User login
Key issues in the management of gastrointestinal immune-related adverse events associated with ipilimumab administration
Ipilimumab is an anticytotoxic T lymphocyte antigen-4 (CTLA-4) monoclonal antibody that attenuates negative signaling from CTLA-4 and potentiates T-cell activation and proliferation. Two phase 3 randomized trials in advanced melanoma demonstrated a significant improvement in overall survival, the first of which led to regulatory approval in the United States and Europe for treatment of unresectable or metastatic melanoma. Ipilimumab administration is associated with immune-related adverse events (irAEs). Gastrointestinal (GI) irAEs are among the most common and although they are typically mild to moderate in severity, if they are left unrecognized or untreated, they can become life-threatening. These toxicities can be managed effectively in almost all patients by using established guidelines that stress vigilance and the use of corticosteroids and other immunosuppressive agents when necessary. The goal of this review is to educate physicians on the recognition and challenges associated with management of GI irAEs.
*Click on the link to the left for a PDF of the full article.
Ipilimumab is an anticytotoxic T lymphocyte antigen-4 (CTLA-4) monoclonal antibody that attenuates negative signaling from CTLA-4 and potentiates T-cell activation and proliferation. Two phase 3 randomized trials in advanced melanoma demonstrated a significant improvement in overall survival, the first of which led to regulatory approval in the United States and Europe for treatment of unresectable or metastatic melanoma. Ipilimumab administration is associated with immune-related adverse events (irAEs). Gastrointestinal (GI) irAEs are among the most common and although they are typically mild to moderate in severity, if they are left unrecognized or untreated, they can become life-threatening. These toxicities can be managed effectively in almost all patients by using established guidelines that stress vigilance and the use of corticosteroids and other immunosuppressive agents when necessary. The goal of this review is to educate physicians on the recognition and challenges associated with management of GI irAEs.
*Click on the link to the left for a PDF of the full article.
Ipilimumab is an anticytotoxic T lymphocyte antigen-4 (CTLA-4) monoclonal antibody that attenuates negative signaling from CTLA-4 and potentiates T-cell activation and proliferation. Two phase 3 randomized trials in advanced melanoma demonstrated a significant improvement in overall survival, the first of which led to regulatory approval in the United States and Europe for treatment of unresectable or metastatic melanoma. Ipilimumab administration is associated with immune-related adverse events (irAEs). Gastrointestinal (GI) irAEs are among the most common and although they are typically mild to moderate in severity, if they are left unrecognized or untreated, they can become life-threatening. These toxicities can be managed effectively in almost all patients by using established guidelines that stress vigilance and the use of corticosteroids and other immunosuppressive agents when necessary. The goal of this review is to educate physicians on the recognition and challenges associated with management of GI irAEs.
*Click on the link to the left for a PDF of the full article.
Anti-PD-1 antibodies in melanoma
The programmed death 1 (PD-1) receptor is a negative regulator of T-cell effector mechanisms that limits immune responses against cancer. Two recent phase 1 studies of anti-PD-1 antibodies indicate that these agents exhibit considerable antitumor activity alone or in combination with ipilimumab in patients with advanced melanoma.
*Click on the links to the left for PDFs of the full article and related Commentary.
The programmed death 1 (PD-1) receptor is a negative regulator of T-cell effector mechanisms that limits immune responses against cancer. Two recent phase 1 studies of anti-PD-1 antibodies indicate that these agents exhibit considerable antitumor activity alone or in combination with ipilimumab in patients with advanced melanoma.
*Click on the links to the left for PDFs of the full article and related Commentary.
The programmed death 1 (PD-1) receptor is a negative regulator of T-cell effector mechanisms that limits immune responses against cancer. Two recent phase 1 studies of anti-PD-1 antibodies indicate that these agents exhibit considerable antitumor activity alone or in combination with ipilimumab in patients with advanced melanoma.
*Click on the links to the left for PDFs of the full article and related Commentary.
Beta-blockers and Melanoma

I have taken beta-blockers for nearly 30 years to combat a non–life-threatening but very disconcerting supraventricular arrhythmia. Also being fair skinned and having suffered through many a blistering sunburn in my youth, I was excited to read the most recent publication regarding the relationship between beta-blocker ingestion and survival from melanoma by De Giorgi et al (Mayo Clin Proc. 2013;88:1196-1203). The Italian investigators collected data from all melanoma patients diagnosed in the dermatology department at the University of Florence (1993-2009). After excluding patients who presented with metastatic disease, they then compared the courses of the remaining patients based on whether or not they had been prescribed beta-blockers for at least 1 year before or after their cutaneous melanoma diagnosis. Of 741 consecutive patients who fit the retrospective study criteria, 79 (11%) had been taking beta-blockers and the remaining 662 (89%) had not been taking beta-blockers.
An analysis based on the multivariate Cox model indicated that the beta-blocker group had improved overall survival after a median follow-up of 4.2 years (P=.005). Looked at in another way, significantly more patients in the untreated group (8%) than the beta-blocker group (3%) experienced disease progression or death (P<.001). The “protective” effect of beta-blockers was so striking that it could be quantified; for each year of beta-blocker use, the risk for death was reduced by 38%. More notable was the fact that the beneficial effect of beta-blocker administration was consistent, even among patients with inherently unfavorable prognostic factors, such as advanced age, thicker lesions, higher frequency of mitoses and ulceration, and nodular melanoma subtype. This large study confirms the results of a much smaller study (N=121) published by the same group in 2011 (Arch Intern Med. 2011;171:779-781).
What’s the issue?
Why might this phenomenon occur? Perhaps blocking sympathetic nervous system neurotransmitters might help avoid the suspected immunosuppression that accompanies stress. Sympathetic neurotransmitters also are known to interact with molecular pathways implicated in abnormal cellular replication, such as the p38/MAPK pathway. Thus, blunting the effects of epinephrine and norepinephrine by blocking some of their receptors might produce a salutatory benefit in the cancer (in this case, melanoma) patient.
Before we all rush out and give all our melanoma patients daily doses of metoprolol, however, De Giorgi et al noted in the most recent study that about two-thirds of the patients were already taking a beta-blocker when their melanoma was diagnosed. Although this study suggests that beta-blockers reduce the risk for recurrence and disease-specific mortality, these drugs clearly do not prevent melanoma in the first place. Furthermore, it should be noted that the number of melanoma patients receiving beta-blockers was small, and conclusions should always be tempered in a retrospective study. The authors duly admit that their investigation indicates the strong need for a truly randomized prospective clinical trial. Of course, administration of beta-blockers is not a totally benign endeavor, as serious hypotension and/or bradycardia may occur, leading to syncope or even worse problems. Finally, the evidence of beta-blocker benefit in melanoma patients is contradictory! A Dutch cohort of 709 melanoma patients recently was studied (Eur J Cancer. 2013;49:3863-3871); this set of investigators found that exposure to beta-blockers did not impact overall melanoma survival regardless of the timing, duration, or dosage of beta-blocker use. In summary, therefore, we still need to determine many things about the relationship between beta-blockers and melanoma survival. That is, if there is a relationship at all. What do you think?

I have taken beta-blockers for nearly 30 years to combat a non–life-threatening but very disconcerting supraventricular arrhythmia. Also being fair skinned and having suffered through many a blistering sunburn in my youth, I was excited to read the most recent publication regarding the relationship between beta-blocker ingestion and survival from melanoma by De Giorgi et al (Mayo Clin Proc. 2013;88:1196-1203). The Italian investigators collected data from all melanoma patients diagnosed in the dermatology department at the University of Florence (1993-2009). After excluding patients who presented with metastatic disease, they then compared the courses of the remaining patients based on whether or not they had been prescribed beta-blockers for at least 1 year before or after their cutaneous melanoma diagnosis. Of 741 consecutive patients who fit the retrospective study criteria, 79 (11%) had been taking beta-blockers and the remaining 662 (89%) had not been taking beta-blockers.
An analysis based on the multivariate Cox model indicated that the beta-blocker group had improved overall survival after a median follow-up of 4.2 years (P=.005). Looked at in another way, significantly more patients in the untreated group (8%) than the beta-blocker group (3%) experienced disease progression or death (P<.001). The “protective” effect of beta-blockers was so striking that it could be quantified; for each year of beta-blocker use, the risk for death was reduced by 38%. More notable was the fact that the beneficial effect of beta-blocker administration was consistent, even among patients with inherently unfavorable prognostic factors, such as advanced age, thicker lesions, higher frequency of mitoses and ulceration, and nodular melanoma subtype. This large study confirms the results of a much smaller study (N=121) published by the same group in 2011 (Arch Intern Med. 2011;171:779-781).
What’s the issue?
Why might this phenomenon occur? Perhaps blocking sympathetic nervous system neurotransmitters might help avoid the suspected immunosuppression that accompanies stress. Sympathetic neurotransmitters also are known to interact with molecular pathways implicated in abnormal cellular replication, such as the p38/MAPK pathway. Thus, blunting the effects of epinephrine and norepinephrine by blocking some of their receptors might produce a salutatory benefit in the cancer (in this case, melanoma) patient.
Before we all rush out and give all our melanoma patients daily doses of metoprolol, however, De Giorgi et al noted in the most recent study that about two-thirds of the patients were already taking a beta-blocker when their melanoma was diagnosed. Although this study suggests that beta-blockers reduce the risk for recurrence and disease-specific mortality, these drugs clearly do not prevent melanoma in the first place. Furthermore, it should be noted that the number of melanoma patients receiving beta-blockers was small, and conclusions should always be tempered in a retrospective study. The authors duly admit that their investigation indicates the strong need for a truly randomized prospective clinical trial. Of course, administration of beta-blockers is not a totally benign endeavor, as serious hypotension and/or bradycardia may occur, leading to syncope or even worse problems. Finally, the evidence of beta-blocker benefit in melanoma patients is contradictory! A Dutch cohort of 709 melanoma patients recently was studied (Eur J Cancer. 2013;49:3863-3871); this set of investigators found that exposure to beta-blockers did not impact overall melanoma survival regardless of the timing, duration, or dosage of beta-blocker use. In summary, therefore, we still need to determine many things about the relationship between beta-blockers and melanoma survival. That is, if there is a relationship at all. What do you think?

I have taken beta-blockers for nearly 30 years to combat a non–life-threatening but very disconcerting supraventricular arrhythmia. Also being fair skinned and having suffered through many a blistering sunburn in my youth, I was excited to read the most recent publication regarding the relationship between beta-blocker ingestion and survival from melanoma by De Giorgi et al (Mayo Clin Proc. 2013;88:1196-1203). The Italian investigators collected data from all melanoma patients diagnosed in the dermatology department at the University of Florence (1993-2009). After excluding patients who presented with metastatic disease, they then compared the courses of the remaining patients based on whether or not they had been prescribed beta-blockers for at least 1 year before or after their cutaneous melanoma diagnosis. Of 741 consecutive patients who fit the retrospective study criteria, 79 (11%) had been taking beta-blockers and the remaining 662 (89%) had not been taking beta-blockers.
An analysis based on the multivariate Cox model indicated that the beta-blocker group had improved overall survival after a median follow-up of 4.2 years (P=.005). Looked at in another way, significantly more patients in the untreated group (8%) than the beta-blocker group (3%) experienced disease progression or death (P<.001). The “protective” effect of beta-blockers was so striking that it could be quantified; for each year of beta-blocker use, the risk for death was reduced by 38%. More notable was the fact that the beneficial effect of beta-blocker administration was consistent, even among patients with inherently unfavorable prognostic factors, such as advanced age, thicker lesions, higher frequency of mitoses and ulceration, and nodular melanoma subtype. This large study confirms the results of a much smaller study (N=121) published by the same group in 2011 (Arch Intern Med. 2011;171:779-781).
What’s the issue?
Why might this phenomenon occur? Perhaps blocking sympathetic nervous system neurotransmitters might help avoid the suspected immunosuppression that accompanies stress. Sympathetic neurotransmitters also are known to interact with molecular pathways implicated in abnormal cellular replication, such as the p38/MAPK pathway. Thus, blunting the effects of epinephrine and norepinephrine by blocking some of their receptors might produce a salutatory benefit in the cancer (in this case, melanoma) patient.
Before we all rush out and give all our melanoma patients daily doses of metoprolol, however, De Giorgi et al noted in the most recent study that about two-thirds of the patients were already taking a beta-blocker when their melanoma was diagnosed. Although this study suggests that beta-blockers reduce the risk for recurrence and disease-specific mortality, these drugs clearly do not prevent melanoma in the first place. Furthermore, it should be noted that the number of melanoma patients receiving beta-blockers was small, and conclusions should always be tempered in a retrospective study. The authors duly admit that their investigation indicates the strong need for a truly randomized prospective clinical trial. Of course, administration of beta-blockers is not a totally benign endeavor, as serious hypotension and/or bradycardia may occur, leading to syncope or even worse problems. Finally, the evidence of beta-blocker benefit in melanoma patients is contradictory! A Dutch cohort of 709 melanoma patients recently was studied (Eur J Cancer. 2013;49:3863-3871); this set of investigators found that exposure to beta-blockers did not impact overall melanoma survival regardless of the timing, duration, or dosage of beta-blocker use. In summary, therefore, we still need to determine many things about the relationship between beta-blockers and melanoma survival. That is, if there is a relationship at all. What do you think?
Thiopurine use ups risk of skin cancer for ulcerative colitis patients
SAN DIEGO – Current thiopurine use is an independent risk factor for nonmelanoma skin cancer in patients with ulcerative colitis, according to a study reported at the annual meeting of the American College of Gastroenterology.
In the retrospective cohort study of more than 14,000 U.S. veterans with ulcerative colitis, current users of thiopurines were more than twice as likely as never users to receive a diagnosis of squamous or basal cell skin cancer. The excess risk, however, disappeared after stopping thiopurines.
"It is crucial to educate physicians and patients about the risk of nonmelanoma skin cancer and possible preventive measures," asserted Dr. Ali Abbas, an internal medicine resident at * University of Florida, Gainesville.
Session comoderator Dr. Stephen B. Hanauer of the University of Chicago noted, "The current quality measures in [inflammatory bowel disease] include a number of measures related to biologic therapy – immunization/vaccination, [tuberculosis] testing, etc. ... Do you believe that yearly skin examination should be added? Is there enough evidence that this should be added to the quality indicators?"
"I think now we have enough evidence to recommend regular skin examination for those on long-term thiopurines," Dr. Abbas replied, while adding that the reversibility of risk after stopping remains controversial.
Dr. Hanauer further noted that number of health care visits also predicted nonmelanoma skin cancer risk. "I would suspect that, while patients are on thiopurines, they are getting more intensive visits, so can you dissociate those factors?"
Multivariate analyses took into account the number of visits, according to Dr. Abbas. "Even after exclusion of this confounding effect, we had a two times increase of the risk," he said. "If you compare our hazard ratio with previously reported hazard ratios, it’s lower – most of them reported were 4 to 5. So I am assuming that we kind of excluded the effect of the detection bias and we present a more independent effect of thiopurines on risk of nonmelanoma skin cancer."
"Do you know what the lag time would be then from exposure to the development of a nonmelanoma? So is it biologically plausible to understand that this would be reversible in patients who stop therapy?" asked Dr. David T. Rubin, codirector of the inflammatory bowel disease center at the University of Chicago, the other session comoderator.
The investigators did not calculate lag time, Dr. Abbas replied. "The biological origin is just the interaction between the UV [ultraviolet] radiation on the skin and the damage that the thiopurine causes to the DNA will prevent the repair. So from a biological point of view, I think it’s understandable to conclude that, once the insult to the DNA or the repair mechanism is gone, the UV radiation effect will also be low."
"Well, I think there is another biologic plausibility," Dr. Hanauer suggested, "which is the potential for viral infections such as [human papillomavirus] contributing to skin cancers, and the known effect of thiopurines on viruses. So certainly, UV is a very strong association, but we are also familiar with individuals who have skin warts."
"You do need to understand lag time between exposure and subsequent neoplasia before you conclude that when you are off therapy, you don’t need screening anymore," Dr. Rubin said.
Upcoming analyses will stratify patients according to the location of skin cancer, for example, looking at anogenital skin cancers separately, Dr. Abbas replied.
In the study, the investigators reviewed pharmacy benefits records for 14,527 veterans with an ulcerative colitis diagnosis who were seen in the Veterans Affairs health care system between 2001 and 2011.
They were 59 years old on average at baseline; 94% were male and 77% were white. Their median follow-up was 8.1 years, according to Dr. Abbas.
Overall, 23% of the patients used thiopurines, for a median of 1.6 years during follow-up, and the median duration of follow-up after stopping these medications was 3.6 years. The median duration of follow-up among thiopurine-unexposed patients was 6.7 years.
Main results showed that the incidence of nonmelanoma skin cancer was 3.7 per 1,000 person-years among unexposed patients, 8.4 per 1,000 person-years during thiopurine use, and 3.0 per 1,000 person-years after stopping thiopurines.
In a multivariate model, patients had a significantly higher risk of nonmelanoma skin cancer while taking thiopurines when compared with never-users (hazard ratio, 2.1). There also was a nonsignificant trend toward reduced risk after stopping, as compared with never users (0.7).
Other factors associated with higher risk included older age, male sex, white race/ethnicity, living in zones with high UV exposure, and more frequent use of the VA health care system.
In stratified analyses, the incidence of nonmelanoma skin cancer during thiopurine use increased with patient age from younger than 40 years to 40-65 years to older than 65 years (0.6, 9.1, and 12.2 per 1,000 person-years); was greater among patients living in high-UV zones versus low- or medium-UV zones (10.3 vs. 6.0 per 1,000 person-years); and increased with number of VA visits annually from fewer than six, to 6-12, and then more than 12 (1.2, 9.4, and 12.3 per 1,000 person-years).
Finally, the rate rose with the cumulative duration of exposure to thiopurines. It increased steadily during the first 2 years of use, stabilized through the fourth year of use, and rose sharply in the fifth year of use to 13.6 per 1,000 person-years.
Dr. Abbas disclosed no relevant conflicts of interest.
*Correction 12/10/13: A previous version of this article incorrectly reported Dr. Ali Abbas' university affiliation. This version has been updated.
SAN DIEGO – Current thiopurine use is an independent risk factor for nonmelanoma skin cancer in patients with ulcerative colitis, according to a study reported at the annual meeting of the American College of Gastroenterology.
In the retrospective cohort study of more than 14,000 U.S. veterans with ulcerative colitis, current users of thiopurines were more than twice as likely as never users to receive a diagnosis of squamous or basal cell skin cancer. The excess risk, however, disappeared after stopping thiopurines.
"It is crucial to educate physicians and patients about the risk of nonmelanoma skin cancer and possible preventive measures," asserted Dr. Ali Abbas, an internal medicine resident at * University of Florida, Gainesville.
Session comoderator Dr. Stephen B. Hanauer of the University of Chicago noted, "The current quality measures in [inflammatory bowel disease] include a number of measures related to biologic therapy – immunization/vaccination, [tuberculosis] testing, etc. ... Do you believe that yearly skin examination should be added? Is there enough evidence that this should be added to the quality indicators?"
"I think now we have enough evidence to recommend regular skin examination for those on long-term thiopurines," Dr. Abbas replied, while adding that the reversibility of risk after stopping remains controversial.
Dr. Hanauer further noted that number of health care visits also predicted nonmelanoma skin cancer risk. "I would suspect that, while patients are on thiopurines, they are getting more intensive visits, so can you dissociate those factors?"
Multivariate analyses took into account the number of visits, according to Dr. Abbas. "Even after exclusion of this confounding effect, we had a two times increase of the risk," he said. "If you compare our hazard ratio with previously reported hazard ratios, it’s lower – most of them reported were 4 to 5. So I am assuming that we kind of excluded the effect of the detection bias and we present a more independent effect of thiopurines on risk of nonmelanoma skin cancer."
"Do you know what the lag time would be then from exposure to the development of a nonmelanoma? So is it biologically plausible to understand that this would be reversible in patients who stop therapy?" asked Dr. David T. Rubin, codirector of the inflammatory bowel disease center at the University of Chicago, the other session comoderator.
The investigators did not calculate lag time, Dr. Abbas replied. "The biological origin is just the interaction between the UV [ultraviolet] radiation on the skin and the damage that the thiopurine causes to the DNA will prevent the repair. So from a biological point of view, I think it’s understandable to conclude that, once the insult to the DNA or the repair mechanism is gone, the UV radiation effect will also be low."
"Well, I think there is another biologic plausibility," Dr. Hanauer suggested, "which is the potential for viral infections such as [human papillomavirus] contributing to skin cancers, and the known effect of thiopurines on viruses. So certainly, UV is a very strong association, but we are also familiar with individuals who have skin warts."
"You do need to understand lag time between exposure and subsequent neoplasia before you conclude that when you are off therapy, you don’t need screening anymore," Dr. Rubin said.
Upcoming analyses will stratify patients according to the location of skin cancer, for example, looking at anogenital skin cancers separately, Dr. Abbas replied.
In the study, the investigators reviewed pharmacy benefits records for 14,527 veterans with an ulcerative colitis diagnosis who were seen in the Veterans Affairs health care system between 2001 and 2011.
They were 59 years old on average at baseline; 94% were male and 77% were white. Their median follow-up was 8.1 years, according to Dr. Abbas.
Overall, 23% of the patients used thiopurines, for a median of 1.6 years during follow-up, and the median duration of follow-up after stopping these medications was 3.6 years. The median duration of follow-up among thiopurine-unexposed patients was 6.7 years.
Main results showed that the incidence of nonmelanoma skin cancer was 3.7 per 1,000 person-years among unexposed patients, 8.4 per 1,000 person-years during thiopurine use, and 3.0 per 1,000 person-years after stopping thiopurines.
In a multivariate model, patients had a significantly higher risk of nonmelanoma skin cancer while taking thiopurines when compared with never-users (hazard ratio, 2.1). There also was a nonsignificant trend toward reduced risk after stopping, as compared with never users (0.7).
Other factors associated with higher risk included older age, male sex, white race/ethnicity, living in zones with high UV exposure, and more frequent use of the VA health care system.
In stratified analyses, the incidence of nonmelanoma skin cancer during thiopurine use increased with patient age from younger than 40 years to 40-65 years to older than 65 years (0.6, 9.1, and 12.2 per 1,000 person-years); was greater among patients living in high-UV zones versus low- or medium-UV zones (10.3 vs. 6.0 per 1,000 person-years); and increased with number of VA visits annually from fewer than six, to 6-12, and then more than 12 (1.2, 9.4, and 12.3 per 1,000 person-years).
Finally, the rate rose with the cumulative duration of exposure to thiopurines. It increased steadily during the first 2 years of use, stabilized through the fourth year of use, and rose sharply in the fifth year of use to 13.6 per 1,000 person-years.
Dr. Abbas disclosed no relevant conflicts of interest.
*Correction 12/10/13: A previous version of this article incorrectly reported Dr. Ali Abbas' university affiliation. This version has been updated.
SAN DIEGO – Current thiopurine use is an independent risk factor for nonmelanoma skin cancer in patients with ulcerative colitis, according to a study reported at the annual meeting of the American College of Gastroenterology.
In the retrospective cohort study of more than 14,000 U.S. veterans with ulcerative colitis, current users of thiopurines were more than twice as likely as never users to receive a diagnosis of squamous or basal cell skin cancer. The excess risk, however, disappeared after stopping thiopurines.
"It is crucial to educate physicians and patients about the risk of nonmelanoma skin cancer and possible preventive measures," asserted Dr. Ali Abbas, an internal medicine resident at * University of Florida, Gainesville.
Session comoderator Dr. Stephen B. Hanauer of the University of Chicago noted, "The current quality measures in [inflammatory bowel disease] include a number of measures related to biologic therapy – immunization/vaccination, [tuberculosis] testing, etc. ... Do you believe that yearly skin examination should be added? Is there enough evidence that this should be added to the quality indicators?"
"I think now we have enough evidence to recommend regular skin examination for those on long-term thiopurines," Dr. Abbas replied, while adding that the reversibility of risk after stopping remains controversial.
Dr. Hanauer further noted that number of health care visits also predicted nonmelanoma skin cancer risk. "I would suspect that, while patients are on thiopurines, they are getting more intensive visits, so can you dissociate those factors?"
Multivariate analyses took into account the number of visits, according to Dr. Abbas. "Even after exclusion of this confounding effect, we had a two times increase of the risk," he said. "If you compare our hazard ratio with previously reported hazard ratios, it’s lower – most of them reported were 4 to 5. So I am assuming that we kind of excluded the effect of the detection bias and we present a more independent effect of thiopurines on risk of nonmelanoma skin cancer."
"Do you know what the lag time would be then from exposure to the development of a nonmelanoma? So is it biologically plausible to understand that this would be reversible in patients who stop therapy?" asked Dr. David T. Rubin, codirector of the inflammatory bowel disease center at the University of Chicago, the other session comoderator.
The investigators did not calculate lag time, Dr. Abbas replied. "The biological origin is just the interaction between the UV [ultraviolet] radiation on the skin and the damage that the thiopurine causes to the DNA will prevent the repair. So from a biological point of view, I think it’s understandable to conclude that, once the insult to the DNA or the repair mechanism is gone, the UV radiation effect will also be low."
"Well, I think there is another biologic plausibility," Dr. Hanauer suggested, "which is the potential for viral infections such as [human papillomavirus] contributing to skin cancers, and the known effect of thiopurines on viruses. So certainly, UV is a very strong association, but we are also familiar with individuals who have skin warts."
"You do need to understand lag time between exposure and subsequent neoplasia before you conclude that when you are off therapy, you don’t need screening anymore," Dr. Rubin said.
Upcoming analyses will stratify patients according to the location of skin cancer, for example, looking at anogenital skin cancers separately, Dr. Abbas replied.
In the study, the investigators reviewed pharmacy benefits records for 14,527 veterans with an ulcerative colitis diagnosis who were seen in the Veterans Affairs health care system between 2001 and 2011.
They were 59 years old on average at baseline; 94% were male and 77% were white. Their median follow-up was 8.1 years, according to Dr. Abbas.
Overall, 23% of the patients used thiopurines, for a median of 1.6 years during follow-up, and the median duration of follow-up after stopping these medications was 3.6 years. The median duration of follow-up among thiopurine-unexposed patients was 6.7 years.
Main results showed that the incidence of nonmelanoma skin cancer was 3.7 per 1,000 person-years among unexposed patients, 8.4 per 1,000 person-years during thiopurine use, and 3.0 per 1,000 person-years after stopping thiopurines.
In a multivariate model, patients had a significantly higher risk of nonmelanoma skin cancer while taking thiopurines when compared with never-users (hazard ratio, 2.1). There also was a nonsignificant trend toward reduced risk after stopping, as compared with never users (0.7).
Other factors associated with higher risk included older age, male sex, white race/ethnicity, living in zones with high UV exposure, and more frequent use of the VA health care system.
In stratified analyses, the incidence of nonmelanoma skin cancer during thiopurine use increased with patient age from younger than 40 years to 40-65 years to older than 65 years (0.6, 9.1, and 12.2 per 1,000 person-years); was greater among patients living in high-UV zones versus low- or medium-UV zones (10.3 vs. 6.0 per 1,000 person-years); and increased with number of VA visits annually from fewer than six, to 6-12, and then more than 12 (1.2, 9.4, and 12.3 per 1,000 person-years).
Finally, the rate rose with the cumulative duration of exposure to thiopurines. It increased steadily during the first 2 years of use, stabilized through the fourth year of use, and rose sharply in the fifth year of use to 13.6 per 1,000 person-years.
Dr. Abbas disclosed no relevant conflicts of interest.
*Correction 12/10/13: A previous version of this article incorrectly reported Dr. Ali Abbas' university affiliation. This version has been updated.
AT THE ACG ANNUAL MEETING
Major Finding: Current users of thiopurines had more than twice the risk of nonmelanoma skin cancer as never users (hazard ratio, 2.1), but former users did not have an elevation of risk.
Data Source: A nationwide retrospective cohort study of 14,527 veterans with ulcerative colitis.
Disclosures: Dr. Abbas disclosed no relevant conflicts of interest.
Zinc oxide
Zinc is a trace element, and it is not synthesized by the human body. The element was identified in the 1960s as being essential for human health and development. Zinc is a cofactor in more than 300 enzymes necessary for cell function. In the dermatologic realm, zinc deficiency has been associated with skin alterations, delayed wound healing, and hair loss.
Zinc oxide (ZnO) is a metal oxide that also has a broad profile in dermatology. It is perhaps best known as a physical sunscreen ingredient. ZnO and titanium dioxide (TiO2) have long been used in this manner. Both ZnO and TiO2 also have been increasingly used to replace large-particle compounds in numerous cosmetics and sunscreens. These two compounds have demonstrated effective protection against UV-induced damage, providing stronger protection against UV radiation while leaving less white residue than previous generations of physical sunscreens.
Particles of ZnO in earlier sunscreens were found to be too large to penetrate the stratum corneum and, thus, were deemed biologically inactive (J. Am. Acad. Dermatol. 1999;40:85-90). However, in novel nanoparticle form, such metal oxides absorb UV radiation, leading to photocatalysis and the release of reactive oxygen species (Australas. J. Dermatol. 2011;52:1-6). Indeed, nanoparticles exhibit new physiochemical properties as a result of increased surface area as compared to large-form products, and the potential adverse effects of the novel nanoparticle formulations in sunscreens cannot be adequately extrapolated from the effects of older-generation larger-particle skin care products (J. Drugs Dermatol. 2010;9:475-81; Int. J. Dermatol. 2011;50:247-54. The relative safety of ZnO nanoparticles will be discussed in a future column. The focus in this column will be a brief comparison with TiO2 and other indications for ZnO.
ZnO and TiO2
While numerous studies explore both TiO2 and ZnO, the latter is noted for greater versatility within the dermatologic armamentarium. In addition, ZnO is less photoactive and is associated with a lower refractive index in visible light than TiO2 (1.9 vs. 2.6, respectively) (J. Am. Acad. Dermatol. 1999;40:85-90); therefore, TiO2 appears whiter and is more difficult to incorporate into transparent products.
Another important difference is the spectrum of action. That is, only avobenzone (butyl methoxydibenzoylmethane) and ZnO are approved in the United States for broad-spectrum protection against UVA wavelengths greater than 360 nm, because TiO2 has been shown to be effective only against UV wavelengths less than 360 nm (UVA is 320-400 nm). In a study by Beasley and Meyer, TiO2 delivered neither the same level of UVA attenuation nor protection from UVA to human skin as did photostabilized formulations of avobenzone or ZnO. Therefore, TiO2 is not a suitable substitute for avobenzone and ZnO for strong UVA protection (Am. J. Clin. Dermatol. 2010;11:413-21).
Indications beyond photoprotection
More than 20 years ago, Hughes and McLean showed that a ZnO tape was effective in dressing fingertip and soft tissue injuries that were resistant to healing (Arch. Emerg. Med. 1988;5:223-7). More recently, Parboteeah and Brown demonstrated the efficacy of treating recalcitrant venous leg ulcers with ZnO paste bandages (Br. J. Nurs. 2008;17:S30, S32, S34-6). In addition, Treadwell has shown that the weekly application of ZnO compression dressings to surgical wounds of the lower leg promotes healing (Dermatol. Surg. 2011;37:166-7).
Micronized zinc oxide is included in a 4% hydroquinone/10% L-ascorbic acid treatment system recently found (in a small study of 34 females) to be effective in alleviating early signs of photodamage in normal to oily skin. Thirty patients, with minimal or mild facial photodamage and hyperpigmentation, completed the 12-week treatment regimen. All the participants were satisfied with the appearance of their skin after the study, with median scores for all assessment parameters significantly improved compared with baseline (J. Drugs Dermatol. 2011;10:1455-61). ZnO also is an active ingredient in formulations intended to support the healing of perianal eczema (Hautarzt. 2010;61:33-8).
A 2001 report on a series of blinded, randomized clinical trials conducted by Baldwin et al. showed that clinical benefits were derived from the continuous topical administration of a ZnO/petrolatum formulation in a diaper introduced at that time. The first study was undertaken to verify that the ZnO/petrolatum formulation was indeed transferred from the diaper to the child’s skin. Stratum corneum (SC) samples were analyzed from each child after the wearing of a single diaper for 3 hours or multiple diapers for 24 hours. The results indicated effective transfer, with ZnO increasing in the SC from 4.2 mcg/cm2 at 3 hours to more than 8 mcg/cm2 at 24 hours.
The second study of the formulation, in an adult arm model, assessed the prevention of irritation and SC damage induced by sodium laureth sulfate. The investigators found that the ZnO/petrolatum combination yielded significant reductions in SC damage and erythema. The third study, a 4-week trial in which 268 infants were assessed, considered the effects of the formulation on erythema and diaper rash. Half of the infants wore the test diaper and half used a control diaper lacking the ZnO/petrolatum product. Significant reductions in erythema and diaper rash were indeed observed in the test group (J. Eur. Acad. Dermatol. Venereol. 2001;15 Suppl 1:5-11).
A 2009 study showed that an unmedicated ZnO/petrolatum paste was effective in restoring the properties of the skin, allowing for balanced transepidermal water loss and water retention by SC previously compromised by diaper dermatitis. This skin condition affects approximately 50% of infants and a small percentage of the bedridden elderly (Int. J. Cosmet. Sci. 2009;31:369-74).
In 2010, a study that assessed the effectiveness of topical ZnO ointment using the rabbit ear hypertrophic scar model showed that the application of 40% ZnO significantly reduced clinical scar hypertrophy scores at 6 weeks compared with placebo. The researchers concluded that these results may suggest clinical applications for ZnO in the treatment of hypertrophic scars in humans (Burns 2010;36:1027-35). In addition, ZnO has demonstrated antibacterial properties, with nanoparticles exhibiting more potent antibacterial activity than bulk ZnO (Sci. Technol. Adv. Mater. 2008;9:1-7).
Products
ZnO is a key ingredient in calamine lotion, an antipruritic compound used to treat various mild conditions such as bites and stings from insects, eczema, poison ivy, rashes, and sunburn. It is also available over the counter in ointment or suppository form for healing hemorrhoids and fissures. In addition, ZnO is used widely in baby powders, barrier creams, moisturizers, antiseptic ointments, antidandruff shampoos, athletic bandage tape, and, of course, sunscreens.
Conclusion
ZnO is a versatile inorganic metal oxide with multiple indications in dermatology. Consequently, it is included in a wide array of skin care products, including shampoos, moisturizers, and sunscreens. Its use in nanoparticle form, along with the similar use of its physical sunscreen counterpart TiO2, represents one of the many subjects debated within the larger context of sunscreen use. The next edition of this column will focus on the relative safety of zinc oxide nanoparticles.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in Miami Beach. She founded the cosmetic dermatology center at the University of Miami in 1997. Dr. Baumann wrote the textbook "Cosmetic Dermatology: Principles and Practice" (McGraw-Hill, April 2002), and a book for consumers, "The Skin Type Solution" (Bantam, 2006). She has contributed to the Cosmeceutical Critique column in Skin & Allergy News since January 2001 and joined the editorial advisory board in 2004. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Galderma, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Stiefel, Topix Pharmaceuticals, and Unilever.
This column, "Cosmeceutical Critique," appears regularly in Skin & Allergy News, a publication of Frontline Medical News.
Zinc is a trace element, and it is not synthesized by the human body. The element was identified in the 1960s as being essential for human health and development. Zinc is a cofactor in more than 300 enzymes necessary for cell function. In the dermatologic realm, zinc deficiency has been associated with skin alterations, delayed wound healing, and hair loss.
Zinc oxide (ZnO) is a metal oxide that also has a broad profile in dermatology. It is perhaps best known as a physical sunscreen ingredient. ZnO and titanium dioxide (TiO2) have long been used in this manner. Both ZnO and TiO2 also have been increasingly used to replace large-particle compounds in numerous cosmetics and sunscreens. These two compounds have demonstrated effective protection against UV-induced damage, providing stronger protection against UV radiation while leaving less white residue than previous generations of physical sunscreens.
Particles of ZnO in earlier sunscreens were found to be too large to penetrate the stratum corneum and, thus, were deemed biologically inactive (J. Am. Acad. Dermatol. 1999;40:85-90). However, in novel nanoparticle form, such metal oxides absorb UV radiation, leading to photocatalysis and the release of reactive oxygen species (Australas. J. Dermatol. 2011;52:1-6). Indeed, nanoparticles exhibit new physiochemical properties as a result of increased surface area as compared to large-form products, and the potential adverse effects of the novel nanoparticle formulations in sunscreens cannot be adequately extrapolated from the effects of older-generation larger-particle skin care products (J. Drugs Dermatol. 2010;9:475-81; Int. J. Dermatol. 2011;50:247-54. The relative safety of ZnO nanoparticles will be discussed in a future column. The focus in this column will be a brief comparison with TiO2 and other indications for ZnO.
ZnO and TiO2
While numerous studies explore both TiO2 and ZnO, the latter is noted for greater versatility within the dermatologic armamentarium. In addition, ZnO is less photoactive and is associated with a lower refractive index in visible light than TiO2 (1.9 vs. 2.6, respectively) (J. Am. Acad. Dermatol. 1999;40:85-90); therefore, TiO2 appears whiter and is more difficult to incorporate into transparent products.
Another important difference is the spectrum of action. That is, only avobenzone (butyl methoxydibenzoylmethane) and ZnO are approved in the United States for broad-spectrum protection against UVA wavelengths greater than 360 nm, because TiO2 has been shown to be effective only against UV wavelengths less than 360 nm (UVA is 320-400 nm). In a study by Beasley and Meyer, TiO2 delivered neither the same level of UVA attenuation nor protection from UVA to human skin as did photostabilized formulations of avobenzone or ZnO. Therefore, TiO2 is not a suitable substitute for avobenzone and ZnO for strong UVA protection (Am. J. Clin. Dermatol. 2010;11:413-21).
Indications beyond photoprotection
More than 20 years ago, Hughes and McLean showed that a ZnO tape was effective in dressing fingertip and soft tissue injuries that were resistant to healing (Arch. Emerg. Med. 1988;5:223-7). More recently, Parboteeah and Brown demonstrated the efficacy of treating recalcitrant venous leg ulcers with ZnO paste bandages (Br. J. Nurs. 2008;17:S30, S32, S34-6). In addition, Treadwell has shown that the weekly application of ZnO compression dressings to surgical wounds of the lower leg promotes healing (Dermatol. Surg. 2011;37:166-7).
Micronized zinc oxide is included in a 4% hydroquinone/10% L-ascorbic acid treatment system recently found (in a small study of 34 females) to be effective in alleviating early signs of photodamage in normal to oily skin. Thirty patients, with minimal or mild facial photodamage and hyperpigmentation, completed the 12-week treatment regimen. All the participants were satisfied with the appearance of their skin after the study, with median scores for all assessment parameters significantly improved compared with baseline (J. Drugs Dermatol. 2011;10:1455-61). ZnO also is an active ingredient in formulations intended to support the healing of perianal eczema (Hautarzt. 2010;61:33-8).
A 2001 report on a series of blinded, randomized clinical trials conducted by Baldwin et al. showed that clinical benefits were derived from the continuous topical administration of a ZnO/petrolatum formulation in a diaper introduced at that time. The first study was undertaken to verify that the ZnO/petrolatum formulation was indeed transferred from the diaper to the child’s skin. Stratum corneum (SC) samples were analyzed from each child after the wearing of a single diaper for 3 hours or multiple diapers for 24 hours. The results indicated effective transfer, with ZnO increasing in the SC from 4.2 mcg/cm2 at 3 hours to more than 8 mcg/cm2 at 24 hours.
The second study of the formulation, in an adult arm model, assessed the prevention of irritation and SC damage induced by sodium laureth sulfate. The investigators found that the ZnO/petrolatum combination yielded significant reductions in SC damage and erythema. The third study, a 4-week trial in which 268 infants were assessed, considered the effects of the formulation on erythema and diaper rash. Half of the infants wore the test diaper and half used a control diaper lacking the ZnO/petrolatum product. Significant reductions in erythema and diaper rash were indeed observed in the test group (J. Eur. Acad. Dermatol. Venereol. 2001;15 Suppl 1:5-11).
A 2009 study showed that an unmedicated ZnO/petrolatum paste was effective in restoring the properties of the skin, allowing for balanced transepidermal water loss and water retention by SC previously compromised by diaper dermatitis. This skin condition affects approximately 50% of infants and a small percentage of the bedridden elderly (Int. J. Cosmet. Sci. 2009;31:369-74).
In 2010, a study that assessed the effectiveness of topical ZnO ointment using the rabbit ear hypertrophic scar model showed that the application of 40% ZnO significantly reduced clinical scar hypertrophy scores at 6 weeks compared with placebo. The researchers concluded that these results may suggest clinical applications for ZnO in the treatment of hypertrophic scars in humans (Burns 2010;36:1027-35). In addition, ZnO has demonstrated antibacterial properties, with nanoparticles exhibiting more potent antibacterial activity than bulk ZnO (Sci. Technol. Adv. Mater. 2008;9:1-7).
Products
ZnO is a key ingredient in calamine lotion, an antipruritic compound used to treat various mild conditions such as bites and stings from insects, eczema, poison ivy, rashes, and sunburn. It is also available over the counter in ointment or suppository form for healing hemorrhoids and fissures. In addition, ZnO is used widely in baby powders, barrier creams, moisturizers, antiseptic ointments, antidandruff shampoos, athletic bandage tape, and, of course, sunscreens.
Conclusion
ZnO is a versatile inorganic metal oxide with multiple indications in dermatology. Consequently, it is included in a wide array of skin care products, including shampoos, moisturizers, and sunscreens. Its use in nanoparticle form, along with the similar use of its physical sunscreen counterpart TiO2, represents one of the many subjects debated within the larger context of sunscreen use. The next edition of this column will focus on the relative safety of zinc oxide nanoparticles.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in Miami Beach. She founded the cosmetic dermatology center at the University of Miami in 1997. Dr. Baumann wrote the textbook "Cosmetic Dermatology: Principles and Practice" (McGraw-Hill, April 2002), and a book for consumers, "The Skin Type Solution" (Bantam, 2006). She has contributed to the Cosmeceutical Critique column in Skin & Allergy News since January 2001 and joined the editorial advisory board in 2004. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Galderma, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Stiefel, Topix Pharmaceuticals, and Unilever.
This column, "Cosmeceutical Critique," appears regularly in Skin & Allergy News, a publication of Frontline Medical News.
Zinc is a trace element, and it is not synthesized by the human body. The element was identified in the 1960s as being essential for human health and development. Zinc is a cofactor in more than 300 enzymes necessary for cell function. In the dermatologic realm, zinc deficiency has been associated with skin alterations, delayed wound healing, and hair loss.
Zinc oxide (ZnO) is a metal oxide that also has a broad profile in dermatology. It is perhaps best known as a physical sunscreen ingredient. ZnO and titanium dioxide (TiO2) have long been used in this manner. Both ZnO and TiO2 also have been increasingly used to replace large-particle compounds in numerous cosmetics and sunscreens. These two compounds have demonstrated effective protection against UV-induced damage, providing stronger protection against UV radiation while leaving less white residue than previous generations of physical sunscreens.
Particles of ZnO in earlier sunscreens were found to be too large to penetrate the stratum corneum and, thus, were deemed biologically inactive (J. Am. Acad. Dermatol. 1999;40:85-90). However, in novel nanoparticle form, such metal oxides absorb UV radiation, leading to photocatalysis and the release of reactive oxygen species (Australas. J. Dermatol. 2011;52:1-6). Indeed, nanoparticles exhibit new physiochemical properties as a result of increased surface area as compared to large-form products, and the potential adverse effects of the novel nanoparticle formulations in sunscreens cannot be adequately extrapolated from the effects of older-generation larger-particle skin care products (J. Drugs Dermatol. 2010;9:475-81; Int. J. Dermatol. 2011;50:247-54. The relative safety of ZnO nanoparticles will be discussed in a future column. The focus in this column will be a brief comparison with TiO2 and other indications for ZnO.
ZnO and TiO2
While numerous studies explore both TiO2 and ZnO, the latter is noted for greater versatility within the dermatologic armamentarium. In addition, ZnO is less photoactive and is associated with a lower refractive index in visible light than TiO2 (1.9 vs. 2.6, respectively) (J. Am. Acad. Dermatol. 1999;40:85-90); therefore, TiO2 appears whiter and is more difficult to incorporate into transparent products.
Another important difference is the spectrum of action. That is, only avobenzone (butyl methoxydibenzoylmethane) and ZnO are approved in the United States for broad-spectrum protection against UVA wavelengths greater than 360 nm, because TiO2 has been shown to be effective only against UV wavelengths less than 360 nm (UVA is 320-400 nm). In a study by Beasley and Meyer, TiO2 delivered neither the same level of UVA attenuation nor protection from UVA to human skin as did photostabilized formulations of avobenzone or ZnO. Therefore, TiO2 is not a suitable substitute for avobenzone and ZnO for strong UVA protection (Am. J. Clin. Dermatol. 2010;11:413-21).
Indications beyond photoprotection
More than 20 years ago, Hughes and McLean showed that a ZnO tape was effective in dressing fingertip and soft tissue injuries that were resistant to healing (Arch. Emerg. Med. 1988;5:223-7). More recently, Parboteeah and Brown demonstrated the efficacy of treating recalcitrant venous leg ulcers with ZnO paste bandages (Br. J. Nurs. 2008;17:S30, S32, S34-6). In addition, Treadwell has shown that the weekly application of ZnO compression dressings to surgical wounds of the lower leg promotes healing (Dermatol. Surg. 2011;37:166-7).
Micronized zinc oxide is included in a 4% hydroquinone/10% L-ascorbic acid treatment system recently found (in a small study of 34 females) to be effective in alleviating early signs of photodamage in normal to oily skin. Thirty patients, with minimal or mild facial photodamage and hyperpigmentation, completed the 12-week treatment regimen. All the participants were satisfied with the appearance of their skin after the study, with median scores for all assessment parameters significantly improved compared with baseline (J. Drugs Dermatol. 2011;10:1455-61). ZnO also is an active ingredient in formulations intended to support the healing of perianal eczema (Hautarzt. 2010;61:33-8).
A 2001 report on a series of blinded, randomized clinical trials conducted by Baldwin et al. showed that clinical benefits were derived from the continuous topical administration of a ZnO/petrolatum formulation in a diaper introduced at that time. The first study was undertaken to verify that the ZnO/petrolatum formulation was indeed transferred from the diaper to the child’s skin. Stratum corneum (SC) samples were analyzed from each child after the wearing of a single diaper for 3 hours or multiple diapers for 24 hours. The results indicated effective transfer, with ZnO increasing in the SC from 4.2 mcg/cm2 at 3 hours to more than 8 mcg/cm2 at 24 hours.
The second study of the formulation, in an adult arm model, assessed the prevention of irritation and SC damage induced by sodium laureth sulfate. The investigators found that the ZnO/petrolatum combination yielded significant reductions in SC damage and erythema. The third study, a 4-week trial in which 268 infants were assessed, considered the effects of the formulation on erythema and diaper rash. Half of the infants wore the test diaper and half used a control diaper lacking the ZnO/petrolatum product. Significant reductions in erythema and diaper rash were indeed observed in the test group (J. Eur. Acad. Dermatol. Venereol. 2001;15 Suppl 1:5-11).
A 2009 study showed that an unmedicated ZnO/petrolatum paste was effective in restoring the properties of the skin, allowing for balanced transepidermal water loss and water retention by SC previously compromised by diaper dermatitis. This skin condition affects approximately 50% of infants and a small percentage of the bedridden elderly (Int. J. Cosmet. Sci. 2009;31:369-74).
In 2010, a study that assessed the effectiveness of topical ZnO ointment using the rabbit ear hypertrophic scar model showed that the application of 40% ZnO significantly reduced clinical scar hypertrophy scores at 6 weeks compared with placebo. The researchers concluded that these results may suggest clinical applications for ZnO in the treatment of hypertrophic scars in humans (Burns 2010;36:1027-35). In addition, ZnO has demonstrated antibacterial properties, with nanoparticles exhibiting more potent antibacterial activity than bulk ZnO (Sci. Technol. Adv. Mater. 2008;9:1-7).
Products
ZnO is a key ingredient in calamine lotion, an antipruritic compound used to treat various mild conditions such as bites and stings from insects, eczema, poison ivy, rashes, and sunburn. It is also available over the counter in ointment or suppository form for healing hemorrhoids and fissures. In addition, ZnO is used widely in baby powders, barrier creams, moisturizers, antiseptic ointments, antidandruff shampoos, athletic bandage tape, and, of course, sunscreens.
Conclusion
ZnO is a versatile inorganic metal oxide with multiple indications in dermatology. Consequently, it is included in a wide array of skin care products, including shampoos, moisturizers, and sunscreens. Its use in nanoparticle form, along with the similar use of its physical sunscreen counterpart TiO2, represents one of the many subjects debated within the larger context of sunscreen use. The next edition of this column will focus on the relative safety of zinc oxide nanoparticles.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in Miami Beach. She founded the cosmetic dermatology center at the University of Miami in 1997. Dr. Baumann wrote the textbook "Cosmetic Dermatology: Principles and Practice" (McGraw-Hill, April 2002), and a book for consumers, "The Skin Type Solution" (Bantam, 2006). She has contributed to the Cosmeceutical Critique column in Skin & Allergy News since January 2001 and joined the editorial advisory board in 2004. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Galderma, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Stiefel, Topix Pharmaceuticals, and Unilever.
This column, "Cosmeceutical Critique," appears regularly in Skin & Allergy News, a publication of Frontline Medical News.
Cutaneous SCC in a Renal Transplant Patient Derived From Donor Kidney Tumor Cells: Should Donor Transplant Organs Undergo Genetic Profiling for Cancer-Associated Mutations?

Squamous cell carcinoma (SCC) on sun-exposed sites following prolonged immunosuppression is one of the main long-term complications of allogeneic transplantations. In an article published in the Journal of Clinical Investigation (2013;123:3797-3801), the investigators demonstrated that the SCC tumor cells from a renal transplant patient had the donor genotype and harbored a TP53 (tumor-suppressing p53) mutation in codon 175; in addition, the same TP53 mutation had previously been documented 7 years earlier in p53+ cells in the renal tubules from a kidney graft biopsy. The observations in this patient provide evidence that the kidney donor can contribute to subsequent SCCs in renal transplant recipients.
What’s the issue?
Donor contribution to the malignant epithelium of cutaneous cancer in organ transplant recipients has several important implications. First, this observation provides additional insight into the initiation and progression of tumor carcinogenesis. Second, because there is longer survival of patients following renal transplant and therefore a prolonged duration of immunosuppression in these individuals, the development of long-term complications such as SCC of sun-exposed skin may be greater. Third, should genetic profiling of kidneys from potential donors to screen for cancer-associated mutations be performed? And fourth, is this phenomenon restricted only to patients receiving kidneys or might it be a possible complication in the recipients of other organs such as hearts, lungs, or even faces?

Squamous cell carcinoma (SCC) on sun-exposed sites following prolonged immunosuppression is one of the main long-term complications of allogeneic transplantations. In an article published in the Journal of Clinical Investigation (2013;123:3797-3801), the investigators demonstrated that the SCC tumor cells from a renal transplant patient had the donor genotype and harbored a TP53 (tumor-suppressing p53) mutation in codon 175; in addition, the same TP53 mutation had previously been documented 7 years earlier in p53+ cells in the renal tubules from a kidney graft biopsy. The observations in this patient provide evidence that the kidney donor can contribute to subsequent SCCs in renal transplant recipients.
What’s the issue?
Donor contribution to the malignant epithelium of cutaneous cancer in organ transplant recipients has several important implications. First, this observation provides additional insight into the initiation and progression of tumor carcinogenesis. Second, because there is longer survival of patients following renal transplant and therefore a prolonged duration of immunosuppression in these individuals, the development of long-term complications such as SCC of sun-exposed skin may be greater. Third, should genetic profiling of kidneys from potential donors to screen for cancer-associated mutations be performed? And fourth, is this phenomenon restricted only to patients receiving kidneys or might it be a possible complication in the recipients of other organs such as hearts, lungs, or even faces?

Squamous cell carcinoma (SCC) on sun-exposed sites following prolonged immunosuppression is one of the main long-term complications of allogeneic transplantations. In an article published in the Journal of Clinical Investigation (2013;123:3797-3801), the investigators demonstrated that the SCC tumor cells from a renal transplant patient had the donor genotype and harbored a TP53 (tumor-suppressing p53) mutation in codon 175; in addition, the same TP53 mutation had previously been documented 7 years earlier in p53+ cells in the renal tubules from a kidney graft biopsy. The observations in this patient provide evidence that the kidney donor can contribute to subsequent SCCs in renal transplant recipients.
What’s the issue?
Donor contribution to the malignant epithelium of cutaneous cancer in organ transplant recipients has several important implications. First, this observation provides additional insight into the initiation and progression of tumor carcinogenesis. Second, because there is longer survival of patients following renal transplant and therefore a prolonged duration of immunosuppression in these individuals, the development of long-term complications such as SCC of sun-exposed skin may be greater. Third, should genetic profiling of kidneys from potential donors to screen for cancer-associated mutations be performed? And fourth, is this phenomenon restricted only to patients receiving kidneys or might it be a possible complication in the recipients of other organs such as hearts, lungs, or even faces?
Electronic Brachytherapy and Superficial Radiation Therapy: Will You Be Adding It to Your Practice?
Low-intensity chemo works for Burkitt’s lymphoma
Two low-intensity chemotherapy regimens proved effective against Burkitt’s lymphoma and markedly less toxic than existing treatments, according to a report published online Nov. 13 in the New England Journal of Medicine.
In a prospective study, 30 consecutive patients with untreated Burkitt’s lymphoma were enrolled over a 10-year period in one of two low-intensity regimens; both included etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (EPOCH-R). A complete remission was achieved in every patient and was sustained for a median of 73-86 months of follow-up, said Dr. Kieron M. Dunleavy of the National Cancer Institute and his associates.
"Low-intensity EPOCH-R–based therapy appears to obviate the need for high-intensity treatment and markedly reduces treatment toxicity while achieving high rates of durable response. Two confirmatory trials [of these regimens] are [now] under way in adults (NCT01092182) and children (NCT01760226)," they noted.
Until now, high-dose methotrexate and cytarabine were considered essential for control of this rare and typically aggressive cancer. "It is accepted that the high growth fraction and short doubling time of Burkitt’s lymphoma make intensive short-course chemotherapy a therapeutic necessity," albeit one that causes severe adverse effects and long-term morbidity. These study findings demonstrate that other less toxic approaches can be at least as effective, Dr. Dunleavy and his colleagues said.
The researchers undertook this study (NCT00001337 and NCT00006436) after hypothesizing that "the exquisite sensitivity of Burkitt’s lymphoma cells to genotoxic stress makes prolonged exposure time, not increased dose, the important therapeutic strategy for maximizing the killing of tumor cells." To test the idea, they studied 17 patients who had the sporadic variant of the malignancy and 13 who had the immunodeficiency-associated variant. Patients were aged 15-88 years, with a median age of 33.
Ten percent of the study population had high-risk disease, 17% had low-risk disease, and 73% had intermediate-risk disease.
The 19 patients who were HIV negative received a standard dose-adjusted EPOCH-R regimen. The 11 HIV-positive patients received a short-course regimen to reduce toxicity even further and got a double-dose of rituximab. The median cumulative dose of doxorubicin-etoposide was 47% lower and that of cyclophosphamide was 57% lower in the latter group.
All patients also were given filgrastim, beginning after the final dose of chemotherapy and continuing until absolute neutrophil recovery.
The HIV-negative group was followed for a median of 86 months and the HIV-positive group for a median of 73 months. At median follow-up, the HIV-negative group had a 95% rate of freedom from disease progression and an overall survival of 100%; the HIV-positive group had a 100% rate of freedom from disease progression and an overall survival of 90%.
Even though the latter group had more advanced Burkitt’s lymphoma as well as immunodeficiency, "they all had complete remissions that have been sustained without additional therapy," Dr. Dunleavy and his associates wrote (N. Engl. J. Med. 2013 Nov. 13 [doi:10.1056/NEJMoa1308392]).
At the most recent follow-up, none of the patients in either group had a disease recurrence or had died from Burkitt’s lymphoma. One HIV-positive patient died of acute myeloid leukemia nearly 3 years after completing the EPOCH-R regimen; it is not yet known whether the chemotherapy may have contributed to the later development of the leukemia, but HIV is a known risk factor for AML, the researchers noted.
There were no treatment-related deaths, and the toxic effects of both low-dose regimens were generally mild. Thrombocytopenia occurred in only 2% of the chemotherapy cycles; fever and neutropenia occurred in 22%. Almost all the chemotherapy infusions were outpatient, with hospital admission from fever and neutropenia occurring in 10% of cycles and only in HIV-positive patients.
This study was funded by the National Cancer Institute. Amgen provides filgrastim to NCI but had no other role in the study. No financial conflicts of interest were reported.
Two low-intensity chemotherapy regimens proved effective against Burkitt’s lymphoma and markedly less toxic than existing treatments, according to a report published online Nov. 13 in the New England Journal of Medicine.
In a prospective study, 30 consecutive patients with untreated Burkitt’s lymphoma were enrolled over a 10-year period in one of two low-intensity regimens; both included etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (EPOCH-R). A complete remission was achieved in every patient and was sustained for a median of 73-86 months of follow-up, said Dr. Kieron M. Dunleavy of the National Cancer Institute and his associates.
"Low-intensity EPOCH-R–based therapy appears to obviate the need for high-intensity treatment and markedly reduces treatment toxicity while achieving high rates of durable response. Two confirmatory trials [of these regimens] are [now] under way in adults (NCT01092182) and children (NCT01760226)," they noted.
Until now, high-dose methotrexate and cytarabine were considered essential for control of this rare and typically aggressive cancer. "It is accepted that the high growth fraction and short doubling time of Burkitt’s lymphoma make intensive short-course chemotherapy a therapeutic necessity," albeit one that causes severe adverse effects and long-term morbidity. These study findings demonstrate that other less toxic approaches can be at least as effective, Dr. Dunleavy and his colleagues said.
The researchers undertook this study (NCT00001337 and NCT00006436) after hypothesizing that "the exquisite sensitivity of Burkitt’s lymphoma cells to genotoxic stress makes prolonged exposure time, not increased dose, the important therapeutic strategy for maximizing the killing of tumor cells." To test the idea, they studied 17 patients who had the sporadic variant of the malignancy and 13 who had the immunodeficiency-associated variant. Patients were aged 15-88 years, with a median age of 33.
Ten percent of the study population had high-risk disease, 17% had low-risk disease, and 73% had intermediate-risk disease.
The 19 patients who were HIV negative received a standard dose-adjusted EPOCH-R regimen. The 11 HIV-positive patients received a short-course regimen to reduce toxicity even further and got a double-dose of rituximab. The median cumulative dose of doxorubicin-etoposide was 47% lower and that of cyclophosphamide was 57% lower in the latter group.
All patients also were given filgrastim, beginning after the final dose of chemotherapy and continuing until absolute neutrophil recovery.
The HIV-negative group was followed for a median of 86 months and the HIV-positive group for a median of 73 months. At median follow-up, the HIV-negative group had a 95% rate of freedom from disease progression and an overall survival of 100%; the HIV-positive group had a 100% rate of freedom from disease progression and an overall survival of 90%.
Even though the latter group had more advanced Burkitt’s lymphoma as well as immunodeficiency, "they all had complete remissions that have been sustained without additional therapy," Dr. Dunleavy and his associates wrote (N. Engl. J. Med. 2013 Nov. 13 [doi:10.1056/NEJMoa1308392]).
At the most recent follow-up, none of the patients in either group had a disease recurrence or had died from Burkitt’s lymphoma. One HIV-positive patient died of acute myeloid leukemia nearly 3 years after completing the EPOCH-R regimen; it is not yet known whether the chemotherapy may have contributed to the later development of the leukemia, but HIV is a known risk factor for AML, the researchers noted.
There were no treatment-related deaths, and the toxic effects of both low-dose regimens were generally mild. Thrombocytopenia occurred in only 2% of the chemotherapy cycles; fever and neutropenia occurred in 22%. Almost all the chemotherapy infusions were outpatient, with hospital admission from fever and neutropenia occurring in 10% of cycles and only in HIV-positive patients.
This study was funded by the National Cancer Institute. Amgen provides filgrastim to NCI but had no other role in the study. No financial conflicts of interest were reported.
Two low-intensity chemotherapy regimens proved effective against Burkitt’s lymphoma and markedly less toxic than existing treatments, according to a report published online Nov. 13 in the New England Journal of Medicine.
In a prospective study, 30 consecutive patients with untreated Burkitt’s lymphoma were enrolled over a 10-year period in one of two low-intensity regimens; both included etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (EPOCH-R). A complete remission was achieved in every patient and was sustained for a median of 73-86 months of follow-up, said Dr. Kieron M. Dunleavy of the National Cancer Institute and his associates.
"Low-intensity EPOCH-R–based therapy appears to obviate the need for high-intensity treatment and markedly reduces treatment toxicity while achieving high rates of durable response. Two confirmatory trials [of these regimens] are [now] under way in adults (NCT01092182) and children (NCT01760226)," they noted.
Until now, high-dose methotrexate and cytarabine were considered essential for control of this rare and typically aggressive cancer. "It is accepted that the high growth fraction and short doubling time of Burkitt’s lymphoma make intensive short-course chemotherapy a therapeutic necessity," albeit one that causes severe adverse effects and long-term morbidity. These study findings demonstrate that other less toxic approaches can be at least as effective, Dr. Dunleavy and his colleagues said.
The researchers undertook this study (NCT00001337 and NCT00006436) after hypothesizing that "the exquisite sensitivity of Burkitt’s lymphoma cells to genotoxic stress makes prolonged exposure time, not increased dose, the important therapeutic strategy for maximizing the killing of tumor cells." To test the idea, they studied 17 patients who had the sporadic variant of the malignancy and 13 who had the immunodeficiency-associated variant. Patients were aged 15-88 years, with a median age of 33.
Ten percent of the study population had high-risk disease, 17% had low-risk disease, and 73% had intermediate-risk disease.
The 19 patients who were HIV negative received a standard dose-adjusted EPOCH-R regimen. The 11 HIV-positive patients received a short-course regimen to reduce toxicity even further and got a double-dose of rituximab. The median cumulative dose of doxorubicin-etoposide was 47% lower and that of cyclophosphamide was 57% lower in the latter group.
All patients also were given filgrastim, beginning after the final dose of chemotherapy and continuing until absolute neutrophil recovery.
The HIV-negative group was followed for a median of 86 months and the HIV-positive group for a median of 73 months. At median follow-up, the HIV-negative group had a 95% rate of freedom from disease progression and an overall survival of 100%; the HIV-positive group had a 100% rate of freedom from disease progression and an overall survival of 90%.
Even though the latter group had more advanced Burkitt’s lymphoma as well as immunodeficiency, "they all had complete remissions that have been sustained without additional therapy," Dr. Dunleavy and his associates wrote (N. Engl. J. Med. 2013 Nov. 13 [doi:10.1056/NEJMoa1308392]).
At the most recent follow-up, none of the patients in either group had a disease recurrence or had died from Burkitt’s lymphoma. One HIV-positive patient died of acute myeloid leukemia nearly 3 years after completing the EPOCH-R regimen; it is not yet known whether the chemotherapy may have contributed to the later development of the leukemia, but HIV is a known risk factor for AML, the researchers noted.
There were no treatment-related deaths, and the toxic effects of both low-dose regimens were generally mild. Thrombocytopenia occurred in only 2% of the chemotherapy cycles; fever and neutropenia occurred in 22%. Almost all the chemotherapy infusions were outpatient, with hospital admission from fever and neutropenia occurring in 10% of cycles and only in HIV-positive patients.
This study was funded by the National Cancer Institute. Amgen provides filgrastim to NCI but had no other role in the study. No financial conflicts of interest were reported.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Major Finding: At median follow-ups of 86 and 73 months, respectively, the HIV-negative group had a 95% rate of freedom from disease progression and an overall survival of 100%; the HIV-positive group had a 100% rate of freedom from disease progression and an overall survival of 90%.
Data Source: A prospective study involving 30 consecutive patients who had untreated Burkitt’s lymphoma and received 2 low-intensity EPOCH-R regimens.
Disclosures: This study was funded by the National Cancer Institute. Amgen provides filgrastim to NCI but had no other role in the study. No financial conflicts of interest were reported.
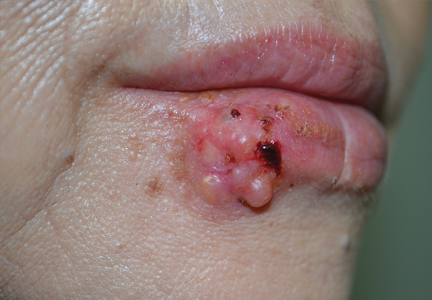
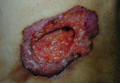
An Amelanotic Malignant Melanoma of the Lip: Unusual Shape and Atypical Location
Test your knowledge on diagnosing cancer with MD-IQ: the medical intelligence quiz. Click here to answer 5 questions.