User login
COVID-19: New guidance to stem mental health crisis in frontline HCPs
A new review offers fresh guidance to help stem the mental health toll of the COVID-19 pandemic on frontline clinicians.
Investigators gathered practice guidelines and resources from a wide range of health care organizations and professional societies to develop a conceptual framework of mental health support for health care professionals (HCPs) caring for COVID-19 patients.
“Support needs to be deployed in multiple dimensions – including individual, organizational, and societal levels – and include training in resilience, stress reduction, emotional awareness, and self-care strategies,” lead author Rachel Schwartz, PhD, health services researcher, Stanford (Calif.) University, said in an interview.
The review was published Aug. 21 in the Annals of Internal Medicine.
An opportune moment
Coauthor Rebecca Margolis, DO, director of well-being in the division of medical education and faculty development, Children’s Hospital of Los Angeles, said that this is “an opportune moment to look at how we treat frontline providers in this country.”
Studies of previous pandemics have shown heightened distress in HCPs, even years after the pandemic, and the unique challenges posed by the COVID-19 pandemic surpass those of previous pandemics, Dr. Margolis said in an interview.
Dr. Schwartz, Dr. Margolis, and coauthors Uma Anand, PhD, LP, and Jina Sinskey, MD, met through the Collaborative for Healing and Renewal in Medicine network, a group of medical educators, leaders in academic medicine, experts in burnout research and interventions, and trainees working together to promote well-being among trainees and practicing physicians.
“We were brought together on a conference call in March, when things were particularly bad in New York, and started looking to see what resources we could get to frontline providers who were suffering. It was great to lean on each other and stand on the shoulders of colleagues in New York, who were the ones we learned from on these calls,” said Dr. Margolis.
The authors recommended addressing clinicians’ basic practical needs, including ensuring essentials like meals and transportation, establishing a “well-being area” within hospitals for staff to rest, and providing well-stocked living quarters so clinicians can safely quarantine from family, as well as personal protective equipment and child care.
Clinicians are often asked to “assume new professional roles to meet evolving needs” during a pandemic, which can increase stress. The authors recommended targeted training, assessment of clinician skills before redeployment to a new clinical role, and clear communication practices around redeployment.
Recognition from hospital and government leaders improves morale and supports clinicians’ ability to continue delivering care. Leadership should “leverage communication strategies to provide clinicians with up-to-date information and reassurance,” they wrote.
‘Uniquely isolated’
Dr. Margolis noted that
“My colleagues feel a sense of moral injury, putting their lives on the line at work, performing the most perilous job, and their kids can’t hang out with other kids, which just puts salt on the wound,” she said.
Additional sources of moral injury are deciding which patients should receive life support in the event of inadequate resources and bearing witness to, or enforcing, policies that lead to patients dying alone.
Leaders should encourage clinicians to “seek informal support from colleagues, managers, or chaplains” and to “provide rapid access to professional help,” the authors noted.
Furthermore, they contended that leaders should “proactively and routinely monitor the psychological well-being of their teams,” since guilt and shame often prevent clinicians from disclosing feelings of moral injury.
“Being provided with routine mental health support should be normalized and it should be part of the job – not only during COVID-19 but in general,” Dr. Schwartz said.
‘Battle buddies’
Dr. Margolis recommended the “battle buddy” model for mutual peer support.
Dr. Anand, a mental health clinician at Mayo Medical School, Rochester, Minn., elaborated.
“We connect residents with each other, and they form pairs to support each other and watch for warning signs such as withdrawal from colleagues, being frequently tearful, not showing up at work or showing up late, missing assignments, making mistakes at work, increased use of alcohol, or verbalizing serious concerns,” Dr. Anand said.
If the buddy shows any of these warning signs, he or she can be directed to appropriate resources to get help.
Since the pandemic has interfered with the ability to connect with colleagues and family members, attention should be paid to addressing the social support needs of clinicians.
Dr. Anand suggested that clinicians maintain contact with counselors, friends, and family, even if they cannot be together in person and must connect “virtually.”
Resilience and strength training are “key” components of reducing clinician distress, but trainings as well as processing groups and support workshops should be offered during protected time, Dr. Margolis advised, since it can be burdensome for clinicians to wake up early or stay late to attend these sessions.
Leaders and administrators should “model self-care and well-being,” she noted. For example, sending emails to clinicians late at night or on weekends creates an expectation of a rapid reply, which leads to additional pressure for the clinician.
“This is of the most powerful unspoken curricula we can develop,” Dr. Margolis emphasized.
Self-care critical
Marcus S. Shaker, MD, MSc, associate professor of pediatrics, medicine, and community and family medicine, Children’s Hospital at Dartmouth-Hitchcock in Lebanon, N.H., and Geisel School of Medicine at Dartmouth, Hanover, N.H., said the study was “a much appreciated, timely reminder of the importance of clinician wellness.”
Moreover, “without self-care, our ability to help our patients withers. This article provides a useful conceptual framework for individuals and organizations to provide the right care at the right time in these unprecedented times,” said Dr. Shaker, who was not involved with the study.
The authors agreed, stating that clinicians “require proactive psychological protection specifically because they are a population known for putting others’ needs before their own.”
They recommended several resources for HCPs, including the Physician Support Line; Headspace, a mindfulness Web-based app for reducing stress and anxiety; the National Suicide Prevention Lifeline; and the Crisis Text Line.
The authors and Dr. Shaker disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A new review offers fresh guidance to help stem the mental health toll of the COVID-19 pandemic on frontline clinicians.
Investigators gathered practice guidelines and resources from a wide range of health care organizations and professional societies to develop a conceptual framework of mental health support for health care professionals (HCPs) caring for COVID-19 patients.
“Support needs to be deployed in multiple dimensions – including individual, organizational, and societal levels – and include training in resilience, stress reduction, emotional awareness, and self-care strategies,” lead author Rachel Schwartz, PhD, health services researcher, Stanford (Calif.) University, said in an interview.
The review was published Aug. 21 in the Annals of Internal Medicine.
An opportune moment
Coauthor Rebecca Margolis, DO, director of well-being in the division of medical education and faculty development, Children’s Hospital of Los Angeles, said that this is “an opportune moment to look at how we treat frontline providers in this country.”
Studies of previous pandemics have shown heightened distress in HCPs, even years after the pandemic, and the unique challenges posed by the COVID-19 pandemic surpass those of previous pandemics, Dr. Margolis said in an interview.
Dr. Schwartz, Dr. Margolis, and coauthors Uma Anand, PhD, LP, and Jina Sinskey, MD, met through the Collaborative for Healing and Renewal in Medicine network, a group of medical educators, leaders in academic medicine, experts in burnout research and interventions, and trainees working together to promote well-being among trainees and practicing physicians.
“We were brought together on a conference call in March, when things were particularly bad in New York, and started looking to see what resources we could get to frontline providers who were suffering. It was great to lean on each other and stand on the shoulders of colleagues in New York, who were the ones we learned from on these calls,” said Dr. Margolis.
The authors recommended addressing clinicians’ basic practical needs, including ensuring essentials like meals and transportation, establishing a “well-being area” within hospitals for staff to rest, and providing well-stocked living quarters so clinicians can safely quarantine from family, as well as personal protective equipment and child care.
Clinicians are often asked to “assume new professional roles to meet evolving needs” during a pandemic, which can increase stress. The authors recommended targeted training, assessment of clinician skills before redeployment to a new clinical role, and clear communication practices around redeployment.
Recognition from hospital and government leaders improves morale and supports clinicians’ ability to continue delivering care. Leadership should “leverage communication strategies to provide clinicians with up-to-date information and reassurance,” they wrote.
‘Uniquely isolated’
Dr. Margolis noted that
“My colleagues feel a sense of moral injury, putting their lives on the line at work, performing the most perilous job, and their kids can’t hang out with other kids, which just puts salt on the wound,” she said.
Additional sources of moral injury are deciding which patients should receive life support in the event of inadequate resources and bearing witness to, or enforcing, policies that lead to patients dying alone.
Leaders should encourage clinicians to “seek informal support from colleagues, managers, or chaplains” and to “provide rapid access to professional help,” the authors noted.
Furthermore, they contended that leaders should “proactively and routinely monitor the psychological well-being of their teams,” since guilt and shame often prevent clinicians from disclosing feelings of moral injury.
“Being provided with routine mental health support should be normalized and it should be part of the job – not only during COVID-19 but in general,” Dr. Schwartz said.
‘Battle buddies’
Dr. Margolis recommended the “battle buddy” model for mutual peer support.
Dr. Anand, a mental health clinician at Mayo Medical School, Rochester, Minn., elaborated.
“We connect residents with each other, and they form pairs to support each other and watch for warning signs such as withdrawal from colleagues, being frequently tearful, not showing up at work or showing up late, missing assignments, making mistakes at work, increased use of alcohol, or verbalizing serious concerns,” Dr. Anand said.
If the buddy shows any of these warning signs, he or she can be directed to appropriate resources to get help.
Since the pandemic has interfered with the ability to connect with colleagues and family members, attention should be paid to addressing the social support needs of clinicians.
Dr. Anand suggested that clinicians maintain contact with counselors, friends, and family, even if they cannot be together in person and must connect “virtually.”
Resilience and strength training are “key” components of reducing clinician distress, but trainings as well as processing groups and support workshops should be offered during protected time, Dr. Margolis advised, since it can be burdensome for clinicians to wake up early or stay late to attend these sessions.
Leaders and administrators should “model self-care and well-being,” she noted. For example, sending emails to clinicians late at night or on weekends creates an expectation of a rapid reply, which leads to additional pressure for the clinician.
“This is of the most powerful unspoken curricula we can develop,” Dr. Margolis emphasized.
Self-care critical
Marcus S. Shaker, MD, MSc, associate professor of pediatrics, medicine, and community and family medicine, Children’s Hospital at Dartmouth-Hitchcock in Lebanon, N.H., and Geisel School of Medicine at Dartmouth, Hanover, N.H., said the study was “a much appreciated, timely reminder of the importance of clinician wellness.”
Moreover, “without self-care, our ability to help our patients withers. This article provides a useful conceptual framework for individuals and organizations to provide the right care at the right time in these unprecedented times,” said Dr. Shaker, who was not involved with the study.
The authors agreed, stating that clinicians “require proactive psychological protection specifically because they are a population known for putting others’ needs before their own.”
They recommended several resources for HCPs, including the Physician Support Line; Headspace, a mindfulness Web-based app for reducing stress and anxiety; the National Suicide Prevention Lifeline; and the Crisis Text Line.
The authors and Dr. Shaker disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A new review offers fresh guidance to help stem the mental health toll of the COVID-19 pandemic on frontline clinicians.
Investigators gathered practice guidelines and resources from a wide range of health care organizations and professional societies to develop a conceptual framework of mental health support for health care professionals (HCPs) caring for COVID-19 patients.
“Support needs to be deployed in multiple dimensions – including individual, organizational, and societal levels – and include training in resilience, stress reduction, emotional awareness, and self-care strategies,” lead author Rachel Schwartz, PhD, health services researcher, Stanford (Calif.) University, said in an interview.
The review was published Aug. 21 in the Annals of Internal Medicine.
An opportune moment
Coauthor Rebecca Margolis, DO, director of well-being in the division of medical education and faculty development, Children’s Hospital of Los Angeles, said that this is “an opportune moment to look at how we treat frontline providers in this country.”
Studies of previous pandemics have shown heightened distress in HCPs, even years after the pandemic, and the unique challenges posed by the COVID-19 pandemic surpass those of previous pandemics, Dr. Margolis said in an interview.
Dr. Schwartz, Dr. Margolis, and coauthors Uma Anand, PhD, LP, and Jina Sinskey, MD, met through the Collaborative for Healing and Renewal in Medicine network, a group of medical educators, leaders in academic medicine, experts in burnout research and interventions, and trainees working together to promote well-being among trainees and practicing physicians.
“We were brought together on a conference call in March, when things were particularly bad in New York, and started looking to see what resources we could get to frontline providers who were suffering. It was great to lean on each other and stand on the shoulders of colleagues in New York, who were the ones we learned from on these calls,” said Dr. Margolis.
The authors recommended addressing clinicians’ basic practical needs, including ensuring essentials like meals and transportation, establishing a “well-being area” within hospitals for staff to rest, and providing well-stocked living quarters so clinicians can safely quarantine from family, as well as personal protective equipment and child care.
Clinicians are often asked to “assume new professional roles to meet evolving needs” during a pandemic, which can increase stress. The authors recommended targeted training, assessment of clinician skills before redeployment to a new clinical role, and clear communication practices around redeployment.
Recognition from hospital and government leaders improves morale and supports clinicians’ ability to continue delivering care. Leadership should “leverage communication strategies to provide clinicians with up-to-date information and reassurance,” they wrote.
‘Uniquely isolated’
Dr. Margolis noted that
“My colleagues feel a sense of moral injury, putting their lives on the line at work, performing the most perilous job, and their kids can’t hang out with other kids, which just puts salt on the wound,” she said.
Additional sources of moral injury are deciding which patients should receive life support in the event of inadequate resources and bearing witness to, or enforcing, policies that lead to patients dying alone.
Leaders should encourage clinicians to “seek informal support from colleagues, managers, or chaplains” and to “provide rapid access to professional help,” the authors noted.
Furthermore, they contended that leaders should “proactively and routinely monitor the psychological well-being of their teams,” since guilt and shame often prevent clinicians from disclosing feelings of moral injury.
“Being provided with routine mental health support should be normalized and it should be part of the job – not only during COVID-19 but in general,” Dr. Schwartz said.
‘Battle buddies’
Dr. Margolis recommended the “battle buddy” model for mutual peer support.
Dr. Anand, a mental health clinician at Mayo Medical School, Rochester, Minn., elaborated.
“We connect residents with each other, and they form pairs to support each other and watch for warning signs such as withdrawal from colleagues, being frequently tearful, not showing up at work or showing up late, missing assignments, making mistakes at work, increased use of alcohol, or verbalizing serious concerns,” Dr. Anand said.
If the buddy shows any of these warning signs, he or she can be directed to appropriate resources to get help.
Since the pandemic has interfered with the ability to connect with colleagues and family members, attention should be paid to addressing the social support needs of clinicians.
Dr. Anand suggested that clinicians maintain contact with counselors, friends, and family, even if they cannot be together in person and must connect “virtually.”
Resilience and strength training are “key” components of reducing clinician distress, but trainings as well as processing groups and support workshops should be offered during protected time, Dr. Margolis advised, since it can be burdensome for clinicians to wake up early or stay late to attend these sessions.
Leaders and administrators should “model self-care and well-being,” she noted. For example, sending emails to clinicians late at night or on weekends creates an expectation of a rapid reply, which leads to additional pressure for the clinician.
“This is of the most powerful unspoken curricula we can develop,” Dr. Margolis emphasized.
Self-care critical
Marcus S. Shaker, MD, MSc, associate professor of pediatrics, medicine, and community and family medicine, Children’s Hospital at Dartmouth-Hitchcock in Lebanon, N.H., and Geisel School of Medicine at Dartmouth, Hanover, N.H., said the study was “a much appreciated, timely reminder of the importance of clinician wellness.”
Moreover, “without self-care, our ability to help our patients withers. This article provides a useful conceptual framework for individuals and organizations to provide the right care at the right time in these unprecedented times,” said Dr. Shaker, who was not involved with the study.
The authors agreed, stating that clinicians “require proactive psychological protection specifically because they are a population known for putting others’ needs before their own.”
They recommended several resources for HCPs, including the Physician Support Line; Headspace, a mindfulness Web-based app for reducing stress and anxiety; the National Suicide Prevention Lifeline; and the Crisis Text Line.
The authors and Dr. Shaker disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Rash, muscle weakness, and confusion
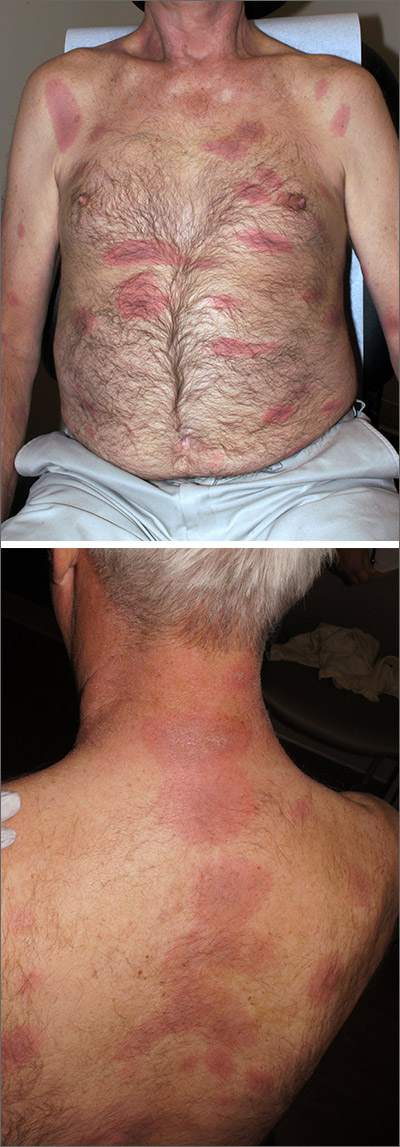
The constellation of symptoms was suggestive of Lyme disease, although connective tissue disease and syphilis were also considered. Two punch biopsies were performed in the office, and erythrocyte sedimentation rate (ESR), complete blood cell count (CBC), international normalized ratio (INR), comprehensive metabolic panel (CMP), Lyme enzyme-linked immunosorbent assay (ELISA) antibody panel, and rapid plasma reagin (RPR) laboratory tests were ordered.
Immediately available laboratory results included ESR, CBC, INR, and CMP. Findings were notable for elevated INR, as well as elevated alanine aminotransferase and aspartate transaminase. The transaminitis suggested myopathy and was consistent with clinical muscle weakness. RPR testing was negative.
Because of the confusion, severity of muscle weakness, and plausibility of early encephalopathy with Lyme disease, the patient was admitted to the hospital for further work-up. Lumbar puncture was delayed until his INR was reduced, but subsequently was found to be normal. He received intravenous (IV) ceftriaxone (2 g/d) empirically for possible early disseminated disease with neurologic complications. His confusion, muscle weakness, and transaminitis rapidly improved.
His Lyme antibody panel was positive for IgM after his third day of hospitalization. A reflexive confirmatory western blot for IgG was not positive on the initial set of labs but was positive when redrawn 4 weeks after this hospitalization, confirming Lyme disease.
Lyme disease is a vector-borne disease caused by the Borrelia genus of spirochete bacteria, most commonly Borrelia burgdorferi in North America. Transmission occurs through prolonged (typically 36-48 hours) attachment of a blacklegged tick.
The disease can be divided into 3 stages:
- localized (3-30 days): erythema migrans rash and flulike illness
- early disseminated (days to weeks; seen in this patient): multiple erythema migrans rashes, early neuroborreliosis, arthritis, carditis, and rarely hepatitis and uveitis
- late disseminated (months to years): chronic Lyme arthritis, chronic neurological disorders (eg, encephalopathy, radicular pain, and chronic neuropathy).
The initial erythema migrans rash is classically red and targetoid; it expands from the site of attachment. Early disseminated patches tend to be smaller and can occur on any body part. The rash is rarely itchy or painful but may be warm to the touch or sensitive. The rash resolves spontaneously within 3 to 4 weeks of onset.
Treatment of all early and early disseminated Lyme disease typically involves a 14- to 28-day course of doxycycline (100 mg bid for adults, 2.2 mg/kg bid [maximum 100 mg bid] for children). Patients with acute neurologic disease often can be treated with doxycycline, but patients who cannot tolerate doxycycline and those with parenchymal disease such as encephalitis should receive IV therapy with ceftriaxone 2 g/d.
In this case, the patient was discharged home on a 3-week course of doxycycline 100 mg bid and cleared without further symptoms.
Text courtesy of Tristan Reynolds, DO, Maine Dartmouth Family Medicine Residency, and Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
Lyme disease. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/lyme/healthcare/index.html. Accessed September 1, 2020.

The constellation of symptoms was suggestive of Lyme disease, although connective tissue disease and syphilis were also considered. Two punch biopsies were performed in the office, and erythrocyte sedimentation rate (ESR), complete blood cell count (CBC), international normalized ratio (INR), comprehensive metabolic panel (CMP), Lyme enzyme-linked immunosorbent assay (ELISA) antibody panel, and rapid plasma reagin (RPR) laboratory tests were ordered.
Immediately available laboratory results included ESR, CBC, INR, and CMP. Findings were notable for elevated INR, as well as elevated alanine aminotransferase and aspartate transaminase. The transaminitis suggested myopathy and was consistent with clinical muscle weakness. RPR testing was negative.
Because of the confusion, severity of muscle weakness, and plausibility of early encephalopathy with Lyme disease, the patient was admitted to the hospital for further work-up. Lumbar puncture was delayed until his INR was reduced, but subsequently was found to be normal. He received intravenous (IV) ceftriaxone (2 g/d) empirically for possible early disseminated disease with neurologic complications. His confusion, muscle weakness, and transaminitis rapidly improved.
His Lyme antibody panel was positive for IgM after his third day of hospitalization. A reflexive confirmatory western blot for IgG was not positive on the initial set of labs but was positive when redrawn 4 weeks after this hospitalization, confirming Lyme disease.
Lyme disease is a vector-borne disease caused by the Borrelia genus of spirochete bacteria, most commonly Borrelia burgdorferi in North America. Transmission occurs through prolonged (typically 36-48 hours) attachment of a blacklegged tick.
The disease can be divided into 3 stages:
- localized (3-30 days): erythema migrans rash and flulike illness
- early disseminated (days to weeks; seen in this patient): multiple erythema migrans rashes, early neuroborreliosis, arthritis, carditis, and rarely hepatitis and uveitis
- late disseminated (months to years): chronic Lyme arthritis, chronic neurological disorders (eg, encephalopathy, radicular pain, and chronic neuropathy).
The initial erythema migrans rash is classically red and targetoid; it expands from the site of attachment. Early disseminated patches tend to be smaller and can occur on any body part. The rash is rarely itchy or painful but may be warm to the touch or sensitive. The rash resolves spontaneously within 3 to 4 weeks of onset.
Treatment of all early and early disseminated Lyme disease typically involves a 14- to 28-day course of doxycycline (100 mg bid for adults, 2.2 mg/kg bid [maximum 100 mg bid] for children). Patients with acute neurologic disease often can be treated with doxycycline, but patients who cannot tolerate doxycycline and those with parenchymal disease such as encephalitis should receive IV therapy with ceftriaxone 2 g/d.
In this case, the patient was discharged home on a 3-week course of doxycycline 100 mg bid and cleared without further symptoms.
Text courtesy of Tristan Reynolds, DO, Maine Dartmouth Family Medicine Residency, and Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).

The constellation of symptoms was suggestive of Lyme disease, although connective tissue disease and syphilis were also considered. Two punch biopsies were performed in the office, and erythrocyte sedimentation rate (ESR), complete blood cell count (CBC), international normalized ratio (INR), comprehensive metabolic panel (CMP), Lyme enzyme-linked immunosorbent assay (ELISA) antibody panel, and rapid plasma reagin (RPR) laboratory tests were ordered.
Immediately available laboratory results included ESR, CBC, INR, and CMP. Findings were notable for elevated INR, as well as elevated alanine aminotransferase and aspartate transaminase. The transaminitis suggested myopathy and was consistent with clinical muscle weakness. RPR testing was negative.
Because of the confusion, severity of muscle weakness, and plausibility of early encephalopathy with Lyme disease, the patient was admitted to the hospital for further work-up. Lumbar puncture was delayed until his INR was reduced, but subsequently was found to be normal. He received intravenous (IV) ceftriaxone (2 g/d) empirically for possible early disseminated disease with neurologic complications. His confusion, muscle weakness, and transaminitis rapidly improved.
His Lyme antibody panel was positive for IgM after his third day of hospitalization. A reflexive confirmatory western blot for IgG was not positive on the initial set of labs but was positive when redrawn 4 weeks after this hospitalization, confirming Lyme disease.
Lyme disease is a vector-borne disease caused by the Borrelia genus of spirochete bacteria, most commonly Borrelia burgdorferi in North America. Transmission occurs through prolonged (typically 36-48 hours) attachment of a blacklegged tick.
The disease can be divided into 3 stages:
- localized (3-30 days): erythema migrans rash and flulike illness
- early disseminated (days to weeks; seen in this patient): multiple erythema migrans rashes, early neuroborreliosis, arthritis, carditis, and rarely hepatitis and uveitis
- late disseminated (months to years): chronic Lyme arthritis, chronic neurological disorders (eg, encephalopathy, radicular pain, and chronic neuropathy).
The initial erythema migrans rash is classically red and targetoid; it expands from the site of attachment. Early disseminated patches tend to be smaller and can occur on any body part. The rash is rarely itchy or painful but may be warm to the touch or sensitive. The rash resolves spontaneously within 3 to 4 weeks of onset.
Treatment of all early and early disseminated Lyme disease typically involves a 14- to 28-day course of doxycycline (100 mg bid for adults, 2.2 mg/kg bid [maximum 100 mg bid] for children). Patients with acute neurologic disease often can be treated with doxycycline, but patients who cannot tolerate doxycycline and those with parenchymal disease such as encephalitis should receive IV therapy with ceftriaxone 2 g/d.
In this case, the patient was discharged home on a 3-week course of doxycycline 100 mg bid and cleared without further symptoms.
Text courtesy of Tristan Reynolds, DO, Maine Dartmouth Family Medicine Residency, and Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
Lyme disease. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/lyme/healthcare/index.html. Accessed September 1, 2020.
Lyme disease. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/lyme/healthcare/index.html. Accessed September 1, 2020.
U.S. tops 500,000 COVID-19 cases in children
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
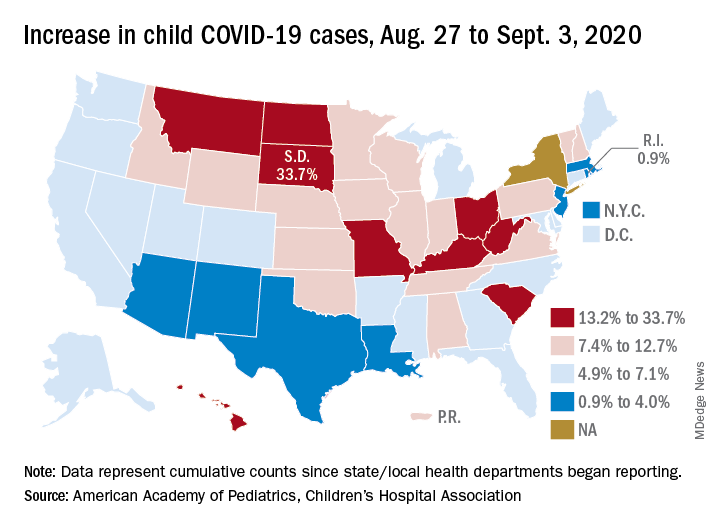
States have reported 513,415 cases of COVID-19 in children since the beginning of the pandemic, with almost 37,000 coming in the last week, the AAP and the CHA said Sept. 8 in the weekly report. That figure includes New York City – the rest of New York State is not reporting ages for COVID-19 patients – as well as Puerto Rico, the District of Columbia, and Guam.
“These numbers are a chilling reminder of why we need to take this virus seriously,” AAP President Sara Goza, MD, said in a written statement.
Children now represent 9.8% of the almost 5.3 million cases that have been reported in Americans of all ages. The proportion of child cases has continued to increase as the pandemic has progressed – it was 8.0% as of mid-July and 5.2% in early June, the data show.
“Throughout the summer, surges in the virus have occurred in Southern, Western, and Midwestern states,” the AAP statement said.
The latest AAP/CHA report shows that, from Aug. 27 to Sept. 3, the total number of child cases jumped by 33.7% in South Dakota, more than any other state. North Dakota was next at 22.7%, followed by Hawaii (18.1%), Missouri (16.8%), and Kentucky (16.4%).
“This rapid rise in positive cases occurred over the summer, and as the weather cools, we know people will spend more time indoors,” said Sean O’Leary, MD, MPH, vice chair of the AAP Committee on Infectious Diseases. “The goal is to get children back into schools for in-person learning, but in many communities, this is not possible as the virus spreads unchecked.”
The smallest increase over the last week, just 0.9%, came in Rhode Island, with Massachusetts just a bit higher at 1.0%. Also at the low end of the increase scale are Arizona (3.3%) and Louisiana (4.0%), two states that have very high rates of cumulative cases: 1,380 per 100,000 children for Arizona and 1,234 per 100,000 for Louisiana, the report said.
To give those figures some context, Tennessee has the highest cumulative count of any state at 1,553 cases per 100,000 children and Vermont has the lowest at 151, based on the data gathered by the AAP and CHA.
“While much remains unknown about COVID-19, we do know that the spread among children reflects what is happening in the broader communities. A disproportionate number of cases are reported in Black and Hispanic children and in places where there is high poverty. We must work harder to address societal inequities that contribute to these disparities,” Dr. Goza said.
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

States have reported 513,415 cases of COVID-19 in children since the beginning of the pandemic, with almost 37,000 coming in the last week, the AAP and the CHA said Sept. 8 in the weekly report. That figure includes New York City – the rest of New York State is not reporting ages for COVID-19 patients – as well as Puerto Rico, the District of Columbia, and Guam.
“These numbers are a chilling reminder of why we need to take this virus seriously,” AAP President Sara Goza, MD, said in a written statement.
Children now represent 9.8% of the almost 5.3 million cases that have been reported in Americans of all ages. The proportion of child cases has continued to increase as the pandemic has progressed – it was 8.0% as of mid-July and 5.2% in early June, the data show.
“Throughout the summer, surges in the virus have occurred in Southern, Western, and Midwestern states,” the AAP statement said.
The latest AAP/CHA report shows that, from Aug. 27 to Sept. 3, the total number of child cases jumped by 33.7% in South Dakota, more than any other state. North Dakota was next at 22.7%, followed by Hawaii (18.1%), Missouri (16.8%), and Kentucky (16.4%).
“This rapid rise in positive cases occurred over the summer, and as the weather cools, we know people will spend more time indoors,” said Sean O’Leary, MD, MPH, vice chair of the AAP Committee on Infectious Diseases. “The goal is to get children back into schools for in-person learning, but in many communities, this is not possible as the virus spreads unchecked.”
The smallest increase over the last week, just 0.9%, came in Rhode Island, with Massachusetts just a bit higher at 1.0%. Also at the low end of the increase scale are Arizona (3.3%) and Louisiana (4.0%), two states that have very high rates of cumulative cases: 1,380 per 100,000 children for Arizona and 1,234 per 100,000 for Louisiana, the report said.
To give those figures some context, Tennessee has the highest cumulative count of any state at 1,553 cases per 100,000 children and Vermont has the lowest at 151, based on the data gathered by the AAP and CHA.
“While much remains unknown about COVID-19, we do know that the spread among children reflects what is happening in the broader communities. A disproportionate number of cases are reported in Black and Hispanic children and in places where there is high poverty. We must work harder to address societal inequities that contribute to these disparities,” Dr. Goza said.
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

States have reported 513,415 cases of COVID-19 in children since the beginning of the pandemic, with almost 37,000 coming in the last week, the AAP and the CHA said Sept. 8 in the weekly report. That figure includes New York City – the rest of New York State is not reporting ages for COVID-19 patients – as well as Puerto Rico, the District of Columbia, and Guam.
“These numbers are a chilling reminder of why we need to take this virus seriously,” AAP President Sara Goza, MD, said in a written statement.
Children now represent 9.8% of the almost 5.3 million cases that have been reported in Americans of all ages. The proportion of child cases has continued to increase as the pandemic has progressed – it was 8.0% as of mid-July and 5.2% in early June, the data show.
“Throughout the summer, surges in the virus have occurred in Southern, Western, and Midwestern states,” the AAP statement said.
The latest AAP/CHA report shows that, from Aug. 27 to Sept. 3, the total number of child cases jumped by 33.7% in South Dakota, more than any other state. North Dakota was next at 22.7%, followed by Hawaii (18.1%), Missouri (16.8%), and Kentucky (16.4%).
“This rapid rise in positive cases occurred over the summer, and as the weather cools, we know people will spend more time indoors,” said Sean O’Leary, MD, MPH, vice chair of the AAP Committee on Infectious Diseases. “The goal is to get children back into schools for in-person learning, but in many communities, this is not possible as the virus spreads unchecked.”
The smallest increase over the last week, just 0.9%, came in Rhode Island, with Massachusetts just a bit higher at 1.0%. Also at the low end of the increase scale are Arizona (3.3%) and Louisiana (4.0%), two states that have very high rates of cumulative cases: 1,380 per 100,000 children for Arizona and 1,234 per 100,000 for Louisiana, the report said.
To give those figures some context, Tennessee has the highest cumulative count of any state at 1,553 cases per 100,000 children and Vermont has the lowest at 151, based on the data gathered by the AAP and CHA.
“While much remains unknown about COVID-19, we do know that the spread among children reflects what is happening in the broader communities. A disproportionate number of cases are reported in Black and Hispanic children and in places where there is high poverty. We must work harder to address societal inequities that contribute to these disparities,” Dr. Goza said.
We are all in this together: Lessons learned on a COVID-19 unit
Like most family medicine residencies, our teaching nursing home was struck with a COVID-19 outbreak. Within 10 days, I was the sole physician responsible for 15 patients with varying degrees of illness, quarantined behind the fire doors of a wing of a Memory Support Unit. My daily work there over the course of the next month prompted me to reflect on some of the core principles of family medicine, and health care, that are vital to effective patient care during a pandemic. My experience provided the following reminders:
Work as a team. Gowned, gloved, and masked behind the fire doors, our world shrank to our patients and a 4-person team comprised of a nurse, 2 nursing assistants, and me. For the first time in the 10+ years I’ve worked at that facility, I actually asked for and memorized the names of everyone I was working with that day. Without an intercom or other telecommunications system, it became important for me to be able to call for my team members by name for immediate help. We had to depend on one another to make sure all patients were hydrated and fed, to avert falls whenever possible, to intervene early when dementia-associated behaviors were escalating, and to recognize when patients were crashing.
We also had to depend on each other to ensure that our personal protective equipment remained properly placed, to combat the psychological sense of isolation that quarantine environments engender, and to placate a gnawing undercurrent of unease while working around a potentially deadly pathogen.
Develop clinical routines. Having listened to other medical directors whose nursing homes were affected by the pandemic earlier than we were, and hearing about potentially avoidable complications, we developed clinical routines. This began with identifying any patients with diabetes whose poor appetites while acutely ill could send them into hypoglycemia. We devised a daily clinical report sheet that included vital signs, date of positive COVID-19 test, global clinical status, and advance directives. Unlike the usual mode of working almost in parallel, I began my workday with a “sign-out” from the nurse, then started examining each patient.
Under the strain of this unusual environment and novel circumstances, we communicated more and more often. This allowed us to quickly recognize and communicate emerging changes in the clinical status of a patient by sharing our observations of subtle, nonspecific “sub-threshold” indicators.
Clarify the goals of care. Since most of the patients in the COVID-19 unit were under the long-term care of other attending physicians, it was important for me to understand the wishes of the patient or surrogate decision maker, should life-threatening complications occur. While all affected patients were long-term residents of a memory support unit, some had full-code advance directives. I quickly realized that what was first necessary was to develop rapport and trust with the families who didn’t know me, then discuss goals of care, and finally assure that the advance directives were in congruence with their stated goals. What helped families gain trust in me was knowing that I was seeing their loved one daily, that I was committed to helping the patient survive this infection, and that I was willing to come back to the facility if a crisis occurred—even at night, if necessary.
Appreciate the daily work of team members. One of my greatest worries was dehydration. When elders were acutely ill and eating and drinking poorly, I would assist with feeding and offering liquids. I quickly came to appreciate how complex and subtle this seemingly mundane task can be. Learning the proper pace and portion size, even choosing the right conversation topic and tone, could make the difference between a patient “shutting down” and refusing all nourishment and successfully drinking a 360-cc cup of a high-nutrient shake.
Continue to: In the disrupted routines...
In the disrupted routines and altered physical environments of the COVID-19 unit, the psychological and behavioral complications of dementia intensified for some patients. I observed first-hand the great patience, kindness, and finesse that nurses and nursing assistants display in their efforts to de-escalate and prevent disruptive behaviors.
Empathize with (and appreciate) families. Families tearfully reminded me that they had been suffering from the absence of contact with their loved ones for months; COVID-19 added to that trauma for many of them. They talked about the missed graduations, birthdays, and other precious times together that were lost because of the quarantine.
Families also prevented me from making mistakes. When I ordered nitrofurantoin for a patient with a urinary tract infection, her son called me and respectfully requested I “just check and make sure” it would not cause a problem, given her G6PD deficiency. He prevented me from prescribing an antibiotic contraindicated in that condition.
Bring forward the lessons learned. The COVID-19 outbreak has passed through our nursing home—at least for now. I perceive a subtle shift in how we continue to interact with one another. Behind the masks, we make a little more eye contact; we more often address each other by name; and we acknowledge a greater mutual respect.
The shared experience of COVID-19 has brought us all a little closer together, and in the end, our patients have benefitted.
Like most family medicine residencies, our teaching nursing home was struck with a COVID-19 outbreak. Within 10 days, I was the sole physician responsible for 15 patients with varying degrees of illness, quarantined behind the fire doors of a wing of a Memory Support Unit. My daily work there over the course of the next month prompted me to reflect on some of the core principles of family medicine, and health care, that are vital to effective patient care during a pandemic. My experience provided the following reminders:
Work as a team. Gowned, gloved, and masked behind the fire doors, our world shrank to our patients and a 4-person team comprised of a nurse, 2 nursing assistants, and me. For the first time in the 10+ years I’ve worked at that facility, I actually asked for and memorized the names of everyone I was working with that day. Without an intercom or other telecommunications system, it became important for me to be able to call for my team members by name for immediate help. We had to depend on one another to make sure all patients were hydrated and fed, to avert falls whenever possible, to intervene early when dementia-associated behaviors were escalating, and to recognize when patients were crashing.
We also had to depend on each other to ensure that our personal protective equipment remained properly placed, to combat the psychological sense of isolation that quarantine environments engender, and to placate a gnawing undercurrent of unease while working around a potentially deadly pathogen.
Develop clinical routines. Having listened to other medical directors whose nursing homes were affected by the pandemic earlier than we were, and hearing about potentially avoidable complications, we developed clinical routines. This began with identifying any patients with diabetes whose poor appetites while acutely ill could send them into hypoglycemia. We devised a daily clinical report sheet that included vital signs, date of positive COVID-19 test, global clinical status, and advance directives. Unlike the usual mode of working almost in parallel, I began my workday with a “sign-out” from the nurse, then started examining each patient.
Under the strain of this unusual environment and novel circumstances, we communicated more and more often. This allowed us to quickly recognize and communicate emerging changes in the clinical status of a patient by sharing our observations of subtle, nonspecific “sub-threshold” indicators.
Clarify the goals of care. Since most of the patients in the COVID-19 unit were under the long-term care of other attending physicians, it was important for me to understand the wishes of the patient or surrogate decision maker, should life-threatening complications occur. While all affected patients were long-term residents of a memory support unit, some had full-code advance directives. I quickly realized that what was first necessary was to develop rapport and trust with the families who didn’t know me, then discuss goals of care, and finally assure that the advance directives were in congruence with their stated goals. What helped families gain trust in me was knowing that I was seeing their loved one daily, that I was committed to helping the patient survive this infection, and that I was willing to come back to the facility if a crisis occurred—even at night, if necessary.
Appreciate the daily work of team members. One of my greatest worries was dehydration. When elders were acutely ill and eating and drinking poorly, I would assist with feeding and offering liquids. I quickly came to appreciate how complex and subtle this seemingly mundane task can be. Learning the proper pace and portion size, even choosing the right conversation topic and tone, could make the difference between a patient “shutting down” and refusing all nourishment and successfully drinking a 360-cc cup of a high-nutrient shake.
Continue to: In the disrupted routines...
In the disrupted routines and altered physical environments of the COVID-19 unit, the psychological and behavioral complications of dementia intensified for some patients. I observed first-hand the great patience, kindness, and finesse that nurses and nursing assistants display in their efforts to de-escalate and prevent disruptive behaviors.
Empathize with (and appreciate) families. Families tearfully reminded me that they had been suffering from the absence of contact with their loved ones for months; COVID-19 added to that trauma for many of them. They talked about the missed graduations, birthdays, and other precious times together that were lost because of the quarantine.
Families also prevented me from making mistakes. When I ordered nitrofurantoin for a patient with a urinary tract infection, her son called me and respectfully requested I “just check and make sure” it would not cause a problem, given her G6PD deficiency. He prevented me from prescribing an antibiotic contraindicated in that condition.
Bring forward the lessons learned. The COVID-19 outbreak has passed through our nursing home—at least for now. I perceive a subtle shift in how we continue to interact with one another. Behind the masks, we make a little more eye contact; we more often address each other by name; and we acknowledge a greater mutual respect.
The shared experience of COVID-19 has brought us all a little closer together, and in the end, our patients have benefitted.
Like most family medicine residencies, our teaching nursing home was struck with a COVID-19 outbreak. Within 10 days, I was the sole physician responsible for 15 patients with varying degrees of illness, quarantined behind the fire doors of a wing of a Memory Support Unit. My daily work there over the course of the next month prompted me to reflect on some of the core principles of family medicine, and health care, that are vital to effective patient care during a pandemic. My experience provided the following reminders:
Work as a team. Gowned, gloved, and masked behind the fire doors, our world shrank to our patients and a 4-person team comprised of a nurse, 2 nursing assistants, and me. For the first time in the 10+ years I’ve worked at that facility, I actually asked for and memorized the names of everyone I was working with that day. Without an intercom or other telecommunications system, it became important for me to be able to call for my team members by name for immediate help. We had to depend on one another to make sure all patients were hydrated and fed, to avert falls whenever possible, to intervene early when dementia-associated behaviors were escalating, and to recognize when patients were crashing.
We also had to depend on each other to ensure that our personal protective equipment remained properly placed, to combat the psychological sense of isolation that quarantine environments engender, and to placate a gnawing undercurrent of unease while working around a potentially deadly pathogen.
Develop clinical routines. Having listened to other medical directors whose nursing homes were affected by the pandemic earlier than we were, and hearing about potentially avoidable complications, we developed clinical routines. This began with identifying any patients with diabetes whose poor appetites while acutely ill could send them into hypoglycemia. We devised a daily clinical report sheet that included vital signs, date of positive COVID-19 test, global clinical status, and advance directives. Unlike the usual mode of working almost in parallel, I began my workday with a “sign-out” from the nurse, then started examining each patient.
Under the strain of this unusual environment and novel circumstances, we communicated more and more often. This allowed us to quickly recognize and communicate emerging changes in the clinical status of a patient by sharing our observations of subtle, nonspecific “sub-threshold” indicators.
Clarify the goals of care. Since most of the patients in the COVID-19 unit were under the long-term care of other attending physicians, it was important for me to understand the wishes of the patient or surrogate decision maker, should life-threatening complications occur. While all affected patients were long-term residents of a memory support unit, some had full-code advance directives. I quickly realized that what was first necessary was to develop rapport and trust with the families who didn’t know me, then discuss goals of care, and finally assure that the advance directives were in congruence with their stated goals. What helped families gain trust in me was knowing that I was seeing their loved one daily, that I was committed to helping the patient survive this infection, and that I was willing to come back to the facility if a crisis occurred—even at night, if necessary.
Appreciate the daily work of team members. One of my greatest worries was dehydration. When elders were acutely ill and eating and drinking poorly, I would assist with feeding and offering liquids. I quickly came to appreciate how complex and subtle this seemingly mundane task can be. Learning the proper pace and portion size, even choosing the right conversation topic and tone, could make the difference between a patient “shutting down” and refusing all nourishment and successfully drinking a 360-cc cup of a high-nutrient shake.
Continue to: In the disrupted routines...
In the disrupted routines and altered physical environments of the COVID-19 unit, the psychological and behavioral complications of dementia intensified for some patients. I observed first-hand the great patience, kindness, and finesse that nurses and nursing assistants display in their efforts to de-escalate and prevent disruptive behaviors.
Empathize with (and appreciate) families. Families tearfully reminded me that they had been suffering from the absence of contact with their loved ones for months; COVID-19 added to that trauma for many of them. They talked about the missed graduations, birthdays, and other precious times together that were lost because of the quarantine.
Families also prevented me from making mistakes. When I ordered nitrofurantoin for a patient with a urinary tract infection, her son called me and respectfully requested I “just check and make sure” it would not cause a problem, given her G6PD deficiency. He prevented me from prescribing an antibiotic contraindicated in that condition.
Bring forward the lessons learned. The COVID-19 outbreak has passed through our nursing home—at least for now. I perceive a subtle shift in how we continue to interact with one another. Behind the masks, we make a little more eye contact; we more often address each other by name; and we acknowledge a greater mutual respect.
The shared experience of COVID-19 has brought us all a little closer together, and in the end, our patients have benefitted.
45-year-old man • fever • generalized rash • recent history of calcaneal osteomyelitis • Dx?
THE CASE
A 45-year-old man was admitted to the hospital with a fever and generalized rash. For the previous 2 weeks, he had been treated at a skilled nursing facility with IV vancomycin and cefepime for left calcaneal osteomyelitis. He reported that the rash was pruritic and started 2 days prior to hospital admission.
His past medical history was significant for type 2 diabetes mellitus and polysubstance drug abuse. Medical and travel history were otherwise unremarkable. The patient was taking the following medications at the time of presentation: hydrocodone-acetaminophen, cyclobenzaprine, melatonin, and metformin.
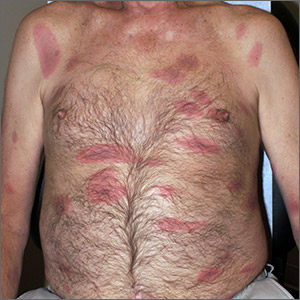
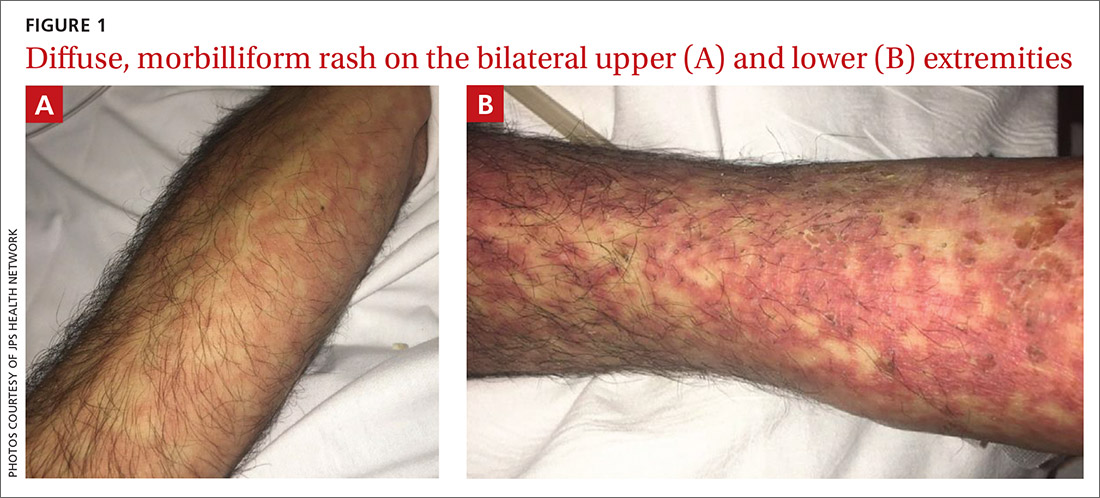
Initial vital signs included a temperature of 102.9°F; respiratory rate, 22 breaths/min; heart rate, 97 beats/min; and blood pressure, 89/50 mm Hg. Physical exam was notable for left anterior cervical and axillary lymphadenopathy. The patient had no facial edema, but he did have a diffuse, morbilliform rash on his bilateral upper and lower extremities, encompassing about 54% of his body surface area (FIGURE 1).
Laboratory studies revealed a white blood cell count of 4.7/mcL, with 3.4% eosinophils and 10.9% monocytes; an erythrocyte sedimentation rate of 60 mm/h; and a C-reactive protein level of 1 mg/dL. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels were both elevated (AST: 95 U/L [normal range, 8 - 48 U/L]; ALT: 115 U/L [normal range: 7 - 55 U/L]). A chest x-ray was obtained and showed new lung infiltrates (FIGURE 2).

Linezolid and meropenem were initiated for a presumed health care–associated pneumonia, and a sepsis work-up was initiated.
THE DIAGNOSIS
The patient’s rash and pruritus worsened after meropenem was introduced. A hepatitis panel was nonreactive except for prior hepatitis A exposure. Ultrasound of the liver and spleen was normal. Investigation of pneumonia pathogens including Legionella, Streptococcus, Mycoplasma, and Chlamydia psittaci did not reveal any causative agents. A skin biopsy revealed perivascular neutrophilic dermatitis with dyskeratosis.
The patient was diagnosed with DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome based on his fever, worsening morbilliform rash, lymphadenopathy, and elevated liver transaminase levels. Although he did not have marked eosinophilia, atypical lymphocytes were present. Serologies for human herpesvirus (HHV), Epstein-Barr virus (EBV), and cytomegalovirus (CMV) were all unremarkable.
Continue to: During discussions...
During discussions with an infectious disease specialist, it was concluded that the patient’s DRESS syndrome was likely secondary to beta-lactam antibiotics. The patient had been receiving cefepime prior to hospitalization. Meropenem was discontinued and aztreonam was started, with continued linezolid. This patient did not have a reactivation of a herpesvirus (HHV-6, HHV-7, EBV, or CMV), which has been previously reported in cases of DRESS syndrome.
DISCUSSION
DRESS syndrome is a challenging diagnosis to make due to the multiplicity of presenting symptoms. Skin rash, lymphadenopathy, hepatic involvement, and hypereosinophilia are characteristic findings.1 Accurate diagnosis reduces fatal disease outcomes, which are estimated to occur in 5%-10% of cases.1,2
Causative agents. DRESS syndrome typically occurs 2 to 6 weeks after the introduction of the causative agent, commonly an aromatic anticonvulsant or antibiotic.3 The incidence of DRESS syndrome in patients using carbamazepine and phenytoin is estimated to be 1 to 5 per 10,000 patients. The incidence of DRESS syndrome in patients using antibiotics is unknown. Frequently, the inducing antibiotic is a beta-lactam, as in this case.4,5
The pathogenesis of DRESS syndrome is not well understood, although there appears to be an immune-mediated reaction that occurs in certain patients after viral reactivation, particularly with herpesviruses. In vitro studies have demonstrated that the culprit drug is able to induce viral reactivation leading to T-lymphocyte response and systemic inflammation, which occurs in multiple organs.6,7 Reported long-term sequelae of DRESS syndrome include immune-mediated diseases such as thyroiditis and type 1 diabetes. In addition, it is hypothesized that there is a genetic predisposition involving human leukocyte antigens that increases the likelihood that individuals will develop DRESS syndrome.5,8
Diagnosis. The
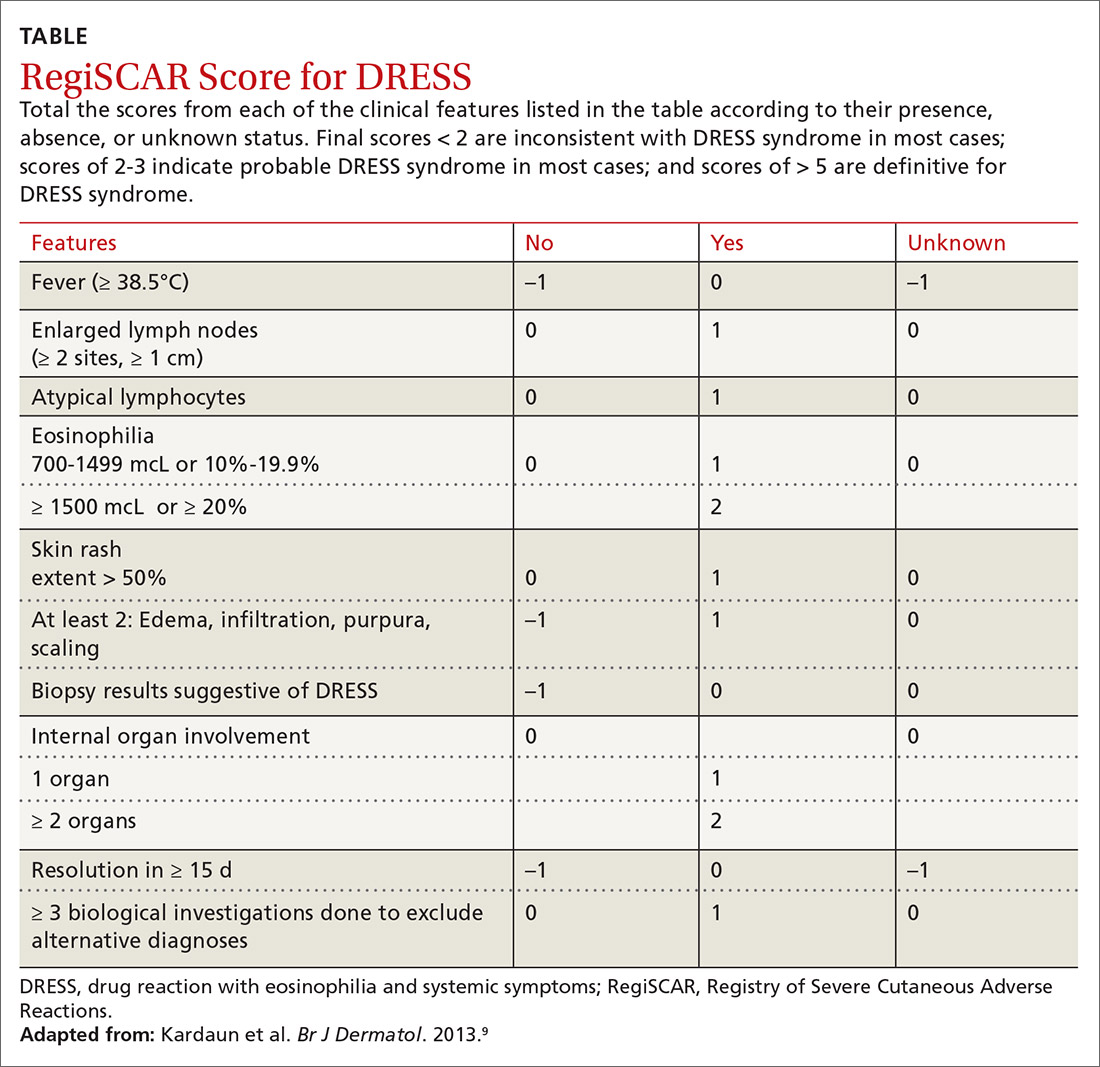
Continue to: Treatment
Treatment is aimed at stopping the causative agent and starting moderate- to high-dose systemic corticosteroids (from 0.5 to 2 mg/kg/d). If symptoms continue to progress, cyclosporine can be used. N-acetylcysteine may also be beneficial due to its ability to neutralize drug metabolites that can stimulate T-cell response.7 There has not been sufficient evidence to suggest that antiviral medication should be initiated.1,7
Our patient was treated with 2 mg/kg/d of prednisone, along with triamcinolone cream, diphenhydramine, and N-acetylcysteine. His rash improved dramatically during his hospital stay and at the subsequent 1-month follow-up was completely resolved.
THE TAKEAWAY
DRESS syndrome should be suspected in patients presenting with fever, rash, lymphadenopathy, pulmonary infiltrates, and liver involvement after initiation of drugs commonly associated with this syndrome. Our case reinforces previous clinical evidence that beta-lactam antibiotics are a common cause of DRESS syndrome; patients taking these medications should be closely monitored. Cross-reactions are frequent, and it is imperative that patients avoid related drugs to prevent recurrence. Although glucocorticoids are the mainstay of treatment, further studies are needed to assess the benefits of N-acetylcysteine.
CORRESPONDENCE
W. Jacob Cobb, MD, JPS Health Network, 1500 South Main Street, Fort Worth, TX, 76104; [email protected]
1. Cacoub P, Musette P, Descamps V, et al. The DRESS syndrome: a literature review. Am J Med. 2011;124:588-597.
2. Chen Y, Chiu H, Chu C. Drug reaction with eosinophilia and systemic symptoms: a retrospective study of 60 cases. Arch Dermatol. 2010;146:1373-1379.
3. Jeung Y-J, Lee J-Y, Oh M-J, et al. Comparison of the causes and clinical features of drug rash with eosinophilia and systemic symptoms and Stevens-Johnson syndrome. Allergy Asthma Immunol Res. 2010;2:123–126.
4. Shiohara T, Iijima M, Ikezawa Z, et al. The diagnosis of a DRESS syndrome has been sufficiently established on the basis of typical clinical features and viral reactivations [commentary]. Br J Dermatol. 2006;156:1083-1084.
5. Ben-Said B, Arnaud-Butel S, Rozières A, et al. Allergic delayed drug hypersensitivity is more frequently diagnosed in drug reaction, eosinophilia and systemic symptoms (DRESS) syndrome than in exanthema induced by beta lactam antibiotics. J Dermatol Sci. 2015;80:71-74.
6. Schrijvers R, Gilissen L, Chiriac AM, et al. Pathogenesis and diagnosis of delayed-type drug hypersensitivity reactions, from bedside to bench and back. Clin Transl Allergy. 2015;5:31.
7. Moling O, Tappeiner L, Piccin A, et al. Treatment of DIHS/DRESS syndrome with combined N-acetylcysteine, prednisone and valganciclovir—a hypothesis. Med Sci Monit. 2012;18:CS57-CS62.
8. Cardoso CS, Vieira AM, Oliveira AP. DRESS syndrome: a case report and literature review. BMJ Case Rep. 2011;2011:bcr0220113898.
9. Kardaun SH, Sekula P, Valeyrie-Allanore L, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071-1080.
10. Bernard L, Eichenfield L. Drug-associated rashes. In: Zaoutis L, Chiang V, eds. Comprehensive Pediatric Hospital Medicine. Philadelphia, PA: Elsevier; 2010: 1005-1011.
11. Grover S. Severe cutaneous adverse reactions. Indian J Dermatol Venereol Leprol. 2011;77:3-6.
THE CASE
A 45-year-old man was admitted to the hospital with a fever and generalized rash. For the previous 2 weeks, he had been treated at a skilled nursing facility with IV vancomycin and cefepime for left calcaneal osteomyelitis. He reported that the rash was pruritic and started 2 days prior to hospital admission.
His past medical history was significant for type 2 diabetes mellitus and polysubstance drug abuse. Medical and travel history were otherwise unremarkable. The patient was taking the following medications at the time of presentation: hydrocodone-acetaminophen, cyclobenzaprine, melatonin, and metformin.
Initial vital signs included a temperature of 102.9°F; respiratory rate, 22 breaths/min; heart rate, 97 beats/min; and blood pressure, 89/50 mm Hg. Physical exam was notable for left anterior cervical and axillary lymphadenopathy. The patient had no facial edema, but he did have a diffuse, morbilliform rash on his bilateral upper and lower extremities, encompassing about 54% of his body surface area (FIGURE 1).

Laboratory studies revealed a white blood cell count of 4.7/mcL, with 3.4% eosinophils and 10.9% monocytes; an erythrocyte sedimentation rate of 60 mm/h; and a C-reactive protein level of 1 mg/dL. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels were both elevated (AST: 95 U/L [normal range, 8 - 48 U/L]; ALT: 115 U/L [normal range: 7 - 55 U/L]). A chest x-ray was obtained and showed new lung infiltrates (FIGURE 2).

Linezolid and meropenem were initiated for a presumed health care–associated pneumonia, and a sepsis work-up was initiated.
THE DIAGNOSIS
The patient’s rash and pruritus worsened after meropenem was introduced. A hepatitis panel was nonreactive except for prior hepatitis A exposure. Ultrasound of the liver and spleen was normal. Investigation of pneumonia pathogens including Legionella, Streptococcus, Mycoplasma, and Chlamydia psittaci did not reveal any causative agents. A skin biopsy revealed perivascular neutrophilic dermatitis with dyskeratosis.
The patient was diagnosed with DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome based on his fever, worsening morbilliform rash, lymphadenopathy, and elevated liver transaminase levels. Although he did not have marked eosinophilia, atypical lymphocytes were present. Serologies for human herpesvirus (HHV), Epstein-Barr virus (EBV), and cytomegalovirus (CMV) were all unremarkable.
Continue to: During discussions...
During discussions with an infectious disease specialist, it was concluded that the patient’s DRESS syndrome was likely secondary to beta-lactam antibiotics. The patient had been receiving cefepime prior to hospitalization. Meropenem was discontinued and aztreonam was started, with continued linezolid. This patient did not have a reactivation of a herpesvirus (HHV-6, HHV-7, EBV, or CMV), which has been previously reported in cases of DRESS syndrome.
DISCUSSION
DRESS syndrome is a challenging diagnosis to make due to the multiplicity of presenting symptoms. Skin rash, lymphadenopathy, hepatic involvement, and hypereosinophilia are characteristic findings.1 Accurate diagnosis reduces fatal disease outcomes, which are estimated to occur in 5%-10% of cases.1,2
Causative agents. DRESS syndrome typically occurs 2 to 6 weeks after the introduction of the causative agent, commonly an aromatic anticonvulsant or antibiotic.3 The incidence of DRESS syndrome in patients using carbamazepine and phenytoin is estimated to be 1 to 5 per 10,000 patients. The incidence of DRESS syndrome in patients using antibiotics is unknown. Frequently, the inducing antibiotic is a beta-lactam, as in this case.4,5
The pathogenesis of DRESS syndrome is not well understood, although there appears to be an immune-mediated reaction that occurs in certain patients after viral reactivation, particularly with herpesviruses. In vitro studies have demonstrated that the culprit drug is able to induce viral reactivation leading to T-lymphocyte response and systemic inflammation, which occurs in multiple organs.6,7 Reported long-term sequelae of DRESS syndrome include immune-mediated diseases such as thyroiditis and type 1 diabetes. In addition, it is hypothesized that there is a genetic predisposition involving human leukocyte antigens that increases the likelihood that individuals will develop DRESS syndrome.5,8
Diagnosis. The

Continue to: Treatment
Treatment is aimed at stopping the causative agent and starting moderate- to high-dose systemic corticosteroids (from 0.5 to 2 mg/kg/d). If symptoms continue to progress, cyclosporine can be used. N-acetylcysteine may also be beneficial due to its ability to neutralize drug metabolites that can stimulate T-cell response.7 There has not been sufficient evidence to suggest that antiviral medication should be initiated.1,7
Our patient was treated with 2 mg/kg/d of prednisone, along with triamcinolone cream, diphenhydramine, and N-acetylcysteine. His rash improved dramatically during his hospital stay and at the subsequent 1-month follow-up was completely resolved.
THE TAKEAWAY
DRESS syndrome should be suspected in patients presenting with fever, rash, lymphadenopathy, pulmonary infiltrates, and liver involvement after initiation of drugs commonly associated with this syndrome. Our case reinforces previous clinical evidence that beta-lactam antibiotics are a common cause of DRESS syndrome; patients taking these medications should be closely monitored. Cross-reactions are frequent, and it is imperative that patients avoid related drugs to prevent recurrence. Although glucocorticoids are the mainstay of treatment, further studies are needed to assess the benefits of N-acetylcysteine.
CORRESPONDENCE
W. Jacob Cobb, MD, JPS Health Network, 1500 South Main Street, Fort Worth, TX, 76104; [email protected]
THE CASE
A 45-year-old man was admitted to the hospital with a fever and generalized rash. For the previous 2 weeks, he had been treated at a skilled nursing facility with IV vancomycin and cefepime for left calcaneal osteomyelitis. He reported that the rash was pruritic and started 2 days prior to hospital admission.
His past medical history was significant for type 2 diabetes mellitus and polysubstance drug abuse. Medical and travel history were otherwise unremarkable. The patient was taking the following medications at the time of presentation: hydrocodone-acetaminophen, cyclobenzaprine, melatonin, and metformin.
Initial vital signs included a temperature of 102.9°F; respiratory rate, 22 breaths/min; heart rate, 97 beats/min; and blood pressure, 89/50 mm Hg. Physical exam was notable for left anterior cervical and axillary lymphadenopathy. The patient had no facial edema, but he did have a diffuse, morbilliform rash on his bilateral upper and lower extremities, encompassing about 54% of his body surface area (FIGURE 1).

Laboratory studies revealed a white blood cell count of 4.7/mcL, with 3.4% eosinophils and 10.9% monocytes; an erythrocyte sedimentation rate of 60 mm/h; and a C-reactive protein level of 1 mg/dL. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels were both elevated (AST: 95 U/L [normal range, 8 - 48 U/L]; ALT: 115 U/L [normal range: 7 - 55 U/L]). A chest x-ray was obtained and showed new lung infiltrates (FIGURE 2).

Linezolid and meropenem were initiated for a presumed health care–associated pneumonia, and a sepsis work-up was initiated.
THE DIAGNOSIS
The patient’s rash and pruritus worsened after meropenem was introduced. A hepatitis panel was nonreactive except for prior hepatitis A exposure. Ultrasound of the liver and spleen was normal. Investigation of pneumonia pathogens including Legionella, Streptococcus, Mycoplasma, and Chlamydia psittaci did not reveal any causative agents. A skin biopsy revealed perivascular neutrophilic dermatitis with dyskeratosis.
The patient was diagnosed with DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome based on his fever, worsening morbilliform rash, lymphadenopathy, and elevated liver transaminase levels. Although he did not have marked eosinophilia, atypical lymphocytes were present. Serologies for human herpesvirus (HHV), Epstein-Barr virus (EBV), and cytomegalovirus (CMV) were all unremarkable.
Continue to: During discussions...
During discussions with an infectious disease specialist, it was concluded that the patient’s DRESS syndrome was likely secondary to beta-lactam antibiotics. The patient had been receiving cefepime prior to hospitalization. Meropenem was discontinued and aztreonam was started, with continued linezolid. This patient did not have a reactivation of a herpesvirus (HHV-6, HHV-7, EBV, or CMV), which has been previously reported in cases of DRESS syndrome.
DISCUSSION
DRESS syndrome is a challenging diagnosis to make due to the multiplicity of presenting symptoms. Skin rash, lymphadenopathy, hepatic involvement, and hypereosinophilia are characteristic findings.1 Accurate diagnosis reduces fatal disease outcomes, which are estimated to occur in 5%-10% of cases.1,2
Causative agents. DRESS syndrome typically occurs 2 to 6 weeks after the introduction of the causative agent, commonly an aromatic anticonvulsant or antibiotic.3 The incidence of DRESS syndrome in patients using carbamazepine and phenytoin is estimated to be 1 to 5 per 10,000 patients. The incidence of DRESS syndrome in patients using antibiotics is unknown. Frequently, the inducing antibiotic is a beta-lactam, as in this case.4,5
The pathogenesis of DRESS syndrome is not well understood, although there appears to be an immune-mediated reaction that occurs in certain patients after viral reactivation, particularly with herpesviruses. In vitro studies have demonstrated that the culprit drug is able to induce viral reactivation leading to T-lymphocyte response and systemic inflammation, which occurs in multiple organs.6,7 Reported long-term sequelae of DRESS syndrome include immune-mediated diseases such as thyroiditis and type 1 diabetes. In addition, it is hypothesized that there is a genetic predisposition involving human leukocyte antigens that increases the likelihood that individuals will develop DRESS syndrome.5,8
Diagnosis. The

Continue to: Treatment
Treatment is aimed at stopping the causative agent and starting moderate- to high-dose systemic corticosteroids (from 0.5 to 2 mg/kg/d). If symptoms continue to progress, cyclosporine can be used. N-acetylcysteine may also be beneficial due to its ability to neutralize drug metabolites that can stimulate T-cell response.7 There has not been sufficient evidence to suggest that antiviral medication should be initiated.1,7
Our patient was treated with 2 mg/kg/d of prednisone, along with triamcinolone cream, diphenhydramine, and N-acetylcysteine. His rash improved dramatically during his hospital stay and at the subsequent 1-month follow-up was completely resolved.
THE TAKEAWAY
DRESS syndrome should be suspected in patients presenting with fever, rash, lymphadenopathy, pulmonary infiltrates, and liver involvement after initiation of drugs commonly associated with this syndrome. Our case reinforces previous clinical evidence that beta-lactam antibiotics are a common cause of DRESS syndrome; patients taking these medications should be closely monitored. Cross-reactions are frequent, and it is imperative that patients avoid related drugs to prevent recurrence. Although glucocorticoids are the mainstay of treatment, further studies are needed to assess the benefits of N-acetylcysteine.
CORRESPONDENCE
W. Jacob Cobb, MD, JPS Health Network, 1500 South Main Street, Fort Worth, TX, 76104; [email protected]
1. Cacoub P, Musette P, Descamps V, et al. The DRESS syndrome: a literature review. Am J Med. 2011;124:588-597.
2. Chen Y, Chiu H, Chu C. Drug reaction with eosinophilia and systemic symptoms: a retrospective study of 60 cases. Arch Dermatol. 2010;146:1373-1379.
3. Jeung Y-J, Lee J-Y, Oh M-J, et al. Comparison of the causes and clinical features of drug rash with eosinophilia and systemic symptoms and Stevens-Johnson syndrome. Allergy Asthma Immunol Res. 2010;2:123–126.
4. Shiohara T, Iijima M, Ikezawa Z, et al. The diagnosis of a DRESS syndrome has been sufficiently established on the basis of typical clinical features and viral reactivations [commentary]. Br J Dermatol. 2006;156:1083-1084.
5. Ben-Said B, Arnaud-Butel S, Rozières A, et al. Allergic delayed drug hypersensitivity is more frequently diagnosed in drug reaction, eosinophilia and systemic symptoms (DRESS) syndrome than in exanthema induced by beta lactam antibiotics. J Dermatol Sci. 2015;80:71-74.
6. Schrijvers R, Gilissen L, Chiriac AM, et al. Pathogenesis and diagnosis of delayed-type drug hypersensitivity reactions, from bedside to bench and back. Clin Transl Allergy. 2015;5:31.
7. Moling O, Tappeiner L, Piccin A, et al. Treatment of DIHS/DRESS syndrome with combined N-acetylcysteine, prednisone and valganciclovir—a hypothesis. Med Sci Monit. 2012;18:CS57-CS62.
8. Cardoso CS, Vieira AM, Oliveira AP. DRESS syndrome: a case report and literature review. BMJ Case Rep. 2011;2011:bcr0220113898.
9. Kardaun SH, Sekula P, Valeyrie-Allanore L, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071-1080.
10. Bernard L, Eichenfield L. Drug-associated rashes. In: Zaoutis L, Chiang V, eds. Comprehensive Pediatric Hospital Medicine. Philadelphia, PA: Elsevier; 2010: 1005-1011.
11. Grover S. Severe cutaneous adverse reactions. Indian J Dermatol Venereol Leprol. 2011;77:3-6.
1. Cacoub P, Musette P, Descamps V, et al. The DRESS syndrome: a literature review. Am J Med. 2011;124:588-597.
2. Chen Y, Chiu H, Chu C. Drug reaction with eosinophilia and systemic symptoms: a retrospective study of 60 cases. Arch Dermatol. 2010;146:1373-1379.
3. Jeung Y-J, Lee J-Y, Oh M-J, et al. Comparison of the causes and clinical features of drug rash with eosinophilia and systemic symptoms and Stevens-Johnson syndrome. Allergy Asthma Immunol Res. 2010;2:123–126.
4. Shiohara T, Iijima M, Ikezawa Z, et al. The diagnosis of a DRESS syndrome has been sufficiently established on the basis of typical clinical features and viral reactivations [commentary]. Br J Dermatol. 2006;156:1083-1084.
5. Ben-Said B, Arnaud-Butel S, Rozières A, et al. Allergic delayed drug hypersensitivity is more frequently diagnosed in drug reaction, eosinophilia and systemic symptoms (DRESS) syndrome than in exanthema induced by beta lactam antibiotics. J Dermatol Sci. 2015;80:71-74.
6. Schrijvers R, Gilissen L, Chiriac AM, et al. Pathogenesis and diagnosis of delayed-type drug hypersensitivity reactions, from bedside to bench and back. Clin Transl Allergy. 2015;5:31.
7. Moling O, Tappeiner L, Piccin A, et al. Treatment of DIHS/DRESS syndrome with combined N-acetylcysteine, prednisone and valganciclovir—a hypothesis. Med Sci Monit. 2012;18:CS57-CS62.
8. Cardoso CS, Vieira AM, Oliveira AP. DRESS syndrome: a case report and literature review. BMJ Case Rep. 2011;2011:bcr0220113898.
9. Kardaun SH, Sekula P, Valeyrie-Allanore L, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071-1080.
10. Bernard L, Eichenfield L. Drug-associated rashes. In: Zaoutis L, Chiang V, eds. Comprehensive Pediatric Hospital Medicine. Philadelphia, PA: Elsevier; 2010: 1005-1011.
11. Grover S. Severe cutaneous adverse reactions. Indian J Dermatol Venereol Leprol. 2011;77:3-6.
Asymptomatic children may transmit COVID-19 in communities
About 22% of children with COVID-19 infections were asymptomatic, and 66% of the symptomatic children had unrecognized symptoms at the time of diagnosis, based on data from a case series of 91 confirmed cases.
Although recent reports suggest that COVID-19 infections in children are generally mild, data on the full spectrum of illness and duration of viral RNA in children are limited, wrote Mi Seon Han, MD, PhD, of Seoul (South Korea) Metropolitan Government–Seoul National University Boramae Medical Center, and colleagues.
To examine the full clinical course and duration of COVID-19 RNA detectability in children with confirmed infections, the researchers reviewed data from 91 individuals with confirmed infections. The children ranged in age from 27 days to 18 years, and 58% were male. The children were monitored at 20 hospitals and 2 isolation facilities for a mean 21.9 days. The findings were published in JAMA Pediatrics.
Overall, COVID-19 viral RNA was present in the study population for a mean 17.6 days, with testing done at a median interval of 3 days. A total of 20 children (22%) were asymptomatic throughout the study period. In these children, viral RNA was detected for a mean 14 days.
“The major hurdle implicated in this study in diagnosing and treating children with COVID-19 is that the researchers noted.
Of the 71 symptomatic children, 47 (66%) had unrecognized symptoms prior to diagnosis, 18 (25%) developed symptoms after diagnosis, and 6 (9%) were diagnosed at the time of symptom onset. The symptomatic children were symptomatic for a median of 11 days; 43 (61%) remained symptomatic at 7 days’ follow-up after the study period, 27 (38%) were symptomatic at 14 days, and 7 (10%) were symptomatic at 21 days.
A total of 41 children had upper respiratory infections (58%) and 22 children (24%) had lower respiratory tract infections. No difference in the duration of virus RNA was detected between children with upper respiratory tract infections and lower respiratory tract infections (average, 18.7 days vs. 19.9 days).
Among the symptomatic children, 46 (65%) had mild cases and 20 (28%) had moderate cases.
For treatment, 14 children (15%) received lopinavir-ritonavir and/or hydroxychloroquine. Two patients had severe illness and received oxygen via nasal prong, without the need for mechanical ventilation. All the children in the case series recovered from their infections with no fatalities.
The study’s main limitation was the inability to analyze the transmission potential of the children because of the quarantine and isolation policies in Korea, the researchers noted. In addition, the researchers did not perform follow-up testing at consistent intervals, so the duration of COVID-19 RNA detection may be inexact.
However, the results suggest “that suspecting and diagnosing COVID-19 in children based on their symptoms without epidemiologic information and virus testing is very challenging,” the researchers emphasized.
“Most of the children with COVID-19 have silent disease, but SARS-CoV-2 RNA can still be detected in the respiratory tract for a prolonged period,” they wrote. More research is needed to explore the potential for disease transmission by children in the community, and increased surveillance with laboratory screening can help identify children with unrecognized infections.
The study is the first known to focus on the frequency of asymptomatic infection in children and the duration of symptoms in both asymptomatic and symptomatic children, Roberta L. DeBiasi, MD, and Meghan Delaney, DO, both affiliated with Children’s National Hospital and Research Institute, Washington, and George Washington University, Washington, wrote in an accompanying editorial. The structure of the Korean public health system “allowed for the sequential observation, testing (median testing interval of every 3 days), and comparison of 91 asymptomatic, presymptomatic, and symptomatic children with mild to moderate upper and lower respiratory tract infection, identified primarily by contact tracing from laboratory-proven cases.”
Two take-home points from the study are that not all infected children are symptomatic, and the duration of symptoms in those who are varies widely, they noted. “Interestingly, this study aligns with adult data in which up to 40% of adults may remain asymptomatic in the face of infection.”
However, “The third and most important take-home point from this study relates to the duration of viral shedding in infected pediatric patients,” Dr. DeBiasi and Dr. Delaney said (JAMA Pediatr. 2020 Aug 28. doi: 10.1001/jamapediatrics.2020.3996).
“Fully half of symptomatic children with both upper and lower tract disease were still shedding virus at 21 days. These are striking data, particularly since 86 of 88 diagnosed children (98%) either had no symptoms or mild or moderate disease,” they explained. The results highlight the need for improvements in qualitative molecular testing and formal studies to identify differences in results from different testing scenarios, such as hospital entry, preprocedure screening, and symptomatic testing. In addition, “these findings are highly relevant to the development of public health strategies to mitigate and contain spread within communities, particularly as affected communities begin their recovery phases.”
The study is important because “schools are opening, and we don’t know what is going to happen,” Michael E. Pichichero, MD, of Rochester General Hospital, N.Y., said in an interview.
“Clinicians, parents, students, school administrators and politicians are worried,” he said. “This study adds to others recently published, bringing into focus the challenges to several suppositions that existed when the COVID-19 pandemic began and over the summer.”
“This study of 91 Korean children tells us that taking a child’s temperature as a screening tool to decide if they may enter school will not be a highly successful strategy,” he said. “Many children are without fever and asymptomatic when infected and contagious. The notion that children shed less virus or shed it for shorter lengths of time we keep learning from this type of research is not true. In another recent study the authors found that children shed as much of the SARS-CoV-2 virus as an adult in the ICU on a ventilator.”
Dr. Pichichero said he was not surprised by the study findings. “A similar paper was published last week in the Journal of Pediatrics from Massachusetts General Hospital, so the findings in the JAMA paper are similar to what has been reported in the United States.”
“Availability of testing will continue to be a challenge in some communities,” said Dr. Pichichero. “Here in the Rochester, New York, area we will use a screening questionnaire based on the CDC [Centers for Disease Control and Prevention] symptom criteria of SARS-CoV-2 infections to decide whom to test.”
As for additional research, “We have so much more to learn about SARS-CoV-2 in children,” he emphasized. “The focus has been on adults because the morbidity and mortality has been greatest in adults, especially the elderly and those with compromised health.”
“The National Institutes of Health has issued a call for more research in children to characterize the spectrum of SARS-CoV-2 illness, including the multisystem inflammatory syndrome in children [MIS-C] and try to identify biomarkers and/or biosignatures for a prognostic algorithm to predict the longitudinal risk of disease severity after a child is exposed to and may be infected with SARS-CoV-2,” said Dr. Pichichero. “NIH has asked researchers to answer the following questions.”
- Why do children have milder illness?
- Are there differences in childhood biology (e.g., gender, puberty, etc.) that contribute to illness severity?
- Are there genetic host differences associated with different disease severity phenotypes, including MIS-C?
- Are there innate mucosal, humoral, cellular and other adaptive immune profiles that are associated with reduced or increased risk of progressive disease, including previous coronavirus infections?
- Will SARS-CoV-2 reinfection cause worse disease as seen with antibody-dependent enhancement (ADE) in other viral infections (e.g., dengue)? Will future vaccines carry a risk of the ADE phenomenon?
- Does substance use (e.g., nicotine, marijuana) exacerbate or trigger MIS-C through immune activation?
“We have no knowledge yet about SARS-CoV-2 vaccination of children, especially young children,” Dr. Pichichero emphasized. “There are different types of vaccines – messenger RNA, adenovirus vector and purified spike proteins of the virus – among others, but questions remain: Will the vaccines work in children? What about side effects? Will the antibodies and cellular immunity protect partially or completely?”
The researchers and editorialists had no financial conflicts to disclose. Dr. Pichichero had no financial conflicts to disclose.
SOURCE: Han MS et al. JAMA Pediatr. 2020 Aug 28. doi:10.1001/jamapediatrics.2020.3988.
About 22% of children with COVID-19 infections were asymptomatic, and 66% of the symptomatic children had unrecognized symptoms at the time of diagnosis, based on data from a case series of 91 confirmed cases.
Although recent reports suggest that COVID-19 infections in children are generally mild, data on the full spectrum of illness and duration of viral RNA in children are limited, wrote Mi Seon Han, MD, PhD, of Seoul (South Korea) Metropolitan Government–Seoul National University Boramae Medical Center, and colleagues.
To examine the full clinical course and duration of COVID-19 RNA detectability in children with confirmed infections, the researchers reviewed data from 91 individuals with confirmed infections. The children ranged in age from 27 days to 18 years, and 58% were male. The children were monitored at 20 hospitals and 2 isolation facilities for a mean 21.9 days. The findings were published in JAMA Pediatrics.
Overall, COVID-19 viral RNA was present in the study population for a mean 17.6 days, with testing done at a median interval of 3 days. A total of 20 children (22%) were asymptomatic throughout the study period. In these children, viral RNA was detected for a mean 14 days.
“The major hurdle implicated in this study in diagnosing and treating children with COVID-19 is that the researchers noted.
Of the 71 symptomatic children, 47 (66%) had unrecognized symptoms prior to diagnosis, 18 (25%) developed symptoms after diagnosis, and 6 (9%) were diagnosed at the time of symptom onset. The symptomatic children were symptomatic for a median of 11 days; 43 (61%) remained symptomatic at 7 days’ follow-up after the study period, 27 (38%) were symptomatic at 14 days, and 7 (10%) were symptomatic at 21 days.
A total of 41 children had upper respiratory infections (58%) and 22 children (24%) had lower respiratory tract infections. No difference in the duration of virus RNA was detected between children with upper respiratory tract infections and lower respiratory tract infections (average, 18.7 days vs. 19.9 days).
Among the symptomatic children, 46 (65%) had mild cases and 20 (28%) had moderate cases.
For treatment, 14 children (15%) received lopinavir-ritonavir and/or hydroxychloroquine. Two patients had severe illness and received oxygen via nasal prong, without the need for mechanical ventilation. All the children in the case series recovered from their infections with no fatalities.
The study’s main limitation was the inability to analyze the transmission potential of the children because of the quarantine and isolation policies in Korea, the researchers noted. In addition, the researchers did not perform follow-up testing at consistent intervals, so the duration of COVID-19 RNA detection may be inexact.
However, the results suggest “that suspecting and diagnosing COVID-19 in children based on their symptoms without epidemiologic information and virus testing is very challenging,” the researchers emphasized.
“Most of the children with COVID-19 have silent disease, but SARS-CoV-2 RNA can still be detected in the respiratory tract for a prolonged period,” they wrote. More research is needed to explore the potential for disease transmission by children in the community, and increased surveillance with laboratory screening can help identify children with unrecognized infections.
The study is the first known to focus on the frequency of asymptomatic infection in children and the duration of symptoms in both asymptomatic and symptomatic children, Roberta L. DeBiasi, MD, and Meghan Delaney, DO, both affiliated with Children’s National Hospital and Research Institute, Washington, and George Washington University, Washington, wrote in an accompanying editorial. The structure of the Korean public health system “allowed for the sequential observation, testing (median testing interval of every 3 days), and comparison of 91 asymptomatic, presymptomatic, and symptomatic children with mild to moderate upper and lower respiratory tract infection, identified primarily by contact tracing from laboratory-proven cases.”
Two take-home points from the study are that not all infected children are symptomatic, and the duration of symptoms in those who are varies widely, they noted. “Interestingly, this study aligns with adult data in which up to 40% of adults may remain asymptomatic in the face of infection.”
However, “The third and most important take-home point from this study relates to the duration of viral shedding in infected pediatric patients,” Dr. DeBiasi and Dr. Delaney said (JAMA Pediatr. 2020 Aug 28. doi: 10.1001/jamapediatrics.2020.3996).
“Fully half of symptomatic children with both upper and lower tract disease were still shedding virus at 21 days. These are striking data, particularly since 86 of 88 diagnosed children (98%) either had no symptoms or mild or moderate disease,” they explained. The results highlight the need for improvements in qualitative molecular testing and formal studies to identify differences in results from different testing scenarios, such as hospital entry, preprocedure screening, and symptomatic testing. In addition, “these findings are highly relevant to the development of public health strategies to mitigate and contain spread within communities, particularly as affected communities begin their recovery phases.”
The study is important because “schools are opening, and we don’t know what is going to happen,” Michael E. Pichichero, MD, of Rochester General Hospital, N.Y., said in an interview.
“Clinicians, parents, students, school administrators and politicians are worried,” he said. “This study adds to others recently published, bringing into focus the challenges to several suppositions that existed when the COVID-19 pandemic began and over the summer.”
“This study of 91 Korean children tells us that taking a child’s temperature as a screening tool to decide if they may enter school will not be a highly successful strategy,” he said. “Many children are without fever and asymptomatic when infected and contagious. The notion that children shed less virus or shed it for shorter lengths of time we keep learning from this type of research is not true. In another recent study the authors found that children shed as much of the SARS-CoV-2 virus as an adult in the ICU on a ventilator.”
Dr. Pichichero said he was not surprised by the study findings. “A similar paper was published last week in the Journal of Pediatrics from Massachusetts General Hospital, so the findings in the JAMA paper are similar to what has been reported in the United States.”
“Availability of testing will continue to be a challenge in some communities,” said Dr. Pichichero. “Here in the Rochester, New York, area we will use a screening questionnaire based on the CDC [Centers for Disease Control and Prevention] symptom criteria of SARS-CoV-2 infections to decide whom to test.”
As for additional research, “We have so much more to learn about SARS-CoV-2 in children,” he emphasized. “The focus has been on adults because the morbidity and mortality has been greatest in adults, especially the elderly and those with compromised health.”
“The National Institutes of Health has issued a call for more research in children to characterize the spectrum of SARS-CoV-2 illness, including the multisystem inflammatory syndrome in children [MIS-C] and try to identify biomarkers and/or biosignatures for a prognostic algorithm to predict the longitudinal risk of disease severity after a child is exposed to and may be infected with SARS-CoV-2,” said Dr. Pichichero. “NIH has asked researchers to answer the following questions.”
- Why do children have milder illness?
- Are there differences in childhood biology (e.g., gender, puberty, etc.) that contribute to illness severity?
- Are there genetic host differences associated with different disease severity phenotypes, including MIS-C?
- Are there innate mucosal, humoral, cellular and other adaptive immune profiles that are associated with reduced or increased risk of progressive disease, including previous coronavirus infections?
- Will SARS-CoV-2 reinfection cause worse disease as seen with antibody-dependent enhancement (ADE) in other viral infections (e.g., dengue)? Will future vaccines carry a risk of the ADE phenomenon?
- Does substance use (e.g., nicotine, marijuana) exacerbate or trigger MIS-C through immune activation?
“We have no knowledge yet about SARS-CoV-2 vaccination of children, especially young children,” Dr. Pichichero emphasized. “There are different types of vaccines – messenger RNA, adenovirus vector and purified spike proteins of the virus – among others, but questions remain: Will the vaccines work in children? What about side effects? Will the antibodies and cellular immunity protect partially or completely?”
The researchers and editorialists had no financial conflicts to disclose. Dr. Pichichero had no financial conflicts to disclose.
SOURCE: Han MS et al. JAMA Pediatr. 2020 Aug 28. doi:10.1001/jamapediatrics.2020.3988.
About 22% of children with COVID-19 infections were asymptomatic, and 66% of the symptomatic children had unrecognized symptoms at the time of diagnosis, based on data from a case series of 91 confirmed cases.
Although recent reports suggest that COVID-19 infections in children are generally mild, data on the full spectrum of illness and duration of viral RNA in children are limited, wrote Mi Seon Han, MD, PhD, of Seoul (South Korea) Metropolitan Government–Seoul National University Boramae Medical Center, and colleagues.
To examine the full clinical course and duration of COVID-19 RNA detectability in children with confirmed infections, the researchers reviewed data from 91 individuals with confirmed infections. The children ranged in age from 27 days to 18 years, and 58% were male. The children were monitored at 20 hospitals and 2 isolation facilities for a mean 21.9 days. The findings were published in JAMA Pediatrics.
Overall, COVID-19 viral RNA was present in the study population for a mean 17.6 days, with testing done at a median interval of 3 days. A total of 20 children (22%) were asymptomatic throughout the study period. In these children, viral RNA was detected for a mean 14 days.
“The major hurdle implicated in this study in diagnosing and treating children with COVID-19 is that the researchers noted.
Of the 71 symptomatic children, 47 (66%) had unrecognized symptoms prior to diagnosis, 18 (25%) developed symptoms after diagnosis, and 6 (9%) were diagnosed at the time of symptom onset. The symptomatic children were symptomatic for a median of 11 days; 43 (61%) remained symptomatic at 7 days’ follow-up after the study period, 27 (38%) were symptomatic at 14 days, and 7 (10%) were symptomatic at 21 days.
A total of 41 children had upper respiratory infections (58%) and 22 children (24%) had lower respiratory tract infections. No difference in the duration of virus RNA was detected between children with upper respiratory tract infections and lower respiratory tract infections (average, 18.7 days vs. 19.9 days).
Among the symptomatic children, 46 (65%) had mild cases and 20 (28%) had moderate cases.
For treatment, 14 children (15%) received lopinavir-ritonavir and/or hydroxychloroquine. Two patients had severe illness and received oxygen via nasal prong, without the need for mechanical ventilation. All the children in the case series recovered from their infections with no fatalities.
The study’s main limitation was the inability to analyze the transmission potential of the children because of the quarantine and isolation policies in Korea, the researchers noted. In addition, the researchers did not perform follow-up testing at consistent intervals, so the duration of COVID-19 RNA detection may be inexact.
However, the results suggest “that suspecting and diagnosing COVID-19 in children based on their symptoms without epidemiologic information and virus testing is very challenging,” the researchers emphasized.
“Most of the children with COVID-19 have silent disease, but SARS-CoV-2 RNA can still be detected in the respiratory tract for a prolonged period,” they wrote. More research is needed to explore the potential for disease transmission by children in the community, and increased surveillance with laboratory screening can help identify children with unrecognized infections.
The study is the first known to focus on the frequency of asymptomatic infection in children and the duration of symptoms in both asymptomatic and symptomatic children, Roberta L. DeBiasi, MD, and Meghan Delaney, DO, both affiliated with Children’s National Hospital and Research Institute, Washington, and George Washington University, Washington, wrote in an accompanying editorial. The structure of the Korean public health system “allowed for the sequential observation, testing (median testing interval of every 3 days), and comparison of 91 asymptomatic, presymptomatic, and symptomatic children with mild to moderate upper and lower respiratory tract infection, identified primarily by contact tracing from laboratory-proven cases.”
Two take-home points from the study are that not all infected children are symptomatic, and the duration of symptoms in those who are varies widely, they noted. “Interestingly, this study aligns with adult data in which up to 40% of adults may remain asymptomatic in the face of infection.”
However, “The third and most important take-home point from this study relates to the duration of viral shedding in infected pediatric patients,” Dr. DeBiasi and Dr. Delaney said (JAMA Pediatr. 2020 Aug 28. doi: 10.1001/jamapediatrics.2020.3996).
“Fully half of symptomatic children with both upper and lower tract disease were still shedding virus at 21 days. These are striking data, particularly since 86 of 88 diagnosed children (98%) either had no symptoms or mild or moderate disease,” they explained. The results highlight the need for improvements in qualitative molecular testing and formal studies to identify differences in results from different testing scenarios, such as hospital entry, preprocedure screening, and symptomatic testing. In addition, “these findings are highly relevant to the development of public health strategies to mitigate and contain spread within communities, particularly as affected communities begin their recovery phases.”
The study is important because “schools are opening, and we don’t know what is going to happen,” Michael E. Pichichero, MD, of Rochester General Hospital, N.Y., said in an interview.
“Clinicians, parents, students, school administrators and politicians are worried,” he said. “This study adds to others recently published, bringing into focus the challenges to several suppositions that existed when the COVID-19 pandemic began and over the summer.”
“This study of 91 Korean children tells us that taking a child’s temperature as a screening tool to decide if they may enter school will not be a highly successful strategy,” he said. “Many children are without fever and asymptomatic when infected and contagious. The notion that children shed less virus or shed it for shorter lengths of time we keep learning from this type of research is not true. In another recent study the authors found that children shed as much of the SARS-CoV-2 virus as an adult in the ICU on a ventilator.”
Dr. Pichichero said he was not surprised by the study findings. “A similar paper was published last week in the Journal of Pediatrics from Massachusetts General Hospital, so the findings in the JAMA paper are similar to what has been reported in the United States.”
“Availability of testing will continue to be a challenge in some communities,” said Dr. Pichichero. “Here in the Rochester, New York, area we will use a screening questionnaire based on the CDC [Centers for Disease Control and Prevention] symptom criteria of SARS-CoV-2 infections to decide whom to test.”
As for additional research, “We have so much more to learn about SARS-CoV-2 in children,” he emphasized. “The focus has been on adults because the morbidity and mortality has been greatest in adults, especially the elderly and those with compromised health.”
“The National Institutes of Health has issued a call for more research in children to characterize the spectrum of SARS-CoV-2 illness, including the multisystem inflammatory syndrome in children [MIS-C] and try to identify biomarkers and/or biosignatures for a prognostic algorithm to predict the longitudinal risk of disease severity after a child is exposed to and may be infected with SARS-CoV-2,” said Dr. Pichichero. “NIH has asked researchers to answer the following questions.”
- Why do children have milder illness?
- Are there differences in childhood biology (e.g., gender, puberty, etc.) that contribute to illness severity?
- Are there genetic host differences associated with different disease severity phenotypes, including MIS-C?
- Are there innate mucosal, humoral, cellular and other adaptive immune profiles that are associated with reduced or increased risk of progressive disease, including previous coronavirus infections?
- Will SARS-CoV-2 reinfection cause worse disease as seen with antibody-dependent enhancement (ADE) in other viral infections (e.g., dengue)? Will future vaccines carry a risk of the ADE phenomenon?
- Does substance use (e.g., nicotine, marijuana) exacerbate or trigger MIS-C through immune activation?
“We have no knowledge yet about SARS-CoV-2 vaccination of children, especially young children,” Dr. Pichichero emphasized. “There are different types of vaccines – messenger RNA, adenovirus vector and purified spike proteins of the virus – among others, but questions remain: Will the vaccines work in children? What about side effects? Will the antibodies and cellular immunity protect partially or completely?”
The researchers and editorialists had no financial conflicts to disclose. Dr. Pichichero had no financial conflicts to disclose.
SOURCE: Han MS et al. JAMA Pediatr. 2020 Aug 28. doi:10.1001/jamapediatrics.2020.3988.
FROM JAMA PEDIATRICS
Latest report adds almost 44,000 child COVID-19 cases in 1 week
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
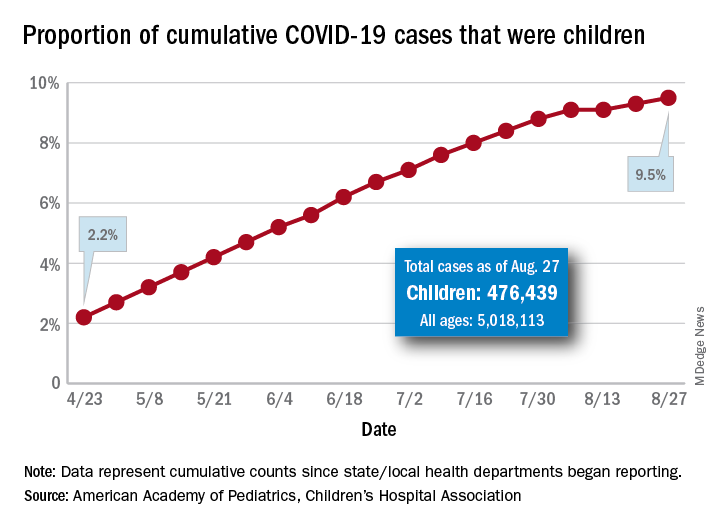
The new cases bring the cumulative number of infected children to over 476,000, and that figure represents 9.5% of the over 5 million COVID-19 cases reported among all ages, the AAP and the CHA said in their weekly report. The cumulative number of children covers 49 states (New York is not reporting age distribution), the District of Columbia, New York City, Puerto Rico, and Guam.
From lowest to highest, the states occupying opposite ends of the cumulative proportion spectrum are New Jersey at 3.4% – New York City was lower with a 3.2% figure but is not a state – and Wyoming at 18.3%, the report showed.
Children represent more than 15% of all reported COVID-19 cases in five other states: Tennessee (17.1%), North Dakota (16.0%), Alaska (15.9%), New Mexico (15.7%), and Minnesota (15.1%). The states just above New Jersey are Florida (5.8%), Connecticut (5.9%), and Massachusetts (6.7%). Texas has a rate of 5.6% but has reported age for only 8% of confirmed cases, the AAP and CHA noted.
Children make up a much lower share of COVID-19 hospitalizations – 1.7% of the cumulative number for all ages – although that figure has been slowly rising over the course of the pandemic: it was 1.2% on July 9 and 0.9% on May 8. Arizona (4.1%) is the highest of the 22 states reporting age for hospitalizations and Hawaii (0.6%) is the lowest, based on the AAP/CHA data.
Mortality figures for children continue to be even lower. Nationwide, 0.07% of all COVID-19 deaths occurred in children, and 19 of the 43 states reporting age distributions have had no deaths yet. Pediatric deaths totaled 101 as of Aug. 27, the two groups reported.
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

The new cases bring the cumulative number of infected children to over 476,000, and that figure represents 9.5% of the over 5 million COVID-19 cases reported among all ages, the AAP and the CHA said in their weekly report. The cumulative number of children covers 49 states (New York is not reporting age distribution), the District of Columbia, New York City, Puerto Rico, and Guam.
From lowest to highest, the states occupying opposite ends of the cumulative proportion spectrum are New Jersey at 3.4% – New York City was lower with a 3.2% figure but is not a state – and Wyoming at 18.3%, the report showed.
Children represent more than 15% of all reported COVID-19 cases in five other states: Tennessee (17.1%), North Dakota (16.0%), Alaska (15.9%), New Mexico (15.7%), and Minnesota (15.1%). The states just above New Jersey are Florida (5.8%), Connecticut (5.9%), and Massachusetts (6.7%). Texas has a rate of 5.6% but has reported age for only 8% of confirmed cases, the AAP and CHA noted.
Children make up a much lower share of COVID-19 hospitalizations – 1.7% of the cumulative number for all ages – although that figure has been slowly rising over the course of the pandemic: it was 1.2% on July 9 and 0.9% on May 8. Arizona (4.1%) is the highest of the 22 states reporting age for hospitalizations and Hawaii (0.6%) is the lowest, based on the AAP/CHA data.
Mortality figures for children continue to be even lower. Nationwide, 0.07% of all COVID-19 deaths occurred in children, and 19 of the 43 states reporting age distributions have had no deaths yet. Pediatric deaths totaled 101 as of Aug. 27, the two groups reported.
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

The new cases bring the cumulative number of infected children to over 476,000, and that figure represents 9.5% of the over 5 million COVID-19 cases reported among all ages, the AAP and the CHA said in their weekly report. The cumulative number of children covers 49 states (New York is not reporting age distribution), the District of Columbia, New York City, Puerto Rico, and Guam.
From lowest to highest, the states occupying opposite ends of the cumulative proportion spectrum are New Jersey at 3.4% – New York City was lower with a 3.2% figure but is not a state – and Wyoming at 18.3%, the report showed.
Children represent more than 15% of all reported COVID-19 cases in five other states: Tennessee (17.1%), North Dakota (16.0%), Alaska (15.9%), New Mexico (15.7%), and Minnesota (15.1%). The states just above New Jersey are Florida (5.8%), Connecticut (5.9%), and Massachusetts (6.7%). Texas has a rate of 5.6% but has reported age for only 8% of confirmed cases, the AAP and CHA noted.
Children make up a much lower share of COVID-19 hospitalizations – 1.7% of the cumulative number for all ages – although that figure has been slowly rising over the course of the pandemic: it was 1.2% on July 9 and 0.9% on May 8. Arizona (4.1%) is the highest of the 22 states reporting age for hospitalizations and Hawaii (0.6%) is the lowest, based on the AAP/CHA data.
Mortality figures for children continue to be even lower. Nationwide, 0.07% of all COVID-19 deaths occurred in children, and 19 of the 43 states reporting age distributions have had no deaths yet. Pediatric deaths totaled 101 as of Aug. 27, the two groups reported.
Beyond microcephaly: Zika-affected children near school age
In 2020, “the virus” has come to mean one thing: SARS-CoV-2. But just a few years ago, Zika had the world's attention, as one news report after another described children with microcephaly born to women who'd been infected while pregnant.
It can be difficult for physicians to determine whether a birth defect is the result of Zika. Most infections have few or no symptoms, and mothers may not know if they’ve been exposed. Karin Nielsen, MD, remembers one child in particular, a 9-month-old boy born with microcephaly whose parents brought the infant to her in 2018 because he had started having seizures.
The child was born in Mexico in 2017, when the Zika virus was still known to be circulating in the Americas, said Dr. Nielsen, a pediatric infectious disease specialist at the University of California, Los Angeles. Brain imaging revealed calcifications and other signs in the boy’s brain that were consistent with exposure. But his mother said she was never sick during pregnancy.
Because Zika is transmitted not just via mosquito and from mother to fetus but also sexually, Dr. Nielsen thinks the mother probably contracted an asymptomatic infection from her husband, who recalled having a rash when she was 4 months pregnant. When they participated in a research study, both parents tested positive for Zika antibodies.
“The child had the classic symptoms of congenital Zika syndrome,” Dr. Nielsen said. “He was 9 months old, he had microcephaly, and he was having mal seizures.”
Researchers have since learned that children with such classic symptoms represent only a small proportion of those affected by prenatal Zika exposure – about 3%-5%. The virus was at its height during the 2016-2016 epidemic and is not currently causing outbreaks. But as researchers have followed cohorts of children exposed to Zika in utero, they have found many subtler effects physicians will need to monitor as the children grow up.
“When we’re seeing hundreds of kids with microcephaly, we had a lot of people infected,” Dr. Nielsen said. “Microcephaly is only the tip of the iceberg.”
Early evidence
Microcephaly may be the most identifiable symptom of fetal Zika infection, but researchers tracking cohorts of exposed children have begun to build a more complete picture of what long-term effects might look like. But hundreds, if not thousands, of children have been exposed to Zika in the womb – it’s not clear how many, Dr. Nielsen said – and many show a range of effects that don’t officially qualify as congenital Zika syndrome.
Current estimates suggest about one third of exposed children have some type of neurologic or neurodevelopmental problem, even though prevalence of visible effects is much lower. Over time, the incidence of these effects has fluctuated; some developmental delays and sensory deficits began manifesting later in childhood whereas others, at least in a few children, have resolved.
“We’re just beginning to have some of the data that we need to think about the full spectrum of outcomes,” said Cindy Moore, MD, chief medical officer in the Division of Congenital and Developmental Disorders in the Centers for Disease Control and Prevention’s National Center on Birth Defects and Developmental Disabilities.
“As we’re learning more and more, we’re learning the spectrum is expanding to less severe forms,” Dr. Moore said. “We do know that with some infections, there are later onset of problems.”
Studies published in 2018 described cohorts of children whose mothers had confirmed or suspected Zika infections during pregnancy in the French Territories of America (Guadalupe, Martinique, and French Guiana) and in Salvador, Brazil. The research provided valuable early data on the incidence of microcephaly and other severe effects in newborns, but noted the need for long-term follow up.
The U.S. Zika Pregnancy and Infant Registry is one of the largest such cohorts. In August 2018, researchers made their first report on data from the registry They looked at 1450 children age 1 or older who had undergone neuroimaging or screenings (developmental, vision, hearing) or both. In 6%, at least one birth defect was linked to Zika, and 9% had at least one neurodevelopmental abnormality.
As these children age past developmental milestones, more effects will likely manifest – even in those children whose appearance and imaging presented as healthy at birth.
Longer-term follow up
Nielsen at UCLA and M. Elisabeth Lopes Moreira, MD, of the Oswaldo Cruz Foundation in Rio de Janeiro, are following a cohort of more than 100 children born in Rio de Janeiro during Brazil’s 2015-2016 epidemic to mothers with symptomatic, PCR-confirmed Zika infections during pregnancy. In December 2018, their team reported that rates of severe neurodevelopmental delay and sensory dysfunction – 14% of 131 children aged 12-18 months – were higher than those found in earlier studies.
In August 2019, the team described neurodevelopmental, vision, and hearing outcomes in 216 Zika-exposed children 2 years after birth. They used the Bayley-III Scales of Infant and Toddler Development to assess cognitive, language and motor skills in 146 of the children. Forty percent of them were below or very below average in development, more than one third (35%) had language delays, 12% percent had hearing loss, and 7% had abnormal eye anatomy, such as underdeveloped retinas.
In two of the eight children in the cohort with microcephaly, the abnormality unexpectedly resolved. Although that finding received a lot of press, Dr. Nielsen pointed out that “not all microcephalies are created equal.”
In one case, a child born small for gestational age had proportional microcephaly: the baby›s head circumference met the criteria for microcephaly, but the infant›s head was proportional to the body so, as the child grew, the apparent microcephaly disappeared.
In the other case, the child was born with craniosynostosis, in which the skull sutures fuse too early – another effect seen with prenatal Zika exposure, Dr. Nielsen said. After corrective surgery, the child’s head circumference no longer met the definition of microcephaly, but the child still had symptoms related to congenital Zika: a developmental delay and calcifications in the brain. Meanwhile, two other children in the Rio cohort developed secondary microcephaly.
In another follow-up study of children up to age 4, Dr. Nielsen and colleagues found that both clinicians and family may think that Zika-exposed infants without microcephaly are developing normally, but that may not be true. Nearly 70% of children without microcephaly had neurologic abnormalities on physical examination, and more than half had failure to thrive because of poor feeding related to neurologic abnormalities.
Initially, some children may be able to mask subtle problems. A study published in January from Sarah B. Mulkey, MD, PhD, of Children’s National Hospital in Washington, DC, and colleagues described neurodevelopmental outcomes in 70 Colombian children up to 18 months old who had been exposed to Zika in utero. The children had a normal head circumference at birth and a normal fetal MRI, but – compared with typically developing peers – their communication, social cognition, and mobility scores on standardized assessments tended to decline as they got older.
“Especially in a very young child, there’s always going to be a possibility that you can compensate for a deficit, and it appears that at least some of these children are doing so,” said William J. Muller, MD, PhD, associate professor of pediatrics at Northwestern University, Chicago. When the children are older, certain behavioral effects will become easier to assess.
“With these children now approaching school age, understanding the full spectrum of neurodevelopmental abnormalities has important public health and educational system implications,” Dr. Muller and Dr. Mulkey wrote in a commentary about one of Dr. Nielsen’s studies.
Researchers face multiple barriers to understanding the long-term effects of fetal Zika infection. Many infants known to have been exposed in utero never received the recommended early assessments and haven’t been followed long-term. Particularly in Brazil, poverty, poor access to healthcare, and overcrowding all complicate surveillance efforts, Dr. Muller said. Stigma related to children’s neurodevelopmental problems also can potentially reduce a mother’s willingness to attend all follow-ups and assessments.
Some children may have been exposed but were never recognized as such, making it difficult for researchers to track their development and assemble a complete picture of prenatal Zika infection outcomes. Asymptomatic infection occurs in about 80% of Zika infections, though it’s not clear if that number holds for infections during pregnancy as well, according to Dr. Muller and Dr. Mulkey. Because nearly all the current research involves children whose mothers had symptomatic infections, the studies’ generalizability may be limited.
Those likely asymptomatic infections are also a major reason none of the cohorts have comparison groups.
“There are literally hundreds of things that can contribute to or cause developmental problems,” said Dr. Moore of the CDC, who noted that it would be nice to have a comparison group so as to know what Zika may not be responsible for. That said, it would be difficult-to-impossible to create a control group with similar geographic and demographic characteristics as the exposed children, a group who researchers can be certain weren’t exposed.
Neurodevelopmental disabilities occur in about 15% of the general population, making it difficult to determine whether Zika causes any or all long-term, less severe developmental findings in exposed children. The difficulty only compounds with time: the older a child is when a developmental problem is recognized, the harder it is to go back and say the problem is a result of something that occurred before birth, Dr. Moore said. “It’s a challenging field to say, this is what caused that outcome.”
Exposed children need continued evaluation
Interpreting the clinical implications of available studies is also challenging. It can be difficult to distinguish between central nervous system damage and peripheral damage, leaving the true etiology of poor vision or hearing elusive. The Zika virus can attack both the optic nerve and the part of the brain that interprets what a person sees: “Are you not seeing well because that part of your brain is not developed, or is it just a problem with the eye?” Dr. Nielsen said.
When problems can’t be precisely identified, successful interventions are harder. If the cochlea is normal, for instance, but the part of the brain that interprets sound or language has deficits, a hearing aid won’t help.
The services and interventions that children need depend on their specific developmental or cognitive deficits, regardless of the cause. But if clinicians know the cause is likely Zika exposure, they also know to look for other deficits.
Children showing likely effects of congenital Zika infection should be further evaluated for other possible birth defects and referred to a developmental specialist, early intervention services, and family support services. Depending on the child, primary care providers might consider referrals to an infectious disease specialist, clinical geneticist, neurologist, or other specialists.
Even with no confirmed infection or visible signs at birth, clinicians should remain vigilant with children who had possible exposure. A recently published study of 120 children conceived during the Zika outbreak in Paraíba, Brazil, assessed as infants and then again at 2 years old, exemplifies why. Researchers identified adverse neurologic outcomes and developmental delays in several children who had no physical evidence of birth defects as newborns, but whose antibody tests showed possible infection.
“In this post-epidemic period, with decreased Zika transmission and less public awareness,” wrote Dr. Mulkey and a colleague, “follow-up of these children is now more important than ever”.
A version of this article originally appeared on Medscape.com.
In 2020, “the virus” has come to mean one thing: SARS-CoV-2. But just a few years ago, Zika had the world's attention, as one news report after another described children with microcephaly born to women who'd been infected while pregnant.
It can be difficult for physicians to determine whether a birth defect is the result of Zika. Most infections have few or no symptoms, and mothers may not know if they’ve been exposed. Karin Nielsen, MD, remembers one child in particular, a 9-month-old boy born with microcephaly whose parents brought the infant to her in 2018 because he had started having seizures.
The child was born in Mexico in 2017, when the Zika virus was still known to be circulating in the Americas, said Dr. Nielsen, a pediatric infectious disease specialist at the University of California, Los Angeles. Brain imaging revealed calcifications and other signs in the boy’s brain that were consistent with exposure. But his mother said she was never sick during pregnancy.
Because Zika is transmitted not just via mosquito and from mother to fetus but also sexually, Dr. Nielsen thinks the mother probably contracted an asymptomatic infection from her husband, who recalled having a rash when she was 4 months pregnant. When they participated in a research study, both parents tested positive for Zika antibodies.
“The child had the classic symptoms of congenital Zika syndrome,” Dr. Nielsen said. “He was 9 months old, he had microcephaly, and he was having mal seizures.”
Researchers have since learned that children with such classic symptoms represent only a small proportion of those affected by prenatal Zika exposure – about 3%-5%. The virus was at its height during the 2016-2016 epidemic and is not currently causing outbreaks. But as researchers have followed cohorts of children exposed to Zika in utero, they have found many subtler effects physicians will need to monitor as the children grow up.
“When we’re seeing hundreds of kids with microcephaly, we had a lot of people infected,” Dr. Nielsen said. “Microcephaly is only the tip of the iceberg.”
Early evidence
Microcephaly may be the most identifiable symptom of fetal Zika infection, but researchers tracking cohorts of exposed children have begun to build a more complete picture of what long-term effects might look like. But hundreds, if not thousands, of children have been exposed to Zika in the womb – it’s not clear how many, Dr. Nielsen said – and many show a range of effects that don’t officially qualify as congenital Zika syndrome.
Current estimates suggest about one third of exposed children have some type of neurologic or neurodevelopmental problem, even though prevalence of visible effects is much lower. Over time, the incidence of these effects has fluctuated; some developmental delays and sensory deficits began manifesting later in childhood whereas others, at least in a few children, have resolved.
“We’re just beginning to have some of the data that we need to think about the full spectrum of outcomes,” said Cindy Moore, MD, chief medical officer in the Division of Congenital and Developmental Disorders in the Centers for Disease Control and Prevention’s National Center on Birth Defects and Developmental Disabilities.
“As we’re learning more and more, we’re learning the spectrum is expanding to less severe forms,” Dr. Moore said. “We do know that with some infections, there are later onset of problems.”
Studies published in 2018 described cohorts of children whose mothers had confirmed or suspected Zika infections during pregnancy in the French Territories of America (Guadalupe, Martinique, and French Guiana) and in Salvador, Brazil. The research provided valuable early data on the incidence of microcephaly and other severe effects in newborns, but noted the need for long-term follow up.
The U.S. Zika Pregnancy and Infant Registry is one of the largest such cohorts. In August 2018, researchers made their first report on data from the registry They looked at 1450 children age 1 or older who had undergone neuroimaging or screenings (developmental, vision, hearing) or both. In 6%, at least one birth defect was linked to Zika, and 9% had at least one neurodevelopmental abnormality.
As these children age past developmental milestones, more effects will likely manifest – even in those children whose appearance and imaging presented as healthy at birth.
Longer-term follow up
Nielsen at UCLA and M. Elisabeth Lopes Moreira, MD, of the Oswaldo Cruz Foundation in Rio de Janeiro, are following a cohort of more than 100 children born in Rio de Janeiro during Brazil’s 2015-2016 epidemic to mothers with symptomatic, PCR-confirmed Zika infections during pregnancy. In December 2018, their team reported that rates of severe neurodevelopmental delay and sensory dysfunction – 14% of 131 children aged 12-18 months – were higher than those found in earlier studies.
In August 2019, the team described neurodevelopmental, vision, and hearing outcomes in 216 Zika-exposed children 2 years after birth. They used the Bayley-III Scales of Infant and Toddler Development to assess cognitive, language and motor skills in 146 of the children. Forty percent of them were below or very below average in development, more than one third (35%) had language delays, 12% percent had hearing loss, and 7% had abnormal eye anatomy, such as underdeveloped retinas.
In two of the eight children in the cohort with microcephaly, the abnormality unexpectedly resolved. Although that finding received a lot of press, Dr. Nielsen pointed out that “not all microcephalies are created equal.”
In one case, a child born small for gestational age had proportional microcephaly: the baby›s head circumference met the criteria for microcephaly, but the infant›s head was proportional to the body so, as the child grew, the apparent microcephaly disappeared.
In the other case, the child was born with craniosynostosis, in which the skull sutures fuse too early – another effect seen with prenatal Zika exposure, Dr. Nielsen said. After corrective surgery, the child’s head circumference no longer met the definition of microcephaly, but the child still had symptoms related to congenital Zika: a developmental delay and calcifications in the brain. Meanwhile, two other children in the Rio cohort developed secondary microcephaly.
In another follow-up study of children up to age 4, Dr. Nielsen and colleagues found that both clinicians and family may think that Zika-exposed infants without microcephaly are developing normally, but that may not be true. Nearly 70% of children without microcephaly had neurologic abnormalities on physical examination, and more than half had failure to thrive because of poor feeding related to neurologic abnormalities.
Initially, some children may be able to mask subtle problems. A study published in January from Sarah B. Mulkey, MD, PhD, of Children’s National Hospital in Washington, DC, and colleagues described neurodevelopmental outcomes in 70 Colombian children up to 18 months old who had been exposed to Zika in utero. The children had a normal head circumference at birth and a normal fetal MRI, but – compared with typically developing peers – their communication, social cognition, and mobility scores on standardized assessments tended to decline as they got older.
“Especially in a very young child, there’s always going to be a possibility that you can compensate for a deficit, and it appears that at least some of these children are doing so,” said William J. Muller, MD, PhD, associate professor of pediatrics at Northwestern University, Chicago. When the children are older, certain behavioral effects will become easier to assess.
“With these children now approaching school age, understanding the full spectrum of neurodevelopmental abnormalities has important public health and educational system implications,” Dr. Muller and Dr. Mulkey wrote in a commentary about one of Dr. Nielsen’s studies.
Researchers face multiple barriers to understanding the long-term effects of fetal Zika infection. Many infants known to have been exposed in utero never received the recommended early assessments and haven’t been followed long-term. Particularly in Brazil, poverty, poor access to healthcare, and overcrowding all complicate surveillance efforts, Dr. Muller said. Stigma related to children’s neurodevelopmental problems also can potentially reduce a mother’s willingness to attend all follow-ups and assessments.
Some children may have been exposed but were never recognized as such, making it difficult for researchers to track their development and assemble a complete picture of prenatal Zika infection outcomes. Asymptomatic infection occurs in about 80% of Zika infections, though it’s not clear if that number holds for infections during pregnancy as well, according to Dr. Muller and Dr. Mulkey. Because nearly all the current research involves children whose mothers had symptomatic infections, the studies’ generalizability may be limited.
Those likely asymptomatic infections are also a major reason none of the cohorts have comparison groups.
“There are literally hundreds of things that can contribute to or cause developmental problems,” said Dr. Moore of the CDC, who noted that it would be nice to have a comparison group so as to know what Zika may not be responsible for. That said, it would be difficult-to-impossible to create a control group with similar geographic and demographic characteristics as the exposed children, a group who researchers can be certain weren’t exposed.
Neurodevelopmental disabilities occur in about 15% of the general population, making it difficult to determine whether Zika causes any or all long-term, less severe developmental findings in exposed children. The difficulty only compounds with time: the older a child is when a developmental problem is recognized, the harder it is to go back and say the problem is a result of something that occurred before birth, Dr. Moore said. “It’s a challenging field to say, this is what caused that outcome.”
Exposed children need continued evaluation
Interpreting the clinical implications of available studies is also challenging. It can be difficult to distinguish between central nervous system damage and peripheral damage, leaving the true etiology of poor vision or hearing elusive. The Zika virus can attack both the optic nerve and the part of the brain that interprets what a person sees: “Are you not seeing well because that part of your brain is not developed, or is it just a problem with the eye?” Dr. Nielsen said.
When problems can’t be precisely identified, successful interventions are harder. If the cochlea is normal, for instance, but the part of the brain that interprets sound or language has deficits, a hearing aid won’t help.
The services and interventions that children need depend on their specific developmental or cognitive deficits, regardless of the cause. But if clinicians know the cause is likely Zika exposure, they also know to look for other deficits.
Children showing likely effects of congenital Zika infection should be further evaluated for other possible birth defects and referred to a developmental specialist, early intervention services, and family support services. Depending on the child, primary care providers might consider referrals to an infectious disease specialist, clinical geneticist, neurologist, or other specialists.
Even with no confirmed infection or visible signs at birth, clinicians should remain vigilant with children who had possible exposure. A recently published study of 120 children conceived during the Zika outbreak in Paraíba, Brazil, assessed as infants and then again at 2 years old, exemplifies why. Researchers identified adverse neurologic outcomes and developmental delays in several children who had no physical evidence of birth defects as newborns, but whose antibody tests showed possible infection.
“In this post-epidemic period, with decreased Zika transmission and less public awareness,” wrote Dr. Mulkey and a colleague, “follow-up of these children is now more important than ever”.
A version of this article originally appeared on Medscape.com.
In 2020, “the virus” has come to mean one thing: SARS-CoV-2. But just a few years ago, Zika had the world's attention, as one news report after another described children with microcephaly born to women who'd been infected while pregnant.
It can be difficult for physicians to determine whether a birth defect is the result of Zika. Most infections have few or no symptoms, and mothers may not know if they’ve been exposed. Karin Nielsen, MD, remembers one child in particular, a 9-month-old boy born with microcephaly whose parents brought the infant to her in 2018 because he had started having seizures.
The child was born in Mexico in 2017, when the Zika virus was still known to be circulating in the Americas, said Dr. Nielsen, a pediatric infectious disease specialist at the University of California, Los Angeles. Brain imaging revealed calcifications and other signs in the boy’s brain that were consistent with exposure. But his mother said she was never sick during pregnancy.
Because Zika is transmitted not just via mosquito and from mother to fetus but also sexually, Dr. Nielsen thinks the mother probably contracted an asymptomatic infection from her husband, who recalled having a rash when she was 4 months pregnant. When they participated in a research study, both parents tested positive for Zika antibodies.
“The child had the classic symptoms of congenital Zika syndrome,” Dr. Nielsen said. “He was 9 months old, he had microcephaly, and he was having mal seizures.”
Researchers have since learned that children with such classic symptoms represent only a small proportion of those affected by prenatal Zika exposure – about 3%-5%. The virus was at its height during the 2016-2016 epidemic and is not currently causing outbreaks. But as researchers have followed cohorts of children exposed to Zika in utero, they have found many subtler effects physicians will need to monitor as the children grow up.
“When we’re seeing hundreds of kids with microcephaly, we had a lot of people infected,” Dr. Nielsen said. “Microcephaly is only the tip of the iceberg.”
Early evidence
Microcephaly may be the most identifiable symptom of fetal Zika infection, but researchers tracking cohorts of exposed children have begun to build a more complete picture of what long-term effects might look like. But hundreds, if not thousands, of children have been exposed to Zika in the womb – it’s not clear how many, Dr. Nielsen said – and many show a range of effects that don’t officially qualify as congenital Zika syndrome.
Current estimates suggest about one third of exposed children have some type of neurologic or neurodevelopmental problem, even though prevalence of visible effects is much lower. Over time, the incidence of these effects has fluctuated; some developmental delays and sensory deficits began manifesting later in childhood whereas others, at least in a few children, have resolved.
“We’re just beginning to have some of the data that we need to think about the full spectrum of outcomes,” said Cindy Moore, MD, chief medical officer in the Division of Congenital and Developmental Disorders in the Centers for Disease Control and Prevention’s National Center on Birth Defects and Developmental Disabilities.
“As we’re learning more and more, we’re learning the spectrum is expanding to less severe forms,” Dr. Moore said. “We do know that with some infections, there are later onset of problems.”
Studies published in 2018 described cohorts of children whose mothers had confirmed or suspected Zika infections during pregnancy in the French Territories of America (Guadalupe, Martinique, and French Guiana) and in Salvador, Brazil. The research provided valuable early data on the incidence of microcephaly and other severe effects in newborns, but noted the need for long-term follow up.
The U.S. Zika Pregnancy and Infant Registry is one of the largest such cohorts. In August 2018, researchers made their first report on data from the registry They looked at 1450 children age 1 or older who had undergone neuroimaging or screenings (developmental, vision, hearing) or both. In 6%, at least one birth defect was linked to Zika, and 9% had at least one neurodevelopmental abnormality.
As these children age past developmental milestones, more effects will likely manifest – even in those children whose appearance and imaging presented as healthy at birth.
Longer-term follow up
Nielsen at UCLA and M. Elisabeth Lopes Moreira, MD, of the Oswaldo Cruz Foundation in Rio de Janeiro, are following a cohort of more than 100 children born in Rio de Janeiro during Brazil’s 2015-2016 epidemic to mothers with symptomatic, PCR-confirmed Zika infections during pregnancy. In December 2018, their team reported that rates of severe neurodevelopmental delay and sensory dysfunction – 14% of 131 children aged 12-18 months – were higher than those found in earlier studies.
In August 2019, the team described neurodevelopmental, vision, and hearing outcomes in 216 Zika-exposed children 2 years after birth. They used the Bayley-III Scales of Infant and Toddler Development to assess cognitive, language and motor skills in 146 of the children. Forty percent of them were below or very below average in development, more than one third (35%) had language delays, 12% percent had hearing loss, and 7% had abnormal eye anatomy, such as underdeveloped retinas.
In two of the eight children in the cohort with microcephaly, the abnormality unexpectedly resolved. Although that finding received a lot of press, Dr. Nielsen pointed out that “not all microcephalies are created equal.”
In one case, a child born small for gestational age had proportional microcephaly: the baby›s head circumference met the criteria for microcephaly, but the infant›s head was proportional to the body so, as the child grew, the apparent microcephaly disappeared.
In the other case, the child was born with craniosynostosis, in which the skull sutures fuse too early – another effect seen with prenatal Zika exposure, Dr. Nielsen said. After corrective surgery, the child’s head circumference no longer met the definition of microcephaly, but the child still had symptoms related to congenital Zika: a developmental delay and calcifications in the brain. Meanwhile, two other children in the Rio cohort developed secondary microcephaly.
In another follow-up study of children up to age 4, Dr. Nielsen and colleagues found that both clinicians and family may think that Zika-exposed infants without microcephaly are developing normally, but that may not be true. Nearly 70% of children without microcephaly had neurologic abnormalities on physical examination, and more than half had failure to thrive because of poor feeding related to neurologic abnormalities.
Initially, some children may be able to mask subtle problems. A study published in January from Sarah B. Mulkey, MD, PhD, of Children’s National Hospital in Washington, DC, and colleagues described neurodevelopmental outcomes in 70 Colombian children up to 18 months old who had been exposed to Zika in utero. The children had a normal head circumference at birth and a normal fetal MRI, but – compared with typically developing peers – their communication, social cognition, and mobility scores on standardized assessments tended to decline as they got older.
“Especially in a very young child, there’s always going to be a possibility that you can compensate for a deficit, and it appears that at least some of these children are doing so,” said William J. Muller, MD, PhD, associate professor of pediatrics at Northwestern University, Chicago. When the children are older, certain behavioral effects will become easier to assess.
“With these children now approaching school age, understanding the full spectrum of neurodevelopmental abnormalities has important public health and educational system implications,” Dr. Muller and Dr. Mulkey wrote in a commentary about one of Dr. Nielsen’s studies.
Researchers face multiple barriers to understanding the long-term effects of fetal Zika infection. Many infants known to have been exposed in utero never received the recommended early assessments and haven’t been followed long-term. Particularly in Brazil, poverty, poor access to healthcare, and overcrowding all complicate surveillance efforts, Dr. Muller said. Stigma related to children’s neurodevelopmental problems also can potentially reduce a mother’s willingness to attend all follow-ups and assessments.
Some children may have been exposed but were never recognized as such, making it difficult for researchers to track their development and assemble a complete picture of prenatal Zika infection outcomes. Asymptomatic infection occurs in about 80% of Zika infections, though it’s not clear if that number holds for infections during pregnancy as well, according to Dr. Muller and Dr. Mulkey. Because nearly all the current research involves children whose mothers had symptomatic infections, the studies’ generalizability may be limited.
Those likely asymptomatic infections are also a major reason none of the cohorts have comparison groups.
“There are literally hundreds of things that can contribute to or cause developmental problems,” said Dr. Moore of the CDC, who noted that it would be nice to have a comparison group so as to know what Zika may not be responsible for. That said, it would be difficult-to-impossible to create a control group with similar geographic and demographic characteristics as the exposed children, a group who researchers can be certain weren’t exposed.
Neurodevelopmental disabilities occur in about 15% of the general population, making it difficult to determine whether Zika causes any or all long-term, less severe developmental findings in exposed children. The difficulty only compounds with time: the older a child is when a developmental problem is recognized, the harder it is to go back and say the problem is a result of something that occurred before birth, Dr. Moore said. “It’s a challenging field to say, this is what caused that outcome.”
Exposed children need continued evaluation
Interpreting the clinical implications of available studies is also challenging. It can be difficult to distinguish between central nervous system damage and peripheral damage, leaving the true etiology of poor vision or hearing elusive. The Zika virus can attack both the optic nerve and the part of the brain that interprets what a person sees: “Are you not seeing well because that part of your brain is not developed, or is it just a problem with the eye?” Dr. Nielsen said.
When problems can’t be precisely identified, successful interventions are harder. If the cochlea is normal, for instance, but the part of the brain that interprets sound or language has deficits, a hearing aid won’t help.
The services and interventions that children need depend on their specific developmental or cognitive deficits, regardless of the cause. But if clinicians know the cause is likely Zika exposure, they also know to look for other deficits.
Children showing likely effects of congenital Zika infection should be further evaluated for other possible birth defects and referred to a developmental specialist, early intervention services, and family support services. Depending on the child, primary care providers might consider referrals to an infectious disease specialist, clinical geneticist, neurologist, or other specialists.
Even with no confirmed infection or visible signs at birth, clinicians should remain vigilant with children who had possible exposure. A recently published study of 120 children conceived during the Zika outbreak in Paraíba, Brazil, assessed as infants and then again at 2 years old, exemplifies why. Researchers identified adverse neurologic outcomes and developmental delays in several children who had no physical evidence of birth defects as newborns, but whose antibody tests showed possible infection.
“In this post-epidemic period, with decreased Zika transmission and less public awareness,” wrote Dr. Mulkey and a colleague, “follow-up of these children is now more important than ever”.
A version of this article originally appeared on Medscape.com.
NYC public hospitals rose to the demands of the COVID-19 crisis
Hospitalists at the center of the storm
New York City Health + Hospitals (NYCH+H), the country’s largest public health care system, encompasses 11 hospitals with 4,354 staffed acute beds during normal times. It serves as the safety net for 1.1 million of the 8.4 million residents of the most populous city in the United States, many of them uninsured, undocumented, covered by Medicaid, or otherwise disadvantaged.
At the very epicenter in the early days of the historic COVID-19 pandemic, NYCH+H transferred patients between its facilities, added medical and ICU beds by the hundreds, mobilized palliative care volunteers, harnessed telemedicine and a clinician hotline, and made other sweeping changes to ensure that the city’s public health system would be able to respond to demand at the peak of the surge. That peak hit in April, when an average of 9,000 new COVID-19 cases were being reported in the city every day.
Through it all, hospitalists have played critical roles in both planning for the system’s response and caring for severely ill COVID-19 patients. Their stories reflect both the unprecedented demands on the system and the dedication of frontline clinicians.
One of those, Carla Saladini-Aponte, MD, who just finished her residency in June 2019, found herself on the firing line in March 2020 as an attending physician at 457-bed NYCH+H/Jacobi Hospital in the Bronx. “I have experienced so much in my first year on the job, dealing with a disease that we’ve never seen before,” she said. “We didn’t grasp the extent of the COVID crisis in the beginning, so we were emotionally unprepared when it first hit.”
Starting on March 30, NYCH+H administration mobilized a centralized incident command structure to coordinate response systemwide to a rapidly changing situation.
Two weeks later Jacobi was a COVID-19 hospital, top to bottom, with its medical ICU beds increased from 12 to more than 100. By mid-April, Dr. Saladini-Aponte’s team, one of 11 medical teams in the hospital, had 26 patients, all of them with COVID-19. There was not a consensus in the early days on how to manage patients with severe respiratory distress. “But by the time the surge came, we had a better understanding of the scope of the situation,” she said.
Learning to be an attending
“They don’t teach you how to be an attending during residency,” Dr. Saladini-Aponte said. “At the beginning I wasn’t such a good teacher. I just wanted to prove myself and stay one step ahead of the residents. But as an academic hospitalist you have to listen to others. I learned to ask questions of the residents every morning, including how they were doing personally.”
Sometimes a visiting consultant would ask on the floor: “‘Where’s your attending?’” not recognizing Dr. Saladini-Aponte, fresh out of residency, filling that role. At times, she felt like a PGY-4 (postgraduate year 4). But she quickly grew into the attending role and was asked to be site coordinator for the mobilization of palliative medicine volunteers at Jacobi.
“We found ourselves having to make tough ethical decisions. Some patients, even if we provided a ventilator and maximum oxygen therapy, would still die. There were difficult discussions when we didn’t know if we had enough dialysis machines, or how to manage other limited resources. The hospital was saying: You decide, if there’s a high degree of certainty about the outcome. But we had never practiced medicine this way before,” she said.
“That’s why our hospital provided daily ethics meetings with our ethics council. There would be eight people sitting 6 feet apart in a conference room, all wearing masks. We’d talk about situations that were giving us trouble. Their role wasn’t to provide answers but to help us see the scope of the situation and the complexities,” she explained.
Dr. Saladini-Aponte said she has had many sleepless nights since the pandemic began. “Sometimes, I would come home from work and lie down on the floor and cry,” she said. “But we had so much support from volunteers helping our little hospitalist service of seven.” It was also important to keep up with the clinical information, and one of her coworkers created “cheat sheets” for the clinicians, regularly updated with the latest essential information on antibiotics, testing, and the like.
“At the peak, I was trying to read everything I could about the virus. I was just pulling myself in too many directions. I asked for help from my boyfriend to remind me not to log onto my computer when I came home from work,” she said. “One of my techniques for preventing burnout was just to avoid social media. I couldn’t deal with what was going on in the news. It just angered me. Even now, seeing people without masks makes me very uncomfortable.”
Organizing the crisis response
As chief value officer for NYCH+H, Hyung (Harry) Cho, MD, FACP, SFHM, typically focuses on issues of patient safety and overuse of medical treatments in the health system. But in the COVID-19 crisis, he found himself at the forefront of organizing its response. “We tried to provide support centrally and to standardize practice in how we test and treat,” he said.
“We were truly at the epicenter of the pandemic,” Dr. Cho said. “All of our hospitals had different experiences, and unique responses. But the system worked well.” Patients were transferred from the more overtaxed hospitals to Bellevue and other NYCH+H hospitals with spare beds. An emergency medical response structure was put in place, and every morning the system’s Tiger Team, with multidisciplinary personnel from administration, operations, logistics, and medical/technical specialists, would gather virtually to discuss needs across the system.
“It was a very open atmosphere and we asked people to report what was happening on the ground,” Dr. Cho said. “We started rapidly reviewing batches of 20 patients at a time for transfer in order to alleviate pressure in the most overtaxed ERs.”
NYCH+H also had to work through concerns about PPE, just like other U.S. hospitals. Treatment guidelines were changing by the day. Medical concerns were relayed at a rapid pace. Another priority was trying to limit unnecessary exposure for staff through a recommendation that only one clinician from a team would go into the room of an infected patient, unless another was absolutely needed.
The reality of public health
NYCH+H was created by the New York State Legislature in 1969 and rebranded in 2015. It includes a low- to no-cost health insurance plan called MetroPlus, along with outpatient centers, comprehensive case management, and social supports in the home.
“What people know about public health systems is that we typically are underresourced. That’s just the reality of public health,” Dr. Cho said. “We help the community, the underserved. The people who truly needed our help are also the ones who have been disproportionately affected by COVID-19. And that is where we really shine as a system.”
Dr. Cho lauded the performance of the health system’s frontline staff. “Watching them come together during the entire pandemic, and do their best every day, was truly inspiring,” he said. “But when they got to the peak, it really took an emotional toll on them.”
NYCH+H’s in-house staff support program, called Helping Healers Heal, was mobilized with specially trained teams at each of its 11 hospitals to provide peer-to-peer support, mental health expertise, and team-debriefing sessions to staff members following traumatic events. Support is provided both over the phone and in person on the floors, Dr. Cho said. “During the surge, everything was happening so quickly, there was no time to take a pause. Now, as we are able to catch our breath, that’s when they most need support.”
The hospitalists at NYCH+H hospitals intended to have goals-of-care conversations with all patients, but everyone was very busy – so having these conversations became harder and harder, Dr. Cho said. Recognizing limited staffing for the quadrupling of patients who needed palliative care at NYCH+H hospitals, he asked the medicine chairs about their palliative care needs and then used social media outreach to ask for help. The message went viral, attracting 413 volunteers from across the country. Sixty-seven telepalliative volunteers were put to work doing goals-of-care conversations remotely with inpatients and their families.1
Expediting transfers
For Ian Fagan, MD, a hospitalist and associate medical director for general internal medicine Inpatient Services at Bellevue Hospital in Manhattan, hospitalist shifts are a normal part of his job. But he had to give them up during the surge to focus on planning, management, and especially scheduling other doctors, with sufficient backups needed to cover last minute changes. Dr. Fagan did that by using the existing pool of hospitalist staff, physicians who were reassigned from other specialties, agency staff, military medical personnel, and volunteer doctors who flew in from around the country to help. He also worked 10- to 12-hour days for 36 consecutive days.
The impact of disparities in access to care in New York City was reflected in the greater demand for care in the hospitals in Brooklyn, Queens, and the Bronx. “With fewer patients and more hospital beds in Manhattan, we had the capacity to share our beds,” Dr. Fagan said. “It was so amazing to me how quickly we could move patients from one hospital to another. We started accepting up to 40 transfers a day. But hey, we were still really busy.”
Bellevue is the nation’s oldest public hospital. “We care for the homeless, for immigrants, and we don’t ask questions. That’s our mission. I’m so proud to work here, and so grateful,” Dr. Fagan said. “If someone is undocumented or without insurance, I will give them exactly the same care. We stepped up in a big way to care for people of New York, but we’ve always been there for them – and we were there for them during the COVID surge.”
The hospitals in the system also worked together in ways Dr. Fagan had never seen. “It helped to have a central command structure with a bird’s eye view from above the level of individual hospitals, to organize and see which hospitals could step up. It’s good to have the data to put it in perspective,” he said. The system also utilized a temporary low-acuity medical center set up by NYCH+H on Roosevelt Island, as well as field hospitals organized at the Jacob K. Javits Convention Center and the USTA Billie Jean King National Tennis Center.
“At Bellevue we tried to stay ready, with the ability to turn former hospital units that were being used as offices back to beds. We always had three units lined up that were fully ready to convert. For example, I was medical director of the preop clinic and one day they gave us 24 hours to pack everything and move out. Three days later, it was a 24-bed unit. We also built a more robust rapid response and code team,” he said.
“It was hard for me not to take hospitalist shifts, because my identity is being a doctor. I eventually came to terms with the importance of the role that I was doing every day. I felt I could protect my colleagues, and if they were having an emotional day, to give them the opportunity to talk to someone. I also did the onboarding, one-on-one, of the new doctors.”
As the crisis in New York City has ebbed, Dr. Fagan was recently able to again take a week of clinical service. “The first day back on the floor I felt that I had forgotten everything. But by the end of the day, I thought, ‘Okay, I do know how to do this, after all.’ Census is down here. It’s quiet. That’s good. We need it now,” he said.
“I think the hardest moment for me was when the head nurse on our trauma unit, Ernesto DeLeon, known to everybody here, died of COVID in our ICU in April,” Dr. Fagan said. When Mr. DeLeon died, 100 hospital personnel gathered in the halls outside the room to pay their respects. “There had been a palpable fear in our lives – and this showed us that the fear was real. Ernesto was the first person I knew well who died, who acquired COVID at work doing what we’re all doing. We haven’t lost any doctors yet, but when this nurse died, we allowed ourselves to realize that this is personal. In that moment, we needed to allow ourselves to be human.”
Joan Curcio, MD, associate director of medicine at Elmhurst Hospital, said Elmhurst was where the story started for New York City and for NYCH+H. “I trained here and have spent my entire career at this hospital. It came to feel like what a battleground must be like, with things coming at you from every direction,” she said. “It was overwhelming in ways I could not have foreseen. I had seen videos from Italy [an early COVID-19 epicenter], but until it happened here, it was just hard to process.”
Things started slowly, with a few patients with severe acute respiratory distress syndrome and a 5- to 7-day turnaround to get results of their viral infection tests. “By week 2, a greater number of patients from our clinics and testing sites were filtering through the emergency department. Then hundreds.”
The normal occupancy rate for the department of medicine at Elmhurst is 110-115%, which typically means full beds plus patients in the emergency department. “We started to grow to 160, then 180, and then a peak of 250% of occupancy. We took over a rehab surgery floor, then a 35-bed surgery and hospice floor, which went to full capacity just like that,” she said. The number of non–critical care service teams increased to 20, working with redeployed staff, volunteers, military, and agency personnel, while ICU beds increased from 20 to 105.
“We were dealing with a much higher acuity level and enduring emotional turmoil with families, trying to carve out time to call them after our shift was over,” Dr. Curcio explained. Elmhurst developed a call-in hotline and a daily call-out service for families. Technology was mobilized to provide video visits and new systems were designed for isolation and for PPE distribution and use.
“I just felt that I couldn’t get everything done. I felt continually overwhelmed, and it didn’t matter how much time I took. I never felt I was able to give enough to anybody in any area, which was hard to take,” Dr. Curcio said. “But I still felt a sense of purpose and that I was making a difference – thanks to lots of support from the central office.”
Patient volume at Elmhurst is now down, lower than Dr. Curcio has ever seen it. “One of the main issues right now, moving forward, is ‘how do we function in a post-crisis mode?’” she said. The process of transitioning back to non-COVID-19 care will be complex. “When we clear a floor and clean it to go back to being a cold [COVID-19-negative] unit, it’s a whole different level of cleaning that takes 7 days.”
One moment that was particularly jarring for Dr. Curcio occurred while she was giving a tour of the hospital to visiting military medical personnel. “We went into the emergency department and I turned around and looked into a shower room, which was full of body bags. They were all full.”
But the experience has also been inspiring. “People gave their all without complaint. We hospitalists, and all those recruited to act as hospitalists, essentially took responsibility for the COVID response,” she said. “This was, hopefully, the experience of a lifetime as a medical professional. I wouldn’t want to ever experience something as daunting as this again.”
Reference
1. Israilov S et al. National outreach of telepalliative medicine volunteers for a New York City safety net system COVID-19 pandemic response. J Pain Symptom Manag. 2020 May 29. doi: 10.1016/j.jpainsymman.2020.05.026.
Hospitalists at the center of the storm
Hospitalists at the center of the storm
New York City Health + Hospitals (NYCH+H), the country’s largest public health care system, encompasses 11 hospitals with 4,354 staffed acute beds during normal times. It serves as the safety net for 1.1 million of the 8.4 million residents of the most populous city in the United States, many of them uninsured, undocumented, covered by Medicaid, or otherwise disadvantaged.
At the very epicenter in the early days of the historic COVID-19 pandemic, NYCH+H transferred patients between its facilities, added medical and ICU beds by the hundreds, mobilized palliative care volunteers, harnessed telemedicine and a clinician hotline, and made other sweeping changes to ensure that the city’s public health system would be able to respond to demand at the peak of the surge. That peak hit in April, when an average of 9,000 new COVID-19 cases were being reported in the city every day.
Through it all, hospitalists have played critical roles in both planning for the system’s response and caring for severely ill COVID-19 patients. Their stories reflect both the unprecedented demands on the system and the dedication of frontline clinicians.
One of those, Carla Saladini-Aponte, MD, who just finished her residency in June 2019, found herself on the firing line in March 2020 as an attending physician at 457-bed NYCH+H/Jacobi Hospital in the Bronx. “I have experienced so much in my first year on the job, dealing with a disease that we’ve never seen before,” she said. “We didn’t grasp the extent of the COVID crisis in the beginning, so we were emotionally unprepared when it first hit.”
Starting on March 30, NYCH+H administration mobilized a centralized incident command structure to coordinate response systemwide to a rapidly changing situation.
Two weeks later Jacobi was a COVID-19 hospital, top to bottom, with its medical ICU beds increased from 12 to more than 100. By mid-April, Dr. Saladini-Aponte’s team, one of 11 medical teams in the hospital, had 26 patients, all of them with COVID-19. There was not a consensus in the early days on how to manage patients with severe respiratory distress. “But by the time the surge came, we had a better understanding of the scope of the situation,” she said.
Learning to be an attending
“They don’t teach you how to be an attending during residency,” Dr. Saladini-Aponte said. “At the beginning I wasn’t such a good teacher. I just wanted to prove myself and stay one step ahead of the residents. But as an academic hospitalist you have to listen to others. I learned to ask questions of the residents every morning, including how they were doing personally.”
Sometimes a visiting consultant would ask on the floor: “‘Where’s your attending?’” not recognizing Dr. Saladini-Aponte, fresh out of residency, filling that role. At times, she felt like a PGY-4 (postgraduate year 4). But she quickly grew into the attending role and was asked to be site coordinator for the mobilization of palliative medicine volunteers at Jacobi.
“We found ourselves having to make tough ethical decisions. Some patients, even if we provided a ventilator and maximum oxygen therapy, would still die. There were difficult discussions when we didn’t know if we had enough dialysis machines, or how to manage other limited resources. The hospital was saying: You decide, if there’s a high degree of certainty about the outcome. But we had never practiced medicine this way before,” she said.
“That’s why our hospital provided daily ethics meetings with our ethics council. There would be eight people sitting 6 feet apart in a conference room, all wearing masks. We’d talk about situations that were giving us trouble. Their role wasn’t to provide answers but to help us see the scope of the situation and the complexities,” she explained.
Dr. Saladini-Aponte said she has had many sleepless nights since the pandemic began. “Sometimes, I would come home from work and lie down on the floor and cry,” she said. “But we had so much support from volunteers helping our little hospitalist service of seven.” It was also important to keep up with the clinical information, and one of her coworkers created “cheat sheets” for the clinicians, regularly updated with the latest essential information on antibiotics, testing, and the like.
“At the peak, I was trying to read everything I could about the virus. I was just pulling myself in too many directions. I asked for help from my boyfriend to remind me not to log onto my computer when I came home from work,” she said. “One of my techniques for preventing burnout was just to avoid social media. I couldn’t deal with what was going on in the news. It just angered me. Even now, seeing people without masks makes me very uncomfortable.”
Organizing the crisis response
As chief value officer for NYCH+H, Hyung (Harry) Cho, MD, FACP, SFHM, typically focuses on issues of patient safety and overuse of medical treatments in the health system. But in the COVID-19 crisis, he found himself at the forefront of organizing its response. “We tried to provide support centrally and to standardize practice in how we test and treat,” he said.
“We were truly at the epicenter of the pandemic,” Dr. Cho said. “All of our hospitals had different experiences, and unique responses. But the system worked well.” Patients were transferred from the more overtaxed hospitals to Bellevue and other NYCH+H hospitals with spare beds. An emergency medical response structure was put in place, and every morning the system’s Tiger Team, with multidisciplinary personnel from administration, operations, logistics, and medical/technical specialists, would gather virtually to discuss needs across the system.
“It was a very open atmosphere and we asked people to report what was happening on the ground,” Dr. Cho said. “We started rapidly reviewing batches of 20 patients at a time for transfer in order to alleviate pressure in the most overtaxed ERs.”
NYCH+H also had to work through concerns about PPE, just like other U.S. hospitals. Treatment guidelines were changing by the day. Medical concerns were relayed at a rapid pace. Another priority was trying to limit unnecessary exposure for staff through a recommendation that only one clinician from a team would go into the room of an infected patient, unless another was absolutely needed.
The reality of public health
NYCH+H was created by the New York State Legislature in 1969 and rebranded in 2015. It includes a low- to no-cost health insurance plan called MetroPlus, along with outpatient centers, comprehensive case management, and social supports in the home.
“What people know about public health systems is that we typically are underresourced. That’s just the reality of public health,” Dr. Cho said. “We help the community, the underserved. The people who truly needed our help are also the ones who have been disproportionately affected by COVID-19. And that is where we really shine as a system.”
Dr. Cho lauded the performance of the health system’s frontline staff. “Watching them come together during the entire pandemic, and do their best every day, was truly inspiring,” he said. “But when they got to the peak, it really took an emotional toll on them.”
NYCH+H’s in-house staff support program, called Helping Healers Heal, was mobilized with specially trained teams at each of its 11 hospitals to provide peer-to-peer support, mental health expertise, and team-debriefing sessions to staff members following traumatic events. Support is provided both over the phone and in person on the floors, Dr. Cho said. “During the surge, everything was happening so quickly, there was no time to take a pause. Now, as we are able to catch our breath, that’s when they most need support.”
The hospitalists at NYCH+H hospitals intended to have goals-of-care conversations with all patients, but everyone was very busy – so having these conversations became harder and harder, Dr. Cho said. Recognizing limited staffing for the quadrupling of patients who needed palliative care at NYCH+H hospitals, he asked the medicine chairs about their palliative care needs and then used social media outreach to ask for help. The message went viral, attracting 413 volunteers from across the country. Sixty-seven telepalliative volunteers were put to work doing goals-of-care conversations remotely with inpatients and their families.1
Expediting transfers
For Ian Fagan, MD, a hospitalist and associate medical director for general internal medicine Inpatient Services at Bellevue Hospital in Manhattan, hospitalist shifts are a normal part of his job. But he had to give them up during the surge to focus on planning, management, and especially scheduling other doctors, with sufficient backups needed to cover last minute changes. Dr. Fagan did that by using the existing pool of hospitalist staff, physicians who were reassigned from other specialties, agency staff, military medical personnel, and volunteer doctors who flew in from around the country to help. He also worked 10- to 12-hour days for 36 consecutive days.
The impact of disparities in access to care in New York City was reflected in the greater demand for care in the hospitals in Brooklyn, Queens, and the Bronx. “With fewer patients and more hospital beds in Manhattan, we had the capacity to share our beds,” Dr. Fagan said. “It was so amazing to me how quickly we could move patients from one hospital to another. We started accepting up to 40 transfers a day. But hey, we were still really busy.”
Bellevue is the nation’s oldest public hospital. “We care for the homeless, for immigrants, and we don’t ask questions. That’s our mission. I’m so proud to work here, and so grateful,” Dr. Fagan said. “If someone is undocumented or without insurance, I will give them exactly the same care. We stepped up in a big way to care for people of New York, but we’ve always been there for them – and we were there for them during the COVID surge.”
The hospitals in the system also worked together in ways Dr. Fagan had never seen. “It helped to have a central command structure with a bird’s eye view from above the level of individual hospitals, to organize and see which hospitals could step up. It’s good to have the data to put it in perspective,” he said. The system also utilized a temporary low-acuity medical center set up by NYCH+H on Roosevelt Island, as well as field hospitals organized at the Jacob K. Javits Convention Center and the USTA Billie Jean King National Tennis Center.
“At Bellevue we tried to stay ready, with the ability to turn former hospital units that were being used as offices back to beds. We always had three units lined up that were fully ready to convert. For example, I was medical director of the preop clinic and one day they gave us 24 hours to pack everything and move out. Three days later, it was a 24-bed unit. We also built a more robust rapid response and code team,” he said.
“It was hard for me not to take hospitalist shifts, because my identity is being a doctor. I eventually came to terms with the importance of the role that I was doing every day. I felt I could protect my colleagues, and if they were having an emotional day, to give them the opportunity to talk to someone. I also did the onboarding, one-on-one, of the new doctors.”
As the crisis in New York City has ebbed, Dr. Fagan was recently able to again take a week of clinical service. “The first day back on the floor I felt that I had forgotten everything. But by the end of the day, I thought, ‘Okay, I do know how to do this, after all.’ Census is down here. It’s quiet. That’s good. We need it now,” he said.
“I think the hardest moment for me was when the head nurse on our trauma unit, Ernesto DeLeon, known to everybody here, died of COVID in our ICU in April,” Dr. Fagan said. When Mr. DeLeon died, 100 hospital personnel gathered in the halls outside the room to pay their respects. “There had been a palpable fear in our lives – and this showed us that the fear was real. Ernesto was the first person I knew well who died, who acquired COVID at work doing what we’re all doing. We haven’t lost any doctors yet, but when this nurse died, we allowed ourselves to realize that this is personal. In that moment, we needed to allow ourselves to be human.”
Joan Curcio, MD, associate director of medicine at Elmhurst Hospital, said Elmhurst was where the story started for New York City and for NYCH+H. “I trained here and have spent my entire career at this hospital. It came to feel like what a battleground must be like, with things coming at you from every direction,” she said. “It was overwhelming in ways I could not have foreseen. I had seen videos from Italy [an early COVID-19 epicenter], but until it happened here, it was just hard to process.”
Things started slowly, with a few patients with severe acute respiratory distress syndrome and a 5- to 7-day turnaround to get results of their viral infection tests. “By week 2, a greater number of patients from our clinics and testing sites were filtering through the emergency department. Then hundreds.”
The normal occupancy rate for the department of medicine at Elmhurst is 110-115%, which typically means full beds plus patients in the emergency department. “We started to grow to 160, then 180, and then a peak of 250% of occupancy. We took over a rehab surgery floor, then a 35-bed surgery and hospice floor, which went to full capacity just like that,” she said. The number of non–critical care service teams increased to 20, working with redeployed staff, volunteers, military, and agency personnel, while ICU beds increased from 20 to 105.
“We were dealing with a much higher acuity level and enduring emotional turmoil with families, trying to carve out time to call them after our shift was over,” Dr. Curcio explained. Elmhurst developed a call-in hotline and a daily call-out service for families. Technology was mobilized to provide video visits and new systems were designed for isolation and for PPE distribution and use.
“I just felt that I couldn’t get everything done. I felt continually overwhelmed, and it didn’t matter how much time I took. I never felt I was able to give enough to anybody in any area, which was hard to take,” Dr. Curcio said. “But I still felt a sense of purpose and that I was making a difference – thanks to lots of support from the central office.”
Patient volume at Elmhurst is now down, lower than Dr. Curcio has ever seen it. “One of the main issues right now, moving forward, is ‘how do we function in a post-crisis mode?’” she said. The process of transitioning back to non-COVID-19 care will be complex. “When we clear a floor and clean it to go back to being a cold [COVID-19-negative] unit, it’s a whole different level of cleaning that takes 7 days.”
One moment that was particularly jarring for Dr. Curcio occurred while she was giving a tour of the hospital to visiting military medical personnel. “We went into the emergency department and I turned around and looked into a shower room, which was full of body bags. They were all full.”
But the experience has also been inspiring. “People gave their all without complaint. We hospitalists, and all those recruited to act as hospitalists, essentially took responsibility for the COVID response,” she said. “This was, hopefully, the experience of a lifetime as a medical professional. I wouldn’t want to ever experience something as daunting as this again.”
Reference
1. Israilov S et al. National outreach of telepalliative medicine volunteers for a New York City safety net system COVID-19 pandemic response. J Pain Symptom Manag. 2020 May 29. doi: 10.1016/j.jpainsymman.2020.05.026.
New York City Health + Hospitals (NYCH+H), the country’s largest public health care system, encompasses 11 hospitals with 4,354 staffed acute beds during normal times. It serves as the safety net for 1.1 million of the 8.4 million residents of the most populous city in the United States, many of them uninsured, undocumented, covered by Medicaid, or otherwise disadvantaged.
At the very epicenter in the early days of the historic COVID-19 pandemic, NYCH+H transferred patients between its facilities, added medical and ICU beds by the hundreds, mobilized palliative care volunteers, harnessed telemedicine and a clinician hotline, and made other sweeping changes to ensure that the city’s public health system would be able to respond to demand at the peak of the surge. That peak hit in April, when an average of 9,000 new COVID-19 cases were being reported in the city every day.
Through it all, hospitalists have played critical roles in both planning for the system’s response and caring for severely ill COVID-19 patients. Their stories reflect both the unprecedented demands on the system and the dedication of frontline clinicians.
One of those, Carla Saladini-Aponte, MD, who just finished her residency in June 2019, found herself on the firing line in March 2020 as an attending physician at 457-bed NYCH+H/Jacobi Hospital in the Bronx. “I have experienced so much in my first year on the job, dealing with a disease that we’ve never seen before,” she said. “We didn’t grasp the extent of the COVID crisis in the beginning, so we were emotionally unprepared when it first hit.”
Starting on March 30, NYCH+H administration mobilized a centralized incident command structure to coordinate response systemwide to a rapidly changing situation.
Two weeks later Jacobi was a COVID-19 hospital, top to bottom, with its medical ICU beds increased from 12 to more than 100. By mid-April, Dr. Saladini-Aponte’s team, one of 11 medical teams in the hospital, had 26 patients, all of them with COVID-19. There was not a consensus in the early days on how to manage patients with severe respiratory distress. “But by the time the surge came, we had a better understanding of the scope of the situation,” she said.
Learning to be an attending
“They don’t teach you how to be an attending during residency,” Dr. Saladini-Aponte said. “At the beginning I wasn’t such a good teacher. I just wanted to prove myself and stay one step ahead of the residents. But as an academic hospitalist you have to listen to others. I learned to ask questions of the residents every morning, including how they were doing personally.”
Sometimes a visiting consultant would ask on the floor: “‘Where’s your attending?’” not recognizing Dr. Saladini-Aponte, fresh out of residency, filling that role. At times, she felt like a PGY-4 (postgraduate year 4). But she quickly grew into the attending role and was asked to be site coordinator for the mobilization of palliative medicine volunteers at Jacobi.
“We found ourselves having to make tough ethical decisions. Some patients, even if we provided a ventilator and maximum oxygen therapy, would still die. There were difficult discussions when we didn’t know if we had enough dialysis machines, or how to manage other limited resources. The hospital was saying: You decide, if there’s a high degree of certainty about the outcome. But we had never practiced medicine this way before,” she said.
“That’s why our hospital provided daily ethics meetings with our ethics council. There would be eight people sitting 6 feet apart in a conference room, all wearing masks. We’d talk about situations that were giving us trouble. Their role wasn’t to provide answers but to help us see the scope of the situation and the complexities,” she explained.
Dr. Saladini-Aponte said she has had many sleepless nights since the pandemic began. “Sometimes, I would come home from work and lie down on the floor and cry,” she said. “But we had so much support from volunteers helping our little hospitalist service of seven.” It was also important to keep up with the clinical information, and one of her coworkers created “cheat sheets” for the clinicians, regularly updated with the latest essential information on antibiotics, testing, and the like.
“At the peak, I was trying to read everything I could about the virus. I was just pulling myself in too many directions. I asked for help from my boyfriend to remind me not to log onto my computer when I came home from work,” she said. “One of my techniques for preventing burnout was just to avoid social media. I couldn’t deal with what was going on in the news. It just angered me. Even now, seeing people without masks makes me very uncomfortable.”
Organizing the crisis response
As chief value officer for NYCH+H, Hyung (Harry) Cho, MD, FACP, SFHM, typically focuses on issues of patient safety and overuse of medical treatments in the health system. But in the COVID-19 crisis, he found himself at the forefront of organizing its response. “We tried to provide support centrally and to standardize practice in how we test and treat,” he said.
“We were truly at the epicenter of the pandemic,” Dr. Cho said. “All of our hospitals had different experiences, and unique responses. But the system worked well.” Patients were transferred from the more overtaxed hospitals to Bellevue and other NYCH+H hospitals with spare beds. An emergency medical response structure was put in place, and every morning the system’s Tiger Team, with multidisciplinary personnel from administration, operations, logistics, and medical/technical specialists, would gather virtually to discuss needs across the system.
“It was a very open atmosphere and we asked people to report what was happening on the ground,” Dr. Cho said. “We started rapidly reviewing batches of 20 patients at a time for transfer in order to alleviate pressure in the most overtaxed ERs.”
NYCH+H also had to work through concerns about PPE, just like other U.S. hospitals. Treatment guidelines were changing by the day. Medical concerns were relayed at a rapid pace. Another priority was trying to limit unnecessary exposure for staff through a recommendation that only one clinician from a team would go into the room of an infected patient, unless another was absolutely needed.
The reality of public health
NYCH+H was created by the New York State Legislature in 1969 and rebranded in 2015. It includes a low- to no-cost health insurance plan called MetroPlus, along with outpatient centers, comprehensive case management, and social supports in the home.
“What people know about public health systems is that we typically are underresourced. That’s just the reality of public health,” Dr. Cho said. “We help the community, the underserved. The people who truly needed our help are also the ones who have been disproportionately affected by COVID-19. And that is where we really shine as a system.”
Dr. Cho lauded the performance of the health system’s frontline staff. “Watching them come together during the entire pandemic, and do their best every day, was truly inspiring,” he said. “But when they got to the peak, it really took an emotional toll on them.”
NYCH+H’s in-house staff support program, called Helping Healers Heal, was mobilized with specially trained teams at each of its 11 hospitals to provide peer-to-peer support, mental health expertise, and team-debriefing sessions to staff members following traumatic events. Support is provided both over the phone and in person on the floors, Dr. Cho said. “During the surge, everything was happening so quickly, there was no time to take a pause. Now, as we are able to catch our breath, that’s when they most need support.”
The hospitalists at NYCH+H hospitals intended to have goals-of-care conversations with all patients, but everyone was very busy – so having these conversations became harder and harder, Dr. Cho said. Recognizing limited staffing for the quadrupling of patients who needed palliative care at NYCH+H hospitals, he asked the medicine chairs about their palliative care needs and then used social media outreach to ask for help. The message went viral, attracting 413 volunteers from across the country. Sixty-seven telepalliative volunteers were put to work doing goals-of-care conversations remotely with inpatients and their families.1
Expediting transfers
For Ian Fagan, MD, a hospitalist and associate medical director for general internal medicine Inpatient Services at Bellevue Hospital in Manhattan, hospitalist shifts are a normal part of his job. But he had to give them up during the surge to focus on planning, management, and especially scheduling other doctors, with sufficient backups needed to cover last minute changes. Dr. Fagan did that by using the existing pool of hospitalist staff, physicians who were reassigned from other specialties, agency staff, military medical personnel, and volunteer doctors who flew in from around the country to help. He also worked 10- to 12-hour days for 36 consecutive days.
The impact of disparities in access to care in New York City was reflected in the greater demand for care in the hospitals in Brooklyn, Queens, and the Bronx. “With fewer patients and more hospital beds in Manhattan, we had the capacity to share our beds,” Dr. Fagan said. “It was so amazing to me how quickly we could move patients from one hospital to another. We started accepting up to 40 transfers a day. But hey, we were still really busy.”
Bellevue is the nation’s oldest public hospital. “We care for the homeless, for immigrants, and we don’t ask questions. That’s our mission. I’m so proud to work here, and so grateful,” Dr. Fagan said. “If someone is undocumented or without insurance, I will give them exactly the same care. We stepped up in a big way to care for people of New York, but we’ve always been there for them – and we were there for them during the COVID surge.”
The hospitals in the system also worked together in ways Dr. Fagan had never seen. “It helped to have a central command structure with a bird’s eye view from above the level of individual hospitals, to organize and see which hospitals could step up. It’s good to have the data to put it in perspective,” he said. The system also utilized a temporary low-acuity medical center set up by NYCH+H on Roosevelt Island, as well as field hospitals organized at the Jacob K. Javits Convention Center and the USTA Billie Jean King National Tennis Center.
“At Bellevue we tried to stay ready, with the ability to turn former hospital units that were being used as offices back to beds. We always had three units lined up that were fully ready to convert. For example, I was medical director of the preop clinic and one day they gave us 24 hours to pack everything and move out. Three days later, it was a 24-bed unit. We also built a more robust rapid response and code team,” he said.
“It was hard for me not to take hospitalist shifts, because my identity is being a doctor. I eventually came to terms with the importance of the role that I was doing every day. I felt I could protect my colleagues, and if they were having an emotional day, to give them the opportunity to talk to someone. I also did the onboarding, one-on-one, of the new doctors.”
As the crisis in New York City has ebbed, Dr. Fagan was recently able to again take a week of clinical service. “The first day back on the floor I felt that I had forgotten everything. But by the end of the day, I thought, ‘Okay, I do know how to do this, after all.’ Census is down here. It’s quiet. That’s good. We need it now,” he said.
“I think the hardest moment for me was when the head nurse on our trauma unit, Ernesto DeLeon, known to everybody here, died of COVID in our ICU in April,” Dr. Fagan said. When Mr. DeLeon died, 100 hospital personnel gathered in the halls outside the room to pay their respects. “There had been a palpable fear in our lives – and this showed us that the fear was real. Ernesto was the first person I knew well who died, who acquired COVID at work doing what we’re all doing. We haven’t lost any doctors yet, but when this nurse died, we allowed ourselves to realize that this is personal. In that moment, we needed to allow ourselves to be human.”
Joan Curcio, MD, associate director of medicine at Elmhurst Hospital, said Elmhurst was where the story started for New York City and for NYCH+H. “I trained here and have spent my entire career at this hospital. It came to feel like what a battleground must be like, with things coming at you from every direction,” she said. “It was overwhelming in ways I could not have foreseen. I had seen videos from Italy [an early COVID-19 epicenter], but until it happened here, it was just hard to process.”
Things started slowly, with a few patients with severe acute respiratory distress syndrome and a 5- to 7-day turnaround to get results of their viral infection tests. “By week 2, a greater number of patients from our clinics and testing sites were filtering through the emergency department. Then hundreds.”
The normal occupancy rate for the department of medicine at Elmhurst is 110-115%, which typically means full beds plus patients in the emergency department. “We started to grow to 160, then 180, and then a peak of 250% of occupancy. We took over a rehab surgery floor, then a 35-bed surgery and hospice floor, which went to full capacity just like that,” she said. The number of non–critical care service teams increased to 20, working with redeployed staff, volunteers, military, and agency personnel, while ICU beds increased from 20 to 105.
“We were dealing with a much higher acuity level and enduring emotional turmoil with families, trying to carve out time to call them after our shift was over,” Dr. Curcio explained. Elmhurst developed a call-in hotline and a daily call-out service for families. Technology was mobilized to provide video visits and new systems were designed for isolation and for PPE distribution and use.
“I just felt that I couldn’t get everything done. I felt continually overwhelmed, and it didn’t matter how much time I took. I never felt I was able to give enough to anybody in any area, which was hard to take,” Dr. Curcio said. “But I still felt a sense of purpose and that I was making a difference – thanks to lots of support from the central office.”
Patient volume at Elmhurst is now down, lower than Dr. Curcio has ever seen it. “One of the main issues right now, moving forward, is ‘how do we function in a post-crisis mode?’” she said. The process of transitioning back to non-COVID-19 care will be complex. “When we clear a floor and clean it to go back to being a cold [COVID-19-negative] unit, it’s a whole different level of cleaning that takes 7 days.”
One moment that was particularly jarring for Dr. Curcio occurred while she was giving a tour of the hospital to visiting military medical personnel. “We went into the emergency department and I turned around and looked into a shower room, which was full of body bags. They were all full.”
But the experience has also been inspiring. “People gave their all without complaint. We hospitalists, and all those recruited to act as hospitalists, essentially took responsibility for the COVID response,” she said. “This was, hopefully, the experience of a lifetime as a medical professional. I wouldn’t want to ever experience something as daunting as this again.”
Reference
1. Israilov S et al. National outreach of telepalliative medicine volunteers for a New York City safety net system COVID-19 pandemic response. J Pain Symptom Manag. 2020 May 29. doi: 10.1016/j.jpainsymman.2020.05.026.
COVID-19 at home: What does optimal care look like?
Marilyn Stebbins, PharmD, fell ill at the end of February 2020. Initially diagnosed with multifocal pneumonia and treated with antibiotics, she later developed severe gastrointestinal symptoms, fatigue, and shortness of breath. She was hospitalized in early March and was diagnosed with COVID-19.
It was still early in the pandemic, and testing was not available for her husband. After she was discharged, her husband isolated himself as much as possible. But that limited the amount of care he could offer.
“When I came home after 8 days in the ICU, I felt completely alone and terrified of not being able to care for myself and not knowing how much care my husband could provide,” said Dr. Stebbins, professor of clinical pharmacy at the University of California, San Francisco.
“I can’t even imagine what it would have been like if I had been home alone without my husband in the house,” she said. “I think about the people who died at home and understand how that might happen.”
Dr. Stebbins is one of tens of thousands of people who, whether hospitalized and discharged or never admitted for inpatient care, needed to find ways to convalesce at home. Data from the Centers for Medicare & Medicaid Services show that, of 326,674 beneficiaries who tested positive for COVID-19 between May 16 and June 11, 2020, 109,607 were hospitalized, suggesting that two-thirds were outpatients.
Most attention has focused on the sickest patients, leaving less severe cases to fall through the cracks. Despite fever, cough, difficulty breathing, and a surfeit of other symptoms, there are few available resources and all too little support to help patients navigate the physical and emotional struggles of contending with COVID-19 at home.
No ‘cookie-cutter’ approach
The speed with which the pandemic progressed caught public health systems off guard, but now, “it is essential to put into place the infrastructure to care for the physical and mental health needs of patients at home because most are in the community and many, if not most, still aren’t receiving sufficient support at home,” said Dr. Stebbins.
said Gary LeRoy, MD, a family physician in Dayton, Ohio. He emphasized that there is “no cookie-cutter formula” for home care, because every patient’s situation is different.
“I begin by having a detailed conversation with each patient to ascertain whether their home environment is safe and to paint a picture of their circumstances,” Dr. LeRoy, who is the president of the American Academy of Family Physicians, said in an interview.
Dr. LeRoy suggested questions that constitute “not just a ‘medical’ checklist but a ‘whole life’ checklist.”
- Do you have access to food, water, medications, sanitation/cleaning supplies, a thermometer, and other necessities? If not, who might assist in providing those?
- Do you need help with activities of daily living and self-care?
- Who else lives in your household? Do they have signs and symptoms of the virus? Have they been tested?
- Do you have enough physical space between you and other household members?
- Do you have children? How are they being cared for?
- What type of work do you do? What are the implications for your employment if you are unable to work for an extended period?
- Do you have an emotional, social, and spiritual support system (e.g., family, friends, community, church)?
- Do you have concerns I haven’t mentioned?
Patients’ responses will inform the management plan and determine what medical and social resources are needed, he said.
Daily check-in
Dr. Stebbins said the nurse case manager from her insurance company called her daily after she came home from the hospital. She was told that a public health nurse would also call, but no one from the health department called for days – a situation she hopes has improved.
One way or another, she said, “health care providers [or their staff] should check in with patients daily, either telephonically or via video.” She noted that video is superior, because “someone who isn’t a family member needs to put eyes on a patient and might be able to detect warning signs that a family member without healthcare training might not notice.”
Dr. LeRoy, who is also an associate professor of medicine at Wright State University, Dayton, Ohio, said that, given his time constraints, a nurse or medical assistant in his practice conducts the daily check-ins and notifies him if the patient has fever or other symptoms.
“Under ordinary circumstances, when a patient comes to see me for some type of medical condition, I get to meet the patient, consider what might be going on, then order a test, wait for the results, and suggest a treatment plan. But these are anything but ordinary circumstances,” said Matthew Exline, MD, a pulmonary and critical care specialist at the Ohio State University Wexner Medical Center, Columbus.
“That traditional structure broke down with COVID-19, when we may have test results without even seeing the patient. And without this interaction, it is harder to know as a physician what course of action to take,” he said in an interview.
Once a diagnosis has been made, the physician has at least some data to help guide next steps, even if there has been no prior meeting with the patient.
For example, a positive test raises a host of issues, not the least of which is the risk of spreading the infection to other household members and questions about whether to go the hospital. Moreover, for patients, positive tests can have serious ramifications.
“Severe shortness of breath at rest is not typical of the flu, nor is loss of taste or smell,” said Dr. Exline. Practitioners must educate patients and families about specific symptoms of COVID-19, including shortness of breath, loss of taste or smell, and gastrointestinal or neurologic symptoms, and when to seek emergency care.
Dr. LeRoy suggests buying a pulse oximeter to gauge blood oxygen levels and pulse rate. Together with a thermometer, a portable blood pressure monitor, and, if indicated, a blood glucose monitor, these devices provide a comprehensive and accurate assessment of vital signs.
Dr. LeRoy also educates patients and their families about when to seek medical attention.
Dr. Stebbins takes a similar approach. “Family members are part of, not apart from, the care of patients with COVID-19, and it’s our responsibility as healthcare providers to consider them in the patient’s care plan.”
Keeping family safe
Beyond care, family members need a plan to keep themselves healthy, too.
“A patient with COVID-19 at home should self-quarantine as much as possible to keep other family members safe, if they continue to live in the same house,” Dr. Exline said.
Ideally, uninfected family members should stay with relatives or friends. When that’s not possible, everyone in the household should wear a mask, be vigilant about hand washing, and wipe down all surfaces – including doorknobs, light switches, faucet handles, cellphones, and utensils – regularly with bleach or an alcohol solution.
Caregivers should also minimize the amount of time they are exposed to the patient.
“Set food, water, and medication on the night table and leave the room rather than spending hours at the bedside, since limiting exposure to viral load reduces the chances of contagion,” said Dr. Exline.
The Centers for Disease Control and Prevention offers guidance for household members caring for COVID-19 patients at home. It provides tips on how to help patients follow the doctor’s instructions and ways to ensure adequate hydration and rest, among others.
Patients with COVID-19 who live alone face more formidable challenges.
Dr. LeRoy says physicians can help patients by educating themselves about available social services in their community so they can provide appropriate referrals and connections. Such initiatives can include meal programs, friendly visit and financial assistance programs, as well as childcare and home health agencies.
He noted that Aunt Bertha, a social care network, provides a guide to social services throughout the United States. Additional resources are available on USA.gov.
Comfort and support
Patients with COVID-19 need to be as comfortable and as supported as possible, both physically and emotionally.
“While I was sick, my dogs curled up next to me and didn’t leave my side, and they were my saving grace. There’s not enough to be said about emotional support,” Dr. Stebbins said.
Although important, emotional support is not enough. For patients with respiratory disorders, such as chronic obstructive pulmonary disease, asthma, heart failure, or pneumonia, their subjective symptoms of shortness of breath, air hunger, or cough may improve with supplemental oxygen at home. Other measures include repositioning of the patient to lessen the body weight over the lungs or the use of lung percussion, Leroy said.
He added that improvement may also come from drainage of sputum from the airway passages, the use of agents to liquefy thick sputum (mucolytics), or aerosolized bronchodilator medications.
However, Dr. LeRoy cautioned, “one remedy does not work for everyone – an individual can improve gradually by using these home support interventions, or their respiratory status can deteriorate rapidly despite all these interventions.”
For this reason, he says patients should consult their personal physician to determine which, if any, of these home treatments would be best for their particular situation.
Patients who need emotional support, psychotherapy, or psychotropic medications may find teletherapy helpful. Guidance for psychiatrists, psychologists, and social workers regarding the treatment of COVID-19 patients via teletherapy can be found on the American Psychiatric Association, the American Psychological Association, and the National Association of Social Workers websites.
Pharmacists can also help ensure patient safety, Dr. Stebbins said.
If a patient has not picked up their usual medications, Dr. Stebbins said, “they may need a check-in call. Some may be ill and alone and may need encouragement to seek medical attention, and some may have no means of getting to the pharmacy and may need medications delivered.”
A home healthcare agency may also be helpful for homebound patients. David Bersson, director of operations at Synergy Home Care of Bergen County, N.J., has arranged in-home caregivers for patients with COVID-19.
The amount of care that professional caregivers provide can range from several hours per week to full-time, depending on the patient’s needs and budget, and can include companionship, Mr. Bersson said in an interview.
Because patient and caregiver safety are paramount, caregivers are thoroughly trained in protection and decontamination procedures and are regularly tested for COVID-19 prior to being sent into a client’s home.
Health insurance companies do not cover this service, Mr. Bersson noted, but the VetAssist program covers home care for veterans and their spouses who meet income requirements.
Caregiving and companionship are both vital pieces of the at-home care puzzle. “It was the virtual emotional support I got from friends, family, coworkers, and healthcare professionals that meant so much to me, and I know they played an important part in my recovery,” Dr. Stebbins said.
Dr. LeRoy agreed, noting that he calls patients, even if they only have mild symptoms and his nurse has already spoken to them. “The call doesn’t take much time – maybe just a 5-minute conversation – but it makes patients aware that I care.”
Dr. Stebbins, Dr. Exline, and Dr. LeRoy report no relevant financial relationships. Mr. Bersson is the director of operations at Synergy Home Care of Bergen County, New Jersey.
This story first appeared on Medscape.com.
Marilyn Stebbins, PharmD, fell ill at the end of February 2020. Initially diagnosed with multifocal pneumonia and treated with antibiotics, she later developed severe gastrointestinal symptoms, fatigue, and shortness of breath. She was hospitalized in early March and was diagnosed with COVID-19.
It was still early in the pandemic, and testing was not available for her husband. After she was discharged, her husband isolated himself as much as possible. But that limited the amount of care he could offer.
“When I came home after 8 days in the ICU, I felt completely alone and terrified of not being able to care for myself and not knowing how much care my husband could provide,” said Dr. Stebbins, professor of clinical pharmacy at the University of California, San Francisco.
“I can’t even imagine what it would have been like if I had been home alone without my husband in the house,” she said. “I think about the people who died at home and understand how that might happen.”
Dr. Stebbins is one of tens of thousands of people who, whether hospitalized and discharged or never admitted for inpatient care, needed to find ways to convalesce at home. Data from the Centers for Medicare & Medicaid Services show that, of 326,674 beneficiaries who tested positive for COVID-19 between May 16 and June 11, 2020, 109,607 were hospitalized, suggesting that two-thirds were outpatients.
Most attention has focused on the sickest patients, leaving less severe cases to fall through the cracks. Despite fever, cough, difficulty breathing, and a surfeit of other symptoms, there are few available resources and all too little support to help patients navigate the physical and emotional struggles of contending with COVID-19 at home.
No ‘cookie-cutter’ approach
The speed with which the pandemic progressed caught public health systems off guard, but now, “it is essential to put into place the infrastructure to care for the physical and mental health needs of patients at home because most are in the community and many, if not most, still aren’t receiving sufficient support at home,” said Dr. Stebbins.
said Gary LeRoy, MD, a family physician in Dayton, Ohio. He emphasized that there is “no cookie-cutter formula” for home care, because every patient’s situation is different.
“I begin by having a detailed conversation with each patient to ascertain whether their home environment is safe and to paint a picture of their circumstances,” Dr. LeRoy, who is the president of the American Academy of Family Physicians, said in an interview.
Dr. LeRoy suggested questions that constitute “not just a ‘medical’ checklist but a ‘whole life’ checklist.”
- Do you have access to food, water, medications, sanitation/cleaning supplies, a thermometer, and other necessities? If not, who might assist in providing those?
- Do you need help with activities of daily living and self-care?
- Who else lives in your household? Do they have signs and symptoms of the virus? Have they been tested?
- Do you have enough physical space between you and other household members?
- Do you have children? How are they being cared for?
- What type of work do you do? What are the implications for your employment if you are unable to work for an extended period?
- Do you have an emotional, social, and spiritual support system (e.g., family, friends, community, church)?
- Do you have concerns I haven’t mentioned?
Patients’ responses will inform the management plan and determine what medical and social resources are needed, he said.
Daily check-in
Dr. Stebbins said the nurse case manager from her insurance company called her daily after she came home from the hospital. She was told that a public health nurse would also call, but no one from the health department called for days – a situation she hopes has improved.
One way or another, she said, “health care providers [or their staff] should check in with patients daily, either telephonically or via video.” She noted that video is superior, because “someone who isn’t a family member needs to put eyes on a patient and might be able to detect warning signs that a family member without healthcare training might not notice.”
Dr. LeRoy, who is also an associate professor of medicine at Wright State University, Dayton, Ohio, said that, given his time constraints, a nurse or medical assistant in his practice conducts the daily check-ins and notifies him if the patient has fever or other symptoms.
“Under ordinary circumstances, when a patient comes to see me for some type of medical condition, I get to meet the patient, consider what might be going on, then order a test, wait for the results, and suggest a treatment plan. But these are anything but ordinary circumstances,” said Matthew Exline, MD, a pulmonary and critical care specialist at the Ohio State University Wexner Medical Center, Columbus.
“That traditional structure broke down with COVID-19, when we may have test results without even seeing the patient. And without this interaction, it is harder to know as a physician what course of action to take,” he said in an interview.
Once a diagnosis has been made, the physician has at least some data to help guide next steps, even if there has been no prior meeting with the patient.
For example, a positive test raises a host of issues, not the least of which is the risk of spreading the infection to other household members and questions about whether to go the hospital. Moreover, for patients, positive tests can have serious ramifications.
“Severe shortness of breath at rest is not typical of the flu, nor is loss of taste or smell,” said Dr. Exline. Practitioners must educate patients and families about specific symptoms of COVID-19, including shortness of breath, loss of taste or smell, and gastrointestinal or neurologic symptoms, and when to seek emergency care.
Dr. LeRoy suggests buying a pulse oximeter to gauge blood oxygen levels and pulse rate. Together with a thermometer, a portable blood pressure monitor, and, if indicated, a blood glucose monitor, these devices provide a comprehensive and accurate assessment of vital signs.
Dr. LeRoy also educates patients and their families about when to seek medical attention.
Dr. Stebbins takes a similar approach. “Family members are part of, not apart from, the care of patients with COVID-19, and it’s our responsibility as healthcare providers to consider them in the patient’s care plan.”
Keeping family safe
Beyond care, family members need a plan to keep themselves healthy, too.
“A patient with COVID-19 at home should self-quarantine as much as possible to keep other family members safe, if they continue to live in the same house,” Dr. Exline said.
Ideally, uninfected family members should stay with relatives or friends. When that’s not possible, everyone in the household should wear a mask, be vigilant about hand washing, and wipe down all surfaces – including doorknobs, light switches, faucet handles, cellphones, and utensils – regularly with bleach or an alcohol solution.
Caregivers should also minimize the amount of time they are exposed to the patient.
“Set food, water, and medication on the night table and leave the room rather than spending hours at the bedside, since limiting exposure to viral load reduces the chances of contagion,” said Dr. Exline.
The Centers for Disease Control and Prevention offers guidance for household members caring for COVID-19 patients at home. It provides tips on how to help patients follow the doctor’s instructions and ways to ensure adequate hydration and rest, among others.
Patients with COVID-19 who live alone face more formidable challenges.
Dr. LeRoy says physicians can help patients by educating themselves about available social services in their community so they can provide appropriate referrals and connections. Such initiatives can include meal programs, friendly visit and financial assistance programs, as well as childcare and home health agencies.
He noted that Aunt Bertha, a social care network, provides a guide to social services throughout the United States. Additional resources are available on USA.gov.
Comfort and support
Patients with COVID-19 need to be as comfortable and as supported as possible, both physically and emotionally.
“While I was sick, my dogs curled up next to me and didn’t leave my side, and they were my saving grace. There’s not enough to be said about emotional support,” Dr. Stebbins said.
Although important, emotional support is not enough. For patients with respiratory disorders, such as chronic obstructive pulmonary disease, asthma, heart failure, or pneumonia, their subjective symptoms of shortness of breath, air hunger, or cough may improve with supplemental oxygen at home. Other measures include repositioning of the patient to lessen the body weight over the lungs or the use of lung percussion, Leroy said.
He added that improvement may also come from drainage of sputum from the airway passages, the use of agents to liquefy thick sputum (mucolytics), or aerosolized bronchodilator medications.
However, Dr. LeRoy cautioned, “one remedy does not work for everyone – an individual can improve gradually by using these home support interventions, or their respiratory status can deteriorate rapidly despite all these interventions.”
For this reason, he says patients should consult their personal physician to determine which, if any, of these home treatments would be best for their particular situation.
Patients who need emotional support, psychotherapy, or psychotropic medications may find teletherapy helpful. Guidance for psychiatrists, psychologists, and social workers regarding the treatment of COVID-19 patients via teletherapy can be found on the American Psychiatric Association, the American Psychological Association, and the National Association of Social Workers websites.
Pharmacists can also help ensure patient safety, Dr. Stebbins said.
If a patient has not picked up their usual medications, Dr. Stebbins said, “they may need a check-in call. Some may be ill and alone and may need encouragement to seek medical attention, and some may have no means of getting to the pharmacy and may need medications delivered.”
A home healthcare agency may also be helpful for homebound patients. David Bersson, director of operations at Synergy Home Care of Bergen County, N.J., has arranged in-home caregivers for patients with COVID-19.
The amount of care that professional caregivers provide can range from several hours per week to full-time, depending on the patient’s needs and budget, and can include companionship, Mr. Bersson said in an interview.
Because patient and caregiver safety are paramount, caregivers are thoroughly trained in protection and decontamination procedures and are regularly tested for COVID-19 prior to being sent into a client’s home.
Health insurance companies do not cover this service, Mr. Bersson noted, but the VetAssist program covers home care for veterans and their spouses who meet income requirements.
Caregiving and companionship are both vital pieces of the at-home care puzzle. “It was the virtual emotional support I got from friends, family, coworkers, and healthcare professionals that meant so much to me, and I know they played an important part in my recovery,” Dr. Stebbins said.
Dr. LeRoy agreed, noting that he calls patients, even if they only have mild symptoms and his nurse has already spoken to them. “The call doesn’t take much time – maybe just a 5-minute conversation – but it makes patients aware that I care.”
Dr. Stebbins, Dr. Exline, and Dr. LeRoy report no relevant financial relationships. Mr. Bersson is the director of operations at Synergy Home Care of Bergen County, New Jersey.
This story first appeared on Medscape.com.
Marilyn Stebbins, PharmD, fell ill at the end of February 2020. Initially diagnosed with multifocal pneumonia and treated with antibiotics, she later developed severe gastrointestinal symptoms, fatigue, and shortness of breath. She was hospitalized in early March and was diagnosed with COVID-19.
It was still early in the pandemic, and testing was not available for her husband. After she was discharged, her husband isolated himself as much as possible. But that limited the amount of care he could offer.
“When I came home after 8 days in the ICU, I felt completely alone and terrified of not being able to care for myself and not knowing how much care my husband could provide,” said Dr. Stebbins, professor of clinical pharmacy at the University of California, San Francisco.
“I can’t even imagine what it would have been like if I had been home alone without my husband in the house,” she said. “I think about the people who died at home and understand how that might happen.”
Dr. Stebbins is one of tens of thousands of people who, whether hospitalized and discharged or never admitted for inpatient care, needed to find ways to convalesce at home. Data from the Centers for Medicare & Medicaid Services show that, of 326,674 beneficiaries who tested positive for COVID-19 between May 16 and June 11, 2020, 109,607 were hospitalized, suggesting that two-thirds were outpatients.
Most attention has focused on the sickest patients, leaving less severe cases to fall through the cracks. Despite fever, cough, difficulty breathing, and a surfeit of other symptoms, there are few available resources and all too little support to help patients navigate the physical and emotional struggles of contending with COVID-19 at home.
No ‘cookie-cutter’ approach
The speed with which the pandemic progressed caught public health systems off guard, but now, “it is essential to put into place the infrastructure to care for the physical and mental health needs of patients at home because most are in the community and many, if not most, still aren’t receiving sufficient support at home,” said Dr. Stebbins.
said Gary LeRoy, MD, a family physician in Dayton, Ohio. He emphasized that there is “no cookie-cutter formula” for home care, because every patient’s situation is different.
“I begin by having a detailed conversation with each patient to ascertain whether their home environment is safe and to paint a picture of their circumstances,” Dr. LeRoy, who is the president of the American Academy of Family Physicians, said in an interview.
Dr. LeRoy suggested questions that constitute “not just a ‘medical’ checklist but a ‘whole life’ checklist.”
- Do you have access to food, water, medications, sanitation/cleaning supplies, a thermometer, and other necessities? If not, who might assist in providing those?
- Do you need help with activities of daily living and self-care?
- Who else lives in your household? Do they have signs and symptoms of the virus? Have they been tested?
- Do you have enough physical space between you and other household members?
- Do you have children? How are they being cared for?
- What type of work do you do? What are the implications for your employment if you are unable to work for an extended period?
- Do you have an emotional, social, and spiritual support system (e.g., family, friends, community, church)?
- Do you have concerns I haven’t mentioned?
Patients’ responses will inform the management plan and determine what medical and social resources are needed, he said.
Daily check-in
Dr. Stebbins said the nurse case manager from her insurance company called her daily after she came home from the hospital. She was told that a public health nurse would also call, but no one from the health department called for days – a situation she hopes has improved.
One way or another, she said, “health care providers [or their staff] should check in with patients daily, either telephonically or via video.” She noted that video is superior, because “someone who isn’t a family member needs to put eyes on a patient and might be able to detect warning signs that a family member without healthcare training might not notice.”
Dr. LeRoy, who is also an associate professor of medicine at Wright State University, Dayton, Ohio, said that, given his time constraints, a nurse or medical assistant in his practice conducts the daily check-ins and notifies him if the patient has fever or other symptoms.
“Under ordinary circumstances, when a patient comes to see me for some type of medical condition, I get to meet the patient, consider what might be going on, then order a test, wait for the results, and suggest a treatment plan. But these are anything but ordinary circumstances,” said Matthew Exline, MD, a pulmonary and critical care specialist at the Ohio State University Wexner Medical Center, Columbus.
“That traditional structure broke down with COVID-19, when we may have test results without even seeing the patient. And without this interaction, it is harder to know as a physician what course of action to take,” he said in an interview.
Once a diagnosis has been made, the physician has at least some data to help guide next steps, even if there has been no prior meeting with the patient.
For example, a positive test raises a host of issues, not the least of which is the risk of spreading the infection to other household members and questions about whether to go the hospital. Moreover, for patients, positive tests can have serious ramifications.
“Severe shortness of breath at rest is not typical of the flu, nor is loss of taste or smell,” said Dr. Exline. Practitioners must educate patients and families about specific symptoms of COVID-19, including shortness of breath, loss of taste or smell, and gastrointestinal or neurologic symptoms, and when to seek emergency care.
Dr. LeRoy suggests buying a pulse oximeter to gauge blood oxygen levels and pulse rate. Together with a thermometer, a portable blood pressure monitor, and, if indicated, a blood glucose monitor, these devices provide a comprehensive and accurate assessment of vital signs.
Dr. LeRoy also educates patients and their families about when to seek medical attention.
Dr. Stebbins takes a similar approach. “Family members are part of, not apart from, the care of patients with COVID-19, and it’s our responsibility as healthcare providers to consider them in the patient’s care plan.”
Keeping family safe
Beyond care, family members need a plan to keep themselves healthy, too.
“A patient with COVID-19 at home should self-quarantine as much as possible to keep other family members safe, if they continue to live in the same house,” Dr. Exline said.
Ideally, uninfected family members should stay with relatives or friends. When that’s not possible, everyone in the household should wear a mask, be vigilant about hand washing, and wipe down all surfaces – including doorknobs, light switches, faucet handles, cellphones, and utensils – regularly with bleach or an alcohol solution.
Caregivers should also minimize the amount of time they are exposed to the patient.
“Set food, water, and medication on the night table and leave the room rather than spending hours at the bedside, since limiting exposure to viral load reduces the chances of contagion,” said Dr. Exline.
The Centers for Disease Control and Prevention offers guidance for household members caring for COVID-19 patients at home. It provides tips on how to help patients follow the doctor’s instructions and ways to ensure adequate hydration and rest, among others.
Patients with COVID-19 who live alone face more formidable challenges.
Dr. LeRoy says physicians can help patients by educating themselves about available social services in their community so they can provide appropriate referrals and connections. Such initiatives can include meal programs, friendly visit and financial assistance programs, as well as childcare and home health agencies.
He noted that Aunt Bertha, a social care network, provides a guide to social services throughout the United States. Additional resources are available on USA.gov.
Comfort and support
Patients with COVID-19 need to be as comfortable and as supported as possible, both physically and emotionally.
“While I was sick, my dogs curled up next to me and didn’t leave my side, and they were my saving grace. There’s not enough to be said about emotional support,” Dr. Stebbins said.
Although important, emotional support is not enough. For patients with respiratory disorders, such as chronic obstructive pulmonary disease, asthma, heart failure, or pneumonia, their subjective symptoms of shortness of breath, air hunger, or cough may improve with supplemental oxygen at home. Other measures include repositioning of the patient to lessen the body weight over the lungs or the use of lung percussion, Leroy said.
He added that improvement may also come from drainage of sputum from the airway passages, the use of agents to liquefy thick sputum (mucolytics), or aerosolized bronchodilator medications.
However, Dr. LeRoy cautioned, “one remedy does not work for everyone – an individual can improve gradually by using these home support interventions, or their respiratory status can deteriorate rapidly despite all these interventions.”
For this reason, he says patients should consult their personal physician to determine which, if any, of these home treatments would be best for their particular situation.
Patients who need emotional support, psychotherapy, or psychotropic medications may find teletherapy helpful. Guidance for psychiatrists, psychologists, and social workers regarding the treatment of COVID-19 patients via teletherapy can be found on the American Psychiatric Association, the American Psychological Association, and the National Association of Social Workers websites.
Pharmacists can also help ensure patient safety, Dr. Stebbins said.
If a patient has not picked up their usual medications, Dr. Stebbins said, “they may need a check-in call. Some may be ill and alone and may need encouragement to seek medical attention, and some may have no means of getting to the pharmacy and may need medications delivered.”
A home healthcare agency may also be helpful for homebound patients. David Bersson, director of operations at Synergy Home Care of Bergen County, N.J., has arranged in-home caregivers for patients with COVID-19.
The amount of care that professional caregivers provide can range from several hours per week to full-time, depending on the patient’s needs and budget, and can include companionship, Mr. Bersson said in an interview.
Because patient and caregiver safety are paramount, caregivers are thoroughly trained in protection and decontamination procedures and are regularly tested for COVID-19 prior to being sent into a client’s home.
Health insurance companies do not cover this service, Mr. Bersson noted, but the VetAssist program covers home care for veterans and their spouses who meet income requirements.
Caregiving and companionship are both vital pieces of the at-home care puzzle. “It was the virtual emotional support I got from friends, family, coworkers, and healthcare professionals that meant so much to me, and I know they played an important part in my recovery,” Dr. Stebbins said.
Dr. LeRoy agreed, noting that he calls patients, even if they only have mild symptoms and his nurse has already spoken to them. “The call doesn’t take much time – maybe just a 5-minute conversation – but it makes patients aware that I care.”
Dr. Stebbins, Dr. Exline, and Dr. LeRoy report no relevant financial relationships. Mr. Bersson is the director of operations at Synergy Home Care of Bergen County, New Jersey.
This story first appeared on Medscape.com.