User login
Teaching Evidence-Based Dermatology Using a Web-Based Journal Club: A Pilot Study and Survey
To the Editor:
With a steady increase in dermatology publications over recent decades, there is an expanding pool of evidence to address clinical questions.1 Residency training is the time when appraising the medical literature and practicing evidence-based medicine is most honed. Evidence-based medicine is an essential component of Practice-based Learning and Improvement, a required core competency of the Accreditation Council for Graduate Medical Education.2 Assimilation of new research evidence is traditionally taught through didactics and journal club discussions in residency.
However, at a time when the demand for information overwhelms safeguards that exist to evaluate its quality, it is more important than ever to be equipped with the proper tools to critically appraise novel literature. Beyond accepting a scientific article at face value, physicians must learn to ask targeted questions of the study design, results, and clinical relevance. These questions change based on the type of study, and organizations such as the Oxford Centre for Evidence-Based Medicine provide guidance through critical appraisal worksheets.3
To investigate the utility of using guided questions to evaluate the reliability, significance, and applicability of clinical evidence, we beta tested a novel web-based application in an academic dermatology setting to design and run a journal club for residents. Six dermatology residents participated in this institutional review board–approved study comprised of 3 phases: (1) independent article appraisal through the web-based application, (2) group discussion, and (3) anonymous postsurvey.
Using this platform, we uploaded a recent article into the interactive reader, which contained an integrated tool for appraisal based on specific questions. Because the article described the results of a randomized clinical trial, we used questions from the Centre for Evidence-Based Medicine’s Randomised Controlled Trials Critical Appraisal Worksheet, which has a series of questions to evaluate internal validity, results, and external validity and applicability.3
Residents used the platform to independently read the article, highlight areas of the text that corresponded to 8 critical appraisal questions, and answer yes or no to these questions. Based on residents’ answers, a final appraisal score (on a scale of 1% to 100%) was generated. Simultaneously, the attending dermatologist leading the journal club (C.W.) also completed the assignment to establish an expert score.
Scores from the residents’ independent appraisal ranged from 75% to 100% (mean, 85.4%). Upon discussing the article in a group setting, the residents established a consensus score of 75%. This consensus score matched the expert score, which suggested to us that both independently reviewing the article using guided questions and conducting a group debriefing were necessary to match the expert level of critical appraisal.
Of note, the residents’ average independent appraisal score was higher than both the consensus and expert scores, indicating that the residents evaluated the article less critically on their own. With more practice using this method, it is possible that the precision and accuracy of the residents’ critical appraisal of scientific articles will improve.
In the postsurvey, we asked residents about the critical appraisal of the medical literature. All residents agreed that evaluating the quality of evidence when reading a scientific article was somewhat important or very important to them; however, only 2 of 6 evaluated the quality of evidence all the time, and the other 4 did so half of the time or less than half of the time.
When critically appraising articles, 2 of 6 residents used specific rubrics half of the time; 4 of 6 less than half of the time. Most important, 5 of 6 residents agreed that the quality of evidence affected their management decisions more than half of the time or all of the time. Although it is clear that residents value evidence-based medicine and understand the importance of evaluating the quality of evidence, doing so currently might not be simple or practical.
An organized framework for appraising articles would streamline the process. Five of 6 residents agreed that the use of specific questions as a guide made it easier to appraise an article for the quality of its evidence. Four of 6 residents found that juxtaposing specific questions with the interactive reader was helpful; 5 of 6 agreed that they would use a web-based journal club platform if given the option.
Lastly, 5 of 6 residents agreed that if such a tool were available, a platform containing all major dermatology publications in an interactive reader format, along with relevant appraisal questions on the side, would be useful.
This pilot study augmented the typical journal club experience by emphasizing goal-directed reading and the importance of analyzing the quality of evidence. The combination of independent appraisal of an article using targeted questions and a group debrief led to better understanding of the evidence and its clinical applicability. The COVID-19 pandemic may be a better time than ever to explore innovative ways to teach evidence-based medicine in residency training.
- Mimouni D, Pavlovsky L, Akerman L, et al. Trends in dermatology publications over the past 15 years. Am J Clin Dermatol. 2010;11:55-58. doi:10.2165/11530190-000000000-00000.
- NEJM Knowledge+ Team. Exploring the ACGME Core Competencies: Practice-Based Learning and Improvement (part 2 of 7). Massachusetts Medical Society. NEJM Knowledge+ website. Published July 28, 2016. Accessed January 15, 2022. https://knowledgeplus.nejm.org/blog/practice-based-learning-and-improvement/
- University of Oxford. Critical appraisal tools. Centre for Evidence-Based Medicine website. Accessed January 2, 2022. www.cebm.ox.ac.uk/resources/ebm-tools/critical-appraisal-tools
To the Editor:
With a steady increase in dermatology publications over recent decades, there is an expanding pool of evidence to address clinical questions.1 Residency training is the time when appraising the medical literature and practicing evidence-based medicine is most honed. Evidence-based medicine is an essential component of Practice-based Learning and Improvement, a required core competency of the Accreditation Council for Graduate Medical Education.2 Assimilation of new research evidence is traditionally taught through didactics and journal club discussions in residency.
However, at a time when the demand for information overwhelms safeguards that exist to evaluate its quality, it is more important than ever to be equipped with the proper tools to critically appraise novel literature. Beyond accepting a scientific article at face value, physicians must learn to ask targeted questions of the study design, results, and clinical relevance. These questions change based on the type of study, and organizations such as the Oxford Centre for Evidence-Based Medicine provide guidance through critical appraisal worksheets.3
To investigate the utility of using guided questions to evaluate the reliability, significance, and applicability of clinical evidence, we beta tested a novel web-based application in an academic dermatology setting to design and run a journal club for residents. Six dermatology residents participated in this institutional review board–approved study comprised of 3 phases: (1) independent article appraisal through the web-based application, (2) group discussion, and (3) anonymous postsurvey.
Using this platform, we uploaded a recent article into the interactive reader, which contained an integrated tool for appraisal based on specific questions. Because the article described the results of a randomized clinical trial, we used questions from the Centre for Evidence-Based Medicine’s Randomised Controlled Trials Critical Appraisal Worksheet, which has a series of questions to evaluate internal validity, results, and external validity and applicability.3
Residents used the platform to independently read the article, highlight areas of the text that corresponded to 8 critical appraisal questions, and answer yes or no to these questions. Based on residents’ answers, a final appraisal score (on a scale of 1% to 100%) was generated. Simultaneously, the attending dermatologist leading the journal club (C.W.) also completed the assignment to establish an expert score.
Scores from the residents’ independent appraisal ranged from 75% to 100% (mean, 85.4%). Upon discussing the article in a group setting, the residents established a consensus score of 75%. This consensus score matched the expert score, which suggested to us that both independently reviewing the article using guided questions and conducting a group debriefing were necessary to match the expert level of critical appraisal.
Of note, the residents’ average independent appraisal score was higher than both the consensus and expert scores, indicating that the residents evaluated the article less critically on their own. With more practice using this method, it is possible that the precision and accuracy of the residents’ critical appraisal of scientific articles will improve.
In the postsurvey, we asked residents about the critical appraisal of the medical literature. All residents agreed that evaluating the quality of evidence when reading a scientific article was somewhat important or very important to them; however, only 2 of 6 evaluated the quality of evidence all the time, and the other 4 did so half of the time or less than half of the time.
When critically appraising articles, 2 of 6 residents used specific rubrics half of the time; 4 of 6 less than half of the time. Most important, 5 of 6 residents agreed that the quality of evidence affected their management decisions more than half of the time or all of the time. Although it is clear that residents value evidence-based medicine and understand the importance of evaluating the quality of evidence, doing so currently might not be simple or practical.
An organized framework for appraising articles would streamline the process. Five of 6 residents agreed that the use of specific questions as a guide made it easier to appraise an article for the quality of its evidence. Four of 6 residents found that juxtaposing specific questions with the interactive reader was helpful; 5 of 6 agreed that they would use a web-based journal club platform if given the option.
Lastly, 5 of 6 residents agreed that if such a tool were available, a platform containing all major dermatology publications in an interactive reader format, along with relevant appraisal questions on the side, would be useful.
This pilot study augmented the typical journal club experience by emphasizing goal-directed reading and the importance of analyzing the quality of evidence. The combination of independent appraisal of an article using targeted questions and a group debrief led to better understanding of the evidence and its clinical applicability. The COVID-19 pandemic may be a better time than ever to explore innovative ways to teach evidence-based medicine in residency training.
To the Editor:
With a steady increase in dermatology publications over recent decades, there is an expanding pool of evidence to address clinical questions.1 Residency training is the time when appraising the medical literature and practicing evidence-based medicine is most honed. Evidence-based medicine is an essential component of Practice-based Learning and Improvement, a required core competency of the Accreditation Council for Graduate Medical Education.2 Assimilation of new research evidence is traditionally taught through didactics and journal club discussions in residency.
However, at a time when the demand for information overwhelms safeguards that exist to evaluate its quality, it is more important than ever to be equipped with the proper tools to critically appraise novel literature. Beyond accepting a scientific article at face value, physicians must learn to ask targeted questions of the study design, results, and clinical relevance. These questions change based on the type of study, and organizations such as the Oxford Centre for Evidence-Based Medicine provide guidance through critical appraisal worksheets.3
To investigate the utility of using guided questions to evaluate the reliability, significance, and applicability of clinical evidence, we beta tested a novel web-based application in an academic dermatology setting to design and run a journal club for residents. Six dermatology residents participated in this institutional review board–approved study comprised of 3 phases: (1) independent article appraisal through the web-based application, (2) group discussion, and (3) anonymous postsurvey.
Using this platform, we uploaded a recent article into the interactive reader, which contained an integrated tool for appraisal based on specific questions. Because the article described the results of a randomized clinical trial, we used questions from the Centre for Evidence-Based Medicine’s Randomised Controlled Trials Critical Appraisal Worksheet, which has a series of questions to evaluate internal validity, results, and external validity and applicability.3
Residents used the platform to independently read the article, highlight areas of the text that corresponded to 8 critical appraisal questions, and answer yes or no to these questions. Based on residents’ answers, a final appraisal score (on a scale of 1% to 100%) was generated. Simultaneously, the attending dermatologist leading the journal club (C.W.) also completed the assignment to establish an expert score.
Scores from the residents’ independent appraisal ranged from 75% to 100% (mean, 85.4%). Upon discussing the article in a group setting, the residents established a consensus score of 75%. This consensus score matched the expert score, which suggested to us that both independently reviewing the article using guided questions and conducting a group debriefing were necessary to match the expert level of critical appraisal.
Of note, the residents’ average independent appraisal score was higher than both the consensus and expert scores, indicating that the residents evaluated the article less critically on their own. With more practice using this method, it is possible that the precision and accuracy of the residents’ critical appraisal of scientific articles will improve.
In the postsurvey, we asked residents about the critical appraisal of the medical literature. All residents agreed that evaluating the quality of evidence when reading a scientific article was somewhat important or very important to them; however, only 2 of 6 evaluated the quality of evidence all the time, and the other 4 did so half of the time or less than half of the time.
When critically appraising articles, 2 of 6 residents used specific rubrics half of the time; 4 of 6 less than half of the time. Most important, 5 of 6 residents agreed that the quality of evidence affected their management decisions more than half of the time or all of the time. Although it is clear that residents value evidence-based medicine and understand the importance of evaluating the quality of evidence, doing so currently might not be simple or practical.
An organized framework for appraising articles would streamline the process. Five of 6 residents agreed that the use of specific questions as a guide made it easier to appraise an article for the quality of its evidence. Four of 6 residents found that juxtaposing specific questions with the interactive reader was helpful; 5 of 6 agreed that they would use a web-based journal club platform if given the option.
Lastly, 5 of 6 residents agreed that if such a tool were available, a platform containing all major dermatology publications in an interactive reader format, along with relevant appraisal questions on the side, would be useful.
This pilot study augmented the typical journal club experience by emphasizing goal-directed reading and the importance of analyzing the quality of evidence. The combination of independent appraisal of an article using targeted questions and a group debrief led to better understanding of the evidence and its clinical applicability. The COVID-19 pandemic may be a better time than ever to explore innovative ways to teach evidence-based medicine in residency training.
- Mimouni D, Pavlovsky L, Akerman L, et al. Trends in dermatology publications over the past 15 years. Am J Clin Dermatol. 2010;11:55-58. doi:10.2165/11530190-000000000-00000.
- NEJM Knowledge+ Team. Exploring the ACGME Core Competencies: Practice-Based Learning and Improvement (part 2 of 7). Massachusetts Medical Society. NEJM Knowledge+ website. Published July 28, 2016. Accessed January 15, 2022. https://knowledgeplus.nejm.org/blog/practice-based-learning-and-improvement/
- University of Oxford. Critical appraisal tools. Centre for Evidence-Based Medicine website. Accessed January 2, 2022. www.cebm.ox.ac.uk/resources/ebm-tools/critical-appraisal-tools
- Mimouni D, Pavlovsky L, Akerman L, et al. Trends in dermatology publications over the past 15 years. Am J Clin Dermatol. 2010;11:55-58. doi:10.2165/11530190-000000000-00000.
- NEJM Knowledge+ Team. Exploring the ACGME Core Competencies: Practice-Based Learning and Improvement (part 2 of 7). Massachusetts Medical Society. NEJM Knowledge+ website. Published July 28, 2016. Accessed January 15, 2022. https://knowledgeplus.nejm.org/blog/practice-based-learning-and-improvement/
- University of Oxford. Critical appraisal tools. Centre for Evidence-Based Medicine website. Accessed January 2, 2022. www.cebm.ox.ac.uk/resources/ebm-tools/critical-appraisal-tools
Practice Points
- A novel web-based application was beta tested in an academic dermatology setting to design and run a journal club for residents.
- Goal-directed reading was emphasized by using guided questions to critically appraise literature based on reliability, significance, and applicability.
- The combination of independent appraisal of an article using targeted questions and a group debrief led to better understanding of the evidence and its clinical applicability.
Tender Papules on the Bilateral Dorsal Hands
The Diagnosis: Interstitial Granulomatous Dermatitis
Interstitial granulomatous dermatitis (IGD) is rare, and the exact incidence is unknown, with only a few cases reported in the literature annually.1 Although IGD may arise in both children and adults, it occurs more commonly in adults, with an age of onset of 52 to 58.5 years. Interstitial granulomatous dermatitis also shows a female predominance.1
Interstitial granulomatous dermatitis may present as annular flesh-colored or erythematous to violaceous papules and plaques, or less commonly erythematous linear cordlike subcutaneous nodules (called the rope sign).1 Lesions often are asymptomatic but may be pruritic or tender. Interstitial granulomatous dermatitis has been associated with autoimmune conditions such as rheumatoid arthritis, systemic lupus erythematosus, and primary biliary cholangitis, and rarely malignancy.2 Interstitial granulomatous drug reactions can occur months to years after initiation of therapy with offending agents, and common causes include calcium channel blockers, statins, and tumor necrosis factor α inhibitors.3
Interstitial granulomatous dermatitis and palisaded neutrophilic and granulomatous dermatitis (PNGD) demonstrate overlapping clinical features and are thought to be part of the same spectrum of granulomatous dermatitis.4 Both IGD and PNGD may present with symmetric flesh-colored to erythematous papules or erythematous annular or linear plaques.5 Interstitial granulomatous dermatitis and PNGD may be differentiated through histopathologic examination.
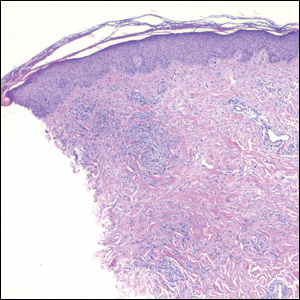
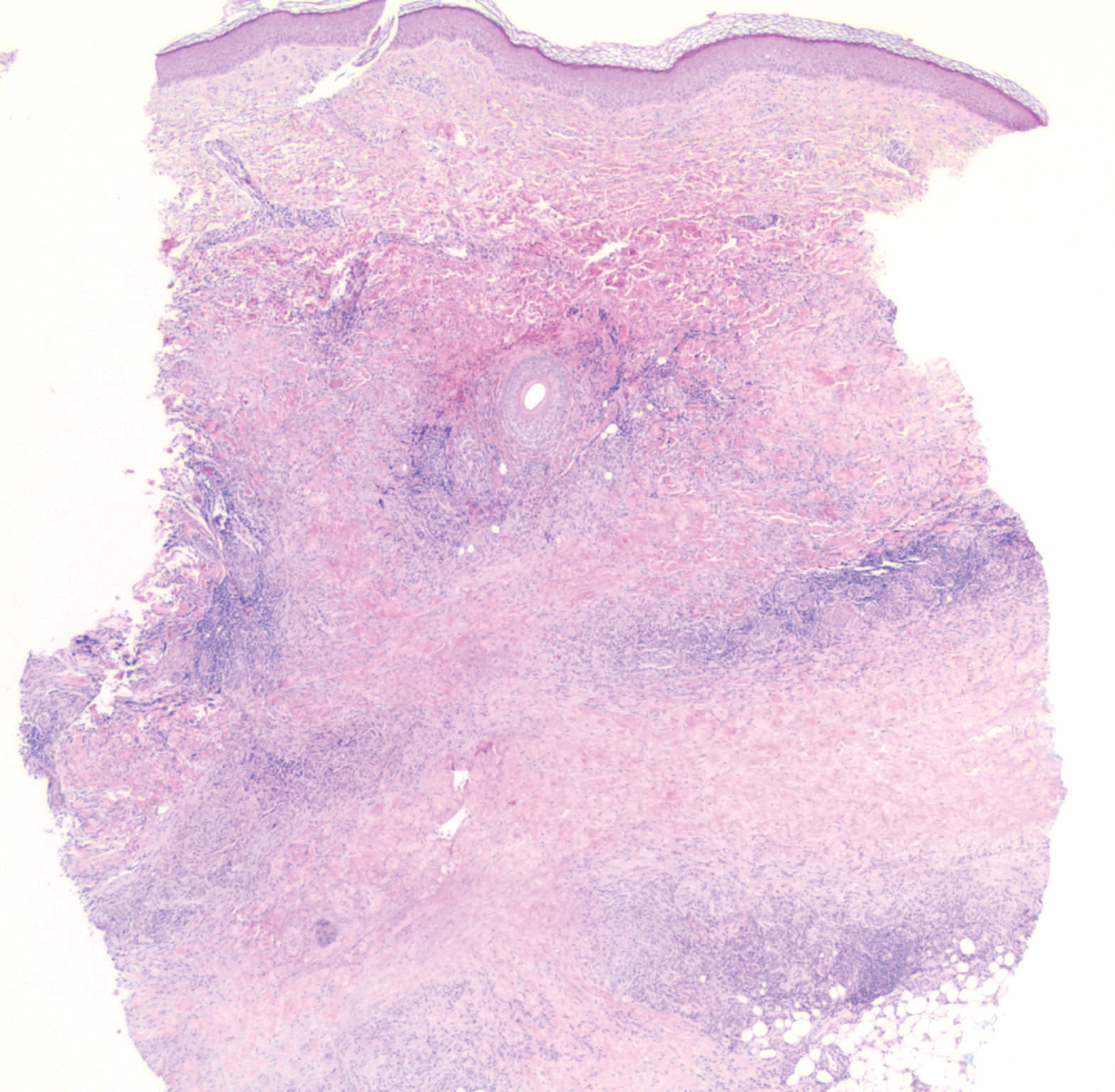
Histopathology of IGD shows an interstitial infiltrate of epithelioid histiocytes in the dermis, often surrounding foci of degenerated collagen resembling palisading granulomas (quiz images).1 Perivascular and interstitial lymphocytic infiltrates also are present in most cases. Epidermal changes are minimal in IGD but can be associated with interstitial granulomatous drug reactions.1 There usually is no vasculitis, and mucin typically is absent, unlike granuloma annulare (GA).3,6 In comparison, histopathologic examination of PNGD shows basophilic degenerated collagen surrounded by palisades of histiocytes, neutrophils, and nuclear debris with focal areas of leukocytoclastic vasculitis and rare mucin.5
No specific treatment is recommended, and lesions may resolve without any therapy. Reported treatments include topical, intralesional, or systemic steroids; nonsteroidal anti-inflammatory drugs; methotrexate; hydroxychloroquine; and cyclosporine.6 Due to the strong association with systemic diseases, it is important to evaluate patients with IGD for autoimmune diseases and conduct age-appropriate cancer screening. Furthermore, a review of medications is warranted to assess the possibility of interstitial granulomatous drug reactions.6 In our patient, rheumatologic workup and age-appropriate cancer screenings were negative, and the rash spontaneously resolved without treatment.
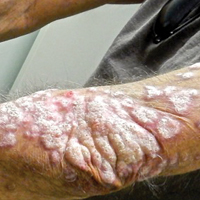
Granuloma annulare presents with asymptomatic flesh-colored to erythematous papules and plaques in an annular configuration. In the localized variant of GA, plaques frequently localize to the distal extremities, especially the dorsal hands, as in our patient. Other variants include generalized GA, subcutaneous GA, and perforating GA. Mucin and a palisading or interstitial pattern of granulomatous inflammation are key features on histopathology in all subtypes of GA (Figure 1).7 Patch GA is a rare variant that presents with asymptomatic erythematous to brown patches, is associated with interstitial-type inflammation on histopathology, and can be difficult to distinguish from IGD.8 Granuloma annulare with interstitial inflammation on histology can be differentiated from IGD by the comparative lack of mucin in IGD.7
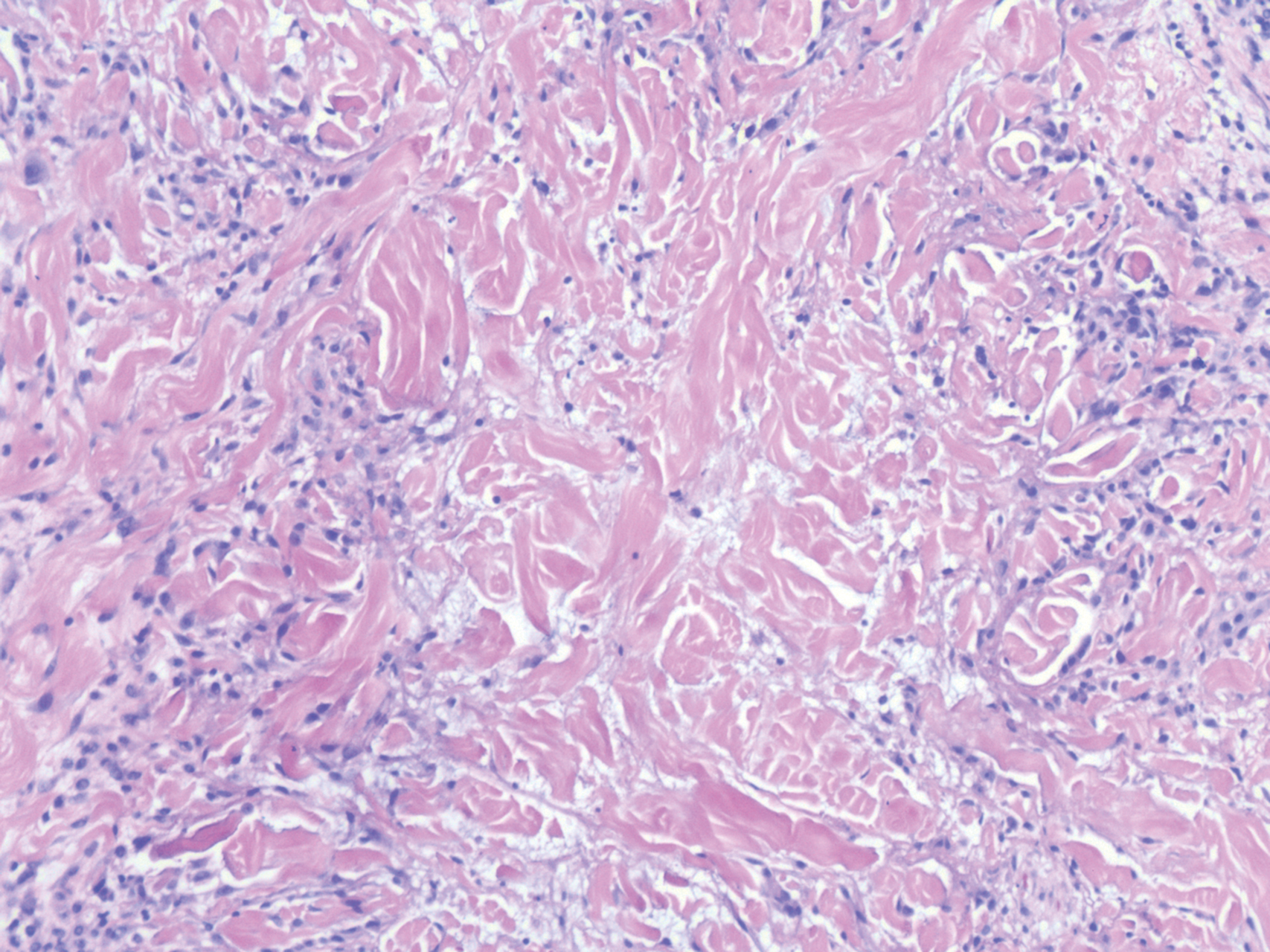
Sweet syndrome (SS) is characterized by sudden-onset, painful, erythematous plaques and/or nodules, commonly associated with fever and leukocytosis. Clinical variants of SS include pustular and bullous SS; giant cellulitis-like SS; necrotizing SS; and neutrophilic dermatosis of the dorsal hands presenting with hemorrhagic bullae, plaques, and pustules.7-9 Histopathologic examination shows dense nodular or perivascular neutrophilic infiltrate in the dermis without evidence of vasculitis (Figure 2).10 Histopathologic variants include histiocytoid, lymphocytic, subcutaneous, and cryptococcoid.9 The classic variant of SS has a bandlike, predominantly neutrophilic infiltrate with marked leukocytoclasia, which can be differentiated from the histiocytoid infiltrate of IGD.11 It has been shown that the infiltrate of the histiocytoid variant of SS is composed of myeloperoxidase-positive, immature myeloid cells rather than true histiocytes, and therefore can be differentiated from IGD.12 Lastly, all variants of SS have dermal edema, which typically is absent in IGD, and SS has no evidence of necrobiosis.
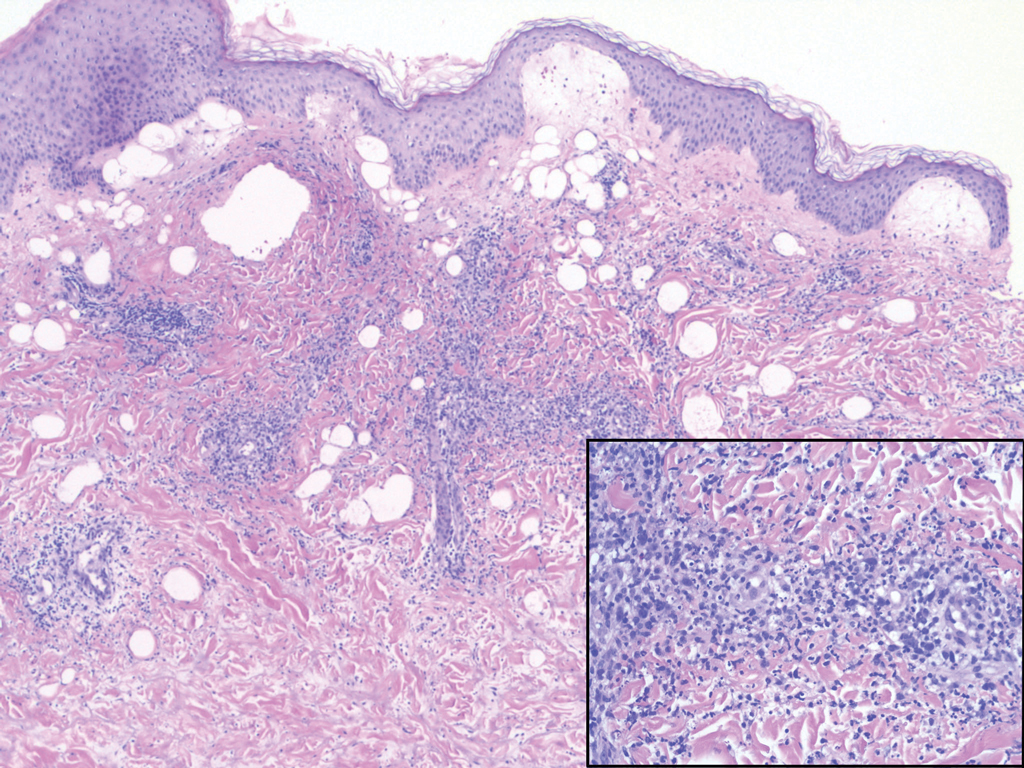
Erythema elevatum diutinum (EED) is a rare disease that presents with bilateral violaceous or erythematous to brown papules, plaques, or nodules. Lesions frequently localize to extensor surfaces, including the hands and fingers, and may be asymptomatic or associated with pruritus, burning, or tingling.13 Early EED lesions are characterized by leukocytoclastic vasculitis of the papillary and mid-dermal vessels with a perivascular neutrophilic infiltrate and perivascular fibrinoid necrosis. With older EED lesions, dermal and perivascular onion skin-like fibrosis become more prominent (Figure 3).14 The neutrophilic infiltrate, dermal fibrosis, and chronic vasculitic changes distinguish EED from IGD.
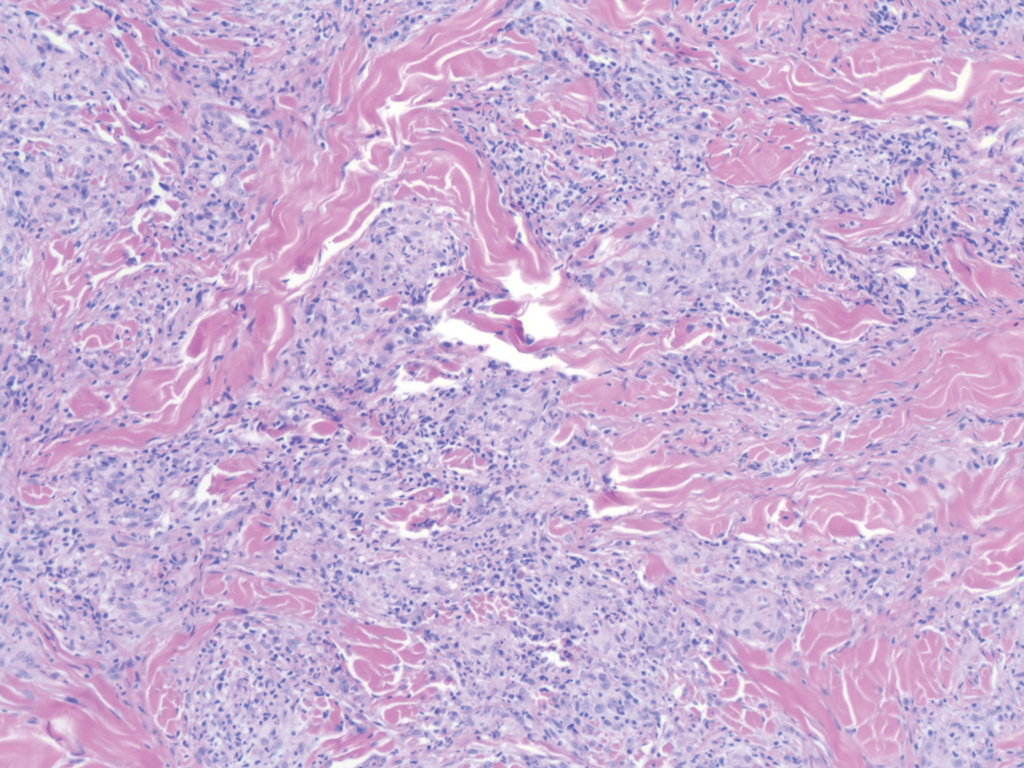
Necrobiosis lipoidica (NL) is a rare disease that presents with well-demarcated, yellow to red-brown papules and nodules most commonly localized to the bilateral lower extremities on the pretibial area. Papules and nodules evolve into plaques over time, and ulceration is common.15 On histopathology, NL primarily exhibits granulomatous inflammation with parallel palisading (Figure 4). The hallmark feature is necrobiosis--or degeneration--of collagen; the alternation of necrobiotic collagen and inflammatory infiltrate creates a layered cake-like appearance on low power.16 The clinical presentation as well as the dermal necrobiotic granuloma consisting of a large confluent area of necrobiosis centered in the superficial dermis and subcutaneous tissue of NL distinguishes it from the histiocytic infiltrate of IGD.
- Peroni A, Colato C, Schena D, et al. Interstitial granulomatous dermatitis: a distinct entity with characteristic histological and clinical pattern. Br J Dermatol. 2012;166:775-783.
- Terziroli Beretta-Piccoli B, Mainetti C, Peeters MA, et al. Cutaneous granulomatosis: a comprehensive review. Clin Rev Allergy Immunol. 2018;54:131-146.
- Rosenbach MA, Wanat KA, Reisenauer A, et al. Non-infectious granulomas. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier Saunders; 2018:1644-1663.
- Chu P, Connolly MK, LeBoit PE. The histopathologic spectrum of palisaded neutrophilic and granulomatous dermatitis in patients with collagen vascular disease. Arch Dermatol. 1994;130:1278-1283.
- Huizenga T, Kado JA, Pellicane B, et al. Interstitial granulomatous dermatitis and palisaded neutrophilic granulomatous dermatitis. Cutis. 2018;101:E19-E21.
- Rosenbach M, English JC 3rd. Reactive granulomatous dermatitis: a review of palisaded neutrophilic and granulomatous dermatitis, interstitial granulomatous dermatitis, interstitial granulomatous drug reaction, and a proposed reclassification. Dermatol Clin. 2015;33:373-387.
- Piette EW, Rosenbach M. Granuloma annulare: clinical and histologic variants, epidemiology, and genetics. J Am Acad Dermatol. 2016;75:457-465.
- Mutasim DF, Bridges AG. Patch granuloma annulare: clinicopathologic study of 6 patients. J Am Acad Dermatol. 2000;42:417-421.
- Nelson CA, Stephen S, Ashchyan HJ, et al. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018;79:987-1006.
- Dabade TS, Davis MD. Diagnosis and treatment of the neutrophilic dermatoses (pyoderma gangrenosum, Sweet's syndrome). Dermatol Ther. 2011;24:273-284.
- Davis M, Moschella L. Neutrophilic dermatoses. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier Saunders; 2018:2102-2112.
- Requena L, Kutzner H, Palmedo G, et al. Histiocytoid Sweet syndrome: a dermal infiltration of immature neutrophilic granulocytes. Arch Dermatol. 2005;141:834-842.
- Gibson LE, el-Azhary RA. Erythema elevatum diutinum. Clin Dermatol. 2000;18:295-299.
- Sardiña LA, Jour G, Piliang MP, et al. Erythema elevatum diutinum a rare and poorly understood cutaneous vasculitis: a single institution experience. J Cutan Pathol. 2019;46:97-101.
- Reid SD, Ladizinski B, Lee K, et al. Update on necrobiosis lipoidica: a review of etiology, diagnosis, and treatment options. J Am Acad Dermatol. 2013;69:783-791.
- Sibbald C, Reid S, Alavi A. Necrobiosis lipoidica. Dermatol Clin. 2015;33:343-360.
The Diagnosis: Interstitial Granulomatous Dermatitis
Interstitial granulomatous dermatitis (IGD) is rare, and the exact incidence is unknown, with only a few cases reported in the literature annually.1 Although IGD may arise in both children and adults, it occurs more commonly in adults, with an age of onset of 52 to 58.5 years. Interstitial granulomatous dermatitis also shows a female predominance.1
Interstitial granulomatous dermatitis may present as annular flesh-colored or erythematous to violaceous papules and plaques, or less commonly erythematous linear cordlike subcutaneous nodules (called the rope sign).1 Lesions often are asymptomatic but may be pruritic or tender. Interstitial granulomatous dermatitis has been associated with autoimmune conditions such as rheumatoid arthritis, systemic lupus erythematosus, and primary biliary cholangitis, and rarely malignancy.2 Interstitial granulomatous drug reactions can occur months to years after initiation of therapy with offending agents, and common causes include calcium channel blockers, statins, and tumor necrosis factor α inhibitors.3
Interstitial granulomatous dermatitis and palisaded neutrophilic and granulomatous dermatitis (PNGD) demonstrate overlapping clinical features and are thought to be part of the same spectrum of granulomatous dermatitis.4 Both IGD and PNGD may present with symmetric flesh-colored to erythematous papules or erythematous annular or linear plaques.5 Interstitial granulomatous dermatitis and PNGD may be differentiated through histopathologic examination.
Histopathology of IGD shows an interstitial infiltrate of epithelioid histiocytes in the dermis, often surrounding foci of degenerated collagen resembling palisading granulomas (quiz images).1 Perivascular and interstitial lymphocytic infiltrates also are present in most cases. Epidermal changes are minimal in IGD but can be associated with interstitial granulomatous drug reactions.1 There usually is no vasculitis, and mucin typically is absent, unlike granuloma annulare (GA).3,6 In comparison, histopathologic examination of PNGD shows basophilic degenerated collagen surrounded by palisades of histiocytes, neutrophils, and nuclear debris with focal areas of leukocytoclastic vasculitis and rare mucin.5
No specific treatment is recommended, and lesions may resolve without any therapy. Reported treatments include topical, intralesional, or systemic steroids; nonsteroidal anti-inflammatory drugs; methotrexate; hydroxychloroquine; and cyclosporine.6 Due to the strong association with systemic diseases, it is important to evaluate patients with IGD for autoimmune diseases and conduct age-appropriate cancer screening. Furthermore, a review of medications is warranted to assess the possibility of interstitial granulomatous drug reactions.6 In our patient, rheumatologic workup and age-appropriate cancer screenings were negative, and the rash spontaneously resolved without treatment.
Granuloma annulare presents with asymptomatic flesh-colored to erythematous papules and plaques in an annular configuration. In the localized variant of GA, plaques frequently localize to the distal extremities, especially the dorsal hands, as in our patient. Other variants include generalized GA, subcutaneous GA, and perforating GA. Mucin and a palisading or interstitial pattern of granulomatous inflammation are key features on histopathology in all subtypes of GA (Figure 1).7 Patch GA is a rare variant that presents with asymptomatic erythematous to brown patches, is associated with interstitial-type inflammation on histopathology, and can be difficult to distinguish from IGD.8 Granuloma annulare with interstitial inflammation on histology can be differentiated from IGD by the comparative lack of mucin in IGD.7

Sweet syndrome (SS) is characterized by sudden-onset, painful, erythematous plaques and/or nodules, commonly associated with fever and leukocytosis. Clinical variants of SS include pustular and bullous SS; giant cellulitis-like SS; necrotizing SS; and neutrophilic dermatosis of the dorsal hands presenting with hemorrhagic bullae, plaques, and pustules.7-9 Histopathologic examination shows dense nodular or perivascular neutrophilic infiltrate in the dermis without evidence of vasculitis (Figure 2).10 Histopathologic variants include histiocytoid, lymphocytic, subcutaneous, and cryptococcoid.9 The classic variant of SS has a bandlike, predominantly neutrophilic infiltrate with marked leukocytoclasia, which can be differentiated from the histiocytoid infiltrate of IGD.11 It has been shown that the infiltrate of the histiocytoid variant of SS is composed of myeloperoxidase-positive, immature myeloid cells rather than true histiocytes, and therefore can be differentiated from IGD.12 Lastly, all variants of SS have dermal edema, which typically is absent in IGD, and SS has no evidence of necrobiosis.

Erythema elevatum diutinum (EED) is a rare disease that presents with bilateral violaceous or erythematous to brown papules, plaques, or nodules. Lesions frequently localize to extensor surfaces, including the hands and fingers, and may be asymptomatic or associated with pruritus, burning, or tingling.13 Early EED lesions are characterized by leukocytoclastic vasculitis of the papillary and mid-dermal vessels with a perivascular neutrophilic infiltrate and perivascular fibrinoid necrosis. With older EED lesions, dermal and perivascular onion skin-like fibrosis become more prominent (Figure 3).14 The neutrophilic infiltrate, dermal fibrosis, and chronic vasculitic changes distinguish EED from IGD.

Necrobiosis lipoidica (NL) is a rare disease that presents with well-demarcated, yellow to red-brown papules and nodules most commonly localized to the bilateral lower extremities on the pretibial area. Papules and nodules evolve into plaques over time, and ulceration is common.15 On histopathology, NL primarily exhibits granulomatous inflammation with parallel palisading (Figure 4). The hallmark feature is necrobiosis--or degeneration--of collagen; the alternation of necrobiotic collagen and inflammatory infiltrate creates a layered cake-like appearance on low power.16 The clinical presentation as well as the dermal necrobiotic granuloma consisting of a large confluent area of necrobiosis centered in the superficial dermis and subcutaneous tissue of NL distinguishes it from the histiocytic infiltrate of IGD.

The Diagnosis: Interstitial Granulomatous Dermatitis
Interstitial granulomatous dermatitis (IGD) is rare, and the exact incidence is unknown, with only a few cases reported in the literature annually.1 Although IGD may arise in both children and adults, it occurs more commonly in adults, with an age of onset of 52 to 58.5 years. Interstitial granulomatous dermatitis also shows a female predominance.1
Interstitial granulomatous dermatitis may present as annular flesh-colored or erythematous to violaceous papules and plaques, or less commonly erythematous linear cordlike subcutaneous nodules (called the rope sign).1 Lesions often are asymptomatic but may be pruritic or tender. Interstitial granulomatous dermatitis has been associated with autoimmune conditions such as rheumatoid arthritis, systemic lupus erythematosus, and primary biliary cholangitis, and rarely malignancy.2 Interstitial granulomatous drug reactions can occur months to years after initiation of therapy with offending agents, and common causes include calcium channel blockers, statins, and tumor necrosis factor α inhibitors.3
Interstitial granulomatous dermatitis and palisaded neutrophilic and granulomatous dermatitis (PNGD) demonstrate overlapping clinical features and are thought to be part of the same spectrum of granulomatous dermatitis.4 Both IGD and PNGD may present with symmetric flesh-colored to erythematous papules or erythematous annular or linear plaques.5 Interstitial granulomatous dermatitis and PNGD may be differentiated through histopathologic examination.
Histopathology of IGD shows an interstitial infiltrate of epithelioid histiocytes in the dermis, often surrounding foci of degenerated collagen resembling palisading granulomas (quiz images).1 Perivascular and interstitial lymphocytic infiltrates also are present in most cases. Epidermal changes are minimal in IGD but can be associated with interstitial granulomatous drug reactions.1 There usually is no vasculitis, and mucin typically is absent, unlike granuloma annulare (GA).3,6 In comparison, histopathologic examination of PNGD shows basophilic degenerated collagen surrounded by palisades of histiocytes, neutrophils, and nuclear debris with focal areas of leukocytoclastic vasculitis and rare mucin.5
No specific treatment is recommended, and lesions may resolve without any therapy. Reported treatments include topical, intralesional, or systemic steroids; nonsteroidal anti-inflammatory drugs; methotrexate; hydroxychloroquine; and cyclosporine.6 Due to the strong association with systemic diseases, it is important to evaluate patients with IGD for autoimmune diseases and conduct age-appropriate cancer screening. Furthermore, a review of medications is warranted to assess the possibility of interstitial granulomatous drug reactions.6 In our patient, rheumatologic workup and age-appropriate cancer screenings were negative, and the rash spontaneously resolved without treatment.
Granuloma annulare presents with asymptomatic flesh-colored to erythematous papules and plaques in an annular configuration. In the localized variant of GA, plaques frequently localize to the distal extremities, especially the dorsal hands, as in our patient. Other variants include generalized GA, subcutaneous GA, and perforating GA. Mucin and a palisading or interstitial pattern of granulomatous inflammation are key features on histopathology in all subtypes of GA (Figure 1).7 Patch GA is a rare variant that presents with asymptomatic erythematous to brown patches, is associated with interstitial-type inflammation on histopathology, and can be difficult to distinguish from IGD.8 Granuloma annulare with interstitial inflammation on histology can be differentiated from IGD by the comparative lack of mucin in IGD.7

Sweet syndrome (SS) is characterized by sudden-onset, painful, erythematous plaques and/or nodules, commonly associated with fever and leukocytosis. Clinical variants of SS include pustular and bullous SS; giant cellulitis-like SS; necrotizing SS; and neutrophilic dermatosis of the dorsal hands presenting with hemorrhagic bullae, plaques, and pustules.7-9 Histopathologic examination shows dense nodular or perivascular neutrophilic infiltrate in the dermis without evidence of vasculitis (Figure 2).10 Histopathologic variants include histiocytoid, lymphocytic, subcutaneous, and cryptococcoid.9 The classic variant of SS has a bandlike, predominantly neutrophilic infiltrate with marked leukocytoclasia, which can be differentiated from the histiocytoid infiltrate of IGD.11 It has been shown that the infiltrate of the histiocytoid variant of SS is composed of myeloperoxidase-positive, immature myeloid cells rather than true histiocytes, and therefore can be differentiated from IGD.12 Lastly, all variants of SS have dermal edema, which typically is absent in IGD, and SS has no evidence of necrobiosis.

Erythema elevatum diutinum (EED) is a rare disease that presents with bilateral violaceous or erythematous to brown papules, plaques, or nodules. Lesions frequently localize to extensor surfaces, including the hands and fingers, and may be asymptomatic or associated with pruritus, burning, or tingling.13 Early EED lesions are characterized by leukocytoclastic vasculitis of the papillary and mid-dermal vessels with a perivascular neutrophilic infiltrate and perivascular fibrinoid necrosis. With older EED lesions, dermal and perivascular onion skin-like fibrosis become more prominent (Figure 3).14 The neutrophilic infiltrate, dermal fibrosis, and chronic vasculitic changes distinguish EED from IGD.

Necrobiosis lipoidica (NL) is a rare disease that presents with well-demarcated, yellow to red-brown papules and nodules most commonly localized to the bilateral lower extremities on the pretibial area. Papules and nodules evolve into plaques over time, and ulceration is common.15 On histopathology, NL primarily exhibits granulomatous inflammation with parallel palisading (Figure 4). The hallmark feature is necrobiosis--or degeneration--of collagen; the alternation of necrobiotic collagen and inflammatory infiltrate creates a layered cake-like appearance on low power.16 The clinical presentation as well as the dermal necrobiotic granuloma consisting of a large confluent area of necrobiosis centered in the superficial dermis and subcutaneous tissue of NL distinguishes it from the histiocytic infiltrate of IGD.

- Peroni A, Colato C, Schena D, et al. Interstitial granulomatous dermatitis: a distinct entity with characteristic histological and clinical pattern. Br J Dermatol. 2012;166:775-783.
- Terziroli Beretta-Piccoli B, Mainetti C, Peeters MA, et al. Cutaneous granulomatosis: a comprehensive review. Clin Rev Allergy Immunol. 2018;54:131-146.
- Rosenbach MA, Wanat KA, Reisenauer A, et al. Non-infectious granulomas. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier Saunders; 2018:1644-1663.
- Chu P, Connolly MK, LeBoit PE. The histopathologic spectrum of palisaded neutrophilic and granulomatous dermatitis in patients with collagen vascular disease. Arch Dermatol. 1994;130:1278-1283.
- Huizenga T, Kado JA, Pellicane B, et al. Interstitial granulomatous dermatitis and palisaded neutrophilic granulomatous dermatitis. Cutis. 2018;101:E19-E21.
- Rosenbach M, English JC 3rd. Reactive granulomatous dermatitis: a review of palisaded neutrophilic and granulomatous dermatitis, interstitial granulomatous dermatitis, interstitial granulomatous drug reaction, and a proposed reclassification. Dermatol Clin. 2015;33:373-387.
- Piette EW, Rosenbach M. Granuloma annulare: clinical and histologic variants, epidemiology, and genetics. J Am Acad Dermatol. 2016;75:457-465.
- Mutasim DF, Bridges AG. Patch granuloma annulare: clinicopathologic study of 6 patients. J Am Acad Dermatol. 2000;42:417-421.
- Nelson CA, Stephen S, Ashchyan HJ, et al. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018;79:987-1006.
- Dabade TS, Davis MD. Diagnosis and treatment of the neutrophilic dermatoses (pyoderma gangrenosum, Sweet's syndrome). Dermatol Ther. 2011;24:273-284.
- Davis M, Moschella L. Neutrophilic dermatoses. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier Saunders; 2018:2102-2112.
- Requena L, Kutzner H, Palmedo G, et al. Histiocytoid Sweet syndrome: a dermal infiltration of immature neutrophilic granulocytes. Arch Dermatol. 2005;141:834-842.
- Gibson LE, el-Azhary RA. Erythema elevatum diutinum. Clin Dermatol. 2000;18:295-299.
- Sardiña LA, Jour G, Piliang MP, et al. Erythema elevatum diutinum a rare and poorly understood cutaneous vasculitis: a single institution experience. J Cutan Pathol. 2019;46:97-101.
- Reid SD, Ladizinski B, Lee K, et al. Update on necrobiosis lipoidica: a review of etiology, diagnosis, and treatment options. J Am Acad Dermatol. 2013;69:783-791.
- Sibbald C, Reid S, Alavi A. Necrobiosis lipoidica. Dermatol Clin. 2015;33:343-360.
- Peroni A, Colato C, Schena D, et al. Interstitial granulomatous dermatitis: a distinct entity with characteristic histological and clinical pattern. Br J Dermatol. 2012;166:775-783.
- Terziroli Beretta-Piccoli B, Mainetti C, Peeters MA, et al. Cutaneous granulomatosis: a comprehensive review. Clin Rev Allergy Immunol. 2018;54:131-146.
- Rosenbach MA, Wanat KA, Reisenauer A, et al. Non-infectious granulomas. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier Saunders; 2018:1644-1663.
- Chu P, Connolly MK, LeBoit PE. The histopathologic spectrum of palisaded neutrophilic and granulomatous dermatitis in patients with collagen vascular disease. Arch Dermatol. 1994;130:1278-1283.
- Huizenga T, Kado JA, Pellicane B, et al. Interstitial granulomatous dermatitis and palisaded neutrophilic granulomatous dermatitis. Cutis. 2018;101:E19-E21.
- Rosenbach M, English JC 3rd. Reactive granulomatous dermatitis: a review of palisaded neutrophilic and granulomatous dermatitis, interstitial granulomatous dermatitis, interstitial granulomatous drug reaction, and a proposed reclassification. Dermatol Clin. 2015;33:373-387.
- Piette EW, Rosenbach M. Granuloma annulare: clinical and histologic variants, epidemiology, and genetics. J Am Acad Dermatol. 2016;75:457-465.
- Mutasim DF, Bridges AG. Patch granuloma annulare: clinicopathologic study of 6 patients. J Am Acad Dermatol. 2000;42:417-421.
- Nelson CA, Stephen S, Ashchyan HJ, et al. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018;79:987-1006.
- Dabade TS, Davis MD. Diagnosis and treatment of the neutrophilic dermatoses (pyoderma gangrenosum, Sweet's syndrome). Dermatol Ther. 2011;24:273-284.
- Davis M, Moschella L. Neutrophilic dermatoses. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier Saunders; 2018:2102-2112.
- Requena L, Kutzner H, Palmedo G, et al. Histiocytoid Sweet syndrome: a dermal infiltration of immature neutrophilic granulocytes. Arch Dermatol. 2005;141:834-842.
- Gibson LE, el-Azhary RA. Erythema elevatum diutinum. Clin Dermatol. 2000;18:295-299.
- Sardiña LA, Jour G, Piliang MP, et al. Erythema elevatum diutinum a rare and poorly understood cutaneous vasculitis: a single institution experience. J Cutan Pathol. 2019;46:97-101.
- Reid SD, Ladizinski B, Lee K, et al. Update on necrobiosis lipoidica: a review of etiology, diagnosis, and treatment options. J Am Acad Dermatol. 2013;69:783-791.
- Sibbald C, Reid S, Alavi A. Necrobiosis lipoidica. Dermatol Clin. 2015;33:343-360.
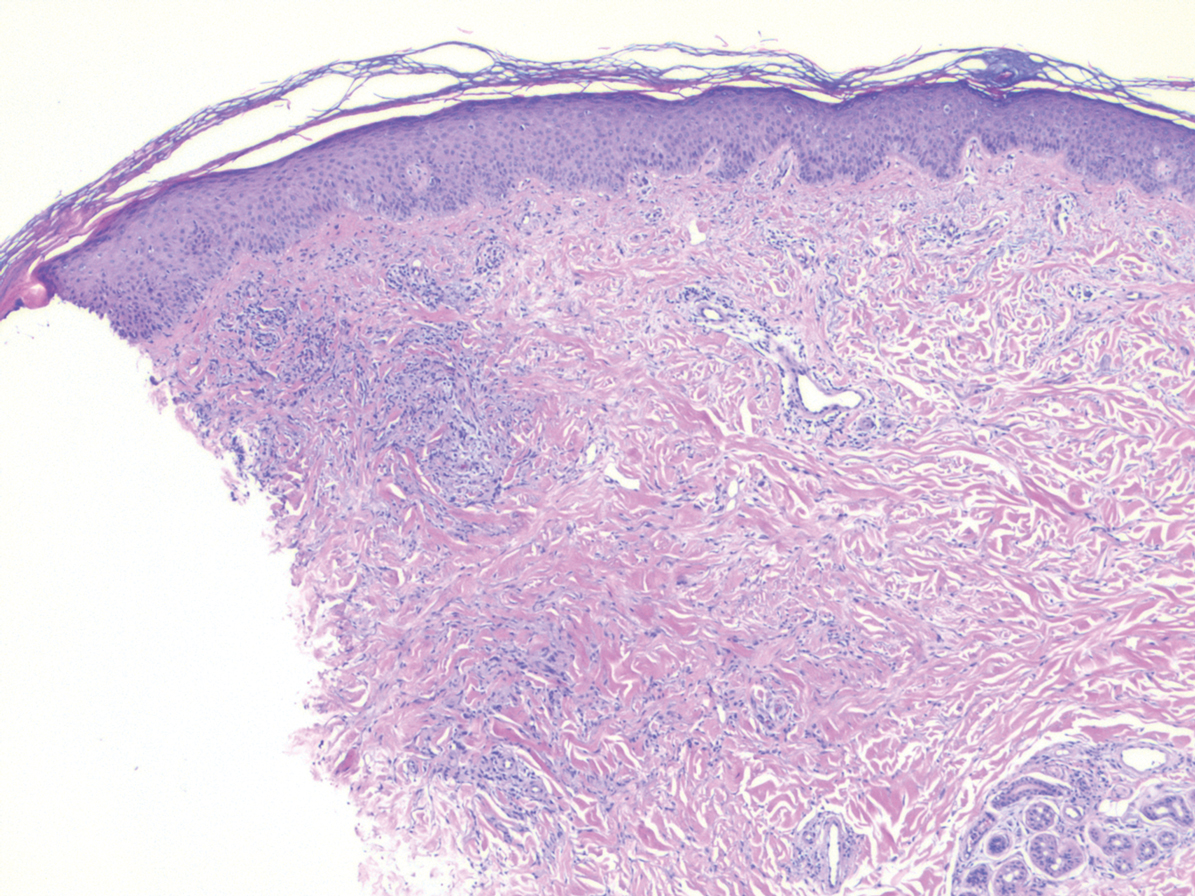
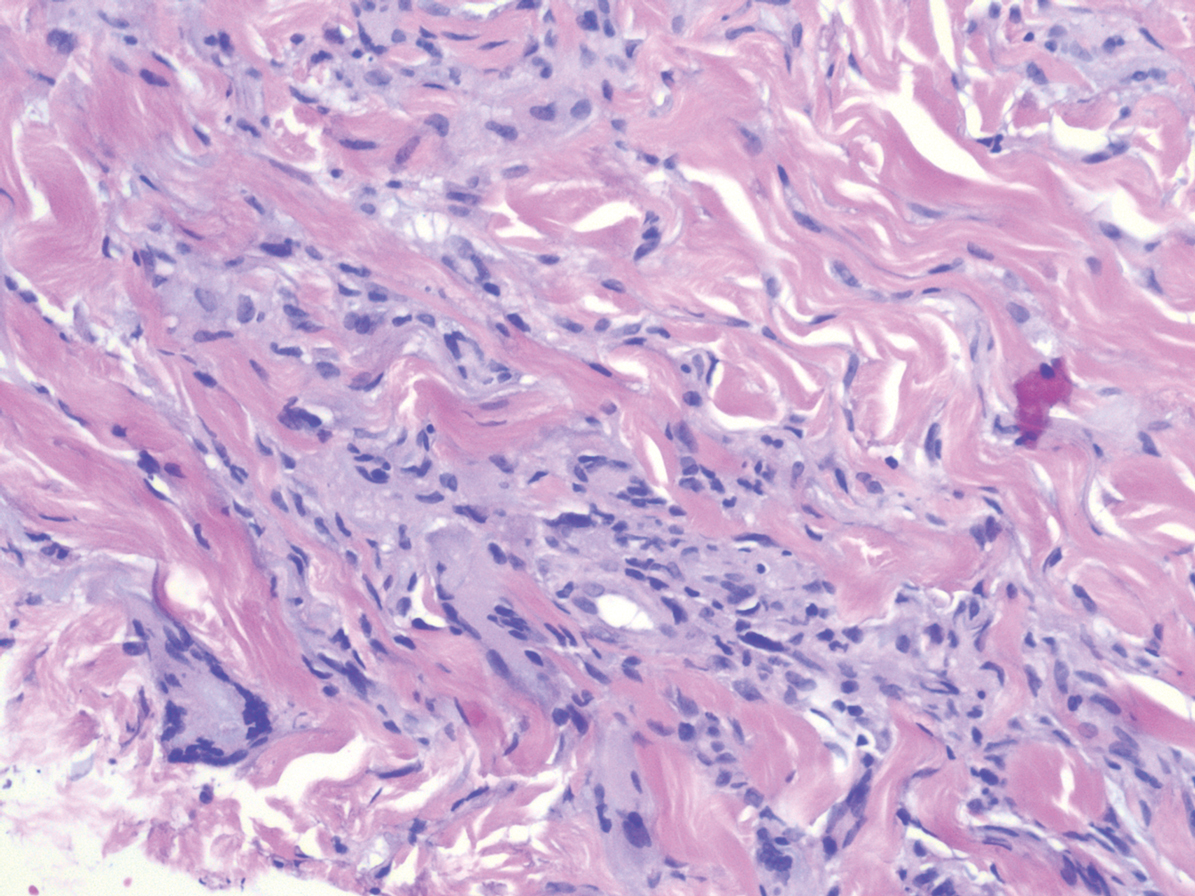
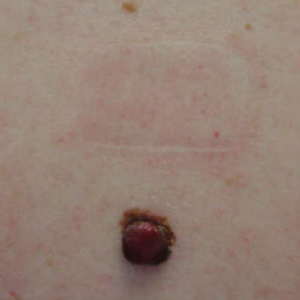
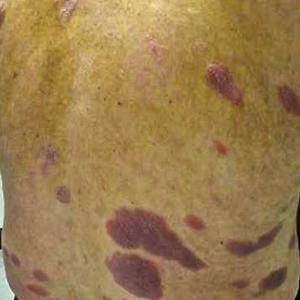
A 58-year-old woman with a medical history of asthma, hypertension, hypothyroidism, and hyperlipidemia presented with a painful rash of 10 days' duration. The rash was associated with fever at home (temperature, 38.5.2 °C), and a review of systems was positive for joint pain. Physical examination revealed numerous 8- to 10-mm, erythematous, discus-shaped papules on the bilateral dorsal hands, bilateral palms, right knee, and right dorsal foot with slight tenderness to palpation. A papule on the right dorsal hand was biopsied.
Sniffing Out Malignant Melanoma: A Case of Canine Olfactory Detection
To the Editor:
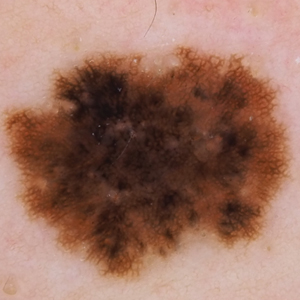
A 43-year-old woman presented with a mole on the central back that had been present since childhood and had changed and grown over the last few years. The patient reported that her 2-year-old rescue dog frequently sniffed the mole and would subsequently get agitated and try to scratch and bite the lesion. This behavior prompted the patient to visit a dermatologist.
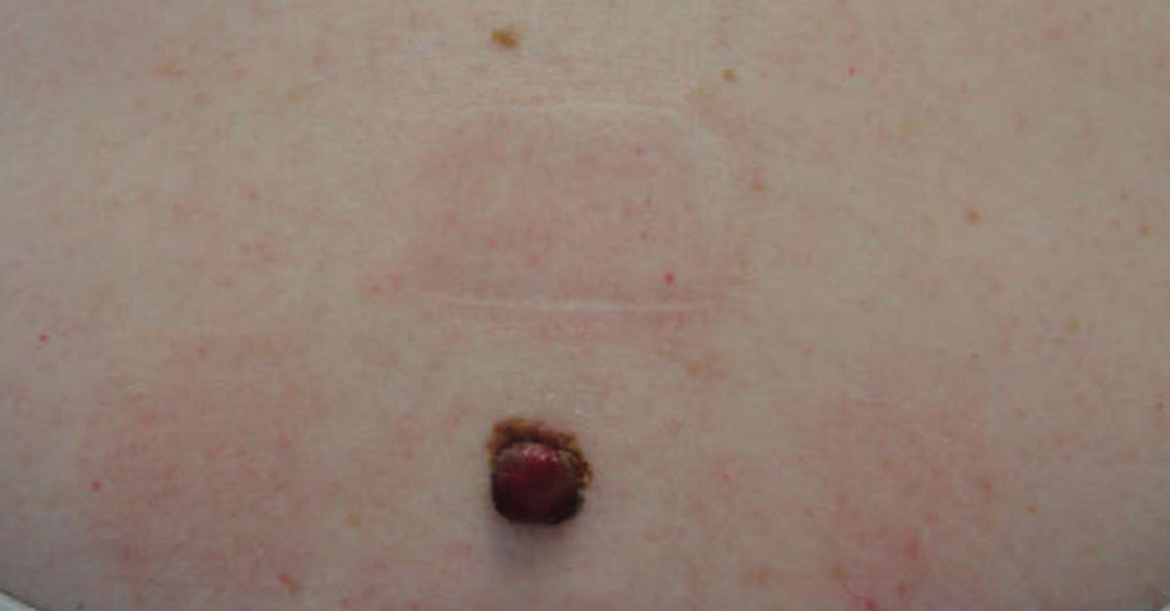
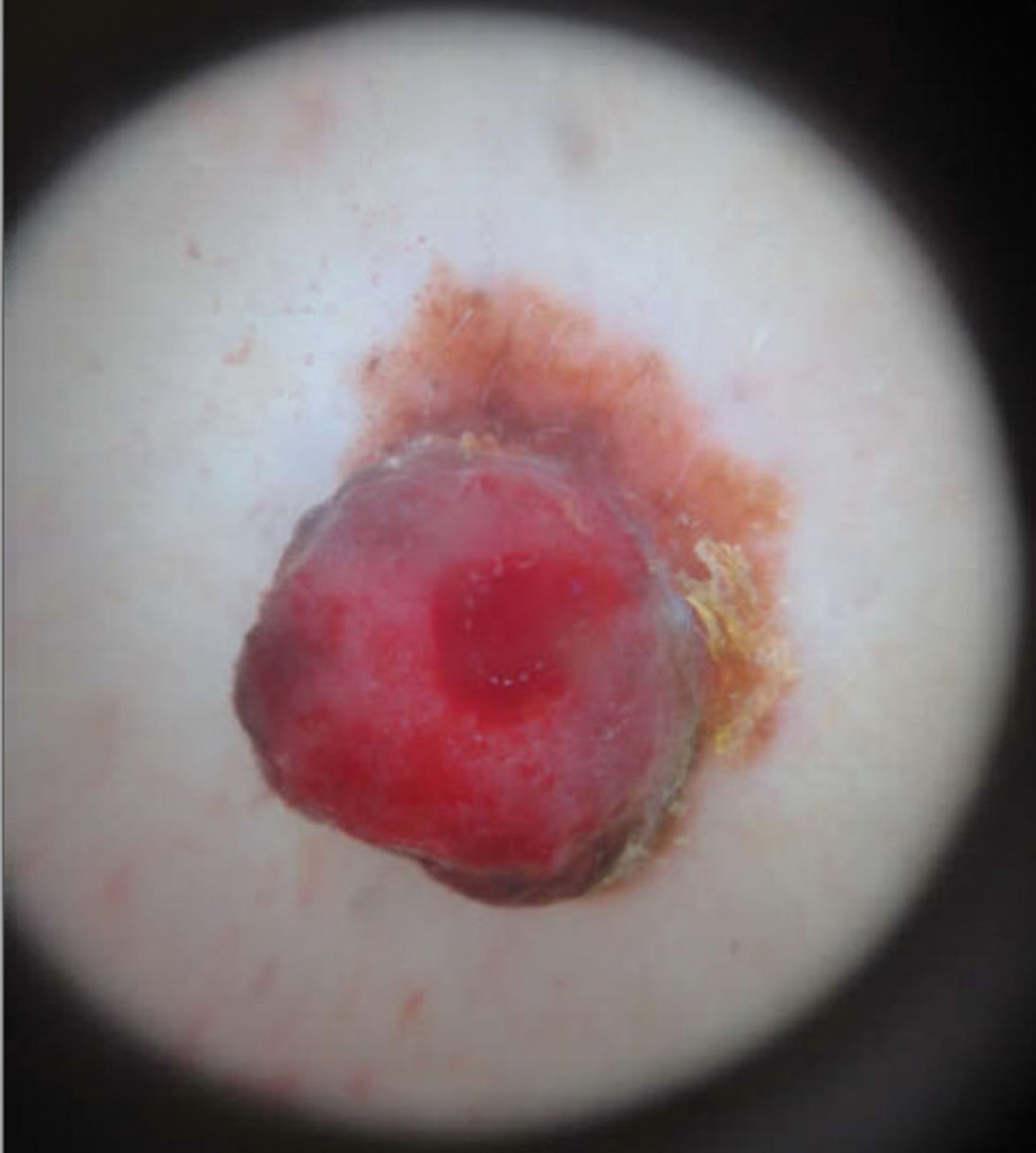
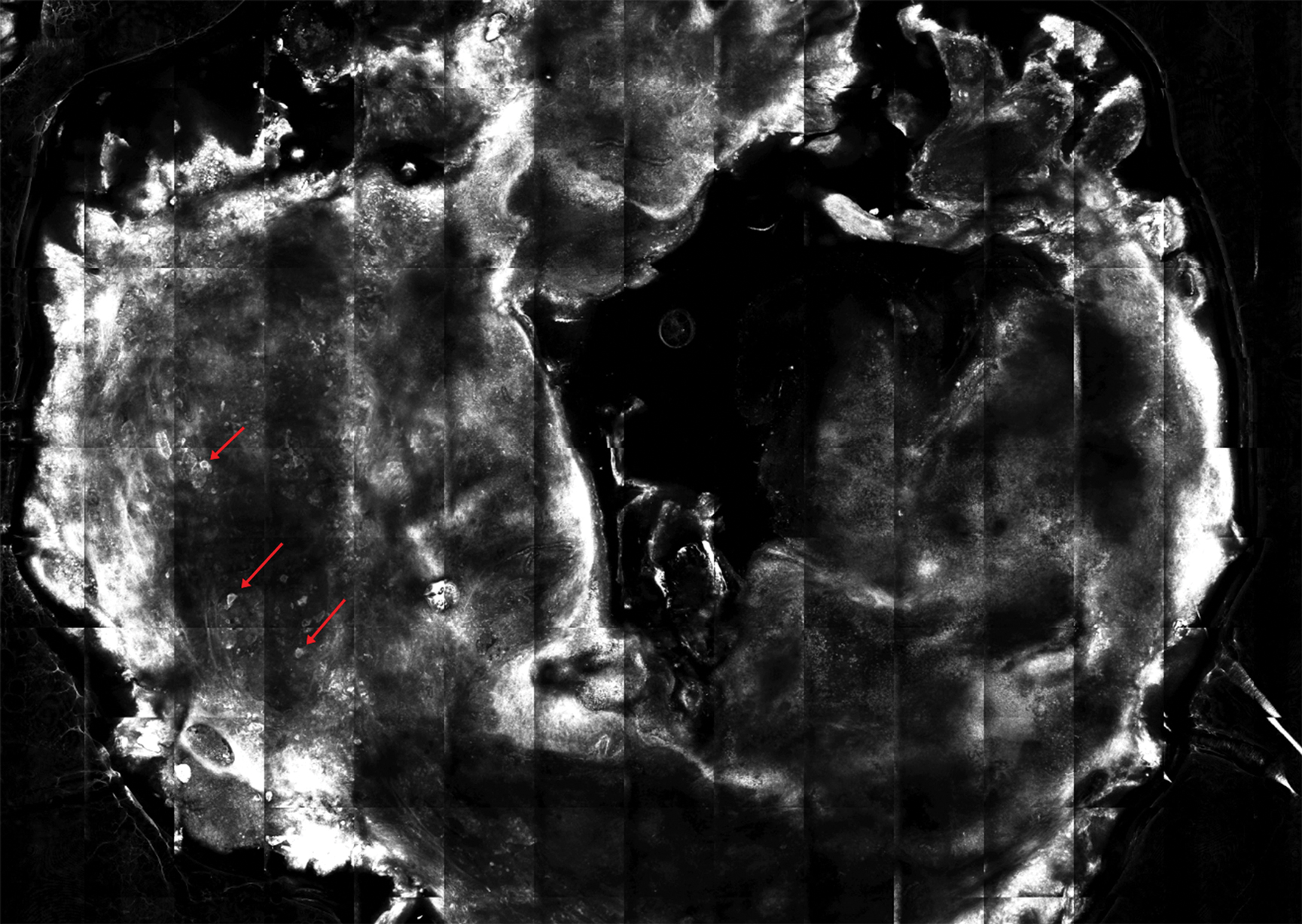
She reported no personal history of melanoma or nonmelanoma skin cancer, tanning booth exposure, blistering sunburns, or use of immunosuppressant medications. Her family history was remarkable for basal cell carcinoma in her father but no family history of melanoma. Physical examination revealed a 1.2×1.5-cm brown patch along with a 1×1-cm ulcerated nodule on the lower aspect of the lesion (Figure 1). Dermoscopy showed a blue-white veil and an irregular vascular pattern (Figure 2). No cervical, axillary, or inguinal lymphadenopathy was appreciated on physical examination. Reflectance confocal microscopy showed pagetoid spread of atypical round melanocytes as well as melanocytes in the stratum corneum (Figure 3).
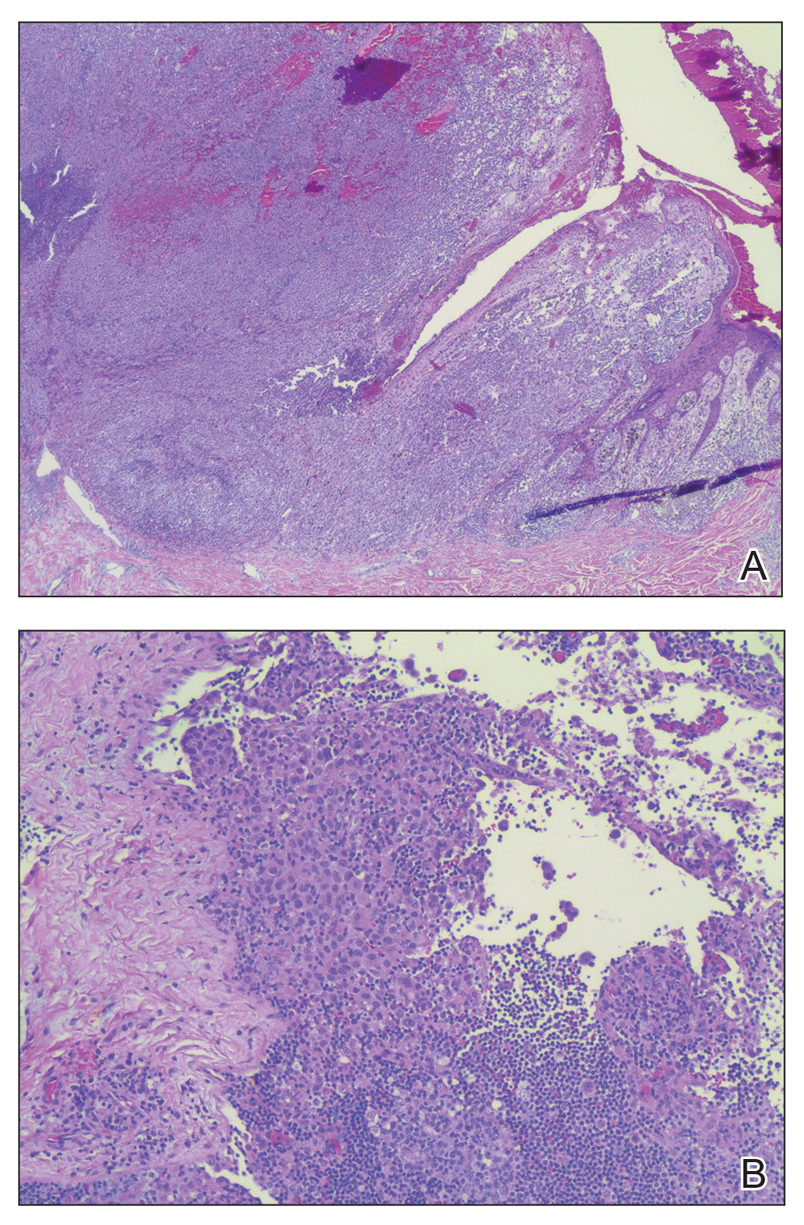
The patient was referred to a surgical oncologist for wide local excision and sentinel lymph node biopsy. Pathology showed a 4-mm-thick melanoma with numerous positive lymph nodes (Figure 4). The patient subsequently underwent a right axillary lymphadenectomy and was diagnosed with stage IIIB malignant melanoma. After surgery, the patient reported that her dog would now sniff her back and calmly rest his head in her lap.
She was treated with ipilimumab but subsequently developed panhypopituitarism, so she was taken off the ipilimumab. Currently, the patient is doing well. She follows up annually for full-body skin examinations and has not had any recurrence in the last 7 years. The patient credits her dog for prompting her to see a dermatologist and saving her life.
Both anecdotal and systematic evidence have emerged on the role of canine olfaction in the detection of lung, breast, colorectal, ovarian, prostate, and skin cancers, including malignant melanoma.1-6 A 1989 case report described a woman who was prompted to seek dermatologic evaluation of a pigmented lesion because her dog consistently targeted the lesion. Excision and subsequent histopathologic examination of the lesion revealed that it was malignant melanoma.5 Another case report described a patient whose dog, which was not trained to detect cancers in humans, persistently licked a lesion behind the patient’s ear that eventually was found to be malignant melanoma.6 These reports have inspired considerable research interest regarding canine olfaction as a potential method to noninvasively screen for and even diagnose malignant melanomas in humans.
Both physiologic and pathologic metabolic processes result in the production of volatile organic compounds (VOCs), or small odorant molecules that evaporate at normal temperatures and pressures.1 Individual cells release VOCs in extremely low concentrations into the blood, urine, feces, and breath, as well as onto the skin’s surface, but there are methods for detecting these VOCs, including gas chromatography–mass spectrometry and canine olfaction.7,8 Pathologic processes, such as infection and malignancy, result in irregular protein synthesis and metabolism, producing new VOCs or differing concentrations of VOCs as compared to normal processes.1
Dimethyl disulfide and dimethyl trisulfide compounds have been identified in malignant melanoma, and these compounds are not produced by normal melanocytes.7 Furthermore, malignant melanoma produces differing quantities of these compounds as compared to normal melanocytes, including isovaleric acid, 2-methylbutyric acid, isoamyl alcohol (3-methyl-1-butanol), and 2-methyl-1-butanol, resulting in a distinct odorant profile that previously has been detected via canine olfaction.7 Canine olfaction can identify odorant molecules at up to 1 part per trillion (a magnitude more sensitive than the currently available gas chromatography–mass spectrometry technologies) and can detect the production of new VOCs or altered VOC ratios due to pathologic processes.1 Systematic studies with dogs that are trained to detect cancers in humans have shown that canine olfaction correctly identified malignant melanomas against healthy skin, benign nevi, and even basal cell carcinomas at higher rates than what would have been expected by chance alone.2,3
Canine olfaction can identify new or altered ratios of odorant VOCs associated with pathologic metabolic processes, and canines can be trained to target odor profiles associated with specific diseases.1 Canine olfaction for melanoma screening and diagnosis may seem appealing, as it provides an easily transportable, real-time, low-cost method compared to other techniques such as gas chromatography–mass spectrometry.1 Although preliminary results have shown that canine olfaction detects melanoma at higher rates than would be expected by chance alone, these findings have not approached clinical utility for the widespread use of canine olfaction as a screening method for melanoma.2,3,9 Further studies are needed to understand the role of canine olfaction in melanoma screening and diagnosis as well as to explore methods to optimize sensitivity and specificity. Until then, patients and dermatologists should not ignore the behavior of dogs toward skin lesions. Dogs may be beneficial in the detection of melanoma and help save lives, as was seen in our case.
- Angle C, Waggoner LP, Ferrando A, et al. Canine detection of the volatilome: a review of implications for pathogen and disease detection. Front Vet Sci. 2016;3:47.
- Pickel D, Mauncy GP, Walker DB, et al. Evidence for canine olfactory detection of melanoma. Applied Animal Behaviour Science. 2004;89:107-116.
- Willis CM, Britton LE, Swindells MA, et al. Invasive melanoma in vivo can be distinguished from basal cell carcinoma, benign naevi and healthy skin by canine olfaction: a proof‐of‐principle study of differential volatile organic compound emission. Br J Dermatol. 2016;175:1020-1029.
- Jezierski T, Walczak M, Ligor T, et al. Study of the art: canine olfaction used for cancer detection on the basis of breath odour. perspectives and limitations. J Breath Res. 2015;9:027001.
- Williams H, Pembroke A. Sniffer dogs in the melanoma clinic? Lancet. 1989;1:734.
- Campbell LF, Farmery L, George SM, et al. Canine olfactory detection of malignant melanoma. BMJ Case Rep. 2013. doi:10.1136/bcr-2013-008566.
- Kwak J, Gallagher M, Ozdener MH, et al. Volatile biomarkers from human melanoma cells. J Chromotogr B Analyt Technol Biomed Life Sci. 2013;931:90-96.
- D’Amico A, Bono R, Pennazza G, et al. Identification of melanoma with a gas sensor array. Skin Res Technol. 2008;14:226-236.
- Elliker KR, Williams HC. Detection of skin cancer odours using dogs: a step forward in melanoma detection training and research methodologies. Br J Dermatol. 2016;175:851-852.
To the Editor:
A 43-year-old woman presented with a mole on the central back that had been present since childhood and had changed and grown over the last few years. The patient reported that her 2-year-old rescue dog frequently sniffed the mole and would subsequently get agitated and try to scratch and bite the lesion. This behavior prompted the patient to visit a dermatologist.
She reported no personal history of melanoma or nonmelanoma skin cancer, tanning booth exposure, blistering sunburns, or use of immunosuppressant medications. Her family history was remarkable for basal cell carcinoma in her father but no family history of melanoma. Physical examination revealed a 1.2×1.5-cm brown patch along with a 1×1-cm ulcerated nodule on the lower aspect of the lesion (Figure 1). Dermoscopy showed a blue-white veil and an irregular vascular pattern (Figure 2). No cervical, axillary, or inguinal lymphadenopathy was appreciated on physical examination. Reflectance confocal microscopy showed pagetoid spread of atypical round melanocytes as well as melanocytes in the stratum corneum (Figure 3).



The patient was referred to a surgical oncologist for wide local excision and sentinel lymph node biopsy. Pathology showed a 4-mm-thick melanoma with numerous positive lymph nodes (Figure 4). The patient subsequently underwent a right axillary lymphadenectomy and was diagnosed with stage IIIB malignant melanoma. After surgery, the patient reported that her dog would now sniff her back and calmly rest his head in her lap.

She was treated with ipilimumab but subsequently developed panhypopituitarism, so she was taken off the ipilimumab. Currently, the patient is doing well. She follows up annually for full-body skin examinations and has not had any recurrence in the last 7 years. The patient credits her dog for prompting her to see a dermatologist and saving her life.
Both anecdotal and systematic evidence have emerged on the role of canine olfaction in the detection of lung, breast, colorectal, ovarian, prostate, and skin cancers, including malignant melanoma.1-6 A 1989 case report described a woman who was prompted to seek dermatologic evaluation of a pigmented lesion because her dog consistently targeted the lesion. Excision and subsequent histopathologic examination of the lesion revealed that it was malignant melanoma.5 Another case report described a patient whose dog, which was not trained to detect cancers in humans, persistently licked a lesion behind the patient’s ear that eventually was found to be malignant melanoma.6 These reports have inspired considerable research interest regarding canine olfaction as a potential method to noninvasively screen for and even diagnose malignant melanomas in humans.
Both physiologic and pathologic metabolic processes result in the production of volatile organic compounds (VOCs), or small odorant molecules that evaporate at normal temperatures and pressures.1 Individual cells release VOCs in extremely low concentrations into the blood, urine, feces, and breath, as well as onto the skin’s surface, but there are methods for detecting these VOCs, including gas chromatography–mass spectrometry and canine olfaction.7,8 Pathologic processes, such as infection and malignancy, result in irregular protein synthesis and metabolism, producing new VOCs or differing concentrations of VOCs as compared to normal processes.1
Dimethyl disulfide and dimethyl trisulfide compounds have been identified in malignant melanoma, and these compounds are not produced by normal melanocytes.7 Furthermore, malignant melanoma produces differing quantities of these compounds as compared to normal melanocytes, including isovaleric acid, 2-methylbutyric acid, isoamyl alcohol (3-methyl-1-butanol), and 2-methyl-1-butanol, resulting in a distinct odorant profile that previously has been detected via canine olfaction.7 Canine olfaction can identify odorant molecules at up to 1 part per trillion (a magnitude more sensitive than the currently available gas chromatography–mass spectrometry technologies) and can detect the production of new VOCs or altered VOC ratios due to pathologic processes.1 Systematic studies with dogs that are trained to detect cancers in humans have shown that canine olfaction correctly identified malignant melanomas against healthy skin, benign nevi, and even basal cell carcinomas at higher rates than what would have been expected by chance alone.2,3
Canine olfaction can identify new or altered ratios of odorant VOCs associated with pathologic metabolic processes, and canines can be trained to target odor profiles associated with specific diseases.1 Canine olfaction for melanoma screening and diagnosis may seem appealing, as it provides an easily transportable, real-time, low-cost method compared to other techniques such as gas chromatography–mass spectrometry.1 Although preliminary results have shown that canine olfaction detects melanoma at higher rates than would be expected by chance alone, these findings have not approached clinical utility for the widespread use of canine olfaction as a screening method for melanoma.2,3,9 Further studies are needed to understand the role of canine olfaction in melanoma screening and diagnosis as well as to explore methods to optimize sensitivity and specificity. Until then, patients and dermatologists should not ignore the behavior of dogs toward skin lesions. Dogs may be beneficial in the detection of melanoma and help save lives, as was seen in our case.
To the Editor:
A 43-year-old woman presented with a mole on the central back that had been present since childhood and had changed and grown over the last few years. The patient reported that her 2-year-old rescue dog frequently sniffed the mole and would subsequently get agitated and try to scratch and bite the lesion. This behavior prompted the patient to visit a dermatologist.
She reported no personal history of melanoma or nonmelanoma skin cancer, tanning booth exposure, blistering sunburns, or use of immunosuppressant medications. Her family history was remarkable for basal cell carcinoma in her father but no family history of melanoma. Physical examination revealed a 1.2×1.5-cm brown patch along with a 1×1-cm ulcerated nodule on the lower aspect of the lesion (Figure 1). Dermoscopy showed a blue-white veil and an irregular vascular pattern (Figure 2). No cervical, axillary, or inguinal lymphadenopathy was appreciated on physical examination. Reflectance confocal microscopy showed pagetoid spread of atypical round melanocytes as well as melanocytes in the stratum corneum (Figure 3).



The patient was referred to a surgical oncologist for wide local excision and sentinel lymph node biopsy. Pathology showed a 4-mm-thick melanoma with numerous positive lymph nodes (Figure 4). The patient subsequently underwent a right axillary lymphadenectomy and was diagnosed with stage IIIB malignant melanoma. After surgery, the patient reported that her dog would now sniff her back and calmly rest his head in her lap.

She was treated with ipilimumab but subsequently developed panhypopituitarism, so she was taken off the ipilimumab. Currently, the patient is doing well. She follows up annually for full-body skin examinations and has not had any recurrence in the last 7 years. The patient credits her dog for prompting her to see a dermatologist and saving her life.
Both anecdotal and systematic evidence have emerged on the role of canine olfaction in the detection of lung, breast, colorectal, ovarian, prostate, and skin cancers, including malignant melanoma.1-6 A 1989 case report described a woman who was prompted to seek dermatologic evaluation of a pigmented lesion because her dog consistently targeted the lesion. Excision and subsequent histopathologic examination of the lesion revealed that it was malignant melanoma.5 Another case report described a patient whose dog, which was not trained to detect cancers in humans, persistently licked a lesion behind the patient’s ear that eventually was found to be malignant melanoma.6 These reports have inspired considerable research interest regarding canine olfaction as a potential method to noninvasively screen for and even diagnose malignant melanomas in humans.
Both physiologic and pathologic metabolic processes result in the production of volatile organic compounds (VOCs), or small odorant molecules that evaporate at normal temperatures and pressures.1 Individual cells release VOCs in extremely low concentrations into the blood, urine, feces, and breath, as well as onto the skin’s surface, but there are methods for detecting these VOCs, including gas chromatography–mass spectrometry and canine olfaction.7,8 Pathologic processes, such as infection and malignancy, result in irregular protein synthesis and metabolism, producing new VOCs or differing concentrations of VOCs as compared to normal processes.1
Dimethyl disulfide and dimethyl trisulfide compounds have been identified in malignant melanoma, and these compounds are not produced by normal melanocytes.7 Furthermore, malignant melanoma produces differing quantities of these compounds as compared to normal melanocytes, including isovaleric acid, 2-methylbutyric acid, isoamyl alcohol (3-methyl-1-butanol), and 2-methyl-1-butanol, resulting in a distinct odorant profile that previously has been detected via canine olfaction.7 Canine olfaction can identify odorant molecules at up to 1 part per trillion (a magnitude more sensitive than the currently available gas chromatography–mass spectrometry technologies) and can detect the production of new VOCs or altered VOC ratios due to pathologic processes.1 Systematic studies with dogs that are trained to detect cancers in humans have shown that canine olfaction correctly identified malignant melanomas against healthy skin, benign nevi, and even basal cell carcinomas at higher rates than what would have been expected by chance alone.2,3
Canine olfaction can identify new or altered ratios of odorant VOCs associated with pathologic metabolic processes, and canines can be trained to target odor profiles associated with specific diseases.1 Canine olfaction for melanoma screening and diagnosis may seem appealing, as it provides an easily transportable, real-time, low-cost method compared to other techniques such as gas chromatography–mass spectrometry.1 Although preliminary results have shown that canine olfaction detects melanoma at higher rates than would be expected by chance alone, these findings have not approached clinical utility for the widespread use of canine olfaction as a screening method for melanoma.2,3,9 Further studies are needed to understand the role of canine olfaction in melanoma screening and diagnosis as well as to explore methods to optimize sensitivity and specificity. Until then, patients and dermatologists should not ignore the behavior of dogs toward skin lesions. Dogs may be beneficial in the detection of melanoma and help save lives, as was seen in our case.
- Angle C, Waggoner LP, Ferrando A, et al. Canine detection of the volatilome: a review of implications for pathogen and disease detection. Front Vet Sci. 2016;3:47.
- Pickel D, Mauncy GP, Walker DB, et al. Evidence for canine olfactory detection of melanoma. Applied Animal Behaviour Science. 2004;89:107-116.
- Willis CM, Britton LE, Swindells MA, et al. Invasive melanoma in vivo can be distinguished from basal cell carcinoma, benign naevi and healthy skin by canine olfaction: a proof‐of‐principle study of differential volatile organic compound emission. Br J Dermatol. 2016;175:1020-1029.
- Jezierski T, Walczak M, Ligor T, et al. Study of the art: canine olfaction used for cancer detection on the basis of breath odour. perspectives and limitations. J Breath Res. 2015;9:027001.
- Williams H, Pembroke A. Sniffer dogs in the melanoma clinic? Lancet. 1989;1:734.
- Campbell LF, Farmery L, George SM, et al. Canine olfactory detection of malignant melanoma. BMJ Case Rep. 2013. doi:10.1136/bcr-2013-008566.
- Kwak J, Gallagher M, Ozdener MH, et al. Volatile biomarkers from human melanoma cells. J Chromotogr B Analyt Technol Biomed Life Sci. 2013;931:90-96.
- D’Amico A, Bono R, Pennazza G, et al. Identification of melanoma with a gas sensor array. Skin Res Technol. 2008;14:226-236.
- Elliker KR, Williams HC. Detection of skin cancer odours using dogs: a step forward in melanoma detection training and research methodologies. Br J Dermatol. 2016;175:851-852.
- Angle C, Waggoner LP, Ferrando A, et al. Canine detection of the volatilome: a review of implications for pathogen and disease detection. Front Vet Sci. 2016;3:47.
- Pickel D, Mauncy GP, Walker DB, et al. Evidence for canine olfactory detection of melanoma. Applied Animal Behaviour Science. 2004;89:107-116.
- Willis CM, Britton LE, Swindells MA, et al. Invasive melanoma in vivo can be distinguished from basal cell carcinoma, benign naevi and healthy skin by canine olfaction: a proof‐of‐principle study of differential volatile organic compound emission. Br J Dermatol. 2016;175:1020-1029.
- Jezierski T, Walczak M, Ligor T, et al. Study of the art: canine olfaction used for cancer detection on the basis of breath odour. perspectives and limitations. J Breath Res. 2015;9:027001.
- Williams H, Pembroke A. Sniffer dogs in the melanoma clinic? Lancet. 1989;1:734.
- Campbell LF, Farmery L, George SM, et al. Canine olfactory detection of malignant melanoma. BMJ Case Rep. 2013. doi:10.1136/bcr-2013-008566.
- Kwak J, Gallagher M, Ozdener MH, et al. Volatile biomarkers from human melanoma cells. J Chromotogr B Analyt Technol Biomed Life Sci. 2013;931:90-96.
- D’Amico A, Bono R, Pennazza G, et al. Identification of melanoma with a gas sensor array. Skin Res Technol. 2008;14:226-236.
- Elliker KR, Williams HC. Detection of skin cancer odours using dogs: a step forward in melanoma detection training and research methodologies. Br J Dermatol. 2016;175:851-852.
Practice Points
- Physiologic and pathologic processes produce volatile organic compounds in the skin and other tissues.
- Malignant melanocytes release unique volatile organic compounds (VOCs) as well as differing combinations and quantities of VOCs as compared to normal melanocytes.
- Volatile organic compounds released at the skin’s surface can be detected by various methods, including canine olfaction; therefore, unusual canine behavior toward skin lesions should not be ignored.
Noninvasive Imaging Tools in Dermatology
Traditionally, diagnosis of skin disease relies on clinical inspection, often followed by biopsy and histopathologic examination. In recent years, new noninvasive tools have emerged that can aid in clinical diagnosis and reduce the number of unnecessary benign biopsies. Although there has been a surge in noninvasive diagnostic technologies, many tools are still in research and development phases, with few tools widely adopted and used in regular clinical practice. In this article, we discuss the use of dermoscopy, reflectance confocal microscopy (RCM), and optical coherence tomography (OCT) in the diagnosis and management of skin disease.
Dermoscopy
Dermoscopy, also known as epiluminescence light microscopy and previously known as dermatoscopy, utilizes a ×10 to ×100 microscope objective with a light source to magnify and visualize structures present below the skin’s surface, such as melanin and blood vessels. There are 3 types of dermoscopy: conventional nonpolarized dermoscopy, polarized contact dermoscopy, and nonpolarized contact dermoscopy (Figure 1). Traditional nonpolarized dermoscopy requires a liquid medium and direct contact with the skin, and it relies on light reflection and refraction properties.1 Cross-polarized light sources allow visualization of deeper structures, either with or without a liquid medium and contact with the skin surface. Although there is overall concurrence among the different types of dermoscopy, subtle differences in the appearance of color, features, and structure are present.1
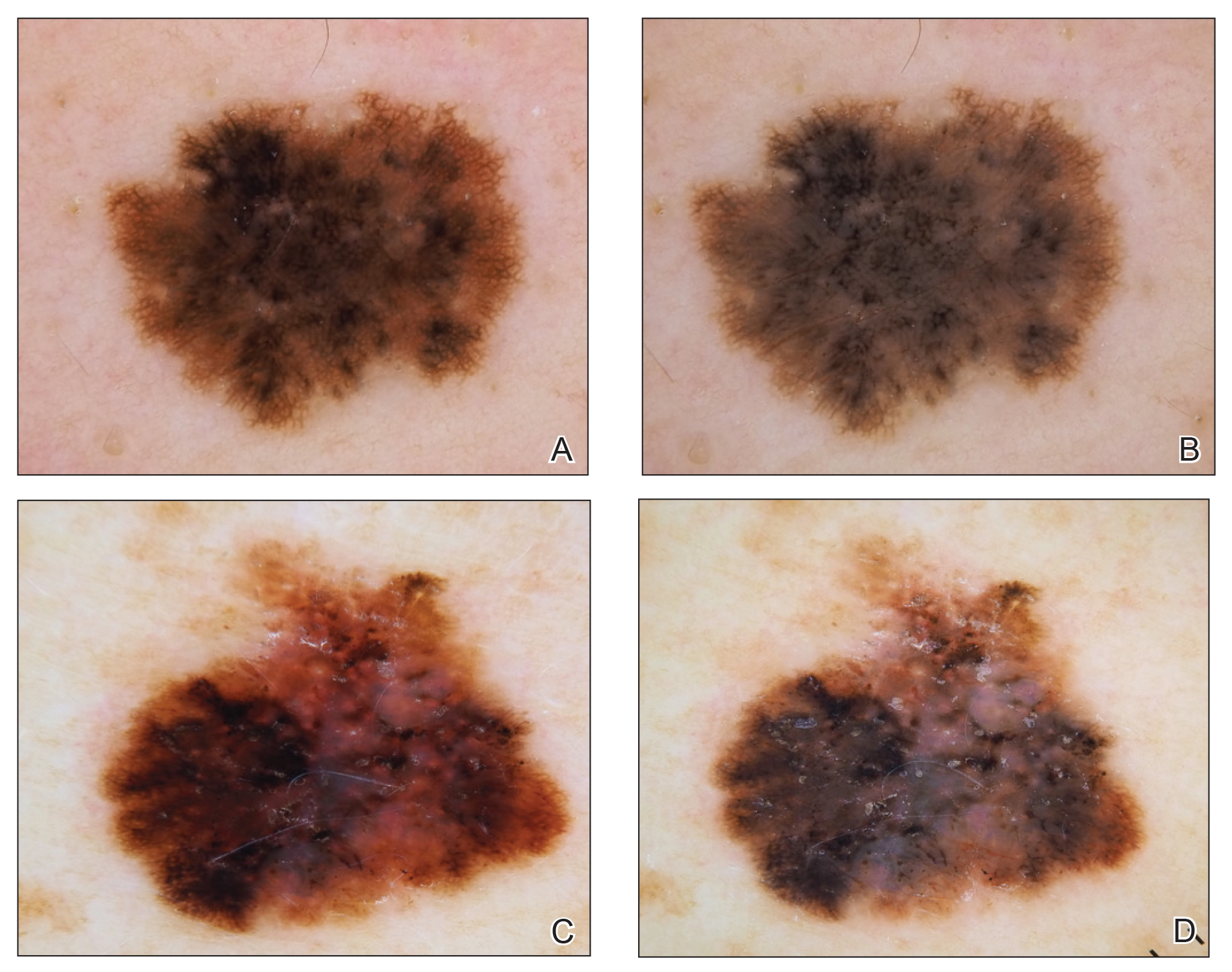
Dermoscopy offers many benefits for dermatologists and other providers. It can be used to aid in the diagnosis of cutaneous neoplasms and other skin diseases. Numerous low-cost dermatoscopes currently are commercially available. The handheld, easily transportable nature of dermatoscopes have resulted in widespread practice integration. Approximately 84% of attending dermatologists in US academic settings reported using dermoscopy, and many refer to the dermatoscope as “the dermatologist’s stethoscope.”2 In addition, 6% to 15% of other US providers, including family physicians, internal medicine physicians, and plastic surgeons, have reported using dermoscopy in their clinical practices. Limitations of dermoscopy include visualization of the skin surface only and not deeper structures within the tissue, the need for training for adequate interpretation of dermoscopic images, and lack of reimbursement for dermoscopic examination.3
Many dermoscopic structures that correspond well with histopathology have been described. Dermoscopy has a sensitivity of 79% to 96% and specificity of 69% to 99% in the diagnosis of melanoma.4 There is variable data on the specificity of dermoscopy in the diagnosis of melanoma, with one meta-analysis finding no statistically significant difference in specificity compared to naked eye examination,5 while other studies report increased specificity and subsequent reduction in biopsy of benign lesions.6,7 Dermoscopy also can aid in the diagnosis of keratinocytic neoplasms, and dermoscopy also results in a sensitivity of 78.6% to 100% and a specificity of 53.8% to 100% in the diagnosis of basal cell carcinoma (BCC).8 Limitations of dermoscopy include false-positive diagnoses, commonly seborrheic keratoses and nevi, resulting in unnecessary biopsies, as well as false-negative diagnoses, commonly amelanotic and nevoid melanoma, resulting in delays in skin cancer diagnosis and resultant poor outcomes.9 Dermoscopy also is used to aid in the diagnosis of inflammatory and infectious skin diseases, as well as scalp, hair, and nail disorders.10
Reflectance Confocal Microscopy
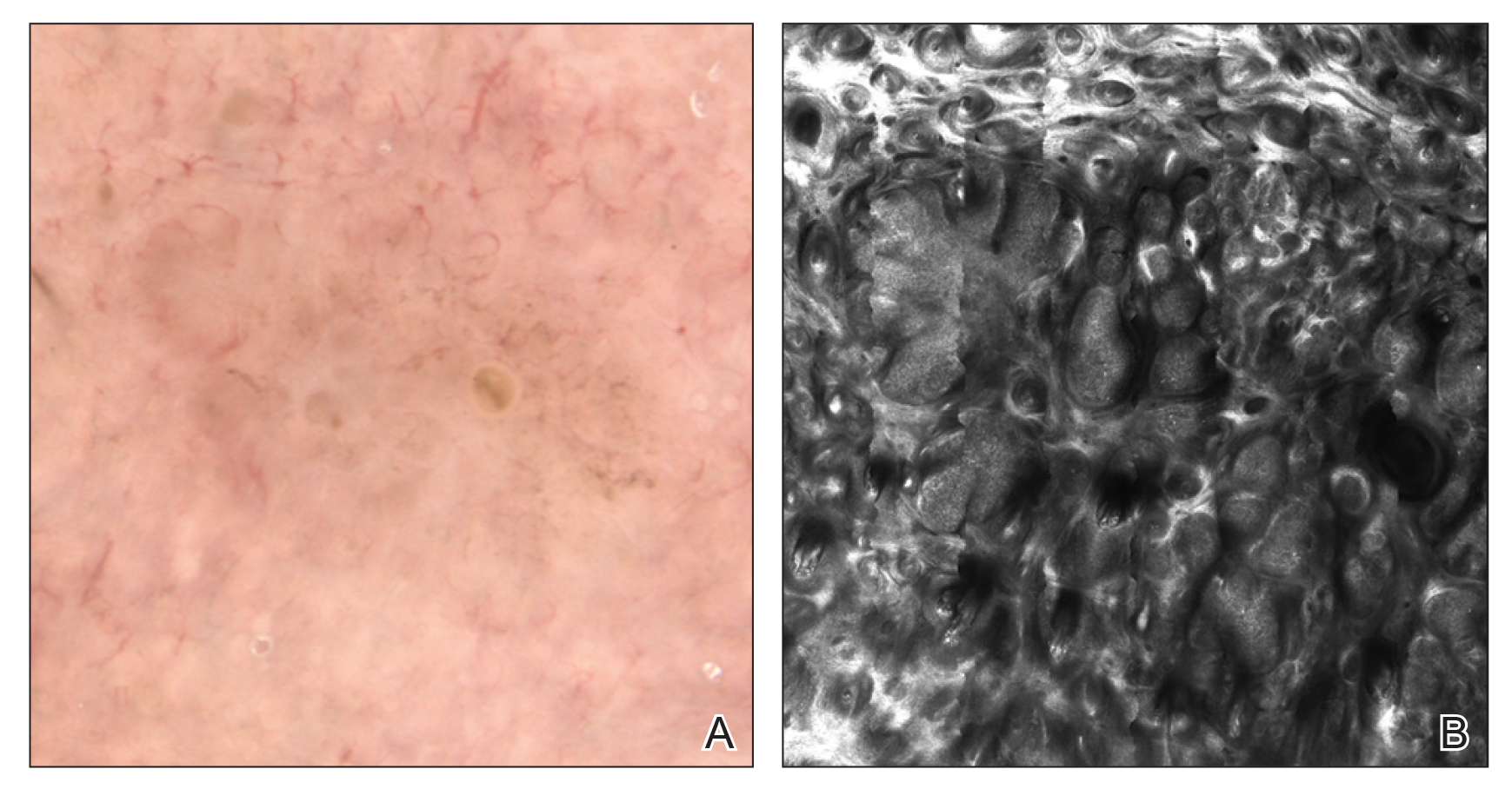
Reflectance confocal microscopy utilizes an 830-nm laser to capture horizontal en face images of the skin with high resolution. Different structures of the skin have varying indices of refraction: keratin, melanin, and collagen appear bright white, while other components appear dark, generating black-and-white RCM images.11 Currently, there are 2 reflectance confocal microscopes that are commercially available in the United States. The Vivascope 1500 (Caliber ID) is the traditional model that captures 8×8-mm images, and the Vivascope 3000 (Caliber ID) is a smaller handheld model that captures 0.5×0.5-mm images. The traditional model provides the advantages of higher-resolution images and the ability to capture larger surface areas but is best suited to image flat areas of skin to which a square window can be adhered. The handheld model allows improved contact with the varying topography of skin; does not require an adhesive window; and can be used to image cartilaginous, mucosal, and sensitive surfaces. However, it can be difficult to correlate individual images captured by the handheld RCM with the location relative to the lesion, as it is exquisitely sensitive to motion and also is operator dependent. Although complex algorithms are under development to stitch individual images to provide better correlation with the geography of the lesion, such programs are not yet widely available.12
Reflectance confocal microscopy affords many benefits for patients and providers. It is noninvasive and painless and is capable of imaging in vivo live skin as compared to clinical examination and dermoscopy, which only allow for visualization of the skin’s surface. Reflectance confocal microscopy also is time efficient, as imaging of a single lesion can be completed in 10 to 15 minutes. This technology generates high-resolution images, and RCM diagnosis has consistently demonstrated high sensitivity and specificity when compared to histopathology.13 Additionally, RCM imaging can spare biopsy and resultant scarring on cosmetically sensitive areas. Recently, RCM imaging of the skin has been granted Category I Current Procedural Terminology reimbursement codes that allow provider reimbursement and integration of RCM into daily practice14; however, private insurance coverage in the United States is variable. Limitations of RCM include a maximum depth of 200 to 300 µm, high cost to procure a reflectance confocal microscope, and the need for considerable training and practice to accurately interpret grayscale en face images.15
There has been extensive research regarding the use of RCM in the evaluation of cutaneous neoplasms and other skin diseases. Numerous features and patterns have been identified and described that correspond with different skin diseases and correspond well with histopathology (Figure 2).13,16,17 Reflectance confocal microscopy has demonstrated consistently high accuracy in the diagnosis of melanocytic lesions, with a sensitivity of 93% to 100% and a specificity of 75% to 99%.18-21 Reflectance confocal microscopy is especially useful in the evaluation of clinically or dermoscopically equivocal pigmented lesions due to greater specificity, resulting in a reduction of unnecessary biopsies.22,23 It also has high accuracy in the diagnosis of keratinocytic neoplasms, with a sensitivity of 82% to 100% and a specificity of 78% to 97% in the diagnosis of BCC,24 and a sensitivity of 74% to 100% and specificity of 78% to 100% in the diagnosis of squamous cell carcinoma (SCC).25,26 Evaluation of SCC and actinic keratosis (AK) using RCM may be limited by considerable hyperkeratosis and ulceration. In addition, it can be challenging to differentiate AK and SCC on RCM, and considerable expertise is required to accurately grade cytologic and architectural atypia.27 However, RCM has been used to discriminate between in situ and invasive proliferations.28 Reflectance confocal microscopy has wide applications in the diagnosis and management of cutaneous infections29,30 and inflammatory skin diseases.29,31-33 Recent RCM research explored the use of RCM to identify biopsy sites,34 delineate presurgical tumor margins,35,36 and monitor response to noninvasive treatments.37,38
Optical Coherence Tomography
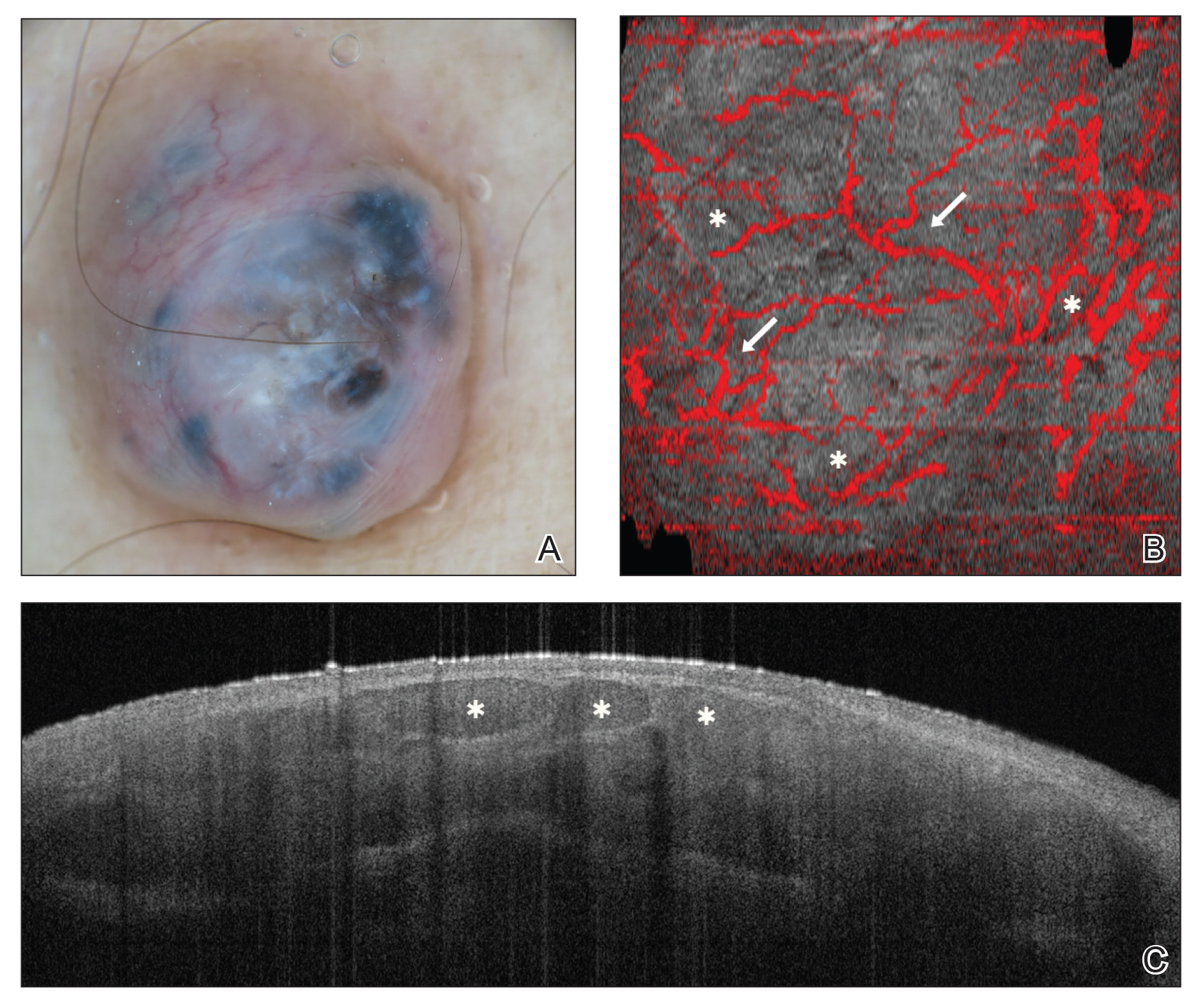
Optical coherence tomography is an imaging modality that utilizes light backscatter from infrared light to produce grayscale cross-sectional or vertical images and horizontal en face images.39 Optical coherence tomography can visualize structures in the epidermis, dermoepidermal junction, and upper dermis.40 It can image boundaries of structures but cannot visualize individual cells.
There are different types of OCT devices available, including frequency-domain OCT (FD-OCT), or conventional OCT, and high-definition OCT (HD-OCT). With FD-OCT, images are captured at a maximum depth of 1 to 2 mm but with limited resolution. High-definition OCT has superior resolution compared to FD-OCT but is restricted to a shallower depth of 750 μm.39 The main advantage of OCT is the ability to noninvasively image live tissue and visualize 2- to 5-times greater depth as compared to RCM. Several OCT devices have obtained US Food and Drug Administration approval; however, OCT has not been widely adopted into clinical practice and is available only in tertiary academic centers. Additionally, OCT imaging in dermatology is rarely reimbursed. Other limitations of OCT include poor resolution of images, high cost to procure an OCT device, and the need for advanced training and experience to accurately interpret images.40,41
Optical coherence tomography primarily is used to diagnose cutaneous neoplasms. The best evidence of the diagnostic accuracy of OCT is in the setting of BCC, with a recent systematic review reporting a sensitivity of 66% to 96% and a specificity of 75% to 86% for conventional FD-OCT.42 The use of FD-OCT results in an increase in specificity without a significant change in sensitivity when compared to dermoscopy in the diagnosis of BCC.43 Melanoma is difficult to diagnose via FD-OCT, as the visualization of architectural features often is limited by poor resolution.44 A study of HD-OCT in the diagnosis of melanoma with a limited sample size reported a sensitivity of 74% to 80% and a specificity of 92% to 93%.45 Similarly, a study of HD-OCT used in the diagnosis of AK and SCC revealed a sensitivity and specificity of 81.6% and 92.6%, respectively, for AK and 93.8% and 98.9%, respectively, for SCC.46
Numerous algorithms and scoring systems have been developed to further explore the utility of OCT in the diagnosis of cutaneous neoplasms.47,48 Recent research investigated the utility of dynamic OCT, which can evaluate microvasculature in the diagnosis of cutaneous neoplasms (Figure 3)49; the combination of OCT with other imaging modalities50,51; the use of OCT to delineate presurgical margins52,53; and the role of OCT in the diagnosis and monitoring of inflammatory and infectious skin diseases.54,55
Final Thoughts
In recent years, there has been a surge of interest in noninvasive techniques for diagnosis and management of skin diseases; however, noninvasive tools exist on a spectrum in dermatology. Dermoscopy provides low-cost imaging of the skin’s surface and has been widely adopted by dermatologists and other providers to aid in clinical diagnosis. Reflectance confocal microscopy provides reimbursable in vivo imaging of live tissue with cellular-level resolution but is limited by depth, cost, and need for advanced training; thus, RCM has only been adopted in some clinical practices. Optical coherence tomography offers in vivo imaging of live tissue with substantial depth but poor resolution, high cost, need for advanced training, and rare reimbursement for providers. Future directions include combination of complementary imaging modalities, increased clinical practice integration, and education and reimbursement for providers.
- Benvenuto-Andrade C, Dusza SW, Agero AL, et al. Differences between polarized light dermoscopy and immersion contact dermoscopy for the evaluation of skin lesions. Arch Dermatol. 2007;143:329-338.
- Terushkin V, Oliveria SA, Marghoob AA, et al. Use of and beliefs about total body photography and dermatoscopy among US dermatology training programs: an update. J Am Acad Dermatol. 2010;62:794-803.
- Morris JB, Alfonso SV, Hernandez N, et al. Use of and intentions to use dermoscopy among physicians in the United States. Dermatol Pract Concept. 2017;7:7-16.
- Yélamos O, Braun RP, Liopyris K, et al. Dermoscopy and dermatopathology correlates of cutaneous neoplasms. J Am Acad Dermatol. 2019;80:341-363.
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676.
- Carli P, de Giorgi V, Chiarugi A, et al. Addition of dermoscopy to conventional naked-eye examination in melanoma screening: a randomized study. J Am Acad Dermatol. 2004;50:683-668.
- Lallas A, Zalaudek I, Argenziano G, et al. Dermoscopy in general dermatology. Dermatol Clin. 2013;31:679-694.
- Reiter O, Mimouni I, Gdalvevich M, et al. The diagnostic accuracy of dermoscopy for basal cell carcinoma: a systematic review and meta-analysis. J Am Acad Dermatol. 2019;80:1380-1388.
- Papageorgiou V, Apalla Z, Sotiriou E, et al. The limitations of dermoscopy: false-positive and false-negative tumours. J Eur Acad Dermatol Venereol. 2018;32:879-888.
- Micali G, Verzì AE, Lacarrubba F. Alternative uses of dermoscopy in daily clinical practice: an update. J Am Acad Dermatol. 2018;79:1117-1132.e1.
- Rajadhyaksha M, Grossman M, Esterowitz D, et al. In vivo confocal scanning laser microscopy of human skin: melanin provides strong contrast. J Invest Dermatol. 1995;104:946-952.
- Kose K, Gou M, Yélamos O, et al. Automated video-mosaicking approach for confocal microscopic imaging in vivo: an approach to address challenges in imaging living tissue and extend field of view. Sci Rep. 2017;7:10759.
- Rao BK, John AM, Francisco G, et al. Diagnostic accuracy of reflectance confocal microscopy for diagnosis of skin lesions [published online October 8, 2018]. Arch Pathol Lab Med. 2019;143:326-329.
- Current Procedural Terminology, Professional Edition. Chicago IL: American Medical Association; 2016. The preliminary physician fee schedule for 2017 is available at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/PFS-Federal-Regulation-Notices-Items/CMS-1654-P.html.
- Jain M, Pulijal SV, Rajadhyaksha M, et al. Evaluation of bedside diagnostic accuracy, learning curve, and challenges for a novice reflectance confocal microscopy reader for skin cancer detection in vivo. JAMA Dermatol. 2018;154:962-965.
- Rao BK, Pellacani G. Atlas of Confocal Microscopy in Dermatology: Clinical, Confocal, and Histological Images. New York, NY: NIDIskin LLC; 2013.
- Scope A, Benvenuto-Andrande C, Agero AL, et al. In vivo reflectance confocal microscopy imaging of melanocytic skin lesions: consensus terminology glossary and illustrative images. J Am Acad Dermatol. 2007;57:644-658.
- Gerger A, Hofmann-Wellenhof R, Langsenlehner U, et al. In vivo confocal laser scanning microscopy of melanocytic skin tumours: diagnostic applicability using unselected tumour images. Br J Dermatol. 2008;158:329-333.
- Stevenson AD, Mickan S, Mallett S, et al. Systematic review of diagnostic accuracy of reflectance confocal microscopy for melanoma diagnosis in patients with clinically equivocal skin lesions. Dermatol Pract Concept. 2013;3:19-27.
- Alarcon I, Carrera C, Palou J, et al. Impact of in vivo reflectance confocal microscopy on the number needed to treat melanoma in doubtful lesions. Br J Dermatol. 2014;170:802-808.
- Lovatto L, Carrera C, Salerni G, et al. In vivo reflectance confocal microscopy of equivocal melanocytic lesions detected by digital dermoscopy follow-up. J Eur Acad Dermatol Venereol. 2015;29:1918-1925.
- Guitera P, Pellacani G, Longo C, et al. In vivo reflectance confocal microscopy enhances secondary evaluation of melanocytic lesions. J Invest Dermatol. 2009;129:131-138.
- Xiong YQ, Ma SJ, Mo Y, et al. Comparison of dermoscopy and reflectance confocal microscopy for the diagnosis of malignant skin tumours: a meta-analysis. J Cancer Res Clin Oncol. 2017;143:1627-1635.
- Kadouch DJ, Schram ME, Leeflang MM, et al. In vivo confocal microscopy of basal cell carcinoma: a systematic review of diagnostic accuracy. J Eur Acad Dermatol Venereol. 2015;29:1890-1897.
- Dinnes J, Deeks JJ, Chuchu N, et al; Cochrane Skin Cancer Diagnostic Test Accuracy Group. Reflectance confocal microscopy for diagnosing keratinocyte skin cancers in adults. Cochrane Database Syst Rev. 2018;12:CD013191.
- Nguyen KP, Peppelman M, Hoogedoorn L, et al. The current role of in vivo reflectance confocal microscopy within the continuum of actinic keratosis and squamous cell carcinoma: a systematic review. Eur J Dermatol. 2016;26:549-565.
- Pellacani G, Ulrich M, Casari A, et al. Grading keratinocyte atypia in actinic keratosis: a correlation of reflectance confocal microscopy and histopathology. J Eur Acad Dermatol Venereol. 2015;29:2216-2221.
- Manfredini M, Longo C, Ferrari B, et al. Dermoscopic and reflectance confocal microscopy features of cutaneous squamous cell carcinoma. J Eur Acad Dermatol Venereol. 2017;31:1828-1833.
- Hoogedoorn L, Peppelman M, van de Kerkhof PC, et al. The value of in vivo reflectance confocal microscopy in the diagnosis and monitoring of inflammatory and infectious skin diseases: a systematic review. Br J Dermatol. 2015;172:1222-1248.
- Cinotti E, Perrot JL, Labeille B, et al. Reflectance confocal microscopy for cutaneous infections and infestations. J Eur Acad Dermatol Venereol. 2016;30:754-763.
- Ardigo M, Longo C, Gonzalez S; International Confocal Working Group Inflammatory Skin Diseases Project. Multicentre study on inflammatory skin diseases from The International Confocal Working Group: specific confocal microscopy features and an algorithmic method of diagnosis. Br J Dermatol. 2016;175:364-374.
- Ardigo M, Agozzino M, Franceschini C, et al. Reflectance confocal microscopy algorithms for inflammatory and hair diseases. Dermatol Clin. 2016;34:487-496.
- Manfredini M, Bettoli V, Sacripanti G, et al. The evolution of healthy skin to acne lesions: a longitudinal, in vivo evaluation with reflectance confocal microscopy and optical coherence tomography [published online April 26, 2019]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.15641.
- Navarrete-Dechent C, Mori S, Cordova M, et al. Reflectance confocal microscopy as a novel tool for presurgical identification of basal cell carcinoma biopsy site. J Am Acad Dermatol. 2019;80:e7-e8.
- Pan ZY, Lin JR, Cheng TT, et al. In vivo reflectance confocal microscopy of basal cell carcinoma: feasibility of preoperative mapping of cancer margins. Dermatol Surg. 2012;38:1945-1950.
- Venturini M, Gualdi G, Zanca A, et al. A new approach for presurgical margin assessment by reflectance confocal microscopy of basal cell carcinoma. Br J Dermatol. 2016;174:380-385.
- Sierra H, Yélamos O, Cordova M, et al. Reflectance confocal microscopy‐guided laser ablation of basal cell carcinomas: initial clinical experience. J Biomed Opt. 2017;22:1-13.
- Maier T, Kulichova D, Ruzicka T, et al. Noninvasive monitoring of basal cell carcinomas treated with systemic hedgehog inhibitors: pseudocysts as a sign of tumor regression. J Am Acad Dermatol. 2014;71:725-730.
- Levine A, Wang K, Markowitz O. Optical coherence tomography in the diagnosis of skin cancer. Dermatol Clin. 2017;35:465-488.
- Schneider SL, Kohli I, Hamzavi IH, et al. Emerging imaging technologies in dermatology: part I: basic principles. J Am Acad Dermatol. 2019;80:1114-1120.
- Mogensen M, Joergensen TM, Nümberg BM, et al. Assessment of optical coherence tomography imaging in the diagnosis of non‐melanoma skin cancer and benign lesions versus normal skin: observer‐blinded evaluation by dermatologists and pathologists. Dermatol Surg. 2009;35:965-972.
- Ferrante di Ruffano L, Dinnes J, Deeks JJ, et al. Optical coherence tomography for diagnosing skin cancer in adults. Cochrane Database Syst Rev. 2018;12:CD013189.
- Ulrich M, von Braunmuehl T, Kurzen H, et al. The sensitivity and specificity of optical coherence tomography for the assisted diagnosis of nonpigmented basal cell carcinoma: an observational study. Br J Dermatol. 2015;173:428-435.
- Wessels R, de Bruin DM, Relyveld GM, et al. Functional optical coherence tomography of pigmented lesions. J Eur Acad Dermatol Venereol. 2015;29:738‐744.
- Gambichler T, Schmid-Wendtner MH, Plura I, et al. A multicentre pilot study investigating high‐definition optical coherence tomography in the differentiation of cutaneous melanoma and melanocytic naevi. J Eur Acad Dermatol Venereol. 2015;29:537‐541.
- Marneffe A, Suppa M, Miyamoto M, et al. Validation of a diagnostic algorithm for the discrimination of actinic keratosis from normal skin and squamous cell carcinoma by means of high-definition optical coherence tomography. Exp Dermatol. 2016;25:684-687.
- Boone MA, Suppa M, Dhaenens F, et al. In vivo assessment of optical properties of melanocytic skin lesions and differentiation of melanoma from non-malignant lesions by high-definition optical coherence tomography. Arch Dermatol Res. 2016;308:7-20.
- Boone MA, Suppa M, Marneffe A, et al. A new algorithm for the discrimination of actinic keratosis from normal skin and squamous cell carcinoma based on in vivo analysis of optical properties by high-definition optical coherence tomography. J Eur Acad Dermatol Venereol. 2016;30:1714-1725.
- Themstrup L, Pellacani G, Welzel J, et al. In vivo microvascular imaging of cutaneous actinic keratosis, Bowen’s disease and squamous cell carcinoma using dynamic optical coherence tomography. J Eur Acad Dermatol Venereol. 2017;31:1655-1662.
- Alex A, Weingast J, Weinigel M, et al. Three-dimensional multiphoton/optical coherence tomography for diagnostic applications in dermatology. J Biophotonics. 2013;6:352-362.
- Iftimia N, Yélamos O, Chen CJ, et al. Handheld optical coherence tomography-reflectance confocal microscopy probe for detection of basal cell carcinoma and delineation of margins. J Biomed Opt. 2017;22:76006.
- Wang KX, Meekings A, Fluhr JW, et al. Optical coherence tomography-based optimization of Mohs micrographic surgery of basal cell carcinoma: a pilot study. Dermatol Surg. 2013;39:627-633.
- Chan CS, Rohrer TE. Optical coherence tomography and its role in Mohs micrographic surgery: a case report. Case Rep Dermatol. 2012;4:269-274.
- Gambichler T, Jaedicke V, Terras S. Optical coherence tomography in dermatology: technical and clinical aspects. Arch Dermatol Res. 2011;303:457-473.
- Manfredini M, Greco M, Farnetani F, et al. Acne: morphologic and vascular study of lesions and surrounding skin by means of optical coherence tomography. J Eur Acad Dermatol Venereol. 2017;31:1541-1546.
Traditionally, diagnosis of skin disease relies on clinical inspection, often followed by biopsy and histopathologic examination. In recent years, new noninvasive tools have emerged that can aid in clinical diagnosis and reduce the number of unnecessary benign biopsies. Although there has been a surge in noninvasive diagnostic technologies, many tools are still in research and development phases, with few tools widely adopted and used in regular clinical practice. In this article, we discuss the use of dermoscopy, reflectance confocal microscopy (RCM), and optical coherence tomography (OCT) in the diagnosis and management of skin disease.
Dermoscopy
Dermoscopy, also known as epiluminescence light microscopy and previously known as dermatoscopy, utilizes a ×10 to ×100 microscope objective with a light source to magnify and visualize structures present below the skin’s surface, such as melanin and blood vessels. There are 3 types of dermoscopy: conventional nonpolarized dermoscopy, polarized contact dermoscopy, and nonpolarized contact dermoscopy (Figure 1). Traditional nonpolarized dermoscopy requires a liquid medium and direct contact with the skin, and it relies on light reflection and refraction properties.1 Cross-polarized light sources allow visualization of deeper structures, either with or without a liquid medium and contact with the skin surface. Although there is overall concurrence among the different types of dermoscopy, subtle differences in the appearance of color, features, and structure are present.1

Dermoscopy offers many benefits for dermatologists and other providers. It can be used to aid in the diagnosis of cutaneous neoplasms and other skin diseases. Numerous low-cost dermatoscopes currently are commercially available. The handheld, easily transportable nature of dermatoscopes have resulted in widespread practice integration. Approximately 84% of attending dermatologists in US academic settings reported using dermoscopy, and many refer to the dermatoscope as “the dermatologist’s stethoscope.”2 In addition, 6% to 15% of other US providers, including family physicians, internal medicine physicians, and plastic surgeons, have reported using dermoscopy in their clinical practices. Limitations of dermoscopy include visualization of the skin surface only and not deeper structures within the tissue, the need for training for adequate interpretation of dermoscopic images, and lack of reimbursement for dermoscopic examination.3
Many dermoscopic structures that correspond well with histopathology have been described. Dermoscopy has a sensitivity of 79% to 96% and specificity of 69% to 99% in the diagnosis of melanoma.4 There is variable data on the specificity of dermoscopy in the diagnosis of melanoma, with one meta-analysis finding no statistically significant difference in specificity compared to naked eye examination,5 while other studies report increased specificity and subsequent reduction in biopsy of benign lesions.6,7 Dermoscopy also can aid in the diagnosis of keratinocytic neoplasms, and dermoscopy also results in a sensitivity of 78.6% to 100% and a specificity of 53.8% to 100% in the diagnosis of basal cell carcinoma (BCC).8 Limitations of dermoscopy include false-positive diagnoses, commonly seborrheic keratoses and nevi, resulting in unnecessary biopsies, as well as false-negative diagnoses, commonly amelanotic and nevoid melanoma, resulting in delays in skin cancer diagnosis and resultant poor outcomes.9 Dermoscopy also is used to aid in the diagnosis of inflammatory and infectious skin diseases, as well as scalp, hair, and nail disorders.10
Reflectance Confocal Microscopy
Reflectance confocal microscopy utilizes an 830-nm laser to capture horizontal en face images of the skin with high resolution. Different structures of the skin have varying indices of refraction: keratin, melanin, and collagen appear bright white, while other components appear dark, generating black-and-white RCM images.11 Currently, there are 2 reflectance confocal microscopes that are commercially available in the United States. The Vivascope 1500 (Caliber ID) is the traditional model that captures 8×8-mm images, and the Vivascope 3000 (Caliber ID) is a smaller handheld model that captures 0.5×0.5-mm images. The traditional model provides the advantages of higher-resolution images and the ability to capture larger surface areas but is best suited to image flat areas of skin to which a square window can be adhered. The handheld model allows improved contact with the varying topography of skin; does not require an adhesive window; and can be used to image cartilaginous, mucosal, and sensitive surfaces. However, it can be difficult to correlate individual images captured by the handheld RCM with the location relative to the lesion, as it is exquisitely sensitive to motion and also is operator dependent. Although complex algorithms are under development to stitch individual images to provide better correlation with the geography of the lesion, such programs are not yet widely available.12
Reflectance confocal microscopy affords many benefits for patients and providers. It is noninvasive and painless and is capable of imaging in vivo live skin as compared to clinical examination and dermoscopy, which only allow for visualization of the skin’s surface. Reflectance confocal microscopy also is time efficient, as imaging of a single lesion can be completed in 10 to 15 minutes. This technology generates high-resolution images, and RCM diagnosis has consistently demonstrated high sensitivity and specificity when compared to histopathology.13 Additionally, RCM imaging can spare biopsy and resultant scarring on cosmetically sensitive areas. Recently, RCM imaging of the skin has been granted Category I Current Procedural Terminology reimbursement codes that allow provider reimbursement and integration of RCM into daily practice14; however, private insurance coverage in the United States is variable. Limitations of RCM include a maximum depth of 200 to 300 µm, high cost to procure a reflectance confocal microscope, and the need for considerable training and practice to accurately interpret grayscale en face images.15
There has been extensive research regarding the use of RCM in the evaluation of cutaneous neoplasms and other skin diseases. Numerous features and patterns have been identified and described that correspond with different skin diseases and correspond well with histopathology (Figure 2).13,16,17 Reflectance confocal microscopy has demonstrated consistently high accuracy in the diagnosis of melanocytic lesions, with a sensitivity of 93% to 100% and a specificity of 75% to 99%.18-21 Reflectance confocal microscopy is especially useful in the evaluation of clinically or dermoscopically equivocal pigmented lesions due to greater specificity, resulting in a reduction of unnecessary biopsies.22,23 It also has high accuracy in the diagnosis of keratinocytic neoplasms, with a sensitivity of 82% to 100% and a specificity of 78% to 97% in the diagnosis of BCC,24 and a sensitivity of 74% to 100% and specificity of 78% to 100% in the diagnosis of squamous cell carcinoma (SCC).25,26 Evaluation of SCC and actinic keratosis (AK) using RCM may be limited by considerable hyperkeratosis and ulceration. In addition, it can be challenging to differentiate AK and SCC on RCM, and considerable expertise is required to accurately grade cytologic and architectural atypia.27 However, RCM has been used to discriminate between in situ and invasive proliferations.28 Reflectance confocal microscopy has wide applications in the diagnosis and management of cutaneous infections29,30 and inflammatory skin diseases.29,31-33 Recent RCM research explored the use of RCM to identify biopsy sites,34 delineate presurgical tumor margins,35,36 and monitor response to noninvasive treatments.37,38

Optical Coherence Tomography
Optical coherence tomography is an imaging modality that utilizes light backscatter from infrared light to produce grayscale cross-sectional or vertical images and horizontal en face images.39 Optical coherence tomography can visualize structures in the epidermis, dermoepidermal junction, and upper dermis.40 It can image boundaries of structures but cannot visualize individual cells.
There are different types of OCT devices available, including frequency-domain OCT (FD-OCT), or conventional OCT, and high-definition OCT (HD-OCT). With FD-OCT, images are captured at a maximum depth of 1 to 2 mm but with limited resolution. High-definition OCT has superior resolution compared to FD-OCT but is restricted to a shallower depth of 750 μm.39 The main advantage of OCT is the ability to noninvasively image live tissue and visualize 2- to 5-times greater depth as compared to RCM. Several OCT devices have obtained US Food and Drug Administration approval; however, OCT has not been widely adopted into clinical practice and is available only in tertiary academic centers. Additionally, OCT imaging in dermatology is rarely reimbursed. Other limitations of OCT include poor resolution of images, high cost to procure an OCT device, and the need for advanced training and experience to accurately interpret images.40,41
Optical coherence tomography primarily is used to diagnose cutaneous neoplasms. The best evidence of the diagnostic accuracy of OCT is in the setting of BCC, with a recent systematic review reporting a sensitivity of 66% to 96% and a specificity of 75% to 86% for conventional FD-OCT.42 The use of FD-OCT results in an increase in specificity without a significant change in sensitivity when compared to dermoscopy in the diagnosis of BCC.43 Melanoma is difficult to diagnose via FD-OCT, as the visualization of architectural features often is limited by poor resolution.44 A study of HD-OCT in the diagnosis of melanoma with a limited sample size reported a sensitivity of 74% to 80% and a specificity of 92% to 93%.45 Similarly, a study of HD-OCT used in the diagnosis of AK and SCC revealed a sensitivity and specificity of 81.6% and 92.6%, respectively, for AK and 93.8% and 98.9%, respectively, for SCC.46
Numerous algorithms and scoring systems have been developed to further explore the utility of OCT in the diagnosis of cutaneous neoplasms.47,48 Recent research investigated the utility of dynamic OCT, which can evaluate microvasculature in the diagnosis of cutaneous neoplasms (Figure 3)49; the combination of OCT with other imaging modalities50,51; the use of OCT to delineate presurgical margins52,53; and the role of OCT in the diagnosis and monitoring of inflammatory and infectious skin diseases.54,55

Final Thoughts
In recent years, there has been a surge of interest in noninvasive techniques for diagnosis and management of skin diseases; however, noninvasive tools exist on a spectrum in dermatology. Dermoscopy provides low-cost imaging of the skin’s surface and has been widely adopted by dermatologists and other providers to aid in clinical diagnosis. Reflectance confocal microscopy provides reimbursable in vivo imaging of live tissue with cellular-level resolution but is limited by depth, cost, and need for advanced training; thus, RCM has only been adopted in some clinical practices. Optical coherence tomography offers in vivo imaging of live tissue with substantial depth but poor resolution, high cost, need for advanced training, and rare reimbursement for providers. Future directions include combination of complementary imaging modalities, increased clinical practice integration, and education and reimbursement for providers.
Traditionally, diagnosis of skin disease relies on clinical inspection, often followed by biopsy and histopathologic examination. In recent years, new noninvasive tools have emerged that can aid in clinical diagnosis and reduce the number of unnecessary benign biopsies. Although there has been a surge in noninvasive diagnostic technologies, many tools are still in research and development phases, with few tools widely adopted and used in regular clinical practice. In this article, we discuss the use of dermoscopy, reflectance confocal microscopy (RCM), and optical coherence tomography (OCT) in the diagnosis and management of skin disease.
Dermoscopy
Dermoscopy, also known as epiluminescence light microscopy and previously known as dermatoscopy, utilizes a ×10 to ×100 microscope objective with a light source to magnify and visualize structures present below the skin’s surface, such as melanin and blood vessels. There are 3 types of dermoscopy: conventional nonpolarized dermoscopy, polarized contact dermoscopy, and nonpolarized contact dermoscopy (Figure 1). Traditional nonpolarized dermoscopy requires a liquid medium and direct contact with the skin, and it relies on light reflection and refraction properties.1 Cross-polarized light sources allow visualization of deeper structures, either with or without a liquid medium and contact with the skin surface. Although there is overall concurrence among the different types of dermoscopy, subtle differences in the appearance of color, features, and structure are present.1

Dermoscopy offers many benefits for dermatologists and other providers. It can be used to aid in the diagnosis of cutaneous neoplasms and other skin diseases. Numerous low-cost dermatoscopes currently are commercially available. The handheld, easily transportable nature of dermatoscopes have resulted in widespread practice integration. Approximately 84% of attending dermatologists in US academic settings reported using dermoscopy, and many refer to the dermatoscope as “the dermatologist’s stethoscope.”2 In addition, 6% to 15% of other US providers, including family physicians, internal medicine physicians, and plastic surgeons, have reported using dermoscopy in their clinical practices. Limitations of dermoscopy include visualization of the skin surface only and not deeper structures within the tissue, the need for training for adequate interpretation of dermoscopic images, and lack of reimbursement for dermoscopic examination.3
Many dermoscopic structures that correspond well with histopathology have been described. Dermoscopy has a sensitivity of 79% to 96% and specificity of 69% to 99% in the diagnosis of melanoma.4 There is variable data on the specificity of dermoscopy in the diagnosis of melanoma, with one meta-analysis finding no statistically significant difference in specificity compared to naked eye examination,5 while other studies report increased specificity and subsequent reduction in biopsy of benign lesions.6,7 Dermoscopy also can aid in the diagnosis of keratinocytic neoplasms, and dermoscopy also results in a sensitivity of 78.6% to 100% and a specificity of 53.8% to 100% in the diagnosis of basal cell carcinoma (BCC).8 Limitations of dermoscopy include false-positive diagnoses, commonly seborrheic keratoses and nevi, resulting in unnecessary biopsies, as well as false-negative diagnoses, commonly amelanotic and nevoid melanoma, resulting in delays in skin cancer diagnosis and resultant poor outcomes.9 Dermoscopy also is used to aid in the diagnosis of inflammatory and infectious skin diseases, as well as scalp, hair, and nail disorders.10
Reflectance Confocal Microscopy
Reflectance confocal microscopy utilizes an 830-nm laser to capture horizontal en face images of the skin with high resolution. Different structures of the skin have varying indices of refraction: keratin, melanin, and collagen appear bright white, while other components appear dark, generating black-and-white RCM images.11 Currently, there are 2 reflectance confocal microscopes that are commercially available in the United States. The Vivascope 1500 (Caliber ID) is the traditional model that captures 8×8-mm images, and the Vivascope 3000 (Caliber ID) is a smaller handheld model that captures 0.5×0.5-mm images. The traditional model provides the advantages of higher-resolution images and the ability to capture larger surface areas but is best suited to image flat areas of skin to which a square window can be adhered. The handheld model allows improved contact with the varying topography of skin; does not require an adhesive window; and can be used to image cartilaginous, mucosal, and sensitive surfaces. However, it can be difficult to correlate individual images captured by the handheld RCM with the location relative to the lesion, as it is exquisitely sensitive to motion and also is operator dependent. Although complex algorithms are under development to stitch individual images to provide better correlation with the geography of the lesion, such programs are not yet widely available.12
Reflectance confocal microscopy affords many benefits for patients and providers. It is noninvasive and painless and is capable of imaging in vivo live skin as compared to clinical examination and dermoscopy, which only allow for visualization of the skin’s surface. Reflectance confocal microscopy also is time efficient, as imaging of a single lesion can be completed in 10 to 15 minutes. This technology generates high-resolution images, and RCM diagnosis has consistently demonstrated high sensitivity and specificity when compared to histopathology.13 Additionally, RCM imaging can spare biopsy and resultant scarring on cosmetically sensitive areas. Recently, RCM imaging of the skin has been granted Category I Current Procedural Terminology reimbursement codes that allow provider reimbursement and integration of RCM into daily practice14; however, private insurance coverage in the United States is variable. Limitations of RCM include a maximum depth of 200 to 300 µm, high cost to procure a reflectance confocal microscope, and the need for considerable training and practice to accurately interpret grayscale en face images.15
There has been extensive research regarding the use of RCM in the evaluation of cutaneous neoplasms and other skin diseases. Numerous features and patterns have been identified and described that correspond with different skin diseases and correspond well with histopathology (Figure 2).13,16,17 Reflectance confocal microscopy has demonstrated consistently high accuracy in the diagnosis of melanocytic lesions, with a sensitivity of 93% to 100% and a specificity of 75% to 99%.18-21 Reflectance confocal microscopy is especially useful in the evaluation of clinically or dermoscopically equivocal pigmented lesions due to greater specificity, resulting in a reduction of unnecessary biopsies.22,23 It also has high accuracy in the diagnosis of keratinocytic neoplasms, with a sensitivity of 82% to 100% and a specificity of 78% to 97% in the diagnosis of BCC,24 and a sensitivity of 74% to 100% and specificity of 78% to 100% in the diagnosis of squamous cell carcinoma (SCC).25,26 Evaluation of SCC and actinic keratosis (AK) using RCM may be limited by considerable hyperkeratosis and ulceration. In addition, it can be challenging to differentiate AK and SCC on RCM, and considerable expertise is required to accurately grade cytologic and architectural atypia.27 However, RCM has been used to discriminate between in situ and invasive proliferations.28 Reflectance confocal microscopy has wide applications in the diagnosis and management of cutaneous infections29,30 and inflammatory skin diseases.29,31-33 Recent RCM research explored the use of RCM to identify biopsy sites,34 delineate presurgical tumor margins,35,36 and monitor response to noninvasive treatments.37,38

Optical Coherence Tomography
Optical coherence tomography is an imaging modality that utilizes light backscatter from infrared light to produce grayscale cross-sectional or vertical images and horizontal en face images.39 Optical coherence tomography can visualize structures in the epidermis, dermoepidermal junction, and upper dermis.40 It can image boundaries of structures but cannot visualize individual cells.
There are different types of OCT devices available, including frequency-domain OCT (FD-OCT), or conventional OCT, and high-definition OCT (HD-OCT). With FD-OCT, images are captured at a maximum depth of 1 to 2 mm but with limited resolution. High-definition OCT has superior resolution compared to FD-OCT but is restricted to a shallower depth of 750 μm.39 The main advantage of OCT is the ability to noninvasively image live tissue and visualize 2- to 5-times greater depth as compared to RCM. Several OCT devices have obtained US Food and Drug Administration approval; however, OCT has not been widely adopted into clinical practice and is available only in tertiary academic centers. Additionally, OCT imaging in dermatology is rarely reimbursed. Other limitations of OCT include poor resolution of images, high cost to procure an OCT device, and the need for advanced training and experience to accurately interpret images.40,41
Optical coherence tomography primarily is used to diagnose cutaneous neoplasms. The best evidence of the diagnostic accuracy of OCT is in the setting of BCC, with a recent systematic review reporting a sensitivity of 66% to 96% and a specificity of 75% to 86% for conventional FD-OCT.42 The use of FD-OCT results in an increase in specificity without a significant change in sensitivity when compared to dermoscopy in the diagnosis of BCC.43 Melanoma is difficult to diagnose via FD-OCT, as the visualization of architectural features often is limited by poor resolution.44 A study of HD-OCT in the diagnosis of melanoma with a limited sample size reported a sensitivity of 74% to 80% and a specificity of 92% to 93%.45 Similarly, a study of HD-OCT used in the diagnosis of AK and SCC revealed a sensitivity and specificity of 81.6% and 92.6%, respectively, for AK and 93.8% and 98.9%, respectively, for SCC.46
Numerous algorithms and scoring systems have been developed to further explore the utility of OCT in the diagnosis of cutaneous neoplasms.47,48 Recent research investigated the utility of dynamic OCT, which can evaluate microvasculature in the diagnosis of cutaneous neoplasms (Figure 3)49; the combination of OCT with other imaging modalities50,51; the use of OCT to delineate presurgical margins52,53; and the role of OCT in the diagnosis and monitoring of inflammatory and infectious skin diseases.54,55

Final Thoughts
In recent years, there has been a surge of interest in noninvasive techniques for diagnosis and management of skin diseases; however, noninvasive tools exist on a spectrum in dermatology. Dermoscopy provides low-cost imaging of the skin’s surface and has been widely adopted by dermatologists and other providers to aid in clinical diagnosis. Reflectance confocal microscopy provides reimbursable in vivo imaging of live tissue with cellular-level resolution but is limited by depth, cost, and need for advanced training; thus, RCM has only been adopted in some clinical practices. Optical coherence tomography offers in vivo imaging of live tissue with substantial depth but poor resolution, high cost, need for advanced training, and rare reimbursement for providers. Future directions include combination of complementary imaging modalities, increased clinical practice integration, and education and reimbursement for providers.
- Benvenuto-Andrade C, Dusza SW, Agero AL, et al. Differences between polarized light dermoscopy and immersion contact dermoscopy for the evaluation of skin lesions. Arch Dermatol. 2007;143:329-338.
- Terushkin V, Oliveria SA, Marghoob AA, et al. Use of and beliefs about total body photography and dermatoscopy among US dermatology training programs: an update. J Am Acad Dermatol. 2010;62:794-803.
- Morris JB, Alfonso SV, Hernandez N, et al. Use of and intentions to use dermoscopy among physicians in the United States. Dermatol Pract Concept. 2017;7:7-16.
- Yélamos O, Braun RP, Liopyris K, et al. Dermoscopy and dermatopathology correlates of cutaneous neoplasms. J Am Acad Dermatol. 2019;80:341-363.
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676.
- Carli P, de Giorgi V, Chiarugi A, et al. Addition of dermoscopy to conventional naked-eye examination in melanoma screening: a randomized study. J Am Acad Dermatol. 2004;50:683-668.
- Lallas A, Zalaudek I, Argenziano G, et al. Dermoscopy in general dermatology. Dermatol Clin. 2013;31:679-694.
- Reiter O, Mimouni I, Gdalvevich M, et al. The diagnostic accuracy of dermoscopy for basal cell carcinoma: a systematic review and meta-analysis. J Am Acad Dermatol. 2019;80:1380-1388.
- Papageorgiou V, Apalla Z, Sotiriou E, et al. The limitations of dermoscopy: false-positive and false-negative tumours. J Eur Acad Dermatol Venereol. 2018;32:879-888.
- Micali G, Verzì AE, Lacarrubba F. Alternative uses of dermoscopy in daily clinical practice: an update. J Am Acad Dermatol. 2018;79:1117-1132.e1.
- Rajadhyaksha M, Grossman M, Esterowitz D, et al. In vivo confocal scanning laser microscopy of human skin: melanin provides strong contrast. J Invest Dermatol. 1995;104:946-952.
- Kose K, Gou M, Yélamos O, et al. Automated video-mosaicking approach for confocal microscopic imaging in vivo: an approach to address challenges in imaging living tissue and extend field of view. Sci Rep. 2017;7:10759.
- Rao BK, John AM, Francisco G, et al. Diagnostic accuracy of reflectance confocal microscopy for diagnosis of skin lesions [published online October 8, 2018]. Arch Pathol Lab Med. 2019;143:326-329.
- Current Procedural Terminology, Professional Edition. Chicago IL: American Medical Association; 2016. The preliminary physician fee schedule for 2017 is available at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/PFS-Federal-Regulation-Notices-Items/CMS-1654-P.html.
- Jain M, Pulijal SV, Rajadhyaksha M, et al. Evaluation of bedside diagnostic accuracy, learning curve, and challenges for a novice reflectance confocal microscopy reader for skin cancer detection in vivo. JAMA Dermatol. 2018;154:962-965.
- Rao BK, Pellacani G. Atlas of Confocal Microscopy in Dermatology: Clinical, Confocal, and Histological Images. New York, NY: NIDIskin LLC; 2013.
- Scope A, Benvenuto-Andrande C, Agero AL, et al. In vivo reflectance confocal microscopy imaging of melanocytic skin lesions: consensus terminology glossary and illustrative images. J Am Acad Dermatol. 2007;57:644-658.
- Gerger A, Hofmann-Wellenhof R, Langsenlehner U, et al. In vivo confocal laser scanning microscopy of melanocytic skin tumours: diagnostic applicability using unselected tumour images. Br J Dermatol. 2008;158:329-333.
- Stevenson AD, Mickan S, Mallett S, et al. Systematic review of diagnostic accuracy of reflectance confocal microscopy for melanoma diagnosis in patients with clinically equivocal skin lesions. Dermatol Pract Concept. 2013;3:19-27.
- Alarcon I, Carrera C, Palou J, et al. Impact of in vivo reflectance confocal microscopy on the number needed to treat melanoma in doubtful lesions. Br J Dermatol. 2014;170:802-808.
- Lovatto L, Carrera C, Salerni G, et al. In vivo reflectance confocal microscopy of equivocal melanocytic lesions detected by digital dermoscopy follow-up. J Eur Acad Dermatol Venereol. 2015;29:1918-1925.
- Guitera P, Pellacani G, Longo C, et al. In vivo reflectance confocal microscopy enhances secondary evaluation of melanocytic lesions. J Invest Dermatol. 2009;129:131-138.
- Xiong YQ, Ma SJ, Mo Y, et al. Comparison of dermoscopy and reflectance confocal microscopy for the diagnosis of malignant skin tumours: a meta-analysis. J Cancer Res Clin Oncol. 2017;143:1627-1635.
- Kadouch DJ, Schram ME, Leeflang MM, et al. In vivo confocal microscopy of basal cell carcinoma: a systematic review of diagnostic accuracy. J Eur Acad Dermatol Venereol. 2015;29:1890-1897.
- Dinnes J, Deeks JJ, Chuchu N, et al; Cochrane Skin Cancer Diagnostic Test Accuracy Group. Reflectance confocal microscopy for diagnosing keratinocyte skin cancers in adults. Cochrane Database Syst Rev. 2018;12:CD013191.
- Nguyen KP, Peppelman M, Hoogedoorn L, et al. The current role of in vivo reflectance confocal microscopy within the continuum of actinic keratosis and squamous cell carcinoma: a systematic review. Eur J Dermatol. 2016;26:549-565.
- Pellacani G, Ulrich M, Casari A, et al. Grading keratinocyte atypia in actinic keratosis: a correlation of reflectance confocal microscopy and histopathology. J Eur Acad Dermatol Venereol. 2015;29:2216-2221.
- Manfredini M, Longo C, Ferrari B, et al. Dermoscopic and reflectance confocal microscopy features of cutaneous squamous cell carcinoma. J Eur Acad Dermatol Venereol. 2017;31:1828-1833.
- Hoogedoorn L, Peppelman M, van de Kerkhof PC, et al. The value of in vivo reflectance confocal microscopy in the diagnosis and monitoring of inflammatory and infectious skin diseases: a systematic review. Br J Dermatol. 2015;172:1222-1248.
- Cinotti E, Perrot JL, Labeille B, et al. Reflectance confocal microscopy for cutaneous infections and infestations. J Eur Acad Dermatol Venereol. 2016;30:754-763.
- Ardigo M, Longo C, Gonzalez S; International Confocal Working Group Inflammatory Skin Diseases Project. Multicentre study on inflammatory skin diseases from The International Confocal Working Group: specific confocal microscopy features and an algorithmic method of diagnosis. Br J Dermatol. 2016;175:364-374.
- Ardigo M, Agozzino M, Franceschini C, et al. Reflectance confocal microscopy algorithms for inflammatory and hair diseases. Dermatol Clin. 2016;34:487-496.
- Manfredini M, Bettoli V, Sacripanti G, et al. The evolution of healthy skin to acne lesions: a longitudinal, in vivo evaluation with reflectance confocal microscopy and optical coherence tomography [published online April 26, 2019]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.15641.
- Navarrete-Dechent C, Mori S, Cordova M, et al. Reflectance confocal microscopy as a novel tool for presurgical identification of basal cell carcinoma biopsy site. J Am Acad Dermatol. 2019;80:e7-e8.
- Pan ZY, Lin JR, Cheng TT, et al. In vivo reflectance confocal microscopy of basal cell carcinoma: feasibility of preoperative mapping of cancer margins. Dermatol Surg. 2012;38:1945-1950.
- Venturini M, Gualdi G, Zanca A, et al. A new approach for presurgical margin assessment by reflectance confocal microscopy of basal cell carcinoma. Br J Dermatol. 2016;174:380-385.
- Sierra H, Yélamos O, Cordova M, et al. Reflectance confocal microscopy‐guided laser ablation of basal cell carcinomas: initial clinical experience. J Biomed Opt. 2017;22:1-13.
- Maier T, Kulichova D, Ruzicka T, et al. Noninvasive monitoring of basal cell carcinomas treated with systemic hedgehog inhibitors: pseudocysts as a sign of tumor regression. J Am Acad Dermatol. 2014;71:725-730.
- Levine A, Wang K, Markowitz O. Optical coherence tomography in the diagnosis of skin cancer. Dermatol Clin. 2017;35:465-488.
- Schneider SL, Kohli I, Hamzavi IH, et al. Emerging imaging technologies in dermatology: part I: basic principles. J Am Acad Dermatol. 2019;80:1114-1120.
- Mogensen M, Joergensen TM, Nümberg BM, et al. Assessment of optical coherence tomography imaging in the diagnosis of non‐melanoma skin cancer and benign lesions versus normal skin: observer‐blinded evaluation by dermatologists and pathologists. Dermatol Surg. 2009;35:965-972.
- Ferrante di Ruffano L, Dinnes J, Deeks JJ, et al. Optical coherence tomography for diagnosing skin cancer in adults. Cochrane Database Syst Rev. 2018;12:CD013189.
- Ulrich M, von Braunmuehl T, Kurzen H, et al. The sensitivity and specificity of optical coherence tomography for the assisted diagnosis of nonpigmented basal cell carcinoma: an observational study. Br J Dermatol. 2015;173:428-435.
- Wessels R, de Bruin DM, Relyveld GM, et al. Functional optical coherence tomography of pigmented lesions. J Eur Acad Dermatol Venereol. 2015;29:738‐744.
- Gambichler T, Schmid-Wendtner MH, Plura I, et al. A multicentre pilot study investigating high‐definition optical coherence tomography in the differentiation of cutaneous melanoma and melanocytic naevi. J Eur Acad Dermatol Venereol. 2015;29:537‐541.
- Marneffe A, Suppa M, Miyamoto M, et al. Validation of a diagnostic algorithm for the discrimination of actinic keratosis from normal skin and squamous cell carcinoma by means of high-definition optical coherence tomography. Exp Dermatol. 2016;25:684-687.
- Boone MA, Suppa M, Dhaenens F, et al. In vivo assessment of optical properties of melanocytic skin lesions and differentiation of melanoma from non-malignant lesions by high-definition optical coherence tomography. Arch Dermatol Res. 2016;308:7-20.
- Boone MA, Suppa M, Marneffe A, et al. A new algorithm for the discrimination of actinic keratosis from normal skin and squamous cell carcinoma based on in vivo analysis of optical properties by high-definition optical coherence tomography. J Eur Acad Dermatol Venereol. 2016;30:1714-1725.
- Themstrup L, Pellacani G, Welzel J, et al. In vivo microvascular imaging of cutaneous actinic keratosis, Bowen’s disease and squamous cell carcinoma using dynamic optical coherence tomography. J Eur Acad Dermatol Venereol. 2017;31:1655-1662.
- Alex A, Weingast J, Weinigel M, et al. Three-dimensional multiphoton/optical coherence tomography for diagnostic applications in dermatology. J Biophotonics. 2013;6:352-362.
- Iftimia N, Yélamos O, Chen CJ, et al. Handheld optical coherence tomography-reflectance confocal microscopy probe for detection of basal cell carcinoma and delineation of margins. J Biomed Opt. 2017;22:76006.
- Wang KX, Meekings A, Fluhr JW, et al. Optical coherence tomography-based optimization of Mohs micrographic surgery of basal cell carcinoma: a pilot study. Dermatol Surg. 2013;39:627-633.
- Chan CS, Rohrer TE. Optical coherence tomography and its role in Mohs micrographic surgery: a case report. Case Rep Dermatol. 2012;4:269-274.
- Gambichler T, Jaedicke V, Terras S. Optical coherence tomography in dermatology: technical and clinical aspects. Arch Dermatol Res. 2011;303:457-473.
- Manfredini M, Greco M, Farnetani F, et al. Acne: morphologic and vascular study of lesions and surrounding skin by means of optical coherence tomography. J Eur Acad Dermatol Venereol. 2017;31:1541-1546.
- Benvenuto-Andrade C, Dusza SW, Agero AL, et al. Differences between polarized light dermoscopy and immersion contact dermoscopy for the evaluation of skin lesions. Arch Dermatol. 2007;143:329-338.
- Terushkin V, Oliveria SA, Marghoob AA, et al. Use of and beliefs about total body photography and dermatoscopy among US dermatology training programs: an update. J Am Acad Dermatol. 2010;62:794-803.
- Morris JB, Alfonso SV, Hernandez N, et al. Use of and intentions to use dermoscopy among physicians in the United States. Dermatol Pract Concept. 2017;7:7-16.
- Yélamos O, Braun RP, Liopyris K, et al. Dermoscopy and dermatopathology correlates of cutaneous neoplasms. J Am Acad Dermatol. 2019;80:341-363.
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676.
- Carli P, de Giorgi V, Chiarugi A, et al. Addition of dermoscopy to conventional naked-eye examination in melanoma screening: a randomized study. J Am Acad Dermatol. 2004;50:683-668.
- Lallas A, Zalaudek I, Argenziano G, et al. Dermoscopy in general dermatology. Dermatol Clin. 2013;31:679-694.
- Reiter O, Mimouni I, Gdalvevich M, et al. The diagnostic accuracy of dermoscopy for basal cell carcinoma: a systematic review and meta-analysis. J Am Acad Dermatol. 2019;80:1380-1388.
- Papageorgiou V, Apalla Z, Sotiriou E, et al. The limitations of dermoscopy: false-positive and false-negative tumours. J Eur Acad Dermatol Venereol. 2018;32:879-888.
- Micali G, Verzì AE, Lacarrubba F. Alternative uses of dermoscopy in daily clinical practice: an update. J Am Acad Dermatol. 2018;79:1117-1132.e1.
- Rajadhyaksha M, Grossman M, Esterowitz D, et al. In vivo confocal scanning laser microscopy of human skin: melanin provides strong contrast. J Invest Dermatol. 1995;104:946-952.
- Kose K, Gou M, Yélamos O, et al. Automated video-mosaicking approach for confocal microscopic imaging in vivo: an approach to address challenges in imaging living tissue and extend field of view. Sci Rep. 2017;7:10759.
- Rao BK, John AM, Francisco G, et al. Diagnostic accuracy of reflectance confocal microscopy for diagnosis of skin lesions [published online October 8, 2018]. Arch Pathol Lab Med. 2019;143:326-329.
- Current Procedural Terminology, Professional Edition. Chicago IL: American Medical Association; 2016. The preliminary physician fee schedule for 2017 is available at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/PFS-Federal-Regulation-Notices-Items/CMS-1654-P.html.
- Jain M, Pulijal SV, Rajadhyaksha M, et al. Evaluation of bedside diagnostic accuracy, learning curve, and challenges for a novice reflectance confocal microscopy reader for skin cancer detection in vivo. JAMA Dermatol. 2018;154:962-965.
- Rao BK, Pellacani G. Atlas of Confocal Microscopy in Dermatology: Clinical, Confocal, and Histological Images. New York, NY: NIDIskin LLC; 2013.
- Scope A, Benvenuto-Andrande C, Agero AL, et al. In vivo reflectance confocal microscopy imaging of melanocytic skin lesions: consensus terminology glossary and illustrative images. J Am Acad Dermatol. 2007;57:644-658.
- Gerger A, Hofmann-Wellenhof R, Langsenlehner U, et al. In vivo confocal laser scanning microscopy of melanocytic skin tumours: diagnostic applicability using unselected tumour images. Br J Dermatol. 2008;158:329-333.
- Stevenson AD, Mickan S, Mallett S, et al. Systematic review of diagnostic accuracy of reflectance confocal microscopy for melanoma diagnosis in patients with clinically equivocal skin lesions. Dermatol Pract Concept. 2013;3:19-27.
- Alarcon I, Carrera C, Palou J, et al. Impact of in vivo reflectance confocal microscopy on the number needed to treat melanoma in doubtful lesions. Br J Dermatol. 2014;170:802-808.
- Lovatto L, Carrera C, Salerni G, et al. In vivo reflectance confocal microscopy of equivocal melanocytic lesions detected by digital dermoscopy follow-up. J Eur Acad Dermatol Venereol. 2015;29:1918-1925.
- Guitera P, Pellacani G, Longo C, et al. In vivo reflectance confocal microscopy enhances secondary evaluation of melanocytic lesions. J Invest Dermatol. 2009;129:131-138.
- Xiong YQ, Ma SJ, Mo Y, et al. Comparison of dermoscopy and reflectance confocal microscopy for the diagnosis of malignant skin tumours: a meta-analysis. J Cancer Res Clin Oncol. 2017;143:1627-1635.
- Kadouch DJ, Schram ME, Leeflang MM, et al. In vivo confocal microscopy of basal cell carcinoma: a systematic review of diagnostic accuracy. J Eur Acad Dermatol Venereol. 2015;29:1890-1897.
- Dinnes J, Deeks JJ, Chuchu N, et al; Cochrane Skin Cancer Diagnostic Test Accuracy Group. Reflectance confocal microscopy for diagnosing keratinocyte skin cancers in adults. Cochrane Database Syst Rev. 2018;12:CD013191.
- Nguyen KP, Peppelman M, Hoogedoorn L, et al. The current role of in vivo reflectance confocal microscopy within the continuum of actinic keratosis and squamous cell carcinoma: a systematic review. Eur J Dermatol. 2016;26:549-565.
- Pellacani G, Ulrich M, Casari A, et al. Grading keratinocyte atypia in actinic keratosis: a correlation of reflectance confocal microscopy and histopathology. J Eur Acad Dermatol Venereol. 2015;29:2216-2221.
- Manfredini M, Longo C, Ferrari B, et al. Dermoscopic and reflectance confocal microscopy features of cutaneous squamous cell carcinoma. J Eur Acad Dermatol Venereol. 2017;31:1828-1833.
- Hoogedoorn L, Peppelman M, van de Kerkhof PC, et al. The value of in vivo reflectance confocal microscopy in the diagnosis and monitoring of inflammatory and infectious skin diseases: a systematic review. Br J Dermatol. 2015;172:1222-1248.
- Cinotti E, Perrot JL, Labeille B, et al. Reflectance confocal microscopy for cutaneous infections and infestations. J Eur Acad Dermatol Venereol. 2016;30:754-763.
- Ardigo M, Longo C, Gonzalez S; International Confocal Working Group Inflammatory Skin Diseases Project. Multicentre study on inflammatory skin diseases from The International Confocal Working Group: specific confocal microscopy features and an algorithmic method of diagnosis. Br J Dermatol. 2016;175:364-374.
- Ardigo M, Agozzino M, Franceschini C, et al. Reflectance confocal microscopy algorithms for inflammatory and hair diseases. Dermatol Clin. 2016;34:487-496.
- Manfredini M, Bettoli V, Sacripanti G, et al. The evolution of healthy skin to acne lesions: a longitudinal, in vivo evaluation with reflectance confocal microscopy and optical coherence tomography [published online April 26, 2019]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.15641.
- Navarrete-Dechent C, Mori S, Cordova M, et al. Reflectance confocal microscopy as a novel tool for presurgical identification of basal cell carcinoma biopsy site. J Am Acad Dermatol. 2019;80:e7-e8.
- Pan ZY, Lin JR, Cheng TT, et al. In vivo reflectance confocal microscopy of basal cell carcinoma: feasibility of preoperative mapping of cancer margins. Dermatol Surg. 2012;38:1945-1950.
- Venturini M, Gualdi G, Zanca A, et al. A new approach for presurgical margin assessment by reflectance confocal microscopy of basal cell carcinoma. Br J Dermatol. 2016;174:380-385.
- Sierra H, Yélamos O, Cordova M, et al. Reflectance confocal microscopy‐guided laser ablation of basal cell carcinomas: initial clinical experience. J Biomed Opt. 2017;22:1-13.
- Maier T, Kulichova D, Ruzicka T, et al. Noninvasive monitoring of basal cell carcinomas treated with systemic hedgehog inhibitors: pseudocysts as a sign of tumor regression. J Am Acad Dermatol. 2014;71:725-730.
- Levine A, Wang K, Markowitz O. Optical coherence tomography in the diagnosis of skin cancer. Dermatol Clin. 2017;35:465-488.
- Schneider SL, Kohli I, Hamzavi IH, et al. Emerging imaging technologies in dermatology: part I: basic principles. J Am Acad Dermatol. 2019;80:1114-1120.
- Mogensen M, Joergensen TM, Nümberg BM, et al. Assessment of optical coherence tomography imaging in the diagnosis of non‐melanoma skin cancer and benign lesions versus normal skin: observer‐blinded evaluation by dermatologists and pathologists. Dermatol Surg. 2009;35:965-972.
- Ferrante di Ruffano L, Dinnes J, Deeks JJ, et al. Optical coherence tomography for diagnosing skin cancer in adults. Cochrane Database Syst Rev. 2018;12:CD013189.
- Ulrich M, von Braunmuehl T, Kurzen H, et al. The sensitivity and specificity of optical coherence tomography for the assisted diagnosis of nonpigmented basal cell carcinoma: an observational study. Br J Dermatol. 2015;173:428-435.
- Wessels R, de Bruin DM, Relyveld GM, et al. Functional optical coherence tomography of pigmented lesions. J Eur Acad Dermatol Venereol. 2015;29:738‐744.
- Gambichler T, Schmid-Wendtner MH, Plura I, et al. A multicentre pilot study investigating high‐definition optical coherence tomography in the differentiation of cutaneous melanoma and melanocytic naevi. J Eur Acad Dermatol Venereol. 2015;29:537‐541.
- Marneffe A, Suppa M, Miyamoto M, et al. Validation of a diagnostic algorithm for the discrimination of actinic keratosis from normal skin and squamous cell carcinoma by means of high-definition optical coherence tomography. Exp Dermatol. 2016;25:684-687.
- Boone MA, Suppa M, Dhaenens F, et al. In vivo assessment of optical properties of melanocytic skin lesions and differentiation of melanoma from non-malignant lesions by high-definition optical coherence tomography. Arch Dermatol Res. 2016;308:7-20.
- Boone MA, Suppa M, Marneffe A, et al. A new algorithm for the discrimination of actinic keratosis from normal skin and squamous cell carcinoma based on in vivo analysis of optical properties by high-definition optical coherence tomography. J Eur Acad Dermatol Venereol. 2016;30:1714-1725.
- Themstrup L, Pellacani G, Welzel J, et al. In vivo microvascular imaging of cutaneous actinic keratosis, Bowen’s disease and squamous cell carcinoma using dynamic optical coherence tomography. J Eur Acad Dermatol Venereol. 2017;31:1655-1662.
- Alex A, Weingast J, Weinigel M, et al. Three-dimensional multiphoton/optical coherence tomography for diagnostic applications in dermatology. J Biophotonics. 2013;6:352-362.
- Iftimia N, Yélamos O, Chen CJ, et al. Handheld optical coherence tomography-reflectance confocal microscopy probe for detection of basal cell carcinoma and delineation of margins. J Biomed Opt. 2017;22:76006.
- Wang KX, Meekings A, Fluhr JW, et al. Optical coherence tomography-based optimization of Mohs micrographic surgery of basal cell carcinoma: a pilot study. Dermatol Surg. 2013;39:627-633.
- Chan CS, Rohrer TE. Optical coherence tomography and its role in Mohs micrographic surgery: a case report. Case Rep Dermatol. 2012;4:269-274.
- Gambichler T, Jaedicke V, Terras S. Optical coherence tomography in dermatology: technical and clinical aspects. Arch Dermatol Res. 2011;303:457-473.
- Manfredini M, Greco M, Farnetani F, et al. Acne: morphologic and vascular study of lesions and surrounding skin by means of optical coherence tomography. J Eur Acad Dermatol Venereol. 2017;31:1541-1546.
Practice Points
- There are several new noninvasive imaging tools in dermatology that can be utilized to aid in the diagnosis and management of skin disease, including dermoscopy, reflectance confocal microscopy, and optical coherence tomography.
- Among these tools, there are several differences in cost, clinical integration, reimbursement, and accuracy.
Back to the Future: Integrating Technology to Improve Patient-Provider Interactions
The advent of electronic medical records (EMRs) is arguably the most important technological revolution in modern medicine. The transition from paper documentation to EMRs has improved organization of medical records, consolidating all physician notes, orders, consultations, laboratory test results, and radiologic studies into a single accessible location.1 However, this revolution has led to mixed consequences for patients, especially in the outpatient setting. The use of EMRs can facilitate questions, clarification, and discussion between patients and health care providers, prompted by the sections of the EMR. Unfortunately, patients too often encounter pressed-for-time, documentation-focused providers who may not even look up from the computer. Provider behaviors such as making eye contact, stopping typing during discussion of sensitive topics, and allowing patients to view the computer screen and using it as an educational tool are important for patients to have a positive care experience.2 We envision further integration of current and future technology to overcome the challenges of outpatient care. We use a hypothetical patient encounter to illustrate what the future may hold.
Hypothetical Patient Encounter
An established patient, Ms. PS, comes to the dermatology clinic for a follow-up appointment and walks into an examination room (Figure). Prior to entering the room, the provider, Dr. FT, reviews Ms. PS’s history via a dermatology-specific EMR and reads that Ms. PS has a 1.5-year history of psoriasis and is considering other therapeutic options.
Upon entering the room, Dr. FT tells Ms. PS that the visit is being recorded and transcribed. A large interactive screen is a key component of the examination room. A remote medical assistant is virtually present via video to transcribe and document the patient-provider interaction. There is potential for artificial intelligence to replace the remote medical assistant in the future. Wearable technology, including a smartwatch and Bluetooth headphones, allow the provider to record audio of the visit as well as through microphones on the interactive screen.
As the interaction begins, Ms. PS reports that her psoriasis is poorly controlled with her current regimen of topical steroids. Dr. FT inquires about Ms. PS’s current symptoms and psychosocial well-being. Dr. FT then performs a skin examination and is easily able to evaluate her skin vs prior visits, as clinical images from prior visits are automatically displayed on the interactive screen. Dr. FT also closely examines Ms. PS’s nails and conducts a joint examination, reminded by a notification on his wearable technology. After capturing clinical images of Ms. PS’s skin and nails with a secure EMR-connected tablet, Dr. FT briefly steps out of the room to allow Ms. PS to get dressed and feel more comfortable in the discussion to follow.
Once he reenters the examination room, Dr. FT initiates a discussion on next steps. Ms. PS’s pathology report and clinical images are displayed on the interactive screen, along with her most recent laboratory test results, which were completed prior to the visit in anticipation of changing therapies. Dr. FT presents Ms. PS with several evidence-based therapeutic options for psoriasis, and she expresses interest in methotrexate. Following the discussion, the remote medical assistant displays information about methotrexate on the interactive screen, including evidence for treatment of psoriasis, contraindications, laboratory monitoring requirements, and possible adverse effects for both the patient and provider to review together. Dr. FT reviews the laboratory test results displayed on the screen, specifically her transaminase levels, and confirms that methotrexate is an appropriate therapeutic option. After a full discussion of risks and benefits, Ms. PS chooses to initiate methotrexate treatment. Reminded by a notification on his wearable technology, Dr. FT follows evidence-based dosing guidelines and sends the prescription electronically to Ms. PS’s pharmacy, which concludes Ms. PS’s visit.
Analysis of the Patient Encounter
In this interaction, Dr. FT was able to fully engage with the patient, unencumbered by the demands of documentation. There were only a few instances when the provider looked at or touched the interactive screen. Furthermore, joint decision-making was optimized by allowing both the patient and provider to review diagnostic test results and current evidence-based therapeutic guidelines together through the interactive screen. Ms. PS goes home feeling satisfied that she received her provider’s complete attention and that they selected a therapeutic option supported by evidence. After the visit, the remote medial assistant’s transcript populates a patient note template, which Dr. FT reviews and amends to create the final note. Reducing the time required to write patient notes increases the speed at which Dr. FT can complete patient encounters and may improve clinic flow and productivity. In addition, a patient summary is generated from Dr. FT’s final note, with an emphasis on patient instructions, and is sent to Ms. PS.
Final Thoughts
Our proposed integration of currently available and future technology can help minimize documentation burdens on providers and improve patient-provider communication in the age of the EMR, thus optimizing patient satisfaction and outcomes.
- Evans RS. Electronic health records: then, now, and in the future. Yearb Med Inform. 2016;(suppl 1):S48-S61.
- Alkureishi MA, Lee WW, Lyons M, et al. Impact of electronic medical record use on the patient-doctor relationship and communication: a systematic review. J Gen Intern Med. 2016;31:548-560.
The advent of electronic medical records (EMRs) is arguably the most important technological revolution in modern medicine. The transition from paper documentation to EMRs has improved organization of medical records, consolidating all physician notes, orders, consultations, laboratory test results, and radiologic studies into a single accessible location.1 However, this revolution has led to mixed consequences for patients, especially in the outpatient setting. The use of EMRs can facilitate questions, clarification, and discussion between patients and health care providers, prompted by the sections of the EMR. Unfortunately, patients too often encounter pressed-for-time, documentation-focused providers who may not even look up from the computer. Provider behaviors such as making eye contact, stopping typing during discussion of sensitive topics, and allowing patients to view the computer screen and using it as an educational tool are important for patients to have a positive care experience.2 We envision further integration of current and future technology to overcome the challenges of outpatient care. We use a hypothetical patient encounter to illustrate what the future may hold.
Hypothetical Patient Encounter
An established patient, Ms. PS, comes to the dermatology clinic for a follow-up appointment and walks into an examination room (Figure). Prior to entering the room, the provider, Dr. FT, reviews Ms. PS’s history via a dermatology-specific EMR and reads that Ms. PS has a 1.5-year history of psoriasis and is considering other therapeutic options.

Upon entering the room, Dr. FT tells Ms. PS that the visit is being recorded and transcribed. A large interactive screen is a key component of the examination room. A remote medical assistant is virtually present via video to transcribe and document the patient-provider interaction. There is potential for artificial intelligence to replace the remote medical assistant in the future. Wearable technology, including a smartwatch and Bluetooth headphones, allow the provider to record audio of the visit as well as through microphones on the interactive screen.
As the interaction begins, Ms. PS reports that her psoriasis is poorly controlled with her current regimen of topical steroids. Dr. FT inquires about Ms. PS’s current symptoms and psychosocial well-being. Dr. FT then performs a skin examination and is easily able to evaluate her skin vs prior visits, as clinical images from prior visits are automatically displayed on the interactive screen. Dr. FT also closely examines Ms. PS’s nails and conducts a joint examination, reminded by a notification on his wearable technology. After capturing clinical images of Ms. PS’s skin and nails with a secure EMR-connected tablet, Dr. FT briefly steps out of the room to allow Ms. PS to get dressed and feel more comfortable in the discussion to follow.
Once he reenters the examination room, Dr. FT initiates a discussion on next steps. Ms. PS’s pathology report and clinical images are displayed on the interactive screen, along with her most recent laboratory test results, which were completed prior to the visit in anticipation of changing therapies. Dr. FT presents Ms. PS with several evidence-based therapeutic options for psoriasis, and she expresses interest in methotrexate. Following the discussion, the remote medical assistant displays information about methotrexate on the interactive screen, including evidence for treatment of psoriasis, contraindications, laboratory monitoring requirements, and possible adverse effects for both the patient and provider to review together. Dr. FT reviews the laboratory test results displayed on the screen, specifically her transaminase levels, and confirms that methotrexate is an appropriate therapeutic option. After a full discussion of risks and benefits, Ms. PS chooses to initiate methotrexate treatment. Reminded by a notification on his wearable technology, Dr. FT follows evidence-based dosing guidelines and sends the prescription electronically to Ms. PS’s pharmacy, which concludes Ms. PS’s visit.
Analysis of the Patient Encounter
In this interaction, Dr. FT was able to fully engage with the patient, unencumbered by the demands of documentation. There were only a few instances when the provider looked at or touched the interactive screen. Furthermore, joint decision-making was optimized by allowing both the patient and provider to review diagnostic test results and current evidence-based therapeutic guidelines together through the interactive screen. Ms. PS goes home feeling satisfied that she received her provider’s complete attention and that they selected a therapeutic option supported by evidence. After the visit, the remote medial assistant’s transcript populates a patient note template, which Dr. FT reviews and amends to create the final note. Reducing the time required to write patient notes increases the speed at which Dr. FT can complete patient encounters and may improve clinic flow and productivity. In addition, a patient summary is generated from Dr. FT’s final note, with an emphasis on patient instructions, and is sent to Ms. PS.
Final Thoughts
Our proposed integration of currently available and future technology can help minimize documentation burdens on providers and improve patient-provider communication in the age of the EMR, thus optimizing patient satisfaction and outcomes.
The advent of electronic medical records (EMRs) is arguably the most important technological revolution in modern medicine. The transition from paper documentation to EMRs has improved organization of medical records, consolidating all physician notes, orders, consultations, laboratory test results, and radiologic studies into a single accessible location.1 However, this revolution has led to mixed consequences for patients, especially in the outpatient setting. The use of EMRs can facilitate questions, clarification, and discussion between patients and health care providers, prompted by the sections of the EMR. Unfortunately, patients too often encounter pressed-for-time, documentation-focused providers who may not even look up from the computer. Provider behaviors such as making eye contact, stopping typing during discussion of sensitive topics, and allowing patients to view the computer screen and using it as an educational tool are important for patients to have a positive care experience.2 We envision further integration of current and future technology to overcome the challenges of outpatient care. We use a hypothetical patient encounter to illustrate what the future may hold.
Hypothetical Patient Encounter
An established patient, Ms. PS, comes to the dermatology clinic for a follow-up appointment and walks into an examination room (Figure). Prior to entering the room, the provider, Dr. FT, reviews Ms. PS’s history via a dermatology-specific EMR and reads that Ms. PS has a 1.5-year history of psoriasis and is considering other therapeutic options.

Upon entering the room, Dr. FT tells Ms. PS that the visit is being recorded and transcribed. A large interactive screen is a key component of the examination room. A remote medical assistant is virtually present via video to transcribe and document the patient-provider interaction. There is potential for artificial intelligence to replace the remote medical assistant in the future. Wearable technology, including a smartwatch and Bluetooth headphones, allow the provider to record audio of the visit as well as through microphones on the interactive screen.
As the interaction begins, Ms. PS reports that her psoriasis is poorly controlled with her current regimen of topical steroids. Dr. FT inquires about Ms. PS’s current symptoms and psychosocial well-being. Dr. FT then performs a skin examination and is easily able to evaluate her skin vs prior visits, as clinical images from prior visits are automatically displayed on the interactive screen. Dr. FT also closely examines Ms. PS’s nails and conducts a joint examination, reminded by a notification on his wearable technology. After capturing clinical images of Ms. PS’s skin and nails with a secure EMR-connected tablet, Dr. FT briefly steps out of the room to allow Ms. PS to get dressed and feel more comfortable in the discussion to follow.
Once he reenters the examination room, Dr. FT initiates a discussion on next steps. Ms. PS’s pathology report and clinical images are displayed on the interactive screen, along with her most recent laboratory test results, which were completed prior to the visit in anticipation of changing therapies. Dr. FT presents Ms. PS with several evidence-based therapeutic options for psoriasis, and she expresses interest in methotrexate. Following the discussion, the remote medical assistant displays information about methotrexate on the interactive screen, including evidence for treatment of psoriasis, contraindications, laboratory monitoring requirements, and possible adverse effects for both the patient and provider to review together. Dr. FT reviews the laboratory test results displayed on the screen, specifically her transaminase levels, and confirms that methotrexate is an appropriate therapeutic option. After a full discussion of risks and benefits, Ms. PS chooses to initiate methotrexate treatment. Reminded by a notification on his wearable technology, Dr. FT follows evidence-based dosing guidelines and sends the prescription electronically to Ms. PS’s pharmacy, which concludes Ms. PS’s visit.
Analysis of the Patient Encounter
In this interaction, Dr. FT was able to fully engage with the patient, unencumbered by the demands of documentation. There were only a few instances when the provider looked at or touched the interactive screen. Furthermore, joint decision-making was optimized by allowing both the patient and provider to review diagnostic test results and current evidence-based therapeutic guidelines together through the interactive screen. Ms. PS goes home feeling satisfied that she received her provider’s complete attention and that they selected a therapeutic option supported by evidence. After the visit, the remote medial assistant’s transcript populates a patient note template, which Dr. FT reviews and amends to create the final note. Reducing the time required to write patient notes increases the speed at which Dr. FT can complete patient encounters and may improve clinic flow and productivity. In addition, a patient summary is generated from Dr. FT’s final note, with an emphasis on patient instructions, and is sent to Ms. PS.
Final Thoughts
Our proposed integration of currently available and future technology can help minimize documentation burdens on providers and improve patient-provider communication in the age of the EMR, thus optimizing patient satisfaction and outcomes.
- Evans RS. Electronic health records: then, now, and in the future. Yearb Med Inform. 2016;(suppl 1):S48-S61.
- Alkureishi MA, Lee WW, Lyons M, et al. Impact of electronic medical record use on the patient-doctor relationship and communication: a systematic review. J Gen Intern Med. 2016;31:548-560.
- Evans RS. Electronic health records: then, now, and in the future. Yearb Med Inform. 2016;(suppl 1):S48-S61.
- Alkureishi MA, Lee WW, Lyons M, et al. Impact of electronic medical record use on the patient-doctor relationship and communication: a systematic review. J Gen Intern Med. 2016;31:548-560.
Practice Points
- Electronic medical records afford many benefits, but documentation burdens on health care providers can impede positive patient-provider interactions.
- Integration of current and future technology can shift the focus back to the patient and facilitate shared decision-making.
The Dayanara Effect: Increasing Skin Cancer Awareness in the Hispanic Community
In February 2019, Dayanara Torres announced that she had been diagnosed with metastatic melanoma. Ms. Torres, a Puerto Rican–born former Miss Universe who has more than 1 million followers on Instagram (@dayanarapr), seemed an unlikely candidate for skin cancer, which often is associated with fair-skinned and light-eyed individuals. She shared the news of her diagnosis in an Instagram video that has now received more than 850,000 views. In the video, Ms. Torres described a new mole with uneven surface that had developed on her leg and noted that she had ignored it, even though it had been growing for years. Ultimately, she was diagnosed with melanoma that had already metastasized to regional lymph nodes in her leg. Ms. Torres concluded the video by urging fans and viewers to be mindful of new or changing skin lesions and to be aware of the seriousness of skin cancer. In March 2019, Ms. Torres posted a follow-up educational video on Instagram highlighting the features of melanoma that has now received more than 300,000 views.
Since her announcement, we have noticed that more Hispanic patients with concerns about skin cancer are presenting to our dermatology clinic, which is located in a highly diverse city (New Brunswick, New Jersey) with approximately 50% of residents identifying as Hispanic.1 Most Hispanic patients typically present to our dermatology clinic for non–skin cancer–related concerns, such as acne, rash, and dyschromia; however, following Ms. Torres’ announcement, many have cited her diagnosis of metastatic melanoma as a cause for concern and a motivating factor in having their skin examined. The diagnosis in a prominent celebrity and Hispanic woman has given a new face to metastatic melanoma.
Although melanoma most commonly occurs in white patients, Hispanic patients experience disproportionately greater morbidity and mortality when diagnosed with melanoma.2 Poor prognosis in patients with skin of color is multifactorial and may be due to poor use of sun protection, misconceptions about melanoma risk, atypical clinical presentation, impaired access to care, and delay in diagnosis. The Hispanic community encompasses a wide variety of individuals with varying levels of skin pigmentation and sun sensitivity.3 However, Hispanics report low levels of sun-protective behaviors. They also may have misconceptions that sunscreen is ineffective in preventing skin cancer and that little can be done to decrease the risk for developing skin cancer.4,5 Additionally, Hispanic patients often have lower perceptions of their personal risk for melanoma and report low rates of clinical and self-examinations compared to non-Hispanic white patients.6-8 Many Hispanic patients have reported that they were not instructed to perform self-examinations of their skin regularly by dermatologists or other providers and did not know the signs of skin cancer.7 Furthermore, a language barrier also may impede communication and education regarding melanoma risk.9
Similar to white patients, superficial spreading melanoma is the most common histologic subtype in Hispanic patients, followed by acral lentiginous melanoma, which is the most common subtype in black and Asian patients.2,4 Compared to non-Hispanic white patients, who most commonly present with truncal melanomas, Hispanic patients (particularly those from Puerto Rico, such as Ms. Torres) are more likely to present with melanoma on the lower extremities.4,10 Additionally, Hispanic patients have high rates of head, neck, and mucosal melanomas compared to all other racial and ethnic groups.2
Hispanic patients diagnosed with melanoma are more likely to present with thicker primary tumors, later stages of disease, and distant metastases compared to non-Hispanic white patients, all of which are associated with poor prognosis.2,4,11 Five-year survival rates for melanoma are lower in Hispanic patients compared to non-Hispanic white patients.12 Although the Hispanic community is diverse in socioeconomic and immigration status as well as occupation, lack of insurance also may contribute to decreased access to care, delayed diagnosis, and ultimately worse survival.
These disparities have spurred suggestions for increased education about skin cancer and the signs and symptoms of melanoma, encouragement of self-examinations, and routine clinical skin examinations for Hispanic patients by dermatologists and other providers.8 There is evidence that knowledge-based interventions, especially when presented in Spanish, produce statistically significant improvements in knowledge of skin cancer risk and sun-protective behavior among Hispanic patients.12 Similarly, we have observed that the videos shared by Ms. Torres regarding her melanoma diagnosis and the features of melanoma, in which she spoke in Spanish, have compelled many Hispanic patients to examine their own skin and have led to increased concern for skin cancer in this patient population. In our practice, we refer to the increase in spot checks and skin examinations requested by Hispanic patients as “The Dayanara Effect,” and we hypothesize that this same effect may be taking place throughout the dermatology community.
- New Brunswick, NJ. Data USA website. https://datausa.io/profile/geo/new-brunswick-nj. Accessed April 17, 2019.
- Higgins S, Nazemi A, Feinstein S, et al. Clinical presentations of melanoma in African Americans, Hispanics, and Asians [published online January 4, 2019]. Dermatol Surg. doi:10.1097/dss.0000000000001759.
- Robinson JK, Penedo FJ, Hay JL, et al. Recognizing Latinos’ range of skin pigment and phototypes to enhance skin cancer prevention [published online July 4, 2017]. Pigment Cell Melanoma Res. 2017;30:488-492.
- Garnett E, Townsend J, Steele B, et al. Characteristics, rates, and trends of melanoma incidence among Hispanics in the USA. Cancer Causes Control. 2016;27:647-659.
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762.
- Andreeva VA, Cockburn MG. Cutaneous melanoma and other skin cancer screening among Hispanics in the United States: a review of the evidence, disparities, and need for expanding the intervention and research agendas. Arch Dermatol. 2011;147:743-745.
- Roman C, Lugo-Somolinos A, Thomas N. Skin cancer knowledge and skin self-examinations in the Hispanic population of North Carolina: the patient’s perspective. JAMA Dermatol. 2013;149:103-104.
- Jaimes N, Oliveria S, Halpern A. A cautionary note on melanoma screening in the Hispanic/Latino population. JAMA Dermatol. 2013;149:396-397.
- Wich LG, Ma MW, Price LS, et al. Impact of socioeconomic status and sociodemographic factors on melanoma presentation among ethnic minorities. J Community Health. 2011;36:461-468.
- Rouhani P, Hu S, Kirsner RS. Melanoma in Hispanic and black Americans. Cancer Control. 2008;15:248-253.
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991.
- Kailas A, Botwin AL, Pritchett EN, et al. Assessing the effectiveness of knowledge-based interventions in increasing skin cancer awareness, knowledge, and protective behaviors in skin of color populations. Cutis. 2017;100:235-240.
In February 2019, Dayanara Torres announced that she had been diagnosed with metastatic melanoma. Ms. Torres, a Puerto Rican–born former Miss Universe who has more than 1 million followers on Instagram (@dayanarapr), seemed an unlikely candidate for skin cancer, which often is associated with fair-skinned and light-eyed individuals. She shared the news of her diagnosis in an Instagram video that has now received more than 850,000 views. In the video, Ms. Torres described a new mole with uneven surface that had developed on her leg and noted that she had ignored it, even though it had been growing for years. Ultimately, she was diagnosed with melanoma that had already metastasized to regional lymph nodes in her leg. Ms. Torres concluded the video by urging fans and viewers to be mindful of new or changing skin lesions and to be aware of the seriousness of skin cancer. In March 2019, Ms. Torres posted a follow-up educational video on Instagram highlighting the features of melanoma that has now received more than 300,000 views.
Since her announcement, we have noticed that more Hispanic patients with concerns about skin cancer are presenting to our dermatology clinic, which is located in a highly diverse city (New Brunswick, New Jersey) with approximately 50% of residents identifying as Hispanic.1 Most Hispanic patients typically present to our dermatology clinic for non–skin cancer–related concerns, such as acne, rash, and dyschromia; however, following Ms. Torres’ announcement, many have cited her diagnosis of metastatic melanoma as a cause for concern and a motivating factor in having their skin examined. The diagnosis in a prominent celebrity and Hispanic woman has given a new face to metastatic melanoma.
Although melanoma most commonly occurs in white patients, Hispanic patients experience disproportionately greater morbidity and mortality when diagnosed with melanoma.2 Poor prognosis in patients with skin of color is multifactorial and may be due to poor use of sun protection, misconceptions about melanoma risk, atypical clinical presentation, impaired access to care, and delay in diagnosis. The Hispanic community encompasses a wide variety of individuals with varying levels of skin pigmentation and sun sensitivity.3 However, Hispanics report low levels of sun-protective behaviors. They also may have misconceptions that sunscreen is ineffective in preventing skin cancer and that little can be done to decrease the risk for developing skin cancer.4,5 Additionally, Hispanic patients often have lower perceptions of their personal risk for melanoma and report low rates of clinical and self-examinations compared to non-Hispanic white patients.6-8 Many Hispanic patients have reported that they were not instructed to perform self-examinations of their skin regularly by dermatologists or other providers and did not know the signs of skin cancer.7 Furthermore, a language barrier also may impede communication and education regarding melanoma risk.9
Similar to white patients, superficial spreading melanoma is the most common histologic subtype in Hispanic patients, followed by acral lentiginous melanoma, which is the most common subtype in black and Asian patients.2,4 Compared to non-Hispanic white patients, who most commonly present with truncal melanomas, Hispanic patients (particularly those from Puerto Rico, such as Ms. Torres) are more likely to present with melanoma on the lower extremities.4,10 Additionally, Hispanic patients have high rates of head, neck, and mucosal melanomas compared to all other racial and ethnic groups.2
Hispanic patients diagnosed with melanoma are more likely to present with thicker primary tumors, later stages of disease, and distant metastases compared to non-Hispanic white patients, all of which are associated with poor prognosis.2,4,11 Five-year survival rates for melanoma are lower in Hispanic patients compared to non-Hispanic white patients.12 Although the Hispanic community is diverse in socioeconomic and immigration status as well as occupation, lack of insurance also may contribute to decreased access to care, delayed diagnosis, and ultimately worse survival.
These disparities have spurred suggestions for increased education about skin cancer and the signs and symptoms of melanoma, encouragement of self-examinations, and routine clinical skin examinations for Hispanic patients by dermatologists and other providers.8 There is evidence that knowledge-based interventions, especially when presented in Spanish, produce statistically significant improvements in knowledge of skin cancer risk and sun-protective behavior among Hispanic patients.12 Similarly, we have observed that the videos shared by Ms. Torres regarding her melanoma diagnosis and the features of melanoma, in which she spoke in Spanish, have compelled many Hispanic patients to examine their own skin and have led to increased concern for skin cancer in this patient population. In our practice, we refer to the increase in spot checks and skin examinations requested by Hispanic patients as “The Dayanara Effect,” and we hypothesize that this same effect may be taking place throughout the dermatology community.
In February 2019, Dayanara Torres announced that she had been diagnosed with metastatic melanoma. Ms. Torres, a Puerto Rican–born former Miss Universe who has more than 1 million followers on Instagram (@dayanarapr), seemed an unlikely candidate for skin cancer, which often is associated with fair-skinned and light-eyed individuals. She shared the news of her diagnosis in an Instagram video that has now received more than 850,000 views. In the video, Ms. Torres described a new mole with uneven surface that had developed on her leg and noted that she had ignored it, even though it had been growing for years. Ultimately, she was diagnosed with melanoma that had already metastasized to regional lymph nodes in her leg. Ms. Torres concluded the video by urging fans and viewers to be mindful of new or changing skin lesions and to be aware of the seriousness of skin cancer. In March 2019, Ms. Torres posted a follow-up educational video on Instagram highlighting the features of melanoma that has now received more than 300,000 views.
Since her announcement, we have noticed that more Hispanic patients with concerns about skin cancer are presenting to our dermatology clinic, which is located in a highly diverse city (New Brunswick, New Jersey) with approximately 50% of residents identifying as Hispanic.1 Most Hispanic patients typically present to our dermatology clinic for non–skin cancer–related concerns, such as acne, rash, and dyschromia; however, following Ms. Torres’ announcement, many have cited her diagnosis of metastatic melanoma as a cause for concern and a motivating factor in having their skin examined. The diagnosis in a prominent celebrity and Hispanic woman has given a new face to metastatic melanoma.
Although melanoma most commonly occurs in white patients, Hispanic patients experience disproportionately greater morbidity and mortality when diagnosed with melanoma.2 Poor prognosis in patients with skin of color is multifactorial and may be due to poor use of sun protection, misconceptions about melanoma risk, atypical clinical presentation, impaired access to care, and delay in diagnosis. The Hispanic community encompasses a wide variety of individuals with varying levels of skin pigmentation and sun sensitivity.3 However, Hispanics report low levels of sun-protective behaviors. They also may have misconceptions that sunscreen is ineffective in preventing skin cancer and that little can be done to decrease the risk for developing skin cancer.4,5 Additionally, Hispanic patients often have lower perceptions of their personal risk for melanoma and report low rates of clinical and self-examinations compared to non-Hispanic white patients.6-8 Many Hispanic patients have reported that they were not instructed to perform self-examinations of their skin regularly by dermatologists or other providers and did not know the signs of skin cancer.7 Furthermore, a language barrier also may impede communication and education regarding melanoma risk.9
Similar to white patients, superficial spreading melanoma is the most common histologic subtype in Hispanic patients, followed by acral lentiginous melanoma, which is the most common subtype in black and Asian patients.2,4 Compared to non-Hispanic white patients, who most commonly present with truncal melanomas, Hispanic patients (particularly those from Puerto Rico, such as Ms. Torres) are more likely to present with melanoma on the lower extremities.4,10 Additionally, Hispanic patients have high rates of head, neck, and mucosal melanomas compared to all other racial and ethnic groups.2
Hispanic patients diagnosed with melanoma are more likely to present with thicker primary tumors, later stages of disease, and distant metastases compared to non-Hispanic white patients, all of which are associated with poor prognosis.2,4,11 Five-year survival rates for melanoma are lower in Hispanic patients compared to non-Hispanic white patients.12 Although the Hispanic community is diverse in socioeconomic and immigration status as well as occupation, lack of insurance also may contribute to decreased access to care, delayed diagnosis, and ultimately worse survival.
These disparities have spurred suggestions for increased education about skin cancer and the signs and symptoms of melanoma, encouragement of self-examinations, and routine clinical skin examinations for Hispanic patients by dermatologists and other providers.8 There is evidence that knowledge-based interventions, especially when presented in Spanish, produce statistically significant improvements in knowledge of skin cancer risk and sun-protective behavior among Hispanic patients.12 Similarly, we have observed that the videos shared by Ms. Torres regarding her melanoma diagnosis and the features of melanoma, in which she spoke in Spanish, have compelled many Hispanic patients to examine their own skin and have led to increased concern for skin cancer in this patient population. In our practice, we refer to the increase in spot checks and skin examinations requested by Hispanic patients as “The Dayanara Effect,” and we hypothesize that this same effect may be taking place throughout the dermatology community.
- New Brunswick, NJ. Data USA website. https://datausa.io/profile/geo/new-brunswick-nj. Accessed April 17, 2019.
- Higgins S, Nazemi A, Feinstein S, et al. Clinical presentations of melanoma in African Americans, Hispanics, and Asians [published online January 4, 2019]. Dermatol Surg. doi:10.1097/dss.0000000000001759.
- Robinson JK, Penedo FJ, Hay JL, et al. Recognizing Latinos’ range of skin pigment and phototypes to enhance skin cancer prevention [published online July 4, 2017]. Pigment Cell Melanoma Res. 2017;30:488-492.
- Garnett E, Townsend J, Steele B, et al. Characteristics, rates, and trends of melanoma incidence among Hispanics in the USA. Cancer Causes Control. 2016;27:647-659.
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762.
- Andreeva VA, Cockburn MG. Cutaneous melanoma and other skin cancer screening among Hispanics in the United States: a review of the evidence, disparities, and need for expanding the intervention and research agendas. Arch Dermatol. 2011;147:743-745.
- Roman C, Lugo-Somolinos A, Thomas N. Skin cancer knowledge and skin self-examinations in the Hispanic population of North Carolina: the patient’s perspective. JAMA Dermatol. 2013;149:103-104.
- Jaimes N, Oliveria S, Halpern A. A cautionary note on melanoma screening in the Hispanic/Latino population. JAMA Dermatol. 2013;149:396-397.
- Wich LG, Ma MW, Price LS, et al. Impact of socioeconomic status and sociodemographic factors on melanoma presentation among ethnic minorities. J Community Health. 2011;36:461-468.
- Rouhani P, Hu S, Kirsner RS. Melanoma in Hispanic and black Americans. Cancer Control. 2008;15:248-253.
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991.
- Kailas A, Botwin AL, Pritchett EN, et al. Assessing the effectiveness of knowledge-based interventions in increasing skin cancer awareness, knowledge, and protective behaviors in skin of color populations. Cutis. 2017;100:235-240.
- New Brunswick, NJ. Data USA website. https://datausa.io/profile/geo/new-brunswick-nj. Accessed April 17, 2019.
- Higgins S, Nazemi A, Feinstein S, et al. Clinical presentations of melanoma in African Americans, Hispanics, and Asians [published online January 4, 2019]. Dermatol Surg. doi:10.1097/dss.0000000000001759.
- Robinson JK, Penedo FJ, Hay JL, et al. Recognizing Latinos’ range of skin pigment and phototypes to enhance skin cancer prevention [published online July 4, 2017]. Pigment Cell Melanoma Res. 2017;30:488-492.
- Garnett E, Townsend J, Steele B, et al. Characteristics, rates, and trends of melanoma incidence among Hispanics in the USA. Cancer Causes Control. 2016;27:647-659.
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762.
- Andreeva VA, Cockburn MG. Cutaneous melanoma and other skin cancer screening among Hispanics in the United States: a review of the evidence, disparities, and need for expanding the intervention and research agendas. Arch Dermatol. 2011;147:743-745.
- Roman C, Lugo-Somolinos A, Thomas N. Skin cancer knowledge and skin self-examinations in the Hispanic population of North Carolina: the patient’s perspective. JAMA Dermatol. 2013;149:103-104.
- Jaimes N, Oliveria S, Halpern A. A cautionary note on melanoma screening in the Hispanic/Latino population. JAMA Dermatol. 2013;149:396-397.
- Wich LG, Ma MW, Price LS, et al. Impact of socioeconomic status and sociodemographic factors on melanoma presentation among ethnic minorities. J Community Health. 2011;36:461-468.
- Rouhani P, Hu S, Kirsner RS. Melanoma in Hispanic and black Americans. Cancer Control. 2008;15:248-253.
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991.
- Kailas A, Botwin AL, Pritchett EN, et al. Assessing the effectiveness of knowledge-based interventions in increasing skin cancer awareness, knowledge, and protective behaviors in skin of color populations. Cutis. 2017;100:235-240.
Reflectance Confocal Microscopy as a First-Line Diagnostic Technique for Mycosis Fungoides
Case Report
A 60-year-old man with a history of Hodgkin lymphoma that had been treated with chemotherapy 6 years prior presented to our dermatology clinic with a persistent pruritic rash on the back, abdomen, and bilateral arms and legs. The eruption initially began as localized discrete lesions on the lower back 1 year prior to the current presentation; at that time a diagnosis of psoriasis was made at an outside dermatology clinic, and treatment with mometasone furoate cream was initiated. Despite the patient’s compliance with this treatment, the lesions did not resolve and began spreading to the arms, legs, chest, and abdomen. His current medications included lisinopril, escitalopram, aspirin, and omeprazole.
On presentation to our clinic, physical examination revealed round, scaly, pink plaques and tumors of variable sizes (3–10 cm) distributed asymmetrically on the chest, back, abdomen, arms, and legs (Figure 1). The lesions were grouped in well-defined areas encompassing approximately 30% of the body surface area. No lymphadenopathy was appreciated. In vivo reflectance confocal microscopy (RCM) performed on one of the lesions revealed disarray of the epidermis with small, weakly refractile, round to oval cells scattered within the spinous layer and dermoepidermal junction (Figure 2). Additionally, these weakly refractile, round to oval cells also were seen in vesiclelike dark spaces, and hyporefractile basal cells were appreciated surrounding the dermal papillae. Mycosis fungoides (MF) was diagnosed following correlation of the RCM findings with the clinical picture.
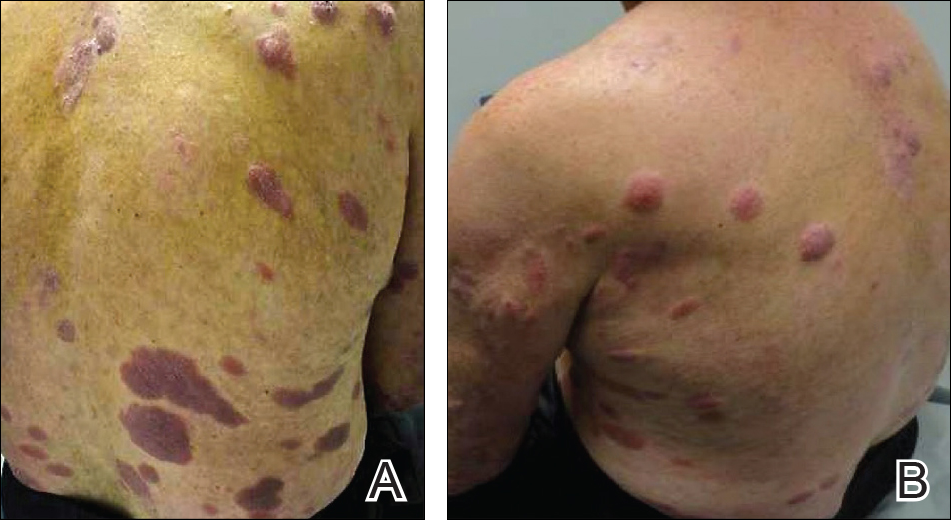
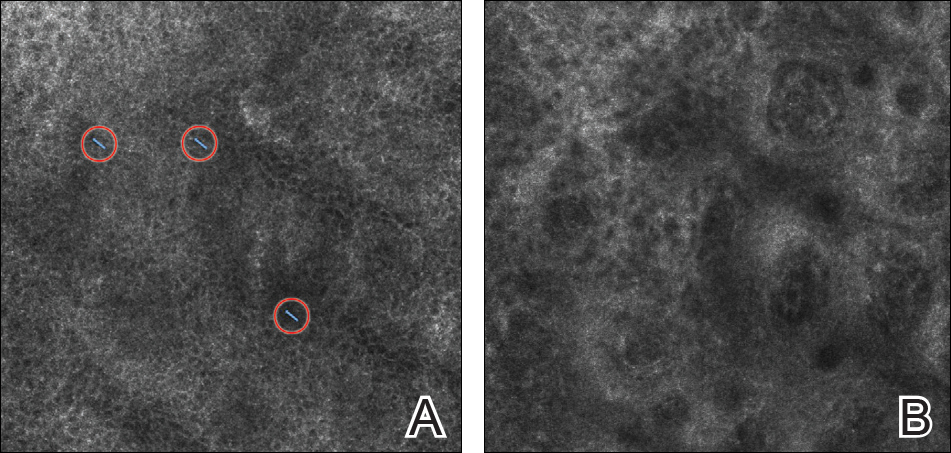
A biopsy was performed, with pathologic examination confirming the diagnosis of tumor-stage MF. Parakeratosis with epidermotropism of lymphocytes was noted along the basal layer and into the spinous layer of the epidermis (Figure 3). Underlying the epidermis there was a dense mononuclear infiltrate and conspicuous eosinophils extending to the deeper reticular dermis. The infiltrating cells had cerebriform nuclei and large pale cytoplasm. On immunostaining, the lymphocytes were positive for CD3 and CD4, and negative for CD5, CD7, and CD8. The patient was referred to the oncology department for disease management. Staging workup including computed tomography, flow cytometry, and T-cell receptor gene rearrangement were consistent with tumor-stage MF (T3N0M0B0).
![Atypical enlarged lymphocytes in the epidermis with hyperchromatic irregular nuclei of cells (inset) as well as a dense infiltrate in the dermis (A)(H&E, original magnifications ×10 and ×50 [inset])... Figure3](https://cdn.mdedge.com/files/s3fs-public/Image/July-2018/ct102001056_fig3.png)
Comment
Clinical Presentation of MF
Mycosis fungoides, a non-Hodgkin lymphoma of T-cell origin, is the most commonly diagnosed cutaneous lymphoma worldwide.1 It has an annual incidence of approximately 0.36 per 100,000 persons, and this number continues to rise.2,3 The median age of diagnosis is 55 to 60 years, and MF occurs twice as often in men versus women.4
The clinical presentation of MF varies and is classified by stages including patches, plaques, tumors, and erythroderma.5 Classically, MF is slowly progressive and begins as pruritic erythematous patches that have a predilection for non–sun-exposed areas of the skin. Over time, these patches may evolve into plaques and tumors. Early or patch-stage MF often presents as well-demarcated lesions of various sizes and shapes that tend to enlarge.6 These lesions may resemble eczema or psoriasis if there is scaling, such as in our patient. At the tumor stage, flat or dome-shaped nodules that may vary in color and are deeper than plaques begin to appear. Ulcerations, which were absent in our case, may often be seen.
Because of the diverse clinical manifestations of MF, which can mimic other common dermatoses, diagnosis often is challenging for clinicians. Furthermore, histology can yield nonspecific diagnostic results and may even resemble chronic inflammatory dermatoses.7 As a result, patients frequently are subjected to multiple skin biopsies to establish the diagnosis,8 and diagnosis may be delayed, with the median time from onset of skin symptoms to diagnosis being approximately 6 years.9
Reflectance Confocal Microscopy
In vivo RCM is a noninvasive technique that allows visualization of the skin at a cellular level and recently has been evaluated as a diagnostic tool for many skin conditions.10,11 Reflectance confocal microscopy findings have been well established for many cutaneous malignancies as well as inflammatory conditions such as psoriasis and atopic dermatitis.12,13 Specifically, 2 preliminary descriptive studies utilized RCM to visualize the characteristic features of MF in vivo.14,15 These studies reported the histopathologic correlation of RCM findings in biopsy-proven MF lesions. Consistent in all stages of MF is the presence of small, weakly refractile, round to oval cells within the spinous layer that correlate with atypical lymphocytes, in addition to hyporefractile basal cells surrounding the dermal papillae. Patch-stage MF lesions have more subtle epidermal findings compared to plaque-stage lesions, which tend to have more prominent vesiclelike dark spaces filled with collections of monomorphous, weakly refractile, round to oval cells corresponding with Pautrier microabscesses and evidence of spongiosis.14,15 The first descriptive study of RCM in the diagnosis of MF failed to identify features of tumor-stage MF that would distinguish it from patch- or plaque-stage disease. The investigators also stated that deep nodular collections of atypical lymphocytes seen on histopathology in tumor-stage MF were missed on RCM evaluation.14 Furthermore, the second descriptive study of RCM and MF, which included 2 patients with tumor-stage disease, also failed to differentiate tumor-stage MF from the patch or plaque stages.15
Because of these 2 descriptive studies, a pilot study was conducted to determine the applicability and reproducibility of RCM findings for MF diagnosis.16 Two blinded confocalists were asked to diagnose RCM images as MF when compared to either normal skin or a variety of lymphoproliferative disorders. Of 15 patients, the confocalists correctly diagnosed MF in 84% and 90% of cases, respectively. Additionally, they reported the specificity and sensitivity of the following RCM features in the diagnosis of MF: spongiosis, 88.9% and 94.7%; loss of demarcation, 88.9% and 94.7%; disarray of the epidermis, 77.8% and 89.5%; hyporefractile rings, 88.9% and 78.9%; junctional atypical lymphocytes, 100% and 73.7%; and vesiclelike structures (Pautrier microabscesses), 100% and 73.7%. Importantly, this study did not evaluate the specificity and sensitivity of MF diagnosis compared to other eczematous or inflammatory conditions that may share similar RCM findings; therefore, these results are not generalizable, and many of the RCM findings characteristically seen in MF are not specific to its diagnosis.16
One study assessed the diagnostic accuracy of RCM in evaluating erythematosquamous diseases including MF, psoriasis, contact dermatitis, discoid lupus, and subacute cutaneous lupus.17 In this study, 3 blinded confocalists achieved a 95.41% and 92.89% specificity and 89.13% and 63.33% sensitivity for psoriasis and MF, respectively. Typical features of psoriasis on RCM included parakeratosis, reduction or absence of the granular layer, papillomatosis, acanthosis with normal honeycomb pattern of the epidermis, and dilated vessels in the upper dermis. Features that were more specific to MF included epidermotropic atypical lymphocytes, interface dermatitis, pleomorphic tumor cells, and dendritic cells.17 However, atypical lymphocytes and interface dermatitis also may be seen in cutaneous lupus; therefore, additional studies are still needed to validate RCM’s utility in differentiating between erythematosquamous skin diseases, including psoriasis, cutaneous lupus, and MF. Currently, RCM findings must be interpreted in conjunction with the clinical and histologic picture.
Importantly, RCM also is limited when evaluating MF due to its limited depth of visualization, as it allows imaging only to the superficial papillary dermis. Furthermore, any infiltrative process such as epidermal hyperplasia, spongiosis, or scaling, which can be seen in MF, may further impair the imaging quality of the deeper dermis.
Conclusion
Despite its limitations, RCM has the potential to be advantageous in evaluating skin lesions suspicious for MF in real time and is a promising technology for a quick noninvasive bedside adjunct tool. Its utility in selecting the optimal site for biopsy for better yield of histopathologic results in suspected MF cases has been demonstrated.16 However, large-scale studies still are needed to evaluate RCM in the diagnosis of the wide diversity of MF lesions as well as its efficacy in selecting optimal biopsy sites.
- Lutzner M, Edelson R, Schein P, et al. Cutaneous T-cell lymphomas: the Sézary syndrome, mycosis fungoides, and related disorders. Ann Intern Med. 1975;83:534-552.
- Akinbami AA, Osikomaiya BI, John-Olabode SO, et al. Mycosis fungoides: case report and literature review. Clin Med Insights Case Rep. 2014;7:95-98.
- Criscione VD, Weinstock MA. Incidence of cutaneous T-cell lymphoma in the United States, 1973-2002. Arch Dermatol. 2007;143:854-959.
- Bradford PT, Devesa SS, Anderson WF, et al. Cutaneous lymphoma incidence patterns in the United States: a population-based study of 3884 cases. Blood. 2009;113:5064-5073.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Nashan D, Faulhaber D, Stander S. Mycosis fungoides: a dermatological masquerader. Br J Dermatol. 2007;157:1-10.
- Santucci M, Biggeri A, Feller AC, et al. Efficacy of histologic criteria for diagnosing early mycosis fungoides: an EORTC cutaneous lymphoma study group investigation. European Organization for Research and Treatment of Cancer. Am J Surg Pathol. 2000;24:40-50.
- Glass LF, Keller KL, Messina JL, et al. Cutaneous T-cell lymphoma. Cancer Control. 1998;5:11-18.
- Hoppe RT, Wood GS, Abel EA. Mycosis fungoides and the Sézary syndrome: pathology, staging, and treatment. Curr Probl Cancer. 1990;14:293-371.
- Tannous ZS, Mihm MC, Flotte TJ, et al. In vivo examination of lentigo maligna and malignant melanoma in situ, lentigo maligna type by near-infrared reflectance confocal microscopy: comparison of in vivo confocal images with histologic sections. J Am Acad Dermatol. 2002;46:260-263.
- Gerger A, Koller S, Weger W, et al. Sensitivity and specificity of confocal laser-scanning microscopy for in vivo diagnosis of malignant skin tumors. Cancer. 2006;107:193-200.
- Branzan AL, Landthaler M, Szeimies RM. In vivo confocal scanning laser microscopy in dermatology [published online November 18, 2006]. Lasers Med Sci. 2007;22:73-82.
- González S. Confocal reflectance microscopy in dermatology: promise and reality of non-invasive diagnosis and monitoring. Actas Dermosifiliogr. 2009;100(suppl 2):59-69.
- Agero AL, Gill M, Ardigo M, et al. In vivo reflectance confocal microscopy of mycosis fungoides: a preliminary study [published online April 16, 2007]. J Am Acad Dermatol. 2007;57:435-441.
- Wi L, Dai H, Li Z, et al. Reflectance confocal microscopy for the characteristics of mycosis fungoides and correlation with histology: a pilot study [published online April 18, 2013]. Skin Res Technol. 2013;19:352-355.
- Lange-Asschenfeldt S, Babilli J, Beyer M, et al. Consistency and distribution of reflectance confocal microscopy features for diagnosis of cutaneous T cell lymphoma. J Biomed Opt. 2012;17:016001.
- Koller S, Gerger A, Ahlgrimm-Siess V. In vivo reflectance confocal microscopy of erythematosquamous skin diseases [published online March 6, 2009]. Exp Dermatol. 2009;18:536-540.
Case Report
A 60-year-old man with a history of Hodgkin lymphoma that had been treated with chemotherapy 6 years prior presented to our dermatology clinic with a persistent pruritic rash on the back, abdomen, and bilateral arms and legs. The eruption initially began as localized discrete lesions on the lower back 1 year prior to the current presentation; at that time a diagnosis of psoriasis was made at an outside dermatology clinic, and treatment with mometasone furoate cream was initiated. Despite the patient’s compliance with this treatment, the lesions did not resolve and began spreading to the arms, legs, chest, and abdomen. His current medications included lisinopril, escitalopram, aspirin, and omeprazole.
On presentation to our clinic, physical examination revealed round, scaly, pink plaques and tumors of variable sizes (3–10 cm) distributed asymmetrically on the chest, back, abdomen, arms, and legs (Figure 1). The lesions were grouped in well-defined areas encompassing approximately 30% of the body surface area. No lymphadenopathy was appreciated. In vivo reflectance confocal microscopy (RCM) performed on one of the lesions revealed disarray of the epidermis with small, weakly refractile, round to oval cells scattered within the spinous layer and dermoepidermal junction (Figure 2). Additionally, these weakly refractile, round to oval cells also were seen in vesiclelike dark spaces, and hyporefractile basal cells were appreciated surrounding the dermal papillae. Mycosis fungoides (MF) was diagnosed following correlation of the RCM findings with the clinical picture.


A biopsy was performed, with pathologic examination confirming the diagnosis of tumor-stage MF. Parakeratosis with epidermotropism of lymphocytes was noted along the basal layer and into the spinous layer of the epidermis (Figure 3). Underlying the epidermis there was a dense mononuclear infiltrate and conspicuous eosinophils extending to the deeper reticular dermis. The infiltrating cells had cerebriform nuclei and large pale cytoplasm. On immunostaining, the lymphocytes were positive for CD3 and CD4, and negative for CD5, CD7, and CD8. The patient was referred to the oncology department for disease management. Staging workup including computed tomography, flow cytometry, and T-cell receptor gene rearrangement were consistent with tumor-stage MF (T3N0M0B0).
![Atypical enlarged lymphocytes in the epidermis with hyperchromatic irregular nuclei of cells (inset) as well as a dense infiltrate in the dermis (A)(H&E, original magnifications ×10 and ×50 [inset])... Figure3](https://cdn.mdedge.com/files/s3fs-public/Image/July-2018/ct102001056_fig3.png)
Comment
Clinical Presentation of MF
Mycosis fungoides, a non-Hodgkin lymphoma of T-cell origin, is the most commonly diagnosed cutaneous lymphoma worldwide.1 It has an annual incidence of approximately 0.36 per 100,000 persons, and this number continues to rise.2,3 The median age of diagnosis is 55 to 60 years, and MF occurs twice as often in men versus women.4
The clinical presentation of MF varies and is classified by stages including patches, plaques, tumors, and erythroderma.5 Classically, MF is slowly progressive and begins as pruritic erythematous patches that have a predilection for non–sun-exposed areas of the skin. Over time, these patches may evolve into plaques and tumors. Early or patch-stage MF often presents as well-demarcated lesions of various sizes and shapes that tend to enlarge.6 These lesions may resemble eczema or psoriasis if there is scaling, such as in our patient. At the tumor stage, flat or dome-shaped nodules that may vary in color and are deeper than plaques begin to appear. Ulcerations, which were absent in our case, may often be seen.
Because of the diverse clinical manifestations of MF, which can mimic other common dermatoses, diagnosis often is challenging for clinicians. Furthermore, histology can yield nonspecific diagnostic results and may even resemble chronic inflammatory dermatoses.7 As a result, patients frequently are subjected to multiple skin biopsies to establish the diagnosis,8 and diagnosis may be delayed, with the median time from onset of skin symptoms to diagnosis being approximately 6 years.9
Reflectance Confocal Microscopy
In vivo RCM is a noninvasive technique that allows visualization of the skin at a cellular level and recently has been evaluated as a diagnostic tool for many skin conditions.10,11 Reflectance confocal microscopy findings have been well established for many cutaneous malignancies as well as inflammatory conditions such as psoriasis and atopic dermatitis.12,13 Specifically, 2 preliminary descriptive studies utilized RCM to visualize the characteristic features of MF in vivo.14,15 These studies reported the histopathologic correlation of RCM findings in biopsy-proven MF lesions. Consistent in all stages of MF is the presence of small, weakly refractile, round to oval cells within the spinous layer that correlate with atypical lymphocytes, in addition to hyporefractile basal cells surrounding the dermal papillae. Patch-stage MF lesions have more subtle epidermal findings compared to plaque-stage lesions, which tend to have more prominent vesiclelike dark spaces filled with collections of monomorphous, weakly refractile, round to oval cells corresponding with Pautrier microabscesses and evidence of spongiosis.14,15 The first descriptive study of RCM in the diagnosis of MF failed to identify features of tumor-stage MF that would distinguish it from patch- or plaque-stage disease. The investigators also stated that deep nodular collections of atypical lymphocytes seen on histopathology in tumor-stage MF were missed on RCM evaluation.14 Furthermore, the second descriptive study of RCM and MF, which included 2 patients with tumor-stage disease, also failed to differentiate tumor-stage MF from the patch or plaque stages.15
Because of these 2 descriptive studies, a pilot study was conducted to determine the applicability and reproducibility of RCM findings for MF diagnosis.16 Two blinded confocalists were asked to diagnose RCM images as MF when compared to either normal skin or a variety of lymphoproliferative disorders. Of 15 patients, the confocalists correctly diagnosed MF in 84% and 90% of cases, respectively. Additionally, they reported the specificity and sensitivity of the following RCM features in the diagnosis of MF: spongiosis, 88.9% and 94.7%; loss of demarcation, 88.9% and 94.7%; disarray of the epidermis, 77.8% and 89.5%; hyporefractile rings, 88.9% and 78.9%; junctional atypical lymphocytes, 100% and 73.7%; and vesiclelike structures (Pautrier microabscesses), 100% and 73.7%. Importantly, this study did not evaluate the specificity and sensitivity of MF diagnosis compared to other eczematous or inflammatory conditions that may share similar RCM findings; therefore, these results are not generalizable, and many of the RCM findings characteristically seen in MF are not specific to its diagnosis.16
One study assessed the diagnostic accuracy of RCM in evaluating erythematosquamous diseases including MF, psoriasis, contact dermatitis, discoid lupus, and subacute cutaneous lupus.17 In this study, 3 blinded confocalists achieved a 95.41% and 92.89% specificity and 89.13% and 63.33% sensitivity for psoriasis and MF, respectively. Typical features of psoriasis on RCM included parakeratosis, reduction or absence of the granular layer, papillomatosis, acanthosis with normal honeycomb pattern of the epidermis, and dilated vessels in the upper dermis. Features that were more specific to MF included epidermotropic atypical lymphocytes, interface dermatitis, pleomorphic tumor cells, and dendritic cells.17 However, atypical lymphocytes and interface dermatitis also may be seen in cutaneous lupus; therefore, additional studies are still needed to validate RCM’s utility in differentiating between erythematosquamous skin diseases, including psoriasis, cutaneous lupus, and MF. Currently, RCM findings must be interpreted in conjunction with the clinical and histologic picture.
Importantly, RCM also is limited when evaluating MF due to its limited depth of visualization, as it allows imaging only to the superficial papillary dermis. Furthermore, any infiltrative process such as epidermal hyperplasia, spongiosis, or scaling, which can be seen in MF, may further impair the imaging quality of the deeper dermis.
Conclusion
Despite its limitations, RCM has the potential to be advantageous in evaluating skin lesions suspicious for MF in real time and is a promising technology for a quick noninvasive bedside adjunct tool. Its utility in selecting the optimal site for biopsy for better yield of histopathologic results in suspected MF cases has been demonstrated.16 However, large-scale studies still are needed to evaluate RCM in the diagnosis of the wide diversity of MF lesions as well as its efficacy in selecting optimal biopsy sites.
Case Report
A 60-year-old man with a history of Hodgkin lymphoma that had been treated with chemotherapy 6 years prior presented to our dermatology clinic with a persistent pruritic rash on the back, abdomen, and bilateral arms and legs. The eruption initially began as localized discrete lesions on the lower back 1 year prior to the current presentation; at that time a diagnosis of psoriasis was made at an outside dermatology clinic, and treatment with mometasone furoate cream was initiated. Despite the patient’s compliance with this treatment, the lesions did not resolve and began spreading to the arms, legs, chest, and abdomen. His current medications included lisinopril, escitalopram, aspirin, and omeprazole.
On presentation to our clinic, physical examination revealed round, scaly, pink plaques and tumors of variable sizes (3–10 cm) distributed asymmetrically on the chest, back, abdomen, arms, and legs (Figure 1). The lesions were grouped in well-defined areas encompassing approximately 30% of the body surface area. No lymphadenopathy was appreciated. In vivo reflectance confocal microscopy (RCM) performed on one of the lesions revealed disarray of the epidermis with small, weakly refractile, round to oval cells scattered within the spinous layer and dermoepidermal junction (Figure 2). Additionally, these weakly refractile, round to oval cells also were seen in vesiclelike dark spaces, and hyporefractile basal cells were appreciated surrounding the dermal papillae. Mycosis fungoides (MF) was diagnosed following correlation of the RCM findings with the clinical picture.


A biopsy was performed, with pathologic examination confirming the diagnosis of tumor-stage MF. Parakeratosis with epidermotropism of lymphocytes was noted along the basal layer and into the spinous layer of the epidermis (Figure 3). Underlying the epidermis there was a dense mononuclear infiltrate and conspicuous eosinophils extending to the deeper reticular dermis. The infiltrating cells had cerebriform nuclei and large pale cytoplasm. On immunostaining, the lymphocytes were positive for CD3 and CD4, and negative for CD5, CD7, and CD8. The patient was referred to the oncology department for disease management. Staging workup including computed tomography, flow cytometry, and T-cell receptor gene rearrangement were consistent with tumor-stage MF (T3N0M0B0).
![Atypical enlarged lymphocytes in the epidermis with hyperchromatic irregular nuclei of cells (inset) as well as a dense infiltrate in the dermis (A)(H&E, original magnifications ×10 and ×50 [inset])... Figure3](https://cdn.mdedge.com/files/s3fs-public/Image/July-2018/ct102001056_fig3.png)
Comment
Clinical Presentation of MF
Mycosis fungoides, a non-Hodgkin lymphoma of T-cell origin, is the most commonly diagnosed cutaneous lymphoma worldwide.1 It has an annual incidence of approximately 0.36 per 100,000 persons, and this number continues to rise.2,3 The median age of diagnosis is 55 to 60 years, and MF occurs twice as often in men versus women.4
The clinical presentation of MF varies and is classified by stages including patches, plaques, tumors, and erythroderma.5 Classically, MF is slowly progressive and begins as pruritic erythematous patches that have a predilection for non–sun-exposed areas of the skin. Over time, these patches may evolve into plaques and tumors. Early or patch-stage MF often presents as well-demarcated lesions of various sizes and shapes that tend to enlarge.6 These lesions may resemble eczema or psoriasis if there is scaling, such as in our patient. At the tumor stage, flat or dome-shaped nodules that may vary in color and are deeper than plaques begin to appear. Ulcerations, which were absent in our case, may often be seen.
Because of the diverse clinical manifestations of MF, which can mimic other common dermatoses, diagnosis often is challenging for clinicians. Furthermore, histology can yield nonspecific diagnostic results and may even resemble chronic inflammatory dermatoses.7 As a result, patients frequently are subjected to multiple skin biopsies to establish the diagnosis,8 and diagnosis may be delayed, with the median time from onset of skin symptoms to diagnosis being approximately 6 years.9
Reflectance Confocal Microscopy
In vivo RCM is a noninvasive technique that allows visualization of the skin at a cellular level and recently has been evaluated as a diagnostic tool for many skin conditions.10,11 Reflectance confocal microscopy findings have been well established for many cutaneous malignancies as well as inflammatory conditions such as psoriasis and atopic dermatitis.12,13 Specifically, 2 preliminary descriptive studies utilized RCM to visualize the characteristic features of MF in vivo.14,15 These studies reported the histopathologic correlation of RCM findings in biopsy-proven MF lesions. Consistent in all stages of MF is the presence of small, weakly refractile, round to oval cells within the spinous layer that correlate with atypical lymphocytes, in addition to hyporefractile basal cells surrounding the dermal papillae. Patch-stage MF lesions have more subtle epidermal findings compared to plaque-stage lesions, which tend to have more prominent vesiclelike dark spaces filled with collections of monomorphous, weakly refractile, round to oval cells corresponding with Pautrier microabscesses and evidence of spongiosis.14,15 The first descriptive study of RCM in the diagnosis of MF failed to identify features of tumor-stage MF that would distinguish it from patch- or plaque-stage disease. The investigators also stated that deep nodular collections of atypical lymphocytes seen on histopathology in tumor-stage MF were missed on RCM evaluation.14 Furthermore, the second descriptive study of RCM and MF, which included 2 patients with tumor-stage disease, also failed to differentiate tumor-stage MF from the patch or plaque stages.15
Because of these 2 descriptive studies, a pilot study was conducted to determine the applicability and reproducibility of RCM findings for MF diagnosis.16 Two blinded confocalists were asked to diagnose RCM images as MF when compared to either normal skin or a variety of lymphoproliferative disorders. Of 15 patients, the confocalists correctly diagnosed MF in 84% and 90% of cases, respectively. Additionally, they reported the specificity and sensitivity of the following RCM features in the diagnosis of MF: spongiosis, 88.9% and 94.7%; loss of demarcation, 88.9% and 94.7%; disarray of the epidermis, 77.8% and 89.5%; hyporefractile rings, 88.9% and 78.9%; junctional atypical lymphocytes, 100% and 73.7%; and vesiclelike structures (Pautrier microabscesses), 100% and 73.7%. Importantly, this study did not evaluate the specificity and sensitivity of MF diagnosis compared to other eczematous or inflammatory conditions that may share similar RCM findings; therefore, these results are not generalizable, and many of the RCM findings characteristically seen in MF are not specific to its diagnosis.16
One study assessed the diagnostic accuracy of RCM in evaluating erythematosquamous diseases including MF, psoriasis, contact dermatitis, discoid lupus, and subacute cutaneous lupus.17 In this study, 3 blinded confocalists achieved a 95.41% and 92.89% specificity and 89.13% and 63.33% sensitivity for psoriasis and MF, respectively. Typical features of psoriasis on RCM included parakeratosis, reduction or absence of the granular layer, papillomatosis, acanthosis with normal honeycomb pattern of the epidermis, and dilated vessels in the upper dermis. Features that were more specific to MF included epidermotropic atypical lymphocytes, interface dermatitis, pleomorphic tumor cells, and dendritic cells.17 However, atypical lymphocytes and interface dermatitis also may be seen in cutaneous lupus; therefore, additional studies are still needed to validate RCM’s utility in differentiating between erythematosquamous skin diseases, including psoriasis, cutaneous lupus, and MF. Currently, RCM findings must be interpreted in conjunction with the clinical and histologic picture.
Importantly, RCM also is limited when evaluating MF due to its limited depth of visualization, as it allows imaging only to the superficial papillary dermis. Furthermore, any infiltrative process such as epidermal hyperplasia, spongiosis, or scaling, which can be seen in MF, may further impair the imaging quality of the deeper dermis.
Conclusion
Despite its limitations, RCM has the potential to be advantageous in evaluating skin lesions suspicious for MF in real time and is a promising technology for a quick noninvasive bedside adjunct tool. Its utility in selecting the optimal site for biopsy for better yield of histopathologic results in suspected MF cases has been demonstrated.16 However, large-scale studies still are needed to evaluate RCM in the diagnosis of the wide diversity of MF lesions as well as its efficacy in selecting optimal biopsy sites.
- Lutzner M, Edelson R, Schein P, et al. Cutaneous T-cell lymphomas: the Sézary syndrome, mycosis fungoides, and related disorders. Ann Intern Med. 1975;83:534-552.
- Akinbami AA, Osikomaiya BI, John-Olabode SO, et al. Mycosis fungoides: case report and literature review. Clin Med Insights Case Rep. 2014;7:95-98.
- Criscione VD, Weinstock MA. Incidence of cutaneous T-cell lymphoma in the United States, 1973-2002. Arch Dermatol. 2007;143:854-959.
- Bradford PT, Devesa SS, Anderson WF, et al. Cutaneous lymphoma incidence patterns in the United States: a population-based study of 3884 cases. Blood. 2009;113:5064-5073.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Nashan D, Faulhaber D, Stander S. Mycosis fungoides: a dermatological masquerader. Br J Dermatol. 2007;157:1-10.
- Santucci M, Biggeri A, Feller AC, et al. Efficacy of histologic criteria for diagnosing early mycosis fungoides: an EORTC cutaneous lymphoma study group investigation. European Organization for Research and Treatment of Cancer. Am J Surg Pathol. 2000;24:40-50.
- Glass LF, Keller KL, Messina JL, et al. Cutaneous T-cell lymphoma. Cancer Control. 1998;5:11-18.
- Hoppe RT, Wood GS, Abel EA. Mycosis fungoides and the Sézary syndrome: pathology, staging, and treatment. Curr Probl Cancer. 1990;14:293-371.
- Tannous ZS, Mihm MC, Flotte TJ, et al. In vivo examination of lentigo maligna and malignant melanoma in situ, lentigo maligna type by near-infrared reflectance confocal microscopy: comparison of in vivo confocal images with histologic sections. J Am Acad Dermatol. 2002;46:260-263.
- Gerger A, Koller S, Weger W, et al. Sensitivity and specificity of confocal laser-scanning microscopy for in vivo diagnosis of malignant skin tumors. Cancer. 2006;107:193-200.
- Branzan AL, Landthaler M, Szeimies RM. In vivo confocal scanning laser microscopy in dermatology [published online November 18, 2006]. Lasers Med Sci. 2007;22:73-82.
- González S. Confocal reflectance microscopy in dermatology: promise and reality of non-invasive diagnosis and monitoring. Actas Dermosifiliogr. 2009;100(suppl 2):59-69.
- Agero AL, Gill M, Ardigo M, et al. In vivo reflectance confocal microscopy of mycosis fungoides: a preliminary study [published online April 16, 2007]. J Am Acad Dermatol. 2007;57:435-441.
- Wi L, Dai H, Li Z, et al. Reflectance confocal microscopy for the characteristics of mycosis fungoides and correlation with histology: a pilot study [published online April 18, 2013]. Skin Res Technol. 2013;19:352-355.
- Lange-Asschenfeldt S, Babilli J, Beyer M, et al. Consistency and distribution of reflectance confocal microscopy features for diagnosis of cutaneous T cell lymphoma. J Biomed Opt. 2012;17:016001.
- Koller S, Gerger A, Ahlgrimm-Siess V. In vivo reflectance confocal microscopy of erythematosquamous skin diseases [published online March 6, 2009]. Exp Dermatol. 2009;18:536-540.
- Lutzner M, Edelson R, Schein P, et al. Cutaneous T-cell lymphomas: the Sézary syndrome, mycosis fungoides, and related disorders. Ann Intern Med. 1975;83:534-552.
- Akinbami AA, Osikomaiya BI, John-Olabode SO, et al. Mycosis fungoides: case report and literature review. Clin Med Insights Case Rep. 2014;7:95-98.
- Criscione VD, Weinstock MA. Incidence of cutaneous T-cell lymphoma in the United States, 1973-2002. Arch Dermatol. 2007;143:854-959.
- Bradford PT, Devesa SS, Anderson WF, et al. Cutaneous lymphoma incidence patterns in the United States: a population-based study of 3884 cases. Blood. 2009;113:5064-5073.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Nashan D, Faulhaber D, Stander S. Mycosis fungoides: a dermatological masquerader. Br J Dermatol. 2007;157:1-10.
- Santucci M, Biggeri A, Feller AC, et al. Efficacy of histologic criteria for diagnosing early mycosis fungoides: an EORTC cutaneous lymphoma study group investigation. European Organization for Research and Treatment of Cancer. Am J Surg Pathol. 2000;24:40-50.
- Glass LF, Keller KL, Messina JL, et al. Cutaneous T-cell lymphoma. Cancer Control. 1998;5:11-18.
- Hoppe RT, Wood GS, Abel EA. Mycosis fungoides and the Sézary syndrome: pathology, staging, and treatment. Curr Probl Cancer. 1990;14:293-371.
- Tannous ZS, Mihm MC, Flotte TJ, et al. In vivo examination of lentigo maligna and malignant melanoma in situ, lentigo maligna type by near-infrared reflectance confocal microscopy: comparison of in vivo confocal images with histologic sections. J Am Acad Dermatol. 2002;46:260-263.
- Gerger A, Koller S, Weger W, et al. Sensitivity and specificity of confocal laser-scanning microscopy for in vivo diagnosis of malignant skin tumors. Cancer. 2006;107:193-200.
- Branzan AL, Landthaler M, Szeimies RM. In vivo confocal scanning laser microscopy in dermatology [published online November 18, 2006]. Lasers Med Sci. 2007;22:73-82.
- González S. Confocal reflectance microscopy in dermatology: promise and reality of non-invasive diagnosis and monitoring. Actas Dermosifiliogr. 2009;100(suppl 2):59-69.
- Agero AL, Gill M, Ardigo M, et al. In vivo reflectance confocal microscopy of mycosis fungoides: a preliminary study [published online April 16, 2007]. J Am Acad Dermatol. 2007;57:435-441.
- Wi L, Dai H, Li Z, et al. Reflectance confocal microscopy for the characteristics of mycosis fungoides and correlation with histology: a pilot study [published online April 18, 2013]. Skin Res Technol. 2013;19:352-355.
- Lange-Asschenfeldt S, Babilli J, Beyer M, et al. Consistency and distribution of reflectance confocal microscopy features for diagnosis of cutaneous T cell lymphoma. J Biomed Opt. 2012;17:016001.
- Koller S, Gerger A, Ahlgrimm-Siess V. In vivo reflectance confocal microscopy of erythematosquamous skin diseases [published online March 6, 2009]. Exp Dermatol. 2009;18:536-540.
Practice Points
- Mycosis fungoides (MF) can be a challenging diagnosis to establish and often requires multiple biopsies.
- Reflectance confocal microscopy (RCM) may be helpful as a bedside noninvasive diagnostic technique.
- In suspected MF cases, RCM may assist in selecting the optimal biopsy site for better yield of histopathologic results.
Evaluating Dermatology Apps for Patient Education
Mobile Medical Apps for Patient Education: A Graded Review of Available Dermatology Apps
According to industry estimates, roughly 64% of US adults were smartphone users in 2015.1 Smartphones enable users to utilize mobile applications (apps) that can perform a variety of functions in many categories, including business, music, photography, entertainment, education, social networking, travel, and lifestyle. The widespread adoption and use of mobile apps has implications for medical practice. Mobile apps have the capability to serve as information sources for patients, educational tools for students, and diagnostic aids for physicians.2 Consequently, a number of medical and health care–oriented apps have already been developed3 and are increasingly utilized by patients and providers.4
Given its visual nature, dermatology is particularly amenable to the integration of mobile medical apps. A study by Brewer et al5 identified more than 229 dermatology-related apps in categories ranging from general dermatology reference, self-surveillance and diagnosis, disease guides, educational aids, sunscreen and UV recommendations, and teledermatology. Patients served as the target audience and principal consumers of more than half of these dermatology apps.5
Mobile medical and health care apps demonstrate great potential for serving as valuable information sources for patients with dermatologic conditions; however, the content, functions, accuracy, and educational value of dermatology mobile apps are not well characterized, making it difficult for patients and health care providers to select and recommend appropriate apps.6 In this study, we created a rubric to objectively grade 44 publicly available mobile dermatology apps with the primary focus of patient education.
Methods
We conducted a search of dermatology-related educational mobile apps that were publicly available via the App Store (Apple Inc) from January 2016 to November 2016. (The pricing, availability, and other features of these apps may have changed since the study period.) The following search terms were used: dermatology, dermoscopy, melanoma, skin cancer, psoriasis, rosacea, acne, eczema, dermal fillers, and Mohs surgery. We excluded apps that were not in English; had a solely commercial focus; were mobile textbooks or scientific journals; were used to provide teledermatology services with no educational purpose; were solely focused on homeopathic, alternative, and/or complementary medicine; or were intended primarily as a reference for students or health care professionals. Our search yielded 44 apps with patient education as a primary objective. The apps were divided into 6 categories based on their focus: general dermatology, cosmetic dermatology, acne, eczema, psoriasis, and skin cancer.
Each app was reviewed using a quantified grading rubric developed by the researchers. In a prior evaluation, Handel7 reviewed 35 health and wellness mobile apps utilizing the categories of ease of use, reliability, quality, scope of information, and aesthetics.4 These criteria were modified and adapted for the purposes of this study, and a 4-point scale was applied to each criterion. The final criteria were (1) educational objectives, (2) content, (3) accuracy, (4) design, and (5) conflict of interest. The quantified grading rubric is described in Table 1.
Results
The possible range of scores based on the grading rubric was 5 to 20. The actual range of scores was 8 to 19 (Table 2). The 44 reviewed apps were categorized by topic as acne, cosmetic dermatology, eczema, general dermatology, psoriasis, or skin cancer. A sample of 15 apps selected to represent the distribution of scores and their grading on the rubric are presented in Table 3.
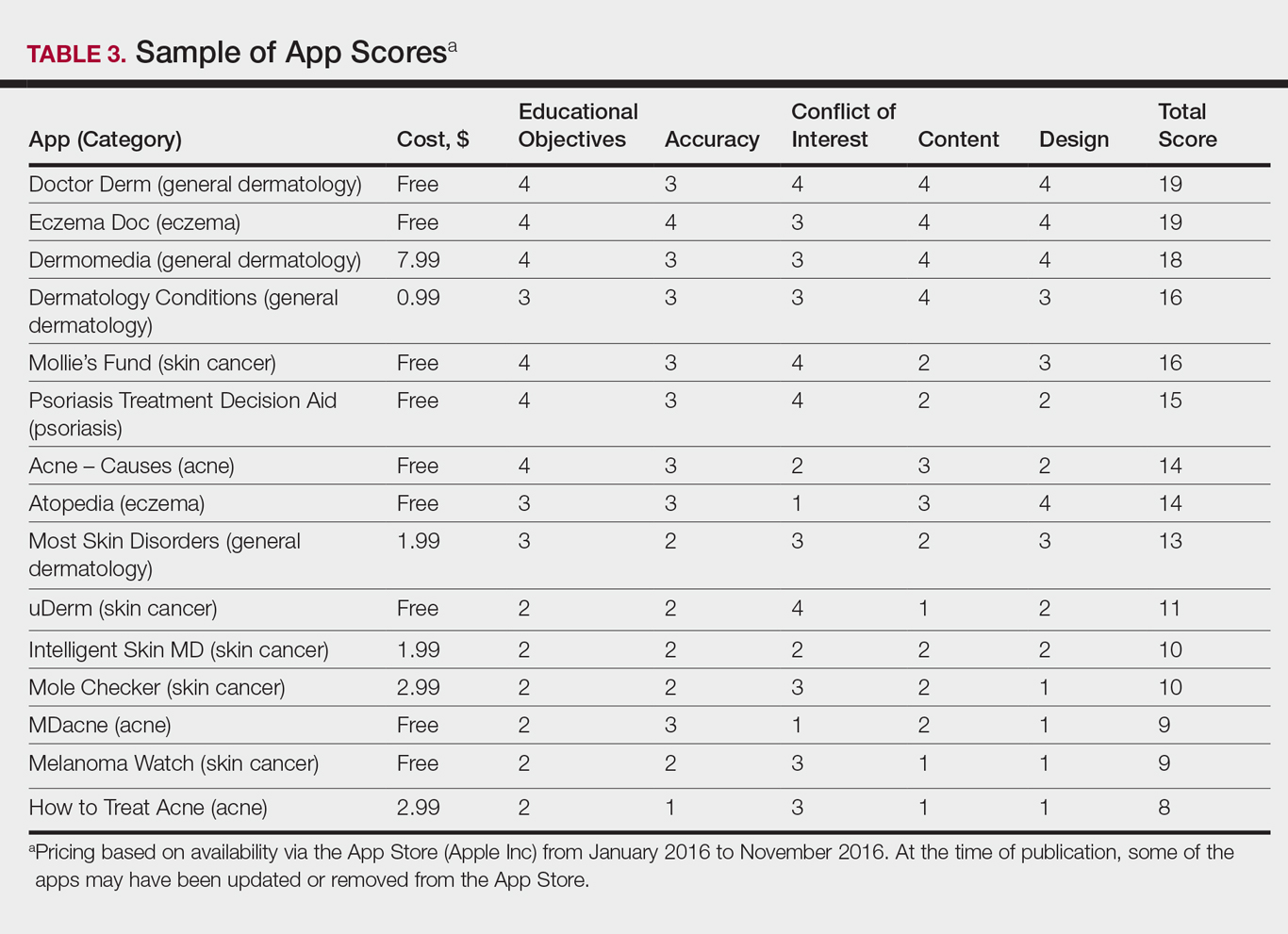
Comment
The number of dermatology-related apps available to mobile users continues to grow at an increasing rate.8 The apps vary in many aspects, including their purpose, scope, intended audience, and goals of the app publisher. In turn, more individuals are turning to mobile apps for medical information,4 especially in dermatology, thus it is necessary to create a systematic way to evaluate the quality and utility of each app to assist users in making informed decisions about which apps will best meet their needs in the midst of a wide array of choices.
For the purpose of this study, an objective rubric was created that can be used to evaluate the quality of medical apps for patient education in dermatology. An app’s adequacy and usefulness for patient education was thought to depend on 3 possible score ranges into which the app could fall based on the grading rubric. An app with a total score in the range of 5 to 10 was not thought to be useful and may even be detrimental to patients. An app with a total score in the range of 11 to 15 may be used for patient education with some reservations based on shortcomings for certain criteria. An app with a score in the range of 16 to 20 was thought to be valuable and adequate for patient education. For example, the How to Treat Acne app received a total score of 8 and therefore would not be recommended to patients based on the grading rubric used in this study. This particular app provided sparse and sometimes inaccurate information, had a confusing user interface, and contained many obstructive advertisements. In contrast, the Eczema Doc app received a total score of 19, which indicates a quality app deemed to be useful for patient information based on the established rubric. This app met all the objectives that it advertised, contained accurate information with verified citation of sources, and was very easy for users to navigate.
Of the 44 graded apps, only 9 (20.5%) received scores in the highest range of 16 to 20, which indicates a need for improvements in mobile dermatology apps intended for patient education. Adopting the grading rubric developed in this study as a standard in the creation of medical apps could have beneficial implications in disseminating accurate, safe, unbiased, and easy-to-understand information to patients.
- Smith A. U.S. smartphone use in 2015. Pew Research Center website. http://www.pewinternet.org/2015/04/01/us-smartphone-use-in-2015. Published April 1, 2015. Accessed August 29, 2017.
- Nilsen W, Kumar S, Shar A, et al. Advancing the science of mHealth. J Health Commun. 2012;17(suppl 1):5-10.
- West DM. How mobile devices are transforming healthcare issues in technology innovation. Issues Technol Innov. 2012;18:1-14.
- Boudreaux ED, Waring ME, Hayes RB, et al. Evaluating and selecting mobile health apps: strategies for healthcare providers and healthcare organizations. Transl Behav Med. 2014;4:363-371.
- Brewer AC, Endly DC, Henley J, et al. Mobile applications in dermatology. JAMA Dermatol. 2013;149:1300-1304.
- Cummings E, Borycki E, Roehrer E. Issues and considerations for healthcare consumers using mobile applications. Stud Health Technol Inform. 2013;183:227-231.
- Handel MJ. mHealth (mobile health)-using apps for health and wellness. Explore. 2011;7:256-261.
- Boulos MN, Brewer AC, Karimkhani C, et al. Mobile medical and health apps: state of the art, concerns, regulatory control and certification. Online J Public Health Inform. 2014;5:229.
According to industry estimates, roughly 64% of US adults were smartphone users in 2015.1 Smartphones enable users to utilize mobile applications (apps) that can perform a variety of functions in many categories, including business, music, photography, entertainment, education, social networking, travel, and lifestyle. The widespread adoption and use of mobile apps has implications for medical practice. Mobile apps have the capability to serve as information sources for patients, educational tools for students, and diagnostic aids for physicians.2 Consequently, a number of medical and health care–oriented apps have already been developed3 and are increasingly utilized by patients and providers.4
Given its visual nature, dermatology is particularly amenable to the integration of mobile medical apps. A study by Brewer et al5 identified more than 229 dermatology-related apps in categories ranging from general dermatology reference, self-surveillance and diagnosis, disease guides, educational aids, sunscreen and UV recommendations, and teledermatology. Patients served as the target audience and principal consumers of more than half of these dermatology apps.5
Mobile medical and health care apps demonstrate great potential for serving as valuable information sources for patients with dermatologic conditions; however, the content, functions, accuracy, and educational value of dermatology mobile apps are not well characterized, making it difficult for patients and health care providers to select and recommend appropriate apps.6 In this study, we created a rubric to objectively grade 44 publicly available mobile dermatology apps with the primary focus of patient education.
Methods
We conducted a search of dermatology-related educational mobile apps that were publicly available via the App Store (Apple Inc) from January 2016 to November 2016. (The pricing, availability, and other features of these apps may have changed since the study period.) The following search terms were used: dermatology, dermoscopy, melanoma, skin cancer, psoriasis, rosacea, acne, eczema, dermal fillers, and Mohs surgery. We excluded apps that were not in English; had a solely commercial focus; were mobile textbooks or scientific journals; were used to provide teledermatology services with no educational purpose; were solely focused on homeopathic, alternative, and/or complementary medicine; or were intended primarily as a reference for students or health care professionals. Our search yielded 44 apps with patient education as a primary objective. The apps were divided into 6 categories based on their focus: general dermatology, cosmetic dermatology, acne, eczema, psoriasis, and skin cancer.
Each app was reviewed using a quantified grading rubric developed by the researchers. In a prior evaluation, Handel7 reviewed 35 health and wellness mobile apps utilizing the categories of ease of use, reliability, quality, scope of information, and aesthetics.4 These criteria were modified and adapted for the purposes of this study, and a 4-point scale was applied to each criterion. The final criteria were (1) educational objectives, (2) content, (3) accuracy, (4) design, and (5) conflict of interest. The quantified grading rubric is described in Table 1.

Results
The possible range of scores based on the grading rubric was 5 to 20. The actual range of scores was 8 to 19 (Table 2). The 44 reviewed apps were categorized by topic as acne, cosmetic dermatology, eczema, general dermatology, psoriasis, or skin cancer. A sample of 15 apps selected to represent the distribution of scores and their grading on the rubric are presented in Table 3.


Comment
The number of dermatology-related apps available to mobile users continues to grow at an increasing rate.8 The apps vary in many aspects, including their purpose, scope, intended audience, and goals of the app publisher. In turn, more individuals are turning to mobile apps for medical information,4 especially in dermatology, thus it is necessary to create a systematic way to evaluate the quality and utility of each app to assist users in making informed decisions about which apps will best meet their needs in the midst of a wide array of choices.
For the purpose of this study, an objective rubric was created that can be used to evaluate the quality of medical apps for patient education in dermatology. An app’s adequacy and usefulness for patient education was thought to depend on 3 possible score ranges into which the app could fall based on the grading rubric. An app with a total score in the range of 5 to 10 was not thought to be useful and may even be detrimental to patients. An app with a total score in the range of 11 to 15 may be used for patient education with some reservations based on shortcomings for certain criteria. An app with a score in the range of 16 to 20 was thought to be valuable and adequate for patient education. For example, the How to Treat Acne app received a total score of 8 and therefore would not be recommended to patients based on the grading rubric used in this study. This particular app provided sparse and sometimes inaccurate information, had a confusing user interface, and contained many obstructive advertisements. In contrast, the Eczema Doc app received a total score of 19, which indicates a quality app deemed to be useful for patient information based on the established rubric. This app met all the objectives that it advertised, contained accurate information with verified citation of sources, and was very easy for users to navigate.
Of the 44 graded apps, only 9 (20.5%) received scores in the highest range of 16 to 20, which indicates a need for improvements in mobile dermatology apps intended for patient education. Adopting the grading rubric developed in this study as a standard in the creation of medical apps could have beneficial implications in disseminating accurate, safe, unbiased, and easy-to-understand information to patients.
According to industry estimates, roughly 64% of US adults were smartphone users in 2015.1 Smartphones enable users to utilize mobile applications (apps) that can perform a variety of functions in many categories, including business, music, photography, entertainment, education, social networking, travel, and lifestyle. The widespread adoption and use of mobile apps has implications for medical practice. Mobile apps have the capability to serve as information sources for patients, educational tools for students, and diagnostic aids for physicians.2 Consequently, a number of medical and health care–oriented apps have already been developed3 and are increasingly utilized by patients and providers.4
Given its visual nature, dermatology is particularly amenable to the integration of mobile medical apps. A study by Brewer et al5 identified more than 229 dermatology-related apps in categories ranging from general dermatology reference, self-surveillance and diagnosis, disease guides, educational aids, sunscreen and UV recommendations, and teledermatology. Patients served as the target audience and principal consumers of more than half of these dermatology apps.5
Mobile medical and health care apps demonstrate great potential for serving as valuable information sources for patients with dermatologic conditions; however, the content, functions, accuracy, and educational value of dermatology mobile apps are not well characterized, making it difficult for patients and health care providers to select and recommend appropriate apps.6 In this study, we created a rubric to objectively grade 44 publicly available mobile dermatology apps with the primary focus of patient education.
Methods
We conducted a search of dermatology-related educational mobile apps that were publicly available via the App Store (Apple Inc) from January 2016 to November 2016. (The pricing, availability, and other features of these apps may have changed since the study period.) The following search terms were used: dermatology, dermoscopy, melanoma, skin cancer, psoriasis, rosacea, acne, eczema, dermal fillers, and Mohs surgery. We excluded apps that were not in English; had a solely commercial focus; were mobile textbooks or scientific journals; were used to provide teledermatology services with no educational purpose; were solely focused on homeopathic, alternative, and/or complementary medicine; or were intended primarily as a reference for students or health care professionals. Our search yielded 44 apps with patient education as a primary objective. The apps were divided into 6 categories based on their focus: general dermatology, cosmetic dermatology, acne, eczema, psoriasis, and skin cancer.
Each app was reviewed using a quantified grading rubric developed by the researchers. In a prior evaluation, Handel7 reviewed 35 health and wellness mobile apps utilizing the categories of ease of use, reliability, quality, scope of information, and aesthetics.4 These criteria were modified and adapted for the purposes of this study, and a 4-point scale was applied to each criterion. The final criteria were (1) educational objectives, (2) content, (3) accuracy, (4) design, and (5) conflict of interest. The quantified grading rubric is described in Table 1.

Results
The possible range of scores based on the grading rubric was 5 to 20. The actual range of scores was 8 to 19 (Table 2). The 44 reviewed apps were categorized by topic as acne, cosmetic dermatology, eczema, general dermatology, psoriasis, or skin cancer. A sample of 15 apps selected to represent the distribution of scores and their grading on the rubric are presented in Table 3.


Comment
The number of dermatology-related apps available to mobile users continues to grow at an increasing rate.8 The apps vary in many aspects, including their purpose, scope, intended audience, and goals of the app publisher. In turn, more individuals are turning to mobile apps for medical information,4 especially in dermatology, thus it is necessary to create a systematic way to evaluate the quality and utility of each app to assist users in making informed decisions about which apps will best meet their needs in the midst of a wide array of choices.
For the purpose of this study, an objective rubric was created that can be used to evaluate the quality of medical apps for patient education in dermatology. An app’s adequacy and usefulness for patient education was thought to depend on 3 possible score ranges into which the app could fall based on the grading rubric. An app with a total score in the range of 5 to 10 was not thought to be useful and may even be detrimental to patients. An app with a total score in the range of 11 to 15 may be used for patient education with some reservations based on shortcomings for certain criteria. An app with a score in the range of 16 to 20 was thought to be valuable and adequate for patient education. For example, the How to Treat Acne app received a total score of 8 and therefore would not be recommended to patients based on the grading rubric used in this study. This particular app provided sparse and sometimes inaccurate information, had a confusing user interface, and contained many obstructive advertisements. In contrast, the Eczema Doc app received a total score of 19, which indicates a quality app deemed to be useful for patient information based on the established rubric. This app met all the objectives that it advertised, contained accurate information with verified citation of sources, and was very easy for users to navigate.
Of the 44 graded apps, only 9 (20.5%) received scores in the highest range of 16 to 20, which indicates a need for improvements in mobile dermatology apps intended for patient education. Adopting the grading rubric developed in this study as a standard in the creation of medical apps could have beneficial implications in disseminating accurate, safe, unbiased, and easy-to-understand information to patients.
- Smith A. U.S. smartphone use in 2015. Pew Research Center website. http://www.pewinternet.org/2015/04/01/us-smartphone-use-in-2015. Published April 1, 2015. Accessed August 29, 2017.
- Nilsen W, Kumar S, Shar A, et al. Advancing the science of mHealth. J Health Commun. 2012;17(suppl 1):5-10.
- West DM. How mobile devices are transforming healthcare issues in technology innovation. Issues Technol Innov. 2012;18:1-14.
- Boudreaux ED, Waring ME, Hayes RB, et al. Evaluating and selecting mobile health apps: strategies for healthcare providers and healthcare organizations. Transl Behav Med. 2014;4:363-371.
- Brewer AC, Endly DC, Henley J, et al. Mobile applications in dermatology. JAMA Dermatol. 2013;149:1300-1304.
- Cummings E, Borycki E, Roehrer E. Issues and considerations for healthcare consumers using mobile applications. Stud Health Technol Inform. 2013;183:227-231.
- Handel MJ. mHealth (mobile health)-using apps for health and wellness. Explore. 2011;7:256-261.
- Boulos MN, Brewer AC, Karimkhani C, et al. Mobile medical and health apps: state of the art, concerns, regulatory control and certification. Online J Public Health Inform. 2014;5:229.
- Smith A. U.S. smartphone use in 2015. Pew Research Center website. http://www.pewinternet.org/2015/04/01/us-smartphone-use-in-2015. Published April 1, 2015. Accessed August 29, 2017.
- Nilsen W, Kumar S, Shar A, et al. Advancing the science of mHealth. J Health Commun. 2012;17(suppl 1):5-10.
- West DM. How mobile devices are transforming healthcare issues in technology innovation. Issues Technol Innov. 2012;18:1-14.
- Boudreaux ED, Waring ME, Hayes RB, et al. Evaluating and selecting mobile health apps: strategies for healthcare providers and healthcare organizations. Transl Behav Med. 2014;4:363-371.
- Brewer AC, Endly DC, Henley J, et al. Mobile applications in dermatology. JAMA Dermatol. 2013;149:1300-1304.
- Cummings E, Borycki E, Roehrer E. Issues and considerations for healthcare consumers using mobile applications. Stud Health Technol Inform. 2013;183:227-231.
- Handel MJ. mHealth (mobile health)-using apps for health and wellness. Explore. 2011;7:256-261.
- Boulos MN, Brewer AC, Karimkhani C, et al. Mobile medical and health apps: state of the art, concerns, regulatory control and certification. Online J Public Health Inform. 2014;5:229.
Practice Points
- Mobile dermatology apps for educational purposes should be objectively reviewed before being used by patients.
- In our study, only 9 (20.5%) of the 44 dermatology apps evaluated were considered adequate for patient information based on our grading criteria.
Recalcitrant Hyperkeratotic Plaques
The Diagnosis: Hypertrophic Lupus Erythematosus
Physical examination at initial presentation revealed well-demarcated, 2- to 3-cm plaques with scale distributed most extensively on the elbows and shins with lesser involvement of the chest and abdomen. After treatment with topical steroids, adalimumab, methotrexate, and narrowband UVB phototherapy, new annular, erythematous, and edematous lesions began to appear on the chest and abdomen (Figure 1). These new lesions appeared less hyperkeratotic than the older ones.
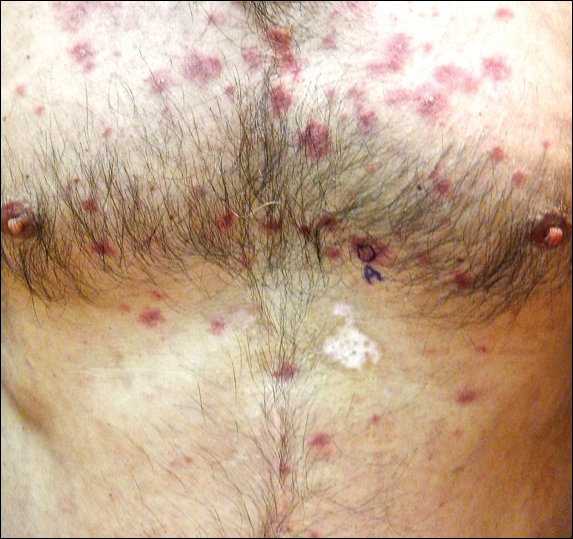
Biopsy of a hyperkeratotic lesion from the patient's arm revealed marked hyperkeratosis, parakeratosis, epidermal hyperplasia, focal vacuolar change, solar elastosis, and transepidermal elastotic elimination (Figure 2A). A second biopsy performed on a newer chest lesion revealed interface changes, degeneration of the basal layer, follicular plugging, and dermal mucin (Figure 2B). Serology revealed an antinuclear antibody (ANA) titer of 1:1280 (reference range, <1:40 dilution) and hemoglobin of 11.5 g/dL (reference range, 14.0-17.5 g/dL). On the basis of clinical, histologic, and serologic findings, hypertrophic lupus erythematosus (LE) was diagnosed. The patient was treated with oral prednisone, which resulted in rapid improvement.
Hypertrophic LE is a rare subset of chronic cutaneous lupus first described by Behcet1 in 1942. Lesions are identified as verrucous keratotic plaques with a characteristic erythematous indurated border.2 Patients predominantly are middle-aged women with lesions distributed on sun-exposed areas. Most often, hypertrophic LE is seen in association with the classic lesions of discoid LE; however, patients may present exclusively with the cutaneous manifestations of hypertrophic LE. More rarely, as seen in this case, hypertrophic LE may present in conjunction with systemic features.3 The diagnosis of systemic LE requires 4 of the following criteria be fulfilled: malar rash; discoid rash; photosensitivity; oral ulcers; arthritis; cardiopulmonary serositis; renal involvement; positive ANA titer; and neurologic, hematologic, or immunologic disorders.4 Our patient qualified for discoid rash, photosensitivity, cardiopulmonary involvement with mitral valve defects and pulmonary pleuritis, hematologic disorder (anemia), and a positive ANA titer. Furthermore, in patients with only cutaneous discoid LE, serology generally reveals negative or low-titer ANA and negative anti-Ro antibodies.5
Hypertrophic LE is characterized histologically by irregular epidermal hyperplasia in association with features of classic cutaneous LE. Distinctive features of cutaneous LE include interface changes, follicular plugging, dermal mucin, and angiocentric lymphocytic inflammation.6 Notably, additional biopsies of the less hyperkeratotic lesions on our patient's chest and abdomen were performed, which revealed classic cutaneous LE features (Figure 2B).
Hypertrophic LE has 2 histological variants: lichen planus-like and keratoacanthoma (KA)-like patterns. Most cases are described as lichen planus-like, with a dense bandlike infiltrate in association with irregular epidermal hyperplasia, vacuolar interface changes, and reactive squamous atypia.5 In contrast, the less common KA-like lesions consist of a keratinous center with vigorous squamous epithelial proliferation.6
Clinically, hypertrophic LE may resemble hypertrophic psoriasis, lichen planus, KA, or squamous cell carcinoma (SCC). Due to the presence of pseudocarcinomatous hyperplasia, the histopathologic differential includes hypertrophic lichen planus, SCC, KA, and deep fungal infections. However, these other diseases lack the classic features of cutaneous LE, which include interface changes, follicular plugging, dermal mucin, and perivascular lymphocytic inflammation. Additionally, transepidermal elastotic elimination (Figure 2A) helps distinguish hypertrophic LE from other diagnoses.7 One of the most important tasks is distinguishing hypertrophic LE from SCC. Hypertrophic LE does not typically display eosinophil infiltrates, which differentiates it from SCC and KA. Additionally, studies report that CD123 positivity can be useful.6 Positive plasmacytoid dendritic cells are abundant at the dermoepidermal junction in hypertrophic LE, while only single or rare clusters of CD123+ cells are seen in SCC.8 Also, SCC has been found to arise in long-standing cutaneous LE lesions including both discoid and hypertrophic LE. Therefore, clinical and sometimes histological follow-up is required.
Hypertrophic LE often is challenging to treat and frequently is resistant to antimalarial drugs. The primary goals of treatment involve reducing inflammatory infiltrate and minimizing hyperkeratinization. Topical corticosteroids and calcineurin inhibitors often are inadequate as monotherapy due to reduced penetrance through the thick lesions; however, intralesional corticosteroids may be beneficial in patients with localized disease.9 Unfortunately, topical or intralesional treatments are impractical in patients with extensive lesions, as seen in our patient, in which case systemic corticosteroids can be beneficial.
Topical retinoids also have been found to be highly effective.10 Specifically, retinoids such as acitretin and isotretinoin, in some cases combined with antimalarial drugs, are effective in reducing the keratinization of these lesions. Successful treatment also has been reported with ustekinumab, thalidomide, mycophenolate mofetil, and pulsed dye laser.11 As in other types of cutaneous LE, hyperkeratotic LE is photosensitive; avoidance of prolonged sun exposure should be advised.8
- Bechet PE. Lupus erythematosus hypertrophicus et profundus. Arch Derm Syphilol. 1942;45:33-39.
- Bernardi M, Bahrami S, Callen JP. Hypertrophic lupus erythematous complicating long-standing systemic lupus erythematous. Lupus. 2011;20:549-550.
- Spann CR, Callen JP, Klein JB, et al. Clinical, serologic and immunogenetic studies in patients with chronic cutaneous (discoid) lupus erythematosus who have verrucous and/or hypertrophic skin lesions. J Rheumatol. 1988;15:256-261.
- Yu C, Gershwin E, Chang C. Diagnostic criteria for systemic lupus erythematosus: a critical review [published online January 21, 2014]. J Autoimmun. 2014;48-49:10-13.
- Provost TT. The relationship between discoid and systemic lupus erythematous. Arch Dermatol. 1994;130:1308-1310.
- Arps DP, Patel RM. Cutaneous hypertrophic lupus erythematous: a challenging histopathologic diagnosis in the absence of clinical information. Arch Pathol Lab Med. 2013;137:1205-1210.
- Daldon PE, De Souza EM, Cintra ML. Hypertrophic lupus erythematous: a clinicopathological study of 14 cases. J Cutan Pathol. 2003;30:443-448.
- Ko CJ, Srivastava B, Braverman I, et al. Hypertrophiclupus erythematous: the diagnostic utility of CD123 staining. J Cutan Pathol. 2011;38:889-892.
- Walling HW, Sontheimer RD. Cutaneous lupus erythematosus. issues in diagnosis and treatment. Am J Clin Dermatol. 2009;10:366-381.
- Al-Mutairi N, Rijhwani M, Nour-Eldin O. Hypertrophic lupus erythematosus treated successfully with acitretin as monotherapy. J Dermatol. 2005;32:482-486.
- Winchester D, Duffin KC, Hansen C. Response to ustekinumab in a patient with both severe psoriasis and hypertrophic cutaneous lupus. Lupus. 2012;12:1007-1010.
The Diagnosis: Hypertrophic Lupus Erythematosus
Physical examination at initial presentation revealed well-demarcated, 2- to 3-cm plaques with scale distributed most extensively on the elbows and shins with lesser involvement of the chest and abdomen. After treatment with topical steroids, adalimumab, methotrexate, and narrowband UVB phototherapy, new annular, erythematous, and edematous lesions began to appear on the chest and abdomen (Figure 1). These new lesions appeared less hyperkeratotic than the older ones.

Biopsy of a hyperkeratotic lesion from the patient's arm revealed marked hyperkeratosis, parakeratosis, epidermal hyperplasia, focal vacuolar change, solar elastosis, and transepidermal elastotic elimination (Figure 2A). A second biopsy performed on a newer chest lesion revealed interface changes, degeneration of the basal layer, follicular plugging, and dermal mucin (Figure 2B). Serology revealed an antinuclear antibody (ANA) titer of 1:1280 (reference range, <1:40 dilution) and hemoglobin of 11.5 g/dL (reference range, 14.0-17.5 g/dL). On the basis of clinical, histologic, and serologic findings, hypertrophic lupus erythematosus (LE) was diagnosed. The patient was treated with oral prednisone, which resulted in rapid improvement.

Hypertrophic LE is a rare subset of chronic cutaneous lupus first described by Behcet1 in 1942. Lesions are identified as verrucous keratotic plaques with a characteristic erythematous indurated border.2 Patients predominantly are middle-aged women with lesions distributed on sun-exposed areas. Most often, hypertrophic LE is seen in association with the classic lesions of discoid LE; however, patients may present exclusively with the cutaneous manifestations of hypertrophic LE. More rarely, as seen in this case, hypertrophic LE may present in conjunction with systemic features.3 The diagnosis of systemic LE requires 4 of the following criteria be fulfilled: malar rash; discoid rash; photosensitivity; oral ulcers; arthritis; cardiopulmonary serositis; renal involvement; positive ANA titer; and neurologic, hematologic, or immunologic disorders.4 Our patient qualified for discoid rash, photosensitivity, cardiopulmonary involvement with mitral valve defects and pulmonary pleuritis, hematologic disorder (anemia), and a positive ANA titer. Furthermore, in patients with only cutaneous discoid LE, serology generally reveals negative or low-titer ANA and negative anti-Ro antibodies.5
Hypertrophic LE is characterized histologically by irregular epidermal hyperplasia in association with features of classic cutaneous LE. Distinctive features of cutaneous LE include interface changes, follicular plugging, dermal mucin, and angiocentric lymphocytic inflammation.6 Notably, additional biopsies of the less hyperkeratotic lesions on our patient's chest and abdomen were performed, which revealed classic cutaneous LE features (Figure 2B).
Hypertrophic LE has 2 histological variants: lichen planus-like and keratoacanthoma (KA)-like patterns. Most cases are described as lichen planus-like, with a dense bandlike infiltrate in association with irregular epidermal hyperplasia, vacuolar interface changes, and reactive squamous atypia.5 In contrast, the less common KA-like lesions consist of a keratinous center with vigorous squamous epithelial proliferation.6
Clinically, hypertrophic LE may resemble hypertrophic psoriasis, lichen planus, KA, or squamous cell carcinoma (SCC). Due to the presence of pseudocarcinomatous hyperplasia, the histopathologic differential includes hypertrophic lichen planus, SCC, KA, and deep fungal infections. However, these other diseases lack the classic features of cutaneous LE, which include interface changes, follicular plugging, dermal mucin, and perivascular lymphocytic inflammation. Additionally, transepidermal elastotic elimination (Figure 2A) helps distinguish hypertrophic LE from other diagnoses.7 One of the most important tasks is distinguishing hypertrophic LE from SCC. Hypertrophic LE does not typically display eosinophil infiltrates, which differentiates it from SCC and KA. Additionally, studies report that CD123 positivity can be useful.6 Positive plasmacytoid dendritic cells are abundant at the dermoepidermal junction in hypertrophic LE, while only single or rare clusters of CD123+ cells are seen in SCC.8 Also, SCC has been found to arise in long-standing cutaneous LE lesions including both discoid and hypertrophic LE. Therefore, clinical and sometimes histological follow-up is required.
Hypertrophic LE often is challenging to treat and frequently is resistant to antimalarial drugs. The primary goals of treatment involve reducing inflammatory infiltrate and minimizing hyperkeratinization. Topical corticosteroids and calcineurin inhibitors often are inadequate as monotherapy due to reduced penetrance through the thick lesions; however, intralesional corticosteroids may be beneficial in patients with localized disease.9 Unfortunately, topical or intralesional treatments are impractical in patients with extensive lesions, as seen in our patient, in which case systemic corticosteroids can be beneficial.
Topical retinoids also have been found to be highly effective.10 Specifically, retinoids such as acitretin and isotretinoin, in some cases combined with antimalarial drugs, are effective in reducing the keratinization of these lesions. Successful treatment also has been reported with ustekinumab, thalidomide, mycophenolate mofetil, and pulsed dye laser.11 As in other types of cutaneous LE, hyperkeratotic LE is photosensitive; avoidance of prolonged sun exposure should be advised.8
The Diagnosis: Hypertrophic Lupus Erythematosus
Physical examination at initial presentation revealed well-demarcated, 2- to 3-cm plaques with scale distributed most extensively on the elbows and shins with lesser involvement of the chest and abdomen. After treatment with topical steroids, adalimumab, methotrexate, and narrowband UVB phototherapy, new annular, erythematous, and edematous lesions began to appear on the chest and abdomen (Figure 1). These new lesions appeared less hyperkeratotic than the older ones.

Biopsy of a hyperkeratotic lesion from the patient's arm revealed marked hyperkeratosis, parakeratosis, epidermal hyperplasia, focal vacuolar change, solar elastosis, and transepidermal elastotic elimination (Figure 2A). A second biopsy performed on a newer chest lesion revealed interface changes, degeneration of the basal layer, follicular plugging, and dermal mucin (Figure 2B). Serology revealed an antinuclear antibody (ANA) titer of 1:1280 (reference range, <1:40 dilution) and hemoglobin of 11.5 g/dL (reference range, 14.0-17.5 g/dL). On the basis of clinical, histologic, and serologic findings, hypertrophic lupus erythematosus (LE) was diagnosed. The patient was treated with oral prednisone, which resulted in rapid improvement.

Hypertrophic LE is a rare subset of chronic cutaneous lupus first described by Behcet1 in 1942. Lesions are identified as verrucous keratotic plaques with a characteristic erythematous indurated border.2 Patients predominantly are middle-aged women with lesions distributed on sun-exposed areas. Most often, hypertrophic LE is seen in association with the classic lesions of discoid LE; however, patients may present exclusively with the cutaneous manifestations of hypertrophic LE. More rarely, as seen in this case, hypertrophic LE may present in conjunction with systemic features.3 The diagnosis of systemic LE requires 4 of the following criteria be fulfilled: malar rash; discoid rash; photosensitivity; oral ulcers; arthritis; cardiopulmonary serositis; renal involvement; positive ANA titer; and neurologic, hematologic, or immunologic disorders.4 Our patient qualified for discoid rash, photosensitivity, cardiopulmonary involvement with mitral valve defects and pulmonary pleuritis, hematologic disorder (anemia), and a positive ANA titer. Furthermore, in patients with only cutaneous discoid LE, serology generally reveals negative or low-titer ANA and negative anti-Ro antibodies.5
Hypertrophic LE is characterized histologically by irregular epidermal hyperplasia in association with features of classic cutaneous LE. Distinctive features of cutaneous LE include interface changes, follicular plugging, dermal mucin, and angiocentric lymphocytic inflammation.6 Notably, additional biopsies of the less hyperkeratotic lesions on our patient's chest and abdomen were performed, which revealed classic cutaneous LE features (Figure 2B).
Hypertrophic LE has 2 histological variants: lichen planus-like and keratoacanthoma (KA)-like patterns. Most cases are described as lichen planus-like, with a dense bandlike infiltrate in association with irregular epidermal hyperplasia, vacuolar interface changes, and reactive squamous atypia.5 In contrast, the less common KA-like lesions consist of a keratinous center with vigorous squamous epithelial proliferation.6
Clinically, hypertrophic LE may resemble hypertrophic psoriasis, lichen planus, KA, or squamous cell carcinoma (SCC). Due to the presence of pseudocarcinomatous hyperplasia, the histopathologic differential includes hypertrophic lichen planus, SCC, KA, and deep fungal infections. However, these other diseases lack the classic features of cutaneous LE, which include interface changes, follicular plugging, dermal mucin, and perivascular lymphocytic inflammation. Additionally, transepidermal elastotic elimination (Figure 2A) helps distinguish hypertrophic LE from other diagnoses.7 One of the most important tasks is distinguishing hypertrophic LE from SCC. Hypertrophic LE does not typically display eosinophil infiltrates, which differentiates it from SCC and KA. Additionally, studies report that CD123 positivity can be useful.6 Positive plasmacytoid dendritic cells are abundant at the dermoepidermal junction in hypertrophic LE, while only single or rare clusters of CD123+ cells are seen in SCC.8 Also, SCC has been found to arise in long-standing cutaneous LE lesions including both discoid and hypertrophic LE. Therefore, clinical and sometimes histological follow-up is required.
Hypertrophic LE often is challenging to treat and frequently is resistant to antimalarial drugs. The primary goals of treatment involve reducing inflammatory infiltrate and minimizing hyperkeratinization. Topical corticosteroids and calcineurin inhibitors often are inadequate as monotherapy due to reduced penetrance through the thick lesions; however, intralesional corticosteroids may be beneficial in patients with localized disease.9 Unfortunately, topical or intralesional treatments are impractical in patients with extensive lesions, as seen in our patient, in which case systemic corticosteroids can be beneficial.
Topical retinoids also have been found to be highly effective.10 Specifically, retinoids such as acitretin and isotretinoin, in some cases combined with antimalarial drugs, are effective in reducing the keratinization of these lesions. Successful treatment also has been reported with ustekinumab, thalidomide, mycophenolate mofetil, and pulsed dye laser.11 As in other types of cutaneous LE, hyperkeratotic LE is photosensitive; avoidance of prolonged sun exposure should be advised.8
- Bechet PE. Lupus erythematosus hypertrophicus et profundus. Arch Derm Syphilol. 1942;45:33-39.
- Bernardi M, Bahrami S, Callen JP. Hypertrophic lupus erythematous complicating long-standing systemic lupus erythematous. Lupus. 2011;20:549-550.
- Spann CR, Callen JP, Klein JB, et al. Clinical, serologic and immunogenetic studies in patients with chronic cutaneous (discoid) lupus erythematosus who have verrucous and/or hypertrophic skin lesions. J Rheumatol. 1988;15:256-261.
- Yu C, Gershwin E, Chang C. Diagnostic criteria for systemic lupus erythematosus: a critical review [published online January 21, 2014]. J Autoimmun. 2014;48-49:10-13.
- Provost TT. The relationship between discoid and systemic lupus erythematous. Arch Dermatol. 1994;130:1308-1310.
- Arps DP, Patel RM. Cutaneous hypertrophic lupus erythematous: a challenging histopathologic diagnosis in the absence of clinical information. Arch Pathol Lab Med. 2013;137:1205-1210.
- Daldon PE, De Souza EM, Cintra ML. Hypertrophic lupus erythematous: a clinicopathological study of 14 cases. J Cutan Pathol. 2003;30:443-448.
- Ko CJ, Srivastava B, Braverman I, et al. Hypertrophiclupus erythematous: the diagnostic utility of CD123 staining. J Cutan Pathol. 2011;38:889-892.
- Walling HW, Sontheimer RD. Cutaneous lupus erythematosus. issues in diagnosis and treatment. Am J Clin Dermatol. 2009;10:366-381.
- Al-Mutairi N, Rijhwani M, Nour-Eldin O. Hypertrophic lupus erythematosus treated successfully with acitretin as monotherapy. J Dermatol. 2005;32:482-486.
- Winchester D, Duffin KC, Hansen C. Response to ustekinumab in a patient with both severe psoriasis and hypertrophic cutaneous lupus. Lupus. 2012;12:1007-1010.
- Bechet PE. Lupus erythematosus hypertrophicus et profundus. Arch Derm Syphilol. 1942;45:33-39.
- Bernardi M, Bahrami S, Callen JP. Hypertrophic lupus erythematous complicating long-standing systemic lupus erythematous. Lupus. 2011;20:549-550.
- Spann CR, Callen JP, Klein JB, et al. Clinical, serologic and immunogenetic studies in patients with chronic cutaneous (discoid) lupus erythematosus who have verrucous and/or hypertrophic skin lesions. J Rheumatol. 1988;15:256-261.
- Yu C, Gershwin E, Chang C. Diagnostic criteria for systemic lupus erythematosus: a critical review [published online January 21, 2014]. J Autoimmun. 2014;48-49:10-13.
- Provost TT. The relationship between discoid and systemic lupus erythematous. Arch Dermatol. 1994;130:1308-1310.
- Arps DP, Patel RM. Cutaneous hypertrophic lupus erythematous: a challenging histopathologic diagnosis in the absence of clinical information. Arch Pathol Lab Med. 2013;137:1205-1210.
- Daldon PE, De Souza EM, Cintra ML. Hypertrophic lupus erythematous: a clinicopathological study of 14 cases. J Cutan Pathol. 2003;30:443-448.
- Ko CJ, Srivastava B, Braverman I, et al. Hypertrophiclupus erythematous: the diagnostic utility of CD123 staining. J Cutan Pathol. 2011;38:889-892.
- Walling HW, Sontheimer RD. Cutaneous lupus erythematosus. issues in diagnosis and treatment. Am J Clin Dermatol. 2009;10:366-381.
- Al-Mutairi N, Rijhwani M, Nour-Eldin O. Hypertrophic lupus erythematosus treated successfully with acitretin as monotherapy. J Dermatol. 2005;32:482-486.
- Winchester D, Duffin KC, Hansen C. Response to ustekinumab in a patient with both severe psoriasis and hypertrophic cutaneous lupus. Lupus. 2012;12:1007-1010.
A 53-year-old man presented with a persistent, hyperkeratotic, pruritic rash on the arms, chest, and abdomen. The patient was treated for presumed psoriasis for 9 months by a primary care physician. However, despite an extensive treatment history, which included topical steroids, adalimumab, methotrexate, and narrowband UVB phototherapy, his condition worsened, and new erythematous and edematous lesions with no scale appeared on the back and chest. The patient's history also was notable for splenic rupture and mitral valve defects for which he was maintained on warfarin. In addition, he was evaluated by an allergist for new-onset dyspnea and treated with prednisone, which subsequently resulted in partial resolution of the skin lesions.