultation, the patient became a candidate for CAR T-cell therapy and received axicabtagene ciloleucel. He experienced grade 2 cytokine release syndrome and grade 3 neurologic toxicity, both of which resolved with treatment. Imaging showed complete remission at approximately 3 months that was sustained at 1 year. Additionally, he had an undetectable HIV viral load and was psychiatrically stable.
The second patient was a man with AIDS-associated, non–germinal center B-cell, Epstein-Barr virus–positive DLBCL who was adherent to antiretroviral therapy. His lymphoma had recurred rapidly after initially responding to dose-adjusted EPOCH-R and then was refractory to combination rituximab and lenalidomide. He previously had hepatitis B virus, cytomegalovirus, and Mycobacterium avium complex infections.
Because of prolonged cytopenias and infectious complications after the previous lymphoma treatments, the patient was considered a poor candidate for high-dose chemotherapy. He underwent CAR T-cell therapy with axicabtagene ciloleucel and had a complete remission on day 28. Additionally, his HIV infection remained well controlled.
“Although much remains to be learned regarding CAR T-cell therapy in patients with refractory hematologic malignancies, with or without HIV infection, the cases presented herein demonstrate that patients with chemotherapy-refractory, high-grade B-cell lymphoma can successfully undergo autologous CAR T-cell manufacturing, and subsequently can safely tolerate CAR T-cell therapy and achieve a durable complete remission,” the researchers wrote. “These cases have further demonstrated the proactive, multidisciplinary care required to navigate a patient with high-risk lymphoma through CAR T-cell therapy with attention to significant medical and psychiatric comorbidities.”
Dr. Abramson reported that he has acted as a paid member of the scientific advisory board and as a paid consultant for Kite Pharma, which markets Yescarta, and several other companies.
SOURCE: Abramson JS et al. Cancer. 2019 Sep 10. doi: 10.1002/cncr.32411.
FROM CANCER
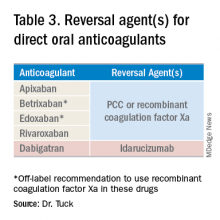
Reversal agents for direct-acting oral anticoagulants
Summary of guidelines published in the Journal of Hospital Medicine
When on call for admissions, a hospitalist receives a request from a colleague to admit an octogenarian man with an acute uncomplicated deep vein thrombosis to start heparin, bridging to warfarin. The patient has no evidence of postphlebitic syndrome, pulmonary embolism, or right-sided heart strain. The hospitalist asks her colleague if he had considered treating the patient in the ambulatory setting using a direct-acting oral anticoagulant (DOAC). After all, this would save the patient an unnecessary hospitalization, weekly international normalized ratio checks, and other important lifestyle changes. In response, the colleague voices concern that the “new drugs don’t have antidotes.”
DOACs have several benefits over vitamin K antagonists (VKAs) and heparins. DOACs have quicker onset of action, can be taken by mouth, in general do not require dosage adjustment, and have fewer dietary and lifestyle modifications, compared with VKAs and heparins. In atrial fibrillation, DOACs have been shown to have lower all-cause and bleeding-related mortality than warfarin (see Table 1).1 Observational studies also suggest less risk of major bleeding with DOACs over warfarin but no difference in overall mortality when used to treat venous thromboembolism (see Table 2).2 Because of these combined advantages, DOACs are increasingly prescribed, accounting for approximately half of all oral anticoagulant prescriptions in 2014.3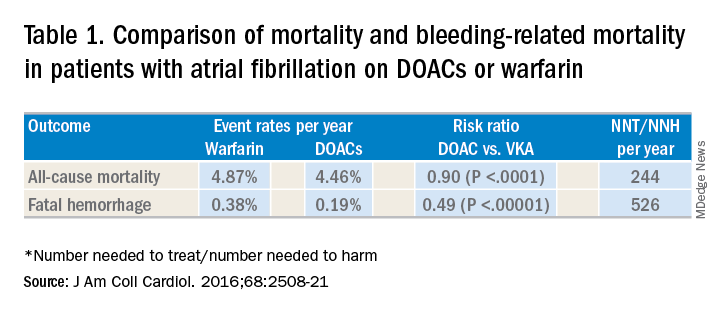
Although DOACs have been shown to be as good if not superior to VKAs and heparins in these circumstances, there are situations where a DOAC should not be used. There is limited data on the safety of DOACs in patients with mechanical heart valves, liver failure, and chronic kidney disease with a creatinine clearance less than 30 mL/min.4 Therefore, warfarin is still the preferred agent in these settings. There is some data that apixaban may be safe in patients with a creatinine clearance of greater than 10 mL/min, but long-term safety studies have not been performed in patients with end-stage renal disease on hemodialysis.5 Finally, in patients requiring concomitant inducers or inhibitors of the P-glycoprotein or cytochrome P450 enzymes like antiepileptics and protease inhibitors, VKAs and heparins are favored.4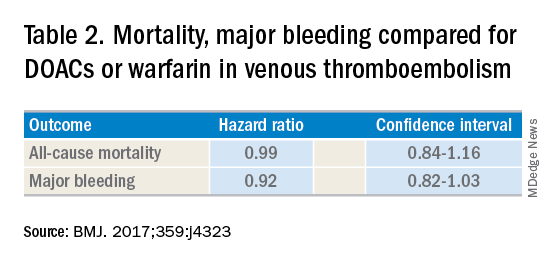
Notwithstanding their advantages, when DOACs first hit the market there were concerns that reversal agents were not available. In the August issue of the Journal of Hospital Medicine’s Clinical Guideline Highlights for the Hospitalist, Emily Gottenborg, MD, and Gregory Misky, MD, summarized guideline recommendations for reversal of the newer agents.6 This includes use of idarucizumab for patients on dabigatran and use of prothrombin complex concentrate (PCC) or recombinant coagulation factor Xa (andexanet alfa) for patients on apixaban or rivaroxaban for the treatment of life-threatening bleeding.
Idarucizumab is a monoclonal antibody developed to reverse the effects of dabigatran, the only DOAC that directly inhibits thrombin. In 2017, researchers reported on a cohort of subjects receiving idarucizumab for uncontrolled bleeding or who were on dabigatran and about to undergo an urgent procedure.7 Of those with uncontrolled bleeding, two-thirds had confirmed bleeding cessation within 24 hours. Periprocedural hemostasis was achieved in 93.4% of patients undergoing urgent procedures. However, it should be noted that use of idarucizumab conferred an increase risk (6.3%) of thrombosis within 90 days. Based on these findings, guidelines recommend use of idarucizumab in patients experiencing life-threatening bleeding, balanced against the risk of thrombosis.8
In 2018, the Food and Drug Administration approved recombinant coagulation factor Xa for treatment of life-threatening or uncontrolled bleeding in patients on apixaban or rivaroxaban.9 The approval came after a study by the ANNEXA-4 investigators showed that recombinant coagulation factor Xa quickly and effectively achieved hemostasis.10 Full study results were published in April 2019, demonstrating 82% of patients receiving the drug attained clinical hemostasis.11 However, as with idarucizumab, up to 10% of patients had a thrombotic event in the follow-up period. Use of recombinant coagulation factor Xa for treatment of life-threatening bleeding related to betrixaban and edoxaban is considered off label but is recommended by guidelines.8 Studies on investigational reversal agents for betrixaban and edoxaban are ongoing.
Both unactivated and activated PCC contain clotting factor X. Their use to control bleeding related to DOAC use is based on observational studies. In a systematic review of the nonrandomized studies, the efficacy of PCC to stem major bleeding was 69% and the risk for thromboembolism was 4%.12 There are no head-to-head studies comparing use of recombinant coagulation factor Xa and PCC. Therefore, guidelines are to use either recombinant factor Xa or PCC for the treatment of life-threatening bleeding related to DOAC use.7
As thrombosis risk heightens after use of any reversal agent, the recommendations are to resume anticoagulation within 90 days if the patient is at moderate or high risk for recurrent thromboembolism.8
After discussion with the hospitalist about the new agents available to reverse anticoagulation, the colleague decided to place the patient on a DOAC and keep the patient in his nursing home. Thankfully, the patient did not thereafter experience sustained bleeding necessitating use of these reversal agents. More importantly for the patient, he was able to stay in the comfort of his home.
Dr. Tuck is associate section chief for hospital medicine at the Veterans Affairs Medical Center in Washington, D.C.
References
1. Gómez-Outes A et al. Causes of death in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol. 2016;68:2508-21.
2. Jun M et al. Comparative safety of direct oral anticoagulants and warfarin in venous thromboembolism: multicentre, population-based, observational study. BMJ. 2017;359:j4323.
3. Barnes GD et al. National trends in ambulatory oral anticoagulant use. Am J Med. 2015;128:(1300-5).e2.
4. Reddy P et al. Practical approach to VTE management in hospitalized patients. Am J Ther. 2017;24(4):e442-67.
5. Kimachi M et al. Direct oral anticoagulants versus warfarin for preventing stroke and systemic embolic events among atrial fibrillation patients with chronic kidney disease. Cochrane Database Syst Rev. 2017 Nov 6;11:CD011373.
6. Gottenborg E et al. Clinical guideline highlights for the hospitalist: The management of anticoagulation in the hospitalized adult. J Hosp Med. 2019; 14(8):499-500.
7. Pollack CV Jr et al. Idarucizumab for dabigatran reversal – full cohort analysis. N Engl J Med. 2017;377(5):431-41.
8. Witt DM et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: Optimal management of anticoagulation therapy. Blood Adv. 2018;2(22):3257-91.
9. Malarky M et al. FDA accelerated approval letter. Retrieved July 15, 2019. https://www.fda.gov/media/113285/download
10. Connolly SJ et al. Andexanet alfa for acute major bleeding associated with factor Xa inhibitors. N Engl J Med. 2016;375(12):1131-41.
11. Connolly SJ et al. Full study report of andexanet alfa for bleeding associated with factor xa inhibitors. N Engl J Med. 2019;380(14):1326-35.
12. Piran S et al. Management of direct factor Xa inhibitor–related major bleeding with prothrombin complex concentrate: A meta-analysis. Blood Adv. 2019;3(2):158-67.
Summary of guidelines published in the Journal of Hospital Medicine
Summary of guidelines published in the Journal of Hospital Medicine
When on call for admissions, a hospitalist receives a request from a colleague to admit an octogenarian man with an acute uncomplicated deep vein thrombosis to start heparin, bridging to warfarin. The patient has no evidence of postphlebitic syndrome, pulmonary embolism, or right-sided heart strain. The hospitalist asks her colleague if he had considered treating the patient in the ambulatory setting using a direct-acting oral anticoagulant (DOAC). After all, this would save the patient an unnecessary hospitalization, weekly international normalized ratio checks, and other important lifestyle changes. In response, the colleague voices concern that the “new drugs don’t have antidotes.”
DOACs have several benefits over vitamin K antagonists (VKAs) and heparins. DOACs have quicker onset of action, can be taken by mouth, in general do not require dosage adjustment, and have fewer dietary and lifestyle modifications, compared with VKAs and heparins. In atrial fibrillation, DOACs have been shown to have lower all-cause and bleeding-related mortality than warfarin (see Table 1).1 Observational studies also suggest less risk of major bleeding with DOACs over warfarin but no difference in overall mortality when used to treat venous thromboembolism (see Table 2).2 Because of these combined advantages, DOACs are increasingly prescribed, accounting for approximately half of all oral anticoagulant prescriptions in 2014.3
Although DOACs have been shown to be as good if not superior to VKAs and heparins in these circumstances, there are situations where a DOAC should not be used. There is limited data on the safety of DOACs in patients with mechanical heart valves, liver failure, and chronic kidney disease with a creatinine clearance less than 30 mL/min.4 Therefore, warfarin is still the preferred agent in these settings. There is some data that apixaban may be safe in patients with a creatinine clearance of greater than 10 mL/min, but long-term safety studies have not been performed in patients with end-stage renal disease on hemodialysis.5 Finally, in patients requiring concomitant inducers or inhibitors of the P-glycoprotein or cytochrome P450 enzymes like antiepileptics and protease inhibitors, VKAs and heparins are favored.4
Notwithstanding their advantages, when DOACs first hit the market there were concerns that reversal agents were not available. In the August issue of the Journal of Hospital Medicine’s Clinical Guideline Highlights for the Hospitalist, Emily Gottenborg, MD, and Gregory Misky, MD, summarized guideline recommendations for reversal of the newer agents.6 This includes use of idarucizumab for patients on dabigatran and use of prothrombin complex concentrate (PCC) or recombinant coagulation factor Xa (andexanet alfa) for patients on apixaban or rivaroxaban for the treatment of life-threatening bleeding.
Idarucizumab is a monoclonal antibody developed to reverse the effects of dabigatran, the only DOAC that directly inhibits thrombin. In 2017, researchers reported on a cohort of subjects receiving idarucizumab for uncontrolled bleeding or who were on dabigatran and about to undergo an urgent procedure.7 Of those with uncontrolled bleeding, two-thirds had confirmed bleeding cessation within 24 hours. Periprocedural hemostasis was achieved in 93.4% of patients undergoing urgent procedures. However, it should be noted that use of idarucizumab conferred an increase risk (6.3%) of thrombosis within 90 days. Based on these findings, guidelines recommend use of idarucizumab in patients experiencing life-threatening bleeding, balanced against the risk of thrombosis.8
In 2018, the Food and Drug Administration approved recombinant coagulation factor Xa for treatment of life-threatening or uncontrolled bleeding in patients on apixaban or rivaroxaban.9 The approval came after a study by the ANNEXA-4 investigators showed that recombinant coagulation factor Xa quickly and effectively achieved hemostasis.10 Full study results were published in April 2019, demonstrating 82% of patients receiving the drug attained clinical hemostasis.11 However, as with idarucizumab, up to 10% of patients had a thrombotic event in the follow-up period. Use of recombinant coagulation factor Xa for treatment of life-threatening bleeding related to betrixaban and edoxaban is considered off label but is recommended by guidelines.8 Studies on investigational reversal agents for betrixaban and edoxaban are ongoing.
Both unactivated and activated PCC contain clotting factor X. Their use to control bleeding related to DOAC use is based on observational studies. In a systematic review of the nonrandomized studies, the efficacy of PCC to stem major bleeding was 69% and the risk for thromboembolism was 4%.12 There are no head-to-head studies comparing use of recombinant coagulation factor Xa and PCC. Therefore, guidelines are to use either recombinant factor Xa or PCC for the treatment of life-threatening bleeding related to DOAC use.7
As thrombosis risk heightens after use of any reversal agent, the recommendations are to resume anticoagulation within 90 days if the patient is at moderate or high risk for recurrent thromboembolism.8
After discussion with the hospitalist about the new agents available to reverse anticoagulation, the colleague decided to place the patient on a DOAC and keep the patient in his nursing home. Thankfully, the patient did not thereafter experience sustained bleeding necessitating use of these reversal agents. More importantly for the patient, he was able to stay in the comfort of his home.
Dr. Tuck is associate section chief for hospital medicine at the Veterans Affairs Medical Center in Washington, D.C.
References
1. Gómez-Outes A et al. Causes of death in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol. 2016;68:2508-21.
2. Jun M et al. Comparative safety of direct oral anticoagulants and warfarin in venous thromboembolism: multicentre, population-based, observational study. BMJ. 2017;359:j4323.
3. Barnes GD et al. National trends in ambulatory oral anticoagulant use. Am J Med. 2015;128:(1300-5).e2.
4. Reddy P et al. Practical approach to VTE management in hospitalized patients. Am J Ther. 2017;24(4):e442-67.
5. Kimachi M et al. Direct oral anticoagulants versus warfarin for preventing stroke and systemic embolic events among atrial fibrillation patients with chronic kidney disease. Cochrane Database Syst Rev. 2017 Nov 6;11:CD011373.
6. Gottenborg E et al. Clinical guideline highlights for the hospitalist: The management of anticoagulation in the hospitalized adult. J Hosp Med. 2019; 14(8):499-500.
7. Pollack CV Jr et al. Idarucizumab for dabigatran reversal – full cohort analysis. N Engl J Med. 2017;377(5):431-41.
8. Witt DM et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: Optimal management of anticoagulation therapy. Blood Adv. 2018;2(22):3257-91.
9. Malarky M et al. FDA accelerated approval letter. Retrieved July 15, 2019. https://www.fda.gov/media/113285/download
10. Connolly SJ et al. Andexanet alfa for acute major bleeding associated with factor Xa inhibitors. N Engl J Med. 2016;375(12):1131-41.
11. Connolly SJ et al. Full study report of andexanet alfa for bleeding associated with factor xa inhibitors. N Engl J Med. 2019;380(14):1326-35.
12. Piran S et al. Management of direct factor Xa inhibitor–related major bleeding with prothrombin complex concentrate: A meta-analysis. Blood Adv. 2019;3(2):158-67.
When on call for admissions, a hospitalist receives a request from a colleague to admit an octogenarian man with an acute uncomplicated deep vein thrombosis to start heparin, bridging to warfarin. The patient has no evidence of postphlebitic syndrome, pulmonary embolism, or right-sided heart strain. The hospitalist asks her colleague if he had considered treating the patient in the ambulatory setting using a direct-acting oral anticoagulant (DOAC). After all, this would save the patient an unnecessary hospitalization, weekly international normalized ratio checks, and other important lifestyle changes. In response, the colleague voices concern that the “new drugs don’t have antidotes.”
DOACs have several benefits over vitamin K antagonists (VKAs) and heparins. DOACs have quicker onset of action, can be taken by mouth, in general do not require dosage adjustment, and have fewer dietary and lifestyle modifications, compared with VKAs and heparins. In atrial fibrillation, DOACs have been shown to have lower all-cause and bleeding-related mortality than warfarin (see Table 1).1 Observational studies also suggest less risk of major bleeding with DOACs over warfarin but no difference in overall mortality when used to treat venous thromboembolism (see Table 2).2 Because of these combined advantages, DOACs are increasingly prescribed, accounting for approximately half of all oral anticoagulant prescriptions in 2014.3
Although DOACs have been shown to be as good if not superior to VKAs and heparins in these circumstances, there are situations where a DOAC should not be used. There is limited data on the safety of DOACs in patients with mechanical heart valves, liver failure, and chronic kidney disease with a creatinine clearance less than 30 mL/min.4 Therefore, warfarin is still the preferred agent in these settings. There is some data that apixaban may be safe in patients with a creatinine clearance of greater than 10 mL/min, but long-term safety studies have not been performed in patients with end-stage renal disease on hemodialysis.5 Finally, in patients requiring concomitant inducers or inhibitors of the P-glycoprotein or cytochrome P450 enzymes like antiepileptics and protease inhibitors, VKAs and heparins are favored.4
Notwithstanding their advantages, when DOACs first hit the market there were concerns that reversal agents were not available. In the August issue of the Journal of Hospital Medicine’s Clinical Guideline Highlights for the Hospitalist, Emily Gottenborg, MD, and Gregory Misky, MD, summarized guideline recommendations for reversal of the newer agents.6 This includes use of idarucizumab for patients on dabigatran and use of prothrombin complex concentrate (PCC) or recombinant coagulation factor Xa (andexanet alfa) for patients on apixaban or rivaroxaban for the treatment of life-threatening bleeding.
Idarucizumab is a monoclonal antibody developed to reverse the effects of dabigatran, the only DOAC that directly inhibits thrombin. In 2017, researchers reported on a cohort of subjects receiving idarucizumab for uncontrolled bleeding or who were on dabigatran and about to undergo an urgent procedure.7 Of those with uncontrolled bleeding, two-thirds had confirmed bleeding cessation within 24 hours. Periprocedural hemostasis was achieved in 93.4% of patients undergoing urgent procedures. However, it should be noted that use of idarucizumab conferred an increase risk (6.3%) of thrombosis within 90 days. Based on these findings, guidelines recommend use of idarucizumab in patients experiencing life-threatening bleeding, balanced against the risk of thrombosis.8
In 2018, the Food and Drug Administration approved recombinant coagulation factor Xa for treatment of life-threatening or uncontrolled bleeding in patients on apixaban or rivaroxaban.9 The approval came after a study by the ANNEXA-4 investigators showed that recombinant coagulation factor Xa quickly and effectively achieved hemostasis.10 Full study results were published in April 2019, demonstrating 82% of patients receiving the drug attained clinical hemostasis.11 However, as with idarucizumab, up to 10% of patients had a thrombotic event in the follow-up period. Use of recombinant coagulation factor Xa for treatment of life-threatening bleeding related to betrixaban and edoxaban is considered off label but is recommended by guidelines.8 Studies on investigational reversal agents for betrixaban and edoxaban are ongoing.
Both unactivated and activated PCC contain clotting factor X. Their use to control bleeding related to DOAC use is based on observational studies. In a systematic review of the nonrandomized studies, the efficacy of PCC to stem major bleeding was 69% and the risk for thromboembolism was 4%.12 There are no head-to-head studies comparing use of recombinant coagulation factor Xa and PCC. Therefore, guidelines are to use either recombinant factor Xa or PCC for the treatment of life-threatening bleeding related to DOAC use.7
As thrombosis risk heightens after use of any reversal agent, the recommendations are to resume anticoagulation within 90 days if the patient is at moderate or high risk for recurrent thromboembolism.8
After discussion with the hospitalist about the new agents available to reverse anticoagulation, the colleague decided to place the patient on a DOAC and keep the patient in his nursing home. Thankfully, the patient did not thereafter experience sustained bleeding necessitating use of these reversal agents. More importantly for the patient, he was able to stay in the comfort of his home.
Dr. Tuck is associate section chief for hospital medicine at the Veterans Affairs Medical Center in Washington, D.C.
References
1. Gómez-Outes A et al. Causes of death in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol. 2016;68:2508-21.
2. Jun M et al. Comparative safety of direct oral anticoagulants and warfarin in venous thromboembolism: multicentre, population-based, observational study. BMJ. 2017;359:j4323.
3. Barnes GD et al. National trends in ambulatory oral anticoagulant use. Am J Med. 2015;128:(1300-5).e2.
4. Reddy P et al. Practical approach to VTE management in hospitalized patients. Am J Ther. 2017;24(4):e442-67.
5. Kimachi M et al. Direct oral anticoagulants versus warfarin for preventing stroke and systemic embolic events among atrial fibrillation patients with chronic kidney disease. Cochrane Database Syst Rev. 2017 Nov 6;11:CD011373.
6. Gottenborg E et al. Clinical guideline highlights for the hospitalist: The management of anticoagulation in the hospitalized adult. J Hosp Med. 2019; 14(8):499-500.
7. Pollack CV Jr et al. Idarucizumab for dabigatran reversal – full cohort analysis. N Engl J Med. 2017;377(5):431-41.
8. Witt DM et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: Optimal management of anticoagulation therapy. Blood Adv. 2018;2(22):3257-91.
9. Malarky M et al. FDA accelerated approval letter. Retrieved July 15, 2019. https://www.fda.gov/media/113285/download
10. Connolly SJ et al. Andexanet alfa for acute major bleeding associated with factor Xa inhibitors. N Engl J Med. 2016;375(12):1131-41.
11. Connolly SJ et al. Full study report of andexanet alfa for bleeding associated with factor xa inhibitors. N Engl J Med. 2019;380(14):1326-35.
12. Piran S et al. Management of direct factor Xa inhibitor–related major bleeding with prothrombin complex concentrate: A meta-analysis. Blood Adv. 2019;3(2):158-67.
Stem cells gene edited to be HIV resistant treat ALL, but not HIV
Gene editing of donor stem cells prior to transplantation into a patient with both HIV infection and acute lymphoblastic leukemia (ALL) was safe and effectively treated the patient’s leukemia, but failed to resolve his HIV, investigators reported.
The 27-year-old man received an HLA-matched transplant of hematopoietic stem and progenitor cells (HSPCs) that had been genetically engineered to lack CCR5, a key gateway for HIV entry into cells.
Although the transplant resulted in complete remission of leukemia with full donor chimerism, only about 9% of the posttransplant lymphocytes showed disruption of CCR5, and during a brief trial of antiretroviral therapy interruption his HIV viral load rebounded, reported Hongkui Deng, PhD, and colleagues from Peking University in China.
Although the experiment did not meet its goal of a drug-free HIV remission, it serves as a proof of concept for the use of CRISPR-Cas9 (clustered regularly interspaced palindromic repeats/CRISPR-associated protein 9) gene editing to treat HIV infection, the authors contend.
“These results show the proof of principle that transplantation and long-term engraftment of CRISPR-edited allogeneic HSPCs can be achieved; however, the efficiency of the response was not adequate to achieve the target of cure of HIV-1 infection,” they wrote in a brief report published in the New England Journal of Medicine.
As previously reported, other research groups have investigated genetic editing to mimic a naturally occurring mutation that effectively disables the CCR5 HIV coreceptor, preventing the retrovirus from entering healthy cells. The mutation was first identified in a man named Timothy Brown who came to be known as “the Berlin patient” after he was apparently cured of HIV infection after a bone marrow transplant from a donor who had the mutation.
Dr. Deng and colleagues took advantage of HSPC transplantation, a standard therapy for ALL to see whether it could also have beneficial effects on concomitant HIV infection.
They treated donor HSPCs with CRISPR-Cas9 to ablate CCR5 and then delivered them to the patient along with additional CD34-depleted donor cells from mobilized peripheral blood.
The transplant was a success, with neutrophil engraftment on day 13 and platelet engraftment on day 27, and the leukemia was in morphologic complete remission at week 4 following transplantation. The patient remained in complete remission from leukemia throughout the 19-month follow-up period, with full donor chimerism .
However, when a planned interruption of antiretroviral therapy was carried out at 7 months post transplant, the serum viral load increased to 3 × 107 copies/ml at week 4 following interruption, and the patient was restarted on the drug. His viral levels gradually decreased to undetectable level during the subsequent months.
The investigators noted that 2 weeks after the drug interruption trial was started there was a small increase in the percentage of CCR5 insertion/deletions.
“The low efficiency of gene editing in the patient may be due to the competitive engraftment of the coinfused HSPCs in CD34-depleted cells and the persistence of donor T cells. To further clarify the anti-HIV effect of CCR5-ablated HSPCs, it will be essential to increase the gene-editing efficiency of our CRISPR-Cas9 system and improve the transplantation protocol,” they wrote.
The study was funded by the Beijing Municipal Science and Technology Commission and others (unspecified). All authors reported having nothing to disclose.
SOURCE: Xu L et al. N Engl J Med. 2019. doi: 10.1056/NEJMoa1817426.
Gene editing of donor stem cells prior to transplantation into a patient with both HIV infection and acute lymphoblastic leukemia (ALL) was safe and effectively treated the patient’s leukemia, but failed to resolve his HIV, investigators reported.
The 27-year-old man received an HLA-matched transplant of hematopoietic stem and progenitor cells (HSPCs) that had been genetically engineered to lack CCR5, a key gateway for HIV entry into cells.
Although the transplant resulted in complete remission of leukemia with full donor chimerism, only about 9% of the posttransplant lymphocytes showed disruption of CCR5, and during a brief trial of antiretroviral therapy interruption his HIV viral load rebounded, reported Hongkui Deng, PhD, and colleagues from Peking University in China.
Although the experiment did not meet its goal of a drug-free HIV remission, it serves as a proof of concept for the use of CRISPR-Cas9 (clustered regularly interspaced palindromic repeats/CRISPR-associated protein 9) gene editing to treat HIV infection, the authors contend.
“These results show the proof of principle that transplantation and long-term engraftment of CRISPR-edited allogeneic HSPCs can be achieved; however, the efficiency of the response was not adequate to achieve the target of cure of HIV-1 infection,” they wrote in a brief report published in the New England Journal of Medicine.
As previously reported, other research groups have investigated genetic editing to mimic a naturally occurring mutation that effectively disables the CCR5 HIV coreceptor, preventing the retrovirus from entering healthy cells. The mutation was first identified in a man named Timothy Brown who came to be known as “the Berlin patient” after he was apparently cured of HIV infection after a bone marrow transplant from a donor who had the mutation.
Dr. Deng and colleagues took advantage of HSPC transplantation, a standard therapy for ALL to see whether it could also have beneficial effects on concomitant HIV infection.
They treated donor HSPCs with CRISPR-Cas9 to ablate CCR5 and then delivered them to the patient along with additional CD34-depleted donor cells from mobilized peripheral blood.
The transplant was a success, with neutrophil engraftment on day 13 and platelet engraftment on day 27, and the leukemia was in morphologic complete remission at week 4 following transplantation. The patient remained in complete remission from leukemia throughout the 19-month follow-up period, with full donor chimerism .
However, when a planned interruption of antiretroviral therapy was carried out at 7 months post transplant, the serum viral load increased to 3 × 107 copies/ml at week 4 following interruption, and the patient was restarted on the drug. His viral levels gradually decreased to undetectable level during the subsequent months.
The investigators noted that 2 weeks after the drug interruption trial was started there was a small increase in the percentage of CCR5 insertion/deletions.
“The low efficiency of gene editing in the patient may be due to the competitive engraftment of the coinfused HSPCs in CD34-depleted cells and the persistence of donor T cells. To further clarify the anti-HIV effect of CCR5-ablated HSPCs, it will be essential to increase the gene-editing efficiency of our CRISPR-Cas9 system and improve the transplantation protocol,” they wrote.
The study was funded by the Beijing Municipal Science and Technology Commission and others (unspecified). All authors reported having nothing to disclose.
SOURCE: Xu L et al. N Engl J Med. 2019. doi: 10.1056/NEJMoa1817426.
Gene editing of donor stem cells prior to transplantation into a patient with both HIV infection and acute lymphoblastic leukemia (ALL) was safe and effectively treated the patient’s leukemia, but failed to resolve his HIV, investigators reported.
The 27-year-old man received an HLA-matched transplant of hematopoietic stem and progenitor cells (HSPCs) that had been genetically engineered to lack CCR5, a key gateway for HIV entry into cells.
Although the transplant resulted in complete remission of leukemia with full donor chimerism, only about 9% of the posttransplant lymphocytes showed disruption of CCR5, and during a brief trial of antiretroviral therapy interruption his HIV viral load rebounded, reported Hongkui Deng, PhD, and colleagues from Peking University in China.
Although the experiment did not meet its goal of a drug-free HIV remission, it serves as a proof of concept for the use of CRISPR-Cas9 (clustered regularly interspaced palindromic repeats/CRISPR-associated protein 9) gene editing to treat HIV infection, the authors contend.
“These results show the proof of principle that transplantation and long-term engraftment of CRISPR-edited allogeneic HSPCs can be achieved; however, the efficiency of the response was not adequate to achieve the target of cure of HIV-1 infection,” they wrote in a brief report published in the New England Journal of Medicine.
As previously reported, other research groups have investigated genetic editing to mimic a naturally occurring mutation that effectively disables the CCR5 HIV coreceptor, preventing the retrovirus from entering healthy cells. The mutation was first identified in a man named Timothy Brown who came to be known as “the Berlin patient” after he was apparently cured of HIV infection after a bone marrow transplant from a donor who had the mutation.
Dr. Deng and colleagues took advantage of HSPC transplantation, a standard therapy for ALL to see whether it could also have beneficial effects on concomitant HIV infection.
They treated donor HSPCs with CRISPR-Cas9 to ablate CCR5 and then delivered them to the patient along with additional CD34-depleted donor cells from mobilized peripheral blood.
The transplant was a success, with neutrophil engraftment on day 13 and platelet engraftment on day 27, and the leukemia was in morphologic complete remission at week 4 following transplantation. The patient remained in complete remission from leukemia throughout the 19-month follow-up period, with full donor chimerism .
However, when a planned interruption of antiretroviral therapy was carried out at 7 months post transplant, the serum viral load increased to 3 × 107 copies/ml at week 4 following interruption, and the patient was restarted on the drug. His viral levels gradually decreased to undetectable level during the subsequent months.
The investigators noted that 2 weeks after the drug interruption trial was started there was a small increase in the percentage of CCR5 insertion/deletions.
“The low efficiency of gene editing in the patient may be due to the competitive engraftment of the coinfused HSPCs in CD34-depleted cells and the persistence of donor T cells. To further clarify the anti-HIV effect of CCR5-ablated HSPCs, it will be essential to increase the gene-editing efficiency of our CRISPR-Cas9 system and improve the transplantation protocol,” they wrote.
The study was funded by the Beijing Municipal Science and Technology Commission and others (unspecified). All authors reported having nothing to disclose.
SOURCE: Xu L et al. N Engl J Med. 2019. doi: 10.1056/NEJMoa1817426.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Donor cells depleted of the HIV coreceptor CCR5 effectively treated ALL, but not HIV.
Major finding: The patient had a sustained complete remission of ALL, but HIV persisted after transplantation.
Study details: Case report of a 27-year-old man with ALL and HIV.
Disclosures: The study was funded by the Beijing Municipal Science and Technology Commission and others (unspecified). All authors reported having nothing to disclose.
Source: Xu L et al. N Engl J Med. 2019. doi: 10.1056/NEJMoa1817426.
Hematocrit (HCT) Levels and Thrombotic Events Among US Veterans With Polycythemia Vera
Background: Thrombotic events (TEs) are a leading cause of death in patients with polycythemia vera (PV), contributing to lower overall survival compared with age/sex-matched controls. This analysis evaluated the relationship between HCT levels and TEs among patients with PV from the Veteran’s Health Administration (VHA) to replicate the findings of the Cytoreductive Therapy in Polycythemia Vera (CYTO-PV) trial with a real-world patient population.
Methods: This retrospective study used VHA medical record and claims data from first PV diagnosis claim (index) until death, disenrollment, or end of study (collected October 1, 2005, through September 30, 2012). Patients were aged ≥ 18 years at index, had ≥ 2 PV claims (ICD-9-CM code, 238.4) ≥ 30 days apart during the identification period, continuous health plan enrollment from 12 months pre-index until end of study, and ≥ 3 HCT measurements/year during follow-up. This analysis focused on patients with no pre-index TE and all HCT values either < 45% or ≥ 45% during follow-up. Patient demographics and disease characteristics were assessed using descriptive statistics. Associations between HCT levels and TE occurrence were analyzed using unadjusted Cox regression models for patients with ≥ 1 HCT before TE (≥ 1 HCT subgroup). A sensitivity analysis including patients with a history of TE before the index period was also performed.
Results: Patients (n=213) were mean (SD) age 68.9 (11.5) years and predominately white (61.5%). TE during follow-up occurred in 44.1% of patients (mean follow-up, 2.3 y; rate per 100 person-years, 18.9). The 3 most common types of TE were ischemic stroke (21.6%), deep vein thrombosis (12.2%), and transient ischemic attack (9.4%). TE rates for patients with HCT values 45% vs < 45% were 54.2% and 40.3%, respectively. In the 1 HCT subgroup (n=208), the TE risk hazard ratio was 1.61 (95% CI, 1.03–2.51; P=0.036). The sensitivity analysis, which included patients with and without pre-index TEs (n=342), had similar results (76.9% vs 55.6%); hazard ratio in the 1 HCT subgroup (n=322):, 1.95 (95% CI, 1.46–2.61; P<0.0001).
Implications: These results in agreement with CYTOPV study findings and further support effective monitoring and management of HCT levels < 45% to reduce the risk of TE in veterans with PV.
Background: Thrombotic events (TEs) are a leading cause of death in patients with polycythemia vera (PV), contributing to lower overall survival compared with age/sex-matched controls. This analysis evaluated the relationship between HCT levels and TEs among patients with PV from the Veteran’s Health Administration (VHA) to replicate the findings of the Cytoreductive Therapy in Polycythemia Vera (CYTO-PV) trial with a real-world patient population.
Methods: This retrospective study used VHA medical record and claims data from first PV diagnosis claim (index) until death, disenrollment, or end of study (collected October 1, 2005, through September 30, 2012). Patients were aged ≥ 18 years at index, had ≥ 2 PV claims (ICD-9-CM code, 238.4) ≥ 30 days apart during the identification period, continuous health plan enrollment from 12 months pre-index until end of study, and ≥ 3 HCT measurements/year during follow-up. This analysis focused on patients with no pre-index TE and all HCT values either < 45% or ≥ 45% during follow-up. Patient demographics and disease characteristics were assessed using descriptive statistics. Associations between HCT levels and TE occurrence were analyzed using unadjusted Cox regression models for patients with ≥ 1 HCT before TE (≥ 1 HCT subgroup). A sensitivity analysis including patients with a history of TE before the index period was also performed.
Results: Patients (n=213) were mean (SD) age 68.9 (11.5) years and predominately white (61.5%). TE during follow-up occurred in 44.1% of patients (mean follow-up, 2.3 y; rate per 100 person-years, 18.9). The 3 most common types of TE were ischemic stroke (21.6%), deep vein thrombosis (12.2%), and transient ischemic attack (9.4%). TE rates for patients with HCT values 45% vs < 45% were 54.2% and 40.3%, respectively. In the 1 HCT subgroup (n=208), the TE risk hazard ratio was 1.61 (95% CI, 1.03–2.51; P=0.036). The sensitivity analysis, which included patients with and without pre-index TEs (n=342), had similar results (76.9% vs 55.6%); hazard ratio in the 1 HCT subgroup (n=322):, 1.95 (95% CI, 1.46–2.61; P<0.0001).
Implications: These results in agreement with CYTOPV study findings and further support effective monitoring and management of HCT levels < 45% to reduce the risk of TE in veterans with PV.
Background: Thrombotic events (TEs) are a leading cause of death in patients with polycythemia vera (PV), contributing to lower overall survival compared with age/sex-matched controls. This analysis evaluated the relationship between HCT levels and TEs among patients with PV from the Veteran’s Health Administration (VHA) to replicate the findings of the Cytoreductive Therapy in Polycythemia Vera (CYTO-PV) trial with a real-world patient population.
Methods: This retrospective study used VHA medical record and claims data from first PV diagnosis claim (index) until death, disenrollment, or end of study (collected October 1, 2005, through September 30, 2012). Patients were aged ≥ 18 years at index, had ≥ 2 PV claims (ICD-9-CM code, 238.4) ≥ 30 days apart during the identification period, continuous health plan enrollment from 12 months pre-index until end of study, and ≥ 3 HCT measurements/year during follow-up. This analysis focused on patients with no pre-index TE and all HCT values either < 45% or ≥ 45% during follow-up. Patient demographics and disease characteristics were assessed using descriptive statistics. Associations between HCT levels and TE occurrence were analyzed using unadjusted Cox regression models for patients with ≥ 1 HCT before TE (≥ 1 HCT subgroup). A sensitivity analysis including patients with a history of TE before the index period was also performed.
Results: Patients (n=213) were mean (SD) age 68.9 (11.5) years and predominately white (61.5%). TE during follow-up occurred in 44.1% of patients (mean follow-up, 2.3 y; rate per 100 person-years, 18.9). The 3 most common types of TE were ischemic stroke (21.6%), deep vein thrombosis (12.2%), and transient ischemic attack (9.4%). TE rates for patients with HCT values 45% vs < 45% were 54.2% and 40.3%, respectively. In the 1 HCT subgroup (n=208), the TE risk hazard ratio was 1.61 (95% CI, 1.03–2.51; P=0.036). The sensitivity analysis, which included patients with and without pre-index TEs (n=342), had similar results (76.9% vs 55.6%); hazard ratio in the 1 HCT subgroup (n=322):, 1.95 (95% CI, 1.46–2.61; P<0.0001).
Implications: These results in agreement with CYTOPV study findings and further support effective monitoring and management of HCT levels < 45% to reduce the risk of TE in veterans with PV.
Abstracts Presented at the 2019 AVAHO Annual Meeting (Digital Edition)
Standardization of the Discharge Process for Inpatient Hematology and Oncology
Background: Hematology/Oncology patients represent a complex population that requires timely follow- up to prevent clinical decompensation and delays in treatment. Previous reports have demonstrated that follow-up within 14 days is associated with decreased 30-day readmissions, and the magnitude of this impact is greater in higher risk patients. This project was designed to standardize the discharge process with the primary goal to reduce average time to hematology/oncology follow-up to 14 days.
Methods: Using Plan-Do-Study-Act (PDSA) quality improvement methodology, a multidisciplinary team of hematology/oncology staff developed and implemented a standardized discharge process. Rotating resident physicians were trained through online and inperson orientation. Additional interventions included the development of a discharge checklist handout and clinical decision support tool including a note template and embedded order set. All patients discharged during the two-month period prior to and discharged after the implementation of the standardized process were reviewed. Patients who followed with hematology/oncology at another facility, enrolled in hospice, or died during admission were excluded. Follow-up appointment scheduling data and communication between inpatient and outpatient providers were reviewed. Data was analyzed using XmR statistical process control chart and Fisher’s Exact Test using GraphPad.
Results: One hundred forty-two consecutive patients were reviewed between May - August 2018 and January - April 2019. The primary endpoint of time to hematology/ oncology follow up appointment improved from a baseline average of 17 days prior to intervention to 13 days in PDSA cycles 1 and 2 and 10 days in PDSA cycle 3. The target of 14 day average time to follow up was achieved. Furthermore, the upper control limit decreased from 58 days at baseline to 21 days in PDSA cycle 3 suggesting a decrease in variation. Outpatient hematology/oncology provider co-signature to discharge summary increased from 20% to 54% after intervention (P=0.01).
Conclusion: Our quality initiative to standardize the discharge process for the hematology & oncology service decreased time to hematology/oncology follow up appointment, improved communication between inpatient and outpatient teams, and decreased process variation. Timelier follow-up for this complex patient population will prevent clinical decompensation and delays in treatment.
Background: Hematology/Oncology patients represent a complex population that requires timely follow- up to prevent clinical decompensation and delays in treatment. Previous reports have demonstrated that follow-up within 14 days is associated with decreased 30-day readmissions, and the magnitude of this impact is greater in higher risk patients. This project was designed to standardize the discharge process with the primary goal to reduce average time to hematology/oncology follow-up to 14 days.
Methods: Using Plan-Do-Study-Act (PDSA) quality improvement methodology, a multidisciplinary team of hematology/oncology staff developed and implemented a standardized discharge process. Rotating resident physicians were trained through online and inperson orientation. Additional interventions included the development of a discharge checklist handout and clinical decision support tool including a note template and embedded order set. All patients discharged during the two-month period prior to and discharged after the implementation of the standardized process were reviewed. Patients who followed with hematology/oncology at another facility, enrolled in hospice, or died during admission were excluded. Follow-up appointment scheduling data and communication between inpatient and outpatient providers were reviewed. Data was analyzed using XmR statistical process control chart and Fisher’s Exact Test using GraphPad.
Results: One hundred forty-two consecutive patients were reviewed between May - August 2018 and January - April 2019. The primary endpoint of time to hematology/ oncology follow up appointment improved from a baseline average of 17 days prior to intervention to 13 days in PDSA cycles 1 and 2 and 10 days in PDSA cycle 3. The target of 14 day average time to follow up was achieved. Furthermore, the upper control limit decreased from 58 days at baseline to 21 days in PDSA cycle 3 suggesting a decrease in variation. Outpatient hematology/oncology provider co-signature to discharge summary increased from 20% to 54% after intervention (P=0.01).
Conclusion: Our quality initiative to standardize the discharge process for the hematology & oncology service decreased time to hematology/oncology follow up appointment, improved communication between inpatient and outpatient teams, and decreased process variation. Timelier follow-up for this complex patient population will prevent clinical decompensation and delays in treatment.
Background: Hematology/Oncology patients represent a complex population that requires timely follow- up to prevent clinical decompensation and delays in treatment. Previous reports have demonstrated that follow-up within 14 days is associated with decreased 30-day readmissions, and the magnitude of this impact is greater in higher risk patients. This project was designed to standardize the discharge process with the primary goal to reduce average time to hematology/oncology follow-up to 14 days.
Methods: Using Plan-Do-Study-Act (PDSA) quality improvement methodology, a multidisciplinary team of hematology/oncology staff developed and implemented a standardized discharge process. Rotating resident physicians were trained through online and inperson orientation. Additional interventions included the development of a discharge checklist handout and clinical decision support tool including a note template and embedded order set. All patients discharged during the two-month period prior to and discharged after the implementation of the standardized process were reviewed. Patients who followed with hematology/oncology at another facility, enrolled in hospice, or died during admission were excluded. Follow-up appointment scheduling data and communication between inpatient and outpatient providers were reviewed. Data was analyzed using XmR statistical process control chart and Fisher’s Exact Test using GraphPad.
Results: One hundred forty-two consecutive patients were reviewed between May - August 2018 and January - April 2019. The primary endpoint of time to hematology/ oncology follow up appointment improved from a baseline average of 17 days prior to intervention to 13 days in PDSA cycles 1 and 2 and 10 days in PDSA cycle 3. The target of 14 day average time to follow up was achieved. Furthermore, the upper control limit decreased from 58 days at baseline to 21 days in PDSA cycle 3 suggesting a decrease in variation. Outpatient hematology/oncology provider co-signature to discharge summary increased from 20% to 54% after intervention (P=0.01).
Conclusion: Our quality initiative to standardize the discharge process for the hematology & oncology service decreased time to hematology/oncology follow up appointment, improved communication between inpatient and outpatient teams, and decreased process variation. Timelier follow-up for this complex patient population will prevent clinical decompensation and delays in treatment.