User login
Data overwhelmingly support use of dermoscopy in practice
LAHAINA, HAWAII – Multiple but there are still some “nonbelievers,” Ashfaq A. Marghoob, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
In fact, not one study has shown that dermoscopy is less sensitive than the naked eye alone, and at clinics where dermoscopy is used, “two-thirds of the melanomas being detected now lack the classic ABCD features of melanoma,” added Dr. Marghoob, director of clinical dermatology at Memorial Sloan Kettering in Hauppauge, N.Y.
Dr. Marghoob cited a meta-analysis of 9 prospective trials, which concluded that dermoscopy was more accurate in diagnosing melanoma than the naked eye alone and did not lower specificity, with a sensitivity and specificity of 90%, compared with 71% and 81%, respectively for the naked eye (Br J Dermatol. 2008 Sep;159[3]:669-76).
“And at least from an evidence-based standpoint ... the Cochrane library has basically endorsed that, yes, dermoscopy does indeed improve your diagnostic accuracy,” Dr. Marghoob said.
He referred to a 2018 Cochrane review on 104 dermoscopy studies, with and without visual inspection, for diagnosing melanoma in adults (Cochrane Database Syst Rev. 2018 Dec 4;12:CD011902). The review authors concluded that “the evidence suggests that melanomas will be missed if visual inspection is used on its own,” and despite limitations in the evidence, “dermoscopy is a valuable tool to support the visual inspection of a suspicious skin lesion for the detection of melanoma and atypical intraepidermal melanocytic variants.”
As for the question of specificity, Dr. Marghoob said studies have found that dermoscopy results in the detection of more melanomas and reduces the number of biopsies of benign lesions and “every study looking into this has shown that, yes, it does improve” specificity.
A 10-year multicenter survey of about 300,000 cases, which included 17,172 melanomas and 283,043 melanocytic nevi, found that the number-needed-to-excise (NNE) values improved over time in specialized clinics where newer diagnostic techniques like dermoscopy were used (from 12.8 to 6.8), but the NNE did not appear to change in the nonspecialized settings (J Am Acad Dermatol. 2012 Jul;67[1]:54-9).
Looking at the benign-to-malignant ratio, there was no change among those not using dermoscopy, where the ratio remained at about 30 to 1. When dermoscopy was used, this ratio started to improve over the 10-year period, to about 5 to 1. In addition, “the dermoscopy users were finding many more melanomas than the non–dermoscopy users,” Dr. Marghoob added. And over the 10 years, the number of nevi being removed did not change among those not using dermoscopy but dropped among those using dermoscopy.
Adding photography with the ability to digitally monitor patients helps bring this ratio down further, Dr. Marghoob noted. He referred to a study that instead evaluated the ratio of melanoma to nonmelanomas diagnosed among dermatologists in three groups: those with no digital dermoscopy with little dermoscopy training (group A, the reference group), no digital dermoscopy but more dermoscopy training (group B), and those using digital dermoscopy (group C). In the group that used digital dermoscopy, that ratio was about 1 to 2.4, compared with about 1 to 8 in group B, and about 1 to 10.7 in group A (Br J Dermatol. 2012;167[4]778-86).
The use of dermoscopy is also associated with thinner tumors. Among dermoscopy users, the thickness of the tumors detected drops, and the proportion of thin to thick lesions detected increases, Dr. Marghoob said.
For example, in one study, the mean thickness in melanomas detected with dermoscopy was 1.4 mm versus 2.59 mm when dermoscopy was not used (J Eur Acad Dermatol Venereol. 2015 Jan; 29[1]:102-8). About 55% of the tumors detected with dermoscopic examination were 1 mm or less in thickness versus 23.4% of those detected without dermoscopy, “so the dermoscopy users from a proportion standpoint were also finding thinner tumors,” Dr. Marghoob said. Dermoscopy was also identified as an independent predictor of finding thinner tumors.
Even without a study, it could be assumed that the use of dermoscopy would reduce costs, since dermoscopy increases sensitivity and the total number of melanomas detected, decreases the number of benign nevi removed, and helps detect disease earlier. But there are data showing that dermoscopy reduces health care costs, Dr. Marghoob said.* For example, a Belgian study that looked at dermoscopy in two cohorts of melanoma patients concluded that adequate dermoscopy training was cost effective (Eur J Cancer. 2016 Nov;67:38-45).
It has also been shown that adding dermoscopy to a primary care setting has cost benefits, Dr. Marghoob said. He cited a study of Dutch general practices that found that the probability of a correct diagnosis was 1.25 times higher when dermoscopy was used to evaluate suspicious skin lesions and concluded that the use of dermoscopy appeared to be cost effective (J Eur Acad Dermatol Venereol. 2014 Nov;28[11]:1442-9).
Dr. Marghoob had no disclosures relevant to this presentation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
*Correction, 2/28/20: An earlier version of this article mischaracterized the cost implications of dermoscopy use.
LAHAINA, HAWAII – Multiple but there are still some “nonbelievers,” Ashfaq A. Marghoob, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
In fact, not one study has shown that dermoscopy is less sensitive than the naked eye alone, and at clinics where dermoscopy is used, “two-thirds of the melanomas being detected now lack the classic ABCD features of melanoma,” added Dr. Marghoob, director of clinical dermatology at Memorial Sloan Kettering in Hauppauge, N.Y.
Dr. Marghoob cited a meta-analysis of 9 prospective trials, which concluded that dermoscopy was more accurate in diagnosing melanoma than the naked eye alone and did not lower specificity, with a sensitivity and specificity of 90%, compared with 71% and 81%, respectively for the naked eye (Br J Dermatol. 2008 Sep;159[3]:669-76).
“And at least from an evidence-based standpoint ... the Cochrane library has basically endorsed that, yes, dermoscopy does indeed improve your diagnostic accuracy,” Dr. Marghoob said.
He referred to a 2018 Cochrane review on 104 dermoscopy studies, with and without visual inspection, for diagnosing melanoma in adults (Cochrane Database Syst Rev. 2018 Dec 4;12:CD011902). The review authors concluded that “the evidence suggests that melanomas will be missed if visual inspection is used on its own,” and despite limitations in the evidence, “dermoscopy is a valuable tool to support the visual inspection of a suspicious skin lesion for the detection of melanoma and atypical intraepidermal melanocytic variants.”
As for the question of specificity, Dr. Marghoob said studies have found that dermoscopy results in the detection of more melanomas and reduces the number of biopsies of benign lesions and “every study looking into this has shown that, yes, it does improve” specificity.
A 10-year multicenter survey of about 300,000 cases, which included 17,172 melanomas and 283,043 melanocytic nevi, found that the number-needed-to-excise (NNE) values improved over time in specialized clinics where newer diagnostic techniques like dermoscopy were used (from 12.8 to 6.8), but the NNE did not appear to change in the nonspecialized settings (J Am Acad Dermatol. 2012 Jul;67[1]:54-9).
Looking at the benign-to-malignant ratio, there was no change among those not using dermoscopy, where the ratio remained at about 30 to 1. When dermoscopy was used, this ratio started to improve over the 10-year period, to about 5 to 1. In addition, “the dermoscopy users were finding many more melanomas than the non–dermoscopy users,” Dr. Marghoob added. And over the 10 years, the number of nevi being removed did not change among those not using dermoscopy but dropped among those using dermoscopy.
Adding photography with the ability to digitally monitor patients helps bring this ratio down further, Dr. Marghoob noted. He referred to a study that instead evaluated the ratio of melanoma to nonmelanomas diagnosed among dermatologists in three groups: those with no digital dermoscopy with little dermoscopy training (group A, the reference group), no digital dermoscopy but more dermoscopy training (group B), and those using digital dermoscopy (group C). In the group that used digital dermoscopy, that ratio was about 1 to 2.4, compared with about 1 to 8 in group B, and about 1 to 10.7 in group A (Br J Dermatol. 2012;167[4]778-86).
The use of dermoscopy is also associated with thinner tumors. Among dermoscopy users, the thickness of the tumors detected drops, and the proportion of thin to thick lesions detected increases, Dr. Marghoob said.
For example, in one study, the mean thickness in melanomas detected with dermoscopy was 1.4 mm versus 2.59 mm when dermoscopy was not used (J Eur Acad Dermatol Venereol. 2015 Jan; 29[1]:102-8). About 55% of the tumors detected with dermoscopic examination were 1 mm or less in thickness versus 23.4% of those detected without dermoscopy, “so the dermoscopy users from a proportion standpoint were also finding thinner tumors,” Dr. Marghoob said. Dermoscopy was also identified as an independent predictor of finding thinner tumors.
Even without a study, it could be assumed that the use of dermoscopy would reduce costs, since dermoscopy increases sensitivity and the total number of melanomas detected, decreases the number of benign nevi removed, and helps detect disease earlier. But there are data showing that dermoscopy reduces health care costs, Dr. Marghoob said.* For example, a Belgian study that looked at dermoscopy in two cohorts of melanoma patients concluded that adequate dermoscopy training was cost effective (Eur J Cancer. 2016 Nov;67:38-45).
It has also been shown that adding dermoscopy to a primary care setting has cost benefits, Dr. Marghoob said. He cited a study of Dutch general practices that found that the probability of a correct diagnosis was 1.25 times higher when dermoscopy was used to evaluate suspicious skin lesions and concluded that the use of dermoscopy appeared to be cost effective (J Eur Acad Dermatol Venereol. 2014 Nov;28[11]:1442-9).
Dr. Marghoob had no disclosures relevant to this presentation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
*Correction, 2/28/20: An earlier version of this article mischaracterized the cost implications of dermoscopy use.
LAHAINA, HAWAII – Multiple but there are still some “nonbelievers,” Ashfaq A. Marghoob, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
In fact, not one study has shown that dermoscopy is less sensitive than the naked eye alone, and at clinics where dermoscopy is used, “two-thirds of the melanomas being detected now lack the classic ABCD features of melanoma,” added Dr. Marghoob, director of clinical dermatology at Memorial Sloan Kettering in Hauppauge, N.Y.
Dr. Marghoob cited a meta-analysis of 9 prospective trials, which concluded that dermoscopy was more accurate in diagnosing melanoma than the naked eye alone and did not lower specificity, with a sensitivity and specificity of 90%, compared with 71% and 81%, respectively for the naked eye (Br J Dermatol. 2008 Sep;159[3]:669-76).
“And at least from an evidence-based standpoint ... the Cochrane library has basically endorsed that, yes, dermoscopy does indeed improve your diagnostic accuracy,” Dr. Marghoob said.
He referred to a 2018 Cochrane review on 104 dermoscopy studies, with and without visual inspection, for diagnosing melanoma in adults (Cochrane Database Syst Rev. 2018 Dec 4;12:CD011902). The review authors concluded that “the evidence suggests that melanomas will be missed if visual inspection is used on its own,” and despite limitations in the evidence, “dermoscopy is a valuable tool to support the visual inspection of a suspicious skin lesion for the detection of melanoma and atypical intraepidermal melanocytic variants.”
As for the question of specificity, Dr. Marghoob said studies have found that dermoscopy results in the detection of more melanomas and reduces the number of biopsies of benign lesions and “every study looking into this has shown that, yes, it does improve” specificity.
A 10-year multicenter survey of about 300,000 cases, which included 17,172 melanomas and 283,043 melanocytic nevi, found that the number-needed-to-excise (NNE) values improved over time in specialized clinics where newer diagnostic techniques like dermoscopy were used (from 12.8 to 6.8), but the NNE did not appear to change in the nonspecialized settings (J Am Acad Dermatol. 2012 Jul;67[1]:54-9).
Looking at the benign-to-malignant ratio, there was no change among those not using dermoscopy, where the ratio remained at about 30 to 1. When dermoscopy was used, this ratio started to improve over the 10-year period, to about 5 to 1. In addition, “the dermoscopy users were finding many more melanomas than the non–dermoscopy users,” Dr. Marghoob added. And over the 10 years, the number of nevi being removed did not change among those not using dermoscopy but dropped among those using dermoscopy.
Adding photography with the ability to digitally monitor patients helps bring this ratio down further, Dr. Marghoob noted. He referred to a study that instead evaluated the ratio of melanoma to nonmelanomas diagnosed among dermatologists in three groups: those with no digital dermoscopy with little dermoscopy training (group A, the reference group), no digital dermoscopy but more dermoscopy training (group B), and those using digital dermoscopy (group C). In the group that used digital dermoscopy, that ratio was about 1 to 2.4, compared with about 1 to 8 in group B, and about 1 to 10.7 in group A (Br J Dermatol. 2012;167[4]778-86).
The use of dermoscopy is also associated with thinner tumors. Among dermoscopy users, the thickness of the tumors detected drops, and the proportion of thin to thick lesions detected increases, Dr. Marghoob said.
For example, in one study, the mean thickness in melanomas detected with dermoscopy was 1.4 mm versus 2.59 mm when dermoscopy was not used (J Eur Acad Dermatol Venereol. 2015 Jan; 29[1]:102-8). About 55% of the tumors detected with dermoscopic examination were 1 mm or less in thickness versus 23.4% of those detected without dermoscopy, “so the dermoscopy users from a proportion standpoint were also finding thinner tumors,” Dr. Marghoob said. Dermoscopy was also identified as an independent predictor of finding thinner tumors.
Even without a study, it could be assumed that the use of dermoscopy would reduce costs, since dermoscopy increases sensitivity and the total number of melanomas detected, decreases the number of benign nevi removed, and helps detect disease earlier. But there are data showing that dermoscopy reduces health care costs, Dr. Marghoob said.* For example, a Belgian study that looked at dermoscopy in two cohorts of melanoma patients concluded that adequate dermoscopy training was cost effective (Eur J Cancer. 2016 Nov;67:38-45).
It has also been shown that adding dermoscopy to a primary care setting has cost benefits, Dr. Marghoob said. He cited a study of Dutch general practices that found that the probability of a correct diagnosis was 1.25 times higher when dermoscopy was used to evaluate suspicious skin lesions and concluded that the use of dermoscopy appeared to be cost effective (J Eur Acad Dermatol Venereol. 2014 Nov;28[11]:1442-9).
Dr. Marghoob had no disclosures relevant to this presentation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
*Correction, 2/28/20: An earlier version of this article mischaracterized the cost implications of dermoscopy use.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Tumor neoantigenicity metric improves prediction of response to immunotherapy
A new tumor neoantigenicity metric may improve prediction of response to immunotherapy in patients with melanoma, lung cancer, and kidney cancer, a retrospective analysis suggests.
The new metric, known as the Cauchy-Schwarz index of neoantigens (CSiN) score, incorporates both immunogenicity and clonality, according to lead study author Tianshi Lu, a PhD candidate at the University of Texas Southwestern Medical Center in Dallas, and colleagues.
“The major biological insight from this study is that the neoantigen clonal structure in each tumor specimen and the immunogenicity of the neoantigens (represented by the MHC-binding strength in our study) are predictive of response to checkpoint inhibitors and prognosis,” the investigators wrote in Science Immunology.
The study involved 2,479 patients with various cancers, including immunogenic types such as renal cell carcinoma (RCC), and nonimmunogenic types, such as pediatric acute lymphocytic leukemia.
The investigators first evaluated CSiN in relation to clinical outcome among patients with immunogenic cancers who received immunotherapy. Drawing data from multiple cohorts, the investigators found that patients who had better responses to therapy were significantly more likely to have above average CSiN scores than those who had worse responses.
In one cohort of patients with melanoma who received anti–CTLA-4 therapy, those with better responses were more likely to have high CSiN scores (P = .009). In another cohort of melanoma patients who received anti–CTLA-4 therapy, those with higher CSiN scores were more likely to achieve durable clinical benefit (response or stable disease for more than 6 months), compared with patients who had lower CSiN scores (P = .033).
Among patients with clear cell RCC treated with anti-PD-1/PD-L1 therapy, there was a significant positive association between higher CSiN scores and better response (P = .036). Among T effector-high patients with metastatic clear cell RCC, there was a significant association between higher CSiN scores and better response to atezolizumab (P = .028) but not sunitinib (P = .890).
In a cohort of patients with non–small cell lung cancer treated with checkpoint inhibitors, those with sustained responses were more likely to have higher CSiN scores than were patients with short-term progression (P = .015).
The investigators also compared the predictive power of CSiN with existing neoantigenicity metrics, ultimately concluding that CSiN was superior.
“Overall, the neoantigen load and neoantigen fitness models were not as strongly predictive of treatment response as CSiN,” the investigators wrote.
Again using data from patients with immunogenic cancers, the investigators looked for an association between CSiN score and overall survival. Indeed, patients with higher-than-average CSiN scores had significantly better survival than that of those with lower scores (P less than .001). This finding was maintained in a multivariate analysis that accounted for disease type, stage, sex, and age.
In contrast with the above findings, CSiN did not predict survival among patients with nonimmunogenic cancer types.
“Overall, our work offers a rigorous methodology of predicting response to immunotherapy and prognosis from routine patient samples and should be useful for personalizing medicine in the modern era of immunotherapy,” the investigators concluded.
The study was funded by the National Institutes of Health, the Cancer Prevention Research Institute of Texas, and the American Cancer Society. The investigators reported no conflicts of interest.
SOURCE: Lu et al. Sci Immunol. 2020 Feb 21. doi: 10.1126/sciimmunol.aaz3199.
A new tumor neoantigenicity metric may improve prediction of response to immunotherapy in patients with melanoma, lung cancer, and kidney cancer, a retrospective analysis suggests.
The new metric, known as the Cauchy-Schwarz index of neoantigens (CSiN) score, incorporates both immunogenicity and clonality, according to lead study author Tianshi Lu, a PhD candidate at the University of Texas Southwestern Medical Center in Dallas, and colleagues.
“The major biological insight from this study is that the neoantigen clonal structure in each tumor specimen and the immunogenicity of the neoantigens (represented by the MHC-binding strength in our study) are predictive of response to checkpoint inhibitors and prognosis,” the investigators wrote in Science Immunology.
The study involved 2,479 patients with various cancers, including immunogenic types such as renal cell carcinoma (RCC), and nonimmunogenic types, such as pediatric acute lymphocytic leukemia.
The investigators first evaluated CSiN in relation to clinical outcome among patients with immunogenic cancers who received immunotherapy. Drawing data from multiple cohorts, the investigators found that patients who had better responses to therapy were significantly more likely to have above average CSiN scores than those who had worse responses.
In one cohort of patients with melanoma who received anti–CTLA-4 therapy, those with better responses were more likely to have high CSiN scores (P = .009). In another cohort of melanoma patients who received anti–CTLA-4 therapy, those with higher CSiN scores were more likely to achieve durable clinical benefit (response or stable disease for more than 6 months), compared with patients who had lower CSiN scores (P = .033).
Among patients with clear cell RCC treated with anti-PD-1/PD-L1 therapy, there was a significant positive association between higher CSiN scores and better response (P = .036). Among T effector-high patients with metastatic clear cell RCC, there was a significant association between higher CSiN scores and better response to atezolizumab (P = .028) but not sunitinib (P = .890).
In a cohort of patients with non–small cell lung cancer treated with checkpoint inhibitors, those with sustained responses were more likely to have higher CSiN scores than were patients with short-term progression (P = .015).
The investigators also compared the predictive power of CSiN with existing neoantigenicity metrics, ultimately concluding that CSiN was superior.
“Overall, the neoantigen load and neoantigen fitness models were not as strongly predictive of treatment response as CSiN,” the investigators wrote.
Again using data from patients with immunogenic cancers, the investigators looked for an association between CSiN score and overall survival. Indeed, patients with higher-than-average CSiN scores had significantly better survival than that of those with lower scores (P less than .001). This finding was maintained in a multivariate analysis that accounted for disease type, stage, sex, and age.
In contrast with the above findings, CSiN did not predict survival among patients with nonimmunogenic cancer types.
“Overall, our work offers a rigorous methodology of predicting response to immunotherapy and prognosis from routine patient samples and should be useful for personalizing medicine in the modern era of immunotherapy,” the investigators concluded.
The study was funded by the National Institutes of Health, the Cancer Prevention Research Institute of Texas, and the American Cancer Society. The investigators reported no conflicts of interest.
SOURCE: Lu et al. Sci Immunol. 2020 Feb 21. doi: 10.1126/sciimmunol.aaz3199.
A new tumor neoantigenicity metric may improve prediction of response to immunotherapy in patients with melanoma, lung cancer, and kidney cancer, a retrospective analysis suggests.
The new metric, known as the Cauchy-Schwarz index of neoantigens (CSiN) score, incorporates both immunogenicity and clonality, according to lead study author Tianshi Lu, a PhD candidate at the University of Texas Southwestern Medical Center in Dallas, and colleagues.
“The major biological insight from this study is that the neoantigen clonal structure in each tumor specimen and the immunogenicity of the neoantigens (represented by the MHC-binding strength in our study) are predictive of response to checkpoint inhibitors and prognosis,” the investigators wrote in Science Immunology.
The study involved 2,479 patients with various cancers, including immunogenic types such as renal cell carcinoma (RCC), and nonimmunogenic types, such as pediatric acute lymphocytic leukemia.
The investigators first evaluated CSiN in relation to clinical outcome among patients with immunogenic cancers who received immunotherapy. Drawing data from multiple cohorts, the investigators found that patients who had better responses to therapy were significantly more likely to have above average CSiN scores than those who had worse responses.
In one cohort of patients with melanoma who received anti–CTLA-4 therapy, those with better responses were more likely to have high CSiN scores (P = .009). In another cohort of melanoma patients who received anti–CTLA-4 therapy, those with higher CSiN scores were more likely to achieve durable clinical benefit (response or stable disease for more than 6 months), compared with patients who had lower CSiN scores (P = .033).
Among patients with clear cell RCC treated with anti-PD-1/PD-L1 therapy, there was a significant positive association between higher CSiN scores and better response (P = .036). Among T effector-high patients with metastatic clear cell RCC, there was a significant association between higher CSiN scores and better response to atezolizumab (P = .028) but not sunitinib (P = .890).
In a cohort of patients with non–small cell lung cancer treated with checkpoint inhibitors, those with sustained responses were more likely to have higher CSiN scores than were patients with short-term progression (P = .015).
The investigators also compared the predictive power of CSiN with existing neoantigenicity metrics, ultimately concluding that CSiN was superior.
“Overall, the neoantigen load and neoantigen fitness models were not as strongly predictive of treatment response as CSiN,” the investigators wrote.
Again using data from patients with immunogenic cancers, the investigators looked for an association between CSiN score and overall survival. Indeed, patients with higher-than-average CSiN scores had significantly better survival than that of those with lower scores (P less than .001). This finding was maintained in a multivariate analysis that accounted for disease type, stage, sex, and age.
In contrast with the above findings, CSiN did not predict survival among patients with nonimmunogenic cancer types.
“Overall, our work offers a rigorous methodology of predicting response to immunotherapy and prognosis from routine patient samples and should be useful for personalizing medicine in the modern era of immunotherapy,” the investigators concluded.
The study was funded by the National Institutes of Health, the Cancer Prevention Research Institute of Texas, and the American Cancer Society. The investigators reported no conflicts of interest.
SOURCE: Lu et al. Sci Immunol. 2020 Feb 21. doi: 10.1126/sciimmunol.aaz3199.
FROM SCIENCE IMMUNOLOGY
Banning indoor tanning devices could save lives and money
according to a study published in JAMA Dermatology.
The study also suggests a ban would result in a collective cost savings of $5.7 billion and productivity gains of $41.3 billion.
Compared with a ban on indoor tanning for minors, the benefits of a full ban on devices were 3.7-fold higher in the United States/Canada and 2.6-fold higher in Europe, according to study author Louisa G. Gordon, PhD, of the QIMR Berghofer Medical Research Institute in Brisbane, Australia, and colleagues.
The researchers noted that indoor tanning is regulated in more than 20 countries. Australia has instituted a ban on commercial indoor tanning devices, and Brazil has banned both commercial and private tanning devices.
In the United States, 19 states have banned the use of indoor tanning beds for minors, and 44 states as well as the District of Columbia have some regulation of tanning facilities for minors, according to the National Conference of State Legislatures.
With this study, Dr. Gordon and colleagues sought to explore what effect an outright ban on indoor tanning devices, a prohibition for minors only, or continuing current levels of indoor tanning would have on the health and economy of the United States, Canada, and Europe.
The researchers created a Markov cohort model of 110,932,523 individuals in the United States/Canada and 141,970,492 individuals in Europe, all aged 12-35 years.
The team used data from epidemiologic studies, cost reports, and official cancer registries to estimate the prevalence of indoor tanning, risk of developing melanoma, and mortality rates from skin cancer and other causes. The researchers also estimated health care costs of melanoma treatment in each region as well as the societal cost of dying prematurely from melanoma, adjusted to 2018 dollars.
Results
The model suggested a ban on indoor tanning in the United States and Canada would result in 244,347 fewer melanomas (–8.7%), 89,193 fewer deaths from melanoma (–6.9%), and 7.3 million fewer keratinocyte carcinomas (–7.8%) than continuing at the current levels of use. The ban would also save 428,781 life-years, have a cost savings of $3.5 billion, and confer productivity gains of $27.5 billion, the researchers said.
When applying the ban in Europe, the model estimated 203,736 fewer melanomas (–4.9%), 98,288 fewer deaths from melanoma (–4.4%), and 2.4 million fewer keratinocyte carcinomas (–4.4%). The researchers also noted that Europe would see a gain of 459,669 life-years, a cost savings of $2.1 billion, and a productivity gain of $13.7 billion.
Dr. Gordon and colleagues acknowledged that their model had some limitations, such as in estimating the prevalence of certain skin cancers across Europe, which can range from 10% to 56% depending on the country. In addition, the model did not account for the money spent in implementing a ban, which could include costs associated with regulation, compliance, and buy-back schemes for tanning devices.
Implications
In an interview, Dr. Gordon said the researchers conducted this study to stress the health benefits and cost savings of regulating indoor tanning devices in North America and Europe. She noted that she had previously published a report in 2009 that helped Australia make the decision to ban such devices there, but she said the tanning industry was in its infancy during that time, which factored into the decision to ban indoor tanning (Health Policy. 2009 Mar;89[3]:303-11).
Any ban by a regulatory agency “should include everyone,” Dr. Gordon said, because “banning minors is a halfway attempt to prevent skin cancers.” The danger isn’t just present in children. “People in their 20s and 30s are still very image conscious,” she said. “The pressure is enormous.”
Anyone interested in tanning should use tanning creams or sprays instead of using indoor tanning devices, Dr. Gordon said. “Consumers can control their UV exposure,” she noted. “Prevention is incredibly important, and skin cancer is one of a few cancers we can almost entirely prevent via protecting our skin. The same can’t be said for other horrible cancers.”
Adam Friedman, MD, a professor at George Washington University, Washington, who was not involved in this study, said it should come as no surprise to dermatologists that preventing artificial UVA heavy exposure reduces the incidence of skin cancer, but the “more compelling component of this study is cost.”
“The lay public is extremely health care cost conscientious,” he said. “This is a commonly debated topic for emerging politicians at every level; not to mention, no one enjoys bleeding money. Dermatologists can use the angle of, ‘save skin now, save money later,’ to target the financial burden of accelerated skin aging and skin cancer as a mechanism for persuading patients not to ‘shake and bake.’ ”
While the Food and Drug Administration has proposed restricting the use of indoor tanning devices for minors nationwide, it has not issued a final rule on the matter, and the prospect of an outright ban in the United States for the general population is less feasible, noted Dr. Friedman.
“I think it would be difficult to expand this [proposed] ban given the financial impact on numerous businesses,” he said. “It would likely take more evidence and support beyond the medical community to make this happen, but here’s hoping,”
This study was funded by the World Health Organization UV Radiation Programme and Cancer Council Victoria. One author disclosed personal fees from Cancer Council Victoria, and one disclosed grants from TrygFonden. The other authors and Dr. Friedman reported no relevant conflicts of interest.
SOURCE: Gordon L et al. JAMA Dermatol. 2020 Feb 19. doi: 10.1001/jamadermatol.2020.0001.
according to a study published in JAMA Dermatology.
The study also suggests a ban would result in a collective cost savings of $5.7 billion and productivity gains of $41.3 billion.
Compared with a ban on indoor tanning for minors, the benefits of a full ban on devices were 3.7-fold higher in the United States/Canada and 2.6-fold higher in Europe, according to study author Louisa G. Gordon, PhD, of the QIMR Berghofer Medical Research Institute in Brisbane, Australia, and colleagues.
The researchers noted that indoor tanning is regulated in more than 20 countries. Australia has instituted a ban on commercial indoor tanning devices, and Brazil has banned both commercial and private tanning devices.
In the United States, 19 states have banned the use of indoor tanning beds for minors, and 44 states as well as the District of Columbia have some regulation of tanning facilities for minors, according to the National Conference of State Legislatures.
With this study, Dr. Gordon and colleagues sought to explore what effect an outright ban on indoor tanning devices, a prohibition for minors only, or continuing current levels of indoor tanning would have on the health and economy of the United States, Canada, and Europe.
The researchers created a Markov cohort model of 110,932,523 individuals in the United States/Canada and 141,970,492 individuals in Europe, all aged 12-35 years.
The team used data from epidemiologic studies, cost reports, and official cancer registries to estimate the prevalence of indoor tanning, risk of developing melanoma, and mortality rates from skin cancer and other causes. The researchers also estimated health care costs of melanoma treatment in each region as well as the societal cost of dying prematurely from melanoma, adjusted to 2018 dollars.
Results
The model suggested a ban on indoor tanning in the United States and Canada would result in 244,347 fewer melanomas (–8.7%), 89,193 fewer deaths from melanoma (–6.9%), and 7.3 million fewer keratinocyte carcinomas (–7.8%) than continuing at the current levels of use. The ban would also save 428,781 life-years, have a cost savings of $3.5 billion, and confer productivity gains of $27.5 billion, the researchers said.
When applying the ban in Europe, the model estimated 203,736 fewer melanomas (–4.9%), 98,288 fewer deaths from melanoma (–4.4%), and 2.4 million fewer keratinocyte carcinomas (–4.4%). The researchers also noted that Europe would see a gain of 459,669 life-years, a cost savings of $2.1 billion, and a productivity gain of $13.7 billion.
Dr. Gordon and colleagues acknowledged that their model had some limitations, such as in estimating the prevalence of certain skin cancers across Europe, which can range from 10% to 56% depending on the country. In addition, the model did not account for the money spent in implementing a ban, which could include costs associated with regulation, compliance, and buy-back schemes for tanning devices.
Implications
In an interview, Dr. Gordon said the researchers conducted this study to stress the health benefits and cost savings of regulating indoor tanning devices in North America and Europe. She noted that she had previously published a report in 2009 that helped Australia make the decision to ban such devices there, but she said the tanning industry was in its infancy during that time, which factored into the decision to ban indoor tanning (Health Policy. 2009 Mar;89[3]:303-11).
Any ban by a regulatory agency “should include everyone,” Dr. Gordon said, because “banning minors is a halfway attempt to prevent skin cancers.” The danger isn’t just present in children. “People in their 20s and 30s are still very image conscious,” she said. “The pressure is enormous.”
Anyone interested in tanning should use tanning creams or sprays instead of using indoor tanning devices, Dr. Gordon said. “Consumers can control their UV exposure,” she noted. “Prevention is incredibly important, and skin cancer is one of a few cancers we can almost entirely prevent via protecting our skin. The same can’t be said for other horrible cancers.”
Adam Friedman, MD, a professor at George Washington University, Washington, who was not involved in this study, said it should come as no surprise to dermatologists that preventing artificial UVA heavy exposure reduces the incidence of skin cancer, but the “more compelling component of this study is cost.”
“The lay public is extremely health care cost conscientious,” he said. “This is a commonly debated topic for emerging politicians at every level; not to mention, no one enjoys bleeding money. Dermatologists can use the angle of, ‘save skin now, save money later,’ to target the financial burden of accelerated skin aging and skin cancer as a mechanism for persuading patients not to ‘shake and bake.’ ”
While the Food and Drug Administration has proposed restricting the use of indoor tanning devices for minors nationwide, it has not issued a final rule on the matter, and the prospect of an outright ban in the United States for the general population is less feasible, noted Dr. Friedman.
“I think it would be difficult to expand this [proposed] ban given the financial impact on numerous businesses,” he said. “It would likely take more evidence and support beyond the medical community to make this happen, but here’s hoping,”
This study was funded by the World Health Organization UV Radiation Programme and Cancer Council Victoria. One author disclosed personal fees from Cancer Council Victoria, and one disclosed grants from TrygFonden. The other authors and Dr. Friedman reported no relevant conflicts of interest.
SOURCE: Gordon L et al. JAMA Dermatol. 2020 Feb 19. doi: 10.1001/jamadermatol.2020.0001.
according to a study published in JAMA Dermatology.
The study also suggests a ban would result in a collective cost savings of $5.7 billion and productivity gains of $41.3 billion.
Compared with a ban on indoor tanning for minors, the benefits of a full ban on devices were 3.7-fold higher in the United States/Canada and 2.6-fold higher in Europe, according to study author Louisa G. Gordon, PhD, of the QIMR Berghofer Medical Research Institute in Brisbane, Australia, and colleagues.
The researchers noted that indoor tanning is regulated in more than 20 countries. Australia has instituted a ban on commercial indoor tanning devices, and Brazil has banned both commercial and private tanning devices.
In the United States, 19 states have banned the use of indoor tanning beds for minors, and 44 states as well as the District of Columbia have some regulation of tanning facilities for minors, according to the National Conference of State Legislatures.
With this study, Dr. Gordon and colleagues sought to explore what effect an outright ban on indoor tanning devices, a prohibition for minors only, or continuing current levels of indoor tanning would have on the health and economy of the United States, Canada, and Europe.
The researchers created a Markov cohort model of 110,932,523 individuals in the United States/Canada and 141,970,492 individuals in Europe, all aged 12-35 years.
The team used data from epidemiologic studies, cost reports, and official cancer registries to estimate the prevalence of indoor tanning, risk of developing melanoma, and mortality rates from skin cancer and other causes. The researchers also estimated health care costs of melanoma treatment in each region as well as the societal cost of dying prematurely from melanoma, adjusted to 2018 dollars.
Results
The model suggested a ban on indoor tanning in the United States and Canada would result in 244,347 fewer melanomas (–8.7%), 89,193 fewer deaths from melanoma (–6.9%), and 7.3 million fewer keratinocyte carcinomas (–7.8%) than continuing at the current levels of use. The ban would also save 428,781 life-years, have a cost savings of $3.5 billion, and confer productivity gains of $27.5 billion, the researchers said.
When applying the ban in Europe, the model estimated 203,736 fewer melanomas (–4.9%), 98,288 fewer deaths from melanoma (–4.4%), and 2.4 million fewer keratinocyte carcinomas (–4.4%). The researchers also noted that Europe would see a gain of 459,669 life-years, a cost savings of $2.1 billion, and a productivity gain of $13.7 billion.
Dr. Gordon and colleagues acknowledged that their model had some limitations, such as in estimating the prevalence of certain skin cancers across Europe, which can range from 10% to 56% depending on the country. In addition, the model did not account for the money spent in implementing a ban, which could include costs associated with regulation, compliance, and buy-back schemes for tanning devices.
Implications
In an interview, Dr. Gordon said the researchers conducted this study to stress the health benefits and cost savings of regulating indoor tanning devices in North America and Europe. She noted that she had previously published a report in 2009 that helped Australia make the decision to ban such devices there, but she said the tanning industry was in its infancy during that time, which factored into the decision to ban indoor tanning (Health Policy. 2009 Mar;89[3]:303-11).
Any ban by a regulatory agency “should include everyone,” Dr. Gordon said, because “banning minors is a halfway attempt to prevent skin cancers.” The danger isn’t just present in children. “People in their 20s and 30s are still very image conscious,” she said. “The pressure is enormous.”
Anyone interested in tanning should use tanning creams or sprays instead of using indoor tanning devices, Dr. Gordon said. “Consumers can control their UV exposure,” she noted. “Prevention is incredibly important, and skin cancer is one of a few cancers we can almost entirely prevent via protecting our skin. The same can’t be said for other horrible cancers.”
Adam Friedman, MD, a professor at George Washington University, Washington, who was not involved in this study, said it should come as no surprise to dermatologists that preventing artificial UVA heavy exposure reduces the incidence of skin cancer, but the “more compelling component of this study is cost.”
“The lay public is extremely health care cost conscientious,” he said. “This is a commonly debated topic for emerging politicians at every level; not to mention, no one enjoys bleeding money. Dermatologists can use the angle of, ‘save skin now, save money later,’ to target the financial burden of accelerated skin aging and skin cancer as a mechanism for persuading patients not to ‘shake and bake.’ ”
While the Food and Drug Administration has proposed restricting the use of indoor tanning devices for minors nationwide, it has not issued a final rule on the matter, and the prospect of an outright ban in the United States for the general population is less feasible, noted Dr. Friedman.
“I think it would be difficult to expand this [proposed] ban given the financial impact on numerous businesses,” he said. “It would likely take more evidence and support beyond the medical community to make this happen, but here’s hoping,”
This study was funded by the World Health Organization UV Radiation Programme and Cancer Council Victoria. One author disclosed personal fees from Cancer Council Victoria, and one disclosed grants from TrygFonden. The other authors and Dr. Friedman reported no relevant conflicts of interest.
SOURCE: Gordon L et al. JAMA Dermatol. 2020 Feb 19. doi: 10.1001/jamadermatol.2020.0001.
FROM JAMA DERMATOLOGY
Smartphone apps for suspicious skin lesions unreliable
Smartphone applications ( said U.K. researchers reporting a systematic review.
These apps are providing information that could lead to “potentially life-or-death decisions,” commented co-lead author Hywel C. Williams, MD, from the Centre of Evidence Based Dermatology, University of Nottingham (England).
“The one thing you mustn’t do in a situation where early diagnosis can make a difference between life and death is you mustn’t miss the melanoma,” he said in an interview.
“These apps were missing melanomas, and that’s very worrisome,” he commented.
The review included nine studies of skin cancer smartphone apps, including two apps, SkinScan and SkinVision, that have been given Conformit Europenne (CE) marks, allowing them to be marketed across Europe. These apps are also available in Australia and New Zealand, but not in the United States.
The review found that SkinScan was not able to identify any melanomas in the one study that assessed this app, while SkinVision had a relatively low sensitivity and specificity, with 12% of cancerous or precancerous lesions missed and 21% of benign lesions wrongly identified as cancerous.
This means that among 1,000 people with a melanoma prevalence of 3%, 4 of 30 melanomas would be missed, and 200 people would be incorrectly told that a mole was of high concern, the authors estimated.
The research was published by The BMJ on Feb. 10.
“Although I was broad minded on the potential benefit of apps for diagnosing skin cancer, I am now worried given the results of our study and the overall poor quality of studies used to test these apps,” Dr. Williams commented in a statement.
Coauthor Jac Dinnes, PhD, from the Institute of Applied Health Research at the University of Birmingham (England), added it is “really disappointing that there is not better quality evidence available to judge the efficacy of these apps.”
“It is vital that health care professionals are aware of the current limitations both in the technologies and in their evaluations,” she added.
The results also highlight the limitations of the regulatory system governing smartphone apps in that they are currently not subject to assessment by bodies such as the U.K.’s Medicines and Healthcare Products Regulatory Agency (MHRA), the authors commented.
“Regulators need to become alert to the potential harm that poorly performing algorithm-based diagnostic or risk monitoring apps create,” said co-lead author Jonathan J. Deeks, PhD, also at the Institute of Applied Health Research.
“We rely on the CE mark as a sign of quality, but the current CE mark assessment processes are not fit for protecting the public against the risks that these apps present.”
Speaking in an interview, Williams lamented the poor quality of the research that had been conducted. “These studies were not good enough,” he said, adding that “there’s no excuse for really poor study design and poor reporting.”
He would like to see the regulations tightened around AI apps purporting to inform decision making for the general public and suggests that these devices should be assessed by the MHRA. “I really do think a CE mark is not enough,” he said.
The team noted that the skin cancer apps “all include disclaimers that the results should only be used as a guide and cannot replace health care advice,” through which the manufacturers “attempt to evade any responsibility for negative outcomes experienced by users.”
Nevertheless, the “poor and variable performance” of the apps revealed by their review indicates that they “have not yet shown sufficient promise to recommend their use,” they concluded.
The “official approval” implied by a CE mark “will give consumers the impression that the apps have been assessed as effective and safe,” wrote Ben Goldacre, DataLab director, Nuffield Department of Primary Care, University of Oxford (England), and colleagues in an accompanying editorial.
“The implicit assumption is that apps are similarly low-risk technology” to devices such as sticking plasters and reading glasses, they comment.
“But shortcomings in diagnostic apps can have serious implications,” they warn. The “risks include psychological harm from health anxiety or ‘cyberchondria,’ and physical harm from misdiagnosis or overdiagnosis; for clinicians there is a risk of increased workload, and changes to ethical or legal responsibilities around triage, referral, diagnosis, and treatment.” There is also potential for “inappropriate resource use, and even loss of credibility for digital technology in general.”
Details of the review
For their review, the authors searched the Cochrane Central Register on Controlled Trials, the MEDLNE, Embase, Cumulative Index to Nursing and Allied Health Literature, Conference Proceedings Citation index, Zetoc, and Science Citation Index databases, and online trial registers for studies published between August 2016 and April 2019.
From 80 studies identified, 9 met the eligibility criteria.
Of those, six studies, evaluating a total of 725 skin lesions, determined the accuracy of smartphone apps in risk stratifying suspicious skin lesions by comparing them against a histopathological reference standard diagnosis or expert follow-up.
Five of these studies aimed to detect only melanoma, while one sought to differentiate between malignant or premalignant lesions (including melanoma, basal cell carcinoma, and squamous cell carcinoma) and benign lesions.
The three remaining studies, which evaluated 407 lesions in all, compared smartphone app recommendations against a reference standard of expert recommendations for further investigation or intervention.
The researchers found the studies had a string of potential biases and limitations.
For example, only four studies recruited a consecutive sample of study participants and lesions, and only two included lesions selected by study participants, whereas five studies used lesions that had been selected by a clinician.
Three studies reported that it took 5-10 attempts to obtain an adequate image. In seven studies, it was the researchers and not the patients who used the app to photograph the lesions, and two studies used images obtained from dermatology databases.
This “raised concerns that the results of the studies were unlikely to be representative of real life use,” the authors comment.
In addition, the exclusion of unevaluable images “might have systematically inflated the diagnostic performance of the tested apps,” they add.
The independent research was supported by the National Institute for Health Research (NIHR) Birmingham Biomedical Research Centre at the University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham and is an update of one of a collection of reviews funded by the NIHR through its Cochrane Systematic Review Programme Grant.
This article first appeared on Medscape.com.
Smartphone applications ( said U.K. researchers reporting a systematic review.
These apps are providing information that could lead to “potentially life-or-death decisions,” commented co-lead author Hywel C. Williams, MD, from the Centre of Evidence Based Dermatology, University of Nottingham (England).
“The one thing you mustn’t do in a situation where early diagnosis can make a difference between life and death is you mustn’t miss the melanoma,” he said in an interview.
“These apps were missing melanomas, and that’s very worrisome,” he commented.
The review included nine studies of skin cancer smartphone apps, including two apps, SkinScan and SkinVision, that have been given Conformit Europenne (CE) marks, allowing them to be marketed across Europe. These apps are also available in Australia and New Zealand, but not in the United States.
The review found that SkinScan was not able to identify any melanomas in the one study that assessed this app, while SkinVision had a relatively low sensitivity and specificity, with 12% of cancerous or precancerous lesions missed and 21% of benign lesions wrongly identified as cancerous.
This means that among 1,000 people with a melanoma prevalence of 3%, 4 of 30 melanomas would be missed, and 200 people would be incorrectly told that a mole was of high concern, the authors estimated.
The research was published by The BMJ on Feb. 10.
“Although I was broad minded on the potential benefit of apps for diagnosing skin cancer, I am now worried given the results of our study and the overall poor quality of studies used to test these apps,” Dr. Williams commented in a statement.
Coauthor Jac Dinnes, PhD, from the Institute of Applied Health Research at the University of Birmingham (England), added it is “really disappointing that there is not better quality evidence available to judge the efficacy of these apps.”
“It is vital that health care professionals are aware of the current limitations both in the technologies and in their evaluations,” she added.
The results also highlight the limitations of the regulatory system governing smartphone apps in that they are currently not subject to assessment by bodies such as the U.K.’s Medicines and Healthcare Products Regulatory Agency (MHRA), the authors commented.
“Regulators need to become alert to the potential harm that poorly performing algorithm-based diagnostic or risk monitoring apps create,” said co-lead author Jonathan J. Deeks, PhD, also at the Institute of Applied Health Research.
“We rely on the CE mark as a sign of quality, but the current CE mark assessment processes are not fit for protecting the public against the risks that these apps present.”
Speaking in an interview, Williams lamented the poor quality of the research that had been conducted. “These studies were not good enough,” he said, adding that “there’s no excuse for really poor study design and poor reporting.”
He would like to see the regulations tightened around AI apps purporting to inform decision making for the general public and suggests that these devices should be assessed by the MHRA. “I really do think a CE mark is not enough,” he said.
The team noted that the skin cancer apps “all include disclaimers that the results should only be used as a guide and cannot replace health care advice,” through which the manufacturers “attempt to evade any responsibility for negative outcomes experienced by users.”
Nevertheless, the “poor and variable performance” of the apps revealed by their review indicates that they “have not yet shown sufficient promise to recommend their use,” they concluded.
The “official approval” implied by a CE mark “will give consumers the impression that the apps have been assessed as effective and safe,” wrote Ben Goldacre, DataLab director, Nuffield Department of Primary Care, University of Oxford (England), and colleagues in an accompanying editorial.
“The implicit assumption is that apps are similarly low-risk technology” to devices such as sticking plasters and reading glasses, they comment.
“But shortcomings in diagnostic apps can have serious implications,” they warn. The “risks include psychological harm from health anxiety or ‘cyberchondria,’ and physical harm from misdiagnosis or overdiagnosis; for clinicians there is a risk of increased workload, and changes to ethical or legal responsibilities around triage, referral, diagnosis, and treatment.” There is also potential for “inappropriate resource use, and even loss of credibility for digital technology in general.”
Details of the review
For their review, the authors searched the Cochrane Central Register on Controlled Trials, the MEDLNE, Embase, Cumulative Index to Nursing and Allied Health Literature, Conference Proceedings Citation index, Zetoc, and Science Citation Index databases, and online trial registers for studies published between August 2016 and April 2019.
From 80 studies identified, 9 met the eligibility criteria.
Of those, six studies, evaluating a total of 725 skin lesions, determined the accuracy of smartphone apps in risk stratifying suspicious skin lesions by comparing them against a histopathological reference standard diagnosis or expert follow-up.
Five of these studies aimed to detect only melanoma, while one sought to differentiate between malignant or premalignant lesions (including melanoma, basal cell carcinoma, and squamous cell carcinoma) and benign lesions.
The three remaining studies, which evaluated 407 lesions in all, compared smartphone app recommendations against a reference standard of expert recommendations for further investigation or intervention.
The researchers found the studies had a string of potential biases and limitations.
For example, only four studies recruited a consecutive sample of study participants and lesions, and only two included lesions selected by study participants, whereas five studies used lesions that had been selected by a clinician.
Three studies reported that it took 5-10 attempts to obtain an adequate image. In seven studies, it was the researchers and not the patients who used the app to photograph the lesions, and two studies used images obtained from dermatology databases.
This “raised concerns that the results of the studies were unlikely to be representative of real life use,” the authors comment.
In addition, the exclusion of unevaluable images “might have systematically inflated the diagnostic performance of the tested apps,” they add.
The independent research was supported by the National Institute for Health Research (NIHR) Birmingham Biomedical Research Centre at the University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham and is an update of one of a collection of reviews funded by the NIHR through its Cochrane Systematic Review Programme Grant.
This article first appeared on Medscape.com.
Smartphone applications ( said U.K. researchers reporting a systematic review.
These apps are providing information that could lead to “potentially life-or-death decisions,” commented co-lead author Hywel C. Williams, MD, from the Centre of Evidence Based Dermatology, University of Nottingham (England).
“The one thing you mustn’t do in a situation where early diagnosis can make a difference between life and death is you mustn’t miss the melanoma,” he said in an interview.
“These apps were missing melanomas, and that’s very worrisome,” he commented.
The review included nine studies of skin cancer smartphone apps, including two apps, SkinScan and SkinVision, that have been given Conformit Europenne (CE) marks, allowing them to be marketed across Europe. These apps are also available in Australia and New Zealand, but not in the United States.
The review found that SkinScan was not able to identify any melanomas in the one study that assessed this app, while SkinVision had a relatively low sensitivity and specificity, with 12% of cancerous or precancerous lesions missed and 21% of benign lesions wrongly identified as cancerous.
This means that among 1,000 people with a melanoma prevalence of 3%, 4 of 30 melanomas would be missed, and 200 people would be incorrectly told that a mole was of high concern, the authors estimated.
The research was published by The BMJ on Feb. 10.
“Although I was broad minded on the potential benefit of apps for diagnosing skin cancer, I am now worried given the results of our study and the overall poor quality of studies used to test these apps,” Dr. Williams commented in a statement.
Coauthor Jac Dinnes, PhD, from the Institute of Applied Health Research at the University of Birmingham (England), added it is “really disappointing that there is not better quality evidence available to judge the efficacy of these apps.”
“It is vital that health care professionals are aware of the current limitations both in the technologies and in their evaluations,” she added.
The results also highlight the limitations of the regulatory system governing smartphone apps in that they are currently not subject to assessment by bodies such as the U.K.’s Medicines and Healthcare Products Regulatory Agency (MHRA), the authors commented.
“Regulators need to become alert to the potential harm that poorly performing algorithm-based diagnostic or risk monitoring apps create,” said co-lead author Jonathan J. Deeks, PhD, also at the Institute of Applied Health Research.
“We rely on the CE mark as a sign of quality, but the current CE mark assessment processes are not fit for protecting the public against the risks that these apps present.”
Speaking in an interview, Williams lamented the poor quality of the research that had been conducted. “These studies were not good enough,” he said, adding that “there’s no excuse for really poor study design and poor reporting.”
He would like to see the regulations tightened around AI apps purporting to inform decision making for the general public and suggests that these devices should be assessed by the MHRA. “I really do think a CE mark is not enough,” he said.
The team noted that the skin cancer apps “all include disclaimers that the results should only be used as a guide and cannot replace health care advice,” through which the manufacturers “attempt to evade any responsibility for negative outcomes experienced by users.”
Nevertheless, the “poor and variable performance” of the apps revealed by their review indicates that they “have not yet shown sufficient promise to recommend their use,” they concluded.
The “official approval” implied by a CE mark “will give consumers the impression that the apps have been assessed as effective and safe,” wrote Ben Goldacre, DataLab director, Nuffield Department of Primary Care, University of Oxford (England), and colleagues in an accompanying editorial.
“The implicit assumption is that apps are similarly low-risk technology” to devices such as sticking plasters and reading glasses, they comment.
“But shortcomings in diagnostic apps can have serious implications,” they warn. The “risks include psychological harm from health anxiety or ‘cyberchondria,’ and physical harm from misdiagnosis or overdiagnosis; for clinicians there is a risk of increased workload, and changes to ethical or legal responsibilities around triage, referral, diagnosis, and treatment.” There is also potential for “inappropriate resource use, and even loss of credibility for digital technology in general.”
Details of the review
For their review, the authors searched the Cochrane Central Register on Controlled Trials, the MEDLNE, Embase, Cumulative Index to Nursing and Allied Health Literature, Conference Proceedings Citation index, Zetoc, and Science Citation Index databases, and online trial registers for studies published between August 2016 and April 2019.
From 80 studies identified, 9 met the eligibility criteria.
Of those, six studies, evaluating a total of 725 skin lesions, determined the accuracy of smartphone apps in risk stratifying suspicious skin lesions by comparing them against a histopathological reference standard diagnosis or expert follow-up.
Five of these studies aimed to detect only melanoma, while one sought to differentiate between malignant or premalignant lesions (including melanoma, basal cell carcinoma, and squamous cell carcinoma) and benign lesions.
The three remaining studies, which evaluated 407 lesions in all, compared smartphone app recommendations against a reference standard of expert recommendations for further investigation or intervention.
The researchers found the studies had a string of potential biases and limitations.
For example, only four studies recruited a consecutive sample of study participants and lesions, and only two included lesions selected by study participants, whereas five studies used lesions that had been selected by a clinician.
Three studies reported that it took 5-10 attempts to obtain an adequate image. In seven studies, it was the researchers and not the patients who used the app to photograph the lesions, and two studies used images obtained from dermatology databases.
This “raised concerns that the results of the studies were unlikely to be representative of real life use,” the authors comment.
In addition, the exclusion of unevaluable images “might have systematically inflated the diagnostic performance of the tested apps,” they add.
The independent research was supported by the National Institute for Health Research (NIHR) Birmingham Biomedical Research Centre at the University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham and is an update of one of a collection of reviews funded by the NIHR through its Cochrane Systematic Review Programme Grant.
This article first appeared on Medscape.com.
FDA: Cell phones still look safe
according to a review by the Food and Drug Administration.
The FDA reviewed the published literature from 2008 to 2018 and concluded that the data don’t support any quantifiable adverse health risks from RFR. However, the evidence is not without limitations.
The FDA’s evaluation included evidence from in vivo animal studies from Jan. 1, 2008, to Aug. 1, 2018, and epidemiologic studies in humans from Jan. 1, 2008, to May 8, 2018. Both kinds of evidence had limitations, but neither produced strong indications of any causal risks from cell phone use.
The FDA noted that in vivo animal studies are limited by variability of methods and RFR exposure, which make comparisons of results difficult. These studies are also impacted by the indirect effects of temperature increases (the only currently established biological effect of RFR) and stress experienced by the animals, which make teasing out the direct effects of RFR difficult.
The FDA noted that strong epidemiologic studies can provide more relevant and accurate information than in vivo studies, but epidemiologic studies are not without limitations. For example, most have participants track and self-report their cell phone use. There’s also no way to directly track certain factors of RFR exposure, such as frequency, duration, or intensity.
Even with those caveats in mind, the FDA wrote that, “based on the studies that are described in detail in this report, there is insufficient evidence to support a causal association between RFR exposure and tumorigenesis. There is a lack of clear dose-response relationship, a lack of consistent findings or specificity, and a lack of biological mechanistic plausibility.”
The full review is available on the FDA website.
according to a review by the Food and Drug Administration.
The FDA reviewed the published literature from 2008 to 2018 and concluded that the data don’t support any quantifiable adverse health risks from RFR. However, the evidence is not without limitations.
The FDA’s evaluation included evidence from in vivo animal studies from Jan. 1, 2008, to Aug. 1, 2018, and epidemiologic studies in humans from Jan. 1, 2008, to May 8, 2018. Both kinds of evidence had limitations, but neither produced strong indications of any causal risks from cell phone use.
The FDA noted that in vivo animal studies are limited by variability of methods and RFR exposure, which make comparisons of results difficult. These studies are also impacted by the indirect effects of temperature increases (the only currently established biological effect of RFR) and stress experienced by the animals, which make teasing out the direct effects of RFR difficult.
The FDA noted that strong epidemiologic studies can provide more relevant and accurate information than in vivo studies, but epidemiologic studies are not without limitations. For example, most have participants track and self-report their cell phone use. There’s also no way to directly track certain factors of RFR exposure, such as frequency, duration, or intensity.
Even with those caveats in mind, the FDA wrote that, “based on the studies that are described in detail in this report, there is insufficient evidence to support a causal association between RFR exposure and tumorigenesis. There is a lack of clear dose-response relationship, a lack of consistent findings or specificity, and a lack of biological mechanistic plausibility.”
The full review is available on the FDA website.
according to a review by the Food and Drug Administration.
The FDA reviewed the published literature from 2008 to 2018 and concluded that the data don’t support any quantifiable adverse health risks from RFR. However, the evidence is not without limitations.
The FDA’s evaluation included evidence from in vivo animal studies from Jan. 1, 2008, to Aug. 1, 2018, and epidemiologic studies in humans from Jan. 1, 2008, to May 8, 2018. Both kinds of evidence had limitations, but neither produced strong indications of any causal risks from cell phone use.
The FDA noted that in vivo animal studies are limited by variability of methods and RFR exposure, which make comparisons of results difficult. These studies are also impacted by the indirect effects of temperature increases (the only currently established biological effect of RFR) and stress experienced by the animals, which make teasing out the direct effects of RFR difficult.
The FDA noted that strong epidemiologic studies can provide more relevant and accurate information than in vivo studies, but epidemiologic studies are not without limitations. For example, most have participants track and self-report their cell phone use. There’s also no way to directly track certain factors of RFR exposure, such as frequency, duration, or intensity.
Even with those caveats in mind, the FDA wrote that, “based on the studies that are described in detail in this report, there is insufficient evidence to support a causal association between RFR exposure and tumorigenesis. There is a lack of clear dose-response relationship, a lack of consistent findings or specificity, and a lack of biological mechanistic plausibility.”
The full review is available on the FDA website.
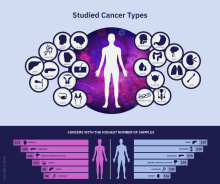
Global project reveals cancer’s genomic playbook
A massive collaborative project spanning four continents and 744 research centers has revealed driver mutations in both protein-coding and noncoding regions of 38 cancer types.
The Pan-Cancer Analysis of Whole Genomes (PCAWG) is an integrative analysis of the whole-genome sequences from 2,658 donors across 38 common tumor types. The findings are expected to add exponentially to what’s currently known about the complex genetics of cancer, and they point to possible strategies for improving cancer prevention, diagnosis, and care.
Six articles summarizing the findings are presented in a series of papers in Nature, and 16 more appear in affiliated publications.
“It’s humbling that it was only 14 years ago that the genomics community sequenced its very first cancer exome, and it was able to identify mutations within the roughly 20,000 protein-coding genes in the human cell,” investigator Lincoln Stein, MD, PhD, of the Ontario Institute for Cancer Research in Toronto, said in a telephone briefing.
Exome sequencing, however, covers only protein-coding genomic regions, which constitute only about 1% of the entire genome, “so assembling an accurate portrait of the cancer genome using just the exome data is like trying to put together a 100,000-piece jigsaw puzzle when you’re missing 99% of the pieces and there’s no puzzle box with a completed picture to guide you,” Dr. Stein said.
Members of the PCAWG from centers in North America, Europe, Asia, and Australia screened 2,658 whole-cancer genomes and matched samples of noncancerous tissues from the same individuals, along with 1,188 transcriptomes cataloging the sequences and expression of RNA transcripts in a given tumor. The 6-year project netted more than 800 terabytes of genomic data, roughly equivalent to the digital holdings of the U.S. Library of Congress multiplied by 11.
The findings are summarized in papers focusing on cancer drivers, noncoding changes, mutational signatures, structural variants, cancer evolution over time, and RNA alterations.
Driver mutations
Investigators found that the average cancer genome contains four or five driver mutations located in both coding and noncoding regions. They also found, however, that in approximately 5% of cases no driver mutations could be identified.
A substantial proportion of tumors displayed “hallmarks of genomic catastrophes.” About 22% of tumors exhibited chromothripsis, a mutational process marked by hundreds or even thousands of clustered chromosomal rearrangements. About 18% showed chromoplexy, which is characterized by scattering and rearrangement of multiple strands of DNA from one or more chromosomes.
Analyzing driver point mutations and structural variants in noncoding regions, the investigators found the usual suspects – previously reported culprits – as well as novel candidates.
For example, they identified point mutations in the five prime region of the tumor suppressor gene TP53 and the three prime untranslated regions of NFKBIZ (a nuclear factor kappa B inhibitor) and TOB1 (an antiproliferative protein), focal deletion in BRD4 (a transcriptional and epigenetic regulator), and rearrangements in chromosomal loci in members of the AKR1C family of enzymes thought to play a role in disease progression.
In addition, investigators identified mutations in noncoding regions of TERT, a telomerase gene. These mutations result in ramped-up expression of telomerase, which in turn promotes uncontrollable division of tumor cells.
Mutational signatures
In a related line of research, PCAWG investigators identified new DNA mutational signatures ranging from single nucleotide polymorphisms to insertions and deletions, as well as to structural variants – rearrangements of large sections of the genome.
“The substantial size of our dataset, compared with previous analyses, enabled the discovery of new signatures, the separation of overlapping signatures, and the decomposition of signatures into components that may represent associated – but distinct – DNA damage, repair, and/or replication mechanisms. By estimating the contribution of each signature to the mutational catalogs of individual cancer genomes, we revealed associations of signatures to exogenous or endogenous exposures, as well as to defective DNA maintenance processes,” the investigators wrote.
They also acknowledged, however, that “many signatures are of unknown cause.”
Cancer evolution
One of the six main studies focused on the evolution of cancer over time. Instead of providing a “snapshot” of the genome as captured by sequencing tissue from a single biopsy, consortium investigators created full-length features of the “life history and evolution of mutational processes and driver mutation sequences.”
They found that early cancer development was marked by relatively few mutations in driver genes and by identifiable copy-number gains, including trisomy 7 in glioblastoma, and an abnormal mirroring of the arms (isochromosome) of chromosome 17 in medulloblastoma.
In 40% of the samples, however, there were significant changes in the mutational spectrum as the cancers grew, leading to a near quadrupling of driver genes and increased genomic instability in later-stage tumors.
“Copy-number alterations often occur in mitotic crises and lead to simultaneous gains of chromosomal segments,” the investigators wrote. “Timing analyses suggest that driver mutations often precede diagnosis by many years, if not decades. Together, these results determine the evolutionary trajectories of cancer and highlight opportunities for early cancer detection.”
Implications for cancer care
“When I used to treat patients with cancer, I was always completely amazed and puzzled by how two patients could have what looked like the same tumor. It would look the same under the microscope, have the same size, and the two patients would receive exactly the same treatment, but the two patients would have completely opposite outcomes; one would survive, and one would die. What this analysis … has done is really laid bare the reasons for that unpredictability in clinical outcomes,” Peter Campbell, MD, PhD, of the Wellcome Sanger Institute in Hinxton, England, said during the telebriefing.
“The most striking finding out of all of the suite of papers is just how different one person’s cancer genome is from another person’s. We see thousands of different combinations of mutations that can cause the cancer, and more than 80 different underlying processes generating the mutations in a cancer, and that leads to very different shapes and patterns in the genome that result,” he added.
On a positive note, the research shows that one or more driver mutations can be identified in about 95% of all cancer patients, and it elucidates the sequence of events leading to oncogenesis and tumor evolution, providing opportunities for earlier identification and potential interventions to prevent cancer, Dr. Campbell said.
The PCAWG was a collaborative multinational effort with multiple funding sources and many investigators.
SOURCE: Nature. 2020 Feb 5. https://www.nature.com/collections/pcawg/
A massive collaborative project spanning four continents and 744 research centers has revealed driver mutations in both protein-coding and noncoding regions of 38 cancer types.
The Pan-Cancer Analysis of Whole Genomes (PCAWG) is an integrative analysis of the whole-genome sequences from 2,658 donors across 38 common tumor types. The findings are expected to add exponentially to what’s currently known about the complex genetics of cancer, and they point to possible strategies for improving cancer prevention, diagnosis, and care.
Six articles summarizing the findings are presented in a series of papers in Nature, and 16 more appear in affiliated publications.
“It’s humbling that it was only 14 years ago that the genomics community sequenced its very first cancer exome, and it was able to identify mutations within the roughly 20,000 protein-coding genes in the human cell,” investigator Lincoln Stein, MD, PhD, of the Ontario Institute for Cancer Research in Toronto, said in a telephone briefing.
Exome sequencing, however, covers only protein-coding genomic regions, which constitute only about 1% of the entire genome, “so assembling an accurate portrait of the cancer genome using just the exome data is like trying to put together a 100,000-piece jigsaw puzzle when you’re missing 99% of the pieces and there’s no puzzle box with a completed picture to guide you,” Dr. Stein said.
Members of the PCAWG from centers in North America, Europe, Asia, and Australia screened 2,658 whole-cancer genomes and matched samples of noncancerous tissues from the same individuals, along with 1,188 transcriptomes cataloging the sequences and expression of RNA transcripts in a given tumor. The 6-year project netted more than 800 terabytes of genomic data, roughly equivalent to the digital holdings of the U.S. Library of Congress multiplied by 11.
The findings are summarized in papers focusing on cancer drivers, noncoding changes, mutational signatures, structural variants, cancer evolution over time, and RNA alterations.
Driver mutations
Investigators found that the average cancer genome contains four or five driver mutations located in both coding and noncoding regions. They also found, however, that in approximately 5% of cases no driver mutations could be identified.
A substantial proportion of tumors displayed “hallmarks of genomic catastrophes.” About 22% of tumors exhibited chromothripsis, a mutational process marked by hundreds or even thousands of clustered chromosomal rearrangements. About 18% showed chromoplexy, which is characterized by scattering and rearrangement of multiple strands of DNA from one or more chromosomes.
Analyzing driver point mutations and structural variants in noncoding regions, the investigators found the usual suspects – previously reported culprits – as well as novel candidates.
For example, they identified point mutations in the five prime region of the tumor suppressor gene TP53 and the three prime untranslated regions of NFKBIZ (a nuclear factor kappa B inhibitor) and TOB1 (an antiproliferative protein), focal deletion in BRD4 (a transcriptional and epigenetic regulator), and rearrangements in chromosomal loci in members of the AKR1C family of enzymes thought to play a role in disease progression.
In addition, investigators identified mutations in noncoding regions of TERT, a telomerase gene. These mutations result in ramped-up expression of telomerase, which in turn promotes uncontrollable division of tumor cells.
Mutational signatures
In a related line of research, PCAWG investigators identified new DNA mutational signatures ranging from single nucleotide polymorphisms to insertions and deletions, as well as to structural variants – rearrangements of large sections of the genome.
“The substantial size of our dataset, compared with previous analyses, enabled the discovery of new signatures, the separation of overlapping signatures, and the decomposition of signatures into components that may represent associated – but distinct – DNA damage, repair, and/or replication mechanisms. By estimating the contribution of each signature to the mutational catalogs of individual cancer genomes, we revealed associations of signatures to exogenous or endogenous exposures, as well as to defective DNA maintenance processes,” the investigators wrote.
They also acknowledged, however, that “many signatures are of unknown cause.”
Cancer evolution
One of the six main studies focused on the evolution of cancer over time. Instead of providing a “snapshot” of the genome as captured by sequencing tissue from a single biopsy, consortium investigators created full-length features of the “life history and evolution of mutational processes and driver mutation sequences.”
They found that early cancer development was marked by relatively few mutations in driver genes and by identifiable copy-number gains, including trisomy 7 in glioblastoma, and an abnormal mirroring of the arms (isochromosome) of chromosome 17 in medulloblastoma.
In 40% of the samples, however, there were significant changes in the mutational spectrum as the cancers grew, leading to a near quadrupling of driver genes and increased genomic instability in later-stage tumors.
“Copy-number alterations often occur in mitotic crises and lead to simultaneous gains of chromosomal segments,” the investigators wrote. “Timing analyses suggest that driver mutations often precede diagnosis by many years, if not decades. Together, these results determine the evolutionary trajectories of cancer and highlight opportunities for early cancer detection.”
Implications for cancer care
“When I used to treat patients with cancer, I was always completely amazed and puzzled by how two patients could have what looked like the same tumor. It would look the same under the microscope, have the same size, and the two patients would receive exactly the same treatment, but the two patients would have completely opposite outcomes; one would survive, and one would die. What this analysis … has done is really laid bare the reasons for that unpredictability in clinical outcomes,” Peter Campbell, MD, PhD, of the Wellcome Sanger Institute in Hinxton, England, said during the telebriefing.
“The most striking finding out of all of the suite of papers is just how different one person’s cancer genome is from another person’s. We see thousands of different combinations of mutations that can cause the cancer, and more than 80 different underlying processes generating the mutations in a cancer, and that leads to very different shapes and patterns in the genome that result,” he added.
On a positive note, the research shows that one or more driver mutations can be identified in about 95% of all cancer patients, and it elucidates the sequence of events leading to oncogenesis and tumor evolution, providing opportunities for earlier identification and potential interventions to prevent cancer, Dr. Campbell said.
The PCAWG was a collaborative multinational effort with multiple funding sources and many investigators.
SOURCE: Nature. 2020 Feb 5. https://www.nature.com/collections/pcawg/
A massive collaborative project spanning four continents and 744 research centers has revealed driver mutations in both protein-coding and noncoding regions of 38 cancer types.
The Pan-Cancer Analysis of Whole Genomes (PCAWG) is an integrative analysis of the whole-genome sequences from 2,658 donors across 38 common tumor types. The findings are expected to add exponentially to what’s currently known about the complex genetics of cancer, and they point to possible strategies for improving cancer prevention, diagnosis, and care.
Six articles summarizing the findings are presented in a series of papers in Nature, and 16 more appear in affiliated publications.
“It’s humbling that it was only 14 years ago that the genomics community sequenced its very first cancer exome, and it was able to identify mutations within the roughly 20,000 protein-coding genes in the human cell,” investigator Lincoln Stein, MD, PhD, of the Ontario Institute for Cancer Research in Toronto, said in a telephone briefing.
Exome sequencing, however, covers only protein-coding genomic regions, which constitute only about 1% of the entire genome, “so assembling an accurate portrait of the cancer genome using just the exome data is like trying to put together a 100,000-piece jigsaw puzzle when you’re missing 99% of the pieces and there’s no puzzle box with a completed picture to guide you,” Dr. Stein said.
Members of the PCAWG from centers in North America, Europe, Asia, and Australia screened 2,658 whole-cancer genomes and matched samples of noncancerous tissues from the same individuals, along with 1,188 transcriptomes cataloging the sequences and expression of RNA transcripts in a given tumor. The 6-year project netted more than 800 terabytes of genomic data, roughly equivalent to the digital holdings of the U.S. Library of Congress multiplied by 11.
The findings are summarized in papers focusing on cancer drivers, noncoding changes, mutational signatures, structural variants, cancer evolution over time, and RNA alterations.
Driver mutations
Investigators found that the average cancer genome contains four or five driver mutations located in both coding and noncoding regions. They also found, however, that in approximately 5% of cases no driver mutations could be identified.
A substantial proportion of tumors displayed “hallmarks of genomic catastrophes.” About 22% of tumors exhibited chromothripsis, a mutational process marked by hundreds or even thousands of clustered chromosomal rearrangements. About 18% showed chromoplexy, which is characterized by scattering and rearrangement of multiple strands of DNA from one or more chromosomes.
Analyzing driver point mutations and structural variants in noncoding regions, the investigators found the usual suspects – previously reported culprits – as well as novel candidates.
For example, they identified point mutations in the five prime region of the tumor suppressor gene TP53 and the three prime untranslated regions of NFKBIZ (a nuclear factor kappa B inhibitor) and TOB1 (an antiproliferative protein), focal deletion in BRD4 (a transcriptional and epigenetic regulator), and rearrangements in chromosomal loci in members of the AKR1C family of enzymes thought to play a role in disease progression.
In addition, investigators identified mutations in noncoding regions of TERT, a telomerase gene. These mutations result in ramped-up expression of telomerase, which in turn promotes uncontrollable division of tumor cells.
Mutational signatures
In a related line of research, PCAWG investigators identified new DNA mutational signatures ranging from single nucleotide polymorphisms to insertions and deletions, as well as to structural variants – rearrangements of large sections of the genome.
“The substantial size of our dataset, compared with previous analyses, enabled the discovery of new signatures, the separation of overlapping signatures, and the decomposition of signatures into components that may represent associated – but distinct – DNA damage, repair, and/or replication mechanisms. By estimating the contribution of each signature to the mutational catalogs of individual cancer genomes, we revealed associations of signatures to exogenous or endogenous exposures, as well as to defective DNA maintenance processes,” the investigators wrote.
They also acknowledged, however, that “many signatures are of unknown cause.”
Cancer evolution
One of the six main studies focused on the evolution of cancer over time. Instead of providing a “snapshot” of the genome as captured by sequencing tissue from a single biopsy, consortium investigators created full-length features of the “life history and evolution of mutational processes and driver mutation sequences.”
They found that early cancer development was marked by relatively few mutations in driver genes and by identifiable copy-number gains, including trisomy 7 in glioblastoma, and an abnormal mirroring of the arms (isochromosome) of chromosome 17 in medulloblastoma.
In 40% of the samples, however, there were significant changes in the mutational spectrum as the cancers grew, leading to a near quadrupling of driver genes and increased genomic instability in later-stage tumors.
“Copy-number alterations often occur in mitotic crises and lead to simultaneous gains of chromosomal segments,” the investigators wrote. “Timing analyses suggest that driver mutations often precede diagnosis by many years, if not decades. Together, these results determine the evolutionary trajectories of cancer and highlight opportunities for early cancer detection.”
Implications for cancer care
“When I used to treat patients with cancer, I was always completely amazed and puzzled by how two patients could have what looked like the same tumor. It would look the same under the microscope, have the same size, and the two patients would receive exactly the same treatment, but the two patients would have completely opposite outcomes; one would survive, and one would die. What this analysis … has done is really laid bare the reasons for that unpredictability in clinical outcomes,” Peter Campbell, MD, PhD, of the Wellcome Sanger Institute in Hinxton, England, said during the telebriefing.
“The most striking finding out of all of the suite of papers is just how different one person’s cancer genome is from another person’s. We see thousands of different combinations of mutations that can cause the cancer, and more than 80 different underlying processes generating the mutations in a cancer, and that leads to very different shapes and patterns in the genome that result,” he added.
On a positive note, the research shows that one or more driver mutations can be identified in about 95% of all cancer patients, and it elucidates the sequence of events leading to oncogenesis and tumor evolution, providing opportunities for earlier identification and potential interventions to prevent cancer, Dr. Campbell said.
The PCAWG was a collaborative multinational effort with multiple funding sources and many investigators.
SOURCE: Nature. 2020 Feb 5. https://www.nature.com/collections/pcawg/
FROM NATURE
Circulating tumor cells at baseline predict recurrence in stage III melanoma
Patients with stage III melanoma who have circulating tumor cells (CTCs) at baseline may benefit from adjuvant therapy, according to investigators.
A prospective study showed that patients with at least one CTC upon first presentation had increased risks of both short-term and long-term recurrence, reported lead author Anthony Lucci, MD, of the University of Texas MD Anderson Cancer Center, Houston, and colleagues.
While previous studies have suggested that CTCs hold prognostic value for melanoma patients, no trials had evaluated the CellSearch CTC Test – a standardized technique approved by the Food and Drug Administration – in patients with stage III disease, the investigators wrote. Their report is in Clinical Cancer Research.
In the present study, the investigators tested the CellSearch system in 243 patients with stage III cutaneous melanoma who were treated at MD Anderson Cancer Center. Patients with uveal or mucosal melanoma, or distant metastatic disease, were excluded.
Baseline blood samples were drawn within 3 months of regional lymph node metastasis, determined by either lymphadenectomy or sentinel lymph node biopsy. CTC assay positivity required that at least one CTC was detected within a single 7.5 mL tube of blood.
Out of 243 patients, 90 (37%) had a positive test. Of these 90 patients, almost one-quarter (23%) relapsed within 6 months, compared with 8% of patients who had a negative CTC assay. Within the full follow-up period, which was as long as 64 months, 48% of patients with CTCs at baseline relapsed, compared with 37% of patients without CTCs.
Multivariable regression analysis, which was adjusted for age, sex, pathological nodal stage, Breslow thickness, ulceration, and lymphovascular invasion, showed that baseline CTC positivity was an independent risk factor for melanoma recurrence, both in the short term and the long term. Compared with patients who lacked CTCs, those who tested positive were three times as likely to have disease recurrence within 6 months (hazard ratio, 3.13; P = .018). For relapse-free survival within 54 months, this hazard ratio decreased to 2.25 (P = .006).
Although a Cochran-Armitage test suggested that recurrence risks increased with CTC count, the investigators noted that a minority of patients (17%) had two or more CTCs, and just 5% had three or more CTCs.
According to the investigators, CTCs at baseline could become the first reliable blood-based biomarker for this patient population.
“[CTCs] clearly identified a group of stage III patients at high risk for relapse,” the investigators wrote. “This would be clinically very significant as an independent risk factor to help identify the stage III patients who would benefit most from adjuvant systemic therapy.”
This study was funded by the Kiefer family, Sheila Prenowitz, the Simon and Linda Eyles Foundation, the Sam and Janna Moore family, and the Wintermann Foundation. The investigators reported no conflicts of interest.
SOURCE: Lucci et al. Clin Cancer Res. doi: 10.1158/1078-0432.CCR-19-2670.
Patients with stage III melanoma who have circulating tumor cells (CTCs) at baseline may benefit from adjuvant therapy, according to investigators.
A prospective study showed that patients with at least one CTC upon first presentation had increased risks of both short-term and long-term recurrence, reported lead author Anthony Lucci, MD, of the University of Texas MD Anderson Cancer Center, Houston, and colleagues.
While previous studies have suggested that CTCs hold prognostic value for melanoma patients, no trials had evaluated the CellSearch CTC Test – a standardized technique approved by the Food and Drug Administration – in patients with stage III disease, the investigators wrote. Their report is in Clinical Cancer Research.
In the present study, the investigators tested the CellSearch system in 243 patients with stage III cutaneous melanoma who were treated at MD Anderson Cancer Center. Patients with uveal or mucosal melanoma, or distant metastatic disease, were excluded.
Baseline blood samples were drawn within 3 months of regional lymph node metastasis, determined by either lymphadenectomy or sentinel lymph node biopsy. CTC assay positivity required that at least one CTC was detected within a single 7.5 mL tube of blood.
Out of 243 patients, 90 (37%) had a positive test. Of these 90 patients, almost one-quarter (23%) relapsed within 6 months, compared with 8% of patients who had a negative CTC assay. Within the full follow-up period, which was as long as 64 months, 48% of patients with CTCs at baseline relapsed, compared with 37% of patients without CTCs.
Multivariable regression analysis, which was adjusted for age, sex, pathological nodal stage, Breslow thickness, ulceration, and lymphovascular invasion, showed that baseline CTC positivity was an independent risk factor for melanoma recurrence, both in the short term and the long term. Compared with patients who lacked CTCs, those who tested positive were three times as likely to have disease recurrence within 6 months (hazard ratio, 3.13; P = .018). For relapse-free survival within 54 months, this hazard ratio decreased to 2.25 (P = .006).
Although a Cochran-Armitage test suggested that recurrence risks increased with CTC count, the investigators noted that a minority of patients (17%) had two or more CTCs, and just 5% had three or more CTCs.
According to the investigators, CTCs at baseline could become the first reliable blood-based biomarker for this patient population.
“[CTCs] clearly identified a group of stage III patients at high risk for relapse,” the investigators wrote. “This would be clinically very significant as an independent risk factor to help identify the stage III patients who would benefit most from adjuvant systemic therapy.”
This study was funded by the Kiefer family, Sheila Prenowitz, the Simon and Linda Eyles Foundation, the Sam and Janna Moore family, and the Wintermann Foundation. The investigators reported no conflicts of interest.
SOURCE: Lucci et al. Clin Cancer Res. doi: 10.1158/1078-0432.CCR-19-2670.
Patients with stage III melanoma who have circulating tumor cells (CTCs) at baseline may benefit from adjuvant therapy, according to investigators.
A prospective study showed that patients with at least one CTC upon first presentation had increased risks of both short-term and long-term recurrence, reported lead author Anthony Lucci, MD, of the University of Texas MD Anderson Cancer Center, Houston, and colleagues.
While previous studies have suggested that CTCs hold prognostic value for melanoma patients, no trials had evaluated the CellSearch CTC Test – a standardized technique approved by the Food and Drug Administration – in patients with stage III disease, the investigators wrote. Their report is in Clinical Cancer Research.
In the present study, the investigators tested the CellSearch system in 243 patients with stage III cutaneous melanoma who were treated at MD Anderson Cancer Center. Patients with uveal or mucosal melanoma, or distant metastatic disease, were excluded.
Baseline blood samples were drawn within 3 months of regional lymph node metastasis, determined by either lymphadenectomy or sentinel lymph node biopsy. CTC assay positivity required that at least one CTC was detected within a single 7.5 mL tube of blood.
Out of 243 patients, 90 (37%) had a positive test. Of these 90 patients, almost one-quarter (23%) relapsed within 6 months, compared with 8% of patients who had a negative CTC assay. Within the full follow-up period, which was as long as 64 months, 48% of patients with CTCs at baseline relapsed, compared with 37% of patients without CTCs.
Multivariable regression analysis, which was adjusted for age, sex, pathological nodal stage, Breslow thickness, ulceration, and lymphovascular invasion, showed that baseline CTC positivity was an independent risk factor for melanoma recurrence, both in the short term and the long term. Compared with patients who lacked CTCs, those who tested positive were three times as likely to have disease recurrence within 6 months (hazard ratio, 3.13; P = .018). For relapse-free survival within 54 months, this hazard ratio decreased to 2.25 (P = .006).
Although a Cochran-Armitage test suggested that recurrence risks increased with CTC count, the investigators noted that a minority of patients (17%) had two or more CTCs, and just 5% had three or more CTCs.
According to the investigators, CTCs at baseline could become the first reliable blood-based biomarker for this patient population.
“[CTCs] clearly identified a group of stage III patients at high risk for relapse,” the investigators wrote. “This would be clinically very significant as an independent risk factor to help identify the stage III patients who would benefit most from adjuvant systemic therapy.”
This study was funded by the Kiefer family, Sheila Prenowitz, the Simon and Linda Eyles Foundation, the Sam and Janna Moore family, and the Wintermann Foundation. The investigators reported no conflicts of interest.
SOURCE: Lucci et al. Clin Cancer Res. doi: 10.1158/1078-0432.CCR-19-2670.
FROM CLINICAL CANCER RESEARCH
Pembrolizumab-Induced Lobular Panniculitis in the Setting of Metastatic Melanoma
To the Editor:
Pembrolizumab is an anti–programmed death receptor 1 humanized monoclonal antibody used for treating advanced or metastatic melanoma.1 It is associated with several immune-related adverse events because it blocks a T-cell receptor checkpoint.2 The most common dermatologic immune-related adverse event seen with anti–programmed death receptor 1 medications is a nonspecific morbilliform rash, usually seen after the second treatment cycle; however, pruritus, vitiligo, bullous disorders, and lichenoid reactions also have been reported.3 We report a case of pembrolizumab-induced, self-limited lobular panniculitis in a patient with metastatic melanoma.
A 37-year-old woman with malignant melanoma presented with tender, erythematous, subcutaneous nodules on the hips and legs of 2 weeks’ duration (Figure 1). Twelve years prior to the current presentation, she was diagnosed with metastases to the cecum, lung, and brain. A review of systems was otherwise negative. She had been receiving pembrolizumab infusions (2 mg/kg every 3 weeks) for the last 2.7 years as second-line therapy after previously undergoing chemotherapy, radiation, and resection. She was not taking oral contraceptives or other hormone-based medications and did not report any new medications.
Laboratory testing was negative for infectious processes including Lyme disease, tuberculosis, and Streptococcus due to recent upper respiratory infection. Punch biopsy of a left shin lesion revealed a lobular panniculitis with lymphohistiocytic inflammation, a focal lymphocytic vasculitis, and small granulomas (Figure 2). Periodic acid–Schiff, Gram, and acid-fast bacilli stains were negative. After ruling out alternative causes, the etiology of the panniculitis was deemed to be a pembrolizumab side effect. The patient was treated conservatively with ibuprofen; pembrolizumab was not discontinued. Two weeks later, the panniculitis had resolved without additional treatment. She remains on pembrolizumab and is doing well.
Panniculitis is known to be associated with certain BRAF inhibitors used for the treatment of melanoma positive for the BRAF V600E mutation, including vemurafenib and dabrafenib.4,5 Reports of panniculitis in the setting of pembrolizumab are limited and are seen within the larger context of sarcoidosis. One patient on pembrolizumab for metastatic melanoma developed granulomatous lobular panniculitis with oligoarthritis, high fever, and hilar/mediastinal adenopathy, consistent with pembrolizumab-induced sarcoidosis. It developed after her second pembrolizumab infusion and resolved with prednisone and temporary pembrolizumab cessation.6 In another case, pembrolizumab triggered a flare of sarcoidosis with similar granulomatous subcutaneous nodules in a patient with stage IV lymphoma who was previously diagnosed with sarcoidosis but lacked cutaneous manifestations. The lesions resolved with prednisone therapy.7
Chest computed tomography was normal in our patient, and she reported no systemic symptoms. Additional laboratory studies to evaluate for sarcoidosis were not obtained. Furthermore, the lesions quickly resolved despite continued use of pembrolizumab. We report this case to highlight that pembrolizumab may induce an isolated, self-limited lobular panniculitis years after medication initiation.
- Poole RM. Pembrolizumab: first global approval. Drugs. 2014;74:1973-1981.
- Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139-148.
- Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2016;27:1362.
- Boussemart L, Routier E, Mateus C, et al. Prospective study of cutaneous side-effects associated with the BRAF inhibitor vemurafenib: a study of 42 patients. Ann Oncol. 2013;24:1691-1697.
- Ramani NS, Curry JL, Kapil J, et al. Panniculitis with necrotizing granulomata in a patient on BRAF inhibitor (dabrafenib) therapy for metastatic melanoma. Am J Dermatopathol. 2015;37:E96-E99.
- Burillo-Martinez S, Morales-Raya C, Prieto-Barrios M, et al. Pembrolizumab-induced extensive panniculitis and nevus regression: two novel cutaneous manifestations of the post-immunotherapy granulomatous reactions spectrum. JAMA Dermatol. 2017;153:721-722.
- Cotliar J, Querfeld C, Boswell WJ, et al. Pembrolizumab-associated sarcoidosis. JAAD Case Rep. 2016;2:290-293.
To the Editor:
Pembrolizumab is an anti–programmed death receptor 1 humanized monoclonal antibody used for treating advanced or metastatic melanoma.1 It is associated with several immune-related adverse events because it blocks a T-cell receptor checkpoint.2 The most common dermatologic immune-related adverse event seen with anti–programmed death receptor 1 medications is a nonspecific morbilliform rash, usually seen after the second treatment cycle; however, pruritus, vitiligo, bullous disorders, and lichenoid reactions also have been reported.3 We report a case of pembrolizumab-induced, self-limited lobular panniculitis in a patient with metastatic melanoma.
A 37-year-old woman with malignant melanoma presented with tender, erythematous, subcutaneous nodules on the hips and legs of 2 weeks’ duration (Figure 1). Twelve years prior to the current presentation, she was diagnosed with metastases to the cecum, lung, and brain. A review of systems was otherwise negative. She had been receiving pembrolizumab infusions (2 mg/kg every 3 weeks) for the last 2.7 years as second-line therapy after previously undergoing chemotherapy, radiation, and resection. She was not taking oral contraceptives or other hormone-based medications and did not report any new medications.

Laboratory testing was negative for infectious processes including Lyme disease, tuberculosis, and Streptococcus due to recent upper respiratory infection. Punch biopsy of a left shin lesion revealed a lobular panniculitis with lymphohistiocytic inflammation, a focal lymphocytic vasculitis, and small granulomas (Figure 2). Periodic acid–Schiff, Gram, and acid-fast bacilli stains were negative. After ruling out alternative causes, the etiology of the panniculitis was deemed to be a pembrolizumab side effect. The patient was treated conservatively with ibuprofen; pembrolizumab was not discontinued. Two weeks later, the panniculitis had resolved without additional treatment. She remains on pembrolizumab and is doing well.

Panniculitis is known to be associated with certain BRAF inhibitors used for the treatment of melanoma positive for the BRAF V600E mutation, including vemurafenib and dabrafenib.4,5 Reports of panniculitis in the setting of pembrolizumab are limited and are seen within the larger context of sarcoidosis. One patient on pembrolizumab for metastatic melanoma developed granulomatous lobular panniculitis with oligoarthritis, high fever, and hilar/mediastinal adenopathy, consistent with pembrolizumab-induced sarcoidosis. It developed after her second pembrolizumab infusion and resolved with prednisone and temporary pembrolizumab cessation.6 In another case, pembrolizumab triggered a flare of sarcoidosis with similar granulomatous subcutaneous nodules in a patient with stage IV lymphoma who was previously diagnosed with sarcoidosis but lacked cutaneous manifestations. The lesions resolved with prednisone therapy.7
Chest computed tomography was normal in our patient, and she reported no systemic symptoms. Additional laboratory studies to evaluate for sarcoidosis were not obtained. Furthermore, the lesions quickly resolved despite continued use of pembrolizumab. We report this case to highlight that pembrolizumab may induce an isolated, self-limited lobular panniculitis years after medication initiation.
To the Editor:
Pembrolizumab is an anti–programmed death receptor 1 humanized monoclonal antibody used for treating advanced or metastatic melanoma.1 It is associated with several immune-related adverse events because it blocks a T-cell receptor checkpoint.2 The most common dermatologic immune-related adverse event seen with anti–programmed death receptor 1 medications is a nonspecific morbilliform rash, usually seen after the second treatment cycle; however, pruritus, vitiligo, bullous disorders, and lichenoid reactions also have been reported.3 We report a case of pembrolizumab-induced, self-limited lobular panniculitis in a patient with metastatic melanoma.
A 37-year-old woman with malignant melanoma presented with tender, erythematous, subcutaneous nodules on the hips and legs of 2 weeks’ duration (Figure 1). Twelve years prior to the current presentation, she was diagnosed with metastases to the cecum, lung, and brain. A review of systems was otherwise negative. She had been receiving pembrolizumab infusions (2 mg/kg every 3 weeks) for the last 2.7 years as second-line therapy after previously undergoing chemotherapy, radiation, and resection. She was not taking oral contraceptives or other hormone-based medications and did not report any new medications.

Laboratory testing was negative for infectious processes including Lyme disease, tuberculosis, and Streptococcus due to recent upper respiratory infection. Punch biopsy of a left shin lesion revealed a lobular panniculitis with lymphohistiocytic inflammation, a focal lymphocytic vasculitis, and small granulomas (Figure 2). Periodic acid–Schiff, Gram, and acid-fast bacilli stains were negative. After ruling out alternative causes, the etiology of the panniculitis was deemed to be a pembrolizumab side effect. The patient was treated conservatively with ibuprofen; pembrolizumab was not discontinued. Two weeks later, the panniculitis had resolved without additional treatment. She remains on pembrolizumab and is doing well.

Panniculitis is known to be associated with certain BRAF inhibitors used for the treatment of melanoma positive for the BRAF V600E mutation, including vemurafenib and dabrafenib.4,5 Reports of panniculitis in the setting of pembrolizumab are limited and are seen within the larger context of sarcoidosis. One patient on pembrolizumab for metastatic melanoma developed granulomatous lobular panniculitis with oligoarthritis, high fever, and hilar/mediastinal adenopathy, consistent with pembrolizumab-induced sarcoidosis. It developed after her second pembrolizumab infusion and resolved with prednisone and temporary pembrolizumab cessation.6 In another case, pembrolizumab triggered a flare of sarcoidosis with similar granulomatous subcutaneous nodules in a patient with stage IV lymphoma who was previously diagnosed with sarcoidosis but lacked cutaneous manifestations. The lesions resolved with prednisone therapy.7
Chest computed tomography was normal in our patient, and she reported no systemic symptoms. Additional laboratory studies to evaluate for sarcoidosis were not obtained. Furthermore, the lesions quickly resolved despite continued use of pembrolizumab. We report this case to highlight that pembrolizumab may induce an isolated, self-limited lobular panniculitis years after medication initiation.
- Poole RM. Pembrolizumab: first global approval. Drugs. 2014;74:1973-1981.
- Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139-148.
- Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2016;27:1362.
- Boussemart L, Routier E, Mateus C, et al. Prospective study of cutaneous side-effects associated with the BRAF inhibitor vemurafenib: a study of 42 patients. Ann Oncol. 2013;24:1691-1697.
- Ramani NS, Curry JL, Kapil J, et al. Panniculitis with necrotizing granulomata in a patient on BRAF inhibitor (dabrafenib) therapy for metastatic melanoma. Am J Dermatopathol. 2015;37:E96-E99.
- Burillo-Martinez S, Morales-Raya C, Prieto-Barrios M, et al. Pembrolizumab-induced extensive panniculitis and nevus regression: two novel cutaneous manifestations of the post-immunotherapy granulomatous reactions spectrum. JAMA Dermatol. 2017;153:721-722.
- Cotliar J, Querfeld C, Boswell WJ, et al. Pembrolizumab-associated sarcoidosis. JAAD Case Rep. 2016;2:290-293.
- Poole RM. Pembrolizumab: first global approval. Drugs. 2014;74:1973-1981.
- Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139-148.
- Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2016;27:1362.
- Boussemart L, Routier E, Mateus C, et al. Prospective study of cutaneous side-effects associated with the BRAF inhibitor vemurafenib: a study of 42 patients. Ann Oncol. 2013;24:1691-1697.
- Ramani NS, Curry JL, Kapil J, et al. Panniculitis with necrotizing granulomata in a patient on BRAF inhibitor (dabrafenib) therapy for metastatic melanoma. Am J Dermatopathol. 2015;37:E96-E99.
- Burillo-Martinez S, Morales-Raya C, Prieto-Barrios M, et al. Pembrolizumab-induced extensive panniculitis and nevus regression: two novel cutaneous manifestations of the post-immunotherapy granulomatous reactions spectrum. JAMA Dermatol. 2017;153:721-722.
- Cotliar J, Querfeld C, Boswell WJ, et al. Pembrolizumab-associated sarcoidosis. JAAD Case Rep. 2016;2:290-293.
Practice Points
- Pembrolizumab may cause lobular panniculitis years after treatment initiation.
- Pembrolizumab-induced lobular panniculitis may self-resolve without discontinuing the medication.
Metastatic Melanoma Mimicking Eruptive Keratoacanthomas
To the Editor:
Melanoma is the third most common skin cancer. It is estimated that 18% of melanoma patients will develop skin metastases, with skin being the first site of involvement in 56% of cases.1 Of all cancers, it is estimated that 5% will develop skin metastases. It is the presenting sign in nearly 1% of visceral cancers.2 Melanoma and nonmelanoma metastases can have sundry presentations. We present a case of metastatic melanoma with multiple keratoacanthoma (KA)–like skin lesions in a patient with a known history of nonmelanoma skin cancer (NMSC) as well as melanoma.
A 76-year-old man with a history of pT2aNXMX melanoma on the left upper back presented for a routine 3-month follow-up and reported several new asymptomatic bumps on the chest, back, and right upper extremity within the last 2 weeks. The melanoma was removed via wide local excision 2 years prior at an outside facility with a Breslow depth of 1.05 mm and a negative sentinel lymph node biopsy. The mitotic rate or ulceration status was unknown. He also had a history of several NMSCs, as well as a medical history of coronary artery disease, myocardial infarction, and ventricular tachycardia with cardiac defibrillator placement. Physical examination revealed 5 pink, volcano-shaped nodules with central keratotic plugs on the upper back (Figure 1), chest, and right upper extremity, in addition to 1 pink pearly nodule on the right side of the chest. The history and appearance of the lesions were suspicious for eruptive KAs. There was no evidence of cancer recurrence at the prior melanoma and NMSC sites.
A deep shave skin biopsy was performed at all 6 sites. Histopathology showed a diffuse dermal infiltrate of elongated nests of melanocytes and nonnested melanocytes. Marked cytologic atypia and ulceration were present. Minimal connection to the overlying epidermis and a lack of junctional nests was noted. Immunohistochemical studies revealed scattered positivity for Melan-A and negative staining for AE1, AE3, cytokeratin 5, and cytokeratin 6 at all 6 sites (Figure 2). A subsequent metastatic workup showed widespread metastatic disease in the liver, bone, lung, and inferior vena cava. Computed tomography of the head was unremarkable. Magnetic resonance imaging of the brain was not performed due to the cardiac defibrillator. The patient’s lactate dehydrogenase level showed a mild increase compared to 2 months prior to the metastatic melanoma diagnosis (144 U/L vs 207 U/L [reference range, 100–200 U/L]).
The patient had no systemic symptoms at follow-up 5 weeks later. He was already evaluated by an oncologist and received his first dose of ipilimumab. He was BRAF-mutation negative. He had developed 2 new skin metastases. Five of 6 initially biopsied metastases returned and were growing; they were tender and friable with intermittent bleeding. He was subsequently referred to surgical oncology for excision of symptomatic nodules as palliative care.
Although melanoma is well known to metastasize years and even decades later, KA-like lesions have not been reported as manifestations of metastatic melanoma.4,5 Our patient likely had a primary amelanotic melanoma, as the medical records from the outside facility stated that basal cell carcinoma was ruled out via biopsy. The amelanotic nature of the primary melanoma may have influenced the amelanotic appearance of the metastases. Our patient had no signs of immunosuppression that could have contributed to the sudden skin metastases.
Depending on the subtype of cutaneous metastases (eg, satellitosis, in-transit disease, distant cutaneous metastases), the location prevalence of the primary melanoma varies. In a study of 4865 melanoma patients who were diagnosed and followed prospectively over a 30-year period, skin metastases were mostly locoregional and presentation on the leg and foot were disproportionate.1 In contrast, the trunk was overrepresented for distant metastases. Distant metastases also were more associated with concurrent metastases to the viscera.1 Accordingly, a patient’s prognosis and management will differ depending on the subtype of cutaneous metastases.
Eruptive or multiple KAs classically have been associated with the Grzybowski variant, the Ferguson-Smith familial variant, and Muir-Torre syndrome. It was reported as a paraneoplastic syndrome associated with colon cancer, ovarian cancer, and once with myelodysplastic syndrome.3 Keratoacanthomas are being classified as well-differentiated squamous cell carcinomas and have metastatic potential. A biopsy is recommended to diagnose KAs as opposed to historically being monitored for resolution. A skin biopsy is the standard of care in management of KAs.
In addition to being associated with Muir-Torre syndrome and classified as a paraneoplastic syndrome,3 eruptive KAs can occur following skin resurfacing for actinic damage, fractional photothermolysis, cryotherapy, Jessner peels, and trichloroacetic acid peels.6 A couple other uncommon settings include a case report of an arc welder with job-associated radiation and multiple reports of tattoo-induced KAs.7,8 There is the new increasingly common association of squamous cell carcinomas with BRAF inhibitors, such as vemurafenib, for metastatic melanoma.9
In a 2012 review article on cutaneous metastases, Riahi and Cohen10 found 8 cases of cutaneous metastases presenting as KA-like lesions; none were metastatic melanoma. All were solitary lesions, not multiple lesions, as in our patient. The sources were lung (3 cases), breast, esophagus, chondrosarcoma, bronchial, and mesothelioma. The most common location was the upper lip. Additionally, similar to our patient, they behaved clinically as KAs with rapid growth and keratotic plugs and were asymptomatic.10
Metastatic melanoma may mimic many other cutaneous processes that may make the diagnosis more difficult. Our case indicates that cutaneous metastases may mimic KAs. Although multiple KA-like lesions can spontaneously occur, a paraneoplastic syndrome and other underlying etiologies should be considered.
- Savoia P, Fava P, Nardò T, et al. Skin metastases of malignant melanoma: a clinical and prognostic study. Melanoma Res. 2009;19:321-326.
- Lookingbill DP, Spangler N, Sexton FM. Skin involvement as the presenting sign of internal carcinoma. J Am Acad Dermatol. 1990;22:19-26.
- Behzad M, Michl C, Pfützner W. Multiple eruptive keratoacanthomas associated with myelodysplastic syndrome. J Dtsch Dermatol Ges. 2012;10:359-360.
- Cheung WL, Patel RR, Leonard A, et al. Amelanotic melanoma: a detailed morphologic analysis with clinicopathologic correlation of 75 cases. J Cutan Pathol. 2012;39:33-39.
- Ferrari A, Piccolo D, Fargnoli MC, et al. Cutaneous amelanotic melanoma metastasis and dermatofibromas showing a dotted vascular pattern. Acta Dermato Venereologica. 2004;84:164-165.
- Mohr B, Fernandez MP, Krejci-Manwaring J. Eruptive keratoacanthoma after Jessner’s and trichloroacetic acid peel for actinic keratosis. Dermatol Surg. 2013;39:331-333.
- Wolfe CM, Green WH, Cognetta AB, et al. Multiple squamous cell carcinomas and eruptive keratoacanthomas in an arc welder. Dermatol Surg. 2013;39:328-330.
- Kluger N, Phan A, Debarbieux S, et al. Skin cancers arising in tattoos: coincidental or not? Dermatology. 2008;217:219-221.
- Mays R, Curry J, Kim K, et al. Eruptive squamous cell carcinomas after vemurafenib therapy. J Cutan Med Surg. 2013;17:419-422.
- Riahi RR, Cohen PR. Clinical manifestations of cutaneous metastases: a review with special emphasis on cutaneous metastases mimicking keratoacanthoma. Am J Clin Dermatol. 2012;13:103-112.
To the Editor:
Melanoma is the third most common skin cancer. It is estimated that 18% of melanoma patients will develop skin metastases, with skin being the first site of involvement in 56% of cases.1 Of all cancers, it is estimated that 5% will develop skin metastases. It is the presenting sign in nearly 1% of visceral cancers.2 Melanoma and nonmelanoma metastases can have sundry presentations. We present a case of metastatic melanoma with multiple keratoacanthoma (KA)–like skin lesions in a patient with a known history of nonmelanoma skin cancer (NMSC) as well as melanoma.
A 76-year-old man with a history of pT2aNXMX melanoma on the left upper back presented for a routine 3-month follow-up and reported several new asymptomatic bumps on the chest, back, and right upper extremity within the last 2 weeks. The melanoma was removed via wide local excision 2 years prior at an outside facility with a Breslow depth of 1.05 mm and a negative sentinel lymph node biopsy. The mitotic rate or ulceration status was unknown. He also had a history of several NMSCs, as well as a medical history of coronary artery disease, myocardial infarction, and ventricular tachycardia with cardiac defibrillator placement. Physical examination revealed 5 pink, volcano-shaped nodules with central keratotic plugs on the upper back (Figure 1), chest, and right upper extremity, in addition to 1 pink pearly nodule on the right side of the chest. The history and appearance of the lesions were suspicious for eruptive KAs. There was no evidence of cancer recurrence at the prior melanoma and NMSC sites.

A deep shave skin biopsy was performed at all 6 sites. Histopathology showed a diffuse dermal infiltrate of elongated nests of melanocytes and nonnested melanocytes. Marked cytologic atypia and ulceration were present. Minimal connection to the overlying epidermis and a lack of junctional nests was noted. Immunohistochemical studies revealed scattered positivity for Melan-A and negative staining for AE1, AE3, cytokeratin 5, and cytokeratin 6 at all 6 sites (Figure 2). A subsequent metastatic workup showed widespread metastatic disease in the liver, bone, lung, and inferior vena cava. Computed tomography of the head was unremarkable. Magnetic resonance imaging of the brain was not performed due to the cardiac defibrillator. The patient’s lactate dehydrogenase level showed a mild increase compared to 2 months prior to the metastatic melanoma diagnosis (144 U/L vs 207 U/L [reference range, 100–200 U/L]).

The patient had no systemic symptoms at follow-up 5 weeks later. He was already evaluated by an oncologist and received his first dose of ipilimumab. He was BRAF-mutation negative. He had developed 2 new skin metastases. Five of 6 initially biopsied metastases returned and were growing; they were tender and friable with intermittent bleeding. He was subsequently referred to surgical oncology for excision of symptomatic nodules as palliative care.
Although melanoma is well known to metastasize years and even decades later, KA-like lesions have not been reported as manifestations of metastatic melanoma.4,5 Our patient likely had a primary amelanotic melanoma, as the medical records from the outside facility stated that basal cell carcinoma was ruled out via biopsy. The amelanotic nature of the primary melanoma may have influenced the amelanotic appearance of the metastases. Our patient had no signs of immunosuppression that could have contributed to the sudden skin metastases.
Depending on the subtype of cutaneous metastases (eg, satellitosis, in-transit disease, distant cutaneous metastases), the location prevalence of the primary melanoma varies. In a study of 4865 melanoma patients who were diagnosed and followed prospectively over a 30-year period, skin metastases were mostly locoregional and presentation on the leg and foot were disproportionate.1 In contrast, the trunk was overrepresented for distant metastases. Distant metastases also were more associated with concurrent metastases to the viscera.1 Accordingly, a patient’s prognosis and management will differ depending on the subtype of cutaneous metastases.
Eruptive or multiple KAs classically have been associated with the Grzybowski variant, the Ferguson-Smith familial variant, and Muir-Torre syndrome. It was reported as a paraneoplastic syndrome associated with colon cancer, ovarian cancer, and once with myelodysplastic syndrome.3 Keratoacanthomas are being classified as well-differentiated squamous cell carcinomas and have metastatic potential. A biopsy is recommended to diagnose KAs as opposed to historically being monitored for resolution. A skin biopsy is the standard of care in management of KAs.
In addition to being associated with Muir-Torre syndrome and classified as a paraneoplastic syndrome,3 eruptive KAs can occur following skin resurfacing for actinic damage, fractional photothermolysis, cryotherapy, Jessner peels, and trichloroacetic acid peels.6 A couple other uncommon settings include a case report of an arc welder with job-associated radiation and multiple reports of tattoo-induced KAs.7,8 There is the new increasingly common association of squamous cell carcinomas with BRAF inhibitors, such as vemurafenib, for metastatic melanoma.9
In a 2012 review article on cutaneous metastases, Riahi and Cohen10 found 8 cases of cutaneous metastases presenting as KA-like lesions; none were metastatic melanoma. All were solitary lesions, not multiple lesions, as in our patient. The sources were lung (3 cases), breast, esophagus, chondrosarcoma, bronchial, and mesothelioma. The most common location was the upper lip. Additionally, similar to our patient, they behaved clinically as KAs with rapid growth and keratotic plugs and were asymptomatic.10
Metastatic melanoma may mimic many other cutaneous processes that may make the diagnosis more difficult. Our case indicates that cutaneous metastases may mimic KAs. Although multiple KA-like lesions can spontaneously occur, a paraneoplastic syndrome and other underlying etiologies should be considered.
To the Editor:
Melanoma is the third most common skin cancer. It is estimated that 18% of melanoma patients will develop skin metastases, with skin being the first site of involvement in 56% of cases.1 Of all cancers, it is estimated that 5% will develop skin metastases. It is the presenting sign in nearly 1% of visceral cancers.2 Melanoma and nonmelanoma metastases can have sundry presentations. We present a case of metastatic melanoma with multiple keratoacanthoma (KA)–like skin lesions in a patient with a known history of nonmelanoma skin cancer (NMSC) as well as melanoma.
A 76-year-old man with a history of pT2aNXMX melanoma on the left upper back presented for a routine 3-month follow-up and reported several new asymptomatic bumps on the chest, back, and right upper extremity within the last 2 weeks. The melanoma was removed via wide local excision 2 years prior at an outside facility with a Breslow depth of 1.05 mm and a negative sentinel lymph node biopsy. The mitotic rate or ulceration status was unknown. He also had a history of several NMSCs, as well as a medical history of coronary artery disease, myocardial infarction, and ventricular tachycardia with cardiac defibrillator placement. Physical examination revealed 5 pink, volcano-shaped nodules with central keratotic plugs on the upper back (Figure 1), chest, and right upper extremity, in addition to 1 pink pearly nodule on the right side of the chest. The history and appearance of the lesions were suspicious for eruptive KAs. There was no evidence of cancer recurrence at the prior melanoma and NMSC sites.

A deep shave skin biopsy was performed at all 6 sites. Histopathology showed a diffuse dermal infiltrate of elongated nests of melanocytes and nonnested melanocytes. Marked cytologic atypia and ulceration were present. Minimal connection to the overlying epidermis and a lack of junctional nests was noted. Immunohistochemical studies revealed scattered positivity for Melan-A and negative staining for AE1, AE3, cytokeratin 5, and cytokeratin 6 at all 6 sites (Figure 2). A subsequent metastatic workup showed widespread metastatic disease in the liver, bone, lung, and inferior vena cava. Computed tomography of the head was unremarkable. Magnetic resonance imaging of the brain was not performed due to the cardiac defibrillator. The patient’s lactate dehydrogenase level showed a mild increase compared to 2 months prior to the metastatic melanoma diagnosis (144 U/L vs 207 U/L [reference range, 100–200 U/L]).

The patient had no systemic symptoms at follow-up 5 weeks later. He was already evaluated by an oncologist and received his first dose of ipilimumab. He was BRAF-mutation negative. He had developed 2 new skin metastases. Five of 6 initially biopsied metastases returned and were growing; they were tender and friable with intermittent bleeding. He was subsequently referred to surgical oncology for excision of symptomatic nodules as palliative care.
Although melanoma is well known to metastasize years and even decades later, KA-like lesions have not been reported as manifestations of metastatic melanoma.4,5 Our patient likely had a primary amelanotic melanoma, as the medical records from the outside facility stated that basal cell carcinoma was ruled out via biopsy. The amelanotic nature of the primary melanoma may have influenced the amelanotic appearance of the metastases. Our patient had no signs of immunosuppression that could have contributed to the sudden skin metastases.
Depending on the subtype of cutaneous metastases (eg, satellitosis, in-transit disease, distant cutaneous metastases), the location prevalence of the primary melanoma varies. In a study of 4865 melanoma patients who were diagnosed and followed prospectively over a 30-year period, skin metastases were mostly locoregional and presentation on the leg and foot were disproportionate.1 In contrast, the trunk was overrepresented for distant metastases. Distant metastases also were more associated with concurrent metastases to the viscera.1 Accordingly, a patient’s prognosis and management will differ depending on the subtype of cutaneous metastases.
Eruptive or multiple KAs classically have been associated with the Grzybowski variant, the Ferguson-Smith familial variant, and Muir-Torre syndrome. It was reported as a paraneoplastic syndrome associated with colon cancer, ovarian cancer, and once with myelodysplastic syndrome.3 Keratoacanthomas are being classified as well-differentiated squamous cell carcinomas and have metastatic potential. A biopsy is recommended to diagnose KAs as opposed to historically being monitored for resolution. A skin biopsy is the standard of care in management of KAs.
In addition to being associated with Muir-Torre syndrome and classified as a paraneoplastic syndrome,3 eruptive KAs can occur following skin resurfacing for actinic damage, fractional photothermolysis, cryotherapy, Jessner peels, and trichloroacetic acid peels.6 A couple other uncommon settings include a case report of an arc welder with job-associated radiation and multiple reports of tattoo-induced KAs.7,8 There is the new increasingly common association of squamous cell carcinomas with BRAF inhibitors, such as vemurafenib, for metastatic melanoma.9
In a 2012 review article on cutaneous metastases, Riahi and Cohen10 found 8 cases of cutaneous metastases presenting as KA-like lesions; none were metastatic melanoma. All were solitary lesions, not multiple lesions, as in our patient. The sources were lung (3 cases), breast, esophagus, chondrosarcoma, bronchial, and mesothelioma. The most common location was the upper lip. Additionally, similar to our patient, they behaved clinically as KAs with rapid growth and keratotic plugs and were asymptomatic.10
Metastatic melanoma may mimic many other cutaneous processes that may make the diagnosis more difficult. Our case indicates that cutaneous metastases may mimic KAs. Although multiple KA-like lesions can spontaneously occur, a paraneoplastic syndrome and other underlying etiologies should be considered.
- Savoia P, Fava P, Nardò T, et al. Skin metastases of malignant melanoma: a clinical and prognostic study. Melanoma Res. 2009;19:321-326.
- Lookingbill DP, Spangler N, Sexton FM. Skin involvement as the presenting sign of internal carcinoma. J Am Acad Dermatol. 1990;22:19-26.
- Behzad M, Michl C, Pfützner W. Multiple eruptive keratoacanthomas associated with myelodysplastic syndrome. J Dtsch Dermatol Ges. 2012;10:359-360.
- Cheung WL, Patel RR, Leonard A, et al. Amelanotic melanoma: a detailed morphologic analysis with clinicopathologic correlation of 75 cases. J Cutan Pathol. 2012;39:33-39.
- Ferrari A, Piccolo D, Fargnoli MC, et al. Cutaneous amelanotic melanoma metastasis and dermatofibromas showing a dotted vascular pattern. Acta Dermato Venereologica. 2004;84:164-165.
- Mohr B, Fernandez MP, Krejci-Manwaring J. Eruptive keratoacanthoma after Jessner’s and trichloroacetic acid peel for actinic keratosis. Dermatol Surg. 2013;39:331-333.
- Wolfe CM, Green WH, Cognetta AB, et al. Multiple squamous cell carcinomas and eruptive keratoacanthomas in an arc welder. Dermatol Surg. 2013;39:328-330.
- Kluger N, Phan A, Debarbieux S, et al. Skin cancers arising in tattoos: coincidental or not? Dermatology. 2008;217:219-221.
- Mays R, Curry J, Kim K, et al. Eruptive squamous cell carcinomas after vemurafenib therapy. J Cutan Med Surg. 2013;17:419-422.
- Riahi RR, Cohen PR. Clinical manifestations of cutaneous metastases: a review with special emphasis on cutaneous metastases mimicking keratoacanthoma. Am J Clin Dermatol. 2012;13:103-112.
- Savoia P, Fava P, Nardò T, et al. Skin metastases of malignant melanoma: a clinical and prognostic study. Melanoma Res. 2009;19:321-326.
- Lookingbill DP, Spangler N, Sexton FM. Skin involvement as the presenting sign of internal carcinoma. J Am Acad Dermatol. 1990;22:19-26.
- Behzad M, Michl C, Pfützner W. Multiple eruptive keratoacanthomas associated with myelodysplastic syndrome. J Dtsch Dermatol Ges. 2012;10:359-360.
- Cheung WL, Patel RR, Leonard A, et al. Amelanotic melanoma: a detailed morphologic analysis with clinicopathologic correlation of 75 cases. J Cutan Pathol. 2012;39:33-39.
- Ferrari A, Piccolo D, Fargnoli MC, et al. Cutaneous amelanotic melanoma metastasis and dermatofibromas showing a dotted vascular pattern. Acta Dermato Venereologica. 2004;84:164-165.
- Mohr B, Fernandez MP, Krejci-Manwaring J. Eruptive keratoacanthoma after Jessner’s and trichloroacetic acid peel for actinic keratosis. Dermatol Surg. 2013;39:331-333.
- Wolfe CM, Green WH, Cognetta AB, et al. Multiple squamous cell carcinomas and eruptive keratoacanthomas in an arc welder. Dermatol Surg. 2013;39:328-330.
- Kluger N, Phan A, Debarbieux S, et al. Skin cancers arising in tattoos: coincidental or not? Dermatology. 2008;217:219-221.
- Mays R, Curry J, Kim K, et al. Eruptive squamous cell carcinomas after vemurafenib therapy. J Cutan Med Surg. 2013;17:419-422.
- Riahi RR, Cohen PR. Clinical manifestations of cutaneous metastases: a review with special emphasis on cutaneous metastases mimicking keratoacanthoma. Am J Clin Dermatol. 2012;13:103-112.
Practice Points
- Cutaneous metastatic melanoma can have variable clinical presentations.
- Patients with a history of melanoma should be monitored closely with a low threshold for biopsy of new skin lesions.
Lenvatinib/pembrolizumab has good activity in advanced RCC, other solid tumors
A combination of the tyrosine kinase inhibitor lenvatinib (Lenvima) and the immune checkpoint inhibitor pembrolizumab (Keytruda) was safe and showed promising activity against advanced renal cell carcinoma and other solid tumors in a phase 1b/2 study.
Overall response rates (ORR) at 24 weeks ranged from 63% for patients with advanced renal cell carcinomas (RCC) to 25% for patients with urothelial cancers, reported Matthew H. Taylor, MD, of Knight Cancer Institute at Oregon Health & Science University in Portland, and colleagues.
The findings from this study sparked additional clinical trials for patients with gastric cancer, gastroesophageal cancer, and differentiated thyroid cancer, and set the stage for larger phase 3 trials in patients with advanced RCC, endometrial cancer, malignant melanoma, and non–small cell lung cancer (NSCLC).
“In the future, we also plan to study lenvatinib plus pembrolizumab in patients with RCC who have had disease progression after treatment with immune checkpoint inhibitors,” they wrote. The report was published in Journal of Clinical Oncology.
Lenvatinib is a multitargeted tyrosine kinase inhibitor (TKI) with action against vascular endothelial growth factor (VEGF) receptors 1-3, fibroblast growth factor (FGF) receptors 1-4, platelet-derived growth factor receptors alpha and the RET and KIT kinases.
“Preclinical and clinical studies suggest that modulation of VEGF-mediated immune suppression via angiogenesis inhibition could potentially augment the immunotherapeutic activity of immune checkpoint inhibitors,” the investigators wrote.
They reported results from the dose finding (1b) phase including 13 patients and initial phase 2 expansion cohorts with a total of 124 patients.
The maximum tolerated dose of lenvatinib in combination with pembrolizumab was established as 20 mg/day.
At 24 weeks of follow-up, the ORR for 30 patients with RCC was 63%; two additional patients had responses after week 24, for a total ORR at study cutoff in this cohort of 70%. The median duration of response for these patients was 20 months, and the median progression-free survival (PFS) was 19.8 months. At the time of data cutoff for this analysis, 9 of the 30 patients with RCC were still on treatment.
For 23 patients with endometrial cancer, the 24-week and overall ORR were 52%, with a median duration of response not reached, and a median PFS of 9.7 months. Seven patients were still on treatment at data cutoff.
For 21 patients with melanoma, the 24-week and overall ORR were 48%, median duration of response was 12.5 months, and median PFS was 5.5 months. Two of the patients were still on treatment at data cutoff.
For the 22 patients with squamous cell cancer of the head and neck, the 24-week ORR was 36%, with two patients having a response after week 24 for a total ORR at data cutoff of 46%. The median duration of response was 8.2 months and the median PFS was 4.7 months. Three patients remained on treatment at data cutoff.
For 21 patients with NSCLC, the 24-week and overall ORR were 33%, the median duration of response was 10.9 months, and median PFS was 5.9 months. Six of the patients were still receiving treatment at data cutoff.
For 20 patients with urothelial cancer, the 24-week and overall ORR were 25%, with a median duration of response not reached, and a median PFS of 5.4 months. Three patients were still receiving the combination at the time of data cutoff.
Treatment related adverse events (TRAEs) occurred in 133 of all 137 patients enrolled in the two study phases. The adverse events were similar across all cohorts, with any grade of events including fatigue in 58%, diarrhea in 52%, hypertension in 47%, hypothyroidism in 42%, and decreased appetite in 39%.
The most frequent grade 3 or 4 TRAEs were hypertension in 20%, fatigue in 12%, diarrhea in 9%, proteinuria in 8%, and increased lipase levels in 7%.
In all, 85% of patients had a TRAE leading to lenvatinib dose reduction and/or interruption, and 13% required lenvatinib discontinuation.
Events leading to pembrolizumab dose interruption occurred in 45% of patients, and pembrolizumab discontinuation in 15%.
The study was sponsored by Eisai with collaboration from Merck Sharp & Dohme. Dr. Taylor disclosed a consulting or advisory role for Bristol-Myers Squibb, Eisai, Array BioPharma, Loxo, Bayer, ArQule, Blueprint Medicines, Novartis, and Sanofi/Genzyme, and speakers bureau activities for BMS and Eisai.
SOURCE: Taylor MH et al. J Clin Oncol. 2020 Jan. 21 doi: 10.1200/JCO.19.01598.
A combination of the tyrosine kinase inhibitor lenvatinib (Lenvima) and the immune checkpoint inhibitor pembrolizumab (Keytruda) was safe and showed promising activity against advanced renal cell carcinoma and other solid tumors in a phase 1b/2 study.
Overall response rates (ORR) at 24 weeks ranged from 63% for patients with advanced renal cell carcinomas (RCC) to 25% for patients with urothelial cancers, reported Matthew H. Taylor, MD, of Knight Cancer Institute at Oregon Health & Science University in Portland, and colleagues.
The findings from this study sparked additional clinical trials for patients with gastric cancer, gastroesophageal cancer, and differentiated thyroid cancer, and set the stage for larger phase 3 trials in patients with advanced RCC, endometrial cancer, malignant melanoma, and non–small cell lung cancer (NSCLC).
“In the future, we also plan to study lenvatinib plus pembrolizumab in patients with RCC who have had disease progression after treatment with immune checkpoint inhibitors,” they wrote. The report was published in Journal of Clinical Oncology.
Lenvatinib is a multitargeted tyrosine kinase inhibitor (TKI) with action against vascular endothelial growth factor (VEGF) receptors 1-3, fibroblast growth factor (FGF) receptors 1-4, platelet-derived growth factor receptors alpha and the RET and KIT kinases.
“Preclinical and clinical studies suggest that modulation of VEGF-mediated immune suppression via angiogenesis inhibition could potentially augment the immunotherapeutic activity of immune checkpoint inhibitors,” the investigators wrote.
They reported results from the dose finding (1b) phase including 13 patients and initial phase 2 expansion cohorts with a total of 124 patients.
The maximum tolerated dose of lenvatinib in combination with pembrolizumab was established as 20 mg/day.
At 24 weeks of follow-up, the ORR for 30 patients with RCC was 63%; two additional patients had responses after week 24, for a total ORR at study cutoff in this cohort of 70%. The median duration of response for these patients was 20 months, and the median progression-free survival (PFS) was 19.8 months. At the time of data cutoff for this analysis, 9 of the 30 patients with RCC were still on treatment.
For 23 patients with endometrial cancer, the 24-week and overall ORR were 52%, with a median duration of response not reached, and a median PFS of 9.7 months. Seven patients were still on treatment at data cutoff.
For 21 patients with melanoma, the 24-week and overall ORR were 48%, median duration of response was 12.5 months, and median PFS was 5.5 months. Two of the patients were still on treatment at data cutoff.
For the 22 patients with squamous cell cancer of the head and neck, the 24-week ORR was 36%, with two patients having a response after week 24 for a total ORR at data cutoff of 46%. The median duration of response was 8.2 months and the median PFS was 4.7 months. Three patients remained on treatment at data cutoff.
For 21 patients with NSCLC, the 24-week and overall ORR were 33%, the median duration of response was 10.9 months, and median PFS was 5.9 months. Six of the patients were still receiving treatment at data cutoff.
For 20 patients with urothelial cancer, the 24-week and overall ORR were 25%, with a median duration of response not reached, and a median PFS of 5.4 months. Three patients were still receiving the combination at the time of data cutoff.
Treatment related adverse events (TRAEs) occurred in 133 of all 137 patients enrolled in the two study phases. The adverse events were similar across all cohorts, with any grade of events including fatigue in 58%, diarrhea in 52%, hypertension in 47%, hypothyroidism in 42%, and decreased appetite in 39%.
The most frequent grade 3 or 4 TRAEs were hypertension in 20%, fatigue in 12%, diarrhea in 9%, proteinuria in 8%, and increased lipase levels in 7%.
In all, 85% of patients had a TRAE leading to lenvatinib dose reduction and/or interruption, and 13% required lenvatinib discontinuation.
Events leading to pembrolizumab dose interruption occurred in 45% of patients, and pembrolizumab discontinuation in 15%.
The study was sponsored by Eisai with collaboration from Merck Sharp & Dohme. Dr. Taylor disclosed a consulting or advisory role for Bristol-Myers Squibb, Eisai, Array BioPharma, Loxo, Bayer, ArQule, Blueprint Medicines, Novartis, and Sanofi/Genzyme, and speakers bureau activities for BMS and Eisai.
SOURCE: Taylor MH et al. J Clin Oncol. 2020 Jan. 21 doi: 10.1200/JCO.19.01598.
A combination of the tyrosine kinase inhibitor lenvatinib (Lenvima) and the immune checkpoint inhibitor pembrolizumab (Keytruda) was safe and showed promising activity against advanced renal cell carcinoma and other solid tumors in a phase 1b/2 study.
Overall response rates (ORR) at 24 weeks ranged from 63% for patients with advanced renal cell carcinomas (RCC) to 25% for patients with urothelial cancers, reported Matthew H. Taylor, MD, of Knight Cancer Institute at Oregon Health & Science University in Portland, and colleagues.
The findings from this study sparked additional clinical trials for patients with gastric cancer, gastroesophageal cancer, and differentiated thyroid cancer, and set the stage for larger phase 3 trials in patients with advanced RCC, endometrial cancer, malignant melanoma, and non–small cell lung cancer (NSCLC).
“In the future, we also plan to study lenvatinib plus pembrolizumab in patients with RCC who have had disease progression after treatment with immune checkpoint inhibitors,” they wrote. The report was published in Journal of Clinical Oncology.
Lenvatinib is a multitargeted tyrosine kinase inhibitor (TKI) with action against vascular endothelial growth factor (VEGF) receptors 1-3, fibroblast growth factor (FGF) receptors 1-4, platelet-derived growth factor receptors alpha and the RET and KIT kinases.
“Preclinical and clinical studies suggest that modulation of VEGF-mediated immune suppression via angiogenesis inhibition could potentially augment the immunotherapeutic activity of immune checkpoint inhibitors,” the investigators wrote.
They reported results from the dose finding (1b) phase including 13 patients and initial phase 2 expansion cohorts with a total of 124 patients.
The maximum tolerated dose of lenvatinib in combination with pembrolizumab was established as 20 mg/day.
At 24 weeks of follow-up, the ORR for 30 patients with RCC was 63%; two additional patients had responses after week 24, for a total ORR at study cutoff in this cohort of 70%. The median duration of response for these patients was 20 months, and the median progression-free survival (PFS) was 19.8 months. At the time of data cutoff for this analysis, 9 of the 30 patients with RCC were still on treatment.
For 23 patients with endometrial cancer, the 24-week and overall ORR were 52%, with a median duration of response not reached, and a median PFS of 9.7 months. Seven patients were still on treatment at data cutoff.
For 21 patients with melanoma, the 24-week and overall ORR were 48%, median duration of response was 12.5 months, and median PFS was 5.5 months. Two of the patients were still on treatment at data cutoff.
For the 22 patients with squamous cell cancer of the head and neck, the 24-week ORR was 36%, with two patients having a response after week 24 for a total ORR at data cutoff of 46%. The median duration of response was 8.2 months and the median PFS was 4.7 months. Three patients remained on treatment at data cutoff.
For 21 patients with NSCLC, the 24-week and overall ORR were 33%, the median duration of response was 10.9 months, and median PFS was 5.9 months. Six of the patients were still receiving treatment at data cutoff.
For 20 patients with urothelial cancer, the 24-week and overall ORR were 25%, with a median duration of response not reached, and a median PFS of 5.4 months. Three patients were still receiving the combination at the time of data cutoff.
Treatment related adverse events (TRAEs) occurred in 133 of all 137 patients enrolled in the two study phases. The adverse events were similar across all cohorts, with any grade of events including fatigue in 58%, diarrhea in 52%, hypertension in 47%, hypothyroidism in 42%, and decreased appetite in 39%.
The most frequent grade 3 or 4 TRAEs were hypertension in 20%, fatigue in 12%, diarrhea in 9%, proteinuria in 8%, and increased lipase levels in 7%.
In all, 85% of patients had a TRAE leading to lenvatinib dose reduction and/or interruption, and 13% required lenvatinib discontinuation.
Events leading to pembrolizumab dose interruption occurred in 45% of patients, and pembrolizumab discontinuation in 15%.
The study was sponsored by Eisai with collaboration from Merck Sharp & Dohme. Dr. Taylor disclosed a consulting or advisory role for Bristol-Myers Squibb, Eisai, Array BioPharma, Loxo, Bayer, ArQule, Blueprint Medicines, Novartis, and Sanofi/Genzyme, and speakers bureau activities for BMS and Eisai.
SOURCE: Taylor MH et al. J Clin Oncol. 2020 Jan. 21 doi: 10.1200/JCO.19.01598.
FROM THE JOURNAL OF CLINICAL ONCOLOGY