User login
FDA Clears AI-Powered Device for Noninvasive Skin Cancer Testing
The handheld wireless tool, which was developed by Miami-based DermaSensor Inc., operates on battery power, uses spectroscopy and algorithms to evaluate skin lesions for potential cancer in a matter of seconds, and is intended for use by primary care physicians. After the device completes the scan of a lesion, a result of “investigate further” (positive result) suggests further evaluation through a referral to a dermatologist, while “monitor” (negative result) suggests that there is no immediate need for a referral to a dermatologist.
In a pivotal trial of the device that evaluated 224 high risk lesions at 18 primary care study sites in the United States and 4 in Australia, the device had an overall sensitivity of 95.5% for detecting malignancy.
In a more recent validation study funded by DermaSensor, investigators tested 333 lesions at four U.S. dermatology offices and found that the overall device sensitivity was 97.04%, with subgroup sensitivity of 96.67% for melanoma, 97.22% for basal cell carcinoma, and 97.01% for squamous cell carcinoma. Overall specificity of the device was 26.22%.
The study authors, led by Tallahassee, Fla.–based dermatologist Armand B. Cognetta Jr., MD, concluded that DermaSensor’s rapid clinical analysis of lesions “allows for its easy integration into clinical practice infrastructures. Proper use of this device may aid in the reduction of morbidity and mortality associated with skin cancer through expedited and enhanced detection and intervention.”
According to marketing material from the DermaSensor website, the device’s AI algorithm was developed and validated with more than 20,000 scans, composed of more than 4,000 benign and malignant lesions. In a statement about the clearance, the FDA emphasized that the device “should not be used as the sole diagnostic criterion nor to confirm a diagnosis of skin cancer.” The agency is requiring that the manufacturer “conduct additional post-market clinical validation performance testing of the DermaSensor device in patients from demographic groups representative of the U.S. population, including populations who had limited representation of melanomas in the premarket studies, due to their having a relatively low incidence of the disease.”
According to a spokesperson for DermaSensor, pricing for the device is based on a subscription model: $199 per month for five patients or $399 per month for unlimited use. DermaSensor is currently commercially available in Europe and Australia.
Asked to comment, Vishal A. Patel, MD, director of cutaneous oncology at the George Washington Cancer Center, Washington, said that the FDA clearance of DermaSensor highlights the growing appreciation of AI-driven diagnostic support for primary care providers and dermatologists. "Skin cancers are a growing epidemic in the US and the ability to accurately identify potential suspicious lesions without immediately reaching for the scalpel is invaluable," Patel told this news organization. He was not involved with DermSensor studies.
"Furthermore, this tool can help address the shortage of dermatologists and long wait times by helping primary care providers accurately risk-stratify patients and identify those who need to be seen immediately for potential biopsy and expert care," he added. "However, just like with any new technology, we must use caution to not overutilize this tool," which he said, could "lead to overdiagnosis and overtreatment of early or innocuous lesions that are better managed with empiric field treatments."
Dr. Cognetta was a paid investigator for the study.
Dr. Patel disclosed that he is chief medical officer for Lazarus AI.
The handheld wireless tool, which was developed by Miami-based DermaSensor Inc., operates on battery power, uses spectroscopy and algorithms to evaluate skin lesions for potential cancer in a matter of seconds, and is intended for use by primary care physicians. After the device completes the scan of a lesion, a result of “investigate further” (positive result) suggests further evaluation through a referral to a dermatologist, while “monitor” (negative result) suggests that there is no immediate need for a referral to a dermatologist.
In a pivotal trial of the device that evaluated 224 high risk lesions at 18 primary care study sites in the United States and 4 in Australia, the device had an overall sensitivity of 95.5% for detecting malignancy.
In a more recent validation study funded by DermaSensor, investigators tested 333 lesions at four U.S. dermatology offices and found that the overall device sensitivity was 97.04%, with subgroup sensitivity of 96.67% for melanoma, 97.22% for basal cell carcinoma, and 97.01% for squamous cell carcinoma. Overall specificity of the device was 26.22%.
The study authors, led by Tallahassee, Fla.–based dermatologist Armand B. Cognetta Jr., MD, concluded that DermaSensor’s rapid clinical analysis of lesions “allows for its easy integration into clinical practice infrastructures. Proper use of this device may aid in the reduction of morbidity and mortality associated with skin cancer through expedited and enhanced detection and intervention.”
According to marketing material from the DermaSensor website, the device’s AI algorithm was developed and validated with more than 20,000 scans, composed of more than 4,000 benign and malignant lesions. In a statement about the clearance, the FDA emphasized that the device “should not be used as the sole diagnostic criterion nor to confirm a diagnosis of skin cancer.” The agency is requiring that the manufacturer “conduct additional post-market clinical validation performance testing of the DermaSensor device in patients from demographic groups representative of the U.S. population, including populations who had limited representation of melanomas in the premarket studies, due to their having a relatively low incidence of the disease.”
According to a spokesperson for DermaSensor, pricing for the device is based on a subscription model: $199 per month for five patients or $399 per month for unlimited use. DermaSensor is currently commercially available in Europe and Australia.
Asked to comment, Vishal A. Patel, MD, director of cutaneous oncology at the George Washington Cancer Center, Washington, said that the FDA clearance of DermaSensor highlights the growing appreciation of AI-driven diagnostic support for primary care providers and dermatologists. "Skin cancers are a growing epidemic in the US and the ability to accurately identify potential suspicious lesions without immediately reaching for the scalpel is invaluable," Patel told this news organization. He was not involved with DermSensor studies.
"Furthermore, this tool can help address the shortage of dermatologists and long wait times by helping primary care providers accurately risk-stratify patients and identify those who need to be seen immediately for potential biopsy and expert care," he added. "However, just like with any new technology, we must use caution to not overutilize this tool," which he said, could "lead to overdiagnosis and overtreatment of early or innocuous lesions that are better managed with empiric field treatments."
Dr. Cognetta was a paid investigator for the study.
Dr. Patel disclosed that he is chief medical officer for Lazarus AI.
The handheld wireless tool, which was developed by Miami-based DermaSensor Inc., operates on battery power, uses spectroscopy and algorithms to evaluate skin lesions for potential cancer in a matter of seconds, and is intended for use by primary care physicians. After the device completes the scan of a lesion, a result of “investigate further” (positive result) suggests further evaluation through a referral to a dermatologist, while “monitor” (negative result) suggests that there is no immediate need for a referral to a dermatologist.
In a pivotal trial of the device that evaluated 224 high risk lesions at 18 primary care study sites in the United States and 4 in Australia, the device had an overall sensitivity of 95.5% for detecting malignancy.
In a more recent validation study funded by DermaSensor, investigators tested 333 lesions at four U.S. dermatology offices and found that the overall device sensitivity was 97.04%, with subgroup sensitivity of 96.67% for melanoma, 97.22% for basal cell carcinoma, and 97.01% for squamous cell carcinoma. Overall specificity of the device was 26.22%.
The study authors, led by Tallahassee, Fla.–based dermatologist Armand B. Cognetta Jr., MD, concluded that DermaSensor’s rapid clinical analysis of lesions “allows for its easy integration into clinical practice infrastructures. Proper use of this device may aid in the reduction of morbidity and mortality associated with skin cancer through expedited and enhanced detection and intervention.”
According to marketing material from the DermaSensor website, the device’s AI algorithm was developed and validated with more than 20,000 scans, composed of more than 4,000 benign and malignant lesions. In a statement about the clearance, the FDA emphasized that the device “should not be used as the sole diagnostic criterion nor to confirm a diagnosis of skin cancer.” The agency is requiring that the manufacturer “conduct additional post-market clinical validation performance testing of the DermaSensor device in patients from demographic groups representative of the U.S. population, including populations who had limited representation of melanomas in the premarket studies, due to their having a relatively low incidence of the disease.”
According to a spokesperson for DermaSensor, pricing for the device is based on a subscription model: $199 per month for five patients or $399 per month for unlimited use. DermaSensor is currently commercially available in Europe and Australia.
Asked to comment, Vishal A. Patel, MD, director of cutaneous oncology at the George Washington Cancer Center, Washington, said that the FDA clearance of DermaSensor highlights the growing appreciation of AI-driven diagnostic support for primary care providers and dermatologists. "Skin cancers are a growing epidemic in the US and the ability to accurately identify potential suspicious lesions without immediately reaching for the scalpel is invaluable," Patel told this news organization. He was not involved with DermSensor studies.
"Furthermore, this tool can help address the shortage of dermatologists and long wait times by helping primary care providers accurately risk-stratify patients and identify those who need to be seen immediately for potential biopsy and expert care," he added. "However, just like with any new technology, we must use caution to not overutilize this tool," which he said, could "lead to overdiagnosis and overtreatment of early or innocuous lesions that are better managed with empiric field treatments."
Dr. Cognetta was a paid investigator for the study.
Dr. Patel disclosed that he is chief medical officer for Lazarus AI.
Coming Soon: The First mRNA Vaccine for Melanoma?
Moderna and Merck have presented promising results from their phase 2b clinical trial that investigated a combination of a messenger RNA (mRNA) vaccine and a cancer drug for the treatment of melanoma.
Is mRNA set to shake up the world of cancer treatment? This is certainly what Moderna seems to think; the pharmaceutical company has published the results of a phase 2b trial combining its mRNA vaccine (mRNA-4157 [V940]) with Merck’s cancer drug KEYTRUDA. While these are not the final results but rather mid-term data from the 3-year follow-up, they are somewhat promising. The randomized KEYNOTE-942/mRNA-4157-P201 clinical trial involves patients with high-risk (stage III/IV) melanoma following complete resection.
Relapse Risk Halved
Treatment with mRNA-4157 (V940) in combination with pembrolizumab led to a clinically meaningful improvement in recurrence-free survival, reducing the risk for recurrence or death by 49%, compared with pembrolizumab alone. T, reducing the risk of developing distant metastasis or death by 62%. “The KEYNOTE-942/mRNA-4157-P201 study was the first demonstration of efficacy for an investigational mRNA cancer treatment in a randomized clinical trial and the first combination therapy to show a significant benefit over pembrolizumab alone in adjuvant melanoma,” said Kyle Holen, MD, Moderna’s senior vice president, after presenting these results.
Side Effects
The combined treatment also did not demonstrate more significant side effects than pembrolizumab alone. The number of patients reporting treatment-related adverse events of grade 3 or greater was similar between the arms (25% for mRNA-4157 [V940] with pembrolizumab vs 20% for KEYTRUDA alone). The most common adverse events of any grade attributed to mRNA-4157 (V940) were fatigue (60.6%), injection site pain (56.7%), and chills (49%). Based on data from the phase 2b KEYNOTE-942/mRNA-4157-P201 study, the US Food and Drug Administration and European Medicines Agency granted breakthrough therapy designation and recognition under the the Priority Medicines scheme, respectively, for mRNA-4157 (V940) in combination with KEYTRUDA for the adjuvant treatment of patients with high-risk melanoma.
Phase 3 Trial
In July, Moderna and Merck announced the launch of a phase 3 trial, assessing “mRNA-4157 [V940] in combination with pembrolizumab as adjuvant treatment in patients with high-risk resected melanoma [stages IIB-IV].” Stéphane Bancel, Moderna’s director general, believes that an mRNA vaccine for melanoma could be available in 2025.
Other Cancer Vaccines
Moderna is not the only laboratory to set its sights on developing a vaccine for cancer. In May, BioNTech, in partnership with Roche, proposed a phase 1 clinical trial of a vaccine targeting pancreatic cancer in Nature. In June, at the American Society of Clinical Oncology›s conference, Transgene presented its conclusions concerning its viral vector vaccines against ENT and papillomavirus-linked cancers. And in September, Ose Immunotherapeutics made headlines with its vaccine for advanced lung cancer.
This article was translated from Univadis France, which is part of the Medscape Professional Network.
Moderna and Merck have presented promising results from their phase 2b clinical trial that investigated a combination of a messenger RNA (mRNA) vaccine and a cancer drug for the treatment of melanoma.
Is mRNA set to shake up the world of cancer treatment? This is certainly what Moderna seems to think; the pharmaceutical company has published the results of a phase 2b trial combining its mRNA vaccine (mRNA-4157 [V940]) with Merck’s cancer drug KEYTRUDA. While these are not the final results but rather mid-term data from the 3-year follow-up, they are somewhat promising. The randomized KEYNOTE-942/mRNA-4157-P201 clinical trial involves patients with high-risk (stage III/IV) melanoma following complete resection.
Relapse Risk Halved
Treatment with mRNA-4157 (V940) in combination with pembrolizumab led to a clinically meaningful improvement in recurrence-free survival, reducing the risk for recurrence or death by 49%, compared with pembrolizumab alone. T, reducing the risk of developing distant metastasis or death by 62%. “The KEYNOTE-942/mRNA-4157-P201 study was the first demonstration of efficacy for an investigational mRNA cancer treatment in a randomized clinical trial and the first combination therapy to show a significant benefit over pembrolizumab alone in adjuvant melanoma,” said Kyle Holen, MD, Moderna’s senior vice president, after presenting these results.
Side Effects
The combined treatment also did not demonstrate more significant side effects than pembrolizumab alone. The number of patients reporting treatment-related adverse events of grade 3 or greater was similar between the arms (25% for mRNA-4157 [V940] with pembrolizumab vs 20% for KEYTRUDA alone). The most common adverse events of any grade attributed to mRNA-4157 (V940) were fatigue (60.6%), injection site pain (56.7%), and chills (49%). Based on data from the phase 2b KEYNOTE-942/mRNA-4157-P201 study, the US Food and Drug Administration and European Medicines Agency granted breakthrough therapy designation and recognition under the the Priority Medicines scheme, respectively, for mRNA-4157 (V940) in combination with KEYTRUDA for the adjuvant treatment of patients with high-risk melanoma.
Phase 3 Trial
In July, Moderna and Merck announced the launch of a phase 3 trial, assessing “mRNA-4157 [V940] in combination with pembrolizumab as adjuvant treatment in patients with high-risk resected melanoma [stages IIB-IV].” Stéphane Bancel, Moderna’s director general, believes that an mRNA vaccine for melanoma could be available in 2025.
Other Cancer Vaccines
Moderna is not the only laboratory to set its sights on developing a vaccine for cancer. In May, BioNTech, in partnership with Roche, proposed a phase 1 clinical trial of a vaccine targeting pancreatic cancer in Nature. In June, at the American Society of Clinical Oncology›s conference, Transgene presented its conclusions concerning its viral vector vaccines against ENT and papillomavirus-linked cancers. And in September, Ose Immunotherapeutics made headlines with its vaccine for advanced lung cancer.
This article was translated from Univadis France, which is part of the Medscape Professional Network.
Moderna and Merck have presented promising results from their phase 2b clinical trial that investigated a combination of a messenger RNA (mRNA) vaccine and a cancer drug for the treatment of melanoma.
Is mRNA set to shake up the world of cancer treatment? This is certainly what Moderna seems to think; the pharmaceutical company has published the results of a phase 2b trial combining its mRNA vaccine (mRNA-4157 [V940]) with Merck’s cancer drug KEYTRUDA. While these are not the final results but rather mid-term data from the 3-year follow-up, they are somewhat promising. The randomized KEYNOTE-942/mRNA-4157-P201 clinical trial involves patients with high-risk (stage III/IV) melanoma following complete resection.
Relapse Risk Halved
Treatment with mRNA-4157 (V940) in combination with pembrolizumab led to a clinically meaningful improvement in recurrence-free survival, reducing the risk for recurrence or death by 49%, compared with pembrolizumab alone. T, reducing the risk of developing distant metastasis or death by 62%. “The KEYNOTE-942/mRNA-4157-P201 study was the first demonstration of efficacy for an investigational mRNA cancer treatment in a randomized clinical trial and the first combination therapy to show a significant benefit over pembrolizumab alone in adjuvant melanoma,” said Kyle Holen, MD, Moderna’s senior vice president, after presenting these results.
Side Effects
The combined treatment also did not demonstrate more significant side effects than pembrolizumab alone. The number of patients reporting treatment-related adverse events of grade 3 or greater was similar between the arms (25% for mRNA-4157 [V940] with pembrolizumab vs 20% for KEYTRUDA alone). The most common adverse events of any grade attributed to mRNA-4157 (V940) were fatigue (60.6%), injection site pain (56.7%), and chills (49%). Based on data from the phase 2b KEYNOTE-942/mRNA-4157-P201 study, the US Food and Drug Administration and European Medicines Agency granted breakthrough therapy designation and recognition under the the Priority Medicines scheme, respectively, for mRNA-4157 (V940) in combination with KEYTRUDA for the adjuvant treatment of patients with high-risk melanoma.
Phase 3 Trial
In July, Moderna and Merck announced the launch of a phase 3 trial, assessing “mRNA-4157 [V940] in combination with pembrolizumab as adjuvant treatment in patients with high-risk resected melanoma [stages IIB-IV].” Stéphane Bancel, Moderna’s director general, believes that an mRNA vaccine for melanoma could be available in 2025.
Other Cancer Vaccines
Moderna is not the only laboratory to set its sights on developing a vaccine for cancer. In May, BioNTech, in partnership with Roche, proposed a phase 1 clinical trial of a vaccine targeting pancreatic cancer in Nature. In June, at the American Society of Clinical Oncology›s conference, Transgene presented its conclusions concerning its viral vector vaccines against ENT and papillomavirus-linked cancers. And in September, Ose Immunotherapeutics made headlines with its vaccine for advanced lung cancer.
This article was translated from Univadis France, which is part of the Medscape Professional Network.
PRAME Expression in Melanocytic Proliferations in Special Sites
The assessment and diagnosis of melanocytic lesions can present a formidable challenge to even a seasoned pathologist, which is especially true when dealing with the subset of nevi occurring at special sites—where baseline variations inherent to particular locations on the body can preclude the use of features routinely used to diagnose malignancy elsewhere. These so-called special-site nevi previously have been described in the literature along with suggested criteria for differentiating malignant lesions from their benign counterparts.1 Locations generally considered to be special sites include the acral skin, anogenital region, breast, ear, and flexural regions.1,2
When evaluating non–special-site melanocytic lesions, general characteristics associated with a malignant diagnosis include confluence or pagetoid spread of melanocytes, nuclear pleomorphism, cytologic atypia, and irregular architecture3; however, these features can be compatible with a benign diagnosis in special-site nevi depending on their extent and the site in question. Although they can be atypical, special-site nevi tend to have the bulk of their architectural distortion and cytologic atypia in the center of the lesion as opposed to the edges.1 If a given lesion is from a special site but lacks this reassuring feature, special care should be taken to rule out malignancy.
Preferentially expressed antigen in melanoma (PRAME) is an antigen first identified in tumor-reactive T-cell populations in patients with malignant melanoma. It is the product of an oncogene that frequently is overexpressed in melanomas, lung squamous cell carcinomas, sarcomas, and acute leukemias.4 It functions as an antagonist of the retinoic acid signaling pathway, which normally serves to induce further cell differentiation, senescence, or apoptosis.5 PRAME inhibits retinoid signaling by forming a complex with both the ligand-bound retinoic acid holoreceptor and the polycomb protein EZH2, which blocks retinoid-dependent gene expression by encouraging chromatin condensation at the RARβ promoter site5; therefore, expressing PRAME allows lesional cells a substantial growth advantage.
PRAME expression has been extensively characterized in non–special-site nevi and has filled the need for a rather specific marker of melanoma.6-10 Although PRAME has been studied in acral nevi,11 the expression pattern in nevi of special sites has yet to be elucidated. Herein, we present a dataset characterizing PRAME expression in these challenging lesions.
Methods
We performed a retrospective case review at the University of Virginia (Charlottesville, Virginia) and collected a panel of 36 special-site nevi that previously were diagnosed as benign by a trained dermatopathologist from January 2020 through December 2022. Special-site nevi were identified using a natural language filter for the following terms: acral, palm, sole, ear, auricular, lip, axilla, armpit, breast, groin, labia, vulva, umbilicus, and penis. This study was approved by the University of Virginia institutional review board.
The original hematoxylin and eosin slides used for primary diagnosis were re-examined to verify the prior diagnosis of benign nevus at a special site. We performed a detailed microscopic examination of all benign nevi in our cohort to determine the frequency of various characteristics at each special site. Sections were prepared from the formalin-fixed and paraffin-embedded tissue blocks and stained with a commercial PRAME antibody (#219650 [Abcam] at a 1:50 dilution) and counterstain. A trained dermatopathologist (S.S.R.) examined the stained sections and recorded the percentage of tumor cells with nuclear PRAME staining. We reported our results using previously established criteria for scoring PRAME immunohistochemistry7: 0 for no expression, 1+ for 1% to 25% expression, 2+ for 26% to 50% expression, 3+ for 51% to 75% expression, and 4+ for diffuse or 76% to 100% expression. Only strong clonal expression within a population of cells was graded.
Data handling and statistical testing were performed using the R Project for Statistical Computing (https://www.r-project.org/). Significance testing was performed using the Fisher exact test. Plot construction was performed using ggplot2 (https://ggplot2.tidyverse.org/).
Results
Our study cohort included 36 special-site nevi, and the control cohort comprised 25 melanoma in situ (MIS) or invasive melanoma (IM) lesions occurring at special sites. Table 1 provides a breakdown of the study and control cohorts by lesion site. Table 2 details the results of our microscopic examination, describing frequency of various characteristics of special-site nevi stratified by site.
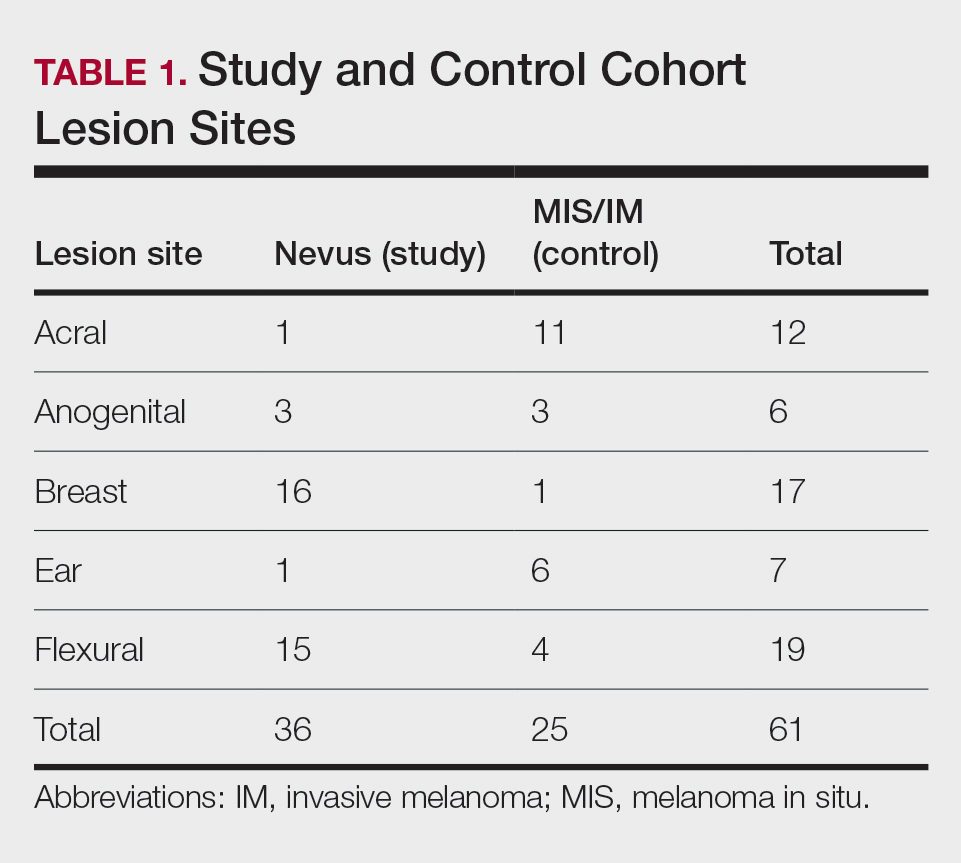
Of the 36 special-site nevi in our cohort, 20 (56%) had no staining (0) for PRAME, 11 (31%) demonstrated 1+ PRAME expression, 3 (8%) demonstrated 2+ PRAME expression, and 2 (6%) demonstrated 3+ PRAME expression. No nevi showed 4+ expression. In the control cohort, 24 of 25 (96%) MIS and IM showed 3+ or 4+ expression, with 21 (84%) demonstrating diffuse/4+ expression. One control case (4%) demonstrated 0 PRAME expression. These data are summarized in Table 3 and Figure 1. There is a significant difference in diffuse (4+) PRAME expression between special-site nevi and MIS/IM occurring at special sites (P=1.039×10-12).
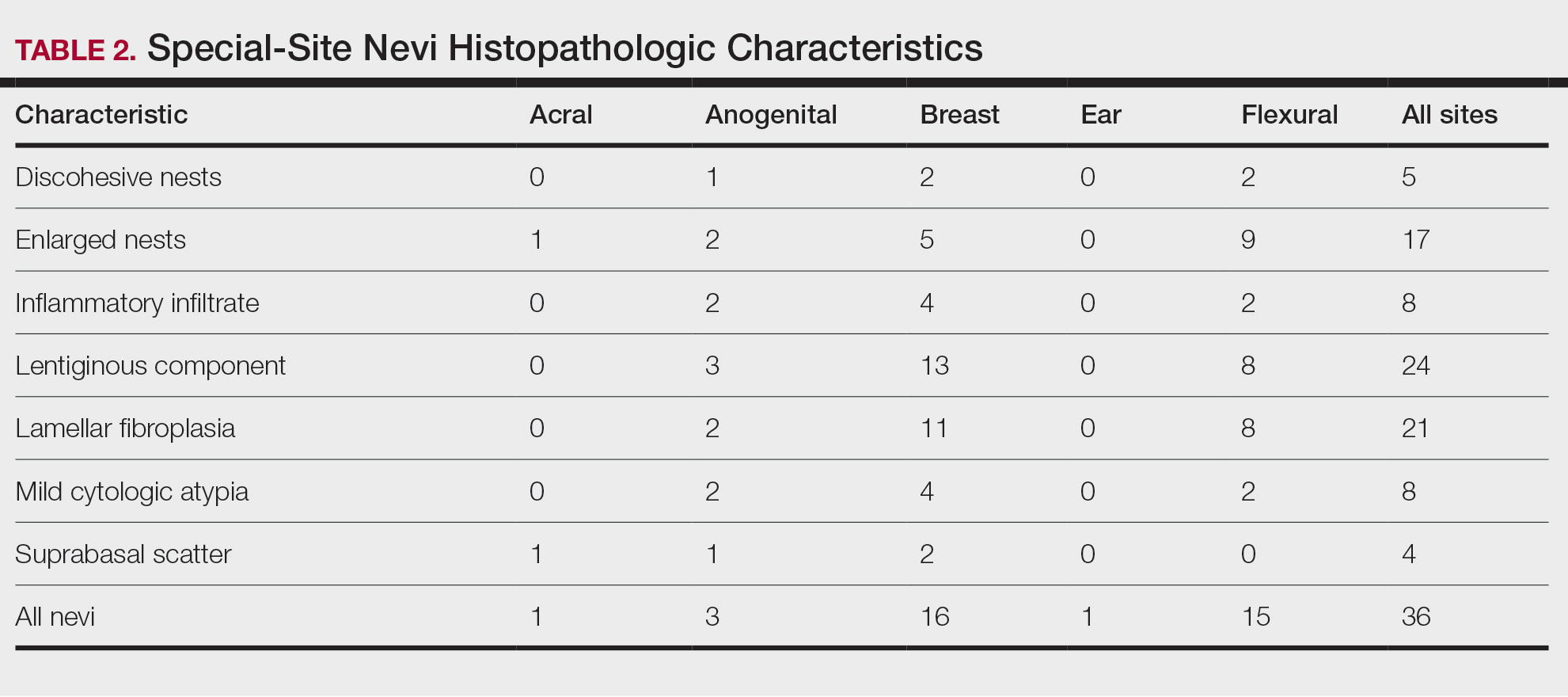
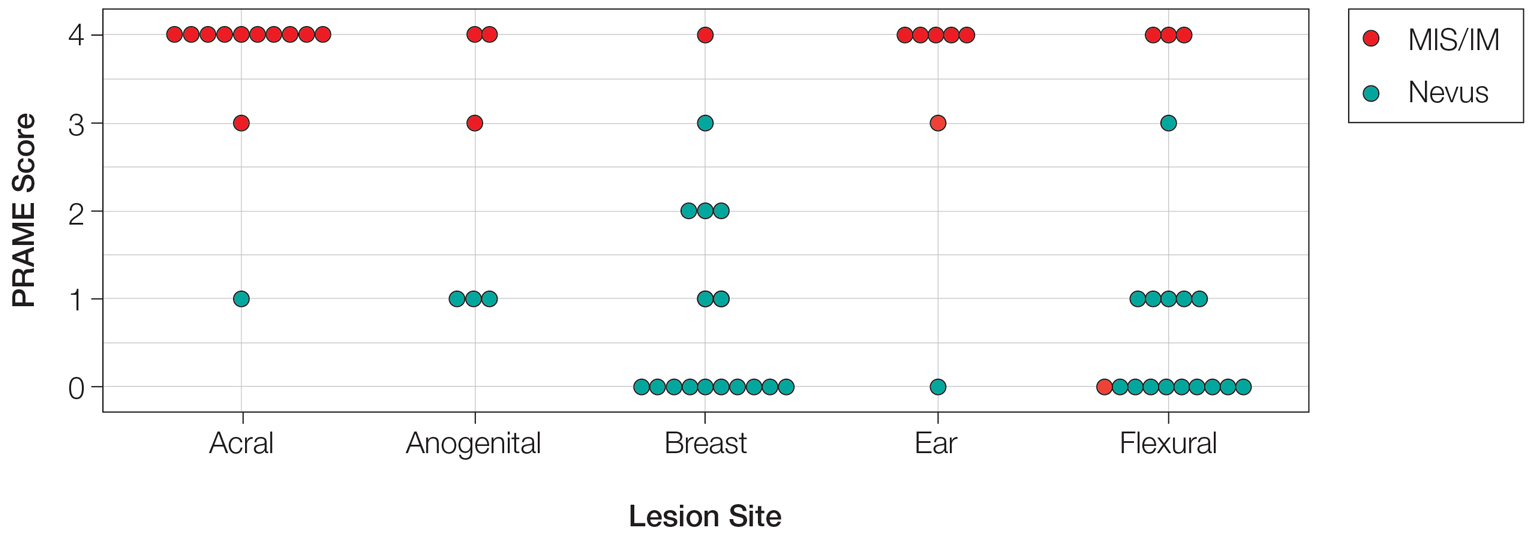
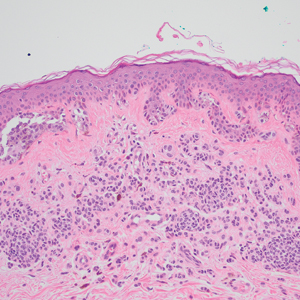
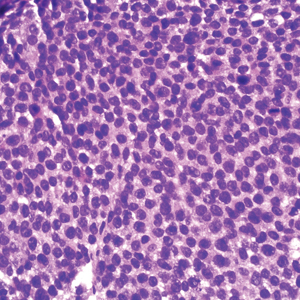
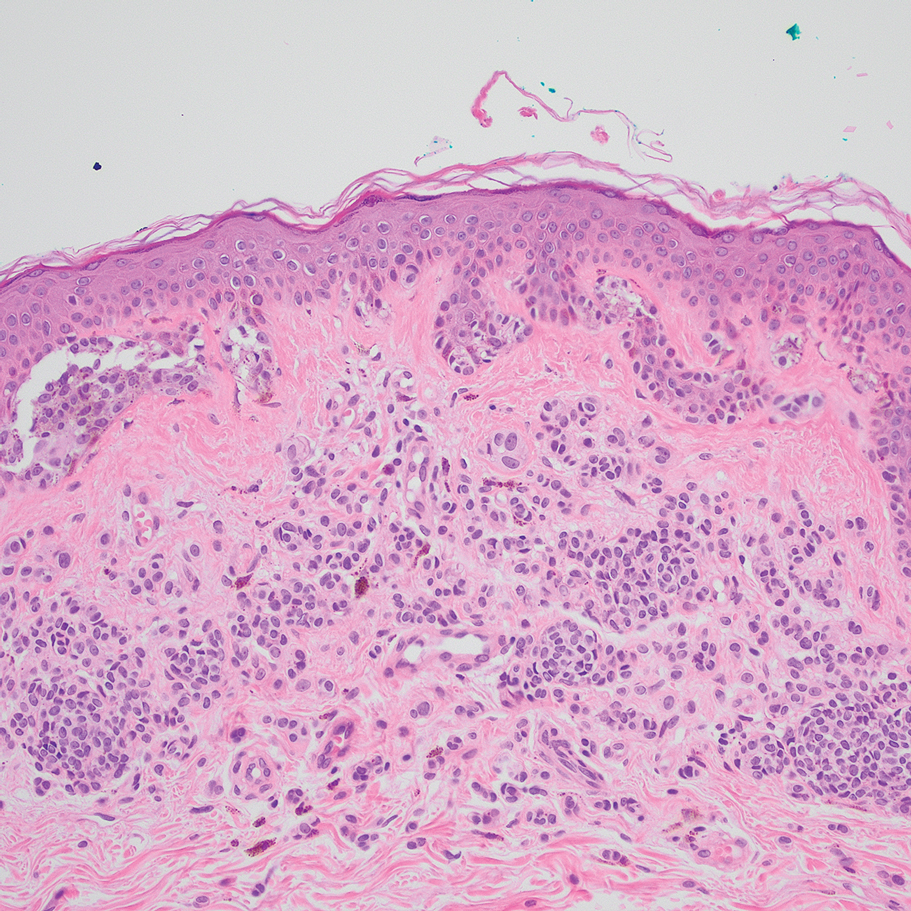
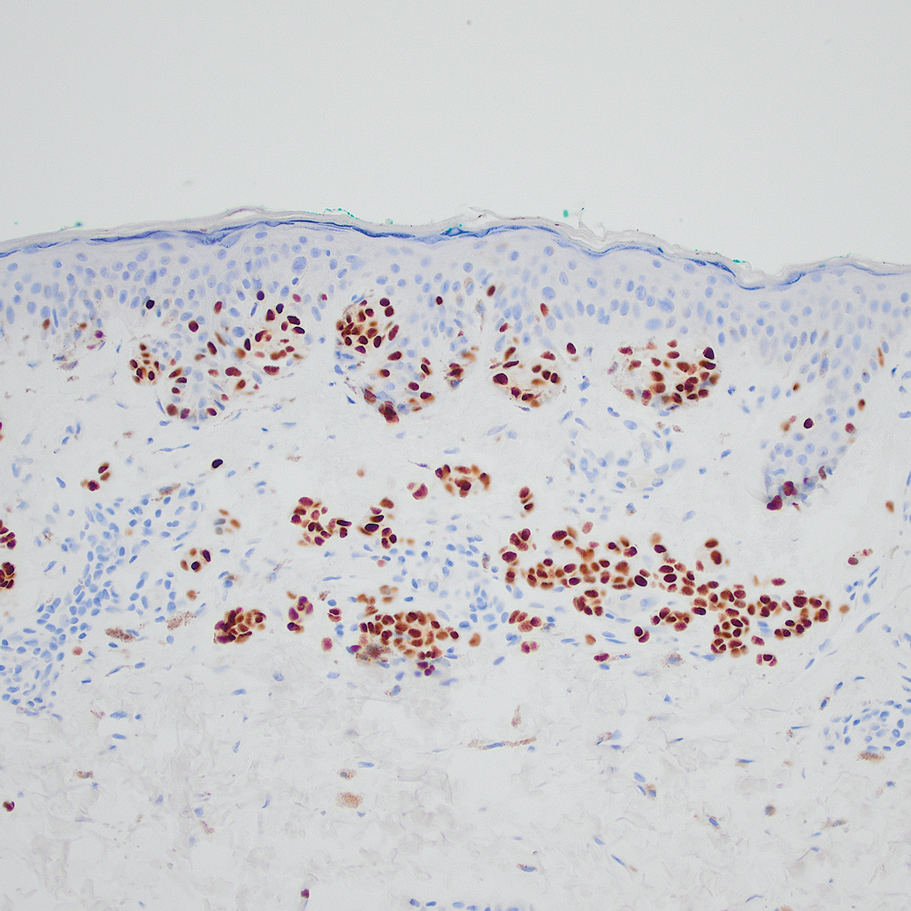
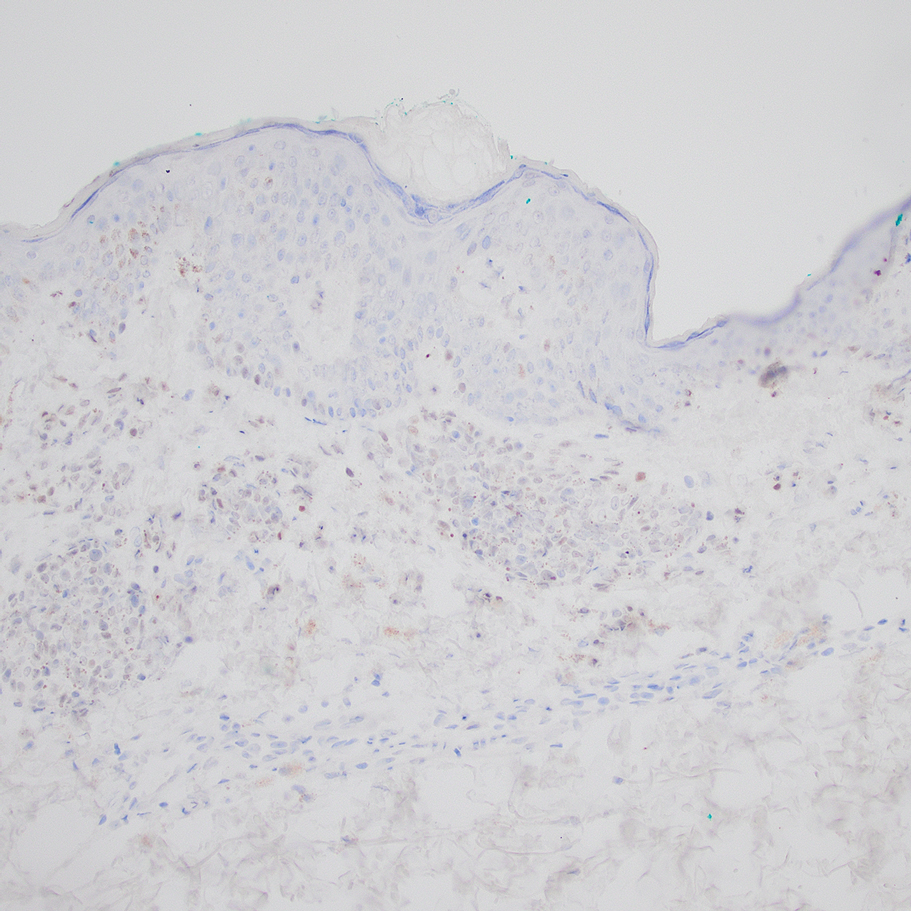
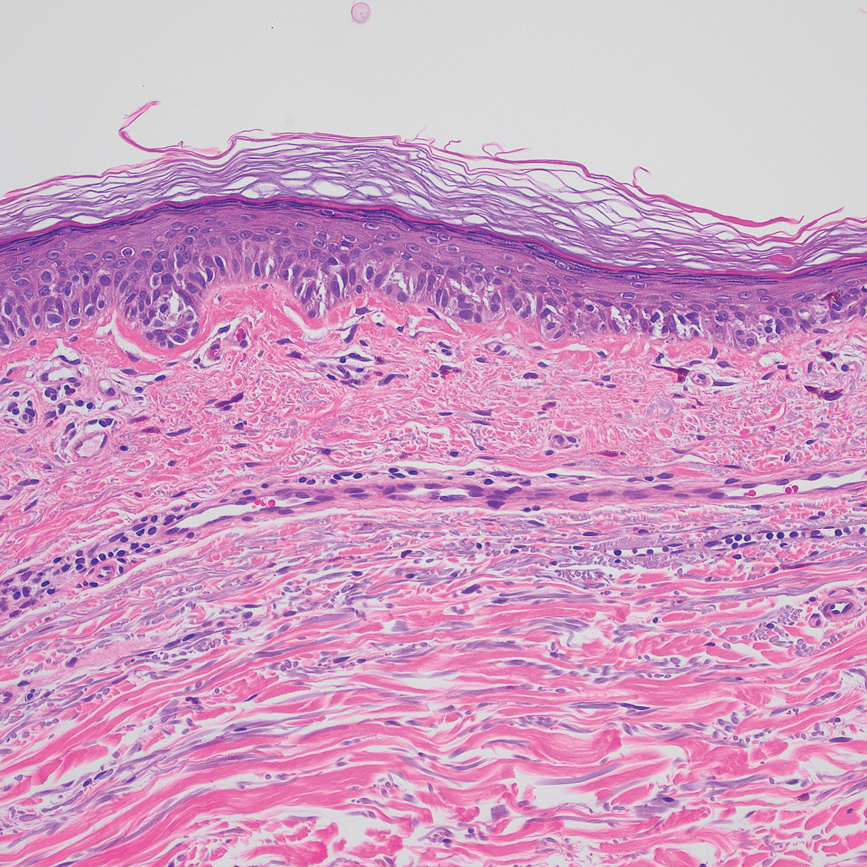
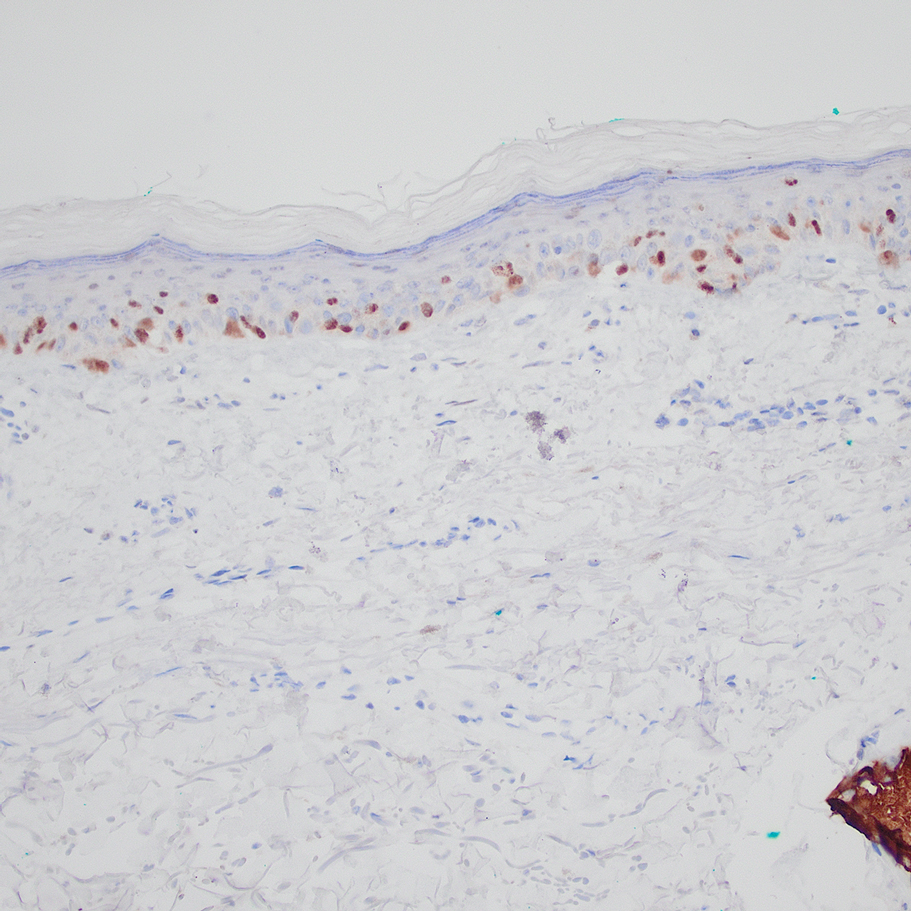
Based on our cohort, a positivity threshold of 3+ for PRAME expression for the diagnosis of melanoma in a special-site lesion would have a sensitivity of 96% and a specificity of 94%, while a positivity threshold of 4+ for PRAME expression would have a sensitivity of 84% and a specificity of 100%. Figures 2 through 4 show photomicrographs of a special-site nevus of the breast, which appropriately does not stain for PRAME; Figures 5 and 6 show an MIS at a special site that appropriately stains for PRAME.

Comment
The distinction between benign and malignant pigmented lesions at special sites presents a fair challenge for pathologists due to the larger degree of leniency for architectural distortion and cytologic atypia in benign lesions at these sites. The presence of architectural distortion or cytologic atypia at the lesion’s edge makes rendering a benign diagnosis especially difficult, and the need for a validated immunohistochemical stain is apparent. In our cohort, strong clonal PRAME expression provided a reliable immunohistochemical marker, allowing for the distinction of malignant lesions from benign nevi at special sites. Diffuse faint PRAME expression was present in several benign nevi within our cohort, and these lesions were considered negative (0) in our analysis.
Given the described test characteristics, we support the implementation of PRAME immunohistochemistry with a positivity threshold of 4+ expression as an ancillary test supporting the diagnosis of IM or MIS in special sites, which would allow clinicians to leverage the high specificity of 4+ PRAME expression to distinguish an IM or MIS from a benign nevus occurring at a special site. We do not recommend the use of 4+ PRAME expression as a screening test for melanoma or MIS among special-site nevi due to its comparatively low sensitivity; however, no one marker is always reliable, and we recommend continued clinicopathologic correlation for all cases.
Although our case series included nevi and MIS/IM from all special sites, we were limited in the number of acrogenital and ear nevi included due to a relative paucity of biopsied benign nevi from these locations at the University of Virginia. Additionally, although the magnitude of the difference in PRAME expression between the study and control groups is sufficient to demonstrate statistical significance, the overall strength of our argument would be increased with a larger study group. We were limited by the number of cases available at our institution, which did not utilize PRAME during the initial diagnosis of the case; including these cases in the study group would have undermined the integrity of our argument because the differentiation of benign vs malignant initially was made using PRAME immunohistochemistry.
Conclusion
Due to their atypical features, special-site nevi can be challenging to assess. In this study, we showed that PRAME expression can be a reliable marker to distinguish benign from malignant lesions. Our results showed that 100% of benign special-site nevi demonstrated 3+ expression or less, with 56% (20/36) demonstrating no expression at all. The presence of diffuse PRAME expression (4+ PRAME staining) appears to be a specific indicator of a malignant lesion, but results should always be interpreted with respect to the patient’s clinical history and the lesion’s histomorphologic features. Further study of a larger sample size would allow refinement of the sensitivity and specificity of diffuse PRAME expression in the determination of malignancy for special-site lesions.
Acknowledgment—The authors thank the pathologistsat the University of Virginia Biorepository and Tissue Research Facility (Charlottesville, Virginia) for their skill and expertise in performing immunohistochemical staining for this study.
- VandenBoom T, Gerami P. Melanocytic nevi of special sites. In: Pathology of Melanocytic Tumors. Elsevier; 2019:90-100. doi:10.1016/B978-0-323-37457-6.00007-9
- Hosler GA, Moresi JM, Barrett TL. Nevi with site-related atypia: a review of melanocytic nevi with atypical histologic features based on anatomic site. J Cutan Pathol. 2008;35:889-898. doi:10.1111/j.1600-0560.2008.01041.x.
- Brenn T. Melanocytic lesions—staying out of trouble. Ann Diagn Pathol. 2018;37:91-102. doi:10.1016/j.anndiagpath.2018.09.010
- Ikeda H, Lethé B, Lehmann F, et al. Characterization of an antigen that is recognized on a melanoma showing partial HLA loss by CTL expressing an NK inhibitory receptor. Immunity. 1997;6:199-208. doi:10.1016/s1074-7613(00)80426-4
- Epping MT, Wang L, Edel MJ, et al. The human tumor antigen PRAME is a dominant repressor of retinoic acid receptor signaling. Cell. 2005;122:835-847. doi:10.1016/j.cell.2005.07.003
- Alomari AK, Tharp AW, Umphress B, et al. The utility of PRAME immunohistochemistry in the evaluation of challenging melanocytic tumors. J Cutan Pathol. 2021;48:1115-1123. doi:10.1111/cup.14000
- Lezcano C, Jungbluth AA, Nehal KS, et al. PRAME expression in melanocytic tumors. Am J Surg Pathol. 2018;42:1456-1465. doi:10.1097/PAS.0000000000001134
- Gill P, Prieto VG, Austin MT, et al. Diagnostic utility of PRAME in distinguishing proliferative nodules from melanoma in giant congenital melanocytic nevi. J Cutan Pathol. 2021;48:1410-1415. doi:10.1111/cup.14091
- Googe PB, Flanigan KL, Miedema JR. Preferentially expressed antigen in melanoma immunostaining in a series of melanocytic neoplasms. Am J Dermatopathol. 2021;43):794-800. doi:10.1097/DAD.0000000000001885
- Raghavan SS, Wang JY, Kwok S, et al. PRAME expression in melanocytic proliferations with intermediate histopathologic or spitzoid features. J Cutan Pathol. 2020;47:1123-1131. doi:10.1111/cup.13818
- McBride JD, McAfee JL, Piliang M, et al. Preferentially expressed antigen in melanoma and p16 expression in acral melanocytic neoplasms. J Cutan Pathol. 2022;49:220-230. doi:10.1111/cup.14130
The assessment and diagnosis of melanocytic lesions can present a formidable challenge to even a seasoned pathologist, which is especially true when dealing with the subset of nevi occurring at special sites—where baseline variations inherent to particular locations on the body can preclude the use of features routinely used to diagnose malignancy elsewhere. These so-called special-site nevi previously have been described in the literature along with suggested criteria for differentiating malignant lesions from their benign counterparts.1 Locations generally considered to be special sites include the acral skin, anogenital region, breast, ear, and flexural regions.1,2
When evaluating non–special-site melanocytic lesions, general characteristics associated with a malignant diagnosis include confluence or pagetoid spread of melanocytes, nuclear pleomorphism, cytologic atypia, and irregular architecture3; however, these features can be compatible with a benign diagnosis in special-site nevi depending on their extent and the site in question. Although they can be atypical, special-site nevi tend to have the bulk of their architectural distortion and cytologic atypia in the center of the lesion as opposed to the edges.1 If a given lesion is from a special site but lacks this reassuring feature, special care should be taken to rule out malignancy.
Preferentially expressed antigen in melanoma (PRAME) is an antigen first identified in tumor-reactive T-cell populations in patients with malignant melanoma. It is the product of an oncogene that frequently is overexpressed in melanomas, lung squamous cell carcinomas, sarcomas, and acute leukemias.4 It functions as an antagonist of the retinoic acid signaling pathway, which normally serves to induce further cell differentiation, senescence, or apoptosis.5 PRAME inhibits retinoid signaling by forming a complex with both the ligand-bound retinoic acid holoreceptor and the polycomb protein EZH2, which blocks retinoid-dependent gene expression by encouraging chromatin condensation at the RARβ promoter site5; therefore, expressing PRAME allows lesional cells a substantial growth advantage.
PRAME expression has been extensively characterized in non–special-site nevi and has filled the need for a rather specific marker of melanoma.6-10 Although PRAME has been studied in acral nevi,11 the expression pattern in nevi of special sites has yet to be elucidated. Herein, we present a dataset characterizing PRAME expression in these challenging lesions.
Methods
We performed a retrospective case review at the University of Virginia (Charlottesville, Virginia) and collected a panel of 36 special-site nevi that previously were diagnosed as benign by a trained dermatopathologist from January 2020 through December 2022. Special-site nevi were identified using a natural language filter for the following terms: acral, palm, sole, ear, auricular, lip, axilla, armpit, breast, groin, labia, vulva, umbilicus, and penis. This study was approved by the University of Virginia institutional review board.
The original hematoxylin and eosin slides used for primary diagnosis were re-examined to verify the prior diagnosis of benign nevus at a special site. We performed a detailed microscopic examination of all benign nevi in our cohort to determine the frequency of various characteristics at each special site. Sections were prepared from the formalin-fixed and paraffin-embedded tissue blocks and stained with a commercial PRAME antibody (#219650 [Abcam] at a 1:50 dilution) and counterstain. A trained dermatopathologist (S.S.R.) examined the stained sections and recorded the percentage of tumor cells with nuclear PRAME staining. We reported our results using previously established criteria for scoring PRAME immunohistochemistry7: 0 for no expression, 1+ for 1% to 25% expression, 2+ for 26% to 50% expression, 3+ for 51% to 75% expression, and 4+ for diffuse or 76% to 100% expression. Only strong clonal expression within a population of cells was graded.
Data handling and statistical testing were performed using the R Project for Statistical Computing (https://www.r-project.org/). Significance testing was performed using the Fisher exact test. Plot construction was performed using ggplot2 (https://ggplot2.tidyverse.org/).
Results
Our study cohort included 36 special-site nevi, and the control cohort comprised 25 melanoma in situ (MIS) or invasive melanoma (IM) lesions occurring at special sites. Table 1 provides a breakdown of the study and control cohorts by lesion site. Table 2 details the results of our microscopic examination, describing frequency of various characteristics of special-site nevi stratified by site.

Of the 36 special-site nevi in our cohort, 20 (56%) had no staining (0) for PRAME, 11 (31%) demonstrated 1+ PRAME expression, 3 (8%) demonstrated 2+ PRAME expression, and 2 (6%) demonstrated 3+ PRAME expression. No nevi showed 4+ expression. In the control cohort, 24 of 25 (96%) MIS and IM showed 3+ or 4+ expression, with 21 (84%) demonstrating diffuse/4+ expression. One control case (4%) demonstrated 0 PRAME expression. These data are summarized in Table 3 and Figure 1. There is a significant difference in diffuse (4+) PRAME expression between special-site nevi and MIS/IM occurring at special sites (P=1.039×10-12).


Based on our cohort, a positivity threshold of 3+ for PRAME expression for the diagnosis of melanoma in a special-site lesion would have a sensitivity of 96% and a specificity of 94%, while a positivity threshold of 4+ for PRAME expression would have a sensitivity of 84% and a specificity of 100%. Figures 2 through 4 show photomicrographs of a special-site nevus of the breast, which appropriately does not stain for PRAME; Figures 5 and 6 show an MIS at a special site that appropriately stains for PRAME.

Comment
The distinction between benign and malignant pigmented lesions at special sites presents a fair challenge for pathologists due to the larger degree of leniency for architectural distortion and cytologic atypia in benign lesions at these sites. The presence of architectural distortion or cytologic atypia at the lesion’s edge makes rendering a benign diagnosis especially difficult, and the need for a validated immunohistochemical stain is apparent. In our cohort, strong clonal PRAME expression provided a reliable immunohistochemical marker, allowing for the distinction of malignant lesions from benign nevi at special sites. Diffuse faint PRAME expression was present in several benign nevi within our cohort, and these lesions were considered negative (0) in our analysis.

Given the described test characteristics, we support the implementation of PRAME immunohistochemistry with a positivity threshold of 4+ expression as an ancillary test supporting the diagnosis of IM or MIS in special sites, which would allow clinicians to leverage the high specificity of 4+ PRAME expression to distinguish an IM or MIS from a benign nevus occurring at a special site. We do not recommend the use of 4+ PRAME expression as a screening test for melanoma or MIS among special-site nevi due to its comparatively low sensitivity; however, no one marker is always reliable, and we recommend continued clinicopathologic correlation for all cases.

Although our case series included nevi and MIS/IM from all special sites, we were limited in the number of acrogenital and ear nevi included due to a relative paucity of biopsied benign nevi from these locations at the University of Virginia. Additionally, although the magnitude of the difference in PRAME expression between the study and control groups is sufficient to demonstrate statistical significance, the overall strength of our argument would be increased with a larger study group. We were limited by the number of cases available at our institution, which did not utilize PRAME during the initial diagnosis of the case; including these cases in the study group would have undermined the integrity of our argument because the differentiation of benign vs malignant initially was made using PRAME immunohistochemistry.

Conclusion
Due to their atypical features, special-site nevi can be challenging to assess. In this study, we showed that PRAME expression can be a reliable marker to distinguish benign from malignant lesions. Our results showed that 100% of benign special-site nevi demonstrated 3+ expression or less, with 56% (20/36) demonstrating no expression at all. The presence of diffuse PRAME expression (4+ PRAME staining) appears to be a specific indicator of a malignant lesion, but results should always be interpreted with respect to the patient’s clinical history and the lesion’s histomorphologic features. Further study of a larger sample size would allow refinement of the sensitivity and specificity of diffuse PRAME expression in the determination of malignancy for special-site lesions.


Acknowledgment—The authors thank the pathologistsat the University of Virginia Biorepository and Tissue Research Facility (Charlottesville, Virginia) for their skill and expertise in performing immunohistochemical staining for this study.
The assessment and diagnosis of melanocytic lesions can present a formidable challenge to even a seasoned pathologist, which is especially true when dealing with the subset of nevi occurring at special sites—where baseline variations inherent to particular locations on the body can preclude the use of features routinely used to diagnose malignancy elsewhere. These so-called special-site nevi previously have been described in the literature along with suggested criteria for differentiating malignant lesions from their benign counterparts.1 Locations generally considered to be special sites include the acral skin, anogenital region, breast, ear, and flexural regions.1,2
When evaluating non–special-site melanocytic lesions, general characteristics associated with a malignant diagnosis include confluence or pagetoid spread of melanocytes, nuclear pleomorphism, cytologic atypia, and irregular architecture3; however, these features can be compatible with a benign diagnosis in special-site nevi depending on their extent and the site in question. Although they can be atypical, special-site nevi tend to have the bulk of their architectural distortion and cytologic atypia in the center of the lesion as opposed to the edges.1 If a given lesion is from a special site but lacks this reassuring feature, special care should be taken to rule out malignancy.
Preferentially expressed antigen in melanoma (PRAME) is an antigen first identified in tumor-reactive T-cell populations in patients with malignant melanoma. It is the product of an oncogene that frequently is overexpressed in melanomas, lung squamous cell carcinomas, sarcomas, and acute leukemias.4 It functions as an antagonist of the retinoic acid signaling pathway, which normally serves to induce further cell differentiation, senescence, or apoptosis.5 PRAME inhibits retinoid signaling by forming a complex with both the ligand-bound retinoic acid holoreceptor and the polycomb protein EZH2, which blocks retinoid-dependent gene expression by encouraging chromatin condensation at the RARβ promoter site5; therefore, expressing PRAME allows lesional cells a substantial growth advantage.
PRAME expression has been extensively characterized in non–special-site nevi and has filled the need for a rather specific marker of melanoma.6-10 Although PRAME has been studied in acral nevi,11 the expression pattern in nevi of special sites has yet to be elucidated. Herein, we present a dataset characterizing PRAME expression in these challenging lesions.
Methods
We performed a retrospective case review at the University of Virginia (Charlottesville, Virginia) and collected a panel of 36 special-site nevi that previously were diagnosed as benign by a trained dermatopathologist from January 2020 through December 2022. Special-site nevi were identified using a natural language filter for the following terms: acral, palm, sole, ear, auricular, lip, axilla, armpit, breast, groin, labia, vulva, umbilicus, and penis. This study was approved by the University of Virginia institutional review board.
The original hematoxylin and eosin slides used for primary diagnosis were re-examined to verify the prior diagnosis of benign nevus at a special site. We performed a detailed microscopic examination of all benign nevi in our cohort to determine the frequency of various characteristics at each special site. Sections were prepared from the formalin-fixed and paraffin-embedded tissue blocks and stained with a commercial PRAME antibody (#219650 [Abcam] at a 1:50 dilution) and counterstain. A trained dermatopathologist (S.S.R.) examined the stained sections and recorded the percentage of tumor cells with nuclear PRAME staining. We reported our results using previously established criteria for scoring PRAME immunohistochemistry7: 0 for no expression, 1+ for 1% to 25% expression, 2+ for 26% to 50% expression, 3+ for 51% to 75% expression, and 4+ for diffuse or 76% to 100% expression. Only strong clonal expression within a population of cells was graded.
Data handling and statistical testing were performed using the R Project for Statistical Computing (https://www.r-project.org/). Significance testing was performed using the Fisher exact test. Plot construction was performed using ggplot2 (https://ggplot2.tidyverse.org/).
Results
Our study cohort included 36 special-site nevi, and the control cohort comprised 25 melanoma in situ (MIS) or invasive melanoma (IM) lesions occurring at special sites. Table 1 provides a breakdown of the study and control cohorts by lesion site. Table 2 details the results of our microscopic examination, describing frequency of various characteristics of special-site nevi stratified by site.

Of the 36 special-site nevi in our cohort, 20 (56%) had no staining (0) for PRAME, 11 (31%) demonstrated 1+ PRAME expression, 3 (8%) demonstrated 2+ PRAME expression, and 2 (6%) demonstrated 3+ PRAME expression. No nevi showed 4+ expression. In the control cohort, 24 of 25 (96%) MIS and IM showed 3+ or 4+ expression, with 21 (84%) demonstrating diffuse/4+ expression. One control case (4%) demonstrated 0 PRAME expression. These data are summarized in Table 3 and Figure 1. There is a significant difference in diffuse (4+) PRAME expression between special-site nevi and MIS/IM occurring at special sites (P=1.039×10-12).


Based on our cohort, a positivity threshold of 3+ for PRAME expression for the diagnosis of melanoma in a special-site lesion would have a sensitivity of 96% and a specificity of 94%, while a positivity threshold of 4+ for PRAME expression would have a sensitivity of 84% and a specificity of 100%. Figures 2 through 4 show photomicrographs of a special-site nevus of the breast, which appropriately does not stain for PRAME; Figures 5 and 6 show an MIS at a special site that appropriately stains for PRAME.

Comment
The distinction between benign and malignant pigmented lesions at special sites presents a fair challenge for pathologists due to the larger degree of leniency for architectural distortion and cytologic atypia in benign lesions at these sites. The presence of architectural distortion or cytologic atypia at the lesion’s edge makes rendering a benign diagnosis especially difficult, and the need for a validated immunohistochemical stain is apparent. In our cohort, strong clonal PRAME expression provided a reliable immunohistochemical marker, allowing for the distinction of malignant lesions from benign nevi at special sites. Diffuse faint PRAME expression was present in several benign nevi within our cohort, and these lesions were considered negative (0) in our analysis.

Given the described test characteristics, we support the implementation of PRAME immunohistochemistry with a positivity threshold of 4+ expression as an ancillary test supporting the diagnosis of IM or MIS in special sites, which would allow clinicians to leverage the high specificity of 4+ PRAME expression to distinguish an IM or MIS from a benign nevus occurring at a special site. We do not recommend the use of 4+ PRAME expression as a screening test for melanoma or MIS among special-site nevi due to its comparatively low sensitivity; however, no one marker is always reliable, and we recommend continued clinicopathologic correlation for all cases.

Although our case series included nevi and MIS/IM from all special sites, we were limited in the number of acrogenital and ear nevi included due to a relative paucity of biopsied benign nevi from these locations at the University of Virginia. Additionally, although the magnitude of the difference in PRAME expression between the study and control groups is sufficient to demonstrate statistical significance, the overall strength of our argument would be increased with a larger study group. We were limited by the number of cases available at our institution, which did not utilize PRAME during the initial diagnosis of the case; including these cases in the study group would have undermined the integrity of our argument because the differentiation of benign vs malignant initially was made using PRAME immunohistochemistry.

Conclusion
Due to their atypical features, special-site nevi can be challenging to assess. In this study, we showed that PRAME expression can be a reliable marker to distinguish benign from malignant lesions. Our results showed that 100% of benign special-site nevi demonstrated 3+ expression or less, with 56% (20/36) demonstrating no expression at all. The presence of diffuse PRAME expression (4+ PRAME staining) appears to be a specific indicator of a malignant lesion, but results should always be interpreted with respect to the patient’s clinical history and the lesion’s histomorphologic features. Further study of a larger sample size would allow refinement of the sensitivity and specificity of diffuse PRAME expression in the determination of malignancy for special-site lesions.


Acknowledgment—The authors thank the pathologistsat the University of Virginia Biorepository and Tissue Research Facility (Charlottesville, Virginia) for their skill and expertise in performing immunohistochemical staining for this study.
- VandenBoom T, Gerami P. Melanocytic nevi of special sites. In: Pathology of Melanocytic Tumors. Elsevier; 2019:90-100. doi:10.1016/B978-0-323-37457-6.00007-9
- Hosler GA, Moresi JM, Barrett TL. Nevi with site-related atypia: a review of melanocytic nevi with atypical histologic features based on anatomic site. J Cutan Pathol. 2008;35:889-898. doi:10.1111/j.1600-0560.2008.01041.x.
- Brenn T. Melanocytic lesions—staying out of trouble. Ann Diagn Pathol. 2018;37:91-102. doi:10.1016/j.anndiagpath.2018.09.010
- Ikeda H, Lethé B, Lehmann F, et al. Characterization of an antigen that is recognized on a melanoma showing partial HLA loss by CTL expressing an NK inhibitory receptor. Immunity. 1997;6:199-208. doi:10.1016/s1074-7613(00)80426-4
- Epping MT, Wang L, Edel MJ, et al. The human tumor antigen PRAME is a dominant repressor of retinoic acid receptor signaling. Cell. 2005;122:835-847. doi:10.1016/j.cell.2005.07.003
- Alomari AK, Tharp AW, Umphress B, et al. The utility of PRAME immunohistochemistry in the evaluation of challenging melanocytic tumors. J Cutan Pathol. 2021;48:1115-1123. doi:10.1111/cup.14000
- Lezcano C, Jungbluth AA, Nehal KS, et al. PRAME expression in melanocytic tumors. Am J Surg Pathol. 2018;42:1456-1465. doi:10.1097/PAS.0000000000001134
- Gill P, Prieto VG, Austin MT, et al. Diagnostic utility of PRAME in distinguishing proliferative nodules from melanoma in giant congenital melanocytic nevi. J Cutan Pathol. 2021;48:1410-1415. doi:10.1111/cup.14091
- Googe PB, Flanigan KL, Miedema JR. Preferentially expressed antigen in melanoma immunostaining in a series of melanocytic neoplasms. Am J Dermatopathol. 2021;43):794-800. doi:10.1097/DAD.0000000000001885
- Raghavan SS, Wang JY, Kwok S, et al. PRAME expression in melanocytic proliferations with intermediate histopathologic or spitzoid features. J Cutan Pathol. 2020;47:1123-1131. doi:10.1111/cup.13818
- McBride JD, McAfee JL, Piliang M, et al. Preferentially expressed antigen in melanoma and p16 expression in acral melanocytic neoplasms. J Cutan Pathol. 2022;49:220-230. doi:10.1111/cup.14130
- VandenBoom T, Gerami P. Melanocytic nevi of special sites. In: Pathology of Melanocytic Tumors. Elsevier; 2019:90-100. doi:10.1016/B978-0-323-37457-6.00007-9
- Hosler GA, Moresi JM, Barrett TL. Nevi with site-related atypia: a review of melanocytic nevi with atypical histologic features based on anatomic site. J Cutan Pathol. 2008;35:889-898. doi:10.1111/j.1600-0560.2008.01041.x.
- Brenn T. Melanocytic lesions—staying out of trouble. Ann Diagn Pathol. 2018;37:91-102. doi:10.1016/j.anndiagpath.2018.09.010
- Ikeda H, Lethé B, Lehmann F, et al. Characterization of an antigen that is recognized on a melanoma showing partial HLA loss by CTL expressing an NK inhibitory receptor. Immunity. 1997;6:199-208. doi:10.1016/s1074-7613(00)80426-4
- Epping MT, Wang L, Edel MJ, et al. The human tumor antigen PRAME is a dominant repressor of retinoic acid receptor signaling. Cell. 2005;122:835-847. doi:10.1016/j.cell.2005.07.003
- Alomari AK, Tharp AW, Umphress B, et al. The utility of PRAME immunohistochemistry in the evaluation of challenging melanocytic tumors. J Cutan Pathol. 2021;48:1115-1123. doi:10.1111/cup.14000
- Lezcano C, Jungbluth AA, Nehal KS, et al. PRAME expression in melanocytic tumors. Am J Surg Pathol. 2018;42:1456-1465. doi:10.1097/PAS.0000000000001134
- Gill P, Prieto VG, Austin MT, et al. Diagnostic utility of PRAME in distinguishing proliferative nodules from melanoma in giant congenital melanocytic nevi. J Cutan Pathol. 2021;48:1410-1415. doi:10.1111/cup.14091
- Googe PB, Flanigan KL, Miedema JR. Preferentially expressed antigen in melanoma immunostaining in a series of melanocytic neoplasms. Am J Dermatopathol. 2021;43):794-800. doi:10.1097/DAD.0000000000001885
- Raghavan SS, Wang JY, Kwok S, et al. PRAME expression in melanocytic proliferations with intermediate histopathologic or spitzoid features. J Cutan Pathol. 2020;47:1123-1131. doi:10.1111/cup.13818
- McBride JD, McAfee JL, Piliang M, et al. Preferentially expressed antigen in melanoma and p16 expression in acral melanocytic neoplasms. J Cutan Pathol. 2022;49:220-230. doi:10.1111/cup.14130
Practice Points
- Special-site nevi are benign melanocytic proliferations at special anatomic sites. Although cytologic atypia and architectural distortion may be present, they are centrally located and should not be present at the borders of the lesion.
- Strong expression of the preferentially expressed antigen in melanoma (PRAME) via immunohistochemistry provides a reliable indicator for benignity in differentiating a special-site nevus from a malignant melanoma occurring at a special site.
New Therapies in Melanoma: Current Trends, Evolving Paradigms, and Future Perspectives
Cutaneous malignant melanoma represents an aggressive form of skin cancer, with 132,000 new cases of melanoma and 50,000 melanoma-related deaths diagnosed worldwide each year.1 In recent decades, major progress has been made in the treatment of melanoma, especially metastatic and advanced-stage disease. Approval of new treatments, such as immunotherapy with anti–PD-1 (pembrolizumab and nivolumab) and anti–CTLA-4 (ipilimumab) antibodies, has revolutionized therapeutic strategies (Figure 1). Molecularly, melanoma has the highest mutational burden among solid tumors. Approximately 40% of melanomas harbor the BRAF V600 mutation, leading to constitutive activation of the mitogen-activated protein kinase (MAPK) signaling pathway.2 The other described genomic subtypes are mutated RAS (accounting for approximately 28% of cases), mutated NF1 (approximately 14% of cases), and triple wild type, though these other subtypes have not been as successfully targeted with therapy to date.3 Dual inhibition of this pathway using combination therapy with BRAF and MEK inhibitors confers high response rates and survival benefit, though efficacy in metastatic patients often is limited by development of resistance. The US Food and Drug Administration (FDA) has approved 3 combinations of targeted therapy in unresectable tumors: dabrafenib and trametinib, vemurafenib and cobimetinib, and encorafenib and binimetinib. The oncolytic herpesvirus talimogene laherparepvec also has received FDA approval for local treatment of unresectable cutaneous, subcutaneous, and nodal lesions in patients with recurrent melanoma after initial surgery.2
In this review, we explore new therapeutic agents and novel combinations that are being tested in early-phase clinical trials (Table). We discuss newer promising tools such as nanotechnology to develop nanosystems that act as drug carriers and/or light absorbents to potentially improve therapy outcomes. Finally, we highlight challenges such as management after resistance and intervention with novel immunotherapies and the lack of predictive biomarkers to stratify patients to targeted treatments after primary treatment failure.
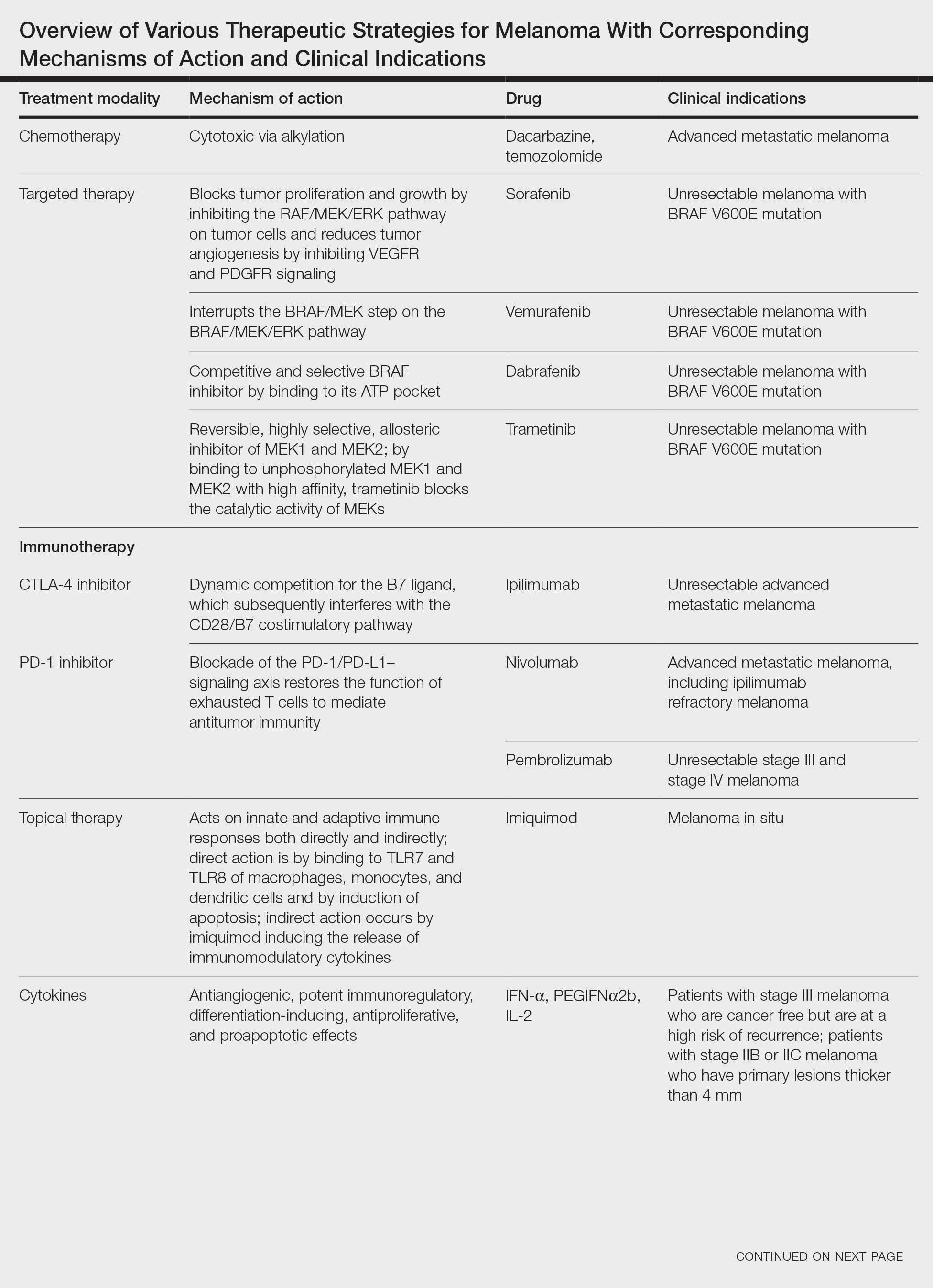
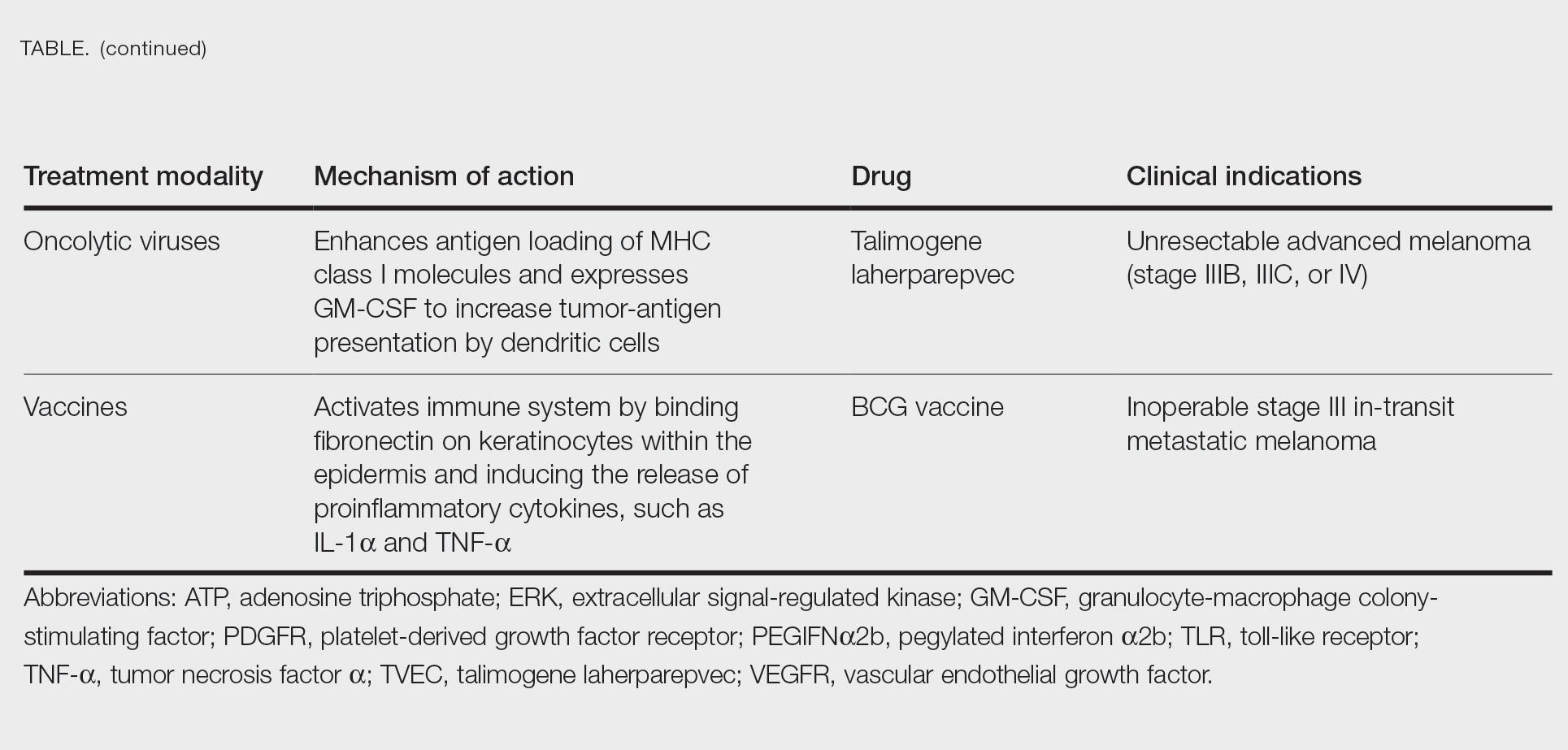
Targeted Therapies
Vemurafenib was approved by the FDA in 2011 and was the first BRAF-targeted therapy approved for the treatment of melanoma based on a 48% response rate and a 63% reduction in the risk for death vs dacarbazine chemotherapy.4 Despite a rapid and clinically significant initial response, progression-free survival (PFS) was only 5.3 months, which is indicative of the rapid development of resistance with monotherapy through MAPK reactivation. As a result, combined BRAF and MEK inhibition was introduced and is now the standard of care for targeted therapy in melanoma. Treatment with dabrafenib and trametinib, vemurafenib and cobimetinib, or encorafenib and binimetinib is associated with prolonged PFS and overall survival (OS) compared to BRAF inhibitor monotherapy, with response rates exceeding 60% and a complete response rate of 10% to 18%.5 Recently, combining atezolizumab with vemurafenib and cobimetinib was shown to improve PFS compared to combined targeted therapy.6 Targeted therapy usually is given as first-line treatment to symptomatic patients with a high tumor burden because the response may be more rapid than the response to immunotherapy. Ultimately, most patients with advanced BRAF-mutated melanoma receive both targeted therapy and immunotherapy.
Mutations of KIT (encoding proto-oncogene receptor tyrosine kinase) activate intracellular MAPK and phosphatidylinositol 3-kinase (PI3K) pathways (Figure 2).7 KIT mutations are found in mucosal and acral melanomas as well as chronically sun-damaged skin, with frequencies of 39%, 36%, and 28%, respectively. Imatinib was associated with a 53% response rate and PFS of 3.9 months among patients with KIT-mutated melanoma but failed to cause regression in melanomas with KIT amplification.8
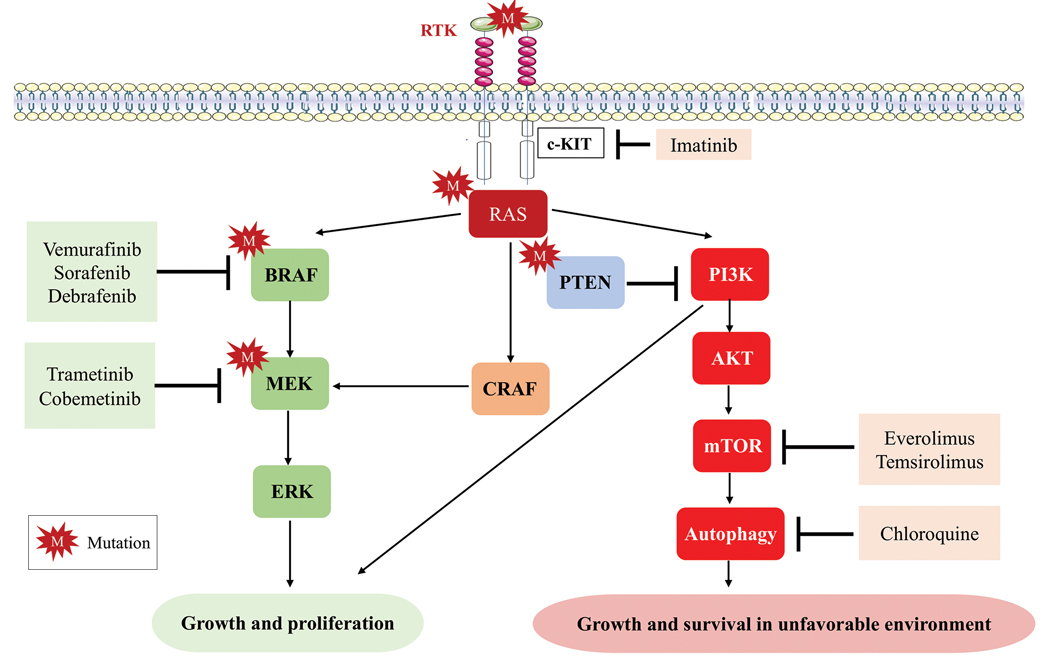
Anti–CTLA-4 Immune Checkpoint Inhibition
CTLA-4 is a protein found on T cells that binds with another protein, B7, preventing T cells from killing cancer cells. Hence, blockade of CTLA-4 antibody avoids the immunosuppressive state of lymphocytes, strengthening their antitumor action.9 Ipilimumab, an anti–CTLA-4 antibody, demonstrated improvement in median OS for management of unresectable or metastatic stage IV melanoma, resulting in its FDA approval.8 A combination of ipilimumab with dacarbazine in stage IV melanoma showed notable improvement of OS.10 Similarly, tremelimumab showed evidence of tumor regression in a phase 1 trial but with more severe immune-related side effects compared with ipilimumab.11 A second study on patients with stage IV melanoma treated with tremelimumab as first-line therapy in comparison with dacarbazine demonstrated differences in OS that were not statistically significant, though there was a longer duration of an objective response in patients treated with tremelimumab (35.8 months) compared with patients responding to dacarbazine (13.7 months).12
Anti–PD-1 Immune Checkpoint Inhibition
PD-1 is a transmembrane protein with immunoreceptor tyrosine-based inhibitory signaling, identified as an apoptosis-associated molecule.13 Upon activation, it is expressed on the cell surface of CD4, CD8, B lymphocytes, natural killer cells, monocytes, and dendritic cells.14 PD-L1, the ligand of PD-1, is constitutively expressed on different hematopoietic cells, as well as on fibroblasts, endothelial cells, mesenchymal cells, neurons, and keratinocytes.15,16 Reactivation of effector T lymphocytes by PD-1:PD-L1 pathway inhibition has shown clinically significant therapeutic relevance.17 The PD-1:PD-L1 interaction is active only in the presence of T- or B-cell antigen receptor cross-link. This interaction prevents PI3K/AKT signaling and MAPK/extracellular signal-regulated kinase pathway activation with the net result of lymphocytic functional exhaustion.18,19 PD-L1 blockade is shown to have better clinical benefit and minor toxicity compared to anti–CTLA-4 therapy. Treatment with anti-PD1 nivolumab in a phase 1b clinical trial (N=107) demonstrated highly specific action, durable tumor remission, and long-term safety in 32% of patients with advanced melanoma.20 These promising results led to the FDA approval of nivolumab for the treatment of patients with advanced and unresponsive melanoma. A recent clinical trial combining ipilimumab and nivolumab resulted in an impressive increase of PFS compared with ipilimumab monotherapy (11.5 months vs 2.9 months).21 Similarly, treatment with pembrolizumab in advanced melanoma demonstrated improvement in PFS and OS compared with anti–CTLA-4 therapy,22,23 which resulted in FDA approval of pembrolizumab for the treatment of advanced melanoma in patients previously treated with ipilimumab or BRAF inhibitors in BRAF V600 mutation–positive patients.24
Lymphocyte-Activated Gene 3–Targeted Therapies
Nanotechnology in Melanoma Therapy
The use of nanotechnology represents one of the newer alternative therapies employed for treatment of melanoma and is especially gaining interest due to reduced adverse effects in comparison with other conventional treatments for melanoma. Nanotechnology-based drug delivery systems precisely target tumor cells and improve the effect of both the conventional and innovative antineoplastic treatment.27,31 Tumor vasculature differs from normal tissues by being discontinuous and having interspersed small gaps/holes that allow nanoparticles to exit the circulation and enter and accumulate in the tumor tissue, leading to enhanced and targeted release of the antineoplastic drug to tumor cells.32 This mechanism is called the enhanced permeability and retention effect.33
Another mechanism by which nanoparticles work is ligand-based targeting in which ligands such as monoclonal antibodies, peptides, and nucleic acids located on the surface of nanoparticles can bind to receptors on the plasma membrane of tumor cells and lead to targeted delivery of the drug.34 Nanomaterials used for melanoma treatment include vesicular systems such as liposomes and niosomes, polymeric nanoparticles, noble metal-based nanoparticles, carbon nanotubes, dendrimers, solid lipid nanoparticles and nanostructures, lipid carriers, and microneedles. In melanoma, nanoparticles can be used to enhance targeted delivery of drugs, including immune checkpoint inhibitors (ICIs). Cai et al35 described usage of scaffolds in delivery systems. Tumor-associated antigens, adjuvant drugs, and chemical agents that influence the tumor microenvironment can be loaded onto these scaffolding agents. In a study by Zhu et al,36 photosensitizer chlorin e6 and immunoadjuvant aluminum hydroxide were used as a novel nanosystem that effectively destroyed tumor cells and induced a strong systemic antitumor response. IL-2 is a cytokine produced by B or T lymphocytes. Its use in melanoma has been limited by a severe adverse effect profile and lack of complete response in most patients. Cytokine-containing nanogels have been found to selectively release IL-2 in response to activation of T-cell receptors, and a mouse model in melanoma showed better response compared to free IL-1 and no adverse systemic effects.37
Nanovaccines represent another interesting novel immunotherapy modality. A study by Conniot et al38 showed that nanoparticles can be used in the treatment of melanoma. Nanoparticles made of biodegradable polymer were loaded with Melan-A/MART-1 (26–35 A27L) MHC class I-restricted peptide (MHC class I antigen), and the limited peptide MHC class II Melan-A/MART-1 51–73 (MHC class II antigen) and grafted with mannose that was then combined with an anti–PD-L1 antibody and injected into mouse models. This combination resulted in T-cell infiltration at early stages and increased infiltration of myeloid-derived suppressor cells. Ibrutinib, a myeloid-derived suppressor cell inhibitor, was added and demonstrated marked tumor remission and prolonged survival.38
Overexpression of certain microRNAs (miRNAs), especially miR-204-5p and miR-199b-5p, has been shown to inhibit growth of melanoma cells in vitro, both alone and in combination with MAPK inhibitors, but these miRNAs are easily degradable in body fluids. Lipid nanoparticles can bind these miRNAs and have been shown to inhibit tumor cell proliferation and improve efficacy of BRAF and MEK inhibitors.39
Triple-Combination Therapy
Immune checkpoint inhibitors such as anti–PD-1 or anti–CTLA-4 drugs have become the standard of care in treatment of advanced melanoma. Approximately 40% to 50% of cases of melanoma harbor BRAF mutations, and patients with these mutations could benefit from BRAF and MEK inhibitors. Data from clinical trials on BRAF and MEK inhibitors even showed initial high objective response rates, but the response was short-lived, and there was frequent acquired resistance.40 With ICIs, the major limitation was primary resistance, with only 50% of patients initially responding.41 Studies on murine models demonstrated that BRAF-mutated tumors had decreased expression of IFN-γ, tumor necrosis factor α, and CD40 ligand on CD4+ tumor-infiltrating lymphocytes and increased accumulation of regulatory T cells and myeloid-derived suppressor cells, leading to a protumor microenvironment. BRAF and MEK pathway inhibition were found to improve intratumoral CD4+ T-cell activity, leading to improved antitumor T-cell responses.42 Because of this enhanced immune response by BRAF and MEK inhibitors, it was hypothesized and later supported by clinical research that a combination of these targeted treatments and ICIs can have a synergistic effect, leading to increased antitumor activity.43 A randomized phase 2 clinical trial (KEYNOTE-022) in which the treatment group was given pembrolizumab, dabrafenib, and trametinib and the control group was treated with dabrafenib and trametinib showed increased medial OS in the treatment group vs the control group (46.3 months vs 26.3 months) and more frequent complete response in the treatment group vs the control group (20% vs 15%).44 In the IMspire150 phase 3 clinical trial, patients with advanced stage IIIC to IV BRAF-mutant melanoma were treated with either a triple combination of the PDL-1 inhibitor atezolizumab, vemurafenib, and cobimetinib or vemurafenib and cobimetinib. Although the objective response rate was similar in both groups, the median duration of response was longer in the triplet group compared with the doublet group (21 months vs 12.6 months). Given these results, the FDA approved the triple-combination therapy with atezolizumab, vemurafenib, and cobimetinib. Although triple-combination therapy has shown promising results, it is expected that there will be an increase in the frequency of treatment-related adverse effects. In the phase 3 COMBi-I study, patients with advanced stage IIIC to IV BRAF V600E mutant cutaneous melanoma were treated with either a combination of spartalizumab, dabrafenib, and trametinib or just dabrafenib and trametinib. Although the objective response rates were not significantly different (69% vs 64%), there was increased frequency of treatment-related adverse effects in patients receiving triple-combination therapy.43 As more follow-up data come out of these ongoing clinical trials, benefits of triple-combination therapy and its adverse effect profile will be more definitely established.
Challenges and Future Perspectives
One of the major roadblocks in the treatment of melanoma is the failure of response to ICI with CTLA-4 and PD-1/PD-L1 blockade in a large patient population, which has resulted in the need for new biomarkers that can act as potential therapeutic targets. Further, the main underlying factor for both adjuvant and neoadjuvant approaches remains the selection of patients, optimizing therapeutic outcomes while minimizing the number of patients exposed to potentially toxic treatments without gaining clinical benefit. Clinical and pathological factors (eg, Breslow thickness, ulceration, the number of positive lymph nodes) play a role in stratifying patients as per risk of recurrence.45 Similarly, peripheral blood biomarkers have been proposed as prognostic tools for high-risk stage II and III melanoma, including markers of systemic inflammation previously explored in the metastatic setting.46 However, the use of these parameters has not been validated for clinical practice. Currently, despite promising results of BRAF and MEK inhibitors and therapeutic ICIs, as well as IL-2 or interferon alfa, treatment options in metastatic melanoma are limited because of its high heterogeneity, problematic patient stratification, and high genetic mutational rate. Recently, the role of epigenetic modifications andmiRNAs in melanoma progression and metastatic spread has been described. Silencing of CDKN2A locus and encoding for p16INK4A and p14ARF by DNA methylation are noted in 27% and 57% of metastatic melanomas, respectively, which enables melanoma cells to escape from growth arrest and apoptosis generated by Rb protein and p53 pathways.47 Demethylation of these and other tumor suppressor genes with proapoptotic function (eg, RASSF1A and tumor necrosis factor–related apoptosis-inducing ligand) can restore cell death pathways, though future clinical studies in melanoma are warranted.48
- Geller AC, Clapp RW, Sober AJ, et al. Melanoma epidemic: an analysis of six decades of data from the Connecticut Tumor Registry. J Clin Oncol. 2013;31:4172-4178.
- Moreira A, Heinzerling L, Bhardwaj N, et al. Current melanoma treatments: where do we stand? Cancers (Basel). 2021;13:221.
- Watson IR, Wu C-J, Zou L, et al. Genomic classification of cutaneous melanoma. Cancer Res. 2015;75(15 Suppl):2972.
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507-2516.
- Hamid O, Cowey CL, Offner M, et al. Efficacy, safety, and tolerability of approved combination BRAF and MEK inhibitor regimens for BRAF-mutant melanoma. Cancers (Basel). 2019;11:1642.
- Gutzmer R, Stroyakovskiy D, Gogas H, et al. Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAFV600 mutation-positive melanoma (IMspire150): primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2020;395:1835-1844.
- Reddy BY, Miller DM, Tsao H. Somatic driver mutations in melanoma. Cancer. 2017;123(suppl 11):2104-2117.
- Hodi FS, Corless CL, Giobbie-Hurder A, et al. Imatinib for melanomas harboring mutationally activated or amplified KIT arising on mucosal, acral, and chronically sun-damaged skin. J Clin Oncol. 2013;31:3182-3190.
- Teft WA, Kirchhof MG, Madrenas J. A molecular perspective of CTLA-4 function. Annu Rev Immunol. 2006;24:65-97.
- Maverakis E, Cornelius LA, Bowen GM, et al. Metastatic melanoma—a review of current and future treatment options. Acta Derm Venereol. 2015;95:516-524.
- Ribas A, Chesney JA, Gordon MS, et al. Safety profile and pharmacokinetic analyses of the anti-CTLA4 antibody tremelimumab administered as a one hour infusion. J Transl Med. 2012;10:1-6.
- Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908-918.
- BG Neel, Gu H, Pao L. The ‘Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci. 2003;28:284-293.
- Ishida Y, Agata Y, Shibahara K, et al. Induced expression of PD‐1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887-3895.
- Yamazaki T, Akiba H, Iwai H, et al. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol. 2002;169:5538-5545.
- Keir ME, Butte MJ, Freeman GJ et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677-704.
- Blank C, Kuball J, Voelkl S, et al. Blockade of PD‐L1 (B7‐H1) augments human tumor‐specific T cell responses in vitro. Int J Cancer. 2006;119:317-327.
- Parry RV, Chemnitz JM, Frauwirth KA, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543-9553.
- Patsoukis N, Brown J, Petkova V, et al. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci Signal. 2012;5:ra46.
- Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020-1030.
- Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375-384.
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320-330.
- Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006-2017.
- Burns MC, O’Donnell A, Puzanov I. Pembrolizumab for the treatment of advanced melanoma. Exp Opin Orphan Drugs. 2016;4:867-873.
- F Triebel. LAG-3: a regulator of T-cell and DC responses and its use in therapeutic vaccination. Trends Immunol. 2003;24:619-622.
- Maruhashi T, Sugiura D, Okazaki I-M, et al. LAG-3: from molecular functions to clinical applications. J Immunother Cancer. 2020;8:e001014.
- Shi J, Kantoff PW, Wooster R, et al. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17:20-37.
- Tawbi HA, Schadendorf D, Lipson EJ, et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med. 2022;386:24-34.
- US Food and Drug Administration approves first LAG-3-blocking antibody combination, Opdualag™ (nivolumab and relatlimab-rmbw), as treatment for patients with unresectable or metastatic melanoma. Press release. Bristol Myers Squibb. March 18, 2022. Accessed November 7, 2023. https://news.bms.com/news/details/2022/U.S.-Food-and-Drug-Administration-Approves-First-LAG-3-Blocking-Antibody-Combination-Opdualag-nivolumab-and-relatlimab-rmbw-as-Treatment-for-Patients-with-Unresectable-or-Metastatic-Melanoma/default.aspx
- Zhao B-W, Zhang F-Y, Wang Y, et al. LAG3-PD1 or CTLA4-PD1 inhibition in advanced melanoma: indirect cross comparisons of the CheckMate-067 and RELATIVITY-047 trials. Cancers (Basel). 2022;14:4975.
- Jin C, Wang K, Oppong-Gyebi A, et al. Application of nanotechnology in cancer diagnosis and therapy-a mini-review. Int J Med Sci. 2020;17:2964-2973.
- Maeda H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv Drug Del Rev. 2015;91:3-6.
- Iyer AK, Khaled G, Fang J, et al. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today. 2006;11:812-818.
- Beiu C, Giurcaneanu C, Grumezescu AM, et al. Nanosystems for improved targeted therapies in melanoma. J Clin Med. 2020;9:318.
- Cai L, Xu J, Yang Z, et al. Engineered biomaterials for cancer immunotherapy. MedComm. 2020;1:35-46.
- Zhu Y, Xue J, Chen W, et al. Albumin-biomineralized nanoparticles to synergize phototherapy and immunotherapy against melanoma. J Control Release. 2020;322:300-311.
- Zhang Y, Li N, Suh H, et al. Nanoparticle anchoring targets immune agonists to tumors enabling anti-cancer immunity without systemic toxicity. Nat Commun. 2018;9:6.
- Conniot J, Scomparin A, Peres C, et al. Immunization with mannosylated nanovaccines and inhibition of the immune-suppressing microenvironment sensitizes melanoma to immune checkpoint modulators. Nat Nanotechnol. 2019;14:891-901.
- Fattore L, Campani V, Ruggiero CF, et al. In vitro biophysical and biological characterization of lipid nanoparticles co-encapsulating oncosuppressors miR-199b-5p and miR-204-5p as potentiators of target therapy in metastatic melanoma. Int J Mol Sci. 2020;21:1930.
- Welti M, Dimitriou F, Gutzmer R, et al. Triple combination of immune checkpoint inhibitors and BRAF/MEK inhibitors in BRAF V600 melanoma: current status and future perspectives. Cancers (Basel). 2022;14:5489.
- Khair DO, Bax HJ, Mele S, et al. Combining immune checkpoint inhibitors: established and emerging targets and strategies to improve outcomes in melanoma. Front Immunol. 2019;10:453.
- Ho P-C, Meeth KM, Tsui Y-C, et al. Immune-based antitumor effects of BRAF inhibitors rely on signaling by CD40L and IFNγBRAF inhibitor-induced antitumor immunity. Cancer Res. 2014;74:3205-3217.
- Dummer R, Sandhu SK, Miller WH, et al. A phase II, multicenter study of encorafenib/binimetinib followed by a rational triple-combination after progression in patients with advanced BRAF V600-mutated melanoma (LOGIC2). J Clin Oncol. 2020;38(15 suppl):10022.
- Ferrucci PF, Di Giacomo AM, Del Vecchio M, et al. KEYNOTE-022 part 3: a randomized, double-blind, phase 2 study of pembrolizumab, dabrafenib, and trametinib in BRAF-mutant melanoma. J Immunother Cancer. 2020;8:e001806.
- Madu MF, Schopman JH, Berger DM, et al. Clinical prognostic markers in stage IIIC melanoma. J Surg Oncol. 2017;116:244-251.
- Davis JL, Langan RC, Panageas KS, et al. Elevated blood neutrophil-to-lymphocyte ratio: a readily available biomarker associated with death due to disease in high risk nonmetastatic melanoma. Ann Surg Oncol. 2017;24:1989-1996.
- Freedberg DE, Rigas SH, Russak J, et al. Frequent p16-independent inactivation of p14ARF in human melanoma. J Natl Cancer Inst. 2008;100:784-795.
- Sigalotti L, Covre A, Fratta E, et al. Epigenetics of human cutaneous melanoma: setting the stage for new therapeutic strategies. J Transl Med. 2010;8:1-22.
Cutaneous malignant melanoma represents an aggressive form of skin cancer, with 132,000 new cases of melanoma and 50,000 melanoma-related deaths diagnosed worldwide each year.1 In recent decades, major progress has been made in the treatment of melanoma, especially metastatic and advanced-stage disease. Approval of new treatments, such as immunotherapy with anti–PD-1 (pembrolizumab and nivolumab) and anti–CTLA-4 (ipilimumab) antibodies, has revolutionized therapeutic strategies (Figure 1). Molecularly, melanoma has the highest mutational burden among solid tumors. Approximately 40% of melanomas harbor the BRAF V600 mutation, leading to constitutive activation of the mitogen-activated protein kinase (MAPK) signaling pathway.2 The other described genomic subtypes are mutated RAS (accounting for approximately 28% of cases), mutated NF1 (approximately 14% of cases), and triple wild type, though these other subtypes have not been as successfully targeted with therapy to date.3 Dual inhibition of this pathway using combination therapy with BRAF and MEK inhibitors confers high response rates and survival benefit, though efficacy in metastatic patients often is limited by development of resistance. The US Food and Drug Administration (FDA) has approved 3 combinations of targeted therapy in unresectable tumors: dabrafenib and trametinib, vemurafenib and cobimetinib, and encorafenib and binimetinib. The oncolytic herpesvirus talimogene laherparepvec also has received FDA approval for local treatment of unresectable cutaneous, subcutaneous, and nodal lesions in patients with recurrent melanoma after initial surgery.2

In this review, we explore new therapeutic agents and novel combinations that are being tested in early-phase clinical trials (Table). We discuss newer promising tools such as nanotechnology to develop nanosystems that act as drug carriers and/or light absorbents to potentially improve therapy outcomes. Finally, we highlight challenges such as management after resistance and intervention with novel immunotherapies and the lack of predictive biomarkers to stratify patients to targeted treatments after primary treatment failure.


Targeted Therapies
Vemurafenib was approved by the FDA in 2011 and was the first BRAF-targeted therapy approved for the treatment of melanoma based on a 48% response rate and a 63% reduction in the risk for death vs dacarbazine chemotherapy.4 Despite a rapid and clinically significant initial response, progression-free survival (PFS) was only 5.3 months, which is indicative of the rapid development of resistance with monotherapy through MAPK reactivation. As a result, combined BRAF and MEK inhibition was introduced and is now the standard of care for targeted therapy in melanoma. Treatment with dabrafenib and trametinib, vemurafenib and cobimetinib, or encorafenib and binimetinib is associated with prolonged PFS and overall survival (OS) compared to BRAF inhibitor monotherapy, with response rates exceeding 60% and a complete response rate of 10% to 18%.5 Recently, combining atezolizumab with vemurafenib and cobimetinib was shown to improve PFS compared to combined targeted therapy.6 Targeted therapy usually is given as first-line treatment to symptomatic patients with a high tumor burden because the response may be more rapid than the response to immunotherapy. Ultimately, most patients with advanced BRAF-mutated melanoma receive both targeted therapy and immunotherapy.
Mutations of KIT (encoding proto-oncogene receptor tyrosine kinase) activate intracellular MAPK and phosphatidylinositol 3-kinase (PI3K) pathways (Figure 2).7 KIT mutations are found in mucosal and acral melanomas as well as chronically sun-damaged skin, with frequencies of 39%, 36%, and 28%, respectively. Imatinib was associated with a 53% response rate and PFS of 3.9 months among patients with KIT-mutated melanoma but failed to cause regression in melanomas with KIT amplification.8

Anti–CTLA-4 Immune Checkpoint Inhibition
CTLA-4 is a protein found on T cells that binds with another protein, B7, preventing T cells from killing cancer cells. Hence, blockade of CTLA-4 antibody avoids the immunosuppressive state of lymphocytes, strengthening their antitumor action.9 Ipilimumab, an anti–CTLA-4 antibody, demonstrated improvement in median OS for management of unresectable or metastatic stage IV melanoma, resulting in its FDA approval.8 A combination of ipilimumab with dacarbazine in stage IV melanoma showed notable improvement of OS.10 Similarly, tremelimumab showed evidence of tumor regression in a phase 1 trial but with more severe immune-related side effects compared with ipilimumab.11 A second study on patients with stage IV melanoma treated with tremelimumab as first-line therapy in comparison with dacarbazine demonstrated differences in OS that were not statistically significant, though there was a longer duration of an objective response in patients treated with tremelimumab (35.8 months) compared with patients responding to dacarbazine (13.7 months).12
Anti–PD-1 Immune Checkpoint Inhibition
PD-1 is a transmembrane protein with immunoreceptor tyrosine-based inhibitory signaling, identified as an apoptosis-associated molecule.13 Upon activation, it is expressed on the cell surface of CD4, CD8, B lymphocytes, natural killer cells, monocytes, and dendritic cells.14 PD-L1, the ligand of PD-1, is constitutively expressed on different hematopoietic cells, as well as on fibroblasts, endothelial cells, mesenchymal cells, neurons, and keratinocytes.15,16 Reactivation of effector T lymphocytes by PD-1:PD-L1 pathway inhibition has shown clinically significant therapeutic relevance.17 The PD-1:PD-L1 interaction is active only in the presence of T- or B-cell antigen receptor cross-link. This interaction prevents PI3K/AKT signaling and MAPK/extracellular signal-regulated kinase pathway activation with the net result of lymphocytic functional exhaustion.18,19 PD-L1 blockade is shown to have better clinical benefit and minor toxicity compared to anti–CTLA-4 therapy. Treatment with anti-PD1 nivolumab in a phase 1b clinical trial (N=107) demonstrated highly specific action, durable tumor remission, and long-term safety in 32% of patients with advanced melanoma.20 These promising results led to the FDA approval of nivolumab for the treatment of patients with advanced and unresponsive melanoma. A recent clinical trial combining ipilimumab and nivolumab resulted in an impressive increase of PFS compared with ipilimumab monotherapy (11.5 months vs 2.9 months).21 Similarly, treatment with pembrolizumab in advanced melanoma demonstrated improvement in PFS and OS compared with anti–CTLA-4 therapy,22,23 which resulted in FDA approval of pembrolizumab for the treatment of advanced melanoma in patients previously treated with ipilimumab or BRAF inhibitors in BRAF V600 mutation–positive patients.24
Lymphocyte-Activated Gene 3–Targeted Therapies
Nanotechnology in Melanoma Therapy
The use of nanotechnology represents one of the newer alternative therapies employed for treatment of melanoma and is especially gaining interest due to reduced adverse effects in comparison with other conventional treatments for melanoma. Nanotechnology-based drug delivery systems precisely target tumor cells and improve the effect of both the conventional and innovative antineoplastic treatment.27,31 Tumor vasculature differs from normal tissues by being discontinuous and having interspersed small gaps/holes that allow nanoparticles to exit the circulation and enter and accumulate in the tumor tissue, leading to enhanced and targeted release of the antineoplastic drug to tumor cells.32 This mechanism is called the enhanced permeability and retention effect.33
Another mechanism by which nanoparticles work is ligand-based targeting in which ligands such as monoclonal antibodies, peptides, and nucleic acids located on the surface of nanoparticles can bind to receptors on the plasma membrane of tumor cells and lead to targeted delivery of the drug.34 Nanomaterials used for melanoma treatment include vesicular systems such as liposomes and niosomes, polymeric nanoparticles, noble metal-based nanoparticles, carbon nanotubes, dendrimers, solid lipid nanoparticles and nanostructures, lipid carriers, and microneedles. In melanoma, nanoparticles can be used to enhance targeted delivery of drugs, including immune checkpoint inhibitors (ICIs). Cai et al35 described usage of scaffolds in delivery systems. Tumor-associated antigens, adjuvant drugs, and chemical agents that influence the tumor microenvironment can be loaded onto these scaffolding agents. In a study by Zhu et al,36 photosensitizer chlorin e6 and immunoadjuvant aluminum hydroxide were used as a novel nanosystem that effectively destroyed tumor cells and induced a strong systemic antitumor response. IL-2 is a cytokine produced by B or T lymphocytes. Its use in melanoma has been limited by a severe adverse effect profile and lack of complete response in most patients. Cytokine-containing nanogels have been found to selectively release IL-2 in response to activation of T-cell receptors, and a mouse model in melanoma showed better response compared to free IL-1 and no adverse systemic effects.37
Nanovaccines represent another interesting novel immunotherapy modality. A study by Conniot et al38 showed that nanoparticles can be used in the treatment of melanoma. Nanoparticles made of biodegradable polymer were loaded with Melan-A/MART-1 (26–35 A27L) MHC class I-restricted peptide (MHC class I antigen), and the limited peptide MHC class II Melan-A/MART-1 51–73 (MHC class II antigen) and grafted with mannose that was then combined with an anti–PD-L1 antibody and injected into mouse models. This combination resulted in T-cell infiltration at early stages and increased infiltration of myeloid-derived suppressor cells. Ibrutinib, a myeloid-derived suppressor cell inhibitor, was added and demonstrated marked tumor remission and prolonged survival.38
Overexpression of certain microRNAs (miRNAs), especially miR-204-5p and miR-199b-5p, has been shown to inhibit growth of melanoma cells in vitro, both alone and in combination with MAPK inhibitors, but these miRNAs are easily degradable in body fluids. Lipid nanoparticles can bind these miRNAs and have been shown to inhibit tumor cell proliferation and improve efficacy of BRAF and MEK inhibitors.39
Triple-Combination Therapy
Immune checkpoint inhibitors such as anti–PD-1 or anti–CTLA-4 drugs have become the standard of care in treatment of advanced melanoma. Approximately 40% to 50% of cases of melanoma harbor BRAF mutations, and patients with these mutations could benefit from BRAF and MEK inhibitors. Data from clinical trials on BRAF and MEK inhibitors even showed initial high objective response rates, but the response was short-lived, and there was frequent acquired resistance.40 With ICIs, the major limitation was primary resistance, with only 50% of patients initially responding.41 Studies on murine models demonstrated that BRAF-mutated tumors had decreased expression of IFN-γ, tumor necrosis factor α, and CD40 ligand on CD4+ tumor-infiltrating lymphocytes and increased accumulation of regulatory T cells and myeloid-derived suppressor cells, leading to a protumor microenvironment. BRAF and MEK pathway inhibition were found to improve intratumoral CD4+ T-cell activity, leading to improved antitumor T-cell responses.42 Because of this enhanced immune response by BRAF and MEK inhibitors, it was hypothesized and later supported by clinical research that a combination of these targeted treatments and ICIs can have a synergistic effect, leading to increased antitumor activity.43 A randomized phase 2 clinical trial (KEYNOTE-022) in which the treatment group was given pembrolizumab, dabrafenib, and trametinib and the control group was treated with dabrafenib and trametinib showed increased medial OS in the treatment group vs the control group (46.3 months vs 26.3 months) and more frequent complete response in the treatment group vs the control group (20% vs 15%).44 In the IMspire150 phase 3 clinical trial, patients with advanced stage IIIC to IV BRAF-mutant melanoma were treated with either a triple combination of the PDL-1 inhibitor atezolizumab, vemurafenib, and cobimetinib or vemurafenib and cobimetinib. Although the objective response rate was similar in both groups, the median duration of response was longer in the triplet group compared with the doublet group (21 months vs 12.6 months). Given these results, the FDA approved the triple-combination therapy with atezolizumab, vemurafenib, and cobimetinib. Although triple-combination therapy has shown promising results, it is expected that there will be an increase in the frequency of treatment-related adverse effects. In the phase 3 COMBi-I study, patients with advanced stage IIIC to IV BRAF V600E mutant cutaneous melanoma were treated with either a combination of spartalizumab, dabrafenib, and trametinib or just dabrafenib and trametinib. Although the objective response rates were not significantly different (69% vs 64%), there was increased frequency of treatment-related adverse effects in patients receiving triple-combination therapy.43 As more follow-up data come out of these ongoing clinical trials, benefits of triple-combination therapy and its adverse effect profile will be more definitely established.
Challenges and Future Perspectives
One of the major roadblocks in the treatment of melanoma is the failure of response to ICI with CTLA-4 and PD-1/PD-L1 blockade in a large patient population, which has resulted in the need for new biomarkers that can act as potential therapeutic targets. Further, the main underlying factor for both adjuvant and neoadjuvant approaches remains the selection of patients, optimizing therapeutic outcomes while minimizing the number of patients exposed to potentially toxic treatments without gaining clinical benefit. Clinical and pathological factors (eg, Breslow thickness, ulceration, the number of positive lymph nodes) play a role in stratifying patients as per risk of recurrence.45 Similarly, peripheral blood biomarkers have been proposed as prognostic tools for high-risk stage II and III melanoma, including markers of systemic inflammation previously explored in the metastatic setting.46 However, the use of these parameters has not been validated for clinical practice. Currently, despite promising results of BRAF and MEK inhibitors and therapeutic ICIs, as well as IL-2 or interferon alfa, treatment options in metastatic melanoma are limited because of its high heterogeneity, problematic patient stratification, and high genetic mutational rate. Recently, the role of epigenetic modifications andmiRNAs in melanoma progression and metastatic spread has been described. Silencing of CDKN2A locus and encoding for p16INK4A and p14ARF by DNA methylation are noted in 27% and 57% of metastatic melanomas, respectively, which enables melanoma cells to escape from growth arrest and apoptosis generated by Rb protein and p53 pathways.47 Demethylation of these and other tumor suppressor genes with proapoptotic function (eg, RASSF1A and tumor necrosis factor–related apoptosis-inducing ligand) can restore cell death pathways, though future clinical studies in melanoma are warranted.48
Cutaneous malignant melanoma represents an aggressive form of skin cancer, with 132,000 new cases of melanoma and 50,000 melanoma-related deaths diagnosed worldwide each year.1 In recent decades, major progress has been made in the treatment of melanoma, especially metastatic and advanced-stage disease. Approval of new treatments, such as immunotherapy with anti–PD-1 (pembrolizumab and nivolumab) and anti–CTLA-4 (ipilimumab) antibodies, has revolutionized therapeutic strategies (Figure 1). Molecularly, melanoma has the highest mutational burden among solid tumors. Approximately 40% of melanomas harbor the BRAF V600 mutation, leading to constitutive activation of the mitogen-activated protein kinase (MAPK) signaling pathway.2 The other described genomic subtypes are mutated RAS (accounting for approximately 28% of cases), mutated NF1 (approximately 14% of cases), and triple wild type, though these other subtypes have not been as successfully targeted with therapy to date.3 Dual inhibition of this pathway using combination therapy with BRAF and MEK inhibitors confers high response rates and survival benefit, though efficacy in metastatic patients often is limited by development of resistance. The US Food and Drug Administration (FDA) has approved 3 combinations of targeted therapy in unresectable tumors: dabrafenib and trametinib, vemurafenib and cobimetinib, and encorafenib and binimetinib. The oncolytic herpesvirus talimogene laherparepvec also has received FDA approval for local treatment of unresectable cutaneous, subcutaneous, and nodal lesions in patients with recurrent melanoma after initial surgery.2

In this review, we explore new therapeutic agents and novel combinations that are being tested in early-phase clinical trials (Table). We discuss newer promising tools such as nanotechnology to develop nanosystems that act as drug carriers and/or light absorbents to potentially improve therapy outcomes. Finally, we highlight challenges such as management after resistance and intervention with novel immunotherapies and the lack of predictive biomarkers to stratify patients to targeted treatments after primary treatment failure.


Targeted Therapies
Vemurafenib was approved by the FDA in 2011 and was the first BRAF-targeted therapy approved for the treatment of melanoma based on a 48% response rate and a 63% reduction in the risk for death vs dacarbazine chemotherapy.4 Despite a rapid and clinically significant initial response, progression-free survival (PFS) was only 5.3 months, which is indicative of the rapid development of resistance with monotherapy through MAPK reactivation. As a result, combined BRAF and MEK inhibition was introduced and is now the standard of care for targeted therapy in melanoma. Treatment with dabrafenib and trametinib, vemurafenib and cobimetinib, or encorafenib and binimetinib is associated with prolonged PFS and overall survival (OS) compared to BRAF inhibitor monotherapy, with response rates exceeding 60% and a complete response rate of 10% to 18%.5 Recently, combining atezolizumab with vemurafenib and cobimetinib was shown to improve PFS compared to combined targeted therapy.6 Targeted therapy usually is given as first-line treatment to symptomatic patients with a high tumor burden because the response may be more rapid than the response to immunotherapy. Ultimately, most patients with advanced BRAF-mutated melanoma receive both targeted therapy and immunotherapy.
Mutations of KIT (encoding proto-oncogene receptor tyrosine kinase) activate intracellular MAPK and phosphatidylinositol 3-kinase (PI3K) pathways (Figure 2).7 KIT mutations are found in mucosal and acral melanomas as well as chronically sun-damaged skin, with frequencies of 39%, 36%, and 28%, respectively. Imatinib was associated with a 53% response rate and PFS of 3.9 months among patients with KIT-mutated melanoma but failed to cause regression in melanomas with KIT amplification.8

Anti–CTLA-4 Immune Checkpoint Inhibition
CTLA-4 is a protein found on T cells that binds with another protein, B7, preventing T cells from killing cancer cells. Hence, blockade of CTLA-4 antibody avoids the immunosuppressive state of lymphocytes, strengthening their antitumor action.9 Ipilimumab, an anti–CTLA-4 antibody, demonstrated improvement in median OS for management of unresectable or metastatic stage IV melanoma, resulting in its FDA approval.8 A combination of ipilimumab with dacarbazine in stage IV melanoma showed notable improvement of OS.10 Similarly, tremelimumab showed evidence of tumor regression in a phase 1 trial but with more severe immune-related side effects compared with ipilimumab.11 A second study on patients with stage IV melanoma treated with tremelimumab as first-line therapy in comparison with dacarbazine demonstrated differences in OS that were not statistically significant, though there was a longer duration of an objective response in patients treated with tremelimumab (35.8 months) compared with patients responding to dacarbazine (13.7 months).12
Anti–PD-1 Immune Checkpoint Inhibition
PD-1 is a transmembrane protein with immunoreceptor tyrosine-based inhibitory signaling, identified as an apoptosis-associated molecule.13 Upon activation, it is expressed on the cell surface of CD4, CD8, B lymphocytes, natural killer cells, monocytes, and dendritic cells.14 PD-L1, the ligand of PD-1, is constitutively expressed on different hematopoietic cells, as well as on fibroblasts, endothelial cells, mesenchymal cells, neurons, and keratinocytes.15,16 Reactivation of effector T lymphocytes by PD-1:PD-L1 pathway inhibition has shown clinically significant therapeutic relevance.17 The PD-1:PD-L1 interaction is active only in the presence of T- or B-cell antigen receptor cross-link. This interaction prevents PI3K/AKT signaling and MAPK/extracellular signal-regulated kinase pathway activation with the net result of lymphocytic functional exhaustion.18,19 PD-L1 blockade is shown to have better clinical benefit and minor toxicity compared to anti–CTLA-4 therapy. Treatment with anti-PD1 nivolumab in a phase 1b clinical trial (N=107) demonstrated highly specific action, durable tumor remission, and long-term safety in 32% of patients with advanced melanoma.20 These promising results led to the FDA approval of nivolumab for the treatment of patients with advanced and unresponsive melanoma. A recent clinical trial combining ipilimumab and nivolumab resulted in an impressive increase of PFS compared with ipilimumab monotherapy (11.5 months vs 2.9 months).21 Similarly, treatment with pembrolizumab in advanced melanoma demonstrated improvement in PFS and OS compared with anti–CTLA-4 therapy,22,23 which resulted in FDA approval of pembrolizumab for the treatment of advanced melanoma in patients previously treated with ipilimumab or BRAF inhibitors in BRAF V600 mutation–positive patients.24
Lymphocyte-Activated Gene 3–Targeted Therapies
Nanotechnology in Melanoma Therapy
The use of nanotechnology represents one of the newer alternative therapies employed for treatment of melanoma and is especially gaining interest due to reduced adverse effects in comparison with other conventional treatments for melanoma. Nanotechnology-based drug delivery systems precisely target tumor cells and improve the effect of both the conventional and innovative antineoplastic treatment.27,31 Tumor vasculature differs from normal tissues by being discontinuous and having interspersed small gaps/holes that allow nanoparticles to exit the circulation and enter and accumulate in the tumor tissue, leading to enhanced and targeted release of the antineoplastic drug to tumor cells.32 This mechanism is called the enhanced permeability and retention effect.33
Another mechanism by which nanoparticles work is ligand-based targeting in which ligands such as monoclonal antibodies, peptides, and nucleic acids located on the surface of nanoparticles can bind to receptors on the plasma membrane of tumor cells and lead to targeted delivery of the drug.34 Nanomaterials used for melanoma treatment include vesicular systems such as liposomes and niosomes, polymeric nanoparticles, noble metal-based nanoparticles, carbon nanotubes, dendrimers, solid lipid nanoparticles and nanostructures, lipid carriers, and microneedles. In melanoma, nanoparticles can be used to enhance targeted delivery of drugs, including immune checkpoint inhibitors (ICIs). Cai et al35 described usage of scaffolds in delivery systems. Tumor-associated antigens, adjuvant drugs, and chemical agents that influence the tumor microenvironment can be loaded onto these scaffolding agents. In a study by Zhu et al,36 photosensitizer chlorin e6 and immunoadjuvant aluminum hydroxide were used as a novel nanosystem that effectively destroyed tumor cells and induced a strong systemic antitumor response. IL-2 is a cytokine produced by B or T lymphocytes. Its use in melanoma has been limited by a severe adverse effect profile and lack of complete response in most patients. Cytokine-containing nanogels have been found to selectively release IL-2 in response to activation of T-cell receptors, and a mouse model in melanoma showed better response compared to free IL-1 and no adverse systemic effects.37
Nanovaccines represent another interesting novel immunotherapy modality. A study by Conniot et al38 showed that nanoparticles can be used in the treatment of melanoma. Nanoparticles made of biodegradable polymer were loaded with Melan-A/MART-1 (26–35 A27L) MHC class I-restricted peptide (MHC class I antigen), and the limited peptide MHC class II Melan-A/MART-1 51–73 (MHC class II antigen) and grafted with mannose that was then combined with an anti–PD-L1 antibody and injected into mouse models. This combination resulted in T-cell infiltration at early stages and increased infiltration of myeloid-derived suppressor cells. Ibrutinib, a myeloid-derived suppressor cell inhibitor, was added and demonstrated marked tumor remission and prolonged survival.38
Overexpression of certain microRNAs (miRNAs), especially miR-204-5p and miR-199b-5p, has been shown to inhibit growth of melanoma cells in vitro, both alone and in combination with MAPK inhibitors, but these miRNAs are easily degradable in body fluids. Lipid nanoparticles can bind these miRNAs and have been shown to inhibit tumor cell proliferation and improve efficacy of BRAF and MEK inhibitors.39
Triple-Combination Therapy
Immune checkpoint inhibitors such as anti–PD-1 or anti–CTLA-4 drugs have become the standard of care in treatment of advanced melanoma. Approximately 40% to 50% of cases of melanoma harbor BRAF mutations, and patients with these mutations could benefit from BRAF and MEK inhibitors. Data from clinical trials on BRAF and MEK inhibitors even showed initial high objective response rates, but the response was short-lived, and there was frequent acquired resistance.40 With ICIs, the major limitation was primary resistance, with only 50% of patients initially responding.41 Studies on murine models demonstrated that BRAF-mutated tumors had decreased expression of IFN-γ, tumor necrosis factor α, and CD40 ligand on CD4+ tumor-infiltrating lymphocytes and increased accumulation of regulatory T cells and myeloid-derived suppressor cells, leading to a protumor microenvironment. BRAF and MEK pathway inhibition were found to improve intratumoral CD4+ T-cell activity, leading to improved antitumor T-cell responses.42 Because of this enhanced immune response by BRAF and MEK inhibitors, it was hypothesized and later supported by clinical research that a combination of these targeted treatments and ICIs can have a synergistic effect, leading to increased antitumor activity.43 A randomized phase 2 clinical trial (KEYNOTE-022) in which the treatment group was given pembrolizumab, dabrafenib, and trametinib and the control group was treated with dabrafenib and trametinib showed increased medial OS in the treatment group vs the control group (46.3 months vs 26.3 months) and more frequent complete response in the treatment group vs the control group (20% vs 15%).44 In the IMspire150 phase 3 clinical trial, patients with advanced stage IIIC to IV BRAF-mutant melanoma were treated with either a triple combination of the PDL-1 inhibitor atezolizumab, vemurafenib, and cobimetinib or vemurafenib and cobimetinib. Although the objective response rate was similar in both groups, the median duration of response was longer in the triplet group compared with the doublet group (21 months vs 12.6 months). Given these results, the FDA approved the triple-combination therapy with atezolizumab, vemurafenib, and cobimetinib. Although triple-combination therapy has shown promising results, it is expected that there will be an increase in the frequency of treatment-related adverse effects. In the phase 3 COMBi-I study, patients with advanced stage IIIC to IV BRAF V600E mutant cutaneous melanoma were treated with either a combination of spartalizumab, dabrafenib, and trametinib or just dabrafenib and trametinib. Although the objective response rates were not significantly different (69% vs 64%), there was increased frequency of treatment-related adverse effects in patients receiving triple-combination therapy.43 As more follow-up data come out of these ongoing clinical trials, benefits of triple-combination therapy and its adverse effect profile will be more definitely established.
Challenges and Future Perspectives
One of the major roadblocks in the treatment of melanoma is the failure of response to ICI with CTLA-4 and PD-1/PD-L1 blockade in a large patient population, which has resulted in the need for new biomarkers that can act as potential therapeutic targets. Further, the main underlying factor for both adjuvant and neoadjuvant approaches remains the selection of patients, optimizing therapeutic outcomes while minimizing the number of patients exposed to potentially toxic treatments without gaining clinical benefit. Clinical and pathological factors (eg, Breslow thickness, ulceration, the number of positive lymph nodes) play a role in stratifying patients as per risk of recurrence.45 Similarly, peripheral blood biomarkers have been proposed as prognostic tools for high-risk stage II and III melanoma, including markers of systemic inflammation previously explored in the metastatic setting.46 However, the use of these parameters has not been validated for clinical practice. Currently, despite promising results of BRAF and MEK inhibitors and therapeutic ICIs, as well as IL-2 or interferon alfa, treatment options in metastatic melanoma are limited because of its high heterogeneity, problematic patient stratification, and high genetic mutational rate. Recently, the role of epigenetic modifications andmiRNAs in melanoma progression and metastatic spread has been described. Silencing of CDKN2A locus and encoding for p16INK4A and p14ARF by DNA methylation are noted in 27% and 57% of metastatic melanomas, respectively, which enables melanoma cells to escape from growth arrest and apoptosis generated by Rb protein and p53 pathways.47 Demethylation of these and other tumor suppressor genes with proapoptotic function (eg, RASSF1A and tumor necrosis factor–related apoptosis-inducing ligand) can restore cell death pathways, though future clinical studies in melanoma are warranted.48
- Geller AC, Clapp RW, Sober AJ, et al. Melanoma epidemic: an analysis of six decades of data from the Connecticut Tumor Registry. J Clin Oncol. 2013;31:4172-4178.
- Moreira A, Heinzerling L, Bhardwaj N, et al. Current melanoma treatments: where do we stand? Cancers (Basel). 2021;13:221.
- Watson IR, Wu C-J, Zou L, et al. Genomic classification of cutaneous melanoma. Cancer Res. 2015;75(15 Suppl):2972.
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507-2516.
- Hamid O, Cowey CL, Offner M, et al. Efficacy, safety, and tolerability of approved combination BRAF and MEK inhibitor regimens for BRAF-mutant melanoma. Cancers (Basel). 2019;11:1642.
- Gutzmer R, Stroyakovskiy D, Gogas H, et al. Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAFV600 mutation-positive melanoma (IMspire150): primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2020;395:1835-1844.
- Reddy BY, Miller DM, Tsao H. Somatic driver mutations in melanoma. Cancer. 2017;123(suppl 11):2104-2117.
- Hodi FS, Corless CL, Giobbie-Hurder A, et al. Imatinib for melanomas harboring mutationally activated or amplified KIT arising on mucosal, acral, and chronically sun-damaged skin. J Clin Oncol. 2013;31:3182-3190.
- Teft WA, Kirchhof MG, Madrenas J. A molecular perspective of CTLA-4 function. Annu Rev Immunol. 2006;24:65-97.
- Maverakis E, Cornelius LA, Bowen GM, et al. Metastatic melanoma—a review of current and future treatment options. Acta Derm Venereol. 2015;95:516-524.
- Ribas A, Chesney JA, Gordon MS, et al. Safety profile and pharmacokinetic analyses of the anti-CTLA4 antibody tremelimumab administered as a one hour infusion. J Transl Med. 2012;10:1-6.
- Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908-918.
- BG Neel, Gu H, Pao L. The ‘Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci. 2003;28:284-293.
- Ishida Y, Agata Y, Shibahara K, et al. Induced expression of PD‐1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887-3895.
- Yamazaki T, Akiba H, Iwai H, et al. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol. 2002;169:5538-5545.
- Keir ME, Butte MJ, Freeman GJ et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677-704.
- Blank C, Kuball J, Voelkl S, et al. Blockade of PD‐L1 (B7‐H1) augments human tumor‐specific T cell responses in vitro. Int J Cancer. 2006;119:317-327.
- Parry RV, Chemnitz JM, Frauwirth KA, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543-9553.
- Patsoukis N, Brown J, Petkova V, et al. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci Signal. 2012;5:ra46.
- Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020-1030.
- Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375-384.
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320-330.
- Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006-2017.
- Burns MC, O’Donnell A, Puzanov I. Pembrolizumab for the treatment of advanced melanoma. Exp Opin Orphan Drugs. 2016;4:867-873.
- F Triebel. LAG-3: a regulator of T-cell and DC responses and its use in therapeutic vaccination. Trends Immunol. 2003;24:619-622.
- Maruhashi T, Sugiura D, Okazaki I-M, et al. LAG-3: from molecular functions to clinical applications. J Immunother Cancer. 2020;8:e001014.
- Shi J, Kantoff PW, Wooster R, et al. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17:20-37.
- Tawbi HA, Schadendorf D, Lipson EJ, et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med. 2022;386:24-34.
- US Food and Drug Administration approves first LAG-3-blocking antibody combination, Opdualag™ (nivolumab and relatlimab-rmbw), as treatment for patients with unresectable or metastatic melanoma. Press release. Bristol Myers Squibb. March 18, 2022. Accessed November 7, 2023. https://news.bms.com/news/details/2022/U.S.-Food-and-Drug-Administration-Approves-First-LAG-3-Blocking-Antibody-Combination-Opdualag-nivolumab-and-relatlimab-rmbw-as-Treatment-for-Patients-with-Unresectable-or-Metastatic-Melanoma/default.aspx
- Zhao B-W, Zhang F-Y, Wang Y, et al. LAG3-PD1 or CTLA4-PD1 inhibition in advanced melanoma: indirect cross comparisons of the CheckMate-067 and RELATIVITY-047 trials. Cancers (Basel). 2022;14:4975.
- Jin C, Wang K, Oppong-Gyebi A, et al. Application of nanotechnology in cancer diagnosis and therapy-a mini-review. Int J Med Sci. 2020;17:2964-2973.
- Maeda H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv Drug Del Rev. 2015;91:3-6.
- Iyer AK, Khaled G, Fang J, et al. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today. 2006;11:812-818.
- Beiu C, Giurcaneanu C, Grumezescu AM, et al. Nanosystems for improved targeted therapies in melanoma. J Clin Med. 2020;9:318.
- Cai L, Xu J, Yang Z, et al. Engineered biomaterials for cancer immunotherapy. MedComm. 2020;1:35-46.
- Zhu Y, Xue J, Chen W, et al. Albumin-biomineralized nanoparticles to synergize phototherapy and immunotherapy against melanoma. J Control Release. 2020;322:300-311.
- Zhang Y, Li N, Suh H, et al. Nanoparticle anchoring targets immune agonists to tumors enabling anti-cancer immunity without systemic toxicity. Nat Commun. 2018;9:6.
- Conniot J, Scomparin A, Peres C, et al. Immunization with mannosylated nanovaccines and inhibition of the immune-suppressing microenvironment sensitizes melanoma to immune checkpoint modulators. Nat Nanotechnol. 2019;14:891-901.
- Fattore L, Campani V, Ruggiero CF, et al. In vitro biophysical and biological characterization of lipid nanoparticles co-encapsulating oncosuppressors miR-199b-5p and miR-204-5p as potentiators of target therapy in metastatic melanoma. Int J Mol Sci. 2020;21:1930.
- Welti M, Dimitriou F, Gutzmer R, et al. Triple combination of immune checkpoint inhibitors and BRAF/MEK inhibitors in BRAF V600 melanoma: current status and future perspectives. Cancers (Basel). 2022;14:5489.
- Khair DO, Bax HJ, Mele S, et al. Combining immune checkpoint inhibitors: established and emerging targets and strategies to improve outcomes in melanoma. Front Immunol. 2019;10:453.
- Ho P-C, Meeth KM, Tsui Y-C, et al. Immune-based antitumor effects of BRAF inhibitors rely on signaling by CD40L and IFNγBRAF inhibitor-induced antitumor immunity. Cancer Res. 2014;74:3205-3217.
- Dummer R, Sandhu SK, Miller WH, et al. A phase II, multicenter study of encorafenib/binimetinib followed by a rational triple-combination after progression in patients with advanced BRAF V600-mutated melanoma (LOGIC2). J Clin Oncol. 2020;38(15 suppl):10022.
- Ferrucci PF, Di Giacomo AM, Del Vecchio M, et al. KEYNOTE-022 part 3: a randomized, double-blind, phase 2 study of pembrolizumab, dabrafenib, and trametinib in BRAF-mutant melanoma. J Immunother Cancer. 2020;8:e001806.
- Madu MF, Schopman JH, Berger DM, et al. Clinical prognostic markers in stage IIIC melanoma. J Surg Oncol. 2017;116:244-251.
- Davis JL, Langan RC, Panageas KS, et al. Elevated blood neutrophil-to-lymphocyte ratio: a readily available biomarker associated with death due to disease in high risk nonmetastatic melanoma. Ann Surg Oncol. 2017;24:1989-1996.
- Freedberg DE, Rigas SH, Russak J, et al. Frequent p16-independent inactivation of p14ARF in human melanoma. J Natl Cancer Inst. 2008;100:784-795.
- Sigalotti L, Covre A, Fratta E, et al. Epigenetics of human cutaneous melanoma: setting the stage for new therapeutic strategies. J Transl Med. 2010;8:1-22.
- Geller AC, Clapp RW, Sober AJ, et al. Melanoma epidemic: an analysis of six decades of data from the Connecticut Tumor Registry. J Clin Oncol. 2013;31:4172-4178.
- Moreira A, Heinzerling L, Bhardwaj N, et al. Current melanoma treatments: where do we stand? Cancers (Basel). 2021;13:221.
- Watson IR, Wu C-J, Zou L, et al. Genomic classification of cutaneous melanoma. Cancer Res. 2015;75(15 Suppl):2972.
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507-2516.
- Hamid O, Cowey CL, Offner M, et al. Efficacy, safety, and tolerability of approved combination BRAF and MEK inhibitor regimens for BRAF-mutant melanoma. Cancers (Basel). 2019;11:1642.
- Gutzmer R, Stroyakovskiy D, Gogas H, et al. Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAFV600 mutation-positive melanoma (IMspire150): primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2020;395:1835-1844.
- Reddy BY, Miller DM, Tsao H. Somatic driver mutations in melanoma. Cancer. 2017;123(suppl 11):2104-2117.
- Hodi FS, Corless CL, Giobbie-Hurder A, et al. Imatinib for melanomas harboring mutationally activated or amplified KIT arising on mucosal, acral, and chronically sun-damaged skin. J Clin Oncol. 2013;31:3182-3190.
- Teft WA, Kirchhof MG, Madrenas J. A molecular perspective of CTLA-4 function. Annu Rev Immunol. 2006;24:65-97.
- Maverakis E, Cornelius LA, Bowen GM, et al. Metastatic melanoma—a review of current and future treatment options. Acta Derm Venereol. 2015;95:516-524.
- Ribas A, Chesney JA, Gordon MS, et al. Safety profile and pharmacokinetic analyses of the anti-CTLA4 antibody tremelimumab administered as a one hour infusion. J Transl Med. 2012;10:1-6.
- Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908-918.
- BG Neel, Gu H, Pao L. The ‘Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci. 2003;28:284-293.
- Ishida Y, Agata Y, Shibahara K, et al. Induced expression of PD‐1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887-3895.
- Yamazaki T, Akiba H, Iwai H, et al. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol. 2002;169:5538-5545.
- Keir ME, Butte MJ, Freeman GJ et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677-704.
- Blank C, Kuball J, Voelkl S, et al. Blockade of PD‐L1 (B7‐H1) augments human tumor‐specific T cell responses in vitro. Int J Cancer. 2006;119:317-327.
- Parry RV, Chemnitz JM, Frauwirth KA, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543-9553.
- Patsoukis N, Brown J, Petkova V, et al. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci Signal. 2012;5:ra46.
- Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020-1030.
- Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375-384.
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320-330.
- Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006-2017.
- Burns MC, O’Donnell A, Puzanov I. Pembrolizumab for the treatment of advanced melanoma. Exp Opin Orphan Drugs. 2016;4:867-873.
- F Triebel. LAG-3: a regulator of T-cell and DC responses and its use in therapeutic vaccination. Trends Immunol. 2003;24:619-622.
- Maruhashi T, Sugiura D, Okazaki I-M, et al. LAG-3: from molecular functions to clinical applications. J Immunother Cancer. 2020;8:e001014.
- Shi J, Kantoff PW, Wooster R, et al. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17:20-37.
- Tawbi HA, Schadendorf D, Lipson EJ, et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med. 2022;386:24-34.
- US Food and Drug Administration approves first LAG-3-blocking antibody combination, Opdualag™ (nivolumab and relatlimab-rmbw), as treatment for patients with unresectable or metastatic melanoma. Press release. Bristol Myers Squibb. March 18, 2022. Accessed November 7, 2023. https://news.bms.com/news/details/2022/U.S.-Food-and-Drug-Administration-Approves-First-LAG-3-Blocking-Antibody-Combination-Opdualag-nivolumab-and-relatlimab-rmbw-as-Treatment-for-Patients-with-Unresectable-or-Metastatic-Melanoma/default.aspx
- Zhao B-W, Zhang F-Y, Wang Y, et al. LAG3-PD1 or CTLA4-PD1 inhibition in advanced melanoma: indirect cross comparisons of the CheckMate-067 and RELATIVITY-047 trials. Cancers (Basel). 2022;14:4975.
- Jin C, Wang K, Oppong-Gyebi A, et al. Application of nanotechnology in cancer diagnosis and therapy-a mini-review. Int J Med Sci. 2020;17:2964-2973.
- Maeda H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv Drug Del Rev. 2015;91:3-6.
- Iyer AK, Khaled G, Fang J, et al. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today. 2006;11:812-818.
- Beiu C, Giurcaneanu C, Grumezescu AM, et al. Nanosystems for improved targeted therapies in melanoma. J Clin Med. 2020;9:318.
- Cai L, Xu J, Yang Z, et al. Engineered biomaterials for cancer immunotherapy. MedComm. 2020;1:35-46.
- Zhu Y, Xue J, Chen W, et al. Albumin-biomineralized nanoparticles to synergize phototherapy and immunotherapy against melanoma. J Control Release. 2020;322:300-311.
- Zhang Y, Li N, Suh H, et al. Nanoparticle anchoring targets immune agonists to tumors enabling anti-cancer immunity without systemic toxicity. Nat Commun. 2018;9:6.
- Conniot J, Scomparin A, Peres C, et al. Immunization with mannosylated nanovaccines and inhibition of the immune-suppressing microenvironment sensitizes melanoma to immune checkpoint modulators. Nat Nanotechnol. 2019;14:891-901.
- Fattore L, Campani V, Ruggiero CF, et al. In vitro biophysical and biological characterization of lipid nanoparticles co-encapsulating oncosuppressors miR-199b-5p and miR-204-5p as potentiators of target therapy in metastatic melanoma. Int J Mol Sci. 2020;21:1930.
- Welti M, Dimitriou F, Gutzmer R, et al. Triple combination of immune checkpoint inhibitors and BRAF/MEK inhibitors in BRAF V600 melanoma: current status and future perspectives. Cancers (Basel). 2022;14:5489.
- Khair DO, Bax HJ, Mele S, et al. Combining immune checkpoint inhibitors: established and emerging targets and strategies to improve outcomes in melanoma. Front Immunol. 2019;10:453.
- Ho P-C, Meeth KM, Tsui Y-C, et al. Immune-based antitumor effects of BRAF inhibitors rely on signaling by CD40L and IFNγBRAF inhibitor-induced antitumor immunity. Cancer Res. 2014;74:3205-3217.
- Dummer R, Sandhu SK, Miller WH, et al. A phase II, multicenter study of encorafenib/binimetinib followed by a rational triple-combination after progression in patients with advanced BRAF V600-mutated melanoma (LOGIC2). J Clin Oncol. 2020;38(15 suppl):10022.
- Ferrucci PF, Di Giacomo AM, Del Vecchio M, et al. KEYNOTE-022 part 3: a randomized, double-blind, phase 2 study of pembrolizumab, dabrafenib, and trametinib in BRAF-mutant melanoma. J Immunother Cancer. 2020;8:e001806.
- Madu MF, Schopman JH, Berger DM, et al. Clinical prognostic markers in stage IIIC melanoma. J Surg Oncol. 2017;116:244-251.
- Davis JL, Langan RC, Panageas KS, et al. Elevated blood neutrophil-to-lymphocyte ratio: a readily available biomarker associated with death due to disease in high risk nonmetastatic melanoma. Ann Surg Oncol. 2017;24:1989-1996.
- Freedberg DE, Rigas SH, Russak J, et al. Frequent p16-independent inactivation of p14ARF in human melanoma. J Natl Cancer Inst. 2008;100:784-795.
- Sigalotti L, Covre A, Fratta E, et al. Epigenetics of human cutaneous melanoma: setting the stage for new therapeutic strategies. J Transl Med. 2010;8:1-22.
Practice Points
- Immune checkpoint inhibition has resulted in a paradigm shift for the treatment of metastatic melanoma.
- Alternative therapies with novel targets such as lymphocyte-activated gene 3 aim to overcome resistance to the usual immune targets such asPD-1/PD-L1 and CTLA-4.
- Newer promising tools such as nanotechnology are being added to the growing armamentarium of melanoma treatment strategies.
Aberrant Expression of CD56 in Metastatic Malignant Melanoma
To the Editor:
Many types of neoplasms can show aberrant immunoreactivity or unexpected expression of markers.1 Malignant melanoma is a tumor that can show not only aberrant immunohistochemical staining patterns but also notable histologic diversity,1,2 which often makes the diagnosis of melanoma challenging and ultimately can lead to diagnostic uncertainty.2
The incidence of malignant melanoma continues to grow.3 Maintaining a high degree of suspicion for this disease, recognizing its heterogeneity and divergent differentiation, and knowing potential aberrant immunohistochemical staining patterns are imperative for accurate diagnosis.
A 36-year-old man presented to a primary care physician with right-sided chest pain, upper and lower back aches, bilateral hip pain, neck pain, headache, night sweats, chills, and nausea. After infectious causes were ruled out, he was placed on a steroid taper without improvement. He presented to the emergency department a few days later with muscle spasms and was found to also have diffuse abdominal tenderness and guarding. The patient’s medical history was noncontributory; he was a lifelong nonsmoker. Laboratory studies revealed elevated levels of alanine aminotransferase and C-reactive protein. Computed tomography of the chest and abdomen revealed innumerable liver and lung lesions that were suspicious for metastatic malignancy. A liver biopsy revealed nests and sheets of metastatic tumor with pleomorphic nuclei, inconspicuous nucleoli, and areas of intranuclear clearing (Figures 1 and 2). Immunohistochemical staining was performed to further characterize the tumor. Neoplastic cells were positive for MART-1 (also known as Melan-A and melanoma-associated antigen recognized by T cells)(Figure 3), SOX10, S-100, HMB-45, and vimentin. Nonspecific staining with CD56 (Figure 4), a neuroendocrine marker, also was noted; however, the neoplasm was negative for synaptophysin, another neuroendocrine marker. Other markers for which staining was negative included pan-keratin, CD138 (syndecan-1), desmin, placental alkaline phosphatase (PLAP), inhibin, OCT-4, cytokeratin 7, and cytokeratin 20. This staining pattern was compatible with metastatic melanoma with aberrant CD56 expression.
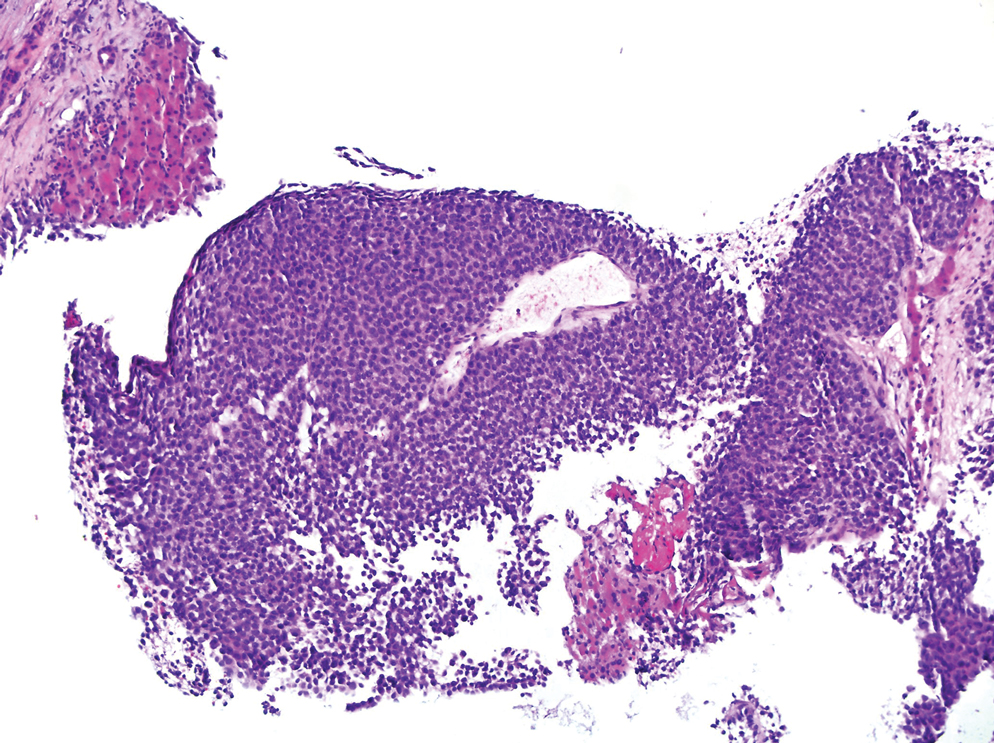
BRAF V600E immunohistochemical staining also was performed and showed strong and diffuse positivity within neoplastic cells. A subsequent positron emission tomography scan revealed widespread metastatic disease involving the lungs, liver, spleen, and bones. The patient did not have a history of an excised skin lesion; no primary cutaneous or mucosal lesions were identified.

The patient was started on targeted therapy with trametinib, a mitogen-activated extracellular signal-related kinase kinase (MEK) inhibitor, and dabrafenib, a BRAF inhibitor. The disease continued to progress; he developed extensive leptomeningeal metastatic disease for which palliative radiation therapy was administered. The patient died 4 months after the initial diagnosis.
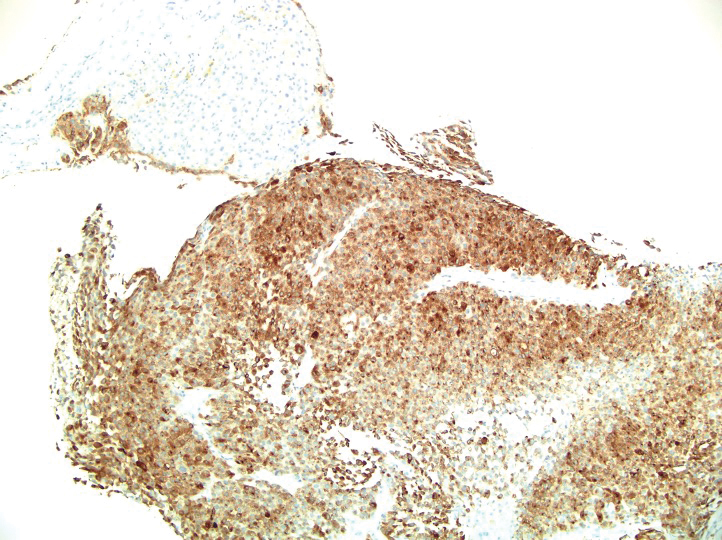
More than 90% of melanoma cases are of cutaneous origin; however, 4% to 8% of cases present as a metastatic lesion in the absence of an identified primary lesion,4 similar to our patient. The diagnosis of melanoma often is challenging; the tumor can show notable histologic diversity and has the potential to express aberrant immunophenotypes.1,2 The histologic diversity of melanoma includes a variety of architectural patterns (eg, nests, trabeculae, fascicular, pseudoglandular, pseudopapillary, or pseudorosette patterns), cytomorphologic features, and stromal changes. Cytomorphologic features of melanoma can be large pleomorphic cells; small cells; spindle cells; clear cells; signet-ring cells; and rhabdoid, plasmacytoid, and balloon cells.5
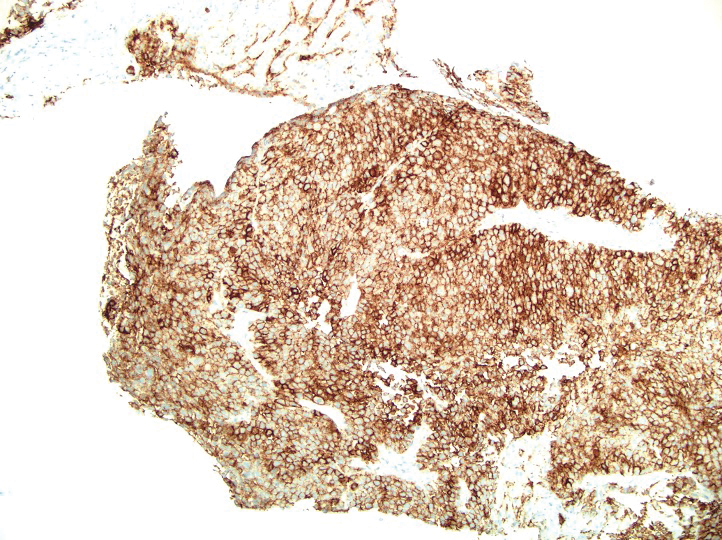
Melanoma can mimic carcinoma, sarcoma, lymphoma, benign stromal tumors, plasmacytoma, and germ-cell tumors.5 Nuclei can binucleated, multinucleated, or lobated and may contain inclusions or grooves. Stroma may become myxoid or desmoplastic in appearance or rarely show granulomatous inflammation or osteoclastic giant cells.5 These variations render the diagnosis of melanoma challenging and ultimately can lead to diagnostic uncertainty.
Melanomas typically express MART-1, HMB-45, S-100, tyrosinase, NK1C3, vimentin, and neuron-specific enolase. However, melanoma is among the many neoplasms that sometimes exhibit aberrant immunoreactivity and differentiation toward nonmelanocytic elements.6 The most commonly expressed immunophenotypic aberration is cytokeratin, especially the low-molecular-weight keratin marker CAM5.2.5 CAM5.2 positivity also is seen more often in metastatic melanoma. Melanomas rarely express other intermediate filaments, including desmin, neurofilament protein, and glial fibrillary acidic protein; expression of smooth-muscle actin is rare.5
Only a few cases of melanoma showing expression of neuroendocrine markers have been reported. However, one study reported synaptophysin positivity in 29% (10/34) of cases of primary and metastatic melanoma, making the stain a relatively common finding.1
In contrast, expression of CD56 (also known as neural-cell adhesion molecule 1) in melanoma has been reported only rarely. CD56 is a nonspecific neuroendocrine marker that normally is expressed on neurons, glial tissue, skeletal muscle, and natural killer cells. Riddle and Bui7 reported a case of metastatic malignant melanoma with focal CD56 positivity and no expression of other neuroendocrine markers, similar to our patient. Suzuki and colleagues4 also reported a case of melanoma metastatic to bone marrow that showed CD56 expression in true nonhematologic tumor cells and negative immunoreactivity with synaptophysin and chromogranin A.
It is important to document cases of melanoma that express neuroendocrine markers to prevent an incorrect diagnosis of a neuroendocrine tumor.1 In some cases, distinguishing amelanotic melanoma from poorly differentiated squamous cell carcinoma, neuroendocrine tumor, and lymphoma can be difficult.5
The term neuroendocrine differentiation is reserved for cases of melanoma that show areas of ultrastructural change consistent with a neuroendocrine tumor.2 Neuroendocrine differentiation in melanoma is not common; its prognostic significance is unknown.8 We do not consider our case to be true neuroendocrine differentiation, as the tumor lacked the morphologic changes of a neuroendocrine tumor. Furthermore, CD56 is a nonspecific neuroendocrine marker, and the tumor was negative for synaptophysin.
Melanoma has the potential to show notable histologic diversity as well as aberrant immunohistochemical staining patterns.1,2 Our patient had metastatic melanoma with aberrant neuroendocrine expression of CD56, which could have been a potential diagnostic pitfall. Because expression of CD56 in melanoma is rare, it is imperative to recognize this potential aberrant staining pattern to ensure the accurate diagnosis of melanoma and appropriate provision of care.
1. Romano RC, Carter JM, Folpe AL. Aberrant intermediate filament and synaptophysin expression is a frequent event in malignant melanoma: an immunohistochemical study of 73 cases. Mod Pathol. 2015;28:1033-1042. doi:10.1038/modpathol.2015.62
2. Eyden B, Pandit D, Banerjee SS. Malignant melanoma with neuroendocrine differentiation: clinical, histological, immunohistochemical and ultrastructural features of three cases. Histopathology. 2005;47:402-409. doi:10.1111/j.1365-2559.2005.02240.x
3. Katerji H, Childs JM, Bratton LE, et al. Primary esophageal melanoma with aberrant CD56 expression: a potential diagnostic pitfall. Case Rep Pathol. 2017;2017:9052637. doi:10.1155/2017/9052637
4. Suzuki T, Kusumoto S, Iida S, et al. Amelanotic malignant melanoma of unknown primary origin metastasizing to the bone marrow: a case report and review of the literature. Intern Med. 2014;53:325-328. doi:10.2169/internalmedicine.53.1412
5. Banerjee SS, Harris M. Morphological and immunophenotypic variations in malignant melanoma. Histopathology. 2000;36:387-402. doi:10.1046/j.1365-2559.2000.00894.x
6. Banerjee SS, Eyden B. Divergent differentiation in malignant melanomas: a review. Histopathology. 2008;52:119-129. doi:10.1111/j.1365-2559.2007.02823.x
7. Riddle ND, Bui MM. When melanoma is negative for S100: diagnostic pitfalls. Arch Pathol Lab Med. 2012;136:237-239. doi:10.5858/arpa.2011-0405-LE
8. Ilardi G, Caroppo D, Varricchio S, et al. Anal melanoma with neuroendocrine differentiation: report of a case. Int J Surg Pathol. 2015;23:329-332. doi:10.1177/1066896915573568
To the Editor:
Many types of neoplasms can show aberrant immunoreactivity or unexpected expression of markers.1 Malignant melanoma is a tumor that can show not only aberrant immunohistochemical staining patterns but also notable histologic diversity,1,2 which often makes the diagnosis of melanoma challenging and ultimately can lead to diagnostic uncertainty.2
The incidence of malignant melanoma continues to grow.3 Maintaining a high degree of suspicion for this disease, recognizing its heterogeneity and divergent differentiation, and knowing potential aberrant immunohistochemical staining patterns are imperative for accurate diagnosis.
A 36-year-old man presented to a primary care physician with right-sided chest pain, upper and lower back aches, bilateral hip pain, neck pain, headache, night sweats, chills, and nausea. After infectious causes were ruled out, he was placed on a steroid taper without improvement. He presented to the emergency department a few days later with muscle spasms and was found to also have diffuse abdominal tenderness and guarding. The patient’s medical history was noncontributory; he was a lifelong nonsmoker. Laboratory studies revealed elevated levels of alanine aminotransferase and C-reactive protein. Computed tomography of the chest and abdomen revealed innumerable liver and lung lesions that were suspicious for metastatic malignancy. A liver biopsy revealed nests and sheets of metastatic tumor with pleomorphic nuclei, inconspicuous nucleoli, and areas of intranuclear clearing (Figures 1 and 2). Immunohistochemical staining was performed to further characterize the tumor. Neoplastic cells were positive for MART-1 (also known as Melan-A and melanoma-associated antigen recognized by T cells)(Figure 3), SOX10, S-100, HMB-45, and vimentin. Nonspecific staining with CD56 (Figure 4), a neuroendocrine marker, also was noted; however, the neoplasm was negative for synaptophysin, another neuroendocrine marker. Other markers for which staining was negative included pan-keratin, CD138 (syndecan-1), desmin, placental alkaline phosphatase (PLAP), inhibin, OCT-4, cytokeratin 7, and cytokeratin 20. This staining pattern was compatible with metastatic melanoma with aberrant CD56 expression.

BRAF V600E immunohistochemical staining also was performed and showed strong and diffuse positivity within neoplastic cells. A subsequent positron emission tomography scan revealed widespread metastatic disease involving the lungs, liver, spleen, and bones. The patient did not have a history of an excised skin lesion; no primary cutaneous or mucosal lesions were identified.

The patient was started on targeted therapy with trametinib, a mitogen-activated extracellular signal-related kinase kinase (MEK) inhibitor, and dabrafenib, a BRAF inhibitor. The disease continued to progress; he developed extensive leptomeningeal metastatic disease for which palliative radiation therapy was administered. The patient died 4 months after the initial diagnosis.

More than 90% of melanoma cases are of cutaneous origin; however, 4% to 8% of cases present as a metastatic lesion in the absence of an identified primary lesion,4 similar to our patient. The diagnosis of melanoma often is challenging; the tumor can show notable histologic diversity and has the potential to express aberrant immunophenotypes.1,2 The histologic diversity of melanoma includes a variety of architectural patterns (eg, nests, trabeculae, fascicular, pseudoglandular, pseudopapillary, or pseudorosette patterns), cytomorphologic features, and stromal changes. Cytomorphologic features of melanoma can be large pleomorphic cells; small cells; spindle cells; clear cells; signet-ring cells; and rhabdoid, plasmacytoid, and balloon cells.5

Melanoma can mimic carcinoma, sarcoma, lymphoma, benign stromal tumors, plasmacytoma, and germ-cell tumors.5 Nuclei can binucleated, multinucleated, or lobated and may contain inclusions or grooves. Stroma may become myxoid or desmoplastic in appearance or rarely show granulomatous inflammation or osteoclastic giant cells.5 These variations render the diagnosis of melanoma challenging and ultimately can lead to diagnostic uncertainty.
Melanomas typically express MART-1, HMB-45, S-100, tyrosinase, NK1C3, vimentin, and neuron-specific enolase. However, melanoma is among the many neoplasms that sometimes exhibit aberrant immunoreactivity and differentiation toward nonmelanocytic elements.6 The most commonly expressed immunophenotypic aberration is cytokeratin, especially the low-molecular-weight keratin marker CAM5.2.5 CAM5.2 positivity also is seen more often in metastatic melanoma. Melanomas rarely express other intermediate filaments, including desmin, neurofilament protein, and glial fibrillary acidic protein; expression of smooth-muscle actin is rare.5
Only a few cases of melanoma showing expression of neuroendocrine markers have been reported. However, one study reported synaptophysin positivity in 29% (10/34) of cases of primary and metastatic melanoma, making the stain a relatively common finding.1
In contrast, expression of CD56 (also known as neural-cell adhesion molecule 1) in melanoma has been reported only rarely. CD56 is a nonspecific neuroendocrine marker that normally is expressed on neurons, glial tissue, skeletal muscle, and natural killer cells. Riddle and Bui7 reported a case of metastatic malignant melanoma with focal CD56 positivity and no expression of other neuroendocrine markers, similar to our patient. Suzuki and colleagues4 also reported a case of melanoma metastatic to bone marrow that showed CD56 expression in true nonhematologic tumor cells and negative immunoreactivity with synaptophysin and chromogranin A.
It is important to document cases of melanoma that express neuroendocrine markers to prevent an incorrect diagnosis of a neuroendocrine tumor.1 In some cases, distinguishing amelanotic melanoma from poorly differentiated squamous cell carcinoma, neuroendocrine tumor, and lymphoma can be difficult.5
The term neuroendocrine differentiation is reserved for cases of melanoma that show areas of ultrastructural change consistent with a neuroendocrine tumor.2 Neuroendocrine differentiation in melanoma is not common; its prognostic significance is unknown.8 We do not consider our case to be true neuroendocrine differentiation, as the tumor lacked the morphologic changes of a neuroendocrine tumor. Furthermore, CD56 is a nonspecific neuroendocrine marker, and the tumor was negative for synaptophysin.
Melanoma has the potential to show notable histologic diversity as well as aberrant immunohistochemical staining patterns.1,2 Our patient had metastatic melanoma with aberrant neuroendocrine expression of CD56, which could have been a potential diagnostic pitfall. Because expression of CD56 in melanoma is rare, it is imperative to recognize this potential aberrant staining pattern to ensure the accurate diagnosis of melanoma and appropriate provision of care.
To the Editor:
Many types of neoplasms can show aberrant immunoreactivity or unexpected expression of markers.1 Malignant melanoma is a tumor that can show not only aberrant immunohistochemical staining patterns but also notable histologic diversity,1,2 which often makes the diagnosis of melanoma challenging and ultimately can lead to diagnostic uncertainty.2
The incidence of malignant melanoma continues to grow.3 Maintaining a high degree of suspicion for this disease, recognizing its heterogeneity and divergent differentiation, and knowing potential aberrant immunohistochemical staining patterns are imperative for accurate diagnosis.
A 36-year-old man presented to a primary care physician with right-sided chest pain, upper and lower back aches, bilateral hip pain, neck pain, headache, night sweats, chills, and nausea. After infectious causes were ruled out, he was placed on a steroid taper without improvement. He presented to the emergency department a few days later with muscle spasms and was found to also have diffuse abdominal tenderness and guarding. The patient’s medical history was noncontributory; he was a lifelong nonsmoker. Laboratory studies revealed elevated levels of alanine aminotransferase and C-reactive protein. Computed tomography of the chest and abdomen revealed innumerable liver and lung lesions that were suspicious for metastatic malignancy. A liver biopsy revealed nests and sheets of metastatic tumor with pleomorphic nuclei, inconspicuous nucleoli, and areas of intranuclear clearing (Figures 1 and 2). Immunohistochemical staining was performed to further characterize the tumor. Neoplastic cells were positive for MART-1 (also known as Melan-A and melanoma-associated antigen recognized by T cells)(Figure 3), SOX10, S-100, HMB-45, and vimentin. Nonspecific staining with CD56 (Figure 4), a neuroendocrine marker, also was noted; however, the neoplasm was negative for synaptophysin, another neuroendocrine marker. Other markers for which staining was negative included pan-keratin, CD138 (syndecan-1), desmin, placental alkaline phosphatase (PLAP), inhibin, OCT-4, cytokeratin 7, and cytokeratin 20. This staining pattern was compatible with metastatic melanoma with aberrant CD56 expression.

BRAF V600E immunohistochemical staining also was performed and showed strong and diffuse positivity within neoplastic cells. A subsequent positron emission tomography scan revealed widespread metastatic disease involving the lungs, liver, spleen, and bones. The patient did not have a history of an excised skin lesion; no primary cutaneous or mucosal lesions were identified.

The patient was started on targeted therapy with trametinib, a mitogen-activated extracellular signal-related kinase kinase (MEK) inhibitor, and dabrafenib, a BRAF inhibitor. The disease continued to progress; he developed extensive leptomeningeal metastatic disease for which palliative radiation therapy was administered. The patient died 4 months after the initial diagnosis.

More than 90% of melanoma cases are of cutaneous origin; however, 4% to 8% of cases present as a metastatic lesion in the absence of an identified primary lesion,4 similar to our patient. The diagnosis of melanoma often is challenging; the tumor can show notable histologic diversity and has the potential to express aberrant immunophenotypes.1,2 The histologic diversity of melanoma includes a variety of architectural patterns (eg, nests, trabeculae, fascicular, pseudoglandular, pseudopapillary, or pseudorosette patterns), cytomorphologic features, and stromal changes. Cytomorphologic features of melanoma can be large pleomorphic cells; small cells; spindle cells; clear cells; signet-ring cells; and rhabdoid, plasmacytoid, and balloon cells.5

Melanoma can mimic carcinoma, sarcoma, lymphoma, benign stromal tumors, plasmacytoma, and germ-cell tumors.5 Nuclei can binucleated, multinucleated, or lobated and may contain inclusions or grooves. Stroma may become myxoid or desmoplastic in appearance or rarely show granulomatous inflammation or osteoclastic giant cells.5 These variations render the diagnosis of melanoma challenging and ultimately can lead to diagnostic uncertainty.
Melanomas typically express MART-1, HMB-45, S-100, tyrosinase, NK1C3, vimentin, and neuron-specific enolase. However, melanoma is among the many neoplasms that sometimes exhibit aberrant immunoreactivity and differentiation toward nonmelanocytic elements.6 The most commonly expressed immunophenotypic aberration is cytokeratin, especially the low-molecular-weight keratin marker CAM5.2.5 CAM5.2 positivity also is seen more often in metastatic melanoma. Melanomas rarely express other intermediate filaments, including desmin, neurofilament protein, and glial fibrillary acidic protein; expression of smooth-muscle actin is rare.5
Only a few cases of melanoma showing expression of neuroendocrine markers have been reported. However, one study reported synaptophysin positivity in 29% (10/34) of cases of primary and metastatic melanoma, making the stain a relatively common finding.1
In contrast, expression of CD56 (also known as neural-cell adhesion molecule 1) in melanoma has been reported only rarely. CD56 is a nonspecific neuroendocrine marker that normally is expressed on neurons, glial tissue, skeletal muscle, and natural killer cells. Riddle and Bui7 reported a case of metastatic malignant melanoma with focal CD56 positivity and no expression of other neuroendocrine markers, similar to our patient. Suzuki and colleagues4 also reported a case of melanoma metastatic to bone marrow that showed CD56 expression in true nonhematologic tumor cells and negative immunoreactivity with synaptophysin and chromogranin A.
It is important to document cases of melanoma that express neuroendocrine markers to prevent an incorrect diagnosis of a neuroendocrine tumor.1 In some cases, distinguishing amelanotic melanoma from poorly differentiated squamous cell carcinoma, neuroendocrine tumor, and lymphoma can be difficult.5
The term neuroendocrine differentiation is reserved for cases of melanoma that show areas of ultrastructural change consistent with a neuroendocrine tumor.2 Neuroendocrine differentiation in melanoma is not common; its prognostic significance is unknown.8 We do not consider our case to be true neuroendocrine differentiation, as the tumor lacked the morphologic changes of a neuroendocrine tumor. Furthermore, CD56 is a nonspecific neuroendocrine marker, and the tumor was negative for synaptophysin.
Melanoma has the potential to show notable histologic diversity as well as aberrant immunohistochemical staining patterns.1,2 Our patient had metastatic melanoma with aberrant neuroendocrine expression of CD56, which could have been a potential diagnostic pitfall. Because expression of CD56 in melanoma is rare, it is imperative to recognize this potential aberrant staining pattern to ensure the accurate diagnosis of melanoma and appropriate provision of care.
1. Romano RC, Carter JM, Folpe AL. Aberrant intermediate filament and synaptophysin expression is a frequent event in malignant melanoma: an immunohistochemical study of 73 cases. Mod Pathol. 2015;28:1033-1042. doi:10.1038/modpathol.2015.62
2. Eyden B, Pandit D, Banerjee SS. Malignant melanoma with neuroendocrine differentiation: clinical, histological, immunohistochemical and ultrastructural features of three cases. Histopathology. 2005;47:402-409. doi:10.1111/j.1365-2559.2005.02240.x
3. Katerji H, Childs JM, Bratton LE, et al. Primary esophageal melanoma with aberrant CD56 expression: a potential diagnostic pitfall. Case Rep Pathol. 2017;2017:9052637. doi:10.1155/2017/9052637
4. Suzuki T, Kusumoto S, Iida S, et al. Amelanotic malignant melanoma of unknown primary origin metastasizing to the bone marrow: a case report and review of the literature. Intern Med. 2014;53:325-328. doi:10.2169/internalmedicine.53.1412
5. Banerjee SS, Harris M. Morphological and immunophenotypic variations in malignant melanoma. Histopathology. 2000;36:387-402. doi:10.1046/j.1365-2559.2000.00894.x
6. Banerjee SS, Eyden B. Divergent differentiation in malignant melanomas: a review. Histopathology. 2008;52:119-129. doi:10.1111/j.1365-2559.2007.02823.x
7. Riddle ND, Bui MM. When melanoma is negative for S100: diagnostic pitfalls. Arch Pathol Lab Med. 2012;136:237-239. doi:10.5858/arpa.2011-0405-LE
8. Ilardi G, Caroppo D, Varricchio S, et al. Anal melanoma with neuroendocrine differentiation: report of a case. Int J Surg Pathol. 2015;23:329-332. doi:10.1177/1066896915573568
1. Romano RC, Carter JM, Folpe AL. Aberrant intermediate filament and synaptophysin expression is a frequent event in malignant melanoma: an immunohistochemical study of 73 cases. Mod Pathol. 2015;28:1033-1042. doi:10.1038/modpathol.2015.62
2. Eyden B, Pandit D, Banerjee SS. Malignant melanoma with neuroendocrine differentiation: clinical, histological, immunohistochemical and ultrastructural features of three cases. Histopathology. 2005;47:402-409. doi:10.1111/j.1365-2559.2005.02240.x
3. Katerji H, Childs JM, Bratton LE, et al. Primary esophageal melanoma with aberrant CD56 expression: a potential diagnostic pitfall. Case Rep Pathol. 2017;2017:9052637. doi:10.1155/2017/9052637
4. Suzuki T, Kusumoto S, Iida S, et al. Amelanotic malignant melanoma of unknown primary origin metastasizing to the bone marrow: a case report and review of the literature. Intern Med. 2014;53:325-328. doi:10.2169/internalmedicine.53.1412
5. Banerjee SS, Harris M. Morphological and immunophenotypic variations in malignant melanoma. Histopathology. 2000;36:387-402. doi:10.1046/j.1365-2559.2000.00894.x
6. Banerjee SS, Eyden B. Divergent differentiation in malignant melanomas: a review. Histopathology. 2008;52:119-129. doi:10.1111/j.1365-2559.2007.02823.x
7. Riddle ND, Bui MM. When melanoma is negative for S100: diagnostic pitfalls. Arch Pathol Lab Med. 2012;136:237-239. doi:10.5858/arpa.2011-0405-LE
8. Ilardi G, Caroppo D, Varricchio S, et al. Anal melanoma with neuroendocrine differentiation: report of a case. Int J Surg Pathol. 2015;23:329-332. doi:10.1177/1066896915573568
Practice Points
- The diagnosis of melanoma often is challenging as tumors can show notable histologic diversity and have the potential to express aberrant immunophenotypes including CD56 expression.
- Because expression of CD56 in melanoma is rare, it is important to be aware of this potential aberrant staining pattern.
- Recognizing this heterogeneity and divergent differentiation as well as knowing potential aberrant immunohistochemical staining patterns are imperative for accurate and timely diagnosis.
Lower-extremity lymphedema associated with more skin cancer risk
TOPLINE:
.
METHODOLOGY:
- In the retrospective cohort study, researchers reviewed reports at Mayo Clinic for all patients who had LE lymphedema, limiting the review to those who had an ICD code for lymphedema.
- 4,437 patients with the ICD code from 2000 to 2020 were compared with 4,437 matched controls.
- The records of patients with skin cancer diagnoses were reviewed manually to determine whether the skin cancer, its management, or both were a cause of lymphedema; cancers that caused secondary lymphedema were excluded.
- This is the first large-scale study evaluating the association between LE lymphedema and LE skin cancer.
TAKEAWAY:
- 211 patients (4.6%) in the LE lymphedema group had any ICD code for LE skin cancer, compared with 89 (2%) in the control group.
- Among those with LE lymphedema, the risk for skin cancer was 1.98 times greater compared with those without lymphedema (95% confidence interval, 1.43-2.74; P < .001). Cases included all types of skin cancer.
- Nineteen of 24 patients with unilateral LE lymphedema had a history of immunosuppression.
- In the group of 24 patients with unilateral LE lymphedema, the lymphedematous LE was more likely to have one or more skin cancers than were the unaffected LE (87.5% vs. 33.3%; P < .05), and skin cancer was 2.65 times more likely to develop on the affected LE than in the unaffected LE (95% CI, 1.17-5.99; P = .02).
IN PRACTICE:
“Our findings suggest the need for a relatively high degree of suspicion of skin cancer at sites with lymphedema,” senior author, Afsaneh Alavi, MD, professor of dermatology at the Mayo Clinic, said in a Mayo Clinic press release reporting the results.
SOURCE:
The study was conducted by researchers at the Mayo Clinic and Meharry Medical College, Nashville. It was published in the November 2023 Mayo Clinic Proceedings.
LIMITATIONS:
This was a single-center retrospective study, and patients with LE lymphedema may be overdiagnosed with LE skin cancer because they have a greater number of examinations.
DISCLOSURES:
Dr. Alavi reports having been a consultant for AbbVie, Boehringer Ingelheim, InflaRx, Novartis, and UCB SA and an investigator for Processa Pharmaceuticals and Boehringer Ingelheim. The other authors had no disclosures.
A version of this article first appeared on Medscape.com.
TOPLINE:
.
METHODOLOGY:
- In the retrospective cohort study, researchers reviewed reports at Mayo Clinic for all patients who had LE lymphedema, limiting the review to those who had an ICD code for lymphedema.
- 4,437 patients with the ICD code from 2000 to 2020 were compared with 4,437 matched controls.
- The records of patients with skin cancer diagnoses were reviewed manually to determine whether the skin cancer, its management, or both were a cause of lymphedema; cancers that caused secondary lymphedema were excluded.
- This is the first large-scale study evaluating the association between LE lymphedema and LE skin cancer.
TAKEAWAY:
- 211 patients (4.6%) in the LE lymphedema group had any ICD code for LE skin cancer, compared with 89 (2%) in the control group.
- Among those with LE lymphedema, the risk for skin cancer was 1.98 times greater compared with those without lymphedema (95% confidence interval, 1.43-2.74; P < .001). Cases included all types of skin cancer.
- Nineteen of 24 patients with unilateral LE lymphedema had a history of immunosuppression.
- In the group of 24 patients with unilateral LE lymphedema, the lymphedematous LE was more likely to have one or more skin cancers than were the unaffected LE (87.5% vs. 33.3%; P < .05), and skin cancer was 2.65 times more likely to develop on the affected LE than in the unaffected LE (95% CI, 1.17-5.99; P = .02).
IN PRACTICE:
“Our findings suggest the need for a relatively high degree of suspicion of skin cancer at sites with lymphedema,” senior author, Afsaneh Alavi, MD, professor of dermatology at the Mayo Clinic, said in a Mayo Clinic press release reporting the results.
SOURCE:
The study was conducted by researchers at the Mayo Clinic and Meharry Medical College, Nashville. It was published in the November 2023 Mayo Clinic Proceedings.
LIMITATIONS:
This was a single-center retrospective study, and patients with LE lymphedema may be overdiagnosed with LE skin cancer because they have a greater number of examinations.
DISCLOSURES:
Dr. Alavi reports having been a consultant for AbbVie, Boehringer Ingelheim, InflaRx, Novartis, and UCB SA and an investigator for Processa Pharmaceuticals and Boehringer Ingelheim. The other authors had no disclosures.
A version of this article first appeared on Medscape.com.
TOPLINE:
.
METHODOLOGY:
- In the retrospective cohort study, researchers reviewed reports at Mayo Clinic for all patients who had LE lymphedema, limiting the review to those who had an ICD code for lymphedema.
- 4,437 patients with the ICD code from 2000 to 2020 were compared with 4,437 matched controls.
- The records of patients with skin cancer diagnoses were reviewed manually to determine whether the skin cancer, its management, or both were a cause of lymphedema; cancers that caused secondary lymphedema were excluded.
- This is the first large-scale study evaluating the association between LE lymphedema and LE skin cancer.
TAKEAWAY:
- 211 patients (4.6%) in the LE lymphedema group had any ICD code for LE skin cancer, compared with 89 (2%) in the control group.
- Among those with LE lymphedema, the risk for skin cancer was 1.98 times greater compared with those without lymphedema (95% confidence interval, 1.43-2.74; P < .001). Cases included all types of skin cancer.
- Nineteen of 24 patients with unilateral LE lymphedema had a history of immunosuppression.
- In the group of 24 patients with unilateral LE lymphedema, the lymphedematous LE was more likely to have one or more skin cancers than were the unaffected LE (87.5% vs. 33.3%; P < .05), and skin cancer was 2.65 times more likely to develop on the affected LE than in the unaffected LE (95% CI, 1.17-5.99; P = .02).
IN PRACTICE:
“Our findings suggest the need for a relatively high degree of suspicion of skin cancer at sites with lymphedema,” senior author, Afsaneh Alavi, MD, professor of dermatology at the Mayo Clinic, said in a Mayo Clinic press release reporting the results.
SOURCE:
The study was conducted by researchers at the Mayo Clinic and Meharry Medical College, Nashville. It was published in the November 2023 Mayo Clinic Proceedings.
LIMITATIONS:
This was a single-center retrospective study, and patients with LE lymphedema may be overdiagnosed with LE skin cancer because they have a greater number of examinations.
DISCLOSURES:
Dr. Alavi reports having been a consultant for AbbVie, Boehringer Ingelheim, InflaRx, Novartis, and UCB SA and an investigator for Processa Pharmaceuticals and Boehringer Ingelheim. The other authors had no disclosures.
A version of this article first appeared on Medscape.com.
Sharps injuries are common among Mohs surgeons, survey finds
TOPLINE:
.
METHODOLOGY:
- Data on the incidence of sharps injuries among dermatologic surgeons is limited.
- In a cross-sectional analysis of anonymous survey responses from members of the American College of , researchers aimed to determine the incidence and types of sharps injuries among Mohs surgeons.
- The researchers used descriptive statistics for continuous and nominal variables (percentage and frequencies) to report survey data and Fisher exact or chi-square analysis of categorical variables to obtain P values.
TAKEAWAY:
- Of the 60 survey respondents, more than half (56.7%) were from single-specialty group practices, 26.6% were from academic practices, and fewer than half (43.3%) had been in practice for 15 or more years.
- In the past year, 56.7% of respondents experienced at least one sharps injury. Of these, 14.7% involved exposure to a blood-borne pathogen, which translated into an annual exposure risk of 7.6% for any given Mohs surgeon.
- The top two types of sharps injuries were self-inflicted suture needlestick (76.5%) and other types of self-inflicted needlestick injuries (26.5%).
- Of respondents who sustained a sharps injury, 44.1% did not report them, while 95% of all survey respondents said they had access to postexposure prophylaxis/protocols at their workplace.
- The researchers determined that the average annual rate of sharps injury was 0.87.
IN PRACTICE:
- “In best practices to prevent sharps injuries, the authors recommend that a standardized sharps handling protocol be developed and disseminated for dermatologic surgeons and their staff,” the researchers wrote.
STUDY DETAILS:
- Faezeh Talebi-Liasi, MD, and Jesse M. Lewin, MD, department of dermatology, Icahn School of Medicine at Mount Sinai, New York, conducted the research. The study was published in Dermatologic Surgery.
LIMITATIONS:
- The study’s cross-sectional observational design and small sample size was skewed toward single-specialty and academic practices.
DISCLOSURES:
- The authors reported having no relevant financial disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
.
METHODOLOGY:
- Data on the incidence of sharps injuries among dermatologic surgeons is limited.
- In a cross-sectional analysis of anonymous survey responses from members of the American College of , researchers aimed to determine the incidence and types of sharps injuries among Mohs surgeons.
- The researchers used descriptive statistics for continuous and nominal variables (percentage and frequencies) to report survey data and Fisher exact or chi-square analysis of categorical variables to obtain P values.
TAKEAWAY:
- Of the 60 survey respondents, more than half (56.7%) were from single-specialty group practices, 26.6% were from academic practices, and fewer than half (43.3%) had been in practice for 15 or more years.
- In the past year, 56.7% of respondents experienced at least one sharps injury. Of these, 14.7% involved exposure to a blood-borne pathogen, which translated into an annual exposure risk of 7.6% for any given Mohs surgeon.
- The top two types of sharps injuries were self-inflicted suture needlestick (76.5%) and other types of self-inflicted needlestick injuries (26.5%).
- Of respondents who sustained a sharps injury, 44.1% did not report them, while 95% of all survey respondents said they had access to postexposure prophylaxis/protocols at their workplace.
- The researchers determined that the average annual rate of sharps injury was 0.87.
IN PRACTICE:
- “In best practices to prevent sharps injuries, the authors recommend that a standardized sharps handling protocol be developed and disseminated for dermatologic surgeons and their staff,” the researchers wrote.
STUDY DETAILS:
- Faezeh Talebi-Liasi, MD, and Jesse M. Lewin, MD, department of dermatology, Icahn School of Medicine at Mount Sinai, New York, conducted the research. The study was published in Dermatologic Surgery.
LIMITATIONS:
- The study’s cross-sectional observational design and small sample size was skewed toward single-specialty and academic practices.
DISCLOSURES:
- The authors reported having no relevant financial disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
.
METHODOLOGY:
- Data on the incidence of sharps injuries among dermatologic surgeons is limited.
- In a cross-sectional analysis of anonymous survey responses from members of the American College of , researchers aimed to determine the incidence and types of sharps injuries among Mohs surgeons.
- The researchers used descriptive statistics for continuous and nominal variables (percentage and frequencies) to report survey data and Fisher exact or chi-square analysis of categorical variables to obtain P values.
TAKEAWAY:
- Of the 60 survey respondents, more than half (56.7%) were from single-specialty group practices, 26.6% were from academic practices, and fewer than half (43.3%) had been in practice for 15 or more years.
- In the past year, 56.7% of respondents experienced at least one sharps injury. Of these, 14.7% involved exposure to a blood-borne pathogen, which translated into an annual exposure risk of 7.6% for any given Mohs surgeon.
- The top two types of sharps injuries were self-inflicted suture needlestick (76.5%) and other types of self-inflicted needlestick injuries (26.5%).
- Of respondents who sustained a sharps injury, 44.1% did not report them, while 95% of all survey respondents said they had access to postexposure prophylaxis/protocols at their workplace.
- The researchers determined that the average annual rate of sharps injury was 0.87.
IN PRACTICE:
- “In best practices to prevent sharps injuries, the authors recommend that a standardized sharps handling protocol be developed and disseminated for dermatologic surgeons and their staff,” the researchers wrote.
STUDY DETAILS:
- Faezeh Talebi-Liasi, MD, and Jesse M. Lewin, MD, department of dermatology, Icahn School of Medicine at Mount Sinai, New York, conducted the research. The study was published in Dermatologic Surgery.
LIMITATIONS:
- The study’s cross-sectional observational design and small sample size was skewed toward single-specialty and academic practices.
DISCLOSURES:
- The authors reported having no relevant financial disclosures.
A version of this article appeared on Medscape.com.
Actinic keratoses may predict skin cancers in older adults
TOPLINE:
.
METHODOLOGY:
- AKs have been associated with a small risk for cutaneous SCC, but associations with risk for other skin cancers have not been well studied.
- AKs may be a marker of overall skin cancer risk, but guidelines for AK management lack recommendations for follow-up cancer surveillance.
- The researchers reviewed data from a random sample of 5 million fee-for-service Medicare beneficiaries treated for AKs from 2009 through 2018 in the United States. Patients with seborrheic keratoses (SKs) were included as comparators, and patients with a history of skin cancer were excluded.
- The primary outcome was the first surgically treated skin cancer, including SCC, BCC, and melanoma.
TAKEAWAY:
- A total of 555,945 adults with AKs and 481,024 with SKs were included. The mean age was approximately 74.0 years. More than half were female. Most were non-Hispanic White.
- Among patients with AKs, the absolute risk for any skin cancer after the first AK was 6.3%, 18.4%, and 28.5% at 1, 3, and 5 years, respectively.
- Patients with AKs had a significantly increased relative risk for any skin cancer compared with those with SKs (adjusted hazard ratio [aHR], 2.17) and separately for keratinocyte carcinoma (aHR, 2.20), SCC (aHR, 2.63), BCC (aHR, 1.85), and melanoma (aHR, 1.67).
- Although AKs are not considered a biological precursor of melanoma or BCC, the results suggest that AKs may be clinical indicators of increased UV exposure that subsequently increases the risk for skin cancer.
IN PRACTICE:
“The present results highlight the importance of developing evidence-based guidelines for follow-up skin cancer surveillance in patients with AKs, optimally including measures of AK burden,” the researchers wrote.
SOURCE:
The lead author on the study was Cassandra Mohr, BS, with corresponding author Mackenzie R. Wehner, MD, MPhil, of The University of Texas MD Anderson Cancer Center, Houston. The study was published online in JAMA Dermatology .
LIMITATIONS:
The study population of Medicare beneficiaries aged 65 years or older may not be a nationally representative sample, and surveillance bias may contribute to the increased risk for skin cancer in patients with AKs. The use of both ICD and CPT codes may underestimate the number of skin cancers because of cases that were treated nonsurgically.
DISCLOSURES:
The study was supported by the National Cancer Institute of the National Institutes of Health, the Cancer Prevention and Research Institute of Texas, and The University of Texas Rising STARS program. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
.
METHODOLOGY:
- AKs have been associated with a small risk for cutaneous SCC, but associations with risk for other skin cancers have not been well studied.
- AKs may be a marker of overall skin cancer risk, but guidelines for AK management lack recommendations for follow-up cancer surveillance.
- The researchers reviewed data from a random sample of 5 million fee-for-service Medicare beneficiaries treated for AKs from 2009 through 2018 in the United States. Patients with seborrheic keratoses (SKs) were included as comparators, and patients with a history of skin cancer were excluded.
- The primary outcome was the first surgically treated skin cancer, including SCC, BCC, and melanoma.
TAKEAWAY:
- A total of 555,945 adults with AKs and 481,024 with SKs were included. The mean age was approximately 74.0 years. More than half were female. Most were non-Hispanic White.
- Among patients with AKs, the absolute risk for any skin cancer after the first AK was 6.3%, 18.4%, and 28.5% at 1, 3, and 5 years, respectively.
- Patients with AKs had a significantly increased relative risk for any skin cancer compared with those with SKs (adjusted hazard ratio [aHR], 2.17) and separately for keratinocyte carcinoma (aHR, 2.20), SCC (aHR, 2.63), BCC (aHR, 1.85), and melanoma (aHR, 1.67).
- Although AKs are not considered a biological precursor of melanoma or BCC, the results suggest that AKs may be clinical indicators of increased UV exposure that subsequently increases the risk for skin cancer.
IN PRACTICE:
“The present results highlight the importance of developing evidence-based guidelines for follow-up skin cancer surveillance in patients with AKs, optimally including measures of AK burden,” the researchers wrote.
SOURCE:
The lead author on the study was Cassandra Mohr, BS, with corresponding author Mackenzie R. Wehner, MD, MPhil, of The University of Texas MD Anderson Cancer Center, Houston. The study was published online in JAMA Dermatology .
LIMITATIONS:
The study population of Medicare beneficiaries aged 65 years or older may not be a nationally representative sample, and surveillance bias may contribute to the increased risk for skin cancer in patients with AKs. The use of both ICD and CPT codes may underestimate the number of skin cancers because of cases that were treated nonsurgically.
DISCLOSURES:
The study was supported by the National Cancer Institute of the National Institutes of Health, the Cancer Prevention and Research Institute of Texas, and The University of Texas Rising STARS program. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
.
METHODOLOGY:
- AKs have been associated with a small risk for cutaneous SCC, but associations with risk for other skin cancers have not been well studied.
- AKs may be a marker of overall skin cancer risk, but guidelines for AK management lack recommendations for follow-up cancer surveillance.
- The researchers reviewed data from a random sample of 5 million fee-for-service Medicare beneficiaries treated for AKs from 2009 through 2018 in the United States. Patients with seborrheic keratoses (SKs) were included as comparators, and patients with a history of skin cancer were excluded.
- The primary outcome was the first surgically treated skin cancer, including SCC, BCC, and melanoma.
TAKEAWAY:
- A total of 555,945 adults with AKs and 481,024 with SKs were included. The mean age was approximately 74.0 years. More than half were female. Most were non-Hispanic White.
- Among patients with AKs, the absolute risk for any skin cancer after the first AK was 6.3%, 18.4%, and 28.5% at 1, 3, and 5 years, respectively.
- Patients with AKs had a significantly increased relative risk for any skin cancer compared with those with SKs (adjusted hazard ratio [aHR], 2.17) and separately for keratinocyte carcinoma (aHR, 2.20), SCC (aHR, 2.63), BCC (aHR, 1.85), and melanoma (aHR, 1.67).
- Although AKs are not considered a biological precursor of melanoma or BCC, the results suggest that AKs may be clinical indicators of increased UV exposure that subsequently increases the risk for skin cancer.
IN PRACTICE:
“The present results highlight the importance of developing evidence-based guidelines for follow-up skin cancer surveillance in patients with AKs, optimally including measures of AK burden,” the researchers wrote.
SOURCE:
The lead author on the study was Cassandra Mohr, BS, with corresponding author Mackenzie R. Wehner, MD, MPhil, of The University of Texas MD Anderson Cancer Center, Houston. The study was published online in JAMA Dermatology .
LIMITATIONS:
The study population of Medicare beneficiaries aged 65 years or older may not be a nationally representative sample, and surveillance bias may contribute to the increased risk for skin cancer in patients with AKs. The use of both ICD and CPT codes may underestimate the number of skin cancers because of cases that were treated nonsurgically.
DISCLOSURES:
The study was supported by the National Cancer Institute of the National Institutes of Health, the Cancer Prevention and Research Institute of Texas, and The University of Texas Rising STARS program. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Incipient ulceration may affect prognosis in primary melanoma
TOPLINE:
Incipient ulceration in primary cutaneous melanoma may represent a more biologically aggressive disease population than truly nonulcerated tumors.
METHODOLOGY:
- The final cohort included 40 cases of incipient ulceration that were matched 1:2 with 80 nonulcerated controls and 80 ulcerated controls.
- The prognostic significance of incipient ulceration in cutaneous melanoma is unclear.
- Current American Joint Committee on Cancer (AJCC) guidelines classify incipient ulceration as nonulcerated.
- In a retrospective case-control study, researchers drew from the Melanoma Institute Australia database to identify resected primary cutaneous melanomas diagnosed between 2005 and 2015 that had slides available at Royal Prince Alfred Hospital in Sydney and a Breslow thickness greater than 0 mm.
- Clinical outcomes compared between cases and controls were recurrence-free survival (RFS), melanoma-specific survival (MSS), and overall survival (OS).
TAKEAWAY:
- The median Breslow depth was 2.8 mm for incipient cases, compared with 1.0 mm for nonulcerated melanomas and 5.3 mm for ulcerated melanomas, while the median tumor mitotic rate was 5.0 per mm2 for incipient cases, compared with 1 per mm2 in nonulcerated controls and 9 per mm2 in ulcerated controls.
- On univariable analyses, compared with patients with incipiently ulcerated cases, patients with nonulcerated tumors had significantly better OS (hazard ratio [HR], 0.49) and RFS (HR, 0.37), while patients with ulcerated tumors showed worse RFS (HR, 1.67).
- On multivariable analyses, no differences in survival outcomes were observed, perhaps due to the moderate number of incipient ulceration cases included in the study, the authors wrote.
IN PRACTICE:
“Future editions of the AJCC staging system should consider acknowledging this interpretive challenge and provide guidance on how primary melanomas with incipient ulceration should be classified,” the researchers wrote.
SOURCE:
Richard A. Scolyer, MD, a pathologist at Royal Prince Alfred Hospital, Camperdown, Australia, is the senior author on the study, which was published online in JAMA Dermatology.
LIMITATIONS:
Limitations of the study include its retrospective design and the relatively small number of cases that met criteria for inclusion.
DISCLOSURES:
Dr. Scolyer disclosed that he has received grants from the Australian National Health and Medical Research Council and personal fees from MetaOptima, F. Hoffmann-La Roche, Evaxion, Provectus, QBiotics, Novartis, Merck Sharp & Dohme, NeraCare, Amgen, Bristol-Myers Squibb, Myriad Genetics, and GlaxoSmithKline, all outside the submitted work. Four coauthors reported having received financial support outside of the submitted work.
A version of this article appeared on Medscape.com.
TOPLINE:
Incipient ulceration in primary cutaneous melanoma may represent a more biologically aggressive disease population than truly nonulcerated tumors.
METHODOLOGY:
- The final cohort included 40 cases of incipient ulceration that were matched 1:2 with 80 nonulcerated controls and 80 ulcerated controls.
- The prognostic significance of incipient ulceration in cutaneous melanoma is unclear.
- Current American Joint Committee on Cancer (AJCC) guidelines classify incipient ulceration as nonulcerated.
- In a retrospective case-control study, researchers drew from the Melanoma Institute Australia database to identify resected primary cutaneous melanomas diagnosed between 2005 and 2015 that had slides available at Royal Prince Alfred Hospital in Sydney and a Breslow thickness greater than 0 mm.
- Clinical outcomes compared between cases and controls were recurrence-free survival (RFS), melanoma-specific survival (MSS), and overall survival (OS).
TAKEAWAY:
- The median Breslow depth was 2.8 mm for incipient cases, compared with 1.0 mm for nonulcerated melanomas and 5.3 mm for ulcerated melanomas, while the median tumor mitotic rate was 5.0 per mm2 for incipient cases, compared with 1 per mm2 in nonulcerated controls and 9 per mm2 in ulcerated controls.
- On univariable analyses, compared with patients with incipiently ulcerated cases, patients with nonulcerated tumors had significantly better OS (hazard ratio [HR], 0.49) and RFS (HR, 0.37), while patients with ulcerated tumors showed worse RFS (HR, 1.67).
- On multivariable analyses, no differences in survival outcomes were observed, perhaps due to the moderate number of incipient ulceration cases included in the study, the authors wrote.
IN PRACTICE:
“Future editions of the AJCC staging system should consider acknowledging this interpretive challenge and provide guidance on how primary melanomas with incipient ulceration should be classified,” the researchers wrote.
SOURCE:
Richard A. Scolyer, MD, a pathologist at Royal Prince Alfred Hospital, Camperdown, Australia, is the senior author on the study, which was published online in JAMA Dermatology.
LIMITATIONS:
Limitations of the study include its retrospective design and the relatively small number of cases that met criteria for inclusion.
DISCLOSURES:
Dr. Scolyer disclosed that he has received grants from the Australian National Health and Medical Research Council and personal fees from MetaOptima, F. Hoffmann-La Roche, Evaxion, Provectus, QBiotics, Novartis, Merck Sharp & Dohme, NeraCare, Amgen, Bristol-Myers Squibb, Myriad Genetics, and GlaxoSmithKline, all outside the submitted work. Four coauthors reported having received financial support outside of the submitted work.
A version of this article appeared on Medscape.com.
TOPLINE:
Incipient ulceration in primary cutaneous melanoma may represent a more biologically aggressive disease population than truly nonulcerated tumors.
METHODOLOGY:
- The final cohort included 40 cases of incipient ulceration that were matched 1:2 with 80 nonulcerated controls and 80 ulcerated controls.
- The prognostic significance of incipient ulceration in cutaneous melanoma is unclear.
- Current American Joint Committee on Cancer (AJCC) guidelines classify incipient ulceration as nonulcerated.
- In a retrospective case-control study, researchers drew from the Melanoma Institute Australia database to identify resected primary cutaneous melanomas diagnosed between 2005 and 2015 that had slides available at Royal Prince Alfred Hospital in Sydney and a Breslow thickness greater than 0 mm.
- Clinical outcomes compared between cases and controls were recurrence-free survival (RFS), melanoma-specific survival (MSS), and overall survival (OS).
TAKEAWAY:
- The median Breslow depth was 2.8 mm for incipient cases, compared with 1.0 mm for nonulcerated melanomas and 5.3 mm for ulcerated melanomas, while the median tumor mitotic rate was 5.0 per mm2 for incipient cases, compared with 1 per mm2 in nonulcerated controls and 9 per mm2 in ulcerated controls.
- On univariable analyses, compared with patients with incipiently ulcerated cases, patients with nonulcerated tumors had significantly better OS (hazard ratio [HR], 0.49) and RFS (HR, 0.37), while patients with ulcerated tumors showed worse RFS (HR, 1.67).
- On multivariable analyses, no differences in survival outcomes were observed, perhaps due to the moderate number of incipient ulceration cases included in the study, the authors wrote.
IN PRACTICE:
“Future editions of the AJCC staging system should consider acknowledging this interpretive challenge and provide guidance on how primary melanomas with incipient ulceration should be classified,” the researchers wrote.
SOURCE:
Richard A. Scolyer, MD, a pathologist at Royal Prince Alfred Hospital, Camperdown, Australia, is the senior author on the study, which was published online in JAMA Dermatology.
LIMITATIONS:
Limitations of the study include its retrospective design and the relatively small number of cases that met criteria for inclusion.
DISCLOSURES:
Dr. Scolyer disclosed that he has received grants from the Australian National Health and Medical Research Council and personal fees from MetaOptima, F. Hoffmann-La Roche, Evaxion, Provectus, QBiotics, Novartis, Merck Sharp & Dohme, NeraCare, Amgen, Bristol-Myers Squibb, Myriad Genetics, and GlaxoSmithKline, all outside the submitted work. Four coauthors reported having received financial support outside of the submitted work.
A version of this article appeared on Medscape.com.
Specialty-trained pathologists more likely to make higher-grade diagnoses for melanocytic lesions
, results from an exploratory study showed.
The findings “could in part play a role in the rising incidence of early-stage melanoma with low risk of progression or patient morbidity, thereby contributing to increasing rates of overdiagnosis,” researchers led by co–senior authors Joann G. Elmore, MD, MPH, of the University of California, Los Angeles, and Raymond L. Barnhill, MD, MBA, of the Institut Curie, Paris, wrote in their study, published online in JAMA Dermatology.
To investigate the characteristics associated with rendering higher-grade diagnoses, including invasive melanoma, the researchers drew from two national data sets: the Melanoma Pathology (M-Path) study, conducted from July 2013 to May 2016, and the Reducing Errors in Melanocytic Interpretations (REMI) study, conducted from August 2018 to March 2021. In both studies, pathologists who interpreted melanocytic lesions in their clinical practices interpreted study cases in glass slide format. For the current study, researchers used logistic regression to examine the association of pathologist characteristics with diagnosis of a study case as higher grade (including severely dysplastic and melanoma in situ) vs. lower grade (including mild to moderately dysplastic nevi) and diagnosis of invasive melanoma vs. any less severe diagnosis.
A total of 338 pathologists were included in the analysis. Of these, 113 were general pathologists and 225 were dermatopathologists (those who were board certified and/or fellowship trained in dermatopathology).
The researchers found that, compared with general pathologists, dermatopathologists were 2.63 times more likely to render higher-grade diagnoses and 1.95 times more likely to diagnose invasive melanoma (P < .001 for both associations). Diagnoses of stage pT1a melanomas with no mitotic activity completely accounted for the difference between dermatopathologists and general pathologists in diagnosing invasive melanoma.
For the analysis limited to the 225 dermatopathologists, those with a higher practice caseload of melanocytic lesions were more likely to assign higher-grade diagnoses (odds ratio for trend, 1.27; P = .02), while those affiliated with an academic center had lower odds of diagnosing invasive melanoma (OR, 0.61; P = .049).
The researchers acknowledged limitations of their analysis, including the lack of data on patient outcomes, “so we could not make conclusions about the clinical outcome of any particular diagnosis by a study participant,” they wrote. “While our analyses revealed pathologist characteristics associated with assigning more vs. less severe diagnoses of melanocytic lesions, we could not conclude that any particular diagnosis by a study participant was overcalling or undercalling. However, the epidemiologic evidence that melanoma is overdiagnosed suggests that overcalling by some pathologists may be contributing to increasing rates of low-risk melanoma diagnoses.”
In an accompanying editorial, authors Klaus J. Busam, MD, of the department of pathology and laboratory medicine at Memorial Sloan Kettering Cancer Center, New York, Pedram Gerami, MD, of the department of dermatology at Northwestern University, Chicago, and Richard A. Scolyer, MD, of the Melanoma Institute, Wollstonecraft, Australia, wrote that the study findings “raise the question of whether subspecialization in dermatopathology may be a factor contributing to the epidemiologic phenomenon of overdiagnosis – that is, the discordance in the rise of melanoma incidence and relatively constant annual mortality rates over many decades. The findings also invite a discussion about strategies to minimize harm from overdiagnosis for both patients and the health care system.”
To minimize misdiagnoses, they continued, efforts to facilitate diagnostic accuracy should be encouraged. “Excisional (rather than partial) biopsies and provision of relevant clinical information would facilitate rendering of the correct histopathologic diagnosis,” they wrote. “When the diagnosis is uncertain, this is best acknowledged. If felt necessary, a reexcision of a lesion with an uncertain diagnosis can be recommended without upgrading the diagnosis.”
In addition, “improvements in prognosis are needed beyond American Joint Committee on Cancer staging,” they noted. “This will likely require a multimodal approach with novel methods, including artificial intelligence and biomarkers that help distinguish low-risk melanomas, for which a conservative approach may be appropriate, from those that require surgical intervention.”
The study was supported by the National Center for Advancing Translational Sciences and by the National Institutes of Health. One author disclosed receiving grants from the National Cancer Institute during the conduct of the study, and another disclosed serving as editor in chief of Primary Care topics at UpToDate; other authors had no disclosures. Dr. Busam reported receiving nonfinancial support from the American Society of Dermatopathology. Dr. Gerami reported receiving consulting fees from Castle Biosciences. Dr. Scolyer reported receiving an investigator grant from the National Health and Medical Research Council of Australia during the conduct of the study and personal fees from several pharmaceutical companies outside the submitted work.
, results from an exploratory study showed.
The findings “could in part play a role in the rising incidence of early-stage melanoma with low risk of progression or patient morbidity, thereby contributing to increasing rates of overdiagnosis,” researchers led by co–senior authors Joann G. Elmore, MD, MPH, of the University of California, Los Angeles, and Raymond L. Barnhill, MD, MBA, of the Institut Curie, Paris, wrote in their study, published online in JAMA Dermatology.
To investigate the characteristics associated with rendering higher-grade diagnoses, including invasive melanoma, the researchers drew from two national data sets: the Melanoma Pathology (M-Path) study, conducted from July 2013 to May 2016, and the Reducing Errors in Melanocytic Interpretations (REMI) study, conducted from August 2018 to March 2021. In both studies, pathologists who interpreted melanocytic lesions in their clinical practices interpreted study cases in glass slide format. For the current study, researchers used logistic regression to examine the association of pathologist characteristics with diagnosis of a study case as higher grade (including severely dysplastic and melanoma in situ) vs. lower grade (including mild to moderately dysplastic nevi) and diagnosis of invasive melanoma vs. any less severe diagnosis.
A total of 338 pathologists were included in the analysis. Of these, 113 were general pathologists and 225 were dermatopathologists (those who were board certified and/or fellowship trained in dermatopathology).
The researchers found that, compared with general pathologists, dermatopathologists were 2.63 times more likely to render higher-grade diagnoses and 1.95 times more likely to diagnose invasive melanoma (P < .001 for both associations). Diagnoses of stage pT1a melanomas with no mitotic activity completely accounted for the difference between dermatopathologists and general pathologists in diagnosing invasive melanoma.
For the analysis limited to the 225 dermatopathologists, those with a higher practice caseload of melanocytic lesions were more likely to assign higher-grade diagnoses (odds ratio for trend, 1.27; P = .02), while those affiliated with an academic center had lower odds of diagnosing invasive melanoma (OR, 0.61; P = .049).
The researchers acknowledged limitations of their analysis, including the lack of data on patient outcomes, “so we could not make conclusions about the clinical outcome of any particular diagnosis by a study participant,” they wrote. “While our analyses revealed pathologist characteristics associated with assigning more vs. less severe diagnoses of melanocytic lesions, we could not conclude that any particular diagnosis by a study participant was overcalling or undercalling. However, the epidemiologic evidence that melanoma is overdiagnosed suggests that overcalling by some pathologists may be contributing to increasing rates of low-risk melanoma diagnoses.”
In an accompanying editorial, authors Klaus J. Busam, MD, of the department of pathology and laboratory medicine at Memorial Sloan Kettering Cancer Center, New York, Pedram Gerami, MD, of the department of dermatology at Northwestern University, Chicago, and Richard A. Scolyer, MD, of the Melanoma Institute, Wollstonecraft, Australia, wrote that the study findings “raise the question of whether subspecialization in dermatopathology may be a factor contributing to the epidemiologic phenomenon of overdiagnosis – that is, the discordance in the rise of melanoma incidence and relatively constant annual mortality rates over many decades. The findings also invite a discussion about strategies to minimize harm from overdiagnosis for both patients and the health care system.”
To minimize misdiagnoses, they continued, efforts to facilitate diagnostic accuracy should be encouraged. “Excisional (rather than partial) biopsies and provision of relevant clinical information would facilitate rendering of the correct histopathologic diagnosis,” they wrote. “When the diagnosis is uncertain, this is best acknowledged. If felt necessary, a reexcision of a lesion with an uncertain diagnosis can be recommended without upgrading the diagnosis.”
In addition, “improvements in prognosis are needed beyond American Joint Committee on Cancer staging,” they noted. “This will likely require a multimodal approach with novel methods, including artificial intelligence and biomarkers that help distinguish low-risk melanomas, for which a conservative approach may be appropriate, from those that require surgical intervention.”
The study was supported by the National Center for Advancing Translational Sciences and by the National Institutes of Health. One author disclosed receiving grants from the National Cancer Institute during the conduct of the study, and another disclosed serving as editor in chief of Primary Care topics at UpToDate; other authors had no disclosures. Dr. Busam reported receiving nonfinancial support from the American Society of Dermatopathology. Dr. Gerami reported receiving consulting fees from Castle Biosciences. Dr. Scolyer reported receiving an investigator grant from the National Health and Medical Research Council of Australia during the conduct of the study and personal fees from several pharmaceutical companies outside the submitted work.
, results from an exploratory study showed.
The findings “could in part play a role in the rising incidence of early-stage melanoma with low risk of progression or patient morbidity, thereby contributing to increasing rates of overdiagnosis,” researchers led by co–senior authors Joann G. Elmore, MD, MPH, of the University of California, Los Angeles, and Raymond L. Barnhill, MD, MBA, of the Institut Curie, Paris, wrote in their study, published online in JAMA Dermatology.
To investigate the characteristics associated with rendering higher-grade diagnoses, including invasive melanoma, the researchers drew from two national data sets: the Melanoma Pathology (M-Path) study, conducted from July 2013 to May 2016, and the Reducing Errors in Melanocytic Interpretations (REMI) study, conducted from August 2018 to March 2021. In both studies, pathologists who interpreted melanocytic lesions in their clinical practices interpreted study cases in glass slide format. For the current study, researchers used logistic regression to examine the association of pathologist characteristics with diagnosis of a study case as higher grade (including severely dysplastic and melanoma in situ) vs. lower grade (including mild to moderately dysplastic nevi) and diagnosis of invasive melanoma vs. any less severe diagnosis.
A total of 338 pathologists were included in the analysis. Of these, 113 were general pathologists and 225 were dermatopathologists (those who were board certified and/or fellowship trained in dermatopathology).
The researchers found that, compared with general pathologists, dermatopathologists were 2.63 times more likely to render higher-grade diagnoses and 1.95 times more likely to diagnose invasive melanoma (P < .001 for both associations). Diagnoses of stage pT1a melanomas with no mitotic activity completely accounted for the difference between dermatopathologists and general pathologists in diagnosing invasive melanoma.
For the analysis limited to the 225 dermatopathologists, those with a higher practice caseload of melanocytic lesions were more likely to assign higher-grade diagnoses (odds ratio for trend, 1.27; P = .02), while those affiliated with an academic center had lower odds of diagnosing invasive melanoma (OR, 0.61; P = .049).
The researchers acknowledged limitations of their analysis, including the lack of data on patient outcomes, “so we could not make conclusions about the clinical outcome of any particular diagnosis by a study participant,” they wrote. “While our analyses revealed pathologist characteristics associated with assigning more vs. less severe diagnoses of melanocytic lesions, we could not conclude that any particular diagnosis by a study participant was overcalling or undercalling. However, the epidemiologic evidence that melanoma is overdiagnosed suggests that overcalling by some pathologists may be contributing to increasing rates of low-risk melanoma diagnoses.”
In an accompanying editorial, authors Klaus J. Busam, MD, of the department of pathology and laboratory medicine at Memorial Sloan Kettering Cancer Center, New York, Pedram Gerami, MD, of the department of dermatology at Northwestern University, Chicago, and Richard A. Scolyer, MD, of the Melanoma Institute, Wollstonecraft, Australia, wrote that the study findings “raise the question of whether subspecialization in dermatopathology may be a factor contributing to the epidemiologic phenomenon of overdiagnosis – that is, the discordance in the rise of melanoma incidence and relatively constant annual mortality rates over many decades. The findings also invite a discussion about strategies to minimize harm from overdiagnosis for both patients and the health care system.”
To minimize misdiagnoses, they continued, efforts to facilitate diagnostic accuracy should be encouraged. “Excisional (rather than partial) biopsies and provision of relevant clinical information would facilitate rendering of the correct histopathologic diagnosis,” they wrote. “When the diagnosis is uncertain, this is best acknowledged. If felt necessary, a reexcision of a lesion with an uncertain diagnosis can be recommended without upgrading the diagnosis.”
In addition, “improvements in prognosis are needed beyond American Joint Committee on Cancer staging,” they noted. “This will likely require a multimodal approach with novel methods, including artificial intelligence and biomarkers that help distinguish low-risk melanomas, for which a conservative approach may be appropriate, from those that require surgical intervention.”
The study was supported by the National Center for Advancing Translational Sciences and by the National Institutes of Health. One author disclosed receiving grants from the National Cancer Institute during the conduct of the study, and another disclosed serving as editor in chief of Primary Care topics at UpToDate; other authors had no disclosures. Dr. Busam reported receiving nonfinancial support from the American Society of Dermatopathology. Dr. Gerami reported receiving consulting fees from Castle Biosciences. Dr. Scolyer reported receiving an investigator grant from the National Health and Medical Research Council of Australia during the conduct of the study and personal fees from several pharmaceutical companies outside the submitted work.
FROM JAMA DERMATOLOGY