User login
Vitamin D pearls
Case: A 56-year-old man with a history of type 2 diabetes, hypertension, hyperlipidemia, and obesity calls clinic to discuss concerns about COVID-19, stating: “I want to do everything I can to reduce my risk of infection.” In addition to physical distancing, mask wearing, hand hygiene, and control of chronic conditions, which of the following supplements would you recommend for this patient?
1. Coenzyme Q10 160 mg twice a day
2. Vitamin D 2,000 IU daily
3. Vitamin E 400 IU daily
4. Vitamin B12 1,000 mcg daily
Of these choices, vitamin D supplementation is likely the best option, based on the limited data that is available.
Risk factors for worse COVID-19 outcome, such as older age, obesity, and more pigmented skin are also risk factors for vitamin D deficiency. This makes the study of vitamin D and COVID-19 both challenging and relevant.
In a recent study of 7,807 people living in Israel, Merzon and colleagues found that low plasma vitamin D level was an independent risk factor for COVID-19 infection. Mean plasma vitamin D level was significantly lower among those who tested positive for COVID-19 (19.00 ng/mL) than negative (20.55 ng/ mL). After controlling for demographic variables and several medical conditions, the adjusted odds ratio of COVID-19 infection in those with lower vitamin D was 1.45 (95% confidence interval, 1.08-1.95; P < .001). However, the odds of hospitalization for COVID-19 was not significantly associated with vitamin D level.1
Prior studies have also looked at vitamin D and respiratory infection. Martineau and colleagues analyzed 25 randomized, controlled trials with a pooled number of 11,321 individuals, including healthy ones and those with comorbidities, and found that oral vitamin D supplementation in daily or weekly doses had a protective effect against acute respiratory infection (adjusted odds ratio, 0.88; 95% CI, 0.81-0.96; P < .001). Patients with vitamin D deficiency (less than 25 nmol/L) experienced the most protective benefit. Vitamin D did not influence respiratory infection outcome.2
These studies suggest an adequate vitamin D level may be protective against infection with COVID-19, but who will benefit from vitamin D supplementation, and in what dose? Per U.S. Preventive Services Task Force guidelines, there is insufficient evidence to recommend screening for vitamin D deficiency in asymptomatic adults. Regarding daily dietary intake, the Institute of Medicine recommends 600 IU for persons aged 1-70, and 800 IU for those aged over 70 years. Salmon (447 IU per 3 oz serving), tuna (154 IU), and fortified milk (116 IU) are among the most vitamin D–rich foods.3 The recommended upper level of intake is 4,000 IU/day.
Too much of a good thing?
Extra vitamin D is stored in adipose tissue. If it builds up over time, storage sites may be overwhelmed, causing a rise in serum D level. While one might expect a subsequent rise in calcium levels, studies have shown this happens inconsistently, and at very high vitamin D levels, over 120 ng/mL.4 Most people would have to take at least 50,000 IU daily for several months to see an effect. The main adverse outcome of vitamin D toxicity is kidney stones, mediated by increased calcium in the blood and urine.
Several animal models have demonstrated hypervitaminosis D–induced aortic and coronary artery calcification. Like with kidney stones, the mechanism appears to be through increased calcium and phosphate levels. Shroff and colleagues studied serum vitamin D levels and vascular disease in children with renal disease on dialysis and found a U-shaped distribution: Children with both low and high vitamin D levels had significantly increased carotid artery intima-media thickness and calcification.5 Given the specialized nature of this population, it’s unclear whether these results can be generalized to most people. More studies are warranted on this topic.
Other benefits
Vitamin D is perhaps most famous for helping to build strong bones. Avenell and colleagues performed a Cochrane meta-analysis of vitamin D supplementation in older adults and found that vitamin D alone did not significantly reduce the risk of hip or other new fracture. Vitamin D plus calcium supplementation did reduce the risk of hip fracture (nine trials, pooled number of individuals was 49,853; relative risk, 0.84; P = .01).6
A lesser-known benefit of vitamin D is muscle protection. A prospective study out of the Jewish Hospital of Cincinnati followed 146 adults who were intolerant to two or more statins because of muscle side effects and found to have a vitamin D level below 32 ng per mL. Subjects were given vitamin D replacement (50,000 units weekly) and followed for 2 years. On statin rechallenge, 88-95% tolerated a statin with vitamin D levels 53-55 ng/mL.7
Pearl
Vitamin D supplementation may protect against COVID-19 infection and has very low chance of harm at daily doses at or below 4,000 IU. Other benefits of taking vitamin D include bone protection and reduction in statin-induced myopathy. The main adverse effect is kidney stones.
Ms. Sharninghausen is a medical student at the University of Washington, Seattle. Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Merzon E et al. Low plasma 25(OH) vitamin D level is associated with increased risk of COVID‐19 infection: An Israeli population‐based study. FEBS J. 2020. doi: 10.1111/febs.15495.
2. Martineau AR et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. doi:10.1136/bmj.i6583
3. “How to Get More Vitamin D From Your Food,” Cleveland Clinic. 2019 Oct 23. https://health.clevelandclinic.org/how-to-get-more-vitamin-d-from-your-food/.
4. Galior K et al. Development of vitamin d toxicity from overcorrection of vitamin D Deficiency: A review of case reports. Nutrients. 2018;10(8):953. doi: 10.3390/nu10080953
5. Shroff R et al. A bimodal association of vitamin D levels and vascular disease in children on dialysis. J Am Soc Nephrol. 2008;19(6):1239-46. doi: 10.1681/ASN.2007090993.
6. Avenell A et al. Vitamin D and vitamin D analogues for preventing fractures in post‐menopausal women and older men. Cochrane Database Syst Rev. 2014 Apr 14;2014(4):CD000227. doi: 10.1002/14651858.CD000227.pub4.
7. Khayznikov M et al. Statin intolerance because of myalgia, myositis, myopathy, or myonecrosis can in most cases be safely resolved by vitamin D supplementation. N Am J Med Sci. 2015;7(3):86-93. doi:10.4103/1947-2714.153919
Case: A 56-year-old man with a history of type 2 diabetes, hypertension, hyperlipidemia, and obesity calls clinic to discuss concerns about COVID-19, stating: “I want to do everything I can to reduce my risk of infection.” In addition to physical distancing, mask wearing, hand hygiene, and control of chronic conditions, which of the following supplements would you recommend for this patient?
1. Coenzyme Q10 160 mg twice a day
2. Vitamin D 2,000 IU daily
3. Vitamin E 400 IU daily
4. Vitamin B12 1,000 mcg daily
Of these choices, vitamin D supplementation is likely the best option, based on the limited data that is available.
Risk factors for worse COVID-19 outcome, such as older age, obesity, and more pigmented skin are also risk factors for vitamin D deficiency. This makes the study of vitamin D and COVID-19 both challenging and relevant.
In a recent study of 7,807 people living in Israel, Merzon and colleagues found that low plasma vitamin D level was an independent risk factor for COVID-19 infection. Mean plasma vitamin D level was significantly lower among those who tested positive for COVID-19 (19.00 ng/mL) than negative (20.55 ng/ mL). After controlling for demographic variables and several medical conditions, the adjusted odds ratio of COVID-19 infection in those with lower vitamin D was 1.45 (95% confidence interval, 1.08-1.95; P < .001). However, the odds of hospitalization for COVID-19 was not significantly associated with vitamin D level.1
Prior studies have also looked at vitamin D and respiratory infection. Martineau and colleagues analyzed 25 randomized, controlled trials with a pooled number of 11,321 individuals, including healthy ones and those with comorbidities, and found that oral vitamin D supplementation in daily or weekly doses had a protective effect against acute respiratory infection (adjusted odds ratio, 0.88; 95% CI, 0.81-0.96; P < .001). Patients with vitamin D deficiency (less than 25 nmol/L) experienced the most protective benefit. Vitamin D did not influence respiratory infection outcome.2
These studies suggest an adequate vitamin D level may be protective against infection with COVID-19, but who will benefit from vitamin D supplementation, and in what dose? Per U.S. Preventive Services Task Force guidelines, there is insufficient evidence to recommend screening for vitamin D deficiency in asymptomatic adults. Regarding daily dietary intake, the Institute of Medicine recommends 600 IU for persons aged 1-70, and 800 IU for those aged over 70 years. Salmon (447 IU per 3 oz serving), tuna (154 IU), and fortified milk (116 IU) are among the most vitamin D–rich foods.3 The recommended upper level of intake is 4,000 IU/day.
Too much of a good thing?
Extra vitamin D is stored in adipose tissue. If it builds up over time, storage sites may be overwhelmed, causing a rise in serum D level. While one might expect a subsequent rise in calcium levels, studies have shown this happens inconsistently, and at very high vitamin D levels, over 120 ng/mL.4 Most people would have to take at least 50,000 IU daily for several months to see an effect. The main adverse outcome of vitamin D toxicity is kidney stones, mediated by increased calcium in the blood and urine.
Several animal models have demonstrated hypervitaminosis D–induced aortic and coronary artery calcification. Like with kidney stones, the mechanism appears to be through increased calcium and phosphate levels. Shroff and colleagues studied serum vitamin D levels and vascular disease in children with renal disease on dialysis and found a U-shaped distribution: Children with both low and high vitamin D levels had significantly increased carotid artery intima-media thickness and calcification.5 Given the specialized nature of this population, it’s unclear whether these results can be generalized to most people. More studies are warranted on this topic.
Other benefits
Vitamin D is perhaps most famous for helping to build strong bones. Avenell and colleagues performed a Cochrane meta-analysis of vitamin D supplementation in older adults and found that vitamin D alone did not significantly reduce the risk of hip or other new fracture. Vitamin D plus calcium supplementation did reduce the risk of hip fracture (nine trials, pooled number of individuals was 49,853; relative risk, 0.84; P = .01).6
A lesser-known benefit of vitamin D is muscle protection. A prospective study out of the Jewish Hospital of Cincinnati followed 146 adults who were intolerant to two or more statins because of muscle side effects and found to have a vitamin D level below 32 ng per mL. Subjects were given vitamin D replacement (50,000 units weekly) and followed for 2 years. On statin rechallenge, 88-95% tolerated a statin with vitamin D levels 53-55 ng/mL.7
Pearl
Vitamin D supplementation may protect against COVID-19 infection and has very low chance of harm at daily doses at or below 4,000 IU. Other benefits of taking vitamin D include bone protection and reduction in statin-induced myopathy. The main adverse effect is kidney stones.
Ms. Sharninghausen is a medical student at the University of Washington, Seattle. Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Merzon E et al. Low plasma 25(OH) vitamin D level is associated with increased risk of COVID‐19 infection: An Israeli population‐based study. FEBS J. 2020. doi: 10.1111/febs.15495.
2. Martineau AR et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. doi:10.1136/bmj.i6583
3. “How to Get More Vitamin D From Your Food,” Cleveland Clinic. 2019 Oct 23. https://health.clevelandclinic.org/how-to-get-more-vitamin-d-from-your-food/.
4. Galior K et al. Development of vitamin d toxicity from overcorrection of vitamin D Deficiency: A review of case reports. Nutrients. 2018;10(8):953. doi: 10.3390/nu10080953
5. Shroff R et al. A bimodal association of vitamin D levels and vascular disease in children on dialysis. J Am Soc Nephrol. 2008;19(6):1239-46. doi: 10.1681/ASN.2007090993.
6. Avenell A et al. Vitamin D and vitamin D analogues for preventing fractures in post‐menopausal women and older men. Cochrane Database Syst Rev. 2014 Apr 14;2014(4):CD000227. doi: 10.1002/14651858.CD000227.pub4.
7. Khayznikov M et al. Statin intolerance because of myalgia, myositis, myopathy, or myonecrosis can in most cases be safely resolved by vitamin D supplementation. N Am J Med Sci. 2015;7(3):86-93. doi:10.4103/1947-2714.153919
Case: A 56-year-old man with a history of type 2 diabetes, hypertension, hyperlipidemia, and obesity calls clinic to discuss concerns about COVID-19, stating: “I want to do everything I can to reduce my risk of infection.” In addition to physical distancing, mask wearing, hand hygiene, and control of chronic conditions, which of the following supplements would you recommend for this patient?
1. Coenzyme Q10 160 mg twice a day
2. Vitamin D 2,000 IU daily
3. Vitamin E 400 IU daily
4. Vitamin B12 1,000 mcg daily
Of these choices, vitamin D supplementation is likely the best option, based on the limited data that is available.
Risk factors for worse COVID-19 outcome, such as older age, obesity, and more pigmented skin are also risk factors for vitamin D deficiency. This makes the study of vitamin D and COVID-19 both challenging and relevant.
In a recent study of 7,807 people living in Israel, Merzon and colleagues found that low plasma vitamin D level was an independent risk factor for COVID-19 infection. Mean plasma vitamin D level was significantly lower among those who tested positive for COVID-19 (19.00 ng/mL) than negative (20.55 ng/ mL). After controlling for demographic variables and several medical conditions, the adjusted odds ratio of COVID-19 infection in those with lower vitamin D was 1.45 (95% confidence interval, 1.08-1.95; P < .001). However, the odds of hospitalization for COVID-19 was not significantly associated with vitamin D level.1
Prior studies have also looked at vitamin D and respiratory infection. Martineau and colleagues analyzed 25 randomized, controlled trials with a pooled number of 11,321 individuals, including healthy ones and those with comorbidities, and found that oral vitamin D supplementation in daily or weekly doses had a protective effect against acute respiratory infection (adjusted odds ratio, 0.88; 95% CI, 0.81-0.96; P < .001). Patients with vitamin D deficiency (less than 25 nmol/L) experienced the most protective benefit. Vitamin D did not influence respiratory infection outcome.2
These studies suggest an adequate vitamin D level may be protective against infection with COVID-19, but who will benefit from vitamin D supplementation, and in what dose? Per U.S. Preventive Services Task Force guidelines, there is insufficient evidence to recommend screening for vitamin D deficiency in asymptomatic adults. Regarding daily dietary intake, the Institute of Medicine recommends 600 IU for persons aged 1-70, and 800 IU for those aged over 70 years. Salmon (447 IU per 3 oz serving), tuna (154 IU), and fortified milk (116 IU) are among the most vitamin D–rich foods.3 The recommended upper level of intake is 4,000 IU/day.
Too much of a good thing?
Extra vitamin D is stored in adipose tissue. If it builds up over time, storage sites may be overwhelmed, causing a rise in serum D level. While one might expect a subsequent rise in calcium levels, studies have shown this happens inconsistently, and at very high vitamin D levels, over 120 ng/mL.4 Most people would have to take at least 50,000 IU daily for several months to see an effect. The main adverse outcome of vitamin D toxicity is kidney stones, mediated by increased calcium in the blood and urine.
Several animal models have demonstrated hypervitaminosis D–induced aortic and coronary artery calcification. Like with kidney stones, the mechanism appears to be through increased calcium and phosphate levels. Shroff and colleagues studied serum vitamin D levels and vascular disease in children with renal disease on dialysis and found a U-shaped distribution: Children with both low and high vitamin D levels had significantly increased carotid artery intima-media thickness and calcification.5 Given the specialized nature of this population, it’s unclear whether these results can be generalized to most people. More studies are warranted on this topic.
Other benefits
Vitamin D is perhaps most famous for helping to build strong bones. Avenell and colleagues performed a Cochrane meta-analysis of vitamin D supplementation in older adults and found that vitamin D alone did not significantly reduce the risk of hip or other new fracture. Vitamin D plus calcium supplementation did reduce the risk of hip fracture (nine trials, pooled number of individuals was 49,853; relative risk, 0.84; P = .01).6
A lesser-known benefit of vitamin D is muscle protection. A prospective study out of the Jewish Hospital of Cincinnati followed 146 adults who were intolerant to two or more statins because of muscle side effects and found to have a vitamin D level below 32 ng per mL. Subjects were given vitamin D replacement (50,000 units weekly) and followed for 2 years. On statin rechallenge, 88-95% tolerated a statin with vitamin D levels 53-55 ng/mL.7
Pearl
Vitamin D supplementation may protect against COVID-19 infection and has very low chance of harm at daily doses at or below 4,000 IU. Other benefits of taking vitamin D include bone protection and reduction in statin-induced myopathy. The main adverse effect is kidney stones.
Ms. Sharninghausen is a medical student at the University of Washington, Seattle. Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Merzon E et al. Low plasma 25(OH) vitamin D level is associated with increased risk of COVID‐19 infection: An Israeli population‐based study. FEBS J. 2020. doi: 10.1111/febs.15495.
2. Martineau AR et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. doi:10.1136/bmj.i6583
3. “How to Get More Vitamin D From Your Food,” Cleveland Clinic. 2019 Oct 23. https://health.clevelandclinic.org/how-to-get-more-vitamin-d-from-your-food/.
4. Galior K et al. Development of vitamin d toxicity from overcorrection of vitamin D Deficiency: A review of case reports. Nutrients. 2018;10(8):953. doi: 10.3390/nu10080953
5. Shroff R et al. A bimodal association of vitamin D levels and vascular disease in children on dialysis. J Am Soc Nephrol. 2008;19(6):1239-46. doi: 10.1681/ASN.2007090993.
6. Avenell A et al. Vitamin D and vitamin D analogues for preventing fractures in post‐menopausal women and older men. Cochrane Database Syst Rev. 2014 Apr 14;2014(4):CD000227. doi: 10.1002/14651858.CD000227.pub4.
7. Khayznikov M et al. Statin intolerance because of myalgia, myositis, myopathy, or myonecrosis can in most cases be safely resolved by vitamin D supplementation. N Am J Med Sci. 2015;7(3):86-93. doi:10.4103/1947-2714.153919
COVID-19 vaccine supply will be limited at first, ACIP says
The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) yesterday held its third meeting this summer to discuss the vaccines and plan how initial vaccines will be allocated, inasmuch as supplies will likely be limited at first. Vaccines are expected to be more available as production ramps up and as more than one vaccine become available, but vaccine allocation initially will need to take place in phases.
Considerations include first getting the vaccine to individuals who need it the most, such as healthcare personnel and essential workers, as well as those at higher risk for severe illness or death, including the elderly, those with underlying conditions, and certain racial and ethnic minorities. Other factors include storage requirements that might be difficult to meet in certain settings and the fact that both vaccines must be given in two doses.
Vaccine allocation models
The group presented two possible models for allocating initial vaccine supplies.
The first population model considers risk status within each age group on the basis of underlying health conditions and occupational group, with priority given to healthcare personnel (paid or unpaid) and essential workers. The model considers partial reopening and social distancing, expected vaccine efficacy, prevaccination immunity, mortality, and the direct and indirect benefits of vaccination.
In this model, COVID-19 infections and deaths were reduced when healthcare personnel, essential workers, or adults with underlying conditions were vaccinated. There were smaller differences between the groups with respect to the impact of vaccination. Declines in infections were “more modest” and declines in deaths were greater when adults aged 65 years and older were vaccinated in comparison with other age groups.
The second model focused on vaccination of nursing home healthcare personnel and residents. Vaccinating nursing home healthcare personnel reduced infections and deaths more than vaccinating nursing home residents.
In settings such as long-term care facilities and correction facilities, where people gather in groups, cases increase first among staff. The vaccine working group suggests that vaccinating staff may also benefit individuals living in those facilities.
The working group expects that from 15 to 45 million doses of vaccine will be available by the end of December, depending on which vaccine is approved by then or whether both are approved.
Supplies won’t be nearly enough to vaccinate everyone: There are approximately 17 to 20 million healthcare workers in the United States and 60 to 80 million essential workers who do not work in healthcare. More than 100 million adults have underlying medical conditions that put them at higher risk for hospitalization and death, such as obesity, cardiovascular disease, diabetes, and chronic obstructive pulmonary disease. And approximately 53 million adults are aged 65 years or older.
The group reviewed promising early data for two vaccines under development.
The mRNA-1273 vaccine, made by Moderna with support from two federal agencies, is moving into phase 3 clinical trials – enrollment into the COVID-19 Efficacy and Safety (COVE) study is ongoing, according to Jacqueline M. Miller, MD, senior vice president and therapeutic area head of infectious diseases. The study’s primary objective will be to determine whether two doses can prevent symptomatic COVID-19, according to an NIH news release.
A second mRNA vaccine, BNT 162b2, made by Pfizer and BioNTech, is entering phase 2/3 trials. Nearly 20% of people enrolled are Black or Hispanic persons, and 4% are Asian persons. The team is also trying to recruit Native American participants, Nicholas Kitchin, MD, senior director in Pfizer’s vaccine clinical research and development group, said in a presentation to the advisory committee.
‘Ultra-cold’ temperatures required for storage
Both vaccines require storage at lower temperatures than is usually needed for vaccines. One vaccine must be distributed and stored at -20° C, and the other must be stored, distributed, and handled at -70° C.
This issue stands out most to ACIP Chair Jose Romero, MD. He says the “ultra-cold” temperatures required for storage and transportation of the vaccines will be a “significant problem” for those in rural areas.
High-risk populations such as meat processors and agricultural workers “may have to wait until we have a more stable vaccine that can be transported and delivered more or less at room temperature,” Romero explained. He is the chief medical officer at the Arkansas Department of Health and is a professor of pediatrics and pediatric infectious diseases at the University of Arkansas for Medical Sciences, both in Little Rock.
The advisory committee will meet again on September 22. At that time, they’ll vote on an interim plan for prioritization of the first COVID-19 vaccine.
This article first appeared on Medscape.com.
The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) yesterday held its third meeting this summer to discuss the vaccines and plan how initial vaccines will be allocated, inasmuch as supplies will likely be limited at first. Vaccines are expected to be more available as production ramps up and as more than one vaccine become available, but vaccine allocation initially will need to take place in phases.
Considerations include first getting the vaccine to individuals who need it the most, such as healthcare personnel and essential workers, as well as those at higher risk for severe illness or death, including the elderly, those with underlying conditions, and certain racial and ethnic minorities. Other factors include storage requirements that might be difficult to meet in certain settings and the fact that both vaccines must be given in two doses.
Vaccine allocation models
The group presented two possible models for allocating initial vaccine supplies.
The first population model considers risk status within each age group on the basis of underlying health conditions and occupational group, with priority given to healthcare personnel (paid or unpaid) and essential workers. The model considers partial reopening and social distancing, expected vaccine efficacy, prevaccination immunity, mortality, and the direct and indirect benefits of vaccination.
In this model, COVID-19 infections and deaths were reduced when healthcare personnel, essential workers, or adults with underlying conditions were vaccinated. There were smaller differences between the groups with respect to the impact of vaccination. Declines in infections were “more modest” and declines in deaths were greater when adults aged 65 years and older were vaccinated in comparison with other age groups.
The second model focused on vaccination of nursing home healthcare personnel and residents. Vaccinating nursing home healthcare personnel reduced infections and deaths more than vaccinating nursing home residents.
In settings such as long-term care facilities and correction facilities, where people gather in groups, cases increase first among staff. The vaccine working group suggests that vaccinating staff may also benefit individuals living in those facilities.
The working group expects that from 15 to 45 million doses of vaccine will be available by the end of December, depending on which vaccine is approved by then or whether both are approved.
Supplies won’t be nearly enough to vaccinate everyone: There are approximately 17 to 20 million healthcare workers in the United States and 60 to 80 million essential workers who do not work in healthcare. More than 100 million adults have underlying medical conditions that put them at higher risk for hospitalization and death, such as obesity, cardiovascular disease, diabetes, and chronic obstructive pulmonary disease. And approximately 53 million adults are aged 65 years or older.
The group reviewed promising early data for two vaccines under development.
The mRNA-1273 vaccine, made by Moderna with support from two federal agencies, is moving into phase 3 clinical trials – enrollment into the COVID-19 Efficacy and Safety (COVE) study is ongoing, according to Jacqueline M. Miller, MD, senior vice president and therapeutic area head of infectious diseases. The study’s primary objective will be to determine whether two doses can prevent symptomatic COVID-19, according to an NIH news release.
A second mRNA vaccine, BNT 162b2, made by Pfizer and BioNTech, is entering phase 2/3 trials. Nearly 20% of people enrolled are Black or Hispanic persons, and 4% are Asian persons. The team is also trying to recruit Native American participants, Nicholas Kitchin, MD, senior director in Pfizer’s vaccine clinical research and development group, said in a presentation to the advisory committee.
‘Ultra-cold’ temperatures required for storage
Both vaccines require storage at lower temperatures than is usually needed for vaccines. One vaccine must be distributed and stored at -20° C, and the other must be stored, distributed, and handled at -70° C.
This issue stands out most to ACIP Chair Jose Romero, MD. He says the “ultra-cold” temperatures required for storage and transportation of the vaccines will be a “significant problem” for those in rural areas.
High-risk populations such as meat processors and agricultural workers “may have to wait until we have a more stable vaccine that can be transported and delivered more or less at room temperature,” Romero explained. He is the chief medical officer at the Arkansas Department of Health and is a professor of pediatrics and pediatric infectious diseases at the University of Arkansas for Medical Sciences, both in Little Rock.
The advisory committee will meet again on September 22. At that time, they’ll vote on an interim plan for prioritization of the first COVID-19 vaccine.
This article first appeared on Medscape.com.
The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) yesterday held its third meeting this summer to discuss the vaccines and plan how initial vaccines will be allocated, inasmuch as supplies will likely be limited at first. Vaccines are expected to be more available as production ramps up and as more than one vaccine become available, but vaccine allocation initially will need to take place in phases.
Considerations include first getting the vaccine to individuals who need it the most, such as healthcare personnel and essential workers, as well as those at higher risk for severe illness or death, including the elderly, those with underlying conditions, and certain racial and ethnic minorities. Other factors include storage requirements that might be difficult to meet in certain settings and the fact that both vaccines must be given in two doses.
Vaccine allocation models
The group presented two possible models for allocating initial vaccine supplies.
The first population model considers risk status within each age group on the basis of underlying health conditions and occupational group, with priority given to healthcare personnel (paid or unpaid) and essential workers. The model considers partial reopening and social distancing, expected vaccine efficacy, prevaccination immunity, mortality, and the direct and indirect benefits of vaccination.
In this model, COVID-19 infections and deaths were reduced when healthcare personnel, essential workers, or adults with underlying conditions were vaccinated. There were smaller differences between the groups with respect to the impact of vaccination. Declines in infections were “more modest” and declines in deaths were greater when adults aged 65 years and older were vaccinated in comparison with other age groups.
The second model focused on vaccination of nursing home healthcare personnel and residents. Vaccinating nursing home healthcare personnel reduced infections and deaths more than vaccinating nursing home residents.
In settings such as long-term care facilities and correction facilities, where people gather in groups, cases increase first among staff. The vaccine working group suggests that vaccinating staff may also benefit individuals living in those facilities.
The working group expects that from 15 to 45 million doses of vaccine will be available by the end of December, depending on which vaccine is approved by then or whether both are approved.
Supplies won’t be nearly enough to vaccinate everyone: There are approximately 17 to 20 million healthcare workers in the United States and 60 to 80 million essential workers who do not work in healthcare. More than 100 million adults have underlying medical conditions that put them at higher risk for hospitalization and death, such as obesity, cardiovascular disease, diabetes, and chronic obstructive pulmonary disease. And approximately 53 million adults are aged 65 years or older.
The group reviewed promising early data for two vaccines under development.
The mRNA-1273 vaccine, made by Moderna with support from two federal agencies, is moving into phase 3 clinical trials – enrollment into the COVID-19 Efficacy and Safety (COVE) study is ongoing, according to Jacqueline M. Miller, MD, senior vice president and therapeutic area head of infectious diseases. The study’s primary objective will be to determine whether two doses can prevent symptomatic COVID-19, according to an NIH news release.
A second mRNA vaccine, BNT 162b2, made by Pfizer and BioNTech, is entering phase 2/3 trials. Nearly 20% of people enrolled are Black or Hispanic persons, and 4% are Asian persons. The team is also trying to recruit Native American participants, Nicholas Kitchin, MD, senior director in Pfizer’s vaccine clinical research and development group, said in a presentation to the advisory committee.
‘Ultra-cold’ temperatures required for storage
Both vaccines require storage at lower temperatures than is usually needed for vaccines. One vaccine must be distributed and stored at -20° C, and the other must be stored, distributed, and handled at -70° C.
This issue stands out most to ACIP Chair Jose Romero, MD. He says the “ultra-cold” temperatures required for storage and transportation of the vaccines will be a “significant problem” for those in rural areas.
High-risk populations such as meat processors and agricultural workers “may have to wait until we have a more stable vaccine that can be transported and delivered more or less at room temperature,” Romero explained. He is the chief medical officer at the Arkansas Department of Health and is a professor of pediatrics and pediatric infectious diseases at the University of Arkansas for Medical Sciences, both in Little Rock.
The advisory committee will meet again on September 22. At that time, they’ll vote on an interim plan for prioritization of the first COVID-19 vaccine.
This article first appeared on Medscape.com.
Bumps on the thighs

The photograph submitted for the telemedicine visit showed 2 classic umbilicated lesions and 1 dome-shaped papule consistent with molluscum contagiosum. Not all skin conditions can be diagnosed or treated via telehealth, but with a careful history, cooperative patients (and parents in this case), and photos taken on newer cell phones or digital cameras, many conditions can be diagnosed and managed appropriately.
Molluscum contagiosum is caused by the Molluscipox genus poxvirus and Is commonly seen in preschool and school-aged children. It can be passed through direct contact with infected individuals or spread by fomites. (In this case, the child may have picked up the virus by sharing a towel with an infected individual.)
The flesh-colored lesions are umbilicated or popular, and occur in clusters on the trunk, face, and extremities. Typically, the lesions will resolve spontaneously, but it may take several weeks to many months for resolution.
Given this lengthy time for spontaneous resolution, the risk of spreading to family members or other contacts, and the skin’s appearance, many patients choose to treat the lesions. Treatment options include curettage, cryosurgery, and laser. Available topical destructive agents include podophyllotoxin, trichloroacetic acid, benzoyl peroxide, potassium hydroxide, and cantharidin (which is from the blister beetle and often difficult to obtain). There also are naturopathic topical products and immune system modulators, including topical imiquimod. These treatments are commonly used, but are off-label for the treatment of molluscum contagiosum.
The family was counseled that there is debate about the effectiveness of imiquimod for molluscum contagiosum, but that some studies find it to be useful. In this case, the mother chose a prescription for imiquimod cream 5%, to be applied 3 times weekly at bedtime until the lesions resolved. (The cream can be used for up to 16 weeks.) The family was advised that erythema and irritation are expected adverse effects at the application site.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Badavanis G, Pasmatzi E, Monastirli A, et al. Topical imiquimod is an effective and safe drug for molluscum contagiosum in children. Acta Dermatovenerol Croat. 2017;25:164-166.

The photograph submitted for the telemedicine visit showed 2 classic umbilicated lesions and 1 dome-shaped papule consistent with molluscum contagiosum. Not all skin conditions can be diagnosed or treated via telehealth, but with a careful history, cooperative patients (and parents in this case), and photos taken on newer cell phones or digital cameras, many conditions can be diagnosed and managed appropriately.
Molluscum contagiosum is caused by the Molluscipox genus poxvirus and Is commonly seen in preschool and school-aged children. It can be passed through direct contact with infected individuals or spread by fomites. (In this case, the child may have picked up the virus by sharing a towel with an infected individual.)
The flesh-colored lesions are umbilicated or popular, and occur in clusters on the trunk, face, and extremities. Typically, the lesions will resolve spontaneously, but it may take several weeks to many months for resolution.
Given this lengthy time for spontaneous resolution, the risk of spreading to family members or other contacts, and the skin’s appearance, many patients choose to treat the lesions. Treatment options include curettage, cryosurgery, and laser. Available topical destructive agents include podophyllotoxin, trichloroacetic acid, benzoyl peroxide, potassium hydroxide, and cantharidin (which is from the blister beetle and often difficult to obtain). There also are naturopathic topical products and immune system modulators, including topical imiquimod. These treatments are commonly used, but are off-label for the treatment of molluscum contagiosum.
The family was counseled that there is debate about the effectiveness of imiquimod for molluscum contagiosum, but that some studies find it to be useful. In this case, the mother chose a prescription for imiquimod cream 5%, to be applied 3 times weekly at bedtime until the lesions resolved. (The cream can be used for up to 16 weeks.) The family was advised that erythema and irritation are expected adverse effects at the application site.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

The photograph submitted for the telemedicine visit showed 2 classic umbilicated lesions and 1 dome-shaped papule consistent with molluscum contagiosum. Not all skin conditions can be diagnosed or treated via telehealth, but with a careful history, cooperative patients (and parents in this case), and photos taken on newer cell phones or digital cameras, many conditions can be diagnosed and managed appropriately.
Molluscum contagiosum is caused by the Molluscipox genus poxvirus and Is commonly seen in preschool and school-aged children. It can be passed through direct contact with infected individuals or spread by fomites. (In this case, the child may have picked up the virus by sharing a towel with an infected individual.)
The flesh-colored lesions are umbilicated or popular, and occur in clusters on the trunk, face, and extremities. Typically, the lesions will resolve spontaneously, but it may take several weeks to many months for resolution.
Given this lengthy time for spontaneous resolution, the risk of spreading to family members or other contacts, and the skin’s appearance, many patients choose to treat the lesions. Treatment options include curettage, cryosurgery, and laser. Available topical destructive agents include podophyllotoxin, trichloroacetic acid, benzoyl peroxide, potassium hydroxide, and cantharidin (which is from the blister beetle and often difficult to obtain). There also are naturopathic topical products and immune system modulators, including topical imiquimod. These treatments are commonly used, but are off-label for the treatment of molluscum contagiosum.
The family was counseled that there is debate about the effectiveness of imiquimod for molluscum contagiosum, but that some studies find it to be useful. In this case, the mother chose a prescription for imiquimod cream 5%, to be applied 3 times weekly at bedtime until the lesions resolved. (The cream can be used for up to 16 weeks.) The family was advised that erythema and irritation are expected adverse effects at the application site.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Badavanis G, Pasmatzi E, Monastirli A, et al. Topical imiquimod is an effective and safe drug for molluscum contagiosum in children. Acta Dermatovenerol Croat. 2017;25:164-166.
Badavanis G, Pasmatzi E, Monastirli A, et al. Topical imiquimod is an effective and safe drug for molluscum contagiosum in children. Acta Dermatovenerol Croat. 2017;25:164-166.
When viruses collide: Flu season during pandemic
The medical community is about to find out how prepared it is for the double whammy of influenza and COVID-19 that has been predicted for the fall of 2020. The complexities of diagnosis, management of vulnerable patients, and overflowing medical centers that have made the COVID-19 crisis so brutal may all be exacerbated by the arrival of seasonal influenza.
Lewis Jay Kaplan, MD, FCCP, a critical care surgeon at the University of Pennsylvania, Philadelphia, has seen his share of critically ill COVID-19 patients in the surgical ICU that he oversees. He’s approaching the upcoming flu season, poised to collide with the ongoing COVID-19 pandemic, ready to listen to each patient’s story to distinguish one from the other and determine treatment.
“The patients that have underlying comorbidities all have a story, and it’s up to you to figure out which chapter you’re in and how far along you happen to be,” he said. “It’s a very interesting approach to care, medical storytelling.”
With flu season closing in, pulmonologists are ruminating about how they’ll distinguish symptoms of COVID-19 and traditional influenza and how they’ll manage the most vulnerable patients, namely those with underlying respiratory disease and children. Influenza kills 12,000-61,000 people a year, according to the Centers for Disease Control, and results in 140,000-810,00 hospitalizations. Having a flu season in the midst of a pandemic of a disease with multiple overlapping symptoms threatens to overwhelm practitioners, hospitals, and the health system.
Dr. Kaplan said each patient’s story can point to the correct clinical approach. “Instead of just sharing data when you are on rounds, you’re really telling someone’s story.” It arises from a series of questions about how the disease has impacted them, specifics of their presentation, how their signs and symptoms differ from the usual, and how they responded to treatment. “It also helps you to then take what you’re doing, which can seem very, very complicated to individuals who are not medically sophisticated, and then help them to understand why you’re doing what you’re doing at this point.”
That can help get through to a patient with respiratory disease who insists he or she has or doesn’t have COVID-19 rather than the flu. “They form a different group that brings with them different fears and concerns, and you have to help them navigate that, too: all of this data and your decision-making around testing and admissions, and what you can omit doing and what you must do help them to navigate their own story,” Dr. Kaplan said.
Benjamin D. Singer, MD, a pulmonologist at Northwestern University, Chicago, authored an editorial in Science Advances that addressed four factors that will determine the scope of flu spread in the upcoming season: rate of transmission; vaccination rates; coinfection rates; and health disparities in minority populations, which are prone to higher rates of flu as well as COVID-19.
Flu vaccine ‘extra important’
The convergence of COVID-19 and influenza has the potential to overwhelm the health system, said Daniel A. Solomon, MD, of Brigham and Women’s in Boston. He coauthored a JAMA Insights clinical update on flu season during the COVID-19 pandemic that lists distinguishing and overlapping signs and symptoms of the two diseases.
The flu vaccine, he said, is “extra important this year,” especially in patients with existing respiratory disease, but COVID-19 has thrown up barriers to vaccination. Telemedicine has supplanted office visits. “People may miss that easy-touch opportunity to get the flu vaccine, so we have to be creative about making the flu vaccine highly accessible, maybe in nontraditional ways,” Dr. Solomon said. Some ideas he offered are pop-up vaccine fairs at schools and churches.
But just as COVID-19 may hinder flu vaccines, it may also be helping to mitigate flu transmission. “The interesting thing about transmission of the flu is that it’s transmitted the same way COVID is, so if we actually know how to decrease transmission of COVID, which we do – we’ve done it – we can actually decrease transmission of influenza as well,” Dr. Solomon said. Studies out of Hong Kong and Japan have reported a reduction in influenza cases during COVID-19 outbreaks in those places (Lancet Public Health. 2020;5:e279-88; JAMA. 2020;323:1969-71).
Risks of coinfection
About one in four COVID-19 patients have been diagnosed with an additional respiratory infection, including influenza (JAMA. 2020:323:2085-6). Pulmonologists must keep that in mind when managing COVID-19 suspects, said Dr. Singer.
“While it is true that most of the time COVID-19 travels alone, we have numerous examples in the literature and in our own experience that COVID-19 is accompanied by either another virus or another bacterial infection, including influenza,” Dr. Singer said. “The distinction is important. One is just for diagnostic reasons and public reporting reasons, but also because flu and COVID-19 have different requirements for how you care for patients in terms of the health system.”
Clinical suspicion for coinfection should remain high if the community spread of both COVID-19 and influenza is high, said Megan Conroy, MD, chief pulmonary and critical care fellow at Ohio State University, Columbus. “As the coronavirus first took hold in the United States in March 2020, we were at the tail end of influenza season, so it’s hard to predict what the upcoming influenza season will really look like with regards to coinfection.”
Distinguishing COVID-19 from flu
Multiple signs and symptoms between COVID-19 and the flu overlap. They include fever, chills, headache, myalgia, cough, and fatigue. Nasal congestion and sore throat are characteristic of the flu; shortness of breath and loss of the sense of smell have been widely reported in COVID-19. “While many upper respiratory infections can result in loss of smell, this may be more prevalent in COVID-19,” Dr. Conroy said. Other symptoms unique to COVID-19 are GI symptoms such as diarrhea and skin rashes such as acral ischemia.
Testing, however, is the cornerstone of the differential diagnosis. “You can’t confidently distinguish between them on symptoms alone,” Dr. Conroy added.
“I think the challenge we’ll face as clinicians, is caring for people with nonspecific symptoms of a respiratory viral illness, especially in the early phase of the illness,” said Dr. Solomon.
But even after that, symptoms can be difficult to distinguish.
“Later in the illness, COVID is more associated with a hypercoagulable state,” he said. “It is more associated with viral pneumonia on chest imaging, like the diffuse ground-glass infiltrates that we’ve all gotten used to seeing – but flu can do both of those things as well. So, without a test, it’s impossible to distinguish between the two infections in the clinic.”
But testing can have its shortcomings when flu season clashes with the COVID-19 pandemic. “Getting the test is not the same as getting the test results,” Dr. Solomon added. “Though a lot of people can get a test, if it takes 7 or 8 days to get the test result back, the result is useless.”
Widespread, rapid testing also depends on having adequate supplies of viral media transport and swabs. “I think that this is what we should be focusing on now: scaling up access to rapid turnaround testing,” he said. Distinguishing between the two is also important to preserve hospital resources. COVID-19 has more rigorous standards than flu for personal protective equipment and isolation of patients within the hospital.
Having chronic lung disease isn’t necessarily a risk factor for contracting COVID-19 or the flu, or both, Dr. Solomon said. “It’s a risk factor for having severe disease.” Again, he noted that flu vaccines are still necessary in these patients, as well as patients of advanced age and underlying medical conditions such as heart disease, diabetes, and obesity.
In managing children, it’s important to keep in mind that they communicate differently about their illnesses than adults, said Dr. Kaplan. “They may not have the words to tell you the same kind of thing that the adult tells you.” That’s where family members can help to flesh out the history. “They may present with an initially much milder form, if you will, where they’re not as critical up front, but then that small proportion of them comes back with the multi-inflammatory syndrome and then they are profoundly ill.”
Younger people make up a larger share of COVID-19 patients now, compared with the initial wave that hit the Northeast in the spring, Dr. Kaplan said. “We don’t know if that’s because the virus is a little different or the people that are getting sick are a little bit different.”
The COVID-19 strain now emerging may be less virulent than the strain that hit in early spring, he said. “That doesn’t mean that there aren’t still profoundly critical ill people with COVID of many different age ranges, that is true, but there are a lot of people that we now see will test positive, but aren’t really as profoundly ill as when it first landed here in the United States.”
That may be somewhat welcome as flu season arrives.
The physicians interviewed have no relevant disclosures.
The medical community is about to find out how prepared it is for the double whammy of influenza and COVID-19 that has been predicted for the fall of 2020. The complexities of diagnosis, management of vulnerable patients, and overflowing medical centers that have made the COVID-19 crisis so brutal may all be exacerbated by the arrival of seasonal influenza.
Lewis Jay Kaplan, MD, FCCP, a critical care surgeon at the University of Pennsylvania, Philadelphia, has seen his share of critically ill COVID-19 patients in the surgical ICU that he oversees. He’s approaching the upcoming flu season, poised to collide with the ongoing COVID-19 pandemic, ready to listen to each patient’s story to distinguish one from the other and determine treatment.
“The patients that have underlying comorbidities all have a story, and it’s up to you to figure out which chapter you’re in and how far along you happen to be,” he said. “It’s a very interesting approach to care, medical storytelling.”
With flu season closing in, pulmonologists are ruminating about how they’ll distinguish symptoms of COVID-19 and traditional influenza and how they’ll manage the most vulnerable patients, namely those with underlying respiratory disease and children. Influenza kills 12,000-61,000 people a year, according to the Centers for Disease Control, and results in 140,000-810,00 hospitalizations. Having a flu season in the midst of a pandemic of a disease with multiple overlapping symptoms threatens to overwhelm practitioners, hospitals, and the health system.
Dr. Kaplan said each patient’s story can point to the correct clinical approach. “Instead of just sharing data when you are on rounds, you’re really telling someone’s story.” It arises from a series of questions about how the disease has impacted them, specifics of their presentation, how their signs and symptoms differ from the usual, and how they responded to treatment. “It also helps you to then take what you’re doing, which can seem very, very complicated to individuals who are not medically sophisticated, and then help them to understand why you’re doing what you’re doing at this point.”
That can help get through to a patient with respiratory disease who insists he or she has or doesn’t have COVID-19 rather than the flu. “They form a different group that brings with them different fears and concerns, and you have to help them navigate that, too: all of this data and your decision-making around testing and admissions, and what you can omit doing and what you must do help them to navigate their own story,” Dr. Kaplan said.
Benjamin D. Singer, MD, a pulmonologist at Northwestern University, Chicago, authored an editorial in Science Advances that addressed four factors that will determine the scope of flu spread in the upcoming season: rate of transmission; vaccination rates; coinfection rates; and health disparities in minority populations, which are prone to higher rates of flu as well as COVID-19.
Flu vaccine ‘extra important’
The convergence of COVID-19 and influenza has the potential to overwhelm the health system, said Daniel A. Solomon, MD, of Brigham and Women’s in Boston. He coauthored a JAMA Insights clinical update on flu season during the COVID-19 pandemic that lists distinguishing and overlapping signs and symptoms of the two diseases.
The flu vaccine, he said, is “extra important this year,” especially in patients with existing respiratory disease, but COVID-19 has thrown up barriers to vaccination. Telemedicine has supplanted office visits. “People may miss that easy-touch opportunity to get the flu vaccine, so we have to be creative about making the flu vaccine highly accessible, maybe in nontraditional ways,” Dr. Solomon said. Some ideas he offered are pop-up vaccine fairs at schools and churches.
But just as COVID-19 may hinder flu vaccines, it may also be helping to mitigate flu transmission. “The interesting thing about transmission of the flu is that it’s transmitted the same way COVID is, so if we actually know how to decrease transmission of COVID, which we do – we’ve done it – we can actually decrease transmission of influenza as well,” Dr. Solomon said. Studies out of Hong Kong and Japan have reported a reduction in influenza cases during COVID-19 outbreaks in those places (Lancet Public Health. 2020;5:e279-88; JAMA. 2020;323:1969-71).
Risks of coinfection
About one in four COVID-19 patients have been diagnosed with an additional respiratory infection, including influenza (JAMA. 2020:323:2085-6). Pulmonologists must keep that in mind when managing COVID-19 suspects, said Dr. Singer.
“While it is true that most of the time COVID-19 travels alone, we have numerous examples in the literature and in our own experience that COVID-19 is accompanied by either another virus or another bacterial infection, including influenza,” Dr. Singer said. “The distinction is important. One is just for diagnostic reasons and public reporting reasons, but also because flu and COVID-19 have different requirements for how you care for patients in terms of the health system.”
Clinical suspicion for coinfection should remain high if the community spread of both COVID-19 and influenza is high, said Megan Conroy, MD, chief pulmonary and critical care fellow at Ohio State University, Columbus. “As the coronavirus first took hold in the United States in March 2020, we were at the tail end of influenza season, so it’s hard to predict what the upcoming influenza season will really look like with regards to coinfection.”
Distinguishing COVID-19 from flu
Multiple signs and symptoms between COVID-19 and the flu overlap. They include fever, chills, headache, myalgia, cough, and fatigue. Nasal congestion and sore throat are characteristic of the flu; shortness of breath and loss of the sense of smell have been widely reported in COVID-19. “While many upper respiratory infections can result in loss of smell, this may be more prevalent in COVID-19,” Dr. Conroy said. Other symptoms unique to COVID-19 are GI symptoms such as diarrhea and skin rashes such as acral ischemia.
Testing, however, is the cornerstone of the differential diagnosis. “You can’t confidently distinguish between them on symptoms alone,” Dr. Conroy added.
“I think the challenge we’ll face as clinicians, is caring for people with nonspecific symptoms of a respiratory viral illness, especially in the early phase of the illness,” said Dr. Solomon.
But even after that, symptoms can be difficult to distinguish.
“Later in the illness, COVID is more associated with a hypercoagulable state,” he said. “It is more associated with viral pneumonia on chest imaging, like the diffuse ground-glass infiltrates that we’ve all gotten used to seeing – but flu can do both of those things as well. So, without a test, it’s impossible to distinguish between the two infections in the clinic.”
But testing can have its shortcomings when flu season clashes with the COVID-19 pandemic. “Getting the test is not the same as getting the test results,” Dr. Solomon added. “Though a lot of people can get a test, if it takes 7 or 8 days to get the test result back, the result is useless.”
Widespread, rapid testing also depends on having adequate supplies of viral media transport and swabs. “I think that this is what we should be focusing on now: scaling up access to rapid turnaround testing,” he said. Distinguishing between the two is also important to preserve hospital resources. COVID-19 has more rigorous standards than flu for personal protective equipment and isolation of patients within the hospital.
Having chronic lung disease isn’t necessarily a risk factor for contracting COVID-19 or the flu, or both, Dr. Solomon said. “It’s a risk factor for having severe disease.” Again, he noted that flu vaccines are still necessary in these patients, as well as patients of advanced age and underlying medical conditions such as heart disease, diabetes, and obesity.
In managing children, it’s important to keep in mind that they communicate differently about their illnesses than adults, said Dr. Kaplan. “They may not have the words to tell you the same kind of thing that the adult tells you.” That’s where family members can help to flesh out the history. “They may present with an initially much milder form, if you will, where they’re not as critical up front, but then that small proportion of them comes back with the multi-inflammatory syndrome and then they are profoundly ill.”
Younger people make up a larger share of COVID-19 patients now, compared with the initial wave that hit the Northeast in the spring, Dr. Kaplan said. “We don’t know if that’s because the virus is a little different or the people that are getting sick are a little bit different.”
The COVID-19 strain now emerging may be less virulent than the strain that hit in early spring, he said. “That doesn’t mean that there aren’t still profoundly critical ill people with COVID of many different age ranges, that is true, but there are a lot of people that we now see will test positive, but aren’t really as profoundly ill as when it first landed here in the United States.”
That may be somewhat welcome as flu season arrives.
The physicians interviewed have no relevant disclosures.
The medical community is about to find out how prepared it is for the double whammy of influenza and COVID-19 that has been predicted for the fall of 2020. The complexities of diagnosis, management of vulnerable patients, and overflowing medical centers that have made the COVID-19 crisis so brutal may all be exacerbated by the arrival of seasonal influenza.
Lewis Jay Kaplan, MD, FCCP, a critical care surgeon at the University of Pennsylvania, Philadelphia, has seen his share of critically ill COVID-19 patients in the surgical ICU that he oversees. He’s approaching the upcoming flu season, poised to collide with the ongoing COVID-19 pandemic, ready to listen to each patient’s story to distinguish one from the other and determine treatment.
“The patients that have underlying comorbidities all have a story, and it’s up to you to figure out which chapter you’re in and how far along you happen to be,” he said. “It’s a very interesting approach to care, medical storytelling.”
With flu season closing in, pulmonologists are ruminating about how they’ll distinguish symptoms of COVID-19 and traditional influenza and how they’ll manage the most vulnerable patients, namely those with underlying respiratory disease and children. Influenza kills 12,000-61,000 people a year, according to the Centers for Disease Control, and results in 140,000-810,00 hospitalizations. Having a flu season in the midst of a pandemic of a disease with multiple overlapping symptoms threatens to overwhelm practitioners, hospitals, and the health system.
Dr. Kaplan said each patient’s story can point to the correct clinical approach. “Instead of just sharing data when you are on rounds, you’re really telling someone’s story.” It arises from a series of questions about how the disease has impacted them, specifics of their presentation, how their signs and symptoms differ from the usual, and how they responded to treatment. “It also helps you to then take what you’re doing, which can seem very, very complicated to individuals who are not medically sophisticated, and then help them to understand why you’re doing what you’re doing at this point.”
That can help get through to a patient with respiratory disease who insists he or she has or doesn’t have COVID-19 rather than the flu. “They form a different group that brings with them different fears and concerns, and you have to help them navigate that, too: all of this data and your decision-making around testing and admissions, and what you can omit doing and what you must do help them to navigate their own story,” Dr. Kaplan said.
Benjamin D. Singer, MD, a pulmonologist at Northwestern University, Chicago, authored an editorial in Science Advances that addressed four factors that will determine the scope of flu spread in the upcoming season: rate of transmission; vaccination rates; coinfection rates; and health disparities in minority populations, which are prone to higher rates of flu as well as COVID-19.
Flu vaccine ‘extra important’
The convergence of COVID-19 and influenza has the potential to overwhelm the health system, said Daniel A. Solomon, MD, of Brigham and Women’s in Boston. He coauthored a JAMA Insights clinical update on flu season during the COVID-19 pandemic that lists distinguishing and overlapping signs and symptoms of the two diseases.
The flu vaccine, he said, is “extra important this year,” especially in patients with existing respiratory disease, but COVID-19 has thrown up barriers to vaccination. Telemedicine has supplanted office visits. “People may miss that easy-touch opportunity to get the flu vaccine, so we have to be creative about making the flu vaccine highly accessible, maybe in nontraditional ways,” Dr. Solomon said. Some ideas he offered are pop-up vaccine fairs at schools and churches.
But just as COVID-19 may hinder flu vaccines, it may also be helping to mitigate flu transmission. “The interesting thing about transmission of the flu is that it’s transmitted the same way COVID is, so if we actually know how to decrease transmission of COVID, which we do – we’ve done it – we can actually decrease transmission of influenza as well,” Dr. Solomon said. Studies out of Hong Kong and Japan have reported a reduction in influenza cases during COVID-19 outbreaks in those places (Lancet Public Health. 2020;5:e279-88; JAMA. 2020;323:1969-71).
Risks of coinfection
About one in four COVID-19 patients have been diagnosed with an additional respiratory infection, including influenza (JAMA. 2020:323:2085-6). Pulmonologists must keep that in mind when managing COVID-19 suspects, said Dr. Singer.
“While it is true that most of the time COVID-19 travels alone, we have numerous examples in the literature and in our own experience that COVID-19 is accompanied by either another virus or another bacterial infection, including influenza,” Dr. Singer said. “The distinction is important. One is just for diagnostic reasons and public reporting reasons, but also because flu and COVID-19 have different requirements for how you care for patients in terms of the health system.”
Clinical suspicion for coinfection should remain high if the community spread of both COVID-19 and influenza is high, said Megan Conroy, MD, chief pulmonary and critical care fellow at Ohio State University, Columbus. “As the coronavirus first took hold in the United States in March 2020, we were at the tail end of influenza season, so it’s hard to predict what the upcoming influenza season will really look like with regards to coinfection.”
Distinguishing COVID-19 from flu
Multiple signs and symptoms between COVID-19 and the flu overlap. They include fever, chills, headache, myalgia, cough, and fatigue. Nasal congestion and sore throat are characteristic of the flu; shortness of breath and loss of the sense of smell have been widely reported in COVID-19. “While many upper respiratory infections can result in loss of smell, this may be more prevalent in COVID-19,” Dr. Conroy said. Other symptoms unique to COVID-19 are GI symptoms such as diarrhea and skin rashes such as acral ischemia.
Testing, however, is the cornerstone of the differential diagnosis. “You can’t confidently distinguish between them on symptoms alone,” Dr. Conroy added.
“I think the challenge we’ll face as clinicians, is caring for people with nonspecific symptoms of a respiratory viral illness, especially in the early phase of the illness,” said Dr. Solomon.
But even after that, symptoms can be difficult to distinguish.
“Later in the illness, COVID is more associated with a hypercoagulable state,” he said. “It is more associated with viral pneumonia on chest imaging, like the diffuse ground-glass infiltrates that we’ve all gotten used to seeing – but flu can do both of those things as well. So, without a test, it’s impossible to distinguish between the two infections in the clinic.”
But testing can have its shortcomings when flu season clashes with the COVID-19 pandemic. “Getting the test is not the same as getting the test results,” Dr. Solomon added. “Though a lot of people can get a test, if it takes 7 or 8 days to get the test result back, the result is useless.”
Widespread, rapid testing also depends on having adequate supplies of viral media transport and swabs. “I think that this is what we should be focusing on now: scaling up access to rapid turnaround testing,” he said. Distinguishing between the two is also important to preserve hospital resources. COVID-19 has more rigorous standards than flu for personal protective equipment and isolation of patients within the hospital.
Having chronic lung disease isn’t necessarily a risk factor for contracting COVID-19 or the flu, or both, Dr. Solomon said. “It’s a risk factor for having severe disease.” Again, he noted that flu vaccines are still necessary in these patients, as well as patients of advanced age and underlying medical conditions such as heart disease, diabetes, and obesity.
In managing children, it’s important to keep in mind that they communicate differently about their illnesses than adults, said Dr. Kaplan. “They may not have the words to tell you the same kind of thing that the adult tells you.” That’s where family members can help to flesh out the history. “They may present with an initially much milder form, if you will, where they’re not as critical up front, but then that small proportion of them comes back with the multi-inflammatory syndrome and then they are profoundly ill.”
Younger people make up a larger share of COVID-19 patients now, compared with the initial wave that hit the Northeast in the spring, Dr. Kaplan said. “We don’t know if that’s because the virus is a little different or the people that are getting sick are a little bit different.”
The COVID-19 strain now emerging may be less virulent than the strain that hit in early spring, he said. “That doesn’t mean that there aren’t still profoundly critical ill people with COVID of many different age ranges, that is true, but there are a lot of people that we now see will test positive, but aren’t really as profoundly ill as when it first landed here in the United States.”
That may be somewhat welcome as flu season arrives.
The physicians interviewed have no relevant disclosures.
As COVID-19 cases increase in children, deaths remain low
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
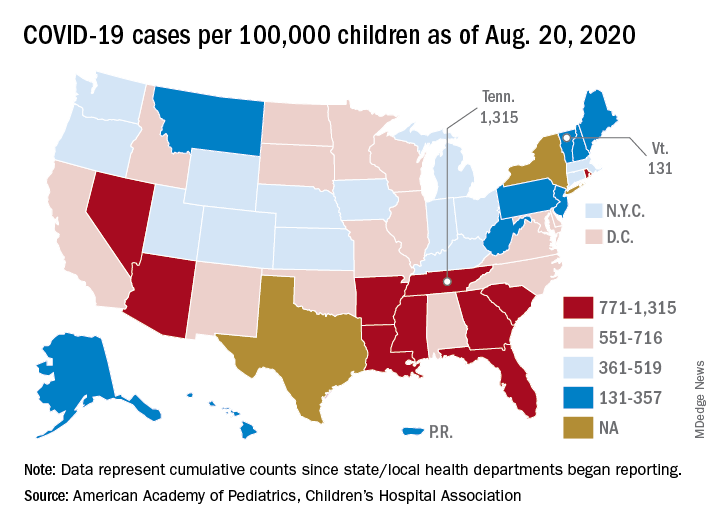
The cumulative number of pediatric cases reported up to that date was 442,785, or 9.3% of the total COVID-19 case load of more than 4.76 million among all ages. There have been only 92 pediatric deaths, however, which works out to just 0.06% of the 154,279 reported for all ages, the AAP and the CHA said Aug. 24 in their most recent update.
Child hospitalizations also were on the low side, representing 1.7% (4,062) of the cumulative total of 234,810 admissions among all ages as of Aug. 20, based on data from 21 states and New York City.
Nationally, the cumulative number of reported child cases is now up to 583 per 100,000 children, and that figure covers 49 states, Washington, D.C., Guam, New York City, and Puerto Rico.
There is some disagreement among the states, though, about the definition of “child.” Most states use an age range of 0-17, 0-18, or 0-19, but Florida and Utah go with a range of 0-14 years while South Carolina and Tennessee consider humans aged 0-20 years to be children. Other data limitations involve Texas, which has reported age distribution for only 8% of all cases, and New York, which is not reporting the age distribution of statewide cases, the AAP/CHA report noted.
The definition of child isn’t the only thing that varies between the states. The cumulative case rate for Tennessee, the highest in the country at 1,315 per 100,000 children, is 10 times that of Vermont, which is the lowest at 131 per 100,000, the AAP and CHA said. Vermont reports child COVID-19 cases using an age range of 0-19 years.
The other states with rates over 1,000 cases per 100,000 children are Arizona (1,300), which had the highest rate a week ago; South Carolina (1,214); Louisiana (1,127); Mississippi (1,120); and Nevada (1,068). Those with rates below 200 cases per 100,000 children are Maine (150), New Hampshire (175), and Hawaii (188), according to this week’s report.
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

The cumulative number of pediatric cases reported up to that date was 442,785, or 9.3% of the total COVID-19 case load of more than 4.76 million among all ages. There have been only 92 pediatric deaths, however, which works out to just 0.06% of the 154,279 reported for all ages, the AAP and the CHA said Aug. 24 in their most recent update.
Child hospitalizations also were on the low side, representing 1.7% (4,062) of the cumulative total of 234,810 admissions among all ages as of Aug. 20, based on data from 21 states and New York City.
Nationally, the cumulative number of reported child cases is now up to 583 per 100,000 children, and that figure covers 49 states, Washington, D.C., Guam, New York City, and Puerto Rico.
There is some disagreement among the states, though, about the definition of “child.” Most states use an age range of 0-17, 0-18, or 0-19, but Florida and Utah go with a range of 0-14 years while South Carolina and Tennessee consider humans aged 0-20 years to be children. Other data limitations involve Texas, which has reported age distribution for only 8% of all cases, and New York, which is not reporting the age distribution of statewide cases, the AAP/CHA report noted.
The definition of child isn’t the only thing that varies between the states. The cumulative case rate for Tennessee, the highest in the country at 1,315 per 100,000 children, is 10 times that of Vermont, which is the lowest at 131 per 100,000, the AAP and CHA said. Vermont reports child COVID-19 cases using an age range of 0-19 years.
The other states with rates over 1,000 cases per 100,000 children are Arizona (1,300), which had the highest rate a week ago; South Carolina (1,214); Louisiana (1,127); Mississippi (1,120); and Nevada (1,068). Those with rates below 200 cases per 100,000 children are Maine (150), New Hampshire (175), and Hawaii (188), according to this week’s report.
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

The cumulative number of pediatric cases reported up to that date was 442,785, or 9.3% of the total COVID-19 case load of more than 4.76 million among all ages. There have been only 92 pediatric deaths, however, which works out to just 0.06% of the 154,279 reported for all ages, the AAP and the CHA said Aug. 24 in their most recent update.
Child hospitalizations also were on the low side, representing 1.7% (4,062) of the cumulative total of 234,810 admissions among all ages as of Aug. 20, based on data from 21 states and New York City.
Nationally, the cumulative number of reported child cases is now up to 583 per 100,000 children, and that figure covers 49 states, Washington, D.C., Guam, New York City, and Puerto Rico.
There is some disagreement among the states, though, about the definition of “child.” Most states use an age range of 0-17, 0-18, or 0-19, but Florida and Utah go with a range of 0-14 years while South Carolina and Tennessee consider humans aged 0-20 years to be children. Other data limitations involve Texas, which has reported age distribution for only 8% of all cases, and New York, which is not reporting the age distribution of statewide cases, the AAP/CHA report noted.
The definition of child isn’t the only thing that varies between the states. The cumulative case rate for Tennessee, the highest in the country at 1,315 per 100,000 children, is 10 times that of Vermont, which is the lowest at 131 per 100,000, the AAP and CHA said. Vermont reports child COVID-19 cases using an age range of 0-19 years.
The other states with rates over 1,000 cases per 100,000 children are Arizona (1,300), which had the highest rate a week ago; South Carolina (1,214); Louisiana (1,127); Mississippi (1,120); and Nevada (1,068). Those with rates below 200 cases per 100,000 children are Maine (150), New Hampshire (175), and Hawaii (188), according to this week’s report.
Famotidine associated with benefits in hospitalized COVID patients in another trial
It also demonstrated lower levels of serum markers for severe disease.
The findings come from an observational study of 83 hospitalized patients that was published in the American Journal of Gastroenterology.
“The mechanism of exactly how famotidine works has yet to be proven,” lead study author Jeffrey F. Mather, MS, said in an interview. “There’s thought that it works directly on the virus, and there is thought that it works through inactivating certain proteases that are required for the virus infection, but I think the most interesting [hypothesis] is by Malone et al. “They’re looking at the blocking of the histamine-2 receptor causing a decrease in the amount of histamine. It’s all speculative, but it will be interesting if that gets worked out.”
In a study that largely mimicked that of an earlier, larger published observational study on the topic (doi: 10.1053/j.gastro.2020.05.053), Mr. Mather and colleagues retrospectively evaluated 878 patients who tested positive for SARS-CoV-2 and who required admission to Hartford (Conn.) Hospital between Feb. 24, 2020, and May 14, 2020. Patients were classified as receiving famotidine if they were treated with either oral or intravenous drug within 1 week of COVID-19 screening and/or hospital admission. Primary outcomes of interest were in-hospital death as recorded in the discharge of the patients, requirement for mechanical ventilation, and the composite of death or requirement for ventilation. Secondary outcomes of interest were several serum markers of disease activity including white blood cell count, lymphocyte count, and eosinophil count.
Famotidine was administered orally in 83% of the patients and intravenously in the remaining 17%. Mr. Mather, director of data management in the division of research management at Hartford Hospital, and his colleagues reported that 83 of the 878 patients studied (9.5%) received famotidine. Compared with patients not treated with famotidine, those who received the drug were slightly younger (a mean of 64 vs. 68 years, respectively; P = .021); otherwise, there were no differences between the two groups in baseline demographics or in preexisting comorbidities.
The use of famotidine was associated with a decreased risk of in-hospital mortality (odds ratio, 0.37; P = .021) as well as combined death or intubation (OR, 0.47; P = .040). The outcomes were similar when the researchers performed propensity score matching to adjust for age differences between groups.
In addition, the use of famotidine was associated with lower levels of serum markers for severe disease including lower median peak C-reactive protein levels (9.4 vs. 12.7 mg/dL; P =. 002), lower median procalcitonin levels (0.16 vs. 0.30 ng/mL; P = .004), and a nonsignificant trend to lower median mean ferritin levels (797.5 vs. 964 ng/mL; P = .076).
Logistic regression analysis revealed that use of famotidine was an independent predictor of both lower mortality and combined death/intubation. In addition, predictors of both adverse outcomes included older age, a body mass index of greater than 30 kg/m2, chronic kidney disease, the national early warning score, and a higher neutrophil-lymphocyte ratio.
“This is an important stepping stone, but until we have a randomized, controlled trial, we really can’t speak about causation; we can only speak about association, and that’s okay,” Brennan Spiegel, MD, MSHS, director of health services research at Cedars-Sinai, Los Angeles, who was not affiliated with the study, said in an interview. “There’s nothing wrong with association because finding associations can raise important hypotheses that can then be tested in prospective randomized trials, for example.”
In July 2020, Dr. Spiegel and his colleagues published a separate paper looking at proton pump inhibitors and the risk of COVID-19. “In that study we did look at H2 blockers, and we did find that they were slightly associated with a reduction in COVID-19,” he said. “It was a small effect, but it was a benefit. When we see consistency among studies, it’s a signal in the noise we can try and follow and see if there is something more to it.”
Mr. Mather acknowledged certain limitations of the study, including the fact that patients who did and did not receive famotidine were propensity-matched for age. “The risk factors that others have shown for adverse events are equivalent in the groups, but anytime you do a retrospective study like this there is the potential for underlying factors that may play a role in the outcomes that you’re not considering,” Mr. Mather said. “That’s why the gold standard is the randomized trial, to wash those effects out. There’s only an association here, and it supports the need for a randomized trial.”
Famotidine is currently being tested in a double-blind randomized clinical trial in combination with either hydroxychloroquine or remdesivir (NCT 04370262).
“It’s fascinating because famotidine is a safe medicine,” added Dr. Spiegel, who is also co–editor in chief of the American Journal of Gastroenterology. “There are very few side effects; it’s something we’ve been using for decades.”
Mr. Mather and his colleagues reported having no financial disclosures. Dr. Spiegel disclosed that he has served on advisory boards for Allergan, Alnylam Pharmaceuticals, Arena Pharmaceuticals, Ironwood Pharmaceuticals, Salix Pharmaceuticals, Synergy Pharmaceuticals, and Takeda Pharmaceuticals.
SOURCE: Mather J et al. 2020 Aug 14. Am J Gastroenterol.
It also demonstrated lower levels of serum markers for severe disease.
The findings come from an observational study of 83 hospitalized patients that was published in the American Journal of Gastroenterology.
“The mechanism of exactly how famotidine works has yet to be proven,” lead study author Jeffrey F. Mather, MS, said in an interview. “There’s thought that it works directly on the virus, and there is thought that it works through inactivating certain proteases that are required for the virus infection, but I think the most interesting [hypothesis] is by Malone et al. “They’re looking at the blocking of the histamine-2 receptor causing a decrease in the amount of histamine. It’s all speculative, but it will be interesting if that gets worked out.”
In a study that largely mimicked that of an earlier, larger published observational study on the topic (doi: 10.1053/j.gastro.2020.05.053), Mr. Mather and colleagues retrospectively evaluated 878 patients who tested positive for SARS-CoV-2 and who required admission to Hartford (Conn.) Hospital between Feb. 24, 2020, and May 14, 2020. Patients were classified as receiving famotidine if they were treated with either oral or intravenous drug within 1 week of COVID-19 screening and/or hospital admission. Primary outcomes of interest were in-hospital death as recorded in the discharge of the patients, requirement for mechanical ventilation, and the composite of death or requirement for ventilation. Secondary outcomes of interest were several serum markers of disease activity including white blood cell count, lymphocyte count, and eosinophil count.
Famotidine was administered orally in 83% of the patients and intravenously in the remaining 17%. Mr. Mather, director of data management in the division of research management at Hartford Hospital, and his colleagues reported that 83 of the 878 patients studied (9.5%) received famotidine. Compared with patients not treated with famotidine, those who received the drug were slightly younger (a mean of 64 vs. 68 years, respectively; P = .021); otherwise, there were no differences between the two groups in baseline demographics or in preexisting comorbidities.
The use of famotidine was associated with a decreased risk of in-hospital mortality (odds ratio, 0.37; P = .021) as well as combined death or intubation (OR, 0.47; P = .040). The outcomes were similar when the researchers performed propensity score matching to adjust for age differences between groups.
In addition, the use of famotidine was associated with lower levels of serum markers for severe disease including lower median peak C-reactive protein levels (9.4 vs. 12.7 mg/dL; P =. 002), lower median procalcitonin levels (0.16 vs. 0.30 ng/mL; P = .004), and a nonsignificant trend to lower median mean ferritin levels (797.5 vs. 964 ng/mL; P = .076).
Logistic regression analysis revealed that use of famotidine was an independent predictor of both lower mortality and combined death/intubation. In addition, predictors of both adverse outcomes included older age, a body mass index of greater than 30 kg/m2, chronic kidney disease, the national early warning score, and a higher neutrophil-lymphocyte ratio.
“This is an important stepping stone, but until we have a randomized, controlled trial, we really can’t speak about causation; we can only speak about association, and that’s okay,” Brennan Spiegel, MD, MSHS, director of health services research at Cedars-Sinai, Los Angeles, who was not affiliated with the study, said in an interview. “There’s nothing wrong with association because finding associations can raise important hypotheses that can then be tested in prospective randomized trials, for example.”
In July 2020, Dr. Spiegel and his colleagues published a separate paper looking at proton pump inhibitors and the risk of COVID-19. “In that study we did look at H2 blockers, and we did find that they were slightly associated with a reduction in COVID-19,” he said. “It was a small effect, but it was a benefit. When we see consistency among studies, it’s a signal in the noise we can try and follow and see if there is something more to it.”
Mr. Mather acknowledged certain limitations of the study, including the fact that patients who did and did not receive famotidine were propensity-matched for age. “The risk factors that others have shown for adverse events are equivalent in the groups, but anytime you do a retrospective study like this there is the potential for underlying factors that may play a role in the outcomes that you’re not considering,” Mr. Mather said. “That’s why the gold standard is the randomized trial, to wash those effects out. There’s only an association here, and it supports the need for a randomized trial.”
Famotidine is currently being tested in a double-blind randomized clinical trial in combination with either hydroxychloroquine or remdesivir (NCT 04370262).
“It’s fascinating because famotidine is a safe medicine,” added Dr. Spiegel, who is also co–editor in chief of the American Journal of Gastroenterology. “There are very few side effects; it’s something we’ve been using for decades.”
Mr. Mather and his colleagues reported having no financial disclosures. Dr. Spiegel disclosed that he has served on advisory boards for Allergan, Alnylam Pharmaceuticals, Arena Pharmaceuticals, Ironwood Pharmaceuticals, Salix Pharmaceuticals, Synergy Pharmaceuticals, and Takeda Pharmaceuticals.
SOURCE: Mather J et al. 2020 Aug 14. Am J Gastroenterol.
It also demonstrated lower levels of serum markers for severe disease.
The findings come from an observational study of 83 hospitalized patients that was published in the American Journal of Gastroenterology.
“The mechanism of exactly how famotidine works has yet to be proven,” lead study author Jeffrey F. Mather, MS, said in an interview. “There’s thought that it works directly on the virus, and there is thought that it works through inactivating certain proteases that are required for the virus infection, but I think the most interesting [hypothesis] is by Malone et al. “They’re looking at the blocking of the histamine-2 receptor causing a decrease in the amount of histamine. It’s all speculative, but it will be interesting if that gets worked out.”
In a study that largely mimicked that of an earlier, larger published observational study on the topic (doi: 10.1053/j.gastro.2020.05.053), Mr. Mather and colleagues retrospectively evaluated 878 patients who tested positive for SARS-CoV-2 and who required admission to Hartford (Conn.) Hospital between Feb. 24, 2020, and May 14, 2020. Patients were classified as receiving famotidine if they were treated with either oral or intravenous drug within 1 week of COVID-19 screening and/or hospital admission. Primary outcomes of interest were in-hospital death as recorded in the discharge of the patients, requirement for mechanical ventilation, and the composite of death or requirement for ventilation. Secondary outcomes of interest were several serum markers of disease activity including white blood cell count, lymphocyte count, and eosinophil count.
Famotidine was administered orally in 83% of the patients and intravenously in the remaining 17%. Mr. Mather, director of data management in the division of research management at Hartford Hospital, and his colleagues reported that 83 of the 878 patients studied (9.5%) received famotidine. Compared with patients not treated with famotidine, those who received the drug were slightly younger (a mean of 64 vs. 68 years, respectively; P = .021); otherwise, there were no differences between the two groups in baseline demographics or in preexisting comorbidities.
The use of famotidine was associated with a decreased risk of in-hospital mortality (odds ratio, 0.37; P = .021) as well as combined death or intubation (OR, 0.47; P = .040). The outcomes were similar when the researchers performed propensity score matching to adjust for age differences between groups.
In addition, the use of famotidine was associated with lower levels of serum markers for severe disease including lower median peak C-reactive protein levels (9.4 vs. 12.7 mg/dL; P =. 002), lower median procalcitonin levels (0.16 vs. 0.30 ng/mL; P = .004), and a nonsignificant trend to lower median mean ferritin levels (797.5 vs. 964 ng/mL; P = .076).
Logistic regression analysis revealed that use of famotidine was an independent predictor of both lower mortality and combined death/intubation. In addition, predictors of both adverse outcomes included older age, a body mass index of greater than 30 kg/m2, chronic kidney disease, the national early warning score, and a higher neutrophil-lymphocyte ratio.
“This is an important stepping stone, but until we have a randomized, controlled trial, we really can’t speak about causation; we can only speak about association, and that’s okay,” Brennan Spiegel, MD, MSHS, director of health services research at Cedars-Sinai, Los Angeles, who was not affiliated with the study, said in an interview. “There’s nothing wrong with association because finding associations can raise important hypotheses that can then be tested in prospective randomized trials, for example.”
In July 2020, Dr. Spiegel and his colleagues published a separate paper looking at proton pump inhibitors and the risk of COVID-19. “In that study we did look at H2 blockers, and we did find that they were slightly associated with a reduction in COVID-19,” he said. “It was a small effect, but it was a benefit. When we see consistency among studies, it’s a signal in the noise we can try and follow and see if there is something more to it.”
Mr. Mather acknowledged certain limitations of the study, including the fact that patients who did and did not receive famotidine were propensity-matched for age. “The risk factors that others have shown for adverse events are equivalent in the groups, but anytime you do a retrospective study like this there is the potential for underlying factors that may play a role in the outcomes that you’re not considering,” Mr. Mather said. “That’s why the gold standard is the randomized trial, to wash those effects out. There’s only an association here, and it supports the need for a randomized trial.”
Famotidine is currently being tested in a double-blind randomized clinical trial in combination with either hydroxychloroquine or remdesivir (NCT 04370262).
“It’s fascinating because famotidine is a safe medicine,” added Dr. Spiegel, who is also co–editor in chief of the American Journal of Gastroenterology. “There are very few side effects; it’s something we’ve been using for decades.”
Mr. Mather and his colleagues reported having no financial disclosures. Dr. Spiegel disclosed that he has served on advisory boards for Allergan, Alnylam Pharmaceuticals, Arena Pharmaceuticals, Ironwood Pharmaceuticals, Salix Pharmaceuticals, Synergy Pharmaceuticals, and Takeda Pharmaceuticals.
SOURCE: Mather J et al. 2020 Aug 14. Am J Gastroenterol.
REPORTING FROM THE AMERICAN JOURNAL OF GASTROENTEROLOGY
Key clinical point: Among hospitalized COVID-19 patients, famotidine use was associated with a reduction in death and either death or intubation.
Major finding: The use of famotidine was associated with a decreased risk of in-hospital mortality (OR, 0.37; P = .021), as well as the combined endpoint of death or intubation (OR, 0.47; P = .040).
Study details: A single-center observational study of 83 patients hospitalized with COVID-19.
Disclosures: The researchers reported having no financial disclosures.
Source: Mather J et al. 2020 Aug 14. Am J Gastroenterol.
Study confirms it’s possible to catch COVID-19 twice
Researchers in Hong Kong say they’ve confirmed that a person can be infected with COVID-19 twice.
The new proof comes from a 33-year-old man in Hong Kong who first caught COVID-19 in March. He was tested for the coronavirus after he developed a cough, sore throat, fever, and a headache for 3 days. He stayed in the hospital until he twice tested negative for the virus in mid-April.
On Aug. 15, the man returned to Hong Kong from a recent trip to Spain and the United Kingdom, areas that have recently seen a resurgence of COVID-19 cases. At the airport, he was screened for COVID-19 with a test that checks saliva for the virus. He tested positive, but this time, had no symptoms. He was taken to the hospital for monitoring. His viral load – the amount of virus he had in his body – went down over time, suggesting that his immune system was taking care of the intrusion on its own.
The special thing about his case is that each time he was hospitalized, doctors sequenced the genome of the virus that infected him. It was slightly different from one infection to the next, suggesting that the virus had mutated – or changed – in the 4 months between his infections. It also proves that it’s possible for this coronavirus to infect the same person twice.
Experts with the World Health Organization responded to the case at a news briefing.
“What we are learning about infection is that people do develop an immune response. What is not completely clear yet is how strong that immune response is and for how long that immune response lasts,” said Maria Van Kerkhove, PhD, an infectious disease epidemiologist with the World Health Organization in Geneva, Switzerland.
A study on the man’s case is being prepared for publication in the journal Clinical Infectious Diseases. Experts say the finding shouldn’t cause alarm, but it does have important implications for the development of herd immunity and efforts to come up with vaccines and treatments.
“This appears to be pretty clear-cut evidence of reinfection because of sequencing and isolation of two different viruses,” said Gregory Poland, MD, an expert on vaccine development and immunology at the Mayo Clinic in Rochester, Minn. “The big unknown is how often is this happening,” he said. More studies are needed to learn whether this was a rare case or something that is happening often.
Past experience guides present
Until we know more, Dr. Poland said, the possibility of getting COVID-19 twice shouldn’t make anyone worry.
This also happens with other kinds of coronaviruses – the ones that cause common colds. Those coronaviruses change slightly each year as they circle the globe, which allows them to keep spreading and causing their more run-of-the-mill kind of misery.
It also happens with seasonal flu. It is the reason people have to get vaccinated against the flu year after year, and why the flu vaccine has to change slightly each year in an effort to keep up with the ever-evolving influenza virus.
“We’ve been making flu vaccines for 80 years, and there are clinical trials happening as we speak to find new and better influenza vaccines,” Dr. Poland said.
There has been other evidence the virus that causes COVID-19 can change this way, too. Researchers at Howard Hughes Medical Center, at Rockefeller University in New York, recently used a key piece of the SARS-CoV-2 virus – the genetic instructions for its spike protein – to repeatedly infect human cells. Scientists watched as each new generation of the virus went on to infect a new batch of cells. Over time, as it copied itself, some of the copies changed their genes to allow them to survive after scientists attacked them with neutralizing antibodies. Those antibodies are among the main weapons used by the immune system to recognize and disable a virus.
Though that study is still a preprint, which means it hasn’t yet been reviewed by outside experts, the authors wrote that their findings suggest the virus can change in ways that help it evade our immune system. If true, they wrote in mid-July, it means reinfection is possible, especially in people who have a weak immune response to the virus the first time they encounter it.
Good news
That seems to be true in the case of the man from Hong Kong. When doctors tested his blood to look for antibodies to the virus, they didn’t find any. That could mean that he either had a weak immune response to the virus the first time around, or that the antibodies he made during his first infection diminished over time. But during his second infection, he quickly developed more antibodies, suggesting that the second infection acted a little bit like a booster to fire up his immune system. That’s probably the reason he didn’t have any symptoms the second time, too.
That’s good news, Dr. Poland said. It means our bodies can get better at fighting off the COVID-19 virus and that catching it once means the second time might not be so bad.
But the fact that the virus can change quickly this way does have some impact on the effort to come up with a vaccine that works well.
“I think a potential implication of this is that we will have to give booster doses. The question is how frequently,” Dr. Poland said. That will depend on how fast the virus is changing, and how often reinfection is happening in the real world.
“I’m a little surprised at 4½ months,” Dr. Poland said, referencing the time between the Hong Kong man’s infections. “I’m not surprised by, you know, I got infected last winter and I got infected again this winter,” he said.
It also suggests that immune-based therapies such as convalescent plasma and monoclonal antibodies may be of limited help over time, since the virus might be changing in ways that help it outsmart those treatments.
Convalescent plasma is essentially a concentrated dose of antibodies from people who have recovered from a COVID-19 infection. As the virus changes, the antibodies in that plasma may not work as well for future infections.
Drug companies have learned to harness the power of monoclonal antibodies as powerful treatments against cancer and other diseases. Monoclonal antibodies, which are mass-produced in a lab, mimic the body’s natural defenses against a pathogen. Just like the virus can become resistant to natural immunity, it can change in ways that help it outsmart lab-created treatments. Some drug companies that are developing monoclonal antibodies to fight COVID-19 have already prepared for that possibility by making antibody cocktails that are designed to disable the virus by locking onto it in different places, which may help prevent it from developing resistance to those therapies.
“We have a lot to learn,” Dr. Poland said. “Now that the proof of principle has been established, and I would say it has with this man, and with our knowledge of seasonal coronaviruses, we need to look more aggressively to define how often this occurs.”
A version of this article originally appeared on WebMD.com.
Researchers in Hong Kong say they’ve confirmed that a person can be infected with COVID-19 twice.
The new proof comes from a 33-year-old man in Hong Kong who first caught COVID-19 in March. He was tested for the coronavirus after he developed a cough, sore throat, fever, and a headache for 3 days. He stayed in the hospital until he twice tested negative for the virus in mid-April.
On Aug. 15, the man returned to Hong Kong from a recent trip to Spain and the United Kingdom, areas that have recently seen a resurgence of COVID-19 cases. At the airport, he was screened for COVID-19 with a test that checks saliva for the virus. He tested positive, but this time, had no symptoms. He was taken to the hospital for monitoring. His viral load – the amount of virus he had in his body – went down over time, suggesting that his immune system was taking care of the intrusion on its own.
The special thing about his case is that each time he was hospitalized, doctors sequenced the genome of the virus that infected him. It was slightly different from one infection to the next, suggesting that the virus had mutated – or changed – in the 4 months between his infections. It also proves that it’s possible for this coronavirus to infect the same person twice.
Experts with the World Health Organization responded to the case at a news briefing.
“What we are learning about infection is that people do develop an immune response. What is not completely clear yet is how strong that immune response is and for how long that immune response lasts,” said Maria Van Kerkhove, PhD, an infectious disease epidemiologist with the World Health Organization in Geneva, Switzerland.
A study on the man’s case is being prepared for publication in the journal Clinical Infectious Diseases. Experts say the finding shouldn’t cause alarm, but it does have important implications for the development of herd immunity and efforts to come up with vaccines and treatments.
“This appears to be pretty clear-cut evidence of reinfection because of sequencing and isolation of two different viruses,” said Gregory Poland, MD, an expert on vaccine development and immunology at the Mayo Clinic in Rochester, Minn. “The big unknown is how often is this happening,” he said. More studies are needed to learn whether this was a rare case or something that is happening often.
Past experience guides present
Until we know more, Dr. Poland said, the possibility of getting COVID-19 twice shouldn’t make anyone worry.
This also happens with other kinds of coronaviruses – the ones that cause common colds. Those coronaviruses change slightly each year as they circle the globe, which allows them to keep spreading and causing their more run-of-the-mill kind of misery.
It also happens with seasonal flu. It is the reason people have to get vaccinated against the flu year after year, and why the flu vaccine has to change slightly each year in an effort to keep up with the ever-evolving influenza virus.
“We’ve been making flu vaccines for 80 years, and there are clinical trials happening as we speak to find new and better influenza vaccines,” Dr. Poland said.
There has been other evidence the virus that causes COVID-19 can change this way, too. Researchers at Howard Hughes Medical Center, at Rockefeller University in New York, recently used a key piece of the SARS-CoV-2 virus – the genetic instructions for its spike protein – to repeatedly infect human cells. Scientists watched as each new generation of the virus went on to infect a new batch of cells. Over time, as it copied itself, some of the copies changed their genes to allow them to survive after scientists attacked them with neutralizing antibodies. Those antibodies are among the main weapons used by the immune system to recognize and disable a virus.
Though that study is still a preprint, which means it hasn’t yet been reviewed by outside experts, the authors wrote that their findings suggest the virus can change in ways that help it evade our immune system. If true, they wrote in mid-July, it means reinfection is possible, especially in people who have a weak immune response to the virus the first time they encounter it.
Good news
That seems to be true in the case of the man from Hong Kong. When doctors tested his blood to look for antibodies to the virus, they didn’t find any. That could mean that he either had a weak immune response to the virus the first time around, or that the antibodies he made during his first infection diminished over time. But during his second infection, he quickly developed more antibodies, suggesting that the second infection acted a little bit like a booster to fire up his immune system. That’s probably the reason he didn’t have any symptoms the second time, too.
That’s good news, Dr. Poland said. It means our bodies can get better at fighting off the COVID-19 virus and that catching it once means the second time might not be so bad.
But the fact that the virus can change quickly this way does have some impact on the effort to come up with a vaccine that works well.
“I think a potential implication of this is that we will have to give booster doses. The question is how frequently,” Dr. Poland said. That will depend on how fast the virus is changing, and how often reinfection is happening in the real world.
“I’m a little surprised at 4½ months,” Dr. Poland said, referencing the time between the Hong Kong man’s infections. “I’m not surprised by, you know, I got infected last winter and I got infected again this winter,” he said.
It also suggests that immune-based therapies such as convalescent plasma and monoclonal antibodies may be of limited help over time, since the virus might be changing in ways that help it outsmart those treatments.
Convalescent plasma is essentially a concentrated dose of antibodies from people who have recovered from a COVID-19 infection. As the virus changes, the antibodies in that plasma may not work as well for future infections.
Drug companies have learned to harness the power of monoclonal antibodies as powerful treatments against cancer and other diseases. Monoclonal antibodies, which are mass-produced in a lab, mimic the body’s natural defenses against a pathogen. Just like the virus can become resistant to natural immunity, it can change in ways that help it outsmart lab-created treatments. Some drug companies that are developing monoclonal antibodies to fight COVID-19 have already prepared for that possibility by making antibody cocktails that are designed to disable the virus by locking onto it in different places, which may help prevent it from developing resistance to those therapies.
“We have a lot to learn,” Dr. Poland said. “Now that the proof of principle has been established, and I would say it has with this man, and with our knowledge of seasonal coronaviruses, we need to look more aggressively to define how often this occurs.”
A version of this article originally appeared on WebMD.com.
Researchers in Hong Kong say they’ve confirmed that a person can be infected with COVID-19 twice.
The new proof comes from a 33-year-old man in Hong Kong who first caught COVID-19 in March. He was tested for the coronavirus after he developed a cough, sore throat, fever, and a headache for 3 days. He stayed in the hospital until he twice tested negative for the virus in mid-April.
On Aug. 15, the man returned to Hong Kong from a recent trip to Spain and the United Kingdom, areas that have recently seen a resurgence of COVID-19 cases. At the airport, he was screened for COVID-19 with a test that checks saliva for the virus. He tested positive, but this time, had no symptoms. He was taken to the hospital for monitoring. His viral load – the amount of virus he had in his body – went down over time, suggesting that his immune system was taking care of the intrusion on its own.
The special thing about his case is that each time he was hospitalized, doctors sequenced the genome of the virus that infected him. It was slightly different from one infection to the next, suggesting that the virus had mutated – or changed – in the 4 months between his infections. It also proves that it’s possible for this coronavirus to infect the same person twice.
Experts with the World Health Organization responded to the case at a news briefing.
“What we are learning about infection is that people do develop an immune response. What is not completely clear yet is how strong that immune response is and for how long that immune response lasts,” said Maria Van Kerkhove, PhD, an infectious disease epidemiologist with the World Health Organization in Geneva, Switzerland.
A study on the man’s case is being prepared for publication in the journal Clinical Infectious Diseases. Experts say the finding shouldn’t cause alarm, but it does have important implications for the development of herd immunity and efforts to come up with vaccines and treatments.
“This appears to be pretty clear-cut evidence of reinfection because of sequencing and isolation of two different viruses,” said Gregory Poland, MD, an expert on vaccine development and immunology at the Mayo Clinic in Rochester, Minn. “The big unknown is how often is this happening,” he said. More studies are needed to learn whether this was a rare case or something that is happening often.
Past experience guides present
Until we know more, Dr. Poland said, the possibility of getting COVID-19 twice shouldn’t make anyone worry.
This also happens with other kinds of coronaviruses – the ones that cause common colds. Those coronaviruses change slightly each year as they circle the globe, which allows them to keep spreading and causing their more run-of-the-mill kind of misery.
It also happens with seasonal flu. It is the reason people have to get vaccinated against the flu year after year, and why the flu vaccine has to change slightly each year in an effort to keep up with the ever-evolving influenza virus.
“We’ve been making flu vaccines for 80 years, and there are clinical trials happening as we speak to find new and better influenza vaccines,” Dr. Poland said.
There has been other evidence the virus that causes COVID-19 can change this way, too. Researchers at Howard Hughes Medical Center, at Rockefeller University in New York, recently used a key piece of the SARS-CoV-2 virus – the genetic instructions for its spike protein – to repeatedly infect human cells. Scientists watched as each new generation of the virus went on to infect a new batch of cells. Over time, as it copied itself, some of the copies changed their genes to allow them to survive after scientists attacked them with neutralizing antibodies. Those antibodies are among the main weapons used by the immune system to recognize and disable a virus.
Though that study is still a preprint, which means it hasn’t yet been reviewed by outside experts, the authors wrote that their findings suggest the virus can change in ways that help it evade our immune system. If true, they wrote in mid-July, it means reinfection is possible, especially in people who have a weak immune response to the virus the first time they encounter it.
Good news
That seems to be true in the case of the man from Hong Kong. When doctors tested his blood to look for antibodies to the virus, they didn’t find any. That could mean that he either had a weak immune response to the virus the first time around, or that the antibodies he made during his first infection diminished over time. But during his second infection, he quickly developed more antibodies, suggesting that the second infection acted a little bit like a booster to fire up his immune system. That’s probably the reason he didn’t have any symptoms the second time, too.
That’s good news, Dr. Poland said. It means our bodies can get better at fighting off the COVID-19 virus and that catching it once means the second time might not be so bad.
But the fact that the virus can change quickly this way does have some impact on the effort to come up with a vaccine that works well.
“I think a potential implication of this is that we will have to give booster doses. The question is how frequently,” Dr. Poland said. That will depend on how fast the virus is changing, and how often reinfection is happening in the real world.
“I’m a little surprised at 4½ months,” Dr. Poland said, referencing the time between the Hong Kong man’s infections. “I’m not surprised by, you know, I got infected last winter and I got infected again this winter,” he said.
It also suggests that immune-based therapies such as convalescent plasma and monoclonal antibodies may be of limited help over time, since the virus might be changing in ways that help it outsmart those treatments.
Convalescent plasma is essentially a concentrated dose of antibodies from people who have recovered from a COVID-19 infection. As the virus changes, the antibodies in that plasma may not work as well for future infections.
Drug companies have learned to harness the power of monoclonal antibodies as powerful treatments against cancer and other diseases. Monoclonal antibodies, which are mass-produced in a lab, mimic the body’s natural defenses against a pathogen. Just like the virus can become resistant to natural immunity, it can change in ways that help it outsmart lab-created treatments. Some drug companies that are developing monoclonal antibodies to fight COVID-19 have already prepared for that possibility by making antibody cocktails that are designed to disable the virus by locking onto it in different places, which may help prevent it from developing resistance to those therapies.
“We have a lot to learn,” Dr. Poland said. “Now that the proof of principle has been established, and I would say it has with this man, and with our knowledge of seasonal coronaviruses, we need to look more aggressively to define how often this occurs.”
A version of this article originally appeared on WebMD.com.
Methotrexate as a Treatment of Palmoplantar Lichen Planus
To the Editor:
Palmoplantar lichen planus (LP) is an uncommon variant of LP that involves the palms and soles. The prevalence of LP is approximately 0.1% to 2% in the general population. It can affect both mucosal and cutaneous surfaces.1 A study of 36 patients with LP showed that 25% (9/36) had palmar and/or plantar involvement.2 Palmoplantar LP is more commonly found in men than women, with an average age of onset of 38 to 65 years.3 It tends to affect the soles more often than the palms, with the most common site being the plantar arch. Itching generally is the most common symptom reported. Lesions often resolve over a few months, but relapses can occur in 10% to 29% of patients.2 The clinical morphology commonly is characterized as erythematous scaly plaques, hyperkeratotic plaques, or ulcerations.4 Due to its rare occurrence, palmoplantar LP often is misdiagnosed as psoriasis, eczematous dermatitis, tinea nigra, or secondary syphilis, making pathology extremely helpful in making the diagnosis.1 Darker skin types can obscure defining characteristics, further impeding a timely diagnosis. We describe a novel case of palmoplantar LP that was successfully treated with methotrexate.
A 38-year-old man with no notable medical history presented for dermatologic evaluation of a palmar and plantar rash of 4 months' duration. The rash was accompanied by intense burning pain and pruritus. Prior to presentation, he had been treated with multiple prednisone tapers starting at 40 mg daily as well as combination therapy of a 2-week course of minocycline 100 mg twice daily and clobetasol ointment twice daily for 4 months, with no notable improvement. Workup prior to presentation included a negative potassium hydroxide fungal preparation and a normal antinuclear antibody titer. A review of symptoms was negative for arthralgia, myalgia, photosensitivity, malar rash, Raynaud phenomenon, pleuritic pain, seizures, and psychosis.
Physical examination revealed focal areas of mildly thick, hyperkeratotic scale with desquamation on the plantar and palmar surfaces of the feet and hands. The underlying skin of the feet consisted of dyspigmented patches of dark brown and hypopigmented skin with erythema, profound scaling, and sparing of the internal plantar arches (Figure 1A). On the palms, thin hyperkeratotic plaques with desquamation and erythematous maceration of the surrounding skin were observed (Figure 2A). Thin white plaques of the posterior bilateral buccal mucosa were appreciated as well as an erosion that extended to the lower lip.
The differential diagnosis included LP, psoriasis, acquired palmoplantar keratoderma, and discoid lupus erythematosus. Tinea pedis and tinea manuum were less likely in the setting of a negative potassium hydroxide fungal preparation.

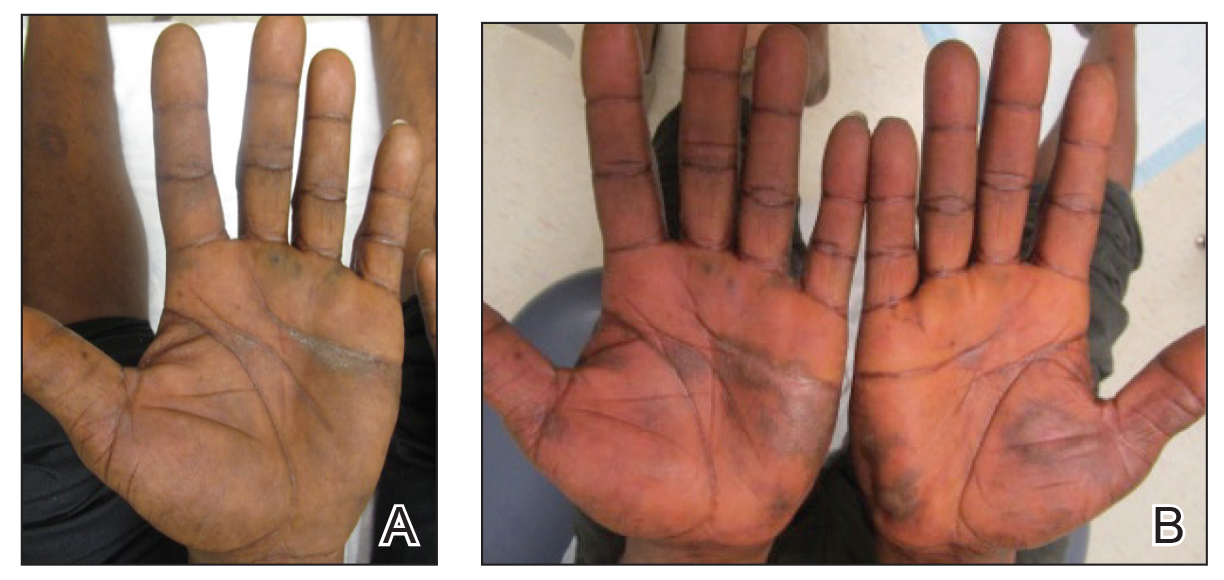
A biopsy of the lateral aspect of the left foot showed a cell-poor interface dermatitis that could resemble partially treated LP or a lichenoid hypersensitivity reaction (Figure 3). Given the clinical and pathologic findings, a diagnosis of palmoplantar LP was favored. The patient was on no medications or over-the-counter supplements prior to the appearance of the rash, making a lichenoid hypersensitivity rash less likely. The histology findings likely were muted, as they were done at the end of the prednisone taper.
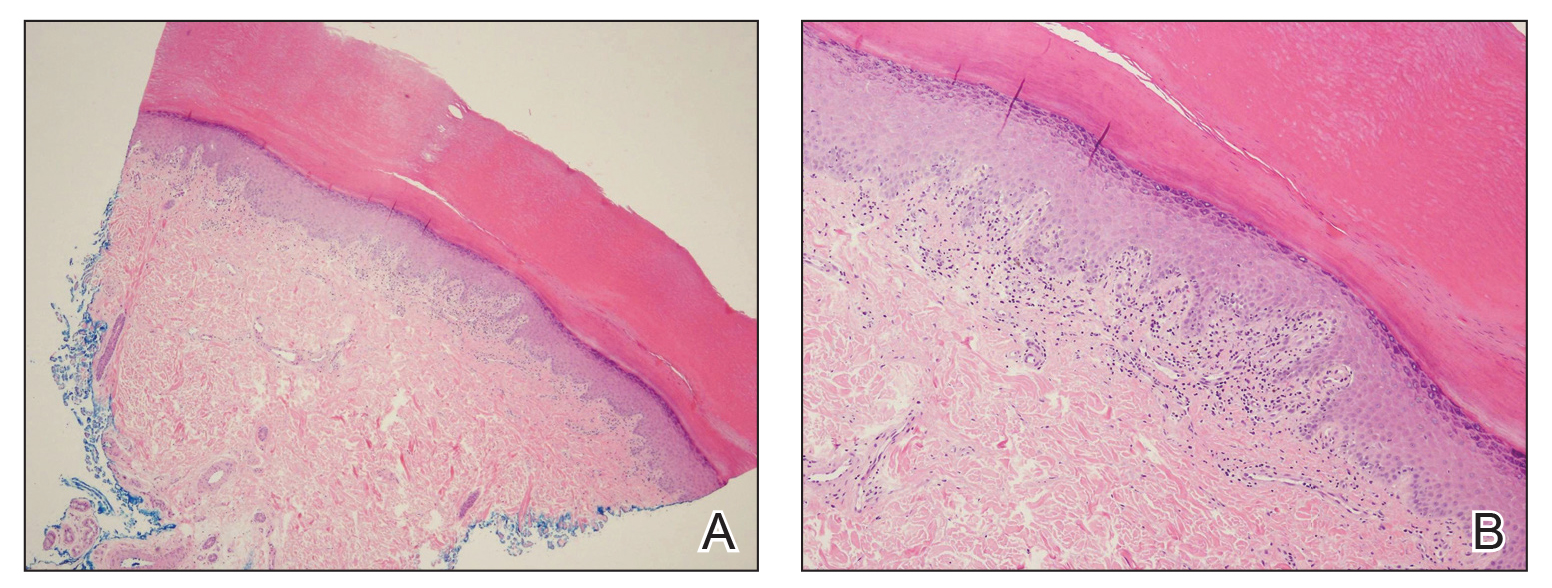
Minocycline and clobetasol ointment were discontinued, and the prednisone taper was completed as originally prescribed. The patient was started on 25 mg daily of acitretin for 4 weeks, then increased to 35 mg daily. Notable improvement in the palmar and plantar lesions was noted after the initial 4 weeks of therapy; however, acitretin treatment was discontinued due to lack of adequate insurance coverage for the medication. The patient became symptomatic several weeks following acitretin cessation and was started on methotrexate 15 mg weekly with triamcinolone acetonide paste 0.1% for the oral lesions. Once again, improvement was seen on both the palmar and plantar surfaces after 4 weeks of therapy (Figures 1B and 2B).
Evidence for treatment of palmoplantar LP is limited to a few case reports and case series. Documented treatments for palmoplantar LP include topical and systemic steroids, tazarotene, acitretin, and immunosuppressive medications.4 One case report described a patient who responded well to prednisone therapy (1 mg/kg daily for 3 weeks, then reduced to 5 mg daily).5 Another report described a patient who responded favorably to cyclosporine 3.5 mg/kg daily for 4 weeks, then tapered over another 4 weeks for a total of 8 weeks of treatment.4 Although the most common treatments described in the literature consist of acitretin as well as topical and systemic steroids, few have discussed the efficacy of methotrexate. In one study, acitretin did not result in clearance, but the patient saw profound improvement with methotrexate (titrated up to 25 mg weekly) over 2 months.1
In our case, treatment with methotrexate was proven successful in a patient who responded to acitretin but was unable to afford treatment. This case highlights a rare variant of a common disease and the possibility of methotrexate as a cost-effective and useful treatment option for LP.
- Rieder E, Hale CS, Meehan SA, et al. Palmoplantar lichen planus. Dermatol Online J. 2015;20:13030/qt1vn9s55z.
- Sánchez-Pérez J, Rios Buceta L, Fraga J, et al. Lichen planus with lesions on the palms and/or soles: prevalence and clinicopathological study of 36 patients. Br J Dermatol. 2000;142:310-314.
- Gutte R, Khopkar U. Predominant palmoplantar lichen planus: a diagnostic challenge. Indian J Dermatol. 2014;59:343-347.
- Karakatsanis G, Patsatsi A, Kastoridou C, et al Palmoplantar lichen planus with umbilicated papules: an atypical case with rapid therapeutic response to cyclosporin. J Eur Acad Dermatol Venereol. 2007;21:1006-1007.
- Goucha S, Khaled A, Bennani Z, et al. Erosive lichen planus of the soles: Effective response to prednisone. Dermatol Ther. 2011;1:20-24.
To the Editor:
Palmoplantar lichen planus (LP) is an uncommon variant of LP that involves the palms and soles. The prevalence of LP is approximately 0.1% to 2% in the general population. It can affect both mucosal and cutaneous surfaces.1 A study of 36 patients with LP showed that 25% (9/36) had palmar and/or plantar involvement.2 Palmoplantar LP is more commonly found in men than women, with an average age of onset of 38 to 65 years.3 It tends to affect the soles more often than the palms, with the most common site being the plantar arch. Itching generally is the most common symptom reported. Lesions often resolve over a few months, but relapses can occur in 10% to 29% of patients.2 The clinical morphology commonly is characterized as erythematous scaly plaques, hyperkeratotic plaques, or ulcerations.4 Due to its rare occurrence, palmoplantar LP often is misdiagnosed as psoriasis, eczematous dermatitis, tinea nigra, or secondary syphilis, making pathology extremely helpful in making the diagnosis.1 Darker skin types can obscure defining characteristics, further impeding a timely diagnosis. We describe a novel case of palmoplantar LP that was successfully treated with methotrexate.
A 38-year-old man with no notable medical history presented for dermatologic evaluation of a palmar and plantar rash of 4 months' duration. The rash was accompanied by intense burning pain and pruritus. Prior to presentation, he had been treated with multiple prednisone tapers starting at 40 mg daily as well as combination therapy of a 2-week course of minocycline 100 mg twice daily and clobetasol ointment twice daily for 4 months, with no notable improvement. Workup prior to presentation included a negative potassium hydroxide fungal preparation and a normal antinuclear antibody titer. A review of symptoms was negative for arthralgia, myalgia, photosensitivity, malar rash, Raynaud phenomenon, pleuritic pain, seizures, and psychosis.
Physical examination revealed focal areas of mildly thick, hyperkeratotic scale with desquamation on the plantar and palmar surfaces of the feet and hands. The underlying skin of the feet consisted of dyspigmented patches of dark brown and hypopigmented skin with erythema, profound scaling, and sparing of the internal plantar arches (Figure 1A). On the palms, thin hyperkeratotic plaques with desquamation and erythematous maceration of the surrounding skin were observed (Figure 2A). Thin white plaques of the posterior bilateral buccal mucosa were appreciated as well as an erosion that extended to the lower lip.
The differential diagnosis included LP, psoriasis, acquired palmoplantar keratoderma, and discoid lupus erythematosus. Tinea pedis and tinea manuum were less likely in the setting of a negative potassium hydroxide fungal preparation.


A biopsy of the lateral aspect of the left foot showed a cell-poor interface dermatitis that could resemble partially treated LP or a lichenoid hypersensitivity reaction (Figure 3). Given the clinical and pathologic findings, a diagnosis of palmoplantar LP was favored. The patient was on no medications or over-the-counter supplements prior to the appearance of the rash, making a lichenoid hypersensitivity rash less likely. The histology findings likely were muted, as they were done at the end of the prednisone taper.

Minocycline and clobetasol ointment were discontinued, and the prednisone taper was completed as originally prescribed. The patient was started on 25 mg daily of acitretin for 4 weeks, then increased to 35 mg daily. Notable improvement in the palmar and plantar lesions was noted after the initial 4 weeks of therapy; however, acitretin treatment was discontinued due to lack of adequate insurance coverage for the medication. The patient became symptomatic several weeks following acitretin cessation and was started on methotrexate 15 mg weekly with triamcinolone acetonide paste 0.1% for the oral lesions. Once again, improvement was seen on both the palmar and plantar surfaces after 4 weeks of therapy (Figures 1B and 2B).
Evidence for treatment of palmoplantar LP is limited to a few case reports and case series. Documented treatments for palmoplantar LP include topical and systemic steroids, tazarotene, acitretin, and immunosuppressive medications.4 One case report described a patient who responded well to prednisone therapy (1 mg/kg daily for 3 weeks, then reduced to 5 mg daily).5 Another report described a patient who responded favorably to cyclosporine 3.5 mg/kg daily for 4 weeks, then tapered over another 4 weeks for a total of 8 weeks of treatment.4 Although the most common treatments described in the literature consist of acitretin as well as topical and systemic steroids, few have discussed the efficacy of methotrexate. In one study, acitretin did not result in clearance, but the patient saw profound improvement with methotrexate (titrated up to 25 mg weekly) over 2 months.1
In our case, treatment with methotrexate was proven successful in a patient who responded to acitretin but was unable to afford treatment. This case highlights a rare variant of a common disease and the possibility of methotrexate as a cost-effective and useful treatment option for LP.
To the Editor:
Palmoplantar lichen planus (LP) is an uncommon variant of LP that involves the palms and soles. The prevalence of LP is approximately 0.1% to 2% in the general population. It can affect both mucosal and cutaneous surfaces.1 A study of 36 patients with LP showed that 25% (9/36) had palmar and/or plantar involvement.2 Palmoplantar LP is more commonly found in men than women, with an average age of onset of 38 to 65 years.3 It tends to affect the soles more often than the palms, with the most common site being the plantar arch. Itching generally is the most common symptom reported. Lesions often resolve over a few months, but relapses can occur in 10% to 29% of patients.2 The clinical morphology commonly is characterized as erythematous scaly plaques, hyperkeratotic plaques, or ulcerations.4 Due to its rare occurrence, palmoplantar LP often is misdiagnosed as psoriasis, eczematous dermatitis, tinea nigra, or secondary syphilis, making pathology extremely helpful in making the diagnosis.1 Darker skin types can obscure defining characteristics, further impeding a timely diagnosis. We describe a novel case of palmoplantar LP that was successfully treated with methotrexate.
A 38-year-old man with no notable medical history presented for dermatologic evaluation of a palmar and plantar rash of 4 months' duration. The rash was accompanied by intense burning pain and pruritus. Prior to presentation, he had been treated with multiple prednisone tapers starting at 40 mg daily as well as combination therapy of a 2-week course of minocycline 100 mg twice daily and clobetasol ointment twice daily for 4 months, with no notable improvement. Workup prior to presentation included a negative potassium hydroxide fungal preparation and a normal antinuclear antibody titer. A review of symptoms was negative for arthralgia, myalgia, photosensitivity, malar rash, Raynaud phenomenon, pleuritic pain, seizures, and psychosis.
Physical examination revealed focal areas of mildly thick, hyperkeratotic scale with desquamation on the plantar and palmar surfaces of the feet and hands. The underlying skin of the feet consisted of dyspigmented patches of dark brown and hypopigmented skin with erythema, profound scaling, and sparing of the internal plantar arches (Figure 1A). On the palms, thin hyperkeratotic plaques with desquamation and erythematous maceration of the surrounding skin were observed (Figure 2A). Thin white plaques of the posterior bilateral buccal mucosa were appreciated as well as an erosion that extended to the lower lip.
The differential diagnosis included LP, psoriasis, acquired palmoplantar keratoderma, and discoid lupus erythematosus. Tinea pedis and tinea manuum were less likely in the setting of a negative potassium hydroxide fungal preparation.


A biopsy of the lateral aspect of the left foot showed a cell-poor interface dermatitis that could resemble partially treated LP or a lichenoid hypersensitivity reaction (Figure 3). Given the clinical and pathologic findings, a diagnosis of palmoplantar LP was favored. The patient was on no medications or over-the-counter supplements prior to the appearance of the rash, making a lichenoid hypersensitivity rash less likely. The histology findings likely were muted, as they were done at the end of the prednisone taper.

Minocycline and clobetasol ointment were discontinued, and the prednisone taper was completed as originally prescribed. The patient was started on 25 mg daily of acitretin for 4 weeks, then increased to 35 mg daily. Notable improvement in the palmar and plantar lesions was noted after the initial 4 weeks of therapy; however, acitretin treatment was discontinued due to lack of adequate insurance coverage for the medication. The patient became symptomatic several weeks following acitretin cessation and was started on methotrexate 15 mg weekly with triamcinolone acetonide paste 0.1% for the oral lesions. Once again, improvement was seen on both the palmar and plantar surfaces after 4 weeks of therapy (Figures 1B and 2B).
Evidence for treatment of palmoplantar LP is limited to a few case reports and case series. Documented treatments for palmoplantar LP include topical and systemic steroids, tazarotene, acitretin, and immunosuppressive medications.4 One case report described a patient who responded well to prednisone therapy (1 mg/kg daily for 3 weeks, then reduced to 5 mg daily).5 Another report described a patient who responded favorably to cyclosporine 3.5 mg/kg daily for 4 weeks, then tapered over another 4 weeks for a total of 8 weeks of treatment.4 Although the most common treatments described in the literature consist of acitretin as well as topical and systemic steroids, few have discussed the efficacy of methotrexate. In one study, acitretin did not result in clearance, but the patient saw profound improvement with methotrexate (titrated up to 25 mg weekly) over 2 months.1
In our case, treatment with methotrexate was proven successful in a patient who responded to acitretin but was unable to afford treatment. This case highlights a rare variant of a common disease and the possibility of methotrexate as a cost-effective and useful treatment option for LP.
- Rieder E, Hale CS, Meehan SA, et al. Palmoplantar lichen planus. Dermatol Online J. 2015;20:13030/qt1vn9s55z.
- Sánchez-Pérez J, Rios Buceta L, Fraga J, et al. Lichen planus with lesions on the palms and/or soles: prevalence and clinicopathological study of 36 patients. Br J Dermatol. 2000;142:310-314.
- Gutte R, Khopkar U. Predominant palmoplantar lichen planus: a diagnostic challenge. Indian J Dermatol. 2014;59:343-347.
- Karakatsanis G, Patsatsi A, Kastoridou C, et al Palmoplantar lichen planus with umbilicated papules: an atypical case with rapid therapeutic response to cyclosporin. J Eur Acad Dermatol Venereol. 2007;21:1006-1007.
- Goucha S, Khaled A, Bennani Z, et al. Erosive lichen planus of the soles: Effective response to prednisone. Dermatol Ther. 2011;1:20-24.
- Rieder E, Hale CS, Meehan SA, et al. Palmoplantar lichen planus. Dermatol Online J. 2015;20:13030/qt1vn9s55z.
- Sánchez-Pérez J, Rios Buceta L, Fraga J, et al. Lichen planus with lesions on the palms and/or soles: prevalence and clinicopathological study of 36 patients. Br J Dermatol. 2000;142:310-314.
- Gutte R, Khopkar U. Predominant palmoplantar lichen planus: a diagnostic challenge. Indian J Dermatol. 2014;59:343-347.
- Karakatsanis G, Patsatsi A, Kastoridou C, et al Palmoplantar lichen planus with umbilicated papules: an atypical case with rapid therapeutic response to cyclosporin. J Eur Acad Dermatol Venereol. 2007;21:1006-1007.
- Goucha S, Khaled A, Bennani Z, et al. Erosive lichen planus of the soles: Effective response to prednisone. Dermatol Ther. 2011;1:20-24.
Practice Points
- Palmoplantar lichen planus (LP) is a rare variant of LP that is resistant to most treatments.
- Methotrexate may be a cost-effective option in patients who cannot tolerate systemic retinoids.
A 36-year-old presents with a mildly pruritic rash consisting of pink papules on his hand
. MG is a dermatophytic folliculitis that classically presents as folliculocentric plaque, in which there are papules, pustules, and nodules, usually found on the lower leg and almost exclusively in adults.1 Wrists are commonly affected as well.
MG is typically caused by mechanical disruption of hair follicles that allows fungi to penetrate deep into dermal tissue.2 Quite often, the source of infection is typically the patient’s skin or nails. Associated risk factors include longstanding fungal infection, shaving or other cutaneous trauma, topical steroids, and immunosuppressive therapy.3,4 Although MG can be caused by other fungal species, it is most often caused by Trichophyton rubrum or Trichophyton tonsurans.1 There are two types of MG, the perifollicular papular form, which is localized and typically occurs in healthy individuals, and the deep subcutaneous plaque or nodular forms that usually occur in immunocompromised individuals.5
MG is an important clinical manifestation to be familiar with because of the increase in the numbers of solid-organ transplants and patients on immunosuppressive therapies. These patients are highly predisposed to opportunistic infections with aggressive clinical courses and will usually require prolonged treatment as relapses are common.3,5
Tissue culture and skin biopsy are often needed to establish the diagnosis. If a topical antifungal has been used, KOH (potassium hydroxide) and culture may be negative. This patient’s tissue culture was positive for T. rubrum. The histopathology revealed hyperkeratosis and acanthosis with focal parakeratosis and a lymphohistiocytic infiltrate in the dermis. On PAS (Periodic acid–Schiff ) stain, PAS-positive hyphae were identified in the keratin layer, confirming a diagnosis of tinea infection.
First line treatment includes systemic antifungals such as griseofulvin, ketoconazole, itraconazole, and terbinafine. Duration of therapy is typically 4-8 weeks or until all lesions are cleared.3,5
This case and photo were submitted by Mr. Hakimi of University of California San Diego School of Medicine and Dr. Sateesh of San Diego Family Dermatology. Donna Bilu Martin, MD, edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1.“Fitzpatrick’s Dermatology in General Medicine” (New York: McGraw-Hill Medical, 2012).
2. Bonifaz A et al. Gac Med Mex. Sep-Oct 2008;144(5):427-33.
3. Romero FA et al. Transpl Infect Dis. 2011 Aug;13(4):424-3. doi:10.1111/j.1399-3062.2010.00596.x
4. Chou WY, Hsu CJ. Medicine (Baltimore). 2016 Jan;95(2):e2245. doi: 10.1097/MD.0000000000002245.
5. Ilkit M et al. Med Mycol. 2102 Jul;50(5):449-57.
. MG is a dermatophytic folliculitis that classically presents as folliculocentric plaque, in which there are papules, pustules, and nodules, usually found on the lower leg and almost exclusively in adults.1 Wrists are commonly affected as well.
MG is typically caused by mechanical disruption of hair follicles that allows fungi to penetrate deep into dermal tissue.2 Quite often, the source of infection is typically the patient’s skin or nails. Associated risk factors include longstanding fungal infection, shaving or other cutaneous trauma, topical steroids, and immunosuppressive therapy.3,4 Although MG can be caused by other fungal species, it is most often caused by Trichophyton rubrum or Trichophyton tonsurans.1 There are two types of MG, the perifollicular papular form, which is localized and typically occurs in healthy individuals, and the deep subcutaneous plaque or nodular forms that usually occur in immunocompromised individuals.5
MG is an important clinical manifestation to be familiar with because of the increase in the numbers of solid-organ transplants and patients on immunosuppressive therapies. These patients are highly predisposed to opportunistic infections with aggressive clinical courses and will usually require prolonged treatment as relapses are common.3,5
Tissue culture and skin biopsy are often needed to establish the diagnosis. If a topical antifungal has been used, KOH (potassium hydroxide) and culture may be negative. This patient’s tissue culture was positive for T. rubrum. The histopathology revealed hyperkeratosis and acanthosis with focal parakeratosis and a lymphohistiocytic infiltrate in the dermis. On PAS (Periodic acid–Schiff ) stain, PAS-positive hyphae were identified in the keratin layer, confirming a diagnosis of tinea infection.
First line treatment includes systemic antifungals such as griseofulvin, ketoconazole, itraconazole, and terbinafine. Duration of therapy is typically 4-8 weeks or until all lesions are cleared.3,5
This case and photo were submitted by Mr. Hakimi of University of California San Diego School of Medicine and Dr. Sateesh of San Diego Family Dermatology. Donna Bilu Martin, MD, edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1.“Fitzpatrick’s Dermatology in General Medicine” (New York: McGraw-Hill Medical, 2012).
2. Bonifaz A et al. Gac Med Mex. Sep-Oct 2008;144(5):427-33.
3. Romero FA et al. Transpl Infect Dis. 2011 Aug;13(4):424-3. doi:10.1111/j.1399-3062.2010.00596.x
4. Chou WY, Hsu CJ. Medicine (Baltimore). 2016 Jan;95(2):e2245. doi: 10.1097/MD.0000000000002245.
5. Ilkit M et al. Med Mycol. 2102 Jul;50(5):449-57.
. MG is a dermatophytic folliculitis that classically presents as folliculocentric plaque, in which there are papules, pustules, and nodules, usually found on the lower leg and almost exclusively in adults.1 Wrists are commonly affected as well.
MG is typically caused by mechanical disruption of hair follicles that allows fungi to penetrate deep into dermal tissue.2 Quite often, the source of infection is typically the patient’s skin or nails. Associated risk factors include longstanding fungal infection, shaving or other cutaneous trauma, topical steroids, and immunosuppressive therapy.3,4 Although MG can be caused by other fungal species, it is most often caused by Trichophyton rubrum or Trichophyton tonsurans.1 There are two types of MG, the perifollicular papular form, which is localized and typically occurs in healthy individuals, and the deep subcutaneous plaque or nodular forms that usually occur in immunocompromised individuals.5
MG is an important clinical manifestation to be familiar with because of the increase in the numbers of solid-organ transplants and patients on immunosuppressive therapies. These patients are highly predisposed to opportunistic infections with aggressive clinical courses and will usually require prolonged treatment as relapses are common.3,5
Tissue culture and skin biopsy are often needed to establish the diagnosis. If a topical antifungal has been used, KOH (potassium hydroxide) and culture may be negative. This patient’s tissue culture was positive for T. rubrum. The histopathology revealed hyperkeratosis and acanthosis with focal parakeratosis and a lymphohistiocytic infiltrate in the dermis. On PAS (Periodic acid–Schiff ) stain, PAS-positive hyphae were identified in the keratin layer, confirming a diagnosis of tinea infection.
First line treatment includes systemic antifungals such as griseofulvin, ketoconazole, itraconazole, and terbinafine. Duration of therapy is typically 4-8 weeks or until all lesions are cleared.3,5
This case and photo were submitted by Mr. Hakimi of University of California San Diego School of Medicine and Dr. Sateesh of San Diego Family Dermatology. Donna Bilu Martin, MD, edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1.“Fitzpatrick’s Dermatology in General Medicine” (New York: McGraw-Hill Medical, 2012).
2. Bonifaz A et al. Gac Med Mex. Sep-Oct 2008;144(5):427-33.
3. Romero FA et al. Transpl Infect Dis. 2011 Aug;13(4):424-3. doi:10.1111/j.1399-3062.2010.00596.x
4. Chou WY, Hsu CJ. Medicine (Baltimore). 2016 Jan;95(2):e2245. doi: 10.1097/MD.0000000000002245.
5. Ilkit M et al. Med Mycol. 2102 Jul;50(5):449-57.
Compression therapy cuts cellulitis risk in chronic leg edema
The effect was so striking that the randomized controlled trial was stopped early and all patients in the study were given the therapy.
“In a climate of increasing antibiotic resistance, we are delighted to have discovered a nondrug management strategy that has such a dramatic impact on the risk of cellulitis,” senior author Bernie Bissett, PhD, from the Discipline of Physiotherapy, Faculty of Health, the University of Canberra, Australia, said in an interview.
“We hope this leads to a shift in preventative medical strategy for patients with chronic edema and cellulitis around the world,” she said.
Lead author Elizabeth Webb, MPH, from the Physiotherapy Department at Calvary Public Hospital Bruce, in Bruce, Australia, and colleagues report their findings in an article published online August 12 in The New England Journal of Medicine.
Dr. Bisset explained that Webb is a “leading lymphedema physiotherapist” and a PhD candidate at the University of Canberra. She added that this is the first study to show that “compression therapy dramatically reduces the risk of cellulitis for patients with chronic edema.”
Penicillin is often given preventively; some research suggests effectiveness wanes after the antibiotic is stopped.
For the current trial, Ms. Webb and colleagues enrolled 84 adults with chronic edema of the leg and recurrent cellulitis. They randomly assigned patients in a 1:1 ratio to receive leg compression therapy plus education about preventing cellulitis (compression group; n = 41) or education only (control group; n = 43).
Compression therapy consisted of wearing knee-high stockings that applied maximum compression at the ankles. The compression gradually decreased up the legs. In addition, 26 patients were treated with “therapist-applied compression bandaging” for 3 to 5 days before receiving the stockings.
Participants underwent follow-up assessments every 6 months for a maximum of 3 years or until 45 episodes of cellulitis, the primary outcome, occurred. Those in the control group crossed over to the compression group once they experienced cellulitis.
The trial was stopped early for reasons of efficacy. “The statistical analysis plan prespecified that after 23 episodes of cellulitis had occurred, an independent data monitoring committee would review the results of the interim analysis and recommend whether the trial should stop early,” the authors write.
At the time of the monitoring committee’s review, six patients (15%) who wore compression stockings and 17 (40%) in the control group had experienced a cellulitis episode (hazard ratio, 0.23; P = .002; relative risk [post hoc analysis], 0.37; P = .02). On the basis of those findings, the researchers stopped the study, and patients in the control group were started on compression therapy.
“Clinicians should definitely consider referring their patients to a skilled lymphedema therapist who can individually prescribe and fit compression garments,” Dr. Bissett said. “In our study, these were well tolerated and reduced the risk of another episode of cellulitis by a huge 77%,” she added.
Secondary outcomes included hospitalization related to cellulitis and quality-of-life assessments.
Three patients (7%) in the compression group and six (14%) in the control group were admitted to the hospital for cellulitis (hazard ratio, 0.38). There were no differences in quality of life outcomes between the treatment groups.
The authors say compression therapy has the potential to decrease cellulitis risk by reducing edema, boosting immune response and skin integrity, and protecting the skin.
“Patients with a history of leg swelling (chronic edema) and previous episodes of cellulitis are ideal candidates for this compression therapy,” Dr. Bissett said.
“Given the lack of side effects of the therapy in our study and the potential to reduce other skin problems in these patients, compression therapy is an ideal prophylactic strategy,” she said.
The authors note several study limitations, including a lack of blinding. In addition, patients in the study had to have access to lymphedema specialists, who might be unavailable to patients outside the study. This could have influenced adherence and limit generalizability. Difficulty putting on and taking off compression garments often leads patients to be less adherent to compression therapy, but 88% of patients in this study wore them at least 4 days per week.
Dr. Bissett said compression therapy would be useful for primary care physicians to consider for patients with chronic edema.
“Primary care physicians are highly likely to encounter patients with chronic edema in their day-to-day practice. We can now confidently say that referral to lymphedema therapists for compression therapy should be a first line of defense against future episodes of cellulitis in this vulnerable patient group,” she explained.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The effect was so striking that the randomized controlled trial was stopped early and all patients in the study were given the therapy.
“In a climate of increasing antibiotic resistance, we are delighted to have discovered a nondrug management strategy that has such a dramatic impact on the risk of cellulitis,” senior author Bernie Bissett, PhD, from the Discipline of Physiotherapy, Faculty of Health, the University of Canberra, Australia, said in an interview.
“We hope this leads to a shift in preventative medical strategy for patients with chronic edema and cellulitis around the world,” she said.
Lead author Elizabeth Webb, MPH, from the Physiotherapy Department at Calvary Public Hospital Bruce, in Bruce, Australia, and colleagues report their findings in an article published online August 12 in The New England Journal of Medicine.
Dr. Bisset explained that Webb is a “leading lymphedema physiotherapist” and a PhD candidate at the University of Canberra. She added that this is the first study to show that “compression therapy dramatically reduces the risk of cellulitis for patients with chronic edema.”
Penicillin is often given preventively; some research suggests effectiveness wanes after the antibiotic is stopped.
For the current trial, Ms. Webb and colleagues enrolled 84 adults with chronic edema of the leg and recurrent cellulitis. They randomly assigned patients in a 1:1 ratio to receive leg compression therapy plus education about preventing cellulitis (compression group; n = 41) or education only (control group; n = 43).
Compression therapy consisted of wearing knee-high stockings that applied maximum compression at the ankles. The compression gradually decreased up the legs. In addition, 26 patients were treated with “therapist-applied compression bandaging” for 3 to 5 days before receiving the stockings.
Participants underwent follow-up assessments every 6 months for a maximum of 3 years or until 45 episodes of cellulitis, the primary outcome, occurred. Those in the control group crossed over to the compression group once they experienced cellulitis.
The trial was stopped early for reasons of efficacy. “The statistical analysis plan prespecified that after 23 episodes of cellulitis had occurred, an independent data monitoring committee would review the results of the interim analysis and recommend whether the trial should stop early,” the authors write.
At the time of the monitoring committee’s review, six patients (15%) who wore compression stockings and 17 (40%) in the control group had experienced a cellulitis episode (hazard ratio, 0.23; P = .002; relative risk [post hoc analysis], 0.37; P = .02). On the basis of those findings, the researchers stopped the study, and patients in the control group were started on compression therapy.
“Clinicians should definitely consider referring their patients to a skilled lymphedema therapist who can individually prescribe and fit compression garments,” Dr. Bissett said. “In our study, these were well tolerated and reduced the risk of another episode of cellulitis by a huge 77%,” she added.
Secondary outcomes included hospitalization related to cellulitis and quality-of-life assessments.
Three patients (7%) in the compression group and six (14%) in the control group were admitted to the hospital for cellulitis (hazard ratio, 0.38). There were no differences in quality of life outcomes between the treatment groups.
The authors say compression therapy has the potential to decrease cellulitis risk by reducing edema, boosting immune response and skin integrity, and protecting the skin.
“Patients with a history of leg swelling (chronic edema) and previous episodes of cellulitis are ideal candidates for this compression therapy,” Dr. Bissett said.
“Given the lack of side effects of the therapy in our study and the potential to reduce other skin problems in these patients, compression therapy is an ideal prophylactic strategy,” she said.
The authors note several study limitations, including a lack of blinding. In addition, patients in the study had to have access to lymphedema specialists, who might be unavailable to patients outside the study. This could have influenced adherence and limit generalizability. Difficulty putting on and taking off compression garments often leads patients to be less adherent to compression therapy, but 88% of patients in this study wore them at least 4 days per week.
Dr. Bissett said compression therapy would be useful for primary care physicians to consider for patients with chronic edema.
“Primary care physicians are highly likely to encounter patients with chronic edema in their day-to-day practice. We can now confidently say that referral to lymphedema therapists for compression therapy should be a first line of defense against future episodes of cellulitis in this vulnerable patient group,” she explained.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The effect was so striking that the randomized controlled trial was stopped early and all patients in the study were given the therapy.
“In a climate of increasing antibiotic resistance, we are delighted to have discovered a nondrug management strategy that has such a dramatic impact on the risk of cellulitis,” senior author Bernie Bissett, PhD, from the Discipline of Physiotherapy, Faculty of Health, the University of Canberra, Australia, said in an interview.
“We hope this leads to a shift in preventative medical strategy for patients with chronic edema and cellulitis around the world,” she said.
Lead author Elizabeth Webb, MPH, from the Physiotherapy Department at Calvary Public Hospital Bruce, in Bruce, Australia, and colleagues report their findings in an article published online August 12 in The New England Journal of Medicine.
Dr. Bisset explained that Webb is a “leading lymphedema physiotherapist” and a PhD candidate at the University of Canberra. She added that this is the first study to show that “compression therapy dramatically reduces the risk of cellulitis for patients with chronic edema.”
Penicillin is often given preventively; some research suggests effectiveness wanes after the antibiotic is stopped.
For the current trial, Ms. Webb and colleagues enrolled 84 adults with chronic edema of the leg and recurrent cellulitis. They randomly assigned patients in a 1:1 ratio to receive leg compression therapy plus education about preventing cellulitis (compression group; n = 41) or education only (control group; n = 43).
Compression therapy consisted of wearing knee-high stockings that applied maximum compression at the ankles. The compression gradually decreased up the legs. In addition, 26 patients were treated with “therapist-applied compression bandaging” for 3 to 5 days before receiving the stockings.
Participants underwent follow-up assessments every 6 months for a maximum of 3 years or until 45 episodes of cellulitis, the primary outcome, occurred. Those in the control group crossed over to the compression group once they experienced cellulitis.
The trial was stopped early for reasons of efficacy. “The statistical analysis plan prespecified that after 23 episodes of cellulitis had occurred, an independent data monitoring committee would review the results of the interim analysis and recommend whether the trial should stop early,” the authors write.
At the time of the monitoring committee’s review, six patients (15%) who wore compression stockings and 17 (40%) in the control group had experienced a cellulitis episode (hazard ratio, 0.23; P = .002; relative risk [post hoc analysis], 0.37; P = .02). On the basis of those findings, the researchers stopped the study, and patients in the control group were started on compression therapy.
“Clinicians should definitely consider referring their patients to a skilled lymphedema therapist who can individually prescribe and fit compression garments,” Dr. Bissett said. “In our study, these were well tolerated and reduced the risk of another episode of cellulitis by a huge 77%,” she added.
Secondary outcomes included hospitalization related to cellulitis and quality-of-life assessments.
Three patients (7%) in the compression group and six (14%) in the control group were admitted to the hospital for cellulitis (hazard ratio, 0.38). There were no differences in quality of life outcomes between the treatment groups.
The authors say compression therapy has the potential to decrease cellulitis risk by reducing edema, boosting immune response and skin integrity, and protecting the skin.
“Patients with a history of leg swelling (chronic edema) and previous episodes of cellulitis are ideal candidates for this compression therapy,” Dr. Bissett said.
“Given the lack of side effects of the therapy in our study and the potential to reduce other skin problems in these patients, compression therapy is an ideal prophylactic strategy,” she said.
The authors note several study limitations, including a lack of blinding. In addition, patients in the study had to have access to lymphedema specialists, who might be unavailable to patients outside the study. This could have influenced adherence and limit generalizability. Difficulty putting on and taking off compression garments often leads patients to be less adherent to compression therapy, but 88% of patients in this study wore them at least 4 days per week.
Dr. Bissett said compression therapy would be useful for primary care physicians to consider for patients with chronic edema.
“Primary care physicians are highly likely to encounter patients with chronic edema in their day-to-day practice. We can now confidently say that referral to lymphedema therapists for compression therapy should be a first line of defense against future episodes of cellulitis in this vulnerable patient group,” she explained.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.