User login
Extraordinary Patients Inspired Father of Cancer Immunotherapy
His pioneering research established interleukin-2 (IL-2) as the first U.S. Food and Drug Administration–approved cancer immunotherapy in 1992.
To recognize his trailblazing work and other achievements, the American Association for Cancer Research (AACR) will award Dr. Rosenberg with the 2024 AACR Award for Lifetime Achievement in Cancer Research at its annual meeting in April.
Dr. Rosenberg, a senior investigator for the Center for Cancer Research at the National Cancer Institute (NCI), and chief of the NCI Surgery Branch, shared the history behind his novel research and the patient stories that inspired his discoveries, during an interview.
Tell us a little about yourself and where you grew up.
Dr. Rosenberg: I grew up in the Bronx. My parents both immigrated to the United States from Poland as teenagers.
As a young boy, did you always want to become a doctor?
Dr. Rosenberg: I think some defining moments on why I decided to go into medicine occurred when I was 6 or 7 years old. The second world war was over, and many of the horrors of the Holocaust became apparent to me. I was brought up as an Orthodox Jew. My parents were quite religious, and I remember postcards coming in one after another about relatives that had died in the death camps. That had a profound influence on me.
How did that experience impact your aspirations?
Dr. Rosenberg: It was an example to me of how evil certain people and groups can be toward one another. I decided at that point, that I wanted to do something good for people, and medicine seemed the most likely way to do that. But also, I was developing a broad scientific interest. I ended up at the Bronx High School of Science and knew that I not only wanted to practice the medicine of today, but I wanted to play a role in helping develop the medicine.
What led to your interest in cancer treatment?
Dr. Rosenberg: Well, as a medical student and resident, it became clear that the field of cancer needed major improvement. We had three major ways to treat cancer: surgery, radiation therapy, and chemotherapy. That could cure about half of the people [who] had cancer. But despite the best application of those three specialties, there were over 600,000 deaths from cancer each year in the United States alone. It was clear to me that new approaches were needed, and I became very interested in taking advantage of the body’s immune system as a source of information to try to make progress.
Were there patients who inspired your research?
Dr. Rosenberg: There were two patients that I saw early in my career that impressed me a great deal. One was a patient that I saw when working in the emergency ward as a resident. A patient came in with right upper quadrant pain that looked like a gallbladder attack. That’s what it was. But when I went through his chart, I saw that he had been at that hospital 12 years earlier with a metastatic gastric cancer. The surgeons had operated. They saw tumor had spread to the liver and could not be removed. They closed the belly, not expecting him to survive. Yet he kept showing up for follow-up visits.
Here he was 12 years later. When I helped operate to take out his gallbladder, there was no evidence of any cancer. The cancer had disappeared in the absence of any external treatment. One of the rarest events in medicine, the spontaneous regression of a cancer. Somehow his body had learned how to destroy the tumor.
Was the second patient’s case as impressive?
Dr. Rosenberg: This patient had received a kidney transplant from a gentleman who died in an auto accident. [The donor’s] kidney contained a cancer deposit, a kidney cancer, unbeknownst to the transplant surgeons. [When the kidney was transplanted], the recipient developed widespread metastatic kidney cancer.
[The recipient] was on immunosuppressive drugs, and so the drugs had to be stopped. [When the immunosuppressive drugs were stopped], the patient’s body rejected the kidney and his cancer disappeared.
That showed me that, in fact, if you could stimulate a strong enough immune reaction, in this case, an [allogeneic] reaction, against foreign tissues from a different individual, that you could make large vascularized, invasive cancers disappear based on immune reactivities. Those were clues that led me toward studying the immune system’s impact on cancer.
From there, how did your work evolve?
Dr. Rosenberg: As chief of the surgery branch at NIH, I began doing research. It was very difficult to manipulate immune cells in the laboratory. They wouldn’t stay alive. But I tried to study immune reactions in patients with cancer to see if there was such a thing as an immune reaction against the cancer. There was no such thing known at the time. There were no cancer antigens and no known immune reactions against the disease in the human.
Around this time, investigators were publishing studies about interleukin-2 (IL-2), or white blood cells known as leukocytes. How did interleukin-2 further your research?
Dr. Rosenberg: The advent of interleukin-2 enabled scientists to grow lymphocytes outside the body. [This] enabled us to grow t-lymphocytes, which are some of the major warriors of the immune system against foreign tissue. After [studying] 66 patients in which we studied interleukin-2 and cells that would develop from it, we finally saw a disappearance of melanoma in a patient that received interleukin-2. And we went on to treat hundreds of patients with that hormone, interleukin-2. In fact, interleukin-2 became the first immunotherapy ever approved by the Food and Drug Administration for the treatment of cancer in humans.
How did this finding impact your future discoveries?
Dr. Rosenberg: [It] led to studies of the mechanism of action of interleukin-2 and to do that, we identified a kind of cell called a tumor infiltrating lymphocyte. What better place, intuitively to look for cells doing battle against the cancer than within the cancer itself?
In 1988, we demonstrated for the first time that transfer of lymphocytes with antitumor activity could cause the regression of melanoma. This was a living drug obtained from melanoma deposits that could be grown outside the body and then readministered to the patient under suitable conditions. Interestingly, [in February the FDA approved that drug as treatment for patients with melanoma]. A company developed it to the point where in multi-institutional studies, they reproduced our results.
And we’ve now emphasized the value of using T cell therapy, t cell transfer, for the treatment of patients with the common solid cancers, the cancers that start anywhere from the colon up through the intestine, the stomach, the pancreas, and the esophagus. Solid tumors such as ovarian cancer, uterine cancer and so on, are also potentially susceptible to this T cell therapy.
We’ve published several papers showing in isolated patients that you could cause major regressions, if not complete regressions, of these solid cancers in the liver, in the breast, the cervix, the colon. That’s a major aspect of what we’re doing now.
I think immunotherapy has come to be recognized as a major fourth arm that can be used to attack cancers, adding to surgery, radiation, and chemotherapy.
What guidance would you have for other physician-investigators or young doctors who want to follow in your path?
Dr. Rosenberg: You have to have a broad base of knowledge. You have to be willing to immerse yourself in a problem so that your mind is working on it when you’re doing things where you can only think. [When] you’re taking a shower, [or] waiting at a red light, your mind is working on this problem because you’re immersed in trying to understand it.
You need to have a laser focus on the goals that you have and not get sidetracked by issues that may be interesting but not directly related to the goals that you’re attempting to achieve.
His pioneering research established interleukin-2 (IL-2) as the first U.S. Food and Drug Administration–approved cancer immunotherapy in 1992.
To recognize his trailblazing work and other achievements, the American Association for Cancer Research (AACR) will award Dr. Rosenberg with the 2024 AACR Award for Lifetime Achievement in Cancer Research at its annual meeting in April.
Dr. Rosenberg, a senior investigator for the Center for Cancer Research at the National Cancer Institute (NCI), and chief of the NCI Surgery Branch, shared the history behind his novel research and the patient stories that inspired his discoveries, during an interview.
Tell us a little about yourself and where you grew up.
Dr. Rosenberg: I grew up in the Bronx. My parents both immigrated to the United States from Poland as teenagers.
As a young boy, did you always want to become a doctor?
Dr. Rosenberg: I think some defining moments on why I decided to go into medicine occurred when I was 6 or 7 years old. The second world war was over, and many of the horrors of the Holocaust became apparent to me. I was brought up as an Orthodox Jew. My parents were quite religious, and I remember postcards coming in one after another about relatives that had died in the death camps. That had a profound influence on me.
How did that experience impact your aspirations?
Dr. Rosenberg: It was an example to me of how evil certain people and groups can be toward one another. I decided at that point, that I wanted to do something good for people, and medicine seemed the most likely way to do that. But also, I was developing a broad scientific interest. I ended up at the Bronx High School of Science and knew that I not only wanted to practice the medicine of today, but I wanted to play a role in helping develop the medicine.
What led to your interest in cancer treatment?
Dr. Rosenberg: Well, as a medical student and resident, it became clear that the field of cancer needed major improvement. We had three major ways to treat cancer: surgery, radiation therapy, and chemotherapy. That could cure about half of the people [who] had cancer. But despite the best application of those three specialties, there were over 600,000 deaths from cancer each year in the United States alone. It was clear to me that new approaches were needed, and I became very interested in taking advantage of the body’s immune system as a source of information to try to make progress.
Were there patients who inspired your research?
Dr. Rosenberg: There were two patients that I saw early in my career that impressed me a great deal. One was a patient that I saw when working in the emergency ward as a resident. A patient came in with right upper quadrant pain that looked like a gallbladder attack. That’s what it was. But when I went through his chart, I saw that he had been at that hospital 12 years earlier with a metastatic gastric cancer. The surgeons had operated. They saw tumor had spread to the liver and could not be removed. They closed the belly, not expecting him to survive. Yet he kept showing up for follow-up visits.
Here he was 12 years later. When I helped operate to take out his gallbladder, there was no evidence of any cancer. The cancer had disappeared in the absence of any external treatment. One of the rarest events in medicine, the spontaneous regression of a cancer. Somehow his body had learned how to destroy the tumor.
Was the second patient’s case as impressive?
Dr. Rosenberg: This patient had received a kidney transplant from a gentleman who died in an auto accident. [The donor’s] kidney contained a cancer deposit, a kidney cancer, unbeknownst to the transplant surgeons. [When the kidney was transplanted], the recipient developed widespread metastatic kidney cancer.
[The recipient] was on immunosuppressive drugs, and so the drugs had to be stopped. [When the immunosuppressive drugs were stopped], the patient’s body rejected the kidney and his cancer disappeared.
That showed me that, in fact, if you could stimulate a strong enough immune reaction, in this case, an [allogeneic] reaction, against foreign tissues from a different individual, that you could make large vascularized, invasive cancers disappear based on immune reactivities. Those were clues that led me toward studying the immune system’s impact on cancer.
From there, how did your work evolve?
Dr. Rosenberg: As chief of the surgery branch at NIH, I began doing research. It was very difficult to manipulate immune cells in the laboratory. They wouldn’t stay alive. But I tried to study immune reactions in patients with cancer to see if there was such a thing as an immune reaction against the cancer. There was no such thing known at the time. There were no cancer antigens and no known immune reactions against the disease in the human.
Around this time, investigators were publishing studies about interleukin-2 (IL-2), or white blood cells known as leukocytes. How did interleukin-2 further your research?
Dr. Rosenberg: The advent of interleukin-2 enabled scientists to grow lymphocytes outside the body. [This] enabled us to grow t-lymphocytes, which are some of the major warriors of the immune system against foreign tissue. After [studying] 66 patients in which we studied interleukin-2 and cells that would develop from it, we finally saw a disappearance of melanoma in a patient that received interleukin-2. And we went on to treat hundreds of patients with that hormone, interleukin-2. In fact, interleukin-2 became the first immunotherapy ever approved by the Food and Drug Administration for the treatment of cancer in humans.
How did this finding impact your future discoveries?
Dr. Rosenberg: [It] led to studies of the mechanism of action of interleukin-2 and to do that, we identified a kind of cell called a tumor infiltrating lymphocyte. What better place, intuitively to look for cells doing battle against the cancer than within the cancer itself?
In 1988, we demonstrated for the first time that transfer of lymphocytes with antitumor activity could cause the regression of melanoma. This was a living drug obtained from melanoma deposits that could be grown outside the body and then readministered to the patient under suitable conditions. Interestingly, [in February the FDA approved that drug as treatment for patients with melanoma]. A company developed it to the point where in multi-institutional studies, they reproduced our results.
And we’ve now emphasized the value of using T cell therapy, t cell transfer, for the treatment of patients with the common solid cancers, the cancers that start anywhere from the colon up through the intestine, the stomach, the pancreas, and the esophagus. Solid tumors such as ovarian cancer, uterine cancer and so on, are also potentially susceptible to this T cell therapy.
We’ve published several papers showing in isolated patients that you could cause major regressions, if not complete regressions, of these solid cancers in the liver, in the breast, the cervix, the colon. That’s a major aspect of what we’re doing now.
I think immunotherapy has come to be recognized as a major fourth arm that can be used to attack cancers, adding to surgery, radiation, and chemotherapy.
What guidance would you have for other physician-investigators or young doctors who want to follow in your path?
Dr. Rosenberg: You have to have a broad base of knowledge. You have to be willing to immerse yourself in a problem so that your mind is working on it when you’re doing things where you can only think. [When] you’re taking a shower, [or] waiting at a red light, your mind is working on this problem because you’re immersed in trying to understand it.
You need to have a laser focus on the goals that you have and not get sidetracked by issues that may be interesting but not directly related to the goals that you’re attempting to achieve.
His pioneering research established interleukin-2 (IL-2) as the first U.S. Food and Drug Administration–approved cancer immunotherapy in 1992.
To recognize his trailblazing work and other achievements, the American Association for Cancer Research (AACR) will award Dr. Rosenberg with the 2024 AACR Award for Lifetime Achievement in Cancer Research at its annual meeting in April.
Dr. Rosenberg, a senior investigator for the Center for Cancer Research at the National Cancer Institute (NCI), and chief of the NCI Surgery Branch, shared the history behind his novel research and the patient stories that inspired his discoveries, during an interview.
Tell us a little about yourself and where you grew up.
Dr. Rosenberg: I grew up in the Bronx. My parents both immigrated to the United States from Poland as teenagers.
As a young boy, did you always want to become a doctor?
Dr. Rosenberg: I think some defining moments on why I decided to go into medicine occurred when I was 6 or 7 years old. The second world war was over, and many of the horrors of the Holocaust became apparent to me. I was brought up as an Orthodox Jew. My parents were quite religious, and I remember postcards coming in one after another about relatives that had died in the death camps. That had a profound influence on me.
How did that experience impact your aspirations?
Dr. Rosenberg: It was an example to me of how evil certain people and groups can be toward one another. I decided at that point, that I wanted to do something good for people, and medicine seemed the most likely way to do that. But also, I was developing a broad scientific interest. I ended up at the Bronx High School of Science and knew that I not only wanted to practice the medicine of today, but I wanted to play a role in helping develop the medicine.
What led to your interest in cancer treatment?
Dr. Rosenberg: Well, as a medical student and resident, it became clear that the field of cancer needed major improvement. We had three major ways to treat cancer: surgery, radiation therapy, and chemotherapy. That could cure about half of the people [who] had cancer. But despite the best application of those three specialties, there were over 600,000 deaths from cancer each year in the United States alone. It was clear to me that new approaches were needed, and I became very interested in taking advantage of the body’s immune system as a source of information to try to make progress.
Were there patients who inspired your research?
Dr. Rosenberg: There were two patients that I saw early in my career that impressed me a great deal. One was a patient that I saw when working in the emergency ward as a resident. A patient came in with right upper quadrant pain that looked like a gallbladder attack. That’s what it was. But when I went through his chart, I saw that he had been at that hospital 12 years earlier with a metastatic gastric cancer. The surgeons had operated. They saw tumor had spread to the liver and could not be removed. They closed the belly, not expecting him to survive. Yet he kept showing up for follow-up visits.
Here he was 12 years later. When I helped operate to take out his gallbladder, there was no evidence of any cancer. The cancer had disappeared in the absence of any external treatment. One of the rarest events in medicine, the spontaneous regression of a cancer. Somehow his body had learned how to destroy the tumor.
Was the second patient’s case as impressive?
Dr. Rosenberg: This patient had received a kidney transplant from a gentleman who died in an auto accident. [The donor’s] kidney contained a cancer deposit, a kidney cancer, unbeknownst to the transplant surgeons. [When the kidney was transplanted], the recipient developed widespread metastatic kidney cancer.
[The recipient] was on immunosuppressive drugs, and so the drugs had to be stopped. [When the immunosuppressive drugs were stopped], the patient’s body rejected the kidney and his cancer disappeared.
That showed me that, in fact, if you could stimulate a strong enough immune reaction, in this case, an [allogeneic] reaction, against foreign tissues from a different individual, that you could make large vascularized, invasive cancers disappear based on immune reactivities. Those were clues that led me toward studying the immune system’s impact on cancer.
From there, how did your work evolve?
Dr. Rosenberg: As chief of the surgery branch at NIH, I began doing research. It was very difficult to manipulate immune cells in the laboratory. They wouldn’t stay alive. But I tried to study immune reactions in patients with cancer to see if there was such a thing as an immune reaction against the cancer. There was no such thing known at the time. There were no cancer antigens and no known immune reactions against the disease in the human.
Around this time, investigators were publishing studies about interleukin-2 (IL-2), or white blood cells known as leukocytes. How did interleukin-2 further your research?
Dr. Rosenberg: The advent of interleukin-2 enabled scientists to grow lymphocytes outside the body. [This] enabled us to grow t-lymphocytes, which are some of the major warriors of the immune system against foreign tissue. After [studying] 66 patients in which we studied interleukin-2 and cells that would develop from it, we finally saw a disappearance of melanoma in a patient that received interleukin-2. And we went on to treat hundreds of patients with that hormone, interleukin-2. In fact, interleukin-2 became the first immunotherapy ever approved by the Food and Drug Administration for the treatment of cancer in humans.
How did this finding impact your future discoveries?
Dr. Rosenberg: [It] led to studies of the mechanism of action of interleukin-2 and to do that, we identified a kind of cell called a tumor infiltrating lymphocyte. What better place, intuitively to look for cells doing battle against the cancer than within the cancer itself?
In 1988, we demonstrated for the first time that transfer of lymphocytes with antitumor activity could cause the regression of melanoma. This was a living drug obtained from melanoma deposits that could be grown outside the body and then readministered to the patient under suitable conditions. Interestingly, [in February the FDA approved that drug as treatment for patients with melanoma]. A company developed it to the point where in multi-institutional studies, they reproduced our results.
And we’ve now emphasized the value of using T cell therapy, t cell transfer, for the treatment of patients with the common solid cancers, the cancers that start anywhere from the colon up through the intestine, the stomach, the pancreas, and the esophagus. Solid tumors such as ovarian cancer, uterine cancer and so on, are also potentially susceptible to this T cell therapy.
We’ve published several papers showing in isolated patients that you could cause major regressions, if not complete regressions, of these solid cancers in the liver, in the breast, the cervix, the colon. That’s a major aspect of what we’re doing now.
I think immunotherapy has come to be recognized as a major fourth arm that can be used to attack cancers, adding to surgery, radiation, and chemotherapy.
What guidance would you have for other physician-investigators or young doctors who want to follow in your path?
Dr. Rosenberg: You have to have a broad base of knowledge. You have to be willing to immerse yourself in a problem so that your mind is working on it when you’re doing things where you can only think. [When] you’re taking a shower, [or] waiting at a red light, your mind is working on this problem because you’re immersed in trying to understand it.
You need to have a laser focus on the goals that you have and not get sidetracked by issues that may be interesting but not directly related to the goals that you’re attempting to achieve.
Why a New Inhalable Lung Cancer Treatment Is So Promising
Cells in the human body chat with each other all the time. One major way they communicate is by releasing tiny spheres called exosomes. These carry fats, proteins, and genetic material that help regulate everything from pregnancy and immune responses to heart health and kidney function.
“Exosomes work like text messages between cells , sending and receiving information,” said lead researcher Ke Cheng, PhD, professor of biomedical engineering at Columbia. “The significance of this study is that exosomes can bring mRNA-based treatment to lung cancer cells locally, unlike systemic chemotherapy that can have side effects throughout the body. And inhalation is totally noninvasive. You don’t need a nurse to use an IV needle to pierce your skin.”
Dr. Cheng expects a human trial could launch within 5 years. For now, his study is attracting attention because it marks an advance in three areas of intense interest by researchers and biotech companies alike: Therapeutic uses of exosomes, inhalable treatments for lung conditions, and the safe delivery of powerful interleukin-12 (IL-12) immunotherapy.
Inside the Study
Dr. Cheng, who has been developing exosome and stem cell therapies for more than 15 years, and his lab team focused on lung cancer because the disease, often detected in later stages, “has a huge mortality rate,” he said. “Therapies have been suboptimal and leave the organ so damaged.”
He wanted to explore new alternatives to systemic treatments. Most are given intravenously, but Dr. Cheng thinks exosomes — also called extracellular vesicles (EVs) — could change that.
“One of the advantages of exosomes is that they are naturally secreted by the body or cultured cells,” he noted. “They have low toxicity and have multiple ways of getting their message into cells.”
The scientists borrowed an approach that captured public attention during the pandemic: Using messenger RNA, which directs cells to make proteins for tasks — including boosting immune response.
IL-12 has shown promise against cancer for decades, but early human trials triggered serious side effects and several deaths. Researchers are now trying new delivery methods that target tumor cells without affecting healthy tissue. Dr. Cheng’s team took a new approach, inserting mRNA for IL-12 into exosomes.
One aim of the study was to compare the effectiveness of inhaled exosomes vs inhaled liposomes, engineered fat droplets also under investigation as drug carriers. The team’s question: Which would work better at introducing IL-12 to the lungs to affect cancer, without triggering side effects?
After lab mice inhaled the particles through the nose, the researchers found that exosomes delivered more mRNA into cancer cells in the lungs and fought lung cancer with few side effects. Three days after treatment, researchers saw an influx of cancer-fighting T cells within tumors — with higher levels for exosome-based treatment. Plus, the exosomes led to more cancer-destroying nature killer cells and more monocytes, a sign of immune-system activation.
Researchers also found the treatment acted as a vaccine, training the immune system to battle newly introduced cancers. Little of the exosome-delivered drug escaped into the bloodstream, and the study found minimal side effects. Inhalation didn’t affect normal breathing, Dr. Cheng added.
The study’s use of inhaled exosomes makes it significant, said Raghu Kalluri, MD, PhD, professor and chair of the Department of Cancer Biology at MD Anderson Cancer Center. “This is an interesting study that explores the inhalable delivery of engineered EVs for the treatment of lung cancer and offers insights into focused delivery of EV-based drugs…with implications for diseases beyond cancer,” he said. Dr. Kalluri is also an exosome researcher.
New Frontiers
Once seen as a “quirky biological phenomenon” or just cellular trash, exosomes are now the subject of intense medical research for their potential as drug carriers, as treatments in their own right for everything from wound healing and pneumonia to heart attacks and bowel disorders, and as measurable biological markers that could lead to new tests for cancer and other conditions. One exosome-based prostate cancer test, the ExoDx Prostate Test, is already on the market.
The explosion in exosome research — the number of published studies has grown from just a handful in the early 1980s to more than 9000 — spotlights a particular focus on cancer. According to a 2021 paper in Annals of Oncology, clinical trials for exosomes in cancer treatments and tests far out-paces those for diabetes, heart disease, or neurologic conditions. Currently, 52 clinical trials using exosomes in cancer diagnosis or treatment have been completed, are underway, or are looking for participants, according to clinicaltrials.gov.
Dr. Cheng’s approach could also be used to deliver other drugs to the lungs and other organs via inhalation. “We’re testing inhalation for a different type of lung disease, acute lung injury,” Dr. Cheng said. Other potential targets include lung disorders like pulmonary hypertension. Inhaled exosomes could potentially reach the brain via the olfactory bulb or the heart as it receives oxygenated blood from the lungs.
Breathing in Medicine
So far, inhalable cancer treatments are not available outside research studies in the United States or Europe , said Remi Rosiere, PhD, a lecturer at the Université libre de Bruxelles in Brussels, Belgium, and chief scientific officer of InhaTarget Therapeutics, a company developing its own inhaled treatments for severe respiratory diseases. “Oncologists are very interested,” he said. “If you concentrate the drug on the tumor site, you can avoid distribution to the body.”
Early research into inhalable chemotherapy began in the 1960s but was unsuccessful because breathing equipment dispersed toxic cancer drugs into the air or delivered only small amounts to the lungs, he said.
New delivery techniques aim to change that. Dr. Rosiere’s company is starting a human trial of a dry powder inhaler with the chemotherapy drug cisplatin for lung cancer. Also in the pipeline is an immunotherapy treatment for lung cancer inserted in lipid nanoparticles, which are tiny fat particles similar to liposomes.
He said Dr. Cheng’s study shows the advantages of sending in exosomes. “The data are very persuasive,” Dr. Rosier said of the study. “Exosomes have a good safety profile and are able to remain in the lung for quite a long time. This prolongs exposure to the drug for greater effectiveness, without causing toxicities.”
Getting from a mouse study to a human trial will take time. “You need to understand this is very early stage,” Dr. Rosiere added. “There will be many challenges to overcome.”
One is purely practical: If the drug approaches human trials, he said, regulators will ask whether the exosomes can be produced in large quantities to meet the huge demand for new lung cancer treatments. “Lung cancer is the number one fatal cancer in the world,” Dr. Rosiere said.
A New Route for ‘Powerful’ Cancer Treatment
Meanwhile, the Columbia University study showed that inhalable exosomes are a unique delivery method for IL-12 — and could help solve a major problem that’s plagued this promising cancer treatment for decades.
Called “one of the most powerful immunotherapy agents ever discovered” in a 2022 literature review, IL-12 showed serious side effects that stalled research in the 1980s , sparking an ongoing search for new delivery methods that continues today. In 2022 and 2023, Big Pharma companies including AstraZenca, Moderna, and Bristol Myers Squib reduced their involvement with IL-12 treatment research, leaving the field open to smaller biotech companies working on a variety of drug-delivery approaches that could make IL-12 safe and effective in humans.
These include injecting it directly into tumors, encasing it in various types of particles, masking the drug so it is activated only in cancer cells, and using IL-12 mRNA, which essentially turns tumor cells into IL-12–producing factories. Another IL-12 mRNA drug, from Pittsburgh-based Krystal Biotech, received a fast-track designation from the US Food and Drug Administration in February 2024 for an inhaled lung cancer treatment that packages mRNA for IL-12 and IL-2 inside an engineered virus.
And of course, there is Dr. Cheng’s inhalable treatment, culminating decades of work across three burgeoning fields.
A version of this article appeared on Medscape.com.
Cells in the human body chat with each other all the time. One major way they communicate is by releasing tiny spheres called exosomes. These carry fats, proteins, and genetic material that help regulate everything from pregnancy and immune responses to heart health and kidney function.
“Exosomes work like text messages between cells , sending and receiving information,” said lead researcher Ke Cheng, PhD, professor of biomedical engineering at Columbia. “The significance of this study is that exosomes can bring mRNA-based treatment to lung cancer cells locally, unlike systemic chemotherapy that can have side effects throughout the body. And inhalation is totally noninvasive. You don’t need a nurse to use an IV needle to pierce your skin.”
Dr. Cheng expects a human trial could launch within 5 years. For now, his study is attracting attention because it marks an advance in three areas of intense interest by researchers and biotech companies alike: Therapeutic uses of exosomes, inhalable treatments for lung conditions, and the safe delivery of powerful interleukin-12 (IL-12) immunotherapy.
Inside the Study
Dr. Cheng, who has been developing exosome and stem cell therapies for more than 15 years, and his lab team focused on lung cancer because the disease, often detected in later stages, “has a huge mortality rate,” he said. “Therapies have been suboptimal and leave the organ so damaged.”
He wanted to explore new alternatives to systemic treatments. Most are given intravenously, but Dr. Cheng thinks exosomes — also called extracellular vesicles (EVs) — could change that.
“One of the advantages of exosomes is that they are naturally secreted by the body or cultured cells,” he noted. “They have low toxicity and have multiple ways of getting their message into cells.”
The scientists borrowed an approach that captured public attention during the pandemic: Using messenger RNA, which directs cells to make proteins for tasks — including boosting immune response.
IL-12 has shown promise against cancer for decades, but early human trials triggered serious side effects and several deaths. Researchers are now trying new delivery methods that target tumor cells without affecting healthy tissue. Dr. Cheng’s team took a new approach, inserting mRNA for IL-12 into exosomes.
One aim of the study was to compare the effectiveness of inhaled exosomes vs inhaled liposomes, engineered fat droplets also under investigation as drug carriers. The team’s question: Which would work better at introducing IL-12 to the lungs to affect cancer, without triggering side effects?
After lab mice inhaled the particles through the nose, the researchers found that exosomes delivered more mRNA into cancer cells in the lungs and fought lung cancer with few side effects. Three days after treatment, researchers saw an influx of cancer-fighting T cells within tumors — with higher levels for exosome-based treatment. Plus, the exosomes led to more cancer-destroying nature killer cells and more monocytes, a sign of immune-system activation.
Researchers also found the treatment acted as a vaccine, training the immune system to battle newly introduced cancers. Little of the exosome-delivered drug escaped into the bloodstream, and the study found minimal side effects. Inhalation didn’t affect normal breathing, Dr. Cheng added.
The study’s use of inhaled exosomes makes it significant, said Raghu Kalluri, MD, PhD, professor and chair of the Department of Cancer Biology at MD Anderson Cancer Center. “This is an interesting study that explores the inhalable delivery of engineered EVs for the treatment of lung cancer and offers insights into focused delivery of EV-based drugs…with implications for diseases beyond cancer,” he said. Dr. Kalluri is also an exosome researcher.
New Frontiers
Once seen as a “quirky biological phenomenon” or just cellular trash, exosomes are now the subject of intense medical research for their potential as drug carriers, as treatments in their own right for everything from wound healing and pneumonia to heart attacks and bowel disorders, and as measurable biological markers that could lead to new tests for cancer and other conditions. One exosome-based prostate cancer test, the ExoDx Prostate Test, is already on the market.
The explosion in exosome research — the number of published studies has grown from just a handful in the early 1980s to more than 9000 — spotlights a particular focus on cancer. According to a 2021 paper in Annals of Oncology, clinical trials for exosomes in cancer treatments and tests far out-paces those for diabetes, heart disease, or neurologic conditions. Currently, 52 clinical trials using exosomes in cancer diagnosis or treatment have been completed, are underway, or are looking for participants, according to clinicaltrials.gov.
Dr. Cheng’s approach could also be used to deliver other drugs to the lungs and other organs via inhalation. “We’re testing inhalation for a different type of lung disease, acute lung injury,” Dr. Cheng said. Other potential targets include lung disorders like pulmonary hypertension. Inhaled exosomes could potentially reach the brain via the olfactory bulb or the heart as it receives oxygenated blood from the lungs.
Breathing in Medicine
So far, inhalable cancer treatments are not available outside research studies in the United States or Europe , said Remi Rosiere, PhD, a lecturer at the Université libre de Bruxelles in Brussels, Belgium, and chief scientific officer of InhaTarget Therapeutics, a company developing its own inhaled treatments for severe respiratory diseases. “Oncologists are very interested,” he said. “If you concentrate the drug on the tumor site, you can avoid distribution to the body.”
Early research into inhalable chemotherapy began in the 1960s but was unsuccessful because breathing equipment dispersed toxic cancer drugs into the air or delivered only small amounts to the lungs, he said.
New delivery techniques aim to change that. Dr. Rosiere’s company is starting a human trial of a dry powder inhaler with the chemotherapy drug cisplatin for lung cancer. Also in the pipeline is an immunotherapy treatment for lung cancer inserted in lipid nanoparticles, which are tiny fat particles similar to liposomes.
He said Dr. Cheng’s study shows the advantages of sending in exosomes. “The data are very persuasive,” Dr. Rosier said of the study. “Exosomes have a good safety profile and are able to remain in the lung for quite a long time. This prolongs exposure to the drug for greater effectiveness, without causing toxicities.”
Getting from a mouse study to a human trial will take time. “You need to understand this is very early stage,” Dr. Rosiere added. “There will be many challenges to overcome.”
One is purely practical: If the drug approaches human trials, he said, regulators will ask whether the exosomes can be produced in large quantities to meet the huge demand for new lung cancer treatments. “Lung cancer is the number one fatal cancer in the world,” Dr. Rosiere said.
A New Route for ‘Powerful’ Cancer Treatment
Meanwhile, the Columbia University study showed that inhalable exosomes are a unique delivery method for IL-12 — and could help solve a major problem that’s plagued this promising cancer treatment for decades.
Called “one of the most powerful immunotherapy agents ever discovered” in a 2022 literature review, IL-12 showed serious side effects that stalled research in the 1980s , sparking an ongoing search for new delivery methods that continues today. In 2022 and 2023, Big Pharma companies including AstraZenca, Moderna, and Bristol Myers Squib reduced their involvement with IL-12 treatment research, leaving the field open to smaller biotech companies working on a variety of drug-delivery approaches that could make IL-12 safe and effective in humans.
These include injecting it directly into tumors, encasing it in various types of particles, masking the drug so it is activated only in cancer cells, and using IL-12 mRNA, which essentially turns tumor cells into IL-12–producing factories. Another IL-12 mRNA drug, from Pittsburgh-based Krystal Biotech, received a fast-track designation from the US Food and Drug Administration in February 2024 for an inhaled lung cancer treatment that packages mRNA for IL-12 and IL-2 inside an engineered virus.
And of course, there is Dr. Cheng’s inhalable treatment, culminating decades of work across three burgeoning fields.
A version of this article appeared on Medscape.com.
Cells in the human body chat with each other all the time. One major way they communicate is by releasing tiny spheres called exosomes. These carry fats, proteins, and genetic material that help regulate everything from pregnancy and immune responses to heart health and kidney function.
“Exosomes work like text messages between cells , sending and receiving information,” said lead researcher Ke Cheng, PhD, professor of biomedical engineering at Columbia. “The significance of this study is that exosomes can bring mRNA-based treatment to lung cancer cells locally, unlike systemic chemotherapy that can have side effects throughout the body. And inhalation is totally noninvasive. You don’t need a nurse to use an IV needle to pierce your skin.”
Dr. Cheng expects a human trial could launch within 5 years. For now, his study is attracting attention because it marks an advance in three areas of intense interest by researchers and biotech companies alike: Therapeutic uses of exosomes, inhalable treatments for lung conditions, and the safe delivery of powerful interleukin-12 (IL-12) immunotherapy.
Inside the Study
Dr. Cheng, who has been developing exosome and stem cell therapies for more than 15 years, and his lab team focused on lung cancer because the disease, often detected in later stages, “has a huge mortality rate,” he said. “Therapies have been suboptimal and leave the organ so damaged.”
He wanted to explore new alternatives to systemic treatments. Most are given intravenously, but Dr. Cheng thinks exosomes — also called extracellular vesicles (EVs) — could change that.
“One of the advantages of exosomes is that they are naturally secreted by the body or cultured cells,” he noted. “They have low toxicity and have multiple ways of getting their message into cells.”
The scientists borrowed an approach that captured public attention during the pandemic: Using messenger RNA, which directs cells to make proteins for tasks — including boosting immune response.
IL-12 has shown promise against cancer for decades, but early human trials triggered serious side effects and several deaths. Researchers are now trying new delivery methods that target tumor cells without affecting healthy tissue. Dr. Cheng’s team took a new approach, inserting mRNA for IL-12 into exosomes.
One aim of the study was to compare the effectiveness of inhaled exosomes vs inhaled liposomes, engineered fat droplets also under investigation as drug carriers. The team’s question: Which would work better at introducing IL-12 to the lungs to affect cancer, without triggering side effects?
After lab mice inhaled the particles through the nose, the researchers found that exosomes delivered more mRNA into cancer cells in the lungs and fought lung cancer with few side effects. Three days after treatment, researchers saw an influx of cancer-fighting T cells within tumors — with higher levels for exosome-based treatment. Plus, the exosomes led to more cancer-destroying nature killer cells and more monocytes, a sign of immune-system activation.
Researchers also found the treatment acted as a vaccine, training the immune system to battle newly introduced cancers. Little of the exosome-delivered drug escaped into the bloodstream, and the study found minimal side effects. Inhalation didn’t affect normal breathing, Dr. Cheng added.
The study’s use of inhaled exosomes makes it significant, said Raghu Kalluri, MD, PhD, professor and chair of the Department of Cancer Biology at MD Anderson Cancer Center. “This is an interesting study that explores the inhalable delivery of engineered EVs for the treatment of lung cancer and offers insights into focused delivery of EV-based drugs…with implications for diseases beyond cancer,” he said. Dr. Kalluri is also an exosome researcher.
New Frontiers
Once seen as a “quirky biological phenomenon” or just cellular trash, exosomes are now the subject of intense medical research for their potential as drug carriers, as treatments in their own right for everything from wound healing and pneumonia to heart attacks and bowel disorders, and as measurable biological markers that could lead to new tests for cancer and other conditions. One exosome-based prostate cancer test, the ExoDx Prostate Test, is already on the market.
The explosion in exosome research — the number of published studies has grown from just a handful in the early 1980s to more than 9000 — spotlights a particular focus on cancer. According to a 2021 paper in Annals of Oncology, clinical trials for exosomes in cancer treatments and tests far out-paces those for diabetes, heart disease, or neurologic conditions. Currently, 52 clinical trials using exosomes in cancer diagnosis or treatment have been completed, are underway, or are looking for participants, according to clinicaltrials.gov.
Dr. Cheng’s approach could also be used to deliver other drugs to the lungs and other organs via inhalation. “We’re testing inhalation for a different type of lung disease, acute lung injury,” Dr. Cheng said. Other potential targets include lung disorders like pulmonary hypertension. Inhaled exosomes could potentially reach the brain via the olfactory bulb or the heart as it receives oxygenated blood from the lungs.
Breathing in Medicine
So far, inhalable cancer treatments are not available outside research studies in the United States or Europe , said Remi Rosiere, PhD, a lecturer at the Université libre de Bruxelles in Brussels, Belgium, and chief scientific officer of InhaTarget Therapeutics, a company developing its own inhaled treatments for severe respiratory diseases. “Oncologists are very interested,” he said. “If you concentrate the drug on the tumor site, you can avoid distribution to the body.”
Early research into inhalable chemotherapy began in the 1960s but was unsuccessful because breathing equipment dispersed toxic cancer drugs into the air or delivered only small amounts to the lungs, he said.
New delivery techniques aim to change that. Dr. Rosiere’s company is starting a human trial of a dry powder inhaler with the chemotherapy drug cisplatin for lung cancer. Also in the pipeline is an immunotherapy treatment for lung cancer inserted in lipid nanoparticles, which are tiny fat particles similar to liposomes.
He said Dr. Cheng’s study shows the advantages of sending in exosomes. “The data are very persuasive,” Dr. Rosier said of the study. “Exosomes have a good safety profile and are able to remain in the lung for quite a long time. This prolongs exposure to the drug for greater effectiveness, without causing toxicities.”
Getting from a mouse study to a human trial will take time. “You need to understand this is very early stage,” Dr. Rosiere added. “There will be many challenges to overcome.”
One is purely practical: If the drug approaches human trials, he said, regulators will ask whether the exosomes can be produced in large quantities to meet the huge demand for new lung cancer treatments. “Lung cancer is the number one fatal cancer in the world,” Dr. Rosiere said.
A New Route for ‘Powerful’ Cancer Treatment
Meanwhile, the Columbia University study showed that inhalable exosomes are a unique delivery method for IL-12 — and could help solve a major problem that’s plagued this promising cancer treatment for decades.
Called “one of the most powerful immunotherapy agents ever discovered” in a 2022 literature review, IL-12 showed serious side effects that stalled research in the 1980s , sparking an ongoing search for new delivery methods that continues today. In 2022 and 2023, Big Pharma companies including AstraZenca, Moderna, and Bristol Myers Squib reduced their involvement with IL-12 treatment research, leaving the field open to smaller biotech companies working on a variety of drug-delivery approaches that could make IL-12 safe and effective in humans.
These include injecting it directly into tumors, encasing it in various types of particles, masking the drug so it is activated only in cancer cells, and using IL-12 mRNA, which essentially turns tumor cells into IL-12–producing factories. Another IL-12 mRNA drug, from Pittsburgh-based Krystal Biotech, received a fast-track designation from the US Food and Drug Administration in February 2024 for an inhaled lung cancer treatment that packages mRNA for IL-12 and IL-2 inside an engineered virus.
And of course, there is Dr. Cheng’s inhalable treatment, culminating decades of work across three burgeoning fields.
A version of this article appeared on Medscape.com.
FROM NATURE NANOTECHNOLOGY
Cancer Data Trends 2024: Lung Cancer
1. Wolf AMD, Oeffinger KC, Shih TYC, et al. Screening for lung cancer: 2023 guideline update from the American Cancer Society. CA Cancer J Clin. 2023;10.3322/caac.21811. doi:10.3322/caac.21811
2. US Department of Veterans Affairs. VA promotes high-quality, patient-centered lung cancer screening for veterans. Published June 15, 2023. Accessed December 18, 2023. http://www.hsrd.research.va.gov/impacts/lcs.cfm
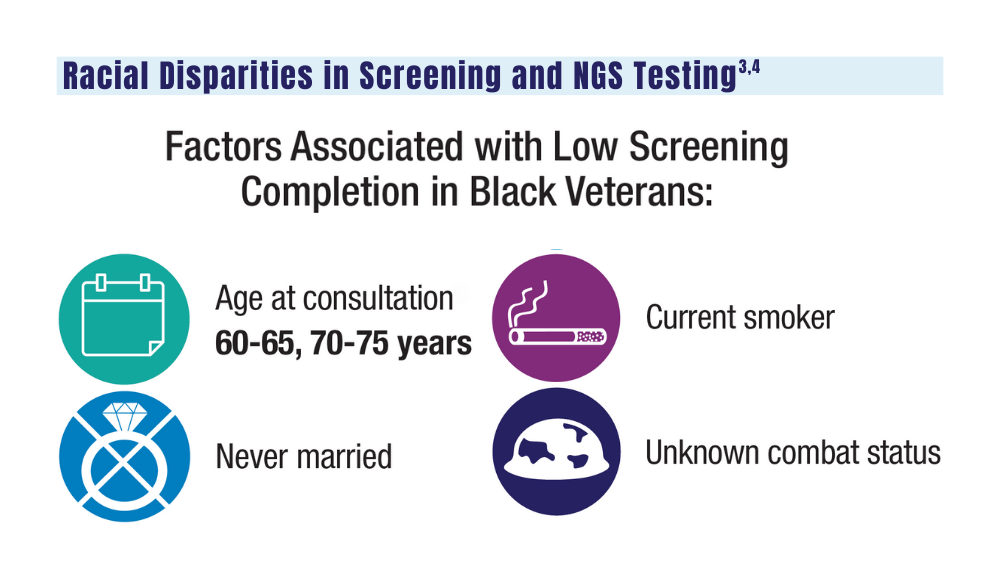
3. Navuluri N, Morrison S, Green CL, et al. Racial disparities in lung cancer screening among veterans, 2013 to 2021. JAMA Netw Open. 2023;6(6):e2318795. doi:10.1001/jamanetworkopen.2023.18795
4. Bruno DS, Hess LM, Li X, Su EW, Patel M. Disparities in biomarker testing and clinical trial enrollment among patients with lung, breast, or colorectal cancers in the United States. JCO Precis Oncol. 2022;6:e2100427. doi:10.1200/PO.21.00427
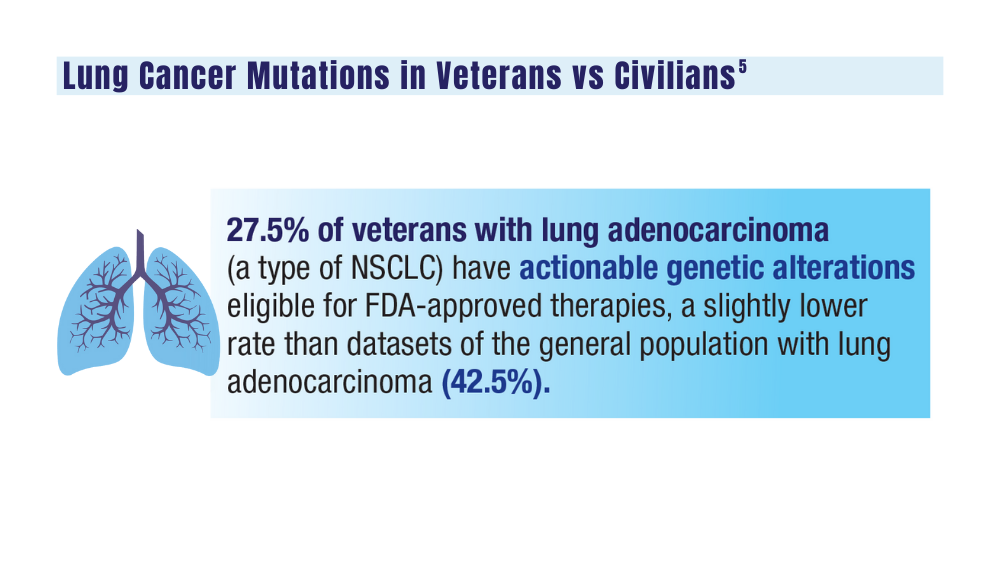
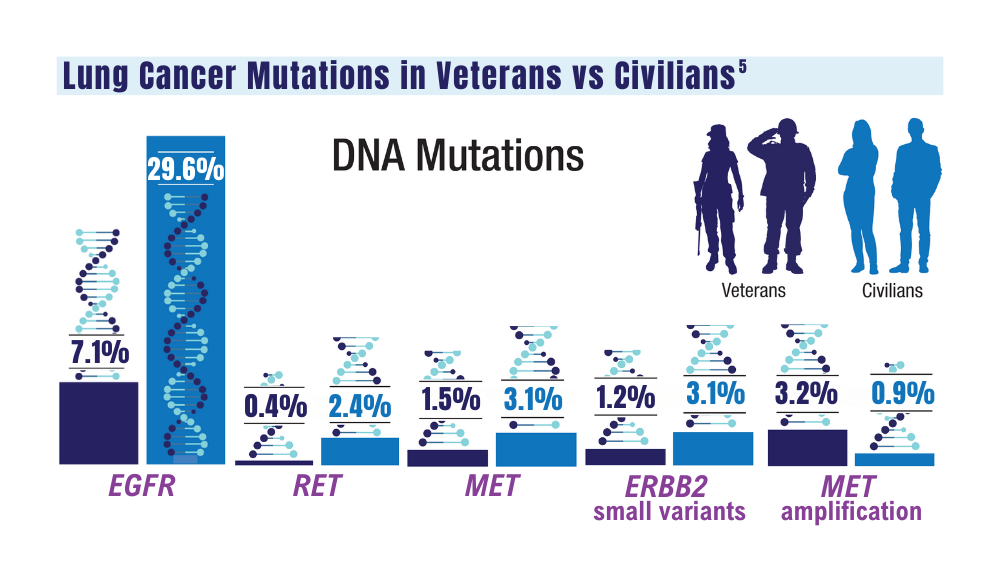
5. Jalal SI, Guo A, Ahmed S, Kelley MJ. Analysis of actionable genetic alterations in lung carcinoma from the VA National Precision Oncology Program. Semin Oncol. 2022;S0093-7754(22)00054-9. doi:10.1053/j.seminoncol.2022.06.014
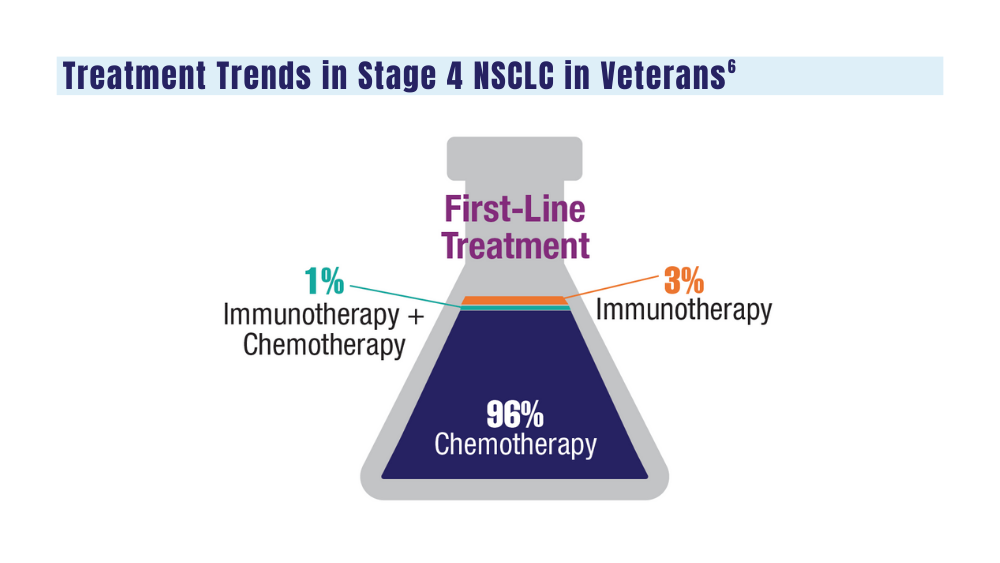
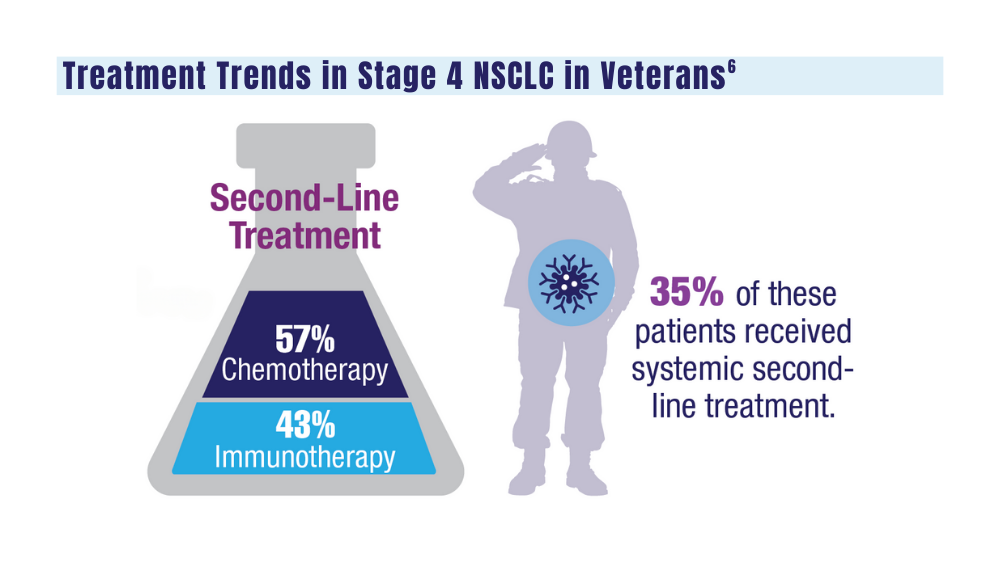
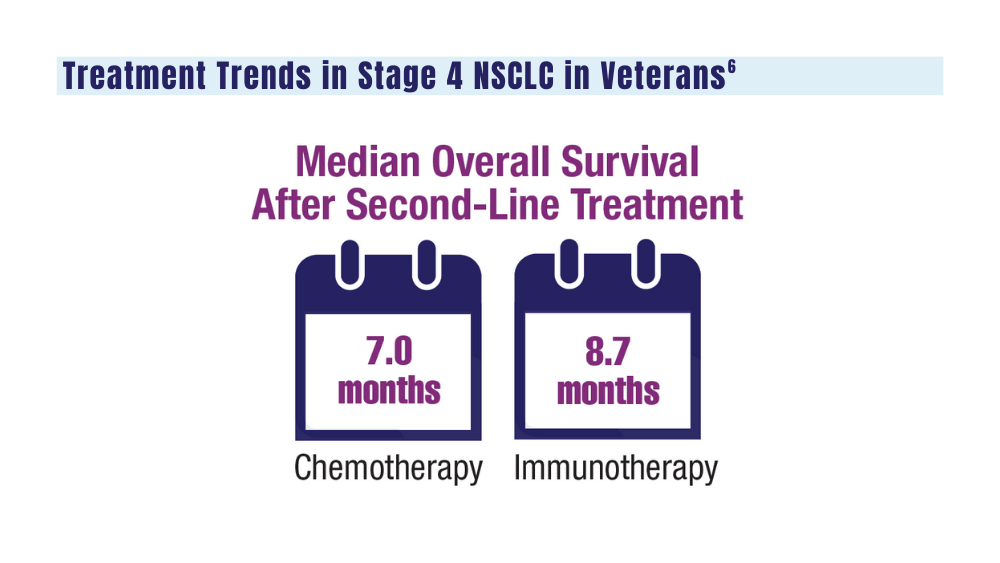
6. Williams CD, Allo MA, Gu L, Vashistha V, Press A, Kelley M. Health outcomes and healthcare resource utilization among veterans with stage IV non-small cell lung cancer treated with second-line chemotherapy versus immunotherapy. PLoS One. 2023;18(2):e0282020. doi:10.1371/journal.pone.0282020
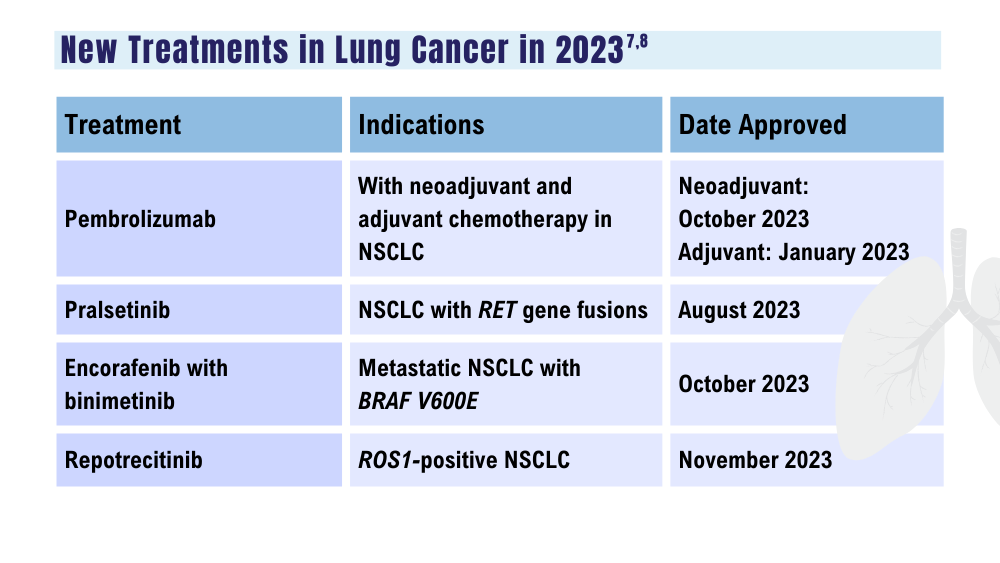
7. US Food and Drug Administration. Oncology (cancer)/hematologic malignancies approval notifications. Updated December 15, 2023. Accessed December 18, 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/oncology-cancer-hematologic-malignancies-approval-notifications
8. Paz-Ares L, Chen Y, Reinmuth N, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer: 3-year overall survival update from CASPIAN. ESMO Open. 2022;7(2):100408. doi:10.1016/j.esmoop.2022.100408
1. Wolf AMD, Oeffinger KC, Shih TYC, et al. Screening for lung cancer: 2023 guideline update from the American Cancer Society. CA Cancer J Clin. 2023;10.3322/caac.21811. doi:10.3322/caac.21811
2. US Department of Veterans Affairs. VA promotes high-quality, patient-centered lung cancer screening for veterans. Published June 15, 2023. Accessed December 18, 2023. http://www.hsrd.research.va.gov/impacts/lcs.cfm
3. Navuluri N, Morrison S, Green CL, et al. Racial disparities in lung cancer screening among veterans, 2013 to 2021. JAMA Netw Open. 2023;6(6):e2318795. doi:10.1001/jamanetworkopen.2023.18795
4. Bruno DS, Hess LM, Li X, Su EW, Patel M. Disparities in biomarker testing and clinical trial enrollment among patients with lung, breast, or colorectal cancers in the United States. JCO Precis Oncol. 2022;6:e2100427. doi:10.1200/PO.21.00427
5. Jalal SI, Guo A, Ahmed S, Kelley MJ. Analysis of actionable genetic alterations in lung carcinoma from the VA National Precision Oncology Program. Semin Oncol. 2022;S0093-7754(22)00054-9. doi:10.1053/j.seminoncol.2022.06.014
6. Williams CD, Allo MA, Gu L, Vashistha V, Press A, Kelley M. Health outcomes and healthcare resource utilization among veterans with stage IV non-small cell lung cancer treated with second-line chemotherapy versus immunotherapy. PLoS One. 2023;18(2):e0282020. doi:10.1371/journal.pone.0282020
7. US Food and Drug Administration. Oncology (cancer)/hematologic malignancies approval notifications. Updated December 15, 2023. Accessed December 18, 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/oncology-cancer-hematologic-malignancies-approval-notifications
8. Paz-Ares L, Chen Y, Reinmuth N, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer: 3-year overall survival update from CASPIAN. ESMO Open. 2022;7(2):100408. doi:10.1016/j.esmoop.2022.100408
1. Wolf AMD, Oeffinger KC, Shih TYC, et al. Screening for lung cancer: 2023 guideline update from the American Cancer Society. CA Cancer J Clin. 2023;10.3322/caac.21811. doi:10.3322/caac.21811
2. US Department of Veterans Affairs. VA promotes high-quality, patient-centered lung cancer screening for veterans. Published June 15, 2023. Accessed December 18, 2023. http://www.hsrd.research.va.gov/impacts/lcs.cfm
3. Navuluri N, Morrison S, Green CL, et al. Racial disparities in lung cancer screening among veterans, 2013 to 2021. JAMA Netw Open. 2023;6(6):e2318795. doi:10.1001/jamanetworkopen.2023.18795
4. Bruno DS, Hess LM, Li X, Su EW, Patel M. Disparities in biomarker testing and clinical trial enrollment among patients with lung, breast, or colorectal cancers in the United States. JCO Precis Oncol. 2022;6:e2100427. doi:10.1200/PO.21.00427
5. Jalal SI, Guo A, Ahmed S, Kelley MJ. Analysis of actionable genetic alterations in lung carcinoma from the VA National Precision Oncology Program. Semin Oncol. 2022;S0093-7754(22)00054-9. doi:10.1053/j.seminoncol.2022.06.014
6. Williams CD, Allo MA, Gu L, Vashistha V, Press A, Kelley M. Health outcomes and healthcare resource utilization among veterans with stage IV non-small cell lung cancer treated with second-line chemotherapy versus immunotherapy. PLoS One. 2023;18(2):e0282020. doi:10.1371/journal.pone.0282020
7. US Food and Drug Administration. Oncology (cancer)/hematologic malignancies approval notifications. Updated December 15, 2023. Accessed December 18, 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/oncology-cancer-hematologic-malignancies-approval-notifications
8. Paz-Ares L, Chen Y, Reinmuth N, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer: 3-year overall survival update from CASPIAN. ESMO Open. 2022;7(2):100408. doi:10.1016/j.esmoop.2022.100408
Cancer Data Trends 2024
The annual issue of Cancer Data Trends, produced in collaboration with the Association of VA Hematology/Oncology (AVAHO), highlights the latest research in some of the top cancers impacting US veterans.
Click to view the Digital Edition.
In this issue:
Hepatocellular Carcinoma
Special care for veterans, changes in staging, and biomarkers for early diagnosis
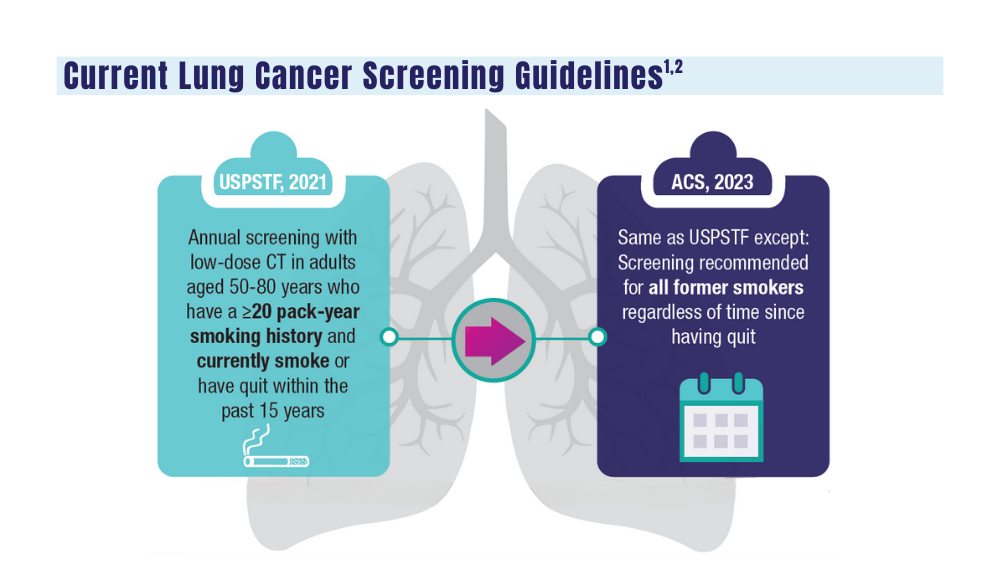
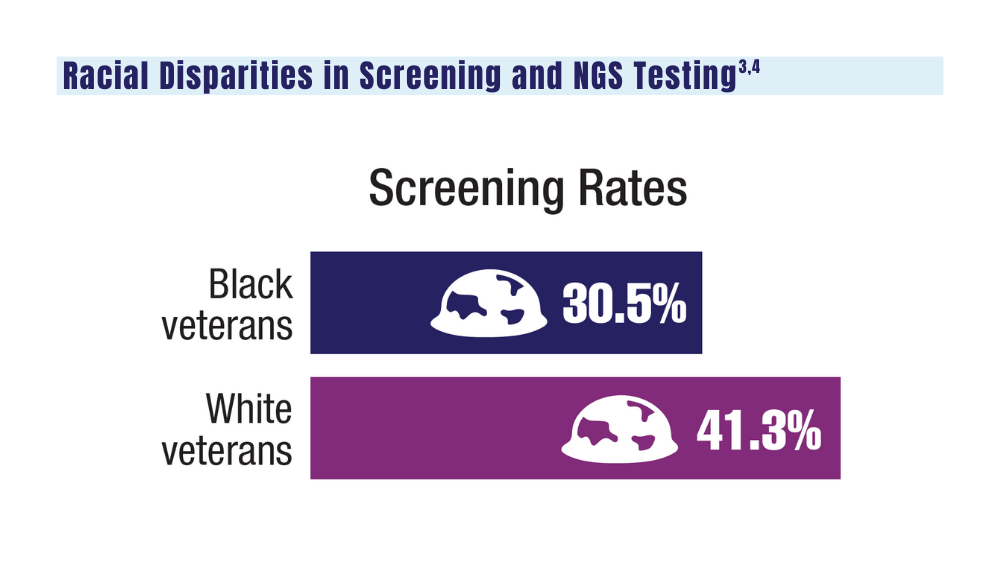
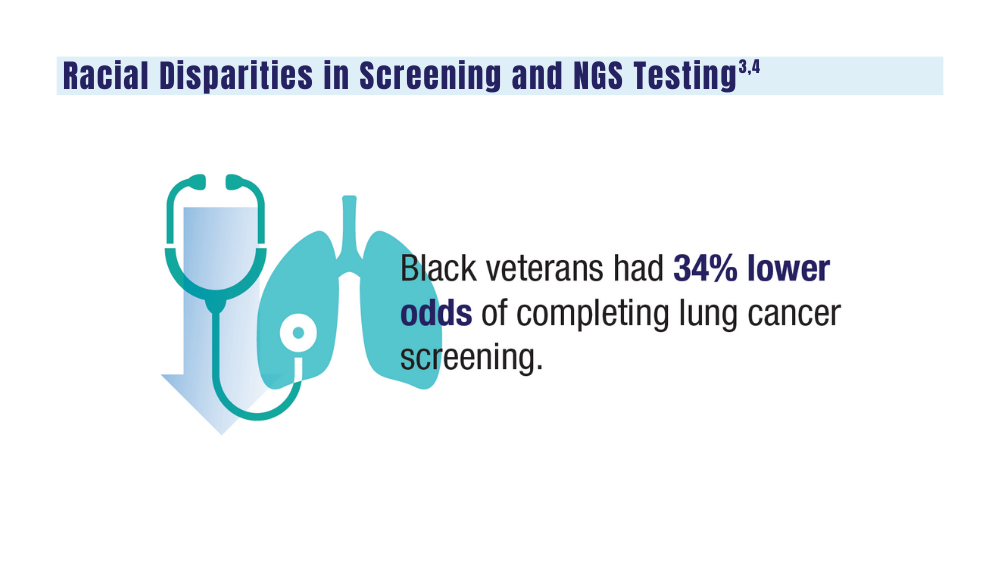
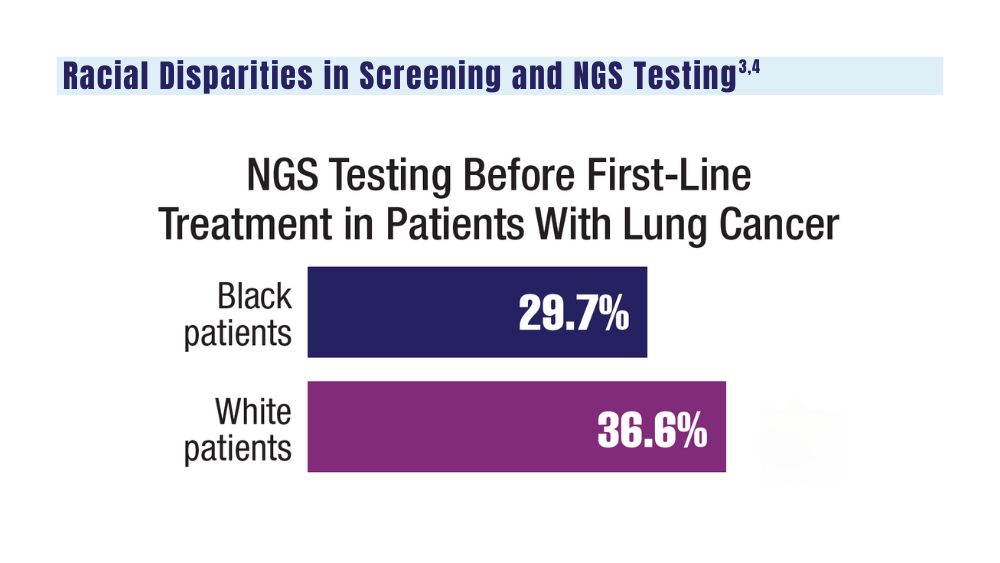
Lung Cancer
Guideline updates and racial disparities in veterans
Multiple Myeloma
Improving survival in the VA
Colorectal Cancer
Barriers to follow-up colonoscopies after FIT testing
B-Cell Lymphomas
Findings from the VA's National TeleOncology Program and recent therapy updates
Breast Cancer
A look at the VA's Risk Assessment Pipeline and incidence among veterans vs the general population
Genitourinary Cancers
Molecular testing in prostate cancer, improving survival for metastatic RCC, and links between bladder cancer and Agent Orange exposure
The annual issue of Cancer Data Trends, produced in collaboration with the Association of VA Hematology/Oncology (AVAHO), highlights the latest research in some of the top cancers impacting US veterans.
Click to view the Digital Edition.
In this issue:
Hepatocellular Carcinoma
Special care for veterans, changes in staging, and biomarkers for early diagnosis
Lung Cancer
Guideline updates and racial disparities in veterans
Multiple Myeloma
Improving survival in the VA
Colorectal Cancer
Barriers to follow-up colonoscopies after FIT testing
B-Cell Lymphomas
Findings from the VA's National TeleOncology Program and recent therapy updates
Breast Cancer
A look at the VA's Risk Assessment Pipeline and incidence among veterans vs the general population
Genitourinary Cancers
Molecular testing in prostate cancer, improving survival for metastatic RCC, and links between bladder cancer and Agent Orange exposure
The annual issue of Cancer Data Trends, produced in collaboration with the Association of VA Hematology/Oncology (AVAHO), highlights the latest research in some of the top cancers impacting US veterans.
Click to view the Digital Edition.
In this issue:
Hepatocellular Carcinoma
Special care for veterans, changes in staging, and biomarkers for early diagnosis
Lung Cancer
Guideline updates and racial disparities in veterans
Multiple Myeloma
Improving survival in the VA
Colorectal Cancer
Barriers to follow-up colonoscopies after FIT testing
B-Cell Lymphomas
Findings from the VA's National TeleOncology Program and recent therapy updates
Breast Cancer
A look at the VA's Risk Assessment Pipeline and incidence among veterans vs the general population
Genitourinary Cancers
Molecular testing in prostate cancer, improving survival for metastatic RCC, and links between bladder cancer and Agent Orange exposure
Consider These Factors in an Academic Radiation Oncology Position
TOPLINE:
— and accept an offer if the practice is “great” in at least two of those areas and “good” in the third, experts say in a recent editorial.
METHODOLOGY:
- Many physicians choose to go into academic medicine because they want to stay involved in research and education while still treating patients.
- However, graduating radiation oncology residents often lack or have limited guidance on what to look for in a prospective job and how to assess their contract.
- This recent editorial provides guidance to radiation oncologists seeking academic positions. The authors advise prospective employees to evaluate three main factors — compensation, daily duties, and location — as well as provide tips for identifying red flags in each category.
TAKEAWAY:
- Compensation: Prospective faculty should assess both direct compensation, that is, salary, and indirect compensation, which typically includes retirement contributions and other perks. For direct compensation, what is the base salary? Is extra work compensated? How does the salary offer measure up to salary data reported by national agencies? Also: Don’t overlook uncompensated duties, such as time in tumor boards or in meetings, which may be time-consuming, and make sure compensation terms are clearly delineated in a contract and equitable among physicians in a specific rank.
- Daily duties: When it comes to daily life on the job, a prospective employee should consider many factors, including the cancer center’s excitement to hire you, the reputation of the faculty and leaders at the organization, employee turnover rates, diversity among faculty, and the time line of career advancement.
- Location: The location of the job encompasses the geography — such as distance from home to work, the number of practices covered, cost of living, and the area itself — as well as the atmosphere for conducting research and publishing.
- Finally, carefully review the job contract. All the key aspects of the job, including compensation and benefits, should be clearly stated in the contract to “improve communication of expectations.”
IN PRACTICE:
“A prospective faculty member can ask 100 questions, but they can’t make 100 demands; consideration of the three domains can help to focus negotiation efforts where the efforts are needed,” the authors noted.
SOURCE:
This editorial, led by Nicholas G. Zaorsky from the Department of Radiation Oncology, University Hospitals Seidman Cancer Center, Case Western Reserve School of Medicine, Cleveland, Ohio, was published online in Practical Radiation Oncology
DISCLOSURES:
The lead author declared being supported by the American Cancer Society and National Institutes of Health. He also reported having ties with many other sources.
A version of this article appeared on Medscape.com.
TOPLINE:
— and accept an offer if the practice is “great” in at least two of those areas and “good” in the third, experts say in a recent editorial.
METHODOLOGY:
- Many physicians choose to go into academic medicine because they want to stay involved in research and education while still treating patients.
- However, graduating radiation oncology residents often lack or have limited guidance on what to look for in a prospective job and how to assess their contract.
- This recent editorial provides guidance to radiation oncologists seeking academic positions. The authors advise prospective employees to evaluate three main factors — compensation, daily duties, and location — as well as provide tips for identifying red flags in each category.
TAKEAWAY:
- Compensation: Prospective faculty should assess both direct compensation, that is, salary, and indirect compensation, which typically includes retirement contributions and other perks. For direct compensation, what is the base salary? Is extra work compensated? How does the salary offer measure up to salary data reported by national agencies? Also: Don’t overlook uncompensated duties, such as time in tumor boards or in meetings, which may be time-consuming, and make sure compensation terms are clearly delineated in a contract and equitable among physicians in a specific rank.
- Daily duties: When it comes to daily life on the job, a prospective employee should consider many factors, including the cancer center’s excitement to hire you, the reputation of the faculty and leaders at the organization, employee turnover rates, diversity among faculty, and the time line of career advancement.
- Location: The location of the job encompasses the geography — such as distance from home to work, the number of practices covered, cost of living, and the area itself — as well as the atmosphere for conducting research and publishing.
- Finally, carefully review the job contract. All the key aspects of the job, including compensation and benefits, should be clearly stated in the contract to “improve communication of expectations.”
IN PRACTICE:
“A prospective faculty member can ask 100 questions, but they can’t make 100 demands; consideration of the three domains can help to focus negotiation efforts where the efforts are needed,” the authors noted.
SOURCE:
This editorial, led by Nicholas G. Zaorsky from the Department of Radiation Oncology, University Hospitals Seidman Cancer Center, Case Western Reserve School of Medicine, Cleveland, Ohio, was published online in Practical Radiation Oncology
DISCLOSURES:
The lead author declared being supported by the American Cancer Society and National Institutes of Health. He also reported having ties with many other sources.
A version of this article appeared on Medscape.com.
TOPLINE:
— and accept an offer if the practice is “great” in at least two of those areas and “good” in the third, experts say in a recent editorial.
METHODOLOGY:
- Many physicians choose to go into academic medicine because they want to stay involved in research and education while still treating patients.
- However, graduating radiation oncology residents often lack or have limited guidance on what to look for in a prospective job and how to assess their contract.
- This recent editorial provides guidance to radiation oncologists seeking academic positions. The authors advise prospective employees to evaluate three main factors — compensation, daily duties, and location — as well as provide tips for identifying red flags in each category.
TAKEAWAY:
- Compensation: Prospective faculty should assess both direct compensation, that is, salary, and indirect compensation, which typically includes retirement contributions and other perks. For direct compensation, what is the base salary? Is extra work compensated? How does the salary offer measure up to salary data reported by national agencies? Also: Don’t overlook uncompensated duties, such as time in tumor boards or in meetings, which may be time-consuming, and make sure compensation terms are clearly delineated in a contract and equitable among physicians in a specific rank.
- Daily duties: When it comes to daily life on the job, a prospective employee should consider many factors, including the cancer center’s excitement to hire you, the reputation of the faculty and leaders at the organization, employee turnover rates, diversity among faculty, and the time line of career advancement.
- Location: The location of the job encompasses the geography — such as distance from home to work, the number of practices covered, cost of living, and the area itself — as well as the atmosphere for conducting research and publishing.
- Finally, carefully review the job contract. All the key aspects of the job, including compensation and benefits, should be clearly stated in the contract to “improve communication of expectations.”
IN PRACTICE:
“A prospective faculty member can ask 100 questions, but they can’t make 100 demands; consideration of the three domains can help to focus negotiation efforts where the efforts are needed,” the authors noted.
SOURCE:
This editorial, led by Nicholas G. Zaorsky from the Department of Radiation Oncology, University Hospitals Seidman Cancer Center, Case Western Reserve School of Medicine, Cleveland, Ohio, was published online in Practical Radiation Oncology
DISCLOSURES:
The lead author declared being supported by the American Cancer Society and National Institutes of Health. He also reported having ties with many other sources.
A version of this article appeared on Medscape.com.
Look Beyond BMI: Metabolic Factors’ Link to Cancer Explained
The new research finds that adults with persistent metabolic syndrome that worsens over time are at increased risk for any type of cancer.
The conditions that make up metabolic syndrome (high blood pressure, high blood sugar, increased abdominal adiposity, and high cholesterol and triglycerides) have been associated with an increased risk of diseases, including heart disease, stroke, and type 2 diabetes, wrote Li Deng, PhD, of Capital Medical University, Beijing, and colleagues.
However, a single assessment of metabolic syndrome at one point in time is inadequate to show an association with cancer risk over time, they said. In the current study, the researchers used models to examine the association between trajectory patterns of metabolic syndrome over time and the risk of overall and specific cancer types. They also examined the impact of chronic inflammation concurrent with metabolic syndrome.
What We Know About Metabolic Syndrome and Cancer Risk
A systematic review and meta-analysis published in Diabetes Care in 2012 showed an association between the presence of metabolic syndrome and an increased risk of various cancers including liver, bladder, pancreatic, breast, and colorectal.
More recently, a 2020 study published in Diabetes showed evidence of increased risk for certain cancers (pancreatic, kidney, uterine, cervical) but no increased risk for cancer overall.
In addition, a 2022 study by some of the current study researchers of the same Chinese cohort focused on the role of inflammation in combination with metabolic syndrome on colorectal cancer specifically, and found an increased risk for cancer when both metabolic syndrome and inflammation were present.
However, the reasons for this association between metabolic syndrome and cancer remain unclear, and the effect of the fluctuating nature of metabolic syndrome over time on long-term cancer risk has not been explored, the researchers wrote.
“There is emerging evidence that even normal weight individuals who are metabolically unhealthy may be at an elevated cancer risk, and we need better metrics to define the underlying metabolic dysfunction in obesity,” Sheetal Hardikar, MBBS, PhD, MPH, an investigator at the Huntsman Cancer Institute, University of Utah, said in an interview.
Dr. Hardikar, who serves as assistant professor in the department of population health sciences at the University of Utah, was not involved in the current study. She and her colleagues published a research paper on data from the National Health and Nutrition Examination Survey in 2023 that showed an increased risk of obesity-related cancer.
What New Study Adds to Related Research
Previous studies have consistently reported an approximately 30% increased risk of cancer with metabolic syndrome, Dr. Hardikar said. “What is unique about this study is the examination of metabolic syndrome trajectories over four years, and not just the presence of metabolic syndrome at one point in time,” she said.
In the new study, published in Cancer on March 11 (doi: 10.1002/cncr.35235), 44,115 adults in China were separated into four trajectories based on metabolic syndrome scores for the period from 2006 to 2010. The scores were based on clinical evidence of metabolic syndrome, defined using the International Diabetes Federation criteria of central obesity and the presence of at least two other factors including increased triglycerides, decreased HDL cholesterol, high blood pressure (or treatment for previously diagnosed hypertension), and increased fasting plasma glucose (or previous diagnosis of type 2 diabetes).
The average age of the participants was 49 years; the mean body mass index ranged from approximately 22 kg/m2 in the low-stable group to approximately 28 kg/m2 in the elevated-increasing group.
The four trajectories of metabolic syndrome were low-stable (10.56% of participants), moderate-low (40.84%), moderate-high (41.46%), and elevated-increasing (7.14%), based on trends from the individuals’ initial physical exams on entering the study.
Over a median follow-up period of 9.4 years (from 2010 to 2021), 2,271 cancer diagnoses were reported in the study population. Those with an elevated-increasing metabolic syndrome trajectory had 1.3 times the risk of any cancer compared with those in the low-stable group. Risk for breast cancer, endometrial cancer, kidney cancer, colorectal cancer, and liver cancer in the highest trajectory group were 2.1, 3.3, 4.5, 2.5, and 1.6 times higher, respectively, compared to the lowest group. The increased risk in the elevated-trajectory group for all cancer types persisted when the low-stable, moderate-low, and moderate-high trajectory pattern groups were combined.
The researchers also examined the impact of chronic inflammation and found that individuals with persistently high metabolic syndrome scores and concurrent chronic inflammation had the highest risks of breast, endometrial, colon, and liver cancer. However, individuals with persistently high metabolic syndrome scores and no concurrent chronic inflammation had the highest risk of kidney cancer.
What Are the Limitations of This Research?
The researchers of the current study acknowledged the lack of information on other causes of cancer, including dietary habits, hepatitis C infection, and Helicobacter pylori infection. Other limitations include the focus only on individuals from a single community of mainly middle-aged men in China that may not generalize to other populations.
Also, the metabolic syndrome trajectories did not change much over time, which may be related to the short 4-year study period.
Using the International Diabetes Federation criteria was another limitation, because it prevented the assessment of cancer risk in normal weight individuals with metabolic dysfunction, Dr. Hardikar noted.
Does Metabolic Syndrome Cause Cancer?
“This research suggests that proactive and continuous management of metabolic syndrome may serve as an essential strategy in preventing cancer,” senior author Han-Ping Shi, MD, PhD, of Capital Medical University in Beijing, noted in a statement on the study.
More research is needed to assess the impact of these interventions on cancer risk. However, the data from the current study can guide future research that may lead to more targeted treatments and more effective preventive strategies, he continued.
“Current evidence based on this study and many other reports strongly suggests an increased risk for cancer associated with metabolic syndrome,” Dr. Hardikar said in an interview. The data serve as a reminder to clinicians to look beyond BMI as the only measure of obesity, and to consider metabolic factors together to identify individuals at increased risk for cancer, she said.
“We must continue to educate patients about obesity and all the chronic conditions it may lead to, but we cannot ignore this emerging phenotype of being of normal weight but metabolically unhealthy,” Dr. Hardikar emphasized.
What Additional Research is Needed?
Looking ahead, “we need well-designed interventions to test causality for metabolic syndrome and cancer risk, though the evidence from the observational studies is very strong,” Dr. Hardikar said.
In addition, a consensus is needed to better define metabolic dysfunction,and to explore cancer risk in normal weight but metabolically unhealthy individuals, she said.
The study was supported by the National Key Research and Development Program of China. The researchers and Dr. Hardikar had no financial conflicts to disclose.
The new research finds that adults with persistent metabolic syndrome that worsens over time are at increased risk for any type of cancer.
The conditions that make up metabolic syndrome (high blood pressure, high blood sugar, increased abdominal adiposity, and high cholesterol and triglycerides) have been associated with an increased risk of diseases, including heart disease, stroke, and type 2 diabetes, wrote Li Deng, PhD, of Capital Medical University, Beijing, and colleagues.
However, a single assessment of metabolic syndrome at one point in time is inadequate to show an association with cancer risk over time, they said. In the current study, the researchers used models to examine the association between trajectory patterns of metabolic syndrome over time and the risk of overall and specific cancer types. They also examined the impact of chronic inflammation concurrent with metabolic syndrome.
What We Know About Metabolic Syndrome and Cancer Risk
A systematic review and meta-analysis published in Diabetes Care in 2012 showed an association between the presence of metabolic syndrome and an increased risk of various cancers including liver, bladder, pancreatic, breast, and colorectal.
More recently, a 2020 study published in Diabetes showed evidence of increased risk for certain cancers (pancreatic, kidney, uterine, cervical) but no increased risk for cancer overall.
In addition, a 2022 study by some of the current study researchers of the same Chinese cohort focused on the role of inflammation in combination with metabolic syndrome on colorectal cancer specifically, and found an increased risk for cancer when both metabolic syndrome and inflammation were present.
However, the reasons for this association between metabolic syndrome and cancer remain unclear, and the effect of the fluctuating nature of metabolic syndrome over time on long-term cancer risk has not been explored, the researchers wrote.
“There is emerging evidence that even normal weight individuals who are metabolically unhealthy may be at an elevated cancer risk, and we need better metrics to define the underlying metabolic dysfunction in obesity,” Sheetal Hardikar, MBBS, PhD, MPH, an investigator at the Huntsman Cancer Institute, University of Utah, said in an interview.
Dr. Hardikar, who serves as assistant professor in the department of population health sciences at the University of Utah, was not involved in the current study. She and her colleagues published a research paper on data from the National Health and Nutrition Examination Survey in 2023 that showed an increased risk of obesity-related cancer.
What New Study Adds to Related Research
Previous studies have consistently reported an approximately 30% increased risk of cancer with metabolic syndrome, Dr. Hardikar said. “What is unique about this study is the examination of metabolic syndrome trajectories over four years, and not just the presence of metabolic syndrome at one point in time,” she said.
In the new study, published in Cancer on March 11 (doi: 10.1002/cncr.35235), 44,115 adults in China were separated into four trajectories based on metabolic syndrome scores for the period from 2006 to 2010. The scores were based on clinical evidence of metabolic syndrome, defined using the International Diabetes Federation criteria of central obesity and the presence of at least two other factors including increased triglycerides, decreased HDL cholesterol, high blood pressure (or treatment for previously diagnosed hypertension), and increased fasting plasma glucose (or previous diagnosis of type 2 diabetes).
The average age of the participants was 49 years; the mean body mass index ranged from approximately 22 kg/m2 in the low-stable group to approximately 28 kg/m2 in the elevated-increasing group.
The four trajectories of metabolic syndrome were low-stable (10.56% of participants), moderate-low (40.84%), moderate-high (41.46%), and elevated-increasing (7.14%), based on trends from the individuals’ initial physical exams on entering the study.
Over a median follow-up period of 9.4 years (from 2010 to 2021), 2,271 cancer diagnoses were reported in the study population. Those with an elevated-increasing metabolic syndrome trajectory had 1.3 times the risk of any cancer compared with those in the low-stable group. Risk for breast cancer, endometrial cancer, kidney cancer, colorectal cancer, and liver cancer in the highest trajectory group were 2.1, 3.3, 4.5, 2.5, and 1.6 times higher, respectively, compared to the lowest group. The increased risk in the elevated-trajectory group for all cancer types persisted when the low-stable, moderate-low, and moderate-high trajectory pattern groups were combined.
The researchers also examined the impact of chronic inflammation and found that individuals with persistently high metabolic syndrome scores and concurrent chronic inflammation had the highest risks of breast, endometrial, colon, and liver cancer. However, individuals with persistently high metabolic syndrome scores and no concurrent chronic inflammation had the highest risk of kidney cancer.
What Are the Limitations of This Research?
The researchers of the current study acknowledged the lack of information on other causes of cancer, including dietary habits, hepatitis C infection, and Helicobacter pylori infection. Other limitations include the focus only on individuals from a single community of mainly middle-aged men in China that may not generalize to other populations.
Also, the metabolic syndrome trajectories did not change much over time, which may be related to the short 4-year study period.
Using the International Diabetes Federation criteria was another limitation, because it prevented the assessment of cancer risk in normal weight individuals with metabolic dysfunction, Dr. Hardikar noted.
Does Metabolic Syndrome Cause Cancer?
“This research suggests that proactive and continuous management of metabolic syndrome may serve as an essential strategy in preventing cancer,” senior author Han-Ping Shi, MD, PhD, of Capital Medical University in Beijing, noted in a statement on the study.
More research is needed to assess the impact of these interventions on cancer risk. However, the data from the current study can guide future research that may lead to more targeted treatments and more effective preventive strategies, he continued.
“Current evidence based on this study and many other reports strongly suggests an increased risk for cancer associated with metabolic syndrome,” Dr. Hardikar said in an interview. The data serve as a reminder to clinicians to look beyond BMI as the only measure of obesity, and to consider metabolic factors together to identify individuals at increased risk for cancer, she said.
“We must continue to educate patients about obesity and all the chronic conditions it may lead to, but we cannot ignore this emerging phenotype of being of normal weight but metabolically unhealthy,” Dr. Hardikar emphasized.
What Additional Research is Needed?
Looking ahead, “we need well-designed interventions to test causality for metabolic syndrome and cancer risk, though the evidence from the observational studies is very strong,” Dr. Hardikar said.
In addition, a consensus is needed to better define metabolic dysfunction,and to explore cancer risk in normal weight but metabolically unhealthy individuals, she said.
The study was supported by the National Key Research and Development Program of China. The researchers and Dr. Hardikar had no financial conflicts to disclose.
The new research finds that adults with persistent metabolic syndrome that worsens over time are at increased risk for any type of cancer.
The conditions that make up metabolic syndrome (high blood pressure, high blood sugar, increased abdominal adiposity, and high cholesterol and triglycerides) have been associated with an increased risk of diseases, including heart disease, stroke, and type 2 diabetes, wrote Li Deng, PhD, of Capital Medical University, Beijing, and colleagues.
However, a single assessment of metabolic syndrome at one point in time is inadequate to show an association with cancer risk over time, they said. In the current study, the researchers used models to examine the association between trajectory patterns of metabolic syndrome over time and the risk of overall and specific cancer types. They also examined the impact of chronic inflammation concurrent with metabolic syndrome.
What We Know About Metabolic Syndrome and Cancer Risk
A systematic review and meta-analysis published in Diabetes Care in 2012 showed an association between the presence of metabolic syndrome and an increased risk of various cancers including liver, bladder, pancreatic, breast, and colorectal.
More recently, a 2020 study published in Diabetes showed evidence of increased risk for certain cancers (pancreatic, kidney, uterine, cervical) but no increased risk for cancer overall.
In addition, a 2022 study by some of the current study researchers of the same Chinese cohort focused on the role of inflammation in combination with metabolic syndrome on colorectal cancer specifically, and found an increased risk for cancer when both metabolic syndrome and inflammation were present.
However, the reasons for this association between metabolic syndrome and cancer remain unclear, and the effect of the fluctuating nature of metabolic syndrome over time on long-term cancer risk has not been explored, the researchers wrote.
“There is emerging evidence that even normal weight individuals who are metabolically unhealthy may be at an elevated cancer risk, and we need better metrics to define the underlying metabolic dysfunction in obesity,” Sheetal Hardikar, MBBS, PhD, MPH, an investigator at the Huntsman Cancer Institute, University of Utah, said in an interview.
Dr. Hardikar, who serves as assistant professor in the department of population health sciences at the University of Utah, was not involved in the current study. She and her colleagues published a research paper on data from the National Health and Nutrition Examination Survey in 2023 that showed an increased risk of obesity-related cancer.
What New Study Adds to Related Research
Previous studies have consistently reported an approximately 30% increased risk of cancer with metabolic syndrome, Dr. Hardikar said. “What is unique about this study is the examination of metabolic syndrome trajectories over four years, and not just the presence of metabolic syndrome at one point in time,” she said.
In the new study, published in Cancer on March 11 (doi: 10.1002/cncr.35235), 44,115 adults in China were separated into four trajectories based on metabolic syndrome scores for the period from 2006 to 2010. The scores were based on clinical evidence of metabolic syndrome, defined using the International Diabetes Federation criteria of central obesity and the presence of at least two other factors including increased triglycerides, decreased HDL cholesterol, high blood pressure (or treatment for previously diagnosed hypertension), and increased fasting plasma glucose (or previous diagnosis of type 2 diabetes).
The average age of the participants was 49 years; the mean body mass index ranged from approximately 22 kg/m2 in the low-stable group to approximately 28 kg/m2 in the elevated-increasing group.
The four trajectories of metabolic syndrome were low-stable (10.56% of participants), moderate-low (40.84%), moderate-high (41.46%), and elevated-increasing (7.14%), based on trends from the individuals’ initial physical exams on entering the study.
Over a median follow-up period of 9.4 years (from 2010 to 2021), 2,271 cancer diagnoses were reported in the study population. Those with an elevated-increasing metabolic syndrome trajectory had 1.3 times the risk of any cancer compared with those in the low-stable group. Risk for breast cancer, endometrial cancer, kidney cancer, colorectal cancer, and liver cancer in the highest trajectory group were 2.1, 3.3, 4.5, 2.5, and 1.6 times higher, respectively, compared to the lowest group. The increased risk in the elevated-trajectory group for all cancer types persisted when the low-stable, moderate-low, and moderate-high trajectory pattern groups were combined.
The researchers also examined the impact of chronic inflammation and found that individuals with persistently high metabolic syndrome scores and concurrent chronic inflammation had the highest risks of breast, endometrial, colon, and liver cancer. However, individuals with persistently high metabolic syndrome scores and no concurrent chronic inflammation had the highest risk of kidney cancer.
What Are the Limitations of This Research?
The researchers of the current study acknowledged the lack of information on other causes of cancer, including dietary habits, hepatitis C infection, and Helicobacter pylori infection. Other limitations include the focus only on individuals from a single community of mainly middle-aged men in China that may not generalize to other populations.
Also, the metabolic syndrome trajectories did not change much over time, which may be related to the short 4-year study period.
Using the International Diabetes Federation criteria was another limitation, because it prevented the assessment of cancer risk in normal weight individuals with metabolic dysfunction, Dr. Hardikar noted.
Does Metabolic Syndrome Cause Cancer?
“This research suggests that proactive and continuous management of metabolic syndrome may serve as an essential strategy in preventing cancer,” senior author Han-Ping Shi, MD, PhD, of Capital Medical University in Beijing, noted in a statement on the study.
More research is needed to assess the impact of these interventions on cancer risk. However, the data from the current study can guide future research that may lead to more targeted treatments and more effective preventive strategies, he continued.
“Current evidence based on this study and many other reports strongly suggests an increased risk for cancer associated with metabolic syndrome,” Dr. Hardikar said in an interview. The data serve as a reminder to clinicians to look beyond BMI as the only measure of obesity, and to consider metabolic factors together to identify individuals at increased risk for cancer, she said.
“We must continue to educate patients about obesity and all the chronic conditions it may lead to, but we cannot ignore this emerging phenotype of being of normal weight but metabolically unhealthy,” Dr. Hardikar emphasized.
What Additional Research is Needed?
Looking ahead, “we need well-designed interventions to test causality for metabolic syndrome and cancer risk, though the evidence from the observational studies is very strong,” Dr. Hardikar said.
In addition, a consensus is needed to better define metabolic dysfunction,and to explore cancer risk in normal weight but metabolically unhealthy individuals, she said.
The study was supported by the National Key Research and Development Program of China. The researchers and Dr. Hardikar had no financial conflicts to disclose.
FROM CANCER
ASTRO Pushes Return to Direct Supervision in RT: Needed or ‘Babysitting’?
Although serious errors during virtual supervision are rare, ASTRO said radiation treatments (RT) should be done with a radiation oncologist on site to ensure high-quality care. But some radiation oncologists do not agree with the proposal to move back to direct in-person supervision only.
Changes to Direct Supervision
Most radiation oncology treatments are delivered in an outpatient setting under a physician’s direction and control.
During the COVID-19 pandemic when social distancing mandates were in place, CMS temporarily changed the definition of “direct supervision” to include telehealth, specifying that a physician must be immediately available to assist and direct a procedure virtually using real-time audio and video. In other words, a physician did not need to be physically present in the room when the treatment was being performed.
CMS has extended this rule until the end of 2024 and is considering making it a permanent change. In the Calendar Year (CY) 2024 Medicare Physician Fee Schedule (PFS) Final Rule, CMS asked for comments on whether to extend the rule.
“We received input from interested parties on potential patient safety or quality concerns when direct supervision occurs virtually, which we will consider for future rulemaking,” a CMS spokesperson told this news organization. “CMS is currently considering the best approach that will protect patient access and safety as well as quality of care and program integrity concerns following CY 2024.”
CMS also noted its concerns that an abrupt transition back to requiring a physician’s physical presence could interrupt care from practitioners who have established new patterns of practice with telehealth.
What Are ASTRO’s Concerns?
Late last month, ASTRO sent CMS a letter, asking the agency to change the rules back to direct in-person supervision for all radiation services, citing that virtual supervision jeopardizes patient safety and quality of care.
Jeff Michalski, MD, MBA, chair of the ASTRO Board of Directors, said in an interview that radiation oncologists should be physically present to supervise the treatments.
“ASTRO is concerned that blanket policies of general or virtual supervision could lead to patients not having direct, in-person access to their doctors’ care,” he said. “While serious errors are rare, real-world experiences of radiation oncologists across practice settings demonstrate how an in-person radiation oncology physician is best suited to ensure high-quality care.”
What Do Radiation Oncologists Think?
According to ASTRO, most radiation oncologists would agree that in-person supervision is best for patients.
But that might not be the case.
Radiation oncologists took to X (formerly Twitter) to voice their opinions about ASTRO’s letter.
Jason Beckta, MD, PhD, of Rutland Regional’s Foley Cancer Center, Vermont, said “the February 26th ASTRO letter reads like an Onion article.”
“I’m struggling to understand the Luddite-level myopia around this topic,” he said in another tweet. “Virtual direct/outpatient general supervision has done nothing but boost my productivity and in particular, face-to-face patient contact.”
Join Y. Luh, MD, with the Providence Medical Network in Eureka, California, said he understands the challenges faced by clinicians working in more isolated rural settings. “For them, it’s either having virtual supervision or closing the center,” Dr. Luh said.
“Virtual care is definitely at my clinic and is not only an option but is critical to my patients who are 2+ snowy, mountainous hours away,” Dr. Luh wrote. “But I’m still in the clinic directly supervising treatments.”
Sidney Roberts, MD, with the CHI St. Luke’s Health-Memorial, Texas, tweeted that supervision does require some face-to-face care but contended that “babysitting trained therapists for every routine treatment is a farce.”
Another issue Dr. Luh brought up is reimbursement for virtual supervision, noting that “the elephant in the room is whether that level of service should be reimbursed at the same rate. Reimbursement has not changed — but will it stay that way?”
ASTRO has acknowledged that radiation oncologists will have varying opinions and says it is working to balance these challenges.
CMS has not reached a decision on whether the change will be implemented permanently. The organization will assess concern, patient safety, and quality of care at the end of the year.
A version of this article first appeared on Medscape.com
Although serious errors during virtual supervision are rare, ASTRO said radiation treatments (RT) should be done with a radiation oncologist on site to ensure high-quality care. But some radiation oncologists do not agree with the proposal to move back to direct in-person supervision only.
Changes to Direct Supervision
Most radiation oncology treatments are delivered in an outpatient setting under a physician’s direction and control.
During the COVID-19 pandemic when social distancing mandates were in place, CMS temporarily changed the definition of “direct supervision” to include telehealth, specifying that a physician must be immediately available to assist and direct a procedure virtually using real-time audio and video. In other words, a physician did not need to be physically present in the room when the treatment was being performed.
CMS has extended this rule until the end of 2024 and is considering making it a permanent change. In the Calendar Year (CY) 2024 Medicare Physician Fee Schedule (PFS) Final Rule, CMS asked for comments on whether to extend the rule.
“We received input from interested parties on potential patient safety or quality concerns when direct supervision occurs virtually, which we will consider for future rulemaking,” a CMS spokesperson told this news organization. “CMS is currently considering the best approach that will protect patient access and safety as well as quality of care and program integrity concerns following CY 2024.”
CMS also noted its concerns that an abrupt transition back to requiring a physician’s physical presence could interrupt care from practitioners who have established new patterns of practice with telehealth.
What Are ASTRO’s Concerns?
Late last month, ASTRO sent CMS a letter, asking the agency to change the rules back to direct in-person supervision for all radiation services, citing that virtual supervision jeopardizes patient safety and quality of care.
Jeff Michalski, MD, MBA, chair of the ASTRO Board of Directors, said in an interview that radiation oncologists should be physically present to supervise the treatments.
“ASTRO is concerned that blanket policies of general or virtual supervision could lead to patients not having direct, in-person access to their doctors’ care,” he said. “While serious errors are rare, real-world experiences of radiation oncologists across practice settings demonstrate how an in-person radiation oncology physician is best suited to ensure high-quality care.”
What Do Radiation Oncologists Think?
According to ASTRO, most radiation oncologists would agree that in-person supervision is best for patients.
But that might not be the case.
Radiation oncologists took to X (formerly Twitter) to voice their opinions about ASTRO’s letter.
Jason Beckta, MD, PhD, of Rutland Regional’s Foley Cancer Center, Vermont, said “the February 26th ASTRO letter reads like an Onion article.”
“I’m struggling to understand the Luddite-level myopia around this topic,” he said in another tweet. “Virtual direct/outpatient general supervision has done nothing but boost my productivity and in particular, face-to-face patient contact.”
Join Y. Luh, MD, with the Providence Medical Network in Eureka, California, said he understands the challenges faced by clinicians working in more isolated rural settings. “For them, it’s either having virtual supervision or closing the center,” Dr. Luh said.
“Virtual care is definitely at my clinic and is not only an option but is critical to my patients who are 2+ snowy, mountainous hours away,” Dr. Luh wrote. “But I’m still in the clinic directly supervising treatments.”
Sidney Roberts, MD, with the CHI St. Luke’s Health-Memorial, Texas, tweeted that supervision does require some face-to-face care but contended that “babysitting trained therapists for every routine treatment is a farce.”
Another issue Dr. Luh brought up is reimbursement for virtual supervision, noting that “the elephant in the room is whether that level of service should be reimbursed at the same rate. Reimbursement has not changed — but will it stay that way?”
ASTRO has acknowledged that radiation oncologists will have varying opinions and says it is working to balance these challenges.
CMS has not reached a decision on whether the change will be implemented permanently. The organization will assess concern, patient safety, and quality of care at the end of the year.
A version of this article first appeared on Medscape.com
Although serious errors during virtual supervision are rare, ASTRO said radiation treatments (RT) should be done with a radiation oncologist on site to ensure high-quality care. But some radiation oncologists do not agree with the proposal to move back to direct in-person supervision only.
Changes to Direct Supervision
Most radiation oncology treatments are delivered in an outpatient setting under a physician’s direction and control.
During the COVID-19 pandemic when social distancing mandates were in place, CMS temporarily changed the definition of “direct supervision” to include telehealth, specifying that a physician must be immediately available to assist and direct a procedure virtually using real-time audio and video. In other words, a physician did not need to be physically present in the room when the treatment was being performed.
CMS has extended this rule until the end of 2024 and is considering making it a permanent change. In the Calendar Year (CY) 2024 Medicare Physician Fee Schedule (PFS) Final Rule, CMS asked for comments on whether to extend the rule.
“We received input from interested parties on potential patient safety or quality concerns when direct supervision occurs virtually, which we will consider for future rulemaking,” a CMS spokesperson told this news organization. “CMS is currently considering the best approach that will protect patient access and safety as well as quality of care and program integrity concerns following CY 2024.”
CMS also noted its concerns that an abrupt transition back to requiring a physician’s physical presence could interrupt care from practitioners who have established new patterns of practice with telehealth.
What Are ASTRO’s Concerns?
Late last month, ASTRO sent CMS a letter, asking the agency to change the rules back to direct in-person supervision for all radiation services, citing that virtual supervision jeopardizes patient safety and quality of care.
Jeff Michalski, MD, MBA, chair of the ASTRO Board of Directors, said in an interview that radiation oncologists should be physically present to supervise the treatments.
“ASTRO is concerned that blanket policies of general or virtual supervision could lead to patients not having direct, in-person access to their doctors’ care,” he said. “While serious errors are rare, real-world experiences of radiation oncologists across practice settings demonstrate how an in-person radiation oncology physician is best suited to ensure high-quality care.”
What Do Radiation Oncologists Think?
According to ASTRO, most radiation oncologists would agree that in-person supervision is best for patients.
But that might not be the case.
Radiation oncologists took to X (formerly Twitter) to voice their opinions about ASTRO’s letter.
Jason Beckta, MD, PhD, of Rutland Regional’s Foley Cancer Center, Vermont, said “the February 26th ASTRO letter reads like an Onion article.”
“I’m struggling to understand the Luddite-level myopia around this topic,” he said in another tweet. “Virtual direct/outpatient general supervision has done nothing but boost my productivity and in particular, face-to-face patient contact.”
Join Y. Luh, MD, with the Providence Medical Network in Eureka, California, said he understands the challenges faced by clinicians working in more isolated rural settings. “For them, it’s either having virtual supervision or closing the center,” Dr. Luh said.
“Virtual care is definitely at my clinic and is not only an option but is critical to my patients who are 2+ snowy, mountainous hours away,” Dr. Luh wrote. “But I’m still in the clinic directly supervising treatments.”
Sidney Roberts, MD, with the CHI St. Luke’s Health-Memorial, Texas, tweeted that supervision does require some face-to-face care but contended that “babysitting trained therapists for every routine treatment is a farce.”
Another issue Dr. Luh brought up is reimbursement for virtual supervision, noting that “the elephant in the room is whether that level of service should be reimbursed at the same rate. Reimbursement has not changed — but will it stay that way?”
ASTRO has acknowledged that radiation oncologists will have varying opinions and says it is working to balance these challenges.
CMS has not reached a decision on whether the change will be implemented permanently. The organization will assess concern, patient safety, and quality of care at the end of the year.
A version of this article first appeared on Medscape.com
Smoking Cessation Before Age 40 Years Brings Great Benefits
Chronic smoking remains a major cause of premature mortality on a global scale. Despite intensified efforts to combat this scourge, a quarter of deaths among middle-aged adults in Europe and North America are attributed to it. However, over the past decades, antismoking campaigns have borne fruit, and many smokers have quit before the age of 40 years, enabling some case-control studies.
Among those abstainers who made the right choice, the excess mortality attributable to smoking over a lifetime would be reduced by 90% compared with controls who continued smoking. The estimated benefit is clear, but the analysis lacks nuance. Is smoking cessation beneficial even at older ages? If so, is the effect measurable in terms of magnitude and speed of the effect? An article published online in The New England Journal of Medicine Evidence provided some answers to these questions.
Four-Cohort Meta-Analysis
The study was a meta-analysis of individual data collected within four national cohort studies that were linked to each country’s death registry. Two of these studies were nationally representative. The National Health Interview Survey involved a sample of US citizens living in the community, aged 20-79 years, who were included annually in the cohort between 1997 and 2018. The second, the Canadian Community Health Survey, included subjects in the same age group, with samples analyzed between 2000 and 2014.
In Norway, three cohort studies conducted between 1974 and 2003, in which participants aged 25-79 years were included, were combined to form the Norwegian Health Screening Survey. These were the Counties Study (1974-1988), the 40 Years Study (1985-1999), and the Cohort of Norway (1994-2003), respectively. The fourth cohort was established through recruitment via the UK Biobank, with adults aged 40-73 years invited to participate in the survey. The data analysis ultimately covered a relatively heterogeneous total population of 1.48 million adults, all from high-income countries and followed for 15 years. It relied on the Cox proportional hazards model applied to each study, considering smoker vs nonsmoker status, as well as the time elapsed since smoking cessation (less than 3 years, between 3 and 9 years, or at least 10 years). Statistical adjustments made in the context of multivariate Cox analysis considered age, education, alcohol consumption, and obesity.
Excess Mortality Confirmed
At the end of follow-up, 122,697 deaths were recorded. The comparison of smokers and nonsmokers confirmed smoking-related excess mortality, with adjusted hazard ratios (HRs) estimated at 2.80 for women and 2.70 for men. Smoking shortened life expectancy in the 40- to 79-year-age group by 12 years for women and 13 years for men, in terms of overall mortality. In terms of smoking-attributable specific mortality, the corresponding figures reached 24 and 26 years, respectively. Respiratory diseases ranked highest in both sexes (HR, 7.6 for women and 6.3 for men), followed by cardiovascular diseases (HR, 3.1 for women and 2.9 for men) and cancers (HR, 2.8 for women and 3.1 for men).
The Earlier, the Better
Smoking cessation halves overall excess mortality. Above all, quitting before age 40 years brings overall mortality back to the level of nonsmokers as early as the third year after quitting. The excess mortality decreases even more as the cessation period is prolonged, even after age 40 years. Thus, cessation ≥ 10 years in smokers aged 40-49 years almost cancels out overall excess mortality (-99% in women, -96% in men). The trend is almost as favorable in the older age group (50-59 years), with corresponding figures of -95% and -92%, respectively.
Long-term survival increases in the early years after cessation, especially if it occurs at a younger age, but the benefit remains tangible even in older smokers. Thus, cessation of less than 3 years, effective in patients aged 50-59 years, reduces overall excess mortality by 63% in women and 54% in men. In patients aged 60-79 years, the figures are -40% and -33%, respectively.
Naturally, the earlier the cessation, the greater the number of years gained. It is 12 years for cessation before age 40 years, reduced to 6 years for cessation between 40 and 49 years, and 2.5 years when it is even later (50-59 years). These quantitative results are approximate, given the methodology (a meta-analysis) and some heterogeneity in the studies, as well as the multitude of potential confounding factors that have not all been considered. Nevertheless, the results probably contain a kernel of truth, and their optimistic implications should be highlighted to encourage smokers to abstain, even older ones. Better late than never, even if the benefit of cessation is maximal when it occurs as early as possible, knowing that a minimum of 3 years of cessation would be sufficient to gain years of life.
This story was translated from JIM, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Chronic smoking remains a major cause of premature mortality on a global scale. Despite intensified efforts to combat this scourge, a quarter of deaths among middle-aged adults in Europe and North America are attributed to it. However, over the past decades, antismoking campaigns have borne fruit, and many smokers have quit before the age of 40 years, enabling some case-control studies.
Among those abstainers who made the right choice, the excess mortality attributable to smoking over a lifetime would be reduced by 90% compared with controls who continued smoking. The estimated benefit is clear, but the analysis lacks nuance. Is smoking cessation beneficial even at older ages? If so, is the effect measurable in terms of magnitude and speed of the effect? An article published online in The New England Journal of Medicine Evidence provided some answers to these questions.
Four-Cohort Meta-Analysis
The study was a meta-analysis of individual data collected within four national cohort studies that were linked to each country’s death registry. Two of these studies were nationally representative. The National Health Interview Survey involved a sample of US citizens living in the community, aged 20-79 years, who were included annually in the cohort between 1997 and 2018. The second, the Canadian Community Health Survey, included subjects in the same age group, with samples analyzed between 2000 and 2014.
In Norway, three cohort studies conducted between 1974 and 2003, in which participants aged 25-79 years were included, were combined to form the Norwegian Health Screening Survey. These were the Counties Study (1974-1988), the 40 Years Study (1985-1999), and the Cohort of Norway (1994-2003), respectively. The fourth cohort was established through recruitment via the UK Biobank, with adults aged 40-73 years invited to participate in the survey. The data analysis ultimately covered a relatively heterogeneous total population of 1.48 million adults, all from high-income countries and followed for 15 years. It relied on the Cox proportional hazards model applied to each study, considering smoker vs nonsmoker status, as well as the time elapsed since smoking cessation (less than 3 years, between 3 and 9 years, or at least 10 years). Statistical adjustments made in the context of multivariate Cox analysis considered age, education, alcohol consumption, and obesity.
Excess Mortality Confirmed
At the end of follow-up, 122,697 deaths were recorded. The comparison of smokers and nonsmokers confirmed smoking-related excess mortality, with adjusted hazard ratios (HRs) estimated at 2.80 for women and 2.70 for men. Smoking shortened life expectancy in the 40- to 79-year-age group by 12 years for women and 13 years for men, in terms of overall mortality. In terms of smoking-attributable specific mortality, the corresponding figures reached 24 and 26 years, respectively. Respiratory diseases ranked highest in both sexes (HR, 7.6 for women and 6.3 for men), followed by cardiovascular diseases (HR, 3.1 for women and 2.9 for men) and cancers (HR, 2.8 for women and 3.1 for men).
The Earlier, the Better
Smoking cessation halves overall excess mortality. Above all, quitting before age 40 years brings overall mortality back to the level of nonsmokers as early as the third year after quitting. The excess mortality decreases even more as the cessation period is prolonged, even after age 40 years. Thus, cessation ≥ 10 years in smokers aged 40-49 years almost cancels out overall excess mortality (-99% in women, -96% in men). The trend is almost as favorable in the older age group (50-59 years), with corresponding figures of -95% and -92%, respectively.
Long-term survival increases in the early years after cessation, especially if it occurs at a younger age, but the benefit remains tangible even in older smokers. Thus, cessation of less than 3 years, effective in patients aged 50-59 years, reduces overall excess mortality by 63% in women and 54% in men. In patients aged 60-79 years, the figures are -40% and -33%, respectively.
Naturally, the earlier the cessation, the greater the number of years gained. It is 12 years for cessation before age 40 years, reduced to 6 years for cessation between 40 and 49 years, and 2.5 years when it is even later (50-59 years). These quantitative results are approximate, given the methodology (a meta-analysis) and some heterogeneity in the studies, as well as the multitude of potential confounding factors that have not all been considered. Nevertheless, the results probably contain a kernel of truth, and their optimistic implications should be highlighted to encourage smokers to abstain, even older ones. Better late than never, even if the benefit of cessation is maximal when it occurs as early as possible, knowing that a minimum of 3 years of cessation would be sufficient to gain years of life.
This story was translated from JIM, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Chronic smoking remains a major cause of premature mortality on a global scale. Despite intensified efforts to combat this scourge, a quarter of deaths among middle-aged adults in Europe and North America are attributed to it. However, over the past decades, antismoking campaigns have borne fruit, and many smokers have quit before the age of 40 years, enabling some case-control studies.
Among those abstainers who made the right choice, the excess mortality attributable to smoking over a lifetime would be reduced by 90% compared with controls who continued smoking. The estimated benefit is clear, but the analysis lacks nuance. Is smoking cessation beneficial even at older ages? If so, is the effect measurable in terms of magnitude and speed of the effect? An article published online in The New England Journal of Medicine Evidence provided some answers to these questions.
Four-Cohort Meta-Analysis
The study was a meta-analysis of individual data collected within four national cohort studies that were linked to each country’s death registry. Two of these studies were nationally representative. The National Health Interview Survey involved a sample of US citizens living in the community, aged 20-79 years, who were included annually in the cohort between 1997 and 2018. The second, the Canadian Community Health Survey, included subjects in the same age group, with samples analyzed between 2000 and 2014.
In Norway, three cohort studies conducted between 1974 and 2003, in which participants aged 25-79 years were included, were combined to form the Norwegian Health Screening Survey. These were the Counties Study (1974-1988), the 40 Years Study (1985-1999), and the Cohort of Norway (1994-2003), respectively. The fourth cohort was established through recruitment via the UK Biobank, with adults aged 40-73 years invited to participate in the survey. The data analysis ultimately covered a relatively heterogeneous total population of 1.48 million adults, all from high-income countries and followed for 15 years. It relied on the Cox proportional hazards model applied to each study, considering smoker vs nonsmoker status, as well as the time elapsed since smoking cessation (less than 3 years, between 3 and 9 years, or at least 10 years). Statistical adjustments made in the context of multivariate Cox analysis considered age, education, alcohol consumption, and obesity.
Excess Mortality Confirmed
At the end of follow-up, 122,697 deaths were recorded. The comparison of smokers and nonsmokers confirmed smoking-related excess mortality, with adjusted hazard ratios (HRs) estimated at 2.80 for women and 2.70 for men. Smoking shortened life expectancy in the 40- to 79-year-age group by 12 years for women and 13 years for men, in terms of overall mortality. In terms of smoking-attributable specific mortality, the corresponding figures reached 24 and 26 years, respectively. Respiratory diseases ranked highest in both sexes (HR, 7.6 for women and 6.3 for men), followed by cardiovascular diseases (HR, 3.1 for women and 2.9 for men) and cancers (HR, 2.8 for women and 3.1 for men).
The Earlier, the Better
Smoking cessation halves overall excess mortality. Above all, quitting before age 40 years brings overall mortality back to the level of nonsmokers as early as the third year after quitting. The excess mortality decreases even more as the cessation period is prolonged, even after age 40 years. Thus, cessation ≥ 10 years in smokers aged 40-49 years almost cancels out overall excess mortality (-99% in women, -96% in men). The trend is almost as favorable in the older age group (50-59 years), with corresponding figures of -95% and -92%, respectively.
Long-term survival increases in the early years after cessation, especially if it occurs at a younger age, but the benefit remains tangible even in older smokers. Thus, cessation of less than 3 years, effective in patients aged 50-59 years, reduces overall excess mortality by 63% in women and 54% in men. In patients aged 60-79 years, the figures are -40% and -33%, respectively.
Naturally, the earlier the cessation, the greater the number of years gained. It is 12 years for cessation before age 40 years, reduced to 6 years for cessation between 40 and 49 years, and 2.5 years when it is even later (50-59 years). These quantitative results are approximate, given the methodology (a meta-analysis) and some heterogeneity in the studies, as well as the multitude of potential confounding factors that have not all been considered. Nevertheless, the results probably contain a kernel of truth, and their optimistic implications should be highlighted to encourage smokers to abstain, even older ones. Better late than never, even if the benefit of cessation is maximal when it occurs as early as possible, knowing that a minimum of 3 years of cessation would be sufficient to gain years of life.
This story was translated from JIM, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Does worsening metabolic syndrome increase the risk of developing cancer?
The conditions that comprise metabolic syndrome (high blood pressure, high blood sugar, increased abdominal adiposity, and high cholesterol and triglycerides) have been associated with an increased risk of diseases, including heart disease, stroke, and type 2 diabetes, wrote Li Deng, PhD, of Capital Medical University, Beijing, China, and colleagues.
A systematic review and meta-analysis published in Diabetes Care in 2012 showed an association between the presence of metabolic syndrome and an increased risk of various cancers including liver, bladder, pancreatic, breast, and colorectal.
More recently, a 2019 study published in Diabetes showed evidence of increased risk for certain cancers (pancreatic, kidney, uterine, cervical) but no increased risk for cancer overall.
However, the reasons for this association between metabolic syndrome and cancer remain unclear, and the effect of the fluctuating nature of metabolic syndrome over time on long-term cancer risk has not been explored, the researchers wrote.
What Does New Study Add to Other Research on Metabolic Syndrome and Cancer Risk?
In the new study, published in Cancer on March 11 (doi: 10.1002/cncr.35235), 44,115 adults in China were separated into four trajectories based on metabolic syndrome scores for the period from 2006 to 2010. The scores were based on clinical evidence of metabolic syndrome, defined using the International Diabetes Federation criteria of central obesity and the presence of at least two other factors including increased triglycerides, decreased HDL cholesterol, high blood pressure (or treatment for previously diagnosed hypertension), and increased fasting plasma glucose (or previous diagnosis of type 2 diabetes).
The average age of the participants was 49 years. The four trajectories of metabolic syndrome were low-stable (10.56% of participants), moderate-low (40.84%), moderate-high (41.46%), and elevated-increasing (7.14%), based on trends from the individuals’ initial physical exams on entering the study.
Over a median follow-up period of 9.4 years (from 2010 to 2021), 2,271 cancer diagnoses were reported in the study population. Those with an elevated-increasing metabolic syndrome trajectory had 1.3 times the risk of any cancer compared with those in the low-stable group. Risk for breast cancer, endometrial cancer, kidney cancer, colorectal cancer, and liver cancer in the highest trajectory group were 2.1, 3.3, 4.5, 2.5, and 1.6 times higher, respectively, compared to the lowest group. The increased risk in the elevated-trajectory group for all cancer types persisted when the low-stable, moderate-low, and moderate-high trajectory pattern groups were combined.
The researchers also examined the impact of chronic inflammation and found that individuals with persistently high metabolic syndrome scores and concurrent chronic inflammation had the highest risks of breast, endometrial, colon, and liver cancer. However, individuals with persistently high metabolic syndrome scores and no concurrent chronic inflammation had the highest risk of kidney cancer.
What Are the Limitations of This Research?
The researchers of the current study acknowledged the lack of information on other causes of cancer, including dietary habits, hepatitis C infection, and Helicobacter pylori infection. Other limitations include the focus only on individuals from a single community of mainly middle-aged men in China that may not generalize to other populations.
Also, the metabolic syndrome trajectories did not change much over time, which may be related to the short 4-year study period.
What Is the Takeaway Message for Clinical Practice?
The results suggest that monitoring and managing metabolic syndrome could help reduce cancer risk, the researchers concluded.
“This research suggests that proactive and continuous management of metabolic syndrome may serve as an essential strategy in preventing cancer,” senior author Han-Ping Shi, MD, PhD, of Capital Medical University in Beijing, said in a press release accompanying the study.
More research is needed to assess the impact of these interventions on cancer risk, he noted. However, the data from the current study can guide future research that may lead to more targeted treatments and more effective preventive strategies, he said in a statement.
The study was supported by the National Key Research and Development Program of China. The researchers had no financial conflicts to disclose.
The conditions that comprise metabolic syndrome (high blood pressure, high blood sugar, increased abdominal adiposity, and high cholesterol and triglycerides) have been associated with an increased risk of diseases, including heart disease, stroke, and type 2 diabetes, wrote Li Deng, PhD, of Capital Medical University, Beijing, China, and colleagues.
A systematic review and meta-analysis published in Diabetes Care in 2012 showed an association between the presence of metabolic syndrome and an increased risk of various cancers including liver, bladder, pancreatic, breast, and colorectal.
More recently, a 2019 study published in Diabetes showed evidence of increased risk for certain cancers (pancreatic, kidney, uterine, cervical) but no increased risk for cancer overall.
However, the reasons for this association between metabolic syndrome and cancer remain unclear, and the effect of the fluctuating nature of metabolic syndrome over time on long-term cancer risk has not been explored, the researchers wrote.
What Does New Study Add to Other Research on Metabolic Syndrome and Cancer Risk?
In the new study, published in Cancer on March 11 (doi: 10.1002/cncr.35235), 44,115 adults in China were separated into four trajectories based on metabolic syndrome scores for the period from 2006 to 2010. The scores were based on clinical evidence of metabolic syndrome, defined using the International Diabetes Federation criteria of central obesity and the presence of at least two other factors including increased triglycerides, decreased HDL cholesterol, high blood pressure (or treatment for previously diagnosed hypertension), and increased fasting plasma glucose (or previous diagnosis of type 2 diabetes).
The average age of the participants was 49 years. The four trajectories of metabolic syndrome were low-stable (10.56% of participants), moderate-low (40.84%), moderate-high (41.46%), and elevated-increasing (7.14%), based on trends from the individuals’ initial physical exams on entering the study.
Over a median follow-up period of 9.4 years (from 2010 to 2021), 2,271 cancer diagnoses were reported in the study population. Those with an elevated-increasing metabolic syndrome trajectory had 1.3 times the risk of any cancer compared with those in the low-stable group. Risk for breast cancer, endometrial cancer, kidney cancer, colorectal cancer, and liver cancer in the highest trajectory group were 2.1, 3.3, 4.5, 2.5, and 1.6 times higher, respectively, compared to the lowest group. The increased risk in the elevated-trajectory group for all cancer types persisted when the low-stable, moderate-low, and moderate-high trajectory pattern groups were combined.
The researchers also examined the impact of chronic inflammation and found that individuals with persistently high metabolic syndrome scores and concurrent chronic inflammation had the highest risks of breast, endometrial, colon, and liver cancer. However, individuals with persistently high metabolic syndrome scores and no concurrent chronic inflammation had the highest risk of kidney cancer.
What Are the Limitations of This Research?
The researchers of the current study acknowledged the lack of information on other causes of cancer, including dietary habits, hepatitis C infection, and Helicobacter pylori infection. Other limitations include the focus only on individuals from a single community of mainly middle-aged men in China that may not generalize to other populations.
Also, the metabolic syndrome trajectories did not change much over time, which may be related to the short 4-year study period.
What Is the Takeaway Message for Clinical Practice?
The results suggest that monitoring and managing metabolic syndrome could help reduce cancer risk, the researchers concluded.
“This research suggests that proactive and continuous management of metabolic syndrome may serve as an essential strategy in preventing cancer,” senior author Han-Ping Shi, MD, PhD, of Capital Medical University in Beijing, said in a press release accompanying the study.
More research is needed to assess the impact of these interventions on cancer risk, he noted. However, the data from the current study can guide future research that may lead to more targeted treatments and more effective preventive strategies, he said in a statement.
The study was supported by the National Key Research and Development Program of China. The researchers had no financial conflicts to disclose.
The conditions that comprise metabolic syndrome (high blood pressure, high blood sugar, increased abdominal adiposity, and high cholesterol and triglycerides) have been associated with an increased risk of diseases, including heart disease, stroke, and type 2 diabetes, wrote Li Deng, PhD, of Capital Medical University, Beijing, China, and colleagues.
A systematic review and meta-analysis published in Diabetes Care in 2012 showed an association between the presence of metabolic syndrome and an increased risk of various cancers including liver, bladder, pancreatic, breast, and colorectal.
More recently, a 2019 study published in Diabetes showed evidence of increased risk for certain cancers (pancreatic, kidney, uterine, cervical) but no increased risk for cancer overall.
However, the reasons for this association between metabolic syndrome and cancer remain unclear, and the effect of the fluctuating nature of metabolic syndrome over time on long-term cancer risk has not been explored, the researchers wrote.
What Does New Study Add to Other Research on Metabolic Syndrome and Cancer Risk?
In the new study, published in Cancer on March 11 (doi: 10.1002/cncr.35235), 44,115 adults in China were separated into four trajectories based on metabolic syndrome scores for the period from 2006 to 2010. The scores were based on clinical evidence of metabolic syndrome, defined using the International Diabetes Federation criteria of central obesity and the presence of at least two other factors including increased triglycerides, decreased HDL cholesterol, high blood pressure (or treatment for previously diagnosed hypertension), and increased fasting plasma glucose (or previous diagnosis of type 2 diabetes).
The average age of the participants was 49 years. The four trajectories of metabolic syndrome were low-stable (10.56% of participants), moderate-low (40.84%), moderate-high (41.46%), and elevated-increasing (7.14%), based on trends from the individuals’ initial physical exams on entering the study.
Over a median follow-up period of 9.4 years (from 2010 to 2021), 2,271 cancer diagnoses were reported in the study population. Those with an elevated-increasing metabolic syndrome trajectory had 1.3 times the risk of any cancer compared with those in the low-stable group. Risk for breast cancer, endometrial cancer, kidney cancer, colorectal cancer, and liver cancer in the highest trajectory group were 2.1, 3.3, 4.5, 2.5, and 1.6 times higher, respectively, compared to the lowest group. The increased risk in the elevated-trajectory group for all cancer types persisted when the low-stable, moderate-low, and moderate-high trajectory pattern groups were combined.
The researchers also examined the impact of chronic inflammation and found that individuals with persistently high metabolic syndrome scores and concurrent chronic inflammation had the highest risks of breast, endometrial, colon, and liver cancer. However, individuals with persistently high metabolic syndrome scores and no concurrent chronic inflammation had the highest risk of kidney cancer.
What Are the Limitations of This Research?
The researchers of the current study acknowledged the lack of information on other causes of cancer, including dietary habits, hepatitis C infection, and Helicobacter pylori infection. Other limitations include the focus only on individuals from a single community of mainly middle-aged men in China that may not generalize to other populations.
Also, the metabolic syndrome trajectories did not change much over time, which may be related to the short 4-year study period.
What Is the Takeaway Message for Clinical Practice?
The results suggest that monitoring and managing metabolic syndrome could help reduce cancer risk, the researchers concluded.
“This research suggests that proactive and continuous management of metabolic syndrome may serve as an essential strategy in preventing cancer,” senior author Han-Ping Shi, MD, PhD, of Capital Medical University in Beijing, said in a press release accompanying the study.
More research is needed to assess the impact of these interventions on cancer risk, he noted. However, the data from the current study can guide future research that may lead to more targeted treatments and more effective preventive strategies, he said in a statement.
The study was supported by the National Key Research and Development Program of China. The researchers had no financial conflicts to disclose.
FROM CANCER
Does Exercise Reduce Cancer Risk? It’s Just Not That Simple
“Exercise is medicine” has become something of a mantra, with good reason. There’s no doubt that regular physical activity has a broad range of health benefits. Exercise can improve circulation, help control weight, reduce stress, and boost mood — take your pick.
Lower cancer risk is also on the list — with exercise promoted as a risk-cutting strategy in government guidelines and in recommendations from professional groups such as the American Cancer Society.
The bulk of the data hangs on less rigorous, observational studies that have linked physical activity to lower risks for certain cancers, but plenty of questions remain.
What are the cancer types where exercise makes a difference? How significant is that impact? And what, exactly, defines a physical activity pattern powerful enough to move the needle on cancer risk?
Here’s an overview of the state of the evidence.
Exercise and Cancer Types: A Mixed Bag
When it comes to cancer prevention strategies, guidelines uniformly endorse less couch time and more movement. But a deeper look at the science reveals a complex and often poorly understood connection between exercise and cancer risk.
For certain cancer types, the benefits of exercise on cancer risk seem fairly well established.
The latest edition of the Physical Activity Guidelines for Americans, published in 2018, cites “strong evidence” that regular exercise might curb the risks for breast and colon cancers as well as bladder, endometrial, esophageal, kidney, and gastric cancers. These guidelines also point to “moderate”-strength evidence of a protective association with lung cancer.
The evidence of a protective effect, however, is strongest for breast and colon cancers, said Jennifer Ligibel, MD, senior physician in the Breast Oncology Center at Dana-Farber Cancer Institute, Boston, . “But,” she pointed out, “that may be because they’re some of the most common cancers, and it’s been easier to detect an association.”
Guidelines from the American Cancer Society, published in 2020, align with the 2018 recommendations.
“We believe there’s strong evidence to suggest at least eight different types of cancer are associated with physical activity,” said Erika Rees-Punia, PhD, MPH, senior principal scientist, epidemiology and behavioral research at the American Cancer Society.
That view is not universal, however. Current recommendations from the World Cancer Research Fund and American Institute for Cancer Research, for example, are more circumspect, citing only three cancers with good evidence of a protective effect from exercise: Breast (postmenopausal), colon, and endometrial.
“We definitely can’t say exercise reduces the risk of all cancers,” said Lee Jones, PhD, head of the Exercise Oncology Program at Memorial Sloan Kettering Cancer Center in New York City. “The data suggest it’s just not that simple.”
And it’s challenging to put all the evidence together, Dr. Jones added.
The physical activity guidelines are based on published systematic reviews, meta-analyses, and pooled analyses of data from observational studies that examined the relationship between physical activity — aerobic exercise, specifically — and cancer incidence. That means the evidence comes with all the limitations observational studies entail, such as how they collect information on participants’ exercise habits — which, Dr. Jones noted, is typically done via “monster questionnaires” that gauge physical activity in broad strokes.
Pooling all those findings into a meta-analysis is tricky, Dr. Jones added, because individual studies vary in important ways — from follow-up periods to how they quantify exercise and track cancer incidence.
In a study published in February in Cancer Cell, Dr. Jones and his colleagues attempted to address some of those issues by leveraging data from the PLCO screening trial.
The PLCO was a prospective study of over 60,000 US adults that compared the effects of annual screening vs usual care on cancer mortality. At enrollment, participants completed questionnaires that included an assessment of “vigorous” exercise. Based on that, Dr. Jones and his colleagues classified 55% as “exercisers” — meaning they reported 2 or more hours of vigorous exercise per week. The remaining 45%, who were in the 0 to 1 hour per week range, were deemed non-exercisers.
Over a median of 18 years, nearly 16,000 first-time invasive cancers were diagnosed, and some interesting differences between exercisers and non-exercisers emerged. The active group had lower risks for three cancers: Head and neck, with a 26% lower risk (hazard ratio [HR], 0.74), lung (a 20% lower risk), and breast (an 11% lower risk).
What was striking, however, was the lack of connection between exercise and many cancers cited in the guidelines, including colon, gastric, bladder, endometrial, and renal cancers.
Perhaps even more surprising — exercisers had higher risks for prostate cancer (12%) and melanoma (20%). This finding, Dr. Jones said, is in line with a previous pooled analysis of data from 12 US and European prospective cohorts. In this study, the most physically active participants (90th percentile) had higher risks for melanoma and prostate cancer, compared with the least active group (10th percentile).
The melanoma findings do make sense, Dr. Jones said, given that highly active people may spend a lot of time in the sun. “My advice,” Dr. Jones said, “is, if you’re exercising outside, wear sunscreen.” The prostate cancer findings, however, are more puzzling and warrant further research, he noted.
But the bottom line is that the relationship between exercise and cancer types is mixed and far from nailed down.
How Big Is the Effect?
Even if exercise reduces the risk for only certain cancers, that’s still important, particularly when those links appear strongest for common cancer types, such as breast and colon.
But how much of a difference can exercise make?
Based on the evidence, it may only be a modest one. A 2019 systematic review by the Physical Activity Guidelines Advisory Committee provided a rough estimate: Across hundreds of epidemiological studies, people with the highest physical activity levels had a 10%-20% lower risk for the cancers cited in the 2018 exercise guidelines compared with people who were least active.
These figures, however, are probably an underestimate, said Anne McTiernan, MD, PhD, a member of the advisory committee and professor of epidemiology, at Fred Hutchinson Cancer Center, Seattle.
“This is what we usually see when a factor is not measured very well,” said Dr. McTiernan, explaining that the individual studies differed in their categories of “highest” and “lowest” physical activity, such that one study’s “highest” could be another’s mid-range.
“In other words, the effects of physical activity are likely larger” than the review found, Dr. McTiernan said.
The next logical question is whether a bigger exercise “dose” — more time or higher intensity — would have a greater impact on cancer risk. A 2019 study published in the Journal of Clinical Oncology tried to clarify that by pooling data on over 750,000 participants from nine prospective cohorts.
Overall, people meeting government recommendations for exercise — equivalent to about 2.5-5 hours of weekly moderate activity, such as a brisk walk, or about 1.25-2.5 hours of more vigorous activities, like running — had lower risks for seven of 15 cancer types studied compared with less active people.
For cancers with positive findings, being on the higher end of the recommended 2.5- to 5-hour weekly range was better. Risk reductions for breast cancer, for instance, were 6% at 2.5 hours of physical activity per week and 10% at 5 hours per week. Similar trends emerged for other cancer types, including colon (8%-14%), endometrial (10%-18%), liver cancer (18%-27%), and non-Hodgkin lymphoma in women (11%-18%).
But there may be an exercise sweet spot that maximizes the cancer risk benefit.
Among people who surpassed the recommendations — exercising for more time or more intensely — the risk reduction benefit did not necessarily improve in a linear fashion. For certain cancer types, such as colon and endometrial, the benefits of more vigorous exercise “eroded at higher levels of activity,” the authors said.
The issue here is that most studies have not dug deeply into aerobic exercise habits. Often, studies present participants with a list of activities — walking, biking, and running — and ask them to estimate how often and for what duration they do each.
Plus, “we’ve usually lumped moderate and vigorous activities together,” Dr. Rees-Punia said, which means there’s a lack of “granular data” to say whether certain intensities or frequencies of exercise are optimal and for whom.
Why Exercise May Lower Cancer Risk
Exercise habits do not, of course, exist in a vacuum. Highly active people, Dr. Ligibel said, tend to be of higher socioeconomic status, leaner, and have generally healthier lifestyles than sedentary people.
Body weight is a big confounder as well. However, Dr. Rees-Punia noted, it’s also probably a reason that exercise is linked to lower cancer risks, particularly by preventing weight gain. Still, studies have found that the association between exercise and many cancers remains significant after adjusting for body mass index.
The why remains unclear, though some studies offer clues.
“There’s been some really interesting mechanistic research, suggesting that exercise may help inhibit tumor growth or upregulate the immune system,” Dr. Ligibel said.
That includes not only lab research but small intervention studies. While these studies have largely involved people who already have cancer, some have also focused on healthy individuals.
A 2019 study from Dr. Ligibel and her colleagues, which randomly assigned 49 women newly diagnosed with breast cancer to start either an exercise program or mind-body practices ahead of surgery, found exercisers, who had been active for about a month at the time of surgery, showed signs of immune system upregulation in their tumors, while the control group did not.
Among healthy postmenopausal women, a meta-analysis of six clinical trials from Dr. McTiernan and her colleagues found that exercise plus calorie reduction can reduce levels of breast cancer-related endogenous hormones, more so than calorie-cutting alone. And a 2023 study found that high-intensity exercise boosted the ranks of certain immune cells and reduced inflammation in the colon among people at high risk for colon and endometrial cancers due to Lynch syndrome.
Defining an Exercise ‘Prescription’
Despite the gaps and uncertainties in the research, government guidelines as well as those from the American Cancer Society and other medical groups are in lockstep in their exercise recommendations: Adults should strive for 150-300 minutes of moderate-intensity aerobic exercise (like brisk walking), 75-150 minutes of vigorous activity (like running), or some combination each week.
The guidelines also encourage strength training twice a week — advice that’s based on research tying those activity levels to lower risks for heart disease, diabetes, and other chronic conditions.
But there’s no “best” exercise prescription for lowering cancer risk specifically. Most epidemiological studies have examined only aerobic activity, Dr. Rees-Punia said, and there’s very little known about whether strength conditioning or other moderate heart rate-elevating activities, such as daily household chores, may reduce the risk for cancer.
Given the lack of nuance in the literature, it’s hard to say what intensities, types, or amounts of exercise are best for each individual.
Going forward, device-based measurements of physical activity could “help us sort out the effects of different intensities of exercise and possibly types,” Dr. Rees-Punia said.
But overall, Dr. McTiernan said, the data do show that the risks for several cancers are lower at the widely recommended activity levels.
“The bottom-line advice is still to exercise at least 150 minutes per week at a moderate-intensity level or greater,” Dr. McTiernan said.
Or put another way, moving beats being sedentary. It’s probably wise for everyone to sit less, noted Dr. Rees-Punia, for overall health and based on evidence tying sedentary time to the risks for certain cancers, including colon, endometrial, and lung.
There’s a practical element to consider in all of this: What physical activities will people actually do on the regular? In the big epidemiological studies, Dr. McTiernan noted, middle-aged and older adults most often report walking, suggesting that’s the preferred, or most accessible activity, for many.
“You can only benefit from the physical activity you’ll actually do,” Dr. Rees-Punia said.
Dr. Ligibel echoed that sentiment, saying she encourages patients to think about physical activity as a process: “You need to find things you like to do and work them into your daily life, in a sustainable way.
“People often talk about exercise being medicine,” Dr. Ligibel said. “But I think you could take that too far. If we get too prescriptive about it, that could take the joy away.”
A version of this article appeared on Medscape.com.
“Exercise is medicine” has become something of a mantra, with good reason. There’s no doubt that regular physical activity has a broad range of health benefits. Exercise can improve circulation, help control weight, reduce stress, and boost mood — take your pick.
Lower cancer risk is also on the list — with exercise promoted as a risk-cutting strategy in government guidelines and in recommendations from professional groups such as the American Cancer Society.
The bulk of the data hangs on less rigorous, observational studies that have linked physical activity to lower risks for certain cancers, but plenty of questions remain.
What are the cancer types where exercise makes a difference? How significant is that impact? And what, exactly, defines a physical activity pattern powerful enough to move the needle on cancer risk?
Here’s an overview of the state of the evidence.
Exercise and Cancer Types: A Mixed Bag
When it comes to cancer prevention strategies, guidelines uniformly endorse less couch time and more movement. But a deeper look at the science reveals a complex and often poorly understood connection between exercise and cancer risk.
For certain cancer types, the benefits of exercise on cancer risk seem fairly well established.
The latest edition of the Physical Activity Guidelines for Americans, published in 2018, cites “strong evidence” that regular exercise might curb the risks for breast and colon cancers as well as bladder, endometrial, esophageal, kidney, and gastric cancers. These guidelines also point to “moderate”-strength evidence of a protective association with lung cancer.
The evidence of a protective effect, however, is strongest for breast and colon cancers, said Jennifer Ligibel, MD, senior physician in the Breast Oncology Center at Dana-Farber Cancer Institute, Boston, . “But,” she pointed out, “that may be because they’re some of the most common cancers, and it’s been easier to detect an association.”
Guidelines from the American Cancer Society, published in 2020, align with the 2018 recommendations.
“We believe there’s strong evidence to suggest at least eight different types of cancer are associated with physical activity,” said Erika Rees-Punia, PhD, MPH, senior principal scientist, epidemiology and behavioral research at the American Cancer Society.
That view is not universal, however. Current recommendations from the World Cancer Research Fund and American Institute for Cancer Research, for example, are more circumspect, citing only three cancers with good evidence of a protective effect from exercise: Breast (postmenopausal), colon, and endometrial.
“We definitely can’t say exercise reduces the risk of all cancers,” said Lee Jones, PhD, head of the Exercise Oncology Program at Memorial Sloan Kettering Cancer Center in New York City. “The data suggest it’s just not that simple.”
And it’s challenging to put all the evidence together, Dr. Jones added.
The physical activity guidelines are based on published systematic reviews, meta-analyses, and pooled analyses of data from observational studies that examined the relationship between physical activity — aerobic exercise, specifically — and cancer incidence. That means the evidence comes with all the limitations observational studies entail, such as how they collect information on participants’ exercise habits — which, Dr. Jones noted, is typically done via “monster questionnaires” that gauge physical activity in broad strokes.
Pooling all those findings into a meta-analysis is tricky, Dr. Jones added, because individual studies vary in important ways — from follow-up periods to how they quantify exercise and track cancer incidence.
In a study published in February in Cancer Cell, Dr. Jones and his colleagues attempted to address some of those issues by leveraging data from the PLCO screening trial.
The PLCO was a prospective study of over 60,000 US adults that compared the effects of annual screening vs usual care on cancer mortality. At enrollment, participants completed questionnaires that included an assessment of “vigorous” exercise. Based on that, Dr. Jones and his colleagues classified 55% as “exercisers” — meaning they reported 2 or more hours of vigorous exercise per week. The remaining 45%, who were in the 0 to 1 hour per week range, were deemed non-exercisers.
Over a median of 18 years, nearly 16,000 first-time invasive cancers were diagnosed, and some interesting differences between exercisers and non-exercisers emerged. The active group had lower risks for three cancers: Head and neck, with a 26% lower risk (hazard ratio [HR], 0.74), lung (a 20% lower risk), and breast (an 11% lower risk).
What was striking, however, was the lack of connection between exercise and many cancers cited in the guidelines, including colon, gastric, bladder, endometrial, and renal cancers.
Perhaps even more surprising — exercisers had higher risks for prostate cancer (12%) and melanoma (20%). This finding, Dr. Jones said, is in line with a previous pooled analysis of data from 12 US and European prospective cohorts. In this study, the most physically active participants (90th percentile) had higher risks for melanoma and prostate cancer, compared with the least active group (10th percentile).
The melanoma findings do make sense, Dr. Jones said, given that highly active people may spend a lot of time in the sun. “My advice,” Dr. Jones said, “is, if you’re exercising outside, wear sunscreen.” The prostate cancer findings, however, are more puzzling and warrant further research, he noted.
But the bottom line is that the relationship between exercise and cancer types is mixed and far from nailed down.
How Big Is the Effect?
Even if exercise reduces the risk for only certain cancers, that’s still important, particularly when those links appear strongest for common cancer types, such as breast and colon.
But how much of a difference can exercise make?
Based on the evidence, it may only be a modest one. A 2019 systematic review by the Physical Activity Guidelines Advisory Committee provided a rough estimate: Across hundreds of epidemiological studies, people with the highest physical activity levels had a 10%-20% lower risk for the cancers cited in the 2018 exercise guidelines compared with people who were least active.
These figures, however, are probably an underestimate, said Anne McTiernan, MD, PhD, a member of the advisory committee and professor of epidemiology, at Fred Hutchinson Cancer Center, Seattle.
“This is what we usually see when a factor is not measured very well,” said Dr. McTiernan, explaining that the individual studies differed in their categories of “highest” and “lowest” physical activity, such that one study’s “highest” could be another’s mid-range.
“In other words, the effects of physical activity are likely larger” than the review found, Dr. McTiernan said.
The next logical question is whether a bigger exercise “dose” — more time or higher intensity — would have a greater impact on cancer risk. A 2019 study published in the Journal of Clinical Oncology tried to clarify that by pooling data on over 750,000 participants from nine prospective cohorts.
Overall, people meeting government recommendations for exercise — equivalent to about 2.5-5 hours of weekly moderate activity, such as a brisk walk, or about 1.25-2.5 hours of more vigorous activities, like running — had lower risks for seven of 15 cancer types studied compared with less active people.
For cancers with positive findings, being on the higher end of the recommended 2.5- to 5-hour weekly range was better. Risk reductions for breast cancer, for instance, were 6% at 2.5 hours of physical activity per week and 10% at 5 hours per week. Similar trends emerged for other cancer types, including colon (8%-14%), endometrial (10%-18%), liver cancer (18%-27%), and non-Hodgkin lymphoma in women (11%-18%).
But there may be an exercise sweet spot that maximizes the cancer risk benefit.
Among people who surpassed the recommendations — exercising for more time or more intensely — the risk reduction benefit did not necessarily improve in a linear fashion. For certain cancer types, such as colon and endometrial, the benefits of more vigorous exercise “eroded at higher levels of activity,” the authors said.
The issue here is that most studies have not dug deeply into aerobic exercise habits. Often, studies present participants with a list of activities — walking, biking, and running — and ask them to estimate how often and for what duration they do each.
Plus, “we’ve usually lumped moderate and vigorous activities together,” Dr. Rees-Punia said, which means there’s a lack of “granular data” to say whether certain intensities or frequencies of exercise are optimal and for whom.
Why Exercise May Lower Cancer Risk
Exercise habits do not, of course, exist in a vacuum. Highly active people, Dr. Ligibel said, tend to be of higher socioeconomic status, leaner, and have generally healthier lifestyles than sedentary people.
Body weight is a big confounder as well. However, Dr. Rees-Punia noted, it’s also probably a reason that exercise is linked to lower cancer risks, particularly by preventing weight gain. Still, studies have found that the association between exercise and many cancers remains significant after adjusting for body mass index.
The why remains unclear, though some studies offer clues.
“There’s been some really interesting mechanistic research, suggesting that exercise may help inhibit tumor growth or upregulate the immune system,” Dr. Ligibel said.
That includes not only lab research but small intervention studies. While these studies have largely involved people who already have cancer, some have also focused on healthy individuals.
A 2019 study from Dr. Ligibel and her colleagues, which randomly assigned 49 women newly diagnosed with breast cancer to start either an exercise program or mind-body practices ahead of surgery, found exercisers, who had been active for about a month at the time of surgery, showed signs of immune system upregulation in their tumors, while the control group did not.
Among healthy postmenopausal women, a meta-analysis of six clinical trials from Dr. McTiernan and her colleagues found that exercise plus calorie reduction can reduce levels of breast cancer-related endogenous hormones, more so than calorie-cutting alone. And a 2023 study found that high-intensity exercise boosted the ranks of certain immune cells and reduced inflammation in the colon among people at high risk for colon and endometrial cancers due to Lynch syndrome.
Defining an Exercise ‘Prescription’
Despite the gaps and uncertainties in the research, government guidelines as well as those from the American Cancer Society and other medical groups are in lockstep in their exercise recommendations: Adults should strive for 150-300 minutes of moderate-intensity aerobic exercise (like brisk walking), 75-150 minutes of vigorous activity (like running), or some combination each week.
The guidelines also encourage strength training twice a week — advice that’s based on research tying those activity levels to lower risks for heart disease, diabetes, and other chronic conditions.
But there’s no “best” exercise prescription for lowering cancer risk specifically. Most epidemiological studies have examined only aerobic activity, Dr. Rees-Punia said, and there’s very little known about whether strength conditioning or other moderate heart rate-elevating activities, such as daily household chores, may reduce the risk for cancer.
Given the lack of nuance in the literature, it’s hard to say what intensities, types, or amounts of exercise are best for each individual.
Going forward, device-based measurements of physical activity could “help us sort out the effects of different intensities of exercise and possibly types,” Dr. Rees-Punia said.
But overall, Dr. McTiernan said, the data do show that the risks for several cancers are lower at the widely recommended activity levels.
“The bottom-line advice is still to exercise at least 150 minutes per week at a moderate-intensity level or greater,” Dr. McTiernan said.
Or put another way, moving beats being sedentary. It’s probably wise for everyone to sit less, noted Dr. Rees-Punia, for overall health and based on evidence tying sedentary time to the risks for certain cancers, including colon, endometrial, and lung.
There’s a practical element to consider in all of this: What physical activities will people actually do on the regular? In the big epidemiological studies, Dr. McTiernan noted, middle-aged and older adults most often report walking, suggesting that’s the preferred, or most accessible activity, for many.
“You can only benefit from the physical activity you’ll actually do,” Dr. Rees-Punia said.
Dr. Ligibel echoed that sentiment, saying she encourages patients to think about physical activity as a process: “You need to find things you like to do and work them into your daily life, in a sustainable way.
“People often talk about exercise being medicine,” Dr. Ligibel said. “But I think you could take that too far. If we get too prescriptive about it, that could take the joy away.”
A version of this article appeared on Medscape.com.
“Exercise is medicine” has become something of a mantra, with good reason. There’s no doubt that regular physical activity has a broad range of health benefits. Exercise can improve circulation, help control weight, reduce stress, and boost mood — take your pick.
Lower cancer risk is also on the list — with exercise promoted as a risk-cutting strategy in government guidelines and in recommendations from professional groups such as the American Cancer Society.
The bulk of the data hangs on less rigorous, observational studies that have linked physical activity to lower risks for certain cancers, but plenty of questions remain.
What are the cancer types where exercise makes a difference? How significant is that impact? And what, exactly, defines a physical activity pattern powerful enough to move the needle on cancer risk?
Here’s an overview of the state of the evidence.
Exercise and Cancer Types: A Mixed Bag
When it comes to cancer prevention strategies, guidelines uniformly endorse less couch time and more movement. But a deeper look at the science reveals a complex and often poorly understood connection between exercise and cancer risk.
For certain cancer types, the benefits of exercise on cancer risk seem fairly well established.
The latest edition of the Physical Activity Guidelines for Americans, published in 2018, cites “strong evidence” that regular exercise might curb the risks for breast and colon cancers as well as bladder, endometrial, esophageal, kidney, and gastric cancers. These guidelines also point to “moderate”-strength evidence of a protective association with lung cancer.
The evidence of a protective effect, however, is strongest for breast and colon cancers, said Jennifer Ligibel, MD, senior physician in the Breast Oncology Center at Dana-Farber Cancer Institute, Boston, . “But,” she pointed out, “that may be because they’re some of the most common cancers, and it’s been easier to detect an association.”
Guidelines from the American Cancer Society, published in 2020, align with the 2018 recommendations.
“We believe there’s strong evidence to suggest at least eight different types of cancer are associated with physical activity,” said Erika Rees-Punia, PhD, MPH, senior principal scientist, epidemiology and behavioral research at the American Cancer Society.
That view is not universal, however. Current recommendations from the World Cancer Research Fund and American Institute for Cancer Research, for example, are more circumspect, citing only three cancers with good evidence of a protective effect from exercise: Breast (postmenopausal), colon, and endometrial.
“We definitely can’t say exercise reduces the risk of all cancers,” said Lee Jones, PhD, head of the Exercise Oncology Program at Memorial Sloan Kettering Cancer Center in New York City. “The data suggest it’s just not that simple.”
And it’s challenging to put all the evidence together, Dr. Jones added.
The physical activity guidelines are based on published systematic reviews, meta-analyses, and pooled analyses of data from observational studies that examined the relationship between physical activity — aerobic exercise, specifically — and cancer incidence. That means the evidence comes with all the limitations observational studies entail, such as how they collect information on participants’ exercise habits — which, Dr. Jones noted, is typically done via “monster questionnaires” that gauge physical activity in broad strokes.
Pooling all those findings into a meta-analysis is tricky, Dr. Jones added, because individual studies vary in important ways — from follow-up periods to how they quantify exercise and track cancer incidence.
In a study published in February in Cancer Cell, Dr. Jones and his colleagues attempted to address some of those issues by leveraging data from the PLCO screening trial.
The PLCO was a prospective study of over 60,000 US adults that compared the effects of annual screening vs usual care on cancer mortality. At enrollment, participants completed questionnaires that included an assessment of “vigorous” exercise. Based on that, Dr. Jones and his colleagues classified 55% as “exercisers” — meaning they reported 2 or more hours of vigorous exercise per week. The remaining 45%, who were in the 0 to 1 hour per week range, were deemed non-exercisers.
Over a median of 18 years, nearly 16,000 first-time invasive cancers were diagnosed, and some interesting differences between exercisers and non-exercisers emerged. The active group had lower risks for three cancers: Head and neck, with a 26% lower risk (hazard ratio [HR], 0.74), lung (a 20% lower risk), and breast (an 11% lower risk).
What was striking, however, was the lack of connection between exercise and many cancers cited in the guidelines, including colon, gastric, bladder, endometrial, and renal cancers.
Perhaps even more surprising — exercisers had higher risks for prostate cancer (12%) and melanoma (20%). This finding, Dr. Jones said, is in line with a previous pooled analysis of data from 12 US and European prospective cohorts. In this study, the most physically active participants (90th percentile) had higher risks for melanoma and prostate cancer, compared with the least active group (10th percentile).
The melanoma findings do make sense, Dr. Jones said, given that highly active people may spend a lot of time in the sun. “My advice,” Dr. Jones said, “is, if you’re exercising outside, wear sunscreen.” The prostate cancer findings, however, are more puzzling and warrant further research, he noted.
But the bottom line is that the relationship between exercise and cancer types is mixed and far from nailed down.
How Big Is the Effect?
Even if exercise reduces the risk for only certain cancers, that’s still important, particularly when those links appear strongest for common cancer types, such as breast and colon.
But how much of a difference can exercise make?
Based on the evidence, it may only be a modest one. A 2019 systematic review by the Physical Activity Guidelines Advisory Committee provided a rough estimate: Across hundreds of epidemiological studies, people with the highest physical activity levels had a 10%-20% lower risk for the cancers cited in the 2018 exercise guidelines compared with people who were least active.
These figures, however, are probably an underestimate, said Anne McTiernan, MD, PhD, a member of the advisory committee and professor of epidemiology, at Fred Hutchinson Cancer Center, Seattle.
“This is what we usually see when a factor is not measured very well,” said Dr. McTiernan, explaining that the individual studies differed in their categories of “highest” and “lowest” physical activity, such that one study’s “highest” could be another’s mid-range.
“In other words, the effects of physical activity are likely larger” than the review found, Dr. McTiernan said.
The next logical question is whether a bigger exercise “dose” — more time or higher intensity — would have a greater impact on cancer risk. A 2019 study published in the Journal of Clinical Oncology tried to clarify that by pooling data on over 750,000 participants from nine prospective cohorts.
Overall, people meeting government recommendations for exercise — equivalent to about 2.5-5 hours of weekly moderate activity, such as a brisk walk, or about 1.25-2.5 hours of more vigorous activities, like running — had lower risks for seven of 15 cancer types studied compared with less active people.
For cancers with positive findings, being on the higher end of the recommended 2.5- to 5-hour weekly range was better. Risk reductions for breast cancer, for instance, were 6% at 2.5 hours of physical activity per week and 10% at 5 hours per week. Similar trends emerged for other cancer types, including colon (8%-14%), endometrial (10%-18%), liver cancer (18%-27%), and non-Hodgkin lymphoma in women (11%-18%).
But there may be an exercise sweet spot that maximizes the cancer risk benefit.
Among people who surpassed the recommendations — exercising for more time or more intensely — the risk reduction benefit did not necessarily improve in a linear fashion. For certain cancer types, such as colon and endometrial, the benefits of more vigorous exercise “eroded at higher levels of activity,” the authors said.
The issue here is that most studies have not dug deeply into aerobic exercise habits. Often, studies present participants with a list of activities — walking, biking, and running — and ask them to estimate how often and for what duration they do each.
Plus, “we’ve usually lumped moderate and vigorous activities together,” Dr. Rees-Punia said, which means there’s a lack of “granular data” to say whether certain intensities or frequencies of exercise are optimal and for whom.
Why Exercise May Lower Cancer Risk
Exercise habits do not, of course, exist in a vacuum. Highly active people, Dr. Ligibel said, tend to be of higher socioeconomic status, leaner, and have generally healthier lifestyles than sedentary people.
Body weight is a big confounder as well. However, Dr. Rees-Punia noted, it’s also probably a reason that exercise is linked to lower cancer risks, particularly by preventing weight gain. Still, studies have found that the association between exercise and many cancers remains significant after adjusting for body mass index.
The why remains unclear, though some studies offer clues.
“There’s been some really interesting mechanistic research, suggesting that exercise may help inhibit tumor growth or upregulate the immune system,” Dr. Ligibel said.
That includes not only lab research but small intervention studies. While these studies have largely involved people who already have cancer, some have also focused on healthy individuals.
A 2019 study from Dr. Ligibel and her colleagues, which randomly assigned 49 women newly diagnosed with breast cancer to start either an exercise program or mind-body practices ahead of surgery, found exercisers, who had been active for about a month at the time of surgery, showed signs of immune system upregulation in their tumors, while the control group did not.
Among healthy postmenopausal women, a meta-analysis of six clinical trials from Dr. McTiernan and her colleagues found that exercise plus calorie reduction can reduce levels of breast cancer-related endogenous hormones, more so than calorie-cutting alone. And a 2023 study found that high-intensity exercise boosted the ranks of certain immune cells and reduced inflammation in the colon among people at high risk for colon and endometrial cancers due to Lynch syndrome.
Defining an Exercise ‘Prescription’
Despite the gaps and uncertainties in the research, government guidelines as well as those from the American Cancer Society and other medical groups are in lockstep in their exercise recommendations: Adults should strive for 150-300 minutes of moderate-intensity aerobic exercise (like brisk walking), 75-150 minutes of vigorous activity (like running), or some combination each week.
The guidelines also encourage strength training twice a week — advice that’s based on research tying those activity levels to lower risks for heart disease, diabetes, and other chronic conditions.
But there’s no “best” exercise prescription for lowering cancer risk specifically. Most epidemiological studies have examined only aerobic activity, Dr. Rees-Punia said, and there’s very little known about whether strength conditioning or other moderate heart rate-elevating activities, such as daily household chores, may reduce the risk for cancer.
Given the lack of nuance in the literature, it’s hard to say what intensities, types, or amounts of exercise are best for each individual.
Going forward, device-based measurements of physical activity could “help us sort out the effects of different intensities of exercise and possibly types,” Dr. Rees-Punia said.
But overall, Dr. McTiernan said, the data do show that the risks for several cancers are lower at the widely recommended activity levels.
“The bottom-line advice is still to exercise at least 150 minutes per week at a moderate-intensity level or greater,” Dr. McTiernan said.
Or put another way, moving beats being sedentary. It’s probably wise for everyone to sit less, noted Dr. Rees-Punia, for overall health and based on evidence tying sedentary time to the risks for certain cancers, including colon, endometrial, and lung.
There’s a practical element to consider in all of this: What physical activities will people actually do on the regular? In the big epidemiological studies, Dr. McTiernan noted, middle-aged and older adults most often report walking, suggesting that’s the preferred, or most accessible activity, for many.
“You can only benefit from the physical activity you’ll actually do,” Dr. Rees-Punia said.
Dr. Ligibel echoed that sentiment, saying she encourages patients to think about physical activity as a process: “You need to find things you like to do and work them into your daily life, in a sustainable way.
“People often talk about exercise being medicine,” Dr. Ligibel said. “But I think you could take that too far. If we get too prescriptive about it, that could take the joy away.”
A version of this article appeared on Medscape.com.