User login
Melanoma Regression: A Quandary of Progression, Progress, and Prognosis
The British Journal of Dermatology recently described prognostic data regarding 1693 consecutive melanoma patients (American Joint Committee on Cancer stage I to II). Based on disease-free and overall survival and sentinel lymph node biopsy (SLNB) characteristics, the study found that the majority of regional lymph node metastases were in patients who did not undergo SLNB and that histologic regression was considered protective. The authors concluded that regression should not be an indication for SLNB in thin melanomas; in fact, it may be a favorable prognostic factor.
What’s the issue?
The art of medicine and its gray areas are respected and in full effect for melanoma, as clinicians always have to take into account the patient’s clinical history, comorbidities, and a fair amount of “gut instinct” in addition to the pathology specimen characteristics when deciding on surgical margins, imaging/laboratory tests, and particularly SLNB. How do you approach cases with regression? Although not an official upstaging factor anymore and now with evidence presented in the above study, how much do we worry and account for the mysterious route a particular melanoma took clinically and molecularly before the patient presented to us?
The British Journal of Dermatology recently described prognostic data regarding 1693 consecutive melanoma patients (American Joint Committee on Cancer stage I to II). Based on disease-free and overall survival and sentinel lymph node biopsy (SLNB) characteristics, the study found that the majority of regional lymph node metastases were in patients who did not undergo SLNB and that histologic regression was considered protective. The authors concluded that regression should not be an indication for SLNB in thin melanomas; in fact, it may be a favorable prognostic factor.
What’s the issue?
The art of medicine and its gray areas are respected and in full effect for melanoma, as clinicians always have to take into account the patient’s clinical history, comorbidities, and a fair amount of “gut instinct” in addition to the pathology specimen characteristics when deciding on surgical margins, imaging/laboratory tests, and particularly SLNB. How do you approach cases with regression? Although not an official upstaging factor anymore and now with evidence presented in the above study, how much do we worry and account for the mysterious route a particular melanoma took clinically and molecularly before the patient presented to us?
The British Journal of Dermatology recently described prognostic data regarding 1693 consecutive melanoma patients (American Joint Committee on Cancer stage I to II). Based on disease-free and overall survival and sentinel lymph node biopsy (SLNB) characteristics, the study found that the majority of regional lymph node metastases were in patients who did not undergo SLNB and that histologic regression was considered protective. The authors concluded that regression should not be an indication for SLNB in thin melanomas; in fact, it may be a favorable prognostic factor.
What’s the issue?
The art of medicine and its gray areas are respected and in full effect for melanoma, as clinicians always have to take into account the patient’s clinical history, comorbidities, and a fair amount of “gut instinct” in addition to the pathology specimen characteristics when deciding on surgical margins, imaging/laboratory tests, and particularly SLNB. How do you approach cases with regression? Although not an official upstaging factor anymore and now with evidence presented in the above study, how much do we worry and account for the mysterious route a particular melanoma took clinically and molecularly before the patient presented to us?
Fewer than 1% of doctors mention sunscreen to patients
Physicians miss 99.9% of their opportunities to counsel patients about use of sunscreen, and dermatologists pass up 98% of those teaching moments, based on data from approximately 18 billion patient visits recorded between January 1989 and December 2010. The findings were published online on Sept. 4 in JAMA Dermatology.
Despite the rising incidence of skin cancer and recommendations from medical organizations that clinicians counsel patients about sun-protective behaviors, "sun-protection counseling ranks among the lowest topics of primary prevention discussed between physicians and patients," said Dr. Kristie Akamine of Wake Forest University in Winston-Salem, N.C., and her colleagues.
To identify trends in sunscreen recommendations by different specialties, the researchers reviewed data from the National Ambulatory Medical Care Survey (NAMCS), an ongoing survey conducted by the National Center for Health Statistics (JAMA Dermatol. 2013 Sept. 4 [doi:10.1001/jamadermatol.2013.4741]).
Overall, sunscreen was recommended at only 12.9 million of 18.3 billion patient visits (0.07%). Most of the appointments at which sunscreen was recommended were visits to a dermatologist (86%), followed by visits to family physicians or general practitioners (10%), pediatricians (1.4%), other specialists (1.4%), and internists (1.1%).
Although dermatologists were the most frequent recommenders of sunscreen, the mention of sunscreen was recorded at only 1.6% of all dermatology visits and 11% of visits associated with a diagnosis of skin cancer, the researchers said. Of note, dermatologists recommended use of sunscreen to skin cancer patients less frequently than did general/family physicians (11% vs. 56%), they added.
Overall, sunscreen was least often recommended for children younger than 10 years; by contrast, patients in their 70s were most likely to receive a recommendation for sunscreen use.
In addition, white patients were nine times more likely than black patients to receive a recommendation for sunscreen use.
Across all specialties, patients with a diagnosis of actinic keratosis accounted for 21% of the visits at which sunscreen was recommended.
The study was limited by several factors, including the cross-sectional nature of the data, which included both new and follow-up visits, and the lack of information about whether sunscreen was discussed at an earlier visit or not documented by the physician on the survey report, Dr. Akamine and her associates noted.
The results, however, suggest that sunscreen use recommendation is less frequent than advised by multiple medical organizations. "The high incidence and morbidity of skin cancer can be greatly reduced with the implementation of sun-protection behaviors, which patients should be counseled about at outpatient visits," the researchers said.
Lead author Dr. Akamine had no financial conflicts to disclose. Corresponding author Dr. Steven Feldman disclosed financial relationships with multiple pharmaceutical companies, but this study was not sponsored by a pharmaceutical company.
On Twitter @hsplete
Physicians miss 99.9% of their opportunities to counsel patients about use of sunscreen, and dermatologists pass up 98% of those teaching moments, based on data from approximately 18 billion patient visits recorded between January 1989 and December 2010. The findings were published online on Sept. 4 in JAMA Dermatology.
Despite the rising incidence of skin cancer and recommendations from medical organizations that clinicians counsel patients about sun-protective behaviors, "sun-protection counseling ranks among the lowest topics of primary prevention discussed between physicians and patients," said Dr. Kristie Akamine of Wake Forest University in Winston-Salem, N.C., and her colleagues.
To identify trends in sunscreen recommendations by different specialties, the researchers reviewed data from the National Ambulatory Medical Care Survey (NAMCS), an ongoing survey conducted by the National Center for Health Statistics (JAMA Dermatol. 2013 Sept. 4 [doi:10.1001/jamadermatol.2013.4741]).
Overall, sunscreen was recommended at only 12.9 million of 18.3 billion patient visits (0.07%). Most of the appointments at which sunscreen was recommended were visits to a dermatologist (86%), followed by visits to family physicians or general practitioners (10%), pediatricians (1.4%), other specialists (1.4%), and internists (1.1%).
Although dermatologists were the most frequent recommenders of sunscreen, the mention of sunscreen was recorded at only 1.6% of all dermatology visits and 11% of visits associated with a diagnosis of skin cancer, the researchers said. Of note, dermatologists recommended use of sunscreen to skin cancer patients less frequently than did general/family physicians (11% vs. 56%), they added.
Overall, sunscreen was least often recommended for children younger than 10 years; by contrast, patients in their 70s were most likely to receive a recommendation for sunscreen use.
In addition, white patients were nine times more likely than black patients to receive a recommendation for sunscreen use.
Across all specialties, patients with a diagnosis of actinic keratosis accounted for 21% of the visits at which sunscreen was recommended.
The study was limited by several factors, including the cross-sectional nature of the data, which included both new and follow-up visits, and the lack of information about whether sunscreen was discussed at an earlier visit or not documented by the physician on the survey report, Dr. Akamine and her associates noted.
The results, however, suggest that sunscreen use recommendation is less frequent than advised by multiple medical organizations. "The high incidence and morbidity of skin cancer can be greatly reduced with the implementation of sun-protection behaviors, which patients should be counseled about at outpatient visits," the researchers said.
Lead author Dr. Akamine had no financial conflicts to disclose. Corresponding author Dr. Steven Feldman disclosed financial relationships with multiple pharmaceutical companies, but this study was not sponsored by a pharmaceutical company.
On Twitter @hsplete
Physicians miss 99.9% of their opportunities to counsel patients about use of sunscreen, and dermatologists pass up 98% of those teaching moments, based on data from approximately 18 billion patient visits recorded between January 1989 and December 2010. The findings were published online on Sept. 4 in JAMA Dermatology.
Despite the rising incidence of skin cancer and recommendations from medical organizations that clinicians counsel patients about sun-protective behaviors, "sun-protection counseling ranks among the lowest topics of primary prevention discussed between physicians and patients," said Dr. Kristie Akamine of Wake Forest University in Winston-Salem, N.C., and her colleagues.
To identify trends in sunscreen recommendations by different specialties, the researchers reviewed data from the National Ambulatory Medical Care Survey (NAMCS), an ongoing survey conducted by the National Center for Health Statistics (JAMA Dermatol. 2013 Sept. 4 [doi:10.1001/jamadermatol.2013.4741]).
Overall, sunscreen was recommended at only 12.9 million of 18.3 billion patient visits (0.07%). Most of the appointments at which sunscreen was recommended were visits to a dermatologist (86%), followed by visits to family physicians or general practitioners (10%), pediatricians (1.4%), other specialists (1.4%), and internists (1.1%).
Although dermatologists were the most frequent recommenders of sunscreen, the mention of sunscreen was recorded at only 1.6% of all dermatology visits and 11% of visits associated with a diagnosis of skin cancer, the researchers said. Of note, dermatologists recommended use of sunscreen to skin cancer patients less frequently than did general/family physicians (11% vs. 56%), they added.
Overall, sunscreen was least often recommended for children younger than 10 years; by contrast, patients in their 70s were most likely to receive a recommendation for sunscreen use.
In addition, white patients were nine times more likely than black patients to receive a recommendation for sunscreen use.
Across all specialties, patients with a diagnosis of actinic keratosis accounted for 21% of the visits at which sunscreen was recommended.
The study was limited by several factors, including the cross-sectional nature of the data, which included both new and follow-up visits, and the lack of information about whether sunscreen was discussed at an earlier visit or not documented by the physician on the survey report, Dr. Akamine and her associates noted.
The results, however, suggest that sunscreen use recommendation is less frequent than advised by multiple medical organizations. "The high incidence and morbidity of skin cancer can be greatly reduced with the implementation of sun-protection behaviors, which patients should be counseled about at outpatient visits," the researchers said.
Lead author Dr. Akamine had no financial conflicts to disclose. Corresponding author Dr. Steven Feldman disclosed financial relationships with multiple pharmaceutical companies, but this study was not sponsored by a pharmaceutical company.
On Twitter @hsplete
FROM JAMA DERMATOLOGY
Major Finding: Overall, physicians recommended sunscreen at 12.9 million of 18.3 billion patient visits (0.07%).
Data Source: The National Ambulatory Medical Care Survey, an ongoing survey conducted by the National Center for Health Statistics.
Disclosures: Lead author Dr. Akamine had no financial conflicts to disclose. Corresponding author Dr. Steven Feldman disclosed financial relationships with multiple pharmaceutical companies, but this study was not sponsored by a pharmaceutical company.
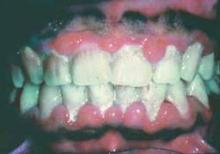
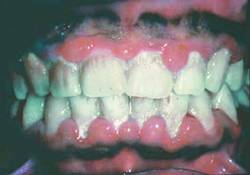
Poor oral health a risk factor in oncogenic HPV infection
Poor oral health is an independent risk factor of oral human papillomavirus infection regardless of smoking and oral sex practices, analysis of a large, nationally representative sample showed.
Poor oral health was associated with a 56% higher prevalence of oral HPV infection in 3,439 study participants aged 30-69 years, Thanh Cong Bui, Dr.P.H., of the University of Texas Health Sciences Center, Houston, and his colleagues wrote in Cancer Prevention Research.
For those with gum disease and dental problems, prevalence of oral HPV infection was 51% and 28% higher, respectively. More men (12%) than women (3%) were infected with oral HPV (Cancer Prev. Res. 2013 Aug. 21 [doi:10.1158/1940-6207.CAPR-13-008]).
Marijuana use also was linked to higher rates of infection: 5% in nonusers, 8% in former users, and 14% in current users.
The findings were derived from the 2009-2010 National Health and Nutrition Examination Survey (NHANES), a nationally representative sampling conducted by the Centers for Disease Control and Prevention. Oral health data were taken from participants’ self-assessments of overall oral health, presence of gum disease, use of mouthwash to treat dental problems within past 7 days of the survey, and number of teeth lost.
"The good news is, this risk factor is modifiable – by maintaining good oral hygiene and good oral health, one can prevent HPV infection and subsequent HPV-related cancers," Dr. Bui said in a statement.
Previous studies have linked oral sex, number of sex partners, and cigarettes smoked per day with infection with high-risk oral HPV. Because oral sex and smoking previously had been found to have similar predictive value, Dr. Bui and colleagues examined NHANES data for both low-risk and high-risk HPV type. Using multivariable logistic regression models, they determined oral HPV infection was significantly linked with self-rated overall poor oral health, independent of tobacco use and oral sex.
According to the researchers, the study was limited because the temporal relationship between variables could not be established because of the cross-sectional nature of the data; however, because oral HPV infection normally is asymptomatic, "it is unlikely to affect self-reported oral health." Still, they noted that the data, based on self-report, could be subject to recall bias or under-reporting.
"Public health interventions may aim to promote oral hygiene and oral health as additional preventative measures for HPV-related oral cancers," Dr. Bui and his colleagues said.
The study was supported by a grant from the University of Texas Health Innovation for Cancer Prevention Research. The authors reported no conflicts of interest.
Poor oral health is an independent risk factor of oral human papillomavirus infection regardless of smoking and oral sex practices, analysis of a large, nationally representative sample showed.
Poor oral health was associated with a 56% higher prevalence of oral HPV infection in 3,439 study participants aged 30-69 years, Thanh Cong Bui, Dr.P.H., of the University of Texas Health Sciences Center, Houston, and his colleagues wrote in Cancer Prevention Research.
For those with gum disease and dental problems, prevalence of oral HPV infection was 51% and 28% higher, respectively. More men (12%) than women (3%) were infected with oral HPV (Cancer Prev. Res. 2013 Aug. 21 [doi:10.1158/1940-6207.CAPR-13-008]).
Marijuana use also was linked to higher rates of infection: 5% in nonusers, 8% in former users, and 14% in current users.
The findings were derived from the 2009-2010 National Health and Nutrition Examination Survey (NHANES), a nationally representative sampling conducted by the Centers for Disease Control and Prevention. Oral health data were taken from participants’ self-assessments of overall oral health, presence of gum disease, use of mouthwash to treat dental problems within past 7 days of the survey, and number of teeth lost.
"The good news is, this risk factor is modifiable – by maintaining good oral hygiene and good oral health, one can prevent HPV infection and subsequent HPV-related cancers," Dr. Bui said in a statement.
Previous studies have linked oral sex, number of sex partners, and cigarettes smoked per day with infection with high-risk oral HPV. Because oral sex and smoking previously had been found to have similar predictive value, Dr. Bui and colleagues examined NHANES data for both low-risk and high-risk HPV type. Using multivariable logistic regression models, they determined oral HPV infection was significantly linked with self-rated overall poor oral health, independent of tobacco use and oral sex.
According to the researchers, the study was limited because the temporal relationship between variables could not be established because of the cross-sectional nature of the data; however, because oral HPV infection normally is asymptomatic, "it is unlikely to affect self-reported oral health." Still, they noted that the data, based on self-report, could be subject to recall bias or under-reporting.
"Public health interventions may aim to promote oral hygiene and oral health as additional preventative measures for HPV-related oral cancers," Dr. Bui and his colleagues said.
The study was supported by a grant from the University of Texas Health Innovation for Cancer Prevention Research. The authors reported no conflicts of interest.
Poor oral health is an independent risk factor of oral human papillomavirus infection regardless of smoking and oral sex practices, analysis of a large, nationally representative sample showed.
Poor oral health was associated with a 56% higher prevalence of oral HPV infection in 3,439 study participants aged 30-69 years, Thanh Cong Bui, Dr.P.H., of the University of Texas Health Sciences Center, Houston, and his colleagues wrote in Cancer Prevention Research.
For those with gum disease and dental problems, prevalence of oral HPV infection was 51% and 28% higher, respectively. More men (12%) than women (3%) were infected with oral HPV (Cancer Prev. Res. 2013 Aug. 21 [doi:10.1158/1940-6207.CAPR-13-008]).
Marijuana use also was linked to higher rates of infection: 5% in nonusers, 8% in former users, and 14% in current users.
The findings were derived from the 2009-2010 National Health and Nutrition Examination Survey (NHANES), a nationally representative sampling conducted by the Centers for Disease Control and Prevention. Oral health data were taken from participants’ self-assessments of overall oral health, presence of gum disease, use of mouthwash to treat dental problems within past 7 days of the survey, and number of teeth lost.
"The good news is, this risk factor is modifiable – by maintaining good oral hygiene and good oral health, one can prevent HPV infection and subsequent HPV-related cancers," Dr. Bui said in a statement.
Previous studies have linked oral sex, number of sex partners, and cigarettes smoked per day with infection with high-risk oral HPV. Because oral sex and smoking previously had been found to have similar predictive value, Dr. Bui and colleagues examined NHANES data for both low-risk and high-risk HPV type. Using multivariable logistic regression models, they determined oral HPV infection was significantly linked with self-rated overall poor oral health, independent of tobacco use and oral sex.
According to the researchers, the study was limited because the temporal relationship between variables could not be established because of the cross-sectional nature of the data; however, because oral HPV infection normally is asymptomatic, "it is unlikely to affect self-reported oral health." Still, they noted that the data, based on self-report, could be subject to recall bias or under-reporting.
"Public health interventions may aim to promote oral hygiene and oral health as additional preventative measures for HPV-related oral cancers," Dr. Bui and his colleagues said.
The study was supported by a grant from the University of Texas Health Innovation for Cancer Prevention Research. The authors reported no conflicts of interest.
FROM CANCER PREVENTION RESEARCH
Major finding: Poor oral health was associated with a 56% higher prevalence of oral infection with human papillomavirus.
Data source: A nationally representative sampling of 3,439 people aged 30-69 years for whom reliable oral health data were available.
Disclosures: The study was supported by a grant from the University of Texas. The researchers reported no relevant conflicts of interest.
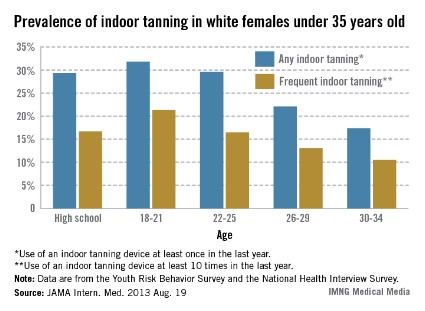
Approximately 25% of young, white women report indoor tanning
Almost one-third of white female high school students and one-quarter of white women aged 18-34 years used indoor tanning devices over a 12-month period, researchers at the Centers for Disease Control and Prevention reported.
Data from the 2011 Youth Risk Behavior Survey showed that 29.3% of the 2,527 non-Hispanic white female high school respondents reported using a sunlamp, sun bed, or tanning booth in the previous 12 months, and 16.7% reported frequent (at least 10 times in the previous year) use of tanning devices, said Gery P. Guy Jr., Ph.D., of the CDC’s Division of Cancer Prevention and Control and his associates (JAMA Intern. Med. 2013 Aug. 19 [doi:10.1001/jamainternmed.2013.10013]).
Among white women aged 18-34 years, 24.9% had used a tanning device at least once in the previous 12 months and 15.1% had used one at least 10 times, according to data from the 2010 National Health Interview Survey. The younger women in that age range were more likely to use tanning devices; 31.8% of women aged 18-21 years reported any use and 21.3% reported frequent use, compared with 17.4% and 10.7%, respectively, in women aged 30-34 years, the investigators wrote.

Almost one-third of white female high school students and one-quarter of white women aged 18-34 years used indoor tanning devices over a 12-month period, researchers at the Centers for Disease Control and Prevention reported.
Data from the 2011 Youth Risk Behavior Survey showed that 29.3% of the 2,527 non-Hispanic white female high school respondents reported using a sunlamp, sun bed, or tanning booth in the previous 12 months, and 16.7% reported frequent (at least 10 times in the previous year) use of tanning devices, said Gery P. Guy Jr., Ph.D., of the CDC’s Division of Cancer Prevention and Control and his associates (JAMA Intern. Med. 2013 Aug. 19 [doi:10.1001/jamainternmed.2013.10013]).
Among white women aged 18-34 years, 24.9% had used a tanning device at least once in the previous 12 months and 15.1% had used one at least 10 times, according to data from the 2010 National Health Interview Survey. The younger women in that age range were more likely to use tanning devices; 31.8% of women aged 18-21 years reported any use and 21.3% reported frequent use, compared with 17.4% and 10.7%, respectively, in women aged 30-34 years, the investigators wrote.

Almost one-third of white female high school students and one-quarter of white women aged 18-34 years used indoor tanning devices over a 12-month period, researchers at the Centers for Disease Control and Prevention reported.
Data from the 2011 Youth Risk Behavior Survey showed that 29.3% of the 2,527 non-Hispanic white female high school respondents reported using a sunlamp, sun bed, or tanning booth in the previous 12 months, and 16.7% reported frequent (at least 10 times in the previous year) use of tanning devices, said Gery P. Guy Jr., Ph.D., of the CDC’s Division of Cancer Prevention and Control and his associates (JAMA Intern. Med. 2013 Aug. 19 [doi:10.1001/jamainternmed.2013.10013]).
Among white women aged 18-34 years, 24.9% had used a tanning device at least once in the previous 12 months and 15.1% had used one at least 10 times, according to data from the 2010 National Health Interview Survey. The younger women in that age range were more likely to use tanning devices; 31.8% of women aged 18-21 years reported any use and 21.3% reported frequent use, compared with 17.4% and 10.7%, respectively, in women aged 30-34 years, the investigators wrote.

FROM JAMA INTERNAL MEDICINE
Illinois bans tanning beds for youngsters
Illinois has become the sixth state to ban indoor tanning for youth younger than 18 years. The bill, which was introduced earlier this year and signed into law by Gov. Patrick Quinn on Aug. 15, will take effect on Jan. 1, 2014.
"The state’s willingness to follow the examples set by the cities of Springfield and Chicago exemplifies a true commitment to protecting teens from the dangers of indoor tanning," Dr. Dirk M. Elston, president of the American Academy of Dermatology Association, said in a statement.
The academy said in a news release that the law is based on "significant scientific evidence that links indoor tanning to increased risk of developing melanoma and other forms of skin cancer."
Compared with previous years, 2013 has been an active year for tanning bed laws.
In a video interview earlier this month, Dr. Bruce A. Brod, the AAD’s State Policy Committee Chair, spoke about the Food and Drug Administration’s proposal to place stricter regulations on indoor tanning beds and issue stronger recommendations against their use by anyone younger than 18 years. The agency is considering the comments and may issue its final ruling by the end of 2013.
Meanwhile, in June, the Internal Revenue Service issued the final regulation on collecting a 10% tax on tanning salon receipts. The regulation was first proposed in 2010 and exempts "qualified physical fitness facilities" that offer tanning services from collecting the tax.
Groups such as the Indoor Tanning Association and the American Suntanning Association continue to lobby against the tanning ban laws.
Illinois joins California, Vermont, Oregon, Nevada, and Texas in passing age-related restrictions on the use of tanning beds. New York, New Jersey, and Connecticut have bans for youth under 17 years of age, while the District of Columbia and West Virginia have tanning bed bans for individuals younger than 14 years.
On Twitter @naseemsmiller
Illinois has become the sixth state to ban indoor tanning for youth younger than 18 years. The bill, which was introduced earlier this year and signed into law by Gov. Patrick Quinn on Aug. 15, will take effect on Jan. 1, 2014.
"The state’s willingness to follow the examples set by the cities of Springfield and Chicago exemplifies a true commitment to protecting teens from the dangers of indoor tanning," Dr. Dirk M. Elston, president of the American Academy of Dermatology Association, said in a statement.
The academy said in a news release that the law is based on "significant scientific evidence that links indoor tanning to increased risk of developing melanoma and other forms of skin cancer."
Compared with previous years, 2013 has been an active year for tanning bed laws.
In a video interview earlier this month, Dr. Bruce A. Brod, the AAD’s State Policy Committee Chair, spoke about the Food and Drug Administration’s proposal to place stricter regulations on indoor tanning beds and issue stronger recommendations against their use by anyone younger than 18 years. The agency is considering the comments and may issue its final ruling by the end of 2013.
Meanwhile, in June, the Internal Revenue Service issued the final regulation on collecting a 10% tax on tanning salon receipts. The regulation was first proposed in 2010 and exempts "qualified physical fitness facilities" that offer tanning services from collecting the tax.
Groups such as the Indoor Tanning Association and the American Suntanning Association continue to lobby against the tanning ban laws.
Illinois joins California, Vermont, Oregon, Nevada, and Texas in passing age-related restrictions on the use of tanning beds. New York, New Jersey, and Connecticut have bans for youth under 17 years of age, while the District of Columbia and West Virginia have tanning bed bans for individuals younger than 14 years.
On Twitter @naseemsmiller
Illinois has become the sixth state to ban indoor tanning for youth younger than 18 years. The bill, which was introduced earlier this year and signed into law by Gov. Patrick Quinn on Aug. 15, will take effect on Jan. 1, 2014.
"The state’s willingness to follow the examples set by the cities of Springfield and Chicago exemplifies a true commitment to protecting teens from the dangers of indoor tanning," Dr. Dirk M. Elston, president of the American Academy of Dermatology Association, said in a statement.
The academy said in a news release that the law is based on "significant scientific evidence that links indoor tanning to increased risk of developing melanoma and other forms of skin cancer."
Compared with previous years, 2013 has been an active year for tanning bed laws.
In a video interview earlier this month, Dr. Bruce A. Brod, the AAD’s State Policy Committee Chair, spoke about the Food and Drug Administration’s proposal to place stricter regulations on indoor tanning beds and issue stronger recommendations against their use by anyone younger than 18 years. The agency is considering the comments and may issue its final ruling by the end of 2013.
Meanwhile, in June, the Internal Revenue Service issued the final regulation on collecting a 10% tax on tanning salon receipts. The regulation was first proposed in 2010 and exempts "qualified physical fitness facilities" that offer tanning services from collecting the tax.
Groups such as the Indoor Tanning Association and the American Suntanning Association continue to lobby against the tanning ban laws.
Illinois joins California, Vermont, Oregon, Nevada, and Texas in passing age-related restrictions on the use of tanning beds. New York, New Jersey, and Connecticut have bans for youth under 17 years of age, while the District of Columbia and West Virginia have tanning bed bans for individuals younger than 14 years.
On Twitter @naseemsmiller
Debunking sun protection myths for skin of color patients
Fair-skinned people are known to be at higher risk for skin cancer and other problems associated with too much exposure to the sun. But people with skin of color are also vulnerable to the harmful effects of ultraviolet rays emitted by both the sun and indoor tanning beds, said Dr. Adam Friedman, director of dermatologic research, division of dermatology, Montefiore Medical Center in New York.
"Darker skin has more reactive melanocytes, or pigment-making cells, and has more and stronger melanosomes, which are small packets that contain skin pigment, both of which provide some inherent protection against UV rays but not enough, he said. "This unique biological difference in darker skin causes the harmful effects of UV exposure to occur more slowly in people of color, and the effects require more direct sun exposure. But the damage does happen, from cosmetic problems such as premature aging of the skin to serious conditions such as skin cancer."
"There are three major misconceptions that I encounter with my patients in the Bronx," Dr. Friedman said.
• Darker skin makes one immune to the harmful effects of the sun.
• There is no risk of exposure on cloudy days.
• One needs to get vitamin D via sun exposure.
"All three are completely false, and ultimately they perpetuate and result in improper skin protection," he noted.
"Skin cancer is rarer in people with skin of color, but it does occur and can be extremely serious when diagnosis is delayed," Dr. Friedman said. For example, melanoma, the most deadly form of skin cancer, is more than 20 times more common in whites than in African Americans, but people with darker skin are at greater risk of late diagnosis with advanced, thicker melanomas and lower survival rates. In fact, the overall 5-year melanoma survival rate for African Americans is only 77%, versus 91% for Caucasians, he said.
"I advise all of my patients to routinely check their own skin for any changes in appearance, and to see a dermatologist annually for a full body exam," Dr. Friedman emphasized.
Traditional sunscreens, especially those containing mineral-based agents such as titanium dioxide and zinc oxide, do not blend well when used on darker skin. The resulting chalky appearance is unacceptable to many patients, Dr. Friedman said. Fortunately, new formulations are changing that.
The newer sunscreens combine several agents to both decrease the concentration needed of each and allow for a synergistic effect between them, "ultimately offering a better sunscreen formula that can blend well into any skin type," said Dr. Friedman. Patients should look for products containing micronized or nanosized zinc oxide or titanium dioxide, which do not scatter light in the visible spectrum (to which older versions of these products owe their chalky appearance) but are highly effective at blocking UV radiation, he advised. Products that combine these mineral agents with multiple chemical blockers such as ecamsule, avobenzone, and cinoxate are particularly effective and cosmetically acceptable. Patients may be most likely to use sunscreens that utilize vehicles that enhance dispersal of these ingredients to limit clumping and phase separation, such as talc and Bentone gel.
In addition, pH stabilizers such as dimethicone are important, as the skin acidity in skin of color is lower and needs to be maintained to prevent degradation of skin adhesion proteins. Dr. Friedman recommends that his skin of color patients use SPF 30 broad-spectrum sunscreen, generously applied, and lip balm with an SPF of at least 30.
Dr. Friedman often hears from patients that they avoid sunscreen because it prevents them from getting vitamin D from the sun, which they believe is the best source. "You can enjoy the best of both worlds – use sunscreen when you spend time outdoors and take a vitamin D supplement, which is a very effective way to get adequate daily intake," he said. He advises his patients to remain vigilant about how much sun exposure they get. "Sunscreen alone is not enough to protect you from skin cancer, especially between the hours of 10:00 a.m. and 2:00 p.m. I encourage all of my patients to seek shade during that time of day and wear hats, sunglasses, and protective clothing if possible," he said.
Dr. Friedman had no financial conflicts to disclose.
[email protected]
On Twitter @hsplete
Fair-skinned people are known to be at higher risk for skin cancer and other problems associated with too much exposure to the sun. But people with skin of color are also vulnerable to the harmful effects of ultraviolet rays emitted by both the sun and indoor tanning beds, said Dr. Adam Friedman, director of dermatologic research, division of dermatology, Montefiore Medical Center in New York.
"Darker skin has more reactive melanocytes, or pigment-making cells, and has more and stronger melanosomes, which are small packets that contain skin pigment, both of which provide some inherent protection against UV rays but not enough, he said. "This unique biological difference in darker skin causes the harmful effects of UV exposure to occur more slowly in people of color, and the effects require more direct sun exposure. But the damage does happen, from cosmetic problems such as premature aging of the skin to serious conditions such as skin cancer."
"There are three major misconceptions that I encounter with my patients in the Bronx," Dr. Friedman said.
• Darker skin makes one immune to the harmful effects of the sun.
• There is no risk of exposure on cloudy days.
• One needs to get vitamin D via sun exposure.
"All three are completely false, and ultimately they perpetuate and result in improper skin protection," he noted.
"Skin cancer is rarer in people with skin of color, but it does occur and can be extremely serious when diagnosis is delayed," Dr. Friedman said. For example, melanoma, the most deadly form of skin cancer, is more than 20 times more common in whites than in African Americans, but people with darker skin are at greater risk of late diagnosis with advanced, thicker melanomas and lower survival rates. In fact, the overall 5-year melanoma survival rate for African Americans is only 77%, versus 91% for Caucasians, he said.
"I advise all of my patients to routinely check their own skin for any changes in appearance, and to see a dermatologist annually for a full body exam," Dr. Friedman emphasized.
Traditional sunscreens, especially those containing mineral-based agents such as titanium dioxide and zinc oxide, do not blend well when used on darker skin. The resulting chalky appearance is unacceptable to many patients, Dr. Friedman said. Fortunately, new formulations are changing that.
The newer sunscreens combine several agents to both decrease the concentration needed of each and allow for a synergistic effect between them, "ultimately offering a better sunscreen formula that can blend well into any skin type," said Dr. Friedman. Patients should look for products containing micronized or nanosized zinc oxide or titanium dioxide, which do not scatter light in the visible spectrum (to which older versions of these products owe their chalky appearance) but are highly effective at blocking UV radiation, he advised. Products that combine these mineral agents with multiple chemical blockers such as ecamsule, avobenzone, and cinoxate are particularly effective and cosmetically acceptable. Patients may be most likely to use sunscreens that utilize vehicles that enhance dispersal of these ingredients to limit clumping and phase separation, such as talc and Bentone gel.
In addition, pH stabilizers such as dimethicone are important, as the skin acidity in skin of color is lower and needs to be maintained to prevent degradation of skin adhesion proteins. Dr. Friedman recommends that his skin of color patients use SPF 30 broad-spectrum sunscreen, generously applied, and lip balm with an SPF of at least 30.
Dr. Friedman often hears from patients that they avoid sunscreen because it prevents them from getting vitamin D from the sun, which they believe is the best source. "You can enjoy the best of both worlds – use sunscreen when you spend time outdoors and take a vitamin D supplement, which is a very effective way to get adequate daily intake," he said. He advises his patients to remain vigilant about how much sun exposure they get. "Sunscreen alone is not enough to protect you from skin cancer, especially between the hours of 10:00 a.m. and 2:00 p.m. I encourage all of my patients to seek shade during that time of day and wear hats, sunglasses, and protective clothing if possible," he said.
Dr. Friedman had no financial conflicts to disclose.
[email protected]
On Twitter @hsplete
Fair-skinned people are known to be at higher risk for skin cancer and other problems associated with too much exposure to the sun. But people with skin of color are also vulnerable to the harmful effects of ultraviolet rays emitted by both the sun and indoor tanning beds, said Dr. Adam Friedman, director of dermatologic research, division of dermatology, Montefiore Medical Center in New York.
"Darker skin has more reactive melanocytes, or pigment-making cells, and has more and stronger melanosomes, which are small packets that contain skin pigment, both of which provide some inherent protection against UV rays but not enough, he said. "This unique biological difference in darker skin causes the harmful effects of UV exposure to occur more slowly in people of color, and the effects require more direct sun exposure. But the damage does happen, from cosmetic problems such as premature aging of the skin to serious conditions such as skin cancer."
"There are three major misconceptions that I encounter with my patients in the Bronx," Dr. Friedman said.
• Darker skin makes one immune to the harmful effects of the sun.
• There is no risk of exposure on cloudy days.
• One needs to get vitamin D via sun exposure.
"All three are completely false, and ultimately they perpetuate and result in improper skin protection," he noted.
"Skin cancer is rarer in people with skin of color, but it does occur and can be extremely serious when diagnosis is delayed," Dr. Friedman said. For example, melanoma, the most deadly form of skin cancer, is more than 20 times more common in whites than in African Americans, but people with darker skin are at greater risk of late diagnosis with advanced, thicker melanomas and lower survival rates. In fact, the overall 5-year melanoma survival rate for African Americans is only 77%, versus 91% for Caucasians, he said.
"I advise all of my patients to routinely check their own skin for any changes in appearance, and to see a dermatologist annually for a full body exam," Dr. Friedman emphasized.
Traditional sunscreens, especially those containing mineral-based agents such as titanium dioxide and zinc oxide, do not blend well when used on darker skin. The resulting chalky appearance is unacceptable to many patients, Dr. Friedman said. Fortunately, new formulations are changing that.
The newer sunscreens combine several agents to both decrease the concentration needed of each and allow for a synergistic effect between them, "ultimately offering a better sunscreen formula that can blend well into any skin type," said Dr. Friedman. Patients should look for products containing micronized or nanosized zinc oxide or titanium dioxide, which do not scatter light in the visible spectrum (to which older versions of these products owe their chalky appearance) but are highly effective at blocking UV radiation, he advised. Products that combine these mineral agents with multiple chemical blockers such as ecamsule, avobenzone, and cinoxate are particularly effective and cosmetically acceptable. Patients may be most likely to use sunscreens that utilize vehicles that enhance dispersal of these ingredients to limit clumping and phase separation, such as talc and Bentone gel.
In addition, pH stabilizers such as dimethicone are important, as the skin acidity in skin of color is lower and needs to be maintained to prevent degradation of skin adhesion proteins. Dr. Friedman recommends that his skin of color patients use SPF 30 broad-spectrum sunscreen, generously applied, and lip balm with an SPF of at least 30.
Dr. Friedman often hears from patients that they avoid sunscreen because it prevents them from getting vitamin D from the sun, which they believe is the best source. "You can enjoy the best of both worlds – use sunscreen when you spend time outdoors and take a vitamin D supplement, which is a very effective way to get adequate daily intake," he said. He advises his patients to remain vigilant about how much sun exposure they get. "Sunscreen alone is not enough to protect you from skin cancer, especially between the hours of 10:00 a.m. and 2:00 p.m. I encourage all of my patients to seek shade during that time of day and wear hats, sunglasses, and protective clothing if possible," he said.
Dr. Friedman had no financial conflicts to disclose.
[email protected]
On Twitter @hsplete
EXPERT ANALYSIS FROM THE SKIN OF COLOR SEMINAR SERIES
Melanoma screening initiatives reveal complex ramifications
NEW YORK – A national, population-based melanoma screening program underway in Germany may provide the data to drive similar initiatives elsewhere, including the United States, but complex issues surround cancer screening programs of any kind, Dr. Allan C. Halpern said in a key address at the American Academy of Dermatology summer meeting.
Even if reduced mortality from the melanoma screening program in Germany equals the numbers seen in the regional initiative that prompted the national program, Dr. Halpern, chief of the dermatology service and co-leader of Memorial Sloan-Kettering Cancer Center (New York) melanoma disease management team, cautioned that U.S. policy makers will face several issues before adopting a similar program.
"We have begun to understand that no matter what you are screening for, you end up finding a lot of indolent disease, or what we now call overdiagnosis," Dr. Halpern said. In these cases, clinicians diagnose cancer or precancerous conditions "in patients who were never going to be hurt by their disease" but may incur harm from treatments or from the psychosocial stress of the diagnosis.
Melanoma is the only cancer for which mortality is increasing, despite simple and effective screening strategies, Dr. Halpern said. One problem is that only a proportion of those patients known to be at high risk for melanoma, such as those with a personal or family history of this disease, undergo regular surveillance. Despite a clear need for rigorous screening in high risk individuals, he suggested that "[dermatology] as a profession has not figured out how to do this consistently."
However, the program in Germany is not restricted to high-risk individuals. It was initiated after a screening program in Schleswig-Holstein, one of 16 German states, was credited with reducing melanoma mortality by 47% in women and 49% in men, based on rates 5 years after screening, compared with rates 4 years before screening (Cancer 2012;118:5395-402). Mortality rates in adjacent states over this period were unchanged.
In the German program, approximately 1,700 general practitioners were trained to provide whole body assessments for melanoma. Individuals aged 20 years and older were eligible, and more than 360,000 residents of Schleswig-Holstein were screened. Dr. Halpern said that the development of the program was largely because of the initiative of Dr. Eckhard W. Breitbart, a Schleswig-Holstein dermatologist who convinced public health authorities to provide funding.
The evidence of benefit was sufficient to generate a national program. So far, 13 million Germans have already been screened, Dr. Halpern said. Although the mortality reduction from the national program may not reach the magnitude seen at the regional level, any large reduction would provide "a huge endorsement for melanoma screening," Dr. Halpern noted.
Melanoma screening data are needed, Dr. Halpern added. The U.S. Preventive Services Task Force "specifically does not recommend melanoma screening for the population at large" because of lack of randomized, controlled trial evidence that it would provide an overall benefit, he said. Such trials have been proposed, and a pilot study was completed in Australia, Dr. Halpern said, but he said he does not believe a large scale trial is forthcoming. Rather, he said he believes that a study similar to the German study may be the best opportunity to show a benefit from screening.
Similar policy changes occurred after a Scandinavian initiative to screen cervical cancer demonstrated a large mortality benefit, according to Dr. Halpern, who called that experience the "poster child" for cancer screening initiatives. The mortality benefit data from the population-based program was so compelling that screening programs for cervical cancer are now broadly accepted worldwide, although a randomized controlled trial was never conducted. If the German data provide similar evidence of the benefits of melanoma screening, "this may be how we get to melanoma screening in this country," Dr. Halpern said.
Melanoma screening is "intuitively attractive," Dr. Halpern added, but he acknowledged the rationale for caution. While he said he believes there is a need to increase screening in high-risk populations, the risk of harm, including psychosocial harm, from population-based screening is not trivial. The German experience may provide the data to help determine whether population-based screening makes sense.
Dr. Halpern disclosed financial relationships with multiple companies including Canfield Scientific, DermTech International, Quintiles, Roche, and SciBase.
NEW YORK – A national, population-based melanoma screening program underway in Germany may provide the data to drive similar initiatives elsewhere, including the United States, but complex issues surround cancer screening programs of any kind, Dr. Allan C. Halpern said in a key address at the American Academy of Dermatology summer meeting.
Even if reduced mortality from the melanoma screening program in Germany equals the numbers seen in the regional initiative that prompted the national program, Dr. Halpern, chief of the dermatology service and co-leader of Memorial Sloan-Kettering Cancer Center (New York) melanoma disease management team, cautioned that U.S. policy makers will face several issues before adopting a similar program.
"We have begun to understand that no matter what you are screening for, you end up finding a lot of indolent disease, or what we now call overdiagnosis," Dr. Halpern said. In these cases, clinicians diagnose cancer or precancerous conditions "in patients who were never going to be hurt by their disease" but may incur harm from treatments or from the psychosocial stress of the diagnosis.
Melanoma is the only cancer for which mortality is increasing, despite simple and effective screening strategies, Dr. Halpern said. One problem is that only a proportion of those patients known to be at high risk for melanoma, such as those with a personal or family history of this disease, undergo regular surveillance. Despite a clear need for rigorous screening in high risk individuals, he suggested that "[dermatology] as a profession has not figured out how to do this consistently."
However, the program in Germany is not restricted to high-risk individuals. It was initiated after a screening program in Schleswig-Holstein, one of 16 German states, was credited with reducing melanoma mortality by 47% in women and 49% in men, based on rates 5 years after screening, compared with rates 4 years before screening (Cancer 2012;118:5395-402). Mortality rates in adjacent states over this period were unchanged.
In the German program, approximately 1,700 general practitioners were trained to provide whole body assessments for melanoma. Individuals aged 20 years and older were eligible, and more than 360,000 residents of Schleswig-Holstein were screened. Dr. Halpern said that the development of the program was largely because of the initiative of Dr. Eckhard W. Breitbart, a Schleswig-Holstein dermatologist who convinced public health authorities to provide funding.
The evidence of benefit was sufficient to generate a national program. So far, 13 million Germans have already been screened, Dr. Halpern said. Although the mortality reduction from the national program may not reach the magnitude seen at the regional level, any large reduction would provide "a huge endorsement for melanoma screening," Dr. Halpern noted.
Melanoma screening data are needed, Dr. Halpern added. The U.S. Preventive Services Task Force "specifically does not recommend melanoma screening for the population at large" because of lack of randomized, controlled trial evidence that it would provide an overall benefit, he said. Such trials have been proposed, and a pilot study was completed in Australia, Dr. Halpern said, but he said he does not believe a large scale trial is forthcoming. Rather, he said he believes that a study similar to the German study may be the best opportunity to show a benefit from screening.
Similar policy changes occurred after a Scandinavian initiative to screen cervical cancer demonstrated a large mortality benefit, according to Dr. Halpern, who called that experience the "poster child" for cancer screening initiatives. The mortality benefit data from the population-based program was so compelling that screening programs for cervical cancer are now broadly accepted worldwide, although a randomized controlled trial was never conducted. If the German data provide similar evidence of the benefits of melanoma screening, "this may be how we get to melanoma screening in this country," Dr. Halpern said.
Melanoma screening is "intuitively attractive," Dr. Halpern added, but he acknowledged the rationale for caution. While he said he believes there is a need to increase screening in high-risk populations, the risk of harm, including psychosocial harm, from population-based screening is not trivial. The German experience may provide the data to help determine whether population-based screening makes sense.
Dr. Halpern disclosed financial relationships with multiple companies including Canfield Scientific, DermTech International, Quintiles, Roche, and SciBase.
NEW YORK – A national, population-based melanoma screening program underway in Germany may provide the data to drive similar initiatives elsewhere, including the United States, but complex issues surround cancer screening programs of any kind, Dr. Allan C. Halpern said in a key address at the American Academy of Dermatology summer meeting.
Even if reduced mortality from the melanoma screening program in Germany equals the numbers seen in the regional initiative that prompted the national program, Dr. Halpern, chief of the dermatology service and co-leader of Memorial Sloan-Kettering Cancer Center (New York) melanoma disease management team, cautioned that U.S. policy makers will face several issues before adopting a similar program.
"We have begun to understand that no matter what you are screening for, you end up finding a lot of indolent disease, or what we now call overdiagnosis," Dr. Halpern said. In these cases, clinicians diagnose cancer or precancerous conditions "in patients who were never going to be hurt by their disease" but may incur harm from treatments or from the psychosocial stress of the diagnosis.
Melanoma is the only cancer for which mortality is increasing, despite simple and effective screening strategies, Dr. Halpern said. One problem is that only a proportion of those patients known to be at high risk for melanoma, such as those with a personal or family history of this disease, undergo regular surveillance. Despite a clear need for rigorous screening in high risk individuals, he suggested that "[dermatology] as a profession has not figured out how to do this consistently."
However, the program in Germany is not restricted to high-risk individuals. It was initiated after a screening program in Schleswig-Holstein, one of 16 German states, was credited with reducing melanoma mortality by 47% in women and 49% in men, based on rates 5 years after screening, compared with rates 4 years before screening (Cancer 2012;118:5395-402). Mortality rates in adjacent states over this period were unchanged.
In the German program, approximately 1,700 general practitioners were trained to provide whole body assessments for melanoma. Individuals aged 20 years and older were eligible, and more than 360,000 residents of Schleswig-Holstein were screened. Dr. Halpern said that the development of the program was largely because of the initiative of Dr. Eckhard W. Breitbart, a Schleswig-Holstein dermatologist who convinced public health authorities to provide funding.
The evidence of benefit was sufficient to generate a national program. So far, 13 million Germans have already been screened, Dr. Halpern said. Although the mortality reduction from the national program may not reach the magnitude seen at the regional level, any large reduction would provide "a huge endorsement for melanoma screening," Dr. Halpern noted.
Melanoma screening data are needed, Dr. Halpern added. The U.S. Preventive Services Task Force "specifically does not recommend melanoma screening for the population at large" because of lack of randomized, controlled trial evidence that it would provide an overall benefit, he said. Such trials have been proposed, and a pilot study was completed in Australia, Dr. Halpern said, but he said he does not believe a large scale trial is forthcoming. Rather, he said he believes that a study similar to the German study may be the best opportunity to show a benefit from screening.
Similar policy changes occurred after a Scandinavian initiative to screen cervical cancer demonstrated a large mortality benefit, according to Dr. Halpern, who called that experience the "poster child" for cancer screening initiatives. The mortality benefit data from the population-based program was so compelling that screening programs for cervical cancer are now broadly accepted worldwide, although a randomized controlled trial was never conducted. If the German data provide similar evidence of the benefits of melanoma screening, "this may be how we get to melanoma screening in this country," Dr. Halpern said.
Melanoma screening is "intuitively attractive," Dr. Halpern added, but he acknowledged the rationale for caution. While he said he believes there is a need to increase screening in high-risk populations, the risk of harm, including psychosocial harm, from population-based screening is not trivial. The German experience may provide the data to help determine whether population-based screening makes sense.
Dr. Halpern disclosed financial relationships with multiple companies including Canfield Scientific, DermTech International, Quintiles, Roche, and SciBase.
EXPERT ANALYSIS FROM THE AAD SUMMER ACADEMY 2013
Health Care Reform Produces Both Heat and Light With the Indoor Tanning Tax
IRS issues final rule on tanning tax
The Internal Revenue Service has issued the final regulation on collecting a 10% tax on tanning salon receipts as called for by the Affordable Care Act.
The tax first went into effect in July 2010 under temporary regulations while the IRS collected comments on the proposal. Updated regulations were issued and opened for comments in 2012.
Tanning salons have widely complained about the tax, which they said would help drive many of them out of business.
It’s not clear whether that has come to pass, Dr. Jack Resneck Jr., a member of the American Academy of Dermatology board of directors, said in a statement.
"It’s too early to assess the full impact of the tanning tax, but we know it has provided countless opportunities to raise awareness about the dangers of indoor tanning," said Dr. Resneck, vice-chair of clinical dermatology at the University of California, San Francisco.
In 2011, the Indoor Tanning Association was able to rally Republicans in the House and Senate to sponsor a bill to repeal the tax. The campaign did not gain much ground.
"Efforts by the tanning industry to fight this important public health measure have only furthered our efforts to communicate through the media about the overwhelming evidence on the risks of indoor UV tanning," Dr. Resneck said.
"Combined with the FDA’s recent proposal to reclassify tanning beds, as well as efforts in numerous states to restrict minors’ access to indoor tanning, I am optimistic that we are witnessing a real turning point in public awareness and sentiment," he added.
The final rule exempts "qualified physical fitness facilities" that offer tanning services from collecting the tax, as was proposed in 2010.
Phototherapy performed by, and on the premises of, a licensed medical professional also is exempted.
[email protected] On Twitter @aliciaault
The Internal Revenue Service has issued the final regulation on collecting a 10% tax on tanning salon receipts as called for by the Affordable Care Act.
The tax first went into effect in July 2010 under temporary regulations while the IRS collected comments on the proposal. Updated regulations were issued and opened for comments in 2012.
Tanning salons have widely complained about the tax, which they said would help drive many of them out of business.
It’s not clear whether that has come to pass, Dr. Jack Resneck Jr., a member of the American Academy of Dermatology board of directors, said in a statement.
"It’s too early to assess the full impact of the tanning tax, but we know it has provided countless opportunities to raise awareness about the dangers of indoor tanning," said Dr. Resneck, vice-chair of clinical dermatology at the University of California, San Francisco.
In 2011, the Indoor Tanning Association was able to rally Republicans in the House and Senate to sponsor a bill to repeal the tax. The campaign did not gain much ground.
"Efforts by the tanning industry to fight this important public health measure have only furthered our efforts to communicate through the media about the overwhelming evidence on the risks of indoor UV tanning," Dr. Resneck said.
"Combined with the FDA’s recent proposal to reclassify tanning beds, as well as efforts in numerous states to restrict minors’ access to indoor tanning, I am optimistic that we are witnessing a real turning point in public awareness and sentiment," he added.
The final rule exempts "qualified physical fitness facilities" that offer tanning services from collecting the tax, as was proposed in 2010.
Phototherapy performed by, and on the premises of, a licensed medical professional also is exempted.
[email protected] On Twitter @aliciaault
The Internal Revenue Service has issued the final regulation on collecting a 10% tax on tanning salon receipts as called for by the Affordable Care Act.
The tax first went into effect in July 2010 under temporary regulations while the IRS collected comments on the proposal. Updated regulations were issued and opened for comments in 2012.
Tanning salons have widely complained about the tax, which they said would help drive many of them out of business.
It’s not clear whether that has come to pass, Dr. Jack Resneck Jr., a member of the American Academy of Dermatology board of directors, said in a statement.
"It’s too early to assess the full impact of the tanning tax, but we know it has provided countless opportunities to raise awareness about the dangers of indoor tanning," said Dr. Resneck, vice-chair of clinical dermatology at the University of California, San Francisco.
In 2011, the Indoor Tanning Association was able to rally Republicans in the House and Senate to sponsor a bill to repeal the tax. The campaign did not gain much ground.
"Efforts by the tanning industry to fight this important public health measure have only furthered our efforts to communicate through the media about the overwhelming evidence on the risks of indoor UV tanning," Dr. Resneck said.
"Combined with the FDA’s recent proposal to reclassify tanning beds, as well as efforts in numerous states to restrict minors’ access to indoor tanning, I am optimistic that we are witnessing a real turning point in public awareness and sentiment," he added.
The final rule exempts "qualified physical fitness facilities" that offer tanning services from collecting the tax, as was proposed in 2010.
Phototherapy performed by, and on the premises of, a licensed medical professional also is exempted.
[email protected] On Twitter @aliciaault
Selumetinib is first therapy to shrink uveal melanomas
CHICAGO – Selumetinib, an investigational MEK 1/2 inhibitor, shrank uveal melanomas in half of all treated patients, with a duration of disease control that was more than twice that achieved with the comparator therapy temozolomide, based on the final analysis of data from 98 patients in a phase II crossover study.
This is the first clinical trial to identify a drug that improves clinical outcome in patients with advanced melanoma of the eye, said Dr. Richard D. Carvajal, who presented the data at the annual meeting of the American Society of Clinical Oncology.
"Selumetinib is a new standard of therapy for uveal metastatic melanoma," he said at a press conference announcing the results. Before this study, there was no evidence that any systemic therapy was truly effective in this disease. In eight different clinical trials conducted in the last decade, 2 of 157 patients have responded to potential new therapies, including chemotherapy, targeted therapy, and immunotherapy.
“Right now, there are no plans for an official compassionate use program; however, we are reopening up our trial without the randomization such that additional patients can receive selumetinib through this mechanism,” he said in an interview after the meeting.
With about 2,000 cases diagnosed each year in the United States, uveal melanoma is an orphan disease that is biologically distinct from cutaneous melanoma. Treatment consists of surgery to remove the tumor or eye, as well as radiation therapy or chemotherapy. About half of cases metastasize, and survival for these patients is 9-12 months.
In this study, 98 patients with metastatic melanoma of the eye were randomly assigned to receive selumetinib or temozolomide (Temodar), with 48 receiving selumetinib and 50 receiving temozolomide. Based on imaging studies using RECIST (Response Evaluation Criteria in Solid Tumors), patients whose disease worsened on temozolomide were permitted to cross over to selumetinib.
Looking at the response patterns, tumors regressed in 11% (RECIST response, 0%) of 46 evaluable patients in the temozolomide arm and in 50% (RECIST response, 15%) of 46 evaluable patients in the selumetinib arm, for a highly significant hazard ratio of 0.46, reported Dr. Carvajal of Memorial Sloan-Kettering Cancer Center in New York.
Progression-free survival was improved to nearly 16 weeks for selumetinib as compared with 7 weeks for temozolomide. Overall survival was 10.8 months for selumetinib and 9.4 months with temozolomide.
More than 90% of patients in both arms of the study had metastatic liver disease and more than 50% had an elevated lactate dehydrogenase, Dr. Carvajal noted. Further, the study allowed for crossover to selumetinib by temozolomide-treated patients with no evidence of response at 4 weeks; 80% of patients in the temozolomide arm crossed over to selumetinib.
Selumetinib blocks the MEK protein, a key component of the MAPK pathway, which is activated by Gnaq and Gna11 gene mutations, found in 84% of the uveal melanoma patients in the study. Selumetinib also is being investigated for the treatment of cancers of the thyroid and lung, and trials are underway with selumetinib in combination with other drugs.
Dr. Carvajal noted that another MEK inhibitor, trametinib (Mekinist, GlaxoSmithKline), recently became the first MEK inhibitor to be approved by the Food and Drug Administration. Trametinib was approved in May 2013 as a single-agent oral treatment for unresectable or metastatic melanoma in adult patients with BRAF V600E or V600K mutations.
AstraZeneca is developing selumetinib under a licensing agreement with Array Biopharma.
The selumetinib study was supported in part by a Conquer Cancer Foundation of ASCO Career Development Award, the National Institutes of Health, Cycle for Survival, and the Fund for Ophthalmic Knowledge. Dr. Carvajal had no relevant financial disclosures.
On Twitter @maryjodales
CHICAGO – Selumetinib, an investigational MEK 1/2 inhibitor, shrank uveal melanomas in half of all treated patients, with a duration of disease control that was more than twice that achieved with the comparator therapy temozolomide, based on the final analysis of data from 98 patients in a phase II crossover study.
This is the first clinical trial to identify a drug that improves clinical outcome in patients with advanced melanoma of the eye, said Dr. Richard D. Carvajal, who presented the data at the annual meeting of the American Society of Clinical Oncology.
"Selumetinib is a new standard of therapy for uveal metastatic melanoma," he said at a press conference announcing the results. Before this study, there was no evidence that any systemic therapy was truly effective in this disease. In eight different clinical trials conducted in the last decade, 2 of 157 patients have responded to potential new therapies, including chemotherapy, targeted therapy, and immunotherapy.
“Right now, there are no plans for an official compassionate use program; however, we are reopening up our trial without the randomization such that additional patients can receive selumetinib through this mechanism,” he said in an interview after the meeting.
With about 2,000 cases diagnosed each year in the United States, uveal melanoma is an orphan disease that is biologically distinct from cutaneous melanoma. Treatment consists of surgery to remove the tumor or eye, as well as radiation therapy or chemotherapy. About half of cases metastasize, and survival for these patients is 9-12 months.
In this study, 98 patients with metastatic melanoma of the eye were randomly assigned to receive selumetinib or temozolomide (Temodar), with 48 receiving selumetinib and 50 receiving temozolomide. Based on imaging studies using RECIST (Response Evaluation Criteria in Solid Tumors), patients whose disease worsened on temozolomide were permitted to cross over to selumetinib.
Looking at the response patterns, tumors regressed in 11% (RECIST response, 0%) of 46 evaluable patients in the temozolomide arm and in 50% (RECIST response, 15%) of 46 evaluable patients in the selumetinib arm, for a highly significant hazard ratio of 0.46, reported Dr. Carvajal of Memorial Sloan-Kettering Cancer Center in New York.
Progression-free survival was improved to nearly 16 weeks for selumetinib as compared with 7 weeks for temozolomide. Overall survival was 10.8 months for selumetinib and 9.4 months with temozolomide.
More than 90% of patients in both arms of the study had metastatic liver disease and more than 50% had an elevated lactate dehydrogenase, Dr. Carvajal noted. Further, the study allowed for crossover to selumetinib by temozolomide-treated patients with no evidence of response at 4 weeks; 80% of patients in the temozolomide arm crossed over to selumetinib.
Selumetinib blocks the MEK protein, a key component of the MAPK pathway, which is activated by Gnaq and Gna11 gene mutations, found in 84% of the uveal melanoma patients in the study. Selumetinib also is being investigated for the treatment of cancers of the thyroid and lung, and trials are underway with selumetinib in combination with other drugs.
Dr. Carvajal noted that another MEK inhibitor, trametinib (Mekinist, GlaxoSmithKline), recently became the first MEK inhibitor to be approved by the Food and Drug Administration. Trametinib was approved in May 2013 as a single-agent oral treatment for unresectable or metastatic melanoma in adult patients with BRAF V600E or V600K mutations.
AstraZeneca is developing selumetinib under a licensing agreement with Array Biopharma.
The selumetinib study was supported in part by a Conquer Cancer Foundation of ASCO Career Development Award, the National Institutes of Health, Cycle for Survival, and the Fund for Ophthalmic Knowledge. Dr. Carvajal had no relevant financial disclosures.
On Twitter @maryjodales
CHICAGO – Selumetinib, an investigational MEK 1/2 inhibitor, shrank uveal melanomas in half of all treated patients, with a duration of disease control that was more than twice that achieved with the comparator therapy temozolomide, based on the final analysis of data from 98 patients in a phase II crossover study.
This is the first clinical trial to identify a drug that improves clinical outcome in patients with advanced melanoma of the eye, said Dr. Richard D. Carvajal, who presented the data at the annual meeting of the American Society of Clinical Oncology.
"Selumetinib is a new standard of therapy for uveal metastatic melanoma," he said at a press conference announcing the results. Before this study, there was no evidence that any systemic therapy was truly effective in this disease. In eight different clinical trials conducted in the last decade, 2 of 157 patients have responded to potential new therapies, including chemotherapy, targeted therapy, and immunotherapy.
“Right now, there are no plans for an official compassionate use program; however, we are reopening up our trial without the randomization such that additional patients can receive selumetinib through this mechanism,” he said in an interview after the meeting.
With about 2,000 cases diagnosed each year in the United States, uveal melanoma is an orphan disease that is biologically distinct from cutaneous melanoma. Treatment consists of surgery to remove the tumor or eye, as well as radiation therapy or chemotherapy. About half of cases metastasize, and survival for these patients is 9-12 months.
In this study, 98 patients with metastatic melanoma of the eye were randomly assigned to receive selumetinib or temozolomide (Temodar), with 48 receiving selumetinib and 50 receiving temozolomide. Based on imaging studies using RECIST (Response Evaluation Criteria in Solid Tumors), patients whose disease worsened on temozolomide were permitted to cross over to selumetinib.
Looking at the response patterns, tumors regressed in 11% (RECIST response, 0%) of 46 evaluable patients in the temozolomide arm and in 50% (RECIST response, 15%) of 46 evaluable patients in the selumetinib arm, for a highly significant hazard ratio of 0.46, reported Dr. Carvajal of Memorial Sloan-Kettering Cancer Center in New York.
Progression-free survival was improved to nearly 16 weeks for selumetinib as compared with 7 weeks for temozolomide. Overall survival was 10.8 months for selumetinib and 9.4 months with temozolomide.
More than 90% of patients in both arms of the study had metastatic liver disease and more than 50% had an elevated lactate dehydrogenase, Dr. Carvajal noted. Further, the study allowed for crossover to selumetinib by temozolomide-treated patients with no evidence of response at 4 weeks; 80% of patients in the temozolomide arm crossed over to selumetinib.
Selumetinib blocks the MEK protein, a key component of the MAPK pathway, which is activated by Gnaq and Gna11 gene mutations, found in 84% of the uveal melanoma patients in the study. Selumetinib also is being investigated for the treatment of cancers of the thyroid and lung, and trials are underway with selumetinib in combination with other drugs.
Dr. Carvajal noted that another MEK inhibitor, trametinib (Mekinist, GlaxoSmithKline), recently became the first MEK inhibitor to be approved by the Food and Drug Administration. Trametinib was approved in May 2013 as a single-agent oral treatment for unresectable or metastatic melanoma in adult patients with BRAF V600E or V600K mutations.
AstraZeneca is developing selumetinib under a licensing agreement with Array Biopharma.
The selumetinib study was supported in part by a Conquer Cancer Foundation of ASCO Career Development Award, the National Institutes of Health, Cycle for Survival, and the Fund for Ophthalmic Knowledge. Dr. Carvajal had no relevant financial disclosures.
On Twitter @maryjodales
AT THE ASCO ANNUAL MEETING 2013
Major finding: Tumors regressed in 11% (RECIST response, 0%) of 46 evaluable patients in the temozolomide arm and in 50% (RECIST response, 15%) of 46 evaluable patients in the selumetinib arm, for a significant hazard ratio of 0.46.
Data source: A randomized phase II crossover study of 98 patients with metastatic uveal melanoma
Disclosures: The selumetinib study was supported in part by a Conquer Cancer Foundation of ASCO Career Development Award, the National Institutes of Health, Cycle for Survival, and the Fund for Ophthalmic Knowledge. Dr. Carvajal had no relevant financial disclosures.