User login
Signals of gut microbiome interaction with experimental Alzheimer’s drug prompt new trial
LOS ANGELES – A single look at the gut microbiome of patients with Alzheimer’s disease (AD) suggests an interaction between anti-inflammatory gut bacteria and long-term exposure to an investigational sigma 1 receptor agonist.
After up to 148 weeks treatment with Anavex 2-73, patients with stable or improved functional scores showed significantly higher levels of both Ruminococcaceae and Porphyromonadaceae, compared with patients who had declining function. Both bacterial families produce butyrate, an anti-inflammatory short-chain fatty acid.
Conversely, poor response was associated with a low level of Verrucomicrobia, a mucin-degrading phylum thought to be important in gut homeostasis. These bacteria live mainly in the intestinal mucosa – the physical interface between the microbiome and the rest of the body.
The data, presented at the Alzheimer’s Association International Conference, represent the first microbiome measurements reported in a clinical trial of an investigational Alzheimer’s therapy. Because they come from a single sample taken from a small group in an extension study, without a baseline comparator, it’s impossible to know what these associations mean. But the findings are enough to nudge Anavex Life Sciences into adding microbiome changes to its new study of Anavex 2-73, according to Christopher Missling, PhD, president and chief executive officer of the company.
The study, ramping up now, aims to recruit 450 patients with mild AD. They will be randomized to high-dose or mid-dose Anavex 2-73 for 48 weeks. The primary outcomes are measures of cognition and function. Stool sampling at baseline and at the end of the study will be included as well, Dr. Missling said in an interview.
Anavex 2-73 is a sigma-1 receptor agonist. A chaperone protein, sigma-1 is activated in response to acute and chronic cellular stressors, several which are important in neurodegeneration. The sigma-1 receptor is found on neurons and glia in many areas of the central nervous system. It modulates several processes implicated in neurodegenerative diseases, including glutamate and calcium activity, reaction to oxidative stress, and mitochondrial function. There is some evidence that sigma-1 receptor activation can induce neuronal regrowth and functional recovery after stroke. It also appears to play a role in helping cells clear misfolded proteins – a pathway that makes it an attractive drug target in Alzheimer’s disease, as well as other neurodegenerative diseases with aberrant proteins, such as Parkinson’s and Huntington’s diseases.
Anavex 2-73’s phase 2 development started with a 5-week crossover trial of 32 patients. This was followed by a 52-week, open-label extension trial of 10, 20, 30, and 50 mg/day orally, in which each patient was titrated to the maximum tolerated dose. The main endpoints were change on the Mini Mental State Exam and change on the Alzheimer’s Disease Cooperative Study-activities of daily living (ADCS-ADL) scale.
At 57 weeks, six patients had improved on the Mini Mental State Exam score: four with high plasma levels and two with low plasma levels, correlating to the dosage obtained. On the functional measure of activities of daily living, nine patients had improved, including five with high plasma levels, three with moderate levels, and one with a low level. One patient, with a moderate level, remained stable. The remaining 14 patients declined.
The company then enrolled 21 of the cohort in a 208-week extension trial, primarily because of patient request, Dr. Missling said. “They know they are doing better. Their families know they’re doing better. They did not want to give this up.”
Last fall, the company released 148-week functional and cognitive data confirming the initial findings: Patients with higher plasma levels (correlating with higher doses) declined about 2 points on the ADCS-ADL scale, compared with a mean decline of about 25 points among those with lower blood levels – an 88% difference in favor of treatment. Cognition scores showed a similar pattern, with the high-concentration group declining 64% less than the low-concentration group.
Sixteen patients consented to stool sampling. A sophisticated computer algorithm characterized the microbiome of each, measuring the relative abundance of phyla. Microbiome analysis wasn’t included as an endpoint in the original study design because, at that time, the idea of a connection between AD and the gut microbiome was barely on the research radar.
Things shifted dramatically in 2017, with a seminal paper finding that germ-free mice inoculated with stool from Parkinson’s patients developed Parkinson’s symptoms. This study was widely heralded as a breakthrough in the field – the first time any neurodegenerative disease had been conclusively linked to dysregulations in the human microbiome.
Last year, Vo Van Giau, PhD, of Gachon University, South Korea, and his colleagues published an extensive review of the data suggesting a similar link with Alzheimer’s disease.
Dr. Giau and his coauthors laid out a potential pathogenic pathway for this interaction.
“The microbiota is closely related to neurological dysfunction and plays a significant role in neuroinflammation through the secretion of proinflammatory cytokines. Changes in the homeostatic state of the microbiota lead to increased intestinal permeability, which may promote the translocation of bacteria and endotoxins across the epithelial barrier, inducing an immunological response associated with the production of proinflammatory cytokines. The activation of both enteric neurons and glial cells may result in various neurological disorders,” including Alzheimer’s, he wrote.
Dr. Missling said this paper, and smaller studies appearing at Alzheimer’s meetings, prompted the company to add the stool sampling as a follow-up measure.
“It’s something of great interest, we think, and deserves to be investigated.”
SOURCE: Missling C et al. AAIC 2019, Abstract 32260.
LOS ANGELES – A single look at the gut microbiome of patients with Alzheimer’s disease (AD) suggests an interaction between anti-inflammatory gut bacteria and long-term exposure to an investigational sigma 1 receptor agonist.
After up to 148 weeks treatment with Anavex 2-73, patients with stable or improved functional scores showed significantly higher levels of both Ruminococcaceae and Porphyromonadaceae, compared with patients who had declining function. Both bacterial families produce butyrate, an anti-inflammatory short-chain fatty acid.
Conversely, poor response was associated with a low level of Verrucomicrobia, a mucin-degrading phylum thought to be important in gut homeostasis. These bacteria live mainly in the intestinal mucosa – the physical interface between the microbiome and the rest of the body.
The data, presented at the Alzheimer’s Association International Conference, represent the first microbiome measurements reported in a clinical trial of an investigational Alzheimer’s therapy. Because they come from a single sample taken from a small group in an extension study, without a baseline comparator, it’s impossible to know what these associations mean. But the findings are enough to nudge Anavex Life Sciences into adding microbiome changes to its new study of Anavex 2-73, according to Christopher Missling, PhD, president and chief executive officer of the company.
The study, ramping up now, aims to recruit 450 patients with mild AD. They will be randomized to high-dose or mid-dose Anavex 2-73 for 48 weeks. The primary outcomes are measures of cognition and function. Stool sampling at baseline and at the end of the study will be included as well, Dr. Missling said in an interview.
Anavex 2-73 is a sigma-1 receptor agonist. A chaperone protein, sigma-1 is activated in response to acute and chronic cellular stressors, several which are important in neurodegeneration. The sigma-1 receptor is found on neurons and glia in many areas of the central nervous system. It modulates several processes implicated in neurodegenerative diseases, including glutamate and calcium activity, reaction to oxidative stress, and mitochondrial function. There is some evidence that sigma-1 receptor activation can induce neuronal regrowth and functional recovery after stroke. It also appears to play a role in helping cells clear misfolded proteins – a pathway that makes it an attractive drug target in Alzheimer’s disease, as well as other neurodegenerative diseases with aberrant proteins, such as Parkinson’s and Huntington’s diseases.
Anavex 2-73’s phase 2 development started with a 5-week crossover trial of 32 patients. This was followed by a 52-week, open-label extension trial of 10, 20, 30, and 50 mg/day orally, in which each patient was titrated to the maximum tolerated dose. The main endpoints were change on the Mini Mental State Exam and change on the Alzheimer’s Disease Cooperative Study-activities of daily living (ADCS-ADL) scale.
At 57 weeks, six patients had improved on the Mini Mental State Exam score: four with high plasma levels and two with low plasma levels, correlating to the dosage obtained. On the functional measure of activities of daily living, nine patients had improved, including five with high plasma levels, three with moderate levels, and one with a low level. One patient, with a moderate level, remained stable. The remaining 14 patients declined.
The company then enrolled 21 of the cohort in a 208-week extension trial, primarily because of patient request, Dr. Missling said. “They know they are doing better. Their families know they’re doing better. They did not want to give this up.”
Last fall, the company released 148-week functional and cognitive data confirming the initial findings: Patients with higher plasma levels (correlating with higher doses) declined about 2 points on the ADCS-ADL scale, compared with a mean decline of about 25 points among those with lower blood levels – an 88% difference in favor of treatment. Cognition scores showed a similar pattern, with the high-concentration group declining 64% less than the low-concentration group.
Sixteen patients consented to stool sampling. A sophisticated computer algorithm characterized the microbiome of each, measuring the relative abundance of phyla. Microbiome analysis wasn’t included as an endpoint in the original study design because, at that time, the idea of a connection between AD and the gut microbiome was barely on the research radar.
Things shifted dramatically in 2017, with a seminal paper finding that germ-free mice inoculated with stool from Parkinson’s patients developed Parkinson’s symptoms. This study was widely heralded as a breakthrough in the field – the first time any neurodegenerative disease had been conclusively linked to dysregulations in the human microbiome.
Last year, Vo Van Giau, PhD, of Gachon University, South Korea, and his colleagues published an extensive review of the data suggesting a similar link with Alzheimer’s disease.
Dr. Giau and his coauthors laid out a potential pathogenic pathway for this interaction.
“The microbiota is closely related to neurological dysfunction and plays a significant role in neuroinflammation through the secretion of proinflammatory cytokines. Changes in the homeostatic state of the microbiota lead to increased intestinal permeability, which may promote the translocation of bacteria and endotoxins across the epithelial barrier, inducing an immunological response associated with the production of proinflammatory cytokines. The activation of both enteric neurons and glial cells may result in various neurological disorders,” including Alzheimer’s, he wrote.
Dr. Missling said this paper, and smaller studies appearing at Alzheimer’s meetings, prompted the company to add the stool sampling as a follow-up measure.
“It’s something of great interest, we think, and deserves to be investigated.”
SOURCE: Missling C et al. AAIC 2019, Abstract 32260.
LOS ANGELES – A single look at the gut microbiome of patients with Alzheimer’s disease (AD) suggests an interaction between anti-inflammatory gut bacteria and long-term exposure to an investigational sigma 1 receptor agonist.
After up to 148 weeks treatment with Anavex 2-73, patients with stable or improved functional scores showed significantly higher levels of both Ruminococcaceae and Porphyromonadaceae, compared with patients who had declining function. Both bacterial families produce butyrate, an anti-inflammatory short-chain fatty acid.
Conversely, poor response was associated with a low level of Verrucomicrobia, a mucin-degrading phylum thought to be important in gut homeostasis. These bacteria live mainly in the intestinal mucosa – the physical interface between the microbiome and the rest of the body.
The data, presented at the Alzheimer’s Association International Conference, represent the first microbiome measurements reported in a clinical trial of an investigational Alzheimer’s therapy. Because they come from a single sample taken from a small group in an extension study, without a baseline comparator, it’s impossible to know what these associations mean. But the findings are enough to nudge Anavex Life Sciences into adding microbiome changes to its new study of Anavex 2-73, according to Christopher Missling, PhD, president and chief executive officer of the company.
The study, ramping up now, aims to recruit 450 patients with mild AD. They will be randomized to high-dose or mid-dose Anavex 2-73 for 48 weeks. The primary outcomes are measures of cognition and function. Stool sampling at baseline and at the end of the study will be included as well, Dr. Missling said in an interview.
Anavex 2-73 is a sigma-1 receptor agonist. A chaperone protein, sigma-1 is activated in response to acute and chronic cellular stressors, several which are important in neurodegeneration. The sigma-1 receptor is found on neurons and glia in many areas of the central nervous system. It modulates several processes implicated in neurodegenerative diseases, including glutamate and calcium activity, reaction to oxidative stress, and mitochondrial function. There is some evidence that sigma-1 receptor activation can induce neuronal regrowth and functional recovery after stroke. It also appears to play a role in helping cells clear misfolded proteins – a pathway that makes it an attractive drug target in Alzheimer’s disease, as well as other neurodegenerative diseases with aberrant proteins, such as Parkinson’s and Huntington’s diseases.
Anavex 2-73’s phase 2 development started with a 5-week crossover trial of 32 patients. This was followed by a 52-week, open-label extension trial of 10, 20, 30, and 50 mg/day orally, in which each patient was titrated to the maximum tolerated dose. The main endpoints were change on the Mini Mental State Exam and change on the Alzheimer’s Disease Cooperative Study-activities of daily living (ADCS-ADL) scale.
At 57 weeks, six patients had improved on the Mini Mental State Exam score: four with high plasma levels and two with low plasma levels, correlating to the dosage obtained. On the functional measure of activities of daily living, nine patients had improved, including five with high plasma levels, three with moderate levels, and one with a low level. One patient, with a moderate level, remained stable. The remaining 14 patients declined.
The company then enrolled 21 of the cohort in a 208-week extension trial, primarily because of patient request, Dr. Missling said. “They know they are doing better. Their families know they’re doing better. They did not want to give this up.”
Last fall, the company released 148-week functional and cognitive data confirming the initial findings: Patients with higher plasma levels (correlating with higher doses) declined about 2 points on the ADCS-ADL scale, compared with a mean decline of about 25 points among those with lower blood levels – an 88% difference in favor of treatment. Cognition scores showed a similar pattern, with the high-concentration group declining 64% less than the low-concentration group.
Sixteen patients consented to stool sampling. A sophisticated computer algorithm characterized the microbiome of each, measuring the relative abundance of phyla. Microbiome analysis wasn’t included as an endpoint in the original study design because, at that time, the idea of a connection between AD and the gut microbiome was barely on the research radar.
Things shifted dramatically in 2017, with a seminal paper finding that germ-free mice inoculated with stool from Parkinson’s patients developed Parkinson’s symptoms. This study was widely heralded as a breakthrough in the field – the first time any neurodegenerative disease had been conclusively linked to dysregulations in the human microbiome.
Last year, Vo Van Giau, PhD, of Gachon University, South Korea, and his colleagues published an extensive review of the data suggesting a similar link with Alzheimer’s disease.
Dr. Giau and his coauthors laid out a potential pathogenic pathway for this interaction.
“The microbiota is closely related to neurological dysfunction and plays a significant role in neuroinflammation through the secretion of proinflammatory cytokines. Changes in the homeostatic state of the microbiota lead to increased intestinal permeability, which may promote the translocation of bacteria and endotoxins across the epithelial barrier, inducing an immunological response associated with the production of proinflammatory cytokines. The activation of both enteric neurons and glial cells may result in various neurological disorders,” including Alzheimer’s, he wrote.
Dr. Missling said this paper, and smaller studies appearing at Alzheimer’s meetings, prompted the company to add the stool sampling as a follow-up measure.
“It’s something of great interest, we think, and deserves to be investigated.”
SOURCE: Missling C et al. AAIC 2019, Abstract 32260.
REPORTING FROM AAIC 2019
Sleep disorder treatment tied to lower suicide attempt risk in veterans
in a large case-control matched study of patients in the Veterans Health Administration database.
However, treatment for sleep disorders was correlated to a reduced risk for suicide attempts.
Todd M. Bishop, PhD, of the Center of Excellence for Suicide Prevention, Canandaigua (N.Y.) VA Medical Center, and the department of psychiatry, University of Rochester (N.Y.) Medical Center, and his colleagues wrote that suicide is the 10th most frequent cause of death in the United States, and “nowhere is the suicide rate more alarming than among military veterans, who after adjusting for age and gender, have an approximately 1.5 times greater risk for suicide as compared to the civilian population.”
Previous research has explored the link between sleep disturbances and suicide attempts. But less has been done to look at specific sleep problems, and little research has examined the role of sleep medicine interventions and suicide attempt risk.
The investigators conducted a study to establish the association between suicide attempts and specific sleep disorders, and to examine the correlation between sleep medicine treatment and suicide attempts. Their sample consisted of 60,102 veterans who had received care within the VHA between Oct. 1, 2012, and Sept. 20, 2014. Half of the sample had a documented suicide attempt in the medical record (n = 30,051) and half did not (n = 30,051). The overall sample was predominately male (87.1%) with a mean age of 48.6 years. More than half the sample identified as white (67.4%).
Suicide attempts, sleep disturbance, and medical and mental health comorbidities were identified via ICD codes and prescription records. The predominant sleep disorders studied were insomnia, sleep-related breathing disorder (SRBD), and nightmares. The first suicide attempt in the study period was determined to be the index date for the case-control matching.
Overall, sleep disturbances were much more prevalent among cases than controls (insomnia, 46.2% vs. 12.6%), sleep-related breathing disorder (8.6% vs. 4.8%), and nightmares (7.1% vs. 1.6%). A logistic regression analysis was undertaken to examine the relationship between specific sleep disorders and suicide attempts. Insomnia, nightmares, and SRBD were each associated with increased odds of a suicide attempt with the following odds ratios: insomnia (odds ratio, 5.62; 95% confidence interval, 5.39-5.86), nightmares (OR, 2.49; 95% CI, 2.23-2.77), and sleep-related breathing disorder (OR, 1.37; 95% CI, 1.27-1.48).
A second model included known drivers of suicide attempts (PTSD, depression, anxiety disorders, schizophrenia, bipolar disorder, substance use disorder, medical comorbidity, and obesity). But after controlling for these factors, neither nightmares (OR, 0.96; 95% CI, 0.85-1.09) nor sleep-related breathing disorders (OR, 0.87, 95% CI, 0.79-0.94) remained positively associated with suicide attempt, but the association of insomnia with suicide attempt was maintained (OR, 1.51; 95% CI, 1.43-1.59).
The question of the impact of sleep medicine interventions on suicide attempts was studied with a third regression model adding the number of sleep medicine clinic visits in the 180 days prior to the suicide attempt index date as an independent variable. The variables in this model were limited to insomnia, SRBD, and nightmares. The investigators found that “for each sleep medicine clinic visit within the 6 months prior to index date the likelihood of suicide attempt is 11% less (OR, 0.89; 95% CI, 0.82-0.97).”
The limitations of the study include the lack of information on sleep treatment modalities or medications provided during the clinic visits, and the overlapping of sleep disturbance with other mental health conditions, such as alcohol dependence and PTSD. In addition, “some insomnia medications are labeled for risk of suicidal ideation and behavior, so there is some chance that the medications rather than insomnia itself were associated with the increased suicidal behavior,” the investigators wrote.
In addition to an analysis of specific types of sleep disorders associated with suicide attempts, the study showed that treatment of sleep disorders may have an important role in suicide prevention. The investigators concluded: “Identifying populations at risk for suicide prior to a first attempt is an important, but difficult task of suicide prevention. Prevention efforts can be aimed at modifiable risk factors that arise early on a patient’s trajectory toward a suicide attempt.”
The study was supported by the VISN 2 Center of Excellence for Suicide Prevention, Canandaigua VAMC. The authors had no disclosures.
SOURCE: Bishop TM et al. Sleep Med. 2019 Jul 25. doi: 10.1016/j.sleep.2019.07.016.
in a large case-control matched study of patients in the Veterans Health Administration database.
However, treatment for sleep disorders was correlated to a reduced risk for suicide attempts.
Todd M. Bishop, PhD, of the Center of Excellence for Suicide Prevention, Canandaigua (N.Y.) VA Medical Center, and the department of psychiatry, University of Rochester (N.Y.) Medical Center, and his colleagues wrote that suicide is the 10th most frequent cause of death in the United States, and “nowhere is the suicide rate more alarming than among military veterans, who after adjusting for age and gender, have an approximately 1.5 times greater risk for suicide as compared to the civilian population.”
Previous research has explored the link between sleep disturbances and suicide attempts. But less has been done to look at specific sleep problems, and little research has examined the role of sleep medicine interventions and suicide attempt risk.
The investigators conducted a study to establish the association between suicide attempts and specific sleep disorders, and to examine the correlation between sleep medicine treatment and suicide attempts. Their sample consisted of 60,102 veterans who had received care within the VHA between Oct. 1, 2012, and Sept. 20, 2014. Half of the sample had a documented suicide attempt in the medical record (n = 30,051) and half did not (n = 30,051). The overall sample was predominately male (87.1%) with a mean age of 48.6 years. More than half the sample identified as white (67.4%).
Suicide attempts, sleep disturbance, and medical and mental health comorbidities were identified via ICD codes and prescription records. The predominant sleep disorders studied were insomnia, sleep-related breathing disorder (SRBD), and nightmares. The first suicide attempt in the study period was determined to be the index date for the case-control matching.
Overall, sleep disturbances were much more prevalent among cases than controls (insomnia, 46.2% vs. 12.6%), sleep-related breathing disorder (8.6% vs. 4.8%), and nightmares (7.1% vs. 1.6%). A logistic regression analysis was undertaken to examine the relationship between specific sleep disorders and suicide attempts. Insomnia, nightmares, and SRBD were each associated with increased odds of a suicide attempt with the following odds ratios: insomnia (odds ratio, 5.62; 95% confidence interval, 5.39-5.86), nightmares (OR, 2.49; 95% CI, 2.23-2.77), and sleep-related breathing disorder (OR, 1.37; 95% CI, 1.27-1.48).
A second model included known drivers of suicide attempts (PTSD, depression, anxiety disorders, schizophrenia, bipolar disorder, substance use disorder, medical comorbidity, and obesity). But after controlling for these factors, neither nightmares (OR, 0.96; 95% CI, 0.85-1.09) nor sleep-related breathing disorders (OR, 0.87, 95% CI, 0.79-0.94) remained positively associated with suicide attempt, but the association of insomnia with suicide attempt was maintained (OR, 1.51; 95% CI, 1.43-1.59).
The question of the impact of sleep medicine interventions on suicide attempts was studied with a third regression model adding the number of sleep medicine clinic visits in the 180 days prior to the suicide attempt index date as an independent variable. The variables in this model were limited to insomnia, SRBD, and nightmares. The investigators found that “for each sleep medicine clinic visit within the 6 months prior to index date the likelihood of suicide attempt is 11% less (OR, 0.89; 95% CI, 0.82-0.97).”
The limitations of the study include the lack of information on sleep treatment modalities or medications provided during the clinic visits, and the overlapping of sleep disturbance with other mental health conditions, such as alcohol dependence and PTSD. In addition, “some insomnia medications are labeled for risk of suicidal ideation and behavior, so there is some chance that the medications rather than insomnia itself were associated with the increased suicidal behavior,” the investigators wrote.
In addition to an analysis of specific types of sleep disorders associated with suicide attempts, the study showed that treatment of sleep disorders may have an important role in suicide prevention. The investigators concluded: “Identifying populations at risk for suicide prior to a first attempt is an important, but difficult task of suicide prevention. Prevention efforts can be aimed at modifiable risk factors that arise early on a patient’s trajectory toward a suicide attempt.”
The study was supported by the VISN 2 Center of Excellence for Suicide Prevention, Canandaigua VAMC. The authors had no disclosures.
SOURCE: Bishop TM et al. Sleep Med. 2019 Jul 25. doi: 10.1016/j.sleep.2019.07.016.
in a large case-control matched study of patients in the Veterans Health Administration database.
However, treatment for sleep disorders was correlated to a reduced risk for suicide attempts.
Todd M. Bishop, PhD, of the Center of Excellence for Suicide Prevention, Canandaigua (N.Y.) VA Medical Center, and the department of psychiatry, University of Rochester (N.Y.) Medical Center, and his colleagues wrote that suicide is the 10th most frequent cause of death in the United States, and “nowhere is the suicide rate more alarming than among military veterans, who after adjusting for age and gender, have an approximately 1.5 times greater risk for suicide as compared to the civilian population.”
Previous research has explored the link between sleep disturbances and suicide attempts. But less has been done to look at specific sleep problems, and little research has examined the role of sleep medicine interventions and suicide attempt risk.
The investigators conducted a study to establish the association between suicide attempts and specific sleep disorders, and to examine the correlation between sleep medicine treatment and suicide attempts. Their sample consisted of 60,102 veterans who had received care within the VHA between Oct. 1, 2012, and Sept. 20, 2014. Half of the sample had a documented suicide attempt in the medical record (n = 30,051) and half did not (n = 30,051). The overall sample was predominately male (87.1%) with a mean age of 48.6 years. More than half the sample identified as white (67.4%).
Suicide attempts, sleep disturbance, and medical and mental health comorbidities were identified via ICD codes and prescription records. The predominant sleep disorders studied were insomnia, sleep-related breathing disorder (SRBD), and nightmares. The first suicide attempt in the study period was determined to be the index date for the case-control matching.
Overall, sleep disturbances were much more prevalent among cases than controls (insomnia, 46.2% vs. 12.6%), sleep-related breathing disorder (8.6% vs. 4.8%), and nightmares (7.1% vs. 1.6%). A logistic regression analysis was undertaken to examine the relationship between specific sleep disorders and suicide attempts. Insomnia, nightmares, and SRBD were each associated with increased odds of a suicide attempt with the following odds ratios: insomnia (odds ratio, 5.62; 95% confidence interval, 5.39-5.86), nightmares (OR, 2.49; 95% CI, 2.23-2.77), and sleep-related breathing disorder (OR, 1.37; 95% CI, 1.27-1.48).
A second model included known drivers of suicide attempts (PTSD, depression, anxiety disorders, schizophrenia, bipolar disorder, substance use disorder, medical comorbidity, and obesity). But after controlling for these factors, neither nightmares (OR, 0.96; 95% CI, 0.85-1.09) nor sleep-related breathing disorders (OR, 0.87, 95% CI, 0.79-0.94) remained positively associated with suicide attempt, but the association of insomnia with suicide attempt was maintained (OR, 1.51; 95% CI, 1.43-1.59).
The question of the impact of sleep medicine interventions on suicide attempts was studied with a third regression model adding the number of sleep medicine clinic visits in the 180 days prior to the suicide attempt index date as an independent variable. The variables in this model were limited to insomnia, SRBD, and nightmares. The investigators found that “for each sleep medicine clinic visit within the 6 months prior to index date the likelihood of suicide attempt is 11% less (OR, 0.89; 95% CI, 0.82-0.97).”
The limitations of the study include the lack of information on sleep treatment modalities or medications provided during the clinic visits, and the overlapping of sleep disturbance with other mental health conditions, such as alcohol dependence and PTSD. In addition, “some insomnia medications are labeled for risk of suicidal ideation and behavior, so there is some chance that the medications rather than insomnia itself were associated with the increased suicidal behavior,” the investigators wrote.
In addition to an analysis of specific types of sleep disorders associated with suicide attempts, the study showed that treatment of sleep disorders may have an important role in suicide prevention. The investigators concluded: “Identifying populations at risk for suicide prior to a first attempt is an important, but difficult task of suicide prevention. Prevention efforts can be aimed at modifiable risk factors that arise early on a patient’s trajectory toward a suicide attempt.”
The study was supported by the VISN 2 Center of Excellence for Suicide Prevention, Canandaigua VAMC. The authors had no disclosures.
SOURCE: Bishop TM et al. Sleep Med. 2019 Jul 25. doi: 10.1016/j.sleep.2019.07.016.
REPORTING FROM SLEEP MEDICINE
Sleep aids and dementia: Studies find both risks and benefits
LOS ANGELES – While a large number of older adults take prescription and nonprescription medications to help them sleep, the effect of these medications on dementia risk is unclear, with most researchers advocating a cautious and conservative approach to prescribing.
Research is increasingly revealing a bidirectional relationship between sleep and dementia. Poor sleep – especially from insomnia, sleep deprivation, or obstructive sleep apnea – is known to increase dementia risk. Dementias, meanwhile, are associated with serious circadian rhythm disturbances, leading to nighttime sleep loss and increasing the likelihood of institutionalization.
At the Alzheimer’s Association International Conference, researchers presented findings assessing the links between sleep medication use and dementia and also what agents or approaches might safely improve sleep in people with sleep disorders who are at risk for dementia or who have been diagnosed with dementia.
Sex- and race-based differences in risk
Yue Leng, PhD, of the University of California, San Francisco, reported a link between frequent sleep medication use and later dementia – but only in white adults. Dr. Leng presented findings from the National Institutes of Health–funded Health, Aging, and Body Composition Study, which recruited 3,068 subjects aged 70-79 and followed them for 15 years. At baseline, 2.7% of African Americans and 7.7% of whites in the study reported taking sleep medications “often” or “almost always.”
Dr. Leng and her colleagues found that white subjects who reported taking sleep aids five or more times a month at baseline had a nearly 80% higher risk of developing dementia during the course of the study (hazard ratio, 1.79; 95% confidence interval, 1.21-2.66), compared with people who reported never taking sleep aids or taking them less frequently.
The researchers saw no between-sex differences for this finding, and adjusted for a variety of genetic and lifestyle confounders. Importantly, no significant increase in dementia risk was seen for black subjects, who made up more than one-third of the cohort.
Dr. Leng told the conference that the researchers could not explain why black participants did not see similarly increased dementia risk. Also, she noted, researchers did not have information on the specific sleep medications people used: benzodiazepines, antihistamines, antidepressants, or other types of drugs. Nonetheless, she told the conference, the findings ratified the cautious approach many dementia experts are already stressing.
“Do we really need to prescribe so many sleep meds to older adults who are already at risk for cognitive impairment?” Dr. Leng said, adding: “I am a big advocate of behavioral sleep interventions.” People with clinical sleep problems “should be referred to sleep centers” for a fuller assessment before medication is prescribed, she said.
Findings from another cohort study, meanwhile, suggest that there could be sex-related differences in how sleep aids affect dementia risk. Investigators at Utah State University in Logan used data from some 3,656 older adults in the Cache County Study on Memory and Aging, an NIH-backed cohort study of white adults in Utah without dementia at baseline who were followed for 12 years.
The investigators, led by doctoral student Elizabeth Vernon, found that men reporting use of sleep medication saw more than threefold higher risk of developing Alzheimer’s disease than did men who did not use sleep aids (HR, 3.604; P = .0001).
Women who did not report having sleep disturbance but used sleep-inducing medications were at nearly fourfold greater risk for developing Alzheimer’s disease (HR, 3.916; P = .0001). Women who self-reported sleep disturbances at baseline, meanwhile, saw a reduction in Alzheimer’s risk of about one-third associated with the use of sleep medications.
Ms. Vernon told the conference that, despite the finding of risk reduction for this particular group of women, caution in prescribing sleep aids was warranted.
Common sleep drugs linked to cognitive aging
Chris Fox, MD, a researcher at the University of East Anglia in Norwich, England, and his colleagues demonstrated in 2018 that long-term exposure to anticholinergic drugs, a class that includes some antidepressants and antihistamines used to promote sleep, was associated with a higher risk of dementia, while use of benzodiazepines, a class of sedatives used commonly in older people as sleep aids, was not. (Whether benzodiazepine exposure relates to dementia remains controversial.)
At AAIC 2019, Dr. Fox presented findings from a study of 337 brains in a U.K. brain bank, of which 17% and 21% came from users of benzodiazepines and anticholinergic drugs, whose usage history was well documented. Dr. Fox and his colleagues found that, while neither anticholinergic nor benzodiazepine exposure was associated with brain pathology specific to that seen in Alzheimer’s disease, both classes of drugs were associated with “slight signals in neuronal loss” in one brain region, the nucleus basalis of Meynert. Dr. Fox described the drugs as causing “an increase in cognitive aging” which could bear on Alzheimer’s risk without being directly causative.
Newer sleep drugs may help Alzheimer’s patients
Scientists working for drug manufacturers presented findings on agents to counter the circadian rhythm disturbances seen in people with Alzheimer’s disease. Margaret Moline, PhD, of Eisai in Woodcliff Lake, N.J., showed some results from a phase 2, dose-ranging, placebo-controlled study of the experimental agent lemborexant in 62 subjects aged 60-90 with mild to moderate Alzheimer’s disease and sleep disturbances. (Lemborexant, an orexin receptor agonist that acts to regulate wakefulness, is being investigated in a broad range of sleep disorders.) Patients were randomized to one of four doses of lemborexant or placebo and wore a device for sleep monitoring. Nighttime activity indicating arousal was significantly lower for people in two dosage arms, 5 mg and 10 mg, compared with placebo, and treatment groups saw trends toward less sleep fragmentation and higher total sleep time, Dr. Moline told the conference.
Suvorexant (Belsomra), the only orexin receptor antagonist currently licensed as a sleep aid, is also being tested in people with Alzheimer’s disease. At AAIC 2019, Joseph Herring, MD, PhD, of Merck in Kenilworth, N.J., presented results from a placebo-controlled trial of 277 patients with Alzheimer’s disease and insomnia, and reported that treatment with 10 or 20 mg of suvorexant over 4 weeks was associated with about an extra half hour of total nightly sleep, with a 73-minute mean increase from baseline, compared with 45 minutes for patients receiving placebo (95% CI, 11-45; P less than .005).
Trazodone linked to slower cognitive decline
An inexpensive antidepressant used in low doses as a sleep aid, including in people with Alzheimer’s disease, was associated with a delay in cognitive decline in older adults, according to results from a retrospective study. Elissaios Karageorgiou, MD, PhD, of the University of California, San Francisco, and the Neurological Institute of Athens presented results derived from two cohorts: patients enrolled at the UCSF Memory and Aging Center and women enrolled in the Study for Osteoporotic Fractures (SOF) in Women. The investigators were able to identify trazodone users in the studies (with two or more contiguous study visits reporting trazodone use) and match them with control patients from the same cohorts who did not use trazodone.
Trazodone was studied because previous research suggests it increases total sleep time in patients with Alzheimer’s disease without affecting next-day cognitive performance.
Trazodone-using patients in the UCSF cohort (n = 25) saw significantly less decline in Mini Mental State Exam (MMSE) scores over 4 years, compared with nonusers (0.27 vs. 0.70 points per year; P = .023), an effect that remained statistically significant even after adjusting for sedative and stimulant use and the expected progression of Alzheimer’s disease pathology. Importantly, the slower decline was seen only among subjects with sleep complaints at baseline and especially those whose sleep improved over time, suggesting that the cognitive benefit was mediated by improved sleep.
In the SOF cohort of 46 trazodone users matched with 148 nonusers, no significant protective or negative effect related to long-term trazodone use was found using the MMSE or the Trails Making Test. In this analysis, however, baseline and longitudinal sleep quality was not captured in the group-matching process, neither was the use of other medications. The patient group was slightly older, and all patients were women.
Dr. Karageorgiou said in an interview that the link between improved sleep, trazodone, and cognition needs to be validated in prospective intervention studies. Trazodone, he said, appears to work best in people with a specific type of insomnia characterized by cortical and behavioral hyperarousal, and its cognitive effect appears limited to people whose sleep improves with treatment. “You’re not going to see long-term cognitive benefits if it’s not improving your sleep,” Dr. Karageorgiou said. “So, whether trazodone improves sleep or not in a patient after a few months can be an early indicator for the clinician to continue using it or suspend it, because it is unlikely to help their cognition otherwise.”
He stressed that physicians need to be broadly focused on improving sleep to help patients with, or at risk for, dementia by consolidating their sleep rhythms.
“Trazodone is not the magic bullet, and I don’t think we will ever have a magic bullet,” Dr. Karageorgiou said. “Because when our brain degenerates, it’s not just one chemical, or one system, it’s many. And our body changes as well. The important thing is to help the patient consolidate their rhythms, whether through light therapy, daily exercise, cognitive behavioral therapy for insomnia, or other evidence-based interventions and their combination. The same applies for a person with dementia as for the rest of us.”
None of the investigators outside of the industry-sponsored studies had relevant disclosures.
LOS ANGELES – While a large number of older adults take prescription and nonprescription medications to help them sleep, the effect of these medications on dementia risk is unclear, with most researchers advocating a cautious and conservative approach to prescribing.
Research is increasingly revealing a bidirectional relationship between sleep and dementia. Poor sleep – especially from insomnia, sleep deprivation, or obstructive sleep apnea – is known to increase dementia risk. Dementias, meanwhile, are associated with serious circadian rhythm disturbances, leading to nighttime sleep loss and increasing the likelihood of institutionalization.
At the Alzheimer’s Association International Conference, researchers presented findings assessing the links between sleep medication use and dementia and also what agents or approaches might safely improve sleep in people with sleep disorders who are at risk for dementia or who have been diagnosed with dementia.
Sex- and race-based differences in risk
Yue Leng, PhD, of the University of California, San Francisco, reported a link between frequent sleep medication use and later dementia – but only in white adults. Dr. Leng presented findings from the National Institutes of Health–funded Health, Aging, and Body Composition Study, which recruited 3,068 subjects aged 70-79 and followed them for 15 years. At baseline, 2.7% of African Americans and 7.7% of whites in the study reported taking sleep medications “often” or “almost always.”
Dr. Leng and her colleagues found that white subjects who reported taking sleep aids five or more times a month at baseline had a nearly 80% higher risk of developing dementia during the course of the study (hazard ratio, 1.79; 95% confidence interval, 1.21-2.66), compared with people who reported never taking sleep aids or taking them less frequently.
The researchers saw no between-sex differences for this finding, and adjusted for a variety of genetic and lifestyle confounders. Importantly, no significant increase in dementia risk was seen for black subjects, who made up more than one-third of the cohort.
Dr. Leng told the conference that the researchers could not explain why black participants did not see similarly increased dementia risk. Also, she noted, researchers did not have information on the specific sleep medications people used: benzodiazepines, antihistamines, antidepressants, or other types of drugs. Nonetheless, she told the conference, the findings ratified the cautious approach many dementia experts are already stressing.
“Do we really need to prescribe so many sleep meds to older adults who are already at risk for cognitive impairment?” Dr. Leng said, adding: “I am a big advocate of behavioral sleep interventions.” People with clinical sleep problems “should be referred to sleep centers” for a fuller assessment before medication is prescribed, she said.
Findings from another cohort study, meanwhile, suggest that there could be sex-related differences in how sleep aids affect dementia risk. Investigators at Utah State University in Logan used data from some 3,656 older adults in the Cache County Study on Memory and Aging, an NIH-backed cohort study of white adults in Utah without dementia at baseline who were followed for 12 years.
The investigators, led by doctoral student Elizabeth Vernon, found that men reporting use of sleep medication saw more than threefold higher risk of developing Alzheimer’s disease than did men who did not use sleep aids (HR, 3.604; P = .0001).
Women who did not report having sleep disturbance but used sleep-inducing medications were at nearly fourfold greater risk for developing Alzheimer’s disease (HR, 3.916; P = .0001). Women who self-reported sleep disturbances at baseline, meanwhile, saw a reduction in Alzheimer’s risk of about one-third associated with the use of sleep medications.
Ms. Vernon told the conference that, despite the finding of risk reduction for this particular group of women, caution in prescribing sleep aids was warranted.
Common sleep drugs linked to cognitive aging
Chris Fox, MD, a researcher at the University of East Anglia in Norwich, England, and his colleagues demonstrated in 2018 that long-term exposure to anticholinergic drugs, a class that includes some antidepressants and antihistamines used to promote sleep, was associated with a higher risk of dementia, while use of benzodiazepines, a class of sedatives used commonly in older people as sleep aids, was not. (Whether benzodiazepine exposure relates to dementia remains controversial.)
At AAIC 2019, Dr. Fox presented findings from a study of 337 brains in a U.K. brain bank, of which 17% and 21% came from users of benzodiazepines and anticholinergic drugs, whose usage history was well documented. Dr. Fox and his colleagues found that, while neither anticholinergic nor benzodiazepine exposure was associated with brain pathology specific to that seen in Alzheimer’s disease, both classes of drugs were associated with “slight signals in neuronal loss” in one brain region, the nucleus basalis of Meynert. Dr. Fox described the drugs as causing “an increase in cognitive aging” which could bear on Alzheimer’s risk without being directly causative.
Newer sleep drugs may help Alzheimer’s patients
Scientists working for drug manufacturers presented findings on agents to counter the circadian rhythm disturbances seen in people with Alzheimer’s disease. Margaret Moline, PhD, of Eisai in Woodcliff Lake, N.J., showed some results from a phase 2, dose-ranging, placebo-controlled study of the experimental agent lemborexant in 62 subjects aged 60-90 with mild to moderate Alzheimer’s disease and sleep disturbances. (Lemborexant, an orexin receptor agonist that acts to regulate wakefulness, is being investigated in a broad range of sleep disorders.) Patients were randomized to one of four doses of lemborexant or placebo and wore a device for sleep monitoring. Nighttime activity indicating arousal was significantly lower for people in two dosage arms, 5 mg and 10 mg, compared with placebo, and treatment groups saw trends toward less sleep fragmentation and higher total sleep time, Dr. Moline told the conference.
Suvorexant (Belsomra), the only orexin receptor antagonist currently licensed as a sleep aid, is also being tested in people with Alzheimer’s disease. At AAIC 2019, Joseph Herring, MD, PhD, of Merck in Kenilworth, N.J., presented results from a placebo-controlled trial of 277 patients with Alzheimer’s disease and insomnia, and reported that treatment with 10 or 20 mg of suvorexant over 4 weeks was associated with about an extra half hour of total nightly sleep, with a 73-minute mean increase from baseline, compared with 45 minutes for patients receiving placebo (95% CI, 11-45; P less than .005).
Trazodone linked to slower cognitive decline
An inexpensive antidepressant used in low doses as a sleep aid, including in people with Alzheimer’s disease, was associated with a delay in cognitive decline in older adults, according to results from a retrospective study. Elissaios Karageorgiou, MD, PhD, of the University of California, San Francisco, and the Neurological Institute of Athens presented results derived from two cohorts: patients enrolled at the UCSF Memory and Aging Center and women enrolled in the Study for Osteoporotic Fractures (SOF) in Women. The investigators were able to identify trazodone users in the studies (with two or more contiguous study visits reporting trazodone use) and match them with control patients from the same cohorts who did not use trazodone.
Trazodone was studied because previous research suggests it increases total sleep time in patients with Alzheimer’s disease without affecting next-day cognitive performance.
Trazodone-using patients in the UCSF cohort (n = 25) saw significantly less decline in Mini Mental State Exam (MMSE) scores over 4 years, compared with nonusers (0.27 vs. 0.70 points per year; P = .023), an effect that remained statistically significant even after adjusting for sedative and stimulant use and the expected progression of Alzheimer’s disease pathology. Importantly, the slower decline was seen only among subjects with sleep complaints at baseline and especially those whose sleep improved over time, suggesting that the cognitive benefit was mediated by improved sleep.
In the SOF cohort of 46 trazodone users matched with 148 nonusers, no significant protective or negative effect related to long-term trazodone use was found using the MMSE or the Trails Making Test. In this analysis, however, baseline and longitudinal sleep quality was not captured in the group-matching process, neither was the use of other medications. The patient group was slightly older, and all patients were women.
Dr. Karageorgiou said in an interview that the link between improved sleep, trazodone, and cognition needs to be validated in prospective intervention studies. Trazodone, he said, appears to work best in people with a specific type of insomnia characterized by cortical and behavioral hyperarousal, and its cognitive effect appears limited to people whose sleep improves with treatment. “You’re not going to see long-term cognitive benefits if it’s not improving your sleep,” Dr. Karageorgiou said. “So, whether trazodone improves sleep or not in a patient after a few months can be an early indicator for the clinician to continue using it or suspend it, because it is unlikely to help their cognition otherwise.”
He stressed that physicians need to be broadly focused on improving sleep to help patients with, or at risk for, dementia by consolidating their sleep rhythms.
“Trazodone is not the magic bullet, and I don’t think we will ever have a magic bullet,” Dr. Karageorgiou said. “Because when our brain degenerates, it’s not just one chemical, or one system, it’s many. And our body changes as well. The important thing is to help the patient consolidate their rhythms, whether through light therapy, daily exercise, cognitive behavioral therapy for insomnia, or other evidence-based interventions and their combination. The same applies for a person with dementia as for the rest of us.”
None of the investigators outside of the industry-sponsored studies had relevant disclosures.
LOS ANGELES – While a large number of older adults take prescription and nonprescription medications to help them sleep, the effect of these medications on dementia risk is unclear, with most researchers advocating a cautious and conservative approach to prescribing.
Research is increasingly revealing a bidirectional relationship between sleep and dementia. Poor sleep – especially from insomnia, sleep deprivation, or obstructive sleep apnea – is known to increase dementia risk. Dementias, meanwhile, are associated with serious circadian rhythm disturbances, leading to nighttime sleep loss and increasing the likelihood of institutionalization.
At the Alzheimer’s Association International Conference, researchers presented findings assessing the links between sleep medication use and dementia and also what agents or approaches might safely improve sleep in people with sleep disorders who are at risk for dementia or who have been diagnosed with dementia.
Sex- and race-based differences in risk
Yue Leng, PhD, of the University of California, San Francisco, reported a link between frequent sleep medication use and later dementia – but only in white adults. Dr. Leng presented findings from the National Institutes of Health–funded Health, Aging, and Body Composition Study, which recruited 3,068 subjects aged 70-79 and followed them for 15 years. At baseline, 2.7% of African Americans and 7.7% of whites in the study reported taking sleep medications “often” or “almost always.”
Dr. Leng and her colleagues found that white subjects who reported taking sleep aids five or more times a month at baseline had a nearly 80% higher risk of developing dementia during the course of the study (hazard ratio, 1.79; 95% confidence interval, 1.21-2.66), compared with people who reported never taking sleep aids or taking them less frequently.
The researchers saw no between-sex differences for this finding, and adjusted for a variety of genetic and lifestyle confounders. Importantly, no significant increase in dementia risk was seen for black subjects, who made up more than one-third of the cohort.
Dr. Leng told the conference that the researchers could not explain why black participants did not see similarly increased dementia risk. Also, she noted, researchers did not have information on the specific sleep medications people used: benzodiazepines, antihistamines, antidepressants, or other types of drugs. Nonetheless, she told the conference, the findings ratified the cautious approach many dementia experts are already stressing.
“Do we really need to prescribe so many sleep meds to older adults who are already at risk for cognitive impairment?” Dr. Leng said, adding: “I am a big advocate of behavioral sleep interventions.” People with clinical sleep problems “should be referred to sleep centers” for a fuller assessment before medication is prescribed, she said.
Findings from another cohort study, meanwhile, suggest that there could be sex-related differences in how sleep aids affect dementia risk. Investigators at Utah State University in Logan used data from some 3,656 older adults in the Cache County Study on Memory and Aging, an NIH-backed cohort study of white adults in Utah without dementia at baseline who were followed for 12 years.
The investigators, led by doctoral student Elizabeth Vernon, found that men reporting use of sleep medication saw more than threefold higher risk of developing Alzheimer’s disease than did men who did not use sleep aids (HR, 3.604; P = .0001).
Women who did not report having sleep disturbance but used sleep-inducing medications were at nearly fourfold greater risk for developing Alzheimer’s disease (HR, 3.916; P = .0001). Women who self-reported sleep disturbances at baseline, meanwhile, saw a reduction in Alzheimer’s risk of about one-third associated with the use of sleep medications.
Ms. Vernon told the conference that, despite the finding of risk reduction for this particular group of women, caution in prescribing sleep aids was warranted.
Common sleep drugs linked to cognitive aging
Chris Fox, MD, a researcher at the University of East Anglia in Norwich, England, and his colleagues demonstrated in 2018 that long-term exposure to anticholinergic drugs, a class that includes some antidepressants and antihistamines used to promote sleep, was associated with a higher risk of dementia, while use of benzodiazepines, a class of sedatives used commonly in older people as sleep aids, was not. (Whether benzodiazepine exposure relates to dementia remains controversial.)
At AAIC 2019, Dr. Fox presented findings from a study of 337 brains in a U.K. brain bank, of which 17% and 21% came from users of benzodiazepines and anticholinergic drugs, whose usage history was well documented. Dr. Fox and his colleagues found that, while neither anticholinergic nor benzodiazepine exposure was associated with brain pathology specific to that seen in Alzheimer’s disease, both classes of drugs were associated with “slight signals in neuronal loss” in one brain region, the nucleus basalis of Meynert. Dr. Fox described the drugs as causing “an increase in cognitive aging” which could bear on Alzheimer’s risk without being directly causative.
Newer sleep drugs may help Alzheimer’s patients
Scientists working for drug manufacturers presented findings on agents to counter the circadian rhythm disturbances seen in people with Alzheimer’s disease. Margaret Moline, PhD, of Eisai in Woodcliff Lake, N.J., showed some results from a phase 2, dose-ranging, placebo-controlled study of the experimental agent lemborexant in 62 subjects aged 60-90 with mild to moderate Alzheimer’s disease and sleep disturbances. (Lemborexant, an orexin receptor agonist that acts to regulate wakefulness, is being investigated in a broad range of sleep disorders.) Patients were randomized to one of four doses of lemborexant or placebo and wore a device for sleep monitoring. Nighttime activity indicating arousal was significantly lower for people in two dosage arms, 5 mg and 10 mg, compared with placebo, and treatment groups saw trends toward less sleep fragmentation and higher total sleep time, Dr. Moline told the conference.
Suvorexant (Belsomra), the only orexin receptor antagonist currently licensed as a sleep aid, is also being tested in people with Alzheimer’s disease. At AAIC 2019, Joseph Herring, MD, PhD, of Merck in Kenilworth, N.J., presented results from a placebo-controlled trial of 277 patients with Alzheimer’s disease and insomnia, and reported that treatment with 10 or 20 mg of suvorexant over 4 weeks was associated with about an extra half hour of total nightly sleep, with a 73-minute mean increase from baseline, compared with 45 minutes for patients receiving placebo (95% CI, 11-45; P less than .005).
Trazodone linked to slower cognitive decline
An inexpensive antidepressant used in low doses as a sleep aid, including in people with Alzheimer’s disease, was associated with a delay in cognitive decline in older adults, according to results from a retrospective study. Elissaios Karageorgiou, MD, PhD, of the University of California, San Francisco, and the Neurological Institute of Athens presented results derived from two cohorts: patients enrolled at the UCSF Memory and Aging Center and women enrolled in the Study for Osteoporotic Fractures (SOF) in Women. The investigators were able to identify trazodone users in the studies (with two or more contiguous study visits reporting trazodone use) and match them with control patients from the same cohorts who did not use trazodone.
Trazodone was studied because previous research suggests it increases total sleep time in patients with Alzheimer’s disease without affecting next-day cognitive performance.
Trazodone-using patients in the UCSF cohort (n = 25) saw significantly less decline in Mini Mental State Exam (MMSE) scores over 4 years, compared with nonusers (0.27 vs. 0.70 points per year; P = .023), an effect that remained statistically significant even after adjusting for sedative and stimulant use and the expected progression of Alzheimer’s disease pathology. Importantly, the slower decline was seen only among subjects with sleep complaints at baseline and especially those whose sleep improved over time, suggesting that the cognitive benefit was mediated by improved sleep.
In the SOF cohort of 46 trazodone users matched with 148 nonusers, no significant protective or negative effect related to long-term trazodone use was found using the MMSE or the Trails Making Test. In this analysis, however, baseline and longitudinal sleep quality was not captured in the group-matching process, neither was the use of other medications. The patient group was slightly older, and all patients were women.
Dr. Karageorgiou said in an interview that the link between improved sleep, trazodone, and cognition needs to be validated in prospective intervention studies. Trazodone, he said, appears to work best in people with a specific type of insomnia characterized by cortical and behavioral hyperarousal, and its cognitive effect appears limited to people whose sleep improves with treatment. “You’re not going to see long-term cognitive benefits if it’s not improving your sleep,” Dr. Karageorgiou said. “So, whether trazodone improves sleep or not in a patient after a few months can be an early indicator for the clinician to continue using it or suspend it, because it is unlikely to help their cognition otherwise.”
He stressed that physicians need to be broadly focused on improving sleep to help patients with, or at risk for, dementia by consolidating their sleep rhythms.
“Trazodone is not the magic bullet, and I don’t think we will ever have a magic bullet,” Dr. Karageorgiou said. “Because when our brain degenerates, it’s not just one chemical, or one system, it’s many. And our body changes as well. The important thing is to help the patient consolidate their rhythms, whether through light therapy, daily exercise, cognitive behavioral therapy for insomnia, or other evidence-based interventions and their combination. The same applies for a person with dementia as for the rest of us.”
None of the investigators outside of the industry-sponsored studies had relevant disclosures.
REPORTING FROM AAIC 2019
CDC finds that too little naloxone is dispensed
Although the CDC recommends that clinicians consider prescribing naloxone, which can reverse the effects of an opioid overdose, to patients who receive high-dose opioid prescriptions, one naloxone prescription was dispensed in 2018 for every 69 such patients, according to a Vital Signs investigation published Aug. 6 in the Morbidity and Mortality Weekly Report.
Approximately 9 million more naloxone prescriptions could have been dispensed in 2018 if every patient with a high-dose opioid prescription were offered the drug, according to the agency. In addition, the rate at which naloxone is dispensed varies significantly according to region.
“Thousands of Americans are alive today thanks to the use of naloxone,” said Alex M. Azar, secretary of Health and Human Services, in a press release. “Giving people a chance to survive an opioid overdose and safely enter recovery is one of the five key pillars of our HHS strategy for ending the overdose epidemic. With help from Congress, the private sector, state, and local governments and communities, targeted access to naloxone has expanded dramatically over the last several years, but today’s CDC report is a reminder that there is much more all of us need to do to save lives.”
Investigators examined retail pharmacy data
In 2017, 47,600 (67.8%) drug overdose deaths in the United States involved opioids. For decades, emergency medical service providers have administered naloxone to patients with suspected drug overdose. A major focus of public health initiatives intended to address the opioid overdose crisis has been to increase access to naloxone through clinician prescribing and pharmacy dispensing. The CDC recommends considering prescribing naloxone to patients with a history of overdose or substance use disorder, those receiving opioid dosages of 50 morphine milligram equivalents per day or greater (that is, high-dose prescriptions), and those who are using benzodiazepines concurrently.
Investigators at the CDC examined retail pharmacy data from IQVIA, a company that maintains information on prescriptions from approximately 50,400 retail pharmacies. They extracted data from 2012 through 2018 to analyze naloxone dispensing by region, urban versus rural status, prescriber specialty, and recipient characteristics (for example, age group, sex, out-of-pocket costs, and method of payment).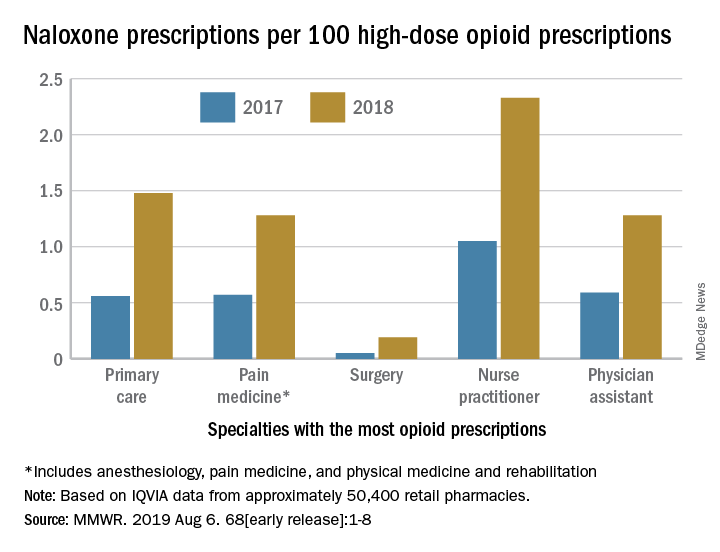
Dispensations doubled from 2017 to 2018
Naloxone dispensing from retail pharmacies increased from 0.4 prescriptions per 100,000 in 2012 to 170.2 prescriptions per 100,000 in 2018. From 2017 to 2018 alone, the number of prescriptions dispensed increased by 106%.
Despite consistency among state laws, naloxone dispensation varied by region. The average rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 0.2 in the lowest quartile to 2.9 in the highest quartile. In 2018, the rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 1.5 in metropolitan counties and 1.6 in the Northeast to 1.2 in rural counties and 1.3 in the Midwest. Rural counties were nearly three times more likely to be low-dispensing counties, compared with metropolitan counties.
The rate of naloxone prescriptions per 100 high-dose opioid prescriptions also varied by provider specialty. This rate was lowest among surgeons (0.2) and highest among psychiatrists (12.9).
Most naloxone prescriptions entailed out-of-pocket costs. About 71% of prescriptions paid for by Medicare entailed out-of-pocket costs, compared with 43.8% of prescriptions paid for by Medicaid, and 41.5% of prescriptions paid for by commercial insurance.
 Centers for Disease Control and Prevention
Centers for Disease Control and Prevention
More can be done

“It is clear from the data that there is still much needed education around the important role naloxone plays in reducing overdose deaths,” said Robert R. Redfield, MD, director of the CDC, in a press release. “The time is now to ensure all individuals who are prescribed high-dose opioids also receive naloxone as a potential life-saving intervention. As we aggressively confront what is the public health crisis of our time, CDC will continue to stress with health care providers the benefit of making this overdose-reversing medicine available to patients.”
“While we’ve seen these important increases [in naloxone prescriptions], we are not as far along as we’d like to be,” said Anne Schuchat, MD, principal deputy director of the CDC, during a press conference. “Cost is one of the issues, but I think awareness is another.” These data should prompt pharmacies to make sure that they stock naloxone and remind clinicians to consider naloxone when they prescribe opioids, she added. Patients and their family members should be aware of naloxone and ask their health care providers about it. “We’d really like to see the increase [in naloxone prescriptions] move much more rapidly,” she concluded.
The investigators disclosed no potential conflicts of interest.
SOURCE: Guy GP et al. MMWR Morb Mortal Wkly Rep. 2019 Aug 6.
Although the CDC recommends that clinicians consider prescribing naloxone, which can reverse the effects of an opioid overdose, to patients who receive high-dose opioid prescriptions, one naloxone prescription was dispensed in 2018 for every 69 such patients, according to a Vital Signs investigation published Aug. 6 in the Morbidity and Mortality Weekly Report.
Approximately 9 million more naloxone prescriptions could have been dispensed in 2018 if every patient with a high-dose opioid prescription were offered the drug, according to the agency. In addition, the rate at which naloxone is dispensed varies significantly according to region.
“Thousands of Americans are alive today thanks to the use of naloxone,” said Alex M. Azar, secretary of Health and Human Services, in a press release. “Giving people a chance to survive an opioid overdose and safely enter recovery is one of the five key pillars of our HHS strategy for ending the overdose epidemic. With help from Congress, the private sector, state, and local governments and communities, targeted access to naloxone has expanded dramatically over the last several years, but today’s CDC report is a reminder that there is much more all of us need to do to save lives.”
Investigators examined retail pharmacy data
In 2017, 47,600 (67.8%) drug overdose deaths in the United States involved opioids. For decades, emergency medical service providers have administered naloxone to patients with suspected drug overdose. A major focus of public health initiatives intended to address the opioid overdose crisis has been to increase access to naloxone through clinician prescribing and pharmacy dispensing. The CDC recommends considering prescribing naloxone to patients with a history of overdose or substance use disorder, those receiving opioid dosages of 50 morphine milligram equivalents per day or greater (that is, high-dose prescriptions), and those who are using benzodiazepines concurrently.
Investigators at the CDC examined retail pharmacy data from IQVIA, a company that maintains information on prescriptions from approximately 50,400 retail pharmacies. They extracted data from 2012 through 2018 to analyze naloxone dispensing by region, urban versus rural status, prescriber specialty, and recipient characteristics (for example, age group, sex, out-of-pocket costs, and method of payment).
Dispensations doubled from 2017 to 2018
Naloxone dispensing from retail pharmacies increased from 0.4 prescriptions per 100,000 in 2012 to 170.2 prescriptions per 100,000 in 2018. From 2017 to 2018 alone, the number of prescriptions dispensed increased by 106%.
Despite consistency among state laws, naloxone dispensation varied by region. The average rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 0.2 in the lowest quartile to 2.9 in the highest quartile. In 2018, the rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 1.5 in metropolitan counties and 1.6 in the Northeast to 1.2 in rural counties and 1.3 in the Midwest. Rural counties were nearly three times more likely to be low-dispensing counties, compared with metropolitan counties.
The rate of naloxone prescriptions per 100 high-dose opioid prescriptions also varied by provider specialty. This rate was lowest among surgeons (0.2) and highest among psychiatrists (12.9).
Most naloxone prescriptions entailed out-of-pocket costs. About 71% of prescriptions paid for by Medicare entailed out-of-pocket costs, compared with 43.8% of prescriptions paid for by Medicaid, and 41.5% of prescriptions paid for by commercial insurance.
 Centers for Disease Control and Prevention
Centers for Disease Control and Prevention
More can be done

“It is clear from the data that there is still much needed education around the important role naloxone plays in reducing overdose deaths,” said Robert R. Redfield, MD, director of the CDC, in a press release. “The time is now to ensure all individuals who are prescribed high-dose opioids also receive naloxone as a potential life-saving intervention. As we aggressively confront what is the public health crisis of our time, CDC will continue to stress with health care providers the benefit of making this overdose-reversing medicine available to patients.”
“While we’ve seen these important increases [in naloxone prescriptions], we are not as far along as we’d like to be,” said Anne Schuchat, MD, principal deputy director of the CDC, during a press conference. “Cost is one of the issues, but I think awareness is another.” These data should prompt pharmacies to make sure that they stock naloxone and remind clinicians to consider naloxone when they prescribe opioids, she added. Patients and their family members should be aware of naloxone and ask their health care providers about it. “We’d really like to see the increase [in naloxone prescriptions] move much more rapidly,” she concluded.
The investigators disclosed no potential conflicts of interest.
SOURCE: Guy GP et al. MMWR Morb Mortal Wkly Rep. 2019 Aug 6.
Although the CDC recommends that clinicians consider prescribing naloxone, which can reverse the effects of an opioid overdose, to patients who receive high-dose opioid prescriptions, one naloxone prescription was dispensed in 2018 for every 69 such patients, according to a Vital Signs investigation published Aug. 6 in the Morbidity and Mortality Weekly Report.
Approximately 9 million more naloxone prescriptions could have been dispensed in 2018 if every patient with a high-dose opioid prescription were offered the drug, according to the agency. In addition, the rate at which naloxone is dispensed varies significantly according to region.
“Thousands of Americans are alive today thanks to the use of naloxone,” said Alex M. Azar, secretary of Health and Human Services, in a press release. “Giving people a chance to survive an opioid overdose and safely enter recovery is one of the five key pillars of our HHS strategy for ending the overdose epidemic. With help from Congress, the private sector, state, and local governments and communities, targeted access to naloxone has expanded dramatically over the last several years, but today’s CDC report is a reminder that there is much more all of us need to do to save lives.”
Investigators examined retail pharmacy data
In 2017, 47,600 (67.8%) drug overdose deaths in the United States involved opioids. For decades, emergency medical service providers have administered naloxone to patients with suspected drug overdose. A major focus of public health initiatives intended to address the opioid overdose crisis has been to increase access to naloxone through clinician prescribing and pharmacy dispensing. The CDC recommends considering prescribing naloxone to patients with a history of overdose or substance use disorder, those receiving opioid dosages of 50 morphine milligram equivalents per day or greater (that is, high-dose prescriptions), and those who are using benzodiazepines concurrently.
Investigators at the CDC examined retail pharmacy data from IQVIA, a company that maintains information on prescriptions from approximately 50,400 retail pharmacies. They extracted data from 2012 through 2018 to analyze naloxone dispensing by region, urban versus rural status, prescriber specialty, and recipient characteristics (for example, age group, sex, out-of-pocket costs, and method of payment).
Dispensations doubled from 2017 to 2018
Naloxone dispensing from retail pharmacies increased from 0.4 prescriptions per 100,000 in 2012 to 170.2 prescriptions per 100,000 in 2018. From 2017 to 2018 alone, the number of prescriptions dispensed increased by 106%.
Despite consistency among state laws, naloxone dispensation varied by region. The average rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 0.2 in the lowest quartile to 2.9 in the highest quartile. In 2018, the rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 1.5 in metropolitan counties and 1.6 in the Northeast to 1.2 in rural counties and 1.3 in the Midwest. Rural counties were nearly three times more likely to be low-dispensing counties, compared with metropolitan counties.
The rate of naloxone prescriptions per 100 high-dose opioid prescriptions also varied by provider specialty. This rate was lowest among surgeons (0.2) and highest among psychiatrists (12.9).
Most naloxone prescriptions entailed out-of-pocket costs. About 71% of prescriptions paid for by Medicare entailed out-of-pocket costs, compared with 43.8% of prescriptions paid for by Medicaid, and 41.5% of prescriptions paid for by commercial insurance.
 Centers for Disease Control and Prevention
Centers for Disease Control and Prevention
More can be done

“It is clear from the data that there is still much needed education around the important role naloxone plays in reducing overdose deaths,” said Robert R. Redfield, MD, director of the CDC, in a press release. “The time is now to ensure all individuals who are prescribed high-dose opioids also receive naloxone as a potential life-saving intervention. As we aggressively confront what is the public health crisis of our time, CDC will continue to stress with health care providers the benefit of making this overdose-reversing medicine available to patients.”
“While we’ve seen these important increases [in naloxone prescriptions], we are not as far along as we’d like to be,” said Anne Schuchat, MD, principal deputy director of the CDC, during a press conference. “Cost is one of the issues, but I think awareness is another.” These data should prompt pharmacies to make sure that they stock naloxone and remind clinicians to consider naloxone when they prescribe opioids, she added. Patients and their family members should be aware of naloxone and ask their health care providers about it. “We’d really like to see the increase [in naloxone prescriptions] move much more rapidly,” she concluded.
The investigators disclosed no potential conflicts of interest.
SOURCE: Guy GP et al. MMWR Morb Mortal Wkly Rep. 2019 Aug 6.
FROM MORBIDITY AND MORTALITY WEEKLY REPORT
Vaccination is not associated with increased risk of MS
(MS), according to an analysis published July 30 in Neurology. Although the results suggest that vaccination is associated with a lower likelihood of incident MS within the following 5 years, “these data alone do not allow for any conclusion regarding a possible protective effect of vaccinations regarding the development of MS,” wrote Alexander Hapfelmeier, PhD, of the Technical University of Munich and colleagues.
In recent years, researchers have proposed and investigated various potential environmental risk factors for the development of MS. Vaccination is one proposed environmental risk factor, but case reports and small studies have yielded conflicting results about its association with incident MS.
To examine this question more closely, Dr. Hapfelmeier and colleagues performed a systematic retrospective analysis of ambulatory claims data held by the Bavarian Association of Statutory Health Insurance Physicians. They reviewed the data to identify patients with new-onset MS and at least two ICD-10 diagnoses of the disorder. They next identified two control cohorts of participants diagnosed with other autoimmune diseases: Crohn’s disease and psoriasis. Finally, they randomly selected a third control cohort of patients without any of these diagnoses and matched them by age, sex, and district to patients with MS in a 5:1 ratio. Eligible participants were younger than 70 years.
Dr. Hapfelmeier and colleagues reviewed the incidence and frequency of vaccinations (such as those targeting tick-borne encephalitis, human papillomavirus, and influenza virus) in all cohorts. They created unconditional logistic regression models to assess the association between vaccination and MS. They also created separate models to contrast the MS cohort with each of the control cohorts.
The researchers included 12,262 patients with MS, 19,296 patients with Crohn’s disease, 112,292 patients with psoriasis, and 79,185 participants without these autoimmune diseases in their analysis. They found 456 participants with Crohn’s disease and psoriasis, 216 participants with MS and psoriasis, 48 participants with Crohn’s disease and MS, and 2 participants with Crohn’s disease, psoriasis, and MS. Dr. Hapfelmeier and colleagues allocated these participants to each of the respective cohorts and did not analyze them differently because of the comparatively small sample sizes.
The investigators analyzed the occurrence of vaccination in all participants during the 5 years before first diagnosis. Among patients who received vaccination, the odds ratio of MS was 0.870 in participants without autoimmune disease, 0.919 in participants with Crohn’s disease, and 0.973 in participants with psoriasis. Decreased risk of MS was most notable for vaccinations against influenza and tick-borne encephalitis. The results were consistent regardless of time frame, control cohort, and definition of MS.
The subjective definition of the MS cohort was a limitation of the study, but the authors addressed it by also using several strict definitions of that cohort. Another limitation is that the source data may reflect entry errors and incorrect coding.
A grant from the German Federal Ministry of Education and Research Competence Network MS supported the study. The authors had no conflicts that were relevant to the topic of the study.
SOURCE: Hapfelmeier A et al. Neurology. 2019 Jul 30. doi: 10.1212/WNL.0000000000008012.
The analysis by Hapfelmeier et al. provides important evidence that vaccinations are not associated with multiple sclerosis (MS), said E. Ann Yeh, MD, a neurologist at the Hospital for Sick Children in Toronto, and Jennifer Graves, MD, PhD, a neurologist at the University of California, San Diego, in an accompanying editorial. On the contrary, the evidence supports a potential protective effect of vaccines on the risk of developing MS, they said.
“The reasons for this [finding] cannot be gleaned from this study and may range from biological to sociocultural/demographic reasons,” the authors added. “Infection, rather than vaccination, may be an MS trigger, or individuals obtaining vaccinations may be practicing other healthy behaviors protective for MS. These possibilities should be the subject of future studies.”
Until future studies are completed and their results published, the findings of Hapfelmeier et al. offer “strong evidence to share with worried patients and families when faced with the question of whether a vaccine in the recent or relatively distant past triggered the individual’s MS,” said Dr. Yeh and Dr. Graves.
The authors had various relationships with industry, including serving on advisory boards for and receiving funding from pharmaceutical companies.
The analysis by Hapfelmeier et al. provides important evidence that vaccinations are not associated with multiple sclerosis (MS), said E. Ann Yeh, MD, a neurologist at the Hospital for Sick Children in Toronto, and Jennifer Graves, MD, PhD, a neurologist at the University of California, San Diego, in an accompanying editorial. On the contrary, the evidence supports a potential protective effect of vaccines on the risk of developing MS, they said.
“The reasons for this [finding] cannot be gleaned from this study and may range from biological to sociocultural/demographic reasons,” the authors added. “Infection, rather than vaccination, may be an MS trigger, or individuals obtaining vaccinations may be practicing other healthy behaviors protective for MS. These possibilities should be the subject of future studies.”
Until future studies are completed and their results published, the findings of Hapfelmeier et al. offer “strong evidence to share with worried patients and families when faced with the question of whether a vaccine in the recent or relatively distant past triggered the individual’s MS,” said Dr. Yeh and Dr. Graves.
The authors had various relationships with industry, including serving on advisory boards for and receiving funding from pharmaceutical companies.
The analysis by Hapfelmeier et al. provides important evidence that vaccinations are not associated with multiple sclerosis (MS), said E. Ann Yeh, MD, a neurologist at the Hospital for Sick Children in Toronto, and Jennifer Graves, MD, PhD, a neurologist at the University of California, San Diego, in an accompanying editorial. On the contrary, the evidence supports a potential protective effect of vaccines on the risk of developing MS, they said.
“The reasons for this [finding] cannot be gleaned from this study and may range from biological to sociocultural/demographic reasons,” the authors added. “Infection, rather than vaccination, may be an MS trigger, or individuals obtaining vaccinations may be practicing other healthy behaviors protective for MS. These possibilities should be the subject of future studies.”
Until future studies are completed and their results published, the findings of Hapfelmeier et al. offer “strong evidence to share with worried patients and families when faced with the question of whether a vaccine in the recent or relatively distant past triggered the individual’s MS,” said Dr. Yeh and Dr. Graves.
The authors had various relationships with industry, including serving on advisory boards for and receiving funding from pharmaceutical companies.
(MS), according to an analysis published July 30 in Neurology. Although the results suggest that vaccination is associated with a lower likelihood of incident MS within the following 5 years, “these data alone do not allow for any conclusion regarding a possible protective effect of vaccinations regarding the development of MS,” wrote Alexander Hapfelmeier, PhD, of the Technical University of Munich and colleagues.
In recent years, researchers have proposed and investigated various potential environmental risk factors for the development of MS. Vaccination is one proposed environmental risk factor, but case reports and small studies have yielded conflicting results about its association with incident MS.
To examine this question more closely, Dr. Hapfelmeier and colleagues performed a systematic retrospective analysis of ambulatory claims data held by the Bavarian Association of Statutory Health Insurance Physicians. They reviewed the data to identify patients with new-onset MS and at least two ICD-10 diagnoses of the disorder. They next identified two control cohorts of participants diagnosed with other autoimmune diseases: Crohn’s disease and psoriasis. Finally, they randomly selected a third control cohort of patients without any of these diagnoses and matched them by age, sex, and district to patients with MS in a 5:1 ratio. Eligible participants were younger than 70 years.
Dr. Hapfelmeier and colleagues reviewed the incidence and frequency of vaccinations (such as those targeting tick-borne encephalitis, human papillomavirus, and influenza virus) in all cohorts. They created unconditional logistic regression models to assess the association between vaccination and MS. They also created separate models to contrast the MS cohort with each of the control cohorts.
The researchers included 12,262 patients with MS, 19,296 patients with Crohn’s disease, 112,292 patients with psoriasis, and 79,185 participants without these autoimmune diseases in their analysis. They found 456 participants with Crohn’s disease and psoriasis, 216 participants with MS and psoriasis, 48 participants with Crohn’s disease and MS, and 2 participants with Crohn’s disease, psoriasis, and MS. Dr. Hapfelmeier and colleagues allocated these participants to each of the respective cohorts and did not analyze them differently because of the comparatively small sample sizes.
The investigators analyzed the occurrence of vaccination in all participants during the 5 years before first diagnosis. Among patients who received vaccination, the odds ratio of MS was 0.870 in participants without autoimmune disease, 0.919 in participants with Crohn’s disease, and 0.973 in participants with psoriasis. Decreased risk of MS was most notable for vaccinations against influenza and tick-borne encephalitis. The results were consistent regardless of time frame, control cohort, and definition of MS.
The subjective definition of the MS cohort was a limitation of the study, but the authors addressed it by also using several strict definitions of that cohort. Another limitation is that the source data may reflect entry errors and incorrect coding.
A grant from the German Federal Ministry of Education and Research Competence Network MS supported the study. The authors had no conflicts that were relevant to the topic of the study.
SOURCE: Hapfelmeier A et al. Neurology. 2019 Jul 30. doi: 10.1212/WNL.0000000000008012.
(MS), according to an analysis published July 30 in Neurology. Although the results suggest that vaccination is associated with a lower likelihood of incident MS within the following 5 years, “these data alone do not allow for any conclusion regarding a possible protective effect of vaccinations regarding the development of MS,” wrote Alexander Hapfelmeier, PhD, of the Technical University of Munich and colleagues.
In recent years, researchers have proposed and investigated various potential environmental risk factors for the development of MS. Vaccination is one proposed environmental risk factor, but case reports and small studies have yielded conflicting results about its association with incident MS.
To examine this question more closely, Dr. Hapfelmeier and colleagues performed a systematic retrospective analysis of ambulatory claims data held by the Bavarian Association of Statutory Health Insurance Physicians. They reviewed the data to identify patients with new-onset MS and at least two ICD-10 diagnoses of the disorder. They next identified two control cohorts of participants diagnosed with other autoimmune diseases: Crohn’s disease and psoriasis. Finally, they randomly selected a third control cohort of patients without any of these diagnoses and matched them by age, sex, and district to patients with MS in a 5:1 ratio. Eligible participants were younger than 70 years.
Dr. Hapfelmeier and colleagues reviewed the incidence and frequency of vaccinations (such as those targeting tick-borne encephalitis, human papillomavirus, and influenza virus) in all cohorts. They created unconditional logistic regression models to assess the association between vaccination and MS. They also created separate models to contrast the MS cohort with each of the control cohorts.
The researchers included 12,262 patients with MS, 19,296 patients with Crohn’s disease, 112,292 patients with psoriasis, and 79,185 participants without these autoimmune diseases in their analysis. They found 456 participants with Crohn’s disease and psoriasis, 216 participants with MS and psoriasis, 48 participants with Crohn’s disease and MS, and 2 participants with Crohn’s disease, psoriasis, and MS. Dr. Hapfelmeier and colleagues allocated these participants to each of the respective cohorts and did not analyze them differently because of the comparatively small sample sizes.
The investigators analyzed the occurrence of vaccination in all participants during the 5 years before first diagnosis. Among patients who received vaccination, the odds ratio of MS was 0.870 in participants without autoimmune disease, 0.919 in participants with Crohn’s disease, and 0.973 in participants with psoriasis. Decreased risk of MS was most notable for vaccinations against influenza and tick-borne encephalitis. The results were consistent regardless of time frame, control cohort, and definition of MS.
The subjective definition of the MS cohort was a limitation of the study, but the authors addressed it by also using several strict definitions of that cohort. Another limitation is that the source data may reflect entry errors and incorrect coding.
A grant from the German Federal Ministry of Education and Research Competence Network MS supported the study. The authors had no conflicts that were relevant to the topic of the study.
SOURCE: Hapfelmeier A et al. Neurology. 2019 Jul 30. doi: 10.1212/WNL.0000000000008012.
FROM NEUROLOGY
Benzodiazepines, hypnotics don’t increase Alzheimer’s pathology
LOS ANGELES – Benzodiazepines and hypnotics, including the so-called “Z drugs,” don’t significantly increase the pathological features typical of Alzheimer’s disease but long-term users may experience some neuronal loss in the nucleus basalis, Chris Fox, MD, reported at the Alzheimer’s Association International Conference.
The nucleus basalis is rich in cholinergic neurons and associated with arousing stimuli, including positive and aversive appetite, sustained attention, and the interplay of reality and visual perception.
“Neuronal loss in the nucleus basalis offers mechanisms for the impact of benzodiazepine and anticholinergic drug use on the aging brain and highlights important areas for future research,” said Dr. Fox, professor of clinical psychiatry at the University of East Anglia, Norwich, England.
“The risk [for taking a Z drug] in the United Kingdom is high, with about 7.5 million older adults using potentially inappropriately prescribed anticholinergic and/or Z-drug medications. Despite well-documented cognitive impairment associated with these medicines, hypnotics are still used for long durations and exceed the recommended limits,” Dr. Fox said. “There’s no association with better cognition, quality of life, or improved behavior when they are given to people with dementia. In fact, we’ve seen a 60% increased risk of hip fractures – an increase from a 3% to a 15% yearly risk.”
Dr. Fox and colleagues studied the brains of 337 subjects who were included in the U.K. Medical Research Council’s Cognitive Function and Ageing Studies (CFAS). The study was intended to explore the incidence of dementia in the United Kingdom, examine incidence variation among regions, and explore factors increasing dementia risk and rate of progression.
The first study, which began in 1989 and lasted until 2015, followed subjects older than 65 years for up to 12 years. Each subject was regularly interviewed and underwent cognitive testing about every 1.5 years. Benzodiazepine use was considered an especially important aspect, because the medications are frequently used in the elderly and seem linked to injuries and cognitive status at last follow-up.
In CFAS, 21% of subjects reported at least one incidence of anticholinergic use, and 12% reported recurrent use. Another 17% reported any hypnotic use, and 11% reported recurrent use. The main indications were as an antidepressant (13%), for urological issues (4%), as antiparkinsonism drugs (1%), as antipsychotics (3%), and as antihistamines (3%). Overall, 18% reported concurrent use of benzodiazepines and hypnotics. At time of death, 46% had a diagnosis of dementia.
“Those reporting benzodiazepine use were more likely to be women and to have depression or sleep problems,” Dr. Fox noted, although he didn’t give specific hazard ratios. After adjustment for numerous factors, including age, sex, stroke, hypertension, depression, anxiety, asthma, Parkinson’s disease, duration of sleep problems, education, and smoking, he found no statistically increased risk of amyloid brain plaques or tau tangles, the pathologic hallmarks of Alzheimer’s disease.
Anticholinergic use was associated with a significant 60% reduction in cortical atrophy (odds ratio, 0.40) and recurrent use with a 61% reduction in amyloid angiopathy (OR, 0.39).
However, both medication classes were associated with greater neuronal loss in the nucleus basalis. Recurrent use of anticholinergic drugs increased neuronal loss by 300% (OR, 4.12), while any use nearly tripled it (OR, 2.87). Recurrent use of benzodiazepines was associated with increased neuronal loss in the region (OR, 3.76) as well. However, these associations did not reach statistical significance. But there was a statistically significant association with any use of benzodiazepines and neuronal loss in the nucleus basalis (OR, 6.84).
“We did find greater neuronal loss in the nucleus basalis associated with benzodiazepine and anticholinergic drugs use,” Dr. Fox said. “The nucleus basalis is rich in neurons that stimulate the cholinergic system of the neocortex. Neuronal loss in this region is thought to occur in the early stages of Alzheimer’s. Other studies have suggested that volume loss in the basal forebrain cholinergic site leads to widespread cortical atrophy in patients with mild cognitive impairment. We did not observe the widespread cortical atrophy, however.
“Given that the strongest associations were observed for benzodiazepines and neuronal loss in the nucleus basalis, it may be that the drugs were prescribed to treat the symptoms of ‘cholinergic deficiency syndrome,’ Our findings suggest that the symptoms of dementia lead to an increase of benzodiazepines as opposed to the medications actually causing Alzheimer’s disease,” he said.
Dr. Fox reported no financial disclosures.
SOURCE: Fox C et al. AAIC 2019, Abstract 34017.
LOS ANGELES – Benzodiazepines and hypnotics, including the so-called “Z drugs,” don’t significantly increase the pathological features typical of Alzheimer’s disease but long-term users may experience some neuronal loss in the nucleus basalis, Chris Fox, MD, reported at the Alzheimer’s Association International Conference.
The nucleus basalis is rich in cholinergic neurons and associated with arousing stimuli, including positive and aversive appetite, sustained attention, and the interplay of reality and visual perception.
“Neuronal loss in the nucleus basalis offers mechanisms for the impact of benzodiazepine and anticholinergic drug use on the aging brain and highlights important areas for future research,” said Dr. Fox, professor of clinical psychiatry at the University of East Anglia, Norwich, England.
“The risk [for taking a Z drug] in the United Kingdom is high, with about 7.5 million older adults using potentially inappropriately prescribed anticholinergic and/or Z-drug medications. Despite well-documented cognitive impairment associated with these medicines, hypnotics are still used for long durations and exceed the recommended limits,” Dr. Fox said. “There’s no association with better cognition, quality of life, or improved behavior when they are given to people with dementia. In fact, we’ve seen a 60% increased risk of hip fractures – an increase from a 3% to a 15% yearly risk.”
Dr. Fox and colleagues studied the brains of 337 subjects who were included in the U.K. Medical Research Council’s Cognitive Function and Ageing Studies (CFAS). The study was intended to explore the incidence of dementia in the United Kingdom, examine incidence variation among regions, and explore factors increasing dementia risk and rate of progression.
The first study, which began in 1989 and lasted until 2015, followed subjects older than 65 years for up to 12 years. Each subject was regularly interviewed and underwent cognitive testing about every 1.5 years. Benzodiazepine use was considered an especially important aspect, because the medications are frequently used in the elderly and seem linked to injuries and cognitive status at last follow-up.
In CFAS, 21% of subjects reported at least one incidence of anticholinergic use, and 12% reported recurrent use. Another 17% reported any hypnotic use, and 11% reported recurrent use. The main indications were as an antidepressant (13%), for urological issues (4%), as antiparkinsonism drugs (1%), as antipsychotics (3%), and as antihistamines (3%). Overall, 18% reported concurrent use of benzodiazepines and hypnotics. At time of death, 46% had a diagnosis of dementia.
“Those reporting benzodiazepine use were more likely to be women and to have depression or sleep problems,” Dr. Fox noted, although he didn’t give specific hazard ratios. After adjustment for numerous factors, including age, sex, stroke, hypertension, depression, anxiety, asthma, Parkinson’s disease, duration of sleep problems, education, and smoking, he found no statistically increased risk of amyloid brain plaques or tau tangles, the pathologic hallmarks of Alzheimer’s disease.
Anticholinergic use was associated with a significant 60% reduction in cortical atrophy (odds ratio, 0.40) and recurrent use with a 61% reduction in amyloid angiopathy (OR, 0.39).
However, both medication classes were associated with greater neuronal loss in the nucleus basalis. Recurrent use of anticholinergic drugs increased neuronal loss by 300% (OR, 4.12), while any use nearly tripled it (OR, 2.87). Recurrent use of benzodiazepines was associated with increased neuronal loss in the region (OR, 3.76) as well. However, these associations did not reach statistical significance. But there was a statistically significant association with any use of benzodiazepines and neuronal loss in the nucleus basalis (OR, 6.84).
“We did find greater neuronal loss in the nucleus basalis associated with benzodiazepine and anticholinergic drugs use,” Dr. Fox said. “The nucleus basalis is rich in neurons that stimulate the cholinergic system of the neocortex. Neuronal loss in this region is thought to occur in the early stages of Alzheimer’s. Other studies have suggested that volume loss in the basal forebrain cholinergic site leads to widespread cortical atrophy in patients with mild cognitive impairment. We did not observe the widespread cortical atrophy, however.
“Given that the strongest associations were observed for benzodiazepines and neuronal loss in the nucleus basalis, it may be that the drugs were prescribed to treat the symptoms of ‘cholinergic deficiency syndrome,’ Our findings suggest that the symptoms of dementia lead to an increase of benzodiazepines as opposed to the medications actually causing Alzheimer’s disease,” he said.
Dr. Fox reported no financial disclosures.
SOURCE: Fox C et al. AAIC 2019, Abstract 34017.
LOS ANGELES – Benzodiazepines and hypnotics, including the so-called “Z drugs,” don’t significantly increase the pathological features typical of Alzheimer’s disease but long-term users may experience some neuronal loss in the nucleus basalis, Chris Fox, MD, reported at the Alzheimer’s Association International Conference.
The nucleus basalis is rich in cholinergic neurons and associated with arousing stimuli, including positive and aversive appetite, sustained attention, and the interplay of reality and visual perception.
“Neuronal loss in the nucleus basalis offers mechanisms for the impact of benzodiazepine and anticholinergic drug use on the aging brain and highlights important areas for future research,” said Dr. Fox, professor of clinical psychiatry at the University of East Anglia, Norwich, England.
“The risk [for taking a Z drug] in the United Kingdom is high, with about 7.5 million older adults using potentially inappropriately prescribed anticholinergic and/or Z-drug medications. Despite well-documented cognitive impairment associated with these medicines, hypnotics are still used for long durations and exceed the recommended limits,” Dr. Fox said. “There’s no association with better cognition, quality of life, or improved behavior when they are given to people with dementia. In fact, we’ve seen a 60% increased risk of hip fractures – an increase from a 3% to a 15% yearly risk.”
Dr. Fox and colleagues studied the brains of 337 subjects who were included in the U.K. Medical Research Council’s Cognitive Function and Ageing Studies (CFAS). The study was intended to explore the incidence of dementia in the United Kingdom, examine incidence variation among regions, and explore factors increasing dementia risk and rate of progression.
The first study, which began in 1989 and lasted until 2015, followed subjects older than 65 years for up to 12 years. Each subject was regularly interviewed and underwent cognitive testing about every 1.5 years. Benzodiazepine use was considered an especially important aspect, because the medications are frequently used in the elderly and seem linked to injuries and cognitive status at last follow-up.
In CFAS, 21% of subjects reported at least one incidence of anticholinergic use, and 12% reported recurrent use. Another 17% reported any hypnotic use, and 11% reported recurrent use. The main indications were as an antidepressant (13%), for urological issues (4%), as antiparkinsonism drugs (1%), as antipsychotics (3%), and as antihistamines (3%). Overall, 18% reported concurrent use of benzodiazepines and hypnotics. At time of death, 46% had a diagnosis of dementia.
“Those reporting benzodiazepine use were more likely to be women and to have depression or sleep problems,” Dr. Fox noted, although he didn’t give specific hazard ratios. After adjustment for numerous factors, including age, sex, stroke, hypertension, depression, anxiety, asthma, Parkinson’s disease, duration of sleep problems, education, and smoking, he found no statistically increased risk of amyloid brain plaques or tau tangles, the pathologic hallmarks of Alzheimer’s disease.
Anticholinergic use was associated with a significant 60% reduction in cortical atrophy (odds ratio, 0.40) and recurrent use with a 61% reduction in amyloid angiopathy (OR, 0.39).
However, both medication classes were associated with greater neuronal loss in the nucleus basalis. Recurrent use of anticholinergic drugs increased neuronal loss by 300% (OR, 4.12), while any use nearly tripled it (OR, 2.87). Recurrent use of benzodiazepines was associated with increased neuronal loss in the region (OR, 3.76) as well. However, these associations did not reach statistical significance. But there was a statistically significant association with any use of benzodiazepines and neuronal loss in the nucleus basalis (OR, 6.84).
“We did find greater neuronal loss in the nucleus basalis associated with benzodiazepine and anticholinergic drugs use,” Dr. Fox said. “The nucleus basalis is rich in neurons that stimulate the cholinergic system of the neocortex. Neuronal loss in this region is thought to occur in the early stages of Alzheimer’s. Other studies have suggested that volume loss in the basal forebrain cholinergic site leads to widespread cortical atrophy in patients with mild cognitive impairment. We did not observe the widespread cortical atrophy, however.
“Given that the strongest associations were observed for benzodiazepines and neuronal loss in the nucleus basalis, it may be that the drugs were prescribed to treat the symptoms of ‘cholinergic deficiency syndrome,’ Our findings suggest that the symptoms of dementia lead to an increase of benzodiazepines as opposed to the medications actually causing Alzheimer’s disease,” he said.
Dr. Fox reported no financial disclosures.
SOURCE: Fox C et al. AAIC 2019, Abstract 34017.
REPORTING FROM AAIC 2019
Researchers examine potential causes of dementia in CTE
, according to a cross-sectional study published online Aug. 5 in JAMA Neurology.
The study of older, deceased former American football players with CTE showed that more years of play were associated with more severe white matter rarefaction and greater burden of neurofibrillary tau tangles in the dorsolateral frontal cortex, wrote Michael L. Alosco, PhD, assistant professor of neurology at Boston University’s CTE Center, and colleagues.
An analysis of donated brains
Repetitive head impacts are associated with CTE. The clinical presentation of CTE includes cognitive, behavioral, and mood changes that can progress to dementia. The contributions of pathologic changes in phosphorylated tau, white matter degeneration, and cerebrovascular disease to dementia in the context of CTE are poorly understood. Dr. Alosco and colleagues examined arteriosclerosis, infarcts, microinfarcts, microbleeds, and white matter rarefaction in donated brains to illuminate these contributions.
The researchers examined data from the Understanding Neurologic Injury and Traumatic Encephalopathy (UNITE) Study and Veterans Affairs–Boston University–Concussion Legacy Foundation brain bank. The population included deceased men who had played football and had received a neuropathologic diagnosis of CTE. Eligible participants had a history of repetitive head impacts. Brains that had been donated after a prolonged time postmortem and those with poor tissue quality were excluded.
Neuropathologists blinded to clinical data analyzed patients’ CTE stage and severity of neurofibrillary tangle burden in the dorsolateral frontal cortex as semiquantitative scales of phosphorylated tau severity. Neurofibrillary tangle burden was dichotomized as none or mild versus moderate or severe. The neuropathologists also rated white matter rarefaction and arteriolosclerosis severity using a scale of 0 points (i.e., none) to 3 points (i.e., severe changes). The investigators obtained clinical data through online surveys and retrospective telephone interviews with informants. They adjudicated consensus diagnoses of dementia based on modified criteria from DSM-IV.
White matter rarefaction was common
Dr. Alosco and colleagues included 180 individuals in their analysis, excluding those aged younger than 40 years because of low pathologic burden and minimal presence of dementia. Mean age at death was nearly 68 years. Fifty patients had no or mild neurofibrillary tangle burden, and 130 had moderate to severe burden. Thirty-five patients had CTE at stage I or II, and 145 had CTE at stage III or IV. In all, 120 patients were determined to have had dementia. About 47% of the sample had moderate to severe white matter rarefaction, and about 47% had arteriolosclerosis. Infarcts, microinfarcts, and microbleeds were uncommon.
When the investigators created a simultaneous equations regression model and controlled for age and race, they found that more years of play was associated with more severe white matter rarefaction, greater phosphorylated tau accumulation, and high CTE stage. Furthermore, white matter rarefaction and dorsolateral frontal cortex neurofibrillary tangles were associated with dementia. The association of years of play with dementia was mediated by white matter rarefaction and neurofibrillary tangle burden. Arteriolosclerosis was not associated with years of play, but arteriolosclerosis was independently associated with dementia.
The odds ratio for dementia was 1.69 among participants with more severe white matter rarefaction and 1.81 among patients with arteriolosclerosis. After the researchers controlled for age and race, the odds ratio of dementia was 2.65 among participants with a high neurofibrillary tangle burden, compared with participants with a low burden.
“Studies that include direct cardiovascular disease and repetitive head impacts metrics and refined measures of white matter integrity are needed to improve understanding of the pathogenesis of white matter rarefaction and cerebral small vessel changes in CTE,” Dr. Alosco and colleagues wrote.
The study was funded by grants from the National Institute on Aging, National Institute of Neurological Disorders and Stroke, the Department of Veterans Affairs, the Nick and Lynn Buoniconti Foundation, and the National Center for Advancing Translational Sciences. Some of the authors reported financial ties to the pharmaceutical industry and serving on professional sports committees.
SOURCE: Alosco ML et al. JAMA Neurol. 2019 Aug 5. doi: 10.1001/jamaneurol.2019.2244.
The study by Alosco et al. provides new insights into the pathogenesis of dementia in deceased former football players with chronic traumatic encephalopathy (CTE), Julie A. Schneider, MD, professor of neuropathology at Rush University, Chicago, wrote in an accompanying editorial (JAMA Neurol. 2019 Aug 5. doi: 10.1001/jamaneurol.2019.1089).
Significant and widespread white matter injury is an established result of head trauma resulting from acceleration-deceleration injuries. In addition, studies of single and repetitive traumatic brain injury have shown disruption of axons and white matter. The findings of Alosco et al. “underscore the importance of studying the risk factors and mechanisms for the white matter rarefaction, in addition to the tauopathy, in individuals who have played U.S. football and have CTE,” Dr. Schneider wrote.
The comprehensive neuropathologic examinations, advanced statistical techniques, and multiple sensitivity analyses that the investigators performed are among the study’s strengths. An important limitation, however, is selection bias. “The frequency of pathologic characteristics in this group should not be generalized to estimate the prevalence of neuropathologic conditions in living individuals who have played or are playing U.S. football,” Dr. Schneider wrote. “Moreover, individuals who played football who were selected for autopsy and found to have CTE may differ in other important ways from those who did not undergo autopsy or did not have CTE.” Recall bias could alter associations between years of play and dementia diagnosis, and the study’s semiquantitative assessments could result in decreased power to observe relevant associations, she said.
“In spite of these limitations, the authors should be applauded for elegant work and compelling support for multiple pathologic pathways to dementia in football players with CTE,” Dr. Schneider concluded.
Dr. Schneider is with the Rush Alzheimer’s Disease Center at Rush University, Chicago. She has been an expert consultant for the National Football League and the National Hockey League.
The study by Alosco et al. provides new insights into the pathogenesis of dementia in deceased former football players with chronic traumatic encephalopathy (CTE), Julie A. Schneider, MD, professor of neuropathology at Rush University, Chicago, wrote in an accompanying editorial (JAMA Neurol. 2019 Aug 5. doi: 10.1001/jamaneurol.2019.1089).
Significant and widespread white matter injury is an established result of head trauma resulting from acceleration-deceleration injuries. In addition, studies of single and repetitive traumatic brain injury have shown disruption of axons and white matter. The findings of Alosco et al. “underscore the importance of studying the risk factors and mechanisms for the white matter rarefaction, in addition to the tauopathy, in individuals who have played U.S. football and have CTE,” Dr. Schneider wrote.
The comprehensive neuropathologic examinations, advanced statistical techniques, and multiple sensitivity analyses that the investigators performed are among the study’s strengths. An important limitation, however, is selection bias. “The frequency of pathologic characteristics in this group should not be generalized to estimate the prevalence of neuropathologic conditions in living individuals who have played or are playing U.S. football,” Dr. Schneider wrote. “Moreover, individuals who played football who were selected for autopsy and found to have CTE may differ in other important ways from those who did not undergo autopsy or did not have CTE.” Recall bias could alter associations between years of play and dementia diagnosis, and the study’s semiquantitative assessments could result in decreased power to observe relevant associations, she said.
“In spite of these limitations, the authors should be applauded for elegant work and compelling support for multiple pathologic pathways to dementia in football players with CTE,” Dr. Schneider concluded.
Dr. Schneider is with the Rush Alzheimer’s Disease Center at Rush University, Chicago. She has been an expert consultant for the National Football League and the National Hockey League.
The study by Alosco et al. provides new insights into the pathogenesis of dementia in deceased former football players with chronic traumatic encephalopathy (CTE), Julie A. Schneider, MD, professor of neuropathology at Rush University, Chicago, wrote in an accompanying editorial (JAMA Neurol. 2019 Aug 5. doi: 10.1001/jamaneurol.2019.1089).
Significant and widespread white matter injury is an established result of head trauma resulting from acceleration-deceleration injuries. In addition, studies of single and repetitive traumatic brain injury have shown disruption of axons and white matter. The findings of Alosco et al. “underscore the importance of studying the risk factors and mechanisms for the white matter rarefaction, in addition to the tauopathy, in individuals who have played U.S. football and have CTE,” Dr. Schneider wrote.
The comprehensive neuropathologic examinations, advanced statistical techniques, and multiple sensitivity analyses that the investigators performed are among the study’s strengths. An important limitation, however, is selection bias. “The frequency of pathologic characteristics in this group should not be generalized to estimate the prevalence of neuropathologic conditions in living individuals who have played or are playing U.S. football,” Dr. Schneider wrote. “Moreover, individuals who played football who were selected for autopsy and found to have CTE may differ in other important ways from those who did not undergo autopsy or did not have CTE.” Recall bias could alter associations between years of play and dementia diagnosis, and the study’s semiquantitative assessments could result in decreased power to observe relevant associations, she said.
“In spite of these limitations, the authors should be applauded for elegant work and compelling support for multiple pathologic pathways to dementia in football players with CTE,” Dr. Schneider concluded.
Dr. Schneider is with the Rush Alzheimer’s Disease Center at Rush University, Chicago. She has been an expert consultant for the National Football League and the National Hockey League.
, according to a cross-sectional study published online Aug. 5 in JAMA Neurology.
The study of older, deceased former American football players with CTE showed that more years of play were associated with more severe white matter rarefaction and greater burden of neurofibrillary tau tangles in the dorsolateral frontal cortex, wrote Michael L. Alosco, PhD, assistant professor of neurology at Boston University’s CTE Center, and colleagues.
An analysis of donated brains
Repetitive head impacts are associated with CTE. The clinical presentation of CTE includes cognitive, behavioral, and mood changes that can progress to dementia. The contributions of pathologic changes in phosphorylated tau, white matter degeneration, and cerebrovascular disease to dementia in the context of CTE are poorly understood. Dr. Alosco and colleagues examined arteriosclerosis, infarcts, microinfarcts, microbleeds, and white matter rarefaction in donated brains to illuminate these contributions.
The researchers examined data from the Understanding Neurologic Injury and Traumatic Encephalopathy (UNITE) Study and Veterans Affairs–Boston University–Concussion Legacy Foundation brain bank. The population included deceased men who had played football and had received a neuropathologic diagnosis of CTE. Eligible participants had a history of repetitive head impacts. Brains that had been donated after a prolonged time postmortem and those with poor tissue quality were excluded.
Neuropathologists blinded to clinical data analyzed patients’ CTE stage and severity of neurofibrillary tangle burden in the dorsolateral frontal cortex as semiquantitative scales of phosphorylated tau severity. Neurofibrillary tangle burden was dichotomized as none or mild versus moderate or severe. The neuropathologists also rated white matter rarefaction and arteriolosclerosis severity using a scale of 0 points (i.e., none) to 3 points (i.e., severe changes). The investigators obtained clinical data through online surveys and retrospective telephone interviews with informants. They adjudicated consensus diagnoses of dementia based on modified criteria from DSM-IV.
White matter rarefaction was common
Dr. Alosco and colleagues included 180 individuals in their analysis, excluding those aged younger than 40 years because of low pathologic burden and minimal presence of dementia. Mean age at death was nearly 68 years. Fifty patients had no or mild neurofibrillary tangle burden, and 130 had moderate to severe burden. Thirty-five patients had CTE at stage I or II, and 145 had CTE at stage III or IV. In all, 120 patients were determined to have had dementia. About 47% of the sample had moderate to severe white matter rarefaction, and about 47% had arteriolosclerosis. Infarcts, microinfarcts, and microbleeds were uncommon.
When the investigators created a simultaneous equations regression model and controlled for age and race, they found that more years of play was associated with more severe white matter rarefaction, greater phosphorylated tau accumulation, and high CTE stage. Furthermore, white matter rarefaction and dorsolateral frontal cortex neurofibrillary tangles were associated with dementia. The association of years of play with dementia was mediated by white matter rarefaction and neurofibrillary tangle burden. Arteriolosclerosis was not associated with years of play, but arteriolosclerosis was independently associated with dementia.
The odds ratio for dementia was 1.69 among participants with more severe white matter rarefaction and 1.81 among patients with arteriolosclerosis. After the researchers controlled for age and race, the odds ratio of dementia was 2.65 among participants with a high neurofibrillary tangle burden, compared with participants with a low burden.
“Studies that include direct cardiovascular disease and repetitive head impacts metrics and refined measures of white matter integrity are needed to improve understanding of the pathogenesis of white matter rarefaction and cerebral small vessel changes in CTE,” Dr. Alosco and colleagues wrote.
The study was funded by grants from the National Institute on Aging, National Institute of Neurological Disorders and Stroke, the Department of Veterans Affairs, the Nick and Lynn Buoniconti Foundation, and the National Center for Advancing Translational Sciences. Some of the authors reported financial ties to the pharmaceutical industry and serving on professional sports committees.
SOURCE: Alosco ML et al. JAMA Neurol. 2019 Aug 5. doi: 10.1001/jamaneurol.2019.2244.
, according to a cross-sectional study published online Aug. 5 in JAMA Neurology.
The study of older, deceased former American football players with CTE showed that more years of play were associated with more severe white matter rarefaction and greater burden of neurofibrillary tau tangles in the dorsolateral frontal cortex, wrote Michael L. Alosco, PhD, assistant professor of neurology at Boston University’s CTE Center, and colleagues.
An analysis of donated brains
Repetitive head impacts are associated with CTE. The clinical presentation of CTE includes cognitive, behavioral, and mood changes that can progress to dementia. The contributions of pathologic changes in phosphorylated tau, white matter degeneration, and cerebrovascular disease to dementia in the context of CTE are poorly understood. Dr. Alosco and colleagues examined arteriosclerosis, infarcts, microinfarcts, microbleeds, and white matter rarefaction in donated brains to illuminate these contributions.
The researchers examined data from the Understanding Neurologic Injury and Traumatic Encephalopathy (UNITE) Study and Veterans Affairs–Boston University–Concussion Legacy Foundation brain bank. The population included deceased men who had played football and had received a neuropathologic diagnosis of CTE. Eligible participants had a history of repetitive head impacts. Brains that had been donated after a prolonged time postmortem and those with poor tissue quality were excluded.
Neuropathologists blinded to clinical data analyzed patients’ CTE stage and severity of neurofibrillary tangle burden in the dorsolateral frontal cortex as semiquantitative scales of phosphorylated tau severity. Neurofibrillary tangle burden was dichotomized as none or mild versus moderate or severe. The neuropathologists also rated white matter rarefaction and arteriolosclerosis severity using a scale of 0 points (i.e., none) to 3 points (i.e., severe changes). The investigators obtained clinical data through online surveys and retrospective telephone interviews with informants. They adjudicated consensus diagnoses of dementia based on modified criteria from DSM-IV.
White matter rarefaction was common
Dr. Alosco and colleagues included 180 individuals in their analysis, excluding those aged younger than 40 years because of low pathologic burden and minimal presence of dementia. Mean age at death was nearly 68 years. Fifty patients had no or mild neurofibrillary tangle burden, and 130 had moderate to severe burden. Thirty-five patients had CTE at stage I or II, and 145 had CTE at stage III or IV. In all, 120 patients were determined to have had dementia. About 47% of the sample had moderate to severe white matter rarefaction, and about 47% had arteriolosclerosis. Infarcts, microinfarcts, and microbleeds were uncommon.
When the investigators created a simultaneous equations regression model and controlled for age and race, they found that more years of play was associated with more severe white matter rarefaction, greater phosphorylated tau accumulation, and high CTE stage. Furthermore, white matter rarefaction and dorsolateral frontal cortex neurofibrillary tangles were associated with dementia. The association of years of play with dementia was mediated by white matter rarefaction and neurofibrillary tangle burden. Arteriolosclerosis was not associated with years of play, but arteriolosclerosis was independently associated with dementia.
The odds ratio for dementia was 1.69 among participants with more severe white matter rarefaction and 1.81 among patients with arteriolosclerosis. After the researchers controlled for age and race, the odds ratio of dementia was 2.65 among participants with a high neurofibrillary tangle burden, compared with participants with a low burden.
“Studies that include direct cardiovascular disease and repetitive head impacts metrics and refined measures of white matter integrity are needed to improve understanding of the pathogenesis of white matter rarefaction and cerebral small vessel changes in CTE,” Dr. Alosco and colleagues wrote.
The study was funded by grants from the National Institute on Aging, National Institute of Neurological Disorders and Stroke, the Department of Veterans Affairs, the Nick and Lynn Buoniconti Foundation, and the National Center for Advancing Translational Sciences. Some of the authors reported financial ties to the pharmaceutical industry and serving on professional sports committees.
SOURCE: Alosco ML et al. JAMA Neurol. 2019 Aug 5. doi: 10.1001/jamaneurol.2019.2244.
FROM JAMA NEUROLOGY
Quality of Care for Veterans With In-Hospital Stroke
Stroke is a leading cause of death and long-term disability in the US.1 Quality improvement efforts for acute stroke care delivery have successfully led to increased rates of thrombolytic utilization.2 Increasing attention is now being paid to additional quality metrics for stroke care, including hospital management and initiation of appropriate secondary stroke prevention measures at discharge. Many organizations, including the Veterans Health Administration (VHA), use these measures to monitor health care quality and certify centers that are committed to excellence in stroke care.3-6 It is anticipated that collection, evaluation, and feedback from these data may lead to improvements in outcomes after stroke.7
Patients who experience onset of stroke symptoms while already admitted to a hospital may be uniquely suited for quality improvement strategies. In-hospital strokes (IHS) are not uncommon and have been associated with higher stroke severity and increased mortality compared with patients with stroke symptoms prior to arriving at the emergency department (ED).8-10 A potential reason for the higher observed mortality is that patients with IHS may have poorer access to acute stroke resources, such as stroke teams and neuroimaging, as well as increased rates of medical comorbidities.9,11,12 Furthermore, stroke management protocols are typically created based on ED resources, which may not be equivalent to resources available on inpatient settings.
Although many studies have examined clinical characteristics of patients with IHS, few studies have looked at the quality of stroke care for IHS. Information on stroke quality data is even more limited in VHA hospitals due to the small number of admitted patients with stroke.13 VHA released a directive on Acute Stroke Treatment (Directive 2011-03) in 2011 with a recent update in 2018, which aimed to implement quality improvement strategies for stroke care in VHA hospitals.14 Although focusing primarily on acute stroke care in the ED, this directive has led to increased awareness of areas for improvement, particularly among larger VHA hospitals. Prior to this directive, although national stroke guidelines were well-defined, more variability likely existed in stroke protocols and the manner in which stroke care was delivered across care settings. As efforts to measure and improve stroke care evolve, it is important to ensure that strategies used in ED settings also are implemented for patients already admitted to the hospital. This study seeks to define the quality of care in VHA hospitals between patients having an in-hospital ischemic stroke compared with those presenting to the ED.
Methods
As a secondary analysis, we examined stroke care quality data from an 11-site VHA stroke quality improvement study.15 Sites participating in this study were high stroke volume VHA hospitals from various geographic regions of the US. This study collected data on ICD-9 discharge diagnosis-defined ischemic stroke admissions between January 2009 and June 2012. Patient charts were reviewed by a group of central, trained abstractors who collected information on patient demographics, clinical history, and stroke characteristics. Stroke severity was defined using the National Institutes of Health Stroke Scale (NIHSS), assessed by standardized retrospective review of admission physical examination documentation.16 A multidisciplinary team defined 11 stroke quality indicators (QIs; the 8 Joint Commission indictors and 3 additional indicators: smoking cessation and dysphagia screening, and NIHSS assessment), and the chart abstractors’ data were used to evaluate eligibility and passing rates for each QI.
For our analysis, patients were stratified into 2 categories: patients admitted to the hospital for another diagnosis who developed an IHS, and patients presenting with stroke to the ED. We excluded patients transferred from other facilities. We then compared the demographic and clinical features of the 2 groups as well as eligibility and passing rates for each of the 11 QIs. Patients were recorded as eligible if they did not have any clinical contraindication to receiving the assessment or intervention measured by the quality metric. Passing rates were defined by the presence of clear documentation in the patient record that the quality metric was met or fulfilled. Comparisons were made using nonparametric Mann-Whitney U tests and chi-square tests. All tests were performed at α .05 level.
Results
A total of 1823 patients were included in this analysis: 35 IHS and 1788 ED strokes. The 2 groups did not differ with respect to age, race, or sex (Table 1). Patients with IHS had higher stroke severity (mean NIHSS 11.3 vs 5.1, P <.01) and longer length of stay than did ED patients with stroke (mean 12.8 vs 7.3 days, P < .01). Patients with IHS also were less likely to be discharged home when compared with ED patients with stroke (34.3% vs 63.8%, P < .01).
Table 2 summarizes our findings on eligibility and passing rates for the 11 QIs. For acute care metrics, we found that stroke severity documentation rates did not differ but were low for each patient group (51% vs 48%, P = .07). Patients with IHS were more likely to be eligible for IV tissue plasminogen activator (tPA; P < .01) although utilization rates did not differ. Only 2% of ED patients met eligibility criteria to receive tPA (36 of 1788), and among these patients only 16 actually received the drug. By comparison, 5 of 6 of eligible patients with IHS received tPA. Rates of dysphagia screening also were low for both groups, and patients with IHS were less likely to receive this screen prior to initiation of oral intake than were ED patients with stroke (27% vs 50%, P = .01).
Beyond the acute period, we found that patients with IHS were less likely than were ED patients with stroke to be eligible to receive antithrombotic therapy by 2 days after their initial stroke evaluation (74% vs 96%, P < .01), although treatment rates were similar between the 2 groups (P = .99). In patients with documented atrial fibrillation, initiation of anticoagulation therapy also did not differ (P = .99). The 2 groups were similar with respect to initiation of venous thromboembolism (VTE) prophylaxis (P = .596) and evaluation for rehabilitation needs (P = .42). Although rates of smoking cessation counseling and stroke education prior to discharge did not differ, overall rates of stroke education were very low for both groups (25% vs 36%, P = .55).
Similar to initiation of antithrombotic therapy in the hospital, we found lower rates of eligibility to receive antithrombotic therapy on discharge in the IHS group when compared with the ED group (77% vs 93%, P = .04). However, actual treatment initiation rates did not differ (P = .12). Use of lipid-lowering agents was similar for the 2 groups (P = .12).
Discussion
Our study found that veterans who develop an IHS received similar quality of care as did those presenting to the ED with stroke symptoms for many QIs, although there were some notable differences. We were pleased to find that overall rates of secondary stroke prevention initiation (antithrombotic and statin therapy), VTE prophylaxis, rehabilitation evaluations, and smoking cessation counseling were high for both groups, in keeping with evidence-based guidelines.17 This likely reflected the fact that these metrics typically involve care outside of the acute period and are less likely to be influenced by the location of initial stroke evaluation. Furthermore, efforts to improve smoking cessation and VTE prophylaxis are not exclusive to stroke care and have been the target of several nonstroke quality projects in the VHA. Many aspects of acute stroke care did differ, and present opportunities for quality improvement in the future.
In our sample, patients with IHS had higher IV thrombolytic eligibility, which has not typically been reported in other samples.10,11,18 In these studies, hospitalized patients have been reported to more often have contraindications to tPA, such as recent surgery or lack of stroke symptom recognition due to delirium or medication effects. Interestingly, patients presenting to VHA EDs had extremely low rates of tPA eligibility (2%), which is lower than many reported estimates of tPA eligibility outside of the VHA.19,20 This may be due to multiple influences, such as geographic barriers, patient perceptions about stroke symptoms, access to emergency medical services (EMS), EMS routing patterns, and social/cultural factors. Although not statistically significant due to small sample size, tPA use also was twice as high in the IHS group.
Given that a significant proportion of patients with IHS in the VHA system may be eligible for acute thrombolysis, our findings highlight the need for acute stroke protocols to ensure that patients with IHS receive the same rapid stroke assessment and access to thrombolytics as do patients evaluated in the ED. Further investigation is needed to determine whether there are unique features of patients with IHS in VHA hospitals, which may make them more eligible for IV thrombolysis.
Dysphagia is associated with increased risks for aspiration pneumonia in stroke patients.21 We found that patients with IHS were less likely to receive dysphagia screening compared with that of stroke patients admitted through the ED. This finding is consistent with the fact that care for patients with IHS is less frequently guided by specific stroke care protocols and algorithms that are more often used in EDs.8,11 Although attention to swallowing function may lead to improved outcomes in stroke, this can be easily overlooked in patients with IHS.22 However, low dysphagia screening also was found in patients admitted through the ED, suggesting that low screening rates cannot be solely explained by differences in where the initial stroke evaluation is occurring. These findings suggest a need for novel approaches to dysphagia screening in VHA stroke patients that can be universally implemented throughout the hospital.
Finally, we also found very low rates of stroke education prior to discharge for both groups. Given the risk of stroke recurrence and the overall poor level of public knowledge about stroke, providing patients with stroke with formal oral and written information on stroke is a critical component of secondary prevention.23,24 Educational tools, including those that are veteran specific, are now available for use in VHA hospitals and should be incorporated into quality improvement strategies for stroke care in VHA hospitals.
In 2012, the VHA Acute Stroke Treatment Directive was published in an effort to improve stroke care systemwide. Several of the metrics examined in this study are addressed in this directive. The data presented in this study is one of the only samples of stroke quality metrics within the VHA that largely predates the directive and can serve as a baseline comparator for future work examining stroke care after release of the directive. At present, although continuous internal reviews of quality data are ongoing, longitudinal description of stroke care quality since publication of the directive will help to inform future efforts to improve stroke care for veterans.
Limitations
Despite the strength of being a multicenter sampling of stroke care in high volume VHA hospitals, our study had several limitations. The IHS sample size was small, which limited our ability to evaluate differences between the groups, to evaluate generalizability, and account for estimation error.13 It is possible that differences existed between the groups that could not be observed in this sample due to small size (type II error) or that patient-specific characteristics not captured by these data could influence these metrics. Assessments of eligibility and passing were based on retrospective chart review and post hoc coding. Our sample assessed only patients who presented to larger VHA hospitals with higher stroke volumes, thus these findings may not be generalizable to smaller VHA hospitals with less systematized stroke care. This sample did not describe the specialty care services that were received by each patient, which may have influenced their stroke care. Finally, this study is an analysis of use of QIs in stroke care and did not examine how these indicators affect outcomes.
Conclusion
Despite reassuring findings for several inpatient ischemic stroke quality metrics, we found several differences in stroke care between patients with IHS compared with those presenting to the ED, emphasizing the need for standardized approaches to stroke care regardless of care setting. Although patients with IHS may be more likely to be eligible for tPA, these patients received dysphagia screening and less often than did ED patients with stroke. Ongoing quality initiatives should continue to place emphasis on improving all quality metrics (particularly dysphagia screening, stroke severity documentation, and stroke education) for patients with stroke at VHA hospitals across all care settings. Future work will be needed to examine how specific patient characteristics and revisions to stroke protocols may affect stroke quality metrics and outcomes between patients with IHS and those presenting to the ED.
Acknowledgments
The authors would like to thank Danielle Sager for her contributions to this project.
1. Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129:e28-e292.
2. Schwamm LH, Ali SF, Reeves MJ, et al. Temporal trends in patient characteristics and treatment with intravenous thrombolysis among acute ischemic stroke patients at Get With the Guidelines—Stroke hospitals. Circ Cardiovasc Qual Outcomes. 2013;6(5):543-549.
3. Reeves MJ, Parker C, Fonarow GC, Smith EE, Schwamm LH. Development of stroke performance measures: definitions, methods, and current measures. Stroke. 2010;41(7):1573-1578.
4. The Joint Commission. Certificate of distinction for primary stroke centers. https://www.jointcommission.org/certificate_of_distinction_for_primary_stroke_centers_/.Published April 30, 2012. Accessed July 9, 2019.
5. US Department of Veterans Affairs. Center highlight: acute ischemic stroke care for veterans. https://www.queri.research.va.gov/center_highlights/stroke.cfm. Updated February 20, 2014. Accessed July 16, 2019.
6. Chumbler NR, Jia H, Phipps MS, et al. Does inpatient quality of care differ by age among US veterans with ischemic stroke? J Stroke Cerebrovasc Dis. 2012;21(8):844-851.
7. Katzan IL, Spertus J, Bettger JP, et al; American Heart Association Stroke Council; Council on Quality of Care and Outcomes Research; Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Surgery and Anesthesia; Council on Clinical Cardiology. Risk adjustment of ischemic stroke outcomes for comparing hospital performance: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(3):918-944.
8. Cumbler E, Wald H, Bhatt DL, et al. Quality of care and outcomes for in-hospital ischemic stroke: findings from the National Get With the Guidelines—Stroke. Stroke. 2014;45(1):231-238.
9. Blacker DJ. In-hospital stroke. Lancet Neurol. 2003;2(12):741-746.
10. Farooq MU, Reeves MJ, Gargano J, Wehner S, Hickenbottom S, Majid A; Paul Coverdell National Acute Stroke Registry Michigan Prototype Investigators. In-hospital stroke in a statewide stroke registry. Cerebrovascular Dis. 2008;25(1-2):12-20.
11. Bhalla A, Smeeton N, Rudd AG, Heuschmann P, Wolfe CD. A comparison of characteristics and resource use between in-hospital and admitted patients with stroke. J Stroke Cerebrovasc Dis. 2010;19:(5)357-363.
12. Garcia-Santibanez R, Liang J, Walker A, Matos-Diaz I, Kahkeshani K, Boniece I. Comparison of stroke codes in the emergency room and inpatient setting. J Stroke Cerebrovasc Dis. 2015;24(8):1948-1950.
13. Arling G, Reeves M, Ross J, et al. Estimating and reporting on the quality of inpatient stroke care by Veterans Health Administration medical centers. Circ Cardiovasc Qual Outcomes. 2012;5(1):44-51.
14. US Department of Veterans Affairs. Treatment of Acute Ischemic Stroke (AIS). VHA Directive 2011-038. https://www.hsrd.research.va.gov/news/feature/stroke.cfm. Updated January 20, 2014. Accessed July 17, 2019.
15. Williams LS, Daggett V, Slaven J, et al. Abstract 18: Does quality improvement training add to audit and feedback for inpatient stroke care processes? [International Stroke Conference abstract 18] Stroke. 2014;45(suppl 1):A18.
16. Williams LS, Yilmaz EY, Lopez-Yunez AM. Retrospective assessment of initial stroke severity with the NIH Stroke Scale. Stroke. 2000;31(4):858-862.
17. Jauch EC, Saver JL, Adams HP Jr, et al; American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870-947.
18. Park HJ, Cho HJ, Kim YD, et al. Comparison of the characteristics for in-hospital and out-of-hospital ischaemic strokes. Eur J Neurol. 2009;16(5):582-588.
19. Messé SR, Fonarow GC, Smith EE, et al. Use of tissue-type plasminogen activator before and after publication of the European Cooperative Acute Stroke Study III in Get With the Guidelines-Stroke. Circ Cardiovasc Qual Outcomes. 2012;5(3):321-326.
20. Allen NB, Kaltenbach L, Goldstein LB, et al. Regional variation in recommended treatments for ischemic stroke and TIA: Get With the Guidelines—Stroke 2003-2010. Stroke. 2012;43(7):1858-1864.
21. Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke. 2005;36(12):2756-2763.
22. Bravata DM, Wells CK, Lo AC, et al. Processes of care associated with acute stroke outcomes. Arch Intern Med. 2010;170(9):804-810.
23. Mosley I, Nicol M, Donnan G, Patrick I, Dewey H. Stroke symptoms and the decision to call for an ambulance. Stroke; a journal of cerebral circulation. 2007;38(2):361-366.
24. Jurkowski JM, Maniccia DM, Dennison BA, Samuels SJ, Spicer DA. Awareness of necessity to call 9-1-1 for stroke symptoms, upstate New York. Prev Chronic Dis. 2008;5(2):A41.
Stroke is a leading cause of death and long-term disability in the US.1 Quality improvement efforts for acute stroke care delivery have successfully led to increased rates of thrombolytic utilization.2 Increasing attention is now being paid to additional quality metrics for stroke care, including hospital management and initiation of appropriate secondary stroke prevention measures at discharge. Many organizations, including the Veterans Health Administration (VHA), use these measures to monitor health care quality and certify centers that are committed to excellence in stroke care.3-6 It is anticipated that collection, evaluation, and feedback from these data may lead to improvements in outcomes after stroke.7
Patients who experience onset of stroke symptoms while already admitted to a hospital may be uniquely suited for quality improvement strategies. In-hospital strokes (IHS) are not uncommon and have been associated with higher stroke severity and increased mortality compared with patients with stroke symptoms prior to arriving at the emergency department (ED).8-10 A potential reason for the higher observed mortality is that patients with IHS may have poorer access to acute stroke resources, such as stroke teams and neuroimaging, as well as increased rates of medical comorbidities.9,11,12 Furthermore, stroke management protocols are typically created based on ED resources, which may not be equivalent to resources available on inpatient settings.
Although many studies have examined clinical characteristics of patients with IHS, few studies have looked at the quality of stroke care for IHS. Information on stroke quality data is even more limited in VHA hospitals due to the small number of admitted patients with stroke.13 VHA released a directive on Acute Stroke Treatment (Directive 2011-03) in 2011 with a recent update in 2018, which aimed to implement quality improvement strategies for stroke care in VHA hospitals.14 Although focusing primarily on acute stroke care in the ED, this directive has led to increased awareness of areas for improvement, particularly among larger VHA hospitals. Prior to this directive, although national stroke guidelines were well-defined, more variability likely existed in stroke protocols and the manner in which stroke care was delivered across care settings. As efforts to measure and improve stroke care evolve, it is important to ensure that strategies used in ED settings also are implemented for patients already admitted to the hospital. This study seeks to define the quality of care in VHA hospitals between patients having an in-hospital ischemic stroke compared with those presenting to the ED.
Methods
As a secondary analysis, we examined stroke care quality data from an 11-site VHA stroke quality improvement study.15 Sites participating in this study were high stroke volume VHA hospitals from various geographic regions of the US. This study collected data on ICD-9 discharge diagnosis-defined ischemic stroke admissions between January 2009 and June 2012. Patient charts were reviewed by a group of central, trained abstractors who collected information on patient demographics, clinical history, and stroke characteristics. Stroke severity was defined using the National Institutes of Health Stroke Scale (NIHSS), assessed by standardized retrospective review of admission physical examination documentation.16 A multidisciplinary team defined 11 stroke quality indicators (QIs; the 8 Joint Commission indictors and 3 additional indicators: smoking cessation and dysphagia screening, and NIHSS assessment), and the chart abstractors’ data were used to evaluate eligibility and passing rates for each QI.
For our analysis, patients were stratified into 2 categories: patients admitted to the hospital for another diagnosis who developed an IHS, and patients presenting with stroke to the ED. We excluded patients transferred from other facilities. We then compared the demographic and clinical features of the 2 groups as well as eligibility and passing rates for each of the 11 QIs. Patients were recorded as eligible if they did not have any clinical contraindication to receiving the assessment or intervention measured by the quality metric. Passing rates were defined by the presence of clear documentation in the patient record that the quality metric was met or fulfilled. Comparisons were made using nonparametric Mann-Whitney U tests and chi-square tests. All tests were performed at α .05 level.
Results
A total of 1823 patients were included in this analysis: 35 IHS and 1788 ED strokes. The 2 groups did not differ with respect to age, race, or sex (Table 1). Patients with IHS had higher stroke severity (mean NIHSS 11.3 vs 5.1, P <.01) and longer length of stay than did ED patients with stroke (mean 12.8 vs 7.3 days, P < .01). Patients with IHS also were less likely to be discharged home when compared with ED patients with stroke (34.3% vs 63.8%, P < .01).
Table 2 summarizes our findings on eligibility and passing rates for the 11 QIs. For acute care metrics, we found that stroke severity documentation rates did not differ but were low for each patient group (51% vs 48%, P = .07). Patients with IHS were more likely to be eligible for IV tissue plasminogen activator (tPA; P < .01) although utilization rates did not differ. Only 2% of ED patients met eligibility criteria to receive tPA (36 of 1788), and among these patients only 16 actually received the drug. By comparison, 5 of 6 of eligible patients with IHS received tPA. Rates of dysphagia screening also were low for both groups, and patients with IHS were less likely to receive this screen prior to initiation of oral intake than were ED patients with stroke (27% vs 50%, P = .01).
Beyond the acute period, we found that patients with IHS were less likely than were ED patients with stroke to be eligible to receive antithrombotic therapy by 2 days after their initial stroke evaluation (74% vs 96%, P < .01), although treatment rates were similar between the 2 groups (P = .99). In patients with documented atrial fibrillation, initiation of anticoagulation therapy also did not differ (P = .99). The 2 groups were similar with respect to initiation of venous thromboembolism (VTE) prophylaxis (P = .596) and evaluation for rehabilitation needs (P = .42). Although rates of smoking cessation counseling and stroke education prior to discharge did not differ, overall rates of stroke education were very low for both groups (25% vs 36%, P = .55).
Similar to initiation of antithrombotic therapy in the hospital, we found lower rates of eligibility to receive antithrombotic therapy on discharge in the IHS group when compared with the ED group (77% vs 93%, P = .04). However, actual treatment initiation rates did not differ (P = .12). Use of lipid-lowering agents was similar for the 2 groups (P = .12).
Discussion
Our study found that veterans who develop an IHS received similar quality of care as did those presenting to the ED with stroke symptoms for many QIs, although there were some notable differences. We were pleased to find that overall rates of secondary stroke prevention initiation (antithrombotic and statin therapy), VTE prophylaxis, rehabilitation evaluations, and smoking cessation counseling were high for both groups, in keeping with evidence-based guidelines.17 This likely reflected the fact that these metrics typically involve care outside of the acute period and are less likely to be influenced by the location of initial stroke evaluation. Furthermore, efforts to improve smoking cessation and VTE prophylaxis are not exclusive to stroke care and have been the target of several nonstroke quality projects in the VHA. Many aspects of acute stroke care did differ, and present opportunities for quality improvement in the future.
In our sample, patients with IHS had higher IV thrombolytic eligibility, which has not typically been reported in other samples.10,11,18 In these studies, hospitalized patients have been reported to more often have contraindications to tPA, such as recent surgery or lack of stroke symptom recognition due to delirium or medication effects. Interestingly, patients presenting to VHA EDs had extremely low rates of tPA eligibility (2%), which is lower than many reported estimates of tPA eligibility outside of the VHA.19,20 This may be due to multiple influences, such as geographic barriers, patient perceptions about stroke symptoms, access to emergency medical services (EMS), EMS routing patterns, and social/cultural factors. Although not statistically significant due to small sample size, tPA use also was twice as high in the IHS group.
Given that a significant proportion of patients with IHS in the VHA system may be eligible for acute thrombolysis, our findings highlight the need for acute stroke protocols to ensure that patients with IHS receive the same rapid stroke assessment and access to thrombolytics as do patients evaluated in the ED. Further investigation is needed to determine whether there are unique features of patients with IHS in VHA hospitals, which may make them more eligible for IV thrombolysis.
Dysphagia is associated with increased risks for aspiration pneumonia in stroke patients.21 We found that patients with IHS were less likely to receive dysphagia screening compared with that of stroke patients admitted through the ED. This finding is consistent with the fact that care for patients with IHS is less frequently guided by specific stroke care protocols and algorithms that are more often used in EDs.8,11 Although attention to swallowing function may lead to improved outcomes in stroke, this can be easily overlooked in patients with IHS.22 However, low dysphagia screening also was found in patients admitted through the ED, suggesting that low screening rates cannot be solely explained by differences in where the initial stroke evaluation is occurring. These findings suggest a need for novel approaches to dysphagia screening in VHA stroke patients that can be universally implemented throughout the hospital.
Finally, we also found very low rates of stroke education prior to discharge for both groups. Given the risk of stroke recurrence and the overall poor level of public knowledge about stroke, providing patients with stroke with formal oral and written information on stroke is a critical component of secondary prevention.23,24 Educational tools, including those that are veteran specific, are now available for use in VHA hospitals and should be incorporated into quality improvement strategies for stroke care in VHA hospitals.
In 2012, the VHA Acute Stroke Treatment Directive was published in an effort to improve stroke care systemwide. Several of the metrics examined in this study are addressed in this directive. The data presented in this study is one of the only samples of stroke quality metrics within the VHA that largely predates the directive and can serve as a baseline comparator for future work examining stroke care after release of the directive. At present, although continuous internal reviews of quality data are ongoing, longitudinal description of stroke care quality since publication of the directive will help to inform future efforts to improve stroke care for veterans.
Limitations
Despite the strength of being a multicenter sampling of stroke care in high volume VHA hospitals, our study had several limitations. The IHS sample size was small, which limited our ability to evaluate differences between the groups, to evaluate generalizability, and account for estimation error.13 It is possible that differences existed between the groups that could not be observed in this sample due to small size (type II error) or that patient-specific characteristics not captured by these data could influence these metrics. Assessments of eligibility and passing were based on retrospective chart review and post hoc coding. Our sample assessed only patients who presented to larger VHA hospitals with higher stroke volumes, thus these findings may not be generalizable to smaller VHA hospitals with less systematized stroke care. This sample did not describe the specialty care services that were received by each patient, which may have influenced their stroke care. Finally, this study is an analysis of use of QIs in stroke care and did not examine how these indicators affect outcomes.
Conclusion
Despite reassuring findings for several inpatient ischemic stroke quality metrics, we found several differences in stroke care between patients with IHS compared with those presenting to the ED, emphasizing the need for standardized approaches to stroke care regardless of care setting. Although patients with IHS may be more likely to be eligible for tPA, these patients received dysphagia screening and less often than did ED patients with stroke. Ongoing quality initiatives should continue to place emphasis on improving all quality metrics (particularly dysphagia screening, stroke severity documentation, and stroke education) for patients with stroke at VHA hospitals across all care settings. Future work will be needed to examine how specific patient characteristics and revisions to stroke protocols may affect stroke quality metrics and outcomes between patients with IHS and those presenting to the ED.
Acknowledgments
The authors would like to thank Danielle Sager for her contributions to this project.
Stroke is a leading cause of death and long-term disability in the US.1 Quality improvement efforts for acute stroke care delivery have successfully led to increased rates of thrombolytic utilization.2 Increasing attention is now being paid to additional quality metrics for stroke care, including hospital management and initiation of appropriate secondary stroke prevention measures at discharge. Many organizations, including the Veterans Health Administration (VHA), use these measures to monitor health care quality and certify centers that are committed to excellence in stroke care.3-6 It is anticipated that collection, evaluation, and feedback from these data may lead to improvements in outcomes after stroke.7
Patients who experience onset of stroke symptoms while already admitted to a hospital may be uniquely suited for quality improvement strategies. In-hospital strokes (IHS) are not uncommon and have been associated with higher stroke severity and increased mortality compared with patients with stroke symptoms prior to arriving at the emergency department (ED).8-10 A potential reason for the higher observed mortality is that patients with IHS may have poorer access to acute stroke resources, such as stroke teams and neuroimaging, as well as increased rates of medical comorbidities.9,11,12 Furthermore, stroke management protocols are typically created based on ED resources, which may not be equivalent to resources available on inpatient settings.
Although many studies have examined clinical characteristics of patients with IHS, few studies have looked at the quality of stroke care for IHS. Information on stroke quality data is even more limited in VHA hospitals due to the small number of admitted patients with stroke.13 VHA released a directive on Acute Stroke Treatment (Directive 2011-03) in 2011 with a recent update in 2018, which aimed to implement quality improvement strategies for stroke care in VHA hospitals.14 Although focusing primarily on acute stroke care in the ED, this directive has led to increased awareness of areas for improvement, particularly among larger VHA hospitals. Prior to this directive, although national stroke guidelines were well-defined, more variability likely existed in stroke protocols and the manner in which stroke care was delivered across care settings. As efforts to measure and improve stroke care evolve, it is important to ensure that strategies used in ED settings also are implemented for patients already admitted to the hospital. This study seeks to define the quality of care in VHA hospitals between patients having an in-hospital ischemic stroke compared with those presenting to the ED.
Methods
As a secondary analysis, we examined stroke care quality data from an 11-site VHA stroke quality improvement study.15 Sites participating in this study were high stroke volume VHA hospitals from various geographic regions of the US. This study collected data on ICD-9 discharge diagnosis-defined ischemic stroke admissions between January 2009 and June 2012. Patient charts were reviewed by a group of central, trained abstractors who collected information on patient demographics, clinical history, and stroke characteristics. Stroke severity was defined using the National Institutes of Health Stroke Scale (NIHSS), assessed by standardized retrospective review of admission physical examination documentation.16 A multidisciplinary team defined 11 stroke quality indicators (QIs; the 8 Joint Commission indictors and 3 additional indicators: smoking cessation and dysphagia screening, and NIHSS assessment), and the chart abstractors’ data were used to evaluate eligibility and passing rates for each QI.
For our analysis, patients were stratified into 2 categories: patients admitted to the hospital for another diagnosis who developed an IHS, and patients presenting with stroke to the ED. We excluded patients transferred from other facilities. We then compared the demographic and clinical features of the 2 groups as well as eligibility and passing rates for each of the 11 QIs. Patients were recorded as eligible if they did not have any clinical contraindication to receiving the assessment or intervention measured by the quality metric. Passing rates were defined by the presence of clear documentation in the patient record that the quality metric was met or fulfilled. Comparisons were made using nonparametric Mann-Whitney U tests and chi-square tests. All tests were performed at α .05 level.
Results
A total of 1823 patients were included in this analysis: 35 IHS and 1788 ED strokes. The 2 groups did not differ with respect to age, race, or sex (Table 1). Patients with IHS had higher stroke severity (mean NIHSS 11.3 vs 5.1, P <.01) and longer length of stay than did ED patients with stroke (mean 12.8 vs 7.3 days, P < .01). Patients with IHS also were less likely to be discharged home when compared with ED patients with stroke (34.3% vs 63.8%, P < .01).
Table 2 summarizes our findings on eligibility and passing rates for the 11 QIs. For acute care metrics, we found that stroke severity documentation rates did not differ but were low for each patient group (51% vs 48%, P = .07). Patients with IHS were more likely to be eligible for IV tissue plasminogen activator (tPA; P < .01) although utilization rates did not differ. Only 2% of ED patients met eligibility criteria to receive tPA (36 of 1788), and among these patients only 16 actually received the drug. By comparison, 5 of 6 of eligible patients with IHS received tPA. Rates of dysphagia screening also were low for both groups, and patients with IHS were less likely to receive this screen prior to initiation of oral intake than were ED patients with stroke (27% vs 50%, P = .01).
Beyond the acute period, we found that patients with IHS were less likely than were ED patients with stroke to be eligible to receive antithrombotic therapy by 2 days after their initial stroke evaluation (74% vs 96%, P < .01), although treatment rates were similar between the 2 groups (P = .99). In patients with documented atrial fibrillation, initiation of anticoagulation therapy also did not differ (P = .99). The 2 groups were similar with respect to initiation of venous thromboembolism (VTE) prophylaxis (P = .596) and evaluation for rehabilitation needs (P = .42). Although rates of smoking cessation counseling and stroke education prior to discharge did not differ, overall rates of stroke education were very low for both groups (25% vs 36%, P = .55).
Similar to initiation of antithrombotic therapy in the hospital, we found lower rates of eligibility to receive antithrombotic therapy on discharge in the IHS group when compared with the ED group (77% vs 93%, P = .04). However, actual treatment initiation rates did not differ (P = .12). Use of lipid-lowering agents was similar for the 2 groups (P = .12).
Discussion
Our study found that veterans who develop an IHS received similar quality of care as did those presenting to the ED with stroke symptoms for many QIs, although there were some notable differences. We were pleased to find that overall rates of secondary stroke prevention initiation (antithrombotic and statin therapy), VTE prophylaxis, rehabilitation evaluations, and smoking cessation counseling were high for both groups, in keeping with evidence-based guidelines.17 This likely reflected the fact that these metrics typically involve care outside of the acute period and are less likely to be influenced by the location of initial stroke evaluation. Furthermore, efforts to improve smoking cessation and VTE prophylaxis are not exclusive to stroke care and have been the target of several nonstroke quality projects in the VHA. Many aspects of acute stroke care did differ, and present opportunities for quality improvement in the future.
In our sample, patients with IHS had higher IV thrombolytic eligibility, which has not typically been reported in other samples.10,11,18 In these studies, hospitalized patients have been reported to more often have contraindications to tPA, such as recent surgery or lack of stroke symptom recognition due to delirium or medication effects. Interestingly, patients presenting to VHA EDs had extremely low rates of tPA eligibility (2%), which is lower than many reported estimates of tPA eligibility outside of the VHA.19,20 This may be due to multiple influences, such as geographic barriers, patient perceptions about stroke symptoms, access to emergency medical services (EMS), EMS routing patterns, and social/cultural factors. Although not statistically significant due to small sample size, tPA use also was twice as high in the IHS group.
Given that a significant proportion of patients with IHS in the VHA system may be eligible for acute thrombolysis, our findings highlight the need for acute stroke protocols to ensure that patients with IHS receive the same rapid stroke assessment and access to thrombolytics as do patients evaluated in the ED. Further investigation is needed to determine whether there are unique features of patients with IHS in VHA hospitals, which may make them more eligible for IV thrombolysis.
Dysphagia is associated with increased risks for aspiration pneumonia in stroke patients.21 We found that patients with IHS were less likely to receive dysphagia screening compared with that of stroke patients admitted through the ED. This finding is consistent with the fact that care for patients with IHS is less frequently guided by specific stroke care protocols and algorithms that are more often used in EDs.8,11 Although attention to swallowing function may lead to improved outcomes in stroke, this can be easily overlooked in patients with IHS.22 However, low dysphagia screening also was found in patients admitted through the ED, suggesting that low screening rates cannot be solely explained by differences in where the initial stroke evaluation is occurring. These findings suggest a need for novel approaches to dysphagia screening in VHA stroke patients that can be universally implemented throughout the hospital.
Finally, we also found very low rates of stroke education prior to discharge for both groups. Given the risk of stroke recurrence and the overall poor level of public knowledge about stroke, providing patients with stroke with formal oral and written information on stroke is a critical component of secondary prevention.23,24 Educational tools, including those that are veteran specific, are now available for use in VHA hospitals and should be incorporated into quality improvement strategies for stroke care in VHA hospitals.
In 2012, the VHA Acute Stroke Treatment Directive was published in an effort to improve stroke care systemwide. Several of the metrics examined in this study are addressed in this directive. The data presented in this study is one of the only samples of stroke quality metrics within the VHA that largely predates the directive and can serve as a baseline comparator for future work examining stroke care after release of the directive. At present, although continuous internal reviews of quality data are ongoing, longitudinal description of stroke care quality since publication of the directive will help to inform future efforts to improve stroke care for veterans.
Limitations
Despite the strength of being a multicenter sampling of stroke care in high volume VHA hospitals, our study had several limitations. The IHS sample size was small, which limited our ability to evaluate differences between the groups, to evaluate generalizability, and account for estimation error.13 It is possible that differences existed between the groups that could not be observed in this sample due to small size (type II error) or that patient-specific characteristics not captured by these data could influence these metrics. Assessments of eligibility and passing were based on retrospective chart review and post hoc coding. Our sample assessed only patients who presented to larger VHA hospitals with higher stroke volumes, thus these findings may not be generalizable to smaller VHA hospitals with less systematized stroke care. This sample did not describe the specialty care services that were received by each patient, which may have influenced their stroke care. Finally, this study is an analysis of use of QIs in stroke care and did not examine how these indicators affect outcomes.
Conclusion
Despite reassuring findings for several inpatient ischemic stroke quality metrics, we found several differences in stroke care between patients with IHS compared with those presenting to the ED, emphasizing the need for standardized approaches to stroke care regardless of care setting. Although patients with IHS may be more likely to be eligible for tPA, these patients received dysphagia screening and less often than did ED patients with stroke. Ongoing quality initiatives should continue to place emphasis on improving all quality metrics (particularly dysphagia screening, stroke severity documentation, and stroke education) for patients with stroke at VHA hospitals across all care settings. Future work will be needed to examine how specific patient characteristics and revisions to stroke protocols may affect stroke quality metrics and outcomes between patients with IHS and those presenting to the ED.
Acknowledgments
The authors would like to thank Danielle Sager for her contributions to this project.
1. Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129:e28-e292.
2. Schwamm LH, Ali SF, Reeves MJ, et al. Temporal trends in patient characteristics and treatment with intravenous thrombolysis among acute ischemic stroke patients at Get With the Guidelines—Stroke hospitals. Circ Cardiovasc Qual Outcomes. 2013;6(5):543-549.
3. Reeves MJ, Parker C, Fonarow GC, Smith EE, Schwamm LH. Development of stroke performance measures: definitions, methods, and current measures. Stroke. 2010;41(7):1573-1578.
4. The Joint Commission. Certificate of distinction for primary stroke centers. https://www.jointcommission.org/certificate_of_distinction_for_primary_stroke_centers_/.Published April 30, 2012. Accessed July 9, 2019.
5. US Department of Veterans Affairs. Center highlight: acute ischemic stroke care for veterans. https://www.queri.research.va.gov/center_highlights/stroke.cfm. Updated February 20, 2014. Accessed July 16, 2019.
6. Chumbler NR, Jia H, Phipps MS, et al. Does inpatient quality of care differ by age among US veterans with ischemic stroke? J Stroke Cerebrovasc Dis. 2012;21(8):844-851.
7. Katzan IL, Spertus J, Bettger JP, et al; American Heart Association Stroke Council; Council on Quality of Care and Outcomes Research; Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Surgery and Anesthesia; Council on Clinical Cardiology. Risk adjustment of ischemic stroke outcomes for comparing hospital performance: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(3):918-944.
8. Cumbler E, Wald H, Bhatt DL, et al. Quality of care and outcomes for in-hospital ischemic stroke: findings from the National Get With the Guidelines—Stroke. Stroke. 2014;45(1):231-238.
9. Blacker DJ. In-hospital stroke. Lancet Neurol. 2003;2(12):741-746.
10. Farooq MU, Reeves MJ, Gargano J, Wehner S, Hickenbottom S, Majid A; Paul Coverdell National Acute Stroke Registry Michigan Prototype Investigators. In-hospital stroke in a statewide stroke registry. Cerebrovascular Dis. 2008;25(1-2):12-20.
11. Bhalla A, Smeeton N, Rudd AG, Heuschmann P, Wolfe CD. A comparison of characteristics and resource use between in-hospital and admitted patients with stroke. J Stroke Cerebrovasc Dis. 2010;19:(5)357-363.
12. Garcia-Santibanez R, Liang J, Walker A, Matos-Diaz I, Kahkeshani K, Boniece I. Comparison of stroke codes in the emergency room and inpatient setting. J Stroke Cerebrovasc Dis. 2015;24(8):1948-1950.
13. Arling G, Reeves M, Ross J, et al. Estimating and reporting on the quality of inpatient stroke care by Veterans Health Administration medical centers. Circ Cardiovasc Qual Outcomes. 2012;5(1):44-51.
14. US Department of Veterans Affairs. Treatment of Acute Ischemic Stroke (AIS). VHA Directive 2011-038. https://www.hsrd.research.va.gov/news/feature/stroke.cfm. Updated January 20, 2014. Accessed July 17, 2019.
15. Williams LS, Daggett V, Slaven J, et al. Abstract 18: Does quality improvement training add to audit and feedback for inpatient stroke care processes? [International Stroke Conference abstract 18] Stroke. 2014;45(suppl 1):A18.
16. Williams LS, Yilmaz EY, Lopez-Yunez AM. Retrospective assessment of initial stroke severity with the NIH Stroke Scale. Stroke. 2000;31(4):858-862.
17. Jauch EC, Saver JL, Adams HP Jr, et al; American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870-947.
18. Park HJ, Cho HJ, Kim YD, et al. Comparison of the characteristics for in-hospital and out-of-hospital ischaemic strokes. Eur J Neurol. 2009;16(5):582-588.
19. Messé SR, Fonarow GC, Smith EE, et al. Use of tissue-type plasminogen activator before and after publication of the European Cooperative Acute Stroke Study III in Get With the Guidelines-Stroke. Circ Cardiovasc Qual Outcomes. 2012;5(3):321-326.
20. Allen NB, Kaltenbach L, Goldstein LB, et al. Regional variation in recommended treatments for ischemic stroke and TIA: Get With the Guidelines—Stroke 2003-2010. Stroke. 2012;43(7):1858-1864.
21. Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke. 2005;36(12):2756-2763.
22. Bravata DM, Wells CK, Lo AC, et al. Processes of care associated with acute stroke outcomes. Arch Intern Med. 2010;170(9):804-810.
23. Mosley I, Nicol M, Donnan G, Patrick I, Dewey H. Stroke symptoms and the decision to call for an ambulance. Stroke; a journal of cerebral circulation. 2007;38(2):361-366.
24. Jurkowski JM, Maniccia DM, Dennison BA, Samuels SJ, Spicer DA. Awareness of necessity to call 9-1-1 for stroke symptoms, upstate New York. Prev Chronic Dis. 2008;5(2):A41.
1. Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129:e28-e292.
2. Schwamm LH, Ali SF, Reeves MJ, et al. Temporal trends in patient characteristics and treatment with intravenous thrombolysis among acute ischemic stroke patients at Get With the Guidelines—Stroke hospitals. Circ Cardiovasc Qual Outcomes. 2013;6(5):543-549.
3. Reeves MJ, Parker C, Fonarow GC, Smith EE, Schwamm LH. Development of stroke performance measures: definitions, methods, and current measures. Stroke. 2010;41(7):1573-1578.
4. The Joint Commission. Certificate of distinction for primary stroke centers. https://www.jointcommission.org/certificate_of_distinction_for_primary_stroke_centers_/.Published April 30, 2012. Accessed July 9, 2019.
5. US Department of Veterans Affairs. Center highlight: acute ischemic stroke care for veterans. https://www.queri.research.va.gov/center_highlights/stroke.cfm. Updated February 20, 2014. Accessed July 16, 2019.
6. Chumbler NR, Jia H, Phipps MS, et al. Does inpatient quality of care differ by age among US veterans with ischemic stroke? J Stroke Cerebrovasc Dis. 2012;21(8):844-851.
7. Katzan IL, Spertus J, Bettger JP, et al; American Heart Association Stroke Council; Council on Quality of Care and Outcomes Research; Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Surgery and Anesthesia; Council on Clinical Cardiology. Risk adjustment of ischemic stroke outcomes for comparing hospital performance: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(3):918-944.
8. Cumbler E, Wald H, Bhatt DL, et al. Quality of care and outcomes for in-hospital ischemic stroke: findings from the National Get With the Guidelines—Stroke. Stroke. 2014;45(1):231-238.
9. Blacker DJ. In-hospital stroke. Lancet Neurol. 2003;2(12):741-746.
10. Farooq MU, Reeves MJ, Gargano J, Wehner S, Hickenbottom S, Majid A; Paul Coverdell National Acute Stroke Registry Michigan Prototype Investigators. In-hospital stroke in a statewide stroke registry. Cerebrovascular Dis. 2008;25(1-2):12-20.
11. Bhalla A, Smeeton N, Rudd AG, Heuschmann P, Wolfe CD. A comparison of characteristics and resource use between in-hospital and admitted patients with stroke. J Stroke Cerebrovasc Dis. 2010;19:(5)357-363.
12. Garcia-Santibanez R, Liang J, Walker A, Matos-Diaz I, Kahkeshani K, Boniece I. Comparison of stroke codes in the emergency room and inpatient setting. J Stroke Cerebrovasc Dis. 2015;24(8):1948-1950.
13. Arling G, Reeves M, Ross J, et al. Estimating and reporting on the quality of inpatient stroke care by Veterans Health Administration medical centers. Circ Cardiovasc Qual Outcomes. 2012;5(1):44-51.
14. US Department of Veterans Affairs. Treatment of Acute Ischemic Stroke (AIS). VHA Directive 2011-038. https://www.hsrd.research.va.gov/news/feature/stroke.cfm. Updated January 20, 2014. Accessed July 17, 2019.
15. Williams LS, Daggett V, Slaven J, et al. Abstract 18: Does quality improvement training add to audit and feedback for inpatient stroke care processes? [International Stroke Conference abstract 18] Stroke. 2014;45(suppl 1):A18.
16. Williams LS, Yilmaz EY, Lopez-Yunez AM. Retrospective assessment of initial stroke severity with the NIH Stroke Scale. Stroke. 2000;31(4):858-862.
17. Jauch EC, Saver JL, Adams HP Jr, et al; American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870-947.
18. Park HJ, Cho HJ, Kim YD, et al. Comparison of the characteristics for in-hospital and out-of-hospital ischaemic strokes. Eur J Neurol. 2009;16(5):582-588.
19. Messé SR, Fonarow GC, Smith EE, et al. Use of tissue-type plasminogen activator before and after publication of the European Cooperative Acute Stroke Study III in Get With the Guidelines-Stroke. Circ Cardiovasc Qual Outcomes. 2012;5(3):321-326.
20. Allen NB, Kaltenbach L, Goldstein LB, et al. Regional variation in recommended treatments for ischemic stroke and TIA: Get With the Guidelines—Stroke 2003-2010. Stroke. 2012;43(7):1858-1864.
21. Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke. 2005;36(12):2756-2763.
22. Bravata DM, Wells CK, Lo AC, et al. Processes of care associated with acute stroke outcomes. Arch Intern Med. 2010;170(9):804-810.
23. Mosley I, Nicol M, Donnan G, Patrick I, Dewey H. Stroke symptoms and the decision to call for an ambulance. Stroke; a journal of cerebral circulation. 2007;38(2):361-366.
24. Jurkowski JM, Maniccia DM, Dennison BA, Samuels SJ, Spicer DA. Awareness of necessity to call 9-1-1 for stroke symptoms, upstate New York. Prev Chronic Dis. 2008;5(2):A41.
Antiepileptic drug outcomes have remained flat for 3 decades
BANGKOK – Since founding the Epilepsy Unit at Glasgow’s Western Infirmary 37 years ago, Martin J. Brodie, MD, has seen many changes in the field, including the introduction of more than a dozen new antiepileptic drugs (AEDs) in the past 2 decades.
And based upon this vast clinical experience coupled with his leadership of landmark studies, he has a message for his physician colleagues and their epilepsy patients. And it’s not pretty.
“Has the probability of achieving seizure freedom increased significantly in the last 3 decades? Regrettably, the answer is no,” he declared at the International Epilepsy Congress.
“Over all these years, in terms of seizure freedom there has been no real difference in outcome. There’s really quite a long way to go before we can say that we are doing all that well for people,” he said at the congress sponsored by the International League Against Epilepsy.
In the year 2000, he and his coinvestigators published a prospective, longitudinal, observational cohort study of 470 newly diagnosed patients with epilepsy treated at the Western Infirmary during 1982-1997, all with a minimum of 2 years’ follow-up. Sixty-one percent achieved complete freedom from seizures for at least 1 year on monotherapy, and another 3% did so on polytherapy, for a total rate of 64% (N Engl J Med. 2000 Feb 3;342[5]:314-19).
But these were patients who by and large were treated with older AEDs such as carbamazepine, which has since fallen by the wayside because of toxicities. Scottish neurologists now generally turn to lamotrigine (Lamictal), levetiracetam (Spritam), and other, newer AEDs. So Dr. Brodie and his coworkers recently published a follow-up study, this one featuring 30 years of longitudinal follow-up of 1,795 patients newly treated for epilepsy with AEDs, new and old, during 1982-2012. The investigators demonstrated that the seizure-free survival curves over time were virtually superimposable. In the larger, more recent study, remission was achieved in 55% of patients with AED monotherapy and in another 9% with polytherapy, for a total rate of 64%, identical to the rate in the 2000 study, and as was the case in the earlier study, 36% of patients remained uncontrolled (JAMA Neurol. 2018 Mar 1;75[3]:279-86).
“Overall, the way this population behaves, there’s no difference in efficacy and no difference in tolerability whether you’re using old drugs used properly or new drugs used properly,” said Dr. Brodie, professor of neurology at the University of Glasgow (Scotland).
It’s noteworthy that Sir William R. Gowers, the Londoner who has been called the greatest neurologist of all time, reported a 70% seizure-free rate in 1881, while Dr. Brodie and workers achieved a 64% rate in their 30-year study. “It’s interesting that the numbers are so bad, really, I suppose,” Dr. Brodie commented.
How about outcomes in pediatric epilepsy?
Dr. Brodie and coworkers recently published a 30-year prospective cohort study of 332 adolescent epilepsy patients newly diagnosed and treated at the Western Infirmary during 1982-2012. At the end of the study, 67% were seizure-free for at least the past year, a feat accomplished via monotherapy in 83% of cases. The seizure-free rate was 72% in those with generalized epilepsy, significantly better than the 60% figure in those with focal epilepsy. The efficacy rate was 74% with newer AED monotherapy and similar at 77% with monotherapy older drugs. Adverse event rates ranged from a low of 12% with lamotrigine to 56% with topiramate (Topamax), according to the findings published in Epilepsia (2019 Jun;60[6]:1083-90).
Roughly similar outcomes have been reported from Norway in a study of 600 children with epilepsy, median age 7 years, with a median follow-up of 5.8 years that is considerably shorter than that in the Glasgow pediatric study. Overall, 59% of the Norwegian children remained seizure free for at least 1 year, 30% developed drug-resistant epilepsy, and 11% followed an intermediate remitting/relapsing course (Pediatrics. 2018 Jun. doi: 10.1542/peds.2017-4016).
Why the decades of flat pharmacologic outcomes?
The consistently suboptimal seizure-free outcomes obtained over the past 30 years shouldn’t really be surprising, according to Dr. Brodie.
“Although we think we have lots of mechanisms of action and lots of differences between the drugs, they’re arguably all antiseizure drugs and not antiepilepsy drugs. We don’t treat the whale; we treat the spout. We don’t treat what we cannot see; we treat what we can see, which is the seizures, but we’re not influencing the long-term outcome,” the neurologist explained.
The compelling case for early epilepsy surgery
Epilepsy surgery remains underutilized, according to Dr. Brodie and other experts.
The International League Against Epilepsy defines drug-resistant epilepsy as failure to achieve sustained seizure freedom after adequate trials of two tolerated and appropriately chosen and used AED schedules. Dr. Brodie’s work was influential in creating that definition because his data demonstrated the sharply diminishing returns of additional drug trials.
“When do we consider epilepsy surgery? Arguably, the earlier, the better. After two drugs have failed appropriately, I don’t think anybody in this room would argue about that, although people in some of the other rooms might,” he said at the congress.
Influential in his thinking on this score were the impressive results of an early study, the first-ever randomized trial of surgery for epilepsy. In 80 patients with a 21-year history of drug-refractory temporal lobe epilepsy who were randomized to surgery or 1 year of AED therapy, at 1 year of follow-up blinded epileptologists rated 58% of surgically treated patients as free from seizures that impair awareness of self and surroundings, compared with just 8% in the AED group (N Engl J Med. 2001 Aug 2;345[5]:311-8).
“That’s a big outcome, and I’m very keen to ensure that my data continue to drive the push for early surgery,” according to the neurologist.
A Cochrane review of 177 studies totaling more than 16,000 patients concluded that 65% of epilepsy patients had good outcomes following surgery. Prognostic factors associated with better surgical outcomes included complete surgical resection of the epileptogenic focus, the presence of mesial temporal sclerosis, concordance of MRI and EEG findings, and an absence of cortical dysplasia (Cochrane Database Syst Rev. 2019;6:CD010541. doi: 10.1002/14651858.CD010541.pub3).
In addition, a systematic review and meta-analysis by Canadian investigators found that 72% of adults with lesional epilepsy identified by MRI or histopathology were seizure-free after surgery, compared with 36% of those with nonlesional epilepsy. The disparity in outcomes was similar in pediatric epilepsy patients, with seizure freedom after surgery in 74% of those with lesional disease versus 45% with nonlesional epilepsy (Epilepsy Res. 2010 May;89[2-3]:310-8).
Whither are neurostimulatory device therapies headed?
Dr. Brodie was quick to admit that as a pharmacologic researcher, device modalities including vagus nerve stimulation, responsive neurostimulation, and deep brain stimulation are outside his area of expertise. But he’s been following developments in the field with interest.
“These device therapies have shown efficacy in short-term randomized trials, but very few patients attain long-term seizure freedom. I think these are largely palliative techniques. I gave up on these techniques a long time ago because I felt it was a very costly way of reducing seizures by a relatively small margin, and really we need to go a little bit further than that. But I know there’s a lot of work going on at the moment,” he said.
Dr. Brodie reported serving on the scientific advisory boards of more than a half dozen pharmaceutical companies.
BANGKOK – Since founding the Epilepsy Unit at Glasgow’s Western Infirmary 37 years ago, Martin J. Brodie, MD, has seen many changes in the field, including the introduction of more than a dozen new antiepileptic drugs (AEDs) in the past 2 decades.
And based upon this vast clinical experience coupled with his leadership of landmark studies, he has a message for his physician colleagues and their epilepsy patients. And it’s not pretty.
“Has the probability of achieving seizure freedom increased significantly in the last 3 decades? Regrettably, the answer is no,” he declared at the International Epilepsy Congress.
“Over all these years, in terms of seizure freedom there has been no real difference in outcome. There’s really quite a long way to go before we can say that we are doing all that well for people,” he said at the congress sponsored by the International League Against Epilepsy.
In the year 2000, he and his coinvestigators published a prospective, longitudinal, observational cohort study of 470 newly diagnosed patients with epilepsy treated at the Western Infirmary during 1982-1997, all with a minimum of 2 years’ follow-up. Sixty-one percent achieved complete freedom from seizures for at least 1 year on monotherapy, and another 3% did so on polytherapy, for a total rate of 64% (N Engl J Med. 2000 Feb 3;342[5]:314-19).
But these were patients who by and large were treated with older AEDs such as carbamazepine, which has since fallen by the wayside because of toxicities. Scottish neurologists now generally turn to lamotrigine (Lamictal), levetiracetam (Spritam), and other, newer AEDs. So Dr. Brodie and his coworkers recently published a follow-up study, this one featuring 30 years of longitudinal follow-up of 1,795 patients newly treated for epilepsy with AEDs, new and old, during 1982-2012. The investigators demonstrated that the seizure-free survival curves over time were virtually superimposable. In the larger, more recent study, remission was achieved in 55% of patients with AED monotherapy and in another 9% with polytherapy, for a total rate of 64%, identical to the rate in the 2000 study, and as was the case in the earlier study, 36% of patients remained uncontrolled (JAMA Neurol. 2018 Mar 1;75[3]:279-86).
“Overall, the way this population behaves, there’s no difference in efficacy and no difference in tolerability whether you’re using old drugs used properly or new drugs used properly,” said Dr. Brodie, professor of neurology at the University of Glasgow (Scotland).
It’s noteworthy that Sir William R. Gowers, the Londoner who has been called the greatest neurologist of all time, reported a 70% seizure-free rate in 1881, while Dr. Brodie and workers achieved a 64% rate in their 30-year study. “It’s interesting that the numbers are so bad, really, I suppose,” Dr. Brodie commented.
How about outcomes in pediatric epilepsy?
Dr. Brodie and coworkers recently published a 30-year prospective cohort study of 332 adolescent epilepsy patients newly diagnosed and treated at the Western Infirmary during 1982-2012. At the end of the study, 67% were seizure-free for at least the past year, a feat accomplished via monotherapy in 83% of cases. The seizure-free rate was 72% in those with generalized epilepsy, significantly better than the 60% figure in those with focal epilepsy. The efficacy rate was 74% with newer AED monotherapy and similar at 77% with monotherapy older drugs. Adverse event rates ranged from a low of 12% with lamotrigine to 56% with topiramate (Topamax), according to the findings published in Epilepsia (2019 Jun;60[6]:1083-90).
Roughly similar outcomes have been reported from Norway in a study of 600 children with epilepsy, median age 7 years, with a median follow-up of 5.8 years that is considerably shorter than that in the Glasgow pediatric study. Overall, 59% of the Norwegian children remained seizure free for at least 1 year, 30% developed drug-resistant epilepsy, and 11% followed an intermediate remitting/relapsing course (Pediatrics. 2018 Jun. doi: 10.1542/peds.2017-4016).
Why the decades of flat pharmacologic outcomes?
The consistently suboptimal seizure-free outcomes obtained over the past 30 years shouldn’t really be surprising, according to Dr. Brodie.
“Although we think we have lots of mechanisms of action and lots of differences between the drugs, they’re arguably all antiseizure drugs and not antiepilepsy drugs. We don’t treat the whale; we treat the spout. We don’t treat what we cannot see; we treat what we can see, which is the seizures, but we’re not influencing the long-term outcome,” the neurologist explained.
The compelling case for early epilepsy surgery
Epilepsy surgery remains underutilized, according to Dr. Brodie and other experts.
The International League Against Epilepsy defines drug-resistant epilepsy as failure to achieve sustained seizure freedom after adequate trials of two tolerated and appropriately chosen and used AED schedules. Dr. Brodie’s work was influential in creating that definition because his data demonstrated the sharply diminishing returns of additional drug trials.
“When do we consider epilepsy surgery? Arguably, the earlier, the better. After two drugs have failed appropriately, I don’t think anybody in this room would argue about that, although people in some of the other rooms might,” he said at the congress.
Influential in his thinking on this score were the impressive results of an early study, the first-ever randomized trial of surgery for epilepsy. In 80 patients with a 21-year history of drug-refractory temporal lobe epilepsy who were randomized to surgery or 1 year of AED therapy, at 1 year of follow-up blinded epileptologists rated 58% of surgically treated patients as free from seizures that impair awareness of self and surroundings, compared with just 8% in the AED group (N Engl J Med. 2001 Aug 2;345[5]:311-8).
“That’s a big outcome, and I’m very keen to ensure that my data continue to drive the push for early surgery,” according to the neurologist.
A Cochrane review of 177 studies totaling more than 16,000 patients concluded that 65% of epilepsy patients had good outcomes following surgery. Prognostic factors associated with better surgical outcomes included complete surgical resection of the epileptogenic focus, the presence of mesial temporal sclerosis, concordance of MRI and EEG findings, and an absence of cortical dysplasia (Cochrane Database Syst Rev. 2019;6:CD010541. doi: 10.1002/14651858.CD010541.pub3).
In addition, a systematic review and meta-analysis by Canadian investigators found that 72% of adults with lesional epilepsy identified by MRI or histopathology were seizure-free after surgery, compared with 36% of those with nonlesional epilepsy. The disparity in outcomes was similar in pediatric epilepsy patients, with seizure freedom after surgery in 74% of those with lesional disease versus 45% with nonlesional epilepsy (Epilepsy Res. 2010 May;89[2-3]:310-8).
Whither are neurostimulatory device therapies headed?
Dr. Brodie was quick to admit that as a pharmacologic researcher, device modalities including vagus nerve stimulation, responsive neurostimulation, and deep brain stimulation are outside his area of expertise. But he’s been following developments in the field with interest.
“These device therapies have shown efficacy in short-term randomized trials, but very few patients attain long-term seizure freedom. I think these are largely palliative techniques. I gave up on these techniques a long time ago because I felt it was a very costly way of reducing seizures by a relatively small margin, and really we need to go a little bit further than that. But I know there’s a lot of work going on at the moment,” he said.
Dr. Brodie reported serving on the scientific advisory boards of more than a half dozen pharmaceutical companies.
BANGKOK – Since founding the Epilepsy Unit at Glasgow’s Western Infirmary 37 years ago, Martin J. Brodie, MD, has seen many changes in the field, including the introduction of more than a dozen new antiepileptic drugs (AEDs) in the past 2 decades.
And based upon this vast clinical experience coupled with his leadership of landmark studies, he has a message for his physician colleagues and their epilepsy patients. And it’s not pretty.
“Has the probability of achieving seizure freedom increased significantly in the last 3 decades? Regrettably, the answer is no,” he declared at the International Epilepsy Congress.
“Over all these years, in terms of seizure freedom there has been no real difference in outcome. There’s really quite a long way to go before we can say that we are doing all that well for people,” he said at the congress sponsored by the International League Against Epilepsy.
In the year 2000, he and his coinvestigators published a prospective, longitudinal, observational cohort study of 470 newly diagnosed patients with epilepsy treated at the Western Infirmary during 1982-1997, all with a minimum of 2 years’ follow-up. Sixty-one percent achieved complete freedom from seizures for at least 1 year on monotherapy, and another 3% did so on polytherapy, for a total rate of 64% (N Engl J Med. 2000 Feb 3;342[5]:314-19).
But these were patients who by and large were treated with older AEDs such as carbamazepine, which has since fallen by the wayside because of toxicities. Scottish neurologists now generally turn to lamotrigine (Lamictal), levetiracetam (Spritam), and other, newer AEDs. So Dr. Brodie and his coworkers recently published a follow-up study, this one featuring 30 years of longitudinal follow-up of 1,795 patients newly treated for epilepsy with AEDs, new and old, during 1982-2012. The investigators demonstrated that the seizure-free survival curves over time were virtually superimposable. In the larger, more recent study, remission was achieved in 55% of patients with AED monotherapy and in another 9% with polytherapy, for a total rate of 64%, identical to the rate in the 2000 study, and as was the case in the earlier study, 36% of patients remained uncontrolled (JAMA Neurol. 2018 Mar 1;75[3]:279-86).
“Overall, the way this population behaves, there’s no difference in efficacy and no difference in tolerability whether you’re using old drugs used properly or new drugs used properly,” said Dr. Brodie, professor of neurology at the University of Glasgow (Scotland).
It’s noteworthy that Sir William R. Gowers, the Londoner who has been called the greatest neurologist of all time, reported a 70% seizure-free rate in 1881, while Dr. Brodie and workers achieved a 64% rate in their 30-year study. “It’s interesting that the numbers are so bad, really, I suppose,” Dr. Brodie commented.
How about outcomes in pediatric epilepsy?
Dr. Brodie and coworkers recently published a 30-year prospective cohort study of 332 adolescent epilepsy patients newly diagnosed and treated at the Western Infirmary during 1982-2012. At the end of the study, 67% were seizure-free for at least the past year, a feat accomplished via monotherapy in 83% of cases. The seizure-free rate was 72% in those with generalized epilepsy, significantly better than the 60% figure in those with focal epilepsy. The efficacy rate was 74% with newer AED monotherapy and similar at 77% with monotherapy older drugs. Adverse event rates ranged from a low of 12% with lamotrigine to 56% with topiramate (Topamax), according to the findings published in Epilepsia (2019 Jun;60[6]:1083-90).
Roughly similar outcomes have been reported from Norway in a study of 600 children with epilepsy, median age 7 years, with a median follow-up of 5.8 years that is considerably shorter than that in the Glasgow pediatric study. Overall, 59% of the Norwegian children remained seizure free for at least 1 year, 30% developed drug-resistant epilepsy, and 11% followed an intermediate remitting/relapsing course (Pediatrics. 2018 Jun. doi: 10.1542/peds.2017-4016).
Why the decades of flat pharmacologic outcomes?
The consistently suboptimal seizure-free outcomes obtained over the past 30 years shouldn’t really be surprising, according to Dr. Brodie.
“Although we think we have lots of mechanisms of action and lots of differences between the drugs, they’re arguably all antiseizure drugs and not antiepilepsy drugs. We don’t treat the whale; we treat the spout. We don’t treat what we cannot see; we treat what we can see, which is the seizures, but we’re not influencing the long-term outcome,” the neurologist explained.
The compelling case for early epilepsy surgery
Epilepsy surgery remains underutilized, according to Dr. Brodie and other experts.
The International League Against Epilepsy defines drug-resistant epilepsy as failure to achieve sustained seizure freedom after adequate trials of two tolerated and appropriately chosen and used AED schedules. Dr. Brodie’s work was influential in creating that definition because his data demonstrated the sharply diminishing returns of additional drug trials.
“When do we consider epilepsy surgery? Arguably, the earlier, the better. After two drugs have failed appropriately, I don’t think anybody in this room would argue about that, although people in some of the other rooms might,” he said at the congress.
Influential in his thinking on this score were the impressive results of an early study, the first-ever randomized trial of surgery for epilepsy. In 80 patients with a 21-year history of drug-refractory temporal lobe epilepsy who were randomized to surgery or 1 year of AED therapy, at 1 year of follow-up blinded epileptologists rated 58% of surgically treated patients as free from seizures that impair awareness of self and surroundings, compared with just 8% in the AED group (N Engl J Med. 2001 Aug 2;345[5]:311-8).
“That’s a big outcome, and I’m very keen to ensure that my data continue to drive the push for early surgery,” according to the neurologist.
A Cochrane review of 177 studies totaling more than 16,000 patients concluded that 65% of epilepsy patients had good outcomes following surgery. Prognostic factors associated with better surgical outcomes included complete surgical resection of the epileptogenic focus, the presence of mesial temporal sclerosis, concordance of MRI and EEG findings, and an absence of cortical dysplasia (Cochrane Database Syst Rev. 2019;6:CD010541. doi: 10.1002/14651858.CD010541.pub3).
In addition, a systematic review and meta-analysis by Canadian investigators found that 72% of adults with lesional epilepsy identified by MRI or histopathology were seizure-free after surgery, compared with 36% of those with nonlesional epilepsy. The disparity in outcomes was similar in pediatric epilepsy patients, with seizure freedom after surgery in 74% of those with lesional disease versus 45% with nonlesional epilepsy (Epilepsy Res. 2010 May;89[2-3]:310-8).
Whither are neurostimulatory device therapies headed?
Dr. Brodie was quick to admit that as a pharmacologic researcher, device modalities including vagus nerve stimulation, responsive neurostimulation, and deep brain stimulation are outside his area of expertise. But he’s been following developments in the field with interest.
“These device therapies have shown efficacy in short-term randomized trials, but very few patients attain long-term seizure freedom. I think these are largely palliative techniques. I gave up on these techniques a long time ago because I felt it was a very costly way of reducing seizures by a relatively small margin, and really we need to go a little bit further than that. But I know there’s a lot of work going on at the moment,” he said.
Dr. Brodie reported serving on the scientific advisory boards of more than a half dozen pharmaceutical companies.
EXPERT ANALYSIS FROM IEC 2019
Click for Credit: Predicting preeclampsia; MI & stroke post-cancer Dx; more
Here are 5 articles from the August issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Smoking cessation could delay or prevent rheumatoid arthritis
To take the posttest, go to: https://bit.ly/2YguN2r
Expires February 22, 2020
2. No increased pregnancy loss risk for women conceiving soon after stillbirth
To take the posttest, go to: https://bit.ly/2ZnMaLc
Expires March 4, 2020
3. Total plasma tau correlates with dementia onset, Alzheimer’s disease
To take the posttest, go to: https://bit.ly/2YeglYV
Expires March 9, 2020
4. MI, strokes spike during 30 days after cancer diagnosis
To take the posttest, go to: https://bit.ly/2GCKZAv
Expires March 12, 2020
5. Combination model predicts imminent preeclampsia
To take the posttest, go to: https://bit.ly/2LTohrO
Expires February 21, 2020
Here are 5 articles from the August issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Smoking cessation could delay or prevent rheumatoid arthritis
To take the posttest, go to: https://bit.ly/2YguN2r
Expires February 22, 2020
2. No increased pregnancy loss risk for women conceiving soon after stillbirth
To take the posttest, go to: https://bit.ly/2ZnMaLc
Expires March 4, 2020
3. Total plasma tau correlates with dementia onset, Alzheimer’s disease
To take the posttest, go to: https://bit.ly/2YeglYV
Expires March 9, 2020
4. MI, strokes spike during 30 days after cancer diagnosis
To take the posttest, go to: https://bit.ly/2GCKZAv
Expires March 12, 2020
5. Combination model predicts imminent preeclampsia
To take the posttest, go to: https://bit.ly/2LTohrO
Expires February 21, 2020
Here are 5 articles from the August issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Smoking cessation could delay or prevent rheumatoid arthritis
To take the posttest, go to: https://bit.ly/2YguN2r
Expires February 22, 2020
2. No increased pregnancy loss risk for women conceiving soon after stillbirth
To take the posttest, go to: https://bit.ly/2ZnMaLc
Expires March 4, 2020
3. Total plasma tau correlates with dementia onset, Alzheimer’s disease
To take the posttest, go to: https://bit.ly/2YeglYV
Expires March 9, 2020
4. MI, strokes spike during 30 days after cancer diagnosis
To take the posttest, go to: https://bit.ly/2GCKZAv
Expires March 12, 2020
5. Combination model predicts imminent preeclampsia
To take the posttest, go to: https://bit.ly/2LTohrO
Expires February 21, 2020