User login
For MD-IQ use only
Antibiotics Pre-Appendectomy Don’t Lower Perforation Risk, But Reduce Infections
, according to a new study.
While the percentage of surgical site infections (SSIs) was small for both groups, patients who received antibiotics during the waiting period had lower rates of these infections.
The trial — titled PERFECT-Antibiotics — was a substudy embedded in a larger PERFECT clinical trial, which aimed to determine whether an in-hospital delay of appendectomy resulted in increased risk for appendiceal perforation when compared to emergent surgery.
The trial “concluded that appendectomy does not need to be performed promptly in acute uncomplicated appendicitis and can be scheduled within 24 hours without increasing complications,” senior author Panu Mentula, MD, of the Department of Gastroenterological Surgery, Helsinki University Hospital, Helsinki, Finland, and colleagues wrote in the study. “The next question is whether preoperatively started antibiotic treatment reduces the risk of appendiceal perforations.”
The findings were published online in JAMA Surgery on May 14, 2025.
Trial Design
PERFECT-Antibiotics was an open-label, randomized trial conducted at two hospitals in Finland and one hospital in Norway. Researchers enrolled 1774 individuals diagnosed with acute uncomplicated appendicitis, diagnosed clinically or via imaging. Patients were placed in one of two groups: The antibiotic group received intravenous (IV) cefuroxime (1500 mg) and metronidazole (500 mg) every 8 hours until surgery, while the nonantibiotic group waited for surgery without antibiotics.
All patients received one dose of IV cefuroxime (1500 mg) and metronidazole (500 mg) during anesthesia induction. The primary outcome was perforated appendicitis and secondary outcomes included complication rate and SSIs within 30 days of follow-up.
The median age of patients was 35 years (interquartile range [IQR], 28-46 years), and 55% of patients were men. Patients waited a median time of 9 hours (IQR, 4.3-15.5) from study randomization to undergoing surgery.
No Difference in Appendiceal Perforation
Of the 888 patients in the preoperative antibiotic group, 26.2% received one dose, 38.7% received two doses, 22.6% received three doses, and 11.8% received four or more doses of antibiotics, including the antibiotic dose given during anesthesia. A total of 74 patients (8.3%) in this group had a perforated appendix.
Of the 886 patients not given preoperative antibiotics, 79 (8.9%) had a perforated appendix, which met the predetermined noninferiority threshold.
The groups had similar complication rates over the 30-day follow-up, though SSIs were lower in the antibiotic group (1.6%) than the no antibiotic group (3.2%).
The researchers estimated that the number needed to treat for antibiotic therapy was 63 for SSIs, 83 for intra-abdominal SSI, and 125 for reintervention.
“Although longer preoperative antibiotic treatment resulted in slightly lower rate of postoperative infectious complications, the actual difference was very small and probably clinically not significant to justify longer preoperative antibiotic treatment,” Mentula and colleagues wrote.
Lower Infection Rates With Antibiotics
Commenting on the study for GI & Hepatology News, Theodore Pappas, MD, professor of surgery at Duke University School of Medicine in Durham, North Carolina, placed greater importance on these secondary outcomes.
Intra-abdominal infections, a subset of SSIs, were more than twice as common in the no-antibiotic group (1.9%) than in the antibiotic group (0.7%; P = .02). Positive blood cultures were also more common in the no-antibiotic group than the antibiotic group (P = .02).
While the authors qualified these results, “the reality was it was better to use antibiotics,” he said.
There was also a “big overlap between the two groups,” he said, which may have muted differences between the two groups. For example, one fourth of patients in the antibiotic group received only one dose of antibiotics, the same treatment regimen as the no-antibiotic group.
“Although protocol required prophylaxis in all patients in the induction of anesthesia, some clinicians thought that it was unnecessary, because antibiotics had already been given only a couple of hours ago” in patients in the antibiotic group, Mentula told GI & Hepatology News. She did not think that would affect the study’s results.
The PERFECT trial and the antibiotics subtrial answer two important questions that have been asked for years, Pappas continued: Whether appendectomy for uncomplicated acute appendicitis needs to be performed emergently and if antibiotics administered while waiting for surgery improve outcomes.
“Basically, the study shows that you probably should keep them on antibiotics while you’re waiting,” he said.
The study was funded by Finnish Medical Foundation, the Mary and Georg Ehrnrooth Foundation, the Biomedicum Helsinki Foundation, and The Norwegian Surveillance Programme for Antimicrobial Resistance and research funds from the Finnish government. Mentula received grants from the Finnish government during the conduct of the study and personal fees from Pfizer outside the submitted work. Pappas reported no relevant disclosures.
A version of this article appeared on Medscape.com.
, according to a new study.
While the percentage of surgical site infections (SSIs) was small for both groups, patients who received antibiotics during the waiting period had lower rates of these infections.
The trial — titled PERFECT-Antibiotics — was a substudy embedded in a larger PERFECT clinical trial, which aimed to determine whether an in-hospital delay of appendectomy resulted in increased risk for appendiceal perforation when compared to emergent surgery.
The trial “concluded that appendectomy does not need to be performed promptly in acute uncomplicated appendicitis and can be scheduled within 24 hours without increasing complications,” senior author Panu Mentula, MD, of the Department of Gastroenterological Surgery, Helsinki University Hospital, Helsinki, Finland, and colleagues wrote in the study. “The next question is whether preoperatively started antibiotic treatment reduces the risk of appendiceal perforations.”
The findings were published online in JAMA Surgery on May 14, 2025.
Trial Design
PERFECT-Antibiotics was an open-label, randomized trial conducted at two hospitals in Finland and one hospital in Norway. Researchers enrolled 1774 individuals diagnosed with acute uncomplicated appendicitis, diagnosed clinically or via imaging. Patients were placed in one of two groups: The antibiotic group received intravenous (IV) cefuroxime (1500 mg) and metronidazole (500 mg) every 8 hours until surgery, while the nonantibiotic group waited for surgery without antibiotics.
All patients received one dose of IV cefuroxime (1500 mg) and metronidazole (500 mg) during anesthesia induction. The primary outcome was perforated appendicitis and secondary outcomes included complication rate and SSIs within 30 days of follow-up.
The median age of patients was 35 years (interquartile range [IQR], 28-46 years), and 55% of patients were men. Patients waited a median time of 9 hours (IQR, 4.3-15.5) from study randomization to undergoing surgery.
No Difference in Appendiceal Perforation
Of the 888 patients in the preoperative antibiotic group, 26.2% received one dose, 38.7% received two doses, 22.6% received three doses, and 11.8% received four or more doses of antibiotics, including the antibiotic dose given during anesthesia. A total of 74 patients (8.3%) in this group had a perforated appendix.
Of the 886 patients not given preoperative antibiotics, 79 (8.9%) had a perforated appendix, which met the predetermined noninferiority threshold.
The groups had similar complication rates over the 30-day follow-up, though SSIs were lower in the antibiotic group (1.6%) than the no antibiotic group (3.2%).
The researchers estimated that the number needed to treat for antibiotic therapy was 63 for SSIs, 83 for intra-abdominal SSI, and 125 for reintervention.
“Although longer preoperative antibiotic treatment resulted in slightly lower rate of postoperative infectious complications, the actual difference was very small and probably clinically not significant to justify longer preoperative antibiotic treatment,” Mentula and colleagues wrote.
Lower Infection Rates With Antibiotics
Commenting on the study for GI & Hepatology News, Theodore Pappas, MD, professor of surgery at Duke University School of Medicine in Durham, North Carolina, placed greater importance on these secondary outcomes.
Intra-abdominal infections, a subset of SSIs, were more than twice as common in the no-antibiotic group (1.9%) than in the antibiotic group (0.7%; P = .02). Positive blood cultures were also more common in the no-antibiotic group than the antibiotic group (P = .02).
While the authors qualified these results, “the reality was it was better to use antibiotics,” he said.
There was also a “big overlap between the two groups,” he said, which may have muted differences between the two groups. For example, one fourth of patients in the antibiotic group received only one dose of antibiotics, the same treatment regimen as the no-antibiotic group.
“Although protocol required prophylaxis in all patients in the induction of anesthesia, some clinicians thought that it was unnecessary, because antibiotics had already been given only a couple of hours ago” in patients in the antibiotic group, Mentula told GI & Hepatology News. She did not think that would affect the study’s results.
The PERFECT trial and the antibiotics subtrial answer two important questions that have been asked for years, Pappas continued: Whether appendectomy for uncomplicated acute appendicitis needs to be performed emergently and if antibiotics administered while waiting for surgery improve outcomes.
“Basically, the study shows that you probably should keep them on antibiotics while you’re waiting,” he said.
The study was funded by Finnish Medical Foundation, the Mary and Georg Ehrnrooth Foundation, the Biomedicum Helsinki Foundation, and The Norwegian Surveillance Programme for Antimicrobial Resistance and research funds from the Finnish government. Mentula received grants from the Finnish government during the conduct of the study and personal fees from Pfizer outside the submitted work. Pappas reported no relevant disclosures.
A version of this article appeared on Medscape.com.
, according to a new study.
While the percentage of surgical site infections (SSIs) was small for both groups, patients who received antibiotics during the waiting period had lower rates of these infections.
The trial — titled PERFECT-Antibiotics — was a substudy embedded in a larger PERFECT clinical trial, which aimed to determine whether an in-hospital delay of appendectomy resulted in increased risk for appendiceal perforation when compared to emergent surgery.
The trial “concluded that appendectomy does not need to be performed promptly in acute uncomplicated appendicitis and can be scheduled within 24 hours without increasing complications,” senior author Panu Mentula, MD, of the Department of Gastroenterological Surgery, Helsinki University Hospital, Helsinki, Finland, and colleagues wrote in the study. “The next question is whether preoperatively started antibiotic treatment reduces the risk of appendiceal perforations.”
The findings were published online in JAMA Surgery on May 14, 2025.
Trial Design
PERFECT-Antibiotics was an open-label, randomized trial conducted at two hospitals in Finland and one hospital in Norway. Researchers enrolled 1774 individuals diagnosed with acute uncomplicated appendicitis, diagnosed clinically or via imaging. Patients were placed in one of two groups: The antibiotic group received intravenous (IV) cefuroxime (1500 mg) and metronidazole (500 mg) every 8 hours until surgery, while the nonantibiotic group waited for surgery without antibiotics.
All patients received one dose of IV cefuroxime (1500 mg) and metronidazole (500 mg) during anesthesia induction. The primary outcome was perforated appendicitis and secondary outcomes included complication rate and SSIs within 30 days of follow-up.
The median age of patients was 35 years (interquartile range [IQR], 28-46 years), and 55% of patients were men. Patients waited a median time of 9 hours (IQR, 4.3-15.5) from study randomization to undergoing surgery.
No Difference in Appendiceal Perforation
Of the 888 patients in the preoperative antibiotic group, 26.2% received one dose, 38.7% received two doses, 22.6% received three doses, and 11.8% received four or more doses of antibiotics, including the antibiotic dose given during anesthesia. A total of 74 patients (8.3%) in this group had a perforated appendix.
Of the 886 patients not given preoperative antibiotics, 79 (8.9%) had a perforated appendix, which met the predetermined noninferiority threshold.
The groups had similar complication rates over the 30-day follow-up, though SSIs were lower in the antibiotic group (1.6%) than the no antibiotic group (3.2%).
The researchers estimated that the number needed to treat for antibiotic therapy was 63 for SSIs, 83 for intra-abdominal SSI, and 125 for reintervention.
“Although longer preoperative antibiotic treatment resulted in slightly lower rate of postoperative infectious complications, the actual difference was very small and probably clinically not significant to justify longer preoperative antibiotic treatment,” Mentula and colleagues wrote.
Lower Infection Rates With Antibiotics
Commenting on the study for GI & Hepatology News, Theodore Pappas, MD, professor of surgery at Duke University School of Medicine in Durham, North Carolina, placed greater importance on these secondary outcomes.
Intra-abdominal infections, a subset of SSIs, were more than twice as common in the no-antibiotic group (1.9%) than in the antibiotic group (0.7%; P = .02). Positive blood cultures were also more common in the no-antibiotic group than the antibiotic group (P = .02).
While the authors qualified these results, “the reality was it was better to use antibiotics,” he said.
There was also a “big overlap between the two groups,” he said, which may have muted differences between the two groups. For example, one fourth of patients in the antibiotic group received only one dose of antibiotics, the same treatment regimen as the no-antibiotic group.
“Although protocol required prophylaxis in all patients in the induction of anesthesia, some clinicians thought that it was unnecessary, because antibiotics had already been given only a couple of hours ago” in patients in the antibiotic group, Mentula told GI & Hepatology News. She did not think that would affect the study’s results.
The PERFECT trial and the antibiotics subtrial answer two important questions that have been asked for years, Pappas continued: Whether appendectomy for uncomplicated acute appendicitis needs to be performed emergently and if antibiotics administered while waiting for surgery improve outcomes.
“Basically, the study shows that you probably should keep them on antibiotics while you’re waiting,” he said.
The study was funded by Finnish Medical Foundation, the Mary and Georg Ehrnrooth Foundation, the Biomedicum Helsinki Foundation, and The Norwegian Surveillance Programme for Antimicrobial Resistance and research funds from the Finnish government. Mentula received grants from the Finnish government during the conduct of the study and personal fees from Pfizer outside the submitted work. Pappas reported no relevant disclosures.
A version of this article appeared on Medscape.com.
Gut Microbiome Changes in Chronic Pain — Test and Treat?
A new study adds to what has been emerging in the literature — namely that — suggesting that microbiome-based diagnostics and therapeutics may one day be routine for a broad range of pain conditions.
“There is now a whole list of pain conditions that appear to have these signatures, including postoperative pain, arthritis, neuropathy and migraine to name a few,” Robert Bonakdar, MD, director of pain management, Scripps Center for Integrative Medicine, San Diego, told GI & Hepatology News.
Fibromyalgia and complex regional pain syndrome (CRPS) are also on the list.
A team led by Amir Minerbi, MD, PhD, director of the Institute for Pain Medicine, Haifa, Israel, and colleagues published one of the first articles on gut changes in fibromyalgia. They noted that the gut microbiome could be utilized to determine which individuals had the condition and which did not — with about a 90% accuracy.
The team went on to show that transplanting gut microbiota from patients with fibromyalgia into germ-free mice was sufficient to induce pain-like behaviors in the animals — “effects that were reversed when healthy human microbiota were transplanted instead,” Minerbi told GI & Hepatology News.
Further, in a pilot clinical study, the researchers showed that transplanting microbiota from healthy donors led to a reduction in pain and other symptoms in women with treatment-resistant fibromyalgia.
Most recently, they found significant differences in the composition of the gut microbiome in a cohort of patients with CRPS from Israel, compared to matched pain-free control individuals.
Notably, two species — Dialister succinatiphilus and Phascolarctobacterium faecium – were enriched in patients with CRPS, while three species — Ligilactobacillus salivarius, Bifidobacterium dentium, and Bifidobacterium adolescentis – were increased in control samples, according to their report published last month in Anesthesiology.
“Importantly,” these findings were replicated in an independent cohort of patients with CRPS from Canada, “suggesting that the observed microbiome signature is robust and consistent across different environments,” Minerbi told GI & Hepatology News.
Causal Role?
“These findings collectively suggest a causal role for the gut microbiome in at least some chronic pain conditions,” Minerbi said.
However, the co-authors of a linked editorial cautioned that it’s “unclear if D succinatiphilus or P faecium are functionally relevant to CRPS pathophysiology or if the bacteria increased in healthy control samples protect against CRPS development.”
Minerbi and colleagues also observed that fecal concentrations of all measured short chain fatty acids (SCFA) in patients with CRPS were lower on average compared to pain-free control individuals, of which butyric, hexanoic, and valeric acid showed significant depletion.
Additionally, plasma concentrations of acetic acid showed significant depletion in patients with CRPS vs control individuals, while propionate, butyrate, isobutyrate and 2-methyl-butyric acid showed a trend toward lower concentrations.
The quantification of SCFA in patient stool and serum is a “notable advance” in this study, Zulmary Manjarres, PhD; Ashley Plumb, PhD; and Katelyn Sadler, PhD; with the Center for Advanced Pain Studies at The University of Texas at Dallas, wrote in their editorial.
SCFA are produced by bacteria as a byproduct of dietary fiber fermentation and appropriate levels of these compounds are important to maintain low levels of inflammation in the colon and overall gut health, they explained.
This begs the question of whether administering probiotic bacteria — many of which are believed to exert health benefits through SCFA production — can be used to treat CRPS-associated pain. It’s something that needs to be studied, the editorialists wrote.
Yet, in their view, the “most notable achievement” of Minerbi and colleagues is the development of a machine learning model that accurately, specifically and sensitively categorized individuals as patients with CRPS or control individuals based on their fecal microbiome signature.
The model, trained on exact sequence variant data from the Israeli patients, achieved 89.5% accuracy, 90.0% sensitivity, and 88.9% specificity in distinguishing patients with CRPS from control individuals in the Canadian cohort.
Interestingly, in three patients with CRPS who underwent limb amputation and recovered from their pain, their gut microbiome signature remained unchanged, suggesting that microbiome alterations might precede or persist beyond symptomatic phases.
Test and Treat: Are We There Yet?
The gut microbiome link to chronic pain syndromes is a hot area of research, but for now gut microbial testing followed by treatment aimed at “fixing” the microbiome remains largely experimental.
At this point, comprehensive gut-microbiome sequencing is not a routine, guideline-supported part of care for fibromyalgia or any chronic pain condition.
“Unfortunately, even for doctors interested in this area, we are not quite at the state of being able to diagnose and treat pain syndrome based on microbiome data,” Bonakdar told GI & Hepatology News.
He said there are many reasons for this including that this type of microbiome analysis is not commonly available at a routine lab. If patients do obtain testing, then the results are quite complex and may not translate to a diagnosis or a simple microbiome intervention.
“I think the closest option we have now is considering supplementing with commonly beneficial probiotic in pain conditions,” Bonakdar said.
One example is a preliminary fibromyalgia trial which found that supplementing with Lactobacillus, Bifidobacterium, and Saccharomyces boulardii appeared to have benefit.
“Unfortunately, this is hit or miss as other trials such as one in low back pain did not find benefit,” Bonakdar said.
Addressing gut microbiome changes will become “more actionable when microbiome analysis is more commonplace as well as is the ability to tailor treatment to the abnormalities seen on testing in a real-world manner,” Bonakdar said.
“Until then, there is no harm in promoting an anti-inflammatory diet for our patients with pain which we know can improve components of the microbiome while also supporting pain management,” he concluded.
Minerbi, Bonakdar, and the editorial writers had no relevant disclosures.
A version of this article appeared on Medscape.com.
A new study adds to what has been emerging in the literature — namely that — suggesting that microbiome-based diagnostics and therapeutics may one day be routine for a broad range of pain conditions.
“There is now a whole list of pain conditions that appear to have these signatures, including postoperative pain, arthritis, neuropathy and migraine to name a few,” Robert Bonakdar, MD, director of pain management, Scripps Center for Integrative Medicine, San Diego, told GI & Hepatology News.
Fibromyalgia and complex regional pain syndrome (CRPS) are also on the list.
A team led by Amir Minerbi, MD, PhD, director of the Institute for Pain Medicine, Haifa, Israel, and colleagues published one of the first articles on gut changes in fibromyalgia. They noted that the gut microbiome could be utilized to determine which individuals had the condition and which did not — with about a 90% accuracy.
The team went on to show that transplanting gut microbiota from patients with fibromyalgia into germ-free mice was sufficient to induce pain-like behaviors in the animals — “effects that were reversed when healthy human microbiota were transplanted instead,” Minerbi told GI & Hepatology News.
Further, in a pilot clinical study, the researchers showed that transplanting microbiota from healthy donors led to a reduction in pain and other symptoms in women with treatment-resistant fibromyalgia.
Most recently, they found significant differences in the composition of the gut microbiome in a cohort of patients with CRPS from Israel, compared to matched pain-free control individuals.
Notably, two species — Dialister succinatiphilus and Phascolarctobacterium faecium – were enriched in patients with CRPS, while three species — Ligilactobacillus salivarius, Bifidobacterium dentium, and Bifidobacterium adolescentis – were increased in control samples, according to their report published last month in Anesthesiology.
“Importantly,” these findings were replicated in an independent cohort of patients with CRPS from Canada, “suggesting that the observed microbiome signature is robust and consistent across different environments,” Minerbi told GI & Hepatology News.
Causal Role?
“These findings collectively suggest a causal role for the gut microbiome in at least some chronic pain conditions,” Minerbi said.
However, the co-authors of a linked editorial cautioned that it’s “unclear if D succinatiphilus or P faecium are functionally relevant to CRPS pathophysiology or if the bacteria increased in healthy control samples protect against CRPS development.”
Minerbi and colleagues also observed that fecal concentrations of all measured short chain fatty acids (SCFA) in patients with CRPS were lower on average compared to pain-free control individuals, of which butyric, hexanoic, and valeric acid showed significant depletion.
Additionally, plasma concentrations of acetic acid showed significant depletion in patients with CRPS vs control individuals, while propionate, butyrate, isobutyrate and 2-methyl-butyric acid showed a trend toward lower concentrations.
The quantification of SCFA in patient stool and serum is a “notable advance” in this study, Zulmary Manjarres, PhD; Ashley Plumb, PhD; and Katelyn Sadler, PhD; with the Center for Advanced Pain Studies at The University of Texas at Dallas, wrote in their editorial.
SCFA are produced by bacteria as a byproduct of dietary fiber fermentation and appropriate levels of these compounds are important to maintain low levels of inflammation in the colon and overall gut health, they explained.
This begs the question of whether administering probiotic bacteria — many of which are believed to exert health benefits through SCFA production — can be used to treat CRPS-associated pain. It’s something that needs to be studied, the editorialists wrote.
Yet, in their view, the “most notable achievement” of Minerbi and colleagues is the development of a machine learning model that accurately, specifically and sensitively categorized individuals as patients with CRPS or control individuals based on their fecal microbiome signature.
The model, trained on exact sequence variant data from the Israeli patients, achieved 89.5% accuracy, 90.0% sensitivity, and 88.9% specificity in distinguishing patients with CRPS from control individuals in the Canadian cohort.
Interestingly, in three patients with CRPS who underwent limb amputation and recovered from their pain, their gut microbiome signature remained unchanged, suggesting that microbiome alterations might precede or persist beyond symptomatic phases.
Test and Treat: Are We There Yet?
The gut microbiome link to chronic pain syndromes is a hot area of research, but for now gut microbial testing followed by treatment aimed at “fixing” the microbiome remains largely experimental.
At this point, comprehensive gut-microbiome sequencing is not a routine, guideline-supported part of care for fibromyalgia or any chronic pain condition.
“Unfortunately, even for doctors interested in this area, we are not quite at the state of being able to diagnose and treat pain syndrome based on microbiome data,” Bonakdar told GI & Hepatology News.
He said there are many reasons for this including that this type of microbiome analysis is not commonly available at a routine lab. If patients do obtain testing, then the results are quite complex and may not translate to a diagnosis or a simple microbiome intervention.
“I think the closest option we have now is considering supplementing with commonly beneficial probiotic in pain conditions,” Bonakdar said.
One example is a preliminary fibromyalgia trial which found that supplementing with Lactobacillus, Bifidobacterium, and Saccharomyces boulardii appeared to have benefit.
“Unfortunately, this is hit or miss as other trials such as one in low back pain did not find benefit,” Bonakdar said.
Addressing gut microbiome changes will become “more actionable when microbiome analysis is more commonplace as well as is the ability to tailor treatment to the abnormalities seen on testing in a real-world manner,” Bonakdar said.
“Until then, there is no harm in promoting an anti-inflammatory diet for our patients with pain which we know can improve components of the microbiome while also supporting pain management,” he concluded.
Minerbi, Bonakdar, and the editorial writers had no relevant disclosures.
A version of this article appeared on Medscape.com.
A new study adds to what has been emerging in the literature — namely that — suggesting that microbiome-based diagnostics and therapeutics may one day be routine for a broad range of pain conditions.
“There is now a whole list of pain conditions that appear to have these signatures, including postoperative pain, arthritis, neuropathy and migraine to name a few,” Robert Bonakdar, MD, director of pain management, Scripps Center for Integrative Medicine, San Diego, told GI & Hepatology News.
Fibromyalgia and complex regional pain syndrome (CRPS) are also on the list.
A team led by Amir Minerbi, MD, PhD, director of the Institute for Pain Medicine, Haifa, Israel, and colleagues published one of the first articles on gut changes in fibromyalgia. They noted that the gut microbiome could be utilized to determine which individuals had the condition and which did not — with about a 90% accuracy.
The team went on to show that transplanting gut microbiota from patients with fibromyalgia into germ-free mice was sufficient to induce pain-like behaviors in the animals — “effects that were reversed when healthy human microbiota were transplanted instead,” Minerbi told GI & Hepatology News.
Further, in a pilot clinical study, the researchers showed that transplanting microbiota from healthy donors led to a reduction in pain and other symptoms in women with treatment-resistant fibromyalgia.
Most recently, they found significant differences in the composition of the gut microbiome in a cohort of patients with CRPS from Israel, compared to matched pain-free control individuals.
Notably, two species — Dialister succinatiphilus and Phascolarctobacterium faecium – were enriched in patients with CRPS, while three species — Ligilactobacillus salivarius, Bifidobacterium dentium, and Bifidobacterium adolescentis – were increased in control samples, according to their report published last month in Anesthesiology.
“Importantly,” these findings were replicated in an independent cohort of patients with CRPS from Canada, “suggesting that the observed microbiome signature is robust and consistent across different environments,” Minerbi told GI & Hepatology News.
Causal Role?
“These findings collectively suggest a causal role for the gut microbiome in at least some chronic pain conditions,” Minerbi said.
However, the co-authors of a linked editorial cautioned that it’s “unclear if D succinatiphilus or P faecium are functionally relevant to CRPS pathophysiology or if the bacteria increased in healthy control samples protect against CRPS development.”
Minerbi and colleagues also observed that fecal concentrations of all measured short chain fatty acids (SCFA) in patients with CRPS were lower on average compared to pain-free control individuals, of which butyric, hexanoic, and valeric acid showed significant depletion.
Additionally, plasma concentrations of acetic acid showed significant depletion in patients with CRPS vs control individuals, while propionate, butyrate, isobutyrate and 2-methyl-butyric acid showed a trend toward lower concentrations.
The quantification of SCFA in patient stool and serum is a “notable advance” in this study, Zulmary Manjarres, PhD; Ashley Plumb, PhD; and Katelyn Sadler, PhD; with the Center for Advanced Pain Studies at The University of Texas at Dallas, wrote in their editorial.
SCFA are produced by bacteria as a byproduct of dietary fiber fermentation and appropriate levels of these compounds are important to maintain low levels of inflammation in the colon and overall gut health, they explained.
This begs the question of whether administering probiotic bacteria — many of which are believed to exert health benefits through SCFA production — can be used to treat CRPS-associated pain. It’s something that needs to be studied, the editorialists wrote.
Yet, in their view, the “most notable achievement” of Minerbi and colleagues is the development of a machine learning model that accurately, specifically and sensitively categorized individuals as patients with CRPS or control individuals based on their fecal microbiome signature.
The model, trained on exact sequence variant data from the Israeli patients, achieved 89.5% accuracy, 90.0% sensitivity, and 88.9% specificity in distinguishing patients with CRPS from control individuals in the Canadian cohort.
Interestingly, in three patients with CRPS who underwent limb amputation and recovered from their pain, their gut microbiome signature remained unchanged, suggesting that microbiome alterations might precede or persist beyond symptomatic phases.
Test and Treat: Are We There Yet?
The gut microbiome link to chronic pain syndromes is a hot area of research, but for now gut microbial testing followed by treatment aimed at “fixing” the microbiome remains largely experimental.
At this point, comprehensive gut-microbiome sequencing is not a routine, guideline-supported part of care for fibromyalgia or any chronic pain condition.
“Unfortunately, even for doctors interested in this area, we are not quite at the state of being able to diagnose and treat pain syndrome based on microbiome data,” Bonakdar told GI & Hepatology News.
He said there are many reasons for this including that this type of microbiome analysis is not commonly available at a routine lab. If patients do obtain testing, then the results are quite complex and may not translate to a diagnosis or a simple microbiome intervention.
“I think the closest option we have now is considering supplementing with commonly beneficial probiotic in pain conditions,” Bonakdar said.
One example is a preliminary fibromyalgia trial which found that supplementing with Lactobacillus, Bifidobacterium, and Saccharomyces boulardii appeared to have benefit.
“Unfortunately, this is hit or miss as other trials such as one in low back pain did not find benefit,” Bonakdar said.
Addressing gut microbiome changes will become “more actionable when microbiome analysis is more commonplace as well as is the ability to tailor treatment to the abnormalities seen on testing in a real-world manner,” Bonakdar said.
“Until then, there is no harm in promoting an anti-inflammatory diet for our patients with pain which we know can improve components of the microbiome while also supporting pain management,” he concluded.
Minerbi, Bonakdar, and the editorial writers had no relevant disclosures.
A version of this article appeared on Medscape.com.
Experiencing DDW as an Early Career GI
Dear Friends,
Like many readers, I just returned from Digestive Disease Week® (DDW) in San Diego, California. For the first time in my early career, my experience was not just overwhelming and exhausting. Before, I wanted to do everything – lectures, posters, meetings with friends, prospective research collaborators, and more! This year, I acknowledged that instead of spreading myself thin and not fully engaging, I made a focused daily schedule mixed with productivity and social events, selecting only what was most important to me at this time in my career. This time, after DDW, instead of giving in to my inner introvert and holing myself in my house for a week to recover, I am invigorated by what I learned and the people I met. I can’t wait to see what’s to come next year!
In this issue’s “In Focus”, Dr. Evan Dellon describes his diagnostic approach, including a clear history, endoscopic evaluation with biopsy, and ruling out other causes of esophageal eosinophilia. He emphasizes that treatment should target both inflammation and fibrostenosis and reviews the guidelines and evidence behind first-line treatments, surveillance, and long-term maintenance.
In the second of a two-part series in the “Short Clinical Review” section, Dr. Christopher Vélez, Dr. Rosa L. Yu, and Dr. Jennifer Dimino discuss care for patients with disorders of brain-gut interaction from historically marginalized communities. They highlight ways to improve care for these patients in day-to-day clinical practice.
The transition from trainee to a practicing gastroenterologist may bring with it responsibilities of giving feedback to trainees and/or colleagues to improve. In the “Early Career” section, Dr. Michelle Baliss and Dr. Christine Hachem give practical tips on how best to deliver feedback, with a focus on creating time, building rapport, bidirectional communication, and more.
Lastly, in the “Finance/Legal” section, John S. Gardner, a financial advisor, guides trainees and early career gastroenterologists through estate planning – why it’s important, how to do it effectively, and long-term benefits to starting early.
If you are interested in contributing or have ideas for future TNG topics, please contact me ([email protected]) or Danielle Kiefer ([email protected]), Communications/Managing Editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are now without appreciating where we were: the first case of eosinophilic esophagitis was only first described in 1978 and became a distinct entity in the early 1990s.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Assistant Professor of Medicine
Interventional Endoscopy, Division of Gastroenterology
Washington University School of Medicine in St. Louis
Dear Friends,
Like many readers, I just returned from Digestive Disease Week® (DDW) in San Diego, California. For the first time in my early career, my experience was not just overwhelming and exhausting. Before, I wanted to do everything – lectures, posters, meetings with friends, prospective research collaborators, and more! This year, I acknowledged that instead of spreading myself thin and not fully engaging, I made a focused daily schedule mixed with productivity and social events, selecting only what was most important to me at this time in my career. This time, after DDW, instead of giving in to my inner introvert and holing myself in my house for a week to recover, I am invigorated by what I learned and the people I met. I can’t wait to see what’s to come next year!
In this issue’s “In Focus”, Dr. Evan Dellon describes his diagnostic approach, including a clear history, endoscopic evaluation with biopsy, and ruling out other causes of esophageal eosinophilia. He emphasizes that treatment should target both inflammation and fibrostenosis and reviews the guidelines and evidence behind first-line treatments, surveillance, and long-term maintenance.
In the second of a two-part series in the “Short Clinical Review” section, Dr. Christopher Vélez, Dr. Rosa L. Yu, and Dr. Jennifer Dimino discuss care for patients with disorders of brain-gut interaction from historically marginalized communities. They highlight ways to improve care for these patients in day-to-day clinical practice.
The transition from trainee to a practicing gastroenterologist may bring with it responsibilities of giving feedback to trainees and/or colleagues to improve. In the “Early Career” section, Dr. Michelle Baliss and Dr. Christine Hachem give practical tips on how best to deliver feedback, with a focus on creating time, building rapport, bidirectional communication, and more.
Lastly, in the “Finance/Legal” section, John S. Gardner, a financial advisor, guides trainees and early career gastroenterologists through estate planning – why it’s important, how to do it effectively, and long-term benefits to starting early.
If you are interested in contributing or have ideas for future TNG topics, please contact me ([email protected]) or Danielle Kiefer ([email protected]), Communications/Managing Editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are now without appreciating where we were: the first case of eosinophilic esophagitis was only first described in 1978 and became a distinct entity in the early 1990s.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Assistant Professor of Medicine
Interventional Endoscopy, Division of Gastroenterology
Washington University School of Medicine in St. Louis
Dear Friends,
Like many readers, I just returned from Digestive Disease Week® (DDW) in San Diego, California. For the first time in my early career, my experience was not just overwhelming and exhausting. Before, I wanted to do everything – lectures, posters, meetings with friends, prospective research collaborators, and more! This year, I acknowledged that instead of spreading myself thin and not fully engaging, I made a focused daily schedule mixed with productivity and social events, selecting only what was most important to me at this time in my career. This time, after DDW, instead of giving in to my inner introvert and holing myself in my house for a week to recover, I am invigorated by what I learned and the people I met. I can’t wait to see what’s to come next year!
In this issue’s “In Focus”, Dr. Evan Dellon describes his diagnostic approach, including a clear history, endoscopic evaluation with biopsy, and ruling out other causes of esophageal eosinophilia. He emphasizes that treatment should target both inflammation and fibrostenosis and reviews the guidelines and evidence behind first-line treatments, surveillance, and long-term maintenance.
In the second of a two-part series in the “Short Clinical Review” section, Dr. Christopher Vélez, Dr. Rosa L. Yu, and Dr. Jennifer Dimino discuss care for patients with disorders of brain-gut interaction from historically marginalized communities. They highlight ways to improve care for these patients in day-to-day clinical practice.
The transition from trainee to a practicing gastroenterologist may bring with it responsibilities of giving feedback to trainees and/or colleagues to improve. In the “Early Career” section, Dr. Michelle Baliss and Dr. Christine Hachem give practical tips on how best to deliver feedback, with a focus on creating time, building rapport, bidirectional communication, and more.
Lastly, in the “Finance/Legal” section, John S. Gardner, a financial advisor, guides trainees and early career gastroenterologists through estate planning – why it’s important, how to do it effectively, and long-term benefits to starting early.
If you are interested in contributing or have ideas for future TNG topics, please contact me ([email protected]) or Danielle Kiefer ([email protected]), Communications/Managing Editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are now without appreciating where we were: the first case of eosinophilic esophagitis was only first described in 1978 and became a distinct entity in the early 1990s.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Assistant Professor of Medicine
Interventional Endoscopy, Division of Gastroenterology
Washington University School of Medicine in St. Louis
Landmark 20-Year Study Reshapes Understanding of PTSD
A large 20-year study — the longest and most detailed of its kind — shows that posttraumatic stress disorder (PTSD) symptoms can endure for decades, challenging conventional timelines for recovery and offering new insights to guide future treatment.
Researchers analyzed data from the World Trade Center Health Program (WTCHP), which is administered by the US CDC’s National Institute for Occupational Safety and Health (NIOSH), and found symptoms of PTSD persisted for as long as two decades in 10% of first responders involved in the World Trade Center disaster of September 2001.
Participation in the WTCHP is voluntary, but those who enroll receive free assessments, monitoring, and treatment, including psychiatric and behavioral healthcare. It is the longest and most detailed analysis of PTSD and includes 81,298 observations from 12,822 WTC responders.
Participants entered the WTCHP at different timepoints and were assessed annually. Not every enrollee was assessed every year, but the sheer number of participants and observations “just provides much greater density of data over that 20-year course than any previous study,” lead author Frank D. Mann, PhD, told this news organization.
The study was published online on May 27 in Nature Mental Health.
Filling the PTSD Knowledge Gap
Most PTSD research has focused on the short term, with limited insight into how symptoms evolve over the long haul. Without long-term data, it’s been difficult to understand whether PTSD resolves, persists, or worsens — hindering efforts to guide treatment and support. This study aimed to fill that gap by tracking symptom patterns over two decades.
Responders were assessed regularly using the PTSD Checklist for a Specific Stressor, a standardized tool that measures symptom severity on an 85-point scale. On average, each participant completed 6.3 assessments over the course of the study.
A score of ≥ 44 was considered indicative of clinically elevated PTSD symptoms. Between 2002 and 2022, the crude prevalence of elevated symptoms ranged from 8% to 15%. At the same time, 16% to 34% of responders each year reported little to no symptoms, scoring at or near the minimum on the scale.
The researchers found that symptom trajectories varied widely. Nearly as many participants experienced worsening symptoms as those who improved. As a result, the overall population average remained relatively flat over the 20-year period.
Among responders who met the threshold for PTSD, the median time to symptom improvement was 8.9 years — and by year 20, about 76% had shown improvement.
New Insights
Mann, a senior research scientist at Stony Brook University Renaissance School of Medicine, Stony Brook, New York, said the study not only reinforced existing knowledge about PTSD in responders but also uncovered new insights.
Most notably, it showed that PTSD symptoms tended to peak around a decade after 9/11 — significantly later than delayed-onset patterns reported in previous trauma studies.
He also noted a surprising outcome — the top 10% of responders who experienced worsening symptoms over the long term accounted for the majority of mental health costs. These individuals, Mann said, represent a critical gap in care, with current interventions proving largely ineffective for them.
Mann suggested that ongoing trauma exposure — especially for responders still in high-risk jobs — and potential genetic susceptibility may contribute to late-emerging or persistent symptoms.
“These individuals are an urgent priority for health systems, as available resources have not been effective for them,” the study authors wrote.
Mann and his colleagues also found that occupation offered the strongest protection against developing PTSD. Police officers and firefighters benefit from training designed to help them cope with trauma, and repeated exposure may build a degree of resilience.
In contrast, responders without such training — like construction workers — faced a 50% to 55% higher risk of developing PTSD symptoms. Mann emphasized that occupational status was a more powerful predictor of PTSD risk than the severity of the traumatic exposures themselves.
A Valuable Contribution
Commenting on the research for this news organization, Sandra Lowe, MD, medical director of the Mount Sinai WTCMH program, noted that while the study largely confirms what has been known about responders — such as the significant variability in symptom trajectories over time — it still makes a valuable contribution.
“Extending observations for up to 20 years is rare in any study, especially in a cohort this large,” said Lowe, an associate professor of psychiatry at the Icahn School of Medicine at Mount Sinai, New York City, who was not involved in the study.
Also commenting, James West, MD, chair of the American Psychiatric Association’s Committee on the Psychiatric Dimensions of Disaster, described the finding that 10% of responders continued to experience symptoms two decades after exposure as “sobering.”
However, he emphasized that it aligns with observations in the disaster recovery community, where the psychological impact “goes way beyond what most people see as the immediate aftermath and recovery.” West stressed the urgent need to develop effective treatments that enable those affected to live fuller, less impaired lives.
“We still need to be finding the effective treatments that can help these people live fuller lives without impairment from their trauma symptoms,” said West.
Lowe pointed out that the symptom peak around 10 years post-exposure is often linked to external factors. Some responders who had been managing symptoms might lose resilience due to major life changes such as retirement.
“One of the things that was able to keep them engaged is now lost,” she said. “They begin to spend more time reflecting on recollections, and symptoms can worsen.”
West agreed, adding that retirement or job loss often leads to symptom increases because it removes a primary coping mechanism. Both Lowe and Mann also highlighted that 9/11 memorial events can trigger new symptoms or exacerbate existing ones.
Lowe noted that responders with stronger coping skills tended to fare better over time. Effective coping strategies include maintaining regular schedules — especially for eating and sleeping — leading a structured life, and employing stress management techniques like meditation, yoga, or enjoyable hobbies. Social connection and being part of a community are also critical for resilience. She added that clinicians should always inquire about trauma history.
Lowe, West, and Mann all pointed out that PTSD is often accompanied by physical health issues, particularly cardiovascular problems, which tend to be worse in those with the disorder.
Responders with stronger coping skills tended to do better over time, said Lowe. Coping skills that can help make a difference include having a regular schedule, especially for eating and sleeping; having a structured life; and stress management tools, such as meditation or yoga or an enjoyable hobby. Social connection — being part of a community — is also critical, Lowe said.
Clinicians should always inquire about trauma, she said. Lowe, West, and Mann all noted that people with PTSD often have physical illness and that cardiovascular outcomes in particular are worse for those individuals.
WTCHP Future Uncertain
However, despite advances in understanding PTSD and the importance of ongoing care, the future of the program supporting World Trade Center responders remains uncertain.
Some 140,000 people are now enrolled in the WTCHP, which was established as a federal program in 2010. Congress has generally reauthorized the program whenever its funding came up for renewal.
However, earlier this year, the Trump administration dismissed two thirds of the NIOSH workforce, including John Howard, MD, the administrator of the WTCHP.
In response, members of Congress and advocates for 9/11 survivors urged the US Department of Health and Human Services (HHS) to reinstate Howard and the affected employees. Howard is listed as back on the job has since returned to his position, and HHS reportedly reinstated hundreds of NIOSH workers in May.
An HHS spokesperson told this news organization that the WTCHP continues to provide services and is actively “accepting, reviewing, and processing new enrollment applications and certification requests.”
Meanwhile, the Trump administration’s fiscal year 2026 budget proposal seeks to reduce CDC funding by $3.5 billion — approximately 40% — with a shift in focus toward infectious diseases. It remains unclear how the WTCHP will be affected by this new direction.
Mann said he is not involved in the program’s funding details but added, “Presumably, as long as some funding continues to keep the program alive, we will continue monitoring responders and providing free treatment until the very last World Trade Center responder passes.”
The study was partially funded through National Institutes of Health and CDC grants, the SUNY Research Foundation, and the CDC’s World Trade Center Health Program. Mann, Lowe, and West reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A large 20-year study — the longest and most detailed of its kind — shows that posttraumatic stress disorder (PTSD) symptoms can endure for decades, challenging conventional timelines for recovery and offering new insights to guide future treatment.
Researchers analyzed data from the World Trade Center Health Program (WTCHP), which is administered by the US CDC’s National Institute for Occupational Safety and Health (NIOSH), and found symptoms of PTSD persisted for as long as two decades in 10% of first responders involved in the World Trade Center disaster of September 2001.
Participation in the WTCHP is voluntary, but those who enroll receive free assessments, monitoring, and treatment, including psychiatric and behavioral healthcare. It is the longest and most detailed analysis of PTSD and includes 81,298 observations from 12,822 WTC responders.
Participants entered the WTCHP at different timepoints and were assessed annually. Not every enrollee was assessed every year, but the sheer number of participants and observations “just provides much greater density of data over that 20-year course than any previous study,” lead author Frank D. Mann, PhD, told this news organization.
The study was published online on May 27 in Nature Mental Health.
Filling the PTSD Knowledge Gap
Most PTSD research has focused on the short term, with limited insight into how symptoms evolve over the long haul. Without long-term data, it’s been difficult to understand whether PTSD resolves, persists, or worsens — hindering efforts to guide treatment and support. This study aimed to fill that gap by tracking symptom patterns over two decades.
Responders were assessed regularly using the PTSD Checklist for a Specific Stressor, a standardized tool that measures symptom severity on an 85-point scale. On average, each participant completed 6.3 assessments over the course of the study.
A score of ≥ 44 was considered indicative of clinically elevated PTSD symptoms. Between 2002 and 2022, the crude prevalence of elevated symptoms ranged from 8% to 15%. At the same time, 16% to 34% of responders each year reported little to no symptoms, scoring at or near the minimum on the scale.
The researchers found that symptom trajectories varied widely. Nearly as many participants experienced worsening symptoms as those who improved. As a result, the overall population average remained relatively flat over the 20-year period.
Among responders who met the threshold for PTSD, the median time to symptom improvement was 8.9 years — and by year 20, about 76% had shown improvement.
New Insights
Mann, a senior research scientist at Stony Brook University Renaissance School of Medicine, Stony Brook, New York, said the study not only reinforced existing knowledge about PTSD in responders but also uncovered new insights.
Most notably, it showed that PTSD symptoms tended to peak around a decade after 9/11 — significantly later than delayed-onset patterns reported in previous trauma studies.
He also noted a surprising outcome — the top 10% of responders who experienced worsening symptoms over the long term accounted for the majority of mental health costs. These individuals, Mann said, represent a critical gap in care, with current interventions proving largely ineffective for them.
Mann suggested that ongoing trauma exposure — especially for responders still in high-risk jobs — and potential genetic susceptibility may contribute to late-emerging or persistent symptoms.
“These individuals are an urgent priority for health systems, as available resources have not been effective for them,” the study authors wrote.
Mann and his colleagues also found that occupation offered the strongest protection against developing PTSD. Police officers and firefighters benefit from training designed to help them cope with trauma, and repeated exposure may build a degree of resilience.
In contrast, responders without such training — like construction workers — faced a 50% to 55% higher risk of developing PTSD symptoms. Mann emphasized that occupational status was a more powerful predictor of PTSD risk than the severity of the traumatic exposures themselves.
A Valuable Contribution
Commenting on the research for this news organization, Sandra Lowe, MD, medical director of the Mount Sinai WTCMH program, noted that while the study largely confirms what has been known about responders — such as the significant variability in symptom trajectories over time — it still makes a valuable contribution.
“Extending observations for up to 20 years is rare in any study, especially in a cohort this large,” said Lowe, an associate professor of psychiatry at the Icahn School of Medicine at Mount Sinai, New York City, who was not involved in the study.
Also commenting, James West, MD, chair of the American Psychiatric Association’s Committee on the Psychiatric Dimensions of Disaster, described the finding that 10% of responders continued to experience symptoms two decades after exposure as “sobering.”
However, he emphasized that it aligns with observations in the disaster recovery community, where the psychological impact “goes way beyond what most people see as the immediate aftermath and recovery.” West stressed the urgent need to develop effective treatments that enable those affected to live fuller, less impaired lives.
“We still need to be finding the effective treatments that can help these people live fuller lives without impairment from their trauma symptoms,” said West.
Lowe pointed out that the symptom peak around 10 years post-exposure is often linked to external factors. Some responders who had been managing symptoms might lose resilience due to major life changes such as retirement.
“One of the things that was able to keep them engaged is now lost,” she said. “They begin to spend more time reflecting on recollections, and symptoms can worsen.”
West agreed, adding that retirement or job loss often leads to symptom increases because it removes a primary coping mechanism. Both Lowe and Mann also highlighted that 9/11 memorial events can trigger new symptoms or exacerbate existing ones.
Lowe noted that responders with stronger coping skills tended to fare better over time. Effective coping strategies include maintaining regular schedules — especially for eating and sleeping — leading a structured life, and employing stress management techniques like meditation, yoga, or enjoyable hobbies. Social connection and being part of a community are also critical for resilience. She added that clinicians should always inquire about trauma history.
Lowe, West, and Mann all pointed out that PTSD is often accompanied by physical health issues, particularly cardiovascular problems, which tend to be worse in those with the disorder.
Responders with stronger coping skills tended to do better over time, said Lowe. Coping skills that can help make a difference include having a regular schedule, especially for eating and sleeping; having a structured life; and stress management tools, such as meditation or yoga or an enjoyable hobby. Social connection — being part of a community — is also critical, Lowe said.
Clinicians should always inquire about trauma, she said. Lowe, West, and Mann all noted that people with PTSD often have physical illness and that cardiovascular outcomes in particular are worse for those individuals.
WTCHP Future Uncertain
However, despite advances in understanding PTSD and the importance of ongoing care, the future of the program supporting World Trade Center responders remains uncertain.
Some 140,000 people are now enrolled in the WTCHP, which was established as a federal program in 2010. Congress has generally reauthorized the program whenever its funding came up for renewal.
However, earlier this year, the Trump administration dismissed two thirds of the NIOSH workforce, including John Howard, MD, the administrator of the WTCHP.
In response, members of Congress and advocates for 9/11 survivors urged the US Department of Health and Human Services (HHS) to reinstate Howard and the affected employees. Howard is listed as back on the job has since returned to his position, and HHS reportedly reinstated hundreds of NIOSH workers in May.
An HHS spokesperson told this news organization that the WTCHP continues to provide services and is actively “accepting, reviewing, and processing new enrollment applications and certification requests.”
Meanwhile, the Trump administration’s fiscal year 2026 budget proposal seeks to reduce CDC funding by $3.5 billion — approximately 40% — with a shift in focus toward infectious diseases. It remains unclear how the WTCHP will be affected by this new direction.
Mann said he is not involved in the program’s funding details but added, “Presumably, as long as some funding continues to keep the program alive, we will continue monitoring responders and providing free treatment until the very last World Trade Center responder passes.”
The study was partially funded through National Institutes of Health and CDC grants, the SUNY Research Foundation, and the CDC’s World Trade Center Health Program. Mann, Lowe, and West reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A large 20-year study — the longest and most detailed of its kind — shows that posttraumatic stress disorder (PTSD) symptoms can endure for decades, challenging conventional timelines for recovery and offering new insights to guide future treatment.
Researchers analyzed data from the World Trade Center Health Program (WTCHP), which is administered by the US CDC’s National Institute for Occupational Safety and Health (NIOSH), and found symptoms of PTSD persisted for as long as two decades in 10% of first responders involved in the World Trade Center disaster of September 2001.
Participation in the WTCHP is voluntary, but those who enroll receive free assessments, monitoring, and treatment, including psychiatric and behavioral healthcare. It is the longest and most detailed analysis of PTSD and includes 81,298 observations from 12,822 WTC responders.
Participants entered the WTCHP at different timepoints and were assessed annually. Not every enrollee was assessed every year, but the sheer number of participants and observations “just provides much greater density of data over that 20-year course than any previous study,” lead author Frank D. Mann, PhD, told this news organization.
The study was published online on May 27 in Nature Mental Health.
Filling the PTSD Knowledge Gap
Most PTSD research has focused on the short term, with limited insight into how symptoms evolve over the long haul. Without long-term data, it’s been difficult to understand whether PTSD resolves, persists, or worsens — hindering efforts to guide treatment and support. This study aimed to fill that gap by tracking symptom patterns over two decades.
Responders were assessed regularly using the PTSD Checklist for a Specific Stressor, a standardized tool that measures symptom severity on an 85-point scale. On average, each participant completed 6.3 assessments over the course of the study.
A score of ≥ 44 was considered indicative of clinically elevated PTSD symptoms. Between 2002 and 2022, the crude prevalence of elevated symptoms ranged from 8% to 15%. At the same time, 16% to 34% of responders each year reported little to no symptoms, scoring at or near the minimum on the scale.
The researchers found that symptom trajectories varied widely. Nearly as many participants experienced worsening symptoms as those who improved. As a result, the overall population average remained relatively flat over the 20-year period.
Among responders who met the threshold for PTSD, the median time to symptom improvement was 8.9 years — and by year 20, about 76% had shown improvement.
New Insights
Mann, a senior research scientist at Stony Brook University Renaissance School of Medicine, Stony Brook, New York, said the study not only reinforced existing knowledge about PTSD in responders but also uncovered new insights.
Most notably, it showed that PTSD symptoms tended to peak around a decade after 9/11 — significantly later than delayed-onset patterns reported in previous trauma studies.
He also noted a surprising outcome — the top 10% of responders who experienced worsening symptoms over the long term accounted for the majority of mental health costs. These individuals, Mann said, represent a critical gap in care, with current interventions proving largely ineffective for them.
Mann suggested that ongoing trauma exposure — especially for responders still in high-risk jobs — and potential genetic susceptibility may contribute to late-emerging or persistent symptoms.
“These individuals are an urgent priority for health systems, as available resources have not been effective for them,” the study authors wrote.
Mann and his colleagues also found that occupation offered the strongest protection against developing PTSD. Police officers and firefighters benefit from training designed to help them cope with trauma, and repeated exposure may build a degree of resilience.
In contrast, responders without such training — like construction workers — faced a 50% to 55% higher risk of developing PTSD symptoms. Mann emphasized that occupational status was a more powerful predictor of PTSD risk than the severity of the traumatic exposures themselves.
A Valuable Contribution
Commenting on the research for this news organization, Sandra Lowe, MD, medical director of the Mount Sinai WTCMH program, noted that while the study largely confirms what has been known about responders — such as the significant variability in symptom trajectories over time — it still makes a valuable contribution.
“Extending observations for up to 20 years is rare in any study, especially in a cohort this large,” said Lowe, an associate professor of psychiatry at the Icahn School of Medicine at Mount Sinai, New York City, who was not involved in the study.
Also commenting, James West, MD, chair of the American Psychiatric Association’s Committee on the Psychiatric Dimensions of Disaster, described the finding that 10% of responders continued to experience symptoms two decades after exposure as “sobering.”
However, he emphasized that it aligns with observations in the disaster recovery community, where the psychological impact “goes way beyond what most people see as the immediate aftermath and recovery.” West stressed the urgent need to develop effective treatments that enable those affected to live fuller, less impaired lives.
“We still need to be finding the effective treatments that can help these people live fuller lives without impairment from their trauma symptoms,” said West.
Lowe pointed out that the symptom peak around 10 years post-exposure is often linked to external factors. Some responders who had been managing symptoms might lose resilience due to major life changes such as retirement.
“One of the things that was able to keep them engaged is now lost,” she said. “They begin to spend more time reflecting on recollections, and symptoms can worsen.”
West agreed, adding that retirement or job loss often leads to symptom increases because it removes a primary coping mechanism. Both Lowe and Mann also highlighted that 9/11 memorial events can trigger new symptoms or exacerbate existing ones.
Lowe noted that responders with stronger coping skills tended to fare better over time. Effective coping strategies include maintaining regular schedules — especially for eating and sleeping — leading a structured life, and employing stress management techniques like meditation, yoga, or enjoyable hobbies. Social connection and being part of a community are also critical for resilience. She added that clinicians should always inquire about trauma history.
Lowe, West, and Mann all pointed out that PTSD is often accompanied by physical health issues, particularly cardiovascular problems, which tend to be worse in those with the disorder.
Responders with stronger coping skills tended to do better over time, said Lowe. Coping skills that can help make a difference include having a regular schedule, especially for eating and sleeping; having a structured life; and stress management tools, such as meditation or yoga or an enjoyable hobby. Social connection — being part of a community — is also critical, Lowe said.
Clinicians should always inquire about trauma, she said. Lowe, West, and Mann all noted that people with PTSD often have physical illness and that cardiovascular outcomes in particular are worse for those individuals.
WTCHP Future Uncertain
However, despite advances in understanding PTSD and the importance of ongoing care, the future of the program supporting World Trade Center responders remains uncertain.
Some 140,000 people are now enrolled in the WTCHP, which was established as a federal program in 2010. Congress has generally reauthorized the program whenever its funding came up for renewal.
However, earlier this year, the Trump administration dismissed two thirds of the NIOSH workforce, including John Howard, MD, the administrator of the WTCHP.
In response, members of Congress and advocates for 9/11 survivors urged the US Department of Health and Human Services (HHS) to reinstate Howard and the affected employees. Howard is listed as back on the job has since returned to his position, and HHS reportedly reinstated hundreds of NIOSH workers in May.
An HHS spokesperson told this news organization that the WTCHP continues to provide services and is actively “accepting, reviewing, and processing new enrollment applications and certification requests.”
Meanwhile, the Trump administration’s fiscal year 2026 budget proposal seeks to reduce CDC funding by $3.5 billion — approximately 40% — with a shift in focus toward infectious diseases. It remains unclear how the WTCHP will be affected by this new direction.
Mann said he is not involved in the program’s funding details but added, “Presumably, as long as some funding continues to keep the program alive, we will continue monitoring responders and providing free treatment until the very last World Trade Center responder passes.”
The study was partially funded through National Institutes of Health and CDC grants, the SUNY Research Foundation, and the CDC’s World Trade Center Health Program. Mann, Lowe, and West reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NATURE MENTAL HEALTH
Posttraumatic Stress Disorder May Increase Morbidity Risk in Veterans With HIV
TOPLINE:
Posttraumatic stress disorder (PTSD) among veterans living with HIV significantly increased the risk for AIDS and multiple comorbidities, particularly arthritis, cardiovascular disease (CVD), chronic obstructive pulmonary disease (COPD), chronic kidney disease (CKD), and multimorbidity — with the greatest impact seen in the first decade after diagnosis.
METHODOLOGY:
- Researchers conducted a prospective cohort study to assess whether PTSD is associated with increased risk for adverse clinical outcomes in veterans with HIV who received care at the Department of Veterans Affairs.
- They included 3206 veterans (97.4% men; median age at HIV diagnosis, 31.7 years; 42.1% with PTSD) who were deployed in Iraq and Afghanistan while serving in the military and initiated antiretroviral therapy before December 31, 2020.
- Participants were followed-up until December 2022, with censoring at death, the last health care visit, or study termination. The association between PTSD with morbidity and mortality, considering the number of deployments and levels of combat exposure were determined.
TAKEAWAY:
- PTSD significantly increased the overall risks for AIDS by 11% (adjusted hazard ratio [aHR], 1.11), CKD by 21% (aHR, 1.21), COPD by 46% (aHR, 1.46), multimorbidity by 49% (aHR, 1.49), CVD by 57% (aHR, 1.57), and arthritis by two folds (aHR, 1.95; P <.05 for all).
- Among veterans with a single deployment, those with PTSD had 92%, 87%, 80%, 53%, 44%, 32%, and 27% higher risks for asthma, CVD, arthritis, multimorbidity, COPD, liver disease, and AIDS, respectively, than those without PTSD.
- Veterans with PTSD and combat exposure had a lower risk for AIDS but higher risks for multimorbidity, asthma, CVD, and arthritis than those never diagnosed with PTSD and unexposed to combat.
- The associations of PTSD with mortality and morbidity appeared most pronounced in the first decade post-diagnosis, followed by a gradual decline in association strength; however, risks remained elevated.
IN PRACTICE:
“It is recommended that providers who work with VWH [veterans with HIV] consider adopting a trauma-informed model of HIV care and that providers screen veterans for PTSD, so that their unique trauma history can help guide medical decisions and treatment,” the authors wrote.
SOURCE:
This study was led by Kartavya J. Vyas, PhD, Department of Epidemiology, Rollins School of Public Health, Emory University, Atlanta. It was published online in AIDS .
LIMITATIONS:
The data could not capture each individual’s true index trauma or the severity of their PTSD. Additionally, the study was limited by considerable loss to follow-up, potential uncontrolled confounding related to homelessness, and a lack of generalizability to veterans with HIV who were not receiving antiretroviral therapy.
DISCLOSURES:
The study did not receive any specific funding. Two authors reported receiving federal research support — one from the Emory Center for AIDS Research and the National Institute of Allergy and Infectious Diseases, and the other from the National Institutes of Health and the CDC — in addition to investigator-initiated grants and consulting fees from various pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Posttraumatic stress disorder (PTSD) among veterans living with HIV significantly increased the risk for AIDS and multiple comorbidities, particularly arthritis, cardiovascular disease (CVD), chronic obstructive pulmonary disease (COPD), chronic kidney disease (CKD), and multimorbidity — with the greatest impact seen in the first decade after diagnosis.
METHODOLOGY:
- Researchers conducted a prospective cohort study to assess whether PTSD is associated with increased risk for adverse clinical outcomes in veterans with HIV who received care at the Department of Veterans Affairs.
- They included 3206 veterans (97.4% men; median age at HIV diagnosis, 31.7 years; 42.1% with PTSD) who were deployed in Iraq and Afghanistan while serving in the military and initiated antiretroviral therapy before December 31, 2020.
- Participants were followed-up until December 2022, with censoring at death, the last health care visit, or study termination. The association between PTSD with morbidity and mortality, considering the number of deployments and levels of combat exposure were determined.
TAKEAWAY:
- PTSD significantly increased the overall risks for AIDS by 11% (adjusted hazard ratio [aHR], 1.11), CKD by 21% (aHR, 1.21), COPD by 46% (aHR, 1.46), multimorbidity by 49% (aHR, 1.49), CVD by 57% (aHR, 1.57), and arthritis by two folds (aHR, 1.95; P <.05 for all).
- Among veterans with a single deployment, those with PTSD had 92%, 87%, 80%, 53%, 44%, 32%, and 27% higher risks for asthma, CVD, arthritis, multimorbidity, COPD, liver disease, and AIDS, respectively, than those without PTSD.
- Veterans with PTSD and combat exposure had a lower risk for AIDS but higher risks for multimorbidity, asthma, CVD, and arthritis than those never diagnosed with PTSD and unexposed to combat.
- The associations of PTSD with mortality and morbidity appeared most pronounced in the first decade post-diagnosis, followed by a gradual decline in association strength; however, risks remained elevated.
IN PRACTICE:
“It is recommended that providers who work with VWH [veterans with HIV] consider adopting a trauma-informed model of HIV care and that providers screen veterans for PTSD, so that their unique trauma history can help guide medical decisions and treatment,” the authors wrote.
SOURCE:
This study was led by Kartavya J. Vyas, PhD, Department of Epidemiology, Rollins School of Public Health, Emory University, Atlanta. It was published online in AIDS .
LIMITATIONS:
The data could not capture each individual’s true index trauma or the severity of their PTSD. Additionally, the study was limited by considerable loss to follow-up, potential uncontrolled confounding related to homelessness, and a lack of generalizability to veterans with HIV who were not receiving antiretroviral therapy.
DISCLOSURES:
The study did not receive any specific funding. Two authors reported receiving federal research support — one from the Emory Center for AIDS Research and the National Institute of Allergy and Infectious Diseases, and the other from the National Institutes of Health and the CDC — in addition to investigator-initiated grants and consulting fees from various pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Posttraumatic stress disorder (PTSD) among veterans living with HIV significantly increased the risk for AIDS and multiple comorbidities, particularly arthritis, cardiovascular disease (CVD), chronic obstructive pulmonary disease (COPD), chronic kidney disease (CKD), and multimorbidity — with the greatest impact seen in the first decade after diagnosis.
METHODOLOGY:
- Researchers conducted a prospective cohort study to assess whether PTSD is associated with increased risk for adverse clinical outcomes in veterans with HIV who received care at the Department of Veterans Affairs.
- They included 3206 veterans (97.4% men; median age at HIV diagnosis, 31.7 years; 42.1% with PTSD) who were deployed in Iraq and Afghanistan while serving in the military and initiated antiretroviral therapy before December 31, 2020.
- Participants were followed-up until December 2022, with censoring at death, the last health care visit, or study termination. The association between PTSD with morbidity and mortality, considering the number of deployments and levels of combat exposure were determined.
TAKEAWAY:
- PTSD significantly increased the overall risks for AIDS by 11% (adjusted hazard ratio [aHR], 1.11), CKD by 21% (aHR, 1.21), COPD by 46% (aHR, 1.46), multimorbidity by 49% (aHR, 1.49), CVD by 57% (aHR, 1.57), and arthritis by two folds (aHR, 1.95; P <.05 for all).
- Among veterans with a single deployment, those with PTSD had 92%, 87%, 80%, 53%, 44%, 32%, and 27% higher risks for asthma, CVD, arthritis, multimorbidity, COPD, liver disease, and AIDS, respectively, than those without PTSD.
- Veterans with PTSD and combat exposure had a lower risk for AIDS but higher risks for multimorbidity, asthma, CVD, and arthritis than those never diagnosed with PTSD and unexposed to combat.
- The associations of PTSD with mortality and morbidity appeared most pronounced in the first decade post-diagnosis, followed by a gradual decline in association strength; however, risks remained elevated.
IN PRACTICE:
“It is recommended that providers who work with VWH [veterans with HIV] consider adopting a trauma-informed model of HIV care and that providers screen veterans for PTSD, so that their unique trauma history can help guide medical decisions and treatment,” the authors wrote.
SOURCE:
This study was led by Kartavya J. Vyas, PhD, Department of Epidemiology, Rollins School of Public Health, Emory University, Atlanta. It was published online in AIDS .
LIMITATIONS:
The data could not capture each individual’s true index trauma or the severity of their PTSD. Additionally, the study was limited by considerable loss to follow-up, potential uncontrolled confounding related to homelessness, and a lack of generalizability to veterans with HIV who were not receiving antiretroviral therapy.
DISCLOSURES:
The study did not receive any specific funding. Two authors reported receiving federal research support — one from the Emory Center for AIDS Research and the National Institute of Allergy and Infectious Diseases, and the other from the National Institutes of Health and the CDC — in addition to investigator-initiated grants and consulting fees from various pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Vital Partners in GI Care
Demand for specialized GI care has skyrocketed in recent years, eclipsing the supply of gastroenterologists and impairing patient access to high-quality GI care, particularly in rural and other underserved areas. In this environment,
Across specialties, APPs are estimated to constitute roughly a third of the US clinical workforce, and demand is only growing. A June 2024 MGMA Stat poll found that 63% of medical groups planned to add new APP roles in the next year. As the GI APP workforce grows, so too will demand for advanced training tailored to the APP role.
AGA has invested heavily in professional development opportunities for NPs and PAs, in recognition of their vital role in providing high-quality GI care. The newly formed AGA NPPA Task Force, co-chaired by Abigail Meyers (who we featured in GIHN’s April issue) and Kimberly Kearns, works closely with the Education and Training Committee to develop education programs to meet the specific needs of NPs and PAs, and advocate for more APP involvement in AGA programming. One example of this is AGA’s 2025 Principles of GI for the NP and PA course, which will be held in Chicago in early August – I encourage you to spread the word and support your APP colleagues in getting involved in these important initiatives as our vital partners in GI care delivery.
In this month’s issue of GIHN, we present the exciting results of the BOSS trial, showing no survival difference between regular and at need surveillance for Barrett’s esophagus, suggesting that at need endoscopy may be a safe alternative for low-risk patients. Continuing our coverage of potentially practice-changing research from DDW, we highlight another recent RCT challenging the use of papillary sphincterotomy as a treatment for pancreas divisum.
In our July Member Spotlight, Eric Shah, MD, MBA (University of Michigan), a past AGA Research Scholar Award recipient, highlights how this critical research support aided him in his journey to develop a now FDA-approved point-of care screening tool used to evaluate patients with chronic constipation for pelvic floor dysfunction during a routine clinic visit. In our quarterly Perspectives column, Dr. David Wan (a GI hospitalist) and Dr. Zeyed Metwalli (an interventional radiologist) discuss best practices in management of lower GI bleeding. We hope you have a restful summer!
Megan A. Adams, MD, JD, MSc
Editor in Chief
Demand for specialized GI care has skyrocketed in recent years, eclipsing the supply of gastroenterologists and impairing patient access to high-quality GI care, particularly in rural and other underserved areas. In this environment,
Across specialties, APPs are estimated to constitute roughly a third of the US clinical workforce, and demand is only growing. A June 2024 MGMA Stat poll found that 63% of medical groups planned to add new APP roles in the next year. As the GI APP workforce grows, so too will demand for advanced training tailored to the APP role.
AGA has invested heavily in professional development opportunities for NPs and PAs, in recognition of their vital role in providing high-quality GI care. The newly formed AGA NPPA Task Force, co-chaired by Abigail Meyers (who we featured in GIHN’s April issue) and Kimberly Kearns, works closely with the Education and Training Committee to develop education programs to meet the specific needs of NPs and PAs, and advocate for more APP involvement in AGA programming. One example of this is AGA’s 2025 Principles of GI for the NP and PA course, which will be held in Chicago in early August – I encourage you to spread the word and support your APP colleagues in getting involved in these important initiatives as our vital partners in GI care delivery.
In this month’s issue of GIHN, we present the exciting results of the BOSS trial, showing no survival difference between regular and at need surveillance for Barrett’s esophagus, suggesting that at need endoscopy may be a safe alternative for low-risk patients. Continuing our coverage of potentially practice-changing research from DDW, we highlight another recent RCT challenging the use of papillary sphincterotomy as a treatment for pancreas divisum.
In our July Member Spotlight, Eric Shah, MD, MBA (University of Michigan), a past AGA Research Scholar Award recipient, highlights how this critical research support aided him in his journey to develop a now FDA-approved point-of care screening tool used to evaluate patients with chronic constipation for pelvic floor dysfunction during a routine clinic visit. In our quarterly Perspectives column, Dr. David Wan (a GI hospitalist) and Dr. Zeyed Metwalli (an interventional radiologist) discuss best practices in management of lower GI bleeding. We hope you have a restful summer!
Megan A. Adams, MD, JD, MSc
Editor in Chief
Demand for specialized GI care has skyrocketed in recent years, eclipsing the supply of gastroenterologists and impairing patient access to high-quality GI care, particularly in rural and other underserved areas. In this environment,
Across specialties, APPs are estimated to constitute roughly a third of the US clinical workforce, and demand is only growing. A June 2024 MGMA Stat poll found that 63% of medical groups planned to add new APP roles in the next year. As the GI APP workforce grows, so too will demand for advanced training tailored to the APP role.
AGA has invested heavily in professional development opportunities for NPs and PAs, in recognition of their vital role in providing high-quality GI care. The newly formed AGA NPPA Task Force, co-chaired by Abigail Meyers (who we featured in GIHN’s April issue) and Kimberly Kearns, works closely with the Education and Training Committee to develop education programs to meet the specific needs of NPs and PAs, and advocate for more APP involvement in AGA programming. One example of this is AGA’s 2025 Principles of GI for the NP and PA course, which will be held in Chicago in early August – I encourage you to spread the word and support your APP colleagues in getting involved in these important initiatives as our vital partners in GI care delivery.
In this month’s issue of GIHN, we present the exciting results of the BOSS trial, showing no survival difference between regular and at need surveillance for Barrett’s esophagus, suggesting that at need endoscopy may be a safe alternative for low-risk patients. Continuing our coverage of potentially practice-changing research from DDW, we highlight another recent RCT challenging the use of papillary sphincterotomy as a treatment for pancreas divisum.
In our July Member Spotlight, Eric Shah, MD, MBA (University of Michigan), a past AGA Research Scholar Award recipient, highlights how this critical research support aided him in his journey to develop a now FDA-approved point-of care screening tool used to evaluate patients with chronic constipation for pelvic floor dysfunction during a routine clinic visit. In our quarterly Perspectives column, Dr. David Wan (a GI hospitalist) and Dr. Zeyed Metwalli (an interventional radiologist) discuss best practices in management of lower GI bleeding. We hope you have a restful summer!
Megan A. Adams, MD, JD, MSc
Editor in Chief
Recommendations for Empiric Antibiotic Therapy in Hidradenitis Suppurativa
Recommendations for Empiric Antibiotic Therapy in Hidradenitis Suppurativa
Hidradenitis suppurativa (HS) is a chronic scarring inflammatory skin condition of the follicular epithelium that impacts 1% to 4% of the general population (eFigure).1-3 This statistic likely is an underrepresentation of the affected population due to missed and delayed diagnoses.1 Hidradenitis suppurativa has been identified as having one of the strongest negative impacts on patients’ lives based on studied skin diseases.4 Its recurrent nature can negatively impact both the patient’s physical and mental state.3 Due to the debilitating effects of HS, we aimed to create updated recommendations for empiric antibotics based on affected anatomic locations in an effort to improve patient quality of life.
Methods
An institutional review board–approved retrospective medical chart review of 485 patients diagnosed with HS and evaluated at the University of Texas Medical Branch in Galveston from January 2006 to December 2021 was conducted. Males and females of all ages (including pregnant and pediatric patients) were included. Only patients for whom anatomic locations of HS lesions or culture sites were not documented were excluded from the analysis. Locations of cultures were categorized into 5 groups: axilla; groin; buttocks; inframammary; and multiple sites of involvement, which included any combination of 2 or more sites. Types of bacteria collected from cultures and recorded included Escherichia coli, Enterococcus species, Proteus mirabilis, Pseudomonas aeruginosa, Staphylococcus aureus, coagulase-negative staphylococci (CoNS), and other Gram-negative species. Sensitivity profiles also were analyzed for the most commonly cultured bacteria to create recommendations on antibiotic use based on the anatomic location of the lesions. Data analysis was conducted using descriptive statistics and bivariate analysis.
Results
The analysis included 485 patients comprising 600 visits. Seventy-five percent (363/485) of the study population was female. The axilla was the most common anatomic location for HS lesions followed by multiple sites of involvement. In total, 283 cultures were performed; males were 1.1 times more likely than females to be cultured. While cultures were most frequently obtained in patients with axillary lesions only (93/262 [35%]) or from multiple sites of involvement (83/179 [46%]) as this was the most common presentation of HS in our patient population, cultures were more likely to be obtained when patients presented with only buttock (32/38 [84%]) and inframammary (20/25 [80%]) lesions (Table).
Staphylococcus aureus was the most commonly cultured bacteria in general (53/283 [19%]) as well as for HS located the axilla (24/56 [43%]) and in multiple sites (16/51 [31%]). Proteus mirabilis (29/283 [10%]) was the second most commonly cultured bacteria overall and was cultured most often in the axilla (15/56 [27%]) and inframammary region (6/14 [43%]). These were followed by beta-hemolytic Streptococcus species (26/283 [9%]) and Enterococcus species (21/283 [7%]), which was second to P mirabilis as the most commonly cultured bacteria in the inframammary region (6/14 [43%])(eTable 1).
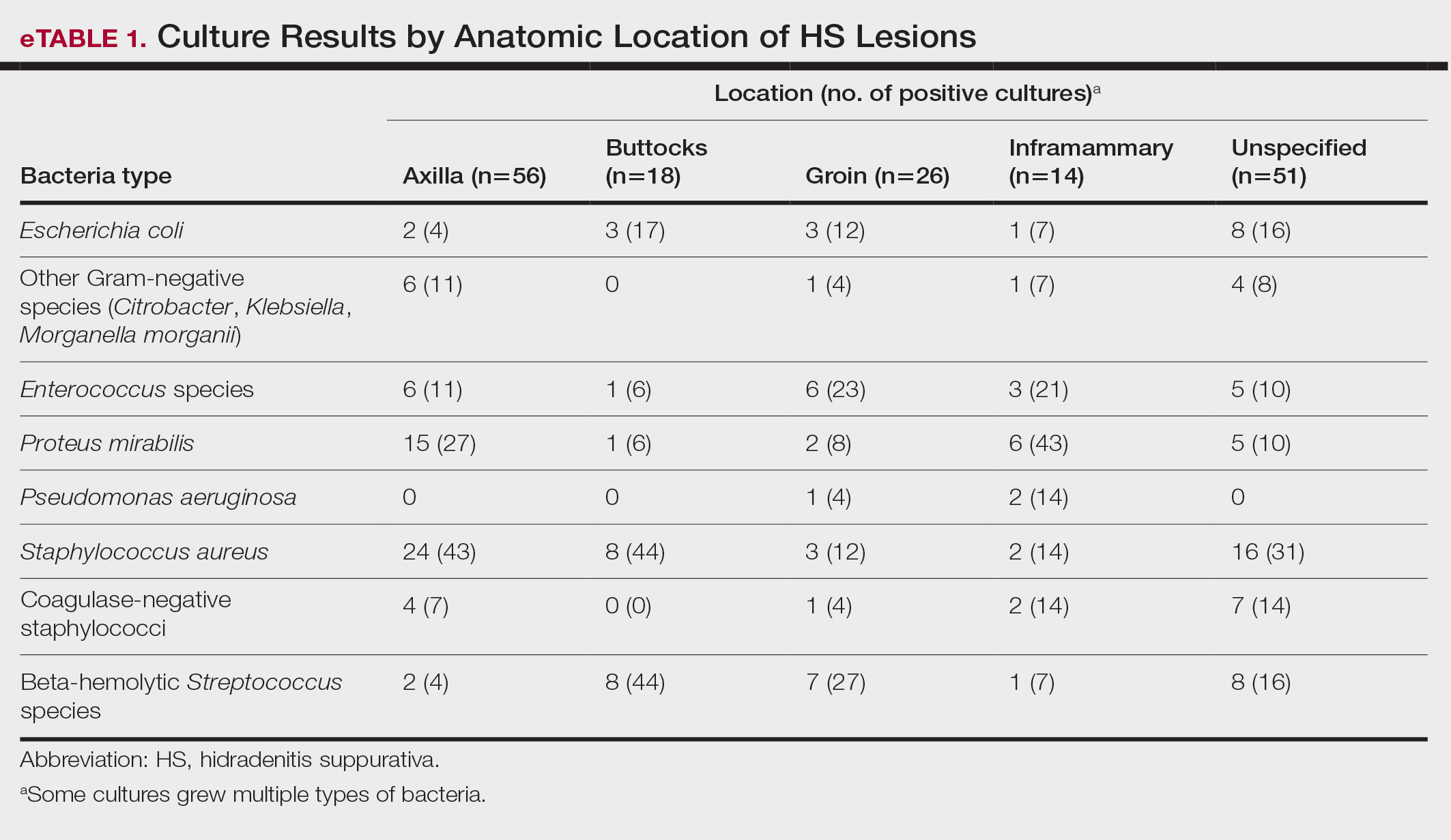
eTable 2 shows the sensitivity profiles for the most commonly cultured bacteria: S aureus, P mirabilis, and Enterococcus species. Staphylococcus aureus located in the axilla, buttocks, and groin was most sensitive to rifampin (41/44 [93%]), TMP/SMX (41/44 [93%]), and tetracycline (39/44 [89%]) and most resistant to erythromycin (26/44 [59%]) and oxacillin (24/44 [55%]). Proteus mirabilis in the inframammary region was most sensitive to ampicillin (27/27 [100%]), gentamicin (27/27 [100%]), levofloxacin (27/27 [100%]), and TMP/SMX (26/27 [96%]). Enterococcus species were most sensitive to vancomycin (20/20 [100%]) and ampicillin (19/20 [95%]) and most resistant to gentamicin (5/20 [25%]).
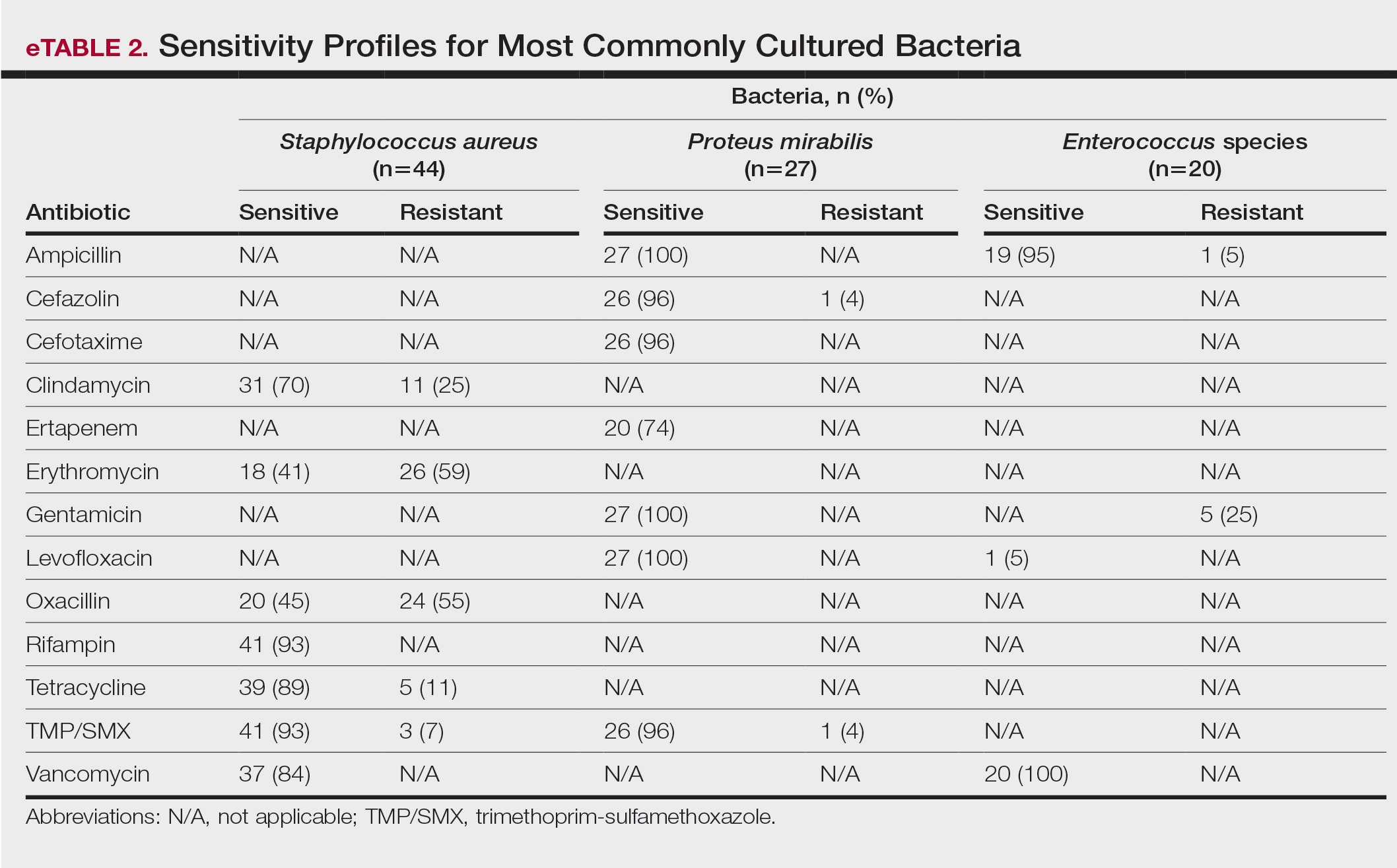
Comment
To treat HS, it is important to understand the cause of the condition. Although the pathogenesis of HS has many unknowns, bacterial colonization and biofilms are thought to play a role. Lipopolysaccharides found in the outer membrane of Gram-negative bacteria are pathogen-associated molecular patterns that present to the toll-like receptors of the human immune system. Once the toll-like receptors recognize the pathogen-associated molecular patterns, macrophages and keratinocytes are activated and release proinflammatory and anti-inflammatory cytokines and chemokines. Persistent presentation of bacteria to the immune system increases immune-cell recruitment and worsens chronic inflammation in patients with HS. Evidence has revealed that bacteria initiate and sustain the inflammation seen in patients with HS; therefore, reducing the amount of bacteria could alleviate some of the symptoms of HS.5 It is important to continue learning about the pathophysiology of this disease as well as formulating tailored treatments to minimize patient discomfort and improve quality of life.
Based on the findings of the current study and the safety profile of the medication, tetracyclines may be considered for first-line empiric therapy in patients with HS involving the axilla only, buttocks only, or multiple sites. For additional coverage of P mirabilis in the axilla or inframammary region, TMP/SMX monotherapy or tetracycline plus ampicillin may be considered. For inframammary lesions only, empiric treatment with ampicillin or TMP/ SMX is recommended. For HS lesions in the groin area, coverage of Enterococcus species with ampicillin should be considered. Patients with multiple sites of involvement that include the inframammary or groin regions similarly should receive empiric antibiotics that cover both S aureus and Gram-negative bacteria, such as TMP/SMX or tetracycline and ampicillin, respectively; if the multiple sites do not include the inframammary or groin regions, Gram-negative coverage may not be indicated. Based on our findings, standardization of treatment for patients with HS can allow for earlier and potentially more effective treatment.
In a similar study conducted in 2016, bacteria species were isolated from the axilla, groin, and gluteus/perineum in patients with HS.5 In that study, the most prominent bacteria in the axilla was CoNS; in the groin, P mirabilis and E coli; and in the gluteus/perineum, E coli and CoNS. These results differed from ours, which found S aureus as the abundant bacteria in these areas. In the 2016 study, the highest rates of resistance were found for penicillin G, erythromycin, clindamycin, and ampicillin.5 In contrast, the current study found high sensitivities for clindamycin and ampicillin, but our results support the finding of high resistance for erythromycin. These differences could be accounted for by the lower sample size of patients in the 2016 study: 68 patients were analyzed for sensitivity results, and 171 patients were analyzed for frequency of bacterial species in patients with HS.5
Our study is limited by its relatively small sample size. Additionally, all patients were seen at 1 of 2 clinic sites, located in League City and Galveston, Texas, and the data from this geographic area may not be applicable to patients seen in different climates.
Conclusion
Outcomes for patients with HS improve with early intervention; however, HS treatment may be delayed by selection of ineffective antibiotic therapy. Our study provides clinicians with recommendations for empiric antibiotic treatment based on anatomic location of HS lesions and culture sensitivity profiles. Utilizing tailored antibiotic therapy on initial clinical evaluation may increase early disease control and improve morbidity and disease outcomes, thereby increasing patient quality of life.
- Vinkel C, Thomsen SF. Hidradenitis suppurativa: causes, features, and current treatments. J Clin Aesthet Dermatol. 2018;11:17-23.
- Lee EY, Alhusayen R, Lansang P, et al. What is hidradenitis suppurativa? Can Fam Physician. 2017;63:114-120.
- Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol. 2009;60:539-561; quiz 562-563.
- Yazdanyar S, Jemec GBE. Hidradenitis suppurativa: a review of cause and treatment. Curr Opin Infect Dis. 2011;24:118-123.
- Hessam S, Sand M, Georgas D, et al. Microbial profile and antimicrobial susceptibility of bacteria found in inflammatory hidradenitis suppurativa lesions. Skin Pharmacol Physiol. 2016; 29:161-167.
Hidradenitis suppurativa (HS) is a chronic scarring inflammatory skin condition of the follicular epithelium that impacts 1% to 4% of the general population (eFigure).1-3 This statistic likely is an underrepresentation of the affected population due to missed and delayed diagnoses.1 Hidradenitis suppurativa has been identified as having one of the strongest negative impacts on patients’ lives based on studied skin diseases.4 Its recurrent nature can negatively impact both the patient’s physical and mental state.3 Due to the debilitating effects of HS, we aimed to create updated recommendations for empiric antibotics based on affected anatomic locations in an effort to improve patient quality of life.

Methods
An institutional review board–approved retrospective medical chart review of 485 patients diagnosed with HS and evaluated at the University of Texas Medical Branch in Galveston from January 2006 to December 2021 was conducted. Males and females of all ages (including pregnant and pediatric patients) were included. Only patients for whom anatomic locations of HS lesions or culture sites were not documented were excluded from the analysis. Locations of cultures were categorized into 5 groups: axilla; groin; buttocks; inframammary; and multiple sites of involvement, which included any combination of 2 or more sites. Types of bacteria collected from cultures and recorded included Escherichia coli, Enterococcus species, Proteus mirabilis, Pseudomonas aeruginosa, Staphylococcus aureus, coagulase-negative staphylococci (CoNS), and other Gram-negative species. Sensitivity profiles also were analyzed for the most commonly cultured bacteria to create recommendations on antibiotic use based on the anatomic location of the lesions. Data analysis was conducted using descriptive statistics and bivariate analysis.
Results
The analysis included 485 patients comprising 600 visits. Seventy-five percent (363/485) of the study population was female. The axilla was the most common anatomic location for HS lesions followed by multiple sites of involvement. In total, 283 cultures were performed; males were 1.1 times more likely than females to be cultured. While cultures were most frequently obtained in patients with axillary lesions only (93/262 [35%]) or from multiple sites of involvement (83/179 [46%]) as this was the most common presentation of HS in our patient population, cultures were more likely to be obtained when patients presented with only buttock (32/38 [84%]) and inframammary (20/25 [80%]) lesions (Table).

Staphylococcus aureus was the most commonly cultured bacteria in general (53/283 [19%]) as well as for HS located the axilla (24/56 [43%]) and in multiple sites (16/51 [31%]). Proteus mirabilis (29/283 [10%]) was the second most commonly cultured bacteria overall and was cultured most often in the axilla (15/56 [27%]) and inframammary region (6/14 [43%]). These were followed by beta-hemolytic Streptococcus species (26/283 [9%]) and Enterococcus species (21/283 [7%]), which was second to P mirabilis as the most commonly cultured bacteria in the inframammary region (6/14 [43%])(eTable 1).

eTable 2 shows the sensitivity profiles for the most commonly cultured bacteria: S aureus, P mirabilis, and Enterococcus species. Staphylococcus aureus located in the axilla, buttocks, and groin was most sensitive to rifampin (41/44 [93%]), TMP/SMX (41/44 [93%]), and tetracycline (39/44 [89%]) and most resistant to erythromycin (26/44 [59%]) and oxacillin (24/44 [55%]). Proteus mirabilis in the inframammary region was most sensitive to ampicillin (27/27 [100%]), gentamicin (27/27 [100%]), levofloxacin (27/27 [100%]), and TMP/SMX (26/27 [96%]). Enterococcus species were most sensitive to vancomycin (20/20 [100%]) and ampicillin (19/20 [95%]) and most resistant to gentamicin (5/20 [25%]).

Comment
To treat HS, it is important to understand the cause of the condition. Although the pathogenesis of HS has many unknowns, bacterial colonization and biofilms are thought to play a role. Lipopolysaccharides found in the outer membrane of Gram-negative bacteria are pathogen-associated molecular patterns that present to the toll-like receptors of the human immune system. Once the toll-like receptors recognize the pathogen-associated molecular patterns, macrophages and keratinocytes are activated and release proinflammatory and anti-inflammatory cytokines and chemokines. Persistent presentation of bacteria to the immune system increases immune-cell recruitment and worsens chronic inflammation in patients with HS. Evidence has revealed that bacteria initiate and sustain the inflammation seen in patients with HS; therefore, reducing the amount of bacteria could alleviate some of the symptoms of HS.5 It is important to continue learning about the pathophysiology of this disease as well as formulating tailored treatments to minimize patient discomfort and improve quality of life.
Based on the findings of the current study and the safety profile of the medication, tetracyclines may be considered for first-line empiric therapy in patients with HS involving the axilla only, buttocks only, or multiple sites. For additional coverage of P mirabilis in the axilla or inframammary region, TMP/SMX monotherapy or tetracycline plus ampicillin may be considered. For inframammary lesions only, empiric treatment with ampicillin or TMP/ SMX is recommended. For HS lesions in the groin area, coverage of Enterococcus species with ampicillin should be considered. Patients with multiple sites of involvement that include the inframammary or groin regions similarly should receive empiric antibiotics that cover both S aureus and Gram-negative bacteria, such as TMP/SMX or tetracycline and ampicillin, respectively; if the multiple sites do not include the inframammary or groin regions, Gram-negative coverage may not be indicated. Based on our findings, standardization of treatment for patients with HS can allow for earlier and potentially more effective treatment.
In a similar study conducted in 2016, bacteria species were isolated from the axilla, groin, and gluteus/perineum in patients with HS.5 In that study, the most prominent bacteria in the axilla was CoNS; in the groin, P mirabilis and E coli; and in the gluteus/perineum, E coli and CoNS. These results differed from ours, which found S aureus as the abundant bacteria in these areas. In the 2016 study, the highest rates of resistance were found for penicillin G, erythromycin, clindamycin, and ampicillin.5 In contrast, the current study found high sensitivities for clindamycin and ampicillin, but our results support the finding of high resistance for erythromycin. These differences could be accounted for by the lower sample size of patients in the 2016 study: 68 patients were analyzed for sensitivity results, and 171 patients were analyzed for frequency of bacterial species in patients with HS.5
Our study is limited by its relatively small sample size. Additionally, all patients were seen at 1 of 2 clinic sites, located in League City and Galveston, Texas, and the data from this geographic area may not be applicable to patients seen in different climates.
Conclusion
Outcomes for patients with HS improve with early intervention; however, HS treatment may be delayed by selection of ineffective antibiotic therapy. Our study provides clinicians with recommendations for empiric antibiotic treatment based on anatomic location of HS lesions and culture sensitivity profiles. Utilizing tailored antibiotic therapy on initial clinical evaluation may increase early disease control and improve morbidity and disease outcomes, thereby increasing patient quality of life.
Hidradenitis suppurativa (HS) is a chronic scarring inflammatory skin condition of the follicular epithelium that impacts 1% to 4% of the general population (eFigure).1-3 This statistic likely is an underrepresentation of the affected population due to missed and delayed diagnoses.1 Hidradenitis suppurativa has been identified as having one of the strongest negative impacts on patients’ lives based on studied skin diseases.4 Its recurrent nature can negatively impact both the patient’s physical and mental state.3 Due to the debilitating effects of HS, we aimed to create updated recommendations for empiric antibotics based on affected anatomic locations in an effort to improve patient quality of life.

Methods
An institutional review board–approved retrospective medical chart review of 485 patients diagnosed with HS and evaluated at the University of Texas Medical Branch in Galveston from January 2006 to December 2021 was conducted. Males and females of all ages (including pregnant and pediatric patients) were included. Only patients for whom anatomic locations of HS lesions or culture sites were not documented were excluded from the analysis. Locations of cultures were categorized into 5 groups: axilla; groin; buttocks; inframammary; and multiple sites of involvement, which included any combination of 2 or more sites. Types of bacteria collected from cultures and recorded included Escherichia coli, Enterococcus species, Proteus mirabilis, Pseudomonas aeruginosa, Staphylococcus aureus, coagulase-negative staphylococci (CoNS), and other Gram-negative species. Sensitivity profiles also were analyzed for the most commonly cultured bacteria to create recommendations on antibiotic use based on the anatomic location of the lesions. Data analysis was conducted using descriptive statistics and bivariate analysis.
Results
The analysis included 485 patients comprising 600 visits. Seventy-five percent (363/485) of the study population was female. The axilla was the most common anatomic location for HS lesions followed by multiple sites of involvement. In total, 283 cultures were performed; males were 1.1 times more likely than females to be cultured. While cultures were most frequently obtained in patients with axillary lesions only (93/262 [35%]) or from multiple sites of involvement (83/179 [46%]) as this was the most common presentation of HS in our patient population, cultures were more likely to be obtained when patients presented with only buttock (32/38 [84%]) and inframammary (20/25 [80%]) lesions (Table).

Staphylococcus aureus was the most commonly cultured bacteria in general (53/283 [19%]) as well as for HS located the axilla (24/56 [43%]) and in multiple sites (16/51 [31%]). Proteus mirabilis (29/283 [10%]) was the second most commonly cultured bacteria overall and was cultured most often in the axilla (15/56 [27%]) and inframammary region (6/14 [43%]). These were followed by beta-hemolytic Streptococcus species (26/283 [9%]) and Enterococcus species (21/283 [7%]), which was second to P mirabilis as the most commonly cultured bacteria in the inframammary region (6/14 [43%])(eTable 1).

eTable 2 shows the sensitivity profiles for the most commonly cultured bacteria: S aureus, P mirabilis, and Enterococcus species. Staphylococcus aureus located in the axilla, buttocks, and groin was most sensitive to rifampin (41/44 [93%]), TMP/SMX (41/44 [93%]), and tetracycline (39/44 [89%]) and most resistant to erythromycin (26/44 [59%]) and oxacillin (24/44 [55%]). Proteus mirabilis in the inframammary region was most sensitive to ampicillin (27/27 [100%]), gentamicin (27/27 [100%]), levofloxacin (27/27 [100%]), and TMP/SMX (26/27 [96%]). Enterococcus species were most sensitive to vancomycin (20/20 [100%]) and ampicillin (19/20 [95%]) and most resistant to gentamicin (5/20 [25%]).

Comment
To treat HS, it is important to understand the cause of the condition. Although the pathogenesis of HS has many unknowns, bacterial colonization and biofilms are thought to play a role. Lipopolysaccharides found in the outer membrane of Gram-negative bacteria are pathogen-associated molecular patterns that present to the toll-like receptors of the human immune system. Once the toll-like receptors recognize the pathogen-associated molecular patterns, macrophages and keratinocytes are activated and release proinflammatory and anti-inflammatory cytokines and chemokines. Persistent presentation of bacteria to the immune system increases immune-cell recruitment and worsens chronic inflammation in patients with HS. Evidence has revealed that bacteria initiate and sustain the inflammation seen in patients with HS; therefore, reducing the amount of bacteria could alleviate some of the symptoms of HS.5 It is important to continue learning about the pathophysiology of this disease as well as formulating tailored treatments to minimize patient discomfort and improve quality of life.
Based on the findings of the current study and the safety profile of the medication, tetracyclines may be considered for first-line empiric therapy in patients with HS involving the axilla only, buttocks only, or multiple sites. For additional coverage of P mirabilis in the axilla or inframammary region, TMP/SMX monotherapy or tetracycline plus ampicillin may be considered. For inframammary lesions only, empiric treatment with ampicillin or TMP/ SMX is recommended. For HS lesions in the groin area, coverage of Enterococcus species with ampicillin should be considered. Patients with multiple sites of involvement that include the inframammary or groin regions similarly should receive empiric antibiotics that cover both S aureus and Gram-negative bacteria, such as TMP/SMX or tetracycline and ampicillin, respectively; if the multiple sites do not include the inframammary or groin regions, Gram-negative coverage may not be indicated. Based on our findings, standardization of treatment for patients with HS can allow for earlier and potentially more effective treatment.
In a similar study conducted in 2016, bacteria species were isolated from the axilla, groin, and gluteus/perineum in patients with HS.5 In that study, the most prominent bacteria in the axilla was CoNS; in the groin, P mirabilis and E coli; and in the gluteus/perineum, E coli and CoNS. These results differed from ours, which found S aureus as the abundant bacteria in these areas. In the 2016 study, the highest rates of resistance were found for penicillin G, erythromycin, clindamycin, and ampicillin.5 In contrast, the current study found high sensitivities for clindamycin and ampicillin, but our results support the finding of high resistance for erythromycin. These differences could be accounted for by the lower sample size of patients in the 2016 study: 68 patients were analyzed for sensitivity results, and 171 patients were analyzed for frequency of bacterial species in patients with HS.5
Our study is limited by its relatively small sample size. Additionally, all patients were seen at 1 of 2 clinic sites, located in League City and Galveston, Texas, and the data from this geographic area may not be applicable to patients seen in different climates.
Conclusion
Outcomes for patients with HS improve with early intervention; however, HS treatment may be delayed by selection of ineffective antibiotic therapy. Our study provides clinicians with recommendations for empiric antibiotic treatment based on anatomic location of HS lesions and culture sensitivity profiles. Utilizing tailored antibiotic therapy on initial clinical evaluation may increase early disease control and improve morbidity and disease outcomes, thereby increasing patient quality of life.
- Vinkel C, Thomsen SF. Hidradenitis suppurativa: causes, features, and current treatments. J Clin Aesthet Dermatol. 2018;11:17-23.
- Lee EY, Alhusayen R, Lansang P, et al. What is hidradenitis suppurativa? Can Fam Physician. 2017;63:114-120.
- Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol. 2009;60:539-561; quiz 562-563.
- Yazdanyar S, Jemec GBE. Hidradenitis suppurativa: a review of cause and treatment. Curr Opin Infect Dis. 2011;24:118-123.
- Hessam S, Sand M, Georgas D, et al. Microbial profile and antimicrobial susceptibility of bacteria found in inflammatory hidradenitis suppurativa lesions. Skin Pharmacol Physiol. 2016; 29:161-167.
- Vinkel C, Thomsen SF. Hidradenitis suppurativa: causes, features, and current treatments. J Clin Aesthet Dermatol. 2018;11:17-23.
- Lee EY, Alhusayen R, Lansang P, et al. What is hidradenitis suppurativa? Can Fam Physician. 2017;63:114-120.
- Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol. 2009;60:539-561; quiz 562-563.
- Yazdanyar S, Jemec GBE. Hidradenitis suppurativa: a review of cause and treatment. Curr Opin Infect Dis. 2011;24:118-123.
- Hessam S, Sand M, Georgas D, et al. Microbial profile and antimicrobial susceptibility of bacteria found in inflammatory hidradenitis suppurativa lesions. Skin Pharmacol Physiol. 2016; 29:161-167.
Recommendations for Empiric Antibiotic Therapy in Hidradenitis Suppurativa
Recommendations for Empiric Antibiotic Therapy in Hidradenitis Suppurativa
PRACTICE POINTS
- The inflammation seen in patients with hidradenitis suppurativa (HS) is initiated and sustained by bacteria; therefore, reducing the number of bacteria may alleviate some of the symptoms of HS.
- For HS involving the axillae or buttocks, tetracyclines should be recommended as first-line empiric therapy.
- Patients with HS with multiple sites affected that include the inframammary or groin regions should receive empiric antibiotics that cover both Staphylococcus aureus and Gram-negative bacteria, such as trimethoprim-sulfamethoxazole or tetracycline plus ampicillin.
Less Invasive Screening May Identify Barrett’s Esophagus Earlier
A new combination modality demonstrated excellent sensitivity and negative predictive value compared with endoscopy in a prospective study of at-risk veterans screened for Barrett’s esophagus (BE) and esophageal adenocarcinoma (EAC), a small comparative study in US veterans found.
BE is up to three times more prevalent in veterans than in the general population.
This and other minimally invasive approaches may reduce patient anxiety and increase screening rates, according to investigators led by Katarina B. Greer, MD, MS, of the VA Northeast Ohio Healthcare System and Case Western University in Cleveland. Such screening platforms are expected to open a window on improved prognosis for EAC by offering well-tolerated, office-based testing, the authors wrote in The American Journal of Gastroenterology.
Greer and colleagues compared standard upper endoscopy with EsoCheck (EC), a nonendoscopic esophageal balloon cell-sampling device coupled with EsoGuard (EG), a DNA-based precancer screening assay, with standard upper endoscopy, an FDA-approved minimally invasive alternative.
Sensitivity and specificity of combined EC/EG for esophagogastroduodenoscopy (EGD)-detected BE/EAC were 92.9% (95% CI, 66.1-99.8) and 72.2% (95% CI, 62.1-80.8), respectively. Positive and negative predictive values were 32.5% (95% CI, 18.6-49.1) and 98.6% (95% CI, 92.4-100), respectively.
“With its strong negative predictive power, this screening modality could be a first-line tool available to a greater number of patients,” Greer and associates wrote. “Data from this test support the notion that EC could be performed as a triaging test to increase the yield of diagnostic upper endoscopy 2.5-fold.”
The US rates of EAC have increased more than six-fold in the past four decades and continue to rise. In 2023, 21,560 cases of EAC were diagnosed here. The prognosis for EAC is still poor, with fewer than 22% of patients surviving beyond 5 years.
Current guidelines recommend sedated EGD for patients with chronic gastroesophageal reflux disease (GERD) and additional BE risk factors such as smoking, obesity, and family history. This strategy, however, often fails to detect BE when symptoms are well controlled with over-the-counter or physician-prescribed therapies, Greer and colleagues noted. It also fails to detect BE in individuals without GERD, who comprise 40% of those who develop EAC.
Fewer than 5% of EACs are diagnosed as early-stage lesions caught by surveillance of patients with previously detected BE.
Study Details
The researchers recruited veterans meeting American College of Gastroenterology criteria for endoscopic BE and EAC screening at the Louis Stokes Cleveland Veterans Affairs Medical Center.
Of 782 eligible veterans, 130 (16.6%) entered the study and 124 completed screening. Common reasons for nonparticipation included completion of upper endoscopy outside of the VA healthcare system, lack of interest in joining a research study, and no recommendation for screening from referring gastroenterology or primary care providers. Eligible candidates had gastroesophageal reflux disorder plus three additional risk factors, such as smoking, higher BMI, male sex, age 50 years or older, and family history. The mean number of risk factors was 4.1.
“Available data suggest that family history is the strongest predictor of BE diagnosis, as prevalence of BE among those with family history was 23%,” Greer’s group wrote. “This points to high priority of pursuing screening in patients with family history of the condition, followed by patients who share multiple risk factors.”
All participants completed unsedated EC-guided distal esophageal sampling followed by a sedated EGD on the same day. The prevalence of BE/EAC was 12.9% (n = 14/2), based on standard EGD.
“The study was not powered to prospectively determine EC diagnostic accuracy for subgroups of nondysplastic and dysplastic BE and EAC. These data are reported for this device in development studies but not available for our study population,” the authors wrote. In comparison, they noted, the Cytosponge-TFF3, another nonendoscopic screening device for EAC and BE, exhibited lower sensitivity of 79.5%-87.2%, depending on lesion length, but higher specificity of 92.4%.
Procedural Anxiety
Baseline scores on the short-form six-item Spielberger State-Trait Anxiety Inventory-6 (STAI-6) revealed notable levels of periprocedural anxiety. STAI-6 scores range from 20 to 80, with higher scores indicating more severe anxiety. In the VA study, scores ranged from 20 to 60, and most domains constituting the scores were the same before and after the procedure. Participants did, however, report a statistically significant decrease in sense of worry after EC and reported good tolerability for both EC and EG.
Offering an outsider’s perspective on the study, Joshua Sloan, DO, an esophageal gastroenterologist at University of Minnesota Medical Center in Minneapolis, said that with the acceleration of US rates of EAC, developing a nonendoscopic screening tool to improve identification of Barrett’s and perhaps early EAC is important. “The study by Greer et al helps support the use of nonendoscopic screening with EsoCheck and EsoGuard to identify these conditions,” he told this news organization. “It will be interesting to see similar studies in the non-VA population as well. As the study notes, veterans are an enriched population with a higher prevalence of Barrett’s esophagus.”
Ultimately, Sloan added, “the hope is to increase our ability to identify and manage BE before it becomes EAC. Nonendoscopic screening tools have the potential to increase diagnosis and funnel the appropriate patients for endoscopic surveillance.”
The Bottom Line
“Calculations regarding effectiveness of the two-step screening strategy afforded by EC indicate that the burden of screening would be reduced by at least half (53%),” the authors wrote. Since the estimated size of the US screen-eligible population ranges from 19.7 million to 120.1 million, noninvasive tools could significantly decrease EGD procedures. A formal cost effectiveness analysis is being conducted and will be published separately.
This study was funded by a Department of Defense award.
Co-Author Chak reported device patents assigned to Case Western Reserve University and licensed to Lucid Diagnostics. The other authors had no competing interests to declare. Sloan disclosed speaking and/or advisory work for Sanofi-Regeneron, Phathom Pharmaceuticals, and Takeda Pharmaceuticals unrelated to his comments.
A version of this article first appeared on Medscape.com.
A new combination modality demonstrated excellent sensitivity and negative predictive value compared with endoscopy in a prospective study of at-risk veterans screened for Barrett’s esophagus (BE) and esophageal adenocarcinoma (EAC), a small comparative study in US veterans found.
BE is up to three times more prevalent in veterans than in the general population.
This and other minimally invasive approaches may reduce patient anxiety and increase screening rates, according to investigators led by Katarina B. Greer, MD, MS, of the VA Northeast Ohio Healthcare System and Case Western University in Cleveland. Such screening platforms are expected to open a window on improved prognosis for EAC by offering well-tolerated, office-based testing, the authors wrote in The American Journal of Gastroenterology.
Greer and colleagues compared standard upper endoscopy with EsoCheck (EC), a nonendoscopic esophageal balloon cell-sampling device coupled with EsoGuard (EG), a DNA-based precancer screening assay, with standard upper endoscopy, an FDA-approved minimally invasive alternative.
Sensitivity and specificity of combined EC/EG for esophagogastroduodenoscopy (EGD)-detected BE/EAC were 92.9% (95% CI, 66.1-99.8) and 72.2% (95% CI, 62.1-80.8), respectively. Positive and negative predictive values were 32.5% (95% CI, 18.6-49.1) and 98.6% (95% CI, 92.4-100), respectively.
“With its strong negative predictive power, this screening modality could be a first-line tool available to a greater number of patients,” Greer and associates wrote. “Data from this test support the notion that EC could be performed as a triaging test to increase the yield of diagnostic upper endoscopy 2.5-fold.”
The US rates of EAC have increased more than six-fold in the past four decades and continue to rise. In 2023, 21,560 cases of EAC were diagnosed here. The prognosis for EAC is still poor, with fewer than 22% of patients surviving beyond 5 years.
Current guidelines recommend sedated EGD for patients with chronic gastroesophageal reflux disease (GERD) and additional BE risk factors such as smoking, obesity, and family history. This strategy, however, often fails to detect BE when symptoms are well controlled with over-the-counter or physician-prescribed therapies, Greer and colleagues noted. It also fails to detect BE in individuals without GERD, who comprise 40% of those who develop EAC.
Fewer than 5% of EACs are diagnosed as early-stage lesions caught by surveillance of patients with previously detected BE.
Study Details
The researchers recruited veterans meeting American College of Gastroenterology criteria for endoscopic BE and EAC screening at the Louis Stokes Cleveland Veterans Affairs Medical Center.
Of 782 eligible veterans, 130 (16.6%) entered the study and 124 completed screening. Common reasons for nonparticipation included completion of upper endoscopy outside of the VA healthcare system, lack of interest in joining a research study, and no recommendation for screening from referring gastroenterology or primary care providers. Eligible candidates had gastroesophageal reflux disorder plus three additional risk factors, such as smoking, higher BMI, male sex, age 50 years or older, and family history. The mean number of risk factors was 4.1.
“Available data suggest that family history is the strongest predictor of BE diagnosis, as prevalence of BE among those with family history was 23%,” Greer’s group wrote. “This points to high priority of pursuing screening in patients with family history of the condition, followed by patients who share multiple risk factors.”
All participants completed unsedated EC-guided distal esophageal sampling followed by a sedated EGD on the same day. The prevalence of BE/EAC was 12.9% (n = 14/2), based on standard EGD.
“The study was not powered to prospectively determine EC diagnostic accuracy for subgroups of nondysplastic and dysplastic BE and EAC. These data are reported for this device in development studies but not available for our study population,” the authors wrote. In comparison, they noted, the Cytosponge-TFF3, another nonendoscopic screening device for EAC and BE, exhibited lower sensitivity of 79.5%-87.2%, depending on lesion length, but higher specificity of 92.4%.
Procedural Anxiety
Baseline scores on the short-form six-item Spielberger State-Trait Anxiety Inventory-6 (STAI-6) revealed notable levels of periprocedural anxiety. STAI-6 scores range from 20 to 80, with higher scores indicating more severe anxiety. In the VA study, scores ranged from 20 to 60, and most domains constituting the scores were the same before and after the procedure. Participants did, however, report a statistically significant decrease in sense of worry after EC and reported good tolerability for both EC and EG.
Offering an outsider’s perspective on the study, Joshua Sloan, DO, an esophageal gastroenterologist at University of Minnesota Medical Center in Minneapolis, said that with the acceleration of US rates of EAC, developing a nonendoscopic screening tool to improve identification of Barrett’s and perhaps early EAC is important. “The study by Greer et al helps support the use of nonendoscopic screening with EsoCheck and EsoGuard to identify these conditions,” he told this news organization. “It will be interesting to see similar studies in the non-VA population as well. As the study notes, veterans are an enriched population with a higher prevalence of Barrett’s esophagus.”
Ultimately, Sloan added, “the hope is to increase our ability to identify and manage BE before it becomes EAC. Nonendoscopic screening tools have the potential to increase diagnosis and funnel the appropriate patients for endoscopic surveillance.”
The Bottom Line
“Calculations regarding effectiveness of the two-step screening strategy afforded by EC indicate that the burden of screening would be reduced by at least half (53%),” the authors wrote. Since the estimated size of the US screen-eligible population ranges from 19.7 million to 120.1 million, noninvasive tools could significantly decrease EGD procedures. A formal cost effectiveness analysis is being conducted and will be published separately.
This study was funded by a Department of Defense award.
Co-Author Chak reported device patents assigned to Case Western Reserve University and licensed to Lucid Diagnostics. The other authors had no competing interests to declare. Sloan disclosed speaking and/or advisory work for Sanofi-Regeneron, Phathom Pharmaceuticals, and Takeda Pharmaceuticals unrelated to his comments.
A version of this article first appeared on Medscape.com.
A new combination modality demonstrated excellent sensitivity and negative predictive value compared with endoscopy in a prospective study of at-risk veterans screened for Barrett’s esophagus (BE) and esophageal adenocarcinoma (EAC), a small comparative study in US veterans found.
BE is up to three times more prevalent in veterans than in the general population.
This and other minimally invasive approaches may reduce patient anxiety and increase screening rates, according to investigators led by Katarina B. Greer, MD, MS, of the VA Northeast Ohio Healthcare System and Case Western University in Cleveland. Such screening platforms are expected to open a window on improved prognosis for EAC by offering well-tolerated, office-based testing, the authors wrote in The American Journal of Gastroenterology.
Greer and colleagues compared standard upper endoscopy with EsoCheck (EC), a nonendoscopic esophageal balloon cell-sampling device coupled with EsoGuard (EG), a DNA-based precancer screening assay, with standard upper endoscopy, an FDA-approved minimally invasive alternative.
Sensitivity and specificity of combined EC/EG for esophagogastroduodenoscopy (EGD)-detected BE/EAC were 92.9% (95% CI, 66.1-99.8) and 72.2% (95% CI, 62.1-80.8), respectively. Positive and negative predictive values were 32.5% (95% CI, 18.6-49.1) and 98.6% (95% CI, 92.4-100), respectively.
“With its strong negative predictive power, this screening modality could be a first-line tool available to a greater number of patients,” Greer and associates wrote. “Data from this test support the notion that EC could be performed as a triaging test to increase the yield of diagnostic upper endoscopy 2.5-fold.”
The US rates of EAC have increased more than six-fold in the past four decades and continue to rise. In 2023, 21,560 cases of EAC were diagnosed here. The prognosis for EAC is still poor, with fewer than 22% of patients surviving beyond 5 years.
Current guidelines recommend sedated EGD for patients with chronic gastroesophageal reflux disease (GERD) and additional BE risk factors such as smoking, obesity, and family history. This strategy, however, often fails to detect BE when symptoms are well controlled with over-the-counter or physician-prescribed therapies, Greer and colleagues noted. It also fails to detect BE in individuals without GERD, who comprise 40% of those who develop EAC.
Fewer than 5% of EACs are diagnosed as early-stage lesions caught by surveillance of patients with previously detected BE.
Study Details
The researchers recruited veterans meeting American College of Gastroenterology criteria for endoscopic BE and EAC screening at the Louis Stokes Cleveland Veterans Affairs Medical Center.
Of 782 eligible veterans, 130 (16.6%) entered the study and 124 completed screening. Common reasons for nonparticipation included completion of upper endoscopy outside of the VA healthcare system, lack of interest in joining a research study, and no recommendation for screening from referring gastroenterology or primary care providers. Eligible candidates had gastroesophageal reflux disorder plus three additional risk factors, such as smoking, higher BMI, male sex, age 50 years or older, and family history. The mean number of risk factors was 4.1.
“Available data suggest that family history is the strongest predictor of BE diagnosis, as prevalence of BE among those with family history was 23%,” Greer’s group wrote. “This points to high priority of pursuing screening in patients with family history of the condition, followed by patients who share multiple risk factors.”
All participants completed unsedated EC-guided distal esophageal sampling followed by a sedated EGD on the same day. The prevalence of BE/EAC was 12.9% (n = 14/2), based on standard EGD.
“The study was not powered to prospectively determine EC diagnostic accuracy for subgroups of nondysplastic and dysplastic BE and EAC. These data are reported for this device in development studies but not available for our study population,” the authors wrote. In comparison, they noted, the Cytosponge-TFF3, another nonendoscopic screening device for EAC and BE, exhibited lower sensitivity of 79.5%-87.2%, depending on lesion length, but higher specificity of 92.4%.
Procedural Anxiety
Baseline scores on the short-form six-item Spielberger State-Trait Anxiety Inventory-6 (STAI-6) revealed notable levels of periprocedural anxiety. STAI-6 scores range from 20 to 80, with higher scores indicating more severe anxiety. In the VA study, scores ranged from 20 to 60, and most domains constituting the scores were the same before and after the procedure. Participants did, however, report a statistically significant decrease in sense of worry after EC and reported good tolerability for both EC and EG.
Offering an outsider’s perspective on the study, Joshua Sloan, DO, an esophageal gastroenterologist at University of Minnesota Medical Center in Minneapolis, said that with the acceleration of US rates of EAC, developing a nonendoscopic screening tool to improve identification of Barrett’s and perhaps early EAC is important. “The study by Greer et al helps support the use of nonendoscopic screening with EsoCheck and EsoGuard to identify these conditions,” he told this news organization. “It will be interesting to see similar studies in the non-VA population as well. As the study notes, veterans are an enriched population with a higher prevalence of Barrett’s esophagus.”
Ultimately, Sloan added, “the hope is to increase our ability to identify and manage BE before it becomes EAC. Nonendoscopic screening tools have the potential to increase diagnosis and funnel the appropriate patients for endoscopic surveillance.”
The Bottom Line
“Calculations regarding effectiveness of the two-step screening strategy afforded by EC indicate that the burden of screening would be reduced by at least half (53%),” the authors wrote. Since the estimated size of the US screen-eligible population ranges from 19.7 million to 120.1 million, noninvasive tools could significantly decrease EGD procedures. A formal cost effectiveness analysis is being conducted and will be published separately.
This study was funded by a Department of Defense award.
Co-Author Chak reported device patents assigned to Case Western Reserve University and licensed to Lucid Diagnostics. The other authors had no competing interests to declare. Sloan disclosed speaking and/or advisory work for Sanofi-Regeneron, Phathom Pharmaceuticals, and Takeda Pharmaceuticals unrelated to his comments.
A version of this article first appeared on Medscape.com.
FROM AMERICAN JOURNAL OF GASTROENTEROLOGY
Workforce Shortage of Pediatric Dermatologists: A Medical Student’s Perspective
Workforce Shortage of Pediatric Dermatologists: A Medical Student’s Perspective
There is a shortage of pediatric dermatologists in the United States, with fewer than 2% of practicing dermatologists specializing in pediatrics.1 Pediatric dermatology has the third highest referral rate by pediatricians but also is the third most challenging specialty to access, with an average appointment wait time of 92 days.2,3 Another factor leading to increased appointment wait times is the specificity of care required for pediatric patients. Frequently, pediatric patients evaluated by a general dermatologist will be referred to their pediatric dermatology colleagues. As medical students, we were introduced to the field of pediatric dermatology through different avenues—personal experience, research mentorship, or a clinical rotation in medical school. We found ourselves curious about the discrepancy between the supply of and demand for pediatric dermatologists and wondered what could be done to increase awareness of this subspecialty among medical students. We believe this workforce shortage can be ameliorated by improving early exposure to pediatric dermatology. In this article, we explore the existing framework surrounding pediatric dermatology in medical education and offer feasible recommendations and solutions to realistically combat this problem.
Pediatric dermatologists are essential to the greater dermatology community. Pediatric dermatologists receive advanced training in complex pediatric skin conditions that often is lacking in general dermatology residency. A large percentage of pediatric dermatology patients seen in academic medical centers have already been seen by general dermatologists who subsequently referred them to specialty care. In one study, 9.6% (10/108) of practicing pediatric dermatologists noted that their referrals were from general dermatologists.4 In another study, 42% (19/45) of referrals to a multidisciplinary pediatric dermatology-genetics were from general dermatologists.5 Given the shortage of pediatric dermatologists, these referrals undoubtedly overwhelm the system, and the results of these studies underscore the reality that general dermatologists do not necessarily feel adequately trained in complex pediatric conditions, creating an intrinsic need for pediatric dermatologists.
Admani et al6 reported that early mentorship was the single most important factor to 84% (91/109) of survey respondents who pursued pediatric dermatology. Forty percent (40/100) of survey respondents chose their specialty of pediatric dermatology during pediatrics residency, 34% (34/100) during medical school, 17% (17/100) during dermatology residency, and 5% (5/100) during internship, indicating that medical school is a crucial time for recruitment.6 It has been noted in the literature that more medical students matched to dermatology residency from schools with dermatology clerkships built into the curriculum than from schools without dedicated dermatology rotations, suggesting that early clinical exposure to dermatology fields has a predictable influence in matching.7 Currently, only about 10% (15/155) of allopathic medical schools in the United States offer a formal elective in pediatric dermatology via the Association of American Medical College’s Visiting Student Learning Opportunities program.8 When this information was cross-referenced with the most recently matched pediatric dermatology fellowship class (2023-2024), provided by the Fellowship Directors Chair of the Society for Pediatric Dermatology, we found that 17% (4/24) of the matched fellows attended one of these 15 medical schools. We also found that the 2023-2024 pediatric dermatology fellowship class had 12 unmatched spots out of 36 total positions nationwide (33%), highlighting a gap in pediatric dermatology care and placing further strain on an already underserved subspecialty. These data suggest that, while dermatologists may decide to pursue pediatric dermatology fellowships during residency, there is an opportunity to foster interest during medical school training and improve the fellowship match rate.
Several medical schools in the United States incorporate pediatric dermatology into their curricula, including lectures in preclinical courses and career panels to pediatric dermatology electives in the third and fourth years. These institutions can serve as models for other medical schools. Within preclinical content, we recommend creating a designated dermatology unit that can incorporate common pediatric dermatology pathologies also seen by general practitioners, such as common childhood rashes, atopic dermatitis, alopecia areata, seborrheic dermatitis, and acne. Rare pediatric diseases such as epidermolysis bullosa, tuberous sclerosis, and Ehlers-Danlos syndrome also may be included in the unit. If schools are not able to offer a stand-alone dermatology preclinical course, this content can be added to the immunology, musculoskeletal, infectious diseases, or genetics courses to account for the multisystemic effects of some of these conditions. Ideally, schools would offer elective exposure to pediatric dermatology during the clinical years of medical school to increase knowledge of the field; for example, pediatric dermatology materials could be included in core clerkships, as much of this content is applicable to the general pediatrics rotation. In particular, a lecture on common rashes in pediatric patients could be given before starting the core pediatric rotation. Additionally, problem-based pediatric dermatology cases could be implemented during the core pediatrics rotation. If students are offered an independent dermatology clinical elective, the already formatted 2- and 4-week basic dermatology courses designed by the American Academy of Dermatology could serve as suggested teaching guides or as self-teaching resources that could complement the dermatology rotation.9,10 Pediatric topics (eg, pediatric cutaneous fungal infections) are included within the American Academy of Dermatology basic dermatology curriculum.8,9
Increasing access to pediatric dermatology resources such as lecture series and mentorship opportunities could further broaden the pediatric dermatology knowledge base of medical students. Within medical school dermatology interest groups, there is an opportunity to have a pediatric dermatology lead to help coordinate lecture series and journal club sessions for interested students. The Society for Pediatric Dermatology and the Pediatric Dermatology Research Alliance have created programs to support students, and we encourage schools to raise awareness of these organizations as well as conference and grant opportunities. These initiatives foster meaningful mentor-mentee relationships, and more medical students may be interested if they are aware of these support networks.
There also may be opportunities to create residency tracks that increase the number of dermatology residency applicants. Programs such as the newly implemented pediatric dermatology track at the University of Pennsylvania and New York University allow medical students who are interested in pursuing pediatric dermatology to have a more focused and linear training path.11,12 Due to the inherent competition in matching into dermatology, we surmise that many students with interest in pediatric dermatology are lost to pediatric residencies. Given the large percentage of pediatric residents who ultimately develop an interest in pediatric dermatology, holding a spot for pediatric dermatology applicants—akin to the combined medical-dermatology spots—may be an avenue to increase the pool of pediatric dermatology fellows.1,6 Another avenue is to encourage the development of first-year pediatric internship tracks that lead directly into dermatology residency, such as newly established programs at the University of Pennsylvania and New York University.11,12
As a group of both aspiring and practicing pediatric dermatologists, we have identified opportunities for formalized education in and early exposure to this subspecialty during medical training instead of leaving the discovery of the field to chance. The gaps in medical education that we have identified have already led us to present potential curricular changes to the medical education committee at our home institution. We hope to inspire the development of strong pediatric dermatology education at the medical school level.
While the solution to the pediatric dermatology workforce shortage is complex and multifaceted, there is a unique opportunity to target medical students through mentorship, access to education, and clinical experiences. We recommend that medical schools implement these educational methods and track the efficacy of these interventions to quantify the predicted association between an increased workforce and early exposure to pediatric dermatology. Addressing a lack of exposure to the field and increasing support of students pursuing pediatric dermatology can help to alleviate the shortage at the earliest point in training.
- Prindaville B, Antaya RJ, Siegfried EC. Pediatric dermatology: past, present, and future. Pediatr Dermatol. 2015;32:1-12. doi:10.1111/pde.12362
- Wright TS. Update on the pediatric dermatology workforce shortage. Cutis. 2021;108:237-238. doi:10.12788/cutis.0379
- Stephens MR, Murthy AS, McMahon PJ. Wait times, health care touchpoints, and nonattendance in an academic pediatric dermatology clinic. ediatr Dermatol. 2019;36:893-897. doi:10.1111/pde.13943
- Fogel AL, Teng JM. A survey to assess perceived differences in referral pathways to board-certified pediatric dermatologists. Pediatr Dermatol. 2015;32:e314-e315. doi:10.1111/pde.12703
- Parker JC, Rangu S, Grand KL, et al. Genetic skin disorders: the value of a multidisciplinary clinic. Am J Med Genet A. 2021;185:1159-1167. doi:10.1002/ajmg.a.62095
- Admani S, Caufield M, Kim SS, et al. Understanding the pediatric dermatology workforce shortage: mentoring matters. J Pediatr. 2014;164:372-5.e1. doi:10.1016/j.jpeds.2013.10.004
- Ogidi P, Ahmed F, Cahn BA, et al. Medical schools as gatekeepers: a survey and analysis of factors predicting dermatology residency placement. J Am Acad Dermatol. 2022;86:490-492. doi:10.1016 /j.jaad.2021.09.027
- Visiting Student Learning Opportunities (VSLO). Accessed May 30, 2025. https://students-residents.aamc.org/visiting-student-learning-opportunities/visiting-student-learning-opportunities-vslo
- American Academy of Dermatology Association. AAD Learning Center. Basic dermatology curriculum (2-week rotation). Accessed May 12, 2025. https://learning.aad.org/Listing/Basic-Dermatology-Curriculum-2-Week-Rotation-5395
- American Academy of Dermatology Association. AAD Learning Center. Basic dermatology curriculum (4-week rotation). Accessed May 12, 2025. https://learning.aad.org/Public/Catalog/Details.aspx?id=YPssTVIbBO3Zb%2bOuf%2fM7Kg%3d%3d&returnurl=%2fUsers%2fUserOnlineCourse.aspx%3fLearningActivityID%3dYPssTVIbBO3Zb%252bOuf%252fM7Kg%253d%253d
- Penn Medicine Dermatology Residency Training Program. Residency tracks. Accessed May 12, 2025. https://dermatology.upenn.edu/residents/residency-tracks/
- Pediatric Dermatology Residency Track at NYU Grossman School of Medicine. Pediatric Track. Accessed May 30, 2025. https://med.nyu.edu/departments-institutes/dermatology/education/residency/pediatric-track
There is a shortage of pediatric dermatologists in the United States, with fewer than 2% of practicing dermatologists specializing in pediatrics.1 Pediatric dermatology has the third highest referral rate by pediatricians but also is the third most challenging specialty to access, with an average appointment wait time of 92 days.2,3 Another factor leading to increased appointment wait times is the specificity of care required for pediatric patients. Frequently, pediatric patients evaluated by a general dermatologist will be referred to their pediatric dermatology colleagues. As medical students, we were introduced to the field of pediatric dermatology through different avenues—personal experience, research mentorship, or a clinical rotation in medical school. We found ourselves curious about the discrepancy between the supply of and demand for pediatric dermatologists and wondered what could be done to increase awareness of this subspecialty among medical students. We believe this workforce shortage can be ameliorated by improving early exposure to pediatric dermatology. In this article, we explore the existing framework surrounding pediatric dermatology in medical education and offer feasible recommendations and solutions to realistically combat this problem.
Pediatric dermatologists are essential to the greater dermatology community. Pediatric dermatologists receive advanced training in complex pediatric skin conditions that often is lacking in general dermatology residency. A large percentage of pediatric dermatology patients seen in academic medical centers have already been seen by general dermatologists who subsequently referred them to specialty care. In one study, 9.6% (10/108) of practicing pediatric dermatologists noted that their referrals were from general dermatologists.4 In another study, 42% (19/45) of referrals to a multidisciplinary pediatric dermatology-genetics were from general dermatologists.5 Given the shortage of pediatric dermatologists, these referrals undoubtedly overwhelm the system, and the results of these studies underscore the reality that general dermatologists do not necessarily feel adequately trained in complex pediatric conditions, creating an intrinsic need for pediatric dermatologists.
Admani et al6 reported that early mentorship was the single most important factor to 84% (91/109) of survey respondents who pursued pediatric dermatology. Forty percent (40/100) of survey respondents chose their specialty of pediatric dermatology during pediatrics residency, 34% (34/100) during medical school, 17% (17/100) during dermatology residency, and 5% (5/100) during internship, indicating that medical school is a crucial time for recruitment.6 It has been noted in the literature that more medical students matched to dermatology residency from schools with dermatology clerkships built into the curriculum than from schools without dedicated dermatology rotations, suggesting that early clinical exposure to dermatology fields has a predictable influence in matching.7 Currently, only about 10% (15/155) of allopathic medical schools in the United States offer a formal elective in pediatric dermatology via the Association of American Medical College’s Visiting Student Learning Opportunities program.8 When this information was cross-referenced with the most recently matched pediatric dermatology fellowship class (2023-2024), provided by the Fellowship Directors Chair of the Society for Pediatric Dermatology, we found that 17% (4/24) of the matched fellows attended one of these 15 medical schools. We also found that the 2023-2024 pediatric dermatology fellowship class had 12 unmatched spots out of 36 total positions nationwide (33%), highlighting a gap in pediatric dermatology care and placing further strain on an already underserved subspecialty. These data suggest that, while dermatologists may decide to pursue pediatric dermatology fellowships during residency, there is an opportunity to foster interest during medical school training and improve the fellowship match rate.
Several medical schools in the United States incorporate pediatric dermatology into their curricula, including lectures in preclinical courses and career panels to pediatric dermatology electives in the third and fourth years. These institutions can serve as models for other medical schools. Within preclinical content, we recommend creating a designated dermatology unit that can incorporate common pediatric dermatology pathologies also seen by general practitioners, such as common childhood rashes, atopic dermatitis, alopecia areata, seborrheic dermatitis, and acne. Rare pediatric diseases such as epidermolysis bullosa, tuberous sclerosis, and Ehlers-Danlos syndrome also may be included in the unit. If schools are not able to offer a stand-alone dermatology preclinical course, this content can be added to the immunology, musculoskeletal, infectious diseases, or genetics courses to account for the multisystemic effects of some of these conditions. Ideally, schools would offer elective exposure to pediatric dermatology during the clinical years of medical school to increase knowledge of the field; for example, pediatric dermatology materials could be included in core clerkships, as much of this content is applicable to the general pediatrics rotation. In particular, a lecture on common rashes in pediatric patients could be given before starting the core pediatric rotation. Additionally, problem-based pediatric dermatology cases could be implemented during the core pediatrics rotation. If students are offered an independent dermatology clinical elective, the already formatted 2- and 4-week basic dermatology courses designed by the American Academy of Dermatology could serve as suggested teaching guides or as self-teaching resources that could complement the dermatology rotation.9,10 Pediatric topics (eg, pediatric cutaneous fungal infections) are included within the American Academy of Dermatology basic dermatology curriculum.8,9
Increasing access to pediatric dermatology resources such as lecture series and mentorship opportunities could further broaden the pediatric dermatology knowledge base of medical students. Within medical school dermatology interest groups, there is an opportunity to have a pediatric dermatology lead to help coordinate lecture series and journal club sessions for interested students. The Society for Pediatric Dermatology and the Pediatric Dermatology Research Alliance have created programs to support students, and we encourage schools to raise awareness of these organizations as well as conference and grant opportunities. These initiatives foster meaningful mentor-mentee relationships, and more medical students may be interested if they are aware of these support networks.
There also may be opportunities to create residency tracks that increase the number of dermatology residency applicants. Programs such as the newly implemented pediatric dermatology track at the University of Pennsylvania and New York University allow medical students who are interested in pursuing pediatric dermatology to have a more focused and linear training path.11,12 Due to the inherent competition in matching into dermatology, we surmise that many students with interest in pediatric dermatology are lost to pediatric residencies. Given the large percentage of pediatric residents who ultimately develop an interest in pediatric dermatology, holding a spot for pediatric dermatology applicants—akin to the combined medical-dermatology spots—may be an avenue to increase the pool of pediatric dermatology fellows.1,6 Another avenue is to encourage the development of first-year pediatric internship tracks that lead directly into dermatology residency, such as newly established programs at the University of Pennsylvania and New York University.11,12
As a group of both aspiring and practicing pediatric dermatologists, we have identified opportunities for formalized education in and early exposure to this subspecialty during medical training instead of leaving the discovery of the field to chance. The gaps in medical education that we have identified have already led us to present potential curricular changes to the medical education committee at our home institution. We hope to inspire the development of strong pediatric dermatology education at the medical school level.
While the solution to the pediatric dermatology workforce shortage is complex and multifaceted, there is a unique opportunity to target medical students through mentorship, access to education, and clinical experiences. We recommend that medical schools implement these educational methods and track the efficacy of these interventions to quantify the predicted association between an increased workforce and early exposure to pediatric dermatology. Addressing a lack of exposure to the field and increasing support of students pursuing pediatric dermatology can help to alleviate the shortage at the earliest point in training.
There is a shortage of pediatric dermatologists in the United States, with fewer than 2% of practicing dermatologists specializing in pediatrics.1 Pediatric dermatology has the third highest referral rate by pediatricians but also is the third most challenging specialty to access, with an average appointment wait time of 92 days.2,3 Another factor leading to increased appointment wait times is the specificity of care required for pediatric patients. Frequently, pediatric patients evaluated by a general dermatologist will be referred to their pediatric dermatology colleagues. As medical students, we were introduced to the field of pediatric dermatology through different avenues—personal experience, research mentorship, or a clinical rotation in medical school. We found ourselves curious about the discrepancy between the supply of and demand for pediatric dermatologists and wondered what could be done to increase awareness of this subspecialty among medical students. We believe this workforce shortage can be ameliorated by improving early exposure to pediatric dermatology. In this article, we explore the existing framework surrounding pediatric dermatology in medical education and offer feasible recommendations and solutions to realistically combat this problem.
Pediatric dermatologists are essential to the greater dermatology community. Pediatric dermatologists receive advanced training in complex pediatric skin conditions that often is lacking in general dermatology residency. A large percentage of pediatric dermatology patients seen in academic medical centers have already been seen by general dermatologists who subsequently referred them to specialty care. In one study, 9.6% (10/108) of practicing pediatric dermatologists noted that their referrals were from general dermatologists.4 In another study, 42% (19/45) of referrals to a multidisciplinary pediatric dermatology-genetics were from general dermatologists.5 Given the shortage of pediatric dermatologists, these referrals undoubtedly overwhelm the system, and the results of these studies underscore the reality that general dermatologists do not necessarily feel adequately trained in complex pediatric conditions, creating an intrinsic need for pediatric dermatologists.
Admani et al6 reported that early mentorship was the single most important factor to 84% (91/109) of survey respondents who pursued pediatric dermatology. Forty percent (40/100) of survey respondents chose their specialty of pediatric dermatology during pediatrics residency, 34% (34/100) during medical school, 17% (17/100) during dermatology residency, and 5% (5/100) during internship, indicating that medical school is a crucial time for recruitment.6 It has been noted in the literature that more medical students matched to dermatology residency from schools with dermatology clerkships built into the curriculum than from schools without dedicated dermatology rotations, suggesting that early clinical exposure to dermatology fields has a predictable influence in matching.7 Currently, only about 10% (15/155) of allopathic medical schools in the United States offer a formal elective in pediatric dermatology via the Association of American Medical College’s Visiting Student Learning Opportunities program.8 When this information was cross-referenced with the most recently matched pediatric dermatology fellowship class (2023-2024), provided by the Fellowship Directors Chair of the Society for Pediatric Dermatology, we found that 17% (4/24) of the matched fellows attended one of these 15 medical schools. We also found that the 2023-2024 pediatric dermatology fellowship class had 12 unmatched spots out of 36 total positions nationwide (33%), highlighting a gap in pediatric dermatology care and placing further strain on an already underserved subspecialty. These data suggest that, while dermatologists may decide to pursue pediatric dermatology fellowships during residency, there is an opportunity to foster interest during medical school training and improve the fellowship match rate.
Several medical schools in the United States incorporate pediatric dermatology into their curricula, including lectures in preclinical courses and career panels to pediatric dermatology electives in the third and fourth years. These institutions can serve as models for other medical schools. Within preclinical content, we recommend creating a designated dermatology unit that can incorporate common pediatric dermatology pathologies also seen by general practitioners, such as common childhood rashes, atopic dermatitis, alopecia areata, seborrheic dermatitis, and acne. Rare pediatric diseases such as epidermolysis bullosa, tuberous sclerosis, and Ehlers-Danlos syndrome also may be included in the unit. If schools are not able to offer a stand-alone dermatology preclinical course, this content can be added to the immunology, musculoskeletal, infectious diseases, or genetics courses to account for the multisystemic effects of some of these conditions. Ideally, schools would offer elective exposure to pediatric dermatology during the clinical years of medical school to increase knowledge of the field; for example, pediatric dermatology materials could be included in core clerkships, as much of this content is applicable to the general pediatrics rotation. In particular, a lecture on common rashes in pediatric patients could be given before starting the core pediatric rotation. Additionally, problem-based pediatric dermatology cases could be implemented during the core pediatrics rotation. If students are offered an independent dermatology clinical elective, the already formatted 2- and 4-week basic dermatology courses designed by the American Academy of Dermatology could serve as suggested teaching guides or as self-teaching resources that could complement the dermatology rotation.9,10 Pediatric topics (eg, pediatric cutaneous fungal infections) are included within the American Academy of Dermatology basic dermatology curriculum.8,9
Increasing access to pediatric dermatology resources such as lecture series and mentorship opportunities could further broaden the pediatric dermatology knowledge base of medical students. Within medical school dermatology interest groups, there is an opportunity to have a pediatric dermatology lead to help coordinate lecture series and journal club sessions for interested students. The Society for Pediatric Dermatology and the Pediatric Dermatology Research Alliance have created programs to support students, and we encourage schools to raise awareness of these organizations as well as conference and grant opportunities. These initiatives foster meaningful mentor-mentee relationships, and more medical students may be interested if they are aware of these support networks.
There also may be opportunities to create residency tracks that increase the number of dermatology residency applicants. Programs such as the newly implemented pediatric dermatology track at the University of Pennsylvania and New York University allow medical students who are interested in pursuing pediatric dermatology to have a more focused and linear training path.11,12 Due to the inherent competition in matching into dermatology, we surmise that many students with interest in pediatric dermatology are lost to pediatric residencies. Given the large percentage of pediatric residents who ultimately develop an interest in pediatric dermatology, holding a spot for pediatric dermatology applicants—akin to the combined medical-dermatology spots—may be an avenue to increase the pool of pediatric dermatology fellows.1,6 Another avenue is to encourage the development of first-year pediatric internship tracks that lead directly into dermatology residency, such as newly established programs at the University of Pennsylvania and New York University.11,12
As a group of both aspiring and practicing pediatric dermatologists, we have identified opportunities for formalized education in and early exposure to this subspecialty during medical training instead of leaving the discovery of the field to chance. The gaps in medical education that we have identified have already led us to present potential curricular changes to the medical education committee at our home institution. We hope to inspire the development of strong pediatric dermatology education at the medical school level.
While the solution to the pediatric dermatology workforce shortage is complex and multifaceted, there is a unique opportunity to target medical students through mentorship, access to education, and clinical experiences. We recommend that medical schools implement these educational methods and track the efficacy of these interventions to quantify the predicted association between an increased workforce and early exposure to pediatric dermatology. Addressing a lack of exposure to the field and increasing support of students pursuing pediatric dermatology can help to alleviate the shortage at the earliest point in training.
- Prindaville B, Antaya RJ, Siegfried EC. Pediatric dermatology: past, present, and future. Pediatr Dermatol. 2015;32:1-12. doi:10.1111/pde.12362
- Wright TS. Update on the pediatric dermatology workforce shortage. Cutis. 2021;108:237-238. doi:10.12788/cutis.0379
- Stephens MR, Murthy AS, McMahon PJ. Wait times, health care touchpoints, and nonattendance in an academic pediatric dermatology clinic. ediatr Dermatol. 2019;36:893-897. doi:10.1111/pde.13943
- Fogel AL, Teng JM. A survey to assess perceived differences in referral pathways to board-certified pediatric dermatologists. Pediatr Dermatol. 2015;32:e314-e315. doi:10.1111/pde.12703
- Parker JC, Rangu S, Grand KL, et al. Genetic skin disorders: the value of a multidisciplinary clinic. Am J Med Genet A. 2021;185:1159-1167. doi:10.1002/ajmg.a.62095
- Admani S, Caufield M, Kim SS, et al. Understanding the pediatric dermatology workforce shortage: mentoring matters. J Pediatr. 2014;164:372-5.e1. doi:10.1016/j.jpeds.2013.10.004
- Ogidi P, Ahmed F, Cahn BA, et al. Medical schools as gatekeepers: a survey and analysis of factors predicting dermatology residency placement. J Am Acad Dermatol. 2022;86:490-492. doi:10.1016 /j.jaad.2021.09.027
- Visiting Student Learning Opportunities (VSLO). Accessed May 30, 2025. https://students-residents.aamc.org/visiting-student-learning-opportunities/visiting-student-learning-opportunities-vslo
- American Academy of Dermatology Association. AAD Learning Center. Basic dermatology curriculum (2-week rotation). Accessed May 12, 2025. https://learning.aad.org/Listing/Basic-Dermatology-Curriculum-2-Week-Rotation-5395
- American Academy of Dermatology Association. AAD Learning Center. Basic dermatology curriculum (4-week rotation). Accessed May 12, 2025. https://learning.aad.org/Public/Catalog/Details.aspx?id=YPssTVIbBO3Zb%2bOuf%2fM7Kg%3d%3d&returnurl=%2fUsers%2fUserOnlineCourse.aspx%3fLearningActivityID%3dYPssTVIbBO3Zb%252bOuf%252fM7Kg%253d%253d
- Penn Medicine Dermatology Residency Training Program. Residency tracks. Accessed May 12, 2025. https://dermatology.upenn.edu/residents/residency-tracks/
- Pediatric Dermatology Residency Track at NYU Grossman School of Medicine. Pediatric Track. Accessed May 30, 2025. https://med.nyu.edu/departments-institutes/dermatology/education/residency/pediatric-track
- Prindaville B, Antaya RJ, Siegfried EC. Pediatric dermatology: past, present, and future. Pediatr Dermatol. 2015;32:1-12. doi:10.1111/pde.12362
- Wright TS. Update on the pediatric dermatology workforce shortage. Cutis. 2021;108:237-238. doi:10.12788/cutis.0379
- Stephens MR, Murthy AS, McMahon PJ. Wait times, health care touchpoints, and nonattendance in an academic pediatric dermatology clinic. ediatr Dermatol. 2019;36:893-897. doi:10.1111/pde.13943
- Fogel AL, Teng JM. A survey to assess perceived differences in referral pathways to board-certified pediatric dermatologists. Pediatr Dermatol. 2015;32:e314-e315. doi:10.1111/pde.12703
- Parker JC, Rangu S, Grand KL, et al. Genetic skin disorders: the value of a multidisciplinary clinic. Am J Med Genet A. 2021;185:1159-1167. doi:10.1002/ajmg.a.62095
- Admani S, Caufield M, Kim SS, et al. Understanding the pediatric dermatology workforce shortage: mentoring matters. J Pediatr. 2014;164:372-5.e1. doi:10.1016/j.jpeds.2013.10.004
- Ogidi P, Ahmed F, Cahn BA, et al. Medical schools as gatekeepers: a survey and analysis of factors predicting dermatology residency placement. J Am Acad Dermatol. 2022;86:490-492. doi:10.1016 /j.jaad.2021.09.027
- Visiting Student Learning Opportunities (VSLO). Accessed May 30, 2025. https://students-residents.aamc.org/visiting-student-learning-opportunities/visiting-student-learning-opportunities-vslo
- American Academy of Dermatology Association. AAD Learning Center. Basic dermatology curriculum (2-week rotation). Accessed May 12, 2025. https://learning.aad.org/Listing/Basic-Dermatology-Curriculum-2-Week-Rotation-5395
- American Academy of Dermatology Association. AAD Learning Center. Basic dermatology curriculum (4-week rotation). Accessed May 12, 2025. https://learning.aad.org/Public/Catalog/Details.aspx?id=YPssTVIbBO3Zb%2bOuf%2fM7Kg%3d%3d&returnurl=%2fUsers%2fUserOnlineCourse.aspx%3fLearningActivityID%3dYPssTVIbBO3Zb%252bOuf%252fM7Kg%253d%253d
- Penn Medicine Dermatology Residency Training Program. Residency tracks. Accessed May 12, 2025. https://dermatology.upenn.edu/residents/residency-tracks/
- Pediatric Dermatology Residency Track at NYU Grossman School of Medicine. Pediatric Track. Accessed May 30, 2025. https://med.nyu.edu/departments-institutes/dermatology/education/residency/pediatric-track
Workforce Shortage of Pediatric Dermatologists: A Medical Student’s Perspective
Workforce Shortage of Pediatric Dermatologists: A Medical Student’s Perspective
PRACTICE POINTS
- Addressing a lack of exposure to pediatric dermatology in medical school and increasing support for students who are interested in the field can help alleviate the shortage of physicians at the earliest point in training.
- Increasing access to pediatric dermatology resources, such as lecture series and mentorship opportunities, could further broaden the medical student knowledge base.
- There is an opportunity to create residency tracks that increase the number of dermatology residency applicants who are medical students interested in pursuing pediatric dermatology.
Eruptive Erythematous Papules on the Forearms
Eruptive Erythematous Papules on the Forearms
THE DIAGNOSIS: Acral Eruptive Syringoma
Syringomas are small, benign, often asymptomatic eccrine tumors that originate in the intraepidermal portion of eccrine sweat ducts.1 Clinically, they present as multiple symmetric white-to-yellow or discrete flesh-colored papules measuring 1 to 3 mm in diameter, often located on the face (most commonly on the eyelids), with a greater prevalence in middle-aged women. Occasionally, they manifest in other locations such as the cheeks, chest, axillae, abdomen, and groin.2
In 1987, Friedman and Butler3 developed a classification system categorizing syringomas into 4 clinical subtypes: familial syringoma, localized syringoma, Down syndrome–related syringoma, and generalized syringoma. The fourth subtype includes the variant of eruptive syringoma,3 a rare clinical manifestation that often develops before or during puberty with several flesh-colored or lightly pigmented papules on the neck, anterior chest, upper abdomen, axillae, periumbilical region, and/or genital region.1,4,5 The etiology of eruptive syringomas is unclear, although it has been linked to abnormal proliferation of sweat glands due to an underlying local inflammatory process.6
Acral distribution of syringomas is a rare variant that can manifest as part of generalized eruptive syringoma with consequent involvement of the arms and other areas.5,7 There are limited case reports on eruptive syringomas with predominant acral distribution.8 Compared to classic syringomas, the acral variant is associated with an older age of onset as well as a similar prevalence between men and women.9 Acral eruptive syringoma (AES) usually is isolated to the distal arms and legs. The most commonly affected region is the anterior surface of the forearms, although involvement of the dorsal hands, wrists, and feet also has been reported.10-16
The first known case of AES, which was reported in 1977, described eruptive syringomas on the dorsal hands of a healthy 31-year-old man.17 Several cases have been reported since then, mostly in patients aged 30 to 60 years, with predominant involvement of the dorsal hands and forearms.18-24 A review of Embase as well as PubMed articles indexed for MEDLINE using the search terms syringoma OR eccrine ductal tumor and eruptive OR acral OR arms OR forearms OR extremities identified 19 reported cases of AES between 1977 and 2023. For the reported AES cases, the mean (SD) age at diagnosis was 45.1 years (15.96 years), with patient ages ranging from 19 to 76 years. Notably, most cases occurred in individuals aged between 30 and 60 years, which deviates from the typical age of onset of localized syringomas, commonly seen during puberty or early adulthood.
Currently, AES is categorized within the clinical presentation of eruptive syringoma. Nevertheless, some authors have proposed classifying it as a distinct fifth clinical group due to specific features that distinguish it from generalized eruptive syringoma.9 This reclassification has considerable implications for the differential diagnosis, particularly because exclusive acral involvement poses a substantial diagnostic challenge and often requires histologic confirmation.
As shown in the Figure, histopathologic examination revealed tubular structures in the upper dermis with characteristic comma-shaped extensions. Some of these structures were lined with cuboidal cells and contained eosinophilic material within the lumen. There was no involvement of the epidermis or deeper dermis. The histologic features were consistent with syringoma, which is distinguished by its predominant involvement of the upper dermis and the presence of enlarged, dilated eccrine ducts, as observed in our case.
Treatment of syringomas often is challenging due to the high rate of recurrence and the risk for postinflammatory hyperpigmentation. Since the condition is benign, treatment typically is pursued for aesthetic reasons. Various therapeutic approaches have been reported, each with diverse response rates. The most common method involves surgical intervention, either with electrodesiccation or CO2 laser—both of which have shown satisfactory resolution of lesions without recurrence at 1-year follow-up, with no major scarring reported.25,26 Alternatively, topical management with retinoids daily over a 4-month period leads to flattening of the tumors with no further appearance of new lesions.27 Despite the availability of numerous management options, establishing a first-line treatment remains controversial due to the high risk for recurrence and the variability in the number and location of lesions among individual patients. In our case, given the benign nature of syringomas, the asymptomatic nature of the lesions, the involvement of noncritical aesthetic areas, and the limited response to noninvasive therapeutic options, the patient was informed of the diagnosis, and no further pharmacologic or surgical intervention was pursued.
- Williams K, Shinkai K. Evaluation and management of the patient with multiple syringomas: a systematic review of the literature. J Am Acad Dermatol. 2016;74:1234-1240.E9. doi:10.1016 /j.jaad.2015.12.006
- Resende C, Araújo C, Santos R, et al. Late-onset of eruptive syringomas: a diagnostic challenge. An Bras Dermatol. 2015;90(3 suppl 1):239-241. doi:10.1590/abd1806-4841.20153899
- Friedman SJ, Butler DF. Syringoma presenting as milia. J Am Acad Dermatol. 1987;16:310-314.
- Avhad G, Ghuge P, Jerajani HR. Generalized eruptive syringoma. Indian J Dermatol. 2015;60:214. doi:10.4103/0019-5154.152586
- Ning WV, Bashey S, Cole C, et al. Multiple eruptive syringomas on the penis. Cutis. 2019;103:E15-E16.
- Cohen PR, Tschen JA, Rapini RP. Penile syringoma: reports and review of patients with syringoma located on the penis. J Clin Aesthet Dermatol. 2013;6:38-42.
- Jamalipour M, Heidarpour M, Rajabi P. Generalized eruptive syringomas. Indian J Dermatol. 2009;54:65-67. doi:10.4103/0019-5154.48992
- Mohaghegh F, Amiri A, Fatemi Naeini F, et al. Acral eruptive syringoma: an unusual presentation with misdiagnosis. Case Rep Dermatol Med. 2020;2020:5416285. doi:10.1155/2020/5416285
- Valdivielso-Ramos M, de la Cueva P, Gimeno M, et al. Acral syringomas. Actas Dermosifiliogr. 2010;101:458-460.
- Patel K, Lundgren AD, Ahmed AM, et al. Disseminated syringomas of the upper extremities in a young woman. Cureus. 2018;10:E3619. doi:10.7759/cureus.3619
- Balci DD, Atik E, Altintas S. Coexistence of acral syringomas and multiple trichoepitheliomas on the face. J Cutan Med Surg. 2009;13:169-171. doi:10.2310/7750.2008.08011
- Martín-García RF, Muñoz CM. Acral syringomas presenting as a photosensitive papular eruption. Cutis. 2006;77:33-36.
- Varas-Meis E, Prada-García C, Samaniego-González E, et al. Acral syringomas associated with hematological neoplasm. Indian J Dermatol Venereol Leprol. 2017;83:136. doi:10.4103/0378-6323.192961
- Berbis P, Fabre JF, Jancovici E, et al. Late-onset syringomas of the upper extremities associated with a carcinoid tumor. Arch Dermatol. 1989;125:848-849.
- Metze D, Jurecka W, Gebhart W. Disseminated syringomas of the upper extremities. case history and immunohistochemical and ultrastructural study. Dermatologica. 1990;180:228-235. doi:10.1159/000248036
- Gómez-de Castro C, Vivanco Allende B, García-García B. Multiple acral syringomas. siringomas acrales múltiples. Actas Dermosifiliogr (Engl Ed). 2018;109:834-836. doi:10.1016/j.ad.2017.10.014
- Hughes PS, Apisarnthanarax P. Acral syringoma. Arch Dermatol. 1977;113:1435-1436.
- Asai Y, Ishii M, Hamada T. Acral syringoma: electron microscopic studies on its origin. Acta Derm Venereol. 1982;62:64-68.
- van den Broek H, Lundquist CD. Syringomas of the upper extremities with onset in the sixth decade. J Am Acad Dermatol. 1982,6:534-536. doi:10.1016/S0190-9622(82)80368-X
- Garcia C, Krunic AL, Grichnik J, et al. Multiple acral syringomata with uniform involvement of the hands and feet. Cutis. 1997;59:213-214, 216.
- Patrizi A, Neri I, Marzaduri S, et al. Syringoma: a review of twenty-nine cases. Acta Derm Venereol. 1998;78:460-462.
- Iglesias Sancho M, Serra Llobet J, Salleras Redonnet M, et al. Siringomas disem- inados de inicio acral, aparecidos en la octava década. Actas Dermosifiliofr. 1999;90:253-257.
- Muniesa C, Fortuño Y, Moreno A, et al. Papules on the dorsum of the fingers. Actas Dermosifiliogr. 2008;99:812-813. doi:10.1016 /S1578-2190(08)70371-8
- Koh MJ. Multiple acral syringomas involving the hands. Clin Exp Dermatol. 2009;34:E438. doi:10.1111/j.1365-2230.2009.03462.x
- Karam P, Benedetto AV. Syringomas: new approach to an old technique. Int J Dermatol. 1996;35:219-220. doi:10.1111/j.1365-4362 .1996.tb01647.x
- Wang JI, Roenigk HH. Treatment of multiple facial syringomas with the carbon dioxide (CO2) laser. Dermatol Surg. 1999;25:136-139. doi:10.1046/j.1524-4725.1999.08111.x
- Gómez MI, Pérez B, Azaña JM, et al. Eruptive syringoma: treatment with topical tretinoin. Dermatology. 2009;189:105-106. doi:10.1159/000246803
THE DIAGNOSIS: Acral Eruptive Syringoma
Syringomas are small, benign, often asymptomatic eccrine tumors that originate in the intraepidermal portion of eccrine sweat ducts.1 Clinically, they present as multiple symmetric white-to-yellow or discrete flesh-colored papules measuring 1 to 3 mm in diameter, often located on the face (most commonly on the eyelids), with a greater prevalence in middle-aged women. Occasionally, they manifest in other locations such as the cheeks, chest, axillae, abdomen, and groin.2
In 1987, Friedman and Butler3 developed a classification system categorizing syringomas into 4 clinical subtypes: familial syringoma, localized syringoma, Down syndrome–related syringoma, and generalized syringoma. The fourth subtype includes the variant of eruptive syringoma,3 a rare clinical manifestation that often develops before or during puberty with several flesh-colored or lightly pigmented papules on the neck, anterior chest, upper abdomen, axillae, periumbilical region, and/or genital region.1,4,5 The etiology of eruptive syringomas is unclear, although it has been linked to abnormal proliferation of sweat glands due to an underlying local inflammatory process.6
Acral distribution of syringomas is a rare variant that can manifest as part of generalized eruptive syringoma with consequent involvement of the arms and other areas.5,7 There are limited case reports on eruptive syringomas with predominant acral distribution.8 Compared to classic syringomas, the acral variant is associated with an older age of onset as well as a similar prevalence between men and women.9 Acral eruptive syringoma (AES) usually is isolated to the distal arms and legs. The most commonly affected region is the anterior surface of the forearms, although involvement of the dorsal hands, wrists, and feet also has been reported.10-16
The first known case of AES, which was reported in 1977, described eruptive syringomas on the dorsal hands of a healthy 31-year-old man.17 Several cases have been reported since then, mostly in patients aged 30 to 60 years, with predominant involvement of the dorsal hands and forearms.18-24 A review of Embase as well as PubMed articles indexed for MEDLINE using the search terms syringoma OR eccrine ductal tumor and eruptive OR acral OR arms OR forearms OR extremities identified 19 reported cases of AES between 1977 and 2023. For the reported AES cases, the mean (SD) age at diagnosis was 45.1 years (15.96 years), with patient ages ranging from 19 to 76 years. Notably, most cases occurred in individuals aged between 30 and 60 years, which deviates from the typical age of onset of localized syringomas, commonly seen during puberty or early adulthood.
Currently, AES is categorized within the clinical presentation of eruptive syringoma. Nevertheless, some authors have proposed classifying it as a distinct fifth clinical group due to specific features that distinguish it from generalized eruptive syringoma.9 This reclassification has considerable implications for the differential diagnosis, particularly because exclusive acral involvement poses a substantial diagnostic challenge and often requires histologic confirmation.
As shown in the Figure, histopathologic examination revealed tubular structures in the upper dermis with characteristic comma-shaped extensions. Some of these structures were lined with cuboidal cells and contained eosinophilic material within the lumen. There was no involvement of the epidermis or deeper dermis. The histologic features were consistent with syringoma, which is distinguished by its predominant involvement of the upper dermis and the presence of enlarged, dilated eccrine ducts, as observed in our case.

Treatment of syringomas often is challenging due to the high rate of recurrence and the risk for postinflammatory hyperpigmentation. Since the condition is benign, treatment typically is pursued for aesthetic reasons. Various therapeutic approaches have been reported, each with diverse response rates. The most common method involves surgical intervention, either with electrodesiccation or CO2 laser—both of which have shown satisfactory resolution of lesions without recurrence at 1-year follow-up, with no major scarring reported.25,26 Alternatively, topical management with retinoids daily over a 4-month period leads to flattening of the tumors with no further appearance of new lesions.27 Despite the availability of numerous management options, establishing a first-line treatment remains controversial due to the high risk for recurrence and the variability in the number and location of lesions among individual patients. In our case, given the benign nature of syringomas, the asymptomatic nature of the lesions, the involvement of noncritical aesthetic areas, and the limited response to noninvasive therapeutic options, the patient was informed of the diagnosis, and no further pharmacologic or surgical intervention was pursued.
THE DIAGNOSIS: Acral Eruptive Syringoma
Syringomas are small, benign, often asymptomatic eccrine tumors that originate in the intraepidermal portion of eccrine sweat ducts.1 Clinically, they present as multiple symmetric white-to-yellow or discrete flesh-colored papules measuring 1 to 3 mm in diameter, often located on the face (most commonly on the eyelids), with a greater prevalence in middle-aged women. Occasionally, they manifest in other locations such as the cheeks, chest, axillae, abdomen, and groin.2
In 1987, Friedman and Butler3 developed a classification system categorizing syringomas into 4 clinical subtypes: familial syringoma, localized syringoma, Down syndrome–related syringoma, and generalized syringoma. The fourth subtype includes the variant of eruptive syringoma,3 a rare clinical manifestation that often develops before or during puberty with several flesh-colored or lightly pigmented papules on the neck, anterior chest, upper abdomen, axillae, periumbilical region, and/or genital region.1,4,5 The etiology of eruptive syringomas is unclear, although it has been linked to abnormal proliferation of sweat glands due to an underlying local inflammatory process.6
Acral distribution of syringomas is a rare variant that can manifest as part of generalized eruptive syringoma with consequent involvement of the arms and other areas.5,7 There are limited case reports on eruptive syringomas with predominant acral distribution.8 Compared to classic syringomas, the acral variant is associated with an older age of onset as well as a similar prevalence between men and women.9 Acral eruptive syringoma (AES) usually is isolated to the distal arms and legs. The most commonly affected region is the anterior surface of the forearms, although involvement of the dorsal hands, wrists, and feet also has been reported.10-16
The first known case of AES, which was reported in 1977, described eruptive syringomas on the dorsal hands of a healthy 31-year-old man.17 Several cases have been reported since then, mostly in patients aged 30 to 60 years, with predominant involvement of the dorsal hands and forearms.18-24 A review of Embase as well as PubMed articles indexed for MEDLINE using the search terms syringoma OR eccrine ductal tumor and eruptive OR acral OR arms OR forearms OR extremities identified 19 reported cases of AES between 1977 and 2023. For the reported AES cases, the mean (SD) age at diagnosis was 45.1 years (15.96 years), with patient ages ranging from 19 to 76 years. Notably, most cases occurred in individuals aged between 30 and 60 years, which deviates from the typical age of onset of localized syringomas, commonly seen during puberty or early adulthood.
Currently, AES is categorized within the clinical presentation of eruptive syringoma. Nevertheless, some authors have proposed classifying it as a distinct fifth clinical group due to specific features that distinguish it from generalized eruptive syringoma.9 This reclassification has considerable implications for the differential diagnosis, particularly because exclusive acral involvement poses a substantial diagnostic challenge and often requires histologic confirmation.
As shown in the Figure, histopathologic examination revealed tubular structures in the upper dermis with characteristic comma-shaped extensions. Some of these structures were lined with cuboidal cells and contained eosinophilic material within the lumen. There was no involvement of the epidermis or deeper dermis. The histologic features were consistent with syringoma, which is distinguished by its predominant involvement of the upper dermis and the presence of enlarged, dilated eccrine ducts, as observed in our case.

Treatment of syringomas often is challenging due to the high rate of recurrence and the risk for postinflammatory hyperpigmentation. Since the condition is benign, treatment typically is pursued for aesthetic reasons. Various therapeutic approaches have been reported, each with diverse response rates. The most common method involves surgical intervention, either with electrodesiccation or CO2 laser—both of which have shown satisfactory resolution of lesions without recurrence at 1-year follow-up, with no major scarring reported.25,26 Alternatively, topical management with retinoids daily over a 4-month period leads to flattening of the tumors with no further appearance of new lesions.27 Despite the availability of numerous management options, establishing a first-line treatment remains controversial due to the high risk for recurrence and the variability in the number and location of lesions among individual patients. In our case, given the benign nature of syringomas, the asymptomatic nature of the lesions, the involvement of noncritical aesthetic areas, and the limited response to noninvasive therapeutic options, the patient was informed of the diagnosis, and no further pharmacologic or surgical intervention was pursued.
- Williams K, Shinkai K. Evaluation and management of the patient with multiple syringomas: a systematic review of the literature. J Am Acad Dermatol. 2016;74:1234-1240.E9. doi:10.1016 /j.jaad.2015.12.006
- Resende C, Araújo C, Santos R, et al. Late-onset of eruptive syringomas: a diagnostic challenge. An Bras Dermatol. 2015;90(3 suppl 1):239-241. doi:10.1590/abd1806-4841.20153899
- Friedman SJ, Butler DF. Syringoma presenting as milia. J Am Acad Dermatol. 1987;16:310-314.
- Avhad G, Ghuge P, Jerajani HR. Generalized eruptive syringoma. Indian J Dermatol. 2015;60:214. doi:10.4103/0019-5154.152586
- Ning WV, Bashey S, Cole C, et al. Multiple eruptive syringomas on the penis. Cutis. 2019;103:E15-E16.
- Cohen PR, Tschen JA, Rapini RP. Penile syringoma: reports and review of patients with syringoma located on the penis. J Clin Aesthet Dermatol. 2013;6:38-42.
- Jamalipour M, Heidarpour M, Rajabi P. Generalized eruptive syringomas. Indian J Dermatol. 2009;54:65-67. doi:10.4103/0019-5154.48992
- Mohaghegh F, Amiri A, Fatemi Naeini F, et al. Acral eruptive syringoma: an unusual presentation with misdiagnosis. Case Rep Dermatol Med. 2020;2020:5416285. doi:10.1155/2020/5416285
- Valdivielso-Ramos M, de la Cueva P, Gimeno M, et al. Acral syringomas. Actas Dermosifiliogr. 2010;101:458-460.
- Patel K, Lundgren AD, Ahmed AM, et al. Disseminated syringomas of the upper extremities in a young woman. Cureus. 2018;10:E3619. doi:10.7759/cureus.3619
- Balci DD, Atik E, Altintas S. Coexistence of acral syringomas and multiple trichoepitheliomas on the face. J Cutan Med Surg. 2009;13:169-171. doi:10.2310/7750.2008.08011
- Martín-García RF, Muñoz CM. Acral syringomas presenting as a photosensitive papular eruption. Cutis. 2006;77:33-36.
- Varas-Meis E, Prada-García C, Samaniego-González E, et al. Acral syringomas associated with hematological neoplasm. Indian J Dermatol Venereol Leprol. 2017;83:136. doi:10.4103/0378-6323.192961
- Berbis P, Fabre JF, Jancovici E, et al. Late-onset syringomas of the upper extremities associated with a carcinoid tumor. Arch Dermatol. 1989;125:848-849.
- Metze D, Jurecka W, Gebhart W. Disseminated syringomas of the upper extremities. case history and immunohistochemical and ultrastructural study. Dermatologica. 1990;180:228-235. doi:10.1159/000248036
- Gómez-de Castro C, Vivanco Allende B, García-García B. Multiple acral syringomas. siringomas acrales múltiples. Actas Dermosifiliogr (Engl Ed). 2018;109:834-836. doi:10.1016/j.ad.2017.10.014
- Hughes PS, Apisarnthanarax P. Acral syringoma. Arch Dermatol. 1977;113:1435-1436.
- Asai Y, Ishii M, Hamada T. Acral syringoma: electron microscopic studies on its origin. Acta Derm Venereol. 1982;62:64-68.
- van den Broek H, Lundquist CD. Syringomas of the upper extremities with onset in the sixth decade. J Am Acad Dermatol. 1982,6:534-536. doi:10.1016/S0190-9622(82)80368-X
- Garcia C, Krunic AL, Grichnik J, et al. Multiple acral syringomata with uniform involvement of the hands and feet. Cutis. 1997;59:213-214, 216.
- Patrizi A, Neri I, Marzaduri S, et al. Syringoma: a review of twenty-nine cases. Acta Derm Venereol. 1998;78:460-462.
- Iglesias Sancho M, Serra Llobet J, Salleras Redonnet M, et al. Siringomas disem- inados de inicio acral, aparecidos en la octava década. Actas Dermosifiliofr. 1999;90:253-257.
- Muniesa C, Fortuño Y, Moreno A, et al. Papules on the dorsum of the fingers. Actas Dermosifiliogr. 2008;99:812-813. doi:10.1016 /S1578-2190(08)70371-8
- Koh MJ. Multiple acral syringomas involving the hands. Clin Exp Dermatol. 2009;34:E438. doi:10.1111/j.1365-2230.2009.03462.x
- Karam P, Benedetto AV. Syringomas: new approach to an old technique. Int J Dermatol. 1996;35:219-220. doi:10.1111/j.1365-4362 .1996.tb01647.x
- Wang JI, Roenigk HH. Treatment of multiple facial syringomas with the carbon dioxide (CO2) laser. Dermatol Surg. 1999;25:136-139. doi:10.1046/j.1524-4725.1999.08111.x
- Gómez MI, Pérez B, Azaña JM, et al. Eruptive syringoma: treatment with topical tretinoin. Dermatology. 2009;189:105-106. doi:10.1159/000246803
- Williams K, Shinkai K. Evaluation and management of the patient with multiple syringomas: a systematic review of the literature. J Am Acad Dermatol. 2016;74:1234-1240.E9. doi:10.1016 /j.jaad.2015.12.006
- Resende C, Araújo C, Santos R, et al. Late-onset of eruptive syringomas: a diagnostic challenge. An Bras Dermatol. 2015;90(3 suppl 1):239-241. doi:10.1590/abd1806-4841.20153899
- Friedman SJ, Butler DF. Syringoma presenting as milia. J Am Acad Dermatol. 1987;16:310-314.
- Avhad G, Ghuge P, Jerajani HR. Generalized eruptive syringoma. Indian J Dermatol. 2015;60:214. doi:10.4103/0019-5154.152586
- Ning WV, Bashey S, Cole C, et al. Multiple eruptive syringomas on the penis. Cutis. 2019;103:E15-E16.
- Cohen PR, Tschen JA, Rapini RP. Penile syringoma: reports and review of patients with syringoma located on the penis. J Clin Aesthet Dermatol. 2013;6:38-42.
- Jamalipour M, Heidarpour M, Rajabi P. Generalized eruptive syringomas. Indian J Dermatol. 2009;54:65-67. doi:10.4103/0019-5154.48992
- Mohaghegh F, Amiri A, Fatemi Naeini F, et al. Acral eruptive syringoma: an unusual presentation with misdiagnosis. Case Rep Dermatol Med. 2020;2020:5416285. doi:10.1155/2020/5416285
- Valdivielso-Ramos M, de la Cueva P, Gimeno M, et al. Acral syringomas. Actas Dermosifiliogr. 2010;101:458-460.
- Patel K, Lundgren AD, Ahmed AM, et al. Disseminated syringomas of the upper extremities in a young woman. Cureus. 2018;10:E3619. doi:10.7759/cureus.3619
- Balci DD, Atik E, Altintas S. Coexistence of acral syringomas and multiple trichoepitheliomas on the face. J Cutan Med Surg. 2009;13:169-171. doi:10.2310/7750.2008.08011
- Martín-García RF, Muñoz CM. Acral syringomas presenting as a photosensitive papular eruption. Cutis. 2006;77:33-36.
- Varas-Meis E, Prada-García C, Samaniego-González E, et al. Acral syringomas associated with hematological neoplasm. Indian J Dermatol Venereol Leprol. 2017;83:136. doi:10.4103/0378-6323.192961
- Berbis P, Fabre JF, Jancovici E, et al. Late-onset syringomas of the upper extremities associated with a carcinoid tumor. Arch Dermatol. 1989;125:848-849.
- Metze D, Jurecka W, Gebhart W. Disseminated syringomas of the upper extremities. case history and immunohistochemical and ultrastructural study. Dermatologica. 1990;180:228-235. doi:10.1159/000248036
- Gómez-de Castro C, Vivanco Allende B, García-García B. Multiple acral syringomas. siringomas acrales múltiples. Actas Dermosifiliogr (Engl Ed). 2018;109:834-836. doi:10.1016/j.ad.2017.10.014
- Hughes PS, Apisarnthanarax P. Acral syringoma. Arch Dermatol. 1977;113:1435-1436.
- Asai Y, Ishii M, Hamada T. Acral syringoma: electron microscopic studies on its origin. Acta Derm Venereol. 1982;62:64-68.
- van den Broek H, Lundquist CD. Syringomas of the upper extremities with onset in the sixth decade. J Am Acad Dermatol. 1982,6:534-536. doi:10.1016/S0190-9622(82)80368-X
- Garcia C, Krunic AL, Grichnik J, et al. Multiple acral syringomata with uniform involvement of the hands and feet. Cutis. 1997;59:213-214, 216.
- Patrizi A, Neri I, Marzaduri S, et al. Syringoma: a review of twenty-nine cases. Acta Derm Venereol. 1998;78:460-462.
- Iglesias Sancho M, Serra Llobet J, Salleras Redonnet M, et al. Siringomas disem- inados de inicio acral, aparecidos en la octava década. Actas Dermosifiliofr. 1999;90:253-257.
- Muniesa C, Fortuño Y, Moreno A, et al. Papules on the dorsum of the fingers. Actas Dermosifiliogr. 2008;99:812-813. doi:10.1016 /S1578-2190(08)70371-8
- Koh MJ. Multiple acral syringomas involving the hands. Clin Exp Dermatol. 2009;34:E438. doi:10.1111/j.1365-2230.2009.03462.x
- Karam P, Benedetto AV. Syringomas: new approach to an old technique. Int J Dermatol. 1996;35:219-220. doi:10.1111/j.1365-4362 .1996.tb01647.x
- Wang JI, Roenigk HH. Treatment of multiple facial syringomas with the carbon dioxide (CO2) laser. Dermatol Surg. 1999;25:136-139. doi:10.1046/j.1524-4725.1999.08111.x
- Gómez MI, Pérez B, Azaña JM, et al. Eruptive syringoma: treatment with topical tretinoin. Dermatology. 2009;189:105-106. doi:10.1159/000246803
Eruptive Erythematous Papules on the Forearms
Eruptive Erythematous Papules on the Forearms
A 44-year-old man presented to the dermatology department with multiple eruptive, nonconfluent, erythematous papules on the anterior forearms of 2 years’ duration. The patient’s medical history was notable for right-sided testicular cancer diagnosed in childhood and 3 excised basal cell carcinomas, the most recent of which was concurrent with the present case. The patient denied any recent pruritus, exposure to irritants, or use of over-the-counter medications. Physical examination was remarkable for numerous monomorphic, symmetric, nonconfluent, flesh-colored to slightly pigmented papules on the dorsal aspect of the forearms. No involvement of the fingers or lower extremities was observed. Two punch biopsies of representative lesions on the right and left forearms were taken. Histopathologic examination revealed eccrine ductal proliferations lined by cuboidal cells embedded within bundles of sclerotic collagen.