User login
Gigapixel Photography for Skin Cancer Surveillance: A Novel Alternative to Total-Body Photography
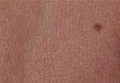
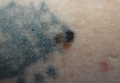
Melanoma Arising in a Tattoo: Case Report and Review of the Literature
Melanoma in Childhood: Changing Our Mind-set
Novel PDT method proves superior for difficult AKs
ISTANBUL – A novel form of photodynamic therapy achieved a much higher cure rate than did conventional PDT, based on a randomized controlled trial of organ transplant recipients with numerous actinic keratoses.
The novel approach combines pretreatment with ablative fractional laser resurfacing followed immediately by application of a photosensitizing agent and natural light exposure. In addition to its efficacy, the novel PDT approach was painless. Conventional PDT can be particularly painful for organ transplant recipients as they tend to have particularly thick and widespread lesions located at pain-sensitive sites including the underarms, dorsal hands, and face, Dr. Merete Haedersdal reported at the annual congress of the European Academy of Dermatology and Venereology.
"From a clinical point of view, we are struggling with these patients. ... Now we can individualize PDT. We already have a wonderful conventional PDT, but now we’re better off with these combined techniques, especially when we are going to treat larger areas as in this high-risk group of patients," said Dr. Haedersdal, professor of dermatology at the University of Copenhagen.
As a result of their long-term immunosuppressive therapy, organ transplant recipients have a 100-fold higher risk than the general population for squamous cell carcinoma. Further, their skin tumors grow aggressively; 6%-8% of organ transplant recipients die due to nonmelanoma skin cancers.
The novel PDT approach begins with ablative fractional laser resurfacing to create tiny holes in the skin, allowing increased uptake of the photosensitizer. Dr. Haedersdal and her coworkers used methyl aminolevulinate 16% cream, applied immediately after laser treatment. It takes 30 minutes to start the biochemical process of protoporphyrin-9 production, and the patient then goes outside for a full 2 hours of natural light exposure. This photoactivation period averaged 62,583 lux in the study. The patient then covers the treated area or stays inside for the rest of the day.
Dr. Haedersdal reported on 16 patients with a total of 542 actinic keratoses (AKs) on the scalp, chest, and extremities. The patients had received a donor organ a median of 14 years earlier. In each patient, four symmetrical skin areas within the same anatomic region were mapped out and randomized to receive a single treatment with either the novel laser-assisted daylight PDT, conventional PDT with a red light–emitting diode light source, PDT with daylight rather than the LED light source, or ablative fractional laser therapy alone.
The primary endpoint was the cure rate, or complete lesion response of all AKs at 3 months post treatment. The cure rate was 74% in the laser-assisted daylight PDT group compared with 50% with daylight PDT, 48% with conventional PDT, and 0 with laser-only treatment. When the analysis was restricted to the thicker, grade II and III AKs, the cure rate was 67% with the novel therapy, 50% with conventional PDT, 40% with daylight PDT, and 0 with laser-only treatment.
Median pain scores on a 0-10 visual analog scale were 0 for the areas treated with laser-assisted daylight PDT, laser only, and daylight PDT compared with 4.5 for the portion of skin targeted with conventional PDT. The key to pain-free PDT, according to Dr. Haedersdal, is to do the laser resurfacing at a very low fluence, allowing for continuous daylight photobleaching. The fractional laser therapy is delivered at a total of 5.6 mJ using 4.6 mJ per pulse, 1.15 W, a 50-microsecond pulse duration, 500 Hz, and 2.4% density using a 2,940-nm erbium laser. The laser holes are drilled into the epidermal layer, but not below the basement membrane.
Crusting and erythema developed in all PDT-treated patients but were slightly more intense in the areas treated with laser-assisted PDT. One patient in the laser-assisted PDT group had transient hypopigmentation.
Based on blinded assessments at 3 months, the laser-assisted daylight PDT areas displayed significantly greater skin smoothening and rejuvenation with less dyschromia than the other treatment zones. Cosmesis was rated "excellent" in the laser-treated zones of 10 out of 16 patients, a much higher rate than seen in the other treatment areas.
Dr. Haedersdal reported having received research grants from roughly half a dozen medical device and pharmaceutical companies.
ISTANBUL – A novel form of photodynamic therapy achieved a much higher cure rate than did conventional PDT, based on a randomized controlled trial of organ transplant recipients with numerous actinic keratoses.
The novel approach combines pretreatment with ablative fractional laser resurfacing followed immediately by application of a photosensitizing agent and natural light exposure. In addition to its efficacy, the novel PDT approach was painless. Conventional PDT can be particularly painful for organ transplant recipients as they tend to have particularly thick and widespread lesions located at pain-sensitive sites including the underarms, dorsal hands, and face, Dr. Merete Haedersdal reported at the annual congress of the European Academy of Dermatology and Venereology.
"From a clinical point of view, we are struggling with these patients. ... Now we can individualize PDT. We already have a wonderful conventional PDT, but now we’re better off with these combined techniques, especially when we are going to treat larger areas as in this high-risk group of patients," said Dr. Haedersdal, professor of dermatology at the University of Copenhagen.
As a result of their long-term immunosuppressive therapy, organ transplant recipients have a 100-fold higher risk than the general population for squamous cell carcinoma. Further, their skin tumors grow aggressively; 6%-8% of organ transplant recipients die due to nonmelanoma skin cancers.
The novel PDT approach begins with ablative fractional laser resurfacing to create tiny holes in the skin, allowing increased uptake of the photosensitizer. Dr. Haedersdal and her coworkers used methyl aminolevulinate 16% cream, applied immediately after laser treatment. It takes 30 minutes to start the biochemical process of protoporphyrin-9 production, and the patient then goes outside for a full 2 hours of natural light exposure. This photoactivation period averaged 62,583 lux in the study. The patient then covers the treated area or stays inside for the rest of the day.
Dr. Haedersdal reported on 16 patients with a total of 542 actinic keratoses (AKs) on the scalp, chest, and extremities. The patients had received a donor organ a median of 14 years earlier. In each patient, four symmetrical skin areas within the same anatomic region were mapped out and randomized to receive a single treatment with either the novel laser-assisted daylight PDT, conventional PDT with a red light–emitting diode light source, PDT with daylight rather than the LED light source, or ablative fractional laser therapy alone.
The primary endpoint was the cure rate, or complete lesion response of all AKs at 3 months post treatment. The cure rate was 74% in the laser-assisted daylight PDT group compared with 50% with daylight PDT, 48% with conventional PDT, and 0 with laser-only treatment. When the analysis was restricted to the thicker, grade II and III AKs, the cure rate was 67% with the novel therapy, 50% with conventional PDT, 40% with daylight PDT, and 0 with laser-only treatment.
Median pain scores on a 0-10 visual analog scale were 0 for the areas treated with laser-assisted daylight PDT, laser only, and daylight PDT compared with 4.5 for the portion of skin targeted with conventional PDT. The key to pain-free PDT, according to Dr. Haedersdal, is to do the laser resurfacing at a very low fluence, allowing for continuous daylight photobleaching. The fractional laser therapy is delivered at a total of 5.6 mJ using 4.6 mJ per pulse, 1.15 W, a 50-microsecond pulse duration, 500 Hz, and 2.4% density using a 2,940-nm erbium laser. The laser holes are drilled into the epidermal layer, but not below the basement membrane.
Crusting and erythema developed in all PDT-treated patients but were slightly more intense in the areas treated with laser-assisted PDT. One patient in the laser-assisted PDT group had transient hypopigmentation.
Based on blinded assessments at 3 months, the laser-assisted daylight PDT areas displayed significantly greater skin smoothening and rejuvenation with less dyschromia than the other treatment zones. Cosmesis was rated "excellent" in the laser-treated zones of 10 out of 16 patients, a much higher rate than seen in the other treatment areas.
Dr. Haedersdal reported having received research grants from roughly half a dozen medical device and pharmaceutical companies.
ISTANBUL – A novel form of photodynamic therapy achieved a much higher cure rate than did conventional PDT, based on a randomized controlled trial of organ transplant recipients with numerous actinic keratoses.
The novel approach combines pretreatment with ablative fractional laser resurfacing followed immediately by application of a photosensitizing agent and natural light exposure. In addition to its efficacy, the novel PDT approach was painless. Conventional PDT can be particularly painful for organ transplant recipients as they tend to have particularly thick and widespread lesions located at pain-sensitive sites including the underarms, dorsal hands, and face, Dr. Merete Haedersdal reported at the annual congress of the European Academy of Dermatology and Venereology.
"From a clinical point of view, we are struggling with these patients. ... Now we can individualize PDT. We already have a wonderful conventional PDT, but now we’re better off with these combined techniques, especially when we are going to treat larger areas as in this high-risk group of patients," said Dr. Haedersdal, professor of dermatology at the University of Copenhagen.
As a result of their long-term immunosuppressive therapy, organ transplant recipients have a 100-fold higher risk than the general population for squamous cell carcinoma. Further, their skin tumors grow aggressively; 6%-8% of organ transplant recipients die due to nonmelanoma skin cancers.
The novel PDT approach begins with ablative fractional laser resurfacing to create tiny holes in the skin, allowing increased uptake of the photosensitizer. Dr. Haedersdal and her coworkers used methyl aminolevulinate 16% cream, applied immediately after laser treatment. It takes 30 minutes to start the biochemical process of protoporphyrin-9 production, and the patient then goes outside for a full 2 hours of natural light exposure. This photoactivation period averaged 62,583 lux in the study. The patient then covers the treated area or stays inside for the rest of the day.
Dr. Haedersdal reported on 16 patients with a total of 542 actinic keratoses (AKs) on the scalp, chest, and extremities. The patients had received a donor organ a median of 14 years earlier. In each patient, four symmetrical skin areas within the same anatomic region were mapped out and randomized to receive a single treatment with either the novel laser-assisted daylight PDT, conventional PDT with a red light–emitting diode light source, PDT with daylight rather than the LED light source, or ablative fractional laser therapy alone.
The primary endpoint was the cure rate, or complete lesion response of all AKs at 3 months post treatment. The cure rate was 74% in the laser-assisted daylight PDT group compared with 50% with daylight PDT, 48% with conventional PDT, and 0 with laser-only treatment. When the analysis was restricted to the thicker, grade II and III AKs, the cure rate was 67% with the novel therapy, 50% with conventional PDT, 40% with daylight PDT, and 0 with laser-only treatment.
Median pain scores on a 0-10 visual analog scale were 0 for the areas treated with laser-assisted daylight PDT, laser only, and daylight PDT compared with 4.5 for the portion of skin targeted with conventional PDT. The key to pain-free PDT, according to Dr. Haedersdal, is to do the laser resurfacing at a very low fluence, allowing for continuous daylight photobleaching. The fractional laser therapy is delivered at a total of 5.6 mJ using 4.6 mJ per pulse, 1.15 W, a 50-microsecond pulse duration, 500 Hz, and 2.4% density using a 2,940-nm erbium laser. The laser holes are drilled into the epidermal layer, but not below the basement membrane.
Crusting and erythema developed in all PDT-treated patients but were slightly more intense in the areas treated with laser-assisted PDT. One patient in the laser-assisted PDT group had transient hypopigmentation.
Based on blinded assessments at 3 months, the laser-assisted daylight PDT areas displayed significantly greater skin smoothening and rejuvenation with less dyschromia than the other treatment zones. Cosmesis was rated "excellent" in the laser-treated zones of 10 out of 16 patients, a much higher rate than seen in the other treatment areas.
Dr. Haedersdal reported having received research grants from roughly half a dozen medical device and pharmaceutical companies.
AT THE EADV CONGRESS
Major finding: Three months post treatment, the actinic keratosis cure rate was 74% in organ transplant recipients who underwent a novel form of ablative fractional resurfacing–assisted photodynamic therapy with daylight as a photoactivator, compared with 48% with conventional PDT.
Data source: A randomized trial including 16 organ transplant recipients with a total of 542 actinic keratoses.
Disclosures: This study was funded by a Danish research foundation. The presenter has received research grants from half a dozen medical device and pharmaceutical companies.
Ipilimumab’s long-term survival edge confirmed in melanoma
AMSTERDAM – Immunotherapy with ipilimumab provides a durable, long-term survival benefit in patients with metastatic or locally advanced melanoma, a pooled analysis confirms.
Median overall survival was 11.4 months and 3-year survival 22% among 1,861 patients receiving ipilimumab (Yervoy) in a mixture of 12 clinical trials.
When data from 2,985 additional patients in the expanded access program were included, median overall survival was 9.5 months and 3-year survival 21%, Dr. F. Stephen Hodi Jr., director of the Melanoma Center at the Dana-Farber Cancer Institute, Boston, said at the European Cancer Congress 2013.
The pooled analysis, involving eight prospective phase II, two prospective phase III, and two retrospective, observational trials, is the largest survival analysis of ipilimumab to date and provides the most precise estimate yet of its survival benefit.
The analysis (LBA24) also confirms the observation that the survival benefit of ipilimumab plateaus around 3 years and that patients who are alive at this point maintain a long-term survival benefit, Dr. Hodi said. No deaths have been reported after 7 years, with some patients surviving for up to 10 years.
"A few years ago, we would never imagine using the ‘C’ word, cure, and to see some patients living long term," he said at a press briefing. "Our goal as clinical investigators is to find something that cures patients of their disease, but at least what we’re showing here is that we’re having a great paradigm shift of maybe curing a subset of patients; it’s hard to use that term, but at least turning their disease into a chronic illness, which is a huge paradigm shift from where we were just a few years ago."
The survival benefit was not affected by prior therapy, ipilimumab dose (3 mg vs. 10 mg), or inclusion of the expanded access program (EAP) data, Dr. Hodi said.
Prof. Martin Gore, medical director of the Royal Marsden Hospital and professor of cancer medicine at the Institute of Cancer Research, both in London, who was invited to discuss the study, disagreed with this conclusion. He highlighted a 6% difference in 3-year overall survival between treatment-naïve and previously treated patients (20% vs. 26%) and a 3% difference in 3-year overall survival between those receiving the licensed 3-mg/kg and 10-mg/kg doses (21% vs. 24%). While these differences probably don’t make a difference in the clinic, they could be relevant with large numbers of patients in clinical trials, he said.
The EAP data would have been better utilized as a validation set to evaluate "real world" toxicity with ipilimumab, rather than being included in the pooled analysis, he said.
Prefacing further remarks with the comment, "I have not treated a patient with IL-2 [interleukin-2] for 15 years, so I’m not an apologist for it," Prof. Gore also pointed out that the 9.5-month median survival in the pooled analysis is "within the bounds" of what IL-2 provided 20 years ago when it posted a median survival of 10.5 months and 3-year survival of 15% in 631 patients with stage IV melanoma (J. Clin. Oncol. 1998;16:2921-9).
Still, Prof. Gore said clinicians should advise their patients that ipilimumab produces a 10% improvement in survival over conventional therapy and to say "that if you reach 3 years, you may well be home and dry ...
"I think there’s a potential cure for some patients, I think we can say that," he added. "But it’s not a competition with targeted agents. That’s really, really important. This is an adjunctive treatment in the same way as targeted agents are an adjunctive treatment for those with BRAF mutations. We have to learn to put these together."
The big question going forward is who may be unsuitable for ipilimumab and whether the "old rules for immunotherapy" apply, such as poor performance status, high disease volume, and rapidly progressive disease, Prof. Gore concluded.
It’s currently not possible to predict which patients will respond to ipilimumab, but this issue is an area of very active investigation, along with how to combine or sequence immunotherapy and targeted agents, Dr. Hodi said.
Prof. Alexander Eggermont, past president of the European Cancer Organization and director of the Institut Gustave Roussy Comprehensive Cancer Center in Villejuif, France, said in a statement that the pooled analysis demonstrates that with a response rate of only 10%-15%, one can achieve survival of more than 3-10 years in 17%-25% of patients who have received only a few doses of ipilimumab.
"These survival results could even double or triple with anti-PD1/PDL1 [programmed death 1 protein and its ligand] monoclonal antibodies; and metastatic melanoma could become a curable disease for perhaps more than 50% of patients over the coming 5 to 10 years," he commented.
Dr. Hodi reported having no financial disclosures and no outside funding for the pooled analysis. The National Cancer Institute conducted three of the phase II studies, and Bristol-Myers Squibb conducted the remaining studies. Prof. Gore reported serving on the speakers bureaus for Pfizer, Roche, Novartis, and Bristol-Myers Squibb, and on the advisory boards for Pfizer, Roche, and Astellas.
AMSTERDAM – Immunotherapy with ipilimumab provides a durable, long-term survival benefit in patients with metastatic or locally advanced melanoma, a pooled analysis confirms.
Median overall survival was 11.4 months and 3-year survival 22% among 1,861 patients receiving ipilimumab (Yervoy) in a mixture of 12 clinical trials.
When data from 2,985 additional patients in the expanded access program were included, median overall survival was 9.5 months and 3-year survival 21%, Dr. F. Stephen Hodi Jr., director of the Melanoma Center at the Dana-Farber Cancer Institute, Boston, said at the European Cancer Congress 2013.
The pooled analysis, involving eight prospective phase II, two prospective phase III, and two retrospective, observational trials, is the largest survival analysis of ipilimumab to date and provides the most precise estimate yet of its survival benefit.
The analysis (LBA24) also confirms the observation that the survival benefit of ipilimumab plateaus around 3 years and that patients who are alive at this point maintain a long-term survival benefit, Dr. Hodi said. No deaths have been reported after 7 years, with some patients surviving for up to 10 years.
"A few years ago, we would never imagine using the ‘C’ word, cure, and to see some patients living long term," he said at a press briefing. "Our goal as clinical investigators is to find something that cures patients of their disease, but at least what we’re showing here is that we’re having a great paradigm shift of maybe curing a subset of patients; it’s hard to use that term, but at least turning their disease into a chronic illness, which is a huge paradigm shift from where we were just a few years ago."
The survival benefit was not affected by prior therapy, ipilimumab dose (3 mg vs. 10 mg), or inclusion of the expanded access program (EAP) data, Dr. Hodi said.
Prof. Martin Gore, medical director of the Royal Marsden Hospital and professor of cancer medicine at the Institute of Cancer Research, both in London, who was invited to discuss the study, disagreed with this conclusion. He highlighted a 6% difference in 3-year overall survival between treatment-naïve and previously treated patients (20% vs. 26%) and a 3% difference in 3-year overall survival between those receiving the licensed 3-mg/kg and 10-mg/kg doses (21% vs. 24%). While these differences probably don’t make a difference in the clinic, they could be relevant with large numbers of patients in clinical trials, he said.
The EAP data would have been better utilized as a validation set to evaluate "real world" toxicity with ipilimumab, rather than being included in the pooled analysis, he said.
Prefacing further remarks with the comment, "I have not treated a patient with IL-2 [interleukin-2] for 15 years, so I’m not an apologist for it," Prof. Gore also pointed out that the 9.5-month median survival in the pooled analysis is "within the bounds" of what IL-2 provided 20 years ago when it posted a median survival of 10.5 months and 3-year survival of 15% in 631 patients with stage IV melanoma (J. Clin. Oncol. 1998;16:2921-9).
Still, Prof. Gore said clinicians should advise their patients that ipilimumab produces a 10% improvement in survival over conventional therapy and to say "that if you reach 3 years, you may well be home and dry ...
"I think there’s a potential cure for some patients, I think we can say that," he added. "But it’s not a competition with targeted agents. That’s really, really important. This is an adjunctive treatment in the same way as targeted agents are an adjunctive treatment for those with BRAF mutations. We have to learn to put these together."
The big question going forward is who may be unsuitable for ipilimumab and whether the "old rules for immunotherapy" apply, such as poor performance status, high disease volume, and rapidly progressive disease, Prof. Gore concluded.
It’s currently not possible to predict which patients will respond to ipilimumab, but this issue is an area of very active investigation, along with how to combine or sequence immunotherapy and targeted agents, Dr. Hodi said.
Prof. Alexander Eggermont, past president of the European Cancer Organization and director of the Institut Gustave Roussy Comprehensive Cancer Center in Villejuif, France, said in a statement that the pooled analysis demonstrates that with a response rate of only 10%-15%, one can achieve survival of more than 3-10 years in 17%-25% of patients who have received only a few doses of ipilimumab.
"These survival results could even double or triple with anti-PD1/PDL1 [programmed death 1 protein and its ligand] monoclonal antibodies; and metastatic melanoma could become a curable disease for perhaps more than 50% of patients over the coming 5 to 10 years," he commented.
Dr. Hodi reported having no financial disclosures and no outside funding for the pooled analysis. The National Cancer Institute conducted three of the phase II studies, and Bristol-Myers Squibb conducted the remaining studies. Prof. Gore reported serving on the speakers bureaus for Pfizer, Roche, Novartis, and Bristol-Myers Squibb, and on the advisory boards for Pfizer, Roche, and Astellas.
AMSTERDAM – Immunotherapy with ipilimumab provides a durable, long-term survival benefit in patients with metastatic or locally advanced melanoma, a pooled analysis confirms.
Median overall survival was 11.4 months and 3-year survival 22% among 1,861 patients receiving ipilimumab (Yervoy) in a mixture of 12 clinical trials.
When data from 2,985 additional patients in the expanded access program were included, median overall survival was 9.5 months and 3-year survival 21%, Dr. F. Stephen Hodi Jr., director of the Melanoma Center at the Dana-Farber Cancer Institute, Boston, said at the European Cancer Congress 2013.
The pooled analysis, involving eight prospective phase II, two prospective phase III, and two retrospective, observational trials, is the largest survival analysis of ipilimumab to date and provides the most precise estimate yet of its survival benefit.
The analysis (LBA24) also confirms the observation that the survival benefit of ipilimumab plateaus around 3 years and that patients who are alive at this point maintain a long-term survival benefit, Dr. Hodi said. No deaths have been reported after 7 years, with some patients surviving for up to 10 years.
"A few years ago, we would never imagine using the ‘C’ word, cure, and to see some patients living long term," he said at a press briefing. "Our goal as clinical investigators is to find something that cures patients of their disease, but at least what we’re showing here is that we’re having a great paradigm shift of maybe curing a subset of patients; it’s hard to use that term, but at least turning their disease into a chronic illness, which is a huge paradigm shift from where we were just a few years ago."
The survival benefit was not affected by prior therapy, ipilimumab dose (3 mg vs. 10 mg), or inclusion of the expanded access program (EAP) data, Dr. Hodi said.
Prof. Martin Gore, medical director of the Royal Marsden Hospital and professor of cancer medicine at the Institute of Cancer Research, both in London, who was invited to discuss the study, disagreed with this conclusion. He highlighted a 6% difference in 3-year overall survival between treatment-naïve and previously treated patients (20% vs. 26%) and a 3% difference in 3-year overall survival between those receiving the licensed 3-mg/kg and 10-mg/kg doses (21% vs. 24%). While these differences probably don’t make a difference in the clinic, they could be relevant with large numbers of patients in clinical trials, he said.
The EAP data would have been better utilized as a validation set to evaluate "real world" toxicity with ipilimumab, rather than being included in the pooled analysis, he said.
Prefacing further remarks with the comment, "I have not treated a patient with IL-2 [interleukin-2] for 15 years, so I’m not an apologist for it," Prof. Gore also pointed out that the 9.5-month median survival in the pooled analysis is "within the bounds" of what IL-2 provided 20 years ago when it posted a median survival of 10.5 months and 3-year survival of 15% in 631 patients with stage IV melanoma (J. Clin. Oncol. 1998;16:2921-9).
Still, Prof. Gore said clinicians should advise their patients that ipilimumab produces a 10% improvement in survival over conventional therapy and to say "that if you reach 3 years, you may well be home and dry ...
"I think there’s a potential cure for some patients, I think we can say that," he added. "But it’s not a competition with targeted agents. That’s really, really important. This is an adjunctive treatment in the same way as targeted agents are an adjunctive treatment for those with BRAF mutations. We have to learn to put these together."
The big question going forward is who may be unsuitable for ipilimumab and whether the "old rules for immunotherapy" apply, such as poor performance status, high disease volume, and rapidly progressive disease, Prof. Gore concluded.
It’s currently not possible to predict which patients will respond to ipilimumab, but this issue is an area of very active investigation, along with how to combine or sequence immunotherapy and targeted agents, Dr. Hodi said.
Prof. Alexander Eggermont, past president of the European Cancer Organization and director of the Institut Gustave Roussy Comprehensive Cancer Center in Villejuif, France, said in a statement that the pooled analysis demonstrates that with a response rate of only 10%-15%, one can achieve survival of more than 3-10 years in 17%-25% of patients who have received only a few doses of ipilimumab.
"These survival results could even double or triple with anti-PD1/PDL1 [programmed death 1 protein and its ligand] monoclonal antibodies; and metastatic melanoma could become a curable disease for perhaps more than 50% of patients over the coming 5 to 10 years," he commented.
Dr. Hodi reported having no financial disclosures and no outside funding for the pooled analysis. The National Cancer Institute conducted three of the phase II studies, and Bristol-Myers Squibb conducted the remaining studies. Prof. Gore reported serving on the speakers bureaus for Pfizer, Roche, Novartis, and Bristol-Myers Squibb, and on the advisory boards for Pfizer, Roche, and Astellas.
AT THE EUROPEAN CANCER CONGRESS 2013
Major finding: Median overall survival was 11.4 months and 3-year survival 22% among 1,861 patients receiving ipilimumab in clinical trials.
Data source: Pooled analysis of 4,846 patients with advanced melanoma treated with ipilimumab.
Disclosures: Dr. Hodi reported having no financial disclosures and no outside funding for the pooled analysis. The National Cancer Institute conducted three of the phase II studies, and Bristol-Myers Squibb conducted the remaining studies. Prof. Gore reported serving on the speakers bureaus for Pfizer, Roche, Novartis, and Bristol-Myers Squibb, and on the advisory boards for Pfizer, Roche, and Astellas.
Inpatient dermatologist offers three rules to diagnose by
SAN FRANCISCO – Three rules can guide inpatient dermatology for the most effective practice, according to Dr. Lindy P. Fox, director of the dermatology consultation service at the University of California, San Francisco.
First, more than one infection or etiology may account for the clinical picture, especially in immunocompromised patients. If there’s more than one morphology present, work up each one separately, she said.
Second, most cases require some degree of clinicopathologic correlation.
Third, for inpatient dermatology it helps to think of "zebras," because that’s how you’ll make the rare diagnosis, she said at the annual meeting of the Pacific Dermatologic Association.
She provided tips on some of the more common morphologies encountered in hospital dermatology:
• Morbilliform. Most commonly, morbilliform brings to mind a drug eruption, viral exanthema, graft vs. host disease (GVHD), and other entities that can present in this pattern. Rarely in immunocompromised patients, disseminated histoplasmosis, cryptococcosis, or coccidiomycosis can mimic a drug eruption, so it’s important in those situations to biopsy what you think might be a drug eruption.
Acute graft vs. host disease on average occurs 25 days after a transplant, but hyperacute GVHD occurs 14 days post transplant, so think of this before your oncologist tells you the patient’s graft hasn’t taken, she said. Two features of GVHD morbilliform eruption distinguish it from other differential diagnoses: a perifollicular accentuation, and an acral distribution around the ears, hand, and periungual areas. A patient’s nausea, vomiting, diarrhea, and hyperbilirubinemia support the diagnosis.
• Papules/papulonodules. The differential diagnosis is long – fungal diseases, septic emboli, Sweet’s syndrome, leukemia or lymphoma cutis, Kaposi sarcoma, cutaneous metastasis, and sarcoidosis, among others. But if you see purple plumb-colored papules, the list shrinks to lymphoma, Kaposi sarcoma, bacillary angiomatosis, melanoma, or cutaneous metastasis.
• Cellulitic plaques. These bring to mind cellulitis or its mimic, stasis dermatitis, as well as some deep fungal infections, carcinoma erysipeloides, neutrophilic diseases, and acute inflammatory edema of the ICU. Keep in mind that cryptococcal cellulitis is the most common presentation of Cryptococcus in an organ transplant recipient. Unlike bacterial cellulitis, it tends to be bilateral. It is almost never purely a cutaneous disease. "If you ever see Cryptococcus on the skin, you are obligated to look for the systemic source of infection," she said.
• Palpable purpura. This typically points to small or mixed-vessel vasculitis. But you can also have secondary hemorrhage into a papular process; it can be due to leukemia, lymphoma cutis, or Sweet’s syndrome; or it can be a cutaneous reaction to cytarabine. What Dr. Fox said she commonly sees are purpuric papules on the lower extremities. If looked at closely, there’s a morbilliform eruption that can spread to the trunk in a few days. Biopsy typically demonstrates spongiosis, which is a "very reassuring" sign that this is a benign and self-limiting reaction to cytarabine. The reaction may or may not recur if the patient takes the drug again. "Steroids do help, and patients do very well," she said.
• Retiform purpura. Another very long differential diagnosis can be organized by thinking about potential causes as vascular (in the vessel wall) or intravascular (in the lumen of the wall). Lumen involvement is a sign of embolization or thrombosis. "If you see concomitant thrombosis and vasculitis truly on the same day early on in lesion development, that differential diagnosis shrinks considerably. There are very few things that can do that: cryoglobulinemia, septic vasculitis, and exposure to levamisole," Dr. Fox said.
• Sclerodermoid. The long differential diagnosis includes carcinoma en cuirasse, radiation dermatitis, GVHD, scleroderma, paraffinoma, lipodermatosclerosis, scleredema, and scleromyxedema, among others. Dr. Fox said she saw an unusual presentation in a woman with "antibiotic refractory cellulitis" who had developed a migrating paraffinoma after mineral oil injections to her calves for cosmetic purposes.
• Necrotic papules/plaques. One of the many potential causes – calciphylaxis – can be thought of as analogous to a myocardial infarction that occurs in the skin, she said. Patients more often than not have a long history of vascular stenosis and medial arterial calcification. An acute thrombotic event occurs, and the patient presents with calciphylaxis with tissue ischemia, pain, and stellate purpuric plaques. "This implies that we need to think about our therapeutics differently," she said. Address the calcium, phosphorous, and parathyroid hormone, as usual, but also consider treating the thrombus with tissue plasminogen activator. Don’t use warfarin; many cases of calciphylaxis are triggered by warfarin, and the drug promotes calcification, Dr. Fox said at the annual meeting of the Pacific Dermatologic Association.
• Ulcers. The most common causes in the hospital are venous insufficiency, pyoderma gangrenosum, deep fungal infection, and chronic viral infections. Chronic herpes simplex virus frequently is the culprit in bedridden, immunosuppressed patients. "Any buttock ulcer or any decubitis ulcer that I see gets ruled out for concomitant herpes simplex virus because I find it probably more than 50% of the time," she said.
• Pustules. One cause – disseminated candidiasis – can mimic acne in the immunosuppressed patient. The lesions tend to have a pale center that sometimes is pustular. "You can KOH that center to prove that you’ve got candidiasis," she said. If there’s no pustule, diagnosing disseminated candidiasis can be difficult because the collection of organisms is so small that it’s easily missed on serial sectioning by dermatopathology. Ask for serial sectioning and staining to identify the organism.
• Erythema. Dr. Fox said she divides erythemas into macular, subacute, and erosive. Macular erythema can be a toxin-mediated erythema or due to drug eruption, GVHD, viral exanthema, or Kawasaki disease. Eosinophilia is a common finding in toxin-mediated erythema. "I use it to help rule [erythema] in, rather than to help rule it out," she said.
When a subacute or chronic erythroderma is due to drug hypersensitivity reaction, be aware of two long-term sequellae: thyroid disruption and late-onset cardiac involvement presenting as life-threatening heart failure in people you wouldn’t expect to have heart problems. Dr. Fox said she checks thyroid-stimulating hormone levels monthly for 6 months and warns patients to go to the emergency department if they develop heart symptoms and tell the staff there to call the dermatologist.
One of the lesser-known causes of erosive erythroderma is toxic erythema of chemotherapy. It can take a lot of charts and talking with the primary medical team to make sure this is not toxic erythema necrolysislike GVHD, she said.
Dr. Fox reported having no relevant financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – Three rules can guide inpatient dermatology for the most effective practice, according to Dr. Lindy P. Fox, director of the dermatology consultation service at the University of California, San Francisco.
First, more than one infection or etiology may account for the clinical picture, especially in immunocompromised patients. If there’s more than one morphology present, work up each one separately, she said.
Second, most cases require some degree of clinicopathologic correlation.
Third, for inpatient dermatology it helps to think of "zebras," because that’s how you’ll make the rare diagnosis, she said at the annual meeting of the Pacific Dermatologic Association.
She provided tips on some of the more common morphologies encountered in hospital dermatology:
• Morbilliform. Most commonly, morbilliform brings to mind a drug eruption, viral exanthema, graft vs. host disease (GVHD), and other entities that can present in this pattern. Rarely in immunocompromised patients, disseminated histoplasmosis, cryptococcosis, or coccidiomycosis can mimic a drug eruption, so it’s important in those situations to biopsy what you think might be a drug eruption.
Acute graft vs. host disease on average occurs 25 days after a transplant, but hyperacute GVHD occurs 14 days post transplant, so think of this before your oncologist tells you the patient’s graft hasn’t taken, she said. Two features of GVHD morbilliform eruption distinguish it from other differential diagnoses: a perifollicular accentuation, and an acral distribution around the ears, hand, and periungual areas. A patient’s nausea, vomiting, diarrhea, and hyperbilirubinemia support the diagnosis.
• Papules/papulonodules. The differential diagnosis is long – fungal diseases, septic emboli, Sweet’s syndrome, leukemia or lymphoma cutis, Kaposi sarcoma, cutaneous metastasis, and sarcoidosis, among others. But if you see purple plumb-colored papules, the list shrinks to lymphoma, Kaposi sarcoma, bacillary angiomatosis, melanoma, or cutaneous metastasis.
• Cellulitic plaques. These bring to mind cellulitis or its mimic, stasis dermatitis, as well as some deep fungal infections, carcinoma erysipeloides, neutrophilic diseases, and acute inflammatory edema of the ICU. Keep in mind that cryptococcal cellulitis is the most common presentation of Cryptococcus in an organ transplant recipient. Unlike bacterial cellulitis, it tends to be bilateral. It is almost never purely a cutaneous disease. "If you ever see Cryptococcus on the skin, you are obligated to look for the systemic source of infection," she said.
• Palpable purpura. This typically points to small or mixed-vessel vasculitis. But you can also have secondary hemorrhage into a papular process; it can be due to leukemia, lymphoma cutis, or Sweet’s syndrome; or it can be a cutaneous reaction to cytarabine. What Dr. Fox said she commonly sees are purpuric papules on the lower extremities. If looked at closely, there’s a morbilliform eruption that can spread to the trunk in a few days. Biopsy typically demonstrates spongiosis, which is a "very reassuring" sign that this is a benign and self-limiting reaction to cytarabine. The reaction may or may not recur if the patient takes the drug again. "Steroids do help, and patients do very well," she said.
• Retiform purpura. Another very long differential diagnosis can be organized by thinking about potential causes as vascular (in the vessel wall) or intravascular (in the lumen of the wall). Lumen involvement is a sign of embolization or thrombosis. "If you see concomitant thrombosis and vasculitis truly on the same day early on in lesion development, that differential diagnosis shrinks considerably. There are very few things that can do that: cryoglobulinemia, septic vasculitis, and exposure to levamisole," Dr. Fox said.
• Sclerodermoid. The long differential diagnosis includes carcinoma en cuirasse, radiation dermatitis, GVHD, scleroderma, paraffinoma, lipodermatosclerosis, scleredema, and scleromyxedema, among others. Dr. Fox said she saw an unusual presentation in a woman with "antibiotic refractory cellulitis" who had developed a migrating paraffinoma after mineral oil injections to her calves for cosmetic purposes.
• Necrotic papules/plaques. One of the many potential causes – calciphylaxis – can be thought of as analogous to a myocardial infarction that occurs in the skin, she said. Patients more often than not have a long history of vascular stenosis and medial arterial calcification. An acute thrombotic event occurs, and the patient presents with calciphylaxis with tissue ischemia, pain, and stellate purpuric plaques. "This implies that we need to think about our therapeutics differently," she said. Address the calcium, phosphorous, and parathyroid hormone, as usual, but also consider treating the thrombus with tissue plasminogen activator. Don’t use warfarin; many cases of calciphylaxis are triggered by warfarin, and the drug promotes calcification, Dr. Fox said at the annual meeting of the Pacific Dermatologic Association.
• Ulcers. The most common causes in the hospital are venous insufficiency, pyoderma gangrenosum, deep fungal infection, and chronic viral infections. Chronic herpes simplex virus frequently is the culprit in bedridden, immunosuppressed patients. "Any buttock ulcer or any decubitis ulcer that I see gets ruled out for concomitant herpes simplex virus because I find it probably more than 50% of the time," she said.
• Pustules. One cause – disseminated candidiasis – can mimic acne in the immunosuppressed patient. The lesions tend to have a pale center that sometimes is pustular. "You can KOH that center to prove that you’ve got candidiasis," she said. If there’s no pustule, diagnosing disseminated candidiasis can be difficult because the collection of organisms is so small that it’s easily missed on serial sectioning by dermatopathology. Ask for serial sectioning and staining to identify the organism.
• Erythema. Dr. Fox said she divides erythemas into macular, subacute, and erosive. Macular erythema can be a toxin-mediated erythema or due to drug eruption, GVHD, viral exanthema, or Kawasaki disease. Eosinophilia is a common finding in toxin-mediated erythema. "I use it to help rule [erythema] in, rather than to help rule it out," she said.
When a subacute or chronic erythroderma is due to drug hypersensitivity reaction, be aware of two long-term sequellae: thyroid disruption and late-onset cardiac involvement presenting as life-threatening heart failure in people you wouldn’t expect to have heart problems. Dr. Fox said she checks thyroid-stimulating hormone levels monthly for 6 months and warns patients to go to the emergency department if they develop heart symptoms and tell the staff there to call the dermatologist.
One of the lesser-known causes of erosive erythroderma is toxic erythema of chemotherapy. It can take a lot of charts and talking with the primary medical team to make sure this is not toxic erythema necrolysislike GVHD, she said.
Dr. Fox reported having no relevant financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – Three rules can guide inpatient dermatology for the most effective practice, according to Dr. Lindy P. Fox, director of the dermatology consultation service at the University of California, San Francisco.
First, more than one infection or etiology may account for the clinical picture, especially in immunocompromised patients. If there’s more than one morphology present, work up each one separately, she said.
Second, most cases require some degree of clinicopathologic correlation.
Third, for inpatient dermatology it helps to think of "zebras," because that’s how you’ll make the rare diagnosis, she said at the annual meeting of the Pacific Dermatologic Association.
She provided tips on some of the more common morphologies encountered in hospital dermatology:
• Morbilliform. Most commonly, morbilliform brings to mind a drug eruption, viral exanthema, graft vs. host disease (GVHD), and other entities that can present in this pattern. Rarely in immunocompromised patients, disseminated histoplasmosis, cryptococcosis, or coccidiomycosis can mimic a drug eruption, so it’s important in those situations to biopsy what you think might be a drug eruption.
Acute graft vs. host disease on average occurs 25 days after a transplant, but hyperacute GVHD occurs 14 days post transplant, so think of this before your oncologist tells you the patient’s graft hasn’t taken, she said. Two features of GVHD morbilliform eruption distinguish it from other differential diagnoses: a perifollicular accentuation, and an acral distribution around the ears, hand, and periungual areas. A patient’s nausea, vomiting, diarrhea, and hyperbilirubinemia support the diagnosis.
• Papules/papulonodules. The differential diagnosis is long – fungal diseases, septic emboli, Sweet’s syndrome, leukemia or lymphoma cutis, Kaposi sarcoma, cutaneous metastasis, and sarcoidosis, among others. But if you see purple plumb-colored papules, the list shrinks to lymphoma, Kaposi sarcoma, bacillary angiomatosis, melanoma, or cutaneous metastasis.
• Cellulitic plaques. These bring to mind cellulitis or its mimic, stasis dermatitis, as well as some deep fungal infections, carcinoma erysipeloides, neutrophilic diseases, and acute inflammatory edema of the ICU. Keep in mind that cryptococcal cellulitis is the most common presentation of Cryptococcus in an organ transplant recipient. Unlike bacterial cellulitis, it tends to be bilateral. It is almost never purely a cutaneous disease. "If you ever see Cryptococcus on the skin, you are obligated to look for the systemic source of infection," she said.
• Palpable purpura. This typically points to small or mixed-vessel vasculitis. But you can also have secondary hemorrhage into a papular process; it can be due to leukemia, lymphoma cutis, or Sweet’s syndrome; or it can be a cutaneous reaction to cytarabine. What Dr. Fox said she commonly sees are purpuric papules on the lower extremities. If looked at closely, there’s a morbilliform eruption that can spread to the trunk in a few days. Biopsy typically demonstrates spongiosis, which is a "very reassuring" sign that this is a benign and self-limiting reaction to cytarabine. The reaction may or may not recur if the patient takes the drug again. "Steroids do help, and patients do very well," she said.
• Retiform purpura. Another very long differential diagnosis can be organized by thinking about potential causes as vascular (in the vessel wall) or intravascular (in the lumen of the wall). Lumen involvement is a sign of embolization or thrombosis. "If you see concomitant thrombosis and vasculitis truly on the same day early on in lesion development, that differential diagnosis shrinks considerably. There are very few things that can do that: cryoglobulinemia, septic vasculitis, and exposure to levamisole," Dr. Fox said.
• Sclerodermoid. The long differential diagnosis includes carcinoma en cuirasse, radiation dermatitis, GVHD, scleroderma, paraffinoma, lipodermatosclerosis, scleredema, and scleromyxedema, among others. Dr. Fox said she saw an unusual presentation in a woman with "antibiotic refractory cellulitis" who had developed a migrating paraffinoma after mineral oil injections to her calves for cosmetic purposes.
• Necrotic papules/plaques. One of the many potential causes – calciphylaxis – can be thought of as analogous to a myocardial infarction that occurs in the skin, she said. Patients more often than not have a long history of vascular stenosis and medial arterial calcification. An acute thrombotic event occurs, and the patient presents with calciphylaxis with tissue ischemia, pain, and stellate purpuric plaques. "This implies that we need to think about our therapeutics differently," she said. Address the calcium, phosphorous, and parathyroid hormone, as usual, but also consider treating the thrombus with tissue plasminogen activator. Don’t use warfarin; many cases of calciphylaxis are triggered by warfarin, and the drug promotes calcification, Dr. Fox said at the annual meeting of the Pacific Dermatologic Association.
• Ulcers. The most common causes in the hospital are venous insufficiency, pyoderma gangrenosum, deep fungal infection, and chronic viral infections. Chronic herpes simplex virus frequently is the culprit in bedridden, immunosuppressed patients. "Any buttock ulcer or any decubitis ulcer that I see gets ruled out for concomitant herpes simplex virus because I find it probably more than 50% of the time," she said.
• Pustules. One cause – disseminated candidiasis – can mimic acne in the immunosuppressed patient. The lesions tend to have a pale center that sometimes is pustular. "You can KOH that center to prove that you’ve got candidiasis," she said. If there’s no pustule, diagnosing disseminated candidiasis can be difficult because the collection of organisms is so small that it’s easily missed on serial sectioning by dermatopathology. Ask for serial sectioning and staining to identify the organism.
• Erythema. Dr. Fox said she divides erythemas into macular, subacute, and erosive. Macular erythema can be a toxin-mediated erythema or due to drug eruption, GVHD, viral exanthema, or Kawasaki disease. Eosinophilia is a common finding in toxin-mediated erythema. "I use it to help rule [erythema] in, rather than to help rule it out," she said.
When a subacute or chronic erythroderma is due to drug hypersensitivity reaction, be aware of two long-term sequellae: thyroid disruption and late-onset cardiac involvement presenting as life-threatening heart failure in people you wouldn’t expect to have heart problems. Dr. Fox said she checks thyroid-stimulating hormone levels monthly for 6 months and warns patients to go to the emergency department if they develop heart symptoms and tell the staff there to call the dermatologist.
One of the lesser-known causes of erosive erythroderma is toxic erythema of chemotherapy. It can take a lot of charts and talking with the primary medical team to make sure this is not toxic erythema necrolysislike GVHD, she said.
Dr. Fox reported having no relevant financial disclosures.
On Twitter @sherryboschert
EXPERT ANALYSIS FROM THE PDA ANNUAL MEETING
Vismodegib prolongs positive response in advanced skin cancer
ISTANBUL – Vismodegib-treated patients with locally advanced basal cell carcinoma showed triple the median response duration after 24 months’ follow-up beyond the primary analysis of the drug, Dr. Luc Dirix reported at the annual congress of the European Academy of Dermatology and Venereology.
Vismodegib, the first-in-class oral medication for treatment of advanced BCC, showed a greatly improved duration of response and no new safety signals in updated results from the ERIVANCE BCC trial, which earlier had earned the drug marketing approval from the Food and Drug Administration and European Medicines Agency.
After 24 months beyond what was included in the primary analysis of ERIVANCE BCC (N. Engl. J. Med. 2012;366:2171-9), the median duration of response by investigator assessment in patients with locally advanced BCC increased from 7.6 to 26.2 months, and increased from 7.6 to 12.9 months in those with metastatic BCC.
"The main new fact is that the median duration of response has increased importantly. That’s a very impressive improvement in disease control, especially in patients with locally advanced disease. It’s a major change from what you’ll find in the New England Journal of Medicine publication," observed Dr. Dirix of Sint-AugustinusHospital, Antwerp, Belgium.
Dr. Dirix reported that, with the additional 24 months of follow-up in the ERIVANCE BCC study, median progression-free survival now stands at 9.3 months in the group with metastatic BCC and 12.9 months in those with locally advanced disease. The objective response rate, which was 43% in patients with locally advanced BCC and 30% in those with metastatic disease in the primary analysis, hasn’t changed significantly with the additional follow-up, he said.
The median overall survival was 33 months. Twelve of the original 33 patients in the cohort with metastatic BCC have died of progressive disease, as have 4 of 63 with locally advanced BCC.
The ERIVANCE BCC trial is an open-label, phase II study in which participants were placed on vismodegib at 150 mg once daily until the occurrence of disease progression, intolerable toxicity, or patient request to withdraw. At the latest follow-up, only 13% of the original study population remained on the drug, Dr. Dirix said. Half of the patients with metastatic BCC who discontinued the drug did so because of disease progression, but only 16% of those with locally advanced BCC stopped treatment. Instead, side effects were the main reason locally advanced BCC patients discontinued therapy.
"The additional 24 months of follow-up didn’t result in any new safety signal, but there are numerous side effects, especially muscle spasms, alopecia, dysgeusia, and others, mostly mild or moderate," said Dr. Dirix.
An estimated 1.6 million new cases of BCC were diagnosed in the United States in 2006, making it the most common form of cancer in the country. Most BCCs are readily treated. However, some cases can progress to locally advanced or metastatic BCC, a disfiguring and potentially life-threatening condition that is not treatable surgically or via radiation therapy, and for which no effective therapy existed before. Vismodegib (Erivedge) provides an oral drug option that works as an inhibitor of the hedgehog pathway, which plays a key role in the pathogenesis of most BCCs.
With vismodegib intended as lifelong treatment in patients with advanced BCC, and considerable research interest in exploring the drug in other forms of BCC, reliable safety data are needed. Also at the EADV Congress, Dr. Rainer Kunstfeld presented new safety information in an interim analysis from an ongoing study known as STEVIE (Safety Events in Vismodegib), the largest study of the hedgehog pathway inhibitor. His report included the first 300 of a planned 1,200 patients with advanced BCC to be placed on vismodegib.
The data underscore the point that advanced BCC is primarily a disease of the elderly. The mean age of the 278 patients with locally advanced BCC is 70 years, and 65 years in the 22 with metastatic BCC. The median treatment duration as of the data cutoff for this analysis was 5.8 months.
Forty-five percent of patients in the locally advanced BCC group have discontinued treatment, as have 32% of those with metastatic disease, Dr. Kunstfeld said. The reasons differed for the two groups. Discontinuation due to disease progression or death was more than twice as common in patients with metastatic BCC. Patients with locally advanced disease were much more likely to halt treatment because of adverse events.
Ninety-three percent of STEVIE participants experienced at least one adverse event. Roughly two-thirds of these side effects were mild or moderate – that is, grade 1 or 2 – while the remainder were grade 3. The incidence of the most common adverse events were muscle spasms in 59% of participants, alopecia in 49%, distorted sense of taste in 41%, loss of the sense of taste in 26%, asthenia in 23%, and weight loss in 16%. These data were consistent with the safety profile seen in the ERIVANCE BCC study, observed Dr. Kunstfeld, a dermatologist at the Medical University of Vienna.
Of the 251 patients with tumor assessments available to date, more than 95% showed some degree of therapeutic benefit. A complete response was documented in 17.5% of patients, a partial response in 39.8%, and 39% had stable disease. Fewer than 3% have experienced disease progression during this early follow-up period.
The ERIVANCE BCC and STEVIE studies are funded by Genentech. Dr. Dirix and Dr. Kunstfeld reported having no financial conflicts of interest.
ISTANBUL – Vismodegib-treated patients with locally advanced basal cell carcinoma showed triple the median response duration after 24 months’ follow-up beyond the primary analysis of the drug, Dr. Luc Dirix reported at the annual congress of the European Academy of Dermatology and Venereology.
Vismodegib, the first-in-class oral medication for treatment of advanced BCC, showed a greatly improved duration of response and no new safety signals in updated results from the ERIVANCE BCC trial, which earlier had earned the drug marketing approval from the Food and Drug Administration and European Medicines Agency.
After 24 months beyond what was included in the primary analysis of ERIVANCE BCC (N. Engl. J. Med. 2012;366:2171-9), the median duration of response by investigator assessment in patients with locally advanced BCC increased from 7.6 to 26.2 months, and increased from 7.6 to 12.9 months in those with metastatic BCC.
"The main new fact is that the median duration of response has increased importantly. That’s a very impressive improvement in disease control, especially in patients with locally advanced disease. It’s a major change from what you’ll find in the New England Journal of Medicine publication," observed Dr. Dirix of Sint-AugustinusHospital, Antwerp, Belgium.
Dr. Dirix reported that, with the additional 24 months of follow-up in the ERIVANCE BCC study, median progression-free survival now stands at 9.3 months in the group with metastatic BCC and 12.9 months in those with locally advanced disease. The objective response rate, which was 43% in patients with locally advanced BCC and 30% in those with metastatic disease in the primary analysis, hasn’t changed significantly with the additional follow-up, he said.
The median overall survival was 33 months. Twelve of the original 33 patients in the cohort with metastatic BCC have died of progressive disease, as have 4 of 63 with locally advanced BCC.
The ERIVANCE BCC trial is an open-label, phase II study in which participants were placed on vismodegib at 150 mg once daily until the occurrence of disease progression, intolerable toxicity, or patient request to withdraw. At the latest follow-up, only 13% of the original study population remained on the drug, Dr. Dirix said. Half of the patients with metastatic BCC who discontinued the drug did so because of disease progression, but only 16% of those with locally advanced BCC stopped treatment. Instead, side effects were the main reason locally advanced BCC patients discontinued therapy.
"The additional 24 months of follow-up didn’t result in any new safety signal, but there are numerous side effects, especially muscle spasms, alopecia, dysgeusia, and others, mostly mild or moderate," said Dr. Dirix.
An estimated 1.6 million new cases of BCC were diagnosed in the United States in 2006, making it the most common form of cancer in the country. Most BCCs are readily treated. However, some cases can progress to locally advanced or metastatic BCC, a disfiguring and potentially life-threatening condition that is not treatable surgically or via radiation therapy, and for which no effective therapy existed before. Vismodegib (Erivedge) provides an oral drug option that works as an inhibitor of the hedgehog pathway, which plays a key role in the pathogenesis of most BCCs.
With vismodegib intended as lifelong treatment in patients with advanced BCC, and considerable research interest in exploring the drug in other forms of BCC, reliable safety data are needed. Also at the EADV Congress, Dr. Rainer Kunstfeld presented new safety information in an interim analysis from an ongoing study known as STEVIE (Safety Events in Vismodegib), the largest study of the hedgehog pathway inhibitor. His report included the first 300 of a planned 1,200 patients with advanced BCC to be placed on vismodegib.
The data underscore the point that advanced BCC is primarily a disease of the elderly. The mean age of the 278 patients with locally advanced BCC is 70 years, and 65 years in the 22 with metastatic BCC. The median treatment duration as of the data cutoff for this analysis was 5.8 months.
Forty-five percent of patients in the locally advanced BCC group have discontinued treatment, as have 32% of those with metastatic disease, Dr. Kunstfeld said. The reasons differed for the two groups. Discontinuation due to disease progression or death was more than twice as common in patients with metastatic BCC. Patients with locally advanced disease were much more likely to halt treatment because of adverse events.
Ninety-three percent of STEVIE participants experienced at least one adverse event. Roughly two-thirds of these side effects were mild or moderate – that is, grade 1 or 2 – while the remainder were grade 3. The incidence of the most common adverse events were muscle spasms in 59% of participants, alopecia in 49%, distorted sense of taste in 41%, loss of the sense of taste in 26%, asthenia in 23%, and weight loss in 16%. These data were consistent with the safety profile seen in the ERIVANCE BCC study, observed Dr. Kunstfeld, a dermatologist at the Medical University of Vienna.
Of the 251 patients with tumor assessments available to date, more than 95% showed some degree of therapeutic benefit. A complete response was documented in 17.5% of patients, a partial response in 39.8%, and 39% had stable disease. Fewer than 3% have experienced disease progression during this early follow-up period.
The ERIVANCE BCC and STEVIE studies are funded by Genentech. Dr. Dirix and Dr. Kunstfeld reported having no financial conflicts of interest.
ISTANBUL – Vismodegib-treated patients with locally advanced basal cell carcinoma showed triple the median response duration after 24 months’ follow-up beyond the primary analysis of the drug, Dr. Luc Dirix reported at the annual congress of the European Academy of Dermatology and Venereology.
Vismodegib, the first-in-class oral medication for treatment of advanced BCC, showed a greatly improved duration of response and no new safety signals in updated results from the ERIVANCE BCC trial, which earlier had earned the drug marketing approval from the Food and Drug Administration and European Medicines Agency.
After 24 months beyond what was included in the primary analysis of ERIVANCE BCC (N. Engl. J. Med. 2012;366:2171-9), the median duration of response by investigator assessment in patients with locally advanced BCC increased from 7.6 to 26.2 months, and increased from 7.6 to 12.9 months in those with metastatic BCC.
"The main new fact is that the median duration of response has increased importantly. That’s a very impressive improvement in disease control, especially in patients with locally advanced disease. It’s a major change from what you’ll find in the New England Journal of Medicine publication," observed Dr. Dirix of Sint-AugustinusHospital, Antwerp, Belgium.
Dr. Dirix reported that, with the additional 24 months of follow-up in the ERIVANCE BCC study, median progression-free survival now stands at 9.3 months in the group with metastatic BCC and 12.9 months in those with locally advanced disease. The objective response rate, which was 43% in patients with locally advanced BCC and 30% in those with metastatic disease in the primary analysis, hasn’t changed significantly with the additional follow-up, he said.
The median overall survival was 33 months. Twelve of the original 33 patients in the cohort with metastatic BCC have died of progressive disease, as have 4 of 63 with locally advanced BCC.
The ERIVANCE BCC trial is an open-label, phase II study in which participants were placed on vismodegib at 150 mg once daily until the occurrence of disease progression, intolerable toxicity, or patient request to withdraw. At the latest follow-up, only 13% of the original study population remained on the drug, Dr. Dirix said. Half of the patients with metastatic BCC who discontinued the drug did so because of disease progression, but only 16% of those with locally advanced BCC stopped treatment. Instead, side effects were the main reason locally advanced BCC patients discontinued therapy.
"The additional 24 months of follow-up didn’t result in any new safety signal, but there are numerous side effects, especially muscle spasms, alopecia, dysgeusia, and others, mostly mild or moderate," said Dr. Dirix.
An estimated 1.6 million new cases of BCC were diagnosed in the United States in 2006, making it the most common form of cancer in the country. Most BCCs are readily treated. However, some cases can progress to locally advanced or metastatic BCC, a disfiguring and potentially life-threatening condition that is not treatable surgically or via radiation therapy, and for which no effective therapy existed before. Vismodegib (Erivedge) provides an oral drug option that works as an inhibitor of the hedgehog pathway, which plays a key role in the pathogenesis of most BCCs.
With vismodegib intended as lifelong treatment in patients with advanced BCC, and considerable research interest in exploring the drug in other forms of BCC, reliable safety data are needed. Also at the EADV Congress, Dr. Rainer Kunstfeld presented new safety information in an interim analysis from an ongoing study known as STEVIE (Safety Events in Vismodegib), the largest study of the hedgehog pathway inhibitor. His report included the first 300 of a planned 1,200 patients with advanced BCC to be placed on vismodegib.
The data underscore the point that advanced BCC is primarily a disease of the elderly. The mean age of the 278 patients with locally advanced BCC is 70 years, and 65 years in the 22 with metastatic BCC. The median treatment duration as of the data cutoff for this analysis was 5.8 months.
Forty-five percent of patients in the locally advanced BCC group have discontinued treatment, as have 32% of those with metastatic disease, Dr. Kunstfeld said. The reasons differed for the two groups. Discontinuation due to disease progression or death was more than twice as common in patients with metastatic BCC. Patients with locally advanced disease were much more likely to halt treatment because of adverse events.
Ninety-three percent of STEVIE participants experienced at least one adverse event. Roughly two-thirds of these side effects were mild or moderate – that is, grade 1 or 2 – while the remainder were grade 3. The incidence of the most common adverse events were muscle spasms in 59% of participants, alopecia in 49%, distorted sense of taste in 41%, loss of the sense of taste in 26%, asthenia in 23%, and weight loss in 16%. These data were consistent with the safety profile seen in the ERIVANCE BCC study, observed Dr. Kunstfeld, a dermatologist at the Medical University of Vienna.
Of the 251 patients with tumor assessments available to date, more than 95% showed some degree of therapeutic benefit. A complete response was documented in 17.5% of patients, a partial response in 39.8%, and 39% had stable disease. Fewer than 3% have experienced disease progression during this early follow-up period.
The ERIVANCE BCC and STEVIE studies are funded by Genentech. Dr. Dirix and Dr. Kunstfeld reported having no financial conflicts of interest.
AT THE EADV CONGRESS
Major finding: With an additional 24 months of follow-up of vismodegib-treated patients with advanced basal cell carcinoma beyond previous results from the ERIVANCE study, the median response duration in patients with locally advanced disease climbed from 7.6 to 26.2 months.
Data source: The ERIVANCE BCC study is an open-label trial in which 63 patients with locally advanced BCC and 33 with metastatic BCC were placed on oral vismodegib at 150 mg/day.
Disclosures: The ERIVANCE BCC and STEVIE studies are funded by Genentech. Dr. Dirix and Dr. Kunstfeld reported having no financial conflicts of interest.
Don’t rush to lymph node biopsy for thin melanomas, expert says
SAN FRANCISCO – Don’t recommend sentinel lymph node biopsy in patients with a melanoma depth of 0.75 mm or thinner unless there are true high-risk features, Dr. Susan Swetter advised at the annual meeting of the Pacific Dermatologic Association.
Increasing numbers of patients with thin melanomas being referred for consideration of sentinel lymph node biopsy inspired some revisions in the 2013 National Comprehensive Cancer Network melanoma guidelines regarding clinical stage, patient work-up, and treatment, said Dr. Swetter, professor of dermatology at Stanford (Calif.) University.
"Because of the issue of upstaging T1a and T1b melanomas based on the mitotic rate, we have seen a dramatic rise in cases referred to the academic centers for sentinel lymph node biopsy consideration," Dr. Swetter said.
The experts convened by the National Comprehensive Cancer Network (NCCN) in 2012 to revise the guidelines reviewed the evidence and found that thickness is the only consistent predictor of sentinel lymph node positivity for thin melanomas 1 mm or less in depth. They revised the stratification of stage IA and IB melanomas according to their risk for sentinel node metastasis rather than on the basis of the American Joint Committee on Cancer stage.
Essentially, tumors up to 0.75 mm in thickness have a low risk of metastasis (2%-2.5%) regardless of the mitotic rate. Tumors with a thickness of 0.76-1mm, particularly those with a high mitotic rate, have a higher risk of metastasis (approximately 5%), and are "very eligible" for sentinel lymph node biopsy, said Dr. Swetter, who was a member of the guidelines committee. "The 0.75-mm cutoff does not change the stage of the patient" but is a way of stratifying the risk for a positive sentinel node biopsy (J. Natl. Compr. Canc. Netw. 2013;11:395-407).
Guidelines from the American Academy of Dermatology in 2011 did not recommend sentinel lymph node biopsy for any T1a tumor up to 1 mm in depth because the AAD committee felt that the risk of a positive biopsy result should be at least 10% to justify the procedure, said Dr. Swetter, who also was on the committee that drafted those guidelines.
A key footnote in the NCCN 2013 recommendations: Sentinel node biopsy is not recommended for primary melanomas up to 0.75 mm unless there is significant uncertainty about the adequacy of microstaging, "meaning it’s a transected tumor or a nonrepresentative biopsy," Dr. Swetter explained. Sentinel node biopsy may be considered for melanomas 0.76-1 mm thick in the appropriate clinical context.
There is little consensus on what puts a thin melanoma (up to 1 mm) at high risk for metastasis, other than primary tumor thickness. Risk factors include a high mitotic rate (though the NCCN guidelines don’t specify a number), Dr. Swetter noted. Ulceration and lymphovascular invasion increase the risk but are rare.
"Consider sentinel lymph node biopsy in thin melanomas on an individual basis," Dr. Swetter said.
In general, don’t recommend sentinel lymph node biopsy for primary melanomas up to 0.75 mm thick unless there is a very high mitotic rate, lymphovascular invasion, ulceration, a nonrepresentative biopsy (with a large clinical residual lesion), or a partial biopsy (particularly one with deep transection of the tumor and inadequate microstaging), she said. A Clark level IV or V also might be cause for a sentinel node biopsy for a thin tumor, but "we only use Clark level when the mitotic rate can’t be assessed," she noted. "I can’t think of a case in the last 4 years where I’ve paid attention to the Clark level as a reason to pursue treatment in a patient."
One other "risk factor" for sentinel lymph node biopsy, even in a patient with a very thin melanoma and low mitotic rate, is a strong patient preference for the procedure, Dr. Swetter added. Dermatologists should try to educate patients and explain that their chances of a positive result may be fairly negligible, but if "they are determined to have sentinel lymph node biopsy, they will have it done," she said.
Dr. Swetter reported having no relevant financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – Don’t recommend sentinel lymph node biopsy in patients with a melanoma depth of 0.75 mm or thinner unless there are true high-risk features, Dr. Susan Swetter advised at the annual meeting of the Pacific Dermatologic Association.
Increasing numbers of patients with thin melanomas being referred for consideration of sentinel lymph node biopsy inspired some revisions in the 2013 National Comprehensive Cancer Network melanoma guidelines regarding clinical stage, patient work-up, and treatment, said Dr. Swetter, professor of dermatology at Stanford (Calif.) University.
"Because of the issue of upstaging T1a and T1b melanomas based on the mitotic rate, we have seen a dramatic rise in cases referred to the academic centers for sentinel lymph node biopsy consideration," Dr. Swetter said.
The experts convened by the National Comprehensive Cancer Network (NCCN) in 2012 to revise the guidelines reviewed the evidence and found that thickness is the only consistent predictor of sentinel lymph node positivity for thin melanomas 1 mm or less in depth. They revised the stratification of stage IA and IB melanomas according to their risk for sentinel node metastasis rather than on the basis of the American Joint Committee on Cancer stage.
Essentially, tumors up to 0.75 mm in thickness have a low risk of metastasis (2%-2.5%) regardless of the mitotic rate. Tumors with a thickness of 0.76-1mm, particularly those with a high mitotic rate, have a higher risk of metastasis (approximately 5%), and are "very eligible" for sentinel lymph node biopsy, said Dr. Swetter, who was a member of the guidelines committee. "The 0.75-mm cutoff does not change the stage of the patient" but is a way of stratifying the risk for a positive sentinel node biopsy (J. Natl. Compr. Canc. Netw. 2013;11:395-407).
Guidelines from the American Academy of Dermatology in 2011 did not recommend sentinel lymph node biopsy for any T1a tumor up to 1 mm in depth because the AAD committee felt that the risk of a positive biopsy result should be at least 10% to justify the procedure, said Dr. Swetter, who also was on the committee that drafted those guidelines.
A key footnote in the NCCN 2013 recommendations: Sentinel node biopsy is not recommended for primary melanomas up to 0.75 mm unless there is significant uncertainty about the adequacy of microstaging, "meaning it’s a transected tumor or a nonrepresentative biopsy," Dr. Swetter explained. Sentinel node biopsy may be considered for melanomas 0.76-1 mm thick in the appropriate clinical context.
There is little consensus on what puts a thin melanoma (up to 1 mm) at high risk for metastasis, other than primary tumor thickness. Risk factors include a high mitotic rate (though the NCCN guidelines don’t specify a number), Dr. Swetter noted. Ulceration and lymphovascular invasion increase the risk but are rare.
"Consider sentinel lymph node biopsy in thin melanomas on an individual basis," Dr. Swetter said.
In general, don’t recommend sentinel lymph node biopsy for primary melanomas up to 0.75 mm thick unless there is a very high mitotic rate, lymphovascular invasion, ulceration, a nonrepresentative biopsy (with a large clinical residual lesion), or a partial biopsy (particularly one with deep transection of the tumor and inadequate microstaging), she said. A Clark level IV or V also might be cause for a sentinel node biopsy for a thin tumor, but "we only use Clark level when the mitotic rate can’t be assessed," she noted. "I can’t think of a case in the last 4 years where I’ve paid attention to the Clark level as a reason to pursue treatment in a patient."
One other "risk factor" for sentinel lymph node biopsy, even in a patient with a very thin melanoma and low mitotic rate, is a strong patient preference for the procedure, Dr. Swetter added. Dermatologists should try to educate patients and explain that their chances of a positive result may be fairly negligible, but if "they are determined to have sentinel lymph node biopsy, they will have it done," she said.
Dr. Swetter reported having no relevant financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – Don’t recommend sentinel lymph node biopsy in patients with a melanoma depth of 0.75 mm or thinner unless there are true high-risk features, Dr. Susan Swetter advised at the annual meeting of the Pacific Dermatologic Association.
Increasing numbers of patients with thin melanomas being referred for consideration of sentinel lymph node biopsy inspired some revisions in the 2013 National Comprehensive Cancer Network melanoma guidelines regarding clinical stage, patient work-up, and treatment, said Dr. Swetter, professor of dermatology at Stanford (Calif.) University.
"Because of the issue of upstaging T1a and T1b melanomas based on the mitotic rate, we have seen a dramatic rise in cases referred to the academic centers for sentinel lymph node biopsy consideration," Dr. Swetter said.
The experts convened by the National Comprehensive Cancer Network (NCCN) in 2012 to revise the guidelines reviewed the evidence and found that thickness is the only consistent predictor of sentinel lymph node positivity for thin melanomas 1 mm or less in depth. They revised the stratification of stage IA and IB melanomas according to their risk for sentinel node metastasis rather than on the basis of the American Joint Committee on Cancer stage.
Essentially, tumors up to 0.75 mm in thickness have a low risk of metastasis (2%-2.5%) regardless of the mitotic rate. Tumors with a thickness of 0.76-1mm, particularly those with a high mitotic rate, have a higher risk of metastasis (approximately 5%), and are "very eligible" for sentinel lymph node biopsy, said Dr. Swetter, who was a member of the guidelines committee. "The 0.75-mm cutoff does not change the stage of the patient" but is a way of stratifying the risk for a positive sentinel node biopsy (J. Natl. Compr. Canc. Netw. 2013;11:395-407).
Guidelines from the American Academy of Dermatology in 2011 did not recommend sentinel lymph node biopsy for any T1a tumor up to 1 mm in depth because the AAD committee felt that the risk of a positive biopsy result should be at least 10% to justify the procedure, said Dr. Swetter, who also was on the committee that drafted those guidelines.
A key footnote in the NCCN 2013 recommendations: Sentinel node biopsy is not recommended for primary melanomas up to 0.75 mm unless there is significant uncertainty about the adequacy of microstaging, "meaning it’s a transected tumor or a nonrepresentative biopsy," Dr. Swetter explained. Sentinel node biopsy may be considered for melanomas 0.76-1 mm thick in the appropriate clinical context.
There is little consensus on what puts a thin melanoma (up to 1 mm) at high risk for metastasis, other than primary tumor thickness. Risk factors include a high mitotic rate (though the NCCN guidelines don’t specify a number), Dr. Swetter noted. Ulceration and lymphovascular invasion increase the risk but are rare.
"Consider sentinel lymph node biopsy in thin melanomas on an individual basis," Dr. Swetter said.
In general, don’t recommend sentinel lymph node biopsy for primary melanomas up to 0.75 mm thick unless there is a very high mitotic rate, lymphovascular invasion, ulceration, a nonrepresentative biopsy (with a large clinical residual lesion), or a partial biopsy (particularly one with deep transection of the tumor and inadequate microstaging), she said. A Clark level IV or V also might be cause for a sentinel node biopsy for a thin tumor, but "we only use Clark level when the mitotic rate can’t be assessed," she noted. "I can’t think of a case in the last 4 years where I’ve paid attention to the Clark level as a reason to pursue treatment in a patient."
One other "risk factor" for sentinel lymph node biopsy, even in a patient with a very thin melanoma and low mitotic rate, is a strong patient preference for the procedure, Dr. Swetter added. Dermatologists should try to educate patients and explain that their chances of a positive result may be fairly negligible, but if "they are determined to have sentinel lymph node biopsy, they will have it done," she said.
Dr. Swetter reported having no relevant financial disclosures.
On Twitter @sherryboschert
EXPERT ANALYSIS FROM THE PDA ANNUAL MEETING
Mohs Appropriate Use Criteria
What is it?
The Mohs Appropriate Use Criteria (AUC) app is a decision support tool based on the Mohs micrographic surgery AUC published in 2012.1 The app helps to determine if Mohs surgery is appropriate for your patients at the point of care.
How does it work?
The app walks you through a short series of selections based on tumor and patient characteristics (ie, cancer type, location, recurrent or primary, histologic features, tumor size, if the patient has immunocompromise or a genetic syndrome) and returns a rating on the appropriateness of Mohs surgery using a scale of 1 to 9. The rating indicates if Mohs surgery is appropriate; not appropriate; or uncertain, meaning that there is not enough published evidence to support if Mohs surgery is appropriate. The whole process takes seconds to complete.
How can it help me?
Compared to the 20-page Mohs surgery AUC publication,1 this application is extremely easy to use at the point of care. It frees me from having to memorize the Mohs AUC or having to wade through an extensive report when I only want to know if Mohs surgery is appropriate. It also will be useful in educating patients who believe every skin cancer requires Mohs surgery.
How can I get it?
Because Mohs AUC is free, every dermatologist should use this mobile app. It can be downloaded from the Apple App Store. It is not available for Android devices at this time.
Reference
1. Connolly SM, Baker DR, Coldiron BM, et al; Ad Hoc Task Force. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
What is it?
The Mohs Appropriate Use Criteria (AUC) app is a decision support tool based on the Mohs micrographic surgery AUC published in 2012.1 The app helps to determine if Mohs surgery is appropriate for your patients at the point of care.
How does it work?
The app walks you through a short series of selections based on tumor and patient characteristics (ie, cancer type, location, recurrent or primary, histologic features, tumor size, if the patient has immunocompromise or a genetic syndrome) and returns a rating on the appropriateness of Mohs surgery using a scale of 1 to 9. The rating indicates if Mohs surgery is appropriate; not appropriate; or uncertain, meaning that there is not enough published evidence to support if Mohs surgery is appropriate. The whole process takes seconds to complete.
How can it help me?
Compared to the 20-page Mohs surgery AUC publication,1 this application is extremely easy to use at the point of care. It frees me from having to memorize the Mohs AUC or having to wade through an extensive report when I only want to know if Mohs surgery is appropriate. It also will be useful in educating patients who believe every skin cancer requires Mohs surgery.
How can I get it?
Because Mohs AUC is free, every dermatologist should use this mobile app. It can be downloaded from the Apple App Store. It is not available for Android devices at this time.
What is it?
The Mohs Appropriate Use Criteria (AUC) app is a decision support tool based on the Mohs micrographic surgery AUC published in 2012.1 The app helps to determine if Mohs surgery is appropriate for your patients at the point of care.
How does it work?
The app walks you through a short series of selections based on tumor and patient characteristics (ie, cancer type, location, recurrent or primary, histologic features, tumor size, if the patient has immunocompromise or a genetic syndrome) and returns a rating on the appropriateness of Mohs surgery using a scale of 1 to 9. The rating indicates if Mohs surgery is appropriate; not appropriate; or uncertain, meaning that there is not enough published evidence to support if Mohs surgery is appropriate. The whole process takes seconds to complete.
How can it help me?
Compared to the 20-page Mohs surgery AUC publication,1 this application is extremely easy to use at the point of care. It frees me from having to memorize the Mohs AUC or having to wade through an extensive report when I only want to know if Mohs surgery is appropriate. It also will be useful in educating patients who believe every skin cancer requires Mohs surgery.
How can I get it?
Because Mohs AUC is free, every dermatologist should use this mobile app. It can be downloaded from the Apple App Store. It is not available for Android devices at this time.
Reference
1. Connolly SM, Baker DR, Coldiron BM, et al; Ad Hoc Task Force. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
Reference
1. Connolly SM, Baker DR, Coldiron BM, et al; Ad Hoc Task Force. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
Restrict Mohs surgery or risk drop in reimbursement
SAN FRANCISCO – Dermatologists and dermatologic surgeons risk a decreased reimbursement value for Mohs surgery if they don’t review the published criteria on its appropriate use, according to Dr. Sumaira Aasi.
Although it can be difficult, it is worth the effort to convince some patients that electrodessication, a curettage procedure, or some other management strategy is appropriate for their skin lesions, she said at the annual meeting of the Pacific Dermatologic Association.
"The reason is, at the end of the day, we might be killing the goose that laid the golden egg ourselves" if too many Mohs surgeries are done for inappropriate reasons, said Dr. Aasi of Stanford (Calif.) University.
SDr. Aasi urged the audience at the meeting to study criteria published in 2012 for the appropriate use of Mohs surgery, and to pay particular attention to scenarios deemed inappropriate for the procedure (J. Am. Acad. Dermatol. 2012;67:531-50).
There are an estimated 4 million nonmelanoma skin cancers in the United States each year, and the use of Mohs surgery increased by 400% from 1995 to 2009, Dr. Aasi said (Dermatol. Clin. 2012;30:167-75).
Some experts argue that the increase is a response to the increase in skin cancer cases, and others say the increasing number of Mohs surgeons is driving the rising rate of the procedure. "Regardless, it’s a problem," Dr. Aasi said.
Federal reimbursement programs don’t care if there is an epidemic of skin cancer, she said. If a surgery is being "overused," it is considered overvalued, and reimbursement rates are subject to change, she explained.
The top two CPT codes flagged by the federal government as potentially misvalued in the July 19, 2013, Federal Register were codes 17311 for Mohs surgery on the face, head, and neck and 17313 for Mohs surgery on the trunk and extremities, Dr. Aasi noted.
The 2012 appropriate-use criteria were created to help maintain the value of Mohs surgery in Medicare reimbursements, Dr. Aasi said. The American Academy of Dermatology, the American Society of Dermatologic Surgery, the American College of Mohs Surgery, and the American Society of Mohs Surgery convened a consensus panel of eight Mohs surgeons and nine non-Mohs surgeons. The panel considered 270 clinical scenarios and rated 74% appropriate for Mohs surgery, 17% inappropriate, and 9% uncertain, meaning there were conflicting data in the literature or not enough information to make a determination of the appropriateness of Mohs surgery in that scenario.
"I still think that Mohs surgeons in this panel were a little highly represented," Dr. Aasi said. Overall, the indications the panel deemed appropriate for Mohs surgery were "pretty generous," she added.
The consensus panel was asked to implicitly consider cost in deciding on the appropriateness of Mohs surgery for each scenario. "It’s an implicit consideration but a very important consideration," said Dr. Aasi.
Invasive melanoma was not included in the scenarios because if its complexity, but some scenarios did include melanoma in situ or lentigo maligna melanoma. The scenarios included different body areas, aggressive or nonaggressive skin cancers, tumor size, and the patient’s immune status. Agreement by at least 12 panel members was considered a consensus on the use of Mohs surgery.
Examples of scenarios that were considered inappropriate for Mohs surgery included nonaggressive basal cell or squamous cell carcinomas smaller than 2 cm on the trunk or extremities. Other lesions deemed inappropriate for Mohs included primary actinic keratosis (AK) with focal squamous cell cancer in situ, bowenoid AK, or squamous cell carcinoma in situ AK-type, labels that Dr. Aasi found "worrisome" because their definitions are unclear.
"As a Mohs surgeon, I found it a little surprising that they had to consider scenarios where actinic keratoses were being considered a possibility for Mohs surgery," she said.
Physicians can use the appropriate-use criteria to talk with other health care providers, third-party payers, and patients, said Dr. Aasi. Talking with patients can be most difficult, because if they’ve heard of Mohs surgery, they often want "the technique where I [the patient] know right away that I’m going to be clear," she said.
When Mohs surgery is inappropriate, "I try to convince them that, no, you don’t need it. It’s like an atomic bomb being used to kill a flea," she said.
The appropriate-use criteria do not compare different treatment modalities with Mohs surgery, Dr. Aasi emphasized, but they aim to state simply whether or not Mohs surgery would be appropriate in selected scenarios.
"I urge everyone to read" the criteria, she said.
Dr. Aasi reported having no financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – Dermatologists and dermatologic surgeons risk a decreased reimbursement value for Mohs surgery if they don’t review the published criteria on its appropriate use, according to Dr. Sumaira Aasi.
Although it can be difficult, it is worth the effort to convince some patients that electrodessication, a curettage procedure, or some other management strategy is appropriate for their skin lesions, she said at the annual meeting of the Pacific Dermatologic Association.
"The reason is, at the end of the day, we might be killing the goose that laid the golden egg ourselves" if too many Mohs surgeries are done for inappropriate reasons, said Dr. Aasi of Stanford (Calif.) University.
SDr. Aasi urged the audience at the meeting to study criteria published in 2012 for the appropriate use of Mohs surgery, and to pay particular attention to scenarios deemed inappropriate for the procedure (J. Am. Acad. Dermatol. 2012;67:531-50).
There are an estimated 4 million nonmelanoma skin cancers in the United States each year, and the use of Mohs surgery increased by 400% from 1995 to 2009, Dr. Aasi said (Dermatol. Clin. 2012;30:167-75).
Some experts argue that the increase is a response to the increase in skin cancer cases, and others say the increasing number of Mohs surgeons is driving the rising rate of the procedure. "Regardless, it’s a problem," Dr. Aasi said.
Federal reimbursement programs don’t care if there is an epidemic of skin cancer, she said. If a surgery is being "overused," it is considered overvalued, and reimbursement rates are subject to change, she explained.
The top two CPT codes flagged by the federal government as potentially misvalued in the July 19, 2013, Federal Register were codes 17311 for Mohs surgery on the face, head, and neck and 17313 for Mohs surgery on the trunk and extremities, Dr. Aasi noted.
The 2012 appropriate-use criteria were created to help maintain the value of Mohs surgery in Medicare reimbursements, Dr. Aasi said. The American Academy of Dermatology, the American Society of Dermatologic Surgery, the American College of Mohs Surgery, and the American Society of Mohs Surgery convened a consensus panel of eight Mohs surgeons and nine non-Mohs surgeons. The panel considered 270 clinical scenarios and rated 74% appropriate for Mohs surgery, 17% inappropriate, and 9% uncertain, meaning there were conflicting data in the literature or not enough information to make a determination of the appropriateness of Mohs surgery in that scenario.
"I still think that Mohs surgeons in this panel were a little highly represented," Dr. Aasi said. Overall, the indications the panel deemed appropriate for Mohs surgery were "pretty generous," she added.
The consensus panel was asked to implicitly consider cost in deciding on the appropriateness of Mohs surgery for each scenario. "It’s an implicit consideration but a very important consideration," said Dr. Aasi.
Invasive melanoma was not included in the scenarios because if its complexity, but some scenarios did include melanoma in situ or lentigo maligna melanoma. The scenarios included different body areas, aggressive or nonaggressive skin cancers, tumor size, and the patient’s immune status. Agreement by at least 12 panel members was considered a consensus on the use of Mohs surgery.
Examples of scenarios that were considered inappropriate for Mohs surgery included nonaggressive basal cell or squamous cell carcinomas smaller than 2 cm on the trunk or extremities. Other lesions deemed inappropriate for Mohs included primary actinic keratosis (AK) with focal squamous cell cancer in situ, bowenoid AK, or squamous cell carcinoma in situ AK-type, labels that Dr. Aasi found "worrisome" because their definitions are unclear.
"As a Mohs surgeon, I found it a little surprising that they had to consider scenarios where actinic keratoses were being considered a possibility for Mohs surgery," she said.
Physicians can use the appropriate-use criteria to talk with other health care providers, third-party payers, and patients, said Dr. Aasi. Talking with patients can be most difficult, because if they’ve heard of Mohs surgery, they often want "the technique where I [the patient] know right away that I’m going to be clear," she said.
When Mohs surgery is inappropriate, "I try to convince them that, no, you don’t need it. It’s like an atomic bomb being used to kill a flea," she said.
The appropriate-use criteria do not compare different treatment modalities with Mohs surgery, Dr. Aasi emphasized, but they aim to state simply whether or not Mohs surgery would be appropriate in selected scenarios.
"I urge everyone to read" the criteria, she said.
Dr. Aasi reported having no financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – Dermatologists and dermatologic surgeons risk a decreased reimbursement value for Mohs surgery if they don’t review the published criteria on its appropriate use, according to Dr. Sumaira Aasi.
Although it can be difficult, it is worth the effort to convince some patients that electrodessication, a curettage procedure, or some other management strategy is appropriate for their skin lesions, she said at the annual meeting of the Pacific Dermatologic Association.
"The reason is, at the end of the day, we might be killing the goose that laid the golden egg ourselves" if too many Mohs surgeries are done for inappropriate reasons, said Dr. Aasi of Stanford (Calif.) University.
SDr. Aasi urged the audience at the meeting to study criteria published in 2012 for the appropriate use of Mohs surgery, and to pay particular attention to scenarios deemed inappropriate for the procedure (J. Am. Acad. Dermatol. 2012;67:531-50).
There are an estimated 4 million nonmelanoma skin cancers in the United States each year, and the use of Mohs surgery increased by 400% from 1995 to 2009, Dr. Aasi said (Dermatol. Clin. 2012;30:167-75).
Some experts argue that the increase is a response to the increase in skin cancer cases, and others say the increasing number of Mohs surgeons is driving the rising rate of the procedure. "Regardless, it’s a problem," Dr. Aasi said.
Federal reimbursement programs don’t care if there is an epidemic of skin cancer, she said. If a surgery is being "overused," it is considered overvalued, and reimbursement rates are subject to change, she explained.
The top two CPT codes flagged by the federal government as potentially misvalued in the July 19, 2013, Federal Register were codes 17311 for Mohs surgery on the face, head, and neck and 17313 for Mohs surgery on the trunk and extremities, Dr. Aasi noted.
The 2012 appropriate-use criteria were created to help maintain the value of Mohs surgery in Medicare reimbursements, Dr. Aasi said. The American Academy of Dermatology, the American Society of Dermatologic Surgery, the American College of Mohs Surgery, and the American Society of Mohs Surgery convened a consensus panel of eight Mohs surgeons and nine non-Mohs surgeons. The panel considered 270 clinical scenarios and rated 74% appropriate for Mohs surgery, 17% inappropriate, and 9% uncertain, meaning there were conflicting data in the literature or not enough information to make a determination of the appropriateness of Mohs surgery in that scenario.
"I still think that Mohs surgeons in this panel were a little highly represented," Dr. Aasi said. Overall, the indications the panel deemed appropriate for Mohs surgery were "pretty generous," she added.
The consensus panel was asked to implicitly consider cost in deciding on the appropriateness of Mohs surgery for each scenario. "It’s an implicit consideration but a very important consideration," said Dr. Aasi.
Invasive melanoma was not included in the scenarios because if its complexity, but some scenarios did include melanoma in situ or lentigo maligna melanoma. The scenarios included different body areas, aggressive or nonaggressive skin cancers, tumor size, and the patient’s immune status. Agreement by at least 12 panel members was considered a consensus on the use of Mohs surgery.
Examples of scenarios that were considered inappropriate for Mohs surgery included nonaggressive basal cell or squamous cell carcinomas smaller than 2 cm on the trunk or extremities. Other lesions deemed inappropriate for Mohs included primary actinic keratosis (AK) with focal squamous cell cancer in situ, bowenoid AK, or squamous cell carcinoma in situ AK-type, labels that Dr. Aasi found "worrisome" because their definitions are unclear.
"As a Mohs surgeon, I found it a little surprising that they had to consider scenarios where actinic keratoses were being considered a possibility for Mohs surgery," she said.
Physicians can use the appropriate-use criteria to talk with other health care providers, third-party payers, and patients, said Dr. Aasi. Talking with patients can be most difficult, because if they’ve heard of Mohs surgery, they often want "the technique where I [the patient] know right away that I’m going to be clear," she said.
When Mohs surgery is inappropriate, "I try to convince them that, no, you don’t need it. It’s like an atomic bomb being used to kill a flea," she said.
The appropriate-use criteria do not compare different treatment modalities with Mohs surgery, Dr. Aasi emphasized, but they aim to state simply whether or not Mohs surgery would be appropriate in selected scenarios.
"I urge everyone to read" the criteria, she said.
Dr. Aasi reported having no financial disclosures.
On Twitter @sherryboschert
EXPERT ANALYSIS FROM THE PDA ANNUAL MEETING